Abstract
Background
Antiplatelet agents are recommended for people with myocardial infarction and acute coronary syndromes, transient ischaemic attack or stroke, and for those in whom coronary stents have been inserted. People who take antiplatelet agents are at increased risk of adverse events when undergoing non‐cardiac surgery because of these indications. However, taking antiplatelet therapy also introduces risk to the person undergoing surgery because the likelihood of bleeding is increased. Discontinuing antiplatelet therapy before surgery might reduce this risk but subsequently it might make thrombotic problems, such as myocardial infarction, more likely.
Objectives
To compare the effects of continuation versus discontinuation for at least five days of antiplatelet therapy on the occurrence of bleeding and ischaemic events in adults undergoing non‐cardiac surgery under general, spinal or regional anaesthesia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1), MEDLINE (1946 to January 2018), and Embase (1974 to January 2018). We searched clinical trials registers for ongoing studies, and conducted backward and forward citation searching of relevant articles.
Selection criteria
We included randomized controlled trials of adults who were taking single or dual antiplatelet therapy, for at least two weeks, and were scheduled for elective non‐cardiac surgery. Included participants had at least one cardiac risk factor. We planned to include quasi‐randomized studies.
We excluded people scheduled for minor surgeries under local anaesthetic or sedation in which bleeding that required transfusion or additional surgery was unlikely. We included studies which compared perioperative continuation of antiplatelet therapy versus discontinuation of antiplatelet therapy or versus substitution of antiplatelet therapy with a placebo for at least five days before surgery.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data, assessed risk of bias and synthesized findings. Our primary outcomes were: all‐cause mortality at longest follow‐up (up to six months); all‐cause mortality (up to 30 days). Secondary outcomes included: blood loss requiring transfusion of blood products; blood loss requiring further surgical intervention; risk of ischaemic events. We used GRADE to assess the quality of evidence for each outcome
Main results
We included five RCTs with 666 randomized adults. We identified three ongoing studies.
All study participants were scheduled for elective general surgery (including abdominal, urological, orthopaedic and gynaecological surgery) under general, spinal or regional anaesthesia. Studies compared continuation of single or dual antiplatelet therapy (aspirin or clopidogrel) with discontinuation of therapy for at least five days before surgery.
Three studies reported adequate methods of randomization, and two reported methods to conceal allocation. Three studies were placebo‐controlled trials and were at low risk of performance bias, and three studies reported adequate methods to blind outcome assessors to group allocation. Attrition was limited in four studies and two studies had reported prospective registration with clinical trial registers and were at low risk of selective outcome reporting bias.
We reported mortality at two time points: the longest follow‐up reported by study authors up to six months, and time point reported by study authors up to 30 days. Five studies reported mortality up to six months (of which four studies had a longest follow‐up at 30 days, and one study at 90 days) and we found that either continuation or discontinuation of antiplatelet therapy may make little or no difference to mortality up to six months (risk ratio (RR) 1.21, 95% confidence interval (CI) 0.34 to 4.27; 659 participants; low‐certainty evidence); the absolute effect is three more deaths per 1000 with continuation of antiplatelets (ranging from eight fewer to 40 more). Combining the four studies with a longest follow‐up at 30 days alone showed the same effect estimate, and we found that either continuation or discontinuation of antiplatelet therapy may make little or no difference to mortality at 30 days after surgery (RR 1.21, 95% CI 0.34 to 4.27; 616 participants; low‐certainty evidence); the absolute effect is three more deaths per 1000 with continuation of antiplatelets (ranging from nine fewer to 42 more).
We found that either continuation or discontinuation of antiplatelet therapy probably makes little or no difference in incidences of blood loss requiring transfusion (RR 1.37, 95% CI 0.83 to 2.26; 368 participants; absolute effect of 42 more participants per 1000 requiring transfusion in the continuation group, ranging from 19 fewer to 119 more; four studies; moderate‐certainty evidence); and may make little or no difference in incidences of blood loss requiring additional surgery (RR 1.54, 95% CI 0.31 to 7.58; 368 participants; absolute effect of six more participants per 1000 requiring additional surgery in the continuation group, ranging from seven fewer to 71 more; four studies; low‐certainty evidence). We found that either continuation or discontinuation of antiplatelet therapy may make little or no difference to incidences of ischaemic events (to include peripheral ischaemia, cerebral infarction, and myocardial infarction) within 30 days of surgery (RR 0.67, 95% CI 0.25 to 1.77; 616 participants; absolute effect of 17 fewer participants per 1000 with an ischaemic event in the continuation group, ranging from 39 fewer to 40 more; four studies; low‐certainty evidence).
We used the GRADE approach to downgrade evidence for all outcomes owing to limited evidence from few studies. We noted a wide confidence in effect estimates for mortality at the end of follow‐up and at 30 days, and for blood loss requiring transfusion which suggested imprecision. We noted visual differences in study results for ischaemic events which suggested inconsistency.
Authors' conclusions
We found low‐certainty evidence that either continuation or discontinuation of antiplatelet therapy before non‐cardiac surgery may make little or no difference to mortality, bleeding requiring surgical intervention, or ischaemic events. We found moderate‐certainty evidence that either continuation or discontinuation of antiplatelet therapy before non‐cardiac surgery probably makes little or no difference to bleeding requiring transfusion. Evidence was limited to few studies with few participants, and with few events. The three ongoing studies may alter the conclusions of the review once published and assessed.
Plain language summary
To continue taking or to stop taking antiplatelet drugs for a few days before non‐cardiac surgery in adults
Review question
We set out to determine whether continuing to take antiplatelet drugs before non‐cardiac surgery that requires general, spinal or regional anaesthesia increases the risk of experiencing serious bleeding, ischaemic event or death in adults, when compared with stopping antiplatelet drugs for at least five days before non‐cardiac surgery.
Background
Antiplatelet drugs such as aspirin or clopidogrel reduce the risk of people getting blood clots, and are routinely prescribed for people who have had coronary stents inserted. They are also recommended for people with unstable angina or heart disease, or people who have had a heart attack, heart surgery or a stroke. Taking antiplatelet therapy introduces an increased risk of bleeding, which could lead to problems if a person needs non‐cardiac surgery. Stopping usual antiplatelet therapy a few days before surgery might reduce the risk of serious bleeding during surgery. Not taking these antiplatelet drugs could, however, increase the risk of a heart attack, stroke, or death.
Study characteristics
The evidence from randomized controlled trials is current to January 2018. We included five trials with 666 adults in the review. Three studies are ongoing. All participants were taking antiplatelet therapy (aspirin or clopidogrel) at the start of the study. Two studies stopped antiplatelet drugs for at least five days before surgery, and three studies gave participants a placebo instead of antiplatelet therapy during this time.
Key results
We found low‐certainty evidence that either continuing or stopping antiplatelet therapy may make little or no difference to the number of people who died up to 30 days or six months after surgery (five studies, 659 participants). We found moderate‐certainty evidence that either continuing or stopping antiplatelet therapy probably makes little or no difference to incidences of bleeding serious enough to need a blood transfusion during or immediately after surgery (four studies, 368 participants). We found low‐certainty evidence that either continuing or stopping antiplatelet therapy may make little or no difference to bleeding serious enough to need further surgery (four studies, 368 participants), and may make little or no difference to the number of ischaemic events such as stroke or heart attack (four studies, 616 participants).
Quality of the evidence
Some studies had low risk of bias because they had clearly reported their methods for randomizing people to each group, and three studies used a placebo agent so that people did not know whether or not they were continuing their usual antiplatelet therapy. However, we found few studies with few events, with wide variation in results. To continue or stop taking antiplatelet drugs for a few days before non‐cardiac surgery might make little or no difference to the number of people who died, who had bleeding that needed further surgery or who had ischaemic events, and it probably makes little or no difference to bleeding that needed a blood transfusion. We found three ongoing studies which will increase certainty in the effect in future updates of the review.
Summary of findings
Summary of findings for the main comparison. Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non‐cardiac surgery.
| Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non‐cardiac surgery | ||||||
| Population: adults undergoing non‐cardiac surgery (including abdominal, urological, orthopaedic, and gynaecological surgery) Setting: hospitals in: Denmark, France, Germany, Sweden, USA Intervention: continuation of antiplatelet therapy (aspirin, clopidogrel, ticlopidine, or others) Comparison: discontinuation of antiplatelet therapy; or replacement of antiplatelet therapy with a placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of randomized participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with discontinuation | Risk with continuation | |||||
| All‐cause mortality (at longest follow‐up reported by study authors up to 6 months) |
Study population | RR 1.21 (0.34 to 4.27) | 659 (5 studies) | ⊕⊕⊝⊝ Low1 | 4 studies reported longest follow‐up at 30 days; 1 study reported longest follow‐up at 90 days | |
| 12 per 1,000 | 15 per 1,000 (4 to 52) | |||||
| All‐cause mortality (up to 30 days) |
Study population | RR 1.21 (0.34 to 4.27) | 616 (4 studies) | ⊕⊕⊝⊝ Low1 | 4 studies reported mortality at 30 days | |
| 13 per 1,000 | 16 per 1,000 (4 to 55) | |||||
| Blood loss requiring transfusion of blood products (intraoperatively and postoperatively) |
Study population | RR 1.37 (0.83 to 2.26) | 368 (4 studies) | ⊕⊕⊕⊝ Moderate2 |
||
| 114 per 1,000 | 156 per 1,000 (94 to 257) | |||||
| Blood loss requiring further surgery (intraoperatively and postoperatively) |
Study population | RR 1.54 (0.31 to 7.58) | 368 (4 studies) | ⊕⊕⊝⊝ Low1 | ||
| 11 per 1,000 | 17 per 1,000 (3 to 82) | |||||
| Ischaemic events: peripheral thrombosis, cerebral myocardial infarction (within 30 days of surgery) |
Study population | RR 0.67 (0.25 to 1.77) | 616 (4 studies) | ⊕⊕⊝⊝ Low3 | 2 studies reported data for major thrombotic events (defined as acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, stroke, transient ischaemic attack, acute coronary syndrome, peripheral arterial ischaemia, mesenteric arterial ischaemia, deep proximal or distal venous thrombosis, and pulmonary embolism); 1 study reported data for myocardial infarction; and 1 study reported data for cardio‐cerebrovascular events (which included acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, transient ischaemic attack or stroke) | |
| 52 per 1,000 | 35 per 1,000 (13 to 91) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect | ||||||
1 We downgraded by 2 levels for imprecision because we noted a wide CI for this effect, and because event data were limited to few studies 2We downgraded by 1 level for imprecision because event data were limited to few studies 3We downgraded by 1 level for imprecision because event data were limited to few studies and by 1 level for inconsistency because we noted visual differences in results
Background
Description of the condition
Platelets play a major role in the pathogenesis of atherosclerotic and thrombotic diseases. Drugs intended to prevent or treat these diseases are widely used. Antiplatelet agents are recommended for people with myocardial infarction and acute coronary syndromes, transient ischaemic attack or stroke, and for those in whom coronary stents have been inserted (NICE 2010). Many people are prescribed two antiplatelet drugs (typically aspirin plus another drug), especially after acute coronary syndromes and coronary artery stenting. In the latter context, these drugs are needed to prevent clots from forming and blocking the stent until normal vascular endothelium grows over the metal of the stent. Stents of different types (‘bare metal’ or ‘drug‐eluting’) require different durations of treatment; for instance, dual antiplatelet therapy with aspirin and clopidogrel is recommended for at least one month after insertion of a bare metal stent, and for at least 12 months after insertion of a drug‐eluting stent (Levine 2016). Interfering with platelet function naturally increases risk of bleeding, but in general this risk is low enough to be acceptable (Sorensen 2009). Anticoagulants such as warfarin are often used to prevent and treat thrombosis, and can also cause bleeding significant enough to warrant pharmacological reversal (Johanson 2015); these are outside the scope of this review.
