Abstract
Background
The use of anaesthetics in the elderly surgical population (more than 60 years of age) is increasing. Postoperative delirium, an acute condition characterized by reduced awareness of the environment and a disturbance in attention, typically occurs between 24 and 72 hours after surgery and can affect up to 60% of elderly surgical patients. Postoperative cognitive dysfunction (POCD) is a new‐onset of cognitive impairment which may persist for weeks or months after surgery.
Traditionally, surgical anaesthesia has been maintained with inhalational agents. End‐tidal concentrations require adjustment to balance the risks of accidental awareness and excessive dosing in elderly people. As an alternative, propofol‐based total intravenous anaesthesia (TIVA) offers a more rapid recovery and reduces postoperative nausea and vomiting. Using TIVA with a target controlled infusion (TCI) allows plasma and effect‐site concentrations to be calculated using an algorithm based on age, gender, weight and height of the patient.
TIVA is a viable alternative to inhalational maintenance agents for surgical anaesthesia in elderly people. However, in terms of postoperative cognitive outcomes, the optimal technique is unknown.
Objectives
To compare maintenance of general anaesthesia for elderly people undergoing non‐cardiac surgery using propofol‐based TIVA or inhalational anaesthesia on postoperative cognitive function, mortality, risk of hypotension, length of stay in the postanaesthesia care unit (PACU), and hospital stay.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 11), MEDLINE (1946 to November 2017), Embase (1974 to November 2017), PsycINFO (1887 to November 2017). We searched clinical trials registers for ongoing studies, and conducted backward and forward citation searching of relevant articles.
Selection criteria
We included randomized controlled trials (RCTs) with participants over 60 years of age scheduled for non‐cardiac surgery under general anaesthesia. We planned to also include quasi‐randomized trials. We compared maintenance of anaesthesia with propofol‐based TIVA versus inhalational maintenance of anaesthesia.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data, assessed risk of bias, and synthesized findings.
Main results
We included 28 RCTs with 4507 randomized participants undergoing different types of surgery (predominantly cardiovascular, laparoscopic, abdominal, orthopaedic and ophthalmic procedures). We found no quasi‐randomized trials. Four studies are awaiting classification because we had insufficient information to assess eligibility.
All studies compared maintenance with propofol‐based TIVA versus inhalational maintenance of anaesthesia. Six studies were multi‐arm and included additional TIVA groups, additional inhalational maintenance or both. Inhalational maintenance agents included sevoflurane (19 studies), isoflurane (eight studies), and desflurane (three studies), and was not specified in one study (reported as an abstract). Some studies also reported use of epidural analgesia/anaesthesia, fentanyl and remifentanil.
We found insufficient reporting of randomization methods in many studies and all studies were at high risk of performance bias because it was not feasible to blind anaesthetists to study groups. Thirteen studies described blinding of outcome assessors. Three studies had a high of risk of attrition bias, and we noted differences in the use of analgesics between groups in six studies, and differences in baseline characteristics in five studies. Few studies reported clinical trials registration, which prevented assessment of risk of selective reporting bias.
We found no evidence of a difference in incidences of postoperative delirium according to type of anaesthetic maintenance agents (odds ratio (OR) 0.59, 95% confidence interval (CI) 0.15 to 2.26; 321 participants; five studies; very low‐certainty evidence); we noted during sensitivity analysis that using different time points in one study may influence direction of this result. Thirteen studies (3215 participants) reported POCD, and of these, six studies reported data that could not be pooled; we noted no difference in scores of POCD in four of these and in one study, data were at a time point incomparable to other studies. We excluded one large study from meta‐analysis because study investigators had used non‐standard anaesthetic management and this study was not methodologically comparable to other studies. We combined data for seven studies and found low‐certainty evidence that TIVA may reduce POCD (OR 0.52, 95% CI 0.31 to 0.87; 869 participants).
We found no evidence of a difference in mortality at 30 days (OR 1.21, 95% CI 0.33 to 4.45; 271 participants; three studies; very low‐certainty evidence). Twelve studies reported intraoperative hypotension. We did not perform meta‐analysis for 11 studies for this outcome. We noted visual inconsistencies in these data, which may be explained by possible variation in clinical management and medication used to manage hypotension in each study (downgraded to low‐certainty evidence); one study reported data in a format that could not be combined and we noted little or no difference between groups in intraoperative hypotension for this study. Eight studies reported length of stay in the PACU, and we did not perform meta‐analysis for seven studies. We noted visual inconsistencies in these data, which may be explained by possible differences in definition of time points for this outcome (downgraded to very low‐certainty evidence); data were unclearly reported in one study. We found no evidence of a difference in length of hospital stay according to type of anaesthetic maintenance agent (mean difference (MD) 0 days, 95% CI ‐1.32 to 1.32; 175 participants; four studies; very low‐certainty evidence).
We used the GRADE approach to downgrade the certainty of the evidence for each outcome. Reasons for downgrading included: study limitations, because some included studies insufficiently reported randomization methods, had high attrition bias, or high risk of selective reporting bias; imprecision, because we found few studies; inconsistency, because we noted heterogeneity across studies.
Authors' conclusions
We are uncertain whether maintenance with propofol‐based TIVA or with inhalational agents affect incidences of postoperative delirium, mortality, or length of hospital stay because certainty of the evidence was very low. We found low‐certainty evidence that maintenance with propofol‐based TIVA may reduce POCD. We were unable to perform meta‐analysis for intraoperative hypotension or length of stay in the PACU because of heterogeneity between studies. We identified 11 ongoing studies from clinical trials register searches; inclusion of these studies in future review updates may provide more certainty for the review outcomes.
Plain language summary
Injected versus inhaled medicines to maintain general anaesthesia during non‐cardiac surgery for cognitive outcomes in elderly people
Background
Anaesthesia during surgery in elderly people (more than 60 years of age) is increasing.
Traditionally, general anaesthesia is maintained with an inhaled drug (a vapour which the patient breathes in) which needs to be adjusted to ensure that the patient remains unconscious during surgery without receiving too much anaesthetic. An alternative method is to use propofol which is injected into a vein throughout the anaesthetic procedure; this is called total intravenous anaesthesia (TIVA).
Elderly people are more likely to experience confusion or problems with thinking following surgery, which can occur up to several days postoperatively. These cognitive problems can last for weeks or months, and can affect the patients' ability to plan, focus, remember, or undertake activities of daily living. We looked at two types of postoperative confusion: delirium (a problem with awareness and attention which is often temporary) and cognitive dysfunction (a persistent problem with brain function).
TIVA with propofol may be a good alternative to inhaled drugs, and it is known that patients who have TIVA experience less nausea and vomiting, and wake up more quickly after anaesthesia. However, it is unknown which is the better anaesthetic technique in terms of postoperative cognitive outcomes.
Review question
To compare maintenance of general anaesthesia for elderly people undergoing non‐cardiac surgery using TIVA or inhalational anaesthesia on postoperative cognitive function, number of deaths, risk of low blood pressure during the operation, length of stay in the postanaesthesia care unit (PACU), and hospital stay.
Study characteristics
The evidence is current to November 2017. We included 28 randomized studies with 4507 participants in the review. We are awaiting sufficient information for the classification of four studies.
All studies included elderly people undergoing non‐cardiac surgery and compared use of propofol‐based TIVA versus inhalational agents during maintenance of general anaesthesia.
Key results
We found little or no difference in postoperative delirium according to the type of anaesthetic maintenance agents from five studies (321 participants). We found that fewer people experienced postoperative cognitive dysfunction when TIVA with propofol was used in seven studies (869 participants). We excluded one study from analysis of this outcome because study authors had used methods to anaesthetize people which were not standard.
We found little or no difference in the number of deaths from three studies (271 participants). We did not combine data for low blood pressure during the operation or length of stay in the PACU because we noted differences in studies, which may be explained by differences in patient management (for low blood pressure), and differences in how length of stay in the PACU is defined in each study . We found little or no difference in length of hospital stay from four studies (175 participants).
Quality of the evidence
Many studies did not report randomization methods adequately and all studies were at high risk of bias from anaesthetists, who needed to be aware of which anaesthetic agent they used. Outcome assessors in some studies were aware of which study group participants were in. We noted a large loss of participants in three studies, and some studies had differences between groups in the types of drugs used for pain, the types of monitors used to assess how deeply‐unconscious the patients were, and participant characteristics at the start of the studies; these factors may have influenced the results. Few studies had reported clinical trials registration. We found few studies for two outcomes (mortality and length of hospital stay), which made the results less precise. We judged evidence for postoperative delirium, number of deaths, length of stay in the PACU, and length of hospital stay to be very low certainty, and evidence for postoperative cognitive dysfunction, and low blood pressure during the operation to be low certainty.
TIVA with propofol may reduce postoperative cognitive dysfunction. We are uncertain whether the choice of anaesthetic agents (TIVA with propofol, or inhalational agents) affects postoperative delirium, mortality and length of hospital stay. We found 11 ongoing studies in database and clinical trials register searches. Inclusion of these studies in future review updates will provide more certainty for the review outcomes.
Summary of findings
Summary of findings for the main comparison. Summary of findings TIVA versus inhalational maintenance of anaesthesia.
| Intravenous maintenance of anaesthesia compared with inhalational maintenance of anaesthesia in elderly people undergoing non‐cardiac surgery | ||||||
|
Participants: elderly people, aged 60 years and above, undergoing non‐cardiac surgery under general anaesthesia Settings: hospitals in: Belgium, Canada, China, Egypt, France, Germany, Greece, Ireland, Japan, Norway, South Korea, Spain, Sweden, Turkey, UK, USA Intervention: intravenous maintenance of anaesthesia with: propofol Comparison: inhalational maintenance of anaesthesia with: sevoflurane, isoflurane, or desflurane | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with Inhalational maintenance | Risk with TIVA | |||||
|
Postoperative delirium (One study used DRS, three studies used CAM and in one study diagnostic tool was not reported) Time points were up to 4 days postoperatively |
Study population | OR 0.59 (0.15 to 2.26) | 321 (5 studies) | very lowa | ||
| 61 per 1,000 | 37 per 1,000 (10 to 129) | |||||
|
Postoperative cognitive dysfunction (9 studies used MMSE, and 2 of these studies used additional diagnostic tools; 1 study used Trail Making Test and additional diagnostic tools; 3 studies did not report diagnostic tools) Time points were up to 30 days postoperatively |
Study population | OR 0.52 (0.31 to 0.87) | 869 (7 studies) | lowb | Overall, 13 studies (3215 participants) reported data for this outcome. We performed meta‐analysis on 7 studies. We excluded 1 large study from this analysis which used non‐standard anaesthetic management. 5 studies reported data in formats that could not be combined. Of these 5: we noted no apparent differences in mean MMSE scores in 3 studies; 1 study reported similar scores in each group; 1 study included data at 2 years and was not comparable with our other data |
|
| 285 per 1,000 | 172 per 1,000 (110 to 257) | |||||
|
Mortality At 30 days |
Study population | OR 1.21, (95% CI 0.33 to 4.45) | 271 (3 studies) | very lowc | Overall, 4 studies reported mortality. We did not include 1 study in analysis because number of deaths (3 in total) were not reported by group. | |
| 29 per 1,000 | 35 per 1,000 (10 to 119) | |||||
|
Intraoperative hypotension (defined by study authors as change in MAP from baseline) |
‐ | See comment | ‐ | 1145 (12 studies) | lowd | Overall, 12 studies (1145 participants) reported intraoperative hypotension. 1 study reported data in a format that could not be combined with other study data (we noted little or no apparent difference in hypotension in this study). We did not pool data in 11 studies; we noted inconsistencies in visual inspection of the data which could be explained by variation in clinical management and medication used to manage hypotension in each study |
|
Length of stay in PACU (measured in minutes) |
‐ | see comment | ‐ | 567 (8 studies) | very lowe | We did not pool data in seven studies: we noted inconsistencies in visual inspection of the data and we expected that studies used different definitions of time points to assess length of time in the PACU. Data were unclearly reported in one study |
|
Length of hospital stay (measured in days) |
‐ | MD 0 days higher (1.32 days lower to 1.32 days higher) | ‐ | 175 (4 studies) | very lowf | Overall, 6 studies (375 participants) reported data for this outcome. Of 4 combined studies, mean scores in the inhalational maintenance group ranged from 1.3 days to 15 days. 2 studies reported data that could not be combined with other studies (we noted little or no difference in median length of stay between groups). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CAM: Confusion Assessment Method; CI: confidence interval; DRS: Delirium Rating Scale; MAP: mean arterial pressure; MD: mean difference; MMSE: Mini‐Mental State Examination; OR: odds ratio; PACU: postanaesthesia care unit | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aWe downgraded by one level for study limitations; we noted few included studies for this outcome had sufficiently reported methods of randomization and we were concerned by high risk of attrition bias in two studies and high risk of selective outcome reporting bias in one study. We downgraded by two levels for inconsistency; we could not be certain whether measurements of delirium, and time points of measurement, were equivalent between studies, and we used sensitivity analysis to show that choice of time point in one study may influence direction of this result
bWe downgraded by one level for study limitations; we noted that some studies had insufficiently reported methods of randomization and we were concerned by high risk of attrition bias in one study. We downgraded by one level for inconsistency; we noted a moderate level of statistical heterogeneity (I² = 41%) which we were unable to explain in subgroup analysis
cWe downgraded by one level for study limitations; we noted that some studies had insufficiently reported methods of randomization. Analysis included few studies with few participants and, because deaths due to anaesthesia are rare we would require a large sample size to show evidence of a difference; we downgraded by two levels for imprecision.
dWe downgraded by one level for study limitations; we noted some studies reported insufficient methods of randomization. We downgraded by one level for inconsistency because of statistical heterogeneity (I² = 63%) and noted differences in visual inspection of results; this could be explained by possible variation in clinical management and medication used to manage hypotension in each study
eWe downgraded by one level for study limitations; we noted some studies reported insufficient methods of randomization. We downgraded by two levels for inconsistency; we noted substantial statistical heterogeneity (I2 = 94%) and differences in visual inspection of results which may be explained by likely differences in study designs related to definitions of time points of measurement for this outcome
fFew studies with few participants; we downgraded by two levels for imprecision. We noted a moderate level of statistical heterogeneity (I2 = 41%) and noted differences in visual inspection of results; we downgraded by one level for inconsistency
Background
Description of the condition
There are an estimated 187 million to 281 million surgical procedures worldwide each year (Weiser 2008). Alongside an aging population, the global use of anaesthetics in the elderly (> 60 years of age) is increasing (Mandal 2009). Surgery and anaesthesia have a pronounced effect on elderly people, which can result in an increased risk of postoperative confusion and functional decline (Rundshagen 2014). Complications such as these have adverse effects on postoperative recovery and are associated with an increased length of hospital stay and an increased risk of mortality. It is hypothesized that the direct effect of anaesthesia on the brain, hypotension, and hypoxia may all have an influence on their development (Ballard 2012; Wang 2015).
Postoperative delirium is an acute condition, characterized by reduced awareness of the environment and a disturbance in attention (Deiner 2009). It typically occurs between 24 and 72 hours after surgery, following an initial lucid phase (Ballard 2012). It is thought to occur in around 10% of elderly patients (Rudolph 2011), although this can rise to 60% following certain types of surgery, such as hip fracture fixation (Ansaloni 2010; Bitsch 2004). Postoperative delirium is a defined condition according to the International Classification of Diseases (WHO 2016a), and there are a number of validated tools to assist in diagnosis and severity scoring, such as the confusion assessment method (CAM) (Inouye 1990).
Postoperative cognitive dysfunction is characterized by a chronic reduction in cognitive function, lasting weeks or months, compared with an individual’s normal cognitive state (Newman 2007). It presents a diagnostic challenge as it has not been formally defined and diagnostic criteria are yet to be developed, but can include changes to circadian rhythm, psychomotor state, and memory deficit. The incidence of postoperative cognitive dysfunction varies depending on the surgery type and the definition of postoperative cognitive dysfunction used (Krenk 2011); it is associated with an inability to return to normal lifestyle following surgery (Monk 2005; Steinmetz 2016).
Description of the intervention
There are three phases involved in the provision of general anaesthesia: induction, maintenance, and emergence. Induction of anaesthesia is often undertaken using intravenous (IV) agents, typically propofol. This has the advantage of rapid onset, and therefore airway control can be quickly obtained. Inhalational induction of anaesthesia (which may be given at high or low initial concentrations; Boonmak 2016), using a non‐irritant volatile agent such as sevoflurane is an alternative which, though slower in onset, offers benefits in terms of the maintenance of spontaneous ventilation and increased cardiovascular stability. In many patients, anaesthesia is maintained by the inhalation of volatile agents (typically sevoflurane, desflurane, or isoflurane, historically also enflurane and halothane). The alternative technique for the maintenance of anaesthesia is the continuous administration of an IV infusion of an anaesthetic drug, typically propofol. This is known as total intravenous anaesthesia (TIVA). Neither maintenance technique provides analgesia, and this may be co‐administered through a variety of techniques which may be used in combination. These include boluses or an infusion of opioid medication, the inhalation of nitrous oxide, or regional anaesthetic techniques. In this review, we will compare inhalational anaesthesia involving maintenance with sevoflurane, desflurane, isoflurane, or halothane, with or without nitrous oxide (Hounsome 2016), (referred to as inhalational anaesthesia) with propofol‐based TIVA (referred to as TIVA).
How the intervention might work
The mechanism of action of anaesthetic agents has not been fully elucidated. However, it is known that both IV and inhalational agents act at multiple receptor sites within the central nervous system to reduce neuronal activity (Koblin 2000). Both propofol and volatile agents are thought to act predominantly though the activation of the gamma‐aminobutyric acid (GABA)‐A receptor, with variable effects on other receptors. Of these, the nicotinic acetylcholine receptor may be of particular relevance to the subject of this review, as it has a role in cognition, and is inhibited by volatile agents at therapeutic levels, but by propofol only in high doses (Fodale 2010).
Inhalational anaesthesia has been associated with lower rates of postoperative cognitive dysfunction in the setting of cardiac surgery (Royse 2011; Schoen 2011), and inhalational induction has been shown to induce less hypotension than IV induction (Luntz 2004; Thwaites 1997). In inhalational anaesthesia, the end‐tidal concentration of anaesthetic agent is measured and this can be compared to a known value at which 50% of patients move in response to a standard surgical stimulus, known as the minimum alveolar concentration (MAC). In order to prevent awareness, it is suggested that the end‐tidal volatile concentration should exceed 0.7 MAC (Pandit 2013). MAC is age‐dependant, decreasing with advancing age, and should therefore be adjusted using nomograms or algorithms in order to reduce the risk of excessive dosing in the elderly population (Griffiths 2014).
There are a number of proposed benefits to the use of TIVA, including a more rapid recovery and a decreased incidence of postoperative nausea and vomiting (Weilbach 2005). However, propofol is associated with hypotension, thought to be mediated by the inhibition of sympathetic outflow, and this may be particularly pronounced in the elderly or those with cardiovascular disease (Robinson 1997). In TIVA, the anaesthetic agent is not measured, but the plasma and effect‐site concentration may be calculated using an algorithm built in to the infusion pump; the anaesthetic can then be administered to a target effect‐site concentration, and this is known as a target‐controlled infusion (TCI). The algorithm is dependant on the gender, age, height, and weight of the patient, but is less reliable in certain patient groups, including the elderly. As the concentration of anaesthetic agent is calculated rather than measured, it has been proposed that the depth of anaesthesia should be monitored using electroencephalogram (EEG)‐based devices in patients undergoing TIVA in order to reduce the risk of accidental awareness (Checketts 2016).
Monitors of anaesthetic depth have been widely available for some years. They enable titration of dose of general anaesthetic both to avoid unnecessarily high doses and also the risk of accidental awareness if too little anaesthetic is given (Chhabra 2016; Messina 2016; Punjasawadwong 2014). The use of EEG‐based depth of anaesthesia monitoring in the elderly population, in order to minimize the risk of the administration of excessive doses of sedative or anaesthetic agents, has been shown to reduce the incidence of postoperative cognitive complications and hypotension (Ballard 2012; Chan 2013; Sieber 2010). As a result of this, its use is advocated for general anaesthesia for the elderly, regardless of technique, in national and international guidelines (Griffiths 2014; NICE 2012).
Why it is important to do this review
Traditionally, surgical anaesthesia has been maintained with inhalational agents, however the introduction of new technologies has made IV maintenance a viable alternative technique which presents a number of possible advantages. In terms of postoperative cognitive outcomes, the optimal technique remains unknown. This review aims to help identify the anaesthetic technique that is optimal for elderly surgical patients in terms of postoperative cognitive function, cardiovascular stability, mortality, and length of stay in hospital in order to optimize the use of healthcare resources and reduce the overall healthcare costs.
Objectives
To compare maintenance of general anaesthesia for elderly people undergoing non‐cardiac surgery using propofol‐based TIVA or inhalational anaesthesia on postoperative cognitive function, mortality, risk of hypotension, length of stay in the postanaesthesia care unit (PACU), and hospital stay.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs), and aimed to include quasi‐randomized studies (for example, in which the method of assignment is by alternation, date of birth, or medical record number).
Types of participants
The United Nations defines the older population as 60 years of age and above (WHO 2016b). We therefore included participants aged 60 years and above, undergoing surgery under general anaesthesia. We excluded participants undergoing cardiac surgery due to the differences in the provision of general anaesthesia whilst on bypass, and the additional risk of postoperative cognitive complications associated with extracorporal support. If studies included participants less than 60 years of age, we included the study if it was possible to identify the ratio of participants who were more than 60 years of age; if the ratio was more than 75%, and this was distributed evenly between intervention groups, we included these studies.
Types of interventions
We included studies that compared maintenance of anaesthesia with propofol‐based TIVA versus inhalational anaesthesia. Comparisons of inhalational maintenance anaesthesia included both inhalational and IV induction of anaesthesia.
Types of outcome measures
We aimed to establish if one type of maintenance of anaesthesia reduces postoperative delirium and postoperative cognitive dysfunction in participants, as these are associated with both an increased length of hospital stay and risk of mortality. Our secondary outcomes establish if one method reduces the incidence of hypotension (a proposed cause of postoperative delirium and postoperative cognitive dysfunction), mortality, length of stay in the PACU, and overall hospital admission time, as these have significant cost implications to healthcare settings.
We excluded studies that did not measure any of the review outcomes. See Differences between protocol and review.
Primary outcomes
Postoperative delirium; as measured by a validated tool or diagnostic criteria, e.g. Diagnostic and Statistical Manual of Mental Disorders (DSM‐5 2013), confusion assessment method (CAM) (Inouye 1990), International Classification of Diseases‐10 (WHO 2016a).
Postoperative cognitive dysfunction; as defined and measured by the study authors.
Secondary outcomes
Mortality at 30 days.
Intraoperative hypotension as defined by the study authors (for example, mean arterial pressure (MAP) < 65 mmHg, drop in MAP > 20% from baseline value).
Length of stay in the PACU (measured as minutes).
Length of hospital stay (measured as days).
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011). We applied no restrictions to language or publication status. We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 11)
MEDLINE (Ovid SP, 1946 to 20 November 2017)
Embase (Ovid SP, 1974 to 20 November 2017)
PsycINFO (EBSCO, 1887 to 21 November 2017)
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other listed databases. The search strategy was developed in consultation with the Information Specialist. Search strategies can be found in Appendix 1, Appendix 2, Appendix 3, Appendix 4.
We scanned the following trials registries for ongoing and unpublished trials (20 November 2017).
The World Health Organization International Clinical Trials Registry Platform (WHOICTRP) (who.int/ictrp/network/en)
Searching other resources
We carried out citation searching of identified included studies in Web of Science (apps.webofknowledge.com), and Google Scholar (scholar.google.co.uk), on 23 November 2017 and conducted a search of grey literature through ’Opengrey’ (www.opengrey.eu./), on 5 December 2017. We carried out backward citation searching of key reviews identified from the searches.
Data collection and analysis
Two review authors (SRL and DM, OSR, or MP) independently assessed trial quality and extracted data. Consensus was reached through discussion. We used standard Cochrane methodological procedures, including assessment of risk of bias for all studies.
Selection of studies
We used reference management software to collate the results of the searches and to remove duplicates (Endnote 2011). We used Covidence software to screen the results of the search from the titles and abstracts and identify any potentially relevant studies from this information alone (Covidence 2016). We sourced the full texts of all those potentially relevant studies and considered whether they met the inclusion criteria. We included abstracts at this stage. However, we only included these in the review if they contained sufficient information and relevant results that included denominator figures for each intervention/comparison group. We recorded the number of papers retrieved at each stage and reported this using a PRISMA flow chart (Moher 2009). We reported brief details of closely‐related, but excluded papers in the review.
Data extraction and management
We used Covidence software to extract data from individual studies (Covidence 2016). A basic template of the data extraction forms are available at www.covidence.org. We adapted the template to include the following information.
Methods: type of study design, setting, dates of study, funding sources.
Participants: number randomized to each group, baseline characteristics (age, urgency of surgery, American Society of Anesthesiologists (ASA) grade and type of surgery).
Intervention: details of anaesthetic techniques (induction technique, type of volatile agents used, use of depth of anaesthesia monitoring, dose of anaesthetic agents given (i.e. minimum alveolar concentration (MAC)/target‐controlled infusion (TCI)/manual infusion), use and dose of concomitant drugs (i.e. analgesics, anticholinergics, antiemetics, hypnotics, vasoactive drugs), use of regional anaesthesia in addition to general anaesthesia).
Outcomes: data for all reported review outcomes to include study author definitions, measurement tools, and time points.
We considered the applicability of information from individual studies and generalizability of the data to our intended study population (i.e. the potential for indirectness in our review). If there were associated publications from the same study, we created a composite data set from all the eligible publications.
Assessment of risk of bias in included studies
We assessed study quality, study limitations, and the extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We considered the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants, personnel, and outcomes assessors (performance and detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other ‐ use of concomitant drugs.
It is not feasible to blind personnel to the study intervention, and we acknowledge that this introduces an unavoidable risk of performance bias in any eligible study. However, it is feasible for outcome assessors to be blinded for all outcomes, except hypotension. In addition to the standard risk of bias domains, we also collected data on the use of concomitant drugs such as opiate analgesics, anticholinergics, antiemetics, and benzodiazapines, which are known or suspected to increase the risk of delirium (Clegg 2011).
For each domain, two review authors (SRL and DM, OSR, or MP) judged whether study authors made sufficient attempts to minimize bias in their study design. We made judgements using three measures ‐ high, low, or unclear risk of bias. We recorded this in 'Risk of bias' tables and presented a summary 'Risk of bias' figure.
Measures of treatment effect
We collected dichotomous data for 30‐day mortality. We anticipated that postoperative delirium and postoperative cognitive dysfunction would be measured using a scale, either validated (e.g. CAM) or determined by the study authors. We planned to establish an appropriate cut‐off on such scales (delirium versus no delirium), so that the data could be recorded as dichotomous. We recorded data for hypotension as dichotomous using cut‐offs defined by the study authors. We collected length of recovery in the PACU and length of hospital stay as continuous data.
Unit of analysis issues
It was possible that studies may have compared TIVA against different anaesthetic induction and maintenance strategies in multi‐arm study designs. For example, TIVA could be compared against an IV induction with inhalational maintenance, and also against an inhalational induction with inhalational maintenance within the same study. For our primary analysis, we combined the two comparison groups for comparison with TIVA. In subgroup analysis, however, we analysed these comparison groups separately against TIVA, and used the 'halving' method for the TIVA group to ensure that no double‐counting occurred (Higgins 2011).
Dealing with missing data
In the event that study authors reported loss of participants during follow‐up, we did not impute values but reported data as analysed by study authors. We used sensitivity analysis to explore the effect of including studies with high risk of attrition bias. See Differences between protocol and review, and sensitivity analysis in Effects of interventions.
