Abstract
Background
Cystic fibrosis is an inherited life‐threatening multisystem disorder with lung disease characterized by abnormally thick airway secretions and persistent bacterial infection. Chronic, progressive lung disease is the most important cause of morbidity and mortality in the condition and is therefore the main focus of clinical care and research. Staphylococcus aureus is a major cause of chest infection in people with cystic fibrosis. Early onset, as well as chronic, lung infection with this organism in young children and adults results in worsening lung function, poorer nutrition and increases the airway inflammatory response, thus leading to a poor overall clinical outcome. There are currently no evidence‐based guidelines for chronic suppressive therapy for Staphylococcus aureus infection in cystic fibrosis such as those used for Pseudomonas aeruginosa infection. This is an update of a previously published review.
Objectives
To assess the evidence regarding the effectiveness of long‐term antibiotic treatment regimens for chronic infection with methicillin‐sensitive Staphylococcus aureus (MSSA) infection in people with cystic fibrosis and to determine whether this leads to improved clinical and microbiological outcomes.
Search methods
Trials were identified by searching the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register, MEDLINE, Embase, handsearching article reference lists and through contact with local and international experts in the field. Date of the last search of the Group's Cystic Fibrosis Trials Register: 09 February 2018.
We also searched ongoing trials databases. Date of latest search: 20 May 2018.
Selection criteria
Randomised or quasi‐randomised controlled trials comparing any combinations of topical, inhaled, oral or intravenous antimicrobials used as suppressive therapy for chronic infection with methicillin‐sensitive Staphylococcus aureus compared with placebo or no treatment.
Data collection and analysis
The authors independently assessed all search results for eligibility. No eligible trials were identified.
Main results
The searches identified 58 trials, but none were eligible for inclusion in the current version of this review.
Authors' conclusions
No randomised controlled trials were identified which met the inclusion criteria for this review. Although methicillin‐sensitive Staphylococcus aureus is an important and common cause of lung infection in people with cystic fibrosis, there is no agreement on how best to treat long‐term infection. The review highlights the need to organise well‐designed trials that can provide evidence to support the best management strategy for chronic methicillin‐sensitive Staphylococcus aureus infection in people with cystic fibrosis.
Plain language summary
Treatment for chronic Staphylococcus aureus chest infection in people with cystic fibrosis
Review question
We looked for evidence to see whether long‐term antibiotic treatment for chronic infection with methicillin‐sensitive Staphylococcus aureus (MSSA) in people with cystic fibrosis would lead to improved clinical outcomes and better results for measures of infection
Background
Cystic fibrosis is an inherited condition that causes thick mucus to build up in the lungs leading to persistent infection with bacteria. Methicillin‐sensitive Staphylococcus aureus (also known as MSSA), is the name given to a particular bacteria which is a common cause of lung infection in people with cystic fibrosis. It can cause long‐term infection in people with cystic fibrosis which leads to worsening lung function and poor overall clinical outcome. There are currently no guidelines based on trial results to inform clinicians how best to treat this infection in people with cystic fibrosis. This is an updated version of the review.
Search date
The evidence is current to: 09 February 2018.
Study characteristics
We found 58 trials in our searches, but could not find any which compared different treatments for this condition in people with cystic fibrosis. Therefore, none of these trials were eligible for inclusion in the current version of this review.
Key results
Although methicillin‐sensitive Staphylococcus aureus is an important and common cause of lung infection in people with cystic fibrosis, there is no agreement on how best to treat long‐term infection. The review highlights the need to organise well‐designed trials to decide the best management strategy for chronic methicillin‐sensitive Staphylococcus aureus infection in people with cystic fibrosis.
Background
Please refer to the glossary for an explanation of terms (Appendix 1).
Description of the condition
Cystic fibrosis (CF) is an inherited life‐threatening multisystem disorder caused by a mutation in the cystic fibrosis trans‐membrane conductance regulator (CFTR) gene located on the long arm of chromosome 7 (Lommatzsch 2009). It is the most common autosomal recessive inherited condition in people of Northen European descent, with a gene carrier rate of 1 in 25 people and affecting around 1 in 2500 newborns in the UK and 1 in every 3500 in the USA (Farrell 2008; Ratjen 2003). The most important clinical feature of this genetic abnormality is lung disease, which is characterised by abnormally thick airway secretions, persistent bacterial infection and lung inflammation. Chronic, progressive lung disease is the main cause of morbidity and mortality in CF and is therefore the main focus of clinical care and research (Accurso 2007).
Staphylococcus aureus is a major cause of chest infection in people with CF. It is a ubiquitous commensal bacterium and is a frequent benign coloniser of the anterior nares, being present in approximately 37% of children aged 1 to 19 years (Kuehnert 2006). People with CF carry S aureus mostly in the oropharynx (Ridder‐Schaphorn 2007).
S aureus is categorised into two groups, methicillin‐sensitive Staphylococcus aureus (MSSA) and methicillin‐resistant Staphylococcus aureus (MRSA). (Please note: methicillin is now the international non‐proprietary name of the drug formerly known as meticillin). The reported prevalence of chronic MSSA (defined as three or more recorded isolates) in the UK is about 15% in people with CF, while MRSA (defined as any single isolate) accounted for 2.7% (CF Trust 2016). In the USA the prevalence was far greater with 70% and 26% for MSSA and MRSA, respectively (CF Foundation 2016). The CF Foundation data shows that the prevalence of S aureus has been increasing over the last two decades, some of this increase in prevalence may be due to improvement in the detecting and reporting of S aureus.
Lung infection with S aureus is a frequent problem in people with CF, particularly during the first decade of life (Szaff 1982) and causes chronic, recurrent endobronchial infections (Kahl 2010; Razvi 2009). Early lower airway infection with this organism in young children with CF results in worsening lung function, poorer nutrition and increases the airway inflammatory response (Gangell 2011; Wong 2013). Furthermore, there is an increase in the incidence of co‐infection with Pseudomonas aeruginosa as the individual ages (CF Foundation 2013). The presence of both P aeruginosa and S aureus increases the concentrations of lower airway inflammatory markers and contributes to morbidity (Sagel 2009a).
Description of the intervention
Appropriate antibiotic therapy against the bacterial pathogens in the respiratory tract is vital in managing CF lung disease (Ratjen 2006). Various antibiotics have been used to treat S aureus including oxacillin, amoxicillin‐clavulanic acid, linezolid, vancomycin, rifampicin, cephalosporins and fusidic acid. Ivacaftor, a CFTR potentiator, has also been shown to have antimicrobial properties against S aureus (Hubert 2018; Thakare 2017).
During chronic infection, S aureus is subjected to additional selective pressures resulting from antibiotic interventions, host immunity and the presence of other microbes in the airways.
Currently in the UK, children are prescribed prophylactic anti‐staphylococcal antibiotics (flucloxacillin) from diagnosis until three years of age to reduce the incidence of infection with MSSA. The prophylactic therapy against S aureus has not been adopted by clinical practice guidelines in the USA in anticipation that this may lead to an increase in colonisation of P aeruginosa (Flume 2007; Stutman 2002; Ratjen 2001). However, there is currently no reliable evidence that flucloxacillin prophylaxis increases the incidence of P aeruginosa (Smyth 2012; Smyth 2005). At present there are no antibiotic regimens in place for chronic suppressive therapy.
How the intervention might work
Antibiotics are the mainstay of management of CF. There has been a significant increase in the life expectancy of people with CF, due partly to the aggressive use of antibiotics in the treatment of respiratory infections (Gibson 2003). The aim of treatment in chronic infection is to reduce the microbial load in the lung, thereby reducing lung damage and the rate of worsening of lung function. These outcomes should be associated with improvement in quality of life.
The use of long‐term antibiotics, which includes the use of macrolides like azithromycin and inhaled tobramycin, has been proved to be helpful in managing chronic P aeruginosa infection (Ramsey 1999). Macrolides such as azithromycin also have a potential anti‐staphylococcal activity (in people who are P aeruginosa‐ naive as well as those with chronic infection).
The effects of chronic suppressive antibiotic therapy on the CF lung microbiome and related clinical outcomes are unclear.
Why it is important to do this review
Data on prevalence of S aureus suggests an increase of S aureus prevalence in people with CF in the USA (CF Foundation 2016) and a variable prevalence of chronic S aureus infection in Europe ranging between 15% and 67% (Zolin 2015). However, there continues to be a lack of any description of chronic suppressive therapy for S aureus infection in CF such as that used for P aeruginosa infection. There are no available guidelines on which antibiotics to use for long‐term treatment, the duration of treatment, the mode of delivery of antibiotics and the associated adverse effects; therefore, current clinical practice is greatly divergent. This review is made particularly relevant in the light of new information from recent studies suggesting correlation of growth of MSSA with accelerated decline in lung function (Cogen 2015).
A systematic review of the evidence for maintenance anti‐staphylococcal therapy in CF will pool together any relevant clinical data to permit clinical decision‐making, influence the design of future clinical trials and provide a scientific basis for the development of treatment guidelines.This is an update of a previously published review (Ahmed 2016).
Objectives
To assess the evidence regarding the effectiveness of long‐term antibiotic treatment regimens for chronic MSSA infection in people with CF and to determine whether this leads to improved clinical and microbiological outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs, published or unpublished.
