Abstract
Background
Cystic fibrosis (CF) is a common life‐shortening condition caused by mutation in the gene that codes for that codes for the cystic fibrosis transmembrane conductance regulator (CFTR) protein, which functions as a salt transporter. F508del, the most common CFTR mutation that causes CF, is found in up to 80% to 90% of people with CF. In people with this mutation, a full length of protein is transcribed, but recognised as misfolded by the cell and degraded before reaching the cell membrane, where it needs to be positioned to effect transepithelial salt transport. This severe mutation is associated with no meaningful CFTR function. A corrective therapy for this mutation could positively impact on an important proportion of the CF population.
Objectives
To evaluate the effects of CFTR correctors on clinically important outcomes, both benefits and harms, in children and adults with CF and class II CFTR mutations (most commonly F508del).
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Cystic Fibrosis Trials Register. We also searched reference lists of relevant articles and online trials registries. Most recent search: 24 February 2018.
Selection criteria
Randomised controlled trials (RCTs) (parallel design) comparing CFTR correctors to placebo in people with CF with class II mutations. We also included RCTs comparing CFTR correctors combined with CFTR potentiators to placebo.
Data collection and analysis
Two authors independently extracted data, assessed risk of bias and quality of the evidence using the GRADE criteria. Study authors were contacted for additional data.
Main results
We included 13 RCTs (2215 participants), lasting between 1 day and 24 weeks. Additional safety data from an extension study of two lumacaftor‐ivacaftor studies were available at 96 weeks (1029 participants). We assessed monotherapy in seven RCTs (317 participants) (4PBA (also known as Buphenyl), CPX, lumacaftor or cavosonstat) and combination therapy in six RCTs (1898 participants) (lumacaftor‐ivacaftor or tezacaftor‐ivacaftor) compared to placebo. Twelve RCTs recruited individuals homozygous for F508del, one RCT recruited participants with one F508del mutation and a second mutation with residual function.
Risk of bias varied in its impact on the confidence we have in our results across different comparisons. Some findings were based on single RCTs that were too small to show important effects. For five RCTs, results may not be applicable to all individuals with CF due to age limits of recruited populations (i.e. adults only, children only) or non‐standard design of converting from monotherapy to combination therapy.
Monotherapy versus placebo
No deaths were reported and there were no clinically relevant improvements in quality of life in any RCT. There was insufficient evidence available from individual studies to determine the effect of any of the correctors examined on lung function outcomes.
No placebo‐controlled study of monotherapy demonstrated a difference in mild, moderate or severe adverse effects; however, it is difficult to assess the clinical relevance of these events with the variety of events and the small number of participants.
Combination therapy versus placebo
No deaths were reported during any RCT (moderate‐ to high‐quality evidence). The quality of life scores (respiratory domain) favoured combination therapy (both lumacaftor‐ivacaftor and tezacaftor‐ivacaftor) compared to placebo at all time points. At six months lumacaftor (600 mg once daily or 400 mg once daily) plus ivacaftor improved Cystic Fibrosis Questionnaire (CFQ) scores by a small amount compared with placebo (mean difference (MD) 2.62 points (95% confidence interval (CI) 0.64 to 4.59); 1061 participants; high‐quality evidence). A similar effect size was observed for twice‐daily lumacaftor (200 mg) plus ivacaftor (250 mg) although the quality of evidence was low (MD 2.50 points (95% CI 0.10 to 5.10)). The mean increase in CFQ scores with twice‐daily tezacaftor (100 mg) and ivacaftor (150 mg) was approximately five points (95% CI 3.20 to 7.00; 504 participants; moderate‐quality evidence). Lung function measured by relative change in forced expiratory volume in one second (FEV1) % predicted improved with both combination therapies compared to placebo at six months, by 5.21% with once daily lumacaftor‐ivacaftor (95% CI 3.61% to 6.80%; 504 participants; high‐quality evidence) and by 2.40% with twice‐daily lumacaftor‐ivacaftor (95% CI 0.40% to 4.40%; 204 participants; low‐quality evidence). One study reported an increase in FEV1 with tezacaftor‐ivacaftor of 6.80% (95% CI 5.30 to 8.30%; 520 participants; moderate‐quality evidence).
More participants receiving the lumacaftor‐ivacaftor combination reported early transient breathlessness, odds ratio 2.05 (99% CI 1.10 to 3.83; 739 participants; high‐quality evidence). In addition, participants allocated to the 400 mg twice‐daily dose of lumacaftor‐ivacaftor experienced a rise in blood pressure over the 120‐week period of the initial studies and the follow‐up study of 5.1 mmHg (systolic blood pressure) and 4.1 mmHg (diastolic blood pressure) (80 participants; high‐quality evidence). These adverse effects were not reported in the tezacaftor‐ivacaftor studies.
The rate of pulmonary exacerbations decreased for participants receiving and additional therapies to ivacaftor compared to placebo: lumacaftor 600 mg hazard ratio (HR) 0.70 (95% CI 0.57 to 0.87; 739 participants); lumacaftor 400 mg, HR 0.61 (95% CI 0.49 to 0.76; 740 participants); and tezacaftor, HR 0.64 (95% CI, 0.46 to 0.89; 506 participants) (moderate‐quality evidence).
Authors' conclusions
There is insufficient evidence that monotherapy with correctors has clinically important effects in people with CF who have two copies of the F508del mutation.
Combination therapies (lumacaftor‐ivacaftor and tezacaftor‐ivacaftor) each result in similarly small improvements in clinical outcomes in people with CF; specifically improvements quality of life (moderate‐quality evidence), in respiratory function (high‐quality evidence) and lower pulmonary exacerbation rates (moderate‐quality evidence). Lumacaftor‐ivacaftor is associated with an increase in early transient shortness of breath and longer‐term increases in blood pressure (high‐quality evidence). These adverse effects were not observed for tezacaftor‐ivacaftor. Tezacaftor‐ivacaftor has a better safety profile, although data are not available for children younger than 12 years. In this age group, lumacaftor‐ivacaftor had an important impact on respiratory function with no apparent immediate safety concerns, but this should be balanced against the increase in blood pressure and shortness of breath seen in longer‐term data in adults when considering this combination for use in young people with CF.
Plain language summary
CFTR correctors, a therapy for cystic fibrosis targeted at specific mutations
Review question
We looked at drugs (or combination of drugs) that aim to correct the basic defect for the commonest mutation (F508del) that causes cystic fibrosis (CF). We assessed the impact on clinical outcomes that are important to people with CF (e.g., survival, quality of life, lung function and safety).
Background
The CF gene makes a protein that has important function in many parts of the body by helping the movement of salts across cells. People with CF are either not able to make this protein or make a protein that is defective. A common mutation of the CF gene is F508del and over 80% of people with CF have at least one copy of this gene variant. When the CF gene is affected by F508del, a full length of protein is made but it is not able to move through the cell correctly. Laboratory experiments suggest that if this protein can be transported to the cell wall then it may be able to function, restore salt movement and correct the chronic problems experienced by people with CF. We examined a number of agents that might correct the F508del mutation.
Search date
Evidence is current to: 24 February 2018.
Study characteristics
We included 13 studies (2215 children and adults with CF) which lasted between 1 day and 24 weeks (with an extension study of two studies up to 96 weeks). Seven studies (317 participants) looked at single agents (monotherapy: 4PBA (also known as Buphenyl), CPX, lumacaftor and cavosonstat) versus placebo (a dummy treatment containing no active medicine) and six recent studies (1898 participants) assessed combination therapy (lumacaftor‐ivacaftor or tezacaftor‐ivacaftor) versus placebo. In 12 studies participants had two copies of the F508del mutation and in one study they had one F508del mutation and a second different mutation.
Key results
Monotherapy versus placebo
These studies did not report any deaths or any clinically relevant improvements in quality of life scores. There was not enough evidence to show an effect on lung function. Side effects were reported in all studies, but it is difficult to assess their relevance due to the range of effects and the small number of participants in the studies.
Combination therapy versus placebo
No deaths were reported in either the lumacaftor‐ivacaftor or tezacaftor‐ivacaftor studies in people with two copies of the F508del mutation and there were improvements in quality of life and lung function. Rates of pulmonary exacerbations (a flare up of symptoms) were also lower. Neither combination therapy was associated with severe side effects, although people on lumacaftor‐ivacaftor regularly experienced shortness of breath for one to two weeks at the start of treatment, which usually resolved without further intervention. Of more concern was that in longer studies some people receiving lumacaftor‐ivacaftor experienced a rise in blood pressure; of these, two people (out of more than 500 who received the combination in these studies) discontinued lumacaftor‐ivacaftor treatment because of high blood pressure. These side effects were not reported for the tezacaftor‐ivacaftor combination. Tezacaftor‐ivacaftor therapy has not yet been assessed in children with CF younger than 12 years of age.
Quality of the evidence
We judged the overall quality of the evidence for the outcomes measured to vary from low to high. Study design was generally poorly reported, which did not allow us to make clear judgements on any potential bias, but we had fewer concerns with the six larger and more recent studies. We did find that some results were omitted from the analysis or not reported in seven studies. Some findings were based on single studies that were too small to show important effects and for five studies the results may not be applicable to all individuals with CF due to the ages of people recruited into the studies (i.e. adults only, children only) or an unusual design used in which people receive monotherapy and then combination therapy.
Summary of findings
Background
Description of the condition
Cystic fibrosis (CF) is the most common inherited life‐shortening illness with a prevalence of 1 in 2000 at birth in Northern Europeans (Bobadilla 2002) and varying prevalence in other populations depending on ethnic composition. The affected gene codes for a protein called the cystic fibrosis transmembrane conductance regulator (CFTR) (Riordan 1989; Southern 1997). CFTR protein is transported to the outer cell membrane, where it has a role in the transport of salts (anions, chloride and bicarbonate) in and out of the cell (Rogan 2011). This role is important in all epithelial cells; particularly those lining the airways, pancreatic ducts, sweat gland, bile ducts in the liver and vas deferens.
In the lungs of people with CF (pwCF), defective salt transport leads to a reduction in airway surface liquid volume. This, in turn, leads to compromised mucociliary clearance, which makes the airway susceptible to infection, which initiates a cycle of inflammation, chronic infection and progressive lung damage. Eventually this causes respiratory failure, which is the commonest cause of premature death for pwCF. In addition to the airway problems, the abnormal transepithelial salt transport can lead to complications in other organs. This can result in malnutrition and diabetes (through pancreatic damage), salt depletion (through excess loss in sweat) and subfertility.
Over 2000 mutations have been identified in the CFTR gene. These mutations are classified according to the impact they have on the synthesis, processing, or function of the CFTR gene (CFMD 2013). Classes of CFTR mutation are described in more detail in the additional tables (Table 20) (Rowntree 2003; Southern 2007). Most CFTR mutations are associated with a complete loss of CFTR protein and result in a classical CF phenotype. Some CFTR mutations are associated with residual function and these tend to be associated with less severe phenotype, e.g. patients may be pancreatic sufficient and not require pancreatic replacement therapy.
1. Classes of mutations affecting CFTR production, structure and function.
| Class | Example mutation | Impact on CFTR structure and function |
| I | G542X | Synthesis of CFTR is critically impaired, and no functional protein is produced. This is due to the presence of a premature stop codon in the nucleotide sequence. Individuals have minimal CFTR function. |
| II | F508del | A full length of CFTR is produced, but this is structurally abnormal and destroyed by the cell before it reaches the cell membrane. This is called a defect in the intracellular trafficking pathway. Small amounts of CFTR do reach the cell membrane; however here, they display defective ion transport, demonstrating that the phe508del mutation is more than just a processing defect. Individuals have minimal CFTR function. |
| III | G551D | CFTR is produced and embedded in the cell membrane, but the chloride channel does not respond ('switch on') to normal stimulation from the cell. This means there is no meaningful ion transport across the protein. Individuals have minimal CFTR function. |
| IV | R347P | CFTR is transported to the outer cell membrane, and responds to normal stimulation, but functions at a low level because chloride ions do not cross the channel appropriately. Individuals have some residual CFTR function. |
| V | A455E | Normal CFTR is produced, but the amount of protein is reduced. Individuals have some residual CFTR function. |
CFTR: cystic fibrosis transmembrane regulator
The commonest CF causing mutation, F508del (also known as ΔF508 or phe508del), is found in the majority of pwCF (up to 80% to 90% of some populations, e.g. pwCF from a Northern European heritage). For individuals with F508del, a full length of protein is transcribed but recognised as misfolded by the cell and is degraded before reaching the cell membrane, where it needs to be positioned to effect transepithelial salt transport. Hence this is a severe mutation associated with no meaningful CFTR function. This type of mutation is called a class II mutation (or trafficking defect) and much research has explored masking the molecular defect, bypassing the cellular mechanisms and enabling the F508del protein to traffic to the cell membrane, where it may have some normal salt transport capability.
Description of the intervention
Increasing understanding of how different mutations affect the production, structure, and function of CFTR has led to the concept of mutation‐specific therapies (Table 20). For class II mutations a full length of protein is produced, but recognised as abnormal by the cell and degraded before reaching the cell membrane. This is called a defect in intracellular trafficking. Scientists have recognised that certain laboratory manoeuvres can affect this process, e.g. reducing cell temperature, and the trafficking defect can be overcome (Colledge 1995). In such circumstances the F508del protein may reach the cell membrane, where it has some ability to transport salt. This has lead to the search for molecules that can overcome the F508del trafficking defect and these drugs have been called 'correctors'.
Two distinct scientific approaches have resulted in the recognition of candidate drugs with this mode of action (Amaral 2007):
testing of compounds known to affect CFTR or other ion channels (either pharmaceutical drugs or chemicals which occur naturally in plants, herbs, fruits or food components);
high throughput screening, which involves testing large numbers of diverse chemicals, on laboratory cell lines, to identify which of these may overcome the intracellular trafficking defect.
These approaches have resulted in the identification of small molecules that may be taken orally, and have been examined in phase 1 and phase 2 clinical trials (Rubenstein 1997; Van Goor 2011).
How the intervention might work
Correction of the basic CF defect may lead to normalisation of airway surface liquid, and correction of mucociliary clearance, reducing the susceptibility to airway infection and inflammation.
In addition to correctors, other drugs which aim to treat the CFTR defect are also under investigation. These include potentiators for class III and IV mutations, which enhance the function of mutated CFTR protein embedded in the cell membrane by increasing the time the CFTR salt channel remains open and therapies for class I mutations, which act to prevent structural abnormalities of CFTR that occur when premature stop codons terminate protein synthesis. Cochrane Reviews assessing these interventions are published (Aslam 2017; Patel 2015).
If correctors are successful at facilitating the F508del protein to reach the cell membrane, it may still have sub‐optimal function. It is possible that CFTR correctors may need to be combined with other agents, such as potentiators to achieve a clinical benefit pwCF who have the F508del mutation. This review examines both correctors on their own and in combination with other agents.
Why it is important to do this review
CFTR correctors are novel therapies and it is important that randomised controlled trials (RCTs) are conducted and critically appraised. This will provide clear evidence to assess the benefits and harms of CFTR correctors. It is important that funding bodies have a clear evidence base on which to assess new therapies for CF that aim to correct the basic defect. In addition, critical appraisal of studies will help inform future study design.
New therapies that correct the F508del mutation will have a positive impact on an important proportion of the CF population (Southern 1997). Given the number of pwCF who will be prescribed this treatment, there will be an important healthcare cost. Experience from other licensed agents that correct the underlying CF defect, suggests that these costs may be considerable (NICE 2016).
This review aims to collate evidence from RCTs that have evaluated the benefits and harms of CFTR correctors in pwCF and class II CFTR mutations.
Objectives
To evaluate the effects of CFTR correctors on clinically important outcomes, both benefits and harms, in children and adults with CF and class II CFTR mutations (most commonly F508del).
Methods
Criteria for considering studies for this review
Types of studies
We have included RCTs of parallel design (published or unpublished). We have not included quasi‐RCTs. Additionally, we have not included cross‐over studies as we do not feel this study design is appropriate given that the intervention aims to correct the underlying defect. If the intervention is effective it will have an important impact on the course of the disease. This has been established from the data from trials examining ivacaftor for people with class III mutations.
Types of participants
We have included studies involving children or adults with CF, as confirmed either by the presence of two disease‐causing mutations, or by a combination of positive sweat test and recognised clinical features of CF. We have included studies that include participants with any level of disease severity. Participants should have at least one class II mutation.
Types of interventions
A CFTR corrector is defined as a drug which aims to increase the amount of CFTR expressed at the epithelial cell apical membrane, by reducing or preventing degradation of CFTR by normal intracellular mechanisms. The main mutation targeted by this approach is F508del.
We have included studies in which CFTR correctors are compared with either placebo or another intervention. We have also included studies in which CFTR correctors are administered alongside another class of drug that also aims to improve CFTR function (e.g. potentiators).
Types of outcome measures
Primary outcomes
Survival
-
Quality of life (QoL) (measured using validated quantitative scales or scores (e.g. Cystic Fibrosis Questionnaire‐Revised (CFQ‐R) (Quittner 2009))
total QoL score
different sub‐domains which may be reported
-
Physiological measures of lung function (L or per cent (%) predicted for age, sex and height)
forced expiratory flow rate at one second (FEV1) (relative change from baseline)
FEV1 absolute values (rather than change from baseline)
forced vital capacity (FVC) (absolute values and change from baseline)
lung clearance index (LCI) (post hoc change)
other relevant physiological measures of lung function
Secondary outcomes
-
Adverse effects
graded by review authors as mild (therapy does not need to be discontinued)
graded by review authors as moderate (therapy is discontinued, and the adverse effect ceases)
graded by review authors as severe (life‐threatening or debilitating, or which persists even after treatment is discontinued)
other adverse effects of therapy (of any severity) that are not classifiable according to these categories
-
Hospitalisation
number of days
number of episodes
time to next hospitalisation
School or work attendance (i.e. number of days missed)
-
Extra courses of antibiotics (measured as time to the next course of antibiotics and the total number of courses of antibiotics)
oral
intravenous
inhaled
Sweat chloride (change from baseline) as a measure of CFTR function
-
Radiological measures of lung disease (assessed using any scoring system)
chest radiograph scores
computerised tomogram (CT) score
-
Acquisition of respiratory pathogens
Pseudomonas aeruginosa
Staphylococcus aureus
Haemophilus influenzae
other pathogen clinically relevant in CF
-
Eradication of respiratory pathogens (as defined by study authors)
P aeruginosa
S aureus
H influenzae
other pathogen clinically relevant in CF
-
Nutrition and growth (measured as relative change from baseline) (including z scores or centiles)
weight
body mass index (BMI)
height
Search methods for identification of studies
We searched for all relevant published and unpublished studies without restrictions on language (we did not exclude studies reported in a language other than English), year or publication status.
Electronic searches
We identified relevant studies from the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register using the terms: 'drugs that correct defects in CFTR transcription, translation or processing'. Relevant studies have been tagged with these terms for indexing purposes in the Group's Cystic Fibrosis Trials Register.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work was identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group website.
Date of the most recent search: 24 February 2018.
We also searched clinical trial registries maintained by the European Medicines Agency, the US National Institutes of Health (NIH) and the WHO; further details are presented in the appendices (Appendix 1).
Date of the most recent search: 25 January 2018.
Searching other resources
We screened references of included studies to identify additional potentially relevant studies. We also contacted authors of included studies, leaders in the field, and companies known to be developing and investigating CFTR correctors, to identify any studies which may have been missed by this search. We recorded response rates from this contact process below (Results of the search).
Data collection and analysis
Selection of studies
Two authors (IS and SP or IS and KWS) independently assessed the suitability of each potential study identified by the search. If disagreement arose on the suitability of a study for inclusion in the review, we attempted to reach a consensus by discussion, failing which, a third author arbitrated.
Data extraction and management
Two authors (IS and SP or IS and SJN) independently extracted relevant data from each included study. If disagreement arose on data extraction, we attempted to reach a consensus by discussion, failing which, a third author (KWS) arbitrated. Two authors (SP and SN) entered the data into RevMan for analysis.
If studies had reported data on our primary outcome (survival), we planned to report these as a binary outcome or a time‐to‐event outcome. We planned on extracting QoL scores as relative change from baseline ((measurement at end of treatment ‐ measurement at baseline) / measurement at baseline) x 100). We extracted data presented as post‐treatment values or change from baseline when this was not possible.
With regards to the secondary outcome 'Extra courses of antibiotics', we planned to extract data as time‐to‐the‐next course of antibiotics and the total number of courses of antibiotics. We noted whether episodes of pulmonary exacerbations were physician‐defined or protocol‐defined. If studies reported baseline and post‐treatment sweat chloride concentration values, we calculated the relative change from baseline values ((measurement at end of treatment ‐ measurement at baseline) / measurement at baseline) x 100).
We reported data as immediate (up to and including one month), short‐term (over one month and up to six months) and longer‐term (over six months).
We attempted to extract the most precise data as possible for each outcome; extraction of tabulated data was preferred. If data were presented only graphically, two authors (SP and SJN) estimated the relevant data from graphs and compared estimations for accuracy.
Assessment of risk of bias in included studies
Two authors (IS and SP or IS and SJN) assessed the risk of bias for each study using the Cochrane risk of bias tool (Higgins 2011a). This includes assessment of the following methodological aspects of the included studies:
procedure for randomisation (selection bias);
allocation concealment (selection bias);
masking (blinding) of the intervention from participants, clinicians, and trial personnel evaluating outcomes (performance bias);
missing outcome data (attrition bias);
selective outcome reporting (reporting bias);
other sources of bias (e.g. the influence of funding sources or industry on trial characteristics and presented results).
We also assessed whether all participants were included in an intention‐to‐treat analysis, regardless of whether they completed the treatment schedule or not. If disagreement arose on the assessment of risk of bias of a study, we attempted to reach a consensus by discussion, failing which, a third author (KWS) arbitrated.
Measures of treatment effect
For binary outcomes, we calculated a pooled estimate of the treatment effect for each outcome using the pooled odds ratio (OR) and 95% confidence intervals (CIs) or 99% CIs for analysis of separate adverse events.
For continuous outcomes, we calculated the mean change from baseline for each group or the mean post‐intervention values and standard deviation (SD) for each group. We converted standard errors (SEs) to SDs. We produced a pooled estimate of treatment effect by calculating the mean difference (MD) and 95% CIs.
In future updates of this review, if different trials present data for the same outcomes in different forms (e.g. absolute values of lung function measures, or change in these measures from a baseline), we will combine these in a meta‐analysis where appropriate.
Where the studies did not report change data, but instead presented absolute post‐treatment data without baseline data (so it was not possible to calculate change data) we planned to use absolute post‐treatment data instead of change from baseline. However, if the report presented baseline and post‐treatment data for any outcome, we calculated SDs for the change from baseline, for example if the CI was available. If there was not enough information available to calculate the SDs for the changes, we planned to impute them from other trials in the review, where data were available and trials were similar (i.e. when they used the same measurement scale, had the same degree of measurement error and had the same time periods between baseline and final value measurement). If neither of these methods were possible, we planned to calculate a change‐from‐baseline SD, making use of an imputed correlation coefficient (methods described in section 16.1.3.2 in the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011b)).
Where time‐to‐event data were reported (e.g. survival time, time to next hospitalisation, time to first exacerbation), we reported a hazard ration (HR) and 95% CIs.
When reporting on outcomes we used the following subheadings to describe the time points: immediate (up to and including one month); short term (over one month and up to six months); and longer term (over six months).
Unit of analysis issues
Within this review, we only included results from RCTs of parallel design in which individual study participants are randomised. We have not included cross‐over studies as we do not feel this study design is appropriate given that the intervention aims to correct the underlying defect. If the intervention is effective it will have an important impact on the course of the disease. This has been established from the data from trials examining ivacaftor for people with class III mutations (Patel 2015).
In one included study, continuous outcomes were analysed via a mixed model repeated measures analysis (MMRM) based on the average effect across the measured time points (Ratjen 2017). Such an analysis is longitudinal and uses all available data at every visit and allows adjustment for covariates such as the baseline measurement of the outcome. All analyses were also adjusted for baseline weight (less than 25 kg versus 25 kg or over) and baseline FEV1 (% predicted ‐ less than 90% versus 90% or above). Results provided by this model can be interpreted as treatment effect averaged from each study visit until week 24. Within this review, results are entered into the analysis via generic inverse variance and are not pooled with other studies, due to the different approaches to analysis.
Dealing with missing data
In order to allow an intention‐to‐treat analysis, we extracted data on the number of participants with each outcome event, by allocated treated group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up. If any data were missing or unclear, we contacted the primary investigators for clarification.
Assessment of heterogeneity
We assessed heterogeneity through visual examination of the combined data presented in the forest plots, and by considering overlap of study‐specific CIs, and the I² statistic (Higgins 2003) together with Chi² values (Deeks 2011). The I² statistic reflects the likelihood that variation of results across studies are due to heterogeneity rather than by chance, and we interpreted this statistic using the following simple classification:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
In order to identify selective outcome reporting, where possible, we have compared outcomes described in the study protocol with those reported in the publication. We have requested protocols for specific studies from the primary investigators and recorded the proportion of protocols that were available to us. If a protocol was not available, we searched for information about outcomes from trial registry databases. We also compared outcomes listed in the 'Methods' section of the final paper with those presented in the 'Results' section. If the published papers reported negative findings either only partially, or not at all, we contacted primary investigators for these data.
We would have assessed publication bias by constructing and assessing the symmetry of a funnel plot. This would have been possible if we included more than 10 studies in a meta‐analysis in the review. We would have plotted the number of participants in the study against a measure of treatment effect. If the funnel plot was asymmetrical, we would consider whether this was due to publication bias, or whether methodology or small sample size caused results of certain studies to show exaggerated treatment effects.
Data synthesis
As we intended to assess different CFTR correctors within this review, we assumed that there would not be a single common true effect. We also anticipated participants in each study would vary due to different eligibility criteria. Therefore, regardless of I² value, we intended to use a random‐effects model to analyse data from studies.
As the review progressed, we included a number of early‐phase studies of interventions (which were ultimately not taken forward) in addition to large Phase 3 studies of combination therapies; therefore, we felt it more appropriate to employ separate comparisons within the review. As only a relatively small number of studies were included in each comparison (and when meta‐analysis was undertaken), it was considered more appropriate to employ a fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We would have investigated any heterogeneity that we identified using subgroup analyses of potential confounding factors, if sufficient numbers (at least 10 studies included in a meta‐analysis) were available. For this review, these confounding factors would be:
age (children (defined as younger than 18 years of age) versus adults);
gender;
different mutation classes (Table 20).
As we did not seek individual patient data from study investigators and such information was not available within published reports, we did not undertake a subgroup analysis on the basis of disease severity. We may incorporate such an analysis in future updates of this review.
Sensitivity analysis
In future updates of this review, if sufficient data are available, we will examine the impact of bias on the results by comparing meta‐analyses including and excluding studies with concerns of high risk of selection or reporting bias due to issues relating to randomisation, allocation concealment, or masking of interventions from participants or study personnel.
Summary of findings and quality of the evidence (GRADE)
In a post hoc change from protocol, we have presented six summary of findings tables (Table 1; Table 2; Table 3; Table 4; Table 5; Table 6).
Summary of findings for the main comparison. Summary of findings ‐ Lumacaftor monotherapy compared to placebo.
| Lumacaftor compared with placebo for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: lumacaftor Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lumacaftor | |||||
|
Survival Follow‐up: 14 to 28 days |
No deaths reported. | No deaths reported. | NA | 147 (2 studies) |
⊕⊕⊝⊝ low1 | |
|
Quality of life ‐ total score Follow‐up: 14 to 28 days |
Outcome not reported. | NA | A higher score indicates a better outcome. | |||
|
Quality of life ‐ CFQ‐R respiratory domain: absolute change from baseline Follow‐up: 14 to 28 days |
There was a statistically significant decrease in the CFQ‐R respiratory domain in the 50 mg lumacaftor group compared to placebo. No differences were found in the other dose groups (25 mg, 100 mg, 200 mg) compared to placebo. | NA | 85 (1 study) |
⊕⊕⊝⊝ low1 | A higher score indicates a better outcome. | |
|
FEV1 % predicted: relative change from baseline Follow‐up: 14 to 28 days |
Outcome not reported. | NA | ||||
|
FEV1 % predicted: absolute change from baseline Follow‐up: 14 to 28 days |
The mean change from baseline was 1.7% predicted. | The mean change from baseline was 1.90% predicted lower (4.13 lower to 0.33 higher). | NA | 61 (1 study) |
⊕⊕⊕⊝ moderate2 | |
|
Adverse events Follow‐up: 14 to 28 days |
There were no statistically significant differences between groups in terms of participants experiencing any specific adverse event. In 1 of the studies, 1 participant from each of the lumacaftor arms ‐ 1 participant in each of the discontinued the study drug due to respiratory adverse effects. No participants discontinued from the placebo group. |
NA | 115 (2 studies) |
⊕⊝⊝⊝ very low1,2,3 | ||
|
Time to first pulmonary exacerbation Follow‐up: 14 to 28 days |
Outcome not reported (see comment). | NA | Time to first pulmonary exacerbation was not reported. There was no statistically significant difference between groups in the number of participants experiencing pulmonary exacerbations. | |||
| *The basis for the assumed risk is the mean placebo group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFQ‐R: Cystic Fibrosis Questionnaire‐Revised; CI: confidence interval, EQ‐5D‐3L: 5‐Dimension‐3 Level, FEV1: forced expiratory volume at one second; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded twice due to risk of bias: in one study data were selectively reported and often presentation of data did not allow for inclusion in analysis (Clancy 2012). There are also incomplete outcome data in the study with participants unaccounted for in analysis.
2. Downgraded once due to indirectness: design of the study means that monotherapy treatment was measured for only 14 days before a combination therapy phase was started (Boyle 2014).
3. Downgraded once due to imprecision: few events occurred therefore CIs for occurrence of specific events are very wide (Analysis 1.2; Analysis 1.3).
Summary of findings 2. Summary of findings ‐ Cavosonstat compared with placebo for cystic fibrosis.
| Cavosonstat compared with placebo for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: cavosonstat 200 mg Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Cavosonstat | |||||
|
Survival Follow‐up: 28 days |
No deaths reported. | No deaths reported. | NA | 26 (1 study) |
⊕⊕⊝⊝ low1,2 | |
|
Quality of life: total score Follow‐up: NA |
Outcome not reported. | NA | A higher score indicates a better outcome. | |||
|
Quality of life: CFQ‐R respiratory domain: absolute change from baseline Follow‐up: 28 days |
The mean absolute change from baseline in CFQ‐R respiratory domain was ‐4.6 points in the placebo group. | The mean absolute change from baseline in CFQ‐R respiratory domain was 3.80 higher (11.30 lower to 18.90 higher) in the Cavosonstat group than the placebo group. | NA | 26 (1 study) |
⊕⊝⊝⊝ very low1,2,3 | A higher score indicates a better outcome. |
|
FEV1 % predicted: relative change from baseline Follow‐up: NA |
Outcome not reported. | NA | ||||
|
FEV1 % predicted: absolute change from baseline Follow‐up: 28 days |
There were no treatment‐related changes in FEV1 (% predicted) compared to placebo. | NA | 26 (1 study) |
⊕⊕⊝⊝ low1,2 | A graphical figure of change from baseline in FEV1 (% predicted) is provided but numerical data cannot be extracted to include in analysis due to overlapping lines. | |
|
Adverse events: occurring in at least 10% of cavosonstat treated participants Follow‐up: 28 days |
There was no statistically significant difference between groups in terms of cough, pulmonary exacerbation, chest discomfort and fatigue. | NA | 26 (1 study) |
⊕⊝⊝⊝ very low1,2,4 | ||
|
Time to first pulmonary exacerbation Follow‐up: NA |
Outcome not reported. | NA | ||||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFQ‐R: Cystic Fibrosis Questionnaire‐Revised; CI: confidence interval; NA: not applicable. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded once due to potential risk of bias: unclear details related to methodological design and some unbalanced baseline characteristics.
2. Downgraded once due to indirectness: adults only were recruited into the study, therefore, results are not applicable to children.
3. Downgraded once due to imprecision: wide CIs around the result.
4. Downgraded once due to imprecision: very wide CIs around results (due to small event numbers).
Summary of findings 3. Summary of findings ‐ Lumacaftor plus ivacaftor (once daily) compared with placebo for cystic fibrosis (short term).
| Lumacaftor plus ivacaftor compared with placebo for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: lumacaftor (600 mg once daily or 400 mg once daily) plus ivacaftor (250 mg twice daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lumacaftor plus ivacaftor | |||||
|
Survival Follow‐up: 6 months |
No deaths reported. | No deaths reported. | NA | 1108 (2 studies) |
⊕⊕⊕⊕ high | |
|
Quality of life ‐ (EuroQol) EQ‐5D‐3L Index Score (total score): absolute change from baseline Follow‐up: 6 months |
The mean absolute change from baseline ranged from 0.0006 to 0.0017 points. | The mean absolute change from baseline was 0.00 points higher (0.01 lower to 0.01 higher). | NA | 1061 (2 studies) |
⊕⊕⊕⊕ high | A higher score indicates a better outcome. |
|
Quality of life ‐ CFQ‐R respiratory domain: absolute change from baseline Follow‐up: 6 months |
The mean absolute change from baseline ranged from 1.1 to 2.81 points. | The mean absolute change from baseline was 2.62 points higher (0.64 higher to 4.59). | NA | 1076 (2 studies) |
⊕⊕⊕⊝ moderate1 | A higher score indicates a better outcome. There was also a statistically significant difference between groups at 28 days, MD 3.70 points (95% CI 1.81 to 5.58). |
|
FEV1 % predicted: relative change from baseline Follow‐up: 6 months |
The mean relative change from baseline ranged from ‐0.34% to 0%. | The mean relative change from baseline was 5.21% higher (3.61% higher to 6.80% higher). | NA | 1072 (2 studies) |
⊕⊕⊕⊕ high | |
|
FEV1 % predicted: absolute change from baseline Follow‐up: 6 months |
The mean absolute change from baseline ranged from ‐0.44 to ‐0.15% predicted. | The mean absolute change from baseline was 3.07% predicted higher (2.17 higher to 3.97 higher). | NA | 1072 (2 studies) |
⊕⊕⊕⊝ moderate1 | There was also a statistically significant difference between groups at 28 days, MD 2.37% predicted (95% CI 1.52 to 3.22). |
|
Adverse events Follow‐up: 6 months |
Cough was statistically significantly more common in the placebo group compared to the lumacaftor‐ivacaftor group. Dyspnoea was statistically significantly more comment in the lumacaftor‐ivacaftor group compared to the placebo group. There were no statistically significant differences between groups in terms of number of participants experiencing adverse events, serious adverse events or other adverse events. Long‐term open‐label follow‐up data of the 2 studies showed a statistically significant increase in early transient shortness of breath. In participants allocated a 400 mg twice‐daily dose, there was a statistically significant rise in blood pressure. |
NA | 1108 (2 studies) |
⊕⊕⊕⊕ high | ||
|
Time to first pulmonary exacerbation Follow‐up: 6 months |
Time to first pulmonary exacerbation was statistically significantly longer in both in the lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily and the lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily groups | NA | 1108 (2 studies) |
⊕⊕⊕⊝ moderate1 | Presentation of data did not allow an analysis of the lumacaftor doses pooled. | |
| *The basis for the assumed risk is the mean placebo group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFQ‐R: Cystic Fibrosis Questionnaire‐Revised; CI: confidence interval; EQ‐5D‐3L: 5‐Dimension‐3 Level; EuroQol: Euro Quality of Life Scale; FEV1: forced expiratory volume at one second; MD: mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded once due to risk of bias from selective reporting: data contributing to analyses were extrapolated from published graphs or estimated. We have requested confirmation of the exact data from the study investigators. Any unpublished information we receive will be included in a future update and this judgement will be reconsidered.
Summary of findings 4. Summary of findings ‐ Lumacaftor plus ivacaftor (twice daily) compared with placebo for cystic fibrosis (short term).
| Lumacaftor plus ivacaftor compared with placebo for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
|
Survival Follow‐up: 24 weeks |
No deaths reported. | No deaths reported. | NA | 204 (1 study) | ⊕⊕⊕⊝ moderate1 | |
|
Quality of life ‐ total score Follow‐up: 24 weeks |
Outcome not reported. | NA | A higher score indicates a better outcome. | |||
|
Quality of life ‐ CFQ‐R respiratory domain: absolute change from baseline Follow‐up: 24 weeks |
See comment. | The mean change in the CFQ‐R respiratory domain was 2.50 points higher in the lumacaftor‐ivacaftor group compared to the placebo group, ranging from 0.10 lower to 5.10 higher. | NA | 204 (1 study) | ⊕⊕⊝⊝ low1,2 | A higher score indicates a better outcome. Data were analysed via a MMRM. Results provided by this model can be interpreted as treatment effect averaged from each study visit until week 24. |
|
FEV1 % predicted: relative change from baseline Follow‐up: 24 weeks |
Outcome not reported. | NA | Relative change from baseline in FEV1 was listed in the methods of the study but no numerical results were presented. if numerical data becomes available at a later date, it will be included in an update of this review. |
|||
|
FEV1 % predicted: absolute change from baseline Follow‐up: 24 weeks |
See comment. | The mean change in FEV1 % predicted was 2.40 higher in the lumacaftor‐ivacaftor group compared to the placebo group, ranging from 0.40 higher to 4.40 higher. | NA | 204 (1 study) | ⊕⊕⊝⊝ low1,2 | Data were analysed via a MMRM. Results provided by this model can be interpreted as treatment effect averaged from each study visit until week 24. |
|
Adverse events Follow‐up: 24 weeks |
There was no statistically significant difference between the groups in terms of productive cough, nasal congestion, oropharyngeal pain, upper abdominal pain, rhinorrhoea, increased sputum, cough, pyrexia, headache, upper respiratory tract infection, abdominal pain, nausea, vomiting, fatigue and respiratory events (such as wheezing, dyspnoea, asthma and chest discomfort). | NA | 204 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
|
Time to first pulmonary exacerbation Follow‐up: 24 weeks |
Outcome not reported. | NA | Time to first pulmonary exacerbation was listed in the methods of the study but no numerical results were presented. If numerical data become available at a later date, they will be included in an update of this review. |
|||
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CFQ‐R: Cystic Fibrosis Questionnaire‐Revised; CI: confidence interval; FEV1: forced expiratory volume at 1 second; MMRM: mixed model for repeated measures; NA: not applicable. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded once due to indirectness: children aged 6 ‐ 11 years were recruited in this study, therefore, results are not applicable to other age groups.
2. Downgraded once due to risk of bias from selective reporting: limited data available which is adjusted for all visits. Further graphical data were available in the publication but could not be accurately extracted. We have requested confirmation of the exact data from the study investigators. Any unpublished information we receive will be included in a future update and this judgement will be reconsidered
3. Downgraded once due to imprecision; few events occurred therefore CIs for occurrence of specific events are very wide (Analysis 11.4).
Summary of findings 5. Summary of findings ‐ Lumacaftor plus ivacaftor compared with placebo for cystic fibrosis (immediate term).
| Lumacaftor plus ivacaftor compared with placebo for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: lumacaftor (200 mg) plus ivacaftor (150 mg or 250 mg twice daily)1 Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lumacaftor plus ivacaftor1 | |||||
|
Survival Follow‐up: 21 days1 |
No deaths reported. | No deaths reported. | NA | 62 (1 study) |
⊕⊕⊕⊝ moderate2 | |
|
Quality of life: total score Follow‐up: 21 days1 |
Outcome not reported. | NA | A higher score indicates a better outcome. | |||
|
Quality of life: respiratory domain Follow‐up: 21 days1 |
Outcome not reported. | NA | A higher score indicates a better outcome. | |||
|
FEV1 % predicted: relative change from baseline Follow‐up: 21 days1 |
Outcome not reported. | NA | ||||
|
FEV1 % predicted: absolute change from baseline Follow‐up: 21 days1 |
The mean change from baseline was 0.3. | The mean change from baseline was 1.57% predicted higher (‐2.13 lower to 5.27 higher). | NA | 59 (1 study) |
⊕⊕⊕⊝ moderate2 | |
|
Adverse events Follow‐up: 21 days1 |
There were no statistically significant differences between groups in terms of participants experiencing: cough, oropharyngeal pain, nasal congestion, dizziness, a prolonged prothrombin time, and upper respiratory tract infection. | NA | 61 (1 study) |
⊕⊕⊝⊝ low2,3 | ||
|
Time to first pulmonary exacerbation Follow‐up: 21 days1 |
Outcome not reported (see comment). | NA | Time to first pulmonary exacerbation was not reported. There was no statistically significant difference between groups in the number of participants experiencing pulmonary exacerbations. | |||
| *The basis for the assumed risk is the mean placebo group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume at 1 second. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. The design of the study was 14 days of lumacaftor monotherapy (200 mg once daily) then a dose of ivacaftor (150 mg or 250 mg once daily) was added on for 7 days of combination therapy. Results presented in this table are from the combination treatment period only.
2. Downgraded once due to indirectness: design of the study means that combination treatment was measured for only 7 days and prior lumacaftor monotherapy phase (see footnote 1) may have influenced results of the combination phase.
3. Downgraded once due to imprecision: few events occurred therefore CIs for occurrence of specific events are very wide (Analysis 12.2).
