Abstract
The objective of this study was to evaluate the feasibility and safety of implementing two methods of in-bed mobilization in critically ill children. This prospective cohort trial was conducted at McMaster Children's Hospital, Pediatric Critical Care Unit (PCCU). Hemodynamically stable patients aged 3 to 17 years with a longer than 24-hour PCCU stay were eligible to participate in the study. Children with cardiorespiratory instability, already mobilizing well or at their baseline mobility, anticipated death during this PCCU admission, and those with contraindications to mobilization were excluded. Two methods of mobilization were applied for a maximum of 2 days, respectively, depending on the level of consciousness and cognitive ability of the participant. In-bed cycling was used for passive mobilization and interactive video games (VG) were used for active mobilization. The primary outcomes were safety and feasibility. Secondary outcomes were physical activity during the study period, as reflected by accelerometer measurements. A total of 406 patients were screened over 1 year, 35 of who were eligible and 31 (89%) consented to participate. Median age of participants was 11 years (quartile 1 is 6 years and quartile 3 is 14 years), and 15 (48%) were male. Twenty-five (81%) participants received the study intervention, 22 (88%) of who received the intervention within 24 hours of consent. Twenty-one (84%) participants received in-bed cycling, five (20%) received VG, and only one received both. Fifteen (60%) completed the prescribed 2-day intervention, while in 11 (44%) the intervention was interrupted or not applied, most commonly because the patient was transferred out of the PCCU. Physical activity was greater during the intervention compared with nonintervention times with in-bed cycling, but not with VG. There were no adverse events attributable to the intervention. This pilot reveals that in-bed cycling can enhance physical activity, and appears to be safe and feasible in this group of critically ill children. VG was feasible only in a minority of patients who were cooperative and age appropriate. Further research is necessary to evaluate the efficacy and most appropriate methods of enhancing mobility and rehabilitation in this population.
Keywords: critical care, pediatrics, mobilization, rehabilitation, interactive video game, cycle ergometry, feasibility, safety
Introduction
There is an accumulating body of evidence supporting the efficacy of mobility-based physical therapy in the prevention of intensive care unit–acquired morbidities and improving functional outcomes in critically ill adults.1 2 Several systematic reviews of randomized controlled trials have demonstrated that early mobilization using in-bed cycling in critically ill adults is safe, feasible, cost-effective, and improves short- and long-term patient outcomes.3 4 These findings have prompted recommendations that early mobilization should be implemented as a practice priority among adult intensive care units.5
In contrast, there is limited research on mobilization interventions in the critically ill pediatric population. Published evidence is limited to case reports and pilot data.6 7 The majority of physical therapy in critically ill children is nonmobility based, most commonly in the form of chest physiotherapy, which has not been shown to improve outcomes in critically ill children.8 9 Barriers to mobilizing critically ill children include patient safety concerns, the feasibility of applying interventions to enhance mobilization, when mobilization should occur, and who may benefit most from early mobilization.10 Pediatric Critical Care Unit (PCCU) clinicians do not feel comfortable mobilizing critically ill children in bed who are sedated, on mechanical ventilation, and have invasive devices in situ. Further challenges are that these children represent a heterogeneous population with diverse cognitive and functional abilities, as such their ability to comply with mobilization activities is variable. The primary objective of this study was to evaluate the feasibility and safety of implementing mobility-based rehabilitation using a combination of individualized active and passive interventions to enhance upper and lower limb mobility, in critically ill children.
Materials and Methods
Study Design and Participants
This prospective cohort trial was conducted at McMaster Children's Hospital PCCU following approval from the Hamilton Health Sciences Research Ethics Board. Consecutive patients aged 3 to 17 years admitted to the PCCU with an anticipated length of stay of longer than 24 hours were screened for eligibility and approached for informed consent and or assent where appropriate. We excluded patients with cardiorespiratory instability (e.g., unstable airway, progressive shock), those who were already mobilizing well or at their baseline level of function, patients in whom death was anticipated during this PCCU admission and/or withholding of life-sustaining therapy was considered, and those with a clear contraindication to mobilization (e.g., suspected or actual evidence of cerebral edema, elevated intracranial pressure [ICP], unstable spinal cord injuries, musculoskeletal injuries, surgery/fixed deformities on the extremities).
Our primary outcomes were feasibility and safety. Feasibility was defined as the ability to screen, enroll, and apply the intervention within 24 hours of consent. Safety was evaluated by the rate of adverse events attributable to the intervention, such as cardiorespiratory instability (i.e., sustained hypo/hypertension, bradycardia, oxygen desaturation), accidental tube dislodgment, pain, discomfort, or injury attributable to the intervention. Secondary outcomes were the degree of mobility during the intervention, as measured by the change in limb activity during the intervention compared with nonintervention time. We also measured the cardiorespiratory response during the intervention.
