Abstract
Background
Metastatic extradural spinal cord compression (MESCC) is treated with radiotherapy, corticosteroids, and surgery, but there is uncertainty regarding their comparative effects. This is an updated version of the original Cochrane review published in theCochrane Database of Systematic Reviews (Issue 4, 2008).
Objectives
To determine the efficacy and safety of radiotherapy, surgery and corticosteroids in MESCC.
Search methods
In March 2015, we updated previous searches (July 2008 and December 2013) of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, LILACS, CANCERLIT, clinical trials registries, conference proceedings, and references, without language restrictions. We also contacted experts for relevant published, unpublished and ongoing trials.
Selection criteria
Randomised controlled trials (RCTs) of radiotherapy, surgery and corticosteroids in adults with MESCC.
Data collection and analysis
Three authors independently screened and selected trials, assessed risk of bias, and extracted data. We sought clarifications from trial authors. Where possible, we pooled relative risks with their 95% confidence intervals, using a random effects model if heterogeneity was significant. We assessed overall evidence‐quality using the GRADE approach.
Main results
This update includes seven trials involving 876 (723 evaluable) adult participants (19 to 87 years) in high‐income countries. Most were free of the risk of bias.
Different radiotherapy doses and schedules
Two equivalence trials in people with MESCC and a poor prognosis evaluated different radiotherapy doses and schedules. In one, a single dose (8 Gray (Gy)) of radiotherapy (RT) was as effective as short‐course RT (16 Gy in two fractions over one week) in enhancing ambulation in the short term (65% versus 69%; risk ratio (RR) was 0.93, (95% confidence interval (CI) 0.82 to 1.04); 303 participants; moderate quality evidence). The regimens were also equally effective in reducing analgesic and narcotic use (34% versus 40%; RR 0.85, 95% CI 0.62 to 1.16; 271 participants), and in maintaining urinary continence (90% versus 87%; RR 1.03, 95% CI 0.96 to 1.1; 303 participants) in the short term (moderate quality evidence). In the other trial, split‐course RT (30 Gy in eight fractions over two weeks) was no different from short‐course RT in enhancing ambulation (70% versus 68%; RR 1.02, 95% CI 0.9 to 1.15; 276 participants); reducing analgesic and narcotic use (49% versus 38%; RR 1.27, 95% CI 0.96 to 1.67; 262 participants); and in maintaining urinary continence (87% versus 90%; RR 0.97, 0.93 to 1.02; 275 participants) in the short term (moderate quality evidence). Median survival was similar with the three RT regimens (four months). Local tumour recurrence may be more common with single‐dose compared to short‐course RT (6% versus 3%; RR 2.21, 95% CI 0.69 to 7.01; 303 participants) and with short‐course compared to split‐course RT (4% versus 0%; RR 0.1, 95% CI 0.01 to 1.72; 276 participants), but these differences were not statistically significant (low quality evidence). Gastrointestinal adverse effects were infrequent with the three RT regimens (moderate quality evidence), and serious adverse events or post‐radiotherapy myelopathy were not noted.
We did not find trials comparing radiotherapy schedules in people with MESCC and a good prognosis.
Surgery plus radiotherapy compared to radiotherapy
Laminectomy plus RT offered no advantage over RT in one small trial with 29 participants (very low quality evidence). In another trial that was stopped early for apparent benefit, decompressive surgery plus RT resulted in better ambulatory rates (84% versus 57%; RR 1.48, 95% CI 1.16 to 1.90; 101 participants, low quality evidence). Narcotic use may also be lower, and bladder control may also be maintained longer than with than RT in selected patients (low quality evidence). Median survival was longer after surgery (126 days versus 100 days), but the proportions surviving at one month (94% versus 86%; RR 1.09, 95% CI 0.96 to 1.24; 101 participants) did not differ significantly (low quality evidence). Serious adverse events were not noted. Significant benefits with surgery occurred only in people younger than 65 years.
High dose corticosteroids compared to moderate dose or no corticosteroids
Data from three small trials suggest that high‐dose steroids may not differ from moderate‐dose or no corticosteroids in enhancing ambulation (60% versus 55%; RR 1.08, 95% CI 0.81 to 1.45; 3 RCTs, 105 participants); survival over two years (11% versus 10%; RR 1.11, 95% CI 0.24 to 5.05; 1 RCT, 57 participants); pain reduction (78% versus 91%; RR 0.86, 95% CI 0.62 to 1.20; 1 RCT, 25 participants); or urinary continence (63% versus 53%; RR 1.18, 95% CI 0.66 to 2.13; 1 RCT, 34 participants; low quality evidence). Serious adverse effects were more frequent with high‐dose corticosteroids (17% versus 0%; RR 8.02, 95% CI 1.03 to 62.37; 2 RCTs, 77 participants; moderate quality evidence).
None of the trials reported satisfaction with care or quality of life in participants.
Authors' conclusions
Based on current evidence, ambulant adults with MESCC with stable spines and predicted survival of less than six months will probably benefit as much from one dose of radiation (8 Gy) as from two doses (16 Gy) or eight doses (30 Gy). We are unsure if a single dose is as effective as two or more doses in preventing local tumour recurrence. Laminectomy preceding radiotherapy may offer no benefits over radiotherapy alone. Decompressive surgery followed by radiotherapy may benefit ambulant and non‐ambulant adults younger than 65 years of age, with poor prognostic factors for radiotherapy, a single area of compression, paraplegia for less than 48 hours, and a predicted survival of more than six months. We are uncertain whether high doses of corticosteroids offer any benefits over moderate doses or indeed no corticosteroids; but high‐dose steroids probably significantly increases the risk of serious adverse effects. Early detection; and treatment based on neurological status, age and estimated survival, are crucial with all treatment modalities. Most of the evidence was of low quality. High‐quality evidence from more trials is needed to clarify current uncertainties, and some studies are in progress.
Plain language summary
Interventions for the treatment of spinal cord compression due to the spread of cancer
Metastatic extradural spinal cord compression (MESCC) due to cancer from other parts of the body affecting the spine and causing compression of the spinal cord often results in pain, impaired functioning including reduced ability to walk, incontinence, and shortened survival. Radiation is the mainstay of treatment, but surgery, and corticosteroids are also used to treat people with MESCC. This update of a previous review published in 2008 evaluates the clinical trial evidence up to 3 March 2015 to determine how effective radiotherapy, surgery and corticosteroids are in improving functioning and survival, and in reducing pain; and how well tolerated they are in adults with MESCC.
We found seven studies conducted in high‐income countries including 876 adults (aged 19 to 87 years) with MESCC. Follow‐up ranged from one month to three years, and the number evaluated in each varied from 29 to 303. Two studies compared different doses of radiation, two compared surgery before radiation versus radiation alone, and three small trials evaluated the effects of high‐dose corticosteroids versus moderate‐dose steroids or placebo.
The key results are: 1. For different doses of radiation: one dose of radiation was as effective as two doses and two doses were as effective as eight doses of radiation in adults with spinal cord compression with stable spines who are expected to live for less than six months. Adults with a better prognosis may require the longer radiation course to prevent local cancer recurrence, but the immediate benefits of shorter courses might be important for people with MESCC who have only a short time to live. No serious adverse events were noted, and the incidence of diarrhoea, nausea and vomiting was low and no different with the different radiation doses. 2. For surgery before radiation: removing part of the vertebra to enlarge the spinal canal (laminectomy) before radiation offered no advantages over radiation alone. Direct decompressive surgery (directly accessing and removing affected parts of the vertebrae and, if required, fixing the spines using bone grafts and instruments) followed by radiation treatment was more effective than radiation alone in carefully selected adults younger than 65 years. Surgery plus radiation did not cause more harmful effects than radiation alone. 3. For high dose steroids: beneficial effects were not significantly different with high‐dose versus moderate‐dose steroids or placebo, but serious adverse effects were more frequent with high‐dose steroids.
None of the studies reported on satisfaction with care or quality of life. We also did not find trials comparing different radiation doses in adults with MESCC with a good prognosis. We lacked full confidence in many results since they came from single trials or a few small trials. Also, in the study of decompressive surgery, some of the adults given radiation alone had cancers that were only moderately sensitive to radiation, and a third of patients in both intervention arms had unstable spines. In usual clinical practice, surgery, not radiation, is the preferred option in such instances. The overall GRADE quality of evidence was moderate for all outcomes for the comparisons of different radiation doses and for the adverse effects of high doses of corticosteroids, indicating reasonable confidence in the results, though future research could alter the estimates in this review and our confidence in the estimates. The GRADE quality of evidence was very low for all outcomes in the comparison of laminectomy, and low for the outcome of local tumour recurrence with different radiation doses, for all outcomes in the comparison with decompressive surgery, and for the efficacy outcomes in the comparison of high‐dose corticosteroids. This indicates less confidence in these results, and acknowledges that future research is likely to alter the estimates in this review. More studies are required to clarify these uncertainties and some are in progress.
Summary of findings
Background
This review is an update of a previously published review titled, 'Interventions for the treatment of metastatic extradural spinal cord compression in adults' that was published in theCochrane Database of Systematic Reviews (Issue 4, 2008) in The Cochrane Library (George 2008).
Description of the condition
Cancer that spreads to the spinal column, if untreated, can result in back pain, paraplegia, tetraplegia, urinary and bowel incontinence (Loblaw 1998; Holt 2012). Metastatic extradural spinal cord compression (MESCC) is defined as "the compression of the dural sac and its contents, spinal cord or cauda equina, or both, by an extradural tumour mass. The minimum radiologic evidence for cord compression is indentation of the theca, at the level of clinical features" (Laperriere 1996; Loblaw 1998). In this definition, and in clinical practice, the term metastatic spinal cord compression refers to compression of the cord itself, or of the cauda equina more distally within the spinal canal.
Up to 40% of all people with cancer develop spinal metastases (Wong 1990); and in 10% to 20% this can progress to symptomatic cord compression (Schaberg 1985; Klimo 2004). A population‐based study in Canada estimated that at least 2.5% of all people with cancer experienced one or more episodes of spinal cord compression in the five years preceding death (Loblaw 2003). In this study, the cumulative five‐year incidence of cord compression in different types of cancer were: myeloma 8%; prostate 7%; nasopharynx 6.5%; lung 6%; breast 5.5%; kidney 5%; cervix 2.5%; head and neck 0.9%; colorectum 0.8% and stomach 0.6% (Loblaw 2003). The vast majority of spinal metastases in people with MESCC are found in the vertebral body with or without extension into the posterior elements; and also into paravertebral regions and the epidural space; these metastases most commonly affect the thoracic spine (70%), followed by the lumbar spine (20%), and less commonly the cervical spine, and sacrum (Sciubba 2010). MESCC occurs when tumour elements or bone fragments displace the spinal cord within the canal.
MESCC is a condition that can cause significant morbidity and disability in large numbers of people across a spectrum of cancers. However there is surprisingly little evidence to guide clinicians and patients in choosing appropriate treatments.
Description of the intervention
Early detection, with magnetic resonance imaging (MRI) as the preferred imaging modality; and measures to avoid delays in treatment are recommended in the management of people with MESCC (Loblaw 2005). Corticosteroids, radiotherapy and surgery are the treatments currently used for MESCC. The management of cancer pain usually involves the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) therapy with mild and strong opioids, titrated to the severity of pain.
How the intervention might work
Corticosteroids
Corticosteroids reduce inflammation, oedema and pain; and are recommended both for immediate treatment and as an adjunct to radiotherapy or surgery, especially in patients with neurologic deficits (NICE 2008; Loblaw 2012). Corticosteroids have demonstrated neurological improvement in human studies (Sorensen 1994); and in rat experimental models of epidural tumours (Ushio 1977). The British and Canadian guidelines recommend starting with doses of around 16 mg per day (NICE 2008; L’espérance 2012; Loblaw 2012) although some authors use doses up to 100 mg (Patchell 2005). In clinical practice corticosteroid doses vary widely. Studies have found a significant increase in serious adverse effects with high‐dose corticosteroids. In a case control study, 14% of patients receiving 96 mg per day of dexamethasone developed serious adverse events, as compared to 0% of those receiving 16 mg per day (Delattre 1989; Heimdal 1992).
Radiotherapy
Radiotherapy is the most commonly used definitive treatment modality. Radiation therapy can cause the tumour to shrink thereby reducing pressure; pain relief may also occur due to reduced pressure as well through neuropathic components. The outcome of radiotherapy depends on the pre‐ treatment neurologic status. Awareness, early detection and rapid access to treatment are crucial (Loblaw 2005, Maranzano 1991; NICE 2008). A review of prospective non‐randomised studies found that 94% of those who could walk unassisted before radiotherapy and 63% of those who needed assistance for walking, retained ambulation after radiotherapy. Much poorer outcomes were seen in patients who were already paraplegic (12%) or paraparetic (38%) (Loblaw 2005).
The total dose and the number of radiotherapy treatment fractions vary widely (Falkmer 2003; Loblaw 2005; Loblaw 2012; Rades 2004; Rades 2006; Rades 2008; Rades 2011a). In a prospective non‐randomised study that recruited patients with motor deficits in Holland and Germany (‘SCORE‐1’), patients in Holland received short‐course radiotherapy (1 × 8 Gray (Gy) or 5 × 4 Gy) completing treatment within a day or one week. Patients in Germany were treated for two to four weeks with long‐course RT (10 × 3 Gy, 15 × 2.5 Gy or 20 × 2 Gy). Improvement in motor function (37% versus 39%) and one–year overall survival rates (23% versus 30%) were similar, but local control, (61% versus 81%) was significantly better in those who received long‐course radiotherapy (Rades 2011a). The median survival was less than six months. The investigators concluded that short courses would suffice in patients with a poor prognosis. Longer courses and close follow‐up would be needed in those who were likely to live long enough to develop a local recurrence.
Surgery
The main goals of surgery in metastatic spine disease are relief of pain, spine stabilisation and preservation or improvement in neurological function. In addition, many clinicians would consider surgery only if the expected survival was greater than three months (Sciubba 2010). Early surgical treatments such as laminectomy were abandoned as laminectomy alone does not address metastatic disease that is primarily in the vertebral body and the ensuing instability after laminectomy does not make the procedure worthwhile. Ventral approaches on the other hand directly deal with the tumour and provide adequate opportunity for interbody fusion.
A review of studies published from 1964 to 2005 clearly showed that outcomes improved with passing years with the introduction of more aggressive surgical approaches; and the addition of posterior stabilisation after laminectomy followed by RT resulted in substantial improvement in motor function and pain relief with similar mortality rates as laminectomy alone; whereas there was no difference in outcomes in studies using RT alone and in those comparing laminectomy with RT (Witham 2006). However, using anterior decompression with stabilisation demonstrated better motor outcomes but with higher mortality. Klimo 2005, in a meta‐analysis of non‐randomised single‐arm radiotherapy and surgical case series, demonstrated that overall ambulatory success rates for surgery and radiation were 85% and 64% respectively. People who had recently lost the ability to walk were more than twice as likely to regain mobility with surgery compared to radiotherapy.
In people with MESCC with an unknown primary, either a percutaneous CT‐guided biopsy or open surgery and RT are recommended. In patients with radioresistant tumours causing extradural cord compression and instability, surgical decompression with fixation is recommended (Sciubba 2010).
Minimally invasive surgical procedures such as Video‐Assisted Thoracostomy (VAST), Endoscopy‐Assisted Posterior Decompression, and Minimal Access Spine Surgery (MASS), have been reported to offer benefit in people in whom aggressive surgeries are not feasible due to comorbid conditions such as malnourishment and diminished immune systems that make extensive surgical procedures unfeasible. Decreased complication rates, blood loss, and length of stay are considered to be among the benefits of minimally invasive surgery compared to open surgery, but their effects have to date been evaluated only by case reports, case series or retrospective study designs and these designs cannot accurately quantify their efficacy of effectiveness (Molina 2011). Their relative efficacy and safety compared to external radiotherapy is also unclear.
The relationship between treatment modality and prognosis
It is important to identify patients who would benefit from major spine surgery, minimally invasive surgery, or long courses of radiotherapy to optimise ambulation, pain relief and survival. In the earlier version of this review, we had discussed the need for good prognostic scoring systems, preferably based on hazard ratios from prospective studies or randomised trials (George 2008).
Important prognostic factors for survival can be classified into the following domains:
1. The cancer: primary site, visceral metastases, skeletal metastases;
2.The patient’s condition: Karnofsky performance status, ability to walk;
3. The time course: the rate of tumour growth or rapidity of motor deficits, interval from the diagnosis of cancer (Maranzano 1991; Helweg‐Larsen 2000; Rades 2000; Rades 2004; Wang 2004; Van der Linden 2005).
A number of prognostic scoring systems are available:
The Global Spine Tumour Study Group, an international group of spine surgeons, recommends the use of the Tomita and the Tokuhashi scoring systems (Tokuhashi 2005; Tokuhashi 2009; Tomita 2001). The group have also initiated prospective data recording to refine a better score for surgical decision making (Choi 2010). For patients in whom radiotherapy is planned, prognostic scores can help to choose between long‐course radiotherapy, short‐course radiotherapy and supportive care (Rades 2010a; Rades 2013).
Why it is important to do this review
The original review identified six trials that randomised 544 adults with MESCC to different treatment regimens: short‐course radiotherapy (16 Gy in two doses over a week) versus split‐course radiotherapy (30 Gy in eight doses over two weeks); laminectomy followed by radiotherapy versus radiotherapy; decompressive surgery followed by radiotherapy versus conventional radiotherapy; and different doses of corticosteroids or no steroids in people undergoing radiotherapy for MESCC (George 2008). The results showed equal benefit with short‐course RT and the longer split courses of radiotherapy. There also was suggestive evidence for greater benefit with decompressive surgery and post‐operative radiation over radiotherapy alone, though uncertainties remained about the specific patient subgroups that decompression surgery was best indicated in. The evidence also suggested that serious adverse events were less frequent with moderate‐dose over high‐dose steroids in people undergoing radiation for MESCC.
In this Cochrane review update we searched for additional trials evaluating the relative benefits and harms of surgery, radiotherapy, and corticosteroids. We incorporated the standard methods recommended for Cochrane Systematic Reviews (MECIR 2011) that includes more detailed 'risk of bias' assessments than were undertaken in the previous version of this review. We also summarised findings for comparisons using the GRADE approach (Schünemann 2011) that links the effect estimates for important outcomes with the overall confidence one can place in these estimates. As in the previous review, we noted, where reported, the prognostic factors that predicted survival and ambulatory outcomes, in order to discriminate between people who clearly benefit from combined modalities of treatment, those who do well with single modalities of treatment alone, and those with poor prognosis who should be spared complex or prolonged courses of treatment.
Objectives
Our primary objective was to compare the efficacy and harm of treating extradural spinal cord compression for the following:
different schedules of radiation therapy;
surgery with or without radiation therapy versus radiation therapy alone;
the administration of high‐dose corticosteroids (more than 32 mg of dexamethasone equivalent), versus moderate dose (less than 32 mg), or no corticosteroids; with or without surgery/radiotherapy.
Our secondary objectives were:
to compare the adverse effects of surgery, radiotherapy and corticosteroids for metastatic spinal cord compression;
to ascertain if the clinical benefit, if any, was influenced by neurological and oncological factors such as ambulatory status, primary tumour type, duration of cord compression and the presence of visceral metastases, spinal instability or bony collapse.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of surgery, radiotherapy or corticosteroids for spinal cord compression.
Types of participants
People with clinical or radiological evidence of extradural spinal cord compression or cauda equina compression caused by metastatic cancer, or both. We sought trials involving adults (aged eighteen years or more), but would have included reports with younger people where the majority (> 90%) of participants were adults. We included trials with participants who were ambulatory, or with paresis and paraplegia. We excluded trials of primary tumours of the spinal cord.
Types of interventions
Conventional radiation treatment using any dose or fractionation schedule.
Surgery (e.g. laminectomy, decompressive surgery, corpectomy, minimally invasive surgery) with or without radiotherapy versus radiotherapy alone.
High‐dose corticosteroids versus moderate‐dose or no corticosteroids, with or without surgery/radiotherapy.
Types of outcome measures
Primary outcomes
1. Ambulation
Overall ambulatory rates
Proportion of patients maintaining ambulation
Proportion of non‐ambulant patients regaining ambulation
Secondary outcomes
2. Survival
3. Pain relief
Scores using validated pain scales
Use of concomitant analgesics
4. Urinary incontinence
Proportion of patients with bladder control
Percentage of patients maintaining bladder control (absence of urinary catheter)
Percentage of patients regaining bladder control (absence of urinary catheter)
5. Local recurrence
6. Adverse effects as reported for:
Radiotherapy
Surgery
Corticosteroids
7. Quality of life (participant‐ or caregiver‐rated)
8. Participant and caregiver satisfaction
9. Characteristics of participants who benefit from treatment
All outcomes were subgrouped as short term (less than four months), medium term (four months to a year) and long term (more than one year).
Search methods for identification of studies
We attempted to identify all relevant trials, regardless of language or publication status (published, unpublished, or ongoing).
Electronic searches
Databases
On 3 March 2015, we updated previous searches (run in July 2008 and 17 December 2013) of the following electronic databases:
The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 2 of 12, 2015);
MEDLINE & Medline In‐Process (OVID) (1947 to 2nd March 2015);
EMBASE (OVID) (1974 to 2nd March 2015);
CINAHL (|EBSCO) (1982 to March 2015);
LILACS (BIREME) (1982 to March 2015);
CancerLit (PubMED) (2007 to March 2015).
The search strategies used to search these databases are detailed in Appendix 1. The strategies used for the 2008 search are provided in Appendix 2.
Clinical trials registries
We also searched clinical trials registers (http://www.clinicaltrials.gov/; www.trialscentral.org; www.controlled‐trials.com; www.nrr.nhs.uk; http://apps.who.int/trialsearch/) for ongoing trials.
Searching other resources
Conference abstracts
The electronic search included the following conference abstracts
The 19th Annual Meeting of the American College of Radiation Oncology. Cancer Clinical Trials Conference: Las Vegas, United States 2009
The European Association of Neurosurgical Societies, EANS Annual Meeting, Groningen, Netherlands, 2010.
