Abstract
Background
Infantile haemangiomas (previously known as strawberry birthmarks) are soft, raised swellings of the skin that occur in 3% to 10% of infants. These benign vascular tumours are usually uncomplicated and tend to regress spontaneously. However, when haemangiomas occur in high‐risk areas, such as near the eyes, throat, or nose, impairing their function, or when complications develop, intervention may be necessary. This is an update of a Cochrane Review first published in 2011.
Objectives
To assess the effects of interventions for the management of infantile haemangiomas in children.
Search methods
We updated our searches of the following databases to February 2017: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, PsycINFO, AMED, LILACS, and CINAHL. We also searched five trials registries and checked the reference lists of included studies for further references to relevant trials.
Selection criteria
Randomised controlled trials (RCTs) of all types of interventions, versus placebo, active monitoring, or other interventions, in any child with single or multiple infantile haemangiomas (IHs) located on the skin.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. The primary outcome measures were clearance, a subjective measure of improvement, and adverse events. Secondary outcomes were other measures of resolution; proportion of parents or children who consider there is still a problem; aesthetic appearance; and requirement for surgical correction. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results
We included 28 RCTs, with a total of 1728 participants, assessing 12 different interventions, including lasers, beta blockers (e.g. propranolol, timolol maleate), radiation therapy, and steroids. Comparators included placebo, an active monitoring approach, sham radiation, and interventions given alone or in combination.
Studies were conducted in a number of countries, including China, Egypt, France, and Australia. Participant age ranged from 12 weeks to 13.4 years. Most studies (23/28) included a majority of females and different types of IHs. Duration of follow‐up ranged from 7 days to 72 months.
We considered most of the trials as at low risk of random sequence generation, attrition bias, and selective reporting bias. Domains such as allocation concealment and blinding were not clearly reported in general. We downgraded evidence for issues related to risk of bias and imprecision.
We report results for the three most important comparisons, which we chose on the basis of current use. Outcome measurement of these comparisons was at 24 weeks' follow‐up.
Oral propranolol versus placebo
Compared with placebo, oral propranolol 3 mg/kg/day probably improves clinician‐assessed clearance (risk ratio (RR) 16.61, 95% confidence interval (CI) 4.22 to 65.34; 1 study; 156 children; moderate‐quality evidence) and probably leads to a clinician‐assessed reduction in mean haemangioma volume of 45.9% (95% CI 11.60 to 80.20; 1 study; 40 children; moderate‐quality evidence). We found no evidence of a difference in terms of short‐ or long‐term serious adverse events (RR 1.05, 95% CI 0.33 to 3.39; 3 studies; 509 children; low‐quality evidence), nor in terms of bronchospasm, hypoglycaemia, or serious cardiovascular adverse events. The results relating to clearance and resolution for this comparison were based on one industry‐sponsored study.
Topical timolol maleate versus placebo
The chance of reduction of redness, as a measure of clinician‐assessed resolution, may be improved with topical timolol maleate 0.5% gel applied twice daily when compared with placebo (RR 8.11, 95% CI 1.09 to 60.09; 1 study; 41 children;low‐quality evidence). Regarding short‐ or long‐term serious cardiovascular events, we found no instances of bradycardia (slower than normal heart rate) or hypotension in either group (1 study; 41 children; low‐quality evidence). No other safety data were assessed, and clearance was not measured.
Oral propranolol versus topical timolol maleate
When topical timolol maleate (0.5% eye drops applied twice daily) was compared with oral propranolol (via a tablet taken once per day, at a 1.0 mg/kg dose), there was no evidence of a difference in haemangioma size (as a measure of resolution) when measured by the proportion of patients with a clinician‐assessed reduction of 50% or greater (RR 1.13, 95% CI 0.64 to 1.97; 1 study; 26 participants; low‐quality evidence). Although there were more short‐ or long‐term general adverse effects (such as severe diarrhoea, lethargy, and loss of appetite) in the oral propranolol group, there was no evidence of a difference between groups (RR 7.00, 95% CI 0.40 to 123.35; 1 study; 26 participants; very low‐quality evidence). This comparison did not measure clearance.
None of our key comparisons evaluated, at any follow‐up, a subjective measure of improvement assessed by the parent or child; proportion of parents or children who consider there is still a problem; or physician‐, child‐, or parent‐assessed aesthetic appearance.
Authors' conclusions
We found there to be a limited evidence base for the treatment of infantile haemangiomas: a large number of interventions and outcomes have not been assessed in RCTs.
Our key results indicate that in the management of IH in children, oral propranolol and topical timolol maleate are more beneficial than placebo in terms of clearance or other measures of resolution, or both, without an increase in harms. We found no evidence of a difference between oral propranolol and topical timolol maleate with regard to reducing haemangioma size, but we are uncertain if there is a difference in safety. Oral propranolol is currently the standard treatment for this condition, and our review has not found evidence to challenge this. However, these results are based on moderate‐ to very low‐quality evidence.
The included studies were limited by small sample sizes and risk of bias in some domains. Future trials should blind personnel and participants; describe trials thoroughly in publications; and recruit a sufficient number of children to deduce meaningful results. Future trials should assess patient‐reported outcomes, as well as objective outcomes of benefit, and should report adverse events comprehensively. Propranolol and timolol maleate require further assessment in RCTs of all types of IH, including those considered problematic, as do other lesser‐used interventions and new interventions. All treatments should be compared against propranolol and timolol maleate, as beta blockers are approved as standard care.
Plain language summary
Treatments for haemangiomas (a cluster of small blood vessels that form a lump) of the skin in children
What is the aim of this review?
This Cochrane Review aimed to assess the benefits and harms of treatments for haemangiomas of the skin in infants and children (known as 'infantile haemangiomas'). We collected and analysed 28 relevant clinical trials to answer this question.
Key messages
Only one of our key comparisons (propranolol versus placebo) measured clearance of the haemangioma, with moderate‐quality evidence supporting this result. We found low‐ or moderate‐quality evidence for the following specific measures of resolution: reduction in volume, redness, and size. We found very low‐ and low‐quality evidence for results concerning side effects, meaning we were unable to draw definitive conclusions about safety.
Oral propranolol is currently the standard treatment for this condition, and we did not find evidence to contest this treatment in terms of efficacy and safety. However, potential biases in the design of many of the included trials affect our confidence in the results of the review. High‐quality future research should assess the effects of propranolol and timolol maleate, as well as other new and older medications, on outcomes that are important to patients.
What was studied in the review?
Infantile haemangiomas are soft, raised swellings on the skin, often with a bright‐red surface caused by a non‐cancerous overgrowth of blood vessels in the skin. The majority of lesions are uncomplicated and will shrink on their own by age seven; however, some require treatment if they occur in high‐risk areas (e.g. near the eyes) or cause psychological distress.
We included all types of treatment for infantile haemangiomas, which could have been given alone or in combination, or compared to each other, to a 'placebo' (i.e. a treatment with no active agent), or against children whose haemangiomas were untreated but observed.
What are the main results of the review?
We included 28 studies, with a total of 1728 participants, which assessed lasers, beta blockers (e.g. propranolol), steroids, radiation therapy, and other treatments. Treatments were compared against an active monitoring approach (observation), placebo, sham radiation, or other interventions (given alone or in combination with another treatment). Studies were conducted in multiple countries; participant age ranged from 12 weeks to 13.4 years; and most studies included more girls than boys (23/28). Children had different types of haemangioma. Duration of follow‐up ranged from 7 days to 72 months.
The following results were measured 24 weeks after the beginning of treatment. All non‐safety outcomes presented here were clinician assessed (i.e. assessed by the physician in charge of a patient).
When compared with placebo treatment, propranolol taken by mouth at a dose of 3 mg/kg/day is probably more beneficial in terms of complete or almost‐complete clearance of swelling and reduction in volume of the haemangioma (moderate‐quality evidence). We found no evidence of a difference between the two treatments in terms of short‐ or long‐term serious or other side effects (low‐quality evidence). Most of the evidence for this comparison was based on an industry‐sponsored study.
Timolol maleate 0.5% gel applied topically twice daily may reduce redness as a measure of resolution when assessed against placebo (low‐quality evidence). Short‐ or long‐term serious cardiovascular events were not reported in either group. There were no other safety data for timolol maleate compared with placebo (low‐quality evidence). This comparison did not assess clearance of the swelling.
There was no evidence of a difference between propranolol taken by mouth (via a tablet once per day, at a 1.0 mg/kg dose) and topical timolol maleate (0.5% eye drops applied twice daily) in terms of their effect on reducing haemangioma size by 50% or more (low‐quality evidence). There were more general short‐ or long‐term side effects (such as severe diarrhoea, tiredness, and decreased appetite) with propranolol, but due to very low‐quality evidence, these results are uncertain. This comparison did not assess clearance of the swelling.
Most of the comparisons assessed, including those described above, did not report on the following outcomes: parent or child's opinion of improvement; the proportion of parents or children who consider there is still a problem; and cosmetic appearance.
How up‐to‐date is this review?
We searched for studies up to February 2017.
Summary of findings
Background
Please refer to the following website for definitions of technical terms: www.ncbi.nlm.nih.gov/mesh.
Description of the condition
Infantile haemangiomas (IH) are the most common vascular tumours among children, occurring in 3% to 10% of infants (Léauté‐Labrèze 2015). They were previously known as 'strawberry birthmarks' or 'strawberry naevi', or 'capillary haemangiomas', terms currently withdrawn by current classifications about vascular tumours (www.issva.org/classification). They are benign and of endothelial cellular origin, characterised by a rapid pattern of propagation in the first months of life, then followed by a period of involution that can take several years (Bruckner 2006). Sometimes the IH is characterised by a precursor lesion at birth. Infantile haemangiomas undergo a phase of rapid growth within the first few months of the first year (Baselga 2016; Wang 2017). Regression is completed in 60% of patients by their fourth birthday, 76% by their seventh birthday, and approximately 90% by their ninth birthday (Zimmermann 2010). However, it has been observed in some retrospective studies that the complete regression has been achieved at 3.5 years (Baselga 2016), and also at four years of follow‐up (Darrow 2015). While most lesions develop in a straightforward way, about 12% of cases result in clinically significant complications requiring referral (Leaute‐Labreze 2015). In addition, IH can result in lifelong sequelae, which can cause psychological distress (Léauté‐Labrèze 2015). More than 50% of untreated IH leave permanent sequelae that may cause disfigurement (Baselga 2016). Bauland and colleagues found residual lesions in 69% of 137 IH studied (Bauland 2011), and Baselga and colleagues in 54.9% out of 184 IH studied (Baselga 2016).
Infantile haemangiomas appear more commonly among Caucasians (understood to be white individuals), being evident in up to 12% of all children (Zimmermann 2010). Infantile haemangiomas affect females in a ratio of 3:1 (Zimmermann 2010). Sixty per cent of IH are located in the head and neck area, whereas 25% occur on the trunk and 15% on the extremities (Zimmermann 2010). Infantile haemangiomas can be divided by their morphology into superficial haemangiomas, subcutaneous (deep) haemangiomas, and mixed haemangiomas (www.issva.org/classification) (Sethuraman 2014). Superficial haemangiomas appear as a bright‐red vascular plaque with an irregular surface. Subcutaneous or deep haemangiomas present as protruding vascular swelling under normal or bluish skin. Mixed or combined haemangiomas show a combination of both superficial and deep characteristics. Infantile haemangiomas usually present as single lesions, although 20% of affected infants develop multiple tumours (van de Kerkhof 1998; Zimmermann 2010). The skin covering haemangiomas may become ulcerated, exposing the underlying blood vessels and making them more liable to bleed from minor trauma and become infected,
Infantile haemangiomas are not normally present at birth, or they are present only as a precursive mark, in the form of a pink macule, telangiectatic patches, or areas that appear bruised (Vega 2017). Infantile haemangiomas generally proliferate during the first year of life, with most growth being completed by age six to nine months. Eighty per cent of haemangioma growth is completed by age three months, and 80% of haemangiomas have completed growth by age five months (Tollefson 2012). Despite the self limiting nature of most IH, several complications have been observed, including bleeding, ulceration and infection, deformation and disfigurement, impairment of vision, and airway obstruction (Achauer 1997; Syed 1999). Children under the age of three are seldom aware of their haemangiomas. Most IH resolve spontaneously; however, when they cause complications, they can be dangerous or present a risk to a person's life.
The diagnosis of an IH is typically made clinically, based on its appearance and characteristic behaviour. When there is doubt in the diagnosis, additional studies such as Doppler ultrasound or skin biopsy may be performed (Holland 2013).
Studies have shown that blood vessel cells (positive for glucose transporter 1 (GLUT1)) in IH are similar to those found in the placenta (Ma 2017); this has raised the possibility that placental cells may become dislodged during pregnancy, travel into the foetus, and grow postnatally to form a haemangioma (Ma 2017). Some mechanisms have been studied in the pathogenesis of infantile haemangiomas such as tissue hypoxia and pathologic vasculogenesis leading to endothelial cell proliferation (Ma 2017). Predisposing factors have also been described in some studies, including low birth weight, advanced maternal age, multiple gestations, pre‐eclampsia, and gestational diabetes mellitus (Castren 2016). Infantile haemangiomas have also been more frequently noted in children whose mothers had chorionic villus sampling (CVS) compared with the general population (Kaplan 1990).
Management of IH can be challenging. Each option involves significant drawbacks or side effects, or both. Although most haemangiomas are self limited and do not need treatment, some indications for treatment include the following: high‐output cardiac failure, bleeding, ulceration, risk of permanent disfigurement, or airway or visual obstruction. Location, age of the patient, risk of complications, and growth rate are all factors that physicians must consider in managing patients with IH (Holland 2013).
Recently, Léauté‐Labrèze 2015 described their chance observation of an antiproliferative effect of propranolol (PR) on IH. Since the introduction of propranolol in 2008, this drug has showed a highly effective profile with tolerable adverse events, in comparison with previous recommended interventions used for IH (e.g. steroids, interferon, chemotherapy) (Zou 2013). Minimal or no side effects have been reported with propranolol, and the response rate has approached 100% (Léauté‐Labrèze 2015). Propranolol is now the first‐line treatment for IH and has been approved for this indication (Baselga 2016; Chinnadurai 2016a).
Description of the intervention
The diagnosis of an IH is typically made clinically, based on appearance and characteristic behaviour. When the diagnosis is uncertain, additional tests such as a Doppler ultrasound or skin biopsy may be performed (Holland 2013). The vast majority of infantile haemangiomas will regress on their own and require no further treatment; therefore, an active monitoring approach is usually implemented (Darrow 2015). However, IH can occur in high‐risk areas, such as near the eyes, throat, and nose, impairing their function. If vision is obscured at a critical stage in brain development, complications such as failure to develop binocular vision can result (Darrow 2015). A large variety of treatments have been used historically, and many are still in use.
Beta blockers, for example oral propranolol, are the current standard care, approved both by the US Food and Drug Administration and the European Medicines Agency, with complete regression without sequelae after six months of treatment in 60% of cases (Darrow 2015). Propranolol has also been assessed for intralesional and topical administration (Zaher 2013). The recommended dose of propranolol in oral administration is 3 mg/kg/day divided into two doses, for at least six months (Leaute‐Labreze 2015). Reported adverse effects include hypoglycaemia, bradycardia, hypotension, bronchospasm, sleep disturbance, and gastrointestinal disorders (Ji 2015). Propranolol interacts with other medications such as insulin, non‐steroidal anti‐inflammatories, antiarrhythmics, and calcium channel blockers (Holland 2013). In practice, when medication is warranted for infantile haemangioma, propranolol is the first‐choice drug. Parents and healthcare professionals must monitor infants closely for adverse effects (Leaute‐Labreze 2015).
In addition, topical timolol maleate, a non‐selective beta blocker, is available in a 0.25% and 0.5% solution, as well as an extended release 0.5% (5 mg/mL) gel‐forming solution. Frequency and method of application have varied from once daily under occlusion to twice daily without occlusion; 1 to 2 drops have typically been used and are usually given for 2 to 6 months (Zheng 2018). Adverse effects of timolol maleate in the paediatric population, especially in high‐risk premature infants, include bradycardia and bronchospasm (Holland 2013).
The following other treatments besides oral propranolol and topical timolol maleate have been assessed and might still be in use (Glassberg 1989).
Atenolol is a cardioselective beta blocker; it is a large, lipophobic molecule and has limited ability to cross the blood–brain barrier (Bayart 2017). Its use may sometimes be preferred if patients experience side effects with propranolol. Treatment with atenolol is recommended in an oral dose of 1 mg/kg/day for three to six months, depending on the positive response or the presence of adverse events such as bradycardia, hypotension, dizziness, and lethargy (Abarzua‐Araya 2014; Raphael 2011). Some interactions with other medicaments have been suggested, including verapamil, clonidine, and ibuprofen (Doshan 1986; Hansson 1975).
Bleomycin, a well‐known anticancer and sclerosing agent, has been used to treat haemangiomas (Luo 2011). Recommended dosage and duration of treatment depends on the age of the patient and the size of the lesion. Some clinicians have used a standard injection of bleomycin of 0.3 to 0.6 mg/kg per injection, and others have used a mixture of 5 mL 2% lidocaine, 5 mg dexamethasone, and 8 mg bleomycin A5 (Pienaar 2006). Some documented adverse events for this agent include oedema and ulceration (Luo 2011). No interactions with other drugs have been reported.
Captopril, an angiotensin‐converting enzyme inhibitor, has been suggested for potential use in the treatment of IH, due to its effects in inhibition of angiogenesis and vasculogenesis (Christou 2012). Dosage and duration of administration have not yet been standardised; Zaher and colleagues used oral captopril at 0.5 to 1 mg/kg/day, in a titrating dose, while Tan and colleagues used a dose of 0.1 to 0.5 mg/kg under response (Tan 2012; Zaher 2016). Cardiac side effects requiring dose reduction or suspension have been documented for its use in IH (Zaher 2016). Treatments known to have interactions with captopril include aliskiren, everolimus, sirolimus, and lithium (Medicines.ie 2018).
High‐intensity focused ultrasound (HIFU) is a non‐invasive surgical option with rapid evolvement in recent years that is mainly used in the management of solid tumours (Fu 2012). Documented side effects include damage in the focal point, endothelial cell loss, necrosis, and vascular discontinuity (Fu 2012). Different levels of energy have been used for different purposes, ranging from 2.6 to 4.5 W (Fu 2012). Lesions in the Fu 2012 studies were small‐ to medium‐sized (from 0.8 cm x 0.6 cm to 6.0 cm x 5.0 cm). No interactions have been reported.
Interferon, an inhibitor of angiogenesis, developed as an antiviral agent, has been suggested as a potential intervention for IH (Ezekowitz 1992; Greinwald 1999), especially for infants with life‐threatening haemangiomas unresponsive to corticosteroids. Some studies have suggested a dosage of interferon alpha‐2b of 3 million units/m² subcutaneously, from daily to 5 times per week for 6 to 24 months (Ezekowitz 1992; Greinwald 1999). Reported side effects include fever, malaise, transient neutropenia, and liver disease (Holland 2013). Known interactions of interferon alpha‐2 include use of theophylline, acalabrutinib, and lamivudine, among others (EMC 2018).
Methylene blue is an inhibitor of nitric oxide synthase and guanylate cyclase and is used in the management of vasoplegia syndrome, septic shock, hepato‐pulmonary syndrome, and malaria, among others (Ginimuge 2010). This intervention has not been widely evaluated in the management of IH. The mechanism of action of methylene blue in photodynamic therapy has shown effects in the elimination of bacterial agents in superficial and deep excisional wounds, as well as the treatment of resistant plaque psoriasis. Reported adverse effects in high doses include cardiac arrhythmias, coronary vasoconstriction, decreased cardiac output, renal blood flow, and mesenteric blood flow (Ginimuge 2010). As methylene is a monoamine oxidase (MAO) inhibitor, it could interplay with MAO inhibitors as well as selective serotonin reuptake inhibitor (SSRI) to produce serious serotonin toxicity (Ginimuge 2010).
Imiquimod is an immune‐response modifier (a substance that changes the way the immune system works), which has been used in the management of condyloma, actinic keratoses, and basal cell carcinoma (McCuaig 2009). It has been suggested that imiquimod 5% cream, applied once daily for up to 16 weeks, can induce involution of superficial IH (McCuaig 2009). Reported side effects of imiquimod include local erythema, crusting, and contact dermatitis (McCuaig 2009).
Laser treatments (including pulsed dye, argon, carbon dioxide, neodymium‐doped yttrium aluminum garnet (Nd:YAG), sequential/concurrent dual‐wavelength laser and erbium) should be considered if there is a contraindication for systemic treatment, such as a history of sensitivity to beta blockers, asthma, renal disease, heart disease, or hypoglycaemia (Chinnadurai 2016a; Chinnadurai 2016b). Some reported side effects of laser treatment include purpura, swelling, blisters, hypopigmentation, bleeding, infection, and atrophic or hypertrophic scarring (Chinnadurai 2016a; Chinnadurai 2016b). Protocols of administrations are multiple and depend on the laser pulse width, age of the patient, IH anatomical location, cooling materials, and size of the tumour (Chinnadurai 2016a; Chinnadurai 2016b). A study recently assessed the concurrent or sequential administration of laser with other potential interventions (Lu 2016). Infants require a general anaesthetic for treatment because laser treatment can be painful. Early childhood anaesthesia carries the usual risks of complications associated with anaesthesia and as well as with neurocognitive impairment.
Oral ibuprofen plus oral paracetamol, as a combination of non‐steroidal anti‐inflammatory and analgesic drugs (NSAIDs), has a role in the management of ulcerated IH located in the head and neck region (Tiwari 2016). Recently, Tawfik 2015 assessed a combination of oral ibuprofen and paracetamol in doses of 10 and 16.2 mg/kg 8‐hourly versus oral propranolol for up to 6 months.
Radiation therapy has been conventionally used for treating life‐ or function‐threatening haemangiomas that have been unresponsive to treatment with corticosteroids. Side effects of using ionising radiation include blisters, infection, and ulcers, while possible lasting complications include pigmentation restricted to certain areas or hypopigmentation, the creation of scars, soft tissue dysplasia, and the retardation of bone growth (Fragu 1991; Probert 1975). Radiation therapy for haemangiomas includes ⁹⁰SR‐⁹⁰Y radiation and soft X‐ray radiation. Adverse effects include radionecrosis (acute) and scarring and skin cancer (long term).
Rapamycin is a macrolide compound with immunosuppression and antiangiogenic activity (Li 2017). Oral and local administration have been assessed. Reported side effects include hyperlipidaemia, impaired glucose tolerance, anaemia, and acute renal toxicity (Li 2017).
Steroids (administered topically, intralesionally, or systemically). Intralesional corticosteroids may be used for the treatment of small haemangiomas, usually involving the facial area. However, many dermatologists prefer systemic corticosteroids for periocular lesions, since intralesional administration has resulted in serious side effects including retinal artery occlusion and eyelid necrosis (Shorr 1986). Prednisolone is the most frequent steroid assessed for management of IH (Aly 2015), at a dosage of 2 mg/kg/day for six months. There is an interaction of steroids with concomitant administration of phenytoin, phenobarbital, ephedrine, estrogens, and diuretics (Bauman 2014). Steroid side effects include growth retardation, increased susceptibility to infectious disease, and hypertension (George 2004). Other minor, reversible complications associated with the administration of steroids include haematomas, periocular calcification, and eyelid pigmentation.
Surgery is indicated for lesions that interfere with function if pharmacologic therapy fails or is contraindicated, as well as where ulceration or bleeding has occurred (Liang 2014). Surgical excision might also be used to improve the final cosmetic appearance if loose skin is left after IH regression (Smolinski 2005). In addition, cryotherapy can be used for small and flat haemangiomas, in order to accelerate haemangioma involution (shrinkage) (Grantzow 2001). Surgical intervention is commonly used for the correction of scarring as well as removal of residual tissue, but it can be used also for excision of life‐threatening haemangiomas (Holland 2013).
Vincristine is a vinca alkaloid that has been assessed in the treatment of IH, especially those IH unaffected by corticosteroids or in patients who cannot bear corticosteroids (Glade 2010). Single weekly doses of 1 to 1.5 mg/m² have been assessed (median of three cycles). Potential serious adverse events included constipation, neuromyopathy,(Glade 2010), and risk associated with placement of the central line (Holland 2013).
Active monitoring. This approach has been shown to produce the best cosmetic outcomes for uncomplicated infantile haemangiomas (Dinehart 2001). In general, most haemangiomas resolve spontaneously without significant sequelae and follow‐up can be the clinician's choice (Liang 2014).
How the intervention might work
Propranolol
Propranolol has been to shown to restrict the haemangioma's capillaries, causing a subsequent decrease in blood flow within the tumour. In addition, it has been suggested that propranolol can hinder angiogenesis and encourage apoptosis in IH cells (Wnek 2017). Potential mechanisms of action of propranolol include the stimulation of apoptosis in haemangioma endothelial cells through GLUT1 receptor antagonism; the prevention of catecholamine‐induced angiogenesis; the constraint of the renin‐angiotensin axis; and the interruption of signalling pathways that regulate progenitor cells (Wnek 2017). In contrast with corticosteroids, propranolol is effective during the proliferative phase of growth. Studies have recently suggested that propranolol administration in IH therapy generates a biological response involving changes in the expression of chosen apoptosis‐regulating factors (Wnek 2017).
Timolol maleate
Topical timolol hinders IH growth and encourages the regression of superficial IH, although some studies have raised concerns about the effect of systemic absorption, as well side effects such as sleep disturbance (Danarti 2016).
Atenolol
The main effects of atenolol involve the beta‐1 receptors with minor beta‐2 effects (Abarzua‐Araya 2014). Because atenolol does not act on pulmonary beta‐2 receptors, it can be used in infants with pulmonary conditions, including reactive airway disease. Likewise, atenolol does not act on pancreatic beta‐2 receptors and thus does not interfere with regulation of gluconeogenesis, glycogenolysis, and lipolysis (Bayart 2017).
Bleomycin
The action of bleomycin involves a decrease in the production of vascular endothelial cells, as well as the tempering of angiogenesis of the infantile haemangiomas (Luo 2011; Qiu 2015). In addition, its administration has an effect in the G2 and S phases of endothelial cells by inducing DNA deterioration and preventing its reconstruction, resulting in collapse, shrinkage, and fibrosis (Luo 2011; Qiu 2015).
Captopril
Because some components of the renin‐angiotensin system (RAS) are expressed in proliferating IH, modulation of downstream products of RAS, including angiotensin‐converting enzymes, could have a role in the treatment of IH (Zaher 2016). Similar to propranolol, captopril leads to a decrease in vascular endothelial growth factor production via downregulation of angiotensin II (Itinteang 2011). In addition, captopril can inhibit the effect of kininase II and increase the plasma bradykinin levels (Waeber 1980).
High‐intensity focused ultrasound (HIFU)
It has been suggested that the effectiveness of HIFU in the treatment of IH, as well as superficial skin lesions, could be similar to that shown in treating body parts and tumour (Fu 2012; Orsi 2010). High‐intensity focused ultrasound produces coagulative necrosis in a focal point without effects in adjacent structures by means of ultrasonic tissue penetration. Biological effects of HIFU include coagulative necrosis, nuclei damage, membrane disruption, and apoptosis (Kennedy 2005).
Interferon
Interferon therapy works by inhibiting locomotion of capillary endothelium in vitro (Ezekowitz 1992). Although research from case series reports indicate similar results for interferon alpha‐2a and interferon alpha‐2b (Chang 1997), other research has indicated that the body may produce neutralising antibodies that reduce the efficacy of interferon alpha‐2a as compared to interferon alpha‐2b (Antonelli 1991). Earlier research on interferon showed it to have an effect on vascular tumours such as Kaposi's sarcoma (Ezekowitz 1992).
Intralesional methylene blue
Feng and colleagues have suggested that methylene has a role in the management of IH due to its endothelial effects by induced thrombosis of the lesion, blocking the vascular supply and accelerating the necrosis of the haemangioma (Feng 2000).
Imiquimod
Imiquimod has a pro‐apoptotic and antiangiogenic activity. It activates the immune response system via the Toll‐like receptor‐7 on dendritic cells (Hu 2015; McCuaig 2009), resulting in induction of cytokines and interferon‐gamma, as well as matrix metalloproteinase (McCuaig 2009).
Laser
Lasers generally work by destroying blood vessels in the IH, with the equivalent wavelength of light absorbed by IH haemoglobin (Anderson 1981). The light from the laser is transformed into heat, and it is transmitted to the vessel wall, producing coagulation and vessel closure (Tawfik 2015). Oxyhaemoglobin has been the classic target chromophore for vascular lesions, due to its absorption peaks at 418, 542, and 577 nm. Oxyhaemoglobin contained within vascular lumina absorb the light energy emitted from pulsed dye laser (PDL) devices, minimising collateral damage (Rothfleisch 2002).
In order to increase the tissue penetration of PDL, the original wavelength of 577 nm, which corresponds to the third absorption peak of oxyhaemoglobin, has been increased over the past decade to 585 nm, and the pulse duration has also been increased from 450 microseconds to 1.5 milliseconds. Cryogen‐cooling devices have been added in order to reduce pain and related adverse events (Rothfleisch 2002).
Argon laser devices radiate blue‐green light, with emissions between 488 to 514 nanometers on the electromagnetic spectrum (Rothfleisch 2002). Argon laser energy is emitted by a continuous beam, which penetrates tissue at a depth of 1 mm to 2 mm. Emissions are absorbed mainly by oxyhaemoglobin, although epidermal and dermal melanin also have a degree of absorption (Rothfleisch 2002). The popularity of the argon laser has markedly declined over the past decade because of its associated limitations and the development of the pulse dye laser (Rothfleisch 2002).
Carbon dioxide laser emits light in the infrared spectrum (10,600 nm), which is primarily absorbed by water molecules. Its action involves the excision or debulking of vascular tissue or actinically damaged facial skin (Al 2003; Krupa 2009).
Neodymium‐doped yttrium aluminum garnet (Nd:YAG) laser has been reported to have a weaker melanin absorption and a deeper effect on lesions (Rothfleisch 2002). In addition, Nd:YAG has a high absorption coefficient of methaemoglobin and deoxyhaemoglobin, both of which are major parts of blue veins. Due to the long pulsed duration feature, Nd:YAG has a slower and more uniformed heat effect in the IH vessels, which generates coagulation without rupturing the vessel or causing purpura or hyperpigmentation (Rothfleisch 2002).
Dual‐wavelength laser combines PDL laser with YAG laser, allowing oxygenated haemoglobin to be transformed into methaemoglobin, and significantly increasing the Nd:YAG laser absorption rate. Local heat is reduced by a cooling system of ‐4 °C at the lesion site (Lu 2016).
The erbium laser (Er:YAG laser) produces a smaller zone of thermal injury, removing the epidermis in two or three passes, compared with the conventional CO2 resurfacing lasers (McDaniel 1997). The 2940‐nanometre wavelength of erbium produces a collagen absorption peak at 3030 nm (McDaniel 1997).
Oral ibuprofen plus oral paracetamol
In general, the mechanism of action of NSAIDs involves the inhibition of prostanoid biosynthesis (Abramson 1989). NSAIDs in combination with analgesics can have a role in the management of ulcerated IH, especially for pain relief (Tiwari 2016).
Radiation
Ionising radiation could be preferred due to its low chance of causing local scarring (Zhu 2015). In general, the pathophysiology of radiation‐induced changes in the skin involves: 1) a transient early erythema, which remits after 24 to 48 hours; 2) a main erythematous reaction related to the severe loss of epidermal basal cells; 3) a subsequent phase of erythema combined with dermal ischaemia and possible necrosis; and 4) the appearance of dermal atrophy, telangiectasia, and necrosis (Hopewell 1990).
