Abstract
Background
This is an updated version of the original Cochrane review published in 2005 on selective serotonin reuptake inhibitors (SSRIs) for preventing migraine and tension‐type headache. The original review has been split in two parts and this review now only regards migraine prevention. Another updated review is under development to cover tension‐type headache.
Migraine is a common disorder. The chronic forms are associated with disability and have a high economic impact. In view of discoveries about the role of serotonin and other neurotransmitters in pain mechanisms, selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs) have been evaluated for the prevention of migraine.
Objectives
To determine the efficacy and tolerability of SSRIs and SNRIs compared to placebo and other active interventions in the prevention of episodic and chronic migraine in adults.
Search methods
For the original review, we searched MEDLINE (1966 to January 2004), EMBASE (1994 to May 2003), the Cochrane Central Register of Controlled Trials (CENTRAL 2003, Issue 4), and Headache Quarterly (1990 to 2003). For this update, we applied a revised search strategy to reflect the broader type of intervention (SSRIs and SNRIs). We searched CENTRAL (2014, Issue 10), MEDLINE (1946 to November 2014), EMBASE (1980 to November 2014), and PsycINFO (1987 to November 2014). We also checked the reference lists of retrieved articles and searched trial registries for ongoing trials.
Selection criteria
We included randomised controlled trials comparing SSRIs or SNRIs with any type of control intervention in participants 18 years and older of either sex with migraine.
Data collection and analysis
Two authors independently extracted data (migraine frequency, index, intensity, and duration; use of symptomatic/analgesic medication; days off work; quality of life; mood improvement; cost‐effectiveness; and adverse events) and assessed the risk of bias of trials. The primary outcome of this updated review is migraine frequency.
Main results
The original review included eight studies on migraine. Overall, we now include 11 studies on five SSRIs and one SNRI with a total of 585 participants. Six studies were placebo‐controlled, four compared a SSRI or SNRI to amitriptyline, and one was a head‐to‐head comparison (escitalopram versus venlafaxine). Most studies had methodological or reporting shortcomings (or both): all studies were at unclear risk of selection and reporting bias. Follow‐up rarely extended beyond three months. The lack of adequate power of most of the studies is also a major concern.
Few studies explored the effect of SSRIs or SNRIs on migraine frequency, the primary endpoint. Two studies with unclear reporting compared SSRIs and SNRIs to placebo, suggesting a lack of evidence for a difference. Two studies compared SSRIs or SNRIs versus amitriptyline and found no evidence for a difference in terms of migraine frequency (standardised mean difference (SMD) 0.04, 95% confidence interval (CI) ‐0.72 to 0.80; I2 = 72%), or other secondary outcomes such as migraine intensity and duration.
SSRIs or SNRIs were generally more tolerable than tricyclics. However, the two groups did not differ in terms of the number of participants who withdrew due to adverse advents or for other reasons (one study, odds ratio (OR) 0.39, 95% CI 0.10 to 1.50 and OR 0.42, 95% CI 0.13 to 1.34).
We did not find studies comparing SSRIs or SNRIs with pharmacological treatments other than antidepressants (e.g. antiepileptics and anti‐hypertensives).
Authors' conclusions
Since the last version of this review, the new included studies have not added high quality evidence to support the use of SSRIs or venlafaxine as preventive drugs for migraine. There is no evidence to consider SSRIs or venlafaxine as more effective than placebo or amitriptyline in reducing migraine frequency, intensity, and duration over two to three months of treatment. No reliable information is available at longer‐term follow‐up. Our conclusion is that the use of SSRIs and SNRIs for migraine prophylaxis is not supported by evidence.
Keywords: Adult, Humans, Amitriptyline, Amitriptyline/therapeutic use, Citalopram, Citalopram/therapeutic use, Migraine Disorders, Migraine Disorders/drug therapy, Migraine Disorders/prevention & control, Randomized Controlled Trials as Topic, Selective Serotonin Reuptake Inhibitors, Selective Serotonin Reuptake Inhibitors/therapeutic use, Serotonin and Noradrenaline Reuptake Inhibitors, Serotonin and Noradrenaline Reuptake Inhibitors/therapeutic use, Venlafaxine Hydrochloride, Venlafaxine Hydrochloride/therapeutic use
Plain language summary
Selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs) for preventing migraine
Migraine is a common condition that can significantly impair people's quality of life. Individuals who experience frequent or severe migraine may benefit from preventive medications taken prior to an attack and before the pain starts. Studies have suggested the potential role of neurotransmitters in the genesis of headache. Accordingly, drugs that inhibit the passage of neurotransmitters in brain cells and, therefore, increase their levels, have been examined for their potential benefit in preventing migraine. Two classes of inhibitors, the selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs), typically used to treat depression, are evaluated in this review.
This is an update of a previous review that included studies on migraine and tension‐type headache. This original review has been split in two separate reviews: this update addresses only studies on migraine, while a second focuses on tension‐type headache. In November 2014, we identified three new studies. Eight studies were already included in the previous version of the review. Overall, we analysed a total of 585 participants. All the studies had a small number of participants and were conducted over a period of two to three months. Only a few were of high quality.
The results suggest that SSRIs and SNRIs are no better than placebo (sugar pill) for reducing the number of migraine attacks. There were no differences in minor side effects between participants treated with SSRIs or SNRIs versus those treated with placebo. SSRIs and SNRIs seem not to offer advantages when compared to other active treatments, specifically the tricyclic antidepressant amitriptyline. The participants treated with SSRIs or SNRIs suffered fewer minor side effects than those who took amitriptyline, however the number of people who stopped taking one drug or the other due to side effects was approximately equal. These results are based on short‐term trials (no more than three months), which are not properly sized and feature serious methodological deficiencies. We did not find studies comparing SSRIs or SNRIs with pharmacological treatments other than antidepressants (e.g. antiepileptics and anti‐hypertensives).
Summary of findings
Summary of findings for the main comparison. SSRIs or SNRIs compared to placebo for migraine prevention in adults.
| SSRIs or SNRIs compared to placebo for migraine prophylaxis in adults | ||||||
| Patient or population: patients for whom migraine preventive interventions are indicated Intervention: SSRIs or SNRIs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | SSRIs or SNRIs | |||||
| Migraine frequency Number of attacks Follow‐up: 2 to 3 months | See comment | See comment | — | 113 (2 studies) | ⊕⊕⊝⊝ low1 | Studies not pooled, inconclusive data |
| Migraine intensity Score Follow‐up: 2 to 3 months | See comment | See comment | — | 113 (2 studies) | ⊕⊕⊝⊝ low1 | Studies not pooled, inconclusive data |
|
Migraine duration Hours Follow‐up: 2 to 3 months |
See comment | See comment | — | 60 (1 study) |
See comment | Data reported as median, no statistically significant difference |
|
Symptomatic/analgesic medication use for acute headache attacks Follow‐up: 2 to 3 months |
See comment | See comment | — | 113 (2 studies) | ⊕⊕⊝⊝ low1 | Studies not pooled, inconclusive data |
| Migraine index Follow‐up: mean 2 months | The mean migraine index ranged across control groups from 24 to 77.2 points | The mean migraine index in the intervention groups was 0.14 SD lower (0.57 lower to 0.3 higher) | — | 86 (3 studies) | ⊕⊝⊝⊝ very low2 | As a rule of thumb, 0.2 SD represents a small difference, 0.5 moderate and 0.8 large (Cohen 1988) |
| Quality of life | See comment | See comment | Not estimable | — | See comment | Not measured |
| Withdrawn (due to adverse events) | Study population | OR 1.95 (0.70 to 5.44) | 221 (5 studies) | ⊕⊝⊝⊝ very low2 | — | |
| 53 per 1000 | 99 per 1000 (38 to 234) | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; SD: standard deviation; SNRI: serotonin‐norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Limitations in study design, imprecision (insufficient data).
2Limitations in study design, imprecision (insufficient data), indirectness (lack of generalisability).
Summary of findings 2. SSRIs or SNRIs compared to amitriptyline for migraine prevention.
| SSRIs or SNRIs compared to amitriptyline for migraine prophylaxis in adults | ||||||
| Patient or population: patients for whom migraine preventive interventions are indicated Intervention: SSRIs or SNRIs Comparison: amitriptyline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Amitriptyline | SSRIs or SNRIs | |||||
| Migraine frequency Number of attacks Follow‐up: 3 to 4 months | The mean migraine frequency ranged across control groups from 0.09 to 1.23 number of attacks | The mean migraine frequency in the intervention groups was 0.04 SD higher (0.72 lower to 0.80 higher) | — | 96 (2 studies) | ⊕⊝⊝⊝ very low1 | As a rule of thumb, 0.2 SD represents a small difference, 0.5 moderate and 0.8 large (Cohen 1988) |
| Migraine intensity Score Follow‐up: 3 to 4 months | See comment | See comment | — | 104 (2 studies) | ⊕⊕⊝⊝ low2 | Studies not pooled, inconclusive data |
| Migraine duration Hours Follow‐up: 3 to 4 months | See comment | See comment | — | 104 (2 studies) | ⊕⊕⊝⊝ low2 | Studies not pooled, inconclusive data |
| Symptomatic/analgesic medication use for acute headache attacks | See comment | See comment | Not estimable | — | See comment | Not measured |
| Migraine index Score Follow‐up: 3 months | See comment | See comment | Not estimable | 62 (1 study) | ⊕⊝⊝⊝ very low3 | Reported only within‐group analyses |
| Quality of life | See comment | See comment | Not estimable | — | See comment | Not measured |
| Withdrawn (for any reasons and due to adverse events) | Study population | OR 0.39 (0.1 to 1.50) | 64 (1 study) | ⊕⊕⊝⊝ low2 | — | |
| 219 per 1000 | 98 per 1000 (27 to 296) | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; SD: standard deviation; SNRI: serotonin‐norepinephrine reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Limitations in study design, imprecision (insufficient data), inconsistency (heterogeneity).
2Limitations in study design, imprecision (insufficient data).
3Limitations in study design, imprecision (insufficient data), indirectness (lack of generalisability).
Background
This updated systematic review considers the evidence for the efficacy and tolerability of selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs) for the prevention of migraine. It is an update of a systematic review on SSRIs for the prevention of migraine and tension‐type headache previously published in the Cochrane Database of Systematic Reviews (Cusi 2000; Moja 2005). Another review is under development to cover the prevention of tension‐type headache (in press).
Description of the condition
Migraine is a common neurological disorder with episodic manifestations that may persist or progress in frequency over time as a function of biologic, psychologic, and environmental influences (Lipton 2004). The Global Burden of Disease Survey 2010 ranked migraine as the third most prevalent disorder and seventh‐highest specific cause of disability worldwide (Vos 2012). Published estimates of migraine prevalence vary widely, with a lifetime prevalence of 20% to 25%; it is reported that 36 million people in the United States suffer from repeated attacks of migraine (American Migraine Foundation 2013). However, according to three epidemiological studies conducted in the United States, the prevalence of migraine is rather stable: migraine occurs in about 12% of people, with an almost three‐fold higher prevalence in adult women compared to adult men (Buse 2012; Lipton 2001; Steward 1992). An episodic migraine tends to evolve into a chronic form, often in relation to an overuse of drugs for acute treatment or in the absence of adequate preventive therapy (Colas 2004; Wiendels 2006). According to a systematic review of population‐based studies the prevalence of chronic migraine is 0% to 5.1%, with estimates typically ranging from 1.4% to 2.2% (Natoli 2010).
Migraine is defined as a recurrent primary headache disorder. Migraine without aura is the most common subtype. It is characterised by attacks lasting four to 72 hours, with a unilateral location, pulsating quality, moderate or severe intensity, aggravation by routine physical activity, and association with nausea and/or photophobia and phonophobia. Migraine with aura is primarily characterised by focal neurological symptoms that usually precede or sometimes accompany the headache. The premonitory phase may occur hours or days before the migraine and includes symptoms such as hyperactivity, hypoactivity, depression, craving for particular foods, and repetitive yawning. As one or a few migraine attacks may be difficult to distinguish from symptomatic migraine‐like attacks, at least five attacks are required to confirm the diagnosis. Migraine frequency is often classified as episodic or chronic. Chronic migraine occurs when the headache lasts 15 or more days per month for more than three months, and has the features of migraine on at least eight days per month. The most common risk factor for episodic migraine to progress to chronic migraine is medication overuse (IHS 2013).
Migraine is associated with significant burden, including functional impairment, disability, and reduced quality of life and well‐being (Buse 2012; Steiner 2013; Vos 2012). Costs of the disease for patients and healthcare systems are also an issue. The substantial economic cost and social impact of migraine has been documented across diverse settings (Bloudek 2012; Cull 1992; Clarke 1996; Hu 1999; Serrano 2013; Von Korff 1998). The estimates vary across countries due to differences in available therapies and they way in which they are delivered, and structural differences in healthcare systems. A recent cost‐of‐illness survey conducted as part of the Eurolight project in six European countries reported an annual direct and indirect cost of migraine per person of Euro 1222, and a total annual cost for the European countries of Euro 111 billion for adults aged 18 to 65 years (Linde 2012). A survey conducted in the US reported a similar figure for episodic migraine and costs up to more than USD 7000 for chronic migraine (Munakata 2009). More than 50% of working persons with migraine report a loss of at least two days of work per month and, among people with chronic migraine, a daily use of analgesic drugs (Zwart 2004).
The recognition of the social and economic burden of migraine calls for an accurate analysis of the efficacy and safety of preventive and treatment options currently available, as well as the development of new effective strategies.
While migraine is no longer considered a vascular‐based phenomena, the pathogenesis is still uncertain. The importance of sensitisation of pain pathways and the possibility that attacks may originate in the central nervous system have gained increasing attention over recent decades. Messenger molecules such as nitric oxide (NO), 5‐hydroxytryptamine (5‐HT), and calcitonin gene‐related peptide (CGRP) may be involved (Hoffmann 2014). Highly receptor‐specific acute medications such as the triptans have demonstrated efficacy in the acute treatment of attacks. They have high receptor specificity, therefore their mechanism of action provides new insight into migraine mechanisms and it is now clear that migraine is a neurobiological disorder (IHS 2013).
