Abstract
Background
Children who undergo surgical procedures in ambulatory and inpatient settings are at risk of experiencing acute pain. Nonsteroidal anti‐inflammatory drugs (NSAIDs) can reduce moderate to severe pain without many of the side effects associated with opioids. However, NSAIDs may cause bleeding, renal and gastrointestinal toxicity, and potentially delay wound and bone healing. Intravenous administration of ketorolac for postoperative pain in children has not been approved in many countries, but is routinely administered in clinical practise.
Objectives
To assess the efficacy and safety of ketorolac for postoperative pain in children.
Search methods
We searched the following databases, without language restrictions, to November 2017: CENTRAL (The Cochrane Library 2017, Issue 10); MEDLINE, Embase, and LILACS. We also checked clinical trials registers and reference lists of reviews, and retrieved articles for additional studies.
Selection criteria
We included randomised controlled trials that compared the analgesic efficacy of ketorolac (in any dose, administered via any route) with placebo or another active treatment, in treating postoperative pain in participants zero to 18 years of age following any type of surgery.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently considered trials for inclusion in the review, assessed risk of bias, and extracted data. We analyzed trials in two groups; ketorolac versus placebo, and ketorolac versus opioid. However, we performed limited pooled analyses. We assessed the overall quality of the evidence for each outcome using GRADE, and created a 'Summary of findings' table.
Main results
We included 13 studies, involving 920 randomised participants. There was considerable heterogeneity among study designs, including the comparator arms (placebo, opioid, another NSAID, or a different regimen of ketorolac), dosing regimens (routes and timing of administration, single versus multiple dose), outcome assessment methods, and types of surgery. Mean study population ages ranged from 356 days to 13.9 years. The majority of studies chose a dose of either 0.5 mg/kg (as a single or multiple dose regimen) or 1 mg/kg (single dose with 0.5 mg/kg for any subsequent doses). One study administered interventions intraoperatively; the remainder administered interventions postoperatively, often after the participant reported moderate to severe pain.
There were insufficient data to perform meta‐analysis for either of our primary outcomes: participants with at least 50% pain relief; or mean postoperative pain intensity. Four studies individually reported statistically significant reductions in pain intensity when comparing ketorolac with placebo, but the studies were small and had various risks of bias, primarily due to incomplete outcome data and small sample sizes.
We found limited data available for the secondary outcomes of participants requiring rescue medication and opioid consumption. For the former, we saw no clear difference between ketorolac and placebo; 74 of 135 (55%) participants receiving ketorolac required rescue analgesia in the post‐anaesthesia care unit (PACU) versus 81 of 127 (64%) receiving placebo (relative risk (RR) 0.85, 95% confidence interval (CI) 0.71 to 1.00, P = 0.05; 4 studies, 262 participants). For opioid consumption in the PACU, we saw no clear difference between ketorolac and placebo (P = 0.61). For the time period zero to four hours after administration of the interventions, participants receiving ketorolac received 1.58 mg less intravenous morphine equivalents than those receiving placebo (95% CI ‐2.58 mg to ‐0.57 mg, P = 0.002; 2 studies, 129 participants). However, we are uncertain whether ketorolac has an important effect on opioid consumption, as the data were sparse and the results were inconsistent. Only one study reported data for opioid consumption when comparing ketorolac with an opioid. There were no clear differences between the ketorolac and opioid group at any time point. There were no data assessing this outcome for the comparison of ketorolac with another NSAID.
There were insufficient data to allow us to analyze overall adverse event or serious adverse event rates. Although the majority of serious adverse events reported in those receiving ketorolac involved bleeding, the number of events was too low to conclude that bleeding risk was increased in those receiving ketorolac perioperatively. There was not a statistically significant increase in event rates for any specific adverse event, either in pooled analysis or in single studies, when comparing ketorolac and placebo. When comparing ketorolac with opioids or other NSAIDs, there were too few data to make any conclusions regarding event rates. Lastly, withdrawals due to adverse events were vary rare in all groups, reflecting the acute nature of such studies.
We assessed the quality of evidence for all outcomes for each comparison (placebo or active) as very low, due to issues with risk of bias in individual studies, imprecision, heterogeneity between studies, and low overall numbers of participants and events.
Authors' conclusions
Due to the lack of data for our primary outcomes, and the very low‐quality evidence for secondary outcomes, the efficacy and safety of ketorolac in treating postoperative pain in children were both uncertain. The evidence was insufficient to support or reject its use.
Plain language summary
Ketorolac for short‐term pain after surgery in children
Bottom line There is no good evidence from studies to support or reject the suggestion that ketorolac is beneficial, or that it is associated with serious side effects in treating children's pain after surgery.
Background Children are at risk of experiencing pain in the short term after surgery. Nonsteroidal anti‐inflammatory drugs (NSAIDs, e.g. aspirin) can reduce moderate to severe pain without many of the side effects associated with opioids (drugs like morphine). However, NSAIDs may cause bleeding and injury to the kidneys and gut. Ketorolac is an NSAID that can be given by injection into a vein, which may be useful when patients are not able to take medicines by mouth. Despite the fact that ketorolac has not been approved for use in children by many government agencies, it is often used after surgery, because of a lack of alternative options.
Study characteristics In November 2017, we searched for clinical trials where ketorolac was used to treat pain after surgery in children. We found 13 studies, enrolling 920 children, that met our requirements for the review. The studies were quite different in their design, the dose of ketorolac, the timing (during or after surgery) and number of doses given, the type of surgery, and to what ketorolac was compared (either a placebo (a dummy treatment, such as a bag of fluid) or another drug).
Key findings
There was not enough information for a statistical analysis of the assessments in which we were most interested, that is, the number of children with at least 50% pain relief; or the average pain intensity (a measure of a patient's pain that asks the patient to rate how much pain they have, often on a scale of 0 for 'no pain' to 10 for 'worst pain imaginable'). Four studies individually reported that ketorolac was better at reducing pain intensity than placebo, but the studies were small and had various design issues. There was more information for other assessments, such as the number of children who needed rescue medication (additional pain medication that is given if the study medication is not helping the person's pain sufficiently), and how much of this rescue medication was used. Fewer children needed rescue medication in the ketorolac group than those who received placebo, although the result was not statistically different. During the four hours after they received study medications, children receiving ketorolac needed slightly less rescue pain medication than those who had received placebo. There was not enough information about ketorolac in direct comparisons with other medications.
There was also not enough information in the studies for us to make a good assessment of side effects and serious side effects when ketorolac was used in this setting. Serious side effects in those receiving ketorolac included bleeding, but it didn't occur often enough for us to make any firm conclusions. Very few children dropped out of the studies because of side effects. This is normal in studies where participants are only in the study for a short period of time.
Quality of the evidence We rated the quality of the evidence as very low, due to methodological issues with many of the studies, differences in study designs, and low overall numbers of children enrolled. Very low‐quality evidence means that we are very uncertain about the results.
Summary of findings
Summary of findings 1. Ketorolac versus placebo for postoperative pain in children.
| Ketorolac versus placebo for postoperative pain in children | ||||||
|
Patient or population: children with postoperative
pain
Settings: hospital
Intervention: ketorolac Comparison: placebo | ||||||
| Outcomes | Probable outcome with: | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Placebo | Ketorolac (95% CI) | |||||
| Number of participants with 50% or greater postoperative pain relief | No data | No data | Not estimable | 0 (0) |
See comment | No studies reported this outcome |
| Postoperative pain intensity | No data | No data | Not estimable | 0 (0) | very low | Data not suitable for pooling due to low number of studies reporting and differences in dosing regimens, assessment tools, and times of assessment |
| Participants using rescue medication in the PACU | 638 per 1000 | 542 per 1000 (453 to 638) | RR 0.85 (0.71 to 1) | 262 (4 studies) | ⊕⊝⊝⊝ very lowa,b,c | Insufficient data for analysis of participants using rescue medication at other time points |
| Opioid consumption (mg IV morphine equivalents): PACU | The mean opioid consumption (IV morphine equivalents) in the intervention groups was 0.17 mg lower (0.8 lower to 0.47 higher) | N/A | 162 (3 studies) | ⊕⊝⊝⊝ very lowa,c,d | Insufficient data for analysis of opioid consumption at time points other than in the PACU and over 0‐4 h. | |
| Opioid consumption (mg IV morphine equivalents): 0 to 4 h | The mean opioid consumption (IV morphine equivalents) in the intervention groups was 1.58 mg lower (2.58 lower to 0.57 lower) | N/A | 129 (2 studies) | ⊕⊝⊝⊝ very lowa,b,c | Insufficient data for analysis of opioid consumption at time points other than in the PACU and over 0‐4 h. | |
| Serious adverse events | 1/63 (2%) | 3/65 (5%) | N/A | 128 (2 studies) |
⊕⊝⊝⊝ very lowa,c | All events in ketorolac groups were bleeding |
| Participants experiencing bleeding events | 198 per 1000 | 196 per 1000 (127 to 301) | RR 0.99 (0.64 to 1.52) | 258 (5 studies) | ⊕⊝⊝⊝ very lowa,e | Differences in incidence between studies likely due to differences in methods of assessment. |
| CI: Confidence interval; RR: Risk ratio; IV: intravenous; PACU = post‐anaesthesia care unit | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a Serious (−1) limitation to study quality (unclear or high risk of bias in multiple domains) b Important inconsistency (substantial heterogeneity (I² > 50%)) c Sparse data (total number of participants < 400) d Imprecision (wide confidence intervals) e Very serious (‐2) limitations to study quality (unclear or high risk of bias in multiple domains; high risk of reporting bias for this outcome)
Background
Description of the condition
Children who undergo surgical procedures in ambulatory and inpatient settings are at risk of experiencing acute pain. A recent study reported rates of moderate to severe pain of over 50% on postoperative day three in children undergoing adenotonsillectomy (Stanko 2013). In the short‐term, inadequately treated postoperative pain may negatively affect quality of life, functioning, and functional recovery, and may increase the risk of postsurgical complications (Chou 2016). Long‐term negative sequelae may include pain sensitisation and an increased risk of developing persistent postsurgical pain (Brasher 2014; Chou 2016). There are many reasons for the under treatment of pain in children, which include: lack of paediatric‐specific training and experience among providers caring for children postoperatively, fear of adverse reactions from analgesics, lack of understanding of pharmacodynamic and pharmacokinetic differences in paediatric patients, and limited studies in this population (Brasher 2014; Schultz‐Machata 2014). Conversely, unnecessarily aggressive analgesia may be administered if agitation that occurs postanaesthesia is mistaken for pain (Somaini 2015).
Description of the intervention
While opioids are considered the cornerstone of management of severe acute pain, they have been associated with a number of adverse events (AEs) in hospitalised paediatric patients (Holdsworth 2003; Kaushal 2001; Takata 2008). A recent study reported that 24% of children experienced an adverse drug event related to postoperative opioid use that required intervention, rescue (breakthrough) doses, or escalation in care (Voepel‐Lewis 2008). Non‐steroidal anti‐inflammatory drugs (NSAIDs) are frequently used as a strategy for reducing opioid requirements in children after surgery. A reduction in opioid use may, in turn, reduce the incidence or severity of AEs, or both, associated with this class of analgesics (Jitpakdee 2014; Voepel‐Lewis 2012). NSAIDs can reduce moderate to severe pain without the nausea and vomiting, sedation, urinary retention, or the potential for respiratory depression associated with opioids (Forrest 1997). The most concerning adverse effects of NSAIDs, such as ketorolac, include renal and gastrointestinal toxicity, antithrombotic effects, and potentially delayed wound and bone healing (Brasher 2014). While there is also concern for serious AEs associated with the use of NSAIDs in paediatric patients, most of the evidence for these events has been reported in neonates who received NSAIDs for the closure of patent ductus arteriosus (Johnston 2012). The incidence of serious side effects in non‐neonatal paediatric patients is estimated to be closer to 0.08% to 0.24% when NSAIDs are used for acute pain (Standing 2009a; Standing 2009b). Tonsillectomy is one of the most common paediatric surgical procedures, along with appendectomy, orchiopexy, and orthopaedic procedures (Schultz‐Machata 2014). There is conflicting evidence regarding the risk of bleeding with NSAID use in otolaryngeal and orthopaedic populations (Chan 2014; Lewis 2013; Riggin 2013).
Ketorolac was the first NSAID to be approved in the USA for intravenous (IV) use for postoperative pain in adults (Bookstaver 2010; Mak 2011). Ketorolac is used in children in the postoperative setting for procedures, such as general surgery, otolaryngological surgery, urological surgery, cardiac surgery, trauma surgery, orthopaedic surgeries, ophthalmology procedures, and chest tube placement and removal (Kossowsky 2015; Tobias 2014). It can be administered by mouth, or parenterally, as either IV or intramuscular (IM) injection (Lexicomp 2015). While oral bioavailability is estimated at 100%, oral administration in the postoperative setting is generally reserved for continuation of therapy initiated with IM or IV ketorolac. Parenteral administration of analgesics may be preferred in the immediate postoperative period, as the gastrointestinal tract may be compromised as a result of postoperative ileus, or because patients experience nausea and vomiting after they receive anaesthetic agents during surgery (Pasero 2011). Also, the patient's ability to maintain his or her airway and to follow commands may dictate the route of administration. While recommended maximum duration of therapy varies by country, a combined therapy duration (oral and parenteral) of five days should not be exceeded (Lexicomp 2015).
While the use of ketorolac injection in children is not approved by most government agencies, several studies have been conducted in this population, including in those under the age of two years (Cohen 2011; Gupta 2004; Papacci 2004). In the UK, IV administration of ketorolac is not approved for children under 16 years old (eMC 2017).
Other FDA‐approved parenteral NSAIDs include ibuprofen (approved for children as young as six months old) and diclofenac (approved in adults only). In the UK, injectable diclofenac is only approved for use in adults, and injectable ibuprofen is unavailable.
How the intervention might work
NSAIDs possess analgesic, anti‐inflammatory, antiplatelet, and antipyretic properties. The analgesic effect of NSAIDs is thought to be predominately mediated by a reduction of prostaglandin synthesis via inhibition of the enzyme cyclo‐oxygenase (COX). Prostaglandins cause pain via sensitisation and stimulation of peripheral nociceptors (Kokki 2003).
Ketorolac is available as a racaemic mixture of stereoisomers. Both isomers may have different analgesic effects. Limited evidence demonstrates similar pharmacokinetic variables for children one to 16 years when normalised for weight (Dsida 2002). Pharmacodynamic differences between adults and children are not well understood (Kokki 2003). Due to serious AEs related to renal function and to the gastrointestinal system, use of ketorolac has been limited to five days duration. Concern for altered bone and ligament healing, gastric mucosal damage, and Reye’s syndrome may also cause providers to avoid the use of ketorolac (Kokki 2003).
Why it is important to do this review
Ketorolac may offer an effective low‐cost analgesic option and decrease or avoid the use of opioids in postoperative paediatric patients. However, providers may avoid its use due to concerns about potential toxicities, including bleeding, gastrointestinal events, and renal dysfunction (Brasher 2014). While the incidence and severity of these AEs has been established in adults, evidence is less robust in the paediatric population.
