Abstract
β-amyloid precursor protein (APP) can be cleaved by α-, and γ-secretase at plasma membrane producing soluble ectodomain fragment (sAPPα). Alternatively, following endocytosis, APP is cleaved by β-, and γ-secretase at early endosomes generating β-amyloid (Aβ), the main culprit in Alzheimer’s disease (AD). Thus, APP endocytosis is critical for Aβ production. Recently, we reported that Monsonia angustifolia, the indigenous vegetables consumed in Tanzania, improved cognitive function and decreased Aβ production. In this study, we examined the underlying mechanism of justicidin A, the active compound of M. angustifolia, on Aβ production. We found that justicidin A reduced endocytosis of APP, increasing sAPPα level, while decreasing Aβ level in HeLa cells overexpressing human APP with the Swedish mutation. The effect of justicidin A on Aβ production was blocked by endocytosis inhibitors, indicating that the decreased APP endocytosis by justicidin A is the underlying mechanism. Thus, justicidin A, the active compound of M. angustifolia, may be a novel agent for AD treatment.
Keywords: Alzheimer’s disease, β-amyloid precursor protein, Justicidin A, Endocytosis, β-amyloid
INTRODUCTION
The pathological hallmark of Alzheimer’s disease (AD) is the formation of extracellular senile plaques in the brain. The major constituent of these plaques is the neurotoxic β-amyloid (Aβ) peptides, which is derived from the β-amyloid precursor protein (APP). APP, a type I transmembrane protein, is first cleaved within its extracellular domain by α- or β-secretases followed by the cleavage within its transmembrane domain by γ-secretase (Shoji et al., 1992; Yoon and Jo, 2012). The non-amyloidogenic processing of APP by α-secretase produces soluble ectodomain fragment (sAPPα) and C-terminal fragment (CTFα). CTFα can be further cleaved by γ-secretase to produce APP intracellular domain (AICD) and an N-terminally truncated Aβ peptide called p3 (Thinakaran and Koo, 2008). The A disintegrin and metalloproteinases (ADAM) family species, ADAM9, 10, and 17, are known as α-secretases (Buxbaum et al., 1998; Lammich et al., 1999). The alternative amyloidogenic pathway involves the cleavage of APP by β-secretase producing soluble ectodomain fragment (sAPPβ) and C-terminal fragment (CTFβ). The cleavage of CTFβ by γ-secretase produces AICD and Aβ peptide. β-site amyloid precursor protein cleaving enzyme 1 (BACE1) is the major β-secretase (Sinha et al., 1999; Vassar et al., 1999).
The synthesized APP at the endoplasmic reticulum (ER) is transported through the secretory pathway via trans-Golgi network (TGN) to the cell surface. APP at the cell surface is subject to rapid clathrin-dependent endocytosis. Internalized APP enters the early endosomes, where it is cleaved by-secretase (Koo and Squazzo, 1994). It is known that blocking of endocytosis increases the amount of cell surface APP and decreases Aβ production, since APP is predominantly cleaved by α-secretase at the cell surface (Koo and Squazzo, 1994; Carey et al., 2005; Cirrito et al., 2008). In contrast, overexpression of Rab5, which drives the maturation of endosomes, increases APP internalization and Aβ secretion (Grbovic et al., 2003). Thus, these results suggest that an inhibition of APP internalization can contribute to lowering of Aβ secretion, which evidently has major implications for treatment of AD.
Monsonia angustifolia is an indigenous vegetables consumed in Tanzania (Lyimo et al., 2003). We recently identified the effect of M. angustifolia on Aβ production and spatial learning ability in vivo (Chun et al., 2017). M. angustifolia’s active compound, justicidin A (Fig. 1), potently decreased Aβ levels. We also reported the neuroprotective effects of justicidin A, an arylnaphthalide lignan, as an inhibitor of tau hyperphosphorylation in Aβ25-35-induced neuronal cell death (Gu et al., 2016). In this study, we examined the effect of justicidin A on Aβ production and its underlying mechanism. We found that justicidin A reduced Aβ production, while increased sAPPα production through inhibiting APP endocytosis.
Fig. 1.
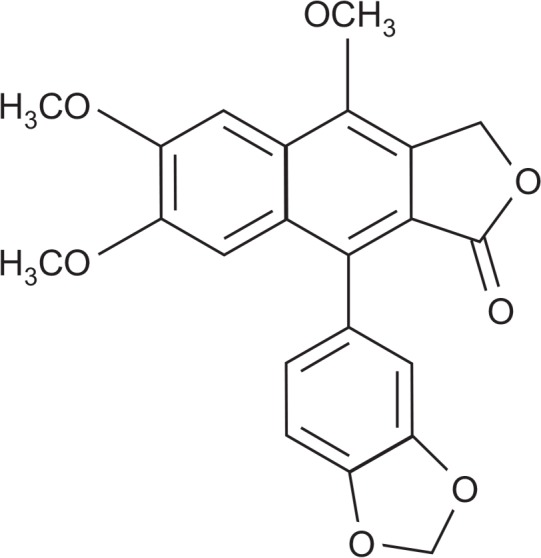
Chemical structure of justicidin A.
