Abstract
Sphingosine 1-phosphate (S1P) levels are often found to be elevated in serum, bronchoalveolar lavage, and lung tissue of idiopathic pulmonary fibrosis patients and experimental mouse models. Although the roles of sphingosine kinase 1 and S1P receptors have been implicated in fibrosis, the underlying mechanism of fibrosis via Sphingosine 1-phosphate receptor 2 (S1P2) has not been fully investigated. Therefore, in this study, the roles of S1P2 in lung inflammation and fibrosis was investigated by means of a bleomycin-induced lung fibrosis model and lung epithelial cells. Bleomycin was found to induce lung inflammation on day 7 and fibrosis on day 28 of treatment. On the 7th day after bleomycin administration, S1P2 deficient mice exhibited significantly less pulmonary inflammation, including cell infiltration and pro-inflammatory cytokine induction, than the wild type mice. On the 28th day after bleomycin treatment, severe inflammation and fibrosis were observed in lung tissues from wild type mice, while lung tissues from S1P2 deficient mice showed less inflammation and fibrosis. Increase in TGF-β1-induced extracellular matrix accumulation and epithelial-mesenchymal transition were inhibited by JTE-013, a S1P2 antagonist, in A549 lung epithelial cells. Taken together, pro-inflammatory and pro-fibrotic functions of S1P2 were elucidated using a bleomycin-induced fibrosis model. Notably, S1P2 was found to mediate epithelial-mesenchymal transition in fibrotic responses. Therefore, the results of this study indicate that S1P2 could be a promising therapeutic target for the treatment of pulmonary fibrosis.
Keywords: Fibrosis, Inflammation, Lung, Sphingosine 1-phosphate, S1P2
INTRODUCTION
Pulmonary fibrosis is a lung disease that occurs when lung tissue is damaged and scarred, and presents a pathological feature of most chronic inflammatory diseases. Thickening stiffness of the tissue hampers proper functioning of the lungs; the stiffened lung tissue makes breathing difficult and reduces oxygen supply to the blood. If highly progressive, the fibrotic process eventually leads to organ malfunction and death (Wynn and Ramalingam, 2012). Scarring associated with pulmonary fibrosis can be caused by a multitude of factors; autoimmune diseases, chronic inflammation, environmental pollutants, infections, and radiation exposure all contribute towards pulmonary fibrosis. However, in most cases, doctors are unable to identify the exact causative factors responsible for the development of pulmonary fibrosis. In such cases, the condition is termed idiopathic pulmonary fibrosis (IPF) (Bourke, 2006). Often, lung damage caused by pulmonary fibrosis is not completely curable. However, medication and therapies help to ease symptoms and improve the quality of life; lung transplant might prove to be beneficial in some patients.
Numerous dysregulated mechanisms can promote fibrosis. At the cellular level, a seminal step involves the accumulation/activation of fibroblasts and myofibroblasts in the lung, with the subsequent release of pro-fibrotic factors and extracellular matrix proteins, including proteoglycans and collagens (Leask and Abraham, 2004). This excessive lung scarring compromises the structural integrity of alveoli, increases lung stiffness, decreases lung volumes, and ultimately leads to severe impairment of gas exchange (Berend, 2014). At the molecular level, elevated levels of growth factors, including transforming growth factor (TGF)-β and connective tissue growth factor (CTGF) are considered major, sometimes sufficient, inducers of fibrosis (Sonnylal et al., 2010). However, the mechanisms regulating lung fibrosis are not completely understood and it is difficult to predict whether new experimental interventions will alleviate or exacerbate fibrosis.
Epithelial-mesenchymal transition (EMT) has become widely accepted as a mechanism by which injured epithelial cells transform into mesenchymal cells that contribute to the development of fibrosis (Kage and Borok, 2012). EMT is one of several proposed mechanisms through which fibroblasts and myofibroblasts are generated (Kalluri and Neilson, 2003). There are two main observations that support the contribution of EMT to pulmonary fibrosis: results of lineage tracing studies in mouse models and co-expression of epithelial and mesenchymal markers in human lung samples with IPF (Willis et al., 2005; Willis and Borok, 2007; Masszi et al., 2010). Advances have been made in elucidating the causes and mechanisms of EMT, potentially leading to new treatment options, although contribution of EMT to lung fibrosis in vivo remains controversial.
Sphingosine 1-phosphate (S1P) is a specific ligand for five G protein-coupled receptors, S1P1–5 (Park and Im, 2017). S1P and S1P signaling may contribute to the development and progression of IPF and pulmonary fibrosis in animal models (Dhami et al., 2010). S1P levels were found to be elevated in bronchoalveolar lavage fluid (BALF) of IPF patients compared to controls, and correlated with poor lung prognosis in IPF patients (Milara et al., 2012). Elevated S1P levels in a murine model of bleomycin-induced pulmonary fibrosis resulted from enhanced sphingosine kinase 1 (Dhami et al., 2010). In IPF patients, increased sphingosine kinase 1 levels contributed to EMT through S1P2 and S1P3 in alveolar type II cells (Milara et al., 2012). Buildup of extracellular matrix proteins (ECM), a characteristic feature of fibrosis, has also been attributed to S1P signaling via S1P2 or S1P3 in agonist-based studies on human lung fibroblasts (Sobel et al., 2013). Furthermore, S1P3 knockout mice showed decreased inflammation and fibrosis in bleomycin-induced murine model of fibrosis (Murakami et al., 2014). However, underlying mechanism of fibrosis via S1P2 has not been fully investigated. Therefore, in this study, we investigated the role of S1P2 in lung inflammation and fibrosis, by using bleomycin-induced lung fibrosis model and lung epithelial cells.