Although reviews that assess the postoperative use of NSAIDs have been conducted, we are unaware of any current systematic review that has specifically assessed ketorolac for postoperative pain in children.
Objectives
To assess the efficacy and safety of ketorolac for postoperative pain in children.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) that evaluated the analgesic efficacy of enteral or parenteral ketorolac to treat postoperative pain following any type of surgery, including dental. We required full‐text journal publication with the exception of online clinical trial results, summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis. We excluded short abstracts (usually meeting reports). Due to the paucity of clinical trials in this age group, we included both blinded and unblinded trials.
We excluded studies that were non‐randomised, studies of experimental pain, case reports, and clinical observations. We excluded cross‐over studies because the intensity of postoperative pain changes over time.
Types of participants
We included studies that assessed paediatric patients (birth to 18 years) regardless of sex or type of surgery. We included studies that assessed both paediatric and adult populations, but only if the study authors presented data separately for children.
Types of interventions
Ketorolac in any dose, by any route, administered for the relief of postoperative pain, and compared to placebo or an active comparator. We included studies in which the interventions were administered intraoperatively or postoperatively alone, or in addition to other analgesic treatment. We included studies that examined the effects of single dose or multiple‐dose.
Types of outcome measures
We included any study that reported any of the following outcome measures. We included studies in which participants self‐reported pain relief or pain intensity, or in which clinicians assessed pain using validated behavioural scales.
Primary outcomes
Pain relief. We had intended to extract the proportion of participants with 50% or greater postoperative pain relief in each treatment arm over various time periods postintervention. However, no studies provided usable data.
Pain intensity. We extracted mean postoperative pain intensity over various time periods in each treatment arm and their corresponding standard deviations (SD).
We anticipated that studies would use a variety of outcome measures for pain intensity, based on participant age, development, and ability to participate. We expected that most outcomes would use standard subjective scales, both self‐report measures (Poker Chip Tool, Faces Pain Scale‐Revised, Visual Analogue Scale (VAS)) and observational measures (Faces, Legs, Arms, Cry, Consolability (FLACC), Children's Hospital of Eastern Ontario Pain Scale (CHEOPS), Parents' Postoperative Pain Measure (PPPM), COMFORT Scale, Toddler‐Preschooler Postoperative Pain Scale), as recommended by PedIMMPACT (McGrath 2008).
Secondary outcomes
We identified the following outcomes, based on PedIMMPACT recommendations (McGrath 2008).
Global judgement of satisfaction with treatment (either participant, using the Patients' Global Impression of Change (PGIC), or caregiver; Hurst 2004). We had intended to extract dichotomous information derived from categorical global evaluations (number of participants who report the top two categories, e.g. good, satisfied or excellent, very satisfied). For VAS ratings, we had intended to extract mean values of each intervention. However, no studies provided data.
Adverse events (AEs). We extracted validated scales when used. When the only available information was subjective or observational for specific adverse effects (such as nausea or vomiting), or determined through asking general questions or merely noting the presence or absence of AEs, without any attempt at quantification, we documented these outcomes as such. We noted the number of participants who withdrew from the study due to both AEs and to lack of efficacy of the intervention, when adequately described.
Serious adverse events (serious AEs). We extracted the number of participants in each arm who experienced a serious AE.
Time‐to‐onset of meaningful (50%) pain relief. We had intended to extract the mean time to achieve this degree of relief in each treatment arm and the corresponding SD. However, no studies provided data.
Number of participants who required rescue medication. We extracted the proportion of participants who received additional analgesic medication during various time periods after administration of the study drugs in each treatment arm.
Time‐to‐rescue medication. We had intended to extract the mean time to requiring rescue medication in each treatment arm and the corresponding SD. However, no studies provided data.
Opioid consumption. In studies that allowed coadministration of opioids (including patient‐controlled analgesia (PCA)), we extracted the mean opioid consumption (in mg) over various time periods in each treatment arm and the corresponding SD. Where opioid consumption was reported as mg/kg, we converted this to mg by multiplying reported values by the mean weight of each study arm. We converted opioid requirements into IV morphine‐equivalents, using commonly used and widely accepted opioid conversion tables (Jacox 1994).
Search methods for identification of studies
Electronic searches
We searched the following databases without language restrictions.
The Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) in the Cochrane Library, (searched 2 September 2016)
MEDLINE Ovid (1946 to August week 4 2016)
Embase Ovid (1974 to 2016 week 35)
LILACS Birme (searched 2 September 2016)
We searched databases for a combination of indexed and free‐text terms incorporating ‘ketorolac’, ‘postoperative pain’ and ‘children’. We modified the search terms based on the restrictions of each database. The search strategies for CENTRAL, MEDLINE, Embase, and LILACS are in Appendix 1; Appendix 2; Appendix 3; and Appendix 4, respectively.
We updated the above searches on 8 November 2017.
Searching other resources
We searched clinicaltrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/) for ongoing trials up to 8 November 2017. In addition, we checked the reference lists of reviews and retrieved articles for additional studies, and we performed citation searches on key articles. We contacted experts in the field for unpublished and ongoing trials. Where necessary, we contacted the study authors for additional information.
Data collection and analysis
Selection of studies
Two review authors (two of EM, ER, or TC) independently determined eligibility by reading the abstract of each study identified by the search. They eliminated studies that clearly did not satisfy the inclusion criteria of the review, and obtained full‐text copies of the remaining studies. Two review authors (two of EM, ER, or TC) independently read these studies and selected relevant studies for inclusion. In the event of disagreement, a third review author adjudicated. We did not anonymise the studies in any way before assessment. We have included a PRISMA flow chart, which shows the status of identified studies (Moher 2009), as recommended in Part 2, Section 11.2.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We included studies in the review regardless of whether measured outcome data were reported in a ‘usable’ way. We listed all studies excluded after full‐text assessment in a 'Characteristics of excluded studies' table and listed the reasons for exclusion.
Data extraction and management
Two review authors (two of EM, ER, or TC) independently extracted data using a standardised form, and checked for agreement before data entry into Review Manager 5 (RevMan 5; Review Manager 2014). We considered different pain scales (VAS, Numerical Rating Scale (NRS), Revised Faces scale) equivalent if they were based on a zero to 10 scale, or we normalised them to such a scale. We included self‐reported and clinician‐assessed pain scores. Where the included studies reported both, we used self‐report scores in the analyses. We resolved any discrepancies between the two review authors at every step of data extraction by discussion. If disagreement persisted, we consulted a third review author (one of EM, ER, or TC). We collated multiple reports of the same study, so that each study, rather than each report, was the unit of interest in the review. If an included study only presented data graphically, we extracted data using WebPlotDigitizer software (Version 3.7, Rohatgi 2016, arohatgi.info/WebPlotDigitizer). We recorded the characteristics of the included studies in sufficient detail in a 'Characteristics of included studies' table.
Assessment of risk of bias in included studies
Two review authors (two of EM, ER, or TC) independently assessed the risk of bias for each included study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions, and adapted from those used by the Cochrane Pregnancy and Childbirth Group (Higgins 2011). We resolved any disagreements by discussion. We completed a 'Risk of bias' table for each included study using the 'Risk of bias' tool in RevMan 5(Review Manager 2014).
We assessed the following for each included study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as at low risk of bias (any truly random process, e.g. random number table, computer random number generator), or unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies that used a non‐random process (e.g. odd or even date of birth, hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as at low risk of bias (e.g. telephone or central randomization, consecutively numbered sealed opaque envelopes), or unclear risk of bias (method not clearly stated). We rated studies that did not conceal allocation as high risk of bias (e.g. open list).
Blinding of outcome assessment (checking for possible detection bias). We included both blinded and unblinded trials, due to the paucity of clinical trials in this age group. We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as at low risk of bias (study stated that it was blinded and described the method used to achieve blinding, e.g. identical tablets, matched in appearance and smell), unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved), or high risk of bias (study stated that it was not blinded, or described inadequate methods).
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as at low risk of bias (less than 10% of participants did not complete the study, or used baseline observation carried forward analysis, or both), unclear risk of bias (used last observation carried forward analysis), or high risk of bias (used completer analysis).
Selective reporting (checking for reporting bias). We assessed whether primary and secondary outcome measures were prespecified and whether they were consistent with those reported. We assessed reporting of results as having low risk of bias (e.g. the study protocol was available and all of the study’s prespecified outcomes of interest in the review were reported in the prespecified way; the study protocol was not available but it was clear that published reports included all expected outcomes, including those that were prespecified); high risk of bias (e.g. not all of the study’s prespecified primary outcomes were reported; one or more primary outcomes were reported using measurements, analysis methods, or subsets of data that were not prespecified); or unclear risk of bias (information insufficient to permit judgement of ‘low risk’ or ‘high risk’).
Size of study (checking for possible biases confounded by small size (Dechartres 2013; Dechartres 2014; Moore 1998; Nüesch 2010; Thorlund 2011)). We assessed studies as being at low risk of bias (200 participants or more per treatment arm), unclear risk of bias (50 to 199 participants per treatment arm), or high risk of bias (less than 50 participants per treatment arm).
Measures of treatment effect
Dichotomous data
We used discrete events, such as the number of participants who required rescue analgesia, or experienced AEs, to calculate the risk difference (RD), or risk ratio (RR), or both, using RevMan 5 (Review Manager 2014). When there was a statistically significant risk difference between interventions, we derived the number needed to treat for an additional beneficial outcome (NNTB), or number needed to treat for an additional harmful outcome (NNTH; Cook 1995). In addition, we presented dichotomous outcomes in terms of both raw numbers and percentages of participants in each study arm who benefited from therapy, or suffered AEs.
Continuous data
We undertook meta‐analyses when comparable data were available from continuous outcomes, such as pain intensity, analgesic consumption in mg of intravenous morphine equivalents, or intensity of a specific AE, using mean differences (MD). Morphine equivalents were calculated by using an equianalgesic conversion table (APS 2008).
Unit of analysis issues
Randomisation was to the individual participant. When a study compared two active treatment arms with a placebo arm within the same analysis, we had intended to avoid double counting of participants in the placebo arm by splitting the total number between the active arms. However, this was not necessary, as we did not combine treatment arms in any analysis.
Dealing with missing data
We attempted to contact study authors to obtain further information for any doubts about missing data (participant dropouts, selective outcome reporting, etc.). We completed an intention‐to‐treat (ITT) analysis if we were able to obtain full information. We performed a completed‐case analysis if we were unable to obtain full information.
Assessment of heterogeneity
We dealt with clinical heterogeneity by combining studies that examined similar populations and administered similar dosing regimens. We assessed statistical heterogeneity by visually examining forest plots and quantified it using the I² statistic. The I² statistic is a reliable and robust test to quantify heterogeneity, since it does not depend on the number of trials or on the between‐study variance. The I² statistic measures the extent of inconsistency among studies' results, and can be interpreted as the proportion of total variation in study estimates that is due to heterogeneity rather than sampling error. We considered an I² statistic value greater than 50% to indicate substantial heterogeneity (Deeks 2011).
Assessment of reporting biases
We had intended to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a NNTB of 10 or higher; Moore 2008). We defined the addition of four comparisons of typical size (400 participants in total) with zero effect as making the result potentially subject to publication bias, and therefore unreliable. However, there were no meta‐analyses of dichotomous outcomes that produced statistically significant results. We also attempted to mitigate the potential for publication bias by searching the ClinicalTrials.gov website (www.clinicaltrials.gov) and the WHO ICTRP (apps.who.int/trialsearch/).
Data synthesis
We performed all meta‐analyses in duplicate using RevMan 5 (Review Manager 2014). We reported summary statistics, including summary RRs and MDs with 95% confidence intervals (CIs) using RevMan 5 (Review Manager 2014). We considered a RR with the range of the lower and upper bounds of the 95% CI not crossing one as statistically significant, and MDs with the range of the lower and upper bounds of the 95% CIs not crossing zero as statistically significant. We used a fixed‐effect model (Deeks 2011).
Quality of the evidence
We assessed the overall quality of the evidence for each outcome using the GRADE system (GRADEpro GDT 2015). We presented these results in a 'Summary of findings' table, so the main findings of the review were in a transparent and simple tabular format. In particular, we included key information that concerned the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
The GRADE system uses the following criteria for assigning grade of evidence.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
We downgraded the quality of the evidence for the following reasons.
Serious (−1) or very serious (−2) limitation to study quality.
Important inconsistency (−1).
Some (−1) or major (−2) uncertainty about directness.
Imprecise or sparse data (−1).
High probability of reporting bias (−1).
'Summary of findings' table
We included a 'Summary of findings' table as set out in the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group author guide (PaPaS 2012), and recommended in the Cochrane Handbook for Systematic Reviews of Interventions, chapter 4.6.6 (Higgins 2011). We present the main findings for the main comparison (ketorolac versus placebo) for the outcomes of pain relief, pain intensity, number of participants that required rescue medication, opioid consumption, serious AEs, and bleeding events.
Subgroup analysis and investigation of heterogeneity
We had intended to perform subgroup analysis to evaluate the effects of clinical heterogeneity, by calculating the RR or MD with the corresponding CI for each subgroup. We had intended to use a fixed‐effect heterogeneity Chi² test to compare subgroups. We considered non‐overlapping CIs to be consistent with a statistically‐significant difference.
If present, we had intended to analyze the following subgroups.
Type of surgery (otolaryngologic, urologic, general, head and neck, thoracic, cardiovascular, or orthopaedic).
Different drug doses and route of administration (enteral, parenteral) of ketorolac.
Different ages of included children (less than one month, one to 12 months, one to nine years, 10 to 18 years).
However, there were insufficient data to perform subgroup analysis.
Sensitivity analysis
We had intended to perform sensitivity analyses by removing studies with non‐self‐reported pain scores. However, there were insufficient data for sensitivity analysis to be necessary.
Results
Description of studies
See 'Characteristics of included studies'; 'Characteristics of excluded studies'; 'Characteristics of studies awaiting classification'; and 'Characteristics of ongoing studies' tables.
Results of the search
Our literature search to 8 November 2017 yielded 459 references from CENTRAL, 296 references from MEDLINE, 204 studies from Embase, and nine studies from LILACS. Our review of the abstracts associated with these references identified 23 potentially relevant studies, one of which was identified in our updated search. The remaining references clearly did not meet inclusion criteria, without the need to obtain a full‐text for confirmation. We excluded nine studies that did not meet the inclusion criteria. Our search of clinical trial websites yielded 59 ongoing or completed trials from clinicaltrials.gov and 13 studies from the WHO ICTRP. From these, we found four relevant ongoing studies (Figure 1).
1.