MATERIALS AND METHODS
Cell culture and experimental treatments
HeLa cells stably transfected with APP carrying the Swedish mutation (APPsw) were cultured at 37°C and 5% CO2 in Dulbecco’s Modified Eagle Medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum (FBS) containing 100 units/ml penicillin, 100 µg/ml streptomycin, 260 µg/ml Zeocin, and 400 µg/ml G418. SH-SY5Y cells stably transfected with APP carrying the wide type (APPwt) were cultured with 260 µg/ml Zeocin. Dynasore (Tocris Bioscience, MN, USA), chlorpromazine (Sigma-Aldrich, MO, USA) were pre-treated 10 min before justicidin A treatment.
sAPPα, sAPPβ, Aβ peptide assay
APPsw-transfected HeLa cells were incubated with justicidin A or DMSO for 8 h. The conditioned medium was analyzed by specific ELISA for detection of sAPPα (IBL, Hamburg, Germany), sAPPβ-sw (IBL), Aβ42 (Invitrogen, CA, USA), and Aβ40 (Invitrogen), according to the manufacturer’s instructions. Aβ42 level from APPwt-transfected SH-SY5Y cells in the medium was measured using a specific high-sensitivity ELISA (Millipore, MN, USA).
sAPPα, and sAPPβ immunoprecipitation
Cells were incubated with justicidin A for 8 h, and the media were concentrated using Amicon Ultra 30K centrifugal filters (Millipore). The concentrated media was immunoprecipitated with an APP antibody recognizing the N-terminus (abcam, MA, USA) and Protein G Agarose (Millipore). The immunoprecipitated samples were washed with PBS, and probed for sAPPα (Covance, NJ, USA) and sAPPβ (Covance) using Western blot.
Protein extraction and Western blotting
Cells were washed with PBS and homogenized with lysis buffer (50 mM HEPES, pH 7.2, 100 mM NaCl, 1% Triton X-100, and 1 mM sodium orthovandate, and protease inhibitors). Lysates were centrifuged at 10,000×g for 10 min at 4°C. The protein concentration in the supernatant was determined using the Bradford assay (Bio-Rad, CA, USA). Protein was resolved with SDS-PAGE and transferred onto a nitrocellulose membrane. Membranes were blocked in Tris-buffered saline/Tween-20 (TBST) with 5% non-fat milk powder for 1 h at room temperature, and incubated overnight at 4°C with anti-APP (6E10; Covance), BACE1 (Millipore), ADAM9 (Cell Signaling Technology, MA, USA), ADAM10 (Calbiochem, CA, USA), ADAM17 (Chemicon, GA, USA), β-actin (Sigma), and β-tubulin (Sigma). Membranes were washed in TBST, and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG, goat anti-rabbit IgG, or goat anti-mouse IgM (µ) antibodies (Invitrogen) for 1 h at room temperature. Peroxidase activity was visualized with enhanced chemiluminescence. The detected signals were quantified with the Multi Gauge software using a LAS-3000 system (Fujifilm, Tokyo, Japan).
Cell surface biotinylation
Cells were washed with PBS, and incubated in PBS with 0.25 mg/ml Sulfo-NHS-SS-biotin (Thermo, CA, USA) for 10 min at 4°C. After washing with PBS to remove the excessive biotinylating reagent, cells were lysed with lysis buffer for 1 h at 4°C. Biotinylated proteins were pulled down using streptavidin-agarose slurry (Sigma) at 4°C for 3 h. The bound material was analyzed using Western blot.
Internalization assay
Cells were placed on ice, washed with ice-cold PBS, and incubated in PBS with 0.25 mg/ml Sulfo-NHS-SS-biotin for 10 min at 4°C. Excessive biotin was washed out with ice-cold PBS, and cells were incubated with 1% BSA in PBS for 15 min at 4°C. After washing with PBS, cells were incubated at 37°C for appropriate time or kept at 4°C as control. The remaining cell surface biotin was cleaved by incubating twice with the reducing agent (50 mM sodium-2-mercapoethanesulfomate, 150 mM NaCl, 1 mM EDTA, 0.2% BSA, 20 mM Tris-HCl, pH 8.6) for 25 min at 4°C. This reaction was quenched by ice-cold 5 mg/ml iodoacetamide (Sigma) in 1% BSA for 10 min. After washing in PBS, cells were extracted in lysis buffer (50 mM HEPES, pH 7.2, 100 mM NaCl, 1% Triton X-100, 1 mM sodium orthovandate, with protease inhibitor mixture). Biotinylated proteins were pulled down using streptavidin-agarose slurry (Sigma) (4°C for 3 h). After washing the agarose beads, the bound material was analyzed by Western blot.
