Abstract
Background
Osteoarthritis (OA) is the most common form of arthritis of the temporomandibular joint (TMJ), and can often lead to severe pain in the orofacial region. Management options for TMJ OA include reassurance, occlusal appliances, physical therapy, medication in addition to several surgical modalities.
Objectives
To investigate the effects of different surgical and non‐surgical therapeutic options for the management of TMJ OA in adult patients.
Search methods
We searched the following databases: Cochrane Oral Health's Trials Register (to 26 September 2011); CENTRAL (The Cochrane Library 2011, Issue 3); MEDLINE via Ovid (1950 to 26 September 2011); Embase via Ovid (1980 to 26 September 2011); and PEDro (1929 to 26 September 2011). There were no language restrictions.
Selection criteria
Randomised controlled trials (RCTs) comparing any form of non‐surgical or surgical therapy for TMJ OA in adults over the age of 18 with clinical and/or radiological diagnosis of TMJ OA according to the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) guideline or compatible criteria.
Primary outcomes considered were pain/tenderness/discomfort in the TMJs or jaw muscles, self assessed range of mandibular movement and TMJ sounds. Secondary outcomes included the measurement of quality of life or patient satisfaction evaluated with a validated questionnaire, morphological changes of the TMJs assessed by imaging, TMJ sounds assessed by auscultation and any adverse effects.
Data collection and analysis
Two review authors screened and extracted information and data from, and independently assessed the risk of bias in the included trials.
Main results
Although three RCTs were included in this review, pooling of data in a meta‐analysis was not possible due to wide clinical diversity between the studies. The reports indicate a not dissimilar degree of effectiveness with intra‐articular injections consisting of either sodium hyaluronate or corticosteroid preparations, and an equivalent pain reduction with diclofenac sodium as compared with occlusal splints. Glucosamine appeared to be just as effective as ibuprofen for the management of TMJ OA.
Authors' conclusions
In view of the paucity of high level evidence for the effectiveness of interventions for the management of TMJ OA, small parallel group RCTs which include participants with a clear diagnosis of TMJ OA should be encouraged and especially studies evaluating some of the possible surgical interventions.
Plain language summary
Interventions for managing osteoarthritis in the temporomandibular joint
Review question
We conducted this review to assess different interventions for managing osteoarthritis in the temporomandibular joint.
Background
The temporomandibular joint (TMJ) or jaw joint is located in front of the ear on either side of the face. However, it is the only joint that the dentists and maxillofacial surgeons predominantly have to deal with. As with many of the other joints, the TMJ can be affected by osteoarthritis (OA). This is characterized by progressive destruction of the internal surfaces of the joint which can result in debilitating pain and joint noises. Several disorders other than OA may affect the TMJ and the correct diagnosis is important such that it can be matched with appropriate therapy. A range of therapeutic options are available for TMJ OA, which include non‐surgical modalities such as control of contributory factors, occlusal appliances, cold or warm packs applied to the joint, pharmacological interventions as well as physiotherapy. Surgical treatment options include intra‐articular injections, arthrocentesis (lavage of the joint) as well as attempts at repair or replacement of portions of the TMJ.
Study characteristics
Authors working with Cochrane Oral Health carried out this review of existing studies, which includes evidence current up to 26 September 2011. This review includes three studies: two conducted in Europe and one in North America. All participants (114) were recruited in university clinics. One study compared intra‐articular injections of sodium hyaluronate (a natural constituent of cartilage) with corticosteroids (betamethasone (an anti‐inflammatory steroid)); the second study compared diclofenac sodium (a non‐steroid anti‐inflammatory drug) with occlusal splint therapy; and the third study compared glucosamine sulfate or ibuprofen (a non‐steroid anti‐inflammatory).
Key results and quality of the evidence
This review found weak evidence indicating that intra‐articular injections of sodium hyaluronate and betamethasone had equivalent effectiveness in reducing pain and discomfort. Occlusal appliances when compared with diclofenac sodium showed a similar pain reduction, as did a comparison between the food supplement glucosamine and ibuprofen.
Future studies should aim to provide reliable information about which therapeutic modality is likely to be more effective for the reduction of pain and other symptoms (e.g. joint sounds) of TMJ OA. Moreover, because the limited evidence available only covers a restricted number of interventions, comparisons with other therapeutic modalities should be encouraged. One of the authors' concerns was the large number of trials which included mixed groups of participants diagnosed with TMJ OA, in addition to other disorders of the TMJ, which could not be considered in this review.
Background
Osteoarthritis (OA) is a chronic degenerative condition that often affects the hands and the large weight‐bearing joints like feet or spine. However, any other joint can also be affected. It is characterized by progressive destruction and loss of the articular cartilage which becomes soft, frayed and thinned (Mercuri 2008; Milam 2006). It also results in a decrease in the synovial fluid that lubricates those joints and can result in severe and debilitating pain (Israel 1997) and loss of joint function. The word OA is derived from the Greek 'osteo', meaning 'of the bone', 'arthro', meaning 'joint', and 'itis', meaning 'inflammation', which is the classical name of the disease. OA has been defined as a 'low‐inflammatory arthritic condition' as opposed to a 'high‐inflammatory arthritic condition' such as rheumatoid arthritis and gout. It is either primary or secondary to trauma or other acute or chronic overload situations (Mercuri 2008; Milam 2006).
The synonym of OA, 'osteoarthrosis', emphasizes the degenerative nature of the disease, but OA draws attention to the secondary inflammatory changes that are superimposed on this degenerative process. The term 'osteoarthrosis' in the medical orthopaedic literature is identified with any low‐inflammatory arthritic condition that results in similar degenerative changes as in OA (Mercuri 2008). In the dental literature, however, osteoarthrosis has also come to be identified with disc displacement or unsuccessful adaptation of the temporomandibular joint (TMJ) to the mechanical forces placed on it with disc derangement or disc interference disorders (Mercuri 2008; Stegenga 2006). Because the basic aetiology and management involved are the same, the terms OA and osteoarthrosis will be used synonymously in this review.
Temporomandibular joint (TMJ) OA is the most common form of arthritis occurring in the TMJs, and the main symptom is pain. Crepitus (joint noise) during functions like chewing is often present. Clinical signs include joint tenderness, crepitus, radiographic bony changes, and joint space narrowing (Kang 2007).
Aetiology and prevalence
Some of the circumstances that are suggested to lead to TMJ OA are as follow: functional overload and parafunction (like tooth grinding during sleeping), unstable occlusion (for example due to a too high restored teeth that does not interact properly with the others) or trauma (microtrauma and macrotrauma). It could also be a consequence of a dysfunctional articular remodeling due to a decreased adaptive capacity of the articulating structures of the joint (Arnett 1996; Nitzan 2001a; Stegenga 1989).
Depending on the diagnostic method used, the prevalence of degenerative TMJ diseases can vary from 1% to 84%. The wide discrepancy illustrates that diagnosis is frequently guided by the presence or absence of rather non‐specific signs and symptoms (Haskin 1995). The Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) guidelines have been shown to be valuable in assessing the prevalence of signs and symptoms and in classifying patients with temporomandibular joint disorders (TMDs) (Dworkin 1992). Therefore, we would use this diagnostic criteria in this review.
Diagnosis
Clinical diagnosis of OA is based on the presence of a number of signs and symptoms: pain (most commonly described as a deep ache) in the pre‐auricular area with or without associated earache, coarse crepitus in the joint with or without clicking, pain in one or both joints during palpation; and usually complemented with radiological evidence of arthrosis (Manfredini 2006). The RDC/TMD guideline (Dworkin 1992) can be a useful aid to diagnosis and is capable of discriminating TMD‐related diagnoses. However, reliability is not good for TMJ OA when compared with other diagnoses (John 2005).
Although panoramic radiographs are a simple and low‐cost method often used for diagnosis of TMJ lesions (Epstein 2001), they do provide little information that influences the diagnosis or management for TMJ OA at early stages (Huumonen 2007), because dramatic changes are only seen in advanced disease. A history of joint overload because of habits (e.g. excessive gum chewing) or parafunction (e.g. bruxism) and clinical examination are extremely important in early manifestations. Because of the lack of correlation between the signs and symptoms and the history and physical findings, however, the most helpful approach to diagnosis may be derived from information provided by appropriate imaging (Mercuri 2008). The characteristic imaging features of low‐inflammation osteoarthritis in which normal joint mechanics have been disrupted (e.g. disc derangement, disc interference) are focal degeneration and the appearance of osteophytes. The image may be characterized by hypertrophic changes about the affected joint rather than atrophic changes seen in high‐inflammatory types of arthritis. Subchondral focal degeneration, the so‐called 'Eli cyst' may be seen in low‐inflammatory arthritis (Mercuri 2008). Radiological examination might also include tomograms or computerised tomography (CT) scan (Dimitroulis 2005), and magnetic resonance imaging (MRI) scans in specific cases. MRIs are useful in detecting changes not readily revealed by clinical examination or other imaging techniques (Larheim 2005). In the near future, sophisticated image methods may become indicated more frequently for the diagnosis of TMJ degeneration due to the recent development of cone beam CT scans and digital volume tomography. This new technique produces the same type of image as CT for a very much reduced X‐ray dose, and at low cost (Rouas 2006). Diagnostic arthroscopic surgery can also provide great insight into the potential problems causing pain to the TMJ at the same time providing treatment (Israel 1998).
Therapeutic options
A TMJ with progressive OA that is clinical detectable cannot be restored in its original structure. Therefore the management strategy either aims to decrease symptoms, stop the progress of the disease or restore the functions:
(a) non‐invasive, (b) minimally invasive, (c) invasive or surgical modalities (d) salvage modalities (Mercuri 2008; Tanaka 2008).
The decision for surgical management of TMJ OA is based on evaluation of the individuals response to non‐invasive management, mandibular form and function, and the effect the condition has on the individuals quality of life (Mercuri 2006). The management goals in TMJ OA are: to (1) decrease joint pain, swelling, and reflex masticatory muscle spasm/pain; (2) increase joint function; (3) prevent further joint damage; and (4) disability and disease‐related morbidity (Tanaka 2008).
Non‐invasive modalities
Non‐surgical management is one of the potential approaches in the therapy of TMJ OA and has been recommended as a first choice approach (de Leeuw 1995). Management should include reassurance, and emphasize rest and clear instructions to avoid loading of the joint as well as the control of contributing factors such as parafunction (de Bont 1997).
Occlusal appliances (e.g. stabilization splint (relaxation splint)) have been shown to be beneficial (Ismail 2007). Full coverage soft occlusal splints have also been suggested for the protection of TMJ from involuntary overloading, and for the reduction of the muscle hyperactivity and articular strain due to bruxism (Tanaka 2008). A controlled study on the effects of occlusal splint therapy in individuals with severe TMJ OA indicated a reduction of clinical signs and symptoms (Kuttila 2002). However, the clinical effectiveness of splints in relieving pain seems modest when compared with that of pain management methods in general (Forssell 2004;Mercuri 2008). None of the occlusal adjustment studies provided evidence supporting the use of this therapeutic method. In terms of medications, non‐steroidal anti‐inflammatory agents, such as ibuprofen, should be used on a time contingent basis to take advantage of their pharmacokinetics. Muscle relaxants may be helpful in controlling the reflex masticatory muscle spasm/pain (Dionne 2006). Oral orthotics, while assisting in the control of parafunctional habits in many persons, can also provide relief from masticatory muscle spasm/pain and, along with a soft diet, will decrease the loads delivered across the TMJ under function. Reconstruction of the occlusion to provide bilateral occlusal stability, temporarily during the early stages of management, may also decrease the potential for unilateral joint overload (Clark 1984).
Physical therapeutic modalities act as counter‐irritants to reduce inflammation and pain. Superficial warm and moist heat or localized cold may relieve pain sufficiently to permit exercise. Therapeutic exercises are designed to increase muscle strength, reduce joint contractures, and maintain a functional range of motion. Ultrasound, electrogalvanic stimulation, and massage techniques may also be helpful in reducing inflammation and pain (De Laat 2003). Active and passive jaw movements, manual therapy techniques, and relaxation techniques were used in the management of 20 consecutive persons with TMJ OA. After intervention (mean 46 days), pain at rest was reduced 80%, and there was no impairment in 37% (Nicolakis 2001).
Minimally invasive modalities
The effectiveness of intra‐articular injections of hyaluronic acid (linear glycosaminoglycan) has been examined in other body joints, but no significant differences were noted in radiographic progression of the disease (Lohmander 1996). Moreover, to date hyaluronic acid has not been approved by the United States Food and Drug Administration as a safe and effective medication in the management of arthritic disease in the TMJ (Tanaka 2008).
Intra‐articular injections of corticosteroids appear to have limited benefit in other joints of the body (Gray 1983). The main limitations of repeated intra‐articular steroid injections are the risks of infection and the destruction of articular cartilage. Repeated intra‐articular corticosteroid injections have been implicated in what is described as the 'chemical condylectomy' phenomenon in the TMJ (Toller 1977).
Arthrocentesis and arthroscopy have also been used as part of the management strategy of TMJ OA. Nitzan and Price presented a 20‐month follow‐up study of 36 persons with 38 dysfunctional joints that had not responded to non‐surgical management, to determine the efficacy of arthrocentesis in restoring functional capacity to the osteoarthrosis joints (Nitzan 2001b). They concluded that arthrocentesis is a rapid and safe procedure that may result in the TMJ OA returning to a 'functional state'.
