Abstract
Mast cells are the most prominent effector cells of Type 1 hypersensitivity immune responses. CYC116 [4-(2-amino-4-methyl-1,3-thiazol-5-yl)-N-[4-(morpholin-4-yl)phenyl] pyrimidin-2-amine] is under development to be used as an anti-cancer drug, but the inhibitory effects of CYC116 on the activation of mast cells and related allergy diseases have not reported as of yet. In this study, we demonstrated, for the first time, that CYC116 inhibited the degranulation of mast cells by antigen stimulation (IC50, ∼1.42 µM). CYC116 also inhibited the secretion of pro-inflammatory cytokines including TNF-α (IC50, ∼1.10 µM), and IL-6 (IC50, ∼1.24 µM). CYC116 inhibited the mast cell-mediated allergic responses, passive cutaneous anaphylaxis (ED50, ∼22.5 mg/kg), and passive systemic anaphylaxis in a dose-dependent manner in laboratory experiments performed on mice. Specifically, CYC116 inhibited the activity of Fyn in mast cells and inhibited the activation of Syk and Syk-dependent signaling proteins including LAT, PLCγ, Akt, and MAP kinases. Our results suggest that CYC116 could be used as an alternative therapeutic medication for mast cell-mediated allergic disorders, such as atopic dermatitis and allergic rhinitis.
Keywords: CYC116, Mast cells, Allergy, Immunoglobulin (Ig) E, Fyn, Syk
INTRODUCTION
Allergic disorders such as allergic rhinitis, food allergies, allergic asthma, and atopic dermatitis are increasing globally (Pawankar et al., 2013). When an allergen, an antigen which causes an allergic response, first enters our body, the antigen-presenting cell stimulates Th2 cells under atopic conditions. IL-4 secreted from Th2 cells leads B cells to produce allergen-specific Immunoglobulin (Ig)E (Ozdemir et al., 2010). The IgE binds to the α subunit of the high-affinity IgE receptor (FcεRI) of mast cells, which are present throughout the body via circulation through blood and lymphatic vessels. When the same allergen then enters the body again, FcεRI’s on the surface of mast cells aggregate and various bioactive allergic mediators present in mast cells are secreted. First, histamine, serotonin, and platelet activation factors, which are present in cytoplasmic granules in mast cells, are secreted as degranulation occurs (Theoharides et al., 2012). In addition, leukotrienes and prostaglandins are synthesized and secreted to induce immediate hypersensitivity, resulting in local or systemic anaphylaxis (Theoharides et al., 2012). Mast cells also synthesize and secrete pro-inflammatory cytokines such as TNF-α, IL-4, IL-5, IL-6, and chemokines and, which play a critical role in chronic allergic responses (Metcalfe et al., 1997). Therefore, we sought to develop anti-allergic drugs, based on the inhibition of mast cells.
In the FcεRI-mediated signaling cascade of mast cells, Src-family kinases, Lyn and Fyn, are initially activated by the antigen (Parravicini et al., 2002; Gilfillan and Rivera, 2009). Fyn activated by the antigen stimulates the Gab2-PI3K-Akt signaling the beginning of a cascade. In addition, the activated Lyn then activates Syk and the active Syk induces the activation of linkers for the activation of T cells (LAT) and phospholipase C (PLC) γ. Subsequently, calcium-mediated signals and mitogen-activated protein (MAP) kinases are activated, and mast cells eventually perform degranulation and secrete cytokines (Gilfillan and Tkaczyk, 2006; Rivera and Gilfillan, 2006). Therefore, Src-family kinases, which are initially activated in mast cells by antigen stimulation, are considered to be good target proteins for the development of anti-allergic drugs.
2-Aminothiazole derivative CYC116, 4-(2-amino-4-methyl-1,3-thiazol-5-yl)-N-[4-(morpholin-4-yl)phenyl]pyrimidin-2-amine is an inhibitor of Aurora kinase and it is an anti-cancer drug candidate, undergoing a phase I clinical trial (Griffiths et al., 2008; Hajduch et al., 2008). In addition to the inhibition of Aurora kinase, CYC116 also inhibited various protein tyrosine kinases, including Src and Lck (Fu et al., 2007). Src-family protein tyrosine kinases play a pivotal role in the initial activation of mast cell signals. Thus, it is plausible that CYC116 may have a suppressive effect on mast cells, and in turn IgE-mediated allergic responses. Several studies previously demonstrated that the compounds derived from 2-aminothiazole derivatives have an antimicrobial effect (Göblyös et al., 2005; Hang and Honek, 2005), an antifungal effect (Beuchet et al., 1999), and an anti-inflammatory effect in ovalbumin-induced allergic asthma in animals (Das et al., 2006). However, the inhibitory effects of CYC116 on mast cells and IgE-mediated allergic responses have not yet been adequately explained.
In the present study, we demonstrate that CYC116 inhibited mast cell degranulation and cytokine release by antigen stimulation. CYC116 inhibited mast cell-mediated PCA and PSA responses in vivo. These effects of CYC116 on mast cells and allergic responses were obtained by the direct inhibition of Fyn kinase in mast cells.
MATERIALS AND METHODS
Reagents
CYC116 [4-(2-amino-4-methyl-1,3-thiazol-5-yl)-N-[4-(morpholin-4-yl)phenyl] pyrimidin-2-amine)] (Fig. 1) was obtained from Selleckchem (catalog no. S1171; Houston, TX, USA). Monoclonal dinitrophenyl (DNP)-specific monoclonal IgE (catalog no. D8406), DNP-human serum albumin (HSA, catalog no. A6661), calcium ionomycin (catalog no. I0634), Evans blue (catalog no. E2129), cetirizine (catalog no. C3618), and toluidine blue (catalog no. 198161) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies for the detection of phosphorylation of ZAP70/Syk (catalog no. 2701), LAT (catalog no. 3584), PLCγ1 (catalog no. 2821), ERK1/2 (catalog no. 9106), JNK (catalog no. 9251), p38 (catalog no. 9211), and Akt (catalog no. 9271) were purchased from Cell Signaling Technology Inc (Danvers, MA, USA). Antibodies against Lyn (catalog no. sc-7274), Fyn (catalog no. sc-434), Syk (catalog no. sc-51703), and PLCγ1 (catalog no. sc-7290) were from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against LAT (catalog no. 06-807) and Actin (catalog no. MAB1501) were purchased from EMD Millipore (Billerica, MA, USA). Cell culture reagents, including Roswell Park Memorial Institute (RPMI) 1640 medium and fetal bovine serum (FBS) were purchased from GIBCO/Life Technologies Inc (Rockville, MD, USA).