Primary antibody uptake
Cells were washed with PBS, and incubated in PBS with 6E10 antibody (1:100 dilution) for 45 min at 4°C to label surface APP. Cells were washed in ice-cold PBS and incubated at 37°C for the required time. They were fixed in 4% paraformaldehyde at room temperature for 15 min, washed, and permeabilized in 0.1% Triton X-100 for 5 min. They were blocked with 2% goat serum in PBS for 1 h at room temperature, and incubated with goat anti-mouse antibodies conjugated with Alexa Fluor 488 in blocking buffer for overnight. Finally, cells were washed in PBS and mounted with medium (DakoCytomation, Glostrup, Denmark) and left overnight at 4°C to dry. Immunofluorescence staining was captured on a confocal microscope (LSM510, Zeiss, Oberkochen, Germany). Images were analyzed using the Image J program (ImageJ, NIH, USA) to quantify the mean fluorescence intensity values. Fluorescence intensities corresponding to the plasma membrane were measured from the edge of the cell to 500 nm inside.
Statistical analysis
Data was expressed as mean ± SEM. Statistical comparisons between controls and treated experimental groups were performed using the Student’s t-test. p<0.05 was considered statistically significant.
RESULTS
Justicidin A decreases amyloidogenic processing of APP
We tested whether justicidin A would affect Aβ levels in APPsw-transfected HeLa cells. Cells were incubated with 0.05, 0.1, 0.5, and 1 µM justicidin A for 8 h. Aβ levels in the medium were significantly decreased by justicidin A (Fig. 2A), consistent with our previous results (Chun et al., 2017). Secreted Aβ42 was decreased by 48.1 ± 0.8% and 58.2 ± 0.7% with 0.5 and 1 µM justicidin A, respectively. Secreted Aβ40 was also reduced by 31.7 ± 6.2% and 50.1 ± 2.8% with 0.5 and 1 µM justicidin A, respectively. We also tested the effect of justicidin A on APPwt-transfected neuronal SH-SY5Y cells. Secreted Aβ42 was significantly decreased by 51 ± 3.7% and 56.6 ± 5% (n=4) at 0.5 and 1 µM justicidin A, respectively (Fig. 2B). These results suggested that the effect of justicidin A was not cell-type specific.
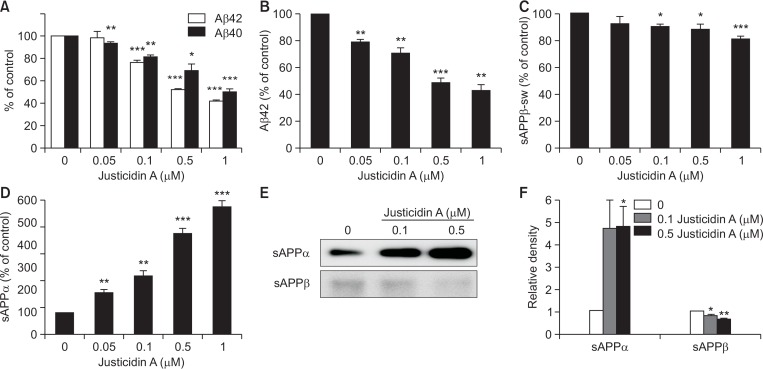
Fig. 2.
Justicidin A decreased secreted Aβ level. (A) APPsw-transfected HeLa cells were incubated with indicated concentrations of justicidin A for 8 h. The levels of Aβ in the medium were measured using ELISA methods. % of control is obtained by normalizing to the control for each experimental condition. Justicidin A decreased the levels of Aβ42 (open bars, n=6) and Aβ40 (closed bar, n=6). (B) APPwt-transfected SH-SY5Y cells were incubated with indicated concentrations of justicidin A for 4 h, and the Aβ42 level was measured from the conditioned media using ELISA methods. Justicidin A decreased secreted Aβ42 level (n=4). (C, D) APPsw-transfected HeLa cells were incubated with indicated concentrations of justicidin A for 8 h. The levels of sAPPβ-sw, and sAPPα in the medium were measured using ELISA methods. sAPPβ-sw level (C) was decreased and sAPPα level (D) was increased by justicidin A (n=5). (E) Levels of sAPPα and sAPPβ in the medium were measured using Western blot. (F) Bars indicate the levels of sAPPα and sAPPβ obtained from densitometric analysis of Western bands in (E) (n=4). *p<0.05, **p<0.01, ***p<0.001.