Description of the intervention
The pharmacological therapy of migraine includes the treatment of acute attacks, usually using simple analgesics, triptans, and antiemetics, and a prophylactic approach that aims to reduce the frequency, severity, and duration of migraine attacks. Preventive treatment is especially well‐suited to patients with very frequent or severe migraine. It encompasses both episodic and chronic forms, causing significant headache‐related disability, and that are resistant to acute therapy (IHS 2013). For instance, migraine prophylactic therapy can be appropriate if, despite appropriate use of acute medications and trigger management/lifestyle modification strategies, patients still experience attacks that highly affect daily activities, or when the frequency of migraine attacks is such that patients are at risk of medication overuse (rebound) migraine (Pringsheim 2012).
Although epidemiologic studies suggest that approximately 38% of migraineurs need preventive therapy, only 3% to 13% currently use it (Lipton 2007). Several pharmacological strategies are currently approved or used off‐label for migraine prevention and thought to affect various aspects of migraine pathophysiology (Sprenger 2009). Preventive treatments aim to eliminate headache pain without intolerable harms and they are also expected to reduce the use of acute drugs and improve quality of life. In clinical practice the choice of a drug over another is based on many drug‐related factors such as familiarity, efficacy, and adverse effects, as well as many patient characteristics such as headache frequency, presence of aura, comorbid conditions, and patient preference.
SSRIs and SNRIs are a class of compounds typically used as antidepressants in the treatment of depression, anxiety disorders, and some personality disorders. SSRIs increase the extracellular level of neurotransmitters such as serotonin by inhibiting its reuptake into the presynaptic cell. Depending on their chemical structure, these compounds have varying degrees of selectivity for the other monoamine transporters, with pure SSRIs having only weak affinity for the noradrenaline transporter and non‐selective compounds also blocking the reuptake of noradrenaline and dopamine.
How the intervention might work
The serotonergic system from the brainstem raphe nucleus seems to be implicated in migraine pathophysiology. Several studies have documented a central neurochemical imbalance and changes in the serotonin metabolism and in the processing of serotonin‐mediated responses during and in between migraine attacks. How the abnormal serotonergic neurotransmission is linked to the manifestation of head pain and the accompanying symptoms has yet to be fully understood. However, evidence suggests that low serotonin levels facilitate the activation of the trigeminovascular nociceptive pathway, as induced by cortical spreading depression (Hamel 2007).
Similar to migraine, depression is also considered to be a disorder of low brain serotonergic activity, and epidemiological studies have reported comorbidity of migraine with psychiatric disorders (Buse 2013). Most antidepressant drugs are aimed at enhancing and stabilising 5‐HT neurotransmission and some antidepressants have been shown to be effective in migraine prophylaxis at lower doses than those used to treat depression.
In view of the discoveries about the role of serotonin and other neurotransmitters in pain mechanisms, SSRIs and SNRIs have also been evaluated for their potential benefit in the treatment of migraine.
Why it is important to do this review
Clinical guidelines often mention SSRIs and SNRIs as possible preventive treatments for migraine. However, the role of these antidepressants for migraine prophylaxis is not completely established. The American Society of Internal Medicine recommends the use of some SSRIs (paroxetine and fluvoxamine) to prevent migraine, while emphasising that this recommendation is based on expert consensus and clinical experiences (Snow 2002). According to the American Headache Society (AHS) and the American Academy of Neurology (AAN) data from migraine prevention guidelines to support or refute the use of SSRIs such as fluoxetine and fluvoxamine for migraine prophylaxis are insufficient (Loder 2012). More recent guidelines by the European Federation of Neurological Societies and Canadian Headache Society do not consider venlafaxine and other antidepressant drugs as effective treatments for migraine prophylaxis (Evers 2009; Pringsheim 2012).
Objectives
To determine the efficacy and tolerability of SSRIs and SNRIs compared to placebo and other active interventions in the prevention of episodic and chronic migraine in adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of SSRIs or SNRIs taken regularly to prevent the occurrence of migraine attacks, reduce the intensity of those attacks, or both. We included published and unpublished trials in any language provided that enough information about eligibility was available.
Types of participants
Participants of either sex, aged 18 and older, diagnosed with migraine, both episodic and chronic forms. Migraine diagnoses were based on the diagnostic criteria of the International Headache Society (IHS 2013 and its previous editions ICHD‐II 2004; IHS 1988) and the Ad Hoc Committee on the Classification of Headache (Ad Hoc 1962). Where no such criteria were specified, the diagnosis of migraine had to be based on at least some of its distinctive features, (e.g. nausea/vomiting; severe pain; pulsating/throbbing pain; mainly unilateral pain; and the presence of photophobia, phonophobia, and/or aura). Patients with episodic migraine usually have it two to eight times per month. Chronic migraine is defined as that occurring with a frequency of at least 15 days/month (180 days/year) for at least a three‐month period.
We included studies in which participants were described as having 'combination' or 'mixed' migraine and tension‐type headaches only if data on migraine participants could be extracted. We excluded trials including patients with a secondary headache.
Types of interventions
To be considered for inclusion, trials were required to have at least one treatment arm in which patients were treated with one of the SSRIs or SNRIs commercially available or under development (fluoxetine, paroxetine, fluvoxamine, citalopram, escitalopram, milnacipran, sertraline, venlafaxine, desvenlafaxine, duloxetine, dapoxetine). We considered any dosage or any dosing regimen lasting for at least four weeks. Acceptable comparator groups included placebo, no intervention, other drug treatments, and behavioural or physical therapies. It was expected that patients were free to take medication for acute migraine attacks as needed during the trial period.
Types of outcome measures
In this update, we reconsidered the outcome measures, taking into consideration patients' preferences, scientific rigour, and the availability of data. In line with the guidelines for controlled trials of drugs in migraine issued by the IHS (Tfelt‐Hansen 2012), the main outcomes to be considered were:
Primary outcomes
Migraine frequency.
We considered the following ways of measuring migraine frequency, listed in the preferred order:
number of migraine attacks per evaluation period;
number of days with migraine per evaluation period;
responders, i.e. patients with ≥ 50% reduction in headache frequency.
Secondary outcomes
Migraine intensity, measured using a numerical or verbal scale.
Migraine duration (hours).
Symptomatic/analgesic medication use for migraine attacks.
Migraine index: we preferred those that incorporated frequency as a component (along with intensity and/or duration), but considered other types of indexes when these were not available. The formula used to calculate the headache index is recorded in the text below and in the table describing the Characteristics of included studies whenever it was reported by investigators.
Quality of life, measured using validated instruments.
Withdrawals (for any reasons and due to adverse events).
Minor adverse events.
We sought migraine‐associated symptoms (nausea, photophobia, phonophobia) and other outcome measures (e.g. workdays lost, mood improvement, and cost‐effectiveness).
We initially recorded the outcomes for all of the assessment periods reported, then, once all of the data had been collected, decided upon which time points to consider in the analysis: we preferred the last periods of the follow‐up, usually eight and 12 weeks. The analyses considered only outcomes obtained directly from the patient, excluding those judged by the treating physician or study personnel.
We included the following outcome measures in the 'Summary of findings' table (Table 1; Table 2).
Migraine frequency.
Migraine intensity.
Migraine duration.
Symptomatic/analgesic medication use for migraine attacks.
Migraine index.
Quality of life.
Withdrawals due to adverse events.
Search methods for identification of studies
The search strategies used for this review are common to a review on SSRIs and SNRIs for tension‐type headache prophylaxis in adults (in press).
Electronic searches
For the original review, we searched MEDLINE (1966 to January 2004), EMBASE (1994 to May 2003), the Cochrane Central Register of Controlled Trials (CENTRAL 2003, Issue 4), andHeadache Quarterly (1990 to 2003). For this update, we applied a revised search strategy to reflect the broader type of intervention (SSRIs and SNRIs). We searched CENTRAL (2014, Issue 10), MEDLINE (1946 to November 2014), EMBASE (1980 to November 2014), and PsycINFO (1987 to November 2014). We also searched trial registries (the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com/mrct), clinicaltrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) for ongoing trials (November 2014). Details of the search strategies are provided in Appendix 1.
Searching other resources
Additional strategies for identifying trials included searching the reference lists of review articles and included studies, searching books related to headache, consulting experts in the field of headache, contacting the authors of trial reports, and contacting pharmaceutical companies to identify additional published or unpublished data.
Data collection and analysis
Compared to the previous version of the review, we revised the assessment of methodological quality of the included trials to include the most recent 'Risk of bias' approach (Assessment of risk of bias in included studies).
Selection of studies
Two review authors independently screened titles and abstracts from the search and judged whether trials fulfilled the inclusion or exclusion criteria. We resolved disagreements through discussion with a third author and by contacting the study authors, if needed. Review authors were not blinded to the names of the study authors, their institutions, the journal of publication, or the results. We retrieved all potentially relevant articles for the assessment of the full publication.
Data extraction and management
Two authors independently abstracted information on study methods (design, duration, randomisation, blinding, withdrawals), participants (age, sex, type of headache, duration of disease, co‐existing depression and other psychiatric illnesses, and concomitant drugs), interventions (type of drug, route of administration, and dosage), outcomes, and adverse events using specially designed, pre‐tested, electronic extraction forms. We resolved disagreements through discussion with a third author. We entered data into Review Manager for analysis (RevMan 2014).
When outcomes were reported in dichotomous form (success/failure), we required that the threshold for distinguishing between success and failure be clinically significant (for instance, more than a 50% reduction in frequency or intensity).
When outcome data were reported on an ordinal scale, we selected a threshold based on the definition of clinically significant improvement and converted the data into a dichotomous form. If categorical data could not be split into dichotomous outcomes meeting our a priori definition, we did not include the data in the analysis.
When a trial used pre‐ and post‐treatment scores to calculate a change score for each patient, and then used these within‐patient change scores to calculate a group mean change score, we recorded and analysed the group mean change scores. When only post‐treatment data were available, we used these, relying on allocation to achieve between‐group balance.
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias for each of the included studies using the 'Risk of bias' tool developed by The Cochrane Collaboration (Higgins 2011). This includes five domains of bias: selection, performance, attrition, detection and reporting, as well as an 'other bias' category to capture other potential threats to validity.
Selection bias included an assessment of adequate sequence generation as well as allocation concealment. We assessed sequence generation to be at low risk when studies clearly specified a method for generating a truly random sequence. We assessed allocation concealment to be at low risk if the method used to ensure that investigators enrolling participants could not predict group assignment was described. Performance and detection bias were incorporated under the blinding domain in the 'Risk of bias' tool: we did not consider them separately as the large majority of outcomes were self reported by the patients (i.e. using diaries). We assessed this to be low risk for studies that reported blinding of participants and study personnel. We assessed studies as low risk for attrition bias if an adequate description of participant flow through the study was provided, the proportion of missing outcome data was relatively balanced across groups, and the reasons for missing outcome data were provided, relatively balanced between groups, and considered unlikely to bias the results.
We assessed studies as having low risk of reporting bias when a published protocol was available and all specified outcomes were included in the study report; we assessed studies without a published protocol as unclear. When an outcome measure was specified and the results were not reported either at baseline or at follow‐up, we considered the study to be at high risk of reporting bias.
We assessed other potential threats to validity, including early trial discontinuation for benefit and trial sponsorship.
Review authors were not blinded with respect to study authors, institution, or journal. We resolved disagreements through discussion with a third author.
Measures of treatment effect
In order to assess efficacy, we extracted raw data for outcomes of interest (means and standard deviations for continuous outcomes and number of events for dichotomous outcomes) when available in the published reports.
For dichotomous outcomes, we calculated odds ratios (ORs) along with 95% confidence intervals. Where outcomes were measured on standard scales, we calculated mean differences (MDs). Where different scales were used to measure the same or similar outcomes, we calculated standardised mean differences (SMDs).
We calculated numbers needed to treat to benefit (NNTB) if possible, although this was a rare circumstance due to the large number of statistically insignificant comparisons. We analysed toxicity for total withdrawals due to adverse events. We calculated numbers needed to treat to harm (NNTH) if possible.
Unit of analysis issues
Cross‐over trials
In randomised cross‐over studies, individuals receive each intervention sequentially in a random order. Cross‐over studies usually contain a washout period, which is a stage after the first treatment but before the second treatment, where time is given for the active effects of the first treatment to wear off before the new treatment begins (that is to reduce the carryover effect). A concern with the cross‐over design is the risk of a carryover effect when the first treatment affects the second. Inadequate washouts are seen when the carryover effect exceeds the washout period. For this review, we considered an adequate washout period for cross‐over studies to be a minimum of one week. When including cross‐over studies with an inadequate washout period we used only the first arm data. Even though this method does not consider all of the information provided it avoids inappropriate consideration of correlated information.
Cluster trials
We assessed whether the unit of analysis was appropriate for the unit of randomisation. If we were to include cluster‐RCTs, we would use the intra‐class correlation coefficient (ICC) to convert trials to their effective sample size before incorporating them into the meta‐analysis.
Dealing with missing data
We described missing data and the drop‐outs/attrition from each included study in the Characteristics of included studies. We planned the analysis of outcomes on an intention‐to‐treat basis; in other words, we included all of the participants randomised to each group in the analyses, regardless of whether or not they received the allocated intervention, and irrespective of how the original study authors analysed the data. However, because only a few studies reported the needed data, we analysed the studies according to an 'available case' approach.
We contacted study authors by email to clarify any missing data. For outcomes reported on a continuous scale, we anticipated that many trials would report pre‐ and post‐treatment group means without reporting data on the variance associated with these means. We attempted to calculate or estimate variances based on primary data or test statistics whenever precise P values or test statistics were provided in sufficient detail.