In the year after stent insertion, about 4% of people will have to undergo non‐cardiac surgery (Berger 2010). Such procedures carry increased risk of an adverse outcome, as both myocardial infarction (Hawn 2013; Wijeysundera 2012), and significant bleeding (Singla 2012), are more likely. However, premature discontinuation of dual antiplatelet therapy can be fatal (Korte 2011), in people with coronary stents. On the other hand, dual antiplatelet therapy used for primary prevention of cardiac or cerebrovascular events can be stopped before surgery without major consequences (Oprea 2013).
Description of the intervention
Commonly used antiplatelet agents fall into three pharmacological classes: thromboxane A2 inhibitors (aspirin), thienopyridines (clopidogrel, prasugrel and ticlopidine) and cyclopentyltriazolopyrimidines (ticagrelor).
Aspirin irreversibly inhibits the enzyme cyclo‐oxygenase 1, leading to loss of platelet aggregation. This effect persists for the lifespan of the platelet, which is seven to 10 days (Oprea 2013). The remaining drugs act on the P2Y12 receptor on the platelet surface, preventing it from binding with adenosine diphosphate (ADP) and thus inhibiting aggregation (Oprea 2013). Again, for clopidogrel, this effect is irreversible and lasts as long as the platelet itself. Thus it is necessary to discontinue these agents for at least one week to allow their effects to wear off. Ticagrelor, on the other hand, is a reversible antagonist at the ADP receptor, although reversal of its clinical effect might not be straightforward.
How the intervention might work
The person on antiplatelet agents undergoing surgery is therefore at risk for two types of complications: bleeding and thrombosis. Which carries greater risk will depend on the indications for the antiplatelet agent and on the type of surgery proposed; these and other factors can be incorporated into a ‘matrix’ to help balance the risks for an individual patient (Korte 2011; Rossini 2014). Discontinuing the antiplatelet agent might make thrombotic problems such as myocardial infarction more likely; on the other hand, performing surgery when antiplatelet drugs are still active in the body is likely to increase bleeding. To complicate matters, the haemodynamic instability caused by severe bleeding might in itself lead to myocardial ischaemia and infarction (Devereaux 2016). Some recent studies (Mantz 2011; Oscarsson 2010), have investigated the effects of continuing or discontinuing aspirin during the perioperative period; results demonstrate no difference in the number of bleeding events (Mantz 2011; Oscarsson 2010), but a reduction in the number of major adverse cardiac events when aspirin is continued during the perioperative period (Oscarsson 2010).
Why it is important to do this review
Current recommendations are to usually continue antiplatelet therapy for people with a coronary artery stent (Fleisher 2014; Oprea 2013), and that very low bleeding risk procedures might be undertaken without stopping dual antiplatelet therapy (Keeling 2016). However, very recent evidence suggests that the relationship between continuation of antiplatelet agents and reduced thrombotic complications might not be as simple as one might suppose (Wasowicz 2016). Given this uncertainty and the large numbers of people affected worldwide, a systematic review of available high‐quality evidence is necessary. The results of this review might inform clinical guidelines and might have implications for the costs of health care.
Objectives
To compare the effects of continuation versus discontinuation for at least five days of antiplatelet therapy on the occurrence of bleeding and ischaemic events in adults undergoing non‐cardiac surgery under general, spinal or regional anaesthesia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We planned to include quasi‐randomized studies (e.g. studies in which participants are assigned by alternation, date of birth or medical record number).
Cluster‐RCTs were not eligible for inclusion.
Types of participants
We included studies involving adults who were taking antiplatelet therapy for at least two weeks, and were scheduled for elective surgery. Antiplatelet therapy was prescribed as single or dual therapy to include all antiplatelet agents, such as aspirin, clopidogrel, prasugrel, ticlopidine or ticagrelor. Included participants had at least one cardiac risk factor.
We included studies involving people scheduled for surgery under general, spinal or regional anaesthesia. We excluded people scheduled for cardiac surgery, which requires different clinical management (Wong 2016). We excluded people scheduled for minor surgeries (such as dental extraction) under local anaesthetic or sedation.
Types of interventions
We included studies that compared perioperative continuation of antiplatelet agents (i.e. continuation of dual or single agent therapy during the preoperative, intraoperative and postoperative periods) with discontinuation of antiplatelet therapy for five days or longer before surgery.
We included studies that administered a placebo or no treatment during the discontinuation phase. We planned to exclude studies that continued only one agent in a dual therapy, and also those which assessed the effects of other drugs initiated before surgery (Sanders 2013; Zhao 2016; Zou 2016).
Types of outcome measures
We aimed to establish whether risk of bleeding is affected by continuation of antiplatelet therapy. Therefore, we collected data on two measures of bleeding: the number of patients requiring transfusion of any blood product owing to blood loss during or after surgery; and the number of patients requiring additional surgical intervention for blood loss during or after surgery. Both continuation and discontinuation of antiplatelets might increase the risk of ischaemic events, and we collected composite data on ischaemic events. We recorded ischaemic events for a follow‐up period of 30 days and recorded the number of deaths at two time points: the longest follow‐up time point reported by study authors up to six months, and time points reported by study authors up to 30 days.
Primary outcomes
All‐cause mortality at longest follow‐up (up to six months)
All‐cause mortality (up to 30 days)
Secondary outcomes
Blood loss requiring transfusion of blood products (intraoperatively and postoperatively)
Blood loss requiring further surgical intervention (intraoperatively and postoperatively)
Risk of ischaemic events: peripheral thrombosis, cerebral infarction, myocardial infarction within 30 days
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching with systematic and sensitive search strategies as outlined in Chapter 6.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We applied no restrictions to language or publication status.
We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 1) in the Cochrane Library (searched 2 January 2018)
MEDLINE (OvidSP, 1946 to 2 January 2018)
Embase (OvidSP, 1974 to 2 January 2018)
We developed a subject‐specific search strategy in MEDLINE and used that as the basis for the search strategies in the other listed databases. The search strategy was developed in consultation with the Information Specialist. Search strategies can be found in Appendix 1, Appendix 2, and Appendix 3.
We scanned the following trial registries for ongoing and unpublished trials (24 July 2017).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/AdvSearch.aspx).
ClinicalTrials.gov (ClinicalTrials.gov).
Searching other resources
We carried out citation searching of identified included studies in Web of Science (apps.webofknowledge.com), on 3 January 2018 and conducted a search of grey literature through 'Opengrey' (www.opengrey.eu), on 1 August 2017. We carried out backward citation searching of key reviews identified from the searches. We did not need to contact study authors or organizations.
Data collection and analysis
We (Sharon Lewis (SL) and Oliver Schofield‐Robinson (OSR)) independently completed all data collection and analysis before comparing results and reaching consensus. We consulted a third review author (Phil Alderson (PA) or Andrew Smith (AS)) to resolve conflicts when necessary.
Selection of studies
We used reference management software to collate the results of searches and to remove duplicates (Endnote). We used Covidence software to screen the results of the search from titles and abstracts, identify potentially relevant studies (Covidence) and consider whether they met the inclusion criteria (see Criteria for considering studies for this review). We included abstracts at this stage. However, we planned to include abstracts in the review only if they contained sufficient information and relevant results that included denominator figures for each intervention/comparison group. We recorded the number of papers retrieved at each stage, and reported this information using a PRISMA flow chart (Liberati 2009). We reported in the review brief details of closely related, but excluded, papers.
Data extraction and management
We used Covidence software to extract data from individual studies (Covidence). A basic template of the data extraction forms is available at www.covidence.org. We adapted the template to include the following information.
Methods: the type of study design; setting; dates of study; funding sources.
Participants: the number of participants randomized to each group; baseline characteristics; type of surgery; type of antiplatelet therapy and duration of administration.
Interventions/comparison: type of control (placebo or no treatment); time of discontinuation of antiplatelet therapy before surgery.
Outcomes: all relevant review outcomes measured and reported by study authors.
Outcome data: the results of outcome measures.
We considered the applicability of information from individual studies and the generalizability of data to our intended study population (i.e. the potential for indirectness in our review). Had we identified associated publications from the same study, we planned to create a composite dataset from all eligible publications.
Assessment of risk of bias in included studies
Two review authors (SL and OSR) independently assessed study quality, study limitations and extent of potential bias using the Cochrane 'Risk of bias' tool (Higgins 2011). We considered the following domains.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants, personnel and outcome assessors (performance and detection bias).
Incomplete outcome data (attrition bias).
Selective outcome reporting (reporting bias).
Other.