We next measured the secreted levels of APP proteolytic products using specific ELISA kits for sAPPβ-sw and sAPPα. β-cleavage of APPsw would produce sAPPβ-sw. The level of sAPPβ-sw was reduced by 17.8 ± 0.5% with 1 µM justicidin A (Fig. 2C). Justicidin A significantly increased the level of sAPPα in a dose-dependent manner (Fig. 2D). At 1 µM justicidin A, the secreted level of sAPPα was increased by about 6-fold. Secreted levels of sAPPα and sAPPβ were also measured using Western blots. The conditioned medium was immunoprecipitated with APP antibody and probed with the specific antibodies. A typical result is shown in Fig. 2E, and the relative band densities of sAPPα and sAPPβ are shown in Fig. 2F. Consistent with ELISA results, justicidin A increased sAPPα levels and decreased sAPPβ levels. These results suggested that justicidin A decreased amyloidogenic processing, while increased non-amyloidogenic processing of APP. Justicidin A did not influence the cell viability in our experimental condition as we have shown previously (Chun et al., 2017).
Justicidin A increases the cell surface level of APP
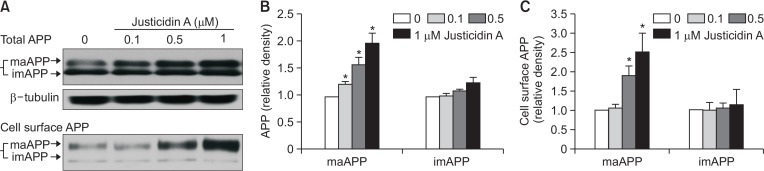
We tested whether justicidin A affected APP levels. APP undergoes post-translational modification such as N- and O-glycosylation, during the transit from the ER to the plasma membrane (Weidemann et al., 1989; Påhlsson et al., 1992; Graebert et al., 1995; Tomita et al., 1998). Accordingly, it exists as an immature form of APP (imAPP, N-glycosylated) or mature form of APP (maAPP, N- and O-glycosylated). Cells were incubated with 0.1, 0.5, and 1 µM justicidin A for 4 h, and APP level was analyzed using Western blot. The level of maAPP was increased by justicidin A in a dose-dependent manner, while the level of imAPP was not changed (upper panel of Fig. 3A). The relative band densities of APP compared to β-tubulin are shown in Fig. 3B. At 1 µM justicidin A, maAPP level was significantly increased by about 2-fold. We next measured the cell surface level of APP using the biotinylation, since it has been reported that APP is transported to the plasma membrane after post-translational modification. Justicidin A increased the cell surface APP level as shown in the lower panel of Fig. 3A. Justicidin A at 1 µM significantly increased the cell surface maAPP level by 2.5-fold (Fig. 3C). In contrast, cell surface imAPP level was not changed by justicidin A. These results demonstrated that justicidin A increases the steady-state level of maAPP at the cell surface.
Fig. 3.
Justicidin A increased the level of cell surface APP. (A) Upper panel. Cells were incubated with indicated concentrations of justicidin A for 4 h, and lysates were obtained for the detection of APP levels. β-tubulin was used to confirm the amount of proteins loaded. Lower panel. Cell surface APP level was measured using biotinylation method. The positions of maAPP and imAPP were indicated. (B) Bars indicate the levels of maAPP and imAPP obtained from densitometric analysis of Western bands in the upper panel in (A) (n=4). (C) Densitometric analysis of Western bands in the lower panel in (A) shows that justicidin A increased the cell surface maAPP level (n=4). *p<0.05.
We observed that justicidin A decreased the cleavage of APP by amyloidogenic β-secretase, while increased the cleavage of APP by non-amyloidogenic α-secretase. Thus, we decided to investigate whether justicidin A affected the level of secretases, such as BACE1, ADAM9, ADAM10, and ADAM17. Incubating cells with justicidin A did not change the levels of these secretases (Supplementary Fig. 1). These results suggest that the effect of justicidin A on APP processing was not due to the changes in the expression level of secretases in our experimental condition.
