Abstract
Background
Non‐alcoholic fatty liver disease (NAFLD) is characterised by fatty deposition in the hepatocytes of patients with minimal or no alcohol intake and without other known cause. NAFLD includes a wide spectrum of histologic abnormalities ranging from hepatic steatosis to non‐alcoholic steatohepatitis (NASH), or even cirrhosis. Antioxidant supplements, therefore, could potentially protect cellular structures against oxidative stress and the resulting lipid peroxidation.
Objectives
To systematically evaluate the beneficial and harmful effects of antioxidant supplements versus no intervention, placebo, or other interventions for patients with NAFLD or NASH.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (June 2006), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 2, 2006), MEDLINE (1966 to June 2006), EMBASE (1980 to June 2006), and The Chinese Biomedical Database (1978 to June 2006). No language restrictions were applied.
Selection criteria
Randomised clinical trials evaluating any antioxidant supplements versus no intervention, placebo, or other interventions in patients with NAFLD or NASH. Our inclusion criteria for NAFLD or NASH were based on history of minimal or no alcohol intake, imaging techniques showing hepatic steatosis, and/or histological evidence of hepatic damage (including simple steatosis, fatty infiltration plus nonspecific inflammation, steatohepatitis, fibrosis, and cirrhosis), and by exclusion of other causes of hepatic steatosis.
Data collection and analysis
We extracted data from the identified trials and contacted authors. We used a random‐effects model and fixed‐effect model with the significant level set at P = 0.05. We evaluated the methodological quality of the randomised trials by looking at how the generation of allocation sequence, allocation concealment, blinding, and follow‐up were performed. We made our analyses following the intention‐to‐treat method by imputing missing data.
Main results
We identified six trials: two were regarded of high methodological quality and four of low methodological quality. None of the trials reported any deaths. Treatment with antioxidant supplements showed a significant, though not clinically relevant, amelioration of aspartate aminotransferase levels, but not of alanine aminotransferase levels, as compared to placebo or other interventions. Gamma‐glutamyl‐transpeptidase was decreased, albeit not significantly, in the treatment arm. Radiological and histological data were too limited to draw any definite conclusions on the effectiveness of these agents. Adverse events were non‐specific and of no major clinical relevance.
Authors' conclusions
There is insufficient data to either support or refute the use of antioxidant supplements for patients with NAFLD. It may be advisable to carry out large prospective randomised clinical trials on this topic.
Plain language summary
No evidence to support or refute antioxidant supplements for patients with non‐alcoholic fatty liver disease and/or steatohepatitis
Non‐alcoholic fatty liver disease is characterised by fatty deposition in the hepatocytes in the absence of excessive alcohol intake and of other known causes of fatty liver. Hepatic injury might be improved by antioxidant supplements. This systematic review identified six randomised clinical trials. No liver‐related or unrelated deaths occurred in any of the included trials. Adverse events were minor and non‐specific. Treatment with antioxidant supplements showed a significant, though not clinically relevant, amelioration of aspartate aminotransferase, but not of alanine aminotransferase, as compared to placebo or other interventions. Data on the radiological and/or histological response were too limited to draw any conclusions. Further placebo‐controlled trials are necessary.
Background
Non‐alcoholic fatty liver disease (NAFLD) is characterised by fatty deposition in the liver cells in patients without excessive alcohol intake. Its prevalence is increasing in the developed world, being now recognised as the most common liver disease in the United States and spreading rapidly to the Asia‐Pacific region (McCullough 2002; Farrell 2003). In the general population, its estimated prevalence ranges from 3% to 24% (McCullough 2002, Clark 2006). Recent studies show that its prevalence may be as high as 29% in healthy Japanese adults (Jimba 2005) and up to 33% in Americans (Farrell 2006). NAFLD includes a wide spectrum of histologic abnormalities ranging from hepatic steatosis, which has a benign clinical course, to nonalcoholic steatohepatitis (NASH) that may progress to cirrhosis, liver failure, and liver‐related death in a significant percentage of patients (McCullough 2002; Farrell 2006; Liou 2006). Indeed, a number of studies suggest that NAFLD may be the cause of cryptogenic cirrhosis (Brolin 1998; Caldwell 1999; Poonawala 2000; Farrell 2003; Liou 2006). Moreover, it remains unclear why only 15% to 20% of patients with NAFLD develop NASH (McCullough 2002).
NAFLD represents the hepatic manifestation of the insulin resistance (or metabolic) syndrome and is particularly associated with obesity, type 2 diabetes mellitus, high triglyceride levels, and low high‐density lipoprotein cholesterol levels (Angulo 2002a; Chitturi 2002a; Younossi 2002; Clark 2006). In addition, NAFLD is extremely common in severely obese patients undergoing bariatric surgery, ranging from 84% to 96% (Clark 2006). NAFLD may also occur in children and normal weight people with normal glucose and lipid metabolism (Bacon 1994).
The pathophysiology of NAFLD is not clear. According to the 'two‐hits' hypothesis, the first hit involves accumulation of excess fat in the liver cells due to insulin resistance and leads to hepatic steatosis. The second hit concerns oxidative stress that causes lipid peroxidation and activates inflammatory cytokines resulting in NASH (Chitturi 2001; McCullough 2002). More precisely, oxidative stress appears to be the result of an imbalance between pro‐oxidant and antioxidant processes in the liver. Such imbalance is likely to be the consequence of: (1) induction of the microsomal cytochrome P450 2E1, which is overexpressed in steatohepatitis because of impaired insulin signalling, (2) mitochondrial release of reactive oxygen species, (3) H2O2 production from peroxisomal β‐oxidation of fatty acids, and (4) cytokine released from activated inflammatory cells (Younossi 2002). Thus, genes for pro‐oxidant (ie, CYP2E1 polymorphism) and antioxidant pathways could also have a role in susceptibility to NASH (Farrell 2003). In addition, these oxidative processes induce a depletion of the potent antioxidants glutathione and vitamin E. The latter is believed to be the 'last antioxidant defence' in lipid membranes. Antioxidant supplements, therefore, could potentially protect cellular structures against damage from oxygen‐free radicals and reactive products of lipid peroxidation.
Since NAFLD is usually associated to a number of insulin resistance related conditions, treatment of comorbidity has been regarded of paramount importance in the management of these patients (Angulo 2002b). However, the benefits of this approach have been inconsistent. Indeed, there is no universal treatment for NAFLD/NASH and the pathogenesis remains poorly understood. So far, therapeutic strategies have been largely empirical and experimental trials have mostly been carried out in uncontrolled settings with small sample sizes. Specific therapeutic interventions include weight reduction, ursodeoxycholic acid, clofibrate, gemfibrozil, atorvastatin, troglitazone, and a number of antioxidants such as vitamin E, betaine, and N‐acetylcysteine (Angulo 2002b; Younossi 2002; Wang 2002; Neuschwander‐T 2003).
We could not find any systematic reviews or meta‐analyses addressing the effects of antioxidant supplements for patients with NAFLD or NASH.
Objectives
To assess beneficial and harmful effects of antioxidants for non‐alcoholic fatty liver disease and/or steatohepatitis.
Methods
Criteria for considering studies for this review
Types of studies
All randomised clinical trials, regardless of publication status, and year, number of patients randomised, language, or blinding.
Types of participants
Participants of any age, sex, or ethnic origin with NAFLD, including NASH and/or cryptogenic cirrhosis diagnosed on the basis of the following criteria: (1) Imaging techniques showing evidence of hepatic steatosis or steatofibrosis. (2) Minimal alcohol intake: preferably a daily alcohol intake less than 20 g in women and 40 g in men (Becker 1996; Neuschwander‐T 2003). (3) Liver biopsy evidence (when available) of histologic damage including simple steatosis, fatty infiltration plus nonspecific inflammation, steatohepatitis, fibrosis, and cirrhosis (Brunt 1999; Kleiner 2005).
We excluded trials enrolling patients with other causes of hepatic steatosis or steatofibrosis, including hepatitis B, hepatitis C, autoimmune hepatitis, and genetic liver disease such as Wilson's disease and haemochromatosis. Studies considering patients with one or more causes commonly associated with secondary NAFLD (drugs, surgical procedures, and miscellaneous disorders such as a‐ or hypo‐betalipoproteinaemia, partial lipodystrophy, environmental toxins, or total parenteral nutrition) were also excluded.