Types of participants
Children and adults with CF who are diagnosed clinically and confirmed by the presence of two disease‐causing mutations, or by a combination of positive sweat test and recognised clinical features of CF and confirmed microbiologic evidence of S aureus (MSSA strains only) in clinically relevant CF respiratory cultures (spontaneous or induced sputum culture, cough or oropharyngeal swab, bronchoalveolar lavage specimen) at least three times over a 12‐month period or more such that 50% of the cultures in a year are positive for MSSA prior to enrolment into the trial. People who are co‐infected with P aeruginosa will also be included.
Types of interventions
Any combinations of topical, inhaled, oral or intravenous (IV) antimicrobials used with the objective of suppressive therapy for chronic infection with S aureus compared with placebo or no treatment.
Types of outcome measures
Primary outcomes
Sputum clearance of S aureus (as determined by negative culture at the end of treatment)
-
Pulmonary function tests
forced expiratory volume at one second (FEV1) per cent (%) predicted or litres
forced vital capacity (FVC) % predicted or litres
any other validated measures of pulmonary function
-
Adverse events
emergence of resistant organisms
other adverse events such as rashes, Stevens‐Johnson type reactions, photosensitivity, tooth discolouration etc
Secondary outcomes
Frequency of respiratory exacerbations (as defined by Fuchs (Fuchs 1994))
-
Hospital admissions secondary to respiratory exacerbation
frequency
duration
School or work attendance
Quality of life (QoL) (as measured by e.g. CF Quality of Life Questionnaire‐Revised (CFQR) (Quittner 2009), or any other validated QoL questionnaire)
Mortality
-
Nutritional parameters (centiles or z scoring)
weight
body mass index (BMI)
height
Chest radiography scores
Days of IV antibiotics
-
New isolation of bacteria
P aeruginosa
MRSA
other
Search methods for identification of studies
There was no restriction on language or publication status.
Electronic searches
We sought relevant trials from the Group's Cystic Fibrosis Trials Register using the terms: (staphylococcus aureus OR mixed infections) AND (maintenance OR unknown). The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group website.
Date of last search of Group's Cystic Fibrosis Trials Register: 09 February 2018.
Searches of ongoing trials databases was undertaken via clinicaltrials.gov (clinicaltrials.gov/), the International Standard Randomised Controlled Trials Number database (www.isrctn.org) and WHO International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/) using the search terms: Cystic fibrosis AND Staphylococcus aureus AND chronic.
Date of latest search: 20 May 2018.
Searching other resources
We will also contact primary authors and research institutions of any future identified trials for unpublished data. We shall contact pharmaceutical companies that manufacture anti‐staphylococcal antibiotics for any information on any relevant trials. If we find any trials in the future, we shall check their reference lists to identify any further relevant trials.
Data collection and analysis
Selection of studies
Both authors (MA, SM) independently applied the selection criteria to determine the trials to be included in the review. There were no disagreements among the authors about the possible inclusion or exclusion of any individual trial.
Data extraction and management
We were not able to include any trials in this version of the review; however, if we include any in the future, we will carry out the following plans for data collection and analysis.
Both authors will use customised data extraction forms for independent data extraction and they will compare outcomes. In case of any disagreements on the suitability of a trial or risk of bias, the authors plan to reach a consensus through discussion.
For long‐term treatment for chronic infection of S aureus, the authors will report outcome data at one month, up to three months, up to six months, up to 12 months and then annually thereafter. For future updates, if outcome data are recorded at other time periods, the authors will consider examining these as well.
In trials where required information is missing, the review authors will contact trial authors to seek this additional information.
Assessment of risk of bias in included studies
Both authors will independently determine the risk of bias using the Cochrane's tool for assessing risk of bias as described in chapter 8 of theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). This tool assists in the assessment of the risk of bias that may be introduced during the process of randomisation; method of allocation concealment; degree of blinding; completeness of outcome data; and selective reporting. The authors will resolve any disagreement over any aspect of the risk of bias for a given trial by discussions.
Measures of treatment effect
The authors plan to assess the distinct types of data that are generated by a wide range of outcome measures using different types of measures. The authors plan to collect data on all participants who took at least the first dose of the drug. For dichotomous data, the authors will summarise the results from the included trials as odds ratios (ORs) with 95% confidence intervals (CIs) according to the Mantel‐Haenszel method. They will assess continuous outcomes (e.g. lung function, QoL) by calculating the mean difference (MD) with 95% CIs. Where trials report multiple measures of the same outcome, such as the percentage change in FEV1 and percentage change in absolute FEV1 volumes, the authors will calculate standardised mean differences (SMDs) with 95% CIs. They will consider absolute changes in FEV1 in context of comparable data being available for each participant before and after the intervention so that the effect size could be calculated.
Unit of analysis issues
In this review, the unit of analysis will be the individual and not the number of episodes of a given event (e.g. infection or adverse reaction). The inclusion criteria used by the authors in this review do not permit the use of cross‐over trials since they are not appropriate in the case of a highly variable and chronic condition such as CF. The review authors will also ensure that the number of participants randomised, and not the number of treatment attempts, is used to calculate CIs in the event of multiple attempts at treating the infection.
Dealing with missing data
The authors will compare trial protocols (where available) to the published report and contact trial authors where such data are missing. When this is not feasible, for continuous variables where standard deviations (SDs) are not published for the mean change from baseline, the authors will impute the missing SD using a correlation coefficient derived from another trial in the same or a different meta‐analysis (where they have been reported) (Higgins 2011b).
Assessment of heterogeneity
With the inclusion of sufficient trials in the review, the authors will assess heterogeneity arising due to clinical and methodological diversity. The authors will attempt to identify statistical heterogeneity by calculating a Chi² test and using this value to compute the I² statistic (Higgins 2003). This measure describes the percentage of total variation across trials that is due to true heterogeneity rather than chance (Higgins 2003). The authors plan to employ a simplified categorisation of heterogeneity where I² values of under 25% are considered to be low, those between 26% and 50% to be moderate, between 51% and 75% to be substantial and those over 75% are considered to be of significant heterogeneity. This test will be used in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Assessment of reporting biases
In order to to assess reporting bias, the authors will compare the published outcome measures of the trials with those in the corresponding protocols (where available) and those mentioned in the 'Methods' sections within the published articles. Where important outcome measures are unaccounted for, they will contact the trial authors for information about the missing data. If the authors are able to include a sufficient number of trials (n = 10), they will use a funnel plot (trial effect against trial size) to assess the publication bias of each trial in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
Data synthesis
The authors will use a fixed‐effect model to analyse the data from the included trials if feasible. However, if they detect at least substantial statistical heterogeneity using the I² statistic (over 50%), the authors will apply a random‐effects method.
Subgroup analysis and investigation of heterogeneity
Where the authors identify substantial heterogeneity among the included studies (I² statistic is at least 50%), it will be further investigated using subgroup analyses as follows:
age of participants (dichotomised into child (under 18 years of age) and adult);
duration of treatment, e.g. up to two weeks, up to one month, up to three months, up to six months, up to one year;
antibiotic therapies used alone or when in combination with other antibiotics and the mode of delivery of antibiotics;
whether or not P aeruginosa was also isolated along with S aureus (co‐infection).
This will be achieved by categorising participants into the related subgroups and conducting meta‐analyses on each of these groups.
Sensitivity analysis
The authors will test the robustness of their results using sensitivity analyses relating to fixed‐effect versus random‐effects analysis, irrespective of the number of studies that are included in the review.
Summary of findings table
The authors will use a summary of findings table to present the following outcomes:
sputum clearance of S aureus;
FEV1;
adverse events;
frequency of respiratory exacerbations;
mortality;
BMI;
isolation of new bacteria.
This table will be used for the main comparison (antibiotics compared to placebo) to present key information about the quality of evidence obtained from trials (using the GRADE approach), the sum of available data on all primary outcomes (listed above) and the magnitude of effect of the interventions examined in this review. If they identify any additional outcomes (desirable or undesirable) during the review process and they deem these to be important, they will include them in the table. The authors will follow the GRADE approach to assess the quality of the body of evidence from the trials, where they will be judged as high, moderate, low or very low. Authors will classify evidence from randomised trials with a low risk of bias as high quality (Schünemann 2011a; Schünemann 2011b).
Results
Description of studies
Results of the search
A total of 81 references to 53 trials were identified from the Cochrane Cystic Fibrosis and Genetic Disorders Group's CF Trials Register. Five references to four additional trials were identified from a separate Embase and MEDLINE search. One trial was identified from the ongoing trials registers.
Details of these trials can be found in the tables (Characteristics of excluded studies). Please also see the PRISMA diagram (Figure 1).
1.
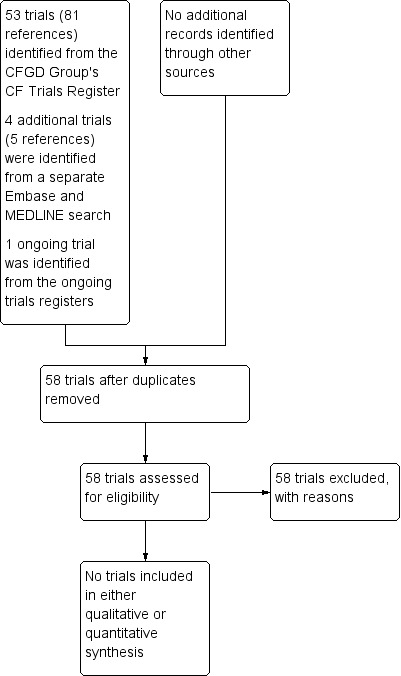
PRISMA Study flow diagram.