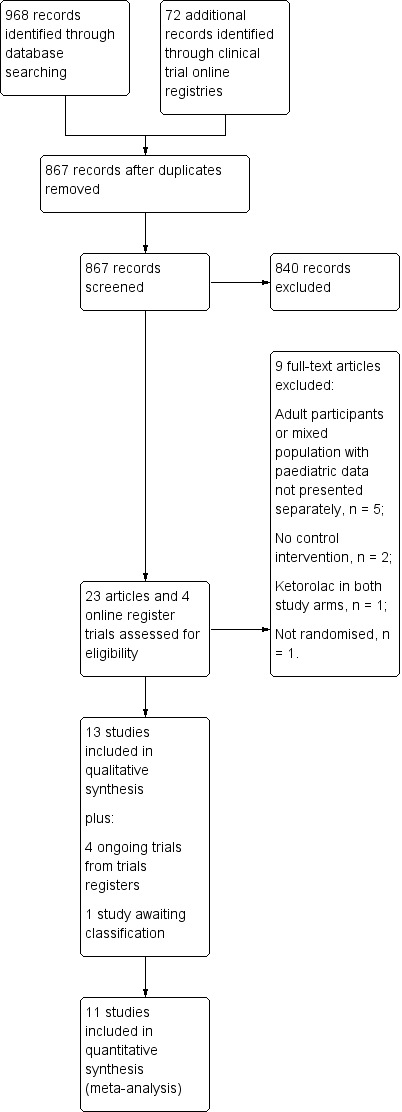
Study flow diagram
Included studies
Thirteen studies with heterogeneous designs fulfilled our inclusion criteria. Two studies administered ketorolac intramuscularly (Hamza 2012; Sutters 1995). The remaining studies administered ketorolac intravenously. Two trials were conducted in Asia (Hamza 2012; Saryazdi 2016), three in Europe (Chiaretti 1997; Maunuksela 1992; Romsing 1998), and eight in North America (Davis 1999; Gunter 1995; Lieh‐Lai 1999; Lynn 2007; Munoz‐Cuevas 1997; Munro 2002; Sutters 1995; Sutters 1999). Enrolment ranged from 37 to 200 participants, with the number of participants receiving ketorolac in each study ranging from 13 to 54. Mean study population ages ranged from 356 days (Lynn 2007), to 13.9 years (Munro 2002); however, the vast majority of participants were elementary (primary) school age children.
Study designs varied. Eight studies administered single doses of each intervention (Davis 1999; Gunter 1995; Lieh‐Lai 1999; Lynn 2007; Munoz‐Cuevas 1997; Romsing 1998; Saryazdi 2016; Sutters 1995); three administered multiple doses (Hamza 2012; Munro 2002; Sutters 1999); and two studies included both single‐ and multiple‐dose arms (Chiaretti 1997; Maunuksela 1992). One study administered interventions intraoperatively (Davis 1999); the remainder administered interventions postoperatively, often after the participant reported moderate to severe pain. Doses also varied; the majority of studies chose a dose of either 0.5 mg/kg (as a single‐ or multiple‐dose regimen), or 1 mg/kg (single dose, with 0.5 mg/kg for any subsequent doses); one study (Chiaretti 1997) administered 1.2 mg/kg every six hours, a dosage regimen higher than that recommended in current guidelines (Lexicomp 2015).
Five studies enrolled participants undergoing otolaryngic surgery (Davis 1999; Gunter 1995; Hamza 2012; Romsing 1998; Sutters 1995); single studies assessed participants undergoing hernia (Saryazdi 2016), ophthalmic (Munoz‐Cuevas 1997), spinal (Munro 2002), and orthopedic procedures (Sutters 1999); and four studies enrolled participants undergoing mixed surgeries, which included neuro‐oncologic, abdominal, thoracic, neurological, urological, and cardiac procedures (Chiaretti 1997; Lieh‐Lai 1999; Lynn 2007; Maunuksela 1992).
Excluded studies
We excluded five studies because they enrolled adult participants (Glickman 1995; Greco 1994; Hernandez 1996; Rossitto 2009), or enrolled both adults and children, but did not report results separately (Hayes 2011). We contacted the investigators in the latter case, but were unable to obtain separate data for child participants. One study was not randomised (Petrov 2009). Two studies did not administer a control intervention (Gupta 2004; Vetter 1994); those not receiving ketorolac received 'usual care'. Lastly, one study administered ketorolac to both arms (Palacio 1997).
Studies awaiting classification
See the 'Characteristics of studies awaiting classification' table. We were unable to obtain the full text for one study from any source (Tariq 2004).
Ongoing studies
See the 'Characteristics of ongoing studies' table. We found four ongoing studies in our search of clinical trial registers (NCT01667120; NCT02653742; NCT02973958; NCT03178539).
Risk of bias in included studies
Our findings are summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Random sequence generation
Five studies described adequate methods of randomization, i.e. via computer generated numbers or a table of random numbers (Davis 1999; Gunter 1995; Romsing 1998; Saryazdi 2016; Sutters 1999). The remaining studies did not describe their methods.
Allocation concealment
Only two studies described adequate allocation concealment via a central randomization process, and by using sealed envelopes, respectively (Lieh‐Lai 1999; Sutters 1995). In the remaining studies, allocation concealment was not mentioned.
Blinding
Four studies described adequate methods of blinding both investigators and participants (Lieh‐Lai 1999; Maunuksela 1992; Romsing 1998; Sutters 1999). Although none of the four studies stated that the interventions appeared identical, they described the methods used to ensure blinding in sufficient detail to lead us to believe that neither the investigator nor the study participant would be able to discriminate interventions based on their appearance. We assessed the remaining studies as having an unclear risk of bias, either because they did not describe methods of blinding in any way, or because their descriptions were inadequate for us to determine whether investigators or participants would be able to distinguish interventions.
Incomplete outcome data
We assessed three studies as having a low risk of attrition bias (Davis 1999; Gunter 1995; Lieh‐Lai 1999). In all studies, it appeared that all participants completed the study and contributed data. While Lieh‐Lai 1999 imputed data using last observation carried forward (LOCF), the numbers of participants in both groups with missing data were similar. We assessed two studies as having a high risk of bias (Sutters 1995; Sutters 1999). In both studies, only participants completing the study contributed data for analysis, and the authors made no mention of how they imputed isolated missing data for those who did complete the study. We assessed the remaining studies as having an unclear risk, primarily because it was unclear how many participants completed the study, or how the investigators imputed missing data.
Selective reporting
Three studies had a low risk of reporting bias (Gunter 1995; Lieh‐Lai 1999; Romsing 1998). While protocols were not available for each study, all outcomes listed in their methods sections were reported, in full, in the results sections. We assessed three studies as having a high risk of bias for various reasons, including not describing primary outcomes, differences in outcomes described in the methods section versus those reported in the results, and incomplete reporting of results, such as only displaying data graphically (Hamza 2012; Munoz‐Cuevas 1997; Munro 2002). We assessed the remaining studies as having unclear risk of bias.
Other potential sources of bias
The major threat to reliability was the small size of the studies. All but two studies had at least one arm that enrolled fewer than 50 participants. In the remaining two studies, each arm enrolled 50 participants (Davis 1999; Hamza 2012).
Effects of interventions
See: Table 1
See Table 1 for outcomes for the main comparison, ketorolac versus placebo.
All 13 included studies provided usable data for analysis. However, not all data were used in meta‐analysis, either because there was only one study for a given outcome, or there were too few participants or events for a given outcome.
Primary outcomes
Pain Relief: proportion of participants with at least 50% postoperative pain relief
No study provided data on the number of participants with at least 50% postoperative pain relief, and no study provided pain intensity or pain relief data in a format that allowed us to derive these numbers. Therefore, there were no data on which to assess quality of evidence for this outcome.
Pain intensity
There were insufficient data to perform a meta‐analysis for this outcome at any time point for the comparison of ketorolac versus either placebo or an active comparator. Few studies reported this outcome, and in those that did, differences in dosing regimens, assessment tools used, and times at which pain intensity was assessed, precluded pooling of data. Therefore, we assessed the quality of evidence for this outcome as very low.
Ketorolac versus placebo
Munro 2002 administered ketorolac every six hours for six doses, with the first dose administered at the completion of surgery. They assessed pain intensity using a 0 to 10 numeric rating scale, but only displayed results graphically. They stated that ketorolac was statistically superior to placebo on postoperative day one and on the afternoon of day two (P < 0.05). They also reported that a greater proportion of participants receiving ketorolac had no discomfort with activity on postoperative day one (59% versus 20%, P < 0.05). Romsing 1998 used a poker chip tool (0 to 4 scale) to assess pain intensity. Mean pain scores for those receiving 1 mg/kg intravenous (IV) single dose of ketorolac immediately after surgery were statistically significantly lower at 1.5 (P = 0.004), 3 (P = 0.002), and 5 (P < 0.001) hours postoperatively, but not at 24 hours, compared to placebo. Sutters 1995 assessed pain intensity with both the CHEOPS and Oucher scales, and reported reduced pain intensity with ketorolac at several time points, but not at others throughout the participant's postanaesthesia (PACU) and Day Surgery stay. Sutters 1999 administered a 1 mg/kg IV loading dose of ketorolac followed by doses of 0.5 mg/kg IV every six hours for a maximum of eight doses. They reported mean pain intensity, assessed by the Faces Pain Scale (0 to 5), every four hours, through to 36 hours, and noted a statistically significant improvement in pain intensity overall in the ketorolac group (P < 0.05), but did not report statistical significance at each time point.
Ketorolac versus opioid
Chiaretti 1997 assessed pain intensity with both the Faces Pain Scale and the CHEOPS. Participants were assigned to four different groups (ketorolac 1.2 mg/kg IV every six hours; ketorolac 1.2 mg/kg IV bolus + 0.21 mg/kg/h continuous infusion; fentanyl 1 mcg/kg/h IV; fentanyl 1 mcg/kg/h + ketorolac 0.21 mg/kg/h IV infusion). They presented both mean and median scores for both assessment tools, at four hours and eight hours post‐administration of interventions, and noted statistically significant improvements in all intervention groups versus baseline for most comparisons (P < 0.05), but noted that ketorolac administered via infusion was superior to ketorolac administered as a bolus only. While those assigned to receive the fentanyl infusion demonstrated similar improvements in pain intensity for items on the CHEOPS scale and Faces Pain Scale scores at four and eight hours as those receiving ketorolac infusion, direct comparisons between the two were not reported. Hamza 2012 compared postoperative ketorolac doses of 0.5 mg/kg intramuscular (IM) every six hours for 24 hours versus meperidine 1 mg/kg administered according to the same schedule. They reported mean pain intensity scores (with ranges), assessed with the Faces Pain Scale, at 1, 6, 12, and 18 hours post‐administration of interventions, and stated that there were no statistically significant differences between ketorolac and meperidine (P > 0.05). Saryazdi 2016 compared a single postoperative dose of 0.5 mg/kg of ketorolac with a single dose of pethidine 1 mg/kg, both administered intravenously. Pain intensity was assessed in the PACU, and at 1, 2, 6, 12, and 24 hours post surgery. There was only a statistical difference between groups at two hours; those receiving ketorolac had a mean pain intensity of 3.88 ± 0.93 versus 5.60 ± 1.41 in the pethidine group (P < 0.001).
Ketorolac versus another NSAID
Only one study compared ketorolac with another NSAID. Munoz‐Cuevas 1997 compared a single postoperative dose of 0.5 mg/kg IV ketorolac versus a single 10 mg/kg dose of dipyrone, administered at the same time. Pain was assessed with the Oucher scale (0 = comfortable child to 100 = crying, extremely restless). At 90 minutes after the administration of the interventions, the authors reported that 21/30 participants (70%) in the ketorolac group had a score of 0 to 30, and 9/30 (30%) had a score between 30 and 80, whereas in the dipyrone group, 12/30 participants (40%) had a score of 0 to 30, and 18/30 participants (60%) had a score between 30 and 80.
Secondary outcomes
Global judgement of satisfaction with treatment
No studies reported data for this outcome for any comparison. Therefore, there were no data on which to assess quality of evidence.
Adverse events
(a) Any adverse event
No studies comparing ketorolac with placebo, or with another NSAID supplied data for this outcome.
Ketorolac versus opioid
Two studies reported data for participants suffering any adverse event (Chiaretti 1997; Maunuksela 1992). Overall, 9/43 participants (21%) receiving ketorolac reported an adverse event versus 11/44 (25%) receiving an opioid (risk ratio (RR) 0.84, 95% confidence interval (CI) 0.40 to 1.79; two studies, 87 participants; Analysis 2.2). We assessed the quality of evidence for this outcome as low. We downgraded quality because the included studies were at moderate or high risk of bias for several outcomes, and because of imprecision (the total number of participants for our analysis was less than 400).
2.2. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 2: Participants reporting any adverse event
(b) Specific adverse events
Reporting of specific AEs was inconsistent across studies, as was the time over which the information was collected. There were insufficient data for meta‐analysis for the vast majority of events.
Ketorolac versus placebo
Six studies reported data for specific AEs, including nausea, vomiting, nausea and vomiting, pruritus, respiratory depression, urinary retention, bleeding, and renal dysfunction (Davis 1999; Lynn 2007; Munro 2002; Romsing 1998; Sutters 1995; Sutters 1999). There were no statistically significant differences between groups for any event in any study. Bleeding was reported in 25/137 (18%) participants receiving ketorolac versus 24/121 (20%) participants receiving placebo (RR 0.99, 95% CI 0.64 to 1.52; five RCTs, 258 participants; Analysis 1.10). This difference was not statistically significant (P = 0.95).
1.10. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 10: Participants experiencing bleeding
Ketorolac versus opioid
Five studies reported AE data for this comparison (Chiaretti 1997; Gunter 1995; Hamza 2012; Lieh‐Lai 1999; Maunuksela 1992). A single study reported a lower incidence of nausea in those receiving ketorolac (3/50, 6%) versus those receiving meperidine (16/50, 32%; RR 0.19, 95% CI 0.06 to 0.60; one RCTs, 100 participants; Analysis 2.4). The overall incidence of vomiting was similar between ketorolac and opioid (RR 1.02, 95% CI 0.78 to 1.32; four RCTs, 359 participants; Analysis 2.5). However, substantial heterogeneity existed between studies (I² = 83%), with one of the studies reporting a statistically significant increase in incidence in those receiving ketorolac (RR 4.89, CI 1.81 to 13.18; 26 participants; Lieh‐Lai 1999). In the three studies that reported incidence of respiratory depression, there was no overall difference in event rates, occurring in 3% of participants in both groups (RR 0.99, 95% CI 0.27 to 3.61; three RCTs, 257 participants; I² = 0%; Analysis 2.7). There were insufficient numbers of participants or events (or both) to allow us to perform meta‐analyses for the remaining AEs (pain on infusion, pruritus, allergy, bleeding, renal dysfunction).
2.4. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 4: Participants reporting nausea
2.5. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 5: Participants reporting vomiting
2.7. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 7: Participants experiencing respiratory depression
Ketorolac versus another NSAID
The single study that compared ketorolac with another NSAID did not report usable data (Munoz‐Cuevas 1997).
We assessed all evidence for this outcome to be very low‐quality, due primarily to risk of bias in included studies (inconsistencies in reporting) and imprecision (low numbers of participants and events). In addition, some analyses demonstrated inconsistency of results (Analysis 2.5; Analysis 2.7).
(c) Withdrawals due to adverse events
Withdrawals due to AEs were rare in those studies that adequately reported data. Where reported, no participants withdrew from any study that compared ketorolac with either opioid or another NSAID. When comparing ketorolac with placebo, none of the 151 participants receiving ketorolac withdrew versus 1/140 who were administered placebo (postoperative edema).
We assessed this as very low‐quality evidence. We downgraded the quality of evidence for all comparisons three times because of limitations to study quality, sparse data, and high risk of reporting bias.
