Abstract
Background
This is an update of a Cochrane Review first published in 2015. The conclusions have not changed.
Hypodermic needles of different sizes (gauges and lengths) can be used for vaccination procedures. The gauge (G) refers to the outside diameter of the needle tubing. The higher the gauge number, the smaller the diameter of the needle (e.g. a 23 G needle is 0.6 mm in diameter, whereas a 25 G needle is 0.5 mm in diameter). Many vaccines are recommended for injection into muscle (intramuscularly), although some are delivered subcutaneously (under the skin) and intradermally (into skin). Choosing an appropriate length and gauge of a needle may be important to ensure that a vaccine is delivered to the appropriate site and produces the maximum immune response while causing the least possible harm. Guidelines conflict regarding the sizes of needles that should be used for vaccinating children and adolescents.
Objectives
To assess the effects of using needles of different sizes for administering vaccines to children and adolescents on vaccine immunogenicity (the ability of the vaccine to elicit an immune response), procedural pain, and other reactogenicity events (adverse events following vaccine administration).
Search methods
We updated our searches of CENTRAL, MEDLINE, Embase, and CINAHL to October 2017. We also searched proceedings of vaccine conferences and two trials registers.
Selection criteria
Randomised controlled trials evaluating the effects of using hypodermic needles of any gauge or length to administer any type of vaccine to people aged from birth to 24 years.
Data collection and analysis
Three review authors independently extracted trial data and assessed the risk of bias. We contacted trial authors for additional information. We rated the quality of evidence using the GRADE system.
Main results
We included five trials involving 1350 participants in the original review. The updated review identified no new trials. The evidence from two small trials (one trial including infants and one including adolescents) was insufficient to allow any definitive statements to be made about the effects of the needles evaluated in the trials on vaccine immunogenicity and reactogenicity.
The remaining three trials (1135 participants) contributed data to comparisons between 25 G 25 mm, 23 G 25 mm, and 25 G 16 mm needles. These trials included infants predominantly aged from two to six months undergoing intramuscular vaccination in the anterolateral thigh using the World Health Organization (WHO) injection technique (skin stretched flat, needle inserted at a 90° angle and up to the needle hub in healthy infants). The vaccines administered were combination vaccines containing diphtheria, tetanus, and whole‐cell pertussis antigens (DTwP). In some trials, the vaccines also contained Haemophilus influenzae type b (DTwP‐Hib) and hepatitis B (DTwP‐Hib‐Hep B) antigen components.
Primary outcomes
Incidence of vaccine‐preventable diseases: No trials reported this outcome.
Procedural pain and crying: Using a wider gauge 23 G 25 mm needle may slightly reduce procedural pain (low‐quality evidence) and probably leads to a slight reduction in the duration of crying time immediately after vaccination (moderate‐quality evidence) compared with a narrower gauge 25 G 25 mm needle (one trial, 320 participants). The effects are probably not large enough to be clinically relevant.
Secondary outcomes
Immune response: There is probably little or no difference in immune response, defined in terms of the proportion of seroprotected infants, between use of 25 G 25 mm, 23 G 25 mm, or 25 G 16 mm needles to administer a series of three doses of a DTwP‐Hib vaccine at ages two, three, and four months (moderate‐quality evidence, one trial, numbers of participants in analyses range from 309 to 402. The immune response to the pertussis antigen was not measured).
Severe and non‐severe local reactions: 25 mm needles (either 25 G or 23 G) probably lead to fewer severe and non‐severe local reactions after DTwP‐Hib vaccination compared with 25 G 16 mm needles (moderate‐quality evidence, one trial, 447 to 458 participants in analyses). We estimate that one fewer infant will experience a severe local reaction (extensive redness and swelling) after the first vaccine dose for every 25 infants vaccinated with the longer rather than the shorter needle (number needed to treat for an additional beneficial outcome (NNTB) with a 25 G 25 mm needle: 25 (95% confidence interval (CI) 15 to 100); NNTB with a 23 G 25 mm needle: 25 (95% CI 17 to 100)). We estimate that one fewer infant will experience a non‐severe local reaction (any redness, swelling, tenderness, or hardness (composite outcome)) at 24 hours after the first vaccine dose for every 5 or 6 infants vaccinated with a 25 mm rather than a 16 mm needle (NNTB with a 25 G 25 mm needle: 5 (95% CI 4 to 10); NNTB with a 23 G 25 mm needle: 6 (95% CI 4 to 13)). The results are similar after the second and third vaccine doses.
Using a narrow gauge 25 G 25 mm needle may produce a small reduction in the incidence of local reactions after each dose of a DTwP vaccine compared with a wider gauge 23 G 25 mm needle, but the effect estimates are imprecise (low‐quality evidence, two trials, 100 to 459 participants in analyses).
Systemic reactions: The comparative effects of 23 G 25 mm, 25 G 25 mm, and 25 G 16 mm needles on the incidence of postvaccination fever and other systemic events such as drowsiness, loss of appetite, and vomiting are uncertain due to the very low quality of the evidence.
Authors' conclusions
Using 25 mm needles (either 23 G or 25 G) for intramuscular vaccination procedures in the anterolateral thigh of infants using the WHO injection technique probably reduces the occurrence of local reactions while achieving a comparable immune response to 25 G 16 mm needles. These findings are applicable to healthy infants aged two to six months receiving combination DTwP vaccines with a reactogenic whole‐cell pertussis antigen component. These vaccines are predominantly used in low‐ and middle‐income countries. The applicability of the findings to vaccines with acellular pertussis components and other vaccines with different reactogenicity profiles is uncertain.
Plain language summary
Needle size for vaccination procedures in children and adolescents
Background
Vaccines contain antigens that make the body's immune system produce antibodies that can protect against disease, which is known as an immune response. Antigens are modified or partial forms of the virus, bacteria, or toxin that cause the disease that the vaccine protects against. Because the antigen is altered from its original form, it cannot cause disease, but it can produce an immune response.
Vaccines can be injected using needles of different lengths and gauges. The needle gauge (G) refers to the width (diameter) of the needle. The higher the gauge number, the narrower the needle. For example, a 25 G needle is approximately 0.5 mm in diameter and is narrower than a 23 G needle, which has a diameter of 0.6 mm. Guidelines conflict regarding the lengths and gauges of needles that should be used for vaccinating children and adolescents.
Review question
We wanted to find out if the length and gauge of needles used to vaccinate children and adolescents has an influence on the:
1) immune response to the injected vaccine;
2) pain experienced during the vaccination procedure;
3) occurrence of reactions such as swelling, tenderness, and redness at the site where the vaccine is given; fever (high temperature); and other side effects that can occur after vaccination.
Quality of the evidence
We included five studies involving 1350 people. We rated the quality of the evidence from studies as very low, low, moderate, or high. Very low‐quality evidence means that we are very uncertain about the results. High‐quality evidence means that we are very confident in the results. There were problems with the design of some studies, and data were insufficient to answer some parts of our review question. The quality of the evidence from two studies was too low to allow us to draw any conclusions about the effects of the needles compared in the studies. However, there was sufficient evidence from the remaining three studies to allow us to reach conclusions.
Study characteristics
The three studies that allowed us to reach conclusions involved 1135 healthy infants aged mostly between two and six months. The infants were vaccinated in the thigh with either 25 G 25 mm (narrow, long needles), 23 G 25 mm (wide, long needles), or 25 G 16 mm needles (narrow, short needles). The needles were inserted at right angles (90° angle) into the skin and pushed down into the muscle of the thigh. The vaccines injected were combination vaccines designed to protect against several diseases including diphtheria (D), tetanus (T), whooping cough (pertussis), and Haemophilus influenzae type b disease (Hib). The vaccines all contained whole‐cell pertussis (wP) vaccine antigens. These vaccines are commonly used in low‐ and middle‐income countries but not in high‐income countries. Our review findings are therefore most relevant to low‐ and middle‐income countries.
Key findings
We found moderate‐quality evidence that infants vaccinated in the thigh with 25 mm needles probably have fewer severe reactions (extensive redness and swelling in the thigh) after DTwP‐Hib vaccination than infants vaccinated with 16 mm needles. We also found that the longer needles probably lead to fewer non‐severe reactions such as mild swelling, tenderness, and redness after vaccination. The immune response to the vaccine is probably similar with the long and the short needles.
We found low‐quality evidence that the wide, long needle may slightly reduce the pain of the vaccination procedure compared with the narrow, long needle. We found moderate‐quality evidence that the wide, long needle probably slightly reduces the duration of crying immediately following vaccination compared with the narrow, long needle. The differences in pain and crying between use of the wide and narrow needles are probably too small to be of any practical importance.
We found low‐quality evidence that infants vaccinated with the narrow, long needle may have slightly fewer non‐severe reactions than infants vaccinated with the wide, long needle.
We do not know if needle size has an effect on fever or other reactions that sometimes occur after vaccination including drowsiness, loss of appetite, and vomiting due to the very low quality of the evidence.
The evidence in our review is current to October 2017.
Summary of findings
Background
This is the first update of the original Cochrane Review published in 2015.
An estimated 16 billion injections are administered by healthcare practitioners worldwide every year, with immunisations accounting for approximately 5% of all injections (WHO 2015a). In the US, the Centers for Disease Control and Prevention (CDC) recommends routine vaccination to prevent 17 vaccine‐preventable diseases (Kroger 2017). Children fully adhering to the US immunisation schedule may receive up to 24 skin‐puncturing injections by the age of two years and up to five injections in a single visit (IOM 2013). In many other high‐income countries, the average child who adheres to recommended immunisation schedules receives at least 18 injections before the age of 16 years, the majority of which are administered during the first six years of life (Curtis 2012). The aim of administering any vaccine should be to ensure the attainment of maximum immunity, with the least possible harm (RCPCH 2002). Important harms are the pain and distress associated with vaccination procedures and other common reactogenicity events that can occur postvaccination such as local reactions (e.g. redness, swelling, and tenderness at the injection site) and systemic reactions (e.g. fever, malaise, irritability, and loss of appetite).
Pain has been defined as "an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage" (IASP 2004). Acute pain during a vaccination procedure results from the stimulation of peripheral nociceptive sensory neurons (pain sensors) during two separate events: 1) needle puncture of the skin and underlying tissues; and 2) injection and deposition of the vaccine constituents into the tissue (Taddio 2009a). Delayed pain following a vaccination procedure may arise due to an inflammatory process in damaged tissue (Gidudu 2012). Pain can be experienced irrespective of the age at which vaccines are administered because the physiological and biochemical prerequisites for nociception are developed in utero, and neonates and infants are able to demonstrate physiological and behavioural pain responses (RCN 2009).
Most vaccines are administered during the developmentally critical first six years of life (Curtis 2012), and pain associated with the procedure may have significant physiological, psychological, and behavioural sequelae. The immediate physical effects of pain are related to the stress response and can affect cardiopulmonary function, metabolic and inflammatory response, and immune competence (Czarnecki 2011). Exposure to painful stimuli in infancy has also been associated with anticipatory fear of future medical procedures, increased sensitivity to pain and heightened responsiveness to painful stimuli, pain avoidance in later life, and negative effects on healthcare behaviour and attitudes (Johnston 1996; Taddio 1997; Andrews 1999; Porter 1999; Taddio 2002; Young 2005; Taddio 2009a; Kassab 2011). In addition, negative experiences with needle‐related procedures, particularly in childhood, can contribute to the development of needle phobias (Hamilton 1995; Wright 2009), which reduce compliance with future immunisation schedules and other preventive healthcare measures (Hogan 2010; Gidudu 2012). Vaccination‐related procedural pain is also a source of anxiety and distress for the parents of children undergoing vaccination and the healthcare workers who administer the injections (Schechter 2007; Taddio 2010; Kassab 2011). Both parents and vaccinators have admitted to non‐compliance with childhood immunisation schedules to reduce pain and distress to children (Woodin 1995; Luthy 2009; Taddio 2012).
In light of the potential adverse short‐ and long‐term consequences of pain related to vaccination procedures, every conceivable effort should be made by healthcare providers to minimise its occurrence. It is also desirable to minimise the occurrence of postvaccination local and systemic reactions. However, efforts to reduce reactogenicity events should not compromise vaccine immunogenicity, that is the ability of the vaccine to elicit an immune response. One aspect of vaccination procedures that has the potential to influence both vaccine immunogenicity and reactogenicity is the size of the needle used to administer the vaccine.
Description of the intervention
Hypodermic needles are available in a wide range of sizes for delivering drugs, vaccines, and other substances into the body or for extracting fluids and tissue samples (Gill 2007). The term 'needle size' is used to refer to two aspects of hypodermic needle geometry, namely gauge (diameter) and length. The gauge refers to the nominal outside diameter of the needle tube, and the length refers to the nominal length of the needle tube (ISO 1993). Both dimensions are typically expressed in millimetres (mm), although in some countries (e.g. the US and the UK) needle length is also expressed in inches. The most commonly used system for describing the gauge of needles is the Stubs Iron Wire Gauge system, developed in England in the early 19th century (Iserson 1987; Ahn 2002). The gauge of a needle is often abbreviated as 'G' or 'ga'; the higher the needle gauge number, the smaller the diameter of the needle lumen (hole) (Pöll 1999). The International Organization for Standardization (ISO) has established ISO standards for the inner and outer diameters of hypodermic medical needles of a specified gauge number (ISO 2001). International standards have also been developed for colour coding of needles to enable rapid visual identification of the outside diameter of single‐use hypodermic needles (ISO 1992). The standards for the most commonly recommended needle gauges for administering vaccines to children and adolescents are presented below.
| International standards pertaining to the gauge and colour coding of hypodermic needles that are commonly recommended for administering vaccines to children and adolescents1 | |||||||
| Gauge number | Nominal outside diameter of needle (mm) | Colour coding | Range of outside diameters (mm) | Inside diameter of tubing (mm) | |||
| Min. | Max. |
Normal‐walled Min. |
Thin‐walled Min. |
Extra‐thin‐walled Min. |
|||
| 22 G | 0.70 | Black | 0.698 | 0.73 | 0.39 | 0.44 | 0.522 |
| 23 G | 0.60 | Deep blue | 0.6 | 0.673 | 0.317 | 0.37 | 0.46 |
| 24 G | 0.55 | Medium purple | 0.55 | 0.58 | 0.28 | 0.343 | ‐ |
| 25 G | 0.50 | Orange | 0.5 | 0.53 | 0.232 | 0.292 | ‐ |
| 26 G | 0.45 | Brown | 0.44 | 0.47 | 0.232 | 0.292 | ‐ |
| 27 G | 0.40 | Medium grey | 0.4 | 0.42 | 0.184 | 0.241 | ‐ |
| Max: maximum; min: minimum. 1Vaccinations typically require injection of less than 1 mL of fluid (Gill 2007), and the viscosity of most vaccines is such that 22 G to 25 G needles are generally recommended for most vaccines that are administered intramuscularly and subcutaneously to children and adolescents (Atkinson 2008; CDC 2011; DoH UK 2012a), and 25 G to 27 G for vaccines administered intradermally (ATAGI 2009; NIAC 2011). Table adapted from the following sources: ISO 1992; ISO 1993; ISO 2001. | |||||||
Different needle lengths are available for a given gauge number. For example, in many countries 25 G (orange) needles are available in lengths of 16 mm (⅝ inch), 25 mm (1 inch), 32 mm (1¼ inches), and 38 mm (1½ inches) (Ajana 2008). Various needle gauges are also available for a given needle length. For example, 25 mm needles are available in 22 G (black), 23 G (deep blue), 24 G (medium purple), and 25 G (orange).
Factors influencing needle size selection for vaccination procedures
It is generally recommended that clinical decisions regarding the choice of an appropriate needle size (gauge and length) for a particular vaccination procedure should take into account the age and body mass of the person receiving the vaccine (see Appendix 1). Obesity increases the subcutaneous tissue thickness, and overweight and obese children and adolescents receiving intramuscular injections may require longer needles to ensure that the vaccine is administered into muscle (Koster 2009). Several other factors influencing needle size selection include the prescribed route of vaccine administration (intramuscular, subcutaneous, or intradermal), the injection site, and the injection technique.