Study Intervention
We evaluated two methods of in-bed mobilization in this feasibility trial, which were applied based on the participant's level of consciousness and cognitive ability at the time of the intervention. Patients not able to comply because of depressed level of consciousness and/or cognitive age received passive lower limb in-bed supine cycling, using a cycle ergometer. Two types of cycle ergometers were used: Ex N' Flex EF-300 for children aged 3 to 7 years and MOTOmed Letto2 for patients aged 8 to 17 years. Cycling may be applied in a passive or active manner with these ergometers. In this feasibility study, we chose to apply in-bed cycling in a passive manner.11 Lower limb range of motion was assessed in each patient by the physiotherapist (H. C.), prior to applying the cycle ergometer. The cycle ergometer was set up and monitored by a member of the research team (M. C., R. W.) and overseen by the physiotherapist (H. C.).
Conscious and cooperative participants were prescribed active upper limb in-bed mobilization using interactive video gaming (VG). For the purposes of this study, we offered games that were age appropriate and could be applied in recumbent patients (Nintendo WiiTM Sport Pack and Mario Kart). Each passive and active mobilization intervention was applied for a maximum of 2 days, respectively. Interventions were applied for a minimum of 10 minutes and up to 20 minutes on the first day to ensure safety and tolerance, and for 20 minutes on day 2. As the ideal duration of exercise has not been established in this population, we based the duration of activity on previous literature in critically ill adults and safety considerations.12 Participants had the option of continuing with the VG for longer than the prescribed duration if they desired. Participants who received the passive intervention first who subsequently became conscious enough for the active intervention received the latter as soon as they were able to comply (Fig. 1). The setup time and total duration of application of each intervention were recorded.
Fig. 1.
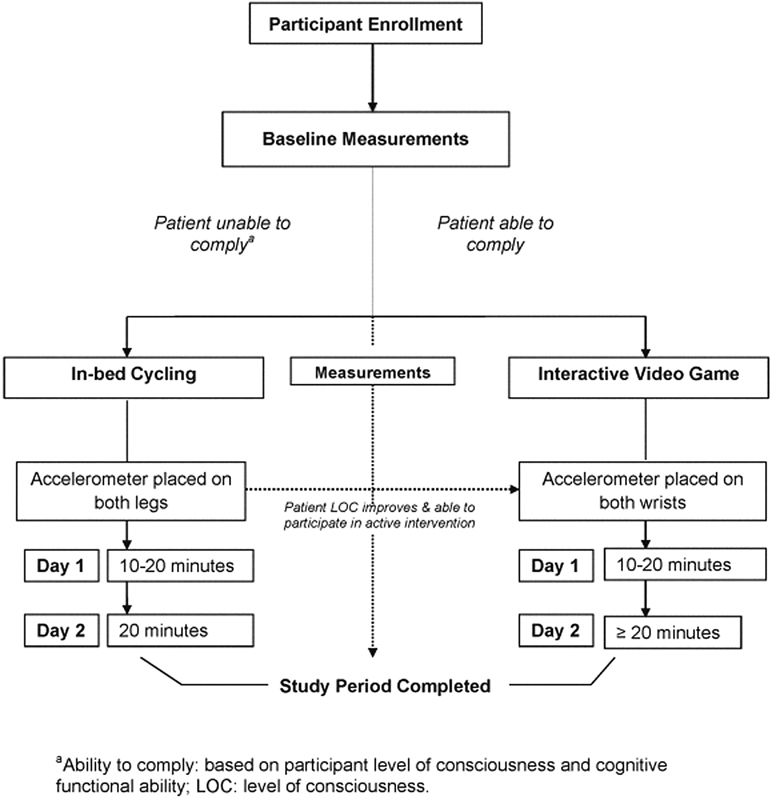
Study intervention. aAbility to comply: based on participant level of consciousness and cognitive functional ability. LOC, level of consciousness.
Predefined safety criteria to interrupt the intervention were as follows: patient intolerance defined by physiological parameters (i.e., persistent hypo- or hypertension, or brady- or tachycardia, oxygen desaturation < 85%, or increased work of breathing), pain and/or discomfort (i.e., change in the pain score or the need for more than one dose of analgesia greater than the patient's usual as needed dosing), and safety (i.e., tube or catheter dislodgement). The study intervention was applied in addition to usual care physical therapy in the PCCU.
Measurements
Demographic and patient outcomes data were collected on standardized case report forms by the investigators (M. C., R. W.). The Pediatric Cerebral Performance Category and Pediatric Overall Performance Category scores were used to determine cognitive and functional ability, respectively.13 Severity of illness was measured using the Pediatric Risk of Mortality and Pediatric Logistic Organ Dysfunction scores.14 15 Concurrent therapies and any additional types of rehabilitation such as physiotherapy, applied in addition to the study interventions, were recorded. We categorized physiotherapy according to mobility and nonmobility interventions (Appendix 1). Physiologic measurements such as heart rate, blood pressure, respiratory rate, pulse oximetry, and fraction of inspired oxygen were recorded at baseline, during and following each intervention period.