The value of TMJ arthroscopy may be in the early diagnosis and management of arthritic processes affecting the TMJ, especially early‐stage arthritic disease, to avoid the complications of open bite and ankylosis (Holmlund 1986).
Invasive (surgical) modalities
While the majority of individuals with TMJ OA can be successfully managed with non‐invasive/minimally invasive procedures, there is a small group with OA (< 20%) who have such severe pathology, pain, and dysfunction that invasive surgical management must be considered (Mercuri 2006).
Arthroplasty (reshaping the articular surfaces to eliminate osteophytes, erosions, and irregularities) found in osteoarthritis refractory to other therapeutic modalities was described in 1966 by Dingman and Grabb (Dingman 1966). While this technique reportedly provided pain relief, concerns about the resultant mandibular dysfunctions, dental malocclusions, facial asymmetries, and the potential for development of further bony articular degeneration, disc disorders or loss, and ankylosis led to the development of techniques for interposing autogenous tissues and alloplastic materials (e.g. the vascularized local temporalis muscle flap appears to present the most applicable data for the management of the arthritic TMJ) (Feinberg 1989).
Individuals with active TMJ OA and either concomitant or resultant maxillofacial skeletal discrepancies, and treated only with orthognathic surgery, often have poor outcomes and significant relapse (Wolford 2003).
Distraction osteogenesis may make its own contribution to TMJ OA in the future (van Strijen 2001).
Salvage modalities
Total joint replacement by grafts or implants, also denominated as salvage modalities, have been recommended for severe TMJ OA (Mercuri 2008). In a 'growing person' affected by either low‐inflammatory or high‐inflammatory arthritic disease, the costochondral graft has been the autogenous bone most frequently recommended for the reconstruction of the TMJ (MacIntosh 2000). However, orthopedists recommend alloplastic reconstruction when total joint replacement is required for the management of a 'non‐growing person' affected by TMJ OA (Chapman 2001).
In the TMJ, alloplastic reconstruction has been discussed at length (McBride 1994; Mercuri 1998; Mercuri 1999). All of these authors agree that when the mandibular condyle is extensively damaged, degenerated, or lost, as in arthritic conditions, replacement with either autogenous graft or alloplastic implant is an acceptable approach to achieve optimal symptomatic and functional improvement (Mercuri 2008; Tanaka 2008).
Objectives
To investigate the effects of different surgical and non‐surgical therapeutic options for the management of TMJ OA in adult patients.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) were considered in this review.
Types of participants
Adult patients over the age of 18 with clinical and/or radiological diagnosis of temporomandibular joint osteoarthritis (TMJ OA) according to the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) guideline were considered. Trials in which the inclusion criteria were compatible with the RDC/TMD diagnosis for OA were also considered. Complementary assessments by means of arthroscopy, computerised tomography (CT) scan or magnetic resonance imaging (MRI) were not a prerequisite for inclusion. We excluded studies which had been conducted on people with polyarthropathies.
We considered studies which included participants with two or more types of TMD only if separate data were provided for patients with TMJ OA.
Types of interventions
Any forms of non‐surgical or surgical therapy for TMJ OA were considered. Studies that evaluated those interventions against each other, placebo or no therapy were included. Trials which permitted any analgesics to be administered as concomitant therapy, provided they were distributed similarly between the two groups, were also considered for inclusion.
Types of outcome measures
Primary outcomes
Pain/tenderness/discomfort (associated with the TMJs or jaw muscles): patient‐assessed using any recognized validated pain scale.
Extent of mandibular movement (maximum jaw opening, lateral movement and protrusion): this could be assessed by a ruler, calliper, kinesiograph either actively (the patients open their jaw themselves) or passively (the clinician opens the jaw of the patient).
Subjective TMJ sounds, as perceived by the patients (if present, attenuated or absent).
Secondary outcomes
Any self assessed quality of life or patient satisfaction evaluated with a validated questionnaire (e.g. Short Form‐36 Health Survey (SF‐36) or Oral Health Impact Profile (OHIP)).
Morphological changes (change in shape) of the TMJs as assessed by imaging (standard radiography, CT or MRI).
TMJ sounds as assessed by auscultation (if present, attenuated or absent).
Number of visits.
Number of days absent from work.
In studies including surgical intervention, we will also consider mean operating time and duration of hospital admission (number of days).
Adverse events
We considered any complications that occurred during or after the therapy. If the report provided any data about the severity of complications this was also recorded.
Costs
Direct costs of therapy and hospital bed.
Search methods for identification of studies
Electronic searches
For the identification of studies included or considered for this review, detailed search strategies were developed for each database to be searched. These were based on the search strategy developed for MEDLINE (Ovid) but revised appropriately for each database. For the MEDLINE search, the subject search was run with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (updated March 2011) (Higgins 2011).
The following databases were searched:
Cochrane Oral Health's Trials Register (to 26 September 2011) (Appendix 1)
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 3) (Appendix 2)
MEDLINE via Ovid (1950 to 26 September 2011) (Appendix 3)
Embase via Ovid (1980 to 26 September 2011) (Appendix 4)
PEDro (1929 to 26 September 2011) (Appendix 5).
There was no language restriction on included studies and we arranged for translation of any relevant papers not in the English language.
Searching other resources
We confirmed from Cochrane Oral Health which journals had already been handsearched as part of the Cochrane worldwide handsearching programme (see the Masterlist of journals searched to date) and handsearched the following journals:
Cranio: the Journal of Craniomandibular Practice (from 1997 to 26 September 2011)
Journal of Orofacial Pain (from 1993 to 26 September 2011)
Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics (from 2003 to 26 September 2011).
The reference lists of any clinical trials that were identified were cross‐checked for additional trials.
We contacted the investigators of the included studies by electronic mail to ask for additional details of their trials and for any information they may have about any further published and unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (Raphael Freitas de Souza (RFS) and Claudia Helena Lovato da Silva (CLS)) independently assessed the abstracts of studies resulting from the searches. Full copies of all relevant and potentially relevant studies, i.e. those appearing to meet the inclusion criteria, or for which there were insufficient data in the title and abstract to make a clear decision, were obtained. The full text papers were assessed independently and in duplicate by two review authors and any disagreement on the eligibility of included studies was resolved through discussion and consensus or if necessary through a third party (Mona Nasser (MN)). All irrelevant records were excluded; details of the studies and the reasons for their exclusion were noted in the Characteristics of excluded studies table in Review Manager 5.1 (RevMan 2011).
Data extraction and management
Study details were entered into the Characteristics of included studies table in RevMan 5.1. The review authors collected outcomes data using a pre‐determined form designed for this purpose. Data were extracted independently and in duplicate by two review authors (Zbys Fedorowicz (ZF) and MN). The review authors only included data if there was an independently reached consensus; any disagreements were resolved by consulting with a third review author (RFS).
The following details were extracted: (1) Trial methods (a) method of allocation (b) masking of participants and outcomes (if feasible) (c) exclusion of participants after randomisation and proportion of losses at follow‐up.
(2) Participants (a) demographic characteristics including symptoms of temporomandibular joint disorders (TMDs) (b) source of recruitment (c) country of origin (d) sample size (e) age (f) sex (g) inclusion and exclusion criteria as described in the Criteria for considering studies for this review section (h) presence of co‐morbidities such as fibromyalgia, irritable bowel syndrome, headache, and mitral valve prolapse.
(3) Intervention (a) type, dose and frequency of the intervention, type of surgical procedure (b) duration and length of follow‐up.
(4) Control (a) type, dose and frequency of the control or placebo or no therapeutic modality (b) duration and length of follow‐up.
(5) Outcomes (a) primary and secondary outcomes as described in the Types of outcome measures section.
If stated, the sources of funding of any of the included studies were recorded. The review authors used this information to help them assess heterogeneity and the external validity of the trials.
Assessment of risk of bias in included studies
Each of two review authors (ZF and MN) graded the selected trials using a simple contingency form and followed the domain‐based evaluation described in theCochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011). The evaluations were compared and any inconsistencies between the review authors were discussed and resolved.
The following domains were assessed as at low risk of bias, unclear risk of bias or high risk of bias:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding (performance bias and detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other bias.
Overall risk of bias in any included study was categorised according to the following:
low risk of bias (plausible bias unlikely to seriously alter the results) if all key domains were assessed as at low risk of bias;
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more key domains were assessed as at unclear risk of bias; or
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more key domains were assessed as at high risk of bias.
These assessments are reported in the Risk of bias in included studies table.
Measures of treatment effect
Data were analysed by RFS and ZF using RevMan 5.1 and reported according to the criteria described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 (Higgins 2011).
For dichotomous data, the estimates of effect of an intervention were expressed as risk ratios together with 95% confidence intervals. For continuous outcomes, mean differences and 95% confidence intervals were used to summarise the data for each group where the mean difference and standard deviations were calculable from the data presented.
Assessment of heterogeneity
The review authors assessed clinical heterogeneity by examining the characteristics of the included studies. This included differences between the types of participants, the interventions and the outcomes across the trials. We planned to assess statistical heterogeneity using a Chi2 test in addition to the I2 statistic, where I2 values over 50% indicate moderate to high heterogeneity (Higgins 2003). However, we were not able to do that due to the limited number of included studies.
Assessment of reporting biases
If we had identified a sufficient number of included studies considering similar interventions and outcomes, we would have attempted to assess publication bias using a funnel plot (Egger 1997).
Data synthesis
Due to the significant clinical heterogeneity between the included studies in terms of the interventions used we were unable to combine the results in a meta‐analysis and therefore present data for individual outcomes and comparisons together with a descriptive analysis as appropriate.
If studies of similar comparisons reporting the same outcome variables are available in future updates, we will use the fixed‐effect and random‐effects models as appropriate for the synthesis and meta‐analysis of any quantitative data. We will use the fixed‐effect model if we establish that the each study is estimating the same quantity, otherwise we will use the random‐effects model. If we establish that there is heterogeneity between the studies, we may use a random‐effects model, but if the heterogeneity between the studies is significant or the studies are too clinically diverse, we will not undertake a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
In future updates if sufficient data are available, we will conduct a subgroup analysis according to age, gender and the degree of severity of OA.
Sensitivity analysis
If there are sufficient included trials in future updates, the review authors plan to conduct sensitivity analyses to assess the robustness of their review results by repeating the analysis after exclusion of lower quality trials. In addition, the authors will undertake sensitivity analyses to examine the effect of allocation concealment, blinded outcome assessment and completeness of follow‐up.
Results
Description of studies
Results of the search
The search strategy retrieved 676 (42 Cochrane Oral Health's Trials Register, 69 CENTRAL, 396 MEDLINE, 81 Embase, 88 PEDro) references to studies and two additional reports were obtained through other sources, which after de‐duplication resulted in 512 potentially eligible studies. After examination of the titles and abstracts of these references, all but 50 were eliminated and excluded from further assessment. Full text copies of these potentially relevant studies were obtained. Only three of the studies matched our inclusion criteria.
See the study flow diagram for further details (Figure 1).
1.
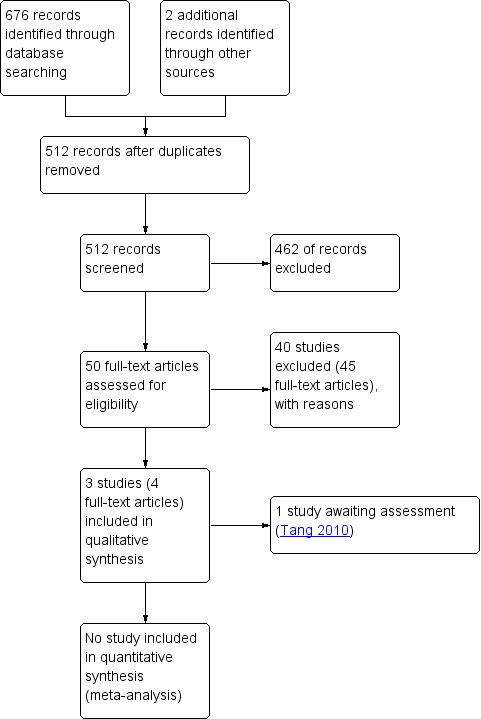
Study flow diagram.
Included studies
We have identified three studies that matched our inclusion criteria (Bjørnland 2007; Mejersjö 2008; Thie 2001). Bjørnland 2007 (Norway) and Mejersjö 2008 (Sweden) were conducted in Europe and Thie 2001 (Canada) was conducted in North America. Two of the included trials recruited participants according to the RDC/TMD guideline (Bjørnland 2007; Mejersjö 2008) and one recruited those according to criteria compatible with the RDC/TMD diagnosis for osteoarthritis (OA) (Thie 2001). Of the three included trials all participants underwent complementary assessment by means of CT scan.
All participants of the three included trials were recruited in university clinics.
Bjørnland 2007 compared intra‐articular injections of sodium hyaluronate with corticosteroids. Mejersjö 2008 compared diclofenac sodium with occlusal splint therapy. Thie 2001 compared glucosamine sulfate or ibuprofen.
All included trials considered pain or tenderness of the temporomandibular joint (TMJ) and masticatory muscles, as well as measures of mandibular movement, as outcomes. Adverse effects or post‐surgical complications were also reported by the three included studies. Less frequent outcome measures were TMJ sounds (Bjørnland 2007) and morphological changes as assessed by CT scans (Mejersjö 2008).
More details are stated in the Characteristics of included studies table.
Excluded studies
All 40 studies which did not match our inclusion criteria were excluded and the reasons for their exclusion were noted in the Characteristics of excluded studies table.