Summary of findings 6. Summary of findings ‐ tezacaftor plus ivacaftor compared with placebo or ivacaftor alone.
| Tezacaftor plus ivacaftor compared with placebo or ivacaftor alone for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: tezacaftor (100 mg daily) plus ivacaftor (150 mg twice daily) Comparison: placebo (i.e. tezacaftor placebo) or ivacaftor (150 mg twice daily) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or ivacaftor alone | Tezacaftor plus ivacaftor | |||||
|
Survival Follow‐up: up to 24 weeks |
No deaths reported. | No deaths reported. | NA | 522 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | |
|
Quality of life: total score Follow‐up: NA |
Outcome not reported. | NA | A higher score indicates a better outcome. | |||
|
Quality of life: CFQ‐R respiratory domain: absolute change from baseline Follow‐up: up to 24 weeks |
See comment. | The mean absolute change from baseline in CFQ‐R respiratory domain score in the tezacaftor‐ivacaftor group was 5.10 points higher (3.20 higher to 7.00 higher) than the placebo group (result from 1 study with 510 individuals). | NA | 522 (2 studies) | ⊕⊕⊕⊝ moderate1, 2 | A higher score indicates a better outcome Difference in absolute change from baseline calculated by least‐squares regression, hence assumed risk not presented. The mean absolute change from baseline in CFQ‐R respiratory domain score in the tezacaftor plus ivacaftor group was also statistically significantly higher than the placebo group at 4 weeks: MD 5.10 (95% CI 2.99 to 7.21) The second study (n = 18) showed that the treatment effect of tezacaftor‐ivacaftor versus placebo was 6.81 points of CFQ‐R respiratory domain (P = 0.2451) up to day 28. |
|
FEV1 % predicted: relative change from baseline Follow‐up: up to 24 weeks |
See comment. | The mean relative change from baseline in FEV1 % predicted in the tezacaftor‐ivacaftor group was 6.80% higher (5.30% higher to 8.30% higher) than the placebo group (result from 1 study with 510 individuals). | NA | 522 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | Difference in relative change from baseline calculated by least‐squares regression, hence assumed risk not presented. The second study (n = 18) showed no statistically significant difference between groups in mean relative change from baseline in FEV1 % predicted MD 3.72 (95% CI ‐7.77 to 15.21). |
|
FEV1 % predicted: absolute change from baseline Follow‐up: up to 24 weeks |
See comment | The mean absolute change from baseline in FEV1 % predicted in the tezacaftor plus ivacaftor group was 4.00 % predicted higher (3.10 higher to 4.90 higher) than the placebo group (result from one study with 510 individuals). | NA | 522 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | Difference in absolute change from baseline calculated by least‐squares regression, hence assumed risk not presented. The mean absolute change from baseline in FEV1 % predicted in the tezacaftor‐ivacaftor group was also statistically significantly higher than the placebo group at 4 weeks, MD 3.59 (95% CI 2.40 to 4.78), 2 studies, n = 528, I² =0%. |
|
Adverse events: most commonly occurring events (occurring in at least 10% of participants) Follow‐up: up to 24 weeks |
The most commonly occurring adverse events in both groups were cough and pulmonary exacerbation. There were no statistically significant differences between groups (99% confidence intervals) in the number of participants experiencing cough, pulmonary exacerbation, headache, nasal congestion or nasopharyngitis, increased sputum, haemoptysis, pyrexia, oropharyngeal pain, nausea or fatigue. |
NA | 527 (2 studies) | ⊕⊕⊕⊝ moderate1,2 | ||
|
Time to first pulmonary exacerbation Follow‐up: up to 24 weeks |
The hazard ratio for pulmonary exacerbation in the tezacaftor plus–ivacaftor group, as compared with the placebo group was 0.64 (95% CI 0.46 to 0.89). | NA | 504 (1 study) |
⊕⊕⊕⊝ moderate1,2 | A hazard ratio below 1 favours the tezacaftor‐ivacaftor group. | |
| *The basis for the assumed risk is the control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; NA: not applicable. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded once due to indirectness: 1 study recruited individuals over the age of 12 (Taylor‐Cousar 2017) and 1 study recruited individuals over the age of 18 with one F508del mutation and one G551D mutation (Donaldson 2018). Therefore, results are not applicable to children under the age of 12 and some results are not applicable to individuals homozygous for F508del.
2. One study has some unclear details related to methodological design and had unbalanced treatment group sizes and baseline characteristics (Donaldson 2018). However, this study contributed a small proportion of the evidence of this comparison (n = 18, 3% of evidence) compared to the second study in the comparison (n = 509, 97% of evidence, overall low risk of bias) (Taylor‐Cousar 2017). Therefore, no downgrading is made due to potential risks of bias in the smaller study.
We have presented two tables under the comparison of 'Correctors (monotherapy) compared to placebo;' where lumacaftor monotherapy is compared to placebo (Table 1). We have presented lumacaftor results only rather than other correctors in the table for this comparison due to the relevance of this particular treatment at the time of writing (NICE 2016). A further table is also provided for cavosonstat compared to placebo (Table 2).
We have presented four tables under the comparison of 'Correctors plus potentiators in combination therapy compared to placebo':
lumacaftor (600 mg once daily or 400 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo reporting short‐term results (one month to six months) (Table 3);
lumacaftor (200 mg twice daily) and ivacaftor (250 mg twice daily) versus placebo reporting immediate‐term results (up to one month (Table 4);
lumacaftor (200 mg) plus ivacaftor (150 mg or 250 mg twice daily) versus placebo reporting immediate‐term results (up to one month) (Table 5).
tezacaftor (100 mg once daily) and ivacaftor (150 mg twice daily) versus placebo or ivacaftor (150 mg twice daily alone) (Table 6).
Tables were presented separately for lumacaftor plus ivacaftor under this comparison due to the differences in doses, measurement times and approaches to analysis.
The following outcomes were reported in all tables (chosen based on relevance to clinicians and consumers): survival, QoL (total score), QoL (respiratory domain), FEV1 (relative and absolute change), adverse events and time to first pulmonary exacerbation. For clarity in the tables, adverse events are not presented according to the sub‐domains in Effects of interventions; instead the authors have inserted a general statement about the summary of findings for these outcomes and the evidence is graded based on all of the sub‐domains combined.
We determined the quality of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We evidence by one level if they considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
The search of specified databases identified 98 unique references corresponding to 38 studies. No further studies were identified from contacting CF researchers or from screening relevant references. There were 13 studies (80 references) which met the eligibility criteria for inclusion in this review (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; Donaldson 2018; PROGRESS 2017; McCarty 2002; Ratjen 2017; Rubenstein 1998; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015; Zeitlin 2002). The results from two of these studies were jointly reported in 15 papers (TRAFFIC 2015; TRANSPORT 2015).
We excluded 11 studies (17 references) (Berkers 2014; Chadwick 1998; Lebecque 2011; Leonard 2012; Chilvers 2017; NCT01899105; Nick 2014; Rowe 2017; Rubenstein 2006; Sumner 2014; Ziady 2015).
We identified 13 relevant ongoing studies (Meijer 2016; NCT02070744; NCT02323100; NCT02412111; NCT02589236; NCT02718495; NCT02730208; NCT02951195; NCT03093714; NCT03150719; NCT03224351; NCT03227471; NCT03258424) and one study is listed as awaiting classification pending further information (Hunt 2017)
Results of the online electronic searches are displayed in a PRISMA diagram (Figure 1).
1.
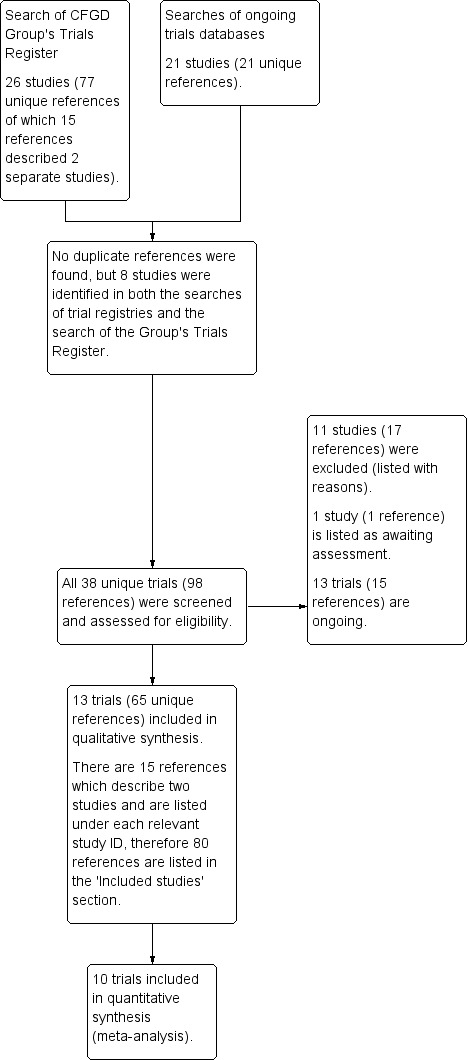
PRISMA study flow diagram
Included studies
Study design
The 13 included studies ranged from Phase 1 to Phase 3 RCTs, and all employed a parallel study design (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; Donaldson 2018; PROGRESS 2017; McCarty 2002; Ratjen 2017; Rubenstein 1998; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015; Zeitlin 2002). The PROGRESS study was an extension study of the TRAFFIC and TRANSPORT studies included in the review (TRAFFIC 2015; TRANSPORT 2015), but with participants in the placebo group from the initial trials randomised to receive the active treatment at one of two doses (PROGRESS 2017).
A total of 2215 randomised participants were included in this review. Study sample sizes ranged from 18 participants (Rubenstein 1998) to 563 participants (TRANSPORT 2015). One study was composed of three cohorts ‐ cohort 1 (n = 62), cohort 2 (n = 109) and cohort 3 (n = 15); any reference to this study is to participants randomised to cohort 1 only, since data for the placebo participants from cohorts 2 and 3 were pooled, undoing the effects of randomisation and rendering them ineligible for inclusion in this review (Boyle 2014). In the Phase 2 study of tezacaftor‐ivacaftor, only data from the heterozygous population are included (n = 18), as the placebo groups in the homozygous arms of the trial were pooled (Donaldson 2018).
The duration of the included studies ranged from 1 day (Phase 1 single‐dose testing) (McCarty 2002) to 24 weeks (Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015) with an extension of two of these studies of 96 weeks (PROGRESS 2017).
Two studies were undertaken at single centres (Rubenstein 1998; Zeitlin 2002), but the remaining studies were conducted at multiple centres, ranging from at four (McCarty 2002) to 191 sites (PROGRESS 2017). Five studies were conducted in the USA only (Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002), four in North America and Europe (Clancy 2012; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017) and the remainder across North America, Europe and Australia (Boyle 2014; PROGRESS 2017; TRAFFIC 2015; TRANSPORT 2015).
Full texts were available for 12 studies (Boyle 2014; Clancy 2012; Donaldson 2017; Donaldson 2018; PROGRESS 2017; McCarty 2002; Ratjen 2017; Rubenstein 1998; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015; Zeitlin 2002) and one as an online summary on Clinicaltrials.gov (Donaldson 2014).
Participants
One study recruited pwCF with one F508del mutation (the other mutation was classified as residual function (ivacaftor responsive)) (Donaldson 2018). All remaining studies recruited participants who were homozygous for F508del.
One study recruited children between the ages of 6 to 11 years (Ratjen 2017), five studies recruited adolescents and adults (PROGRESS 2017; Rubenstein 1998; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015) and the remaining studies recruited only adults (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; Donaldson 2018; McCarty 2002; Zeitlin 2002).
Interventions
Monotherapy
The included studies examined the effects of 4‐Phenylbutyrate (4PBA) (Rubenstein 1998; Zeitlin 2002), 8‐cyclopentyl‐1, 3‐dipropylxanthine (CPX) (McCarty 2002), N6022 (Donaldson 2014), cavosonstat (N91115) (Donaldson 2017), lumacaftor monotherapy (Boyle 2014; Clancy 2012), lumacaftor‐ivacaftor combination therapy (Boyle 2014; PROGRESS 2017; Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015) and tezacaftor‐ivacaftor combination therapy (Donaldson 2018; Taylor‐Cousar 2017).
Two studies compared 4PBA to placebo (Rubenstein 1998; Zeitlin 2002). In the earlier study, participants received either 19 g of 4PBA (split into three daily doses) or placebo for one week (Rubenstein 1998). The subsequent Phase 2 study examined the effects 4PBA given at either 20 g (n = 6), 30 g (n = 6) or 40 g (n = 3), given in three daily doses for one week (Zeitlin 2002).
One study compared escalating doses of CPX to placebo (McCarty 2002). Participants were randomised to receive single doses of either placebo (n = 8) or 1 mg (n = 4), 3 mg (n = 4), 10 mg (n = 4), 30 mg (n = 4), 100 mg (n = 5), 300 mg (n = 4) or 1000 mg (n = 4) of CPX.
One study compared sequential ascending doses of N6022 to placebo (Donaldson 2014). Participants were randomised to receive placebo (n = 19) or the active drug (intravenous solution of N6022 in normal saline) at a dose of either 5 mg (n = 10), 10 mg (n = 9), 20 mg (n = 9), 40 mg (n = 19). Both treatments were administered by infusion pump over one to eight minutes once per day for seven days.
The study of cavosonstat included both healthy volunteers and pwCF (Donaldson 2017). Those with CF were randomised to receive 50 mg placebo (n = 12) or cavosonstat at different doses (50 mg (n = 12), 100 mg (n = 13), or 200 mg (n = 14)) twice daily for 28 days.
One included study compared lumacaftor monotherapy at escalating doses of 25 mg (n = 18), 50 mg (n = 18), 100 mg (n = 17) and 200 mg (n =19), to placebo (n = 17) for 28 days (Clancy 2012).
Combination therapy
Five studies have evaluated lumacaftor‐ivacaftor combination therapy (Boyle 2014; PROGRESS 2017; Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015). In one cohort of a Phase 2 study, participants received 200 mg lumacaftor once daily for 14 days, followed by seven days of 200 mg lumacaftor once daily plus either 150 mg (n = 20) or 250 mg (n = 21) of ivacaftor twice daily (day 15 to 21), or placebo (Boyle 2014). In one Phase 3 study, children received either a combination of lumacaftor 200 mg plus ivacaftor 250 mg every 12 hours or placebo for 24 weeks (Ratjen 2017). Two Phase 3, three‐arm studies (TRAFFIC and TRANSPORT) also compared lumacaftor‐ivacaftor combination therapy to placebo. In these studies, two separate doses of lumacaftor (600 mg once daily and 400 mg twice daily) were combined with twice daily 250 mg of ivacaftor. The placebo group received lumacaftor‐matched placebo every 12 hours in combination with ivacaftor‐matched placebo every 12 hours (TRAFFIC 2015; TRANSPORT 2015). A long‐term extension study (96 weeks) randomised those in the placebo groups of the TRAFFIC and TRANSPORT studies to one of the two lumacaftor‐ivacaftor combination doses; those already receiving an active treatment continued with their existing treatment (PROGRESS 2017).
Two studies have evaluated tezacaftor‐ivacaftor combination therapy (Donaldson 2018; Taylor‐Cousar 2017). A Phase 2 study included a dose‐escalation arm, a comparison of various doses of tezacaftor‐ivacaftor in people homozygous for F508del, and a comparison of tezacaftor‐ivacaftor against ivacaftor alone in people with one F508del mutation and one G551D mutation (Donaldson 2018). The Phase 3 study compared a combination of tezacaftor 100 mg plus ivacaftor 150 mg every 12 hours to a matched placebo for 24 weeks (Taylor‐Cousar 2017).
Outcomes
Lung function using FEV1 was reported in 11 studies (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; Donaldson 2018; PROGRESS 2017; McCarty 2002; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). One study additionally reported LCI (Ratjen 2017). Eight studies reported QoL, all of which utilised the respiratory domain of the CFQ‐R (Clancy 2012; Donaldson 2017; Donaldson 2018; PROGRESS 2017; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
Reporting of the pre‐specified secondary outcomes in this review varied across studies. All included studies monitored the adverse effects of therapy, but the manner in which these safety outcomes were analysed and reported varied considerably. Five studies reported outcomes relating to pulmonary exacerbations (PROGRESS 2017; Rubenstein 1998; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). No studies specifically reported on rates of hospitalisation. Eight studies reported changes in sweat chloride, as a marker of CFTR function (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; Ratjen 2017; Rubenstein 1998; Taylor‐Cousar 2017; Zeitlin 2002). No studies reported radiological outcomes. Two studies reported microbiological outcomes (Taylor‐Cousar 2017; Zeitlin 2002). Five studies reported BMI (PROGRESS 2017; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
Funding sources
Eight studies were funded by pharmaceutical companies (Donaldson 2014; Donaldson 2017; PROGRESS 2017; McCarty 2002; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). Three studies were funded jointly by pharmaceutical companies and other sources (Boyle 2014; Clancy 2012; Donaldson 2018). Two studies were not funded by pharmaceutical companies at all: one was funded by the Cystic Fibrosis Foundation (CFF) (Zeitlin 2002), and one jointly by the CFF and the NIH (Rubenstein 1998).
Further information about the studies is presented in the tables (Characteristics of included studies).
Excluded studies
We excluded 11 studies in total. Six studies were of cross‐over design (Berkers 2014; Lebecque 2011; Leonard 2012; NCT01899105; Nick 2014; Rowe 2017), two studies were single‐assignment studies, i.e. participants were not randomised to different study arms (Chilvers 2017; Rubenstein 2006), one study was not randomised (Chadwick 1998), one study was a pre‐clinical laboratory study (Ziady 2015) and the remaining study was of general gene therapy and not a mutation‐specific therapy (Sumner 2014).
Ongoing studies
There are 13 studies listed as ongoing (Meijer 2016; NCT02070744; NCT02323100; NCT02412111; NCT02589236; NCT02718495; NCT02730208; NCT02951195; NCT03093714; NCT03150719; NCT03224351; NCT03227471; NCT03258424).
Monotherapy
Five ongoing clinical studies are currently evaluating four monotherapy correctors. One study is comparing doses of a corrector known as (R)‐roscovitine to placebo in adults with CF with either one or two copies of the F508del mutation (Meijer 2016). A second study is comparing GPBA to placebo in people who are homozygous for F508del (NCT02323100). A third is comparing a corrector called FDL 169 to placebo in people who are homozygous for F508del (NCT03093714). Two studies are evaluating PTI 428 (a particular type of CFTR corrector called an amplifier, which augment the actions of other CFTR modulators) (NCT02718495; NCT03258424).
Combination therapy
Four ongoing studies are evaluating the safety and efficacy of tezacaftor‐ivacaftor in pwCF; three of these are in people homozygous for F508del (NCT02070744; NCT02730208; NCT03150719), and one in heterozygous people who have one copy of the F508del mutation and one mutation that has been demonstrated to be responsive to ivacaftor therapy (NCT02412111). One of the studies is in people who have previously been taking lumacaftor‐ivacaftor but were not able to continue due to an adverse event or drug reaction (NCT03150719).
Three ongoing placebo‐controlled studies are evaluating triple combination therapies, each adding a drug to a tezacaftor‐ivacaftor combination therapy in pwCF (NCT02951195; NCT03224351; NCT03227471). One study is evaluating VX152 in combination with tezacaftor‐ivacaftor in people homozygous for F508del (NCT02951195). Two studies are evaluating triple therapies, both in people who are homozygous for F508del and people with one F508del mutation and a minimal function mutation that is unlikely to respond to tezacaftor‐ivacaftor, one of these is evaluating VX‐659 (NCT03224351) and the second is evaluating VX‐445 (NCT03227471).
A Phase 2 placebo‐controlled study is assessing the efficacy of cavosonstat when added to pre‐existing lumacaftor‐ivacaftor therapy in adults with CF who are homozygous for the F508del‐CFTR mutation (NCT02589236).
Studies awaiting classification
One study is currently awaiting classification until further information is available (Hunt 2017). In this RCT, 18 adults (11 females) with CF who were homozygous for the F508del mutation, and who were all receiving lumacaftor‐ivacaftor combination therapy were randomised to four weeks of either 40 mg sildenafil three times daily or matched placebo. Outcomes measured include sweat chloride, FEV1 % predicted, BMI, exhaled nitric oxide, CFQ‐R, nasal potential difference and LCI. This study was described in an abstract presented at a conference in 2017 and we will further evaluate for inclusion once the full publication is available.
Risk of bias in included studies
We have summarised our risk of bias judgements in the figures (Figure 2; Figure 3).
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
We judged six studies to have a low risk of bias (Boyle 2014; PROGRESS 2017; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). Of these, one study used a computer‐generated randomisation schedule developed by an independent party (Boyle 2014), and the others randomised participants via an interactive web response system (PROGRESS 2017; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). As none of the remaining seven included studies reported details of random sequence generation we have judged the risk of bias as unclear (Clancy 2012; Donaldson 2014; Donaldson 2017; Donaldson 2018; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
Allocation concealment
We judged six studies to have a low risk of bias (Boyle 2014; PROGRESS 2017; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). In the phase 2 lumacaftor‐ivacaftor study, site pharmacists dispensed drugs on the basis of an interactive voice response system, making it unlikely that participants or study personnel would have been aware of group assignments prior to recruitment into the study (Boyle 2014). The remaining lumacaftor‐ivacaftor studies, and the tezacaftor‐ivacaftor study also employed an interactive web response system to allocate participants to treatment groups (PROGRESS 2017; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). Methods to conceal group allocation were not reported by the remaining seven studies, who also failed to report on random sequence generation, so we judged these as having an unclear risk of bias (Clancy 2012; Donaldson 2014; Donaldson 2017; Donaldson 2018; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
Blinding
Eight studies were judged to have a low risk of performance and detection bias (Boyle 2014; Donaldson 2014; Donaldson 2017; Donaldson 2018; PROGRESS 2017; Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015). In the Boyle study, drug doses were prepared by an independent unmasked pharmacist and dispensed by site pharmacists who were masked to treatment assignment. Site investigators and the study sponsor were also masked to treatment assignment and to sweat chloride levels ‐ data that could have potentially disclosed treatment assignment. Participant blinding was maintained by placebo which was matched to intervention by the quantity of tablets and by size, colour, coating and packaging (Boyle 2014). In the Donaldson study, participants, care givers, investigators and outcome assessors were double‐blinded via intravenous administration of placebo (saline) using the same volume as the active drug groups (Donaldson 2014). In the two Phase 3 lumacaftor‐ivacaftor studies and the extension study, the participants and study team remained blinded to the treatment assignments and the placebo was matched in appearance and packaging to the active intervention. The online protocol further stated that all site personnel, including the investigator, the site monitor and the study team would remain blinded to treatment group (PROGRESS 2017; TRAFFIC 2015; TRANSPORT 2015). In the paediatric lumacaftor‐ivacaftor study, double blinding was achieved by using placebo tablets visually identical to the test product (Ratjen 2017).
In the pilot 4PBA, CPX and the lumacaftor monotherapy studies, there was insufficient information about how participant, study personnel or outcome assessor blinding was maintained and so we judged these three studies to have an unclear risk of performance and detection bias (Clancy 2012; McCarty 2002; Rubenstein 1998).
Participants from the three intervention groups (20 g, 30 g and 40 g) in the Phase 2 4PBA study had different dosing schedules and were given a different number of tablets. Therefore this study was judged to have a high risk of performance bias. Also in this study, there were insufficient data on blinding of outcome assessors and we therefore judged it to have an unclear risk of detection bias (Zeitlin 2002).
Incomplete outcome data
We judged nine studies to have a low risk of bias due to incomplete outcome data (Donaldson 2014; Donaldson 2018; PROGRESS 2017; McCarty 2002; Ratjen 2017; Rubenstein 1998; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
Three studies were judged to have an unclear risk of attrition bias (Boyle 2014; Donaldson 2017; Zeitlin 2002). In the phase 2 lumacaftor‐ivacaftor study, one out of 62 participants withdrew (1.6%) due to an adverse effect, demonstrating a low withdrawal rate. However, in the analysis only participants for whom data were available were included. Although these participants were excluded because of insufficient data rather than for reasons that could potentially lead to the exclusion of participants with unfavourable characteristics, e.g. adverse effects, we judged this study as having an unclear risk of attrition bias because it was unclear how these exclusions would have affected the balance between groups in baseline characteristics (Boyle 2014). The cavosonstat study was judged as having an unclear risk of bias in this domain because two out of 51 participants are unaccounted for in the final analysis, but it is unlikely that these would affect the overall findings (Donaldson 2017). In the phase 2 4PBA study, all 19 randomised participants completed the final study visit, but risk of attrition bias was unclear because there was no report of how many of these participants were included in the analysis (Zeitlin 2002). We approached the primary author to clarify this, but did not receive any additional information.
We judged the study of lumacaftor monotherapy to have a high risk of attrition bias (Clancy 2012). Although only four out of 89 (5%) participants withdrew from the study due to adverse events (demonstrating a low withdrawal rate), data for a number of outcomes were excluded from the analysis. A total of 42 participants were excluded from reports of adverse events; nine participants were excluded from reports on change from baseline in sweat chloride concentration (demonstrated by figure 1b in the full‐text article) and four participants were excluded from the information on CFQ‐R domain scores. Our judgement of a high risk of attrition bias was due firstly to the high level of excluded participant data and secondly to the lack of reasons for the exclusion of these participant data. The study's lead investigator was approached for clarification, but we have received no response to date (Clancy 2012).
Selective reporting
Where study protocols were not available, or there were missing outcome data, we approached the studies' primary authors for additional information.
We judged six studies to have a low risk of reporting bias (Boyle 2014; Donaldson 2017; Donaldson 2018; PROGRESS 2017; McCarty 2002; Rubenstein 1998). For the Phase 2 lumacaftor‐ivacaftor study, the protocol was not available, but outcomes were presented on the NIH trials registry (clinicaltrials.gov/); we did not identify any missing outcomes for the included cohort (Boyle 2014). For the pilot 4PBA and CPX studies, protocols were not available and planned outcomes were not listed on ongoing online trials databases (McCarty 2002; Rubenstein 1998). So, we compared the outcomes reported in the 'Methods' sections to the outcomes reported in the 'Results' sections of the publications and did not identify any missing outcomes (McCarty 2002; Rubenstein 1998). For the extension study of TRAFFIC and TRANSPORT, we compared the list of outcomes provided on the NIH trials registry (clinicaltrials.gov/) to the results reported in the published paper; all listed outcomes were reported (PROGRESS 2017).
One study was judged to be at unclear risk of bias from selective outcome reporting (Donaldson 2014). Only limited results were available from the NIH trials registry (clinicaltrials.gov/) and it was unclear if all relevant information has been made available.
We judged six studies to have a high risk of bias from selective outcome reporting (Clancy 2012; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015; Zeitlin 2002). The protocol for the Clancy lumacaftor study was not available, but the planned outcomes were listed on the NIH trials registry (clinicaltrials.gov/). We compared these outcomes to those reported in the 'Results' section of the published paper and ascertained that no data were reported for FEF25‐75% or FVC at 28 days (Clancy 2012). The study protocol for the phase 2 4PBA study was not available and planned outcomes were not listed on ongoing online trials databases (Zeitlin 2002). We compared the outcomes reported in the 'Methods' section of the paper to the outcomes reported in the 'Results' section and identified that data were not reported for the change from baseline in FEV1 or microbiology scores at day seven (Zeitlin 2002).
In the two Phase 3 lumacaftor‐ivacaftor studies, pre‐specified data were reported on the NIH trials registry (TRAFFIC 2015; TRANSPORT 2015). In these studies, data for the outcomes; absolute change from baseline in FEV1 and relative change from baseline in FEV1 were combined at 16 and 24 weeks. Combination of this data was not pre‐specified and the primary author was contacted from clarification. Furthermore, some results had to be extrapolated from graphical figures and some additional data were only reported on clinicaltrials.gov for outcomes not reported in the final paper. Also, investigators state that they measured FVC (which was not listed as an end‐point) and do not report this in the joint paper (TRAFFIC 2015; TRANSPORT 2015).
In the paediatric combination study, several outcomes which were listed in the methods of the full publication and also on the ClinicalTrials.gov entry for this study were not reported in the results section of the paper (Ratjen 2017). These outcomes include LCI5.0, weight, height and time to first pulmonary exacerbation.
In the tezacaftor‐ivacaftor combination study, a number of outcomes were recorded according to the study protocol but were not reported in the published paper (Taylor‐Cousar 2017). These outcomes were the CF respiratory symptom diary, duration of daily physical activity (number of minutes), the Pittsburgh Sleep Quality Index (PSQI), SF‐12 health survey, sputum microbiology, the time‐to‐first and number of days with an exacerbation, the time to first hospitalisation and the number of days hospitalised with exacerbation, the number of exacerbations requiring IV therapy, the time to the first IV therapy and the number of days on IV therapy.
Other potential sources of bias
We judged there to be a low risk of other bias due to no statistically significant difference between baseline characteristics in six studies (PROGRESS 2017; Rubenstein 1998; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015; Zeitlin 2002) and due to well‐matched baseline characteristics in a further four studies (Boyle 2014; Clancy 2012; Donaldson 2014; Ratjen 2017). Futhermore, in both the TRAFFIC and TRANSPORT studies adherence to treatment was high with similar compliance rates across the different treatment groups (TRAFFIC 2015; TRANSPORT 2015).
In the remaining three studies, there was insufficient detail about baseline characteristics or an apparent imbalance in baseline characteristics, leading to an unclear risk of bias (Donaldson 2017; Donaldson 2018; McCarty 2002).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
As described above, we identified two types of intervention relevant for this review. The first group of studies examined single agents that aimed to correct the F508del trafficking defect (commonly referred to as "correctors"). The second group of studies examined a combination of various correctors with ivacaftor (a drug known to potentiate the function of the CFTR in the membrane). As these interventions have different potential mechanisms of action, we present the results separately for 'Correctors (monotherapy) compared to placebo' and 'Correctors plus potentiators (combination therapy) compared to placebo'. Results are summarised for all doses reported separately and for treatment doses combined where appropriate. In the summary of findings tables, the quality of the evidence has been graded for pre‐defined outcomes (see above) and definitions of these gradings provided.
Correctors (monotherapy) compared to placebo
Seven studies with 317 participants contributed to this comparison (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
Two studies (n = 37) compared 4PBA to placebo (Rubenstein 1998; Zeitlin 2002), one study (n = 66) compared N6022 to placebo (Donaldson 2014), one study (n = 37) compared CPX to placebo (McCarty 2002) and two studies (n = 151) compared varying doses of lumacaftor alone to placebo (Boyle 2014; Clancy 2012). One study (n = 26) compared cavosonstat 200 mg (twice daily) to placebo (Donaldson 2017). We only present this dose comparison (200 mg) from this early‐phase study as this is the only dose that is being studied further and other doses are not relevant to current clinical practice. Participants in one study (n = 64) received lumacaftor monotherapy for 14 days followed by combination therapy with ivacaftor for seven days, therefore this study contributes to both comparisons of this review (Boyle 2014).
Important results for this comparison are summarised in the tables (Table 1; Table 2).
Primary outcomes
1. Survival
No deaths were reported during any of the included studies (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
2. QoL
a. Total QoL score
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
b. Different sub‐domains
i. Immediate term (up to and including one month)
Lumacaftor versus placebo
The study by Clancy (n = 89) reported on the change from baseline scores for all CFQ‐R domains at 28 days (Table 21). We have presented these absolute change from baseline scores as we were unable to calculate the relative change from baseline in CFQ‐R scores since baseline CFQ‐R scores were not reported. Furthermore, no SDs or CIs were reported to allow calculation of SDs for entry into the analysis (Clancy 2012).
2. Change from baseline CFQ‐R domain scores at 28 days (Clancy 2012).
| Lumacaftor | Placebo | ||||
| Domain | 25 mg (n = 17) | 50 mg (n = 17) | 100 mg (n = 16) | 200 mg (n =18) | (n = 17) |
| Body | ‐0.21 | ‐1.63 | 2.61 | 0.06 | ‐1.34 |
| Digestion | 2.28 | ‐0.72 | 0.25 | 2.58 | 4.62 |
| Eating | ‐3.66 | ‐7.27* | 3.24 | ‐2.58 | 2.11 |
| Emotion | ‐3.22 | ‐1.36 | 3.49 | ‐2.62 | 4.86 |
| Health Perceptions | ‐2.84 | ‐6.97* | ‐0.44 | ‐1.9 | 5.03 |
| Physical | ‐5.97 | ‐7.38* | ‐3.46 | ‐0.98 | 1.23 |
| Respiratory | ‐5.22 | ‐6.32* | ‐1.29 | 2.22 | 4.53 |
| Role | ‐5.94* | ‐4.6 | 1.1 | ‐6.53* | 2.21 |
| Social | 0 | ‐1.01 | 0.47 | ‐2.64 | ‐0.55 |
| Treatment Burden | 4.19 | ‐5.96* | 1.42 | ‐0.68 | 2.46 |
| Vitality | ‐4.65 | ‐7.23* | ‐1.52 | 0.73 | ‐2.18 |
| Weight | 5.41 | 2.18 | 8.83 | ‐4.19 | 0.3 |
*statistically significant results versus placebo are highlighted by stars
Participants in the 25 mg group reported statistically significantly lower CFQ‐R scores for the role domain (MD ‐8.15) and respiratory domain (MD ‐9.75) compared participants in the placebo group. Participants in the 50 mg lumacaftor group reported statistically significantly lower CFQ‐R scores for the eating domain (MD ‐9.4), health perceptions domain (MD ‐12.0), respiratory domain (MD ‐10.85) and treatment burden domain (MD ‐8.42) compared to participants assigned to placebo. Participants in the 200 mg group reported statistically significantly lower CFQ‐R scores for the role domain (P < 0.05) compared participants in the placebo group (Clancy 2012).
Cavosonstat versus placebo
Donaldson (n = 51) also reported data for both the respiratory and eating domains of the CFQ‐R at 28 days, but neither result showed any difference between cavosonstat and placebo groups (Analysis 2.1; Analysis 2.2) (Donaldson 2017).
2.1. Analysis.
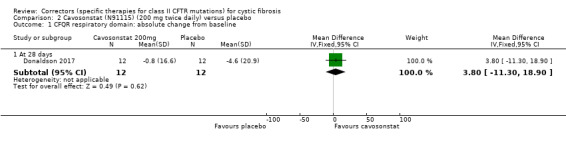
Comparison 2 Cavosonstat (N91115) (200 mg twice daily) versus placebo, Outcome 1 CFQR respiratory domain: absolute change from baseline.
2.2. Analysis.
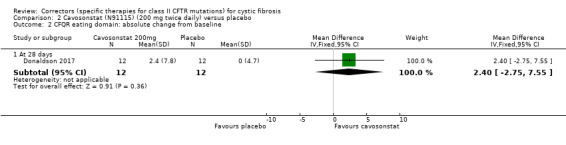
Comparison 2 Cavosonstat (N91115) (200 mg twice daily) versus placebo, Outcome 2 CFQR eating domain: absolute change from baseline.
ii. Short term (over one month and up to and including six months)
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
3. Physiological measures of lung function
a. FEV1 (relative change from baseline)
i. Immediate term (up to and including one month)
Lumacaftor versus placebo
The study by Clancy (n = 89) reported the mean relative change from baseline in FEV1 after 28 days of treatment with escalating doses of lumacaftor, but did not present the corresponding SDs precluding analysis (Clancy 2012). No statistically significant differences were reported between the placebo group and the different lumacaftor dose groups: 25 mg, MD ‐2.53% predicted; 50 mg, MD ‐2.22% predicted; 100 mg, MD 0.25% predicted; and 200 mg, MD 0.40% predicted. No SDs or CIs were reported to allow calculation of SDs for entry into the analysis (Clancy 2012).
Cavosonstat versus placebo
Donaldson (n = 51) presents data for cavosonstat versus placebo pictorially in the graph (supplementary tables), but overlapping SD lines render these data difficult to extract. The paper reports that no treatment‐related changes in FEV1 were seen with cavosonstat compared to placebo (Donaldson 2017).
N6022 versus placebo
The study by Donaldson (n = 66) reported the mean relative change from baseline in FEV1 % predicted after seven days of treatment with sequential ascending doses of N6022 (5 mg, 10 mg, 20 mg or 40 mg per day) (Donaldson 2014). No statistically significant differences were reported between the placebo group and any of the N6022 dose groups (Analysis 3.1).
3.1. Analysis.
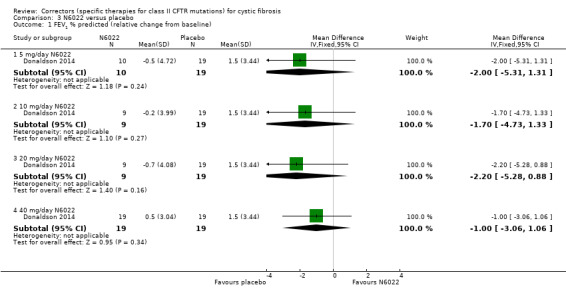
Comparison 3 N6022 versus placebo, Outcome 1 FEV1 % predicted (relative change from baseline).
ii. Short term (over one month and up to and including six months)
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
b. FEV1 (absolute values)
i. Immediate term (up to and including one month)
Lumacaftor versus placebo
The Phase 2 study (n = 62) reported on the absolute change from baseline in FEV1 after lumacaftor monotherapy (day 14) (Boyle 2014); there was no statistically significant difference between treatment groups, MD ‐1.90 (95% CI ‐4.13 to 0.33) (Analysis 1.1) (moderate‐quality evidence).
1.1. Analysis.
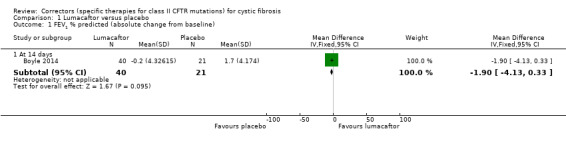
Comparison 1 Lumacaftor versus placebo, Outcome 1 FEV1 % predicted (absolute change from baseline).
Cavosonstat versus placebo
As previously stated, Donaldson (n = 51) reported that no treatment‐related changes in FEV1 were seen with cavosonstat compared to placebo (Donaldson 2017) (low‐quality evidence).
ii. Short term (over one month and up to and including six months)
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
c. FVC
Data for this outcome were not reported by six studies (Boyle 2014; Clancy 2012; Donaldson 2014; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
Cavosonstat versus placebo
Similarly, to FEV1, Donaldson (n = 51) reported that no treatment‐related changes in FVC were seen with cavosonstat compared to placebo (Donaldson 2017).
Secondary outcomes
1. Adverse effects
Adverse effects of therapy were reported by all included studies (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002). The extent and type of adverse event reporting varied between studies.
a. Mild (therapy does not need to be discontinued)
In Phase 2 trials of potential correctors (CPX, 4PBA, N6022, lumacaftor and cavosonstat), there was no evidence of a statistically significant increase in adverse event reporting compared to placebo (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002). However, a large number of events were reported and it is difficult to assess the clinical relevance of these events with the small number of participants in the trials. Further details are given below.
Lumacaftor versus placebo
Adverse events occurring in more than one participant in any lumacaftor dose treatment group in the lumacaftor study by Clancy are presented in the additional tables (Table 22) (Clancy 2012). We have combined the total number of participants with adverse events occurring in the 100 mg and 200 mg lumacaftor groups and compared this to the number of participants experiencing adverse effects in the placebo group (Analysis 1.2). Adverse event data for participants receiving a lower dose (25 or 50 mg of lumacaftor) were not included as there was no evidence of efficacy. The most commonly reported side effect was cough; there was no statistically significant difference in the number of participants who reported cough between the participants assigned to either 100 mg or 200 mg lumacaftor and those assigned to placebo, OR 1.28 (99% CI 0.28 to 5.92) (Analysis 1.2) (Clancy 2012).
3. Frequency of adverse effects occurring in more than one participant in any VX‐809 treatment group (Clancy 2012).
|
Placebo n (%) |
Lumacaftor n (%) |
Total n (%) |
||||
| Adverse effect n (%) | (n = 17) | 25 mg (n = 18) | 50 mg (n = 18) | 100 mg (n = 17) | 200 mg (n = 18) | (n = 45)* |
| Cough | 7 (41.2) | 10 (55.6) | 6 (33.3) | 7 (41.2) | 10 (52.6) | 40 (88.9) |
| Headache | 3 (17.6) | 4 (22.2) | 5 (27.8) | 2 (11.8) | 5 (26.3) | 19 (42.2) |
| Rales | 1 (5.9) | 6 (33.3) | 2 (11.1) | 3 (17.6) | 3 (15.8) | 15 (33.3) |
| Productive cough | 3 (17.6) | 2 (11.1) | 0 (0.0) | 4 (23.5) | 6 (31.6) | 15 (17.8) |
| Dyspnoea | 1 (5.9) | 5 (27.8) | 3 (16.7) | 2 (11.8) | 4 (21.1) | 15 (33.3) |
| Pulmonary exacerbation* | 2 (11.8) | 4 (22.2) | 2 (11.1) | 2 (11.8) | 4 (21.1) | 14 (31.1) |
| Fatigue | 2 (11.8) | 3 (16.7) | 3 (16.7) | 2 (11.8) | 3 (15.8) | 13 (28.9) |
| Fever | 2 (11.8) | 2 (11.1) | 1 (5.6) | 1 (5.9) | 5 (26.3) | 11 (24.4) |
| Nasal congestion | 3 (17.6) | 2 (11.1) | 1 (5.6) | 2 (11.8) | 2 (10.5) | 10 (22.2) |
| Wheezing | 3 (17.6) | 1 (5.6) | 4 (22.2) | 1 (5.9) | 0 (0.0) | 9 (20.0) |
| Diarrhoea | 3 (17.6) | 3 (16.7) | 1 (5.6) | 2 (11.8) | 0 (0.0) | 9 (20) |
| Oropharyngeal pain | 3 (17.6) | 0 (0.0) | 3 (16.7) | 0 (0.0) | 2 (10.5) | 8 (17.8) |
| Upper respiratory tract infection | 1 (5.9) | 2 (11.1) | 1 (5.6 | 3 (17.6) | 0 (0.0) | 7 (15.6) |
| Sinus congestion | 2 (11.8) | 1 (5.6) | 2 (11.1) | 0 (0.0) | 1 (5.3) | 6 (13.3) |
| Respiration abnormal | 0 (0.0) | 1 (5.6) | 1 (5.6) | 0 (0.0) | 4 (21.1) | 6 (13.3) |
| Haemoptysis | 2 (11.8) | 1 (5.6) | 1 (5.6) | 0 (0.0) | 2 (10.5) | 6 (13.3) |
| Constipation | 0 (0.0) | 2 (11.1) | 2 (11.1) | 1 (5.9) | 1 (5.3) | 6 (13.3) |
| Abdominal pain | 1 (5.9) | 3 (16.7) | 1 (5.6) | 0 (0.0) | 1 (5.3) | 6 (13.3) |
| Myalgia | 1 (5.9) | 0 (0.0) | 3 (16.7) | 0 (0.0) | 1 (5.3) | 5 (11.1) |
| Post‐tussive vomiting | 0 (0.0) | 0 (0.0) | 2 (11.1) | 1 (5.9) | 1 (5.3) | 4 (8.9) |
| Nausea | 0 (0.0) | 3 (16.7) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 4 (8.9) |
| Nasopharyngitis | 0 (0.0) | 1 (5.6) | 0 (0.0) | 1 (5.9) | 2 (10.5) | 4 (8.9) |
| Dizziness | 0 (0.0) | 1 (5.6) | 0 (0.0) | 2 (11.8) | 1 (5.3) | 4 (8.9) |
| Back pain | 0 (0.0) | 2 (11.1) | 1 (5.6) | 0 (0.0) | 1 (5.3) | 4 (8.9) |
| Abdominal pain upper | 1 (5.9) | 0 (0.0) | 0 (0.0) | 1 (5.9) | 2 (10.5) | 4 (8.9) |
| Sputum abnormal | 0 (0.0) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 1 (5.3) | 3 (6.7) |
| Epistaxis | 1 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (10.5) | 3 (6.7) |
| C‐reactive protein increased | 0 (0.0) | 1 (5.6) | 0 (0.0) | 2 (11.8) | 0 (0.0) | 3 (6.7) |
| Paranasal sinus hypersecretion | 0 (0.0) | 2 (11.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (4.4) |
| Lung hyperinflation | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (11.8) | 0 (0.0) | 2 (4.4) |
* Unclear why the total number of participants in the study is shown to be 45. The author has been contacted for clarification.
1.2. Analysis.
Comparison 1 Lumacaftor versus placebo, Outcome 2 Adverse effects: 100 mg and 200 mg lumacaftor groups (combined data) versus placebo.
Data for 14 days of lumacaftor monotherapy (200 mg once daily) demonstrated no statistically significant differences between participants treated with lumacaftor therapy and placebo in the number of participants experiencing cough, oropharyngeal pain, nasal congestion, dizziness, a prolonged prothrombin time, and upper respiratory tract infection (Analysis 1.3) (Boyle 2014) (very low‐quality evidence).