Rapamycin
Rapamycin has been reported to have a superior antihaemangioma activity due to its inhibition of the proliferation of haemangioma endothelial cells and vascular endothelial growth factor production (Li 2017).
Steroids
High‐dose intralesional (injected directly into the lesion) or systemic (whole body) steroid therapies work by stopping the growth of the haemangioma through the promotion of stabilisation or regression, and possibly by softening the lesion (Bennett 2001).
Surgery
Surgical management involves removing the haemangioma in order to restore normal facial features (Liang 2014). Several techniques have been proposed including circular excision with purse‐string closure (Mulliken 2002), as well as single‐stage resection (Daramola 2012).
Vincristine
Vincristine is thought to act as an antiangiogenic through its effect on vascular endothelial growth factor (Azzopardi 2012). In addition, vincristine works by reducing the creation of microtubules, triggering mitotic arrest during metaphase, which produces apoptosis of tumour cells in vitro (Glade 2010).
Active monitoring
Despite the fact that it is hypothesised that several growth factors (e.g. hormonal, mechanical) are involved in the abnormal proliferation of endothelial cells in IH, the mechanism of action behind haemangiogenesis remains unknown (Marchuk 2001). In addition, most infantile haemangiomas are reported to involute completely by four years of age (Couto 2012).
Why it is important to do this review
A significant minority of babies (up to 1 in 10) develop infantile haemangiomas (Léauté‐Labrèze 2015). While the majority of IH are non‐problematic and will regress and disappear in five to seven years, a few will become problematic or cause mental distress to children and their parents (Csoma 2017). Some IH may also result in complications, including congestive heart failure, lifelong disfigurement, bleeding, ulceration, and visual and airway‐related obstruction (Csoma 2017). In such cases, medical intervention with a variety of medical treatments may be necessary. Propranolol therapy has been recommended as the most effective way of treating IH, as it inhibits proliferation and incites regression of IH during the proliferative phase (Zhang 2017). However, other methods may still be in use. It was therefore necessary to review the efficacy and potential adverse events of these interventions for the management of IH.
A first assessment of these interventions was published by Leonardi‐Bee in 2011 (Leonardi‐Bee 2011), reporting information from four trials with limited evidence.
Objectives
To assess the effects of interventions for the management of infantile haemangiomas in children.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials.
Types of participants
Any child (usually under 24 months) with single or multiple infantile haemangiomas located on the skin. We excluded participants above the age of 18 years. We excluded studies with a mixture of populations (including children and adults) that did not provide separate information for children. In addition, we excluded children with cases of very rare types of haemangiomas (including congenital haemangioma, haemangiomas associated with Kasabach‐Merritt syndrome, and eruptive neonatal haemangiomatosis) and internal haemangiomas.
Types of interventions
We considered all types of interventions used in the treatment of infantile haemangiomas. Interventions could be given alone or in combination. The most commonly used interventions include the following.
Beta blockers: propranolol, timolol maleate, and atenolol
Lasers: pulsed dye, argon, carbon dioxide, Nd:YAG, and erbium
Steroids: administered topically, intralesionally, or systemically
Surgery: excision or cryotherapy
Other treatments: imiquimod, interferon alpha‐2a, bleomycin, vincristine, and rapamycin (administered topically, intralesionally, orally, or systemically)
Comparators included placebo, active monitoring (i.e. wait‐and‐see), or other interventions (e.g. systemic steroids versus laser therapy). Comparator interventions could be given alone or in combination.
Types of outcome measures
Primary outcomes
Clearance, as assessed by a clinician at any follow‐up: proportion of children with lesions completely cleared or with minimal residual signs (defined as faint macular erythema with no palpable component).
A subjective measure of improvement, as assessed by the parent or child, at any follow‐up.
Adverse events experienced at short (immediately after treatment until 48 hours after) or long term (more than 48 hours after treatment) related to each intervention. These included skin atrophy (scarring where the skin is thinned, with or without depression at the skin surface), skin hypopigmentation (loss of skin pigmentation), and complications (including bleeding, ulceration, infection, deformation, disfigurement, vision impairment, airway obstruction, pain associated with treatment or ulceration, and/or side effects of treatments). We also considered reports of number of adverse events in general, as well as serious/severe adverse events (as defined by trial authors).
Secondary outcomes
Other measures of resolution, as assessed by a clinician, at any follow‐up. These included surface area, height or volume of lesion, and redness of lesion, preferably using an objective measure of assessment, such as photographs.
Proportion of parents who consider their child still has a problem, at any follow‐up.
Proportion of children who consider they still have a problem, at any follow‐up.
Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up.
Requirement for surgical correction, as assessed by a physician, at any follow‐up.
Timing
We considered data recorded for six months or less from baseline to reflect short‐term benefit, and we analysed these data separately from data recorded after six months' follow‐up, apart from adverse events data, where we considered up to 48 hours from baseline short term.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials regardless of language or publication status (published, unpublished, in press, or ongoing).
Electronic searches
For this update, we revised our search strategies in line with current Cochrane Skin Group practices. Details of the previous search strategies are available in Leonardi‐Bee 2011.
We searched the following databases up to 22 February 2017:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2017, Issue 1 in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
Embase via Ovid (from 1974) using the strategy in Appendix 4;
AMED via Ovid (Allied and Complementary Medicine, from 1985) using the strategy in Appendix 5;
PsycINFO via Ovid (from 1806) using the strategy in Appendix 6;
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 7; and
CINAHL via EBSCO (Cumulative Index of Nursing and Allied Health Literature, from 1981) using the strategy in Appendix 8.
Searching other resources
Trials registers
On 22 February 2017 we searched the following ongoing trials databases using the terms 'haemangioma', 'hemangioma', 'strawberry', 'naevi', or 'naevus':
ISRCTN register (www.isrctn.com);
ClinicalTrials.gov (www.clinicaltrials.gov);
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/); and
EU Clinical Trials Register (www.clinicaltrialsregister.eu).
Searching reference lists
We checked the bibliographies of included studies for additional references to relevant trials.
Adverse effects
We did not perform a separate search for adverse effects of interventions used for the treatment of infantile haemangiomas. We considered adverse and side effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (LG and SB) independently selected eligible studies. Authors reviewed titles and abstracts of all articles identified by the search to assess whether they met the inclusion criteria. The full texts of selected studies were further assessed to confirm their relevance for inclusion in the review. An additional third review author was consulted when disagreements arose (IAR). At any stage of the review, review authors were not blinded to the authors’ names and institutions, journal of publication, or study results. All excluded studies and reasons for their exclusion are listed in the Characteristics of excluded studies tables.
Data extraction and management
Two review authors (LG and SB) independently performed data extraction using predesigned data collection spreadsheets. We extracted participant characteristics, methods of randomisation, blinding, comparisons of interest, number of children originally randomised by arm, and follow‐up losses and outcomes. A third review author was consulted when disagreements arose (MN or IAR). We entered extracted data into Review Manager 5 for further analysis (Review Manager 5.3).
Assessment of risk of bias in included studies
As outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), two review authors (LG and SB) independently assessed risk of bias in included trials. We took six domains into consideration: random sequence generation, blinding of participants and personnel, blinding of outcome assessment, allocation concealment, selective reporting, and other biases. We assessed blinding of participants and blinding of personnel separately, as in most cases this information was partially reported (i.e. study authors reported blinding for participants or for personnel, but not both). We judged each domain to be at low, high, or unclear risk of bias. Disagreements were solved in consultation with a third review author (IAR). We assessed the direction and magnitude of bias, as well as its correlational impact on any findings (Higgins 2011). We summarised information in 'Risk of bias' tables in the Characteristics of included studies.
Measures of treatment effect
We expressed results as risk ratios (RR) with 95% confidence intervals (CI) for dichotomous outcomes, and difference in means (MD) with 95% CI for continuous outcomes. Transformation of data was not required.
Unit of analysis issues
Where there were multiple intervention groups within a trial, we made pair‐wise comparisons of similar active interventions versus no treatment, placebo, or other active intervention. Although we did not find any randomised controlled trials that used cross‐over or internally controlled designs, we would have analysed the former using data only from the first phase pooled, where possible, with parallel‐design studies. For the latter, we would have used appropriate techniques for paired designs without pooling with studies of other designs.
Dealing with missing data
In cases where participant dropout led to missing data, we conducted an intention‐to‐treat analysis. For dichotomous outcomes, we regarded children with missing outcome data as treatment failures and included them in the analysis. For continuous outcomes, we would have considered using the last recorded value carried forward for children with missing outcome data; however, these circumstances did not occur. If high levels of missing data were seen within the analyses, we planned to conduct sensitivity analyses to assess the robustness of the results from the approaches described above, by comparing the results with those excluding the missing data from the analyses. However, these circumstances did not occur.
Assessment of heterogeneity
We investigated heterogeneity with close visual examination of the forest plots. Additionally, we assessed statistical heterogeneity of effect sizes by means of the I² statistic. The I² statistic is employed to describe the per cent of total variation across all contributing trials due to heterogeneity rather than sampling error (Higgins 2011). If we identified signs of heterogeneity (I² > 30%), we performed further exploration by prespecified subgroup analysis; furthermore, if we identified considerable per cent of heterogeneity (I² > 80%), we did not present pooled results.
Assessment of reporting biases
We planned to use funnel plots, with respect to primary outcomes, to illustrate whether treatment estimates were related to study size or to determine variability among trials in an attempt to detect publication bias. If 10 or more trials are available, extrapolation based on asymmetry is plausible. However, due to scarcity of data in all comparisons, we were unable to perform a full analysis of reporting bias.
Data synthesis
For studies with a similar type of active intervention, we performed a meta‐analysis to calculate a weighted treatment effect across trials using a random‐effects (DerSimonian and Laird) model. We planned and carried out statistical analyses using Review Manager 5 (Review Manager 5.3). When it was not possible to perform a meta‐analysis, we presented data narratively.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis and to determine interaction tests to check for subgroup differences where meaningful. For the primary outcomes, we considered subgroup analyses for the following factors:
dosage;
duration of treatment;
types of infantile haemangioma (superficial, deep, mixed, others);
location of birthmark (low‐risk or high‐risk areas).
Due to scarcity of data in all comparisons, we were unable to perform a full investigation of heterogeneity.
Sensitivity analysis
We planned to conduct sensitivity analyses using trials classified as having low risk of bias in three core domains: allocation concealment, incomplete outcome data, and blinding of outcome assessment, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). However, due to scarcity of data in all comparisons, we were unable to perform a full sensitivity analysis.
'Summary of findings' tables
We assessed quality of the body of evidence (also known as certainty in the evidence) pertaining to primary and secondary outcomes using the principles of the GRADE system (Guyatt 2008). We also constructed 'Summary of findings' tables. Factors taken into consideration in the evaluation of quality of the evidence are study risk of bias, heterogeneity of data, directness of the evidence, precision of effect estimates, and potential publication bias (Guyatt 2008; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g; Guyatt 2011h). We developed the 'Summary of findings' table using a web‐based version of the GRADEpro software (GRADEpro GDT), according to the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We selected information about currently used treatments for these 'Summary of findings' tables.
We developed 'Summary of findings' tables for the following comparisons:
oral propranolol versus placebo;
topical timolol maleate versus placebo;
oral propranolol versus topical timolol maleate.
We assessed the quality of the evidence for the following outcomes in these comparisons:
clearance (as assessed by a clinician);
subjective measurements of improvement;
adverse events;
other measures of resolution;
proportion of parents who consider their child still has a problem;
proportion of children who consider they still have a problem; and
aesthetic appearance.
For the outcome 'adverse events', we presented in the corresponding table the most frequent or the most important adverse event, or both, related to each intervention. When information about adverse events in general (including serious/severe adverse events) was available, we presented these results instead of individual findings.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
Our updated searches identified 932 new records in addition to the 128 identified in the first version of this review. From the combined total of 1060 records, we screened out 969 records based on titles and abstracts. We examined the remaining 91 records in full text. From these, we excluded 45 studies (16 identified in the previous version of this review and 29 from this update, see Characteristics of excluded studies). We included 28 studies reported in 37 references (see Characteristics of included studies). We classified seven studies as awaiting assessment (see Characteristics of studies awaiting classification) and two further studies as ongoing (see Characteristics of ongoing studies). For a further description of the screening process, see the study flow diagram (Figure 1),
1.
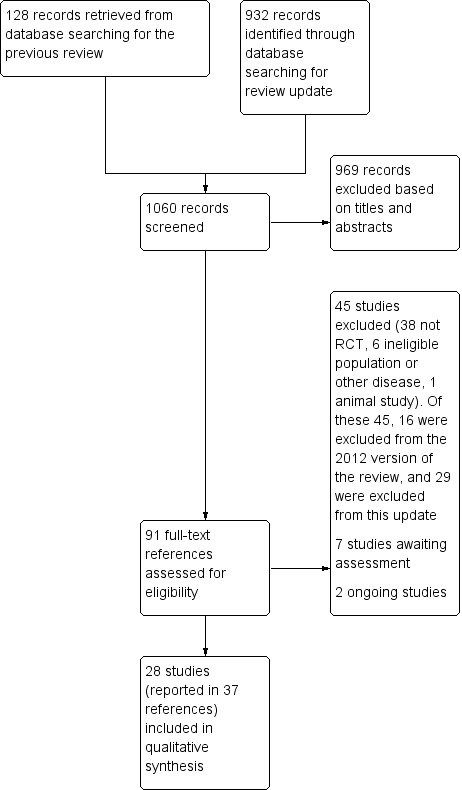
Study flow diagram.
Included studies
Twenty‐eight studies (reported in 37 references) were eligible for inclusion in this updated review. All studies were published. The 28 studies enrolled and randomised a total of 1728 participants. Details of the included studies are provided in the Characteristics of included studies tables. Four of the 28 studies were included in the previous version of this review, with 24 new studies included in this update.
Design
A total of 21 studies used a two‐arm design; six used a three‐arm design; and one used a four‐arm, parallel‐group design.
Sample sizes
Numbers of children in the studies ranged from 12 in Zhang 2013 to 460 in Leaute‐Labreze 2015. Only five studies reported a calculation of sample sizes prior to the beginning of the trial (Bauman 2014; Hogeling 2011; Kessels 2013; Leaute‐Labreze 2015; Pope 2007).
Setting
Studies were conducted in Canada (Pope 2007), China (Feng 2000; Fu 2012; Gong 2015; Li 2016; Lu 2016; Tan 2012; Xu 2006; Zhang 2013; Zhong 2015; Zhu 2015), Germany (Jung 1977), Chile (Abarzua‐Araya 2014), the UK (Batta 2002), the USA (Bauman 2014), Australia (Chan 2013; Hogeling 2011), Iran (Asilian 2015; Ehsani 2014), the Netherlands (Kessels 2013), France (Leaute‐Labreze 2013; Leaute‐Labreze 2015), India (Malik 2013; Tiwari 2016), and Egypt (Aly 2015; Tawfik 2015; Zaher 2013; Zaher 2016).
Participants
Baseline data were reported in 17 trials (Abarzua‐Araya 2014; Asilian 2015; Batta 2002; Bauman 2014; Chan 2013; Ehsani 2014; Gong 2015; Hogeling 2011; Jung 1977; Kessels 2013; Leaute‐Labreze 2013; Leaute‐Labreze 2015; Li 2016; Lu 2016; Pope 2007; Zaher 2013; Zaher 2016). Four trials did not report the number of males and females included (Aly 2015; Malik 2013; Tiwari 2016; Xu 2006). One trial had equal numbers of males and females (Zhang 2013). The remaining 23 trials had a greater number of females than males, ranging from 58% in Li 2016 to 86% in Zaher 2013. The maximum age of enrolment at the beginning of the trial, as an inclusion criterion, ranged from 14 weeks in Batta 2002 to five years in Hogeling 2011; four studies did not clearly state this information (Lu 2016; Tawfik 2015; Zaher 2013; Zaher 2016). Age was heterogeneously reported in the included studies (mean, medians, ranges for total, or subgroups were reported). In 19 studies reporting mean age, this ranged from 12 weeks in Pope 2007 to 13.4 years in Tawfik 2015.
Subtypes of haemangiomas
Different subtypes of infantile haemangiomas were assessed, including children with mixed or deep IH (Li 2016; Lu 2016; Pope 2007; Zhong 2015), ulcerated or problematic IH (Hogeling 2011; Malik 2013; Tiwari 2016; Zaher 2013; Zaher 2016), or high‐risk haemangiomas (Abarzua‐Araya 2014; Aly 2015; Asilian 2015; Hogeling 2011; Lu 2016; Zaher 2013; Zaher 2016; Zhu 2015). One study assessed facial haemangiomas (defined as "periorbital/orbital tumours with visual impairment and/or large size/disfiguring haemangiomas") (Pope 2007). Six studies exclusively assessed superficial haemangiomas (Asilian 2015; Batta 2002; Chan 2013; Gong 2015; Kessels 2013; Zhu 2015), while one trial evaluated mixed haemangiomas only (Li 2016). Jung 1977 assessed planotuberous or tuberocavernous haemangiomas. The remaining trials did not provide additional information about the type of IH included or included a mixture of subtypes (Bauman 2014; Ehsani 2014; Feng 2000; Fu 2012; Leaute‐Labreze 2013; Leaute‐Labreze 2015; Tan 2012; Tawfik 2015; Xu 2006; Zhang 2013).
Interventions
The included trials assessed the following interventions for treating infantile haemangiomas.
Lasers: pulsed dye laser (PDL), Nd:YAG laser, sequential/concurrent dual‐wavelength laser.
Beta blockers: oral/topical propranolol, topical timolol maleate.
Steroids: oral prednisolone.
Other treatments: topical bleomycin, intralesional methylene blue.
High‐intensity focused ultrasound (HIFU).
Radiation therapy: soft X‐ray radiation, ⁹⁰SR‐⁹⁰Y radiation.
Some treatments were used in combination.
Comparators included active monitoring (observation), placebo, sham radiation, and the following interventions (single or in combination with another intervention).
Beta blockers: intralesional/oral/topical propranolol, topical timolol maleate, oral atenolol.
Oral ibuprofen plus oral paracetamol, oral captopril.
Lasers: concurrent dual‐wavelength laser, PDL alone, Nd:YAG laser.
Steroids: oral prednisolone, intralesional triamcinolone, methylprednisolone (infusion).
HIFU.
Radiation therapy: ⁹⁰SR‐⁹⁰Y radiation.
We identified no evidence for argon laser, carbon dioxide laser, erbium laser, excision, cryotherapy, imiquimod, interferon alpha, vincristine, or rapamycin. We found the following treatment comparisons.
PDL versus wait‐and‐see (i.e active monitoring) (Batta 2002; Kessels 2013).
Oral propranolol versus placebo (Hogeling 2011; Leaute‐Labreze 2013; Leaute‐Labreze 2015).
Topical timolol maleate versus placebo (Chan 2013).
Topical bleomycin versus placebo (Xu 2006).
X‐ray radiation versus sham radiation (Jung 1977).
Nd:YAG laser versus topical timolol maleate (Tawfik 2015).
Nd:YAG laser versus oral propranolol (Tan 2012; Zhong 2015).
PDL + topical propranolol versus PDL alone (Ehsani 2014).
PDL + topical timolol maleate versus PDL alone (Asilian 2015).
Nd:YAG laser + oral propranolol versus Nd:YAG laser (Tan 2012; Zhong 2015).
Nd:YAG laser + oral propranolol versus oral propranolol (Tan 2012; Zhong 2015).
⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate versus ⁹⁰SR‐⁹⁰Y radiation (Zhu 2015).
Sequential dual‐wavelength laser + oral propranolol versus concurrent dual‐wavelength laser + oral propranolol (Lu 2016).
Oral propranolol versus topical propranolol (Zaher 2013).
Oral propranolol versus intralesional propranolol (Zaher 2013).
Topical propranolol versus intralesional propranolol (Zaher 2013).
Oral propranolol versus oral atenolol (Abarzua‐Araya 2014).
Oral propranolol versus oral prednisolone (Bauman 2014; Malik 2013).
Oral propranolol versus oral captopril (Zaher 2016).
Oral propranolol versus topical timolol maleate (Gong 2015).
Oral propranolol versus oral propranolol + oral prednisolone (Aly 2015; Malik 2013).
Oral propranolol versus oral ibuprofen + oral paracetamol (Tiwari 2016).
Oral propranolol + topical timolol maleate versus oral propranolol (Gong 2015; Li 2016).
Oral propranolol + topical timolol maleate versus topical timolol maleate (Gong 2015).
Oral propranolol + oral prednisolone versus oral prednisolone (Malik 2013).
Intralesional methylene blue versus intralesional triamcinolone (Feng 2000).
Oral prednisolone versus oral methylprednisolone (Pope 2007).
HIFU at 3.5 W versus HIFU at 4.5 W (Fu 2012).
HIFU at 3.5 W versus HIFU at 4.0 W (Fu 2012).
HIFU at 4.0 W versus HIFU at 4.5 W (Fu 2012).
Duration of treatment and follow‐up
The most common duration of treatment was 24 weeks, found in five trials (Abarzua‐Araya 2014; Aly 2015; Chan 2013; Hogeling 2011; Tan 2012). In six trials, there was no fixed length of intervention for all children; the intervention was stopped for the following reasons: when the lesion was cleared; there was no clear improvement; in the presence of important side effects; or for parent's/clinician request, among other reasons (Batta 2002; Bauman 2014; Gong 2015; Kessels 2013; Zaher 2013; Zaher 2016). In 11 trials, this information was unclear or poorly reported (Asilian 2015; Ehsani 2014; Feng 2000; Fu 2012; Li 2016; Lu 2016; Malik 2013; Pope 2007; Tawfik 2015; Tiwari 2016; Zhang 2013).
Duration of follow‐up ranged from 7 days in Xu 2006 to 72 months in Jung 1977. The most frequent duration of follow‐up was six months, found in nine trials (Abarzua‐Araya 2014; Asilian 2015; Chan 2013; Fu 2012; Hogeling 2011; Tan 2012; Zaher 2013; Zhong 2015; Zhu 2015). In three trials this information was unclear (Feng 2000; Lu 2016; Zhang 2013).
Outcomes
Fourteen trials assessed our primary outcome measure of clearance (Abarzua‐Araya 2014; Asilian 2015; Batta 2002; Ehsani 2014; Feng 2000; Fu 2012; Jung 1977; Leaute‐Labreze 2015; Tan 2012; Tawfik 2015; Tiwari 2016; Zaher 2013; Zaher 2016; Zhu 2015). One trial reported a subjective measure of improvement (Pope 2007). Adverse events were fully reported in 20 trials (Abarzua‐Araya 2014; Aly 2015; Asilian 2015; Batta 2002; Bauman 2014; Chan 2013; Ehsani 2014; Fu 2012; Gong 2015; Hogeling 2011; Kessels 2013; Leaute‐Labreze 2015; Li 2016; Malik 2013; Tan 2012; Tiwari 2016; Zaher 2013; Zaher 2016; Zhong 2015; Zhu 2015). Other measures of resolution such as redness, reduction of volume, colour fading, haemoglobin levels, or mean size reduction were assessed in 16 trials (Aly 2015; Asilian 2015; Batta 2002; Bauman 2014; Chan 2013; Gong 2015; Hogeling 2011; Kessels 2013; Li 2016; Lu 2016; Malik 2013; Pope 2007; Tawfik 2015; Tiwari 2016; Xu 2006; Zhong 2015). One trial reported the proportion of parents who consider their child still has a problem (Batta 2002); the same trial also reported on requirement for surgical correction. No trials reported the proportion of children who consider they still have a problem. In addition, one trial reported findings related to aesthetic appearance (Kessels 2013).
Excluded studies
Among the 45 studies excluded after full‐text assessment, six were excluded due to ineligible population or other diseases (Liu 2009; Midena 2008; Pancar 2011; Rouvas 2009; Tierney 2009; Zhou 2002), and one for being developed in animals (Zhou 2015). The remaining 38 excluded studies were not randomised trials. Details of these studies and reasons for exclusion are listed in the Characteristics of excluded studies tables.
Studies awaiting classification
We assessed seven studies as awaiting classification because only partial information was available for these references (from abstracts, conference proceedings, or registration entries in trial platforms, among other reasons) (Kuang 2014; Maier 2012; NCT00004436; NCT00555464; NCT00744185; NCT01072045; Pandey 2010). Preliminary details are reported in the Characteristics of studies awaiting classification tables.
Ongoing studies
We identified two ongoing trials from the updated searches (NCT01147601; NCT02913612). The designs of the trials are listed below.
NCT01147601: topical 0.5% timolol maleate versus placebo, 2 to 3 drops to cover the haemangioma, twice daily.
NCT02913612: timolol maleate gel forming solution drug versus wait‐and‐see (i.e. active monitoring).
Risk of bias in included studies
We summarised the risk of bias of all the studies in the Characteristics of included studies section. The 'Risk of bias' graph (review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies) is presented in Figure 2. The 'Risk of bias' summary (review authors' judgements about each 'Risk of bias' item for each included study) is presented in Figure 3.
2.
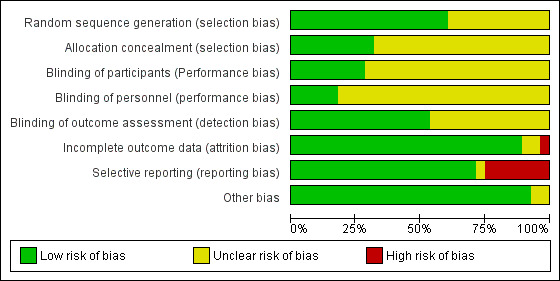
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
3.
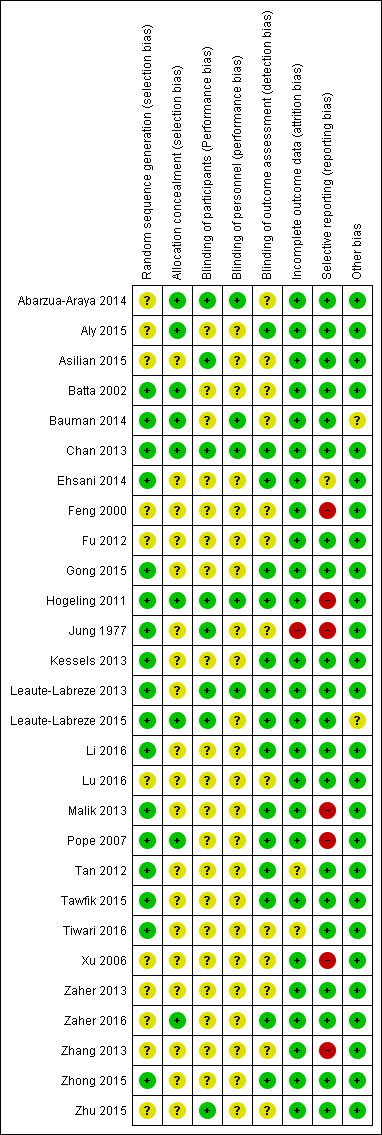
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Seventeen trials used an adequate method of randomisation and were hence rated as at low risk of bias (Batta 2002; Bauman 2014; Chan 2013; Ehsani 2014; Gong 2015; Hogeling 2011; Jung 1977; Kessels 2013; Leaute‐Labreze 2013; Leaute‐Labreze 2015; Li 2016; Malik 2013; Pope 2007; Tan 2012; Tawfik 2015; Tiwari 2016; Zhong 2015), while the other 11 did not provide sufficient information about the sequence generation process to permit judgement (unclear risk of bias) (Abarzua‐Araya 2014; Aly 2015; Asilian 2015; Feng 2000; Fu 2012; Lu 2016; Xu 2006; Zaher 2013; Zaher 2016; Zhang 2013; Zhu 2015).
Nine studies specified adequate methods of allocation concealment and were hence rated as at low risk of bias (Abarzua‐Araya 2014; Aly 2015; Batta 2002; Bauman 2014; Chan 2013; Hogeling 2011; Leaute‐Labreze 2015; Pope 2007; Zaher 2016). The remaining trials (n = 19) did not specify allocation concealment methods and so were assessed as at unclear risk of bias.
Blinding
Eight trials reported blinding of participants (Abarzua‐Araya 2014; Asilian 2015; Chan 2013; Hogeling 2011; Jung 1977; Leaute‐Labreze 2013; Leaute‐Labreze 2015; Zhu 2015), and we judged these trials to be at a low risk of performance bias. The remaining studies (n = 20) did not report this information clearly and so were judged to be at unclear risk of performance bias in relation to participants.
Five trials reported blinding of study personnel and so were rated as at low risk of performance bias (Abarzua‐Araya 2014; Bauman 2014; Chan 2013; Hogeling 2011; Leaute‐Labreze 2013). We rated the remaining 23 trials as at unclear risk of performance bias in relation to personnel.
Fifteen trials reported blinding of outcome assessment and so were rated as at low risk of bias (Aly 2015; Chan 2013; Ehsani 2014; Gong 2015; Hogeling 2011; Kessels 2013; Leaute‐Labreze 2013; Leaute‐Labreze 2015; Li 2016; Malik 2013; Pope 2007; Tan 2012; Tawfik 2015; Zaher 2016; Zhong 2015). The remaining trials (n = 13) did not report this information explicitly and so were rated as at unclear risk of bias.
We rated only three trials as at low risk of bias for all three of the blinding items assessed (Chan 2013; Hogeling 2011; Leaute‐Labreze 2013).
Incomplete outcome data
Risk of attrition bias was high in Jung 1977 because of a high dropout rate (47% in the intervention group and 44% in the control group) with no reasons given. We assessed two trials as at unclear risk of bias (Tan 2012; Tiwari 2016). The risk of attrition bias was low in the remaining 25 trials due to a low or null dropout rate.
Selective reporting
There was evidence of selective omissions of outcomes or critical information from the publications of seven trials (Feng 2000; Hogeling 2011; Jung 1977; Malik 2013; Pope 2007; Xu 2006; Zhang 2013). In the Pope 2007 trial, the scores for a subjective measure of improvement as rated by the parents were not presented; the authors instead presented the correlation between the scores of the parents and the scores of the outcome assessors (intraclass correlation coefficient: 0.92). The choice of selective omission of the outcome did not appear to be based on outcome result, since highly significant findings were seen for the reported outcomes. We judged Ehsani 2014 to be at unclear risk of selective reporting because an outcome in their protocol was not included in their study report. We judged the remaining trials (n = 20) as at low risk of reporting bias.
Other potential sources of bias
There may be other sources of bias in one study related to the role of the sponsors in the development of the research (Leaute‐Labreze 2015); hence we rated this trial as at unclear risk of bias for this domain. We found Bauman 2014 to be at unclear risk of other potential sources of bias due to the termination of the trial, which might generate biases in the results. We identified no additional sources of bias in the remaining studies (n = 26).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Oral propranolol compared to placebo for infantile haemangiomas of the skin.
| Oral propranolol compared to placebo for infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Patient or population: infantile haemangiomas (strawberry birthmarks) of the skin Setting: all settings (outpatient care) Intervention: oral propranolol Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with oral propranolol | |||||
|
Clearance, as assessed by a clinician at any follow‐up ‐ 3 mg/kg/day 24 weeks' follow‐up |
36 per 1000 | 604 per 1000 (153 to 1000) | RR 16.61 (4.22 to 65.34) | 156 (1 RCT) | ⊕⊕⊝⊝ MODERATE1 | |
| A subjective measure of improvement, as assessed by the parent or child, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
|
Adverse events experienced at short or long term ‐ Serious adverse events 24 weeks' follow‐up |
37 per 1000 | 38 per 1000 (12 to 124) | RR 1.05 (0.33 to 3.39) | 509 (3 RCTs) | ⊕⊝⊝⊝ LOW2 | |
| Other measures of resolution, as assessed by a clinician, at any follow‐up ‐ percentage change in mean haemangioma volume at 24 weeks | Mean: ‐14.1% (SD not reported) | Mean: ‐60% (SD not reported) | MD 45.9% lower (80.2% lower to 11.6% lower) |
40 (1 RCT) | ⊕⊕⊕⊝ MODERATE3 | Mean difference was reported by the study authors, but no SDs were reported for group means. |
| Proportion of parents who consider their child still has a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Proportion of children who consider they still have a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (the event rate in the single study or the mean event rate in the meta‐analysis) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level for imprecision (wide confidence interval around the estimate of the effect). 2Downgraded by two levels for imprecision (wide confidence interval around the estimate of the effect and low number of events). 3Downgraded by one level for risk of bias.