For each domain, we judged whether study authors made sufficient attempts to minimize bias in their study design. We made judgements using one of three measures (high, low or unclear risk of bias). We recorded this information in the 'Risk of bias' tables and presented a summary 'Risk of bias'.
Measures of treatment effect
To calculate risk ratios (RR), we collected dichotomous data for outcomes related to mortality, number of participants requiring transfusion of blood products or additional surgery for blood loss and number of participants having an ischaemic event.
Unit of analysis issues
If we had included multi‐arm studies comparing different antiplatelet agents or comparing dual‐ and single‐agent therapies versus a control, we planned to include both comparison groups but split the data for the control group, using a 'halving' method to avoid double‐counting, as recommended by Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We collected risk of ischaemic events as composite data. We reported the number of participants who have had at least one ischaemic event and paid attention to how study authors reported this outcome to avoid a unit of analysis issue in which a participant might have experienced more than one different event.
Dealing with missing data
We assessed whether all measured outcomes had been reported by study authors by comparing, when possible, published reports with protocols or clinical trial registration documents that had been prospectively published.
We assessed whether all randomized participants were included in outcome data. We did not need to contact study authors for missing data.
Assessment of heterogeneity
We assessed evidence of inconsistency within our results through consideration of heterogeneity. We assessed clinical heterogeneity by comparing differences in study design, participants, interventions and outcomes in our included studies using the data we collected during data extraction. We assessed statistical heterogeneity by calculating the Chi² P value or the I² statistic. We judged any heterogeneity above an I² statistic of 60% and a Chi² P value of 0.05 or less to indicate moderate to substantial statistical heterogeneity (Higgins 2011).
As well as looking at the statistical results, we considered point estimates and overlap of confidence intervals (CIs). If the CIs overlap, the results are more consistent. However, combined studies might show a large consistent effect but with significant heterogeneity. Therefore, we planned to interpret heterogeneity with caution (Guyatt 2011).
Assessment of reporting biases
We attempted to source published protocols for each of our included studies using clinical trial registers. We compared published protocols with published study results to assess the risk of selective reporting bias. Had we identified sufficient studies (i.e. more than 10) (Higgins 2011), we planned to generate a funnel plot to assess risk of publication bias in the review; an asymmetrical funnel plot might indicate publication of only positive results (Egger 1997).
Data synthesis
We completed meta‐analysis for outcomes for which we had comparable effect measures from more than one study and when measures of heterogeneity indicated that pooling of results was appropriate. We used Review Manager 5 (Review Manager 2014), to perform meta‐analysis.
For each outcome, we calculated risk ratios (RRs) using the summary data presented in each trial report. We used a Mantel‐Haenszel effects model, unless events were extremely rare (1 per 1000), in which case we planned to use Peto odds ratios (Higgins 2011). We used a random‐effects statistical model to account for the variation in different types of surgical procedures in studies (Borenstein 2010). If evidence suggested moderate statistical or clinical heterogeneity, we planned to investigate this by performing subgroup analyses, as below.
Subgroup analysis and investigation of heterogeneity
We aimed to use subgroup analysis to address potential differences in the population group for which the risk‐benefit ratios might differ according to continuation or discontinuation of the drug; whether people were taking a single or dual antiplatelet therapy; and whether they have coronary stents. We also aimed to address whether there is an optimum point at which antiplatelets can be discontinued; people whose therapy has been discontinued earlier than five days might be at increased risk of ischaemic events (Korte 2011).
We planned to perform subgroup analyses as follows.
Single antiplatelet treatment versus dual therapy.
Coronary stents versus no coronary stents.
Discontinuation of antiplatelet agents within five days before surgery versus discontinuation at more than five days before surgery.
Sensitivity analysis
We planned to explore potential effects of decisions made as part of the review process as follows.
Excluding all studies that we judged to be at high or unclear risk of selection bias.
Using the alternate meta‐analytical effects model (fixed‐effect).
We compared effect estimates from the above results with effect estimates from the main analysis. We planned to report differences that alter interpretation of the effect. We planned to perform sensitivity analysis on all of our outcomes.
In sensitivity analysis, we used trial sequential analysis on our primary outcomes using software from The Copenhagen Trial Unit (www.ctu.dk/); this alternative meta‐analytic method accounted for studies with zero events. See Differences between protocol and review. Also, we assessed decisions made on individual studies as part of the review process.
'Summary of findings' table and GRADE
The GRADE approach incorporates assessment of indirectness, study limitations, inconsistency, publication bias and imprecision. We assessed the certainty of the evidence (high, moderate, low, and very low) using these five GRADE considerations and, if required, downgraded the evidence by one or two levels using assessments at each stage of our analysis (Data collection and analysis; Assessment of risk of bias in included studies; Assessment of heterogeneity; Assessment of reporting biases; Data synthesis). This approach gives an overall measure of how certain we can be that our estimate of effect is correct (Guyatt 2008).
We used the principles of the GRADE system to give an overall assessment of evidence certainty related to each of the following outcomes.
All‐cause mortality at longest follow‐up (up to six months).
All‐cause mortality at longest follow‐up (up to 30 days).
Blood loss requiring transfusion of blood products.
Blood loss requiring further surgical intervention.
Risk of ischaemic events: peripheral thrombosis, cerebral infarction, myocardial infarction within 30 days.
One review author (SL) used GRADEpro software (GRADEpro GDT), to create a 'Summary of findings' table for each comparison (www.guidelinedevelopment.org/). A second author (PA) assessed judgements made and consensus was reached through discussion.
Results
Description of studies
Results of the search
After removing duplicates we screened 10,207 titles and abstracts from database searches, results from clinical trial register searches, grey literature searches, and forward and backward citation searches. We carried out full‐text review of 20 articles. We excluded 10 studies. We identified five eligible studies which were randomized controlled trials (RCTs) (two studies had two publications); we found no quasi‐randomized studies. We found three ongoing studies. See Figure 1.
1.
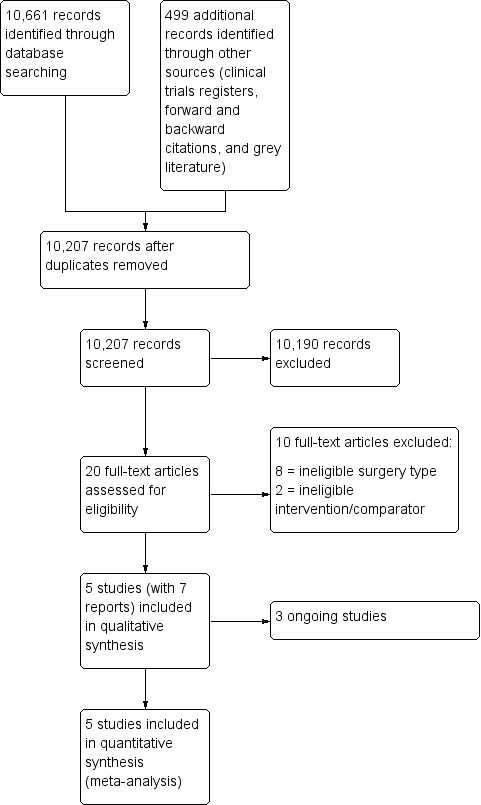
Study flow diagram.
Included studies
We included five RCTs with 666 participants (Antolovic 2012; Chu 2016; Mantz 2011; Nielsen 2000; Oscarsson 2010). All studies were parallel design, three were single‐centre studies (Antolovic 2012; Chu 2016; Nielsen 2000), and two were multi‐centre studies (Mantz 2011; Oscarsson 2010). Two studies had anticipated recruitment of more participants, but stopped early due to recruitment difficulties (Mantz 2011; Oscarsson 2010). In addition, Oscarsson 2010, reported early stopping because of publication during the study period of guidelines about the management of high‐risk patients taking aspirin. See Characteristics of included studies tables.
Study population and setting
Participants were adults scheduled for general elective surgery as follows.
Inguinal hernia repair, cholecystectomy, colonic/colorectal, laparoscopic (Antolovic 2012).
Open inguinal hernia repair, laparoscopic cholecystectomy, laparoscopic or open ventral hernia repair, laparoscopic inguinal repair, Nissen's fundoplication, ostomy closure, open sigmoidectomy and ileostomy (Chu 2016).
Orthopaedic, abdominal, urologic, thoracic, oncologic, ears, nose, and throat (ENT) (Mantz 2011).
Transurethral prostatectomy (Nielsen 2000) and
Abdominal, urologic, orthopaedic, gynaecologic (Oscarsson 2010).
Three studies reported surgery completed under general anaesthesia: in 65.4% participants (Antolovic 2012); in 82% participants (Mantz 2011); and in 22.3% participants (Oscarsson 2010). Surgery was completed using spinal anaesthesia in all participants in Nielsen 2000. One study did not report the type of anaesthesia (Chu 2016).
Three studies reported the number of participants who had coronary stents: 25% participants in Antolovic 2012; 72.1% participants in Chu 2016; and 13.1% participants in Mantz 2011. The studies did not report the length of time since stent insertion. Two studies did not report the number of participants with coronary stents (Nielsen 2000; Oscarsson 2010); one study reported change to study inclusion criteria with a decision to exclude participants with coronary stents in the second year of study (Oscarsson 2010).
Interventions and comparators
In four studies, all participants were on existing antiplatelet therapy (Antolovic 2012; Chu 2016; Mantz 2011; Nielsen 2000), which was: aspirin (100 mg in Antolovic 2012; 150 mg to 300 mg in Nielsen 2000); clopidogrel (75 mg as a single therapy, or in 66.7% participants as dual therapy with aspirin in Chu 2016); and any antiplatelet agent (aspirin, clopidogrel, or ticlopidine and others) in Mantz 2011. In one study, 90% participants were on existing antiplatelet therapy with aspirin (Oscarsson 2010). The studies did not report the length of time that participants had been taking antiplatelet therapy, and we assumed that all participants had been taking antiplatelets for at least two weeks.
Three studies were placebo‐controlled trials (Mantz 2011; Nielsen 2000; Oscarsson 2010). These studies substituted existing therapy with placebo or with aspirin (75 mg aspirin in Mantz 2011 and Oscarsson 2010; 150 mg aspirin in Nielsen 2000).
In two studies, participants continued existing antiplatelet therapy at existing dose or discontinued existing antiplatelet therapy without substitution agent during study period (Antolovic 2012; Chu 2016).