The principal reasons for exclusion of many of the studies were related to the characteristics of the enrolled participants.
Other TMJ disorders than OA (Al‐Badawi 2004; Bertolucci 1995; Di Rienzo Businco 2004; Ekberg 1996; Ekberg 1998; Ekberg 2002; Emshoff 2008; Fikácková 2007; Funch 1984; Ismail 2007; Kopp 1985; Kulekcioglu 2003; Maloney 2002; Maluf 2010; Nguyen 2001; Okeson 1983; Shi 2001; Stegenga 1993; Stowell 2007; Turk 1993; Winocur 2000), whereas a few excluded participants with signs of TMJ OA (Ayesh 2008; Schmid‐Schwap 2006; Truelove 2006).
Use of the RDC/TMD or compatible criteria but the participants were diagnosed with two or more TMDs and did not provide separate data for participants with TMJ OA (Bertolami 1993; List 2001; Marini 2010; Nilsson 2009; Peroz 2004; Reid 1994; Turner 2006).
Study designs that were not eligible for this review (Bjørnland 1995; Haddad 2000; Israel 2010; Jones 2011; Kurita 2007; Machon 2011; Ohnuki 2006).
Incomplete data and poor quality (Guarda Nardini 2004).
Protocol violation (Guarda Nardini 2005).
Risk of bias in included studies
Of the three included studies, two were judged as at unclear risk of bias (Bjørnland 2007; Thie 2001) and one as high risk (Mejersjö 2008). Please seeFigure 2 and Figure 3 for a summary assessment and the risk of bias tables (Characteristics of included studies) for the judgement of each study.
2.
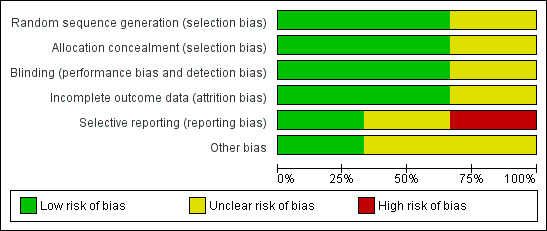
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
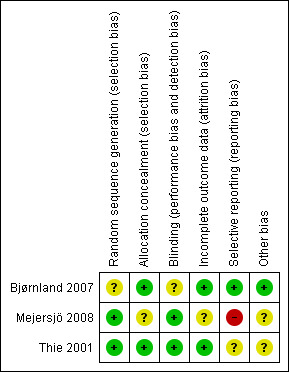
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Comparison 1. Sodium hyaluronate versus corticosteroids
Only Bjørnland 2007 compared these two interventions (40 participants).
Mean differences for reported pain on a VAS were ‐14.00 mm (95% confidence interval (CI): ‐30.80 to 2.80) at 14 days after the first injection (Analysis 1.1), and ‐10.00 mm (95% CI: ‐26.56 to 6.56) after 1 month (Analysis 1.2). After 6 months, both interventions showed significant mean difference (‐17.00, 95% CI: ‐32.60 to ‐1.40), with lower pain observed after injections with sodium hyaluronate (Analysis 1.3; Figure 4). Among the two groups, pain on palpation of the affected and contralateral TMJ and masticatory muscles presented similar frequencies regardless of the time (Analysis 1.4; Analysis 1.5; Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11; Analysis 1.12; Figure 5; Figure 6; Figure 7).
1.1. Analysis.
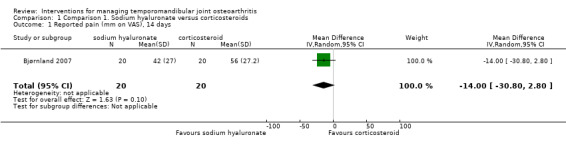
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1 Reported pain (mm on VAS), 14 days.
1.2. Analysis.
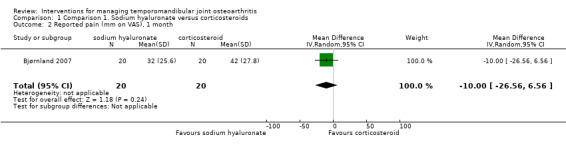
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 2 Reported pain (mm on VAS), 1 month.
1.3. Analysis.
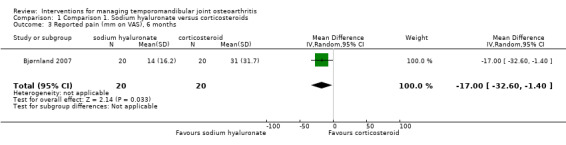
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 3 Reported pain (mm on VAS), 6 months.
4.
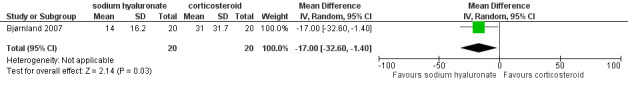
Forest plot of Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1.3. Reported pain (mm on VAS), 6 months.
1.4. Analysis.
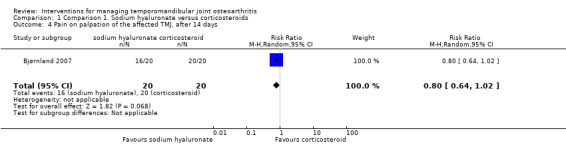
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 4 Pain on palpation of the affected TMJ, after 14 days.
1.5. Analysis.
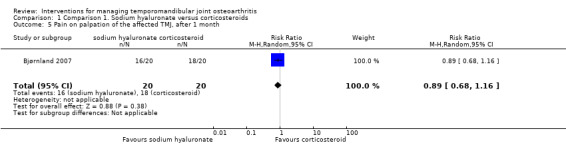
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 5 Pain on palpation of the affected TMJ, after 1 month.
1.6. Analysis.
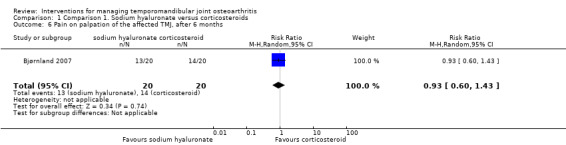
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 6 Pain on palpation of the affected TMJ, after 6 months.
1.7. Analysis.
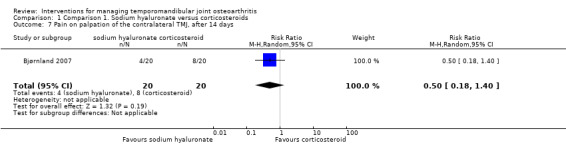
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 7 Pain on palpation of the contralateral TMJ, after 14 days.
1.8. Analysis.
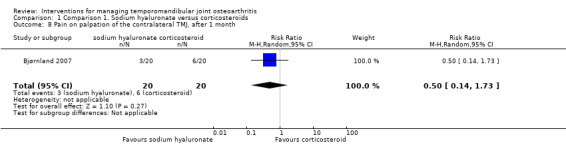
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 8 Pain on palpation of the contralateral TMJ, after 1 month.
1.9. Analysis.
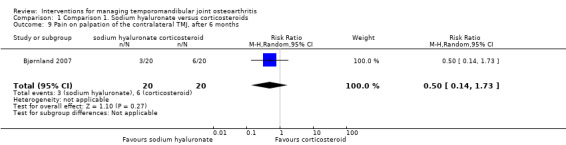
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 9 Pain on palpation of the contralateral TMJ, after 6 months.
1.10. Analysis.
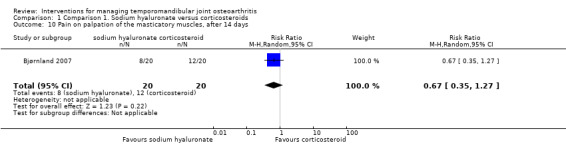
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 10 Pain on palpation of the masticatory muscles, after 14 days.
1.11. Analysis.
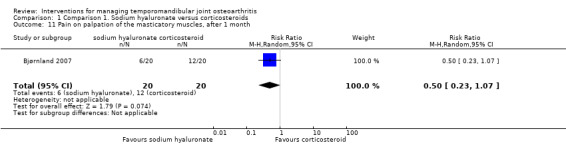
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 11 Pain on palpation of the masticatory muscles, after 1 month.
1.12. Analysis.
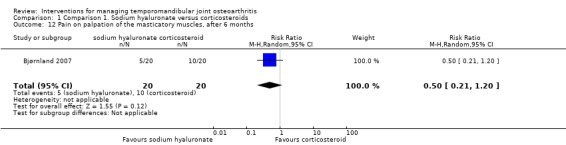
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 12 Pain on palpation of the masticatory muscles, after 6 months.
5.
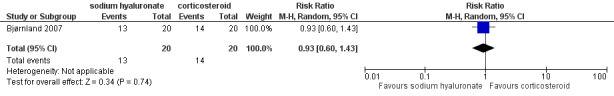
Forest plot of Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1.6. Pain on palpation of the affected TMJ, after 6 months.
6.
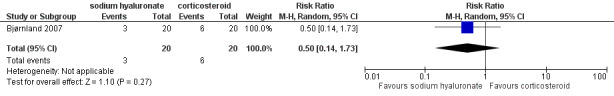
Forest plot of Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1.9. Pain on palpation of the contralateral TMJ, after 6 months.
7.
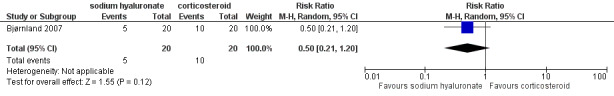
Forest plot of Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1.12. Pain on palpation of the masticatory muscles, after 6 months.
The study found lower frequency of TMJ sounds among the participants receiving sodium hyaluronate after 14 days (risk ratio: 0.59, 95% CI: 0.37 to 0.95) (Analysis 1.13). However, risk ratios at 1 and 6 months of follow‐up are not significant (0.83, 95% CI: 0.47 to 1.47, and 0.55, 95% CI: 0.16 to 1.91, respectively) (Analysis 1.14; Analysis 1.15).
1.13. Analysis.
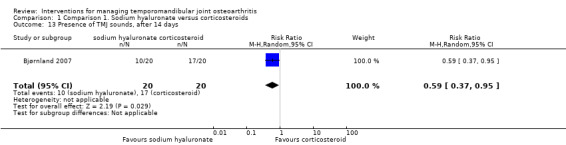
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 13 Presence of TMJ sounds, after 14 days.
1.14. Analysis.
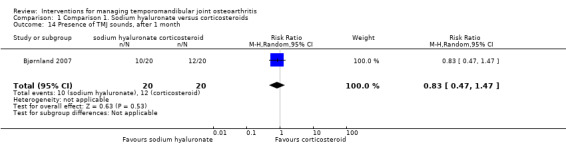
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 14 Presence of TMJ sounds, after 1 month.
1.15. Analysis.
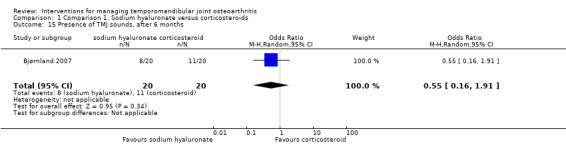
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 15 Presence of TMJ sounds, after 6 months.
None of the mean differences between interventions for the measures of jaw movements showed significance (Analysis 1.16; Analysis 1.17; Analysis 1.18; Analysis 1.19; Analysis 1.20; Analysis 1.21; Analysis 1.22; Analysis 1.23; Analysis 1.24; Analysis 1.25; Analysis 1.26; Analysis 1.27; Figure 8; Figure 9; Figure 10; Figure 11).
1.16. Analysis.
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 16 Maximum jaw opening (mm), 14 days.
1.17. Analysis.
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 17 Maximum jaw opening (mm), 1 month.
1.18. Analysis.
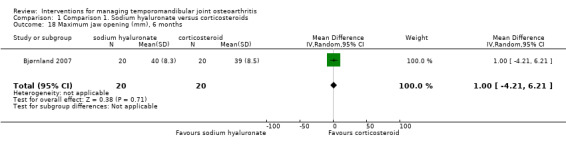
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 18 Maximum jaw opening (mm), 6 months.
1.19. Analysis.
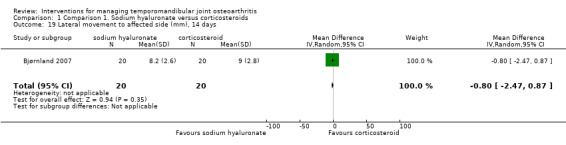
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 19 Lateral movement to affected side (mm), 14 days.
1.20. Analysis.
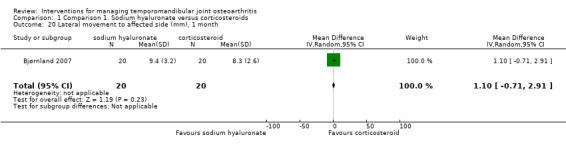
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 20 Lateral movement to affected side (mm), 1 month.
1.21. Analysis.
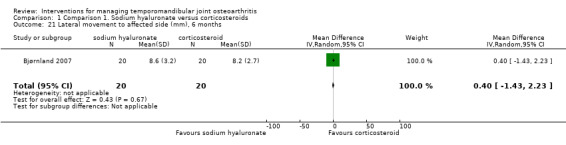
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 21 Lateral movement to affected side (mm), 6 months.
1.22. Analysis.
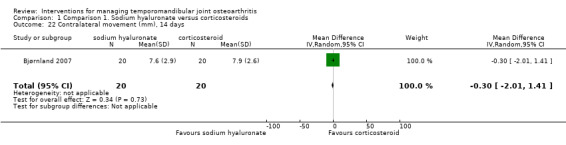
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 22 Contralateral movement (mm), 14 days.