Appendix 1. Definitions for physiotherapy interventions.
| Definitions | Description |
|---|---|
| Nonmobility therapies | |
| Respiratory or “chest physiotherapy” | Physical methods to improve ventilation, ventilation/perfusion matching, breathing mechanics, airway secretions clearance—e.g., percussion techniques, manual facilitation of chest wall movement, and deep breathing exercises (including blowing bubbles and incentive spirometry) |
| Passive range of motion | Includes passive repositioning of patient, or passive stretching of their limbs and joints. Passive = patient does not voluntarily participate in the activity |
| Mobility therapies | |
| Active range of motion or strengthening exercises | Muscle strengthening exercises with therapist. This may be described as “active” or “active-assisted” exercises. “Active” infers patient participation, no matter how little. This may include exercises and stretches that are taught to patient to do independently. Includes neurodevelopmental play: play activities to facilitate fine and gross motor development for infants and developmentally delayed children |
| Mobility device | Activities done with a device that facilitates limb movement, i.e., cycle ergometer. Maybe done while patient is recumbent |
| Bed mobility | Activities done while patient is recumbent—but requires active participation of the patient; e.g., active or active-assisted repositioning in bed; rolling from side to side; and bridging (i.e., pelvic or hip lifts) |
| Transfers | Patient actively transfers from one surface to the other, e.g., from bed to chair/commode, sitting or dangling on edge of bed, unsupported sitting or sitting with trunk control, assisting from a sitting to a standing position. These activities may occur with or without therapist assistance |
| Pre-gait activities | Assisting the patient in exercises prior to ambulation, e.g., weight shifting from foot to foot, stepping in place, and sideways stepping |
| Ambulation | Gait training of the patient, with or without assistance by therapist or device (e.g., walker) |
We measured upper and lower extremity movement during the study intervention, using Actigraph GT3X accelerometers attached to the participants' wrists and ankles. This is a widely accepted method of measuring physical activity in response to rehabilitation interventions.16 Activity counts were recorded in 3-second sampling intervals (i.e., epochs, time period over which activity counts recorded by the accelerometer are summed) and data were downloaded and processed at the end of each study period according to standard procedures previously described by our group.17 Mean activity counts recorded during the 20-minute intervention period were compared with the highest 20 minutes of activity counts achieved during the remainder of the day.
Statistical Analyses
Given our feasibility objectives, we planned to evaluate the number of participants who could be enrolled over a 12-month period. Descriptive summaries were used to present baseline demographics; categorical data are reported as percentages, and continuous data as mean ± standard deviation (SD) or median (quartile 1 [Q1], quartile 3 [Q3]) where appropriate. We reported upper- and lower-limb mobility as raw activity counts. Upper- and lower-limb mobility during the intervention period was compared with nonintervention periods via an independent samples t-test. One-way repeated measures ANOVA was used to assess changes in physiological parameters at baseline, at 5 and 10 minutes of exercise, and at recovery. All statistical analyses were conducted using SPSS (IBM SPSS Statistics for Windows, Version 22.0, IBM Corp, Armonk, New York, United States) and statistical significance was set at p ≤ 0.05.
Results
This trial was conducted from June 2012 to June 2013. During this period, 406 critically ill children were admitted to the PCCU and screened for eligibility. Thirty-five children were eligible and approached, of whom, 31 (89%) consented to participate (Fig. 2). The most common reason for ineligibility was young age. The demographic data of study participants are presented in Table 1. The median age was 11 years (6 years, 14 years), and 15 (48%) participants were male (Table 1). Twelve (39%) participants had preexisting comorbidities, and 20 (65%) had a functional and/or cognitive limitation at baseline (as determined by a Pediatric Overall Performance Category or Pediatric Cerebral Performance Category score of ≥ 1 prior to PCCU admission).
Fig. 2.