Time points for discontinuation of antiplatelet prior to surgery were at: five days before surgery (Antolovic 2012); seven days before surgery (Chu 2016; Oscarsson 2010); and 10 days before surgery (Mantz 2011; Nielsen 2000).
Sources of funding
Four studies reported the sources of funding (Chu 2016; Mantz 2011; Nielsen 2000; Oscarsson 2010). We noted support from pharmaceutical companies in two of these studies (Nielsen 2000; Oscarsson 2010). One study did not report the sources of funding (Antolovic 2012), and the study authors declared no conflict of interest.
Excluded studies
We excluded 10 studies from review of full‐text articles (Ardekian 2000; Assia 1998; Danino 2007; Devereaux 2014; Duygu 2010; Eapen 2017; Engheta 2016; Gaspar 1999; Medeiros 2011; Varghese 2015). In one study, interruption of long‐term antiplatelet therapy was compared with a substitution agent rather than no agent or a placebo (Danino 2007). One study compared antiplatelets with a placebo, and participants were stratified according to whether they were previously taking antiplatelets; participants who were taking antiplatelets were required to stop treatment for at least three days prior to surgery. We excluded this study because of interruption to treatment (Devereaux 2014). We excluded eight studies because surgery was minor and either under local anaesthetic or sedation (Ardekian 2000; Assia 1998; Duygu 2010; Eapen 2017; Engheta 2016; Gaspar 1999; Medeiros 2011; Varghese 2015). These surgery types were not comparable to other non‐cardiac surgical procedures included in the review. We also noted that the risk of bleeding in the studies was limited to use of local haemostatic measures to control bleeding events. See Characteristics of excluded studies.
Awaiting classification
There are no studies awaiting classification.
Ongoing studies
We found three ongoing studies in clinical trials register searches (NCT01806090; NCT02797548; NCT03184805). The studies have an anticipated recruitment of 4616 participants, and compare continuation versus discontinuation of antiplatelet therapy before non‐cardiac surgery in adults. See Characteristics of ongoing studies.
Risk of bias in included studies
See Characteristics of included studies tables, and 'Risk of bias' summary and 'Risk of bias' graph (Figure 2; Figure 3).
2.
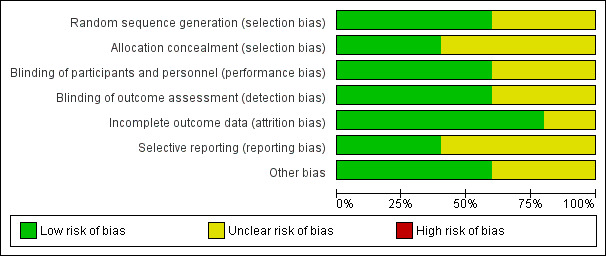
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
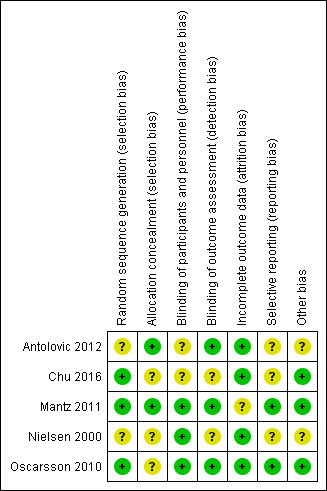
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All included studies were described as randomized, and we judged three of the five studies to have a low risk of bias because they provided sufficient details on the method of randomization (Chu 2016; Mantz 2011; Oscarsson 2010). Two studies reported no details of the method of randomization, and we judged these studies to have an unclear risk of bias (Antolovic 2012; Nielsen 2000).
Two studies reported sufficient detail about methods used to conceal allocation, and we judged these studies to have low risk of bias for allocation concealment (Antolovic 2012; Mantz 2011). The three remaining studies reported no details of allocation concealment, and we judged these studies to have an unclear risk of bias.
Blinding
Three studies were placebo‐controlled trials and study authors reported, or we assumed, that participants and personnel were blinded to group allocation. We judged these three studies to have low risk of performance bias (Mantz 2011; Nielsen 2000; Oscarsson 2010). One study reported that participants were not blinded to group allocation (Chu 2016), and one study reported no information about blinding of participants and personnel (Antolovic 2012).We judged these studies to have and unclear risk of performance bias. However, we acknowledge that it is possible that awareness of group allocation might influence outcome data because some participants might not have adhered to study protocol if requested to discontinue regular antiplatelet therapy.
Three studies reported that outcome assessors were blinded, and we judged these studies to be at low risk of detection bias (Antolovic 2012; Mantz 2011; Oscarsson 2010). The two remaining studies reported insufficient information about blinding of outcome assessors, and we judged these studies to have an unclear risk of bias (Chu 2016; Nielsen 2000).
Incomplete outcome data
Four studies reported no participant losses during the trial or few losses, and we judged these to have low risk of attrition bias (Antolovic 2012; Chu 2016; Nielsen 2000; Oscarsson 2010). One study reported a large number of losses (Mantz 2011), but study authors reported reasons for these losses. We judged this study to have unclear risk of bias because we noted more losses in the continuation group, and we could not be certain of the effect of these losses on the results.
Selective reporting
We found registration with clinical trial registers for two studies, and outcomes were reported according to these documents.We judged these studies to have low risk of selective reporting bias (Mantz 2011; Oscarsson 2010). Two studies reported retrospective clinical trial registration, and it was not feasible to use these documents to assess risk of selective reporting bias (Antolovic 2012; Chu 2016). One study did not report details of a published protocol (Nielsen 2000). We judged these studies to have unclear risk of selective reporting bias.
Other potential sources of bias
One study reported limited baseline characteristics, and details of existing antiplatelet therapies (Nielsen 2000). We noted a difference in ranges of ages between groups in one study (Antolovic 2012). We were unclear whether information that was not reported might have influenced the study results, and we judged these studies to have an unclear of risk of other bias. We noted no other sources of bias in the three remaining studies which we judged to have low risk of bias (Chu 2016; Mantz 2011; Oscarsson 2010).
Effects of interventions
See: Table 1
Primary outcomes
1. All‐cause mortality at longest follow‐up (up to six months)
All five studies reported mortality (Antolovic 2012; Chu 2016; Mantz 2011; Nielsen 2000; Oscarsson 2010). Longest follow‐up time points for data were 30 days after surgery in four studies (Antolovic 2012; Mantz 2011; Nielsen 2000; Oscarsson 2010), and at 90 days in one study (Chu 2016).
We noted no evidence of a difference in deaths according to whether participants continued or discontinued antiplatelet therapy (risk ratio (RR) 1.21, 95% confidence interval (CI) 0.34 to 4.27; 659 participants; Analysis 1.1).
1.1. Analysis.
Comparison 1 Continuation vs discontinuation, Outcome 1 All‐cause mortality at end of follow up (up to six months).
We used the GRADE approach to downgrade the evidence to low quality. We downgraded by two levels for imprecision because we noted a wide CI for this effect and because event data were limited to only three of the five studies. See Table 1.
2. All‐cause mortality (up to 30 days)
Four studies reported mortality at 30 days (Antolovic 2012; Mantz 2011; Nielsen 2000; Oscarsson 2010).
We noted no evidence of a difference in deaths according to whether participants continued or discontinued antiplatelet therapy (RR 1.21, 95% CI 0.34 to 4.27; 616 participants; Analysis 1.2).
1.2. Analysis.
Comparison 1 Continuation vs discontinuation, Outcome 2 All‐cause mortality (up to 30 days).
We used the GRADE approach to downgrade the evidence to low quality. We downgraded by two levels for imprecision because we noted a wide CI for this effect and because event data were limited to only three of the four studies. See Table 1.
Secondary outcomes
1. Blood loss requiring transfusion of blood products (intraoperatively and postoperatively)
Four studies reported blood loss requiring transfusion (Antolovic 2012; Chu 2016; Nielsen 2000; Oscarsson 2010). We interpreted data in Nielsen 2000, from a graph in the published report. One study reported major bleeding events but did not define major bleeding by need for transfusion or surgery and we could not include these data in the analysis (Mantz 2011). We noted no difference between groups in these data and have reported the results separately in Table 2. Bleeding was not monitored in any of these trials (Wikkelsø 2016).
1. Data table.
| Outcome: major bleeding event | |||
| Study | Continuation group; n = 145 | Discontinuation group; n = 146 | Effect estimate* |
| Mantz 2011 | 10 participants had at least 1 major bleeding event | 10 participants had at least 1 major bleeding event | OR 0.99, 95% CI 0.36 to 2.8; P = 1.0 |
* as reported by study authors CI: confidence interval n: number of randomized participants OR: odds ratio
We noted no evidence of a difference in the number of participants requiring blood transfusions according to whether participants continued or discontinued antiplatelets (RR 1.37, 95% CI 0.83 to 2.26; 368 participants; Analysis 1.3).
1.3. Analysis.
Comparison 1 Continuation vs discontinuation, Outcome 3 Blood loss requiring transfusion of blood products (intraoperatively and postoperatively).
We used the GRADE approach to downgrade the evidence to moderate quality. We downgraded by one level for imprecision because event data were limited to only three of four studies. See Table 1.
2. Blood loss requiring further surgical intervention (intraoperatively and postoperatively)
Four studies reported blood loss requiring surgical intervention (Antolovic 2012; Chu 2016; Nielsen 2000; Oscarsson 2010). One study reported major bleeding events but did not define major bleeding by need for transfusion or surgery, and we could not include these data in analysis (Mantz 2011). We noted no difference between groups in these data and have reported it separately in Table 2.
We noted no difference in number of participants with blood loss requiring surgical interventions (RR 1.54, 95% CI 0.31 to 7.58; 368 participants; Analysis 1.4).
1.4. Analysis.
Comparison 1 Continuation vs discontinuation, Outcome 4 Blood loss requiring further surgical intervention (intraoperatively and postoperatively).
We used the GRADE approach to downgrade the evidence to low quality. We downgraded by two levels for imprecision because event data were limited to only three of four studies and because we noted a wide CI for this effect. See Table 1.