MATERIALS AND METHODS
Materials
JTE-013 was purchased from Cayman Chemicals (Ann Arbor, MI, USA) and recombinant human TGF-β1 was obtained from Peprotech, (London, UK). Bleomycin was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Animals
Three S1P2 heterozygous mice were kindly provided by Richard Proia at NIH (Kono et al., 2007a). They had been backcrossed to Balb/c mice for 8 generations. S1P2 wild-type (WT) and knock out (KO) mice were housed in a Laboratory Animal facility in Pusan National University, and provided unrestricted amounts of food and water. The animal protocol used in this study was reviewed and approved by the Pusan National University–Institutional Animal Care Committee (PNU–IACUC) with respect to procedure ethicality and scientific care (Approval Number PNU-2016-1131).
Induction of lung inflammation and fibrosis in Balb/c mice
Eight-week old Balb/c mice were divided into four groups (n=6), that is, a PBS-treated control S1P2 WT group, a bleomycin-treated S1P2 WT group, a PBS-treated control S1P2 KO group, and a bleomycin-treated S1P2 KO group. Mice were anesthetized with Avertin (tribromoethanol, 200 mg/kg body weight) i.p. injection. Lung inflammation was induced by administration of 50 µl of bleomycin (50 mg/kg body weight) by intratracheal instillation. Control group mice received the same volume of sterile saline. On day 7 after bleomycin administration, mice were sacrificed, and lung and BALF were collected for succeeding experiments. For lung fibrosis, 50 µl of bleomycin (20 mg/kg body weight) was administrated the same way as described above. On day 28 after bleomycin administration, mice were sacrificed and lung tissues were examined.
Histological analysis of the lung and cell counting in BALF for fibrosis
After sacrificing the mice on experimental day 7 or 28, left lung tissues were fixed in 10% formalin, embedded in paraffin and sectioned (4 µm). Sections were stained with hematoxylin and eosin (H&E) or by Masson’s trichrome method. For H&E staining, sections were washed in running tap water for 5 min, counterstained with hematoxylin solution for 90 s, washed in running tap water, dehydrated, and coverslipped with Permount. To confirm collagen production by Masson’s trichrome staining, sections were deparaffinized, hydrated with water. Sections were stained in Bouin’s solution for 1 min using microwave and then allowed to stand for 15 min at room temperature. After washing in running tap water, sections were placed in hematoxylin for 10 min, rinsed in running tap water, and stained in Biebrich scarlet for 5 min. Thereafter, sections were stained in phosphotungstic/phosphomolybdic acid for 15 min, transferred directly into aniline blue for 5 min, dehydrated and coverslipped. Flow cytometry was used to obtain total cell counts in BALF samples.
Hydroxyproline assay
Hydroxyproline content of the lungs was determined as follows, according to the manufacturer’s instruction (BioVision, Milpitas, CA, USA): in brief, lung tissue homogenates in H2O were hydrolyzed with concentrated HCl at 120°C for 3 h and the supernatants were collected after centrifuging the hydrolyzed homogenates at 10,000×g for 3 minutes. Followed by a drying step, 10 µl of the supernatants were loaded onto a 96-well plate, and the samples were assayed for hydroxyproline at 550 nm. The data were expressed as µg of hydroxyproline/mg wet tissue.
Western blot
Lung tissues were lysed in RIPA lysis buffer with protease inhibitors. Protein concentration was determined using a BCA protein assay. Proteins (30 µg) were resolved by 10% SDS-polyacrylamide gel electrophoresis and electrophoretically transferred to nitrocellulose paper. After transfer, the nitrocellulose membrane was incubated with specific primary antibodies, recognizing β-actin or α-smooth muscle actin (α-SMA), followed by incubation with an HRP-conjugated secondary antibody. Signals were developed using an enhanced chemiluminescence system.
Reverse transcriptase-PCR
To assess the expression of fibrotic markers by reverse transcription-PCR (RT-PCR), first strand cDNA was synthesized from total RNA, isolated from BALF-associatedcells and lung tissues, using Trizol reagent (Thermo Fisher Scientific, Carlsbad, CA, USA). Synthesized cDNA products, primers for each gene, and Promega Go-Taq DNA polymerase (USA) were used for PCR. Specific primers were summarized in Table 1. PCR was performed over 27–33 amplification cycles of denaturation at 95°C for 30 s, annealing at 49–57°C for 30 s, and elongation at 72°C for 30 s in an Eppendorf Mastercycler PCR machine (Hamburg, Germany). Aliquots (7 µl) were electrophoresed through 1.2% agarose gels and stained with StaySafeTM Nucleic Acid Gel Stain (Real Biotech Corporation, Banqiao, Taiwan).
Table 1.