Fig. 1.
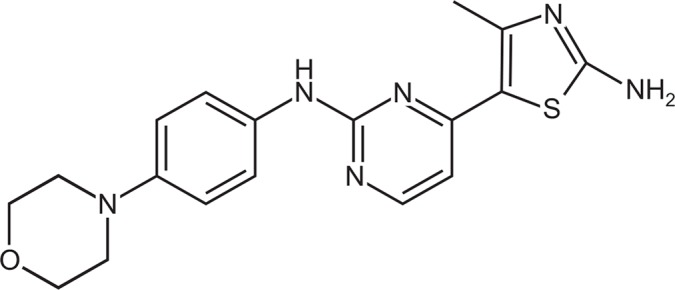
Chemical structure of 4-(2-amino-4-methyl-1,3-thiazol-5-yl)-N-[4-(morpholin-4-yl)phenyl]pyrimidin-2-amine (CYC116).
Animals
Five-week-old male BALB/c mice were obtained from Orient Bio Experimental Animal Center (Gapyeong, Korea). The mice were used for the isolation of bone marrow-derived mast cells (BMMCs) and in the in vivo anaphylaxis study. All animal studies were performed according to institutional guidelines, after receiving approval from the Institutional Animal Care and Use Committee (IACUC) at Konkuk University.
Preparation and stimulation of BMMCs
Bone marrow-derived mast cells (BMMCs) were isolated from the femurs and tibias from the five-week-old male BALB/c mice. They were grown in a complete medium (RPMI 1640, 4 mM L-glutamine, 100 units/ml penicillin, 100 µg/ml streptomycin, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 25 mM HEPES, 10% FBS) with 0.05 mM β-mercaptoethanol and 10 ng/ml IL-3. After 4-6 weeks, BMMCs were used for in vitro assays. For all in vitro experiments, cells were sensitized with 50 ng/ml DNP-specific IgE overnight. The next day, cells were washed twice in ice-cold PBS and treated for 30 min with or without CYC116 or PP2 in Tyrode buffer (20 mM HEPES, pH 7.4, 135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 5.6 mM glucose, and 0.1% BSA). After the CYC116 or PP2 treatment, BMMCs were stimulated with 50 ng/ml DNP-HSA for 10 min or for other indicated times.
Measurement of β-Hexosaminidase release in BMMCs
Cells (2.5×105) were sensitized with 50 ng/ml DNP-specific IgE overnight. The next day, cells were washed twice in ice-cold PBS and treated for 30 min with or without CYC116 or PP2 in Tyrode buffer. After the CYC116 or PP2 treatment, BMMCs were stimulated with 50 ng/ml DNP-HSA for 15 min. The degranulation of mast cells was determined by measuring the ratio of released β-hexosaminidase in supernatants in relation to the total of β-hexosaminidase in the buffer and cell lysates.
Western blot analysis
The cells were chilled on ice to stop the reaction after the CYC116, PP2, and antigen stimulation. Protein extracts were prepared according to Lee et al. (2006). In brief, cells were lysed for 30 min in a RIPA buffer (Thermo Fisher Scientific, Waltham) in 1 mM phenylmethylsulphonyl fluoride, 2.5 mM pnitrophenyl phosphate, 0.7 µg/ml pepstatin, and a protease-inhibitor cocktail tablet while on ice. The protein content of the cellular extracts was quantified by using the Bradford assay (absorbance at 595 nm). The lysates were denatured at 100°C for 5 min in a 4× sample buffer. For western blotting, proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride membranes (Bio-Rad Laboratories, Berkeley, CA, USA). The membranes were blocked in 5% BSA-containing TBS-T buffer for 1 h, and then were incubated with the indicated primary antibodies. The membranes were washed and incubated with horseradish peroxidase-labeled secondary antibodies. Blots were processed using the ImageQuantTMLAS 4000 system (GE Healthcare Life Sciences; Piscataway, NJ, USA).
Measurement of release of TNF-α and IL-6
BMMCs (5.0×106 cells) were stimulated for 3 h as described above, and the amounts of TNF-α and IL-6 in a supernatant of cultured media were measured with ELISA kits (BD Biosciences, San Jose, CA, USA) following the manufacturer’s instructions.
Immunoprecipitation and protein tyrosine kinase assay
Cells were lysed with a lysis buffer for 30 min on ice. The protein content from cell lysates was precleared by adding 50 µl of protein G-agarose. Equal amounts of protein were incubated overnight with the Lyn or Fyn or Syk antibody at 4°C, in turn, protein G-Agarose. The agarose was washed 5 times with a washing buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 0.1% Nonidet P-40, 10% glycerol, 10 mM NaF, 1 mM Na3VO4, 1 mM PMSF, 2.5 mM p-nitrophenyl phosphate, 0.7 µg/ml pepstatin, and protease-inhibitor cocktail tablet). The tyrosine kinase activity was measured with a Universal Tyrosine Kinase Assay Kit (GenWay Biotech Inc., San Diego, CA, USA) following the manufacturer’s instructions.
Passive cutaneous anaphylaxis and passive systemic anaphylaxis
The procedures for the PCA assay were as previously described by (Nam et al., 2018). For PCA, mice were intradermally injected with 50 ng DNP-specific IgE into the skin of the back. After 24 h, either CYC116 (10, 30, or 100 mg/kg) or cetirizine (20 mg/kg) was administered orally to the mice. After 1 h, the mice received an i.v. injection of 100 µg of antigen (DNP-HSA) in 250 µl of PBS containing 5 mg/ml of Evans blue (Sigma-Aldrich). After 1 h, the mice were euthanized, and the ears were removed to measure the amount of dye present. The dye was extracted from the mice back skins in 1.5 ml formamide at 63°C overnight. The absorbance was measured at 620 nm with a microplate reader (Tecan, Männedorf, Switzerland). For PSA, mice were injected with 3 µg of IgE into the tail vein. The following day, CYC116 (10, 30, or 100 mg/kg) or cetirizine (20 mg/kg) was administered orally 1 h prior to the i.v. injection of 100 µg antigen. Then, the rectal temperature was measured every 10 min for 1 h.