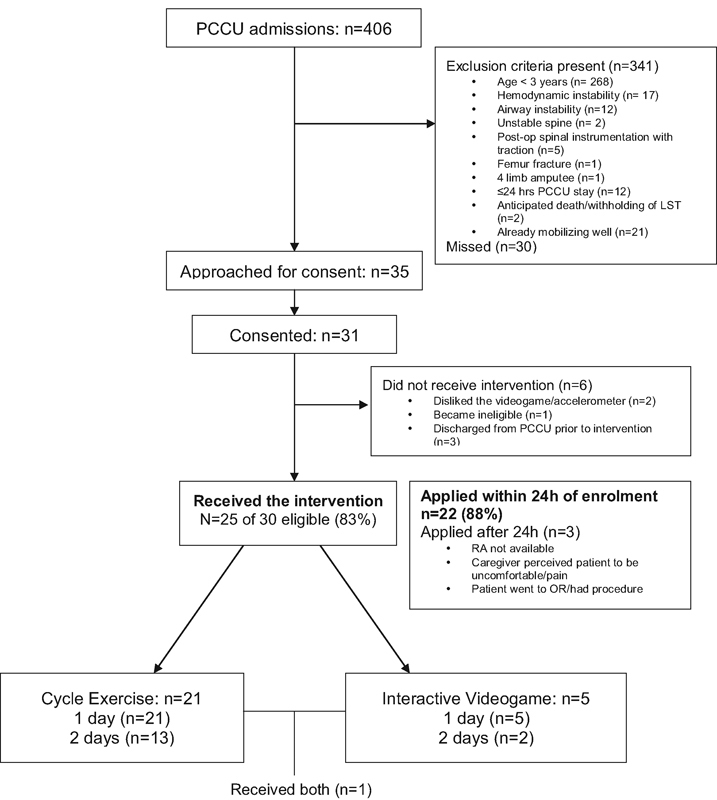
Study flowchart. MSK, musculoskeletal; OR, operating room; PCCU, pediatric critical care unit; RA, research assistant.
Table 1. Participant demographics.
| Demographic variable | Number of participants (n = 31) |
|---|---|
| Age (y); median (quartile 1, quartile 3) | 11 (6, 14) |
| Gender (male); n (%) | 15 (48%) |
| Weight (kg); median (minimum, maximum) | 29.7 (20.1, 47.8) |
| Primary reason for pediatric critical care unit | n (%) |
| Respiratory failure (including respiratory tract infections) | 4 (13) |
| Sepsis | 4 (13) |
| Shock | 4 (13) |
| Trauma | 3 (10) |
| Neurologic | 5 (16) |
| Postsurgical intervention | 7 (23) |
| Other | 4 (13) |
| Severity of illness scores | Median (quartile 1, quartile 3) |
| Pediatric risk of mortality III scorea on admission | 6 (0, 8) |
| Pediatric logistic organ dysfunction scoreb on admission | 2 (1, 11) |
| Pediatric logistic organ dysfunction score at enrolment (n = 25) | 1 (0, 11.5) |
| Preexisting comorbidity/chronic condition; n (%) | 12 (39%) |
| Baselinec pediatric cerebral performance category score; median (quartile 1, quartile 3) | 3 (1, 4) |
| Distribution of pediatric cerebral performance category scores d | n (%) |
| Good overall performance (1) | 11 (35) |
| Mild overall disability (2) | 2 (6.4) |
| Moderate overall disability (3) | 7 (23) |
| Severe disability (4) | 11 (35) |
| Coma or vegetative state (5) | 0 |
| Baseline pediatric overall performance category score; median (quartile 1, quartile 3) | 2 (1, 5) |
| Distribution of pediatric overall performance category scoresd | n (%) |
| Normal | 15 (48) |
| Mild disability | 1 (3) |
| Moderate disability | 3 (10) |
| Severe disability | 10 (32) |
| Coma or vegetative state | 2 (6) |
Assessment range 0–74, with higher scores indicating a greater risk of death.
Assessment range 0–71, with higher scores indicating more severe organ dysfunction.
Baseline denotes their premorbid condition prior to pediatric critical care unit admission.
Assessment range for pediatric overall performance category and pediatric cerebral performance category scores is 1–7, with increasing scores indicating increasing disability (e.g., 1 = normal vs. 7 = cardiorespiratory death). Functional limitation was defined as a pediatric overall performance category score >1; cognitive limitation was defined as a pediatric cerebral performance category score >1.
Feasibility Outcomes
Twenty-five (81%) of the 31 patients who consented to participate received the study intervention, 21 of who (84%) received only in-bed cycling, 5 (20%) received VG, and 1 received both (Fig. 2). Of the six patients who consented but never received the intervention, three were discharged from PCCU prior to the intervention, one did not like the choice of VGs, one did not like applying the accelerometer, and one became hemodynamically unstable and subsequently progressed to brain death and was therefore no longer eligible. The median time from admission to enrolment was 4 days (2 days, 10 days). Of the 25 patients who received the intervention, 22 (88%) did so within 24 hours of consent, while in 3 patients the intervention was delayed until after 24 hours due to patient discomfort, transfer to the operating room, and unavailability of research staff to apply the intervention, respectively. In one patient, the intervention was not applied until 7 days later owing to parental request to delay initiation of the intervention and subsequent research assistant unavailability.
The interventions were applied for the full 2 days in 13 (62%) participants who received in-bed cycling and 2 (40%) participants who received VG. Reasons for why the intervention could not be applied for the full 2 days are outlined in Table 2. In-bed cycling was applied for a median of 20 minutes (range: 10–22 minutes), and VG was applied for 19 minutes (range: 15–21 minutes) during the intervention period. The median time taken to set up and prepare a patient for the intervention was 14 minutes (9 minutes, 17 minutes) for cycle ergometry, and 12 minutes (11 minutes, 14 minutes) for VG, respectively.