Assessment of heterogeneity
We assessed whether there was evidence of inconsistency within our results through consideration of heterogeneity. We assessed clinical heterogeneity by comparing similarities between the participants, the interventions, and outcomes in our included studies. We assessed statistical heterogeneity by calculation of the Chi2 (with an associated P value) or I2 statistic (with an associated percentage). We judged any heterogeneity above 60% as a reason not to pool the data, unless we considered the heterogeneity to be not clinically important. As well as looking at the statistical results, we considered the point estimates and the overlap of confidence intervals (CIs). If the CIs overlap, then the results are more consistent. However, it is also possible for combined studies to show a large consistent effect, but with significant heterogeneity. We therefore interpreted heterogeneity with caution (Guyatt 2011a).
Assessment of reporting biases
We attempted to source published protocols for each of our included studies using clinical trials registers. We compared published protocols with published study results to assess the risk of selective reporting bias. If there were sufficient studies, i.e. more than 10 (Higgins 2011), we planned to generate a funnel plot to assess the risk of publication bias in the review; an asymmetric funnel plot may indicate potential publication of only positive results (Egger 1997).
Data synthesis
We completed a meta‐analysis for outcomes for which we had comparable effect measures from more than one study, and where measures of heterogeneity indicated that pooling of results was appropriate. We used the statistical calculator in Review Manager 5 (Review Manager 2014).
For dichotomous outcomes, for example, mortality rate, we calculated the odds ratio (OR) using the summary data presented in each trial. We used the Mantel‐Haenszel effects model, unless events were extremely rare (1 per 1000), in which case we planned to use the Peto method (Higgins 2011). For continuous outcomes, for example, length of hospital stay, we used mean difference (MD). We used a random‐effects statistical model which allowed for differences between studies (for example, because of different types of surgery (Borenstein 2010).
We calculated CIs at 95% and used a P value of 0.05 or below to judge if a result was statistically significant. We considered whether there was imprecision in the results of analysis by assessing the CI around the relative effects measure; a wide CI suggested a higher level of imprecision in our results. A small number of studies may also reduce the precision (Guyatt 2011b).
Subgroup analysis and investigation of heterogeneity
We undertook a subgroup analysis when there were sufficient studies that reported the relevant characteristic (Higgins 2011). We used RevMan 5 to calculate differences in subgroups, based on the test for heterogeneity Chi2 statistics (Review Manager 2014); we used a P value ≥ 0.05 to indicate a statistically significant difference between subgroups.
The United Nations' definition of old age is over 60 years, however many surgical patients in early old age (under 80 years of age) are fit with few comorbidities, whilst patients 80 years of age and over are at an increased risk of adverse outcomes (NCEPOD 2010). Other sources of potential heterogeneity include the urgency of surgery, with non‐elective surgery being associated with an increased risk of postoperative cognitive problems (Raats 2015), and the use of depth of anaesthesia monitoring, which is associated with a reduction in intra‐ and postoperative complications (Ballard 2012; Chan 2013). We also used subgroup analysis to explore differences in results for the inhalational maintenance group, in which induction was undertaken using either inhalational or IV agents. We only conducted a subgroup analysis based on information presented in the written paper. In summary, subgroups were:
elderly (60 to 79 years of age) versus late elderly (80 years of age or older);
elective versus non‐elective surgery;
inhalational induction versus IV induction (as a subgroup of inhalational maintenance only);
TCI versus non‐TCI maintenance of anaesthesia (as a subgroup of TIVA only); and
use of depth of anaesthesia monitoring.
Sensitivity analysis
We explored the potential effects of decisions made as part of the review process in the following way.
We excluded all studies that we judged to be at high or unclear risk of selection bias.
We excluded studies that we judged to have a high risk of attrition bias because of missing data for a large number of participants that were unevenly distributed or unclearly reported between groups. See Differences between protocol and review.
We conducted a meta‐analysis using the alternate meta‐analytic effects model (fixed‐effect or random‐effects).
We compared effect estimates from the above results with effect estimates from the main analysis. We reported differences that altered interpretation of the effect.
'Summary of findings' tables and GRADE
The GRADE Working Group approach incorporates assessment of indirectness, study limitations, inconsistency, publication bias, and imprecision (Atkins 2004). We made these assessments at each stage of our analysis detailed above (Data collection and analysis; Assessment of risk of bias in included studies; Assessment of heterogeneity; Assessment of reporting biases; Data synthesis). This approach gives an overall measure of how confident we can be that our estimate of effect is correct (Guyatt 2008).
We used the principles of the GRADE system to give an overall assessment of the evidence relating to each of the following outcomes: postoperative delirium, postoperative cognitive dysfunction, mortality within 30 days, intraoperative hypotension, length of stay in the PACU, and overall hospital length of stay. We assessed the certainty of the evidence using one of four judgements (high, moderate, low, and very low).
One review author (SL) used the GRADEpro software to create a 'Summary of findings' table for each comparison (GRADEpro GDT). Consensus was reached with a second author (MP) who checked the table and approved judgements.
Results
Description of studies
Results of the search
We screened 12,313 titles and abstracts from database searches, results from clinical trials register searches, grey literature searches, and forward and backward citation searches. We carried out full‐text review of 440 articles. We excluded 397 studies, and reported details of 46 of these excluded studies. We identified 28 eligible studies, and 11 ongoing studies. We found four studies awaiting classification; we had insufficient information to assess review eligibility for these studies. See Figure 1.
1.
Study flow diagram
Included studies
We included 28 parallel design randomized controlled trials (Ammar 2016; Biboulet 2012; Cai 2012a; Celik 2011; Chan 1996; Demeere 2006; Egawa 2016; Epple 2001; Geng 2017; Gursoy 2015; Ishii 2016; Jellish 2003; Juvin 1997; Kim 2015a; Lindholm 2013; Liu 2013; Longas 2004; Luntz 2004; Micha 2016; Moffat 1995; Nishikawa 2004; Rohan 2005; Tan 2009; Tanaka 2017; Tang 2014; Trembach 2012; Tylman 2011; Zhang 2015). We sourced no quasi‐randomized studies. Included studies had an assumed total of 4507 randomized participants; two studies reported number of participants unclearly and we assumed totals from other data in the study reports (Jellish 2003; Longas 2004). One included study was an abstract with sufficient information regarding number of participants in each group and relevant outcome data (Trembach 2012). See Characteristics of included studies.
Study population and setting
Twenty‐one studies specifically included elderly participants (Biboulet 2012; Cai 2012a; Celik 2011; Chan 1996; Epple 2001; Geng 2017; Gursoy 2015; Ishii 2016; Juvin 1997; Kim 2015a; Liu 2013; Luntz 2004; Micha 2016; Moffat 1995; Nishikawa 2004; Rohan 2005; Tan 2009; Tanaka 2017; Tang 2014; Trembach 2012; Zhang 2015). Seven studies did not report inclusion of elderly participants and we used mean ages reported in the baseline characteristics table to ascertain that more than 75% of participants were > 60 years of age (Ammar 2016; Demeere 2006; Egawa 2016; Jellish 2003; Lindholm 2013; Longas 2004; Tylman 2011).
All participants were undergoing surgery which were typical of elderly patients. Surgery types were:
vascular surgery: abdominal aortic aneurysm (AAA) (Ammar 2016); open abdominal aortic surgery (Lindholm 2013); carotid endarterectomy (Jellish 2003; Longas 2004);
laparoscopic surgery: laparoscopic surgery (choledocholithotomy, colectomy, sigmoidectomy) (Nishikawa 2004); laparoscopic cholecystectomy (Geng 2017; Trembach 2012);
abdominal surgery: abdominal surgery (Tan 2009); laparotomy (Gursoy 2015); radical rectal resection surgery (Tang 2014); colorectal surgery (Tylman 2011); gastrectomy, colectomy, or rectectomy (Ishii 2016);
orthopaedic surgery: total hip replacement (Biboulet 2012; Chan 1996; Demeere 2006); hip arthroplasty, knee arthroplasty, laminectomy, other orthopaedic surgery (Juvin 1997); hip replacement, knee replacement, long bone fracture fixation, spinal surgery (Kim 2015a); spinal surgery (Liu 2013); total knee arthroplasty (Tanaka 2017);
ophthalmic surgery: cataract surgery (Epple 2001), cataract extraction and lens implantation (Moffat 1995); ophthalmic surgery (Luntz 2004); and
mixed surgery to include: oesophagectomy, gastrectomy, nephrectomy and fracture reduction (Cai 2012a); urological surgery (Celik 2011); one‐lung surgery (Egawa 2016); minor urological or gynaecological surgery (Rohan 2005); tumour resection (Micha 2016); radical surgery (Zhang 2015).
We noted American Society of Anesthesiologists (ASA) status reported in studies. Four studies recruited participants with ASA I to II and did not report breakdown per group (Ammar 2016; Ishii 2016; Liu 2013; Tan 2009). Four studies recruited participants with ASA I to II (Juvin 1997; Kim 2015a; Nishikawa 2004; Zhang 2015), and most participants in these studies were ASA II. Eight studies recruited participants with ASA I to III; in four studies most participants were ASA II (Celik 2011; Chan 1996; Egawa 2016; Epple 2001), in one study most participants were ASA II and III (Micha 2016), and four studies did not report breakdown per group (Gursoy 2015; Luntz 2004; Moffat 1995; Tang 2014). One study recruited participants who were ASA II and III; in one study most participants were ASA II (Geng 2017), and in one study ASA status was evenly distributed (Tanaka 2017). Three studies recruited participants who were all ASA III (Jellish 2003; Longas 2004; Trembach 2012), and one study recruited participants who were ASA II, III, and IV, and most were ASA III (Lindholm 2013). One study recruited participants who were ASA III and IV, and most were ASA III (Biboulet 2012); this study recruited participants > 75 years of age. Four studies reported no ASA status (Cai 2012a; Demeere 2006; Rohan 2005; Tylman 2011). One study recruited participants with a body mass index (BMI) > 30 kg/m².
Whilst some studies excluded patients who had existing neurological, psychiatric or cognitive disorders, or had dementia symptoms (Cai 2012a; Egawa 2016; Geng 2017; Gursoy 2015; Kim 2015a; Lindholm 2013; Micha 2016; Nishikawa 2004; Rohan 2005; Tan 2009; Tanaka 2017), we noted two studies included only participants who had existing mild cognitive impairment (Liu 2013; Tang 2014).
Interventions and comparators
All studies compared total intravenous anaesthesia (TIVA) using propofol versus maintenance anaesthesia using inhalational agents. Six studies were multi‐arm studies and included additional TIVA groups or additional inhalational maintenance or both (Demeere 2006; Geng 2017; Juvin 1997; Longas 2004; Luntz 2004; Zhang 2015).
Ten studies described propofol anaesthesia using target‐controlled infusion (TCI) (Biboulet 2012; Demeere 2006; Egawa 2016; Geng 2017; Kim 2015a; Moffat 1995; Nishikawa 2004; Rohan 2005; Tylman 2011; Zhang 2015).
Nineteen studies compared TIVA versus maintenance using sevoflurane (Ammar 2016; Biboulet 2012; Celik 2011; Demeere 2006; Egawa 2016; Geng 2017; Gursoy 2015; Ishii 2016; Kim 2015a; Lindholm 2013; Liu 2013; Longas 2004; Luntz 2004; Micha 2016; Nishikawa 2004; Rohan 2005; Tang 2014; Tylman 2011; Zhang 2015). Eight studies compared TIVA versus maintenance using isoflurane (Cai 2012a; Chan 1996; Epple 2001; Geng 2017; Jellish 2003; Juvin 1997; Moffat 1995; Tan 2009). Three studies compared TIVA versus maintenance using desflurane (Demeere 2006; Juvin 1997; Tanaka 2017). One study described the comparator as volatile induction and maintenance anaesthesia (VIMA) and did not report details of the anaesthetic agents (Trembach 2012).
Seven studies used inhalation agents during induction of participants in the inhalational maintenance groups (Biboulet 2012; Nishikawa 2004; Rohan 2005; Tang 2014; Trembach 2012; Tylman 2011; Zhang 2015). Twenty studies used intravenous agents during induction of participants in the inhalational maintenance groups (Ammar 2016; Cai 2012a; Celik 2011; Chan 1996; Demeere 2006; Egawa 2016; Epple 2001; Geng 2017; Gursoy 2015; Ishii 2016; Jellish 2003; Juvin 1997; Lindholm 2013; Liu 2013; Longas 2004; Luntz 2004; Micha 2016; Moffat 1995; Tan 2009; Tanaka 2017). Two studies used propofol and inhalation agents during induction of participants in the inhalational maintenance groups (Kim 2015a; Luntz 2004); Luntz 2004 was a multi‐arm study that included a group that used only inhalation agents during induction.
Six studies reported use of epidural for anaesthesia and postoperative analgesia in addition to general anaesthesia (Ammar 2016; Egawa 2016; Ishii 2016; Lindholm 2013; Nishikawa 2004; Zhang 2015). We noted 13 studies administered fentanyl (Ammar 2016; Cai 2012a; Chan 1996; Egawa 2016; Ishii 2016; Juvin 1997; Longas 2004; Micha 2016; Rohan 2005; Tan 2009; Tanaka 2017; Tang 2014; Zhang 2015), and three studies administered remifentanil (Biboulet 2012; Celik 2011; Luntz 2004) during induction or maintenance or both. One study administered fentanyl at induction, and remifentanil during maintenance (Geng 2017). Two studies administered remifentanil in only the TIVA group (Gursoy 2015; Kim 2015a), and one study administered fentanyl in only the TIVA group (Trembach 2012). Two studies administered remifentanil to participants in the TIVA group, and fentanyl to participants in the inhalational maintenance group (Epple 2001; Jellish 2003), and two studies administered fentanyl and remifentanil in the TIVA group and only fentanyl in the inhalational maintenance group (Lindholm 2013; Tylman 2011). Two studies administered sufentanil (Demeere 2006; Liu 2013). We have included details of other analgesics and agents as part of routine anaesthetic management in Characteristics of included studies.
Fourteen studies described use of bispectral index (BIS) for monitoring of depth of anaesthesia (Ammar 2016; Biboulet 2012; Cai 2012a; Demeere 2006; Egawa 2016; Geng 2017; Ishii 2016; Kim 2015a; Lindholm 2013; Liu 2013; Longas 2004; Micha 2016; Tang 2014; Zhang 2015), and one study used Sedline for monitoring of depth of anaesthesia (Tanaka 2017). Other studies used standard care (e.g. clinical assessment, vital signs, and end‐tidal concentration of anaesthetic agent (for inhalational agents) or calculated concentrations of anaesthetic agent (for TCI TIVA)), or did not describe monitoring and we assumed standard care was used.
We noted that one study (Cai 2012a) used anaesthetic methods that differed from standard practice. Participants were exposed to a disproportionately high dose of isoflurane (2% to 3% end‐tidal concentration; equivalent to 2.06 to 3.09 minimum alveolar concentration (MAC) at age 70 years) compared to propofol (target concentration 3 µg/mL; a conventional dose for this age group (Al‐Rifai 2016)). This methodological criticism was raised by Deiner 2012, who postulated that participants in Cai 2012a had been exposed to a toxic dose of isoflurane; this was not disputed in the study authors' subsequent response (Cai 2012b).
Funding sources
Ten studies reported department funding or external funding sources that we assumed to be independent (Ammar 2016; Biboulet 2012; Cai 2012a; Egawa 2016; Geng 2017; Kim 2015a; Lindholm 2013; Liu 2013; Rohan 2005; Tang 2014). Four studies reported support from pharmaceutical companies (Epple 2001; Juvin 1997; Luntz 2004; Tanaka 2017). The remaining 14 studies reported no details of funding sources (Celik 2011; Chan 1996; Demeere 2006; Gursoy 2015; Ishii 2016; Jellish 2003; Longas 2004; Micha 2016; Moffat 1995; Nishikawa 2004; Tan 2009; Trembach 2012; Tylman 2011; Zhang 2015).
Excluded studies
We excluded 397 articles following review of full texts where available. See Figure 1.
We excluded 24 articles because they were not RCTs (for example: commentaries; editorials; observational or cohort studies). Many studies did not report participant age within the abstract and therefore, we considered participant age from full texts. We excluded 292 studies in which participants had a mean age less than 60 years, or the study inclusion criteria was 18 to 65 years of age (in which case, these studies had participants with a mean age less than 60 years), or we calculated that fewer than 75% of participants were more than 60 years of age. We excluded five articles that reported details of retracted studies and three studies for which we were unable to access full texts and information in abstracts was insufficient. We excluded 27 studies that did not compare a propofol‐based TIVA versus an inhalational maintenance anaesthetic agent. We did not include references for these studies in the review.
We excluded 46 RCTs that compared propofol‐based TIVA versus an inhalational maintenance anaesthetic agent and did not measure any of our review outcomes (Arar 2005; Arnaoutoglou 2007; But 2003; Carles 2008; Doe 2016; Filipovic 2007; Fredman 2002; Gasowska 1999; Gauger 2008; Guedes 1988; Halberg 1996; Holst 1993; Hosseinzadeh 2013; Ionescu 2009; Ito 2012; Kadoi 2009a; Kim 2015b; Konstantopoulos 2013a; Kvarnstrom 2012; Malcharek 2015; Manolescu 2012; Mets 1992; Murray 1994; Mutch 1995; Ohe 2014; Oikkonen 1992; Passot 2005; Pirttikangas 1996; Polarz 1995; Sal'nikov 2003; Schäfer 2002; Schilling 2007; Schilling 2011; Shao 2013; Sohn 2008; Sugata 2012; Trifu 2011; Tufano 2000; Ueda 1999; Wakabayashi 2014; Weilbach 2005; Wen 2010; Wormald 2005; Yu 2010a; Zabolotskikh 2013; Zhang 2014). It was a post‐hoc decision to exclude studies that did not measure the review outcomes and we have included references and additional details for these 46 studies in Characteristics of excluded studies.
Awaiting classification
We found four studies for which we had insufficient information to assess eligibility or extract data (IRCT2015112925277N1; McDonagh 2012; NCT02766062; Shen 2011). Two studies were described as completed in clinical trials registers; study results were not posted in the register and we were unable to source a published full‐text reports for these studies (IRCT2015112925277N1; NCT02766062). One study was published as an abstract and reported insufficient information to assess eligibility (McDonagh 2012). One study requires translation from Chinese to assess eligibility (Shen 2011). See Characteristics of studies awaiting classification.
Ongoing studies
We found 11 ongoing studies from clinical trials register searches, with an estimated 3704 participants. All studies compare TIVA with inhalation anaesthetic agents. Eight studies specifically include older participants (ChiCTR‐IOR‐16009851; NCT01809041; NCT01995214; NCT02133638; NCT02301676; NCT02458547; NCT02662257; NCT03165396); remaining studies do not specify age and we will ascertain mean age of participants once the studies are completed. Nine studies aim to report data for our postoperative delirium or postoperative cognitive dysfunction (POCD) (ChiCTR‐IOR‐16009851; NCT01809041; NCT01995214; NCT02107170; NCT02133638; NCT02301676; NCT02662257; NCT03165396; NCT03194074). See Characteristics of ongoing studies.
Risk of bias in included studies
See Figure 2 and Figure 3, and Characteristics of included studies.
2.
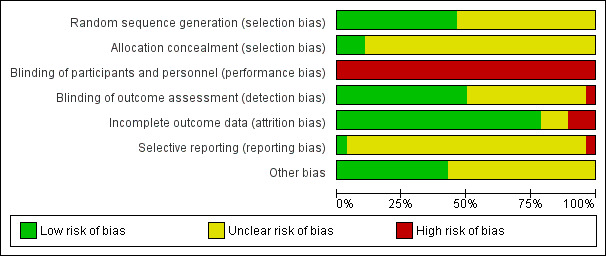
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
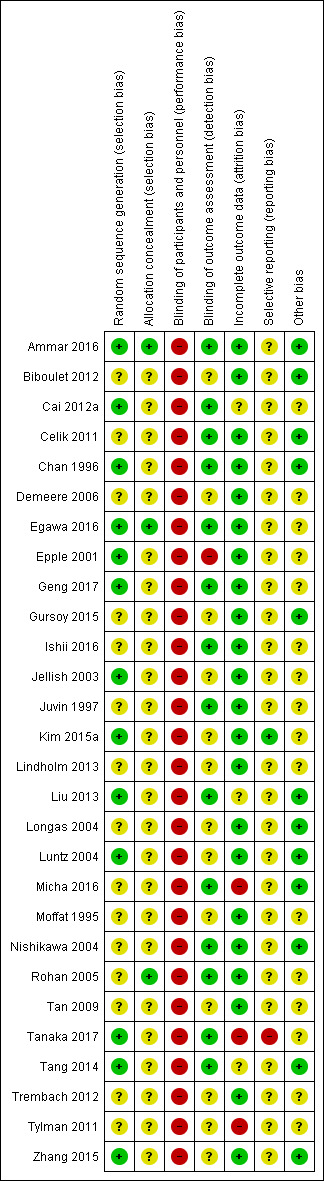
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thirteen studies reported adequate randomization methods and we judged these studies to have low risk of selection bias (Ammar 2016; Cai 2012a; Chan 1996; Egawa 2016; Epple 2001; Geng 2017; Jellish 2003; Kim 2015a; Liu 2013; Luntz 2004; Tanaka 2017; Tang 2014; Zhang 2015). Remaining studies reported insufficient details of randomization methods to judge risk of selection bias.
Only three studies reported adequate methods to conceal allocation and we judged these to have low risk of allocation bias (Ammar 2016; Egawa 2016; Rohan 2005). Remaining studies reported no details and we were unable to judge risk of selection bias.
Blinding
It was not feasible to blind personnel to anaesthetic management and we judged all studies to have high risk of performance bias.
For studies that reported data for more than one outcome we judged risk of detection bias for our primary outcomes. For studies that did not report our primary outcomes, we judged risk of detection bias on our secondary outcomes. Thirteen studies had adequately reported whether personnel responsible for outcome assessment were blinded to the intervention and we judged these studies to have low risk of detection bias (Ammar 2016; Cai 2012a; Celik 2011; Chan 1996; Egawa 2016; Geng 2017; Ishii 2016; Juvin 1997; Micha 2016; Nishikawa 2004; Rohan 2005; Tanaka 2017; Tang 2014). Attempts to blind assessors was not described in Liu 2013; the only review outcome of interest was mortality and we believed assessment of this outcome had low risk of detection bias.
One study reported that assessment of discharge from PACU was completed by personnel aware of group allocation and we judged this study to have high risk of detection bias (Epple 2001).
Remaining studies reported insufficiently whether outcome assessors were blinded to group allocation.
Incomplete outcome data
Twenty‐two studies reported no losses or few losses that were clearly reported and balanced between groups and we judged these studies to have a low risk of bias (Ammar 2016; Biboulet 2012; Celik 2011; Chan 1996; Demeere 2006; Egawa 2016; Epple 2001; Geng 2017; Gursoy 2015; Ishii 2016; Jellish 2003; Juvin 1997; Kim 2015a; Lindholm 2013; Longas 2004; Luntz 2004; Moffat 1995; Nishikawa 2004; Rohan 2005; Tan 2009; Trembach 2012; Zhang 2015). We noted a large number of losses (> 10%) in three studies and were unclear whether risk of attrition bias could influence outcome data (Cai 2012a; Liu 2013; Tang 2014).
We judged three studies to have high risk of attrition bias (Micha 2016; Tanaka 2017; Tylman 2011). Micha 2016 reported loss of participants at nine months but did not include data for these participants at an earlier time point of seven days. Tanaka 2017 reported a large number of losses and reasons for losses were not clearly reported by group. Tylman 2011 reported a post‐hoc decision to exclude participants due to particular conditions; these lost participants belonged to only the inhalational maintenance group.
Selective reporting
Three studies reported retrospective clinical trials registration (Ammar 2016; Geng 2017; Tanaka 2017). It was not feasible to assess risk of selective outcome reporting bias from these documents. We judged Ammar 2016 and Geng 2017 to have unclear risk of bias. In Tanaka 2017, however, we noted that one outcome was listed in the methods section but not reported in the results, and some outcome data were inconsistently reported; therefore, we judged this study to have high risk of selective outcome reporting bias.
Two studies reported prospective clinical trials registration (Kim 2015a; Lindholm 2013). We judged Kim 2015a to have a low risk of selective reporting bias, although we noted that secondary outcomes were not reported as described in the clinical trials register documents (i.e. MAP was reported, rather than hypotension). It was not feasible to assess risk of selective outcome reporting bias in Lindholm 2013 because the clinical trials registration documents did not report intended outcomes.
Remaining studies did not report clinical trials registration or prospectively published study protocols and it was not feasible to assess risk of selective reporting bias for these studies.
Other potential sources of bias
We noted no other sources of bias in 12 studies and judged these to have low risk of other biases (Ammar 2016; Biboulet 2012; Celik 2011; Chan 1996; Gursoy 2015; Liu 2013; Longas 2004; Luntz 2004; Micha 2016; Nishikawa 2004; Tang 2014; Zhang 2015).
Six studies reported differences between groups in administration of fentanyl or remifentanil and it is unclear whether these differences may influence outcome data (Epple 2001; Jellish 2003; Kim 2015a; Lindholm 2013; Trembach 2012; Tylman 2011). We noted baseline imbalances between groups, or differences in length of surgery or duration of anaesthesia in five studies (Demeere 2006; Egawa 2016; Geng 2017; Juvin 1997; Tanaka 2017).
Four full‐text study reports and one abstract contained limited information in the report and it is unclear whether other sources of bias were present (Demeere 2006; Ishii 2016; Rohan 2005; Tan 2009; Trembach 2012).
We noted differences in study design in Moffat 1995, which used a different airway management technique in each group. This difference was related to the study aim which compared the use of neuromuscular blockade in addition to anaesthetic agents for maintenance. We were uncertain whether this may influence data.
Effects of interventions
See: Table 1
Primary outcomes
1. Postoperative delirium
Five studies reported postoperative delirium (Chan 1996; Ishii 2016; Micha 2016; Nishikawa 2004; Tanaka 2017).
Chan 1996 did not report the diagnostic tool used to assess delirium which was reported nine hours postoperatively in one participant (associated with a transient episode of cerebral ischaemia), on the second postoperative day in one participant,and on the fourth postoperative day in one participant (associated with pneumonia). Three studies used the Confusion Assessment Method (CAM) to diagnose postoperative delirium (Ishii 2016; Micha 2016; Tanaka 2017). Micha 2016 made assessments at 48 hours postoperatively, and Ishii 2016 did not report the time point of assessment. Tanaka 2017 made assessments at one, six, 24, and 48 hours postoperatively, although time points for reported data are not clear. We noted differences in data between the published report for Tanaka 2017, and outcome data in the clinical trials register documents; for primary analysis we used the data as reported in the published study report. Nishikawa 2004 used the Delirium Rating Scale (DRS) on the first, second, and third postoperative day; in order to avoid risk of double‐counting participants in this study, we included data only for the third postoperative day.
We noted no difference in postoperative delirium according to whether total intravenous anaesthesia (TIVA )or inhalational maintenance of anaesthesia was used (odds ratio (OR) 0.59, 95% confidence interval (CI) 0.15 to 2.26; 321 = participants; I2 = 17%; Analysis 1.1).
1.1. Analysis.
Comparison 1 TIVA vs Inhalational maintenance, Outcome 1 Postoperative delirium.
We used the GRADE approach to judge the certainty of the evidence for postoperative delirium to be very low. We downgraded by one level for study limitations; we noted few included studies for this outcome had sufficiently reported the methods of randomization and we were concerned by high risk of attrition bias in two studies and high risk of selective outcome reporting bias in one study. We downgraded by two levels for inconsistency; we could not be certain whether measurements of delirium, and time points of measurement, were equivalent between studies, and we used sensitivity analysis to show that choice of time point in one study may influence direction of this result. See Table 1.