Statistical analysis
The data are expressed as means ± SEM from three or more independent experiments. Statistical analysis was performed using one-way ANOVA and unpaired Student’s t-tests. All statistical calculations were performed using Sigma Stat software (Systat Software, Inc., Point Richmond, CA, USA), with differences that were considered statistically significant were set at p<0.05.
RESULTS
Effect of CYC116 on β-hexosaminidase release by antigen in mast cells
The intracellular granules of mast cells contain various allergic mediators including histamine, serotonin, and platelet activating factor that could cause allergic disorders (Theoharides et al., 2012). We measured the extracellular secretion of β-hexosaminidase, an enzyme present in granules, to assess degranulation of mast cells by antigen. The degranulation in BMMCs caused by antigen was inhibited by CYC116 in a dose-dependent manner (IC50, ∼1.42 µM) (Fig. 2A). However, the inhibitory effect of CYC116 on mast cell degranulation disappeared by 5-time washes after 30 min of pre-incubation of CYC116 (Fig. 2B), thus indicating that the inhibitory effect of CYC116 on mast cells is reversible. CYC116 was not cytotoxic to mast cells under the experimental conditions (Fig. 2C).
Fig. 2.
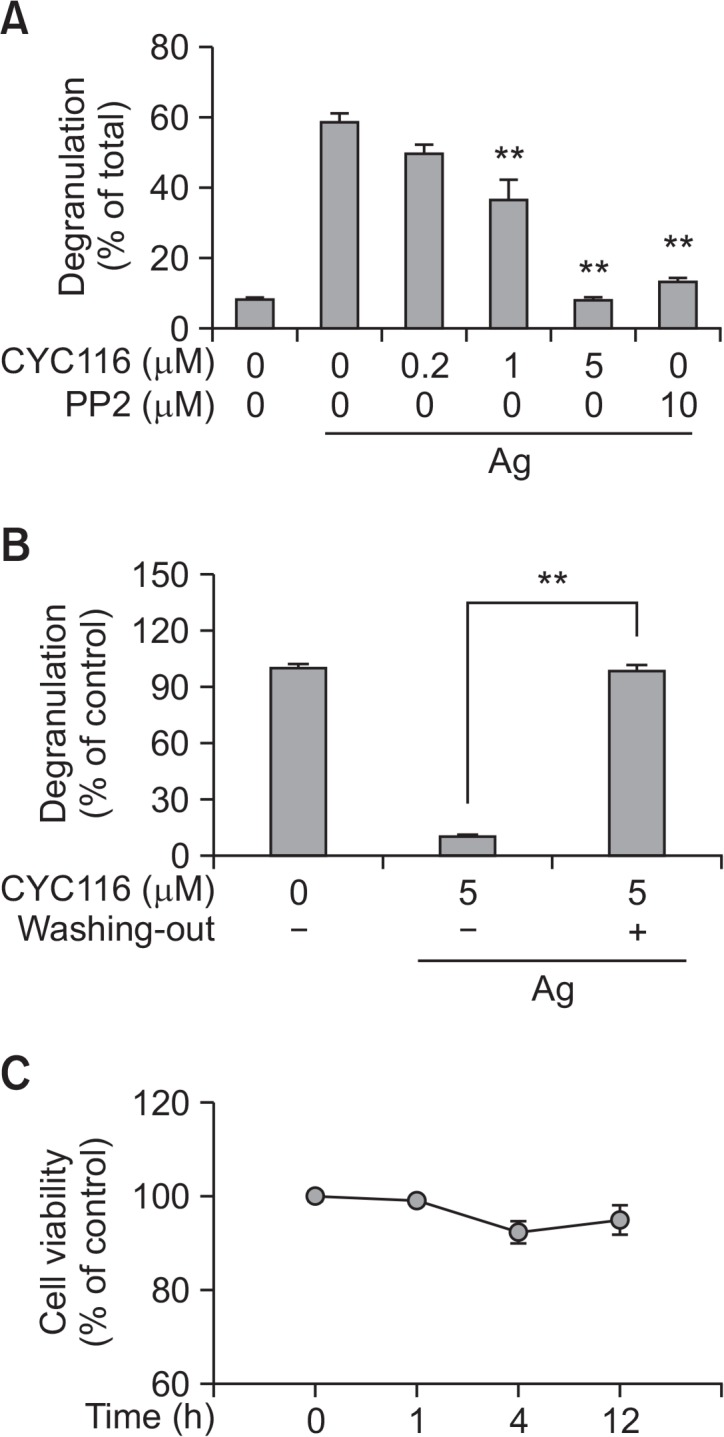
CYC116 reversibly suppresses degranulation in antigen-stimulated Mast cells. (A) The amount of β-hexosaminidase released from BMMCs was determined as described in “Materials and Methods”. (B) BMMCs were washed 3 times after pre-incubation with or without CYC116 (5 µM) or PP2 for 30 min. The cells were stimulated with 50 ng/ml antigen for 15 min. (C) BMMCs were incubated with or without CYC116 for 4 h and cytotoxicity was examined by CCK-8 as described in “Materials and Methods”. The values are presented as the means ± SEM from three independent experiments in triplicate. Significant differences compared to antigen-only group are indicated (A) or as indicated (B), **p<0.01. Ag, antigen; PP2, a typical Src-family kinase inhibitor.