Summary of findings 2. Topical timolol compared to placebo for infantile haemangiomas of the skin.
| Topical timolol maleate compared to placebo for infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Patient or population: infantile haemangiomas (strawberry birthmarks) of the skin Setting: all settings (outpatient care) Intervention: topical timolol maleate Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with topical timolol maleate | |||||
| Clearance, as assessed by a clinician at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| A subjective measure of improvement, as assessed by the parent or child, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
|
Adverse events experienced at short or long term ‐ Serious cardiovascular adverse events ‐ bradycardia 24 weeks' follow‐up |
See comment | See comment | Not estimable | 41 (1 RCT) | ⊕⊕⊝⊝ LOW1 | No events of bradycardia reported in Chan 2013. |
| Other measures of resolution, as assessed by a clinician, at any follow‐up ‐ no redness | 45 per 1000 | 369 per 1000 (50 to 1000) | RR 8.11 (1.09 to 60.09) | 41 (1 RCT) | ⊕⊕⊝⊝ LOW2 | |
| Proportion of parents who consider their child still has a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Proportion of children who consider they still have a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (the event rate in the single study) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by two levels for imprecision (low number of participants and events). 2Downgraded by two levels for imprecision (wide confidence interval around the estimate of the effect and low number of participants and events).
Summary of findings 3. Oral propranolol compared to topical timolol for infantile haemangiomas of the skin.
| Oral propranolol compared to topical timolol maleate for infantile haemangiomas (strawberry birthmarks) of the skin | ||||||
| Patient or population: infantile haemangiomas (strawberry birthmarks) of the skin Setting: all settings (outpatient care) Intervention: oral propranolol Comparison: topical timolol maleate | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with topical timolol maleate | Risk with oral propranolol | |||||
| Clearance, as assessed by a clinician at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| A subjective measure of improvement, as assessed by the parent or child, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
|
Adverse events experienced at short or long term ‐ general adverse events 24 weeks' follow‐up |
See comment | See comment | RR 7.00 (0.40 to 123.35) | 26 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 | There were 3 events in the oral propranolol group and no events in the topical timolol maleate group. Due to no events in the control group, absolute events could not be calculated. |
|
Other measures of resolution, as assessed by a clinician, at any follow‐up ‐ size reduction ≥ 50% 24 weeks' follow up |
615 per 1000 | 695 per 1000 (394 to 1000) | RR 1.13 (0.64 to 1.97) | 26 (1 RCT) | ⊕⊕⊝⊝ LOW2 | |
| Proportion of parents who consider their child still has a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Proportion of children who consider they still have a problem, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | We did not identify any studies reporting this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group (the event rate in the single study) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by three levels: one level due to unclear risk of selection and performance bias and two levels for imprecision (wide confidence interval around the estimate of the effect and low number of participants and events). 2Downgraded by two levels: one level due to unclear risk of selection and performance bias and one level for imprecision (small sample size).
Comparison 1. Interventions versus placebo: PDL versus wait‐and‐see (i.e. active monitoring)
For this comparison, we included the information from two trials with a total of 143 children (Batta 2002; Kessels 2013). Both authors included children with superficial early haemangiomas in the preproliferative or early proliferative growth phase. Batta 2002 used Chromos 585 nm wavelength venous flash‐lamp pulsed dye laser without epidermal cooling (SLS Biophile, Dyfed, Wales, UK) at a pulse duration of 0.45 ms, with spot diameter of 3 to 5 mm, and energy fluence of 6.0 to 7.5 J/cm², and the treatment was repeated every 2 to 4 weeks. Duration of treatment was until the lesion cleared, stopped proliferating, stopped responding, or if the parents discontinued treatment (treatment maximum of one year), and children were followed until six months. Kessels 2013 used 595 nm PDL (Vbeam, Syneron Candela, Wayland, MA, USA) with 7‐millimetre spot diameter, 30/10 to 40/10 epidermal cooling, at fluence range of 7 to 15 J/cm² and a pulse duration of 0.45 to 40.0 ms. The treatment was repeated every two to six weeks. The intervention ended when the child had complete remission, stop of proliferation, or no response of the haemangioma. Children were followed until 12 months.
Primary outcome 1: Clearance, as assessed by a clinician
One trial provided information about this outcome and reported a total of 52 cases of clearance (121 children; percentage of clearance: 42.9%) (Batta 2002): 25 children out of 60 (41.6%) in the PDL group and 27 out of 61 (44.2%) in the wait‐and‐see group reached clearance of lesions. This study found no differences in terms of clearance when comparing PDL with wait‐and‐see (risk ratio (RR) 0.94, 95% confidence interval (CI) 0.62 to 1.42; Analysis 1.1).
1.1. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Two trials provided information for four different adverse events. Batta 2002 provided information about 22 cases of skin atrophy (121 children; percentage of skin atrophy: 18.1%) and 36 cases of skin hypopigmentation (121 children; percentage of skin hypopigmentation: 29.7%). Seventeen children out of 60 (28.3%) in the PDL group and 5 out of 61 (8.1%) in the wait‐and‐see group had skin atrophy. The risk of skin atrophy after PDL was 3.46 times that after wait‐and‐see (RR 3.46, 95% CI 1.36 to 8.77; Analysis 1.2). In addition, 27 children out of 60 (45%) in the PDL group and 9 out of 61 (14.7%) in the wait‐and‐see group had skin hypopigmentation. The risk of skin hypopigmentation after PDL was 3.05 times that after wait‐and‐see (RR 3.05, 95% CI 1.57 to 5.93; Analysis 1.3).
1.2. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 2 Adverse events: skin atrophy.
1.3. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 3 Adverse events: skin hypopigmentation.
Likewise, Kessels 2013 provided information about two cases of minimal crusting (22 children; percentage of minimal crusting: 9.09%) and two cases of pain (22 children; percentage of skin hypopigmentation: 9.09%). Two children out of 11 (18%) in the PDL group and 0 out of 11 (0%) in the wait‐and‐see group had minimal crusting. This study found no clear differences in terms of these adverse events when comparing PDL with wait‐and‐see, due to imprecision (RR 5.00, 95% CI 0.27 to 93.5; Analysis 1.4; and RR 5.00, 95% CI 0.27 to 93.5; Analysis 1.5).
1.4. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 4 Adverse events: minimal crusting.
1.5. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 5 Adverse events: pain.
Secondary outcome 1: Other measures of resolution
Batta 2002 provided information about 23 cases of no‐redness (121 children; percentage of no‐redness: 19%). Nineteen children out of 60 (31.6%) in the PDL group and 4 out of 61 (6.5%) in the wait‐and‐see group had no‐redness. The risk of absence of redness after PDL was 4.83 times that after wait‐and‐see (RR 4.83, 95% CI 1.75 to 13.36; Analysis 1.6). Batta 2002 provided information about surface area after follow‐up, reporting a median of 113 mm² (range 0 to 150) for the PDL group and a median of 146 mm² (range 0 to 2403) for the group under observation. Kessels 2013 provided information about median change in surface area at the age of 1 year, reporting a median of 0.20 cm² (interquartile range (IQR) ‐0.10 to 0.58) for the group receiving PDL versus a median of 0.00 cm² (IQR ‐0.10 to 0.4) for the group under observation.
1.6. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 6 Other measures of resolution: no redness.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
One trial provided information for this outcome with 20 cases (121 participants; percentage of parents: 16.5%) (Batta 2002). Eleven participants out of 60 (18.3%) in the PDL group and 9 out of 61 (14.7%) in the wait‐and‐see group considered their child still has a problem. This study found no clear differences for this outcome when comparing PDL with wait‐and‐see (RR 1.24, 95% CI 0.56 to 2.78; Analysis 1.7).
1.7. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 7 Parents who consider that their child still has a problem.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
One trial provided information for this outcome with nine cases (22 children; percentage of aesthetic appearance: 40.9%) (Kessels 2013). A better cosmetic outcome was reported for 7 children out of 11 (63%) in the PDL group and 4 out of 11 (36%) in the wait‐and‐see group. This study found no clear differences for this outcome when comparing PDL with wait‐and‐see (RR 1.75, 95% CI 0.71 to 4.31; Analysis 1.8).
1.8. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 8 Aesthetic appearance: better cosmetic outcome.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
In Batta 2002, 7 out of 60 children in the PDL group compared with 3 out of 61 children in the wait‐and‐see group required surgical correction at 5 years' follow‐up. There was no significant difference between groups for this outcome (RR 2.37, 95% CI 0.64 to 8.75; Analysis 1.9).
1.9. Analysis.
Comparison 1 Pulsed dye laser versus wait‐and‐see, Outcome 9 Requirement for surgical correction.
Comparison 2. Interventions versus placebo: oral propranolol versus placebo
For this comparison, we included information from three trials (312 children) (Hogeling 2011; Leaute‐Labreze 2013; Leaute‐Labreze 2015). Hogeling 2011 enrolled 40 children between the ages of 9 weeks and 5 years with facial infantile haemangioma or infantile haemangiomas in sites with potential for disfigurement. Children were randomly assigned to receive propranolol or placebo. Administration was initiated at a dosage of 1 mg/kg per day divided 3 times daily and then increased to 2 mg/kg per day divided 3 times daily from weeks 2 to 24. The children were followed up at weeks 0, 4, 8, 12, 16, 20, and 24. Duration of treatment and follow‐up was 24 weeks in both cases. Leaute‐Labreze 2013 enrolled 14 children younger than 16 weeks with one or more non‐threatening infantile haemangiomas of more than 1 cm in diameter, without vital or functional impairment. Children were randomly assigned to receive placebo or propranolol 3 mg/kg daily for 15 days, then 4 mg/kg daily for 15 additional days. Duration of treatment and follow‐up was one month in both cases. Finally, Leaute‐Labreze 2015 enrolled 456 infants between 35 and 150 days old with proliferating infantile haemangioma requiring systemic therapy. Children were randomly assigned to receive propranolol 1 mg/kg per day, 3 mg/kg per day, or placebo for 3 or 6 months. We reported data for the groups followed for six months in this analysis.
Primary outcome 1: Clearance, as assessed by a clinician
One trial provided information for this outcome (Leaute‐Labreze 2015). Fifty children out of 102 (49%) in the oral propranolol group (1 mg/kg/day) and 2 out of 55 (3.6%) in the placebo group reached clearance of lesions. The risk of clearance after oral propranolol 1 mg/kg/day was 13.48 times that after placebo (RR 13.48, 95% CI 3.41 to 53.30; Analysis 2.1). Likewise, 61 children out of 101 (60.3%) in the oral propranolol group (3 mg/kg/day) and 2 out of 55 (3.6%) in the placebo group reached clearance of lesions. The risk of clearance after oral propranolol 3 mg/kg/day was 16.61 times that after placebo (RR 16.61, 95% CI 4.22 to 65.34; Analysis 2.1). We downgraded the quality of the evidence from high to moderate due to imprecision (see Table 1).
2.1. Analysis.
Comparison 2 Oral propranolol versus placebo, Outcome 1 Clearance, as assessed by a clinician.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Three trials reported information about 26 cases of serious adverse events in general, but this was based only on information from a single trial (Leaute‐Labreze 2015; percentage of cases: 5.1%), as the other two trials had zero events in both arms (Hogeling 2011; Leaute‐Labreze 2013). Twenty‐three children out of 427 (5.3%) in the oral propranolol group and 3 out of 82 (3.6%) in the placebo group had serious adverse events. This study found no differences in terms of adverse events when comparing oral propranolol with placebo (RR 1.05, 95% CI 0.33 to 3.39; Analysis 2.2). We downgraded the quality of the evidence from high to low due to imprecision (see Table 1). Likewise, we did not find a significant difference between oral propranolol and placebo at any doses, in terms of serious cardiovascular adverse events (Analysis 2.3) and other adverse events, including bronchospasm and hypoglycaemia (Analysis 2.4).
2.2. Analysis.
Comparison 2 Oral propranolol versus placebo, Outcome 2 Serious adverse events.
2.3. Analysis.
Comparison 2 Oral propranolol versus placebo, Outcome 3 Serious cardiovascular adverse events.
2.4. Analysis.
Comparison 2 Oral propranolol versus placebo, Outcome 4 Other adverse events.
Secondary outcome 1: Other measures of resolution
Hogeling 2011 provided information about other measures of resolution, including per cent change in volume at 24 weeks and redness. For change in volume, the authors reported a reduction of mean haemangioma volume at 24 weeks of 45.9% (95% CI 11.60% to 80.20%) comparing oral propranolol with placebo (Analysis 2.5). We downgraded the quality of the evidence from high to moderate due to imprecision (See Table 1). Likewise, Hogeling 2011 reported improvement in redness at week 24 in 4 children out of 20 (20%) in the oral propranolol group and 0 out of 20 (0%) in the placebo group. This study found no clear difference in terms of redness improvement when comparing oral propranolol with placebo due to imprecision (RR 9.00, 95% CI 0.52 to 156.9; Analysis 2.6).
2.5. Analysis.
Comparison 2 Oral propranolol versus placebo, Outcome 5 Other measures of resolution: change in volume.
2.6. Analysis.
Comparison 2 Oral propranolol versus placebo, Outcome 6 Other measures of resolution: no improvement in redness.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 3. Interventions versus placebo: topical timolol maleate versus placebo
For this comparison, we included the information from one trial (Chan 2013), with 41 children between the ages of 5 weeks and 24 weeks with small, focal superficial infantile haemangiomas. Children were randomly assigned to receive placebo or timolol maleate 0.5% gel. Administration was initiated by applying, with a fingertip, part of one drop of the gel onto the surface of the IH twice a day. Duration of treatment and follow‐up was six months in both cases.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Chan 2013 provided information about serious cardiovascular events, reporting zero events of bradycardia and zero of hypotension (41 children). We downgraded the quality of the evidence from high to low due to imprecision (see Table 2).
Secondary outcome 1: Other measures of resolution
Chan 2013 provided information about absence of redness at the end of follow‐up, reporting eight cases (41 children; percentage of absence of redness: 19.5%). Seven children out of 19 (36.8%) in the topical timolol maleate group and 1 out of 22 (4.5%) in the placebo group reached clearance of lesions. The absence of redness after topical timolol maleate was 8.11 times that after placebo (RR 8.11, 95% CI 1.09 to 60.09; Analysis 3.1). We downgraded the quality of the evidence from high to low due to imprecision (see Table 2). Chan 2013 also provided information about cases of volume reduction (equal to or more than 5%), reporting 11 cases (41 children; percentage of volume reduction: 26.8%). Nine children out of 19 (47.3%) in the topical timolol maleate group and 2 out of 22 (9%) in the placebo group reached clearance of lesions. Volume reduction after topical timolol maleate was 5.21 times that after placebo (RR 5.21, 95% CI 1.28 to 21.21; Analysis 3.1). We downgraded the quality of the evidence from high to low due to imprecision (see Table 2).
3.1. Analysis.
Comparison 3 Topical timolol maleate versus placebo, Outcome 1 Other measures of resolution (6 months).
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 4. Interventions versus placebo: topical bleomycin versus placebo
For this comparison, we included information from one trial with 30 children (Xu 2006). Thirty infants with "capillary hemangioma" at body surface aged less than six months were enrolled in this study. Children were randomly assigned to receive placebo or bleomycin emulsion 2 mg/dL by the ultrasound‐atomised technique three times per day for one week and made a biopsy. Duration of treatment and follow‐up was seven days in both cases.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
We found no information on this outcome for this comparison.
Secondary outcome 1: Other measures of resolution
In Xu 2006, two‐thirds of the 15 haemangiomas treated with bleomycin, a very toxic agent, became deep red and their surfaces began to shrink slightly at day 7 (10 cases; 30 children). Ten children out of 15 (66%) in the topical bleomycin group and 0 out of 15 (0%) in the placebo group reached clearance of lesions. The shrink of lesions after topical bleomycin was 21 times greater than after placebo, but the 95% CI was wide, showing imprecision (RR 21.00, 95% CI 1.34 to 328.86; Analysis 4.1).
4.1. Analysis.
Comparison 4 Topical bleomycin versus placebo, Outcome 1 Other measures of resolution: reduction in size at day 7.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 5. Interventions versus placebo: X‐ray radiation versus sham radiation
For this comparison, we included the information from one trial with 100 children (Jung 1977). The infantile haemangiomas were irradiated 2 or 3 times with 400 rad (rad = unit of absorbed radiation dose) at intervals of 4 to 8 weeks. For more than 4 cm of diameter, the 400 rad was dissolved in 3 x 200 rad at weekly intervals. Follow‐up was performed at the end of each month, then monitored on an outpatient basis in six‐month intervals. Duration of treatment ranged from 4 to 7 weeks; children were followed until 72 months.
Primary outcome 1: Clearance, as assessed by a clinician
Jung 1977 provided information on this outcome, reporting a total of 34 cases of clearance (100 children; percentage of clearance: 34%). Eighteen children out of 51 (35.2%) in the X‐ray radiation group and 16 out of 49 (32.6%) in the sham radiation group reached clearance of lesions. This study found no differences in terms of clearance when comparing X‐ray radiation with sham radiation (RR 1.08, 95% CI 0.63 to 1.87; Analysis 5.1).
5.1. Analysis.
Comparison 5 X‐ray radiation versus sham radiation, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
We found no information on this outcome for this comparison.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 6. Laser comparisons: Nd:YAG laser versus topical timolol maleate
For this comparison, we included information from one trial with 60 children (Tawfik 2015). Children were randomly allocated into two groups. Group 1 was assigned to receive timolol maleate 0.5% drops (5 mg/mL) to apply twice a day. Group 2 was treated using combined sequential dual‐wavelength 585 nm PDL and 1064 nm Nd:YAG laser (Synergy Multiplex, Cynosure, Westford, MA, USA) to flat superficial haemangiomas. The parameters were: PDL with a 7‐millimetre spot size, 6‐millisecond pulse duration, and fluence of 4.5 to 6 J/cm². After a 1‐second delay, Nd:YAG laser was administered at 15‐millisecond pulse duration and fluence of 25 to 35 J/cm². Parameters for children with mixed haemangiomas were: PDL with a 7‐millimetre spot size, 10‐millisecond pulse duration, and fluence of 6 to 7.5 J/cm². After a 1‐second delay, Nd:YAG laser was administered at 15‐millisecond pulse duration and fluence of 30 to 40 J/cm². Interventions were administered in a maximum of six sessions; children were followed until three months.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Tawfik 2015 narratively reported four cases of crusts and hyperpigmentation in the laser group after the first session. One child in the timolol maleate group reported shortness of breath and insomnia.
Secondary outcome 1: Other measures of resolution
Tawfik 2015 provided information on mean haemoglobin level after treatment with timolol maleate or laser, reporting a mean of 1.67 (standard deviation (SD) = 0.54) for the timolol maleate group versus a mean of 2.58 (SD = 0.86) for the laser group. Mean haemoglobin levels after topical timolol maleate were 0.91 units smaller than after Nd:YAG laser treatment (mean difference (MD) ‐0.91, 95% CI ‐1.27 to ‐0.55; Analysis 6.1).
6.1. Analysis.
Comparison 6 Topical timolol maleate versus Nd:YAG laser, Outcome 1 Other measures of resolution (continuous).
Tawfik 2015 also provided information on children with an improvement between 76% and 100% (excellent improvement), reporting 12 cases (60 children; 20%). Nine children out of 30 (30%) in the Nd:YAG laser group and 3 out of 30 (10%) in the topical timolol maleate group reported excellent improvement. No clear differences in terms of this score were found when comparing Nd:YAG laser with topical timolol maleate due to imprecision (RR 3.00, 95% CI 0.90 to 10.01; Analysis 6.2).
6.2. Analysis.
Comparison 6 Topical timolol maleate versus Nd:YAG laser, Outcome 2 Other measures of resolution (dichotomous).
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 7. Laser comparisons: Nd:YAG laser versus oral propranolol
For this comparison, we included information from two trials with a total of 105 children (Tan 2012; Zhong 2015). In Tan 2012, Group B received Nd:YAG laser with spot diameter of 1.5‐ to 3.0‐millimetre handle; energy density at 170 to 240 J/cm² range; width from 20 to 50 each session every 6 weeks. Group C received only twice‐daily dose of 0.5 mg/kg oral propranolol, increased two weeks later to 0.8 mg/kg, and four weeks later to 1.0 mg/kg. Duration of treatment and follow‐up were six months in both cases. In Zhong 2015, Group C received Nd:YAG once, with parameters adjusted according to lesion depth. Group B received propranolol 1.5 mg/kg over 3 divided doses per day for a total of 6 months. Treatment ranged from three to six months. Follow‐up was six months.
Primary outcome 1: Clearance, as assessed by a clinician
Tan 2012 provided information on this outcome, reporting a total of 4 cases of clearance out of 65 children (percentage of clearance: 6.1%). Three children out of 35 (8.5%) in the Nd:YAG laser group and 1 out of 30 (3.3%) in the oral propranolol group reached complete clearance of lesions. This study found no clear differences in terms of clearance when comparing Nd:YAG laser with oral propranolol due to imprecision (RR 2.57, 95% CI 0.28 to 23.44; Analysis 7.1).
7.1. Analysis.
Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Tan 2012 provided information about cases of hyperpigmentation, reporting a total of 4 cases out of 65 children (6.1%). Three children out of 35 (8.5%) in the Nd:YAG laser group and 1 out of 30 (3.3%) in the oral propranolol group reported hyperpigmentation. This study found no clear differences in terms of hyperpigmentation when comparing Nd:YAG laser with oral propranolol (RR 2.57, 95% CI 0.28 to 23.44; Analysis 7.2). Zhong 2015 also provided information about cases of pigmentation and thinning, reporting a total of 12 cases (40 children, 30%). Three children out of 20 (15%) in the Nd:YAG laser group and 9 out of 20 (45%) in the oral propranolol group reported this combined outcome. This study found no clear differences in terms of pigmentation and thinning when comparing Nd:YAG laser with oral propranolol (RR 0.33, 95% CI 0.11 to 1.05; Analysis 7.3).
7.2. Analysis.
Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 2 Adverse events: hyperpigmentation.
7.3. Analysis.
Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 3 Adverse event: pigmentation and thinning.
Both studies provided information about cases of superficial scar, reporting a total of 11 cases out of 105 children (10.4%). Seven children out of 55 (12.2%) in the Nd:YAG laser group and 4 out of 50 (8%) in the oral propranolol group reported superficial scars. These studies found no clear differences in terms of superficial scars when comparing Nd:YAG laser with oral propranolol (RR 1.52, 95% CI 0.24 to 9.58; I² = 48%; Analysis 7.4). Zhong 2015 also reported that no cases of severe hypoglycaemia, hypotension, or Reynauld’s syndrome (extremity coldness) were found.
7.4. Analysis.
Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 4 Adverse events: superficial scar.
Secondary outcome 1: Other measures of resolution
Zhong 2015 provided information about "excellent response", defined as an improvement equal or superior to 95% performed by two clinicians, reporting a total of eight cases (40 children; 20%). Two children out of 20 (10%) in the Nd:YAG laser group and 6 out of 20 (30%) in the oral propranolol group reported this response. This study found no clear differences when comparing Nd:YAG laser with oral propranolol (RR 0.33, 95% CI 0.08 to 1.46; Analysis 7.5).
7.5. Analysis.
Comparison 7 Nd:YAG laser versus oral propranolol, Outcome 5 Other measures of resolution: excellent response.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 8. Laser comparisons: PDL + topical propranolol versus PDL alone
For this comparison, we included information from one trial with 19 children (Ehsani 2014). Children were randomly divided into two groups: the first group (nine children) were treated with PDL (spot size 7 mm, fluence 12 J/cm², pulse duration 1.5 ms, dynamic cooling device 40/40), while second group (10 children) were treated with the same PDL sessions together with topical ointment of propranolol hydrochloride 1% applied twice a day for at least 12 weeks. Duration of treatment was at least 12 weeks; children were followed until four months.
Primary outcome 1: Clearance, as assessed by a clinician
Ehsani 2014 provided information on this outcome, reporting a total of seven cases of clearance (19 children; percentage of clearance: 36.8%). Five children out of 10 (50%) in the PDL + topical propranolol group and 2 out of 9 (22%) in the PDL group reached complete clearance of lesions. This study found no clear differences in terms of clearance when comparing PDL + topical propranolol with PDL alone (RR 2.25, 95% CI 0.57 to 8.86; Analysis 8.1).
8.1. Analysis.
Comparison 8 Pulsed dye laser + topical propranolol versus pulsed dye laser, Outcome 1 Clearance, as assessed by a clinician.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Ehsani 2014 provided information about serious cardiovascular events and other adverse events, reporting zero cases for both outcomes.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 9. Laser comparisons: PDL + topical timolol maleate versus PDL alone
For this comparison, we included information from one trial with 32 children (Asilian 2015). Children were divided into two groups: one group was treated with four sessions of PDL (585 nm, spot size 5 mm, fluence 9 J/cm², pulse duration 450 ms, without cooling, and spot overlap 20%) plus administration of timolol maleate gel 0.5%, while the other group received PDL plus lubricant gel as placebo. Duration of treatment was unclear; children were followed until six months.
Primary outcome 1: Clearance, as assessed by a clinician
Asilian 2015 provided information on this outcome, reporting a total of two cases of clearance (32 children; percentage of clearance: 6.2%). One child out of 16 (6.2%) in the PDL + topical timolol maleate group and 1 out of 16 (6.2%) in the PDL group reached complete clearance of lesions. This study found no differences in terms of clearance when comparing PDL + topical timolol maleate with PDL alone (RR 1.00, 95% CI 0.07 to 14.64; Analysis 9.1).
9.1. Analysis.
Comparison 9 Pulsed dye laser + topical timolol maleate versus pulsed dye laser, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Asilian 2015 provided information about severe adverse events such as hypotension, bradycardia, sleep disturbance, and anxiety, reporting zero cases.
Secondary outcome 1: Other measures of resolution
Asilian 2015 provided information about mean size reduction after treatment, reporting a mean of 17.62 cm (SD = 6.97) for the PDL + timolol maleate group versus a mean of 12 cm (SD = 5.71) for the PDL‐alone group. The mean size reduction after PDL + timolol maleate was 5.62 cm greater than after PDL alone (MD 5.62, 95% CI 1.21 to 10.03; Analysis 9.2).
9.2. Analysis.
Comparison 9 Pulsed dye laser + topical timolol maleate versus pulsed dye laser, Outcome 2 Other measures of resolution: mean size reduction.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 10. Laser comparisons: Nd:YAG laser + oral propranolol versus Nd:YAG laser
For this comparison, we included information from two trials with a total of 107 children (Tan 2012; Zhong 2015). In Tan 2012, one of the groups received Nd:YAG laser using spot diameter of 1.5‐ to 3.0‐millimetre handle; energy density at 170 to 240 J/cm² range; width from 20 to 50 each session every 6 weeks; the first 2 days after laser treatment start oral propranolol 0.5 mg/kg/day (twice daily) increased dosage 2 weeks later to 0.8 mg/kg/day, 4 weeks later increased to 1.0 mg/kg/day. The other group received only Nd:YAG laser with same parameters, one session every six weeks. Duration of treatment and follow‐up was six months in both cases. In Zhong 2015, Group C received Nd:YAG once, with parameters adjusted according to lesion depth. Group B received propranolol 1.5 mg/kg/day over three doses per day for a total of six months. Treatment ranged from three to six months. Follow‐up was six months.
Primary outcome 1: Clearance, as assessed by a clinician
Tan 2012 provided information on this outcome, reporting a total of 12 cases of clearance (67 children; percentage of clearance: 17.9%). Nine children out of 32 (28%) in Nd:YAG laser + oral propranolol group and 3 out of 35 (8.5%) in Nd:YAG laser group reached complete clearance of lesions. This study found no clear differences in terms of clearance when comparing Nd:YAG laser + oral propranolol with Nd:YAG laser alone, as the 95% confidence interval marginally included 1 (RR 3.28, 95% CI 0.97 to 11.06; Analysis 10.1).
10.1. Analysis.
Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Tan 2012 provided information about hyperpigmentation, reporting a total of seven cases (67 children; 10.4%). Four children out of 32 (12.5%) in the Nd:YAG laser + oral propranolol group and 3 out of 35 (8.5%) in the Nd:YAG laser group reported hyperpigmentation. This study found no differences in terms of hyperpigmentation when comparing Nd:YAG laser + oral propranolol with Nd:YAG laser alone (RR 1.46, 95% CI 0.35 to 6.02; Analysis 10.2). In addition, Zhong 2015 provided information on cases of pigmentation and thinning, reporting a total of five cases (40 children; 12.5%). Two children out of 20 (10%) in the Nd:YAG laser + oral propranolol group and 3 out of 20 (15%) in the Nd:YAG laser group reported this combined outcome. This study found no clear differences in terms of pigmentation and thinning when comparing Nd:YAG laser with oral propranolol (RR 0.67, 95% CI 0.12 to 3.57; Analysis 10.3).
10.2. Analysis.
Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 2 Adverse events: hyperpigmentation.
10.3. Analysis.
Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 3 Adverse events: pigmentation and thinning.
Both studies provided information about superficial scars, reporting a total of nine cases (107 children; 8.4%). Two children out of 52 (3.8%) in the Nd:YAG laser + oral propranolol group and 7 out of 55 (12.7%) in the Nd:YAG laser group reported superficial scars. This study found no clear difference in terms of superficial scars when comparing Nd:YAG laser + oral propranolol with Nd:YAG laser alone (RR 0.37, 95% CI 0.09 to 1.48; Analysis 10.4).
10.4. Analysis.
Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 4 Adverse events: superficial scar.
Secondary outcome 1: Other measures of resolution
Zhong 2015 provided information about "excellent response", defined as an improvement equal or superior to 95% performed by two clinicians, reporting a total of eight cases (40 children; 20%). Two children out of 20 (10%) in the Nd:YAG laser+ oral propranolol group and 6 out of 20 (30%) in the Nd:YAG laser‐alone group reported this response. The risk of excellent response after Nd:YAG laser + oral propranolol was 8.5 times that after Nd:YAG laser alone (RR 8.50, 95% CI 2.25 to 32.06; Analysis 10.5).
10.5. Analysis.
Comparison 10 Nd:YAG laser + oral propranolol versus Nd:YAG laser, Outcome 5 Other measures of resolution: excellent response.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 11. Laser comparisons: Nd:YAG laser + oral propranolol versus oral propranolol
For this comparison, we included information from two trials with a total of 102 children (Tan 2012; Zhong 2015). In Tan 2012, one of the groups received Nd:YAG laser using spot diameter of 1.5‐ to 3.0‐millimetre handle; energy density at 170 to 240 J/cm² range; width from 20 to 50 each session every 6 weeks; the first 2 days after laser treatment start oral propranolol 0.5 mg/kg/day (twice daily) increased dosage 2 weeks later to 0.8 mg/kg/day, 4 weeks later increased to 1.0 mg/kg/day. The other group received only oral propranolol with the same scheme. Duration of treatment and follow‐up was six months in both cases. In Zhong 2015, Group C received Nd:YAG once, with parameters adjusted according to lesion depth. Group B received propranolol 1.5 mg/kg/day over three doses per day for a total of six months. Treatment ranged from three to six months. Follow‐up was six months.