Justicidin A decreases the endocytosis rate of APP
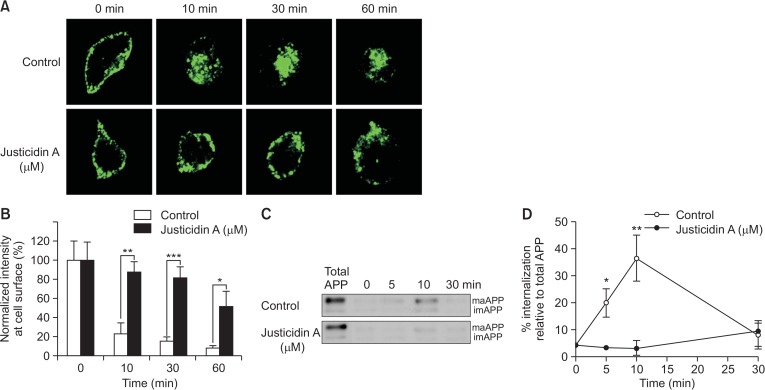
Previous studies have shown that only a small fraction of APP localizes in the plasma membrane at any given time (Haass et al., 1991), and APP rapidly undergoes endocytosis from the plasma membrane. Thus, justicidin A may affect the endocytosis rate of APP, thereby increasing its cell surface levels. To test this possibility, we first measured the endocytosis rate of APP using the antibody uptake method. We labeled the cell surface APP with the 6E10 antibody at 4°C, followed by incubating at 37°C for 10, 30, or 60 min to allow endocytosis. Then, cells were fixed, permeabilized, and APP was visualized using a fluorescent conjugated secondary antibody. Typical fluorescence intensities from APP immunoreactivity are shown in Fig. 4A. Fluorescence intensities at the vicinity of the cell surface were measured and are shown in Fig. 4B. At 0 min before initiating the endocytosis, APP was localized in the cell surface. At 10 min, only 23% of APP was localized in the plasma membrane in control cells, indicating a rapid endocytosis of APP at the plasma membrane (Koo et al., 1996). In contrast, in justicidin A-treated cells, 88% of APP was localized in the plasma membrane at 10 min. At 30 and 60 min, very few APP was located at the cell surface in control cells, while a large amount of APP still remained at the cell surface in justicidin A-treated cells. These results indicate that the endocytosis of APP was significantly inhibited by justicidin A.
Fig. 4.
Endocytosis rate of APP was decreased by justicidin A. (A) Justicidin A decreased the endocytosis rate of APP. Cell surface APP was immunolabeled with 6E10 antibody in the presence of 1 µM justicidin A for 45 min at 4°C. Then, cells were incubated at 37°C for varying time periods, followed by fixation, and permeabilization. Cells were incubated with GFP-tagged secondary antibody and observed under a fluorescence microscope. (B) Fluorescence intensities of APP at the plasma membrane were obtained using Image J software from control (open bars) and justicidin-treated (closed bars) cells (n=10). (C) Cells were incubated with EZ-Link Sulfo-NHS-SS-Biotin at 4°C for 10 min. Next, cells were incubated with 1 µM justicidin A at 4°C for 45 min, followed by incubation at 37°C for various time periods. The remaining biotin at the cell surface was removed by reducing agent, and the internalized biotinylated proteins were pulled down using streptavidin beads. Representative Western blot shows the internalization of APP. (D) Internalized APP levels were quantified by the densitometric analysis of the bands. % of internalization was obtained by comparing to the total cell surface APP (n=5). *p<0.05, **p<0.01, ***p<0.001.
We also used reversible biotinylation method to confirm that justicidin A decreased the endocytosis rate of APP. This method has been used to quantify the endocytosis rate of various receptors (Ehlers, 2000). Proteins at the cell surface were biotinylated and allowed to internalize by incubating cells at 37°C. The remaining biotin at the cell surface was removed using reducing reagent and the internalized biotinylated proteins were pulled down using streptavidin beads. Biotinylated proteins were analyzed by Western blot using the APP antibody. Thus, the biotinylated APP represented the internalized APP by the endocytosis. The total cell surface level of APP was also shown in both control and justicidin A-treated cells (total APP). At 10 min after endocytosis was initiated, 36% of surface APP was internalized in control cells, while almost no APP was internalized in justicidin A-treated cells (Fig. 4C, 4D). These data indicated that justicidin A decreased the endocytosis rate of APP, consistent with our antibody uptake result.
Since the inhibitory effect of justicidin A on APP endocytosis may be due to the general inhibition of endocytosis, we tested the effect of justicidin A on the endocytosis rate of transferrin, which undergoes receptor-mediated endocytosis in various cell types. Similar to APP, transferrin is internalized through clathrin-dependent endocytosis (Doherty and McMahon, 2009). After stripping the surface-residing transferrin by acidic buffer, cells were fixed, permeabilized, and transferrin was visualized under fluorescent microscopy (Supplementary Fig. 2). The endocytosis level of transferrin was not changed by justicidin A, indicating that justicidin A did not inhibit the clathrin-dependent endocytosis in general.