(d) Withdrawals due to lack of efficacy
Withdrawals due to lack of efficacy were also rare. No participants withdrew for this reason in comparisons of ketorolac and placebo. For ketorolac versus opioids, 5/216 participants (2%) overall withdrew, versus 1/175 (<1%) of participants receiving opioids. In the one included study that compared ketorolac with another NSAID, no participants in either arm withdrew due to lack of efficacy (Munoz‐Cuevas 1997).
We assessed this as very low‐quality evidence. We downgraded the quality of evidence for all comparisons three times because of limitations to study quality, sparse data, and high risk of reporting bias.
Serious adverse events
Serious adverse events were rare overall, preventing meta‐analysis.
Ketorolac versus placebo
Two studies reported serious AE data (Romsing 1998; Sutters 1995). Three of 65 (5%) participants receiving ketorolac were assessed as suffering serious AEs versus 1/63 (2%) who were administered placebo. Of note, all of the serious AEs in those receiving ketorolac were bleeding, which required reoperation (Romsing 1998).
Ketorolac versus opioid
Two studies reported SAE data for this comparison (Gunter 1995; Hamza 2012). Four of 99 (4%) participants receiving ketorolac were assessed as suffering SAEs versus 6/97 (6%) receiving an opioid. Two of the reported SAEs were for postoperative bleeding (Gunter 1995). Of note, this study was terminated early due to concerns about increased incidence of major bleeding and a greater number of bleeding episodes in the first 24 hours after surgery in ketorolac subjects.
Ketorolac versus another NSAID
The single study that compared ketorolac with another NSAID reported no severe AEs in either group (Munoz‐Cuevas 1997).
We assessed the quality of evidence as very low for this outcome. We downgraded the quality of evidence for all comparisons three times because of limitations to study quality, sparse data, and high risk of reporting bias.
Time‐to‐onset of meaningful (50%) pain relief
No studies reported data for this outcome for any comparison. Therefore, there were no data on which to assess quality of evidence.
Number of participants requiring rescue medication
Ketorolac versus placebo
Four studies provided data for the comparison of ketorolac versus placebo (Davis 1999; Munro 2002; Romsing 1998; Sutters 1995). In the PACU, 74/135 (55%) participants receiving ketorolac required rescue pain medication versus 81/127 (64%) receiving placebo. The relative risk of requiring rescue medication was 0.85 (95% CI 0.71 to 1.00, P = 0.05; four RCTs, 262 participants; Analysis 1.1; Figure 4). Visual inspection of the forest plot and the I² of 83% suggested substantial heterogeneity between studies. There were insufficient data for analysis at other time points. We judged the quality of evidence for this outcome as very low, based on limitations to study quality, inconsistency (between‐study heterogeneity), and sparse data.
1.1. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 1: Participants using rescue medication in the PACU
4.

Forest plot of comparison: 1 Ketorolac versus placebo, outcome: 1.1 Participants using rescue medication in the PACU
Ketorolac versus opioid
Four studies contributed data for the comparison of ketorolac versus opioid (Gunter 1995; Hamza 2012; Lieh‐Lai 1999; Maunuksela 1992). However, this outcome was assessed over different time periods for each study, thus preventing meta‐analysis. In each study, similar numbers of participants required rescue pain medication in each arm (ketorolac or opioid).
Ketorolac versus another NSAID
There were no data assessing this outcome for the comparison of ketorolac with another NSAID.
Time‐to‐rescue medication
Only one of the 13 included studies reported data for this outcome. Saryazdi 2016 noted similar times to first rescue pain medication when comparing single intravenous doses of ketorolac and pethidine (1.33 ± 0.42 h versus 1.79 ± 0.42 h). Therefore, there were insufficient data on which to assess quality of the evidence.
Opioid consumption
Ketorolac versus placebo
Five studies reported data on opioid consumption (which we converted to, and present here, as IV morphine equivalents) at various time points (Lynn 2007; Munro 2002; Romsing 1998; Sutters 1995; Sutters 1999). Data were available from more than one study at only two time points: in the PACU, and zero to four hours post‐interventions. For the former, the pooled estimate was not statistically different (mean difference (MD) ‐0.17, 95% CI ‐0.80 to 0.47, P = 0.61; three RCTs, 162 participants; Analysis 1.2). For the time period zero to four hours, those in the ketorolac group received 1.58 mg less IV morphine equivalents (95% CI ‐2.58 to ‐0.57, P = 0.002; two RCTs, 129 participants; Analysis 1.3) than those who received placebo.
1.2. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 2: Opioid consumption (mg IV morphine equivalents) in PACU
1.3. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 3: Opioid consumption (mg IV morphine equivalents): 0 to 4 h
Again, we assessed the quality of evidence for this outcome for ketorolac versus placebo as very low, based on included study limitations, inconsistency (between‐study heterogeneity), and sparse data.
Ketorolac versus opioid
Only one study reported data for this outcome. Saryazdi 2016 assessed pethidine consumption in the PACU and at 1, 2, 6, 12, and 24 hours after surgery. The authors reported that there were no statistical differences between the ketorolac and pethidine group at any time point.
Ketorolac versus another NSAID
There were no data assessing this outcome for the comparison of ketorolac with another NSAID.
Sensitivity analysis
While not planned in our protocol, we performed a sensitivity analysis where studies that did not mention that they were blinded were removed from any analysis in which they were initially included (Hamza 2012; Munoz‐Cuevas 1997). Only Hamza 2012 contributed data to our quantitative analysis; removing this study made no difference to the size of effect or the statistical significance in any analysis.
Discussion
Summary of main results
We included 13 studies in this review. There was considerable heterogeneity among study designs, including the comparator arms (placebo, opioid, another nonsteroidal anti‐inflammatory drug (NSAID), or a different regimen of ketorolac), dosing regimens, assessment methods, and types of surgery. This was reflected in the number of outcomes for which meta‐analysis was not possible, and for which substantial statistical heterogeneity was observed for many of the pooled analyses. There were insufficient data to draw conclusions regarding differences in efficacy or safety based on route of administration, time of administration, or dose. The results of the studies available for ketorolac in children were insufficient to allow any conclusions to be drawn about its efficacy or harm in treating postoperative pain. The evidence was insufficient to support or reject its use.
Efficacy
No studies contributed data that enabled us to perform meta‐analysis for either of our primary outcomes: participants with at least 50% pain relief; or mean postoperative pain intensity. There were no data for the former outcome, perhaps reflecting ethical concerns about the risks of subjecting children to unrelieved pain when using this method of assessment (Kossowsky 2015). For the latter, differences in comparator arms, dosing regimens, assessment tools, and times of assessment prevented pooling of data. Four studies individually reported statistically significant reductions in pain intensity at various time points when comparing ketorolac with placebo, but the studies were small and had various risks of bias (Munro 2002; Romsing 1998; Sutters 1995; Sutters 1999). Of the three small studies that compared ketorolac with opioids for this outcome, only Saryazdi 2016 reported a difference in efficacy between treatments, and only at a single time point (Chiaretti 1997; Hamza 2012; Saryazdi 2016). Lastly, a single study comparing ketorolac with the NSAID dipyrone reported that a greater proportion of those receiving ketorolac had mild pain, but again, participant numbers were too small to draw any conclusion (Munoz‐Cuevas 1997).
There were more data available for the outcomes of requirement for, and amount of rescue analgesia. It has been suggested that opioid sparing is a valid pragmatic outcome for analgesic trials in children (Kossowsky 2015). Our pooled analysis of ketorolac versus placebo did not demonstrate a statistically significant difference in the proportion of participants requiring rescue analgesia in the post anaesthesia care unit (PACU). In the single study that did demonstrate superiority versus placebo, ketorolac was administered intraoperatively, which suggests that this was the only study where systemic drug concentrations were sufficiently high to provide analgesia in the immediate postoperative period (Davis 1999). Peak analgesic action of ketorolac occurs between 30 and 60 minutes after administration (Baley 2014). The amount of opioid rescue administered in the PACU was similar between ketorolac and placebo. Pooling of two small studies demonstrated a slight reduction in opioid use versus placebo in the first four hours after administration of interventions, but this reduction was unlikely to be clinically significant (Sutters 1995; Sutters 1999). There were insufficient data to draw any conclusions regarding rescue analgesia use when ketorolac was compared with opioids or other NSAIDs.
There were no data for our secondary efficacy outcomes of global judgement of satisfaction with treatment or time‐to‐meaningful pain relief. Only one study assessed time‐to‐rescue medication, and there were insufficient data for any comparator to assess differences in withdrawal rates due to lack of efficacy.
Safety
There were insufficient data to allow us to analyze overall adverse event rates or serious adverse event rates when comparing ketorolac with either placebo or active controls. Although the majority of serious adverse events reported in those receiving ketorolac involved bleeding events, the number of events was too low to allow us to conclude that bleeding risk was increased in those receiving ketorolac perioperatively. There were also very few data regarding renal dysfunction or gastrointestinal events, two widely recognised adverse effects of NSAIDs (Baley 2014). Concerns regarding the gastrointestinal safety of ketorolac have led to dosing restrictions in the United Kingdom, and withdrawal from the market in some countries (MHRA 2007; Pharma Letter 1993).
As noted, low overall numbers and heterogeneity of assessment methods restricted analysis of specific adverse events. In the few analyses performed, there was not a statistically significant increase in event rates for any specific event, either in pooled analysis or in single studies, when comparing ketorolac and placebo. When comparing ketorolac with opioids or other NSAIDs, there were too few data to make any conclusions regarding event rates.
Lastly, withdrawals due to adverse events were vary rare in all groups, reflecting the acute nature of such studies.
Overall completeness and applicability of evidence
Included studies reported data from comparisons of ketorolac with both placebo and with active controls that are commonly used to treat postoperative pain. The studies covered a range of surgeries commonly performed in children, across a wide age range, but there were few infants enrolled. Doses were typically 0.5 mg/kg or 1 mg/kg, mostly administered intravenously and after surgery. These regimens reflect clinical practice. However, as noted, there was insufficient evidence available for all efficacy and safety outcomes, with no data for one of our primary outcomes.
Quality of the evidence
When assessing the quality of findings using GRADE, we ranked the quality of the evidence as very low across all efficacy and safety outcomes, for all comparisons (placebo, another NSAID, or an opioid). Our GRADE assessments for our main comparison, ketorolac versus placebo, are also shown in Table 1. Very low quality means that this research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different is very high.
A number of individual studies had high risk of bias for issues such as incomplete outcome data, selective reporting, and small size, and none of the trials was unequivocally at low risk of bias for all criteria. For the few outcomes that we were able to perform pooled analysis, the quality of evidence was downgraded due to issues with imprecision, heterogeneity between studies, and low overall numbers of participants and events.
Potential biases in the review process
We attempted to minimise the potential for publication bias related to unpublished or unidentified studies by assessing clinical trial registries and multiple databases, respectively. We combined data from studies with different methodologies, e.g. where ketorolac was administered both intra‐ and post‐operatively. However, we assessed heterogeneity based on these differences, and noted it where we thought it important. We are not aware of any other potential biases.
Agreements and disagreements with other studies or reviews
Baley 2014 assessed efficacy and safety of ketorolac in paediatric surgical pain in a qualitative narrative review. Unlike our review, the authors also included pre‐emptive studies, i.e. where interventions were administered before surgery. In addition to the outcomes we assessed, they also discussed recovery time and length of stay. From the five clinical trials reviewed, they did not note a decrease in opioid consumption when ketorolac was compared with tramadol, morphine, or placebo, but in a study that also included adult participants, ketorolac was shown to provide analgesia without the need for additional analgesics, whereas parenteral acetaminophen was not efficacious as a single agent. Evidence regarding increased risk of bleeding, from both clinical trials and epidemiological studies, was equivocal. The authors further noted that gastrointestinal and renal events are extremely rare with acute use.
Michelet 2012 performed a meta‐analysis of NSAID use for postoperative pain in paediatric patients. In contrast to our review, they included all NSAIDs, including cyclooxygenase‐2 (COX‐2) selective compounds. The review was conducted in a similar manner to ours, i.e. according to guidelines set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Their analysis included 27 studies and found statistically significant reductions in opioid requirements in both the PACU and during the first 24 postoperative hours, and reductions in pain intensity in the PACU, but not during the first 24 hours. The analysis also demonstrated a reduction in postoperative nausea and vomiting during the first 24 hours postoperatively, which the authors determined was dependent on the type of surgery. Subgroup analysis did not demonstrate an effect of the timing of NSAID administration (intra‐ versus postoperative). The most obvious explanation for their finding of reductions in pain intensity and opioid use, where our analysis did not, is that their analysis included more studies.
Authors' conclusions
Implications for practice.
For children with postoperative pain
The amount and quality of evidence for the use of ketorolac for treating postoperative pain in children is very low. The evidence we have indicates that intra‐ and postoperative administration of ketorolac may reduce opioid requirements in some children, but we are very unsure of this. No judgement can be made about adverse events or withdrawals. There are not enough efficacy or safety data to make dosing recommendations.
For clinicians
The amount and quality of evidence for the use of ketorolac for treating postoperative pain in children is very low. The evidence we have indicates that intra‐ and postoperative administration of ketorolac may reduce opioid requirements in some children, but we are very unsure of this. There are insufficient data to determine whether intraoperative administration of ketorolac is more efficacious or has fewer adverse events than postoperative administration. No judgement can be made about adverse events or withdrawals.
For policymakers
The amount and quality of evidence for the use of ketorolac for treating postoperative pain in children is very low, and insufficient to guide policy. The evidence we have indicates that intra‐ and postoperative administration of ketorolac may reduce opioid requirements in some children, but we are very unsure of this. No judgement can be made about adverse events or withdrawals.
For funders
The amount and quality of evidence for the use of ketorolac for treating postoperative pain in children is very low. The evidence we have indicates that intra‐ and postoperative administration of ketorolac may reduce opioid requirements in some children, but we are very unsure of this. No judgement can be made about adverse events or withdrawals. Ketorolac is an inexpensive drug, but we did not search for data assessing indirect costs related to its use (e.g. nursing time), and did not find evidence regarding savings related to reductions in daily expenses or in length of stay.
Implications for research.
General
In common with many interventions used in the paediatric population, there is an insufficient amount of evidence to determine best practice. Only one study that met our inclusion criteria was published since 2010. Many of the included studies had methodological issues. Additional studies that meet current standards for research in paediatric patients are needed.
Design
The studies included in our review were small, and reported on very few participants. Much larger studies of several hundred participants, or more, are needed. Adverse events were generally rare, and serious adverse events very rare. Epidemiological studies may determine the adverse event profile of ketorolac in this setting more accurately, but are lacking in the paediatric population. Such studies are of great importance, given safety concerns raised, based on adult data. Dosing recommendations vary by country; studies that clarify dose, efficacy and dose, and safety ratios are needed.