Types of interventions
Antioxidants (vitamin A, carotenoids, vitamin C, vitamin E, selenium, and other administered antioxidants that we could identify) at any dose, duration, and route of administration, given separately or in combination versus no intervention, placebo, or other interventions (for example, regimens including a reduction of calories intake, increase in physical activity, behaviour modification, or various surgical interventions aiming at reducing weight). Co‐interventions were also considered when used equally in both intervention arms.
Types of outcome measures
Primary outcome measures (1) All‐cause mortality: number of deaths irrespective of cause. (2) Hepatic‐related mortality. (3) Radiological response (degree of fatty liver infiltration assessed by ultrasound, computer tomography scanning, nuclear magnetic resonance or other imaging techniques). (4) Biochemical response (serum activities of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatases, gamma‐glutamyl‐transpeptidase, serum total bilirubin, and ferritin). (5) Histological response (number of patients with histological improvement/deterioration and changes in the degree of fatty liver infiltration, inflammation, and fibrosis).
Secondary outcome measures (6) Adverse events (any adverse events as reported in trials). Depending on availability of data, we attempted to classify adverse events as serious or non‐serious. Serious adverse events were defined as any outward medical occurrence that was life threatening, resulted in death, or persistent or significant disability, or any medical event, which may have jeopardised the patient or required intervention to prevent it. All other adverse events were considered non‐serious. (7) Quality of life measures. (8) Cost‐effectiveness.
Search methods for identification of studies
We identified relevant randomised clinical trials by searching The Cochrane Hepato‐Biliary Group Controlled Trials Register (June 2006), the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Issue 2, 2006), MEDLINE (January 1966 to June 2006), and EMBASE (January 1980 to June 2006). In addition, we also searched The Chinese Biomedical Database (1978 to June 2006). See Appendix 1 for the search strategies we applied to the individual databases.
Data collection and analysis
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2006) and the Cochrane Hepato‐Biliary Group Module (Gluud 2006). Trial selection Two authors (FL and SO) independently assessed whether the identified trials fulfilled the inclusion criteria. Excluded trials were listed in "Characteristics of excluded studies" with the reasons for exclusions.
Data extraction SO extracted data, and FL validated the data extraction. We also wrote to authors of included trials asking them to specify the data of interest, which were not clearly reported in their publications.
Trial characteristics: date, location and funding of the trial, length of follow‐up, use of intention‐to‐treat analyses, as well as the publication status.
Patient characteristics: number of patients randomised, inclusion and exclusion criteria, mean (or median) age, sex ratio.
Intervention characteristics: dose, duration and mode of administration of various antioxidants and/or of additional intervention(s).
Outcome measures: number of events in the intervention group and in the control group (including the number and type of adverse events).
Methodological quality Due to the risk of overestimation of intervention effects in randomised clinical trials with inadequate methodology (Schulz 1995; Moher 1998; Kjaergard 2001), we assessed the influence of methodological quality using the following components:
Generation of the allocation sequence
Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice will be considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described.
Inadequate, if a system involving dates, names, or admittance numbers were used for the allocation of patients. These studies are known as quasi‐randomised and were excluded from the present review when assessing beneficial effects.
Allocation concealment
Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes.
Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding
Adequate, if the trial was described as double blind and the method of blinding involved identical placebo or active drugs.
Unclear, if the trial was described as double blind, but the method of blinding was not described.
Not performed, if the trial was not double blind.
Follow‐up
Adequate, if the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals.
Unclear, if the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated.
Inadequate, if the number or reasons for dropouts and withdrawals were not described.
Statistical methods We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2006). We used the software package RevMan 4.2 (RevMan 2003) provided by The Cochrane Collaboration. We presented dichotomous variables as risk difference (RD) or odds ratios (OR) with 95% confidence interval (CI) and continuous outcome measures as weighted mean differences (WMD) with 95% CI. The fixed‐effect model (DeMets 1987) as well as the random‐effects model were used, with the significant level set at P = 0.05. The analyses included all patients irrespective of compliance or follow‐up following the 'intention‐to‐treat' principle and using the last reported observed response ('carry forward'). In addition, subgroup analyses were performed according to the methodological quality of the trials. We planned to use funnel plot asymmetry to assess the existence of publication bias and other biases (Egger 1997).
Results
Description of studies
Search results In total we identified 106 references: 81 through PUBMED and EMBASE, 2 through The Cochrane Hepato‐Biliary Group Controlled Trials Register, and 23 through the Cochrane Central Register of Controlled Trials in The Cochrane Library (thirteen of these were not present in PUBMED, but none was relevant). Of the 81 references identified by PUBMED and EMBASE, 10 were clearly irrelevant to this review and were excluded on the basis of their titles and abstracts. Of the remaining 71 references, 13 were studies of antioxidants usage for non‐alcoholic fatty liver disease or steatohepatitis. Another randomised trial was an ongoing trial published in an abstract form. Thus, a total of fifteen completed trials were identified: of these, six were selected for inclusion, whereas nine were excluded from the review.
Included trials Six randomised clinical trials of antioxidants usage met the criteria for this review. All were published as full text articles. The included trials differed markedly in their inclusion criteria: two (Miglio 2000; Vajro 2004) assessed hepatic steatosis through ultrasonographic investigation, whereas the remaining four trials (Rui 2001; Harrison 2003; Pamuk 2003; Bugianesi 2005) diagnosed NAFLD/NASH by means of histology. Moreover, in one trial (Vajro 2004), the included patients were children with a mean age of 10 years, whereas the remaining trials enrolled adult patients (age > 18 years). In addition, in four trials (Harrison 2003; Pamuk 2003; Vajro 2004; Bugianesi 2005) hypertransaminasaemia, ie, increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serum levels or increased ALT serum levels only, was the main inclusion criteria (see Table of included studies). Major exclusion criteria were: other causes of chronic liver disease (hepatitis B and/or C, haemochromatosis, alpha1‐antitrypsin deficiency, Wilson's disease, autoimmune liver disease), use of drugs associated with the development of NASH, and alcohol abuse. The latter was defined as alcohol consumption exceeding respectively 10 g/day, 30 g/day and 20 g/day (Harrison 2003; Pamuk 2003; Bugianesi 2005). In half of the trials it was not reported.
The type of intervention in the experimental arm varied greatly among the different trials. Three trials (Harrison 2003; Vajro 2004, Bugianesi 2005) used vitamin E in different formulations and dosages, either alone or in combination with other antioxidants. In one trial (Miglio 2000), a combination of betaine glucuronate, diethanolamine glucuronate, and nicotinamide ascorbate was used. In another (Pamuk 2003), n‐acetylcysteine was administered. Finally, in the Chinese trial (Rui 2001) reduced glutathione was administered intravenously. The duration of treatment ranged from two weeks (Rui 2001) to twelve months (Bugianesi 2005).
The intervention in the control arm was placebo or no treatment in four trials (Miglio 2000; Harrison 2003, Pamuk 2003; Vajro 2004), a mixture of herbs called DanNingPian administered orally in the Chinese trial (Rui 2001), and metformin in the remaining one (Bugianesi 2005).
Additional interventions offered to both intervention arms included a low‐calorie diet and a moderate daily exercise programme in two trials (Harrison 2003; Vajro 2004).
The sample size varied greatly among the included trials: from 28 (Vajro 2004) to 191 (Miglio 2000) patients.
Excluded studies A total of nine studies were excluded (see Table of excluded studies). Six of these studies were not randomised; moreover, the open‐label pilot study by Lavine (Lavine 2000) was excluded also because NASH diagnosis was erroneously made upon ultrasonographic findings and not upon histological features. The study by Sanyal et al (Sanyal 2004) was excluded because it was a comparative study assessing the efficacy of vitamin E alone versus a combination of vitamin E and pioglitazone. In the study by Lu (Lu 2005), there was no control arm, but only a comparison between two different dosages of the same agent. Finally, in the trial by Kugelmans (Kugelmas 2003), data were presented before and after therapy and not based on patients who received or did not receive vitamin E.