43rd Congress of the International Society of Paediatric Oncology, Pediatric Blood and Cancer Conference, SIOP; Auckland, New Zealand, 2011
Proceedings of the 26th Annual Meeting of the North American Spine Society, NASS; Chicago, Illinois, USA, 2011
European Spine Journal Conference: Annual Meeting of the British Association of Spine Surgeons, BASS: Britspine; Newcastle upon Tyne, UK, 2012
Royal College of Radiologists, 2013 Clinical Radiology Annual Scientific Meeting, London, UK, 2013
European Spine Journal Conference: Annual Meeting of the British Association of Spine Surgeons, BASS: Britspine; Norwich, UK, 2013
96th Annual Meeting of the American Radium Society, St. Thomas, US Virgin Islands, 2014
American Society of Clinical Oncology Annual Meeting, Chicago, Illinois, USA, 2014
Contacts
We contacted the first author of included trials for unpublished trials.
Reference lists
We also searched the references of included trials for other relevant trials.
Data collection and analysis
Selection of studies
For this update, RG, JJ, and PT independently screened and selected studies. We resolved differences regarding trial selection by consensus.
Data extraction and management
For this update, RG, JJ, and PT independently extracted data and this was checked by the other authors. We used a standardised form to extract the following information:
Participant characteristics
The number of participants in the trial; age; gender; their performance and ambulatory status; the investigative techniques and definitions used to diagnose cord compression; the types of primary tumours and the presence or absence of visceral metastases; the duration and rapidity of onset of cord compression; the spinal level and the presence of spinal instability or vertebral collapse.
Intervention details
The year, country and setting in which the trial was conducted; surgical procedures used; radiotherapy doses and schedules; names, and doses of corticosteroids; the provision of rehabilitation services; the timing of these interventions in relation to the development of cord compression and the use of opioids or other analgesics.
Outcome data
Short‐term, intermediate and long‐term ambulatory and survival rates; the definition of ambulation used in the study; urinary sphincter function; the proportion of participants with pain relief or reduced analgesic use; the adverse effects of interventions; quality of life as assessed by any validated scale; and participant satisfaction.
Assessment of risk of bias in included studies
Three authors (RG, JJ, PT) independently assessed the risk of bias in the included trial reports. We assessed the standard six components: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other biases. For each of these components we assigned a judgement regarding the risk of bias as 'high risk', 'low risk', or 'unclear risk' (Higgins 2011a). We attempted to contact the trial authors for clarifications if any of the six components were unclear or not stated in the report. We recorded the results in the standard tables in Review Manager (RevMan 5.3), and summarised the assessments in figures, and graphs.
Measures of treatment effect
For binary outcomes, we calculated risk ratios (RR) with their 95% confidence intervals (CIs). For continuous outcomes we planned to calculate pooled mean differences (MD) and CIs for outcomes using similar measures. If dissimilar measures were used for similar outcomes from trials (such as quality of life scales that measured similar domains) data from these would have been combined using pooled standardised mean difference (SMD) and their 95% CIs.
Unit of analysis issues
We analysed data at the participant level. Had included studies randomised participants to multiple treatment arms, we would have excluded data from intervention arms that were not relevant to this review. If all treatment arms were considered relevant, we would have attempted to combine data in meta‐analysis, if possible, using the methods described in Higgins 2011b.
Dealing with missing data
We attempted to obtain missing data from study authors. We conducted an intention‐to‐treat analysis in trials with no loss to follow‐up and complete‐case analysis for trials with incomplete follow‐up. We did not make any assumptions for missing data for outcomes due to difficulties in making valid assumptions about those lost to follow‐up, apart from what was reported in the trials. However, we used this information in making judgements about the risk of attrition bias and in making judgements about the overall quality of the evidence in summarising findings.
Had there been continuous data for our outcomes of interest we would have reported these as presented in the original studies without making any assumptions about those lost to follow‐up. Where possible we would have reported endpoint data, and if both endpoint and change data were available for the same outcomes, then we would have reported only the former.
Assessment of heterogeneity
We assessed heterogeneity between the trials by examining forest plots for inconsistency in the direction or magnitude of the effect estimates and non‐overlapping confidence intervals. We used the Chi² test for heterogeneity with a 10% level of significance to detect inconsistency in study results that excluded random error, and the I2 statistic to denote the percentage of inconsistency in results due to intertrial variability that exceeded random error (Higgins 2003). In general, we interpreted I2 values of 50% or greater to denote significant heterogeneity (Higgins 2003), though we acknowledged that this cut‐off is arbitrary. We therefore interpreted I2 values between 0% to 40% as possibly unimportant, 30% to 60% as possibly significant, 50% to 90% as possibly substantial, and 75% to 100% as possibly considerable; depending on whether the inconsistency in results was due to differences in the direction of effects estimates between trials, rather than due to differences in the magnitude of effects favouring an intervention; as well as the strength of the evidence for heterogeneity from the P value for the Chi² test for heterogeneity (Deeks 2011).
Assessment of reporting biases
Had there been at least 10 trials in a meta‐analysis we would have considered assessing the likelihood of publication bias by examining the funnel plot for asymmetry due to small study effects.
Data synthesis
We synthesised comparable data from more than one trial using the Mantel‐Haenszel method to derive pooled, weighted risk ratios in fixed‐effect meta‐analyses. We used the random‐effects model for data synthesis when heterogeneity was identified as significant (see above) and could not be explained by subgroup analyses (see below). If I2 values had revealed substantial intertrial variability in effect estimates in excess of chance that were thought not to be due to variations in clinical or methodological attributes, we would have suggested caution in interpreting the pooled estimates.
Subgroup analysis and investigation of heterogeneity
If heterogeneity had been identified as significant, and if data had been available, we would have conducted subgroup analyses according to the following categories:
ambulant versus non‐ambulant patients
the presence or absence of unfavourable radiological features such as bony instability, or vertebral collapse
primary tumour type
presence or absence of visceral metastases
screening versus no screening for cord compression
The included trials reported results separately for ambulant versus non‐ambulant participants and the results of these subgroups are reported as the proportions maintaining and regaining ambulation.
Sensitivity analysis
We planned to conduct sensitivity analyses to investigate the robustness of the results of meta‐analyses if included trials were at high risk of bias, for any assumptions made in data analyses, and if trials had more than 20% lost to follow‐up. .
Summarising and interpreting results
We used the GRADE approach to interpret findings (Schünemann 2011); and used GRADE Profiler (Gradepro 2008) to import data from Review Manager (RevMan 5.3) to create 'Summary of findings' tables for each comparison included in this review. These tables provide information concerning the overall quality of the evidence from the trials, the magnitude of effect of the interventions examined, and the sum of available data on the primary outcome and selected secondary outcomes.The GRADE approach integrates evaluations regarding study limitations; unexplained inconsistency in the results; indirectness (how representative of clinical practice the populations studied were; the deviations from accepted practice in the way interventions and comparisons were given; the choice of outcomes as representative of those considered important to clinical decision making; and the methods used in assessing these outcomes); imprecision in the estimates (in terms of statistical significance as well as clinical importance); and the likelihood that publication bias affected the estimates.
We selected the following outcomes that we considered important or critically important to clinical decision making for inclusion in these tables:
Ambulation
Survival
Pain relief
Urinary continence
Local recurrence
Adverse events
Quality of life
Results
Description of studies
Results of the search
The 2008 search yielded 1255 citations (CENTRAL 114, MEDLINE 845, EMBASE 226, CINAHL 24, LILACS 22, CANCERLIT 24) of which we selected seven reports for evaluation; six trials were included in the review; one citation was referenced under excluded studies; and two on‐going studies were identified (George 2008).
The update searches to March 2015 yielded 807 additional citations (CENTRAL 32, MEDLINE 295, EMBASE342, CINAHL 18, LILACS 19, CANCELIT 101), which after de‐duplication left 686 unique citations. Of these, full copies of 17 potentially eligible reports were obtained; the remainder were not relevant to this review. Of the 17 reports, seven studies (described in eight reports) were excluded (see Characteristics of excluded studies). Of the remaining nine reports, three pertaining to two new trials were selected for inclusion in this review update; one trial described in two reports is included in quantitative synthesis in this review (Maranzano 2009); while the other trial reported only as a conference abstract awaits assessment (Hegazy 2012). Maranzano 2005 and Patchell 2005, two of the six trials included in the 2008 review, are referenced along with the primary studies in this update.
The two ongoing studies identified in the 2008 review are still recruiting participants (ISRCTN97555949; NCT00968643).
The process of study selection is described in Figure 1.
1.

PRISMA flow diagram depicting study selection
Included studies
This review update includes seven trials (described in 23 reports) that are detailed in 'Characteristics of included studies' tables. Relevant aspects of these trials are summarised below.
Study design
The seven trials were randomised parallel group trials with two intervention arms. Three were conducted before 1995 (Sorensen 1994; Vecht 1989; Young 1980); and the most recent trial was completed in 2007 (Maranzano 2009). One trial recruited participants over a period of 10 years (1992 to 2002) and was stopped early after recruiting 50% of the planned sample due to perceived benefit with one intervention (Patchell 2005). Another trial also had difficulty in recruiting sufficient participants over a three‐year period (Graham 2006).
Three of the trials used stratified randomisation: Patchell 2005 stratified participants by treating institution, tumour type, ambulatory status and relative stability of the spine; Sorensen 1994 stratified randomisation by primary tumour (breast or other cancers) and gait function (ambulant, non‐ambulant); Vecht 1989 stratified for carcinoma versus reticular malignancy.
Two of the trials were powered to detect equivalence between interventions and achieved the required sample size and response rates within pre‐set precision limits to demonstrate this (Maranzano 2005; Maranzano 2009). Only one included trial was registered in a clinical trials registry, and even this trial was retrospectively registered (Graham 2006). The duration of follow‐up in the trials ranged from one week after treatment (Vecht 1989); to nearly five years (Maranzano 2005; Maranzano 2009); with many trials following participants till death.
Location
Sorensen 1994 was conducted in a single institution while the remainder were multi‐institutional trials in the same country. None of the trials were conducted in low‐ or middle‐income countries. Two trials were conducted in Italy (Maranzano 2005; Maranzano 2009); and two in the USA (Patchell 2005; Young 1980). The other trials were conducted in Australia (Graham 2006); Denmark (Sorensen 1994); and the Netherlands (Vecht 1989).
Participants
The seven trials randomised a total of 876 participants, 723 of whom were evaluable. The sample sizes of these trials ranged from 20 (Graham 2006); to 300 in Maranzano 2005; and 327 in Maranzano 2009. All trials recruited more males than females, except Sorensen 1994 that recruited more females (with the majority having primary breast malignancies). The age of participants in the trials ranged from 19 to 87 years.
The trials used different criteria to select participants with MESCC. All trials required the presence of a primary non‐central nervous system tumour and the demonstration of cord compression by either MRI or CT (Graham 2006; Maranzano 2005; Maranzano 2009; Patchell 2005), or by myelogram in the older studies (Sorensen 1994; Vecht 1989; Young 1980). Two trials specifically selected participants with a poor prognosis (life expectancy of six months or less, as defined by unfavourable histologies; or favourable histologies with poor performance status; or motor or sphincter dysfunction) (Maranzano 2005; Maranzano 2009). The two trials of surgical interventions selected participants with a better prognosis (Graham 2006; and particularly Patchell 2005).
The duration and rapidity of cord compression was not reported in any trial except Patchell 2005, where the onset of total paraplegia of less than 48 hours was an inclusion criterion. All trials except two reported the spinal level of cord compression (Vecht 1989; Young 1980); the thoracic spine was the most commonly reported site of the compression in more than 50% of participants in these trials. Spinal instability was an exclusion criterion in three trials (Maranzano 2005; Maranzano 2009; Sorensen 1994); was a stratifying variable in one trial (Patchell 2005); and was not reported in the other trials.
Interventions and comparisons
The seven trials evaluated different radiotherapy schedules (two trials), different surgical approaches with radiotherapy compared to radiotherapy alone (two trials); and high doses of corticosteroids versus moderate doses of steroids or no steroids (three trials).
1. Different radiotherapy doses and schedules
1.1 Single‐fraction radiotherapy (8 Gy) versus a short course of radiotherapy (two fractions of 8 Gy)
Maranzano 2009, in a multi‐centre trial in Italy, randomised 327 adults with MESCC with no indications for primary surgery (diagnostic doubt, vertebral instability or bony impingement as the cause of cord compression, or previous radiotherapy of the same area) and an estimated survival of six months or less (due to unfavourable histologies; or, if the primary tumour histology was favourable, with motor or sphincter dysfunction, or poor performance status). Participants were randomised to a single dose of radiation (8 Gy) or to a short course of radiotherapy (8 Gy × 2; delivered as 8 Gy, 6 days' rest, and then 8 Gy to a total dose of 16 Gy in 1 week). Screening was actively done for early diagnosis of MESCC with MRI or CT in those with back pain or osteolysis, or a positive bone scan, even in the absence of neurological clinical signs of spinal cord compression. Radiotherapy (RT) was started within 24/48 hours of the radiologic diagnosis and was delivered by a 4 to 18 MV linear accelerator. Parenteral dexamethasone (8 mg twice daily) was administered from the first day of clinical‐radiologic diagnosis until 4 to 5 days after the end of RT in both arms. Parenteral 5‐hydroxytryptamine‐3 receptor antagonists were also given to those in whom radiation included the upper abdomen (30% in each arm). Participants were assessed one month after the end of RT and the follow‐up examination was continued once a month for one year, and four times per year until death. Of the 327 randomised, 303 (93%) were evaluable. The median follow‐up was 36 months (range 4 to 58 months).
Of the 327 randomised, 134 (44%) had bone and visceral metastases (liver, lung, brain or a combination); 271 (89%) had back pain; 199 (65%) were able to walk pre‐treatment (114 (37%) without support and 85 (28%) with support); 78 (26%) were non‐ambulant; and 26 (9%) were paraplegic. Bladder dysfunction was present in 41 (14%) pre‐treatment. In the 303 evaluable participants, 212 (70%) had unfavourable primary tumour histologies, and 91 (30%) had favourable primaries for response to RT. These baseline characteristics were similar in the intervention arms.
1.2 Eight fractions "split‐course regimen" versus two fractions "short‐course regimen"
Maranzano 2005, in an earlier multi‐centre trial in Italy, randomised 300 participants with no indications for surgery and with a poor prognosis (as in Maranzano 2009) to receive eight fractions: "split‐course regimen" (5 Gy x 3, 4 days rest; then 3 Gy x 5, to a total of 30 Gy in 2 weeks); or to two fractions: "short‐course regimen" (8 Gy, 6‐days rest, and then 8 Gy, to a total of 16 Gy in one week). RT was started within 24 hours of the radiologic diagnosis. The timing of intervention in relation to development of cord compression was not reported. Dexamethasone 8 mg twice daily was given to all participants and tapered after completion of RT. Parenteral 5‐hydroxytryptamine‐3 receptor antagonists were also given to those in whom radiation included the upper abdomen (60%). Here, too, assessments were done one month after the end of radiotherapy and follow‐up examinations were continued once a month for one year, and four times per year until death. The median follow‐up was 33 months (range: 4 to 61 months).
As in Maranzano 2009, in the majority (177 of 276 evaluable participants (64%)), the histology of the primary tumour was unfavourable, and 99 (36%) had favourable histological primary tumours. Of the 276 evaluable participants, 262 (95%) had back pain pre‐treatment; 184 (67%) were able to walk (107 (39%) without support and 77 (28%) with support); 75 (27%) were non‐ambulant; and 17 (6%) were paraplegic. Bladder dysfunction was present in 29 (11%) pre‐treatment. Baseline characteristics were similar in the intervention arms.
2. Different surgical approaches with radiotherapy compared to radiotherapy alone
2.1 Laminectomy with postoperative radiotherapy versus radiotherapy alone
Young 1980 was the first randomised trial in people with MESCC and was conducted in two centres in the United States of America (USA). Twenty‐nine participants with haematologic and solid tumours, and a myelographic diagnosis of cord compression, were randomised to laminectomy and post‐operative radiotherapy or radiotherapy alone. Participants were followed up till death, but the average duration of follow‐up was not reported. Complete myelographic block (22/29) was significantly more common in the surgery group (15/16; 94%) than the RT group (7/13; 54%) and was a confounding factor that negatively influenced some outcomes. The authors emphasised the need to stratify patients by clinically significant prognostic factors in future studies.
2.2. Direct decompressive surgery and postoperative radiotherapy versus radiotherapy alone
Patchell 2005 was a randomised multicentric trial conducted in the USA. The trial recruited participants with an MRI diagnosis of a single area of MESCC, an expected survival of at least three months, and non‐radiosensitive primaries. Participants should not have been paraplegic for greater than 48 hours. Over one third of participants had spinal instability or pathologic spine fractures. One hundred and one participants were randomised to surgical decompression and postoperative radiotherapy (with stabilisation if instability was present); or to radiotherapy alone, 30 Gy in 10 fractions. All patients were given high‐dose dexamethasone 100 mg initially that was tapered until completion of radiotherapy. Ten patients from the radiotherapy arm crossed over to surgery because of deteriorating neurological status. After 10 years of recruitment, the study was stopped with 50% recruitment because results in the surgical arm were superior in an interim analysis with prespecified stopping rules.
3. High dose corticosteroids versus moderate dose steroids or no steroids (placebo)
Three trials (Graham 2006; Sorensen 1994; Vecht 1989) with participant numbers ranging from 20 to 57 assessed the role of corticosteroids in patients receiving conventional radiotherapy for MESCC. Sorensen 1994 and Graham 2006 excluded haematologic malignancies. Graham 2006 and Vecht 1989 compared high‐dose boluses of dexamethasone of 96 mg to 100 mg, with moderate doses of 10 mg to 16 mg. Sorensen 1994 compared a single dose of 100 mg dexamethasone with saline placebo. Graham 2006 administered omeprazole and nystatin as prophylaxis to all participants.
Outcomes
Primary outcome
Ambulation
All trials reported ambulation as a primary outcome but used different criteria to define ambulation. Patchell 2005 reported a participant as ambulant if he or she was able to take at least two steps with each foot (four steps in total) either unassisted or with use of a cane or walker at completion of radiotherapy. Maranzano 2005 and Maranzano 2009 used Tomita 2001 grades for ambulation (group I – ability to walk without support; group II – ability to walk with support; group III – inability to walk; and group IV – paraplegic) where participants who were walking with or without support at one month were considered ambulant. The three corticosteroid studies measured ambulation in different ways and at different time points. Graham 2006 used different definitions of ambulation within the trial but did not provide these definitions in the trial report; the report also noted variability in ambulatory outcomes depending on the definition used. Vecht 1989 used five grades of ambulation, with Grade I (walking independently) and Grade II (walking with support) used to denote good ambulation compared to Grade III (walking not possible but both legs can be lifted from the bed), Grade IV (lifting of legs not possible but muscle contraction is present in legs), and Grade V (absence of muscle contractions in legs). Young 1980 defined ambulation (in a similar manner to Patchell 2005) as the ability to take steps (number not specified) alone, even if a cane or walker was required.
Data were provided for proportions ambulant pre‐treatment and overall ambulatory rates after treatment in all trials. The proportions maintaining and regaining ambulation were reported in all trials except two (Graham 2006; Vecht 1989).
Secondary outcomes
Survival: all trials reported survival except Vecht 1989. However, many did not report survival data in a form that could be analysed as they were reported as medians, or as the percentage probability of survival.
Pain relief: five trials provided data on pain relief as reduction in analgesic and narcotic use or as proportions with pain reduction (Maranzano 2005; Maranzano 2009; Patchell 2005; Vecht 1989; Young 1980). In the remaining trials this was not reported or was reported in a form that could not be used.
Urinary incontinence: Maranzano 2005, Maranzano 2009, Vecht 1989, and Young 1980 also provided usable data on urinary continence. This was defined in two as not requiring a urinary catheter (Maranzano 2005; Maranzano 2009). Patchell 2005 reported the median duration of maintaining urinary continence, but not the proportions with urinary continence.
Local recurrence: only two trials provided data on in‐field recurrences (Maranzano 2005; Maranzano 2009).
Adverse events: all trials provided data on adverse events though in some it was unclear if they were systematically ascertained.
Quality of life: none of the trials reported this outcome.
Participant or caregiver satisfaction: none of the trials reported this.
Characteristics of participants who benefit from treatment: four trials provided this information (Maranzano 2005; Maranzano 2009; Patchell 2005; Young 1980).
Excluded studies
This update contains the references to, and reasons for exclusion of, eight studies (described in nine reports) in people with MESCC (see: Excluded studies). Only one was an RCT but the comparisons evaluated were not relevant to this review (Holden 2011).
Ongoing trials
The two ongoing trials (ISRCTN97555949 and NCT00968643) identified by the 2008 search are still ongoing. ISRCTN97555949 is an RCT of single‐fraction radiotherapy compared to multi‐fraction radiotherapy in patients with metastatic spinal cord compression. Although scheduled for completion in August 2009, the contact investigator informed us that they expect to complete recruitment by the end of 2014. NCT00968643 is a randomised phase III trial of two fractionation schemes in the treatment of malignant spinal cord compression that is currently recruiting participants. They are described in more detail in Characteristics of ongoing studies.
Risk of bias in included studies
The details of 'risk of bias' assessments are provided in the 'Characteristics of included studies' table, and are summarised in Figure 2 and Figure 3.
2.