Primer sequences
| Gene | Primer sequence (5′→3′) | Annealing temperature (°C) | Cycles |
|---|---|---|---|
| mouse α-SMA | F : CTGACAGAGGCACCACTGAA | 60 | 25 |
| R : GAAGGAATAGCCACGCTCAG | |||
| mouse ColIα1 | F : CACCCTCAAGAGCCTGAGTC | 60 | 25 |
| R : GTTCGGGCTGATGTACCAGT | |||
| mouse ColIα2 | F : TGGCCCATCTGGTAAAGAAG | 60 | 25 |
| R : ACCTTTGCCACCTTGAACAC | |||
| mouse ColIIIα1 | F : GTCCACGAGGTGACAAAGGT | 60 | 25 |
| R : GTGCCCACTTGTTGGATCT | |||
| mouse fibronectin | F : ACCACCCAGAACTACGATGC | 60 | 25 |
| R : ACGTGTCGTTCACATTG | |||
| mouse GAPDH | F : TTCACCACCATGGAGAAGGC | 60 | 27 |
| R : GGCATGGACTGTGGTCATGA | |||
| mouse IL-1β | F : GGAGAAGCTGTGGCAGCTA | 57 | 22 |
| R : GCTGATGTACCAGTTGGGGA | |||
| mouse IL-6 | F : CCGGAGAGGAGACTTCACAG | 57 | 24 |
| R : TGGTCTTGGTCCTTAGCCAC | |||
| mouse MCP-1 | F : AGGTCCCTGTCATGCTTCTG | 55 | 30 |
| R : TCTGGACCCATTCCTTCTTG | |||
| mouse N-cadherin | F : GGGACAGGAACACTGCAAAT | 60 | 25 |
| R : CGGTTGATGGTCCAGTTTCT | |||
| mouse TGF-β | F : GCCCTGGATACCAACTATTGC | 55 | 30 |
| R : AGCTGCACTTGCAGGAGCG | |||
| mouse TNFα | F : GACCCTCACACTCAGATCAT | 57 | 30 |
| R : TTGAAGAGAACCTGGGAGTA | |||
| human ColIα1 | F : AGACTTTGGTGTGGGTCAGG | 60 | 25 |
| R : CAGGACCAGGAGAAGAGTGC | |||
| human E-cadherin | F : GGTTCAAGCTGCTGACCTTC | 60 | 30 |
| R : AGCCAGTTGGCAGTGTCTCT | |||
| human fibronectin | F : CCAACCTACGGATGACTCGT | 60 | 25 |
| R : TGGCACCGAGATATTCCTTC | |||
| human GAPDH | F : GAGTCAACGGATTTGGTCGT | 52 | 27 |
| R : TTGATTTTGGAGGGATCTCG | |||
| human N-cadherin | F : GGACAGTTCCTGAGGGATCA | 60 | 25 |
| R : GGATTGCCTTCCATGTCTGT | |||
| human ZEB1 | F : GAGAAGCGGAAGAACGTGAC | 60 | 30 |
| R : GCTTGACTTTCAGCCCTGTC |
Statistics
Results are expressed as mean ± standard error (SE) of the indicated number of individual values. Statistical significance of differences were determined by analysis of variance (ANOVA) with Turkey’s post hoc test, and statistical significance was accepted for p-values <0.05. Analyses were performed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA).
RESULTS
Effect of S1P2 deficiency on bleomycin-induced lung inflammation
Bleomycin is an effective chemotherapy drug, used to treat cancer, although it may cause inflammation of the lung that can result in lung scarring (Dhami et al., 2010). Based on this observation, bleomycin has been used to induce pulmonary fibrosis in mice. The bleomycin-induced fibrosis model indicates significant characteristics of human IPF, including an increased inflammatory response, accompanied by epithelial cell injury, basement membrane damage, and interstitial and intra-alveolar fibrosis. By utilizing the bleomycin-mediated fibrosis model, we investigated whether S1P2 mediates proinflammatory and pro-fibrotic responses.
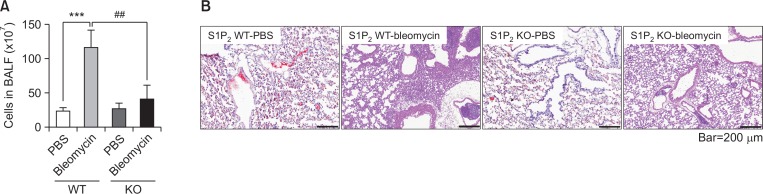
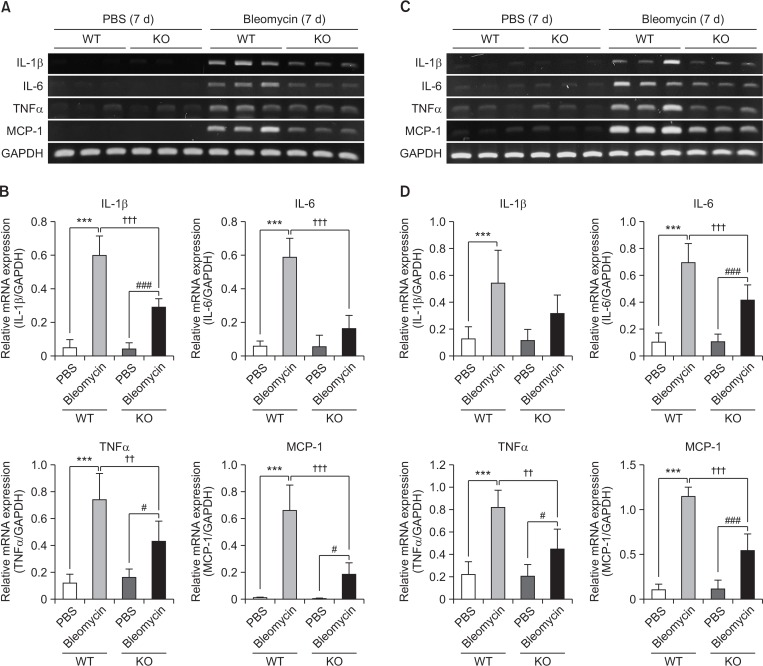
On the 7th day after bleomycin instillation, the number of inflammatory cells in BALF of S1P2 WT mice was found to be higher compared to PBS-treated mice. However, S1P2 deficiency attenuated bleomycin-induced infiltration of cells in BALF, compared to WT mice (Fig. 1A). In the H&E-stained lung tissues, inflammation was apparent with slight progression of fibrosis in the WT mice, indicating infiltration of inflammatory cells, hyperplasia of the alveolar and bronchiolar epithelium, and interstitial collagen deposition. Lung tissues from S1P2 KO mice were less inflamed than those from S1P2 WT mice (Fig. 1B). In addition, the inflammatory responses were assessed by measurements of pro-inflammatory cytokines in lung tissue and BALF. The mRNA expression of pro-inflammatory cytokines, IL-1β, IL-6, TNFα, and monocyte chemoattractant protein-1 (MCP-1), in immune cells of BALF in bleomycin-treated S1P2 WT mice was higher, as compared to the PBS-instilled S1P2 WT mice (Fig. 2A, 2B). The elevated levels in bleomycin-treated S1P2 WT mice was reduced in bleomycin-instilled S1P2 KO mice (Fig. 2A, 2B). Furthermore, the mRNA levels of IL-1β, IL-6, TNFα, and MCP-1 were increased in lung tissues from the bleomycin-instilled WT mice. Those levels of inflammatory cytokines in lung tissues from the bleomycin-instilled KO mice were lower than WT mice (Fig. 2C, 2D).
