Abstract
Background
Almost 358,000 women die each year in childbirth, mainly in low‐income countries. More than half of all maternal deaths occur within 24 hours of giving birth; severe bleeding in the postpartum period is the single most important cause. Depending on the rate of blood loss and other factors, such as pre‐existing anaemia, untreated postpartum haemorrhage (PPH) can lead to hypovolaemic shock, multi‐organ dysfunction, and maternal death, within two to six hours.
This review investigated different methods for estimating blood loss. The most common method of measuring blood loss during the third stage of labour is visual estimation, during which the birth attendant makes a quantitative or semi‐quantitative estimate of the amount of blood lost. In direct blood collection, all blood lost during the third stage of labour (except for the placenta and membranes) is contained in a disposable, funnelled, plastic collector bag, which is attached to a plastic sheet, and placed under the woman's buttocks. When the bleeding stops, there are two options: the bag can be weighed (also called gravimetric technique), or the bag can be calibrated, allowing for a direct measurement. A more precise measurement of blood loss is haemoglobin concentration (Hb) in venous blood sampling and spectrophotometry. With the dye dilution technique, a known quantity of dye is injected into the vein and its plasmatic concentration is monitored after the uterus stops bleeding. Using nuclear medicine, a radioactive tracer is injected, and its concentration is monitored after the uterus stops bleeding. Although hypothetically, these advanced methods could provide a better quantification of blood loss, they are difficult to perform and are not accessible in most settings.
Objectives
To evaluate the effect of alternative methods to estimate blood loss during the third stage of labour, to help healthcare providers reduce the adverse consequences of postpartum haemorrhage after vaginal birth.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (2 February 2018), ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP; 21 March 2018), and reference lists of retrieved studies.
Selection criteria
All randomised trials, including cluster‐randomised trials, evaluating methods for estimating blood loss after vaginal birth.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data, and checked them for accuracy.
Main results
The search retrieved 62 reports in total. Of these, we assessed 12 reports in full, corresponding to six trials. We included three trials and excluded one; two trials are ongoing.
The included trials were conducted in hospital settings. Two trials were conducted in India; the third trial was a large cluster‐randomised trial, which took place in 13 European countries. Overall, we judged the included trials to be at a low risk of bias. One study evaluated the use of calibrated drapes versus visual estimation, another evaluated the use of calibrated drapes versus the gravimetric technique (weight of blood‐soaked materials), therefore, we were unable to pool the data from the two studies. The third study did not measure any of the outcomes of interest, so did not contribute data to the analyses.
Direct measurement using calibrated drapes versus visual estimation
One cluster‐randomised controlled trial in 13 western European countries, with over 25,000 women, examined this comparison.
The trial did not report on postpartum anaemia (defined as Hb lower than 9 mg/dL), blood loss greater than 500 mL, or maternal infection.
Moderate‐quality evidence suggests there is probably little or no difference between groups in: severe morbidity (coagulopathy, organ failure, intensive care unit admission; adjusted risk ratio (RR) 0.82, 95% confidence interval (CI) 0.48 to 1.39); the risk of blood transfusion (adjusted RR 0.82, 95% CI 0.46 to 1.46); the use of plasma expanders (adjusted RR 0.77, 95% CI 0.42 to 1.42); and the use of therapeutic uterotonics (adjusted RR 0.87, 95% CI 0.42 to 1.76).
Direct measurement using calibrated drapes (Excellent BRASSS‐V Drape™) versus gravimetric technique
One randomised controlled trial in India, with 900 women, examined this comparison.
The trial did not report on postpartum anaemia (defined as Hb lower than 9 mg/dL), severe morbidity, or maternal infection.
High‐quality evidence showed that using calibrated drapes improved the detection of blood loss greater than 500 mL when compared with the gravimetric technique (RR 1.86, 95% CI 1.11 to 3.11). Low‐quality evidence suggests there may be little or no difference in the risk of blood transfusion between the two groups (RR 1.00, 95% CI 0.06 to 15.94), or in the use of plasma expanders, reported as intravenous fluids given for PPH treatment (RR 0.67; 95% CI 0.19 to 2.35). High‐quality evidence showed little or no difference in the use of therapeutic uterotonics (RR 1.01, 95% CI 0.90 to 1.13), but the use of therapeutic uterotonics was extremely high in both arms of the study (57% and 56%).
Authors' conclusions
Overall, the evidence in this review is insufficient to support the use of one method over another for blood loss estimation after vaginal birth. In general, the quality of evidence for our predefined outcomes ranged from low to high quality, with downgrading decisions due to imprecision. The included trials did not report on many of our primary and secondary outcomes.
In trials that evaluate methods for estimating blood loss during vaginal birth, we believe it is important to measure their impact on clinical maternal and neonatal outcomes, along with their diagnostic accuracy. This body of knowledge needs further, well designed, appropriately powered, randomised controlled trials that correlate blood loss with relevant clinical outcomes, such as those listed in this review.
Plain language summary
Methods for estimating blood loss after vaginal birth to improve maternal outcomes
What is the issue?
While postpartum haemorrhage (PPH) is one of the leading causes of maternal death worldwide, it mostly occurs in low‐income countries. It frequently occurs during the third stage of labour, the period of time from delivery of the baby to the expulsion of the placenta and membranes. During this period, the birth attendant evaluates how much blood the mother has lost.
Why is this important?
There is always some blood loss after the birth of a baby, but when this loss is excessive, it is called PPH. Severe PPH can lead to poor health for the mother (maternal morbidity), and sometimes even death, particularly in low‐ and middle‐income countries. If excessive blood loss is identified early, interventions to help stem the blood flow can be started sooner, and improve health outcomes for the mother. Therefore, it is important to find the best method to measure blood loss after birth; one that is practical in all birth settings, including those in low‐ to middle‐income countries.
In many instances, the birth attendant assesses blood loss by looking at the amount of blood lost, and estimating its volume (visual estimation). While this method is not very accurate, it is available in all birth settings. In another method, the birth attendant places a shallow bedpan below the mother’s buttocks, and then weighs the collected blood, along with blood that has soaked into any pads and material. This is referred to as an indirect method. In one direct method that has been devised, a 'calibrated delivery drape' is placed under the mother’s buttocks and tied around her waist, with the calibrated funnel portion (that indicates how much blood she has lost) hanging down between her legs. Other methods are also available, such as dye dilutions and radioactive techniques, but these are not practical in many birth settings.
What evidence did we find?
We searched for evidence in February 2018, and found three randomised controlled trials, involving over 26,000 women. Two trials contributed data to our analyses; one study did not provide data for any of the outcomes of interest in this review. All of the trials took place in hospital settings. Two trials took place in India, the other was conducted in 13 different European countries. The trials examined different methods of estimating blood loss.
One trial (conducted in 13 European countries, involving over 25,000 women) compared the use of a calibrated drape (direct estimation) to visual estimation (indirect estimation). Moderate‐quality evidence showed there was probably little or no difference between the methods for the risk of women developing serious conditions (e.g. failure to form clots, poor functioning of the liver, kidneys, and brain, admission to intensive care); their need for blood transfusion; the use of fluids to maintain their blood pressure; or the use of drugs to help their uterus contract to stop the bleeding. The trial did not report the number of women who had anaemia after birth, blood loss of at least 500 mL, or infection.
One trial (conducted in India, involving 900 women) compared the use of a calibrated drape (direct estimation) to weighing and measuring blood and blood‐soaked materials (indirect method). High‐quality evidence showed that calibrated drapes were better than measuring the blood and blood‐soaked materials at detecting blood loss of at least 500 mL. Low‐quality evidence showed there may be little or no difference between methods in the need for blood transfusion or fluids to maintain blood pressure. High‐quality evidence showed little or no difference in the use of drugs to help the uterus contract in order to stop bleeding. The trial did not report the number of women who had anaemia after birth or infection, or the risk of developing serious conditions (such as failure to form clots, poor functioning of the liver, kidneys, and brain, or being admitted to intensive care).
What does this mean?
There was insufficient evidence to support the use of one method over another to estimate blood loss after vaginal birth. There is a need for high quality trials that measure important outcomes, such as those listed in this review.