Outcomes
The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) determined six core outcome domains for consideration in clinical trials of paediatric acute pain (including postoperative pain): pain intensity, global judgment of satisfaction with treatment, symptoms and adverse events, physical recovery, emotional response, and economic factors (McGrath 2008). As noted, the included studies did not report data for several of these outcomes. We found no information for our primary outcome of number of participants with at least 50% reduction in pain, and very few data assessing pain intensity. Concerns have been raised about the enrolment of children in placebo‐controlled trials, where participants are enrolled only when moderate to severe pain is reported, and where access to rescue analgesia is restricted (Kossowsky 2015). It has been suggested that the use of rescue analgesic is a practical and valid surrogate for pain intensity in this population. Although the most clinically important efficacy outcome remains to be determined in the paediatric population, at a minimum, future studies should assess the six core outcomes recommended by IMMPACT, and provide rescue analgesia to all participants.
What's new
| Date | Event | Description |
|---|---|---|
| 29 June 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 7, 2016 Review first published: Issue 7, 2018
| Date | Event | Description |
|---|---|---|
| 18 February 2020 | Amended | Clarification added to Declarations of interest. |
Notes
Assessed for updating in 2020
An updated search in June 2020 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We would like to acknowledge the PaPaS Information Specialist, Joanne Abbott, who developed the search strategy, and ran and compiled all the literature searches for our review.
We would also like to thank Juan Carlos Quijano‐Campos for his translation of Munoz‐Cuevas 1997.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Ketorolac] this term only
#2 ketorolac:ti,ab,kw (Word variations have been searched)
#3 toradol:ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Pain, Postoperative] explode all trees
#6 pain:ti,ab,kw (Word variations have been searched)
#7 #5 or #6
#8 MeSH descriptor: [Adolescent] this term only
#9 MeSH descriptor: [Child] explode all trees
#10 MeSH descriptor: [Infant] explode all trees
#11 (child* or boy* or girl* or baby or babies or teen* or adolescen* or toddler* or infant*):ti,ab,kw (Word variations have been searched)
#12 #8 or #9 or #10 or #11
#13 #4 and #7 and 12
Appendix 2. MEDLINE search strategy
Database: Ovid MEDLINE® Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 Ketorolac/ 2 ketorolac.tw. 3 toradol.tw. 4 or/1‐3 5 exp Pain, Postoperative/ 6 pain.tw. 7 or/5‐6 8 adolescent/ or exp child/ or infant/ or exp infant, newborn/ 9 (child* or boy* or girl* or baby or babies or teen* or adolescen* or toddler* or infant*).tw. 10 8 or 9 11 4 and 7 and 10 12 randomized controlled trial.pt. 13 controlled clinical trial.pt. 14 randomized.ab. 15 placebo.ab. 16 drug therapy.fs. 17 randomly.ab. 18 trial.ab. 19 groups.ab. 20 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21 exp animals/ not humans.sh. 22 20 not 21 23 11 and 22
Appendix 3. Embase search strategy
1 Ketorolac/ (7778)
2 ketorolac.tw. (3320)
3 toradol.tw. (750)
4 or/1‐3 (8600)
5 exp Postoperative Pain/ (49428)
6 pain.tw. (679128)
7 or/5‐6 (692726)
8 adolescent/ or exp child/ or infant/ or exp infant, newborn/ (3035282)
9 (child* or boy* or girl* or baby or babies or teen* or adolescen* or toddler* or infant*).tw. (2012633)
10 8 or 9 (3557531)
11 4 and 7 and 10 (686)
12 random$.tw. (1117645)
13 factorial$.tw. (28453)
14 crossover$.tw. (58783)
15 cross over$.tw. (26173)
16 cross‐over$.tw. (26173)
17 placebo$.tw. (243454)
18 (doubl$ adj blind$).tw. (171695)
19 (singl$ adj blind$).tw. (18162)
20 assign$.tw. (294904)
21 allocat$.tw. (107414)
22 volunteer$.tw. (210917)
23 Crossover Procedure/ (48406)
24 double‐blind procedure.tw. (235)
25 Randomized Controlled Trial/ (417141)
26 Single Blind Procedure/ (22860)
27 or/12‐26 (1743689)
28 (animal/ or nonhuman/) not human/ (5100131)
29 27 not 28 (1549380)
30 11 and 29 (186)
Appendix 4. LILACS search strategy
ketorolac or toradol [Words] and pain [Words] and (child$ or boy$ or girl$ or baby or babies or teen$ or adolescen$ or toddler$ or infant$) [Words]
Data and analyses
Comparison 1. Ketorolac versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Participants using rescue medication in the PACU | 4 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.71, 1.00] |
| 1.2 Opioid consumption (mg IV morphine equivalents) in PACU | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.80, 0.47] |
| 1.3 Opioid consumption (mg IV morphine equivalents): 0 to 4 h | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐2.58, ‐0.57] |
| 1.4 Participants reporting nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.5 Participants reporting vomiting | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.29, 1.67] |
| 1.6 Participants reporting nausea and vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.7 Participants reporting pruritus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.8 Participants experiencing respiratory depression | 2 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.9 Participants reporting urinary retention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.10 Participants experiencing bleeding | 5 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.64, 1.52] |
| 1.11 Participants experiencing renal dysfunction | 2 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
1.4. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 4: Participants reporting nausea
1.5. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 5: Participants reporting vomiting
1.6. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 6: Participants reporting nausea and vomiting
1.7. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 7: Participants reporting pruritus
1.8. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 8: Participants experiencing respiratory depression
1.9. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 9: Participants reporting urinary retention
1.11. Analysis.

Comparison 1: Ketorolac versus placebo, Outcome 11: Participants experiencing renal dysfunction
Comparison 2. Ketorolac versus opioid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Participants using rescue medication in the PACU | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Participants reporting any adverse event | 2 | 87 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.40, 1.79] |
| 2.3 Participants reporting pain on infusion | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.4 Participants reporting nausea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.5 Participants reporting vomiting | 4 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.78, 1.32] |
| 2.6 Participants reporting pruritus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7 Participants experiencing respiratory depression | 3 | 257 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.27, 3.61] |
| 2.8 Participants reporting allergy, rash, or local reaction | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.23, 4.72] |
| 2.9 Participants experiencing bleeding | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.56 [0.60, 185.85] |
| 2.10 Participants experiencing renal dysfunction | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.11, 64.10] |
2.1. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 1: Participants using rescue medication in the PACU
2.3. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 3: Participants reporting pain on infusion
2.6. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 6: Participants reporting pruritus
2.8. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 8: Participants reporting allergy, rash, or local reaction
2.9. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 9: Participants experiencing bleeding
2.10. Analysis.

Comparison 2: Ketorolac versus opioid, Outcome 10: Participants experiencing renal dysfunction
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chiaretti 1997.
| Study characteristics | ||
| Methods | Randomised, multiple‐dose and continuous infusion, single‐centre. Participants treated and assessed until resolution of postoperative pain. Interventions administered after admission to PICU (exact timing not reported) | |
| Participants | Type of surgery: neuro‐oncologic (n = 31), orthopedic (n = 8), abdominal (n
= 8), thoracic (n = 5) Ketorolac bolus group Entered/completing: 13/unclear Age (mean, SD): 72.6 ± 76.8 months Sex (male, %): 7 (54%) Ketorolac continuous infusion group Entered/completing: 13/unclear Age (mean, SD): 50.3 ± 60.9 Sex (male, %): 9 (69%) Fentanyl group Entered/completing: 13/unclear Age (mean, SD): 62.2 ± 61.3 Sex (male, %): 11 (85%) |
|
| Interventions | Timing of first dose not specified for all groups. All interventions
administered until resolution of painful symptoms (average of 31 h; range:
10 to 42 h) Ketorolac bolus: 1.2 mg/kg IV every six hours Ketorolac continuous infusion: 1.2 mg/kg IV bolus + 0.21 mg/kg/h continuous infusion Fentanyl: 1 mcg/kg/h |
|
| Outcomes | Primary (as specified in study): haemodynamic parameters: systolic and
diastolic blood pressure, breathing rate, heart rate, oxygen saturation
(before and after every 4 h of treatment), oxygen arterial pressure (every
12 h) Secondary: child’s behaviour: affective Facial Scale and CHEOPS score; both assessed every 4 h The first 8 h post initiation of interventions were used to assess which intervention was most rapidly efficacious |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, sex, type of surgery, haemoglobin, body temperature | |
| Notes | Fourth group received fentanyl and ketorolac (data not included here). Length of treatment similar among groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all participants completed the study or if any data were imputed |
| Selective reporting (reporting bias) | Unclear risk | All outcomes described in methods reported in results. Mean CHEOPS and Faces Pain Scale scores presented without SDs |
| Sample size | High risk | 52 participants (13 in each group) |
Davis 1999.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, single‐dose. Outcomes
assessed over 24 hours. Single dose administered “while the surgeon was performing the procedure”. |
|
| Participants | Type of surgery: ambulatory bilateral myringotomy and pressure equalization
tube insertion in 1 to 5 year‐olds Ketorolac group Entered/completing: 50/50 Age (mean, SD): 2.1 ± 0.1 Sex (male, %): not reported Placebo group Entered/completing: 50/50 Age (mean, SD): 2.0 ± 0.1 Sex (male, %): not reported |
|
| Interventions | Ketorolac: 1 mg/kg single dose IV during the procedure Placebo: equal volume of saline, as with ketorolac |
|
| Outcomes | Primary (as specified in study): nurse assessment of anaesthetic emergence
on a 3‐point scale: 1 = asleep, calm, or mildly agitated but easily
consolable; to 3 = hysterical, crying inconsolably, or thrashing. Assessed
until discharge from hospital. Secondary: Requirement for rescue analgesia (acetaminophen 15 mg/kg) in the PACU Incidence of emesis in the PACU Incidence of emesis, need for pain medications, and overall quality of the participant’s and family’s surgical experience assessed 24 h after patient discharge via telephone. |
|
| Source of funding | Supported in part by Abbott Laboratories | |
| Were treatment groups comparable at baseline? | Yes: age, weight, previous anaesthetic experience, duration of anaesthesia | |
| Notes | Four groups assessed: Group 1 – halothane and ketorolac; Group 2 – halothane and placebo; Group 3 – sevoflurane and ketorolac; Group 4 – sevoflurane and placebo. Only sevoflurane groups included in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated number code |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “injections were prepared by the hospital pharmacist and placed in
specially labelled syringes. Patients, physicians, and the research nurse
were blinded to the syringe’s contents”. No mention that syringes appeared
identical. “In the recovery room, the anaesthetic emergence was evaluated by the research study nurse who was blinded to the patient’s anaesthetic and analgesic grouping” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appears that all participants completed the study and contributed data. All outcomes were measured over single time periods |
| Selective reporting (reporting bias) | Unclear risk | Overall quality of patient and family experience assessed but not reported |
| Sample size | Unclear risk | 50 participants in each arm |
Gunter 1995.
| Study characteristics | ||
| Methods | Double‐blind, randomised, active‐controlled, single‐dose. Outcomes assessed
for up to 2 weeks post discharge Interventions administered when surgery was complete and haemostasis was achieved |
|
| Participants | Type of surgery: tonsillectomy or adenotonsillectomy in patients aged 1 to
12 years Ketorolac group Entered/completing: 49/49 Age (mean, SD): 70 ± 31 months Sex (male, %): not reported Morphine group Entered/completing: 48/47 Age (mean, SD): 73 ± 30 months Sex (male, %): not reported |
|
| Interventions | Ketorolac: 1 mg/kg IV single bolus after surgery Morphine: 0.1 mg/kg IV single bolus after surgery |
|
| Outcomes | Primary (as specified in study): Not specified Secondary: PACU: duration of oxygen requirement; supplemental analgesic requirements (up to 2 doses of morphine 0.05 mg/kg); emesis; bleeding; time to readiness for discharge Outpatient surgery: emesis; bleeding; time to readiness for discharge home Ward: emesis; duration of oxygen requirement; bleeding Day after surgery and 2 weeks later: emesis (day after only); bleeding; any return to clinic or readmission to hospital |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, weight, ASA status, admission status, diagnosis, tonsil size, obstructive sleep apnoea score, procedure, surgical technique, surgery time, intraoperative bleeding. | |
| Notes | Power calculation estimated total enrolment of 300 required. The first interval analysis of bleeding data was performed after 97 subjects had been recruited; because of number of bleeding episodes in the first 24 h after surgery in ketorolac subjects, the study was terminated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random numbers stratified by location of surgery |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “Study drugs were prepared by a pharmacist or an anaesthesiologist not
involved in the care of the subject. Subjects, families, anaesthesiologists,
nurses, and investigators were blinded to study drug assignment” No mention that interventions appeared identical. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One subject randomised to receive morphine experienced severe
intraoperative arterial bleeding, was not treated with study drug, and was
not included in the analysis. Appears that follow‐up was complete in remaining 96 participants |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods section reported in results section. |
| Sample size | High risk | 96 participants (49 ketorolac, 47 morphine) |
Hamza 2012.
| Study characteristics | ||
| Methods | Randomised, active‐controlled, multiple dose, evaluating outcomes 24 hours
postoperatively Interventions administered in recovery room |
|
| Participants | Type of surgery: tonsillectomy Ketorolac group Entered/completing: 50/50 Age (mean, SD): range for inclusion: 5 to 12. Actual age range of participants not specified Sex (male, %): not reported Pethidine group Entered/completing: 50/50 Age (mean, SD): range 5 to 12 Sex (male, %): not reported |
|
| Interventions | Ketorolac: 0.5 mg/kg IM (gluteal) diluted in 2 mL, every 6 h x 24 hours,
starting in the recovery room Pethidine: 1 mg/kg IM (gluteal) diluted in 2 mL, every 6 h x 24 hours, starting in the recovery room |
|
| Outcomes | Primary and secondary outcomes not noted Outcomes included: postoperative pain (Faces pain scale, 0 to 5: 0 = no pain, 5 = severe pain) every 6 h x 24 h; number of participants requiring analgesia (pethidine 0.5 mg/kg), vital signs, oxygen saturation and requirement for oxygen therapy, AEs |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appears that all participants completed the study, but no mention of methods for dealing with missing data |
| Selective reporting (reporting bias) | High risk | Primary and secondary outcomes not specified in methods. Several outcomes reported in results were not mentioned in methods |
| Sample size | Unclear risk | 50 participants in both groups |
Lieh‐Lai 1999.
| Study characteristics | ||
| Methods | Prospective, randomised, double‐blind, parallel, single dose,
positive‐control Intervention administered at first postoperative complaint of moderate to severe pain |
|
| Participants | Type of surgery: mixed (cardiovascular, spinal fusion, neurosurgical,
reconstructive) requiring admission to an intensive care unit
postoperatively Ketorolac group Entered/completing: 54/54 Age (mean, SD): 10.6 ± 4.2 Sex (male, %): 25 (46%) Morphine group Entered/completing: 48/48 Age (mean, SD): 10.2 ± 4.6 Sex (male, %): 18 (38%) |
|
| Interventions | Ketorolac: 0.6 mg/kg IV single dose at first postoperative complaint of
moderate to severe pain Morphine: 0.1 mg/kg IV as with ketorolac |
|
| Outcomes | Primary (as specified in study): proportion of patients reporting
measurable pain relief at 1 and 2 hrs after dosing as defined by a CHEOPS
score < 7 or an Oucher Analogue Scale score ≤ 60 Secondary: proportion of patients in each group achieving maximum pain relief during the first 2 h after dosing; percentage of patients needing repeat medication within the first 4 hrs after study drug administration; vital signs; AEs |
|
| Source of funding | Ronald McDonald's Children's Charities, by National Institute of Child Health and Human Development Pediatric Pharmacology Research Unit Network grant UO1 HD31313, and by the Children's Hospital of Michigan Research Endowment Fund. | |
| Were treatment groups comparable at baseline? | Yes: age, sex, severity of illness, type of surgery, use of inotropic drugs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | “Only the research pharmacist and the analytical chemist had access to the randomization code” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The appropriate dose of morphine or ketorolac was prepared and dispensed in coded syringes by the research pharmacist. The concentration of morphine was 2 mg/mL, whereas the ketorolac concentration was 15 mg/mL. Therefore, the volume of ketorolac was adjusted to equal the volume of the appropriate dose of morphine for the same patient. As a further precaution to ensure blinding, ICU nurses who were not involved in the study administered the study medication” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Table 2 confirmed that all participants contributed data for primary and
secondary outcomes. LOCF used in participants requiring rescue analgesia, numbers similar between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes from methods section reported in results section |
| Sample size | High risk | 54 in ketorolac arm, 48 in morphine arm |
Lynn 2007.
| Study characteristics | ||
| Methods | Pharmacokinetic double‐blind, placebo‐controlled, single dose. Assessments 12 h post‐dosing Intervention administered on postoperative day 1 | |
| Participants | Type of surgery: various (craniectomy, other neurosurgery, general,
plastic, urologic, cardiac) Infants and toddlers, age 6 to 18 months included. All infants received morphine sulphate by continuous IV infusion at 5 mcg/kg/h to 30 mcg/kg/h with bolus doses (0.05 mg/kg) as needed for pain control Ketorolac group Entered/completing: 16/16 Age (mean, SD): 356 ± 79 days Sex (male, %): 8 (50%) Placebo group Entered/completing: 12/12 Age (mean, SD): 352 ± 106 days Sex (male, %): 5 (42%) |
|
| Interventions | Ketorolac: 1 mg/kg in 2 mL dextrose 5% as a single 10 minute IV infusion.