Fig. 1.
Effect of S1P2 deficiency on bleomycin-induced lung inflammation. Bleomycin or saline was administered intratracheally to S1P2 WT and KO mice. Seven days after bleomycin instillation, cells in BALF were counted by flow cytometry. (A) Total cell counts in the BALF of PBS-treated S1P2 WT mice (S1P2 WT-PBS), bleomycin-treated S1P2 WT mice (S1P2 WT-bleomycin), PBS-treated S1P2 KO mice (S1P2 KO-PBS), and bleomycin-treated S1P2 KO mice (S1P2 KO-bleomycin). (B) Histological analysis of lung tissues from each group was performed by H&E staining. On the 7th day after treatment, lung tissues of bleomycin-treated WT mice showed lung inflammation, but lung tissues from KO mice exhibited a lower inflammatory response compared to the WT mice. Results are presented as mean ± SE (n=6). Statistical significance: ***p<0.001 vs. PBS-treated S1P2 WT mice and ##p<0.01 vs. bleomycin-treated S1P2 WT mice.
Fig. 2.
Suppressive effect of S1P2 deficiency on mRNA expression of pro-inflammatory cytokines in BALF or lung tissues. (A, B) RT-PCR analysis of pro-inflammatory cytokines (IL-1β, IL-6, TNFα, and MCP-1) was performed using mRNA in BALF-associated cells on the 7th day after bleomycin treatment. (C, D) mRNA expression of pro-inflammatory cytokines was checked by RT-PCR using mRNAs isolated from lung tissues. mRNA levels were expressed as ratios with GAPDH mRNA levels. The values shown are mean ± SE (n=6). Statistical significance: ***p<0.001 vs. PBS-treated S1P2 WT mice, #p<0.05, ###p<0.001 vs. PBS-treated S1P2 KO mice, and ††p<0.01, †††p<0.001 vs. bleomycin-treated S1P2 WT mice.
Effect of S1P2 deficiency on bleomycin-induced lung fibrosis
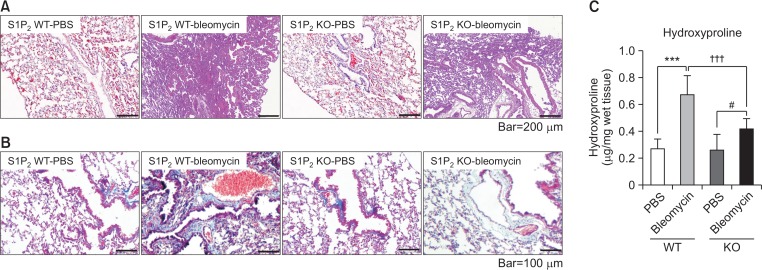
On the 28th day after treatment with bleomycin or saline, lung tissues from S1P2 WT and KO mice were stained with H&E or Masson’s trichrome staining, to measure histological changes during chronic lung fibrosis. Inflammation and fibrosis were apparent in the lung tissues from WT mice, while tissues from S1P2 deficient mice exhibited less inflammation and fibrosis (Fig. 3A). When fibrosis was examined by Masson’s trichrome staining of lung tissues, severe fibrosis was observed in lung tissues from bleomycin-instilled WT mice. However, in the lung of bleomycin-instilled S1P2 KO mice, collagen deposition in fibrotic areas was suppressed (Fig. 3B). In addition, administration of bleomycin was found to increase the hydroxyproline content (a major component of collagen) in lung tissues from S1P2 bleomycin-instilled WT mice, whereas the amount of hydroxyproline in lung tissues from bleomycin-instilled S1P2 KO mice was less than those of S1P2 WT mice (Fig. 3C).
Fig. 3.
Inhibitory effect of S1P2 deficiency on fibrosis in lung. Bleomycin or saline was administered intratracheally to S1P2 WT and KO mice. Twenty-eight days after bleomycin instillation, histological analysis in lungs was performed by H&E staining (A) and Masson’s trichrome staining (B). The amounts of hydroxyproline in lung tissues from PBS- or bleomycin-treated mice were analyzed as quantitation of collagen (C). The values shown are mean ± SE (n = 6). Statistical significance: ***p<0.001 vs. PBS-treated S1P2 WT mice, #p<0.05 vs. PBS-treated S1P2 KO mice, and †††p<0.001 vs. bleomycin-treated S1P2 WT mice.