Summary of findings
Summary of findings for the main comparison. Direct estimation using calibrated drapes compared to indirect estimation using visual estimation for blood loss estimation after vaginal birth.
| Direct estimation using calibrated drapes compared to indirect estimation using visual estimation for estimating blood loss after vaginal birth | ||||||
| Patient or population: women undergoing vaginal birth Setting: 78 maternity units from 13 European countries Intervention: direct estimation using calibrated drapes Comparison: indirect estimation using visual estimation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with visual estimation | Risk with using calibrated drapes | |||||
| Postpartum anaemia | trial did not report outcome | ‐ | ‐ | ‐ | ||
| Severe morbidity | Study population | Adjusted RR 0.82 (0.48 to 1.39) | 2999 (1 RCT) | ⊕⊕⊕⊝ Moderate a | The actual numbers of severe PPH were 189/11,037 vaginal deliveries in the calibrated drapes group, and 295/14,344 vaginal deliveries in the visual estimation group. | |
| 21 per 1000 | 17 per 1000 (10 to 29) | |||||
| Blood loss ≥ 500 mL | trial did not report outcome | ‐ | ‐ | ‐ | ||
| Blood transfusion | Study population | Adjusted RR 0.82 (0.46 to 1.46) | 5561 (1 RCT) | ⊕⊕⊕⊝ Moderate a | ||
| 10 per 1000 | 8 per 1000 (4 to 14) | |||||
| Use of plasma expanders | Study population | Adjusted RR 0.77 (0.42 to 1.42) | 3122 (1 RCT) | ⊕⊕⊕⊝ Moderate a | ||
| 15 per 1000 | 12 per 1000 (6 to 22) | |||||
| Use of therapeutic uterotonics | Study population | Adjusted RR 0.87 (0.42 to 1.76) | 593 (1 RCT) | ⊕⊕⊕⊝ Moderate a | ||
| 54 per 1000 | 47 per 1000 (23 to 95) | |||||
| Maternal infection | trial did not report outcome | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; PPH: postpartum haemorrhage | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Imprecision: wide confidence interval crossing the line of no effect
Summary of findings 2. Direct measurement using calibrated drapes compared to indirect estimation using gravimetric technique for blood loss estimation after vaginal birth.
| Direct estimation using calibrated drapes compared to indirect estimation using gravimetric technique | ||||||
| Patient or population: women undergoing vaginal birth Setting: hospital maternity unit, India Intervention: direct estimation using calibrated drapes (Excellent BRASSS‐V Drape™) Comparison: indirect estimation using gravimetric technique (blood and blood‐soaked materials weighed and measured) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with gravimetric technique | Risk with using calibrated drapes | |||||
| Postpartum anaemia | trial did not report outcome | ‐ | ‐ | ‐ | ||
| Severe morbidity | trial did not report outcome | ‐ | ‐ | ‐ | ||
| Blood loss ≥ 500 mL | Study population | RR 1.86 (1.11 to 3.11) | 900 (1 RCT) | ⊕⊕⊕⊕ High | ||
| 47 per 1000 | 87 per 1000 (52 to 145) | |||||
| Blood transfusion | Study population | RR 1.00 (0.06 to 15.94) | 900 (1 RCT) | ⊕⊕⊝⊝ Low a | ||
| 2 per 1000 | 2 per 1000 (0 to 35) | |||||
| Use of plasma expanders | Study population | RR 0.67 (0.19 to 2.35) | 900 (1 RCT) | ⊕⊕⊝⊝ Low a | ||
| 13 per 1000 | 9 per 1000 (3 to 31) | |||||
| Use of therapeutic uterotonics | Study population | RR 1.01 (0.90 to 1.13) |
900 (1 study) | ⊕⊕⊕⊕ High | ||
| 564 per 1,000 | 570 per 1,000 (508 to 638) |
|||||
| Maternal infection | trial did not report outcome | ‐ | ‐ | ‐ | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Imprecision: wide confidence interval crossing the line of no effect
Background
Globally, it is estimated that every day, more than 800 women die from pregnancy‐related complications, which meant over 300,000 deaths in 2015. Most of these deaths were preventable, and happened in the poorest countries, where comorbidities, such as HIV infection and malnutrition, and barriers to timely access to care increase the risk of death from postpartum haemorrhage (PPH (Alkema 2016; Say 2014)).
At least half of all maternal deaths occur within 24 hours of giving birth, most commonly from excessive blood loss (WHO 2012). Depending on the rate of blood loss and other factors, such as pre‐existing anaemia, untreated PPH can lead to hypovolaemic shock, multi‐organ dysfunction, and maternal death, within two to six hours
Description of the condition
After the baby is born, the placenta and membranes are expelled from the uterine wall. This is called the 'third stage of labour'. While the third stage of labour always involves some bleeding, PPH is a major complication. Uterine atony (failure of the uterus to contract following birth of the baby) is a major cause of PPH; other causes of excessive blood loss include vaginal or cervical tears, uterine rupture, and retained placenta or membranes (WHO 2012).
The World Health Organization (WHO) defines PPH as 'blood loss of 500 mL or more within 24 hours after birth' (WHO 2012). Other authors state that abnormal bleeding during the postpartum period occurs when the volume is over 600 mL (Beischer 1986), or 1000 mL (Burchell 1980). WHO classifies PPH as moderate when the blood loss is between 500 mL and 1000 mL, and severe when the blood loss is over 1000 mL (WHO 2012). Moderate blood loss should not be clinically important, except for women who were anaemic during their pregnancy.
The prevalence of PPH worldwide is around 6% of all births, mostly in low‐income settings (Carroli 2008). However, it may vary from 5% to 18%, even within the same country (CEMACH 2004; Gilbert 1987; Hall 1985).
Besides maternal death, other serious sequelae may occur as a result of acute anaemia (i.e. Sheehan's syndrome, defined as decreased functioning of the pituitary gland, caused by PPH), and as a consequence of the interventions performed to stop severe PPH (i.e. general anaesthesia, manual removal of the placenta, blood transfusion, hysterectomy (Pacagnella 2013)).
Description of the intervention
There are different ways to measure blood loss during the third stage of labour. The most frequently used method is a visual estimation of blood loss (VEBL), during which the birth attendant makes a quantitative or semi‐quantitative estimate of the amount of blood lost. Direct blood collection means that all the blood lost during the third stage of labour (except the placenta and membranes) is contained in a disposable, funnelled, plastic collector bag attached to a plastic sheet and placed under the woman's buttocks (WHO 2010). When the bleeding stops, there are two options: the bag can be weighted (also called gravimetric technique; 1 mL = 1 gr/1.06), or the bag can be calibrated, which allows a direct measurement (Ambardekar 2014). More precise measurements are performed by evaluating haemoglobin concentration (Hb) in venous blood sampling, and spectrophotometry. The dye dilution technique for the measurement of plasma volume is a method that assesses the flow through the venous system by injecting a known quantity of dye into a vein and monitoring its plasmatic concentration after the uterus stops bleeding. Nuclear medicine offers a similar technique, but instead, a radioactive tracer is used and its concentration is measured (Patel 2006). Although hypothetically, they may provide a better quantification of blood loss, these methods are difficult to perform and are not available in most settings (Pacagnella 2013; Patel 2006).
How the intervention might work
Diagnosing PPH during the third stage of labour is not easy. By visually estimating the amount of blood loss, birth attendants can underestimate or overestimate its magnitude (Bose 2006; Didly 2004; Duthie 1991; Newton 2000; Prasertcharoensuk 2000; Razvi 1996; Stafford 2008; Zhang 2010).
We evaluated any alternative method for estimating blood loss compared with visual estimation, not in terms of their measurement and diagnostic accuracy, but for their effectiveness in reducing the adverse consequences of severe PPH. We defined an effective method as one that did not underestimate the amount of blood loss, hence the appropriate therapeutic measure was applied in a timely manner, but also it did not overestimate it, which may have led to unnecessary and potentially aggressive or invasive treatments.
Potential adverse effects
The potential side effects of the interventions may include those produced by invasive methods, such as blood extraction for any measurement, or the injection of any substance to be measured for the quantification of blood loss, among others.
Why it is important to do this review
It is important to find the most accurate method of quantifying blood loss during the third stage of labour in order to prevent, or once commenced, control PPH in a timely manner. Precision was not the only advantage the method should have; it also had to be practical and accessible for all, including minimally trained, healthcare providers.
Objectives
To evaluate the effect of alternative methods to estimate blood loss during the third stage of labour, to help healthcare providers reduce the adverse consequences of postpartum haemorrhage after vaginal birth.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (full text and abstract) comparing methods for estimating blood loss during the third stage of labour. We excluded quasi‐randomised trials and cross‐over trials.
Types of participants
Pregnant women during the third stage of labour, and 24 hours post‐delivery, regardless of other aspects of third stage of labour management, and mode of vaginal delivery (spontaneous or instrumental).
We excluded women who delivered by caesarean section.
Types of interventions
Any method of estimating blood loss compared to any other method of estimating blood loss.
Types of outcome measures
We included relevant studies regardless of whether they reported the outcomes of interest.
Primary outcomes
Postpartum anaemia (defined as a haemoglobin (Hb) lower than 9 mg/dL)
Severe morbidity (including coagulopathy, organ failure, intensive care unit admission, or as defined by authors)
Secondary outcomes
Maternal outcomes
Blood loss ≥ 1000 mL
Blood loss ≥ 500 mL
Blood transfusion
Use of plasma expanders
Use of therapeutic uterotonics
Changes in vital parameters, such as heart rate, blood pressure, urine output, etc
Further operative procedures (curettage, laparotomy, laparoscopy, surgical exploration, manual removal of the placenta, etc)
Hysterectomy due to postpartum haemorrhage (PPH)
Maternal infection
Maternal pre‐ and postdelivery change in Hb concentration
Maternal death
Adverse effects
Any side effect related to the method used (for example, phlebitis at the site of puncture for blood extraction)
Acceptability of intervention
Maternal satisfaction with intervention (as defined by authors)
Providers satisfaction with intervention (as defined by authors)
Search methods for identification of studies
The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (2 February 2018).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register, including the detailed search strategies for CENTRAL, MEDLINE, Embase, and CINAHL; the list of handsearched journals and conference proceedings; and the list of journals reviewed via the current awareness service; please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Information Specialist maintains their Trials Register that contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE Ovid;
weekly searches of Embase Ovid;
monthly searches of CINAHL EBSCO;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals, plus monthly BioMed Central email alerts.