1.23. Analysis.
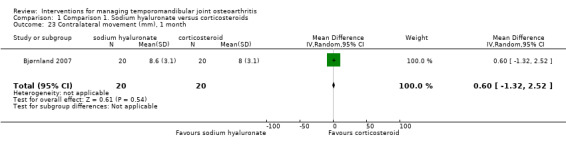
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 23 Contralateral movement (mm), 1 month.
1.24. Analysis.
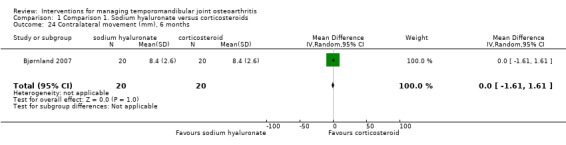
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 24 Contralateral movement (mm), 6 months.
1.25. Analysis.
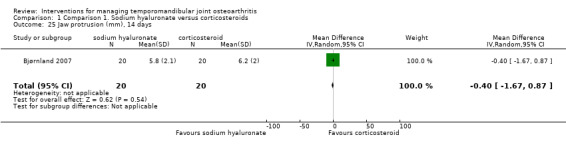
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 25 Jaw protrusion (mm), 14 days.
1.26. Analysis.
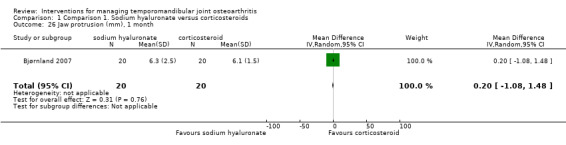
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 26 Jaw protrusion (mm), 1 month.
1.27. Analysis.
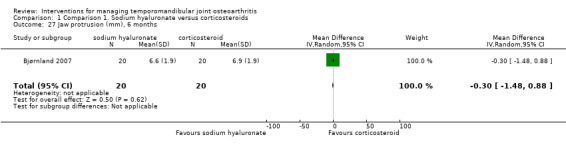
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 27 Jaw protrusion (mm), 6 months.
8.

Forest plot of Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1.18. Maximum jaw opening (mm), 6 months.
9.
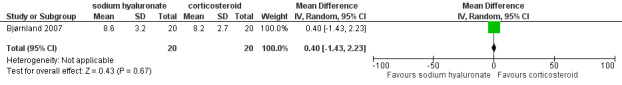
Forest plot of Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1.21. Lateral movement to affected side (mm), 6 months.
10.
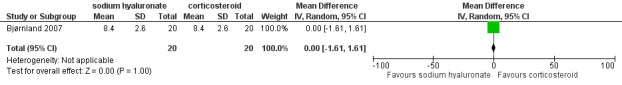
Forest plot of Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1.24. Contralateral movement (mm), 6 months.
11.
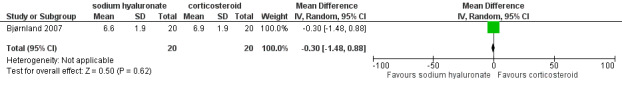
Forest plot of Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 1.27. Jaw protrusion (mm), 6 months.
The risk ratio for complications was 1.20 (95% CI: 0.44 to 3.30), which discloses no difference between the interventions (Analysis 1.28).
1.28. Analysis.
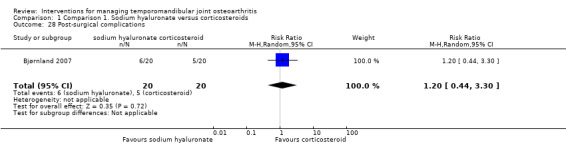
Comparison 1 Comparison 1. Sodium hyaluronate versus corticosteroids, Outcome 28 Post‐surgical complications.
Comparison 2. Diclofenac sodium versus occlusal splint
Only the Mejersjö 2008 trial compared these interventions (29 participants).
Data for pain on movement or pain on palpation of the affected TMJ (both after 1 week and 3 months) could not be extracted, as long as they are described as percentages of an unclear total number. The study reports morphological changes assessed by CT for the entire sample without distinction of group, so extraction was not possible.
The frequency of complications was similar for both groups (risk ratio: 1.07, 95% CI: 0.50 to 2.28) (Analysis 2.1), although their nature differed drastically (diclofenac sodium: gastrointestinal problems 3, sleepiness 3, dizziness 1; occlusal splint: sleeping problems 2, pressure or friction of teeth 3, increased decreased saliva 2).
2.1. Analysis.
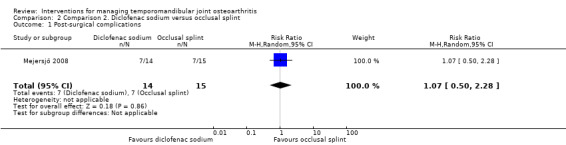
Comparison 2 Comparison 2. Diclofenac sodium versus occlusal splint, Outcome 1 Post‐surgical complications.
Comparison 3. Glucosamine sulfate versus ibuprofen
The Thie 2001 study compared intraoral medication with glucosamine sulfate or ibuprofen (45 participants).
The researchers found that six participants in Group 1 did not experience significant improvement in TMJ pain compared to seven in Group 2 (Analysis 3.1) (Fisher's exact test, P = 0.73, according to the authors). The results for the other variables are described according to the changes from baseline after 90 days of intervention. Mean difference for reported pain on a VAS was ‐4.57 mm (95% CI: ‐9.91 to 0.77) (Analysis 3.2).
3.1. Analysis.
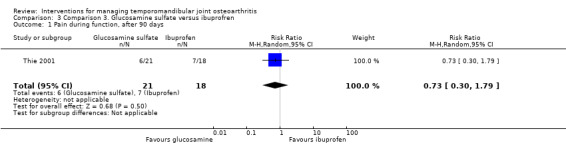
Comparison 3 Comparison 3. Glucosamine sulfate versus ibuprofren, Outcome 1 Pain during function, after 90 days.
3.2. Analysis.
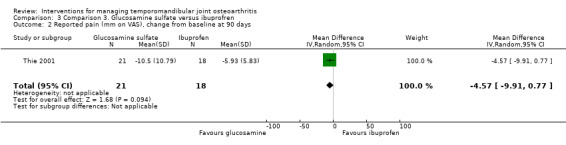
Comparison 3 Comparison 3. Glucosamine sulfate versus ibuprofren, Outcome 2 Reported pain (mm on VAS), change from baseline at 90 days.
Maximum jaw opening was similar for the tested interventions, both in its pain‐free range (1.75, 95% CI: ‐4.10 to 7.60) and at its maximum voluntary movement (3.08, 95% CI: ‐0.97 to 7.13) (Analysis 3.3; Analysis 3.4).
3.3. Analysis.
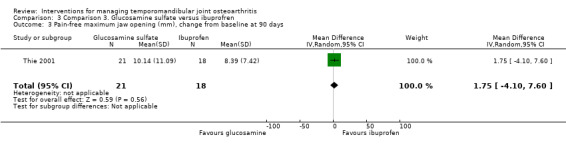
Comparison 3 Comparison 3. Glucosamine sulfate versus ibuprofren, Outcome 3 Pain‐free maximum jaw opening (mm), change from baseline at 90 days.
3.4. Analysis.
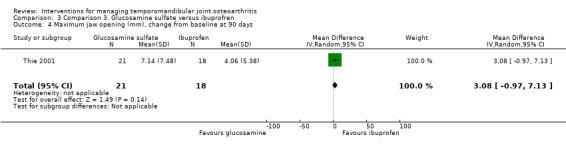
Comparison 3 Comparison 3. Glucosamine sulfate versus ibuprofren, Outcome 4 Maximum jaw opening (mm), change from baseline at 90 days.
BPI scores were similar for both groups, with no difference for the 'pain intensity' and the 'pain interference' domain either (‐2.69, 95% CI: ‐7.38 to 2.00, and ‐6.74, 95% CI: ‐14.70 to 1.22, respectively) (Analysis 3.5; Analysis 3.6). Mean difference for the number of extraoral masticatory muscle tenderness was 0.38 (95% CI: ‐2.30 to 3.06) (Analysis 3.7). After 90 days, the use of acetaminophen was the same for both groups (t test; P = 0.11).
3.5. Analysis.
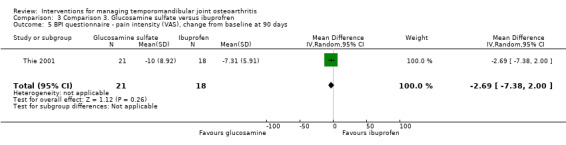
Comparison 3 Comparison 3. Glucosamine sulfate versus ibuprofren, Outcome 5 BPI questionnaire ‐ pain intensity (VAS), change from baseline at 90 days.
3.6. Analysis.
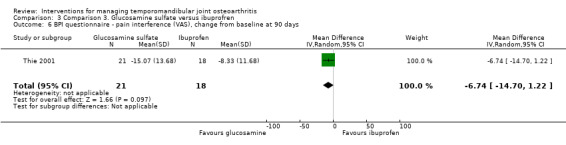
Comparison 3 Comparison 3. Glucosamine sulfate versus ibuprofren, Outcome 6 BPI questionnaire ‐ pain interference (VAS), change from baseline at 90 days.
3.7. Analysis.
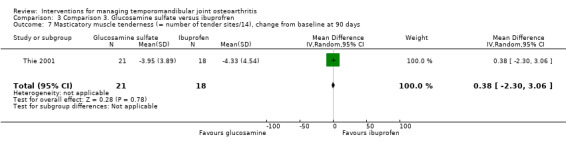
Comparison 3 Comparison 3. Glucosamine sulfate versus ibuprofren, Outcome 7 Masticatory muscle tenderness (= number of tender sites/14), change from baseline at 90 days.
Discussion
Summary of main results
Despite the wide range of therapeutic options available for the management of temporomandibular joint osteoarthritis (TMJ OA), this review included only a few trials which provided data for three comparisons for non‐surgical and minimally invasive surgical modalities.
Intra‐articular injections with sodium hyaluronate appear to be as effective as other modalities. One of the trials (Bjørnland 2007) found that intra‐articular injections of both sodium hyaluronate or corticosteroids have similar effectiveness over several parameters. However, TMJ pain seems to be slightly less after 6 months following injections of sodium hyaluronate, as well as TMJ sounds immediately after treatment.
Comparisons among non‐surgical modalities showed no important differences either. Although diclofenac sodium showed similar benefits when compared with occlusal splints in terms of pain and complications at 3 months (Mejersjö 2008), symptoms were less intense at the 1 month follow‐up for participants medicated with diclofenac sodium. Despite the similar number of complications for both interventions, the nature of these varies quite widely and these should be discussed with patients. Glucosamine was as effective as ibuprofen in terms of pain, discomfort and jaw movements, according to one of the trials (Thie 2001).
Overall completeness and applicability of evidence
The trials in general enrolled participants that were representative of the average TMJ OA patient with respect to gender and age (Kino 2005). However, any result should be interpreted with caution, as long as all comparisons are based on single studies. The external validity of these trials would be further enhanced by involving participants from other nationalities. Moreover, all participants were recruited from specialized university clinics; this way, it is likely that most were referred patients. Other settings such as general practice or emergency facilities may provide participants with different therapeutic responsiveness. Although some of the trials considered a wide range of outcomes, an aspect that should be encouraged, data extraction was not always feasible.
The scarcity of comparative evidence for intra‐articular injections, occlusal splints and pharmacological agents is somewhat disappointing. However, even more important is the noticeable absence for other interventions, i.e. reassurance, physiotherapy, short‐wave diathermy, ultrasound, iontophoretically applied corticosteroids, laser therapy and open surgery. The lack of data from randomised controlled trials (RCTs) makes decision making about the management of TMJ OA strongly dependent on consumers' preferences and clinical expertise.
Quality of the evidence
The review only found three unclear‐high risk of bias studies with a small sample size. Two of the reports did not provide adequate information about important methodological aspects such as sequence generation, allocation concealment or blinding, despite the recommendations of guidelines such as CONSORT. A critical aspect in terms of quality of the studies is selective reporting. One of the included studies did not provide extractable results, possibly because they were not considered as the most relevant by authors or journal or both (Furukawa 2007).
As we had only one study for each comparison and outcome, we were not able to judge about consistency between study results.
We only had three included studies, therefore, we were not able to assess publication bias.
Potential biases in the review process
Before undertaking this review, one of our main concerns was the possible differences in the diagnosis of TMJ disorders between studies. The inclusion of participants with a generic diagnosis (e.g. painful TMJ) might well reduce the validity of our results regarding our clinical question. Moreover, different diagnostic approaches for TMJ OA would insert non‐comparable data in our review. Thus, we considered the RDC/TMD as a standard approach, due to its comprehensiveness and wide use for research about disorders of the TMJ. Although RDC/TMD may not be highly reliable for TMJ OA (John 2005), it provides a list of criteria which can be promptly compared among different studies (Laskin 2007) and is likely to be the best alternative to date (Hasanain 2009).
We employed a search in several databases and without any language restriction, in an attempt to include all eligible studies. A successful attempt to translate non‐English studies (e.g. Shi 2001) was conducted in order to minimize language bias. However, we still had a low number of included studies, which are most likely linked to the restrictive inclusion criteria used.