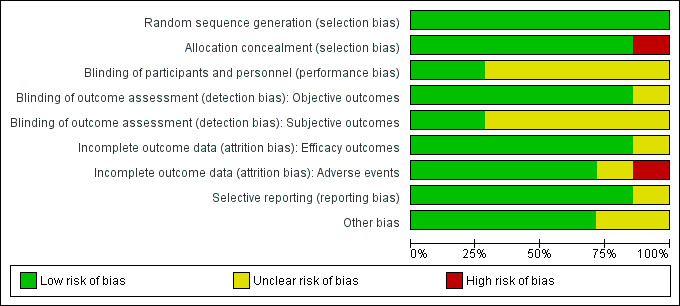
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
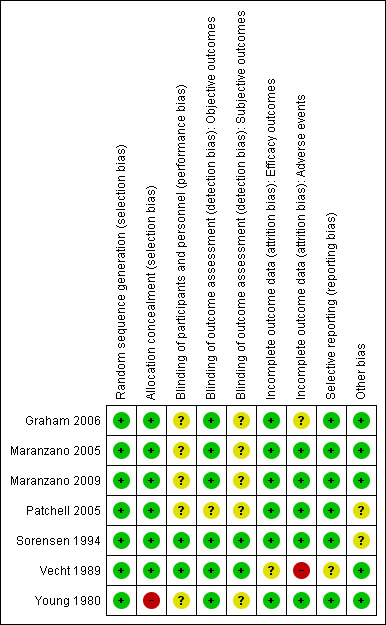
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies were judged at low risk for selection bias except Young 1980, that was judged at high risk of selection bias since allocation concealment was not reported and baseline imbalances in prognostic variables were evident.
Blinding
Because of the nature of the studies, blinding of clinicians and participants was not practical for the radiotherapy and surgical trials. Sorensen 1994 and Vecht 1989 were judged at low risk of performance and detection bias while the remainder were judged at unclear risk on this domain (Figure 3).
Incomplete outcome data
Vecht 1989 was judged unclear for the risk of attrition bias for efficacy outcomes and at high risk of attrition bias for adverse events. Graham 2006 was judged unclear for the risk of attrition bias for reporting adverse events.
Selective reporting
None of the trials were prospectively registered (Graham 2006 was retrospectively registered) and the trial protocols were not available for any trial. However, no evidence of selective reporting was detected.
Other potential sources of bias
Patchell 2005 was prematurely terminated for apparent benefit after only 50% recruitment had been achieved. This is often considered a source of bias, since such truncated RCTs are associated with greater effect sizes than RCTs not stopped early, with this difference being greatest in smaller studies (particularly those that have fewer than 200 events), and independent of the presence of statistical stopping rules, even if they were pre‐determined (Bassler 2010). However, following updated advice in Higgins 2011a (Table 8.5b), we judged this trial as unclear for the risk of bias due to premature stopping. No other sources of bias were evident in any trial.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Single‐fraction radiotherapy (8 Gy) compared to short‐course radiotherapy (16 Gy in two fractions) for adults with metastatic extradural spinal cord compression.
| Single‐fraction radiotherapy (8 Gy) compared to short‐course radiotherapy (8 Gy ‐ two fractions over one week) for adults with metastatic extradural spinal cord compression | ||||||
| Patient or population: adults with metastatic extradural spinal cord compression (poor prognosis with visceral metastasis, and no spinal instability or bony impingement of cord) Intervention: single‐fraction (8 Gy) radiotherapy Comparison: short‐course (8 Gy ‐ two fractions over one week) radiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐course radiotherapy | Single‐fraction radiotherapy | |||||
|
Ambulation One month after radiation |
693 per 1000 | 645 per 1000 (569 to 721) | RR 0.93 (0.82 to 1.04) | 303 (1 study1) | ⊕⊕⊕⊝
moderate2,3 (serious indirectness) |
Single‐fraction and short‐course radiotherapy are probably equally effective in enhancing ambulation (maintaining and regaining ambulation) in the short term. |
| Survival Follow‐up: mean 36 months | See comment | See comment | Not estimable | 0 (1 study) | ⊕⊕⊕⊝
moderate2,3 (serious indirectness) |
Median survival was similar with both radiotherapy schedules (four months). |
|
Reduction in analgesic and narcotic use One month after radiation |
403 per 1000 | 343 per 1000 (250 to 467) | RR 0.85 (0.62 to 1.16) | 271 (1 study) | ⊕⊕⊕⊝
moderate2,3 (serious indirectness) |
Single‐fraction and short‐course radiotherapy are probably equally effective in reducing analgesic and narcotic use in the short term. |
|
Urinary continence One month after radiation |
873 per 1000 | 900 per 1000 (838 to 961) | RR 1.03 (0.96 to 1.1) | 303 (1 study) | ⊕⊕⊕⊝
moderate2,3 (serious indirectness) |
Single‐fraction and short‐course radiotherapy are probably equally effective in enhancing urinary continence overall, and in the proportions maintaining or regaining continence one month after treatment. |
| Local recurrence MRI: follow‐up: median 36 months | 27 per 1000 | 59 per 1000 (18 to 187) | RR 2.21 (0.69 to 7.01) | 303 (1 study) | ⊕⊕⊝⊝
low2,4 (serious indirectness, serious imprecision) |
Short‐course radiotherapy may result in fewer local recurrences than single fraction radiotherapy, but the numbers with local recurrences were too few for the difference to be statistically significant. |
| Adverse events Grade 3 oesophagitis, diarrhoea and nausea | 20 per 1000 | 3 per 1000 (0 to 54) | RR 0.14 (0.01 to 2.69) | 303 (1 study) | ⊕⊕⊕⊝
moderate4 (serious indirectness) |
Single‐fraction and short‐course radiotherapy probably do not differ significantly in the incidence of gastrointestinal adverse effects. Serious adverse events or post‐radiotherapy myelopathy were not noted with either treatment schedule. |
| Quality of life | See comment | See comment | Not estimable | ‐ | ‐ | Not assessed. |
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Maranzano 2009 was conducted in multiple sites in Italy and used an equivalence design with the sample size powered to demonstrate equivalence in response rates separately evaluated for ambulatory status (maintaining or regaining ambulation), urinary continence (not requiring a catheter), and reduction in back pain (not requiring a narcotic) one month after treatment. Parenteral dexamethasone (8 mg twice daily) was administered from the first day of clinical‐radiologic diagnosis for 4–5 days. 2 Serious indirectness: the trial included those usually given short‐course radiotherapy (those with poor prognosis, with visceral metastasis, and not suitable for surgery). However the evidence for the equivalence of single dose and short‐course radiotherapy is from only one trial from a high‐income country, where early diagnosis and early institution of radiotherapy (within 24 to 48 hours after diagnosis) was possible; these may not be possible in many resource constrained settings. Downgraded 1 level. 3 No imprecision: the trial was powered to demonstrate equivalence in response rates post‐treatment and the difference in response rates with the two radiotherapy schedules was within the pre‐set precision limits. Not downgraded. 4 Serious imprecision: the trial was not powered to detect equivalence for this outcome; the 95% CI of the effect estimate includes no difference. The number of events were few and the sample size was smaller than the optimal sample size. Downgraded 1 level.
Summary of findings 2. Split‐course radiotherapy (8 fractions, 30 Gy) compared to short‐course radiotherapy (2 fractions, 16 Gy) for adults with metastatic extradural spinal cord compression.
| Split‐course (8 fractions, 30 Gy) radiotherapy compared to short‐course (2 fractions, 16 Gy) radiotherapy for adults with metastatic extradural spinal cord compression | ||||||
| Patient or population: adults with metastatic extradural spinal cord compression (poor prognosis, no spinal instability or bony impingement of cord) Intervention: split‐course radiotherapy (8 fractions‐ 5 Gy × 3; 3 Gy × 5 = 30 Gy) Comparison: short‐course radiotherapy (2 fractions × 8 Gy = 16 Gy) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Short‐course radiotherapy (2 fractions) | Split‐course radiotherapy (8 fractions) | |||||
| Ambulation One month after treatment | 683 per 1000 | 697 per 1000 (615 to 786) | RR 1.02 (0.9 to 1.15) | 276 (1 study1) | ⊕⊕⊕⊝
moderate2,3 (serious indirectness) |
Split‐course and short‐course radiotherapy are probably equally effective in enhancing ambulation (maintaining and regaining ambulation) in the short term. |
| Survival Follow‐up: median 33 months | See comment | See comment | Not estimable | 0 (1 study) | ⊕⊕⊕⊝
moderate2,3 (serious indirectness) |
Median survival was similar with both treatment schedules (four months). |
|
Reduction in analgesic and narcotic use One month after radiation |
382 per 1000 | 486 per 1000 (367 to 639) | RR 1.27 (0.96 to 1.67) | 262 (1 study) | ⊕⊕⊕⊝
moderate2,3 (serious indirectness) |
Split‐course and short‐course radiotherapy are probably equally effective in reducing analgesic and narcotic use in the short term. |
|
Urinary continence One month after radiation |
901 per 1000 | 874 per 1000 (838 to 919) | RR 0.97 (0.93 to 1.02) | 275 (1 study) | ⊕⊕⊕⊝
moderate2,3 (serious indirectness) |
Split‐course and short‐course radiotherapy are probably equally effective in enhancing urinary continence overall, and in the proportions maintaining or regaining continence one month after treatment. |
| Local recurrence Follow‐up: median 33 months | 35 per 1000 | 4 per 1000 (0 to 61) | RR 0.1 (0.01 to 1.72) | 276 (1 study) | ⊕⊕⊝⊝
low2,4 (serious indirectness, serious imprecision) |
Split‐course radiotherapy probably results in fewer local recurrences than short‐course radiotherapy, but the numbers with local recurrences were too few to demonstrate statistically significant differences. |
| Adverse events Grade 3 oesophagitis, diarrhoea, and nausea | 21 per 1000 | 37 per 1000 (9 to 153) | RR 1.77 (0.43 to 7.25) | 276 (1 study) | ⊕⊕⊕⊝
moderate4 (serious indirectness) |
Split‐course and short‐course radiotherapy probably do not differ significantly in the incidence of gastrointestinal adverse effects. Serious adverse events or post‐radiotherapy myelopathy were not noted with either treatment schedule. |
| Quality of life | See comment | See comment | Not estimable | ‐ | ‐ | Not assessed |
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Maranzano 2005 was conducted in multiple sites in Italy and used an equivalence design with the sample size powered to demonstrate equivalence in response rates separately evaluated for ambulatory status (maintaining or regaining ambulation), urinary continence (not requiring a catheter), and reduction in back pain (not requiring a narcotic) one month after treatment.. Dexamethasone: 8 mg twice daily was given to both arms and tapered after completion of radiotherapy. 2 Serious indirectness: the trial included those usually given short courses of radiotherapy (patients with poor prognosis; no spinal instability or bony impingement causing cord compression). However this trial is from a high‐income country where early diagnosis and early institution of radiotherapy (within 24 hours after diagnosis) was possible; these may not be possible in many low‐ and middle‐income settings. Downgraded 1 level. 3 No imprecision: the trial was powered to demonstrate equivalence in response rates post‐treatment and the difference in response rates with the two radiotherapy schedules was within the pre‐set precision limits. Not downgraded. 4 Serious imprecision: the trial was not powered to detect equivalence for this outcome.. The number of events were few, and the 95% CI of the risk difference indicated non‐appreciable benefits with both schedules. The sample size was smaller than the optimal sample size. Downgraded 1 level.
Summary of findings 3. Laminectomy plus radiotherapy compared to radiotherapy for adults with metastatic extradural spinal cord compression.
| Laminectomy plus radiotherapy compared to radiotherapy for adults with metastatic extradural spinal cord compression | ||||||
| Patient or population: adults with metastatic extradural spinal cord compression (single lesion, no prior radiotherapy, fit for surgery) Intervention: laminectomy plus radiotherapy Comparison: radiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Radiotherapy | Laminectomy plus radiotherapy | |||||
|
Ambulation At four months |
385 per 1000 | 373 per 1000 (146 to 954) | RR 0.98 (0.38 to 2.48) | 29 (1 study1) | ⊕⊝⊝⊝
very low2,3,4 (risk of bias, serious indirectness, very serious imprecision) |
Laminectomy followed by radiotherapy may offer no advantage over radiotherapy alone in enhancing ambulation. . |
|
Survival At four months |
462 per 1000 | 563 per 1000 (272 to 1000) | RR 1.22 (0.59 to 2.53) | 29 (1 study) | ⊕⊝⊝⊝
very low3,4 (serious indirectness, very serious imprecision) |
Laminectomy followed by radiotherapy may offer no advantage over radiotherapy alone in improving survival. |
| Reduction in analgesic use | 500 per 1000 | 440 per 1000 (210 to 905) | RR 0.88 (0.42 to 1.81) | 26 (1 study) | ⊕⊝⊝⊝
very low3,4 (serious indirectness, very serious imprecision) |
Laminectomy followed by radiotherapy may offer no advantage over radiotherapy alone in reducing analgesic use. |
|
Urinary continence One month after treatment |
538 per 1000 | 32 more per 1000 (232 fewer to 538 more) | RR 1.06 (0.57 to 2.00) | 29 (1 study) | ⊕⊝⊝⊝
very low2,3,4 (risk of bias, serious indirectness, very serious imprecision) |
Laminectomy followed by radiotherapy may offer no advantage over radiotherapy alone in enhancing urinary continence. |
| Local recurrence | See comment | See comment | Not estimable | ‐ | ‐ | Not assessed |
| Adverse events | See comment | See comment | Not estimable | 29 (1 study) |
‐ | No adverse events were reported with laminectomy plus RT or with RT, but it is unclear if these were systematically ascertained |
| Quality of life | See comment | See comment | Not estimable | ‐ | ‐ | Not assessed |
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Data from Young 1980: radiotherapy was given as 30 Gy in 10 fractions (4 Gy/day first 3 days, then 18 Gy in 7 fractions over 14 days), Both arms were given dexamethasone 12 mg; followed by 4 mg four times daily till radiotherapy completion.
2 Serious risk of bias. There were baseline imbalances in the proportions with myelographic block and this negatively influenced effect estimates for the outcomes of ambulation and urinary continence. Downgraded 1 level. 3 Serious indirectness: the trial included those likely to be offered a surgical intervention in preference to radiotherapy. However, the data for the comparative effects of laminectomy versus radiotherapy come from only one small trial done over 30 years ago. Downgraded 1 level. 4 Very serious imprecision. The 95% CI of the effect estimate indicates appreciable benefits for both interventions. The number of events and participants were too few to provide reliable estimates. Downgraded by 2 levels.
Summary of findings 4. Decompressive surgery plus radiotherapy compared to radiotherapy for adults with metastatic extradural spinal cord compression.
| Decompressive surgery plus radiotherapy compared to radiotherapy for adults with metastatic extradural spinal cord compression | ||||||
| Patient or population: adults with metastatic extradural spinal cord compression (fit for surgery, paraplegic less than 48 hours, and without multiple discrete compressions or radiosensitive tumours; with cervical or thoracic lesions, and life expectancy three months or more) Intervention: decompressive surgery plus radiotherapy Comparison: radiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Radiotherapy | Decompressive surgery plus radiotherapy | |||||
|
Ambulation Immediately after completing RT |
569 per 1000 | 842 per 1000 (660 to 1000) | RR 1.48 (1.16 to 1.90) | 101 (1 study1) | ⊕⊕⊝⊝
low2,3 (serious indirectness, serious imprecision) |
Surgery plus RT may be superior to RT in enhancing ambulation (maintaining ambulation: and regaining ambulation) in selected patients with MESCC |
|
Survival (short term) At 30 days |
863 per 1000 | 940 per 1000 (828 to 1000) | RR 1.09 (0.96 to 1.24) | 101 (1 study) | ⊕⊕⊝⊝
low2,3 (serious indirectness, serious imprecision) |
Surgery plus RT may not significantly enhance proportions surviving compared to RT (though median survival may be longer with surgery plus RT: 126 days versus 100 days). |
| Reduction in analgesic and narcotic use | See comment | See comment | Not estimable | 101 (1 study) |
See comment | Surgery plus RT may have beneficial effects compared to RT in reducing analgesic use (median daily morphine equivalent dose: 0·4 mg (0 to 60 mg) versus 4·8 mg (0 to 200 mg; P = 0·002). |
| Urinary continence | See comment | See comment | Not estimable | 101 (1 study) |
See comment | Surgery plus RT may have beneficial effects compared to RT in enhancing the maintenance of urinary continence (median duration for maintenance of continence: 156 days versus 17 days; P = 0.016)4. |
| Local recurrence | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Adverse events | See comment | See comment | Not estimable | 101 (1 study) |
See comment | No serious adverse events were seen after surgery or after RT. In 10 participants randomised to RT who subsequently had surgery, 4 (40%) had surgical complications (wound infections‐3; failure of fixation‐1). Other adverse events were not reported. |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk is the risk in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Data from Patchell 2005: surgery ‐ direct circumferential decompression with or without stabilisation within 24 hours of randomisation; Radiotherapy‐ 3 Gy x 10, starting within 24 hours after randomisation (in most), or within 14 days of surgery. All patients were given 100 mg dexamethasone immediately at diagnosis, then 24 mg every 6 hours until the start of radiotherapy or surgery, after which steroids were then reduced and continued until completion of radiotherapy. 2 Serious indirectness: this trial selected patients who were good candidates for surgery and excluded participants with radiosensitive tumours; 18 patients given RT had unstable spines. These biased the results against radiotherapy and the participants given RT were not representative of the usual candidates for RT. Emergency surgery was offered within 24 hours of the diagnosis of cord compression, within 48 hours of onset of paraplegia and within two weeks of the onset of symptoms in most. RT was also offered as an emergency. These may not be feasible outside a clinical trial setting and in healthcare systems in resource poor countries. Downgraded 1 level. 3 Serious imprecision. This trial was stopped early for apparent benefit after recruiting only 50% of the estimates sample. Truncated RCTs are at risk of over‐estimating benefits (Bassler 2010). The evidence in favour of surgery comes from only one trial with 101 participants, and the sample size is smaller than the optimal information size. Downgraded 1 level.
4 Based on secondary analysis in the Patchell 2005 report using a Cox model with all covariates included
Summary of findings 5. High‐dose corticosteroids compared to moderate‐dose or no corticosteroids for adults with metastatic extradural spinal cord compression.
| High‐dose corticosteroids compared to moderate‐dose or no corticosteroids for adults with metastatic extradural spinal cord compression | ||||||
| Patient or population: adults with metastatic extradural spinal cord compression (without peptic ulceration or infection; mixed prognosis) Intervention: high‐dose corticosteroids (96 mg and 100 mg bolus) Comparison: moderate‐dose (10 mg and 16 mg) or no corticosteroids | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Moderate‐dose or no corticosteroids | High dose corticosteroids | |||||
|
Ambulation Follow‐up: one week to three months |
550 per 1000 | 594 per 1000 (446 to 798) | RR 1.08 (0.81 to 1.45) | 105 (3 studies1) | ⊕⊕⊝⊝
low2 (very serious imprecision) |
High dose steroids may not offer any beneficial effects compared to moderate dose steroids in enhancing ambulation. |
|
Survival (long term) Over two years |
100 per 1000 | 111 per 1000 (25 to 505) | RR 1.11 (0.24 to 5.05) | 57 (1 study) | ⊕⊕⊝⊝
low2 (very serious imprecision) |
High dose steroids may offer no beneficial effects compared to no steroids in enhancing long term survival. |
| Pain reduction | 909 per 1000 | 782 per 1000 (564 to 1000) | RR 0.86 (0.62 to 1.20) | 25 (1 study) | ⊕⊕⊝⊝
low3 (very serious imprecision) |
High dose steroids may offer no beneficial effects compared to moderate dose steroids in reducing pain. |
|
Urinary continence One week after treatment |
533 per 1000 | 629 per 1000 (352 to 1000) | RR 1.18 (0.66 to 2.13) | 34 (1 study) | ⊕⊕⊝⊝
low2 (very serious imprecision) |
High dose steroids may offer no beneficial effects compared to moderate dose steroids in enhancing urinary continence. |
| Local recurrence ‐ | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Serious adverse events | See comment | See comment | RR 8.02 (1.03 to 62.37) | 77 (2 studies) | ⊕⊕⊕⊝
moderate3 (serious imprecision) |
Serious adverse events (such as perforated gastric ulcer, psychoses and deaths due to infection) occurred only with high dose steroids: 6/36 (17%) versus 0/41 (0%) on moderate dose (N = 11) or no steroids (N = 30). |
| Quality of life | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| *The basis for the assumed risk is the median control group risk across studies for pooled data or the control group risk in single studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Vecht 1989 and Graham 2006 compared high dose (100 to 96 mg dexamethazone) versus moderate dose steroids (10 to 16 mg); Sorensen 1994 compared a single IV dose of 100 mg dexamethazone with saline placebo. The three trials were free of the risk of serious biases for efficacy outcomes. 2 Very serious imprecision: the 95% CI of the effect estimates indicate appreciable benefit for high dose and moderate dose or no steroids. Downgraded 2 levels.
3 Serious imprecision: the 95% CI of the effect estimate indicates appreciable and non‐appreciable benefits with moderate dose or no steroids; and the number of events and participants were too few to provide reliable estimates. Downgraded 1 level.
1. Different radiotherapy schedules
1.1 Single‐fraction radiotherapy (8 Gy) versus a short course of radiotherapy (two fractions of 8 Gy)
Maranzano 2009 compared a single dose of radiotherapy (8 Gy) versus a short course of radiotherapy (8 Gy × 2, given as 8 Gy, 6 days rest, and then 8 Gy, to a total dose of 16 Gy over one week) in 327 randomised participants with poor prognosis, of whom 303 were evaluable. Parenteral dexamethasone (8 mg twice daily) was administered from the first day of clinical‐radiologic diagnosis for 4 to 5 days. Follow‐up was for a median duration of 31 months (range four to 58 months).
The trial was powered to demonstrate equivalence in the response rate one month after treatment with the two regimens. The response rate was separately evaluated for ambulatory status (maintaining or regaining ambulation), urinary continence (not requiring a catheter), and back pain (not requiring a narcotic). The sample size ensured an 80% probability to detect equivalence in these response rates with a two‐sided precision of 15% if the response rate was similar and approximately 70%.
Since the data for this comparison comes from only a single study, we were not able to perform a meta‐analysis for any of the outcomes assessed.
1.1.1 Ambulation
Overall ambulatory rates post‐treatment were 65% with single‐dose RT and 69% with short‐course RT, and did not significantly differ (303 participants). Single‐dose RT and short‐course RT also did not differ significantly in the proportions who maintained ambulation (88% versus 90%; 199 participants), nor in the proportions who regained ambulation post‐RT (16% versus 26%; 104 participants). The difference in ambulatory rates was within 15% (7% for overall ambulation; 2% for maintaining ambulation; and 10% for regaining ambulation), meeting pre‐set criteria to demonstrate equivalence in enhancing ambulation with the two RT schedules.