Two people screen the search results, and the full text of all relevant trial reports identified through the searching activities described above. Based on the intervention described, they assign a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics) to each trial report, and then add it to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) on 21 March 2018 for unpublished, planned, and ongoing trial reports, using the search terms given in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (Virginia Diaz (VD) and Edgardo Abalos (EA)) independently assessed for inclusion all the potential studies identified. They resolved disagreements through discussion, or if required, by consulting the third review author (Guillermo Carroli (GC)).
Data extraction and management
We designed an ad‐hoc form for extracted data. For eligible studies, two review authors (VD and EA) independently extracted the data onto the agreed form. They resolved discrepancies through discussion, or if required, by consulting the third review author (GC). They entered data into Review Manager 5 software, and checked for accuracy (RevMan 2014).
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors (VD and EA) independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion, or by involving a third assessor (GC).
(1) Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table, computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal allocation to interventions prior to assignment, and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
For each included study, we described the method used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would have been unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the method used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high, or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data
For each included study, and for each outcome or class of outcomes, we described the completeness of data, including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data into the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data, missing outcome data balanced across groups, missing data for less than 20% of participants);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups, ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review were reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported, one or more reported primary outcomes were not pre‐specified, outcomes of interest were reported incompletely and so could not be used, study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
For each included study, we described any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could have put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there was risk of other bias.
(7) Particular biases to consider for cluster‐randomised trials
(i) Recruitment bias
low risk of recruitment bias when the knowledge of whether each cluster was an intervention or control cluster could not affect the types of participants recruited;
high risk of recruitment bias when the knowledge of whether each cluster was an intervention or control cluster could affect the types of participants recruited;
unclear whether there was risk of recruitment bias.
(ii) Baseline imbalance
low risk of baseline imbalance when cluster baseline were comparable, or statistical adjustment done, or stratified or pair‐matched randomisation of cluster used;
high risk of baseline imbalance when cluster baseline were imbalanced, or statistical adjustment was not done, or stratified or pair‐matched randomisation of cluster was not used;
unclear whether there was risk of baseline imbalance.
(iii) Loss of cluster
low risk of loss of cluster when more than 80% of clusters, or patients within clusters, or both, were reported;
high risk of loss of cluster when 20% or more of cluster, or patients within clusters, or both, were not reported;
unclear whether there was risk of loss of cluster.
(iv) Incorrect analysis
low risk of incorrect analysis when clustering effect was taken into account;
high risk of loss of cluster when clustering effect was not taken into account;
unclear whether there was risk of incorrect analysis.
(v) Comparability with individually randomised trials
low risk of comparability with individually randomised trials when contamination and ‘herd effects’ did not affect the direction of the effect of the cluster trial when compared with the individual randomised trials;
high risk of comparability with individually randomised trials when contamination and ‘herd effects’ affected the direction of the effect of the cluster trial when compared with the individual randomised trials;
unclear whether there was risk of comparability with individually randomised trials.
(8) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above (or (1) to (7) for cluster‐randomised trials), we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We had planned to explore the impact of the level of bias by undertaking Sensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
We evaluated the quality of the evidence, using the GRADE approach, as outlined in the GRADE handbook, in order to assess the quality of the body of evidence relating to the main comparisons: direct estimation using calibrated drapes compared to visual estimation for blood loss estimation after vaginal birth; and direct estimation using calibrated drapes compared to gravimetric technique for blood loss estimation after vaginal birth (Schünemann 2013). For GRADE assessment, we considered the main outcomes, postpartum anaemia (defined as a haemoglobin (Hb) lower than 9 mg/dL), and severe morbidity (including coagulopathy, organ failure, intensive care unit admission); and the following secondary outcomes.
Blood loss ≥ 500 mL
Blood transfusion
Use of plasma expander
Use of therapeutic uterotonics
Maternal infection
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data, we attempted to use the mean difference (MD) if outcomes were measured in the same way between trials, and the standardised mean difference (SMD) to combine trials that measured the same outcome, but used different methods, with 95% CI.
Unit of analysis issues
Cluster‐randomised trials
We included one cluster‐randomised trial in the analyses. We did not pool the data as it was the only trial reporting data for this comparison. For each outcome, we used the intraclass correlation coefficient (ICC) reported by the author. We adjusted the data for clustering effect as per the Cochrane Handbook for Systematic Review of Interventions, version 5.1.0, sections 16.3.3 to 16.3.5 (Higgins 2011). We derived the effective sample size by dividing the events and sample sizes in treatment and control groups by the Design Effect (DE) for each outcome. This was calculated according to Higgins 2011, using the intracluster correlation coefficient (ICC), the number of clusters and average cluster size (M): DE = 1 + (M – 1) ICC.
In future updates, if we identify both cluster‐randomised trials and individually‐randomised trials that report our outcomes of interest, we plan to synthesize the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity (less than 30%) between the study designs, and the interaction between the effect of the intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
We noted low levels of attrition in our included studies, so we did not explore its impact on the overall results.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention.
For future updates, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using a sensitivity analysis, in which we will use the number randomised minus any participants whose outcomes are known to be missing as the denominator for each outcome in each trial.
Assessment of heterogeneity
We evaluated statistical heterogeneity in each meta‐analysis using the T², I², and Chi² statistics. We defined heterogeneity as substantial if the I² was greater than 30%, and either the T² was greater than zero, or the P value was less than 0.10 in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analyses, we will investigate reporting biases (such as publication bias) using funnel plots, assessing asymmetry visually.
Data synthesis
We could not combine the data from the included studies in meta‐analysis, as two of the studies reported on different comparisons, and the third study did not report on the outcomes of interest.
In future updates, we will carry out statistical analysis using RevMan 5 software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it is reasonable to assume that studies are estimating the same underlying treatment effect, i.e. where trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If there is clinical heterogeneity sufficient to expect that the underlying treatment effects will differ between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects, and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with 95% confidence intervals, and estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we can pool data and we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses.
We plan to carry out the following subgroup analyses.
Whether or not intervention is part of AMTSL (active management of third stage of labour)
Whether or not oxytocin was used during first stage of labour
Whether or not a uterotonic was used to prevent PPH
Whether or not PPH prevention with oxytocin was given as an intravenous bolus or infusion, or by an intramuscular injection
We will assess the following outcomes in subgroup analysis.
Severe PPH (at least 1000 mL)
Serious maternal morbidity (organ failure, coma, intensive care unit admission, hysterectomy, or as defined by the authors)
We will assess subgroup differences by interaction tests available in Review Manager 5. We will report the results of subgroup analyses quoting the χ² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, we will carry out sensitivity analyses to explore the effect of trial quality on the primary outcomes of this review. We will divide trials into groups according to whether they are at low risk of bias, unclear, or high risk of bias for the primary outcomes (e.g. inadequate allocation concealment or lost to follow‐up in the intervention versus control arms). For cluster‐randomised trials, we will perform sensitivity analysis using a range of values for the intraclass correlation coefficient.
Results
Description of studies
Results of the search
See: Figure 1.
1.
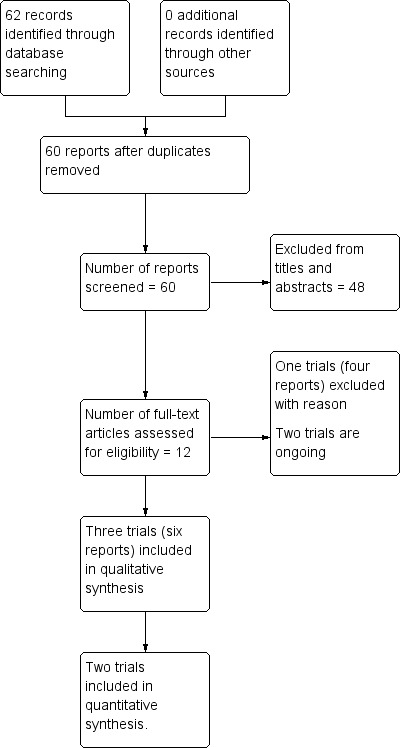
Study flow diagram
The search retrieved 62 reports in total. Of these, we assessed 12 reports in full, corresponding to six trials. From these, we included three trials (six reports (Ambardekar 2014; Patel 2006; Zhang 2010)) and excluded one trial (four reports (Toledo 2007). Two trials are ongoing (ACTRN12613000628741; ChiCTR‐IOR‐17012453).
Included studies
For detailed characteristics of the included studies, see Characteristics of included studies.
Design
Two of the trials were randomised controlled trials including a total of 1023 women, conducted between 2003 and 2007 (Ambardekar 2014; Patel 2006). Zhang 2010 was a cluster‐randomised trial including 78 maternity units with 25,381 women, conducted between 2006 and 2007.
Setting
Ambardekar 2014 and Patel 2006 were conducted in hospitals in India. Zhang 2010 randomised 78 maternity units from 13 European countries: six in Austria, 16 in Belgium, six in Denmark, four in Finland, six in France, eight in Hungary, four in Ireland, six in Italy, two in the Netherlands, two in Norway, five in Portugal, seven in Spain, and six in Switzerland.
