Abstract
Background
This is the second update of the review first published in the Cochrane Library (2010, Issue 2) and later updated (2014, Issue 9).
Despite advances in chemotherapy, the prognosis of ovarian cancer remains poor. Antigen‐specific active immunotherapy aims to induce tumour antigen‐specific anti‐tumour immune responses as an alternative treatment for ovarian cancer.
Objectives
Primary objective • To assess the clinical efficacy of antigen‐specific active immunotherapy for the treatment of ovarian cancer as evaluated by tumour response measured by Response Evaluation Criteria In Solid Tumors (RECIST) and/or cancer antigen (CA)‐125 levels, response to post‐immunotherapy treatment, and survival differences
◦ In addition, we recorded the numbers of observed antigen‐specific humoral and cellular responses Secondary objective • To establish which combinations of immunotherapeutic strategies with tumour antigens provide the best immunological and clinical results
Search methods
For the previous version of this review, we performed a systematic search of the Cochrane Central Register of Controlled Trials (CENTRAL; 2009, Issue 3), in the Cochrane Library, the Cochrane Gynaecological Cancer Group Specialised Register, MEDLINE and Embase databases, and clinicaltrials.gov (1966 to July 2009). We also conducted handsearches of the proceedings of relevant annual meetings (1996 to July 2009).
For the first update of this review, we extended the searches to October 2013, and for this update, we extended the searches to July 2017.
Selection criteria
We searched for randomised controlled trials (RCTs), as well as non‐randomised studies (NRSs), that included participants with epithelial ovarian cancer, irrespective of disease stage, who were treated with antigen‐specific active immunotherapy, irrespective of type of vaccine, antigen used, adjuvant used, route of vaccination, treatment schedule, and reported clinical or immunological outcomes.
Data collection and analysis
Two reviews authors independently extracted the data. We evaluated the risk of bias for RCTs according to standard methodological procedures expected by Cochrane, and for NRSs by using a selection of quality domains deemed best applicable to the NRS.
Main results
We included 67 studies (representing 3632 women with epithelial ovarian cancer). The most striking observations of this review address the lack of uniformity in conduct and reporting of early‐phase immunotherapy studies. Response definitions show substantial variation between trials, which makes comparison of trial results unreliable. Information on adverse events is frequently limited. Furthermore, reports of both RCTs and NRSs frequently lack the relevant information necessary for risk of bias assessment. Therefore, we cannot rule out serious biases in most of the included trials. However, selection, attrition, and selective reporting biases are likely to have affected the studies included in this review. GRADE ratings were high only for survival; for other primary outcomes, GRADE ratings were very low.
The largest body of evidence is currently available for CA‐125‐targeted antibody therapy (17 studies, 2347 participants; very low‐certainty evidence). Non‐randomised studies of CA‐125‐targeted antibody therapy suggest improved survival among humoral and/or cellular responders, with only moderate adverse events. However, four large randomised placebo‐controlled trials did not show any clinical benefit, despite induction of immune responses in approximately 60% of participants. Time to relapse with CA‐125 monoclonal antibody versus placebo, respectively, ranged from 10.3 to 18.9 months versus 10.3 to 13 months (six RCTs, 1882 participants; high‐certainty evidence). Only one RCT provided data on overall survival, reporting rates of 80% in both treatment and placebo groups (three RCTs, 1062 participants; high‐certainty evidence). Other small studies targeting many different tumour antigens have presented promising immunological results. As these strategies have not yet been tested in RCTs, no reliable inferences about clinical efficacy can be made. Given the promising immunological results and the limited side effects and toxicity reported, exploration of clinical efficacy in large well‐designed RCTs may be worthwhile.
Authors' conclusions
We conclude that despite promising immunological responses, no clinically effective antigen‐specific active immunotherapy is yet available for ovarian cancer. Results should be interpreted cautiously, as review authors found a significant dearth of relevant information for assessment of risk of bias in both RCTs and NRSs.
Plain language summary
Antigen‐specific active immunotherapy for ovarian cancer
Background Ovarian cancer is the leading cause of death from gynaecological cancers. Standard therapy consists of surgery and chemotherapy. Responses to chemotherapy are generally good; however, most women experience relapse, for which no curative treatment is available. The presence of certain immune cells in tumours is associated with longer survival. This suggests that stimulation of anti‐tumour immune responses (i.e. immunotherapy) might be a useful approach for improving outcomes among women with ovarian cancer.
Review question This review evaluated the feasibility of antigen‐specific active immunotherapy. Antigen‐specific active immunotherapy aims to induce anti‐tumour immune responses through administration of a tumour antigen ‐ a molecule that is expressed by tumour cells and is hardly expressed by healthy cells. Reviewers collected information on clinical outcomes, immunological responses, and side effects.
Main findings We identified 67 studies, which included 3632 women with ovarian cancer and were published between 1966 and 2017. The most frequently described strategy was administration of antibodies targeting the tumour antigen CA‐125 (2347 participants in 17 studies). Most of these studies primarily evaluated safety and immunological responses. Severe flu‐like and gastrointestinal symptoms occurred in 7% to 30% of participants. Researchers frequently detected antibodies and immune cells recognising the tumour antigen CA‐125, albeit response rates varied between studies. Despite these promising immunological responses, four large studies reported no survival advantage for participants treated with CA‐125‐directed antibody over those given placebo.
For strategies not relying on antibody administration, similar conclusions cannot yet be drawn. Overall, study authors report that treatment was well tolerated and inflammatory side effects at the injection site were most frequently observed. Researchers observed responses of the immune system for most strategies studied, but the clinical benefit of these strategies remains to be evaluated in large trials.
Certainty of the evidence and conclusions Because no high‐certainty evidence of clinical benefit is currently available, antibody therapy targeting CA‐125 should not be incorporated into standard treatment in its current form.
Based on lack of uniformity in included studies, we strongly advocate universal adoption of response definitions, guidelines for adverse events reporting, and directives for trial conduct and reporting. Furthermore, results from ongoing randomised controlled trials (RCTs) are awaited, and further RCTs should be conducted.
Summary of findings
Summary of findings for the main comparison. Antigen‐specific immunotherapy for ovarian carcinoma.
| Antigen‐specific immunotherapy for ovarian carcinoma | |||
| Patient or population: ovarian carcinoma Setting: primary and recurrent ovarian carcinoma Intervention: antigen‐specific immunotherapy | |||
| Outcomes | Impact | № of participants (studies) | Certainty of the evidence (GRADE) |
| Tumour response assessed with: RECIST | In total, 2 participants (0.01%) were defined as having a complete response, 9 (0.03%) had a partial response, and 50 (14%) had stable disease. Twelve participants (0.03%) showed no evidence of disease. Finally, 218 (61%) participants had progressive disease. The remaining 64 (18%) participants were not mentioned. | 355 (17 observational studies) | ⊕⊝⊝⊝ Very lowa,b,c,d |
| Tumour response assessed with: CA‐125 according to GCIG criteria | In total, 8 participants (13%) were reported to have an increase in CA‐125. In 22 patients, CA‐125 was stable or decreasing (34%). The remaining 34 participants (53%) were considered not evaluable or were not mentioned. | 64 (6 observational studies) | ⊕⊝⊝⊝ Very lowa,b,c,d,e |
| Post‐immunotherapy treatment response assessed with: survival | Two studies suggested that antigen‐specific immunotherapy may lead to improved responses to future therapy. Two studies revealed no evidence of a difference. | 88 (4 observational studies) | ⊕⊝⊝⊝ Very lowa,f |
| Survival assessed with: overall survival | None of the 3 RCTs estimating overall survival found a significant difference in overall survival. Two studies of CA‐125 monoclonal antibody vs placebo evaluated overall survival, respectively, at 57.5 vs 48.6 months (95% CI 041 to 1.25) and 80% survival for both groups. | 1062 (3 RCTs) | ⊕⊕⊕⊕ High |
| Survival assessed with: progression‐free survival/time to relapse | None of the 6 RCTs found statistically significant differences in progression‐free survival/time to relapse, including 4 RCTs evaluating CA‐125 monoclonal antibody vs placebo; time to relapse ranged from 10.3 to 18.9 months vs 10.3 to 13 months, respectively. | 1882 (6 RCTs) | ⊕⊕⊕⊕ High |
| Antigen‐specific immunogenicity (humoral response) assessed with: ELISA/Luminex assay | Nine studies evaluated anti‐idiotopic (Ab2) humoral response, with responses ranging from 3% to 100%. Ten studies evaluated anti‐anti‐idiotropic (Ab3) humoral response, with responses ranging from 0% to 100%. Two studies observed no humoral response to other antigen‐specific immunotherapy, and the 9 remaining studies noted large differences in percentages of participants with measurable antigen‐specific antibodies (IgG: 8% to 96%). | 1521 (25 observational studies) | ⊕⊝⊝⊝ Very lowa,d,g |
| Antigen‐specific immunogenicity (cellular response) assessed with: e.g. IFN‐γ ELISPOT/proliferation assay/IFN‐γ secretion assay | A total of 39 studies showed an induced cellular immune response in at least 1 cohort and to at least 1 target antigen; range of positive response varied broadly between 18% and 100%. One study retrospectively compared cellular immune response after CA‐125 monoclonal antibody treatment vs placebo but showed no significant differences (31.8% intervention vs 26.3% control). | 966 (40 observational studies) | ⊕⊝⊝⊝ Very lowa,d,g,h |
| Ab2: anti‐idiotopic; Ab3: anti‐anti‐idiotopic; CA: cancer antigen; CI: confidence interval; ELISA: enzyme‐linked immunosorbent assay; GCIG: Gynecologic Cancer Intergroup; IFN: interferon; RCTs: randomised controlled trials; RECIST: Response Evaluation Criteria In Solid Tumors. | |||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
aMost studies were uncontrolled phase I/II trials. bA large percentage of the included participants were not mentioned or were not evaluable for the analysis. cExplicit descriptions of tumour responses per participant and the time points at which evaluations took place frequently were not available. dDisease status at start of treatment differed among studies. Therefore the likelihood of clinical and immune responses to immunotherapy, especially in uncontrolled studies, which frequently include participants with recurrent disease and previous exposure to different types of therapy, is likely to be affected. eCA‐125 is a biomarker that serves as an indication for response; however CA‐125 does not directly reflect tumour size. fAlthough in one study participants with a complete response had strong humoral responses, similar or stronger antibody responses were observed for participants with stable or progressive disease. gBetween studies, there were broad differences in (1) response definition, (2) number of treatment cycles after which immune responses were measured, and (3) targeted antigens. hExplicit descriptions of immune responses per participant and the time points at which evaluations took place, types of evaluations, and when an evaluation was considered positive often were not available.
Background
Description of the condition
Ovarian cancer is the sixth most common cancer and the seventh most common cause of death from cancer among women worldwide (Torre 2012). It is the second most common gynaecological cancer and the leading cause of death from gynaecological cancers in the Western world. As most ovarian malignancies (80% to 90%) arise from the epithelium, all statements about ovarian cancer presented in the remainder of this review apply to epithelial ovarian cancer only. Worldwide age‐standardised incidence rates range from 5 per 100,000 in less developed areas to 9.1 per 100,000 in developed areas (Torre 2012).
Stage of disease at presentation is the most important prognostic factor. Owing to the asymptomatic course of the disease, most participants have extensive disease at presentation (stage III to IV, according to the International Federation of Gynecology and Obstetrics (FIGO) classification (Prat 2015)). Despite standard treatment, which consists of cytoreductive surgery and platinum‐based chemotherapy, almost all women with advanced‐stage disease at presentation will experience relapse, with median progression‐free survival of only 18 months. When residual or recurrent disease manifests itself, resistance to chemotherapy often prohibits further curative therapy, resulting in disease‐specific five‐year survival for women with advanced‐stage ovarian disease of only 10% to 20% (Agarwal 2006; Thigpen 2000).
Description of the intervention
The immune system seems to play a role in ovarian cancer. This is reflected in the observation that in more than half of women with ovarian cancer, T‐cells are present within tumour islets (Raspollini 2005; Zhang 2003). Women with advanced ovarian cancer, whose tumour is infiltrated by these T‐cells, have better clinical outcomes than women without these tumour‐infiltrating T‐cells (Dong 2006; Raspollini 2005; Zhang 2003). More specifically, higher numbers of cytotoxic T‐cells, which can directly recognise and kill tumour cells, and increased ratios between cytotoxic T‐cells (CD8+) and helper T‐cells (CD4+) within the tumour epithelium are associated with improved survival (Gooden 2011; Sato 2005).
Immunotherapy is one of the novel therapeutic strategies under investigation for ovarian cancer. It aims to induce or enhance active immune responses directed towards the tumour and to consolidate anti‐tumour effects of standard therapy, delaying and possibly preventing disease progression. Antigen‐specific active immunotherapy aims to activate the adaptive immune system directed towards a specific target antigen through administration of a molecularly defined antigen‐specific vaccine to the patient.
How the intervention might work
An antigen is a molecule ‐ usually a protein or a polysaccharide ‐ that can stimulate an immune response. Tumour antigens can be subdivided into different categories such as mutated self‐proteins, products of oncogenes (e.g. Her‐2/Neu), mutated tumour suppressor genes (e.g. p53), and aberrantly expressed self‐proteins (e.g. sperm protein 17, MAGE‐1). Numerous tumour‐associated antigens are known in ovarian cancer. To obtain a tumour‐specific immune response, immunotherapy exploits the differential expression of antigens between normal and tumour cells. A major challenge related to the safety of immunotherapy lies in the prevention of autoimmunity (i.e. induction of immune cells that preferentially recognise and kill tumour cells while avoiding destruction of normal body cells). From a theoretical point of view, other possible side effects include allergic reactions to components of the vaccine and inflammatory reactions at the site of injection.
Why it is important to do this review
Researchers are now employing several immunotherapeutic strategies by using different tumour antigens. However, this research generally has not yet evolved past phase I/II studies. To our knowledge, no systematic review of antigen‐specific active immunotherapy in ovarian cancer has been carried out so far. This review evaluates the immunogenicity and clinical efficacy of antigen‐specific active immunotherapy in ovarian cancer. A systematic review about this topic should prove useful for ascertaining the effectiveness of this treatment modality for ovarian cancer.
Objectives
Primary objective
-
To assess the clinical efficacy of antigen‐specific active immunotherapy for the treatment of ovarian cancer as evaluated by tumour response measured by Response Evaluation Criteria In Solid Tumors (RECIST) and/or cancer antigen (CA)‐125 levels, response to post‐immunotherapy treatment, and survival differences
In addition, we recorded the numbers of observed antigen‐specific humoral and cellular responses
Secondary objective
To establish which combinations of immunotherapeutic strategies with tumour antigens provide the best immunological and clinical results
Methods
Criteria for considering studies for this review
Types of studies
We had anticipated that we would identify limited randomised controlled trials (RCTs) on this topic. Therefore, we included phase I and phase II non‐randomised studies (NRSs) and phase III RCTs. We realise that results from NRSs cannot readily be extrapolated to the general population, but given the lack of RCTs, inclusion of these studies in the review was justifiable.
Types of participants
We included women with a diagnosis of epithelial ovarian cancer, irrespective of stage of disease. However, as patient populations may differ substantially between different types of studies to be included in this review, we documented what type of participant was included in each study (e.g. women with end‐stage disease, women with residual disease).
Because we anticipated that we would find few studies that included women with ovarian cancer only, we also included immunotherapeutic studies in people with cancer that included at least two women with ovarian cancer, with the additional requirement that the results for these individual women were separately identifiable from those of the study publication or could be obtained by communication with the study author, and we extracted only data on these women for inclusion in the review. We are fully aware of the vigilance necessary when conclusions are based on studies with such small numbers, but we believe that given the anticipated lack of large RCTs, inclusion of these studies in this review is justifiable.
Types of interventions
Antigen‐specific active immunotherapy is defined as therapy that aims to induce an adaptive immune response directed towards the tumour through administration of a specific well‐defined tumour antigen. We compared interventions against each other based on the above‐mentioned characteristics.
We included all interventions that aimed to provide antigen‐specific active immunotherapy, irrespective of type of vaccine, antigen, or adjuvant used; route of vaccination; and vaccination schedule.
Types of outcome measures
Primary outcomes
Clinical efficacy
To assess clinical efficacy, we evaluated the following.
-
Tumour responses to immunotherapy (complete/partial response, stable/progressive disease), as measured by:
cancer antigen (CA)‐125 levels according to or transposable to Gynecologic Cancer Intergroup (GCIG) criteria (Rustin 2004); or
tumour response according to World Health Organization (WHO) criteria ‐ WHO 1979 ‐ or Response Evaluation Criteria in Solid Tumors (RECIST) criteria ‐ Therasse 2000.
We evaluated responses to post‐immunotherapy treatment, as evidence suggests that people with small cell lung cancer treated with chemotherapy after immunotherapy have improved survival as opposed to people who do not receive immunotherapy (Antonia 2006).
-
We assessed:
survival differences, including time to relapse or progression‐free survival, based on treatment with immunotherapy.
Antigen‐specific immunogenicity
We recorded the numbers of observed antigen‐specific humoral and cellular responses. When possible, we separately reported responses of cytotoxic (CD8+) T‐lymphocytes and/or helper (CD4+) T‐lymphocytes.
Secondary outcomes
Carrier‐specific immunogenicity
Given that certain immunotherapeutic strategies rely on the use of carriers that may be the target of an immune response besides the intended antigen‐specific immune response, we recorded information on the induction of carrier‐specific immune responses when appropriate.
Adverse events
To obtain information on the toxicity of antigen‐specific immunotherapy, we extracted data on adverse events observed and reported in the different studies. We categorised adverse events as local adverse events at the site of immunisation and systemic adverse events (all other reported adverse events). We subdivided systemic adverse events into autoimmunity, allergic reactions, and other adverse events occurring after immunisation. If sufficient information was available, we classified adverse events according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) (CTCAE 2009).
Search methods for identification of studies
Electronic searches
For the original review (Leffers 2010), we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2013, Issue 9), in the Cochrane Library (Appendix 1), along with the Cochrane Gynaecological Cancer Group Specialised Register, in October 2013. We also searched MEDLINE (1966 to July 2009) and Embase (1974 to July 2009) according to the search strategies listed (Appendix 2; Appendix 3, respectively).
For the first update of the review, we extended the searches to October 2013, and for this update, we extended the searches to July 2017:
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 6), in The Cochrane Library;
MEDLINE via OVID (October 2013 to June week 4 2017);
Embase via OVID (October 2013 to 2017 week 27).
Searching other resources
We also searched the prospective trial register at www.clinicaltrials.gov.
We undertook handsearching of abstracts in the proceedings of annual meetings of the Society of Gynecologic Oncologists, the American Association for Cancer Research, and the International Society for Biological Therapy of Cancer (1996 to July 2009). The International Society for Biological Therapy of Cancer has been renamed the Society for Immunotherapy of Cancer (SITC), thus we also searched the proceedings of the annual meeting of SITC.
We checked the bibliography of each primary reference and of recent reviews on immunotherapy for ovarian cancer for additional study publications. In addition, we wrote to specialists involved in research regarding immunotherapy for ovarian cancer to ask for information about the results of unpublished and ongoing studies. We included relevant data in this review.
Data collection and analysis
Selection of studies
We downloaded to Reference Manager all titles and abstracts retrieved by electronic searching. We applied no language restrictions other than those inherent to the databases surveyed. We removed duplicates, and two review authors (HWN and NL) independently examined the remaining references. We excluded studies that clearly did not meet the review inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors (HWN and NL) independently assessed the eligibility of retrieved papers. We resolved differences by discussion or by appeal to a third review author (TD), if necessary. We documented reasons for exclusion. The second update included all titles and abstracts from October 2013 until July 2017 retrieved by electronic searches of MEDLINE, Embase, and CENTRAL. Two review authors (STP and MB) selected and independently assessed studies using the same procedure that was used in the primary review and the first update. We resolved differences by discussion or by appeal to a third review author (HWN), if necessary.
Data extraction and management
Two review authors (HWN and NL) independently extracted data on characteristics of participants and interventions, study quality, and endpoints for included studies, and entered them onto a data extraction form specially developed for this review (Appendix 4). Two review authors (STP and MB) followed the same procedure for the second update.
When data on clinical efficacy and antigen‐specific immunogenicity were missing from reports, we attempted to contact study authors to obtain the missing information. A third review author (WH or TD; or HWN during the second update) checked the results.
Assessment of risk of bias in included studies
We assessed the risk of bias in RCTs using the Cochrane 'Risk of bias' tool.
No standard tools are available to evaluate validity for non‐RCTs. For these studies, we evaluated the risk of bias using the following four domains (Table 2).
1. Study report to assess quality of non‐randomised, non‐controlled studies.
| Item | Question | Evaluation |
| 1. a. b. c. |
Sample definition and selection Are inclusion and exclusion criteria clearly defined? Is the study population a representative selection of the true population? Are baseline characteristics adequately described? |
Yes No ? Yes No ? Yes No ? |
| 2 a. b. |
Interventions Are the interventions clearly defined (type of vaccine, antigen, adjuvant, route of vaccination, and vaccination schedule)? Did patients receive concurrent/concomitant treatment with immunomodulatory effects? |
Yes No ? Yes No ? |
| 3 a. b. c. |
Outcomes Are the selected outcome measures clearly specified? Are the outcome measures relevant? Are the outcome measures clearly reported? |
Yes No ? Yes No ? Yes No ? |
| 4. a. b. c. |
Statistical analysis Is there an adequate rationale for the number of participants included? Is there an adequate description of withdrawal/exclusion of participants during the study? Is presentation of the results adequate? |
Yes No ? Yes No ? Yes No ? |
-
Sample definition and selection.
Clear definition of inclusion/exclusion criteria.
Representative selection.
Adequate description of baseline characteristics.
-
Interventions.
Clear specification.
Concurrent/concomitant treatment.
-
Outcomes.
Specifications of outcome measures.
Relevance of outcome measures.
Reporting of outcome measures.
-
Statistical analysis.
Adequate rationale for numbers of participants included.
Adequate description of withdrawals/exclusions during the study.
Adequate presentation of results.
We selected these domains as representative for, and applicable to, non‐randomised non‐controlled studies from a list of 12 quality domains and items deemed to be pivotal to the assessment of non‐RCTs (Deeks 2003).
Two review authors (HWN and NL) carried out the 'Risk of bias' assessment. We resolved discrepancies by discussion; if necessary, we consulted a third review author (WH or TD). For the second update, two review authors (STP and MB) carried out the 'Risk of bias' assessment. We resolved discrepancies by discussion; if necessary, we consulted a third review author (HWN).
Data synthesis
This review provides a narrative analysis because the included studies are highly heterogeneous in terms of intervention and outcome measures. Furthermore, publications often presented data with insufficient details (e.g. lack of standard deviations (SDs), presentation of only some of the multiple outcomes), and it was difficult for review authors to obtain additional information from report authors. Therefore we agreed that quantitative meta‐analysis and calculation of effect size estimates would be neither meaningful nor appropriate for this review. We limited analysis to a structured summary and discussion of available studies and findings.
Certainty of the evidence
We assessed the certainty of the evidence for main outcomes using GRADE (Grading of Recommendations Assessment, Development and Evaluation) criteria (Guyatt 2008), and we presented the main findings along with our judgements in a 'Summary of findings' table.
We will present the overall certainty of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2008), which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias for quantitative studies) but also to external validity (directness of results).
We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation.
High‐certainty: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low‐certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low‐certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
For qualitative studies, we would upgrade for large consistent effect, dose response, and confounders that only reduced the effect size.
Results
Description of studies
Results of the search
Initial version of the review
Upon completing electronic searches of MEDLINE and Embase, we selected 56 out of 311 abstracts as potentially compliant with the selection criteria of this review and retrieved the full texts. Evaluation of the retrieved full texts resulted in the exclusion of 26 papers (see Excluded studies). In addition to the 30 selected full texts, we identified another 14 abstracts by handsearching the proceedings of the periodic meetings specified in the Methods section. We contacted study authors for manuscripts but obtained no full texts for these abstracts. Together, the 44 selected full texts and meeting abstracts described a total of 35 studies. A search of the prospective trial register www.clinicaltrials.gov resulted in identification of an additional 26 studies. We could retrieve a full text or meeting abstract for only four of these and found that only one study complied with our inclusion criteria (Sabbatini 2007). The remaining studies were either ongoing (n = 15) or completed but not yet published (n = 6). A search of CENTRAL (2009, Issue 3) yielded no additional studies. Thus, we included a total of 36 studies in this review. Generally, we selected the most recent peer‐reviewed publication as the primary reference.
First update of the review
For the first update of this review, electronic searches of MEDLINE and Embase yielded 158 records, which resulted in an additional 23 included papers and 10 excluded papers (Characteristics of excluded studies). For five studies in the previous version of this review, a full‐text publication, update, or additional paper was now available. A search of CENTRAL (2013, Issue 3) did not yield additional studies. A search of clinicaltrials.gov resulted in two additional published studies. Furthermore, we identified 26 relevant studies without available results (Characteristics of ongoing studies). Twelve studies are currently recruiting participants, four studies are ongoing but not recruiting, nine studies are classified as completed, and for two studies status is unknown. Overall, we included an additional 19 studies in the update of this review, resulting in a total of 55 included studies involving 3051 women (Characteristics of included studies).
Second update of the review
For the second update of the review, an electronic search of CENTRAL, MEDLINE, and Embase yielded 266 records, which resulted in an additional nine included papers and nine excluded papers (Characteristics of excluded studies). For two studies identified in the previous version of this review, a full‐text publication, update, or additional paper was now available.
A search of ongoing studies identified from the last update in clinicaltrials.gov revealed four additional published studies, three of which are included in this update. In addition, five studies were completed for which no results were published, four studies are still recruiting, and for one study status remains unknown. We removed four studies from the Ongoing studies section because the study had been terminated, or because studies did not include women with epithelial ovarian cancer. Furthermore, we identified 22 relevant new ongoing studies without available results (Characteristics of ongoing studies).
Overall, we included an additional 12 studies in the update of this review, resulting in a total number of 67 included studies involving 3632 women (Characteristics of included studies).
Included studies
The 67 studies included in this updated review were all published in English (Characteristics of included studies;Table 3).