Second group received 0.5 mg/kg; not reported here, as only 9
participants Placebo: as with ketorolac |
|
| Outcomes | Primary (as specified in study): pharmacokinetic study Secondary: morphine bolus and cumulative doses for 12 h period postintervention Safety data (BUN, serum creatinine, liver function tests, pulse oximetry, blood loss from surgical drains, haemoccult testing of stools and gastric output) |
|
| Source of funding | FDA Orphan Product Development Grant FD‐R‐001815. The population analysis was partially supported by NIH grant P41 EB001975, and safety assessments were partially supported by NIH grant M01‐RR‐00037 | |
| Were treatment groups comparable at baseline? | Reported as similar for age, weight, height and sex | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of methods of imputation or description of dropouts postintervention, but it appeared that all participants completed the study and contributed data. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section reported in results section, but pain scores only used to determine opioid dosing and not assessed as an outcome |
| Sample size | High risk | 37 participants (12 placebo, 9 ketorolac 0.5 mg/kg (not included in extraction), 16 ketorolac 1 mg/kg) |
Maunuksela 1992.
| Study characteristics | ||
| Methods | Two centre, double‐blind, randomised, parallel, active‐comparator trial
lasting up to 8 h after last dose of study medication (up to 8.5 h
total) First dose of interventions administered at first observation of at least moderate pain after surgery. The second and third doses were administered in the event of incomplete pain relief and were given at 15 and 30 minutes after dosing. However, if the clinician thought that further analgesia was required earlier, the second dose was administered at 5 or 10 minutes and the third dose at 15 minutes after the first dose |
|
| Participants | Type of surgery: Elective (squint repair, circumcision, other), age range 3
to 12 years Ketorolac bolus group Entered/completing: 30/30 Age (median, range): 7 (4 to 12) Sex (male, %): 15, 50% Ketorolac titration group Entered/completing: 31/30 Age (median, range): 7 (3 to 12) Sex (male, %): 19, 61% Morphine group Entered/completing: 31/30 Age (median, range): 7 (4 to 12) Sex (male, %): 12 (39%) |
|
| Interventions | Ketorolac bolus: initial bolus of 0.5 mg/kg IV followed by two doses of
placebo Ketorolac titration: 0.2 + 0.2 + 0.1 mg/kg IV Morphine: three successive doses of 0.1 mg/kg IV All interventions administered as 0.1 mL/kg via syringe. The participant received the first dose of the study medication at first observation of at least moderate pain. The clinical observer visited the participant at 5 and 10 minutes after dosing to record vital signs and assess degree of sedation and pain intensity. After the 15‐minute assessment, the following action was taken: pain intensity score of 0, no further medication, score of 1 to 3, medication was given on the basis of the decision of the clinician and the child, and score > 4, further medication was given. Participants who received a second dose of study medication continued to be assessed at 5‐minute intervals. If no further study medication was needed, the participant entered phase II and the clinical observer continued the assessment visits at 15‐minute intervals up to 8 hours or until the participant needed a rescue medication |
|
| Outcomes | Primary (as specified in study): SPID from baseline until the time that 50%
of participants had withdrawn (data from all participants); and SPID from
the time at which pain relief was achieved (the final assessment in phase I)
until the time at which 50% of these participants had withdrawn (only data
from subjects who achieved pain relief). Pain intensity assessed using
behavioral pain intensity (BPI) scale Secondary: number of analgesic doses used (morphine 0.1 mg/kg); duration of analgesia (time from administration of the final dose of test medication to administration of a rescue analgesic, or withdrawal, or 8 h); sedation (0 = completely awake; 4 = asleep and does not respond to any gentle stimulus) |
|
| Source of funding | Supported by a grant from Syntex Research Europe (Maidenhead, England) | |
| Were treatment groups comparable at baseline? | Yes: mean BPI scores, sedation scores. Other demographic variables not reported as similar and P values not reported, but appear similar between groups | |
| Notes | Rescue analgesia of 0.1 mg/kg morphine was available if required because the participant did not achieve analgesia after the study medication or because of a return of pain after the participant had initially obtained analgesia from the study medication. Participants requiring rescue analgesia were withdrawn from the study at that time | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Ketorolac … was provided as a 10 mg/mL‐1 solution in 1 mL ampoules with matching placebo. Morphine was also supplied as a 10 mg/mL‐1 solution in 1 mL ampoules…10 identical ampoules of 1 mL each were provided for each dose” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | To calculate SPIDs for participants who withdrew from the study before the time specified, the last recorded BPI score was carried forward. Two participants excluded from efficacy analyses due to protocol violations. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes from methods section reported in results section, but additional outcomes reported that were not outlined in methods |
| Sample size | High risk | 91 participants (31 morphine, 31 ketorolac titration, 30 ketorolac bolus) |
Munoz‐Cuevas 1997.
| Study characteristics | ||
| Methods | randomised, single centre, single dose. Participants assessed for 90 minutes post intervention. Intervention administered in the immediate postoperative period | |
| Participants | Type of surgery: ophthalmic: strabismus correction (n = 33), cataract
extraction (n = 9), and others (n = 18) Ketorolac group Entered/completing: 30/30 Age (mean, SD): 7.4 (no SD reported) Sex (male, %): 17 (57%) Dipyrone group Entered/completing: 30/30 Age (mean, SD): 7.7 Sex (male, %): 18 (60%) |
|
| Interventions | Ketorolac: 0.5 mg/kg in 10 ml sterile water IV slow injection, single dose
in immediate postoperative period Dipyrone: 10 mg/kg in 10 ml sterile water IV slow injection, single dose in immediate postoperative period |
|
| Outcomes | Primary (as specified in study): not specified Hemodynamic variables (systolic and diastolic blood pressure, respiratory rate), sedation scales (from I to V), and pain (Oucher scale); every 10 minutes for 90 minutes post intervention |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, height | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants completing study not reported, although appears that all did. Methods for dealing with dropouts and missing data not reported |
| Selective reporting (reporting bias) | High risk | Hemodynamic variables presented graphically only, without SDs. Anchors for sedation scale not specified. Oucher scale data presented as number of participants with scores of 0 to 30 and with scores of 30 to 80; Oucher scale usually scored on 0 to 10 scale |
| Sample size | High risk | 60 participants (30 in each group) |
Munro 2002.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled, multiple dose study. Acute
outcomes assessed over 3 days postoperatively First dose administered at completion of surgery |
|
| Participants | Type of surgery: posterior spinal fusion for correction of idiopathic
scoliosis Ketorolac group Entered/completing: 20/20 Age (mean, SD): 13.9 ± 1.3 Sex (male, %): 2 (10%) Placebo group Entered/completing: 18/15 Age (mean, SD): 14.1 ± 1.2 Sex (male, %): 0 |
|
| Interventions | Ketorolac: 0.5 mg/kg IV (max dose 15 mg) in 5 mL normal saline. Average
dose = 0.2 mg/kg. Every 6 h x 6 doses Placebo: 5 mL normal saline as with ketorolac |
|
| Outcomes | Primary (as specified in study): not specified, but appeared to be
differences in postoperative NRS pain scores at various time points through
postoperative day 3 Secondary: Pain on activity Sedation (0 to 5 scale) Degree of muscle spasm (0 to 4 scale) Morphine consumption via PCA Acetaminophen, diazepam, and anti‐emetic use per day Hematocrit, intra‐ and postoperative blood loss, and transfusion requirements Curve progression, back pain, and hardware failure at postoperative follow‐up and yearly for at least two years |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | Yes: age, sex, weight, number of levels fused, length of surgery, length of PACU stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “All personnel administering the drugs and evaluating the patients for pain and side effects were blinded to the contents of the syringes”. No further details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants in placebo group excluded due to protocol violations or incomplete data. Data were analyzed for all remaining participants. No mention of how missing data (if any) were imputed |
| Selective reporting (reporting bias) | High risk | Primary outcome unclear. All outcomes mentioned in methods section reported in results, but pain scores reported graphically only |
| Sample size | High risk | 35 participants (15 placebo, 20 ketorolac) |
Romsing 1998.
| Study characteristics | ||
| Methods | Randomised, parallel, placebo‐controlled, single‐dose with outcomes
assessed over 24 h postoperatively Single dose administered immediately after surgery (another group received ketorolac pre‐operatively – not included in this review) |
|
| Participants | Type of surgery: elective tonsillectomy with or without adenoidectomy ASA physical status 1 to 2, aged 5 to 15 years. Ketorolac group Entered/completing: 22/20 Age (mean, SD): 9.7 ± 3.7 Sex (male, %): 6 (30%) Placebo group Entered/completing: 20/20 Age (mean, SD): 8.8 ± 3.2 Sex (male, %): 6 (30%) |
|
| Interventions | Ketorolac: 1 mg/kg IV single dose immediately after surgery Placebo: saline |
|
| Outcomes | Primary (as specified in study): not defined, but presumed to be: rescue analgesia in PACU (fentanyl 0.5 mcg/kg to 1 mcg/kg IV) and on ward (acetaminophen rectally 20 mg/kg). Fentanyl reported as number of participants requiring and mean dose, acetaminophen as number of doses required Pain intensity (Poker Chip Tool) at 1.3, 3, 5, and 24 h postoperatively, both while at rest and while drinking 50 mL of water Secondary: Intra‐ and postoperative bleeding episodes: any bleeding that was noted by the patient or parent and was confirmed by medical evaluation Vomiting: number of participants reporting |
|
| Source of funding | Not reported | |
| Were treatment groups comparable at baseline? | No P values reported but stated to be no significant differences between groups for demographic (age, weight, sex) and clinical (surgery, duration of anaesthesia, duration of surgery) variables | |
| Notes | “After the first 15 patients had been treated, a high incidence of postoperative bleeding (5 patients representing all groups) resulted in an interruption of the study. It appeared that this higher rate of postoperative bleeding occurred in the patients of only one surgeon. Consequently, after re‐approval from the regional Ethics Committee and the National Board of Health, the study was continued without participation of the above‐mentioned surgeon”. All 15 participants included in analysis (10 in our analysis) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “All patients and parents, and personnel involved in patient management and data collection were unaware of drug group assignment”. “Each patient received equal volumes of study medication and saline” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of ITT analysis or imputation methods, but appears that all participants that received an intervention completed the study, and contributed data at all time points |
| Selective reporting (reporting bias) | Low risk | All outcomes in methods reported in full in results |
| Sample size | High risk | 20 participants in each arm at completion of study |
Saryazdi 2016.
| Study characteristics | ||
| Methods | Randomised, double‐blind, active‐controlled, single dose, two centre study
in children aged 1 to 12 years. Outcomes assessed over 24 hours
postoperatively. Interventions administered at completion of surgery. |
|
| Participants | Type of surgery: inguinal hernia surgery Ketorolac group Entered/completing: 33/33 Age (mean, SD): 4.86 ± 2.59 years Sex (male, %): 3 (9%) Pethidine group Entered/completing: 33/33 Age (mean, SD): 4.95 ± 2.58 years Sex (male, %): 5 (15%) |
|
| Interventions | Ketorolac: 0.5 mg/kg IV single dose at completion of surgery and before
extubation Pethidine: 1 mg/kg IV as above |
|
| Outcomes | Primary (as specified in study): not specified, but appeared to be
differences in postoperative NRS pain scores (Wong Baker) at various time
points through 24 h postoperatively. Secondary: Additional analgesia (preferably pethidine 0.5 mg/kg), administered for pain score > 4: time‐to‐first use and total dose Level of consciousness (modified Aldrete score) at various time points through 24 h postoperatively Vital signs (systolic, diastolic and mean arterial blood pressure; heart rate) Medications used and their side effects Vomiting: incidence and amount of metoclopramide used in 24 h |
|
| Source of funding | Not mentioned | |
| Were treatment groups comparable at baseline? | Yes: age, sex, and haemodynamic parameters prior to surgery | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “To ensure blinding, data were collected by one person and the intervention was conducted by another”. No mention of blinding of participants or whether interventions appeared identical. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Appeared that all participants provided data at all time points, but not explicitly stated. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods were reported in results, but units of
measurement were not specified and were unclear. Discussion reported several
outcomes as being statistically superior in ketorolac group, when they were
not statistically different, not superior, or both. Assessment of adverse events unclear. |
| Sample size | High risk | 33 participants in both groups |
Sutters 1995.