Primary outcome 1: Clearance, as assessed by a clinician
Tan 2012 provided information on this outcome, reporting a total of 10 cases of clearance (62 children; percentage of clearance: 16.1%). Nine children out of 32 (28%) in the Nd:YAG laser + oral propranolol group and 1 out of 30 (3.3%) in the oral propranolol group reached complete clearance of lesions. The risk of clearance after Nd:YAG laser + oral propranolol was 8.44 times that after oral propranolol alone (RR 8.44, 95% CI 1.14 to 62.66; Analysis 11.1).
11.1. Analysis.
Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Tan 2012 provided information about hyperpigmentation, reporting a total of five cases (62 children; 8.06%). Four children out of 32 (12.5%) in the Nd:YAG laser + oral propranolol group and 1 out of 30 (3.3%) in the oral propranolol group reported hyperpigmentation. This study found no clear differences in terms of hyperpigmentation when comparing Nd:YAG laser + oral propranolol with oral propranolol (RR 3.75, 95% CI 0.44 to 31.68; Analysis 11.2). In addition, Zhong 2015 provided information on cases of pigmentation and thinning, reporting a total of 11 cases (40 children; 27.5%). Two children out of 20 (10%) in the Nd:YAG laser + oral propranolol group and 9 out of 20 (45%) in the oral propranolol group reported this combined outcome. The risk of this combined outcome after Nd:YAG laser + oral propranolol was 78% lower than after oral propranolol alone (RR 0.22, 95% CI 0.05 to 0.90; Analysis 11.3).
11.2. Analysis.
Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 2 Adverse events: hyperpigmentation.
11.3. Analysis.
Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 3 Adverse events: pigmentation and thinning.
Both studies provided information about superficial scars, reporting a total of six cases (102 children; 5.8%). Two children out of 52 (3.8%) in the Nd:YAG laser + oral propranolol group and 4 out of 50 (8%) in the oral propranolol group reported superficial scars. This study found no clear differences in terms of superficial scars when comparing Nd:YAG laser + oral propranolol with oral propranolol (RR 0.60, 95% CI 0.05 to 7.63; Analysis 11.4).
11.4. Analysis.
Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 4 Adverse events: superficial scar.
Secondary outcome 1: Other measures of resolution
Zhong 2015 provided information about "excellent response", defined as an improvement equal or superior to 95% performed by two clinicians, reporting a total of 23 cases (40 children; 57.5%). Seventeen children out of 20 (85%) in the Nd:YAG laser group and 6 out of 20 (30%) in the oral propranolol group reported this response. Excellent response after Nd:YAG laser + oral propranolol was 2.83 times greater than after oral propranolol alone (RR 2.83, 95% CI 1.42 to 5.67; Analysis 11.5).
11.5. Analysis.
Comparison 11 Nd:YAG laser + oral propranolol versus oral propranolol, Outcome 5 Other measures of resolution: excellent response.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 12. Laser comparisons: ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate versus ⁹⁰SR‐⁹⁰Y radiation
For this comparison, we included information from one trial with 72 children (Zhu 2015). One of the groups received 1 to 2 courses of ⁹⁰SR‐⁹⁰Y (applicator area of 2x2 and a surface‐absorbed dose rate of 2.2 Gy/min) contact therapy and local external application of 0.5% topical timolol maleate solution on the area for 3 to 6 months. Children in the control group received an identical dosage and treatment course of ⁹⁰SR‐⁹⁰Y contact therapy with local topical application of normal saline for three to six months. In cases with a total haemangioma area of < 20 cm², a single course of treatment consisted of a radiation dose of 2 to 2.4 Gy, once per day, for 5 consecutive days. In cases with a total haemangioma area of > 20 cm², a single course of treatment consisted of a radiation dose of 1 to 1.2 Gy, once per day, for 10 consecutive days. Duration of treatment ranged from three to six months; children were followed until six months.
Primary outcome 1: Clearance, as assessed by a clinician
Zhu 2015 provided information on this outcome, reporting a total of 55 cases of clearance (72 children; percentage of clearance: 76.3%). Thirty‐three children out of 37 (89.1%) in the ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate group and 22 out of 35 (62.8%) in the ⁹⁰SR‐⁹⁰Y radiation group reached complete clearance of lesions. Clearance after ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate was 1.42 times greater than after ⁹⁰SR‐⁹⁰Y radiation alone (RR 1.42, 95% CI 1.07 to 1.87; Analysis 12.1).
12.1. Analysis.
Comparison 12 ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate versus ⁹⁰SR‐⁹⁰Y radiation, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Zhu 2015 provided information about adverse events in general, such as mild itching, mild skin flaking, and pruritus, reporting a total of 22 cases (72 children; 30.5%). Twelve children out of 37 (32.4%) in the ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate group and 10 out of 35 (28.5%) in the ⁹⁰SR‐⁹⁰Y radiation group reported adverse events. This study found no differences in terms of adverse events when comparing ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate with ⁹⁰SR‐⁹⁰Y radiation alone (RR 1.14, 95% CI 0.56 to 2.29; Analysis 12.2).
12.2. Analysis.
Comparison 12 ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate versus ⁹⁰SR‐⁹⁰Y radiation, Outcome 2 Adverse events.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 13. Laser comparisons: sequential dual‐wavelength laser + oral propranolol versus concurrent dual‐wavelength laser + oral propranolol
For this comparison, we included information from one trial with 61 children (Lu 2016). One of the groups received dual‐wavelength laser therapy after discontinuation of oral propranolol (1 to 2 mg/kg/d). Propranolol treatment was stopped when maximised treatment effect was achieved. The second group were treated with oral propranolol for one week before laser therapy was added concurrently. Duration of treatment and follow‐up was unclear.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Lu 2016 narratively described general information about adverse events. The authors reported two cases of upper respiratory tract infection, three cases of mild hyperkalaemia, and one case with decreased appetite.
Secondary outcome 1: Other measures of resolution
Lu 2016 provided information about the efficacy rating between the two groups evaluated by means of a 0‐to‐10 scale. Three researchers independently scored the treatment effect (changes of lesions appearance by digital photograph) by each month, and the average of these scores was reported. Complete resolution of the haemangioma was considered if the lesion achieved 1 or 0 points. At 3 months, the sequential‐treatment group obtained a mean score of 6.23 units (SD = 0.99), and the concurrent‐treatment group obtained a mean score of 7 units (SD = 0.46). The mean score after sequential dual‐wavelength laser + oral propranolol was 0.77 units lower than after concurrent dual‐wavelength laser + oral propranolol (MD ‐0.77, 95% CI ‐1.16 to ‐0.38; Analysis 13.1).
13.1. Analysis.
Comparison 13 Sequential dual‐wavelength laser + oral propranolol versus concurrent dual‐wavelength laser + oral propranolol, Outcome 1 Other outcomes of resolution: mean efficacy rating.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 14. Propranolol comparisons: oral propranolol versus topical propranolol
For this comparison, we included information from one trial with 30 children (Zaher 2013). Children with problematic infantile haemangioma (rapidly progressive, compromising vital functions, or causing cosmetic disfigurement) were randomised into three groups. Group A received oral propranolol, 2 mg/kg/day divided into two daily doses. Group B received topical propranolol 1% ointment in a hydrophilic base, applied twice daily. The intervention ended if "complete resolution occurred, if a sustained plateau in the size of the hemangioma was reached for a period of 2 months of treatment or if any intolerable side effects from propranolol developed". Children were followed until six months.
Primary outcome 1: Clearance, as assessed by a clinician
Zaher 2013 provided information on this outcome, reporting a total of 12 cases of clearance (30 children; percentage of clearance: 40%). Nine children out of 15 (60%) in the oral propranolol group and 3 out of 15 (20%) in the topical propranolol group reached complete clearance of lesions. The risk of clearance after oral propranolol was three times that after topical propranolol (RR 3.00, 95% CI 1.01 to 8.95; Analysis 14.1).
14.1. Analysis.
Comparison 14 Oral propranolol versus topical propranolol, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Zaher 2013 provided information about syncopal attack, reporting a total of three cases (30 children; 10%). Three children out of 15 (60%) in the oral propranolol group and 0 out of 15 (0%) in the topical propranolol group reported syncopal attack. This study found no clear differences (due to imprecision) in terms of syncopal attack when comparing oral propranolol with topical propranolol (RR 7.00, 95% CI 0.39 to 124.83; Analysis 14.2).
14.2. Analysis.
Comparison 14 Oral propranolol versus topical propranolol, Outcome 2 Adverse events: syncopal attack.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 15. Propranolol comparisons: oral propranolol versus intralesional propranolol
For this comparison, we included information from one trial with 30 children (Zaher 2013). Children with problematic infantile haemangioma (rapidly progressive, compromising vital functions, or causing cosmetic disfigurement) were randomised into three groups. Group A received oral propranolol, 2 mg/kg/day divided into two daily doses. Group C received intralesional propranolol. The intervention ended if "complete resolution occurred, if a sustained plateau in the size of the hemangioma was reached for a period of 2 months of treatment or if any intolerable side effects from propranolol developed". Children were followed until six months.
Primary outcome 1: Clearance, as assessed by a clinician
Zaher 2013 provided information on this outcome, reporting a total of 11 cases of clearance (30 children; percentage of clearance: 36.6%). Nine children out of 15 (60%) in the oral propranolol group and 2 out of 15 (13.3%) in the intralesional propranolol group reached complete clearance of lesions. The risk of clearance after oral propranolol was 4.5 times that after intralesional propranolol (RR 4.50, 95% CI 1.16 to 17.44; Analysis 15.1).
15.1. Analysis.
Comparison 15 Oral propranolol versus intralesional propranolol, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Zaher 2013 provided information about syncopal attack, reporting a total of three cases (30 children; 10%). Three children out of 15 (20%) in the oral propranolol group and 0 out of 15 (0%) in the intralesional propranolol group reported syncopal attack events. This study found no clear differences (due to imprecision) in terms of syncopal attack when comparing oral propranolol with intralesional propranolol (RR 7.00, 95% CI 0.39 to 124.83; Analysis 15.2).
15.2. Analysis.
Comparison 15 Oral propranolol versus intralesional propranolol, Outcome 2 Adverse events: syncopal attack.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 16. Propranolol comparisons: topical propranolol versus intralesional propranolol
For this comparison, we included information from one trial with 30 children (Zaher 2013). Children with problematic infantile haemangioma (rapidly progressive, compromising vital functions, or causing cosmetic disfigurement) were randomised into three groups. Group B received topical propranolol 1% ointment in a hydrophilic base, applied twice daily. Group C received intralesional propranolol. The intervention ended if "complete resolution occurred, if a sustained plateau in the size of the hemangioma was reached for a period of 2 months of treatment or if any intolerable side effects from propranolol developed". Children were followed until six months.
Primary outcome 1: Clearance, as assessed by a clinician
Zaher 2013 provided information on this outcome, reporting a total of five cases of clearance (30 children; percentage of clearance: 16.6%). Three children out of 15 (20%) in the topical propranolol group and 2 out of 15 (13.3%) in the intralesional propranolol group reached complete clearance of lesions. This study found no clear differences in terms of clearance when comparing topical propranolol with intralesional propranolol (RR 1.50, 95% CI 0.29 to 7.73; Analysis 16.1).
16.1. Analysis.
Comparison 16 Topical propranolol versus intralesional propranolol, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Zaher 2013 provided information about syncopal attack, reporting zero events for this comparison.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 17. Propranolol comparisons: oral propranolol versus oral atenolol
For this comparison, we included information from one trial with 23 children (Abarzua‐Araya 2014). Children were randomised to receive either atenolol or propranolol. Thirteen children receive propranolol in a dose of 2 mg/kg/d in three daily doses for 6 months, and 10 children receive atenolol in a dose of 1 mg/kg/d in a single daily dose for 6 months. Duration of treatment and follow‐up was six months in both cases.
Primary outcome 1: Clearance, as assessed by a clinician
Abarzua‐Araya 2014 provided information on this outcome, reporting a total of 13 cases of clearance (23 children; percentage of clearance: 56.5%). Six children out of 10 (60%) in the oral propranolol group and 7 out of 13 (53.8%) in the oral atenolol group reached complete clearance of lesions. This study found no differences in terms of clearance when comparing oral propranolol with oral atenolol (RR 1.11, 95% CI 0.55 to 2.27; Analysis 17.1).
17.1. Analysis.
Comparison 17 Oral atenolol versus oral propranolol, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Abarzua‐Araya 2014 provided information about adverse events in general and serious cardiovascular events, reporting zero events for this comparison.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 18. Propranolol comparisons: oral propranolol versus oral prednisolone
For this comparison, we included information from two trials with a total of 39 children (Bauman 2014; Malik 2013). In Bauman 2014, participants were infants aged from two weeks to six months with actively proliferating and symptomatic IH. Nineteen were enrolled and randomly assigned to prednisolone (n = 8) or propranolol (n = 11), both treatments given at a dose of 2 mg/kg/d until halted owing to toxic effects or clinical response. Treatment was stopped if IH resolved; no measurable improvement was noted in the lesion at two sequential monthly evaluations; in the presence of severe adverse events; at caretaker's or physician request; or no clinical improvement after one month. Children were followed for at least four months.
In Malik 2013, 30 children aged from one week to eight months with potentially disfiguring or functionally threatening IH were randomised into three equal groups: Group A, propranolol (2 to 3 mg/kg/d); Group B, prednisolone (1 to 4 mg/kg/d); and Group C, receiving both for a minimum duration of three months. After discharge, all children were re‐evaluated after eight days of treatment and then every month for a minimum of three months. Duration of treatment was three months or more; children were followed until 18 months.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Bauman 2014 provided information about severe adverse events, such as Cushingoid appearance and gastrointestinal upset, reporting a total of six cases (19 children; 31.5%). One child out of 11 (9%) in the oral propranolol group and 5 out of 8 (62.5%) in the oral prednisolone group reported severe adverse events, and the corresponding risk ratio favoured the oral propranolol group; however, the confidence interval was wide and did marginally include 1 showing some uncertainty (RR 0.15, 95% CI 0.02 to 1.02; Analysis 18.1).
18.1. Analysis.
Comparison 18 Oral propranolol versus oral prednisolone, Outcome 1 Severe adverse events.
Likewise, Malik 2013 provided information about complications in general, reporting a total of 11 cases (20 children; 55%). Two children out of 10 (20%) in the oral propranolol group and 9 out of 10 (90%) in the oral prednisolone group reported complications. The risk of complications after oral propranolol was 78% lower than after oral prednisolone (RR 0.22, 95% CI 0.06 to 0.78; Analysis 18.2). Malik 2013 also assessed the incidence of serious cardiovascular events such as bradycardia and hypotension, reporting zero events for both adverse events.
18.2. Analysis.
Comparison 18 Oral propranolol versus oral prednisolone, Outcome 2 Adverse events: complications in general.
Secondary outcome 1: Other measures of resolution
Malik 2013 provide information about two measures of resolution. The authors stated that "Measure of assessment for colour and size was based on Visual Analogue Scale (VAS) ranging from −10 to +10 by comparing follow‐up images to the baseline photograph pretreatment. Here, 0 represented the baseline photograph, a decrease resulting in a minus number and an increase in a + number" (Malik 2013). Regarding colour fading, Malik 2013 reported a mean score of ‐9 units (SD = 1.7) in the visual analogue scale for the propranolol group, versus a mean of ‐8 units (SD = 2.9) for the prednisolone group. This study found no clear difference in terms of colour fading score when comparing oral propranolol with oral prednisolone (MD ‐1.00, 95% CI ‐3.08 to 1.08; Analysis 18.3). Malik 2013 also assessed the percentage of mean size reduction after treatment, reporting a mean size of 89.8 (SD = 10.3) for the propranolol group, versus a mean size of 66.6 (SD = 41.6) for the prednisolone group. This study found no clear differences (due to imprecision) in terms of size reduction when comparing oral propranolol with oral prednisolone (MD 23.2, 95% CI ‐3.36 to 49.76; Analysis 18.4).
18.3. Analysis.
Comparison 18 Oral propranolol versus oral prednisolone, Outcome 3 Other measures of resolution: colour fading.
18.4. Analysis.
Comparison 18 Oral propranolol versus oral prednisolone, Outcome 4 Other measures of resolution: mean size reduction.
Likewise, Bauman 2014 reported information about the proportional change in the total surface area at four months. They found a mean of 0.64 (SD = 0.29) for the propranolol group, versus a mean of 0.41 (SD = 0.37) for the prednisolone group. This study found no differences in terms of changes in the total surface area when comparing oral propranolol with oral prednisolone (MD 0.23, 95% CI ‐0.08 to 0.54; Analysis 18.5).
18.5. Analysis.
Comparison 18 Oral propranolol versus oral prednisolone, Outcome 5 Other measures of resolution: proportional change in the total surface area.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 19. Propranolol comparisons: oral propranolol versus oral captopril
For this comparison, we included information from one trial with 30 children (Zaher 2016). Children with problematic infantile haemangiomas were enrolled and randomly divided into two groups. Group A (n = 15) received oral propranolol (2 mg/kg/d, divided into two daily doses). Group B (n = 15) received oral captopril (0.5 to 1 mg/kg/d, in a titrating dose). Children were discharged and followed up on a weekly basis for the first month, every two weeks in the second month, and finally at four‐week intervals until four months after stopping treatment. The intervention ended if there was "complete resolution of IH, no initial or further improvement of IH (for 2 months), or intolerable side effects". Children were followed until four months.
Primary outcome 1: Clearance, as assessed by a clinician
Zaher 2016 provided information on this outcome, reporting a total of seven cases of clearance (30 children; percentage of clearance: 23.3%). Seven children out of 15 (46.6%) in the oral propranolol group and 0 out of 15 (0%) in the oral captopril group reached complete clearance of lesions. Due to the large 95% confidence interval, it is uncertain whether there is a difference in terms of clearance when comparing oral propranolol with oral captopril (RR 15.00, 95% CI 0.93 to 241.2; Analysis 19.1).
19.1. Analysis.
Comparison 19 Oral propranolol versus oral captopril, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Gong 2015 provided information about cardiac side effects such as hypotension and dizziness, reporting a total of four cases (30 children; 13.3%). No children out of 15 (0%) in the oral propranolol group and 4 out of 15 (26.6%) in the oral captopril group reported cardiac side effects. This study found no clear difference (due to imprecision) in terms of cardiac side effects when comparing oral propranolol with oral captopril (RR 0.11, 95% CI 0.01 to 1.90; Analysis 19.2).
19.2. Analysis.
Comparison 19 Oral propranolol versus oral captopril, Outcome 2 Adverse events: cardiac side effects.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 20. Propranolol comparisons: oral propranolol versus topical timolol maleate
For this comparison, we included information from one trial with 26 children (Gong 2015). Children with superficial infantile haemangiomas were randomised into three equal groups: one group received oral propranolol, and a second group received topical timolol maleate. Treatment efficacy was evaluated based on clinical photographs taken at the onset of treatment, during treatment, and at the end of treatment. Treatment ended if lesions had regressed or after 6 months without improvement; children were followed from 3 to 12 months.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Gong 2015 provided information about adverse events in general, such as severe diarrhoea, lethargy, and loss of appetite, reporting a total of three cases (26 children; 11.5%). Three children out of 13 (23%) in the oral propranolol group and 0 out of 13 (0%) in the topical timolol maleate group reported adverse events. This study found no clear difference in terms of adverse events when comparing oral propranolol with topical timolol maleate (RR 7.00, 95% CI 0.40 to 123.35; Analysis 20.1). We downgraded the quality of the evidence from high to very low due to unclear risk of selection and performance bias, as well as imprecision (see Table 3).
20.1. Analysis.
Comparison 20 Oral propranolol versus topical timolol maleate, Outcome 1 Adverse events.
Secondary outcome 1: Other measures of resolution
Gong 2015 provided information about size reduction equal or superior to 50% after treatment, reporting a total of 17 cases (26 children; 65.3%). Nine children out of 13 (69.2%) in the oral propranolol group and 8 out of 13 (61.5%) in the topical timolol maleate group reached size reduction of ≥ 50% . This study found no differences in terms of this measurement when comparing oral propranolol with topical timolol maleate (RR 1.13, 95% CI 0.64 to 1.97; Analysis 20.2). We downgraded the quality of the evidence from high to low due to unclear risk of selection and performance bias, as well as imprecision (see Table 3).
20.2. Analysis.
Comparison 20 Oral propranolol versus topical timolol maleate, Outcome 2 Other measures of resolution: size reduction > 50%.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 21. Propranolol comparisons: oral propranolol versus oral propranolol + oral prednisolone
For this comparison, we included information from two trials with a total of 60 children (Aly 2015; Malik 2013). In Aly 2015, infants aged less than 9 months with cutaneous haemangiomas were randomly assigned into 2 groups: Group A received oral prednisolone 2 mg/kg/day in 2 divided doses for the initial 2 weeks combined with oral propranolol 2 mg/kg/day in 3 divided doses for 6 months, while Group B received oral propranolol alone in the same dose for 6 months. Duration of treatment was 24 weeks; children were followed until 9 months.
In Malik 2013, children aged from 1 week to 8 months with potentially disfiguring or functionally threatening IH were randomised into 3 equal groups: Group A, propranolol (2 to 3 mg/kg/d); Group B, prednisolone (1 to 4 mg/kg/d); and Group C, receiving both for a minimum duration of 3 months. After discharge, all children were re‐evaluated after eight days of treatment and then every month for a minimum of three months. Duration of treatment was three months or more; children were followed until 18 months.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Both studies provided information about adverse events in general, including bronchiolitis, upper respiratory tract infection, and Cushingoid appearance, reporting a total of 13 cases (60 children; 21.6%). Three children out of 30 (10%) in the oral propranolol group and 10 out of 30 (33.3%) in the oral propranolol + oral prednisolone group reported adverse events. The risk of adverse events after oral propranolol was 70% lower that after oral propranolol + oral prednisolone (RR 0.30, 95% CI 0.10 to 0.91; I² = 0%; Analysis 21.1).
21.1. Analysis.
Comparison 21 Oral propranolol versus oral propranolol + oral prednisolone, Outcome 1 Adverse events in general.
Secondary outcome 1: Other measures of resolution
Malik 2013 provided information about the percentage of mean size reduction on a visual analogue scale at 1.5 years' follow‐up, reporting a mean of 89.8 (SD = 10.3) for the propranolol group, versus a mean of 82.6 (SD = 10.4) for the propranolol + prednisolone group. This study found no clear differences in terms of size reduction when comparing oral propranolol with oral propranolol + oral prednisolone (MD 7.20, 95% CI ‐1.87 to 16.27; Analysis 21.2).
21.2. Analysis.
Comparison 21 Oral propranolol versus oral propranolol + oral prednisolone, Outcome 2 Other measures of resolution: mean size reduction.
Likewise, Aly 2015 reported decrease in redness in 23 children (40 children; 57.5%). Ten children out of 20 (50%) in the oral propranolol group and 13 out of 20 (65%) in the oral propranolol + oral prednisolone group reported decrease in redness. This study found no clear differences in terms of redness when comparing oral propranolol with oral propranolol + oral prednisolone (RR 0.77, 95% CI 0.45 to 1.32; Analysis 21.3).
21.3. Analysis.
Comparison 21 Oral propranolol versus oral propranolol + oral prednisolone, Outcome 3 Other measures of resolution: decrease in redness.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 22. Propranolol comparisons: oral propranolol versus oral ibuprofen + oral paracetamol
For this comparison, we included information from one trial with 64 children (Tiwari 2016). Children with ulcerated infantile haemangiomas of the head and neck region, without any prior treatment and with age older than one month, were randomised into two groups. Group A received oral propranolol at a dose of 2 mg/kg per day in three divided doses. Group B received oral ibuprofen and paracetamol in doses of 10 and 16.2 mg/kg 8‐hourly. Duration of treatment was unclear; follow‐up was performed until 12 months.
Primary outcome 1: Clearance, as assessed by a clinician
Tiwari 2016 provided information on this outcome, reporting a total of 11 cases of clearance (64 children; percentage of clearance: 17.1%). Eight children out of 32 (25%) in the oral propranolol group and 3 out of 32 (9.3%) in the oral ibuprofen + oral paracetamol group reached clearance of lesions. This study found no clear differences in terms of clearance when comparing oral propranolol with oral ibuprofen + oral paracetamol (RR 2.67, 95% CI 0.78 to 9.15; Analysis 22.1).
22.1. Analysis.
Comparison 22 Oral propranolol versus oral ibuprofen + oral paracetamol, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Tiwari 2016 provided information about adverse events in general, including maculopapular generalised rash, reporting a total of three cases (64 children; 4.6%). Three children out of 32 (9.3%) in the oral propranolol group and 0 out of 32 (0%) in the oral ibuprofen + oral paracetamol group reported adverse events. This study found no clear differences (due to imprecision) in terms of adverse events when comparing oral propranolol with oral ibuprofen + oral paracetamol (RR 7.00, 95% CI 0.38 to 130.26; Analysis 22.2).
22.2. Analysis.
Comparison 22 Oral propranolol versus oral ibuprofen + oral paracetamol, Outcome 2 Adverse events.
Secondary outcome 1: Other measures of resolution
Tiwari 2016 assessed the mean size of ulceration, reporting a mean of 3.25 cm (SD = 0.75) for the propranolol group, versus a mean of 2.94 cm (SD = 0.42) for the ibuprofen + paracetamol group. The mean size of ulceration after oral propranolol was 0.31 cm greater than after oral ibuprofen + oral paracetamol (MD 0.31, 95% CI 0.01 to 0.61; Analysis 22.3).
22.3. Analysis.
Comparison 22 Oral propranolol versus oral ibuprofen + oral paracetamol, Outcome 3 Other measures of resolution: mean size of ulceration.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 23. Propranolol comparisons: oral propranolol + topical timolol maleate versus oral propranolol
For this comparison, we included information from two trials with a total of 57 children (Gong 2015; Li 2016). In Gong 2015, children with superficial infantile haemangiomas were randomised into three equal groups: one group received topical timolol maleate together with oral propranolol, and a second group received only oral propranolol. Treatment ended if lesions had regressed or after 6 months without improvement; children were followed from 3 to 12 months.
In Li 2016, children with mixed infantile haemangiomas in the oral and maxillofacial regions were randomised into two groups. Children in the experimental group (A) were treated with oral propranolol in combination with topical timolol maleate, and children in the control group (B) were treated with oral propranolol alone. Treatment was administered "for a maximum period of 8 months or complete regression of lesions". Children were followed until eight months.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Gong 2015 provided information about adverse events in general, including severe diarrhoea, lethargy, and loss of appetite, reporting a total of four cases (26 children; 15.3%). One child out of 13 (7.6%) in the oral propranolol + topical timolol maleate group and 3 children out of 13 (23%) in the oral propranolol group reported adverse events. This study found no clear differences in terms of adverse events when comparing oral propranolol + topical timolol maleate with oral propranolol (RR 0.33, 95% CI 0.04 to 2.80; Analysis 23.1). Likewise, Li 2016 assessed the incidence of severe adverse events, finding zero events in both study arms.
23.1. Analysis.
Comparison 23 Oral propranolol + topical timolol maleate versus oral propranolol, Outcome 1 Adverse events in general.
Secondary outcome 1: Other measures of resolution
Li 2016 reported information about colour fading and size reduction, based on visual analogue scale scores. For colour fading, the authors reported a mean of 8.36 (SD = 1.39) for the propranolol + timolol maleate group, versus a mean of 7.18 (SD = 1.71) for the propranolol group. The mean score for colour fading after oral propranolol + topical timolol maleate was 1.18 units greater than after oral propranolol (MD 1.18, 95% CI 0.09 to 2.27; Analysis 23.2). Regarding size reduction, Li 2016 found a mean of 8.0 (SD = 1.75) for the propranolol + timolol maleate group, versus a mean of 7.59 (SD = 1.8) for the propranolol group. This study found no clear difference in terms of size reduction when comparing oral propranolol + topical timolol maleate with oral propranolol (MD 0.41, 95% CI ‐0.84 to 1.66; Analysis 23.3).
23.2. Analysis.
Comparison 23 Oral propranolol + topical timolol maleate versus oral propranolol, Outcome 2 Other measures of resolution: colour fading/visual analogue scale score.
23.3. Analysis.
Comparison 23 Oral propranolol + topical timolol maleate versus oral propranolol, Outcome 3 Other measures of resolution: size reduction/visual analogue scale score.
Likewise, Gong 2015 provided information on the number of cases with size reduction ≥ 50% after treatment, reporting a total of 20 cases (26 children; 76.9%). Eleven children out of 13 (84.6%) in the oral propranolol + topical timolol maleate group and 9 out of 13 (69.2%) in the oral propranolol group reached size reduction ≥ 50%. This study found no significant differences in terms of size reduction when comparing oral propranolol + topical timolol maleate with oral propranolol (RR 1.22, 95% CI 0.79 to 1.88; Analysis 23.4).
23.4. Analysis.
Comparison 23 Oral propranolol + topical timolol maleate versus oral propranolol, Outcome 4 Other measures of resolution: size reduction > 50%.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 24. Propranolol comparisons: oral propranolol + topical timolol maleate versus topical timolol maleate
For this comparison, we included information from one trial with 26 children (Gong 2015). Children with superficial infantile haemangiomas were randomised into three equal groups: one group received topical timolol maleate together with oral propranolol, and a second group received only topical timolol maleate. Treatment ended if lesions had regressed or after 6 months without improvement; children were followed from 3 to 12 months.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Gong 2015 provided information about adverse events in general, including severe diarrhoea, lethargy, and loss of appetite, reporting one case that developed loss of appetite and vomiting (26 children; 3.84%). One child out of 13 (7.6%) in the oral propranolol + topical timolol maleate group and 0 out of 13 (0%) in the topical timolol maleate group reported adverse events. This study found no clear differences (due to imprecision) in terms of adverse events when comparing oral propranolol + topical timolol maleate with topical timolol maleate (RR 3.00, 95% CI 0.13 to 67.51; Analysis 24.1).
24.1. Analysis.
Comparison 24 Oral propranolol + topical timolol maleate versus topical timolol maleate, Outcome 1 Adverse events.
Secondary outcome 1: Other measures of resolution
Gong 2015 provided information on the number of cases with size reduction ≥ 50% after treatment, reporting a total of 19 cases (26 children; 73%). Eleven children out of 13 (84.6%) in the oral propranolol + topical timolol maleate group and 8 out of 13 (61.5%) in the topical timolol maleate group reached size reduction ≥ 50%. This study found no significant differences in terms of size reduction when comparing oral propranolol + topical timolol maleate with topical timolol maleate (RR 1.38, 95% CI 0.84 to 2.24; Analysis 24.2).
24.2. Analysis.
Comparison 24 Oral propranolol + topical timolol maleate versus topical timolol maleate, Outcome 2 Other measures of resolution: size reduction > 50%.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 25. Propranolol comparisons: oral propranolol + oral prednisolone versus oral prednisolone
For this comparison, we included information from one trial with 20 children (Malik 2013). Children aged from 1 week to 8 months with potentially disfiguring or functionally threatening IH were randomised into 3 groups: Group A, propranolol (2 to 3 mg/kg/d); Group B, prednisolone (1 to 4 mg/kg/d); and Group C, propranolol + prednisolone for a minimum duration of 3 months. After discharge, all children were re‐evaluated after eight days of treatment and then every month for a minimum of three months. Duration of treatment was three months or more; children were followed until 18 months.