2. Overview of included studies.
| Study | Design | N | Disease status | Target antigen | Type of intervention |
| Antonilli 2016 | Uncontrolled phase I/II | 10 | No evidence of disease (n = 7) + recurrent disease (n = 3) | MUC1 ± ErbB2 ± CEA | Multi‐peptide vaccine |
| Baumann 2011 | RCT | 45 | Evidence of disease after first‐ and/or second‐line chemotherapy | EpCAM | Antibody (low dose vs high dose) |
| Berek 2001 | RCT | 252 | No evidence of disease after primary surgery and chemotherapy | CA‐125 | Antibody vs placebo |
| Berek 2004 | RCT | 145 | No evidence of disease after primary surgery and chemotherapy | CA‐125 | Antibody vs placebo |
| Berek 2009 | RCT | 317 | No evidence of disease after primary surgery and chemotherapy | CA‐125 | Antibody vs placebo |
| Berinstein 2012 | Uncontrolled phase I | 6 | (No) evidence of disease after primary surgery | Topoisomerase IIα, integrin β8 subunit precursor, ABI‐binding protein C3, TACE/ADAM17, junction plakglobin, EDDR1, BAP31 | Short peptides |
| Berinstein 2013 | Uncontrolled phase I | 19 | Unknown | Survivin | Short peptides |
| Braly 2009 | RCT | 40 | (No) evidence of disease after primary surgery | CA‐125 | Antibody (concurrent or delayed with standard chemotherapy) |
| Brossart 2000 | Uncontrolled phase I/II | 3 | Residual or recurrent disease | Her‐2/Neu or MUC1 | Peptide‐pulsed dendritic cells |
| Buzzonetti 2014 | RCT | 129 | No evidence of disease after primary treatment | CA‐125 | Antibody vs placebo |
| Chianese‐Bullock 2008 | Uncontrolled phase I | 9 | (No) evidence of disease or recurrence after primary therapy | FBP, Her‐2/Neu, MAGE‐A1 | Multi‐peptide vaccine |
| Chu 2012 | RCT | 11 | No evidence of disease after primary therapy or surgery for first recurrence | Her‐2/Neu, hTERT, PADRE | Peptide‐pulsed dendritic cells (with vs without cyclophosphamide) |
| Dhodapkar 2012 | Uncontrolled phase I | 6 | Unknown | NY‐ESO‐1 | Fusion protein |
| Diefenbach 2008 | Uncontrolled phase I | 9 | No evidence of disease after primary surgery and chemotherapy | NY‐ESO‐1 | Short peptide |
| Dijkgraaf 2015 | Uncontrolled phase I/II | 15 | Evidence of disease | P53 | Synthetic long peptides |
| Ehlen 2005 | Uncontrolled phase II | 13 | Measurable recurrent disease | CA‐125 | Antibody |
| Freedman 1998 | RCT | 30 | Unknown | Sialyl‐Tn | KLH conjugate (low dose vs high dose) |
| Galanis 2010 | Uncontrolled phase I | 21 | Persistent, recurrent, or progressive disease after primary therapy | CEA | Recombinant virus |
| Goh 2013 | RCT | 63 | No evidence of disease after first‐ or second‐line therapy | MUC1 | Protein‐pulsed dendritic cells vs standard of care |
| Gordon 2004 | Uncontrolled phase II | 20 | Recurrent disease | CA‐125 | Antibody |
| Gray 2016 | Randomised phase II | 56 | First or second clinical remission | MUC1 | Dendritic cell therapy |
| Gribben 2005 | Uncontrolled phase I | 6 | Evidence of disease | CYP1B1 | Plasmid DNA |
| Gulley 2008 | Uncontrolled phase I/II | 3 | Progressive disease after standard chemotherapy | CEA, MUC1 | Recombinant virus |
| Heiss 2010 | RCT | 129 | Recurrent malignant ascites | EpCAM | Antibody + paracentesis vs paracentesis |
| Imhof 2013 | Uncontrolled phase I | 15 | No evidence of disease after primary therapy | TERT, survivin | mRNA‐ and peptide‐pulsed dendritic cells |
| Kaumaya 2009 | Uncontrolled phase I | 5 | Evidence of disease after prior therapy | Her‐2/Neu | Long peptides |
| Kawano 2014 | Uncontrolled phase II | 42 | Recurrent and persistent disease | Personalised (max 4 out of 31 vaccine candidates) | Peptides |
| Kobayashi 2014 | Uncontrolled trial | 56 | Recurrent disease | WT1 ± MUC1 ± CA‐125 | Peptide‐pulsed DC vaccine |
| Le 2012 | Uncontrolled phase I | 2 | Evidence of disease after prior therapy | Mesothelin | Recombinant bacteria |
| Leffers 2009a | Uncontrolled phase II | 20 | Recurrent disease | p53 | Long peptides |
| Lennerz 2014 | Uncontrolled randomised phase I | 7 | (No) evidence of disease | Survivin | Five short peptides |
| Letsch 2011 | Uncontrolled | 8 | Unknown | WT1 | Short peptide |
| Ma 2002 | Uncontrolled | 4 | Unknown | CA‐125 | Antibody |
| MacLean 1992 | Uncontrolled phase I | 10 | Residual or recurrent disease | Thomsen Friedenreich | KLH conjugate |
| MacLean 1996 | Uncontrolled phase II | 34 | Residual or recurrent disease | Sialyl‐Tn | KLH conjugate |
| Method 2002 | RCT | 102 | Unknown | CA‐125 | Antibody (2 vs 3 vs 6 gifts) |
| Möbus 2003 | Retrospective uncontrolled | 44 | Recurrent disease after primary therapy | CA‐125 | Antibody |
| Mohebtash 2011 | Uncontrolled | 14 | Recurrent or residual disease after therapy | CEA, MUC1 | Recombinant virus |
| Morse 2011 | Uncontrolled phase I | 8 | No evidence of disease after first‐ or second‐line chemotherapy | APC, HHR6A, BAP31, replication protein A, Abl‐binding protein 3c, cyclin I, topoisomerase IIα/β, integrin β 8 subunit precursor, CDC2, TACE, g‐catenin, EEDDR1 | Short peptides |
| Nicholson 2004 | Uncontrolled phase I | 26 | Residual disease after primary therapy or second complete remission | MUC1 | Antibody |
| Nishikawa 2006 | Uncontrolled phase II | 4 | Unknown | NY‐ESO‐1 | Short peptide |
| Noujaim 2001 | Retrospective uncontrolled | 184 | Recurrent disease | CA‐125 | Antibody |
| O'Cearbhaill 2016 | Uncontrolled phase I | 24 | No evidence of disease | Globo‐H, GM2, sTn, TF, and Tn | Unimolecular pentavalent vaccine |
| Odunsi 2007 | Uncontrolled phase I | 18 | (No) evidence of disease after chemotherapy for primary or recurrent disease | NY‐ESO‐1 | Short peptide |
| Odunsi 2012 | Uncontrolled phase I/II | 22 | No evidence of disease after primary therapy | NY‐ESO‐1 | Recombinant virus |
| Odunsi 2014 | Uncontrolled phase I/II | 12 | Recurrent epithelial cancer | NY‐ESO‐1 | Protein vaccine with Montanide |
| Ohno 2009 | Uncontrolled phase II | 6 | Unknown | WT1 | Short peptide |
| Peethambaram 2009 | Uncontrolled phase II | 4 | Progressive disease after therapy |
Her‐2/Neu | Fusion protein pulsed antigen‐presenting cells |
| Pfisterer 2006 | Uncontrolled phase I | 36 | Unknown | CA‐125 | Antibody |
| Rahma 2012 | Uncontrolled phase II | 21 | No evidence of disease | p53 | Short peptide vs peptide‐pulsed dendritic cells |
| Reinartz 2004 | Uncontrolled phase Ib/II | 119 | Unknown | CA‐125 | Antibody |
| Sabbatini 2000 | Uncontrolled phase I | 25 | No evidence of disease after chemotherapy for primary or recurrent disease | MUC1 | KLH conjugate |
| Sabbatini 2006 | RCT | 42 | (No) evidence of disease (< 2 cm) after chemotherapy for recurrent disease | CA‐125 | Antibody (intramuscular vs subcutaneous) |
| Sabbatini 2007 | Uncontrolled phase I/II | 11 | No evidence of disease after chemotherapy for primary or recurrent disease | GM2, Globo‐H, Lewis Y, Tn‐MUC1, Tn(c), sTN(c), TF(c) | Heptavalent KLH conjugate |
| Sabbatini 2012 | Uncontrolled phase I | 28 | No evidence of disease after second‐ or third‐line therapy | NY‐ESO‐1 | Long peptides |
| Sabbatini 2013 | RCT | 888 | No evidence of disease after primary therapy | CA‐125 | Antibody vs placebo |
| Sabbatini 2017 | RCT | 171 | No evidence of disease after second‐ or third‐line therapy | Globo‐H, GM2, MUC1‐TN, TF | Polyvalent antigen‐KLH vaccine |
| Sandmaier 1999 | Uncontrolled phase II | 7 | Unknown | Sialyl‐Tn | KLH conjugate |
| Schultes 1998 | Retrospective uncontrolled | 75 | Unknown | CA‐125 | Antibody |
| Ströhlein 2009 | Uncontrolled phase I | 2 | Progressive disease | EpCAM or Her‐2/Neu | Trifunctional antibody |
| Suzuki 2016 | Uncontrolled phase II | 32 | Unknown | Glypican‐3 (GCP3) | Peptide vaccine |
| Takeoka 2017 | Uncontrolled phase I | 2 | Advanced cancer | NY‐ESO‐1 | Whole protein vaccine |
| Takeuchi 2013 | Uncontrolled phase I/II | 38 | Unknown | HLA‐A24: FOXM1, MELK, HJURP, VEGFR1, VEGFR2; HLA‐A02: HIG2, VEGFR1, VEGFR2 | Short peptides |
| Tsuda 2004 | Uncontrolled phase I/II | 7 | (No) evidence of disease | Patient‐tailored cocktail | Multi‐peptide vaccine |
| van Zanten‐Przybysz 2002 | Uncontrolled phase I/II | 5 | Residual or recurrent disease after prior chemotherapy | Membrane folate receptor | Antibody |
| Vermeij 2012 | Uncontrolled phase II | 12 | Recurrent disease | p53 | Long peptides |
| Wagner 1993 | Retrospective uncontrolled | 58 | Unknown | CA‐125 | Antibody |
APC: Adenomatous polyposis coli. CA‐125: cancer antigen‐125. CDC2: Cell division control protein 2. CEA: carcinoembryonic antigen. ED: Evidence of disease. EPCAM: epithelial cell adhesion molecule. ERbB2: Human Epidermal growth factor Receptor 2. FBP: Folate binding protein. HLA: human leucocyte antigen. hTERT: telomerase reverse transcriptase. MAGE‐A1: melanoma‐associated antigen A1. MUC1: Mucin‐1. NED: No evidence of disease. NY‐ESO‐1: New York esophageal squamous cell carcinoma 1. PADRE: DR‐restricted Th helper epitope. RCT: randomised controlled trial. sTn: sialyl Tn. TERT: Telomerase Reverse Transcriptase. TF: Thompson Friedreich.
Design
As we expected, most studies were uncontrolled phase I or II studies (52/67). Only four studies were randomised placebo‐controlled studies (Berek 2001; Berek 2004; Berek 2009; Sabbatini 2013). Eleven studies randomly allocated participants to different regimens (Baumann 2011; Braly 2009; Chu 2012; Freedman 1998; Goh 2013; Gray 2016; Heiss 2010; Lennerz 2014; Method 2002; Sabbatini 2006; Sabbatini 2017). Five studies retrospectively studied the immunogenicity of a previously applied immunoscintigraphic agent (Buzzonetti 2014; Möbus 2003; Noujaim 2001; Schultes 1998; Wagner 1993).
Sample sizes
The median number of women with epithelial ovarian cancer treated per study was 20 (range 2 to 888). Twenty‐one studies included fewer than 10 participants. Twenty studies also included participants with other types of cancer (Antonilli 2016; Berinstein 2012; Brossart 2000; Dhodapkar 2012; Gribben 2005; Gulley 2008; Heiss 2010; Kaumaya 2009; Le 2012; Lennerz 2014; Letsch 2011; Mohebtash 2011; Morse 2011; Odunsi 2012; Ohno 2009; Peethambaram 2009; Sandmaier 1999; Ströhlein 2009; Takeoka 2017; Tsuda 2004). Only 13 studies provided a sample size calculation or rationale (Baumann 2011; Berek 2004; Berek 2009; Braly 2009; Gribben 2005; Heiss 2010; Leffers 2009a; Rahma 2012; Sabbatini 2006; Sabbatini 2007; Sabbatini 2012; Sabbatini 2013; Vermeij 2012).
Participants
As was expected, disease status at study entry varied largely between studies (Table 3). Participants with evidence of residual or recurrent disease after treatment were most frequently included (30/67) (Baumann 2011; Brossart 2000; Dijkgraaf 2015; Ehlen 2005; Galanis 2010; Gordon 2004; Gribben 2005; Gulley 2008; Heiss 2010; Kaumaya 2009; Kawano 2014; Le 2012; Leffers 2009a; MacLean 1992; MacLean 1996; Möbus 2003; Mohebtash 2011; Nicholson 2004; Noujaim 2001; Odunsi 2014; Peethambaram 2009; Ströhlein 2009; van Zanten‐Przybysz 2002; Vermeij 2012). Eight studies included participants with and without evidence of disease after prior therapy (Antonilli 2016; Berinstein 2012; Braly 2009; Chianese‐Bullock 2008; Lennerz 2014; Odunsi 2007; Sabbatini 2006; Tsuda 2004). Seventeen studies included participants with complete response to therapy for primary or recurrent disease (Berek 2001; Berek 2004; Berek 2009; Buzzonetti 2014; Chu 2012; Diefenbach 2008; Goh 2013; Gray 2016; Imhof 2013; Morse 2011; Odunsi 2012; Rahma 2012; Sabbatini 2000; Sabbatini 2007; Sabbatini 2012; Sabbatini 2013; Sabbatini 2017). One study administered treatment together with adjuvant chemotherapy after primary cytoreductive surgery (Braly 2009). The remaining 18 studies did not report disease status at study entry (Berinstein 2013; Dhodapkar 2012; Freedman 1998; Kobayashi 2014; Letsch 2011; Ma 2002; Method 2002; Nishikawa 2006; O'Cearbhaill 2016; Ohno 2009; Pfisterer 2006; Reinartz 2004; Sandmaier 1999; Schultes 1998; Suzuki 2016; Takeoka 2017; Takeuchi 2013; Wagner 1993).
Interventions
Most studies described antibody therapy (22/55), usually targeting cancer antigen (CA)‐125 (17/22 (2347 women)). Most studies included only one target antigen in the vaccine, but 15 studies simultaneously targeted multiple antigens (Antonilli 2016; Berinstein 2012; Chianese‐Bullock 2008; Chu 2012; Gulley 2008; Imhof 2013; Kawano 2014; Kobayashi 2014; Mohebtash 2011; Morse 2011; O'Cearbhaill 2016; Sabbatini 2007; Sabbatini 2017; Takeuchi 2013; Tsuda 2004). Antibodies were usually administered intravenously (12/22). For other vaccine types, subcutaneous injections were most common (29/43).
Fifteen out of 55 studies did not allow concurrent treatment with immunomodulatory drugs. In an additional 20 studies, concomitant immunomodulatory agents were not part of the studied intervention but study authors made no explicit statements in the protocol about prohibition of such drugs. For 27 studies, immunomodulatory drugs were part of the protocol (i.e. carboplatin‐paclitaxel, gemcitabine, doxorubicin and decitabine, cyclophosphamide, interleukin (IL)‐2 ± granulocyte‐macrophage colony‐stimulating factor (GM‐CSF), OK‐432, OPT‐821, PegIntron, toll‐like receptor agonist poly‐ICLC or resiquimod, or diphenhydramine) and one of these allowed interruption of immunotherapy by chemotherapy for progressive disease (Reinartz 2004). Furthermore, two retrospective studies explicitly mentioned that concurrent chemotherapy was allowed at the discretion of the treating clinician (Möbus 2003; Wagner 1993).
Outcomes
Information on immunological responses, clinical responses, survival, and adverse events was available for 63, 43, 44, and 54 studies, respectively.
Excluded studies
A summary of the excluded studies is given in the Characteristics of excluded studies table. Frequent reasons for exclusion were inclusion of too few participants with ovarian cancer, use of antigen non‐specific immunotherapy, and the impossibility of distinguishing results for women with ovarian cancer from results for other study participants.
Risk of bias in included studies
We included GRADE ratings for all primary outcomes. We rated survival as high but all other primary outcomes as very low, as is displayed in Table 1.
We evaluated risk of bias using the Cochrane 'Risk of bias' tool (Higgins 2011). Results of individual studies (both RCTs and NRSs) are available in the Characteristics of included studies table. The fact that for four of 16 RCTs only meeting abstracts were available hindered assessment of risk of bias. The 14 trials for which we could retrieve full texts also did not report on some of the items in the 'Risk of bias' tool. This substantial lack of information means it is highly likely that included studies are subject to biases, and it is therefore difficult to make any statements about the validity of the included RCTs (Figure 1).
1.
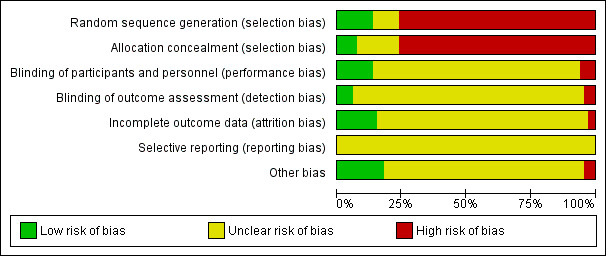
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies. The high risk of selection bias in the majority of included studies is a reflection of the large number of uncontrolled studies included in this review. The risk of remaining biases could not be adequately judged for the included uncontrolled studies, thus explaining the large percentage of missing risk assessments.
In addition to using the 'Risk of bias' tool, we evaluated non‐RCTs using the checklist provided in Table 2. An overview of these results is provided in Table 4. Important observations from this table include lack of clearly defined inclusion/exclusion criteria in 13 out of 51 studies and serious under‐reporting of baseline characteristics in 31 out of 51 studies; this combination makes it impossible to evaluate whether the study populations were representative of the true population. Although most studies carefully described the investigational interventions (47 out of 51), information on allowance or application of concomitant immunomodulatory treatment was frequently absent (24 out of 51). Albeit a clear description of outcome measures was available for 35 studies, adequate calculation of sample size based on a clearly defined primary outcome measure was available for only five studies. Furthermore, the applied checklist shows that justification for withdrawals and exclusions during the study, as well as presentation of study results, requires serious attention in the reports of these non‐randomised studies.
3. Assessment of quality of non‐randomised, (un)controlled studies.
| N | Clear definition of inclusion/exclusion criteria | Representative of true population | Baseline characteristics adequately described | Interventions clearly described | Concomitant/ concurrent immunomodulatory treatment | Outcome measures clearly specified | Outcome measures relevant | Outcome measures clearly reported | Adequate rationale for number of patients | Adequate description of exclusion /withdrawal | Adequate presentation of results | |
| Antonilli 2016 | 10 | yes | unknown | yes | yes | no | yes | yes | yes | no | no | yes |
| Berinstein 2012 | 6 | no | unknown | yes | yes | unknown | yes | yes | yes | no | no | yes |
| Berinstein 2013 | 19 | yesa | unknown | no | yesa | yes | no | yes | no | no | no | no |
| Brossart 2000 | 3 | yes | unknown | no | yes | unknown | yes | yes | yes | no | no | no |
| Chianese‐Bullock 2008 | 9 | yes | no | yes | yes | unknown | yes | yes | yes | no | yes | no |
| Dhodapkar 2012 | 6 | no | unknown | no | no | unknown | no | yes | no | unknown | no | no |
| Diefenbach 2008 | 9 | yes | no | yes | yes | no | yes | yes | yes | no | yes | yes |
| Dijkgraaf 2015 | 6 | yes | no | yes | yes | yes | yes | yes | yes | no | yes | yes |
| Ehlen 2005 | 13 | yes | yes | yes | yes | unknown | yes | yes | yes | no | yes | yes |
| Galanis 2010 | 21 | yes | unknown | no | yes | no | yes | yes | yes | no | yes | yes |
| Goh 2013 | 63 | yesa | unknown | no | no | no | no | yes | no | no | no | no |
| Gribben 2005 | 6 | no | no | no | yes | unknown | no | yes | no | yes | yes | no |
| Gulley 2008 | 3 | yes | unknown | no | yes | unknown | yes | yes | yes | no | yes | no |
| Imhof 2013 | 15 | yesa | unknown | no | yes | no | no | yes | no | no | no | no |
| Kaumaya 2009 | 5 | no | no | no | yes | no | yes | yes | yes | no | no | no |
| Kawano 2014 | 42 | yes | no | yes | yes | yes | yes | yes | yes | no | no | yes |
| Kobayashi 2014 | 56 | yes | unknown | yes | yes | no | no | yes | no | no | yes | no |
| Le 2012 | 2 | yes | no | no | yes | no | yes | yes | yes | no | no | no |
| Leffers 2009a | 20 | yes | unknown | yes | yes | no | yes | yes | yes | yes | yes | yes |
| Letsch 2011 | 8 | unknown | unknown | no | yes | unknown | unknown | unknown | unknown | unknown | unknown | unknown |
| Ma 2002 | 4 | no | unknown | no | no | unknown | no | no | no | no | no | no |
| MacLean 1992 | 10 | no | unknown | no | yes | yes | yes | yes | yes | no | no | yes |
| MacLean 1996 | 34 | yes | unknown | no | yes | yes | no | yes | no | no | yes | no |
| Möbus 2003 | 44 | yes | yes | yes | yes | yes | no | yes | yes | no | no | yes |
| Mohebtash 2011 | 14 | yes | unknown | no | yes | no | yes | yes | yes | no | no | no |
| Morse 2011 | 8 | yes | no | no | yes | unknown | yes | yes | no | no | yes | no |
| Nicholson 2004 | 26 | yes | unknown | no | yes | unknown | yes | yes | yes | no | yes | yes |
| Nishikawa 2006 | 4 | no | unknown | no | no | unknown | yes | yes | yes | no | no | no |
| Noujaim 2001 | 184 | yes | yes | yes | no | unknown | yes | yes | yes | no | no | yes |
| O'Cearbhaill 2016 | 24 | yes | yes | yes | yes | no | no | yes | no | no | no | no |
| Odunsi 2007 | 18 | no | no | yes | yes | unknown | no | yes | yes | no | unknown | yes |
| Odunsi 2012 | 22 | no | yes | yes | yes | no | yes | yes | yes | no | no | yes |
| Odunsi 2014 | 12 | yes | unknown | no | yes | yes | yes | yes | yes | no | no | yes |
| Ohno 2009 | 6 | no | unknown | no | yes | no | yes | yes | yes | no | yes | yes |
| Peethambaram 2009 | 4 | yes | unknown | no | yes | no | yes | yes | no | no | no | no |
| Pfisterer 2006 | 36 | yes | unknown | no | yes | unknown | yes | yes | yes | no | yes | yes |
| Rahma 2012 | 21 | no | unknown | no | yes | yes | yes | no | no | yes | yes | no |
| Reinartz 2004 | 119 | yes | unknown | no | yes | no | yes | yes | yes | no | no | yes |
| Sabbatini 2000 | 25 | yes | yes | yes | yes | unknown | no | yes | yes | no | yes | yes |
| Sabbatini 2007 | 11 | yes | unknown | yes | yes | unknown | yes | yes | yes | yes | yes | no |
| Sabbatini 2012 | 28 | yes | no | yes | yes | no | yes | yes | yes | yes | yes | no |
| Sandmaier 1999 | 7 | yes | unknown | no | yes | no | no | yes | yes | no | yes | yes |
| Schultes 1998 | 75 | no | unknown | no | yes | unknown | no | yes | yes | no | no | yes |
| Ströhlein 2009 | 2 | yes | no | no | yes | unknown | yes | yes | yes | no | yes | yes |
| Suzuki 2016 | 32 | yes | no | yes | yes | no | yes | yes | yes | no | yes | yes |
| Takeoka 2017 | 2 | yes | unknown | no | yes | no | yes | yes | yes | no | yes | yes |
| Takeuchi 2013 | 38 | yes | unknown | no | yes | no | no | yes | no | no | no | no |
| Tsuda 2004 | 5 | yes | no | no | yes | no | yes | yes | no | no | yes | no |
| van Zanten‐Przybysz 2002 | 5 | yes | no | yes | yes | unknown | yes | yes | yes | no | yes | yes |
| Vermeij 2012 | 12 | yes | no | yes | yes | yes | yes | yes | yes | yes | yes | no |
| Wagner 1993 | 58 | no | unknown | no | yes | unknown | no | yes | no | no | no | no |
aSpecified in clinical trial register, not in publication.
Based on the above, the risk of bias of studies included in this systematic review cannot be neglected. Especially selection bias (selection of a treatment population not comparable to the control group or the true population), attrition bias (inadequate reporting of withdrawal and exclusions during the study, resulting in possible overestimation or underestimation of effects), and selective reporting bias are likely to affect the studies included in this review. The effects of interventions described below must therefore be interpreted with prudence.
Allocation
As can be deduced from the Characteristics of included studies table, we were unable to identify the methods of randomisation and allocation used for several randomised studies, which means that we cannot rule out a selection bias for these studies. For the remaining RCTs, selection bias does not seem likely.
However most included studies were early‐phase non‐randomised studies including only a single study arm. Selection bias in these studies may have occurred in two ways: (1) by selective inclusion of participants with no other treatment options owing to end‐stage disease, at which point function of the immune system may also be seriously impaired, thus resulting in an underestimation of immunogenicity and possible clinical benefit of a given vaccine, or (2) via selective recruitment of fairly immunocompetent patients with no evidence of disease, resulting in a possible overestimation of immunogenicity and possible clinical benefit of a given vaccine.
Blinding
Inherent to the study design, no non‐RCTs blinded participants or treating (study) physicians. All participants may have derived benefit from the additional attention awarded to them as participants in a study, and thus performance bias may have influenced the results of these studies. Furthermore, it is unclear whether for these studies, outcome assessors were aware of the clinical condition of patients; thus detection bias may have occurred in these studies.
Only five RCTs described blinding of patients, caregivers, and/or outcome assessors; all compared antibody therapy versus placebo (Berek 2001; Berek 2004; Berek 2009; Sabbatini 2013; Sabbatini 2017). The other RCTs compared dosage levels (Baumann 2011; Freedman 1998; Lennerz 2014), administration route (Sabbatini 2006), number of gifts of a given drug (Method 2002), timing of the intervention in relation to standard chemotherapy (Braly 2009), addition of an immunomodulatory drug (Chu 2012), or immunotherapeutic intervention compared with standard of care (Goh 2013; Gray 2016; Heiss 2010). Given these study designs, we believe that for most of these studies, risk of performance bias is low. Information on blinding of outcome assessors is frequently missing, and risk of detection bias cannot be reliably judged.
Incomplete outcome data
We deemed that only one RCT had high risk of attrition bias based on differences in withdrawals between groups (Heiss 2010). Risk of attrition bias was unclear for nine other RCTs (Berek 2001; Buzzonetti 2014; Freedman 1998; Goh 2013; Gray 2016; Lennerz 2014; Method 2002; Sabbatini 2006; Sabbatini 2017), and risk was low for the remaining RCTs (Baumann 2011; Berek 2004; Berek 2009; Braly 2009; Chu 2012; Sabbatini 2013).
Selective reporting
None of the included studies had a publicly available registered study protocol. It is therefore unclear whether studies selectively reported outcomes.
Other potential sources of bias
Given the elapsed time since publication of the meeting abstract, a publication bias is likely to exist for two out of three RCTs for which only a meeting abstract was available (Berek 2001; Freedman 1998).
Effects of interventions
See: Table 1
Primary outcomes
Clinical efficacy
Tumour responses
Forty‐three studies evaluated clinical responses to therapy (Table 5). No RCTs evaluated tumour response (Berek 2001; Berek 2004; Berek 2009; Gray 2016; Sabbatini 2013; Sabbatini 2017). In reports on these studies, criteria for evaluation and/or explicit descriptions of tumour responses per patient as well as the time point at which the evaluation took place were frequently not available. For studies that did mention evaluation of tumour responses, response outcomes were based on CA‐125 levels combined with tumour imaging (Baumann 2011; Chianese‐Bullock 2008; Chu 2012; Diefenbach 2008; Dijkgraaf 2015; Ehlen 2005; Galanis 2010; Gordon 2004; Gulley 2008; Leffers 2009a; Ohno 2009; Rahma 2012; Sabbatini 2006; Ströhlein 2009; Tsuda 2004; van Zanten‐Przybysz 2002; Vermeij 2012), CA‐125 alone (Nicholson 2004; Wagner 1993), or imaging alone (Le 2012; Odunsi 2007; Peethambaram 2009; Reinartz 2004; Sabbatini 2012; Takeuchi 2013). Eighteen studies explicitly mentioned evaluation of imaging according to the internationally accepted WHO or RECIST criteria (Baumann 2011; Dijkgraaf 2015; Galanis 2010; Kawano 2014; Kobayashi 2014; Leffers 2009a; Lennerz 2014; Odunsi 2014; Ohno 2009; Rahma 2012; Reinartz 2004; Sabbatini 2012; Suzuki 2016; Takeoka 2017; Takeuchi 2013; Tsuda 2004; Vermeij 2012), and only six studies evaluated CA‐125 levels according to GCIG criteria or described CA‐125 levels in such a way that evaluation according to these criteria was possible for at least some participants (Baumann 2011; Dijkgraaf 2015; Galanis 2010; Leffers 2009a; van Zanten‐Przybysz 2002; Vermeij 2012). It is striking that eight studies stated that study authors evaluated tumour responses but did not provide these results in their publications (Dhodapkar 2012; Diefenbach 2008; Gulley 2008; Imhof 2013; Method 2002; Odunsi 2007; Reinartz 2004; Wagner 1993). Only seven studies reported complete or partial tumour responses in a small fraction of patients with evidence of disease at study entry (Baumann 2011; Dijkgraaf 2015; Gordon 2004; Kaumaya 2009; Kawano 2014; Odunsi 2007; Takeuchi 2013). These results must be interpreted with caution, as two of these studies did not define criteria for response evaluation (Gordon 2004; Odunsi 2007).
4. Evaluation of clinical responses to immunotherapy.
| N | Analysed | Method | CA‐125 | Tumour | Overall conclusion | |||
| Response definition | Results | Definition for tumour response | Results | |||||
| Antonilli 2016 | 10 | yes | tumour | unknown | Cohort 1 (baseline status; disease free): 1× PD and 6× NED Cohort 2 (baseline status; recurrent disease): 3× PD |
6× NED 4× PD |
||
| Baumann 2011 | 45 | yes | both | Gynaecologic Cancer Intergroup Guidelines (evaluable patients: cohort 1: 7; cohort 2: 3) | Cohort 1: 7× ↑, Cohort 2: 3× ↑ | RECIST | Cohort 1: 2× SD, 21× PD Cohort 2: 1× PR, 5× SD, 16× PD |
Cohort 1: 2× SD, 21× PD Cohort 2: 1× PR, 5× SD, 16× PD |
| Braly 2009 | 18/22 | yes | unknown | unknown | complete clinical remission 15×/18× | |||
| Brossart 2000 | 3 | yes | unknown | 2× SD, 1× PD | ||||
| Chianese‐Bullock 2008 | 9 | yes | both | unknown | unknown | 1× NED, 8× PD | ||
| Chu 2012 | 11 | yes | both | unknown | unknown | 3× PD, 7× NED | ||
| Dhodapkar 2012 | 6 | yes | unknown | not reported | ||||
| Diefenbach 2008 | 9 | yes | both | unknown | unknown | not reported | ||
| Dijkgraaf 2015 | 6 | yes | both | Gynaecologic Cancer Intergroup Guidelines | Cohort 3 (n = 6): 4× PD, 2× PR | RECIST | Cohort 3 (n = 6): 2× PR, 3× PD, 1× SD | Cohort 3: 2× PR, 3× PD, 1× SD |
| Ehlen 2005 | 13 | yes | both | decrease > 15% (↓); < 15% change (=) stable; > 15% increase (↑) | 4× ↓, 1× =, 6× ↑ | unknown | 3× SD, 10× PD | |
| Freedman 1998 | 30 | yes | unknown | 18× SD, 10× PD | ||||
| Galanis 2010 | 21 | yes | both | Gynaecologic Cancer Intergroup Guidelines | 2× ↓, 3× =, 16× ^? | RECIST | 14× SD, 7× PD | 14× SD, 7× PD |
| Gordon 2004 | 20 | yes | both | unknown | 6× ↓ | unknown | 2× NED, 2× CR, 1× PR, 1× SD, 9× PD | |
| Gribben 2005 | 6 | yes | unknown | 6× PD | ||||
| Gulley 2008 | 3 | yes | both | unknown | unknown | not reported | ||
| Imhof 2013 | 15 | yes | unknown | not reported | ||||
| Kaumaya 2009 | 5 | yes | unknown | 2× SD, 3× PD | ||||
| Kawano 2014 | 42 | yes | tumour | RECIST | 1× CR, 3× SD, 21× PD | 1× CR, 3× SD, 21× PD | ||
| Kobayashi 2014 | 56 | yes | tumour | RECIST 3 months after first vaccination | 2× PR, 14× SD, 32× PD Disease controle rate: 29% Objective response rate: 3.6% |
PR: 3.6%, SD: 25%, PD: 57% | ||
| Le 2012 | 2 | yes | tumour | RECIST | 2× PD | 2× PD | ||
| Leffers 2009a | 20 | yes | both | Gynaecologic Cancer Intergroup Guidelines | not reported | RECIST | not reported | 2× SD, 18× PD |
| Lennerz 2014 | 7 | yes | tumour | RECIST | 5× PD, 2× NE | 5× PD | ||
| Letsch 2011 | 8 | yes | unknown | 4× SD, 4× PD | ||||
| MacLean 1992 | 10 | yes | unknown | 3× SD, 7× PD | ||||
| Method 2002 | 102 | yes | unknown | not reported | ||||
| Mohebtash 2011 | 14 | yes | unknown | 1× SD, 11× PD | ||||
| Nicholson 2004 | 26 | yes | CA‐125 | unknown | 21× PD, 1× SD, 1× lost to follow‐up, 3× unknown | |||
| Odunsi 2007 | 18 | yes | tumour | unknown | 1× CR, 17× unknown | |||
| Odunsi 2014 | 12 | yes | tumour | RECIST | 1× PD, 5× SD | PD: 10%, SD:50% | ||
| Ohno 2009 | 6 | yes | both | unknown | not reported | RECIST | 1× SD, 3× PD | 1× SD, 4× PD, 1× withdrawal |
| Peethambaram 2009 | 4 | yes | tumour | unknown | 2× SD, 2× PD | 2× SD, 2× PD | ||
| Rahma 2012 | 21 | yes | both | unknown | not reported | RECIST | Cohort 1: 2× NED, 11× PD Cohort 2: 2× NED, 5× PD |
Cohort 1: 2× NED, 11× PD Cohort 2: 2× NED, 5× PD |
| Reinartz 2004 | 119 | yes | tumour | WHO | not reported | |||
| Sabbatini 2006 | 42 | yes | both | unknown | unknown | 12× SD, 21× PD, 9× withdrawal (6× PD) | ||
| Sabbatini 2012 | 28 | yes | tumour | RECIST | Cohort 1: 1× NED, 3× PD Cohort 2: 3× NED, 10× PD Cohort 3: 2× NED, 9× PD |
Cohort 1: 1× NED, 3× PD Cohort 2: 3× NED, 10× PD Cohort 3: 2× NED, 9× PD |
||
| Ströhlein 2009 | 2 | yes | both | unknown | unknown | 1× PD, 1× PR or SD | ||
| Suzuki 2016 | 32 | yes | tumour | RECIST | 12 months: PR: 2/32, PD: 28/32 | 2× PR, 28× PD | ||
| Takeoka 2017 | 2 | yes | tumour | RECIST | 2× PD | 2× PD | ||
| Takeuchi 2013 | 38 | yes | tumour | RECIST | 1× CR, 2× PR, 10× SD, 9× PD | 1× CR, 2× PR, 10× SD, 9× PD | ||
| Tsuda 2004 | 5 | yes | both | unknown | WHO | 4× PD, 1× SD | ||
| van Zanten‐Przybysz 2002 | 5 | yes | both | unknown | 1× ↓, 1× =, 1× ↑, 2× unknown | unknown | 1× NED, 1× SD, 2× PD, 1× unknown | 3× PD, 2× SD |
| Vermeij 2012 | 12 | yes | both | Gynaecologic Cancer Intergroup Guidelines | 7× ↓/=, 3× ↑ | RECIST | not reported | 2× SD, 8× PD |
| Wagner 1993 | 58 | yes | CA‐125 | unknown | not reported | |||
| Berek 2001 | 252 | no | ||||||
| Berek 2004 | 145 | no | ||||||
| Berek 2009 | 371 | no | ||||||
| Berinstein 2012 | 6 | no | ||||||
| Berinstein 2013 | 19 | no | ||||||
| Buzzonetti 2014 | 129 | no | ||||||
| Goh 2013 | 63 | no | ||||||
| Gray 2016 | 56 | no | ||||||
| Heiss 2010 | 129 | no | ||||||
| Ma 2002 | 4 | no | ||||||
| MacLean 1996 | 34 | no | ||||||
| Möbus 2003 | 44 | no | ||||||
| Morse 2011 | 8 | no | ||||||
| Nishikawa 2006 | 4 | no | ||||||
| Noujaim 2001 | 184 | no | ||||||
| O'Cearbhaill 2016 | 24 | no | ||||||
| Odunsi 2012 | 22 | no | ||||||
| Pfisterer 2006 | 36 | no | ||||||
| Sabbatini 2000 | 25 | no | ||||||
| Sabbatini 2007 | 11 | no | ||||||
| Sabbatini 2013 | 888 | no | ||||||
| Sabbatini 2017 | 171 | no | ||||||
| Sandmaier 1999 | 7 | no | ||||||
| Schultes 1998 | 75 | no | ||||||
C1: cohort 1. C2: cohort 2. C3: cohort 3. CA‐125: cancer antigen‐125. CR: complete response. GCIG: Gynecologic Cancer Intergroup. NED: no evidence of disease. PD: progressive disease. PR: partial response. RECIST: Response Evaluation Criteria In Solid Tumors. SD: stable disease. WHO: World Health Organization.