| Study characteristics | ||
| Methods | randomised, double‐blind, placebo controlled, single dose at time of completion of surgery. Patients assessed until discharge from day surgery | |
| Participants | Type of surgery: tonsillectomy ± adenoidectomy ± myringotomy Ketorolac group Entered/completing: 45/45 Age (mean, SD): 84.7 ± 28.9 months Sex (male, %): 26 (58%) Placebo group Entered/completing: 43?/42 Age (mean, SD): 85.0 ± 26.7 months Sex (male, %): 22 (52%) |
|
| Interventions | Ketorolac: 1 mg/kg IM single dose at completion of surgery Placebo: saline |
|
| Outcomes | Primary and secondary outcomes not defined Pain intensity via both CHEOPS (behavioural) and Oucher (self‐report) scales in the PACU (before and after administration of fentanyl) and day surgery areas (at 1, 2, 3, and 4 h post‐PACU discharge, and at discharge to home); Requirement for supplemental analgesia (fentanyl 0.5 mcg/kg/dose) in the PACU and day surgery areas; Length of stay; Severity (0 = no bleeding, 3 = severe bleeding) and incidence of bleeding |
|
| Source of funding | Supported by Novametrix and Critikon Corporations, the Purdue Frederick Company, and grants from the American Nurse's Foundation, Graduate Research Division, University of California at San Francisco, and Sigma Theta Tau, Alpha Eta and Alpha Gamma Chapters | |
| Were treatment groups comparable at baseline? | Yes: demographics (age, weight, sex, mother’s and father’s ethnicity, household income) and clinical characteristics (last infection, previous hospitalisation, previous surgery) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | “The group assignment was sealed in a manila envelope and accompanied the patient to surgery. The anaesthesiologist opened the envelope and administered the study drug in the deltoid muscle at the completion of surgery” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “The identity of the injection was not revealed to the PACU nurses, the
parents, or to the investigator who performed the clinical assessments.” No mention of interventions appearing identical |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis only for all outcomes |
| Selective reporting (reporting bias) | Unclear risk | Outcomes described in much more detail in results section than in methods. No assessment of AEs other than bleeding |
| Sample size | High risk | 45 in ketorolac group, 42 in placebo group |
Sutters 1999.
| Study characteristics | ||
| Methods | randomised, multiple‐dose, double blind, single‐centre. Participants assessed for 36 hours postoperatively. Interventions administered upon arrival to PACU and at 6‐hour intervals for a maximum of 8 doses | |
| Participants | Type of surgery: orthopedic (spine or pelvis, hip, long bone, shoulder or
knee, ankle or wrist or foot) Ketorolac group Entered/completing: 36?/36? Age (mean, SD): 151.75 ± 42.17 months Sex (male, %): 22 (61%) Placebo group Entered/completing: 32?/32? Age (mean, SD): 152.09 ± 50.68 months Sex (male, %): 22 (69%) |
|
| Interventions | Ketorolac: 1 mg/kg IV loading dose upon arrival to PACU. Subsequent doses
of 0.5 mg/kg IV every 6 hours for a maximum of 8 doses Placebo: Not specified. Administered on same schedule as ketorolac |
|
| Outcomes | Primary (as specified in study): not specified Pain intensity via self‐report (Faces Pain Rating Scale) and nurse assessment (0 to 5 NRS) at 4‐h intervals through 36 h postoperatively. Opioid use via PCA morphine (number of PCA attempts, number of PCA doses received, and total PCA morphine dose) Incidence of AEs. Severity of sedation (1 = awake, 5 = only awakens when aroused) |
|
| Source of funding | An intramural research grant from the study hospital | |
| Were treatment groups comparable at baseline? | Yes, for surgical and demographic variables, with exception of ASA classification (higher in placebo group, P < 0.03) and father’s education status (fewer years in placebo group, P < 0.04) | |
| Notes | Age range not reported, but described as children and mean age of around 150 months in both groups. Unclear how many participants were enrolled or completed the study. Stated that only those completing the study were analyzed, and five withdrawals described, but reported that 68 children participated and data presented for 68 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “To protect the double‐blind study design, all patients received coded unit‐dose syringes of the study drug from the pharmacy” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only participants completing the study were included in the analysis. Five participants withdrew. No mention of imputation methods for missing data in those participants that completed the study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes mentioned in methods reported in results. PACU and overall length of stay reported in results, but not mentioned in methods. Self‐report pain scores presented graphically only, nurse assessed pain scales reported only as P values |
| Sample size | High risk | 68 participants (ketorolac 36, placebo 32) |
ASA: American Society of Anesthesiologists; BUN: blood urea nitrogen; CHEOPS: Children's Hospital of Eastern Ontario pain scale; IM: intramuscular; ITT: intention‐to‐treat; IV: intravenous; LOCF: last observation carried forward; NRS: numeric rating scale; PACU: post‐anaesthesia care unit; PCA: patient‐controlled analgesia; PICU: paediatric intensive care unit; SD: standard deviation; SPID: summed pain intensity difference.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Glickman 1995 | Adult participants |
| Greco 1994 | Adult participants |
| Gupta 2004 | No control intervention |
| Hayes 2011 | Both paediatric and adult participants. Data not presented separately for paediatric participants. |
| Hernandez 1996 | Adult participants |
| Palacio 1997 | Ketorolac administered in both arms |
| Petrov 2009 | Not randomised |
| Rossitto 2009 | Adult participants |
| Vetter 1994 | Control group did not receive any intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Tariq 2004.
| Methods | Unclear |
| Participants | Unclear ‐ no confirmation that participants were children |
| Interventions | 20mL of 0.25% bupivacaine intraperitoneally (group 1) or 0.25% bupivacaine and 30 mg ketorolac intraperitoneally (group 2) |
| Outcomes | VAS pain scores at rest, on movement, and on coughing at 3, 6, and 12 h postoperatively. Time‐to‐first demand analgesia, total analgesic requirement, and recovery variables |
| Notes | Unable to obtain full article from any source |
VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
NCT01667120.
| Study name | The use of ketorolac in surgical neonates |
| Methods | randomised, single canter, double‐blinded, parallel, placebo‐controlled, multiple dose study |
| Participants | Infants gestational age > 37 weeks and ≥ one week of age to 3 months of age undergoing a surgical procedure on the abdomen |
| Interventions | Ketorolac group: ketorolac 0.5mg/kg IV every 8 h x 72 h Placebo group: saline as with ketorolac group |
| Outcomes | Primary: bleeding events Secondary: daily creatinine levels, pain scores, urine output per shift, platelet counts, haemoglobin levels, number of days on the ventilator, amount of opioid administered, blood pressure, and reintubation events |
| Starting date | July 2012 |
| Contact information | Jennifer H Aldrink, MD, Jennifer.aldrink@nationwidechildrens.org |
| Notes | Unclear when intervention first administered. The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years |
NCT02653742.
| Study name | Ketorolac sublingual vs. fentanyl intranasal in pain control for bilateral myringotomy and tubes (BMT) placement in children |
| Methods | randomised, single centre, double‐blinded, parallel, active‐controlled, single dose study |
| Participants | Children 8 months to 7 years undergoing bilateral myringotomy and tube placement |
| Interventions | Ketorolac group: ketorolac 1mg/kg sublingual administered after induction
of general anaesthesia Fentanyl group: fentanyl 2mg/kg intranasal administered after induction of general anaesthesia |
| Outcomes | Primary: pain intensity via CHEOPS score, through approximately 1 h
postoperatively Secondary: administration of additional pain medication |
| Starting date | May 2015 |
| Contact information | Farzana Afroze, MD, afrozef@mail.amc.edu |
| Notes | Unclear if interventions are administered preoperatively or intraoperatively |
NCT02973958.
| Study name | Evaluating pain outcomes of ketorolac administration in children undergoing circumcision |
| Methods | randomised, single centre, single‐blinded, parallel, single dose study |
| Participants | Male children (< 18 years) undergoing circumcision |
| Interventions | Ketorolac group: 0.5 mg/kg IV ketorolac at the beginning of the
circumcision, once the patient is asleep Control group: unclear |
| Outcomes | Postoperative pain evaluated through the use of the Face, Legs, Activity, Cry, Consolability (FLACC) pain score or Wong‐Baker FACES scale at various time points postoperatively, through 24 h |
| Starting date | February 1, 2017 |
| Contact information | Bryce Weber, MD FRCSC, bryce.weber@albertahealthservices.ca |
| Notes | Unclear if control group receives placebo or only no intervention |
NCT03178539.
| Study name | Comparative study between the effect of diclofanic and ketorolac in post‐tonsillectomy pain management |
| Methods | randomised, single centre, double‐blinded, parallel, active‐controlled, multiple dose study |
| Participants | Children scheduled for elective tonsillectomy or adenotonsillectomy for chronic or recurrent tonsillitis and aged between 6 to 12 years |
| Interventions | Ketoroloac group: intra‐operative ketorolac tromethamine 0.5 mg/kg IV, then
postoperatively (frequency and duration not specified) Diclofenac group: intra‐operative diclofenac sodium 0.3 mg/kg IV, then postoperatively (frequency and duration not specified) |
| Outcomes | Primary: pain intensity via 4‐point VRS immediately postoperatively and 3,
6, 12, and 24 h postoperatively Secondary: bleeding, dysphagia (time to resume normal diet) |
| Starting date | May 31, 2017 |
| Contact information | Ahmed H Hussein Khalifa, MBBCH, ahmed_hussein20172017@yahoo.com |
| Notes |
VRS: verbal rating scale
Differences between protocol and review
We did not specify which adverse events we would be analysing in the protocol. In the review we assessed nausea; vomiting; nausea and vomiting; pruritus; respiratory depression; urinary retention; allergy, rash, local reaction; bleeding; and renal dysfunction.
We assessed risk of bias due to selective reporting, but did not mention this in the protocol.
We did not add the following outcomes to our 'Summary of findings' table: global judgement of satisfaction with treatment; overall number of participants with an adverse event; and time to onset of pain relief. There were no data available for these outcomes in the included studies. We stated that we would analyze opioid consumption over various time periods; however, we did not specify what these time periods would be. In the 'Summary of findings' tables, we assessed the evidence for opioid consumption over two periods: participant stay in the post‐anaesthesia care unit; and in the period of zero to four hours after the administration of interventions. In addition, we assessed the quality of evidence for the outcome of participants experiencing bleeding, although we did not explicitly mention this in our protocol.
Contributions of authors
Searched for studies: EM, ER, TC Obtained copies of studies: EM, TC Selected which studies to include (two plus one arbiter): EM, ER, TC Extracted data from studies: EM, ER, TC Entered data into Review Manager 5: EM Carried out the analysis: EM Interpreted the analysis: EM Drafted the final review: EM Edited the final review: EM, ER
Sources of support
Internal sources
-
Saltonstall Fund for Pain Research, USA
Supports Ewan McNicol
External sources
No sources of support supplied
Declarations of interest
EM: none known. EM is a pharmacist with a Master's degree in Pain Research, Education and Policy, and manages patients with acute pain.
ER has received support from Pfizer for travel and accommodation (2015). ER is a pharmacist with a Master's degree in Pain Research, Education and Policy, and manages paediatric patients with acute pain.
TC: none known.
The protocol for this review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. A new author team fully compliant with the 2014 policy completed the review. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Chiaretti 1997 {published data only}
- Chiaretti A, Simeone E, Langer A, Butera G, Piastra M, Tortorolo L, et al. Comparison of ketorolac and fentanyl for pain relief in pediatric intensive care [Efficacia analgesica del ketorolac e del fentanyl in terapia intensiva pediatrica]. La Pediatria Medica e Chirugica 1997;19(6):419-24. [PubMed] [Google Scholar]
Davis 1999 {published data only}
- Davis PJ, Greenberg JA, Gendelman M, Fertal K. Recovery characteristics of sevoflurane and halothane in preschool-aged children undergoing bilateral myringotomy and pressure equalization tube insertion. Anesthesia and Analgesia 1999;88(1):34-8. [DOI] [PubMed] [Google Scholar]
Gunter 1995 {published data only}
- Gunter JB, Varughese AM, Harrington JF, Wittkugel EP, Patankar SS, Matar MM, et al. Recovery and complications after tonsillectomy in children: a comparison of ketorolac and morphine. Anesthesia and Analgesia 1995;81(6):1136-41. [DOI] [PubMed] [Google Scholar]
Hamza 2012 {published data only}
- Hamza A, Hayat U, Khan Q, Khan M. To compare the efficacy of ketorolac and pethidine for postoperative pain relief in first 24 hours after tonsillectomy. Pakistan Journal of Medical and Health Sciences 2012;6(2):326-8. [Google Scholar]
Lieh‐Lai 1999 {published data only}
- Lieh-Lai MW, Kauffman RE, Uy HG, Danjin M, Simpson PM. A randomized comparison of ketorolac tromethamine and morphine for postoperative analgesia in critically ill children. Critical Care Medicine 1999;27(12):2786-91. [DOI] [PubMed] [Google Scholar]
Lynn 2007 {published data only}
- Lynn AM, Bradford H, Kantor ED, Seng KY, Salinger DH, Chen J, et al. Postoperative ketorolac tromethamine use in infants aged 6-18 months: the effect on morphine usage, safety assessment, and stereo-specific pharmacokinetics. Anesthesia and Analgesia 2007;104(5):1040-51. [DOI] [PubMed] [Google Scholar]
Maunuksela 1992 {published data only}
- Maunuksela EL, Kokki H, Bullingham RE. Comparison of intravenous ketorolac with morphine for postoperative pain in children. Clinical Pharmacology & Therapeutics 1992;52(4):436-43. [DOI] [PubMed] [Google Scholar]
Munoz‐Cuevas 1997 {published data only}
- Muñoz Cuevas H, Fernández L, Carmen M, Martínez Segura RT, Zavala de la Rosa JE, Zinher Espino E. Postoperative pain control in children postoperated of ophthalmic surgery [Control del dolor con Ketorolac en niños postoperados de cirugía oftalmológica]. Revista Mexicana de Anestesiologia 1997;20(4):180-3. [Google Scholar]
Munro 2002 {published data only}
- Munro HM, Walton SR, Malviya S, Merkel S, Voepel-Lewis T, Loder RT, et al. Low-dose ketorolac improves analgesia and reduces morphine requirements following posterior spinal fusion in adolescents. Canadian Journal of Anaesthesia 2002;49(5):461-6. [DOI] [PubMed] [Google Scholar]
Romsing 1998 {published data only}
- Romsing J, Ostergaard D, Walther-Larsen S, Valentin N. Analgesic efficacy and safety of preoperative versus postoperative ketorolac in paediatric tonsillectomy. Acta Anaesthesiologica Scandinavica 1998;42(7):770-5. [DOI] [PubMed] [Google Scholar]
Saryazdi 2016 {published data only}
- Saryazdi HH, Aghadavoudi OM, Shafa AM, Masoumi AM, Saberian PA. A comparative study of the analgesic effect of intravenous pethidine vs. ketorolac after inguinal hernia surgery in children under general anesthesia. Middle East Journal of Anaesthesiology 2016;23(5):527-33. [PubMed] [Google Scholar]
Sutters 1995 {published data only}
- Sutters KA, Levine JD, Dibble S, Savedra M, Miaskowski C. Analgesic efficacy and safety of single-dose intramuscular ketorolac for postoperative pain management in children following tonsillectomy. Pain 1995;61(1):145-53. [DOI] [PubMed] [Google Scholar]
Sutters 1999 {published data only}
- Sutters KA, Shaw BA, Gerardi JA, Hebert D. Comparison of morphine patient-controlled analgesia with and without ketorolac for postoperative analgesia in pediatric orthopedic surgery. The American Journal of Orthopedics 1999;28(6):351-8. [PubMed] [Google Scholar]
References to studies excluded from this review
Glickman 1995 {published data only}
- Glickman G, Olazabal A, Corcoran J. Comparative effects of Toradol® and Ibuprofen in the control of endodontic postoperative pain. Journal of Dental Research 1995;74(Suppl):27. [Google Scholar]
Greco 1994 {published data only}
- Greco R, Piastra M, Iacovacci V, Belcastro F, Forastiere AMS, Proietti S, et al. Continuous venous infusion of buprenorphine with autonomous elastomeric system in the control of postoperative pain. Minerva Anestesiologica 1994;60(Suppl 2):1-8. [PubMed] [Google Scholar]
Gupta 2004 {published data only}
- Gupta A, Daggett C, Drant S, Rivero N, Lewis A. Prospective randomized trial of ketorolac after congenital heart surgery. Journal of Cardiothoracic and Vascular Anesthesia 2004;18(4):454-7. [DOI] [PubMed] [Google Scholar]
Hayes 2011 {published data only}
- Hayes JA, Forrest CR, Walsh W, Petroz GC, Adeli K, Bissonnette B. Continuous bupivacaine infusion post-iliac crest bone graft harvesting in pediatric cleft surgery: role and comparison with ketorolac. Cleft Palate-Craniofacial Journal 2011;48(5):532-7. [DOI] [PubMed] [Google Scholar]
Hernandez 1996 {published data only}
- Hernández Márquez VM, Toranzo Fernández JM, Guevara L, Javier F. A comparative study among ibuprofen, ketorolac and buprenorphine in postoperative pain control of third molar removal [Estudio comparativo entre ibuprofeno, buprenorfina y ketorolac en el control postoperatorio del dolor en la remoción de terceros molares]. Revista Asociación Dental Mexicana 1996;53(2):99-102. [Google Scholar]
Palacio 1997 {published data only}
- Palacio MA, Castejon J, Galvez R, Garcia-Sanchez MJ, Vazquez-Alonso E, Peran F, et al. Analgesia controlled by the patient with ketorolac in the treatment of postoperative pain in pediatric surgery [Analgesia controlada por el paciente con ketorolaco en el tratamiento del dolor postoperatorio en cirugia pediatrica]. Revista de la Sociedad Espanola del Dolor 1997;4(Suppl I):12-7. [Google Scholar]
Petrov 2009 {published data only}
- Petrov VI, Sabanov AV, Medvedev VG, Semenov PA, Tyrsin OI. Efficacy of lornoxicam and ketorolac in the prevention and treatment of postoperative pain syndrome in neurosurgical patients. Khirurgiia Moskva 2009;2:64-70. [PubMed] [Google Scholar]
Rossitto 2009 {published data only}
- Rossitto M, Pante S, Manfre A, Ciccolo A. Post-operative analgesia in case of ano-rectal diseases. Annali Italiani di Chirurgia 2009;80(6):459-61. [PubMed] [Google Scholar]
Vetter 1994 {published data only}
- Vetter TR, Heiner EJ. Intravenous ketorolac as an adjuvant to pediatric patient-controlled analgesia with morphine. Journal of Clinical Anethesia 1994;6(2):110-3. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Tariq 2004 {published data only}
- Tariq GR, Mian MA, Chaudry IA. Comparison of intraperitoneal analgesia with bupivacaine/bupivacaine with ketorolac after laparoscopic cholecystectomy. Journal of Surgery Pakistan 2004;9(3):9-13. [Google Scholar]
References to ongoing studies
NCT01667120 {published data only}
- NCT01667120. The use of ketorolac in surgical neonates. clinicaltrials.gov/ct2/show/NCT01667120 (first received 15 August 2012).