Participants
Ambardekar 2014 included 900 women, 18 years old or older, who presenting at the maternity unit for imminent vaginal birth. Patel 2006 included 123 women who were recruited early in labour, and had no contraindications for a vaginal delivery. Zhang 2010 included all 25,381 women with vaginal deliveries who were admitted in labour to the 78 maternity units participating in the study.
Interventions and comparisons
Ambardekar 2014 evaluated two different measurements techniques: a direct method, the Excellent BRASSS‐V Drape™, whereby the amount of blood was measured at the time of bleeding by a calibrated drape, versus an indirect method in which personnel weighed blood‐soaked materials and measured the blood following the cessation of bleeding after vaginal delivery (also called gravimetric technique). The author described the methods as follows; “calibrated drapes: direct technique that allows the provider to assess the blood loss as it accumulates in a calibrated receptacle (usually a drape) beneath the woman" and "gravimetric technique: the collection of blood and blood‐soaked material into a vessel. The vessel is then weighed and an equivalent volume calculated".
In Patel 2006, blood loss during third stage of labour was evaluated by two methods: visual estimation or calibrated drape estimation, as in Ambardekar 2014.
In Zhang 2010, blood loss was measured by using a calibrated collector bag for direct estimation (intervention group) or by visually assessing postpartum blood loss (control group).
Outcomes
Ambardekar 2014 only included our secondary pre‐defined outcomes: blood loss, maternal pre‐ and post‐delivery changes in Hb concentration, use of therapeutic uterotonics (Oxytocin, Misoprost, Carboprost), further operative procedures (internal iliac ligation, resuturing of episiotomy), and blood transfusion.
Zhang 2010 included one of our primary outcomes, severe morbidity, defined by authors as severe postpartum haemorrhage (PPH) after vaginal delivery. This composite outcome included all women who experienced one or more of: blood transfusion, intravenous plasma expansion, arterial embolization, surgical procedure, admission to an intensive care unit, treatment with recombinant factor VII, and death. Secondary outcomes were blood transfusion, intravenous plasma expansion, arterial embolisation, surgical procedures, admission to intensive care unit, treatment with recombinant factor VII, death, manual removal of the placenta, and administration of prostaglandin after delivery.
Patel 2006 did not evaluate any of our outcomes of interest.
Sources of trial funding
Ambardekar 2014 was funded by the Bill and Melinda Gates Foundation, and Zhang 2010 was funded by a grant from the Foundation Philippe Wiener‐Maurice Anspach and the European Union under framework 5. Patel 2006 did not report sources of trial funding.
Trial authors' declarations of interest
Ambardekar 2014 and Zhang 2010 reported that they had no competing interests; Patel 2006 did not provide any information about declarations of interest.
Excluded studies
For detailed characteristics of the excluded trials, see Characteristics of excluded studies.
Toledo 2007 was a randomised controlled trial conducted among health practitioners in simulated vaginal deliveries.
Ongoing studies
ACTRN12613000628741 and ChiCTR‐IOR‐17012453 were both protocols, registered on trial registers. ACTRN12613000628741 is not yet recruiting. See Characteristics of ongoing studies.
Risk of bias in included studies
Overall, we judged the trials to be at low risk of bias. See Figure 2.
2.
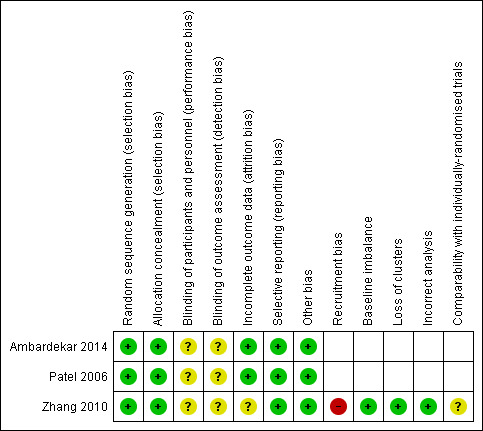
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Sequence generation
Ambardekar 2014 and Patel 2006 used a computer‐generated sequence. Zhang 2010 was a cluster‐RCT, in which the unit of randomisation was the maternity unit; random generation was performed centrally with stratification by country and size of the maternity unit. We assessed all three trials at low risk of bias.
Allocation concealment
Ambardekar 2014 used opaque, sealed, sequentially numbered envelopes, and Patel 2006 used a sequence of letters V (visual) and B (drape). In the Zhang 2010 study, the maternity units were randomly allocated to either use of a collector bag after vaginal delivery (intervention) or no use of the bag (control).
Blinding
We assessed all three included studies as having an unclear risk of performance and detection bias. Blinding of the intervention was not possible for either the participants or the trial personnel, given the characteristic of the intervention. Blinding of outcome assessments was not possible as they were evaluated immediately after the intervention. There might have been overestimation or underestimation of the outcomes. Blinding of analyses was not reported.
Incomplete outcome data
The included studies were not at risk of attrition bias, mainly due to the short intervention period. However, Zhang 2010 did not report the total number of vaginal deliveries in the participating maternity units during the study period. This trial also reported the characteristics of the population in a period previous to the initiation of the trial (baseline data), instead of describing the characteristic of the population participating in the study. Therefore, we assessed Ambardekar 2014 and Patel 2006 at low risk of attrition bias, Zhang 2010 at unclear risk of attrition bias.
Selective reporting
All three included trials were judged to be at low risk of reporting bias, as trial protocols were available and all proposed outcomes were reported.
Particular biases to consider for cluster‐randomised trials
As Zhang 2010 was a cluster‐randomised trial, we evaluated other potential sources of bias. Overall, we considered this trial to be at low risk of bias.
(i) recruitment bias (differential participant recruitment in clusters for different interventions); we assessed Zhang 2010 at high risk of recruitment bias. Given the logistics of clinical practice on the delivery suite, contamination seemed to be inevitable in an individual patient randomised trial setting.
(ii) baseline imbalance: they used a stratified design to ensure that the two arms were as similar as possible, so we assessed Zhang 2010 at low risk of bias for baseline imbalance.
(iii) loss of clusters: there was no loss of clusters, and we assessed Zhang 2010 at low risk of bias.
(iv) incorrect analysis: we assessed Zhang 2010 at low risk of bias for incorrect analysis.
(v) comparability with individually randomised trials: we assessed Zhang 2010 at low risk of bias, as it was the only study that contributed to the comparison.
Other potential sources of bias
We judged all three included trials at low risk of other potential sources of bias.
Effects of interventions
We did not perform meta‐analysis, as the two studies contributing data used different study methods, and examined different comparisons (Ambardekar 2014; Zhang 2010). Patel 2006 did not contribute any data as it did not report on any outcomes of interest for this review.
Comparison 1: Direct estimation using calibrated drapes versus visual estimation (indirect estimation)
This comparison includes data from one cluster‐RCT with a total of 25,381 women. Although the unit of randomisation was the delivery suite (N = 78), outcomes were measured and reported by women analysed (Zhang 2010).
Primary outcomes
Postpartum anaemia
Zhang 2010 did not report on postpartum anaemia (defined as Hb lower than 9 mg/dL).
Severe morbidity (including coagulopathy, organ failure, intensive care unit admission)
Moderate‐quality evidence showed little or no difference in severe morbidity (adjusted risk ratio (RR) 0.82, 95% confidence interval (CI) 0.48 to 1.39; intraclass correlation coefficient (ICC) 0.023; Analysis 1.1).
1.1. Analysis.
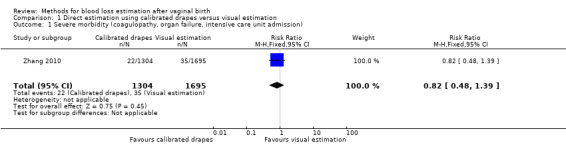
Comparison 1 Direct estimation using calibrated drapes versus visual estimation, Outcome 1 Severe morbidity (coagulopathy, organ failure, intensive care unit admission).
Zhang 2010 classified this outcome as severe PPH (defined as one or more of: maternal death, transfusion, plasma expansion, surgery or embolisation, admission to intensive care unit, or treatment with recombinant factor VII). The crude incidence of severe PPH was 1.71% (189/11,037 vaginal deliveries) in the calibrated group, and 2.06% (295/14,344 vaginal deliveries) in the visual estimation group.
Secondary outcomes
Blood transfusion
Moderate‐quality evidence showed there is probably little or no difference between groups in the risk of receiving a blood transfusion (adjusted RR 0.82, 95% CI 0.46 to 1.46; ICC 0.011; Analysis 1.2). The actual number of women who received blood transfusion was 86/11,037 deliveries in the calibrated group, and 135/14,344 deliveries in the visual estimation group.
1.2. Analysis.
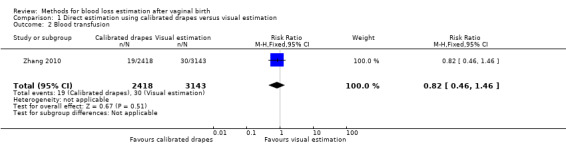
Comparison 1 Direct estimation using calibrated drapes versus visual estimation, Outcome 2 Blood transfusion.