Route of administration and injection site
The recommended routes of administration (intramuscular, subcutaneous, or intradermal) for injectable vaccines are specified in manufacturers' summaries of product characteristics (SPCs) and in recommendations published by National Immunization Technical Advisory Groups (NITAGs) in different countries (Atkinson 2008). Injectable vaccines should be administered in sites where local, neural, vascular, or tissue injury is unlikely, and where they will elicit the desired immune response (Atkinson 2008; CDC 2011).
The intramuscular route is recommended for most vaccines administered to children and adolescents (CDC 2011; DoH UK 2012a). The vastus lateralis muscle in the anterolateral thigh (located on the outside of the leg in the mid to upper thigh) is the generally recommended site for infants under one year old, and the deltoid muscle of the upper arm for older children and adolescents (Diggle 2007). Many NITAGs have issued needle size recommendations for intramuscular vaccinations that take into account the age or size (body mass) of the vaccine recipient and the injection site (see Appendix 1). However, these recommendations are not consistent between countries. For example, in the UK, a needle 25 mm in length with a gauge of 23 G or 25 G is recommended for intramuscular injections in the deltoid of children older than one year of age (DoH UK 2012a). By contrast, in New Zealand, 23 G to 25 G 16 mm needles are recommended for deltoid site injections in children aged 15 months to seven years (MoH NZ 2017). In the US, the recommended needle gauges and lengths for intramuscular deltoid site injections in children and adolescents aged three to 18 years range from 22 G to 25 G and from 16 to 25 mm, depending on injection technique (Kroger 2017).
Vaccines recommended for subcutaneous delivery include some formulations of Japanese encephalitis vaccine (e.g. 'Green Cross' vaccine, Imojev) and varicella vaccines (DoH UK 2012a; ATAGI 2013). Subcutaneous vaccine injections are usually administered into the anterolateral thigh area of infants aged less than 12 months, and the upper, outer triceps area of people aged 12 months or older (Atkinson 2008; CDC 2011). Some NITAGs have recommended using needles 16 mm in length for administering vaccines subcutaneously with gauges ranging from 23 G to 25 G (NIAC 2016; Kroger 2017), or from 25 G to 26 G (ATAGI 2016). By contrast, the World Health Organization (WHO) has suggested that 23 G 25 mm needles can be used for subcutaneous administration of measles and yellow fever vaccines (WHO 2004).
Only a small number of vaccines are administered intradermally using hypodermic needles. Bacille Calmette‐Guérin (BCG) vaccine against tuberculosis is administered using the Mantoux method, and concentrated and purified cell‐culture rabies vaccines can also be delivered intradermally using the same technique (WHO 2010; Kim 2012). The preferred site for intradermal injection of the BCG vaccine is over the insertion of the left deltoid muscle, avoiding the tip of the shoulder due to an increased risk of keloid scar formation at this site (DoH UK 2012a). Needles of between 10 mm and 20 mm in length with gauges ranging from 25 G to 27 G have been advocated for administering intradermal injections (Kroger 2013).
Injection technique for intramuscular vaccinations
For an intramuscular vaccination procedure, two aspects of injection technique may influence the length of needle chosen: 1) the angle of needle insertion; and 2) whether the skin is bunched or stretched before needle insertion.
Angle of insertion
National Immunization Technical Advisory Groups in several countries (including Ireland, the UK, Australia, the US, and New Zealand) recommend that intramuscular injections should be administered at a 90° angle to the skin (NIAC 2011; DoH UK 2012a; ATAGI 2013; Kroger 2017; MoH NZ 2017). However, recommendations on injection angle have varied over time. For example, before 2005, New Zealand endorsed a 45° angle and Australia a 45° to 60° angle (NHMRC 2000; NHMRC 2003; Petousis‐Harris 2008). The angle of insertion will impact on the depth of needle penetration, and an insertion angle that deviates from the perpendicular may require the use of a longer needle to ensure that the vaccine is administered into muscle (Petousis‐Harris 2008).
Bunching or stretching
One technique for intramuscular injections entails gently bunching the muscle using the free hand while inserting the needle perpendicular to the skin. A second technique involves stretching the skin flat over the injection site and then inserting the needle perpendicular to the skin. A longer needle may be required to reach the muscle with the bunching rather than the stretching technique.
Injection technique for subcutaneous vaccinations
For subcutaneous injections, it is generally recommended that the needle be inserted into the subcutaneous tissues below the dermal layer at a 45° angle to the skin (DoH UK 2012a; Kroger 2017). To avoid administering the vaccine into muscle, some NITAGs recommend that the skin and subcutaneous tissue should be bunched or pinched‐up to raise these tissues from the muscle layer before inserting the needle into the resulting skinfold (DoH UK 2012a; NIAC 2016). Other NITAGs make no specific recommendation in this regard (MoH NZ 2017).
Injection technique for intradermal vaccinations
Intradermal injection technique requires special training and should only be administered by a trained provider (DoH UK 2012a; ATAGI 2016). It is generally recommended that the skin should be stretched between the thumb and forefinger on one hand and the needle inserted almost parallel to the skin surface with the bevel facing upwards into the superficial layers of the dermis. The recommended insertion depth varies from approximately 2 mm to 5 mm (DoH UK 2012a; NIAC 2016).
How the intervention might work
Unintentional deviation from the prescribed route of administration (intramuscular, subcutaneous, intradermal) for an injectable vaccine can occur if a needle of an inappropriate length is used. This can affect both vaccine immunogenicity (the ability of the vaccine to elicit an immune response) and reactogenicity (adverse events following vaccine administration). The majority of vaccines administered to children and adolescents are given via the intramuscular route, and the needle used must be sufficiently long to reach the muscle mass, but not excessively long as to involve underlying nerves, blood vessels, or bone (Zimmerman 2006; CDC 2011; Kroger 2017). If the needle used is too short, the vaccine may inadvertently be administered into the layer of subcutaneous or deep fatty tissue rather than muscle, which may compromise immune response and vaccine efficacy (Zuckerman 2000). Inadvertent subcutaneous or intradermal administration, particularly of adjuvant‐containing vaccines, can also increase the risk of reactogenicity events including pain, local irritation, induration (hardening of the tissue at or near the injection site), skin discolouration, inflammation, and abscess formation (Atkinson 2008; CDC 2011). If the needle used is too long, there is a risk of overpenetration of the muscle, which can result in pain and damage to the underlying bone or periosteum (a fibrous membrane covering the surface of bones) (Lippert 2008).
Needle gauge may also influence the pain experienced during a vaccination procedure. Progressive decreases in the frequency of pain and bleeding on needle insertion into human skin have been recorded when needles of successively smaller outer diameters (23 G, 27 G, 30 G, 32 G) were used in an automated needle injection system where velocity, angle of insertion, and depth of injection were controlled (Arendt‐Nielsen 2006). However, any reduction in insertion pain associated with using a higher gauge (narrow) needle may potentially be offset during the subsequent injection procedure. It has been hypothesised that the passage of the vaccine through a narrow bore needle may produce an 'injection jet' under high pressure, thereby inducing more severe local reactions at the injection site (Watson 2001). By contrast, although a wider‐bore needle may be associated with greater pain on needle insertion, the vaccine may be dissipated over a wider area, potentially resulting in less severe local reactions (Zuckerman 2000; RCN 2001). Needle size (length and gauge) may therefore influence both vaccine immunogenicity and reactogenicity, and clinicians should endeavour to select a needle for performing a specific vaccination procedure that will ensure the attainment of maximum immunity with the least possible harm.
Further indirect evidence to support the hypothesis that needle size may have an impact on vaccination‐related procedural pain and the incidence of other reactogenicity events is provided by trials that have reported differences in injection‐related pain scores and injection‐related adverse effects (including bleeding and bruising) when using needles of different sizes to perform Mantoux skin testing for tuberculosis (Flynn 1994), breast fine‐needle aspiration cytology (Daltrey 2000), and when administering insulin subcutaneously (Schwartz 2004; Kreugel 2007; Hirsch 2010), onabotulinumtoxinA (Botox) intradermally into the axilla (Skiveren 2010), and lidocaine intradermally into the volar surface of the forearm (Palmon 1998). Although the gauges and lengths of the needles used for many of the aforementioned procedures are different to those typically recommended for vaccinations, it is reasonable to postulate that similar effects may be observed when needles of different sizes are used to administer vaccines via intramuscular, subcutaneous, and intradermal routes.
Why it is important to do this review
There are inconsistencies in the recommendations made by NITAGs in different countries regarding the sizes of needles that should be used when administering vaccines to children and adolescents of specific ages or body masses at preferred injection sites via intramuscular, subcutaneous, and intradermal routes. There is also some evidence of variation in clinician adherence to these recommendations. For example, surveys conducted in Australia, Cook 2001, Scotland, McKinstry 2004, and the US, Schechter 2010, have documented that, contrary to guideline recommendations, some clinicians prefer to use a shorter (16 mm) rather than a longer (25 mm) needle when administering intramuscular vaccinations to children. This reluctance to use longer needles may be due to concerns about the possibility of damaging deep tissue and bone and causing more discomfort to the child (Zuckerman 2000; McKinstry 2004).
The inconsistencies in NITAG recommendations, coupled with the evidence of variable clinician compliance with these recommendations, suggest medical uncertainty in this area. This review may help to reduce uncertainty by providing a critical summary and synthesis of the evidence from randomised controlled trials (RCTs) on the beneficial and adverse effects of using needles of different sizes to administer vaccines to children and adolescents. The review may also help to improve outcomes for people undergoing vaccination, by assisting clinicians in making well‐informed decisions regarding the choice of needle size (gauge and length) for specific vaccination procedures that will minimise pain and discomfort while ensuring that an optimum immune response is attained. Reducing the pain associated with vaccine injections has the potential to improve parental, child, and adolescent satisfaction with the vaccination experience, thereby enhancing vaccine uptake and compliance with recommended immunisation schedules. This is critically important in light of global concerns regarding suboptimal vaccine uptake and outbreaks of vaccine‐preventable diseases in many countries (WHO 2009; Barret 2010; Roehr 2010; WHO 2011; Diekema 2012; HPSC 2012; Kmietowicz 2012; Wise 2013).
This review may also help to reduce international variations in manufacturers’ packaging and presentation of vaccines, which may influence clinicians' decisions regarding the size of needle to use for specific vaccination procedures. For example, packages of the human papillomavirus vaccine Gardasil currently supplied in Ireland include two needles: a 23 G 25 mm needle and a 25 G 16 mm needle (Kiersey 2016). However, some presentations of Gardasil available in other countries offer clinicians no choice when selecting a needle, as only one 25 G 25 mm needle is included in the packaging (Merck 2007). Our review may help inform manufacturers' decisions regarding the gauges and lengths of hypodermic needles that are supplied with specific vaccines.
Finally, this review complements existing reviews published in the Cochrane Library that have evaluated the effects of other interventions for needle‐related procedural pain in children and adolescents, including sweet‐tasting solutions, Harrison 2015; Kassab 2012, and psychological interventions (Uman 2013).
Objectives
To assess the effects of using needles of different lengths and gauges for administering vaccines to children and adolescents on vaccine immunogenicity (the ability of the vaccine to elicit an immune response), procedural pain, and other reactogenicity events (adverse events following vaccine administration).
Methods
Criteria for considering studies for this review
Types of studies
We only included RCTs in this review. We excluded quasi‐randomised trials due to the increased risk of systematic differences between comparison groups (i.e. selection bias) if allocation is performed on the basis of a pseudo‐random sequence (e.g. odd/even hospital number or date of birth, alternation).
Types of participants
We included trials involving children and adolescents, from birth to 24 years of age, undergoing vaccination with any type of vaccine(s) administered via intramuscular, subcutaneous, or intradermal routes using hypodermic needles in any setting (e.g. hospital or community). For the purposes of this review, we defined a child as a person aged less than 10 years and an adolescent as a person aged 10 to 24 years. We chose the upper limit of 24 years because "many researchers and developmental specialists in the U.S. use the age span 10 ‐ 24 years as a working definition of adolescence" (Kaplan 2004; DHHS 2013).
Types of interventions
We included trials evaluating the effects of hypodermic needles of any size (i.e. any gauge or length) used to administer any type of injectable vaccine to children and adolescents.
We included trials making any of the following needle size comparisons:
needles with the same gauge but different lengths (e.g. 25 G 25 mm needle versus 25 G 16 mm needle);
needles with different gauges but the same length (e.g. 25 G 25 mm needle versus 23 G 25 mm needle);
needles with different gauges and different lengths (e.g. 23 G 25 mm needle versus 25 G 16 mm needle).
We excluded trials where the comparison arms of the trial intentionally differed as part of the trial design with regard to factors other than needle size that could influence immunogenicity and reactogenicity outcomes. These factors included:
different vaccines administered to participants in the comparison groups (e.g. one group was given an acellular pertussis‐containing vaccine and one group was given a whole‐cell pertussis‐containing vaccine (these vaccines have different reactogenicity profiles));
different volumes of vaccine administered to participants in the comparison groups (e.g. one group was given 1.0 mL of a vaccine and one group was given 0.5 mL);
vaccines administered by different routes (e.g. one group was vaccinated by the subcutaneous route and one group was vaccinated by the intramuscular route);
vaccines administered at different sites (e.g. one group was vaccinated in the anterolateral thigh area and one group was vaccinated in the deltoid region of the upper arm);
vaccines administered using different injection techniques (e.g. one group was vaccinated using the WHO technique (skin stretched flat and the needle inserted at a 90° angle), and one group was vaccinated using a bunching technique whereby the skin and subcutaneous tissue was bunched/pinched and the needle inserted at a 45° angle).
We also excluded trials evaluating the effects of:
microneedle devices using solid or hollow, dissolvable or non‐dissolvable microneedles for intradermal vaccine delivery;
jet injectors;
devices for administering vaccines via intranasal injection;
bifurcated needles used to administer smallpox vaccine.
Types of outcome measures
We included all outcomes reported by trial authors that were deemed likely to be meaningful to clinicians, patients (consumers), parents, and policymakers. In the review protocol (Beirne 2013), we prespecified the following primary and secondary outcomes that we would consider in the review.
Primary outcomes
Postvaccination incidence of vaccine‐preventable diseases: in the protocol, we stated that the diagnosis of these diseases should be made using one or a combination of standard clinical or bacteriological or serological criteria (e.g. a diagnosis of pertussis (whooping cough) should be based on a characteristic clinical history as well as isolation of Bordetella pertussis from a clinical specimen or positive polymerase chain reaction (PCR) assay for B pertussis. A diagnosis of hepatitis B infection should be based on detection of the surface antigen of the hepatitis B virus (HBsAg), hepatitis B e antigen (HBeAg), hepatitis B virus (HBV) DNA, or antibody to hepatitis B core (HBc) antigen in serum (anti‐HBc) with or without clinical or laboratory features of hepatitis or its complications).
-
Pain, experienced during the vaccination procedure or at any time point postvaccination measured via self report, observer global reports, or behavioural measures using any age‐appropriate pain assessment tool with established validity and reliability (see Appendix 2):
-
self report measures of pain:
visual analogue scales (VAS);
numerical rating scales (NRS);
verbal rating scales (VRS);
other scales with established validity and reliability (see Appendix 2).
observer global reports: observer versions of the self report measures listed above, completed by parents, researchers, healthcare professionals, or other observers (see Appendix 2).
-
behavioural measures:
Face Legs Activity Crying Consolability scale (FLACC) (Merkel 1997);
Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) (McGrath 1985);
COMFORT scale (Ambuel 1992);
Neonatal Infant Pain Scale (NIPS) (Lawrence 1993);
Modified Behavioural Pain Scale (MBPS) (Taddio 1995);
other scales with established validity and reliability (see Appendix 2).