One of the limitations of our review was the bad reporting of the trial publications. We attempted to contact the authors of the included studies for clarification regarding unclear aspects and missing data in order to minimize this condition, but were not successful. Therefore, some criteria from the risk of bias table remained unclear. Moreover, there were a number of studies that included also other patients in the trial and did not provide separate data for patients with TMJ OA and therefore, we had to exclude those studies too.
Agreements and disagreements with other studies or reviews
The results of this and other published reviews reinforce the idea that we have a restricted number of RCTs about the management of TMJ disorders. A systematic review of surgical therapy of the TMJ classified 3 out of 22 included studies as RCT, and none of the trials included participants with TMJ OA (Reston 2003). The number of studies included in other Cochrane reviews investigating TMJ disorders was not dissimilar and ranged from two to seven (Guo 2009; Koh 2003; Shi 2003).
Authors' conclusions
Implications for practice.
The available evidence regarding the management of temporomandibular joint osteoarthritis (TMJ OA) continues to present challenges to clinical decision making. The scarce evidence available suggests that certain non‐surgical and minimally invasive interventions might be equally effective, but any finding should be interpreted with caution.
Implications for research.
In light of the paucity of evidence for the effectiveness of interventions for the management of TMJ OA, further parallel group randomised controlled trials (RCTs) comparing different interventions may provide useful data. The characteristics of participants is of concern, as long as several excluded studies did not classify TMJ problems according to their different clinical forms (e.g. TMJ OA), thus the use of RDC/TMD should be encouraged as a tool that can be used in the comparison of different trials. RCTs investigating modalities of open surgery for treating TMJ OA would provide valuable data for both clinicians and consumers. Placebo or sham interventions as comparators should provide valuable information about the effectiveness of management methods for TMJ OA as well. Future studies on the management of TMJ OA should consider a comprehensive set of outcomes in addition to a longer follow‐up period.
For more information as to the detailed design of research recommended please see Additional Table 1.
1. Research recommendations based on a gap in the management of temporomandibular joint osteoarthritis .
| Core elements | Issues to consider | Status of research for this review |
| Evidence (E) | What is the current state of evidence? | A systematic review identified three RCTs which matched the eligibility criteria, but two were inadequately reported and assessed as 'unclear risk of bias' |
| Population (P) | Diagnosis, disease stage, comorbidity, risk factor, sex, age, ethnic group, specific inclusion or exclusion criteria, clinical setting | Adult patients of any gender or age, diagnosed with TMJ OA according to RDC/TMD or compatible criteria. Pain or tenderness of masticatory muscles should be considered as an important comorbidity |
| Intervention (I) | Type, frequency, dose, duration, prognostic factor | Any non‐surgical or surgical therapy for TMJ OA. No study evaluated open surgery or arthrocentesis, which should be explored in future trials |
| Comparison (C) | Type, frequency, dose, duration, prognostic factor | Placebo/sham or other therapeutic modality with frequency, dose and duration comparable to the intervention. Comparison with any inactive treatment to be compared in future trials |
| Outcome (O) | Which clinical or patient related outcomes will the researcher need to measure, improve, influence or accomplish? Which methods of measurement should be used? | Pain/tenderness/discomfort in TMJs or jaw muscles (VAS)
Extent of mandibular movement
TMJ sounds (as perceived by the patients or auscultation)
Health‐related quality of life or patient satisfaction
Morphological changes of the TMJs
Number of visits or days absent from work Adverse events Costs |
| Time stamp (T) | Date of literature search or recommendation | March 2010 |
| Study type | What is the most appropriate study design to address the proposed question? | Randomised controlled trial Methods: concealment of allocation sequence Blinding: participants, researchers, outcomes assessors, data analysts Setting: primary care and orofacial pain clinics |
RCTs = randomised controlled trials; RDC/TMD = Research Diagnostic Criteria for Temporomandibular Disorders; TMJ OA = temporomandibular joint osteoarthritis.
What's new
| Date | Event | Description |
|---|---|---|
| 18 June 2018 | Review declared as stable | This review has had low usage and is not a priority for updating. |
Notes
No update planned. This review has had low usage and is not a priority for updating.
Acknowledgements
The review authors would like to thank Cochrane Oral Health for their help in developing this systematic review. They also want to thank Dr Chang Ching I for undertaking translation of Chinese texts (Shi 2001).
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
((osteoarthrit* OR arthrit* OR arthalgi* OR "degenerative disorder*" OR "degenerative condition*") AND (temporomandibular* OR "myofacial pain" OR craniomandibular OR (#4 CONTAINS TMJ) OR (#43 CONTAINS TMJ) OR (#4 CONTAINS CMD) OR (#43 CONTAINS CMD) OR (#4 CONTAINS TMD) OR (#43 CONTAINS TMD) OR "temporo mandibular" OR temporo‐mandibular OR "cranio mandibular" OR cranio‐mandibular OR "joint diseases"))
(#4 relates to the Title field within the Cochrane Oral Health Group Trials Register. #43 relates to the abstract field)
Appendix 2. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 OSTEOARTHRITIS Single MeSH term #2 osteoarthriti* #3 (arthrit* OR arthralgi* OR (degenerative near/6 disorder*) OR (degenerative* near/6 condition*)) #4 TEMPOROMANDIBULAR JOINT Exploded MeSH term #5 TEMPOROMANDIBULAR JOINT DISORDERS Exploded MeSH term #6 (temporomandibular OR temporo‐mandibular) #7 ("TMJ" in Title, Abstract or Keywords or "TMD" in Title, Abstract or Keywords or "CMD" in Title, Abstract or Keywords) #8 (#1 or #2 or #3) #9 (#4 or #5 or #6 or #7) #10 (#8 and #9)
Appendix 3. MEDLINE (Ovid) search strategy
1. Osteoarthritis/ 2. osteoarthrit$.mp. 3. (arthrit$ or arthralgi$ or (degenerative adj6 disorder$) or (degenerative$ adj6 condition$)).mp. 4. exp Temporomandibular Joint/ 5. exp Temporomandibular Joint Disorders/ 6. (temporomandibular or temporo‐mandibular).ab,sh,ti. 7. ("TMJ" or "TMD" or "CMD").ab,sh,ti. 8. or/1‐3 9. or/4‐7 10. 8 and 9
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase (Ovid) search strategy
1. Osteoarthritis/ 2. osteoarthrit$.mp. 3. (arthrit$ or arthralgi$ or (degenerative adj6 disorder$) or (degenerative$ adj6 condition$)).mp. 4. exp Temporomandibular Joint/ 5. exp Temporomandibular Joint Disorders/ 6. (temporomandibular or temporo‐mandibular).ab,sh,ti. 7. ("TMJ" or "TMD" or "CMD").ab,sh,ti. 8. or/1‐3 9. or/4‐7 10. 8 and 9
The above subject search was linked to Cochrane Oral Health filter for identifying RCTs in EMBASE via OVID:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ 16. HUMAN/ 17. 16 and 15 18. 15 not 17 19. 14 not 18
Appendix 5. PEDro search strategy
The Physiotherapy Evidence Database (PEDro) was searched via the Internet: http://search.pedro.org.au/:
temporomandibular AND osteoarthritis
Data and analyses
Comparison 1. Comparison 1. Sodium hyaluronate versus corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reported pain (mm on VAS), 14 days | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐12.00 [‐30.80, 2.80] |
| 2 Reported pain (mm on VAS), 1 month | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐26.56, 6.56] |
| 3 Reported pain (mm on VAS), 6 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐17.0 [‐32.60, ‐1.40] |
| 4 Pain on palpation of the affected TMJ, after 14 days | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.64, 1.02] |
| 5 Pain on palpation of the affected TMJ, after 1 month | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.68, 1.16] |
| 6 Pain on palpation of the affected TMJ, after 6 months | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.60, 1.43] |
| 7 Pain on palpation of the contralateral TMJ, after 14 days | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.18, 1.40] |
| 8 Pain on palpation of the contralateral TMJ, after 1 month | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.14, 1.73] |
| 9 Pain on palpation of the contralateral TMJ, after 6 months | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.14, 1.73] |
| 10 Pain on palpation of the masticatory muscles, after 14 days | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.35, 1.27] |
| 11 Pain on palpation of the masticatory muscles, after 1 month | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.23, 1.07] |
| 12 Pain on palpation of the masticatory muscles, after 6 months | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.21, 1.20] |
| 13 Presence of TMJ sounds, after 14 days | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.37, 0.95] |
| 14 Presence of TMJ sounds, after 1 month | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.47, 1.47] |
| 15 Presence of TMJ sounds, after 6 months | 1 | 40 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.16, 1.91] |
| 16 Maximum jaw opening (mm), 14 days | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐7.10, 3.50] |
| 17 Maximum jaw opening (mm), 1 month | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.60 [‐7.84, 2.64] |
| 18 Maximum jaw opening (mm), 6 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐4.21, 6.21] |
| 19 Lateral movement to affected side (mm), 14 days | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.47, 0.87] |
| 20 Lateral movement to affected side (mm), 1 month | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐0.71, 2.91] |
| 21 Lateral movement to affected side (mm), 6 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐1.43, 2.23] |
| 22 Contralateral movement (mm), 14 days | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.01, 1.41] |
| 23 Contralateral movement (mm), 1 month | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐1.32, 2.52] |
| 24 Contralateral movement (mm), 6 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.61, 1.61] |
| 25 Jaw protrusion (mm), 14 days | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐1.67, 0.87] |
| 26 Jaw protrusion (mm), 1 month | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐1.08, 1.48] |
| 27 Jaw protrusion (mm), 6 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.48, 0.88] |
| 28 Post‐surgical complications | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.44, 3.30] |
Comparison 2. Comparison 2. Diclofenac sodium versus occlusal splint.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Post‐surgical complications | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.50, 2.28] |
Comparison 3. Comparison 3. Glucosamine sulfate versus ibuprofren.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain during function, after 90 days | 1 | 39 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.30, 1.79] |
| 2 Reported pain (mm on VAS), change from baseline at 90 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐4.57 [‐9.91, 0.77] |
| 3 Pain‐free maximum jaw opening (mm), change from baseline at 90 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 1.75 [‐4.10, 7.60] |
| 4 Maximum jaw opening (mm), change from baseline at 90 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 3.08 [‐0.97, 7.13] |
| 5 BPI questionnaire ‐ pain intensity (VAS), change from baseline at 90 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐2.69 [‐7.38, 2.00] |
| 6 BPI questionnaire ‐ pain interference (VAS), change from baseline at 90 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | ‐6.74 [‐14.70, 1.22] |
| 7 Masticatory muscle tenderness (= number of tender sites/14), change from baseline at 90 days | 1 | 39 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐2.30, 3.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bjørnland 2007.
| Methods | Parallel group trial in Norway, which compared sodium hyaluronate (S) and corticosteroids (C) for intra‐articular injections in TMJ OA patients. No information about sequence generation methods. Reports cite sealed envelopes with codes, opened after confirming participants' eligibility and informed consent. Unawareness of participants and outcome assessors about the drug used. Participants were 40 out of 52 TMJ patients (12 presented no radiographic evidence of OA and were excluded before allocation). No drop‐outs for the study (clinical assessment at follow‐up), although a few participants did not return for radiographic examination (Group S: n = 3; Group C: n = 1). |
|
| Participants | 40 patients of the Department of Oral Surgery and Oral Medicine, University of Oslo, divided into two groups (n = 20 each). All fulfilled the RDC/TMD criteria for TMJ OA. Group S: 19 women and 1 man, mean age (±SD): 53.4±12.9 years, mean duration of TMJ symptoms: 5.9±7.7 years. Group C: 15 women and 5 men, mean age (±SD): 50.0±13.3 years; mean duration of TMJ symptoms: 5.8±10.7 years. Inclusion criteria: (1) reported TMJ pain at function and rest form >1 year; (2) restricted mandibular function; (3) evidences of TMJ OA by CT scan, i.e. erosions, flattening and sclerosis/osteophytes of the condyle or articular fossa; (4) previous attempt of non‐surgical treatment without success; (5) age >20 years. Exclusion criteria: (1) injections of corticosteroids or hyaluronate preparation within the previous 12 months; (2) history of general arthritis or other connective tissue disease; (3) known hypersensitivity to chicken, eggs or other avian products; (4) other systemic conditions (treatment with immunosuppressive drugs, general infection, and pregnant or breastfeeding women). |
|
| Interventions | Sodium hyaluronate (S) compared with a betamethasone sodium phosphate/betamethasone acetate combination (C). Two intra‐articular injections (0.7‐1.0 mL), 14 days apart. Follow‐up: 14 days (before the 2nd injection), 1 month and 6 months. |
|
| Outcomes | (a) Pain on palpation of the affected and contralateral TMJ and masticatory muscles (VAS values and yes/no). (b) Extent of active mandibular movement (maximum jaw opening, lateral movement and protrusion). (c) TMJ sounds (crepitation and clicking) assessed by clinical exam. (d) Post‐surgical complications. |
|
| Notes | Supported by grants from the Institute of Clinical Dentistry, Faculty of Dentistry, University of Oslo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated into two groups". Comment: No clear description provided. |
| Allocation concealment (selection bias) | Low risk | Quote: "Forty sealed envelopes contained the code for participation in the two treatment groups, and the envelopes were not opened before it was determined the patient was eligible for study inclusion". Comment: Participants and investigators enrolling participants were unable to foresee the assignment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | (a) Participants: Quote: "The patients were given information about the two drugs to be used for this study, without knowledge of which they were given". Comment: Yes. (b) Researcher: Unclear. (c) Outcome assessor: Unclear. (d) Data analyst: Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "..there were no drop‐outs for the clinical re‐examinations". Comment: All outcome data appeared to be completely addressed. |
| Selective reporting (reporting bias) | Low risk | Quote: "..the aim of the present study was, therefore, to compare the efficacy and the complications of intra‐articular injections of corticosteroids and sodium hyaluronate. We tested the hypothesis that there were no significant differences between intra‐articular injections of the two drugs in terms of pain relief, joint sounds, function and complications". Comment: The stated objectives matched the listed outcomes. |
| Other bias | Low risk | The study appeared to be free of other sources of bias. |
Mejersjö 2008.