Use of plasma expanders
Moderate‐quality evidence showed there is probably little or no difference between groups in the risk of receiving plasma expanders (adjusted RR 0.77, 95% CI 0.42 to 1.42; ICC 0.022; Analysis 1.3). The actual number of women who received plasma expanders was 127/11,037 deliveries in the calibrated group, and 222/14,344 deliveries in the visual estimation group.
1.3. Analysis.
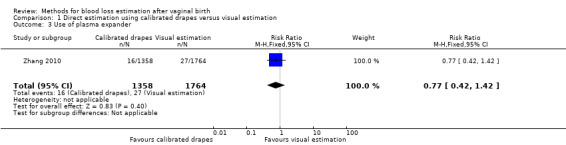
Comparison 1 Direct estimation using calibrated drapes versus visual estimation, Outcome 3 Use of plasma expander.
Use of therapeutic uterotonics
Moderate‐quality evidence showed there is probably little or no difference between groups in the use of therapeutic uterotonics (adjusted RR 0.87, 95% CI 0.42 to 1.76; ICC 0.129; Analysis 1.4). The actual number of women who received prostaglandins as therapeutic uterotonics was 501/11,037 deliveries in the in the calibrated group, compared to 766/14,344 deliveries in the visual estimation group.
1.4. Analysis.
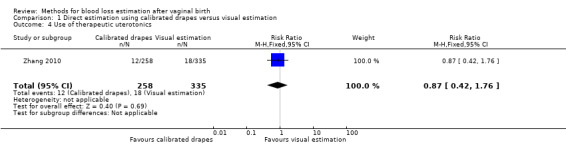
Comparison 1 Direct estimation using calibrated drapes versus visual estimation, Outcome 4 Use of therapeutic uterotonics.
Further operative procedures
Moderate‐quality evidence showed there is probably little or no difference between groups for the risk of manual removal of the placenta (adjusted RR 1.17, 95% CI 0.81 to 1.68; ICC 0.016; Analysis 1.5). The actual number of women had their placenta manually removed was 326/11,037 deliveries in the calibrated drape group, and 366/14,344 deliveries in the visual estimation group.
1.5. Analysis.
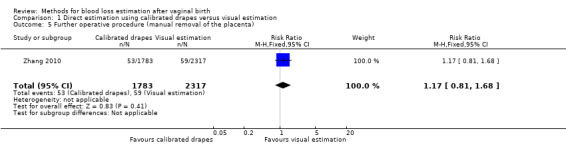
Comparison 1 Direct estimation using calibrated drapes versus visual estimation, Outcome 5 Further operative procedure (manual removal of the placenta).
Moderate‐quality evidence showed little or no difference between groups for the risk of surgical procedures or embolisation (adjusted RR 0.81, 95% CI 0.37 to 1.79; ICC 0.012; Analysis 1.6). The actual number of women who underwent surgical procedures or embolisation was 50/11,037 deliveries in the calibrated drape group, and 76/14,344 deliveries in the visual estimation group.
1.6. Analysis.
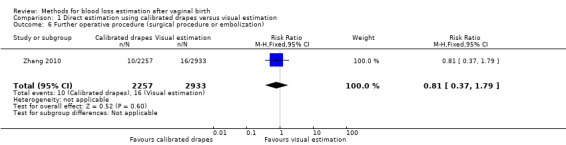
Comparison 1 Direct estimation using calibrated drapes versus visual estimation, Outcome 6 Further operative procedure (surgical procedure or embolization).
Maternal death
There were no maternal deaths reported in the study (Analysis 1.7).
1.7. Analysis.
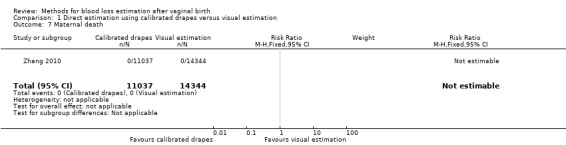
Comparison 1 Direct estimation using calibrated drapes versus visual estimation, Outcome 7 Maternal death.
The study did not report on the following secondary outcomes of interest in this review.
Blood loss greater than 1000 mL
Blood loss greater than 500 mL
Changes in vital parameters, such as heart rate
Hysterectomy due to PPH
Maternal infection
Maternal pre‐ and postdelivery change in Hb concentration
Adverse effects related to the method used
-
Acceptability of intervention
Maternal satisfaction with the intervention
Provider satisfaction with the intervention
Comparison 2: Direct estimation using calibrated drapes versus gravimetric techniques (the weight of blood‐soaked materials)
One trial (900 women) contributed data to this comparison (Ambardekar 2014).
Primary outcomes
Postpartum anaemia (defined as Hb lower than 9 mg/dL)
Ambardekar 2014 did not report on postpartum anaemia (defined as Hb lower than 9 mg/dL).
Severe morbidity (including coagulopathy, organ failure, intensive care unit admission)
Ambardekar 2014 did not report on severe morbidity (including coagulopathy, organ failure, intensive care unit admission).
Secondary outcomes
Blood loss greater than 500 mL
High‐quality evidence showed that calibrated drapes were better at detecting blood loss greater than 500 mL than the gravimetric technique (RR 1.86, 95% CI 1.11 to 3.11; Analysis 2.1). This outcome was detected in 39 women/450 deliveries in the calibrated drape group and in 21 women/450 deliveries in the gravimetric technique group.
2.1. Analysis.
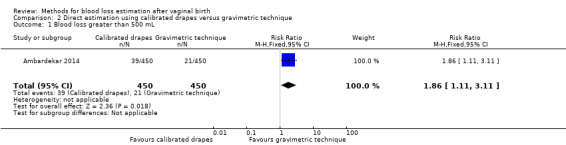
Comparison 2 Direct estimation using calibrated drapes versus gravimetric technique, Outcome 1 Blood loss greater than 500 mL.
Blood transfusion
Low‐quality evidence showed that there was little or no difference between groups in the risk of blood transfusion (RR 1.00, 95% CI 0.06 to 15.94; Analysis 2.2). There was just one blood transfusion in each group.
2.2. Analysis.
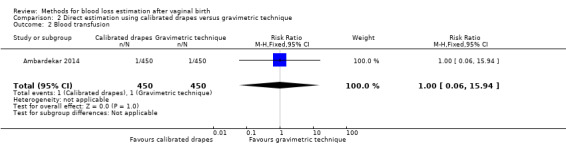
Comparison 2 Direct estimation using calibrated drapes versus gravimetric technique, Outcome 2 Blood transfusion.
Use of plasma expanders
Low‐quality evidence showed there may be little or no difference between groups in the use of plasma expanders, reported as intravenous fluids given to treat PPH (RR 0.67, 95% CI 0.19 to 2.35; Analysis 2.3). Four women in the calibrated drape group received intravenous fluids, while six women in the gravimetric group did.
2.3. Analysis.
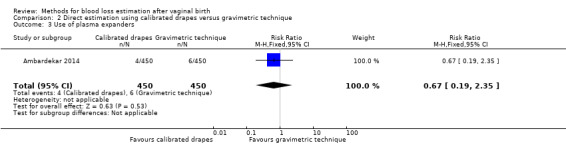
Comparison 2 Direct estimation using calibrated drapes versus gravimetric technique, Outcome 3 Use of plasma expanders.
Use of therapeutic uterotonics
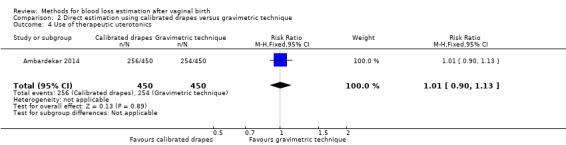
High‐quality evidence showed little or no difference between groups in the use of therapeutic uterotonics (RR 1.01, 95% CI 0.90 to 1.13; Analysis 2.4). Therapeutic uterotonics, which included oxytocin, misoprostol, and carboprost, were needed by 256 women in the calibrated drape group, and 254 women in the gravimetric group.
2.4. Analysis.
Comparison 2 Direct estimation using calibrated drapes versus gravimetric technique, Outcome 4 Use of therapeutic uterotonics.
Further operative procedures
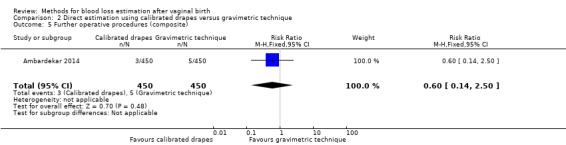
Low‐quality evidence showed there may be little or no difference between groups in the risk of further operative procedures, which included manual removal of the placenta, internal iliac ligation, and resuturing of the episiotomy (RR 0.60, 95% CI 0.14 to 2.50; Analysis 2.5). Three women in the calibrated drape group, and five women in the gravimetric group underwent further procedures.
2.5. Analysis.
Comparison 2 Direct estimation using calibrated drapes versus gravimetric technique, Outcome 5 Further operative procedures (composite).