-
-
Crying measures:
crying incidence following vaccination;
persistent crying incidence following vaccination (defined as the presence of crying that is continuous (not episodic) and unaltered for three hours or more) (Bonhoeffer 2004);
total cry duration (onset of first cry to cessation of all crying (seconds));
duration of crying (in seconds) during a specified time period (e.g. three minutes) following vaccination;
percentage of time spent crying during a specified time period (e.g. three minutes) following vaccination.
Secondary outcomes
Surrogate measures of vaccine efficacy or correlates of vaccine‐induced immunity including measures of serum antibody responses to the administered vaccine such as geometric mean concentration (GMC), geometric mean titre (GMT), geometric mean fold increase (GMFI), or percentage of people with a predefined antibody level (e.g. for yellow fever vaccine, the proportion of people with a log neutralisation index (LNI) of 0.7 or higher). In the protocol for the review, we stated that antibody responses to core vaccine antigens must be assessed using standard tests, as described in Plotkin 2013. We have listed thresholds of vaccine‐induced correlates and surrogates of protection for selected vaccines in Appendix 3.
-
Physiological measures including the following adverse cardio‐respiratory events measured by standard cardio‐respiratory monitors (e.g. monitors that detect central apnoea using thoracic impedance and bradycardia employing electrocardiography and beat‐to‐beat heart rate recording) or observation by trained healthcare professionals or researchers or other personnel or both standard monitoring and observation:
episodes of oxygen desaturation, defined as a spontaneous fall in peripheral capillary oxygen saturation (SpO2) of 85% for 10 seconds or longer in duration as measured with pulse oximetry;
episodes of bradycardia, defined as a fall in heart rate of more than 30% below the baseline;
episodes of apnoea, defined as a cessation of breathing for more than 20 seconds or a shorter pause associated with bradycardia or cyanosis.
Incidence of common adverse events following vaccine administration: fever, erythema (redness), swelling, induration, tenderness at the site of injection, local hypersensitivity reactions, malaise, irritability, headache, and loss of appetite. In the protocol for the review, we stated that we would include trials reporting any of these adverse events, irrespective of how the events had been defined and measured or recorded by the trial authors. Where the information was available, we reported the case definitions of adverse events used by trial authors and explained how these events were measured by trial researchers in the Characteristics of included studies table. We included trials where data on common adverse events were reported separately or combined as composite outcomes. Where trial authors combined data on various adverse events, we reported precisely what events (e.g. erythema, swelling, induration, etc.) were included in the aggregated data.
-
Incidence of other local, systemic, or allergic adverse events following vaccine administration reported by trial authors, including:
local reactions: injection site nodule, granuloma, cyst, haematoma, rash, abscess, cellulitis, ulceration (necrosis), warmth, or any other reported morphological or physiological change at or near the injection site;
other adverse events: disturbed sleeping, drowsiness/tiredness, nausea, vomiting, diarrhoea, syncope (vasovagal or vasodepressor reaction), anaphylaxis, febrile convulsions, hypotonic‐hyporesponsive episode (HHE), generalised rash, paraesthesia, brachial neuritis (see Appendix 4 for explanations of selected terms).
In the protocol for the review, we stated that we would also report in our review any adverse events related to the equipment used to deliver vaccines including, but not limited to, needle bending, needle breakage, or detachment of the needle from the syringe.
Search methods for identification of studies
Electronic searches
For this review update we searched the following databases, with no language restrictions, using the search strategies in Appendix 5:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 9) via the Cochrane Register of Studies Online (CRSO) (searched 24 October 2017);
MEDLINE and MEDLINE in Progress via Ovid (November 2014 to 23 October 2017);
Embase via Ovid (November 2014 to 2017 week 43);
CINAHL (Cumulative Index of Nursing and Allied Health Literature) via EBSCOhost (November 2014 to 24 October 2017).
Details of the search strategy for the original review are available in Beirne 2015.
Searching other resources
For this review update we searched the Annual Meeting Abstract Archives of the Infectious Diseases Society of America (IDSA) (2015), the proceedings of the 9th to the 11th Vaccine and International Society for Vaccines (ISV) congresses (2015 to 2017), and the online library of the European Society of Clinical Microbiology and Infectious Diseases (on 16 January 2018). In addition, we searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the WHO International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/) to 17 January 2018.
Details of the search strategy for the original review are available in Beirne 2015.
Data collection and analysis
Selection of studies
The PRISMA flow diagram in Figure 1 summarises the screening and selection process for the updated review. Two review authors (PB, SH) independently screened the titles and abstracts of the search results.
1.
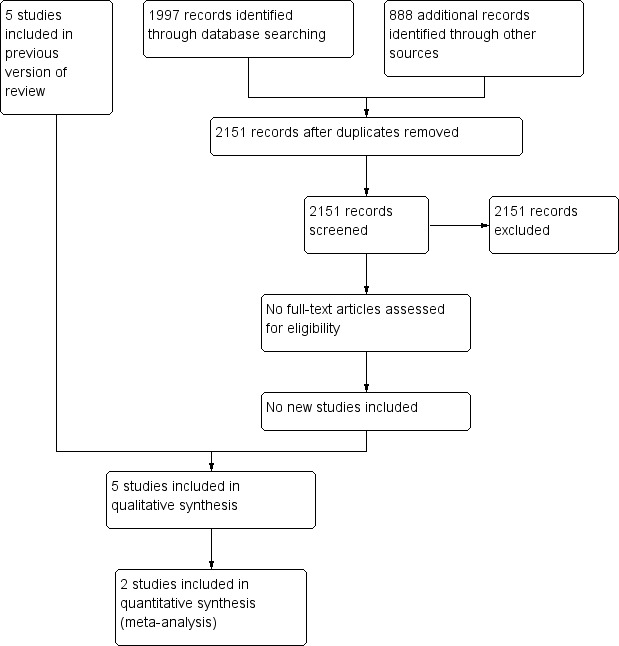
Study flow diagram.
For the original review (Beirne 2015), three review authors (PB, FS, SH) independently screened the titles and abstracts of the search results to identify trials that met the selection criteria. We retrieved the full texts of any potentially relevant papers and corresponded with trial authors where necessary to clarify study eligibility. The PRISMA flow diagram for the original review is available in Beirne 2015.
Data extraction and management
Three review authors (PB, SH, SC) independently extracted data from the included trials using a predesigned data collection form developed by one of the review authors (PB). The three review authors independently piloted the data extraction form on one of the included trials, Diggle 2006, before proceeding with data extraction for the remaining included trials. Following completion of the data extraction process, the three review authors compared the details recorded in the three independently completed data collection forms for each trial. In instances where details were missing from included trials (e.g. details regarding colour coding of needle hubs and the precise type and formulation of the vaccines administered), we contacted the trial authors to obtain the required information. Any disagreements regarding the details recorded on the data extraction forms were resolved by discussion and consensus.
The information recorded on the data extraction form included:
general trial information: trial ID, title of publication, source of publication, year of publication, country where the trial was conducted, details of trial authors, contact addresses, or other contact details (e.g. email addresses) for trial authors;
-
characteristics of the study: trial design (e.g. parallel group), trial setting (e.g. general practice), details necessary for assessing the risk of bias as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions including (Higgins 2011a):
methods used to generate a random allocation sequence;
methods used to conceal the allocation sequence;
details of all measures used, if any, to blind participants and personnel;
details of all measures used, if any, to blind outcome assessors;
details of the completeness of data for each outcome, including attrition and exclusions from the analysis;
details of any other concerns about bias.
characteristics of the trial participants: details of the inclusion and exclusion criteria for the trial; baseline characteristics of trial participants in the study groups including age, gender, and weight; and the numbers randomised to each group;
characteristics of the interventions: needle size (length and gauge) used to administer the vaccine to different study groups; details of any colour coding on the needle hubs; and details of the needle composition, needle coating, needle bevel, and type of needle hub. Type and formulation of vaccine administered, including details of the 'biological' characteristics of the vaccine and the composition of the vaccine (e.g. presence or absence of adjuvant). The volume of vaccine administered and details of the vaccine manufacturer. Details of the personnel who administered the vaccination. Details of the injection technique used including bunching or stretching of skin and underlying tissues before needle insertion, angle of needle insertion, depth of needle insertion (e.g. needle inserted to full depth (i.e. to the needle hub), 2 mm of needle exposed between the skin and needle hub). Several of these issues were either not reported or were incompletely reported in the included trials, and we corresponded with all trial authors to obtain the missing information;
characteristics of the outcome measures: details of all outcomes measured, definitions of outcomes, and time points of measurements. Details of the outcome assessors and methods/instruments used to measure outcomes. Units of measurement (where relevant), upper and lower limits for any scales used;
trial results: for each outcome, we recorded details of the numbers in each study group for whom outcome data were available at each time point and details of, and reasons for, any attrition or exclusions and any re‐inclusions in analyses performed by the trial authors. For dichotomous outcomes, we recorded the numbers of participants experiencing the outcome of interest in each study group at each time point. For continuous outcomes, we recorded the mean value and standard deviation of the outcome measurements in each study group at each time point or the medians and interquartile ranges (IQR) for skewed outcome distributions. Where trial authors log‐transformed the data for analysing antibody concentrations after vaccination, we recorded geometric means and accompanying 95% confidence intervals (CI) and the standard deviations of measurements on a natural log scale where reported;
miscellaneous information: source of funding for the trial, key conclusions of the trial authors, miscellaneous comments made by the trial authors, and references to other relevant studies.
One review author (PB) entered all relevant data into Review Manager 5 (RevMan 2014); two review authors (SH, SC) checked data entries. We considered contextual factors recorded in the data extraction form for each trial (i.e. conditions and circumstances relevant to the application of the intervention such as the country (e.g. low‐ and middle‐income, high‐income) where the trial was conducted and the trial setting (e.g. general practice, other setting) when interpreting the overall results of the review. We also considered the applicability, transferability, and external validity of findings for disadvantaged groups, as recommended in the "Equity checklist for systematic review authors" (Ueffing 2012).
Assessment of risk of bias in included studies
Three review authors (PB, SH, SC) independently assessed and discussed the risk of bias in trials meeting the selection criteria. Any disagreements were resolved by discussion and consensus. The review authors were not blinded to the authors of each trial, the trial location/setting, sources of funding for the trial, or trial acknowledgements.
We assessed the following domains for each trial:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias);
incomplete outcome data (attrition bias);
selective reporting (reporting bias);
other sources of bias (other bias).
For each domain, we reached a judgement of low risk of bias, high risk of bias, or unclear risk of bias, and provided justification for all judgements in the 'Risk of bias' tables and in the Risk of bias in included studies section of the review. In reaching our judgements, we considered the risk of material bias, defined as "bias of sufficient magnitude to have a notable impact on the results or conclusions of the trial" (Higgins 2011a), rather than the risk of any bias. We produced a separate 'Risk of bias' table for each trial as described in Section 8.6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Measures of treatment effect
The review team statistician (TF) monitored all statistical analyses in our review.
Dichotomous data
We calculated risk ratios (RR), risk differences (RD), and numbers needed to treat for an additional beneficial outcome (NNTB) as effect measures for dichotomous outcomes. We performed any meta‐analyses of dichotomous data using RR (see Data synthesis). None of the trials included in the review reported on the incidence of vaccine‐preventable diseases postvaccination, and we based our analyses of the effects of needle size on vaccine immunogenicity on differences in seroprotection rates between needle size groups. The term 'seroprotection' refers to antibody titre levels above a predefined threshold level that correlates with protection from disease. We used the following threshold levels for seroprotection against diphtheria, tetanus, and Haemophilus influenzae type b (Hib) disease: diphtheria antitoxin levels 0.01 IU/mL or greater, tetanus antitoxin levels 0.01 IU/mL or greater, and Hib antibody titre levels 1.0 μg/mL or greater (see Appendix 3).
Continuous data
We calculated mean differences (MD) with 95% CIs as effect measures for continuous outcomes. Medians and ranges in needle size groups were reported in tables. We did not perform any meta‐analyses of continuous data in this review.
In accordance with guidance proposed by the Cochrane Infectious Diseases Group (Donegan 2010), where continuous data (e.g. antibody titres) were summarised using geometric means, we reported geometric mean ratios (GMRs) as effect measures. We calculated the GMRs from the information provided in trial reports as follows: the reported geometric means in the needle size groups being compared were log‐transformed to obtain the estimated mean log concentrations. The standard errors (SE) on the log scale were calculated from the quoted standard deviations on the log scale and the sample size. We combined the mean log concentrations and corresponding SEs to obtain the difference in estimated log concentrations and the 95% CI for the difference in mean log concentrations. Finally, the estimate of the difference in the mean log concentrations and the corresponding 95% CI were exponentiated to obtain the ratio of the geometric means and the 95% CI for the ratio of the geometric means.
Unit of analysis issues
All trials included in the review were parallel‐group trials where participants were individually randomised to the intervention groups. Two trials had three groups and therefore contributed multiple comparisons of relevance to the review (Diggle 2006; Nirupam 2008). For example, Diggle 2006 contributed data for:
needles of the same lengths but different gauges (25 G 25 mm versus 23 G 25 mm);
needles with the same gauges but different lengths (25 G 25 mm versus 25 G 16 mm);
needles with different lengths and gauges (23 G 25 mm versus 25 G 16 mm).
A unit of analysis error could have arisen if several comparisons from this trial had been entered into a meta‐analysis when these comparisons had intervention groups and hence participants in common. However, this issue did not arise as data from only one comparison in Diggle 2006 (23 G 25 mm versus 25 G 16 mm) were entered into the meta‐analyses conducted as part of this review (see Data synthesis). We did not include data from Nirupam 2008 in any meta‐analyses.
Several trials included in the review presented multiple local reaction outcomes at multiple time points raising a 'multiplicity' issue in the analysis (as described in Section 16.7.2 of the Cochrane Handbook for Systematic Reviews of Interventions) (Higgins 2011b). For example, in one trial each local reaction (swelling, tenderness, redness, hardness) was measured at four time points (six hours, days one, two, and three) after vaccination with each of three doses of the two vaccines administered in the trial, giving a total of 96 separate analyses (4 outcomes x 4 time points x 3 doses x 2 vaccines) (Diggle 2006). The existence of multiple analyses can be a source of bias in systematic reviews if review authors selectively highlight some analyses in the review (e.g. selective presentation of results at time points where the analysis yielded statistically significant findings). This issue of selectivity could have been problematic in our review as we did not identify in the review protocol specific time points at which we would record and report local reaction outcomes. We adopted two approaches to reduce the risk of any potential bias arising from selectivity. First, we conducted sensitivity analyses to investigate if the results of our review varied according to time point selection. Second, when presenting the results of analyses for local reaction outcomes in Effects of interventions, we followed the advice specified in Section 16.7.2 of the Cochrane Handbook for Systematic Reviews of Interventions, namely "If there is a choice of time‐points for an outcome, attempts should be made to present a summary effect over all time‐points, or to choose one time‐point that is the most appropriate one (although availability of suitable data from all trials may be a problem)" (our emphasis) (Higgins 2011b). Where suitable data were available from trials, we decided through discussion and consensus to present the results of local reaction analyses at 24 hours after vaccination or the nearest approximation to this time point (e.g. day one postvaccination). There were several reasons for selecting this time point. First, we considered that the most common local reactions after vaccination (redness, swelling, and tenderness) would have manifested by 24 hours with only a minority appearing de novo after this time point. Second, we reasoned that an analysis at an earlier time point (e.g. six hours postvaccination) would undoubtedly capture numerous immediate but potentially very transient local reactions (e.g. minor redness at the injection site that could potentially dissipate shortly after the six‐hour time point). We considered that parents and clinicians would be less concerned about such transient reactions and would be more concerned about local reactions that persisted at 24 hours. Finally, one previous systematic review and meta‐analysis evaluating the effect of needle size on vaccine reactogenicity also used the 24‐hour time point for analyses (Davenport 2003).
In instances where local reaction outcomes were not reported at 24 hours in a trial, we presented summary effects over all time points. We also adopted this approach for analysing systemic reactions (such as fever, irritability, and malaise) following vaccination. These systemic reactions can appear at any time point postvaccination, therefore we considered that it would be inappropriate to use the 24‐hour time point.