Primary outcome 1: Clearance, as assessed by a clinician
We found no clear information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Malik 2013 provided information about complications in general, including Cushingoid appearance and gastrointestinal upset, reporting a total of 16 cases (20 children; 80%). Nine children out of 10 (90%) in the oral propranolol + oral prednisolone group and 7 out of 10 (70%) in the oral prednisolone group reported adverse events. This study found no significant differences in terms of adverse events when comparing oral propranolol + oral prednisolone with oral prednisolone (RR 1.29, 95% CI 0.82 to 2.03; Analysis 25.1).
25.1. Analysis.
Comparison 25 Oral prednisolone versus oral prednisolone + oral propranolol, Outcome 1 Adverse events: complications.
Secondary outcome 1: Other measures of resolution
Malik 2013 provided information on two measures of resolution. Regarding colour fading, Malik 2013 reported a mean score of ‐8 units (SD = 2.9) in the visual analogue scale for the propranolol + prednisolone group, versus a mean of ‐9 units (SD = 1.5) for the prednisolone group. This study found no differences in terms of colour‐fading score when comparing oral propranolol + oral prednisolone with oral prednisolone (MD 1.00, 95% CI ‐1.02 to 3.02; Analysis 25.2). Malik 2013 also assessed the percentage of mean size reduction after treatment, reporting a mean size of 66.6 (SD = 41.6) for the propranolol + prednisolone group, versus a mean size of 82.6 (SD = 10.4) for the prednisolone group. This study found no clear differences (due to imprecision) in terms of size reduction when comparing oral propranolol + oral prednisolone with oral prednisolone (MD ‐16.00, 95% CI ‐42.58 to 10.58; Analysis 25.3).
25.2. Analysis.
Comparison 25 Oral prednisolone versus oral prednisolone + oral propranolol, Outcome 2 Other measures of resolution: colour fading.
25.3. Analysis.
Comparison 25 Oral prednisolone versus oral prednisolone + oral propranolol, Outcome 3 Other measures of resolution: mean size reduction.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 26. Other comparisons: intralesional methylene blue versus intralesional triamcinolone
For this comparison, we included information from one trial with 268 children (Feng 2000). In this trial, children were randomised into two groups. Group A received methylene blue 1% injection doses from 10 to 20 mg, once a week for four weeks. Group B received triamcinolone injection doses from 20 to 50 mg, once a week for four weeks. Duration of treatment was unclear; children were followed from one to three years.
Primary outcome 1: Clearance, as assessed by a clinician
Fu 2012 provided information on this outcome, reporting a total of 172 cases of clearance (268 children; percentage of clearance: 64.1%). In this study, 129 children out of 150 (86%) in the intralesional methylene blue group and 43 out of 118 (36.4%) in the intralesional triamcinolone group reached clearance of lesions. Clearance after intralesional methylene blue was 2.36 times greater than after intralesional triamcinolone (RR 2.36, 95% CI 1.84 to 3.02; Analysis 26.1).
26.1. Analysis.
Comparison 26 Intralesional methylene blue versus intralesional triamcinolone, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
We found no information on this outcome for this comparison.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 27. Other comparisons: oral prednisolone versus intravenous methylprednisolone
For this comparison, we included information from one trial with 20 children (Pope 2007). Children with problematic haemangiomas from one to four months of age were randomised into two groups. In the oral group, infants received oral prednisolone, 2 mg/kg per day, in two divided doses for 3 months. This dose was followed by a tapering schedule (decreasing the dose by 1 mg per month) over 6 to 9 months to prevent rebound. The intravenous group received pulses of intravenous high‐dose corticosteroids monthly for three months. A pulse consisted of methylprednisolone in doses of 30 mg/kg per day infused over 1 hour daily for 3 days. Treatment was administered for at least three months; children were followed from three months to one year of life.
Primary outcome 1: Clearance, as assessed by a clinician
We found no information on this outcome for this comparison.
Primary outcome 2: A subjective measurement of improvement
The trial by Pope 2007 found a good correlation between the visual analogue scale scores of the size of the haemangioma as reported by the parents of the children and the blinded outcome assessors (correlation coefficient = 0.92).
Primary outcome 3: Adverse events
In the Pope 2007 trial, adverse events in the 20 children were recorded by the parents (parent diaries) and the clinicians (monitoring). Parental reports of adverse events were reported as median and range along with a P value for the following eight types.
Irritability: median 1 (range 0 to 3) for oral group and 1.5 (0 to 3) for IV group (P = 0.85).
Crying: median 1 (range 0 to 3) for oral group and 0.5 (0 to 2) for IV group (P = 0.58).
Hyperactivity: both groups had a median 0 (range 0 to 2) (P = 1.00).
Apathy: median 0 (range 0) for oral group and 0 (0 to 1) for IV group (P = 0.32).
Insomnia: median 1 (range 0 to 3) for oral group and 0 (0 to 1) for IV group (P = 0.08).
Vomiting: both groups had a median 0 (range 0 to 2) (P = 1.00).
Abdominal pains: median 0 (range 0 to 2) for both groups (P = 0.34).
Behavioural changes: median 0 (range 0) for oral group and 0 (0 to 1) for IV group (P = 0.32).
One child in the oral prednisolone group required antihypertensive medication for persistent high blood pressure, and one child in each of the treatment groups experienced serious respiratory distress, but both made a full recovery. Although no difference in the children's growth factors (height and weight) were seen at 3 months, by 1 year children in the oral prednisolone group had evidence of growth retardation as compared to the intravenous methylprednisolone group (weight, P = 0.003; height, P < 0.001).
Secondary outcome 1: Other measures of resolution
In the Pope 2007 trial, the 10 children in the oral prednisolone group had significantly greater reductions in the size of the haemangioma when compared to the 10 children in the intravenous methylprednisolone group at 1 year of age (MD 51.50 mm, 95% CI 21.49 to 81.51; Analysis 27.1).
27.1. Analysis.
Comparison 27 Oral prednisolone versus intravenous methylprednisolone, Outcome 1 Other measures of resolution: haemangioma size.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 28. Other comparisons: HIFU at 3.5 W versus HIFU at 4.5 W
For this comparison, we included information from one trial with 40 children (Fu 2012). In Fu 2012, the lesion surface was irradiated with 3 to 5 mm/second continuously by ultrasonic therapeutic apparatus at frequency of 9 MHz, impulse of 1000, and 10% of scanning overlap. The ultrasound was used three times as a course of treatment, with one‐month interval. Duration of treatment was unclear; children were followed until six months.
Primary outcome 1: Clearance, as assessed by a clinician
Fu 2012 provided information on this outcome, reporting a total of 15 cases of clearance (40 children; percentage of clearance: 37.5%). Seven children out of 20 (35%) in HIFU at 3.5 W group and 8 out of 20 (40%) in HIFU at 4.5 W group reached clearance of lesions. This study found no significant differences in terms of clearance when comparing HIFU at 3.5 W with HIFU at 4.5 W (RR 0.88, 95% CI 0.39 to 1.95; Analysis 28.1).
28.1. Analysis.
Comparison 28 HIFU 3.5 W versus HIFU 4.5 W, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Fu 2012 provided information about cases of ulceration or scars, reporting a total of seven cases (40 children; Analysis 28.2). No children out of 20 (0%) in HIFU at 3.5 W group and 7 out of 20 (35%) in HIFU at 4.5 W group reported ulcerations or scars. This study found no clear differences (due to imprecision) in terms of adverse events when comparing HIFU at 3.5 W with HIFU at 4.5 W (RR 0.07, 95% CI 0.00 to 1.09; Analysis 28.2).
28.2. Analysis.
Comparison 28 HIFU 3.5 W versus HIFU 4.5 W, Outcome 2 Adverse events: ulceration or scars.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 29. Other comparisons: HIFU at 3.5 W versus HIFU at 4.0 W
For this comparison, we included information from one trial with 40 children (Fu 2012). In Fu 2012, the lesion surface was irradiated with 3 to 5 mm/second continuously by ultrasonic therapeutic apparatus at frequency of 9 MHz, impulse of 1000, and 10% of scanning overlap. The ultrasound was used three times as a course of treatment, with one‐month interval. Duration of treatment was unclear; children were followed until six months.
Primary outcome 1: Clearance, as assessed by a clinician
Fu 2012 provided information on this outcome, reporting a total of 16 cases of clearance (40 children; percentage of clearance: 40%). Seven children out of 20 (35%) in HIFU at 3.5 W group and 9 out of 20 (45%) in HIFU at 4.0 W group reached clearance of lesions. This study found no significant differences in terms of clearance when comparing HIFU at 3.5 W with HIFU at 4.0 W (RR 0.78, 95% CI 0.36 to 1.68; Analysis 29.1).
29.1. Analysis.
Comparison 29 HIFU 3.5 W versus HIFU 4.0 W, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Fu 2012 provided information about cases of ulceration or scars, reporting a total of four cases (40 children; 10%). No children out of 20 (0%) in HIFU at 3.5 W group and 4 out of 20 (20%) in HIFU at 4.0 W group reported ulceration or scars. This study found no clear differences (due to imprecision) in terms of adverse events when comparing HIFU at 3.5 W with HIFU at 4.0 W (RR 0.11, 95% CI 0.01 to 1.94; Analysis 29.2).
29.2. Analysis.
Comparison 29 HIFU 3.5 W versus HIFU 4.0 W, Outcome 2 Adverse events: ulceration or scars.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Comparison 30. Other comparisons: HIFU at 4.0 W versus HIFU at 4.5 W
For this comparison, we included information from one trial with 40 children (Fu 2012). In Fu 2012, the lesion surface was irradiated with 3 to 5 mm/second continuously by ultrasonic therapeutic apparatus at frequency of 9 MHz, impulse of 1000, and 10% of scanning overlap. The ultrasound was used three times as a course of treatment, with one‐month interval. Duration of treatment was unclear; children were followed until six months.
Primary outcome 1: Clearance, as assessed by a clinician
Fu 2012 provided information on this outcome, reporting a total of 17 cases of clearance (40 children; percentage of clearance: 42.5%). Nine children out of 20 (45%) in HIFU at 4.0 W group and 8 out of 20 (40%) in HIFU at 4.5 W group reached clearance of lesions. This study found no differences in terms of clearance when comparing HIFU at 4.0 W with HIFU at 4.5 W (RR 1.13, 95% CI 0.55 to 2.32; Analysis 30.1).
30.1. Analysis.
Comparison 30 HIFU 4.0 W versus HIFU 4.5 W, Outcome 1 Clearance.
Primary outcome 2: A subjective measurement of improvement
We found no information on this outcome for this comparison.
Primary outcome 3: Adverse events
Fu 2012 provided information about cases of ulceration or scars, reporting a total of 11 cases (40 children; 27.5%). Four children out of 20 (20%) in HIFU at 4.0 W group and 7 out of 20 (35%) in HIFU at 4.5 W group reported ulceration or scars. This study found no clear differences in terms of adverse events when comparing HIFU at 4.0 W with HIFU at 4.5 W (RR 0.57, 95% CI 0.20 to 1.65; Analysis 30.2).
30.2. Analysis.
Comparison 30 HIFU 4.0 W versus HIFU 4.5 W, Outcome 2 Adverse events: ulceration or scars.
Secondary outcome 1: Other measures of resolution
We found no information on this outcome for this comparison.
Secondary outcome 2: Proportion of parents who consider their child still has a problem
We found no information on this outcome for this comparison.
Secondary outcome 3: Proportion of children who consider they still have a problem, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 4: Aesthetic appearance as assessed by physician, child, or parent, at any follow‐up
We found no information on this outcome for this comparison.
Secondary outcome 5: Requirement for surgical correction, as assessed by a physician, at any follow‐up
We found no information on this outcome for this comparison.
Discussion
Summary of main results
This systematic review identified 28 controlled trials with 1728 children enrolled and randomised.
We selected information for three important comparisons, which we summarised in three tables: Table 1; Table 2; and Table 3.
Oral propranolol versus placebo (3 parallel studies; 312 children)
Moderate‐quality evidence showed that compared with placebo, 3 mg/kg/day of propranolol probably improves clinician‐assessed clearance at 24 weeks (based on 156 children, from one study) and probably leads to a clinician‐assessed change in mean haemangioma volume at 24 weeks, as another measure of resolution (based on 40 children, from one study). We found no evidence of a difference in terms of short‐ or long‐term severe adverse events between the two groups (based on 509 children, from three studies) or in terms of cardiovascular adverse events, bronchospasm, or hypoglycaemia events (based on low‐quality evidence).
Topical timolol maleate versus placebo (1 parallel study; 41 children)
Clinician‐assessed clearance was not reported for this comparison. Low‐quality evidence indicated that achieving clinician‐confirmed reduction of redness at 24 weeks (used as a measure of resolution) may be increased with topical timolol maleate 0.5% gel applied twice daily, as opposed to placebo (based on 41 children, from one study). Regarding short‐ or long‐term serious cardiovascular events, in both groups there were zero events of bradycardia and hypotension (based on low‐quality evidence from 41 children in one study). No other safety data were assessed.
Oral propranolol versus topical timolol maleate (1 parallel study; 26 children)
Clinician‐assessed clearance was not reported for this comparison. There was no evidence of a difference between oral propranolol (via a tablet, at a 1.0 mg/kg dose, taken once per day) and topical timolol maleate (0.5% eye drops applied twice daily) in producing a 50% or greater reduction in haemangioma size at 24 weeks (based on 26 children from one study; low‐quality evidence). Regarding short‐ or long‐term general adverse events, although there were more events in the oral propranolol group such as severe diarrhoea, lethargy, and loss of appetite, this result was based on a low number of events and very low‐quality evidence; therefore, we are uncertain about the safety implications (based on 26 children from one study).
For the three comparisons just discussed, the following outcomes, measured at any follow‐up, were not reported.
A subjective measure of improvement, as assessed by the parent or child.
Proportion of parents who consider their child still has a problem.
Proportion of children who consider they still have a problem.
Aesthetic appearance as assessed by physician, child, or parent.
We found little evidence for a number of outcomes, especially patient‐reported outcomes, such as subjective measure of improvement, proportion of children who consider they still have a problem, proportion of parents who consider their child still has a problem, and aesthetic appearance. Furthermore, we included no trials assessing a large number of haemangioma treatments, such as argon laser, carbon dioxide laser, erbium laser, excision, cryotherapy, imiquimod, interferon alpha, vincristine, and rapamycin.
Overall completeness and applicability of evidence
We aimed to include all interventions recommended for the management of infantile haemangiomas in children, and found a wide variety of different interventions and comparisons. We included a total of 12 interventions (not including combinations of these interventions) and 30 comparisons. Most of the included interventions were evaluated in single studies, precluding meta‐analysis. Due to the low quality of the evidence found for our key comparisons and incomplete coverage in terms of outcomes of interest and interventions, the external validity of the review is poor.
Propranolol (oral, topical, and intralesional), currently the first‐line recommended intervention for IH, was the most assessed intervention (in 16 studies). However, some trials included in this systematic review assessed the efficacy of less used, and perhaps less important, treatments, such as bleomycin and radiation, whilst we found no evidence from randomised trials related to efficacy and safety of potential interventions such as argon laser, carbon dioxide laser, erbium laser, excision, cryotherapy, imiquimod, interferon alpha, vincristine, or rapamycin. Information about ongoing trials may be useful in clarifying the role of these potential treatments in the management of infantile haemangiomas in children (NCT01147601; NCT02913612).
Data were lacking for a number of outcomes, especially patient‐relevant outcomes, such as reports of improvement by parents/children or aesthetic/cosmetic assessment, among others. Results for these outcomes could provide critical information about the patient/carer perspective on the benefits and harms of the proposed interventions for the treatment of this condition.
Furthermore, it is difficult to ascertain the applicability of the results in terms of population because the age of the children was reported inconsistently; numerous subtypes of haemangiomas were included; and some trials did not provide information on haemangioma type. Lack of data also meant we were unable to undertake subgroup analysis by haemangioma type, although we did note that evidence was scarce for complicated scenarios such as ulcerated or problematic IH.
Quality of the evidence
This systematic review included only randomised controlled trials, as this design is considered the gold‐standard for assessing the efficacy of an intervention. However, although we included 28 trials that were eligible according to the inclusion criteria, we considered the quality of the evidence for the three most important comparisons mainly moderate or low. One of the reasons for this rating was the unclear risk of bias in several elements related to the methodological quality of included trials. For example, blinding of outcomes assessment was reported for 13 trials (Aly 2015; Chan 2013; Ehsani 2014; Gong 2015; Hogeling 2011; Kessels 2013; Leaute‐Labreze 2013; Leaute‐Labreze 2015; Li 2016; Malik 2013; Pope 2007; Tan 2012; Zaher 2016), whereas the remaining trials did not report this information in a clear way. We considered only three studies at low risk of bias for the three blinding items (Chan 2013; Hogeling 2011; Leaute‐Labreze 2013). Likewise, there was some evidence of selective omissions of outcomes from publications for six trials (Feng 2000; Hogeling 2011; Jung 1977; Malik 2013; Pope 2007; Xu 2006; Zhang 2013), especially concerning the safety of the assessed interventions. In addition, one of the factors that led to us downgrading the quality of evidence was small sample sizes. As mentioned above, most of the comparisons were based on a single trial with a small number of recruited children; only four trials recruited more than 100 children (Batta 2002; Feng 2000; Jung 1977; Leaute‐Labreze 2015). Due to these limitations, our confidence in the effect estimate is limited, and we considered that the true effect may be different from the estimate of the effect showed in this present review.
Potential biases in the review process
We aimed to minimise potential biases during the development of this review. We followed the methodology for systematic reviews outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We feel that this review was comprehensive in identifying clinical trials addressing the issue of efficacy and safety of suggested interventions for the management of infantile haemangiomas. However, insufficient information was available for seven studies to classify them as included or excluded, because they were published only as conference proceedings, or because we did not have access to the full texts. The fact that these studies have not yet been incorporated into the review may be a potential source of bias. In addition, we considered two of the studies as 'ongoing' due to their date of publication as abstracts. We may be able to decide whether or not to include them once they are published as full texts.
We modified some of the methods planned for and used in the original review published by Leonardi‐Bee 2011: changes included the need to add current interventions used in the treatment of IH (such as oral propranolol), modification of primary and secondary outcomes, and assessment of evidence using the GRADE approach, among other changes (see Differences between protocol and review for more information). These modifications could be a potential source of bias in the review process. In addition, due to scarcity of data for most of the comparisons, we did not perform the planned investigation of heterogeneity and sensitivity analysis.
Agreements and disagreements with other studies or reviews
We identified and selected five reviews published in the last five years on the management of IH in children. A recent systematic review of the literature highlighted several potential pharmacotherapy treatments for management of IH (Chinnadurai 2016b); the authors of this review included randomised trials as well as observational evidence to address several potential interventions for the management of infantile haemangiomas, and found evidence that propranolol had the highest clearance rate, with high variability, under a network meta‐analysis approach. These findings are also in agreement with Izadpanah 2013, a systematic review and meta‐analysis, which suggests that propranolol therapy could potentially be superior to other alternatives of management of IH in children. Our results also found propranolol to be one of the interventions with clear evidence of benefits, a comprehensive assessment of adverse events, as well as a considerable number of studies focused on its efficacy and safety. However, unlike Chinnadurai 2016b and Izadpanah 2013, we added an assessment of the evidence using the GRADE approach, finding issues related to potential risk of bias and imprecision, which raise uncertainty in the estimated effect, especially those effects related to adverse events (see Quality of the evidence).
Regarding laser treatments, other authors have remarked that evidence is limited by low sample size, lack of comparisons of the same modalities, and variations in the used laser settings including wavelength and cooling protocols (Chinnadurai 2016a). However, in an additional systematic review and meta‐analysis, Shen 2015 found that despite the few trials included in the review, PDL should be considered as a treatment modality, especially for superficial haemangiomas. Our findings from Batta 2002, an included study, suggested that there was no evidence of a difference in achieving clearance of the haemangioma between PDL and wait‐and‐see (i.e. active monitoring), but fewer adverse events were generally observed with wait‐and‐see.
Xu 2014 gathered evidence under a systematic approach related to the efficacy and safety of propranolol versus corticosteroids for the treatment of periorbital infantile haemangiomas, finding only case series addressing this comparison. The authors concluded that propranolol may be an effective and even safer intervention for periorbital IHs compared with corticosteroids. This conclusion was based on the results of studies some of which were neither randomised nor controlled. We did not find evidence from randomised trials addressing this issue.
Authors' conclusions
Implications for practice.
Our Cochrane Review updated the evidence on the effects of different interventions for the management of infantile haemangiomas (IHs). We included 12 interventions, 28 studies, and 30 comparisons. We assessed the quality of the evidence underlining three key treatment comparisons, and have been able to draw the following conclusions.
There is moderate‐quality evidence that, when compared with placebo, oral propranolol is probably beneficial in terms of complete or almost complete clearance and probably reduces haemangioma volume more than placebo. We found no evidence of a difference in terms of short‐ or long‐term adverse events between the groups (low‐quality evidence).
Low‐quality evidence indicates that topical timolol maleate may reduce IH redness more than placebo, with possibly no accompanying cardiovascular events, although no other safety data were assessed for this comparison.
There was no evidence of a difference between oral propranolol and topical timolol maleate in their ability to generate a 50% or greater reduction in IH size, based on low‐quality evidence. We were unable to draw conclusions about adverse events for this comparison due to very low‐quality evidence.
All outcomes reported for these comparisons were measured at 24 weeks’ follow‐up and were clinician assessed, except for the safety outcomes. We are unable to present evidence on the following key outcomes for our key comparisons because they were not reported.
A subjective measure of improvement, as assessed by the parent or child.
Proportion of parents who consider their child still has a problem.
Proportion of children who consider they still have a problem.
Aesthetic appearance as assessed by physician, child, or parent.
As the evidence underlying our results for propranolol and timolol maleate was allocated a GRADE rating no higher than moderate, we cannot make qualitative statements with high certainty. However, propranolol remains the standard treatment for infantile haemangiomas, and clinicians should be aware that clinical management of haemangiomas depends on the following risk factors, amongst others: the grade of complication, presence of comorbidities, clinician experience, need for hospitalisation versus ambulatory care, and patient factors.
A large number of interventions were not assessed by any included study: argon laser, carbon dioxide laser, erbium laser, excision, cryotherapy, imiquimod, interferon alpha, vincristine, and rapamycin.
Implications for research.
Despite the fact that there was a considerable increase in the number of trials since the publication of the first version of this review (from 4 trials/271 children to 28 trials/1728 children), the certainty of the evidence was reduced due to issues such as the sample size of the included studies and risk of bias in, for example, blinding and selective reporting domains. Furthermore, scarce or non‐existent evidence for certain interventions and outcomes means that there is still a need for high‐quality randomised controlled trials (RCTs) to assess interventions for IH.
Participants
Randomised controlled trials are needed for all types of haemangiomas, especially complicated scenarios such as ulcerated or problematic IH, where evidence is lacking. It is important that the haemangioma subtype is clearly reported in trial publications.
Interventions
There is a need for RCTs related to the efficacy and safety of the following interventions:
oral propranolol and topical timolol maleate (assessed separately);
combination of oral propranolol with other interventions (such as laser or corticosteroids);
combination of topical timolol maleate with other interventions;
other potential interventions, such as imiquimod, interferon alpha, excision, cryotherapy, vincristine, and rapamycin; and
new interventions, such as beta blockers.
The evaluation of different dosages and duration, which will vary according to treatment, should also be taken into account.
Comparators
High‐quality trials should assess oral propranolol and topical timolol maleate against each other. Other interventions should also be compared against oral propranolol and topical timolol maleate, as beta blockers are currently approved as standard care both by the US Food and Drug Administration and the European Medicines Agency.
Outcomes
Important outcomes to assess include the incidence and types of adverse events experienced by trial participants, as well as other patient‐reported outcomes, or those pertaining to parents and carers, such as the proportion of participants that feel they still have a problem (either reported by the child or the parent/carer), the requirement for surgical correction, and aesthetic appearance. These outcomes should ensure follow‐up in both the short term and the long term. Furthermore, objective outcomes such as resolution need further assessment.
Methodology
A sufficient sample size to enable the detection of a clinically important effect size is crucial when conducting future trials; this could perhaps be achieved using a multicentre approach. Trials must be rigorously reported to help overcome methodological issues associated with poor RCTs in this field, such as selective reporting of outcomes and unclear blinding (outcomes, participants, and personnel). Furthermore, thorough reporting with regard to the nature of the interventions, the age of participants, and the type of haemangiomas included will ensure the applicability of future trial results.
What's new
| Date | Event | Description |
|---|---|---|
| 4 April 2018 | New citation required and conclusions have changed | This update included studies of many more interventions, including beta blockers, which are currently the standard treatment for infantile haemangiomas. |
| 4 April 2018 | New search has been performed | A new search led to the addition of 24 new included studies, and we updated the review in line with MECIR standards. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 5, 2011
| Date | Event | Description |
|---|---|---|
| 15 March 2012 | Amended | Corrected a 'What's new' event on a previous version of the review |
| 1 August 2008 | Amended | Converted to new review format |
Acknowledgements
We would like to thank Cochrane Skin for their help during all stages of this review. Cochrane Skin wishes to thank Urbá González, who was the Dermatology Editor for this review; Thomas Chu and Ching‐Chi Chi, who were the Statistical and Methods Editors, respectively; the clinical referees, Lea Solman and another who wishes to remain anonymous; and the consumer referee, Anne Lyddiatt. We would also like to thank Aidan Tan for his help with papers in data extraction of Zhong 2015.
Appendices
Appendix 1. Skin Group Specialised Register (CRS) search strategy
#1 MeSH DESCRIPTOR Hemangioma #2 MeSH DESCRIPTOR Hemangioma, Capillary #3 MeSH DESCRIPTOR Hemangioma, Cavernous #4 (hemangioma* or haemangioma*) #5 (capillary and (naev* or nev*)) #6 (strawberry and (naev* or nev*)) #7 (strawberry birthmark*) #8 (strawberry mark*) #9 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 hemangioma* or haemangioma*:ti,ab,kw #2 MeSH descriptor: [Hemangioma] explode all trees #3 MeSH descriptor: [Hemangioma, Capillary] explode all trees #4 capillary and (naev* or nev*):ti,ab,kw #5 strawberry and (naev* or nev*):ti,ab,kw #6 strawberry birthmark*:ti,ab,kw #7 MeSH descriptor: [Hemangioma, Cavernous] explode all trees #8 strawberry mark*:ti,ab,kw #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
Appendix 3. MEDLINE (Ovid) search strategy
1. exp Hemangioma, Capillary/ or exp Hemangioma/ or exp Hemangioma, Cavernous/ 2. (haemangioma$ or hemangioma$).mp. 3. (strawberry naev$ or strawberry nev$).mp. 4. (capillary naev$ or capillary nev$).mp. 5. (superficial angiomatous naev$ or superficial angiomatous nev$).mp. 6. strawberry birthmark$.mp. 7. strawberry mark$.mp. 8. 1 or 2 or 3 or 4 or 5 or 6 or 7 9. randomized controlled trial.pt. 10. controlled clinical trial.pt. 11. randomized.ab. 12. placebo.ab. 13. clinical trials as topic.sh. 14. randomly.ab. 15. trial.ti. 16. 9 or 10 or 11 or 12 or 13 or 14 or 15 17. exp animals/ not humans.sh. 18. 16 not 17 19. 8 and 18
Lines 9‐18: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision).
Appendix 4. Embase (Ovid) search strategy
1. exp skin hemangioma/ or exp capillary hemangioma/ or exp hemangioma/ or exp cavernous hemangioma/ 2. (haemangioma$ or hemangioma$).mp. 3. (strawberry naev$ or strawberry nev$).mp. 4. (capillary naev$ or capillary nev$).mp. 5. (superficial angiomatous naev$ or superficial angiomatous nev$).mp. 6. strawberry birthmark$.mp. 7. strawberry mark$.mp. 8. or/1‐7 9. crossover procedure.sh. 10. double‐blind procedure.sh. 11. single‐blind procedure.sh. 12. (crossover$ or cross over$).tw. 13. placebo$.tw. 14. (doubl$ adj blind$).tw. 15. allocat$.tw. 16. trial.ti. 17. randomized controlled trial.sh. 18. random$.tw. 19. or/9‐18 20. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 21. human/ or normal human/ 22. 20 and 21 23. 20 not 22 24. 19 not 23 25. 8 and 24
Appendix 5. AMED (Ovid) search strategy
1. (haemangioma$ or hemangioma$).mp. 2. strawberry birthmark$.mp. 3. strawberry mark$.mp. 4. or/1‐3 5. randomized controlled trial$/ 6. random allocation/ 7. double blind method/ 8. single blind method.mp. 9. exp Clinical trials/ 10. (clin$ adj25 trial$).mp. 11. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$ or dummy)).mp. 12. (placebo$ or random$).mp. 13. research design/ or clinical trials/ or comparative study/ or double blind method/ or random allocation/ 14. prospective studies.mp. 15. cross over studies.mp. 16. Follow up studies/ 17. control$.mp. 18. (multicent$ or multi‐cent$).mp. 19. ((stud or design$) adj25 (factorial or prospective or intervention or crossver or cross‐over or quasi‐experiment$)).mp. 20. 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. 4 and 20
Appendix 6. PsycINFO (Ovid) search strategy
1. (haemangioma$ or hemangioma$).mp. 2. strawberry birthmark$.mp. 3. strawberry mark$.mp. 4. or/1‐3 5. double‐blind.tw. 6. random$ assigned.tw. 7. control.tw. 8. 5 or 6 or 7 9. 4 and 8
Lines 5‐8: therapy filter for PsycINFO (Ovid) created by the Health Information Research Unit at McMaster University.
Appendix 7. LILACS search strategy
In LILACS we searched using the Controlled clinical trials topic‐specific query filter and the following terms: hemangioma$ or haemangioma$ or nevi or nevus
Appendix 8. CINAHL (EBSCO) search strategy
S1 TX strawberry birthmark* S2 TX strawberry mark* S3 (MM "Hemangioma+") OR (MM "Hemangioma, Cavernous") S4 (MH "Clinical Trials+") S5 PT clinical trial S6 TX (clinic* n1 trial*) S7 (MH "Random Assignment") S8 TX random* allocat* S9 TX placebo* S10 (MH "Placebos") S11 (MH "Quantitative Studies") S12 TX allocat* random* S13 "randomi#ed control* trial*" S14 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) S15 S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 S16 TI ( haemangioma* or hemangioma* ) OR AB ( haemangioma* or hemangioma* ) S17 S1 OR S2 OR S3 OR S16 S18 S15 AND S17
Lines S4 to S15: based on the SIGN filter for RCTs in CINAHL via EBSCO.