Post‐immunotherapy treatment response
Although studies generally report a period of follow‐up to obtain information on survival, most studies provide no report on subsequent treatment with and response to secondary chemotherapy. Nine studies mention that participants were treated with chemotherapy after immunotherapy (Berek 2004; Gordon 2004; Gribben 2005; Leffers 2009a; Möbus 2003; Odunsi 2007; Reinartz 2004; Ströhlein 2009; van Zanten‐Przybysz 2002), but only four non‐comparative phase I/II studies report response to secondary chemotherapy in relation to immunological responses to immunotherapy (Gordon 2004; Gribben 2005; Leffers 2009a; Reinartz 2004).
Reinartz 2004 provided a preliminary report on clinical responses of 28 out of 42 participants treated with chemotherapy for clinically relevant progression during or after antibody therapy in conjunction with the induction of human‐anti‐mouse and anti‐anti‐idiotype antibodies. Although both types of participants with a complete response had strong humoral responses, researchers observed similar or stronger antibody responses for participants with stable or progressive disease. In another study, shortly after monotherapy with a monoclonal antibody, 13 out of 20 participants received chemotherapy combined with the monoclonal antibody. Researchers in this study observed clinical responses to chemo‐immunotherapy only in patients with cellular responses to CA‐125 and/or autologous tumour (Gordon 2004). A study of synthetic long peptides targeting p53 showed no improvement in survival or tumour responses to secondary chemotherapy (Leffers 2009a). Finally, the authors of a study investigating plasmid DNA vaccination targeting CYP1B1 suggest that treatment has led to improved responses to third‐line therapy but included no control group, nor do we find this observation convincing when only patients with ovarian cancer are considered (Gribben 2005).
Survival and time to relapse
Definitions of survival used in the different studies varied greatly (Table 6 and Table 7). Furthermore, reliable statements about survival (dis)advantages can be made only on the basis of RCT findings. Only six studies were designed to primarily evaluate survival; however, investigators found no statistically significant differences in time to relapse and/or overall survival between patients treated with a monoclonal antibody and those given placebo (Berek 2001; Berek 2004; Berek 2009; Sabbatini 2013). Another study compared antigen‐specific immunotherapy versus a non‐specific immunotherapy and noted no significant differences in progression‐free survival (Sabbatini 2017). Another study compared MUC1 dendritic cell therapy versus standard of care and reported no significant differences in progression‐free survival and overall survival. However, when patients were divided into two subgroups (first and second clinical remission), a significant difference in overall survival and progression‐free survival was evident among those with a second clinical remission. Researchers included a small number of participants in the trial and median overall survival of the treated group has not yet been reached; therefore these results must be interpreted with caution (Gray 2016). Many non‐RCTs also evaluated survival, frequently by comparing survival of patients with robust immunological responses versus that of patients with no or weak immunological responses to treatment (Table 6 and Table 7). These results should be interpreted with great caution, as shorter survival among non‐responders could merely be a reflection of the general condition of these patients and might reflect well‐known clinical and pathological prognostic parameters. Patient numbers in the non‐comparative groups were often too low to permit a reliable conclusion.
5. Definitions and results of survival and/or relapse analysis in antigen‐specific antibody studies.
| Study | Analysed | Definition | Results |
| Baumann 2011 | yes | progression‐free survival/overall survival | median progression‐free survival: low dose 70 days (95% CI 63 to 91), high dose 68 days (95% CI 58 to 77) median overall survival: low dose 137 days (95% CI 99 to 218), high dose 185 days (95% CI 134 to 472) |
| Berek 2001 | yes | time to relapse | median time to relapse: placebo 11.3, robust HAMA 16.4, robust Ab2 18.9 months |
| Berek 2004 | yes | time to relapse | all patients: time to relapse: oregovomab 13.3 vs placebo 10.3 months (P = 0.71) (HR 0.881, 95% CI 0.578 to 1.349) successful front‐line therapy patients: time to relapse: oregovomab 24 vs placebo 10.8 months (P = 0.71) (HR 0.543, 95% CI 0.287 to 1.025) |
| Berek 2009 | yes | time to relapse (randomisation to relapse) | median time to relapse: oregovomab 10.3 months (95% CI 9.7 to 13.0 months) vs placebo 12.9 months (95% CI 10.1 to 17.4 months) (P = .29) |
| Braly 2009 | yes | progression‐free survival | median progression‐free survival: simultaneous administration 17.9 months vs delayed administration 16.1 months |
| Buzzonetti 2014 | no | ||
| Ehlen 2005 | yes | time to progression/survival (first dose to death) | time to progression: median 8.4 weeks (range 2 to 61 weeks); survival 37 weeks (range 11 to 110) |
| Gordon 2004 | yes | time to progression/survival (first dose to death) | time to progression: median 11 weeks (T‐cell responders vs non‐responders; P < 0.0001; HR 0.150, 95% CI 0.006 to 0.168); survival: median 70.4 weeks (T‐cell responders vs non‐responders; P < 0.002; HR 0.157, 95% CI 0.009 to 0.347) |
| Heiss 2010 | yes | puncture‐free survival (first dose to therapeutic puncture or death)/overall survival (first dose to death) | Median puncture‐free survival: paclitaxel + catumaxomab 52 days (95% CI 38 to 62) vs catumaxomab 11 days (95% CI 9 to 20) Median overall survival: paclitaxel + catumaxomab 110 days (95% CI 70 to 164) vs catumaxomab 81 days (95% CI 68 to 134) |
| Ma 2002 | no | ||
| Method 2002 | no | ||
| Möbus 2003 | yes | survival (first dose to death)/overall survival (diagnosis to death) | survival: median 16.8 months (95% CI 10.3 to 22.6) (Ab3 responders vs non‐responders 18.2 vs 13.1, P = 0.0896; HAMA responders vs non‐responders 22.6 months vs 7.6 months, P = 0.0016); overall survival: median 34.4 months |
| Nicholson 2004 | no | ||
| Noujaim 2001 | yes | survival (first dose to death) | median survival and 3‐year survival: Ab3 responders vs non‐responders 22.9 vs 13.5 months, P = 0.0089, 38% vs 8%; T‐cell responders vs non‐responders (n = 16) > 84 vs 13.2 months, P = 0.0202, 75% vs 0% |
| Pfisterer 2006 | no | ||
| Reinartz 2004 | yes | survival (first dose to death) | median survival: 19.4 months, Ab3 responders vs non‐responders: 23.4 vs 4.9 months, P < 0.0001 |
| Sabbatini 2006 | yes | time to progression | time to progression: 4 months (95% CI 3 to 5 months) |
| Sabbatini 2013 | yes | recurrence‐free survival (randomisation to recurrence)/overall survival (randomisation to death) | median recurrence‐free survival: abagovomab 403 days (95% CI 323 to 414) vs placebo 402 days (95% CI 323 to 487) 2‐year overall survival rate: abagovomab 80% (SE 1.71) vs placebo 80% (SE 2.43) |
| Schultes 1998 | yes | overall survival (diagnosis to death) | median overall survival: robust Ab3 responders vs non‐robust responders 49 vs 38 months, P = 0.0029; Ab2 robust vs non‐robust responders 30.0 vs 44.0 months, P = 0.0475 |
| Ströhlein 2009 | yes | overall survival | not described separately for ovarian cancer patients |
| van Zanten‐Przybysz 2002 | yes | survival (first dose to death) | median survival: 22.0 months |
| Wagner 1993 | yes | not described | survival: robust Ab2 vs non‐robust Ab2 responders: NS |
Ab2: anti‐idiotype antibody. Ab3: anti‐anti‐idiotype antibody. CI: confidence interval. HAMA: human‐anti‐mouse antibody. HR: hazard ratio. SE: standard error.
6. Definitions and results of survival and/or relapse analysis in other antigen‐specific immunotherapy studies.
| Study | Analysed | Definition | Results |
| Antonilli 2016 | yes | recurrence rate | recurrence rate: n = 2 |
| Berinstein 2012 | yes | time to progression (study day 0 to relapse) | median time to progression > 8 months (range 4 to > 9) |
| Berinstein 2013 | no | ||
| Brossart 2000 | no | ||
| Chianese‐Bullock 2008 | no | ||
| Chu 2012 | yes | progression‐free survival (first vaccination to relapse)/overall survival (first vaccination to death/last follow‐up) | 3‐year progression‐free survival: arm 1 vs arm 2, 40% vs 80% (P = 0.17) 3‐year overall survival: arm 1 vs arm 2, 80% vs 100% (P = 1.00) |
| Diefenbach 2008 | yes | time to progression (last chemo to relapse) | median time to progression 13.0 months (95% CI 11.2 to not reached) |
| Dijkgraaf 2015 | yes | progression‐free survival: time from start of therapy until progression in weeks overall survival: time from start of therapy until death in weeks |
Progression‐free survival cohort 3: 8 to 36 (median 13) Overall survival cohort 3: 12 to 48 (median 37) |
| Dhodapkar 2012 | no | ||
| Freedman 1998 | yes | progression‐free interval; survival | median progression‐free interval: 4 months (95% CI 1.9 to 7.6) median survival: 13.3. months (95% CI 1.5 to 30.8) |
| Galanis 2010 | yes | overall survival | median overall survival: 12.2 months (range 1.3 to 38.4) |
| Goh 2013 | yes | progression‐free survival; overall survival | median progression‐free survival vaccine vs standard of care 365 days vs 321 days overall survival: not reported |
| Gray 2016 | yes | progression‐free survival overall survival |
progression‐free survival: 13 months (Cvac) vs 9 months (standard of care) overall survival: median not reached at 43 months in both study arms. |
| Gribben 2005 | no | ||
| Gulley 2008 | yes | progression‐free survival; overall survival | progression‐free survival: 9, 18, 19+ months; OS: 6, 19+, 21 months |
| Imhof 2013 | yes | time to progression (first vaccination to relapse)/overall survival (first vaccination to death) | not reported |
| Kaumaya 2009 | no | ||
| Kawano 2014 | yes | median survival time | median survival time overall (n = 42): 19.1 months median survival time platinum‐sensitive (n = 17): 39.3 months median survival time platinum‐resistant (n = 25): 16.2 months |
| Kobayashi 2014 | yes | median survival time from first vaccination | median survival time 14.5 months |
| Le 2012 | no | ||
| Leffers 2009a | yes | disease‐specific survival (diagnosis to death of ovarian cancer) | median disease‐specific survival participants vs historical controls: 44.0 months vs 47.4 months |
| Lennerz 2014 | no | ||
| Letsch 2011 | no | ||
| MacLean 1996 | yes | survival (trial entry to death) | median survival: 12.7 months |
| MacLean 1992 | no | ||
| Mohebtash 2011 | yes | progression‐free survival/overall survival | median progression‐free survival: 2 months (range 1 to 36) median overall survival: 15.5 months (range 1.5 to > 57.0) |
| Morse 2011 | yes | overall survival | median overall survival: not reached (range 289 to 1115+ days) |
| Nishikawa 2006 | no | ||
| O'Cearbhaill 2016 | yes | progression‐free survival: time from the end of adjuvant chemotherapy until disease progression | not adequately described |
| Odunsi 2007 | yes | time to progression (first vaccination to relapse) | median time to progression: 19.0 months (95% CI 9.0 to not reached) |
| Odunsi 2012 | yes | progression‐free survival/overall survival | median progression‐free survival: 21 months (95% CI 16 to 29 months) median overall survival: 48 months (95% CI not estimable) |
| Odunsi 2014 | no | ||
| Ohno 2009 | no | ||
| Peethambaram 2009 | yes | time to progression | median time to progression: 14.0 (range 12.1 to 18.3) |
| Rahma 2012 | yes | progression‐free survival (date on study to date of progression) overall survival (date on study to date of death or last follow‐up) |
median progression‐free survival: 4.2 vs 8.7 months median overall survival: 40.8 vs 29.6 months |
| Sabbatini 2000 | yes | time to progression (trial entry to relapse) | median time to progression: 6 months (range 2 to 17) |
| Sabbatini 2007 | yes | time to progression (first vaccination to relapse) | median time to progression: 4.2 months (95% CI 2.7 to 8.5) |
| Sabbatini 2012 | yes | time to progression | no differences between cohorts (numbers not reported) |
| Sabbatini 2017 | yes | progression‐free survival: time from randomisation to first clinical, biochemical, or radiological evidence of progression overall survival: time from study untill death. |
progression‐free survival: 5.9 months vaccine + OPT‐821 vs 6.5 months OPT‐821 only overall survival: 46.5 months vaccine + OPT‐821 vs 46.2 months OPT‐821 only |
| Sandmaier 1999 | no | ||
| Suzuki 2016 | yes | time to progression/overall survival | time of progression: not reported overall survival after 12 months of all patients: 20.6% |
| Takeoka 2017 | no | ||
| Takeuchi 2013 | yes | overall survival | median overall survival: HLA‐A24 5 months (range 30 to 623 days), HLA‐A02 9 months (range 54 to 921 days) |
| Tsuda 2004 | no | ||
| Vermeij 2012 | no |
CI: confidence interval.
Antigen‐specific immunogenicity
Humoral responses
Monoclonal antibodies may induce anti‐idiotype antibodies (Ab2), directed primarily against the administered monoclonal antibody, as well as anti‐anti‐idiotype antibodies (Ab3), directed towards the target antigen. Anti‐idiotype and anti‐anti‐idiotype antibodies were evaluated in 10 out of 22 studies and 9 out of 22 studies, respectively (Table 8 and Table 9). Response percentages varied greatly (Ab2: 3% to 100%, Ab3: 0% to 100%).
7. Definitions and results of anti‐idiotypic (Ab2) humoral responses in antigen‐specific monoclonal antibody studies.
| Study | N | Dose | Target antigen | Analysed | Positive if: | % positive | Robust if: | % robust |
| Baumann 2011 | 45 | C1: 10‐10‐10‐10 μg C2: 10‐20‐50‐100 g |
EpCAM | no | ||||
| Berek 2001 | 252 | 2 mg | CA‐125 | yes | > 50 ng/mL | 63% | > 100 ng/mL | |
| Berek 2004 | 145 | 2 mg | CA‐125 | yes | > 100 ng/mL | 67% | ||
| Berek 2009 | 371 | 2 mg | CA‐125 | no | ||||
| Braly 2009 | 40 | unknown | CA‐125 | yes | > 100 ng/mL | 94% vs 74% | ||
| Buzzonetti 2014 | 129 | 2 mg | CA‐125 | no | ||||
| Ehlen 2005 | 13 | 2 mg | CA‐125 | yes | > 50 ng/mL | 45% | ||
| Gordon 2004 | 20 | 2 mg | CA‐125 | yes | > 50 ng/mL | > 100 ng/mL | 79% | |
| Heiss 2010 | 129 | 10‐20‐50‐150 μg | EpCAM | no | ||||
| Ma 2002 | 4 | unknown | CA‐125 | no | ||||
| Method 2002 | 102 | 2 mg | CA‐125 | yes | > 100 ng/mL | 13% vs 31% vs 67% | ||
| Möbus 2003 | 44 | 2 mg | CA‐125 | yes | > 50 ng/mL | 77% | ||
| Nicholson 2004 | 26 | 25 mg | MUC1 | yes | unknown | 100% | ||
| Noujaim 2001 | 184 | 2 mg | CA‐125 | yes | ||||
| Pfisterer 2006 | 36 | 2 mg | CA‐125 | no | ||||
| Reinartz 2004 | 119 | 2 mg | CA‐125 | no | ||||
| Sabbatini 2006 | 42 | 2 mg/0.2 mg | CA‐125 | no | ||||
| Sabbatini 2013 | 888 | 2 mg | CA‐125 | no | ||||
| Schultes 1998 | 75 | 2 mg | CA‐125 | yes | > 50 ng/mL | 64% | > 250 ng/mL | |
| Ströhlein 2009 | 2 | 10/20/40 μg 10/40/80 μg |
EpCAM Her‐2/Neu |
no | ||||
| van Zanten‐Przybysz 2002 | 5 | 50 mg | membrane folate receptor | no | ||||
| Wagner 1993 | 58 | 1 mg | CA‐125 | yes | > 0 μ/L | 64% | > 10 μ/L | 32% |
8. Definitions and results of anti‐anti‐idiotypic (Ab3) humoral responses in antigen‐specific antibody studies.
| Study | N | Dose | Target antigen | Analysed | Positive if: | % positive | Robust if: | % robust |
| Baumann 2011 | 45 | C1: 10‐10‐10‐10 μg C2: 10‐20‐50‐100 μg |
EpCAM | no | ||||
| Berek 2001 | 252 | 2 mg | CA‐125 | no | ||||
| Berek 2004 | 145 | 2 mg | CA‐125 | no | ||||
| Berek 2009 | 371 | 2 mg | CA‐125 | no | ||||
| Braly 2009 | 40 | unknown | CA‐125 | no | ||||
| Buzzonetti 2014 | 129 | 2 mg | CA‐125 | yes; reported in Sabbatini 2013 | ||||
| Ehlen 2005 | 13 | 2 mg | CA‐125 | yes | > 100 ng/mL | > 3× baseline | 0% | |
| Gordon 2004 | 20 | 2 mg | CA‐125 | yes | > 100 ng/mL | > 3× baseline | 10.5% | |
| Heiss 2010 | 129 | 10‐20‐50‐150 μg | EpCAM | no | ||||
| Ma 2002 | 4 | unknown | CA‐125 | no | ||||
| Method 2002 | 102 | 2 mg | CA‐125 | no | ||||
| Möbus 2003 | 44 | 2 mg | CA‐125 | yes | > 3× baseline | 28% | ||
| Nicholson 2004 | 26 | 25 mg | MUC1 | yes | > 0.015 μg/mL | 38% | ||
| Noujaim 2001 | 184 | 2 mg | CA‐125 | yes | > 3× baseline | 43% | ||
| Pfisterer 2006 | 36 | 2 mg | CA‐125 | yes | > 1000 ng/mL | L vs S: 100% vs 100% | ||
| Reinartz 2004 | 119 | 2 mg | CA‐125 | yes | > 1000 μ/mL | 68% | ||
| Sabbatini 2006 | 42 | 2 mg/0.2 mg | CA‐125 | yes | > 1000 μ/mL | 100% | ||
| Sabbatini 2013 | 888 | 2 mg | CA‐125 | yes | unknown | placebo: stable abagovomab: increase |
||
| Schultes 1998 | 75 | 2 mg | CA‐125 | yes | > 200 ng/mL | 24% | > 3× baseline | |
| Ströhlein 2009 | 2 | 10/20/40 μg 10/40/80 μg |
EpCAM Her‐2/Neu |
no | ||||
| van Zanten‐Przybysz 2002 | 5 | 50 mg | membrane folate receptor | no | ||||
| Wagner 1993 | 58 | 1 mg | CA‐125 | no |
Twenty‐one studies of other vaccine types evaluated the induction of antigen‐specific antibodies as shown by enzyme‐linked immunosorbent assay (ELISA) or luminex assay; however only 11 of these studies clearly defined when an antibody titre or concentration was considered positive (Table 10) (Diefenbach 2008; Galanis 2010; Kaumaya 2009; Kawano 2014; O'Cearbhaill 2016; Odunsi 2014; Sabbatini 2007; Sabbatini 2012; Sabbatini 2017; Sandmaier 1999; Takeoka 2017). In addition, the study combining an NY‐ESO‐1 vaccine with chemotherapy and an anti‐methylation agent tested humoral response with ELISA to 22 recombinant proteins that were not included in the vaccine and showed de novo serum reactivity to at least one of those proteins in all analysed participants (n = 3), suggesting that combination regimens may lead to a broadened profile of anti‐tumour immune response in vivo (Odunsi 2014). Results show large differences in percentages of patients with measurable antigen‐specific antibodies (IgG: 0% to 96%). Possible explanations for these broad ranges include differences in (1) response definition, (2) number of treatment cycles after which humoral responses were measured, and (3) targeted antigens.
9. Definitions and results of humoral response evaluation in other antigen‐specific immunotherapy studies.
| Study | N | Target antigen(s) | Analysed | Assay | Positive if: | % positive |
| Antonilli 2016 | 10 | MUC1 ± ErbB1 ± CEA | no | |||
| Berinstein 2012 | 6 | topoisomerase IIα, integrin β8 subunit precursor, ABI‐binding protein C3, TACE/ADAM17, junction plakglobin, EDDR1, BAP31 | no | |||
| Berinstein 2013 | 19 | survivin | no | |||
| Brossart 2000 | 3 | Her‐2/Neu, MUC1 | no | |||
| Chianese‐Bullock 2008 | 9 | FBP, Her‐2/Neu, MAGE‐A1 | no | |||
| Chu 2012 | 11 | Her‐2/Neu, hTERT, PADRE | no | |||
| Diefenbach 2008 | 6 | NY‐ESO‐1 | yes | unknown | unknown | not reported |
| Dijkgraaf 2015 | 6 | p53 | no | |||
| Dhodapkar 2012 | 9 | NY‐ESO‐1 | yes | ELISA | > 100 | 0% |
| Freedman 1998 | 21 | CEA | yes | ELISA | ≥ 2× pretreatment and > mean + 2 SD of 10 normal sera | 0% |
| Galanis 2010 | 63 | MUC1 | yes | unknown | unknown | 0% |
| Goh 2013 | 6 | CYP1B1 | no | |||
| Gray 2016 | 56 | MUC1 | yes | ELISA | unknown | No response measured |
| Gribben 2005 | 3 | CEA, MUC1 | no | |||
| Gulley 2008 | 30 | Sialyl‐Tn | no | |||
| Imhof 2013 | 15 | TERT, survivin | no | |||
| Kaumaya 2009 | 5 | Her‐2/Neu | yes | ELISA | high response: > 0.6 intermediate response: 0.2 to 0.6 |
60% high responses, 40% intermediate responses |
| Kawano 2014 | 42 | personalised (max 4 out of 31 vacinne candidates) | yes | Luminex assay | 1 out of 4 vaccine‐specific IgG titers is 2‐fold higher than pre‐vaccination | 6 vaccinations: 16/42 12 vaccinations: 29/30 |
| Kobayashi 2014 | 56 | WT1 ± MUC1 ± CA‐125 | no | |||
| Le 2012 | 2 | mesothelin | no | |||
| Leffers 2009a | 20 | p53 | yes | unknown | unknown | pre‐imm: 40%, post‐imm: 45% |
| Lennerz 2014 | 7 | survivin | no | |||
| Letsch 2011 | 8 | WT1 | no | |||
| MacLean 1996 | 10 | Thomsen Friedenreich | yes | ELISA | unknown | 80% IgA, 90% IgM, 90% IgG, 0% IgE |
| MacLean 1992 | 34 | Sialyl‐Tn | yes | ELISA | unknown | 96% |
| Mohebtash 2011 | 14 | MUC1, CEA | no | |||
| Morse 2011 | 8 | APC, HHR6A, BAP31, replication protein A, Abl‐binding protein 3c, cyclin I, toposiomerase IIα/β, integrin β 8 subunit precursor, CDC2, TACE, g‐catenin, EEDDR1 | no | |||
| Nishikawa 2006 | 4 | NY‐ESO‐1 | no | |||
| O'Cearbhaill 2016 | 24 | GM2, Globo‐H, Tn, TF, sTN | yes | ELISA | IgM titer > 1:80 or at least 4‐fold increase from baseline | IgM: GM2 25%, Globo‐H 8%, Tn 58%, TF 67%, sTn 92% IgG: GM2 17%, Globo‐H 58%, Tn 83%, TF 25%, sTN 67% 20/24 responded to at least 3 antigens |
| Odunsi 2007 | 18 | NY‐ESO‐1 | yes | ELISA | unknown | 22% |
| Odunsi 2012 | 22 | NY‐ESO‐1 | yes | ELISA | unknown | 50% |
| Odunsi 2014 | 12 | NY‐ESO‐1 | yes | ELISA | reciprocal titer > 100 | 4 patients remained seropositive 5/6 became seropositive no differences between cohorts. |
| Ohno 2009 | 6 | WT1 | no | |||
| Peethambaram 2009 | 4 | Her‐2/Neu | yes | ELISA | unknown | unknown |
| Rahma 2012 | 21 | p53 | no | |||
| Sabbatini 2000 | 25 | Lewis Y | yes | ELISA | unknown | 67% |
| Sabbatini 2007 | 11 | GM2, Globo‐H, Lewis Y, Tn‐MUC1, Tn(c), sTN(c), TF(c) | yes | ELISA | negative to ≥ 1:40 or 8‐fold increase | 89% ≥ 3 antigens; 22% GM2, 33% Globo‐H, 11% Lewis Y, 100% Tn‐MUC1, 44% Tn(c), 44% sTN(c), 78% TF(c) |
| Sabbatini 2012 | 28 | NY‐ESO‐1 | yes | ELISA | ≥ 100 | cohort 1: 25%, C2: 46%, C3: 91% |
| Sabbatini 2017 | 86 | Globo‐H, GM2, MUC1‐TN, TF | yes | unknown | 1:40 or 2‐fold increase | IgG: GLOBO‐H 7%, GM2 8%, MUC1‐TN 32%, MUC1 45%, TF 13% IgM: GLOBO‐H 21%, GM2 26%, MUC1‐TN 40%, MUC1 49%, TF 22% |
| Sandmaier 1999 | 7 | Sialyl‐Tn | yes | ELISA | ≥ 1:20 | 100% IgM, 80% IgG |
| Suzuki 2016 | 32 | GPC3 | no | |||
| Takeoka 2017 | 2 | NY‐ESO‐1 | yes | ELISA | optical density cutoff value 0.47 | > 2 |
| Takeuchi 2013 | 38 | HLA‐A24: FOXM1, MELK, HJURP, VEGFR1, VEGFR2 HLA‐A02: HIG2, VEGFR1, VEGFR2 |
no | |||
| Tsuda 2004 | 5 | patient‐tailored cocktail | yes | ELISA | unknown | 67% |
| Vermeij 2012 | 12 | p53 | no |
SD: standard deviation.
Cellular responses
Thirteen out of 20 monoclonal antibody studies investigated induction of T‐cells against the target antigen (Table 11). Investigators evaluated the presence of antigen‐specific T‐cells using commonly applied tests, such as interferon‐gamma (IFN‐γ) ELISPOT (Ehlen 2005; Gordon 2004; Method 2002; Sabbatini 2006), proliferation assay (Ma 2002; Noujaim 2001; van Zanten‐Przybysz 2002), cytokine profiling (Noujaim 2001; Pfisterer 2006), IFN‐γ secretion assay (Ströhlein 2009), and IFN‐γ intracellular staining assay (Buzzonetti 2014). One study used the leucocyte migration inhibition assay, which nowadays is rarely used (Wagner 1993). As described above for humoral responses, response definitions were frequently lacking or inadequate. Nevertheless, results showed cellular immunity against CA‐125 for 21% to 80% of participants. One study retrospectively compared cellular immune response after CA‐125 monoclonal antibody treatment versus placebo but noted no significant differences (31.8% intervention vs 26.3% control) (Buzzonetti 2014). Antibody treatment targeting the membrane folate receptor did not however induce cellular responses (van Zanten‐Przybysz 2002). Only two studies reported recognition of autologous tumour cells by induced T‐cells, describing positive responses in five out of eight and one out of two patients, respectively (Gordon 2004; Ströhlein 2009).