3. Risk of ischaemic events: peripheral thrombosis, cerebral infarction, myocardial infarction within 30 days
Four studies reported ischaemic events (Antolovic 2012; Mantz 2011; Nielsen 2000; Oscarsson 2010). We reported data collected for major thrombotic events (defined as acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, severe pulmonary embolism, transient ischaemic attack, or stroke in Antolovic 2012; and defined as stroke, transient ischaemic attack, acute coronary syndrome, peripheral arterial ischaemia, mesenteric arterial ischaemia, deep proximal and distal venous thrombosis, and pulmonary embolism in Mantz 2011); for myocardial infarction in Nielsen 2000; and for cardio‐cerebrovascular events (which included acute myocardial infarction, severe arrhythmia, cardiac arrest, cardiovascular death, transient ischaemic attack or stroke) in Oscarsson 2010.
We noted no evidence of a difference in number of participants with an ischaemic event (RR 0.67, 95% CI 0.25 to 1.77; 616 participants; Analysis 1.5).
1.5. Analysis.
Comparison 1 Continuation vs discontinuation, Outcome 5 Risk of ischaemic events (within 30 days).
We used the GRADE approach to downgrade the evidence to low quality. We downgraded by one level for imprecision because event data were limited to only three of four studies. We downgraded by one level for inconsistency because we noted visual differences in results. See Table 1.
Subgroup analysis
We did not conduct subgroup analysis because we included insufficient studies for each outcome to justify meaningful subgroup analysis (Higgins 2011).
Sensitivity analysis
We completed the following sensitivity analyses which were specified in the protocol.
Studies at high or unclear risk of selection bias. We judged only one study to have low risk of selection bias in both domains (sequence generation and allocation concealment), and sensitivity analysis was not feasible.
Meta‐analytical effects model. We assessed whether using a fixed‐effect model would alter effect estimates for our outcomes. We found no difference in interpretation of effect estimates depending on meta‐analytical effects model for each of our outcomes.
We completed the following sensitivity analyses which were performed as posthoc decisions (see Differences between protocol and review).
Assessment of the primary outcomes using trial sequential analysis. We used a conventional test boundary and a random‐effects model (DerSimonian‐Laird method) to perform trial sequential analysis using software from The Copenhagen Trial Unit (www.ctu.dk/) with continuity corrected for studies with zero events. We found no difference in interpretation of the result for all‐cause mortality at the end of follow‐up (up to six months), and all‐cause mortality (up to 30 days) when analysis accounted for studies with zero events (RR 1.17, 95% CI 0.37 to 3.67).
Decisions made during the review process. We included one study in which 10% of participants were not on antiplatelet therapy before the start of the study (Oscarsson 2010). In sensitivity analysis, we excluded this study. We noted no difference to interpretation of effect estimates for each outcome. In one study, we interpreted data for the number of participants with a bleeding event requiring transfusion from a graph (Nielsen 2000). In sensitivity analysis, we excluded this study for this outcome and noted no difference to interpretation of the effect estimate.
Discussion
Summary of main results
We included five studies comparing continuation of antiplatelet therapy with discontinuation of antiplatelet before non‐cardiac surgery. The five studies included 666 adults. We found three ongoing studies.
We found no evidence of a difference in mortality up to six months, and up to 30 days, after surgery, and no evidence of a difference in bleeding requiring further surgery or in ischaemic events. We used the GRADE approach to downgrade evidence for these outcomes to low certainty. We found no evidence of a difference in bleeding requiring transfusion, and we used the GRADE approach to downgrade evidence to moderate certainty.
Overall completeness and applicability of evidence
We included studies of adults who were taking antiplatelet drugs and were scheduled for a variety of different surgical procedures. The study authors did not report the length of time that the participants had been on antiplatelet therapy. Three studies reported the number of people who had a coronary stent, and this was balanced between the groups. However, the length of time since insertion of stent was not reported by study authors. We noted that the number of people with coronary stents differed between these three studies.
Evidence was limited to five studies. Because data were limited, we did not conduct subgroup analysis to explore whether potential differences between studies (e.g. whether participants were on single or dual therapy, had coronary stents, or discontinuation of therapy was before or after five days) could influence the results.
Quality of the evidence
Three studies reported adequate methods of randomization, and two reported methods to conceal allocation. Three studies were placebo‐controlled trials and were at low risk of performance bias, and three studies reported adequate methods to blind outcome assessors to group allocation. Attrition was limited in four studies and two studies had reported prospective registration with clinical trial registers and were at low risk of selective outcome reporting bias.
We used the GRADE approach to downgrade evidence for this review. We noted limited available data for each outcome, a wide confidence interval in evidence for mortality and for blood loss requiring further surgery, and visual differences noted between studies for ischaemic events; we used these reasons to downgrade evidence to low certainty, or moderate certainty.
Potential biases in the review process
We conducted a thorough search and used two review authors to assess study eligibility, to extract data, and to assess risk of bias in included studies, and believe that we reduced potential bias in the review process by using two review authors. However, we did not contact study authors to clarify missing information (such as length of follow‐up, funding sources, or missing information for 'Risk of bias' assessments), and judgements made in the review were based only on information in published reports.
We included one study in which some participants were not on antiplatelets before the start of the trial (Oscarsson 2010). We carried out sensitivity analysis and results were unaffected if this study was excluded from analysis; we believed that including this study did not introduce bias to the results.
We made a post‐hoc decision to exclude studies in which people were scheduled for minor surgeries under local anaesthetic or sedation. These type of surgeries would introduce considerable clinically heterogeneity in the review, and are unlikely to lead to major bleeding requiring transfusion or additional surgery. For this review, eight of the 10 excluded studies were studies of minor surgeries under local anaesthetic or sedation (dental extraction, cataract surgery, removal of skin tumour). These studies did not measure outcome data or provide useful information for this review and, therefore, exclusion of these studies did not influence effect estimates for this review. See Differences between protocol and review.
Agreements and disagreements with other studies or reviews
We found no systematic reviews that considered only randomized controlled trials (RCTs) for non‐cardiac surgery. One review recommended discontinuation of clopidogrel for at least five days before surgery, but this judgement was based on limited evidence from observational studies of non‐cardiac surgery, and evidence from studies of cardiac surgery (Au 2012). Another review concluded no evidence of excessive bleeding from continuation of aspirin using limited evidence from both RCTs and observational studies (Sahebally 2014). Burger 2005 noted an increase in the risk of bleeding complications with aspirin use but without an increase in the severity of the bleeding event; this review included both RCTs and observation studies. We excluded one large RCT from this review because participants taking aspirin were required to stop at least three days before surgery, and was not comparable with other studies in this review (Devereaux 2014). We noted that this large study of non‐cardiac surgery similarly found little or no difference in all‐cause mortality at 30 days or in ischaemic events. Devereaux 2014 found an increased risk of major bleeding in participants who were taking aspirin in the perioperative period.
Authors' conclusions
Implications for practice.
We found low‐certainty evidence that either continuation or discontinuation of antiplatelet therapy before non‐cardiac surgery may make little or no difference to mortality, bleeding requiring surgical intervention, or ischaemic events. We found moderate‐certainty evidence that either continuation or discontinuation of antiplatelet therapy before non‐cardiac surgery probably makes little or no difference to bleeding requiring transfusion. Evidence was limited to few studies with few participants, and with few events. The three ongoing studies may alter the conclusions of the review once published and assessed.
Implications for research.
Additional randomized controlled trials (RCTs) of antiplatelet therapy management in adults undergoing non‐cardiac surgery are required to add certainty to the review outcomes. We found three ongoing studies with an anticipated large sample size. These studies will increase certainty in the effect in future updates.
What's new
| Date | Event | Description |
|---|---|---|
| 4 October 2018 | Amended | Acknowledgement section amended to include Sign‐off Editor |
Acknowledgements
We would like to thank Jane Cracknell (Managing Editor), Arash Afshari (Content Editor), Jing Xie (Statistical Editor), Gianni Virgili, Michael J Desborough, Ben Gibbison (Peer Reviewers), Janet Wale (Consumer Editor), Harald Herkner (Sign‐off Editor), for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Arash Afshari (Content Editor), Jing Xie (Statistical Editor), Michael Desborough, Domenico Prisco (Peer Reviewers) for their help and editorial advice during the preparation of the protocol for the systematic review (Lewis 2017).
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Anticoagulants] explode all trees |
| #2 | MeSH descriptor: [Platelet Aggregation Inhibitors] explode all trees |
| #3 | MeSH descriptor: [Aspirin] explode all trees |
| #4 | MeSH descriptor: [Thienopyridines] explode all trees |
| #5 | #1 or #2 or #3 or #4 |
| #6 | anti?platelet* or anti platelet* or anti?thrombo* or anti thrombo* or anti?coagulant or anti coagulant or aspirin* or clopidogrel or prasugrel or ticagrelor or ticlopidine or (platelet and aggregation and inhibitor*) or (anti and thrombosis) |
| #7 | #5 or #6 |
| #8 | MeSH descriptor: [General Surgery] explode all trees |
| #9 | surgery or (operative and procedure*) or (operative and surgical and procedure*) or (surgical and procedure*) |
| #10 | #8 or #9 |
| #11 | MeSH descriptor: [Hemorrhage] explode all trees |
| #12 | bleeding or ischemi* or ischaemic* |
| #13 | #11 or #12 |
| #14 | #7 and #10 and #13 |
Appendix 2. MEDLINE search strategy
| 1 | Anticoagulants/ or Platelet Aggregation Inhibitors/ or Aspirin/ or Thienopyridines/ |
| 2 | (anti?platelet* or anti platelet* or anti?thrombo* or anti thrombo* or anti?coagulant or anti coagulant or aspirin* or clopidogrel or prasugrel or ticagrelor or ticlopidine or (platelet and aggregation and inhibitor*) or (anti and thrombosis)).mp. |
| 3 | 1 or 2 |
| 4 | general surgery/ |
| 5 | (surgery or (operative and procedure*) or (operative and surgical and procedure*) or (surgical and procedure*)).mp. |
| 6 | 4 or 5 |
| 7 | Hemorrhage/ |
| 8 | (bleeding or isch?emia).mp. |
| 9 | 7 or 8 |
| 10 | 3 and 6 and 9 |
| 11 | ((randomized controlled trial or controlled clinical trial).pt. or randomi*.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. |
| 12 | 10 and 11 |
Appendix 3. Embase search strategy
| 1 | antithrombocytic agent/ or acetylsalicylic acid/ or anticoagulant agent/ |
| 2 | (anti?platelet* or anti platelet* or anti?thrombo* or anti thrombo* or anti?coagulant or anti coagulant or aspirin* or clopidogrel or prasugrel or ticagrelor or ticlopidine or (platelet and aggregation and inhibitor*) or (anti and thrombosis)).mp. |
| 3 | 1 or 2 |
| 4 | general surgery/ |
| 5 | (surgery or (operative and procedure*) or (operative and surgical and procedure*) or (surgical and procedure*)).mp. |
| 6 | 4 or 5 |
| 7 | exp bleeding/ or bleeding.mp. or ischaemic.mp. |
| 8 | 3 and 6 and 7 |
| 9 | ((crossover procedure or double blind procedure or single blind procedure).sh. or (crossover* or cross over*).ti,ab. or placebo*.ti,ab,sh. or (doubl* adj blind*).ti,ab. or (controlled adj3 (study or design or trial)).ti,ab. or allocat*.ti,ab. or trial*.ti,ab. or randomized controlled trial.sh. or random*.ti,ab.) not ((exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.)) |
| 10 | 8 and 9 |
Data and analyses
Comparison 1. Continuation vs discontinuation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality at end of follow up (up to six months) | 5 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.34, 4.27] |
| 2 All‐cause mortality (up to 30 days) | 4 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.34, 4.27] |
| 3 Blood loss requiring transfusion of blood products (intraoperatively and postoperatively) | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.83, 2.26] |
| 4 Blood loss requiring further surgical intervention (intraoperatively and postoperatively) | 4 | 368 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.31, 7.58] |
| 5 Risk of ischaemic events (within 30 days) | 4 | 616 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.25, 1.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antolovic 2012.