1.1.2 Other outcomes
The median survival was four months in both intervention arms (excluding three early deaths and 21 lost to follow‐up).
Reduction in analgesic or narcotic use was seen in both treatment arms (34% with single‐dose RT and 40% with short‐course RT) and did not significantly differ between the RT regimens (271 participants). The difference in response rate for pain of 6% was within the bounds pre‐set to demonstrate equivalence.
Similar proportions (90% given single‐dose RT versus 87% given short‐course RT) had urinary continence after treatment (303 participants), with the difference of 3% again demonstrating equivalence in response with the two RT schedules .
Local recurrence was diagnosed by MRI in people with symptomatic progression in 4% of patients, at a median interval between the end of the first RT and diagnosis of 8 months (range two to 31 months). In‐field recurrences were higher in the single‐fraction arm (6% versus 3%), but the numbers with in‐field recurrences were too few to rule out random error. Seven of the 13 with in‐field recurrences were re‐irradiated as decided by their treating clinicians and outside of the trial protocol. Those ambulant at the time of recurrence survived longer after irradiation compared to those non‐ambulant; and maintained their ability to walk till the time of death. None who were non‐ambulant at the time of recurrence regained ambulatory status after re‐irradiation. Median survival after irradiation was six months (range: 1 to 13 months). None developed serious adverse events or radiation‐induced myelopathy. In those re‐irradiated, the cumulative BED Gy2 (Biologically Effective Dose with an α/β ratio of 2 Gy) did not exceed 120 Gy2.
1.1.3 Adverse events
Early
Gastrointestinal adverse events of Grade 3 severity was seen only in three people given short‐course RT. Less severe grades of oesophagitis, diarrhoea, nausea and vomiting were not significantly different with both RT regimens. No participant developed serious adverse events.
Late
No radiation myelopathy was noted over a median follow‐up of 31 months (range 4 to 58 months).
1,1.4. Outcomes not reported
Quality of life, and participant and caregiver satisfaction were not reported.
1.1.5. Subgroup analysis
Although meta‐analysis was not possible, and the planned subgroup analyses to investigate heterogeneity were not indicated, subgroup analyses in Maranzano 2009 indicated that the duration of ambulation after treatment was influenced by tumour histology and was significantly longer with favourable histologies (median duration 11 months) than with unfavourable histologies (four months).
Survival was also associated with pre‐treatment and post‐treatment ambulatory status; median survival was five months and two months for pre‐treatment ambulant and non‐ambulant patients respectively, (P = 0.001); and six months and one month for post‐treatment ambulant and no‐ambulant patients respectively (P < 0.001). The median survival was also influenced by tumour histology and was 9.5 months for favourable cancers and three months for unfavourable ones (P < 0.001).
Ambulatory outcomes were also better in those ambulant pre‐treatment, the majority of whom maintained ambulatory status (88% and 90% with single dose and two doses respectively), while only 16% and 28% of those non‐ambulant pre‐treatment regained ambulation with the two RT regimens.
Outcomes for those with and without visceral metastases were not available. Vertebral instability and bony impingement were exclusion criteria in this trial. All participants were detected to have MESCC after active screening by MRI or CT and the results of this trial pertain to those who are actively screened to enable an early diagnosis of cord compression and early RT within 24/48 hours after diagnosis.
1.1.6.Sensitivity analysis
Overall loss to follow‐up was only 7%, and there was no differential loss in the two arms. Attrition was considered unlikely to have influenced outcome estimates.
1.2 Split‐course (eight fractions) radiotherapy versus short‐course (two fractions) radiotherapy
Maranzano 2005 was also powered to detect equivalence in response rates for the outcomes of ambulation, urinary continence and pain reduction based on narcotic use, using the same assumptions (80% power to detect equivalence in response rates with a precision of 15% if the response rates were roughly similar and around 70%) as described above in Maranzano 2009.
Data were from only one trial and we were unable to perform a meta‐analysis for any outcome. The results are summarised below and there were no statistically significant differences between the two arms for any of the outcomes analysed.
1.2.1 Ambulation
Overall ambulatory rates were 70% for eight fractions versus 68% for two fractions and did not significantly differ (276 participants). There were no significant differences in the proportions who maintained ambulation (91% versus 89%; 184 participants), or who regained ambulation (28% versus 29%; 92 participants). The median duration of ambulation (excluding 17 early deaths) was 3.5 months in both arms. The difference in ambulatory rates with the two RT schedules were less than 15% (3% for overall ambulation and maintaining ambulation, and 1% for regaining ambulation), demonstrating equivalence in enhancing ambulation with split‐course (eight fractions) and short‐course (two fractions) radiotherapy.
1.2.2 Other outcomes
The median survival was four months in both intervention arms (excluding 17 early deaths).
The proportion of participants with reduction in the use of analgesics and narcotics was not significantly different (49% versus 38%; 262 participants); and the difference in response rates (9%) was within the pre‐set limits to demonstrate equivalence in response rates between the split‐course and short‐course RT regimens.
The overall proportion of participants with urinary continence after treatment was also not significantly different (87% versus 90%; 275 participants) for split‐course versus short‐course RT, and the difference in urinary continence rates (3%) again demonstrated equivalence.
Local recurrence was diagnosed on MRI in 5/142 (4%) given the short‐course RT regimen and none of 134 given the longer‐course RT regimen, but this difference was not statistically significant given the small number of local recurrences. The five with recurrences were re‐irradiated with different RT schedules at the discretion of their treating clinicians and outside the trial protocol. The two non‐ambulant before irradiation continued to be non‐ambulant till death (three and four months later) while the three ambulant maintained ambulation till death in two (one and seven months later) and at 20 months' follow‐up in the third. Radiation‐induced myelopathy was not detected in any of the five. In this trial, as in Maranzano 2009, the cumulative BED Gy2 did not exceed 120 Gy2 in those with in‐field recurrences who were irradiated.
1.2.3 Adverse effects
Early
Grade 3 acute gastrointestinal mucositis attributable to radiation was reported in 4% of patients who had eight fractions of radiotherapy and in 2% of those who had two fractions of radiotherapy; 6/276 (2%) of participants had Grade 3 vomiting, and 5/276 (2%) had Grade 3 nausea; the incidence was similar with both the RT regimens. No other serious adverse events were noted.
Late
There was no documented instance of late radiation myelopathy over a median follow‐up of 33 months (range: 4 to 61 months).
1.2.4 Outcomes not reported
Survival rates, quality of life, and participant and caregiver satisfaction were not reported.
1.2.5 Subgroup analysis
Data were from only a single trial but subgroup analyses in Maranzano 2005 revealed that the duration of ambulation post‐treatment was influenced by tumour histology (six months with favourable histologies and three months for unfavourable histologies). Survival was also significantly correlated with ambulant status. Median survival was five months for those ambulant pre‐treatment and three months for those non‐ambulant pre‐treatment; median survival was five months for those ambulant after treatment and two months for those non‐ambulant post‐treatment. Median survival was also influenced by tumour histology and was significantly longer (six months) with favourable histologies compared to unfavourable histologies (three months).
Ambulatory outcomes were also better in those ambulant pre‐treatment, the majority of whom maintained ambulatory status (91% and 89% with eight doses and two doses respectively), while only 28% and 29% of those non‐ambulant pre‐treatment regained ambulation with the two RT regimens.
Outcomes for those with and without visceral metastases were not available. Vertebral instability and bony impingement were exclusion criteria in this trial. As in Maranzano 2009, participants in Maranzano 2005 with a primary tumour and with local or radicular pain even in the absence of neurological symptoms were screened actively with MRI or CT to establish an early diagnosis of cord compression, and early institution of RT within 24 hours of the diagnosis.
1.2.5 Sensitivity analyses
Maranzano 2005 reported that the 24 patients excluded from analyses (those lost to follow‐up or who experienced early deaths) were well balanced between the two arms and an intent‐to‐treat analysis did not significantly alter the results.
2. Different surgical procedures plus radiotherapy compared to radiotherapy alone
2.1 Laminectomy plus radiotherapy versus radiotherapy alone
The results for laminectomy plus radiotherapy versus radiotherapy alone are from a single trial with 29 participants and we were unable to perform a meta‐analysis for any outcome (Young 1980). The results for laminectomy plus RT were not significantly different from RT alone for the primary and secondary outcomes.
2.1.1 Ambulation
Overall ambulatory rates for laminectomy versus radiotherapy were 44% and 54% immediately after treatment; and 37% versus 39% at four months; and did not significantly differ at both time‐points. Half of laminectomy patients versus all radiotherapy patients maintained ambulation; while 40% of surgical patients and 25% of radiotherapy patients regained ambulation immediately after treatment; these differences were also not statistically significant.
2.1.2 Other outcomes
Short‐term survival (100% versus 77%; 29 participants) and intermediate‐term survival (56% versus 46%; 29 participants) were not significantly different with laminectomy or radiation. Reduction in analgesic use in those with pain pre‐treatment (50% versus 57%; 26 participants); and urinary continence in the short term (44% versus 54%; 29 participants) or in the intermediate term in survivors (67% versus 100%; 15 participants) also did not significantly differ between the two intervention arms.
2.1.3 Adverse effects
There were no surgery‐ or radiotherapy‐related complications reported.
2.1.4 Outcomes not reported
Local recurrence, quality of life, participant and caregiver satisfaction were not assessed.
2.1.5 Subgroup analysis
Although data were only from a single trial, a post‐hoc subgroup analysis in Young 1980 revealed that those with a complete myelographic block fared significantly poorer than those with an incomplete block for ambulation and sphincter function immediately after treatment; with this trend persisting in survivors four months after treatment.
2.1.6 Sensitivity analysis
None of the planned sensitivity analyses were relevant in this single trial.
2.2. Direct decompressive surgery with radiotherapy versus radiotherapy
Patchell 2005 recruited 101 participants with an MRI diagnosis of a single area of MESCC, an expected survival of at least three months, and non‐radiosensitive primaries. All had a duration of paraplegia less than 48 hours; and were immediately randomised to emergency surgical decompression and postoperative radiotherapy (with stabilisation if instability was present) or radiotherapy alone (30 Gy in 10 fractions). All patients were given high‐dose dexamethasone, 100 mg, initially that was tapered by completion of radiotherapy. We were unable to perform a meta‐analysis since the data for all outcomes were only from one trial.
2.2.1 Ambulation
The overall ambulatory rates and the proportion of participants maintaining or regaining ambulation were significantly better in the surgery plus radiotherapy group. At completion of treatment, overall ambulatory rates were 84% versus 57% (RR 1.48, 95% CI 1.16 to 1.90; 101 participants). Of them, 94% versus 74% previously ambulant patients maintained ambulation (RR 1.27, 95% CI 1.02 to 1.57; 69 participants). Of those in the decompressive surgery followed by RT arm, 63% versus 19% given RT alone who were previously non‐ambulant regained ambulation; (RR 3.33, 95% CI 1.12 to 9.90; 32 participants). The median duration of ambulation also significantly favoured surgical decompression (122 days versus 13 days; P = 0.003). For maintaining ambulation, medians were 153 days versus 54 days; and for regaining ambulation 59 versus 0 days in the surgery and radiotherapy arms respectively.
2.2.2 Other outcomes
Of the 101 participants, similar proportions (94% after surgical decompression and 86% after RT) were alive at one month. However, median survival was statistically significantly longer for surgery plus radiotherapy compared to RT alone (126 days versus 100 days; P = 0.033).
The authors reported that the median daily morphine equivalent dose was significantly lower in the surgery plus RT arm than in the RT arm (0.4 mg, range 0 mg to 60 mg versus 4.8 mg, range 0 mg to 200 mg; P = 0.002).
The authors also reported that urinary continence was maintained significantly longer in those who were surgically decompressed and given RT compared to those given RT alone (after surgery plus RT the median duration for maintaining continence was 156 days compared to 17 days with RT; P = 0.016).
2.2.3 Adverse events
Surgery did not prolong hospitalisation significantly compared to RT. Extended hospital stays (greater than 20 days) occurred in seven patients in those given surgery plus RT compared to 11 given RT alone. Ten patients (20%) in the RT group who had a substantial decline in motor strength during radiotherapy crossed over to receive surgery. Four of them (40%) had surgical complications; three had wound infections and one had a failure of spinal fixation that required additional surgery.
2.2.4 Outcomes not reported
Quality of life, and participant and care‐giver satisfaction were not assessed. Local recurrence, participant‐rated pain relief, adverse effects, dichotomous data for analgesic reduction and urinary continence were not reported.
2.2.5 Subgroup analysis
Although data were from a single trial, multivariate analysis in Patchell 2005 showed surgery, Frankel score, and breast primary tumour to be associated with longer ambulatory times. In a secondary data analysis of prognostic factors using multivariate modelling and Kaplan‐Meier curves for stratified treatment groups from this trial, there was no difference in outcome between treatments for patients older than 65 years of age; and the benefits for decompressive surgery were apparent for ambulation and survival only in those less than 65 years of age (Chi 2009).
2.2.6 Sensitivity analysis
Attirition was negligible and hence the planned sensitivity analysis was not undertaken.
3. High dose corticosteroids versus no or moderate dose corticosteroids
3.1 Ambulation
Three small trials did not show significant benefit for ambulation with high‐dose corticosteroids compared to no steroids or to moderate‐dose steroids given as adjuvant to conventional doses of RT (60% versus 55%; 3 RCTs, 105 participants; Analysis 1.1) (Graham 2006; Sorensen 1994; Vecht 1989). In Sorensen 1994, the proportions maintaining ambulation (100% versus 90%) and regaining ambulation (50% versus 18%) were not statistically significantly different between high‐dose steroids versus no steroids (1 RCT, 57 participants).
1.1. Analysis.

Comparison 1 High dose versus no or moderate dose corticosteroids, Outcome 1 Overall ambulation (short term).
3.2 Other outcomes
No significant difference was seen between high‐dose versus no corticosteroids for two‐year survival (11% versus 10%, 1 RCT, 57 participants); or versus moderate‐dose steroids for pain reduction (78% versus 91%; 1 RCT, 25 participants); or for urinary continence (63% versus 53%; 1 RCT, 34 participants).
3.3 Adverse effects
There was a significant increase in the incidence of serious drug‐related adverse effects such as perforated gastric ulcer, psychoses and deaths due to infection in those who received high‐dose corticosteroids. Seventeen percent of this group developed serious adverse effects as compared to 0% of those who received moderate‐ or low‐dose corticosteroids (RR 8.02, 95% CI 1.03 to 62.37; two RCTs, 77 participants; Analysis 1.2; Figure 4)
1.2. Analysis.

Comparison 1 High dose versus no or moderate dose corticosteroids, Outcome 2 Serious drug related adverse effects.
4.
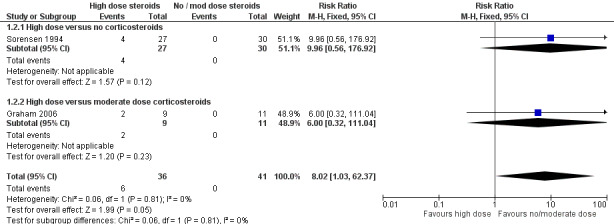
Forest plot of comparison: 1 High dose versus no or moderate dose corticosteroids, outcome: 1.2 Serious drug related adverse effects.
3.4 Outcomes not reported
Local recurrence, quality of life, and participant‐rated or caregiver‐rated satisfaction were not reported in any of the three corticosteroid trials.
3.5 Subgroup analysis
Data were not heterogenous where meta‐analysis was possible, and subgroup analyses were therefore not indicated. Favourable histologies (breast and prostate cancer) and baseline ambulant status predicted longer survival with high‐ and low‐dose steroids in Graham 2006,. Those with breast cancers also had significantly better ambulatory outcomes than those with other primary tumours in Sorensen 1994,
3.5 Sensitivy analysis
Vecht 1989 was judged at high risk for attrition bias for adverse events but did not contribute data to the meta‐analysis for adverse events; hence no sensitivity analysis was indicated.
Discussion
Summary of main results
Although metastatic extradural spinal cord compression (MESCC) is a common and distressing problem in many people with cancer, there is limited evidence from RCTs to inform optimal treatment. We found only seven eligible trials with a total of 876 (723 evaluable) participants that compared three different sets of comparisons in adults with MESCC: different radiotherapy schedules; surgery plus RT versus RT; and high‐dose steroids versus moderate‐dose steroids versus no steroids. Meta‐analysis was not possible for most outcomes.
1. Radiotherapy doses and schedules
Radiotherapy (combined with corticosteroids) is the most widely used treatment for cord compression, and was part of the treatment protocol in all the seven trials reviewed. Only Maranzano 2005 and Maranzano 2009 directly compared the effects of different radiotherapy fractionation schedules combined with steroids in people with MESCC with a poor prognosis.
These two Italian trials were powered to demonstrate equivalence and showed that in patients with MESCC with no indications for primary surgery and an estimated survival of six months or less, the short‐term results were similar with a single dose of 8 Gray (Gy) to two fractions of 8 Gy given over a week (moderate quality evidence; Table 1); as were two fractions of 8 Gy compared to eight fractions (30 Gy) of RT (moderate quality evidence; Table 2). In both trials, those who were ambulant pre‐treatment and who had favourable histologies were ambulant longer and survived longer post‐treatment than those not ambulant pre‐treatment or with unfavourable histologies.
With the three radiotherapy fractionation regimens, around 90% of ambulant patients maintained ambulation; around 3% had a documented overall in‐field recurrence rate; median survival was four months; and toxicity was minimal. This suggests that shorter courses of radiotherapy are probably justified in patients with MESCC and a poor prognosis, who constitute a significant proportion of the MESCC population, particularly in many resource‐constrained settings.
The incidence of in‐field recurrences with single‐dose RT was 6%. With two doses of RT recurrences ranged from 2.5% to 3.5% in the two trials where short‐course RT was a comparator, and was 0% with eight doses. Though not statistically significant due to the small number of recurrences, these differences in recurrence rates suggest that people with an expected survival more than three to six months who decline, or are not suitable candidates for surgical interventions, should perhaps be offered courses longer than a single dose or even two doses of RT, if local tumour recurrence is to be delayed or averted. Further comparative studies with larger samples would be required to confirm this impression. Data from a prospective non‐randomised study also suggests that local control is poorer (61% versus 81%) in those given short courses of RT (8 Gy or 20 Gy in five fractions) compared to longer courses of RT (37.7 Gy in 15 fractions or 40 Gy in 20 fractions) (Rades 2011a). In those who develop recurrences, re‐irradiation may be considered, particularly in those ambulant; while ensuring that the cumulative BED does not exceed 120Gy2 in order to reduce the chances of developing radiation‐induced myelopathy (Rades 2008).
The results in non‐ambulant patients with the short‐course (16 Gy in two fractions) or the split‐course ( 30 Gy in eight fractions) RT regimens were similar to rates in reports from non‐randomised prospective studies with 28% to 29% of non‐ambulant patients regaining ambulation (Rades 2011a). Though the results in non‐ambulant participants in the comparison of short‐course RT with a single dose of 8 Gy did not differ significantly, the smaller proportion regaining ambulation with a single dose (9/55; 16%) in Maranzano 2009 is worrying. It is possible that the proportions that will regain ambulation with single‐dose RT may be similar to that with a short course or with longer RT courses, since the 95% CI for this estimate in Maranzano 2009 ranged from 9% to 29%. This would need confirmation in future trials; until which time, non‐ambulant people with MESCC who are considered for RT could be offered two or more doses of treatment.
We did not find trials comparing different radiotherapy doses, schedules or techniques in people with MESCC and a good prognosis; and no conclusions can be drawn regarding the optimal radiotherapy dose or schedules in this population.
2. Surgery plus radiotherapy versus radiotherapy alone
Laminectomy followed by radiotherapy
Data from Young 1980 comparing outcomes in 16 people with a single lesion, no prior RT and who were fit to undergo surgery indicate that ambulation and survival at four months and urinary continence one month after treatment may not differ significantly with the addition of laminectomy prior to RT compared to RT alone (low quality evidence; Table 3). Laminectomy for a ventrally located tumour is undesirable as direct decompression of the cord through removal of the compressive element is not achieved. Moreover, removal of the laminae and posterior ligaments of the spine in the setting of vertebral body disease quite often further destabilises the spine (Klimo 2005). In two separate retrospective matched pair analyses of people with radiosensitive and radio‐resistant tumours, outcomes with laminectomy were not different to radiation alone (Rades 2010b); or were poorer than with radiotherapy (Rades 2011b). Thus, though we are uncertain about the results of Young 1980, they are indicative of the lack of benefit expected with laminectomy and RT without spinal stabilisation compared to RT alone in metastatic spine disease.
Direct decompressive surgical resection followed by radiotherapy
Progress in spine surgery, particularly with regard to sophisticated instrumentation and ventral approaches through the neck, thorax and abdomen, provides an opportunity for direct decompression of the cord and stabilisation of the spine. In Patchell 2005 surgery was undertaken by experienced teams and was tailored to the location of the compression: that is, a ventral approach was employed for vertebral body disease, a lateral approach for predominantly laterally located compression and a posterior approach when the posterior elements were primarily involved. When required the spines were fixed using bone grafts and instrumentation.
The results of Patchell 2005 suggest that surgery may result in an incremental benefit of 27% in short‐term ambulatory rates compared to RT, in selected people with MESCC. For those regaining ambulation this benefit may be as much as 43%. With ambulation as a primary endpoint, Patchell 2005 reported that 63% of non‐ambulant patients regained the ability to walk with surgery and radiotherapy as compared with only 19% in those receiving radiotherapy alone. Median survival was short in both treatment arms although statistically significantly longer in the surgery group versus radiotherapy alone. However, the overall quality of the evidence was graded as low quality, with full confidence in these estimates limited by the highly selected sample that was biased in favour of better outcomes in those undergoing decompression, and by the evidence for this intervention limited to one trial from a high‐income country that was stopped prematurely for perceived benefit.