Ongoing trials A two‐year multicentre randomised placebo‐controlled trial by Dufour et al (Dufour 2005), published in abstract form, is an ongoing trial assessing the efficacy of ursodeoxycholic acid in combination with vitamin E to treat NASH.
Risk of bias in included studies
None of the included trials reported power calculations to assess sample size.
Allocation sequence was considered adequate in two trials (Harrison 2003; Bugianesi 2005): it was computer‐generated in the former and based on a random sequence in the latter. In the remaining four trials (Miglio 2000; Rui 2001; Pamuk 2003; Vajro 2004) it was either not described or unclear.
Allocation concealment was considered adequate in three trials (Harrison 2003; Vajro 2004; Bugianesi 2005): it was performed by the pharmacy in the first one and by means of sealed envelopes in the other two. In the remaining three trials (Miglio 2000; Rui 2001; Pamuk 2003) it was either not described or unclear.
Blinding was considered adequate in three trials (Miglio 2000; Harrison 2003; Vajro 2004), whereas it was either not performed or unclear in the remaining three (Rui 2001; Pamuk 2003; Bugianesi 2005).
Follow‐up was considered adequate in all the included trials except the Chinese trial (Rui 2001), in which it was unclear.
The trials with all four components adequate, ie, generation of the allocation sequence, allocation concealment, blinding and follow‐up, were regarded as trials of high‐methodological quality (Miglio 2000; Harrison 2003). The remaining trials, with one or more unclear or inadequate quality components, were classified as low methodological quality and, therefore, regarded as high‐bias risk trials (Rui 2001; Harrison 2003; Vajro 2004; Bugianesi 2005).
Effects of interventions
All‐cause mortality and liver‐related mortality None of the included trials reported any fatalities related or unrelated to liver disease.
Radiological response Radiological response was assessed by means of ultrasound in two trials (Miglio 2000; Vajro 2004). In the trial by Miglio et al, ultrasonography (US) steatosis score was reduced by 0.49 (SD = 0.63) points in the antioxidant group, whereas it remained virtually unchanged in the control group (‐0.05, SD = 0.66). WMD was ‐0.44 IU/L (95% CI ‐0.63 to ‐0.25). In the trial by Vajro et al, bright liver on US persisted in 11/14 patients in the antioxidant group and in 8/14 in the control arm. The odds ratio was 2.75 (95% CI 0.52 to 14.44).
Biochemical response Biochemical response was assessed by measuring the serum activities of aspartate aminotransferase, alanine aminotransferase, gamma‐glutamyltranspeptidase, and serum total bilirubin levels.
Aspartate aminotransferase (AST) activity was evaluated in only one high‐methodological quality trial (Miglio 2000) and in two low‐methodological quality ones (Rui 2001; Pamuk 2003). Overall, antioxidants‐treated patients showed only a slight, but significant (P = 0.004) decrease in AST activity (WMD ‐5.28 IU/L, 95% CI ‐8.84 to ‐1.72) compared to placebo‐treated subjects.
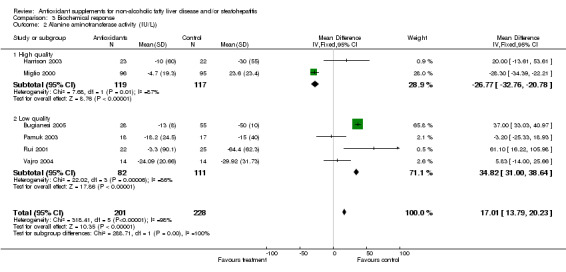
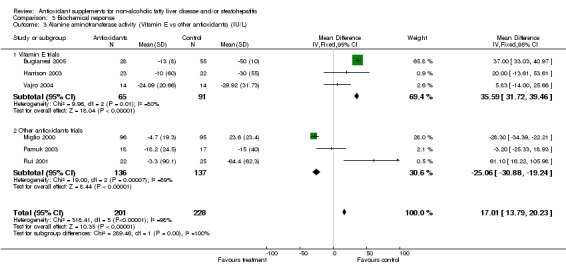
Alanine aminotransferase (ALT) activity was evaluated in all six trials. In the high‐quality group WMD was ‐26.77 IU/L (95% CI ‐32.76 to ‐20.78), whereas in the low‐quality group WMD was 34.82 IU/L (95% CI 31.00 to 38.64). Overall, antioxidants significantly increased ALT activity (P < 0.00001): WMD was 17.01 IU/L (95% CI 13.79 to 20.23). In order to clarify whether the type of intervention could have affected the results, we further divided the trials into two sub‐groups (vitamin E compared to other antioxidants) and assessed again the effect on ALT activity (Comparison 02‐03). The vitamin E interventions were associated with a significant increase in ALT activity (Harrison 2003; Vajro 2004; Bugianesi 2005), whereas the other types of antioxidants seemed to significantly decrease ALT activity (Miglio 2000; Rui 2001; Pamuk 2003).
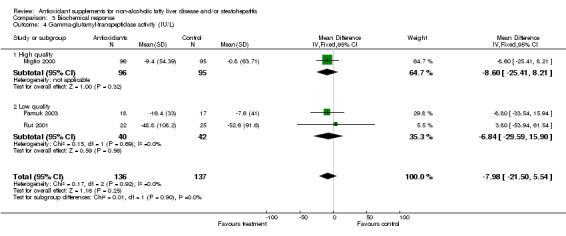
Gamma‐glutamyl‐transpeptidase (GGT) activity was evaluated in one high‐quality trial (Miglio 2000) and in two low‐quality ones (Rui 2001; Pamuk 2003). In both sub‐groups, GGT activity seemed to decrease slightly, but not significantly (WMD ‐8.60 IU/L, 95% CI ‐25.41 to 8.21) and (WMD ‐6.84 IU/L , 95% CI ‐29.59 to 15.90), respectively.
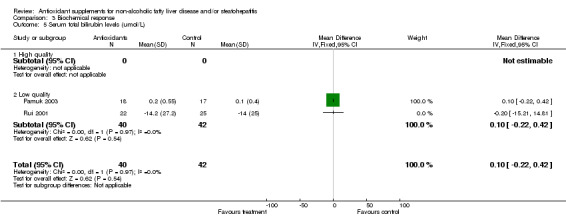
Serum total bilirubin levels were assessed only in two low‐quality trials (Rui 2001; Pamuk 2003), showing virtually no change (WMD 0.10 IU/L, 95% CI ‐0.22 to 0.42).
No trials investigated changes in ALP and ferritin levels following the administration of antioxidant supplements, placebo, or in respect to other interventions.
Histological response Histological response was assessed only in the trial by Harrison et al (Harrison 2003) who evaluated the scores of inflammation/necrosis and fibrosis before and after treatment. The combined inflammation/necrosis score did not change in the placebo or vitamin group with time. In 11/23 patients in the antioxidant group and in 9/22 patients in the control group there was an improvement in the fibrosis score (OR = 0.76, 95% CI 0.23 to 2.46), by a mean of ‐0.50 (SD = 1.00) and ‐0.25 points (SD = 1.25), respectively (WMD ‐0.25 IU/L, 95% CI ‐0.91 to 0.41). However, these results were not significantly different. In the trial by Bugianesi et al (Bugianesi 2005), a post‐treatment biopsy was performed only in 17/55 metformin‐treated patients, whereas none of the patients included in the vitamin E group underwent a second liver biopsy.
Adverse events Among the six trials, four provided information on adverse events. In the trials by Harrison et al (Harrison 2003) and Bugianesi et al (Bugianesi 2005) no adverse events were reported. Vajro et al (Vajro 2004) reported an increase in serum transaminase after starting the administration of vitamin E in one patient. In the trial by Miglio (Miglio 2000) there were 10 cases of adverse events among 96 patients in the antioxidant group and 6 cases among 95 patients in the control group: all adverse events were not serious and included mild to moderate headache, nausea, diarrhoea, heartburn, and meteorism. In the trial by Pamuk (Pamuk 2003) no information about adverse events was provided, and in the Chinese trial (Rui 2001) this issue could not be evaluated.
Quality‐of‐life measures and cost effectiveness Quality of life measures and cost effectiveness were not reported in any of the trials.
Bias exploration We were unable to perform the funnel plot analysis as stated in the protocol, as both visual examination and statistical analysis of funnel plots have limited power to detect bias if the number of trials is small. We, therefore, could not assess the existence of bias by funnel plot.