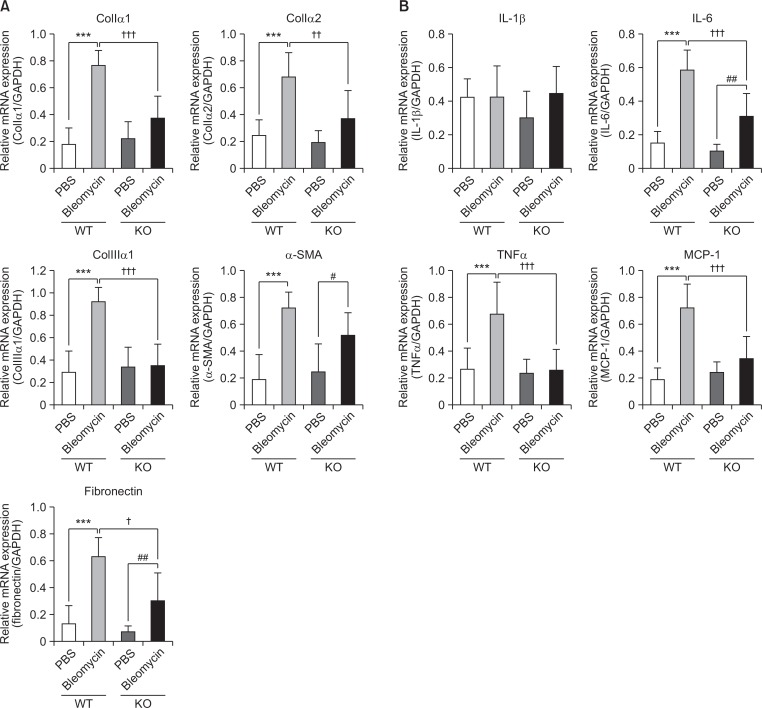
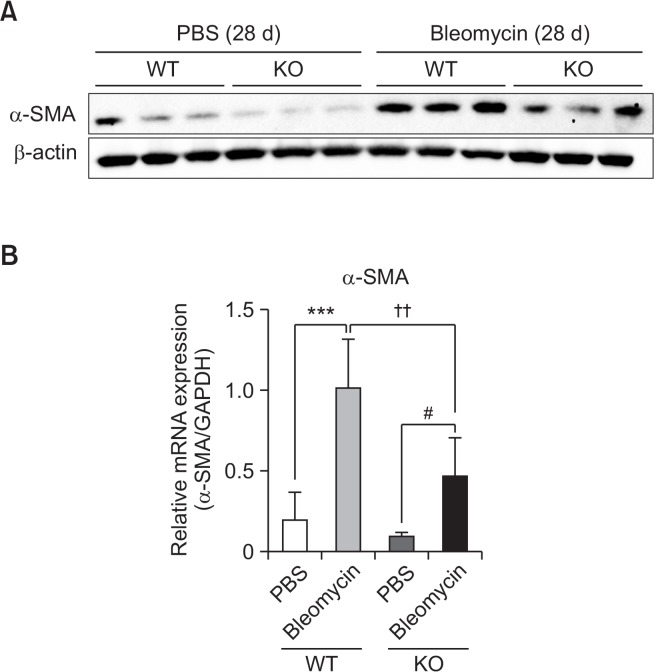
Next, changes in the mRNA levels of pro-inflammatory cytokines as well as pro-fibrotic markers in lung tissues were measured in S1P2 WT and KO mice. The mRNA expression of pro-fibrotic markers, collagen Iα1, collagen Iα2, collagen IIIα1, α-SMA, and fibronectin, in bleomycin-instilled lung tissues were markedly lower in S1P2 deficient mice than in WT mice (Fig. 4A). Furthermore, the mRNA levels of IL-1β, IL-6, TNFα, and MCP-1 were elevated in lungs of bleomycin-treated WT mice, but these bleomycin-induced increases were suppressed in S1P2 KO mice (Fig. 4B). In addition, the protein levels of α-SMA were increased in lung tissues of bleomycin-treated WT mice but not in bleomycin-treated KO mice (Fig. 5A, 5B).
Fig. 4.
Inhibitory effect of S1P2 deficiency on mRNA expression of pro-fibrotic or pro-inflammatory cytokines in lung tissues. (A) RT-PCR analysis of pro-fibrotic proteins (collagen Iα1, collagen Iα2, collagen IIIα1, α-SMA, and fibronectin) was performed using mRNA isolated from lung tissues on 28th day after bleomycin treatment. (B) mRNA expression of pro-inflammatory cytokines (IL-1β, IL-6, TNFα, and MCP-1) was determined by RT-PCR in lung tissues. mRNA levels were expressed as ratios with GAPDH mRNA levels. The values shown are mean ± SE (n=6). Statistical significance: ***p<0.001 vs. PBS-treated S1P2 WT mice, #p<0.05, ##p<0.01 vs. PBS-treated S1P2 KO mice, and †p<0.05, ††p<0.01, †††p<0.001 vs. bleomycin-treated S1P2 WT mice.
Fig. 5.