1.3. Analysis.
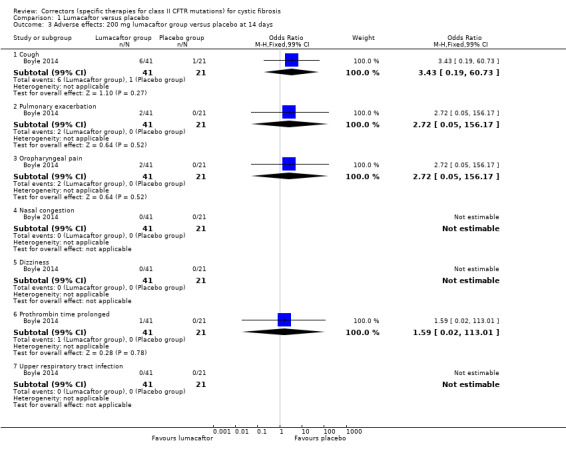
Comparison 1 Lumacaftor versus placebo, Outcome 3 Adverse effects: 200 mg lumacaftor group versus placebo at 14 days.
Cavosonstat versus placebo
In the cavosonstat study, there was no statistically significant difference in cough, pulmonary exacerbation, chest discomfort, fatigue in the treatment group compared to placebo (Analysis 2.3) (Donaldson 2017) (very low‐quality evidence). All adverse events observed in this study were reported to be 'mild or moderate' in severity.
2.3. Analysis.
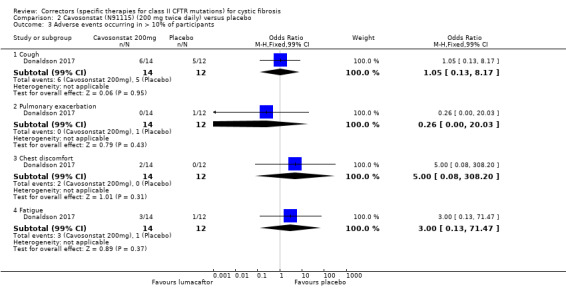
Comparison 2 Cavosonstat (N91115) (200 mg twice daily) versus placebo, Outcome 3 Adverse events occurring in > 10% of participants.
N6022 versus placebo
The number of Grade 1 (mild) adverse events across all N6022 doses and placebo were reported (Donaldson 2014). There was no statistically significant difference between any of the N6022 doses and placebo in terms of the number of mild adverse events, specific events were not reported (Analysis 3.2).
3.2. Analysis.
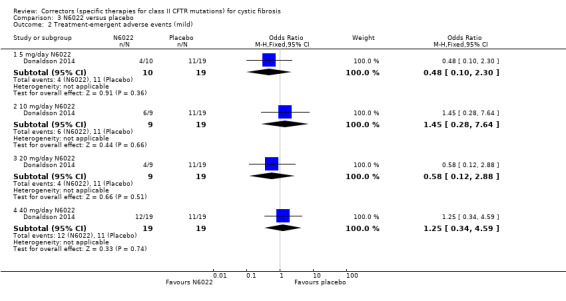
Comparison 3 N6022 versus placebo, Outcome 2 Treatment‐emergent adverse events (mild).
CPX versus placebo
Participants received a single dose of the assigned CPX dose level (1 mg, 3 mg, 10 mg, 30 mg, 100 mg, 300 mg or 1000 mg) (McCarty 2002). Adverse events were recorded on the day of dosing (day one), day two and followed up one week post‐dosing. Adverse effects that occurred in more than 3% of participants are shown in the additional tables (Table 23). Combined data from all CPX groups versus placebo demonstrated that the following events were less common in the placebo group: abdominal pain, OR 0.45 (99% CI 0.01 to 24.92); asthenia, OR 0.65 (99% CI 0.01 to 39.69); headache, OR 0.33 (99% CI 0.01 to 17.72); pain, OR 0.45 (99% CI 0.01 to 24.92); diarrhoea, OR 0.65 (99% CI 0.01 to 39.69); lung disease, OR 0.45 (99% CI 0.01 to 24.92); and rhinitis, OR 0.45 (99% CI 0.01 to 24.92) (Analysis 4.1). Dizziness was more common amongst participants in the placebo group, OR 9.33 (99% CI 0.32 to 268.92) (Analysis 4.1). The difference between CPX groups (combined data) and placebo was not statistically significant for any adverse event (McCarty 2002).
4. Frequency of occurrence of adverse effects occurring in more than 3% of participants in any CPX treatment group in McCarty 2002.
| Placebo | CPX | |||||||
| Adverse effects, n | (n = 8) | 1 mg (n = 4) | 3 mg (n = 4) | 10 mg (n = 4) | 30 mg (n = 4) | 100 mg (n = 5) | 300 mg (n = 4) | 1000 mg (n = 4) |
| Abdominal pain | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 |
| Asthenia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
| Headache | 0 | 0 | 0 | 2 | 1 | 0 | 1 | 0 |
| Pain | 0 | 0 | 0 | 1 | 0 | 0 | 2 | 0 |
| Diarrhoea | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Dizziness | 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Lung Disease | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 |
| Rhinitis | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 |
CPX: 8‐cyclopentyl‐1, 3‐dipropylxanthine
4.1. Analysis.
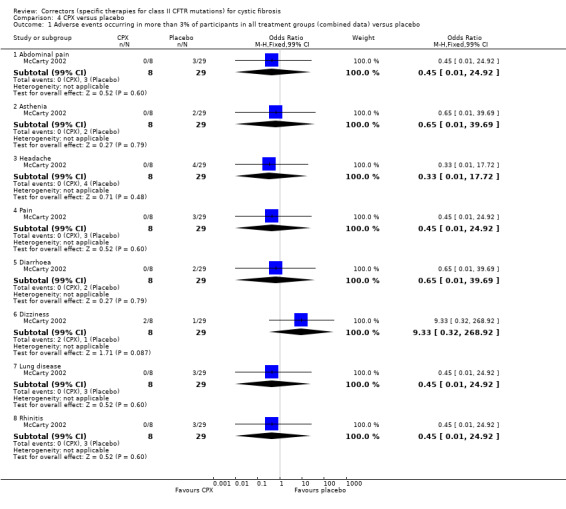
Comparison 4 CPX versus placebo, Outcome 1 Adverse events occurring in more than 3% of participants in all treatment groups (combined data) versus placebo.
4PBA versus placebo
In the pilot 4PBA study (n = 18) (Rubenstein 1998), the differences between groups in the number of participants who reported episodes of bad taste in their mouth and diarrhoea were not statistically significant, OR 0.44 (99% CI 0.01 to 13.44) and OR 3.35 (99% CI 0.04 to 267.31) respectively (Analysis 5.1).
5.1. Analysis.
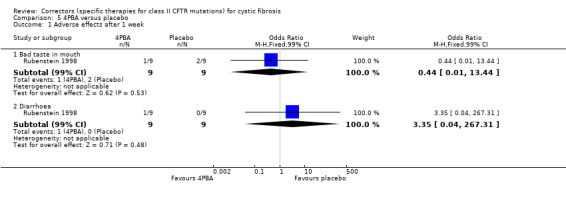
Comparison 5 4PBA versus placebo, Outcome 1 Adverse effects after 1 week.
In the Phase 2 4PBA study (n = 19), participants randomised to the 20 g cohort reported episodes of transient nausea, headache, sleepiness and body odour after the initial dose; the transient nausea, sleepiness and headache resolved with a dose of Tylenol® (acetominophen). No numerical data were reported regarding adverse events, therefore no data can be entered into analysis for this study (Zeitlin 2002).
b. Moderate (therapy is discontinued, and the adverse effect ceases)
None of the participants in the Phase 2 lumacaftor‐ivacaftor study, the pilot 4PBA study or the CPX study the required study drug interruption for the adverse effects of therapy (Boyle 2014; McCarty 2002; Rubenstein 1998).
Lumacaftor versus placebo
There were no statistically significant differences in terms of any lumacaftor dose compared to placebo in the number of adverse events requiring study drug discontinuation up to day 28 (Analysis 1.4) (Clancy 2012).
1.4. Analysis.
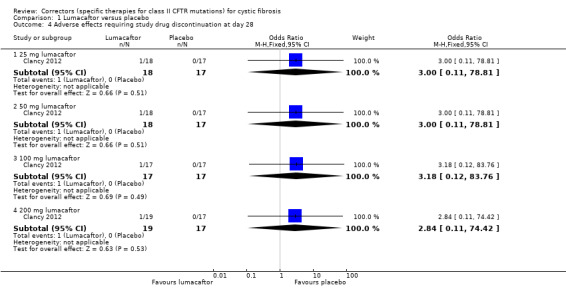
Comparison 1 Lumacaftor versus placebo, Outcome 4 Adverse effects requiring study drug discontinuation at day 28.
Cavosonstat versus placebo
In the cavosonstat study, there was no statistically significant difference in cough, pulmonary exacerbation, chest discomfort, fatigue in the treatment group compared to placebo (Analysis 2.3) (Donaldson 2017) (very low‐quality evidence). All adverse events observed in this study were reported to be 'mild or moderate' in severity.
N6022 versus placebo
The number of Grade 2 (moderate) adverse events across all N6022 doses and placebo were reported (Donaldson 2014). There was no statistically significant differences between any of the N6022 doses and placebo in terms of the number of Grade 2 adverse events; specific events were not reported (Analysis 3.3).
3.3. Analysis.
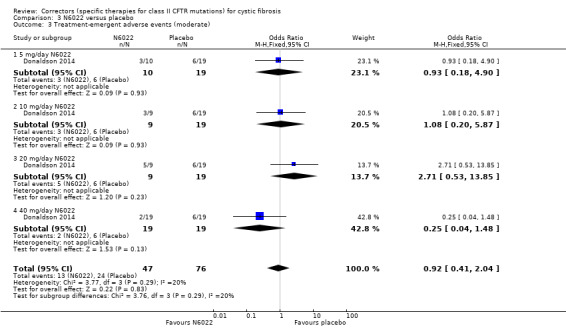
Comparison 3 N6022 versus placebo, Outcome 3 Treatment‐emergent adverse events (moderate).
4PBA versus placebo
In the phase 4 4PBA study, participants who were discontinued from a particular study dose were assigned a reduced dose and this is discussed under severe adverse effects (Zeitlin 2002).
c. Severe (life‐threatening or debilitating, or which persists even after treatment is discontinued)
None of the participants from the CPX study or the cavosonstat study required study drug termination (Rubenstein 1998; Donaldson 2017).
Lumacaftor versus placebo
In the Clancy study, adverse effects in eight participants were considered severe: fatigue (n = 1); sinus congestion (n = 1); musculoskeletal discomfort (n = 1); cough (n = 2); and pulmonary exacerbation (n = 3). It is not stated which arm these participants were randomised to. Four out of 89 participants (5%) ‐ one participant from each of the lumacaftor arms ‐ discontinued the study drug due to respiratory adverse effects. No participants discontinued from the placebo group (Clancy 2012).
N6022 versus placebo
The number of Grade 3 or above (serious or life‐threatening) adverse events across all N6022 doses and placebo were reported (Donaldson 2014). There was no statistically significant differences between any of the N6022 doses and placebo in terms of the number of Grade 3 or above adverse events (Analysis 3.4). The events were as follows: one participant with appendicitis in the 5 mg/day N6022 group and three participants with a pulmonary exacerbation of CF one each in the placebo, 5 mg/day and 40 mg/day N6022 groups.
3.4. Analysis.
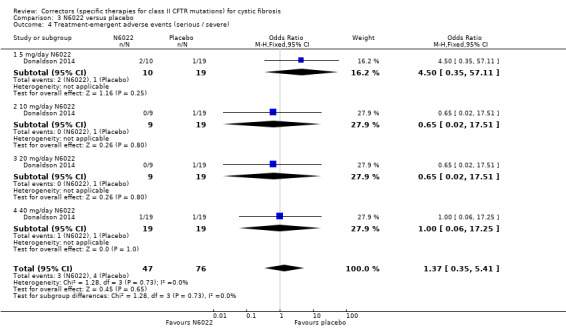
Comparison 3 N6022 versus placebo, Outcome 4 Treatment‐emergent adverse events (serious / severe).
4PBA versus placebo
None of the participants from the pilot 4PBA study required study drug termination (McCarty 2002). In the Phase 2 4PBA study, none of the participants in the 20 g group required study drug termination (Zeitlin 2002). "Several" participants (exact number not stated) in the 30 g group reported episodes of transient nausea, headache, sleepiness and transient visual disturbances after the initial dose. Two participants from the 30 g cohort required dose reduction to 20 g due to headache (n = 1) and for an unknown reason (n = 1). One participant who started in this group had to discontinue medication after developing acute distal intestinal obstruction syndrome on day two, but was replaced by another participant. The three participants assigned 40 g of 4PBA reported episodes of nausea, headache and visual disturbances and one participant reported cramp in hands and fingers. One participant tolerated the dose whilst splitting the 40 g into six daily doses, one participant had a dose reduction to 30 g daily and another participant in this group was discontinued from the study due to intolerable symptoms (nausea, headache and visual disturbances). The 40 g cohort was terminated early following analysis of the data by the safety monitoring committee (Zeitlin 2002).
d. Other adverse effects of therapy (of any severity) that are not classifiable according to these categories
Lumacaftor versus placebo
Two studies also reported on the number of participants who experienced episodes of pulmonary exacerbations described as adverse events (Boyle 2014; Clancy 2012). Results are presented in the analyses and described below (see 'Extra courses of antibiotics') (Analysis 1.2; Analysis 1.3).
N6022 versus placebo
Donaldson reported on "none serious" adverse events on each dose of N6022 and placebo (Donaldson 2014). Due to the small numbers of participants experiencing different adverse events, these results are not entered into analysis and are reported in the additional tables (Table 24).
5. Adverse events (non‐serious) reported in Donaldson 2014 (N6022 versus placebo).
| Placebo | N6022 | Total | ||||
| Adverse events, n | (n = 19) | 5 mg (n = 10) | 10 mg (n = 9) | 20 mg (n = 9) | 40 mg (n = 19) | (n = 66) |
| Lymphadenopathy | 1 | 0 | 0 | 0 | 0 | 1 |
| Chest tightness | 1 | 2 | 0 | 0 | 2 | 5 |
| Atrioventricular block second degree | 0 | 0 | 1 | 0 | 0 | 1 |
| Nodal rhythm | 0 | 0 | 0 | 1 | 0 | 1 |
| Supraventricular extrasystoles | 0 | 0 | 0 | 1 | 0 | 1 |
| Supraventricular tachycardia | 0 | 0 | 1 | 0 | 0 | 1 |
| Ventricular extrasystoles | 1 | 0 | 0 | 0 | 0 | 1 |
| Ventricular tachycardia | 0 | 0 | 0 | 1 | 0 | 1 |
| Diarrhoea | 2 | 0 | 1 | 0 | 0 | 3 |
| Nausea | 1 | 1 | 0 | 0 | 1 | 3 |
| Vomiting | 0 | 0 | 0 | 0 | 2 | 2 |
| Flatulence | 0 | 0 | 1 | 0 | 0 | 1 |
| Parosmia | 0 | 0 | 0 | 2 | 0 | 2 |
| Night sweats | 0 | 0 | 2 | 0 | 0 | 2 |
| Fatigue | 1 | 1 | 0 | 0 | 2 | 4 |
| Pyrexia | 0 | 1 | 0 | 0 | 2 | 3 |
| Infective pulmonary exacerbations of CF | 1 | 1 | 0 | 0 | 1 | 3 |
| Upper respiratory tract infection | 1 | 0 | 0 | 0 | 0 | 1 |
| Headache | 1 | 1 | 1 | 2 | 1 | 6 |
| Cough | 7 | 3 | 1 | 3 | 2 | 16 |
| Increased bronchial secretion | 3 | 2 | 2 | 2 | 1 | 10 |
| Nasal congestion | 1 | 3 | 0 | 0 | 1 | 5 |
| Rales | 0 | 3 | 0 | 1 | 0 | 4 |
| Total participants with at least one adverse event, n (%) |
18 (95%) | 9 (90%) | 9 (100%) | 9 (100%) | 15 (79%) | 60 (91%) |
CF: cystic fibrosis
2. Hospitalisation
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
3. School or work attendance
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017McCarty 2002; Rubenstein 1998; Zeitlin 2002).
4. Extra courses of antibiotics
a. Time‐to the next course of antibiotics
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
b. Total number of courses of antibiotics
Lumacaftor versus placebo
In the lumacaftor study (n = 89), pulmonary exacerbations were physician‐defined and there was no statistically significant difference in the frequency of participants who developed pulmonary exacerbations between those in the lumacaftor groups and the placebo group, OR 1.50 (99% CI 0.16 to 14.31) (Analysis 1.2) (Clancy 2012).
In the Boyle study (n = 62), it was unclear whether the reported exacerbations were protocol‐defined or physician‐defined. At day 14, exacerbations were more common in participants receiving 200 mg lumacaftor once daily in comparison to participants receiving placebo, OR 2.72 (99% CI 0.05 to 156.17) (Analysis 1.3). However, the difference between groups was not statistically significant (Boyle 2014).
5. Sweat chloride (change from baseline) as a measure of CFTR function
All included studies reported on sweat chloride concentration (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
i. Immediate (up to one month)
Lumacaftor versus placebo
In the Clancy study (n = 89), data at seven days demonstrated small reductions in the change from baseline in sweat chloride concentration compared to placebo for the participants taking 25 mg lumacaftor, MD 1.7 mmol/L; 50 mg lumacaftor, MD ‐1.5 mmol/L; 100 mg lumacaftor, MD ‐0.1mmol/L; and 200 mg lumacaftor, MD ‐4.4 mmol/L (Clancy 2012). No SDs or CIs were reported to allow the inclusion of these results into the analysis (Clancy 2012). At 28 days, participants in the 25 mg lumacaftor group demonstrated a marginal increase in sweat chloride concentration compared to placebo, MD 0.1 mmol/L and those in the 50 mg lumacaftor group demonstrated a decreased sweat chloride concentration compared to placebo, MD ‐4.61 mmol/L (Clancy 2012). These differences were not statistically significant and no SDs or CIs were reported for inclusion of these results into the analysis (Clancy 2012). Data at one month demonstrated statistically significant reductions in sweat chloride concentration compared to placebo for participants in the once daily 100 mg lumacaftor group, MD ‐6.13 mmol/L (95% CI ‐12.25 to ‐0.01) and once daily 200 mg lumacaftor group, MD ‐8.21 (95% CI ‐14.30 to ‐2.12) (Analysis 1.5) (Clancy 2012).
1.5. Analysis.
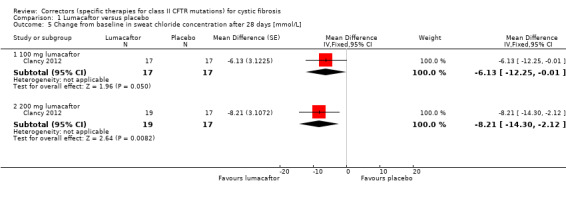
Comparison 1 Lumacaftor versus placebo, Outcome 5 Change from baseline in sweat chloride concentration after 28 days [mmol/L].
Boyle reported that at day 14, there was a small reduction in sweat chloride concentration reported in participants taking 200 mg lumacaftor once daily compared to placebo, MD ‐2.75 mmol/L (95% CI ‐7.65 to 2.15) which was not statistically significant (Analysis 1.6) (Boyle 2014). Results for up to 21 days (monotherapy and combination therapy) are reported above (see 'Correctors plus potentiators in combination therapy compared to placebo').
1.6. Analysis.
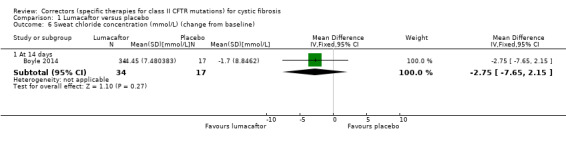
Comparison 1 Lumacaftor versus placebo, Outcome 6 Sweat chloride concentration (mmol/L) (change from baseline).
Cavosonstat versus placebo
There was no statistically significant difference in sweat chloride concentration between cavosonstat and placebo at 28 days (n = 51), MD ‐3.30 mmol/L (95% CI ‐9.13 to 2.53) (Analysis 2.4) (Donaldson 2017).
2.4. Analysis.
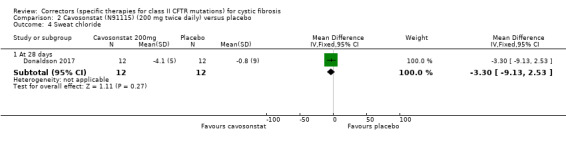
Comparison 2 Cavosonstat (N91115) (200 mg twice daily) versus placebo, Outcome 4 Sweat chloride.
CPX versus placebo
In the CPX study (n = 37), McCarty reported post‐treatment sweat chloride concentration values at the end of treatment on day one (McCarty 2002). The baseline sweat chloride values in the CPX group and the placebo group appear to have been pooled. By calculating the values for relative change from baseline, we have assumed that the baseline sweat chloride value represents the baseline sweat chloride concentration value for each arm. At the end of treatment on day one, there were no statistically significant differences in sweat chloride concentration between the placebo group and the 1 mg CPX group, MD 12.8%; the 3 mg CPX group, MD 7.5%; the 10 CPX mg group, MD 11.3%; the 30 CPX mg group, MD 5.4%; the 100 CPX mg group, MD 5.1%; the 300 CPX mg group, MD 14.7%; and the 1000 CPX mg group, MD ‐8.2%. No SDs or CIs were reported to allow calculation of SDs for entry into the analysis (McCarty 2002).
4BPA versus placebo
In the pilot 4PBA study by Rubenstein (n = 18), there was no statistically significant difference in sweat chloride concentration at one week between participants in the 4PBA group and the placebo group (P = 0.387). Data were plotted on a graph and could not be extracted with accuracy (Rubenstein 1998).
The Phase 2 4PBA study by Zeitlin reported post‐treatment sweat chloride concentration values at day two, day three, day four and day seven; we calculated the relative change from baseline values at each time‐point. There was no statistically significant difference in sweat chloride concentration between the 20 g 4PBA group and the placebo group after two days of treatment, MD ‐7.8%; three days, MD ‐4.9%; four days, MD ‐3.3% and seven days, MD ‐8.7%. Furthermore, there was no statistically significant difference in sweat chloride concentration between the 30 g 4PBA group and the placebo group after two days of treatment, MD ‐25.9%; three days, MD 0.5%; four days, MD ‐6.4% and seven days, MD ‐3.9% (Zeitlin 2002). No SDs or CIs were reported to allow calculation of SDs for entry into the analysis. Due to insufficient reporting of data by both 4PBA studies, we were unable to include the results in the analysis (Rubenstein 1998; Zeitlin 2002).
6. Radiological measures of lung disease
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
7. Acquisition of respiratory pathogens
Data for the acquisition of S aureus, H influenzae or any other clinically relevant pathogens except P aeruginosa, were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
a. P aeruginosa
This was a pre‐defined outcome of interest in the Phase 1/2 4PBA study, but no study results were reported (Zeitlin 2002). Data for this outcome were not reported the other studies (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998).
8. Eradication of respiratory pathogens
Data for this outcome were not reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
9. Nutrition and growth
No data for this outcome, either in terms of weight, BMI or height, were reported by any study (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002).
Correctors plus potentiators (combination therapy) compared to placebo
Six studies with 1898 participants contributed to the efficacy results in this comparison (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). A further study contributed additional safety data to this comparison (see below) (PROGRESS 2017).
Four studies with 1376 participants compared lumacaftor plus ivacaftor to placebo (Boyle 2014; Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015) and two studies with 522 participants compared tezacaftor plus ivacaftor to placebo or ivacaftor alone (i.e. tezacaftor placebo) (Donaldson 2018; Taylor‐Cousar 2017).
Two three‐arm studies (n = 1108) compared 600 mg once daily lumacaftor plus 250 mg twice daily ivacaftor to 400 mg twice daily lumacaftor plus 250 mg twice daily ivacaftor and to placebo (TRAFFIC 2015; TRANSPORT 2015). One study (n = 64) compared lumacaftor 200 mg once daily plus 150 mg or 250 mg twice daily ivacaftor to placebo (Boyle 2014). Participants in this study received lumacaftor monotherapy for 14 days followed by combination therapy with ivacaftor for seven days, therefore this study contributes to both comparisons of this review (Boyle 2014).
The paediatric combination study (n = 204) compared 200 mg lumacaftor twice daily plus 250mg ivacaftor twice daily to placebo for 24 weeks (Ratjen 2017). Primary and secondary outcomes of this study were analysed via a mixed model for repeated measures (MMRM), further details of this analysis approach are provided in the tables (Characteristics of included studies). Results provided by this model can be interpreted as treatment effect averaged from each study visit until week 24.
The PROGRESS study was an extension to the TRAFFIC and TRANSPORT studies (TRAFFIC 2015; TRANSPORT 2015), in which participants from the original placebo groups were randomised to one of the two interventions (PROGRESS 2017). Due to the overlap of participants in these three studies, we have not included efficacy data for the PROGRESS study under a comparison of lumacaftor (plus ivacaftor) doses. We have included safety data from this study as these are important longer‐term results for participants on this intervention; results for the PROGRESS study are presented in the tables for information (PROGRESS 2017; Table 25; Table 26).
6. Adverse events with an incidence of ≥ 0.20 events per patient‐year in Konstan 2017.
| Event | Lumacaftor 400 mg twice daily/ivacaftor 250 twice daily (n = 340) | Placebo transitioned to lumacaftor 400 mg twice daily/ivacaftor 250 mg twice daily (n = 176) | Lumacaftor 600 mg once daily/ivacaftor 250 mg twice daily (n = 335) | Placebo transitioned to lumacaftor 600 mg once daily/ ivacaftor 250 mg twice daily (n = 178) |
| Total exposure in patient‐years | 570 | 290 | 570 | 300 |
| Infective pulmonary exacerbation | 0.980 | 1.035 | 1.157 | 1.080 |
| Cough | 0.510 | 0.573 | 0.627 | 0.609 |
| Haemoptysis | 0.266 | 0.200 | 0.235 | 0.239 |
| Increased sputum | 0.208 | 0.207 | 0.224 | 0.175 |
| Nasopharyngitis | 0.194 | 0.169 | Not reported | Not reported |
| Headache | 0.140 | 0.107 | 0.129 | 0.101 |
| Dyspnoea | 0.124 | 0.166 | 0.117 | 0.128 |
| Pyrexia | 0.114 | 0.152 | 0.148 | 0.148 |
| Upper respiratory tract infection | 0.129 | 0.131 | Not reported | Not reported |
| Diarrhoea | 0.093 | 0.145 | 0.111 | 0.101 |
| Abnormal respiration | 0.077 | 0.128 | 0.088 | 0.145 |
| Nausea | 0.072 | 0.104 | Not reported | Not reported |
| Fatigue | 0.084 | 0.090 | Not reported | Not reported |
| Abdominal pain | 0.087 | 0.066 | 0.087 | 0.084 |
| Oropharyngeal pain | Not reported | Not reported | 0.101 | 0.081 |
| Nasal congestion | Not reported | Not reported | 0.104 | 0.091 |
| Rhinitis | Not reported | Not reported | 0.064 | 0.030 |
| Any adverse event: n (%) | 333 (97.9) | 176 (100) | 331 (98.8) | 177 (99.4) |
| Any serious adverse event: n (%) | 143 (42.1) | 89 (50.6) | 156 (46.6) | 77 (43.3) |
| Any treatment emergent respiratory event: n (%) | 99 (29) | 67 (38) | 102 (30) | 67 (38) |
7. Secondary efficacy outcomes reported in Konstan 2017.
| Outcome | Lumacaftor 400 mg twice daily/ivacaftor 250 mg twice daily (n = 340) | Placebo transitioned to lumacaftor 400 mg twice daily/ivacaftor 250 mg twice daily (n = 176) | Lumacaftor 600 mg once daily/ivacaftor 250 mg twice daily (n = 335) | Placebo transitioned to lumacaftor 600 mg once daily/ivacaftor 250 mg twice daily (n = 178) |
| FEV1 (% predicted):1 Week 72 |
0.5 (95% CI –0.4 to 1.5) P = 0.2806 |
1.5 (95% CI 0.2, 2.9) P = 0.0254 |
1.2 (95% CI 0.3 to 2.2) P = 0.0127 |
1.9 (95% CI 0.6 to 3.2) P = 0.0037 |
| FEV1 (% predicted):1 Week 96 |
0.5 (95% CI –0.7 to 1.6) P = 0.4231 |
0.8 (95% CI –0.8, 2.3) P = 0.3495 |
0.0 (95% CI ‐1.1 to 1.1) P = 0.9682 |
1.6 (95% CI ‐0.1 to 3.2) P = 0.0632 |
| FEV1 (% predicted):2 Week 72 |
0.9 (95% CI 0.0 to 1.9) P = 0.0500 |
1.9 (95% CI 0.6 to 3.2) P = 0.0040 |
1.7 (95% CI 0.8 to 2.7) P = 0.0003 |
2.2 (95% CI 1.0 to 3.5) P = 0.0005 |
| FEV1 (% predicted):2 Week 96 |
1.1 (95% CI 0.0 to 2.2) P = 0.0535 |
1.1 (95% CI –0.5 to 2.6) P = 0.1696 |
0.7 (95% CI ‐0.4 to 1.8) P = 0.1966 |
2.0 (95% CI 0.4 to 3.6) P = 0.0149 |
| FEV1 (% predicted):1 Relative change Week 72 |
1.4 (95% CI –0.3 to 3.2) P = 0.1074 |
2.6 (95% CI 0.2 to 5.0) P = 0.0332 |
2.4 (95% CI 0.6 to 4.1) P = 0.0080 |
3.8 (95% CI 1.4 to 6.1) P = 0.0017 |
| FEV1 (% predicted):1 Relative change Week 96 |
1.2 (95% CI –0·8 to 3·3) P = 0·2372 |
1·1 (95% CI –1·7 to 3·9) P = 0·4415 |
0.1 (95% CI ‐1.9 to 2.1) P = 0.9297 |
3.6 (95% CI 0.6 to 6.6) P = 0.0172 |
| BMI Week 72 |
0.69 (95% CI 0.56 to 0.81) P < 0.0001 |
0.62 (95% CI 0.45 to 0.79) P < 0.0001 |
0.72 (95% CI 0.60 to 0.84) P < 0.0001 |
0.52 (95% CI 0.36 to 0.69) P < 0.0001 |
| BMI Week 96 |
0.96 (95% CI 0.81 to 1.11) P < 0.0001 |
0.76 (95% CI 0.56 to 0.97) P < 0.0001 |
0.81 (95% CI 0.66 to 0.95) P < 0.0001 |
0.55 (95% CI 0.34 to 0.76) P < 0.0001 |
| CFQ‐R respiratory domain Week 72 |
5.7 (95% CI 3.8 to 7.5) P < 0.0001 |
3.3 (95% CI 0.7 to 5.9) P = 0.0124 |
3.2 (95% CI 1.4 to 5.1) P = 0.0007 |
3.3 (95% CI 0.7 to 5.8) P = 0.0116 |
| CFQ‐R respiratory domain Week 96 |
3.5 (95% CI 1.3 to 5.8) P = 0·0018 |
0.5 (95% CI –2.7 to 3.6) P = 0·7665 |
1.1 (95% CI ‐1.1 to 3.2) P = 0.3339 |
2.0 (95% CI ‐1.1 to 5.1) P = 0.2033 |
| Pulmonary exacerbations: events per patient year |
0.65 (95% CI 0.56 to 0.75) | 0.69 (95% CI 0·56 to 0.85) | 0.80 (95% CI 0.70 to 0.92) | 0.76 (95% CI 0.62 to 0.93) |
| Pulmonary exacerbations: events requiring hospital admission per patient year |
0.24 (95% CI 0.19 to 0.29) | 0.30 (95% CI 0.22 to 0.40) | 0.31 (95% CI 0.25 to 0.38) | 0.35 (95% CI 0.26 to 0.47) |
| Pulmonary exacerbations: events requiring IV antibiotics per patient year |
0.32 (95% CI 0.26 to 0.38) | 0.37 (95% CI 0.29 to 0.49) | 0.38 (95% CI 0.32 to 0.46) | 0.42 (95% CI 0.33 to 0.54) |
BMI: body mass index CFQ‐R: cystic fibrosis questionnaire‐revised CI: confidence interval FEV1: forced expiratory volume at one second IV: intravenous
Unless otherwise stated, all outcomes reported are the mean (95% CI) absolute change from baseline. P values correspond to the within‐group change compared to baseline.
1. Calculated using Wang‐Hankinson equations. 2. Calculated using Global Lungs Initiative equations.
One study (n = 510) compared a combination of tezacaftor 100 mg plus ivacaftor 150 mg every 12 hours to a matched placebo for 24 weeks (Taylor‐Cousar 2017) and one study compared tezacaftor (100 mg per day) plus ivacaftor (150 mg twice daily) against placebo (150 mg twice daily ivacaftor alone) in people with one F508del mutation and one G551D mutation (Donaldson 2018).
Important results for this comparison are summarised in the tables (Table 3; Table 4; Table 5; Table 6).
Primary outcomes
1. Survival
No deaths were reported during any of the included studies (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015) (high to moderate‐quality evidence).
2. QoL
Five studies reported on QoL (Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
a. Total QoL score
Lumacaftor plus ivacaftor versus placebo
Two studies reported QoL according to the Euro Quality of Life Scale (EuroQol) 5‐Dimension‐3 Level (EQ‐5D‐3L) Index Score at six months (TRAFFIC 2015; TRANSPORT 2015). This information was not reported in the primary journal article, but is available from the study record on ClinicalTrials.gov (TRAFFIC 2015; TRANSPORT 2015).
There was no statistically significant improvement in the absolute change from baseline of EQ‐5D‐3L index score between the lumacaftor 600 mg once daily plus 250 mg ivacaftor twice daily group and placebo group, MD 0.00 (95% CI ‐0.01 to 0.02) (Analysis 6.1), or the lumacaftor 400 mg twice daily plus 250 mg ivacaftor twice daily group and placebo group, MD 0.00 (95% CI ‐0.01 to 0.02) (Analysis 7.1), or when the two lumacaftor doses were pooled at six months, MD 0.00 (95% CI ‐0.01 to 0.01) (Analysis 8.1) (high‐quality evidence).
6.1. Analysis.
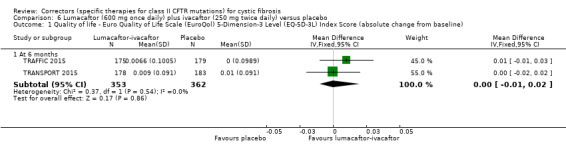
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 1 Quality of life ‐ Euro Quality of Life Scale (EuroQol) 5‐Dimension‐3 Level (EQ‐5D‐3L) Index Score (absolute change from baseline).
7.1. Analysis.
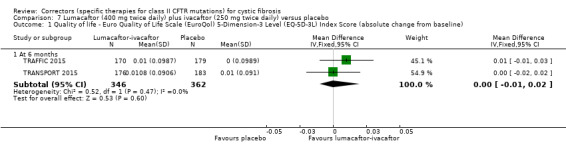
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 1 Quality of life ‐ Euro Quality of Life Scale (EuroQol) 5‐Dimension‐3 Level (EQ‐5D‐3L) Index Score (absolute change from baseline).
8.1. Analysis.
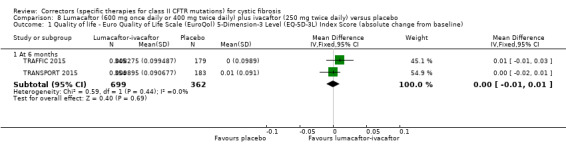
Comparison 8 Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 1 Quality of life ‐ Euro Quality of Life Scale (EuroQol) 5‐Dimension‐3 Level (EQ‐5D‐3L) Index Score (absolute change from baseline).
b. QoL sub‐domains
i. Immediate term (up to and including one month)
Lumacaftor plus ivacaftor versus placebo
In the TRAFFIC and TRANSPORT studies (n = 1108), at 28 days participants in the both the lumacaftor 600 mg once daily plus 250 mg ivacaftor twice daily group and the lumacaftor 400 mg twice daily plus 250 mg ivacaftor twice daily group experienced statistically significantly higher absolute changes from baseline in the CFQ‐R respiratory domain compared to the placebo group, MD 3.32 (95% CI 1.13 to 5.51) (Analysis 6.2) and MD 4.13 (95% CI 1.94 to 6.31) (Analysis 7.2), respectively (TRAFFIC 2015; TRANSPORT 2015). There was also a statistically significantly higher absolute change from baseline in the CFQ‐R respiratory domain compared to the placebo group when the two lumacaftor doses were pooled, MD 3.70 (95% CI 1.81 to 5.58) (Analysis 8.2).
6.2. Analysis.
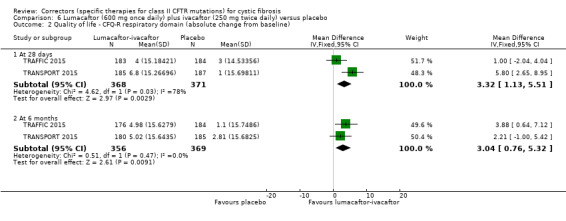
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 2 Quality of life ‐ CFQ‐R respiratory domain (absolute change from baseline).
7.2. Analysis.
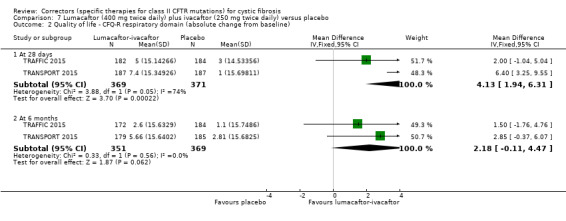
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 2 Quality of life ‐ CFQ‐R respiratory domain (absolute change from baseline).
8.2. Analysis.
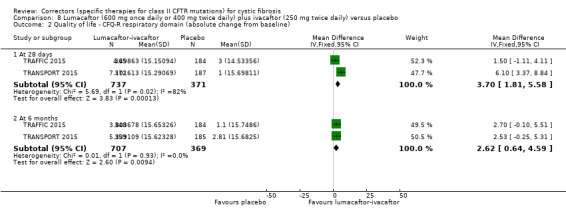
Comparison 8 Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 2 Quality of life ‐ CFQ‐R respiratory domain (absolute change from baseline).
Tezacaftor plus ivacaftor versus control
Taylor‐Cousar (n = 510) reported on the CFQ‐R respiratory domain at four weeks (Taylor‐Cousar 2017) and found a statistically significant difference in favour of the treatment group, MD 5.10 (95% CI 2.99 to 7.21) (Analysis 13.1).
13.1. Analysis.
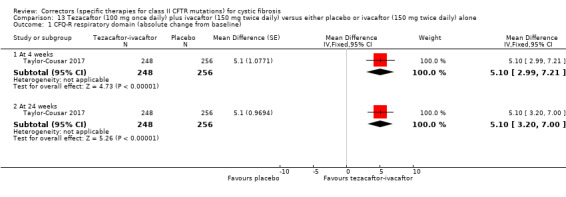
Comparison 13 Tezacaftor (100 mg once daily) plus ivacaftor (150 mg twice daily) versus either placebo or ivacaftor (150 mg twice daily) alone, Outcome 1 CFQ‐R respiratory domain (absolute change from baseline).
Donaldson (n= 18) presents the within‐group change from baseline to Day 28 for the CFQ‐R respiratory domain and at the end of the study the difference in treatment effect between tezacaftor‐ivacaftor and placebo was 6.81 points (P = 0.2451) (Donaldson 2018). These data were extrapolated and we have requested confirmation of the exact data from the study investigators. Any unpublished information we receive will be included in a future update.
ii. Short term (over one month and up to and including six months)
Lumacaftor plus ivacaftor versus placebo
In the two studies (n = 1108) (TRAFFIC 2015; TRANSPORT 2015), the statistically significant difference in the absolute change from baseline in the CFQ‐R respiratory domain was maintained to six months in the 600 mg lumacaftor group compared to placebo, MD 3.04 (95% CI 0.76 to 5.32) (Analysis 6.2), but not in the 400 mg lumacaftor group compared to placebo, MD 2.18 (95% CI ‐0.11 to 4.47) (Analysis 7.2). There was a statistically significantly higher absolute change from baseline in the CFQ‐R respiratory domain compared to the placebo group when the two lumacaftor doses were pooled, MD 2.62 (95% CI 0.64 to 4.59) (Analysis 8.2) (moderate‐quality evidence). These data were extrapolated and we have requested confirmation of the exact data from the study investigators. Any unpublished information we receive will be included in a future update.
The EQ‐5D‐3L Visual Analog Scale (VAS) domain score was also reported at six months in two studies (TRAFFIC 2015; TRANSPORT 2015). Participants in the both the lumacaftor 600 mg group and the lumacaftor 400 mg group experienced statistically significantly higher absolute changes from baseline in the EQ‐5D‐3L VAS domain compared to the placebo group, MD 2.24 (95% CI 0.18 to 4.31) (Analysis 6.3) and MD 2.30 (95% CI 0.25 to 4.36) (Analysis 7.3) respectively. There was also a statistically significantly higher absolute change from baseline in the EQ‐5D‐3L VAS domain compared to the placebo group when the two lumacaftor doses were pooled, MD 2.28 (95% CI 0.50 to 4.06) (Analysis 8.3). This information was not reported in the primary journal article, but is available from the study record on ClinicalTrials.gov (TRAFFIC 2015; TRANSPORT 2015). Immediate‐term data for this domain have been requested from the study investigators and any unpublished information we receive will be included in a future update.
6.3. Analysis.
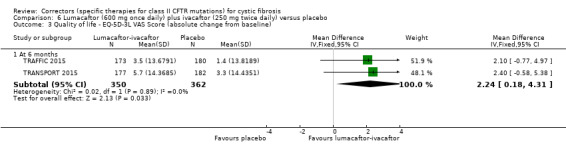
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 3 Quality of life ‐ EQ‐5D‐3L VAS Score (absolute change from baseline).
7.3. Analysis.
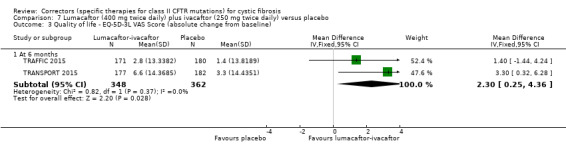
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 3 Quality of life ‐ EQ‐5D‐3L VAS Score (absolute change from baseline).
8.3. Analysis.
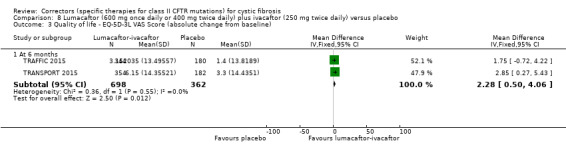
Comparison 8 Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 3 Quality of life ‐ EQ‐5D‐3L VAS Score (absolute change from baseline).
The paediatric combination study also reported the absolute change from baseline (up to and including 24 weeks) of the CFQ‐R respiratory domain (Ratjen 2017). The change within the lumacaftor plus ivacaftor group was higher compared to the placebo group, but this difference did not reach statistical significance, MD 2.50 (95% CI ‐0.10 to 5.10) (Analysis 11.1) (low‐quality evidence). Additional results at earlier time points (day 15, week 4, and week 16) were published graphically in the full study publication, but the graphical plots were too small to allow for accurate extraction of data (Ratjen 2017). Numerical data for these time points have been requested from the study investigators and any unpublished information we receive will be included in a future update.