Discussion
We identified six randomised clinical trials assessing the effects of antioxidant supplements for patients with NAFLD and NASH. There was a considerable heterogeneity among these trials in respect to inclusion criteria, sample size, age (adults and children), type of interventions, type of control interventions, duration of treatment, and methods of outcome assessment. It could very well be argued that we should have excluded the Bugianesi 2005 et al trial as they administered metformin in the control group. We decided to keep it included because there is no evidence that metformin significantly affects prognosis in non‐alcoholic fatty liver disease and/or non‐alcoholic steatohepatitis (Angelico 2007) and the exclusion would not dramatically have an effect on our results. Moreover, the included trials varied substantially regarding methodological quality and hence bias risk.
Based on allocation sequence generation, allocation concealment, blinding, and follow‐up, two trials were regarded as being of high methodological quality (Miglio 2000; Harrison 2003). Of these, one (Harrison 2003) had a small sample size (45 patients), whereas the trial by Miglio et al (Miglio 2000) included a total of 191 patients. The remaining four trials (Rui 2001; Pamuk 2003; Vajro 2004; Bugianesi 2005) resulted mostly as inadequate or unclear in at least one of the four components used to assess methodological quality.
No fatalities were recorded in any of the six trials. This was partly due to the fact that all patients enrolled in all, but one (Bugianesi 2005) trial, did not have an advanced liver disease. Also, the duration of therapy with a maximum of twelve months was not long enough to detect long‐term complications of the disease, such as decompensation, hepatocellular carcinoma, or death. Indeed, the few cases of cirrhosis included in the trials were diagnosed by means of liver biopsy and not on clinical grounds, suggesting that these patients were in a stable and compensated condition.
Biochemical response was evaluated by means of the serum activities of different hepatic enzymes and total bilirubin. Three trials assessed the effect of antioxidant supplements on aspartate aminotransferase (AST) activity; only one of these being regarded of high methodological quality. Overall, they showed a slight, but significant reduction of AST levels in the experimental arm. Nevertheless, the high degree of heterogeneity measured with the I2 test makes the interpretation of these findings difficult. Serum alanine aminotransferase (ALT) activity was the main outcome measured in all the six trials. The pattern of results for ALT activity in the low‐methodological quality sub‐group showed a significant beneficial effect in the control arm. However, this could have been biased by the large standard deviation observed in one trial (Rui 2001) and, again, by the high degree of heterogeneity (I2 = 86.4%) observed among the four low‐methodological quality trials. In the high‐methodological quality sub‐group, the analysis seemed to favour the treatment arm, although heterogeneity was high (I2 = 87.0). Overall, the analysis showed a beneficial effect in the control arm, although there was a high degree of heterogeneity (I2 = 98.4%). Thus, the beneficial effect of antioxidant supplements on hepatic cytolysis cannot be evaluated on the basis of the identified trials. As regards the effect of antioxidant supplements on the indices of cholestasis, the pattern of results for GGT was similar in both the high‐ and the low‐methodological quality trials (Miglio 2000; Rui 2001; Pamuk 2003); a slight, but not significant decrease of the activity of this enzyme in the experimental arm was observed. Serum total bilirubin remained virtually unchanged amongst the two trials evaluating this marker (Rui 2001; Pamuk 2003).
As regards the radiological response, this was assessed in only two trials (Miglio 2000; Vajro 2004) with contradictory results. In the first trial (Miglio 2000), carried out in adults, an eight‐week treatment with betaine glucuronate, combined with diethanolamine glucuronate and nicotinamide ascorbate, caused a 25% reduction of hepatic steatosis evaluated by US scanning, which resulted highly significant. By contrast, the trial by Vajro et al (Vajro 2004), which was carried out in 28 children with obesity‐related hypertransaminasaemia, treated for five months with either vitamin E or placebo, showed that disappearance of the bright liver occurred only in patients who lost weight and was twice as common in the children taking the placebo. Therefore, no conclusion can be drawn on the efficacy of antioxidant supplements in respect to changes of US hepatic steatosis, due to the limited number of trials, the different age of the participants, the type of interventions, the duration of treatment, and the sample size.
As to the histological response, only Harrison et al (Harrison 2003) included this outcome measure in their trial. Although there was a trend showing a beneficial effect on hepatic fibrosis (both in terms of number of patients with less fibrosis and improvement of the fibrosis score), there is not enough data to adequately examine the effect of antioxidant supplements on this histological feature. Besides, there was no significant effect of vitamins E and C on hepatic inflammation and necrosis.
Finally, if one considers the type of intervention used in the different trials, antioxidant supplements other than vitamin E seem to exert a beneficial effect on the activity of ALT (Comparison 02‐03) (Miglio 2000; Rui 2001; Pamuk 2003) as well as on US steatosis (Miglio 2000). The concept that different antioxidants can exhibit different effects on liver structure and function is emphasised. However, these findings need to be confirmed by further randomised clinical trials, comparing specifically the effect of vitamin E versus other antioxidants. A further issue for discussion relates to the dose of vitamin E administered in the trials by Harrison et al (Harrison 2003), Bugianesi et al (Bugianesi 2005), and Vajro et al (Vajro 2004). In all three trials a daily dose of vitamin E ranging from 600 IU to 1000 IU was used, either alone on in combination with vitamin C. A major recent meta‐analysis study (Miller 2005) assessed the risk of high dosage of vitamin E supplementation in 19 clinical trials for a total of 136,000 patients. The dosage of vitamin E ranged from 16.5 IU/day to 2000 IU/day (median 400 IU/day). The dose response analysis showed a statistically significant relationship between vitamin E dosage and all‐cause mortality, with an increased risk of all‐cause mortality for dosages greater than 400 IU/day. It is, therefore, not possible that the dosages of vitamin E may have been too high in the assessed trials (Harrison 2003; Vajro 2004; Bugianesi 2005), having caused the increased serum ALT activities.
No trials used validated assessments of symptoms. We performed an adverse events analysis. Harrison et al (Harrison 2003) found that no patient experienced any adverse events. Bugianesi et al (Bugianesi 2005) reported that no patient had to discontinue the trial because of adverse events to medication. Vajro et al (Vajro 2004) found that one of their young patients showed an increase in serum transaminase after starting treatment with vitamin E. Miglio et al (Miglio 2000) reported a number of non‐specific symptoms (including nausea, diarrhoea, headache, heartburn and meteorism), regarded as non‐serious, in 10% of patients of the experimental group and in 6% of the patients of the control group. Considering the individual symptoms, none of these resulted significantly different between the two trial arms. Moreover, it is difficult to assess a cause‐effect relationship with the experimental interventions since all reported adverse events are common symptoms in patients with liver disorders.
In conclusion, the results of the randomised trials identified in this review do not allow us to provide evidence pros or cons antioxidant supplements in NAFLD or NASH, due to the small number of trials, their low methodological quality, the variability in clinical and histological assessment, and the number of different tested antioxidant supplements.
Authors' conclusions
Implications for practice.
There is not enough evidence to recommend or refute antioxidant supplements in patients with NAFLD or NASH. Vitamin E may increase the activity of alanine aminotransferase in these patients.
Implications for research.