Inhibitory effect of S1P2 deficiency on level of pro-fibrotic protein in lung tissues. (A) Western blotting results of pro-fibrotic proteins (α-SMA), performed using protein isolated from lung tissues, on the 28th day after bleomycin treatment. (B) Protein levels were expressed as ratios with β-actin levels. The values shown are mean ± SE (n=6). Statistical significance: ***p<0.001 vs. PBS-treated S1P2 WT mice, #p<0.05 vs. PBS-treated S1P2 KO mice, and ††p<0.01 vs. bleomycin-treated S1P2 WT mice.
Effect of JTE-013 on TGF-β1-mediated EMT signaling inA549 cells
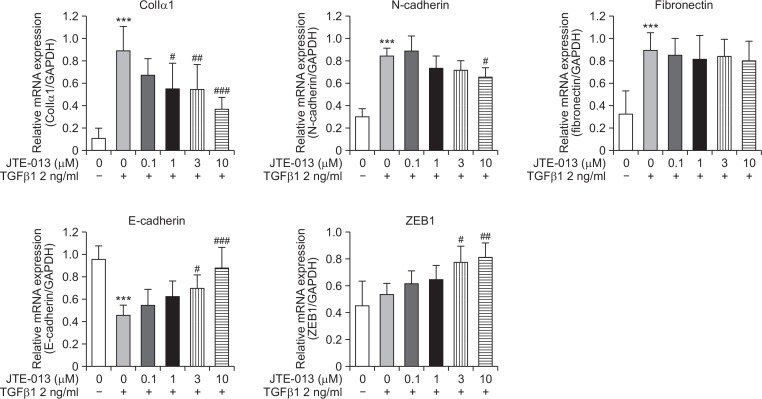
Repeated cycles of inflammation and repair initiate tissue damage and may progress to fibrotic changes, resulting in excessive matrix deposition and scar formation in tissues. In this process, TGF-β signaling is quite important and EMT is a critical phenomenon. To confirm the effect of S1P2 on TGF-β1– induced EMT, S1P2 antagonist, JTE-013 was used in A549 human alveolar basal epithelial cells. TGF-β1 (2 ng/ml, for 24 h) activated the expression levels of EMT and ECM accumulation markers including collagen Iα1, N-cadherin, and fibronectin, but decreased the mRNA expression levels of epithelial markers such as E-cadherin (Fig. 6). The mRNA expression of zinc finger E-box-binding homeobox 1 (ZEB1), which is an E-cadherin repressor, was not changed by TGF-β1. However, JTE-013 pretreatment attenuated the increased collagen Iα1 and N-cadherin expression (except fibronectin) and elevated the E-cadherin and ZEB1 expression in A549 cells (Fig. 6). Our results indicate that although activation of E-cadherin could be induced via suppression of ZEB1, increased expression of E-cadherin is probably mediated by other inducers or repressors, but not ZEB1. Rather, these results suggest that S1P2 regulates TGF-β1–induced EMT and ECM accumulation, hence contributing to the anti-fibrotic activity in vivo.
Fig. 6.
Effect of JTE-013 on TGF-β1-induced ECM expression and EMT in A549 cells. Expression of ECM and EMT associated proteins (collagen Iα1, N-cadherin, fibronectin, E-cadherin, and ZEB1) confirmed in A549 cells by RT-PCR analysis. A549 cells were cultured for 24 h in media containing 0.5% FBS and then treated with JTE-013 and TGF-β1 for 24 h. mRNA levels were expressed as ratios with GAPDH mRNA levels. The values shown are mean ± SE (n=3). Statistical significance: ***p<0.001 vs. vehicle-treated cells and #p<0.05, ##p<0.01, ###p<0.001 vs. TGF-β1-treated cells.
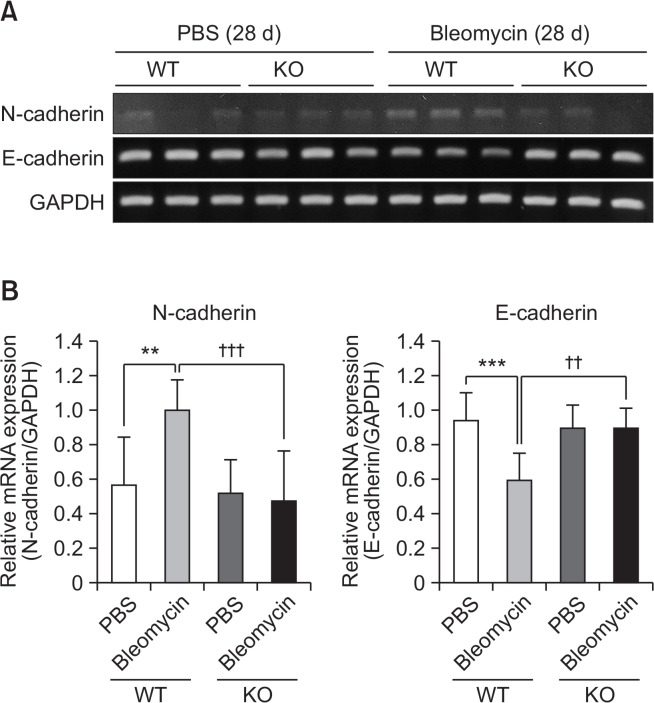
Bleomycin instillation increased N-cadherin expression and suppressed E-cadherin in lung tissues, but these bleomycin-induced changes were suppressed in S1P2 deficient mice (Fig. 7).
Fig. 7.