Dealing with missing data
We contacted the authors of all included trials to obtain missing data or for data clarification. We recorded details of any discrepancies between the numbers of participants randomised and the numbers analysed in each treatment group for each outcome and reported this information in the 'Risk of bias' table for each trial. If more than 20% of the data for a particular outcome were missing from a trial, we planned to exclude the trial from any meta‐analysis relating to that outcome. However, this issue did not arise in relation to the meta‐analyses we performed in the review (see Data synthesis). In instances where missing outcome data could reasonably be assumed to be missing at random, we analysed only the available data (i.e. we conducted an available‐case analysis). We adopted this analysis strategy in our review for all missing outcome data. Where appropriate, we explained the reasons why we deemed it reasonable to assume that data were missing at random in the 'Risk of bias' table for each included trial (see Characteristics of included studies).
Assessment of heterogeneity
We quantified inconsistency between the results of individual studies included in meta‐analyses using the I2 statistic (Deeks 2011), which describes the percentage of variability in effect estimates that is due to heterogeneity rather than to chance. We interpreted the values of the I2 statistic in accordance with the following approximate guide as specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Where heterogeneity was present, we investigated the heterogeneity by performing further quality control checks of data extraction from included studies and data entry into Review Manager 5 (RevMan 2014). We had also planned to investigate heterogeneity by conducting the subgroup analyses specified in the review protocol (Beirne 2013). However, the number of included trials was insufficient to conduct these analyses (see Differences between protocol and review).
Assessment of reporting biases
Publication bias
In the review protocol (Beirne 2013), we stated that we would only conduct tests for funnel plot asymmetry if at least 10 studies were included in the meta‐analysis (Sterne 2011). As our review included only five trials, we did not investigate the likelihood of publication bias by producing a funnel plot.
Outcome reporting bias
Three review authors (PB, SH, SC) examined the reports of all included trials for evidence of selective outcome reporting. We contacted all trial authors for additional information. We judged trials as having a low risk of bias due to selective outcome reporting if they fulfilled the following criteria specified in the Cochrane 'Risk of bias' assessment tool (Higgins 2011c):
study protocol was available and all of the trial's prespecified outcomes (primary and secondary) that were of interest in our review were reported in the prespecified way;
study protocol was not available, but we judged that the published reports included all expected outcomes, including those that were prespecified.
Data synthesis
We conducted all statistical analyses and data syntheses using Review Manager 5 (RevMan 2014), in consultation with the review team statistician (TF) where necessary. We only conducted statistical syntheses of the results from individual trials if we deemed the trials to be sufficiently similar in terms of the participants, interventions, comparisons, and outcomes to render calculation of a pooled estimate meaningful. In this context, we only included two trials in the meta‐analyses conducted in this review (Diggle 2000a; Diggle 2006). Both trials involved comparisons of the same needle sizes (23 G 25 mm versus 25 G 16 mm), and similar doses (third dose) of a DTwP‐Hib vaccine were administered using similar injection techniques. In addition, the trial participants were of a similar age, and local reaction outcomes (redness, swelling, and tenderness) were measured using similar techniques at similar time points. Where we deemed meta‐analysis inappropriate or not feasible due to the heterogeneity of the included trials, we presented a narrative summary of the results of individual trials at appropriate time points as described in the Unit of analysis issues section of the review.
In accordance with the review protocol (Beirne 2013), we used the RR as the summary statistic in meta‐analyses of dichotomous outcomes and pooled the RRs using the random‐effects Mantel‐Haenszel method (Higgins 2011c). We did not conduct any meta‐analyses of continuous outcomes in this review.
'Summary of findings' tables
We used 'Summary of findings' tables to summarise the results for the main comparisons (Schünemann 2011a). We created these tables by exporting data from Review Manager 5, RevMan 2014, into GRADEprofiler (GRADE 2011). We then exported the tables into Microsoft Word for additional editing before finally transferring the information into 'Summary of findings' tables created using the table editor in Review Manager 5.
We created three 'Summary of findings' tables for comparisons between the following needles that were used to administer combination vaccines with diphtheria, tetanus, and whole‐cell pertussis (DTwP) vaccine antigen components to infants:
25 G 25 mm versus 25 G 16 mm (comparison between needles with different lengths but with the same gauge);
25 G 25 mm versus 23 G 25 mm (comparison between needles with different gauges but with the same length);
23 G 25 mm versus 25 G 16 mm (comparison between needles with different gauges and different lengths).
One trial evaluated the effects on vaccine immunogenicity and reactogenicity of using these needle sizes to administer a meningitis C conjugate (MenC) vaccine. We did not incorporate these results into a 'Summary of findings' table as the vaccine was administered using a schedule (timing and spacing of vaccine doses) that is no longer recommended. We summarised the results of any analyses pertaining to the administration of the MenC vaccine in the main text of the review.
Two small trials compared 38 mm versus 25 mm needles and 22 G 25 mm versus 23 G 25 mm versus 24 G 25 mm needles; we did not construct separate 'Summary of findings' tables for these comparisons. We presented a narrative summary of the results of these trials in the Effects of interventions section.
We included the following outcomes in the 'Summary of findings' tables.
-
Immunogenicity outcomes
postvaccination incidence of vaccine‐preventable diseases
proportion of seroprotected vaccine recipients: this substitute (surrogate) immunogenicity outcome refers to the proportion of vaccine recipients who responded in a prescribed manner by reaching predefined antibody titre (or antitoxin) 'threshold levels' of protection against disease (e.g. the proportion of vaccine recipients with diphtheria antitoxin levels 0.01 IU/mL or greater) (see Appendix 3 for the threshold levels used in this review)
-
Reactogenicity outcomes
pain: experienced during the vaccination procedure or at any time point postvaccination measured using an age‐appropriate pain assessment tool with established validity and reliability (see Appendix 2)
crying: any measures of crying during and immediately after the vaccination procedure or at any time point postvaccination
severe local reactions (redness and swelling covering more than two‐thirds of the anterolateral thigh)
non‐severe local reactions on the day after vaccination: this composite outcome refers to any local reaction (e.g. any redness, swelling, tenderness, or hardness/induration) at the injection site
fever: experienced at any time point postvaccination
Not all of these outcomes were explicitly prespecified in our protocol for inclusion in 'Summary of findings' tables. We have therefore explained (below) our rationale for selecting some entries in the 'Summary of findings' tables.
It is generally recommended that outcomes included in the 'Summary of findings' tables should be those that are essential for decision‐making and that the emphasis should be on patient‐important outcomes (Guyatt 2013). In this context, our decision to include a substitute (surrogate) immunogenicity outcome in the 'Summary of findings' tables could be criticised. However, we considered that the substitute outcome should be included because no trials reported on the incidence of vaccine‐preventable diseases. This was not entirely unexpected given the low incidence of many of these diseases, particularly in countries with well‐established vaccination programmes, which would mean that trials with disease endpoints would require unfeasibly large sample sizes and duration of follow‐up. Under these circumstances, the use of substitute outcomes was the only realistic way of measuring the immune response to an administered vaccine. In accordance with the recommendations specified in GRADE guidelines (Guyatt 2013), we clearly indicated in 'Summary of findings' tables where inferences regarding intervention effects were based on the results of substitute endpoints, and we downgraded the quality of evidence level for indirectness.
In the review protocol (Beirne 2013), we listed two substitute immunogenicity endpoints that are commonly used in vaccine trials: 1) the proportion of vaccine recipients who reached a predefined antibody level following vaccination; this endpoint specifies "a threshold level of an immune marker above which subjects are assumed to be protected and below which they are not" (WHO 2013a); and 2) GMC or GMT of antibody; this endpoint uses antibody titres as continuous variables to predict vaccine efficacy. In the review protocol, we did not specify which of these substitute outcomes would be highlighted in 'Summary of findings' tables. We decided through discussion and consensus to highlight the proportion of vaccine recipients who reached a predefined threshold level of protection (as defined in Appendix 3). This decision took into account the use of this outcome in other Cochrane Reviews (e.g. Bar‐On 2012), and the fact that threshold endpoints are considered to be particularly meaningful when evaluating the immune response to specific components of some combination vaccines (e.g. the Hib component of DTwP‐Hib vaccines) (Horne 2001). For completeness, we included the results of analyses for GMCs and GMTs of antibodies in the footnotes of the 'Summary of findings' tables.
In the review protocol (Beirne 2013), we did not prespecify that the outcome 'severe local reactions' would be included in 'Summary of findings' tables. However, there was unanimous agreement within the review team that this outcome should be included, considering the extent and clinical severity of these reactions and their perceived importance to patients, their parents, and clinicians.
In the review protocol (Beirne 2013), we did not specify precisely what non‐severe local reactions we would include in the 'Summary of findings' tables and the time point(s) at which we would report these outcomes. As previously described in the Unit of analysis issues section, some of the included trials reported on multiple individual local reactions at the injection sites (swelling, tenderness, redness, and hardness) at multiple time points postvaccination (six hours, days one, two, and three). In one trial, these outcomes were also reported after each dose (first, second, and third) of the vaccine series administered to trial participants. This presentation of trial data posed a challenge in terms of compiling user‐friendly 'Summary of findings' tables that contained no more than the recommended seven outcomes (Guyatt 2013). We decided through discussion and consensus among the review team to include composite local reaction outcomes (any redness, swelling, tenderness, or hardness/induration at the vaccination site) experienced at 24 hours (day one) after vaccination in the 'Summary of findings' tables. The reason for selecting the 24‐hour (day one) time point is explained in the Unit of analysis issues section. We also decided to present in 'Summary of findings' tables the findings for local reactions after each dose of the vaccine(s) administered in the trials because it is well established that the same vaccine given as a primary dose (first dose) may have a different reactogenicity profile than when it is given as a booster dose (WHO 2013b).
We based the inclusion of fever in the 'Summary of findings' tables on a review of reports to the US Vaccine Adverse Events Reporting System (VAERS) (CDC 2003). Postvaccination fever and injection site (non‐severe) local reactions such as skin redness and oedema were the most frequently reported adverse events. Reports to VAERS are typically submitted by all relevant stakeholders in vaccination programmes, including healthcare providers, vaccine recipients (or their parents/guardians), and vaccine manufacturers. The Vaccine Adverse Events Reporting System "encourages the reporting of any significant adverse event occurring after the administration of any vaccine licensed in the United States" (our emphasis). By implication, events reported to this system could be deemed significant events for stakeholders, thereby meriting inclusion in 'Summary of findings' tables.
Methods used to assess the quality of the evidence for outcomes included in 'Summary of findings' tables
We assessed the quality of the evidence in relation to each outcome included in the 'Summary of findings' tables using the GRADE evidence grading system (Schünemann 2009), as described in Section 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011b). One review author (PB) initially applied the GRADE system and then discussed the quality of evidence ratings for each outcome with two other members of the review team (SH, SC). Final decisions on the ratings were reached through discussion and consensus. We took the following factors into account when deciding whether or not to downgrade the quality of evidence in relation to each outcome:
risk of bias;
inconsistency of results;
indirectness of evidence;
imprecision of results;
publication bias.
Our review included only RCTs, and we downgraded the evidence for each outcome from high quality by one level if we considered that there was a serious limitation in relation to a particular factor or by two levels if we considered there was a very serious limitation. We included footnotes in the 'Summary of findings' tables to explain our reasons for downgrading the evidence. We also included footnotes to justify some of our decisions not to downgrade the quality of the evidence, particularly in instances where we considered that users of our review might reasonably disagree with our decisions.
Some of the outcomes included in 'Summary of findings' tables were reported from a single trial, which created problems in terms of evaluating the quality of the evidence as it related to the criterion of consistency/inconsistency of results. The Agency for Healthcare Research and Quality (AHRQ) in the US has noted that "evaluation of consistency ideally requires an evidence‐base with independent replication of findings" and that we "cannot be certain that a single trial, no matter how large or well‐designed, presents the definitive picture of any particular clinical benefit or harm for a given treatment" (Owens 2009). The AHRQ has suggested that consideration should be given to decreasing the strength of evidence grade in instances where evidence is from a single study and where consistency is therefore unknown (Owens 2010). We adopted this approach of downgrading evidence for outcomes with a single trial evidence base, with two exceptions.
Some trials reported two substitute endpoints for immunogenicity: the proportion of vaccine recipients who reached a predefined antibody level (seroprotection) following vaccination, and the GMC or GMT of antibody. In evaluating the immunogenicity evidence base for consistency, we considered the consistency of the effect sizes for these different endpoints.
One included trial reported on some reactogenicity outcomes (redness, swelling, tenderness, and hardness) after each of three doses of the vaccines that were administered in the trial using needles of different sizes. These doses of the vaccines were administered to the same trial participants when they were aged two months (first dose), three months (second dose), and four months (third dose). In evaluating the evidence base for consistency for these reactogenicity outcomes, we considered the consistency of the effect sizes after each dose of the vaccine.
We acknowledge that neither point 1 nor 2 above represents truly independent replication of findings. Nevertheless, we considered that this was a reasonable approach to adopt when evaluating the quality of evidence for some of the local reaction and immunogenicity outcomes reported in single trials in our review.
Identification and definitions of minimum important differences
It has been recommended that systematic reviewers should endeavour to identify an appropriate minimum important difference (MID) for the outcomes of interest in the review (AHRQ 2012). The MID has been defined as "The smallest difference in score of the outcome of interest that informed what patients or proxies perceive as important and which would lead the patient or clinician to consider a change in the management" (Schünemann 2005, cited in AHRQ 2012). The MID can facilitate the interpretation of the results of a systematic review and the evaluation of statistical significance in the context of clinical relevance (AHRQ 2012).
In order to determine and define MIDs for the immunogenicity and pain outcomes reported in our review, we adopted approaches suggested by the AHRQ including: reviews of the literature to locate already‐conducted empirical studies to identify the smallest change in a particular outcome that people perceive as important; using MIDs specified by prominent authorities; and using MIDs specified in the power calculations of relevant studies (AHRQ 2012). When reviewing the literature on MIDs in immune responses, we considered the power calculations in non‐inferiority trials of combination vaccines. The rationale for this approach was that most of the trials included in our review involved using needles of different sizes to administer combination vaccines, and trials designed to evaluate combination vaccines are customarily designed and analysed as non‐inferiority studies (Horne 2001).
For differences in seroprotection rates between needle size groups, we used an RD of 10% as the MID. We considered needle sizes to have comparable effects on immune response if the 95% CI accompanying the RD effect estimate was sufficiently narrow to exclude a 10% difference in seroprotection rates in either direction. The selection of this MID was based on the recommended non‐inferiority 10% protection rate for vaccines specified by the Committee for Proprietary Medicinal Products (CPMP 1999), and cited in a systematic review of margins for equivalence and non‐inferiority in biomedical research (Lange 2005). This MID was also used in several non‐inferiority trials of combination vaccines (Guerra 2009; Kosalaraksa 2011; Thierry‐Carstensen 2012; EMA 2013).
When choosing an MID for the outcome of pain, we examined the literature on pain scales for children and adolescents for information on differences in scores considered to be clinically significant. In general, reported estimates of the minimum clinically important difference ranged from 10% to 20% (e.g. a change of one face on the Faces Pain Scale‐Revised, or a change of 10 to 20 mm on a 100‐millimetre VAS) (von Baeyer 2006). These MIDs are commensurate with those specified in studies measuring pain response to vaccinations using the 10‐point MBPS. For example, we identified one study that specified in a power calculation a "clinically important difference in mean MBPS between groups of 2 units" (Ipp 2004). Another study specified that "only differences greater than 1 point on the 10‐point MBPS were considered clinically significant" and that "this is in line with recently published meta‐analytic work determining the effect of a known analgesic agent on immunization pain using MBPS" (Pillai Riddell 2013). We selected as an MID the more conservative estimate of 1 point on the 10‐point MBPS scale or its equivalent on other scales.