11.1. Analysis.
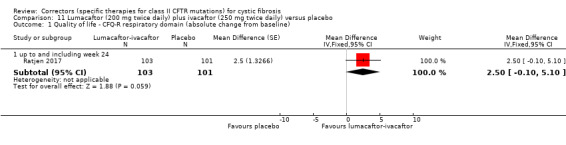
Comparison 11 Lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 1 Quality of life ‐ CFQ‐R respiratory domain (absolute change from baseline).
Investigators in this study also list the absolute change in Treatment Satisfaction Questionnaire for Medication (TSQM) domains as a secondary outcome of the study, but results for this outcome are not presented (Ratjen 2017). Numerical data for this outcome have also been requested from the study investigators and any unpublished information we receive will be included in a future update.
Tezacaftor plus ivacaftor versus control
Taylor‐Cousar (n = 510) also reported on the CFQ‐R respiratory domain at 24 weeks (Taylor‐Cousar 2017); there was a statistically significant difference in favour of tezacaftor‐ivacaftor, MD 5.10 (95% CI 3.20 to 7.00) (Analysis 13.1) (moderate‐quality evidence).
3. Physiological measures of lung function
a. FEV1 (relative change from baseline)
i. Immediate term (up to and including one month)
Lumacaftor plus ivacaftor versus placebo
Immediate‐term data for this domain have been requested from the investigators of two studies (TRAFFIC 2015; TRANSPORT 2015). Any unpublished information we receive will be included in a future update.
Tezacaftor plus ivacaftor versus control
There was no statistically significant difference between tezacaftor plus ivacaftor compared to ivacaftor alone at four weeks (n = 504), MD 3.72 (95% CI ‐7.77 to 15.21) (Analysis 13.2) (Donaldson 2018).
13.2. Analysis.
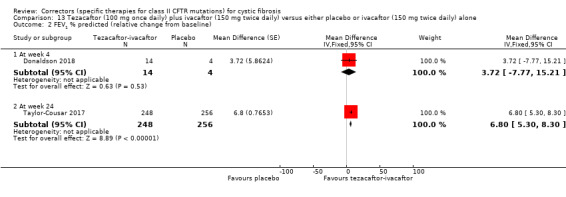
Comparison 13 Tezacaftor (100 mg once daily) plus ivacaftor (150 mg twice daily) versus either placebo or ivacaftor (150 mg twice daily) alone, Outcome 2 FEV1 % predicted (relative change from baseline).
ii. Short term (over one month and up to and including six months)
Lumacaftor plus ivacaftor versus placebo
At six months, participants in the TRAFFIC and TRANSPORT studies (n = 1108) in the both the lumacaftor 600 mg group and the lumacaftor 400 mg group experienced statistically significantly higher relative changes from baseline in FEV1 (% predicted) compared to the placebo group, MD 5.63 (95% CI 3.80 to 7.47) (Analysis 6.4) and MD 4.77 (95% CI 2.93 to 6.61) (Analysis 7.4) respectively (TRAFFIC 2015; TRANSPORT 2015). There was also a statistically significantly higher relative change from baseline in FEV1 (% predicted) compared to the placebo group when the two lumacaftor doses were pooled, MD 5.21 (95% CI 3.61 to 6.80) (Analysis 8.4) (high‐quality evidence).
6.4. Analysis.
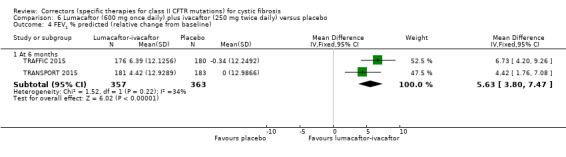
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 4 FEV1 % predicted (relative change from baseline).
7.4. Analysis.
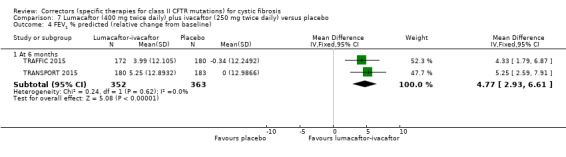
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 4 FEV1 % predicted (relative change from baseline).
8.4. Analysis.
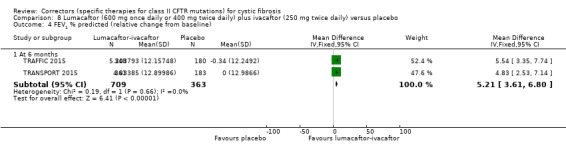
Comparison 8 Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 4 FEV1 % predicted (relative change from baseline).
Tezacaftor plus ivacaftor versus control
At 24 weeks, a statistically significantly higher relative change from baseline in FEV1 (% predicted) compared to the placebo group in the tezacaftor‐ivacaftor study (n = 510), MD 6.80 (95% CI 5.30 to 8.30) (Analysis 13.2) (Taylor‐Cousar 2017) (moderate‐quality evidence).
b. FEV1 absolute values
i. Immediate term (up to and including one month)
Lumacaftor plus ivacaftor versus placebo
In two studies (n = 1108), participants in the both the lumacaftor 600 mg group and the lumacaftor 400 mg group experienced statistically significantly higher absolute changes from baseline in FEV1 (% predicted) at 28 days compared to the placebo group, MD 2.32 (95% CI 1.34 to 3.31) (Analysis 6.5) and MD 2.42 (95% CI 1.43 to 3.40) (Analysis 7.5), respectively (TRAFFIC 2015; TRANSPORT 2015). There was also a statistically significantly higher absolute change from baseline in FEV1 compared to the placebo group when the two lumacaftor doses were pooled, MD 2.37 (95% CI 1.52 to 3.22) (Analysis 8.5). These data were extrapolated and we have requested confirmation of the exact data from the study investigators. Any unpublished information we receive will be included in a future update.
6.5. Analysis.
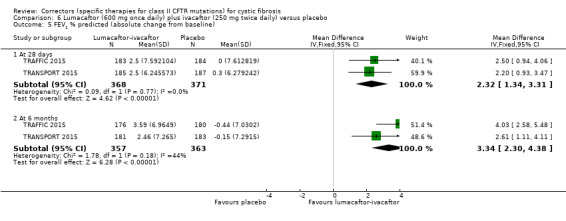
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 5 FEV1 % predicted (absolute change from baseline).
7.5. Analysis.
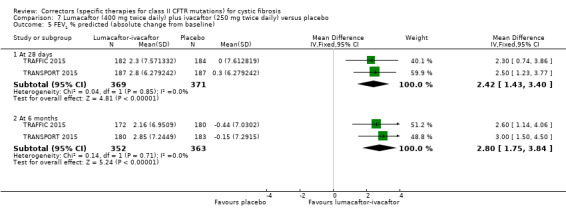
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 5 FEV1 % predicted (absolute change from baseline).
8.5. Analysis.
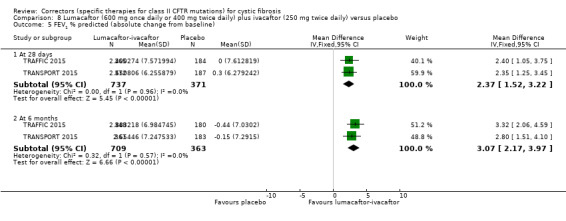
Comparison 8 Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 5 FEV1 % predicted (absolute change from baseline).
The Phase 2 lumacaftor‐ivacaftor study (n = 62) reported on the absolute change from baseline in FEV1 after lumacaftor monotherapy (day 14) and lumacaftor (200 mg daily) and ivacaftor (150 mg or 250 mg twice daily) combination therapy (day 21) (Boyle 2014). Results for lumacaftor monotherapy are discussed above (see 'Correctors (monotherapy) compared to placebo').
Small, but non‐statistically significant improvements in FEV1 were reported at day 21 for participants treated with 200 mg lumacaftor once daily (day 1 to 21) and either 150 mg ivacaftor twice daily (day 15 to 21), MD 2.80 (95% CI ‐1.39 to 6.99) (Analysis 9.1) or 250 mg ivacaftor twice daily (day 15 to 21), MD 0.20 (95% CI ‐4.20 to 4.60) (Analysis 10.1) respectively and when ivacaftor doses were combined, MD 1.57 (95% CI ‐2.13 to 5.27) (Analysis 12.1) (Boyle 2014) (moderate‐quality evidence).
9.1. Analysis.
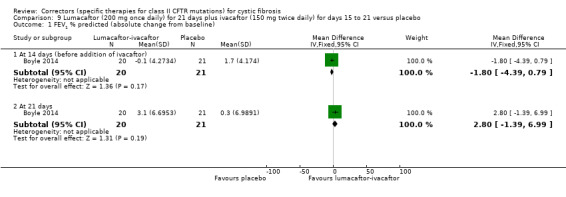
Comparison 9 Lumacaftor (200 mg once daily) for 21 days plus ivacaftor (150 mg twice daily) for days 15 to 21 versus placebo, Outcome 1 FEV1 % predicted (absolute change from baseline).
10.1. Analysis.
Comparison 10 Lumacaftor (200 mg once daily) for 21 days plus ivacaftor (250 mg twice daily) for days 15 to 21 versus placebo, Outcome 1 FEV1 % predicted (absolute change from baseline).
12.1. Analysis.
Comparison 12 Lumacaftor (200 mg once daily monotherapy for 14 days) plus ivacaftor (150 mg or 250 mg twice daily for days 15 to 21) for 21 days, Outcome 1 FEV1 % predicted (absolute change from baseline).
Tezacaftor plus ivacaftor versus control
At four weeks, there was a statistically significantly higher absolute change from baseline in FEV1 (% predicted) compared to the control groups in the two tezacaftor‐ivacaftor studies (n = 528), pooled MD 3.59 (95% CI 2.40 to 4.78) (Analysis 13.3) (Taylor‐Cousar 2017).
13.3. Analysis.
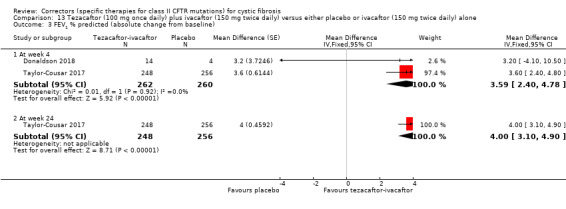
Comparison 13 Tezacaftor (100 mg once daily) plus ivacaftor (150 mg twice daily) versus either placebo or ivacaftor (150 mg twice daily) alone, Outcome 3 FEV1 % predicted (absolute change from baseline).
ii. Short term (over one month and up to and including six months)
Lumacaftor plus ivacaftor versus placebo
In the TRAFFIC and TRANSPORT studies (n = 1108), at six months the statistically significant difference in absolute changes from baseline in FEV1 (% predicted) were maintained in the both the lumacaftor 600 mg group and the lumacaftor 400 mg groups, MD 3.34 (95% CI 2.30 to 4.38) (Analysis 6.5) and MD 2.80 (95% CI 1.75 to 3.84) (Analysis 7.5) respectively (TRAFFIC 2015; TRANSPORT 2015). There was also a statistically significantly higher absolute change from baseline in FEV1 (% predicted) compared to the placebo group when the two lumacaftor doses were pooled, MD 3.07 (95% CI 2.17 to 3.97) (Analysis 8.5) (moderate‐quality evidence).
In the paediatric combination study, investigators reported a statistically significantly higher absolute change from baseline in FEV1 (% predicted) in the lumacaftor plus ivacaftor group compared to the placebo group up to and including 24 weeks (n = 204) (Ratjen 2017), MD 2.40 (95% CI 0.40 to 4.40) (Analysis 11.2) (low‐quality evidence). Additional results at earlier time points (day 15, week 4, and week 16) were published graphically in the study report, but the graphical plots were too small to allow for accurate extraction of data (Ratjen 2017). Numerical data for these time points have been requested from the study investigators and any unpublished information we receive will be included in a future update. Investigators in this study also reported early post‐drug dose declines in FEV1 (% predicted) at day one in the lumacaftor plus ivacaftor group (Ratjen 2017). A markedly smaller decline was observed post‐dose at day 15, and no decline was observed by week 16. These data are not available for all participants so are not entered into analysis for this review; instead these results are presented in the additional tables (Table 27).
11.2. Analysis.
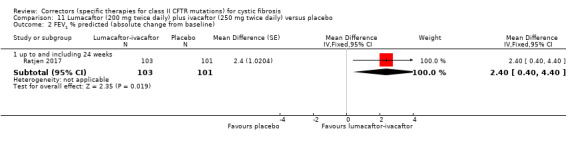
Comparison 11 Lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 2 FEV1 % predicted (absolute change from baseline).
8. Acute changes in FEV1 (% predicted) following study drug administration in Ratjen 2017.
|
Lumacaftor plus ivacaftor mean (SD) |
Placebo mean (SD) |
|
| Day 1, ≤ 2 hours post dose | n = 91 ‐5.5 (8.2) |
n = 97 ‐0.1 (5.1) |
| Day 1, 4 to 6 hours post dose | n = 92 ‐7.7 (7.3) |
n = 96 ‐1.4 (7.1) |
| Day 1, 24 hours post dose | n = 38 ‐4.1 (10.1) |
n = 44 ‐1.7 (6.8) |
| Day 15, ≤ 2 hours post dose | n = 88 ‐1.4 (7.0) |
n = 87 0.9 (5.5) |
| Day 15, 4 to 6 hours post dose | n = 86 ‐1.3 (6.4) |
n = 87 0.1 (5.2) |
| Week 16, ≤ 2 hours post dose | n = 33 1.7 (4.8) |
n = 42 0.8 (5.8) |
| Week 16, 4 to 6 hours post dose | n = 33 0.5 (7.4) |
n = 42 0.6 (7.1) |
| Week 24, ≤ 2 hours post dose | n = 25 0.3 (4.1) |
n = 23 0.0 (3.4) |
| Week 24, 4 to 6 hours post dose | n = 24 ‐2.8 (4.0) |
n = 24 0.1 (4.3) |
SD: standard deviation
Tezacaftor plus ivacaftor versus control
At 24 weeks, there was a statistically significantly higher absolute change from baseline in FEV1 (% predicted) compared to the placebo group in the tezacaftor‐ivacaftor study, MD 4.00 (95% CI 3.10 to 4.90) (Analysis 13.3) (Taylor‐Cousar 2017) (moderate‐quality evidence).
c. FVC (absolute values and change from baseline)
Data for this outcome were not reported by any study in this comparison (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). However, it is stated in the protocol of the TRAFFIC and TRANSPORT studies that FVC data was collected (although not considered as an outcome). Any recorded data relevant to this outcome has been requested from the study investigators and any unpublished information we receive will be included in a future update.
d. LCI
Lumacaftor plus ivacaftor versus placebo
Only one study (n = 204) reported this outcome and its primary outcome was LCI2.5, i.e. the number of lung volume turnovers required to reach 2.5% of starting tracer gas concentration (Ratjen 2017). There was a statistically significantly larger reduction in LCI2.5 in the lumacaftor plus ivacaftor group compared to the placebo group up to and including 24 weeks, MD ‐1.10 (95% CI ‐1.40 to ‐0.80) (Analysis 11.3). Additional results at earlier time points (day 15, week 4, and week 16) were published graphically in the full study report, but the graphical plots were too small to allow for accurate extraction of data (Ratjen 2017). Numerical data for these time points have been requested from the study investigators and any unpublished information we receive will be included in a future update.
11.3. Analysis.
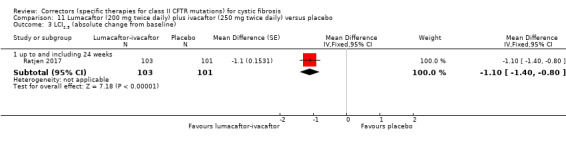
Comparison 11 Lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 3 LCI2.5 (absolute change from baseline).
The study investigators also list LCI5.0 as a secondary outcome of the study, i.e. number of lung volume turnovers required to reach 5% of starting tracer gas concentration (Ratjen 2017). However, results for this outcome are not presented. Numerical data for this outcome have been requested from the study investigators and any unpublished information we receive will be included in a future update.
Secondary outcomes
1. Adverse events
Adverse events were reported by all studies examining combination therapies (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). The type and extent of adverse event reporting was not consistent across studies making comparison between different treatment regimens and interventions a challenge. For the Phase 3 studies a common adverse events was defined by the researchers as one that occurred in more than 10% of participants.
Lumacaftor plus ivacaftor versus placebo
The TRAFFIC and TRANSPORT studies reported no statistically significant differences in the number of participants experiencing adverse events during the study, either by lumacaftor dose or when lumacaftor doses were pooled, OR 1.00 (99% CI 0.37 to 2.71) (Analysis 6.6), OR 0.77 (99% CI 0.30 to 1.96) (Analysis 7.6) and OR 0.87 (99% CI 0.38 to 2.02) (Analysis 8.6), respectively (TRAFFIC 2015; TRANSPORT 2015) (high‐quality evidence).
6.6. Analysis.
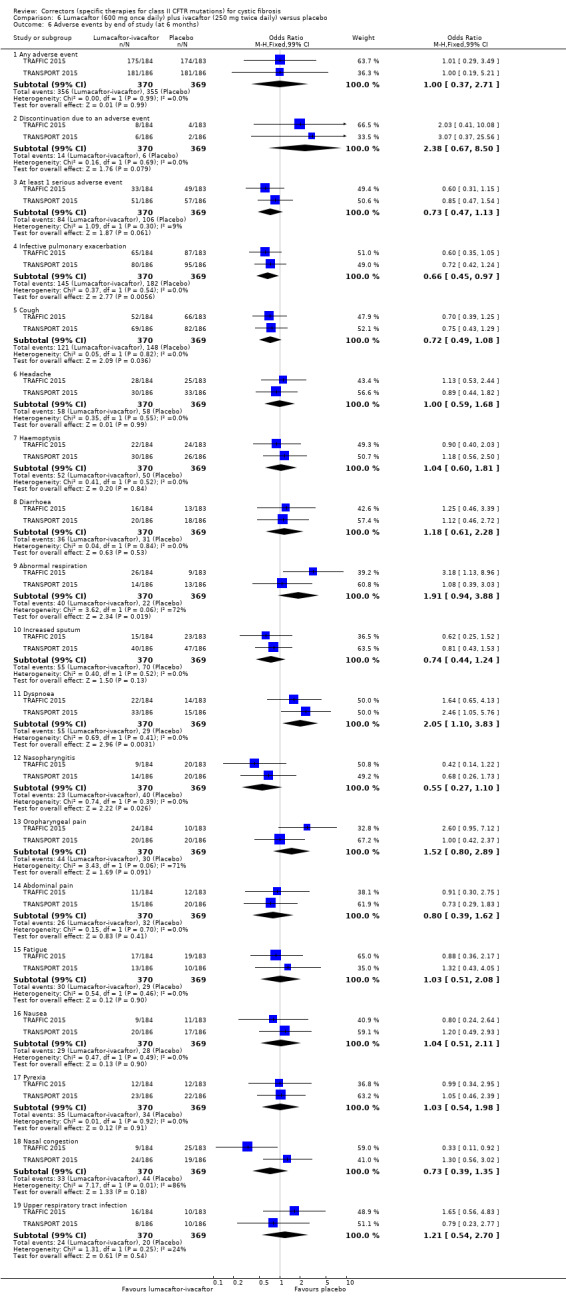
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 6 Adverse events by end of study (at 6 months).
7.6. Analysis.
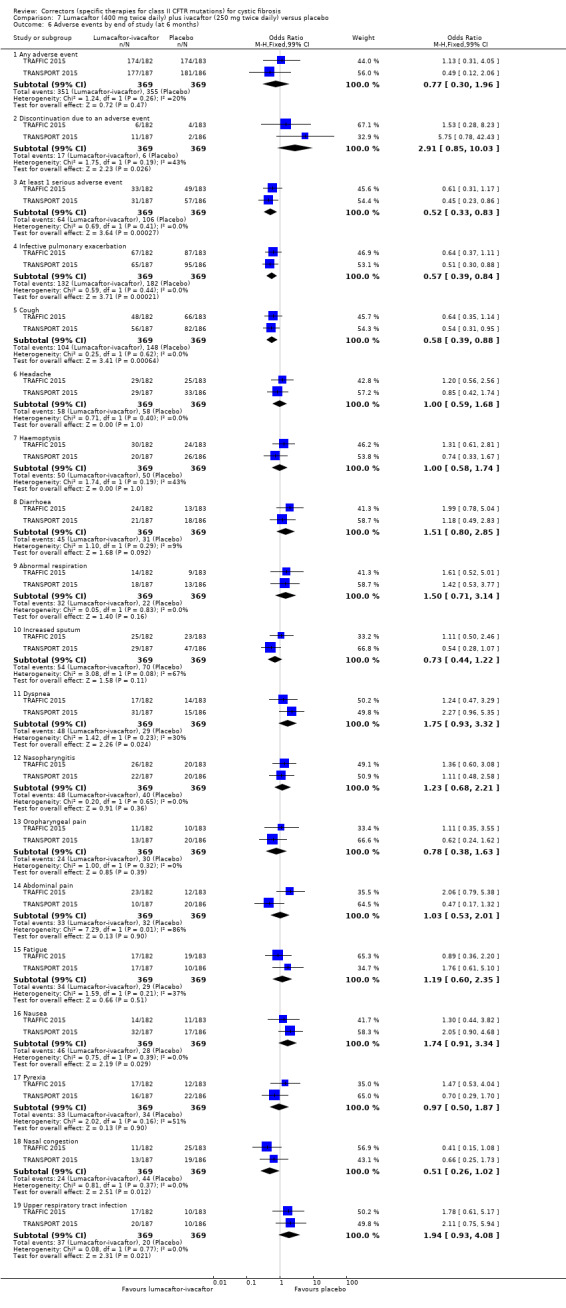
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 6 Adverse events by end of study (at 6 months).
8.6. Analysis.
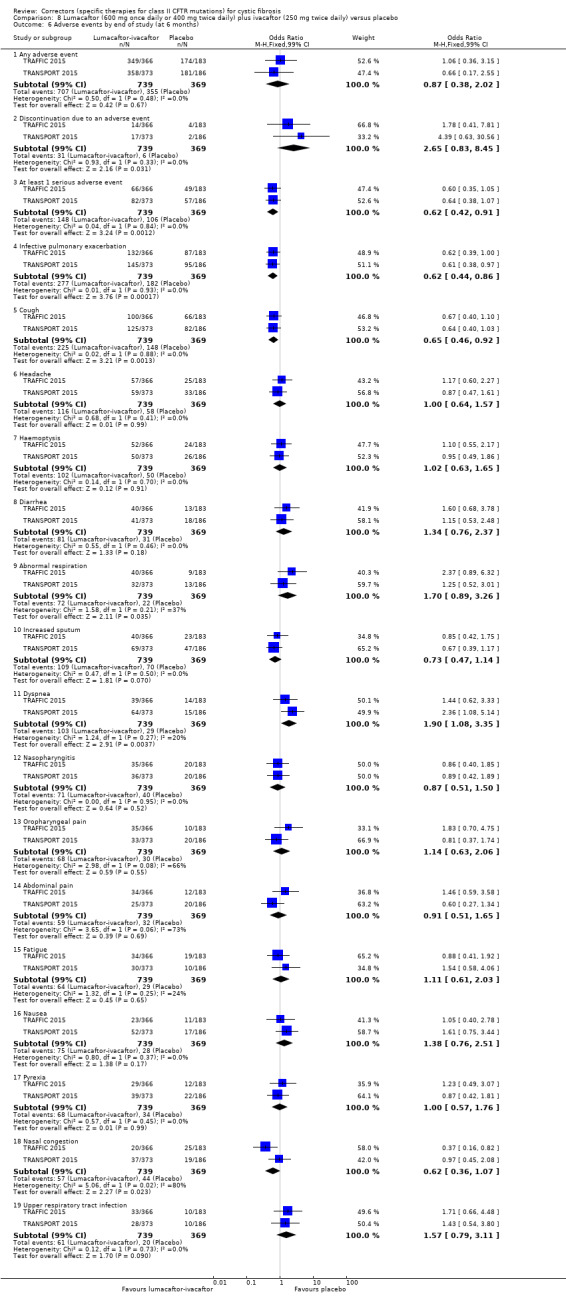
Comparison 8 Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 6 Adverse events by end of study (at 6 months).
In the paediatric lumacaftor‐ivacaftor study the overall rate of reporting of adverse events was lower than for the TRAFFIC and TRANSPORT studies with a similar profile, including increased reporting of chest tightness on starting the lumacaftor‐ivacaftor intervention compared to placebo (Ratjen 2017). The studies also reported no statistically significant difference between the lumacaftor plus ivacaftor group compared to placebo in the number of participants experiencing adverse events during the study, OR 0.60 (99% CI 0.09 to 4.08) (Analysis 11.4) (low‐quality evidence).
11.4. Analysis.
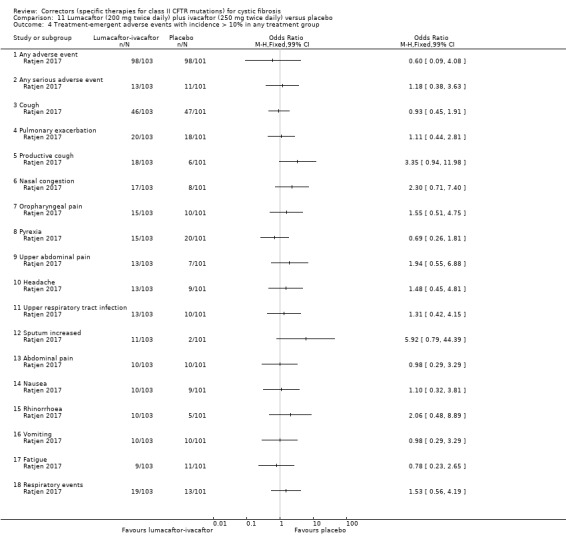
Comparison 11 Lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 4 Treatment‐emergent adverse events with incidence > 10% in any treatment group.
Tezacaftor plus ivacaftor versus control
For the tezacaftor‐ivacaftor studies, we present the most common adverse events which occurred in at least 10% of participants in either study (Analysis 13.4). Further less commonly occurring adverse events are presented in the original trial reports; none of which showed any difference between groups (Donaldson 2018; Taylor‐Cousar 2017) (moderate‐quality evidence).
13.4. Analysis.
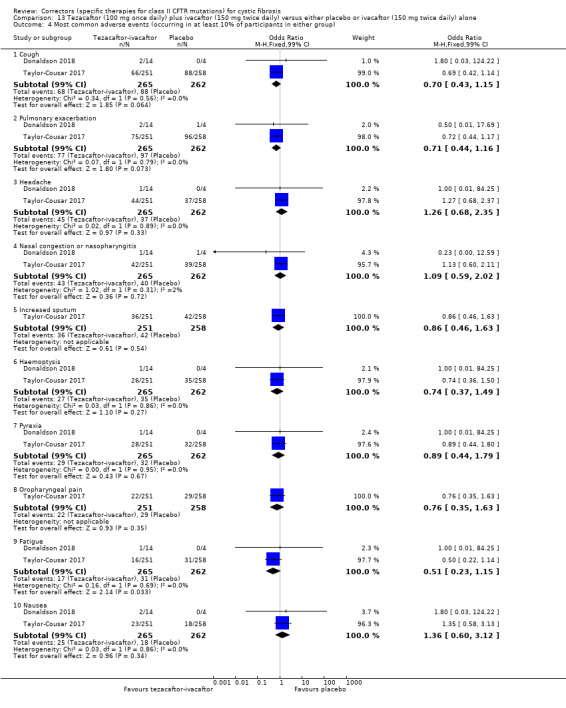
Comparison 13 Tezacaftor (100 mg once daily) plus ivacaftor (150 mg twice daily) versus either placebo or ivacaftor (150 mg twice daily) alone, Outcome 4 Most common adverse events (occurring in at least 10% of participants in either group).
a. Mild (therapy does not need to be discontinued)
Lumacaftor plus ivacaftor versus placebo
Boyle reported data for lumacaftor‐ivacaftor combination therapy at 21 days (day 14 to 21) (Boyle 2014). The combined analysis showed no statistically significant differences between participants treated with lumacaftor‐ivacaftor combination therapy and placebo in the number of participants experiencing cough, oropharyngeal pain, nasal congestion, dizziness, a prolonged prothrombin time, and upper respiratory tract infection (Analysis 9.2; Analysis 10.2; Analysis 12.2) (low‐quality evidence).
9.2. Analysis.
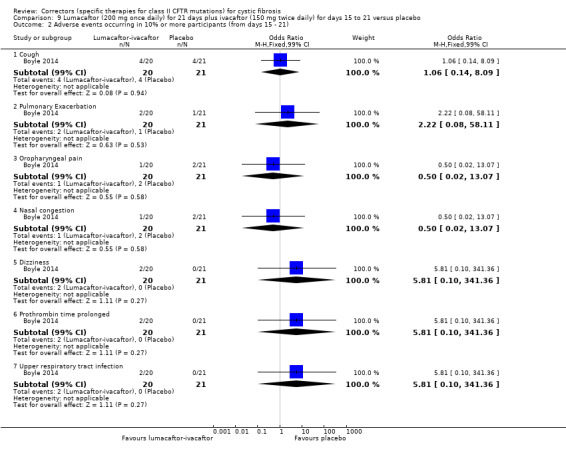
Comparison 9 Lumacaftor (200 mg once daily) for 21 days plus ivacaftor (150 mg twice daily) for days 15 to 21 versus placebo, Outcome 2 Adverse events occurring in 10% or more participants (from days 15 ‐ 21).
10.2. Analysis.
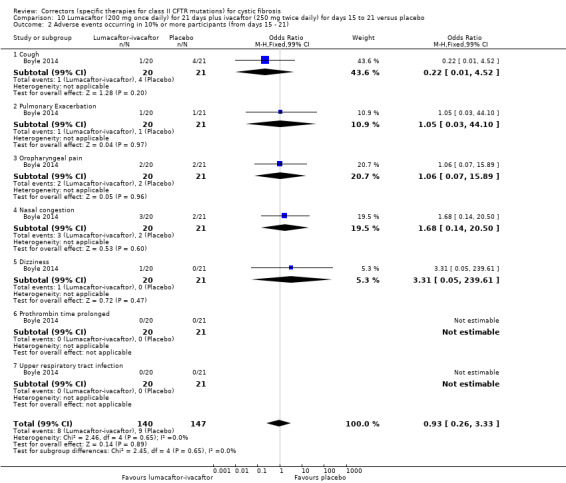
Comparison 10 Lumacaftor (200 mg once daily) for 21 days plus ivacaftor (250 mg twice daily) for days 15 to 21 versus placebo, Outcome 2 Adverse events occurring in 10% or more participants (from days 15 ‐ 21).
12.2. Analysis.
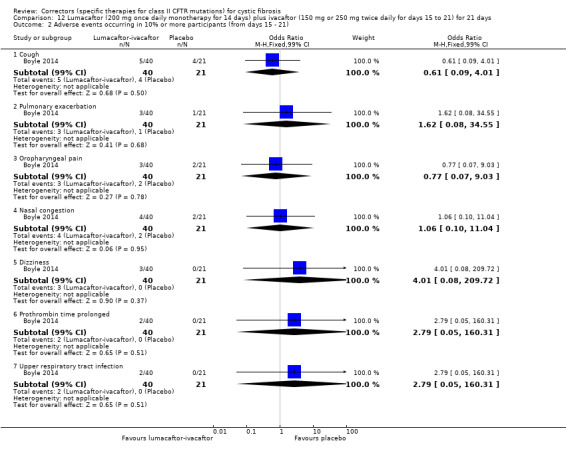
Comparison 12 Lumacaftor (200 mg once daily monotherapy for 14 days) plus ivacaftor (150 mg or 250 mg twice daily for days 15 to 21) for 21 days, Outcome 2 Adverse events occurring in 10% or more participants (from days 15 ‐ 21).
For participants in the TRAFFIC and TRANSPORT studies receiving the lumacaftor‐ivacaftor therapy, the most regularly reported adverse events were respiratory in nature (e.g. chest tightness) (TRAFFIC 2015; TRANSPORT 2015). Most respiratory adverse events occurred shortly after starting lumacaftor‐ivacaftor therapy and for those who continued with the intervention they were reported to be transient in nature. Dyspnoea was statistically significantly more common in the lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily group compared to placebo, OR 2.05 (99% CI 1.10 to 3.83) (Analysis 6.6) and when lumacaftor doses were combined, OR 1.90 (99% CI 1.08 to 3.35) (Analysis 8.6). Cough was statistically significantly less common in the lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily group compared to placebo, OR 0.58 (99% CI 0.39 to 0.88) (Analysis 7.6) and when lumacaftor doses were combined, OR 0.65 (99% CI 0.46 to 0.92) (Analysis 8.6).
There were no statistically significant differences between lumacaftor 600 mg once daily, lumacaftor 400 mg twice daily plus 250 mg ivacaftor twice daily or lumacaftor doses combined compared to placebo in terms of the number of participants experiencing other adverse events: infective pulmonary exacerbation, headache, haemoptysis, diarrhoea, abnormal respiration, increased sputum, nasopharyngitis, oropharyngeal pain, abdominal pain, fatigue, nausea, pyrexia, nasal congestion, upper respiratory tract infection (Analysis 6.6; Analysis 7.6, Analysis 8.6).
In the TRAFFIC and TRANSPORT studies, in seven participants receiving lumacaftor‐ivacaftor therapy abnormal liver function (elevated liver enzyme) results led to a temporary discontinuation of the intervention, after which liver function improved (to baseline in six participants). Treatment was re‐started in six of these participants; in one participant abnormal liver function was associated with hepatitis E infection (TRAFFIC 2015; TRANSPORT 2015).
For the paediatric lumacaftor‐ivacaftor study, the number of treatment‐emergent adverse events with an incidence over 10% were reported (Ratjen 2017). Productive cough, nasal congestion, oropharyngeal pain, upper abdominal pain, rhinorrhoea and increased sputum were observed more frequently in the lumacaftor‐ivacaftor group compared to the placebo group, but there was no statistically significant difference between the groups (Analysis 11.4). There was also no statistically significant differences between the groups in terms of cough, pyrexia, headache, upper respiratory tract infection, abdominal pain, nausea, vomiting, fatigue and respiratory events (such as wheezing, dyspnoea, asthma and chest discomfort) (Analysis 11.4).
Tezacaftor plus ivacaftor versus control
There were no statistically significant differences between tezacaftor‐ivacaftor and control groups (99% confidence intervals) in the number of participants experiencing cough, pulmonary exacerbation, headache, nasal congestion or nasopharyngitis, increased sputum, haemoptysis, pyrexia, oropharyngeal pain, nausea or fatigue (Analysis 13.4) (Donaldson 2018; Taylor‐Cousar 2017). Taylor‐Cousar specified respiratory compromise on initiation as an adverse event in light of the reports from the TRAFFIC and TRANSPORT studies, but there was no increased reporting of this event (Taylor‐Cousar 2017).
b. Moderate (therapy is discontinued, and the adverse effect ceases)
Lumacaftor plus ivacaftor versus placebo
None of the participants in the Phase 2 lumacaftor‐ivacaftor study required study drug interruption for the adverse effects of therapy (Boyle 2014). It was not stated in the TRAFFIC and TRANSPORT studies whether study drug interruption for the adverse effects of therapy was required for any participants (TRAFFIC 2015; TRANSPORT 2015). The combined safety data demonstrated similar rates of serious adverse event reporting for participants receiving placebo (28.6%) and those receiving the lumacaftor‐ivacaftor combination therapy (17.3% to 22.8%); however, the characteristics of these events were different (Analysis 6.6; Analysis 7.6; Analysis 8.6).
In two studies, 14 participants on lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily and 17 participants on lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily discontinued the study due to adverse events. In total 31 of 738 (4.2%) of participants receiving lumacaftor‐ivacaftor discontinued compared to six of 370 (1.6%) participants receiving placebo (TRAFFIC 2015; TRANSPORT 2015). The differences in the discontinuation rates in the treatment groups were not statistically significant compared to placebo at the 1% statistical significance level to allow for multiple analyses related to adverse events, OR 2.38 (99% CI 0.67 to 8.50) (Analysis 6.6), OR 2.91 (99% CI 0.85 to 10.03) (Analysis 7.6) and OR 2.65 (99% CI 0.83 to 8.45) (Analysis 8.6) respectively.
In the same studies, 84 participants on lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily, 64 participants on lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily and 106 participants on placebo experienced at least one 'serious' adverse event (TRAFFIC 2015; TRANSPORT 2015). A 'serious' adverse event was defined as "death, life threatening adverse experience, in‐patient hospitalization/prolongation of hospitalization, persistent/significant disability or incapacity, congenital anomaly/birth defect, important medical event". The following adverse events, which occurred on more than one occasion, were reported to have resulted in discontinuation of the lumacaftor‐ivacaftor therapy: elevated serum creatinine kinase level (n = 4), haemoptysis (n = 3), bronchospasm (n = 2), dyspnoea (n = 2), pulmonary exacerbation (n = 2) (see below), and rash (n = 2). One participant developed hypertension and discontinued the study (not included in the initial reports of this study); other reasons for discontinuation were not recorded (TRAFFIC 2015; TRANSPORT 2015).
There was no statistically significant difference between the number of adverse events between participants on lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily compared to placebo, OR 0.73 (99% CI 0.47 to 1.13) (Analysis 6.6). Statistically significantly fewer participants experienced serious adverse events on lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily compared to placebo, OR 0.52 (99% CI 0.33 to 0.83) (Analysis 7.6); this was also true when lumacaftor doses were combined, OR 0.62 (99% CI 0.42 to 0.91) (Analysis 8.6).
For the paediatric study of lumacaftor‐ivacaftor, there was no statistically significant difference reported between groups in the number of serious adverse events reported, OR 1.18 (99% CI 0.38 to 3.63) (Analysis 11.4). There were 13 participants who had serious adverse events in the lumacaftor plus ivacaftor group; these were considered to be treatment‐related in two participants (one drug interaction and one obstructive airways disorder). In the placebo group, 11 participants had serious adverse events; these were considered to be treatment‐related in three participants (one distal intestinal obstruction syndrome, two elevated aminotransferases) (Ratjen 2017). In this study six out of 103 participants discontinued, three due to adverse events. One participant discontinued due to an early respiratory event, a second due to persistently abnormal liver function tests and the reasons for the remaining four who discontinued were not recorded (Ratjen 2017).
In the longer‐term follow‐up study to the TRAFFIC and TRANSPORT studies (PROGRESS), in which participants were randomised to two different lumacaftor‐ivacaftor dose regimens, the paper reported that 7% of the participants withdrew because of adverse events during the 96 week‐study period; in one participant this was due to hypertension (PROGRESS 2017).
Tezacaftor plus ivacaftor versus control
In the Phase 3 tezacaftor‐ivacaftor study, seven out of 251 participants receiving tezacaftor‐ivacaftor discontinued compared to eight out of 258 participants in the placebo group (Taylor‐Cousar 2017). Reasons for discontinuation in the tezacaftor‐ivacaftor group included abdominal pain (n = 2), raised serum creatinine phosphokinase (n = 1), raised liver enzymes (n = 1) and a generalised tonic‐clonic seizure (n = 1).
c. Severe (life‐threatening or debilitating, or which persists even after treatment is discontinued)
Lumacaftor plus ivacaftor versus placebo
For trials evaluating lumacaftor‐ivacaftor, there were no adverse events reported, that in our assessment, were life‐threatening or debilitating. When treatments were discontinued the reported adverse events resolved (Boyle 2014; Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015).
Tezacaftor plus ivacaftor versus control
In the large tezacaftor‐ivacaftor study (n = 510) one life‐threatening adverse event (haemoptysis) was reported in a participant in the tezacaftor‐ivacaftor treatment group (Taylor‐Cousar 2017).
d. Other adverse effects of therapy (of any severity) that are not classifiable according to these categories
All studies reported on the number of participants who experienced episodes of pulmonary exacerbations described as adverse events (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). Results are presented in the analyses (Analysis 6.6; Analysis 7.6; Analysis 8.6; Analysis 9.2; Analysis 10.2; Analysis 12.2; Analysis 11.4; Analysis 13.4) and described below (see 'Extra courses of antibiotics').
Lumacaftor plus ivacaftor versus placebo
After a second participant on lumacaftor‐ivacaftor was withdrawn with hypertension, the researchers for the TRAFFIC and TRANSPORT and PROGRESS studies reported the blood pressure measurements for the participants over the total study period (PROGRESS 2017; TRAFFIC 2015; TRANSPORT 2015). Average blood pressure data were presented from participants over the total 120‐week study period of TRAFFIC and TRANSPORT and PROGRESS for participants who continued on lumacaftor‐ivacaftor, but only for those who received the 400 mg twice daily dose, as this was the dose for which the company received a product licence (PROGRESS 2017). There was a statistically significant mean (SE) increase in systolic blood pressure of 5.1 (1.5) mm Hg and in diastolic blood pressure of 4.1 (1.2) mm Hg (n = 80) (PROGRESS 2017).
2. Hospitalisation
Data for this outcome were reported in two lumacaftor‐ivacaftor studies (TRAFFIC 2015; TRANSPORT 2015) and for the tezacaftor‐ivacaftor study (Taylor‐Cousar 2017), but not by the remaining studies in this comparison (Boyle 2014; Donaldson 2018; Ratjen 2017).
Lumacaftor plus ivacaftor versus placebo
Excerbations were protocol‐defined in the TRAFFIC and TRANSPORT studies as exacerbations leading to hospitalisation or treatment with intravenous antibiotics (TRAFFIC 2015; TRANSPORT 2015). We present information relating to events leading to hospitalisation here and information relating to all pulmonary exacerbations below (see 'Extra courses of antibiotics').
The study publications reported on the rate of events per participant leading to hospitalisation over 48 weeks, graphically pooled across both studies (TRAFFIC 2015; TRANSPORT 2015). We estimate that the event rate per participant over 48 weeks in the placebo group was 0.45. The corresponding event rate in the lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily group was 0.27 (equal to a 39% decrease compared to the placebo group, P = 0.003). In the lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily the event rate was 0.18 (equal to a 61% decrease compared to the placebo group, P < 0.001). The presentation of data did not allow us to estimate the rate over the two lumacaftor doses combined. These data were extrapolated and we have requested confirmation of the exact data from the study investigators. Any unpublished information we receive will be included in a future update.
Tezacaftor plus ivacaftor versus control
The rate of pulmonary exacerbations that led to hospitalisation or treatment with intravenous antibiotic agents (or both) was also lower in the tezacaftor–ivacaftor group than in the placebo group (0.29 versus 0.54 events per year; rate ratio, 0.53; 95% CI, 0.34 to 0.82) (Taylor‐Cousar 2017).
3. School or work attendance
Data for this outcome were not reported by any study (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
4. Extra courses of antibiotics
Excerbations were protocol‐defined in two studies as exacerbations leading to hospitalisation or treatment with intravenous antibiotics (TRAFFIC 2015; TRANSPORT 2015). Therefore we present information relating to pulmonary exacerbations (as well as information specifically relating to antibiotic use) here. In the other studies, it was unclear whether reported exacerbations were protocol‐defined or physician‐defined (Boyle 2014; Ratjen 2017).
a. Time‐to the next course of antibiotics
The paediatric combination study listed the time to first pulmonary exacerbation as a secondary outcome of the study, but results for this outcome are not presented (Ratjen 2017). Numerical data for this outcome have been requested from the trial investigators and any unpublished information we receive will be included in a future update.