Data and analyses
Comparison 1. Pulsed dye laser versus wait‐and‐see.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.62, 1.42] |
| 2 Adverse events: skin atrophy | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 3.46 [1.36, 8.77] |
| 3 Adverse events: skin hypopigmentation | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [1.57, 5.93] |
| 4 Adverse events: minimal crusting | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.27, 93.55] |
| 5 Adverse events: pain | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.27, 93.55] |
| 6 Other measures of resolution: no redness | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 4.83 [1.75, 13.36] |
| 7 Parents who consider that their child still has a problem | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.56, 2.78] |
| 8 Aesthetic appearance: better cosmetic outcome | 1 | 22 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [0.71, 4.31] |
| 9 Requirement for surgical correction | 1 | 121 | Risk Ratio (M‐H, Random, 95% CI) | 2.37 [0.64, 8.75] |
Comparison 2. Oral propranolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance, as assessed by a clinician | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 At 1 mg/kg/day | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 13.48 [3.41, 53.30] |
| 1.2 At 3 mg/kg/day | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 16.61 [4.22, 65.34] |
| 2 Serious adverse events | 3 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.33, 3.39] |
| 3 Serious cardiovascular adverse events | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Bradycardia | 3 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.03, 14.32] |
| 3.2 Hypotension | 3 | 509 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.10, 6.71] |
| 4 Other adverse events | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Bronchospasm | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.04, 3.89] |
| 4.2 Bronchitis | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 5.62 [0.79, 40.07] |
| 4.3 Bronchiolitis | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.42, 4.21] |
| 4.4 Hypoglycaemia | 1 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.03, 14.32] |
| 4.5 Sleep disturbance | 2 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.79, 3.00] |
| 5 Other measures of resolution: change in volume | 1 | Mean Difference (Random, 95% CI) | ‐45.9 [‐80.20, ‐11.60] | |
| 6 Other measures of resolution: no improvement in redness | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 9.00 [0.52, 156.91] |
Comparison 3. Topical timolol maleate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other measures of resolution (6 months) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 No redness | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 8.11 [1.09, 60.09] |
| 1.2 IH volume reduction of ≥ 5% | 1 | 41 | Risk Ratio (M‐H, Random, 95% CI) | 5.21 [1.28, 21.21] |
Comparison 4. Topical bleomycin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other measures of resolution: reduction in size at day 7 | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 21.0 [1.34, 328.86] |
Comparison 5. X‐ray radiation versus sham radiation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.63, 1.87] |
Comparison 6. Topical timolol maleate versus Nd:YAG laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other measures of resolution (continuous) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.27, ‐0.55] |
| 1.1 Haemoglobin level (redness) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.91 [‐1.27, ‐0.55] |
| 2 Other measures of resolution (dichotomous) | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.90, 10.01] |
| 2.1 Excellent improvement | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.90, 10.01] |
Comparison 7. Nd:YAG laser versus oral propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.28, 23.44] |
| 2 Adverse events: hyperpigmentation | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.28, 23.44] |
| 3 Adverse event: pigmentation and thinning | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.11, 1.05] |
| 4 Adverse events: superficial scar | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [0.24, 9.58] |
| 5 Other measures of resolution: excellent response | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.08, 1.46] |
Comparison 8. Pulsed dye laser + topical propranolol versus pulsed dye laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance, as assessed by a clinician | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.57, 8.86] |
Comparison 9. Pulsed dye laser + topical timolol maleate versus pulsed dye laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.07, 14.64] |
| 2 Other measures of resolution: mean size reduction | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 5.62 [1.21, 10.03] |
Comparison 10. Nd:YAG laser + oral propranolol versus Nd:YAG laser.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 3.28 [0.97, 11.06] |
| 2 Adverse events: hyperpigmentation | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.35, 6.02] |
| 3 Adverse events: pigmentation and thinning | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.57] |
| 4 Adverse events: superficial scar | 2 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.09, 1.48] |
| 5 Other measures of resolution: excellent response | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 8.5 [2.25, 32.06] |
Comparison 11. Nd:YAG laser + oral propranolol versus oral propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 8.44 [1.14, 62.66] |
| 2 Adverse events: hyperpigmentation | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 3.75 [0.44, 31.68] |
| 3 Adverse events: pigmentation and thinning | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.05, 0.90] |
| 4 Adverse events: superficial scar | 2 | 102 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.05, 7.63] |
| 5 Other measures of resolution: excellent response | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.83 [1.42, 5.67] |
Comparison 12. ⁹⁰SR‐⁹⁰Y radiation + topical timolol maleate versus ⁹⁰SR‐⁹⁰Y radiation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.07, 1.87] |
| 2 Adverse events | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.56, 2.29] |
Comparison 13. Sequential dual‐wavelength laser + oral propranolol versus concurrent dual‐wavelength laser + oral propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other outcomes of resolution: mean efficacy rating | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.16, ‐0.38] |
Comparison 14. Oral propranolol versus topical propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [1.01, 8.95] |
| 2 Adverse events: syncopal attack | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 124.83] |
Comparison 15. Oral propranolol versus intralesional propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 4.5 [1.16, 17.44] |
| 2 Adverse events: syncopal attack | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 124.83] |
Comparison 16. Topical propranolol versus intralesional propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 1.5 [0.29, 7.73] |
Comparison 17. Oral atenolol versus oral propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.27] |
Comparison 18. Oral propranolol versus oral prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe adverse events | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.02, 1.02] |
| 2 Adverse events: complications in general | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.78] |
| 3 Other measures of resolution: colour fading | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐3.08, 1.08] |
| 4 Other measures of resolution: mean size reduction | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 23.20 [‐3.36, 49.76] |
| 5 Other measures of resolution: proportional change in the total surface area | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.08, 0.54] |
Comparison 19. Oral propranolol versus oral captopril.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 15.0 [0.93, 241.20] |
| 2 Adverse events: cardiac side effects | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.90] |
Comparison 20. Oral propranolol versus topical timolol maleate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.40, 123.35] |
| 2 Other measures of resolution: size reduction > 50% | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.64, 1.97] |
Comparison 21. Oral propranolol versus oral propranolol + oral prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events in general | 2 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.91] |
| 2 Other measures of resolution: mean size reduction | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 7.20 [‐1.87, 16.27] |
| 3 Other measures of resolution: decrease in redness | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.45, 1.32] |
Comparison 22. Oral propranolol versus oral ibuprofen + oral paracetamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [0.78, 9.15] |
| 2 Adverse events | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.38, 130.26] |
| 3 Other measures of resolution: mean size of ulceration | 1 | 64 | Mean Difference (IV, Random, 95% CI) | 0.31 [0.01, 0.61] |
Comparison 23. Oral propranolol + topical timolol maleate versus oral propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events in general | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.04, 2.80] |
| 2 Other measures of resolution: colour fading/visual analogue scale score | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 1.18 [0.09, 2.27] |
| 3 Other measures of resolution: size reduction/visual analogue scale score | 1 | 31 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.84, 1.66] |
| 4 Other measures of resolution: size reduction > 50% | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.79, 1.88] |
Comparison 24. Oral propranolol + topical timolol maleate versus topical timolol maleate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 67.51] |
| 2 Other measures of resolution: size reduction > 50% | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.84, 2.24] |
Comparison 25. Oral prednisolone versus oral prednisolone + oral propranolol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events: complications | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.82, 2.03] |
| 2 Other measures of resolution: colour fading | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐1.02, 3.02] |
| 3 Other measures of resolution: mean size reduction | 1 | 20 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐42.58, 10.58] |
Comparison 26. Intralesional methylene blue versus intralesional triamcinolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [1.84, 3.02] |
Comparison 27. Oral prednisolone versus intravenous methylprednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Other measures of resolution: haemangioma size | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 51.5 [21.49, 81.51] |
Comparison 28. HIFU 3.5 W versus HIFU 4.5 W.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.39, 1.95] |
| 2 Adverse events: ulceration or scars | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.09] |
Comparison 29. HIFU 3.5 W versus HIFU 4.0 W.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.36, 1.68] |
| 2 Adverse events: ulceration or scars | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.94] |
Comparison 30. HIFU 4.0 W versus HIFU 4.5 W.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clearance | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.55, 2.32] |
| 2 Adverse events: ulceration or scars | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.20, 1.65] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abarzua‐Araya 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients who met inclusion criteria were randomised by simple randomisation (...)." Page 1046 Comment: There was insufficient information to rate this item as low or high risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was respected." Page 1046 Comment: Authors reported information about allocation concealment. |
| Blinding of participants (Performance bias) | Low risk | Quote: "The drugs were similar in aspect and the patients and main investigators were blind." Page 1046 Comment: Authors reported information about adequate blinding of participants and personnel. |
| Blinding of personnel (performance bias) | Low risk | Quote: "The drugs were similar in aspect and the patients and main investigators were blind." Page 1046 Comment: Authors reported information about adequate blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to rate this item as low or high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting of information was not detected. |
| Other bias | Low risk | No other biases were detected. |
Aly 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "All patients were randomly assigned into one of the two groups at equal probability using sealed envelope method." Page 1504 Comment: Insufficient information to rate this item as low or high risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "All patients were randomly assigned into one of the two groups at equal probability using sealed envelope method." Page 1504 Comment: Authors reported information about adequate allocation concealment. |
| Blinding of participants (Performance bias) | Unclear risk | It is highly probable that participants were aware of intervention group assigned, but it is unclear whether this had an impact or not in trial results. |
| Blinding of personnel (performance bias) | Unclear risk | It is highly probable that researchers were aware of intervention group assigned, but it is unclear whether this had an impact or not in trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Response to therapy was measured by blinded volume estimations at weeks (...)." Page 1504 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting of information was not detected. |
| Other bias | Low risk | No other biases were detected. |
Asilian 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Then, patients were divided into two groups in [a] double blind manner." Comment: There was insufficient information to rate this item as low or high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to rate this item as low or high risk of bias. |
| Blinding of participants (Performance bias) | Low risk | Quote: " (...) Group B with 585 PDL plus lubricant gel as placebo." Page 2 Comment: Placebo intervention was used. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to rate this item as low or high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to rate this item as low or high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting of information was not detected. |
| Other bias | Low risk | No other biases were detected. |
Batta 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotation: "A block telephone randomisation was provided by the Birmingham Clinical trial unit. We stratified randomisation by completely flat and raised lesions (...)." Page 522 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quotation: "A block telephone randomisation was provided by the Birmingham Clinical trial unit. We stratified randomisation by completely flat and raised lesions (...)." Page 522 Comment: Authors reported information about adequate allocation concealment. |
| Blinding of participants (Performance bias) | Unclear risk | It is highly probable that participants were aware of the intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of personnel (performance bias) | Unclear risk | It is highly probable that researchers were aware of the intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Despite the fact that standardised methods were used, the lead author treated children with PDL and also assessed the primary outcome at 1 year. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors performed intention‐to‐treat analysis; all children were analysed for primary outcome. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported on in the results. |
| Other bias | Low risk | No other biases were identified. |
Bauman 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: " ...were randomised ... using a CNMC institutional tamper‐proof, pregenerated encrypted schedule..." Page 324 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quotes: " ...were randomised ... using a CNMC institutional tamper‐proof, pregenerated encrypted schedule..." Page 324. "The CNMC research pharmacy dispensed study drugs." Page 324 Comment: Authors reported information about adequate allocation concealment. |
| Blinding of participants (Performance bias) | Unclear risk | Quote: "Unblinded caretakers received counselling and written instructions to administer the medication 15 minutes before meals. (...) Caretakers were trained to recognize signs of hypoglycaemia, hypotension, and bradycardia that would warrant withholding of medication." Page 324 Comment: Unclear information was reported regarding the impact of lack of blinding for caretakers in the development of this trial. |
| Blinding of personnel (performance bias) | Low risk | Quote: "At enrolment, the blinded investigators assigned a number (...). The same blinded investigator repeated the measurements of size and skin involvement monthly." Page 324 Comment: Authors reported information about adequate blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Unblinded caretakers received counselling and written instructions to administer the medication 15 minutes before meals. (...) Caretakers were trained to recognize signs of hypoglycaemia, hypotension, and bradycardia that would warrant withholding of medication." Page 324 Comment: Unclear information was reported regarding the impact of lack of blinding for caretakers in the development of this trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors performed intention‐to‐treat analysis; all children were analysed for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported on in the results. |
| Other bias | Unclear risk | Quote: "The study was terminated prior to targeted enrolment at the DSMB's recommendation owing to severe AEs described herein that prompted early withdrawal of 6 of the 8 prednisolone participants." Page 325 Comment: Reason for termination of trial could be a potential source of bias. |
Chan 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were enrolled in the trial by 1 of 2 study physicians and randomly assigned (by using a method of minimization) by the clinical trials pharmacist into 4 groups: age between 5 and 15 weeks or between 16 and 24 weeks and size of lesion, < or > 25 mm”. Page e1740 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were enrolled in the trial by 1 of 2 study physicians and randomly assigned (by using a method of minimization) by the clinical trials pharmacist into 4 groups: age between 5 and 15 weeks or between 16 and 24 weeks and size of lesion, < or > 25 mm”. Page e1740 Comment: Authors reported information about adequate allocation concealment. |
| Blinding of participants (Performance bias) | Low risk | Quote: “Participants, caregivers, and physicians were blinded to group status”. Page e1740 Comment: Authors reported information about adequate blinding of participants. |
| Blinding of personnel (performance bias) | Low risk | Quote: “Participants, caregivers, and physicians were blinded to group status”. Page e1740 Comment: Authors reported information about adequate blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Response to therapy was measured by (1) blinded predicted volume estimation at weeks 0, 1, 2, 3, 4, 8, 12, 16, 20, and 24 and (2) blinded scoring of clinical photographs at 0, 12, and 24 weeks”. Page e1741 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed using intention‐to‐treat approach. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported on in the results. |
| Other bias | Low risk | No other biases were identified. |
Ehsani 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "(...) and randomly divided into two treatment groups using a randomised number table." Page 658 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to classify this item as high or low risk of bias. |
| Blinding of participants (Performance bias) | Unclear risk | It is highly probable that participants were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of personnel (performance bias) | Unclear risk | It is highly probable that researchers were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The clinical improvements of IH lesions were quantified using a visual score system after comparing pre‐ and post‐treatment lesion photographs by two dermatologists who were blind to treatment regimens”. Page 659 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “All the 19 patients who were included to the study completed the treatment course.” Page 659 Comment: Authors reported complete data for all included children. |
| Selective reporting (reporting bias) | Unclear risk | In the protocol, the authors specified an outcome called “improvement of overall health of patient”. However, this outcome is not reported in the manuscript. |
| Other bias | Low risk | No other biases were identified. |
Feng 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "268 cases were randomly divided into two groups (...)." Page 284 Comment: Authors reported insufficient information about random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to classify this item as high or low. |
| Blinding of participants (Performance bias) | Unclear risk | Insufficient information to classify this item as high or low. |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information to classify this item as high or low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to classify this item as high or low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | High risk | Adverse events not reported. |
| Other bias | Low risk | No other biases were identified. |
Fu 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 60 infants were randomly divided into 3 groups (...)." Page 1477 Comment: Authors reported insufficient information about random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to classify this item as high or low. |
| Blinding of participants (Performance bias) | Unclear risk | Insufficient information to classify this item as high or low. |
| Blinding of personnel (performance bias) | Unclear risk | Insufficient information to classify this item as high or low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to classify this item as high or low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting of data was not detected. |
| Other bias | Low risk | No other biases were identified. |
Gong 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention A (number of children: 13): propranolol oral (regimen below) + timolol maleate topical (regimen below) Intervention B (number of children: 13): propranolol oral: 10 mg tablet, at a 1.0 mg/kg dose orally once a day with food Intervention C (number of children: 13): timolol maleate topical: 0.5% timolol maleate eye drops to the lesion twice daily (25 mg/5 mL) with medical cotton swabs |
|
| Outcomes | Outcome A: the improvement in size after treatment was graded on a 4‐point scale as proposed by Achauer and colleagues: class I (poor) ‐ reduction in size of < 25%; class II (moderate) ‐ reduction in size of 25% to 50%; class III (good) ‐ reduction in size of 50% to 75%; and class IV (excellent) ‐ reduction in size of 75% to 100%. Classes I and II were considered ineffective treatment, and classes III and IV effective treatment. | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a random sequence was generated using a computer program to assign patients in a 1:1:1 ratio to three groups of 13 patients each." Page 837 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to classify this item as high or low. |
| Blinding of participants (Performance bias) | Unclear risk | It is highly probable that participants were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of personnel (performance bias) | Unclear risk | It is highly probable that researchers were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A panel of three surgeons who were unaware of which treatment the infant had been given and the response rates, assessed the outcomes." Page 837 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting of data was not detected. |
| Other bias | Low risk | No other biases were identified. |
Hogeling 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were enrolled in the trial by study physicians and randomised into 4 groups using minimization by the clinical trials pharmacist. The study physician telephoned the clinical trials pharmacist who then assigned sequence of randomisation.” Page 260 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were enrolled in the trial by study physicians and randomised into 4 groups using minimization by the clinical trials pharmacist. The study physician telephoned the clinical trials pharmacist who then assigned sequence of randomisation.” Page 260 Comment: Authors reported information about adequate allocation concealment. |
| Blinding of participants (Performance bias) | Low risk | Quotes: “Participants, caregivers, and physicians were blinded to group status.” Page 260 “The placebo oral solution had a similar taste and smell and an identical dispensing bottle.” Page 260 Comment: Authors reported information about adequate blinding of participants. |
| Blinding of personnel (performance bias) | Low risk | Quotes: “Participants, caregivers, and physicians were blinded to group status.” Page 260 "The placebo oral solution had a similar taste and smell and an identical dispensing bottle." Page 260 Comment: Authors reported information about adequate blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “The IH colour (redness or blueness) and elevation were assessed by the blinded investigator and were given scores by the investigators.” Page 261 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% of children were lost at follow‐up. Page 261 |
| Selective reporting (reporting bias) | High risk | All outcomes were predefined and reported. However, information about variance of information (standard deviations) associated to mean estimation were ommited. |
| Other bias | Low risk | No other biases were identified. |
Jung 1977.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes | Translation of original publication (article in German)
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation was according to "table of chance". |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to classify this item as high or low. |
| Blinding of participants (Performance bias) | Low risk | Participants were blinded by means of pseudo‐radiation intervention. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to classify this item as high or low. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to classify this item as high or low. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis not used:
|
| Selective reporting (reporting bias) | High risk | Adverse events were not reported in this trial. |
| Other bias | Low risk | No other biases were identified. |
Kessels 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "were randomised using a computer program to two groups (...)." Page 415 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to classify this item as high or low. |
| Blinding of participants (Performance bias) | Unclear risk | It is highly probable that participants were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of personnel (performance bias) | Unclear risk | It is highly probable that researchers were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "At the end of the study, we asked a panel consisting of a dermatologist, physician assistant, dermatology resident, dermatology nurse, and plastic surgery resident to score improvement on a scale from 1 to 3 (...) The panel was blinded to treatment group and when the photographs were taken." Page 416 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 children were lost at follow‐up (13%) for such reasons as long travel distance between hospital and home and dissatisfaction with final group randomisation. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods were reported on in the results. |
| Other bias | Low risk | No other biases were identified. |
Leaute‐Labreze 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Once enrolled, patients were subsequently randomised into two groups to receive either oral propranolol (3 mg kg 1 daily for 15 days then 4 mg kg 1 daily for 15 additional days) or a placebo, according to a predefined randomisation list.” Page 169 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to classify this item as high or low. |
| Blinding of participants (Performance bias) | Low risk | Quote: “Participants, caregivers and physicians were blinded to group status.” Page 169 Comment: Authors reported information about adequate blinding of participants. |
| Blinding of personnel (performance bias) | Low risk | Quote: “Participants, caregivers and physicians were blinded to group status.” Page 169 Comment: Authors reported information about adequate blinding of personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Participants, caregivers and physicians were blinded to group status.” Page 169 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14% were lost at follow‐up: 1 child in the propranolol group and 1 child in placebo group. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the trial protocol were reported. |
| Other bias | Low risk | No other biases were identified. |
Leaute‐Labreze 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
Primary
Secondary
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were assigned to treatment through an interactive voice‐response system, with the use of block randomisation stratified according to age group (35 to 90 days vs. 91 to 150 days) and hemangioma location (facial vs. non‐facial) and applied in a 2:2:2:2:1 ratio (propranolol at 1 mg per kilogram per day for 3 months, propranolol at 1 mg per kilogram per day for 6 months, propranolol at 3 mg per kilogram per day for 3 months, propranolol at 3 mg per kilogram per day for 6 months, and placebo, respectively).” Page 736 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: “Patients were assigned to treatment through an interactive voice‐response system, with the use of block randomisation stratified according to age group (35 to 90 days vs. 91 to 150 days) and hemangioma location (facial vs. non‐facial) and applied in a 2:2:2:2:1 ratio (propranolol at 1 mg per kilogram per day for 3 months, propranolol at 1 mg per kilogram per day for 6 months, propranolol at 3 mg per kilogram per day for 3 months, propranolol at 3 mg per kilogram per day for 6 months, and placebo, respectively).” Page 736 Comment: Authors reported information about adequate allocation concealment. |
| Blinding of participants (Performance bias) | Low risk | Quote: “Different concentrations of propranolol were used (1.25, 2.50, or 3.75 mg per millilitre) in order to administer the same volume to each patient and thereby maintain blinding; patients assigned to 3‐month propranolol regimens received placebo for the second 3 months.” Page 737 Comment: Authors reported information about adequate blinding of participants. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Primary efficacy was assessed by centralized evaluation of standardized digital photographs (taken by investigators at each visit) by two independent, trained, validated readers who were unaware of the study‐group assignments, with adjudication for discrepancies; inter‐reader and intra‐reader reliability were assessed.” Page 737 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The percentage lost at follow‐up was between 1% and 4%, respectively (intention‐to‐treat analysis with overrun). |
| Selective reporting (reporting bias) | Low risk | All outcomes were predefined and reported. |
| Other bias | Unclear risk | Quote: “The sponsor (Pierre Fabre Dermatologie) was involved in the study design in collaboration with three of the academic authors and was responsible for trial management, analysis and interpretation of data, and the decision to submit the manuscript for publication.” Page 736 Comment: The industry sponsor was involved in the analysis and interpretation of the data, as well as the decision to submit the manuscript for publication. It is unclear what effect this may have on the study results. |
Li 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention A (number of children: 14): oral propranolol in combination with topical timolol maleate Intervention B (number of children: 17): oral propranolol treatment alone |
|
| Outcomes |
Primary Outcome A: Changes of size and colour of the haemangioma were assessed by B‐ultrasound and photographs at the onset of treatment, between treatment intervals, and at the conclusion of treatment. Secondary Outcome B: The visual analogue scale was compared with the response to treatment. |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly divided into experimental and control groups using a randomised number table." Page 56 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | Despite participants being aware of intervention group assigned, it is unclear whether this had an impact or not on the trial results. |
| Blinding of personnel (performance bias) | Unclear risk | Despite researchers being aware of intervention group assigned, it is unclear whether this had an impact or not on the trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Three independent surgeons blind to the patients assessed the efficacy by analysing the clinical photograph at baseline and the end of the treatment." Page 57 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting was not detected. |
| Other bias | Low risk | No other biases were identified. |
Lu 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the patients were randomised into two groups.” Page 16,135 Comment: There was insufficient information to rate this item as low or high risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to rate this item as low or high risk of bias. |
| Blinding of participants (Performance bias) | Unclear risk | There was insufficient information to rate this item as low or high risk of bias. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to rate this item as low or high risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to rate this item as low or high risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting of information was not detected. |
| Other bias | Low risk | No other biases were detected. |
Malik 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
Primary outcome
Secondary outcomes
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Random sequence was generated using a computer program in a 1:1:1 ratio.” Page 2454 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The images were evaluated by two independent blinded examiners who scored the improvement (...)." Page 2454 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | High risk | The primary outcome was not clearly reported in the Results section. |
| Other bias | Low risk | No other biases were detected. |
Pope 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were allocated randomly to each group by the research pharmacist who prepared blocks of 4." Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "Subjects were allocated randomly to each group by the research pharmacist who prepared blocks of 4." Comment: Authors reported information about adequate allocation concealment. |
| Blinding of participants (Performance bias) | Unclear risk | Despite participants being aware of the intervention group assigned, it is unclear whether this had an impact or not on the trial results. |
| Blinding of personnel (performance bias) | Unclear risk | Despite researchers being aware of the intervention group assigned, it is unclear whether this had an impact or not on the trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "However, the assessors who measured the primary outcome were blinded to the patient's intervention allocation" Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children lost to follow‐up. |
| Selective reporting (reporting bias) | High risk | Evidence of selective omissions of outcomes from the report (a subjective measure of improvement, as assessed by the parent or child), where only an interclass correlation is presented. |
| Other bias | Low risk | No other biases were detected. |
Tan 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned based on random number table.” Page 165 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The outcome assessor was blinded." Page 165 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 (16%) children in the propranolol group left the study early; no intention‐to‐treat analysis was conducted. |
| Selective reporting (reporting bias) | Low risk | Selective reporting was not detected. |
| Other bias | Low risk | No other biases were identified. |
Tawfik 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcomes were not classified as primary or secondary:
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated into two groups using a simple coin toss method." Page 370 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | It is highly probable that participants were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of personnel (performance bias) | Unclear risk | It is highly probable that researchers were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two independent doctors, a paediatrician and a dermatologist blind to the treatment protocol, evaluated the efficacy of the two modes of treatment as follows (...)." Page 372 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting bias was not detected. |
| Other bias | Low risk | No other biases were detected. |
Tiwari 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention A (number of participants: 28): participants were given oral propranolol at a dose of 2 mg/kg per day in 3 divided doses as outpatients. Intervention B (number of participants: 24): participants were given oral ibuprofen at a dose of 10 mg/kg 8‐hourly and paracetamol at a dose of 16.2 mg/kg 8‐hourly. |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The division was performed using [a] computer‐generated random number table." Page 74 Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | It is highly probable that participants were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of personnel (performance bias) | Unclear risk | It is highly probable that participants were aware of intervention group assigned, but it is unclear whether this had an impact or not on the trial results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess this item as low or high. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18.7% of participants were excluded due to insufficient follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting bias was not detected. |
| Other bias | Low risk | No other biases were detected. |
Xu 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "30 cases were randomly divided into A or B group (...)." Comment: There was insufficient information to assess this item as low or high. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess this item as low or high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts were reported. |
| Selective reporting (reporting bias) | High risk | Important patient‐reported outcomes, such as adverse events, were not reported. |
| Other bias | Low risk | No other biases were identified. |
Zaher 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "(...) and those fit for inclusion were subsequently randomly divided into three groups as follows (...)." Page 647 Comment: There was insufficient information to assess this item as low or high. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess this item as low or high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting bias was not detected. |
| Other bias | Low risk | No other biases were detected. |
Zaher 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Only infants meeting the preset inclusion criteria were enrolled and randomly divided (using envelope concealment method) into (...)." Page 500 Comment: There was insufficient information to assess this item as low or high. |
| Allocation concealment (selection bias) | Low risk | Quote: "Only infants meeting the preset inclusion criteria were enrolled and randomly divided (using envelope concealment method) into (...)." Page 500 Comment: Authors reported information about adequate allocation concealment. |
| Blinding of participants (Performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The final response to treatment was evaluated by 3 blinded investigators, by comparing (...)." Page 500 Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting bias was not detected. |
| Other bias | Low risk | No other biases were detected. |
Zhang 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Outcome A: plasma PRN concentrations at 2, 6, 10, and 24 hours | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “12 patients were randomly divided into 2 groups, qd (n=6) and bid (n=6)...” Page 343 Comment: There was insufficient information to assess this item as low or high. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess this item as low or high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | High risk | Patient‐important outcomes were not addressed in this study. |
| Other bias | Low risk | No other biases were identified. |
Zhong 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “60 participants were given group allocations according to a random number table.” Comment: Authors reported information about adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two unrelated doctors not involved in the study were given the results for analysis, including all clinical, follow up, imaging and laboratory results. They were blinded to the group allocation for the participants.” Comment: Authors reported information about adequate blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting bias was not detected. |
| Other bias | Low risk | No other biases were identified. |
Zhu 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary: "Cure": the haemangioma subsided completely, and the skin returned to normal or exhibited barely visible decolouration. | |
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The 72 infants were allocated at random into the observation or control group." Page 1014 Comment: There was insufficient information to assess this item as low or high. |
| Allocation concealment (selection bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of participants (Performance bias) | Low risk | Quote: "Control group patients received an identical dosage and treatment course of ⁹⁰SR‐⁹⁰Y contact therapy, combined with local topical application of normal saline (NS) for 3‐6 months." Page 1014 Comment: Authors reported information about adequate blinding of participants. |
| Blinding of personnel (performance bias) | Unclear risk | There was insufficient information to assess this item as low or high. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information to assess this item as low or high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No children were lost at follow‐up. |
| Selective reporting (reporting bias) | Low risk | Selective reporting bias was not detected. |
| Other bias | Low risk | No other biases were identified. |
Abbreviations: AE: adverse events; AV block: atrioventricular block; bid: two times a day; CBC count: complete blood cell count; CIHs: complicated infantile haemangiomas; CT imaging: computed tomography imaging; HIFU: high‐intensity focused ultrasound; IH: infantile haemangioma; Nd:YAG laser: neodymium‐doped yttrium aluminium garnet laser; PDL: pulse dye laser; PHACE syndrome: posterior fossa malformations–haemangiomas–arterial anomalies–cardiac defects–eye abnormalities syndrome; plasma PRN concentrations: plasma propranolol concentrations; qd: one a day; rad: unit of absorbed radiation dose.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahn 2004 | Study design not eligible (non‐randomised trial). |
| Ambika 2013 | Study design not eligible (case series). |
| Anonymous 2002 | Study design not eligible (review). |
| Anonymous 2011 | Study design not eligible (review). |
| Baselga 2014 | Study design not eligible (review). |
| Bozena 2012 | Comment about propranolol trials |
| Branco 2008 | Study design not eligible (case series). |
| Chang 2008 | Disease type not eligible (port‐wine stain). |
| Chen 2013 | Study design not eligible (case series). |
| Costinescu 1981 | Report of clinical experience with cryotherapy |
| Dalby 2013 | Study design not eligible (review). |
| Ferahbas 2008 | Study design not eligible (non‐randomised trial). |
| Frieden 2009 | Study design not eligible (review). |
| Gajbhiye 2011 | Study design not eligible (review). |
| Goelz 2014 | Study design not eligible (non‐randomised trial). |
| Incesoy 2011 | Study design not eligible (case series). |
| Jalil 2006 | Study design not eligible (allocated participants in sequence: every 1st to control, every 2nd to intervention 1, and every 3rd to intervention 2). |
| Jesitus 2011 | Study design not eligible (review). |
| Jha 2012 | Study design not eligible (case report). |
| Jiang 2011 | Study design not eligible (self controlled trial). |
| Kunzi‐Rapp 2012 | Study design not eligible (non‐randomised trial). |
| Liu 2009 | Age of participants not eligible (aged 3 to 55 years (mean 17 years), thus likely to include adult‐acquired haemangiomas). |
| McCuaig 2009 | Study design not eligible (no comparison group, all received same treatment). |
| Menezes 2011 | Study design not eligible (review). |
| Michel 1998 | Study design not eligible (non‐randomised trial). |
| Midena 2008 | Disease type not eligible (internal haemangioma). |
| Miranda 2005 | Study design not eligible (non‐randomised study). |
| NCT01074437 | Retrospective review of charts |
| Pancar 2011 | Patient population not eligible. |
| Poetke 2000 | Study design not eligible (non‐randomised trial). |
| Pope 2013 | Study design not eligible (non‐randomised trial). |
| Rouvas 2009 | Age of participants not eligible (aged 50+ years). |
| Sadan 1996 | Study design not eligible (non‐randomised study). |
| Schlosser 2009 | Study design not eligible (review). |
| Smit 2005 | Study design not eligible (review). |
| Song 2015 | Study design not eligible (non‐randomised study). |
| Thaivalappil 2013 | Study design not eligible (non‐randomised study). |
| Tierney 2009 | Disease type not eligible (not haemangiomas). |
| Weienstein 2012 | Study design not eligible (review). |
| Weissenstein 2015 | Study design not eligible (case report). |
| Zhao 1997 | Study design not eligible (non‐randomised study). |
| Zhong 2014 | Study design not eligible (non‐randomised study). |
| Zhou 2000 | Study design not eligible (non‐randomised study). |
| Zhou 2002 | Age of participants not eligible (3 months to 62 years, thus likely to include adult‐acquired haemangioma). |
| Zhou 2015 | Animal study (white rabbits) |
Characteristics of studies awaiting assessment [ordered by study ID]
Kuang 2014.