Table 2. Feasibility of applying each intervention.
| Reasons why intervention could not be re-applied on day 2 | In-bed cycling (n = 8) n (%) |
Interactive video game (n = 3) n (%) |
|---|---|---|
| Transferred out of the pediatric critical care unit | 4 (50) | 2 (67) |
| Technical difficultiesa | 2 (25) | |
| Parent perceived patient to be uncomfortable | 1 (12.5) | |
| Patient resisting the device | 1 (12.5) | |
| Patient taken to operating room | 1 (33) |
Technical difficulties were due to patient body size (i.e., too small or too large).
Concurrent Physiotherapy
All 25 participants who received the intervention also received physiotherapy while in the PCCU, and 22 (88%) participants received physiotherapy prior to the study intervention. The most common type of physiotherapy was chest physiotherapy (n = 14, 56%) and passive range of motion (n = 20, 80%). Only five (20%) participants received mobility-based physiotherapy during the study period. The time from PCCU admission to initiation of physiotherapy in the study cohort is outlined in Table 3.
Table 3. Time from pediatric critical care unit admission to physiotherapy; days [median, (quartile 1, quartile 3)].
| Physiotherapy modality | All patients (n = 25) | In-bed cycling (n = 21) | Video gamea (n = 4) |
|---|---|---|---|
| Nonmobilityb physiotherapy (n = 20) | 2.5 (2, 3.75) | 3 (2, 4.5) | 2 (0, 2) |
| Mobilityc physiotherapy (n = 5) | 4 (0, 6) | 5 (4, 6) | 0 |
| Time to study intervention | 5 (3, 10) | 5 (3, 10) | 3 (2.5, 47.5) |
Includes the total number of participants who used each specific intervention including one patient who completed both interventions.
Nonmobility physiotherapy includes chest physiotherapy and passive range of motion.
Physiotherapy includes bed mobility, sitting at the edge of bed, transfers to chair or ambulation (Appendix 1). Participants may have received more than one type of physiotherapy.
Safety
Concurrent therapies at the time of the intervention are presented in Table 4. Of note, 19 (90%) participants were sedated, 12 (57%) were mechanically ventilated, 3 (14%) were receiving continuous renal replacement therapy, and 2 (10%) were on vasoactive infusions at the time of in-bed cycling. Two (10%) participants had ICP monitors in situ during in-bed cycling, which did not record any elevations in their ICP during the intervention. Two additional participants with stimulus-sensitive seizures were receiving continuous electroencephalographic monitoring during in-bed cycling and did not experience any seizures during the intervention. There were no accidental extubations, tube dislodgements, cardiorespiratory instability, and physical or cutaneous injuries that occurred as a result of the study. Three mechanically ventilated patients required a single bolus of sedation during in-bed cycling, reportedly for “excessive movements of their limbs.” After a period of VG, two postoperative patients complained of some pain at their surgical site, and one patient with rectal prolapse complained of fatigue and discomfort. There were no statistical differences in blood pressure, heart rate, respiratory rate, or pulse oximetry measurements during exercise or recovery, compared with baseline.
Table 4. Cointerventions.
| Present during intervention | In-bed cycling (n = 21) n (%) |
Video game (n = 4) n (%) |
|---|---|---|
| Mechanically ventilated | 12 (57) | 1 (25) |
| Continuous sedative infusion | 19 (90) | 4 (100) |
| Receiving neuromuscular blockers | 1 (5) | 0 (0) |
| Receiving vasoactive infusions | 2 (10) | 1 (25) |
| Total parenteral nutrition | 3 (14) | 2 (50) |
| Continuous renal replacement therapy | 3 (14) | 0 (0) |
| Tubes/drains in situ | 21 (100) | 4 (100) |
| External ventricular drain/intracranial pressure monitor | 2 (10) | 0 (0) |
| Chest tube | 1 (5) | 1 (25) |
| Central venous line | 9 (43) | 3 (75) |
| Arterial line | 4 (19) | 1 (25) |
| Feeding tube | 15 (71) | 3 (75) |
Activity
Upper- and lower-limb activity during intervention and nonintervention periods is presented in Fig. 3. Mean lower-limb activity was significantly greater during in-bed cycling compared with the highest 20 minutes of activity during nonintervention time (mean ± SD: 266.47 ± 166.12 vs. 20.94 ± 15.26 counts/20 minutes, p < 0.001). However, mean upper-limb activity was significantly lower during VG as compared with the highest 20 minutes of activity during nonintervention time (mean ± SD: 27.31 ± 12.55 vs. 51.32 ± 23.68 counts/20 minutes, p < 0.05).
Fig. 3.