| Methods | Parallel group RCT in Sweden, which compared oral medication (diclofenac sodium ‐ DS) versus occlusal splints (OS). Sequence generated according to computer generated tables and stratified according to the duration of symptoms (two strata: <6 months or >6 months). No information about allocation concealment was provided. The study employed blinded outcome assessors. Eligible participants were 29 out of 1417 orofacial pain patients. Reasons for exclusion were: did not fulfil inclusion criteria (n = 1373); refused to participate before or after randomisation (n = 11 and 3, respectively). Four participants (1 in DS and 3 in OS) stopped the treatment before 3 months. |
|
| Participants | 29 TMJ OA patients referred to the Department of Stomatognatic Physiology of the University of Göteborg (27 women and 2 men; mean age: 62 years, range: 39‐76 years). All had unilateral TMJ OA (right TMJ: 16; left TMJ: 13). 2/3 of the participants had some general pharmacological treatment, often related to circulatory disorders. Inclusion criteria: TMJ OA diagnosis according to the RDC/TMD. Exclusion criteria: systemic joint disease, known sensitivity to acetylsalicylic acid, impaired coagulation, gastric ulcers, kidney or liver problems, and any previous treatment for the TMJ (except analgesics). Group DS: n = 15; group OS: n = 14; instructions to take paracetamol tablets if additional analgesics were needed. |
|
| Interventions | Group DS: diclofenac sodium 50 mg, 3 times daily. Group OS: acrylic flat occlusal splint covering all the upper teeth. The report does not clarify the regimen of use (e.g. continuous or during sleep only). The period of intervention for both groups was 3 months. |
|
| Outcomes | (a) Pain on movement and palpation. (b) Morphological changes of the TMJs as assessed by CT scans. (c) Adverse effects. |
|
| Notes | We considered the 3 months follow‐up for this review, which coincides with the duration of interventions. The 1 year follow‐up had several disagreements with the protocol and a large amount of drop‐outs. No funding reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The mode of treatment was decided according to two computer generated tables randomly produced in advance depending on whether the duration of symptoms was acute (<6 months) or chronic (>6 months)..." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit a clear judgement. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | (a) Participants: N/A. (b) Researcher: N/A. (c) Outcome assessor: Yes. (d) Data analyst: Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The report describes the frequency of drop‐outs but not their reasons. |
| Selective reporting (reporting bias) | High risk | The study assessed a comprehensive set of outcomes; however, they were incompletely reported. |
| Other bias | Unclear risk | Participants were allowed to take paracetamol during the trial in order to achieve additional analgesia. However, the quantity and doses used by each group were unreported. |
Thie 2001.
| Methods | Parallel group trial in Canada about oral medications, with a 7 days run‐in period. Several aspects associated with control of bias are well described. Sequence generated by means of block randomisation by a statistician. Identical clear capsules prepared and coded by a pharmacist. Neither participants nor researchers knew the administered medication before the end of the study. Eligible participants: 45 out of 176 interviewed volunteers. Reasons for exclusion were: no radiographic evidence of TMJ OA (n = 45); low pain levels (n = 33); allergy to NSAID (n = 12); participant did not proceed to radiographic assessment (n = 31). Withdrawals and losses:
|
|
| Participants | 45 orofacial pain patients of the University of Alberta or respondents to advertisement in the Edmonton area (40 women and 5 men). Inclusion criteria:
Mean age (SD): Group 1 (n = 21): 36.6 (10.3); Group 2 (n = 18): 38.7 (13.3). |
|
| Interventions | Glucosamine sulfate 500 mg (Group 1) compared with ibuprofen 400 mg (Group 2). Participants used both medications 3 times daily, during 90 days. |
|
| Outcomes | Primary outcome: reduction of 20% or more in joint pain during function, assessed by a VAS. Secondary outcomes: (1) pain free and voluntary maximum incisal opening; (2) the Brief Pain Inventory (BPI) questionnaire; (3) extraoral masticatory muscle tenderness on 14 sites (= number of tender sites/14). Frequency of adverse effects. |
|
| Notes | Medications were donated by private companies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician generated the randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Medications were prepared and coded as identical clear capsules by a pharmacist. Investigators did not know which of the two medications was administered until the end of the trial. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | (a) Participants: Yes. (b) Researcher: Yes. (c) Outcome assessor: Yes. (d) Data analyst: Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The report describes the frequency of drop‐outs and their reasons. Although some missing participants reported gastric problems (expected in association with NSAID), the reasons were balanced across groups. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit a clear judgement. |
| Other bias | Unclear risk | It is unclear how the sponsorship of this study could have influenced results. Although the study used block randomisation, blinding was adequate and the report states that assignments were revealed after the end of the trial. Thus, it is unlikely that assignments were predictable. |
DJD = degenerative joint disease; RCT = randomised controlled trial; RDC/TMD = Research Diagnostic Criteria for Temporomandibular Disorders; SD = standard deviation; TMJ OA = temporomandibular joint osteoarthritis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Badawi 2004 | Participants presented arthralgia according to the RDC/TMD. |
| Ayesh 2008 | Participants with TMJ OA were excluded. |
| Bertolami 1993 | No separate data provided for patients with TMJ OA. |
| Bertolucci 1995 | Participants were not diagnosed according to criteria compatible with RDC/TMD, and presented with disk displacement without reduction. |
| Bjørnland 1995 | Non‐RCT. |
| Di Rienzo Businco 2004 | Participants received no specific diagnosis for TMJ pain. |
| Ekberg 1996 | Participants received no specific diagnosis for TMJ pain. |
| Ekberg 1998 | Participants received no specific diagnosis for TMJ pain. |
| Ekberg 2002 | Participants received no specific diagnosis for TMJ pain. |
| Emshoff 2008 | Participants with TMJ disorders according to the RDC/TMD were excluded. |
| Fikácková 2007 | Participants with TMJ OA were excluded. |
| Funch 1984 | Participants received no specific diagnosis of TMJ pain. |
| Guarda Nardini 2004 | Incomplete data on the allocation of participants and masking of investigators. Important imbalance in group size in addition to differences in the frequency of delivery of intervention and comparator. Very limited information about the characteristics of the participants and no usable data in the report. |
| Guarda Nardini 2005 | Lack of detail about the inclusion criteria or the characteristics of participants and interventions and the overall conduct of the trial. Parallel arms study (intra‐articular injections of sodium hyaluronate versus occlusal splints) with a third group consisting of participants who had refused any therapy for TMJ OA. |
| Haddad 2000 | Non‐RCT. |
| Ismail 2007 | No separate data provided for patients with TMJ OA. |
| Israel 2010 | Non‐RCT. |
| Jones 2011 | Non‐RCT. |
| Kopp 1985 | Participants received no specific diagnosis for TMJ pain. |
| Kulekcioglu 2003 | Diagnostic criteria not compatible with the RDC/TMD, no separate data provided for patients with TMJ OA. |
| Kurita 2007 | Non‐RCT. |
| List 2001 | No separate data provided for patients with TMJ OA. |
| Machon 2011 | Non‐RCT. |
| Maloney 2002 | No separate data provided for patients with TMJ OA. |
| Maluf 2010 | Participants received no specific diagnosis for TMJ pain. |
| Marini 2010 | No separate data provided for patients with TMJ OA. |
| Nguyen 2001 | Participants received no specific diagnosis for TMJ pain. |
| Nilsson 2009 | No separate data provided for patients with TMJ OA. |
| Ohnuki 2006 | Non‐RCT.. |
| Okeson 1983 | Participants received no specific diagnosis for TMJ pain. |
| Peroz 2004 | No separate data provided for patients with TMJ OA. |
| Reid 1994 | No separate data provided for patients with TMJ OA. |
| Schmid‐Schwap 2006 | Participants received no specific diagnosis for TMJ pain. |
| Shi 2001 | Diagnostic criteria not compatible with the RDC/TMD, no separate data provided for patients with TMJ OA. |
| Stegenga 1993 | Diagnostic criteria not compatible with the RDC/TMD, no separate data provided for patients with TMJ OA. |
| Stowell 2007 | Participants received no specific diagnosis for TMJ pain. |
| Truelove 2006 | Participants with TMJ OA were excluded. |
| Turk 1993 | Participants received no specific diagnosis for TMJ pain. |
| Turner 2006 | No separate data provided for patients with TMJ OA. |
| Winocur 2000 | Participants received no specific diagnosis for TMJ pain. |
RCT = randomised controlled trial; RDC/TMD = Research Diagnostic Criteria for Temporomandibular Disorders; TMJ OA = temporomandibular joint osteoarthritis.
Characteristics of studies awaiting assessment [ordered by study ID]
Tang 2010.
| Methods | Parallel group trial in China: intra‐articular injections of sodium hyaluronate versus placebo. Very limited information about trial conduct. No information about how the generation of allocation sequence (Quote: "Forty OA patients were randomly divided into groups"). The report cites blinding of both patients and researchers ("operator"), but gives no further information about blinding or allocation concealment, i.e. delivery of unidentified vials with the tested substances. No reference made to the exclusion of participants after sequence generation. |
| Participants | 40 participants, divided into two groups (n = 20 each). All were patients of the West China Hospital of Stomatology and presented TMJ OA from 1/2 to 1 year. Group 1: 11 women and 9 men, mean age: 43 years, range: 28‐57 years. Group 2: 10 women and 10 men, mean age: 44 years, range: 25‐63 years. Inclusion criteria: TMJ OA diagnosis according to the RDC/TMD, history of unsuccessful treatment by non‐surgical therapy (jaw exercises, physiotherapy and occlusal splints). Exclusion criteria: systemic joint disease, treatment with immunosuppressive drugs, any organ disease, general infection or trauma‐induced joint disorder. |
| Interventions | Group 1: intra‐articular injections of sodium hyaluronate (1 mL, molecular weight: 1,500 ‐ 2,500 kDa). Group 2: intra‐articular injections of physiologic saline solution. Both groups received five injections (1/week for 5 weeks). Follow‐up: 1 week. |
| Outcomes | TMJ pain (VAS value, 0 to 10). |
| Notes | We contacted the authors in order to check if they employed any form of randomisation. |
RDC/TMD = Research Diagnostic Criteria for Temporomandibular Disorders; TMJ OA = temporomandibular joint osteoarthritis.