Lumacaftor plus ivacaftor versus placebo
In the TRAFFIC and TRANSPORT studies, when compared to placebo the time‐to‐first pulmonary exacerbation was statistically significantly longer in both in the lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily group, hazard ratio (HR) 0.70 (95% CI 0.57 to 0.87) (Analysis 6.7) and the lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily group, HR 0.61 (95% CI 0.49 to 0.76) (Analysis 7.7) (both moderate‐quality evidence). Similarly, the rate of exacerbations was statistically significantly lower in both the active intervention groups compared to placebo; the lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily group, rate ratio 0.70 (95% CI 0.57 to 0.87) (Analysis 6.8) and the lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily group, rate ratio 0.61 (95% CI 0.49 to 0.76) (Analysis 7.8). This information was not reported in the primary journal article, but is available from the study record on ClinicalTrials.gov (TRAFFIC 2015; TRANSPORT 2015). Information regarding time to first pulmonary exacerbation was reported only as a hazard ratio and P value; the SE used in this analysis was estimated using the methods of Parmar (Parmar 1998).
6.7. Analysis.
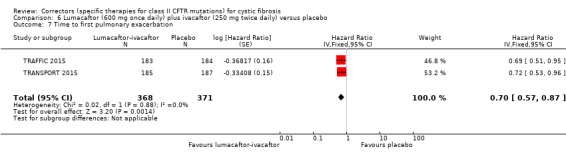
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 7 Time to first pulmonary exacerbation.
7.7. Analysis.
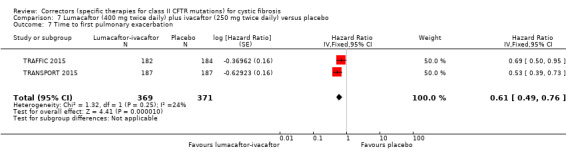
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 7 Time to first pulmonary exacerbation.
6.8. Analysis.
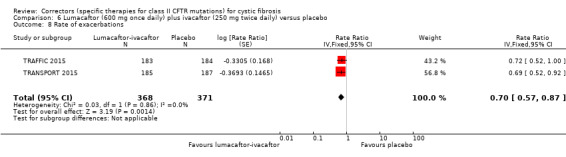
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 8 Rate of exacerbations.
7.8. Analysis.
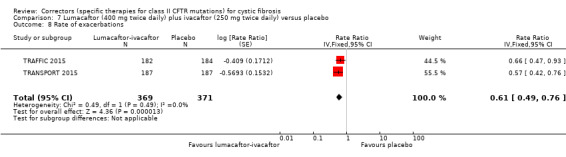
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 8 Rate of exacerbations.
Tezacaftor plus ivacaftor versus control
The time‐to‐first pulmonary exacerbation was statistically significantly longer in the tezacaftor‐ivacaftor group compared to the placebo group, HR 0.64 (95% CI, 0.46 to 0.89) (Taylor‐Cousar 2017) (Analysis 13.5) (moderate‐quality evidence).
13.5. Analysis.
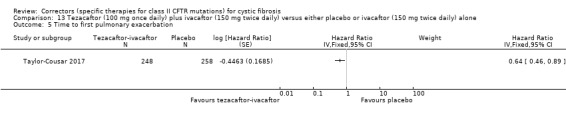
Comparison 13 Tezacaftor (100 mg once daily) plus ivacaftor (150 mg twice daily) versus either placebo or ivacaftor (150 mg twice daily) alone, Outcome 5 Time to first pulmonary exacerbation.
b. Total number of courses of antibiotics
Lumacaftor plus ivacaftor versus placebo
In two studies, both the lumacaftor 600 mg once daily plus 250 mg ivacaftor twice daily and 400 mg twice daily plus 250 mg ivacaftor twice daily groups experienced statistically significantly fewer pulmonary exacerbations than the placebo group, OR 0.66 (99% CI 0.45 to 0.97) (Analysis 6.6) and OR 0.57 (99% CI 0.39 to 0.84) (Analysis 7.6), respectively (TRAFFIC 2015; TRANSPORT 2015). This statistically significant difference was also observed for the two lumacaftor doses combined compared to placebo, OR 0.62 (99% CI 0.44 to 0.86) (Analysis 8.6).
These studies also reported the rate of events per participant leading to intravenous antibiotic treatment over 48 weeks graphically pooled across the two studies (TRAFFIC 2015; TRANSPORT 2015). These data were extrapolated and we have requested confirmation of the exact data from the study investigators. Any unpublished information we receive will be included in a future update.
We estimate that the event rate per participant over 48 weeks in the placebo group was 0.58. The corresponding event rate in the lumacaftor 600 mg once daily plus ivacaftor 250 mg twice daily group was 0.32 (equal to a 45% decrease compared to the placebo group, P < 0.001) and in the lumacaftor 400 mg twice daily plus ivacaftor 250 mg twice daily was 0.18 (equal to a 56% decrease compared to the placebo group, P < 0.001).
The presentation of data did not allow us to estimate the time to first pulmonary exacerbation or rate of exacerbations over the two lumacaftor doses combined. These data were extrapolated and we have requested confirmation of the exact data from the study investigators. Any unpublished information we receive will be included in a future update.
Data from the Phase 2 lumacaftor‐ivacaftor study on the number of exacerbations were reported at day 21 (Boyle 2014). These data demonstrated no statistically significant differences between treatment groups from participants receiving 200 mg lumacaftor once daily plus either 150 mg or 250 mg of ivacaftor twice daily or ivacaftor doses combined compared to placebo, OR 2.22 (99% CI 0.08 to 58.11) (Analysis 9.2), OR 1.05 (99% CI 0.03 to 44.10) (Analysis 10.2) and OR 1.62 (99% CI 0.08 to 34.55) (Analysis 12.2), respectively.
There were no statistically significance differences between treatment groups in the number of pulmonary exacerbations experienced in the paediatric combination study, OR 1.11 (99% CI 0.44 to 2.81) (Ratjen 2017) (Analysis 11.4).
Tezacaftor plus ivacaftor versus control
The larger study (n = 510) also reported rate of pulmonary exacerbations that led to hospitalisation or treatment with intravenous antibiotic agents (or both); see secondary outcome 'Hospitalisation' above for further details (Taylor‐Cousar 2017).
5. Sweat chloride (change from baseline) as a measure of CFTR function
Lumacaftor plus ivacaftor versus placebo
Two studies did not report this outcome (TRAFFIC 2015; TRANSPORT 2015).
Boyle reported that following lumacaftor (day 1 to 21) and ivacaftor (day 15 to 21) combination therapy, data at 21 days demonstrated reductions in sweat chloride concentration in the 150 mg ivacaftor group, MD ‐5.00 mmol/L (95% CI ‐11.60 to 1.60) (Analysis 9.3) and 250 mg ivacaftor group, MD ‐10.90 mmol/L (95% CI ‐17.60 to ‐4.20) (Analysis 10.3), the latter of which was statistically significant (Boyle 2014). There was also a statistically significant reduction in sweat chloride concentration when ivacaftor doses were combined MD ‐7.95 (95% CI ‐13.81 to ‐2.09) (Analysis 12.3)
9.3. Analysis.
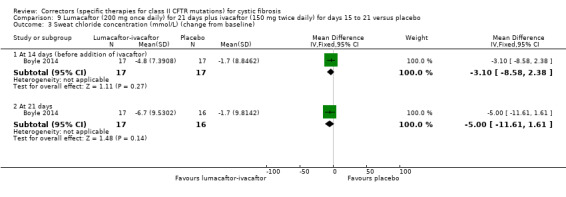
Comparison 9 Lumacaftor (200 mg once daily) for 21 days plus ivacaftor (150 mg twice daily) for days 15 to 21 versus placebo, Outcome 3 Sweat chloride concentration (mmol/L) (change from baseline).
10.3. Analysis.
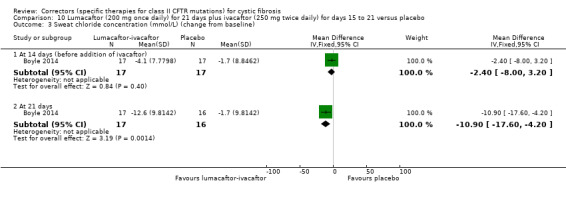
Comparison 10 Lumacaftor (200 mg once daily) for 21 days plus ivacaftor (250 mg twice daily) for days 15 to 21 versus placebo, Outcome 3 Sweat chloride concentration (mmol/L) (change from baseline).
12.3. Analysis.
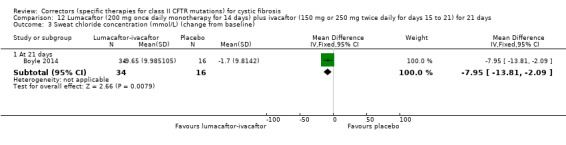
Comparison 12 Lumacaftor (200 mg once daily monotherapy for 14 days) plus ivacaftor (150 mg or 250 mg twice daily for days 15 to 21) for 21 days, Outcome 3 Sweat chloride concentration (mmol/L) (change from baseline).
The paediatric combination study reported a statistically significantly greater absolute reduction in sweat chloride concentration from baseline in the lumacaftor plus ivacaftor group compared to the placebo group, up to and including four weeks, MD ‐20.80 (95% CI ‐23.40 to ‐18.20) (Analysis 11.5). Additional results (at time points day 15, week 16, and week 24) were published graphically in the study publication, but the graphical plots were too small to allow for accurate extraction of data (Ratjen 2017). Numerical data for these time points have been requested from the study investigators and any unpublished information we receive will be included in a future update.
11.5. Analysis.
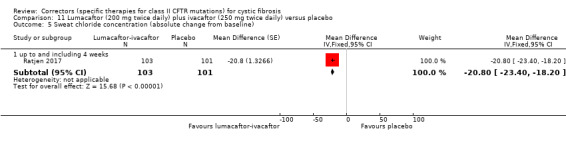
Comparison 11 Lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 5 Sweat chloride concentration (absolute change from baseline).
Tezacaftor plus ivacaftor versus control
There was a statistically significant reduction in sweat chloride concentration in the tezacaftor‐ivacaftor groups at four weeks compared to the control groups, pooled MD ‐9.24 mmol/L (95% CI ‐11.12 to ‐7.35) (Analysis 13.6) (Donaldson 2018; Taylor‐Cousar 2017); this was maintained at 24 weeks in the Taylor‐Cousar study, MD ‐10.10 mmol/L (95% CI ‐11.40 to ‐8.80) (Analysis 13.6) (Taylor‐Cousar 2017).
13.6. Analysis.
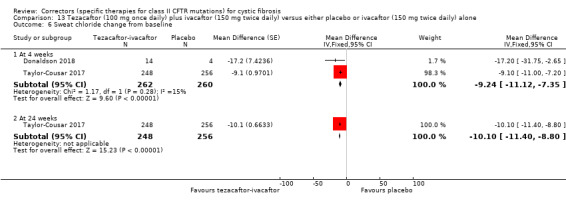
Comparison 13 Tezacaftor (100 mg once daily) plus ivacaftor (150 mg twice daily) versus either placebo or ivacaftor (150 mg twice daily) alone, Outcome 6 Sweat chloride change from baseline.
6. Radiological measures of lung disease (assessed using any scoring system)
Data for this outcome were not reported by any study (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
7. Acquisition of respiratory pathogens
No study reported on the acquisition of any respiratory pathogens (P aeruginosa, S aureus, H influenzae, or any other clinically relevant pathogen in CF) (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
8. Eradication of respiratory pathogens
Data for this outcome were not reported by any study (Boyle 2014; Donaldson 2018; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
9. Nutrition and growth
a. Weight
Lumacaftor plus ivacaftor versus placebo
Data for this outcome were reported by three studies (Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015).
Two studies presented results for the absolute change from baseline in weight (kg) at six months (TRAFFIC 2015; TRANSPORT 2015). Participants in both the lumacaftor 600 mg once daily plus 250 mg ivacaftor twice daily group and the 400 mg twice daily plus 250 mg ivacaftor twice daily group experienced a statistically significantly higher absolute weight gain from baseline compared to the placebo group, MD 0.80 kg (95% CI 0.42 to 1.18) (Analysis 6.9) and MD 0.65 kg (95% CI 0.27 to 1.03) (Analysis 7.9), respectively. There was also a statistically significantly higher absolute weight gain from baseline compared to the placebo group when the two lumacaftor doses were pooled, MD 0.72 kg (95% CI 0.39 to 1.05) (Analysis 8.7).
6.9. Analysis.
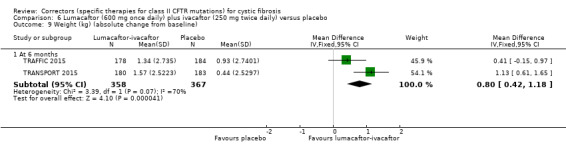
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 9 Weight (kg) (absolute change from baseline).
7.9. Analysis.
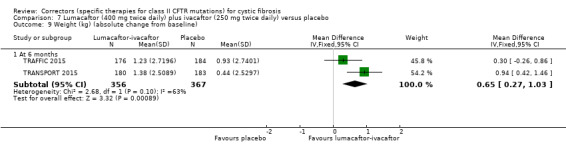
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 9 Weight (kg) (absolute change from baseline).
8.7. Analysis.
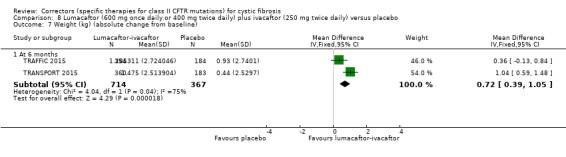
Comparison 8 Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 7 Weight (kg) (absolute change from baseline).
The paediatric combination study listed absolute change in weight and absolute change in weight‐for‐age z score as a secondary outcome of the study, but results for this outcome are not presented (Ratjen 2017). Numerical data for this outcome have been requested from the study investigators and any unpublished information we receive will be included in a future update.
b. BMI
Data for the absolute change from baseline in BMI were reported by four studies (Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015); one study additionally reported absolute change in BMI‐for‐age z score (Ratjen 2017).
i. Immediate term (up to and including one month)
Lumacaftor plus ivacaftor versus placebo
At 28 days, there was no statistically significant difference in the absolute change in BMI from baseline between the lumacaftor 600 mg once daily plus 250 mg ivacaftor twice daily and placebo groups, MD 0.01 (95% CI ‐0.07 to 0.09) (Analysis 6.10) or the lumacaftor 400 mg twice daily plus 250 mg ivacaftor twice daily and placebo groups, MD 0.02 (95% CI ‐0.06 to 0.10) (Analysis 7.10). There was also no statistically significant difference in absolute change in BMI from baseline compared to the placebo group when the two lumacaftor doses were pooled, MD 0.02 (95% CI ‐0.05 to 0.08) (Analysis 8.8).
6.10. Analysis.
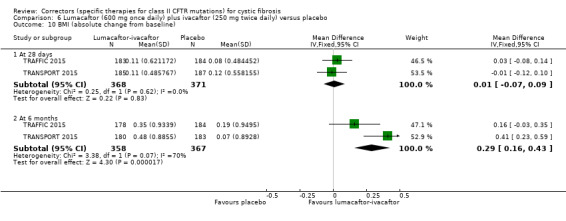
Comparison 6 Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 10 BMI (absolute change from baseline).
7.10. Analysis.
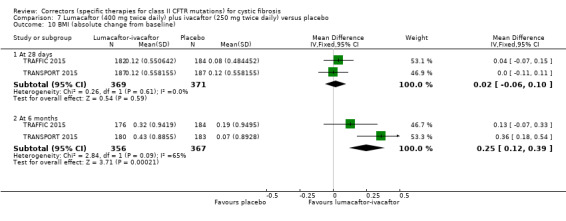
Comparison 7 Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 10 BMI (absolute change from baseline).
8.8. Analysis.
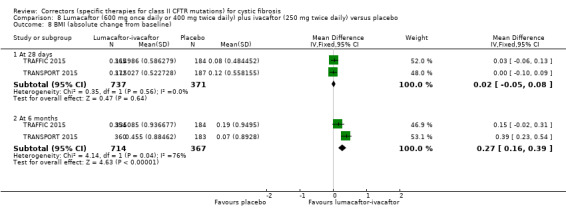
Comparison 8 Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 8 BMI (absolute change from baseline).
Tezacaftor plus ivacaftor versus control
There was no statistically significant difference between tezacaftor‐ivacaftor and placebo in terms of change from baseline in BMI in the Taylor‐Cousar study at four weeks, MD ‐0.03 (95% CI ‐0.13 to 0.07) (Analysis 13.7) (Taylor‐Cousar 2017).
13.7. Analysis.
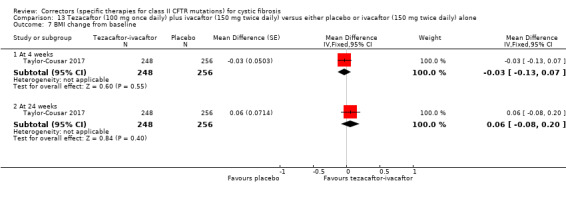
Comparison 13 Tezacaftor (100 mg once daily) plus ivacaftor (150 mg twice daily) versus either placebo or ivacaftor (150 mg twice daily) alone, Outcome 7 BMI change from baseline.
ii. Short term (over one month and up to and including six months)
Lumacaftor plus ivacaftor versus placebo
Despite no immediate differences between treatment groups, at six months participants in the both the lumacaftor 600 mg once daily plus 250 mg ivacaftor twice daily and 400 mg twice daily plus 250 mg ivacaftor twice daily groups experienced a statistically significantly higher absolute change in BMI from baseline compared to the placebo group, MD 0.29 (95% CI 0.16 to 0.43) (Analysis 6.10) and MD 0.25 (95% CI 0.12 to 0.39) (Analysis 7.10) respectively. There was also a statistically significantly higher absolute change in BMI from baseline compared to the placebo group when the two lumacaftor doses were pooled; MD 0.27 (95% CI 0.16 to 0.39) (Analysis 8.8)
At six months Rajten reported no statistically significant difference between groups in the absolute change in BMI or the absolute change in BMI‐for‐age z score, MD 0.10 (95% CI ‐0.10 to 0.30) (Analysis 11.6) and MD 0.00 (95% CI ‐0.10 to 0.10) (Analysis 11.7) respectively. Additional results for BMI at earlier time points (day 15, week 4, and week 16) were published graphically in the full paper, but the graphical plots were too small to allow for accurate extraction of data (Ratjen 2017). Numerical data for these time points have been requested from the study investigators and any unpublished information we receive will be included in a future update.
11.6. Analysis.
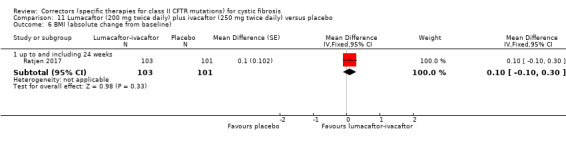
Comparison 11 Lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 6 BMI (absolute change from baseline).
11.7. Analysis.
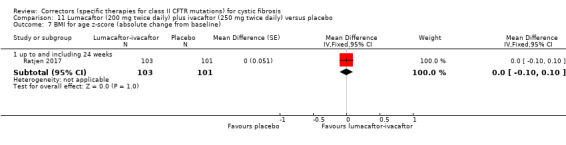
Comparison 11 Lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo, Outcome 7 BMI for age z‐score (absolute change from baseline).
Tezacaftor plus ivacaftor versus control
There was no statistically significant difference between tezacaftor‐ivacaftor and placebo in terms of change from baseline in BMI in the Taylor‐Cousar study at 24 weeks, MD ‐0.06 (95% CI ‐0.08 to 0.20) (Analysis 13.7) (Taylor‐Cousar 2017).
c. Height
Lumacaftor plus ivacaftor versus placebo
This outcome was not reported in three studies (Boyle 2014; TRAFFIC 2015; TRANSPORT 2015). One study listed the absolute change in height and absolute change in height‐for‐age z score as a secondary outcome of the study, but results for this outcome are not presented (Ratjen 2017). Numerical data for this outcome have been requested from the study investigators and any unpublished information we receive will be included in a future update.
Discussion
The class II mutation of the CFTR gene, F508del, is prevalent (particularly in the Northern European population) and is the commonest cause of CF. A therapy that corrects the F508del molecular defect would potentially have a profound impact on the field of CF, providing a therapeutic option for many pwCF.
Summary of main results
We identified 13 eligible RCTs evaluating correctors for pwCF and class II CFTR mutations (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; Donaldson 2018; PROGRESS 2017; McCarty 2002; Ratjen 2017; Rubenstein 1998; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015; Zeitlin 2002). Seven studies examined monotherapy of a number of different correctors (Boyle 2014; Clancy 2012; Donaldson 2014; Donaldson 2017; McCarty 2002; Rubenstein 1998; Zeitlin 2002). Seven studies (including the multi‐arm Boyle trial) examined combination therapy of either lumacaftor‐ivacaftor or tezacaftor‐ivacaftor (Boyle 2014; Donaldson 2018; PROGRESS 2017; Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015). Four of these were well‐powered Phase 3 studies that enrolled pwCF (including one of children aged 6 to 11 years) with two copies of the F508del mutation (F508del homozygotes) (Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANSPORT 2015).
Monotherapy versus placebo
Early phase trials evaluated various potential molecules 4PBA (Rubenstein 1998; Zeitlin 2002), CPX (McCarty 2002), N6022 (Donaldson 2014), cavosonstat (Donaldson 2017) and lumacaftor (Clancy 2012; Boyle 2014). There was no statistically significant impact on clinical outcomes (including sweat chloride) with either 4BPA, CPX or N6022 and phase 3 studies of these drugs were not conducted. In one small Phase 1 study of cavosonstat monotherapy, there was a reduction in sweat chloride of ‐4.1 mmol (P = 0.032) at the highest dose (200 mg), and there were no important safety concerns (Donaldson 2017); and we have identified an ongoing study of this agent (NCT02589236). There was a modest improvement in sweat chloride with lumacaftor alone compared with placebo after four weeks, MD ‐8.21 mmol/L (95% CI ‐14.30 to ‐2.12) (Clancy 2012), but not sufficient to warrant investigation of this agent as monotherapy in later phase studies. Although there was not a statistically significant increase in adverse events the size of the studies was not sufficient to provide confidence on the safety profiles of these agents.
Combination therapy versus placebo
Early phase studies of lumacaftor combined with ivacaftor demonstrated a greater magnitude of effect with a reduction in sweat chloride of MD ‐10.9 mmol/L (95% CI ‐17.6 to ‐4.2) compared to lumacaftor monotherapy (Boyle 2014). The efficacy and safety of the lumacaftor‐ivacaftor combination therapy were examined in two multicentre placebo‐controlled RCTs, enrolling over 1000 adults and young pwCF, homozygous for F508del (TRAFFIC 2015; TRANSPORT 2015). In participants allocated to the lumacaftor‐ivacaftor combination, combined trial data demonstrated no difference with regards to change in a generic measure of QoL (measured by the EQ‐5L‐3D tool). There was a statistically significant improvement in the respiratory domain of the CF‐specific QoL measure (CFQ‐R) at 24 weeks, MD 2.62 (95% CI 0.64 to 4.59). An improvement of four points on this scale is considered the minimal clinically important difference (MCID) for this outcome measure (Quittner 2009; Ramsey 2011).
With respect to respiratory function (as measured by % predicted FEV1), there were statistically significant differences from baseline in favour of the lumacaftor‐ivacaftor combination over placebo in both relative change, MD 5.21 (95% CI 3.61 to 6.80) and absolute change, MD 3.07 (95% CI 2.17 to 3.97). Improvement in FEV1 is considered an important surrogate measure for pwCF. The European Medicines Agency (EMA) has suggested that "as FEV1 is linked to mortality, any statistically significant difference between placebo and active treatment is potentially clinically relevant" (EMA 2012). In the study protocol, the MCID in absolute change in FEV1 used to calculate the sample sizes for the TRAFFIC and TRANSPORT was 5% (TRAFFIC 2015; TRANSPORT 2015). This magnitude of improvement in respiratory function was not achieved with the lumacaftor‐ivacaftor combination. In a post hoc change to the protocol, the primary outcome for TRAFFIC and TRANSPORT was altered from absolute change from baseline in FEV1 at 24 weeks to an average of the FEV1 values at 16 and 24 weeks.
A number of important secondary outcomes were reported that were included in this review. In the TRAFFIC and TRANSPORT studies, pulmonary exacerbations were reported more frequently in participants allocated to placebo compared to those receiving lumacaftor‐ivacaftor combination therapy (TRAFFIC 2015; TRANSPORT 2015); pulmonary exacerbations are a challenging outcome to record accurately, but one that is important to pwCF. Additionally, BMI improved in participants allocated to the lumacaftor‐ivacaftor combination therapy after 24 weeks (Analysis 1.10; Analysis 2.10; Analysis 3.8). Data on school or work attendance, acquisition or eradication of microbial pathogens, or radiological outcomes were not reported.
Overall the safety data reported for the lumacaftor‐ivacaftor combination were reassuring, but there was clear evidence of increased reporting of early respiratory compromise, OR 2.05 (99% CI 1.1 to 3.8) (TRAFFIC 2015; TRANSPORT 2015). The aetiology of this event is unclear and it was reported to settle after a few weeks if the intervention was continued (TRAFFIC 2015; TRANSPORT 2015). Two participants were withdrawn because of hypertension (including one in the follow‐up study (PROGRESS 2017)). For participants (n = 80) receiving 400 mg twice a day of lumacaftor there was a statistically significant mean (SE) increase in systolic blood pressure of 5.1 (1.5) mm Hg and in diastolic blood pressure of 4.1 (1.2) mm Hg (PROGRESS 2017).
For the children (aged 6 to 11 years) enrolled in the Phase 3 study of lumacaftor‐ivacaftor combination therapy, the safety profile reported was similar to the TRAFFIC and TRANSPORT studies, including transient early respiratory compromise and infrequent elevation in serum transaminases (liver enzymes) (Ratjen 2017). The efficacy data for this study are discussed below in the section on applicability.
The efficacy outcomes (primary and secondary) for the tezacaftor‐ivacaftor Phase 3 study were very similar to those reported with lumacaftor‐ivacaftor (Taylor‐Cousar 2017). After 24 weeks the participants allocated to tezacaftor‐ivacaftor had a 4.0% improvement in their absolute change in FEV1 compared to those who received placebo (Analysis 12.1). There was no increased reporting of adverse events, in particular the early transient dyspnoea reported with the lumacaftor‐ivacaftor combination, and no increased withdrawals of tezacaftor‐ivacaftor participants compared to those receiving placebo.
Overall completeness and applicability of evidence
Monotherapy versus placebo
The single‐agent studies have enrolled participants with two copies of the F508del mutation. These studies have not been taken forward on larger more representative populations in Phase 3 studies. New agents (such as cavosonstat) are currently being assessed in early phase studies (Donaldson 2017).
Combination therapy versus placebo
The Phase 3 studies of both lumacaftor and tezacaftor combined with ivacaftor have examined this therapy for people with two copies of the F508del mutation (F508del homozygotes) (Ratjen 2017; Taylor‐Cousar 2017; TRAFFIC 2015; TRANS{ORT 2015). There have been no Phase 3 studies for pwCF who are compound heterozygotes of F508del with another CF‐causing mutation. One cross‐over study has examined compound heterozygotes who have F508del with a ivacaftor‐sensitive residual function mutation, to evaluate any potential additive impact of tezacaftor on the recognised ivacaftor benefit (Rowe 2017). We excluded this study because of concerns over study design, in particular carryover effects of an intervention (ivacaftor) that has been shown to correct the basic defect in CF.
The Phase 3 studies of both the lumacaftor and ivacaftor combination therapy are well‐powered and provide clear statistical evidence of improvement in clinical outcomes, even if these are limited in magnitude compared to the changes anticipated in the protocols and to those reported for individuals with G551D receiving ivacaftor (Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015). These Phase 3 studies were conducted across a large number of CF centres in North America, Europe and Australia, and the results are applicable to pwCF who are homozygous for F508del in these regions with mild to moderate lung disease.
The Phase 3 studies of lumacaftor‐ivacaftor enrolled children and adults (age range 6 to 64 years) (Ratjen 2017; TRAFFIC 2015; TRANSPORT 2015)). For lumacaftor‐ivacaftor, results were consistent across age groups, although for the study of 6 to 11 year olds the absolute change in FEV1 was less marked, MD 2.4% (95% CI 0.4 to 4.4) (Ratjen 2017). The primary outcome for the study on children was change in LCI; although this measure is a well‐validated research outcome assessing respiratory function, it is not yet routinely used in clinical practice. The children allocated to lumacaftor‐ivacaftor demonstrated a statistically significant reduction in LCI compared to those receiving placebo, least squares mean difference ‐1.09 (95% CI ‐1.43 to ‐0.75). Although this difference is statistically significant, it is difficult to assess the clinical relevance of this result for young pwCF.
The tezacaftor‐ivacaftor study enrolled adults and young pwCF (from 12 years and with a mean age of approximately 26 years) (Taylor‐Cousar 2017).
This review has examined evidence for efficacy and safety. We have not included outcomes relating to cost‐effectiveness.
Quality of the evidence
Monotherapy versus placebo
The quality of the evidence of the early phase studies included in this review were difficult to appraise and interpret due to limited reporting and complex study design, including numerous dose regimens. For the early phase studies of 4PBA, CPX an N6022; relevant outcome data to this review were limited and the risk of bias for various domains was difficult to judge.
The quality of the evidence from a short‐term study of cavosonstat compared to placebo was low to very low due to concerns over unclear methodological design, indirectness (lack of applicability of results to children) and limited outcome data resulting in wide CIs around effect sizes (Table 2).
Combination therapy versus placebo
The studies included in this review were difficult to appraise and interpret due to very complex study designs that incorporated several drug doses.
We judged the quality of the evidence from the three large multicentre RCTs of lumacaftor‐ivacaftor combination therapy to be moderate to high (Table 3). Not all outcomes were reported in the final study publication; some were available in the online supplement, some were extrapolated from graphical figures and others were available on the NIH database (ClinicalTrials.gov). Although the time‐point for assessment of the primary outcome changed after the data had been collected, from FEV1 at 24 weeks to an aggregate of 16 and 24 weeks (which was in fact a larger treatment effect), we did not judge this to reflect a high risk of bias. This was because the results at 24 weeks were also statistically significant, and the amended protocol states that "This change was made during final review by senior management. It is important to note that this change was made based on theoretical considerations alone. No data analysis was used to support this change and, in fact, the spirometry data were maintained at the designated vendor and were not available to any Vertex personnel".
We judged the quality of the evidence from an additional large multicentre RCT of lumacaftor‐ivacaftor combination therapy to be moderate to low (Table 4). The study recruited children aged 6 to 11 years, so results are not applicable to other age groups. Not all outcomes were reported in the final study report and additional data could not be extracted from graphical figures. Furthermore the analysis approach taken within this review adjusted for earlier time points in the analysis at 24 weeks, therefore results should be interpreted as the treatment effect averaged from each study visit until week 24.
We judged the quality of the evidence from two tezacaftor‐ivacaftor combination therapy studies (including one large multicentre RCT) to be of moderate quality (Table 6); results are not applicable to children under the age of 12 and some results are not applicable to individuals homozygous for F508del. Furthermore, in the large tezacaftor‐ivacaftor combination study, a number of outcomes which were not presented in the summary of findings table of this review were recorded according to the study protocol, but not presented in the published study report (Taylor‐Cousar 2017).
We judged the quality of the evidence from a small, very short‐term study of lumacaftor‐ivacaftor combination therapy and from a small study of lumacaftor monotherapy to be very low to moderate due to concerns over incomplete outcome data, selective reporting and limited outcome data resulting in wide CIs around effect sizes (Table 1; Table 5).
Potential biases in the review process
The review authors conducted a comprehensive literature search of online journal databases using the Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register and the ongoing online trials databases (Appendix 1) and also of manual searching of journal conference abstracts. Two authors individually applied the inclusion and exclusion criteria to the identified studies and excluded studies that were not relevant. Included studies were appraised more thoroughly and data extracted independently using a pre‐determined form. The authors assessed the risk of bias of the included studies and if they failed to reach a consensus on the risk of bias, a third author (KWS) arbitrated. The analyses were undertaken by two review authors (SP, IS) and checked for appropriateness by the review statistician (SN). This approach minimized the risks of bias in the review process.
None of the authors have received direct or indirect payments from the companies responsible for the development of any agents included in this review; however, KWS has attended and has organised educational events that have received financial support from Vertex, the company that has developed and is evaluating some of the agents included in this review.
Not all results were reported in a format from which they could be accurately extracted, and so we have had to extrapolate data for several important outcomes from graphs and figures. We are awaiting confirmation from Vertex that these estimates are accurate.
This review has assessed all available published study data. Study authors were contacted for relevant unpublished information and individual participant data. None have been made available to date. We are not aware of any unpublished trials.
Agreements and disagreements with other studies or reviews
The National Institute for Health and Care Excellence (NICE) in the UK has undertaken a health technology appraisal for lumacaftor‐ivacaftor which was published on 27 July 2016 (NICE 2016). The appraisal included the TRAFFIC, TRANSPORT and PROGRESS studies (PROGRESS 2017; TRAFFIC 2015; TRANSPORT 2015); the report concluded that the quality of these studies was generally good and that the results were generalisable to a UK population with mild‐moderate disease severity. The evidence review group (ERG) noted that there were statistically significant effects on key outcomes compared with standard care alone, but it was unclear how clinically significant the effects were. Adverse event data were recorded as per the published papers, but withdrawals due to hypertension and the overall increase in blood pressure in participants receiving 400 mg twice a day were not recorded. In addition, the ERG examined a detailed cost‐effectiveness assessment (including an estimate of incremental cost‐effectiveness ratio) and concluded, on that basis, that lumacaftor‐ivacaftor is not recommended, within its marketing authorisation, for treating CF in people 12 years and older who are homozygous for F508del mutation of the CFTR gene.
An evaluation of the safety of lumacaftor and ivacaftor highlighted the finding of "transaminitis" (raised transaminases) in ivacaftor and combination studies (Talamo Guervara 2017). In addition, the review reported non‐congenital cataracts identified in pre‐clinical studies and in children taking ivacaftor and combined therapy. The review also highlighted that lumacaftor is a strong inducer of the liver enzyme, cytochrome P3A and the implications for co‐prescribing of drugs metabolised through this route.
Authors' conclusions
Implications for practice.
Data from the Phase 3 studies of both lumacaftor and tezacaftor combined with ivacaftor suggest that these compounds are influencing the basic defect associated with the F508del mutation; with small but consistent and statistically significant improvements in key clinical outcomes. The size of these studies and the low‐ to moderate‐quality evidence from the studies gives us good confidence in the validity of these results. Overall the drugs appeared well‐tolerated, but there were some important adverse effects, in particular with the lumacaftor‐ivacaftor combination. The adverse events noted with lumacaftor‐ivacaftor were not recorded in the tezacaftor‐ivacaftor study and, from the available data, this combination appears to have a better safety profile.
From our appraisal of the available data, the combination of lumacaftor or tezacaftor with ivacaftor can be considered for the management of people with cystic fibrosis (pwCF) homozygous for F508del, with mild to moderate lung disease. These results cannot be translated automatically to pwCF with more severe lung disease. This agrees with the NICE appraisal of lumacaftor‐ivacaftor, although the evidence review group did not support the routine use of lumacaftor‐ivacaftor on the basis of their detailed cost‐effectiveness assessment (NICE 2016). Lumacaftor‐ivacaftor (trade name, Orkambi™) has been approved for use in other countries, including the USA, Canada and Germany. It is useful to compare the results of the available studies of correctors with studies examining ivacaftor for pwCF with one class 3 mutation (G551D). The absolute change in forced expiratory volume in one second (FEV1) after ivacaftor therapy compared to placebo was 16.9% (95% confidence interval 13.6 to 20.2) (Patel 2015).
The evidence from this systematic review demonstrates that tezacaftor‐ivacaftor for treating pwCF with two F508del mutations and mild to moderate lung disease results in similar clinical benefit but with an improved safety profile compared to lumacaftor‐ivacaftor.
In children younger than 12 years of age, there are no data to assess tezacaftor‐ivacaftor. In a study of lumacaftor‐ivacaftor in children aged 6 to 11 years, there was some evidence of clinical efficacy (decreasing lung clearance index (LCI) value), but the clinical relevance of these changes is not clear. The reports of increased adverse events for lumacaftor‐ivacaftor in this age group and in older pwCF should be taken into account when considering this intervention for this age group until further data or an alternative agent (e.g., tezacaftor‐ivacaftor) is available.
Implications for research.
It is important that post‐market surveillance is undertaken for all agents that correct the F508del mutation. It is clear that lumacaftor‐ivacaftor is associated with a number of important adverse effects, some of which necessitated the withdrawal of therapy. Although tezacaftor‐ivacaftor appears to have a more favourable safety profile there are no data in children and close monitoring is required for all individuals on this drug combination.
Evidence of efficacy for the population of pwCF who have two copies of the F508del mutation cannot be automatically translated to pwCF who have one copy of F508del or another class II mutation (such as G85E) and research strategies need to be developed that assess impact on these individuals. Small numbers of potential participants for these studies makes this a challenge.
As new mutation‐specific therapies emerge, it is important that lessons learnt from this review are taken on board. Investigators should report clearly on methodological approaches to reduce the risk of bias, in particular with regards to random sequence generation, allocation concealment and blinding; they should also ensure that randomisation is maintained when analysing data. It is important that future studies examine and clearly report on outcomes relevant to pwCF and their families.
With novel therapies and approaches, reporting of adverse events is critical and this should be undertaken in a robust, comprehensive and consistent manner.
What's new
| Date | Event | Description |
|---|---|---|
| 28 August 2018 | Amended | Wording in Background section of the Abstract revised with regard to the populations where CF occurs. |
Acknowledgements
The review authors would like to acknowledge help and support of the CF and Genetic Disorders Review Group in particular Nikki Jahnke and Tracey Remmington. Toby Lasserson and Newton Opiyo, from the Cochrane Editorail Unit, provided valuable and precise editorial oversight. We would also like to acknowledge the study investigators, Professors Elborn, Boyle, Clancy, McCarty, Ratjen, Rubenstein and Zeitlin, who engaged constructively with this review and thank them for retrieving additional requested information.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Electronic search strategies
| Database/resource | Date searched | Seach strategy |
| US National Institutes of Health database (clinicaltrials.gov/) |
25 January 2018 | Cystic fibrosis AND (VX OR corrector) |
| WHO ICTRP (www.who.int/ictrp/en/) |
25 January 2018 | Cystic fibrosis AND (VX OR corrector) |
| European Medicines Agency (www.clinicaltrialsregister.eu/) |
25 January 2018 | Cystic fibrosis AND (VX OR corrector) |
Data and analyses
Comparison 1. Lumacaftor versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 14 days | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐4.13, 0.33] |
| 2 Adverse effects: 100 mg and 200 mg lumacaftor groups (combined data) versus placebo | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 2.1 Cough | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.28 [0.28, 5.92] |
| 2.2 Headache | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.13 [0.16, 8.04] |
| 2.3 Rales | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 3.2 [0.18, 57.82] |
| 2.4 Productive cough | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.79 [0.27, 11.98] |
| 2.5 Dyspnoea | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 3.2 [0.18, 57.82] |
| 2.6 Pulmonary exacerbation | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.5 [0.16, 14.31] |
| 2.7 Fatigue | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.21 [0.12, 12.09] |
| 2.8 Fever | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.5 [0.16, 14.31] |
| 2.9 Nasal congestion | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.58 [0.07, 4.93] |
| 2.10 Wheezing | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.13 [0.01, 2.91] |
| 2.11 Diarrhoea | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.27 [0.02, 3.31] |
| 2.12 Oropharyngeal pain | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.27 [0.02, 3.31] |
| 2.13 Upper respiratory tract infection | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.45 [0.07, 31.52] |
| 2.14 Sinus congestion | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.21 [0.01, 5.55] |
| 2.15 Respiration abnormal | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 4.85 [0.10, 243.04] |
| 2.16 Haemoptysis | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.44 [0.03, 6.54] |
| 2.17 Constipation | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.54 [0.04, 147.25] |
| 2.18 Abdominal pain | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.46 [0.01, 18.95] |
| 2.19 Myalgia | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.46 [0.01, 18.95] |
| 2.20 Post‐tussive vomiting | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.54 [0.04, 147.25] |
| 2.21 Nausea | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.48 [0.02, 106.10] |
| 2.22 Nasopharyngitis | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 3.66 [0.07, 193.30] |
| 2.23 Dizziness | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 3.66 [0.07, 193.30] |
| 2.24 Back pain | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.48 [0.02, 106.10] |
| 2.25 Upper abdominal pain | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.45 [0.07, 31.52] |
| 2.26 Sputum abnormal | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.48 [0.02, 106.10] |
| 2.27 Epistaxis | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.94 [0.04, 24.27] |
| 2.28 C‐reactive protein increased | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.54 [0.04, 147.25] |
| 2.29 Paranasal sinus hypersecretion | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 2.30 Lung hyperinflation | 1 | 53 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.54 [0.04, 147.25] |
| 3 Adverse effects: 200 mg lumacaftor group versus placebo at 14 days | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 3.1 Cough | 1 | 62 | Odds Ratio (M‐H, Fixed, 99% CI) | 3.43 [0.19, 60.73] |
| 3.2 Pulmonary exacerbation | 1 | 62 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.72 [0.05, 156.17] |
| 3.3 Oropharyngeal pain | 1 | 62 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.72 [0.05, 156.17] |
| 3.4 Nasal congestion | 1 | 62 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 3.5 Dizziness | 1 | 62 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 3.6 Prothrombin time prolonged | 1 | 62 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.59 [0.02, 113.01] |
| 3.7 Upper respiratory tract infection | 1 | 62 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 4 Adverse effects requiring study drug discontinuation at day 28 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 25 mg lumacaftor | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.11, 78.81] |
| 4.2 50 mg lumacaftor | 1 | 35 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.11, 78.81] |
| 4.3 100 mg lumacaftor | 1 | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.18 [0.12, 83.76] |
| 4.4 200 mg lumacaftor | 1 | 36 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.84 [0.11, 74.42] |
| 5 Change from baseline in sweat chloride concentration after 28 days [mmol/L] | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 100 mg lumacaftor | 1 | 34 | Mean Difference (Fixed, 95% CI) | ‐6.13 [‐12.25, ‐0.01] |
| 5.2 200 mg lumacaftor | 1 | 36 | Mean Difference (Fixed, 95% CI) | ‐8.21 [‐14.30, ‐2.12] |
| 6 Sweat chloride concentration (mmol/L) (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 At 14 days | 1 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐2.75 [‐7.65, 2.15] |
Comparison 2. Cavosonstat (N91115) (200 mg twice daily) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CFQR respiratory domain: absolute change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 28 days | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 3.8 [‐11.30, 18.90] |
| 2 CFQR eating domain: absolute change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 28 days | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 2.4 [‐2.75, 7.55] |
| 3 Adverse events occurring in > 10% of participants | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 3.1 Cough | 1 | 26 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.05 [0.13, 8.17] |
| 3.2 Pulmonary exacerbation | 1 | 26 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.26 [0.00, 20.03] |
| 3.3 Chest discomfort | 1 | 26 | Odds Ratio (M‐H, Fixed, 99% CI) | 5.0 [0.08, 308.20] |
| 3.4 Fatigue | 1 | 26 | Odds Ratio (M‐H, Fixed, 99% CI) | 3.0 [0.13, 71.47] |
| 4 Sweat chloride | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 28 days | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐3.30 [‐9.13, 2.53] |
Comparison 3. N6022 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (relative change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 5 mg/day N6022 | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐5.31, 1.31] |
| 1.2 10 mg/day N6022 | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐1.7 [‐4.73, 1.33] |
| 1.3 20 mg/day N6022 | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐5.28, 0.88] |
| 1.4 40 mg/day N6022 | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.06, 1.06] |
| 2 Treatment‐emergent adverse events (mild) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 5 mg/day N6022 | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.10, 2.30] |
| 2.2 10 mg/day N6022 | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.28, 7.64] |
| 2.3 20 mg/day N6022 | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.12, 2.88] |
| 2.4 40 mg/day N6022 | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.34, 4.59] |
| 3 Treatment‐emergent adverse events (moderate) | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.04] |
| 3.1 5 mg/day N6022 | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.18, 4.90] |
| 3.2 10 mg/day N6022 | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.20, 5.87] |
| 3.3 20 mg/day N6022 | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.53, 13.85] |
| 3.4 40 mg/day N6022 | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.04, 1.48] |
| 4 Treatment‐emergent adverse events (serious / severe) | 1 | 123 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.35, 5.41] |
| 4.1 5 mg/day N6022 | 1 | 29 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.5 [0.35, 57.11] |
| 4.2 10 mg/day N6022 | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.02, 17.51] |
| 4.3 20 mg/day N6022 | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.02, 17.51] |
| 4.4 40 mg/day N6022 | 1 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.25] |
Comparison 4. CPX versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events occurring in more than 3% of participants in all treatment groups (combined data) versus placebo | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 1.1 Abdominal pain | 1 | 37 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.45 [0.01, 24.92] |
| 1.2 Asthenia | 1 | 37 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.65 [0.01, 39.69] |
| 1.3 Headache | 1 | 37 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.33 [0.01, 17.72] |
| 1.4 Pain | 1 | 37 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.45 [0.01, 24.92] |
| 1.5 Diarrhoea | 1 | 37 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.65 [0.01, 39.69] |
| 1.6 Dizziness | 1 | 37 | Odds Ratio (M‐H, Fixed, 99% CI) | 9.33 [0.32, 268.92] |
| 1.7 Lung disease | 1 | 37 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.45 [0.01, 24.92] |
| 1.8 Rhinitis | 1 | 37 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.45 [0.01, 24.92] |
Comparison 5. 4PBA versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects after 1 week | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 1.1 Bad taste in mouth | 1 | 18 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.44 [0.01, 13.44] |
| 1.2 Diarrhoea | 1 | 18 | Odds Ratio (M‐H, Fixed, 99% CI) | 3.35 [0.04, 267.31] |
| 2 Participants requiring study drug termination or a reduced dosage | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 30 g 4PBA | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.2. Analysis.