2. Postoperative cognitive dysfunction (POCD)
Thirteen studies reported on POCD (Cai 2012a; Egawa 2016; Geng 2017; Gursoy 2015; Juvin 1997; Lindholm 2013; Liu 2013; Micha 2016; Moffat 1995; Rohan 2005; Tan 2009; Tanaka 2017; Tang 2014). Nine studies used the Mini‐Mental State Examination (MMSE) or Mini Mental Test (MMT) (Cai 2012a; Egawa 2016; Geng 2017; Gursoy 2015; Juvin 1997; Liu 2013; Micha 2016; Rohan 2005; Tan 2009); two of these studies used additional tools, which are reported in Characteristics of included studies (Egawa 2016; Geng 2017). Tanaka 2017 assessed postoperative cognitive function with the Digit Symbol Substitution Test (DSST), Digit Span, and Trail Making tests.The remaining studies did not report diagnostic tools used to measure POCD.
Seven studies (2869 participants) reported data as number of participants who had POCD: Cai 2012a at three days postoperatively; Egawa 2016 at five days postoperatively; Geng 2017 at one and three days postoperatively, and we used data at three days; Lindholm 2013 up to 30 days postoperatively; Micha 2016 and Tanaka 2017 at 48 hours postoperatively; Rohan 2005 on the day following surgery; Tang 2014 at seven days postoperatively. Geng 2017 reported data for two inhalational maintenance arms (isoflurane and sevoflurane) and we combined data for these groups. In Tanaka 2017, we used data provided from study authors (following email communication) for Trail Making (part A). Owing to concern about methodology in Cai 2012a, in particular that participants may have been exposed to a toxic dose of inhalational agent, we did not include this large study in the primary analysis. We found fewer incidences of POCD in participants following use of TIVA (OR 0.52, 95% CI 0.31 to 0.87; 869 participants; I2 = 41%; Analysis 1.2).
1.2. Analysis.
Comparison 1 TIVA vs Inhalational maintenance, Outcome 2 Postoperative cognitive dysfunction.
Three studies (160 participants) reported data as mean (standard deviation (SD)), or mean (range), scores for POCD and we reported these data in Table 2; we used time points at 24 hours postoperatively (Gursoy 2015; Tan 2009), and two hours postoperatively (Moffat 1995). We noted no apparent differences in these scores from visual inspection.
1. Study data reported in different formats.
| Outcome: postoperative cognitive dysfunction | |||
| Study | Measurement |
Data* TIVA group |
Data* Inhalational maintenance group |
| Gursoy 2015 | Using MMT (higher scores indicate improved cognitive function); 24 hours | Mean (SD): 24.5 (± 2.4); n = 30 | Mean (SD): 23.7 (± 3.1); n = 30 |
| Moffat 1995 | Using MMSE (higher scores indicate improved cognitive function); 2 hours | Mean (range): 28 (25 to 30); n = 20 | Mean (range): 27 (25 to 30); n = 20 |
| Tan 2009 | Using MMSE (higher scores indicate improved cognitive function); 24 hours | Mean (SD): 26.2 (± 2.9); n = 30 | Mean (SD): 25.8 (± 3.7); n = 30 |
| Outcome: intraoperative hypotension | |||
| Study | Measurement |
Data* TIVA group |
Data* Inhalational maintenance group |
| Lindholm 2013 | Episodes lasting > 2 minutes | Median (25 to 75% percentiles): 4 (2 to 6) | Median (25 to 75% percentiles): 5 (2 to 6) |
| Outcome: length of hospital stay | |||
| Study | Measurement |
Data* TIVA group |
Data* Inhalational maintenance group |
| Lindholm 2013 | Number of days | Median (25 to 75% percentiles): 9 (8 to 12) days; n = 96 | Median (25 to 75% percentiles): 9 (8 to 12) days; n = 97 |
| Tylman 2011 | Number of days | Median (25 to 75% percentiles): 8 (6 to 12) days; n = 25 | Median (25 to 75% percentiles): 8 (6 to 10) days; n = 21 |
*data as reported by study authors;
n: number of analysed participants MMSE: mini‐mental state examination MMT: mini‐mental test SD: standard deviation TIVA: total intravenous anaesthesia
One study reported data in a figure, which we were unable to interpret for this outcome; study authors reported that postoperative psychometric evaluations were similar in each groups (Juvin 1997).
One study included participants with amnesic mild cognitive impairment (aMCI) and assessed progression at two years postoperatively using the MMSE; we did not include data for this study in the analysis because this time point was not comparable to other included studies (Liu 2013). Study authors reported that 30/55 participants in the sevoflurane group had aMCI at two years, and 17/52 participants in the propofol group had aMCI.
We used the GRADE approach to judge the certainty of the evidence for POCD to be low. We downgraded by one level for study limitations; we noted that some studies had insufficiently reported methods of randomization and we were concerned by high risk of attrition bias in one study. We downgraded by one level for inconsistency; we noted a moderate level of statistical heterogeneity (I² = 41%) which we could not explain. See Table 1.
Secondary outcomes
1. Mortality at 30 days
Four studies reported on mortality (Ammar 2016; Biboulet 2012; Lindholm 2013; Liu 2013). Liu 2013 reported the number of participants who were lost to follow‐up because of death; three participants died but these deaths were not reported by group.
We included Ammar 2016, Biboulet 2012 and Lindholm 2013 in the analysis which demonstrated no difference in the number of deaths at 30 days according to whether TIVA or inhalational maintenance of anaesthesia was used (OR 1.21, 95% CI 0.33 to 4.45; 271 participants; I2 = 0%; Analysis 1.3).
1.3. Analysis.
Comparison 1 TIVA vs Inhalational maintenance, Outcome 3 Mortality.
We used the GRADE approach to judge certainty of the evidence for mortality to be very low. We downgraded by one level for study limitations because we noted that some studies had insufficiently reported methods of randomization. We downgraded by two levels for imprecision because the analysis included only three studies with few participants and, because deaths due to anaesthesia are rare, we would require a large sample size to show evidence of a difference. See Table 1.
2. Intraoperative hypotension
Twelve studies reported data for intraoperative hypotension (Biboulet 2012; Chan 1996; Geng 2017; Jellish 2003; Lindholm 2013; Longas 2004; Luntz 2004; Micha 2016; Nishikawa 2004; Tang 2014; Trembach 2012; Zhang 2015). We included data for 11 studies in the analysis; one study (Lindholm 2013), reported data as median number of episodes lasting more than two minutes and we reported these data in Table 2.
We included hypotension as defined by study authors, which was reported as a change from baseline in mean arterial pressure.
We included three multi‐arm studies in analysis (Longas 2004; Luntz 2004; Zhang 2015). For Luntz 2004, we combined data from the two inhalational maintenance groups (one that used total sevoflurane anaesthesia, and one that used propofol induction with sevoflurane maintenance). For Longas 2004, we combined data from the two inhalational maintenance groups (one used sevoflurane 1 MAC, and one used sevoflurane 1.5 MAC). For Zhang 2015, we combined the two TIVA groups (one used additional epidural anaesthesia) versus combined data for the two sevoflurane groups (one used additional epidural anaesthesia).
We noted a high level of statistical heterogeneity (I2 = 63%), and because we expected that studies had clinical variation in the management strategy and medication used to manage hypotension, we did not combine data in a meta‐analysis. Visual inspection of data demonstrated inconsistencies in results and we could not be certain whether TIVA or inhalational maintenance anaesthesia reduces episodes of intraoperative hypotension. Unpooled data for 11 studies (945 participants) are presented in Analysis 1.4.
1.4. Analysis.
Comparison 1 TIVA vs Inhalational maintenance, Outcome 4 Intraoperative hypotension.
We used the GRADE approach to judge certainty of the evidence for intraoperative hypotension to be low. We downgraded by one level for study limitations; we noted some studies reported insufficient methods of randomization. We downgraded by one level for inconsistency because of possible variation in clinical management of participants in each study. See Table 1.
3. Length of stay in the postoperative anaesthesia care unit (PACU)
Eight studies reported the length of stay in the PACU (Celik 2011; Chan 1996; Demeere 2006; Epple 2001; Jellish 2003; Juvin 1997; Kim 2015a; Tanaka 2017). Two of these studies were multi‐arm studies and reported data for TIVA versus maintenance using sevoflurane and TIVA versus maintenance using desflurane (Demeere 2006), and TIVA versus maintenance using isoflurane and TIVA versus maintenance using desflurane (Juvin 1997). For the primary analysis, we included data for the sevoflurane and isoflurane groups; we assessed this decision in a sensitivity analysis using data for the desflurane groups in each study. Data for length of stay in the PACU were not clearly reported in Tanaka 2017, and we noted discrepancies between the published study report and the clinical trials registration documents; we did not report data for this study.
We noted a substantial level of statistical heterogeneity between studies (I2 = 94%), and we expected that there were differences in study methods for this outcome (e.g. whether length of stay in the PACU was reported as time until ready for discharge or time until discharge occurred). We did not conduct meta‐analysis for this outcome because of these differences. Visual inspection of data demonstrated inconsistencies in results and we could not be certain whether TIVA or inhalational maintenance anaesthesia reduces length of time in the PACU. Unpooled data for seven studies (467 participants) are presented in Analysis 1.5.
1.5. Analysis.
Comparison 1 TIVA vs Inhalational maintenance, Outcome 5 Length of stay in PACU.
We used the GRADE approach to judge the certainty of the evidence for length of time in the PACU to be very low. We downgraded the evidence by one level for study limitations; we noted some studies reported insufficient methods of randomization. We downgraded the evidence by two levels because of inconsistency; we expected likely differences in study methods related to definitions of time points of measurement of this outcome. See Table 1.
4. Length of hospital stay
Six studies reported length of hospital stay (Ammar 2016; Demeere 2006; Jellish 2003; Juvin 1997; Lindholm 2013; Tylman 2011). Two of these studies were multi‐arm studies and reported data for TIVA versus maintenance using sevoflurane and TIVA versus maintenance using desflurane (Demeere 2006), and TIVA versus maintenance using isoflurane and TIVA versus maintenance using desflurane (Juvin 1997). For the primary analysis we included data for the sevoflurane and isoflurane groups; we assessed this decision in sensitivity analysis using data for the desflurane groups in each study. Two studies reported data as median values with little or no difference between median number of days in each group, therefore we did not include these data in analysis (Lindholm 2013; Tylman 2011); data for these studies are reported in Table 2.
We included four studies in meta‐analysis and noted no difference between participants given TIVA and participants given inhalational maintenance anaesthesia in length of hospital stay (mean difference (MD) ‐0.00, 95% CI ‐1.32 to 1.32; participants = 175; I2 = 41%; Analysis 1.6).
1.6. Analysis.
Comparison 1 TIVA vs Inhalational maintenance, Outcome 6 Length of hospital stay.
We used the GRADE approach to judge the certainty of the evidence for length of hospital stay to be very low. We downgraded by two levels for imprecision because we included few studies with few participants, and we downgraded by one level for inconsistency because we noted moderate statistical heterogeneity and visual differences in the results. See Table 1.
Subgroup analysis
We performed pre‐planned subgroup analysis as follows.
1. Elderly (60 to 79 years of age) versus late elderly (80 years of age or older)
We included no studies recruiting participants who were > 80 years of age.
2. Elective versus non‐elective surgery
We identified no studies that described surgery as non‐elective.
3. Inhalational induction versus intravenous (IV) induction (as a subgroup of inhalational maintenance only)
Postoperative delirium: one study used inhalational agents at induction (Nishikawa 2004), and four studies used propofol at induction (Chan 1996; Ishii 2016; Micha 2016; Tanaka 2017). We noted little or no difference in postoperative delirium in participants who had anaesthesia with TIVA versus anaesthesia induction with propofol and inhalational maintenance (OR 0.42, 95% CI 0.11 to 1.67; 271 participants; 4 studies; Analysis 2.1). We noted little or no difference between subgroups according to agents used during induction (P = 0.27).
2.1. Analysis.
Comparison 2 TIVA vs inhalational maintenance: subgroup analysis (induction agents; and TCI vs non‐TCI), Outcome 1 Postoperative delirium (induction agents; and TCI vs non‐TCI).
POCD: two studies used inhalational agents at induction (Rohan 2005; Tang 2014), and this analysis showed little or no difference in incidences of POCD between groups (OR 0.87, 95% CI 0.50 to 1.50; 230 participants). Five studies used intravenous agents at induction and we found less POCD in participants when IV agents had been used (OR 0.38, 95% CI 0.20 to 0.75; 639 participants). We noted little or no difference between subgroups according to agents used during induction (P = 0.07). See Analysis 2.2.
2.2. Analysis.
Comparison 2 TIVA vs inhalational maintenance: subgroup analysis (induction agents; and TCI vs non‐TCI), Outcome 2 Postoperative cognitive dysfunction (induction agents).
Mortality: one study used inhalational agents at induction (Biboulet 2012) and two studies used propofol for induction (Ammar 2016; Lindholm 2013). We noted little or no difference between subgroups according to agents used during induction (P = 0.53). See Analysis 2.3.
2.3. Analysis.
Comparison 2 TIVA vs inhalational maintenance: subgroup analysis (induction agents; and TCI vs non‐TCI), Outcome 3 Mortality (induction agents; and TCI vs non‐TCI).
Intraoperative hypotension: we noted visual inconsistencies in the data during our primary assessment of this outcome, which we expected could be explained by differences in the clinical management of hypotension between studies and we did not conduct meta‐analysis. We used pre‐planned subgroup analysis to assess whether induction agents may explain inconsistencies in data between studies. However, we noted visual inconsistencies in one of the subgroups (when induction was given with inhalational agents), and expected that differences in clinical management between studies continued to affect the data such that subgroup analysis was not appropriate. See Analysis 2.4.
2.4. Analysis.
Comparison 2 TIVA vs inhalational maintenance: subgroup analysis (induction agents; and TCI vs non‐TCI), Outcome 4 Intraoperative hypotension (induction agents).
Length of stay in the PACU: we could not perform subgroup analysis because we included no studies using inhalational agents for induction.
Length of hospital stay: we could not perform subgroup analysis because we included no studies using inhalational agents for induction.
4. Target‐controlled infusion (TCI) versus non‐TCI maintenance of anaesthesia (as a subgroup of TIVA only)
Postoperative delirium: one study used TCI (Nishikawa 2004), and four studies did not report use of TCI for maintenance of TIVA (Chan 1996; Ishii 2016; Micha 2016; Tanaka 2017). We noted no difference in postoperative delirium when TCI had not been used (OR 0.42, 95% CI 0.11 to 1.67; 271 participants; Analysis 2.1). We noted little or no difference between subgroups according to whether TCI had been used (P = 0.27).
POCD: we noted little or no difference between subgroups (P = 0.38). Whilst effect estimates in each subgroup favoured use of TIVA, we found little or no difference in POCD when studies used TCI (OR 0.31, 95% CI 0.07 to 1.38; 294 participants), or when studies did not use TCI (OR 0.63, 95% CI 0.36 to 1.10; 575 participants). We noted a high level of statistical heterogeneity (I² = 71%) between the studies that used TCI which we could not explain. See Analysis 2.5.
2.5. Analysis.
Comparison 2 TIVA vs inhalational maintenance: subgroup analysis (induction agents; and TCI vs non‐TCI), Outcome 5 Postoperative cognitive dysfunction (TCI vs non‐TCI).
Mortality: one study used TCI for maintenance of anaesthesia (Biboulet 2012). We noted no difference between subgroups according to whether TCI had been used (P = 0.53). See Analysis 2.3.
Intraoperative hypotension: we noted visual inconsistencies in the data during our primary assessment of this outcome, which we expected could be explained by differences in the clinical management of hypotension between studies and therefore, we did not conduct meta‐analysis. We used pre‐planned subgroup analysis to assess whether use of TCI maintenance may explain inconsistence in data between studies. However, we noted visual inconsistencies in each subgroup (TCI, and non‐TCI) and expected that differences in clinical management between studies continued to affect the data such that subgroup analysis was not appropriate. See Analysis 2.6.
2.6. Analysis.
Comparison 2 TIVA vs inhalational maintenance: subgroup analysis (induction agents; and TCI vs non‐TCI), Outcome 6 Intraoperative hypotension (TCI vs non‐TCI).
Length of stay in the PACU: we noted visual inconsistencies in the data during our primary assessment of this outcome, which we expected could be explained by differences in the definition of time point for length of stay in PACU between studies and we did not conduct meta‐analysis. We used pre‐planned subgroup analysis to assess whether use of TCI maintenance may explain inconsistence in data between studies. However, we noted visual inconsistencies in one of the subgroups (non‐TCI) and expected that possible differences in time point definitions between studies continued to affect the data such that subgroup analysis was not appropriate. See Analysis 2.7.
2.7. Analysis.
Comparison 2 TIVA vs inhalational maintenance: subgroup analysis (induction agents; and TCI vs non‐TCI), Outcome 7 Length of stay in the PACU (TCI vs non‐TCI).
Length of hospital stay: no studies used TCI for maintenance of anaesthesia.
5. Use of depth of anaesthesia monitoring
We considered the use of any processed electroencephalogram (EEG) for depth of monitoring. Fourteen studies described use of bispectral index (BIS) for monitoring of depth of anaesthesia (Ammar 2016; Biboulet 2012; Cai 2012a; Demeere 2006; Egawa 2016; Geng 2017; Ishii 2016; Kim 2015a; Lindholm 2013; Liu 2013; Longas 2004; Micha 2016; Tang 2014; Zhang 2015), and one study used Sedline for monitoring of depth of anaesthesia (Tanaka 2017). We compared studies that reported use any processed EEG versus studies that used standard care for monitoring (e.g. clinical assessment, vital signs, and end‐tidal concentration of anaesthetic agent (for inhalational agents) or calculated concentrations of anaesthetic agent (for TCI TIVA)).
Postoperative delirium: three studies used processed EEG (Ishii 2016; Micha 2016; Tanaka 2017) and when combined, we noted little or no difference in whether anaesthesia was maintained with TIVA or inhalation agents (OR 0.56, 95% CI 0.04 to 7.44; 211 participants). Two studies used standard care (Chan 1996; Nishikawa 2004) and when combined we noted little or no difference in whether anaesthesia was maintained with TIVA or inhalation agents (OR 1.00, 95% CI 0.14 to 7.06; 110 participants). We noted no differences between subgroups (P = 0.73). See Analysis 3.1.
3.1. Analysis.
Comparison 3 TIVA vs inhalational maintenance: subgroup analysis, monitoring with processed EEG vs standard care, Outcome 1 Postoperative delirium.
POCD: one study used standard care (Rohan 2005); this single study showed no difference in POCD depending on whether anaesthesia was maintained with TIVA or inhalation agents (OR 1.00, 95% CI 0.24 to 4.20; 30 participants). Six studies used processed EEG or Sedline for depth of monitoring and when combined we noted that fewer participants had experiences of POCD when TIVA was used (OR 0.47, 95% CI 0.27 to 0.84; 839 participants). We noted little or no difference between subgroups (P = 0.35). See Analysis 3.2.
3.2. Analysis.
Comparison 3 TIVA vs inhalational maintenance: subgroup analysis, monitoring with processed EEG vs standard care, Outcome 2 Postoperative cognitive dysfunction.
Mortality: all included studies used processed EEG for depth of anaesthesia monitoring.
Intraoperative hypotension: we noted visual inconsistencies in the data during our primary assessment of this outcome, which we expected could be explained by differences in the clinical management of hypotension between studies and we did not conduct meta‐analysis. We used pre‐planned subgroup analysis to assess whether use of processed EEG may explain inconsistence in data between studies. However, we noted visual inconsistencies in each subgroup and expected that differences in clinical management between studies continued to affect the data such that subgroup analysis was not appropriate. See Analysis 3.3.
3.3. Analysis.
Comparison 3 TIVA vs inhalational maintenance: subgroup analysis, monitoring with processed EEG vs standard care, Outcome 3 Intraoperative hypotension.
Length of stay in the PACU: we noted visual inconsistencies in the data during our primary assessment of this outcome, which we expected could be explained by differences in the definition of time point for length of stay in PACU between studies and we did not conduct meta‐analysis. We used pre‐planned subgroup analysis to assess whether use of processed EEG may explain inconsistence in data between studies. However, we noted visual inconsistencies in one of the subgroups (use of processed EEG) and expected that possible differences in time point definitions between studies continued to affect the data such that subgroup analysis was not appropriate. See Analysis 3.4.
3.4. Analysis.
Comparison 3 TIVA vs inhalational maintenance: subgroup analysis, monitoring with processed EEG vs standard care, Outcome 4 Length of stay in PACU.
Length of hospital stay: one study used processed EEG, and for studies which used standard care; we noted little or no difference in length of hospital stay depending on whether anaesthesia was maintained with TIVA or inhalation agents (OR ‐0.27 minutes, 95% CI ‐1.40 to 0.86; 138 participants; Analysis 3.5). We noted little or no difference between subgroups (P = 0.10).
3.5. Analysis.
Comparison 3 TIVA vs inhalational maintenance: subgroup analysis, monitoring with processed EEG vs standard care, Outcome 5 Length of hospital stay.
Sensitivity analysis
1. Risk of bias judgements. In sensitivity analysis, we excluded studies that we judged to be at high or unclear risk of selection bias. We performed sensitivity analysis on studies that were pooled in primary analysis.
Postoperative delirium: we excluded three studies from the analysis, which did not alter interpretation of the effect (Ishii 2016; Micha 2016; Nishikawa 2004).
POCD: we excluded three studies from analysis, which did not alter interpretation of the effect (Lindholm 2013; Micha 2016; Rohan 2005).
Mortality: we excluded two studies from analysis (Biboulet 2012; Lindholm 2013), the remaining study reported no deaths in either group.
Length of hospital stay: we excluded two studies (Demeere 2006; Juvin 1997). We noted that the effect remained the same but statistical heterogeneity was reduced (I² = 0%).
2. Decisions made for missing data. In sensitivity analysis, we excluded studies that we judged to be at high risk of attrition bias.
Postoperative delirium: we excluded two studies which did not alter interpretation of the effect (Micha 2016; Tanaka 2017).
POCD: we excluded one study from analysis which did not alter interpretation of the effect (Micha 2016).
3. Effects model. In sensitivity analysis, we used the alternate meta‐analytic effects model for those outcomes in which we pooled data.
Postoperative delirium: we used a fixed‐effect model which did not alter interpretation of the result.
POCD: we used a fixed‐effect model which did not alter interpretation of the result.
Length of hospital stay: we used a fixed‐effect model which did not alter interpretation of the result.
Additional sensitivity analysis
We made decisions during the review process that may have influenced our review results. In sensitivity analysis, we assessed the following decisions for each outcome.
1. In primary analysis, we included studies in which we used mean ages reported in the baseline characteristics table to ascertain that > 75% of participants were > 60 years of age (Ammar 2016; Demeere 2006; Egawa 2016; Jellish 2003; Lindholm 2013; Longas 2004; Tylman 2011). It was feasible that some participants in these studies were not elderly.
Postoperative delirium: we included no studies in primary analysis that may have included participants that were not elderly.
POCD: in sensitivity analysis, we removed Egawa 2016 and Lindholm 2013 from analysis and this did not alter interpretation of the effect.
Mortality: in sensitivity analysis, we removed Ammar 2016 and Lindholm 2013. One remaining study reported one death in the TIVA group.
Length of hospital stay: in sensitivity analysis, we removed three studies (Ammar 2016; Demeere 2006; Jellish 2003); it was not possible to pool data because only one study remained.
2. In primary analysis, we included studies in which participants had an existing neurological impairment at baseline (Liu 2013; Tang 2014).
Postoperative delirium: we included no studies in primary analysis that recruited participants with an existing neurological impairment.
POCD: in sensitivity analysis, we removed Tang 2014 from analysis. This did not alter our interpretation of the effect.
Mortality: we included no studies in primary analysis that recruited participants with an existing neurological impairment.
Length of hospital stay: we included no studies in primary analysis that recruited participants with an existing neurological impairment.
3. In primary analysis, we made decisions to include data for one time point when the study reported different time points (Nishikawa 2004 reported postoperative delirium for the first and second postoperative day, which we did not include in primary analysis; Geng 2017 reported POCD for the first postoperative day that we did not include in analysis).
Postoperative delirium: in sensitivity analysis, we used data for the first postoperative day in Nishikawa 2004 and, whilst we found no statistically significant difference in incidences of delirium between groups, we noted a change in the direction of effect and a reduced level of statistical heterogeneity (OR 0.41, 95% CI 0.13 to 1.29; 321 participants; 5 studies; I² = 11%). This result was similar when we used data for the second postoperative day in Nishikawa 2004 (OR 0.54, 95% CI 0.19 to 1.50; participants = 321; studies = 5; I² = 17%).
POCD: in sensitivity analysis, we used data for the first postoperative day in Geng 2017.This did not alter interpretation of the effect.
Mortality: we included no studies in which different time points were reported.
4. In primary analysis, we made decisions to manage data for multi‐arm studies. We combined groups for POCD and intraoperative hypotension (Geng 2017; Longas 2004; Luntz 2004; Zhang 2015), and we used one inhalational maintenance group for length of PACU stay, and length of hospital stay (sevoflurane in Demeere 2006; isoflurane in Juvin 1997).
Postoperative delirium: we included no multi‐arm studies in analysis of this outcome.
POCD: in sensitivity analysis, we included data separately for each inhalational maintenance group for Geng 2017. This did not alter interpretation of the effect.
Mortality: we included no multi‐arm studies in analysis of this outcome.
Length of hospital stay: in sensitivity analysis, we included data for the desflurane groups in Demeere 2006 and Juvin 1997. We noted a change in the effect estimate which showed that participants who had anaesthesia maintained with inhalational agents had a shorter length of hospital stay (MD 0.10 days, 95% CI 0.00 to 0.20; 175 participants; I² = 9%). However, this result demonstrated only a small change in time and is unlikely to be clinically important.
5. In primary analysis, we excluded one large study (because of methodological differences that were inconsistent with usual anaesthetic practice) in analysis of POCD (Cai 2012a).
POCD: in sensitivity analysis, we included Cai 2012a. This increased statistical heterogeneity from I² = 41% to I² = 90%. The direction of effect was not altered by including this study in analysis (OR 0.32, 95% CI 0.11 to 0.93; 2869 participants; I2 = 90%).
Discussion
Summary of main results
We included 28 studies with 4507 randomized participants. Four studies are awaiting classification because we had insufficient information to assess eligibility. All included studies compared maintenance with propofol‐based total intravenous anaesthesia (TIVA) versus inhalational maintenance of anaesthesia.
We found little or no evidence of a difference in incidences of postoperative delirium according to type of anaesthetic maintenance agents from five studies (Chan 1996; Ishii 2016; Micha 2016; Nishikawa 2004; Tanaka 2017). We used sensitivity analysis to explore including different time points of outcome assessment reported by one study (Nishikawa 2004), which may influence direction of effect for postoperative delirium. We found that fewer people may experience postoperative cognitive dysfunction (POCD) with propofol‐based TIVA in seven studies. We excluded one large study from analysis for POCD because study investigators had used a non‐standard method of anaesthetic management. Five additional studies reported data for POCD, which we were unable to pool and we noted little or no difference in scores of POCD in five of these studies, and in the remaining study the time point was not comparable to other studies.
We found little or no evidence of a difference in mortality from three studies (Ammar 2016; Biboulet 2012; Lindholm 2013). We did not combine data in meta‐analysis for intraoperative hypotension or length of stay in the postanaesthesia care unit (PACU); we noted visual inconsistencies in the data and expected that these might be explained by clinical differences between studies in the management of hypotension and methodological differences in definition of time points before discharge from the PACU. We found little or no evidence of a difference in length of hospital stay according to type of anaesthetic maintenance agent from four studies.