Further randomised clinical trials are needed if the potential beneficial and harmful effects of antioxidant supplements for NAFLD and NASH is to be evaluated. In such trials, a sound methodology with careful consideration of the inclusion criteria and of the outcome measures will be necessary. Moreover, it is advisable to design randomised clinical trials with a large enough sample size, including a well‐defined type of antioxidant supplements, given for longer periods of time and compared with placebo. Such randomised trials ought to be reported according to the CONSORT guidelines (http://www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 13 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Dimitrinka Nikolova and Christian Gluud of The Cochrane Hepato‐Biliary Group for their help in the development of the review.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | June 2006. | (antioxidant* OR vitamin* OR N‐acetylcysteine OR betaine OR glutathione OR S‐adenosyl‐methionine OR SAMe OR caroten* OR retinol OR silymarin OR selenium OR lycopene OR 'ascorbic acid' OR tocopherol OR flavonoid* OR polyphenol* OR ginseng) AND (('non*alcoholic fatty liver' OR NAFL OR NASH) OR steato*hepatitis) |
| Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 2, 2006. | #1 ANTIOXIDANTS explode all trees (MeSH) #2 VITAMINS explode all trees (MeSH) #3 BETAINE explode all trees (MeSH) #4 GLUTATHIONE explode all trees (MeSH) #5 SILYMARIN explode all trees (MeSH) #6 SELENIUM explode all trees (MeSH) #7 ASCORBIC ACID explode all trees (MeSH) #8 GINSENG explode all trees (MeSH) #9 (antioxidant* or vitamin* or n‐acetylcysteine or betaine or glutathione or s‐adenosyl‐methionine or same or caroten* or retinol or silymarin or selenium or lycopene or (ascorbic next acid) or tocopherol or flavonoid* or polyphenol* or ginseng) #10 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #11 ((non next alcoholic next fatty next liver) or nafl or nash) #12 steatohepatitis #13 (#10 and (#11 or #12)) |
| MEDLINE | 1966 to June 2006. | (antioxidant* OR vitamin* OR vitamin A OR "vitamin E" OR N‐acetylcysteine OR betaine OR glutathione OR S‐adenosyl‐methionine OR SAMe OR carotene OR carotenoid* OR retinol OR silymarin OR selenium OR lycopene OR ascorbic acid OR tocopherol OR flavonoid* OR polyphenol* OR ginseng OR thioredoxin OR glutaredoxin OR 'black tea') AND ("non*alcoholic fatty liver" OR "non alcoholic fatty liver" OR "nonalcoholic fatty liver" OR "NAFL*" OR "NASH" OR (non*alcoholic steato*hepatitis OR (non alcoholic steato*hepatitis) OR (nonalcoholic steato*hepatitis) OR "bright liver" OR "liver disease")) AND (trial* OR stud* OR random* OR allocat* OR double*blind* OR placebo) NOT "NASH A" NOT "NASH AA" NOT "NASH AD" NOT "NASH AS" NOT "NASH B" NOT "NASH BR" NOT "NASH BW" NOT "NASH C" NOT "NASH CB" NOT "NASH CH" NOT "NASH CL" NOT "NASH CR" NOT "NASH CW" NOT "NASH D" NOT "NASH DC" NOT "NASH DF" NOT "NASH DL" NOT "NASH DR" NOT "NASH DT" NOT "NASH E" NOT "NASH EC" NOT "NASH EJ" NOT "NASH ES" NOT "NASH FD" NOT "NASH FW" NOT "NASH G" NOT "NASH GB" NOT "NASH General Hospital" NOT "NASH GL" NOT "NASH GS" NOT "NASH GV" NOT "NASH H" NOT "NASH HA" NOT "NASH Hall" NOT "NASH HD" NOT "NASH HM" NOT "NASH HW" NOT "NASH J" NOT "NASH JB" NOT "NASH JE" NOT "NASH JF" NOT "NASH JH" NOT "NASH JJ" NOT "NASH JM" NOT "NASH JQ" NOT "NASH JR" NOT "NASH JW" NOT "NASH K" NOT "NASH KA" NOT "NASH KL" NOT "NASH L" NOT "NASH LD" NOT "NASH LJ" NOT "NASH M" NOT "NASH MA" NOT "NASH MC" NOT "NASH ML" NOT "NASH MM" NOT "NASH MW" NOT "NASH N" NOT "NASH P" NOT "NASH PB" NOT "NASH PJ" NOT "NASH PP" NOT "NASH R" NOT "NASH RA" NOT "NASH reaction" NOT "NASH reagent" NOT "NASH RJ" NOT "NASH RS" NOT "NASH RW" NOT "NASH S" NOT "NASH SA" NOT "NASH SM" NOT "NASH solution" NOT "NASH SV" NOT "NASH T" NOT "NASH TA" NOT "NASH TC" NOT "NASH TD" NOT "NASH TE" NOT "NASH TG" NOT "NASH TL" NOT "NASH TW" NOT "NASH University" NOT "NASH W" NOT "NASH WA" NOT "NASH WG" |
| EMBASE | 1980 to June 2006. | (antioxidant OR vitamin OR 'vitamin A' OR 'vitamin E' OR N‐acetylcysteine OR betaine OR glutathione OR S‐adenosyl‐methionine OR SAMe OR carotene OR carotenoid OR retinol OR silymarin OR selenium OR lycopene OR 'ascorbic acid' OR tocopherol OR flavonoid OR polyphenol OR ginseng OR thioredoxin OR glutaredoxin OR 'black tea') AND ("non*alcoholic fatty liver" OR "non alcoholic fatty liver" OR "nonalcoholic fatty liver" OR "NAFL*" OR "NASH" OR (non*alcoholic steato*hepatitis) OR (non alcoholic steato*hepatitis) OR (nonalcoholic steato*hepatitis) OR (bright liver) OR liver disease)) AND (trial* OR stud* OR random* OR allocat* OR double*blind* OR placebo) |
| The Chinese Biomedical Database | 1978 to June 2006. | Search strategy in Chinese. Available at a request from the Chinese Cochrane Center. |
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All cause mortality | 6 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 1.1 High quality | 2 | 236 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.02, 0.02] |
| 1.2 Low quality | 4 | 193 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.04, 0.04] |
1.1. Analysis.
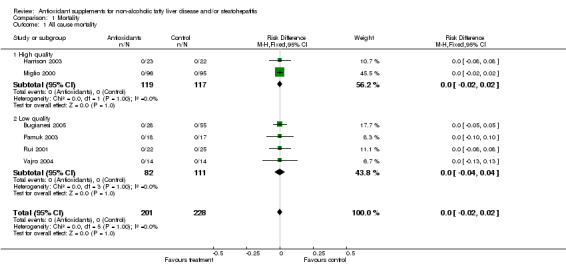
Comparison 1 Mortality, Outcome 1 All cause mortality.
Comparison 2. Radiological response.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 US pattern improvement | 1 | 186 | Mean Difference (IV, Fixed, 95% CI) | ‐0.44 [‐0.63, ‐0.25] |
| 2 US bright liver persistence | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.52, 14.44] |
2.1. Analysis.
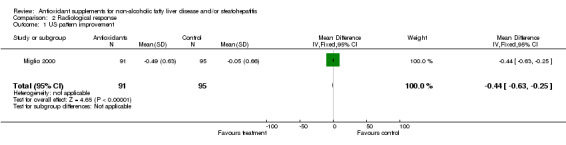
Comparison 2 Radiological response, Outcome 1 US pattern improvement.
2.2. Analysis.
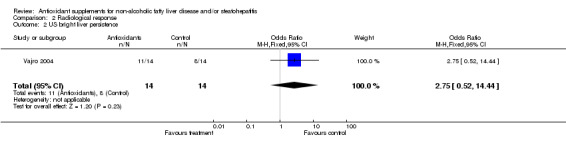
Comparison 2 Radiological response, Outcome 2 US bright liver persistence.
Comparison 3. Biochemical response.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Aspartate aminotransferase activity (IU/L) | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐5.28 [‐8.84, ‐1.72] |
| 1.1 High quality | 1 | 191 | Mean Difference (IV, Fixed, 95% CI) | ‐6.9 [‐10.67, ‐3.13] |
| 1.2 Low quality | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 8.18 [‐2.68, 19.05] |
| 2 Alanine aminotransferase activity (IU/L)) | 6 | 429 | Mean Difference (IV, Fixed, 95% CI) | 17.01 [13.79, 20.23] |
| 2.1 High quality | 2 | 236 | Mean Difference (IV, Fixed, 95% CI) | ‐26.77 [‐32.76, ‐20.78] |
| 2.2 Low quality | 4 | 193 | Mean Difference (IV, Fixed, 95% CI) | 34.82 [31.00, 38.64] |
| 3 Alanine aminotransferase activity (Vitamin E vs other antioxidants) (IU/L) | 6 | 429 | Mean Difference (IV, Fixed, 95% CI) | 17.01 [13.79, 20.23] |
| 3.1 Vitamin E trials | 3 | 156 | Mean Difference (IV, Fixed, 95% CI) | 35.59 [31.72, 39.46] |
| 3.2 Other antioxidants trials | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐25.06 [‐30.88, ‐19.24] |
| 4 Gamma‐glutamyl‐transpeptidase activity (IU/L) | 3 | 273 | Mean Difference (IV, Fixed, 95% CI) | ‐7.98 [‐21.50, 5.54] |
| 4.1 High quality | 1 | 191 | Mean Difference (IV, Fixed, 95% CI) | ‐8.6 [‐25.41, 8.21] |
| 4.2 Low quality | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐6.84 [‐29.59, 15.90] |
| 5 Serum total bilirubin levels (umol/L) | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
| 5.1 High quality | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low quality | 2 | 82 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.22, 0.42] |
3.1. Analysis.
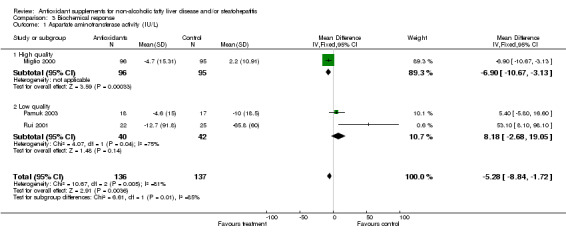
Comparison 3 Biochemical response, Outcome 1 Aspartate aminotransferase activity (IU/L).