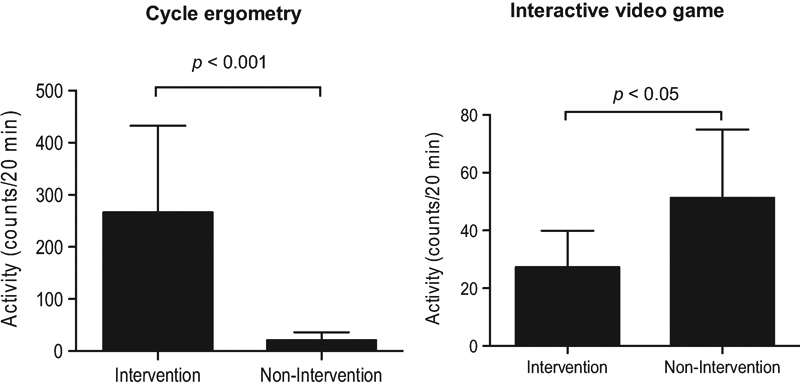
Limb activity during intervention and nonintervention periods. Data are presented as mean ± SD.
Discussion
Enhancing physical activity in critically ill patients has been a subject of great interest in the adult literature since the evidence of its safety and feasibility emerged in 2007, and subsequent efficacy data on improving short- and long-term functional recovery in adults were demonstrated in 2009.11 18 19 Interventions studied to date have focused on mobilizing these patients as soon as possible, progressing from passive in-bed cycling in the unconscious, uncooperative patient through graded increases in active and/or resistance training and ultimately ambulation in increasingly cooperative patients.20 This study is the first, to our knowledge, to prospectively evaluate the safety and feasibility of enhancing in-bed mobilization using two strategies in critically ill children. We selected cycling and interactive VG, as these are two effective methods of rehabilitation used in other inpatient adult and pediatric populations.21 22 We determined that the 20-minute maximum duration for the interventions based on safety considerations and evidenced from critically ill adults.23 Our results demonstrate the following: (1) in-bed cycling increases lower-limb activity and can be safely applied in a variety of uncooperative, supine, critically ill children; (2) interactive VG used to enhance upper-limb mobility is feasible only in a minority of PCCU patients; and (3) by the time VG can be applied, it may not enhance upper-limb activity nor supplement mobility physiotherapy in this population. Almost all patients had invasive lines in situ or were sedated, and more than half of them were intubated and mechanically ventilated during the study intervention. In-bed cycling did not cause any cardiorespiratory instability or adverse events in this study cohort. We were also able to observe that during continuous monitoring, in-bed cycling did not increase ICP, interfere with continuous renal replacement therapy, or exacerbate seizures in a small sample of patients.
Our study cohort consisted of children with significant severity of illness on admission, and a significant proportion had preexisting comorbid conditions, functional or cognitive disabilities. These pose significant challenges when trying to encourage any form of mobilization in the PCCU,10 and explains why only a minority of patients were able to comply with the VG portion in this study. Children need to be cognitively alert and able to comply with VG. By the time this occurs, these children are ready for mobility physiotherapy and are close to PCCU discharge, which explains why most of these patients did not complete their second day of this intervention, and why VG did not increase upper-limb mobility compared with nonintervention periods of the day. Equipment was the major limitation to the feasibility of in-bed cycling in this trial—we could not enroll 66% of screened patients due to their small size. We used a cycle ergometer designed for adults (Letto2) in this study, as there are currently no supine models specifically designed for pediatrics. We therefore adapted a pediatric model for supine use (EF-300) in children younger than 8 years. Furthermore, these ergometers were appropriate only for lower limb use. While setup time decreased as the study progressed, most of the time was spent in appropriately positioning the child.
Almost all of the participants received physiotherapy prior to the intervention, which was most commonly nonmobility based. We were therefore able to demonstrate that in the majority of participants, we could quantifiably enhance mobilization using in-bed cycling, but not VG. In-bed cycling appeared to be implemented earlier than mobility physiotherapy (PT), but we interpret these results with caution in this small pilot, and are unsure of how PT practice was influenced in this open-label study as usual care was not standardized.
While this may be the first prospective trial to evaluate interventions to enhance in-bed mobilization in critically ill children, we acknowledge the following limitations. First, we did not design this as an early intervention. Adult evidence suggests that the benefits of mobilization on functional recovery may be more significant the earlier it is applied during critical illness.2 While we were able to apply the mobilization intervention promptly after enrollment in the majority of participants, we did not assess the feasibility of applying mobilization as early as possible following PCCU admission, for reasons of safety. As we did not mandate the timing of intervention following consent, the majority of our delays were related to caregiver concerns, physician preference, and availability of research staff. As safety in this population has not previously been established, we did not start the intervention until both physician and caregivers were comfortable. Subsequently, the study intervention was applied a median of 5 days after PCCU admission, and six participants were discharged prior to completing the second day of their intervention. This study was a first step in evaluating appropriate patient selection criteria, cardiorespiratory stability, and potential adverse events during in-bed exercise interventions in critically ill children. Now that our results have provided some evidence of safety and feasibility, future pediatric interventional trials may be designed to evaluate the efficacy of early initiation of mobilization in this population.