Included studies
The authors did not identify any eligible trials for inclusion in the current version of this review.
Excluded studies
All of the 53 trials identified in the search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's CF Trials Register were excluded; one was a trial in MRSA and not MSSA, 16 were pharmacokinetic trials, four trials were tolerability trials and the remaining 32 were excluded because the participants or interventions, or both, were not relevant to our review. None of the 53 trials that were identified by the searches of the Group's CF Trials Register had the primary goal of chronic suppressive therapy for the treatment of established MSSA infection in people with CF (seeCharacteristics of excluded studies).
Of the five additional trials identified, two had relevant participants, interventions and outcomes; but none were included as they were not randomised or controlled trials.
Risk of bias in included studies
No trials were identified which were eligible for inclusion in this review.
Effects of interventions
No trials were identified which were eligible for inclusion in this review.
Discussion
Summary of main results
No RCTs were identified which met the inclusion criteria for this review. Although MSSA is an important and frequently encountered respiratory pathogen in CF, there is no consensus on suppressive therapy of chronic infection.
Quality of the evidence
For this review, there is no available evidence in the form of published RCTs and currently there are only a few observational studies.
Potential biases in the review process
No bias was encountered. We used broad search terms in this review which identified a large number of trials listed on the Cochrane Cystic Fibrosis and Genetic Disorders Group's CF Trials Register and therefore the likelihood of missing eligible trials for inclusion during searches is negligible.
Agreements and disagreements with other studies or reviews
There is a paucity of trials proposing suppressive therapy for chronic infection with MSSA.
The authors identified one small observational non‐randomised and non‐controlled trial, in which 13 individuals with CF and symptoms of chronic bronchopulmonary infection due to MSSA were treated with nebulized ampicillin (age range 3 to 34 years, with a mean (standard deviation) age of 14.8 (7.6) years) (Máiz 2012).This trial did not show eradication of MSSA or evidence of co‐colonisation with P aeruginosa. There was a significant reduction in hospitalisations, sputum volume and purulence in all participants with no statistically significant differences for lung function.
One case report reported a successful long‐term aerosolised ampicillin treatment of a 14‐year‐old girl with chronic symptomatic S aureus lung infection (Máiz 2009).
A cross‐sectional study of microbiomes and clinical outcomes in individuals with CF colonized with MRSA compared to MSSA showed no significant difference in pulmonary function between the two groups, with significant differences in the number of hospitalisations and number of antibiotic courses over one year prior to sputum sample collection (Yenduri 2013). However, maintenance therapy of chronic infection was not reviewed during the trial.
A clinical trial examining the use of Aurexis® ‐ a humanized monoclonal antibody that is designed to combat S aureus (NCT00198289) has been completed, the results have not been published. Results will include its pharmacokinetics, changes in the bacterial load of S aureus in sputum and changes in pulmonary function tests. However, we have already excluded the trial from our review as it is a non‐randomised drug safety and pharmacokinetic trial.
Authors' conclusions
Implications for practice.
There is currently no published evidence from randomised controlled trials (RCTs) to support any regimen for chronic suppressive therapy of methicillin‐sensitive Staphylococcus aureus (MSSA) in people with cystic fibrosis (CF). There are reports of long‐term antibiotic treatment and successful eradication of MSSA; however, there is no evidence of improved patient outcomes. In the absence of any adequately‐powered RCTs, the treatment protocols for those with chronic infection should be based on any available non‐RCT evidence, individual clinician preference and a person's characteristics.
Implications for research.
This review has shown that there is a lack of evidence for maintenance therapy of chronic MSSA infection in people with CF and highlights the need for well‐designed and adequately‐powered RCTs.
We recommend that the following questions need to be answered.
What is the optimal duration of long‐term antibiotic treatment of people with chronic MSSA infection in CF?
Does long‐term suppressive therapy for chronic MSSA infection in CF improve the prognosis in these individuals?
Does long‐term antibiotic treatment of people with chronic MSSA infection in CF have any adverse effects (i.e. emergence of resistant organisms, colonisation with other pathogens including Pseudomonas aeruginosa, MRSA)?
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2018 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register identified five new references which were potentially eligible for inclusion in the review. Three references were additional references to already excluded studies (Dasenbrook 2015; Geller 2011b; Prayle 2016) and two new studies each with a single reference have been excluded (Khorasani 2009; Sharma 2016). Additional searches identified a single study which was excluded (Autry 2016). |
| 23 July 2018 | New citation required but conclusions have not changed | We have not included any new data in this update, hence our conclusions remain the same. |
Acknowledgements
We gratefully acknowledge the support of Nikki Jahnke for her critical review and management of the review process.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Glossary
| Term | Explanation |
| autosomal recessive | autosomal recessive is one of several ways that a trait, disorder, or disease can be passed down through family genes; in an autosomal recessive disorder two copies of an abnormal gene must be present in order for the disease or trait to develop |
| bacterial pathogen | a bacteria that can produce disease |
| bronchoalveolar lavage | a procedure in which a bronchoscope (a tube) is passed through the mouth or nose into the lungs and fluid is squirted into a small part of the lung and then collected for examination |
| chi‐squared test | a statistical test to ascertain whether the association between two variables is true |
| correlation coefficient | a statistical measure of the degree of association between two continuous variables |
| endobronchial | located within a bronchus (one of two large air tubes that begins at the end of the windpipe and branch into the lungs) |
| GRADE | GRADE is a systematic and explicit approach to making judgements about quality of evidence and strength of recommendations |
| microbiome | the micro‐organisms found in a particular body or part of the body |
| morbidity | illness, diseased state |
| oropharynx | the part of the throat that is at the back of the mouth |
| oropharyngeal swab | a swab taken from the throat at the back of the mouth |
| photosensitivity | how the skin reacts to light |
| prophylactic | preventative |
| pulmonary exacerbations | flare ups of lung disease |
| respiratory cultures | a test to detect and identify bacteria or fungi that infect the lungs or breathing passages |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adeboyeku 2001 | Not a relevant intervention ‐ tolerability trial of differing dosages of nebulised colistin. |
| Amelina 2000 | Not a relevant intervention or participants ‐ difference in quality of life between home versus hospital IV treatment. No MSSA. |
| App 2000 | Pharmacokinetic trial. |
| Autry 2016 | Pharmacokinetic study, no MSSA |
| Carswell 1987 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Chua 1990 | Not a relevant intervention ‐ used differing tonicities of inhaled antibiotics to assess airway responsiveness. |
| Coates 2011 | Pharmacokinetic trial. |
| Conway 1996 | Not relevant, no chronic MSSA. |
| Cooper 1985 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Dalbøge 2013 | Observational cohort study |
| Dasenbrook 2015 | Treatment for chronic MRSA not MSSA. |
| Davis 1987 | Pharmacokinetic trial. |
| Davis 1990 | Safety and efficacy study. No microbiologic correlation. |
| Degg 1996 | Not a relevant intervention or relevant participants ‐ trial of long‐term effects of gentamicin on hearing. |
| Dodd 1997 | Not relevant ‐ tolerability trial of nebulised colistin. |
| Dodd 1998 | Not a relevant intervention or relevant participants ‐ a compliance study. |