Assessment of heterogeneity
We assessed statistical heterogeneity by examining the I2 statistic (Deeks 2011), a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than sampling error.
We interpreted the I2 statistic as suggested by the latest version of Higgins 2011:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
In addition, we used a Chi2 test of homogeneity to determine the strength of evidence that heterogeneity is genuine.
We explored clinical variation across studies by comparing the distribution of important participant factors among trials (for example, age) and trial factors (randomisation concealment, blinding of outcome assessment, losses to follow‐up, treatment type, and co‐interventions).
Data synthesis
We performed the analyses using Review Manager (RevMan 2014). We assumed a considerable clinical heterogeneity and usually combined the studies using the random‐effects model. When including both parallel and cross‐over studies with an adequate washout period, we used the inverse variance method, as recommended by Elbourne 2002. In the meta‐analysis, the weight of each study is inversely proportional to the variance (one over the square of the standard error) (Deeks 2011).
'Summary of findings' table
We synthesised the main outcome measures (see also Types of outcome measures) in two 'Summary of findings' tables, comparing SSRIs or SNRIs to placebo (Table 1) or to other active comparators (Table 2). Whenever possible, we used the control arm to calculate the 'assumed risk' values. We assessed the overall quality of the evidence for each outcome using the GRADE approach, as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), against five factors: study design and limitations, consistency of results, directness (generalisability), precision (sufficient data), and reporting of the results across all studies that measure that particular outcome. The quality starts at high when high quality RCTs provide results for the outcome, and reduces by a level for each of the factors not met.
High quality evidence: there are consistent findings among at least 75% of RCTs with no limitations of the study design, consistent, direct and precise data, and no known or suspected publication biases. Further research is unlikely to change either the estimate or our confidence in the results.
Moderate quality evidence: one of the domains is not met. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality evidence: two of the domains are not met. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality evidence: three of the domains are not met. We are very uncertain about the results.
No evidence: no RCTs were identified that addressed this outcome.
Subgroup analysis and investigation of heterogeneity
We investigated the effects in two subgroup analyses:
Trials in which patients were depressed (as determined by a rating scale or clinical interview) versus trials in which patients were not depressed.
Trials evaluating the various SSRIs and SNRIs separately.
Sensitivity analysis
We did not plan any sensitivity analysis.
Results
Description of studies
Results of the search
The new electronic search up to November 2014 retrieved a total of 4508 results after discarding duplicates. After retrieving full‐text articles, we included three new studies (253 participants) (Bulut 2004; Ozyalcin 2005; Tarlaci 2009), along with the eight studies already included. We classified three studies, which were published only as poster presentations (Dzagnidze 2009; Stanic 2009; Togha 2014), and one Chinese publication (He 2004), as awaiting classification (see Characteristics of studies awaiting classification). After searching clinical trial registries, we found four ongoing studies that were potentially eligible: two on duloxetine and two on milnacipran. Of these, we excluded three because they were not controlled trials, while we classified one assessing the efficacy of milnacipran in headache pain reduction in patients with chronic migraine without fibromyalgia as 'ongoing' (see Characteristics of ongoing studies).
See Figure 1.
1.
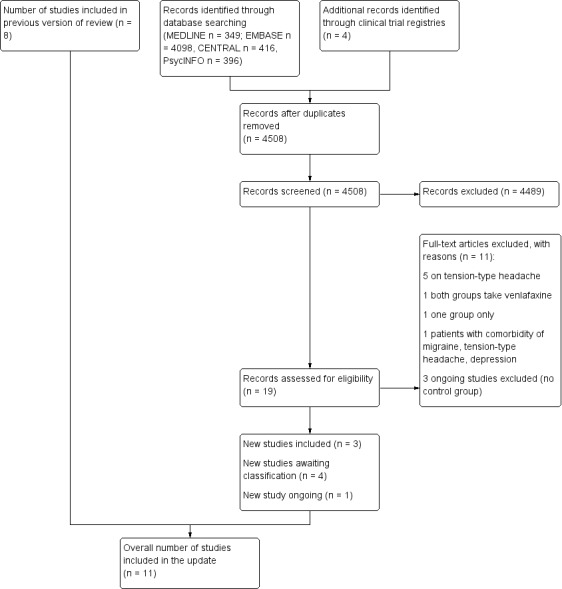
PRISMA flow diagram
Included studies
Overall, we included 11 studies published between 1991 and 2009 in this updated review (Adly 1992; Bank 1994; Bulut 2004; Colucci d'Amato 1999a; Krymchantowski 2002; Landy 1999; Oguzhanoglu 1999; Ozyalcin 2005; Polisca 1992; Steiner 1998; Tarlaci 2009).
All but one, Bulut 2004, were parallel studies. Steiner 1998 was a multicentre trial. The mean length of studies was 13 weeks (range: eight to 24 weeks).
A description of an initial run‐in washout period from the previous preventive drug was given in six studies (Adly 1992; Bank 1994; Bulut 2004; Colucci d'Amato 1999a; Landy 1999; Steiner 1998).
See Characteristics of included studies.
Participants
The included studies enrolled a total of 585 participants, with women more represented than men (73% versus 27%). Four studies did not report the sex of non‐completers (Adly 1992; Bulut 2004; Oguzhanoglu 1999; Tarlaci 2009). The mean age of the patients ranged from 31 (Tarlaci 2009) to 43.5 (Polisca 1992) years old.
Eight studies (N = 461) enrolled patients with migraine mainly diagnosed following the IHS classification (Adly 1992; Bank 1994; Bulut 2004; Colucci d'Amato 1999a; Landy 1999; Ozyalcin 2005; Steiner 1998; Tarlaci 2009). One (N = 39) included patients with migraine transformed into chronic daily headache due to symptomatic medication overuse (Krymchantowski 2002) and one (N = 60) with chronic daily headache caused by underlying chronic migraine, with episodic tension‐type attacks (Polisca 1992). One study included a mixed population, e.g. patients with migraine, chronic tension‐type headache, and episodic tension‐type headache (Oguzhanoglu 1999, N = 52).
One study included depressed patients (Adly 1992), while in two other studies depressed patients were clearly excluded (Bulut 2004; Oguzhanoglu 1999). The remaining studies excluded patients suffering from generic neurological or psychiatric conditions, or on treatment with antidepressant drugs.
The median number of patients randomised in the included studies was 53 and ranged from 27 (Landy 1999) to 105 (Tarlaci 2009). Losses to follow‐up were greater than 30% in three studies (Bulut 2004; Krymchantowski 2002; Steiner 1998) and greater than 40% in two (Adly 1992; Landy 1999).
Interventions and controls
Five studies compared SSRIs with placebo (fluoxetine four studies: Adly 1992; Colucci d'Amato 1999a; Polisca 1992; Steiner 1998; sertraline one study: Landy 1999). Two studies compared SSRIs (fluoxetine one study: Oguzhanoglu 1999; fluvoxamine one study: Bank 1994) with amitriptyline. Krymchantowski 2002 compared a regime of fluoxetine plus amitriptyline with amitriptyline alone. Three studies compared SNRIs (venlafaxine) with placebo (Ozyalcin 2005), amitriptyline (Bulut 2004), and escitalopram (Tarlaci 2009), respectively.
Four studies used a fixed dose of fluoxetine (20 mg or 40 mg daily) (Colucci d'Amato 1999a; Oguzhanoglu 1999; Polisca 1992; Steiner 1998), while in two trials dose ranged up to 40 mg/day (Adly 1992; Krymchantowski 2002). Similarly, one study used fixed doses of venlafaxine (75 mg/day or 150 mg/day) (Ozyalcin 2005), while two used dose escalations from 37.5 mg/day to 150 mg/day (Bulut 2004; Tarlaci 2009). In the active comparator trials, amitriptyline was increased progressively over the first two weeks of treatment.
For the other SSRIs the average doses were: sertraline 50 mg/day (Landy 1999), and fluvoxamine 50 mg/day (Bank 1994).
We did not identify any study comparing SSRIs or SNRIs with a drug treatment other than antidepressants or with a non‐pharmacological treatment (behavioural or physical therapy).
Country and language of publication
Four studies were carried out in Turkey (Bulut 2004; Oguzhanoglu 1999; Ozyalcin 2005; Tarlaci 2009), two in Italy (Colucci d'Amato 1999a; Polisca 1992), two in the US (Adly 1992; Landy 1999), and one each in Brazil (Krymchantowski 2002), Hungary (Bank 1994), and the UK (Steiner 1998). The only non‐English language paper included was published in Italian (Polisca 1992).
Excluded studies
In the original review, we excluded nine studies because they were not randomised, four because the license of the SSRI studied (femoxetine) has been discontinued by drug companies (company communication, Knoll, February 1988, and Martec, February 1990). In the original review, we excluded two studies because it was impossible to separate data on patients with migraine from data on patients with chronic daily headache or chronic tension‐type headache (Bussone 1991; Saper 1994). We contacted the authors, who confirmed that study data and analyses are no longer available. In this update, we excluded one study because it recruited patients with comorbidity of depression, migraine, and tension‐type headache (Rampello 2004). We excluded one trial previously classified as 'awaiting for classification' as it did not report any comparison between treatment groups (Amelin 2000). We also excluded one trial among those screened in this update as both groups were treated with venlafaxine (Centonze 2000).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 2 and summarised in Figure 3. The majority of included trials had methodological or reporting shortcomings, or both. We cannot exclude the fact that poor reporting could have hampered our assessment.
2.
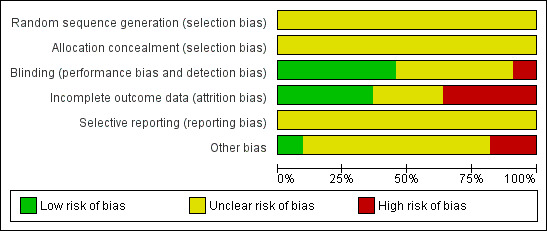
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
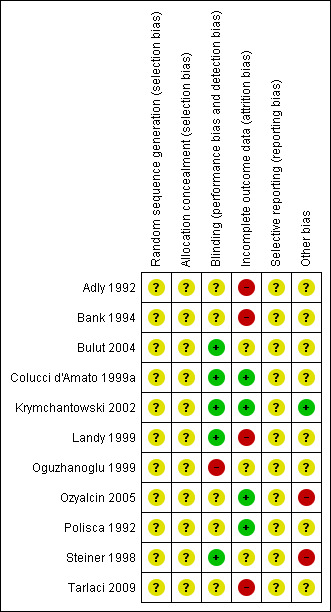
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
None of the included studies reported information on how the random sequence was generated and concealed from the study personnel.
Blinding
Five studies were double‐blind and reported information on how participants or physicians, or both, were blinded to the study treatments (Bulut 2004; Colucci d'Amato 1999a; Krymchantowski 2002; Landy 1999; Steiner 1998). Four trials were claimed to be double‐blinded but no additional information was reported (Adly 1992; Bank 1994; Ozyalcin 2005; Polisca 1992). One study did not report any information on blinding (Tarlaci 2009), and one was open‐label (Oguzhanoglu 1999).
Incomplete outcome data
We judged four studies at low risk of attrition bias (Colucci d'Amato 1999a; Krymchantowski 2002; Ozyalcin 2005; Polisca 1992), and four at high risk: Adly 1992 and Landy 1999 due to a high drop‐out rate (more than 40% lost at follow‐up); Bank 1994 and Tarlaci 2009 due to a moderate drop‐out rate but imbalanced among arms and with reasons for drop‐out not fully reported. We judged the remaining three studies at unclear risk of bias as we did not have sufficient information on drop‐outs (Bulut 2004; Oguzhanoglu 1999; Steiner 1998).
Selective reporting
We did not find any information useful to assess the possible selective reporting of studies and outcomes. None of the trials included in this version of the review are registered nor have a publicly available protocol for consultation. All the studies used multiple outcomes without a predefined primary outcome and multiple time points for the assessment. This suggests possible selective outcome reporting.
Other potential sources of bias
Seven studies did not provide any information about financial sponsorship (Adly 1992; Bank 1994; Bulut 2004; Colucci d'Amato 1999a; Oguzhanoglu 1999; Polisca 1992; Tarlaci 2009). Two were supported by the manufacturer of the SSRI or SNRI being tested (Ozyalcin 2005; Steiner 1998), one by a charitable organisation (Landy 1999), and one was apparently without any financial or other type of support (Krymchantowski 2002).
We have particular doubts about one study, which reported extremely positive results in favour of fluoxetine but was characterised by a general lack of detail in reporting (Polisca 1992). Our concerns include the following: (1) lack of any clear evidence that the patients were truly randomised; (2) 100% of patients (N = 60) completed the study; (3) the adopted statistical analysis is insufficiently described; and (4) identical means and standard deviations are reported for two different outcomes (frequency and symptomatic/analgesic medication use).
Only one study reported an adequate sample size calculation (Steiner 1998), with most of the studies clearly underpowered and, therefore, more prone to be inconclusive (e.g. not enabled to find a statistically significant difference that is true) (Altman 1990; Hotopf 1997; Hotopf 1999). The median sample size was 53 and ranged from 27 to 105. The mean drop‐out rate was 22% of all randomised patients, leading to much smaller sample size across studies.
With respect to the previous version of this review, the median sample size per arm increased by 20% (from 25 to 30). However, concerns remain about the fact that many studies are likely to be underpowered to detect any difference (Moja 2005).
The lack of statistical power is reflected in the use of a large number of rating scales to measure outcomes. Furthermore the majority of trials analysed the multiple outcomes at many different time intervals (four weeks, eight weeks, etc.), increasing exponentially the number of comparisons. Performing multiple comparisons easily leads to detect statistically significant differences that are spurious (Thornley 1998).