| Methods | Randomised controlled trial |
| Participants | 61 RIH patients Age: unclear Sex: unclear Country: China |
| Interventions | Children in Group A (sequential therapy, 30 cases) were treated with oral propranolol (1 to 2 mg/kg/d) until maximal reduction of tumour size was achieved, after which laser treatment was initiated. Children in Group B (concurrent therapy, 31 cases) were treated with oral propranolol (1 to 2 mg/kg/d) for 1 week before laser was applied concurrently. |
| Outcomes | Size and colour of tumours were observed and recorded to assess treatment efficacy. |
| Notes | Abstract |
Maier 2012.
| Methods | Randomised trial |
| Participants | 182 infants (55 males/127 females) with 1 (n = 124) or several (n = 58) superficial haemangiomas in the early progressive or the indifferent phase with a maximum diameter of 30 mm Age: unclear Sex: unclear Country: unclear |
| Interventions | Pulsed dye laser (n = 70), cryotherapy (n = 54), and observation (n = 58) |
| Outcomes | Authors defined the following evaluation criteria: complete remission, partial remission, stop of growth, progression; blistering, crust, scar, hypo‐ or hyperpigmentation. Furthermore, there was a grading of the cosmetic appearance of the vascular tumour by the children's parents: 1 (cosmetically acceptable) to 4 (cosmetically not acceptable). |
| Notes | Abstract |
NCT00004436.
| Methods | Parallel‐group, phase III, RCT |
| Participants | 1 month to 8 months of age, male and female. Presence of haemangioma meeting at least 1 of the following criteria: vision threatening, severe anatomic distortion, or other complications Age: 1 to 8 months Sex: all Country: USA |
| Interventions | All children received oral prednisone daily for 3 weeks. Children were then randomised to receive either placebo or leuprolide intramuscularly every 3 weeks, whilst continuing oral prednisone. If the tumour did not respond, the leuprolide was administered every 2 weeks. |
| Outcomes | Safety and efficacy: tumours were assessed at 1, 3, 6 weeks and 3 and 6 months |
| Notes | The study was declared completed in 2005; however, we were unable to locate a report of the findings. |
NCT00555464.
| Methods | Cross‐over design, phase II, RCT |
| Participants | Up to 6 months of age, male and female, infants with haemangiomas with complication that required systemic therapy to control their growth. Clinical diagnosis of infantile haemangioma confirmed by tissue biopsy positive for GLUT1. Size must be greater than or equal to 50 cm², adequate liver function. Age: 0.15 years (standard deviation = 0.06) Sex: 5 female, 3 male Country: USA |
| Interventions | Vincristine (0.05 mg/kg/dose) administered into a vein (PICC line) every week for 12 weeks, versus prednisone (3 mg/kg/day) administered by mouth for 12 weeks. If there is evidence of disease progression (larger haemangioma) at 6 weeks, then the child is switched to the other intervention. |
| Outcomes |
Primary outcome:
Secondary outcome:
|
| Notes | The limitations of this trial include early termination of enrolment resulting in small numbers of children analysed. |
NCT00744185.
| Methods | Parallel‐group, phase II and III, RCT |
| Participants | Up to 4 months of age, male and female, 1 or more haemangiomas sized more than 1 cm in diameter, social insurance, infant not threatened for vital or functional structure and for which no treatment would be proposed Age: up to 4 months Sex: all Country: France |
| Interventions | Propranolol (3 mg/kg for 15 days then 4 mg/kg for 15 days) versus placebo (for 30 days) |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Notes | This study has been terminated. (Study halted prematurely due to difficulties in recruitment of participants.) |
NCT01072045.
| Methods | Parallel‐group, phase II, RCT |
| Participants | Up to 2 years of age, male and female, absence of cardiopathy, clinically diagnosed haemangioma in proliferative or involutive phase with relative indication for clinical treatment, itemised as follows:
Age: up to 2 years Sex: all Country: Brazil |
| Interventions | Propranolol (2 mg/kg/day) given orally, divided into 2 doses for initial 60 days, versus prednisone (2 mg/kg/day) given orally, divided into 2 doses for initial 60 days |
| Outcomes |
Primary outcome:
Secondary outcome:
|
| Notes | This study has been completed. Study completion date: December 2014 |
Pandey 2010.
| Methods | Parallel RCT |
| Participants | Fewer than 2 superficial haemangiomas less than 5 cm Age: unclear Sex: unclear Country: unclear |
| Interventions | Topical steroid (mometasone furoate) given twice daily versus intralesional steroid (triamcinolone acetonide), injected at monthly intervals using 24‐gauge needle at doses of 1 to 2 mg/kg |
| Outcomes |
Primary outcome:
Secondary outcome:
Complications (including irritation, hypopigmentation, pain, bleeding, infection, cutaneous atrophy, Cushingoid facies, and growth retardation) |
| Notes |
GLUT1: glucose transporter 1; MRI: magnetic resonance imaging; PHACE syndrome: posterior fossa malformations–haemangiomas–arterial anomalies–cardiac defects–eye abnormalities syndrome; PICC line: peripherally inserted central catheter; RCT: randomised controlled trial; RIH: refractory infantile haemangiomas.
Characteristics of ongoing studies [ordered by study ID]
NCT01147601.
| Trial name or title | Topical timolol maleate 0.5% solution for proliferating infantile hemangiomas: a prospective double blinded placebo controlled study |
| Methods | Parallel randomised controlled trial |
| Participants | Boys and girls, aged 1 to 8 months with haemangiomas 3 cm or less on the scalp, trunk, or extremities Exclusion: facial, genital, hand, finger, feet, or toe haemangiomas; proven or suspected PHACE syndrome; ulcerated haemangiomas; hypersensitivity to beta blockers; history of asthma; known renal impairment; cardiac conditions that may predispose to heart block; hypoglycaemia; medication that could interact with beta blockers |
| Interventions | Topical 0.5% timolol maleate versus placebo, 2 to 3 drops to cover the haemangioma, twice daily |
| Outcomes |
Primary outcome:
Secondary outcomes:
Assessments: 6 months |
| Starting date | March 2010 |
| Contact information | Alfons L Krol (503‐494‐9993, krola@ohsu.edu) and Lindsay K Severson (503‐494‐6009, seversol@ohsu.edu) |
| Notes | The recruitment status of this study is unknown because the information has not been recently verified. |
NCT02913612.
| Trial name or title | Efficacy, safety and pharmacokinetics of topical timolol in infants with infantile hemangioma (IH) (TIM01) |
| Methods | Multicentre, double‐masked randomised, efficacy, safety, and pharmacokinetic study |
| Participants | 110 children up to 60 days |
| Interventions | Drug: 0.25% timolol maleate gel forming solution Drug: 0.5% timolol maleate gel forming solution Wait‐and‐see |
| Outcomes |
Primary outcomes:
|
| Starting date | 12 August 2016 |
| Contact information | Chiara Melloni, Principal Investigator, Duke University Medical Center |
| Notes | This study is not yet open for participant recruitment. |
PHACE syndrome: posterior fossa malformations–haemangiomas–arterial anomalies–cardiac defects–eye abnormalities syndrome.
Differences between protocol and review
This is an update of the review published by Leonardi‐Bee 2011. We made the following modifications to the original version.
Background: We incorporated additional information about current findings about infantile haemangiomas (IH), as well as the current treatment for this condition.
Methods/Types of participants: We decided we would exclude internal haemangiomas due to the fact that most of them are not symptomatic and are usually treated in the presence of other IH.
Methods/Types of participants: We clarified that we excluded studies with a mixture of populations that did not provide separate information for children, due to our review focusing on children only.
Methods/Types of interventions: We updated the list of interventions used to manage infantile haemangiomas in line with current practice. We also included studies comparing combinations of interventions, as interventions may be used in combination for complicated IH or in special circumstances.
Methods/Types of outcome measures: We modified the list of primary and secondary outcomes, including making adverse events a primary outcome in line with the Methodological Expectations of Cochrane Intervention Reviews (MECIR) conduct standard 14. We reported outcome data at any follow‐up, but still considered six months or less to be short term. Finally, we considered 'economic data' as additional information and removed it from this list.
Methods/Types of outcome measures/adverse events: Due to some studies reporting adverse events in general (i.e. not specifying which were the serious adverse events), we presented total number of adverse events along with information about single adverse events related to each intervention. In addition, we included information about what we defined as short‐term adverse events (those presented until 48 hours after treatment) and long‐term adverse events (those presented after 48 hours following treatment).
Methods/Search methods: We updated the search strategies in line with current Cochrane Skin practices and searched currently recommended trials registers.
Methods/Search methods: We did not handsearch conference proceedings, as many are now available online in Embase.
Methods/Search methods: We did not correspond with pharmaceutical companies that manufacture specific treatments, or companies that produce laser‐based therapies, in order to identify relevant trials, since it is probable that these randomised controlled trials would be registered with one of the databases of ongoing trials already included in the search methods.
Methods/Data collection and analysis: We updated the methods according to the current guidelines to develop systematic reviews of interventions (Higgins 2011). We used the Cochrane 'Risk of bias' tool for each study, including those identified in the first version of this review.
Methods/Measures of treatment effect: We omitted the estimation of the number needed to treat, as we believe this figure is mostly helpful in the presence of high‐quality evidence, and there were a scarcity of data to calculate these numbers.
Methods/Assessment of heterogeneity: We updated this section according to the current Cochrane guidelines (Higgins 2011).
Methods/Data synthesis: We added information about the software employed to perform the statistical analysis (Review Manager 5.3).
Methods/Data collection and analysis: We included the assessment of the quality/certainty of evidence, following the principles of the GRADE system, including the development of 'Summary of findings' tables (Guyatt 2008).
Methods/'Summary of findings' tables: For the outcome 'adverse events', we presented in the corresponding table the most frequent or the most important adverse event, or both, related to each intervention. When information about adverse events in general (including serious/severe adverse events) was available, we presented these results instead of individual findings.
We checked all ongoing studies and classified them as 'included' or 'excluded' when the full publication was available.
We selected information about the most currently used treatments to report as main findings in the abstract, summary of main results, and 'Summary of findings' tables. This included oral propranolol and topical timolol maleate.
Due to scarcity of data in all comparisons, we were unable to perform a full analysis of reporting bias, subgroup analysis, sensitivity analysis, or investigation of heterogeneity.
Contributions of authors
IAR was the contact person with the editorial base. IAR and MN co‐ordinated the contributions from the coauthors and wrote the final draft of the protocol. IAR, MN, SB, and LG worked on the methods sections. MN, EB, SB, AS, and LG drafted the clinical sections of the Background and responded to the clinical comments of the referees. MN and IAR responded to the methodology and statistics comments of the referees. HPH was the consumer coauthor and checked the protocol for readability and clarity. He also ensured that the outcomes are relevant to consumers. IAR is the guarantor of the final review.
Disclaimer
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Sources of support
Internal sources
University of Nottingham, UK.
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Monica Novoa: nothing to declare. Eulalia Baselga: I have been the principal investigator for propranolol trials in infantile haemangioma, which included reviewing the final data, and for this I received honorarium/a consulting fee from Pierre Fabre Dermatology. I have received financial support for travel to meetings from Pierre Fabre Dermatology. I have received consultancy fees from the following: Pierre Fabre Dermatology and Sanofi‐Regeneron. I have received payment for lectures from the following: Ordesa, Meda, Cantabria, and Almirall. I have given paid educational presentations for the following companies: Pierre Fabre Dermatology, Almirall, ISDIN, Novartis, Ferrer, Leo, IFC (Cantabria), and Ordesa. I have received travel/accommodation funding to attend the European Academy of Dermatology & Venereology and Spanish Academy of Dermatology meeting from the following: Novartis, Almirall, Ferrer, ISDIN, Cantabria, and Lao. Sandra Beltrán: nothing to declare. Lucia Giraldo: nothing to declare. Ali Shahbaz: nothing to declare. Hector Pardo‐Hernandez: nothing to declare. Ingrid Arevalo‐Rodriguez: nothing to declare.
Dr Baselga was involved in the development of Leaute‐Labreze 2015. She did not participate in the 'Risk of bias' assessment and data extraction for this study.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Abarzua‐Araya 2014 {published data only}
- Abarzua‐Araya A, Navarrete‐Dechent CP, Heusser F, Retamal J, Zegpi‐Trueba MS. Atenolol versus propranolol for the treatment of infantile hemangiomas: a randomized controlled study. Journal of the American Academy of Dermatology 2014;70(6):1045‐9. [CENTRAL: CN‐00993183; PUBMED: 24656727] [DOI] [PubMed] [Google Scholar]
Aly 2015 {published data only}
- Aly MMD, Hamza AF, Abdel Kader HM, Saafan HA, Ghazy MS, Ragab IA. Therapeutic superiority of combined propranolol with short steroids course over propranolol monotherapy in infantile hemangioma. European Journal of Pediatrics 2015;174(11):1503‐9. [CENTRAL: CN‐01106020; PUBMED: 25982338] [DOI] [PubMed] [Google Scholar]
Asilian 2015 {published data only}
- Asilian A, Mokhtari F, Kamali AS, Abtahi‐Naeini B, Nilforoushzadeh MA, Mostafaie S. Pulsed dye laser and topical timolol gel versus pulse dye laser in treatment of infantile hemangioma: A double‐blind randomized controlled trial. Advanced Biomedical Research. 2015;4:257. [PUBMED: 26918239] [DOI] [PMC free article] [PubMed] [Google Scholar]
Batta 2002 {published data only}
- Batta K, Goodyear H, Moss C, Williams H, Hiller L, Waters R. Randomized controlled study of early pulsed dye laser treatment of uncomplicated infantile haemangiomas: results of a 5‐year analysis. British Journal of Dermatology 2008;159(Suppl 1):113‐27. [CENTRAL: CN‐00784022] [DOI] [PubMed] [Google Scholar]
- Batta K, Goodyear HM, Moss C, Williams HC, Hiller L, Waters R. Randomised controlled study of early pulsed dye laser treatment of uncomplicated childhood haemangiomas: results of a 1‐year analysis. Lancet 2002;360(9332):521‐7. [CENTRAL: CN‐00410133] [DOI] [PubMed] [Google Scholar]
- Batta K, Waters R, Titley M, Goodyear H, Moss C. Experience in the very early treatment of childhood haemangiomas with the 585 nm pulsed dye laser (PDL). British Journal of Dermatology 2000;143(Suppl 57):42‐85. [CENTRAL: CN‐00783264] [Google Scholar]
Bauman 2014 {published data only}
- Bauman NM, McCarter RJ, Guzzetta PC, Shin JJ, Oh AK, Preciado DA, et al. Propranolol vs prednisolone for symptomatic proliferating infantile hemangiomas: A randomized clinical trial. JAMA Otolaryngology, Head & Neck Surgery. 2014;140(4):323‐30. [CENTRAL: CN‐00988156; PUBMED: 24526257] [DOI] [PubMed] [Google Scholar]
- NCT00967226. Propranolol versus prednisolone for treatment of symptomatic hemangiomas. clinicaltrials.gov/ct2/show/NCT00967226 (accessed 23 April 2010).
Chan 2013 {published data only}
- Chan H, McKay C, Adams S, Wargon O. RCT of timolol maleate gel for superficial infantile hemangiomas in 5‐ to 24‐week‐olds. Pediatrics 2013;131(6):e1739‐47. [CENTRAL: CN‐00876607] [DOI] [PubMed] [Google Scholar]
Ehsani 2014 {published data only}
- Ehsani AH, Noormohammadpoor P, Abdolreza M, Balighi K, Arianian Z, Daklan S. Combination therapy of infantile hemangioma with pulsed dye laser with topical propranolol: a randomized clinical trial. Archives of Iranian Medicine 2014;17(10):657‐60. [CENTRAL: CN‐01042472; PUBMED: 25305763] [PubMed] [Google Scholar]
Feng 2000 {published data only}
- Feng LL, Wang XJ, Sun LQ, Zhang ZR, Ma CH. Clinical observation on efficacy of methylthionine chloride in the treatment of hemangiomas in children. Chinese Journal of Dermatology 2000;33(4):284. [CENTRAL: CN‐00843680] [Google Scholar]
Fu 2012 {published data only}
- Fu S, Wang B, Huang H, Huang L. Primary clinical application of high‐intensity focused ultrasound on infant hemangiomas. Chung‐Kuo Hsiu Fu Chung Chien Wai Ko Tsa Chih/Chinese Journal of Reparative & Reconstructive Surgery 2011;25(12):1477‐80. [CENTRAL: CN‐00896632] [PubMed] [Google Scholar]
- Fu SZ, Wang B, Huang HP, Huang LL. Clinical study on hemangiomas treatment with high‐intensity focused ultrasound (60 cases). Zhonghua Zheng Xing Wai Ke za Zhi = Zhonghua Zhengxing Waike Zazhi [Chinese Journal of Plastic Surgery] 2012;28(4):252‐5. [CENTRAL: CN‐00966758] [PubMed] [Google Scholar]
Gong 2015 {published data only}
- Gong H, Xu D‐P, Li Y‐X, Cheng C, Li G, Wang X‐K. Evaluation of the efficacy and safety of propranolol, timolol maleate, and the combination of the two, in the treatment of superficial infantile haemangiomas. British Journal of Oral & Maxillofacial Surgery 2015;53(9):836‐40. [CENTRAL: CN‐01125564; PUBMED: 26427968] [DOI] [PubMed] [Google Scholar]
Hogeling 2011 {published data only}
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of Propranolol for infantile haemangiomas. Australasian Journal of Dermatology 2011;52(Suppl 2):4. [EMBASE: 70558759] [DOI] [PubMed] [Google Scholar]
- Hogeling M, Adams S, Wargon O. A randomized controlled trial of propranolol for infantile hemangiomas. Pediatrics 2011;128(2):e259‐66. [PUBMED: 21788220] [DOI] [PubMed] [Google Scholar]
- Wargon O. Randomised placebo controlled trial: Safety and efficacy of topical timolol maleate gel vs placebo for small superficial infantile haemangiomas. Australasian Journal of Dermatology 2013;54(Suppl 2):22‐3. [EMBASE: 71067708] [Google Scholar]
Jung 1977 {published data only}
- Jung EG. X‐ray therapy for hemangiomatas [Die strahlentherapie der hamangiome]. Dermatologica 1976;153(2):86‐7. [CENTRAL: CN‐00784697] [PubMed] [Google Scholar]
- Jung EG, Kohler U. Regression of haemangiomata in infants after x‐ray treatment and mock‐radiation [Rückbildung frühkindlicher hämangiome nach röntgen‐ und pseudobestrahlung]. Archives of Dermatological Research 1977;259(1):21‐8. [CENTRAL: CN‐00614355] [DOI] [PubMed] [Google Scholar]
Kessels 2013 {published data only}
- Kessels JP, Hamers ET, Ostertag JU. Superficial hemangioma: pulsed dye laser versus wait‐and‐see. Dermatologic Surgery 2013;39(3 Pt 1):414‐21. [CENTRAL: CN‐00966183; PUBMED: 23279058] [DOI] [PubMed] [Google Scholar]
Leaute‐Labreze 2013 {published data only}
- Leaute‐Labreze C, Dumas de la Roque E, Nacka F, Abouelfath A, Grenier N, Rebola M, et al. Double‐blind randomized pilot trial evaluating the efficacy of oral propranolol on infantile haemangiomas in infants < 4 months of age. British Journal of Dermatology 2013;169(1):181‐3. [CENTRAL: CN‐00977604; PUBMED: 23301692] [DOI] [PubMed] [Google Scholar]
Leaute‐Labreze 2015 {published data only}
- Léauté‐Labrèze C, Hoeger P, Mazereeuw‐Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. New England Journal of Medicine. 2015;372(8):735‐46. [CENTRAL: CN‐01052642; PUBMED: 25693013] [DOI] [PubMed] [Google Scholar]
Li 2016 {published data only}
- Li G, Xu DP, Tong S, Xue L, Sun NN, Wang XK. Oral propranolol with topical timolol maleate therapy for mixed infantile hemangiomas in oral and maxillofacial regions. Journal of Craniofacial Surgery 2016;27(1):56‐60. [CENTRAL: CN‐01195472; PUBMED: 26716547] [DOI] [PubMed] [Google Scholar]
Lu 2016 {published data only}
- Lu J, Kuang X, Zhao J, Xiang Y, Huang J, Zeng Q, et al. Concurrent versus sequential combination of propranolol and dual‐wavelength laser (585 nm and 1064 nm) to treat complicated infantile hemangiomas. International Journal of Clinical and Experimental Medicine 2016;9(8):16132‐16138. [EMBASE: 20160650632] [Google Scholar]
Malik 2013 {published data only}
- Malik MA, Menon P, Rao KL, Samujh R. Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: A randomized controlled study. Journal of Pediatric Surgery 2013;48(12):2453‐9. [CENTRAL: CN‐00921325; PUBMED: 24314186] [DOI] [PubMed] [Google Scholar]
Pope 2007 {published data only}
- Pope E, Krafchik BR, Macarthur C, Stempak D, Stephens D, Weinstein M, et al. Oral versus high‐dose pulse corticosteroids for problematic infantile hemangiomas: a randomized controlled trial. Pediatrics 2007;119(6):e1239‐e1247. [CENTRAL: CN‐00588641; PUBMED: 17485449] [DOI] [PubMed] [Google Scholar]
Tan 2012 {published data only}
- Tan M, Duan B, Zhou CM, Gong H. The therapeutic effect of propranolol with 1064 nm Nd: YAG laser on proliferating hemangioma in body surface. Zhonghua Zheng Xing Wai Ke za Zhi = Zhonghua Zhengxing Waike Zazhi [Chinese Journal of Plastic Surgery] 2012;28(3):164‐8. [CENTRAL: CN‐01124671; PUBMED: 22870700] [PubMed] [Google Scholar]
Tawfik 2015 {published data only}
- Tawfik AA, Alsharnoubi J. Topical timolol solution versus laser in treatment of infantile hemangioma: a comparative study. Pediatric dermatology 2015;32(3):369‐76. [CENTRAL: CN‐01102313; PUBMED: 25740672] [DOI] [PubMed] [Google Scholar]
Tiwari 2016 {published data only}
- Tiwari P, Pandey V, Gangopadhyay AN, Sharma SP, Gupta DK. Role of propranolol in ulcerated haemangioma of head and neck: a prospective comparative study. Oral & Maxillofacial Surgery 2016;20(1):73‐7. [PUBMED: 26481918] [DOI] [PubMed] [Google Scholar]
Xu 2006 {published data only}
- Xu WL. The research of pingyangmycin emulsion on proliferation of tumor cell apoptosis in capillary hemangiomas [translation]. Shijiazhuang, Hebei, China: Hebei Medical University, 2003. [Google Scholar]
- Xu WL, Niu AG, Li SL, Li ZD. Pingyangmycin emulsion inducing apoptosis in infantile proliferating capillary hemangiomas. Zhonghua Zheng Xing Wai Ke za Zhi = Zhonghua Zhengxing Waike Zazhi [Chinese Journal of Plastic Surgery] 2006;22(5):362‐4. [CENTRAL: CN‐00730309] [PubMed] [Google Scholar]
- Xu WL, Niu AG, Li ZD, Li SL, Shi BJ, Zhang YB, et al. Effect of pingyangmycin emulsion on the microenvironment of infantile proliferating capillary hemangioma. World Journal of Pediatrics 2006;2(3):217‐22. [Google Scholar]
Zaher 2013 {published data only}
- Zaher H, Rasheed H, Esmat S, Gawdat HI, Hegazy RA, El‐Komy M, et al. Propranolol and infantile hemangiomas: Different routes of administration, a randomized clinical trial. European Journal of Dermatology 2013;23(5):646‐52. [CENTRAL: CN‐00961393; PUBMED: 24135427] [DOI] [PubMed] [Google Scholar]
Zaher 2016 {published data only}
- Zaher H, Rasheed H, El‐Komy MM, Hegazy RA, Gawdat HI, Abdel Halim DM, et al. Propranolol versus captopril in the treatment of infantile hemangioma (IH): A randomized controlled trial. Journal of the American Academy of Dermatology 2016;74(3):499‐505. [CENTRAL: CN‐01138323; PUBMED: 26685718] [DOI] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang L, Mai HM, Zheng J, Zheng JW, Chen ZG, Wang YA, et al. Preliminary study on plasma RPN concentration of patients with infantile hemangioma treated with propranolol. International journal of clinical and experimental medicine 2013;6(5):342‐5. [CENTRAL: CN‐00906420; PUBMED: 23724152] [PMC free article] [PubMed] [Google Scholar]
Zhong 2015 {published data only}
- Zhong S, Tao Y, Zhou J, Yao L, Uu Y, Yan D, et al. Evaluation on efficacy of low dose propranolol combined with 1 064 nm Nd: YAG laser on mixed and deeper infantile hemangioma. Journal of Jilin University Medicine Edition 2015;41(5):1032‐5. [CENTRAL: CN‐01177799] [Google Scholar]
Zhu 2015 {published data only}
- Zhu HJ, Liu Q, Deng XL, Guan YX. Efficacy of low‐dose 90Sr‐90Y therapy combined with topical application of 0.5% timolol maleate solution for the treatment of superficial infantile hemangiomas. Experimental and Therapeutic Medicine 2015;10(3):1013‐8. [PUBMED: 26622431] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Ahn 2004 {published data only}
- Ahn H, Kim YJ, Hwang ES, Kim IH. Clinical trial of 5% imiquimod cream for eleven cases of infantile hemangioma. Korean Journal of Dermatology 2004;42(6):718‐23. [EMBASE: 2004354455] [Google Scholar]
Ambika 2013 {published data only}
- Ambika H, Sujatha C, Kumar YH. Topical timolol: A safer alternative for complicated and un‐complicated infantile hemangiomas. Indian Journal of Dermatology 2013;58(4):330. [PUBMED: 23919041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2002 {published data only}
- Anonymous. Assessing early laser treatment of strawberry naevi. Medicine Today 2002;3(12):10. [Google Scholar]
Anonymous 2011 {published data only}
- Anonymous. Propranolol effective in shrinking infantile hemangioma. Contemporary Pediatrics 2011;28:11‐2. [Google Scholar]
Baselga 2014 {published data only}
- Baselga E. IS‐065: Hemangiomas: Oral propanolol and beyond. Archives of Disease in Childhood 2014;99:A20‐A21. [Google Scholar]
Bozena 2012 {published data only}
- Bozena DB. HEMANGIOL Study: The first worldwide dose‐effect study concerning propranolol in infantile hemangiomas [Badanie HEMANGIOL ‐ pierwsze ogolnoswiatowe badanie zaleznosci dawka‐efekt dotyczace stosowania propranololu u pacjentow z naczyniakami niemowlecymi]. Przeglad Dermatologiczny 2012;99(4):419. [CENTRAL: CN‐01028347] [Google Scholar]
Branco 2008 {published data only}
- Branco DFR, Goldenberg DC, Heitor BS, Bastos EO, Alonso N. Early surgical resection of nasal hemangiomas: indications and results [Ressecção cirúrgica precoce de hemangiomas nasais: indicações e resultados]. Revista da Sociedade Brasileira de Cirurgia Craniomaxilofacial. 2008;11(3 Suppl):23. [Google Scholar]
Chang 2008 {published data only}
- Chan CJ, Hsiao YC, Mihm MC Jr, Nelson JS. Pilot study examining the combined use of pulsed dye laser and topical Imiquimod versus laser alone for treatment of port wine stain birthmarks. Lasers in Surgery & Medicine 2008;40(9):605‐10. [CENTRAL: CN‐00708211; PUBMED: 18951427] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen XD, Ma G, Huang JL, Chen H, Jin YB, Ye XX, et al. Serum‐level changes of vascular endothelial growth factor in children with infantile hemangioma after oral propranolol therapy. Pediatric Dermatology 2013;30(5):549‐53. [CENTRAL: CN‐01122228; PUBMED: 23909679] [DOI] [PubMed] [Google Scholar]
Costinescu 1981 {published data only}
- Costinescu V, Dinu C, Martu D. Clinical trial of sodium morrhuate in the treatment of ORL angiomas [Experimentarea clinica a moruatului de sodiu in tratamentul angioamelor din sfera O.R.L]. Revista de Chirurgie, Oncologie, Radiologie, O.r.l., Oftalmologie, Stomatologie. Oto‐rino‐laringologia 1981;26(1):61‐2. [PUBMED: 6454221] [PubMed] [Google Scholar]
Dalby 2013 {published data only}
- Dalby TK, Lester‐Smith D. Propranolol for the treatment of infantile haemangioma. Journal of Paediatrics & Child Health 2013;49(2):148‐51. [PUBMED: 23418706] [DOI] [PubMed] [Google Scholar]
Ferahbas 2008 {published data only}
- Ferahbas A, Kartal D, Taslider N, Utas S. The effectiveness of contact cryotherapy in treatment of infantile hemangiomas. Turkish Journal of Dermatology 2008;2(4):107‐10. [Google Scholar]
Frieden 2009 {published data only}
- Frieden IJ, Drolet BA. Propranolol for infantile hemangiomas: promise, peril, pathogenesis. Pediatric Dermatology 2009;26(5):642‐4. [PUBMED: 19840341] [DOI] [PubMed] [Google Scholar]
Gajbhiye 2011 {published data only}
- Gajbhiye V, Nath S, Chatterjee S, De A, Ghosh D, Das SK. Role of propranolol in hemangiomas. Journal of Indian Association of Pediatric Surgeons 2011;16(4):173‐4. [PUBMED: 22121324] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goelz 2014 {published data only}
- Goelz R, Moll M, Meisner C, Rocken M, Poets CF, Moehrle MC. Prospective controlled study to evaluate cryocontact therapy for infantile haemangioma in preterm infants. Archives of Disease in Childhood Fetal & Neonatal Edition 2014;99(4):F345‐6. [CENTRAL: CN‐01042486] [DOI] [PubMed] [Google Scholar]
Incesoy 2011 {published data only}
- Incesoy Ozdemir S, Bozkurt C, Orun UA, Sahin G, Yuksek N, Cetinkaya S, et al. Successful treatment of pulmonary arteriovenous malformation and infantile hepatic hemangioendothelioma with alpha‐interferon. Anadolu Kardiyoloji Dergisi [Anatolian Journal of Cardiology] 2011;11(2):181‐3. [PUBMED: 21362599] [DOI] [PubMed] [Google Scholar]
Jalil 2006 {published data only}
- Jalil S, Akhtar J, Ahmed S. Corticosteroids therapy in the management of infantile cutaneous hemangiomas. Journal of the College of Physcians & Surgeons ‐ Pakistan 2006;16(10):662‐665. [CENTRAL: CN‐00608871; PUBMED: 17007757] [DOI] [PubMed] [Google Scholar]
Jesitus 2011 {published data only}
- Jesitus J. Lack of data: More controlled trials needed for infantile hemangioma therapies, experts say. Dermatology Times 2011;32:28‐34. [Google Scholar]
Jha 2012 {published data only}
- Jha AK, Mallik SK, Raihan M. Topical ophthalmic solution in infantile hemangioma. Journal of Postgraduate Medicine 2012;58(2):163‐5. [PUBMED: 22718070] [DOI] [PubMed] [Google Scholar]
Jiang 2011 {published data only}
- Jiang C, Hu X, Ma G, Chen D, Jin Y, Chen H, et al. A prospective self‐controlled phase II study of imiquimod 5% cream in the treatment of infantile hemangioma. Pediatric Dermatology 2011;28(3):259‐66. [CENTRAL: CN‐00799727; PUBMED: 21615472] [DOI] [PubMed] [Google Scholar]
Kunzi‐Rapp 2012 {published data only}
- Kunzi‐Rapp K. Topical propranolol therapy for infantile hemangiomas. Pediatric Dermatology 2012;29(2):154‐9. [CENTRAL: CN‐00900917] [DOI] [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu XJ, Quin ZP, Tai MZ. Angiographic classification and sclerotic therapy of maxillofacial cavernous haemangiomas: a report of 204 cases. Journal of International Medical Research 2009;37(5):1285‐92. [PUBMED: 19930833] [DOI] [PubMed] [Google Scholar]
McCuaig 2009 {published data only}
- McCuaig CC, Dubois J, Powell J, Belleville C, David M, Rousseau E, et al. A phase II, open‐label study of the efficacy and safety of imiquimod in the treatment of superficial and mixed infantile hemangioma. Pediatric Dermatology 2009;26(2):203‐12. [PUBMED: 19419474] [DOI] [PubMed] [Google Scholar]
Menezes 2011 {published data only}
- Menezes MD, McCarter R, Greene EA, Bauman NM. Status of propranolol for treatment of infantile hemangioma and description of a randomized clinical trial. Annals of Otology, Rhinology, and Laryngology 2011;120(10):686‐95. [CENTRAL: CN‐00804792] [DOI] [PubMed] [Google Scholar]
Michel 1998 {published data only}
- Michel S, Wlotzke U, Hohenleutner U, Landthaler M. Laser and cryotherapy of hemangioma in infants in a direct comparison [Laser‐ und kryotherapie der Sauglingshamangiome im direckten vergleich]. Hautarzt 1998;49(3):192‐6. [CENTRAL: CN‐00509698] [DOI] [PubMed] [Google Scholar]
Midena 2008 {published data only}
- Midena E, Pilotto E, Radin PP, Belvis V. Bolus vs standard photodynamic therapy in the treatment of circumscribed choroidal hemangioma: a randomized, masked study. Investigative Ophthalmology & Visual Science 2004;45:3154. [CENTRAL: CN‐00598352] [Google Scholar]
- Urban F, Pilotto E, Parrozzani R, Midena E. Photodynamic therapy of circumscribed choroidal hemangioma: comparison of dosage and timing. Acta Ophthalmologica 2008;86:s243. [Google Scholar]
Miranda 2005 {published data only}
- Miranda Madinya Ricardo, Vásquez Beckmann Carlos. Importance of absolute alcohol in the treatment of infantile capillary hemangiomas. Dr. Francisco de Ycaza Bustamanteö Hospital, Guayaquil [Valor del alcohol absoluto en el tratamiento de los hemangiomas capilares infantiles. Hospital Dr. Francisco de Ycaza Bustamante, Guayaquil]. Medicina 2005;10:203‐6. [Google Scholar]
NCT01074437 {published data only}
- NCT01074437. Corticosteroids with placebo versus corticosteroids with propranolol treatment of infantile hemangiomas. clinicaltrials.gov/ct2/results?term=NCT01074437 (accessed 23 April 2010).