We did not specify MIDs for other reactogenicity outcomes (such as local reactions, fever, irritability, etc.) reported in our review. We could identify no consensus in the international literature on differences in event rates for these outcomes that would be considered clinically important. Furthermore, MIDs for reactogenicity events are rarely specified in power calculations in vaccine trials, which are typically based on immunogenicity rather than reactogenicity endpoints. We therefore reported all observed differences between needle size groups for these outcomes.
Subgroup analysis and investigation of heterogeneity
The number of trials included in the review was insufficient to conduct subgroup analyses (see Differences between protocol and review).
Sensitivity analysis
We performed meta‐analyses using both fixed‐effect and random‐effects models. Due to the small number of trials included in the meta‐analyses, we did not conduct any of the other sensitivity analyses prespecified in our protocol (see Differences between protocol and review) (Beirne 2013).
During the review process we made several post hoc decisions including the time points at which we would analyse trial data, the selection of MID in seroprotection rates between needle size groups, and the presentation in 'Summary of findings' tables of the results pertaining to the effects of needle size on a composite local reaction outcome rather than on the individual components of the composite. We conducted sensitivity analyses to investigate the impact of these decisions on the review findings. We presented the results of these analyses in the Effects of interventions section.
Results
Description of studies
Results of the search
As shown in Figure 1 and Appendix 6, our updated searches identified 2151 records. We screened out all 2151 records based on the titles and abstracts and therefore identified no new studies for this review update.
In the original review (Beirne 2015), our searches yielded 8058 records. We screened out 8013 references based on titles and abstracts. We examined the remaining 45 records in full text, and excluded 33 records (see Excluded studies). We excluded for this review update one trial originally assigned to 'studies awaiting classification' (see Characteristics of excluded studies) (Ozdemir 2012).
Included studies
Five RCTs (reported in 11 articles) met the inclusion criteria of the review and were included. Full details of the included trials are provided in the Characteristics of included studies table.
Design and sample sizes
All of the trials were parallel‐group trials. Three of the trials had two groups (Diggle 2000a; Pathak 2007; Middleman 2010), and two trials had three groups (Diggle 2006; Nirupam 2008). The total number of randomised participants in the five trials was 1350, with individual trial sample sizes ranging from 65, in Middleman 2010, to 696 participants, in Diggle 2006.
Settings
The vaccinations were administered in general medical practices in England in two trials (Diggle 2000a; Diggle 2006); in tertiary paediatric hospitals in India in two trials (Pathak 2007; Nirupam 2008); and at city clinics or in the participants' homes in the USA in one trial (Middleman 2010).
Participants
One trial involved obese adolescents aged 14 to 24 years (Middleman 2010). All participants in the remaining trials were infants under the age of six months and included:
healthy infants attending for routine vaccinations due at four months of age (Diggle 2000a);
healthy infants attending for routine vaccinations due at two, three, and four months of age (Diggle 2006);
infants up to 24 weeks of age attending for routine vaccinations, 14% of whom were "malnourished", with the remainder being of "normal weight" (categorised as per WHO growth standards) (WHO 2006; Pathak 2007);
healthy infants aged six to 10 weeks attending for prescribed routine vaccinations (Nirupam 2008).
One trial included only two males (one in each of the comparison groups) in the final data analysis (Middleman 2010). The proportions of male participants in the remaining trials ranged from 51% to 59%.
Interventions and comparisons
Needle sizes compared in the trials
Two trials compared needles with the same gauges but different lengths:
25 G 25 mm versus 25 G 16 mm (Diggle 2006);
38 mm versus 25 mm (the precise gauge number is unknown, but we received confirmation from the trial authors that the needles had the same gauge) (Middleman 2010).
Three trials compared needles with different gauges but the same length:
25 G 25 mm versus 23 G 25 mm (Diggle 2006; Pathak 2007);
24 G 25 mm versus 23 G 25 mm versus 22 G 25 mm (Nirupam 2008).
Two trials compared needles with different gauges and different lengths:
23 G 25 mm versus 25 G 16 mm (Diggle 2000a; Diggle 2006).
Vaccines used in the trials
The vaccines administered to trial participants were:
the first, second, and third doses of a recombinant hepatitis B (Hep B) vaccine (Middleman 2010);
the third dose of a combined DTwP‐Hib vaccine (Diggle 2000a);
the first, second, and third doses of a combined DTwP‐Hib vaccine and the first, second, and third doses of a MenC vaccine (Diggle 2006);
either the first, second, or third doses of i) a combined DTwP vaccine; ii) a combined DTwP‐Hib vaccine; or iii) a combined DTwP‐Hib‐Hep B vaccine (Pathak 2007);
the first dose of a combined DTwP vaccine and the first dose of a recombinant hepatitis B (Hep B) vaccine (Nirupam 2008).
The volume of the vaccine(s) administered to trial participants was:
0.5 mL (Diggle 2006; Pathak 2007; Nirupam 2008);
0.5 mL to 15% and 1 mL to 85% of the trial participants (see Characteristics of included studies table for additional details) (Diggle 2000a);
0.5 mL to trial participants aged less than 19 years and 1 mL to trial participants aged 19 years or greater (Middleman 2010).
The vaccination procedures were performed by general medical practice nurses in one trial (Diggle 2000a); by paediatric research nurses in one trial (Diggle 2006); by hospital nurses in two trials (Pathak 2007; Nirupam 2008); and by a faculty paediatrician and a trained medical student in one trial (Middleman 2010).
Route of administration, injection site, and injection technique
The vaccines used in all trials were intended to be administered via the intramuscular route. The injection site was the anterolateral thigh in four trials (Diggle 2000a; Diggle 2006; Pathak 2007; Nirupam 2008), and the deltoid region of the upper arm in one trial (Middleman 2010). The skin was stretched flat and the needle was inserted into the skin at a 90° angle in all trials (WHO injection technique). In three trials, the needle was inserted to its full length up to the needle hub (Diggle 2000a; Diggle 2006; Nirupam 2008). In one trial, some of the infants were malnourished, and it is possible that the staff nurses may not have inserted the full length of the needle when vaccinating these infants (personal communication with trial author) (Pathak 2007). In the trial involving obese adolescents, 2 to 3 mm of needle was left visible between the skin and the needle hub (Middleman 2010).
Outcomes
Details of the definitions of all outcomes (where provided by the trial authors) and the time points at which the outcomes were measured are provided in the Characteristics of included studies tables.
Two trials reported immunogenicity outcomes:
failed immunogenicity (vaccine non‐response): the numbers of vaccinated participants who failed to reach a predefined protective antibody concentration threshold (Diggle 2006; Middleman 2010). In this review, we reported the numbers who reached (rather than failed to reach) predefined thresholds, as specified in the review protocol (see Appendix 3 for details of the threshold levels used in this review);
antibody titres to HBsAg (Middleman 2010);
geometric mean concentrations (GMCs) of diphtheria, tetanus, and Hib antibodies and geometric mean titres (GMTs) of serogroup C meningococcal glycoconjugate antibodies (Diggle 2006). The immune response to the pertussis (whooping cough) component of the combined vaccine administered in the trial was not measured as there is no well‐established immune correlate or surrogate of protection against pertussis.
Four trials reported reactogenicity outcomes:
pain (Pathak 2007);
-
crying:
procedural crying (Pathak 2007);
persistent inconsolable crying (four hours or greater in Diggle 2006; greater than three hours in Nirupam 2008).
severe local reaction (Diggle 2006);
-
common local reactions at the injection site:
redness (Diggle 2000a; Diggle 2006; Pathak 2007; Nirupam 2008);
swelling (Diggle 2000a; Diggle 2006; Pathak 2007; Nirupam 2008);
tenderness (Diggle 2000a; Diggle 2006; Pathak 2007; Nirupam 2008);
hardness (Diggle 2006).
-
common systemic reactions:
fever (Diggle 2006; Pathak 2007; Nirupam 2008);
irritability (Nirupam 2008);
eating less than usual/refusal to feed (Diggle 2006; Nirupam 2008);
sleepier than usual/drowsiness (Diggle 2006; Nirupam 2008).
-
other local, systemic, or allergic adverse events following vaccine administration:
restricted movement (Pathak 2007);
vomiting (Diggle 2006; Nirupam 2008);
use of analgesics (Diggle 2006; Pathak 2007);
needle contact with bone Diggle 2006);
seizures (Nirupam 2008).
Three trials reported composite reactogenicity outcomes:
any local reaction (any redness, swelling, or tenderness) after vaccination (Diggle 2000a);
any local reaction (any redness, swelling, hardness, or tenderness) after vaccination (Diggle 2006);
any local reaction (any redness, swelling, tenderness, or restricted movement) after vaccination (Pathak 2007).
Excluded studies
Of the 34 excluded full‐text articles, seven articles reported the results of studies comparing groups that had been vaccinated with needles of different sizes on the rates of local and systemic reactions (Ipp 1989; Cook 2005; Jackson 2008; Fateh 2014), or the immune response (Shaw 1989; Johnsen 1995; Ozdemir 2012). Details of these studies are provided in the Characteristics of excluded studies table. Briefly, we excluded four of the studies because the participants were either not randomised to the comparison groups or insufficient details were available about the precise method of allocation to study groups and insufficient outcome data were available (Ipp 1989; Johnsen 1995; Jackson 2008; Ozdemir 2012). The remaining three studies were RCTs; we excluded one because the trial participants were not children or adolescents (Shaw 1989), one because the injection techniques used in the comparison groups were different (Cook 2005), and one because different types of syringe were used to administer the vaccine to participants in the comparison groups (Fateh 2014).
We excluded the remaining 27 full‐text articles for various reasons, including that the article:
was a narrative review, an editorial, or opinion piece;
was a summary of, or commentary on, one of the trials that met the selection criteria;
described a trial where there was no comparison of needle size when administering vaccines;
described a trial where the injections administered were not vaccinations;
described a trial comparing hypodermic needle versus jet injector.
Risk of bias in included studies
We did not conduct formal assessments of interrater reliability between review authors for each domain in the Cochrane 'Risk of bias' tool. The three review authors (PB, SH, SC) were in complete agreement for the domains of random sequence generation (selection bias) and allocation concealment (selection bias). Judgements about all other domains, in particular regarding the risks of performance bias and detection bias, were reached through discussion and consensus. We have summarised the salient aspects of these discussions in the relevant sections below. Figure 2 summarises our decisions regarding the risk of bias for all included trials. The empty fields in Figure 2 pertaining to detection bias and attrition bias for specific outcomes indicate that these outcomes were not measured in trials.
2.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study. Blank cells in the table indicate outcomes that were not measured in studies.
Allocation
Random sequence generation
All trials described a random component in the sequence generation process. Four trials used computer‐generated randomisation schemes (Diggle 2000a; Diggle 2006; Pathak 2007; Nirupam 2008), and one trial used random number tables (Middleman 2010).
Allocation concealment
In four trials, we considered that participants and investigators enrolling participants could not have foreseen needle size allocations in advance of, or during, enrolment due to the use of sequentially numbered, sealed, opaque envelopes to conceal allocation (Diggle 2000a; Diggle 2006; Pathak 2007; Nirupam 2008). We judged one trial that did not conceal allocation as at high risk of bias for this domain (Middleman 2010).
Blinding
As specified in the review protocol (Beirne 2013), when reaching judgements about the risk of bias due to lack of blinding or incomplete blinding we considered the risk of material bias rather than the risk of any bias. The Cochrane Handbook for Systematic Reviews of Interventions defines material bias as "bias of sufficient magnitude to have a notable impact on the results or conclusions of the trial, recognizing that subjectivity is involved in any such judgement" (our emphasis) (Higgins 2011a). Overall, we were less concerned about the potential for material bias due to lack of blinding of participants and personnel in trials (performance bias), and more concerned about the potential for material bias due to lack of blinding of outcome assessors, particularly for subjective outcomes. We have explained the reasons for this below.
Performance bias (blinding of participants, their parents, and trial personnel)
One trial did not blind participants (obese adolescents) (Middleman 2010). In the remaining trials, the participants were all infants, and we deemed blinding of their parents or guardians to be adequate if any one of the following conditions were fulfilled:
parents or guardians were not present when the child was vaccinated, and they were not informed by trial personnel of the needle size used to administer the vaccine;
parents or guardians were present when the child was vaccinated but they did not view the procedure and were not informed by trial personnel of the needle size used to administer the vaccine;
in trials or trial comparisons where the effect of needle gauge only was being assessed (i.e. the needles being compared were of the same length), parents or guardians were present and viewed the procedure, but the needle hubs were not colour‐coded, and they were not informed by trial personnel of the gauge of needle used to administer the vaccine.
No trials completely fulfilled these conditions. In two trials, parents or guardians were present and viewed the vaccination procedures (Diggle 2000a; Diggle 2006), with the exception of parents known to be health professionals in the latter trial who were specifically asked not to view the procedure. The needles were colour‐coded in both trials, but parents were not told by study nurses which needle size was being used or how the different‐coloured hubs of the needles related to needle size. In the remaining trials, parents or guardians were present during the vaccination procedure, and the needle hubs were colour‐coded (Pathak 2007; Nirupam 2008). We therefore judged blinding of parents to be either inadequate or incomplete in all trials.
Blinding of the personnel administering the vaccinations to trial participants was not possible in the three trials that compared needles of different lengths (Diggle 2000a; Diggle 2006; Middleman 2010), as these differences would have been obvious to trial personnel experienced in performing vaccination procedures. In the three trials that compared the effects of needles with the same lengths but different gauges (Diggle 2006; Pathak 2007; Nirupam 2008), the needle hubs were colour‐coded, therefore the trial personnel administering the vaccines were not blinded to needle gauge.
As recommended in Section 8.11.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), when considering the risk of performance bias arising from lack of blinding or incomplete blinding of participants and personnel, it is important to consider the "risk of bias in actual outcomes due to lack of blinding during the study (e.g. due to co‐intervention or differential behaviour)." In this context, we had to consider the likelihood that knowledge of needle size would have resulted in trial personnel or parents differentially providing care or co‐interventions to participants in the comparison groups and the likelihood that this would impact on immunogenicity and reactogenicity outcomes. We judged that such performance bias was unlikely to have occurred for the following reasons. First, standardised injection procedures were used by the trial personnel in all trials, and the same injection procedures were reportedly used in the comparison arms of the trials (see Characteristics of included studies table). We considered it unlikely that knowledge of needle size would have resulted in trial personnel deviating from the standardised injection technique or otherwise behaving in a manner that could produce systematic differences between comparison groups in terms of the care provided or in exposure to factors other than the interventions of interest. We also deemed it unlikely that parental or guardian knowledge of needle size would have resulted in systematic differences between study groups in terms of the care provided to infants either during or following the vaccination procedure that would have materially influenced reactogenicity and immunogenicity outcomes. We therefore assessed all trials as being at low risk of performance bias. We acknowledge the subjectivity inherent in this judgement.
Detection bias (blinding of outcome assessors)
In assessing the risk of bias from lack of blinding of outcome assessment, we considered who was assessing the outcome and the objectivity and subjectivity of the immunogenicity and reactogenicity outcomes.
Immunogenicity outcomes
Two trials reported immunogenicity outcomes (Diggle 2006; Middleman 2010). In both trials, outcomes were assessed via assays of serum antibody concentrations performed by laboratory staff who were unaware of the needle size group from which the serum sample originated. We therefore judged that there was a low risk of detection bias.
Reactogenicity outcomes
Four trials reported reactogenicity outcomes (Diggle 2000a; Diggle 2006; Pathak 2007; Nirupam 2008).
Pain was assessed in only one trial using both a Modified Behavioural Pain Scale (MBPS) and a visual analogue scale (VAS) (Pathak 2007). A researcher (nurse) viewed video clips of the vaccination procedure to assess infant pain response using the MBPS. As the needle hubs were colour‐coded and may have been visible on the video recordings, we considered that the researcher was not blinded. Two separate postvaccination pain assessments using the VAS were conducted by a researcher and by parents or guardians. The trial authors reported that the researcher was blinded. We judged that blinding of parents was incomplete due to the colour‐coding of the needle hubs and parental presence during the vaccination procedure. Overall, we considered the potential for detection bias for the pain outcomes reported in this trial to be uncertain, and therefore judged this domain as at unclear risk of bias.