Effects of CYC116 on the secretion of pro-inflammatory cytokines in antigen-stimulated mast cells
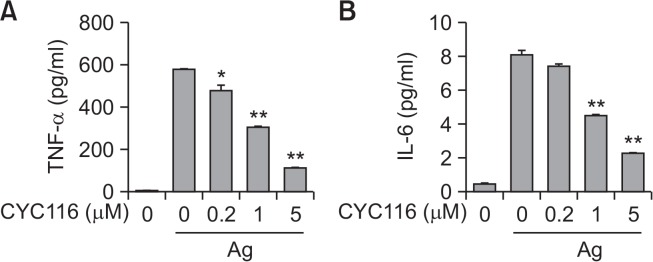
Mast cells release pro-inflammatory cytokines including IL-4, IL-5, IL-6, and TNF-α by antigen stimulation. These cytokines could induce late phase allergic inflammation by recruiting various immune cells into lesion tissues (Bradding et al., 1994; Grimbaldeston et al., 2006). We next measured whether CYC116 inhibited the secretion of TNF-α and IL-6 from mast cells by antigen stimulation. CYC116 dose-dependently inhibited the secretion of TNF-α (IC50, ∼1.10 µM) and IL-6 (IC50, ∼1.24 µM) from mast cells by antigen stimulation (Fig. 3A, 3B). These results suggest that CYC116 could suppress late phase allergic responses caused by mast cells.
Fig. 3.
CYC116 inhibits secretion of TNF-α and IL-6 from BMMCs. BMMCs were stimulated with 50 ng/ml antigen for 3 h with or without CYC116. The cultured media were subjected to ELISA for TNF-α (A) and IL-6 (B). The values are presented as the mean ± SEM from three independent experiments in triplicate. Significant differences compared to the antigen-only group are indicated, *p<0.05 and **p<0.01. Ag, antigen.
Effect of CYC116 on mast cell-mediated anaphylaxis in mice
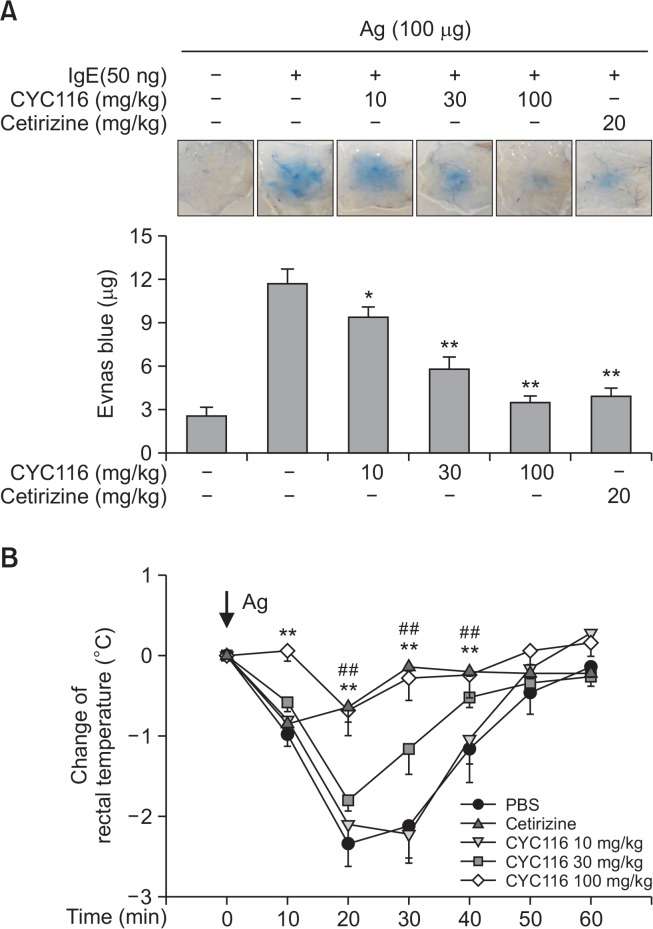
Mast cells are well known as essential effector cells in IgE-mediated passive cutaneous anaphylaxis (PCA) and passive systemic anaphylaxis (PSA) (Maier et al., 2007; Ito et al., 2013). We also examined whether CYC116 inhibits PCA and PSA in mice. CYC116 suppressed PCA response by antigen stimulation in mice in a dose-dependent manner (Fig. 4A, ED50, ∼22.5 mg/kg). Consistent with the PCA results, we also found that CYC116 dose-dependently inhibited the rectal temperature reduction by PSA response in mice stimulated with antigen (Fig. 4B). In particular, the inhibitory effect of PCA and PSA at a dose of 100 mg/kg of CYC116 was similar to that of cetirizine (20 mg/kg), a well-known drug for type I allergic disorders (Portnoy and Dinakar, 2004). These results indicate that CYC116 has a good anti-allergic effect in vivo.
Fig. 4.
CYC116 inhibits PCA and PSA. (A) An intradermal injection of IgE (50 ng) into the back skins of mice was conducted. After 24 h, CYC116 was administered orally 1 h prior to the injection of antigen (100 µg) containing Evans blue into the tail vein. The severity of PCA was evaluated, as described in “Materials and Methods.” Representative images (upper panel) of the back skins and the quantification (lower panel) of extravasated Evans blue are shown. (B) Mice were injected with IgE (3 µg) into tail vein. After 24 h, CYC116 or cetirizine (20 mg/kg, as a reference drug) was administered orally 1 h prior to the i.v. injection of an antigen (100 µg). The severity of PSA is measured by the reduction of rectal temperature as described in “Materials and Methods.” The values are shown as the means ± SEM (n=10 per group). Significant differences compared to IgE-only group (A) or PBS (B) are indicated, *p<0.05 and **p<0.01 (A) or ##p<0.01 for cetirizine or **p<0.01 for CYC116 (B). Ag, antigen.