Overall completeness and applicability of evidence
We included studies that recruited participants who were more than 60 years of age, and studies in which we calculated that more than 75% participants were more than 60 years of age.
The included studies recruited people scheduled for non‐cardiac surgery under general anaesthesia. The surgery types were typical of elderly patients but varied between studies to include: cardiovascular, laparoscopic, abdominal, orthopaedic, ophthalmic, and mixed surgery (oesophagectomy, gastrectomy, nephrectomy, urological surgery, one‐lung surgery, gynaecological surgery, tumour resection, and radical surgery). The ASA status differed between the included studies. Most studies included a majority of participants who were classed as ASA II; however, some studies included only participants who were ASA III, and two studies also included participants with an ASA status up to ASA IV (Biboulet 2012; Lindholm 2013).
Anaesthetic management differed between studies, for example with use of different intraoperative and postoperative analgesic management, use of epidurals, or use of premedication. We also noted differences in studies that used target‐controlled infusion (TCI) for TIVA, that used processed electroencephalogram (EEG) for monitoring of depth of anaesthesia (bispectral index (BIS) or Sedline), and that used inhalation agents only for induction and maintenance.
These differences may introduce inconsistency and reduce the overall applicability of the evidence.
Quality of the evidence
We found insufficient reporting of randomization methods in many studies and all studies were at high risk of performance bias because it was not feasible to blind anaesthetists for this study design. Thirteen studies had described blinding of outcome assessors. Three studies had a high of risk of attrition bias, and we noted differences in use of analgesics between groups in six studies, and differences in baseline characteristics, which may have influenced results in five studies. Few studies reported clinical trials registration and we could not assess risk of selective outcome reporting bias.
We used the GRADE approach and considered study limitations noted during 'Risk of bias' assessment which may influence the certainty of the evidence for each outcome. In addition, we identified few studies with few participants for two outcomes (mortality, and length of hospital stay) which introduced imprecision. We noted visual differences in some results which might be explained by differences in clinical management or methodological designs which prevented pooling of data in meta‐analysis and introduced inconsistency. We judged evidence for postoperative delirium, mortality, length of stay in the PACU, and length of hospital stay to be very low certainty, and evidence for POCD, and intraoperative hypotension to be low certainty.
We explored potential explanations for this heterogeneity in subgroup analysis, in particular with consideration of whether intravenous agents were used during induction in the inhalational maintenance group, whether TIVA was given using TCI, and whether depth of anaesthesia was monitored. Results of subgroup analyses did not appear to explain heterogeneity and we noted that high levels of statistical heterogeneity remained in one or both subgroups in each analysis. We were not confident that these subgroups alone could explain the differences between studies and the levels of heterogeneity that prevented meta‐analysis; we did not explore this in additional subgroup analyses.
Potential biases in the review process
We conducted our review using Cochrane methodology, using two review authors to select studies, extract data, and assess risk of bias according to our published protocol (Miller 2016). We conducted a thorough search that included clinical trials registers, forward and backward citation searching, and grey literature.
We reported changes from the protocol in Differences between protocol and review. In particular, we found that studies did not always define 'elderly' using a cut‐off of 60 years (according to WHO 2016b), and studies typically used an included age category of 18 to 65 years. We excluded studies that used an age category of 18 to 65 years, but we found that these studies had a mean age for participants of less than 60 years and therefore this decision did not affect choice of included studies for this review.
We made a post‐hoc decision to exclude studies that did not measure our review outcomes. We included references for these studies in the review in order to inform readers of other studies that compare intravenous versus inhalational maintenance anaesthesia for different purposes.
We were cautious to assess the impact of decisions that we made during the review process and used sensitivity analysis for this purpose.
In particular, some studies may have included participants that were younger than 60 years of age. When sufficient studies allowed sensitivity analysis, we considered whether results differed if we excluded these studies; we found no differences in the interpretation of effect estimates. In addition, we considered the effect of including studies in which participants had an existing cognitive impairment, and, again, found excluding relevant studies did not alter the effect.
We considered the effect of decisions regarding which time point to use in studies that reported more than one time point. For delirium, we noted that, whilst there remained no statistical evidence of a difference according to type of anaesthetic maintenance agent, direction of effect changed when we used different time points reported in one study. We believed that our decisions on which time point to use may have the potential to affect interpretation of the data and we used GRADE to downgrade the certainty of the evidence for postoperative delirium.
We noted one large study which had methodological differences in anaesthetic management that were not consistent with standard anaesthetic management (Cai 2012a). For this reason, we excluded Cai 2012a from analysis of POCD. We assessed this decision during sensitivity, by including the study in analysis of POCD. The direction of effect was not altered and we believed that the decision to exclude Cai 2012a from primary analysis did not affect the conclusion of the review.
Also, we were unable to assess eligibility of four studies (see Studies awaiting classification); inclusion of these studies may have influenced the results (IRCT2015112925277N1; McDonagh 2012; NCT02766062; Shen 2011).
Agreements and disagreements with other studies or reviews
We found no reviews that specifically looked at intravenous versus inhalational maintenance of anaesthesia in elderly surgical patients.
One Cochrane Review considered intravenous versus inhalation agents for transabdominal robotic assisted laparoscopic surgery (Herling 2017). This review did not specifically include elderly patients and no included randomized controlled trials measured cognitive function, mortality, or length of stay. Another Cochrane Review compared the two types of anaesthetic for emergence from anaesthesia after brain tumour surgery (Prabhakar 2016). Again, the patients were not specifically elderly and the review authors did not seek the outcomes specified in our review. Another Cochrane Review considered general anaesthesia versus regional anaesthesia for hip fracture (a surgery which would typically include an older patient population), however this review did not measure outcomes related to cognitive function (Guay 2016). This review does serve to remind us, however, that general anaesthesia is not the only option and can be avoided for many operations (Lewis 2015).
Authors' conclusions
Implications for practice.
We are uncertain whether maintenance with propofol‐based total intravenous anaesthesia (TIVA) or with inhalational agents affect incidences of postoperative delirium, mortality, or length of hospital stay. We identified 28 studies which assessed the effects of propofol‐based TIVA versus inhalational maintenance in elderly surgical patients. Few of the included studies reported the effect on postoperative delirium.
We found no evidence of a difference in postoperative delirium according to type of anaesthetic agents used and we judged this evidence to be very low certainty. We found low‐certainty evidence that propofol‐based TIVA may reduce postoperative cognitive dysfunction (POCD). We were unable to ascertain any effects on length of stay in postanaesthesia care unit (PACU); we judged this evidence to be very low certainty, and we were unable to ascertain any effects on intraoperative hypotension for which we judged the evidence to be low certainty. We found little or no evidence of a difference in mortality and length of hospital stay, but this evidence was very low certainty.
Implications for research.
We identified a large number of ongoing studies (11), which assess the effects of propofol‐based TIVA versus inhalational agents in elderly surgical patients. This demonstrates continuing interest in this research field and including these studies in future review updates would increase certainty of the effect. The studies included in this review did not separate data for participants that were frail elderly (or more than 80 years of age), and no studies specifically included non‐elective surgical patients. These are important subgroups and evidence for these groups of patients in future research would be useful. We focused our review outcomes on postoperative cognitive outcomes and length of stay; however we propose that future review updates consider postoperative nausea and vomiting as an additional relevant outcome.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | Amended | Acknowledgement section amended to include Sign‐off Editor |
Acknowledgements
We would like to thank Anna Lee (Content Editor), Jane Cracknell (Managing Editor), Nathan Pace (Statistical Editor), Harriet W Hopf, Michel Struys, Emmanuel Boselli (Peer reviewers), Janne Vendt (Information Specialist), Janet Wale (Consumer Editor), and Harald Herkner (Sign‐off Editor), for their help and editorial advice during the preparation of this systematic review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Anesthesia, Intravenous] explode all trees #2 MeSH descriptor: [Anesthesia, Inhalation] explode all trees #3 MeSH descriptor: [Anesthetics, Inhalation] explode all trees #4 MeSH descriptor: [Anesthetics, Intravenous] explode all trees #5( an?esthe* near/2 (iv or intravenous or inhalation* or volatile)) or (TIVA or propofol or halothane or enflurane or isoflurane or desflurane) #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor: [Geriatrics] explode all trees #8 MeSH descriptor: [Aged] explode all trees #9 (Geriatric* or Elder* or old‐age or pensioner*) or ((aging or aged or elderly or senior or old) near/2 (wom?n or m?n or lady or ladies or adult* or citizen* or population* or people or person)) #10 #7 or #8 or #9 #11 #6 and #10
Appendix 2. MEDLINE (Ovid) search strategy
Anesthesia, Intravenous/ or Anesthesia, Inhalation/ or (an?esthe* adj2 (iv or intravenous or inhalation* or volatile)).mp. or (TIVA or propofol or halothane or enflurane or isoflurane or desflurane).mp.
(Geriatric* or Elder* or old‐age* or pensioner*).ti,ab.
((Aging or aged or senior or old*) adj2 (wom#n or m#n or lady or ladies or adult* or citizen* or population*1 or people or person)).ti,ab.
exp Aged/ or exp geriatrics/
2 or 3 or 4
1 and 5
((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh.
6 and 7
Appendix 3. Embase (Ovid) search strategy
intravenous anesthesia/ or inhalation anesthesia.mp. or (an?esthe* adj2 (iv or intravenous or inhalation* or volatile)).mp. or (TIVA or propofol or halothane or enflurane or isoflurane or desflurane).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word]
(geriatric* or elder* or old‐age* or pensioner*).ti,ab.
((aging or aged or senior or old*) adj2 (wom#n or m#n or lady or ladies or adult* or citizen* or population*1 or people or person)).ti,ab.
aged/ or geriatrics/
2 or 3 or 4
1 and 5
((crossover procedure or double blind procedure or single blind procedure).sh. or (crossover* or cross over*).ti,ab. or placebo*.ti,ab,sh. or (doubl* adj blind*).ti,ab. or (controlled adj3 (study or design or trial)).ti,ab. or allocat*.ti,ab. or trial*.ti,ab. or randomized controlled trial.sh. or random*.ti,ab.) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.))
6 and 7
Appendix 4. PsycINFO (EBSCO) search strategy
S1 MM "Anesthesiology" S2 ((an?esthe* N2 (iv or intravenous or inhalation* or volatile)) S3 TIVA or propofol or halothane or enflurane or isoflurane or desflurane S4 S1 OR S2 OR S3 S5 MM "Geriatrics" S6 Geriatric* or Elder* or old‐age or pensioner* S7 ((aging or aged or elderly or senior or old) N2 (wom?n or m?n or lady or ladies or adult* or citizen* or population* or people or person)) S8 S5 OR S6 OR S7 S9 ((MM "Randomized Controlled Trials") OR (MM "Random Assignment") OR (MH "Clinical Trials") OR (MH "Placebos")) OR (random* or (trial* and (clinical or controlled)) or multicenter or prospective) S10 S4 AND S8 AND S9
Data and analyses
Comparison 1. TIVA vs Inhalational maintenance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative delirium | 5 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.15, 2.26] |
| 2 Postoperative cognitive dysfunction | 7 | 869 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 3 Mortality | 3 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.33, 4.45] |
| 4 Intraoperative hypotension | 11 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Length of stay in PACU | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6 Length of hospital stay | 4 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐1.32, 1.32] |
Comparison 2. TIVA vs inhalational maintenance: subgroup analysis (induction agents; and TCI vs non‐TCI).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative delirium (induction agents; and TCI vs non‐TCI) | 5 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.15, 2.26] |
| 1.1 Induction with inhalational agents, and TCI | 1 | 50 | Odds Ratio (M‐H, Random, 95% CI) | 3.12 [0.12, 80.39] |
| 1.2 Induction with intravenous agents, and non‐TCI | 4 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.67] |
| 2 Postoperative cognitive dysfunction (induction agents) | 7 | 869 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 2.1 Induction with inhalational agents | 2 | 230 | Odds Ratio (M‐H, Random, 95% CI) | 0.87 [0.50, 1.50] |
| 2.2 Induction with intravenous agents | 5 | 639 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.20, 0.75] |
| 3 Mortality (induction agents; and TCI vs non‐TCI) | 3 | 271 | Odds Ratio (M‐H, Random, 95% CI) | 1.21 [0.33, 4.45] |
| 3.1 Induction with inhalational agents, and TCI | 1 | 28 | Odds Ratio (M‐H, Random, 95% CI) | 3.22 [0.12, 86.09] |
| 3.2 Induction with intravenous agents, and non‐TCI | 2 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.25, 4.16] |
| 4 Intraoperative hypotension (induction agents) | 11 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 Induction with inhalational agents | 5 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Induction with intravenous agents | 6 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Postoperative cognitive dysfunction (TCI vs non‐TCI) | 7 | 869 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 5.1 TCI | 2 | 294 | Odds Ratio (M‐H, Random, 95% CI) | 0.31 [0.07, 1.38] |
| 5.2 non‐TCI | 5 | 575 | Odds Ratio (M‐H, Random, 95% CI) | 0.63 [0.36, 1.10] |
| 6 Intraoperative hypotension (TCI vs non‐TCI) | 11 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.1 TCI | 4 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 non‐TCI | 7 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Length of stay in the PACU (TCI vs non‐TCI) | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 TCI | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 non‐TCI | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. TIVA vs inhalational maintenance: subgroup analysis, monitoring with processed EEG vs standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Postoperative delirium | 5 | 321 | Odds Ratio (M‐H, Random, 95% CI) | 0.59 [0.15, 2.26] |
| 1.1 Monitoring with processed EEG | 3 | 211 | Odds Ratio (M‐H, Random, 95% CI) | 0.56 [0.04, 7.44] |
| 1.2 Monitoring with standard care | 2 | 110 | Odds Ratio (M‐H, Random, 95% CI) | 1.00 [0.14, 7.06] |
| 2 Postoperative cognitive dysfunction | 7 | 869 | Odds Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.87] |
| 2.1 Monitoring with processed EEG | 6 | 839 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.27, 0.84] |
| 2.2 Monitoring with standard care | 1 | 30 | Odds Ratio (M‐H, Random, 95% CI) | 1.0 [0.24, 4.20] |
| 3 Intraoperative hypotension | 11 | Odds Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 Monitoring with processed EEG | 6 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Monitoring with standard care | 5 | Odds Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Length of stay in PACU | 7 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1 Monitoring with processed EEG | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Monitoring with standard care | 6 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Length of hospital stay | 4 | 175 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐1.32, 1.32] |
| 5.1 Monitoring with processed EEG | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐0.50, 5.10] |
| 5.2 Monitoring with standard care | 3 | 138 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.40, 0.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ammar 2016.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 50 Inclusion criteria
Exclusion criteria
Type of surgery: elective infrarenal AAA repair Baseline characteristics TIVA group
Inhalational maintenance group
Country: Egypt Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 25; 0 losses Induction details: propofol 1.5 mg/kg to 2 mg/kg, fentanyl 3 µg/kg, cisatracurium 0.1 mg/kg Maintenance details: continuous infusion of propofol 4 mg/kg/hour to 6 mg/kg/hour, and cisatracurium 2 µg/kg/min. BIS kept between 45 and 55 Additional regional anaesthesia: epidural analgesia before starting anaesthesia at T8‐T10. Epidural block with 12 mL bupivacaine hydrochloride 0.25%. 4 mL bupivacaine injected 2 hours later as maintenance and every hour thereafter for postoperative epidural analgesia Other information: fluid loading was performed with 1.0 L of 6% 130/0.4 hydroxyethyl starch (Voluven) infusion. Fluid and blood replacements were adjusted to maintain participant haematocrit value above 30%. Norepinephrine and nicardipine were used if required (if MAP changed by > 20%) to maintain haemodynamic stability. Normothermia maintained. Acetaminophen IV postoperatively if required Inhalational maintenance group Participants: n = 25; 0 losses Induction details: propofol 1.5 mg/kg to 2 mg/kg, fentanyl 3 µg/kg, cisatracurium 0.1 mg/kg Maintenance details: sevoflurane 1 MAC, cisatracurium 2 µg/kg/min. BIS kept between 45 and 55 Additional regional anaesthesia and other information: epidural analgesia, epidural block and all other fluid management etc. was the same as the TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: university funding. No conflicts of interest Study dates: February 2012 to April 2014 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated random number table |
| Allocation concealment (selection bias) | Low risk | Quote: "an independent statistician was assigned to perform central randomization to ensure proper concealment of the study management from the patients and investigators until the release of the final statistical results." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "one analyst was blinded in respect to the drug under study during the procedure by covering the lines, infusion pump, gas analyzer, and by numeric codes during the whole process of data evaluation. Furthermore, physicians who were charged for postoperative care of patients and for their discharges from intensive care unit (ICU) and hospital were effectively blinded to the study design." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Retrospective registration with clinical trials register (PACTR201505001095139). Not feasible to assess risk of selective outcome reporting bias with these documents |
| Other bias | Low risk | No other sources of bias identified |
Biboulet 2012.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria
Exclusion criteria
Type of surgery: total hip replacement Baseline characteristics: TIVA group (characteristics for 14 participants)
Inhalational maintenance group (characteristics for 15 participants)
Country: France Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 15; 1 loss (change to surgical technique which warranted study exclusion); 14 analysed Induction details: initial target plasma concentration 1.5 µg/mL propofol, gradually increased by increments of 0.5 µg/mL every 2 minutes until BIS of 50. Remifentanil 0.25 µg/kg for 2 minutes, with repeated boluses if required to maintain BIS of 50 or HR and MAP no more than 20% of baseline Maintenance details: after intubation, propofol TCI decreased to 0.5 µg/mL, and titrated to maintain BIS of 50. Remifentanil infusion 0.1 µg/kg/min, preceded by bolus of 0.25 µg/kg for 2 minutes Other information: femoral nerve block with 30 mL ropivacaine 0.5% on arrival in operating theatre Inhalational maintenance group Participants: n = 15; 1 loss (cardiac arrest during induction); 14 analysed Induction details: sevoflurane, initially at 6%, decreased to 3% when BIS fell to 50. Remifentanil 0.25 µg/kg for 2 minutes, with repeated boluses if required to maintain BIS of 50 or HR and MAP no more than 20% of baseline Maintenance details: after intubation, sevoflurane decreased to FiO2 0.5%, to maintain BIS of 50. Remifentanil infusion 0.1 µg/kg/min, preceded by bolus of 0.25 µg/kg for 2 minutes Other information: femoral nerve block with 30 mL ropivacaine 0.5% on arrival in operating theatre. 1 g paracetamol given in recovery room, and, if score on VAS > 3, 1 mg IV morphine given every 5 minutes up to 10 mg |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: Department of Anaesthsia and Critical Care Unit, Lapeyronie University Hospital, France. Study authors declare no conflicts of interest Study dates: not reported Note: study includes a group with continuous spinal anaesthesia. We have not included data for this group in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses, unlikely to influence outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Low risk | No other sources of bias identified |
Cai 2012a.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 2216 Inclusion criteria
Exclusion criteria
Type of surgery: oesophagectomy, gastrectomy, nephrectomy, fracture reduction Baseline characteristics TIVA group
Inhalational maintenance group
Country: China Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 1106; 106 losses (anastomotic leaks, bleeding, respiratory failure, heart failure, inflammation); 1106 analysed using ITT: 1000 analysed PP Induction details: loading doses of fentanyl 4 µg/kg, propofol 3 mg/kg and vecuronium 0.08 mg/kg Maintenance details: fentanyl continuous infusion 0.03 µg/kg/min, propofol continuous infusion at a rate of 53.8 µg/kg/min injected with gradual increases in concentration of 0.4 µg/mL with initial target level of 1 µg/mL. Continuous infusion of vecuronium 0.5 µg/kg/min. BIS maintained at 40 to 60 Other information: premedication with 10 mg diazepam, 0.5 mg atropine im 30 minutes before GA Inhalational maintenance group Participants: n = 1110; 110 losses (anastomotic leaks, bleeding, respiratory failure, heart failure, inflammation); 1110 analysed using ITT; 1000 analysed PP Induction details: loading doses of fentanyl 4 µg/kg, propofol 3 mg/kg and vecuronium 0.08 mg/kg Maintenance details: continuous inhalation 2% to 3% end‐tidal concentration isoflurane. Continuous infusion of vecuronium 0.5 µg/kg/min. BIS maintained at 40 to 60 Other information: premedication same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported by grants from National Nature Science Foundation of China, and by Doctor funding Study dates: 2005 to 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computerized random number generator and block randomization |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperative assessment of MMSE was carried out by psychiatrists who were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for losses are described and balanced between group but number of losses is large (> 10%) and we were unclear whether this could influence outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | We noted a discrepancy between table 2 and the text in results section of the study report. Table 2 reports a big difference in MMSE scores at baseline, with very low scores in the inhalation group, and text reports no difference at baseline. We have assumed that table 2 has a typo, because baseline MMSE score is unusually low. We noted that data in this study differed from other studies. We did not identify any differences that could explain this, and we could not be certain whether other sources of unidentified bias were present |
Celik 2011.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 100 Inclusion criteria
Exclusion criteria
Type of surgery: urological surgery Baseline characteristics TIVA group
Inhalational maintenance group
Country: Turkey Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 50; 0 losses Induction details: premedicated with 0.06 mg/kg midazolam 45 minutes before surgery. Prior to induction 5 mL/kg of IV fluid. Bolus dose 1 µg/kg remifentanil (over 30 to 60 seconds), and infusion of remifentanil at rate of 0.5µg/kg/min added simultaneously. Propofol starting dose of 0.5 mg/kg and titrated thereafter at 10 mg every 10 seconds until participant was unresponsive to verbal commands. Rocuronium 0.6 mg/kg. Maintentance details: remifentanil 0.25 µg/kg/min. Propofol 2 mg/kg/hour to 8 mg/kg/hour. Fresh gas flow with 4 L/min oxygen 35% in air. Depth of anaesthesia adjusted according to haemodynamic parameters Other: tramadol 2 mg/kg administered for hyperalgesia 30 minutes before end of surgery Inhalational maintenance group Participants: n = 50; 0 losses Induction details: premedicated with 0.06 mg/kg midazolam 45 minutes before surgery. Prior to induction 5 mL/kg of IV fluid. Bolus dose 1 µg/kg remifentanil (over 30 to 60 seconds), and infusion of remifentanil at rate of 0.5µg/kg/min added simultaneously. Propofol starting dose of 0.5 mg/kg and titrated thereafter at 10 mg every 10 seconds until participant was unresponsive to verbal commands. Rocuronium 0.6 mg/kg Maintenance details: remifentanil 0.25 µg/kg/min. Sevoflurane end expiratory levels 0 to 4% and MAC values at 0.5 to 1. Fresh gas flow with 4 L/min oxygen 35% in air. Depth of anaesthesia adjusted according to haemodynamic parameters Other: tramadol 2 mg/kg administered for hyperalgesia 30 minutes before end of surgery |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly divided into groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants assessed in recovery room by an investigator who was blinded to group allocations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Low risk | No other sources of bias identified |
Chan 1996.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria
Exclusion criteria
Type of surgery: total hip replacement Baseline characteristics TIVA group
Inhalational maintenance group
Country: Canada Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 29; 0 losses Induction details: propofol at 0.75 mg/kg/min via electronic pump. Succinylcholine 1.0 mg/kg to 1.5 mg/kg to facilitate tracheal intubation Maintenance details: 60% N2O in O2. Propofol increased/decreased by 50% in response to 25% change in baseline BP or HR. Fentanyl 1 µg/kg (to a maximum of 4 µg/kg) with increase of propofol. Intraoperative muscle relaxation maintained with vecuronium. Propofol discontinued 5 minutes before end of surgery, N2O and O2 continued until end of surgery. Postoperative pain management with IV morphine as required. Use of clinical parameters (HR and BP) to monitor depth of anaesthesia Other information: evening before surgery, participants were given triazolam 0.125 mg to 0.25 mg, if required. Participants usual medication was withheld on morning of surgery. Then as premedication given 10 mL/kg IV crystalloid, then vecuronium 1 mg, and fentanyl 0.75 µg/kg Inhalational maintenance groups Participants: n = 31; 0 losses Induction details : bolus of 2 mg/kg thiopental, titrated to 4 mg/kg within 60 seconds as necessary. Succinylcholine 1.0 mg/kg to 1.5 mg/kg to facilitate tracheal intubation Maintenance details: 60% N2O in O2. 0.5% to 1.5% isoflurane end‐tidal concentration increased/decreased by 50% in response to 25% change in baseline BP or HR. Fentanyl 1 µg/kg (to a maximum of 4 µg/kg) with increase of propofol. Intraoperative muscle relaxation maintained with vecuronium. Isoflurane discontinued 5 minutes before end of surgery, N2O and O2 continued until end of surgery. Postoperative pain management with IV morphine as required Other information: premedication etc. same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated random number list |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Discharge from the PACU was assessed by a blinded independent investigator. Study authors do not report whether assessment of hypotension was done by a blinded investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Low risk | N2O in O2 used in both groups in addition to other agents. However, unlikely to affect results. |
Demeere 2006.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria
Exclusion criteria
Type of surgery: total hip replacement surgery Baseline characteristics (table reported by study authors appears to include data for number analysed not number randomized) TIVA group
Inhalational maintenance group (sevoflurane)
Inhalational maintenance group (desflurane)
Country: Belgium Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 20; 1 loss (reasons for losses described only as 'methodological problems'); 19 analysed Induction details: propofol 1% 50 mL, TCI 4 µg/mL via a Diprivusor, 3 µg/kg sufentanil. Atracurium 0.5 µg/kg Maintenance details: 50% N2O and 50% O2. Propofol TCI, 10 mL atracurium, and 10 µg sufentanil as necessary. To maintain BIS 'around 40' Other information: oral premedication with 0.25 or 0.5 mg alprazolam. BP maintained above 80 mmHg with ephedrine as required Inhalational maintenance group (sevoflurane) Participants: n = 20; 2 losses (reasons for losses described only as 'methodological problems'); 18 analysed Induction details: propofol 1% 20 mL (1 mg/kg/body weight to 2 mg/kg/body weight), 3 µg/kg sufentanil. Atracurium 0.5 µg/kg Maintenance details: 50% N2O and 50% O2. 10 mL atracurium, and 10 µg sufentanil as necessary. Sevoflurane to maintain BIS 'around 40' Other information: oral premedication with 0.25 mg or 0.5 mg alprazolam. BP maintained above 80 mmHg with ephedrine as required Inhalational maintenance group (desflurane) Participants: n = 20; 0 losses Induction details: propofol 1% 20 mL (1 mg/kg/body weight to 2 mg/kg/body weight), 3 µg/kg sufentanil. Atracurium 0.5 µg/kg Maintenance details: 50% N2O and 50% O2. 10 mL atracurium, and 10 µg sufentanil as necessary. Desflurane to maintain BIS 'around 40' Other information: oral premedication with 0.25 mg or 0.5 mg alprazolam. BP maintained above 80 mmHg with ephedrine as required |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Although reasons for losses are not well described, loss is small and unlikely to influence outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | Limited detail in paper ‐ does not include inclusion/exclusion criteria. We noted a difference in gender balance between groups. |
Egawa 2016.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 148 Inclusion criteria
Exclusion criteria
Type of surgery: one‐lung surgery Baseline characteristics TIVA group
Inhalational maintenance group
Country: Japan Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 74; 2 losses (1 withdrew prior to surgery; 1 had surgery cancelled); 72 analysed (at 5 days postoperatively) Induction details: propofol TCI 3 µg/mL to 4 µg/mL, bolus of fentanyl 2.0 µg/kg, to 2.5 µg/kg, Rocuronium 0.6 mg/kg to 0.9 mg/kg Maintenance details: TCI propofol, plus fentanyl, and epidural Other information: epidural inserted between thoracic 5 to 6 and 7 to 8 intervertebral spaces. No additional details Inhalational maintenance groups Participants: n = 74; 2 losses (1 withdrew prior to surgery; 1 had unsuccessful jugular vein cannulation); 72 analysed (at 5 days postoperatively) Induction details : propofol 1 mg/kg to 2 mg/kg and fentanyl 2.0 µg/kg to 2.5 µg/kg Maintenance details: sevoflurane, plus fentanyl and epidural. To maintain BIS 40 to 60 Other information: epidural same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: department funding. Study authors declared no conflicts of interest. Study dates: March 2007 to January 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated randomization list |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was assured by the use of numbered sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome was assessed by the same anaesthesiologist blinded to group allocation and not involved in intraoperative management |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses which were well reported |
| Selective reporting (reporting bias) | Unclear risk | Study authors report that clinical trials registration was not required in Japan at the time of the start of the study. Not feasible to judge risk of selective reporting bias |
| Other bias | Unclear risk | Participants in the sevoflurane groups appeared to have shorter duration of surgery and anaesthesia |
Epple 2001.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 124 Inclusion criteria
Exclusion criteria
Type of surgery: cataract surgery Baseline characteristics TIVA group
Inhalational maintenance group
Country: Germany Setting: PACU in hospital |
|
| Interventions |
TIVA group Participants: n = 62; 0 losses Induction details: propofol 1.5 mg/kg and remifentanil 1.5 µg/kg over 3 minutes, 0.15 mg/kg mivacurium Maintenance details: continuous infusion of propofol 0.05 mg/kg/min to 0.1 mg/kg/min and remifentanil 0.15 µg/kg/min to 0.3 µg/kg/min. Haemodynamic parameters used to monitor depth of anaesthesia Other information: received no medication before surgery Inhalational maintenance group Participants: n = 62; 0 losses Induction details: etomidate 0.1 mg/kg to 0.3 mg/kg and fentanyl 1.5 µg/kg, 0.15 mg/kg mivacurium Maintenance details: isoflurane 0.8 to 2.5 MAC and bolus of 0.1 mg fentanyl. Haemodynamic parameters used to monitor depth of anaesthesia |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported by a grant from Glaxo Wellcome GmbH Co., Hamburg, Germany Study dates: not reported Note: we identified an associated reference for this study (Kubitz 2001) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated randomization list |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Discharge from the PACU judged by unblinded anaesthetist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | Use of remifentanil and fentanyl differs between groups |
Geng 2017.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 150 Inclusion criteria
Exclusion criteria
Type of surgery: laparoscopic cholecystectomy Baseline characteristics TIVA group
Inhalational maintenance group (isoflurane)
Inhalational maintenance group (sevoflurane)
Country: China Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 50; 0 losses Induction details: 5 minutes of pre‐oxygenation, then midazolam 0.05 mg/kg, fentanyl 4 µg/kg, rocuronium 0.6 mg/kg. TCI 3.0 µg/kg propofol Maintenance details: propofol with target concentration 2.5 µg/mL to 3.0 µg/mL. Remifentanil 0.2 µg/kg/min to 0.3 µg/kg/min. To maintain BIS 40 to 50 Other information: all patients given crystalloids as required. All patients were given flurbiprofen 100 mg and granisetron 3 mg at beginning of operation, and 0.25% ropivacaine via local infiltration for postoperative analgesia Inhalational maintenance groups Participants: n = 50; 0 losses Induction details: 5 minutes of pre‐oxygenation, then midazolam 0.05 mg/kg, fentanyl 4 µg/kg, rocuronium 0.6 mg/kg. TCI 3.0 µg/kg propofol Maintenance details: isoflurane 1.0 MAC to 1.5 MAC. Remifentanil 0.2 µg/kg/min to 0.3 µg/kg/min. To maintain BIS 40 to 50 Other information: fluids and analgesics same as TIVA group Inhalational maintenance groups Participants: n = 50; 0 losses Induction details: 5 minutes of pre‐oxygenation, then midazolam 0.05 mg/kg, fentanyl 4 µg/kg, rocuronium 0.6 mg/kg. TCI 3.0 µg/kg propofol Maintenance details: sevoflurane 1.0 MAC to 1.5 MAC. Remifentanil 0.2 µg/kg/min to 0.3 µg/kg/min. To maintain BIS 40 to 50 Other information: fluids and analgesics same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: no funding and authors declare no conflicts of interest Study dates: December 2010 to June 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A blinded anaesthetist evaluated cognitive scores |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trials registration (ChiCTR‐OCC‐11001411). Not feasible to assess risk of selective reporting bias |
| Other bias | Unclear risk | Some differences in duration of anaesthesia, surgery times, and time to emergence from anaesthesia. We were not certain whether these differences were clinically significant. Also note that no ages were reported in baseline characteristics |
Gursoy 2015.