In the same trial, a researcher assessed crying time from digital camera recordings. Although the researcher was reportedly blinded to needle size, the colour‐coded needle hubs may have been visible on the digital recordings, and we considered blinding to be incomplete. Nevertheless, we judged that procedural crying was a more objective outcome than pain and that assessment of crying time was unlikely to be influenced by incomplete blinding, and therefore considered there to be a low risk of detection bias for this outcome. We reached a similar judgement with regard to the two trials that reported persistent inconsolable crying (Diggle 2006; Nirupam 2008), as we considered that outcome assessment was unlikely to be influenced by knowledge of needle size.
The remaining reactogenicity outcomes in all trials (redness, tenderness, swelling, irritability, etc.) were assessed by the parents of trial participants. We deemed blinding of parents as inadequate or incomplete in all trials (see the above section on performance bias), and there was considerable debate within the review team about the potential for material bias arising from parental assessment of subjective reactogenicity outcomes. Some members of the review team noted that efforts were made in some trials to ensure that parents were 'as blind as possible' (e.g. in trials where parents were not informed how the different‐coloured hubs of the needles related to needle size). Furthermore, they suggested that knowledge of needle size allocation would be unlikely to influence parental assessment of outcomes such as redness, particularly in trials where a ruler was used to measure the diameter of any redness, as this would have reduced the level of subjectivity inherent in the assessment. Some review authors argued on these grounds that a low risk of bias could be assigned in relation to the assessment of some subjective reactogenicity outcomes. Other review authors disagreed and suggested that complete blinding of outcome assessors for all subjective outcomes should be ensured to justify assigning a low risk of bias. These review authors also noted that most of the trials included in the review reported binary subjective outcomes (i.e. outcome present or absent), and that there is empirical evidence from meta‐epidemiological studies illustrating that randomised trials with non‐blinded assessment of such outcomes generate substantially biased estimates of treatment effects (Hróbjartsson 2012). There is also empirical evidence that the failure to blind outcome assessors in randomised trials with subjective measurement scale outcomes results in a high risk of substantial bias (Hróbjartsson 2013). We ultimately considered that this debate within the review team reflected uncertainty over the potential for bias and agreed to assign an unclear risk of bias in the detection bias domain for all subjective reactogenicity outcomes assessed by parents. This is in accordance with recommendations in the Cochrane Handbook for Systematic Reviews of Interventions, whereby the unclear risk category indicates “either lack of information or uncertainty over the potential for bias” (Higgins 2011a).
Incomplete outcome data
Details regarding any disparities between the numbers of participants randomised and analysed in each trial can be found in the Characteristics of included studies tables. We assessed the risk of attrition bias separately for immunogenicity and reactogenicity outcomes.
Immunogenicity outcomes
In one trial, missing immunogenicity outcome data were balanced in numbers across intervention groups with similar reasons for missing data across groups, therefore we assigned a judgement of low risk of bias (Diggle 2006). In another trial, there was a notable disparity between the numbers of participants randomised (65) and the number analysed for immunogenicity (24) (Middleman 2010). Due to the magnitude of this disparity, we considered that there was uncertainty over the potential for bias, even though the missing outcome data were balanced in numbers across the two needle size groups with similar reasons for missing data across groups. We therefore judged there to be an unclear risk of attrition bias in this trial.
Reactogenicity outcomes
We judged that there was a low risk of attrition bias in all four trials that reported reactogenicity outcomes (Diggle 2000a; Diggle 2006; Pathak 2007; Nirupam 2008). In Pathak 2007, follow‐up for pain and crying outcomes was 100% complete, as these outcomes were assessed at the time of vaccination. For the remaining postvaccination reactogenicity outcomes assessed in this trial, data were missing for 35% of the randomised participants, but the missing data were balanced in numbers across the needle size groups and there were similar reasons for missing data across groups (see the Characteristics of included studies table for this trial). In Nirupam 2008, only one trial participant was lost to follow‐up. In Diggle 2000a, outcome data were missing for 8% of the randomised participants, and the numbers and reasons for missing data were balanced across groups. In Diggle 2006, reactogenicity outcome data were missing after the first, second, and third doses of the vaccine for 2%, 6%, and 10% of the randomised participants, respectively. Missing outcome data were balanced in numbers across groups. There were also similar reasons for missing data across groups, with the exception of trial withdrawals due to severe local reactions. During the trial, 11 infants experienced redness and swelling covering more than two‐thirds of the anterolateral thigh, contraindicating receipt of further whole‐cell pertussis‐containing vaccine and, therefore, necessitating withdrawal of these infants from the trial. Ten of the infants were vaccinated using the narrow, short (25 G 16 mm) needle, and one was vaccinated using the wide, long (23 G 25 mm) needle. Although these infants were withdrawn from the trial, we did not treat the data for these 11 infants as missing outcome data. We analysed and reported severe local reactions separately from other reactogenicity outcomes in the Effects of interventions section of the review.
With regard to all trials, we judged that missing outcome data were likely to be missing at random (i.e. the fact that these data were missing was probably unrelated to actual values of the missing data). The rationale for this judgement for each trial is provided in the 'Risk of bias' tables (see Characteristics of included studies table). In the review protocol, we specified that where missing outcome data could reasonably be assumed to be missing at random, we would conduct available‐case analyses of the trial data. The results of these analyses are presented in the Effects of interventions section of the review.
Selective reporting
We reached a judgement of unclear risk of bias for four trials because we did not examine the trial protocols and were therefore unable to confirm whether the trial reports contained all expected outcomes, including those that were prespecified (Diggle 2000a; Pathak 2007; Nirupam 2008; Middleman 2010). We did not examine the protocol for the remaining trial (Diggle 2006), but we were given access by the principal trial author to all relevant original trial data. We were confident that we had access to the trial results for all of the prespecified primary and secondary outcomes that were of interest in our review. We therefore judged that there was a low risk of reporting bias for this trial.
Other potential sources of bias
We considered that all trials appeared to be free of other potential sources of bias. In one trial, there was a potential source of bias related to imbalances in the ages of the trial participants who were analysed in the two groups, which resulted in differences between the groups in the dose of the vaccine administered (Middleman 2010). In this trial, participants aged less than 19 years received a 0.5 mL dose of the vaccine, whereas older participants received 1.0 mL. In the 38 mm (1.5 inch) needle group, 36% (5/14) of the participants included in the final analysis were aged less than 19 years compared with 20% (2/10) of the participants analysed in the 25 mm (1 inch) needle group. Due to the small sample size in the trial, this imbalance may have occurred by chance rather than failure of randomisation. One would anticipate lower antibody titres to be recorded in participants receiving the smaller dose of the vaccine and, therefore, the imbalance between the groups may have biased the estimate of intervention effect (the difference between the median titre levels in the groups). In the trial report, individual participant titres were reported for each trial participant, but it was unclear which titres corresponded to the individuals who received 0.5 mL of the vaccine. We obtained these details from the principal trial author and reanalysed the data excluding the individuals from each group who received 0.5 mL of the vaccine. The trial results were essentially the same (albeit with reduced power due to the exclusions). We therefore considered that a judgement of low risk of bias was appropriate for this domain.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Comparison between needles with different lengths but with the same gauge.
| 25 G 25 mm needles compared with 25 G 16 mm needles for vaccination procedures | ||||||
|
Patient or population: infants aged approximately 2 to 6 months undergoing vaccination in the anterolateral thigh with a DTwP‐Hib vaccine Intervention: 25 G 25 mm needles; injection technique ‐ skin stretched flat between thumb and forefinger and needle inserted at a 90° angle to skin (WHO injection technique) and up to the needle hub in healthy infants Comparison: 25 G 16 mm needles; injection technique ‐ same as above | ||||||
| Outcomes (1 to 7) | Probable outcome with 25 G 16 mm needles* | Probable outcome with 25 G 25 mm needles (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| 1. Incidence of diphtheria, tetanus, pertussis, Haemophilus influenzae type b (Hib) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 2a. Adequate immune response (seroprotection) against diphtheria (surrogate outcome)2 | 1000 per 1000 | 1000 per 1000 (990 to 1000)** |
RR 1.00 (0.99 to 1.01) |
312 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 2b. Adequate immune response (seroprotection) against tetanus (surrogate outcome)2 | 1000 per 1000 | 1000 per 1000 (990 to 1000)** |
RR 1.00 (0.99 to 1.01) |
390 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 2c. Adequate immune response (seroprotection) against pertussis (not measured)2 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 2d. Adequate immune response (seroprotection) against Haemophilus influenzae type b disease (surrogate outcome)2 | 804 per 1000 | 885 per 1000 (812 to 965) |
RR 1.10 (1.01 to 1.2) |
400 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 3. Pain (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 4a. Procedural crying (during and immediately after the vaccination procedure) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 4b. Persistent inconsolable crying4 | 9 per 1000 |
22 per 1000 (4 to 114) |
RR 2.49 (0.49 to 12.7) |
447 (1 study) |
⊕⊝⊝⊝ Very low5,6,7 |
‐ |
| 5. Severe local reaction8 | 40 per 1000 |
2 per 1000 (0 to 36) |
RR 0.05 (0 to 0.89) |
447 (1 study) |
⊕⊕⊕⊝ Moderate7,9 |
NNTB*** 25 (95% CI 15 to 100) |
| 6. Non‐severe local reaction10 | 560 per 1000 |
359 per 1000 (291 to 443) |
RR 0.64 (0.52 to 0.79)11 |
447 (1 study) |
⊕⊕⊕⊝ Moderate12,13 |
NNTB**** 5 (95% CI 4 to 10) |
| 7. Fever14 | 179 per 1000 |
258 per 1000 (179 to 369) |
RR 1.44 (1.01 to 2.06) |
447 (1 study) |
⊕⊝⊝⊝ Very low5,7,12 |
‐ |
| *The basis for the assumed risk (i.e. the probable outcome with 25 G 16 mm needles) and the corresponding risk (ie the probable outcome with 25 G 25 mm needles) is provided in footnote 1. **Due to bounding the upper limit of the confidence interval for the absolute effect does not match exactly the upper limit of the confidence interval for the relative effect. ***NNTB = the expected number of infants who would need to be vaccinated with the 25 mm rather than the 16 mm needle for 1 additional infant to avoid a severe local reaction event. ****NNTB = the expected number of infants who would need to be vaccinated with the 25 mm rather than the 16 mm needle for 1 additional infant to avoid a non‐severe local reaction event. CI: confidence interval; DTwP‐Hib vaccine: a combination vaccine containing diphtheria, tetanus, whole‐cell pertussis, and Haemophilus influenzae type b vaccine antigen components; RR: risk ratio; WHO: World Health Organization. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
Please see the Data collection and analysis section of the review for comprehensive details regarding the methods we used to assess the quality of evidence for outcomes included in 'Summary of findings' tables. See the Effects of interventions section of the review for a full explanation of the rationale for our judgements regarding the quality of evidence for each outcome.
1Only one trial provided data for the comparison between 25 G 25 mm and 25 G 16 mm needles (Diggle 2006). The assumed and corresponding risks for all outcomes are based on the event rates in the needle size groups in this trial. In Diggle 2006, a DTwP‐Hib vaccine and a meningitis C conjugate (MenC) vaccine were concurrently administered in the right (DTwP‐Hib) and left (MenC) anterolateral thighs of infants aged 2 months (first vaccine dose), 3 months (second dose), and 4 months (third dose). The MenC vaccine was administered in a schedule (time between vaccine doses) that is no longer recommended, and the results pertaining to the effects of needle size on the immunogenicity and reactogenicity of this vaccine are not presented in this 'Summary of findings' table or in the Abstract or Plain language summary for this Cochrane Review (the results are, however, presented in the Effects of interventions section). 2The term 'seroprotection' refers to antibody titre levels above a predefined threshold level that correlates with protection from disease (after completion of a series of 3 doses of a DTwP‐Hib vaccine). The threshold levels used in this review were: diphtheria antitoxin levels ≥ 0.01 IU/mL; tetanus antitoxin levels ≥ 0.01 IU/mL; Hib antibody titre levels ≥ 1.0 μg/mL. There is no well‐established immune correlate or surrogate of protection against pertussis. 3We downgraded the quality of evidence by one level for indirectness due to the use of a substitute (surrogate) seroprotection endpoint in place of the patient‐important outcome of interest. Although the seroprotection endpoints were reported in only one trial, thus precluding an evaluation of the consistency or inconsistency of results across trials, we did not downgrade the quality of evidence. We considered the consistency of the results of the seroprotection analyses reported in the trial and the results of the analyses of the ratios of the antibody/antitoxin geometric mean concentrations (GMCs) between the needle size groups. The GMC ratios (25 mm versus 16 mm) were: diphtheria: 1.05 (95% CI 0.85 to 1.29); tetanus: 0.97 (95% CI 0.81 to 1.17); Hib: 1.35 (95% CI 1.02 to 1.79). (NOTE: a ratio > 1.0 indicates a higher antibody level (better immune response) after vaccination with the 25 mm compared with the 16 mm needle.) 4This term refers to a persistent inconsolable crying event lasting for ≥ 4 hours. The data presented in the table relate to persistent inconsolable crying recorded at any time point (6 hours, day 1, day 2, or day 3) after concurrent administration of any dose (first, second, or third) of a DTwP‐Hib vaccine and a MenC vaccine. 5We downgraded the quality of evidence by one level due to imprecision, taking into account the width of the 95% confidence interval accompanying the effect estimate. 6We downgraded the quality of evidence by one level for indirectness. The definition of persistent inconsolable crying (≥ 4 hours' duration) used in the trial differed from the case definition specified in the protocol for our review (≥ 3 hours' duration). The reported effect size may have differed if the latter definition had been used in the trial, and we considered that this uncertainty merited downgrading the quality of evidence. 7We downgraded the quality of evidence by one level as this outcome was reported in only one trial, thus precluding any evaluation of the consistency or inconsistency of results across trials. 8'Severe local reaction' refers to redness and swelling covering more than two‐thirds of the anterolateral thigh after the first dose of a DTwP‐Hib vaccine. 9Although blinding of outcome assessment was incomplete, we did not downgrade the quality of evidence for risk of bias. We considered that the clinical severity of the reaction reduced the level of subjectivity in outcome assessment. 10'Non‐severe local reaction' refers to any redness, swelling, tenderness, or hardness (i.e. a composite outcome) at the injection site on the day after the first dose of a DTwP‐Hib vaccine. 11Similar effect sizes were observed in the trial after the second and third doses of the DTwP‐Hib vaccine (second dose RR 0.67, 95% CI 0.54 to 0.83; third dose RR 0.65, 95% CI 0.52 to 0.80). The corresponding NNTBs were 6 (95% CI 4 to 12) for the second dose and 5 (95% CI 4 to 10) for the third dose. 12We downgraded the quality of evidence by one level due to incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias. 13Although this outcome was reported in only one trial, thus precluding an evaluation of the consistency or inconsistency of results across trials, we did not downgrade the quality of evidence, taking into account the consistency of the effect sizes after the first, second, and third doses of the DTwP‐Hib vaccine (see footnote 11). 14The data presented in the table relate to fever (temperature ≥ 38 °C) experienced at any time point (6 hours, day 1, day 2, or day 3) after concurrent administration of any dose (first, second, or third) of a DTwP‐Hib vaccine and a MenC vaccine.