The effect of justicidin A on Aβ production is prevented by the inhibition of clathrin-dependent endocytosis
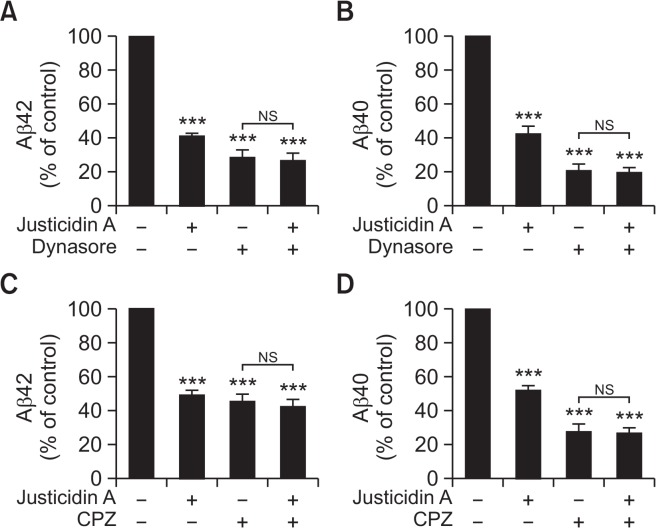
APP is preferentially cleaved by α-secretase at the plasma membrane, while internalized APP enters the early endosomes where it is cleaved by β-secretase (Parvathy et al., 1999). Consistent with this model, inhibition of dynamin, which is an essential component for clathrin-dependent endocytosis, induces the increase of sAPPα production and the decrease of Aβ production (Carey et al., 2005). Thus, the reduced endocytosis rate of APP by justicidin A might lead to a decreased Aβ production. To test this possibility, we used dynasore, which acts as inhibitor for endocytic pathways by blocking clathrin-coated vesicle formation (Urrutia et al., 1997). Cells were incubated with or without 0.5 µM justicidin A and 150 µM dynasore for 2 h, and Aβ levels were measured from the conditioned media using ELISA kits. Aβ42 level was decreased by 59.6 ± 1.8% with 0.5 µM justicidin A and by 72.0 ± 4.2% with dynasore treatment, respectively (Fig. 5A). When cells were incubated with both justicidin A and dynasore, Aβ42 level was decreased by 73.7 ± 4.7%. Also, the secreted Aβ40 level was decreased by 57.8 ± 4.2% and 79.7 ± 4% when cells were incubated with justicidin A and dynasore, respectively (Fig. 5B). In the presence of both justicidin A and dynasore, Aβ40 level was decreased by 80.9 ± 3.4%, a level similar to that of dynasore alone. Thus, dynasore effectively blocked the inhibitory effect of justicidin A on Aβ production.
Fig. 5.
The effects of justicidin A on Aβ secretion were prevented by endocytosis inhibitors. (A, B) Cells were incubated with or without 0.5 µM justicidin A containing 150 µM dynasore for 2 h. The effects of justicidin A on Aβ42 (A, n=5) and Aβ40 (B, n=4) were blocked by a selective dynamin inhibitor, dynasore. (C, D) Cells were incubated with or without 0.5 µM justicidin A containing 20 µM chlorpromazine (CPZ), the clathrin-mediated endocytosis inhibitor, for 1 h. The effects of justicidin A on Aβ42 (C, n=7) and Aβ40 (D, n=6) were blocked by the presence of CPZ. ***p<0.001.
Next, as another way to inhibit endocytic pathways, chlorpromazine (CPZ) was used. CPZ disrupts endocytosis through a redistribution of clathrin-coated vesicle component (Wang et al., 1993). Cells were incubated with or without 0.5 µM justicidin A and 20 µM CPZ for 1 h. The secreted Aβ42 level was decreased by 50.6 ± 2.7% with justicidin A and 54.3 ± 3.9% with CPZ, respectively (Fig. 5C). In presence of both justicidin A and CPZ, Aβ42 level was decreased by 57.6 ± 4.6%. The secreted Aβ40 level was decreased by 47.9 ± 2.9% and 71.8 ± 4.4% when cells were incubated with justicidin A and CPZ, respectively (Fig. 5D). In the presence of both justicidin A and CPZ, Aβ40 level was decreased by 73.0 ± 3.6%. Thus, the inhibitory effect of justicidin A on Aβ secretion was blocked by CPZ, consistent with our results obtained with dynasore. Together, these data supported the conclusion that the decrease of Aβ secretion by justicidin A was mainly mediated by decreased APP endocytosis.