Patchell 2005 acknowledges the restrictive selection criteria used in the trial and partly concedes that the results do not apply to the entire MESCC population. Subsequently the same group performed a secondary analysis of their data, stratifying patients into two groups based on age greater or less than 65. They concluded that patients over 65 years of age did not benefit from surgery, in terms of ambulation or survival (Chi 2009). This conclusion is supported by Rades 2012, an independent matched‐pair analysis where data from 42 elderly people over 65 years of age from Europe offered surgery plus radiotherapy were retrospectively matched 1:2 to 84 people given radiotherapy alone for 10 potential prognostic factors and compared regarding motor function, local control, and survival. Additional matched‐pair analyses were performed for the subgroups of patients receiving direct decompressive surgery plus stabilisation of involved vertebrae (N = 81) and receiving laminectomy alone (N = 45). Rades 2012 concluded that elderly people with MSCC did not benefit from surgery (decompressive surgery plus stabilisation or laminectomy) in addition to radiotherapy for functional outcomes, local control, or survival.
We feel that the case for the routine use of direct decompressive surgery in patients with good neurological function and stable spines is not established based on current evidence. We suggest that in a patient with localised cord compression, clinicians need to consider the following questions:
Are there prognostic features to suggest that the patient would have poor ambulatory outcomes with radiotherapy?
Will the patient survive long enough to benefit from major surgery?
In summary, the evidence from this review indicates that non‐ambulant adults with MESCC below 65 years of age, with an onset of paraplegia less than 48 hours, a single site of compression, radio‐resistant primary tumours, and an expected survival of more than three months, may benefit more from decompressive surgery followed by radiotherapy compared to radiotherapy alone. Radiotherapy alone may suffice for many ambulant patients with stable spines and radiosensitive tumours, while decompressive surgery followed by RT may be offered as first‐line therapy in those ambulant patients with factors predicting a poor outcome with radiotherapy and with good predicted survivals.
3. High dose corticosteroids versus no or moderate dose corticosteroids
Although corticosteroids have been used in the treatment of MESCC for many years, we found only three small trials that were inadequately powered to determine clinical benefit and optimal dosage (Graham 2006; Sorensen 1994; Vecht 1989). No significant differences in ambulation, survival, and urinary continence, or for pain reduction were seen with high‐dose steroids compared to moderate‐dose or no steroids (low quality evidence). However, high‐dose corticosteroids were associated with serious drug‐related adverse effects (moderate quality evidence; Table 5).
Considering the widespread clinical use of corticosteroids in MESCC, it is unlikely that a trial comparing corticosteroids to placebo will be undertaken in the future; but the serious adverse effects reported with high‐dose steroids should be noted.
Adverse effects
One of our secondary objectives was to compare the adverse effects between different interventions but these were not always systematically recorded or graded. Serious adverse effects were infrequent in both arms of the Maranzano 2005 and Maranzano 2009 studies evaluating radiotherapy schedules. Patchell 2005 reported more adverse effects in patients who had preoperative rather than postoperative radiotherapy after surgical decompression. In the corticosteroid dose comparison trials, serious gastrointestinal, infectious and central nervous system adverse effects were reported in 15% to 22% of participants receiving high‐dose corticosteroids.
Overall completeness and applicability of evidence
Completeness
We had planned to evaluate radiotherapy schedules, surgical interventions and adjunctive therapies. However, high‐dose corticosteroids versus moderate‐dose corticosteroids versus no corticosteroids was the only comparison for which there was more than one trial to allow meta‐analysis. We did not find trials eligible for inclusion that evaluated corticosteroid doses intermediate between 16 mg to 20 mg and 96 mg to 100 mg, or that evaluated the optimal duration of steroid therapy. We also found no trials comparing different doses of radiotherapy in people with MESCC with a good prognosis; nor did we find trials evaluating the role of decompressive surgery in health care settings outside that in a high‐income country. Trials evaluating newer treatment modalities such as minimally invasive surgical techniques, intensity modulated RT, and radiosurgery in MESCC were also not available for inclusion. The modern management of MESCC involves a variety of interventions, and a multidisciplinary approach; but trials that included education, rehabilitation, screening and supportive care as components of the interventions used were also lacking. Although we had not specified these in our search terms, our broad search strategy should have detected such RCTs.
We assessed different comparisons in a heterogeneous population of adult patients with MESCC. Many of the trials included here had small numbers of participants making it difficult to draw precise or firm conclusions. Nor can our conclusions apply to all subgroups: patients with haematologic tumours, post‐RT recurrences, or paraplegia longer than 48 hours constituted a very small (or undeterminable) proportion of participants in the trials included in this review.
Clinically important outcomes such as patient‐reported pain relief, satisfaction with treatment, and quality of life were not assessed in the included studies, and survival rates were not always adequately reported. Local recurrence was not reported in any of the trials apart from those evaluating different radiotherapy schedules. Not all reported data could be reliably used for analysis, for example when results are reported as medians, or as percentage probability of survival.
The body of evidence to date is therefore incomplete to fully answer questions of current clinical relevance to the optimal management of people with MESCC.
Applicability
Differences in inclusion criteria, and in the definition of ambulation used in the studies limit the applicability of the results of this review to clinical settings. Evidence for the superiority of surgical decompression over radiotherapy in Patchell 2005; and the equivalence of single dose over two doses in Maranzano 2009 and of two doses and eight doses of radiotherapy in Maranzano 2005 were from trials done in high‐income countries. The early detection of MESCC and the early institution of interventions that were critical in achieving the high response rates seen in these trials may not always be possible in many resource‐constrained settings,
Differences in inclusion criteria
Within the seven trials inclusion criteria varied and while Maranzano 2005 and Maranzano 2009 recruited patients with a poor prognosis, Patchell 2005 required an estimated survival of at least three months. Maranzano 2005, and Maranzano 2009 excluded patients with spinal instability but such patients comprised over a third of the Patchell 2005 population. While Graham 2006,Patchell 2005 and Sorensen 1994 excluded radiosensitive tumours other trials included haematologic and germ cell tumours (Maranzano 2005; Maranzano 2009; Vecht 1989; Young 1980). Patchell 2005 also excluded patients with cauda equina lesions. These differences in inclusion criteria are likely to impact on the expected outcomes for interventions used in people with MESCC in clinical settings.
Differences in the definition of ambulation
Although ambulation was the primary outcome in all seven trials, there were differences in the definition of ambulation. Patchell 2005 reported a participant as ambulant if he or she was able to take at least two steps with each foot (four steps in total) either unassisted or with use of a cane or walker at completion of radiotherapy. In Maranzano 2005 and Maranzano 2009, participants who were walking with or without support at one month were considered ambulant, but this was not quantified. The three corticosteroid studies measured ambulation at different time points and in different ways. It is important that ambulation be defined in a manner that is uniform, meaningful and worthwhile, for patients considering the intervention.
Surgical decompression versus external radiotherapy
The assertion by Patchell 2005 that, "However, the results of our trial do not lend support to the use of radiation alone as first‐line treatment"; and the concluding remarks, "..the best treatment for spinal cord compression caused by metastatic cancer is surgery as initial treatment followed by radiotherapy," are important to address from a clinical decision‐making perspective. A cost effectiveness analysis from this group also supported this assertion (Thomas 1996). Another cost‐utility analysis suggested that adopting surgery plus RT as standard for patients with MSCC would result in improved outcome but would increase health care costs (Furlan 2012).
There are a number of reasons why surgery may not uniformly offer the best option as first line treatment in people with MESCC. They include the potential selection bias favouring better outcomes with decompressive surgery in Patchell 2005; the applicability of the results of this trial to resource‐constrained settings; and the potential biases arising from the premature termination of the trial. The overall quality of the evidence for ambulatory and survival outcomes for which comparative data were available was rated as of low quality (Table 4).
One reason for our lack of confidence in the reported effect estimates translating to real world settings was the bias in the trial in the selection of participants. Patchell 2005 selected patients who were good candidates for surgery and excluded participants who were good candidates for radiotherapy (those with radiosensitive tumours). Most common carcinomas in adults are (moderately) radiosensitive. Radio‐resistant tumours like melanomas and osteogenic/soft tissue sarcomas are uncommon. Some highly radio‐sensitive tumours such as hematologic, round cell or germ cell tumours are generally treated with chemotherapy. However, as also noted by Sciubba 2010, other radiosensitive tumours such as small cell lung carcinoma were excluded from both RT and surgical groups in Patchell 2005. Moreover, 18/51 patients (35%) given RT had unstable spines, precluding them from being mobilised early; and the secondary effects of non‐ambulation were significantly associated with 30‐day morbidity and mortality (due to infections and deep vein thrombosis etc) in the trial. Only 45% of those treated with RT maintained or regained ambulation compared to 75% treated with surgery and RT. This contrasts with the 66% to 70% ambulatory rates seen in clinical practice (Maranzano 2007; Rades 2010a; Rades 2011b), and in trials after RT alone (Maranzano 2005; Maranzano 2009). Patchell 2005 did not provide the results in patients with unstable spines treated with radiotherapy alone, but unstable spines were a predictor for poor outcome in the study population as a whole. Twenty per cent of ambulant patients randomised to the radiotherapy arm crossed over to surgery on the occurrence of neurological deterioration and lost the ability to walk. Thirty per cent of them regained the ability to walk, but the complication rate was higher in this group. Patchell 2005 therefore justified the use of surgery as the first line of therapy even in ambulant patients. However, the selection criteria appear to have biased the results in Patchell 2005 against radiotherapy. It also took 10 years in Patchell 2005 to recruit 50% of their estimated sample size, adding support to the contention that those selected for this trial represent only a fraction (probably around 15%) of those with MESCC seen in clinical practice, and do not represent the usual candidates given RT for MESCC (Maranzano 2007; Rades 2010b; Rades 2011b), since radiotherapy does not relieve compressions caused by bone fragments; nor does it correct deformities such as vertebral collapse (Wise 1999; Klimo 2005).
The secondary analysis of data from Patchell 2005 revealed that patients over 65 years of age did not benefit from surgery, compared to younger patients, for ambulation or survival (Chi 2009). This observation was supported by the matched‐pair analysis in Rades 2012. In two other retrospective matched‐pair analyses from Europe that currently constitute the largest available database of malignant spinal cord compression, Rades 2010b first demonstrated in 324 patients with MESCC from a variety of tumour types, who were matched on 11 prognostic variables, that surgery (decompressive surgery plus vertebral stabilisation and laminectomy) followed by RT did not significantly differ from RT alone in the proportions who maintained or regained ambulation; or for local control, or survival. Rades 2011b further evaluated outcomes with surgery followed by RT (N = 67) and RT alone (N = 134) in a retrospective matched‐pair analysis of people with MESCC with an unfavourable primary tumour (non‐small cell lung cancers, cancer of unknown primary, renal cell carcinoma and colorectal cancer), and concluded that decompressive surgery plus RT (but not laminectomy) improved ambulation compared to RT alone, especially if the interval between surgery and RT did not exceed two weeks. These observations add to the body of evidence indicating that decompressive surgery followed immediately by radiotherapy primarily benefits people with MESCC with poor prognostic factors for RT. Both studies called for further adequately powered definitive RCTs to evaluate the role of surgical decompression in people with MESCC representative of those usually seen in clinical practice (Rades 2010b; Rades 2011b).
In Patchell 2005, emergency surgery was offered within 24 hours of the diagnosis of cord compression and within 48 hours of onset of paraplegia and within two weeks of the onset of symptoms in the majority. RT was also offered as an emergency treatment. These may not be feasible for all people with MESCC in many healthcare settings outside a clinical trial, and particularly in resource‐poor settings. The effect estimates in Patchell 2005 are therefore not likely to reflect the effects achievable in practice in many parts of the world, if surgery were to be routinely offered as first‐line treatment for people with MESCC. The results of the Rades 2011b matched‐pair analysis of people with unfavourable primaries indicate that optimal outcomes with decompressive surgery also depend on RT being given within two weeks of decompressive surgery, as was done in Patchell 2005. Delays in offering RT within this time period may also occur in different parts of the world, due to resource constraints, delays in referral, and due to post‐operative complications (as was seen in 53% of 17 patients with RT delayed beyond two weeks after surgery in Rades 2011b).
Another reason that limited our confidence in the results of Patchell 2005 was that this trial was stopped early for apparent benefit after recruiting only 50% of the estimated sample. Truncated RCTs are at high risk of over‐estimating benefits (Guyatt 2012), particularly when the total number of events are less than 500, and even more so when events number less than 200 (Bassler 2010). The evidence in favour of surgery as a potential first‐line treatment in MESCC comes from only one RCT with 101 participants. It is also likely that the premature termination of this trial due to apparent benefit prevented the conduct of more definitive trials, since it would have been considered unethical to do so (Guyatt 2012).
Finally, circumferential decompression or combined (anterior, posterior, and lateral) approaches are only feasible in healthier patients and are not feasible for the majority of patients with MESCC who often have numerous comorbid conditions precluding such aggressive interventions (Molina 2011).
Current evidence suggests that decompressive surgery with external fixation, if needed, is indicated in people (younger than 65 years) with MESC who are expected to live for three months or more, are medically fit for surgery, have spinal instability or bony impingement and a recent onset of paraplegia; or who have tumours not sensitive to radiation; and particularly when RT can be offered within two weeks of surgery. Evidence of moderate or high quality is currently lacking to recommend the routine use of decompressive surgery for all people with MESCC. The optimal dose, schedules and duration of post‐operative RT are currently unclear.
Quality of the evidence
We assessed the overall quality of the evidence using the GRADE approach (Schünemann 2011), that considers ‘quality’ to be a judgement of the extent to which we can be confident that the estimates of effect are correct. Evidence from randomised controlled studies is initially graded as high and downgraded by one or two levels on each of five domains after full consideration of: limitations in the design of the studies, the directness (or applicability) of the evidence, the consistency and precision of the results, and the possibility of publication bias. A GRADE quality level of 'high' reflects confidence that the true effect lies close to that of the estimate of the effect for an outcome. A judgement of 'moderate' quality indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges the possibility that it could be substantially different, and that future research could alter the effect estimates. Low and very low quality evidence limit our confidence in the effect estimates, and indicates that future research is likely to alter the effect estimates and our confidence in the estimates (Balshem 2011).
The evidence for key outcomes in the comparisons of different radiotherapy regimens was rated as moderate quality (Table 1; Table 2) with the factor limiting full confidence being that each comparison was represented by only one trial conducted in a high‐income setting. The evidence for most outcomes for the surgical intervention trials was rated as low orvery low quality (Table 3; Table 4), with indirectness and imprecision being reasons for limiting full confidence in the effect estimates. The evidence for most outcomes for the steroid comparisons were rated as low quality due to imprecision in effect estimates resulting from the small numbers evaluated, though we had greater confidence in estimates indicating an increased risk of adverse events with high dose steroids (moderate quality evidence;Table 5).
Potential biases in the review process
We used standard methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and also ensured compliance with the Cochrane standards for the conduct of reviews of interventions (MECIR 2011). These should have minimised biases in the review process.
Agreements and disagreements with other studies or reviews
The findings in this review are in concordance with current guidance for the treatment of MESCC and with more recent reviews on this topic (NICE 2008; Sciubba 2010; Holt 2012; Kim 2012; Loblaw 2012; L’espérance 2012).
Authors' conclusions
Implications for practice.
General implications
Early detection and institution of treatment are crucial. Patients and clinicians should be educated about the symptoms of spinal cord compression, and patients should ideally be assessed by a multidisciplinary team, with treatment decisions taking into account the neurologic status, age and estimated survival. Prognostic scores for survival and spinal instability are available.
Radiotherapy
Radiotherapy is an essential component of treatment in adults with MESCC. Radiotherapy could be considered as the primary treatment for ambulant people with MESCC and stable spines and for those who do not meet the criteria for decompressive surgery listed below.
Short courses of radiotherapy (one to two fractions) could be considered for people with MESCC with a predicted survival of less than three to six months, particularly if they are ambulant, and have radio‐sensitive tumours. Evidence from this review indicates that non‐ambulant patients have only a 16% chance of regaining ambulation with a single dose of radiotherapy and 29% with short courses of radiotherapy.
Since short courses of radiotherapy (8 Gy to 16 Gy in one or two fractions) may be associated with a higher risk of local recurrence than longer courses (8 fractions; 30 Gy) or more, patients with a good prognosis could be considered for longer courses of radiotherapy. In people with MESCC who only have a short time to live, the benefits with the shorter courses may be important.
The optimum dose and fractionation for RT in people with MESCC who have a good prognosis is currently uncertain.
Since the overall GRADE quality of evidence for the efficacy and safety outcomes with different radiation doses was only of moderate quality (and low quality for local recurrence), it is possible that future research may impact on our confidence in the estimates of effect, and alter these estimates and the implications for practice.
Decompressive surgery
Decompressive surgery could be considered in people younger than 65 years with MESCC, who are fit to undergo surgery, have lost motor function for less than 48 hours, have localised cord compression, unfavourable histologies, and an estimated survival of greater than three months.
Decompressive surgery could also be considered in ambulant patients with poor prognostic factors for radiotherapy (e.g. spinal instability, bony compression, rapidly progressive neurologic deficits, tumours not sensitive to radiation), provided good prognostic factors for survival are present.
Decompressive surgery should ideally be followed immediately by postoperative radiotherapy for optimal ambulatory and survival outcomes. Postoperative RT was instituted within two weeks after surgery in the trial in this review and the evidence from another observational study also indicates that optimal outcomes with decompressive surgery depend on RT being given within two weeks of decompressive surgery (Rades 2011b). The optimal fractionation schedules and dose for postoperative RT are uncertain.
Since the overall GRADE quality of evidence for all outcomes with decompressive surgery was of low quality, it is likely that future research could affect the implications for practice.
Corticosteroids
High doses of corticosteroids (96 mg to 100 mg dexamethasone) carry a significant risk of serious adverse effects.
It is uncertain if they offer any additional benefit over moderate doses of steroids (16 mg to 32 mg of dexamethasone) or no steroids.
The optimal dose and duration of corticosteroid treatment to be given with radiation or decompressive surgery is currently unclear.
The overall GRADE quality of evidence was moderate for the risk of serious adverse effects and low for all other outcomes; hence it is possible that future research may affect the implications for practice.
Implications for research.
The two ongoing trials (ISRCTN97555949; NCT00968643), when completed and reported, will provide more data to help clarify the effects of different short RT regimens in people with MESCC not selected for having a poor prognosis. The first, being conducted in the UK, is comparing a single fraction of 8 Gy to 20 Gy in five fractions (ISRCTN97555949; SCORAD). The second, underway in Ireland, is comparing a single fraction of 10 Gy to 20 Gy in five fractions (NCT00968643; IRCOG).
Adequately powered, multinational RCTs are needed to:
define appropriate radiotherapy schedules for good‐prognosis patients with MESCC (if deemed necessary after the results of the ongoing trials are included in an update of this review);
clarify the role of decompressive surgery in different prognostic groups and health care settings;
determine the optimal radiotherapy dose and fractionation regimens after surgical interventions;
determine the optimal dosage and duration of corticosteroids. (If future studies comparing different doses of corticosteroids are conducted, they should be adequately powered to ascertain the balance between benefits and adverse effects with different doses of corticosteroids, but should also optimally select patients for their suitability for RT or decompressive surgical interventions as the primary treatment modalities);
determine the efficacy and safety of minimally invasive surgical techniques, intensity modulated RT, and radiosurgery in the primary treatment of MESCC; and in those with local recurrences.
Trials should report results in accordance with the CONSORT 2010 guidelines. Various prognostic scales are currently available; and stratification by prognostic factors is important in future RCTs comparing different interventions. These trials would need to be adequately powered to detect differences between subgroups. Where appropriate, outcomes and endpoints should be patient‐ and caregiver‐defined.
Additionally, there is need for both qualitative and quantitative research into education, rehabilitation, screening and supportive care for patients with MESCC.
What's new
| Date | Event | Description |
|---|---|---|
| 28 June 2018 | Review declared as stable | See Published notes |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 19 August 2015 | Review declared as stable | The authors and editors agreed to re‐assess this review for further updating in 2018. |
| 5 March 2015 | New citation required and conclusions have changed | The additional data and the new approach to summarizing and interpreting results have altered the conclusions of this review. Readers may wish to re‐read this review update. |
| 3 March 2015 | New search has been performed | This review has been updated to include the results of a new search. 518 unique citations were screened, Data from one new study for a new comparison and data for a secondary outcome (local recurrence) from two studies were added. This review update also includes risk of bias tables, and summary of findings tables. |
| 17 December 2013 | New search has been performed | Search updated; 501 reports were not relevant, one RCT added to included studies; one study awaits assessment; eight reports added to excluded studies; six additional citations added to previously included studies. Mhoira Leng left review team. |
| 9 November 2009 | Amended | Contact details updated. |
| 27 August 2008 | Amended | Contact details updated |
Notes
A restricted search in June 2018 did not identify any potentially relevant studies likely to change the conclusions. Therefore this review has now been stabilised following discussion with the authors and editors. The review will be assessed for updating in five years time. If appropriate we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Acknowledgements
We thank Jessica Thomas and Anna Hobson from the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS) and Jabez Paul and Richard Kirubakaran, staff at the South Asian Cochrane Centre, for administrative and technical support; Joanne Abbott, Sylvia Bickley and Caroline Struthers, the Trials Search Co‐ordinators from PaPaS, for undertaking all the searches for the current review; and Manuel Raj and Roja Rekha for assistance in obtaining papers. We also acknowledge Angela Melder, and Dr Peter Hoskin for constructive suggestions during peer review.