| Geller 2004 | Pharmacokinetic trial. |
| Geller 2008 | Pharmacokinetic trial. |
| Geller 2011a | Pharmacokinetic and tolerability study. |
| Geller 2011b | Not relevant participants ‐ trial of chronic P. aeruginosa treatment, no MSSA and no information about chronicity of S. aureus infection. |
| Goldfarb 1986 | Pharmacokinetic trial. |
| Gulliver 2003 | Not a relevant intervention or relevant participants ‐ testing whether nebulised IV tobramycin solution induces cough or bronchospasm, or both. |
| Heininger 1993 | Not relevant participants. |
| Hjelte 1988 | Not relevant participants ‐ investigated affect of home IV antibiotics for P. aeruginosa on quality of life. |
| Huang 1979 | Not relevant participants – no chronic MSSA and trial of P. aeruginosa treatment. |
| Junge 2001 | Not a relevant intervention or relevant participants ‐ effects of IV tobramycin on hearing. No MSSA. |
| Kapranov 1995 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Keel 2011 | Pharmacokinetic trial. |
| Khorasani 2009 | Not relevant ‐ No MSSA |
| Knight 1979 | Not relevant ‐ trial of P. aeruginosa treatment. |
| Kun 1984 | Not relevant ‐ no chronic MSSA. |
| Kuti 2004 | Pharmacokinetic trial. |
| Labiris 2004 | Not a relevant intervention or relevant participants, no microbiological correlation, no MSSA. |
| Loening ‐Bauke 1979 | Not a relevant intervention ‐ used cephalexin as prophylaxis. |
| Máiz 2009 | A case report of one 14‐year old boy. |
| Máiz 2012 | Observational study. |
| Nathanson 1985 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| NCT00198289 | Non randomised pharmacokinetic trial |
| Pai 2006 | Pharmacokinetic trial. |
| Popa 2001 | Not relevant – no MSSA. |
| Postnikov 2000 | Not a relevant intervention or relevant participants – an efficacy and tolerability study of Pefloxacin. No chronic MSSA. |
| Postnikov 2001a | Not a relevant intervention or relevant participants ‐ trial on chondrotoxicity of fluoroquinolones. No MSSA. |
| Postnikov 2001b | Not a relevant intervention or relevant participants ‐ study of arthrotoxicity of fluoroquinolones. |
| Prayle 2016 | MSSA not present, pharmacokinetic trial. |
| Ramstrom 2000 | Not a relevant intervention or relevant participants ‐ a compliance study. |
| Roberts 1993 | Pharmacokinetic trial. |
| Romano 1992 | Not relevant participants ‐ trial of P. aeruginosa treatment. |
| Rosenfeld 2006 | Pharmacokinetic study |
| Sagel 2009 | Not a relevant intervention, tolerability trial. |
| Salh 1992 | Not relevant participants ‐ MSSA not required for entry into trial. |
| Sharma 2016 | Not relevant ‐ no MSSA |
| Singh 2013 | No chronic MSSA. No relevant outcomes. |
| Smith 1997 | Pharmacokinetic trial. |
| Stutman 1987 | Pharmacokinetic trial. Not relevant participants, chronic MSSA not a requirement for entry. |
| Vitti 1975 | Pharmacokinetic trial. |
| Willekens 2013 | Not relevant participants, no relevant outcomes |
| Wood 1996 | Not a relevant intervention or participants, trial of toxic effects of long‐term gentamicin therapy. |
| Yenduri 2013 | Participants in the trial and outcomes were not relevant to review. |
IV: intravenous MRSA: methicillin‐resistant Staphylococcus aureus MSSA: methicillin‐sensitive Staphylococcus aureus P. aeruginosa: Pseudomonas aeruginosa S. aureus: Staphylococcus aureus
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO WILL UNDERTAKE THE TASK? |
| Protocol stage: draft the protocol | Molla Imaduddin Ahmed Saptarshi Mukherjee |
| Review stage: select which trials to include | Molla Imaduddin Ahmed Saptarshi Mukherjee |
| Review stage: extract data from trials | Molla Imaduddin Ahmed Saptarshi Mukherjee |
| Review stage: enter data into RevMan | Molla Imaduddin Ahmed Saptarshi Mukherjee |
| Review stage: carry out the analysis | Molla Imaduddin Ahmed Saptarshi Mukherjee |
| Review stage: interpret the analysis | Molla Imaduddin Ahmed Saptarshi Mukherjee |
| Review stage: draft the final review | Molla Imaduddin Ahmed Saptarshi Mukherjee |
| Update stage: update the review | Molla Imaduddin Ahmed Saptarshi Mukherjee |
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Molla Imaduddin Ahmed declares no known potential conflict of interest.
Saptarshi Mukherjee declares no known potential conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Adeboyeku 2001 {published data only}
- Adeboyeku DU, Agent P, Jackson V, Hodson M. A double blind randomised study to compare the safety and tolerance of differing concentrations of nebulised colistin administered using HaloLite in cystic fibrosis (CF) patients [abstract]. Pediatric Pulmonology 2001;Suppl 22:288. [CENTRAL: 362164; CFGD Register: PI165 ; CRS: 5500100000001976] [Google Scholar]
Amelina 2000 {published data only}
- Amelina E, Senkevich N, Cherniak A, Cherniaev A, Chuchalin A. Home intravenous therapy in adult cystic fibrosis patients. The impact on lung fuction and quality of life [abstract]. European Respiratory Journal 2000;16(Suppl 31):123s. [CENTRAL: 415175; CFGD Register: PI181 ; CRS: 5500100000002262] [Google Scholar]
App 2000 {published data only}
- App EM, Huls G, Bittner‐Dersch P, Stolz S, Lindemann H, Matthys H. Impared lung function influences the serum concentration of inhaled drugs in cystic fibrosis [abstract]. Pediatric Pulmonology 2000;Suppl 20:279‐80. [CENTRAL: 315347; CFGD Register: PI156b ; CRS: 5500100000001731] [Google Scholar]
- Huls G, App EM, Bittner‐Dersch P, Stolz S, Lindemann H. Impaired lung function influences the serum concentration of inhaled drugs in cystic fibrosis [abstract]. 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:177. [CENTRAL: 302957; CFGD Register: PI156a ; CRS: 5500100000001691]
Autry 2016 {published data only}
- Autry EB, Rybak JM, Leung NR, Gardner BM, Burgess DR, Anstead MI, et al. Pharmacokinetic and pharmacodynamic analyses of ceftaroline in adults with cystic fibrosis. Pharmacotherapy 2016;36(1):13‐8. [DOI] [PubMed] [Google Scholar]
Carswell 1987 {published data only}
- Carswell F, Ward C, Cook DA, Speller DC. A controlled trial of nebulized aminoglycoside and oral flucloxacillin versus placebo in the outpatient management of children with cystic fibrosis. British Journal of Diseases of the Chest 1987;81(4):356‐60. [CENTRAL: 53621; CFGD Register: PI54 ; CRS: 5500100000000343; PUBMED: 3329531] [DOI] [PubMed] [Google Scholar]
Chua 1990 {published data only}
- Chua H, Collis G, Souef P. Bronchial response of children with cystic fibrosis to nebulised antibiotics [abstract]. Australian and New Zealand Journal of Medicine 1990;20:537. [CENTRAL: 320152; CFGD Register: PI66b ; CRS: 5500100000001769] [Google Scholar]
- Chua HL, Collis GG, Souef PN. Bronchial response to nebulized antibiotics in children with cystic fibrosis. European Respiratory Journal 1990;3(10):1114‐6. [CENTRAL: 74980; CFGD Register: PI66a ; CRS: 5500100000000468; PUBMED: 2090472] [PubMed] [Google Scholar]
Coates 2011 {published data only}
- Coates AL, Denk O, Leung K, Ribeiro N, Chan J, Green M, et al. Higher tobramycin concentration and vibrating mesh technology can shorten antibiotic treatment time in cystic fibrosis. Pediatric Pulmonology 2011;46(4):401‐8. [CENTRAL: 786190; CFGD Register: PI241b ; CRS: 5500100000006333] [DOI] [PubMed] [Google Scholar]
- Denk O, Coates AL, Keller M, Leung K, Green M, Chan J, et al. Lung delivery of a new tobramycin nebuliser solution (150mg/1.5ml) by an investigational eFlow® nebuliser is equivalent to TOBI® but in a fraction of time [abstract]. Journal of Cystic Fibrosis 2009;8 Suppl 2:S66, Abstract no: 264. [CENTRAL: 794467; CFGD Register: PI241c ; CRS: 5500100000003576] [Google Scholar]
- Keller M, Coates AL, Griese M, Denk O, Schierholz J, Knoch M. In‐vivo data support equivalent therapeutic efficacy of a new tobramycin inhalation solution (150mg/1.5ml) administered by the eFlow® electronic nebuliser compared to TOBI® in the PARI LC PLUS® [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S22, Abstract no: 84. [CENTRAL: 794286; CFGD Register: PI241a ; CRS: 5500100000003569] [Google Scholar]
Conway 1996 {published data only}
- Conway SP. Ceftazidime 3G BD is as effective as ceftazidime 2G TDS in the treatment of respiratory exacerbations in cystic fibrosis [abstract]. Israel Journal of Medical Sciences 1996;32(Suppl):S256. [CENTRAL: 291256; CFGD Register: PI78 ; CRS: 5500100000001321] [Google Scholar]
Cooper 1985 {published data only}
- Cooper DM, Harris M, Mitchell I. Comparison of intravenous and inhalation antibiotic therapy in acute pulmonary deterioration in cystic fibrosis [abstract]. American Review of Respiratory Disease 1985;131:A242. [CENTRAL: 208500; CFGD Register: PI129 ; CRS: 5500100000001084] [Google Scholar]
Dalbøge 2013 {published data only}