Only one trial stated that the analysis was done by intention‐to‐treat but no additional details were provided (Tarlaci 2009). The number of patients in the final analysis (when reported) rarely matched that which was reported at baseline. Only two studies analysed the patients on the basis of the randomised group, but this was related to the fact that there were no drop‐outs (Colucci d'Amato 1999a; Polisca 1992).
Effects of interventions
Whenever possible, for each efficacy outcome, we focused on outcomes at two different follow‐up time points in the same analysis graph (eight and 12 weeks).
SSRIs or SNRIs versus placebo
None of the new trials included in this update compared SSRIs to placebo. One study compared venlafaxine, 75 mg or 150 mg administered once a day, to placebo (Ozyalcin 2005). This study reported variables as medians (e.g. number of days with migraine) while other outcomes were reported with categorical data (e.g. daily activities deteriorated, remained unchanged, improved). One study reported continuous data on several efficacy outcomes, but did not report variance data (Steiner 1998). We contacted the authors to obtain the missing data, however we did not receive any additional information. Due to incomplete reporting and other shortcomings, these two trials could not be pooled in meta‐analyses.
Primary outcome
Migraine frequency
Steiner 1998 assessed changes from baseline in both (i) number of attacks and (ii) number of days with migraine per month. For number of attacks, there was no significant difference between fluoxetine and placebo after two months of treatment: mean frequency decreased from 3.3 to 1.8 with fluoxetine, and from 4.1 to 2.4 with placebo (no F or P values reported). After three months of treatment, the mean frequency was 1.6 attacks in the fluoxetine group compared to 3.0 in the placebo group (F value = 4.55; P value = 0.041). The mean number of days with migraine per month decreased from 7.2 to 4.1 with fluoxetine, and from 8.8 to 6.6 with placebo (no F or P values reported).
Ozyalcin 2005 reported that the number of days with migraine was reduced only by venlafaxine 150 mg (median over placebo: four days less per month).
Secondary outcomes
Migraine intensity
Steiner 1998 reported results for this outcome. Investigators used a three‐point scale (1 = mild, 2 = moderate, 3 = severe) and a Patient's Global Impression of Disease Severity scale (100 mm visual analogue scale) to measure headache intensity. After three months of treatment, there was no significant difference between the two groups on the three‐point scale (mean scores at baseline and three months: 1.7 and 1.9 with fluoxetine, 1.7 and 1.7 with placebo). The Patient's Global Impression of Disease Severity scale score decreased with fluoxetine at two months (F value = 5.75; P value = 0.033) and three months (F value = 3.83; P value = 0.060).
Ozyalcin 2005 did not find any difference in terms of pain intensity among the three groups (venlafaxine 75 mg or 150 mg, or placebo).
Migraine duration
Only Ozyalcin 2005 reported data on this outcome. This study did not find any statistically significant difference in terms of migraine duration among the three groups (venlafaxine 75 mg, 150 mg, or placebo).
Symptomatic/analgesic medication use
Steiner 1998 reported that the mean number of doses taken per attack increased slightly from baseline to three months in both groups, from 2.4 to 2.9 with fluoxetine, and from 2.0 to 2.3 with placebo. There was no significant difference between the two groups (no F or P values reported).
Ozyalcin 2005 reported a "statistically significant decrease" in analgesic drug consumption (median decrease of five units, venlafaxine 150 mg over placebo
Migraine index
Three trials provided data in an unambiguous format (Adly 1992; Colucci d'Amato 1999a; Landy 1999). Adly 1992 utilised a migraine score, based on patient diaries, which combined a subjective record of intensity, duration of migraine, and amount of medication used to abort the attack. Colucci d'Amato 1999a and Landy 1999 calculated a migraine index combining levels of pain intensity and duration of pain in each level.
SSRIs did not improve the migraine score at eight weeks (three studies, N = 86) compared to placebo. The combined standardised mean difference (SMD) was ‐0.14 (95% confidence interval (CI) ‐0.57 to 0.30; I2 = 0%), which is not statistically significant. One study, Colucci d'Amato 1999a, also reported data at 12 weeks: the SMD was ‐0.32 (95% CI ‐0.88 to 0.25) (Analysis 1.1; Figure 4).
1.1. Analysis.

Comparison 1 SSRI or SNRI versus placebo, Outcome 1 Migraine index.
4.
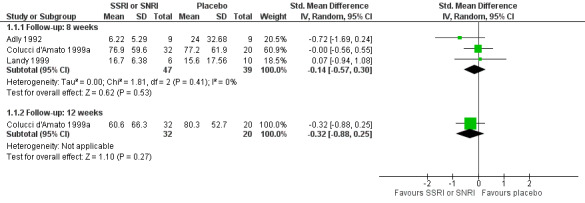
Forest plot of comparison: 1 SSRI or SNRI versus placebo, outcome: 1.1 Migraine index.
Quality of life
None of the included studies reported data on quality of life.
Withdrawals (for any reasons and due to adverse events)
Overall, similar rates of withdrawals were found in the five studies where patients were treated with SSRI or SNRI (N = 127) or placebo (N = 94). We found no significant difference between the two treatments (Peto odds ratio (OR) 1.37, 95% CI 0.73 to 2.56; I2 = 0%) in terms of the number of patients who withdrew from treatment for any reason (Analysis 1.2).
1.2. Analysis.

Comparison 1 SSRI or SNRI versus placebo, Outcome 2 Withdrawals ‐ any reason.
Of patients receiving a SSRI or SNRI, 9.4% (12/127) withdrew from treatment due to adverse events, compared with 5.3% (5/94) of patients treated with placebo (Peto OR 1.95, 95% CI 0.70 to 5.44; I2 = 0%) (Analysis 1.3; Figure 5).
1.3. Analysis.

Comparison 1 SSRI or SNRI versus placebo, Outcome 3 Withdrawals due to adverse events.
5.
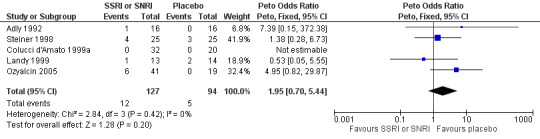
Forest plot of comparison: 1 SSRI or SNRI versus placebo, outcome: 1.3 Withdrawals due to adverse events.
Minor adverse events
The number of patients with minor adverse effects was reported in two studies (Adly 1992; Colucci d'Amato 1999a). In Adly 1992, 3/16 patients treated with fluoxetine and 3/16 treated with placebo experienced minor adverse events (fluoxetine: insomnia and anxiety, strange skin sensations, excitement and insomnia; placebo: insomnia and anxiety, weakness, and problems sleeping). In Colucci d'Amato 1999a, 8/32 patients taking fluoxetine and 3/20 patients on placebo reported minor adverse events (fluoxetine: pyrosis, asthenia, excitement, insomnia; placebo: asthenia, sleepiness). There was no significant difference between the two treatments (OR 1.46, 95% CI 0.47 to 4.52; Analysis 1.4).
1.4. Analysis.

Comparison 1 SSRI or SNRI versus placebo, Outcome 4 Number of patients with minor adverse events.
SSRIs and SNRIs versus another active drug (amitriptyline)
Two studies compared a SSRI (fluoxetine, fluvoxamine) to amitriptyline in patients with migraine (Bank 1994; Oguzhanoglu 1999). Oguzhanoglu 1999 did not report any quantitative data that could be used in our analyses.
One cross‐over study compared different regimens of venlafaxine to amitriptyline (Bulut 2004). We included data on migraine attacks, duration, and intensity considering the first period only.
Primary outcome
Migraine frequency
Bank 1994 used a frequency measure called the Headache Unit Index (HUI), defined as the number of migraine attacks divided by the number of days in the visit period. The author presented only within‐group analyses and reported that the HUI decreased from baseline to the end of treatment (three months). Bulut 2004 reported the number of attacks per month at four and three months, respectively. No significant difference between SSRI or SNRI and amitriptyline was found (SMD 0.04, 95% CI ‐0.72 to 0.80; I2 = 72% Analysis 2.1; Figure 6). Oguzhanoglu 1999 described only within‐group analyses and reported no quantitative data. The investigators stated that no significant reduction was found in migraine frequency (number of days with headache per month) at three months in migraine patients (N = 15) receiving either fluoxetine or amitriptyline.
2.1. Analysis.

Comparison 2 SSRI or SNRI versus another active drug (amitriptyline), Outcome 1 Migraine frequency (number of migraine attacks).
6.
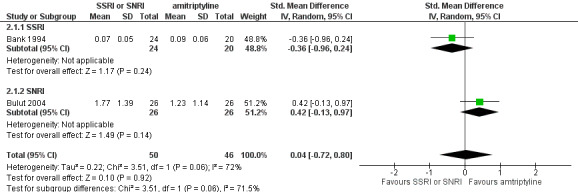
Forest plot of comparison: 2 SSRI or SNRI versus another active drug (amitriptyline), outcome: 2.1 Migraine frequency (number of migraine attacks).
Secondary outcomes
Migraine intensity
This outcome was considered by Bulut 2004 and Oguzhanoglu 1999. Bulut 2004 used a 0‐ to 3‐point scale, where 0 = able to work throughout the attack and 3 = staying in bed. Venlafaxine and amitriptyline were similar in reducing migraine intensity (mean difference (MD) 0.52, 95% CI ‐0.04 to 1.07). Oguzhanoglu 1999 did not report quantitative data but stated that neither fluoxetine nor amitriptyline significantly reduced migraine intensity over the three‐month treatment period; there was no clear definition of the intensity measure used.
Migraine duration
This outcome was considered by Bulut 2004 and Oguzhanoglu 1999. Bulut 2004 reported that venlafaxine and amitriptyline were similar in reducing migraine duration (MD 1.41, 95% CI ‐0.03 to 2.85). Oguzhanoglu 1999 did not report quantitative data but stated that fluoxetine reduced attack duration at two months (P value = 0.015) and at three months (P value = 0.013). No significant differences were found within the amitriptyline group.
Symptomatic/analgesic medication use
None of the included studies reported data on symptomatic/analgesic medication use.
Migraine index
Bank 1994 provided data using two different indexes and we preferred the one that incorporated frequency. This was called the Headache Index (HI) and was defined as the number of migraine attacks times the intensity of attacks (mild, moderate, or severe) divided by the number of days in the visit period. The author presented only within‐group analyses and reported that the HI decreased from baseline to the end of treatment (three months) with both fluvoxamine and amitriptyline.
Withdrawals (for any reasons and due to adverse events)
Oguzhanoglu 1999 reported two drop‐outs but did not specify their treatment group. Bank 1994 reported that 15.6% (5/32) of patients receiving fluvoxamine withdrew from treatment, compared with 31.3% (10/32) of patients treated with amitriptyline; the difference between the two treatments was not statistically significant (Peto OR 0.42, 95% CI 0.13 to 1.34).
In Bank 1994, 9.4% of patients receiving fluvoxamine (3/32) withdrew from treatment due to adverse events, compared to 21.9% of patients treated with amitriptyline (7/32). The difference was not statistically significant (Peto OR 0.39, 95% CI 0.10 to 1.50). Bulut 2004 reported drop‐out for both periods together: five (8.6%) patients withdrew from treatment due to hypersomnia in the amitriptyline group and one (1.7%) due to nausea and vomiting in the venlafaxine group. Reasons for withdrawals are specified in the Characteristics of included studies.
Minor adverse events
Oguzhanoglu 1999 reported only aggregated data on adverse events. In Bank 1994, 12.5% of patients treated with fluvoxamine (4/32) experienced drowsiness, dry mouth, nausea, or general weakness during the first week; 15.6% of patients receiving amitriptyline (5/32) experienced drowsiness. The difference between the two treatments was not statistically significant (OR 0.77, 95% CI 0.19 to 3.18).
Chronic daily headache
In this section, we reported the results of two studies that included participants with chronic daily headache or transformed migraine (Krymchantowski 2002; Polisca 1992). These definitions are no longer used in clinical practice and, in fact, we found no new studies. Furthermore, the rigour of the study design was far from optimal. The findings of this section are likely to be of little relevance and are no longer reported in this update (for details see Moja 2005).
Polisca 1992 described the headache syndrome of included patients with a chronic type of migraine with tension‐type episodes, and compared fluoxetine versus placebo. We have major concerns about the methodological quality of this study. This study reported that fluoxetine was more effective than placebo in terms of headache frequency, headache index, and symptomatic/analgesic medication use after eight and 12 weeks of follow‐up. No drop‐outs were observed and no adverse events reported.
Krymchantowski 2002 included participants suffering from transformed migraine with symptomatic medication overuse treated with fluoxetine and amitriptyline or amitriptyline alone. No difference in terms of headache index, total withdrawals, and withdrawals due to adverse events were reported.
Planned subgroup analyses
Only Adly 1992 included patients with depression, thus we lacked sufficient data to compare trials enrolling depressed patients versus trials in which patients were not depressed.
Due to the low number of trials included we could not analyse the various SSRIs and SNRIs separately.
Prevention of transformation to a chronic headache syndrome
We did not find trials focusing on whether SSRIs or SNRIs could prevent the progression of an episodic migraine into a chronic headache syndrome.
Head‐to‐head comparison
One study compared escitalopram to venlafaxine, a serotonin and weak nor‐adrenaline reuptake inhibitor in 93 patients with migraine without depression or anxiety (Tarlaci 2009). The authors described mainly within‐group analyses and did not report a statistical comparison between two tested drugs. Venlafaxine and escitalopram appear to have similar efficacy in terms of migraine frequency (5.1 versus 6.6 attack/months) and migraine duration (6.7 versus 4.6 hours), while escitalopram appears to have a better safety profile.