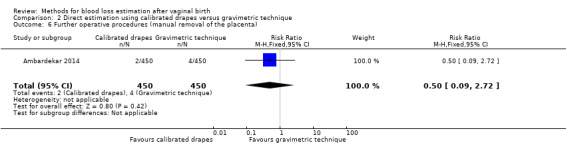
Low‐quality evidence showed little or no difference between groups in the risk of manual removal of placenta (RR 0.50, 95% CI 0.09 to 2.72; Analysis 2.6). Two women in the calibrated drape group and four in the gravimetric group had their placenta manually removed.
2.6. Analysis.
Comparison 2 Direct estimation using calibrated drapes versus gravimetric technique, Outcome 6 Further operative procedures (manual removal of the placenta).
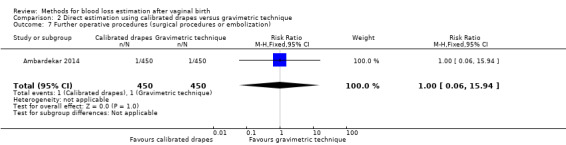
Low‐quality evidence showed there may be little or no difference between groups in the risk of surgical procedures or embolisation (RR 1.00, 95% CI 0.06 to 15.94; Analysis 2.7). One woman in each group underwent a surgical procedure or embolisation.
2.7. Analysis.
Comparison 2 Direct estimation using calibrated drapes versus gravimetric technique, Outcome 7 Further operative procedures (surgical procedures or embolization).
Maternal pre‐ and postdelivery change in Hb concentration
Moderate‐quality evidence showed there is probably little or no difference between groups in the maternal pre‐ and postdelivery change in Hb concentration (mean difference (MD) ‐0.10, 95% CI ‐0.30 to 0.10; Analysis 2.8).
2.8. Analysis.
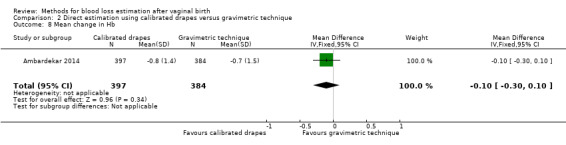
Comparison 2 Direct estimation using calibrated drapes versus gravimetric technique, Outcome 8 Mean change in Hb.
The study did not report on the following secondary outcomes of interest in this review.
Blood loss greater than 1000 mL
Changes in vital parameters such as heart rate, blood pressure, urine output, etc
Hysterectomy due to PPH
Maternal infection
Maternal death
Adverse effects related to the method used
-
Acceptability of intervention
Maternal satisfaction with the intervention
Provider satisfaction with the intervention
Discussion
We included three trials in this review. We were unable to conduct meta‐analysis as two trials used different comparisons: one evaluated the use of calibrated drapes versus visual estimation, another evaluated the use of calibrated drapes versus the weight of blood‐soaked materials (gravimetric technique). The third trial did not measure any outcomes of interest, so did not contribute data.
Summary of main results
One large cluster‐RCT (including over 26,000 women) provided data for our comparison of direct estimation using calibrated drapes versus visual estimation. Moderate‐quality evidence showed there is probably little or no difference between methods in the risk of developing severe morbidity (our primary outcome). The trial did not report on postpartum anaemia (our other primary outcome). For the remaining outcomes reported (blood transfusion, use of plasma expanders, use of therapeutic uterotonics, manual removal of the placenta, and surgical procedures or embolization), moderate‐quality evidence found that any statistical significance favouring one technique or the other disappeared after we adjusting for clustering effect. The trial did not report on a number of our secondary outcomes of interest: blood loss ≥ 500 mL or ≥ 1000 mL, changes in vital parameters, hysterectomy due to PPH, maternal infection, maternal pre‐ and postdelivery changes in haemoglobin concentration, adverse events related to the method used, and maternal and provider satisfaction. The trial reported there were no maternal deaths.
One trial (including 900 women) provided data for our comparison of direct estimation using calibrated drapes versus indirect estimate, using the gravimetric technique. The trial did not report on our two primary outcomes, postpartum anaemia and severe morbidity. High‐quality evidence found that fewer women, for which blood loss was evaluated using the gravimetrics technique, were found to have blood loss ≥ 500 mL than those in which the evaluation was performed using calibrated drapes. The fact that a higher rate of blood loss ≥ 500 mL was detected did not mean that there was necessarily a higher rate of blood loss ≥ 500 mL. We could not discern whether direct estimation by calibrated drapes truly overestimated blood loss, or whether this method failed to discriminate between blood and other types of fluid, such as amniotic fluid or urine. Similarly, we were unsure whether the gravimetric technique underestimated the real amount of blood loss, because it only took into account blood‐soaked materials, which may not have captured all of the blood lost. The trial was underpowered to detect significant differences between groups for blood transfusion, the use of plasma expanders, further operative procedures, manual removal of the placenta, surgical procedures or embolization, and for maternal pre‐ and postdelivery changes in haemoglobin concentration. High‐quality evidence showed little or no difference between groups in the use of therapeutic uterotonics, but it is worth noting that their use was extremely high in both arms of the study (57% and 56%), and we could not find a clinical reason justifying this intervention in the paper. The trial did not report on a number of our secondary outcomes: blood loss ≥ 1000 mL, changes in vital parameters, hysterectomy due to PPH, maternal infection, maternal death, adverse effects related to the method used, and maternal and provider satisfaction.
Overall completeness and applicability of evidence
The included studies only considered vaginal births, and stopped collecting blood 'as soon as the loss was considered normal'. It is important to take these facts into consideration, because it means that women with 'trickling loss', who are most often missed, are also those who would be missed by the use of drapes. As a consequence, the intervention would not be applicable to these women.
It is important to highlight that the trial comparing calibrated drapes with visual estimation of blood loss, failed to report on one of our primary outcome of interest, postpartum anaemia, as well as secondary outcomes: blood loss ≥ 1000 mL, changes in vital parameters, hysterectomy due to PPH, maternal infection, maternal death, and maternal and provider satisfaction.
The other two studies compared calibrated drapes versus a gravimetric technique (collecting and weighting blood, pads, and gauze), but failed to report on our two primary outcomes, postpartum anaemia and severe morbidity, as well as secondary outcomes: blood loss ≥ 1000 mL, changes in vital parameters, hysterectomy due to PPH, maternal infection, maternal death, and maternal and provider satisfaction. None of the included studies reported adverse effects due to the intervention.
Visual estimation was not compared against the gravimetric technique. Other, more sophisticated, methods of blood loss estimation were not evaluated in properly randomised controlled trials.
As previously described, one of the interventions was tested only in high‐income countries, while the other two were evaluated in single hospitals in India.
Quality of the evidence
Overall, we considered the included trials to be at a low risk of bias.
Comparison 1: for our pre‐defined primary outcome severe morbidity, we assessed the quality of the evidence using GRADE as moderate. We downgraded the quality for this outcome due to wide confidence intervals that crossed the line of no effect. The trial did not report on postpartum anaemia (the other pre‐defined primary outcome). We also assessed the evidence for the reported secondary outcomes as moderate. We also downgraded the quality of these outcomes due to imprecision. See Table 1.
Comparison 2: neither trial reported on the primary outcomes. We assessed the evidence as high‐quality for blood loss ≥ 500 mL and the use of therapeutic uterotonics, and as low‐quality for the use of blood transfusions and plasma expanders. See Table 2.
Potential biases in the review process
We followed Cochrane Pregnancy and Childbirth's search strategies and used Cochrane methodology to minimise the potential for bias. This included having at least two review authors independently assessing identified studies, extracting data and evaluating risk of bias.
Agreements and disagreements with other studies or reviews
A non‐systematic review of the literature concluded that visual estimation of blood loss was inaccurate, and that direct estimations and indirect estimation by gravimetric techniques were both accurate and feasible in clinical practice (Schorn 2010).
Another non‐systematic review of the literature stated that there was little evidence that improved accuracy of blood loss volume measurements could improve maternal outcomes (Hancock 2015). Blood volume is largely used retrospectively, to manage volume replacement and transfusion, but not to determine when to treat. The diagnosis may rely on factors other than volume, such as speed of blood flow, the nature of the loss, or other clinical signs.
Clinical signs suggesting decompensation usually begin with greater amounts of blood loss: an increase in heart rate to above 120 beats per minute, a decrease in blood pressure to less than 90 mmHg systolic blood pressure or 60 mmHg diastolic blood pressure, an increase in respiratory rate to above 30 breaths per minute, or a combination, could indicate a blood loss greater than 30% of the total blood volume, which is suggestive of shock (ATLS 2013). However, these signs in isolation are not diagnostic. The shock index, calculated as the heart rate divided by the systolic blood pressure, is a more accurate predictor of hypovolaemia, although this is indirect evidence, based mainly on data from trauma patients, not from obstetrical populations (Pacagnella 2013). There is now a published protocol that states the purpose of the study is to evaluate how the amount of blood lost during the third stage of labour modifies the shock index (NCT03135158).
Authors' conclusions
Implications for practice.
Overall, the findings of this review are insufficient to support the use of one method of estimating blood loss after vaginal birth over another. The quality of evidence for our predefined outcomes was downgraded due to imprecision, or small sample size with few or no events. Many of our primary and secondary outcomes were not reported by trial authors.
Implications for research.