Second, our selection of interactive VG games may be responsible for the lack of increase in physical activity. Previous evidence suggests that activity levels during VG are highly variable, and may be lower in those promoting primarily upper body movements compared with those that engage the lower body.24 In a previous study, we observed that performance and intensity of activity using VG was dependent on the child's ability to understand the game, previous exposure, and the degree of enjoyment with the particular VG.7 It was challenging to find age-appropriate games that encouraged bilateral upper-limb exercise. While there are certain games that facilitate greater limb activity such as those in the Sport Pack, we balanced this with games that we felt children would be familiar with and therefore enjoy, yet may be applied in the recumbent position. We did assess other available forms of interactive VG (e.g., Xbox Kinect) prior to the start of this trial; however, we chose the WiiTM, as this console provided the most reliable interface in a recumbent patient. In-bed cycling technology is advancing and can be used both in a passive and active modes.
We were able to demonstrate in this small cohort that in-bed cycling produced higher activity than nonactivity periods even when patients become more alert and begin mobility PT. However, we did not assess the use of in-bed cycling as an active mode of exercise. We feel, therefore, that in-bed cycling should not be restricted to a passive mode in future trials, as it may be effective in enhancing and engaging active participation in exercise as they start to recover from their critical illness. As rehabilitation is a continuum that should begin early in one's PCCU stay but extend beyond the PCCU, exercise-based physical therapy should continue after PCCU discharge.
Finally, we did not evaluate clinically important outcomes in this pilot trial, as our primary objective was to evaluate feasibility and safety. We therefore chose not to perform measurements of muscle power or strength, as this has poor feasibility in the majority of critically ill children, and has not been demonstrated to be a good surrogate measure for functional recovery.25 26 The question regarding the efficacy of early mobilization on functional outcomes in critically ill children remains to be answered. Our results do, however, provide us with some insights into patient selection, and informs the design of future trials on early mobilization. Once we understand the nature and predictors of functional recovery in children, then we can risk stratify patients and evaluate those who may benefit most from early mobilization.
Conclusion
Critically ill children are a heterogeneous population with significant comorbidities. Their need for physical therapy and rehabilitation is high; however, the resources available to support their needs are limited.27 While functional outcome is increasingly recognized an important patient-centered outcome that should be the focus of pediatric trials, there is a paucity of prospective research on physical rehabilitation interventions in critically ill children.28 In-bed cycling can be used to facilitate activity and mobilization in critically ill children. This trial allowed us to identify safety and feasibility issues while piloting novel methods of mobilizing critically ill children. Through gaining more evidence on the safety, appropriateness, and efficacy of different physical therapy modalities, we can then educate clinicians and affect a culture change among PCCUs to embrace early exercise in critically ill children. Pediatric-specific research is needed to risk stratify children who may be at the greatest risk of PCCU-acquired morbidities, evaluate appropriate patient-centered outcomes, select the most appropriate forms of rehabilitation, and implement these early in the course of critical illness, when there may the best opportunity for benefit.
Acknowledgments
We sincerely thank patients and families for their participation in this study, PCCU staff and nurses for their assistance during the recruitment process, and Dr. Haima Etorki for her assistance with the data collection.
Authors' Contributions
K. C. and M. D. P. C. were responsible for the study concept, design, and methodology and both oversaw the conduct and execution of the study. M. D. P. C. and R. G. W. were responsible for implementation of the study protocol, coordinating the data collection, and performing statistical analyses. K. C., M. C., and R. W. drafted and revised the manuscript. All authors contributed to revisions and approval of the final version of the manuscript.