Discussion
Summary of main results
Evidence supporting the use of selective serotonin reuptake inhibitors (SSRIs) or serotonin‐norepinephrine reuptake inhibitors (SNRIs) to ameliorate the most relevant clinical outcome in adult patients with migraine ‐ frequency ‐ is scarce. The only new study included in this update that analysed a SNRI, venlafaxine, versus placebo, reported a decrease of migraine frequency. However, it is questionable to rely on the evidence originated by a single sponsored study with poor reporting. SSRIs and SNRIs may be useful, not useful, or detrimental for attacks of migraine. Our analysis does not exclude any of these possibilities. However, the interpretation most likely to emerge in revising all the evidence is that, when compared to placebo, the SSRIs and SNRIs did not seem to reduce migraine frequency at two‐ or three‐month follow‐up. Neither SSRIs nor venlafaxine were better than amitriptyline. Among other secondary outcomes, again SSRIs or SNRIs did not ameliorate migraine intensity, duration, or migraine index, and did not reduce the consumption of analgesic drugs when compared to placebo. Amitriptyline appeared to be similar to venlafaxine in reducing migraine intensity and duration. The data on the safety profiles of SSRIs and SNRIs derived from the included studies are also limited. No differences in terms of withdrawals due to adverse events or minor adverse events were detected, but the number of events were few and their reporting generally unclear. SSRIs and SNRIs appear to be better tolerated than amitriptyline but no firm conclusions can be drawn on the basis of the included trials.
We included three new trials in this update, which did not provide any substantial new evidence in the field. Overall, these results are based on 10 studies comparing SSRIs or SNRIs with placebo or other antidepressants (amitriptyline), and one head‐to‐head comparison. We did not find studies comparing SSRIs and SNRIs with non‐antidepressant drug treatments for preventing migraine (beta‐blockers, antiepileptics, etc.) or with physical or behavioural treatments for migraine.
Many indexes, scales, and sub‐scales were used in this relatively small number of trials. The clinical relevance of some of these rating tools is questionable (Thornley 1998). The only scales that have been formally evaluated are those that assess patients for depressed mood (e.g. Zung Depression Scale or Montgomery and Asberg Depression Scale), a secondary outcome in the management of chronic pain conditions (Snow 2002). Furthermore, the working hypothesis of these trials is that the overall effect of SSRIs or SNRIs is not due to a direct antidepressant effect (Sindrup 2000). Thus, SSRIs or SNRIs need to be compared with non‐antidepressant prophylactic drugs or non‐pharmacologic preventive treatments in order to avoid the confounding effects of the antidepressant therapy.
Only two studies considered symptomatic/analgesic medication use as an outcome of interest (Ozyalcin 2005; Steiner 1998). Medication use may be more difficult to interpret than migraine frequency (as measured by daily self report) because it is based on a behavioural response on the part of the patient (taking medication for acute relief) to the occurrence of a headache, which introduces an extra layer of variability. Accordingly, while the use of medications may be a less direct measure of their effect on migraine compared to attack frequency, we believe it is a desirable secondary outcome, particularly since the overuse of acute medication may perpetuate or increase chronic migraines (Kaniecki 2003).
Pain associated with migraine affects many aspects of an individual's life, including both social and occupational roles. We did not find any mention of workdays lost or any data pertaining to cost‐effectiveness or quality of life. Workdays lost is a specific, strong measure to assess headache improvement from a more social‐economic perspective. Several quality of life scales have been validated and are now available. They assess the impact of migraine on daily activities and include many items related to individuals' general well‐being such as pain and mood states. Many headache indexes replicate a sub‐scale or items already included in quality of life scales. Finally, quality of life is a global measure capable of making useful comparisons between adverse events of drugs (Hotopf 1997).
Only one study compared a SSRI to a SNRI and it reported no difference between escitalopram and venlafaxine in terms of migraine frequency (Tarlaci 2009). We cannot draw any conclusion about the fact that differences in selectivity (serotonin or nor adrenaline reuptake) could be related to differences in efficacy.
Overall completeness and applicability of evidence
The data that inform this review are few and generally poor, in terms of the quality of the trials that originated them. Only five studies reported data on the most relevant clinical outcome, migraine frequency (two placebo‐ and three amitriptyline‐controlled) for a total of fewer than 300 participants. Reporting was often incomplete, making some studies uninformative. The applicability of this scarce evidence is also an issue, mainly because the analysed studies used short follow‐up and outcomes with a small clinical value. However, the findings of this review suggest that SSRIs and SNRIs do not show benefits for the outcomes that may matter to patients.
Quality of the evidence
The majority of the included trials can be considered to be at unclear risk of bias (see Risk of bias in included studies). None of the trials reported any information on allocation concealment, or the blinding of the treatment allocation. Blinding of participants and study personnel was described in only a few studies. Many trials were likely to be underpowered, had missing intention‐to‐treat analysis and had a strong inclination to perform multiple testing. The small study size is a consistent marker of overestimation of treatment effects. Finally, there were frequently ambiguities in the presentation of the results of the analyses, emphasising within‐group comparisons. Some readers may find these methodological problems surprising; we did not. Previous methodological work showed that these are common problems in studies of SSRIs and related antidepressants (Hotopf 1997; Hotopf 1999; Thornley 1998) and, more generally, can be found across many medical specialties (Altman 1990). Methodological quality did not seem to have improved in the more recent trials.
Even though we did not formally explore outcome reporting bias, the use of multiple outcomes without a predefined primary outcome, with no prespecified priorities among outcomes, and without knowing whether outcomes are equally correlated, may have increased the risk of data dredging and distorted reporting in an attempt to demonstrate post hoc differences between interventions (Pocock 1997). This problem is magnified by the limited size of most included trials.
We rated the overall quality of the evidence for clinically relevant efficacy and safety outcomes as 'low' or 'very low' (Table 1 and Table 2). We downgraded the overall quality of the evidence for each outcome because of limitations in the study designs and imprecision. We also downgraded the outcomes 'migraine index' and 'withdrawals (for any reasons and due to adverse events)' for indirectness. Our choice was driven by the fact that migraine indexes, as well as the definitions of adverse events, varied among the trials, and their applicability and appropriateness to the clinical context might be questionable.
Agreements and disagreements with other studies or reviews
Other systematic reviews (Tomkins 2001 and its update in Jackson 2010) have examined antidepressant medication for migraine prophylaxis. On the basis of four studies, which were also included in our review, the authors concluded that tricyclic antidepressants reduced the pain from migraine, citing some evidence about their superiority over SSRIs. The studies included in our review suggested a similar trend, however we cannot conclude that one class of drug is a better option than the other.
Recent clinical guidelines from the USA considered the role of antidepressants in migraine prophylaxis (Silberstein 2012). It was suggested that SSRIs and venlafaxine were probably effective, although the authors cautiously assessed the overall evidence supporting this recommendation as either negative or equivocal. This recommendation, possibly favouring the use of SSRIs and SNRIs as a preventive strategy, seems to be generous. A more balanced message is provided by the National Institute for Health and Care Excellence (NICE) and Canadian guidelines in which SSRIs and SNRIs are either not considered at all or are not indicated as a suitable option (NICE 2012; Pringsheim 2012), in preference to more effective strategies (e.g. topiramate or propranolol). Even in patients with migraine, concomitant depression, and/or anxiety, the role of SSRIs and SNRIs should be considered as limited given the paucity of evidence.
Authors' conclusions
Implications for practice.
Since the last version of this review, we included three new relevant studies, which have provided little new evidence on the effectiveness of selective serotonin reuptake inhibitors (SSRIs) and serotonin‐norepinephrine reuptake inhibitors (SNRIs) in patients with migraine. SSRIs or SNRIs are no more effective than placebo and are likely to be less effective than amitriptyline in preventing migraine. Fluoxetine was the most studied SSRI, while venlafaxine was the only SNRI under investigation.
The usefulness of SSRIs or SNRIs for preventing migraine is obscure and the best guess is that these drugs are unlikely to be effective for the majority of patients. When compared to placebo or amitriptyline, SSRIs and SNRIs did not show any superiority on relevant outcomes (migraine frequency, intensity, duration). There was some evidence that SSRIs are better tolerated than amitriptyline and venlafaxine. The issue of long‐term treatment (more than three months) with respect to efficacy and tolerability should still be addressed because in real‐life conditions, patients with chronic migraine receive treatment for more than a few weeks.
No conclusion can be drawn on the use of antidepressants with respect to other prophylactic pharmacological treatments, such as antihypertensives (e.g. angiotensin‐converting enzyme (ACE) inhibitors and angiotensin II receptor antagonists, beta‐blockers, calcium channel antagonists) or antiepileptics.
Implications for research.
Overall, the standards in terms of design and reporting of randomised clinical trials still need to be improved. For example, open designs are not acceptable in this context. Migraine frequency should be the primary outcome measure in any new trial. Migraine is a recurrent condition that persists over long periods of time/whole parts of the lifespan, therefore longer follow‐up is needed and harder outcomes that relate to real‐life should be assessed (e.g. migraine frequency, acute medication use, days off work, and quality of life) (Tfelt‐Hansen 2012). This will also avoid the use of non‐validated indexes, discouraging multiple comparisons at different time points, with the warning that multiple data testing easily results in misleading statistically significant findings that appear by chance (Moja 2005; Thornley 1998). Standardised collection of outcomes as suggested by the COMET (Core Outcome Measures in Effectiveness Trials) Initiative, which is engaged in developing, applying, and promoting core outcomes sets (COS), using rigorous consensus methods, for effectiveness trials (Williamson 2012) could be helpful. The sample size should be carefully estimated on the basis of the available evidence and the expected effect, in order to protect the study against random error.
During the current update, we noticed a clear reduction in the number of publications testing SSRIs and SNRIs in the prophylaxis of migraine. For several clinically relevant outcomes, we reported a low level of evidence. This indicates that "further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate" (Guyatt 2008). Under this assumption, amitriptyline could still be suggested as a reference comparator to be used in clinical trials comparing antidepressants for prevention of migraine. The limited number of studies on SNRIs retrieved by this review may suggest that further exploration of the role of these drugs, such as duloxetine, is needed in migraine prevention. However, overall, we think that the value of new studies comparing different antidepressants in this setting is questionable. A randomised controlled trial comparing a SSRI or a SNRI versus another drug or another non‐pharmacological intervention is not a priority in the migraine research pipeline and might not exert a significant impact on the overall evidence. Other preventive strategies are likely to be the target of future research. In the field of antidepressants, exploring the efficacy and safety SSRIs or SNRIs in depressed patients with migraine might be of greater interest, as optimal treatments and the role of weak antidepressants are still debatable.
What's new
| Date | Event | Description |
|---|---|---|
| 15 November 2019 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 8 April 2016 | Amended | Affiliation added for LM. |
| 30 April 2015 | Amended | Minor spelling inconsistencies corrected. |
| 1 April 2015 | Review declared as stable | This review will be assessed for further updating in 2020. |
| 24 January 2014 | New citation required and conclusions have changed | This is an update of the review 'Selective serotonin re‐uptake inhibitors (SSRIs) for preventing migraine and tension‐type headaches'. We have implemented the following major changes:
We included three new studies (253 participants) in this update (Bulut 2004; Ozyalcin 2005; Tarlaci 2009). Overall, we included 11 studies and 585 participants. We recommend that previous readers of the review should re‐read this update. |
| 14 November 2013 | New search has been performed | We have updated this review to include the results of a new search and to include a new class of antidepressants (serotonin‐norepinephrine reuptake inhibitors). We have added a study flow chart, 'Risk of bias' tables, and 'Summary of findings' tables. |
| 10 August 2009 | Amended | Contact details updated. |
| 28 October 2008 | Amended | Converted to new review format. |
Notes
We performed a restricted updated search in October 2019, but we did not identify any potentially relevant studies likely to change the conclusions. The research area is no longer active and we do not expect new RCTs for this intervention and population to be published. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be reassessed for updating in five years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The authors gratefully acknowledge Joanne Abbott and Jane Hayes for the search strategies; Claudio Canepari for the handsearching; Svetlana Kobrina for translations; Valentina Pecoraro and Koren Kwag for manuscript revision; and the Cochrane Pain, Palliative and Supportive Care review group for its helpful assistance during all the phases of the preparation of the review.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Search strategies for identification of studies
MEDLINE (Ovid)
1 Headache/
2 exp Headache Disorders/
3 (headach* or migrain* or cephalgi* or cephalalgi*).mp.
4 1 or 2 or 3
5 exp Serotonin Uptake Inhibitors/
6 (SSRI* or SNRI*).mp.
7 (serotonin* and (reuptake or re‐uptake) and inhibitor*).mp.
8 (citalopram or dapoxetin* or escitalopram or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or desvenlafaxin* or duloxetin* or milnacipran or venlafaxin*).mp.
9 5 or 6 or 7 or 8
10 4 and 9
11 randomized controlled trial.pt.
12 controlled clinical trial.pt.
13 randomized.ab.
14 placebo.ab.
15 clinical trials as topic.sh.
16 randomly.ab.
17 trial.ti.
18 11 or 12 or 13 or 14 or 15 or 16 or 17
19 10 and 18
20 exp animals/ not humans.sh.
21 19 not 20
key:
mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier
pt=publication type
ab=abstract
fs=floating subheading
EMBASE (Ovid)
1 exp "headache and facial pain"/
2 (headach* or migrain* or cephalgi* or cephalalgi*).mp.
3 1 or 2
4 exp serotonin uptake inhibitor/
5 exp serotonin noradrenalin reuptake inhibitor/
6 (SSRI* or SNRI*).mp.
7 (serotonin* and (reuptake or re‐uptake) and inhibitor*).mp.
8 (citalopram or dapoxetin* or escitalopram or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or desvenlafaxin* or duloxetin* or milnacipran or venlafaxin*).mp.
9 4 or 5 or 6 or 7 or 8
10 3 and 9
11 crossover procedure/
12 double‐blind procedure/
13 randomized controlled trial/
14 single‐blind procedure/
15 random*.mp.
16 factorial*.mp.
17 (crossover* or cross over* or cross‐over*).mp.
18 placebo*.mp.
19 (double* adj blind*).mp.
20 (singl* adj blind*).mp.
21 assign*.mp.
22 allocat*.mp.
23 volunteer*.mp.