| Methods | RCT, single‐centre, parallel study | |
| Participants |
Total number of randomized participants: 52 Inclusion criteria
Exclusion criteria
Type of surgery
Baseline characteristics Continuation group
Discontinuation group
Overall
Country: Germany Setting: hospital |
|
| Interventions |
Continuation group
Discontinuation group
|
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: funding not reported. Study authors declare no conflicts of interest Study dates: January 2009 to January 2010 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized, but no additional details provided in the published study report |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed in opaque, sequentially numbered envelopes supplied by external trial centre |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants are not blinded which might influence adherence to study protocol for participants in the discontinuation group. Study authors do not state if personnel were aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent observers performed data collection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No apparent losses |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trial registration ISRCTN45810007. Not feasible to assess risk of selective outcome reporting bias |
| Other bias | Unclear risk | Baseline characteristics well reported. We noted that mean age of participants was similar between groups, but age ranges showed that the youngest participant in the intervention group was considerably younger than in the control group |
Chu 2016.
| Methods | RCT, single‐centre, parallel study | |
| Participants |
Total number of randomized participants: 48 Inclusion criteria
Exclusion criteria
Type of surgery
Baseline characteristics Continuation group
Discontinuation group
Country: USA Setting: hospital |
|
| Interventions |
Continuation group
Discontinuation group
|
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: grant from Doris Duke Foundation Study dates: January 2011 to May 2013 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computerized software for randomization method |
| Allocation concealment (selection bias) | Unclear risk | No details describing allocation concealment were provided in the published study report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and personnel were not blinded to group allocation and we did not know whether this would influence performance |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details describing blinding of outcome assessment were provided in the published study report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few losses, explained by study authors |
| Selective reporting (reporting bias) | Unclear risk | Retrospective clinical trial registration NCT01960296. Not feasible to make judgement on risk of selective reporting bias |
| Other bias | Low risk | No other sources of bias identified |
Mantz 2011.
| Methods | RCT, multi‐centre, parallel study | |
| Participants |
Total number of randomized participants: 291 Inclusion criteria
Exclusion criteria
Types of surgery
Baseline characteristics Continuation group
Discontinuation group
Country: France Setting: hospital |
|
| Interventions |
Continuation group
Discontinuation group
|
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: grant from French Ministry of Health and sponsored by the Department of Clinical Research and Development, Assistance Publique des Hȏpitaux de Paris Study dates: June 2005 to September 2007. Note
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated randomization system |
| Allocation concealment (selection bias) | Low risk | Allocation maintained through use of a centralized system, accessed by telephone |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial. Investigators, patients, and healthcare providers were not aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators collecting outcome data were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Use of ITT analysis, but high number of losses with more losses in the continuation group; losses explained by study authors |
| Selective reporting (reporting bias) | Low risk | Clinical trial registration NCT00190307. Registered shortly after start of recruitment. Outcomes reported according to registration documents |
| Other bias | Low risk | No other sources of bias identified |
Nielsen 2000.
| Methods | RCT, single‐centre, parallel study | |
| Participants |
Total number of randomized participants: 55 Inclusion criteria
Exclusion criteria
Type of surgery
Baseline characteristics Continuation group
Discontinuation group
Country: Denmark Setting: hospital |
|
| Interventions |
Continuation group
Discontinuation group
|
|
| Outcomes |
|
|
| Notes |
Funding/declarations of interest: Leo Pharmaceutical Products, Ballerup, Denmark. Donated medication Study dates: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomized but no additional details describing random sequence generation were provided in the published study report |
| Allocation concealment (selection bias) | Unclear risk | Allocation list was kept at the pharmaceutical company which provided the drugs. No additional details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated as double‐blind and placebo was used to blind the participants. We assumed that personnel were also blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details describing blinding of outcome assessment were provided in the published study report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two losses from each group. Study authors do not report to which group these participants belonged. However, few losses and unlikely to influence outcome data |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration or prospectively published protocol not reported. Not feasible to assess risk of selective reporting bias |
| Other bias | Unclear risk | Study is supported by pharmaceutical company. Study authors do not report level of involvement in trial design and management by the company. Baseline characteristics not reported |
Oscarsson 2010.
| Methods | RCT, multi‐centre, parallel study | |
| Participants |
Total number of randomized participants: 220 Inclusion criteria
Exclusion criteria
Type of surgery
Baseline characteristics Continuation group
Discontinuation group
Country: Sweden Setting: hospitals |
|
| Interventions |
Continuation group
Discontinuation group
|
|
| Outcomes |
All assessed within first 30 postoperative days |
|
| Notes |
Funding/declarations of interest: supported by grants from: the Health Research Council in South‐East of Sweden; European Society of Anaesthesiology; county council of Östergötland, Weden; Stina and Birger Johansson Foundation, Sweden; The Heart Foundation, Sweden; Linköping Medical Association; Pfizer Inc., Stokholm Study dates: November 2005 and December 2008. Note
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization |
| Allocation concealment (selection bias) | Unclear risk | No details describing methods to conceal allocation were provided in the published study report |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo controlled trial, and we assume participants are unlikely to be aware of group allocation. We assumed that personnel were also unaware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Signs of myocardial ischaemia were assessed by a clinical physiologist who was blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of 17 participants. Low number unlikely to influence outcome data. We noted that although ITT was used, study authors assumed that lost participants had no events |
| Selective reporting (reporting bias) | Low risk | Prospective clinical trial registration (Eudract CT no. 2004‐005136‐76). No evidence of selective reporting based on trial protocol |
| Other bias | Low risk | No other sources of bias identified |
ASA: American Society of Anesthesiologists BMI: body mass index CAD: coronary artery disease CT: computerized tomography CVA: cerebrovascular accident DAPT: dual antiplatelet therapy ENT: ears, nose, and throat GA: general anaesthesia IQR: interquartile range ITT: intention to treat LA: local anaesthesia M/F: male/female MACE: major adverse cardiovascular event n: number of participants NSAIDs: nonsteroidal anti‐inflammatory drugs RCT: randomized control trial SD: standard deviation SPA: spinal anaesthesia TIA: transient ischaemic attack TURP: transurethral resection of the prostate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ardekian 2000 | RCT. Participants scheduled for dental extraction under local anaesthetic. Continuation of 100 mg aspirin versus discontinuation of aspirin 7 days before procedure. Outcomes included bleeding (mild: < 20 mL; moderate: 20 mL to 50 mL; severe: > 50 mL). We excluded this study because there was minimal bleeding risk in this type of surgery. |
| Assia 1998 | RCT. Participants scheduled for cataract surgery under local anaesthetic. Continuation of aspirin, versus discontinuation of aspirin for 2 to 5 days before procedure versus discontinuation of aspirin for 7 to 10 days before procedure. We excluded this study because there was minimal bleeding risk in this type of surgery. |
| Danino 2007 | RCT. Participants undergoing surgery for skin carcinoma. Continuation of antiplatelet agents compared with discontinuation; however participants in discontinuation group were given an alternative agent (flurbiprofen or isocoagulant fractionated heparin). |
| Devereaux 2014 | RCT. Participants undergoing non‐cardiac surgery were assigned to receive aspirin or placebo, or clonidine or placebo. Participants stratified according to whether they had been taking aspirin before the study. However, participants in the continuation group were required to stop taking aspirin at least 3 days before surgery. We excluded this because this was not comparable to other studies in which 'continuation' did not involve any interruption of antiplatelet agent before surgery. |
| Duygu 2010 | RCT. Participant scheduled for dental surgery under local anaesthetic. Continuation of aspirin (75 mg to 300 mg) versus discontinuation for 7 days before procedure. Outcomes measured were bleeding time and haemostatic measures to control bleeding (e.g. gauze soaked with resorbable gelatin). We excluded this study because there was minimal bleeding risk in this type of surgery. |
| Eapen 2017 | RCT. Participants scheduled for dental extraction under local anaesthetic. Continuation of 75 mg aspirin or discontinuation of aspirin for 5 days before procedure. Outcomes measured were bleeding times, prolonged bleeding, and need for local haemostatic measures to control bleeding. We excluded this study because there was minimal bleeding risk in this type of surgery. |
| Engheta 2016 | RCT. Participants scheduled for skin tumour surgery under sedation. Continuation of aspirin (80 mg) versus placebo for 7 days before procedure. Outcomes measured volume of bleeding, need for early changing of dressings, development of haematoma or local anticoagulation disorders. We excluded this study because there was minimal bleeding risk in this type of surgery. |
| Gaspar 1999 | RCT. Participants scheduled for dental surgery. Continuation of low‐dose aspirin or discontinuation of aspirin for 1 week before procedure. Outcomes measured bleeding times, and use of haemostatic measures to control bleeding. We excluded this study because there was minimal bleeding risk in this type of surgery. |
| Medeiros 2011 | RCT. Participants scheduled for single tooth extraction under local anaesthetic. Continuation of 100 mg aspirin of discontinuation of aspirin for 7 days before procedure. Outcomes measured volume of bleeding, need for haemostatic measures to control bleeding (e.g. use of sutures). We excluded this study because there was minimal bleeding risk in this type of surgery. |
| Varghese 2015 | RCT. Participants scheduled for dental extraction under local anaesthetic. Continuation of antiplatelet therapy (aspirin or clopidogrel, or both) or discontinuation of antiplatelet therapy for 5 days before procedure. Outcomes measured bleeding (oozing or active) using haemostatic measures to control bleeding. We excluded this study because there was minimal bleeding risk in this type of surgery. |
RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01806090.