Contributions of authors
Raphael Freitas de Souza (RFS), Zbys Fedorowicz (ZF), Mona Nasser (MN) and Claudia Helena Lovato da Silva (CLS) were responsible for designing and co‐ordinating the review. RFS and CLS organised the retrieval of papers. RFS, CLS and MN were responsible for: writing to authors of papers for additional information; screening search results; screening retrieved papers against inclusion criteria; appraising the quality of papers; data collection for the review; and obtaining and screening data on unpublished studies. ZF and MN were responsible for: obtaining copies of trials; extracting data from papers; and entering the data into Review Manager. All review authors contributed to analysis and interpretation of the data, and to writing the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; AS‐Akademie, Germany; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK; and the Swiss Society for Endodontology, Switzerland.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
Declarations of interest
There are no financial conflicts of interest and the review authors declare that they do not have any associations with any parties who may have vested interests in the results of this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Bjørnland 2007 {published data only}
- Bjørnland T, Gjaerum AA, Møystad A. Osteoarthritis of the temporomandibular joint: an evaluation of the effects and complications of corticosteroid injection compared with injection with sodium hyaluronate. Journal of Oral Rehabilitation 2007;34(8):583‐9. [DOI] [PubMed] [Google Scholar]
- Møystad A, Mork‐Knutsen BB, Bjørnland T. Injection of sodium hyaluronate compared to a corticosteroid in the treatment of patients with temporomandibular joint osteoarthritis: a CT evaluation. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2008;105(2):e53‐60. [DOI] [PubMed] [Google Scholar]
Mejersjö 2008 {published data only}
- Mejersjö C, Wenneberg B. Diclofenac sodium and occlusal splint therapy in TMJ osteoarthritis: a randomized controlled trial. Journal of Oral Rehabilitation 2008;35(10):729‐38. [DOI] [PubMed] [Google Scholar]
Thie 2001 {published data only}
- Thie NM, Prasad NG, Major PW. Evaluation of glucosamine sulfate compared to ibuprofen for the treatment of temporomandibular joint osteoarthritis: a randomized double blind controlled 3 month clinical trial. Journal of Rheumatology 2001;28(6):1347‐55. [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Badawi 2004 {published data only}
- Al‐Badawi EA, Mehta N, Forgione AG, Lobo SL, Zawawi KH. Efficacy of pulsed radio frequency energy therapy in temporomandibular joint pain and dysfunction. Cranio 2004;22(1):10‐20. [DOI] [PubMed] [Google Scholar]
Ayesh 2008 {published data only}
- Ayesh EE, Jensen TS, Svensson P. Effects of intra‐articular ketamine on pain and somatosensory function in temporomandibular joint arthralgia patients. Pain 2008;137(2):286‐94. [DOI] [PubMed] [Google Scholar]
Bertolami 1993 {published data only}
- Bertolami CN, Gay T, Clark GT, Rendell J, Shetty V, Liu C, Swann DA. Use of sodium hyaluronate in treating temporomandibular joint disorders: a randomized, double‐blind, placebo‐controlled clinical trial. Journal of Oral and Maxillofacial Surgery 1993;51(3):232‐42. [DOI] [PubMed] [Google Scholar]
Bertolucci 1995 {published data only}
- Bertolucci LE, Grey T. Clinical analysis of mid‐laser versus placebo treatment of arthralgic TMJ degenerative joints. Cranio 1995;13(1):26‐9. [DOI] [PubMed] [Google Scholar]
- Bertolucci LE, Grey T. Clinical comparative study of microcurrent electrical stimulation to mid‐laser and placebo treatment in degenerative joint disease of the temporomandibular joint. Cranio 1995;13(2):116‐20. [DOI] [PubMed] [Google Scholar]
Bjørnland 1995 {published data only}
- Bjørnland T, Larheim TA. Synovectomy and diskectomy of the temporomandibular joint in patients with chronic arthritic disease compared with diskectomies in patients with internal derangement. A 3‐year follow‐up study. European Journal of Oral Sciences 1995;103(1):2‐7. [DOI] [PubMed] [Google Scholar]
Di Rienzo Businco 2004 {published data only}
- Rienzo Businco L, Rienzo Businco A, D'Emilia M, Lauriello M, Coen Tirelli G. Topical versus systemic diclofenac in the treatment of temporo‐mandibular joint dysfunction symptoms. Acta Otorhinolaryngologica Italica 2004;24(5):279‐83. [PubMed] [Google Scholar]
Ekberg 1996 {published data only}
- Ekberg EC, Kopp S, Akerman S. Diclofenac sodium as an alternative treatment of temporomandibular joint pain. Acta Odontologica Scandinavica 1996;54(3):154‐9. [DOI] [PubMed] [Google Scholar]
Ekberg 1998 {published data only}
- Ekberg EC, Vallon D, Nilner M. Occlusal appliance therapy in patients with temporomandibular disorders. A double‐blind controlled study in a short‐term perspective. Acta Odontologica Scandinavica 1998;56(2):122‐8. [DOI] [PubMed] [Google Scholar]
Ekberg 2002 {published data only}
- Ekberg E, Vallon D, Nilner M. Treatment outcome of headache after occlusal appliance therapy in a randomised controlled trial among patients with temporomandibular disorders of mainly arthrogenous origin. Swedish Dental Journal 2002;26(3):115‐24. [PubMed] [Google Scholar]
Emshoff 2008 {published data only}
- Emshoff R, Bösch R, Pümpel E, Schöning H, Strobl H. Low‐level laser therapy for treatment of temporomandibular joint pain: a double‐blind and placebo‐controlled trial. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2008;105(4):452‐6. [DOI] [PubMed] [Google Scholar]
Fikácková 2007 {published data only}
- Fikácková H, Dostálová T, Navrátil L, Klaschka J. Effectiveness of low‐level laser therapy in temporomandibular joint disorders: a placebo‐controlled study. Photomedicine and Laser Surgery 2007;25(4):297‐303. [DOI] [PubMed] [Google Scholar]
Funch 1984 {published data only}
- Funch DP, Gale EN. Biofeedback and relaxation therapy for chronic temporomandibular joint pain: predicting successful outcomes. Journal of Consulting and Clinical Psychology 1984;52(6):928‐35. [DOI] [PubMed] [Google Scholar]
Guarda Nardini 2004 {published data only}
- Guarda Nardini L, Oliviero F, Ramonda R, Ferronato G. Influence of intra‐articular injections of sodium hyaluronate on clinical features and synovial fluid nitric oxide levels of temporomandibular osteoarthritis [Influenza delle infiltrazioni intra‐articolari con acido ialuronico sugli indici clinici e sui livelli di ossido nitrico nell’artrosi temporomandibolare]. Reumatismo 2004;56(4):272‐7. [DOI] [PubMed] [Google Scholar]
Guarda Nardini 2005 {published data only}
- Guarda‐Nardini L, Masiero S, Marioni G. Conservative treatment of temporomandibular joint osteoarthrosis: intra‐articular injection of sodium hyaluronate. Journal of Oral Rehabilitation 2005;32(10):729‐34. [DOI] [PubMed] [Google Scholar]
Haddad 2000 {published data only}
- Haddad IK. Temporomandibular joint osteoarthrosis. Histopathological study of the effects of intra‐articular injection of triamcinolone acetonide. Saudi Medical Journal 2000;21(7):675‐9. [PubMed] [Google Scholar]
Ismail 2007 {published data only}
- Ismail F, Demling A, Hessling K, Fink M, Stiesch‐Scholz M. Short‐term efficacy of physical therapy compared to splint therapy in treatment of arthrogenous TMD. Journal of Oral Rehabilitation 2007;34(11):807‐13. [DOI] [PubMed] [Google Scholar]
Israel 2010 {published data only}
- Israel HA, Behrman DA, Friedman JM, Silberstein J. Rationale for early versus late intervention with arthroscopy for treatment of inflammatory/degenerative temporomandibular joint disorders. Journal of Oral and Maxillofacial Surgery 2010;68(11):2661‐7. [DOI] [PubMed] [Google Scholar]
Jones 2011 {published data only}
- Jones RH. Temporomandibular joint reconstruction with total alloplastic joint replacement. Australian Dental Journal 2011;56(1):85‐91. [DOI] [PubMed] [Google Scholar]
Kopp 1985 {published data only}
- Kopp S, Carlsson GE, Haraldson T, Wenneberg B. Long‐term effect of intra‐articular injections of sodium hyaluronate and corticosteroid on temporomandibular joint arthritis. Journal of Oral and Maxillofacial Surgery 1987;45(11):929‐35. [DOI] [PubMed] [Google Scholar]
- Kopp S, Wenneberg B, Haraldson T, Carlsson GE. The short‐term effect of intra‐articular injections of sodium hyaluronate and corticosteroid on temporomandibular joint pain and dysfunction. Journal of Oral and Maxillofacial Surgery 1985;43(6):429‐35. [DOI] [PubMed] [Google Scholar]
Kulekcioglu 2003 {published data only}
- Kulekcioglu S, Sivrioglu K, Ozcan O, Parlak M. Effectiveness of low‐level laser therapy in temporomandibular disorder. Scandinavian Journal of Rheumatology 2003;32(2):114‐8. [DOI] [PubMed] [Google Scholar]
Kurita 2007 {published data only}
- Kurita H, Chen Z, Uehara S, Miyazawa H, Kurashina K. Comparison of imaging follow‐up between joints with arthroscopic surgery (lysis and lavage) and those with nonsurgical treatment. Journal of Oral and Maxillofacial Surgery 2007;65(7):1309‐14. [DOI] [PubMed] [Google Scholar]
List 2001 {published data only}
- List T, Tegelberg A, Haraldson T, Isacsson G. Intra‐articular morphine as analgesic in temporomandibular joint arthralgia/osteoarthritis. Pain 2001;94(3):275‐82. [DOI] [PubMed] [Google Scholar]
Machon 2011 {published data only}
- Machon V, Hirjak D, Lukas J. Therapy of the osteoarthritis of the temporomandibular joint. Journal of Craniomaxillofacial Surgery 2011;39(2):127‐30. [DOI] [PubMed] [Google Scholar]
Maloney 2002 {published data only}
- Maloney GE, Mehta N, Forgione AG, Zawawi KH, Al‐Badawi EA, Driscoll SE. Effect of a passive jaw motion device on pain and range of motion in TMD patients not responding to flat plane intraoral appliances. Cranio 2002;20(1):55‐66. [DOI] [PubMed] [Google Scholar]
Maluf 2010 {published data only}
- Maluf SA, Moreno BG, Crivello O, Cabral CM, Bortolotti G, Marques AP. Global postural reeducation and static stretching exercises in the treatment of myogenic temporomandibular disorders: a randomized study. Journal of Manipulative and Physiological Therapeutics 2010;33(7):500‐7. [DOI] [PubMed] [Google Scholar]
Marini 2010 {published data only}
- Marini I, Gatto MR, Bonetti GA. Effects of superpulsed low‐level laser therapy on temporomandibular joint pain. Clinical Journal of Pain 2010;26(7):611‐6. [DOI] [PubMed] [Google Scholar]
Nguyen 2001 {published data only}
- Nguyen P, Mohamed SE, Gardiner D, Salinas T. A randomized double‐blind clinical trial of the effect of chondroitin sulfate and glucosamine hydrochloride on temporomandibular joint disorders: a pilot study. Cranio 2001;19(2):130‐9. [DOI] [PubMed] [Google Scholar]
Nilsson 2009 {published data only}
- Nilsson H. Resilient appliance therapy of temporomandibular disorders. Subdiagnoses, sense of coherence and treatment outcome. Swedish Dental Journal. Supplement 2010;(206):9‐88. [PubMed] [Google Scholar]
- Nilsson H, Limchaichana N, Nilner M, Ekberg EC. Short‐term treatment of a resilient appliance in TMD pain patients: a randomized controlled trial. Journal of Oral Rehabilitation 2009;36(8):547‐55. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Vallon D, Ekberg EC. Long‐term efficacy of resilient appliance therapy in TMD pain patients: a randomised, controlled trial. Journal of Oral Rehabilitation 2011;38(10):713‐21. [DOI] [PubMed] [Google Scholar]
Ohnuki 2006 {published data only}
- Ohnuki T, Fukuda M, Nakata A, Nagai H, Takahashi T, Sasano T, et al. Evaluation of the position, mobility, and morphology of the disc by MRI before and after four different treatments for temporomandibular joint disorders. Dentomaxillofacial Radiology 2006;35(2):103‐9. [DOI] [PubMed] [Google Scholar]
Okeson 1983 {published data only}
- Okeson JP, Moody PM, Kemper JT, Haley JV. Evaluation of occlusal splint therapy and relaxation procedures in patients with temporomandibular disorders. Journal of the American Dental Association 1983;107(3):420‐4. [DOI] [PubMed] [Google Scholar]
Peroz 2004 {published data only}
- Peroz I, Chun YH, Karageorgi G, Schwerin C, Bernhardt O, Roulet JF, et al. A multicenter clinical trial on the use of pulsed electromagnetic fields in the treatment of temporomandibular disorders. Journal of Prosthetic Dentistry 2004;91(2):180‐7. [DOI] [PubMed] [Google Scholar]
Reid 1994 {published data only}
- Reid KI, Dionne RA, Sicard‐Rosenbaum L, Lord D, Dubner RA. Evaluation of iontophoretically applied dexamethasone for painful pathologic temporomandibular joints. Oral Surgery, Oral Medicine, and Oral Pathology 1994;77(6):605‐9. [DOI] [PubMed] [Google Scholar]
Schmid‐Schwap 2006 {published data only}
- Schmid‐Schwap M, Simma‐Kletschka I, Stockner A, Sengstbratl M, Gleditsch J, Kundi M, et al. Oral acupuncture in the therapy of craniomandibular dysfunction syndrome ‐ a randomized controlled trial. Wiener Klinische Wochenschrift 2006;118(1‐2):36‐42. [DOI] [PubMed] [Google Scholar]
Shi 2001 {published data only}
- Shi Z, Yang F, Shi B. Randomized controlled trial to assess effect of hyaluronate for degenerative disorders of the temporomandibular joint. Third National Symposium on Temporomandibular Disorders. 2001.