NCT02653742 {published data only}
- NCT02653742. Ketorolac sublingual vs. fentanyl intranasal in pain control for bilateral myringotomy and tubes (BMT) placement in children. clinicaltrials.gov/ct2/show/NCT02653742 (first received 8 January 2016).
NCT02973958 {published data only}
- NCT02973958. Evaluating pain outcomes of ketorolac administration in children undergoing circumcision. clinicaltrials.gov/show/NCT02973958 (first received 18 November 2016).
NCT03178539 {published data only}
- NCT03178539. Comparative study between the effect of diclofanic and ketorolac in post tonsillectomy pain management. clinicaltrials.gov/ct2/show?id=NCT01363076 (first received 5 June 2017).
Additional references
APS 2008
- American Pain Society (APS). Management of acute pain and cancer pain with analgesics. In: Principles of analgesic use in the treatment of acute pain and cancer pain. 6th edition. Glenview, IL: American Pain Society, 2008. [Google Scholar]
Baley 2014
- Baley K, Michalov K, Kossick MA, McDowell M. Intravenous acetaminophen and intravenous ketorolac for management of pediatric surgical pain: a literature review. AANA Journal 2014;82(1):53-64. [PubMed] [Google Scholar]
Bookstaver 2010
- Bookstaver PB, Miller AD, Rudisill CN, Norris LB. Intravenous ibuprofen: the first injectable product for the treatment of pain and fever. Journal of Pain Research 2010;3:67-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brasher 2014
- Brasher C, Gafsous B, Dugue S, Thiollier A, Kinderf J, Nivoche Y, et al. Postoperative pain management in children and infants: an update. Pediatric Drugs 2014;16(2):129-40. [DOI: 10.1007/s40272-013-0062-0] [DOI] [PubMed] [Google Scholar]
Chan 2014
- Chan DK, Parikh SR. Perioperative ketorolac increases post-tonsillectomy hemorrhage in adults but not children. The Laryngoscope 2014;124(8):1789-93. [DOI: 10.1002/lary.24555] [DOI] [PubMed] [Google Scholar]
Chou 2016
- Chou R, Gordon DB, Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. The Journal of Pain 2016;17(2):131-57. [DOI] [PubMed] [Google Scholar]
Cohen 2011
- Cohen MN, Christians U, Henthorn T, Vu Tran Z, Moll V, Zuk J, et al. Pharmacokinetics of single-dose intravenous ketorolac in infants aged 2-11 months. Anesthesia and Analgesia 2011;112(3):655-60. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452-4. [PMID: PMC2548824] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2014
- Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta-analyses. JAMA 2013;312:623-30. [DOI: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses [Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011]. Available from handbook.cochrane.org.
Dsida 2002
- Dsida RM, Wheeler M, Birmingham PK, Wang Z, Heffner CL, Coté CJ, et al. Age-stratified pharmacokinetics of ketorolac tromethamine in pediatric surgical patients. Anesthesia and Analgesia 2002;94(2):266-70. [DOI: 10.1213/00000539-2002020000-00007] [DOI] [PubMed] [Google Scholar]
eMC 2017
- UK Medicines and Healthcare Products Regulatory Agency (MHRA) and the European Medicines Agency (EMA). Ketorolac 30mg/ml solution for injection. In: Datapharm Communications Ltd. electronic Medicines Compendium (eMC 1999; updated 17 February 2017). www.medicines.org.uk/emc/medicine/20922 (accessed June 2018).
Forrest 1997
- Forrest JB, Heitlinger EL, Revell S. Ketorolac for postoperative pain management in children. Drug Safety 1997;16(5):309-29. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.) GRADEpro GDT. Version (accessed June 26, 2018). Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.Available at gradepro.org.
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holdsworth 2003
- Holdsworth MT, Fichtl RE, Behta M, Raisch DW, Mendez-Rico E, Adams A, et al. Incidence and impact of adverse drug events in pediatric inpatients. Archives of Pediatrics & Adolescent Medicine 2003;157(1):60-5. [DOI: 10.1001/archpedi.157.1.60] [DOI] [PubMed] [Google Scholar]
Hurst 2004
- Hurst H, Bolton J. Assessing the clinical significance of change scores recorded on subjective outcome measures. Journal of Manipulative and Physiological Therapeutics 2004;27(1):26-35. [DOI] [PubMed] [Google Scholar]
Jacox 1994
- Jacox A, Carr DB, Payne R, Berde CB, Brietbart W, Cain JM, et al. Management of Cancer Pain. Clinical Practice Guideline Number 9. AHCPR Publication no. 94-0592. Rockville, MD: Agency for Health Care Policy and Research, 1994. [Google Scholar]
Jitpakdee 2014
- Jitpakdee T, Mandee S. Strategies for preventing side effects of systemic opioid in postoperative pediatric patients. Pediatric Anesthesia 2014;24(6):561-8. [DOI: 10.1111/pan.12420] [DOI] [PubMed] [Google Scholar]
Johnston 2012
- Johnston PG, Gillam-Krakauer M, Fuller MP, Reese J. Evidence-based use of indomethacin and ibuprofen in the neonatal intensive care unit. Clinics in Perinatology 2012;39(1):111-36. [DOI: 10.1016/j.clp.2011.12.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaushal 2001
- Kaushal R, Bates DW, Landrigan C, McKenna KJ, Clapp MD, Federico F, et al. Medication errors and adverse drug events in pediatric inpatients. JAMA 2001;285(16):2114-20. [DOI: 10.1001/jama.285.16.2114] [DOI] [PubMed] [Google Scholar]
Kokki 2003
- Kokki H. Nonsteroidal anti-inflammatory drugs for postoperative pain: a focus on children. Pediatric Drugs 2003;5(2):103-23. [DOI: 10.2165/00128072-200305020-00004] [DOI] [PubMed] [Google Scholar]
Kossowsky 2015
- Kossowsky J, Donado C, Berde CB. Immediate rescue designs in pediatric analgesic trials: a systematic review and meta-analysis. Anesthesiology 2015;122(1):150-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2013
- Lewis SR, Nicholson A, Cardwell ME, Siviter G, Smith AF. Nonsteroidal anti-inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003591.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lexicomp 2015 [Computer program]
- Wolters Kluwer Clinical Drug Information, Inc Ketorolac (systemic). In: Lexicomp Online, Pediatric and Neonatal Lexi-Drugs Online, Hudson, Ohio: Lexi-Comp, Inc. Wolters Kluwer Clinical Drug Information, Inc, (accessed June 26, 2018).
Mak 2011
- Mak WY, Yuen V, Irwin M, Hui T. Pharmacotherapy for acute pain in children: current practice and recent advances. Expert Opinion in Pharamacotherapy 2011;12(6):865-81. [DOI: 10.1517/14656566.2011.542751] [DOI] [PubMed] [Google Scholar]
McGrath 2008
- McGrath PH, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, et al. Core outcome domains and measures for acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. The Journal of Pain 2008;9(9):771-83. [DOI] [PubMed] [Google Scholar]
MHRA 2007
- Medicines and Healthcare Products Regulatory Agency. Ketoprofen and ketorolac: gastrointestinal risk. www.gov.uk/drug-safety-update/ketoprofen-and-ketorolac-gastrointestinal-risk (accessed 24 January 2018).
Michelet 2012
- Michelet D, Andreu-Gallien J, Bensalah T, Hilly J, Wood C, Nivoche Y, et al. A meta-analysis of the use of nonsteroidal antiinflammatory drugs for pediatric postoperative pain. Anesthesia & Analgesia 2012;114(2):393-406. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78:209-16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15-24. [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta-analyses of osteoarthritis trials: meta-epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papacci 2004
- Papacci P, De Francisci G, Iacobucci T, Giannantonio C, De Carolis MP, Zecca E, et al. Use of intravenous ketorolac in the neonate and premature babies. Paediatric Anesthesia 2004;14(6):487-92. [DOI] [PubMed] [Google Scholar]
PaPaS 2012
- Cochrane Pain, Palliative and Supportive Care Review Group. PaPaS Author and Referee Guidance. papas.cochrane.org/papas-documents (accessed 6 November 2015).
Pasero 2011
- Pasero C, McCaffery M. Perioperative nonopioid use. In: Pain Assessment and Pharmacologic Management. St Louis, Missouri: Mosby, 2011. [Google Scholar]
Pharma Letter 1993
- Syntex' ketorolac withdrawn in Germany. In: The Pharma Letter 21 June 1993. www.thepharmaletter.com/article/syntex-ketorolac-withdrawn-in-germany (accessed 24 January 2018).
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riggin 2013
- Riggin L, Ramakrishna J, Sommer DD, Koren G. A 2013 updated systematic review & meta-analysis of 36 randomized controlled trials; no apparent effects of non steriodal anti-inflammatory agents on the risk of bleeding after tonsillectomy. Clinical Otolaryngology 2013;38(2):115-29. [DOI: 10.1111/coa.12106] [DOI] [PubMed] [Google Scholar]
Rohatgi 2016 [Computer program]
- WebPlotDigitizer. Version 3.10. Austin, Texas, USA: Ankit Rohatgi, May 2016.automeris.io/WebPlotDigitizer/.
Schultz‐Machata 2014
- Schultz-Machata AM, Weiss M, Karin B. What's new in pediatric acute pain therapy? Current Opinion in Anaesthesiology 2014;27(3):316-22. [DOI: 10.1097/ACO.0000000000000074] [DOI] [PubMed] [Google Scholar]
Somaini 2015
- Somaini M, Sahillioğlu E, Marzorati C, Lovisari F, Engelhardt T, Ingelmo PM. Emergence delirium, pain or both? A challenge for clinicians. Paediatric Anesthesia 2015;25(5):524-9. [DOI] [PubMed] [Google Scholar]
Standing 2009a
- Standing JF, Ooi K, Keady S, Howard RF, Savage I, Wong ICK. Prospective observational study of adverse reactions to diclofenac in children. British Journal of Clinical Pharmacology 2009;68(2):243-51. [DOI: 10.1111/j.1365-2125.2009.03447.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Standing 2009b
- Standing JF, Savage I, Pritchard D, Waddington M. Diclofenac for acute pain in children. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005538.pub2] [DOI] [PubMed] [Google Scholar]
Stanko 2013
- Stanko D, Bergesio R, Davies K, Hegarty M, Ungern-Sternberg BS. Postoperative pain, nausea and vomiting following adeno-tonsillectomy - a long-term follow-up. Pediatric Anesthesia 2013;23(8):690-6. [DOI] [PubMed] [Google Scholar]
Takata 2008
- Takata GS, Mason W, Taketomo C, Logsdon T, Sharek PJ. Development, testing, and findings of a pediatric-focused trigger tool to identify medication-related harm in US children's hospitals. Pediatrics 2008;121(4):e927-35. [DOI: 10.1542/peds.2007-1779] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta-analysis - a simulation study. PLOS One 2011;6:e25491. [DOI: 10.1371/journal.pone.0025491] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tobias 2014
- Tobias JD. Acute pain management in infants and children - Part 2: Intravenous opioids, intravenous nonsteroidal anti-inflammatory drugs, and managing adverse effects. Pediatric Annals 2014;43(7):e169-75. [DOI] [PubMed] [Google Scholar]
Voepel‐Lewis 2008
- Voepel-Lewis T, Marinkovic A, Kostrzewa A, Tait A, Malviya S. The prevalence of an risk factors for adverse events in children receiving patient-controlled analgesia by proxy or patient-controlled analgesia after surgery. Anesthesia and Analgesia 2008;107(1):70-5. [DOI: 10.1213/ane.0b013e318172fa9e] [DOI] [PubMed] [Google Scholar]
Voepel‐Lewis 2012
- Voepel-Lewis T, Wagner D, Burke C, Tait AR, Hemberg J, Pechlivanidis E, et al. Early adjuvant use of nonopioids associated with reduced odds of serious postoperative opioid adverse events and need for rescue in children. Pediatric Anesthesia 2012;23(2):162-9. [DOI: 10.1111/pan.12026] [DOI] [PubMed] [Google Scholar]