Effect of CYC116 on calcium influx-induced degranulation
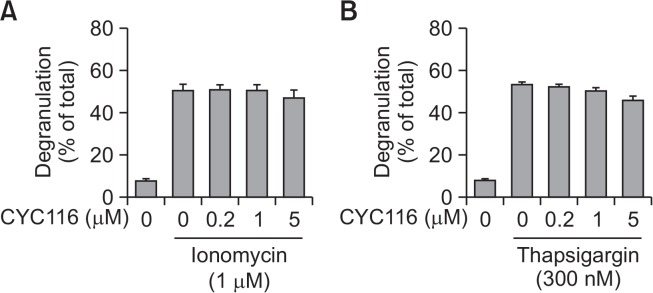
We next conducted a mechanism study of how CYC116 controls mast cells. The increase of the cytosolic Ca2+ concentration is critical for the process of degranulation through the activation of FcεRI signaling of mast cells (Gilffillan et al., 2006). Cross-linking of FcεRI by antigen stimulation activates the Lyn/Syk pathway followed by PLCγ in the mast cells. Activated PLCγ stimulates the degradation of phosphatidylinositol 4,5-bisphosphate (PIP2) to 1,4,5-trisphosphate (IP3) in the membrane. IP3 binds to IP3 receptor of endoplasmic reticulum (ER) and releases Ca2+ from ER into cytosol to increase the cytosolic Ca2+ concentration. In addition, the activation of ER membrane proteins such as STIM1 (Stromal Interaction Molecule 1) and CRAC (Calcium release-activated channels) induces an intracellular entry of extracellular Ca2+, thereby further inducing an increase in cytosolic Ca2+. This cytosolic concentration of Ca2+ is very crucial for the degranulation of mast cells by antigen (Baba et al., 2006; Luik et al., 2006). Therefore, we conducted the following experiments to determine whether CYC116 inhibits the upstream or downstream signaling molecules of Ca2+ in the degranulation process of mast cells by antigen. We used ionomycin (Ca2+ ionophore) and thapsigargin (an inhibitor of the sarco/ER Ca2+ ATPase) to induce mast cell degranulation via increasing the cytosolic Ca2+ concentration. It was evident that degranulation was induced by both ionomycin and thapsigargin in mast cells, but there was no inhibitory effect from the CYC116 (Fig. 5A, 5B). These results suggest that the inhibitory effects of CYC116 on antigen-induced degranulation comes from the control of upstream signaling molecules rather than the control of Ca2+-dependent signals in mast cells.
Fig. 5.
CYC116 has no effects on calcium mobilization-induced mast cell degranulation. BMMCs were pre-incubated with or without CYC116 for 30 min and then stimulated with ionomycin (A) or thapsigargin (B) for 15 min. The data are presented as the mean ± SEM from three independent experiments in triplicate.
Effect of CYC116 on the FcεRI signaling pathways
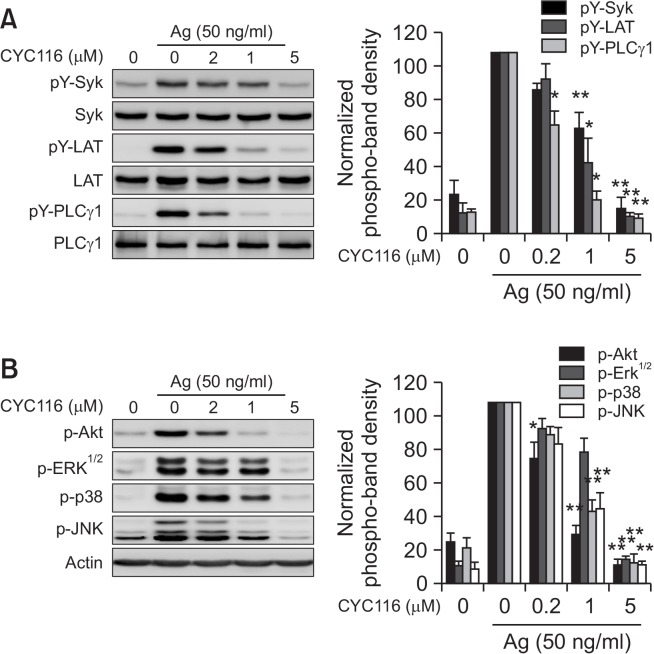
The Lyn-Syk-LAT signaling axis is a central signaling pathway for degranulation in mast cells by antigen. Akt and three typical MAP kinases (ERK1/2, p38, and JNK) are closely associated with the synthesis and secretion of cytokines and eicosanoids (Zhang et al., 1997; Furumoto et al., 2006). Thus, we measured whether CYC116 inhibited antigen-activated Syk, LAT, PLCγ, Akt, and three MAP kinases in mast cells. CYC116 inhibited the phosphorylations of Syk, LAT, and PLCγ in a dose-dependent manner (Fig. 6A). In addition, it was observed that CYC116 inhibited phosphorylations of Erk1/2, p38, JNK, and Akt, a downstream molecule of PI3K signaling (Fig. 6B). Taken together, these results suggest that CYC116 inhibits the initial signaling molecule earlier than the activation of Syk by antigen stimulation.
Fig. 6.
CYC116 inhibits phosphorylations of Syk and Syk-mediated downstream proteins in BMMCs. BMMCs were stimulated with 50 ng/ml antigen for 10 min with or without CYC116. The cell lysates were subjected to Western blot analysis. (A, B) Representative Western blot images (upper panels) and band densities as the mean ± SEM (lower panels) are shown from three independent experiments. Significant differences compared to antigen-only group are indicated, *p<0.05 and **p<0.01. Ag, antigen.
Effects of CYC116 on the activity of Lyn, Fyn, and Syk kinase in vitro
The activation of FcεRI signaling by antigen begins with the activation of Src family kinases, such as Lyn and Fyn (Gilfillan and Rivera, 2009). In addition to the Lyn-Syk-LAT pathway in mast cells, Fyn kinase, one of the Src family kinases, has also been reported as a kinase that regulates Syk activity as a complementary signaling pathway that induces mast cell degranulation (Parravicini et al., 2002). In the above results (Fig. 6A), CYC116 suppressed the phosphorylations of Syk and LAT by antigen. It was next tested in vitro whether or not CYC116 directly inhibits the kinase activity of Lyn, Fyn, and Syk. CYC116 inhibited the activity of Fyn, but not those of Lyn and Syk (Fig. 7). These results suggest that the inhibition of mast cell activation by CYC116 was mediated by directly inhibiting the activity of Fyn.
Fig. 7.