10. Definitions and results of cellular responses in antigen‐specific antibody studies.
| Study | N | Dose | Target antigen | Analysed | Assay | Positive if: | % positive |
| Baumann 2011 | 45 | C1: 10‐10‐10‐10 μg C2: 10‐20‐50‐100 μg |
EpCAM | no | |||
| Berek 2001 | 252 | 2 mg | CA‐125 | no | |||
| Berek 2004 | 145 | 2 mg | CA‐125 | no | |||
| Berek 2009 | 371 | 2 mg | CA‐125 | no | |||
| Braly 2009 | 40 | unknown | CA‐125 | yes | ELISPOT | permutation test | 44% vs 21% |
| Buzzonetti 2014 | 129 | 2 mg | CA‐125 | yes | flow cytometry | patients with a CA‐125‐CTL count above 0.410 × 10^6 (=90th percentile level of CA‐125‐specific CTL count in the placebo arm) for at least 1 of the time points throughout the study | 31.8% (treatment arm) vs 26.3% (placebo arm) |
| Ehlen 2005 | 13 | 2 mg | CA‐125 | yes | ELISPOT | permutation test | n = 4 CA‐125: 75%; n = 3 oregovomab 67% |
| Gordon 2004 | 20 | 2 mg | CA‐125 | yes | ELISPOT | permutation test | n = 18 CA‐125: 39%; n = 8 oregovomab 50%; n = 8 autologous tumour cells 63% |
| Heiss 2010 | 129 | 10‐20‐5‐150 μg | EpCAM | no | |||
| Ma 2002 | 4 | unknown | CA‐125 | yes | proliferation assay | unknown | n = 4: 50% |
| Method 2002 | 102 | 2 mg | CA‐125 | yes | ELISPOT | not reported | not reported |
| Möbus 2003 | 44 | 2 mg | CA‐125 | no | |||
| Nicholson 2004 | 26 | 25 mg | MUC1 | no | |||
| Noujaim 2001 | 184 | 2 mg | CA‐125 | yes | proliferation assay/ cytokine ELISA | proliferation assay: Wilcoxon signed rank test; cytokine ELISA: unknown | n = 17 CA‐125 53%; Th1 cytokines 41%, Th2 cytokines 94% |
| Pfisterer 2006 | 36 | 2 mg | CA‐125 | yes | cytokine flow cytometry | > 2‐fold increase in IFN‐γ‐expressing T‐cells | L vs S: n = 12 vs 17, CD4: 58% vs 29%; CD8 75% vs 18% |
| Reinartz 2004 | 119 | 2 mg | CA‐125 | no | |||
| Sabbatini 2006 | 42 | 2 mg/0.2 mg | CA‐125 | yes | ELISPOT | spots experimental wells ‐ control wells > 20 and experimental wells/control wells > 1.5× | n = 5: 80% |
| Sabbatini 2013 | 888 | 2 mg | CA‐125 | yes | not reported | not reported | |
| Schultes 1998 | 75 | 2 mg | CA‐125 | no | |||
| Ströhlein 2009 | 2 | 10/20/40 μg 10/40/80 μg |
EpCAM Her‐2/Neu |
yes | IFN‐γ secretion assay | unknown | EpCAM n = 1 (100%) Her‐2/Neu n = 1 (0%) |
| van Zanten‐Przybysz 2002 | 5 | 50 mg | membrane folate receptor | yes | proliferation assay | unknown | 0% |
| Wagner 1993 | 58 | 1 mg | CA‐125 | yes | leucocyte migration inhibition assay | unknown | 21% |
CTL: cytotoxic T‐cell.
A total of 35 out of 44 studies evaluated antigen‐specific cellular immune responses with the use of other vaccine types (Table 12). The most frequently used assay was the IFN‐γ ELISPOT assay, which sometimes was used to separately analyse CD4+ and/or CD8+ cells. Again, response definitions for positive and/or vaccine‐induced responses were frequently absent or unclear (15 out of 44). Six of eight studies targeting NY‐ESO‐1 induced antigen‐specific T‐cells, with percentages of patients with NY‐ESO‐1‐specific CD8+ ranging from 33% to 92% (Dhodapkar 2012; Diefenbach 2008; Nishikawa 2006; Odunsi 2007; Odunsi 2012; Odunsi 2014; Sabbatini 2012), and one study did not report the results for ovarian cancer participants separately (Dhodapkar 2012). Another study showed a positive NY‐ESO‐1‐specific CD8+ T‐cell induction by IFN‐γ catch assay (1% to 5% positive CD8+ T‐cells) (Takeoka 2017). After treatment with vaccines targeting p53, investigators observed p53‐specific T‐cells in 64% to 100% of patients, irrespective of the type of vaccine (Leffers 2009a; Rahma 2012; Vermeij 2012). One study compared p53‐specific T‐cell responses between treatment with a p53‐targeting vaccine plus chemotherapy and PegIntron versus chemotherapy and PegIntron versus chemotherapy alone. Immune response rates were 100%, 22%, and 0%, respectively (Dijkgraaf 2015), indicating that applying chemotherapy and PegIntron at the same time as antigen‐targeted immunotherapy may induce a stronger immune response. Studies targeting multiple antigens demonstrated antigen‐specific cellular immunity with varying immunogenicity of the different antigens targeted (Antonilli 2016; Berinstein 2012; Brossart 2000; Chianese‐Bullock 2008; Chu 2012; Gray 2016; Kaumaya 2009; Kawano 2014; Lennerz 2014; Mohebtash 2011; Morse 2011; Suzuki 2016; Tsuda 2004). Finally, a study testing dendritic cell‐based immunotherapy showed no induction of IFN‐γ‐specific CD4+ and CD8+ cells by flow cytometry, although tetramer staining of WT1‐specific cytotoxic T‐lymphocytes did show an increase in 12 out of 17 patients (70.6%) (Kobayashi 2014).
11. Definitions and results of cellular responses in other antigen‐specific immunotherapy studies.
| Study | N | Target antigen(s) | Analysed | Assay | Positive if: | % positive |
| Antonilli 2016 | 10 | MUC1 ± ErbB1 ± CEA | yes | IFN‐γ ELISPOT delayed hypersensitvity test |
ELISPOT: 2‐fold increase in IFN‐γ production Delayed hypersensitvity test: unknown |
ELISPOT: 6/7 + 0/3 Delayed hypersensitvity test: 3/7 |
| Berinstein 2012 | 6 | topoisomerase IIα, integrin β8 subunit precursor, ABI‐binding protein C3, TACE/ADAM17, junction plakglobin, EDDR1, BAP31 | yes | pentamer staining (CD8) | > 2× increase in pentamer‐positive CD8‐cells | 83% against at least 1 peptide |
| Berinstein 2013 | 19 | survivin | yes | ELISPOT tetramer staining intracellular cytokine staining |
unknown | combined results cohort 2 + 3: 92% on ≥ 2 assays |
| Brossart 2000 | 3 | Her‐2/Neu, MUC1 | yes | intracellular IFN‐γ staining (CD8) | unknown | n = 1: Her‐2/Neu 100%; n = 2: MUC1 50% |
| Chianese‐Bullock 2008 | 9 | FBP, Her‐2/Neu, MAGE‐A1 | yes | ELISPOT (CD8) | unknown | n = 9: FBP 40%, Her‐2/Neu 83%, MAGE‐A1 83% |
| Chu 2012 | 14 | Her‐2/Neu, hTERT, PADRE | yes | ELISPOT tetramer staining (CD8) |
unknown | hTERT: cohort 1: 100%, cohort 2: 100% Her‐2/Neu: cohort 1: 60%, cohort 2: 0% PADRE: cohort 1: 60%, cohort 2: 60% |
| Diefenbach 2008 | 6 | NY‐ESO‐1 | yes | ELISPOT intracellular cytokine staining |
unknown | not reported |
| Dijkgraaf 2015 | 6 | Cohort 3: gemcitabine, PegIntron, and p53 SLP vaccine | yes | IFN‐γ ELISPOT | > 3‐fold change compared to baseline | cohort 3: 6/6 |
| Dhodapkar 2012 | 9 | NY‐ESO‐1 | yes | ELISPOT/Tetramer staining (CD8) | specific spots > 30 and > 3× spots irrelevant control > 0.1% tetramer‐positive CD8‐cells | both assays n = 9: 67% |
| Freedman 1998 | 30 | Sialyl‐Tn | no | |||
| Galanis 2010 | 21 | CEA | no | |||
| Goh 2013 | 63 | MUC1 | yes | unknown | not reported | |
| Gray 2016 | 56 | MUC1 | yes | intracellular cytokine staining (CD4/CD8) | unknown | inadequately reported |
| Gribben 2005 | 6 | CYP1B1 | yes | ELISPOT | spots minus negative control > 20/10⁶ PBMC and > 2× baseline | n = 5: 20% |
| Gulley 2008 | 3 | CEA, MUC1 | yes | ELISPOT (CD8)/IFN‐γ ELISA (CD4) | ELISPOT: ≥ 2‐fold increase in IFN‐γ‐secreting cells IFN‐γ ELISA: unknown |
n = 3: 100% CEA n = 3: 33% CEA |
| Imhof 2013 | 15 | TERT, survivin | yes | intracellular cytokine staining | unknown | overall > 90% |
| Kaumaya 2009 | 5 | Her‐2/Neu | no | |||
| Kawano 2014 | 42 | personalised (max 4 out of 31 vaccine candidates) | yes | ELISPOT | 2‐fold higher values post‐vaccination than pre‐vaccination | 6 vaccinations: 18/42 12 vaccinations: 19/42 |
| Kobayashi 2014 | 56 | WT1 ± MUC1 ± CA‐125 | yes | Flow cytometry (CD4/CD8/NK) Tetramer staining (WT‐1 CTLs) |
unknown | flow cytometry: no chances in CD4+, CD8+, and NK cell frequency tetramer staining: 12/17 increased |
| Le 2012 | 2 | mesothelin | yes | ELISPOT (CD8) | specific spots > 2× baseline and ≥ 1 per 10⁵ PBMC | n = 1 evaluable, mesothelin‐specific CD8 cells present |
| Leffers 2009a | 20 | p53 | yes | ELISPOT proliferation assay intracellular cytokine staining (CD4/CD8) |
specific spots ≥ 10/10⁵ PBMC and ≥ 3× pre‐immunisation cpm > 1000/min, SI ≥ 3, and ≥ 2× pre‐immunisation ≥ 3 pre‐immunisation |
n = 18: 100% n = 17: 82% n = 5: CD8 0%, CD4 100% |
| Lennerz 2014 | 7 | survivin | yes | ELISPOT/HLA‐multimer staining | ELISPOT (CD8): spot number > 10 and 2‐fold higher than background and 2‐fold higher than standard deviation of all combined negative values HLA‐multimer staining: detection of > 50 cells in the multimer gate, minimum percentage of 0.03% CD8+ cells |
ex vivo ELISPOT: n = 0/7 in vivo ELISPOT: n = 1/2 ex vivo multimer: n = 2/5 in vivo multimer: n = 3/4 |
| Letsch 2011 | 8 | WT1 | yes | tetramer staining | unknown | not reported |
| MacLean 1996 | 10 | Sialyl‐Tn | no | |||
| MacLean 1992 | 34 | Thomsen Friedenreich | no | |||
| Mohebtash 2011 | 14 | MUC1, CEA | yes | ELISPOT (CD8) | ≥ 2× pre‐immunisation | n = 2: 0%; MUC1‐specific CD8 cells 50%, CEA‐specific CD8 cells |
| Morse 2011 | 8 | APC, HHR6A, BAP31, replication protein A, Abl‐binding protein 3c, cyclin I, toposiomerase IIα/β, integrin β 8 subunit precursor, CDC2, TACE, g‐catenin, EEDDR1 | yes | ELISPOT | > 40 spots/10⁶ PBMC over pre‐vaccination | n = 8: 63% |
| Nishikawa 2006 | 4 | NY‐ESO‐1 | yes | ELISPOT (CD4) | unknown | n = 4: 75% |
| O'Cearbhaill 2016 | 24 | GM2, Globo‐H, Tn, TF, sTN | no | |||
| Odunsi 2007 | 18 | NY‐ESO‐1 | yes | ELISPOT (CD4/CD8) | mean ± 3 SD | n = 18; CD4: 83%, CD8: 33% |
| Odunsi 2012 | 22 | NY‐ESO‐1 | yes | ELISPOT (CD4/CD8) intracellular cytokine staining (CD8) |
unknown | CD4: 91% CD8: 45% |
| Odunsi 2014 | 12 | NY‐ESO‐1 | yes | ELISPOT (CD4/CD8) tetramer staining |
ELISPOT: spot numbers in the presence of target cells exceeded cutoff value (> 50 spots/50,000 cells) + at least 3 times more spots than unpulsed target cells tetramer: > 0.1% tetramer‐positive cells are CD8+ T‐cells and at least 3 times more than the percentage obtained with control tetramer. |
CD8: 5/11 (45%), of which 3 de novo inductions CD4: 7/10 (70%), of which 2 de novo responses tetramer staining: 2× NY‐ESO‐1 CD8 cell expansion |
| Ohno 2009 | 6 | WT1 | no | |||
| Peethambaram 2009 | 4 | Her‐2/Neu | yes | proliferation assay ELISPOT assay |
unknown | not reported separately for ovarian cancer patients |
| Rahma 2012 | 21 | p53 | yes | ELISPOT tetramer staining |
≥ 2× pre‐immunisation | cohort 1: 64%, cohort 2: 83% |
| Sabbatini 2000 | 25 | Lewis Y | no | |||
| Sabbatini 2007 | 11 | GM2, Globo‐H, Lewis Y, Tn‐MUC1, Tn(c), sTN(c), TF(c) | no | |||
| Sabbatini 2012 | 28 | NY‐ESO‐1 | yes | ELISPOT (CD4/CD8) | > 50 spots/5 × 10⁴ cells and > 3× unstimulated cells | CD4: 100% in cohort 1, 2, and 3 CD8: cohort 1: 0%, cohort 2: 62%, cohort 3: 92% |
| Sabbatini 2017 | 171 | Globo‐H, GM2, MUC1‐TN, TF | no | |||
| Sandmaier 1999 | 7 | Sialyl‐Tn | yes | proliferation assaya | > upper limit of normal (SI 2.35) | n = 4: 50% |
| Suzuki 2016 | 32 | GPC3 | yes | ELISPOT (CD8) | unknown | n = 15/24: 62.5% |
| Takeoka 2017 | 2 | NY‐ESO‐I | yes | IFN‐γ catch assay (CD4/CD8) | > 0.5% | CD4: n = 2; > 5% CD8: n = 2; 1% to 5% |
| Takeuchi 2013 | 38 | HLA‐A24: FOXM1, MELK, HJURP, VEGFR1, VEGFR2 HLA‐A02: HIG2, VEGFR1, VEGFR2 |
yes | unknown | unknown | inadequately reported |
| Tsuda 2004 | 5 | patient‐tailored cocktail | yes | IFN‐γ ELISA | unclear | n = 2 after 6 vacc 100%; n = 1 after 12 vacc 100% |
| Vermeij 2012 | 12 | p53 | yes | ELISPOT proliferation assay |
specific spots ≥ 10/10⁵ PBMC and ≥ 3× pre‐immunisation cpm > 1000/min, SI ≥ 3, and ≥ 2× pre‐immunisation |
90% after 2 vacc, 87.5% after 4 vacc 80% after 2 vacc, 62.5% after 4 vacc |
aas measured after at least three immunisations. C1: cohort 1. SD: standard deviation. SI: stimulation index.
Secondary outcomes
Carrier‐specific immunogenicity
Most studies using a monoclonal antibody (18/22) used a murine antibody, two studies used a trifunctional rat‐mouse hybrid (Baumann 2011; Heiss 2010), and one study used a chimeric antibody construct (van Zanten‐Przybysz 2002). Next to antigen‐specific immunity, 16 studies assessed the induction of human‐anti‐mouse antibodies (HAMAs) using HAMA‐specific ELISA assays (Table 13). HAMAs were present in 4% to 97% of participants immunised (Baumann 2011; Berek 2004; Braly 2009; Ehlen 2005; Gordon 2004; Method 2002; Möbus 2003; Pfisterer 2006; Reinartz 2004; Sabbatini 2006; Schultes 1998). It seems that this large variation between studies cannot be attributed to differences in dosage but is best ascribed to different definitions of a HAMA response (i.e. some studies report only robust responses, whereas others report all responses above a certain threshold). Furthermore, the point in time at which HAMA titres were measured is of importance, as responses increase in frequency and strength with repeated administration of the antibody (Baumann 2011; Gordon 2004; Method 2002; Möbus 2003).
12. Definitions and results of human‐anti‐mouse antibody (HAMA) evaluation in antigen‐specific antibody studies.
| Study | N | Dose | Target antigen | Analysed | Positive if: | % positive | Robust if: | % robust |
| Baumann 2011 | 45 | C1: 10‐10‐10‐10 μg C2: 10‐20‐50‐100 μg |
EpCAM | yes | unknown | C1: 61%, C2: 100% | ||
| Berek 2001 | 252 | 2 mg | CA‐125 | yes | > 5000 ng/mL | 51% | ||
| Berek 2004 | 145 | 2 mg | CA‐125 | yes | > 200 ng/mL | unknown | > 5000 ng/mL | 59% |
| Berek 2009 | 371 | 2 mg | CA‐125 | yes | unknown | n.r. | ||
| Braly 2009 | 40 | unknown | CA‐125 | yes | unknown | SIM vs OWD: 100% vs 80% | > 3000 ng/mL | SIM vs OWD: 88% vs 74% |
| Buzzonetti 2014 | 129 | 2 mg | CA‐125 | yes; reported in Sabbatini 2013 | ||||
| Ehlen 2005 | 13 | 2 mg | CA‐125 | yes | > 200 ng/mL | 100% | > 5000 ng/mL | 58% |
| Gordon 2004 | 20 | 2 mg | CA‐125 | yes | > 200 ng/mL | unknown | > 5000 ng/mL | 79% |
| Heiss 2010 | 129 | 10‐20‐50‐150 μg | EpCAM | yes | unknown | not reported | ||
| Ma 2002 | 4 | unknown | CA‐125 | no | ||||
| Method 2002 | 102 | 2 mg | CA‐125 | yes | > 200 ng/mL | unknown | unknown | 4% vs 36% vs 39% |
| Möbus 2003 | 44 | 2 mg | CA‐125 | yes | > 5000 ng/mL | 68% | ||
| Nicholson 2004 | 26 | 25 mg | MUC1 | no | ||||
| Noujaim 2001 | 184 | 2 mg | CA‐125 | no | ||||
| Pfisterer 2006 | 36 | 2 mg | CA‐125 | yes | > 15 ng/mL | L vs S: 94% vs 100% | ||
| Reinartz 2004 | 119 | 2 mg | CA‐125 | yes | > 100 ng/mL | 78% | ||
| Sabbatini 2006 | 42 | 2 mg/0.2 mg | CA‐125 | yes | > 100 ng/mL | 90% | ||
| Sabbatini 2013 | 888 | 2 mg | CA‐125 | yes | unknown | inadequately reported | ||
| Schultes 1998 | 75 | 2 mg | CA‐125 | yes | > 200 ng/mL | 90% | ||
| Ströhlein 2009 | 2 | 10/20/40 μg 10/40/80 μg |
EpCAM Her2/Neu |
yes | unknown | 100% (n = 1) | ||
| van Zanten‐Przybysz 2002 | 5 | 50 mg | membrane folate receptor | n.a. | ||||
| Wagner 1993 | 58 | 1 mg | CA‐125 | no |
n.r.: not reported.
Although eight studies investigated synthetic carbohydrate antigens conjugated to the keyhole limpet haemocyanin (KLH) carrier protein (Freedman 1998; MacLean 1992; MacLean 1996; O'Cearbhaill 2016; Sabbatini 2000; Sabbatini 2007; Sabbatini 2017; Sandmaier 1999), only one study reported on KLH‐specific immunity (Sandmaier 1999). In this study, proliferative responses to stimulation with KLH and the KLH‐antigen complex were substantially stronger than responses to the synthetic carbohydrate itself in all women with ovarian cancer tested, similar to what has previously been reported for viral vectors.
Five studies reported use of recombinant viruses or bacteria as vectors (Galanis 2010; Gulley 2008; Le 2012; Mohebtash 2011; Odunsi 2012). Three of these studies reported that they investigated anti‐vector immune responses. One study used a recombinant pox‐virus induced anti‐vector immunity for all participants with ovarian cancer (Gulley 2008). Another study used a recombinant measles virus and did not show any differences in anti‐measles‐antibody titres, although inclusion criteria required that included participants must be immune to measles virus (Galanis 2010). In the third study, use of live‐attenuated listeria did result in virus‐specific T‐cells in some cancer patients; however, too few patients with ovarian cancer were tested to permit any conclusions regarding this specific disease entity (Le 2012).
Adverse events
For this review, we defined adverse events as any adverse changes in health or side effects that occurred in a clinical study participant receiving treatment, irrespective of whether the event could be attributed to the treatment received.
Although 56 studies mentioned adverse events; sufficiently detailed information on adverse events that occurred during the study was available for 43 out of 67 studies. Thirty‐four studies explicitly mentioned local adverse events, all of which involved local administration of the vaccine (i.e. intradermal, intramuscular, or subcutaneous injection). When local adverse events were further specified, these were best summarised as pain at the injection site and local inflammatory responses (erythema, induration, pruritis). Researchers observed ulceration and/or abscesses at the injection site in nine of 89 participants with varying types of cancer participating in four studies (Berinstein 2012; Berinstein 2013; Freedman 1998; Gribben 2005). One study described a patient with a grade III infection presenting with lower‐limb lymphoedema at the injection site, which was attributed to the vaccine. This patient underwent a pelvic lymphadenectomy during the primary debulking surgery, suggesting in this case that women who have undergone pelvic lymphadenectomy might be less suitable for vaccination of the lower limbs (Kawano 2014).
Systemic adverse events occurred in 42 studies, and four studies explicitly reported that systemic adverse events did not occur. Two studies explicitly reported autoimmunity. In one study, a patient with strong immunological responses to the vaccine developed symptomatic hypothyroidism necessitating replacement therapy (Diefenbach 2008). Study authors described minor induction of anti‐nuclear antibodies (grade I according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0 (Trotti 2003)) for two patients receiving a multi‐peptide vaccine (Chianese‐Bullock 2008). Allergic reactions occurred in a total of 14 participants (Berek 2009; Braly 2009; Ehlen 2005; MacLean 1992; Möbus 2003; Pfisterer 2006; Ströhlein 2009). Allergic reactions (e.g. hypersensitivity, allergic exanthema, urticaria) were mild and were easily managed. Continuation of study treatment did not result in renewed allergic reactions (Braly 2009; Ehlen 2005; Möbus 2003; Pfisterer 2006). Treatment with chemotherapy, an anti‐methylation agent, and an NY‐ESO‐1‐targeting vaccine resulted in clinically manageable adverse events (Odunsi 2014).
Other reported systemic adverse events, irrespective of whether attributable to the investigated drug, included haematological changes (e.g. anaemia, leucopenia), flu‐like symptoms (including fatigue, myalgia, arthralgia, headache, fever, and chills), and gastrointestinal events (e.g. nausea, vomiting, diarrhoea, abdominal pain), most of which were classified as grade I or II events. Thirty‐three studies reported serious (CTCAE grade III or IV) adverse events that varied from recurrent or progressive disease to local ulceration at the injection site, and from abdominal pain, neutropenia, and fever to elevated liver enzymes. One study compared standard of care versus MUC1 dendritic cell therapy. Respectively, 8% versus 27% of participants suffered an adverse event grade III or IV (Gray 2016). Another study combining vaccination with chemotherapy reported 10 high‐grade adverse events, nine of which were attributed to the chemotherapy (Kawano 2014). In addition, one study comparing chemotherapy alone versus chemotherapy and PegIntron versus chemotherapy, PegIntron, and p53 vaccination reported grade III or IV adverse events in 50% of participants, with no significant differences between treatment groups (Dijkgraaf 2015). A study combining chemotherapy, an anti‐methylation agent, and an NY‐ESO‐1‐targeting vaccine described three serious adverse events, which study authors did not attribute to any of the investigated drugs (Odunsi 2014). Twenty studies reported no serious adverse events. Ten studies did not mention lack or presence of serious adverse events (Berek 2001; Imhof 2013; Ma 2002; MacLean 1996; Möbus 2003; Nishikawa 2006; Noujaim 2001; Sandmaier 1999; Schultes 1998; Wagner 1993).
Discussion
Summary of main results
The aim of this review was to evaluate the clinical and immunological efficacy of antigen‐specific active immunotherapy in ovarian cancer, whilst also obtaining an impression of the safety and tolerability of this treatment modality. The antigen‐specific active immunotherapy described in this review can largely be divided into two strategies: (1) administration of antibodies targeting a specific tumour antigen and (2) administration of, or parts of, a specific tumour antigen itself. As expected, most studies were non‐randomised controlled trials (NRSs).
Data suggest that almost all strategies are capable of inducing an immunological response to some extent. Furthermore, only two studies evaluated recognition of autologous tumour cells in vitro, and no studies evaluated immune responses at the tumour site. Although obtaining autologous tumour material may be burdensome, such assays would be extremely valuable, as they comprise true interactions between induced immunity and tumour cells and as such could provide important information on how immunotherapeutic strategies can continue to be improved to reach clinical effectiveness. Even though comparison between studies is difficult, it seems that most antigen‐specific therapies, independent of the target, are able to induce at least a minimal immune response.
Clinical responses to immunotherapy (i.e. tumour responses, responses to post‐immunotherapy treatment, and survival benefits) were observed only incidentally, and their occurrence cannot be used to draw a reliable conclusion. The indication for immunotherapeutic treatment in the adjuvant setting is supported by the observation of enhanced antigen‐specific responses to immunotherapy when combined with chemotherapeutic agents currently or previously used in the primary treatment of ovarian cancer (i.e. docetaxel or cyclophosphamide) (Garnett 2008; Laheru 2008). However, four large randomised controlled trials (RCTs) using a monoclonal cancer antigen (CA)‐125 antibody in the adjuvant setting after successful primary therapy did not demonstrate any differences in time to relapse and/or overall survival between treatment and placebo arms (Berek 2001; Berek 2004; Berek 2009; Sabbatini 2013), which indicates that despite immunogenicity, CA‐125‐targeted monoclonal antibody therapy is clinically ineffective. For studies of other vaccine types, no such conclusions can be made at this time, as large RCTs and more studies in the adjuvant rather than recurrent setting have yet to be performed to examine the different strategies.
Eighty per cent of studies reported adverse events in sufficient detail for interpretation. Study authors made a distinction between local and systemic events and further subdivided the latter into autoimmunity, allergy, and other adverse events. We did not evaluate whether adverse events could be or were considered attributable to the treatment studied, although for local adverse events, this is indisputably the case. Studies using intradermal, subcutaneous, or intramuscular application have frequently reported inflammatory reactions and pain at the injection site, with ulceration at the most severe side of the spectrum. Severe or life‐threatening systemic adverse events occurred in approximately 50% of studies. Thirty per cent of studies explicitly described the lack of severe adverse events. For monoclonal antibody studies, researchers could identify no pattern suggestive of an underlying treatment‐associated process and often considered events to be associated with ovarian cancer progression.
In summary, this review describes 67 immunotherapy studies including 3632 women with ovarian cancer. It seems that although all strategies described are capable of inducing immunological responses, be it humoral or cellular, clinical effectiveness thus far has not been convincingly demonstrated. The largest body of evidence is available for CA‐125‐directed antibody therapy, which has been studied in 2347 people participating in 17 studies. As only one study reported complete or partial clinical responses and four large RCTs did not demonstrate any clinical benefit of antibody treatment, we believe it is unlikely that the clinical effectiveness of CA‐125‐directed antibody therapy for ovarian cancer will ever be obtained. It is possible that inducing an immunological response alone is not enough to derive clinical benefit owing to immune suppressive characteristics of the tumour. To overcome this suppression, combining antigen‐specific immunotherapy with other forms of immunotherapy (e.g. checkpoint inhibitors, chemotherapy, poly ADP ribose polymerase (PARP) inhibitors, anti‐methylation agents) might be necessary to achieve clinical response. However, in view of the immunological responses and the usually mild side effects reported, we believe that further investigation of other antigen‐specific active immunotherapy strategies in ovarian cancer is worthwhile.
Overall completeness and applicability of evidence
The most striking observations of this review unfortunately do not concern the aim of the review but address lack of uniformity in the conduct and reporting of early‐phase immunotherapy studies.
According to the GRADE rating, only certainty for the primary outcome survival is assessed as 'high', whereas that for all other outcomes is assessed as 'very low' (Table 1). Of note, most of the RCTs that were analysed for survival were investigating a CA‐125 monoclonal antibody. Their results may not be applicable in a similar way for other strategies using antigen‐specific immune therapy for ovarian carcinoma.
Reliability of the results for clinical response to immunotherapy was questionable because clear response definitions were lacking, and because concomitant immunotherapy or administration of additional treatment after immunotherapy often was not described. Furthermore, for studies that used a monoclonal antibody targeting CA‐125, use of CA‐125 as a marker for clinical response is questionable. An additional important comment regarding the likelihood of clinical response to immunotherapy, especially in uncontrolled studies, which frequently include patients with recurrent disease, is the fact that this likelihood may be affected by disease status at the start of treatment (Leffers 2009).
In addition, antigen‐specific humoral and/or cellular immunogenicity of different interventions showed great variation for both monoclonal antibody studies and studies examining other strategies. This variation may be attributed at least in part to variation in the immunological response definitions used by different study authors. Therefore it is not possible to reliably compare studies and infer which intervention and/or immunisation strategy is most promising for the induction of strong anti‐tumour immunity.
A disturbing observation regarding adverse events is the lack of uniformity in adverse event reporting. Reporting of safety and tolerability of new treatment strategies should have high priority in all studies of investigational drugs, especially in uncontrolled phase I and II studies. To promote uniformity in adverse event evaluation and reporting, as well as comparability of adverse events between studies, in addition to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) (Trotti 2003), the Brighton Collaboration has committed itself to developing standardised, widely disseminated, and globally accepted case definitions for an exhaustive number of adverse events following immunisation, as well as guidelines for data collection, analysis, and presentation (Brighton Collaboration 2009). These case definitions and guidelines are freely available, and we strongly recommend that, when applicable, they be used for all immunotherapeutic studies.
This review emphasises an aspect of immunotherapeutic studies that warrants serious attention in the immunotherapeutic scientific community, that is, lack of consensus on (1) what assays should be used to establish immunogenicity of an intervention (Britten 2008), (2) what cutoffs should be used to define true immunological responses, and (3) what response definitions should be used to determine clinical efficacy. Given these large inconsistencies, it is evident that elucidation of which type of immunological response is necessary for and/or is a surrogate marker of clinical activity of an immunotherapeutic intervention is burdensome.
Quality of the evidence
We assessed the included studies for risks of bias, using the Cochrane 'Risk of bias' tool. Risk of bias items, especially selection, attrition, and selective reporting bias, are likely to affect the studies included in this review.
It is interesting to note that for 10 studies described in this review, review authors collected study information only from a meeting abstract that was several years old. The lack of full‐text manuscripts, even after contact was made with abstract authors, strongly suggests the existence of a publication bias. To avoid the disappearance of negative studies, registration of trials in a prospective trial register is widely recommended and is supported by the International Committee of Medical Journal Editors (ICMJE). However, at first, in 2005, registration was requested only for RCTs. Since July 1, 2008, all trials prospectively assigning human participants to one or more health‐related interventions for evaluation of their effects on health outcomes are required to be registered in a clinical trial register approved by the World Health Organization (WHO). From the ongoing studies section, it is apparent that despite registration in a prospective trial register, studies may suffer from publication bias, as several relatively small studies that began more than five years ago have not yet been published to date nor closed according to the trial register. In addition to registration in trial registers, the uniform requirements for manuscripts submitted to biomedical journals drafted by the ICMJE encourage uniformity in reporting of clinical trials by stating ethical principles for the conduct and reporting of research and by providing recommendations related to specific elements of editing and writing. As is obvious from this review, the scientific community might benefit substantially if early‐phase uncontrolled clinical trials would also strive for uniformity in trial conduct and reporting.