Summary of findings 2. Comparison between needles with different gauges but with the same length.
| 25 G 25 mm needles compared with 23 G 25 mm needles for vaccination procedures | ||||||
|
Patient or population: infants aged approximately 2 to 6 months undergoing vaccination in the anterolateral thigh with a DTwP combination vaccine Intervention: 25 G 25 mm needles; injection technique ‐ skin stretched flat between thumb and forefinger and needle inserted at a 90° angle to skin (WHO injection technique) and up to the needle hub in healthy infants Comparison: 23 G 25 mm needles; injection technique ‐ same as above | ||||||
| Outcomes (1 to 7) | Probable outcome with 23 G 25 mm needles* | Probable outcome with 25 G 25 mm needles (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| 1. Incidence of diphtheria, tetanus, pertussis, Haemophilus influenzae type b (Hib) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 2a. Adequate immune response (seroprotection) against diphtheria (surrogate outcome)2 | 1000 per 1000 | 1000 per 1000 (990 to 1000)** |
RR 1.00 (0.99 to 1.01) |
311 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 2b. Adequate immune response (seroprotection) against tetanus (surrogate outcome)2 | 1000 per 1000 | 1000 per 1000 (990 to 1000)** |
RR 1.00 (0.99 to 1.01) |
402 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 2c. Adequate immune response (seroprotection) against pertussis (not measured)2 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 2d. Adequate immune response (seroprotection) against Haemophilus influenzae type b disease (surrogate outcome)2 | 856 per 1000 | 881 per 1000 (822 to 950) |
RR 1.03 (0.96 to 1.11) |
414 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 3. Pain: 0 to 10 on MBPS 0 = no pain; 10 = worst possible pain4 | Mean net pain score 5.9 points | Mean net pain score 0.7 points higher (0.39 higher to 1.01 higher) | N/A | 320 (1 study) |
⊕⊕⊝⊝ Low5,6 | Differences between groups of less than 1 point may not be clinically relevant. |
| 4a. Procedural crying (during and immediately after the vaccination procedure) | Mean crying time 37 seconds | Mean crying time 8 seconds longer (3 longer to 13 longer) | N/A | 320 (1 study) |
⊕⊕⊕⊝ Moderate7 | ‐ |
| 4b. Persistent inconsolable crying8 | 17 per 1000 |
22 per 1000 (6 to 82) |
RR 1.31 (0.36 to 4.8) |
459 (1 study) |
⊕⊝⊝⊝ Very low7,9,10 |
‐ |
| 5. Severe local reaction11 | Estimates not available, but risk very low; see footnote 12 | See footnote 12 | 559 (2 studies)12 |
⊕⊕⊕⊕ High12 | Only 1 severe local reaction event was recorded in the 23 G 25 mm group in 1 trial. | |
| 6. Non‐severe local reaction13 | 387 per 1000 |
356 per 1000 (283 to 453) |
RR 0.92 (0.73 to 1.17)14 |
459 (1 study) |
⊕⊕⊝⊝ low9,15,16 | ‐ |
| 7. Fever | See footnote 17 |
See footnote 17 |
See footnote 17 | 561 (2 studies) |
⊕⊝⊝⊝ Very low9,15,17 |
‐ |
| *The basis for the assumed risk (i.e. the probable outcome with 23 G 25 mm needles) and the corresponding risk (i.e. the probable outcome with 25 G 25 mm needles) is provided in footnote 1. **Due to bounding the upper limit of the confidence interval for the absolute effect does not match exactly the upper limit of the confidence interval for the relative effect. CI: confidence interval; DTwP vaccine: a combination vaccine containing diphtheria, tetanus, and whole‐cell pertussis vaccine antigen components. The vaccine may be combined with other antigen components including Haemophilus influenzae type b (DTwP‐Hib vaccine) and hepatitis B (DTwP‐Hib‐Hep B vaccine); MBPS: Modified Behavioural Pain Scale; N/A: not applicable; RR: risk ratio; WHO: World Health Organization. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
Please see the Data collection and analysis section of the review for comprehensive details regarding the methods we used to assess the quality of evidence for outcomes included in 'Summary of findings' tables. See the Effects of interventions section for a full explanation of the rationale for our judgements regarding the quality of evidence for each outcome.
1Two trials contributed data to this comparison (Diggle 2006; Pathak 2007). The assumed and corresponding risks for the immune response outcomes, persistent inconsolable crying, and non‐severe local reactions are based on the event rates in the needle size groups in the Diggle 2006 trial. The entries in the table for pain and procedural crying are based on the results of the Pathak 2007 trial. The entries for severe local reactions and fever are based on the results from both trials.
In Diggle 2006, a DTwP‐Hib vaccine and a meningitis C conjugate (MenC) vaccine were concurrently administered in the right (DTwP‐Hib) and left (MenC) anterolateral thighs of infants aged 2 months (first vaccine dose), 3 months (second dose), and 4 months (third dose). The MenC vaccine was administered in a schedule (time between vaccine doses) that is no longer recommended, and the results pertaining to the effects of needle size on the immunogenicity and reactogenicity of this vaccine are not presented in this 'Summary of findings' table or in the Abstract or Plain language summary for this Cochrane Review (the results are, however, presented in the Effects of interventions section).
In Pathak 2007, the first, second, or third dose of 1) a DTwP vaccine or 2) a DTwP‐Hib vaccine or 3) a DTwP‐Hib‐Hep B vaccine was administered to infants aged up to 6 months. 2The term 'seroprotection' refers to antibody titre levels above a predefined threshold that correlates with protection from disease (after completion of a series of 3 doses of a DTwP‐Hib vaccine). The threshold levels used in this review were: diphtheria antitoxin levels ≥ 0.01 IU/mL; tetanus antitoxin levels ≥ 0.01 IU/mL; and Hib antibody titre levels ≥ 1.0 μg/mL. There is no well‐established immune correlate or surrogate of protection against pertussis. 3We downgraded the quality of evidence by one level for indirectness due to the use of a substitute (surrogate) seroprotection endpoint in place of the patient‐important outcome of interest. Although the seroprotection endpoints were reported in only one trial, thus precluding an evaluation of the consistency or inconsistency of results across trials, we did not downgrade the quality of evidence. We considered the consistency of the results of the seroprotection analyses reported in the trial and the results of the analyses of the ratios of the antibody/antitoxin geometric mean concentrations (GMCs) between the needle gauge groups. The GMC ratios (25 G versus 23 G) were: diphtheria: 0.93 (95% CI 0.76 to 1.14); tetanus: 0.96 (95% CI 0.80 to 1.15); and Hib: 1.29 (95% CI 0.98 to 1.69). (NOTE: a ratio > 1.0 indicates a higher antibody level (better immune response) after vaccination with the 25 G compared with the 23 G needle.) 4The net pain score on the MBPS = postvaccination MBPS score minus prevaccination (baseline) MBPS score. Pain was also assessed in the trial using a visual analogue scale (see footnote 6). We have highlighted the MBPS pain score in the table because this pain scale was initially developed for use with infants during medical procedures such as vaccinations. 5We downgraded the quality of evidence by one level due to uncertainty over the potential for detection bias. 6In the trial, pain was also assessed by a researcher and by parents using a visual analogue scale (VAS) (0 to 100, 0 = no pain; 100 = worst possible pain). In the 25 G group the researcher‐assessed mean pain scores were 7.3 points higher (3.6 higher to 11 higher), and the parental‐assessed mean pain scores were 1.6 points higher (4 points lower to 7 points higher) compared with the 23 G group. Differences of less than 10 points on the 100‐millimetre VAS may not be clinically important. We downgraded the quality of evidence by one level for inconsistency taking into consideration the difference between the results of the parental and researcher pain assessments using the VAS and the reporting of procedural pain in only one trial, thus precluding any evaluation of the consistency or inconsistency of results across trials. 7We downgraded the quality of evidence by one level because this outcome was reported in only one trial, thus precluding any evaluation of the consistency or inconsistency of results across trials. 8This term refers to a persistent inconsolable crying event lasting for ≥ 4 hours. The data presented in the table are based on the results of a single included trial (Diggle 2006), and relate to persistent inconsolable crying recorded at any time point (6 hours, day 1, day 2, or day 3) after concurrent administration of any dose (first, second, or third) of a DTwP‐Hib vaccine and a MenC vaccine. 9We downgraded the quality of evidence by one level for imprecision taking into account the width of the confidence interval(s) around the effect estimate(s). 10We downgraded the quality evidence by one level for indirectness as the definition of persistent inconsolable crying (≥ 4 hours' duration) used in the trial that reported this outcome differed from the definition specified in the protocol for our review (≥ 3 hours' duration). The reported effect size in the trial may have differed if the latter definition had been used in the trial, and we considered that this uncertainty merited downgrading the quality of evidence. 11'Severe local reaction' refers to redness and swelling covering more than two‐thirds of the anterolateral thigh. 12In Diggle 2006, only one severe local reaction occurred in the 23 G group (1/235) and 0 were reported in the 25 G group (0/224). In Pathak 2007, no severe local reactions were reported in either the 23 G (0/47) or the 25 G group (0/53). As there was only one severe local reaction event, our judgement about the quality of evidence was based on the absolute rather than the relative effect. The high quality rating reflects the fact that a severe local reaction did not occur in 558 out of the 559 participants analysed for reactogenicity in the needle groups in the two trials. Although blinding of outcome assessment was incomplete, we did not downgrade the quality of evidence for risk of bias. We considered that the clinical severity of the reaction reduced the level of subjectivity in outcome assessment. 13'Non‐severe local reaction' refers to any redness, swelling, tenderness, or hardness (i.e. a composite outcome) at the injection site on the day after the first dose of a DTwP‐Hib vaccine. 14Similar effect sizes were observed in Diggle 2006 after the second and third doses of the DTwP‐Hib vaccine (second dose RR 0.89, 95% CI 0.70, 1.12; third dose RR 0.84, 95% CI 0.66 to 1.06). 15We downgraded the quality of evidence by one level due to incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias. 16Although this outcome was reported in only one trial, thus precluding an evaluation of the consistency or inconsistency of results across trials, we did not downgrade the quality of evidence, taking into account the consistency of the effect sizes after the first, second, and third doses of the DTwP‐Hib vaccine (see footnote 14). 17We downgraded the quality of evidence for inconsistency taking into account the variation between the results of the two trials and our inability to definitively explain the reason(s) for this heterogeneity. In Diggle 2006, fever incidence was higher in the group of infants vaccinated with the 25 G needle (26%) versus the 23 G needle (20%) (RR 1.28, 95% CI 0.91 to 1.79). In Pathak 2007, fever incidence on the day after vaccination was lower in the group vaccinated with the 25 G needle (62%) versus the 23 G needle (78%) (RR 0.79, 95% CI 0.61 to 1.02). The confidence intervals accompanying the effect estimates in both trials did not rule out the absence of any difference between the needle size groups. The rates of fever were substantially higher in both needle gauge groups in Pathak 2007 compared with Diggle 2006. The reason for the difference between the results of the two trials is unclear, but it may be due to differences in the definitions of fever used in the two trials, in study populations, in the vaccines administered, or in the risk of bias between the trials. See footnote 1 for details of the vaccines administered in the two trials.
Summary of findings 3. Comparison between needles with different lengths and different gauges.
| 23 G 25 mm needles compared with 25 G 16 mm needles for vaccination procedures | ||||||
|
Patient or population: infants aged approximately 2 to 6 months undergoing vaccination in the anterolateral thigh with a DTwP‐Hib vaccine Intervention: 23 G 25 mm needles; injection technique ‐ skin stretched flat between thumb and forefinger and needle inserted at a 90° angle to skin (WHO injection technique) and up to the needle hub in healthy infants Comparison: 25 G 16 mm needles; injection technique ‐ same as above | ||||||
| Outcomes (1 to 7) | Probable outcome with 25 G 16 mm needles* | Probable outcome with 23 G 25 mm needles (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments |
| 1. Incidence of diphtheria, tetanus, pertussis, Haemophilus influenzae type b (Hib) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 2a. Adequate immune response (seroprotection) against diphtheria (surrogate outcome)2 | 1000 per 1000 | 1000 per 1000 (990 to 1000)** |
RR 1.00 (0.99 to 1.01) |
309 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 2b. Adequate immune response (seroprotection) against tetanus (surrogate outcome)2 | 1000 per 1000 | 1000 per 1000 (990 to 1000)** |
RR 1.00 (0.99 to 1.01) |
394 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 2c. Adequate immune response (seroprotection) against pertussis (not measured)2 | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 2d. Adequate immune response (seroprotection) against Haemophilus influenzae type b disease (surrogate outcome)2 | 804 per 1000 | 852 per 1000 (780 to 933) |
RR 1.06 (0.97 to 1.16) |
402 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| 3. Pain (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 4a. Procedural crying (during and immediately after the vaccination procedure) (not measured) | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| 4b. Persistent inconsolable crying4 | 9 per 1000 |
17 per 1000 (3 to 92) |
RR 1.9 (0.35 to 10.26) |
458 (1 study) |
⊕⊝⊝⊝ Very low5,6,7 |
‐ |
| 5. Severe local reaction8 | 40 per 1000 |
4 per 1000 (0 to 33) |
RR 0.11 (0.01 to 0.83) |
458 (1 study) |
⊕⊕⊕⊝ Moderate9,10 | NNTB*** 25 (95% CI 17 to 100) |
| 6. Non‐severe local reaction11 | 560 per 1000 |
387 per 1000 (320 to 471) |
RR 0.69 (0.57 to 0.84)12 |
458 (1 study) |
⊕⊕⊕⊝ Moderate13,14 | NNTB**** 6 (95% CI 4 to 13) |
| 7. Fever15 | 179 per 1000 |
204 per 1000 (140 to 298) |
RR 1.14 (0.78 to 1.66) |
458 (1 study) |
⊕⊝⊝⊝ Very low5,7,13 |
‐ |
| *The basis for the assumed risk (i.e. the probable outcome with 25 G 16 mm needles) and the corresponding risk (i.e. the probable outcome with 23 G 25 mm needles) is provided in footnote 1. **Due to bounding the upper limit of the confidence interval for the absolute effect does not match exactly the upper limit of the confidence interval for the relative effect. ***NNTB = the expected number of infants who would need to be vaccinated with the 25 mm rather than the 16 mm needle for 1 additional infant to avoid a severe local reaction event. ****NNTB = the expected number of infants who would need to be vaccinated with the 25 mm rather than the 16 mm needle for 1 additional infant to avoid a non‐severe local reaction event. CI: confidence interval; DTwP‐Hib vaccine: a combination vaccine containing diphtheria, tetanus, whole‐cell pertussis, and Haemophilus influenzae type b vaccine antigen components; RR: risk ratio; WHO: World Health Organization. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
Please see the Data collection and analysis section of the review for comprehensive details regarding the methods we used to assess the quality of evidence for outcomes included in 'Summary of findings' tables. See the Effects of interventions section of the review for a full explanation of the rationale for our judgements regarding the quality of evidence for each outcome.