| Methods | RCT, parallel group, single‐centre | |
| Participants |
Total number of participants: 60 Inclusion criteria
Exclusion criteria
Type of surgery: laparotomy Baseline characteristics TIVA group
Inhalational maintenance group
Country: Turkey Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 30; 0 reported losses (study authors report use of ITT analysis) Induction details: propofol 3 mg/kg to 6 mg/kg, remifentanil 1 µg/kg, vecuronium 0.1 mg/kg Maintenance details: propofol infusion of 12 mg/kg/hour, then 9 mg/kg/hour, then 6 mg/kg/hour over 10 minutes. Remifentainil 0.15 µg/kg/hour to 0.30 µg/kg/hour. 67% air and 33% O2 Inhalational maintenance group Participants: n = 30; 0 reported losses (study authors report use of ITT analysis) Induction details: thiopentone 3 mg/kg to 5 mg/kg, vecuronium 0.1 mg/kg IV Maintenance details: 2% sevoflurane, with 67% N2O/33% O2 |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: study authors report no conflict of interest Study dates: not reported Note: study report in Turkish. Review authors used Google translate to assist with translation of key paragraphs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized to groups; no additional details. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Low risk | No other sources of bias identified |
Ishii 2016.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 59 Inclusion criteria
Exclusion criteria
Type of surgery: elective gastrectomy, colectomy, or rectectomy Baseline characteristics TIVA group
Inhalational maintenance group
Country: Japan Setting: single‐centre |
|
| Interventions |
TIVA group Participants: n = 29; 0 losses Induction details: insertion of epidural catheter, then induction with propofol 1 mg/kg to 1.5 mg/kg Maintenance details: propofol to maintain BIS 40 to 60 Other information: intraoperative analgesia given with injection of fentanyl or continuous infusion of 0.25% ropivacaine (6 mL/hour) Inhalational maintenance groups Participants: n = 30; 0 losses Induction details: insertion of epidural catheter, then induction with propofol 1 mg/kg to 1.5 mg/kg Maintenance details: sevoflurane to maintain BIS 40 to 60 Other information: analgesia same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: July 2009 to December 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized to groups; no additional details. |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment done by ICU nurses blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | No other sources of bias noted. However, report is short with limited detail on anaesthetic regimen |
Jellish 2003.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 60 (unclearly reported in paper, possibly 59 randomized participants) Inclusion criteria
Exclusion criteria
Type or surgery: carotid endarterectomy Baseline characteristics TIVA group
Inhalational maintenance group
Country: USA Setting: single‐centre |
|
| Interventions |
TIVA group Participants: n = 30; 0 losses Induction details : propofol 1.0 mg/kg to 1.5 mg/kg IV. Remifentanil infusion started at 0.25 µg/kg/min. Additional propofol 25 mg to 50 mg IV given if necessary to maintain MAP within 10 % pre‐induction values during intubation Maintenance details: propofol 50 µg/kg/min to 75 µg/kg/min. Remifentanil 0.125 µg/kg/min to 0.5 µg/kg/min. Adjusted to maintain haemodynamic parameters within 15% pre‐induction. N2O in O2 mix 60/40 Other information: hypertension non‐responsive to anaesthesia treated with sodium nitroprusside 0.5 µg/kg/min. Hypotension non‐responsive to anaesthesia treated with phenylephrine 40 µg to 80 µg IV. Tachycardia unresponsive to anaesthesia treated with esmolol 10 mg to mg 20 mg IV, bradycardia treated with glycopyrrolate 0.2 mg IV Inhalational maintenance group Participants: number of randomized participants is unclearly reported. We have assumed that 30 participants were randomized, with 1 loss (owing to technical difficulties with transoesophageal probe), and 29 participants were analysed. Induction details: propofol 1.5 mg/kg to 2 mg/kg IV, fentanyl 2 µg/kg. Additional propofol 25 mg to 50 mg IV given if necessary to maintain MAP within 10 % pre‐induction values during intubation Maintenance details: isoflurane 0.5% to 2% end‐tidal. Titrated to maintain MAP 15% pre‐induction values. N2O in O2 mix 60/40 Other information: other drugs to maintain stability same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer generated randomization |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant lost from inhalation group, which is unclearly reported. We have assumed that 30 participants were randomized to the inhalation group, with one loss. We were not concerned by risk of attrition bias because losses were few and unlikely to influence outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | Study includes comparison of remifentanil with fentanyl, which introduces methodological differences between groups. Also note differences in amount of propofol given at induction |
Juvin 1997.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 45 Inclusion criteria
Excluded criteria
Type of surgery: hip arthroplasty, knee arthroplasty, laminectomy, other orthopaedic surgery Baseline characteristics TIVA group
Inhalational maintenance group (isoflurane)
Inhalational maintenance group (desflurane)
Country: France Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 15; 1 loss (excluded owing to intraoperative complication); 14 analysed Induction details: propofol 1 mg/kg to 2 mg/kg, fentanyl 1 µg/kg to 2 µg/kg, vecuronium 0.1 mg/kg Maintenance details: 60% N2O in O2. Propofol titrated to maintain HR and BP within 20% of baseline. Study authors report mean (SD) infusion rates at 2.18 (± 1.24) mg/kg/hour Other information: premedication with oral hydroxyzine 100 mg. Additional fentanyl at 1 µg/kg at 40‐minute intervals depending on length of surgery Inhalational maintenance group (isoflurane) Participants: n = 15; 0 losses Induction details: propofol 1 mg/kg to 2 mg/kg, fentanyl 1 µg/kg to 2 µg/kg, vecuronium 0.1 mg/kg Maintenance details: 60% N2O in O2. Isoflurane titrated to maintain HR and BP within 20% of baseline. Fresh gas flow of 1.5 L/min. Study authors report mean (SD) concentration isoflurane at 0.33% (± 0.21%) Other info: premedication and use of fentanyl same as TIVA group Inhalational maintenance group (desflurane) Participants: n = 15; 1 loss (owing to sudden vaporizer failure); 14 analysed Induction details: propofol 1 mg/kg to 2 mg/kg, fentanyl 1 µg/kg to 2 µg/kg, vecuronium 0.1 mg/kg Maintenance details: 60% N2O in O2. Desflurane titrated to maintain HR and BP within 20% of baseline. Fresh gas flow of 1.5 L/min. Study authors report mean (SD) concentration desflurane 1.59% (± 1.02) Other information: premedication and use of fentanyl same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported by Pharmacia and Upjohn Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to groups; no additional information |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes assessed by a single investigator who was blinded to participants' group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants losses (1 participant in desflurane group, and 1 in propofol group); unlikely to influence outcome data |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | Some differences between groups in numbers for each type of surgery. Note balance of gender, with more female participants; balanced between groups and not a risk of bias within the study |
Kim 2015a.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria
Exclusion criteria
Type of surgery: orthopaedic surgery (hip replacement, knee replacement, long bone fracture fixation, spinal surgery) Baseline characteristics TIVA group
Inhalational maintenance group
Country: South Korea Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 30; 0 losses Induction details: premedication with midazolam 0.05 mg/kg im. Remifentanil and propofol based on Minto and Marsh pharmacokinetic model using TCI. Target effect‐site concentration 3 µg/mL propofol, 2.5 ng/mL remifentanil. Rocuronium 1.0 mg/kg Maintenance details: propofol‐remifentanil with 50% O2 and 50% air mix. Target effect‐site concentration 3 µg/mL propofol, 2.5 ng/mL remifentanil. Rocuronium 1.0 mg/kg. To maintain BIS near 50 (range 40 to 60) Other information: after surgery fentanyl administration using PCI Inhalational maintenance group Participants: n = 30; 2 losses (owing to surgery lasting more than 2 hours); 27 analysed Induction details: premedication with midazolam 0.05 mg/kg im. Propofol 1.5 mg/kg to 2.0 mg/kg, 3% to 4 % sevoflurane and 50% O2‐ air mixture. Rocuronium 1.0 mg/kg Maintenance details: sevoflurane with 50% O2 and 50% air mix. Adjusted to maintain BIS near 50 (range 40 to 60) Other information: fentanyl after surgery same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: grants from Chosun University Medical Research Institute. Study authors declare no competing interests Study dates: not reported Note: study has four comparison groups ‐ sevoflurane vs TIVA, with and without dexmedetomidine. For the review, we have only used the comparison groups without dexmedetomidine |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 2 participants in the inhalation group; few losses unlikely to influence outcome data |
| Selective reporting (reporting bias) | Low risk | Prospective trial registration (NCT01851005). Most outcomes were reported, although we noted that adverse events (secondary outcomes) were not included in the written report. For the purpose of our review, MAP was reported but not in terms of hypotension. |
| Other bias | Unclear risk | Differences between groups in use of remifentanil and fentanyl. Also, a higher ratio of female to male participants; however, this is balanced between groups |
Lindholm 2013.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 200 Inclusion criteria
Excluded criteria
Type of surgery: open abdominal aortic surgery Baseline characteristics TIVA group
Inhalational maintenance group
Country: Norway Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 100; losses unclearly reported; 96 analysed (PP) Induction details : premedication with paracetamol. Fentanyl 0.1 mg to 0.3 mg IV, and propofol 1 mg/kg to 2 mg/kg IV. Vecuronium 0.1 mg/kg, and 0.01 mg/kg to 0.02 mg/kg based on train‐of‐four Maintenance details: propofol 1 mg/kg/hour to 10 mg/kg/hour IV, and remifentanil 0.1 mg/kg/min to 0.7 mg/kg/min. Aim to maintain BIS 40 to 60. Additional regional anaesthesia: epidural 3 mL/hour to 12 mL/hour (bupivacaine 1 mg/mL, fentanyl 2 µg/mL, adrenaline 2 µg/mL) Other information: morphine 1 mg to 10 mg IV as rescue analgesia Inhalational maintenance group Participants: n = 100; losses unclearly reported; 97 analysed (PP) Induction details : premedication with paracetamol as for TIVA. Fentanyl 0.1 mg to 0.3 mg IV and thiopental sodium 3 mg/kg to 6 mg/kg IV. Vecuronium as for TIVA Maintenance details: balanced anaesthesia with sevoflurane at 0.7 MAC to 1.5 MAC, and repeated doses of fentanyl 0.05 mg to 0.1 mg IV. Aim to maintain BIS 40 to 60 Additional regional anaesthesia: epidural 3 mL/hour to 12 mL/hour (bupivacaine 1 mg/mL, fentanyl 2 µg/mL, adrenaline 2 µg/mL) Other information: morphine same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: institution or department funding. One author received fees for presentations at Baxter AS Norway Study dates: February 2008 to February 2012 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Quote: "after informed consent was given, patients selected a blank envelope with the randomization code inside from a box containing envelopes for all remaining patients to be included." Study does not report if envelopes were opaque and sealed. Unclear if this is a sufficient method to conceal group allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Postoperative care was blinded. However, study authors do not report who collected data for POCD |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small loss of participant data. Reasons for losses are unclearly reported, however loss is < 10% and balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Prospective registration with clinical trials register (NCT00538421). However, outcomes are not reported in trials register documents; not feasible to assess risk of selective outcome reporting bias |
| Other bias | Unclear risk | Groups differ in use of fentanyl and remifentanil which presents methodological differences between groups |
Liu 2013.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 120 Inclusion criteria
Exclusion criteria
Type of surgery: spinal surgery Baseline characteristics TIVA group
Inhalational maintenance group
Country: China Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 60; 8 losses (reasons reported overall, not by group, to include: 'lost to follow‐up', death, other surgeries before 2‐year follow‐up time point); 52 analysed Induction details: midazolam 0.05 mg/kg, sufentanil 0.5 µg/kg, vecuronium 0.5 µg/kg, propofol 1.0 mg/kg Maintenance details: propofol 4 mg/kg/hour to 6 mg/kg/hour continuously, intermittent vecuronium 0.5 mg/kg. To maintain BIS 40 to 50 Other information: during surgery, patients given lactated Ringer's solution and hetastarch. Continuous infusion of sufentanil 0.6 µg/kg/hour, tropisetron 6 µg/kg/hour, single bolus of sufentanil 0.015 µg/kg and tropisetron 1.5 µg/kg over a 15‐minute interval for postoperative pain relief Inhalational maintenance group Participants: n = 60; 5 losses (reasons reported overall, not by group, to include: 'lost to follow‐up', death, other surgeries before 2‐year follow‐up time point); 55 analysed Induction details: midazolam 0.05 mg/kg, sufentanil 0.5 µg/kg, vecuronium 0.5 µg/kg, propofol 1.0 mg/kg Maintenance details: sevoflurane 2% to 3 % in pure O2. Adjusted to maintain BIS 40 to 50 Other information: fluids and analgesic management etc. same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported by the Department of Anesthesiology, Beijing Military General Hospital. The authors have no financial or other conflicts of interest to disclose Study dates: January 2007 to January 2009 Note: study has 3 arms: propofol vs sevoflurane vs lidocaine epidural. We have not included data for the lidocaine comparison arm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only review outcome of interest is mortality. Blinding of assessors is not described but lack of blinding is unlikely to influence mortality data |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High number of losses, which are reported with reasons. We have used this as data for mortality outcome |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. It is not feasible to assess risk of selective outcome reporting |
| Other bias | Low risk | No other sources of bias identified |
Longas 2004.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria
Exclusion criteria
Type of surgery: carotid endarterectomy Baseline characteristics TIVA group
Inhalational maintenance group (sevoflurane MAC 1.0)
Inhalational maintenance group (sevoflurane MAC 1.5)
Country: Spain Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 20; 0 losses Induction details: premedication the night before surgery with diazepam 10 mg given orally, then 30 minutes before surgery with midazolam 0.1 mg/kg im. Induction with propofol 2 mg/kg, cisatracurium 0.2 mg/kg, and fentanyl 3 µg/kg to 4 µg/kg Maintenance details: mix of O2 and air, FiO2 of 0.4. Fentanyl 0.05 mg, cisatracurium 0.1 mg/kg IV. Propofol 5 mg/kg/hour. To maintain a BIS 40 to 60 Other information: for postoperative analgesia methadone 0.1 mg/kg, and metamizole in doses of 2 g IV every 8 hours. Analgesia started 30 minutes before end of surgery Inhalational maintenance group (sevoflurane MAC 1.0) Participants: n = 20; 0 losses Induction details: premedication the night before surgery with diazepam 10 mg given orally, then 30 minutes before surgery with midazolam 0.1 mg/kg im. Then induction with propofol 2 mg/kg, cisatracurium 0.2 mg/kg, and fentanyl 3 µg/kg to 4 µg/kg Maintenance details: mix of O2 and air, FiO2 of 0.4. Fentanyl 0.05 mg, cisatracurium 0.1 mg/kg IV. Sevoflurane MAC 1.0. To maintain a BIS 40 to 60 Other information: postoperative analgesia same as TIVA group Inhalational maintenance group (sevoflurane MAC 1.5) Participants: n = 20; 0 losses Induction details: premedication the night before surgery with diazepam 10 mg given orally, then 30 minutes before surgery with midazolam 0.1 mg/kg im. Then induction with propofol 2 mg/kg, cisatracurium 0.2 mg/kg, and fentanyl 3 µg/kg to 4 µg/kg Maintenance details: mix of O2 and air, FiO2 of 0.4. Fentanyl 0.05 mg, cisatracurium 0.1 mg/kg IV. Sevoflurane MAC 1.5. To maintain a BIS 40 to 60 Other information: postoperative analgesia same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note: the study included a 4th comparison group of remifentanil. We did not include this group in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to assess risk of selective outcome reporting |
| Other bias | Low risk | No other sources of bias identified |
Luntz 2004.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 96 Inclusion criteria
Exclusion criteria
Type of surgery: ophthalmic surgery Baseline characteristics TIVA group
Inhalational maintenance group (propofol/sevoflurane)
Inhalational maintenance group (total sevoflurane)
Note: table of baseline characteristics is not reported. Study authors report "There were no significant differences between the patient groups with regard to age, gender, height, weight and ASA physical status" Country: Germany Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 32; 0 losses Induction details: propofol 2 mg/kg, continuous infusion of remifentanil 20 µg/ kg/hour. Atracurium 0.3 mg/kg to 0.5 mg/kg Maintenance details: continuous infusion of propofol 4 mg/kg/hour to 8 mg/kg/hour. Remifentanil at 10 µg/kg/hour Inhalational maintenance group (propofol/sevoflurane) Participants: n = 32; 0 losses Induction details: propofol 2 mg/kg, continuous infusion of remifentanil 20 µg/ kg/hour. Atracurium 0.3 mg/kg to 0.5 mg/kg Maintenance details: sevoflurane end‐tidal concentration 0.6% to 1.2%. Remifentanil 10µg/kg/hour Inhalational maintenance group (total sevoflurane) Participants: n = 32; 0 losses Induction details : continuous infusion of remifentanil 20 µg/ kg/hour. Atracurium 0.3 mg/kg to 0.5 mg/kg. After 1 minute pre‐oxygenation, vaporizer adjusted stepwise up to 8% sevoflurane until eyelash reflex was abolished, then reduced to 5% Maintenance details: sevoflurane end‐tidal concentration 0.6% to 1.2%. Remifentanil 10µg/kg/hour |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: supported in part by a grant from Abbott Laboratories, Wiesbaden, Germany Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Relevant reported outcome is for hypotension. Study authors do not report who collected this data and whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Low risk | Baseline characteristics table not reported, but study authors reported no differences. No other sources of bias identified |
Micha 2016.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria
Exclusion criteria
Type of surgery: tumour resection (non‐cardiovascular or neurosurgical) Baseline characteristics TIVA group
Inhalational maintenance group
Country: Greece Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 40; 4 losses (2 patients had operations cancelled; 2 were haemodynamically unstable); 36 analysed Induction details: propofol 2 mg/kg, and fentanyl 2 µg/kg Maintenance details: propofol 6 mg/kg/hour to 10 mg/kg/hour. To maintain BIS 40 to 60 Other information: postoperative analgesia with morphine to achieve a VAS score ≤ 3 Inhalational maintenance groups Participants: n = 40; 3 losses (no data available at 9 months); 37 analysed = 37 Induction details: propofol 2 mg/kg, and fentanyl 2 µg/kg Maintenance details: sevoflurane 2% to 3%. To maintain BIS 40 to 60 Other information: postoperative analgesia same as TIVA group |
|
| Outcomes |
Notes
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: June 2010 to July 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used; no additional details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessment of cognitive function completed by personnel blinded to study groups |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reason for losses in sevoflurane group owing to loss of data at 9 months; however, data time points are at 7 days as well as 9 months postoperatively |
| Selective reporting (reporting bias) | Unclear risk | Clinical trials registration not reported. Not feasible to assess risk of selective outcome reporting bias |
| Other bias | Low risk | No other sources of bias identified |
Moffat 1995.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 40 Inclusion criteria
Exclusion criteria
Type of surgery: cataract extraction and lens implantation Baseline characteristics TIVA group
Inhalational maintenance group
Country: Scotland, UK Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 20; 0 losses Induction details: premedication with metoclopramide 10 mg 1 hour before surgery. Topical anaesthesia (1% amethocaine) applied to non‐operative eye. Propofol with initial plasma concentration of 6 µg/mL reducing to 4 µg/mL after 10 minutes. Mix of 70% N2O in O2 throughout the procedure Maintenance details: 4 µg/mL propofol TCI Other information: topical anaesthesia with 1% amethocaine in operative eye before surgical incision. Airway maintained with LMA Inhalational maintenance group Participants: n = 20; 0 losses Induction details: premedication with metoclopramide 10 mg 1 hour before surgery. Topical anaesthesia (1% amethocaine) applied to non‐operative eye. Induction with etomidate 0.25 mg/kg and vecuronium 0.075 mg/kg. Maintenance details: Mix of 70% N2O in oxygen, and 0.5% to 1% isoflurane Other information: topical anaesthesia with 1% amethocaine in operative eye before surgical incision. Airway maintained with intubation |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | We noted use of different types of airway management which was because of the study aim to assess anaesthetic management using neuromuscular blockade vs no neuromuscular blockade for intraocular pressure |
Nishikawa 2004.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 50 Inclusion criteria
Exclusion criteria
Type of surgery: laparoscopic surgery (choledocholithotomy, colectomy, sigmoidectomy) Baseline characteristics TIVA group
Inhalational maintenance group
Country: Japan Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 25; 0 losses Induction details: 100% O2 via face mask for 3 minutes prior to induction. Induction with propofol using 4 µg/mL TCI. Use of 2% lidocaine solution for injection pain Maintenance details: 4 µg/mL propofol TCI. Study authors report mean (SD) range of 1.2 (± 0.2) µg/mL to 2.7 (± 0.2) µg/mL propofol. Use of clinical signs to maintain anaesthesia Additional regional anaesthesia: epidural anaesthesia: 6 mL to 8 mL of 1.5% lidocaine, followed by continuous epidural administration at a rate of 4 mL/hour to 6 mL/hour throughout surgery Inhalational maintenance group Participants: n = 25; 0 losses Induction details: 100% oxygen via face mask for 3 minutes prior to induction. 5% sevoflurane and 100% oxygen at 6 L/min until inspired limb‐drug concentration was > 4%. Vecuronium 0.1 mg/kg. Maintenance details: sevoflurane with O2/air mix at total gas flow of 3 L/min. Vecuronium 1 mg to 2 mg IV boluses as required. Study authors report mean (SD) range of 0.9% (± 0.1%) to 1.7% (± 0.4%) sevoflurane |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned by a sealed envelope technique". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Described as "randomly assigned by a sealed envelope technique". Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Delirium was assessed by a psychiatrist blinded to intervention group. Data on emergence times was assessed by a nurse who was blinded to intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Low risk | No other source of bias identified |
Rohan 2005.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 30 Inclusion criteria:
Exclusion criteria
Type of surgery: minor urological or gynaecological surgery Baseline characteristics TIVA group
Inhalational maintenance group
Country: Ireland Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 15; 0 losses Induction details: 500 mL crystalloid solution, fentanyl 1 µg/kg IV, propofol TCI using a Deprifusor Maintenance details: TCI propofol adjusted to maintain adequate depth of anaesthesia, at discretion of attending anaesthetist. 50% O2 and 50% air Inhalational maintenance group Participants: n = 15; 0 losses Induction details: 500 mL crystalloid solution, fentanyl 1 µg/kg IV. Incremental dose of sevoflurane by tidal volume inhalation induction technique Maintenance details: 50% O2 and 50% air. No additional information for maintenance |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: funded entirely from the resources of the Department of Anesthesia, Critical Care and Pain Medicine, Mater Misericordiae Hospital Study dates: not reported Note: study also includes an age‐matched control group of participants which we did not include in the review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized to groups; no additional details |
| Allocation concealment (selection bias) | Low risk | Use of sequentially numbered sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the investigator who undertook patient enrolment, neuropsychological tests and blood tests did not deliver anaesthesia to the patient and, therefore, was unaware of study group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to assess risk of selective outcome reporting |
| Other bias | Unclear risk | No detail on doses of anaesthetic drugs. Unable to assess whether groups were equivalent |
Tan 2009.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 60 Inclusion criteria
Exclusion criteria
Type of surgery: abdominal surgery Baseline characteristics TIVA group
Inhalational maintenance group
Note: Study authors do not report a baseline characteristics table. Study authors report no differences between group in age, weight, height and general condition Country: China Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 30; 0 losses Induction details: propofol IV 1.5 mg/kg to 2 mg/kg, fentanyl 2 µg/kg to 4 µg/kg, vecuronium 0.1 mg/kg Maintenance details: propofol IV 100 µg/kg/min to 150 µg/kg/min, fentanyl and vecuronium as required Inhalational maintenance group Participants: n = 30; 0 losses Induction details: propofol IV 1.5 mg/kg to 2 mg/kg, fentanyl 2 µg/kg to 4 µg/kg, vecuronium 0.1 mg/kg Maintenance details: 1% to 2 % isoflurane, fentanyl and vecuronium as required |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Note: study report is in Chinese. We have used Google translate for essential paragraphs. We noted that this study was reported by a single author and may not be the original study report; we checked the study details against other included studies for duplication but found no duplication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomized to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | No baseline characteristics table. Limited information in short report, and we noted that this study was reported by a single author |
Tanaka 2017.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 100 Inclusion criteria
Exclusion criteria
Type of surgery: total knee arthroplasty Baseline characteristics TIVA group
Inhalational maintenance group
Country: US Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 50; 11 losses (3 withdrawn; other reasons include early hospital discharge, oversedation, respiratory distress, PONV, and pain ‐ not reported by group); 39 analysed Induction details: femoral nerve block with initial bolus of 30 mL 0.25% ropivacaine as well as placement of indwelling catheter. Sedation with fentanyl and midazolam provided for femoral nerve block at discretion of regional anaesthesia team. Induction with propofol 1 mg/kg, fentanyl 1 µg/kg to 2 µg/kg, rocuronium 0.4 mg/kg, all dosed according to lean body weight Maintenance details: propofol. Use of Sedline to maintain PSI 30 to 50 Other information: after surgery, a continuous infusion of 0.2% ropivacaine at 6 mL/hour was initiated in recovery room and adjusted to maximum of 10 mL/hour for next 48 hours. PCA device to administer IV hydromorphone with standardized dosing and lock‐out period Inhalational maintenance groups Participants: n = 50; 10 losses (1 withdrawn; other reasons include early hospital discharge, oversedation, respiratory distress, PONV, and pain ‐ not reported by group); 40 analysed Induction details: femoral nerve block with initial bolus of 30 mL 0.25% ropivacaine as well as placement of indwelling catheter. Sedation with fentanyl and midazolam provided for femoral nerve block at discretion of regional anaesthesia team. Induction with propofol 1 mg/kg, fentanyl 1 µg/kg to 2 µg/kg, rocuronium 0.4 mg/kg, all dosed according to lean body weight Maintenance details: desflurane. Use of Sedline to maintain PSI 30 to 50 Other information: after surgery, a continuous infusion of 0.2% ropivacaine at 6 mL/hour was initiated in recovery room and adjusted to maximum of 10 mL/hour for next 48 hours. PCA device to administer IV hydromorphone with standardized dosing and lock‐out period |