Pancar 2011 {published data only}
- Pancar GS, Aydin F, Senturk N, Bek Y, Canturk MT, Turanli AY. Comparison of the 532‐nm KTP and 1064‐nm Nd:YAG lasers for the treatment of cherry angiomas. Journal of Cosmetic and Laser Therapy 2011;13(4):138‐41. [CENTRAL: CN‐00811208] [DOI] [PubMed] [Google Scholar]
Poetke 2000 {published data only}
- Poetke M, Philipp C, Berlien HP. Flashlamp‐pumped pulsed dye laser for hemangiomas in infancy: treatment of superficial vs mixed hemangiomas. Archives of Dermatology 2000;136(5):628‐32. [CENTRAL: CN‐00277983] [DOI] [PubMed] [Google Scholar]
Pope 2013 {published data only}
- Pope E, Chakkittakandiyil A, Lara‐Corrales I, Maki E, Weinstein M. Expanding the therapeutic repertoire of infantile haemangiomas: Cohort‐blinded study of oral nadolol compared with propranolol. British Journal of Dermatology 2013;168(1):222‐4. [CENTRAL: CN‐00912475] [DOI] [PubMed] [Google Scholar]
- Pope E, Chakkittakandiyil A, Weinstein M. Expanding the therapeutic repertoire of infantile hemangiomas: Prospective pilot study using oral nadolol. Pediatric Dermatology 2011;28(5):510. [EMBASE: 70661234] [Google Scholar]
Rouvas 2009 {published data only}
- Rouvas AA, Papakostas TD, Vavvas D, Vergados I, Moschos MM, Kotsolis A, et al. Intravitreal ranibizumab, intravitreal ranibizumab with PDT, and intravitreal triamcinolone with PDT for the treatment of retinal angiomatous proliferation: a prospective study. Retina 2009;29(4):536‐44. [CENTRAL: CN‐00704491] [DOI] [PubMed] [Google Scholar]
Sadan 1996 {published data only}
- Sadan N, Wolach B. Treatment of hemangiomas of infants with high doses of prednisone. Journal of Pediatrics 1996;128(1):141‐6. [CENTRAL: CN‐00122746] [DOI] [PubMed] [Google Scholar]
Schlosser 2009 {published data only}
- Schlosser KA. Infantile hemangioma: how to treat this benign neoplasm of childhood. JAAPA: Journal of the American Academy of Physician Assistants 2009;22(5):46‐9. [PUBMED: 19469391] [DOI] [PubMed] [Google Scholar]
Smit 2005 {published data only}
- Smit JM, Bauland CG, Wijnberg DS, Spauwen PH. Pulsed dye laser treatment, a review of indications and outcome based on published trials. British Journal of Plastic Surgery 2005;58(7):981‐7. [PUBMED: 16039628] [DOI] [PubMed] [Google Scholar]
Song 2015 {published data only}
- Song H, Shi H, Zhang X, Wang J, Yu Y, Chen W, et al. Safety profile of a divided dose of propranolol for heart rate in children with infantile haemangioma during 16 weeks of treatment. British Journal of Dermatology 2015;172(2):444‐9. [CENTRAL: CN‐01052634] [DOI] [PubMed] [Google Scholar]
Thaivalappil 2013 {published data only}
- Thaivalappil S, Bauman N, Saieg A, Movius E, Brown KJ, Preciado D. Propranolol‐mediated attenuation of MMP‐9 excretion in infants with hemangiomas. JAMA Otolaryngology‐‐ Head & Neck Surgery 2013;139(10):1026‐31. [CENTRAL: CN‐00921293] [DOI] [PubMed] [Google Scholar]
Tierney 2009 {published data only}
- Tierney E, Barker A, Ahdout J, Hanke CW, Moy RL, Kouba DJ. Photodynamic therapy for the treatment of cutaneous neoplasia, inflammatory disorders, and photoaging. Dermatologic Surgery 2009;35(5):725‐46. [PUBMED: 19309338] [DOI] [PubMed] [Google Scholar]
Weienstein 2012 {published data only}
- Weissenstein A, Villalon G, Luchter E, Bittmann S. Children's haemangiomas: use of new topical therapies. British Journal of Nursing 2012;21(5):274. [PUBMED: 22398997] [DOI] [PubMed] [Google Scholar]
Weissenstein 2015 {published data only}
- Weissenstein A, Luchter E, Bittmann S. Successful treatment of infantile haemangioma with propranolol. British Journal of Nursing 2015;24(2):96‐7. [PUBMED: 25615994] [DOI] [PubMed] [Google Scholar]
Zhao 1997 {published data only}
- Zhao YL, He CN, Zhi CF, Zhang YX, Wang HJ. Prednisolone acetate mixed up Morrhuate sodium for the treatment of hemangiomas in children. Chinese Journal of Dermatology 1997;30(6):414. [CENTRAL: CN‐00844079] [Google Scholar]
Zhong 2014 {published data only}
- Zhong SX, Tao YC, Zhou JF, Yao L, Li SS. Evaluation on efficacy of different doses of propranolol in treatment of infantile hemangioma. Journal of Jilin University Medicine Edition 2014;40(4):880‐3. [CENTRAL: CN‐01041728] [Google Scholar]
Zhou 2000 {published data only}
- Zhou JY, Fang GJ, Wang XM. Clinical study of microwave operation in treating nasal hemangioma. International and 6th National Head and Neck Cancer Conference. Shanghai, china, 9‐13 June, 2000. 2000:195. [CENTRAL: CN‐00343206]
Zhou 2002 {published data only}
- Zhou K, Liang C, Yang K, Wang L. A randomised controlled study on the efficacy of modified sclerotherapy in treating angioma of ear, nose and throat. Lin Chuang Er Bi Yan Hou Ke za Zhi [Journal of Clinical Otorhinolaryngology] 2002;16(12):681‐3. [CENTRAL: CN‐00436296] [PubMed] [Google Scholar]
Zhou 2015 {published data only}
- Zhou W, He S, Yang Y, Jian D, Chen X, Ding J. Formulation, characterization and clinical evaluation of propranolol hydrochloride gel for transdermal treatment of superficial infantile hemangioma. Drug Development & Industrial Pharmacy 2015;41(7):1109‐19. [CENTRAL: CN‐01254273] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Kuang 2014 {published data only}
- Kuang X, Lu J. Concurrent versus sequential combination therapy of propranolol and dual‐wavelength laser (585nm and 1064nm) to treat refractory infantile hemangiomas. Journal of Dermatology 2014;41:100. [CENTRAL: CN‐01055093] [Google Scholar]
Maier 2012 {published data only}
- Maier H, Wanka A, Schmalwieser AW, Maier B, Dani T, Neumann R, et al. Prospective, randomized, investigator‐blind, controlled therapy study on treatment of haemangioma of infancy: Pulsed dye laser versus cryotherapy versus observation. Experimental Dermatology 2012;21(3):e15. [EMBASE: 70792062] [Google Scholar]
NCT00004436 {published data only}
- NCT00004436. Randomized study of hormonal regulation of infantile hemangioma. clinicaltrials.gov/ct2/show/NCT00004436 (accessed 23 April 2010).
NCT00555464 {published data only}
- NCT00555464. Clinical Trial of Vincristine vs. Prednisolone for Treatment of Complicated Hemangiomas. clinicaltrials.gov/ct2/show/NCT00555464 (accessed 23 April 2010).
NCT00744185 {published data only}
- NCT00744185. Propranolol on capillary hemangiomas. clinicaltrials.gov/ct2/show/NCT00744185 (accessed 23 April 2010).
NCT01072045 {published data only}
- NCT01072045. Comparative Study of the Use of Beta Blocker and Oral Corticosteroid in the Treatment of Infantile Hemangioma. clinicaltrials.gov/ct2/show/NCT01072045 (accessed 23 April 2010).
Pandey 2010 {published data only}
- Pandey A, Gangopadhyay AN, Sharma SP, Kumar V, Gupta DK, Gopal SC. Evaluation of topical steroids in the treatment of superficial hemangioma. SKINmed 2010;8(1):9‐11. [CENTRAL: CN‐00760496] [PubMed] [Google Scholar]
References to ongoing studies
NCT01147601 {published data only}
- NCT01147601. Topical Timolol 0.5% solution for proliferating infantile haemangiomas. clinicaltrials.gov/ct2/show/NCT01147601?term=NCT01147601 (accessed 25 March 2011).
NCT02913612 {published data only}
- NCT02913612. Efficacy, Safety and Pharmacokinetics of Topical Timolol in Infants With Infantile Hemangioma (IH) (TIM01). clinicaltrials.gov/ct2/show/NCT02913612 Date first received: 12 August 2016.
Additional references
Abramson 1989
- Abramson S, Weissmann G. The mechanisms of action of nonsteroidal antiinflammatory drugs. Clinical and experimental rheumatology 1989;7 Suppl 3:S163‐70. [PUBMED: 2557993] [PubMed] [Google Scholar]
Achauer 1997
- Achauer BM, Chang CJ, Vander Kam VM. Management of hemangioma of infancy: review of 245 patients. Plastic & Reconstructive Surgery 1997;99(5):1301‐8. [DOI] [PubMed] [Google Scholar]
Al 2003
- Al Buainian H, Verhaeghe E, Dierckxsens L, Naeyaert JM. Early treatment of hemangiomas with lasers. A review. Dermatology (Basel, Switzerland) 2003;206(4):370‐3. [PUBMED: 12771489] [DOI] [PubMed] [Google Scholar]
Anderson 1981
- Anderson RR, Parrish JA. The optics of human skin. Journal of Investigative Dermatology 1981;77(1):9‐13. [DOI] [PubMed] [Google Scholar]
Antonelli 1991
- Antonelli G, Currenti M, Turriziani O, Dianzani F. Neutralizing antibodies to interferon‐alpha: relative frequency in patients treated with different interferon preparations. Journal of Infectious Diseases 1991;163(4):882‐885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Azzopardi 2012
- Azzopardi S, Wright TC. Novel strategies for managing infantile hemangiomas: a review. Annals of plastic surgery 2012;68(2):226‐8. [PUBMED: 21629088] [DOI] [PubMed] [Google Scholar]
Baselga 2016
- Baselga E, Roe E, Coulie J, Munoz FZ, Boon LM, McCuaig C, et al. Risk factors for degree and type of sequelae after involution of untreated hemangiomas of infancy. JAMA Dermatology 2016;152(11):1239‐43. [PUBMED: 27540637] [DOI] [PubMed] [Google Scholar]
Bauland 2011
- Bauland CG, Luning TH, Smit JM, Zeebregts CJ, Spauwen PH. Untreated hemangiomas: growth pattern and residual lesions. Plastic and Reconstructive Surgery 2011;127(4):1643‐8. [PUBMED: 21460670] [DOI] [PubMed] [Google Scholar]
Bayart 2017
- Bayart CB, Tamburro JE, Vidimos AT, Wang L, Golden AB. Atenolol Versus Propranolol for Treatment of Infantile Hemangiomas During the Proliferative Phase: A Retrospective Noninferiority Study. Pediatric dermatology 2017;34(4):413‐21. [PUBMED: 28556385] [DOI] [PubMed] [Google Scholar]
Bennett 2001
- Bennett ML, Fleischer AB Jr, Chamlin SL, Frieden IJ. Oral corticosteroid use is effective for cutaneous hemangiomas: an evidence‐based evaluation. Archives of Dermatology 2001;137(9):1208‐1213. [DOI] [PubMed] [Google Scholar]
Bruckner 2006
- Bruckner AL, Frieden IJ. Infantile hemangiomas. Journal of the American Academy of Dermatology 2006;55(4):671‐82. [PUBMED: 17010748] [DOI] [PubMed] [Google Scholar]
Castren 2016
- Castren E, Salminen P, Gissler M, Stefanovic V, Pitkaranta A, Klockars T. Risk factors and morbidity of infantile haemangioma: preterm birth promotes ulceration. Acta Paediatrica 2016;105(8):940‐5. [PUBMED: 27146410] [DOI] [PubMed] [Google Scholar]
Chang 1997
- Chang E, Boyd A, Nelson CC, Crowley D, Law T, Keough KM, et al. Successful treatment of infant hemangiomas with interferon alpha‐2b. Journal of Pediatric Hematology/Oncology 1997;19(3):237‐244. [DOI] [PubMed] [Google Scholar]
Chinnadurai 2016a
- Chinnadurai S, Sathe NA, Surawicz T. Laser treatment of infantile hemangioma: A systematic review. Lasers in Surgery & Medicine 2016;48(3):221‐233. [PUBMED: 26711436] [DOI] [PubMed] [Google Scholar]
Chinnadurai 2016b
- Chinnadurai S, Snyder K, Sathe N, Fonnesbeck C, Morad A, Likis FE, et al. In: Agency for Healthcare Research and Quality, editor(s). Diagnosis and Management of Infantile Hemangioma. Vol. AHRQ Publication No.16‐EHC002‐EF, Rockville MD: Agency for Healthcare Research and Quality, 2016. [PubMed] [Google Scholar]
Christou 2012
- Christou EM, Wargon O. Effect of captopril on infantile haemangiomas: a retrospective case series. The Australasian journal of dermatology 2012;53(3):216‐8. [PUBMED: 22671578] [DOI] [PubMed] [Google Scholar]
Couto 2012
- Couto RA, Maclellan RA, Zurakowski D, Greene AK. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plastic and reconstructive surgery 2012;130(3):619‐24. [PUBMED: 22575857] [DOI] [PubMed] [Google Scholar]
Csoma 2017
- Csoma ZR, Dalmady S, Abraham R, Rozsa T, Racz K, Kemeny L. Infantile haemangioma: clinical and demographic characteristics, experiences in the treatment [Infantilis haemangioma: klinikai es demografiai jellemzok, kezelesi‐gondozasi tapasztalatok.]. Orvosi Hetilap 2017;158(39):1535‐44. [PUBMED: 28942665] [DOI] [PubMed] [Google Scholar]
Danarti 2016
- Danarti R, Ariwibowo L, Radiono S, Budiyanto A. Topical timolol maleate 0.5% for infantile hemangioma: its effectiveness compared to ultrapotent topical corticosteroids ‐ a single‐center experience of 278 cases. Dermatology (Basel, Switzerland) 2016;232(5):566‐71. [PUBMED: 27592104] [DOI] [PubMed] [Google Scholar]
Daramola 2012
- Daramola OO, Chun RH, Kerschner JE. Surgical management of auricular infantile hemangiomas. Archives of otolaryngology‐‐head & neck surgery 2012;138(1):72‐5. [PUBMED: 22249633] [DOI] [PubMed] [Google Scholar]
Darrow 2015
- Darrow DH, Greene AK, Mancini AJ, Nopper AJ. Diagnosis and management of infantile hemangioma. Pediatrics 2015;136(4):e1060‐104. [PUBMED: 26416931] [DOI] [PubMed] [Google Scholar]
Dinehart 2001
- Dinehart SM, Kincannon J, Geronemus R. Hemangiomas: evaluation and treatment. Dermatologic Surgery 2001;27(5):475‐85. [PUBMED: 11359498] [DOI] [PubMed] [Google Scholar]
Doshan 1986
- Doshan HD, Rosenthal RR, Brown R, Slutsky A, Applin WJ, Caruso FS. Celiprolol, atenolol and propranolol: a comparison of pulmonary effects in asthmatic patients. Journal of cardiovascular pharmacology 1986;8 Suppl 4:S105‐8. [PUBMED: 2427836] [PubMed] [Google Scholar]
EMC 2018
- EMC. Summary of product characteristics. www.medicines.org.uk/emc/medicine/1730/SPC/Roferon‐A+Pre‐Filled+Syringe/#INTERACTIONS (accessed prior to 21 February 2018).
Ezekowitz 1992
- Ezekowitz RA, Mulliken JB, Folkman J. Interferon alfa‐2a therapy for life‐threatening hemangiomas of infancy. The New England journal of medicine 1992;326(22):1456‐63. [PUBMED: 1489383] [DOI] [PubMed] [Google Scholar]
Fragu 1991
- Fragu P, Lemarchand‐Venencie F, Benhamou S, François P, Jeannel D, Benhamou E, et al. Long‐term effects in skin and thyroid after radiotherapy for skin angiomas: a French retrospective cohort study. European Journal of Cancer 1991;27(10):1215‐22. [PUBMED: 1835589] [DOI] [PubMed] [Google Scholar]
George 2004
- George ME, Sharma V, Jacobson J, Simon S, Nopper AJ. Adverse effects of systemic glucocorticosteroid therapy in infants with hemangiomas. Archives of Dermatology 2004;140(8):963‐9. [PUBMED: 15313812] [DOI] [PubMed] [Google Scholar]
Ginimuge 2010
- Ginimuge PR, Jyothi SD. Methylene blue: revisited. Journal of anaesthesiology, clinical pharmacology 2010;26(4):517‐20. [PUBMED: 21547182] [PMC free article] [PubMed] [Google Scholar]
Glade 2010
- Glade RS, Vinson K, Becton D, Bhutta S, Buckmiller LM. Management of complicated hemangiomas with vincristine/vinblastine: Quantitative response to therapy using MRI. International journal of pediatric otorhinolaryngology 2010;74(11):1221‐5. [PUBMED: 20884067] [DOI] [PubMed] [Google Scholar]
Glassberg 1989
- Glassberg E, Lask G, Rabinowitz LG, Tunnessen WW Jr. Capillary hemangiomas: case study of novel laser treatment and a review of therapeutic options. Journal of Dermatologic Surgery & Oncology 1989;15(11):1214‐23. [PUBMED: 2808890] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed prior to 27/03/2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grantzow 2001
- Grantzow R. [Differential therapy of hemangiomas‐‐when cryotherapy, laser therapy or operation?] [Differentialtherapie von Hamangiomen‐‐Wann Kryo, Laser oder Operation?]. Kongressband. Deutsche Gesellschaft fur Chirurgie. Kongress 2001;118:521‐4. [PUBMED: 11824311] [PubMed] [Google Scholar]
Greinwald 1999
- Greinwald JH Jr, Burke DK, Bonthius DJ, Bauman NM, Smith RJ. An update on the treatment of hemangiomas in children with interferon alfa‐2a. Archives of Otolaryngology ‐ Head and Neck Surgery 1999;125(1):21‐7. [PUBMED: 9932582] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence‐‐publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011h
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Hansson 1975
- Hansson L, Aberg H, Karlberg BE, Westerlund A. Controlled study of atenolol in treatment of hypertension. British medical journal 1975;2(5967):367‐70. [PUBMED: 236810] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration, 2011.
Holland 2013
- Holland KE, Drolet BA. Approach to the patient with an infantile hemangioma. Dermatologic Clinics 2013;31(2):289‐301. [PUBMED: 23557656] [DOI] [PubMed] [Google Scholar]
Hopewell 1990
- Hopewell JW. The skin: its structure and response to ionizing radiation. International journal of radiation biology 1990;57(4):751‐73. [PUBMED: 1969905] [DOI] [PubMed] [Google Scholar]
Hu 2015
- Hu L, Huang HZ, Li X, Lin XX, Li W. Open‐label nonrandomized left‐right comparison of imiquimod 5% ointment and timolol maleate 0.5% eye drops in the treatment of proliferating superficial infantile hemangioma. Dermatology (Basel, Switzerland) 2015;230(2):150‐5. [PUBMED: 25633200] [DOI] [PubMed] [Google Scholar]
Itinteang 2011
- Itinteang T, Brasch HD, Tan ST, Day DJ. Expression of components of the renin‐angiotensin system in proliferating infantile haemangioma may account for the propranolol‐induced accelerated involution. Journal of plastic, reconstructive & aesthetic surgery : JPRAS 2011;64(6):759‐65. [PUBMED: 20870476] [DOI] [PubMed] [Google Scholar]
Izadpanah 2013
- Izadpanah A, Izadpanah A, Kanevsky J, Belzile E, Schwarz K. Propranolol versus corticosteroids in the treatment of infantile hemangioma: a systematic review and meta‐analysis. Plastic Reconstructive Surgery 2013;131(3):601‐13. [PUBMED: 23142941] [DOI] [PubMed] [Google Scholar]
Ji 2015
- Ji Y, Chen S, Xu C, Li L, Xiang B. The use of propranolol in the treatment of infantile haemangiomas: an update on potential mechanisms of action. British Journal of Dermatology 2015;172(1):24‐32. [PUBMED: 25196392] [DOI] [PubMed] [Google Scholar]
Kaplan 1990
- Kaplan P, Normandin J Jr, Wilson GN, Plauchi H, Lippman A, Vekemans M. Malformations and minor anomalies in children whose mothers had prenatal diagnosis: comparison between CVS and amniocentesis. American Journal of Medical Genetics 1990;37(3):366‐70. [PUBMED: 2260567] [DOI] [PubMed] [Google Scholar]
Kennedy 2005
Krupa 2009
- Krupa Shankar D, Chakravarthi M, Shilpakar R. Carbon dioxide laser guidelines. Journal of cutaneous and aesthetic surgery 2009;2(2):72‐80. [PUBMED: 20808594] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2017
- Li B, Li Z, Wang P, Huang Q, Xu L, He R, et al. Mammalian target of rapamycin complex 1 signalling is essential for germinal centre reaction. Immunology 2017;152(2):276‐86. [PUBMED: 28557002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2014
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Seminars in pediatric surgery 2014;23(4):162‐7. [PUBMED: 25241092] [DOI] [PubMed] [Google Scholar]
Luo 2011
- Luo QF, Zhao FY. The effects of Bleomycin A5 on infantile maxillofacial haemangioma. Head & face medicine 2011;7:11. [PUBMED: 21736714] [DOI] [PMC free article] [PubMed] [Google Scholar]
Léauté‐Labrèze 2015
- Léauté‐Labrèze C, Hoeger P, Mazereeuw‐Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. New England Journal of Medicine 2015;372(8):735‐46. [PUBMED: 25693013] [DOI] [PubMed] [Google Scholar]
Ma 2017
- Ma EH, Robertson SJ, Chow CW, Bekhor PS. Infantile hemangioma with minimal or arrested growth: further observations on clinical and histopathologic findings of this unique but underrecognized entity. Pediatric Dermatology 2017;34(1):64‐71. [PUBMED: 27873347] [DOI] [PubMed] [Google Scholar]
Marchuk 2001
- Marchuk DA. Pathogenesis of hemangioma. Journal of Clinical Investigation 2001;107(6):665‐6. [PUBMED: 11254664] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDaniel 1997
- McDaniel DH, Ash K, Lord J, Newman J, Zukowski M. The erbium: YAG laser: a review and preliminary report on resurfacing of the face, neck, and hands. Aesthetic surgery journal 1997;17(3):157‐64. [PUBMED: 19327707] [DOI] [PubMed] [Google Scholar]
Medicines.ie 2018
- Medicines.ie. Summary of product characteristics. www.medicines.ie/medicine/15865/SPC/Capoten+25mg+Tablets/#INTERACTIONS (accessed prior to 21 February 2018).
Mulliken 2002
- Mulliken JB, Rogers GF, Marler JJ. Circular excision of hemangioma and purse‐string closure: the smallest possible scar. Plastic and reconstructive surgery 2002;109(5):1544‐54; discussion 1555. [PUBMED: 11932595] [DOI] [PubMed] [Google Scholar]
Orsi 2010
- Orsi F, Arnone P, Chen W, Zhang L. High intensity focused ultrasound ablation: a new therapeutic option for solid tumors. Journal of cancer research and therapeutics 2010;6(4):414‐20. [PUBMED: 21358073] [DOI] [PubMed] [Google Scholar]
Pienaar 2006
- Pienaar C, Graham R, Geldenhuys S, Hudson DA. Intralesional bleomycin for the treatment of hemangiomas. Plastic and reconstructive surgery 2006;117(1):221‐6. [PUBMED: 16404271] [DOI] [PubMed] [Google Scholar]
Probert 1975
- Probert JC, Parker BR. The effects of radiation therapy on bone growth. Radiology 1975;114(1):115‐62. [PUBMED: 813276] [DOI] [PubMed] [Google Scholar]
Qiu 2015
- Qiu Y, Lin X, Ma G, Chang L, Jin Y, Chen H, et al. Eighteen cases of soft tissue atrophy after intralesional bleomycin a5 injections for the treatment of infantile hemangiomas: a long‐term follow‐up. Pediatric Dermatology 2015;32(2):188‐91. [PUBMED: 25640925] [DOI] [PubMed] [Google Scholar]
Raphael 2011
Review Manager 5.3 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration.. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration., 2014.
Rothfleisch 2002
- Rothfleisch JE, Kosann MK, Levine VJ, Ashinoff R. Laser treatment of congenital and acquired vascular lesions. A review. Dermatologic Clinics 2002;20(1):1‐18. [PUBMED: 11859585] [DOI] [PubMed] [Google Scholar]
Sethuraman 2014
- Sethuraman G, Yenamandra VK, Gupta V. Management of infantile hemangiomas: current trends. Journal of cutaneous and aesthetic surgery 2014;7(2):75‐85. [PUBMED: 25136206] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 2015
- Shen L, Zhou G, Zhao J, Li P, Xu Q, Dong Y, et al. Pulsed dye laser therapy for infantile hemangiomas: a systemic review and meta‐analysis. QJM : Monthly Journal of the Association of Physicians 2015;108(6):473‐80. [PUBMED: 25376585] [DOI] [PubMed] [Google Scholar]
Shorr 1986
- Shorr N, Seiff SR. Central retinal artery occlusion associated with periocular corticosteroid injection for juvenile haemangioma. Ophthalmic Surgery 1986;17(4):229‐31. [PUBMED: 3714192] [PubMed] [Google Scholar]
Smolinski 2005
- Smolinski KN, Yan AC. Hemangiomas of infancy: clinical and biological characteristics. Clinical Pediatrics 2005;44(9):747‐66. [PUBMED: 16327961] [DOI] [PubMed] [Google Scholar]
Syed 1999
- Syed SB. Vascular birthmarks: update on presentation and management. Current Paediatrics 1999;9(1):20‐6. [Google Scholar]
Tollefson 2012
- Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents' photographs tell us. Pediatrics 2012;130(2):e314‐20. [PUBMED: 22826568] [DOI] [PubMed] [Google Scholar]
van de Kerkhof 1998
- Kerkhof PCM, Rooij M, Steijlen PM. Spontaneous course of haemangiomas: facts and speculations. International Journal of Dermatology 1998;37(2):101‐2. [DOI] [PubMed] [Google Scholar]
Vega 2017
- Vega Mata N, Lopez Gutierrez JC, Vivanco Allende B, Fernandez Garcia MS. Different clinical features of acral abortive hemangiomas. Case Reports in Dermatological Medicine 2017;2017:2897617. [PUBMED: 28785492] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waeber 1980
- Waeber B, Brunner HR, Brunner DB, Curtet AL, Turini GA, Gavras H. Discrepancy between antihypertensive effect and angiotensin converting enzyme inhibition by captopril. Hypertension (Dallas, Tex. : 1979) 1980;2(2):236‐42. [PUBMED: 6247269] [DOI] [PubMed] [Google Scholar]
Wang 2017
- Wang C, Li Y, Xiang B, Xiong F, Li K, Yang K, et al. Quality of life in children with infantile hemangioma: a case control study. Health and Quality of Life Outcomes 2017;15(1):221. [PUBMED: 29145889] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wnek 2017
- Wnek A, Andrzejewska E, Kobos J, Taran K, Przewratil P. Molecular and immunohistochemical expression of apoptotic proteins Bax, Bcl‐2 and Caspase 3 in infantile hemangioma tissues as an effect of propranolol treatment. Immunology Letters 2017;185:27‐31. [PUBMED: 28279700] [DOI] [PubMed] [Google Scholar]
Xu 2014
- Xu S, Jia R, Ge S, Lin M, Fan X. Treatment of periorbital infantile haemangiomas: a systematic literature review on propranolol or steroids. Journal of Paediatrics and Child Health 2014;50(4):271‐9. [PUBMED: 24754793] [DOI] [PubMed] [Google Scholar]
Zhang 2017
- Zhang L, Wu HW, Yuan W, Zheng JW. Propranolol therapy for infantile hemangioma: our experience. Drug Design, Development and Therapy 2017;11:1401‐8. [PUBMED: 28507428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2018
Zimmermann 2010
- Zimmermann AP, Wiegand S, Werner JA, Eivazi B. Propranolol therapy for infantile haemangiomas: review of the literature. International Journal of Pediatric Otorhinolaryngology 2010;74(4):338‐42. [PUBMED: 20117846] [DOI] [PubMed] [Google Scholar]
Zou 2013
- Zou HX, Jia J, Zhang WF, Sun ZJ, Zhao YF. Propranolol inhibits endothelial progenitor cell homing: a possible treatment mechanism of infantile hemangioma. Cardiovascular Pathology 2013;22(3):203‐10. [PUBMED: 23151525] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Leonardi‐Bee 2011
- Leonardi‐Bee J, Batta K, O’Brien C, Bath‐Hextall FJ. Interventions for infantile haemangiomas (strawberry birthmarks) of the skin. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD006545.pub2] [DOI] [PubMed] [Google Scholar]