3.2. Analysis.
Comparison 3 Biochemical response, Outcome 2 Alanine aminotransferase activity (IU/L)).
3.3. Analysis.
Comparison 3 Biochemical response, Outcome 3 Alanine aminotransferase activity (Vitamin E vs other antioxidants) (IU/L).
3.4. Analysis.
Comparison 3 Biochemical response, Outcome 4 Gamma‐glutamyl‐transpeptidase activity (IU/L).
3.5. Analysis.
Comparison 3 Biochemical response, Outcome 5 Serum total bilirubin levels (umol/L).
Comparison 4. Histological response.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fibrosis persistence | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.23, 2.46] |
| 2 Fibrosis improvement | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.91, 0.41] |
| 3 Necro‐inflammatory improvement | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.22, 0.62] |
4.1. Analysis.
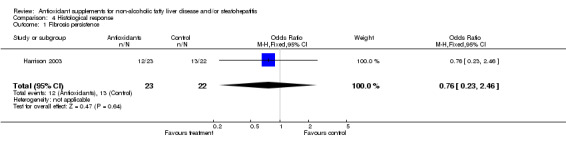
Comparison 4 Histological response, Outcome 1 Fibrosis persistence.
4.2. Analysis.
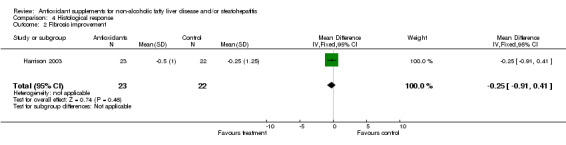
Comparison 4 Histological response, Outcome 2 Fibrosis improvement.
4.3. Analysis.
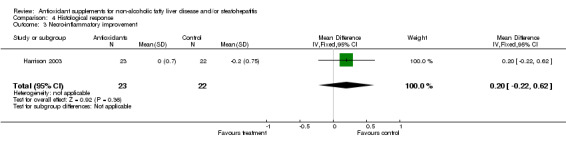
Comparison 4 Histological response, Outcome 3 Necro‐inflammatory improvement.
Comparison 5. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache | 1 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 22.43] |
| 2 Nausea | 1 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 22.43] |
| 3 Diarrhea | 1 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 22.43] |
| 4 Heartburn | 1 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 7.17] |
| 5 Meteorism | 1 | 191 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.18, 22.43] |
| 6 Increase of transaminase | 1 | 28 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.22 [0.12, 86.09] |
5.1. Analysis.
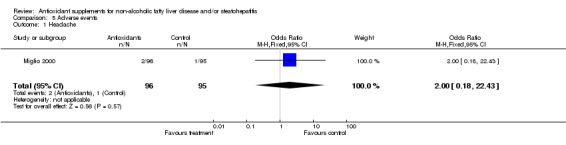
Comparison 5 Adverse events, Outcome 1 Headache.
5.2. Analysis.
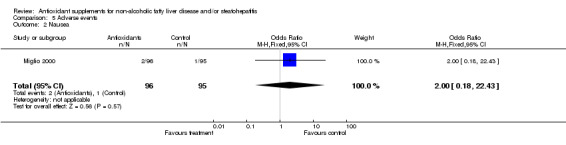
Comparison 5 Adverse events, Outcome 2 Nausea.
5.3. Analysis.
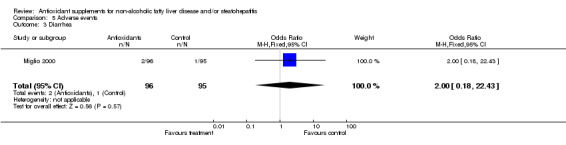
Comparison 5 Adverse events, Outcome 3 Diarrhea.
5.4. Analysis.
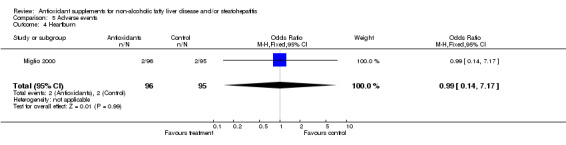
Comparison 5 Adverse events, Outcome 4 Heartburn.
5.5. Analysis.
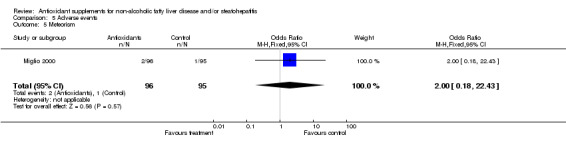
Comparison 5 Adverse events, Outcome 5 Meteorism.
5.6. Analysis.
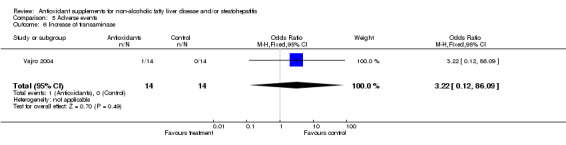
Comparison 5 Adverse events, Outcome 6 Increase of transaminase.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bugianesi 2005.
| Methods | Generation of allocation sequence: adequate, random sequence. Allocation concealment: adequate, sealed envelopes. Blinding: not performed. Follow‐up: adequate. |
|
| Participants | Inclusion criteria:
‐ elevation of ALT > 1.5 times normal values for six months or more
‐ histological diagnosis of NAFLD Exclusion criteria: ‐ other causes for chronic liver disease (hepatitis B and C, hereditary hemochromatosis, autoimmune hepatitis) ‐ diabetes mellitus ‐ celieac disease ‐ BMI >= 35 kg/m2 ‐ a history of alcohol consumption greater than 20 g/day. Characteristics of included patients: n = 83 Mean age = 42 Males = 82% |
|
| Interventions | Vitamin E (800 IU/day) versus metformin (2 g/day) for 12 months. | |
| Outcomes | Assessment of liver enzymes, HOMA‐IR, parameters of the metabolic syndrome at the beginning and at the end of the treatment period. Pre‐ and post‐treatment histology available only in 17/55 patients in the metformin group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Harrison 2003.
| Methods | Generation of allocation sequence: adequate, computer‐generated randomisation table. Allocation concealment: adequate, performed by a pharmacy. Blinding: adequate, double blinding. Follow‐up: adequate. |
|
| Participants | Inclusion criteria:
‐ clinical and histologic diagnosis of NASH
‐ age >18 years
‐ liver biopsy within the past 6 months for elevated aminotransferases
‐ well compensated liver disease (Hb at least 12 g/dl for women and 13 g/dl for men, white blood cell count of greater than 3.000/mm3, neutrophil count of greater than 1500/mm3, platelets greater than 70.000/mm3, serum albumin greater than 1.4 mg/dl and a serum creatinine less than 1.4 mg/dl). Exclusion criteria: ‐ other causes for chronic liver disease (hepatitis B and C, hereditary hemochromatosis, alpha‐1 antitrypsin deficiency, Wilson's disease, or autoimmune liver disease) ‐ use of drugs associated with the development of steatohepatitis ‐ prior surgical procedures ‐ evidence of decompensated liver disease, such as a history of ascites, bleeding varices, hepatic encephalopathy ‐ pregnancy ‐ total parental nutrition within the past 6 months ‐ a history of organ transplant ‐ other conditions that have been known to cause NASH or worsen the disease ‐ a history of alcohol consumption greater than 10 g/day. Characteristics of included patients: n = 49 Mean age = 51 Males = 44% |
|
| Interventions | Vitamin E 1000 IU and Vitamin C 1000 mg per day versus placebo for six months. All patients in both groups were given the same 1600‐calorie diet and written exercise plan as outlined by the National Institutes of Health and the National Heart, Lung and Blood Institute. | |
| Outcomes | Biochemical and histological outcomes were assessed before and after treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Miglio 2000.