We are grateful to Mhoira Leng for her contributions to the 2008 review (and to Cairdeas International Palliative Care Trust, Aberdeen). We are also grateful to Dr Andrew Loblaw for his suggestions on the draft protocol, to Dr Robert Twycross for his helpful comments on the abstract of the 2008 review, to the peer reviewers and to Dr Joseph O'Sullivan, Dr Roy Patchell, Dr Ernesto Maranzano, and Dr. Peter Hoskin for responding to our queries for identifying trials.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Electronic databases: Search strategies (2015 update)
| Database | Search strategy |
|
CENTRAL (2008 to 2015; Issue 2) |
#1 MeSH descriptor: [Spinal Cord Compression] explode all trees #2 MeSH descriptor: [Spinal Cord Neoplasms] this term only #3 ((epidural or extradural or extra‐dural or "spinal cord" or "dural sac" or "cauda equina" or "spinal column") and (neoplasm* or cancer* or tumour* or tumor* or malignan* or metast*) and compress*):ti,ab,kw (Word variations have been searched) #4 (neoplasm* or cancer* or tumour* or tumor* or malignan* or metast*):ti,ab,kw (Word variations have been searched) #5 #1 and #4 #6 #2 or #3 or #5 from 2008 to 2013 |
| MEDLINE (OVID) | 1 SPINAL CORD COMPRESSION/ 2 SPINAL CORD NEOPLASMS/ 3 ((epidural or extradural or extra‐dural or "spinal cord" or "dural sac" or "cauda equina" or "spinal column") and (neoplasm$ or cancer$ or tumour$ or tumor$ or malignan$ or metast$) and compress$).tw. 4 (neoplasm$ or cancer$ or tumour$ or tumor$ or malignan$ or metast$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] 5 1 and 4 6 2 or 3 or 5 7 randomized controlled trial.pt. 8 controlled clinical trial.pt. 9 randomized.ab. 10 placebo.ab. 11 drug therapy.fs. 12 randomly.ab. 13 trial.ab. 14 or/7‐13 15 exp animals/ not humans.sh. 16 14 not 15 17 6 and 16 18 (2008* or 2009* or 2010* or 2011* or 2012* or 2013*).ed. 19 17 and 18 |
| EMBASE (OVID) | 1 SPINAL CORD COMPRESSION/ 2 exp SPINAL CORD TUMOR/ 3 ((epidural or extradural or extra‐dural or "spinal cord" or "dural sac" or "cauda equina" or "spinal column") and (neoplasm$ or cancer$ or tumour$ or tumor$ or malignan$ or metast$) and compress$).tw. 4 (neoplasm$ or cancer$ or tumour$ or tumor$ or malignan$ or metast$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 5 1 and 4 6 2 or 3 or 5 7 random$.tw. 8 factorial$.tw. 9 crossover$.tw. 10 cross over$.tw. 11 cross‐over$.tw. 12 placebo$.tw. 13 (doubl$ adj blind$).tw. 14 (singl$ adj blind$).tw. 15 assign$.tw. 16 allocat$.tw. 17 volunteer$.tw. 18 Crossover Procedure/ 19 double‐blind procedure.tw. 20 Randomized Controlled Trial/ 21 Single Blind Procedure/ 22 or/7‐21 23 (animal/ or nonhuman/) not human/ 24 22 not 23 25 6 and 24 26 (2008* or 2099* or 2010* or 2011* or 2012* or 2013*).dd. 27 25 and 26 |
| CINAHL (EBSCO) | S16 S6 AND S15 S15 S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 S14 (allocat* random*) S13 (MH "Quantitative Studies") S12 (MH "Placebos") S11 placebo* S10 (random* allocat*) S9 (MH "Random Assignment") S8 (Randomi?ed control* trial*) S7 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* ) S6 S1 OR S4 OR S5 S5 S2 AND S3 S4 (MH "Spinal Cord Neoplasms") S3 (MH "Spinal Cord Compression") S2 (neoplasm* or cancer* or tumour* or tumor* or malignan* or metast*) S1 ((epidural or extradural or extra‐dural or "spinal cord" or "dural sac" or "cauda equina" or "spinal column") and (neoplasm* or cancer* or tumour* or tumor* or malignan* or metast*) and compress*) |
| LILACS (Bireme) | "Spinal Cord Compression" [Subject descriptor] or "Spinal Cord neoplasm$" OR ((( epidural OR extradural OR extra‐dural OR " Spinal cord" OR " dural sac" OR " cauda equina" OR "spinal column") AND (neoplasm$ OR Cancer$ OR Tumour$ OR Tumor$ OR Malignan$ OR metast$)) AND compression) [Words] and (Pt ENSAIO CONTROLADO ALEATORIO Or Pt ENSAIO CLINICO CONTROLADO OR Pt ENSAIO CLÍNICO OR Mh ENSAIOS CONTROLADOS ALEATORIOS Or Mh DISTRIBUICAO ALEATORIA Or Mh MÉTODO DUPLO‐CEGO Or Mh MÉTODO SIMPLES‐CEGO OR Ex E05.318.760.535$ OR Mh PLACEBOS OR Mh RESEARCH DESIGN) AND NOT (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)) OR ((Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) or ((Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) and (Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) and (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$)) AND NOT (Pt ENSAIO CONTROLADO ALEATORIO Or Pt ENSAIO CLINICO CONTROLADO OR Pt ENSAIO CLÍNICO OR Mh ENSAIOS CONTROLADOS ALEATORIOS Or Mh DISTRIBUICAO ALEATORIA Or Mh MÉTODO DUPLO‐CEGO Or Mh MÉTODO SIMPLES‐CEGO OR Ex E05.318.760.535$ OR Mh PLACEBOS OR Mh RESEARCH DESIGN) AND NOT (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)) OR ((Ct COMPARATIVE STUDY or Ex E05.337$ or Mh FOLLOW‐UP STUDIES or Mh PROSPECTIVE STUDIES or Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw vol‐unteer$) and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)) and not (Pt ENSAIO CONTROLADO ALEATORIO Or Pt ENSAIO CLINICO CONTROLADO Or Mh ENSAIOS CONTROLADOS ALEATORIOS Or Mh DISTRIBUICAO ALEATORIA Or Mh MÉTODO DUPLO‐CEGO Or Mh MÉTODO SIMPLES‐CEGO) OR ((Pt ENSAIO CLÍNICO or Ex E05.318.760.535$ or (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) and (Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) and (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) or Mh PLACEBOS or Tw placebo$ or (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) or Mh RESEARCH DESIGN)) and not ((Pt ENSAIO CONTROLADO ALEATORIO Or Pt ENSAIO CLINICO CONTROLADO Or Mh ENSAIOS CONTROLADOS ALEATORIOS Or Mh DISTRIBUICAO ALEATORIA Or Mh MÉTODO DUPLO‐CEGO Or Mh MÉTODO SIMPLES‐CEGO)) and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL))) [Words] |
| CancerLit (PubMED) | Search: ("Spinal Cord Compression" OR "Spinal Cord neoplasm" OR (epidural OR extradural OR extra‐dural OR "Spinal cord" OR "dural sac" OR "cauda equina" OR "spinal column") AND (neoplasm* OR Cancer* OR Tumour* OR Tumor*)) AND (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized controlled trials[mh] OR random allocation[mh] OR double‐blind method[mh] OR single‐blind method[mh] OR clinical trial[pt] OR clinical trials[mh] OR random*[tw] NOT (animals[mh])) |
Appendix 2. Electronic databases: search strategies (2008 search)
| Database | Search strategy |
| MEDLINE (1980 |
1. SPINAL CORD COMPRESSION/ 2. SPINAL CORD NEOPLASMS/ 3. ((epidural or extradural or extra‐dural or "spinal cord" or "dural sac" or "cauda equina" or "spinal column") AND (neoplasm$ or cancer$ or tumour$ or tumor$ or malignan$ or metast$) AND compress$) 4. (neoplasm$ or cancer$ or tumour$ or tumor$ or malignan$ or metast$).mp. [mp=ti, ot, ab, nm, hw] 5. 1 AND 4 6. 2 OR 3 OR 5 7. RANDOMIZED CONTROLLED TRIAL.pt. 8. CONTROLLED CLINICAL TRIAL.pt. 9. RANDOMIZED CONTROLLED TRIALS.sh. 10. RANDOM ALLOCATION.sh. 11. DOUBLE BLIND METHOD.sh. 12. SINGLE BLIND METHOD.sh. 13. OR/7‐12 14. (ANIMALS not HUMANS).sh. 15. 13 NOT 14 16. CLINICAL TRIAL.pt. 17. exp CLINICAL TRIALS/ 18. (clin$ adj25 trial$).ti,ab. 19. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 20. placebos.sh. 21. placebo$.ti,ab. 22. random$.ti,ab. 23. research design.sh. 24. or/16‐23 25. 24 not 14 26. 25 not 15 27. 15 or 25 28. 6 AND 27 |
| EMBASE (1980 to July 2008) | 1. Spinal Cord Compression/ 2. exp Spinal Cord Tumor/ 3. ((epidural or extradural or extra‐dural or "spinal cord" or "dural sac" or "cauda equina" or "spinal column") and (neoplasm$ or cancer$ or tumour$ or tumor$ or malignan$ or metast$) and compress$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 4. or/1‐3 5. random$.ti,ab. 6. factorial$.ti,ab. 7. (crossover$ or cross over$ or cross‐over$).ti,ab. 8. placebo$.ti,ab. 9. (doubl$ adj blind$).ti,ab. 10. (singl$ adj blind$).ti,ab. 11. assign$.ti,ab. 12. allocat$.ti,ab. 13. volunteer$.ti,ab. 14. CROSSOVER PROCEDURE.sh. 15. DOUBLE‐BLIND PROCEDURE.sh. 16. RANDOMIZED CONTROLLED TRIAL.sh. 17. SINGLE BLIND PROCEDURE.sh. 18. or/5‐17 19. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 20. HUMAN/ 21. 20 and 19 22. 19 not 21 23. 18 not 22 24. 4 and 23 |
| CANCERLIT (PubMed cancer subset) | ("Spinal Cord Compression" OR "Spinal Cord neoplasm" OR (epidural OR extradural OR extra‐dural OR "Spinal cord" OR "dural sac" OR "cauda equina" OR "spinal column") AND (neoplasm* OR Cancer* OR Tumour* OR Tumor* OR malignan* OR metast*) AND compression*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR random* [tw] NOT (animals [mh]) |
| LILACS (1992 to April 2007) | (Mh "Spinal Cord Compression" OR Tw"Spinal Cord neoplasm$" OR (Tw epidural OR Tw extradural OR Tw extra‐dural OR " Tw Spinal cord" OR " Tw dural sac" OR " Tw cauda equina" OR " Tw spinal column") AND (Tw neoplasm$ OR Tw Cancer$ OR Tw Tumour$ OR Tw Tumor$ OR Tw Malignan$ OR Tw metast$) AND Tw compression$) [Words] and (Pt ENSAIO CONTROLADO ALEATORIO Or Pt ENSAIO CLINICO CONTROLADO OR Pt ENSAIO CLÍNICO OR Mh ENSAIOS CONTROLADOS ALEATORIOS Or Mh DISTRIBUICAO ALEATORIA Or Mh MÉTODO DUPLO‐CEGO Or Mh MÉTODO SIMPLES‐CEGO OR Ex E05.318.760.535$ OR Mh PLACEBOS OR Mh RESEARCH DESIGN) AND NOT (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)) OR ((Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) or ((Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) and (Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) and (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$)) AND NOT (Pt ENSAIO CONTROLADO ALEATORIO Or Pt ENSAIO CLINICO CONTROLADO OR Pt ENSAIO CLÍNICO OR Mh ENSAIOS CONTROLADOS ALEATORIOS Or Mh DISTRIBUICAO ALEATORIA Or Mh MÉTODO DUPLO‐CEGO Or Mh MÉTODO SIMPLES‐CEGO OR Ex E05.318.760.535$ OR Mh PLACEBOS OR Mh RESEARCH DESIGN) AND NOT (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)) OR ((Ct COMPARATIVE STUDY or Ex E05.337$ or Mh FOLLOW‐UP STUDIES or Mh PROSPECTIVE STUDIES or Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw vol‐unteer$) and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)) and not (Pt ENSAIO CONTROLADO ALEATORIO Or Pt ENSAIO CLINICO CONTROLADO Or Mh ENSAIOS CONTROLADOS ALEATORIOS Or Mh DISTRIBUICAO ALEATORIA Or Mh MÉTODO DUPLO‐CEGO Or Mh MÉTODO SIMPLES‐CEGO) OR ((Pt ENSAIO CLÍNICO or Ex E05.318.760.535$ or (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) and (Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) and (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) or Mh PLACEBOS or Tw placebo$ or (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) or Mh RESEARCH DESIGN)) and not ((Pt ENSAIO CONTROLADO ALEATORIO Or Pt ENSAIO CLINICO CONTROLADO Or Mh ENSAIOS CONTROLADOS ALEATORIOS Or Mh DISTRIBUICAO ALEATORIA Or Mh MÉTODO DUPLO‐CEGO Or Mh MÉTODO SIMPLES‐CEGO)) and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL))) [Words] |
| CINAHL (1982 to July 2008) | 1. spinal cord compression/ or spinal cord neoplasms/ 2. ((epidural or extradural or extra‐dural or "spinal cord" or "dural sac" or "cauda equina" or "spinal column") and (neoplasm$ or cancer$ or tumour$ or tumor$ or malignan$ or metast$) and compress$).mp. [mp=title, subject heading word, abstract, instrumentation] 3. or/1‐2 4. Random Assignment/ 5. single‐blind studies/ 6. Double‐Blind Studies/ 7. Triple‐Blind Studies/ 8. Crossover Design/ 9. Factorial Design/ 10. (multicentre study or multicenter study or multi‐centre study or multi‐center study).mp. [mp=title, subject heading word, abstract, instrumentation] 11. random$.ti,ab. 12. latin square.ti,ab. 13. cross‐over.mp. or crossover.ti,ab. [mp=title, subject heading word, abstract, instrumentation] 14. Placebos/ 15. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 16. placebo$.mp. [mp=title, subject heading word, abstract, instrumentation] 17. Clinical Trials/ 18. (clin$ adj25 trial$).mp. [mp=title, subject heading word, abstract, instrumentation] 19. or/4‐18 20. 3 and 19 |
| CENTRAL (Issue 3, 2008 of The Cochrane Library) | #1 "Spinal Cord Compression" (single term MeSH) #2 "Spinal Cord Neoplasms" (single term MeSH) #3 ((epidural OR extradural OR extra‐dural OR "spinal cord" OR "dural sac" OR "cauda equina" OR "spinal column") AND (neoplasm* OR cancer* OR tumour* OR tumor* OR malignan* OR metast*) AND compress*) #4 #1 OR #2 OR #3 |
Data and analyses
Comparison 1. High dose versus no or moderate dose corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall ambulation (short term) | 3 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.81, 1.45] |
| 1.1 High dose versus no corticosteroids | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.93, 1.78] |
| 1.2 High versus moderate corticosteroids | 2 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.46, 1.43] |
| 2 Serious drug related adverse effects | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.02 [1.03, 62.37] |
| 2.1 High dose versus no corticosteroids | 1 | 57 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.96 [0.56, 176.92] |
| 2.2 High dose versus moderate dose corticosteroids | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.0 [0.32, 111.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Graham 2006.
| Methods | Randomised, parallel group, two armed, active controlled, open label, "pilot" multicenter clinical trial | |
| Participants |
Inclusion: MRI evidence of MESCC, pain or weakness or sensory disturbance or sphincter disturbance; histological proof of malignancy; ECOG performance status less than four, minimum survival two months, and minimum power 1/5, estimated minimum survival of less than two months; written informed consent. Exclusion: prior radiotherapy; prior treatment for MESCC, lymphoma and myeloma, people undergoing surgery, multi‐level MESCC or central nervous system disease; ongoing steroid medication, pregnancy, peptic ulcer or cardiac failure Age: 41 to 81 years Gender: males ‐ 14, females ‐ 6 Ambulant pretreatment: 15/20 were ambulant pre‐treatment (high‐dose dexamethasone versus low‐dose dexamethasone: 6/9 ‐ 67% versus 9/11‐ 82%). Performance status: not stated Type of primary tumours: breast/prostate ‐ 11 (5/9 ‐ 56% with high dose, 6/11 ‐ 55% with low dose), lung/GI/renal/others ‐ 9 Visceral metastasis: not stated Duration and rapidity of cord compression: not stated; 9/20 were diagnosed with MESCC > 6 months after the diagnosis of cancer Spinal level: cervical ‐ 1; thoracic ‐ 15; lumbar ‐ 4 Spinal instability: not stated |
|
| Interventions |
Intevention High‐dose dexamethasone 96 mg intravenous on days 0 to 2; (N = 9)* Control Moderate‐dose dexamethasone 16 mg intravenous on days 0 to 2; (N = 11)* Dexamethazone was weaned over 15 days in both arms Timing of intervention in relation to development of cord compression: not stated Concomitant treatment:
|
|
| Outcomes |
Outcomes of interest reported and used:
Outcomes reported but not used :
Outcomes sought but not reported
|
|
| Notes |
Setting:eight hospitals in three states in Australia Period of trial: September 2001 to November 2003 Provision for rehabilitation:not reported Source of funding: Trans‐Tasman Radiation Oncology Group (TROG); Cancer Council New South Wales Comments:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Pilot randomized comparison" Generation of allocation sequence: website‐based randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation ("Patients were randomized via the Superdex web site.") |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open label trial; similar co‐interventions were prescribed for participants in both groups; steroid doses were flexibly used |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Open label design and lack of agreement in the definition of ambulation raises the possibility of bias, but different ambulatory rates based on different interpretations were provided in the report |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Open label trial, unclear if the open label design introduced bias in detection of adverse events; also unclear if all participants were systematically assessed for adverse events |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | Study closed due to low recruitment; 20% were not assessed for primary outcome (had died); intention to treat analysis used (assuming all dead were not ambulant) |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Only serious adverse events were reported; unclear if less serious adverse events were systematically looked for |
| Selective reporting (reporting bias) | Low risk | The study was retrospectively registered (September 2005) but all pre‐stated outcomes were reported |
| Other bias | Low risk | No other sources of bias were detected |
Maranzano 2005.
| Methods | Randomised, parallel group, two arm, active controlled, open label, multi‐centre, equivalence, clinical trial. | |
| Participants |
Inclusion: MRI or CT diagnosis, short life expectancy (≤ six months, as defined by unfavourable histologies or favourable histologies with poor performance status, motor or sphincter dysfunction). Exclusion: diagnostic doubt, spinal instability, bony impingement, previous irradiation, favourable histology with life expectancy greater than or equals 6 months (15% of observed patients). Age: 30 to 89 years Gender: male ‐ 191, female ‐ 85 Pretreatment ambulant: two fractions versus eight fractions: 93 versus 91 Performance status: Karnovsky performance status: </= 40 ‐ 86, 50 to 70 ‐ 143, 80 to 100 ‐ 47 Type of primary tumours: Favourable histology (lymphoma, seminoma, myeloma, breast and prostate cancer) ‐ 99 Unfavourable histology (lung, renal, gastrointestinal, head and neck carcinoma, melanoma, sarcoma) ‐ 177 Visceral metastasis: unclear Duration and rapidity of cord compression: not stated Spinal level: cervical ‐ 8%, thoracic ‐ 50%, lumbar ‐ 23%, sacral ‐ 7%, cervicothoracic ‐ 1%, thoracolumbar ‐ 6%, lumbosacral ‐ 2% Spinal instability: was an exclusion criterion |
|
| Interventions |
Intervention Eight fractions: "Split course regimen" (5 Gy x 3, 4 days rest, then 3 Gy x 5, to a total of 30 Gy in 2 weeks), N = 147 Control Two fractions: "Short course regimen"( 8 Gy, 6‐days rest, and then 8 Gy, to a total of 16 Gy in 1 week ), N = 153 Timing of intervention in relation to development of cord compression not stated Concomitant medications: Dexamethasone: 8 mg twice daily tapered after completion of radiotherapy. Parenteral 5‐hydroxy‐tryptamine‐3 receptor antagonist if radiation included upper abdomen. |
|
| Outcomes |
Outcomes reported and used :
Outcomes reported but not used:
Outcomes sought but not reported:
|
|
| Notes |
Setting: five radiation oncology centres in Italy Period of study: February 1998 to November 2002 Provision for rehabilitation: not reported Source of funding: not reported Comments:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned, allocation was performed by a centralized registration, " |
| Allocation concealment (selection bias) | Low risk | "...investigators were notified of assignment by telephone and fax." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open label trial raises the possibility of differential use of additional interventions |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Ambulatory status was clearly defined and minimised possibility of observer bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | While subjective outcomes were operationally defined, detection bias cannot be ruled out in this open label trial |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | "With regard to the considered principal characteristics of this study, the 24 excluded patients (those lost to follow‐up or who experienced early deaths) were well balanced between the two arms and, in the intent‐to‐treat analysis, did not cause significant changes in results." |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | As above |
| Selective reporting (reporting bias) | Low risk | The trial protocol was not available; but all pre‐stated outcomes were reported |
| Other bias | Low risk | No other sources of bias were detected |
Maranzano 2009.
| Methods | Randomised, allocation concealed, phase III, parallel group, two arm, active controlled, open label, multi‐centre, equivalence trial | |
| Participants |
Inclusion: MRI or CT diagnosis, short life expectancy (less than or equals six months, as defined by unfavourable histologies or favourable histologies with poor performance status, motor or sphincter dysfunction). Exclusion: diagnostic doubt, spinal instability, bony compression, previous irradiation, Age: 33 to 87 years Gender: male ‐ 197, female ‐106 Pretreatment ambulant: short course vs Single dose 101 versus 98 Performance status: Karnovsky performance status: </= 40 ‐ 47, 50 to 70 ‐ 182, 80 to 100 ‐ 74 Type of primary tumours: favourable histology (breast, prostate, myeloma, small cell lung cancer, seminoma and lymphoma) ‐ 91 unfavourable histology (non‐small cell lung cancer, colog‐rectal, kidney, cancer of unknown origin, liver, bladder, gastric, pancreas, melanoma, uterine, head and neck, oesophagus and others) ‐ 212 Visceral metastasis: 134 Duration and rapidity of cord compression: not stated Spinal level: cervical spine (7%), thoracic (56%), lumbar (18%), sacral (3%) cervi co‐thoracic (2%), thoracolumbar (9%) and lumbo‐sacral (5%). Spinal instability: was an exclusion criterion |
|
| Interventions |
Intervention Single dose (8 Gy) N = 153 Control Short course (of two fractions: 8 Gy x 2 given as 8 Gy, 6 days rest, and then 8 Gy, to a total dose of 16 Gy in 1 week) N = 150 Radiotherapy was started within 24/48 hours of the radiologic diagnosis and was delivered by a 4 to 18 MV linear accelerator. Concomitant medications: parenteral dexamethasone (8 mg X 2/day) was administered from the first day of clinical‐radiologic diagnosis until 4 to 5 days |
|
| Outcomes |
Outcomes reported and used:
Outcomes reported but not used: duration of improvement according to pre‐ and post‐treatment walking capacity and histology Outcomes sought but not reported:.