DISCUSSION
M. angustifolia is traditionally used for food and cooked as an indigenous vegetable for daily meals in Tanzania (Lyimo et al., 2003) and it is also traditionally used in South Africa as a medicinal plant to treat erectile dysfunction. Recently, we showed that ethanol extract of M. angustifolia ameliorated behavioral deficits and reduced insoluble Aβ42 level in AD model mouse (Chun et al., 2017). We showed that justicidin A, one of active compound of M. angustifolia, decreased the production of Aβ. Justicidin A with aryl naphthalene structure exhibited strong Aβ decreasing effect starting from low-concentration treatment, while many candidate compounds from the major constituent group failed to show such effect. In this study, we further investigated the effect of justicidin A on Aβ production and its underlying mechanism. Justicidin A inhibits the APP endocytosis and increases cell surface APP level. Concomitantly, it enhances sAPPα secretion and decreases Aβ secretion, which reflect the increased cleavage of APP by α-secretase at the plasma membrane. Justicidin A may be a novel agent for AD therapeutics not only for decreasing Aβ but increasing sAPPα which possesses neuroprotective properties that are beneficial for memory function (Mattson, 1997; Kögel et al., 2012). Furthermore, sAPPα is known to regulate synaptogenesis and stabilize neuronal calcium homeostasis (Morimoto et al., 1998; Guo et al., 1998).
APP is synthesized in the ER and transported to the plasma membrane, where it is predominantly cleaved by α-secretase (Parvathy et al., 1999). Alternatively, following clathrin-dependent endocytosis in the plasma membrane APP is sorted to the endosomes, where it is likely to be cleaved by β-secretase (Koo and Squazzo, 1994). Thus, the endocytosis of APP and the partitioning within intracellular compartments are crucial steps in determining Aβ level. The YENPTY motif in the cytoplasmic region of APP is the sorting signal that regulates APP endocytosis (Perez et al., 1999). Thus, the deletion of this motif results in increased cell surface APP levels and decreased Aβ production in the brain (Ring et al., 2007). In addition, upregulation of the endocytic pathway by overexpressing Rab5 increases Aβ production (Grbovic et al., 2003). These results support the premise that the reduced endocytosis of APP may induce a decreased Aβ production. Our results showed that the inhibition of clathrin-dependent endocytosis effectively prevented the effect of justicidin A on Aβ production, indicating that the effect of justicidin A on Aβ production was mainly due to the decrease of APP endocytosis. However, how justicidin A regulates APP endocytosis needs to be further investigated. We demonstrated that justicidin A did not change clathrin-dependent endocytosis of transferrin receptor and the effect of justicidin A on endocytosis was specific to APP. This finding indicates that justicidin A does not affect clathrin-dependent endocytosis in general. For novel targets in AD research, the mechanisms involved in the action of justicidin A on APP processing merit further study.
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) (2016R1D1A1A099) to S.C. This work was also supported by the Bio-Synergy Research Project (NRF-2012M3A9C4048793) and the Bio & Medical Technology Development Program (NRF-2015M3A9A5030735) of the Ministry of Science, ICT, and Future Planning through the National Research Foundation, Republic of Korea to HOY.
REFERENCES
- Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- Carey RM, Balcz BA, Lopez-Coviella I, Slack BE. Inhibition of dynamin-dependent endocytosis increases shedding of the amyloid precursor protein ectodomain and reduces generation of amyloid beta protein. BMC Cell Biol. 2005;6:30–40. doi: 10.1186/1471-2121-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YS, Kim J, Chung S, Khorombi E, Naidoo D, Nthambeleni R, Harding N, Maharaj V, Fouche G, Yang HO. Protective roles of Monsonia angustifolia and its active compounds in experimental models of Alzheimer’s disease. J Agric Food Chem. 2017;65:3133–3140. doi: 10.1021/acs.jafc.6b04451. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloidbeta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902. doi: 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/S0896-6273(00)00129-X. [DOI] [PubMed] [Google Scholar]
- Graebert KS, Popp GM, Kehlw T, Herzog V. Regulated O-glycosylation of the Alzheimer beta-A4 amyloid precursor protein in thyrocytes. Eur J Cell Biol. 1995;66:39–46. [PubMed] [Google Scholar]
- Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, Summers-Terio NB, Ceresa BP, Nixon RA, Cataldo AM. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J Biol Chem. 2003;278:31261–31268. doi: 10.1074/jbc.M304122200. [DOI] [PubMed] [Google Scholar]
- Gu MY, Kim J, Yang HO. The neuroprotective effects of justicidin A on amyloid beta25-35-induced neuronal cell death through inhibition of tau hyperphosphorylation and induction of autophagy in SH-SY5Y cells. Neurochem Res. 2016;41:1458–1467. doi: 10.1007/s11064-016-1857-5. [DOI] [PubMed] [Google Scholar]