- Dalbøge CS, Pressler T, Høiby N, Nielsen KG, Johansen HK. A cohort study of the Copenhagen CF Centre eradication strategy against Staphylococcus aureus in patients with CF. Journal of Cystic Fibrosis 2013;12(1):42‐8. [DOI: 10.1016/j.jcf.2012.06.005] [DOI] [PubMed] [Google Scholar]
Dasenbrook 2015 {published data only}
- Dasenbrook EC. Emerging therapies in cystic fibrosis: aerovanc for the treatment of chronic MRSA [abstract]. Pediatric Pulmonology 2015;50 Suppl 41:149, Abstract no: S11.4. [CENTRAL: 1092196; CFGD Register: PI289a; CRS: 5500135000001385] [Google Scholar]
- Marich C, Lord J, Dasenbrook EC, Flume PA, Jouhikainen T. Pharmacokinetics of vancomycin in plasma and sputum following pulmonary administration in cystic fibrosis patients with persistent methicillin‐resistant staphylococcus aureus infection. Pediatric Pulmonology 2016;51 Suppl 45:298‐99. [Abstract no.: 282; CFGD Register: PI289b] [Google Scholar]
Davis 1987 {published data only}
- Davis RL, Koup JR, Williams Warren J, Weber A, Heggen L, Stempel D, et al. Pharmacokinetics of ciprofloxacin in cystic fibrosis. Antimicrobial Agents and Chemotherapy 1987;31(6):915‐9. [CENTRAL: 49449; CFGD Register: PI50 ; CRS: 5500100000000303; PUBMED: 3619423] [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1990 {published data only}
- Davis S, Davis P, Mather F, Tankersly P, Waring W. A randomized trial of home intravenous antibiotic therapy (HIVAT) in cystic fibrosis (CF) : Short‐term safety and efficacy [abstract]. Pediatric Pulmonology 1990;Suppl 5:245. [CENTRAL: 291272; CFGD Register: PI91a ; CRS: 5500100000001336] [Google Scholar]
- Davis S, Davis P, Mather F, Waring W, Home IVASG. A randomized trial of home intravenous antibiotic therapy (HIVAT) in cystic fibrosis (CF): Short‐term psychological effects [abstract]. Pediatric Pulmonology 1990;Suppl 5:281‐2. [CENTRAL: 291273; CFGD Register: PI91b ; CRS: 5500100000001337] [Google Scholar]
Degg 1996 {published data only}
- Degg C, Mulheran M. The effect of frequent exposure to gentamicin on distortion product OAEs in patients with cystic fibrosis. British Journal of Audiology 1996;30(2):99‐100. [CENTRAL: 385714; CFGD Register: PI167 ; CRS: 5500100000002095] [Google Scholar]
Dodd 1997 {published data only}
- Dodd M, Maddison J, Abbott J, Webb AK. The effect of the tonicity of nebulised colistin on lung function in adults with cystic fibrosis [abstract]. 18th European Cystic Fibrosis Conference; 1993 May 21‐26; Madrid, Spain. 1993:121. [CENTRAL: 291278; CFGD Register: PI100a ; CRS: 5500100000001342]
- Dodd ME, Abbott J, Maddison J, Moorcroft AJ, Webb AK. Effect of tonicity of nebulised colistin on chest tightness and pulmonary function in adults with cystic fibrosis. Thorax 1997;52(7):656‐8. [CENTRAL: 142169; CFGD Register: PI100b; CRS: 5500100000000830; PUBMED: 9246141] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd ME, Maddison J, Abbot J, Webb AR. The effect of the tonicity of nebulised colistin on chest tightness and lung function in adults with cystic fibrosis [abstract]. European Respiratory Journal 1993;6(Suppl 17):515s. [CENTRAL: 393381; CFGD Register: PI100c ; CRS: 5500100000002149] [Google Scholar]
Dodd 1998 {published data only}
- Dodd ME, Haworth CS, Moorcroft AJ, Miles J, Webb AK. Is medicine evidence‐based when there is discrepancy between patient reported and objective measures of compliance in clinical trials? [abstract]. Pediatric Pulmonology 1998;26(Suppl 17):389‐90. [CENTRAL: 291279; CFGD Register: PI237 ; CRS: 5500100000001343] [Google Scholar]
Geller 2004 {published data only}
- Geller DE, Rodriguez CA, Howenstine M, Murphy T, Voter K, Nickerson B, et al. The effects of doubling concentration of tobramycin solution for inhalation on pharmacokinetics (PK), safety and delivery time in patients with cystic fibrosis (CF) [abstract]. American Journal of American Journal of Respiratory and Critical Care Medicine 2004;169(7):A391. [CENTRAL: 486943; CFGD Register: PI183a ; CRS: 5500100000002627] [Google Scholar]
- Rosenfeld M, Geller DE, Rodriguez CA, Howenstine M, Konstan M, Ordonez C, et al. Serum pharmacokinetics of two preparations of tobramycin solution for inhalation in young cystic fibrosis patients [abstract]. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A386. [CENTRAL: 495351; CFGD Register: PI183b ; CRS: 5500100000002644] [Google Scholar]
Geller 2008 {published data only}
- Geller DE, Flume P, Schwab R, Fornos P, Conrad DJ, Morgan E, et al. A phase 1 safety, tolerability and pharmacokinetic (PK) study of MP‐376 (levofloxacin solution for inhalation) in stable cystic fibrosis (CF) patients [abstract]. Pediatric Pulmonology 2008;43 Suppl 31:315, Abstract no: 321. [CENTRAL: 744131; CFGD Register: PI210b; CRS: 5500100000003459] [Google Scholar]
- Griffith DC, Hansen C, Pressler T, Balchen T, Jensen TJ, Geller DE, et al. Single‐dose pharmacokinetics of aerosol MP‐376 (levofloxacin solution for inhalation) in cystic fibrosis patients: PK‐PD implications [abstract]. Journal of Cystic Fibrosis 2008;7(Suppl 2):S26. [CENTRAL: 643120; CFGD Register: PI210a ; CRS: 5500100000003230] [Google Scholar]
- Kearns GL, Rubino CM, Griffith DC, Geller DE, Forrest A, Bhavnani SM, et al. Levofloxacin pharmacokinetics (PK) after administration of MP‐376 (Levofloxacin inhalation solution; Aeroquin) in children with cystic fibrosis [abstract]. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2011;10 Suppl 1:S23, Abstract no: 88. [CENTRAL: 1053535; CFGD Register: PI210d; CRS: 5500133000000015] [Google Scholar]
- Stockmann C, Hillyard B, Ampofo K, Spigarelli MG, Sherwin CM. Levofloxacin inhalation solution for the treatment of chronic Pseudomonas aeruginosa infection among patients with cystic fibrosis. Expert Review of Respiratory Medicine 2014:1‐10. [CENTRAL: 1053533; CFGD Register: PI210c; CRS: 5500131000000314; JID:: 101278196; PUBMED: 25417708] [DOI] [PubMed] [Google Scholar]
Geller 2011a {published data only}
- Geller DE, Flume PA, Griffith DC, Morgan E, White D, Loutit J, et al. Pharmacokinetics (PK) of aerosol MP‐376 (aeroquin; levofloxacin inhalation solution) in CF patients [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S23, Abstract no: 87. [CENTRAL: 848915; CFGD Register: PI254a ; CRS: 5500100000010626] [Google Scholar]
- Geller DE, Flume PA, Griffith DC, Morgan E, White D, Loutit JS, et al. Pharmacokinetics and safety of MP‐376 (levofloxacin inhalation solution) in cystic fibrosis subjects. Antimicrobial Agents and Chemotherapy 2011;55(6):2636‐40. [CENTRAL: 801007; CFGD Register: PI254b ; CRS: 5500100000010627] [DOI] [PMC free article] [PubMed] [Google Scholar]
Geller 2011b {published data only}
- Conrad D, Flume P, Sindel L, Andrews S, Morgan L, Loutit J, et al. Phase 2b study of inhaled MP‐376 (Aeroquin, levofloxacin inhalation solution) in stable cystic fibrosis (CF) patients with chronic Pseudomonas Aeruginosa (PA) lung infection. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts). [CFGD Register: PI240g] [Google Scholar]
- Flume P, Geller DE, Sindel L, Staab D, Fischer R, Riethmuller J, et al. Effects of inhaled MP‐376 (aeroquin, levofloxacin inhalation solution) on lung function in stable cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa (PA) lung infection [abstract]. Journal of Cystic Fibrosis 2010;9 Suppl 1:S23, Abstract no:86. [CENTRAL: 774683; CFGD Register: PI240a ; CRS: 5500100000003504] [Google Scholar]
- Flume P, VanDevanter DR, Cohen F, Fleming R, Elborn JS. Safety profile of levofloxacin inhalation solution from 3 controlled cystic fibrosis trials [abstract]. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S87, Abstract no: 117. [CENTRAL: 1077213; CFGD Register: PI240f // PI283c ; CRS: 5500135000001302] [Google Scholar]
- Flume PA, Geller DE, Loutit JS, Dudly MN, Conrad D, Mpex 204S group. Effects of inhaled MP‐376 (Aeroquin™ levofloxacin inhalation solution) on cystic fibrosis patients with both Staphylococcus aureus (SA) and Pseudomonas aeruginosa (PA) lung infection [abstract]. Journal of Cystic Fibrosis 2011;10 Suppl 1:S22, Abstract no: 87. [CENTRAL: 849020; CFGD Register: PI240b ; CRS: 5500100000010582] [Google Scholar]
- Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ. Levofloxacin inhalation solution (MP‐376) in patients with cystic fibrosis with Pseudomonas aeruginosa. American Journal of Respiratory and Critical Care Medicine 2011;183(11):1510‐6. [CENTRAL: 800852; CFGD Register: PI240d ; CRS: 5500100000010625] [DOI] [PubMed] [Google Scholar]
- Geller DE, Flume PA, Staab D, Fischer R, Loutit JS, Conrad DJ. Online supplemental methods to "Levofloxacin inhalation solution (MP‐376) in patients with cystic fibrosis with Pseudomonas aeruginosa" [online]. American Journal of Respiratory and Critical Care Medicine 2011;183(11):1510‐1516 online. [CENTRAL: 1073292; CFGD Register: PI240e; CRS: 5500135000000004] [DOI] [PubMed] [Google Scholar]