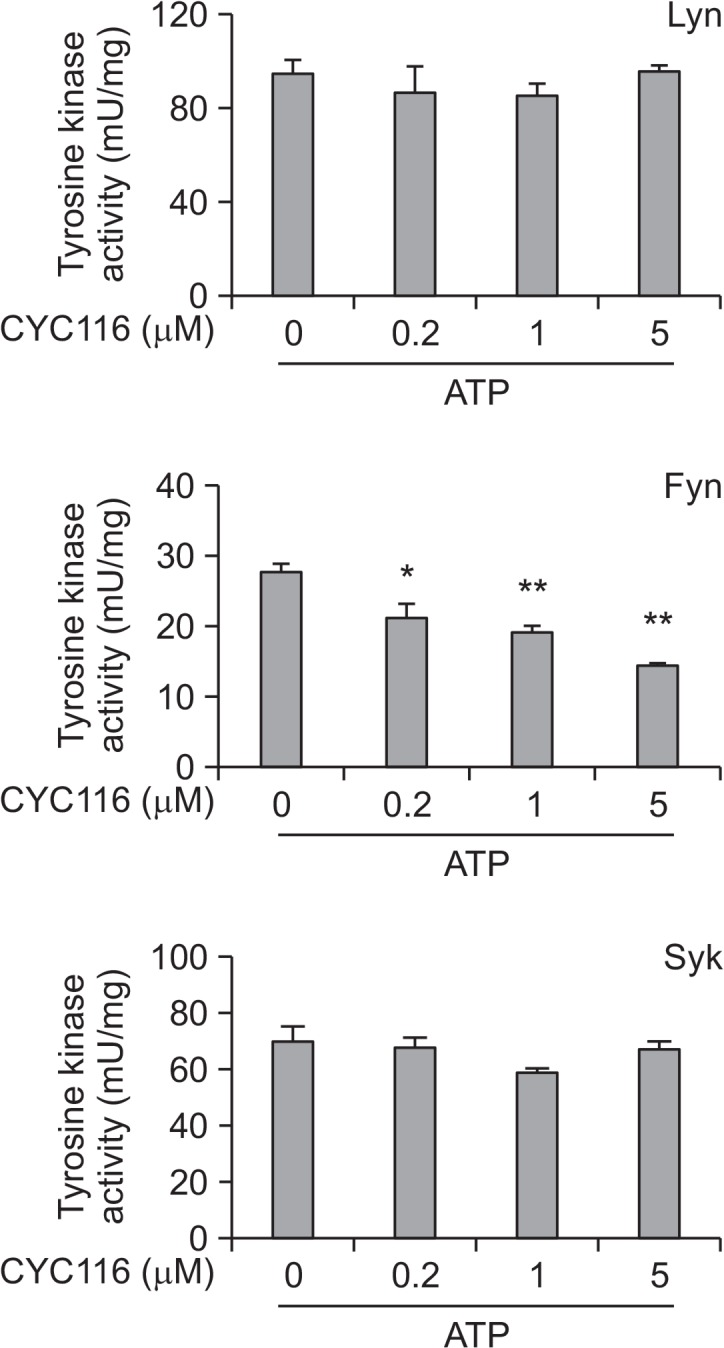
CYC116 inhibits the activity of Fyn, but not Lyn or Syk. The Fyn, Lyn, or Syk from BMMC lysates was immunoprecipitated by using specific antibodies. The kinase activity was determined by a Tyrosine kinase assay kit, as described in “Materials and Methods”. The values are presented as the mean ± SEM from three independent experiments in triplicate. Significant differences compared to each value for ATP-only groups are shown, *p<0.05 and **p<0.01.
DISCCUSION
Mast cells are leukocytes derived from hematopoietic stem cells and characterized by granulocytes containing abundant granules in cytosol (Siebenhaar et al., 2018). The progenitors of mast cells circulate through the bloodstream and migrate to various tissues, including skin, airways, and the gastrointestinal tract, and then differentiate into mature mast cells (Galli et al., 2005; Hallgren and Gurish, 2007). When mast cells are activated by antigen, they could contribute to some inflammatory pathogenesis by secreting various bioactive mediators. Antigen-stimulated mast cells secrete biogenic amines, such as histamine and serotonin, cytokines, such as IL-4, IL-5, and IL-6, and lipid mediators, such as prostaglandin D2, leukotriene B4, and leukotriene C4. These bioactive mediators are involved in the exacerbation of the symptoms of allergic diseases, such as asthma, allergic rhinitis, and atopic dermatitis (Theoharides et al., 2007). Therefore, studies on anti-allergic therapy by targeting mast cells have been actively undertaken. In this study, we investigated the inhibitory effect of CYC116 on the degranulation and cytokine secretion from mast cells by antigen stimulation and the mechanism of its inhibitory activity.
It was reported that CYC116, an inhibitor of Aurora kinase, also inhibited FMS-like tyrosine kinase-3, VEGFR2 kinase, Src, and Lck in vitro (Fu et al., 2007) and CYC116 suppressed tumor growth in animal experiments (Wang et al., 2010). On the other hand, Src-family kinases are crucial for the activation of mast cells by antigen stimulation (Gilfillan and Rivera, 2009). Based on these reports, we performed the experiments in this study to investigate the inhibitory effect of CYC116 on mast cells and mast cell-mediated type I allergic responses. We observed that CYC116 reversibly inhibited the degranulation and the pro-inflammatory cytokine secretion of mast cells by antigen stimulation (Fig. 2A, 3). Furthermore, CYC116 inhibited mast cell-mediated PCA and PSA in mice (Fig. 4A, 4B). These results suggest that CYC116 could be used as an anti-allergic drug in addition to an anti-cancer drug.