|
|
| Notes |
Setting: seven radiation oncology centres in Italy Period of study: November 2002 to September 2007 Provision for rehabilitation: not reported Source of funding: not reported Comments: all assessments were done 1 month after the end of RT and the follow‐up examination was continued once a month for 1 year, and four times per year until death. Median follow‐up 36 months (range 4 to 58 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Patients were randomly assigned" Comment: method of randomisation was not stated but allocation was centralised, suggesting that randomisation may have been adequately performed |
| Allocation concealment (selection bias) | Low risk | Quote from report: "allocation was performed by a centralised registration, and investigators were notified of assignment by telephone and fax." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open label trial raises the possibility of differential use of additional interventions |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Ambulatory status was clearly defined and minimised possibility of observer bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | While subjective outcomes were operationally defined, detection bias cannot be ruled out in this open label trial |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | Quote from report: "327 patients entered the trial, of which 303 (93%) are assessable (150 in arm A, i.e., 8 Gy 2, and 153 in arm B, i.e., 8 Gy). Twenty‐four patients are not assessable because they lost to follow‐up (21 cases, of whom 11 in arm A and 10 in arm B) or for early death (3 cases, of whom 2 died of myocardial infarction 8 and 14 days after the start of RT, respectively, and the other one died of ictus 9 days after the start of RT)". Comment: The lack of differential rates of those lost to follow‐up in the two arms indicates low risk of attrition bias. Overall loss to follow‐up was only 7%. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Adverse events were well reported |
| Selective reporting (reporting bias) | Low risk | The trial protocol is not available but all pre‐stated outcomes were reported fully |
| Other bias | Low risk | The source of funding was not reported but funding is unlikely to have been from conflicted sources. No other sources of bias were identified |
Patchell 2005.
| Methods | Randomised, parallel group, two arm, active controlled, stratified (institution, tumour type, ambulatory status, spinal stability) open label, multi‐institutional trial | |
| Participants |
Inclusion: MRI diagnosis, general medical status good enough to be acceptable surgical candidates, life expectancy three months or greater, total paraplegia less than 48 hours, single compressive lesion in cervical or thoracic lesions Exclusion: total paraplegia more than 48 hours, multiple discrete compression, radiosensitive tumours (haematologic and germ cell tumours), previous irradiation, compression of only cauda equina or spinal roots, preexisting neurological problems Age: median 60 years Gender: males ‐ 70, females ‐ 31 Pretreatment ambulant: surgery plus radiotherapy versus radiotherapy alone: 34 versus 35 Performance status: not reported Type of primary tumours: all except radiosensitive tumours (haematologic and germ cell tumours) Visceral metastasis: not stated Duration of cord compression: less than 48 hours Rapidity of cord compression: not stated Spinal level: cervical ‐ 13, upper thoracic ‐ 38, lower thoracic ‐ 50 Spinal instability: used as a stratifying variable; surgery plus radiotherapy versus radiotherapy alone: 20 versus 18 |
|
| Interventions |
Intervention Surgery with radiotherapy: N = 50 (Surgery‐ direct circumferential decompression with or without stabilisation within 24 hours of randomisation; Radiotherapy‐ 3 Gy x 10, starting within 14 days of surgery) Control radiotherapy ‐ 3 Gy x 10; started within 24 hours after randomisation; N = 51 Timing of intervention in relation to development of cord compression: surgery plus radiotherapy versus radiotherapy alone: median time 10 versus 12 days Concomitant medications: Dexamethasone, both arms 100 mg immediate, 24 mg four times daily till start of radiotherapy or surgery then tapered. |
|
| Outcomes |
Outcomes reported and used:
Outcomes reported but not used:
Outcomes sought but not reported:
|
|
| Notes |
Setting: eight institutions in the United States of America Period of Study: September 1992 to December 2002 Provision for rehabilitation: not reported Source of Funding: National Cancer Institute; National Institute for Neurological Disorders and Stroke Comments:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "Randomisation within strata by permutated blocks was done separately at each institution with a computerised technique, which ensured immediate randomisation at study entry". |
| Allocation concealment (selection bias) | Low risk | Comment: stratified randomisation with central allocation appear to have been done. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open label trial raises the possibility of differential use of additional interventions |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Ambulatory status was clearly defined and minimised possibility of observer bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | While subjective outcomes were operationally defined, detection bias cannot be ruled out in this open label trial |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | There was no attrition |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Adverse events were adequately reported |
| Selective reporting (reporting bias) | Low risk | The trial protocol was not available but all pre‐stated outcomes were reported |
| Other bias | Unclear risk | The trial was stopped early for benefit based on pre‐set stopping rules with only 50% of the estimated samples size recruited. It is unclear if this biased the reliability of the estimates; though it is possible that effect estimates were overstated in favour of surgery. No other sources of bias were identified |
Sorensen 1994.
| Methods | Randomised trial, parallel group, two arm, observer blinded, single centre trial | |
| Participants |
Inclusion: consecutive patients with spinal cord or cauda equina compression by a carcinoma confirmed by myelogram (and in some instances by MRI) referred for RT Exclusion: lymphoma, surgery for cord compression, unstable vertebral lesions, previous treatment for epidural metastasis, carcinomatous meningitis, peptic ulcer, infection Age: median 62 years (range: 25 to 82 years) Gender: males ‐ 18, females 39 Pretreatment ambulant: high‐dose corticosteroids versus no corticosteroids: 17 versus 19 Performance status: not reported Type of primary tumours: all types except lymphoma (majority from breast 34/57) Visceral metastasis: not stated Duration and rapidity of cord compression: interval from diagnosis of cancer to diagnosis of cord compression ranged from 0‐17 years Spinal level: cervical ‐ 3; thoracic ‐ 33; lumbar ‐ 21 Spinal instability: was an exclusion criterion |
|
| Interventions |
Intervention: Dexamethasone 96 mg intravenous stat and per oral for 3 days and taper over 15 days ‐ N = 27* Control: No dexamethasone ‐ N = 30* * Both arms received radiotherapy ‐ 28 Gy in 7 fractions on consecutive days staring within a few hours after myelography (1‐20 hours) Timing of intervention in relation to development of cord compression ‐ not stated Concomitant medications: prophylactic medication in people with peptic ulcer and dyspepsia. |
|
| Outcomes |
Outcomes reported and used:
Outcomes reported but not used:
Outcomes sought but not reported:
|
|
| Notes |
Setting: single centre in Denmark Period of trial: May 1987 to April 1989 Provision for rehabilitation: not reported. Source of funding: Danish Cancer Research Foundation; Dexamethazone provided by Merck, Sharpe & Dhome, Denmark Comments Duration of follow‐up: assessed every three months for two years or until death |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratifed randomisation by primary tumour (breast or other tumour) and gait function (ambulant, non‐ambulant) |
| Allocation concealment (selection bias) | Low risk | Not reported; but treating physician was not aware of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treating physician who also assessed outcomes was unaware of treatment allotment |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Assessor blinded trial |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | No subjective outcomes were reported |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | Two patients in the steroid group were withdrawn after randomisation (ineligible); this is unlikely to have introduced bias |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Intention to treat analysis used |
| Selective reporting (reporting bias) | Low risk | The trial protocol was not available, but pre‐stated outcomes were fully reported |
| Other bias | Unclear risk | The role of the pharmaceutical sponsor is unclear |
Vecht 1989.
| Methods | Randomised,parallel group, two arm, active controlled,participant and assessor blinded, multi‐institutional trial | |
| Participants |
Inclusion: complete obstruction on myelogram, histologically confirmed primary carcinoma or lymphoreticular malignancy Exclusion: not mentioned Age: 22 to 87 years Gender: males ‐ 26, females ‐ 11 Pretreatment ambulant: high‐dose versus moderate‐dose corticosteroids: 14 versus 7 Performance status: not stated Type of primary tumours: carcinoma ‐ 26; lymphoreticular malignancy ‐ 11 Visceral metastasis: not stated Duration and rapidity of cord compression: not stated Spinal level: not stated Spinal instability: not stated |
|
| Interventions |
Intervention Dexamethasone bolus 100 mg IV followed by 4 mg 4 times a day orally(N = 22)* Control Dexamethazone bolus 10 mg IV followed by 4 mg 4 times a day orally (N = 15)* Radiotherapy in both arms: 3 Gy x 7 or 10 fractions* Timing of intervention in relation to development of cord compression ‐ not stated Concomitant medications: not stated |
|
| Outcomes |
Outcomes reported and used:
Outcomes reported and not used:
Outcomes sought but not reported:
|
|
| Notes |
Setting: four centres in the Nethelands Period of study: not reported Provision for rehabilitation: not reported Source of funding: not stated Comments:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were stratified during randomization for carcinoma versus reticular malignancy" |
| Allocation concealment (selection bias) | Low risk | "The code was broken by the statistician at the final analysis" Comment: the method of allocation concealment is not reported but attempts appear to have been made to conceal the randomisation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The interventions were masked |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | The interventions were masked |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | The interventions were masked |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Unclear risk | Data were missing for three participants whose allocated intervention was not reported |
| Incomplete outcome data (attrition bias) Adverse events | High risk | Data on pain were missing for pain outcomes in two participants given low dose dexamethazone and in five participants given high dose dexamethazone |
| Selective reporting (reporting bias) | Unclear risk | The trial protocol was not available and there were missing data |
| Other bias | Low risk | No other sources of bias were detected |
Young 1980.
| Methods | Randomised, parallel group, two arm, active controlled, open label, two‐centre trial | |
| Participants |
Inclusion: tissue diagnosis of a malignant tumour not of central nervous system origin; the presence of clinical symptoms of cord compression; and a myelogram showing extradural lesion or block that correlated with clinical presentation Exclusion: prior radiotherapy, unfit for surgery, more than one lesion, presence of only spinal or radicular pain. Age: 19 to 83 years Gender: not stated Pretreatment ambulant: laminectomy plus radiotherapy versus radiotherapy alone: 6 versus 5 Performance status: not stated Type of primary tumours: all types Visceral metastasis: not stated Duration and rapidity of cord compression: not stated Spinal level: not stated Spinal instability: not stated |
|
| Interventions |
Intervention laminectomy with radiotherapy: 30 Gy in 10 fractions over 14 days + steroids* (N = 16) Control radiotherapy: 30 Gy in 10 fractions (4 Gy/day first 3 days, then 18 Gy in 7 fractions over 14 days) + steroids* (N = 13) Timing of intervention in relation to development of cord compression ‐ not stated Concomitant medications: *Dexamethasone 12 mg stat followed by 4 mg four times daily till radiotherapy completion; other medications ‐not reported |
|
| Outcomes |
Outcomes reported and used:
Outcomes reported but not used:
Outcomes sought but not reported:
|
|
| Notes |
Setting: two centres in USA Period of study: not reported Provision for rehabilitation: not reported Source of funding: not reported Comments: radiotherapy alone: mortality ‐ 24% (due to underlying disease) duration of follow‐up: participants were followed up at regular intervals until death; duration unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out using a "table of random numbers" |
| Allocation concealment (selection bias) | High risk | Not stated; there were also baseline imbalances in prognostic variables that might have occurred due to lack of stratification |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Lack of blinding could have led to differential interventions in terms of pain medication; though no differences were noted with interventions |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Ambulatory status was clearly defined and minimised the possibility of observer bias |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | While subjective outcomes were operationally defined, detection bias cannot be ruled out in this open label trial |
| Incomplete outcome data (attrition bias) Efficacy outcomes | Low risk | There was no attrition |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | As above |
| Selective reporting (reporting bias) | Low risk | The trial protocol was not available but all relevant outcomes were reported |
| Other bias | Low risk | No other sources of bias were identified |
MRI ‐ magnetic resonance imaging CT‐ computed tomography ECOG ‐ Eastern Co‐operative Oncology Group SD ‐ standard deviation Gy ‐ Gray
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aviles 2002 | Not RCT: abstract described it as randomised, but full paper reveals this to be a retrospective rather than a prospective study |
| Chaichana 2008 | Not RCT: retrospective chart review |
| Holden 2011 | RCT; randomised participants with MESCC to erythropoietin or placebo; all patients got radiotherapy and steroids |
| Hunter 2008 | Not a primary RCT: a summary of Patchell 2005 drawing attention to controversial aspects of the study |
| Mannion 2007 | Not RCT: cohort study of patients who presented with metastatic cord or cauda equina compression, and were treated with surgical decompression and fixation where necessary. |
| Rades 2009 | Not RCT: prospective non‐randomised comparison of short courses of RT and long courses of RT in two countries |
| Ryu 2010 | Not RCT: case series |
| Schaefer 2012 | Not RCT: prospective controlled clinical trial comparing open surgery versus minimally invasive surgery in patients needing spinal surgery with instrumentation due to neoplastic instability/pain or spinal cord compression |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Hegazy 2012.
| Methods | Randomised: methods not clear |
| Participants | Patients with metastatic spinal cord compression (MSCC) (N = 285) ; no other details available |
| Interventions | Three different radiotherapy schedules 1. 1 x 8 Gy (N = 95) 2. 10 x 3 Gy (N = 100) 3. 20 x 2 Gy (N = 90) |
| Outcomes | Funtional outcomes, Eastern Cooperative Oncology Group performance status (ECOG‐PS), toxicity, in‐field recurrences, prognostic factors |
| Notes | Only conference abstract available Contact details: Hegazy M, Neurology, (Wahba) Clinical Oncology and Nuclear Medicine, Mansoura University, Mansoura, Egypt |
Characteristics of ongoing studies [ordered by study ID]
ISRCTN97555949.
| Trial name or title | A randomised feasibility study of single fraction radiotherapy compared to multi‐fraction radiotherapy in patients with metastatic spinal cord compression |
| Methods | Allocation: randomised |
| Participants |
Inclusion: 1. Proven diagnosis of spinal cord compression on Magnetic Resonance Imaging (MRI) 2. Histologically or cytologically confirmed malignant disease 3. Life expectancy > 1 month 4. Age 18 years or older 5. Able to give informed consent 6. Willing and able to complete assessment forms Exclusion: 1. Patients for whom surgery or chemotherapy treatment is more appropriate 2. Patient who are known to be pregnant |
| Interventions | Radiotherapy (single or multiple fractions): arm 1: 20 Gy/5 fractions daily for five consecutive days arm 2: 8 Gy/1 fraction |
| Outcomes | Primary outcome measure(S) Patient accrual per centre over a 12 month period Secondary outcome measure(S) 1. Ambulatory status at 1, 4, 8 and 12 weeks from Day 1 of treatment compared to baseline 2. Bladder and bowel function at baseline compared to week 1, 4, 8 and 12 3. Acute side effects at week 1 and 4. assessed using Radiation Therapy Oncology Group (RTOG) scales 4. Quality of life at week 1, 4, 8 and 12, measured by the EORTC QLQ‐C30 questionnaire 5. Further treatment 6. Overall survival at 3, 6 and 12 months 7. Total number of days spent in hospital 8. Preferred place of care 9. Number of patients who were eligible but not randomised and reasons for non‐randomisation |
| Starting date | November 2007 |
| Contact information | Prof Peter J Hoskin, Marie Curie Research Wing, Mount Vernon Hospital, Rickmansworth Road, Northwood, Middlesex, Northwood, United Kingdom, HA6 2RN |
| Notes |
Estimated Enrollment: 100 Study sites: Bristol Haematology and Oncology Centre; Cancer Research UK and University College London Cancer Trials Centre, Northwood; Glan Clwyd Hospital, Rhyl, Denbighshire, Wales; Christie Hospital, Manchester (all in the UK) Acronym (SCORAD) Study due to be completed: August 2009; No results posted; Prof Hoskin contacted by e‐mail on 5 April 2014; Reply "The trial is still ongoing ; we hope to complete accrual by end 2014" |
NCT00968643.
| Trial name or title | A randomised phase III trial of two fractionation schemes in the treatment of malignant spinal cord compression (Spinal Cord Compression. ICORG 05‐03, V6) |
| Methods | Randomised, parallel group, open label trial |
| Participants |
Inclusion
Exclusion
|
| Interventions | Radiotherapy (single or multiple fractions): Arm 1: 20 Gy/5 fractions daily for five consecutive days (Control) Arm 2: 10 Gy/1 fraction |
| Outcomes |
Primary outcome measure(s):
change in motor functioning as measured by the change in physical functioning dimension of the EORTC QLQ‐C30 version 3 quality of life questionnaire, over a four week period Secondary outcome measure(s):
Median survival ‐ calculated on the basis of time from date of randomisation to death |
| Starting date | February 2007 |
| Contact information | Dr Joe O'Sullivan, Senior Lecturer and Consultant in Clinical Oncology, The Northern Ireland Cancer Centre
Belfast City Hospital, Belfast, BT9 7AB, Northern Ireland. Tel: +44 (0)28 90699204 E‐mail: joe.osullivan@Queens‐Belfast.ac.uk |
| Notes |
Estimated enrolment: 126 Study sites: Cork University Hospital; Saint Luke's Radiation Oncology Network (SLRON), Dublin; Galway University Hospital; Whitfield Cancer Centre at Whitfield Clinic, Waterford (all in Ireland) Status: currently recruiting First received: August 28, 2009 (retrospectively registered) Last updated: January 23, 2014 Sponsor: Ireland Cooperative Oncology Research Group (Pierre Thirion‐Principal Investigator) |
Differences between protocol and review
We updated the background section to incorporate important developments published since the 2008 review was published.
We amended the following sections in the methods: Assessment of risk of bias in included studies; Unit of analysis issues; Dealing with missing data; Assessment of heterogeneity; Assessment of reporting biases, to reflect the current standards for Cochrane Reviews (MECIR 2011). We generated 'Risk of bias' for the included studies in this review update using the methods described in Higgins 2011a.
Following recommendations from the editorial group, we removed analyses from single studies presented in the 2008 review and summarised the overall findings from single studies, without effect estimates, in the text of the results section. PaPaS suggests that meta‐analysis is only considered when there are at least two studies with at least 200 total participants. Following editorial suggestions, we retained meta‐analysis for only two outcomes for the comparison of 'high dose versus moderate dose or no steroids'.
We used GRADE profiler (GRADE 2004) and interpreted the evidence for each important and critically important outcome for the comparisons in the included trials using the GRADE approach to create 'Summary of findings' tables for each comparison (Schünemann 2011). We selected outcomes to include in these tables through discussion before evaluating the search results. We described in our methods section the GRADE approach to assessing overall study quality, and the meaning of each grade of quality.
Compared to the 2008 review, this update includes one new study with 303 additional participants, and an additional comparison (single‐dose versus short‐course radiotherapy). This update also has data for one additional secondary outcome (local recurrence) for the new comparison and for one other existing comparison. This update includes a more detailed discussion section than in the 2008 review. Finally, this update also contains 'Summary of findings' tables for five comparisons that link the effect estimates for key outcomes with the confidence one can place in these estimates. These have altered the conclusions as detailed in the results, discussion and conclusions.
Contributions of authors
2015 review update
All authors were involved in updating the review. RG updated the background section of the review, helped update the methods, selected studies, assessed risk of bias, extracted and analysed data, and re‐wrote the results, discussion and conclusions. JJ helped update the background and the methods, selected studies, assessed risk of bias, extracted data, and helped interpret the results. RKG helped interpret the results. AGC helped update the background, and methods, and interpret the results. PT helped revise the background, updated the methods section, checked the selected studies, assessing risk of bias, extracted data, analysed data, helped re‐write the results, discussion, and conclusions, prepared the summary of findings tables, and re‐drafted the abstract and plain language summary. All authors approved the final version of the review.
2008 review:
Conceived review ‐ RG, JJ; devised search strategy ‐ JJ, RG, RKG in collaboration with Sylvia Bickley; wrote protocol ‐ RG, JJ, PT; reviewed protocol ‐ ML, AGC; assessed and selected trials ‐ JJ, RG; obtained reports ‐ RKG, RG; literature search ‐ RKG, RG, JJ; assessed quality ‐ RKG, JJ, RG; extracted data ‐ JJ, RG, RKG, ML; analysed and interpreted data ‐ JJ, RG, RKG, PT; wrote review ‐ RG, JJ, RKG, AGC, PT
Sources of support
Internal sources
-
Christian Medical College, Vellore, India.
Employment for RG, JJ, AC, PT
-
South Asian Cochrane Centre, India.
Protocol and review completion workshops; logistic support
-
Royal Adelaide Hospital, Adelaide, Australia.
Employment for RKG
External sources
-
UKaid: Department for International Development (DFID), UK.