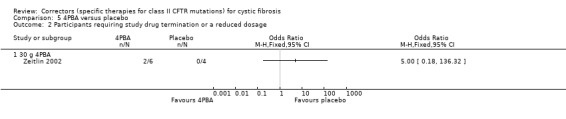
Comparison 5 4PBA versus placebo, Outcome 2 Participants requiring study drug termination or a reduced dosage.
Comparison 6. Lumacaftor (600 mg once daily) plus ivacaftor (250 mg twice daily) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life ‐ Euro Quality of Life Scale (EuroQol) 5‐Dimension‐3 Level (EQ‐5D‐3L) Index Score (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 6 months | 2 | 715 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 2 Quality of life ‐ CFQ‐R respiratory domain (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 28 days | 2 | 739 | Mean Difference (IV, Fixed, 95% CI) | 3.32 [1.13, 5.51] |
| 2.2 At 6 months | 2 | 725 | Mean Difference (IV, Fixed, 95% CI) | 3.04 [0.76, 5.32] |
| 3 Quality of life ‐ EQ‐5D‐3L VAS Score (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 months | 2 | 712 | Mean Difference (IV, Fixed, 95% CI) | 2.24 [0.18, 4.31] |
| 4 FEV1 % predicted (relative change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 6 months | 2 | 720 | Mean Difference (IV, Fixed, 95% CI) | 5.63 [3.80, 7.47] |
| 5 FEV1 % predicted (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 28 days | 2 | 739 | Mean Difference (IV, Fixed, 95% CI) | 2.32 [1.34, 3.31] |
| 5.2 At 6 months | 2 | 720 | Mean Difference (IV, Fixed, 95% CI) | 3.34 [2.30, 4.38] |
| 6 Adverse events by end of study (at 6 months) | 2 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 6.1 Any adverse event | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.00 [0.37, 2.71] |
| 6.2 Discontinuation due to an adverse event | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.38 [0.67, 8.50] |
| 6.3 At least 1 serious adverse event | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.73 [0.47, 1.13] |
| 6.4 Infective pulmonary exacerbation | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.66 [0.45, 0.97] |
| 6.5 Cough | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.72 [0.49, 1.08] |
| 6.6 Headache | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.00 [0.59, 1.68] |
| 6.7 Haemoptysis | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.04 [0.60, 1.81] |
| 6.8 Diarrhoea | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.18 [0.61, 2.28] |
| 6.9 Abnormal respiration | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.91 [0.94, 3.88] |
| 6.10 Increased sputum | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.74 [0.44, 1.24] |
| 6.11 Dyspnoea | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.05 [1.10, 3.83] |
| 6.12 Nasopharyngitis | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.55 [0.27, 1.10] |
| 6.13 Oropharyngeal pain | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.52 [0.80, 2.89] |
| 6.14 Abdominal pain | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.80 [0.39, 1.62] |
| 6.15 Fatigue | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.03 [0.51, 2.08] |
| 6.16 Nausea | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.04 [0.51, 2.11] |
| 6.17 Pyrexia | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.03 [0.54, 1.98] |
| 6.18 Nasal congestion | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.73 [0.39, 1.35] |
| 6.19 Upper respiratory tract infection | 2 | 739 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.21 [0.54, 2.70] |
| 7 Time to first pulmonary exacerbation | 2 | 739 | Hazard Ratio (Fixed, 95% CI) | 0.70 [0.57, 0.87] |
| 8 Rate of exacerbations | 2 | 739 | Rate Ratio (Fixed, 95% CI) | 0.70 [0.57, 0.87] |
| 9 Weight (kg) (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 At 6 months | 2 | 725 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [0.42, 1.18] |
| 10 BMI (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 At 28 days | 2 | 739 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.09] |
| 10.2 At 6 months | 2 | 725 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [0.16, 0.43] |
Comparison 7. Lumacaftor (400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life ‐ Euro Quality of Life Scale (EuroQol) 5‐Dimension‐3 Level (EQ‐5D‐3L) Index Score (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 6 months | 2 | 708 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
| 2 Quality of life ‐ CFQ‐R respiratory domain (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 28 days | 2 | 740 | Mean Difference (IV, Fixed, 95% CI) | 4.13 [1.94, 6.31] |
| 2.2 At 6 months | 2 | 720 | Mean Difference (IV, Fixed, 95% CI) | 2.18 [‐0.11, 4.47] |
| 3 Quality of life ‐ EQ‐5D‐3L VAS Score (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 months | 2 | 710 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [0.25, 4.36] |
| 4 FEV1 % predicted (relative change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 6 months | 2 | 715 | Mean Difference (IV, Fixed, 95% CI) | 4.77 [2.93, 6.61] |
| 5 FEV1 % predicted (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 28 days | 2 | 740 | Mean Difference (IV, Fixed, 95% CI) | 2.42 [1.43, 3.40] |
| 5.2 At 6 months | 2 | 715 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [1.75, 3.84] |
| 6 Adverse events by end of study (at 6 months) | 2 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 6.1 Any adverse event | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.77 [0.30, 1.96] |
| 6.2 Discontinuation due to an adverse event | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.91 [0.85, 10.03] |
| 6.3 At least 1 serious adverse event | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.52 [0.33, 0.83] |
| 6.4 Infective pulmonary exacerbation | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.57 [0.39, 0.84] |
| 6.5 Cough | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.58 [0.39, 0.88] |
| 6.6 Headache | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.00 [0.59, 1.68] |
| 6.7 Haemoptysis | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.00 [0.58, 1.74] |
| 6.8 Diarrhoea | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.51 [0.80, 2.85] |
| 6.9 Abnormal respiration | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.50 [0.71, 3.14] |
| 6.10 Increased sputum | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.73 [0.44, 1.22] |
| 6.11 Dyspnea | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.75 [0.93, 3.32] |
| 6.12 Nasopharyngitis | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.23 [0.68, 2.21] |
| 6.13 Oropharyngeal pain | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.38, 1.63] |
| 6.14 Abdominal pain | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.03 [0.53, 2.01] |
| 6.15 Fatigue | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.19 [0.60, 2.35] |
| 6.16 Nausea | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.74 [0.91, 3.34] |
| 6.17 Pyrexia | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.97 [0.50, 1.87] |
| 6.18 Nasal congestion | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.51 [0.26, 1.02] |
| 6.19 Upper respiratory tract infection | 2 | 738 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.94 [0.93, 4.08] |
| 7 Time to first pulmonary exacerbation | 2 | 740 | Hazard Ratio (Fixed, 95% CI) | 0.61 [0.49, 0.76] |
| 8 Rate of exacerbations | 2 | 740 | Rate Ratio (Fixed, 95% CI) | 0.61 [0.49, 0.76] |
| 9 Weight (kg) (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 At 6 months | 2 | 723 | Mean Difference (IV, Fixed, 95% CI) | 0.65 [0.27, 1.03] |
| 10 BMI (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 At 28 days | 2 | 740 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.06, 0.10] |
| 10.2 At 6 months | 2 | 723 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [0.12, 0.39] |
Comparison 8. Lumacaftor (600 mg once daily or 400 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life ‐ Euro Quality of Life Scale (EuroQol) 5‐Dimension‐3 Level (EQ‐5D‐3L) Index Score (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 6 months | 2 | 1061 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.01, 0.01] |
| 2 Quality of life ‐ CFQ‐R respiratory domain (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 28 days | 2 | 1108 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [1.81, 5.58] |
| 2.2 At 6 months | 2 | 1076 | Mean Difference (IV, Fixed, 95% CI) | 2.62 [0.64, 4.59] |
| 3 Quality of life ‐ EQ‐5D‐3L VAS Score (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 6 months | 2 | 1060 | Mean Difference (IV, Fixed, 95% CI) | 2.28 [0.50, 4.06] |
| 4 FEV1 % predicted (relative change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 6 months | 2 | 1072 | Mean Difference (IV, Fixed, 95% CI) | 5.21 [3.61, 6.80] |
| 5 FEV1 % predicted (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 28 days | 2 | 1108 | Mean Difference (IV, Fixed, 95% CI) | 2.37 [1.52, 3.22] |
| 5.2 At 6 months | 2 | 1072 | Mean Difference (IV, Fixed, 95% CI) | 3.07 [2.17, 3.97] |
| 6 Adverse events by end of study (at 6 months) | 2 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 6.1 Any adverse event | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.87 [0.38, 2.02] |
| 6.2 Discontinuation due to an adverse event | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.65 [0.83, 8.45] |
| 6.3 At least 1 serious adverse event | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.62 [0.42, 0.91] |
| 6.4 Infective pulmonary exacerbation | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.62 [0.44, 0.86] |
| 6.5 Cough | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.65 [0.46, 0.92] |
| 6.6 Headache | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.00 [0.64, 1.57] |
| 6.7 Haemoptysis | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.02 [0.63, 1.65] |
| 6.8 Diarrhea | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.34 [0.76, 2.37] |
| 6.9 Abnormal respiration | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.70 [0.89, 3.26] |
| 6.10 Increased sputum | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.73 [0.47, 1.14] |
| 6.11 Dyspnea | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.90 [1.08, 3.35] |
| 6.12 Nasopharyngitis | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.87 [0.51, 1.50] |
| 6.13 Oropharyngeal pain | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.14 [0.63, 2.06] |
| 6.14 Abdominal pain | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.91 [0.51, 1.65] |
| 6.15 Fatigue | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.11 [0.61, 2.03] |
| 6.16 Nausea | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.38 [0.76, 2.51] |
| 6.17 Pyrexia | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.00 [0.57, 1.76] |
| 6.18 Nasal congestion | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.62 [0.36, 1.07] |
| 6.19 Upper respiratory tract infection | 2 | 1108 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.57 [0.79, 3.11] |
| 7 Weight (kg) (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 At 6 months | 2 | 1081 | Mean Difference (IV, Fixed, 95% CI) | 0.72 [0.39, 1.05] |
| 8 BMI (absolute change from baseline) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 At 28 days | 2 | 1108 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.08] |
| 8.2 At 6 months | 2 | 1081 | Mean Difference (IV, Fixed, 95% CI) | 0.27 [0.16, 0.39] |
Comparison 9. Lumacaftor (200 mg once daily) for 21 days plus ivacaftor (150 mg twice daily) for days 15 to 21 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 14 days (before addition of ivacaftor) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐4.39, 0.79] |
| 1.2 At 21 days | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 2.80 [‐1.39, 6.99] |
| 2 Adverse events occurring in 10% or more participants (from days 15 ‐ 21) | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 2.1 Cough | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.06 [0.14, 8.09] |
| 2.2 Pulmonary Exacerbation | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.22 [0.08, 58.11] |
| 2.3 Oropharyngeal pain | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.5 [0.02, 13.07] |
| 2.4 Nasal congestion | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.5 [0.02, 13.07] |
| 2.5 Dizziness | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 5.81 [0.10, 341.36] |
| 2.6 Prothrombin time prolonged | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 5.81 [0.10, 341.36] |
| 2.7 Upper respiratory tract infection | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 5.81 [0.10, 341.36] |
| 3 Sweat chloride concentration (mmol/L) (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 14 days (before addition of ivacaftor) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐8.58, 2.38] |
| 3.2 At 21 days | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐5.0 [‐11.61, 1.61] |
Comparison 10. Lumacaftor (200 mg once daily) for 21 days plus ivacaftor (250 mg twice daily) for days 15 to 21 versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 14 days (before addition of ivacaftor) | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐4.66, 0.66] |
| 1.2 At 21 days | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | 0.2 [‐4.20, 4.60] |
| 2 Adverse events occurring in 10% or more participants (from days 15 ‐ 21) | 1 | 287 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.93 [0.26, 3.33] |
| 2.1 Cough | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.22 [0.01, 4.52] |
| 2.2 Pulmonary Exacerbation | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.05 [0.03, 44.10] |
| 2.3 Oropharyngeal pain | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.06 [0.07, 15.89] |
| 2.4 Nasal congestion | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.68 [0.14, 20.50] |
| 2.5 Dizziness | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 3.31 [0.05, 239.61] |
| 2.6 Prothrombin time prolonged | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 2.7 Upper respiratory tract infection | 1 | 41 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] |
| 3 Sweat chloride concentration (mmol/L) (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 14 days (before addition of ivacaftor) | 1 | 34 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐6.00, 3.20] |
| 3.2 At 21 days | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐10.9 [‐17.60, ‐4.20] |
Comparison 11. Lumacaftor (200 mg twice daily) plus ivacaftor (250 mg twice daily) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life ‐ CFQ‐R respiratory domain (absolute change from baseline) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 up to and including week 24 | 1 | 204 | Mean Difference (Fixed, 95% CI) | 2.5 [‐0.10, 5.10] |
| 2 FEV1 % predicted (absolute change from baseline) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 up to and including 24 weeks | 1 | 204 | Mean Difference (Fixed, 95% CI) | 2.4 [0.40, 4.40] |
| 3 LCI2.5 (absolute change from baseline) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 up to and including 24 weeks | 1 | 204 | Mean Difference (Fixed, 95% CI) | ‐1.1 [‐1.40, ‐0.80] |
| 4 Treatment‐emergent adverse events with incidence > 10% in any treatment group | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | Totals not selected | |
| 4.1 Any adverse event | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Any serious adverse event | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Cough | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Pulmonary exacerbation | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Productive cough | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Nasal congestion | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Oropharyngeal pain | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Pyrexia | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Upper abdominal pain | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Headache | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.11 Upper respiratory tract infection | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Sputum increased | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.13 Abdominal pain | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.14 Nausea | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.15 Rhinorrhoea | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.16 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.17 Fatigue | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 4.18 Respiratory events | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.0 [0.0, 0.0] | |
| 5 Sweat chloride concentration (absolute change from baseline) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 up to and including 4 weeks | 1 | 204 | Mean Difference (Fixed, 95% CI) | ‐20.8 [‐23.40, ‐18.20] |
| 6 BMI (absolute change from baseline) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 6.1 up to and including 24 weeks | 1 | 204 | Mean Difference (Fixed, 95% CI) | 0.1 [‐0.10, 0.30] |
| 7 BMI for age z‐score (absolute change from baseline) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 up to and including 24 weeks | 1 | 204 | Mean Difference (Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
Comparison 12. Lumacaftor (200 mg once daily monotherapy for 14 days) plus ivacaftor (150 mg or 250 mg twice daily for days 15 to 21) for 21 days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 % predicted (absolute change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 21 days | 1 | 59 | Mean Difference (IV, Fixed, 95% CI) | 1.57 [‐2.13, 5.27] |
| 2 Adverse events occurring in 10% or more participants (from days 15 ‐ 21) | 1 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 2.1 Cough | 1 | 61 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.61 [0.09, 4.01] |
| 2.2 Pulmonary exacerbation | 1 | 61 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.62 [0.08, 34.55] |
| 2.3 Oropharyngeal pain | 1 | 61 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.77 [0.07, 9.03] |
| 2.4 Nasal congestion | 1 | 61 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.06 [0.10, 11.04] |
| 2.5 Dizziness | 1 | 61 | Odds Ratio (M‐H, Fixed, 99% CI) | 4.01 [0.08, 209.72] |
| 2.6 Prothrombin time prolonged | 1 | 61 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.79 [0.05, 160.31] |
| 2.7 Upper respiratory tract infection | 1 | 61 | Odds Ratio (M‐H, Fixed, 99% CI) | 2.79 [0.05, 160.31] |
| 3 Sweat chloride concentration (mmol/L) (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 21 days | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐7.95 [‐13.81, ‐2.09] |
Comparison 13. Tezacaftor (100 mg once daily) plus ivacaftor (150 mg twice daily) versus either placebo or ivacaftor (150 mg twice daily) alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CFQ‐R respiratory domain (absolute change from baseline) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 At 4 weeks | 1 | 504 | Mean Difference (Fixed, 95% CI) | 5.1 [2.99, 7.21] |
| 1.2 At 24 weeks | 1 | 504 | Mean Difference (Fixed, 95% CI) | 5.1 [3.20, 7.00] |
| 2 FEV1 % predicted (relative change from baseline) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 At week 4 | 1 | 18 | Mean Difference (Fixed, 95% CI) | 3.72 [‐7.77, 15.21] |
| 2.2 At week 24 | 1 | 504 | Mean Difference (Fixed, 95% CI) | 6.80 [5.30, 8.30] |
| 3 FEV1 % predicted (absolute change from baseline) | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 At week 4 | 2 | 522 | Mean Difference (Fixed, 95% CI) | 3.59 [2.40, 4.78] |
| 3.2 At week 24 | 1 | 504 | Mean Difference (Fixed, 95% CI) | 4.0 [3.10, 4.90] |
| 4 Most common adverse events (occurring in at least 10% of participants in either group) | 2 | Odds Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 4.1 Cough | 2 | 527 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.70 [0.43, 1.15] |
| 4.2 Pulmonary exacerbation | 2 | 527 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.71 [0.44, 1.16] |
| 4.3 Headache | 2 | 527 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.26 [0.68, 2.35] |
| 4.4 Nasal congestion or nasopharyngitis | 2 | 527 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.09 [0.59, 2.02] |
| 4.5 Increased sputum | 1 | 509 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.86 [0.46, 1.63] |
| 4.6 Haemoptysis | 2 | 527 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.74 [0.37, 1.49] |
| 4.7 Pyrexia | 2 | 527 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.89 [0.44, 1.79] |
| 4.8 Oropharyngeal pain | 1 | 509 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.76 [0.35, 1.63] |
| 4.9 Fatigue | 2 | 527 | Odds Ratio (M‐H, Fixed, 99% CI) | 0.51 [0.23, 1.15] |
| 4.10 Nausea | 2 | 527 | Odds Ratio (M‐H, Fixed, 99% CI) | 1.36 [0.60, 3.12] |
| 5 Time to first pulmonary exacerbation | 1 | Hazard Ratio (Fixed, 95% CI) | Subtotals only | |
| 6 Sweat chloride change from baseline | 2 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 6.1 At 4 weeks | 2 | 522 | Mean Difference (Fixed, 95% CI) | ‐9.24 [‐11.12, ‐7.35] |
| 6.2 At 24 weeks | 1 | 504 | Mean Difference (Fixed, 95% CI) | ‐10.1 [‐11.40, ‐8.80] |
| 7 BMI change from baseline | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 7.1 At 4 weeks | 1 | 504 | Mean Difference (Fixed, 95% CI) | ‐0.03 [‐0.13, 0.07] |
| 7.2 At 24 weeks | 1 | 504 | Mean Difference (Fixed, 95% CI) | 0.06 [‐0.08, 0.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boyle 2014.
| Methods | Phase 2 placebo‐controlled RCT with 3 different cohorts. Only cohort 1 was included in this review (n = 62). (The following information will refer to cohort 1 only ‐ see 'Notes'.) Parallel design. Multicentre study conducted at 69 different sites in North America, Europe and Australia. Duration: Cohort 1 lasted 21 days. |
|
| Participants |
Mutation: participants homozygous for F508del mutation. Age: participants in Cohort 1 have a mean age of 29.1 years. Gender split: 50% of participants are male Lung function: all participants in Cohort 1 have a FEV1 ≥ 40% of predicted normal for age, gender, and height and a mean (range) predicted FEV1 of 66.9% (32.8 ‐ 117.1). Sweat chloride levels: participants in Cohort 1 have a mean (range) level of 101.9 mmol/L (87.5 ‐ 121.0). |
|
| Interventions |
Intervention 1: lumacaftor (also known as VX‐809, a CFTR corrector) alone. Intervention 2: lumacaftor in combination with ivacaftor (also known as VX‐770, a CFTR potentiator). Intervention 3: placebo. Cohort 1 (n = 62) Study drug participants: 200 mg lumacaftor once daily for 14 days; then from day 15, participants continue to take 200 mg of lumacaftor in addition to either 150 mg or 250 mg of ivacaftor twice daily until day 21. Placebo participants: placebo for 21 days. |
|
| Outcomes |
Primary outcomes 1. Change in sweat chloride when ivacaftor is administered in combination with lumacaftor* 2. Safety and tolerability assessments based on adverse events, plasma samples (haematology, clinical chemistry, coagulation), urinalysis, ECGs, and vital signs* Secondary outcomes 1. Change in % predicted FEV1* 2. Change in sweat chloride of increasing doses of lumacaftor administered alone* 3. PK parameters (including exposure, concentration and half‐life) of lumacaftor and metabolite in plasma in the presence and absence of ivacaftor 4. PK parameters (including exposure, concentration and half‐life) of ivacaftor and metabolites in plasma in the presence of lumacaftor |
|
| Funding source | Vertex Pharmaceuticals, and the Cystic Fibrosis Foundation Therapeutics Development Network. | |
| Notes | * denotes outcomes relevant to this review. Only data from Cohort 1 were included in this review. This was because data for placebo participants from Cohorts 2 and 3 were pooled, although randomisation in these cohorts occurred separately. This meant that the effects of randomisation in these cohorts were undone. Data for participants in Cohorts 2 and 3 were excluded. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random sequence was generated by a computer by an independent party. |
| Allocation concealment (selection bias) | Low risk | "Site pharmacists dispensed drugs on the basis of an interactive voice response system". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Drug doses were prepared by an independent unmasked pharmacist and dispensed by site pharmacists who were masked to treatment assignment. Participant blinding was maintained by placebo which was matched to intervention by the quantity of tablets and by size, colour, coating and packaging. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Site investigators and the study sponsor were also masked to treatment assignment and to sweat chloride levels ‐ data that could have potentially disclosed treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participant data were excluded from the analysis due to insufficient data, e.g. participants were excluded from the analysis of sweat chloride concentration if insufficient amount of sweat were provided. We judged this trial as having an unclear risk of attrition bias because it was unclear how these exclusions would have affected the balance between groups in baseline characteristics. |
| Selective reporting (reporting bias) | Low risk | We compared the outcomes reported on the US NIH trials registry (www.clinicaltrials.gov) to the outcomes reported in the results of the published paper as the protocol was not available. No selective outcome reporting was identified. |
| Other bias | Low risk | Similar baseline characteristics. |
Clancy 2012.
| Methods | Phase 2a placebo‐controlled RCT. Parallel design. Multicentre study conducted at 25 study locations over North America and Europe. Duration: 28 days. |
|
| Participants |
Mutation: all 89 randomised participants had a confirmed diagnosis of CF and all but 1 were homozygous for the F508del mutation. Age: median (range) age of 26 (18 ‐ 54) years. Gender split: 60% of the participants were males. Lung function: a baseline FEV1 > 40% predicted was an eligibility criteria; but scores ranged from 34.2 to 126.3 with a median of 71. Sweat chloride levels: 103.5 (66.0 ‐ 129.0) mmol/L. Nutritional status: median (range) baseline BMI of 22 (16 ‐ 34). |
|
| Interventions |
Intervention 1: placebo (n = 17). Intervention 2: lumacaftor (VX‐809) 25 mg once daily (n = 18). Intervention 3: lumacaftor 50 mg once daily (n = 18). Intervention 4: lumacaftor 100 mg once daily (n = 17). Intervention 5: lumacaftor 200 mg once daily (n = 19). |
|
| Outcomes |
Primary outcome
1. Evaluation of safety and tolerability of lumacaftor based on adverse events*, haematology, clinical chemistry, urinalysis, ECGs, vital signs, and physical examinations Secondary outcomes 1. Evaluation of the pharmacodynamic impact of lumacaftor on CFTR function 2. Change from baseline in sweat chloride concentration* 3. Nasal potential difference (optional) 4. Spirometry* (FEV1, FEF25‐75%, FVC) 5. Change from baseline in CFQ‐R score* |
|
| Funding source | Vertex Pharmaceuticals, and grants from the NIH. | |
| Notes | * denotes outcomes relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information on how participant or study personnel blinding were maintained. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information on how outcome assessor blind was maintained. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the adverse events table, the total number of participants shown (n = 45) is less than the total number of participants randomised (n = 89). In Figure 1B the number of participants analysed in the outcome 'Change from baseline in sweat chloride' (n = 63) is less than the total number of participants randomised to the intervention (n = 72). Therefore, 9 participants have been unaccounted for. In the table of results of total CFQ‐R scores, 1 participant appears to be excluded from each of the treatment groups. |
| Selective reporting (reporting bias) | High risk | No results have been presented for FEF25‐75% or FVC despite being stated as an outcome. |
| Other bias | Low risk | Baseline characteristics well matched except for the less severe lung disease in 25 mg and placebo groups. |
Donaldson 2014.
| Methods | Double‐blind, placebo‐controlled RCT. Parallel design. Duration: 7 days. Multicentre: 17 sites in USA. |
|
| Participants | 66 participants. Mean (SD) age: 29 (8) years. Gender split: 40 female and 26 male. Disease severity: mean (SD) % predicted FEV1 70 (21)%, and mean (SD) sweat chloride 101 (11) mmol/L. There were no statistically significant differences among the treatment groups at baseline. |
|
| Interventions |
Intervention: 4 sequential ascending doses of N6022 were assessed (5, 10, 20, and 40 mg/day) given by intravenous infusion once daily. Control: placebo (normal saline). |
|
| Outcomes |
Primary outcome Safety and tolerability (over 7 treatment days and 7 days of follow‐up)* Secondary outcomes Change from baseline in % predicted FEV1 (at Day 7)* Change from baseline in biomarkers of CFTR Function measured as sweat chloride mEq/L (at Day 7) |
|
| Funding source | Sponsored by Nivalis Therapeutics. | |
| Notes | * denotes outcomes relevant to this review. 4 sequential ascending doses of N6022 were assessed (5, 10, 20, and 40 mg/day) followed by a confirmatory cohort of participants at the highest dose. An independent Data Monitoring Committee adjudicated dose escalation at the completion of each cohort after review of unblinded safety data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (participant, caregiver, investigator, outcomes assessor) achieved with intravenous administration of placebo (saline) using the same volume as the active drug groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind (participant, caregiver, investigator, outcomes assessor) achieved with intravenous administration of placebo (saline) using the same volume as the active drug groups. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants completed the 7 days of follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No full text publication of the study available. Limited results (without any statistical analysis) available on the ongoing trials database (www.clinicaltrials.gov). Unclear if all relevant information has been made available. |
| Other bias | Low risk | Baseline characteristics across the 5 treatment groups seem fairly well‐balanced despite small numbers in each group. |
Donaldson 2017.
| Methods | Phase 1 double‐blind RCT. Parallel design. Duration: 28 days treatment. Multicentre (10 centres). |
|
| Participants | 51 adults with CF randomised. Mutation: CF homozygous for the F508del‐CFTR mutation. Age: over 18 years. Gender: 32 out of 51 participants were female. Lung function: FEV1 ≥ 40% of predicted normal for age, gender, and height (Hankinson standards). pre‐ or post‐bronchodilator value, at screening. Sweat chloride: ≥ 60 mEq/L. |
|
| Interventions |
Intervention: cavosonstat 2x daily 50 mg, 100 mg, or 200 mg. Control: placebo 2x daily. |
|
| Outcomes |
Primary outcome (no prespecified sample size) Safety (AE and SAE) * Secondary outcomes (at 28 days) Sweat chloride * FEV1* *CFQ‐R |
|
| Funding source | Sponsored by Nivalis Therapeutics. | |
| Notes | * Denotes an outcome relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of method. |
| Allocation concealment (selection bias) | Unclear risk | No description of method. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Likely low risk as double‐blind and placebo‐controlled but further information about this aspect of methodology not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Likely low risk as double‐blind and placebo‐controlled but further information about this aspect of methodology not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants unaccounted for in analysis, but unlikely to have affected result. |
| Selective reporting (reporting bias) | Low risk | Likely low risk ‐ all outcomes reported, but they appear to have been measured at other time‐points that are not reported (7 days and 14 days). |
| Other bias | Unclear risk | "Approximately two‐thirds of CF subjects were female; however, there was a greater proportion of males in the 200 mg BID dose group. Other baseline characteristics were similar across the treatment groups." Unclear if this gender imbalance may have influenced the results. |
Donaldson 2018.
| Methods | Double‐blind, placebo‐controlled Phase 2 RCT, which included a dose‐ranging arm. Parallel design. Duration: 28 days treatment followed by 28 days observation. Multicentre. |
|
| Participants |
Mutation: participants homozygous for F508del mutation, and heterozygous participants with 1 F508del mutation and 1 G551D mutation. Only the 18 heterozygous participants are included in the analysis of this review (this is because the placebo participants in the homozygous arms of the trial were pooled, and this was judged to negate the effects of randomisation). Age: heterozygous participants ‐ active drug arm mean (SD) age 26.6 (7.0) years, placebo arm mean (SD) age 34.5 (7.6) years. Gender split: heterozygous participants ‐ active drug arm 6/14 (43%) participants female; placebo arm 3/4 (75%) female. Lung function, mean (SD): heterozygous participants ‐ active drug arm baseline FEV1 59.1 (16.6)% predicted, placebo arm baseline FEV1 62.6 (12.7)% predicted. Sweat chloride levels, mean (SD): heterozygous participants ‐ active drug arm baseline 52.9 (19.6); placebo arm baseline 56.7 (22.1). |
|
| Interventions |
Intervention: tezacaftor 100 mg/day and ivacaftor 150 mg. Control: ivacaftor 150 mg (heterozygous arm only). |
|
| Outcomes |
Primary outcome
Safety through day 56* Secondary outcomes Absolute change in FEV1 at day 28* Relative change in FEV1 at day 28* Change in CFQ‐R respiratory domain (day 28)* |
|
| Funding source | Vertex Pharmaceuticals and grants from the NIH. | |
| Notes | * Denotes outcomes relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described. |
| Allocation concealment (selection bias) | Unclear risk | Method not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched placebo ‐ double‐blind RCT. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Matched placebo ‐ double‐blind RCT. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants included in analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes appear to have been reported. |
| Other bias | Unclear risk | Baseline characteristics of heterozygous participants somewhat imbalanced across groups (e.g. sex, age, FEV1). However, this imbalance may be due to small numbers (active drug arm n = 14 and placebo arm n = 4) and unclear if the imbalance has influenced results. |
McCarty 2002.
| Methods | Phase 1, placebo‐controlled RCT. Parallel design. Multicentre conducted at 4 sites in North America. Duration: single‐dose assessment. Participants were monitored for 2 days followed up at 1 week. |
|
| Participants |
Mutation: all 37 participants were homozygous for the F508del mutation and were described as having mild CF. Age: 18 years or over; age range 18 ‐ 38 years. Gender split: 21 males and 16 females. Lung function: participants were eligible if they had a baseline FEV1 ≥ 60% predicted and had not endured pulmonary colonisation by a drug resistant organism within 12 months of screening. |
|
| Interventions |
Intervention 1: placebo. Intervention 2: CPX in the following escalating doses: 1 mg CPX; 3 mg CPX; 10 mg CPX; 30 mg CPX; 100 mg CPX; 300 mg CPX; 1000 mg CPX. |
|
| Outcomes |
Primary outcome 1. Safety profile of CPX including occurrence of adverse events* Secondary outcomes 1. Nasal potential difference values 2. Sweat chloride values (mEq/L) * 3. Analysis of blood haemoglobin and serum potassium |
|
| Funding source | SciClone Pharmaceuticals, and grants from the NIH. | |
| Notes | * denotes outcomes relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was insufficient information on how participant or study personnel blinding were maintained. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information on how outcome assessor blind was maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No report of withdrawals and all originally randomised participants were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol not available and outcomes not reported on the ongoing online database (www.clinicaltrials.gov). Reported results corresponded to outcomes listed in methods section. |
| Other bias | Unclear risk | Unclear whether baseline characteristics were well matched. |
PROGRESS 2017.
| Methods | Phase 3 RCT.
Double‐blinded rollover study (participants on active treatment continued their treatment, participants on placebo were randomised to 1 of the 2 active interventions).
Parallel design. Multicentre: 191 sites in 15 countries across North America, Australia and Europe Duration: 96 weeks. |
|
| Participants |
Mutation: homozygous or heterozygous for the F508del mutation. Age: 12 years and older. Gender: both males and females. Confirmed diagnosis of CF. Participants have previously participated in TRAFFIC or TRANSPORT and completed 24 weeks of treatment. |
|
| Interventions |
Intervention 1: 600 mg lumacaftor once daily + 250 mg ivacaftor every 12 hours (continued treatment). Intervention 2: 600 mg lumacaftor once daily + 250 mg ivacaftor every 12 hours (rolled over from placebo). Intervention 3: 400 mg lumacaftor every 12 hours + 250 mg ivacaftor every 12 hours (continued treatment). Intervention 4: 400 mg lumacaftor every 12 hours + 250 mg ivacaftor every 12 hours (rolled over from placebo). |
|
| Outcomes |
Primary outcome measure
Treatment cohorts: safety of long‐term treatment based on AEs, clinical laboratory values (serum chemistry, haematology, coagulation studies, and urinalysis), standard digital ECGs, vital signs, and pulse oximetry at 100 weeks
Secondary outcome measures 1. Absolute change from baseline in % predicted FEV1 at 96 weeks 2. Relative change from baseline in % predicted FEV1 at 96 weeks 3. Absolute change from baseline in CFQ‐R respiratory domain score at 96 weeks 4. Absolute change from baseline in BMI at 100 weeks 5. Number of pulmonary exacerbations starting from the previous study through 96 weeks 8. Event of having at least 1 pulmonary exacerbation in the current study through 96 weeks |
|
| Funding source | Sponsored by Vertex Pharmaceuticals Inc. | |
| Notes | Long‐term extension of the TRAFFIC and TRANSPORT studies in which participants receiving an active treatment continued with this treatment and those receiving placebo were randomised to receive 1 of the 2 active treatments from the TRAFFIC and TRANSPORT. Additional analyses were conducted comparing participants receiving 400 mg lumacaftor every 12 hours + 250 mg ivacaftor every 12 hours to an observational registry cohort of matched controls. These analyses are not reported in this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Only the placebo groups from the previous studies were randomised. Participants randomly assigned (in a 1:1:1 ratio) to 1 of 3 study groups; the randomisation was established by an interactive web response system. Randomisation was stratified according to age (< 18 years versus ≥ 18 years), sex, and pulmonary function (% predicted FEV1 at screening, < 70 versus ≥ 70). |
| Allocation concealment (selection bias) | Low risk | Only the placebo groups from the previous studies were randomised. The randomisation was established by an interactive web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | These were double‐blind studies in which the participant and study team remained blinded to the treatment assignments. Interventions were matched in appearance and packaging. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | These were double‐blind studies in which the participant and study team remained blinded to the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates reported, all participants randomised who received at least 1 dose of study medication were included in analysis. Missing data were investigated in sensitivity analyses. |
| Selective reporting (reporting bias) | Low risk | All listed outcomes reported in the results. |
| Other bias | Low risk | A 'rate of change' analysis showed that baseline characteristics across the groups were well balanced. |
Ratjen 2017.
| Methods | Phase 3, placebo‐controlled RCT. Parallel design. Multicentre: 54 sites in 9 countries (USA, Australia, Belgium, Canada, Denmark, France, Germany, Sweden, and the UK). Duration: 24 weeks. |
|
| Participants |
Mutation: all participants were homozygous for the F508del‐CFTR mutation. Age: eligibility criteria 6 ‐ 11 years, mean (SD) age was 8.8 (1.6) years. Gender split: 83 males and 121 females. Lung function: participants must have a FEV1 (% predicted) of 70 or more, and LCI2.5 of 7.5 or more. |
|
| Interventions |
Intervention: lumacaftor 200 mg every 12 hours in combination with ivacaftor 250 mg every 12 hours. Control: matched placebo. |
|
| Outcomes |
Primary outcome
Mean absolute change in LCI2.5 from baseline at all study visits up to and including week 24* Secondary outcomes Absolute change in BMI up to and including week 24* Absolute change in CFQ‐R respiratory domain score up to and including week 24* Absolute change in LCI5.0 up to and including week 24* Absolute change in sweat chloride up to and including week 24* Absolute change in FEV1 (% predicted) up to and including week 24* Relative change in FEV1 (% predicted) up to and including week 24* Absolute change in BMI‐for‐age z score up to and including week 24* Absolute change in weight up to and including week 24* Absolute change in weight‐for‐age z score up to and including week 24* Absolute change in height up to and including week 24* Absolute change in height‐for‐age z score up to and including week 24* Absolute change in TSQM domains up to and including week 24 Time‐to‐first pulmonary exacerbation up to and including week 24 Event of having at least 1 pulmonary exacerbation up to and including week 24 Number of pulmonary exacerbations up to and including week 24 Number of participants with adverse events and serious adverse events up to week 24* |
|
| Funding source | Vertex Pharmaceuticals. | |
| Notes | * denotes outcomes relevant to this review. Analyses were performed as the absolute change from baseline (including all measurements up to and including week 24, both on‐treatment measurements and measurements after treatment discontinuation) ‐ was based on a MMRM, adjusted for the baseline measurement of the outcome, baseline weight (less than 25 kg versus 25 kg or over and baseline FEV1 (% predicted) (less than 90% compared to 90% or more), with treatment‐by‐visit interaction as fixed effects, participant as a random effect. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation was performed via an interactive web response system, stratified by baseline weight and FEV1 (% predicted). |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed centrally via the interactive web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding was achieved by using placebo tablets visually identical to the test product. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was not stated whether outcome assessment was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were reported and an ITT approach was taken to analysis, with all randomised participants who received at least 1 dose of the study drug included in analysis (1 participant in each group was randomised but did not receive the study drug). |
| Selective reporting (reporting bias) | High risk | Several outcomes which are listed in the methods (e.g. LCI5.0, time‐to‐first pulmonary exacerbation, absolute change in TSQM domains) but are not presented in the results. |
| Other bias | Low risk | Baseline characteristics were similar across the 2 groups. |
Rubenstein 1998.
| Methods | A pilot, placebo‐controlled RCT. Parallel design. Single centre. Duration: 1 week. |
|
| Participants |
Mutation: homozygous for F508del mutation. Age, mean (SD): participants were eligible if 14 years or older, placebo group 24.8 (4.9) years; intervention group: 22.3 (5.9) years. Gender split: placebo group 4 males and 5 females; intervention group 5 males and 4 females. Lung function: baseline mean (SD) FVC % predicted placebo group: 65.5 (18.6); intervention group 73.4 (20.3). Baseline mean (SD) FEV1 % predicted placebo group 47.5 (22.1); intervention group 57.8 (27.2). |
|
| Interventions | 18 participants were allocated to either intervention or placebo group (9 participants in each group). Intervention 1: placebo. Intervention 2: sodium 4‐phenylbutyrate (also known as Buphenyl or 4PBA) 19 g, orally administered, in 3 daily doses of 6 g, 6 g, and 7 g. |
|
| Outcomes | 1. Changes from baseline in nasal potential difference in 1 week 2. Change from baseline in sweat chloride in 1 week* 3. 4BPA metabolites in plasma and urine after 1 week 4. Side effects* |
|
| Funding source | NIH and Cystic Fibrosis Foundation. | |
| Notes | * denotes outcomes relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study article states that "randomization and blinding were performed by the Johns Hopkins Hospital Investigational Drug Pharmacy" but exact method of randomisation has not been described. |
| Allocation concealment (selection bias) | Unclear risk | Study article states that "randomization and blinding were performed by the Johns Hopkins Hospital Investigational Drug Pharmacy" but exact method of allocation concealment has not been described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind. Study article states that "randomization and blinding were performed by the Johns Hopkins Hospital Investigational Drug Pharmacy" but exact method of blinding has not been described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind. Study article states that "randomization and blinding were performed by the Johns Hopkins Hospital Investigational Drug Pharmacy" but exact method of blinding has not been described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed with 9 participants each group, equivalent to the number originally randomised. |
| Selective reporting (reporting bias) | Low risk | Protocol not available and outcome not presented on the ongoing trials database (www.clinicaltrials.gov/). Outcomes reported in the 'methods' were reported in the 'results' so selective reporting bias is low. |
| Other bias | Low risk | "... baseline characteristics between the groups were similar with respect to age, gender, pancreatic sufficiency, and baseline pulmonary function." |
Taylor‐Cousar 2017.