The signaling pathway of antigen-IgE-FcεRI-mediated mast cells begins with the activation of the Src-family kinases. For the first time, when Lyn phosphorylates the immunoreceptor tyrosine-based activation motifs (ITAMs) of β and γ subunits of FcεRIs, cytosolic Syk is recruited to the ITAMs and then Syk is fully activated. The activated Syk stimulates the key signaling molecules such as LAT and PLCγ1, and subsequently activates PI3K-Akt and MAP kinases (Furumoto et al., 2004; Gilfillan and Tkaczyk, 2006). In this study, CYC116 inhibited the activations of the signaling proteins including Syk, LAT, PLCγ1, Akt, and three MAP kinases by antigen in a concentration-dependent manner (Fig. 6). These results suggest that CYC116 inhibited the early signaling proteins above Syk in mast cells. Another Src-family kinase, Fyn, in addition to Lyn was reported to activate Syk and subsequent Gab2-PI3K-Akt signaling cascade (Parravicini et al., 2002). In our in vitro kinase assay, CYC116 inhibited Fyn, but not Lyn or Syk (Fig. 7). These results indicate that the inhibitory effect of CYC116 on mast cell activation was derived from the inhibition of Fyn activity among the signal transduction proteins initially activated by antigen stimulation.
The secretion of inflammatory cytokines such as TNF-α and IL-6 is closely related to the activation of Akt and MAP kinase in antigen-stimulated mast cells. Akt activation stimulates the transcription of cytokines through the activations of NFκB or NFAT (Kitakura et al., 2000). In addition, MAP kinases are also involved in cytokine expression by regulating the transcription of cytokines (Furumoto et al., 2004; Siraganian et al., 2010). We observed that CYC116 inhibited the activation of Akt and MAP kinase by antigen stimulation in mast cells (Fig. 6B). These results suggest that CYC116 inhibited the production and secretion of cytokines by inhibiting the activation of Akt and MAP kinases in mast cells.
Aurora kinase is a serine/threonine kinase important for the mitosis process such as centrosome maturation, chromosome alignment, chromosome segregation, and cytokinesis (Carmena and Earnshaw, 2003). For this reason, when CYC116 is administered for a long time to treat allergic diseases, it may cause a unwanted cell death of normal cells through the nonspecific cell cycle arrest and lead to some side effects. It is well known that a large portion of mast cells in patients with allergic diseases such as atopic dermatitis and allergic rhinitis are located in skin or mucosal tissues (Galli et al., 2005). Therefore, for the purpose of suppressing allergic inflammation of skin or mucosa, it would be reasonable to apply CYC116 as the topical medication of ointment or cream for a short period of time rather than oral administration. Further studies on the toxicity of CYC116 and derivatization to reduce its toxicity should be further carried out.
In summary, CYC116 reversibly inhibited the activation of mast cells and passive anaphylaxes including PCA and PSA in mice. For the mechanism of action, CYC116 inhibited Syk and Syk-mediated downstream signaling proteins through the inhibition of Fyn in mast cells (Fig. 8). In conclusion, we report for the first time that CYC116 could be used as an alternative medication for mast cell-mediated allergic disorders.
Fig. 8.
A schematic diagram for the inhibitory mechanism of CYC116 in mast cell activation. CYC116 directly inhibits Fyn kinase in mast cell signaling pathway stimulated by antigen.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant (NRF-2016R1A2B3015840) and in part by Basic Research Laboratory Program (No. 2013R1A4A1069575) funded by the Korea government.
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests.
REFERENCES
- Baba Y, Hayashi K, Fujii Y, Mizushima A, Watarai H, Wakamori M, Numaga T, Mori Y, Iino M, Hikida M, Kurosaki T. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc Natl Acad Sci USA. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuchet P, Varache-Lembège M, Neveu A, Léger JM, Vercauteren J, Larrouture S, Deffieux G, Nuhrich A. New 2-sulfonamidothiazoles substituted at C-4: synthesis of polyoxy-genated aryl derivatives and in vitro evaluation of antifungal activity. Eur. J. Med. Chem. 1999;34:773–779. doi: 10.1016/S0223-5234(99)00215-9. [DOI] [Google Scholar]
- Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, -5, and -6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–480. doi: 10.1165/ajrcmb.10.5.8179909. [DOI] [PubMed] [Google Scholar]
- Carmena M, Earnshaw WC. The cellular geography of aurora kinases. Nat Rev Mol Cell Biol. 2003;4:842–854. doi: 10.1038/nrm1245. [DOI] [PubMed] [Google Scholar]
- Das J, Furch JA, Liu C, Moquin RV, Lin J, Spergel SH, McIntyre KW, Shuster DJ, O’Day KD, Penhallow B, Hung CY. Discovery and SAR of 2-amino-5-(thioaryl) thiazoles as potent and selective Itk inhibitors. Bioorg Med Chem Lett. 2006;16:3706–3712. doi: 10.1016/j.bmcl.2006.04.060. [DOI] [PubMed] [Google Scholar]
- Fu J, Bian M, Jiang Q, Zhang C. Roles of Aurora Kinases in Mitosis and Tumorigenesis. Mol Cancer Res. 2007;5:1–10. doi: 10.1158/1541-7786.MCR-06-0208. [DOI] [PubMed] [Google Scholar]
- Furumoto Y, Nunomura S, Terada T, Rivera J, Ra C. The FcepsilonRIBeta immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IkappaB kinase phosphorylation and mast cell cytokine production. J Biol Chem. 2004;279:49177–49187. doi: 10.1074/jbc.M404730200. [DOI] [PubMed] [Google Scholar]