- Geller DFPA, Sindel L, Staab D, Fischer R, Loutit J, Conrad D. Effects of inhaled MP‐376 (aeroquin, levofloxacin inhalation solution) on the need for other anti‐pseudomonal antimicrobials in stable CF patients with chronic pseudomonas aeruginosa lung infection [abstract]. Pediatric Pulmonology 2010;45 Suppl 33:301, Abstract no: 232. [CENTRAL: 848914; CFGD Register: PI240c ; CRS: 5500100000010624] [Google Scholar]
Goldfarb 1986 {published data only}
- Goldfarb J, Wormser GP, Inchiosa MAJ, Guideri G, Diaz M, Gandhi R, et al. Single‐dose pharmacokinetics of oral ciprofloxacin in patients with cystic fibrosis. Journal of Clinical Pharmacology 1986;26(3):222‐6. [CENTRAL: 42124; CFGD Register: PI44 ; CRS: 5500100000000255; PUBMED: 2937812] [DOI] [PubMed] [Google Scholar]
Gulliver 2003 {published data only}
- Gulliver T, Wilson S, Williams G, Harris M, Cooper D. Nebulized tobramycin (intravenous solution) is tolerated without inducing cough and wheeze in cystic fibrosis patients [abstract]. Proceedings of the Thoracic Society of Australia & New Zealand Annual Scientific Meeting; 2003 April 4‐9; Adelaide, Australia. 2003:Abstract no: P139. [CENTRAL: 593086; CFGD Register: PI184 ; CRS: 5500100000003041]
Heininger 1993 {published data only}
- Heininger U, Bowing B, Stehr K, Solbach W. Aminoglycosides in patients with mucoviscidosis and pulmonary exacerbation. Comparison of once or three times daily administration [Aminoglykoside bei Patienten mit Mukoviszidose und pulmonaler Exazerbation: Vergleich von Einmal‐ und Dreimalgabe]. Klinische Padiatrie 1993;205(1):18‐22. [CENTRAL: 91490; CFGD Register: PI74 ; CRS: 5500100000000564; PUBMED: 8445848] [DOI] [PubMed] [Google Scholar]
Hjelte 1988 {published data only}
- Hjelte L, Widen B, Malmborg AS, Freyschuss U, Strandvik B. [Intravenous administration of antibiotics at home in patients with cystic fibrosis improves quality of life] [Intravenos antibiotikabehandling i hemmet vid cystisk fibros ger okad livskvalitet]. Lakartidningen 1988;85(18):1614‐7. [CENTRAL: 53458; CFGD Register: PI206 ; CRS: 5500100000000341; PUBMED: 3283482] [PubMed] [Google Scholar]
Huang 1979 {published data only}
- Huang N, Palmer J, Schidlow D, Hsuan F, Hsu C, Goldberg M, et al. Evaluation of antibiotic therapy in patients with cystic fibrosis [abstract]. Chest 1979;76(3):354‐5. [CENTRAL: 291362; CFGD Register: PI113a ; CRS: 5500100000001409] [Google Scholar]
- Huang NN, Palmer J, Braverman S, Keith HH, Schidlow D. Therapeutic efficacy of ticarcillin and carbenicillin in patients with cystic fibrosis: a double blind study [abstract]. 23rd Annual Meeting Cystic Fibrosis Club Abstracts; 1982 May 14; Washington D.C. 1982:124. [CENTRAL: 291363; CFGD Register: PI113b ; CRS: 5500100000001410]
Junge 2001 {published data only}
- Junge S, Kruger K, Schonweiler R, Ptok M, Ballmann M. Once daily dosage of intravenous trobramycin ‐ increased risk for cochlea damage in children with cystic fibrosis (CF)? [abstract]. Pediatric Pulmonology 2001;Suppl 22:291. [CENTRAL: 362190; CFGD Register: PI160b ; CRS: 5500100000001990] [Google Scholar]
- Kruger K, Junge S, Schonweiler R, Ptok M, Ballmann M. Once daily dosage of intravenous trobramycin in patients with cystic fibrosis ‐ increased risk for cochlea damage? [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P191. [CENTRAL: 354425; CFGD Register: PI160a ; CRS: 5500100000001945]
Kapranov 1995 {published data only}
- Kapranov NI, Belousov YB, Kashyrskaya NY, Smirnova EY. Quinoline therapy in children with cystic fibrosis [abstract]. 20th European Cystic Fibrosis Conference; 1995 June 18‐21; Brussels, Belgium. 1995:P19. [CENTRAL: 291377; CFGD Register: PI104 ; CRS: 5500100000001422]
Keel 2011 {published data only}
- Keel RA, Schaeftlein A, Kloft C, Pope JS, Knauft RF, Muhlebach M, et al. Pharmacokinetics of intravenous and oral linezolid in adults with cystic fibrosis. Antimicrobial Agents and Chemotherapy 2011;55(7):3393‐8. [CENTRAL: 800442; CFGD Register: PI251 ; CRS: 5500100000005261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khorasani 2009 {published data only}
- Khorasani EN, Mansouri F. Effect of zinc supplementation on respiratory infections in children with cystic fibrosis. European Respiratory Society Annual Congress; 2009; Sept 12‐16; Vienna, Austria. 2009:722s. [Abstract no.: P4032; CFGD Register: GN255]
Knight 1979 {published data only}
- Knight RK, Batten JC, Mearns M. A double blind trial of cephalexin in cystic fibrosis patients with pseudomonas in the sputum [abstract]. 9th Meeting European Working Group for Cystic Fibrosis; 1979 June 12‐13; Noordwijkerhout, the Netherlands. 1979:52. [CENTRAL: 291389; CFGD Register: PI124 ; CRS: 5500100000001431]
Kun 1984 {published data only}
- Kun P, Landau LI, Phelan PD. Nebulized gentamicin in children and adolescents with cystic fibrosis. Australian Paediatric Journal 1984;20:43‐5. [CENTRAL: 208154; CFGD Register: PI106 ; CRS: 5500100000001047] [DOI] [PubMed] [Google Scholar]
Kuti 2004 {published data only}
- Kuti J, Nightingale C, Knauft R, Nicolau D. Pharmacokinetics (PK) of continuously infused meropenem (MEM) in adults with cystic fibrosis (CF) [abstract]. American Journal of Respiratory and Critical Care Medicine 2003:Poster: B2. [CENTRAL: 431304; CFGD Register: PI174a ; CRS: 5500100000002301] [Google Scholar]
- Kuti JL, Nightingale CH, Knauft RF, Nicolau DP. Pharmacokinetic properties and stability of continuous‐infusion meropenem in adults with cystic fibrosis. Clinical Therapeutics 2004;26(4):493‐501. [CENTRAL: 468644; CFGD Register: PI174b ; CRS: 5500100000002549; PUBMED: 15189746] [DOI] [PubMed] [Google Scholar]
Labiris 2004 {published data only}
- Labiris R, Freitag A, Pratt B, Efthimiadis A, Hargreave F, Dolovich M. Does inhalation of preservatives from IV tobramycin preparations (TOB) cause airway inflammation? [abstract]. American Journal of Respiratory and Critical Care Medicine 2004;169(7):A307. [CENTRAL: 494424; CFGD Register: PI182 ; CRS: 5500100000002643] [Google Scholar]
Loening ‐Bauke 1979 {published data only}
- Loening Baucke VA, Mischler E, Myers MG. A placebo‐controlled trial of cephalexin therapy in the ambulatory management of patients with cystic fibrosis. Journal of Pediatrics 1979;95(4):630‐7. [CENTRAL: 21139; CFGD Register: PI19b ; CRS: 5500100000000098; PUBMED: 383934] [DOI] [PubMed] [Google Scholar]
- Loening‐Baucke VA, Mischler EH, Myers MG. Cephalexin in cystic fibrosis: a placebo‐controlled study [abstract]. Pediatric Research 1978;12(4 Pt 2):495. [CENTRAL: 189031; CFGD Register: PI19c ; CRS: 5500100000000980; EMBASE: 1978335753] [Google Scholar]
- Loening‐Bauke V, Mischler EH, Myers MG. Cephalexin compared to placebo in the management of patients with cystic fibrosis [abstract]. 19th Cystic Fibrosis Club Abstracts; 1978. 1978:69. [CENTRAL: 291430; CFGD Register: PI19a ; CRS: 5500100000001461]
Máiz 2009 {published data only}
- Máiz L, Lamas A, Fernández‐Olmos A, Suárez L, Cantón R. Unorthodox long‐term aerosolized ampicillin use for methicillin‐susceptible Staphylococcus aureus lung infection in a cystic fibrosis patient. Pediatric Pulmonology 2009;44(5):512‐5. [DOI: 10.1002/ppul.20983] [DOI] [PubMed] [Google Scholar]
Máiz 2012 {published data only}
- Lamas A, Maiz L, Campo R, Castro M, Gutierrez‐Alonso D, Giron R, et al. Long‐term inhaled ampicillin for the treatment of methicillin‐susceptible Staphylococcus aureus bronchopulmonary infection in cystic fibrosis patients[abstract]. Journal of Cystic Fibrosis 2011;10 Suppl 1:S55. [Google Scholar]
- Máiz L, Campo R, Castro M, Gutiérrez D, Girón R, Cantón Moreno R. Maintenance treatment with inhaled ampicillin in patients with cystic fibrosis and lung infection due to methicillin‐sensitive Staphylococcus aureus. Archivos de Bronconeumologia 2012;48(10):384. [DOI: 10.1016/j.arbr.2012.07.012] [DOI] [PubMed] [Google Scholar]
Nathanson 1985 {published data only}
- Nathanson I, Cropp GJA, Li P, Neter E. Effectiveness of aerosolized gentamicin in cystic fibrosis (CF) [abstract]. Cystic Fibrosis Club Abstracts; 1985. 1985; Vol. 28:145. [CENTRAL: 291475; CFGD Register: PI130; CRS: 5500100000001502]
NCT00198289 {published data only}
- Aurexis® in cystic fibrosis subjects chronically colonized with Staphylococcus aureus in their lungs. clinicaltrials.gov/ct2/show/NCT00198289 (accessed 29 September 2015). [clinicaltrials.gov: NCT00198289]
Pai 2006 {published data only}
- Pai MP, Allen SE, Amsden GW. Altered steady state pharmacokinetics of levofloxacin in adult cystic fibrosis patients receiving calcium carbonate. Journal of Cystic Fibrosis 2006;5(3):153‐7. [CENTRAL: 570220; CFGD Register: PI199 ; CRS: 5500100000002864; EMBASE: 2006340493; PUBMED: 16481224] [DOI] [PubMed] [Google Scholar]