24 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23
25 10 and 24
26 (exp Animal/ or Nonhuman/ or exp animal Experiment/) not Human/
27 25 not 26
key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
CENTRAL (The Cochrane Library)
#1 MeSH descriptor Headache, this term only
#2 MeSH descriptor Headache Disorders explode all trees
#3 (headach* or migrain* or cephalgi* or cephalalgi*)
#4 (#1 OR #2 OR #3)
#5 MeSH descriptor Serotonin Uptake Inhibitors explode all trees
#6 (SSRI* or SNRI*)
#7 (serotonin* and (reuptake or re‐uptake) and inhibitor*)
#8 (citalopram or dapoxetin* or escitalopram or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or desvenlafaxin* or duloxetin* or milnacipran or venlafaxin*)
#9 (#5 OR #6 OR #7 OR #8)
#10 (#4 AND #9)
PsycINFO (Ovid)
1 exp headache/
2 (headach* or migrain* or cephalgi* or cephalalgi*).mp.
3 1 or 2
4 exp serotonin reuptake inhibitors/
5 exp serotonin norepinephrine reuptake inhibitors/
6 (SSRI* or SNRI*).mp.
7 (serotonin* and (reuptake or re‐uptake) and inhibitor*).mp.
8 (citalopram or dapoxetin* or escitalopram or fluoxetin* or fluvoxamin* or paroxetin* or sertralin* or desvenlafaxin* or duloxetin* or milnacipran or venlafaxin*).mp.
9 4 or 5 or 6 or 7 or 8
10 3 and 9
key: [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
Clinicaltrial.gov
(headache or migraine) and serotonin
"Intervention studies"
metaRegister of controlled trials (www.controlled‐trials.com/mrct/search.html)
(headache or migraine) and serotonin
"All registries"
WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/)
(headache or migraine) AND serotonin
Status: "all"
Data and analyses
Comparison 1. SSRI or SNRI versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Migraine index | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Follow‐up: 8 weeks | 3 | 86 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.57, 0.30] |
| 1.2 Follow‐up: 12 weeks | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.88, 0.25] |
| 2 Withdrawals ‐ any reason | 5 | 221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.73, 2.56] |
| 3 Withdrawals due to adverse events | 5 | 221 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.95 [0.70, 5.44] |
| 4 Number of patients with minor adverse events | 2 | 84 | Odds Ratio (M‐H, Random, 95% CI) | 1.46 [0.47, 4.52] |
Comparison 2. SSRI or SNRI versus another active drug (amitriptyline).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Migraine frequency (number of migraine attacks) | 2 | 96 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.72, 0.80] |
| 1.1 SSRI | 1 | 44 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.96, 0.24] |
| 1.2 SNRI | 1 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [‐0.13, 0.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adly 1992.
| Methods | Single‐centre, double‐blind, randomised, parallel study: fluoxetine versus placebo No control for symptomatic/analgesic medications use |
|
| Participants | Country: USA N = 32 participants Sex: 3 M, 15 F (sex not reported for drop‐outs) Mean age: fluoxetine 34, placebo 41 Diagnosis: migraine according to Ad hoc Committee on Classification of Headache (Ad Hoc 1962) Exclusion criteria: less then 1 weekly severe disabling migraine headache, concomitant medical conditions, overuse of alcohol and drugs Recruitment: volunteers solicited through a local newspaper and paid to participate (USD 40) | |
| Interventions | N = 16 fluoxetine to a maximum of 40 mg/day
N = 16 placebo Active treatment: 8 weeks |
|
| Outcomes | 1. Migraine Headache Score = intensity (scale 1 to 10) * duration * amount of medications used to abort attacks 2. Zung Depression Rating Scale | |
| Notes | 14 drop‐outs (44%):
Fluoxetine: 7 (4 did not keep appointments, 2 for lack of efficacy,1 for side effects)
Placebo: 7 (2 lack of efficacy, 4 changed their mind, 1 submitted an incomplete diary)
Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | Prescription for fluoxetine or placebo to be filled by the hospital pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High drop‐out rate (44%) |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Bank 1994.
| Methods | Single‐centre, double‐blind, randomised, parallel study: fluvoxamine versus amitriptyline No control for symptomatic/analgesic medications use | |
| Participants | Country: Hungary N = 64 Sex: 17 M, 47 F Mean age: 34.5 (SD 7.4) fluvoxamine, 33.5 (SD 8.3) amitriptyline Diagnosis: migraine with and without aura (according to International Headache Society Criteria, IHS 1988) Exclusion criteria: tension‐type headache, headache due to any physical cause, severe systemic illness and pregnancy | |
| Interventions | N = 32 fluvoxamine 50 mg/day
N = 32 amitriptyline 25 mg/day Active treatment: 12 weeks |
|
| Outcomes | 1. Headache Unit Index = number of attacks/number of days in visit period 2. Corrected Headache Unit Index = (intensity of attacks ('1 = mild pain' to '3 = severe pain, preventing any activity') * duration (hours))/number of days in visit period 3. Headache Index = (number of attacks * intensity)/number of days in visit period | |
| Notes | 15 drop‐outs (23%):
Fluvoxamine: 5 (2 reasons unknown, 3 for side effects: 1 drowsiness and 2 gastrointestinal problems)
Amitriptyline: 10 (3 reasons unknown, 7 for side effects: all experienced severe drowsiness)
Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Drugs were given to patients in a closed envelope |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Moderate drop‐out rate, unbalanced, reasons not fully reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Bulut 2004.
| Methods | Single‐centre, randomised, double‐blind, cross‐over: venlafaxine + amitriptyline versus amitriptyline + venlafaxine | |
| Participants | Country: Turkey N = 52 Sex: 8 M 44 F Mean age: 31.9 Diagnosis: migraine with and without aura according to International Headache Society (IHS 1988) Exclusion criteria: use of other drugs ordered for prophylactic treatment of migraine in the 4 weeks before randomisation, depression or other psychiatric disorders, allergy to venlafaxine and/or amitriptyline, serious diseases such as hepatic or renal dysfunction, heart disease, and pregnancy or breast feeding |
|
| Interventions | N = 26 venlafaxine + amitriptyline N = 26 amitriptyline + venlafaxine Active treatment: 12 weeks |
|
| Outcomes | 1. Number of attacks per month 2. Attack intensity 3. Attack duration |
|
| Notes | 76 patients involved, 52 completers 24 drop‐outs: 6 due to side effects, 18 due to other reasons Washout period: 4 weeks Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information (randomised) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both drugs were identical in appearance and were packed in identical bottles |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Overall 24/76 (32%) unbalanced for side effects; no other info |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Colucci d'Amato 1999a.
| Methods | Single‐centre, double‐blind, randomised, parallel study: fluoxetine versus placebo No control for symptomatic/analgesic medications use | |
| Participants | Country: Italy N = 52 Sex: 19 M, 33 F Mean age: 36.8 (SD 12.4) fluoxetine; 38.8 (SD 15.6) placebo Diagnosis: migraine without aura, for at least 6 months (according to International Headache Society Criteria) (IHS 1988) Exclusion criteria: patient using medications for migraine prophylaxis, severe concomitant neurological and medical disorders, breast feeding and pregnancy Recruitment: headache service outpatients | |
| Interventions | N = 32 fluoxetine 20 mg/day
N = 20 placebo Active treatment: 24 weeks |
|
| Outcomes | 1. Pain Total Index = pain intensity ('1 = mild pain' to '3 = severe pain, preventing any activity') x duration (number of hours of headache per month). The algorithm was as follows: PTI = (D1 x 1) + (D2 x 2) + (D3 x 3), where 1, 2, and 3 are levels of pain intensity, and D1, D2, and D3 are the hours of migraine per month with intensity 1, 2, and 3, respectively | |
| Notes | No drop‐outs
Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drugs identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Krymchantowski 2002.
| Methods | Single‐centre, double‐blind, randomised, parallel study: amitriptyline versus amitriptyline plus fluoxetine Control for symptomatic/analgesic medications use | |
| Participants | Country: Brazil N = 39 Sex: 13 M, 26 F Mean age: 36.4 (SD 2.5) Diagnosis: transformed migraine with overusing symptomatic medications (according criteria proposed by Silberstein 1996) Exclusion criteria: patients using medications for migraine prophylaxis or chronic treatment for other clinical and psychiatric conditions, women of childbearing potential not using contraceptives Recruitment: no information provided | |
| Interventions | N = 19 amitriptyline to a maximum of 40 mg/day
N = 20 amitriptyline to a maximum of 40 mg/day plus fluoxetine to a maximum of 40 mg/day Active treatment: 9 weeks |
|
| Outcomes | 1. Headache Index = frequency (number of headache days/30 day) x intensity ('0 = no headache' to '4 = bed rest') | |
| Notes | 12 drop‐outs (31%):
Amitriptyline: 6 (4 for incomplete diary, 2 for worsening condition)
Amitriptyline plus fluoxetine: 6 (3 for incomplete diary, 1 for worsening condition, 2 for side effects)
Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drugs identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate drop‐out rate, balanced, reasons fully reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Low risk | No financial support provided by drug companies |
Landy 1999.
| Methods | Single‐centre, double‐blind, randomised, parallel study: sertraline versus placebo No control for symptomatic/analgesic medications use | |
| Participants | Country: USA N = 27 Sex: 2 M, 25 F Mean age: 36 (SD 8.6) Diagnosis: migraine with or without aura for 1 year or longer (according to International Headache Society Criteria, IHS 1988) Exclusion criteria: severe systemic illness, pregnancy, lactation, treated for a concomitant seizures or psychiatric disorder Recruitment: patients of the Wesley Headache Clinic | |
| Interventions | N = 13 sertraline to a maximum of 100 mg/day
N = 14 placebo Active treatment: 8 weeks |
|
| Outcomes | 1. Headache Index = intensity ('1 = mild pain' to '3 = severe') x number of occurrences 2. Impairment Index = impairment ('1 = no impairment' to '3 = bed rest') * number of occurrences | |
| Notes | 11 drop‐outs (41%):
Sertraline: 7 (6 did not complete the study, 1 for side effects: loss of appetite and insomnia)
Placebo: 4 (2 did not return, 2 for side effects: anxiety, nausea, dizziness and sweating)
Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Patient, treating physician, and nurse were unaware of the group assignment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Substantial drop‐out rate, unbalanced, reasons not fully reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Oguzhanoglu 1999.
| Methods | Single‐centre, open‐label, randomised, parallel study: fluoxetine versus amitriptyline No control for symptomatic/analgesic medications use | |
| Participants | Country: Turkey Overall: N = 52; Sex: 6 M, 41 F (sex not reported for drop‐outs) Migraine group: N = 17; Sex: 3 M, 12 F (sex not reported for drop‐outs); mean age: 31 Diagnosis: migraine (N = 17), CTTH (N = 14) and ETTH (N = 21), all defined according to International Headache Society criteria (IHS 1988) Exclusion criteria: antidepressants use in the previous year, score > 17 Hamilton Depression Scale Recruitment: from November 1996 to September 1997, no other information | |
| Interventions | N = 22 amitriptyline to a maximum of 50 mg/day
N = 25 fluoxetine 20 mg/day Duration of active treatment: unclear |
|
| Outcomes | 1. Headache frequency (number of days with headache/30 days) 2. Pain intensity (not defined) 3. Headache duration (not defined) | |
| Notes | Overall, 5 drop‐outs (10%) for side effects (2 in the migraine group)
Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Moderate drop‐out rate (2/17, 11.7%), reasons reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Ozyalcin 2005.
| Methods | Single‐centre, double‐blind, randomised, parallel study: venlafaxine 75 mg versus venlafaxine 150 mg versus placebo | |
| Participants | Country: Turkey N = 60 Sex: 6 M, 54 F Mean age: 34.25 (SD 8.28) venlafaxine 75 mg; 37.19 (SD 12.37) venlafaxine 150 mg; 38.16 (SD 11.24) placebo Diagnosis: migraine without aura according to International Headache Society (IHS 1988) Exclusion criteria: age < 18 or > 70, headache for < 2 years, < 3 attacks or > 10 attacks per month and > 15 headache days per month. Patients taking other prophylactic treatment not stopped 2 weeks prior the start of the study. Women who were breast feeding, patients with major cardiovascular, metabolic, gastrointestinal, neurologic diseases, any primary headache disorder other than migraine without aura, and any secondary headache disorder including drug over use headache disorder |
|
| Interventions | N = 20 venlafaxine 75 mg N = 21 venlafaxine 150 mg N = 19 placebo |
|
| Outcomes | 1. Number of days with headache 2. Pain intensity 3. Headache duration 4. Analgesic consumption 5. Daily activities 6. Patient satisfaction 7. Adverse events 8. Global tolerance |
|
| Notes | Lost at follow‐up 22% (47 completers) Per protocol analysis No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information (randomised) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information (double‐blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Moderate drop‐out rate (13/60 = 21.67%), balanced (placebo 3/19 = 15.8%, venlafaxine 75 mg 5/20 = 25%, venlafaxine 150 mg 5/21 = 23.8%) |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Grant from Wyeth Ilaclari AS, Istanbul, Turkey. Blinded study drugs were prepared by Wyeth Pharmaceuticals, PA, USA |
Polisca 1992.
| Methods | Single‐centre, double‐blind, randomised, parallel study: fluoxetine versus placebo No control for symptomatic/analgesic medications use | |
| Participants | Country: Italy N = 60 Sex: 22 M, 38 F Mean age: 43.5 (SD 2.1) fluoxetine; 39 (SD 0.8) placebo Diagnosis: transformed migraine (chronic daily migraine headache, according to Manzoni and Nappi (Nappi 1985), with a 6‐year or longer history) Exclusion criteria: unclear Recruitment: no information provided | |
| Interventions | N = 30 fluoxetine 20 mg/day
N = 30 placebo Active treatment: 12 weeks |
|
| Outcomes | 1. Pain Total Index (not defined) 2. Migraine Index (not defined) 3. Frequency (number of headache days/30 days) 4. Symptomatic/analgesic drug consumption | |
| Notes | No drop‐outs
Article in Italian The report omits important details No sample size calculation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Neither doctors nor patients were able to foresee the assignment (not specified how) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
Steiner 1998.