In trials that evaluate methods for estimating blood loss during vaginal birth, we believe it is important to measure their impact on clinical maternal and neonatal outcomes, along with their diagnostic accuracy.
A current collaborative project is exploring the development of a minimum set of critically important outcomes. The postpartum haemorrhage (PPH) core outcomes sets (COS) for prevention and treatment of PPH will help to evaluate current and future methods for estimating blood loss, in terms of both accuracy and clinical usefulness, to prevent the complications of under‐diagnosing or over‐diagnosing postpartum haemorrhage.
Acknowledgements
As part of the pre‐publication editorial process, this review was commented on by six peers (an editor and five referees who were external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers, and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors, and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and ICTRP
estimat* AND blood AND loss AND labo(u)r
measur* AND blood AND loss AND labo(u)r
Data and analyses
Comparison 1. Direct estimation using calibrated drapes versus visual estimation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe morbidity (coagulopathy, organ failure, intensive care unit admission) | 1 | 2999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.48, 1.39] |
| 2 Blood transfusion | 1 | 5561 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.46] |
| 3 Use of plasma expander | 1 | 3122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.42] |
| 4 Use of therapeutic uterotonics | 1 | 593 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.42, 1.76] |
| 5 Further operative procedure (manual removal of the placenta) | 1 | 4100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.81, 1.68] |
| 6 Further operative procedure (surgical procedure or embolization) | 1 | 5190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.37, 1.79] |
| 7 Maternal death | 1 | 25381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Direct estimation using calibrated drapes versus gravimetric technique.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Blood loss greater than 500 mL | 1 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.11, 3.11] |
| 2 Blood transfusion | 1 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.94] |
| 3 Use of plasma expanders | 1 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.19, 2.35] |
| 4 Use of therapeutic uterotonics | 1 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.13] |
| 5 Further operative procedures (composite) | 1 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.14, 2.50] |
| 6 Further operative procedures (manual removal of the placenta) | 1 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.09, 2.72] |
| 7 Further operative procedures (surgical procedures or embolization) | 1 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.94] |
| 8 Mean change in Hb | 1 | 781 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.30, 0.10] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ambardekar 2014.
| Methods | This study was a randomised trial to evaluate the measurement of blood loss after delivery, using opaque, sequentially numbered envelopes in blocks of 10. Randomisation sequence was generated by computer. Between January 2006 and September 2007; KEM Hospital, Pune, India |
|
| Participants | All women aged 18 and older, presenting at the study site for an imminent vaginal delivery. 1195 women enrolled and randomised; direct method (intervention group) = 593 women; indirect method (control group) = 602 women; 295 withdrew from the study, mostly because of the provider's decision to perform caesarean section, 35 for clinical reasons, and 4 at women's choice | |
| Interventions | A direct method, the Excellent BRASSS‐V Drape™ versus indirect method, involving the weight and measurement of blood and blood‐soaked materials following the cessation of bleeding | |
| Outcomes | Blood loss; Hb level; amount of intravenous fluids; any transfusion received; time of intravenous removal, time of haemoglobin assessment | |
| Notes | Competing interests: the authors declared that they had no competing interests. This study was funded by the Bill and Melinda Gates Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by computer |
| Allocation concealment (selection bias) | Low risk | Cards indicating group allocation were placed in opaque, sequentially numbered envelopes, randomised in blocks of 10 via a computerised randomisation sequence, generated in New York by Gynuity Health Projects staff. Envelopes were opened by study staff after enrolment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 27.9% women excluded after randomisation, mainly for the intrapartum indication of a caesarean section. No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Trial protocol available |
| Other bias | Low risk | No other bias found |
Patel 2006.
| Methods | A randomised, controlled, hospital‐based trial was conducted from September through December 2003, with 123 women undergoing vaginal delivery at the District Hospital in Belgaum, Karnataka, India. | |
| Participants | Women undergoing vaginal deliveries. Inclusion criteria: (1) the woman was scheduled for vaginal delivery; (2) she was able to give written or verbal consent to participating in the study; and (3) she had no contraindications for vaginal delivery. Exclusion criteria: women in active labour (defined as labour with a cervical dilation > 4 cm). |
|
| Interventions | Visual estimation group or the drape estimation group with photospectrometry measurement | |
| Outcomes | Primary outcome: blood loss | |
| Notes | Conflict of interest and source of trial funding not stated in the article | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The women were randomly assigned to the visual estimation group or the drape estimation group. To ensure balanced randomisation and to conceal the treatment assignment, random block size was used, as well as a computer‐generated randomisation list with a sequence of letters V (visual) and B (drape). |
| Allocation concealment (selection bias) | Low risk | The women were randomly assigned to the visual estimation group or the drape estimation group. To ensure balanced randomisation and to conceal the treatment assignment, random block size was used, as well as a computer‐generated randomisation list with a sequence of letters V (visual) and B (drape). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Trial protocol available |
| Other bias | Low risk | No other bias were found. |
Zhang 2010.
| Methods | Cluster‐randomised trial in 13 western European countries; 78 maternity units; random allocation performed centrally by the National Perinatal Epidemiology Unit; stratified by country and size of maternity unit; from January 2006 to May 2007 | |
| Participants | Women with vaginal deliveries who attended the participating maternity units | |
| Interventions | Use of a calibrated collector bag for direct measurement versus visual assessment of blood loss without collector bag. | |
| Outcomes | Primary outcome: Severe postpartum haemorrhage (composite outcome including: maternal death, blood transfusion, plasma expansion, surgical procedures or embolisation, admission to intensive care unit, treatment with recombinant factor VII) Secondary outcomes: Components of the composite outcome considered individually, plus manual removal of the placenta and use of prostaglandins |
|
| Notes | Competing interests: none declared Authors received a grant from Fondation Philippe Wiener‐Maurice Anspach for the final analysis of the data. The project was funded by the European Union under framework 5 (contract QLG4‐CT‐2001‐01352). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random generation performed centrally by the National Perinatal Epidemiology Unit; stratified by country and size of maternity unit |
| Allocation concealment (selection bias) | Low risk | Was produced centrally by the National Epidemiology Unit, Oxford. A stratified design was used to ensure that the 2 arms of the trial were as similar as possible at baseline. The maternity units were randomly allocated to either systematic use of a collector bag after vaginal delivery (intervention arm) or no use of the bag (control group). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although authors reported no lost to follow‐up, the total number of deliveries that occurred in the study hospital were not reported. |
| Selective reporting (reporting bias) | Low risk | Trial protocol available |
| Other bias | Low risk | No other bias found |
| Recruitment bias | High risk | Given the logistics of clinical practice on the delivery suite, contamination seemed to be inevitable in an individual patient randomised trial setting. |
| Baseline imbalance | Low risk | A stratified design was used to ensure that the 2 arms of the trial were as similar as possible at baseline for the stratification factors of country and size of maternity unit (median split within country). |
| Loss of clusters | Low risk | Lost to follow up = 0 |
| Incorrect analysis | Low risk | Cluster level analysis was carried out only on the primary outcome |
| Comparability with individually‐randomised trials | Unclear risk | This study was the only one that contributed data for this comparison. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Toledo 2007 | Randomised cross‐over study in simulated vaginal deliveries. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12613000628741.
| Trial name or title | A pilot study to evaluate Australian midwives' experiences of using a calibrated blood collection drape to improve estimation of third stage blood loss in labour |
| Methods | Randomised controlled trial. An associate investigator will place an equal number of data sheets for each group in sealed blank envelopes, place them in a sealed box and shuffle them. The chief investigator will, on receipt of signed consent form, draw an envelope from the box and assign the participant to the group in the envelope |
| Participants | Midwives working in birth suite |
| Interventions | A calibrated plastic drape (the Evergrand Underbuttocks Surgical Drape) will be placed under the buttocks of the woman at the commencement of third stage labour. It will measure blood loss in the collection pouch and be removed 30 minutes post birth of the placenta and membranes. Control is a visual estimation of blood loss at 30 minutes post completion of birth of the placenta and membranes |
| Outcomes | Degree of difference in Mean (or Median) total estimated (standard care) and measured (drape) blood loss |
| Starting date | 17 July 2013 |
| Contact information | Dr Sharon Licqurish. sharon.licqurish@acu.edu.au |
| Notes | BLEED trial |
ChiCTR‐IOR‐17012453.
| Trial name or title | Design and application of 2 sets of measurable liquid collection bags based on delivery position |
| Methods | Randomised parallel controlled trial |
| Participants | (1) mothers aged 18 to 35 years; (2) pregnancy 37 to 41 weeks; (3) fetal orientation: singleton head position; (4) normal delivery position |
| Interventions | To prove that liquid bag design can provide a more rapid assessment of postpartum haemorrhage, can better distinguish between blood and amniotic fluid, can effectively reduce the environmental pollution and staff needed. |
| Outcomes | Amount of postpartum haemorrhage; amount of amniotic fluid |
| Starting date | 25 August 2017 |
| Contact information | Fang Wang fangw@zju.edu.cn/Chunxiao Hu huchunxiao12345@163.com |
| Notes | Protocol registration on Chinese clinical trial registry (www.chictr.org.cn/showprojen.aspx?proj=19952) |
Differences between protocol and review
There are some differences between our published protocol and this full review (Diaz 2014).