Potential biases in the review process
We minimised potential biases in the review process by searching the literature from a variety of sources with no restrictions on date of publication. At least two review authors independently extracted and assessed data.
To minimise the chances of error and bias, review authors adhered to Cochrane guidelines for selection of studies, extraction of data, and assessment of the certainty of evidence and potential risks of different types of biases in all included studies.
Agreements and disagreements with other studies or reviews
Our findings are in broad agreement with those presented by most systematic reviews on antigen‐specific active immunotherapy for ovarian cancer (Drerup 2015; Hardwick 2016; Odunsi 2017). However, the focus of current publications leans more towards immunotherapy in general (e.g. whole tumour lysate‐targeting immunotherapy, immune checkpoint blockade, cytokine induction, adoptive cell transfer) and not towards antigen‐specific immunotherapy alone. The general consensus is that antigen‐specific immunotherapy is sufficient for eliciting an immune response, but clinical response to monotherapy is only modest (Drerup 2015; Odunsi 2017). Combining antigen‐specific immunotherapy with other types of immunotherapy, especially immune checkpoint blockade, is a promising approach to be examined by future researchers (Hardwick 2016; Odunsi 2017).
Authors' conclusions
Implications for practice.
At this point, review authors have found no evidence of effective immunotherapy for ovarian cancer. Although promising immunological responses have been observed for most strategies evaluated, they do not coincide with clinical benefits for women with ovarian cancer. Furthermore, no immunological surrogate markers currently correlate with clinical outcomes. Therefore, until evidence of true clinical effectiveness is available, immunotherapy should not be offered as an alternative to standard therapy for primary or recurrent ovarian cancer.
Implications for research.
Our primary recommendation relates to the need for uniformity in trial conduct and reporting. Not until universally accepted immunological and clinical response definitions and guidelines for adverse events reporting are adopted for immunotherapeutic studies will it be possible to make any inferences about the effectiveness of immunotherapy as a treatment for ovarian cancer. Furthermore, expanding evaluation of immunogenicity to include recognition of autologous tumour is advisable. Given the usually mild side effects and the immunological responses witnessed in most studies, we believe that further investigation of antigen‐specific active immunotherapy other than cancer antigen (CA)‐125‐targeted antibody therapy for ovarian cancer in randomised controlled trials is worthwhile. In addition, research combining antigen‐targeted immunotherapy with other forms of immunotherapy to optimise response, and perhaps induce clinical response, is of interest.
What's new
| Date | Event | Description |
|---|---|---|
| 13 March 2018 | New citation required but conclusions have not changed | Review text updated to reflect additional studies, both included and excluded. Overall, conclusions unchanged |
| 1 August 2017 | New search has been performed | Searches re‐run July 2017. New studies included and excluded |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 8 September 2014 | Amended | Author details amended |
| 31 July 2014 | New search has been performed | Searches re‐run October 2013. New studies included and excluded |
| 10 July 2014 | New citation required but conclusions have not changed | Review text updated to reflect additional studies, both included and excluded. Overall, conclusions unchanged |
Acknowledgements
We would like to thank all members of the Cochrane Gynaecological, Neuro‐oncology, and Orphan Cancers Editorial Team for their support.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology, and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Ovarian Neoplasms] explode all trees #2 ovar* near/5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or adenocarcinoma* or malignan*) #3 #1 or #2 #4 MeSH descriptor: [Immunotherapy, Active] explode all trees #5 MeSH descriptor: [Cancer Vaccines] explode all trees #6 immunotherapy or vaccination* or vaccine* or immunization or immunisation #7 #4 or #5 or #6 #8 MeSH descriptor: [Antigens, Neoplasm] explode all trees #9 antigen* #10 #8 or #9 #11 MeSH descriptor: [T‐Lymphocytes] explode all trees #12 (T cell*) or T‐cell* or (T lymphocyte*) or T‐lymphocyte* or CD4* or CD8* #13 #11 or #12 #14 #3 and #7 and #10 and #13
Appendix 2. MEDLINE search strategy
MEDLINE Ovid
1 exp Ovarian Neoplasms/ 2 (ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or adenocarcinoma* or malignan*)).mp. 3 1 or 2 4 exp Immunotherapy, Active/ 5 Cancer Vaccines/ 6 (immunotherapy or vaccination* or vaccine* or immunization or immunisation).mp. 7 4 or 5 or 6 8 exp Antigens, Neoplasm/ 9 antigen*.mp. 10 8 or 9 11 exp T‐Lymphocytes/ 12 (T cell* or T‐cell* or T lymphocyte* or T‐lymphocyte* or CD4* or CD8*).mp. 13 11 or 12 14 3 and 7 and 10 and 13
key: mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier
Appendix 3. Embase search strategy
Embase Ovid
1 exp ovary tumor/ 2 (ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or adenocarcinoma* or malignan*)).mp. 3 1 or 2 4 active immunization/ 5 cancer vaccine/ 6 (immunotherapy or vaccination* or vaccine* or immunization or immunisation).mp. 7 4 or 5 or 6 8 exp tumor antigen/ 9 antigen*.mp. 10 8 or 9 11 exp T lymphocyte/ 12 (T cell* or T‐cell* or T lymphocyte* or T‐lymphocyte* or CD4* or CD8*).mp. 13 11 or 12 14 3 and 7 and 10 and 13
key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 4. Data extraction form
CRITICAL REVIEW & DATA EXTRACTION FORM
Review Title: Antigen‐specific active immunotherapy for ovarian cancer
Date: …………………………… Reviewer: ………………………………
Study Title: ………………………………………………………………….
| First Author | |
| Year of Publication | |
| Country of Publication | |
| Publication Type | Journal/Abstract/Other (specify) |
Study Characteristics
| Study | |
| Study inclusion criteria | |
| Study exclusion criteria | |
| Participants | · Total number of participants: ……………… · Number of patients with EOC: ……………. · Age: o Median + range: …………………… o Mean + SD: ………………………… · FIGO stage: ………………………………… · Histological tumour type: …………………… · Tumour grade: ……………………………… · Previous therapy: …………………………… · Concurrent therapy: ……………………….. |
| Trial intervention | · Type of vaccine: ……………………………… · Antigen used: ………………………………… · Adjuvant used: ………………………………. · Route of vaccination: ………………………… · Vaccination schedule: ………………………. |
Outcomes
| Trial | N + reason |
| Patients excluded during trial | |
| Patients lost to follow‐up |
| Clinical responses | N |
| CA‐125 levels according to GCIG definition | Decreasing: …………………. Stable: ……………………….. Progressing: ………………… Total: …………………………. |
| Tumour response according to RECIST or WHO criteria | Complete remission: …………. Partial remission: …………….. Stable disease: ……………….. Progressive disease: ………….. Total: ……………………………. |
| Post‐immunotherapy treatment | Administered: Yes ? No ? If yes: specify response to post‐immunotherapy treatment: Complete remission: …………. Partial remission: …………….. Stable disease: ……………….. Progressive disease: ………….. Total: ……………………………. |
| Survival | Information on survival available: Yes ? No ? If yes, specify: …………………………………………………………… …………………………………………………………………… |
| Immunogenicity | |
| |
| Humoral responses | Observed Total Assay(s) used: ………………………………………………… |
| Cellular responses | Observed Total Assay(s) used: ………………………………………………… Separate information on cytotoxic T‐lymphocytes and T‐helper lymphocytes available: Yes ? No ? If yes, specify: ……………………………………………………………………… |
| Vaccine‐ or vector‐specific immunogenicity: Applicable Yes ? No ? | |
| Humoral responses | Observed Total Assay(s) used: ………………………………………………… |
| Cellular responses | Observed Total Assay(s) used: ………………………………………………… |
| Adverse events | |
| Type of AEs | · Local events (injection site): Yes ? No ? If yes, specify: ………………… · Systemic: Yes ? No ? If yes: Autoimmunity: Yes ? No ? If yes, specify: …………………………… Allergic reactions: Yes ? No ? If yes, specify: …………………………… Other: Yes ? No ? If yes, specify: …………………………… |
Other
| Contact with primary investigators | Clarify methods ? Clarify results ? |
| Notes |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antonilli 2016.
| Methods | Uncontrolled phase I/II | |
| Participants | 14 high‐risk, disease‐free ovarian cancer (n = 7) or breast carcinoma participants + 3 recurrent OC patients vaccinated for compassionate use | |
| Interventions | Triple peptide (MUC1, ErbB2, and CEA) with Montanide vaccine | |
| Outcomes | Safety Immune response Clinical response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not explicitly stipulated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not explicitly stipulated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not publicly available |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Baumann 2011.
| Methods | Randomised controlled phase II trial | |
| Participants | 45 ovarian cancer patients with evidence of disease after first‐ or second‐line chemotherapy | |
| Interventions | Intraperitoneal trifunctional bispecific antibody (catumaxomab ‐ EpCAM): low dose (10‐10‐10‐10 μg) vs high dose (10‐20‐50‐100 μg) | |
| Outcomes | Tumour responses Survival (progression‐free survival/overall survival) Immune responses: humoral (HAMA) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly stipulated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data insufficient to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not publicly available |
| Other bias | Low risk | No other sources of bias detected |
Berek 2001.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 252 stage III/IV ovarian cancer patients after successful primary surgery and chemotherapy | |
| Interventions | Intravenous monoclonal antibody (oregovomab ‐ CA‐125) vs placebo | |
| Outcomes | Survival (time to relapse) Immune responses: humoral (Ab2, HAMA) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Other bias | High risk | Publication bias possible |
Berek 2004.
| Methods | Randomised placebo‐controlled phase II trial | |
| Participants | 145 stage III/IV ovarian cancer patients with complete clinical response to primary therapy | |
| Interventions | Intravenous monoclonal antibody (oregovomab) vs placebo | |
| Outcomes | Survival (time to relapse/overall survival) Immune responses: humoral (Ab2, HAMA) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other sources of bias detected |
Berek 2009.
| Methods | Randomised placebo‐controlled phase III trial | |
| Participants | 371 stage III/IV ovarian cancer patients with complete clinical response to primary therapy | |
| Interventions | Intravenous monoclonal antibody (oregovomab) vs placebo | |
| Outcomes | Survival (time to relapse) Immune responses Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation procedure |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded to treatment assignment, post‐randomisation immune responses, and CA‐125 measurements |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to treatment assignment, post‐randomisation immune responses, and CA‐125 measurements |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other sources of bias detected |
Berinstein 2012.
| Methods | Uncontrolled phase I study | |
| Participants | 23 late‐stage cancer HLA‐A2+ participants with complete or partial response to primary therapy (ovarian cancer n = 6) | |
| Interventions | Subcutaneous 7 short peptides (topoisomerase IIα, integrin β8 subunit precursor, ABI‐binding protein C3, TACE/ADAM17, junction plakglobin, EDDR1, BAP31) Adjuvant: DepoVax |
|
| Outcomes | Survival (time to progression) Tumour response Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants with OC included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other sources of bias detected |
Berinstein 2013.
| Methods | Uncontrolled phase I study | |
| Participants | 19 women with ovarian cancer with unknown disease status | |
| Interventions | Subcutaneous peptides (survivin) Adjuvant: DepoVax |
|
| Outcomes | Immune responses (cellular) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Braly 2009.
| Methods | Randomised controlled phase II trial | |
| Participants | 40 stage III/IV ovarian cancer patients after primary debulking surgery with or without residual disease | |
| Interventions | Intravenous monoclonal antibody (oregovomab ‐ CA‐125): concurrent (SIM) or delayed (OWD) with standard carboplatin/paclitaxel primary chemotherapy | |
| Outcomes | Survival (progression‐free survival) Clinical responses Immune responses Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other sources of bias detected |
Brossart 2000.
| Methods | Uncontrolled phase I/II study | |
| Participants | 10 participants with measurable residual or recurrent breast or ovarian cancer (3 women with ovarian cancer) | |
| Interventions | Subcutaneous peptide pulsed dendritic cells (n = 1 Her‐2/Neu; n = 2 MUC1) | |
| Outcomes | Tumour responses Immune response Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Buzzonetti 2014.
| Methods | Randomised double‐blind placebo‐controlled trial | |
| Participants | 129 participants (n = 91 treatment arm; n = 38 placebo arm) with ovarian cancer in complete clinical remission after primary treatment | |
| Interventions | Subcutaneous monoclonal antibody (abagovomab ‐ CA‐125) | |
| Outcomes | Immune response Survival |
|
| Notes | Substudy of MIMOSA trial (NCT00418574) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear whether samples used were coded for key study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unknown why and which samples are missing |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other sources of bias detected |
Chianese‐Bullock 2008.
| Methods | Uncontrolled phase I study | |
| Participants | 9 women with ovarian cancer with or without residual or recurrent disease after primary therapy | |
| Interventions | Subcutaneous and intradermal multi‐peptide vaccine (FBP, Her‐2/Neu, MAGE‐A1) Adjuvant: Montanide ISA‐51, GM‐CSF |
|
| Outcomes | Tumour responses Immune response Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Chu 2012.
| Methods | Randomised controlled phase I/II study | |
| Participants | 14 ovarian cancer patients with complete clinical response to primary therapy (10 received treatment so far) | |
| Interventions | Intradermal peptide pulsed dendritic cells (Her‐2/Neu, hTERT, PADRE): vaccine alone vs single dose of cyclophosphamide before first vaccination | |
| Outcomes | Tumour responses Immune response Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding or incomplete blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | High risk | Early termination due to financial limitations |
Dhodapkar 2012.
| Methods | Uncontrolled phase I study | |
| Participants | 45 participants with advanced malignancies; exact disease status unknown (ovarian cancer n = 6) | |
| Interventions | Fusion protein of full‐length tumour antigen and human monoclonal antibody specific for DEC‐205 Adjuvants: TLR agonist resiquimod and/or poly‐ICLC |
|
| Outcomes | Immune responses (cellular and humoral) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Diefenbach 2008.
| Methods | Uncontrolled phase I study | |
| Participants | 9 participants with ovarian cancer with complete clinical response to primary therapy | |
| Interventions | Subcutaneous short peptide (NY‐ESO‐1) Adjuvant: Montanide ISA‐51 |
|
| Outcomes | Survival (time to progression) Tumour responses Immune responses: cellular and humoral Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Dijkgraaf 2015.
| Methods | Controlled phase I/II trial | |
| Participants | 15 participants with platinum‐resistant ovarian cancers expressing 'mutant' p53 | |
| Interventions | C1: 6 cycles of gemcitabine (n = 3) C2: 6 cycles of gemcitabine and interferon alpha‐2b 7 days before and 22 days after first cycle of gemcitabine (n = 6) C3: 6 cycles of gemcitabine and interferon alpha‐2b with p53 SLP vaccine 7 days before and 22 days after first cycle of gemcitabine (n = 6) |
|
| Outcomes | Immune response Safety Clinical response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomised to treatment groups |
| Allocation concealment (selection bias) | High risk | Sequencial allocation to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other sources of bias detected |
Ehlen 2005.
| Methods | Uncontrolled phase II study | |
| Participants | 13 women with ovarian cancer with measurable recurrent disease | |
| Interventions | Intravenous monoclonal antibody (oregovomab ‐ CA‐125) | |
| Outcomes | Survival (time to progression/survival) Tumour responses Immune responses: humoral (Ab2, Ab3, HAMA), cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Freedman 1998.
| Methods | Randomised controlled phase II study | |
| Participants | 30 ovarian cancer patients previously treated with platinum‐based chemotherapy (disease status at study entry not described) | |
| Interventions | Subcutaneous KLH conjugate (Sialyl‐Tn) at 2 different dosages Adjuvant: Detox B |
|
| Outcomes | Survival (progression‐free interval/survival) Tumour responses Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reporting of attrition/exclusions insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Other bias | High risk | Publication bias possible |
Galanis 2010.
| Methods | Uncontrolled phase I study | |
| Participants | 21 ovarian cancer patients with persistent, recurrent, or progressive disease after primary therapy | |
| Interventions | Intraperitoneal recombinant measles virus (CEA) | |
| Outcomes | Tumour responses Immune responses (humoral) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial and sequential allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Goh 2013.
| Methods | Randomised controlled phase IIb trial | |
| Participants | 63 patients in complete remission after primary therapy | |
| Interventions | Protein‐pulsed dendritic cells (MUC1) vs standard of care | |
| Outcomes | Survival Immune responses (cellular) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reporting of attrition/exclusions insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available; study recently completed |
Gordon 2004.
| Methods | Uncontrolled phase II study | |
| Participants | 20 ovarian cancer patients with recurrent disease | |
| Interventions | Intravenous monoclonal antibody (oregovomab ‐ CA‐125) | |
| Outcomes | Survival (time to progression/survival) Tumour responses Immune responses: humoral (Ab2, Ab3, HAMA), cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Gray 2016.
| Methods | Randomized controlled phase II | |
| Participants | 56 participants with epithelial ovarian cancer | |
| Interventions | Mucin 1 targeted dendritic cell vs standard of care | |
| Outcomes | Progression‐free survival Overall survival Immune response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised trial |
| Allocation concealment (selection bias) | Low risk | Randomised trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Gribben 2005.
| Methods | Uncontrolled phase I study | |
| Participants | 17 participants with advanced cancer with progressive disease (ovarian cancer n = 6) | |
| Interventions | Intramuscular plasmid DNA vaccine (CYP1B1) | |
| Outcomes | Tumour responses Immune responses (cellular) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Gulley 2008.
| Methods | Uncontrolled phase I/II study | |
| Participants | 25 participants with CEA or MUC1 overexpressing metastatic cancer with progressive disease following standard chemotherapy (ovarian cancer n = 3) | |
| Interventions | Subcutaneous recombinant pox virus (CEA, MUC1): 1× vaccinia, ≥ 4 fowlpox Adjuvant: local GM‐CSF |
|
| Outcomes | Survival (progression‐free survival/overall survival) Immune responses: cellular, humoral Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Heiss 2010.
| Methods | Randomised controlled open‐label phase II/III trial | |
| Participants | 258 patients with malignant ascites due to epithelial cancer (ovarian cancer n = 129) | |
| Interventions | Intraperitoneal trifunctional antibody (EpCAM) + paracentesis vs paracentesis | |
| Outcomes | Survival (puncture‐free survival/overall survival) Immune responses (HAMA) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reason for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other sources of bias detected |
Imhof 2013.
| Methods | Uncontrolled phase I study | |
| Participants | 15 participants with complete remission after primary therapy | |
| Interventions | Intradermal dendritic cells pulsed with mRNA (TERT) and short peptide (survivin) | |
| Outcomes | Immune responses (cellular) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Kaumaya 2009.
| Methods | Uncontrolled phase I study | |
| Participants | 24 participants with metastatic and/or recurrent solid tumours (ovarian cancer n = 5) | |
| Interventions | Intramuscular synthetic long peptides (Her2) Adjuvant: Montanide ISA720 |
|
| Outcomes | Tumour responses Immune responses (humoral) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Kawano 2014.
| Methods | Uncontrolled phase II study | |
| Participants | 42 participants with platinum‐sensitive (n = 17) and platinum‐resistant (n = 25) recurrent ovarian cancer | |
| Interventions | Personalised peptide vaccine (PPV); max of 4 peptides out of 31 different vaccine candidates + Montanide ± chemotherapy | |
| Outcomes | Safety Immune response Clinical response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Kobayashi 2014.
| Methods | Uncontrolled phase I/II or retrospective? | |
| Participants | 56 participants who received chemotherapy for recurrent ovarian carcinoma | |
| Interventions | Peptide pulsed DC vaccine (WT‐1 ± MUC1 ± CA‐12) + OK‐432 | |
| Outcomes | Safety Immune response Clinical response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Le 2012.
| Methods | Uncontrolled phase I study | |
| Participants | 17 participants with advanced cancers after prior therapy (ovarian cancer n = 2) | |
| Interventions | Intravenous recombinant listeria (mesothelin) | |
| Outcomes | Immune responses (cellular) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Leffers 2009a.
| Methods | Uncontrolled phase II study | |
| Participants | 20 women with epithelial ovarian cancer with (biochemical) recurrence not (yet) eligible for renewed chemotherapy | |
| Interventions | Subcutaneous synthetic long peptides (p53) Adjuvant: Montanide ISA51 |
|
| Outcomes | Survival (disease‐specific survival) Tumour responses Immune responses: humoral, cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | IInformation insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Lennerz 2014.
| Methods | Randomized phase I | |
| Participants | 49 participants with survivin expressing solid tumours (ovarian cancer n = 7) | |
| Interventions | Three dosage groups of EMD640744 vaccine (5 HLA class I‐binding survivin peptides in Montanide ISA 62 VG) | |
| Outcomes | Immune response Safety Clinical response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised between 3 dosage groups |
| Allocation concealment (selection bias) | Unclear risk | Randomised trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other forms of bias detected |
Letsch 2011.
| Methods | Uncontrolled study | |
| Participants | 18 participants with WT1‐expressing solid tumours (disease status unreported) (ovarian cancer n = 8) | |
| Interventions | Short peptide (WT1) Adjuvant: KLH, GM‐CSF |
|
| Outcomes | Tumour responses Immune responses (cellular) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Ma 2002.
| Methods | Uncontrolled study | |
| Participants | 4 women with ovarian cancer (disease status at study entry not described) | |
| Interventions | Monoclonal antibody (MJ01 ‐ CA‐125) | |
| Outcomes | Immune response: cellular | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
MacLean 1992.
| Methods | Uncontrolled phase I study | |
| Participants | 10 women with ovarian cancer and residual or recurrent disease | |
| Interventions | Subcutaneous KLH conjugate (Thomsen Friedenreich) Adjuvant: Detox B |
|
| Outcomes | Tumour responses Immune responses: humoral Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
MacLean 1996.
| Methods | Uncontrolled phase II study | |
| Participants | 34 women with ovarian cancer and evaluable residual or recurrent disease | |
| Interventions | Subcutaneous KLH conjugate (Sialyl‐Tn) Adjuvant: Detox B |
|
| Outcomes | Survival (trial entry to death) Immune response: humoral |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Method 2002.
| Methods | Randomised controlled study | |
| Participants | 102 women with ovarian cancer after primary therapy (disease status at study entry not described) | |
| Interventions | Intravenous monoclonal antibody (oregovomab ‐ CA‐125): 2 gifts vs 3 gifts vs 6 gifts | |
| Outcomes | Tumour responses Immune response: humoral (Ab2, HAMA), cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about the sequence generation process insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding or incomplete blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reporting of attrition/exclusions insufficient to permit judgement of ‘low risk’ or ‘high risk'; only abstract available |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’; only abstract available |
Mohebtash 2011.
| Methods | Uncontrolled study | |
| Participants | 31 metastatic ovarian and breast cancer patients (ovarian cancer n = 14) | |
| Interventions | Subcutaneous recombinant pox virus (MUC1 and CEA) Adjuvant: local GM‐CSF |
|
| Outcomes | Survival: median time to progression 2 months (range 1 to 36) Immune responses (cellular) Adverse events: no severe adverse events, mostly locoregional grade 1 or 2 reactions |
|
| Notes | Max 3 patients overlap with Gulley 2008 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Morse 2011.
| Methods | Uncontrolled phase I study | |
| Participants | 15 ovarian and breast cancer patients with no evidence of disease after prior therapy (ovarian cancer n = 8) | |
| Interventions | Intradermal and subcutaneous short peptides in 2 groups (group 1: APC, HHR6A, BAP31, replication protein A, Abl‐binding protein 3c, cyclin I; group 2: topoisomerase IIα/β, integrin β 8 subunit precursor, CDC2, TACE, g‐catenin, EEDDR1) Adjuvant: Montanide ISA‐51, GM‐CSF |
|
| Outcomes | Survival Immune responses: cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Möbus 2003.
| Methods | Retrospective uncontrolled study | |
| Participants | 44 ovarian cancer patients with clinical recurrence after primary therapy | |
| Interventions | Intravenous monoclonal antibody (oregovomab ‐ CA‐125) | |
| Outcomes | Survival (time from first dose to death/overall survival) Immune response: humoral (Ab2, Ab3, HAMA) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Nicholson 2004.
| Methods | Uncontrolled phase I study | |
| Participants | 26 epithelial ovarian cancer patients with residual disease (n = 19), microscopic disease (n = 3) after chemotherapy, or second complete remission (n = 4) | |
| Interventions | Monoclonal antibody (HMFG1 ‐ MUC1); first gift intraperitoneal (n = 16) or intravenous (n = 10), then ID boosts Adjuvant: aluminium hydroxide |
|
| Outcomes | Tumour responses Immune response: humoral (Ab2) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Nishikawa 2006.
| Methods | Uncontrolled phase II study | |
| Participants | 4 epithelial ovarian cancer patients after primary debulking surgery (disease status at study entry not described) | |
| Interventions | Short peptide (NY‐ESO‐1) Adjuvant: incomplete Freund's adjuvant |
|
| Outcomes | Immune responses: cellular | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’&& |
Noujaim 2001.
| Methods | Retrospective uncontrolled study | |
| Participants | 184 ovarian cancer patients with clinically or radiologically suspected recurrence | |
| Interventions | Intravenous monoclonal antibody (oregovomab ‐ CA‐125) | |
| Outcomes | Survival (overall survival) Immune responses: humoral (Ab3), cellular |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
O'Cearbhaill 2016.
| Methods | Uncontrolled phase I | |
| Participants | 24 participants with advanced‐stage, first‐remission ovarian cancer | |
| Interventions | Dose escalation ‐ 25, 50, 100 mcg ‐ unimolecular pentavalent carbohydrate vaccine (Globo‐H, GM2, sTn, TF and Tn in QS‐21) | |
| Outcomes | Safety Immune response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Odunsi 2007.
| Methods | Uncontrolled phase I study | |
| Participants | 18 ovarian cancer patients after chemotherapy for primary or recurrent disease with or without residual disease | |
| Interventions | Subcutaneous short peptide (NY‐ESO‐1) Adjuvant: incomplete Freund's adjuvant |
|
| Outcomes | Survival: median time to progression: 19.0 months Tumour responses: 1× CR, 17× unknown Immune responses: humoral, cellular Adverse events: well tolerated, no further description |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Odunsi 2012.
| Methods | Uncontrolled phase I/II study | |
| Participants | 22 women with ovarian cancer without evidence of disease after primary therapy | |
| Interventions | intradermal recombinant virus (NY‐ESO‐1); 1× vaccinia virus, 6× fowlpox boost | |
| Outcomes | Survival (disease‐free survival) Immune responses: humoral, cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Odunsi 2014.
| Methods | Uncontrolled phase I/II dose escalation trial | |
| Participants | 12 participants with recurrent epithelial ovarian cancer | |
| Interventions | C1: day 1 decitabine (45 mg/m²), day 8 doxorubicin (40 mg/m²), day 15 NY‐ESO‐I vaccine C2: day 1 decitabine (90 mg/m²), day 8 doxorubicin (40 mg/m²), day 15 NY‐ESO‐I vaccine C3: days 1 to 5 decitabine (10 mg/m²), day 8 doxorubicin (40 mg/m²), day 15 NY‐ESO‐I vaccine |
|
| Outcomes | Immune response Clinical response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Ohno 2009.
| Methods | Uncontrolled phase II study | |
| Participants | 12 patients with gynaecological malignancies resistant to standard therapy (ovarian cancer n = 6) | |
| Interventions | Intradermal short peptide (WT1) Adjuvant: Montanide ISA‐51 |
|
| Outcomes | Tumour responses | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Peethambaram 2009.
| Methods | Uncontrolled phase I study | |
| Participants | 18 patients with refractory metastatic tumours (ovarian cancer n = 4) | |
| Interventions | Intravenous recombinant fusion antigen pulsed antigen‐presenting cells (Her‐2/Neu) Adjuvant: GM‐CSF (included in the recombinant fusion product) |
|
| Outcomes | Survival (time to progression) Tumour responses Immune responses: cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Pfisterer 2006.
| Methods | Uncontrolled phase I study | |
| Participants | 36 stage I‐IV ovarian cancer patients within 6 weeks after completion of chemotherapy for recurrent disease (disease status at study entry not described) | |
| Interventions | Subcutaneous monoclonal antibody (abagovomab ‐ CA‐125) | |
| Outcomes | Immune responses: humoral (Ab3, HAMA), cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Rahma 2012.
| Methods | Uncontrolled phase II study | |
| Participants | 21 ovarian cancer patients without evidence of disease after prior therapy | |
| Interventions | Subcutaneous short peptide (p53) vs intravenous peptide‐pulsed dendritic cells (p53) Adjuvant: Montanide ISA‐51 and GM‐CSF (only in cohort treated with peptide) |
|
| Outcomes | Survival (progression‐free survival, overall survival) Tumour responses Immune responses: cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Reinartz 2004.
| Methods | Uncontrolled multi‐centre phase Ib/II study | |
| Participants | 119 patients with ovarian cancer after at least primary treatment (disease status at entry not described) | |
| Interventions | Intramuscular monoclonal antibody (ACA125 ‐ CA‐125) | |
| Outcomes | Survival (time from first dose to death) Tumour responses Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Sabbatini 2000.
| Methods | Uncontrolled phase I study | |
| Participants | 25 ovarian cancer patients with complete clinical response to chemotherapy after residual or recurrent disease following primary therapy | |
| Interventions | Subcutaneous KLH conjugate (Lewis Y pentasaccharide ‐ MUC1) Adjuvant: QS‐21 |
|
| Outcomes | Survival (time to progression) Immune responses: humoral Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Sabbatini 2006.
| Methods | Randomised open‐label multi‐centre phase I study | |
| Participants | 42 stage II‐IV ovarian cancer patients after chemotherapy for recurrence of disease with complete clinical response or measurable disease (< 2 cm) | |
| Interventions | Intramuscular (IM) or subcutaneous (SC) monoclonal antibody (abagovomab ‐ CA‐125): 4 cohorts (2× IM; 2× SC; 0.2 mg or 2 mg) | |
| Outcomes | Survival (time to progression) Tumour responses Immune response: humoral (Ab3, HAMA), cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Standard 2 × 2 factorial design |
| Allocation concealment (selection bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding or incomplete blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reporting of attrition/exclusions insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other sources of bias detected |
Sabbatini 2007.
| Methods | Uncontrolled phase I/II study | |
| Participants | 11 epithelial ovarian cancer patients with complete clinical remission after primary therapy or chemotherapy for recurrent disease | |
| Interventions | Subcutaneous heptavalent KLH conjugate (GM2, Globo‐H, Lewis Y, Tn‐MUC1, Tn(c), sTN(c), TF(c)) | |
| Outcomes | Survival (time to treatment failure) Immune responses: humoral |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Sabbatini 2012.
| Methods | Uncontrolled phase I study | |
| Participants | 28 ovarian cancer patients in second or third remission | |
| Interventions | Subcutaneous overlapping long peptides (NY‐ESO‐1) Adjuvant: cohort 1 ‐ no (n = 4); cohort 2: Montanide ISA‐51 (n = 13); cohort 3: poly‐ICLC in Montanide ISA‐51 (n = 11) |
|
| Outcomes | Survival (time to progression) Tumour responses Immune responses: cellular and humoral Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Sabbatini 2013.