| Methods | Multi‐centre, double‐blind, randomised, parallel study: S‐fluoxetine versus placebo. Control for symptomatic/analgesic medications use | |
| Participants | Country: United Kingdom N = 53 Sex: 13 M, 40 F Mean age: 37.5 S‐fluoxetine, 39 placebo Diagnosis: migraine (according to International Headache Society Criteria, IHS 1988) with a 1‐year or longer history and 6 to 18 attacks reported in the previous 3 months Exclusion criteria: patient using medications for migraine prophylaxis or chronic treatment for depression, breast feeding and pregnancy, drug or alcohol abuse, participation in previous trials Recruitment: 3 headache centres in the London region. Patients stratified at each centre according to historical attack frequency | |
| Interventions | N = 27 S‐fluoxetine 40 mg/day
N = 26 placebo Active treatment: 12 weeks |
|
| Outcomes | Primary efficacy measures: 1. Headache frequency (attacks/28 days) Secondary efficacy measures: 2. Migraine days/28 days 3. Attack intensity (rating scale from '1 = mild attack' to '3 = severe') 4. Symptomatic/analgesic drug consumption (dose/attack) 5. Patient's Global Impression of Disease intensity (0 to 100 mm visual analogue scale) | |
| Notes | 65 patients recruited, 53 randomised
20 (38%) drop‐outs:
3 for non‐adherence to treatment
S‐fluoxetine: 9 (4 for side effects; 1 for inadequate response; 4 reason not specified)
Placebo: 8 (4 for side effects, 1 for inadequate response, 3 reason not specified)
Per protocol analysis Sample size calculation done, drop‐out rate higher than expected |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drugs identical in appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Substantial drop‐out rate, balanced, reason fully reported |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | High risk | Financial support provided by Sepracor Inc. (Marlborough, MA, USA) |
Tarlaci 2009.
| Methods | Single‐centre, randomised, parallel study: venlafaxine versus escitalopram | |
| Participants | Country: Turkey Overall: N = 93 Sex: 17 M, 76 F (sex not reported for drop‐outs) Mean age: 31.4 ± 7.8 Diagnosis: migraine according to the criteria of the International Headache Society (ICHD‐II 2004) Exclusion criteria: abnormal systemic and neurological examination; < 3 migraine attacks per month; prophylactic medication for the last 2 months |
|
| Interventions | N = 35 venlafaxine ranging from 75 mg/day to 150 mg/day N = 58 escitalopram ranging from 10 mg/day to 20 mg/day |
|
| Outcomes | 1. Number of attacks at 12 weeks 2. VAS 3. Duration at 12 weeks |
|
| Notes | 12 drop‐outs from venlafaxine group due to adverse events Lost at follow‐up 22% (intention‐to‐treat analysis claimed but no further information) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information (randomised) |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Low drop‐out rate (11.4%) but unbalanced drop‐out in 1 group |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | Financial support not reported |
CTTH: Chronic tension‐type headache
ETTH: Episodic tension‐type headache
PTI: Pain Total Index SD: standard deviation VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amelin 2000 | Not controlled, only intra‐group comparison reported |
| Andersson 1981 | SSRI femoxetine no longer produced by drug company |
| Bittman 1992 | Not randomised |
| Bussone 1991 | Data presented aggregate patients with migraine and chronic tension‐type headache |
| Centonze 2000 | Both groups took venlafaxine |
| Colucci d'Amato 1998 | Not randomised |
| Colucci d'Amato 1999b | Not randomised |
| Colucci d'Amato 2000 | Not randomised |
| Diamond 1989 | Not randomised |
| Foster 1994 | Not randomised |
| Iannacchero 1999 | Not randomised |
| Kangasniemi 1983 | SSRI femoxetine no longer produced by drug company |
| Kathpal 1998 | Not randomised |
| Orholm 1986 | SSRI femoxetine no longer produced by drug company |
| Rampello 2004 | Patients with comorbidity of depression, migraine, tension‐type headache |
| Sandrini 1991 | Not randomised |
| Saper 1994 | Data presented aggregate patients with migraine and with chronic daily headache |
| Zeeberg 1981 | SSRI femoxetine no longer produced by drug company |
SSRI: selective serotonin reuptake inhibitor
Characteristics of studies awaiting assessment [ordered by study ID]
Dzagnidze 2009.
| Methods | Randomised controlled trial, 3 arms |
| Participants | 68 patients with migraine (6 to 7 attacks per month) |
| Interventions | Venlafaxine 150 mg/day (N = 20); venlafaxine 150 mg/day and metoprolol 100 mg/day (N = 26); placebo (N = 22) |
| Outcomes | Headache frequency, intensity, and duration; symptomatic/analgesic medication use for acute headache attacks. Outcome assessed but data not provided |
| Notes | Poster presentation |
He 2004.
| Methods | Single‐centre, randomised trial done in China |
| Participants | 60 patients with migraine |
| Interventions | Fluoxetine 20 mg/day (N = 35); placebo (N = 25) |
| Outcomes | Rate of migraine treatment, general cure rate, serum lipid level |
| Notes | Chinese publication, English abstract |
Stanic 2009.
| Methods | Single‐centre, 3‐arm, randomised trial done in Serbia and Montenegro |
| Participants | 300 patients (men 96, women 204); migraine (International Headache Society (IHS)), 3 to 6 attacks per month |
| Interventions | Sertraline 50 mg/day (N = 100); 75 mg cinnarizine 37.5 mg (N = 100); placebo (N = 100) |
| Outcomes | Migraine attacks |
| Notes | Poster presentation |
Togha 2014.
| Methods | Single‐centre, double‐blind, randomised trial done in Iran |
| Participants | Chronic migraine according to IHS. Number of participants not reported |
| Interventions | Venlafaxine 150 mg; topiramate 100 mg |
| Outcomes | Not reported |
| Notes | — |
IHS: International Headache Society
Characteristics of ongoing studies [ordered by study ID]
NCT01393522.
| Trial name or title | SAV‐MD‐25 Title: A randomized double blind placebo control trial of milnacipran for migraine pain |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: double‐blind (subject, investigator) Primary purpose: to evaluate the efficacy of milnacipran in headache pain reduction in subjects with chronic migraine (CM) without fibromyalgia |
| Participants | Inclusion criteria:
Main exclusion criteria:
|
| Interventions | Intervention: milnacipran titration schedule starting with 12.5 mg per day increasing to 50 mg twice a day, starting with day 1 to day 90 and then tapered down Control: placebo titrated 1 tablet once a day increased per protocol to 2 tablets twice a day, with a starting dose of 12.5 mg per day to 50 mg twice a day |
| Outcomes | Evaluating Headache Pain Reduction (time frame: change from baseline after the 90‐day reporting period) Evaluating the improvement of Quality of Life (time frame: change from visit 1 until study completion month 4) |
| Starting date | June 2011 |
| Contact information | Timothy R Smith, MD; mercyhealthresearch@mercy.net |
| Notes | Study sponsor: Mercy Health Research Recruitment status: recruiting (verification date: September 2011) |
ICHD‐2: International Classification of Headache Disorders: 2nd edition PI: principal investigator SNRI: serotonin‐norepinephrine reuptake inhibitor
Differences between protocol and review
This 2015 update excludes tension‐type headache. A separate review on tension‐type headache is in press. Summary of findings table and Risk of bias tables have been added.
Contributions of authors
Study concept, protocol, and first version publication: LM, CC, RS. Selection of studies: RB, CC, CR, DT. Acquisition of data: RB, CC, CR, DT. 'Risk of bias' assessment: RB, CC, CR, DT. Analysis of data: RB, LM. Drafting of the manuscript: RB. Interpretation and critical revision of the manuscript for important intellectual content: RB, CC, CR, DT, RS, LM.
Sources of support
Internal sources
Cochrane Neurological Network, Other.
Italian Cochrane Centre, Italy.
Fondazione Bancaria Monte di Lombardia, Italy.
External sources
International Headache Society (for administrative costs associated with editorial review and peer review), Other.
Declarations of interest
RB has no relevant conflicts of interest to declare. CC has no relevant conflicts of interest to declare. CR has no relevant conflicts of interest to declare. DT has no relevant conflicts of interest to declare. RS has no relevant conflicts of interest to declare. LM has no relevant conflicts of interest to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Adly 1992 {published data only}
- Adly C, Straumanis J, Chesson A. Fluoxetine prophylaxis of migraine. Headache 1992;32(2):101‐4. [DOI] [PubMed] [Google Scholar]
Bank 1994 {published data only}
- Bank J. A comparative study of amitriptyline and fluvoxamine in migraine prophylaxis. Headache 1994;34(8):476‐8. [DOI] [PubMed] [Google Scholar]
Bulut 2004 {published data only}
- Bulut S, Berilgen MS, Baran A, Tekatas A, Atmaca M, Mungen B. Venlafaxine versus amitriptyline in the prophylactic treatment of migraine: randomized, double‐blind, crossover study. Clinical Neurology and Neurosurgery 2004;107:44–8. [DOI] [PubMed] [Google Scholar]
Colucci d'Amato 1999a {published data only}
- Colucci d'Amato C, Pizza V, Marmolo T, Giordano E, Alfano V, Nasta A. Fluoxetine for migraine prophylaxis: a double‐blind trial. Headache 1999;39(10):716‐9. [DOI] [PubMed] [Google Scholar]
Krymchantowski 2002 {published data only}
- Krymchantowski AV, Silva MT, Barbosa JS, Alves LA. Amitriptyline versus amitriptyline combined with fluoxetine in the preventative treatment of transformed migraine: a double‐blind study. Headache 2002;42(6):510‐4. [DOI] [PubMed] [Google Scholar]
Landy 1999 {published data only}
- Landy S, McGinnis J, Curlin D, Laizure SC. Selective serotonin reuptake inhibitors for migraine prophylaxis. Headache 1999;39(1):28‐32. [DOI] [PubMed] [Google Scholar]
Oguzhanoglu 1999 {published data only}
- Oguzhanoglu A, Sahiner T, Kurt T, Akalin O. Use of amitriptyline and fluoxetine in prophylaxis of migraine and tension‐type headaches. Cephalalgia 1999;19(5):531‐2. [DOI] [PubMed] [Google Scholar]
Ozyalcin 2005 {published data only}
- Ozyalcin SN, Talu GK, Kiziltan E, Yucel B, Ertas M, Disci R. The efficacy and safety of venlafaxine in the prophylaxis of migraine. Headache 2005;45(5):144‐52. [DOI] [PubMed] [Google Scholar]
Polisca 1992 {published data only}
- Polisca R, Signoretti P, Marchi P. Fluoxetine in the treatment of migraine with interval headache [La fluoxetina nella terapia della emicrania con cefalea intervallare]. Rassegna Internazionale di Clinica e Terapia 1992;72(9):408‐15. [Google Scholar]
Steiner 1998 {published data only}
- Steiner TJ, Ahmed F, Findley LJ, MacGregor EA, Wilkinson M. S‐fluoxetine in the prophylaxis of migraine: a phase II double‐blind randomized placebo‐controlled study. Cephalalgia 1998;18(5):283‐6. [DOI] [PubMed] [Google Scholar]
Tarlaci 2009 {published data only}
- Tarlaci S. Escitalopram and venlafaxine for the prophylaxis of migraine headache without mood disorders. Clinical Neuropharmacology 2009;32:254‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amelin 2000 {published data only}
- Amelin AV, Skoromets AA, Korenko LA, Tumelevich BCh, Gonchar MA. A comparative efficiency of amitriptyline, fluoxetine and maprotiline in prevention of migraine in attack‐free period [Sravnitel'naia effektivnost' amitriptilina, fluoksetina i maprotilina pri lechenii migreni v mezhpristupnom periode]. Zhurnal Nevrologii i Psikhiatrii Imeni S.S. Korsakova 2000;100(8):20‐3. [PubMed] [Google Scholar]
Andersson 1981 {published data only}
- Andersson PG, Petersen EN. Propranolol and femoxetine, a 5‐HT‐uptake inhibitor, in migraine prophylaxis. A double‐blind crossover study. Acta Neurologica Scandinavica 1981;64(4):280‐8. [DOI] [PubMed] [Google Scholar]
Bittman 1992 {published data only}
- Bittman B, Emanuele S. Fluoxetine: side effects and efficacy in a headache population. Headache Quarterly 1992;3(1):82‐5. [Google Scholar]
Bussone 1991 {published data only}
- Bussone G, Sandrini G, Patruno G, Ruiz L, Tassorelli C, Nappi G. Effectiveness of fluoxetine on pain and depression in chronic headache disorders. In: Nappi G, Bono G, Sandrini G, Martignoni E, Micieli G editor(s). Headache and Depression: Serotonin Pathways as a Common Clue. New York: Raven Press, 1991:265‐72. [Google Scholar]
Centonze 2000 {published data only}
- Centonze V, Bassi A, Cassiano M, Causaran V, Dalfino L, Centonze A, et al. Venlafaxine, serotonin and norepinephrine reuptake inhibitor for preventive migraine without aura treatment: a preliminary study. Medicina Psicosomatica 2000;45(1):3‐9. [Google Scholar]
Colucci d'Amato 1998 {published data only}
- Colucci d'Amato C, Pizza V, Marmolo T, Giordano E, Alfano V. Paroxetine versus flunarizine in the preventive treatment of migraine. Headache Quarterly 1998;9(3):266‐7. [Google Scholar]
Colucci d'Amato 1999b {published data only}
- Colucci d'Amato C, Pizza V, Marmolo T, Giordano E, Alfano V, Ruggiero A, et al. Experience of treatment with SSRI in migraine prophylaxis [Esperienza del trattamento con SSRI nella profilassi dell'emicrania]. Atti del 14° congresso Nazionale della Societa' Italiana per lo studio delle cefalee; 1999 sett 19‐22; Perugia. 1999:79.