Popa 2001 {published data only}
- Popa I, Pascu C, Popa Z, Pop L. Forced ionization of the indoor air ‐ an additional method in the treatment of the respiratory disease in cystic fibrosis [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P69. [CENTRAL: 354440; CFGD Register: PI226 ; CRS: 5500100000001952]
Postnikov 2000 {published data only}
- Postnikov SS, Semykin SI, Kapranov NI, Perederko LV, Polikarpova SV, Khamidullina KF. Evaluation of tolerance and efficacy of pefloxacin in the treatment and prevention of severe infections in children with mucoviscidosis and aplastic anemia [Otsenka effektivnosti i perenosimosti pefloksatsina pri lechenii i profilaktike tiazhelykh infektsii u detei s mukovistsidozom i aplasticheskoi anemiei]. Antibiotiki i Khimioterapiia [Antibiotics and Chemoterapy] 2000;45(8):25‐30. [CENTRAL: 372408; CFGD Register: PI171 ; CRS: 5500100000002038; PUBMED: 10989721] [PubMed] [Google Scholar]
Postnikov 2001a {published data only}
- Postnikov SS, Semykin SJ, Najimov VP. Safety of fluoroquinolones in children [abstract]. 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P213. [CENTRAL: 354442; CFGD Register: PI162 ; CRS: 5500100000001953]
Postnikov 2001b {published data only}
- Postnikov SS, Semiakin SI, Nazhimov VP, Kapranov NI. Comparative yearly growth rate of children with mucoviscidosis treated and not treated with ciprofloxacin:clinicomorphological comparisons [Cravnitel'naiia godovaia skorost' rosta u detei s mukovistsidozom, poluchavshikh i nepolychavshikh tsiprofloksatsin:klinicomorfologicheskie copostavleniia]. Antibiotiki i Khimioterapiia [Antibiotics and Chemoterapy] 2001;46(10):11‐3. [CENTRAL: 404060; CFGD Register: PI169 ; CRS: 5500100000002231] [PubMed] [Google Scholar]
Prayle 2016 {published data only}
- Prayle A, Jain K, Watson A, Smyth AR. Are morning doses of intravenous tobramycin less nephrotoxic than evening? Evidence from urinary biomarkers in the critic study. Pediatric Pulmonology 2013;48 Suppl 36:299. [Abstract no.: 261; CENTRAL: 980338; CFGD Register: CO55a; CRS: 5500125000000420] [Google Scholar]
- Prayle AP, Jain K, Touw DJ, Koch BCP, Knox AJ, Watson A, et al. The pharmacokinetics and toxicity of morning vs. evening tobramycin dosing for pulmonary exacerbations of cystic fibrosis: A randomised comparison. Journal of Cystic Fibrosis 2016;15(4):510‐7. [CFGD Register: CO55b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramstrom 2000 {published data only}
- Ramstrom H, Erwander I, Mared L, Kornfalt R, Seiving B. Pharmaceutical intervention in the care of cystic fibrosis patients. Journal of Clinical Pharmacy and Therapeutics 2000;25(6):427‐34. [CENTRAL: 329927; CFGD Register: PI159 ; CRS: 5500100000001818; PUBMED: 11123496] [DOI] [PubMed] [Google Scholar]
Roberts 1993 {published data only}
- Roberts GW, Nation RL, Jarvinen AO. Measurement of serum tobramycin in the presence of ticarcillin or piperacillin. Australian Journal of Hospital Pharmacy 1992;22(2):152‐4. [CENTRAL: 284020; CFGD Register: PI132b ; CRS: 5500100000001253] [Google Scholar]
- Roberts GW, Nation RL, Jarvinen AO, Martin AJ. An in vivo assessment of the tobramycin/ticarcillin interaction in cystic fibrosis patients. British Journal of Clinical Pharmacology 1993;36(4):372‐5. [CENTRAL: 339167; CFGD Register: PI132a ; CRS: 5500100000001836; PUBMED: 12959319] [DOI] [PMC free article] [PubMed] [Google Scholar]
Romano 1992 {published data only}
- Romano L, Girosi D, Spallone E, Parisi F, Minicucci L, Romano C. The use of ofloxacin in cystic fibrosis patients [Uso dell'ofloxacin nei pazienti con fibrosi cistica]. Minerva Pediatrica 1992;44(3):79‐86. [CENTRAL: 86795; CFGD Register: PI68b ; CRS: 5500100000000544; PUBMED: 1518497] [PubMed] [Google Scholar]
- Romano L, Minicucci L, Spallone E, Girosi D, Campelli A, Fabbri A, et al. [Role of home therapy with ofloxacin in patients with cystic fibrosis (CF)] [Ruolo della terapia domiciliare con ofloxacin in pazienti con fibrosi cistica (FC)]. Giornale Italiano di Chemioterapia 1991;38(1‐3):181‐3. [CENTRAL: 109123; CFGD Register: PI68a ; CRS: 5500100000000661; PUBMED: 1365585] [PubMed] [Google Scholar]
Rosenfeld 2006 {published data only}
- Rosenfeld M, Emerson J, Uh D, Anderson G, Genatossio A, McNamara S, et al. Does tobramycin accumulate in respiratory secretions with repeated aerosol administration: a pilot study [abstract]. Pediatric Pulmonology 2006;41 Suppl 29:327. [CENTRAL: 593081; CFGD Register: PI203 ; CRS: 5500100000003036] [Google Scholar]
Sagel 2009 {published data only}
- Sagel SD, Monchil L, Parker D, Emmett P, Wagner B, Abman S. Safety and antimicrobial effects of inhaled nitric oxide in CF: a pilot study [abstract]. Pediatric Pulmonology 2009;44 Suppl 32:299, Abstract no: 251. [CENTRAL: 921940; CFGD Register: OV21 ; CRS: 5500100000011060] [Google Scholar]
Salh 1992 {published data only}
- Salh B, Bilton D, Dodd M, Abbot J, Webb K. A comparison of aztreonam and ceftazidime in the treatment of respiratory infections in adults with cystic fibrosis. Scandinavian Journal of Infectious Diseases 1992;24(2):215‐8. [CENTRAL: 86010; CFGD Register: PI72 ; CRS: 5500100000000536; PUBMED: 1641599] [DOI] [PubMed] [Google Scholar]
Sharma 2016 {published data only}
- Sharma G, Lodha R, Shastri S, Saini S, Kapil A, Singla M, et al. Zinc supplementation for one year among children with cystic fibrosis does not decrease pulmonary infection. Respiratory care 2016;61(1):78‐84. [CFGD Register: GN256] [DOI] [PubMed] [Google Scholar]
Singh 2013 {published data only}
- Singh SB, Shelton AU, Kotek K, Starner TD. A clinically‐embedded trial to evaluate the efficacy of interventions for pre‐pseudomonal pathogens [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:335, Abstract no: 358. [CENTRAL: 999884; CFGD Register: PI274 ; CRS: 5500127000000006] [Google Scholar]
Smith 1997 {published data only}
- Smith A, Weber A, Pandher R, Williams‐Warren J, Cohen ML, Ramsey B. Utilization of salivary concentrations of ciprofloxacin in subjects with cystic fibrosis. Infection 1997;25(2):106‐8. [CENTRAL: 192506; CFGD Register: PI145 ; CRS: 5500100000000991; EMBASE: 1997101357] [DOI] [PubMed] [Google Scholar]
Stutman 1987 {published data only}
- Shalit I, Stutman HR, Marks MI, Chartrand SA, Hilman BC. Randomized study of two dosage regimens of ciprofloxacin for treating chronic bronchopulmonary infection in patients with cystic fibrosis. American Journal of Medicine 1987;82(Suppl 4A):189‐95. [CENTRAL: 47924; CFGD Register: PI48b ; CRS: 5500100000000293; PUBMED: 3555035] [PubMed] [Google Scholar]
- Stutman HR, Shalit I, Marks MI, Greenwood R, Chartrand SA, Hilman BC. Pharmacokinetics of two dosage regimens of ciprofloxacin during a two‐week therapeutic trial in patients with cystic fibrosis. American Journal of Medicine 1987;82(Suppl 4A):142‐5. [CENTRAL: 47920; CFGD Register: PI48a ; CRS: 5500100000000290; PUBMED: 3555028] [PubMed] [Google Scholar]
Vitti 1975 {published data only}
- Vitti TG, Berg TJ, Pagtakhan RD. The effect of pancreatic enzyme supplement on the intestinal absorption of ampicillin and cloxacillin in children with cystic fibrosis [abstract]. 16th Cystic Fibrosis Club Abstracts; 1975. 1975:56. [CENTRAL: 291631; CFGD Register: PI81 ; CRS: 5500100000001630]
Willekens 2013 {published data only}
- Willekens J, Vanderhelst E, Schutter I, Wachter E, Eyns H, Pierard D, et al. Impact of azithromycin maintenance treatment on Staphylococcus aureus prevalence and macrolide resistance: Experience in one centre [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:329, Abstract no: 342. [Google Scholar]
Wood 1996 {published data only}
- Wood PJ, Ioannides Demos LL, Li SC, Williams TJ, Hickey B, Spicer WJ, et al. Minimisation of aminoglycoside toxicity in patients with cystic fibrosis. Thorax 1996;51(4):369‐73. [CENTRAL: 127097; CFGD Register: PI109 ; CRS: 5500100000000761; EMBASE: 1996127618; PUBMED: 8733487] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yenduri 2013 {published data only}
- Yenduri NJ, Luna RA, Moonnumakal SP, Mann MC, Hiatt PW, Smith EO, Oermann CM. A cross‐sectional study of microbiomes and clinical outcomes in cystic fibrosis patients colonized with MRSA vs MSSA [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:324, Abstract no: 328. [Google Scholar]
Additional references
Accurso 2007
- Accurso FJ. Update in cystic fibrosis 2006. American Journal of Respiratory and Critical Care Medicine 2007;175(8):754‐7. [DOI: 10.1164/rccm.200701-160UP] [DOI] [PMC free article] [PubMed] [Google Scholar]
CF Foundation 2013
- Cystic Fibrosis Foundation. Annual Patient Registry 2013. https://www.cff.org/2013_CFF_Patient_Registry_Annual_Data_Report.pdf.