| Trial name or title | Uninterrupted clopidogrel therapy before elective colonoscopy will increase the risk of post‐polypectomy bleeding |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 216 Inclusion criteria
Exclusion criteria
Type of surgery: colonoscopy Country: China Setting: hospital |
| Interventions |
Continuation group
Discontinuation group
|
| Outcomes |
|
| Starting date | February 2012 |
| Contact information | suenbingyee@surgery.cuhk.edu.hk |
| Notes |
NCT02797548.
| Trial name or title | Perioperative antiplatelet therapy in patients with drug‐eluting stent undergoing noncardiac surgery (ASSURE‐DES) |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 2000 Inclusion criteria
Exclusion criteria
Type of surgery: non‐cardiac surgery Country: Republic of Korea Setting: hospital |
| Interventions |
Continuation group
Discontinuation group
|
| Outcomes |
All outcomes measured at 30 days |
| Starting date | 16 March 2017 |
| Contact information | sjpark@amc.seoul.kr |
| Notes |
NCT03184805.
| Trial name or title | STOPping versus continuing antiplatelet therapy during noncardiac surgery and procedures after next generation drug‐eluting stent implantation |
| Methods | RCT, parallel design |
| Participants |
Target number of randomized participants: 2400 Inclusion criteria
Exclusion criteria
Type of surgery: elective non‐cardiac surgery or invasive procedure Country: Republic of Korea Setting: hospital |
| Interventions |
Continuation group
Discontinuation group
|
| Outcomes |
All outcomes measured at 1 day after hospital discharge |
| Starting date | June 2017 |
| Contact information | mkhong61@yuhs.ac |
| Notes |
BARC: Bleeding Academic Research Consortium BMS: bare‐metal stent CVA: cerebrovascular accident DES: drug‐eluting stent EMR: endoscopic mucosal resection ERCP: endoscopic retrograde cholangiopancreatography ESD: endoscopic submucosal dissection EUS‐FNA: endoscopic ultrasonography‐guided fine‐needle aspiration NYHA: New York Heart Association PCI: percutaneous coronary intervention RCT: randomized controlled trial
Differences between protocol and review
We made the following changes to the published protocol (Lewis 2017).
Review authors: we added two new review authors (Michael W Pritchard and Oliver J Schofield‐Robinson).
Objectives: we edited the objectives to reflect changes made to types of participants, and to specify that discontinuation was required for at least five days before surgery.
Types of participants: we excluded people scheduled for minor surgeries under local anaesthetic or sedation because we anticipated that clinical heterogeneity would be too great between minor surgery under local anaesthetic or sedation and other surgery under general, spinal or regional anaesthesia). We added an inclusion criteria by type of anaesthesia (people scheduled for surgery under general, spinal or regional anaesthesia) in order to distinguish between studies in which minor surgery was performed under local anaesthetic or sedation.
Types of interventions: we clarified exclusion of studies which assessed drugs others than antiplatelet agents because this review aimed to specifically address continuation or discontinuation of antiplatelets therapy.
Types of outcome measures: for clarity, we edited the wording of the time points for mortality data collection for the primary outcomes 1 and 2. We collected mortality data at the longest follow‐up time point reported by study authors (up to six months) for outcome 1, and at a time point up to 30 days for outcome 2.
We added a time point (intraoperatively and postoperatively) to the outcome 'Blood loss requiring further surgical intervention'.
Sensitivity analysis: we added an additional sensitivity analysis using an alternative meta‐analytic tool (trial sequential analysis) on our primary outcomes. We added this analysis because this method accounts for studies with zero events which we did not anticipate during preparation of the protocol.
Contributions of authors
Sharon R Lewis (SL), Oliver Schofield‐Robinson (OSR), Michael W Pritchard (MP), Phil Alderson (PA), Andrew F Smith (AS)
Co‐ordinating the review: SL.
Undertaking manual searches: SL, OSR.
Screening search results: SL, OSR.
Organizing retrieval of papers: SL, OSR.
Screening retrieved papers against inclusion criteria: SL, OSR.
Appraising the quality of papers: SL, OSR.
Abstracting data from papers: SL, OSR.
Managing data for the review: SL.
Entering data into Review Manager 5 (Review Manager 2014): SL.
Analysing RevMan statistical data: SL, PA, AS.
Interpreting data: SL, OSR, PA, AS.
Making statistical inferences: SL, PA, AS.
Writing the review: SL, MP.
Securing funding for the review: AS.
Serving as guarantor for the review (one review author): AS.
Reading and checking the review before submission: SL.
Sources of support
Internal sources
No sources of support supplied
External sources
-
NIHR Cochrane Collaboration Programme Grant, UK.
’Back to normal’: speed and quality of recovery after surgery, major injury and critical care. Project ref. 13/89/16
Declarations of interest
Sharon Lewis: see Sources of support.
Oliver Schofield‐Robinson: see Sources of support.
Michael Pritchard: see Sources of support.
Andrew Smith: see Sources of support.
Phil Alderson: see Sources of support.
Edited (no change to conclusions)
References
References to studies included in this review
Antolovic 2012 {published data only}
- Antolovic D, Rakow A, Contin P, Ulrich A, Rahbari NN, Buchler MW, et al. A randomised controlled pilot trial to evaluate and optimize the use of anti‐platelet agents in the perioperative management in patients undergoing general and abdominal surgery: the APAP trial (ISRCTN45810007). Langenbeck's Archives of Surgery 2012;397(2):297‐306. [PUBMED: 22048442] [DOI] [PubMed] [Google Scholar]
- Antolovic D, Reissfelder C, Rakow A, Contin P, Rahbari NN, Buchler MW, et al. A randomised controlled trial to evaluate and optimize the use of antiplatelet agents in the perioperative management in patients undergoing general and abdominal surgery: the APAP trial (ISRCTN45810007). BMC Surgery 2011;11:7. [PUBMED: 21371292] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chu 2016 {published data only}
- Chu EW, Chernoguz A, Divino CM. The evaluation of clopidogrel use in perioperative general surgery patients: a prospective randomized controlled trial. American Journal of Surgery 2016;211(6):1019‐25. [PUBMED: 27002953] [DOI] [PubMed] [Google Scholar]
Mantz 2011 {published data only}
- Mantz J, Samama CM, Tubach F, Devereaux PJ, Collet JP, Albaladejo P, et al. Impact of preoperative maintenance or interruption of aspirin on thrombotic and bleeding events after elective non‐cardiac surgery: the multicentre, randomized, blinded, placebo‐controlled, STRATAGEM trial. British Journal of Anaesthesia 2011;107(6):899‐910. [PUBMED: 21873632] [DOI] [PubMed] [Google Scholar]
Nielsen 2000 {published data only}
- Nielsen JD, Holm‐Nielsen A, Jespersen J, Vinther CC, Settgast IW, Gram J. The effect of low‐dose acetylsalicylic acid on bleeding after transurethral prostatectomy: a prospective, randomized, double‐blind, placebo‐controlled study. Scandinavian Journal of Urology and Nephrology 2000;34(3):194‐8. [PUBMED: 10961474] [DOI] [PubMed] [Google Scholar]
Oscarsson 2010 {published data only}
- Oscarsson A, Gupta A, Fredrikson M, Jarhult J, Nystrom M, Pettersson E, et al. To continue or discontinue aspirin in the perioperative period: a randomized, controlled clinical trial. British Journal of Anaesthesia 2010;104(3):305‐12. [PUBMED: 20150346] [DOI] [PubMed] [Google Scholar]
- Oscarsson A, Oster S, Fredrikson M, Lindahl TL, Eintrei C. Platelet function assessed by whole‐blood aggregometry in patients undergoing non‐cardiac surgery. European Journal of Anaesthesiology 2011;28(5):363‐9. [PUBMED: 21499200] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ardekian 2000 {published data only}
- Ardekian L, Gaspar R, Peled M, Brener B, Laufer D. Does low‐dose aspirin therapy complicate oral surgical procedures?. Journal of the American Dental Association (1939) 2000;131(3):331‐5. [PUBMED: 10715924 ] [DOI] [PubMed] [Google Scholar]
Assia 1998 {published data only}
- Assia EI, Raskin T, Kaiserman I, Rotenstreich Y, Segev F. Effect of aspirin intake on bleeding during cataract surgery. Journal of Cataract and Refractive Surgery 1998;24(9):1243‐6. [PUBMED: 9768401] [DOI] [PubMed] [Google Scholar]
Danino 2007 {published data only}
- Danino A, Duvernay A, Dautriche R, Duvernay‐Debin R, Cottin Y, Dalac S. Is withdrawal of antiplatelet therapy necessary prior to skin cancer surgery?. Annales de Dermatologie et de Venereologie 2007;134(10 Pt 1):731‐4. [PUBMED: 17978709] [DOI] [PubMed] [Google Scholar]
Devereaux 2014 {published data only}
- Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso‐Coello P, Kurz A, et al. Aspirin in patients undergoing noncardiac surgery. New England Journal of Medicine 2014;370(16):1494‐503. [PUBMED: 24679062] [DOI] [PubMed] [Google Scholar]
Duygu 2010 {published data only}
- Duygu G, Ozcakir‐Tomruk C, Guler N, Sencift K. Assessment of effects of antiplatelet drugs on bleeding risk after teeth extractions. Biotechnology & Biotechnological Equipment 2010;24(3):2040‐3. [DOI: 10.2478/v10133-010-0061-z] [DOI] [Google Scholar]
Eapen 2017 {published data only}
- Eapen BV, Baig MF, Avinash S. An assessment of the incidence of prolonged postoperative bleeding after dental extraction among patients on uninterrupted low dose aspirin therapy and to evaluate the need to stop such medication prior to dental extractions. Journal of Maxillofacial and Oral Surgery 2017;16(1):48‐52. [PUBMED: 28286384] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engheta 2016 {published data only}
- Engheta A, Hadadi Abianeh S, Atri A, Sanatkarfar M. Aspirin use and bleeding volume in skin cancer patients undergoing surgery: a randomized controlled trial. Daru: Journal of Faculty of Pharmacy, Tehran University of Medical Sciences 2016;24(1):20. [PUBMED: 27465859] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaspar 1999 {published data only}
- Gaspar R, Ardekian L, Brenner B, Peled M, Laufer D. Ambulatory oral procedures in patients on low‐dose aspirin. Harefuah 1999;136(2):108‐10. [PUBMED: 10914173] [PubMed] [Google Scholar]
Medeiros 2011 {published data only}
- Medeiros FB, Andrade AC, Angelis GA, Conrado VC, Timerman L, Farsky P, et al. Bleeding evaluation during single tooth extraction in patients with coronary artery disease and acetylsalicylic acid therapy suspension: a prospective, double‐blinded, and randomized study. Journal of Oral & Maxillofacial Surgery 2011;69(12):2949‐55. [PUBMED: 21802823] [DOI] [PubMed] [Google Scholar]
Varghese 2015 {published data only}
- Varghese KG, Manoharan S, Sadhanandan M. Evaluation of bleeding following dental extraction in patients on long‐term antiplatelet therapy: a clinical trial. Indian Journal of Dental Research: Official Publication of Indian Society for Dental Research 2015;26(3):252‐5. [PUBMED: 26275190] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01806090 {published data only}
- NCT01806090. Uninterrupted clopidogrel therapy before elective colonoscopy will increase the risk of post‐polypectomy bleeding. clinicaltrials.gov/show/NCT01806090 (first received 4 March 2013).