| Methods | Randomised placebo‐controlled trial | |
| Participants | 888 ovarian cancer patients in complete clinical remission after primary therapy | |
| Interventions | Subcutaneous monoclonal antibody (abagovomab ‐ CA‐125) | |
| Outcomes | Survival (recurrence‐free survival, overall survival) Immune responses: humoral (Ab3, HAMA), cellular (to be reported in separate paper) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured; unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured; unlikely that the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other forms of bias detected |
Sabbatini 2017.
| Methods | Randomised trial | |
| Participants | 171 participants with epithelial ovarian cancer in second or third clinical remission | |
| Interventions | OPT‐821 (n = 86) + polyvalent vaccine conjugate (Globo‐H‐GM2, MUC1‐TN,TF) vs OPT‐821 alone (n = 85) | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised trial |
| Allocation concealment (selection bias) | Low risk | Randomised allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding of participant and investigator |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinding of participant and investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants analysed for primary endpoint |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Low risk | No other forms of bias detected |
Sandmaier 1999.
| Methods | Uncontrolled phase II study | |
| Participants | 40 breast or ovarian cancer (n = 7) patients who underwent high‐dose chemotherapy and autologous or syngeneic stem cell rescue (disease status at study entry unknown) | |
| Interventions | Subcutaneous KLH conjugate (Sialyl‐Tn) Adjuvant: Detox B |
|
| Outcomes | Immune responses: humoral, cellular | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Schultes 1998.
| Methods | Retrospective uncontrolled study | |
| Participants | 75 stage I‐IV ovarian cancer patients (disease status at study entry not described) | |
| Interventions | Intravenous monoclonal antibody (oregovomab ‐ CA‐125) | |
| Outcomes | Survival (overall survival) Immune responses: humoral (Ab2, Ab3, HAMA) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Ströhlein 2009.
| Methods | Uncontrolled phase I study | |
| Participants | 9 patients with progressive peritoneal carcinomatosis (ovarian cancer n = 2) | |
| Interventions | Intraperitoneal trifunctional antibody targeting EpCAM (n = 1) or Her‐2/Neu (n = 1) | |
| Outcomes | Survival: not reported separately for ovarian cancer patients Tumour responses Immune responses: cellular, humoral (HAMA) Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Suzuki 2016.
| Methods | Uncontrolled phase II | |
| Participants | 32 women with clear cell ovarian carcinoma | |
| Interventions | Antigen glypican‐3 (GPC3) vaccine | |
| Outcomes | Immune response Clinical response Safety |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Takeoka 2017.
| Methods | Uncontrolled phase I | |
| Participants | 15 participants with advanced cancer expressing NY‐ESO‐1 (N = 2 ovarian cancer cohort 3) | |
| Interventions | Cohort 1: NY‐ESO‐1 protein Cohort 2a: NY‐ESO‐1 protein + OK‐432 Cohort 2b: NY‐ESO‐1 protein + poly‐ICLC Cohort 3: NY‐ESO‐1 protein + OK‐432 + poly‐ICLC with Montanide ISA‐51 |
|
| Outcomes | Safety Immune response Clinical response |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All OC patients analysed |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Takeuchi 2013.
| Methods | Uncontrolled phase I/II study | |
| Participants | 38 ovarian cancer patients with advanced/recurrent disease | |
| Interventions | Subcutaneous peptide cocktail (HLA‐A24 ‐ n = 23: FOXM1, MELK, HJURP, VEGFR1, VEGFR2; HLA‐A02 ‐ n = 13: HIG2, VEGFR1, VEGFR2) Adjuvant: Montanide ISA‐51 |
|
| Outcomes | Survival Tumour responses Immune responses (not adequately reported) Adverse events (not adequately reported) |
|
| Notes | Meeting abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Tsuda 2004.
| Methods | Uncontrolled phase I/II study | |
| Participants | 14 patients with gynaecological cancer after primary therapy (ovarian cancer n = 5; NED n = 2) | |
| Interventions | Subcutaneous individualised short peptide cocktail Adjuvant: Montanide ISA‐51 |
|
| Outcomes | Tumour responses Immune responses: humoral, cellular Adverse events: not separately described for ovarian cancer patients |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
van Zanten‐Przybysz 2002.
| Methods | Uncontrolled phase I/II study | |
| Participants | 5 patients with residual or recurrent ovarian cancer after primary debulking surgery and at least 1 course of chemotherapy | |
| Interventions | Intravenous monoclonal antibody (c‐MOv18 ‐ membrane folate receptor) | |
| Outcomes | Survival: median time from first dose to death: 22.0 months Tumour responses: 3× PD, 2× SD Immune responses: cellular Adverse events: max grade I events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Vermeij 2012.
| Methods | Uncontrolled phase II study | |
| Participants | 12 women with epithelial ovarian cancer with (biochemical) recurrence not (yet) eligible for renewed chemotherapy | |
| Interventions | Subcutaneous synthetic long peptides (p53) Adjuvant: Montanide ISA51 Immunomodulation: cyclophosphamide 2 days before each vaccination |
|
| Outcomes | Tumour responses Immunological responses: cellular Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Wagner 1993.
| Methods | Retrospective uncontrolled study | |
| Participants | 58 patients with advanced‐stage ovarian cancer after primary treatment with high preoperative CA‐125 levels (disease status at study entry not described) | |
| Interventions | Intravenous monoclonal antibody fragments (F(Ab)2‐fragments of MAb OC125 ‐ CA‐125) | |
| Outcomes | Survival Tumour responses Immune responses: humoral (Ab2), cellular |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled trial |
| Allocation concealment (selection bias) | High risk | Uncontrolled trial |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Selective reporting (reporting bias) | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
| Other bias | Unclear risk | Information insufficient to permit judgement of ‘low risk’ or ‘high risk’ |
Ab2: anti‐idiotype antibody. Ab3: anti‐anti‐idiotype antibody. CA‐125: cancer antigen‐125. CEA: carcinoembryonic antigen. EpCAM: epithelial cell adhesion molecule. ErbB2: human Epidermal growth factor Receptor 2. FBP: folate binding protein. GM‐CSF: granulocyte‐macrophage colony‐stimulating factor. GPC3: antigen glypican‐3. HAMA: human‐anti‐mouse antibody. HLA: human leucocyte antigen. hTERT: telomerase reverse transcriptase. KLH: keyhole limpet haemocyanin. MAb: monoclonal antibody. MAGE‐A1: melanoma‐associated antigen A1. MUC1: Mucin‐1. NED: no evidence of disease. OC: ovarian carcinoma. OWD: 1‐week delayed PADRE: DR‐restricted Th helper epitope. poly‐ICLC: polyinosinic‐polycytidylic acid complexed with poly‐L‐lysine and carboxymethylcellulose. SIM: simultaneous infusion. SLP: synthetic long peptide.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2000 | Only 1 woman with epithelial ovarian cancer; no ASAI |
| Baek 2015 | No ASAI |
| Bapsy 2014 | No ASAI |
| Bender 2007 | Only 1 woman with epithelial ovarian cancer |
| Bernal 2012 | Only 1 woman with epithelial ovarian cancer; no ASAI |
| Carbone 2005 | Only 1 woman with epithelial ovarian cancer |
| Chiang 2013 | No ASAI |
| Coosemans 2013 | Only 1 woman with epithelial ovarian cancer |
| Dhodapkar 2014 | Impossible to distinguish between other and women with ovarian cancer |
| Disis 1999 | Impossible to distinguish between other and women with ovarian cancer |
| Disis 2000 | Impossible to distinguish between other and women with ovarian cancer |
| Disis 2002 | Impossible to distinguish between other and women with ovarian cancer |
| Disis 2002a | Only 1 woman with epithelial ovarian cancer |
| Disis 2004 | Impossible to distinguish between other and women with ovarian cancer |
| Disis 2004a | Only 1 woman with epithelial ovarian cancer |
| Galanis 2013 | No ASAI |
| Haakenstad 2012 | Impossible to distinguish between other and women with ovarian cancer |
| Hasumi 2011 | No ASAI |
| Hernando 2002 | Autologous tumour lysate vaccine |
| Hernando 2007 | Only 1 woman with epithelial ovarian cancer |
| Holmberg 2000 | Impossible to distinguish between women with breast cancer and women with ovarian cancer |
| Hui 1997 | No ASAI |
| Jackson 2017 | Impossible to distinguish between other and women with ovarian cancer |
| Jager 2006 | Only 1 woman with epithelial ovarian cancer |
| Kandalaft 2010 | Autologous tumour lysate vaccine |
| Karbach 2010 | Only 1 woman with epithelial ovarian cancer |
| Kato 2010 | Impossible to distinguish between other and women with ovarian cancer |
| Khranovska 2011 | Autologous tumour lysate vaccine |
| Knutson 2001 | Only 1 woman with epithelial ovarian cancer |
| Knutson 2002 | Women with epithelial ovarian cancer withdrew before evaluation of immune responses. |
| Letsch 2008 | Impossible to distinguish between other and women with ovarian cancer |
| Loveland 2006 | Only 1 woman with epithelial ovarian cancer |
| Manjunath 2012 | Only 1 woman with epithelial ovarian cancer |
| Marshall 2005 | Only 1 woman with ovarian cancer |
| Matsuzaki 2014 | Additional results to Odunsi 2007; irrelevant for review |
| Miotti 1999 | Autologous T‐cell vaccine |
| Morera 2017 | Only 1 woman with epithelial ovarian cancer |
| Morse 1999 | Impossible to distinguish between other and women with ovarian cancer |
| Morse 2003 | Uncertain if and how many women with ovarian cancer were included |
| Morse 2011a | Impossible to distinguish between other and women with ovarian cancer; unclear number of women with ovarian cancer |
| Murray 2002 | Only 1 woman with epithelial ovarian cancer |
| Oh 2016 | No ASAI |
| Parkhurst 2004 | No women with epithelial ovarian cancer |
| Reddish 1996 | Impossible to distinguish between other and women with ovarian cancer |
| Salazar 2006 | Impossible to distinguish between other and women with ovarian cancer |
| Schiffman 2002 | No immunisations carried out |
| Tsuji 2013 | Additional results to Sabbatini 2013; irrelevant for review |
| Yacyshyn 1995 | Additional results to MacLean 1992; irrelevant for review |
| Zaks 1998 | Impossible to distinguish between other and women with ovarian cancer |
ASAI: antigen‐specific active immunotherapy.
Characteristics of ongoing studies [ordered by study ID]
NCT00003002.
| Trial name or title | Her‐2/Neu vaccine plus GM‐CSF in treating participants with stage III or stage IV breast, ovarian, or non‐small cell lung cancer |
| Methods | Uncontrolled phase I |
| Participants | Participants with stage III or IV Her‐2/Neu‐expressing breast, ovarian, or non‐small cell lung cancer |
| Interventions | Intradermal vaccinations of Her‐2/Neu‐derived peptides with sargramostim (GM‐CSF) |
| Outcomes | Immune responses Adverse events |
| Starting date | April 1996 |
| Contact information | |
| Notes | Completed January 2004; no publication available |
NCT00004604.
| Trial name or title | Biological therapy in treating patients with metastatic cancer |
| Methods | Uncontrolled phase I |
| Participants | 24 participants with histologically confirmed metastatic adenocarcinoma expressing carcinoembryonic antigen (CEA) who has failed conventional therapy |
| Interventions | Intravenous CEA RNA‐pulsed autologous dendritic cells |
| Outcomes | Adverse events Immune responses Clinical and biochemical responses |
| Starting date | February 1998 |
| Contact information | |
| Notes | Completed July 2002; no publication available |
NCT00006041.
| Trial name or title | Vaccine therapy in treating patients with ovarian, fallopian tube, or peritoneal cancer |
| Methods | Uncontrolled phase I |
| Participants | 18 patients with histologically confirmed ovarian, fallopian tube, or peritoneal epithelial cancer (any stage at diagnosis); refractory or recurrent after cytoreductive surgery and at least 1 prior regimen of platinum‐based chemotherapy |
| Interventions | Glycosylated MUC1‐KLH vaccine plus QS21 |
| Outcomes | Adverse events Immune responses |
| Starting date | February 2000 |
| Contact information | |
| Notes | Completed February 2002; no publication available |
NCT00381173.
| Trial name or title | A phase I open‐label study of the safety and feasibility of ZYC300 administration with cyclophosphamide pre‐dosing |
| Methods | Phase I |
| Participants | 22 advanced‐stage malignancies with evidence of disease and no therapeutic options |
| Interventions | IM ZYC300 (a plasmid DNA formulated within biodegradable microencapsulated particles) with IV cyclophosphamide |
| Outcomes | Safety Immune responses Tumour responses |
| Starting date | November 2006 |
| Contact information | |
| Notes | Study completion January 2009; no published records available |
NCT00803569.
| Trial name or title | Phase I study of ALVAC(2)‐NY‐ESO‐1(M)/TRICOM in patients with epithelial ovarian, fallopian tube, or primary peritoneal carcinoma whose tumours express NY‐ESO‐1 or LAGE‐1 antigen |
| Methods | Phase I |
| Participants | 12 stage II‐IV women with ovarian cancer with complete response to primary or secondary (chemo)therapy |
| Interventions | SC ALVAC(2)‐NY‐ESO‐1(M)/TRICOM vaccine plus SC GM‐CSF |
| Outcomes | Safety Tumour responses Immune responses |
| Starting date | November 2008 |
| Contact information | |
| Notes | Completed 2011; no publication available |
NCT01223235.
| Trial name or title | Polyvalent vaccine‐KLH conjugate + Opt‐821 given in combination with bevacizumab |
| Methods | Uncontrolled phase I |
| Participants | 22 participants who have recently completed chemotherapy and/or surgery for recurrent epithelial carcinoma arising from the ovary, fallopian tube, or peritoneum |
| Interventions | Bevacizumab and polyvalent vaccine KLH‐conjugate + OPT‐821 |
| Outcomes | Adverse events Immune responses Survival |
| Starting date | October 2010 |
| Contact information | |
| Notes | Completed September 2017; no publication available |
NCT01322802.
| Trial name or title | Vaccine therapy in treating patients with stage III‐IV or recurrent ovarian cancer |
| Methods | Uncontrolled phase I |
| Participants | 22 participants with advanced‐stage or recurrent ovarian cancer treated to complete remission with standard therapies |
| Interventions | pUMVC3‐hIGFBP‐2 multi‐epitope plasmid DNA vaccine |
| Outcomes | Adverse events Immune responses Survival |
| Starting date | March 2012 |
| Contact information | |
| Notes | Active April 2017; not recruiting |
NCT01376505.
| Trial name or title | Vaccine therapy in treating patients with metastatic solid tumors |
| Methods | Uncontrolled phase I trial |
| Participants | 36 participants with malignant solid tumour, breast cancer, malignant tumour of colon, GIST, or ovarian cancer |
| Interventions | HER‐2 vaccine |
| Outcomes | Immune response Clinical response Adverse events |
| Starting date | June 2011 |
| Contact information | |
| Notes | Recruiting, April 2018 |
NCT01522820.
| Trial name or title | Vaccine therapy with or without sirolimus in treating patients with NY‐ESO‐1‐expressing solid tumours |
| Methods | Uncontrolled phase I |
| Participants | 30 participants with solid NY‐ESO‐1‐ or LAGE‐1‐expressing tumours at high risk of recurrence or with minimal residual disease |
| Interventions | Intranodal injections with DEC‐205‐NY‐ESO‐1 fusion protein vaccine with or without oral sirolimus |
| Outcomes | Adverse events Immune responses Survival |
| Starting date | March 2012 |
| Contact information | |
| Notes | Completed October 2016; no publication available |
NCT01536054.
| Trial name or title | Sirolimus and vaccine therapy in treating patients with stage II‐IV ovarian epithelial, fallopian tube, or primary peritoneal cavity cancer |
| Methods | Uncontrolled phase I |
| Participants | 12 women with completed therapy for primary or recurrent disease with asymptomatic residual disease or complete remission |
| Interventions | Subcutaneous injections with ALVAC(2)‐NY‐ESO‐1 (M)/TRICOM vaccine, subcutaneous GM‐CSF, and oral sirolimus |
| Outcomes | Adverse events Immune responses Survival |
| Starting date | August 2012 |
| Contact information | |
| Notes | Active not recruiting, March 2017 |
NCT01556841.
| Trial name or title | A phase II study to assess the activity of TroVax® (MVA‐5T4) versus placebo in patients with relapsed asymptomatic epithelial ovarian, fallopian tube, or primary peritoneal cancer |
| Methods | Randomised phase II |
| Participants | 97 participants with CA‐125‐relapsed asymptomatic ovarian cancer |
| Interventions | Vaccine targeting 5T4 (TroVax) vs placebo |
| Outcomes | Clinical response Immune response Survival |
| Starting date | November 2013 |
| Contact information | |
| Notes | Active, not recruiting, December 2017 |
NCT01584115.
| Trial name or title | Clinical trial of therapeutic vaccine with NY‐ESO‐1 in combination with the adjuvant monophosphoryl lipid A (MPLA) |
| Methods | Uncontrolled phase I/II |
| Participants | 15 participants with a NY‐ESO‐1‐expressing malignancy after standard treatment |
| Interventions | Intramuscular injection with NY‐ESO‐1 combined with MPLA vaccine |
| Outcomes | Adverse events Immune responses |
| Starting date | July 2012 |
| Contact information | |
| Notes | Status unknown |
NCT01606241.
| Trial name or title | Cyclophosphamide and vaccine therapy in treating patients with stage II‐III breast, ovarian, primary peritoneal, or fallopian tube cancer |
| Methods | Uncontrolled phase I |
| Participants | 24 women in complete remission after systemic treatment of breast, ovarian, primary peritoneal, or fallopian tube cancer |
| Interventions | Oral cyclophosphamide and intradermal multi‐epitope folate receptor alpha peptide vaccine |
| Outcomes | Adverse events Immune responses |
| Starting date | July 2012 |
| Contact information | |
| Notes | Manuscript submitted January 2018 |
NCT01616303.
| Trial name or title | A controlled study of effectiveness of oregovomab (antibody) plus chemotherapy in advanced ovarian cancer |
| Methods | Randomised open‐label phase II |
| Participants | 80 women with newly diagnosed ovarian, tubal, or peritoneal cancer after optimal cytoreductive surgery about to start first‐line chemotherapy |
| Interventions | Carboplatin + paclitaxel vs carboplatin + paclitaxel + oregovomab |
| Outcomes | Adverse events Immune responses Survival Clinical responses |
| Starting date | June 2012 |
| Contact information | |
| Notes | Active not recruiting, September 2017 |
NCT01621542.
| Trial name or title | Clinical study of WT2725 in patients with advanced solid malignancies |
| Methods | Uncontrolled phase I |
| Participants | 80 participants with measurable WT1‐expressing advanced‐stage malignancies |
| Interventions | WT2725 injection |
| Outcomes | Adverse events Immune responses |
| Starting date | July 2012 |
| Contact information | |
| Notes | Completed June 2017; no publication available |
NCT01673217.
| Trial name or title | Decitabine, vaccine therapy, and pegylated liposomal doxorubicin hydrochloride in treating patients with recurrent ovarian epithelial cancer, fallopian tube cancer, or peritoneal cancer |
| Methods | Uncontrolled phase I |
| Participants | 18 women with relapsed epithelial ovarian, fallopian tube, or primary peritoneal cancer who are to receive liposomal doxorubicin as salvage therapy for recurrent disease |
| Interventions | Intravenous decitabine, intravenous liposomal doxorubicin, subcutaneous NY‐ESO‐1 peptide vaccine in Montanide ISA‐51, subcutaneous GM‐CSF |
| Outcomes | Adverse events Immune responses Survival |
| Starting date | April 2009 |
| Contact information | |
| Notes | Study completed June 2013; no publication available |
NCT02111941.
| Trial name or title | A pilot study of the safety and immunogenicity of folate receptor alpha peptide‐loaded dendritic cell vaccination in patients with advanced stage epithelial ovarian cancer |
| Methods | Uncontrolled phase I |
| Participants | 19 women with stage IIIC‐IV ovarian epithelial, fallopian tube, or primary peritoneal cavity cancer following surgery and chemotherapy |
| Interventions | Multi‐epitope folate receptor alpha‐loaded dendritic cell vaccine |
| Outcomes | Adverse events Survival Immune response |
| Starting date | April 2014 |
| Contact information | |
| Notes | Active not recruiting, October 2017 |
NCT02132988.
| Trial name or title | An open labeled phase II trial of active immunotherapy with Globo H‐KLH (OPT‐822/821) in women who have non‐progressive epithelial ovarian, fallopian tube, or primary peritoneal cancer |
| Methods | Phase II |
| Participants | 110 participants with non‐progressive epithelial ovarian, fallopian tube, or primary peritoneal cancer after cytoreductive surgery and platinum‐based chemotherapy as initial treatment for primary disease or as salvage treatment for first relapse |
| Interventions | Globo H‐KLH vaccine (OPT‐822/OPT‐821) |
| Outcomes | Progression‐free survival Disease recurrence rate |
| Starting date | November 2013 |
| Contact information | |
| Notes | Recruiting, May 2014 |
NCT02146313.
| Trial name or title | A phase I, open‐label, dose‐escalation study of the safety, tolerability, and pharmacokinetics of DMUC4064A administered intravenously to patients with platinum‐resistant ovarian cancer or unresectable pancreatic cancer |
| Methods | Non‐randomised phase I |
| Participants | 30 participants with platinum‐resistant ovarian cancer or unresectable pancreatic cancer |
| Interventions | Intravenous DMUC4064A |
| Outcomes | DLT Adverse events Immune response Clinical response |
| Starting date | May 2014 |
| Contact information | |
| Notes | Active not recruiting, March 2018 |
NCT02166905.
| Trial name or title | A phase I/IIb study of DEC205mAb‐NY‐ESO‐1 fusion protein (CDX‐1401) given with adjuvant poly‐ICLC in combination with INCB024360 for patients in remission with epithelial ovarian, fallopian tube, or primary peritoneal carcinoma whose tumors express NY‐ESO‐1 or LAGE‐1 antigen |
| Methods | Phase II and randomised phase IIb |
| Participants | 62 participants with epithelial ovarian, fallopian tube, or primary peritoneal carcinoma whose tumours express NY‐ESO‐1 or LAGE‐1 antigen |
| Interventions | Phase I: DEC‐205/NY‐ESO‐1 fusion protein CDX‐1401, poly ICLC, and IDO1 inhibitor INCB024360 Phase IIb cohort I: DEC‐205/NY‐ESO‐1 fusion protein CDX‐1401 and poly ICLC Phase IIB cohort II: DEC‐205/NY‐ESO‐1 fusion protein CDX‐1401, poly ICLC, and IDO1 inhibitor INCB024360 |
| Outcomes | Adverse events Immune response |
| Starting date | August 2014 |
| Contact information | |
| Notes | Recruiting, May 2018 |
NCT02275039.
| Trial name or title | A phase I study of a p53MVA vaccine in combination with gemcitabine in ovarian cancer |
| Methods | Uncontrolled phase I |
| Participants | 9 participants with recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma |
| Interventions | Vaccinia virus ankara vaccine expressing p53 and gemcitabine hydrochloride |
| Outcomes | Dosage determination Immune response |
| Starting date | October 2014 |
| Contact information | |
| Notes | Completed, April 2018 |
NCT02387125.
| Trial name or title | A phase Ib study evaluating the safety, tolerability and immunogenicity of CMB305 (sequentially administered LV305 and G305) in patients with locally advanced, relapsed, or metastatic cancer expressing NY‐ESO‐1 |
| Methods | Non‐randomised open‐label multi‐centre phase Ib |
| Participants | 69 participants with melanoma, sarcoma, ovarian cancer, or non‐small cell lung cancer that expresses NY‐ESO‐1 |
| Interventions | CMB305 (sequentially administered LV305 (a dendritic cell‐targeting viral vector expressing the NY‐ESO‐1 gene) and G305 (NY‐ESO‐1 recombinant protein plus GLA‐SE)) |
| Outcomes | Adverse events Clinical response Survival Immune response |
| Starting date | March 2015 |
| Contact information | |
| Notes | Recruiting, January 2018 |
NCT02498665.
| Trial name or title | A phase I clinical study of DSP‐7888 dosing emulsion in adult patients with advanced malignancies |
| Methods | Non‐randomised phase I |
| Participants | 96 participants with advanced malignancies |
| Interventions | WT1 protein‐derived peptide vaccine (DSP‐7888) |
| Outcomes | DLT Survival Immune response |
| Starting date | November 2015 |
| Contact information | |
| Notes | Recruiting, April 2018 |
NCT02575807.
| Trial name or title | A phase I/II open‐label safety and efficacy evaluation of CRS‐207 in combination with epacadostat in adults with platinum‐resistant ovarian, fallopian, or peritoneal cancer |
| Methods | Randomised phase I/II |
| Participants | 126 participants with platinum‐resistant ovarian, fallopian, or peritoneal cancer |
| Interventions | Phase I cohort I: CRS‐207/epacadostat Phase I cohort II: CRS‐207 Phase 2 cohort I: CRS‐207, pembrolizumab Phase II cohort II: CRS‐207, pembrolizumab, epacadostat |
| Outcomes | DLT Adverse events Clinical response Survival |
| Starting date | October 2015 |
| Contact information | |
| Notes | Active not recruiting, February 2018 |
NCT02737787.
| Trial name or title | A phase I study of concomitant WT1 analog peptide vaccine with Montanide and GM‐CSF in combination with nivolumab in patients with recurrent ovarian cancer who are in second or greater remission |
| Methods | Uncontrolled phase I |
| Participants | 10 participants with ovarian, fallopian tube, or primary peritoneal cancer |
| Interventions | WT1 vaccine and nivolumab |
| Outcomes | Dose‐limiting toxicity |
| Starting date | April 2016 |
| Contact information | |
| Notes | Active not recruiting, March 2018 |
NCT02764333.
| Trial name or title | A phase II trial of TPIV200/huFR‐1 (a multi‐epitope anti‐folate receptor vaccine) plus anti‐PD‐L1 MEDI4736 (durvalumab) in patients with platinum‐resistant ovarian cancer |
| Methods | Uncontrolled phase II |
| Participants | 40 participants with epithelial ovarian, fallopian tube, or primary peritoneal carcinoma |
| Interventions | Intradermal TPIV200 (vaccine targeting folate receptor alpha mixed with GM‐CSF) and intravenous durvalumab |
| Outcomes | Clinical response |
| Starting date | May 2016 |
| Contact information | |
| Notes | Active not recruiting, March 2018 |
NCT02785250.
| Trial name or title | A phase Ib study of an immunotherapeutic vaccine, DPX‐Survivac with low dose cyclophosphamide and epacadostat (INCB024360), in patients with recurrent ovarian cancer |
| Methods | Uncontrolled phase I |
| Participants | 40 participants with recurrent epithelial ovarian, fallopian tube, or peritoneal cancer |
| Interventions | Survivin vaccine DPX‐Survivac, low‐dose oral cyclophosphamide, and IDO1 inhibitor epacadostat |
| Outcomes | Adverse events Immune response Clinical response Survival |
| Starting date | May 2016 |
| Contact information | |
| Notes | Recruiting, June 2017 |
NCT02833506.
| Trial name or title | A phase I clinical trial of mTOR inhibition with sirolimus for enhancing NY‐ESO‐1 protein with MIS416 vaccine induced anti‐tumor immunity in ovarian, fallopian tube, and primary peritoneal cancer |
| Methods | Non‐randomised phase I |
| Participants | 12 participants with stage II‐IV ovarian, fallopian tube, or primary peritoneal cancer |
| Interventions | Cohort 1: NY‐ESO‐1 protein with MIS416 Cohort 2: sirolimus and NY‐ESO‐1 protein with MIS416 |
| Outcomes | Adverse events Immune response Clinical response |
| Starting date | December 2017 |
| Contact information | |
| Notes |
NCT02933073.
| Trial name or title | A phase I study of OncoImmunome for the treatment of stage III/IV ovarian carcinoma |
| Methods | Uncontrolled phase I |
| Participants | 15 participants |
| Interventions | Personalised vaccine containing a mixture of 7 to 10 peptides, each containing 17 or 18 amino acids (OncoImmunome) |
| Outcomes | Adverse events Immune response Survival |
| Starting date | November 2016 |
| Contact information | |
| Notes | Recruiting, July 2017 |
NCT02978222.
| Trial name or title | A randomized multicenter phase II trial to evaluate the safety, efficacy and immunogenicity of vaccination with folate receptor alpha peptides with GM‐CSF versus GM‐CSF alone in patients with platinum sensitive ovarian cancer and a response or stable disease to platinum therapy |
| Methods | Multi‐centre double‐blind controlled randomised phase II study |
| Participants | 120 participants with platinum‐sensitive ovarian cancer |
| Interventions | FRα peptide vaccine with GM‐CSF or GM‐CSF alone |
| Outcomes | Survival Clinical response |
| Starting date | November 2016 |
| Contact information | |
| Notes | Recruting, April 2018 |
NCT03029403.
| Trial name or title | A phase II study of pembrolizumab (MK‐3475), DPX‐Survivac vaccine and low dose of cyclophosphamide combination in patients with advanced ovarian, primary peritoneal or fallopian tube cancer |
| Methods | Non‐randomised phase II |
| Participants | 42 participants with advanced ovarian, primary peritoneal, or fallopian tube cancer |
| Interventions | Intravenous pembrolizumab, subcutaneous DPX‐Survivac vaccine, and oral low‐dose cyclophosphamide |
| Outcomes | Overall response rate Survival Adverse events |
| Starting date | January 2017 |
| Contact information | |
| Notes | Recruiting, May 2018 |
NCT03029611.
| Trial name or title | A phase II study of concurrent IGFBP‐2 vaccination and neoadjuvant chemotherapy to increase the rate of pathologic complete response at the time of cytoreductive surgery |
| Methods | Uncontrolled phase II |
| Participants | 38 participants with fallopian tube cancer, ovarian cancer, or primary peritoneal cancer |
| Interventions | Intravenous paclitaxel and carboplatin, intradermal IGFBP‐2 vaccine |
| Outcomes | Clinical response Immune response |
| Starting date | April 2017 |
| Contact information | |
| Notes | Recruiting, May 2018 |
NCT03113487.
| Trial name or title | A phase II study of a P53MVA vaccine in combination with pembrolizumab in platinum resistant ovarian cancer |
| Methods | Uncontrolled phase II trial |
| Participants | 28 participants with ovarian, primary peritoneal, or fallopian tube cancer |
| Interventions | Vaccinia virus ankara vaccine expressing p53 (p53MVA) and pembrolizumab |
| Outcomes | Clinical response Survival |
| Starting date | March 2017 |
| Contact information | |
| Notes | Not yet recruiting, April 2018 |
NCT03127098.
| Trial name or title | Phase Ib/II study of ETBX‐011 (Ad5 (E1‐, E2b‐)‐CEA(6D)) vaccine in combination with ALT‐803 (super‐agonist IL‐15) in subjects having CEA‐expressing cancer |
| Methods | Phase Ib/II |
| Participants | 3 participants with locally advanced or metastatic CEA‐expressing cancers |
| Interventions | Subcutaneous ETBX‐011 and subcutaneous ALT‐803. |
| Outcomes | Adverse events Survival |
| Starting date | April 2017 |
| Contact information | |
| Notes | Active not recruiting, June 2018 |
NCT03197584.
| Trial name or title | NANT ovarian cancer vaccine: combination immunotherapy in subjects with epithelial ovarian cancer who have progressed on or after standard‐of‐care (SoC) therapy |
| Methods | Uncontrolled phase Ib/II |
| Participants | 67 participants with epithelial ovarian cancer |
| Interventions | Avelumab, bevacizumab, capecitabine, cyclophosphamide, 5‐fluorouracil, fulvestrant, leucovorin, paclitaxel, omega‐3‐acid ethyl esters, oxaliplatin, stereotactic body radiation therapy, ALT‐803, ETBX‐021, ETBX‐051, ETBX‐061, GI‐4000, GI‐6301, and hank |
| Outcomes | Adverse events Response rate (RECIST) Immune response |
| Starting date | June 2017 |
| Contact information | |
| Notes | Not yet recruiting, October 2017 |
NCT03206047.
| Trial name or title | A randomized phase II trial of atezolizumab (MPDL3280A), SGI‐110 and CDX‐1401 vaccine in recurrent ovarian cancer |
| Methods | Randomised phase I/IIb |
| Participants | 78 participants |
| Interventions | Cohort 1: intravenous atezolizumab Cohort 2: intravenous atezolizumab and subcutaneous guadecitabine Cohort 3: intravenous atezolizumab, subcutaneous guadecitabine, and DEC‐205/NY‐ESO‐1 fusion protein CDX‐1401 |
| Outcomes | Adverse events Survival Immune response Clinical response |
| Starting date | September 2017 |
| Contact information | |
| Notes | Recruiting, June 2018 |
NCT03300843.
| Trial name or title | A phase II trial to evaluate the ability of a dendritic cell vaccine to immunize melanoma or epithelial cancer patients against defined mutated neoantigens expressed by the autologous cancer |
| Methods | Uncontrolled phase II |
| Participants | 86 participants with evaluable metastatic melanoma or epithelial cancer refractory to standard treatment |
| Interventions | Personalised therapeutic dendritic cell vaccine |
| Outcomes | Clinical response Immune response Adverse events |
| Starting date | October 2017 |
| Contact information | |
| Notes | Recruiting, May 2018 |
CA‐125: cancer antigen‐125. CEA: carcinoembryonic antigen. DLT: dose‐limiting toxicity. GIST: gastrointestinal stromal tumour. GM‐CSF: granulocyte‐macrophage colony‐stimulating factor. KLH: keyhole limpet haemocyanin. MPLA: monophosphoryl lipid A. MUC1: Mucin‐1. RECIST: Response Evaluation Criteria In Solid Tumors.