- Guo Q, Robinson N, Mattson MP. Secreted beta-amyloid precursor protein counteracts the proapoptotic action of mutant presenilin-1 by activation of NF-kappaB and stabilization of calcium homeostasis. J Biol Chem. 1998;273:12341–12351. doi: 10.1074/jbc.273.20.12341. [DOI] [PubMed] [Google Scholar]
- Haass C, Hung AY, Selkoe DJ. Processing of beta amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J Neurosci. 1991;11:3783–3793. doi: 10.1523/JNEUROSCI.11-12-03783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kögel D, Deller T, Behl C. Roles of amyloid precursor protein family members in neuroprotection, stress signaling and aging. Exp Brain Res. 2012;217:471–479. doi: 10.1007/s00221-011-2932-4. [DOI] [PubMed] [Google Scholar]
- Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- Koo EH, Squazzo SL, Selkoe DJ, Koo CH. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J. Cell Sci. 1996;109:991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- Lammich S, Kojro E, Postina R, Gilbert S, Pfeiffer R, Jasionowski M, Haass C, Fahrenholz F. Constitutive and regulated alpha-secretase cleavage of Alzheimer’s amyloid precursor protein by a disintegrin metalloprotease. Proc Natl Acad Sci USA. 1999;96:3922–3927. doi: 10.1073/pnas.96.7.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyimo M, Temu RP, Mugula JK. Identification and nutrient composition of indigenous vegetables of Tanzania. Plant Foods Hum Nutr. 2003;58:85–92. doi: 10.1023/A:1024044831196. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Cellular actions of beta-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- Morimoto T, Ohsawa I, Takamura C, Ishiguro M, Kohsaka S. Involvement of amyloid precursor protein in functional synapse formation in cultured hippocampal neurons. J Neurosci Res. 1998;51:185–195. doi: 10.1002/(SICI)1097-4547(19980115)51:2<185::AID-JNR7>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Påhlsson P, Shakin-Eshleman SH, Spitalnik SL. N-linked glycosylation of beta-amyloid precursor protein. Biochem Biophys Res Commun. 1992;189:1667–1673. doi: 10.1016/0006-291X(92)90269-Q. [DOI] [PubMed] [Google Scholar]
- Parvathy S, Hussain I, Karran EH, Turner AJ, Hooper NM. Cleavage of Alzheimer’s amyloid precursor protein by alpha-secretase occurs at the surface of neuronal cells. Biochemistry. 1999;38:9728–9734. doi: 10.1021/bi9906827. [DOI] [PubMed] [Google Scholar]
- Perez RG, Soriano S, Hayes JD, Ostaszewski B, Xia W, Selkoe DJ, Chen X, Stokin GB, Koo EH. Mutagenesis identifies new signals for beta-amyloid precursor protein endocytosis, turnover, and the generation of secreted fragments, including Abeta42. J Biol Chem. 1999;274:18851–18856. doi: 10.1074/jbc.274.27.18851. [DOI] [PubMed] [Google Scholar]
- Ring S, Weyer SW, Kilian SB, Waldron E, Pietrzik CU, Filippov MA, Herms J, Buchholz C, Eckman CB, Korte M, Wolfer DP, Müller UC. The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral, and electrophysiological abnormalities of APP-deficient mice. J Neurosci. 2007;27:7817–7826. doi: 10.1523/JNEUROSCI.1026-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji M, Golde TE, Ghiso J, Cheung TT, Estus S, Shaffer LM, Cai XD, McKay DM, Tintner R, Frangione B, Younkin SG. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- Sinha S, Anderson JP, Barbour R, Basi GS, Caccavello R, Davis D, Doan M, Dovey HF, Frigon N, Hong J, Jacobson-Croak K, Jewett N, Keim P, Knops J, Lieberburg I, Power M, Tan H, Tatsuno G, Tung J, Schenk D, Seubert P, Suomensaari SM, Wang S, Walker D, Zhao J, McConlogue L, John V. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature. 1999;402:537–540. doi: 10.1038/990114. [DOI] [PubMed] [Google Scholar]
- Thinakaran G, Koo EH. Amyloid precursor protein trafficking, processing, and function. J Biol Chem. 2008;283:29615–29619. doi: 10.1074/jbc.R800019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Kirino Y, Suzuki T. Cleavage of Alzheimer’s amyloid precursor protein (APP) by secretases occurs after O-glycosylation of APP in the protein secretory pathway. Identification of intracellular compartments in which APP cleavage occurs without using toxic agents that interfere with protein metabolism. J. Biol. Chem. 1998;273:6277–6284. doi: 10.1074/jbc.273.11.6277. [DOI] [PubMed] [Google Scholar]
- Urrutia R, Henley JR, Cook T, McNiven MA. The dynamins: Redundant or distinct functions for an expanding family of related GTPases? Proc Natl Acad Sci U S A. 1997;94:377–384. doi: 10.1073/pnas.94.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A, König G, Bunke D, Fischer P, Salbaum JM, Masters CL, Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer’s disease A4 amyloid protein. Cell. 1989;57:115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Yoon S-S, Jo SA. Mechanisms of amyloid-β peptide clearance: potential therapeutic targets for Alzheimer’s disease. Biomol. Ther (Seoul) 2012;20:245–255. doi: 10.4062/biomolther.2012.20.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.