Inhibitory effect of S1P2 deficiency on EMT in lung tissues. (A) RT-PCR analysis of N-cadherin and E-cadherin was performed using mRNA isolated from lung tissues on 28th after bleomycin treatment. (B) mRNA levels were expressed as ratios with GAPDH mRNA levels. The values shown are mean ± SE (n=6). Statistical significance: **p<0.01, ***p<0.001 vs. PBS-treated S1P2 WT mice and ††p<0.01, †††p<0.001 vs. bleomycin-treated S1P2 WT mice.
DISCUSSION
S1P and S1P receptors are known to be involved in many different physiological processes, and may contribute to the development and progression of IPF and pulmonary fibrosis in animal models. S1P levels were found to be elevated in the BALF of IPF patients that correlated with poor prognosis (Milara et al., 2012). Furthermore, S1P, S1P receptors, and sphingosine kinase 1 were up-regulated in the serum, bronchoalveolar lavage, and lung tissue of experimental mouse models of bleomycin-induced lung fibrosis, human liver fibrosis, and experimental models of cholestasis-induced liver fibrosis (Kono et al., 2007b; Li et al., 2009; Dhami et al., 2010; Li et al., 2011; Sobel et al., 2013). Long-term treatment with fingolimod (a well-known S1P1 modulator) caused fibrosis of lungs, such as increased lung weight, hypertrophy of smooth muscle, hyperdistension of alveoli, and increased collagen deposition in mice (Shea et al., 2010). It has been reported that S1P2 signaling contributes to the pathogenesis of liver fibrosis. S1P inhibited proliferation through S1P2 in hepatocytes of rats, and S1P2 deficiency inhibited accumulation of hepatic stellate cells and ameliorated CCl4-induced liver fibrosis (Ikeda et al., 2009). Some studies also suggest a correlation between S1P2 and lung inflammation and fibrosis, but the role and function of S1P2 in the pathogenesis of lung diseases has been poorly understood.
In the current study, the role of S1P2 was demonstrated using S1P2 deficient mice in bleomycin-induced pulmonary fibrosis model. First, pro-inflammatory and pro-fibrotic functions of S1P2 were determined in the animal model. During the acute and chronic phase of lung injury, analysis of lung tissue and BALF showed that S1P2 KO mice showed decreased inflammation and reduction in the number of total cells, compared to WT mice. These findings suggest that inactivation of S1P2 may prevent bleomycin-induced acute inflammation and chronic fibrosis, by inhibiting inflammatory and fibrotic signaling. Second, S1P2 regulates TGF-β1-induced EMT and ECM accumulation in lung epithelial cells, indicating that S1P2–mediated blockade of ECM production and EMT (which are possible causes of IPF) could be a strategy to treat IPF.
IPF, the most common interstitial fibrotic pulmonary disease is a chronic and progressive disease, caused by proliferation of fibroblasts and deposition of collagen and ECM proteins, resulting in failure of lung function. However, there is no effective treatment for preventing and curing the development of fibrosis in IPF. Several drugs for IPF have been tested, but currently, lung transplantation is the best available treatment option for improving the survival of IPF patients. There are several key fibrogenic pathways that can be exploited for potential therapeutic approaches, including myofibroblasts and the TGF-β signaling, pro-inflammatory pathways, and pro-fibrotic type 2 immune response. A more multipronged and integrated anti-fibrotic strategy will probably emerge as the most successful way to treat the complex pathogenesis of IPF. In this study, we demonstrate that the blockade of S1P2 attenuates TGF-β signaling and pro-inflammatory pathways triggered by bleomycin, suggesting that the inactivation of S1P2 could be a promising therapeutic target for pulmonary fibrosis disorders, such as IPF.
Recently, the bleomycin-induced lung fibrosis mouse model has been applied for studying the S1P receptor deficient mice. Knock-out mice of S1P3 attenuated inflammation and fibrosis via connective tissue growth factor expression (Murakami et al., 2014). Zhao et al. (2018) reported that S1P2 facilitated pulmonary fibrosis through potentiating IL-13 pathway in macrophages. In their study, bleomycin was administrated repeatedly by intraperitoneal injection into C57BL/6J mice, in contrast to the direct intratracheal instillation of bleomycin into Balb/c mice performed in our study (Zhao et al., 2018). In the lung tissues from S1P2 deficient mice, Zhao et al. (2018) noted reduced fibroblast accumulation and attenuation of fibronectin and collagen1α1 mRNA, supporting our observation. They suggested S1P2 facilitated lung fibrosis through macrophages and IL-13 signaling (Zhao et al., 2018). Using S1pr2LacZ/+ mice, they found S1P2 was expressed in alveolar macrophages, vascular endothelial cells, and alveolar epithelial cells in the lung (Zhao et al., 2018). In their model, S1P2 was found to be involved in the production of IL-13 and STAT-6 phosphorylation response in macrophages, upon bleomycin intraperitoneal administration. In our model, S1P2 was found to play an important role in early inflammatory responses and late fibrotic ECM accumulation and EMT transition in lung epithelial cells, upon bleomycin intratracheal administration.
In summary, this study identifies S1P2 as a molecular target in lung inflammation and fibrosis. The inhibition of S1P2 attenuates fibrotic signaling and pro-inflammatory pathways triggered by bleomycin. Therefore, this study suggests that the inhibition of S1P2 could be a therapeutic target for pulmonary fibrosis such as IPF.
Acknowledgments
This research was supported by the Basic Science Research Program of the Korean National Research Foundation, funded by the Korean Ministry of Education, Science and Technology (NRF-2016R1D1A1A009917086), and by NRF (National Research Foundation of Korea) Grant, funded by the Korean Government (NRF-2015-Fostering Core Leaders of the Future Basic Science Program/Global Ph.D. Fellowship Program). This study was financially supported by the 2018 Post-Doc. Development Program of Pusan National University.