|
| Outcomes |
Note: we interpreted bar charts provided by study authors (from email communication) for cognitive function tests. In meta‐analysis, we used data for Trail Making part A. |
|
| Notes |
Funding/declarations of interest: research grant from Baxter Healthcare Corporation Study dates: October 2010 to August 2014 Note: all participants are obese |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses who administered CAM assessment were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study authors do not report reasons for losses by each group, and data is reported inconsistently between clinical trials register documents and published study report. Overall losses are high |
| Selective reporting (reporting bias) | High risk | Retrospectively registered with clinical trials register (NCT01270620). Not feasible to assess risk of selective reporting bias from this document. However, we noted that MMSE was an outcome in the methods section of the published report but was not reported in results. In addition, we noted a difference in data for postoperative delirium, and length of stay was reported for a different number of participants. Overall, we judged risk of selective reporting bias as high |
| Other bias | Unclear risk | We noted a difference in gender balance between groups; unclear if this is clinically important |
Tang 2014.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 220 Inclusion criteria
Exclusion criteria
Type of surgery: radical rectal resection surgery Baseline characteristics TIVA group
Inhalational maintenance group
Country: China Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 110; 9 losses (declined to participate in follow‐up at day 7); 101 analysed Induction details: midazolam 0.03 mg/kg to 0.04 mg/kg IV, fentanyl 0.002 mg/kg to 0.003 mg/kg IV, vecuronium 0.15 mg/kg to 0.2 mg/kg. Then propofol 1.5 mg/kg to 2 mg/kg IV Maintenance details: propofol 6 mg/kg/hour to 10 mg/kg/hour. To maintain BIS 30 to 60. Remifentanil 9 µg/kg/hour to 12 µg/kg/hour continuous IV infusion, vecuronium intermittent IV infusion Other information: all patients had PCI 150 mL saline with fentanyl 1.5 mg, tropisetron 12 mg, infusion rate 2 mL/hour, with 15‐minute lockout Inhalational maintenance group Participants: n = 110; 11 losses (declined to participate in follow‐up at day 7); 99 analysed Induction details: midazolam 0.03 mg/kg to 0.04 mg/kg IV, fentanyl 0.002 mg/kg to 0.003 mg/kg IV, vecuronium 0.15 mg/kg to 0.2 mg/kg. Then 8% sevoflurane (fresh gas flow 6 L/min, decreased to 3% to 4% after loss of consciousness with fresh gas flow 1 L/min to 2 L/min) Maintenance details: sevoflurane 2% to 3%. To maintain BIS 30 to 60. Remifentanil 9 µg/kg/hour to 12 µg/kg/hour continuous IV infusion, vecuronium intermittent IV infusion Other information: analgesics same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: study authors report that authors received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors Study dates: January 2010 to November 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated, blocked random‐allocation sequence |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetist to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "to ensure blinding, neuropsychological assessment work was carried out by a physician trained in psychology. Neither the physician nor the patient knew which anaesthetic had been used during surgery" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some loss of participant data at about 10%. It is unclear whether this loss could influence outcome data. |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Low risk | No other sources of bias identified. |
Trembach 2012.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number or randomized participants: 99 Included criteria
Excluded criteria
Type of surgery: laparoscopic cholecystectomy Baseline characteristics not reported (abstract only) Country: not reported Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 45; 0 reported losses Described as propofol‐fentanyl TIVA. No additional details in abstract Inhalational maintenance group Participants: n = 44; 0 reported losses Described a VIMA. No additional details in abstract |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported Very limited detail in abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details. Abstract only |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details. Abstract only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No details. Abstract only. We have assumed there were no losses |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | Limited detail in abstract, unable to assess risk of other biases. Description of inhalational maintenance does not include fentanyl/remifentanil |
Tylman 2011.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 50 Inclusion criteria
Exclusion criteria
Types of surgery: colorectal surgery for rectal or colon cancer Baseline characteristics TIVA group
Inhalational maintenance group
Country: Sweden Setting: hospital |
|
| Interventions |
TIVA group Participants: n = 25; 0 losses Induction details : propofol TCI 3 µg/mL. Continuous infusion of remifentanil 0.25 µg/kg/min Maintenance details: propofol 2 µg/mL. Remifentanil 0.15 µg/kg/min Additional regional anaesthesia: epidural anaesthesia of 5 mg/mL bupivacaine, and 5 µg/mL epinephrine at rate of 4 mL to 5 mL during surgery. Postoperatively participants epidural changed to 1 mg/mL bupivacaine, 2 µg/mL fentanyl, 2 µg/mL epinephrine at rate of 5 mL/hour to 12 mL/hour Other information: before induction of anaesthesia participants given 1 µg/kg to 2 µg/kg fentanyl IV, and standard dose of rocuronium Inhalational maintenance group Participants: n = 25; 4 losses (did not meet study inclusion criteria); 21 analysed Induction/maintenance details: sevoflurane with 60% O2 throughout surgery. Concentration not reported. We assume that induction was also with sevoflurane Additional regional anaesthesia: epidural anaesthesia of 5 mg/mL bupivacaine, and 5 µg/mL epinephrine at rate of 4mL to 5 mL during surgery. Postoperatively participants epidural changed to 1 mg/mL bupivacaine, 2 µg/mL fentanyl, 2 µg/mL epinephrine at rate of 5 mL/hour to 12 mL/hour Other information: fentanyl and rocuronium same as TIVA group |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to groups; no additional details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetist to intervention groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of participants (all in inhalation group) after randomization because these participants were diagnosed with additional conditions (ulcerative colitis and Crohn's disease). Decision to remove these participants was to avoid confounding. Post‐hoc decision which is imbalanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Unclear risk | Differences in groups in use of remifentanil and fentanyl. Also, study authors do not report concentration of sevoflurane. Note limited information in baseline characteristics table, and lack of inclusion/exclusion criteria |
Zhang 2015.
| Methods | RCT, parallel design, single‐centre | |
| Participants |
Total number of randomized participants: 80 Inclusion criteria
Exclusion criteria
Type of surgery: radical surgery for gastric cancer Baseline characteristics TIVA group (without epidural)
Inhalational maintenance group (without epidural)
TIVA group (with epidural)
Inhalational maintenance group (with epidural)
Country: China Setting: hospital |
|
| Interventions |
TIVA group (without epidural) Participants: n = 20; 0 losses Induction details: TCI propofol 4.0 µg/mL, 3 µg/kg to 4 µg/kg fentanyl and 0.2 mg/kg cisatracurium IV Maintenance details: fentanyl IV 0.15 µg/kg/min to 0.35 µg/kg/min, TCI propofol 1.5 µg/mL to 3.0 µg/mL. To maintain BIS 40 to 60 Other information: 30 minutes before end of surgery, 0.6 µg to µg 1 µg fentanyl IV Inhalational maintenance group (without epidural) Participants: n = 20; 0 losses Induction details: 8% sevoflurane at high‐flow rate, 8 L/min to 10 L/min. After loss of consciousness, adjusted to 2 L/min to achieve end‐tidal concentration of 2% Maintenance details: continuous inhalation end‐tidal concentration of 1.5% to 3.5%. Cisastracurium 0.05 mg/kg to 0.1 mg/kg. To maintain BIS 40 to 60 Other info: 30 minutes before end of surgery, 0.6 µg to 1 µg fentanyl IV TIVA group (with epidural) Participants: n = 20; 0 losses Induction details: TCI propofol 4.0 µg/mL, 3 µg/kg to 4 µg/kg fentanyl and 0.2 mg/kg cisatracurium IV Maintenance details: fentanyl IV 0.15 µg/kg/min to 0.35 µg/kg/min, TCI propofol 1.5 µg/mL to 3.0 µg/mL. 30 minutes before skin incision: 10 mL ropivacaine and 2 µg/mL, fentanyl injected into epidural space Other info: once epidural puncture was performed, a test dose of 3 mL 2% lidocaine to confirm level and absence of adverse reactions. 30 minutes before end of surgery, 10 mL mixed anaesthesia solution Inhalational maintenance group (with epidural) Participants: n = 20; 0 losses Induction details: 8% sevoflurane at high‐flow rate, 8 L/min to 10 L/min. After loss of consciousness, adjusted to 2 L/min. to achieve end‐tidal concentration of 2% Maintenance details: 30 minutes before skin incision: 10 mL ropivacaine and 2 µg/mL fentanyl injected into epidural space. Continuous inhalation end‐tidal concentration of 1.5% to 3.5% sevoflurane. Cisastracurium 0.05 mg/kg to 0.1 mg/kg. BIS 40 to 60 Other info: once epidural puncture was performed, a test dose of 3 mL 2% lidocaine to confirm level and absence of adverse reactions. 30 minutes before end of surgery, 10 mL mixed anaesthesia solution |
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: not reported Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a random number table |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind anaesthetists to intervention groups |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent loss of study participants |
| Selective reporting (reporting bias) | Unclear risk | Study authors do not report clinical trials registration. Not feasible to judge risk of selective outcome reporting |
| Other bias | Low risk | No other sources of bias identified |
AAA: abdominal aortic aneurysm aMCI: amnesic mild cognitive impairment ApoE: apoliproprotein E ASA: American Society of Anesthesiologists BDI: Beck Depression Inventory BIS: bispectral index BMI: body mass index BP: blood pressure CAM: confusion assessment method DRS: delirium rating scale DSST: Digit Symbol Substitution Test FiO2: fraction of inspired oxygen GA: general anaesthesia HR: heart rate ICU: intensive care unit im: intramuscular IV: intravenous(ly) IQR: interquartile range ITT: intention to treat LMA: laryngeal mask airway MAC: minimum alveolar concentration MAP: mean arterial pressure MCI: mild cognitive impairment M/F: male/female MI: myocardial infarction MMSE: Mini‐Mental State Examination MMT: Mini Mental Test n: number of randomized participants per group N2O: nitrous oxide NYHA: New York Heart Association O2: oxygen PACU: postanaesthesia care unit PCA: patient controlled analgesia PCI: percutaneous coronary intervention POCD: postoperative cognitive dysfunction PONV: postoperative nausea and vomiting PP: per protocol PSI: patient state index RAVLT: Rey Auditory Verbal Learning test RCT: randomized control trial SBP: systolic blood pressure SD: standard deviation SOFA: Sequential Organ Failure Assessment T8‐T10: epidural given between the 8th and 9th, or the 9th and 10th thoracic vertebrae TCI: target‐controlled infusion TDT: Trieger Dot Test TEE: transoesophageal echocardiography TIVA: total intravenous anaesthesia TKA: total knee arthroplasty VAS: visual analogue scale VIMA: volatile induction and maintenance anaesthesia vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arar 2005 | RCT, measuring effects of sevoflurane versus isoflurane versus propofol infusions on postoperative recovery criteria in geriatric participants. Outcomes measured: time to spontaneous eye opening, extubation, response to verbal stimuli, and orientation. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Arnaoutoglou 2007 | RCT, measuring effects of propofol versus sevoflurane on the production of free oxygen radicals during total knee arthroplasty in elderly participants. Outcomes measured: MDA levels. Post‐hoc decision to exclude studies that did not measure review outcomes |
| But 2003 | Unclear if this is an RCT. Measures effects of sevoflurane versus propofol on hepatic and renal functions in participants > 65 years of age. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Carles 2008 | RCT, measuring effects of sevoflurane versus propofol versus spinal anaesthesia on levels of interstitial glycolysis metabolites in elderly participants. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Doe 2016 | RCT, measuring effects of sevoflurane versus propofol on jugular venous bulb oxygenation (SjO2) and regional oxygen saturation in participants undergoing robotic‐assisted laparoscopic prostatectomy. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Filipovic 2007 | RCT, measuring effects of anaesthetics on left ventricular diastolic function in participants aged between 18 and 75. Outcomes measured: haemodynamic parameters. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Fredman 2002 | RCT, measuring the effects of propofol verses sevoflurane on postanaesthesia recovery in geriatric participants. Outcomes measured: emergence time, time to orientation, postanaesthesia recovery scores, and therapeutic interventions. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Gasowska 1999 | RCT, measuring effects of halothane versus isoflurane versus propofol on venous admixture in participants between 28 to 72 years of age. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Gauger 2008 | RCT, measuring effects of propofol on postoperative nausea and vomiting in participants undergoing thyroid and parathyroid operations. Outcomes measured: occurrences of nausea and vomiting. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Guedes 1988 | RCT, measuring effects of propofol versus enflurane on intraocular pressure in elderly participants. Outcomes measured: haemodynamic parameters. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Halberg 1996 | Unclear if this is an RCT. A pharmaco‐economic evaluation of anaesthesia in ambulatory surgery comparing desflurane verses isoflurane and propofol. Unable to source full text. Post‐hoc decision to exclude studies that did not measure review outcomes. Decision made from information in the abstract |
| Holst 1993 | Unclear if this is an RCT. A comparison of the intraoperative sympatho‐adrenergic response and the postoperative vigilance of a propofol/alfentanil anaesthesia to a conventional isoflurane anaesthesia. Unable to source full text. Post‐hoc decision to exclude studies that did not measure review outcomes. Decision made from information in the abstract |
| Hosseinzadeh 2013 | RCT, measuring effects of propofol versus isoflurane on incidence of postoperative nausea and vomiting in participants between 16 to 65 year of age. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Ionescu 2009 | Unclear if this is an RCT. Effects of TIVA versus isoflurane on postoperative nausea and vomiting, and patient satisfaction, in participants undergoing laparoscopic cholecystectomy. Unable to source full text. Post‐hoc decision to exclude studies that did not measure review outcomes. Decision made from information in the abstract |
| Ito 2012 | RCT, measuring effects of TIVA versus desflurane on postoperative emergence in elderly participants. Outcomes measured: presence of spontaneous speech, early recovery time, time to extubation, eye opening, and squeezing fingers on command. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Kadoi 2009a | RCT, measuring effects of propofol versus sevoflurane on cerebrovascular carbon dioxide reactivity in elderly participants. Outcomes measured: cerebral circulation. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Kim 2015b | RCT, measuring effects of propofol versus desflurane on postoperative spirometry in elderly after knee surgery. Outcomes measured: spirometry parameters. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Konstantopoulos 2013a | RCT, measuring effects of sevoflurane versus propofol on recovery characteristics in older participants. Outcomes measured: haemodynamic stability, recovery characteristics, postoperative nausea and vomiting, and pain intensity. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Kvarnstrom 2012 | RCT, measuring effects of sevoflurane versus propofol on complement activation and the release of inflammatory interleukins in participants undergoing major abdominal surgery. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Malcharek 2015 | RCT, measuring effects of desflurane versus propofol on tcMEP amplitudes in participants without PMDs undergoing CEA. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Manolescu 2012 | Unclear if this is an RCT. Evaluation of cardioprotective effects of sevoflurane versus propofol in patients with cardiac risk, undergoing noncardiac surgery. Unable to source full text. Post‐hoc decision to exclude studies that did not measure review outcomes. Decision made from information in the abstract |
| Mets 1992 | RCT, measuring effects of propofol versus isoflurane in elderly participants undergoing ophthalmic surgery. Outcomes measured: haemodynamic parameters. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Murray 1994 | RCT, measuring effects of isoflurane versus propofol on hepatic glutathione‐S‐transferase concentrations. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Mutch 1995 | RCT, measuring effects of propofol versus isoflurane in older patients undergoing carotid endarterectomy. Outcomes measured: haemodynamic parameters. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Ohe 2014 | Unclear if this is an RCT. Compares effects of sevoflurane versus propofol on preventing intraoperative hypothermia. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Oikkonen 1992 | RCT, measuring effects of isoflurane versus alfentanil‐methohexitone verses propofol on arterial pressure or heart rate in geriatric participants. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Passot 2005 | RCT, measuring effects of target‐ versus manually‐controlled infusion of propofol and desflurane in elderly participants undergoing hip fracture surgery. Outcomes measured: haemodynamic parameters. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Pirttikangas 1996 | RCT, measuring effects of propofol versus combined isoflurane in elderly participants undergoing ophthalmic surgery. Outcomes measured: immune responses. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Polarz 1995 | RCT, measuring effects of isoflurane versus propofol on participants undergoing ophthalmic surgery. Outcomes measured: intraocular pressure. Unable to source full text. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Sal'nikov 2003 | Unclear if this is an RCT. A comparative evaluation of "cerebral oximetry" during anaesthesia with xenon and other anaesthetics. Unable to source full text. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Schilling 2007 | RCT, measuring effects of propofol versus desflurane in older participants undergoing open thoracic surgery. Outcomes measured: alveolar inflammatory response to one‐lung ventilation. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Schilling 2011 | RCT, measuring effects of propofol versus desflurane versus sevoflurane in older participants undergoing open thoracic surgery. Outcomes measured: alveolar inflammatory response. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Schäfer 2002 | RCT, measuring effects of propofol versus sevoflurane in participants aged over 50 undergoing cataract surgery. Outcomes measured: intraocular pressure. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Shao 2013 | RCT, measuring effects of propofol versus sevoflurane in elderly participants. Outcomes measured: quality of neuromuscular blockade with cisatracurium. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Sohn 2008 | RCT, measuring effects of propofol versus sevoflurane in elderly participants undergoing total knee arthroplasty. Outcomes measured: haemodynamic parameters. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Sugata 2012 | RCT, measuring effects of propofol versus sevoflurane in participants undergoing prone spine surgery. Outcomes measured: intraocular pressure. Unable to source full text. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Trifu 2011 | RCT, measuring effects of propofol versus sevoflurane in participants aged between 16 and 76 undergoing elective neurosurgery. Unable to source full text. Outcomes measured: cardiovascular stability, recovery characteristics, and side effects. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Tufano 2000 | RCT, measuring effects of propofol versus sevoflurane in participants aged between 18 and 70. Outcomes measured: drug consumption, intraoperative responses, and times of recovery. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Ueda 1999 | RCT, measuring effects of sevoflurane versus propofol combined with thoracic epidural anaesthesia on arterial oxygenation during one‐lung ventilation for thoracotomy. Unable to source full text. Outcomes measured: haemodynamic parameters. Post‐hoc decision to exclude studies that did not measure review outcomes. Decision made from information in the abstract |
| Wakabayashi 2014 | RCT, measuring effects of sevoflurane versus propofol in older participants undergoing oesophagectomy. Outcomes measured: levels of cytokine and chemokine at the airway epithelium. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Weilbach 2005 | RCT, measuring effects of TIVA versus BA in elderly participants undergoing a cataract operation. Outcomes measured: patient satisfaction. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Wen 2010 | RCT, measuring effects of sevoflurane versus propofol on neuromuscular blockade produced by continuous cisatracurium infusion. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Wormald 2005 | RCT, measuring effects of sevoflurane versus propofol on the surgical field. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Yu 2010a | RCT, measuring effects of sevoflurane versus propofol in elderly patients undergoing abdominal surgery. Outcomes measured: haemodynamic parameters. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Zabolotskikh 2013 | Unclear if this is an RCT. Measuring effects of sevoflurane versus propofol on intracerebral and cerebral perfusion pressure. Post‐hoc decision to exclude studies that did not measure review outcomes |
| Zhang 2014 | RCT, measuring effects of propofol versus propofol and sevoflurane versus sevoflurane on immune responses in patients undergoing surgery for tongue cancer. Post‐hoc decision to exclude studies that did not measure review outcomes |
BA: balanced anaesthesia CEA: carotid endarterectomy MDA: malondialdehyde PMDs: pre‐existing motor deficits RCT: randomized control trial SjO2: jugular venous bulb oxygenation saturation tcMEP: transcranial electrical motor evoked potential TIVA: total intravenous anaesthesia
Characteristics of studies awaiting assessment [ordered by study ID]
IRCT2015112925277N1.
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 100 Inclusion criteria
Exclusion criteria
Type of surgery: inguinal herniorrhaphy Country: Iran Setting: hospital |
| Interventions |
TIVA group Maintenance details: 100 mg /kg/minute propofol Inhalational maintenance group Maintenance details: 1 mg/kg/minute isoflurane |
| Outcomes |
|
| Notes | Study is completed, but study results are not posted. Study does not specifically recruit elderly participants. Once published, we would need to ascertain whether mean age of participants is > 60 years of age |
McDonagh 2012.
| Methods | RCT, parallel design |
| Participants |
Number of randomized participants: 200 Inclusion criteria
Exclusion criteria
Type of surgery: orthopaedic Country: not reported Setting: hospital |
| Interventions |
TIVA group Induction details: pre‐medicated with midazolam. Induction with propofol; no additional details Maintenance details: propofol TIVA to maintain BIS 40 to 60 Inhalational maintenance group Induction details: pre‐medicated with midazolam Induction with propofol; no additional details Maintenance details: isoflurane to maintain BIS 40 to 60 |
| Outcomes |
|
| Notes | We only have an abstract for this study. No denominator figures for each group. Not clear whether outcome data is available for immediate postoperative period |
NCT02766062.
| Methods | RCT, parallel design |
| Participants |
Target number of participants: 94 Inclusion criteria
Exclusion criteria
Type of surgery: noncardiac and non‐neural surgery Country: China Setting: General Hospital of Ningxia Medical University |
| Interventions |
TIVA group Maintenance details: propofol Inhalational maintenance group Maintenance details: sevoflurane |
| Outcomes |
|
| Notes | Study is completed, but study results are not posted |
Shen 2011.
| Methods | RCT, parallel design |
| Participants |
Total number of randomized participants: 60 Inclusion criteria: requires translation Exclusion criteria: requires translation Type of surgery: thoracic Country: China Setting: hospital |
| Interventions |
TIVA group Induction details: requires translation Maintenance details: propofol and fentanyl Inhalational maintenance group Induction details: requires translation Maintenance details: sevoflurane and fentanyl |
| Outcomes |
|
| Notes | Unable to extract detailed data due to paper being written in Chinese. All data extracted from abstract |
ASA: American Society of Anesthesiologists BIS: bispectral index GDS: Geriatric Depression scale IRB: institutional review board MMSE: Mini‐Mental State Examination RCT: randomized control trial POCD: postoperative cognitive dysfunction TIVA: total intravenous anaesthesia
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IOR‐16009851.
| Trial name or title | Impact of postoperative cognitive function after sevoflurane‐ or propofol‐anaesthesia in aged cancer patients: a double‐blinded randomized controlled trial |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 220 Inclusion criteria
Exclusion criteria
Type of surgery: major abdominal malignant tumour resection Country: China Setting: hospital |
| Interventions |
TIVA group Details: propofol; no details Inhalational maintenance group Details: sevoflurane; no details |
| Outcomes |
|
| Starting date | 11 July 2016 |
| Contact information | Liang Guo (1159398818@qq.com) or Ling‐Hui Pan (plinghui@hotmail.com) |
| Notes |
EUCTR2014‐004604‐29‐DK.
| Trial name or title | Sevoflurane versus standard general anaesthesia in elective open abdominal aortic aneurism surgery |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 24 Inclusion criteria
Exclusion criteria
Type of surgery: aortic aneurysm repair Country: Denmark Setting: hospital |
| Interventions |
TIVA group Details: propofol; no details Inhalational maintenance group Details: sevoflurane; no details |
| Outcomes |
|
| Starting date | Not clear from the clinical trials register documents |
| Contact information | Peder Bach (pedebach@rm.dk) |
| Notes | Study does not specifically recruit elderly participants. Once completed, we would need to ascertain whether mean age of participants is > 60 years of age |
NCT01809041.
| Trial name or title | Comparison of intravenous anesthetics to volatile anesthetics on postoperative cognitive dysfunction |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 684 Inclusion criteria
Exclusion criteria
Type of surgery: intra‐abdominal and intrapelvic surgery Country: China Setting: hospital |
| Interventions |
TIVA group Maintenance details: propofol (50 ‐ 150 µg/kg/min) and remifentanil (0.1 ‐ 0.5 µg/kg/min) Inhalational maintenance group Maintenance details: sevoflurane at 0.5 to 1.5 MAC plus remifentanil (0.1 ‐ 0.5 µg/kg/min) |
| Outcomes | Number of participants with POCD (at 7 days and 3 months) Time for bowel function return after surgery Degree of increase of stress hormones Length of hospital stay |
| Starting date | March 2013 |
| Contact information | Yujuan Li, MD, PhD (yujuan_04@hotmail.com); or Shulin Peng (pslmzk@yahoo.com.cn) |
| Notes |
NCT01995214.
| Trial name or title | Sevoflurane and propofol anaesthesia on postoperative delirium |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 500 Inclusion criteria
Exclusion criteria
Type of surgery: not specified Country: China Setting: hospital |
| Interventions |
TIVA group Maintenance details: propofol and remifentanil guided by Narcotrend index monitoring Inhalational maintenance group Maintenance details: sevoflurane and remifentanil guided by Narcrotrend index monitoring |
| Outcomes |
|
| Starting date | June 2013 |
| Contact information | Yuke Tian, MD, PhD |
| Notes |
NCT02107170.