References
- 1.Lee C M, Fan E. ICU-acquired weakness: what is preventing its rehabilitation in critically ill patients? BMC Med. 2012;10:115. doi: 10.1186/1741-7015-10-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calvo-Ayala E, Khan B A, Farber M O, Ely E W, Boustani M A. Interventions to improve the physical function of ICU survivors: a systematic review. Chest. 2013;144(5):1469–1480. doi: 10.1378/chest.13-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler J, Malone D. Early mobilization in the intensive care unit: a systematic review. Cardiopulm Phys Ther J. 2012;23(1):5–13. [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Peng X, Zhu B, Zhang Y, Xi X. Active mobilization for mechanically ventilated patients: a systematic review. Arch Phys Med Rehabil. 2013;94(3):551–561. doi: 10.1016/j.apmr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Stiller K. Physiotherapy in intensive care: an updated systematic review. Chest. 2013;144(3):825–847. doi: 10.1378/chest.12-2930. [DOI] [PubMed] [Google Scholar]
- 6.Zebuhr C, Sinha A, Skillman H, Buckvold S. Active rehabilitation in a pediatric extracorporeal membrane oxygenation patient. PM R. 2014;6(5):456–460. doi: 10.1016/j.pmrj.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Abdulsatar F, Walker R G, Timmons B W, Choong K. “Wii-Hab” in critically ill children: a pilot trial. J Pediatr Rehabil Med. 2013;6(4):193–204. doi: 10.3233/PRM-130260. [DOI] [PubMed] [Google Scholar]
- 8.Choong K, Foster G, Fraser D D. et al. Acute rehabilitation practices in critically ill children: a multicenter study. Pediatr Crit Care Med. 2014;15(6):e270–e279. doi: 10.1097/PCC.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 9.Argent A C, Morrow B. Chest physiotherapy: how does it work (if it does)? Pediatr Crit Care Med. 2012;13(2):238–239. doi: 10.1097/PCC.0b013e3182257a6e. [DOI] [PubMed] [Google Scholar]
- 10.Choong K, Koo K K, Clark H. et al. Early mobilization in critically ill children: a survey of Canadian practice. Crit Care Med. 2013;41(7):1745–1753. doi: 10.1097/CCM.0b013e318287f592. [DOI] [PubMed] [Google Scholar]
- 11.Burtin C, Clerckx B, Robbeets C. et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit Care Med. 2009;37(9):2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 12.Kress J P Clinical trials of early mobilization of critically ill patients Crit Care Med 200937(10, Suppl):S442–S447. [DOI] [PubMed] [Google Scholar]
- 13.Fiser D H, Long N, Roberson P K, Hefley G, Zolten K, Brodie-Fowler M. Relationship of pediatric overall performance category and pediatric cerebral performance category scores at pediatric intensive care unit discharge with outcome measures collected at hospital discharge and 1- and 6-month follow-up assessments. Crit Care Med. 2000;28(7):2616–2620. doi: 10.1097/00003246-200007000-00072. [DOI] [PubMed] [Google Scholar]
- 14.Pollack M M, Patel K M, Ruttimann U E. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Leteurtre S, Martinot A, Duhamel A. et al. Validation of the paediatric logistic organ dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 16.Noorkõiv M, Rodgers H, Price C I. Accelerometer measurement of upper extremity movement after stroke: a systematic review of clinical studies. J Neuroeng Rehabil. 2014;11:144. doi: 10.1186/1743-0003-11-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Obeid J, Nguyen T, Gabel L, Timmons B W. Physical activity in Ontario preschoolers: prevalence and measurement issues. Appl Physiol Nutr Metab. 2011;36(2):291–297. doi: 10.1139/h11-002. [DOI] [PubMed] [Google Scholar]
- 18.Bailey P, Thomsen G E, Spuhler V J. et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 19.Schweickert W D, Pohlman M C, Pohlman A S. et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373(9678):1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gosselink R, Clerckx B, Robbeets C, Vanhullebusch T, Vanpee G, Segers J. Physiotherapy in the intensive care unit. Neth J Crit Care. 2011;15(2):66–75. [Google Scholar]
- 21.Kho M E, Damluji A, Zanni J M, Needham D M. Feasibility and observed safety of interactive video games for physical rehabilitation in the intensive care unit: a case series. J Crit Care. 2012;27(2):2190–2.19E8. doi: 10.1016/j.jcrc.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Taylor M J, McCormick D, Shawis T, Impson R, Griffin M. Activity-promoting gaming systems in exercise and rehabilitation. J Rehabil Res Dev. 2011;48(10):1171–1186. doi: 10.1682/jrrd.2010.09.0171. [DOI] [PubMed] [Google Scholar]
- 23.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41(6):1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 24.Biddiss E, Irwin J. Active video games to promote physical activity in children and youth: a systematic review. Arch Pediatr Adolesc Med. 2010;164(7):664–672. doi: 10.1001/archpediatrics.2010.104. [DOI] [PubMed] [Google Scholar]
- 25.Hough C L, Lieu B K, Caldwell E S. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15(1):R43. doi: 10.1186/cc10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waak K, Zaremba S, Eikermann M. Muscle strength measurement in the intensive care unit: not everything that can be counted counts. J Crit Care. 2013;28(1):96–98. doi: 10.1016/j.jcrc.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 27.Cremer R Leclerc F Lacroix J Ploin D; GFRUP/RMEF Chronic Diseases in PICU Study Group. Children with chronic conditions in pediatric intensive care units located in predominantly French-speaking regions: prevalence and implications on rehabilitation care need and utilization Crit Care Med 20093741456–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hartman M, Lin J C. Functional outcomes for children with severe sepsis: is a “good save” good enough? Pediatr Crit Care Med. 2013;14(9):893–894. doi: 10.1097/PCC.0b013e3182a551e9. [DOI] [PubMed] [Google Scholar]