| Methods | Generation of allocation sequence: adequate, computer‐generated. Allocation concealment: adequate, sealed envelopes. Blinding: adequate, double blinding. Follow‐up: adequate. |
|
| Participants | Inclusion criteria:
‐liver enlargement
‐ultrasound steatosis
‐age >18 years. Exclusion criteria: ‐alcoholic past or present abuse ‐viral hepatitis, mononucleosis, cytomegalovirus or spirochete infection ‐Wilson's disease ‐Crohn's disease ‐pregnancy or nursing ‐uncooperative patients or patients not able or not willing to release informed consent Characteristics of included patients: Mean age = 57 Males = 70 % |
|
| Interventions | 2 capsules/day containing betaine glucuronate 150 mg, diethanolamine glucuronate 30 mg, nicotinamide ascorbate 20 mg versus placebo for eight weeks. | |
| Outcomes | Outcomes assessed at the beginning and at the end of the study were: ‐complete clinical examination ‐laboratory investigations ‐ultrasound response. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Pamuk 2003.
| Methods | Generation of allocation sequence: unclear. Allocation concealment: unclear. Blinding: not performed. Follow‐up: adequate. |
|
| Participants | Inclusion criteria:
‐ biopsy proven NASH
‐ high alanine aminotransferase and/or aspartate aminotransferase levels at least 2 occasions in the past 6 months
‐ adults Exclusion criteria: ‐ daily alcohol intake >30 g ‐ patients with steatohepatitis accompanying other liver diseases, or systemic diseases other than obesity, hyperlipidemia, diabetes, intake of hepatotoxic drugs or lipid‐lowering agents Characteristics of included patients: n = 18 Mean age = 49 (SD 10) Males = 53 % |
|
| Interventions | N‐acetylcysteine (600 mg/day) for 4 weeks versus no treatment. | |
| Outcomes | Outcomes assessed at the beginning and at the end of the study were: ‐laboratory investigations ‐BMI. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Rui 2001.
| Methods | Generation of allocation sequence: unclear. Allocation concealment: unclear. Blinding: not performed. Follow‐up: unclear. Sample size: no. |
|
| Participants | Inclusion criteria:
‐ biopsy proven NASH
‐ no history of alcohol abuse
‐ adults. Exclusion criteria: not reported. Characteristics of included patients: n = 22 Mean age = 53.1 (SD 7.9) Males = 41% |
|
| Interventions | DanNingPian (5 tablets TID for 12 weeks) versus reduced glutathione (TAD) (1200 mg with 0.9 NS 500 ml IV infusion QD for 2 to 3 weeks). | |
| Outcomes | Liver function tests assessed at 4 and 12 weeks. Definition of remarkably effective: two or more than two liver function indicators completely back to normal. Definition of effective: two or more than two liver function indicators reduced for more than 50%. Definition of ineffective: no improvement or others. | |
| Notes | Also patients with type 2 diabetes/IGT, hyperlipidemia and/or obesity were included in both groups. The majority of them complained of GI symptoms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vajro 2004.
| Methods | Generation of allocation sequence: unclear. Allocation concealment: adequate, sealed envelopes. Blinding: adequate. Follow‐up: adequate. |
|
| Participants | Inclusion criteria:
‐ body weight >= 120% according to Tanner
‐ BMI > 95th percentile
‐hypertransaminasemia (ALT or AST >= 1.5 times above normal values for more than 6 months
‐ US hepatic steatosis
‐ children. Exclusion criteria: ‐ hepatitis B and/or C ‐ haemochromatosis ‐ alpha1‐antitrypsin deficiency ‐ Wilson's disease ‐ autoimmune liver disease ‐ use of drugs associated with the development of NASH ‐ history of alcohol abuse ‐ cystic fibrosis ‐ hereditary fructose intolerance ‐ aminoacid disorders ‐ malnutrition ‐ atypical celiac disease. Characteristics of included patients: n = 28 Mean age = 43 Males = 88% in Bologna, 77% in Turin Cirrhotics = 3.6 % |
|
| Interventions | Placebo (for 5 months) versus oral alpha‐acetate tocopherol (400 mg/day for 2 months, then 100 mg/day for 3 months). All patients received a balanced low‐calorie diet and underwent a moderate daily exercise programme. | |
| Outcomes | Outcomes assessed at month 2 and month 5 were: ‐ loss of weight ‐ ALT levels ‐ Vitamin E / cholesterol ratio. | |
| Notes | During the study a reallocation of the patients was made from initial groups between diet and vitamin E compliers and noncompliers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
> = more or equal to; greater or equal to. > more than.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelmalek 2001 | Patients were consecutively, but not randomly, enrolled. |
| Dentico 1995 | The study was not randomised. |
| Hasegawa 2001 | Pilot study. No control group. |
| Kugelmas 2003 | Data for all biochemical variables were presented combining patients who did or did not receive vitamin E. |
| Lavine 2000 | Open‐label non‐randomised pilot study. NASH diagnosis was made on ultrasound and not on histology. |
| Lu 2005 | There was no control arm, but only a comparison between two different dosages of the same agent. |
| Merat 2003 | The allocation was not randomised. |
| Sanyal 2004 | This study compared the efficacy of vitamin E alone and a combination of vitamin E and pioglitazone. |
| Trappoliere 2005 | The allocation was not randomised. |
Characteristics of ongoing studies [ordered by study ID]
Dufour 2005.