Differences between protocol and review
TD was added to the team. For the update of this review, we used the Cochrane 'Risk of bias' tool to assess risk of bias in randomised controlled trials, whereas for the protocol and the previous version of this review, we used the Delphi list. We can report no further relevant differences between protocol and review. For the second update, STP and MB were added to the review author team.
Contributions of authors
NL selected relevant studies, assessed study quality, extracted data, and wrote the review. HWN selected relevant studies, assessed study quality, and extracted data. TD and WH checked all rejected titles and resolved disagreements on study selection and data extraction. HMB and BC provided statistical and methodological support. KM was supportive of writing the review as an expert in immunology. STP and MDB selected relevant studies, assessed study quality, extracted data, and wrote the second update of this review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Ninke Leffers, Cornelis Melief, Toos Daemen, and Hans Nijman were investigators in two studies included in this review (Leffers 2009a; Vermeij 2012). No potential conflicts of interest are known for the other contributing review authors (WH, BJC, STP, MDB) .
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Antonilli 2016 {published data only}
- Antonilli M, Rahimi H, Visconti V, Napoletano C, Ruscito I, Zizzari IG, et al. Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: clinical and immunological data of a phase I/II clinical trial. International Journal of Oncology 2016;48:1369‐78. [PUBMED: 26892612] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumann 2011 {published data only}
- Baumann K, Pfisterer J, Wimberger P, Burchardi P, Kurzeder C, du Bois A, et al. Intraperitoneal treatment with the trifunctional bispecific antibody catumaxomab in patients with platinum‐resistant epithelial ovarian cancer: a phase IIa study of the AGO Study Group. Gynecologic Oncology 2011;123:27‐32. [DOI] [PubMed] [Google Scholar]
Berek 2001 {published data only}
- Berek JS, Ehlen TG, Gordon A, Nicodemus CF, Schultes B, Whiteside TL, et al. Interim analysis of a double blind study of Ovared mAb B43.13 (OV) versus placebo (PBO) in patients with ovarian cancer. American Society of Clinical Oncology Annual Meeting. 2001.
Berek 2004 {published data only}
- Berek JS, Taylor PT, Gordon A, Cunningham MJ, Finkler N, Orr J, et al. Randomized, placebo‐controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. Journal of Clinical Oncology 2004;22(17):3507‐16. [DOI] [PubMed] [Google Scholar]
- Berek JS, Taylor PT, Nicodemus CF. CA125 velocity at relapse is a highly significant predictor of survival post relapse: results of a 5‐year follow‐up survey to a randomized placebo‐controlled study of maintenance oregovomab immunotherapy in advanced ovarian cancer. Journal of Immunotherapy 2008;31(2):207‐14. [DOI] [PubMed] [Google Scholar]
Berek 2009 {published data only}
- Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcome in advanced ovarian cancer. Journal of Clinical Oncology 2009;27:418‐25. [DOI] [PubMed] [Google Scholar]
- Berek J, Taylor PT, McGuire WP, Smith LM, Schultes B, Nicodemus CF. Evaluation of maintenance mono‐immunotherapy to improve outcomes in advanced ovarian cancer (OV CA). ASCO Annual Meeting. 2008.
Berinstein 2012 {published data only}
- Berinstein NL, Karkada M, Morse MM, Nemunaitis JJ, Chatta G, Kaufman H, et al. First‐in‐man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi‐functional T cell responses in ovarian, breast and prostate cancer. Journal of Translational Medicine 2012;10:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berinstein 2013 {published data only}
- Berinstein NL, Oza AM, Odunsi K, Karkada M, Villella JA, Nemunaitis JJ, et al. Effect of oral cyclophosphamide on the immunogenicity of DPX‐Survivac in ovarian cancer patients: results of a phase I study. American Society of Clinical Oncology Annual Meeting. 2013.
Braly 2009 {published data only}
- Braly P, Chu C, Collins Y, Edwards R, Gordon A, McGuire W, et al. Prospective evaluation of front‐line chemo‐immunotherapy (C‐IT) with oregovomab (2 alternative dosing schedules) carboplatin‐paclitaxel (C‐P) in advanced ovarian cancer (OC). American Society of Clinical Oncology Annual Meeting. 2007.
- Braly P, Nicodemus CF, Chu C, Collins Y, Edwards R, Gordon A, et al. The immune adjuvant properties of front‐line carboplatin‐paclitaxel: a randomised phase 2 study of alternative schedules of intravenous oregovomab chemo‐immunotherapy in advanced ovarian cancer. Journal of Immunotherapy 2009;32:54‐65. [DOI] [PubMed] [Google Scholar]
- Method M, Gordon A, Smith LM, Nicodemus CF. Oregovomab immune‐modulating antibody therapy concurrent to standard chemotherapy of epithelial ovarian cancer (EOC): feasibility and initial clinical experience. Society of Gynecologic Oncologists Annual Meeting on Women's Cancer. 2006.
Brossart 2000 {published data only}
- Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T‐lymphocyte responses in vivo after vaccinations with peptide‐pulsed dendritic cells. Blood 2000;96(9):3102‐8. [PubMed] [Google Scholar]
Buzzonetti 2014 {published data only}
- Buzzonetti A, Fossati M, Catzola V, Scambia G, Fattorossi A, Battaglia A. Immunological response induced by abagovomab as a maintenance therapy in patients with epithelial ovarian cancer: relationship with survival ‐ a substudy of the MIMOSA trial. Cancer Immunology Immunotherapy 2014;63:1037‐45. [PUBMED: 24952307] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chianese‐Bullock 2008 {published data only}
- Chianese‐Bullock KA, Irvin WP, Petroni GR, Murphy C, Smolkin M, Olson WC, et al. A multipeptide vaccine is safe and elicits T‐cell responses in participants with advanced stage ovarian cancer. Journal of Immunotherapy 2008;31(4):420‐30. [DOI] [PubMed] [Google Scholar]
Chu 2012 {published data only}
- Chu CS, Boyer J, Coukos G, Rubin SC, Morgan MA, Bendig DL, et al. Autologous dendritic cell (IDD‐6) vaccination as consolidation for advanced ovarian cancer. Society of Gynecologic Oncologists Annual Meeting on Women's Cancer. 2008.
- Chu CS, Boyer J, Schullery DS, Gimotty PH, Gamerman V, Bender J, et al. Phase I/II randomized trial of dendritic cell vaccination with or without cyclophosphamide for consolidation therapy of advanced ovarian cancer in first or second remission. Cancer Immunology Immunotherapy 2012;61:629‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhodapkar 2012 {published data only}
- Dhodapkar M, Zhao B, Wang D, Lutzky RD, Carvajal RD, Keohan M, et al. A phase I trial of a novel vaccine targeting NY‐ESO‐1 to the dendritic cell receptor DEC‐205 in combination with toll‐like receptor agonists. Society for Immunotherapy of Cancer Annual Meeting. 2012.
Diefenbach 2008 {published data only}
- Diefenbach CSM, Gnjatic S, Sabbatini P, Aghajanian C, Hensley ML, Spriggs DR, et al. Safety and immunogenicity study of NY‐ESO‐1b peptide and montanide ISA‐51 vaccination of patients with epithelial ovarian cancer in high‐risk first remission. Clinical Cancer Research 2008;14(9):2740‐8. [DOI] [PubMed] [Google Scholar]
Dijkgraaf 2015 {published data only}
- Dijkgraaf EM, Santegoets SJ, Reyners AK, Goedemans R, Nijman HW, Poelgeest MI, et al. A phase 1/2 study combining gemcitabine, Pegintron and p53 SLP vaccine in patients with platinum‐resistant ovarian cancer. Oncotarget 2015;6:32228‐43. [PUBMED: 26334096] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehlen 2005 {published data only}
- Ehlen TG, Hoskins PJ, Miller D, Whiteside TL, Nicodemus CF, Schultes BC, et al. A pilot phase 2 study of oregovomab murine monoclonal antibody to CA125 as an immunotherapeutic agent for recurrent ovarian cancer. International Journal of Gynecological Cancer 2005;15(6):1023‐34. [DOI] [PubMed] [Google Scholar]
Freedman 1998 {published data only}
- Freedman RS, Kudelka AP, Verschraegen CF, Edwards CL, Tomasovic B, Kaplan A, et al. Therapeutic anti‐cancer vaccine: a randomized double blind dose comparison study of sialyl Tn‐KLH with Detox‐B SE adjuvant for active specific immunotherapy of ovarian cancer (OC). American Society of Clinical Oncology Annual Meeting. 1998.
- Termrungruanglert W, Kudelka AP, Verschraegen CF, Freedman RS, Edwards CL, Tomasovic B, et al. Therapeutic anticancer vaccine: a randomized double blind dose comparison study of sialyl TN‐K with detox‐B SE adjuvant for active specific immunotherapy of ovarian cancer (OC). American Society of Clinical Oncology Annual Meeting. 1996.
Galanis 2010 {published data only}
- Galanis E, Hartmann L, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, et al. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer Research 2010;70(3):875‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goh 2013 {published data only}
- Goh J, CAN‐003 Study Team, Gargosky SE, Gray H. Clinical study of autologous dendritic cell therapy targeting mucin 1 for treatment of ovarian cancer patients in first remission. European Cancer Congress. 2013.
Gordon 2004 {published data only}
- Gordon AN, Schultes BC, Gallion H, Edwards R, Whiteside TL, Cermak JM, et al. CA125‐ and tumor‐specific T‐cell responses correlate with prolonged survival in oregovomab‐treated recurrent ovarian cancer patients. Gynecologic Oncology 2004;94(2):340‐51. [DOI] [PubMed] [Google Scholar]
Gray 2016 {published data only}
- Gray HJ, Benigno B, Berek J, Chang J, Mason J, Mileshkin L, et al. Progression‐free and overall survival in ovarian cancer patients treated with CVac, a mucin 1 dendritic cell therapy in a randomized phase 2 trial. Journal for Immunotherapy of Cancer 2016;4:34. [PUBMED: 27330807] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gribben 2005 {published data only}
- Gribben JG, Ryan DP, Urban RG, Hedley ML, Beach K, Nealon P, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clinical Cancer Research 2005;11(12):4430‐6. [DOI] [PubMed] [Google Scholar]
Gulley 2008 {published data only}
- Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, et al. Pilot study of vaccination with recombinant CEA‐MUC‐1‐TRICOM poxviral‐based vaccines in patients with metastatic carcinoma. Clinical Cancer Research 2008;14:3060‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heiss 2010 {published data only}
- Heiss MM, Maruwa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. International Journal of Cancer 2010;127:2209‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imhof 2013 {published data only}
- Imhoff M, Lipovac M, Angleitner‐Boubenizek L, Barta J, Gomez I, Hrdina A, et al. Double‐loaded mature dendritic cell (DC) therapy for non‐HLA‐restricted patients with advanced ovarian cancer: final results of a clinical phase I study. American Society of Clinical Oncolocy Annual Meeting. 2013.
Kaumaya 2009 {published data only}
- Kaumaya PTP, Foy KC, Garrett J, Rawale SV, Vicari D, Thurmond JM, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, b‐cell epitopes fused to a promiscuous T‐cell epitope in patients with metastatic and/or recurrent solid tumors. Journal of Clinical Oncology 2009;27(31):5270‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawano 2014 {published data only}
- Kawano K, Tsuda N, Matsueda S, Sasada T, Watanabe N, Ushijima K, et al. Feasibility study of personalized peptide vaccination for recurrent ovarian cancer patients. Immunopharmacology Immunotoxicology 2014;36:224‐36. [PUBMED: 24773550] [DOI] [PubMed] [Google Scholar]
Kobayashi 2014 {published data only}
- Kobayashi M, Chiba A, Izawa H, Yanagida E, Okamoto M, Shimodaira S, et al. The feasibility and clinical effects of dendritic cell‐based immunotherapy targeting synthesized peptides for recurrent ovarian cancer. Journal of Ovarian Research 2014;7:48. [PUBMED: 25298213] [DOI] [PMC free article] [PubMed] [Google Scholar]
Le 2012 {published data only}
- Le DT, Brockstedt DG, Nir‐Paz R, Hampl J, Mathur S, Nemunaitis J, et al. A live‐attenuated listeria vaccine (ANZ‐100) and a live‐attenuated listeria vaccine expressing mesothelin (CRS‐207) for advanced cancers: phase I studies of safety and immune induction. Clinical Cancer Research 2012;18(3):858‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leffers 2009a {published data only}
- Leffers N, Lambeck AJ, Gooden MJ, Hoogeboom BN, Wolf R, Hamming IE, et al. Immunization with a P53 synthetic long peptide vaccine induces P53‐specific immune responses in ovarian cancer patients, a phase II trial. International Journal of Cancer 2009;May 28:[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Leffers N, Vermeij R, Hoogeboom BN, Schultze UR, Wolf R, Hamming IE, et al. Long‐term clinical and immunological effects of p53‐SLP vaccine in patients with ovarian cancer. International Journal of Cancer 2012;130:105‐12. [DOI] [PubMed] [Google Scholar]
Lennerz 2014 {published data only}
- Lennerz V, Gross S, Gallerani E, Sessa C, Mach N, Boehm S, et al. Immunologic response to the survivin‐derived multi‐epitope vaccine EMD640744 in patients with advanced solid tumors. Cancer Immunology Immunotherapy 2014;63:381‐94. [PUBMED: 24487961] [DOI] [PMC free article] [PubMed] [Google Scholar]
Letsch 2011 {published data only}
- Letsch A, Scheibenbogen C, Asemissen AM, Zimmermann K, Knodler M, Völker‐Call M, et al. Wilms tumor protein (WT) 1 peptide vaccination in patients with WT1 expressing solid tumors demonstrates clinical and immunological efficacy. Onkologie 2011;34(Suppl 6):194. [Google Scholar]
Ma 2002 {published data only}
- Ma J, Zhou L, Wang D. Functional mimicry of an anti‐idiotypic antibody to nominal antigen on cellular response. Japanese Journal of Cancer Research 2002;93(1):78‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
MacLean 1992 {published data only}
- MacLean GD, Bowen‐Yacyshyn MB, Samuel J, Meikle A, Stuart G, Nation J, et al. Active immunization of human ovarian cancer patients against a common carcinoma (Thomsen‐Friedenreich) determinant using a synthetic carbohydrate antigen. Journal of Immunotherapy 1992;11(4):292‐305. [DOI] [PubMed] [Google Scholar]
MacLean 1996 {published data only}
- MacLean GD, Reddish MA, Koganty RR, Longenecker BM. Antibodies against mucin‐associated sialyl‐Tn epitopes correlate with survival of metastatic adenocarcinoma patients undergoing active specific immunotherapy with synthetic STn vaccine. Journal of Immunotherapy With Emphasis on Tumor Immunology 1996;19(1):59‐68. [DOI] [PubMed] [Google Scholar]
Method 2002 {published data only}
- Method M, Gordon A, Finkler N, Fingert H, Nicodemus CF, Whiteside TL, et al. Randomized evaluation of 3 treatment schedules to optimize clinical activity of OvaRex Mab B43.13 (OV) in patients (pts) with epithelial ovarian cancer (EOC). American Society of Clinical Oncology Annual Meeting. 2002.
Möbus 2003 {published data only}
- Mobus VJ, Baum RP, Bolle M, Kreienberg R, Noujaim AA, Schultes BC, et al. Immune responses to murine monoclonal antibody‐B43.13 correlate with prolonged survival of women with recurrent ovarian cancer. American Journal of Obstetrics and Gynecology 2003;189(1):28‐36. [DOI] [PubMed] [Google Scholar]
Mohebtash 2011 {published data only}
- Mohebtash M, Madan R, Tsang K, Arlen Ph, Pazdur M, Jones J, et al. Clinical outcomes following immunotherapy with a MUC1/CEA vaccine in patients with metastatic breast and ovarian cancer. American Association of Cancer Research Annual Meeting. 2009.
- Mohebtash M, Tsang KY, Huen NY, Poole DJ, Jochems C, Jones J, et al. A pilot study of MUC‐1/CEA/TRICOM poxviral‐based vaccine in patients with metastatic breast and ovarian cancer. Clinical Cancer Research 2011;17(22):7164‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morse 2011 {published data only}
- Morse MA, Secord AA, Blackwell K, Hobeika AM, Sinnathamby G, Osada T, et al. MHC class I‐presented tumor antigens identified in ovarian cancer by immunoproteomic analysis are targets for T‐cell responses against breast and ovarian cancer. Clinical Cancer Research 2011;17(10):3408‐19. [DOI] [PubMed] [Google Scholar]
Nicholson 2004 {published data only}
- Nicholson S, Bomphray CC, Thomas H, McIndoe A, Barton D, Gore M, et al. A phase I trial of idiotypic vaccination with HMFG1 in ovarian cancer. Cancer Immunology, Immunotherapy 2004;53(9):809‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishikawa 2006 {published data only}
- Nishikawa H, Qian F, Tsuji T, Ritter G, Old LJ, Gnjatic S, et al. Influence of CD4(+)CD25(+) regulatory T cells on low/high‐avidity CD4(+) T cells following peptide vaccination. Journal of Immunology 2006;176(10):6340‐6. [DOI] [PubMed] [Google Scholar]
Noujaim 2001 {published data only}
- Noujaim AA, Schultes BC, Baum RP, Madiyalakan R. Induction of CA125‐specific B and T cell responses in patients injected with MAb‐B43.13 ‐ evidence for antibody‐mediated antigen‐processing and presentation of CA125 in vivo. Cancer Biotherapy & Radiopharmaceuticals 2001;16(3):187‐203. [DOI] [PubMed] [Google Scholar]
O'Cearbhaill 2016 {published data only}
- O'Cearbhaill RE, Ragupathi G, Zhu J, Wan Q, Mironov S, Yang G, et al. A phase I study of unimolecular pentavalent (Globo‐H‐GM2‐sTn‐TF‐Tn) immunization of patients with epithelial ovarian, fallopian tube, or peritoneal cancer in first remission. Cancers 2016;8:46. [PUBMED: 27110823] [DOI] [PMC free article] [PubMed] [Google Scholar]
Odunsi 2007 {published data only}
- Odunsi K, Qian F, Matsuzaki J, Mhawech‐Fauceglia P, Andrews C, Hoffman EW, et al. Vaccination with an NY‐ESO‐1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America 2007;104(31):12837‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Odunsi 2012 {published data only}
- Odunsi K, Matsuzaki J, Karbach J, Neumann A, Mhawech‐Fauceglia P, Miller M, et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY‐ESO‐1 antigen in ovarian cancer and melanoma patients. PNAS 2012;109(15):5797‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odunsi K, Rodabaugh K, Lele S, Old LJ, Matsuzaki J, Qian F, et al. Diversified prime and boost vaccination using recombinant vaccinia and fowlpox expressing NY‐ESO‐1 efficiently induces antibody, CD4+, and CD8+ antitumor immune responses in patients with ovarian cancer. Society of Gynecologic Oncologists Annual Meeting on Women's Cancer. 2007.
Odunsi 2014 {published data only}
- Odunsi K, Matsuzaki J, James SR, Mhawech‐Fauceglia P, Tsuji T, Miller A, et al. Epigenetic potentiation of NY‐ESO‐1 vaccine therapy in human ovarian cancer. Cancer Immunology, Immunotherapy 2014;2:37‐49. [24535937] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ohno 2009 {published data only}
- Dohi S, Ohno S, Ohno Y, Takakura M, Kyo S, Soma GI, et al. WT1 peptide vaccine stabilized intractable ovarian cancer patient for one year: a case report. Anticancer Research 2001;31:2441‐6. [PubMed] [Google Scholar]
- Ohno S, Kyo S, Myojo S, Dohi S, Ishizaki J, Miyamoto KI, et al. Wilms' tumor 1 (WT1) peptide immunotherapy for gynecological malignancy. Anticancer Research 2009;29:4779‐84. [PubMed] [Google Scholar]
Peethambaram 2009 {published data only}
- Peethambaram PP, Melisko ME, Rinn KJ, Alberts SR, Provost NM, Jones LA, et al. A phase I trial of immunotherapy with Lapuleucel‐T (APC8024) in patients with refractory metastatic tumors that express Her‐2/neu. Clinical Cancer Research 2009;15(18):5937‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfisterer 2006 {published data only}
- Pfisterer J, du Bois A, Sehouli J, Loibl S, Reinartz S, Reuss A, et al. The anti‐idiotypic antibody abagovomab in patients with recurrent ovarian cancer. A phase I trial of the AGO‐OVAR. Annals of Oncology 2006;17(10):1568‐77. [DOI] [PubMed] [Google Scholar]
Rahma 2012 {published data only}
- Herrin V, Achtar M, Steinberg S, Whiteside TL, Wieckowsk E, Czystowska M, et al. A randomized phase II p53 vaccine trial comparing subcutaneous direct administration with intravenous peptide‐pulsed dendritic cells in high risk ovarian cancer patients. American Society of Clinical Oncology Annual Meeting. 2007.
- Herrin V, Behrens RJ, Achtar M, Monahan B, Bernstein S, Brent‐Steele T, et al. Wild‐type p53 peptide vaccine can generate a specific immune response in low burden ovarian adenocarcinom. American Society of Clinical Oncology Annual Meeting. 2003.
- Rahma OE, Ashtar E, Czystowska M, Szajnik ME, Wieckowski E, Bernstein S, et al. A gynecologic oncology group phase II trial of two p53 peptide vaccine approaches: subcutaneous injection and intravenous pulsed dendritic cells in high recurrence risk ovarian cancer patients. Cancer Immunology, Immunotherapy 2012;61:374‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinartz 2004 {published data only}
- Reinartz S, Kohler S, Schlebusch H, Krista K, Giffels P, Renke K, et al. Vaccination of patients with advanced ovarian carcinoma with the anti‐idiotype ACA125: immunological response and survival (phase Ib/II). Clinical Cancer Research 2004;10(5):1580‐7. [DOI] [PubMed] [Google Scholar]
- Wagner U, Kohler S, Reinartz S, Giffels P, Huober J, Renke K, et al. Immunological consolidation of ovarian carcinoma recurrences with monoclonal anti‐idiotype antibody ACA125: immune responses and survival in palliative treatment. Clinical Cancer Research 2001;7(5):1154‐62. [PubMed] [Google Scholar]
Sabbatini 2000 {published data only}
- Sabbatini PJ, Kudryashov V, Ragupathi G, Danishefsky SJ, Livingston PO, Bornmann W, et al. Immunization of ovarian cancer patients with a synthetic Lewis(y)‐protein conjugate vaccine: a phase 1 trial. Clinical Cancer Research 2000;87(1):79‐85. [PubMed] [Google Scholar]
Sabbatini 2006 {published data only}
- Sabbatini P, Dupont J, Aghajanian C, Derosa F, Poynor E, Anderson S, et al. Phase I study of abagovomab in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer. Clinical Cancer Research 2006;12(18):5503‐10. [DOI] [PubMed] [Google Scholar]
Sabbatini 2007 {published data only}
- Sabbatini PJ, Ragupathi G, Hood C, Aghajanian CA, Juretzka M, Iasonos A, et al. Pilot study of a heptavalent vaccine‐keyhole limpet hemocyanin conjugate plus QS21 in patients with epithelial ovarian, fallopian tube, or peritoneal cancer. Clinical Cancer Research 2007;13:4170‐7. [DOI] [PubMed] [Google Scholar]
Sabbatini 2012 {published data only}
- Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, et al. Phase I trial of overlapping long peptides from a tumor self‐antigen and poly‐ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clinical Cancer Research 2012;18(23):6497‐508. [DOI] [PubMed] [Google Scholar]
Sabbatini 2013 {published data only}
- Sabbatini P, Harther P, Scambia G, Sehouli J, Meier W, Wimberger P, et al. Abagovomab as maintenance therapy in patients with epithelial ovarian cancer: a phase III trial of the AGO OVAR, COGI, GINECO and GEICO ‐ the MIMOSA study. Journal of Clinical Oncology 2013;31(12):1554‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabbatini 2017 {published data only}
- Sabbatini P, Chen L, Lucci J, Behbakht K, Spirtos N, Muller C. OPT‐821 with or without vaccine therapy in treating patients with ovarian epithelial cancer, fallopian tube cancer, or peritoneal cancer in second or third complete remission. clinicaltrials.gov 2017. [NCT00857545]
Sandmaier 1999 {published data only}
- Sandmaier BM, Oparin DV, Holmberg LA, Reddish MA, MacLean GD, Longenecker BM. Evidence of a cellular immune response against sialyl‐Tn in breast and ovarian cancer patients after high‐dose chemotherapy, stem cell rescue, and immunization with Theratope STn‐KLH cancer vaccine. Journal of Immunotherapy 1999;22(1):54‐66. [DOI] [PubMed] [Google Scholar]
Schultes 1998 {published data only}
- Schultes B, Yang R, Agopsowicz K, Kuzma M, Dharampaul S, Baum R, et al. Anti‐idiotype induction therapy for ovarian cancer: immune responses in patients injected with OvaRex‐Mab‐B43.13. American Society of Clinical Oncology Annual Meeting. 1999.
- Schultes BC, Baum RP, Niesen A, Noujaim AA, Madiyalakan R. Anti‐idiotype induction therapy: anti‐CA125 antibodies (Ab3) mediated tumor killing in patients treated with Ovarex mAb B43.13 (Ab1). Cancer Immunology, Immunotherapy 1998;46(4):201‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ströhlein 2009 {published data only}
- Ströhlein MA, Siegel R, Jäger M, Lindhofer H, Jauch KW, Heiss MM. Induction of anti‐tumor immunity by trifunctional antibodies in patients with peritoneal carcinomatosis. Journal of Experimental & Clinical Cancer Research 2009;28:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 2016 {published data only}
- Suzuki S, Sakata J, Utsumi F, Sekiya R, Kajiyama H, Shibata K, et al. Efficacy of glypican‐3‐derived peptide vaccine therapy on the survival of patients with refractory ovarian clear cell carcinoma. Oncoimmunology 2016;5(11):1238542. [PUBMED: 27999758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takeoka 2017 {published data only}
- Takeoka T, Nagase H, Kurose K, Ohue Y, Yamasaki M, Takiguchi S, et al. NY‐ESO‐1 protein cancer vaccine with Poly‐ICLC and OK‐432: rapid and strong induction of NY‐ESO‐1‐specific immune responses by Poly‐ICLC. Journal of Immunotherapy 2017;40:140‐7. [PUBMED: 28338507] [DOI] [PubMed] [Google Scholar]
Takeuchi 2013 {published data only}
- Takeuchi S, Shoji T, Kagabu M, Honda T, Miura F, Omi H, et al. A phase I/II study of multiple peptides cocktail vaccine for advanced/recurrent ovarian cancer. American Society of Clinical Oncology Annual Meeting. 2013.