Colucci d'Amato 2000 {published data only}
- Colucci d'Amato C, Pizza V, Marmolo T, Giordano E, Alfano V, Ruggiero A. Migraine prophylactic therapy: citalopram versus flunarizine. Headache Quarterly 2000;11(3):213‐5. [Google Scholar]
Diamond 1989 {published data only}
- Diamond S, Freitag FG. The use of fluoxetine in the treatment of headache. Clinical Journal of Pain 1989;5(2):200‐1. [PubMed] [Google Scholar]
Foster 1994 {published data only}
- Foster CA, Bafaloukos J. Paroxetine in the treatment of chronic daily headache. Headache 1994;34(10):587‐9. [DOI] [PubMed] [Google Scholar]
Iannacchero 1999 {published data only}
- Iannacchero R, Ambrosio A, Rose F, Flippo A, Ambrosio LA. Efficacy and tolerability of citalopram in chronic headache syndrome prophylaxis [Efficacia e tollerabilita' del citaloprom nella profilassi delle sindromi cefalalgiche cronicizzate: comorbilita' emicrania senz'aura e depressione]. Psichiatria 1999:73‐7. [Google Scholar]
Kangasniemi 1983 {published data only}
- Kangasniemi PJ, Nyrke T, Lang AH, Petersen E. Femoxetine ‐ a new 5‐HT uptake inhibitor ‐ and propranolol in the prophylactic treatment of migraine. Acta Neurologica Scandinavica 1983;68(4):262‐7. [DOI] [PubMed] [Google Scholar]
Kathpal 1998 {published data only}
- Kathpal GS. Role of SSRIs in the management of migraine. Headache Quarterly 1998;9(3):265‐6. [Google Scholar]
Orholm 1986 {published data only}
- Orholm M, Honorè PF, Zeeberg I. A randomized general practice group‐comparative study of femoxetine and placebo in the prophylaxis of migraine. Acta Neurologica Scandinavica 1986;74(3):235‐9. [DOI] [PubMed] [Google Scholar]
Rampello 2004 {published data only}
- Rampello L, Alvano A, Chiechio S, Malaguarnera M, Raffaele R, Vecchio I, et al. Evaluation of the prophylactic efficacy of amitriptyline and citalopram, alone or in combination, in patients with comorbidity of depression, migraine, and tension‐type headache. Neuropsychobiology 2004;50:322‐8. [DOI] [PubMed] [Google Scholar]
Sandrini 1991 {published data only}
- Sandrini G, Ruiz L, Patruno G, Bussone G, Verri AP, Nappi G. Antalgic and antidepressant activities of fluoxetine in daily chronic headache. Cephalalgia 1991;11 Suppl 11:280‐1. [Google Scholar]
Saper 1994 {published data only}
- Saper JR, Silberstein SD, Lake AE 3rd, Winters ME. Double‐blind trial of fluoxetine: chronic daily headache and migraine. Headache 1994;34(9):497‐502. [DOI] [PubMed] [Google Scholar]
Zeeberg 1981 {published data only}
- Zeeberg I, Orholm M, Nielsen JD, Honoré PF, Larsen JJ. Femoxetine in the prophylaxis of migraine ‐ a randomised comparison with placebo. Acta Neurologica Scandinavica 1981;64(6):452‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dzagnidze 2009 {published data only}
- Dzagnidze A, Kukava M, Janelidze M, Katsarava M. Selective serotonin reuptake inhibitor venlafaxine as a prophylactic drug for migraine without aura. European Journal of Neurology 2009;16(3):55–334. [Google Scholar]
He 2004 {published data only}
- He WZ, Chen M, Zheng JZ. Curative effect of fluoxetine for adjuvant treatment of migraine and its effect on serum lipid. Chinese Journal of Clinical Rehabilitation 2004;8(25):5254‐5. [Google Scholar]
Stanic 2009 {published data only}
- Stanic SI, Sretenovic SL. The profilaxis of the frequency of migraine attacks with sertraline and cinarizine. Cephalalgia 2009;29(Suppl 1):142. [Google Scholar]
Togha 2014 {published data only}
- Togha M, Taghdiri F, Razeghi Jahromi S. Efficacy and safety of venlafaxine for the treatment of chronic migraine: a randomized, double‐blind, controlled trial. Journal of Headache and Pain 2014;15(Suppl 1):G38. [Google Scholar]
References to ongoing studies
NCT01393522 {published data only}
- Milnacipran for migraine pain. https://clinicaltrials.gov/ct2/show/NCT01393522 (accessed November 2014).
Additional references
Ad Hoc 1962
- Ad Hoc Committee on the Classification of Headache of the National Institute of Neurological Diseases and Blindness. Classification of headache. JAMA 1962;179(9):717‐8. [Google Scholar]
Altman 1990
- Altman DG, Dore CJ. Randomisation and baseline comparisons in clinical trials. Lancet 1990;335(8682):149‐53. [DOI] [PubMed] [Google Scholar]
American Migraine Foundation 2013
- American Migraine Foundation. 36 Million Migraine Campaign. http://www.americanmigrainefoundation.org/support‐the‐foundation/36‐million‐migraine‐campaign/ (accessed January 2015).
Bloudek 2012
- Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). Journal of Headache and Pain 2012;13(5):361‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buse 2012
- Buse D, Manack A, Serrano D, Reed M, Varon S, Turkel C, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 2012;52(1):3‐17. [DOI] [PubMed] [Google Scholar]
Buse 2013
- Buse DC, Silberstein SD, Manack AN, Papapetropoulos S, Lipton RB. Psychiatric comorbidities of episodic and chronic migraine. Journal of Neurology 2013;260(8):1960‐9. [DOI] [PubMed] [Google Scholar]
Clarke 1996
- Clarke CE, MacMillan L, Sondhi S, Wells NEJ. Economic and social impact of migraine. QJM: Monthly Journal of the Association of Physicians 1996;89(1):77‐84. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Colas 2004
- Colas R, Munoz P, Temprano R, Gomez C, Pascual J. Chronic daily headache with analgesic overuse: epidemiology and impact on quality of life. Neurology 2004;62:1338–42. [DOI] [PubMed] [Google Scholar]
Cull 1992
- Cull RE, Wells NEJ, Miochevic ML. The economic cost of migraine. British Journal of Medical Economics 1992;2:103‐15. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Evers 2009
- Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, et al. European Federation of Neurological Societies (EFNS) guideline on the drug treatment of migraine – revised report of an EFNS task force. European Journal of Neurology 2009;16(9):968‐81. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Falck‐Ytter Y, Vist GE, Liberati A, et al. GRADE Working Group. Rating quality of evidence and strength of recommendations: Going from evidence to recommendations. BMJ 2008;336(7652):1049‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamel 2007
- Hamel E. Serotonin and migraine: biology and clinical implications. Cephalalgia 2007;27(11):1293‐300. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hoffmann 2014
- Hoffmann J, Goadsby PJ. Emerging targets in migraine. CNS Drugs 2014;28:11–7. [DOI] [PubMed] [Google Scholar]
Hotopf 1997
- Hotopf M, Lewis G, Normand C. Putting trials on trial ‐ the costs and consequences of small trials in depression: a systematic review of methodology. Journal of Epidemiology & Community Health 1997;51(4):354‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hotopf 1999
- Hotopf M, Churchill R, Lewis G. Pragmatic randomised controlled trials in psychiatry. British Journal of Psychiatry 1999;175:217‐23. [DOI] [PubMed] [Google Scholar]
Hu 1999
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Archives of Internal Medicine 1999;159(8):813‐8. [DOI] [PubMed] [Google Scholar]
ICHD‐II 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl(1):1–160. [DOI] [PubMed] [Google Scholar]
IHS 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl(7):1‐96. [PubMed] [Google Scholar]
IHS 2013
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629–808. [DOI] [PubMed] [Google Scholar]
Jackson 2010
- Jackson JL, Shimeall W, Sessums L, DeZee KJ, Becher D, Diemer M, et al. Tricyclic antidepressants and headaches: systematic review and meta‐analysis. BMJ 2010;341:c5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaniecki 2003
- Kaniecki R. Headache assessment and management. JAMA 2003;289(11):1430‐3. [DOI] [PubMed] [Google Scholar]
Linde 2012
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19(5):703‐11. [DOI] [PubMed] [Google Scholar]
Lipton 2001
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 2001;41(7):646‐57. [DOI] [PubMed] [Google Scholar]
Lipton 2004
- Lipton RB, Pan J. Is migraine a progressive brain disease?. JAMA 2004;291:493–4. [DOI] [PubMed] [Google Scholar]
Lipton 2007
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. [DOI] [PubMed] [Google Scholar]
Loder 2012
- Loder E, Burch R, Rizzoli P. The 2012 AHS/AAN guidelines for prevention of episodic migraine: a summary and comparison with other recent clinical practice guidelines. Headache 2012;52:930‐45. [DOI] [PubMed] [Google Scholar]
Munakata 2009
- Munakata J, Hazard E, Serrano D, Klingman D, Rupnow MF, Tierce J, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2009;49(4):498‐508. [DOI] [PubMed] [Google Scholar]
Nappi 1985
- Nappi G, Savoldi F. Headache: Diagnostic System and Taxonomic Criteria. London, Paris: J. Libbey Eurotext, 1985. [Google Scholar]
Natoli 2010
- Natoli JL, Manack A, Dean B, Butler Q, Turkel CC, Stovner L, et al. Global prevalence of chronic migraine: a systematic review. Cephalalgia 2010;30(5):599–609. [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute of Health and Care Excellence. Headaches: diagnosis and management of headaches in young people and adults. Clinical guidelines, CG150 September 2012:187‐214.
Pocock 1997
- Pocock SJ. Clinical trials with multiple outcomes: a statistical perspective on their design, analysis, and interpretation. Controlled Clinical Trials 1997;18(6):530‐45. [DOI] [PubMed] [Google Scholar]
Pringsheim 2012
- Pringsheim T, Davenport WJ, Mackie G, Worthington I, Aubé M, Christie SN, et al. on behalf of the Canadian Headache Society Prophylactic Guidelines Development Group. Canadian Headache Society Guideline for Migraine Prophylaxis. Canadian Journal of Neurological Sciences 2012;39 Suppl 2:S1‐S2. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Serrano 2013
- Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Value in Health 2013;16(1):31‐8. [DOI] [PubMed] [Google Scholar]
Silberstein 1996
- Silberstein SD, Lipton RB, Sliwinski M. Classification of daily and near‐daily headaches: field trial of revised IHS criteria. Neurology 1996;47:871‐5. [DOI] [PubMed] [Google Scholar]
Silberstein 2012
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E, Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence‐based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012;78(17):1337‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sindrup 2000
- Sindrup SH, Jensen TS. Pharmacologic treatment of pain in polyneuropathy. Neurology 2000;55(7):915‐20. [DOI] [PubMed] [Google Scholar]
Snow 2002
- Snow V, Weiss K, Wall EM, Mottur‐Pilson C, for the American Academy of Family Physicians and the American College of Physicians‐American Society of Internal Medicine. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Annals of Internal Medicine 2002;137(10):840‐9. [DOI] [PubMed] [Google Scholar]
Sprenger 2009
- Sprenger T, Goadsby PJ. Migraine pathogenesis and state of pharmacological treatment options. BMC Medicine 2009;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steiner 2013
- Steiner TJ, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. Journal of Headache and Pain 2013;14(1):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steward 1992
- Stewart WF, Lipton RB, Celentano DD, Reed ML. Prevalence of migraine headache in the United States. Relation to age, income, race, and other sociodemographic factors. JAMA 1992;267(1):64‐9. [PubMed] [Google Scholar]
Tfelt‐Hansen 2012
- Tfelt‐Hansen P, Pascual J, Ramadan N, Dahlöf C, D'Amico D, Diener HC, et al. International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012;32(1):6‐38. [DOI] [PubMed] [Google Scholar]
Thornley 1998
- Thornley B, Adams C. Content and quality of 2000 controlled trials in schizophrenia over 50 years. BMJ 1998;317(7167):1181‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomkins 2001
- Tomkins GE, Jackson JL, O'Malley PG, Balden E, Santoro JE. Treatment of chronic headache with antidepressants: a meta‐analysis. American Journal of Medicine 2001;111(1):54‐63. [DOI] [PubMed] [Google Scholar]
Von Korff 1998
- Korff M, Stewart WF, Simon DJ, Lipton RB. Migraine and reduced work performance: a population‐based study. Neurology 1998;50(6):1741‐5. [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, et al. Years lived with disability (YLD) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet 2012;380:2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiendels 2006
- Wiendels NJ, Knuistingh Neven A, Rosendaal FR, Spinhoven P, Zitman FG, Assendelft WJ, et al. Chronic frequent headache in the general population: prevalence and associated factors. Cephalalgia 2006;26:1434–42. [DOI] [PubMed] [Google Scholar]
Williamson 2012
- Williamson PR, Altman DG, Blazeby JM, Clarke M, Devane D, Gargon E, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zwart 2004
- Zwart JA, Dyb G, Hagen K, Svebak S, Stovner LJ, Holmen J. Analgesic overuse among subjects with headache, neck, and low‐back pain. Neurology 2004;62(9):1540. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cusi 2000
- Cusi C, Sterzi R, Canepari C, Moja L. Selective serotonin re‐uptake inhibitors (SSRIs) for preventing migraine and tension‐type headaches. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD002919.pub2] [DOI] [PubMed] [Google Scholar]
Moja 2005
- Moja L, Cusi C, Sterzi R, Canepari C. Selective serotonin re‐uptake inhibitors (SSRIs) for preventing migraine and tension‐type headaches. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002919.pub2] [DOI] [PubMed] [Google Scholar]