- Shi ZD, Yang F, Zhang JY, Shi B. Randomized controlled trial of sodium hyaluronate for degenerative disorders of the temporomandibular joint. Chung‐Kuo Hsiu Fu Chung Chien Wai Ko Tsa Chih 2002;16(1):11‐5. [PubMed] [Google Scholar]
Stegenga 1993 {published data only}
- Stegenga B, Bont LG, Dijkstra PU, Boering G. Short‐term outcome of arthroscopic surgery of temporomandibular joint osteoarthrosis and internal derangement: a randomized controlled clinical trial. British Journal of Oral and Maxillofacial Surgery 1993;31(1):3‐14. [DOI] [PubMed] [Google Scholar]
Stowell 2007 {published data only}
- Stowell AW, Gatchel RJ, Wildenstein L. Cost‐effectiveness of treatments for temporomandibular disorders: biopsychosocial intervention versus treatment as usual. Journal of the American Dental Association 2007;138(2):202‐8. [DOI] [PubMed] [Google Scholar]
Truelove 2006 {published data only}
- Truelove E, Huggins KH, Mancl L, Dworkin SF. The efficacy of traditional, low‐cost and nonsplint therapies for temporomandibular disorder: a randomized controlled trial. Journal of the American Dental Association 2006;137(8):1099‐107. [DOI] [PubMed] [Google Scholar]
Turk 1993 {published data only}
- Turk DC, Zaki HS, Rudy TE. Effects of intraoral appliance and biofeedback/stress management alone and in combination in treating pain and depression in patients with temporomandibular disorders. Journal of Prosthetic Dentistry 1993;70(2):158‐64. [DOI] [PubMed] [Google Scholar]
Turner 2006 {published data only}
- Turner JA, Mancl L, Aaron LA. Short‐ and long‐term efficacy of brief cognitive‐behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain 2006;121(3):181‐94. [DOI] [PubMed] [Google Scholar]
Winocur 2000 {published data only}
- Winocur E, Gavish A, Halachmi M, Eli I, Gazit E. Topical application of capsaicin for the treatment of localized pain in the temporomandibular joint area. Journal of Orofacial Pain 2000;14(1):31‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Tang 2010 {published data only}
- Tang YL, Zhu GQ, Hu L, Zheng M, Zhang JY, Shi ZD, et al. Effects of intra‐articular administration of sodium hyaluronate on plasminogen activator system in temporomandibular joints with osteoarthritis. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2010;109(4):541‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Arnett 1996
- Arnett GW, Milam SB, Gottesman L. Progressive mandibular retrusion‐idiopathic condylar resorption. Part I. American Journal of Orthodontics and Dentofacial Orthopedics 1996;110:8‐15. [DOI] [PubMed] [Google Scholar]
Chapman 2001
- Chapman MW. Chapman's Orthopaedic Surgery. 3rd Edition. Philadelphia: Lippincott William's & Wilkins, 2001. [Google Scholar]
Clark 1984
- Clark GT. A critical evaluation of orthopedic interocclusal appliance therapy: design, theory, and overall effectiveness. Journal of the American Dental Association 1984;108(3):359‐64. [DOI] [PubMed] [Google Scholar]
de Bont 1997
- Bont LG, Dijkgraaf LC, Stegenga B. Epidemiology and natural progression of articular temporomandibular disorders. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 1997;83(1):72‐6. [DOI] [PubMed] [Google Scholar]
De Laat 2003
- Laat A, Stappaerts K, Papy S. Counseling and physical therapy as treatment for myofascial pain of the masticatory system. Journal of Orofacial Pain 2003;17(1):42‐9. [PubMed] [Google Scholar]
de Leeuw 1995
- Leeuw R, Boering G, Stegenga B, Bont LG. Symptoms of temporomandibular joint osteoarthrosis and internal derangement 30 years after non‐surgical treatment. Cranio 1995;13(2):81‐8. [DOI] [PubMed] [Google Scholar]
Dimitroulis 2005
- Dimitroulis G. The role of surgery in the management of disorders of the temporomandibular joint: a critical review of the literature. Part 2. International Journal of Oral Maxillofacial Surgery 2005;34(3):231‐7. [DOI] [PubMed] [Google Scholar]
Dingman 1966
- Dingman RO, Grabb WC. Intracapsular temporomandibular joint arthroplasty. Plastic and Reconstructive Surgery 1966;38(3):179‐85. [DOI] [PubMed] [Google Scholar]
Dionne 2006
- Dionne RA. Pharmacologic approaches. In: Laskin DM, Greene CS, Hylander WL editor(s). TMDs, an Evidence‐Based Approach to Diagnosis and Treatment. Chicago: Quintessence, 2006. [Google Scholar]
Dworkin 1992
- Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. Journal of Craniomandibular Disorders: Facial & Oral Pain 1992;6(4):301‐55. [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Epstein 2001
- Epstein JB, Caldwell J, Black G. The utility of panoramic imaging of the temporomandibular joint in patients with temporomandibular disorders. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontics 2001;92(2):236‐9. [DOI] [PubMed] [Google Scholar]
Feinberg 1989
- Feinberg SE, Larsen PE. The use of a pedicled temporalis muscle‐pericranial flap for replacement of the TMJ disc: preliminary report. Journal of Oral and Maxillofacial Surgery 1989;47(2):142‐6. [DOI] [PubMed] [Google Scholar]
Forssell 2004
- Forssell H, Kalso E. Application of principles of evidence‐based medicine to occlusal treatment for temporomandibular disorders: are there lessons to be learned?. Journal of Orofacial Pain 2004;18(1):23‐32. [PubMed] [Google Scholar]
Furukawa 2007
- Furukawa TA, Watanabe N, Omori IM, Montori VM, Guyatt GH. Association between unreported outcomes and effect size estimates in Cochrane meta‐analyses. JAMA 2007;297(5):468‐70. [DOI] [PubMed] [Google Scholar]
Gray 1983
- Gray RG, Gottlieb NL. Intra‐articular corticosteroids. An updated assessment. Clinical Orthopaedics and Related Research 1983;177:235‐63. [PubMed] [Google Scholar]
Guo 2009
- Guo C, Shi Z, Revington P. Arthrocentesis and lavage for treating temporomandibular joint disorders. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004973.pub2] [DOI] [PubMed] [Google Scholar]
Hasanain 2009
- Hasanain F, Durham J, Moufti A, Steen IN, Wassell RW. Adapting the diagnostic definitions of the RDC/TMD to routine clinical practice: a feasibility study. Journal of Dentistry 2009;37(12):955‐62. [DOI] [PubMed] [Google Scholar]
Haskin 1995
- Haskin CL, Milam SB, Cameron IL. Pathogenesis of degenerative joint disease in the human temporomandibular joint. Critical Reviews in Oral Biology and Medicine 1995;6(3):248‐77. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmlund 1986
- Holmlund A, Hellsing G, Wredmark T. Arthroscopy of the temporomandibular joint. A clinical study. International Journal of Oral and Maxillofacial Surgery 1986;15(6):715‐21. [DOI] [PubMed] [Google Scholar]
Huumonen 2007
- Huumonen S, Sipila K, Zitting P, Raustia AM. Panoramic findings in 34‐year‐old subjects with facial pain and pain‐free controls. Journal of Oral Rehabilitation 2007;34(6):456‐62. [DOI] [PubMed] [Google Scholar]
Israel 1997
- Israel HA, Diamond BE, Saed‐Nejad F, Ratcliffe A. Correlation between arthroscopic diagnosis of osteoarthritis and synovitis of the human temporomandibular joint and keratan sulfate levels in the synovial fluid. Journal of Oral and Maxillofacial Surgery 1997;55(3):210‐7. [DOI] [PubMed] [Google Scholar]
Israel 1998
- Israel HA, Diamond B, Saed‐Nejad F, Ratcliffe A. Osteoarthritis and synovitis as major pathoses of the temporomandibular joint: comparison of clinical diagnosis with arthroscopic morphology. Journal of Oral and Maxillofacial Surgery 1998;56(9):1023‐7. [DOI] [PubMed] [Google Scholar]
John 2005
- John MT, Dworkin SF, Mancl LA. Reliability of clinical temporomandibular disorder diagnoses. Pain 2005;118(1‐2):61‐9. [DOI] [PubMed] [Google Scholar]
Kang 2007
- Kang SC, Lee DG, Choi JH, Kim ST, Kim YK, Ahn HJ. Association between estrogen receptor polymorphism and pain susceptibility in female temporomandibular joint osteoarthritis patients. International Journal of Oral and Maxillofacial Surgery 2007;36(5):391‐4. [DOI] [PubMed] [Google Scholar]
Kino 2005
- Kino K, Sugisaki M, Haketa T, Amemori Y, Ishikawa T, Shibuya T, et al. The comparison between pains, difficulties in function, and associating factors of patients in subtypes of temporomandibular disorders. Journal of Oral Rehabilitation 2005;32(5):315‐25. [DOI] [PubMed] [Google Scholar]
Koh 2003
- KohH, Robinson P. Occlusal adjustment for treating and preventing temporomandibular joint disorders. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003812] [DOI] [PubMed] [Google Scholar]
Kuttila 2002
- Kuttila M, Bell Y, Savolainen‐Niemi E, Kuttila S, Alanen P. Efficiency of occlusal appliance therapy in secondary otalgia and temporomandibular disorders. Acta Odontologica Scandinavica 2002;60(4):248‐54. [DOI] [PubMed] [Google Scholar]
Larheim 2005
- Larheim TA. Role of magnetic resonance imaging in the clinical diagnosis of the temporomandibular joint. Cells, Tissues, Organs 2005;180(1):6‐21. [DOI] [PubMed] [Google Scholar]
Laskin 2007
- Laskin DM. Temporomandibular disorders: the past, present, and future. Odontology 2007;95(1):10‐5. [DOI] [PubMed] [Google Scholar]
Lohmander 1996
- Lohmander LS, Dalén N, Englund G, Hämäläinen M, Jensen EM, Karlsson K, et al. Intra‐articular hyaluronan injections in the treatment of osteoarthritis of the knee: a randomised, double blind, placebo controlled multicentre trial. Hyaluronan Multicentre Trial Group. Annals of the Rheumatic Diseases 1997;56(7):441. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacIntosh 2000
- MacIntosh RB. The use of autogenous tissues for temporomandibular joint reconstruction. Journal of Oral and Maxillofacial Surgery 2000;58(1):63‐9. [DOI] [PubMed] [Google Scholar]
Manfredini 2006
- Manfredini D, Chiappe G, Bosco M. Research diagnostic criteria for temporomandibular disorders (RDC/TMD) axis I diagnoses in an Italian patient population. Journal of Oral Rehabilitation 2006;33(8):551‐8. [DOI] [PubMed] [Google Scholar]
McBride 1994
- McBride Kl. Total temporomandibular joint reconstruction. In: Bell WH editor(s). Modern Practice in Orthognathic and Reconstructive Surgery. Philadelphia: WB Saunders, 1994:381‐96. [Google Scholar]
Mercuri 1998
- Mercuri LG. Alloplastic temporomandibular joint reconstruction. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology & Endodontics 1998;85(6):631‐7. [DOI] [PubMed] [Google Scholar]
Mercuri 1999
- Mercuri LG. Considering total temporomandibular joint replacement. Cranio 1999;17(1):44‐8. [DOI] [PubMed] [Google Scholar]
Mercuri 2006
- Mercuri LG. Surgical management of TMJ arthritis. In: Laskin DM, Greene CS, Hylander WL editor(s). TMDs, an Evidence‐Based Approach to Diagnosis and Treatment. Chicago: Quintessence, 2006:455‐68. [Google Scholar]
Mercuri 2008
- Mercuri LG. Arthritis, osteoarthrosis, and idiopathic condylar resorption. Oral and Maxillofacial Surgery Clinics of North America 2008;20(2):169‐83. [DOI] [PubMed] [Google Scholar]
Milam 2006
- Milam SB. TMJ osteoarthritis. In: Laskin DM, Greene CS, Hylander WL editor(s). TMDs, an Evidence‐Based Approach to Diagnosis and Treatment. Chicago: Quintessence, 2006. [Google Scholar]
Nicolakis 2001
- Nicolakis P, Burak EC, Kollmitzer J, Kopf A, Piehslinger E, Wiesinger GF, et al. An investigation of the effectiveness of exercise and manual therapy in treating symptoms of TMJ osteoarthritis. Cranio 2001;19(1):26‐32. [DOI] [PubMed] [Google Scholar]
Nitzan 2001a
- Nitzan DW. The process of lubrication impairment and its involvement in temporomandibular joint disc displacement: a theoretical concept. Journal of Oral and Maxillofacial Surgery 2001;59(1):36‐45. [DOI] [PubMed] [Google Scholar]
Nitzan 2001b
- Nitzan DW, Price A. The use of arthrocentesis for the treatment of osteoarthritic temporomandibular joints. Journal of Oral and Maxillofacial Surgery 2001;59(10):1154‐9. [DOI] [PubMed] [Google Scholar]
Reston 2003
- Reston JT, Turkelson CM. Meta‐analysis of surgical treatments for temporomandibular articular disorders. Journal of Oral and Maxillofacial Surgery 2003;61(1):3‐10. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rouas 2006
- Rouas P, Bandon D, Nancy J, Delbos Y, Hauret L, Bar D. Digital volume tomography using the NewTom system: advantages of this new technique in children [La tomographie volumétrique numérisée par le système NewTom : intérêt de ce nouvel examen d'imagerie médicale chez l'enfant]. Archives de Pédiatrie 2006;13(8):1169‐77. [DOI] [PubMed] [Google Scholar]
Shi 2003
- Shi Z, Guo C, Awad M. Hyaluronate for temporomandibular joint disorders. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD002970] [DOI] [PubMed] [Google Scholar]
Stegenga 1989
- Stegenga B, Bont LG, Boering G. Osteoarthrosis as the cause of craniomandibular pain and dysfunction: a unifying concept. Journal of Oral and Maxillofacial Surgery 1989;47(3):249‐56. [DOI] [PubMed] [Google Scholar]
Stegenga 2006
- Stegenga B, Bont LGM. TMJ disc derangements. In: Laskin DM, Greene CS, Hylander WL editor(s). TMDs, an Evidence‐Based Approach to Diagnosis and Treatment. Chicago: Quintessence, 2006. [Google Scholar]
Tanaka 2008
- Tanaka E, Detamore MS, Mercuri LG. Degenerative disorders of the temporomandibular joint: etiology, diagnosis, and treatment. Journal of Dental Research 2008;87(4):296‐307. [DOI] [PubMed] [Google Scholar]
Toller 1977
- Toller PA. Use and misuse of intra‐articular corticosteroids in treatment of temporomandibular joint pain. Proceedings of the Royal Society of Medicine 1977;70(7):461‐3. [PMC free article] [PubMed] [Google Scholar]
van Strijen 2001
- Strijen PJ, Breuning KH, Becking AG, Tuinzing DB. Condylar resorption following distraction osteogenesis: a case report. Journal of Oral and Maxillofacial Surgery 2001;59(9):1104‐7. [DOI] [PubMed] [Google Scholar]
Wolford 2003
- Wolford LM, Reiche‐Fischel O, Mehra P. Changes in temporomandibular joint dysfunction after orthognathic surgery. Journal of Oral and Maxillofacial Surgery 2003;61(6):655‐60. [DOI] [PubMed] [Google Scholar]