NCT02797548 {published data only}
- NCT02797548. Perioperative antiplatelet therapy in patients with drug‐eluting stent undergoing noncardiac surgery [Perioperative antiplatelet therapy in patients with drug‐eluting stent undergoing noncardiac surgery ASSURE‐DES]. clinicaltrials.gov/show/NCT02797548 (first received 8 June 2016).
NCT03184805 {published data only}
- NCT03184805. STOPping versus continuing antiplatelet therapy during noncardiac surgery and procedures after next generation drug‐eluting stent implantation [Randomized controlled comparison: STOPping versus continuing antiplatelet therapy during noncardiac surgery and procedures after next generation drug‐eluting stent implantation (STOP‐ASP trial)]. clinicaltrials.gov/show/NCT03184805 (first received 7 July 2017).
Additional references
Au 2012
- Au AG, Majumdar SR, McAlister FA. Preoperative thienopyridine use and outcomes after surgery: a systematic review. American Journal of Medicine 2012;125(1):87‐99.e1. [PUBMED: 22079019] [DOI] [PubMed] [Google Scholar]
Berger 2010
- Berger PB, Kleiman NS, Pencina MJ, Hsieh WH, Steinhubl SR, Jeremias A, et al. Frequency of major noncardiac surgery and subsequent adverse events in the year after drug‐eluting stent placement: results from the EVENT (Evaluation of Drug‐Eluting Stents and Ischemic Events) Registry. Journal of the American College of Cardiology. Cardiovascular Interventions 2010;3(9):920‐7. [PUBMED: 20850090] [DOI] [PubMed] [Google Scholar]
Borenstein 2010
- Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research Synthesis Methods 2010;1(2):97‐111. [PUBMED: 26061376] [DOI] [PubMed] [Google Scholar]
Burger 2005
- Burger W, Chemnitius JM, Kneissl GD, Rucker G. Low‐dose aspirin for secondary cardiovascular prevention ‐ cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation ‐ review and meta‐analysis. Journal of Internal Medicine 2005;257(5):399‐414. [PUBMED: 15836656] [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence systematic review software. Melbourne (Australia): Veritas Health Innovation, (accessed 24 May 2016).
Devereaux 2016
- Devereaux PJ, Eikelboom J. Insights into myocardial infarction after noncardiac surgery in patients with a prior coronary artery stent. British Journal of Anaesthesia 2016;116(5):584‐6. [PUBMED: 27106960] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Endnote [Computer program]
- Thomson Reuters. Endnote X5. Version accessed 8 September 2016. Philadelphia, PA, USA: Thomson Reuters, 2011.
Fleisher 2014
- Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;130(24):2215‐45. [PUBMED: 25085962] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed August 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ, et al. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical research ed.) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence: inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Hawn 2013
- Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013;310(14):1462‐72. [PUBMED: 24101118] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johanson 2015
- Johansen M, Wikkelsø A, Lunde J, Wetterslev J, Afshari A. Prothrombin complex concentrate for reversal of vitamin K antagonist treatment in bleeding and non‐bleeding patients. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD010555.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keeling 2016
Korte 2011
- Korte W, Cattaneo M, Chassot PG, Eichinger S, Heymann C, Hofmann N, et al. Peri‐operative management of antiplatelet therapy in patients with coronary artery disease: joint position paper by members of the Working Group on Perioperative Haemostasis of the Society on Thrombosis and Haemostasis Research (GTH), the Working Group on Perioperative Coagulation of the Austrian Society for Anesthesiology, Resuscitation and Intensive Care (OGARI) and the Working Group on Thrombosis of the European Society for Cardiology (ESC). Thrombosis and Haemostasis 2011;105(5):743‐9. [PUBMED: 21437351] [DOI] [PubMed] [Google Scholar]
Levine 2016
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST‐elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2016;133(11):1135‐47. [PUBMED: 26490017] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology 2009;62(10):e1‐34. [PUBMED: 19631507] [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Health and Clinical Excellence (NICE). Clopidogrel and modified‐release dipyridamole for the prevention of occlusive vascular events. https://www.nice.org.uk/guidance/ta210; 2010.
Oprea 2013
- Oprea AD, Popescu WM. Perioperative management of antiplatelet therapy. British Journal of Anaesthesia 2013;111 Suppl 1:i3‐17. [PUBMED: 24335397] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rossini 2014
- Rossini R, Musumeci G, Visconti LO, Bramucci E, Castiglioni B, Servi S, et al. Perioperative management of antiplatelet therapy in patients with coronary stents undergoing cardiac and non‐cardiac surgery: a consensus document from Italian cardiological, surgical and anaesthesiological societies. EuroIntervention: Journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology 2014;10(1):38‐46. [PUBMED: 24832636] [DOI] [PubMed] [Google Scholar]
Sahebally 2014
- Sahebally SM, Healy D, Coffey JC, Walsh SR. Should patients taking aspirin for secondary prevention continue or discontinue the medication prior to elective, abdominal surgery? Best evidence topic (BET). International Journal of Surgery 2014;12(5):16‐21. [PUBMED: 24246172] [DOI] [PubMed] [Google Scholar]
Sanders 2013
- Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009971] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singla 2012
- Singla S, Sachdeva R, Uretsky BF. The risk of adverse cardiac and bleeding events following noncardiac surgery relative to antiplatelet therapy in patients with prior percutaneous coronary intervention. Journal of the American College of Cardiology 2012;60(20):2005‐16. [PUBMED: 23083781] [DOI] [PubMed] [Google Scholar]
Sorensen 2009
- Sorensen R, Hansen ML, Abildstrom SZ, Hvelplund A, Andersson C, Jorgensen C, et al. Risk of bleeding in patients with acute myocardial infarction treated with different combinations of aspirin, clopidogrel, and vitamin K antagonists in Denmark: a retrospective analysis of nationwide registry data. Lancet (London, England) 2009;374(9706):1967‐74. [PUBMED: 20006130] [DOI] [PubMed] [Google Scholar]
Wasowicz 2016
- Wasowicz M, Syed S, Wijeysundera DN, Starzyk L, Grewal D, Ragoonanan T, et al. Effectiveness of platelet inhibition on major adverse cardiac events in non‐cardiac surgery after percutaneous coronary intervention: a prospective cohort study. British Journal of Anaesthesia 2016;116(4):493‐500. [PUBMED: 26888800] [DOI] [PubMed] [Google Scholar]
Wijeysundera 2012
- Wijeysundera DN, Wijeysundera HC, Yun L, Wasowicz M, Beattie WS, Velianou JL, et al. Risk of elective major noncardiac surgery after coronary stent insertion: a population‐based study. Circulation 2012;126(11):1355‐62. [PUBMED: 22893606] [DOI] [PubMed] [Google Scholar]
Wikkelsø 2016
- Wikkelsø A, Wetterslev J, Møller AM, Afshari A. Thromboelastography (TEG) or thromboelastometry (ROTEM) to monitor haemostatic treatment versus usual care in adults or children with bleeding. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD007871.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2016
- Wong WT, Lai VKW, Chee YE, Lee A. Fast‐track cardiac care for adult cardiac surgical patients. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD003587] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2016
- Zhao N, Xu J, Singh B, Yu X, Wu T, Huang Y. Nitrates for the prevention of cardiac morbidity and mortality in patients undergoing non‐cardiac surgery. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD010726.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zou 2016
- Zou Z, Yuan HB, Yang B, Xu F, Chen XY, Liu GJ, Shi XY. Perioperative angiotensin‐converting enzyme inhibitors or angiotensin II type 1 receptor blockers for preventing mortality and morbidity in adults. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD009210.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Lewis 2017
- Lewis SR, Alderson P, Smith AF. Continuation versus discontinuation of antiplatelet therapy for bleeding and ischaemic events in adults undergoing non‐cardiac surgery. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD012584] [DOI] [PMC free article] [PubMed] [Google Scholar]