- Furumoto Y, Brooks S, Olivera A, Takagi Y, Miyagishi M, Taira K, Casellas R, Beaven MA, Gilfillan AM, Rivera J. Cutting edge: lentiviral short hairpin RNA silencing of PTEN in human mast cells reveals constitutive signals that promote cytokine secretion and cell survival. J Immunol. 2006;176:5167–5171. doi: 10.4049/jimmunol.176.9.5167. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signaling pathways for mast-cell activation. Nat Rev Immunol. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Rivera J. The tyrosine kinase network regulating mast cell activation. Immunol Rev. 2009;228:149–169. doi: 10.1111/j.1600-065X.2008.00742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göblyös A, Santiago SN, Pietra D, Mulder-Krieger T, von Frijtag, Drabbe, Künzel J, Brussee J, Ijzerman AP. Synthesis and biological evaluation of 2-aminothiazoles and their derivatives on human adenosine receptors. Lack of effect of 2-aminothiazoles as allosteric enhancers. Bioorg. Med. Chem. 2005;13:2079–2087. doi: 10.1016/j.bmc.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Scaerou F, Midgley C, McClue S, Tosh C, Jackson W, MacCallum D, Wang S, Fischer P, Glover D, Zheleva D. Anti-tumor activity of CYC116, a novel small molecule inhibitor of aurora kinases and VEGFR2. Cancer Res. 2008;49:5644. (Abstract). [Google Scholar]
- Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune response. Curr Opin Immunol. 2006;18:751–760. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Hajduch M, Vydra D, Dzubak P, Dziechciarkova M, Stuart I, Zheleva D. In vivo mode of action of CYC116, a novel small molecule inhibitor of aurora kinases and VEGFR2. Cancer Res. 2008;49:5645. (Abstract). [Google Scholar]
- Hallgren J, Gurish MF. Pathways of murine mast cell development and trafficking: tracking the roots and routes of the mast cell. Immunol Rev. 2007;217:8–18. doi: 10.1111/j.1600-065X.2007.00502.x. [DOI] [PubMed] [Google Scholar]
- Hang PC, Honek JF. Electronic structure calculations on the thiazole-containing antibiotic thiostrepton: molecular mechanics, semi-empirical and ab initio analyses. Bioorg Med Chem Lett. 2005;15:1471–1474. doi: 10.1016/j.bmcl.2004.12.076. [DOI] [PubMed] [Google Scholar]
- Ito R, Takahashi T, Katano I, Kawai K, Kamisako T, Ogura T, Ida-Tanaka M, Suemizu H, Nunomura S, Ra C, Mori A, Aiso S, Ito M. Establishment of a human allergy model using human IL-3/GM-CSF-transgenic NOG mice. J Immunol. 2013;191:2890–2899. doi: 10.4049/jimmunol.1203543. [DOI] [PubMed] [Google Scholar]
- Kitakura J, Asai K, Maeda-Yamamoto M, Kawakami Y, Kikkawa U, Kawakami T. Akt-dependent cytokine production in mast cells. J Exp Med. 2000;192:729–740. doi: 10.1084/jem.192.5.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim YM, Kim NW, Kim JW, Her E, Kim BK, Kim JH, Ryu SH, Park JW, Seo DW. Phospholipase D2 acts as an essential adaptor protein in the activation of Syk in antigen-stimulated mast cells. Blood. 2006;108:956–964. doi: 10.1182/blood-2005-10-009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier JV, Brema S, Tuckermann J, Herzer U, Klein M, Stassen M, Moorthy A, Cato AC. Dual specificity phosphatase 1 knockout mice show enhanced susceptibility to anaphylaxis but are sensitive to glucocorticoids. Mol Endocrinol. 2007;21:2663–2671. doi: 10.1210/me.2007-0067. [DOI] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Nam ST, Kim HW, Kim HS, Park YH, Lee D, Lee MB, Min KY, Kim YM, Choi WS. Furaltadone suppresses IgE-mediated allergic response through the inhibition of Lyn/Syk pathway in mast cells. Eur J Pharmacol. 2018;828:119–125. doi: 10.1016/j.ejphar.2018.03.035. [DOI] [PubMed] [Google Scholar]
- Ozdemir C, Akdis M, Akdis CA. T-cell response to allergens. Chem. Immunol Allergy. 2010;95:22–44. doi: 10.1159/000315936. [DOI] [PubMed] [Google Scholar]
- Parravicini V, Gadina M, Kovarova M, Odom S, Gonzalez-Espinosa C, Furumoto Y, Saitoh S, Samelson LE, O’Shea JJ, Rivera J. Fyn kinase initiates complementary signals required for IgE-dependent mast cell degranulation. Nat Immunol. 2002;3:741–748. doi: 10.1038/ni817. [DOI] [PubMed] [Google Scholar]
- Pawankar R, Canonica GW, Holgate GT, Lockey RF, Blaiss MS. WAO White Book on Allergy. World Allergy Organization; Milwaukee: 2013. p. 11. Update 2013. Available from: http://www.worldallergy.org/UserFiles/file/WhiteBook2-2013-v8.pdf/. [Google Scholar]
- Portnoy JM, Dinakar C. Review of cetirizine hydrochloride for the treatment of allergic disorders. Expert Opin Pharmacother. 2004;5:125–135. doi: 10.1517/14656566.5.1.125. [DOI] [PubMed] [Google Scholar]
- Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Siebenhaar F, Redegeld FA, Bischoff SC, Gibbs BF, Maurer M. Mast cells as drivers of disease and therapeutic targets. Trends Immunol. 2018;39:151–162. doi: 10.1016/j.it.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Siraganian RP, de Castro RO, Barbu EA, Zhang J. Mast cell signaling: the role of protein tyrosine kinase Syk, its activation and screening methods for new pathway participants. FEBS Lett. 2010;584:4933–4940. doi: 10.1016/j.febslet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides TC, Kempuraj D, Tagen M, Conti P, Kalogeromitros D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol Rev. 2007;217:65–78. doi: 10.1111/j.1600-065X.2007.00519.x. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sismanopoulos N, Zhang B, Asadi S, Vasiadi M, Weng Z, Miniati A, Kalogeromitros D. Mast cell and inflammation. Biochim. Biophys Acta. 2012;1822:21–33. doi: 10.1016/j.bbadis.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Midgley CA, Scaërou F, Grabarek JB, Griffiths G, Jackson W, Kontopidis G, McClue SJ, McInnes C, Meades C, Mezna M, Plater A, Stuart I, Thomas MP, Wood G, Clarke RG, Blake DG, Zheleva DI, Lane DP, Jackson RC, Glover DM, Fischer PM. Discovery of N-phenyl-4-(thiazol-5-yl)pyrimidin-2-amine aurora kinase inhibitors. J Med Chem. 2010;53:4367–4378. doi: 10.1021/jm901913s. [DOI] [PubMed] [Google Scholar]
- Zhang C, Baumgartner RA, Yamada K, Beaven MA. Mitogen-activated Protein (MAP) kinase regulates production of tumor necrosis factor-α and release of arachidonic acid in mast cells indications of communication between p38 and p42 Map kinases. J Biol Chem. 1997;272:13397–13402. doi: 10.1074/jbc.272.20.13397. [DOI] [PubMed] [Google Scholar]