Footnotes
CONFLICT OF INTEREST
Authors declare there is no conflict of interest.
REFERENCES
- Berend N. Respiratory disease and respiratory physiology: putting lung function into perspective interstitial lung disease. Respirology. 2014;19:952–959. doi: 10.1111/resp.12348. [DOI] [PubMed] [Google Scholar]
- Bourke SJ. Interstitial lung disease: progress and problems. Postgraduate Med J. 2006;82:494–499. doi: 10.1136/pgmj.2006.046417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhami R, He X, Schuchman EH. Acid sphingomyelinase deficiency attenuates bleomycin-induced lung inflammation and fibrosis in mice. Cell Physiol Biochem. 2010;26:749–760. doi: 10.1159/000322342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Watanabe N, Ishii I, Shimosawa T, Kume Y, Tomiya T, Inoue Y, Nishikawa T, Ohtomo N, Tanoue Y, Iitsuka S, Fujita R, Omata M, Chun J, Yatomi Y. Sphingosine 1-phosphate regulates regeneration and fibrosis after liver injury via sphingosine 1-phosphate receptor 2. J Lipid Res. 2009;50:556–564. doi: 10.1194/jlr.M800496-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kage H, Borok Z. EMT and interstitial lung disease: a mysterious relationship. Curr Opin Pulm Med. 2012;18:517–523. doi: 10.1097/MCP.0b013e3283566721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI200320530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M, Belyantseva IA, Skoura A, Frolenkov GI, Starost MF, Dreier JL, Lidington D, Bolz SS, Friedman TB, Hla T, Proia RL. Deafness and stria vascularis defects in S1P2 receptor-null mice. J Biol Chem. 2007a;282:10690–10696. doi: 10.1074/jbc.M700370200. [DOI] [PubMed] [Google Scholar]
- Kono Y, Nishiuma T, Nishimura Y, Kotani Y, Okada T, Nakamura S, Yokoyama M. Sphingosine kinase 1 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-beta1. Am J Respir Cell Mol Biol. 2007b;37:395–404. doi: 10.1165/rcmb.2007-0065OC. [DOI] [PubMed] [Google Scholar]
- Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- Li C, Jiang X, Yang L, Liu X, Yue S, Li L. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am J Pathol. 2009;175:1464–1472. doi: 10.2353/ajpath.2009.090037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zheng S, You H, Liu X, Lin M, Yang L, Li L. Sphingosine 1-phosphate (S1P)/S1P receptors are involved in human liver fibrosis by action on hepatic myofibroblasts motility. J Hepatol. 2011;54:1205–1213. doi: 10.1016/j.jhep.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Masszi A, Speight P, Charbonney E, Lodyga M, Nakano H, Szaszi K, Kapus A. Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J Cell Biol. 2010;188:383–399. doi: 10.1083/jcb.200906155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milara J, Navarro R, Juan G, Peiro T, Serrano A, Ramon M, Morcillo E, Cortijo J. Sphingosine-1-phosphate is increased in patients with idiopathic pulmonary fibrosis and mediates epithelial to mesenchymal transition. Thorax. 2012;67:147–156. doi: 10.1136/thoraxjnl-2011-200026. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kohno M, Kadoya M, Nagahara H, Fujii W, Seno T, Yamamoto A, Oda R, Fujiwara H, Kubo T, Morita S, Nakada H, Hla T, Kawahito Y. Knock out of S1P3 receptor signaling attenuates inflammation and fibrosis in bleomycin-induced lung injury mice model. PLoS ONE. 2014;9:e106792. doi: 10.1371/journal.pone.0106792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Im DS. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol. Ther (Seoul) 2017;25:80–90. doi: 10.4062/biomolther.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD, Tager AM. Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol. 2010;43:662–673. doi: 10.1165/rcmb.2009-0345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel K, Menyhart K, Killer N, Renault B, Bauer Y, Studer R, Steiner B, Bolli MH, Nayler O, Gatfield J. Sphingosine 1-phosphate (S1P) receptor agonists mediate pro-fibrotic responses in normal human lung fibroblasts via S1P2 and S1P3 receptors and Smad-independent signaling. J Biol Chem. 2013;288:14839–14851. doi: 10.1074/jbc.M112.426726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnylal S, Shi-Wen X, Leoni P, Naff K, Van Pelt CS, Nakamura H, Leask A, Abraham D, Bou-Gharios G, de Crombrugghe B. Selective expression of connective tissue growth factor in fibroblasts in vivo promotes systemic tissue fibrosis. Arthritis Rheum. 2010;62:1523–1532. doi: 10.1002/art.27382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis BC, Borok Z. TGF-beta-induced EMT: mechanisms and implications for fibrotic lung disease. Am J Physiol Lung Cell Mol Physiol. 2007;293:L525–534. doi: 10.1152/ajplung.00163.2007. [DOI] [PubMed] [Google Scholar]
- Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/S0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Okamoto Y, Asano Y, Ishimaru K, Aki S, Yoshioka K, Takuwa N, Wada T, Inagaki Y, Takahashi C, Nishiuchi T, Takuwa Y. Sphingosine-1-phosphate receptor-2 facilitates pulmonary fibrosis through potentiating IL-13 pathway in macrophages. PLoS ONE. 2018;13:e0197604. doi: 10.1371/journal.pone.0197604. [DOI] [PMC free article] [PubMed] [Google Scholar]