Funding via a grant to the Effective Health Care Research Consortium (to Paul Garner, Liverpool, UK) in aid of developing countries, of which PT is a programme partner
Declarations of interest
RG: declares no financial or academic conflicts of interest
JJ: declares no financial or academic conflicts of interest
RJG: declares no financial or academic conflicts of interest
ACG: declares no financial or academic conflicts of interest
PT: declares no financial or academic conflicts of interest
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Graham 2006 {published data only}
- Graham PH, Capp A, Delaney G, Goozee G, Hickey B, Turner S, et al. A pilot randomised comparison of dexamethasone 96 mg vs 16 mg per day for malignant spinal‐cord compression treated by radiotherapy: TROG 01.05 Superdex study. Clinical Oncology (R Coll Radiol) 2006;18(1):70‐6. [NCT00193869] [DOI] [PubMed] [Google Scholar]
Maranzano 2005 {published data only}
- Kwok Y, Patchell RA, Regine WF. Journal of Clinical Oncology. 2005; Vol. 23 (32):8272‐4. [DOI] [PubMed]
- Kwok Y, Regine WF, Patchell RA. Radiation therapy alone for spinal cord compression: time to improve upon a relatively ineffective status quo [comment on: Journal of Clinical Oncology 2005;23(15),3358‐65]. Journal of Clinical Oncology 2005;23(15):3308‐10. [DOI] [PubMed] [Google Scholar]
- Macbeth F, Stephens R, Hoskin P. Radiation dose in spinal cord compression. Comment on: Radiation therapy alone for spinal cord compression: time to improve upon a relatively ineffective status quo. [J Clin Oncol. 2005 20;23(15):3308‐10]. Journal of Clinical Oncology 2005;23(32):8270. [DOI] [PubMed] [Google Scholar]
- Maranzano E, Bellavita R, Rossi R. Radiotherapy alone or surgery in spinal cord compression? The choice depends on accurate patient selection. Journal of Clinical Oncology 2005; Vol. 23, issue 32:8270‐2. [DOI] [PubMed]
- Maranzano E, Bellavita R, Rossi R, Angelis V, Frattegiani A, Bagnoli R, et al. Short‐course versus split‐course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. Journal of Clinical Oncology 2005;23(15):3358‐65. [DOI] [PubMed] [Google Scholar]
- Maranzano E, Trippa F, Casale M, Anselmo P, Rossi R. Reirradiation of metastatic spinal cord compression: definitive results of two randomized trials. Radiotherapy and Oncology 2011;98(2):234‐7. [DOI] [PubMed] [Google Scholar]
Maranzano 2009 {published data only}
- Maranzano E, Trippa F, Casale M, Anselmo P, Rossi R. Reirradiation of metastatic spinal cord compression: definitive results of two randomized trials. Radiotherapy and Oncology 2011;98(2):234‐7. [DOI] [PubMed] [Google Scholar]
- Maranzano E, Trippa F, Casale M, Costantini S, Lupattelli M, Bellavita R, et al. 8Gy single‐dose radiotherapy is effective in metastatic spinal cord compression: results of a phase III randomized multicentre Italian trial. Radiotherapy and Oncology 2009;93(2):174‐9. [DOI] [PubMed] [Google Scholar]
Patchell 2005 {published data only}
- Chi GH, Gokaslan Z, McCormick P, Tibbs PA, Kryscio RJ, Patchell RA. Selecting treatment for patients with malignant epidural spinal cord compression‐does age matter?:results from a randomized clinical trial. Spine (Phila Pa 1976) 2009;34(5):431‐5. [DOI] [PubMed] [Google Scholar]
- Furlan JC, Chan KK, Sandoval GA, Lam KC, Klinger CA, Patchell RA, et al. The combined use of surgery and radiotherapy to treat patients with epidural cord compression due to metastatic disease: a cost‐utility analysis. Neuro‐oncology 2012;14(5):631‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan JC, Chan KKW, Sandoval G, Lam KC, Klinger CA, Patchell RA, et al. An Ontario‐based cost‐utility analysis comparing the standard of care with direct decompressive surgical resection followed by radiotherapy in the palliative care of patients with metastatic spinal cord cancer. Canadian Journal of Neurological Sciences. [Conference Abstract]. 2011:1) S12.
- Koch M, Keyser J. Surgical resection in metastatic spinal cord compression. Lancet 2006; Vol. 367, issue 9505:109. [DOI] [PubMed]
- Kunkler I. Surgical resection in metastatic spinal cord compression. Lancet 2006; Vol. 367, issue 9505:109. [DOI] [PubMed]
- Maranzano E, Bellavita R, Rossi R. Radiotherapy alone or surgery in spinal cord compression? The choice depends on accurate patient selection. Comment on Radiation therapy alone for spinal cord compression: time to improve upon a relatively ineffective status quo. [J Clin Oncol. 2005]. Journal of Clinical Oncology 2005;23(32):8270‐2; author reply 8272‐4. [DOI] [PubMed] [Google Scholar]
- Maranzano E, Trippa F. Be careful in getting cost‐effectiveness conclusions from a debatable trial! Comment on Cost‐effectiveness of surgery plus radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression. [International Journal of Radiation Oncology, Biology, Physics, 2006]. International Journal of Radiation Oncology, Biology, Physics 2007;68(1):314; author reply 314‐5. [DOI] [PubMed] [Google Scholar]
- Patchell R, Tibbs PA, Regine F, Payne R, Saris S, Kryscio RJ, et al. A randomized trial of direct decompressive surgical resection in the treatment of spinal cord compression caused by metastasis. Journal of Clinical Oncology 2003;21:237S. [DOI] [PubMed] [Google Scholar]
- Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet 2005;366(9486):643‐8. [DOI] [PubMed] [Google Scholar]
- Thomas KC, Nosyk B, Fisher CG, Dvorak M, Patchell RA, Regine WF, et al. Cost‐effectiveness of surgery plus radiotherapy versus radiotherapy alone for metastatic epidural spinal cord compression. International Journal of Radiation Oncology, Biology, Physics 2006;66(4):1212‐8. [DOI] [PubMed] [Google Scholar]
- Bent MJ. Surgical resection improves outcome in metastatic epidural spinal cord compression [comment on: Lancet 2005;366(9486):643‐8]. Lancet 2005;366(9486):609‐10. [DOI] [PubMed] [Google Scholar]
Sorensen 1994 {published data only}
- Sorensen S, Helweg‐Larsen S, Mouridsen H, Hansen HH. Effect of high‐dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. European Journal of Cancer 1994;30A(1):22‐7. [DOI] [PubMed] [Google Scholar]
Vecht 1989 {published data only}
- Vecht CJ, Haaxma‐Reiche H, Putten WL, Visser M, Vries EP, Twijnstra A. Initial bolus of conventional versus high‐dose dexamethasone in metastatic spinal cord compression. Neurology 1989;39(9):1255‐7. [DOI] [PubMed] [Google Scholar]
Young 1980 {published data only}
- Young RF, Post EM, King GA. Treatment of spinal epidural metastases: randomized prospective comparison of laminectomy and radiotherapy. Journal of Neurosurgery 1980;53:741‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aviles 2002 {published data only}
- Aviles A, Fernandez R, Gonzalez JL, Garcia EL, Neri N, Talavera A, et al. Spinal cord compression as a primary manifestation of aggressive malignant lymphomas: long‐term analysis of treatments with radiotherapy, chemotherapy or combined therapy. Leukemia & Lymphoma 2002;43(2):355‐9. [DOI] [PubMed] [Google Scholar]
Chaichana 2008 {published data only}
- Chaichana KL, Woodworth GF, Sciubba DM, McGirt MJ, Witham TJ, Bydon A, et al. Predictors of ambulatory function after decompressive surgery for metastatic epidural spinal cord compression. Neurosurgery. 2008;62(3):683‐92. [DOI] [PubMed] [Google Scholar]
Holden 2011 {published data only}
- Holden L, Loblaw A. Recombinant human erythropoietin (r‐HuEPO) in the prevention of neurologic sequelae from malignant spinal cord compression (MSCC): A multicenter, placebo‐controlled, phase 2 randomized study. Journal of Medical Imaging and Radiation Sciences. [Conference Abstract] 2011;42(3):155. [Google Scholar]
Hunter 2008 {published data only}
- Hunter RE, Wigfield CC. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomized trial. British Journal of Neurosurgery 2008;22(5):13‐4. [DOI] [PubMed] [Google Scholar]
Mannion 2007 {published data only}
- Mannion RJ, Wilby M, Godward S, Lyratzopoulos G, Laing RJC. The surgical management of metastatic spinal disease: prospective assessment and long‐term follow‐up. British Journal of Neurosurgery. 2007;21(6):593‐8. [DOI] [PubMed] [Google Scholar]
Rades 2009 {published data only}
- Rades D, Douglas S, Veninga T, Stalpers LJ, Hoskin PJ, Bajrovic A, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer 2010;116(15):3670‐3. [DOI] [PubMed] [Google Scholar]
- Rades D, Lange M, Veninga T, Rudat V, Bajrovic A, Stalpers LJ, et al. Preliminary results of spinal cord compression recurrence evaluation (score‐1) study comparing short‐course versus long‐course radiotherapy for local control of malignant epidural spinal cord compression. International Journal of Radiation Oncology, Biology, Physics 2009;73(1):228‐34. [DOI] [PubMed] [Google Scholar]
Ryu 2010 {published data only}
- Ryu S, Rock J, Jain R, Lu M, Anderson J, Jin J‐Y, et al. Radiosurgical decompression of metastatic epidural compression. Cancer 2010;116(9):2250‐7. [DOI] [PubMed] [Google Scholar]
Schaefer 2012 {published data only}
- Schaefer C, Viezens L, Vettorazzi E, Wellbrock J, Hansen‐Algenstaedt N. Prospective clinical trial of minimal‐invasive instrumentation of the spine in spinal metastasis‐benefit or dead end. European Spine Journal. [Conference Abstract]. 2012;21(11):2395‐6. [Google Scholar]
References to studies awaiting assessment
Hegazy 2012 {published data only (unpublished sought but not used)}
- Hegazy, M, Wahba, H. Single‐ versus multi‐fraction radiation treatment for metastatic spinal cord compression: Functional outcome study Neurology. Conference. 64th American Academy of Neurology Annual Meeting New Orleans, LA United States. Conference Start. 20120421 Conference End: 20120428. Conference Publication: (var.pagings). 22 April 2012.
References to ongoing studies
ISRCTN97555949 {unpublished data only}
- A randomised feasibility study of single fraction radiotherapy compared to multi‐fraction radiotherapy in patients with metastatic spinal cord compression. http://controlled‐trials.com, UK Clinical Trials Gateway. [CRUK‐UCL‐BRD‐07‐010; EU‐20863; NCT00727584]
NCT00968643 {unpublished data only}
- A randomised phase III trial of two fractionation schemes in the treatment of malignant spinal cord compression (Spinal Cord Compression. ICORG 05‐03, V6). http://clinicaltrials.gov/show/NCT00968643. [CDR0000644307; EU‐20952; ICORG‐05‐03]
Additional references
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bassler 2010
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta‐regression analysis. JAMA 2010;303(12):1180‐7. [DOI] [PubMed] [Google Scholar]
Chi 2009
- Chi JH, Gokaslan Z, McCormick P, Tibbs PA, Kryscio RJ, Patchell RA. Selecting treatment for patients with malignant epidural spinal cord compression‐does age matter?: results from a randomized clinical trial. Spine (Phila Pa 1976). 2009;34(5):431‐5. [DOI] [PubMed] [Google Scholar]
Choi 2010
- Choi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C, et al. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. European Spine Journal 2010;19(2):215‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONSORT 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. PLoS Medicine 2010;7(3):e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Delattre 1989
- Delattre JY, Arbit E, Thaler HT, Rosenblum MK, Posner JB. A dose‐response study of dexamethasone in a model of spinal cord compression caused by epidural tumor. Journal of Neurosurgery 1989;70(6):920‐5. [DOI] [PubMed] [Google Scholar]
Falkmer 2003
- Falkmer U, Jarhult J, Wersall P, Cavallin‐Stahl E. A systematic overview of radiation therapy effects in skeletal metastases. Acta Oncologica 2003;42(5/6):620‐33. [DOI] [PubMed] [Google Scholar]
Furlan 2012
- Furlan JC, Chan KK, Sandoval GA, Lam KC, Klinger CA, Patchell RA, et al. The combined use of surgery and radiotherapy to treat patients with epidural cord compression due to metastatic disease: a cost‐utility analysis. Neuro‐oncology 2012 2012;14(5):631‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2004 [Computer program]
- GRADE Working Group. GRADE Profiler; version 3.6. GRADE Working Group, 2004.
Gradepro 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.2 for Windows. GRADE Working Group, 2008.
Guyatt 2012
- Guyatt GH, Briel M, Glasziou P, Bassler D, Montori V. Problems of stopping trials early. BMJ 2012;344:e3863. [DOI] [PubMed] [Google Scholar]
Heimdal 1992
- Heimdal K, Hirschberg H, Slettebo H, Watne K, Nome O. High incidence of serious side effects of high‐dose dexamethasone treatment in patients with epidural spinal cord compression. Journal of Neuroncology 1992;12(2):141‐4. [DOI] [PubMed] [Google Scholar]
Helweg‐Larsen 2000
- Helweg‐Larsen S, Sorensen PS, Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables including survival and gait function in 153 patients. International Journal of Radiation Oncology, Biology and Physics 2000;46(5):1163‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Holt 2012
- Holt T, Hoskin P, Maranzano E, Sahgal A, Schild SE, Ryu S, et al. Malignant epidural spinal cord compression: the role of external beam radiotherapy. Current Opinion in Supportive and Palliative Care 2012;6(1):103‐8. [DOI] [PubMed] [Google Scholar]
Kim 2012
- Kim JM, Losina E, Bono CM, Schoenfeld AJ, Collins JE, Katz JN, et al. Clinical outcome of metastatic spinal cord compression treated with surgical excision ± radiation versus radiation therapy alone: a systematic review of literature. Spine (Philadelphia, Pa.: 1976) 2012;37(1):78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klimo 2004
- Klimo P, Schmidt MH. Surgical management of spinal metastases. Oncologist 2004;9(2):188‐96. [DOI] [PubMed] [Google Scholar]
Klimo 2005
- Klimo P, Thompson CJ, Kestle JRW, Schmidt MHS. A meta‐analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro‐oncology 2005;7(1):64‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laperriere 1996
- Laperriere NJ. The management of spinal cord compression. Toronto, Ontario, Canada, Princess Margaret Hospital 1996.
Loblaw 1998
- Loblaw DA, Laperriere NJ. Emergency treatment of malignant spinal cord compression: an evidence based guideline. Journal of Clinical Oncology 1998;16(4):1613‐24. [DOI] [PubMed] [Google Scholar]
Loblaw 2003
- Loblaw DA, Laperriere NJ, Mackillop WJ. A population‐based study of malignant spinal cord compression in Ontario. Clinical Oncology (R Coll Radiol) 2003;15(4):211‐7. [DOI] [PubMed] [Google Scholar]
Loblaw 2005
- Loblaw A, Perry J, Chambers A, Laperriere NJ. Systematic review of the diagnosis and management of malignant extradural spinal cord compression: The Cancer Care Ontario Practice Guidelines Initiative's Neuro‐oncology Disease Site Group. Journal of Clinical Oncology 2005;23:2028‐37. [DOI] [PubMed] [Google Scholar]
Loblaw 2012
- Loblaw DA, Mitera G, Ford M, Laperriere NJ. A 2011 updated systematic review and clinical practice guideline for the management of malignant extradural spinal cord compression. International Journal of Radiation Oncology, Biology, Physics 2012;84(2):312‐7. [DOI] [PubMed] [Google Scholar]
L’espérance 2012
- L'espérance S, Vincent F, Gaudreault M, Ouellet JA, Li M, Tosikyan A, Goulet S, Comité de l’évolution des pratiques en oncologie. Treatment of metastatic spinal cord compression: cepo review and clinical recommendations.. Current Oncology 2012;19(6):e478‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maranzano 1991
- Maranzano E, Latini P, Checcaglini F, Ricci S, Panizza BM, Aristei C, et al. Radiation therapy in metastatic spinal cord compression. A prospective analysis of 105 consecutive patients. Cancer 1991;67(5):1311‐7. [DOI] [PubMed] [Google Scholar]
Maranzano 2007
- Maranzano E, Trippa F. Be careful in getting cost effectiveness conclusions from a debatable trial. International Journal of Radiation Oncology, Biology, Physics 2007;68(1):314. [DOI] [PubMed] [Google Scholar]
MECIR 2011
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Methodological standards for the conduct of new Cochrane Intervention Reviews. Version 2.1, 8 December 2011. Available at: http://www.editorial‐unit.cochrane.org/sites/editorial‐unit.cochrane.org/files/uploads/MECIR_conduct_standards%202.1.pdf (accessed on 27 March 2012).
Molina 2011
- Molina CA, Gokaslan ZL, Sciubba DM. A systematic review of the current role of minimally invasive spine surgery in the management of metastatic spine disease. International Journal of Surgical Oncology 2011;2011:598148. [DOI: 10.1155/2011/598148] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2008
- National Institute for Clinical Excellence (NICE). CG 75 Metastatic spinal cord compression. Available at: http://guidance.nice.org.uk/CG75/NICEGuidance/pdf 2008 (accessed 10 December 2013).
Rades 2000
- Rades D, Blach M, Bremer M, Wildfang I, Karstens JH, Heidenreich F. Prognostic significance of the time of developing motor deficits before radiation therapy in metastatic spinal cord compression: one‐year results of a prospective trial. International Journal of Radiation Oncology Biology Physics 2000;48(5):1403‐8. [DOI] [PubMed] [Google Scholar]
Rades 2004
- Rades D, Fehlauer F, Stalpers LJ, Wildfang I, Zschenker O, Schild SE. A prospective evaluation of two radiotherapy schedules with 10 versus 20 fractions for the treatment of metastatic spinal cord compression: final results of a multicenter study. Cancer 2004;101(11):2687‐92. [DOI] [PubMed] [Google Scholar]
Rades 2006
- Rades D, Fehlauer F, Schulte R, Veninga T, Stalpers LJ, Basic H, et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. Journal of Clinical Oncology 2006;20(24):3388‐93. [DOI] [PubMed] [Google Scholar]
Rades 2008
- Rades D, Rudat V, Veninga T, Stalpers LJ, Hoskin PJ, Schild SE. Prognostic factors for functional outcome and survival after re‐irradiation for in‐field recurrences of metastatic spinal cord compression. Cancer 2008;113:1090‐6. [DOI] [PubMed] [Google Scholar]
Rades 2010a
- Rades D, Douglas S, Veninga T, Stalpers LJ, Hoskin PJ, Bajrovic A, et al. Validation and simplification of a score predicting survival in patients irradiated for metastatic spinal cord compression. Cancer 2010;116(15):3670‐3. [DOI] [PubMed] [Google Scholar]
Rades 2010b
- Rades D, Huttenlocher S, Dunst J, Bajrovic A, Karstens JH, Rudat V, et al. Matched pair analysis comparing surgery followed by radiotherapy and radiotherapy alone for metastatic spinal cord compression. Journal of Clinical Oncology 2010;28(22):3597‐604. [DOI] [PubMed] [Google Scholar]
Rades 2011a
- Rades D, Lange M, Veninga T, Stalpers LJ, Bajrovic A, Adamietz IA, et al. Final results of a prospective study comparing the local control of short‐course and long‐course radiotherapy for metastatic spinal cord compression. International Journal of Radiation Oncology, Biology, Physics 2011;79(2):524‐30. [DOI] [PubMed] [Google Scholar]
Rades 2011b
- Rades D, Huttenlocher S, Bajrovic A, Karstens JH, Adamietz IA, Kazic N, et al. Surgery followed by radiotherapy versus radiotherapy alone for metastatic spinal cord compression from unfavorable tumors. International Journal of Radiation Oncology, Biology, Physics 2011;81(5):e861‐8. [DOI] [PubMed] [Google Scholar]
Rades 2012
- Rades D, Huttenlocher S, Evers JN, Bajrovic A, Karstens JH, Rudat V, et al. Do elderly patients benefit from surgery in addition to radiotherapy for treatment of metastatic spinal cord compression?. Strahlentherapie und Onkologie 2012;188(5):424‐30. [DOI] [PubMed] [Google Scholar]
Rades 2013
- Rades D, Veninga T, Bajrovic A, Karstens JH, Schild SE. A validated scoring system to identify long‐term survivors after radiotherapy for metastatic spinal cord compression. Strahlentherapie und Onkologie 2013;189(6):462‐6. [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Schaberg 1985
- Schaberg J, Gainor BJ. A profile of metastatic carcinoma of the spine. Spine 1985;10:19‐20. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results drawing conclusions. Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Sciubba 2010
- Sciubba DM, Petteys RJ, Dekutoski MB, Fisher CG, Fehlings MG, Ondra SL, et al. Diagnosis and management of metastatic spine disease. A review. Journal of Neurosurgery. Spine 2010;13(1):94‐108. [DOI] [PubMed] [Google Scholar]
Thomas 1996
- Thomas KC, Nosyk B, Fisher CG, Dvorak M, Patchell RA, Regine WF, et al. Cost‐effectiveness of surgery plus radiotherapy vs. radiotherapy alone for treatment of metastatic epidural spinal cord compression. International Journal of Radiation Oncology, Biology, Physics 2006;66:1212‐8. [DOI] [PubMed] [Google Scholar]
Tokuhashi 2005
- Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30(9):2186‐91. [DOI] [PubMed] [Google Scholar]
Tokuhashi 2009
- Tokuhashi Y, Ajiro Y, Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine (Phila Pa 1976) 2009;34(1):69‐73. [DOI] [PubMed] [Google Scholar]
Tomita 2001
- Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine 2001;26(3):298‐306. [DOI] [PubMed] [Google Scholar]
Ushio 1977
Van der Linden 2005
- Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW, Dutch Bone Metastasis Study Group. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer 2005;103(2):320‐8. [DOI] [PubMed] [Google Scholar]
Wang 2004
- Wang JC, Boland P, Mitra N, Yamada Y, Lis E, Stubblefield M, et al. Single‐stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Journal of Neurosurgery. Spine 2004;1(3):287‐98. [DOI] [PubMed] [Google Scholar]
Wise 1999
- Wise JJ, Fischgrund JS, Herkowitz HN, Montgomery D, Kurz LT. Complication, survival rates, and risk factors of surgery for metastatic disease of the spine. Spine 1999;24(18):1943‐51. [DOI] [PubMed] [Google Scholar]
Witham 2006
- Witham TF, Khavkin YA, Gallia GL, Wolinsky JP, Gokaslan ZL. Surgery insight: current management of epidural spinal cord compression from metastatic spine disease. Nature Clinical Practice Neurology 2006;2:87‐94. [DOI] [PubMed] [Google Scholar]
Wong 1990
- Wong DA, Fornasier VL, MacNab I. Spinal metastases: the obvious, the occult, and the impostors. Spine 1990;15:1‐4. [PubMed] [Google Scholar]
References to other published versions of this review
George 2008
- George R, Jeba J, Ramkumar G, Chacko AG, Leng M, Tharyan P. Interventions for the treatment of metastatic extradural spinal cord compression in adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD006716] [DOI] [PubMed] [Google Scholar]