Tsuda 2004 {published data only}
- Tsuda N, Mochizuki K, Harada M, Sukehiro A, Kawano K, Yamada A, et al. Vaccination with predesignated or evidence‐based peptides for patients with recurrent gynecologic cancers. Journal of Immunotherapy 2004;27(1):60‐72. [DOI] [PubMed] [Google Scholar]
van Zanten‐Przybysz 2002 {published data only}
- Zanten‐Przybysz I, Molthoff C, Gebbinck JK, Mensdorff‐Pouilly S, Verstraeten R, Kenemans P, et al. Cellular and humoral responses after multiple injections of unconjugated chimeric monoclonal antibody MOv18 in ovarian cancer patients: a pilot study. Journal of Cancer Research and Clinical Oncology 2002;128(9):484‐92. [DOI] [PubMed] [Google Scholar]
Vermeij 2012 {published data only}
- Vermeij R, Leffers N, Hoogeboom BN, Hamming LE, Wolf R, Reyners AKL, et al. Potentiation of a p53‐SLP vaccine by cyclophosphamide in ovarian cancer: a single‐arm phase II study. International Journal of Cancer 2012;131:E670‐80. [DOI] [PubMed] [Google Scholar]
Wagner 1993 {published data only}
- Wagner U. Antitumor antibodies for immunotherapy of ovarian carcinomas. Hybridoma 1993;12(5):521‐8. [DOI] [PubMed] [Google Scholar]
- Wagner U, Reinsberg J, Schmidt S, Mallmann P, Schmolling J, Schultes B, et al. Monoclonal antibodies and idiotypic network activation for ovarian carcinoma. Cell Biophysics 1994;24‐25:237‐42. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2000 {published data only}
- Anderson BW, Kudelka AP, Honda T, Pollack MS, Gershenson DM, Gillogly MA, et al. Induction of determinant spreading and of Th1 responses by in vitro stimulation with HER‐2 peptides. Cancer Immunology, Immunotherapy 2000;49(9):459‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baek 2015 {published data only}
- Baek S, Kim YM, Kim SB, Kim CS, Kwon SW, Kim Y. Therapeutic DC vaccination with IL‐2 as a consolidation therapy for ovarian cancer patients: a phase I/II trial. Cellular & Molecular Immunology 2015;12:87‐95. [PUBMED: 24976269] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bapsy 2014 {published data only}
- Bapsy PP, Sharan B, Kumar C, Das RP, Rangarajan B, Jain M. Open‐label, multi‐center, non‐randomized, single‐arm study to evaluate the safety and efficacy of dendritic cell immunotherapy in patients with refractory solid malignancies, on supportive care. Cytotherapy 2014;16(2):234‐44. [PUBMED: 24438902] [DOI] [PubMed] [Google Scholar]
Bender 2007 {published data only}
- Bender A, Karbach J, Neumann A, Jager D, Al‐Batran SE, Atmaca A, et al. LUD 00‐009: phase 1 study of intensive course immunization with NY‐ESO‐1 peptides in HLA‐A2 positive patients with NY‐ESO‐1‐expressing cancer. Cancer Immunology, Immunotherapy 2007;7:16. [PMC free article] [PubMed] [Google Scholar]
Bernal 2012 {published data only}
- Bernal SD, Ona ET, Riego‐Javier E, Villa R, Cristal‐Luna GR, Laguatan JB, et al. Anticancer immune reactivity and long‐term survival after treatment of metastatic ovarian cancer with dendritic cells. Oncology Letters 2012;3:66‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbone 2005 {published data only}
- Carbone DP, Ciernik IF, Kelley MJ, Smith MC, Nadaf S, Kavanaugh D, et al. Immunization with mutant p53‐ and K‐ras‐derived peptides in cancer patients: immune response and clinical outcome. Journal of Clinical Oncology 2005;23(22):5099‐107. [DOI] [PubMed] [Google Scholar]
Chiang 2013 {published data only}
- Chiang CL, Kandalaft LE, Tanyi J, Hagemann AR, Motz GT, Svoronos N. A dendritic cell vaccine pulsed with autologous hypochlorous acid‐oxidized ovarian cancer lysate primes effective broad antitumor immunity: from bench to bedside. Clinical Cancer Research 2013;19(17):4801‐15. [PUBMED: 23838316] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coosemans 2013 {published data only}
- Coosemans A, Vanderstraeten A, Tuyaerts S, Verschuere T, Moerman P, Berneman Z. Immunological response after WT1 mRNA‐loaded dendritic cell immunotherapy in ovarian carcinoma and carcinosarcoma. Anticancer Research 2013;33(9):3855‐9. [PUBMED: 24023319] [PubMed] [Google Scholar]
Dhodapkar 2014 {published data only}
- Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, et al. Induction of antigen‐specific immunity with a vaccine targeting NY‐ESO‐1 to the dendritic cell receptor DEC‐205. Science Translational Medicine 2014;6(232):51. [PUBMED: 24739759] [DOI] [PMC free article] [PubMed] [Google Scholar]
Disis 1999 {published data only}
- Disis ML, Grabstein KH, Sleath PR, Cheever MA. Generation of immunity to the HER‐2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide‐based vaccine. Clinical Cancer Research 1999;5(6):1289‐97. [PubMed] [Google Scholar]
Disis 2000 {published data only}
- Disis ML, Schiffman K, Gooley TA, McNeel DG, Rinn K, Knutson KL. Delayed‐type hypersensitivity response is a predictor of peripheral blood T‐cell immunity after HER‐2/neu peptide immunization. Clinical Cancer Research 2000;6(4):1347‐50. [PubMed] [Google Scholar]
Disis 2002 {published data only}
- Disis ML, Gooley TA, Rinn K, Davis D, Piepkorn M, Cheever MA, et al. Generation of T‐cell immunity to the HER‐2/neu protein after active immunization with HER‐2/neu peptide‐based vaccines. Journal of Clinical Oncology 2002;20(11):2624‐32. [DOI] [PubMed] [Google Scholar]
Disis 2002a {published data only}
- Disis ML, Rinn K, Knutson KL, Davis D, Caron D, dela Rosa C, et al. Flt3 ligand as a vaccine adjuvant in association with HER‐2/neu peptide‐based vaccines in patients with HER‐2/neu‐overexpressing cancers. Blood 2002;99(8):2845‐50. [DOI] [PubMed] [Google Scholar]
Disis 2004 {published data only}
- Disis ML, Goodell V, Schiffman K, Knutson KL. Humoral epitope‐spreading following immunization with a HER‐2/neu peptide based vaccine in cancer patients. Journal of Clinical Immunology 2004;24(5):571‐8. [DOI] [PubMed] [Google Scholar]
Disis 2004a {published data only}
- Disis ML, Schiffman K, Guthrie K, Salazar LG, Knutson KL, Goodell V, et al. Effect of dose on immune response in patients vaccinated with an her‐2/neu intracellular domain protein‐‐based vaccine. Journal of Clinical Oncology 2004;22(10):1916‐25. [DOI] [PubMed] [Google Scholar]
Galanis 2013 {published data only}
- Galanis E, Atherton P, Dowdy S, Cliby W, Haluska P, Long H, et al. Intraperitoneal (IP) administration of an oncolytic measles virus (MV) strain expressing the sodium iodine symporter gene in patients (pts) with advanced ovarian cancer (ovca). American Society of Gene & Cell Therapy (ASGCT) Annual Meeting. 2013.
Haakenstad 2012 {published data only}
- Haakenstad H, Suso EMI, Rasmussen AM, Larsen SS, Dueland S, Lilleby W, et al. Clinical use of fast DCs transfected with hTERT and Survivin mRNA ‐ an effective and simplified cancer vaccine approach. European Group for Blood and Marrow Transplantation Annual Meeting. 2012.
- Kvalheim G, Suso E, Rasmussen A, Honnashagen T, Dueland S, Gaudernack G. Fast DCs transfected with hTERT and Survivin mRNA ‐ a novel, effective and simplified cancer vaccine approach. European Group for Blood and Marrow Transplantation Annual Meeting. 2011.
Hasumi 2011 {published data only}
- Hasumi K, Aoki Y, Watanabe R, Hankey KG, Mann DL. Therapeutic response in patients with advanced malignancies treated with combined dendritic cell‐activated T cell based immunotherapy and intensity‐modulated radiotherapy. Cancers 2011;3:2223‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernando 2002 {published data only}
- Hernando JJ, Park TW, Kubler K, Offergeld R, Schlebusch H, Bauknecht T. Vaccination with autologous tumour antigen‐pulsed dendritic cells in advanced gynaecological malignancies: clinical and immunological evaluation of a phase I trial. Cancer Immunology, Immunotherapy 2002;51(1):45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernando 2007 {published data only}
- Hernando JJ, Park T‐W, Fischer H‐P, Zivanovic O, Braun M, Polcher M, et al. Vaccination with dendritic cells transfected with mRNA‐encoded folate‐receptor‐(alpha) for relapsed metastatic ovarian cancer. Lancet Oncology 2007;8(5):451‐4. [DOI] [PubMed] [Google Scholar]
Holmberg 2000 {published data only}
- Holmberg LA, Oparin DV, Gooley T, Lilleby K, Bensinger W, Reddish MA, et al. Clinical outcome of breast and ovarian cancer patients treated with high‐dose chemotherapy, autologous stem cell rescue and THERATOPE STn‐KLH cancer vaccine. Bone Marrow Transplant 2000;25(12):1233‐41. [DOI] [PubMed] [Google Scholar]
Hui 1997 {published data only}
- Hui KM, Ang PT, Huang L, Tay SK. Phase I study of immunotherapy of cutaneous metastases of human carcinoma using allogeneic and xenogeneic MHC DNA‐liposome complexes. Gene Therapy 1997;4(8):783‐90. [DOI] [PubMed] [Google Scholar]
Jackson 2017 {published data only}
- Jackson D, Byrd K, Vreeland T, Hale D, Herbert G, Greene JM, et al. Interim analysis of a phase I/IIa trial assessing E39+GM‐CSF, a folate binding protein vaccine, to prevent recurrence in ovarian and endometrial cancer patients. Oncotarget 2017;8:15912‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jager 2006 {published data only}
- Jager E, Karbach J, Gnjatic S, Neumann A, Bender A, Valmori D, et al. Recombinant vaccinia/fowlpox NY‐ESO‐1 vaccines induce both humoral and cellular NY‐ESO‐1‐specific immune responses in cancer patients. Proceedings of the National Academy of Sciences of the United States of America 2006;103(39):14453‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kandalaft 2010 {published data only}
- Coukos G, Powell D, Kandalaft L, Smith L, Chu C, Rubin S, et al. Autologous whole‐tumor antigen combinatorial immunotherapy for recurrent ovarian cancer. Gynecologic Oncology 2010;116:s130. [Google Scholar]
- Kandalaft LE, Powell DJ, Smith L, Adams S, Liao J, Hageman A, et al. Autologous whole‐tumor anitgen combinatorial immunotherapy for recurrent ovarian cancer. Journal of Immunotherapy 2010;33(8):878. [Google Scholar]
Karbach 2010 {published data only}
- Karbach J, Gnjatic S, Bender A, Neumann A, Weidmann E, Yuan J, et al. Tumor‐reactive CD8+ T‐cell responses after vaccination with NY‐ESO‐1 peptide, CpG 7909 and Montanide ISA‐51: association with survival. International Journal of Cancer 2010;126:909‐18. [DOI] [PubMed] [Google Scholar]
Kato 2010 {published data only}
- Kato Y. WT1 peptide pulsed dendritic cell therapy with activated T lymphocytes therapy for advanced cancers. Gan To Kagaku Ryoho 2010;37(12):2240‐2. [PubMed] [Google Scholar]
Khranovska 2011 {published data only}
- Khranovska NM, Svyntsytsky VS, Potebnya GP, Vorobyova LI, Skachkova OV, Tsyp NP, et al. New dendritic cell vaccine therapy approach ‐ randomized phase I/II study in III‐IV stage ovarian cancer patients. European Multidisciplinary Cancer Conference (ECCO ESMO ESTRO). 2011.
Knutson 2001 {published data only}
- Knutson KL, Schiffman K, Disis ML. Immunization with a HER‐2/neu helper peptide vaccine generates HER‐2/neu CD8 T‐cell immunity in cancer patients. Journal of Clinical Investigation 2001;107(4):477‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Knutson 2002 {published data only}
- Knutson KL, Schiffman K, Cheever MA, Disis ML. Immunization of cancer patients with a HER‐2/neu, HLA‐A2 peptide, p369‐377, results in short‐lived peptide‐specific immunity. Clinical Cancer Research 2002;8(5):1014‐8. [PubMed] [Google Scholar]
Letsch 2008 {published data only}
- Letsch A, Asemissen AM, Zimmermann K, Bauer S, Stather D, Völker‐Call M, et al. Different quality of T cell responses to WT1 peptide vaccination in patients with AML/MDS and patients with solid tumors. Journal of Immunotherapy. 2008; Vol. 31:943‐4.
Loveland 2006 {published data only}
- Loveland BE, Zhao A, White S, Gan H, Hamilton K, Xing PX, et al. Mannan‐MUC1‐pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clinical Cancer Research 2006;12(3 Pt 1):869‐77. [DOI] [PubMed] [Google Scholar]
Manjunath 2012 {published data only}
- Manjunath SR, Ramanan G, Dedeepiya VD, Terunuma H, Deng X, Baskar S, et al. Autologous immune enhancement therapy in recurrent ovarian cancer with metastases: a case report. Case Reports in Oncology 2012;5:114‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marshall 2005 {published data only}
- Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, et al. Phase I study of sequential vaccinations with fowlpox‐CEA(6D)‐TRICOM alone and sequentially with vaccinia‐CEA(6D)‐TRICOM, with and without granulocyte‐macrophage colony‐stimulating factor, in patients with carcinoembryonic antigen‐expressing carcinomas. Journal of Clinical Oncology 2005;23:720‐31. [DOI] [PubMed] [Google Scholar]
Matsuzaki 2014 {published data only}
- Matsuzaki J, Tsuji T, Luescher I, Old LJ, Shrikant P, Gnjatic S. Nonclassical antigen‐processing pathways are required for MHC class II‐restricted direct tumor recognition by NY‐ESO‐1‐specific CD4(+) T cells. Cancer Immunology Research 2014;2(4):341‐50. [PUBMED: 24764581] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miotti 1999 {published data only}
- Miotti S, Negri DR, Valota O, Calabrese M, Bolhuis RL, Gratama JW, et al. Level of anti‐mouse‐antibody response induced by bi‐specific monoclonal antibody OC/TR in ovarian‐carcinoma patients is associated with longer survival. International Journal of Cancer 1999;84(1):62‐8. [DOI] [PubMed] [Google Scholar]
Morera 2017 {published data only}
- Morera Y, Sánchez J, Bequet‐Romero M, Selman‐Housein KH, Torre A, Hernández‐Bernal F. Specific humoral and cellular immune responses in cancer patients undergoing chronic immunization with a VEGF‐based therapeutic vaccine. Vaccine 2017;35:3582‐90. [PUBMED: 28536029] [DOI] [PubMed] [Google Scholar]
Morse 1999 {published data only}
- Morse MA, Deng Y, Coleman D, Hull S, Kitrell‐Fisher E, Nair S, et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP‐1)‐pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen. Clinical Cancer Research 1999;5(6):1331‐8. [PubMed] [Google Scholar]
Morse 2003 {published data only}
- Morse MA, Clay TM, Colling K, Hobeika A, Grabstein K, Cheever MA, et al. Her2 dendritic cell vaccines. Clinical Breast Cancer 2003;3(Suppl 4):S164‐72. [DOI] [PubMed] [Google Scholar]
Morse 2011a {published data only}
- Morse MA, Chapman R, Powerdly J, Blackwell K, Keler T, Green J, et al. Phase I study utilizing a novel antigen‐presenting cell‐targeted vaccine with toll‐like receptor stimulation to induce immunity to self‐antigens in cancer patients. Clinical Cancer Research 2011;17(14):4844‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murray 2002 {published data only}
- Murray JL, Gillogly ME, Przepiorka D, Brewer H, Ibrahim NK, Booser DJ, et al. Toxicity, immunogenicity, and induction of E75‐specific tumor‐lytic CTLs by HER‐2 peptide E75 (369‐377) combined with granulocyte macrophage colony‐stimulating factor in HLA‐A2+ patients with metastatic breast and ovarian cancer. Clinical Cancer Research 2002;8(11):3407‐18. [PubMed] [Google Scholar]
Oh 2016 {published data only}
- Oh J, Barve M, Matthews CM, Koon EC, Heffernan TP, Fine B. Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynaecologic Oncology 2016;143(3):504‐10. [27678295] [DOI] [PubMed] [Google Scholar]
Parkhurst 2004 {published data only}
- Parkhurst MR, Riley JP, Igarashi T, Li Y, Robbins PF, Rosenberg SA. Immunization of patients with the hTERT:540‐548 peptide induces peptide‐reactive T lymphocytes that do not recognize tumors endogenously expressing telomerase. Clinical Cancer Research 2004;10(14):4688‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddish 1996 {published data only}
- Reddish MA, MacLean GD, Poppema S, Berg A, Longenecker BM. Pre‐immunotherapy serum CA27.29 (MUC‐1) mucin level and CD69+ lymphocytes correlate with effects of Theratope sialyl‐Tn‐KLH cancer vaccine in active specific immunotherapy. Cancer Immunology, Immunotherapy 1996;42(5):303‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salazar 2006 {published data only}
- Salazar LG, Murray JL, Disis ML, Cheever M. A phase I vaccine trial of a HER‐2/neu peptide incorporated into PLG microspheres in patients with advanced stage HER2‐expressing cancers. ASCO Annual Meeting. 2006.
Schiffman 2002 {published data only}
- Schiffman K, Rinn K, Disis ML. Delayed type hypersensitivity response to recall antigens does not accurately reflect immune competence in advanced stage breast cancer patients. Breast Cancer Research and Treatment 2002;74(1):17‐23. [DOI] [PubMed] [Google Scholar]
Tsuji 2013 {published data only}
- Tsuji T, Sabbatini P, Jungbluth AA, Ritter E, Pan L, Ritter G, et al. Effect of Montanide and poly‐ICLC adjuvant on human self/tumor antigen‐specific CD4+ T cells in phase I overlapping long peptide vaccine trial. Cancer Immunology Research 2013;1:340‐50. [DOI: 10.1158/2326-6066.CIR-13-0089] [DOI] [PubMed] [Google Scholar]
Yacyshyn 1995 {published data only}
- Yacyshyn MB, Poppema S, Berg A, MacLean GD, Reddish MA, Meikle A, et al. CD69+ and HLA‐DR+ activation antigens on peripheral blood lymphocyte populations in metastatic breast and ovarian cancer patients: correlations with survival following active specific immunotherapy. International Journal of Cancer 1995;61(4):470‐4. [DOI] [PubMed] [Google Scholar]
Zaks 1998 {published data only}
- Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369‐377) from HER‐2/neu leads to peptide‐specific cytotoxic T lymphocytes that fail to recognize HER‐2/neu+ tumors. Cancer Research 1998;58(21):4902‐8. [PubMed] [Google Scholar]
References to ongoing studies
NCT00003002 {unpublished data only}
- University of Washington. Her‐2/Neu vaccine plus GM‐CSF in treating patients with stage III or stage IV breast, ovarian, or non‐small cell lung cancer. clinicaltrials.gov.
NCT00004604 {unpublished data only}
- Duke University. Biological therapy in treating patients with metastatic cancer. clinicaltrials.gov.
NCT00006041 {unpublished data only}
- Memorial Sloan ‐ Kettering Cancer Center. Vaccine therapy in treating patients with ovarian, fallopian tube, or peritoneal cancer. clinicaltrials.gov.
NCT00381173 {unpublished data only}
- Eisai Medical Research Inc. A phase 1 open‐label study of the safety and feasibility of ZYC300 administration with cyclophosphamide pre‐dosing. clinicaltrials.gov.
NCT00803569 {unpublished data only}
- Roswell Park Cancer Institute and National Cancer Institute (NCI). Phase I study of ALVAC(2)‐NY‐ESO‐1(M)/TRICOM in patients with epithelial ovarian, fallopian tube or primary peritoneal carcinoma whose tumors express NY‐ESO‐1 or LAGE‐1 antigen. clinicaltrilas.gov.
NCT01223235 {unpublished data only}
- Memorial Sloan ‐ Kettering Cancer Center. Polyvalent vaccine‐KLH conjugate + Opt‐821 given in combination with bevacuzimab. clinicaltrials.gov.
NCT01322802 {unpublished data only}
- University of Washington. Vaccine therapy in treating patients with stage III‐IV or recurrent ovarian cancer. clinicaltrials.gov.
NCT01376505 {unpublished data only}
- Pravin Kaumaya. Vaccine therapy in treating patients with metastatic solid tumors. clinicaltrials.gov.
NCT01522820 {unpublished data only}
- Roswell Park Cancer Institute. Vaccine therapy with or without sirolimus in treating patients with NY‐ESO‐1 expressing solid tumors. clinicaltrials.gov.
NCT01536054 {unpublished data only}
- Roswell Park Cancer Institute. Sirolimus and vaccine therapy in treating patients with stage II‐IV ovarian epithelial, fallopian tube, or primary peritoneal cavity cancer. clinicaltrials.gov.
NCT01556841 {unpublished data only}
- Oxford BioMedica. The activity of TroVax versus placebo in relapsed asymptomatic ovarian cancer (TRIOC). clinicaltrials.gov.
NCT01584115 {unpublished data only}
- Instituto de Investigacao em Imunologia. Clinical trial of a therapeutic vaccine with NY‐ESO‐1 in combination with the adjuvant monophosphoryl lipid A (MPLA). clinicaltrials.gov.
NCT01606241 {unpublished data only}
- Mayo Clinic. Cyclophosphamide and vaccine therapy in treating patients with stage II‐III breast, ovarian, primary peritoneal, or fallopian tube cancer. clinicaltrials.gov.
NCT01616303 {unpublished data only}
- Quest PharmaTech Inc. A controlled study of the effectiveness of oregovomab (antibody) plus chemotherapy in advanced ovarian cancer. clinicaltrials.gov.
NCT01621542 {unpublished data only}
- Sunovion. Clinical study of WT2725 in patients with advanced solid malignancies. clinicaltrials.gov.
NCT01673217 {unpublished data only}
- Roswell Park Cancer Institute. Decitabine, vaccine therapy, and pegylated liposomal doxorubicin hydrochloride in treating patients with recurrent ovarian epithelial cancer, fallopian tube cancer, or peritoneal cancer. clinicaltrials.gov.
NCT02111941 {unpublished data only}
- Mayo Clinic. Vaccine therapy in treating patients with stage IIIC‐IV ovarian epithelial, fallopian tube, or primary peritoneal cavity cancer following surgery and chemotherapy. clinicaltrials.gov.
NCT02132988 {unpublished data only}
- Mackay Memorial Hospital. Trial of active immunotherapy with Globo H‐KLH (OPT‐822/821) in women who have non‐progressive ovarian cancer. clinicaltrials.gov.
NCT02146313 {unpublished data only}
- Genentech, Inc. A study evaluating the safety and pharmacokinetics of DMUC4064A in participants with platinum‐resistant ovarian cancer or unresectable pancreatic cancer. clinicaltrials.gov.
NCT02166905 {unpublished data only}
- Roswell Park Cancer Institute. DEC‐205/NY‐ESO‐1 fusion protein CDX‐1401, Poly ICLC, and IDO1 inhibitor INCB024360 in treating patients with ovarian, fallopian tube, or primary peritoneal cancer in remission. clinicaltrials.gov.
NCT02275039 {unpublished data only}
- City of Hope Medical Center. P53MVA vaccine and gemcitabine hydrochloride in treating patients with recurrent ovarian epithelial cancer. clinicaltrials.gov.
NCT02387125 {unpublished data only}
- Immune Design. Phase 1b safety study of CMB305 in patients with locally advanced, relapsed, or metastatic cancer expressing NY‐ESO‐1. clinicaltrials.gov. [DOI] [PMC free article] [PubMed]
NCT02498665 {unpublished data only}
- Boston Biomedical, Inc. A study of DSP‐7888 dosing emulsion in adult patients with advanced malignancies. clinicaltrials.gov.
NCT02575807 {unpublished data only}
- Aduro Biotech, Inc. Safety and efficacy of CRS‐207 with Epacadostat in platinum resistant ovarian, fallopian or peritoneal cancer (SEASCAPE). clinicaltrials.gov.
NCT02737787 {unpublished data only}
- Memorial Sloan Kettering Cancer Center. A study of WT1 vaccine and nivolumab for recurrent ovarian cancer. clinicaltrials.gov.
NCT02764333 {unpublished data only}
- Memorial Sloan Kettering Cancer Center. TPIV200/huFR‐1 (a multi‐epitope anti‐folate receptor vaccine) plus anti‐PD‐L1 MEDI4736 (Durvalumab) in patients with platinum resistant ovarian cancer. clinicaltrials.gov.
NCT02785250 {unpublished data only}
- ImmunoVaccine Technologies, Inc. Study of DPX‐Survivac vaccine therapy and Epacadostat in patients with recurrent ovarian cancer. clinicaltrials.gov.
NCT02833506 {unpublished data only}
- Roswell Park Cancer Institute. Sirolimus and vaccine therapy in treating patients with stage II‐IV ovarian, fallopian tube, or primary peritoneal cancer. clinicaltrials.gov.
NCT02933073 {unpublished data only}
- UConn Health. Study of oncoimmunome for the treatment of stage III/IV ovarian carcinoma. clinicaltrials.gov.
NCT02978222 {unpublished data only}
- Tapimmune Inc. Folate receptor alpha peptide vaccine with GM‐CSF versus GM‐CSF alone in patients with platinum sensitive ovarian cancer. Clinicaltrials.gov.
NCT03029403 {unpublished data only}
- University Health Network, Toronto. Phase 2 study of Pembrolizumab, DPX‐Survivac vaccine and Cyclophosphamide in advanced ovarian, primary peritoneal or fallopian tube cancer. clinicaltrials.gov.
NCT03029611 {unpublished data only}
- University of Washington. IGFBP‐2 vaccine and combination chemotherapy in treating patients with stage III‐IV ovarian, fallopian tube, or primary peritoneal cancer undergoing surgery. clinicaltrials.gov.
NCT03113487 {unpublished data only}
- City of Hope Medical Center. P53MVA and Pembrolizumab in treating patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. clinicaltrials.gov.
NCT03127098 {unpublished data only}
- NantCell, Inc. QUILT‐3.040: ETBX‐011 (Ad5 [E1‐, E2b‐]‐CEA(6D)) vaccine in combination with ALT‐803 (super‐agonist IL‐15) in subjects having CEA‐expressing cancer. clinicaltrials.gov.
NCT03197584 {unpublished data only}
- NantKwest, Inc. QUILT‐3.051: NANT ovarian cancer vaccine: combination immunotherapy in subjects with epithelial ovarian cancer who have progressed on or after standard‐of‐care (SOC) therapy. clinicaltrials.gov.
NCT03206047 {unpublished data only}
- National Cancer Institute (NCI). Atezolizumab, Guadecitabine, and CDX‐1401 vaccine in treating patients with recurrent ovarian, fallopian tube, or primary peritoneal cancer. clinicaltrials.gov.
NCT03300843 {unpublished data only}
- National Cancer Institute (NCI). Ability of a dendritic cell vaccine to immunize melanoma or epithelial cancer patients against defined mutated neoantigens expressed by the autologous cancer. clinicaltrials.gov.
Additional references
Agarwal 2006
- Agarwal R, Linch M, Kaye SB. Novel therapeutic agents in ovarian cancer. European Journal of Surgical Oncology 2006;32(8):875‐86. [DOI] [PubMed] [Google Scholar]
Antonia 2006
- Antonia SJ, Mirza N, Fricke I, Chiappori A, Thompson P, Williams N, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clinical Cancer Research 2006;12(3):878‐87. [DOI] [PubMed] [Google Scholar]
Brighton Collaboration 2009
- Brighton Collaboration. www.brightoncollaboration.org.
Britten 2008
- Britten CM, Gouttefangeas C, Welters MJ, Pawelec G, Koch S, Ottensmeier C, et al. The CIMT‐monitoring panel: a two‐step approach to harmonize the enumeration of antigen‐specific CD8+ T lymphocytes by structural and functional assays. Cancer Immunology, Immunotherapy 2008;57(3):289‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTCAE 2009
- Common Terminology Criteria for Adverse Events v4.03. National Institutes of Health, US Department of Health and Human Services, 2009, publication # 09‐7473.
Deeks 2003
- Deeks JJ, Dinnes J, D'Amico R, Sowden AJ, Sakarovitch C, Song F, et al. International Stroke Trial Collaborative Group, European Carotid Surgery Trial Collaborative Group. Evaluating non‐randomised intervention studies. Health Technology Assessment 2003;7(27):1‐173. [DOI] [PubMed] [Google Scholar]
Dong 2006
- Dong HP, Elstrand MB, Holth A, Silins I, Berner A, Trope CG, et al. NK‐ and B‐cell infiltration correlates with worse outcome in metastatic ovarian carcinoma. American Journal of Clinical Pathology 2006;125(3):451‐8. [PubMed] [Google Scholar]
Drerup 2015
- Drerup JM, Liu Y, Padron AS, Murthy K, Hurez V, Zhang B, Curiel TJ. Immunotherapy for ovarian cancer. Current Treatment Options in Oncology 2015;16(1):317. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garnett 2008
- Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T‐cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clinical Cancer Research 2008;14(11):3536‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gooden 2011
- Gooden MJM, Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour‐infiltrating lymphocytes in cancer: a systematic review with meta‐analysis. British Journal of Cancer 2011;105:93‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. Rating quality of evidence and strength of recommendations: what is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardwick 2016
- Hardwick N, Frankel PH, Cristea M. New approaches for immune directed treatment for ovarian cancer. Current Treatment Options in Oncology 2016;17(3):14. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Laheru 2008
- Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B. Allogeneic granulocyte macrophage colony‐stimulating factor‐secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clinical Cancer Research 2008;14(5):1455–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leffers 2009
- Leffers N, Daemen CAHH, Zee AGJ, Nijman HW. Multimodality treatment warranted for ovarian cancer: immunotherapy, a prerequisite to improve prognosis for this vicious disease. Immunotherapy 2009;1(2):163‐5. [DOI] [PubMed] [Google Scholar]
Odunsi 2017
- Odunsi K. Immunotherapy in ovarian cancer. Annals of Oncology 2017;28(8):viii1–viii7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prat 2015
- Prat J. FIGO's staging classification for cancer of the ovary, fallopian tube, and peritoneum: abridged republication. Journal of Gynecologic Oncology 2015;26(2):87‐9. [DOI: 10.3802/jgo.2015.26.2.87] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raspollini 2005
- Raspollini MR, Castiglione F, Rossi Degl'innocenti D, Amunni G, Villanucci A, Garbini F, et al. Tumour‐infiltrating gamma/delta T‐lymphocytes are correlated with a brief disease‐free interval in advanced ovarian serous carcinoma. Annals of Oncology 2005;16(4):590‐6. [DOI] [PubMed] [Google Scholar]
Rustin 2004
- Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade‐Lauraine E, Jakobsen A, et al. Re: new guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). Journal of the National Cancer Institute 2004;96(6):487‐8. [DOI] [PubMed] [Google Scholar]
Sato 2005
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor‐infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favourable prognosis in ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America 2005;102(51):18538‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. [DOI] [PubMed] [Google Scholar]
Thigpen 2000
- Thigpen JT. Chemotherapy for advanced ovarian cancer: overview of randomized trials. Seminars in Oncology 2000;27(3 Suppl 7):11‐6. [PubMed] [Google Scholar]
Torre 2012
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. A Cancer Journal for Clinicians 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
Trotti 2003
- Trotti A, Colevas A, Setser V, Rusch D, Jaques V, Budach C, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Seminars in Radiation Oncology 2003;13(3):176‐81A. [DOI] [PubMed] [Google Scholar]
WHO 1979
- World Health Organization. Handbook for Reporting Results of Cancer Treatment. Geneva: World Health Organization, 1979. [Google Scholar]
Zhang 2003
- Zhang L, Conejo‐Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. New England Journal of Medicine 2003;348(3):203‐13. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Leffers 2010
- Leffers N, Daemen T, Helfrich W, Boezen HM, Cohlen BJ, Melief K, et al. Antigen‐specific active immunotherapy for ovarian cancer. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007287.pub2] [DOI] [PubMed] [Google Scholar]
Leffers 2014
- Leffers N, Daemen T, Helfrich W, Boezen HM, Cohlen BJ, Melief CJM, et al. Antigen‐specific active immunotherapy for ovarian cancer. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD007287.pub3] [DOI] [PubMed] [Google Scholar]