We added a search of ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP).
We updated our methods to include the use of GRADE to assess the quality of the body of evidence, and included 'Summary of findings' tables for the two primary outcomes and five secondary outcomes (Table 1; Table 2); and adding the assessment of risk of bias for cluster‐randomised controlled trials.
Contributions of authors
V Diaz, E Abalos and G Carroli prepared the protocol. V Diaz and E Abalos selected the studies for inclusion, performed the 'Risk of bias' assessment of the study, and extracted the data. G Carroli resolved disagreements when appropriate. V Diaz prepared the final version of this review. All authors commented on, and agreed the final version. V Diaz is the guarantor for the review.
Sources of support
Internal sources
Centro Rosarino de Estudios Perinatales. Rosario, Argentina, Argentina.
External sources
No sources of support supplied
Declarations of interest
V Diaz ‐ none known.
E Abalos ‐ none known.
G Carroli ‐ none known.
New
References
References to studies included in this review
Ambardekar 2014 {published data only}
- Ambardekar S, Coyaji K, Otiv S, Bracken H, Winikoff B. A comparison of drape estimation and standardized weight method for the measurement of postpartum blood loss. International Journal of Gynecology and Obstetrics 2009;107(S2):S106. [DOI: 10.1016/S0020-7292(09)60418-5] [DOI] [Google Scholar]
- Ambardekar S, Shochet T, Bracken H, Coyaji K, Winikoff B. Calibrated delivery drape versus indirect gravimetric technique for the measurement of blood loss after delivery: a randomized trial. BMC Pregnancy and Childbirth 2014;14(1):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01885845. A comparison of the excellent BRASSS‐V drape and an indirect blood measurement protocol for the measurement of blood loss during third stage of vaginal delivery. clinicaltrials.gov/show/NCT01885845 (first received 19 June 2013).
Patel 2006 {published data only}
- Patel A, Goudar SS, Geller SE, Kodkany BS, Edlavitch SA, Wagh K, et al. Drape estimation vs. visual assessment for estimating postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2006;93(3):220‐4. [DOI] [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- ISRCTN66197422. European project on obstetric haemorrhage reduction: attitudes, trial, and early warning system. isrctn.com/ISRCTN66197422 (first received 6 September 2005).
- Zhang WH, Deneux‐Tharaux C, Brocklehurst P, Juszczak E, Joslin M, Alexander S, et al. Effect of a collector bag for measurement of postpartum blood loss after vaginal delivery: cluster randomised trial in 13 countries. BMJ 2010;340:c293. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Toledo 2007 {published data only}
- NCT00462839. The accuracy of blood loss estimation after simulated vaginal delivery. clinicaltrials.gov/show/NCT00462839 (first received 17 April 2007).
- Toledo P, McCarthy RJ, Fitzgerald PC, Wong CA. Accuracy of blood loss estimation after vaginal delivery. Anesthesiology 2007;107:Abstract no: 773. [DOI] [PubMed] [Google Scholar]
- Toledo P, McCarthy RJ, Hewlett BJ, Fitzgerald PC, Wong CA. The accuracy of blood loss estimation after simulated vaginal delivery. Anesthesia and Analgesia 2007;105(6):1736‐40. [DOI] [PubMed] [Google Scholar]
- Toledo P, Wong CA, Fitzgerald P, McCarthy RJ. Accuracy of blood loss estimation after vaginal delivery. Anesthesiology 2007;106(Suppl 1):10. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12613000628741 {published data only}
- ACTRN12613000628741. A pilot study to evaluate Australian midwives' experiences of using a calibrated blood collection drape to improve estimation of third stage blood loss in labour. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364334 (first received 3 June 2013).
ChiCTR‐IOR‐17012453 {published data only}
- ChiCTR‐IOR‐17012453. Design and application of two sets of measurable liquid collection bag based on delivery position. Design and application of two sets of measurable liquid collection bag based on delivery position. chictr.org.cn/showproj.aspx?proj=19952 (first received 23 August 2017).
Additional references
Alkema 2016
- Alkema L, Chou D, Hogan D, Zhang S. National, regional, and global levels and trends in maternal mortality between 1990 and 2015 with scenario‐based projections to 2030: a systematic analysis by the United Nations Maternal Mortality Estimation Inter‐Agency Group. Lancet 2016;387(10017):462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
ATLS 2013
- American College of Surgeons. Advance Trauma Life Support. 9th Edition. American College of Surgeons, 2013. [Google Scholar]
Beischer 1986
- Beischer NA, Mackay EV. Obstetrics and the Newborn. Eastbourne: Bailliere Tindall, 1986. [Google Scholar]
Bose 2006
- Bose P, Regan F, Paterson‐Brown S. Improving the accuracy of estimated blood loss at obstetric haemorrhage using clinical reconstructions. BJOG 2006;113:919‐24. [DOI] [PubMed] [Google Scholar]
Burchell 1980
- Burchell RC. Postpartum haemorrhage. In: Quilligan ES editor(s). Current Therapy in Obstetrics and Gynaecology. Philadelphia: WB Saunders, 1980. [Google Scholar]
Carroli 2008
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum haemorrhage: a systematic review. Best Practice & Research Clinical Obstetrics and Gynaecology 2008;22:999‐1012. [DOI] [PubMed] [Google Scholar]
CEMACH 2004
- Lewis G, editor. The Sixth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom (CEMACH) 2000–2002. RCOG Press at the Royal College of Obstetricians and Gynaecologists 2004.
Diaz 2014
- Diaz V, Abalos E, Carroli G. Methods for blood loss estimation after vaginal birth. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010980] [DOI] [PMC free article] [PubMed] [Google Scholar]
Didly 2004
- Dildy GA 3rd, Paine AR, George NC, Velasco C. Estimating blood loss: can teaching significantly improve visual estimation?. Obstetrics and Gynecology 2004;104:601‐6. [DOI] [PubMed] [Google Scholar]
Duthie 1991
- Duthie SJ, Ven D, Yung GL, Guang DZ, Chan SY, Ma HK. Discrepancy between laboratory determination and visual estimation of blood loss during normal delivery. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1991;38:119‐24. [DOI] [PubMed] [Google Scholar]
Gilbert 1987
- Gilbert L, Porter W, Brown V. Postpartum haemorrhage ‐ a continuing problem. British Journal of Obstetrics and Gynaecology 1987;94:67–71. [DOI] [PubMed] [Google Scholar]
Hall 1985
- Hall M, Halliwell R, Carr‐Hill R. Concomitant and repeated happenings of complications of the third stage of labour. British Journal of Obstetrics and Gynaecology 1985;92:732–8. [DOI] [PubMed] [Google Scholar]
Hancock 2015
- Hancock A, Weeks A, Lavender T. Is accurate and reliable blood loss estimation the 'crucial step' in early detection of postpartum haemorrhage: an integrative review of the literature. BMC Pregnancy and Childbirth 2015;15(230):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
NCT03135158
- NCT03135158. The association between shock index and severity of postpartum blood loss. clinicaltrials.gov/ct2/show/NCT03135158 (first received 1 May 2017). [NCT03135158]
Newton 2000
- Newton M, Mosey LM, Egli GE, Gifford WB, Hull CT. Blood loss during and immediately after delivery. Obstetrics and Gynecology 1961;17:9‐18. [PubMed] [Google Scholar]
Pacagnella 2013
- Pacagnella RC, Souza JP, Durocher J, Perel P, Blum J, Winikoff B, et al. A systematic review of the relationship between blood loss and clinical signs. PLoS One 2013;8(3):e57594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasertcharoensuk 2000
- Prasertcharoensuk W, Swadpanich U, Lumbiganon P. Accuracy of the blood loss estimation in the third stage of labor. International Journal of Gynecology and Obstetrics 2000;71:69‐70. [DOI] [PubMed] [Google Scholar]
Razvi 1996
- Razvi K, Chua S, Arulkumaran S, Ratnam SS. A comparison between visual estimation and laboratory determination of blood loss during the third stage of labour. Obstetrics and Gynecology 1996;36:152‐4. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Say 2014
- Say L, Chou D, Gemmill A, Tunçalp O, Moller A‐B, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Global Health 2014;2:323‐33. [DOI] [PubMed] [Google Scholar]
Schorn 2010
- Schorn MN. Measurement of blood loss: review of the literature. American College of Nurse‐Midwives 2010;55(1):20‐7. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Stafford 2008
- Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. American Journal of Obstetrics and Gynecology 2008;199(5):519.e1‐7. [DOI: 10.1016/j.ajog.2008.04.049] [DOI] [PubMed] [Google Scholar]
WHO 2010
- Velazquez‐Berumen A, Fahlgren B, Barragan J, Gerrard V. Innovative technologies that address global health concerns. Outcome of the call. Global initiative on health technologies. www.who.int/iris/handle/10665/70522 2010.
WHO 2012
- World Health Organization. WHO Recommendations for the Prevention and Treatment of Postpartum Haemorrhage. 2012. apps.who.int/iris/bitstream/handle/10665/75411/9789241548502_eng.pdf;jsessionid=3BB762D5850A807E4FFD86BB24F05C9D?sequence=1. Geneva: WHO.