| Trial name or title | Effects of anesthetics on postoperative cognitive function of patients undergoing endovascular repair of aortic aneurysm and endovascular treatment of arteriosclerosis obliterans of lower extremities |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 400 Inclusion criteria
Exclusion criteria
Type of surgery: endovascular repair of aortic aneurysm, endovascular treatment of arteriosclerosis obliterans of lower extremities Country: China Setting: hospital |
| Interventions |
TIVA group Details: propofol (50 to 150 µg/kg/min) plus remifentanil (0.1 to 0.5 µg/kg/min) during the surgery Inhalational maintenance group Details: sevoflurane at 0.5 to 1.5 MAC plus remifentanil (0.1 to 0.5 µg/kg/min) during the surgery |
| Outcomes |
|
| Starting date | February 2014 |
| Contact information | Tao Zhang, Master of Medicine (zhtao98@aliyun.com) |
| Notes | Study does not specifically recruit elderly participants. Once completed, we would need to ascertain whether mean age of participants is > 60 years of age |
NCT02133638.
| Trial name or title | Sevoflurane decreases the risk of postoperative delirium after cerebral hypoxemia during surgery |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 130 Inclusion criteria
Exclusion criteria
Type of surgery: elective non‐cardiac surgery (hemicolectomy, hernioplasty, laparoscopic cholecystectomy and laparoscopic hysterectomy) Country: Russia Setting: hospital |
| Interventions |
TIVA group Induction details: propofol 2 mg/kg and fentanyl 4 µg/kg Maintenance details: infusion of propofol 8 mg/kg/hour and boluses of fentanyl 3 µg/kg Inhalational maintenance group Induction details: fentanyl 2 µg/kg and a bolus inhalation of 8% sevoflurane in an 8 L/min fresh gas flow Maintenance details: 1 MAC sevoflurane at a low fresh gas flow of 0.6 to 0.8 L/min in a 60% air‐oxygen mixture supplemented with boluses of fentanyl |
| Outcomes |
|
| Starting date | May 2014 |
| Contact information | Yuri V Iljin, Negovsky Reanimatology Research Institute, Moscow, Russia |
| Notes |
NCT02301676.
| Trial name or title | Long term postoperative cognitive dysfunction in the elderly patients |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 190 Inclusion criteria
Exclusion criteria
Type of surgery: laparoscopic cholecystectomy under GA Country: South Korea Setting: hospital |
| Interventions |
TIVA group Details: no details Inhalational maintenance group Details: sevoflurane; no details |
| Outcomes |
|
| Starting date | December 2014 |
| Contact information | Seung‐Hoon Baek, Pusan National University Yangsan Hospital |
| Notes |
NCT02458547.
| Trial name or title | Effect of anaesthesia technique on outcome after hip fracture surgery in elderly adult patients |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 186 Inclusion criteria
Exclusion criteria
Type of surgery: elective or emergency hip fracture surgery Country: South Korea Setting: hospital |
| Interventions |
TIVA group Details: propofol TCI Inhalational maintenance group Details: desflurane at age‐adjusted MAC of 0.8 to 1.0 |
| Outcomes |
|
| Starting date | May 2015 |
| Contact information | Not reported |
| Notes | Study may not report outcomes of interest. Because the study includes elderly surgical patients and compares the anaesthetic agents of interest, we have included this study in our list of ongoing studies. |
NCT02662257.
| Trial name or title | Impact of anaesthesia maintenance methods on incidence of postoperative delirium |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 1200 Inclusion criteria
Exclusion criteria:
Type of surgery: treatment of tumour Country: China Setting: hospital |
| Interventions |
TIVA group Details: propofol adjusted to maintain BIS 40 to 60, with or without 50% nitrous oxide. Remifentanil (administered by continuous infusion), sufentanil (administered by intermittent injection/continuous infusion), or fentanyl (administered by intermittent injection). Towards the end of surgery, propofol infusion rate will be decreased and fentanyl/sufentanil will be administered when necessary Inhalational maintenance group Details: sevoflurane adjusted to maintain BIS 40 to 60, with or without 50% nitrous oxide. Remifentanil (administered by continuous infusion), sufentanil (administered by intermittent injection/continuous infusion), or fentanyl (administered by intermittent injection). Towards the end of surgery, sevoflurane inhalational concentration will be decreased and fentanyl/sufentanil will be administered when necessary |
| Outcomes |
|
| Starting date | April 2015 |
| Contact information | Dong‐Xin Wang, MD, PhD, Peking University FIrst Hospital |
| Notes | Also registered as ChiCTR‐IPR‐15006209 |
NCT03165396.
| Trial name or title | Appropriate compatibility of propofol and sevoflurane for orthopaedic surgery of patients with MCI |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 100 Inclusion criteria
Exclusion criteria
Type of surgery: orthopaedic surgery Country: China Setting: hospital |
| Interventions |
TIVA group Details: propofol TCI 2.0 to 2.5 μg/mL Inhalational maintenance group Details: 1.3 MAC sevoflurane |
| Outcomes |
|
| Starting date | 10 May 2016 |
| Contact information | Haiyun Wang (why@126.com) or Yimeng Chen (chenyimeng5525@163.com) |
| Notes | Compares two additional groups using propofol at different doses combined with sevoflurane |
NCT03194074.
| Trial name or title | Early cognitive function in elderly patients after laser laryngeal surgery: des vs prop |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 70 Inclusion criteria
Exclusion criteria
Type of surgery: laser laryngeal surgery Country: China Setting: hospital |
| Interventions |
TIVA group Maintenance details: propofol at a rate 75 to 150 µg/kg/min and remifentanil at 0.1 to 0.3 µg/kg/min maintained throughout surgery Inhalational maintenance group Maintenance details: desflurane at end‐tidal concentration at 0.7 to 1.0 MAC and remifentanil 0.1 to 0.3 µg/kg/min |
| Outcomes |
|
| Starting date | 15 August 2017 |
| Contact information | Xia Shen, MD (zlsx@yahoo.com) or Hui Qiao, MD (theyellow@163.com) |
| Notes | Study does not specifically recruit elderly participants. Once completed, we would need to ascertain whether mean age of participants is > 60 years of age |
ApoJ: Apolipoprotein J ARDS: acute respiratory distress syndrome ASA: American Society of Anesthesiologists BIS: Bispectral Index BMI: body mass index CAM: confusion assessment method CD: cluster of differentiation EORCT QLQ‐C30: (quality of life questionnaire for cancer patients) GA: general anaesthesia ICU: intensive care unit IL: interleukin MAC: minimum alveolar concentration MCI: mild cognitive impairment MI: myocardial infarction MMSE: Mini‐Mental State Examination MoCA: Montreal Cognitive Assessment PACU: postanaesthesia care unit POCD: postoperative cognitive dysfunction PONV: postoperative nausea and vomiting QOR‐40: quality of recovery questionnaire RCT: randomized control trial TGF: transforming growth factor TCI: target‐controlled infusion TICS‐m: Telephone Interview for Cognitive Status‐Modified TIVA: total intravenous anaesthesia TNF: tumour necrosis factor VEGF: vascular endothelial growth factor
Differences between protocol and review
We made the following changes to the published protocol (Miller 2016).
Authors: we added additional authors during the review, Michael W Pritchard and Oliver J Schofield Robinson.
Title: we edited it to make it clear that our inclusion criteria was 'non cardiac' surgery.
Objectives: we edited the wording of our review objective to reflect our intention at protocol to only include interventions that were propofol‐based TIVA.
Inclusion criteria: we excluded studies in which the inclusion criteria specified a participant age range of 18 to 65 years because we believed these studies were not aiming to specifically recruit elderly patients; we found that these studies had a mean age for participants of < 60 years and therefore this decision did not affect choice of included studies. We found a large number of studies that compared intravenous versus inhalational anaesthetic agents, but only measured outcomes which were outside the scope of this review, e.g. biochemical parameters. We therefore added an exclusion criteria to the review: to exclude studies that did not measure our review outcomes. We reported these studies in Characteristics of excluded studies.
In the protocol, we stated that our final choice of fixed‐effect or random‐effects statistical model was influenced by the level of identified heterogeneity and the number of studies. We selected to use a random‐effects statistical model; this decision was made because a random‐effects model is more appropriate for analysis of studies in which differences (for example, in types of surgery) were most likely.
Dealing with missing data: we did not contact authors to request missing data (except for in Tanaka 2017). In the case that study participants were lost at follow‐up, we included data as analysed by study authors. We did not impute missing values with replacement values. In the case of missing statistics, we did not impute missing values with replacement values. We reported data in the format presented by study authors, and if it was in a format that was not comparable to other data that could be pooled (e.g. median values), we reported these data separately in additional tables. We found high statistical heterogeneity in included studies and noted inconsistencies in visual inspection of results; imputing appropriate values was not appropriate because of heterogeneity. We used sensitivity analysis to explore the effect of including studies in which attrition was high and unbalanced between groups.
'Summary of findings' table and GRADE: only one review author used GRADEpro software to create a 'Summary of findings' table. This was checked and approved by a second review author.
Contributions of authors
David Miller (DM), Sharon R Lewis (SRL), Michael W Pritchard (MP), Oliver Schofield‐Robinson (OSR), Cliff Shelton (CS), Phil Alderson (PA), Andrew F Smith (AS)
Conceiving the review: SRL, PA, AS
Writing the protocol: CS, DM, SRL
Co‐ordinating the review: SRL
Undertaking manual searches: SRL, OSR, MP
Screening search results: DM, SRL, OSR, MP
Organizing retrieval of papers: SRL, OSR
Screening retrieved papers against inclusion criteria: DM, SRL, OSR, MP
Appraising quality of papers: DM, SRL, OSR, MP
Extracting data from papers: DM, SRL, OSR, MP
Data management for the review: SRL
Entering data into Review Manager (Review Manager 2014): SRL, MP
RevMan statistical data: SRL
Interpretation of data: SRL, DM
Statistical inferences: SRL
Writing the review: SRL, MP, DM, CS, PA, AS
Securing funding for the review: AS, DM, CS
Guarantor for the review (one author): AS
Person responsible for reading and checking review before submission: SRL
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR Cochrane Collaboration Programme Grant, UK. 'Back to normal': speed and quality of recovery after surgery, major injury and critical care. Project ref. 13/89/16, UK.
-
NIHR/HEE Integrated academic programme internship, UK.
North West, North East and Yorkshire and the Humber internship programme
Declarations of interest
David Miller: Funded Health Education England internship for the clinical academic programme. This was a six month part time funded post, 30 days in total, to allow an introduction into all aspects and roles across clinical academic research. The internship is designed to provide dedicated time to gain an understanding of the world of health research (Sources of support)
Cliff Shelton has received an NIHR award (DRF‐2015‐08‐208) to fund a qualitative research project investigating anaesthesia for hip fracture surgery as part of his doctoral research fellowship at Lancaster University
Sharon R Lewis see Sources of support
Michael Pritchard: see Sources of support
Oliver Schofield‐Robinson see Sources of support.
Phil Alderson: work on this review is funded, in part, by a UK NIHR Cochrane programme grant for the preparation of reviews relevant to recovery from critical illness (Sources of support)
Andrew F Smith: see Sources of support
Edited (no change to conclusions)
References
References to studies included in this review
Ammar 2016 {published data only}
- Ammar AS, Mahmoud KM. Comparative effect of propofol versus sevoflurane on renal ischemia/reperfusion injury after elective open abdominal aortic aneurysm repair. Saudi Journal of Anaesthesia 2016;10(3):301‐7. [PUBMED: 27375385] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biboulet 2012 {published data only}
- Biboulet P, Jourdan A, Haevre V, Morau D, Bernard N, Bringuier S, et al. Hemodynamic profile of target‐controlled spinal anesthesia compared with 2 target‐controlled general anesthesia techniques in elderly patients with cardiac comorbidities. Regional Anesthesia and Pain Medicine 2012;37(4):433‐40. [PUBMED: 22609644] [DOI] [PubMed] [Google Scholar]
Cai 2012a {published data only}
- Cai Y, Hu H, Liu P, Feng G, Dong W, Yu B, et al. Association between the apolipoprotein E4 and postoperative cognitive dysfunction in elderly patients undergoing intravenous anesthesia and inhalation anesthesia. Anesthesiology 2012;116(1):84‐93. [PUBMED: 22108393] [DOI] [PubMed] [Google Scholar]
- Cai Y, Hu H, Xue R, Liu P, Feng G, Dong W, et al. Cognitive function correlations with apolipoprotein E epsilon 4 single nucleotide polymorphism in 1000 elderly patients following general anesthesia: a randomized controlled study. Neural Regeneration Research 2009;4(4):316‐20. [Google Scholar]
Celik 2011 {published data only}
- Celik JB, Topal A, Erol A, Guven S, Kara I. A comparison of recovery characteristics of sevoflurane and propofol‐remifentanil anesthesia in geriatric patients [Ileri yafi hastalarda sevofluran ve propofol‐remifentanil anestezisinin derlenme ozelliklerinin karfiilafitirilmasi]. Turkish Journal of Geriatrics 2011;14(3):208‐13. [Google Scholar]
Chan 1996 {published data only}
- Chan VW, Chung FF. Propofol infusion for induction and maintenance of anesthesia in elderly patients: recovery and hemodynamic profiles. Journal of Clinical Anesthesia 1996;8(4):317‐23. [PUBMED: 8695136] [DOI] [PubMed] [Google Scholar]
Demeere 2006 {published data only}
- Demeere JL, Merckx Ch, Demeere N. Cost minimisation and cost effectiveness in anaesthesia for total hip replacement surgery, in Belgium? A study comparing three general anaesthesia techniques. Acta Anaesthesiologica Belgica 2006;57(2):145‐51. [PUBMED: 16916184] [PubMed] [Google Scholar]
Egawa 2016 {published data only}
- Egawa J, Inoue S, Nishiwada T, Tojo T, Kimura M, Kawaguchi T, et al. Effects of anesthetics on early postoperative cognitive outcome and intraoperative cerebral oxygen balance in patients undergoing lung surgery: a randomized clinical trial. Canadian Journal of Anaesthesia 2016;63(10):1161‐9. [PUBMED: 27412465] [DOI] [PubMed] [Google Scholar]
Epple 2001 {published data only}
- Epple J, Kubitz J, Schmidt H, Motsch J, Bottiger BW, Martin E, et al. Comparative analysis of costs of total intravenous anaesthesia with propofol and remifentanil vs. balanced anaesthesia with isoflurane and fentanyl. European Journal of Anaesthesiology 2001;18(1):20‐8. [PUBMED: 11270005] [DOI] [PubMed] [Google Scholar]
- Kubitz J, Epple J, Bach A, Motsch J, Martin E, Schmidt H. Psychomotor recovery in very old patients after total intravenous or balanced anaesthesia for cataract surgery. British Journal of Anaesthesia 2001;86(2):203‐8. [PUBMED: 11573660] [DOI] [PubMed] [Google Scholar]
Geng 2017 {published data only}
- Geng YJ, Wu QH, Zhang RQ. Effect of propofol, sevoflurane, and isoflurane on postoperative cognitive dysfunction following laparoscopic cholecystectomy in elderly patients: a randomized controlled trial. Journal of Clinical Anesthesia 2017;38:165‐71. [PUBMED: 28372661] [DOI] [PubMed] [Google Scholar]
Gursoy 2015 {published data only}
- Gursoy T, Saracotlu KT, Dincer PC, Akotlu H, Umurotlu T. The assessment of early postoperative neurocognitive functions in geriatric patients following sevoflurane‐nitrous oxide inhalation anesthesia and intravenous propofol‐remifentanil [Geriatrik Hastalarda Sevofluran‐nitroz Oksit Inhalasyon Anestezisi ve Intravenoz Propofol‐remifentanil Uygulamasi Sonrasinda Erken Postoperatif Norokognitif Fonksiyonlarin Deterlendirilmesi]. Anestezi Dergisi 2015;22(4):202‐6. [Google Scholar]
Ishii 2016 {published data only}
- Ishii K, Makita T, Yamashita H, Matsunaga S, Akiyama D, Toba K, et al. Total intravenous anesthesia with propofol is associated with a lower rate of postoperative delirium in comparison with sevoflurane anesthesia in elderly patients. Journal of Clinical Anesthesia 2016;33:428‐31. [PUBMED: 27555205] [DOI] [PubMed] [Google Scholar]
Jellish 2003 {published data only}
- Jellish WS, Sheikh T, Baker WH, Louie EK, Slogoff S. Hemodynamic stability, myocardial ischemia, and perioperative outcome after carotid surgery with remifentanil/propofol or isoflurane/fentanyl anesthesia. Journal of Neurosurgical Anesthesiology 2003;15(3):176‐84. [PUBMED: 12826964] [DOI] [PubMed] [Google Scholar]
Juvin 1997 {published data only}
- Juvin P, Servin F, Giraud O, Desmonts JM. Emergence of elderly patients from prolonged desflurane, isoflurane, or propofol anesthesia. Anesthesia and Analgesia 1997;85(3):647‐51. [PUBMED: 9296424] [DOI] [PubMed] [Google Scholar]
Kim 2015a {published data only}
- Kim DJ, Kim SH, So KY, Jung KT. Effects of dexmedetomidine on smooth emergence from anaesthesia in elderly patients undergoing orthopaedic surgery. BMC Anesthesiology 2015;15:139. [PUBMED: 26446479] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01851005. Effect of dexmedetomidine on recovery profiles of elderly patients. clinicaltrials.gov/ct2/show/record/NCT01851005 (first received 30 April 2013).
Lindholm 2013 {published data only}
- Lindholm EE, Aune E, Froland G, Kirkeboen KA, Otterstad JE. Analysis of transthoracic echocardiographic data in major vascular surgery from a prospective randomised trial comparing sevoflurane and fentanyl with propofol and remifentanil anaesthesia. Anaesthesia 2014;69(6):558‐72. [PUBMED: 24720268] [DOI] [PubMed] [Google Scholar]
- Lindholm EE, Aune E, Noren CB, Seljeflot I, Hayes T, Otterstad JE, et al. The anesthesia in abdominal aortic surgery (ABSENT) study: a prospective, randomized, controlled trial comparing troponin T release with fentanyl‐sevoflurane and propofol‐remifentanil anesthesia in major vascular surgery. Anesthesiology 2013;119(4):802‐12. [PUBMED: 23838709] [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu Y, Pan N, Ma Y, Zhang S, Guo W, Li H, et al. Inhaled sevoflurane may promote progression of amnestic mild cognitive impairment: a prospective, randomized parallel‐group study. American Journal of the Medical Sciences 2013;345(5):355‐60. [PUBMED: 23044653] [DOI] [PubMed] [Google Scholar]
Longas 2004 {published data only}
- Longás VJ, Guerrero PLM, Gonzalo A, Infantes MM, Rodriguez ZA, Abengochea BJ. Comparison of 4 techniques for general anesthesia for carotid endarterectomy: inflammatory response, cardiocirculatory complications, and postoperative analgesia. Revista Espanola de Anestesiologia y Reanimacion 2004;51(10):568‐75. [PUBMED: 15641601] [PubMed] [Google Scholar]
Luntz 2004 {published data only}
- Luntz SP, Janitz E, MotschJ, Bach A, Martin E, Bottiger BW. Cost‐effectiveness and high patient satisfaction in the elderly: sevoflurane versus propofol anaesthesia. European Journal of Anaesthesiology 2004;21(2):115‐22. [PUBMED: 14977342] [DOI] [PubMed] [Google Scholar]
Micha 2016 {published data only}
- Micha G, Tzimas P, Zalonis J, Kotsis K, Papadopoulos G, Arnaoutoglou E. Propofol vs sevoflurane anaesthesia on postoperative cognitive dysfunction in the elderly. A randomized controlled trial. Acta Anaesthesiologica Belgica 2016;67(3):129‐37. [EMBASE: 614101023] [PubMed] [Google Scholar]
Moffat 1995 {published data only}
- Moffat A, Cullen PM. Comparison of two standard techniques of general anaesthesia for day‐case cataract surgery. British Journal of Anaesthesia 1995;74(2):145‐8. [PUBMED: 7696061] [DOI] [PubMed] [Google Scholar]
Nishikawa 2004 {published data only}
- Nishikawa K, Nakayama M, Omote K, Namiki A. Recovery characteristics and post‐operative delirium after long‐duration laparoscope‐assisted surgery in elderly patients: propofol‐based vs. sevoflurane‐based anesthesia. Acta Anaesthesiologica Scandinavica 2004;48(2):162‐8. [PUBMED: 14995937] [DOI] [PubMed] [Google Scholar]
Rohan 2005 {published data only}
- Rohan D, Buggy DJ, Crowley S, Ling FK, Gallagher H, Regan C, et al. Increased incidence of postoperative cognitive dysfunction 24 hr after minor surgery in the elderly. Canadian Journal of Anaesthesia 2005;52(2):137‐42. [PUBMED: 15684252] [DOI] [PubMed] [Google Scholar]
Tan 2009 {published data only}
- Tan R. Effect of propofol and isoflurane on surgical stress response and postoperative cognitive function in elderly patients. Journal of Southern Medical University 2009;29(6):1247‐8. [PUBMED: 19726376] [PubMed] [Google Scholar]
Tanaka 2017 {published data only}
- NCT01270620. Desflurane or propofol anesthesia in elderly obese patients undergoing total knee replacement [Desflurane or propofol anesthesia in elderly obese patients undergoing total knee replacement: a pilot assessment of short‐term and long‐term differences in outcome]. https://clinicaltrials.gov/ct2/show/record/NCT01270620 first received 14 December 2010.