| Methods | Placebo‐controlled RCT. Parallel design. Duration: 24 weeks. Multicentre in North America and Europe. |
|
| Participants | 510 participants diagnosed with CF. Age: inclusion criteria 12 years and older, 23% were aged 12 ‐ 18 years. Mutation: homozygous for F508del. Gender: 49% female. Mean FEV1 at baseline: 60% (9.4% had baseline FEV1 < 40% predicted, 2% had baseline FEV1 > 90% predicted). Mean baseline sweat chloride: 100.5. Mean BMI: 21. |
|
| Interventions |
Intervention: 100 mg tezacaftor 1x daily and 150 mg ivacaftor 2x daily. Control: placebo. |
|
| Outcomes |
Primary outcome Absolute change in FEV1 % predicted (from baseline through week 24) Secondary outcomes Relative change in FEV1 % predicted (from baseline through week 24) Number of pulmonary exacerbations (through week 24) Absolute change in BMI (from baseline at week 24) Absolute change in CFQ‐R respiratory domain score (from baseline through week 24) Safety and tolerability assessments based on AEs, clinical laboratory values (i.e., haematology, serum chemistry, coagulation studies, vitamin levels, lipid panel, and urinalysis), standard 12‐lead ECGs, vital signs, pulse oximetry, and spirometry Time‐to‐first pulmonary exacerbation (through week 24) Absolute change in sweat chloride (from baseline through week 24) Absolute change in BMI z score (from baseline at week 24 (in participants under 20 years of age at time of screening)) Absolute change in body weight (from baseline at week 24) PK parameters of VX‐661, M1‐661, M2‐661, ivacaftor, and M1‐ivacaftor Absolute change in CFRSD severity score (from baseline through week 24) Absolute change in duration of physical activity during the day (from baseline through week 24) Absolute change in duration of sleep time and sleep quality during the night (from baseline through week 24) Absolute change in PSQI score (from baseline through week 24 (in participants under 18 years of age)) Absolute change in QoL assessment (SF‐12) physical, mental, and utility component scores (at weeks 12 and 24) Absolute change in inflammatory mediators (from baseline at week 24) Absolute change in sputum microbiology (from baseline at week 24) Absolute change in serum IRT (from baseline at week 24) |
|
| Funding source | Vertex Pharmaceuticals. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Statistician separate to study team produced a list of randomisation codes and allocations assigned via web‐based interactive system (information provided in online protocol). |
| Allocation concealment (selection bias) | Low risk | Web‐based interactive system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matched placebo ‐ all relevant people blinded (participants and study personnel). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Matched placebo ‐ all relevant people blinded (participants and study personnel). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant who was randomised but did not receive the trial intervention was excluded from efficacy and safety analyses. A further 5 participants who were randomised and received the trial intervention were found to have an ineligible or unconfirmed CFTR genotype were excluded from efficacy analyses. Small numbers of excluded participants (up to 6 out of 510) unlikely to have introduced bias. |
| Selective reporting (reporting bias) | High risk | The following outcomes were measured (according to the protocol), but not reported.
|
| Other bias | Low risk | Final manuscript written with the assistance of medical writers funded by the sponsor, however, this is unlikely to have introduced bias. |
TRAFFIC 2015.
| Methods | Double‐blind, placebo‐controlled Phase 3 RCT. Parallel design. Multicentre: 90 sites in North America, Australia and Europe. Estimated sample size: 559. Duration: 24 weeks. |
|
| Participants | 549 participants with a confirmed diagnosis of CF and stable disease (as judged by the investigator). Mean age (range): treatment arm 1 24.7 (12 ‐ 54) years; treatment arm 2 25.5 (12 ‐ 57) years; placebo 25.0 (12 to 64 years). Gender: 295 (54%) males; 254 (46%) females. Mutation: homozygous for the F508del mutation. Lung function: FEV1 between ≥ 40% and ≤ 90% of predicted normal for age, sex, and height. |
|
| Interventions |
Intervention 1 (n = 183): 600 mg of lumacaftor 1x daily and 250 mg of ivacaftor every 12 hours. Intervention 2 (n = 182): 400 mg of lumacaftor every 12 hours and 250 mg of ivacaftor every 12 hours. Intervention 3 (n = 184): lumacaftor‐matched placebo every 12 hours in combination with ivacaftor‐matched placebo every 12 hours. |
|
| Outcomes |
Primary outcome measure Absolute change in % predicted FEV1 (% predicted) at 24 weeks Secondary outcome measures 1. Relative change in % predicted FEV1 (% predicted) at 24 weeks 2. Absolute change in BMI at 24 weeks 3. Number of pulmonary exacerbations at 24 weeks 4. Absolute change in CFQ‐R respiratory domain score at 24 weeks 5. Absolute change in BMI z score at 24 weeks 6. Absolute change in body weight at 24 weeks 7. Time‐to‐first pulmonary exacerbation at 24 weeks 8. Event of having at least 1 pulmonary exacerbation through week 24 9. Absolute change in EuroQol 3 Level (EQ 5D 3L) at 24 weeks 10. Absolute change in TSQM domains at 24 weeks 11. Safety and tolerability assessments based on adverse events, clinical laboratory values (haematology, serum chemistry, coagulation studies, and urinalysis), standard digital ECGs, ambulatory ECGs, vital signs, and pulse oximetry up to 28 weeks 12. PK parameters of lumacaftor, M28 lumacaftor, ivacaftor, M1 ivacaftor, and M6 ivacaftor at 16 weeks |
|
| Funding source | Sponsored by Vertex Pharmaceuticals Inc. | |
| Notes | Known as TRAFFIC study. The TRAFFIC and TRANSPORT studies were identical with the following exceptions: TRAFFIC included ambulatory ECG screening at days 1 and 15 in approximately 165 participants in the USA; TRANSPORT included additional pharmacokinetics assessments performed in approximately 28 adolescents in the USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned (in a 1:1:1 ratio) to 1 of 3 study groups; the randomisation was established by an interactive web response system. Randomisation was stratified according to age (< 18 years versus ≥ 18 years), sex, and pulmonary function (% predicted FEV1 (% predicted) at screening, < 70 versus ≥ 70). |
| Allocation concealment (selection bias) | Low risk | The randomisation was established by an interactive web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | These were double‐blind studies in which the participant and study team remained blinded to the treatment assignments. Placebo was matched in appearance and packaging. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | These were double‐blind studies in which the participant and study team remained blinded to the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Correct number of participants included in the analysis (i.e. those who received at least one dose of the study drug – ITT). Prior to first dose 10 out of 559 participants withdrew: 2 withdrew from treatment arm 1; 5 withdrew from treatment arm 2; 3 withdrew from placebo group. Post first dose 25 out of 549 participants withdrew (with reasons): 11 from treatment arm 1; 10 withdrew from treatment arm 2; 4 withdrew from placebo group. |
| Selective reporting (reporting bias) | High risk | Additional data available on www.clinicaltrials.gov for outcomes not reported in the final paper such as: 1. absolute change in EQ‐5D‐3L score from baseline at week 24; 2. absolute change in TSQM domains from baseline at week 24; 3. time to first exacerbation; 4. event of having at least one pulmonary exacerbation. Some results had to be extrapolated from graphical figures, we await confirmation from the study sponsor of the accuracy of the results. Investigators state that they measured FVC (which was not listed as an end‐point) and do not report this in the joint paper. |
| Other bias | Low risk | Adherence to study treatment was high and the mean compliance rate (determined by site personnel and ongoing study drug count) was similar across lumacaftor‐ivacaftor and placebo groups (99.1% versus 98.5%). |
TRANSPORT 2015.
| Methods | Double‐blind, placebo‐controlled Phase 3 RCT.
Parallel design. Multicentre: 82 sites in North America, Australia and Europe. Estimated sample size: 563. Duration: 24 weeks. |
|
| Participants | 559 participants with confirmed diagnosis of CF and with stable disease (as judged by the investigator). Mean age (range): treatment arm 1 24.3 (12 ‐ 48) years; treatment arm 2 25.0 (12 ‐ 54) years; placebo 25.7 (12 ‐ 55) years. Gender: 268 (48%) males; 291 (52%) females. Mutation: homozygous for the F508del mutation. Lung function: FEV1 (% predicted) between ≥ 40% and ≤ 90% of predicted normal for age, sex, and height. |
|
| Interventions |
Intervention 1: 600 mg of lumacaftor 1x daily and 250 mg of ivacaftor every 12 hours for 24 weeks. Intervention 2: 400 mg of lumacaftor every 12 hours and 250 mg of ivacaftor every 12 hours for 24 weeks. Intervention 3: placebo. |
|
| Outcomes |
Primary outcome measure Absolute change in % predicted FEV1 (% predicted) at 24 weeks Secondary outcome measures 1. Relative change in % predicted FEV1 (% predicted) at 24 weeks 2. Absolute change in BMI at 24 weeks 3. Number of pulmonary exacerbations at 24 weeks 4. Absolute change in CFQ‐R respiratory domain score at 24 weeks 5. Absolute change in BMI z score at 24 weeks 6. Absolute change in body weight at 24 weeks 7. Time‐to‐first pulmonary exacerbation at 24 weeks 8. Event of having at least 1 pulmonary exacerbation at week 24 9. Absolute change in EQ 5D 3L at 24 weeks 10. Absolute change in TSQM domains at 24 weeks 11. Safety and tolerability assessments based on adverse events, clinical laboratory values (haematology, serum chemistry, coagulation studies, and urinalysis), standard digital ECGs, ambulatory ECGs, vital signs, and pulse oximetry up to 28 weeks 12. PK parameters of lumacaftor, M28 lumacaftor, ivacaftor, M1 ivacaftor, and M6 ivacaftor at 16 weeks |
|
| Funding source | Sponsored by Vertex Pharmaceuticals Inc. | |
| Notes | Known as TRANSPORT study. The TRAFFIC and TRANSPORT studies were identical with the following exceptions: TRAFFIC included ambulatory ECG screening at days 1 and 15 in approximately 165 participants in the USA; TRANSPORT included additional pharmacokinetics assessments performed in approximately 28 adolescents in the USA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned (in a 1:1:1 ratio) to 1 of 3 study groups; the randomisation was established by an interactive web response system. Randomisation was stratified according to age (< 18 years versus ≥ 18 years), sex, and pulmonary function (% predicted FEV1 (% predicted) at screening, < 70 versus ≥ 70). |
| Allocation concealment (selection bias) | Low risk | The randomisation was established by an interactive web response system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | These were double‐blind studies in which the participant and study team remained blinded to the treatment assignments. Placebo was matched in appearance and packaging. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | These were double‐blind studies in which the participant and study team remained blinded to the treatment assignments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Correct number of participants included in the analysis (i.e. those who received at least one dose of the study drug – ITT). Prior to first dose 4 out of 563 participants withdrew: 2 withdrew from treatment arm 1; 2 withdrew from treatment arm 2; none withdrew from placebo group. Post first dose 29 out of 559 participants withdrew (with reasons): 9 from treatment arm 1; 15 withdrew from treatment arm 2; 5 withdrew from placebo group. |
| Selective reporting (reporting bias) | High risk | Additional data available on www.clinicaltrials.gov for outcomes not reported in the final paper such as: 1. absolute change in EQ‐5D‐3L score from baseline at week 24; 2. absolute change in TSQM domains from baseline at week 24; 3.. time to first exacerbation; 4. event of having at least 1 pulmonary exacerbation. Some results had to be extrapolated from graphical figures, we await confirmation from the study sponsor of the accuracy of the results. Investigators state that they measured FVC (which was not listed as an end‐point) and do not report this in the joint paper. |
| Other bias | Low risk | Adherence to study treatment was high and the mean compliance rate (determined by site personnel and ongoing study drug count) was similar across lumacaftor‐ivacaftor and placebo groups (99.1% versus 98.5%). |
Zeitlin 2002.
| Methods | Phase 1/2 placebo‐controlled RCT. Parallel design. Single centre. Duration: 1 week. This study follows on from a pilot study (see above) (Rubenstein 1998). |
|
| Participants | 19 participants were supposed to be randomised in a 3:1 ratio to either study drug or placebo. Randomisation to 40 g group discontinued due to safety reasons; therefore 6 participants were allocated to the 20 g and 30 g groups, 3 to the 40 g group and 4 to the placebo group. It is unclear why only 4 participants were randomised to the placebo group. Mutation: all participants were homozygous for the F508del mutation. Age: mean (SD) age of 28.5 years. Gender split: 12 males and 7 females. Lung function: mean (SD) FEV1 % predicted of 63.7 (17.0). Nutritional status: mean (SD) weight 62.6 (17.0) kg. |
|
| Interventions |
Intervention 1: placebo. Intervention 2: 4‐phenylbutyrate (4PBA) 20 g. Intervention 3: 4‐phenylbutyrate (4PBA) 30 g. Intervention 4: 4‐phenylbutyrate (4PBA) 40 g. All active interventions split into 3 daily doses. |
|
| Outcomes | 1. Nasal epithelial chloride transport measured by nasal potential difference 2. Adverse events* 3. Absolute values in sweat chloride concentrations 4. Hepatic enzyme profile 5. Uric acid levels 6. Change from baseline in pulmonary function (% predicted FEV1 (% predicted)) 7. Semi‐quantitative scoring of sputum microbiology |
|
| Funding source | Cystic Fibrosis Foundation. | |
| Notes | * denotes outcomes relevant to this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but it is not clear how this was conducted. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Escalation to the next dose level was preceded by an examination of the safety profile of the preceding dose. Therefore, study personnel would have been aware of treatment allocation. Also between the 3 intervention groups, participants received a different number of tablets and had different dosage schedules:
Therefore it is unlikely that study personnel blinding and participant blinding was maintained throughout the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was insufficient information on how outcome assessor blind was maintained. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All 19 participants completed the final study visit, but it is unclear how many participants were used in the analysis. |
| Selective reporting (reporting bias) | High risk | Protocol not available. Pulmonary function or microbiology scores at day 7 not reported. |
| Other bias | Low risk | "There were no significant differences in gender, baseline age, weight, or FEV1 (% predicted) among participants in the four groups." |
AE: adverse event BMI: body mass index CF: cystic fibrosis CFQ‐R: Cystic Fibrosis Questionnaire‐Revised CFRSD: Cystic Fibrosis Respiratory Symptom Diary CPX: 8‐cyclopentyl‐1, 3‐dipropylxanthine ECG: electrocardiograms EQ 5D 3L: EuroQol 3 Level FEF25‐75%: forced expiratory flow FEV1: forced expiratory volume in one second FVC: forced vital capacity IRT: immunoreactive trypsinogen ITT: intention to treat IV: intravenous LCI: lung clearance index mEq/L: millequivalents/L MMRM: mixed effects model for repeated measurements NIH: National Institutes of Health PK: pharmacokinetic PSQI: Pittsburgh Sleep Quality Index QoL: quality of life RCT: randomised controlled trial SAE: serious adverse event TSQM: Treatment Satisfaction Questionnaire for Medication
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berkers 2014 | Cross‐over study with participants with gating defects and not a class II defect. |
| Chadwick 1998 | Investigators informed review authors that study was not randomised. |
| Chilvers 2017 | Single‐group assignment. |
| Lebecque 2011 | Cross‐over design. |
| Leonard 2012 | Cross‐over design. |
| NCT01899105 | Cross‐over design. |
| Nick 2014 | Cross‐over study assessing CFTR mutations eligible for treatment with ivacaftor (not relevant to this review). |
| Rowe 2017 | Cross‐over design. |
| Rubenstein 2006 | Participants were not randomised. |
| Sumner 2014 | Gene therapy study, not a mutation‐specific therapy. |
| Ziady 2015 | Laboratory study conducted within cells donated by CF and non‐CF donors. Not a study of people with CF. |
CF: cystic fibrosis CFTR: cystic fibrosis transmembrane conductance regulator
Characteristics of studies awaiting assessment [ordered by study ID]
Hunt 2017.
| Methods | Randomised placebo‐controlled trial. Duration: 4‐week treatment period. |
| Participants | 18 adults with mild to moderate CF who were homozygous for the f508‐del mutation, and who were receiving lumacaftor‐ivacaftor combination therapy. Baseline characteristics: Mean (SD) age: 28.7 (6.6) years. Gender split: 65% (11/18) were female. Mean (SD) % predicted FEV1: 85.2 (12.9)%. Mean (SD) BMI: 23.2 (6.6). |
| Interventions |
Intervention: 40 mg sildenafil 3x daily. Control: matched placebo. |
| Outcomes | Sweat chloride, % predicted FEV1, BMI, exhaled nitric oxide, CFQR, nasal potential difference, LCI. |
| Notes | Presented as a poster at the 31st Annual North American Cystic Fibrosis Conference, Indianapolis, November 2017. Supported by NIH/NHLBI and NIH/NCRR Colorado CTSI Grant Number UL1 RR025780. NCT01132482. |
BMI: body mass index CF: cystic fibrosis CFQR: cystic fibrosis questionnaire ‐ revised FEV1: forced expiratory volume in one second LCI: lung clearance index
Characteristics of ongoing studies [ordered by study ID]
Meijer 2016.
| Trial name or title | Evaluation of (R)‐Roscovitine Safety and Effects in Subjects With Cystic Fibrosis, Homozygous for the F508del‐CFTR Mutation (ROSCO‐CF) |
| Methods | Phase 2, dose‐ranging, multicentre, double‐blind, placebo‐controlled RCT. Duration: 3 months. Multicentre study conducted in France. |
| Participants | 36 adults with CF carrying 2 CF‐causing mutations with at least 1 F508del‐CFTR mutation and chronically infected with Pseudomonas aeruginosa. |
| Interventions | Roscovitine 200 mg or 400 mg compared to placebo. |
| Outcomes |
Primary outcome measure Safety of increasing doses of roscovitine Secondary outcome measures Change in the concentration of P aeruginosa Change in the concentration (CFU/mL) of P aeruginosa in the sputum at each visit from V1 (screening) up to V7 (completion visit) PK parameters: Cmax, time to reach Cmax, AUC (AUCt and AUCInf), half‐life (t1/2) for roscovitine and its M3 metabolite Pro‐ and anti‐inflammatory cytokines Change in C‐reactive protein at each visit from V1 (screening) up to V7 (completion) Change in CFQ‐R at each visit from V1 up to V8 (safety follow‐up) Change in BMI at each visit from V1 (screening) up to V7 (completion visit) Change in FEV1 at each visit from V1 (screening) up to V7 (completion visit) Change in sweat chloride concentration at V2, V3, V5 and V7 (Completion) Change in nasal potential difference at V1 (screening) and V6 (for participants included in Paris Cochin CF Center) Pain questionnaire |
| Starting date | February 2016. |
| Contact information | Principle investigator: Dr Gilles Rault (gilles.rault@perharidy.fr). |
| Notes | Estimated study completion date: October 2017. Estimated primary completion date: August 2017 (Final data collection date for primary outcome measure). |
NCT02070744.
| Trial name or title | Study to Evaluate Safety and Efficacy of VX‐661 in Combination With Ivacaftor in Subjects With Cystic Fibrosis, Homozygous for the F508del‐CFTR Mutation |
| Methods | Double‐blind, placebo‐controlled, 3‐part Phase 2 RCT. Parallel design. Multicentre: 20 sites. Sample size: expected to enrol 40 participants. Duration: 12 weeks of treatment. |
| Participants | Age: 18 years or older. Gender: both male and female. Mutation: homozygous for the F508del mutation. Lung function: FEV1 ≥ 40% and ≤ 90% of predicted normal for age, sex, and height. Participants must have stable CF disease as judged by the investigator. |
| Interventions |
Experimental Group 1 Treatment: VX‐661 + ivacaftor (every 12 hours schedule). Control: VX‐661 placebo + ivacaftor placebo (every 12 hours schedule). Experimental Group 2 Treatment: VX‐661 + ivacaftor (once daily and schedule). Control: VX‐661 placebo + ivacaftor placebo (once daily and schedule). |
| Outcomes |
Primary outcome measure 1. Safety as determined by AEs, physical examination, clinical laboratory values, standard digital ECGs, vital signs and pulse oximetry at 16 weeks Secondary outcome measures 1. Absolute change in % predicted FEV1 at 12 weeks 2. Relative change in % predicted FEV1 at 12 weeks 3. Absolute change in body weight at 12 weeks 4. Absolute change in BMI at 12 weeks 5. Absolute change in the respiratory domain of the CFQ‐R at 12 weeks 6. PK parameters estimates of VX‐661 and ivacaftor and their respective metabolites, derived from plasma concentration‐time data at 16 weeks 7. Absolute change in sweat chloride at 12 weeks |
| Starting date | March 2014. |
| Contact information | No contact information provided. |
| Notes | This study is listed as completed but no results are available on www.clinicaltrials.gov. Sponsored by Vertex Pharmaceuticals Inc. |
NCT02323100.
| Trial name or title | Glycerol Phenylbutyrate Corrector Therapy For CF (Cystic Fibrosis) (GPBA) |
| Methods | Double‐blind, placebo‐controlled, 3‐part Phase 2 RCT. Parallel design. Multicentre: 3 sites. Sample size: expected to enrol 36 participants. Duration: 7 days of treatment. |
| Participants |
Inclusion criteria Age/gender: male or female ≥ 18 years of age. Mutation: homozygous for F508del, and taking pancreatic enzyme replacement therapy. Lung function: FEV1 > 30% of predicted normal for age, gender, and height (Hankinson standards). |
| Interventions | Low‐dose glycerol phenylbutyrate versus high‐dose glycerol phenylbutyrate versus placebo. |
| Outcomes |
Primary outcome Change in average measurement of nasal potential difference between day 7 and baseline (at 7 days) Secondary outcomes Change from baseline in other nasal potential difference measures (baseline potential difference, change in amiloride, low chloride, and low chloride plus isoproterenol) (at 4 days, 7 days and 14 days) Change from baseline in sodium and chloride transport Change from baseline in average sweat chloride measurement (at 4 days, 7 days and 14 days) Change from baseline in sweat chloride Safety and tolerability (standard safety and tolerability lab values) (at 14 days) |
| Starting date | December 2017. |
| Contact information | Britany Zeglin (bzeglin1@jhmi.edu). |
| Notes |
NCT02412111.
| Trial name or title | A Phase 3 Study of VX‐661 in Combination With Ivacaftor in Subjects Aged 12 Years and Older With Cystic Fibrosis, Who Have One F508del‐CFTR Mutation and a Second Mutation That Has Been Demonstrated to be Clinically Responsive to Ivacaftor |
| Methods | Double‐blind, placebo‐controlled Phase 2 RCT. Parallel design. Multicentre: 68 centres. Sample size: expected to enrol 156 participants. Duration: unclear duration of treatment. |
| Participants | Age: 12 years and older Mutation: heterozygous for F508del‐CFTR mutation and a second CFTR allele with a gating defect that is clinically demonstrated to be ivacaftor responsive Lung function: FEV1 ≥ 40% and ≤ 90% of predicted. |
| Interventions | This study is evaluating VX‐661 in combination with ivacaftor versus placebo with ivacaftor GROUP 1: morning VX‐661 100 mg/ivacaftor 150 mg fixed‐dose tablet with ivacaftor matching placebo tablet; evening ivacaftor 150 mg tablet. GROUP 2: morning VX‐661/ivacaftor matching placebo tablet plus ivacaftor 150 mg tablet; evening ivacaftor 150 mg tablet. |
| Outcomes |
Primary outcome Absolute change in % predicted FEV1 (from baseline through week 8) Secondary outcomes Relative change in % predicted FEV1 (from baseline through week 8) Absolute change in sweat chloride (from baseline through week 8) Absolute change in CFQ‐R respiratory domain score (from baseline through week 8) Number of participants with AEs and serious AEs (up to 4 weeks after receiving last dose) PK parameters of VX‐661, M1‐661, ivacaftor, and M1‐ivacaftor |
| Starting date | June 2015. |
| Contact information | Sponsored by Vertex Pharmaceuticals ‐ no contact details given. |
| Notes |
NCT02589236.
| Trial name or title | Study of Cavosonstat (N91115) in Patients With CF Homozygous for the F508del‐CFTR Mutation (SNO‐6) |
| Methods | Double‐blind, placebo‐controlled, parallel RCT. |
| Participants | Participants must have been treated with lumacaftor‐ivacaftor for at least 8 weeks prior to day 1. Age: 18 years and older. FEV1: 40% ‐ 85% predicted. |
| Interventions | Group 1: cavosonstat 200 mg 2x daily. Group 2: cavosonstat 400 mg 2x daily. Group 3: placebo. |
| Outcomes |
Primary outcome Absolute change in FEV1 % predicted (from baseline to 12 weeks) Secondary outcomes Relative change in FEV1 % predicted (from baseline to 12 weeks) Absolute change in sweat chloride (from baseline to 12 weeks) Absolute change in CFQ‐R (respiratory symptom scale) (from baseline to 16 weeks) Absolute change in BMI (from baseline to 12 weeks) Absolute change in Patient Global Impression of Change (patient‐reported outcome journal) (from baseline to 12 weeks) Incidence of treatment‐emergent AEs (including clinical laboratory values, ECG, pulmonary exacerbations, or vital sign changes) (from baseline to 16 weeks) Number of pulmonary exacerbations (up to 12 weeks) |
| Starting date | November 2015. |
| Contact information | |
| Notes |
NCT02718495.
| Trial name or title | Study Assessing PTI‐428 Safety, Tolerability, and Pharmacokinetics in Subjects With Cystic Fibrosis |
| Methods | Quadruple‐blind, placebo‐controlled, parallel design 3‐arm Phase 2 RCT. Multicentre: 29 centres Sample size: expected to enrol 136 participants. Duration: 28 days of treatment. Part A has 2 groups: the 1st group will enrol adults with CF into a single ascending dose treatment group; the 2nd group will enrol adults with CF, including those on background treatment with ORKAMBI® and those not on a CFTR modulator into a multiple ascending dose treatment group. Part B will enrol adults with CF currently on stable ORKAMBI® background therapy for a minimum of 3 months into a Phase II treatment group consisting of 2 cohorts. Part C will enrol adults with CF, including those on background treatment with KALYDECO® and those not on a CFTR modulator, into a Phase II treatment group consisting of 3 cohorts. |
| Participants | Age: 18 years and older.
Mutation: not specified. Lung function: FEV1 40% ‐ 90% predicted. |
| Interventions | PTI‐428 versus placebo. |
| Outcomes |
Primary outcome Safety and tolerability as assessed by adverse events, pulmonary function tests, safety labs (haematology, chemistry, and urinalysis, ECGs, physical examinations, and vital signs) Secondary outcomes PK and pharmacodynamic parameters Change in FEV1 Change in sweat chloride Change in weight Change in CFQ‐R Change in nasal epithelial CFTR mRNA and protein expression |
| Starting date | November 2017. |
| Contact information | |
| Notes |
NCT02730208.
| Trial name or title | A Phase 2, Randomized, Placebo‐Controlled, Double‐blind Study to Evaluate the Effect of VX‐661 in Combination With Ivacaftor on Chest Imaging Endpoints in Subjects Aged 12 Years and Older With Cystic Fibrosis, Homozygous for the F508del CFTR Mutation |
| Methods | Placebo‐controlled, double‐blind Phase 2 RCT. |
| Participants | 12 years and older. Homozygous for F508del CFTR mutation. Stable CF disease as judged by the investigator. FEV1 ≥ 40% and ≤ 90% of predicted; ≥ 70% of predicted normal for age, sex, and height during screening. |
| Interventions |
Group 1: morning dose VX‐661 100 mg/ivacaftor 150 mg and an evening dose (approximately 12 hours after the morning dose) ivacaftor 150 mg. Group 2: placebo. |
| Outcomes |
Primary outcome Change in CT imaging score (from baseline to week 72) Secondary outcomes Safety and tolerability assessments including number of participants with AEs and serious AEs (up to week 72) |
| Starting date | September 2016. |
| Contact information | Unclear of individual to contact ‐ study conducted by Vertex Pharmaceuticals. |
| Notes |
NCT02951195.
| Trial name or title | A Study Evaluating the Safety of VX‐152 Combination Therapy in Adults With Cystic Fibrosis |
| Methods | Double‐blind, placebo and active‐controlled, parallel group, multicentre Phase 2 RCT. |
| Participants |
Inclusion criteria Body weight ≥ 35 kg. Sweat chloride value ≥ 60 mmol/L from test results obtained during screening. CFTR genotype: Cohorts 1A, 1B, 1C are heterozygous for F508del and a minimal function mutation known or predicted not to respond to tezacaftor and/or ivacaftor; Cohorts 2A, 2B are homozygous for F508del. FEV1 ≥ 40% and ≤ 90% of predicted normal for age, sex, and height at the screening visit. Stable CF disease as judged by the investigator. |
| Interventions |
Initial cohort: VX‐152 100 mg administered every 12 hours, tezacaftor 100 mg 1x daily, ivacaftor 150 mg every 12 hours versus placebo (i.e. no active VX‐152, tezacaftor, or ivacaftor). Subsequent cohorts: same combination with different doses of VX‐152, adjusted as the study progresses. |
| Outcomes |
Primary outcome AEs and serious AEs (up to 8 weeks) Secondary outcomes Absolute change in sweat chloride concentrations (from baseline to day 15) Absolute change in % predicted FEV1 (from baseline to day 15) Relative change in % predicted FEV1 (from baseline to day 15) Absolute change in CFQ‐R respiratory domain score (from baseline to day 15) PK and pharmacodynamic parameters |
| Starting date | November 2016. |
| Contact information | Vertex Pharmaceuticals (medicalinfo@vrtx.com). |
| Notes |
NCT03093714.
| Trial name or title | A Study to Evaluate Safety, PK and PD of FDL169 in Cystic Fibrosis Subjects |
| Methods | Multicentre (14 sites), placebo‐controlled, dose‐escalation RCT. |
| Participants |
Inclusion criteria Male and female participants with CF. Age 18 and above on the date of informed consent. Weight ≥ 40 kg. Homozygous for the F508del‐CFTR mutation. |
| Interventions |
Group 1: FDL 169 test formulation at 3 doses (dose escalation). Group 2: placebo. |
| Outcomes |
Primary outcome Treatment‐emergent AEs (up to 28 days) Secondary outcomes PK parameters |
| Starting date | August 2017. |
| Contact information | Jingwen Chai, Flatley Discovery Laboratory (jingwen.chai@flatleydiscoverylab.com). |
| Notes |
NCT03150719.
| Trial name or title | A Study to Evaluate Safety, Efficacy, and Tolerability of TEZ/IVA in Orkambi® (Lumacaftor/Ivacaftor) ‐ Experienced Subjects With Cystic Fibrosis (CF) |
| Methods | Double‐blind, placebo‐controlled, 3‐part Phase 3b RCT. Parallel design. Multicentre: 32 centres. Sample size: expected to enrol 90 participants. Duration: 28 days of treatment. |
| Participants | Age: 12 years and older. Mutation: homozygous for F508del mutation. Lung function: FEV1 ≥ 25% and ≤ 90% of predicted. |
| Interventions |
Group 1: tezacaftor 100 mg plus ivacaftor 150 mg fixed‐dose combination tablet in the morning plus ivacaftor 150 mg tablet in the evening. Group 2: placebo. |
| Outcomes |
Primary outcome Respiratory AEs (at day 56) Secondary outcomes Absolute change in % predicted FEV1 (from baseline to the average of the day 28 and day 56 measurements) Relative change in % predicted FEV1 (from baseline to the average of the day 28 and day 56 measurements) Absolute change in CFQ‐R respiratory domain score % predicted FEV1 (from baseline to the average of the day 28 and day 56 measurements) Tolerability (defined as the number and proportion of study participants who discontinue treatment) (up to day 56) AEs and serious AEs (AEs, abnormal laboratory values, vital signs or pulse oximetry) (safety follow‐up (up to 28 days after last dose of study drug)) |
| Starting date | April 2017. |
| Contact information | Vertex Pharmaceuticals (medicalinfo@vrtx.com). |
| Notes |
NCT03224351.
| Trial name or title | A Study Evaluating the Safety and Efficacy of VX‐659 Combination Therapy in Subjects With Cystic Fibrosis |
| Methods | Double‐blind, placebo‐ and tezacaftor‐ivacaftor (TEZ/IVA)‐controlled, parallel design, 3‐part, multicentre Phase 2 RCT. |
| Participants |
Inclusion criteria Body weight ≥ 35 kg. CFTR genotype: Part 1 and Part 3 ‐ heterozygous for F508del and an MF mutation (F/MF); Part 2 ‐ homozygous for F508del (F/F). FEV1 value ≥ 40% and ≤ 90% of predicted mean for age, sex, and height. |
| Interventions | Group 1: VX‐659 4x daily with tezacaftor and ivacaftor for 4 weeks (80 mg, 240 mg, or 400 mg). Group 2: placebo. |
| Outcomes |
Primary outcome Safety and tolerability as assessed by number of participants with AEs and serious AEs (up to 20 weeks) Absolute change in FEV1 % predicted (from baseline to day 29) Secondary outcomes Absolute change in sweat chloride concentrations (from baseline through day 29) Relative change in FEV1 % predicted Absolute change in CFQ‐R respiratory domain score (from baseline at day 29) PK and pharmacodynamic parameters |
| Starting date | August 2017. |
| Contact information | Vertex Pharmaceuticals (medicalinfo@vrtx.com). |
| Notes |
NCT03227471.
| Trial name or title | A Study of VX‐445 in Healthy Subjects and Subjects With Cystic Fibrosis |
| Methods | First‐in‐human, proof‐of‐concept RCT. Parallel design. Multicentre: 38 centres Sample size: expected to enrol 224 participants. Duration: 4 weeks of treatment. The study includes 6 parts, of which the first 3 are conducted in healthy participants, and the last 3 in people with CF. |
| Participants |
Inclusion criteria Age: 18 years and older. Mutation: heterozygous for F508del and an MF mutation (F/MF), or Homozygous for F508del (F/F). Lung function: FEV1 value ≥ 40% and ≤ 90% of predicted mean for age, sex, and height. |
| Interventions |
Group 1: VX‐445 in triple combination with tezacaftor and VX‐561 for 4 weeks. Group 2: placebo. |
| Outcomes |
Primary outcomes Safety and tolerability as assessed by number of participants with AEs and serious AEs Absolute change in FEV1 % predicted (Parts D, E, and F only) (from baseline through day 29) Secondary outcomes Absolute change in sweat chloride concentrations (from baseline through day 29) Relative change in FEV1 % predicted (from baseline through day 29) Absolute change in CFQ‐R respiratory domain score (from baseline through day 29) |
| Starting date | January 2017. |
| Contact information | Vertex Pharmaceuticals (medicalinfo@vrtx.com). |
| Notes |
NCT03258424.
| Trial name or title | Study Assessing PTI‐428 Safety, Tolerability, and Pharmacokinetics in Subjects With Cystic Fibrosis on KALYDECO® as Background Therapy |
| Methods | Phase 1 placebo‐controlled RCT. Parallel design. 2 centres. Sample size: expected to enrol 16 participants. Duration: 14 days of treatment. |
| Participants |
Inclusion criteria Age: 18 years and older. Mutation: taking ivacaftor but no specific mutations specified as eligible/ineligible. Lung function: FEV1 40% ‐ 90% predicted. |
| Interventions | Group 1: 1x daily dosing of PTI‐428. Group 2: placebo for 14 days. All participants continue on ivacaftor. |
| Outcomes |
Primary outcome Safety and tolerability as assessed by adverse events, safety labs, ECGs, physical examinations, and vital signs (at day 21) Secondary outcomes t1/2 of multiple oral doses (change from baseline to day 21) Tmax of multiple oral doses (change from baseline to day 21) Cmax of multiple oral doses (change from baseline to day 21) AUC0‐t of multiple oral doses (change from baseline to day 21) Other outcomes Nasal epithelial mRNA and protein expression over time (change from baseline to day 21) Sweat chloride (change from baseline to day 21) FEV1 (change from baseline to day 21) Weight (change from baseline to day 21) |
| Starting date | July 2017. |
| Contact information | Proteostasis Clinical Trials (pticlinicaltrials@proteostasis.com). |
| Notes |
AE: adverse event AUC: area under the curve BMI: body mass index CF: cystic fibrosis CFTR: cystic fibrosis transmembrane conductance regulator CFU: colony forming units CFQ‐R: Cystic Fibrosis Questionnaire‐Revised Cmax: maximum concentration CT: computer tomography ECG: electrocardiograms FEV1: forced expiratory volume in one second PK: pharmacokinetic RCT: randomised controlled trial TSQM: Treatment Satisfaction Questionnaire for Medication
Differences between protocol and review
Lung clearance index (LCI) was added as an outcome due to the increasing use of this outcome as a measure of lung function in the younger population.
We have added a statement to the Methods section that 99% confidence intervals will be used to analyse separate adverse events. This is the most appropriate statistical approach for considering adverse events individually.
Originally, we intended to combine all studies included in the review using a random‐effects approach to meta‐analysis. However, due to the substantial differences in the designs and interventions employed within the studies, we considered it more appropriate to make separate comparisons within the review, and where small numbers of studies of a similar design and intervention were pooled in meta‐analysis, a fixed‐effect approach was appropriate.
In line with current Cochrane guidance, we have included summary of findings tables for all comparisons.
Contributions of authors
| Roles and responsibilities | |
| TASK | WHO WILL UNDERTAKE THE TASK? |
| Protocol stage: draft the protocol | IS, SP with comments from all |
| Review stage: select which trials to include (2 + 1 arbiter) | IS, SP (+ KWS) |
| Review stage: extract data from trials (2 people) | IS, SP |
| Review stage: enter data into RevMan | SP, SJN |
| Review stage: carry out the analysis | SP, SJN |
| Review stage: interpret the analysis | IS, SP, SJN |
| Review stage: draft the final review | IS, SP with comments from all |
| Update stage: update the review | SP |
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Dr Ian Sinha declares no potential conflict of interest.
Dr Sanjay Patel declares no potential conflict of interest.
Professor Kevin Southern has attended and has organised educational events that have received financial support from Vertex, the company that has developed and is evaluating some of the agents included in this review.
Dr Sarah J Nevitt declares no potential conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Boyle 2014 {published data only}
- Boyle M, Bell SC, Konstan M, McColley S, Flume P, Kang L, et al. Lumacaftor, an investigational CFTR corrector, in combination with ivacaftor, a CFTR potentiator, in CF patients with the F508del‐CFTR mutation: phase 2 interim analysis. Journal of Cystic Fibrosis 2013;12 Suppl 1:S14. [Abstract no.: WS7.4; CENTRAL: 921640; CFGD Register: BD169c; CRS: 5500100000011652] [Google Scholar]
- Boyle MP, Bell S, Konstan M, McColley SA, Kang L, Patel N, et al. The investigational CFTR corrector, VX‐809 (lumacaftor) co‐administered with the oral potentiator ivacaftor improved CFTR and lung function in F509‐8DEL homozygous patients: phase II study results. Pediatric Pulmonology 2012;47 Suppl 35:315. [Abstract no.: 260; CENTRAL: 921644; CFGD Register: BD169b; CRS: 5500125000000037] [Google Scholar]
- Boyle MP, Bell S, Konstan MW, McColley SA, Wisseh S, Spencer‐Green G. VX‐809, an investigational CFTR corrector, in combination with VX‐770, an investigational CFTR potentiator, in subjects with CF and homozygous for the F508DEL‐CFTR Mutation [abstract]. Pediatric Pulmonology 2011;46 Suppl 34:287. [Abstract no.: 212; CENTRAL: 848840; CFGD Register: BD169a; CRS: 5500100000006520] [Google Scholar]
- Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, et al. A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: A phase 2 randomised controlled trial. The Lancet. Respiratory Medicine 2014;2(7):527‐38. [CENTRAL: 994993; CFGD Register: BD169d; CRS: 5500050000000081; EMBASE: 2014458184] [DOI] [PubMed] [Google Scholar]
- Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, et al. Supplementary Appendix to "A CFTR corrector (lumacaftor) and a CFTR potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del CFTR mutation: A phase 2 randomised controlled trial." [online]. The Lancet. Respiratory Medicine 2014;2(7):527‐38 online. [CENTRAL: 997711; CFGD Register: BD169e; CRS: 5500125000000703] [DOI] [PubMed] [Google Scholar]
- Rowe SM, McColley SA, Rietschel E, Li X, Bell SC, Konstan MW, et al. Lumacaftor/Ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del‐CFTR. Annals of the American Thoracic Society 2017;14(2):213‐219. Online supplement: data. [CFGD Register: BD169h; CRS: 5500135000002054] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, McColley SA, Rietschel E, Li X, Bell SC, Konstan MW, et al. Lumacaftor/Ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del‐CFTR. Annals of the American Thoracic Society 2017;14(2):213‐219. Online supplement: disclosures. [CFGD Register: BD169g; CRS: 5500135000002053] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, McColley SA, Rietschel E, Li X, Bell SC, Konstan MW, et al. Lumacaftor/Ivacaftor treatment of patients with cystic fibrosis heterozygous for F508del‐CFTR. Annals of the American Thoracic Society 2017;14(2):213‐9. [CFGD Register: BD169f; CRS: 5500135000001985; PUBMED: 27898234] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clancy 2012 {published data only}
- Clancy JP, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, et al. Results of a phase IIa study of VX‐809, an investigational CFTR corrector compound, in subjects with cystic fibrosis homozygous for the F508del‐CFTR mutation. Thorax 2012;67(1):12‐8. [CENTRAL: 806692; CFGD Register: BD166c; CRS: 5500100000006528] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy JP, Rowe SM, Accurso FJ, Ballmann M, Boyle MP, DeBoeck C, et al. A phase II, randomized, placebo‐controlled, clinical trial of four doses of VX‐809 in CF patients homozygous for the F508del CFTR mutation. Pediatric Pulmonology 2010;45 Suppl 33(S33):298. [Abstract no.: 224; CENTRAL: 848845; CFGD Register: BD166b; CRS: 5500100000006530] [Google Scholar]
- Clancy JP, Rowe SM, Liu B, Hathorne H, Dong Q, Wisseh S, et al. Variability of nasal potential difference measurements in clinical testing of CFTR modulators. Pediatric Pulmonology 2011;46 Suppl 34(S34):283. [Abstract no.: 202; CENTRAL: 848842; CFGD Register: BD166d // BD165n; CRS: 5500100000006522] [Google Scholar]
- Clancy JP, Spencer‐Green G, for theVX‐809‐101SG. Clinical evaluation of VX‐809, a novel investigational oral F508del‐CFTR corrector, in subjects with cystic fibrosis homozygous for the F508del‐CFTR mutation. Journal of Cystic Fibrosis 2010;9 Suppl 1:S20. [Abstract no.: 73; CENTRAL: 848846; CFGD Register: BD166a; CRS: 5500100000006531] [Google Scholar]
Donaldson 2014 {published data only}
- Donaldson SH, Shoemaker S, Mandagere A, Troha J. Novel modifiers of CFTR: emerging clinical experience with GSNOR inhibitors. Pediatric Pulmonology 2014;49 Suppl 38:154. [Abstract no.: S10.2; CENTRAL: 1015872; CFGD Register: BD217b; CRS: 5500131000000273] [Google Scholar]
- Donaldson SH, Taylor‐Cousar JL, Rosenbluth D, Zeitlin P, Chmiel J, Jain M, et al. Safety, tolerability, and pharmacokinetics of the intravenous S‐nitrosoglutathione reductase inhibitor N6022: an ascending‐dose study in subjects homozygous for the F508DEL‐CFTR mutation. Pediatric Pulmonology 2014;49 Suppl 38:308. [Abstract no.: 258; CENTRAL: 1012386; CFGD Register: BD217a; CRS: 5500131000000158] [Google Scholar]
- Nivalis Pharmaceuticals Incorporated. Safety and Pharmacokinetic Study of N6022 in Subjects With Cystic Fibrosis Homozygous for the F508del CFTR Mutation (SNO1). https://www.clinicaltrials.gov/ct2/show/NCT01746784 (accessed 16 December 2016). [Clinicaltrials.gov: NCT01746784]
Donaldson 2017 {published data only}
- Study of N91115 in Patients With Cystic Fibrosis Homozygous F508del‐CFTR Mutation (SNO4). https://clinicaltrials.gov/ct2/show/NCT02275936 (accessed 03 July 2017).