| Trial name or title | A 2‐years multicenter randomised placebo‐controlled study testing UDCA in combination with vitamin E to treat NASH. |
| Methods | |
| Participants | 48 patients with elevated aminotransferases, drinking less than 40g alcohol/week with liver biopsy‐proven NASH less than 6 months prior inclusion and without other liver condition were enrolled. |
| Interventions | UDCA 12 mg/kg/d to 15 mg/kg/d with vitamin E 2 X 400 IU/d or UDCA with placebo or placebo/placebo for 2 years. |
| Outcomes | Outcomes assesed at the beginning and at the end of the study were: ‐liver biopsy ‐laboratory investigations. |
| Starting date | 2005 |
| Contact information | |
| Notes |
Contributions of authors
F Lirussi designed the question, wrote the protocol, and supervised the writing of the review text. L Azzalini performed data extraction and statistical analysis, and co‐wrote the review text. S Orando devised the search strategies and selected the trials. R Orlando was responsible for data retrieval. F Angelico provided statistical advice. All authors contributed to the improvement of the review.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bugianesi 2005 {published data only}
- Bugianesi E, Gentilcore E, Manini R, Natale S, Vanni E, Villanova N, et al. A randomized controlled trial of metformin versus Vitamin E or prescriptive diet in nonalcoholic fatty liver disease. American Journal of Gastroenterology 2005;100:1082‐90. [DOI] [PubMed] [Google Scholar]
Harrison 2003 {published data only}
- Harrison S, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. The American Journal of Gastroenterology 2003;98:2485‐90. [DOI] [PubMed] [Google Scholar]
Miglio 2000 {published data only}
- Miglio F, Rovati LC, Santoro A, Setnikar I. Efficacy and safety of oral betaine glucuronate in non‐alcoholic steatohepatitis. Leber‐Therapeutika 2000;50(2):722‐7. [DOI] [PubMed] [Google Scholar]
Pamuk 2003 {published data only}
- Pamuk GE, Sonsuz A. N‐acetylcysteine in the treatment of non‐alcoholic steatohepatitis. Journal of Gastroenterology and Hepatology 2003;18(10):1220‐1. [DOI] [PubMed] [Google Scholar]
Rui 2001 {published data only}
- Rui M, Wang C, Fang J, et al. The clinical comparison of reduced glutathione and DanNingPlan in the treatment of nonalcoholic steatohepatitis. Chinese General Practice 2001;4(4):269‐70. [Google Scholar]
Vajro 2004 {published data only}
- Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello, Savoia M, et al. Vitamin E treatment in pediatric obesity‐related liver disease: a randomized study. Journal of Pediatric Gastroenterology 2004;38:48‐55. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelmalek 2001 {published data only}
- Abdelmalek MF, Angulo P, Jorgensen RA, Sylvestre PB, Lindor KD. Betaine, a promising new agent for patients with nonalcoholic steatohepatitis: results of a pilot study. The American Journal of Gastroenterology 2001;96(9):2711‐7. [DOI] [PubMed] [Google Scholar]
Dentico 1995 {published data only}
- Dentico P, Volpe A, Buongiorno R, Grattagliano I, Altomare E, Tantimonaco G, et al. Glutathione in the treatment of chronic fatty liver diseases [Il glutatione nella terapia dell'epatopatie croniche steatosiche]. Recenti Progressi in Medicina 1995;86(7‐8):290‐3. [PubMed] [Google Scholar]
Hasegawa 2001 {published data only}
- Hasegawa T, Yoneda M, Nakamura K, Makino I, Terano A. Plasma transforming growth factor‐beta1 level and efficacy of alpha‐tocopherol in patients with non‐alcoholic steatohepatitis: a pilot study. Alimentary Pharmacology & Therapeutics 2001;15(10):1667‐72. [DOI] [PubMed] [Google Scholar]
Kugelmas 2003 {published data only}
- Kugelmas M, Hill D, Vivian B, Marsano L, McClain C. Cytokines and NASH: a pilot study of the effect of lifestyle modification and vitamin E. Liver Failure and Liver Disease 2003;38(2):413‐9. [DOI] [PubMed] [Google Scholar]
Lavine 2000 {published data only}
- Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. The Journal of Pediatrics 2000;136(6):734‐42. [PubMed] [Google Scholar]
Lu 2005 {published data only}
- Lu L, Zeng M, Mao Y, Chen C, Fu Q, Wang J. Diisopropylamine dichloroacetate in the treatment of nonalcoholic fatty liver disease: a multicenter random double‐blind controlled trial. Chinese Journal of Hepatology 2005;13(2):92‐5. [PubMed] [Google Scholar]
Merat 2003 {published data only}
- Merat S, Malekzadeh R, Sohrabi MR, Hormazdi M, Naserimoghadam S, Mikaeli J, et al. Probucol in the treatment of nonalcoholic steatohepatitis. Journal of Clinical Gastroenterology 2003;36(3):266‐8. [DOI] [PubMed] [Google Scholar]
Sanyal 2004 {published data only}
- Sanyal A, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clinical Gastroenterology and Hepatology 2004;2:1107‐15. [DOI] [PubMed] [Google Scholar]
Trappoliere 2005 {published data only}
- Trappoliere M, Federico A, Tuccillo C, Sio I, leva A, Niosi M, et al. Effects of new pharmacological complex (sylibin+vitamin E+phospolipids) on some markers of metabolic syndrome and of a liver fibrosis in patients with non‐alcoholic fatty liver disease: a preliminary open pilot study. Minerva Gastroenterologica e Dietologica 2005;51:193‐9. [PubMed] [Google Scholar]
References to ongoing studies
Dufour 2005 {published data only}
- Dufour JF, Oneta C, Gonvers JJ, Bihl F, Cerny A, Cereda JM. A 2‐years multicenter randomized placebo‐controlled study testing UDCA in combination with vitamin E to treat NASH. Journal of Hepatology 2005;12(Supplement 2):4‐5. [Google Scholar]
Additional references
Angelico 2007
Angulo 2002a
- Angulo P. Nonalcoholic fatty liver disease. New England Journal of Medicine 2002;16:1221‐31. [DOI] [PubMed] [Google Scholar]
Angulo 2002b
- Angulo P, Lindor KD. Treatment of non‐alcoholic steatohepatitis. Best Practice & Research. Clinical Gastroenterology 2002;16(5):797‐810. [DOI] [PubMed] [Google Scholar]
Bacon 1994
- Bacon BR, Farahvash MJ, Janney CG, Neuschwander‐Tetri BA. Non‐alcoholic steatohepatitis: an expanded clinical entity. Gastroenterology 1994;107:1103‐9. [DOI] [PubMed] [Google Scholar]
Becker 1996
- Becker U, Deis A, Sorensen TI, Gronbæk M, Borch‐Johnsen K, Florvall Muller C, et al. Prediction of risk of liver disease by alcohol intake, sex and age: a prospective population study. Hepatology 1996;23(5):1025‐9. [DOI] [PubMed] [Google Scholar]
Brolin 1998
- Brolin RE, Bradley LJ, Taliwal RV. Unsuspected cirrhosis discovered during obesity operations. Archives of Surgery 1998;133:84‐8. [DOI] [PubMed] [Google Scholar]
Brunt 1999
- Brunt EM, Janney CG, Bisceglie AM, Neuschwander‐Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. American Journal of Gastroenterology 1999;94(9):2467‐74. [DOI] [PubMed] [Google Scholar]
Caldwell 1999
- Calwell SH, Oelsner DH, Iezzoni J, Hespenheide EE, Battle EH, Driscoll CJ. Cryptogenic cirrhosis: clinical characterization and risk factors for underlying disease. Hepatology 1999;29:664‐9. [DOI] [PubMed] [Google Scholar]
Chitturi 2001
- Chitturi S, Farrell GC. Etiopathogenesis of non‐alcoholic steatohepatitis. Seminars in Liver Diseases 2001;21:27‐41. [DOI] [PubMed] [Google Scholar]
Chitturi 2002a
- Chitturi S, Abeygunasekera S, Farrell GC, Holmes‐Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 2002;35:373‐9. [DOI] [PubMed] [Google Scholar]
Clark 2006
- Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. Journal of Clinical Gastroenterology 2006;40(3 Suppl 1):S5‐S10. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farrell 2003
- Farrell GC. Non‐alcoholic steatohepatitis: what is it, and why is it important in the Asia‐Pacific region?. Journal of Gastroenterology and Hepatology 2003;18:124‐38. [DOI] [PubMed] [Google Scholar]
Farrell 2006
- Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43(2 Suppl 1):S99‐S112. [DOI] [PubMed] [Google Scholar]
Gluud 2006
- Gluud C, Als‐Nielsen B, D'Amico G, Fingerhut A, Gluud LL, Khan S, et al. Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2006, Issue 3. Art. No.: LIVER.
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. The Cochrane Library, Issue 4, 2006. Chichester, UK: John Wiley & sons, Ltd. [Google Scholar]
Jimba 2005
- Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, et al. Prevalence of non‐alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabetic Medicine 2005;22(9):1141‐5. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kleiner 2005
- Kleiner DE, Brunt EM, Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005;41(6):1313‐21. [DOI] [PubMed] [Google Scholar]
Liou 2006
- Liou I, Kowdley KV. Natural history of nonalcoholic steatohepatitis. Journal of Clinical Gastroenterology 2006;40(3 Suppl 1):S11‐S16. [DOI] [PubMed] [Google Scholar]
McCullough 2002
- McCullough AJ. Update on nonalcoholic fatty liver disease. Journal of Clinical Gastroenterology 2002;34(3):255‐62. [DOI] [PubMed] [Google Scholar]
Miller 2005
- Miller ER 3rd, Pastor‐Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E. Meta‐analysis: high‐dosage vitamin E supplementation may increase all‐cause mortality. Annals of Internal Medicine 2005;142(1):37‐46. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Neuschwander‐T 2003
- Neuschwander‐Tetri BA, Caldwell ST. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003;37(5):1202‐19. [DOI] [PubMed] [Google Scholar]
Poonawala 2000
- Poonawala A, Nair S, Thuluvath PJ. Prevalence of obesity and diabetes in patients with cryptogenic cirrhosis: a case‐control study. Hepatology 2000;32:689‐92. [DOI] [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 2002
Younossi 2002
- Younossi ZM, Diehl AM, Ong JP. Nonalcoholic fatty liver disease: an agenda for clinical research. Hepatology 2002;35(4):746‐52. [DOI] [PubMed] [Google Scholar]