- Tanaka P, Goodman S, Sommer BR, Maloney W, Huddleston J, Lemmens HJ. The effect of desflurane versus propofol anesthesia on postoperative delirium in elderly obese patients undergoing total knee replacement: A randomized, controlled, double‐blinded clinical trial. Journal of Clinical Anesthesia 2017;39:17‐22. [PUBMED: 28494898] [DOI] [PubMed] [Google Scholar]
Tang 2014 {published data only}
- Tang N, Ou C, Liu Y, Zuo Y, Bai Y. Effect of inhalational anaesthetic on postoperative cognitive dysfunction following radical rectal resection in elderly patients with mild cognitive impairment. Journal of International Medical Research 2014;42(6):1252‐61. [PUBMED: 25339455] [DOI] [PubMed] [Google Scholar]
Trembach 2012 {published data only}
- Trembach N, Daniljuk P. Comparison of sevoflurane volatile induction and maintenance anesthesia and propofol‐fentanyl total intravenous anesthesia during laparoscopic surgery in elderly. British Journal of Anaesthesia 2012;108:ii156. [Google Scholar]
Tylman 2011 {published data only}
- Tylman M, Sarbinowski R, Bengtson JP, Kvarnstrom A, Bengtsson A. Inflammatory response in patients undergoing colorectal cancer surgery: the effect of two different anesthetic techniques. Minerva Anestesiologica 2011;77(3):275‐82. [PUBMED: 21150855] [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang L, Chen C, Wang L, Cheng G, Wu WW, Li YH. Awakening from anesthesia using propofol or sevoflurane with epidural block in radical surgery for senile gastric cancer. International Journal of Clinical and Experimental Medicine 2015;8(10):19412‐7. [PUBMED: 26770584] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Arar 2005 {published data only}
- Arar C, Kaya G, Karamanlioğlu B, Pamukçu Z, Turan N. Effects of sevoflurane, isoflurane and propofol infusions on post‐operative recovery criteria in geriatric patients. Journal of International Medical Research 2005;33(1):55‐60. [PUBMED: 15651715] [DOI] [PubMed] [Google Scholar]
Arnaoutoglou 2007 {published data only}
- Arnaoutoglou H, Vretzakis G, Souliotis D, Cambili M, Galaris D, Papadopoulos G. The effects of propofol or sevoflurane on free radical production after tourniquet induced ischaemia‐reperfusion injury during knee arthroplasty. Acta Anaesthesiologica Belgica 2007;58(1):3‐6. [PUBMED: 17486917] [PubMed] [Google Scholar]
But 2003 {published data only}
- But AK, Durmus M, Koroglu A, Yucel A, Ulger H, Ersoy MO. The effects of sevoflurane and propofol on hepatic and renal functions in elderly patients [Yasli olgularda sevofluran ve propofolun karaciger ve bobrek fonksiyonlarina etkisi]. Anestezi Dergisi 2003;11(2):111‐6. [Google Scholar]
- But AK, Durmus M, Togal T, Gedik E, Yucel A, Ersoy MO. The effects of sevoflurane and propofol on induction, maintenance and recovery in elderly patients [Yasli olgularda sevofluran ve propofolun induksiyon, idame ve derlenmeye etkisi]. Turk Anesteziyoloji ve Reanimasyon Dernegi Dergisi 2003;31(5):224‐30. [Google Scholar]
Carles 2008 {published data only}
- Carles M, Dellamonica J, Roux J, Lena D, Levraut J, Pittet JF, et al. Sevoflurane but not propofol increases interstitial glycolysis metabolites availability during tourniquet‐induced ischaemia‐reperfusion. British Journal of Anaesthesia 2008;100(1):29‐35. [PUBMED: 18029344] [DOI] [PubMed] [Google Scholar]
Doe 2016 {published data only}
- Doe A, Kumagai M, Tamura Y, Sakai A, Suzuki K. A comparative analysis of the effects of sevoflurane and propofol on cerebral oxygenation during steep Trendelenburg position and pneumoperitoneum for robotic‐assisted laparoscopic prostatectomy. Journal of Anesthesia 2016; Vol. 30, issue 6:949‐55. [PUBMED: 27565964] [DOI] [PMC free article] [PubMed]
Filipovic 2007 {published data only}
- Filipovic M, Michaux I, Wang J, Hunziker P, Skarvan K, Seeberger M. Effects of sevoflurane and propofol on left ventricular diastolic function in patients with pre‐existing diastolic dysfunction. British Journal of Anaesthesia 2007;98(1):12‐8. [PUBMED: 17060331] [DOI] [PubMed] [Google Scholar]
Fredman 2002 {published data only}
- Fredman B, Sheffer O, Zohar E, Paruta I, Richter S, Jedeikin R, et al. Fast‐track eligibility of geriatric patients undergoing short urologic surgery procedures. Anesthesia and Analgesia 2002;94(3):560‐4. [PUBMED: 11867375] [DOI] [PubMed] [Google Scholar]
Gasowska 1999 {published data only}
- Gasowska J, Brzezinski K, Przesmycki K, Nestorowicz A. Effects of halothane, isoflurane, and propofol on venous admixture during one‐lung ventilation in patients undergoing thoracoscopic procedures. Medical Science Monitor 1999;5(5):929‐33. [Google Scholar]
Gauger 2008 {published data only}
- Gauger PG, Shanks A, Morris M, Greenfield ML, Burney RE, O'Reilly M. Propofol decreases early postoperative nausea and vomiting in patients undergoing thyroid and parathyroid operations. World Journal of Surgery 2008;32(7):1525‐34. [PUBMED: 18305999] [DOI] [PubMed] [Google Scholar]
Guedes 1988 {published data only}
- Guedes Y, Rakotoseheno JC, Leveque M, Mimouni F, Egreteau JP. Changes in intra‐ocular pressure in the elderly during anaesthesia with propofol. Anaesthesia 1988;43 Suppl:58‐60. [PUBMED: 3259099] [DOI] [PubMed] [Google Scholar]
Halberg 1996 {published data only}
- Halberg DL, Russell W, Hatton RC, Segal R, Guyton TS, Paulu DA. Pharmacoeconomic evaluation of anesthesia in ambulatory surgery: comparison of desflurane to isoflurane and propofol (Structured abstract). Pharmacy Practice Management Quarterly 1996;16(2):71‐85. [PUBMED: 10172770] [PubMed] [Google Scholar]
Holst 1993 {published data only}
- Holst D, Anger C, Bauch HJ. N2O‐supplemented intravenous anaesthesia versus inhalation anaesthesia. Comparative study of sympathoadrenergic reaction and postoperative vigilance. Anasthesiologie Intensivmedizin Notfallmedizin Schmerztherapie 1993;28(1):18‐22. [PUBMED: 8467026] [DOI] [PubMed] [Google Scholar]
Hosseinzadeh 2013 {published data only}
- Hosseinzadeh B, Zahedian F, Ghorbani R, Forozesfard M. Incidence of post operative nausea and vomiting following induction and maintance of general anesthesia with propofol or induction by propofol and maintenance with isoflurane: a randomized clinical trial. Koomesh 2013;15(1):54‐8. [Google Scholar]
Ionescu 2009 {published data only}
- Ionescu D, Margarit S, Vlad L, Iancu C, Alexe A, Deac D, et al. TIVA‐TCI (total intraVenous anesthesia‐target controlled infusion) versus isoflurane anesthesia for laparoscopic cholecystectomy. Postoperative nausea and vomiting, and patient satisfaction. Chirurgia (Bucuresti) 2009;104(2):167‐72. [PUBMED: 19499659] [PubMed] [Google Scholar]
Ito 2012 {published data only}
- Ito H. Recovery of elderly patients from four or more hours of desflurane or any other anaesthesia. European Journal of Anaesthesiology 2012;29:225. [Google Scholar]
Kadoi 2009a {published data only}
- Kadoi Y, Kawauchi C, Saito S, Takahashi K. The comparative effects of equipotent Bispectral Index dosages of propofol and sevoflurane on cerebrovascular carbon dioxide reactivity in elderly patients. Journal of Clinical Anesthesia 2009;21(3):173‐7. [PUBMED: 19464609] [DOI] [PubMed] [Google Scholar]
Kim 2015b {published data only}
- Kim YS, Lim BG, Kim H, Kong MH, Lee IO. Effects of propofol or desflurane on post‐operative spirometry in elderly after knee surgery: a double‐blind randomised study. Acta Anaesthesiologica Scandinavica 2015;59(6):788‐95. [PUBMED: 25736101] [DOI] [PubMed] [Google Scholar]
Konstantopoulos 2013a {published data only}
- Konstantopoulos K, Makris A, Moustaka A, Karmaniolou I, Konstantopoulos G, Mela A. Sevoflurane versus propofol anesthesia in patients undergoing lumbar spondylodesis: a randomized trial. Journal of Surgical Research 2013;179(1):72‐7. [PUBMED: 23073511] [DOI] [PubMed] [Google Scholar]
Kvarnstrom 2012 {published data only}
- Kvarnstrom AL, Sarbinowski RT, Bengtson JP, Jacobsson LM, Bengtsson AL. Complement activation and interleukin response in major abdominal surgery. Scandinavian Journal of Immunology 2012;75(5):510‐6. [PUBMED: 22229650] [DOI] [PubMed] [Google Scholar]
Malcharek 2015 {published data only}
- Malcharek MJ, Loeffler S, Schiefer D, Manceur MA, Sablotzki A, Gille J, et al. Transcranial motor evoked potentials during anesthesia with desflurane versus propofol ‐ a prospective randomized trial. Clinical Neurophysiology 2015;126(9):1825‐32. [PUBMED: 25541524] [DOI] [PubMed] [Google Scholar]
Manolescu 2012 {published data only}
- Manolescu R, Melnic M, Rotaru R, Florea S, Corneci D, Azamfirei L. Cardioprotective effects of sevoflurane vs propofol for non‐cardiac surgery in patients with an increased cardiac risk [Efectele cardioprotectoare ale sevofluranului vs propofol in chirurgia noncardiaca la pacientii cu risc cardiac crescut]. Romanian Journal of Anaesthesia and Intensive Care 2012;19(2):99‐109. [Google Scholar]
Mets 1992 {published data only}
- Mets B, Salmon JF, James MF. Continuous intravenous propofol with nitrous oxide for ocular surgery. A comparison with etomidate, alfentanil, nitrous oxide and isoflurane. South African Medical Journal 1992;81(10):523‐6. [PUBMED: 1585226] [PubMed] [Google Scholar]
Murray 1994 {published data only}
- Murray JM, Phillips AS, Fee JP. Comparison of the effects of isoflurane and propofol on hepatic glutathione‐S‐transferase concentrations during and after prolonged anaesthesia. British Journal of Anaesthesia 1994;72(5):599‐601. [PUBMED: 8198917] [DOI] [PubMed] [Google Scholar]
Mutch 1995 {published data only}
- Mutch WA, White IW, Donen N, Thomson IR, Rosenbloom M, Cheang M. Haemodynamic instability and myocardial ischaemia during carotid endarterectomy: a comparison of propofol and isoflurane. Canadian Journal of Anaesthesia 1995;42(7):577‐87. [PUBMED: 7553993] [DOI] [PubMed] [Google Scholar]
Ohe 2014 {published data only}
- Ohe Y, Kunimasa K, Watanabe Y. Effects of amino acid infusion in preventing intraoperative hypothermia: comparison between sevoflurane versus propofol. Masui ‐ Japanese Journal of Anesthesiology 2014;63(6):623‐8. [PUBMED: 24979850] [PubMed] [Google Scholar]
Oikkonen 1992 {published data only}
- Oikkonen M. Anaesthesia for geriatric oesophagoscopy: isoflurane vs. alfentanil‐methohexitone vs. propofol. Acta Anaesthesiologica Scandinavica 1992;36(2):195‐200. [PUBMED: 1549942] [DOI] [PubMed] [Google Scholar]
Passot 2005 {published data only}
- Passot S, Servin F, Pascal J, Charret F, Auboyer C, Molliex S. A comparison of target‐ and manually controlled infusion propofol and etomidate/desflurane anesthesia in elderly patients undergoing hip fracture surgery. Anesthesia and Analgesia 2005;100(5):1338‐42. [PUBMED: 15845680] [DOI] [PubMed] [Google Scholar]
Pirttikangas 1996 {published data only}
- Pirttikangas CO, Salo M, Peltola O. Propofol infusion anaesthesia and the immune response in elderly patients undergoing ophthalmic surgery. Anaesthesia 1996;51(4):318‐23. [PUBMED: 8686816] [DOI] [PubMed] [Google Scholar]
Polarz 1995 {published data only}
- Polarz H, Bohrer H, Tabouillot W, Martin E, Tetz M, Volcker HE. Behavior of intraocular pressure in anesthesia with isoflurane in comparison with propofol/alfentanil. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 1995;30(2):96‐8. [PUBMED: 7772663] [DOI] [PubMed] [Google Scholar]
Sal'nikov 2003 {published data only}
- Sal'nikov PS, Burov NE. A comparative evaluation of "cerebral oximetry" during anesthesia with xenon and other anesthetics. Anesteziologiia i Reanimatologiia 2003;May‐June(3):35‐7. [PUBMED: 12918199] [PubMed] [Google Scholar]
Schäfer 2002 {published data only}
- Schäfer R, Klett J, Auffarth G, Polarz H, Volcker HE, Martin E, et al. Intraocular pressure more reduced during anesthesia with propofol than with sevoflurane: both combined with remifentanil. Acta Anaesthesiologica Scandinavica 2002;46(6):703‐6. [PUBMED: 12059895] [DOI] [PubMed] [Google Scholar]
Schilling 2007 {published data only}
- Schilling T, Kozian A, Kretzschmar M, Huth C, Welte T, Buhling F, et al. Effects of propofol and desflurane anaesthesia on the alveolar inflammatory response to one‐lung ventilation. British Journal of Anaesthesia 2007;99(3):368‐75. [PUBMED: 17621602] [DOI] [PubMed] [Google Scholar]
Schilling 2011 {published data only}
- Schilling T, Kozian A, Senturk M, Huth C, Reinhold A, Hedenstierna G, et al. Effects of volatile and intravenous anesthesia on the alveolar and systemic inflammatory response in thoracic surgical patients. Anesthesiology 2011;115(1):65‐74. [PUBMED: 21399490] [DOI] [PubMed] [Google Scholar]
Shao 2013 {published data only}
- Shao JJ, Lu L, Ren ZH, Lu JY. Effect of propofol or sevoflurane on the quality of neuromuscular blockade with cisatracurium during comparable depth of anesthesia in elderly patients. Journal of the American Geriatrics Society 2013;61:S335. [Google Scholar]
Sohn 2008 {published data only}
- Sohn JE, Kim YJ, Baik HJ, Kim JH. Hemodynamic comparison of propofol‐remifentanil and sevoflurane based anesthesia for total knee arthroplasty using tourniquet. Korean Journal of Anesthesiology 2008;55(4):412‐8. [Google Scholar]
Sugata 2012 {published data only}
- Sugata A, Hayashi H, Kawaguchi M, Hasuwa K, Nomura Y, Furuya H. Changes in intraocular pressure during prone spine surgery under propofol and sevoflurane anesthesia. Journal of Neurosurgical Anesthesiology 2012;24(2):152‐6. [PUBMED: 22274735] [DOI] [PubMed] [Google Scholar]
Trifu 2011 {published data only}
- Trifu M, Ratha O, Berteanu C, Gheorghitha E, Ciurea AV, Gorgan M, et al. TIVA‐TCI for the elective neurosurgical procedures ‐ the experience of a Romanian hospital in a prospective, randomized study [Anestezia TIVA‐TCI pentru interventiile neurochirurgicale elective ‐ experienta unui spital din romania intr‐un studio prospectiv si randomizat]. Romanian Journal of Anaesthesia and Intensive Care 2011;18(1):19‐25. [Google Scholar]
Tufano 2000 {published data only}
- Tufano R, Palomba R, Lambiase G, Giurleo LG. The utility of bispectral index monitoring in general anesthesia. Minerva Anestesiologica 2000;66(5):389‐93. [PUBMED: 10965722] [PubMed] [Google Scholar]
Ueda 1999 {published data only}
- Ueda M, Ishibe Y, Yamasaki K. Comparison of sevoflurane and propofol combined with thoracic epidural anesthesia on arterial oxygenation during one‐lung ventilation for thoracotomy. Anesthesia and Resuscitation 1999;35(3‐4):139‐43. [Google Scholar]
Wakabayashi 2014 {published data only}
- Wakabayashi S, Yamaguchi K, Kumakura S, Murakami T, Someya A, Kajiyama Y. Effects of anesthesia with sevoflurane and propofol on the cytokine/chemokine production at the airway epithelium during esophagectomy. International Journal of Molecular Medicine 2014;34(1):137‐44. [PUBMED: 24788377] [DOI] [PubMed] [Google Scholar]
Weilbach 2005 {published data only}
- Weilbach C, Scheinichen D, Raymondos K, Juttner B, Schlosshardt S, Piepenbrock S. Assessment of anesthesia methods in ophthalmologic surgery by patients, surgeons, and anesthesiologists. Ophthalmologe 2005;102(8):783‐6. [PUBMED: 15770505] [DOI] [PubMed] [Google Scholar]
Wen 2010 {published data only}
- Wen L, Lin W, Zhao W, Li G, Bai X, Xiao J. Effect of sevoflurane versus propofol‐remifentanil anesthesia on neuromuscular blockade by continuous cisatracurium infusion. Journal of Southern Medical University 2010;30(1):163‐5. [PUBMED: 20118012] [PubMed] [Google Scholar]
Wormald 2005 {published data only}
- Wormald PJ, Renen G, Perks J, Jones JA, Langton‐Hewer CD. The effect of the total intravenous anesthesia compared with inhalational anesthesia on the surgical field during endoscopic sinus surgery. American Journal of Rhinology 2005;19(5):514‐20. [PUBMED: 16270608] [PubMed] [Google Scholar]
Yu 2010a {published data only}
- Yu H, Zuo M. Effects of sevoflurane or propofol maintained anesthesia in elderly patients undergoing abdominal surgery. Anesthesia and Analgesia 2010;1:S452. [Google Scholar]
Zabolotskikh 2013 {published data only}
- Zabolotskikh IB, Trembach NV, Gormakova EV. Dynamics of intracerebral and cerebral perfusion pressure during major abdominal surgery. Anesteziologiia i Reanimatologiia 2013;May‐Jun(3):21‐4. [PUBMED: 24340991] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang T, Fan Y, Liu K, Wang Y. Effects of different general anaesthetic techniques on immune responses in patients undergoing surgery for tongue cancer. Anaesthesia and Intensive Care 2014;42(2):220‐7. [PUBMED: 24580388] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
IRCT2015112925277N1 {published data only}
- IRCT2015112925277N1. Comparison intravenous anesthesia with propofol and inhalation anesthesia with Isoflurane for pain measurement in post operative patients after inguinal herniorrhaphy. en.irct.ir/trial/21168 (first received 24 December 2015).
McDonagh 2012 {published data only}
- McDonagh D, Attix D, Pieper C, Martin G, Monk T. Preoperative cognitive status, not type of general anesthesia, predicts early postoperative cognitive dysfunction. Journal of Neurosurgical Anesthesiology 2012;24(4):493. [Google Scholar]
NCT02766062 {published data only}
- NCT02766062. Effects of propofol and sevoflurane on early POCD in elderly patients with metabolic syndrome. clinicaltrials.gov/ct2/show/NCT02766062 (first received 9 May 2016).
Shen 2011 {published data only}
- Shen YF, Wu JX, Xu MY. Effects of anesthesia with propofol and sevoflurane on postoperative cognitive function of elderly patients undergoing thoracic surgery. Journal of Shanghai Jiaotong University (Medical Science) 2011;31(3):322‐5. [Google Scholar]
References to ongoing studies
ChiCTR‐IOR‐16009851 {published data only}
- ChiCTR‐IOR‐16009851. Impact of postoperative cognitive function after sevoflurane‐ or propofol‐aneasthesia in aged cancer patients: a double‐blinded randomized controlled trial. www.chictr.org.cn/hvshowproject.aspx?id=10508 (first received 14 November 2016).
EUCTR2014‐004604‐29‐DK {published data only}
- EUCTR2014‐004604‐29‐DK. Sevoflurane versus standard general anesthesia in elective open abdominal aortic aneurism surgery. www.clinicaltrialsregister.eu/ctr‐search/trial/2014‐004604‐29/DK (first received 3 April 2015).
NCT01809041 {published data only}
- NCT01809041. Comparison of intravenous anesthetics to volatile anesthetics on postoperative cognitive dysfunction [Comparison of total intravenous anesthesia with sevoflurane‐based balanced anesthesia on postoperative cognitive dysfunction in elderly patients for major elective intra‐abdominal surgery]. clinicaltrials.gov/ct2/show/record/NCT01809041 (first received 6 March 2013).
NCT01995214 {published data only}
- NCT01995214. Sevoflurane and propofol anesthesia on postoperative delirium [Comparison of sevoflurane and propofol anesthesia on postoperative delirium in geriatric patients]. clinicaltrials.gov/ct2/show/record/NCT01995214 (first received 13 November 2013).
NCT02107170 {published data only}
- NCT02107170. Effects of anesthetics on postoperative cognitive function of patients undergoing endovascular repair of aortic aneurysm and endovascular treatment of arteriosclerosis obliterans of lower extremities [Comparison of intravenous anesthetics and volatile anesthetics on postoperative cognitive dysfunction of patients undergoing endovascular repair of aortic aneurysm and endovascular treatment of arteriosclerosis obliterans of lower extremities]. clinicaltrials.gov/ct2/show/record/NCT02107170 (first received 25 March 2014).
NCT02133638 {published data only}
- NCT02133638. Sevoflurane decreases the risk of postoperative delirium after cerebral hypoxemia during surgery [Sevoflurane‐based volatile induction and maintenance of anaesthesia (VIMA) strategy decreases the risk of postoperative delirium in elderly patients with registered cerebral hypoxemia episodes during general surgery]. clinicaltrials.gov/ct2/show/record/NCT02133638 (first received 6 May 2014).
NCT02301676 {published data only}
- NCT02107170. Long term postoperative cognitive dysfunction in the elderly patients [Phase 4 study of long term postoperative cognitive dysfunction after laparoscopic cholecystectomy in the elderly patients]. clinicaltrials.gov/ct2/show/record/NCT02301676 (first received 8 October 2014).
NCT02458547 {published data only}
- NCT02458547. Effect of anesthesia technique on outcome after hip fracture surgery in elderly adult patients. clinicaltrials.gov/ct2/show/record/NCT02458547 (first received 26 May 2015).
NCT02662257 {published data only}
- NCT02662257. Impact of anesthesia maintenance methods on incidence of postoperative delirium [Impact of inhalational versus intravenous anesthesia maintenance methods on incidence of postoperative delirium in elderly patients after cancer surgery: an open‐label, randomized controlled trial]. clinicaltrials.gov/ct2/show/record/NCT02662257 (first received 5 January 2016).
NCT03165396 {published data only}
- NCT03165396. Appropriate compatibility of propofol and sevoflurane for orthopaedic surgery of patients with MCI [The appropriate compatibility of propofol and sevoflurane for orthopaedic surgery of patients with mild cognitive impairment]. clinicaltrials.gov/ct2/show/record/NCT03165396 (first received 17 May 2017).
NCT03194074 {published data only}
- NCT03194074. Early cognitive function in elderly patients after laser laryngeal surgery: des vs prop [Early cognitive function and recovery in elderly patients after laser laryngeal surgery: desflurane‐based vs propofol‐based anesthesia]. clinicaltrials.gov/ct2/show/record/NCT03194074 (first received 17 June 2017).
Additional references
Al‐Rifai 2016
- Al‐Rifai Z, Mulvey D. Principles of total intravenous anaesthesia: practical aspects of using total intravenous anaesthesia. BJA Education 2016;16(8):276‐80. [Google Scholar]
Ansaloni 2010
- Ansaloni L, Catena F, Chattat R, Fortuna D, Franceschi C, Mascitti P, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. British Journal of Surgery 2010;97(2):273‐80. [PUBMED: 20069607] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [PUBMED: 15205295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballard 2012
- Ballard C, Jones E, Gauge N, Aarsland D, Nilsen OB, Saxby BK, et al. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLOS one 2012;7(6):e37410. [PUBMED: 22719840] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bitsch 2004
- Bitsch M, Foss N, Kristensen B, Kehlet H. Pathogenesis of and management strategies for postoperative delirium after hip fracture: a review. Acta Orthopaedica Scandinavica 2004;75(4):378‐89. [PUBMED: 15370579] [DOI] [PubMed] [Google Scholar]
Boonmak 2016
- Boonmak P, Boonmak S, Pattanittum P. High initial concentration versus low initial concentration sevoflurane for inhalational induction of anaesthesia. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD006837.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borenstein 2010
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research Synthesis Methods 2010;1(2):97‐111. [PUBMED: 26061376] [DOI] [PubMed] [Google Scholar]
Cai 2012b
- Cai YM, Hiu HT, Liu PB, Feng GF, Dong WJ, Yu B, et al. Cognitive dysfunction after inhalation versus intravenous anaesthesia in elderly patients reply. Anesthesiology 2012;117:678. [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan MT, Cheng BC, Lee TM, Gin T, CODA Trial Group. BIS‐guided anesthesia decreases postoperative delirium and cognitive decline. Journal of Neurosurgical Anesthesiology 2013;25(1):33‐42. [PUBMED: 23027226] [DOI] [PubMed] [Google Scholar]
Checketts 2016
- Checketts MR, Alladi R, Ferguson K, Gemmell L, Handy JM, Klein AA, et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2016; Vol. 71, issue 1:85‐93. [PUBMED: 26582586] [DOI] [PMC free article] [PubMed]
Chhabra 2016
- Chhabra A, Subramaniam R, Srivastava A, Prabhakar H, Kalaivani M, Paranjape S. Spectral entropy monitoring for adults and children undergoing general anaesthesia. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD010135.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clegg 2011
- Clegg A, Young JB. Which medications to avoid in people at risk of delirium: a systematic review. Age and Ageing 2011;40(1):23‐9. [PUBMED: 21068014] [DOI] [PubMed] [Google Scholar]
Covidence 2016 [Computer program]
- Veritas Health Innovation. Covidence. Version (accessed March 2016). Melbourne, Australia: Veritas Health Innovation, 2016.
Deiner 2009
- Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. British Journal of Anaesthesia 2009;103 Suppl 1:i41‐6. [PUBMED: 20007989] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deiner 2012
DSM‐5 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. American Psychiatric Publishing, 2013. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote 2011 [Computer program]
- Thomson Reuters. Endnote. Version Endnote X5. Philadelphia, PA, USA: Thomson Reuters, 2011.
Fodale 2010
- Fodale V, Santamaria LB, Schifilliti D, Mandal PK. Anaesthetics and postoperative cognitive dysfunction: a pathological mechanism mimicking Alzheimer's disease. Anaesthesia 2010;65(4):388‐95. [PUBMED: 20136805] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed March 2016). Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Griffiths 2014
- Griffiths R, Beech F, Brown A, Dhesi J, Foo I, Goodall J, et al. Peri‐operative care of the elderly 2014: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2014;69 Suppl 1:81‐98. [PUBMED: 24303864] [DOI] [PubMed] [Google Scholar]
Guay 2016
- Guay J, Parker MJ, Gajendragadkar PR, Kopp S. Anaesthesia for hip fracture surgery in adults. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD000521.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical research edition) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence ‐ inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Herling 2017
- Herling SF, Dreijer B, Wrist Lam G, Thomsen T, Møller AM. Total intravenous anaesthesia versus inhalational anaesthesia for adults undergoing transabdominal robotic assisted laparoscopic surgery. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD011387] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hounsome 2016
- Hounsome J, Nicholson A, Greenhalgh J, Cook TM, Smith AF, Lewis SR. Nitrous oxide‐based versus nitrous oxide‐free general anaesthesia and accidental awareness during general anaesthesia in surgical patients. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD011052.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Inouye 1990
- Inouye SK, Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Annals of Internal Medicine 1990;113(12):941‐8. [PUBMED: 2240918] [DOI] [PubMed] [Google Scholar]
Koblin 2000
- Koblin D. Mechanisms of action of anesthetics. In: Miller RD editor(s). Anesthesia. New York: Churchill‐Livingstone, 2000:48‐73. [Google Scholar]
Krenk 2011
- Krenk L, Rasmussen LS. Postoperative delirium and postoperative cognitive dysfunction in the elderly ‐ what are the differences?. Minerva Anestesiologica 2011;77(7):742‐9. [PUBMED: 21709661] [PubMed] [Google Scholar]
Lewis 2015
- Lewis SR, Price A, Walker KJ, McGrattan K, Smith AF. Ultrasound guidance for upper and lower limb blocks. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD006459.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandal 2009
- Mandal PK, Schifilliti D, Mafrica F, Fodale V. Inhaled anesthesia and cognitive performance. Drugs of Today 2009;45(1):47‐54. [PUBMED: 19271031] [DOI] [PubMed] [Google Scholar]
Messina 2016
- Messina AG, Wang M, Ward MJ, Wilker CC, Smith BB, Vezina DP, et al. Anaesthetic interventions for prevention of awareness during surgery. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD007272.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PUBMED: 19622551] [PMC free article] [PubMed] [Google Scholar]
Monk 2005
- Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one‐year mortality after noncardiac surgery. Anesthesia and Analgesia 2005;100(1):4‐10. [PUBMED: 15616043] [DOI] [PubMed] [Google Scholar]
NCEPOD 2010
- National Confidential Enquiry into Patient Outcome and Death. An age old problem. www.ncepod.org.uk/2010report3/downloads/EESE_fullReport.pdf (accessed 25 February 2016).
Newman 2007
- Newman S, Stygall J, Hirani S, Shaefi S, Maze M. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology 2007;106(3):572‐90. [PUBMED: 17325517] [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Care Excellence. Depth of Anaesthesia Monitors – Bispectral Index (BIS), E‐Entropy and Narcotrend‐Compact M. NICE Diagnostics Guideline 6. National Institute of Health and Care Excellence, 2012. [Google Scholar]
Pandit 2013
- Pandit JJ, Cook TM, Jonker WR, O'Sullivan E. A national survey of anaesthetists (NAP5 baseline) to estimate an annual incidence of accidental awareness during general anaesthesia in the UK. British Journal of Anaesthesia 2013;110(4):501‐9. [PUBMED: 23482998] [DOI] [PubMed] [Google Scholar]
Prabhakar 2016
- Prabhakar H, Singh GP, Mahajan C, Kapoor I, Kalaivani M, Anand V. Intravenous versus inhalational techniques for rapid emergence from anaesthesia in patients undergoing brain tumour surgery. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD010467.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Punjasawadwong 2014
- Punjasawadwong Y, Phongchiewboon A, Bunchungmongkol N. Bispectral index for improving anaesthetic delivery and postoperative recovery. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD003843.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Raats 2015
- Raats JW, Steunenberg SL, Crolla RM, Wijsman JH, Slaa A, Laan L. Postoperative delirium in elderly after elective and acute colorectal surgery: A prospective cohort study. International Journal of Surgery 2015;18:216‐9. [PUBMED: 25937152] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robinson 1997
- Robinson BJ, Ebert TJ, O'Brien TJ, Colinco MD, Muzi M. Mechanisms whereby propofol mediates peripheral vasodilation in humans. Sympathoinhibition or direct vascular relaxation?. Anesthesiology 1997;86(1):64‐72. [PUBMED: 9009941] [DOI] [PubMed] [Google Scholar]
Royse 2011
- Royse CF, Andrews DT, Newman SN, Stygall J, Williams Z, Pang J, et al. The influence of propofol or desflurane on postoperative cognitive dysfunction in patients undergoing coronary artery bypass surgery. Anaesthesia 2011;66(6):455‐64. [PUBMED: 21501129] [DOI] [PubMed] [Google Scholar]
Rudolph 2011
- Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long‐term implications. Anesthesia and Analgesia 2011;112(5):1202‐11. [PUBMED: 21474660] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rundshagen 2014
- Rundshagen I. Postoperative cognitive dysfunction. Deutsches Arzteblatt International 2014;111(8):119‐25. [PUBMED: 24622758] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoen 2011
- Schoen J, Husemann L, Tiemeyer C, Lueloh A, Sedemund‐Adib B, Berger KU, et al. Cognitive function after sevoflurane‐ vs propofol‐based anaesthesia for on‐pump cardiac surgery: a randomized controlled trial. British Journal of Anaesthesia 2011;106(6):840‐50. [PUBMED: 21518736] [DOI] [PubMed] [Google Scholar]
Sieber 2010
- Sieber FE, Zakriya KJ, Gottschalk A, Blute MR, Lee HB, Rosenberg PB, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clinic Proceedings 2010;85(1):18‐26. [PUBMED: 20042557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Steinmetz 2016
- Steinmetz J, Rasmussen LS. Peri‐operative cognitive dysfunction and protection. Anaesthesia 2016;71 Suppl 1:58‐63. [PUBMED: 26620148] [DOI] [PubMed] [Google Scholar]
Thwaites 1997
- Thwaites A, Edmends S, Smith I. Inhalation induction with sevoflurane: a double‐blind comparison with propofol. British Journal of Anaesthesia 1997;78(4):356‐61. [PUBMED: 9135350] [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang NY, Hirao A, Sieber F. Association between intraoperative blood pressure and postoperative delirium in elderly hip fracture patients. PLOS One 2015;10(4):e0123892. [PUBMED: 25860338] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiser 2008
- Weiser TG, Regenbogen SE, Thompson KD, Haynes AB, Lipsitz SR, Berry WR, et al. An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008;372(9633):139‐44. [PUBMED: 18582931] [DOI] [PubMed] [Google Scholar]
WHO 2016a
- World Health Organization. ICD‐10 Version: 2016. apps.who.int/classifications/icd10/browse/2016/en#/F00‐F09 (accessed 25 February 2016).
WHO 2016b
- World Health Orginization. Definition of an older or elderly person. www.who.int/healthinfo/survey/ageingdefnolder/en/ (accessed 25 February 2016).
References to other published versions of this review
Miller 2016
- Miller ID, Shelton CL, Lewis SR, Alderson P, Smith AF. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly surgical patients. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012317] [DOI] [PMC free article] [PubMed] [Google Scholar]