- Donaldson SH. Safety and pharmacokinetics of N91115 in patients with cystic fibrosis homozygous for the F508DEL‐CFTR mutation. Pediatric Pulmonology 2015;50 Suppl 41:293. [Abstract no.: 270; CENTRAL: 1092200; CFGD Register: BD226a; CRS: 5500135000001389] [Google Scholar]
- Donaldson SH, Solomon GM, Zeitlin PL, Flume PA, Casey A, McCoy K, et al. Pharmacokinetics and safety of cavosonstat (N91115) in healthy and cystic fibrosis adults homozygous for F508DEL‐CFTR. Journal of Cystic Fibrosis 2017;16(3):371‐379. Online supplementary tables and figures. [CFGD Register: BD226c] [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Solomon GM, Zeitlin PL, Flume PA, Casey A, McCoy K, et al. Pharmacokinetics and safety of cavosonstat (N91115) in healthy and cystic fibrosis adults homozygous for F508DEL‐CFTR. Journal of Cystic Fibrosis 2017;16(3):371‐9. [CFGD Register: BD226b] [DOI] [PubMed] [Google Scholar]
Donaldson 2018 {published data only}
- Study of VX‐661 Alone and in Combination With Ivacaftor in Subjects Homozygous or Heterozygous to the F508del‐Cystic Fibrosis Transmembrane Conductance Regulator(CFTR) Mutation. https://clinicaltrials.gov/ct2/show/NCT01531673 (accessed 03 July 2017).
- Donaldson S, Pilewski J, Griese M, Dong Q, Lee PS, for theVX11‐661‐101SG. VX‐661, an investigational CFTR corrector, in combination with ivacaftor, a CFTR potentiator, in patients with CF and homozygous for the F508Del‐CFTR mutation: interim analysis. Journal of Cystic Fibrosis 2013;12 Suppl 1:S14. [Abstract no.: WS7.3; CENTRAL: 872941; CFGD Register: BD190a; CRS: 5500100000011651] [Google Scholar]
- Donaldson SH, Pilewski JM, Cooke J, Himes‐Lekstrom J, VX11‐661‐101 SG. Addition of VX‐661, an investigational CFTR corrector, to ivacaftor, a CFTR potentiator, in patients with CF and heterozygous for F508DEL/G551D‐CFTR. Pediatric Pulmonology 2014;49 Suppl 38:308‐309. [Abstract no.: 260; CENTRAL: 1012385; CFGD Register: BD190c; CRS: 5500131000000156] [Google Scholar]
- Donaldson SH, Pilewski JM, Griese M, Cooke J, Viswanathan L, Tullis E, et al. Tezacaftor/Ivacaftor in Subjects with Cystic Fibrosis and F508del/F508del‐CFTR or F508del/G551D‐CFTR. American Journal of Respiratory and Critical Care Medicine 2018;197(2):214‐224. Online data supplement. [CFGD Register: BD190f] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson SH, Pilewski JM, Griese M, Cooke J, Viswanathan L, Tullis E, et al. Tezacaftor/Ivacaftor in Subjects with Cystic Fibrosis and F508del/F508del‐CFTR or F508del/G551D‐CFTR. American Journal of Respiratory and Critical Care Medicine 2018;197(2):214‐24. [CFGD Register: BD190e] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilewski JM, Cooke J, Lekstrom‐Himes J, Donaldson S, for theVX‐661IG. VX‐661 in combination with ivacaftor in patients with cystic fibrosis and the F508del‐CFTR mutation. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S1. [Abstract no.: WS01.4; CENTRAL: 1081479; CFGD Register: BD190d; CRS: 5500135000000009] [Google Scholar]
- Pilewski JM, Donaldson SH, Cooke J, Lekstrom‐Himes J. Phase 2 studies reveal additive effects of VX‐661, an investigational CFTR corrector, and ivacaftor, a CFTR potentiator, in patients with CF who carry the F508Del‐CFTR mutation. Pediatric Pulmonology 2014;49 Suppl 38:157‐9. [CENTRAL: 1015871; CFGD Register: BD190b; CRS: 5500131000000272] [Google Scholar]
McCarty 2002 {published data only}
- Ahrens RC, Standaert TA, Launspach J, Han SH, Teresi ME, Aitken ML, et al. Use of nasal potential difference and sweat chloride as outcome measures in multicenter clinical trials in subjects with cystic fibrosis. Pediatric Pulmonology 2002;33(2):142‐50. [CENTRAL: 385693; CFGD Register : BD136d ; CRS: 5500100000002083] [DOI] [PubMed] [Google Scholar]
- Aitken ML, Ahrens RC, Karlin DA, Konstan MW, McNamara SC, Regelman WE, et al. Safety of a phase I double‐blind placebo‐controlled dose escalation trial of oral CPX in adult CF patients. Pediatric Pulmonology 1998;26 Suppl 17:276. [CENTRAL: 385694; CFGD Register: BD136b; CRS: 5500100000002084] [Google Scholar]
- McCarty NA, Standaert TA, Teresi M, Tuthill C, Launspach J, Kelley TJ, et al. A phase I randomized, multicenter trial of CPX in adult subjects with mild cystic fibrosis. Pediatric Pulmonology 2002;33(2):90‐8. [CENTRAL: 377220; CFGD Register: BD136c; CRS: 5500100000002050; PUBMED: 11802244] [DOI] [PubMed] [Google Scholar]
- McCarty NA, Weatherly MR, Kelley TJ, Konstan MW, Milgram LJH, Teresi M, et al. Multicenter phase I trial of CPX in adults patients with mild CF: results of nasal potential difference measurements [abstract]. Pediatric Pulmonology 1998;26 Suppl 17:276. [CENTRAL: 291449; CFGD Register: BD136a; CRS: 5500100000001478] [Google Scholar]
PROGRESS 2017 {published data only}
- Konstan M, McKone E, Moss R, Marigowda G, Cooke J, Lubarsky B, et al. Evidence of reduction in annual rate of FEV1 decline and sustained benefits with lumacaftor and ivacaftor (LUM/IVA) in patients (pts) with cf homozygous for F508DEL‐CFTR. Pediatric Pulmonology 2016;51 Suppl 45:260. [Abstract no.: 180; CFGD Register: BD213p // BD214p; CRS: 5500135000002058] [Google Scholar]
- Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, et al. Assessment of safety and efficacy of long‐term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del‐CFTR mutation (PROGRESS): a phase 3, extension study. The Lancet. Respiratory Medicine 2017;5(2):107‐118. Online supplementary appendix. [CFGD Register: BD213r // BD214r; CRS: 5500135000002059] [DOI] [PubMed] [Google Scholar]
- Konstan MW, McKone EF, Moss RB, Marigowda G, Tian S, Waltz D, et al. Assessment of safety and efficacy of long‐term treatment with combination lumacaftor and ivacaftor therapy in patients with cystic fibrosis homozygous for the F508del‐CFTR mutation (PROGRESS): a phase 3, extension study. The Lancet. Respiratory Medicine 2017;5(2):107‐18. [CFGD Register: BD213q // BD214q; CRS: 5500135000001984; PUBMED: 28011037] [DOI] [PubMed] [Google Scholar]
- Vertex Pharmaceuticals Incorporated. A Phase 3 Rollover Study of Lumacaftor in Combination With Ivacaftor in Subjects 12 Years and Older With Cystic Fibrosis. https://clinicaltrials.gov/ct2/show/NCT01931839 (accessed 15 July 2014). [Clinicaltrials.gov: NCT01931839]
Ratjen 2017 {published data only}
- Brody A, Nagle SK, Owen C, Marigowda G, Waltz D, Goldin J, et al. Effect of lumacaftor/ ivacaftor on total, bronchiectasis, and air trapping computed tomography scores in children homozygous for F508DEL‐CFTR: exploratory imaging substudy. Pediatric Pulmonology 2017;52 Suppl 47:286. [CFGD Register: BD233d] [Google Scholar]
- Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6‐11 years with cystic fibrosis homozygous for F508del‐CFTR: a randomised, placebo‐controlled phase 3 trial. The Lancet. Respiratory Medicine 2017;5(7):557‐567. Online supplementary appendix. [CFGD Register: BD233c; CRS: 5500135000002065] [DOI] [PubMed] [Google Scholar]
- Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6–11 years with cystic fibrosis homozygous forF508del‐CFTR: a randomised, placebo‐controlled, phase 3 trial. Lancet Respiratory Medicine 2017;5(7):557‐67. [CFGD Register: BD233b] [DOI] [PubMed] [Google Scholar]
- Ratjen F, Tian S, Marigowda G, Hug C, Huang X, Stanojevic S, et al. Efficacy and safety of lumacaftor/ivacaftor (LUM/IVA) in patients (pts) aged 6‐11 years (yrs) with cystic fibrosis (CF) homozygous for F508del‐CFTR: a randomized placebo (PBO)‐controlled phase 3 trial. Journal of Cystic Fibrosis 2017;16 Suppl 1:S24. [Abstract no.: WS13.4; CENTRAL: 1383249; CFGD Register: BD233a; CRS: 5500135000002063] [Google Scholar]
Rubenstein 1998 {published data only}
- Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4‐phenylbutyrate (Buphenyl) in deltaF508‐homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. American Journal of Respiratory and Critical Care Medicine 1998;157(2):484‐90. [CENTRAL: 201485; CFGD Register: BD146b; CRS: 5500100000001017; EMBASE: 1998064104; PUBMED: 9476862] [DOI] [PubMed] [Google Scholar]
- Rubenstein RC, Zeitlin PL. A randomized, double blind, placebo‐controlled trial of sodium 4‐phenylbutyrate (Buphenyl) in deltaF508‐homozygous cystic fibrosis patients: Partial restoration of nasal epithelial CFTR function. Pediatric Pulmonology 1997;Suppl 14:272. [CENTRAL: 291563; CFGD Register: BD146a; CRS: 5500100000001576] [Google Scholar]
Taylor‐Cousar 2017 {published data only}
- Taylor‐Cousar JL, Elborn S. Advances in treating patients homozygous for F508del. Pediatric pulmonology 2017;52 Suppl 47:173‐5. [CFGD Register: BD236c] [Google Scholar]
- Taylor‐Cousar JL, Lekstrom‐Himes J, Wang L, Lu Y, Elborn S. Efficacy and safety of tezacaftor/ ivacaftor in patients aged >=12 years with cf homozygous for f508del‐cftr: a randomized placebo‐controlled phase 3 trial. Pediatric Pulmonology 2017;52 Suppl 47:307. [CFGD Register: BD236a] [Google Scholar]
- Taylor‐Cousar JL, Munck A, McKone EF, Ent CK, Moeller A, Simard C, et al. Tezacaftor‐ivacaftor in patients with cystic fibrosis homozygous for Phe508del. New England Journal of Medicine 2017;377(21):2013‐23. [CFGD Register: BD236b] [DOI] [PubMed] [Google Scholar]
- Taylor‐Cousar JL, Munck A, McKone EF, Ent CK, Moeller A, Simard C, et al. Tezacaftor‐ivacaftor in patients with cystic fibrosis homozygous for Phe508del. New England Journal of Medicine 2017;377(21):2013‐23. [DOI: 10.1056/NEJMoa1709846] [DOI] [PubMed] [Google Scholar]
TRAFFIC 2015 {published data only}
- Anstead M, Tupayachi G, Murphy D, Autry E, Bulkley V, Kuhn R. Lumacaftor/ivacaftor: real world experience in a CF center. Pediatric Pulmonology 2016;51 Suppl:302. [CFGD Register: BD213s // BD214s] [Google Scholar]
- Boeck C. Long‐term clinical effects of CFTR co‐therapy with Lumacaftor/Ivacaftor. Pediatric Pulmonology 2015;50:135‐7. [CENTRAL: 1163954; CFGD Register: BD213m/BD214m; CRS: 5500135000001994; EMBASE: 72081237; Symposium summary: S9.1] [Google Scholar]
- Boeck K, Elborn J, Ramsey B, Boyle MP, Konstan MW, Huang X, et al. Efficacy and safety of lumacaftor+ivacaftor combination therapy in patients with CF homozygous for F508DEL‐CFTR by FEV1 subgroups. Pediatric Pulmonology 2015;50 Suppl 41:283. [Abstract no.: 245; CENTRAL: 1092180; CFGD Register: BD213f/BD214f; CRS: 5500135000001376] [Google Scholar]
- Elborn J, Wainwright CE, Ramsey B, Huang X, Margowda G, Waltz D, et al. Effect of lumacaftor in combination with ivacaftor in patients with cystic fibrosis who are homozygous for F508‐DEL‐CFTR: the TRAFFIC Study. Pediatric Pulmonology 2014;49 Suppl 38:304. [Abstract no.: 249; CENTRAL: 1012382; CFGD Register: BD213a; CRS: 5500131000000150] [Google Scholar]
- Elborn JS, Ramsey B, Boyle MP, Wainwright C, Konstan M, Huang X, et al. Lumacaftor in combination with ivacaftor in patients with cystic fibrosis who are homozygous for the F508del‐CFTR mutation. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S1. [Abstract no.: WS01.3; CENTRAL: 1077209; CFGD Register: BD213e/BD214d; CRS: 5500135000000008] [Google Scholar]
- Elborn JS, Ramsey BW, Boyle MP, Konstan MW, Huang X, Marigowda G, et al. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. The Lancet. Respiratory Medicine 2016;4(8):617‐26. [CENTRAL: 1157425; CFGD Register: BD213i/BD214i; CRS: 5500135000001532; DOI: 10.1016/S2213-2600(16)30121-7; PUBMED: 27298017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn JS, Ramsey BW, Boyle MP, Konstan MW, Huang X, Marigowda G, et al. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. The Lancet. Respiratory Medicine 2016;4(8):617‐626. Online supplementary appendix. [CFGD Register: BD213o/BD214o ; CRS: 5500135000002057] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn JS, Ramsey BW, Boyle MP, Wainwright CE, Konstan MW, Huang X, et al. Lumacaftor/ Ivacaftor combination therapy in CF patients homozygous for F508del‐CFTR with severe lung dysfunction. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S94. [Abstract no.: 143; CENTRAL: 1077207; CFGD Register: BD213c/BD214d; CRS: 5500135000000006] [Google Scholar]
- Konstan M, McKone E, Moss R, Marigowda G, Cooke J, Lubarsky B, et al. Evidence of reduction in annual rate of FEV1 decline and sustained benefits with lumacaftor and ivacaftor (LUM/IVA) in patients (pts) with cf homozygous for F508DEL‐CFTR. Pediatric Pulmonology 2016;51 Suppl 45:260, Abstract no: 180. [CFGD Register: BD213p // BD214p] [Google Scholar]
- Konstan MW, Ramsey BW, Elborn J, Boyle MP, Waltz D, Marigowda G, et al. Safety and efficacy of treatments with lumacaftor in combination with ivacaftor in patients with CF homozygous for F508DEL‐CFTR. Pediatric Pulmonology 2015;50 Suppl 41:269. [Abstract no.: 211; CENTRAL: 1092192; CFGD Register: BD213h/BD214h; CRS: 5500135000001381] [Google Scholar]
- McColley SA, Konstan MW, Ramsey BW, Elborn J, Boyle MP, Wainwright CE, et al. Association between changes in percent predicted FEV1 and incidence of pulmonary exacerbations, including those requiring hospitalization and/ or IV antibiotics, in patients with CF treated with lumacaftor in combination with ivacaftor. Pediatric Pulmonology 2015;50 Suppl 41:282. [Abstract no.: 241; CENTRAL: 1092182; CFGD Register: BD213g/BD214g; CRS: 5500135000001378] [Google Scholar]
- Seliger V, Bai Y, Volkova N, Tian S, Waltz. Prevalance of cataracts in a population of cystic fibrosis patients homozygous for the F508del mutation. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S108. [Abstract no.: 196; CENTRAL: 1077208; CFGD Register: BD213d/BD214c; CRS: 5500135000000007] [Google Scholar]
- Sullivan JC, Accurso FJ, Marigowda G, Beusmans J, Geho D, Zhang E, et al. Combination lumacaftor/ivacaftor therapy improves inflammatory biomarkers in patients with cf homozygous for the f508DEL‐CFTR mutations. Pediatric Pulmonology 2016;51 Suppl 45:274. [Abstract no.: 218; CFGD Register: BD213n/BD214n ; CRS: 5500135000002056] [Google Scholar]
- Sullivan JC, Accurso FJ, Marigowda G, Beusmans J, Geho D, Zhang E, et al. Improvement in inflammatory biomarkers in patients (pts) wth cystic fibrosis (CF) homozygous for the F508del‐CFTR mutation treated with lumacaftor (LUM) and ivacaftor (IVA). Journal of Cystic Fibrosis 2016;15 Suppl 1:S6. [Abstract no.: WS04.2; CENTRAL: 1157463; CFGD Register: BD213k/BD214k; CRS: 5500135000001540] [Google Scholar]
- Suthoff ED, Kosinski M, Sikirica S, Quittner AL, Flume P. Impact of pulmonary exacerbations (PEx) on health‐related quality of life (HRQoL) assessed by the Cystic Fibrosis Questionnaire‐Revised (CFQ‐R) in TRAFFIC and TRANSPORT. Journal of Cystic Fibrosis 2016;15 Suppl 1:S86. [Abstract no.: 138; CENTRAL: 1157462; CFGD Register: BD213j/BD214j; CRS: 5500135000001539] [Google Scholar]
- Vertex Pharmaceuticals Incorporated. A Study of Lumacaftor in Combination With Ivacaftor in Cystic Fibrosis Subjects Aged 12 Years and Older Who Are Homozygous for the F508del‐CFTR Mutation (TRAFFIC). https://clinicaltrials.gov/ct2/show/NCT01807923 (accessed 15 July 2014). [Clinicaltrials.gov: NCT01807923]
- Wainwright CE, Elborn JS, Ramsey B, Huang X, Marigowda G, Waltz D, et al. Effect of lumacaftor in combination with ivacaftor in patients with cf who are homozygous for F508‐CFTR: phase 3 TRAFFIC and TRANSPORT studies. Pediatric Pulmonology 2014;49 Suppl 38:156. [Abstract no.: S10.3; CENTRAL: 1383236; CFGD Register: BD213L/BD214L ; CRS: 5500135000002055] [Google Scholar]
- Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor‐Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. New England Journal of Medicine 2015;373(3):220‐31. [CENTRAL: 1067433; CFGD Register: BD213b/BD214b; CRS: 5500133000000031; 5500133000000031; PUBMED: 25981758]25981758 [Google Scholar]
TRANSPORT 2015 {published data only}
- Anstead M, Tupayachi G, Murphy D, Autry E, Bulkley V, Kuhn R. Lumacaftor/ivacaftor: real world experience in a CF center. Pediatric Pulmonology 2016;51 Suppl:302. [CFGD Register: BD213s // BD214s] [Google Scholar]
- Boeck C. Long‐term clinical effects of CFTR co‐therapy with Lumacaftor/Ivacaftor. Pediatric Pulmonology 2015;50:135‐7. [CENTRAL: 1163954; CFGD Register: BD214m/BD213m; CRS: 5500135000001994; EMBASE: 72081237; Symposium summary: S9.1] [Google Scholar]
- Boeck K, Elborn J, Ramsey B, Boyle MP, Konstan MW, Huang X, et al. Efficacy and safety of lumacaftor+ivacaftor combination therapy in patients with CF homozygous for F508DEL‐CFTR by FEV1 subgroups. Pediatric Pulmonology 2015;50 Suppl 41:283. [Abstract no.: 245; CENTRAL: 1092180; CFGD Register: BD214f/BD213f; CRS: 5500135000001376] [Google Scholar]
- Elborn JS, Ramsey B, Boyle MP, Wainwright C, Konstan M, Huang X, et al. Lumacaftor in combination with ivacaftor in patients with cystic fibrosis who are homozygous for the F508del‐CFTR mutation. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S1. [Abstract no.: WS01.3; CENTRAL: 1077209; CFDG Register: BD214d/BD213e; CRS: 5500135000000008] [Google Scholar]
- Elborn JS, Ramsey BW, Boyle MP, Konstan MW, Huang X, Marigowda G, et al. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. The Lancet. Respiratory Medicine 2016;4(8):617‐26. [CENTRAL: 1157425; CFGD Register: BD214i/BD213i; CRS: 5500135000001532; DOI: 10.1016/S2213-2600(16)30121-7; PUBMED: 27298017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn JS, Ramsey BW, Boyle MP, Konstan MW, Huang X, Marigowda G, et al. Efficacy and safety of lumacaftor/ivacaftor combination therapy in patients with cystic fibrosis homozygous for Phe508del CFTR by pulmonary function subgroup: a pooled analysis. The Lancet. Respiratory Medicine 2016;4(8):617‐626. Online supplementary appendix. [CFGD Register: BD214o/BD213o ; CRS: 5500135000002057] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elborn JS, Ramsey BW, Boyle MP, Wainwright CE, Konstan MW, Huang X, et al. Lumacaftor/ Ivacaftor combination therapy in CF patients homozygous for F508del‐CFTR with severe lung dysfunction. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S94. [Abstract no.: 143; CENTRAL: 1077207; CFGD Register: BD214d/BD213c; CRS: 5500135000000006] [Google Scholar]
- Konstan M, McKone E, Moss R, Marigowda G, Cooke J, Lubarsky B, et al. Evidence of reduction in annual rate of FEV1 decline and sustained benefits with lumacaftor and ivacaftor (LUM/IVA) in patients (pts) with cf homozygous for F508DEL‐CFTR. Pediatric Pulmonology 2016;51 Suppl 45:260, Abstract no: 180. [CFGD Register: BD213p // BD214p] [Google Scholar]
- Konstan MW, Ramsey BW, Elborn J, Boyle MP, Waltz D, Marigowda G, et al. Safety and efficacy of treatments with lumacaftor in combination with ivacaftor in patients with CF homozygous for F508DEL‐CFTR. Pediatric Pulmonology 2015;50 Suppl 41:269. [Abstract no.: 211; CENTRAL: 1092192; CFGD Register: BD214h/BD213h; CRS: 5500135000001381] [Google Scholar]
- McColley SA, Konstan MW, Ramsey BW, Elborn J, Boyle MP, Wainwright CE, et al. Association between changes in percent predicted FEV1 and incidence of pulmonary exacerbations, including those requiring hospitalization and/ or IV antibiotics, in patients with CF treated with lumacaftor in combination with ivacaftor. Pediatric Pulmonology 2015;50 Suppl 41:282. [Abstract no.: 241; CENTRAL: 1092182; CFGD Register: BD214g/BD213g; CRS: 5500135000001378] [Google Scholar]
- Ramsey B, Boyle MP, Elborn J, Huang X, Marigowda G, Waltz D, et al. Effect of lumacaftor in combination with ivacaftor in patients with cystic fibrosis who are homozygous for F508DEL‐CFTR: TRANSPORT Study. Pediatric Pulmonology 2014;49 Suppl 38:305. [Abstract no.: 250; CENTRAL: 1012383; CFGD Register: BD214a; CRS: 5500131000000152] [Google Scholar]
- Seliger V, Bai Y, Volkova N, Tian S, Waltz. Prevalance of cataracts in a population of cystic fibrosis patients homozygous for the F508del mutation. Journal of Cystic Fibrosis : Official Journal of the European Cystic Fibrosis Society 2015;14 Suppl 1:S108. [Abstract no.: 196; CENTRAL: 1077208; CFGD Register: BD214c/BD213d; CRS: 5500135000000007] [Google Scholar]
- Sullivan JC, Accurso FJ, Marigowda G, Beusmans J, Geho D, Zhang E, et al. Combination lumacaftor/ivacaftor therapy improves inflammatory biomarkers in patients with cf homozygous for the f508DEL‐CFTR mutations. Pediatric Pulmonology 2016;51 Suppl 45:274. [Abstract no.: 218; CFGD Register: BD214n/BD213n ; CRS: 5500135000002056] [Google Scholar]
- Sullivan JC, Accurso FJ, Marigowda G, Beusmans J, Geho D, Zhang E, et al. Improvement in inflammatory biomarkers in patients (pts) wth cystic fibrosis (CF) homozygous for the F508del‐CFTR mutation treated with lumacaftor (LUM) and ivacaftor (IVA). Journal of Cystic Fibrosis 2016;15 Suppl 1:S6. [Abstract no.: WS04.2; CENTRAL: 1157463; CFGD Register: BD214k/BD213k; CRS: 5500135000001540] [Google Scholar]
- Suthoff ED, Kosinski M, Sikirica S, Quittner AL, Flume P. Impact of pulmonary exacerbations (PEx) on health‐related quality of life (HRQoL) assessed by the Cystic Fibrosis Questionnaire‐Revised (CFQ‐R) in TRAFFIC and TRANSPORT. Journal of Cystic Fibrosis 2016;15 Suppl 1:S86. [Abstract no.: 138; CENTRAL: 1157462; CFGD Register: BD214j/BD213j; CRS: 5500135000001539] [Google Scholar]
- Vertex Pharmaceuticals Incorporated. A Study of Lumacaftor in Combination With Ivacaftor in Cystic Fibrosis Subjects Aged 12 Years and Older Who Are Homozygous for the F508del‐CFTR Mutation (TRANSPORT). https://clinicaltrials.gov/ct2/show/NCT01807949 (accessed 15 July 2014). [Clinicaltrials.gov: NCT01807949]
- Wainwright CE, Elborn JS, Ramsey B, Huang X, Marigowda G, Waltz D, et al. Effect of lumacaftor in combination with ivacaftor in patients with cf who are homozygous for F508‐CFTR: phase 3 TRAFFIC and TRANSPORT studies. Pediatric Pulmonology 2014;49 Suppl 38:156. [Abstract no.: S10.3; CENTRAL: 1383236; CFGD Register: BD214L/BD213L ; CRS: 5500135000002055] [Google Scholar]
- Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor‐Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. New England Journal of Medicine 2015;373(3):220‐31. [CENTRAL: 1067433; CFGD Register: BD214b/BD213b; CRS: 5500133000000031; 5500133000000031; PUBMED: 25981758]25981758 [Google Scholar]
Zeitlin 2002 {published data only}
- Zeitlin PL, Diener‐West M, Rubenstein RC, Boyle MP, Lee CK, Brass‐Ernst L. Evidence of CFTR function in cystic fibrosis after systemic administration of 4‐phenylbutyrate. Molecular Therapy 2002;6(1):119‐26. [CENTRAL: 409030; CFGD Register: BD148; CRS: 5500100000002247; PUBMED: 12095312] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Berkers 2014 {published data only}
- Berkers G, Vijftigschild L, Bronsveld I, Arets H, Winter‐de Groot K, Heijerman H, et al. A beta‐2 agonist as a CFTR activator in CF; the ABBA study. Pediatric Pulmonology 2014;49 Suppl 38:299‐300. [Abstract no.: 236; CENTRAL: 1012380; CFGD Register: BD212; CRS: 5500131000000147] [Google Scholar]
Chadwick 1998 {published data only}
- Chadwick S, Browning JE, Stern M, Cheng SH, Gruenert DC, Geddes DM, et al. Nasal application of glycerol in DF508 cystic fibrosis patients. Pediatric Pulmonology 1998;Suppl 17:278. [Abstract no.: 275; CENTRAL: 208568; CFGD Register: BD147; CRS: 5500100000001110] [Google Scholar]
Chilvers 2017 {published data only}
- Chilvers M, Tian S, Marigowda G, Bsharat M, Hug C, Solomon M, et al. An open‐label extension (EXT) study of lumacaftor/ivacaftor (LIM/IVA) therapy in patients (pts) aged 6‐11 years (yrs) with cystic fibrosis (CF) homozygous for F508del‐CFTR. Journal of Cystic Fibrosis 2017;16 Suppl 1:S77. [Abstract no: 52; CENTRAL: 1383248; CFGD Register: BD232; clinicaltrials.gov: NCT01897233; CRS: 5500135000002061] [Google Scholar]
- Vertex Pharmaceuticals Incorporated. A phase 1, open‐label study to evaluate the pharmacokinetics and safety of Lumacaftor in combination with Ivacaftor in subjects 6 through 11 years of age with cystic fibrosis, homozygous for the F508del‐CFTR mutation. http://clinicaltrials.gov/ct2/show/NCT01897233 (accessed 15 July 2014). [Clinicaltrials.gov: NCT01897233]
Lebecque 2011 {published data only}
- Lebecque P, Leal T. Does a nasal instillation of miglustat normalize the nasal potential difference in cystic fibrosis patients homozygous for the F508del mutation? A randomized, double blind placebo‐controlled study. http://clinicaltrials.gov/show/NCT00945347 (accessed 15 July 2014).
Leonard 2012 {published data only}
- Leonard A, Dingemanse J, Lebecque P, Leal T. Oral miglustat in homozygous F508del CF patients. Journal of Cystic Fibrosis 2010;9 Suppl 1:S20. [Abstract no.: 75; CENTRAL: 921950; CFGD Register: BD193a; CRS: 5500125000000356] [Google Scholar]
- Leonard A, Lebecque P, Dingemanse J, Leal T. A randomized placebo‐controlled trial of miglustat in cystic fibrosis based on nasal potential difference. Journal of Cystic Fibrosis 2012;11:231‐6. [CENTRAL: 840524; CFGD Register: BD193b; CRS: 5500100000003733; PUBMED: 22281182] [DOI] [PubMed] [Google Scholar]
NCT01899105 {published data only}
- Vertex Pharmaceuticals Incorporated. A phase 1, randomized, single‐dose, open‐label crossover study to investigate the effect of food on the relative bioavailability of 2 fixed‐dose combinations of Lumacaftor and Ivacaftor tablet formulations in healthy adult subjects. http://clinicaltrials.gov/show/NCT01899105 (accessed 15 July 2014). [Clinicaltrials.gov: NCT01899105]
Nick 2014 {published data only}
- Nick JA, Rodman D, Clair C, Jones MC, Li H, Higgins M. Utilization of an "n‐of‐1" study design to test the effect of ivacaftor in CF patients with residual CFTR function and FEV1 >40% of predicted. Pediatric Pulmonology 2014;49:188‐9. [CENTRAL: 1008991; CFGD Register: BD211b; CRS: 5500050000000150; EMBASE: 71616032] [Google Scholar]
- Nick JA, Rodman D, Clair C, Jones MC, Li H, Higgins M, et al. Effect of ivacaftor in patients with cystic fibrosis, residual CFTR function, and FEV1 ≥40% of predicted, N‐of‐1 study. Pediatric Pulmonology 2014;49 Suppl 38:285. [Abstract no.: 196; CENTRAL: 1012379; CFGD Register: BD211a ; CRS: 5500131000000145] [Google Scholar]
Rowe 2017 {published data only}
- Rowe SM, Daines C, Ringshausen FC, Kerem E, Wilson J, Tullis E, et al. Tezacaftor‐ivacaftor in residual‐function heterozygotes with cystic fibrosis. New England Journal of Medicine 2017;377(21):2024‐35. [CFGD Register: BD237c; Clinicaltrials.gov: NCT02392234] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, Davies J. CFTR modulation with tezacaftor/ivacaftor in patients heterozygous for F508del and a residual function mutation. Pediatric Pulmonology 2017;52 Suppl 47:175‐6. [CFGD Register: BD237a; Clinicaltrials.gov: NCT02392234] [Google Scholar]
- Rowe SM, Davies JC, Nair N, Han L, Lekstrom‐Himes J. Efficacy and safety of tezacaftor/ ivacaftor and ivacaftor in patients aged >=12 years with cf heterozygous for f508del and a residual function mutation: a randomized, double‐blind, placebo‐controlled, crossover phase 3 study. Pediatric Pulmonology 2017;52 Suppl 47:317. [CFGD Register: BD237b; Clinicaltrials.gov: NCT02392234] [Google Scholar]
Rubenstein 2006 {published data only}
- Rubenstein RC, Propert KJ, Reenstra WW, Skotleski ML. A pilot trial of the combination of phenylbutyrate and genistein. Pediatric Pulmonology 2006;41 Suppl 29:294. [Abstract no.: 248; CENTRAL: 593141; CFGD Register: BD149; CRS: 5500100000003089] [Google Scholar]
Sumner 2014 {published data only}
- Sumner Jones SG, Alton EW, Boyd A, Chang SH, Davies JC, Davies LA, et al. Molecular analyses of vector delivery and gene expression in a multidose trial of non‐viral gene therapy in patients with CF. Pediatric Pulmonology 2014;49 Suppl 38:302. [Abstract no.: 243; CENTRAL: 1012381; CFGD Register: BD210b; CRS: 5500131000000149] [Google Scholar]
- Waller MD, Harman KM, Boyd A, Chang SH, Gill DR, Griesenbach U, et al. Measurement of CFTR function in cystic fibrosis patients in response to multidose CFTR gene therapy. Pediatric Pulmonology 2014;49 Suppl 38:249. [Abstract no.: 97; CENTRAL: 1012378; CFGD Register: BD210a; CRS: 5500131000000142] [Google Scholar]
Ziady 2015 {published data only}
- Ziady AG, Lin S, Heltshe SL, Kelley T, Muhlebach MS, Accurso FJ, et al. Protein expression in CF primary airway epithelia following treatment with VX‐809 reveals significant changes in pkc‐mediated signalling, proton and iron transport, and lipid metabolism. Pediatric Pulmonology 2015;50 Suppl 41:300. [Abstract no.: 290; CENTRAL: 1092203; CFGD Register: BD225; CRS: 5500135000001392] [Google Scholar]
References to studies awaiting assessment
Hunt 2017 {published data only}
- Hunt K, Clair C, Curran‐Everett D, Solomon GM, Saavedra MT, Nick JA, et al. CFTR effects of oral sildenafil in combination with lumacaftor/ivacaftor in adults with CF. Pediatric Pulmonology 2017;52(Suppl 47):322. [CFGD Register: IB117] [Google Scholar]
References to ongoing studies
Meijer 2016 {published data only}
- Evaluation of (R)‐Roscovitine Safety and Effects in Subjects With Cystic Fibrosis, Homozygous for the F508del‐CFTR Mutation (ROSCO‐CF). https://clinicaltrials.gov/ct2/show/NCT02649751 (accessed 03 July 2017).
- Meijer L, Hery‐Arnaud G, Berre R, Nowak E, Roux L, Gueganton L, et al. ROSCO‐CF, a safety and efficacy clinical trial of (r)‐roscovitine in CF patients. Pediatric Pulmonology 2016;51 Suppl 45:269. [CFGD Register: BD230b] [Google Scholar]
- Meijer L, Hery‐Arnaud G, Berre R, Nowak E, Roux L, Gueganton L, et al. Rosco‐CF, a safety and efficacy clinical trial of (R)‐roscovitine in CF patients. Journal of Cystic Fibrosis 2016;15 Suppl 1:S42. [Abstract no.: ePS03.7; CENTRAL: 1157464; CFGD Register: BD230a; CRS: 5500135000001541] [Google Scholar]
NCT02070744 {published data only}
- Vertex Pharmaceuticals Incorporated. Study to Evaluate Safety and Efficacy of VX‐661 in Combination With Ivacaftor in Subjects With Cystic Fibrosis, Homozygous for the F508del‐CFTR Mutation With an Open‐Label Expansion. https://www.clinicaltrials.gov/ct2/show/NCT02070744 (accessed 03 July 2017). [Clinicaltrials.gov: NCT02070744]
NCT02323100 {published data only}
- NCT02323100. Glycerol Phenylbutyrate Corrector Therapy For CF (Cystic Fibrosis) (GPBA). clinicaltrials.gov/ct2/show/NCT02323100 (first received 23 December 2014).
NCT02412111 {published data only}
- NCT02412111. A Phase 3 Study of VX‐661 in Combination With Ivacaftor in Subjects Aged 12 Years and Older With Cystic Fibrosis, Who Have One F508del‐CFTR Mutation and a Second Mutation That Has Been Demonstrated to be Clinically Responsive to Ivacaftor. clinicaltrials.gov/ct2/show/NCT02412111 (first received 08 April 2015).
NCT02589236 {published data only}
- NCT02589236. Study of Cavosonstat (N91115) in Patients With CF Homozygous for the F508del‐CFTR Mutation (SNO‐6). clinicaltrials.gov/ct2/show/NCT02589236 (first received 28 October 2015).
NCT02718495 {published data only}
- NCT02718495. Study Assessing PTI‐428 Safety, Tolerability, and Pharmacokinetics in Subjects With Cystic Fibrosis. clinicaltrials.gov/ct2/show/NCT02718495 (first received 24 March 2016).
NCT02730208 {published data only}
- NCT02730208. A Study to Evaluate the Effect of VX‐661 in Combination With Ivacaftor on Chest Imaging Endpoints in Subjects With Cystic Fibrosis, Homozygous for the F508del CFTR Mutation. clinicaltrials.gov/ct2/show/NCT02730208 (first received 06 April 2016).
NCT02951195 {published data only}
- NCT02951195. A Study Evaluating the Safety of VX‐152 Combination Therapy in Adults With Cystic Fibrosis. clinicaltrials.gov/ct2/show/NCT02951195 (first received 01 November 2016).
NCT03093714 {published data only}
- NCT03093714. A Study to Evaluate Safety, PK and PD of FDL169 in Cystic Fibrosis Subjects. https://clinicaltrials.gov/ct2/show/NCT03093714 2018.
NCT03150719 {published data only}
- NCT03150719. A Study to Evaluate Safety, Efficacy, and Tolerability of TEZ/IVA in Orkambi® (Lumacaftor/Ivacaftor) ‐ Experienced Subjects With Cystic Fibrosis (CF). clinicaltrials.gov/ct2/show/NCT03150719 (first received 12 May 2017).
NCT03224351 {published data only}
- NCT03224351. A Study Evaluating the Safety and Efficacy of VX‐659 Combination Therapy in Subjects With Cystic Fibrosis. clinicaltrials.gov/ct2/show/NCT03224351 (first received 21 July 2017).
NCT03227471 {published data only}
- NCT03227471. A Study of VX‐445 in Healthy Subjects and Subjects With Cystic Fibrosis. clinicaltrials.gov/ct2/show/NCT03227471 (first received 24 July 2017).
NCT03258424 {published data only}
- NCT03258424. Study Assessing PTI‐428 Safety, Tolerability, and Pharmacokinetics in Subjects With Cystic Fibrosis on KALYDECO® as Background Therapy. clinicaltrials.gov/ct2/show/NCT03258424 (first received 23 August 2017).
Additional references
Amaral 2007
- Amaral MD, Kunzelmann K. Molecular targeting of CFTR as a therapeutic approach to cystic fibrosis. Trends in Pharmacological Sciences 2007;28(7):334‐41. [DOI] [PubMed] [Google Scholar]
Aslam 2017
- Aslam AA, Higgins C, Sinha IP, Southern KW. Ataluren and similar compounds (specific therapies for premature termination codon class I mutations) for cystic fibrosis. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012040.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bobadilla 2002
- Bobadilla JL, Macek M, Fine JP, Farrell PM. Cystic fibrosis: A worldwide analysis of CFTR mutations ‐ Correlation with incidence data and application to screening. Human Mutation 2002;19(6):575‐606. [DOI] [PubMed] [Google Scholar]
CFMD 2013
- Cystic Fibrosis Centre at the Hospital for Sick Children in Toronto, Canada. Cystic Fibrosis Mutation Database. http://www.genet.sickkids.on.ca/app (accessed 23 September 2013).
Colledge 1995
- Colledge WH, Abella BS, Southern KW, Ratcliff R, Jiang C, Cheng SH, et al. Generation and characterization of a delta F508 cystic fibrosis mouse model. Nature Genetics 1995;10(4):445‐52. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks J, Higgins J, Altman D. Chapter 9 Analysing data and undertaking meta‐analysis. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
EMA 2012
- European Medicines Agency. Report of the workshop on endpoints for cystic fibrosis clinical trials (EMA/769571/2012). http://www.ema.europa.eu/docs/en_GB/document_library/Report/2012/12/WC500136159.pdf (accessed 12/09/2016) 29 November 2012.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG on behalf of the CSMG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
NICE 2016
- Cystic fibrosis (F508del mutation) ‐ lumacaftor (with ivacaftor) [ID786]. https://www.nice.org.uk/guidance/indevelopment/gid‐tag530 (accessed 21/06/2016).
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Patel 2015
- Patel S, Sinha IP, Dwan K, Echevarria C, Schechter M, Southern KW. Potentiators (specific therapies for class III and IV mutations) for cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD009841.pub2] [DOI] [PubMed] [Google Scholar]
Quittner 2009
- Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire‐Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest 2009;135(6):1610‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsey 2011
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, Moss R. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. New England Journal of Medicine 2011 Nov 3;365(18):1663‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riordan 1989
- Riordan JR, Rommens JM, Kerem BS, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene – cloning and characterization of complementary. Science 1989;245(4922):1066‐72. [DOI] [PubMed] [Google Scholar]
Rogan 2011
- Rogan MP, Stoltz DA, Hornick DB. Cystic fibrosis transmembrane conductance regulator intracellular processing, trafficking, and opportunities for mutation‐specific treatment. Chest 2011;139(6):1480‐90. [DOI: 10.1378/chest.10-2077] [DOI] [PubMed] [Google Scholar]
Rowntree 2003
- Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Annals of Human Genetics 2003;67(5):471‐85. [DOI] [PubMed] [Google Scholar]
Rubenstein 1997
- Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR‐mediated chloride transport with sodium 4‐phenylbutyrate in cystic fibrosis epithelial cells containing delta F508‐CFTR. Journal of Clinical Investigation 1997;100(10):2457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Southern 1997
- Southern KW. Delta F508 in cystic fibrosis: Willing but not able. Archives of Disease in Childhood 1997;76(3):278‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Southern 2007
- Southern KW. Cystic fibrosis and formes frustes of CFTR‐related disease. Respiration: International Review of Thoracic Diseases 2007;74(3):241‐51. [DOI] [PubMed] [Google Scholar]
Van Goor 2011
- Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, et al. Correction of the F508del‐CFTR protein processing defect in vitro by the investigational drug VX‐809. Proceedings of the National Academy of Sciences of the United States of America 2011;108(46):18843‐8. [DOI: 10.1073/pnas.1105787108] [DOI] [PMC free article] [PubMed] [Google Scholar]