CF Foundation 2016
- Cystic Fibrosis Foundation. Cystic Fibrosis Foundation Patient Registry. 2016 Annual Data Report. www.cff.org/Research/Researcher‐Resources/Patient‐Registry/2016‐Patient‐Registry‐Annual‐Data‐Report.pdf 2017.
CF Trust 2016
- Cystic Fibrosis Trust. UK Cystic Fibrosis Registry Annual Data Report 2016. www.cysticfibrosis.org.uk/the‐work‐we‐do/uk‐cf‐registry/reporting‐and‐resources 2017.
Cogen 2015
- Cogen J, Emerson J, Sanders DB, Ren C, Schechter MS, Gibson RL, et al. Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr Pulmonol 2015 Aug;50(8):763‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group. Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Farrell 2008
- Farrell PM. The prevalence of cystic fibrosis in the European Union. Journal of Cystic Fibrosis 2008;7(5):450‐3. [DOI: 10.1016/j.jcf.2008.03.007] [DOI] [PubMed] [Google Scholar]
Flume 2007
- Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ Jr, Willey‐Courand DB, et al. Cystic fibrosis pulmonary guidelines: chronic medications for maintenance of lung health. Ameican Journal of Respiratory and Critical Care Medicine 2007;176(10):957‐9. [DOI] [PubMed] [Google Scholar]
Fuchs 1994
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. New England Journal of Medicine 1994;331(10):637‐42. [DOI] [PubMed] [Google Scholar]
Gangell 2011
- Gangell C, Gard S, Douglas T, Park J, Klerk N, Keil T, et al. Inflammatory responses to individual microorganisms in the lungs of children with cystic fibrosis. Clin Infect Dis 2011 Sep;53(5):425‐32. [DOI] [PubMed] [Google Scholar]
Gibson 2003
- Gibson RL, Burns JL. Pathophysiology and management of pulmonary infections in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;168(8):918‐51. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG on behalf of the Cochrane Statistical Methods Group (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hubert 2018
- Hubert D, Dehillotte C, Munck A, David V, Baek J, Mely L, et al. Retrospective observational study of French patients with cystic fibrosis and a Gly551Asp‐CFTR mutation after 1 and 2years of treatment with ivacaftor in a real‐world setting. J Cyst Fibros. 2018 Jan;17(1):89‐95. [DOI] [PubMed] [Google Scholar]
Kahl 2010
- Kahl BC. Impact of Staphylococcus aureus on the pathogenesis of chronic cystic fibrosis lung disease. International Journal of Medical Microbiology 2010;300(8):514‐9. [DOI] [PubMed] [Google Scholar]
Kuehnert 2006
- Kuehnert MJ, Kruszon‐Moran D, Hill HA, McQuillan G, McAllister SK, Fosheim G, et al. Prevalence of Staphylococcus aureus nasal colonization in the United States, 2001‐2002. Journal of Infectious Diseases 2006;193(2):172‐9. [DOI] [PubMed] [Google Scholar]
Lommatzsch 2009
- Lommatzsch ST, Aris R. Genetics of cystic fibrosis. Seminars in Respiratory and Critical Care Medicine 2009;30(5):531‐8. [DOI: 10.1055/s-0029-1238911] [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsey 1999
- Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. New England Journal of Medicine 1999;340(1):23‐30. [DOI] [PubMed] [Google Scholar]
Ratjen 2001
- Ratjen F, Comes G, Paul K, Posselt HG, Wagner TO, Harms K, et al. Effect of continuous antistaphylococcal therapy on the rate of P. aeruginosa acquisition in patients with cystic fibrosis. Pediatric Pulmonology 2001;31(1):13. [DOI] [PubMed] [Google Scholar]
Ratjen 2003
- Ratjen F. Cystic fibrosis. Lancet 2003;361(9358):681‐9. [DOI] [PubMed] [Google Scholar]
Ratjen 2006
- Ratjen F. Diagnosing and managing infection in CF. Pediatric Respiratory Reviews 2006;7 Suppl 1:S151‐3. [DOI] [PubMed] [Google Scholar]
Razvi 2009
- Razvi S, Quittell L, Sewall A, Quinton H, Marshall B, Saiman L. Respiratory microbiology of patients with cystic fibrosis in the United States, 1995 to 2005. Chest 2009;136(6):1554‐60. [DOI] [PubMed] [Google Scholar]
Ridder‐Schaphorn 2007
- Ridder‐Schaphorn S, Ratjen F, Dübbers A, Häberle J, Falk S, Küster P, et al. Nasal Staphylococcus aureus carriage is not a risk factor for lower‐airway infection in young cystic fibrosis patients. Journal of Clinical Microbiology 2007;45(9):2979–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sagel 2009a
- Sagel SD, Gibson RL, Emerson J, McNamara S, Burns JL, Wagener JS, et al. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. Journal of Pediatrics 2009;154(2):183‐8. [DOI: 10.1016/j.jpeds.2008.08.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al on behalf of the Cochrane Applicability and Recommendations Methods Group and the Cochrane Statistical Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Smyth 2005
- Smyth A. Prophylactic antibiotics in cystic fibrosis: a conviction without evidence?. Pediatric Pulmonology 2005;40(6):471‐6. [DOI] [PubMed] [Google Scholar]
Smyth 2012
- Smyth AR, Walters S. Prophylactic anti‐staphylococcal antibiotics for cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD001912.pub2] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D on behalf of the Cochrane Bias Methods Group (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Stutman 2002
- Stutman HR, Lieberman JM, Nussbaum E, Marks MI. Antibiotic prophylaxis in infants and young children with cystic fibrosis: A randomised controlled trial. Journal of Pediatrics 2002;140(3):299‐305. [DOI] [PubMed] [Google Scholar]
Szaff 1982
- Szaff M, Hoiby N. Antibiotic treatment of Staphylococcus aureus infection in cystic fibrosis. Acta Paediatrica Scandinavica 1982;71(5):821–6. [DOI] [PubMed] [Google Scholar]
Thakare 2017
- Thakare R, Singh AK, Das S, Vasudevan N, Jachak GR, Reddy DS, et al. Repurposing Ivacaftor for treatment of Staphylococcus aureus infections. Int J Antimicrob Agents 2017 Sep;50(3):389‐392. [DOI] [PubMed] [Google Scholar]
Wong 2013
- Wong JK, Ranganathan SC, Hart E, Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF). Staphylococcus aureus in early cystic fibrosis lung disease. Pediatric Pulmonology 2013;48(12):1151‐9. [DOI: 10.1002/ppul.22863] [DOI] [PubMed] [Google Scholar]
Zolin 2015
- Zolin A, Orenti A, Naehrlich L, Rens J, Fox A, Iansa P, et al. European Cystic Fibrosis Society Patient Registry Annual Report 2015. www.ecfs.eu/news/ecfs‐patient‐registry‐2015‐annual‐report 2017.
References to other published versions of this review
Ahmed 2016
- Ahmed MI, Mukherjee S. Treatment for chronic methicillin‐sensitive Staphylococcus aureus pulmonary infection in people with cystic fibrosis10.1002/14651858.CD011581.pub2. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD011581.pub2] [DOI] [PubMed] [Google Scholar]