1Two trials contributed data to this comparison (Diggle 2000a; Diggle 2006). In Diggle 2000a, the third dose of a DTwP‐Hib vaccine was administered into the anterolateral thigh of infants aged 4 months, and only non‐severe local reaction outcomes (redness, swelling, and tenderness) were reported postvaccination. In Diggle 2006, a DTwP‐Hib vaccine and a meningitis C conjugate (MenC) vaccine were concurrently administered in the right (DTwP‐Hib) and left (MenC) anterolateral thighs of infants aged 2 months (first vaccine dose), 3 months (second dose), and 4 months (third dose). The MenC vaccine was administered in a schedule (time between vaccine doses) that is no longer recommended, and the results pertaining to the effects of needle size on the immunogenicity and reactogenicity of this vaccine are not presented in this 'Summary of findings' table. As the Diggle 2006 trial reported immunogenicity and reactogenicity outcomes after all three doses of the vaccine, the assumed and corresponding risks for all outcomes presented in this table are based on the event rates in the needle size groups in this trial. 2The term 'seroprotection' refers to antibody titre levels above a predefined threshold level that correlates with protection from disease (after completion of a series of three doses of a DTwP‐Hib vaccine). The threshold levels used in this review were: diphtheria antitoxin levels ≥ 0.01 IU/mL; tetanus antitoxin levels ≥ 0.01 IU/mL; and Hib antibody titre levels ≥ 1.0 μg/mL. There is no well‐established immune correlate or surrogate of protection against pertussis. 3We downgraded the quality of evidence by one level for indirectness due to the use of a substitute (surrogate) seroprotection endpoint in place of the patient‐important outcome of interest. Although the seroprotection endpoints were reported in only one trial, thus precluding an evaluation of the consistency or inconsistency of results across trials, we did not downgrade the quality of evidence. We considered the consistency of the results of the seroprotection analyses reported in the trial and the results of the analyses of the ratios of the antibody/antitoxin geometric mean concentrations (GMCs) between the needle size groups. The GMC ratios (23 G 25 mm versus 25 G 16 mm) were: diphtheria: 1.13 (95% CI 0.91 to 1.40); tetanus: 1.01 (95% CI 0.84 to 1.22); and Hib: 1.05 (95% CI 0.78 to 1.42). (NOTE: a ratio value > 1.0 indicates a higher antibody level (better immune response) after vaccination with the 23 G 25 mm compared with the 25 G 16 mm needle.) 4This term refers to a persistent inconsolable crying event lasting for ≥ 4 hours. The data presented in the table relate to persistent inconsolable crying recorded at any time point (6 hours, day 1, day 2, or day 3) after concurrent administration of any dose (first, second, or third) of a DTwP‐Hib vaccine and a MenC vaccine. 5We downgraded the quality of evidence by one level due to imprecision taking into account the width of the 95% confidence interval accompanying the effect estimate. 6We downgraded the quality of evidence by one level for indirectness. The definition of persistent inconsolable crying (≥ 4 hours' duration) used in the trial differed from the case definition specified in the protocol for our review (≥ 3 hours' duration). The reported effect size may have differed if the latter definition had been used in the trial, and we considered that this uncertainty merited downgrading the quality of evidence. 7We downgraded the quality of evidence by one level, as this outcome was reported in only one trial, thus precluding any evaluation of the consistency or inconsistency of results across trials. 8'Severe local reaction' refers to redness and swelling covering more than two‐thirds of the anterolateral thigh after the first dose of a DTwP‐Hib vaccine. 9No severe local reactions were reported in Diggle 2000a, where all infants received the third dose of the vaccine only. Most of the severe local reactions in Diggle 2006 occurred after the first dose of the vaccine. Severe local reactions may be more likely to occur after the first vaccine dose, and as this dose was not administered in Diggle 2000a, we were unable to reach a judgement regarding the consistency or inconsistency of results across trials. We therefore downgraded the quality of evidence by one level. 10Although blinding of outcome assessment was incomplete, we did not downgrade the quality of evidence for risk of bias. We considered that the clinical severity of the reaction reduced the level of subjectivity in outcome assessment. 11'Non‐severe local reaction' refers to any redness, swelling, tenderness, or hardness (i.e. a composite outcome) at the injection site on the day after the first dose of a DTwP‐Hib vaccine. 12Similar effect sizes were observed in Diggle 2006 on the day after the second and third doses of the DTwP‐Hib vaccine (second RR 0.76, 95% CI 0.62 to 0.92; third RR 0.77, 95% CI 0.64 to 0.94). The corresponding NNTBs were 8 (95% CI 5 to 25) for the second dose and 8 (95% CI 5 to 34) for the third dose. In Diggle 2000a (n = 110), data on the day after vaccination were not available, but the incidence of any redness, swelling, or tenderness at any time point postvaccination was significantly lower in infants vaccinated with the 23 G 25 mm versus the 25 G 16 mm needle (62% with 23 G 25 mm needle versus 84% with 25 G 16 mm needle; RR 0.74, 95% CI 0.58 to 0.94). 13We downgraded the quality of evidence by one level due to incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias. 14Although this outcome was reported in only one trial, thus precluding an evaluation of the consistency or inconsistency of results across trials, we did not downgrade the quality of evidence. This decision took into account the consistency of the effect sizes after the first, second, and third doses of the DTwP‐Hib vaccine (see footnote 12). 15The data presented in the table relate to fever (temperature ≥ 38 °C) experienced at any time point (6 hours, day 1, day 2, or day 3) after concurrent administration of any dose (first, second, or third) of a DTwP‐Hib vaccine and a MenC vaccine.
1. Comparisons between needles with different lengths and the same gauge
Two trials provided data for this comparison (Diggle 2006; Middleman 2010). One of the trials compared 25 G 25 mm and 25 G 16 mm needles (Diggle 2006), and one trial compared 38 mm and 25 mm needles (Middleman 2010). We were unable to ascertain the precise gauge of the needles used in the Middleman 2010 trial, but the principal trial author confirmed that the needles had the same gauge. In Diggle 2006, a DTwP‐Hib vaccine and a MenC* vaccine were concurrently administered into the right (DTwP‐Hib vaccine) and left (MenC) anterolateral thighs of infants when they were aged two months (first vaccine dose), three months (second dose), and four months (third dose) using the WHO injection technique, with the needle inserted to its full length up to the needle hub. In Middleman 2010, the first, second, and third doses of a hepatitis B vaccine were administered into the deltoid region of the upper arm of obese adolescents aged 14 to 24 years. The skin was stretched flat before needle insertion and injections were given at a 90° angle to the deltoid muscle, leaving 2 to 3 mm of needle visible between the arm and the needle hub.
*Note: the MenC vaccination schedule used in the trial is no longer recommended. This is discussed in the Overall completeness and applicability of evidence section.
25 G 25 mm versus 25 G 16 mm needles – effects on vaccine immunogenicity
Seroprotection rates
In Diggle 2006, all infants for whom outcome data were available reached antibody titre level thresholds of protection against diphtheria in both the 25 mm (155/155) and 16 mm (157/157) needle groups. Similarly, the seroprotection rates against tetanus were 100% in both the 25 mm (199/199) and 16 mm (191/191) groups. Seroprotection rates against Hib disease were 88% (182/206) in the 25 mm group and 80% (156/194) in the 16 mm group (risk difference (RD) 8%, 95% confidence interval (CI) 1% to 15%). Seroprotection rates against MenC were 99% (188/189) in the 25 mm group and 100% (179/179) in the 16 mm group (RD ‐1%, 95% CI ‐2% to 1%).
Based on an MID in seroprotection rates of 10%, we judged the immune response to the diphtheria, tetanus, and meningitis C vaccine antigen components to be equivalent in the two needle size groups. The longer needle may result in a superior immune response to the Hib component of the combined vaccine, but the evidence is inconclusive, as the lower boundary of the CI accompanying the effect estimate is compatible with little or no difference between the needle size groups.
We judged the quality of evidence for seroprotection to be moderate, downgrading by one level for indirectness due to the use of substitute endpoints in lieu of patient‐important outcomes. Although these endpoints were reported from a single trial without independent replication of results in additional trials, we did not downgrade the quality of evidence for consistency unknown. As described in the Data collection and analysis section, we considered the consistency of the results from the antibody threshold analyses and the results (reported below) of the ratios of the antibody GMCs or GMTs between the needle size groups.
Geometric mean antibody concentrations and geometric mean antibody titres
The ratios (25 mm versus 16 mm) of the GMC of diphtheria and tetanus antibodies were 1.05 (95% CI 0.85 to 1.29) for diphtheria and 0.97 (95% CI 0.81 to 1.17) for tetanus. The GMC of Hib antibodies was higher in the longer needle group than in the shorter needle group (ratio of GMCs: 1.35, 95% CI 1.02 to 1.79). The GMT of serogroup C meningococcal glycoconjugate antibodies was also higher in the longer needle group than in the shorter needle group, although the lower boundary of the CI did not exclude the absence of any difference between the needle size groups (ratio of GMTs 1.20, 95% CI 0.92 to 1.57).
38 mm versus 25 mm needles – effects on vaccine immunogenicity
In Middleman 2010, seroprotection rates against hepatitis B were 93% (14/15) in the 38 mm group and 91% (10/11) in the 25 mm group (RD 2%, 95% CI ‐19% to 24%). Median antibody titres to hepatitis B surface antigen were higher in the 38 mm compared with the 25 mm group (345.4 mIU/mL (interquartile range (IQR) 243 to 464.2) in the 38 mm group versus 189.8 mIU/mL (IQR 143.6 to 324.7) in the 25 mm group; P = 0.03). The latter analysis did not include the two trial participants (one in each needle size group) who failed to reach antibody titre level thresholds of protection against hepatitis B.
We judged the quality of evidence for these immunogenicity outcomes to be very low, downgrading by one level for indirectness due to use of a substitute endpoint in lieu of patient‐important outcomes, one level for imprecision due to the width of the CIs around effect estimates, and one level for risk of bias taking into account the absence of allocation concealment and the disparity between the numbers of participants randomised and analysed in the trial.
25 G 25 mm versus 25 G 16 mm needles – effects on pain, crying, and other reactogenicity events
Only one trial reported data on reactogenicity outcomes, therefore the results presented below are derived from this trial (Diggle 2006).
Pain
The trial did not measure vaccination‐related procedural pain.
Crying
After any dose of the two vaccines administered in the trial, persistent inconsolable crying lasting for four or more hours was reported in 2.2% (5/224) of infants in the 25 mm group and 0.9% (2/223) of infants in the 16 mm group (risk ratio (RR) 2.49, 95% CI 0.49 to 12.7). As the event rates were low in the two needle size groups, the wide CI for the relative effect translated to a small difference in absolute effect (RD 1.3%, 95% CI ‐1% to 4%). We judged the quality of evidence for persistent inconsolable crying to be very low; our reasons for downgrading the quality of evidence are summarised below.
There was a debate within the review team about the GRADE rating for this outcome with regard to the criterion of imprecision. Some review authors considered that the CI accompanying the RD effect estimate excluded important benefit and important harm and that downgrading evidence quality for imprecision was not justified. Other review authors noted that a potential 4% absolute difference (i.e. the upper limit of the CI) for a distressing persistent crying event could potentially be deemed important to parents of infants undergoing vaccination. We decided by a consensus borderline decision to downgrade the evidence rating for this outcome by one level for imprecision. We also downgraded the quality of evidence by one level because the outcome was reported in only one trial, thus precluding any evaluation of the consistency or inconsistency of results across trials. In addition, the definition of persistent inconsolable crying used in the Diggle 2006 trial (four or more hours' duration) differed from the case definition proposed by Bonhoeffer 2004 as specified in the protocol for our review (three or more hours' duration). The impact of using the latter case definition on the effect size reported in the Diggle 2006 trial is unknown. In light of this uncertainty, we also downgraded the quality of evidence by one level for indirectness.
Severe local reaction after DTwP‐Hib vaccination
Ten infants vaccinated with the 25 G 16 mm needle experienced redness and swelling covering more than two‐thirds of the anterolateral thigh necessitating withdrawal from the trial and contraindicating further receipt of DTwP‐Hib vaccine. Nine of these infants had a severe local reaction after the first dose of the vaccine, and the remaining infant experienced the reaction after the second dose. No infants vaccinated with the 25 G 25 mm needle experienced a severe local reaction (RD after first dose ‐4%, 95% CI ‐7% to ‐1%). Based on these data, one additional infant would be prevented from experiencing a severe local reaction after the first dose of DTwP‐Hib vaccine for every 25 infants vaccinated with the longer rather than the shorter needle (number needed to treat for an additional beneficial outcome (NNTB) 25, 95% CI 15 to 100).
We rated the quality of evidence for this outcome as moderate, downgrading by one level because the results were from a single trial; we were thus unable to reach a judgement regarding the consistency or inconsistency of results across trials. We did not downgrade the evidence for risk of bias despite incomplete blinding of outcome assessment in the trial. We considered that the extent and clinical severity of these severe reactions reduced the level of subjectivity in outcome assessment.
Severe local reaction after meningitis C vaccination
No infants in either needle size group experienced a severe local reaction after MenC vaccination.
Non‐severe local reactions (composite outcome) after DTwP‐Hib vaccination
The incidence of any local reaction (composite outcome: any redness, swelling, tenderness, or hardness) on the day after vaccination was consistently lower in the 25 G 25 mm group compared with the 25 G 16 mm group:
-
after first dose: 36% (25 mm) versus 56% (16 mm):
RD ‐20% (95% CI ‐29% to ‐11%);
NNTB 5 (95% CI 4 to 10).
-
after second dose: 37% (25 mm) versus 55% (16 mm):
RD ‐18% (95% CI ‐28% to ‐9%);
NNTB 6 (95% CI 4 to 12).
-
after third dose: 37% (25 mm) versus 57% (16 mm):
RD ‐20% (95% CI ‐29% to ‐11%);
NNTB 5 (95% CI 4 to 10).
We rated the quality of evidence to be moderate for these composite outcomes, downgrading for risk of bias due to incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias. Although these results were from a single trial, we did not downgrade the quality of evidence for this reason. This decision took into account the consistency of the effect estimates after each dose of the vaccine.
Non‐severe local reactions (composite outcome) after meningitis C vaccination
Data on the incidence of any local reaction (composite outcome: any swelling, tenderness, redness, or hardness) were not available at 24 hours (day one). We have therefore presented below summary effects across all time points measured in the trial.
After the first dose of the MenC vaccine, the incidence of any local reaction was lower in the 25 mm group (41%) compared with the 16 mm group (51%), although the upper boundary of the CI accompanying the RD effect estimate was compatible with no difference between the groups (RD ‐10%, 95% CI ‐19% to 0%). The CIs accompanying the effect estimates after the second and third doses of the vaccine were compatible with both reductions and increases in the rates of local reactions after vaccination with the 25 mm compared with the 16 mm needle (second dose: RD 5%, 95% CI ‐5% to 14%; third dose: RD ‐2%, 95% CI ‐12% to 7%). The MenC vaccine was less reactogenic than the DTwP‐Hib vaccine, which was reflected in the lower event rates for local reactions in the needle size groups after each dose of the MenC vaccine compared with the event rates after each dose of the DTwP‐Hib vaccine. For example, the incidence of any local reaction across all time points in the 25 mm group after the first dose of the DTwP‐Hib vaccine was 62% compared with 41% after the first dose of the MenC vaccine.
Fever, other systemic reactions, and use of paracetamol after vaccination
The incidence of postvaccination fever at any time point after concurrent administration of any dose of DTwP‐Hib and MenC vaccines was higher in infants vaccinated using the 25 mm (26%) compared with the 16 mm (18%) needle, although the lower boundary of the CI accompanying the effect estimate was compatible with no difference between the groups (RD 8%, 95% CI 0% to 16%).
There were no statistically significant differences in the incidence of all other systemic outcomes between the needle length groups. The RD effect estimates (25 mm versus 16 mm) were: ‐1% (95% CI ‐9% to 7%) for paracetamol use; ‐3% (95% CI ‐10% to 4%) for sleepier than usual; ‐1% (95% CI ‐9% to 6%) for vomiting more than three times in 24 hours; and 0% (95% CI ‐9% to 9%) for eating less than usual.
We assigned a very low quality of evidence rating for these outcomes. We downgraded for imprecision, taking into account the width of the CIs around the effect estimates; by one level for risk of bias due to incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias; and by one level because these outcomes were reported in a single trial, thus precluding any evaluation of the consistency or inconsistency of results across trials.
Individual non‐severe local reactions (swelling, tenderness, redness, and hardness) after DTwP‐Hib vaccination
The incidence of each individual local reaction on the day after administration of the first dose of the vaccine was lower in infants vaccinated with the 25 mm needle compared with the 16 mm needle. Calculations of the NNTB indicated that the expected number of infants who would need to be vaccinated with the 25 mm rather than the 16 mm needle in order to prevent an additional local reaction at 24 hours were 13 (95% CI 7 to 100) for swelling; 12 (95% CI 7 to 50) for tenderness; 13 (95% CI 7 to 100) for redness; and 7 (95% CI 4 to 13) for hardness. After the second and third doses of the vaccine, the incidence of redness, swelling, and hardness was also significantly lower in infants vaccinated with the longer needle. The CIs accompanying the RD point estimates for tenderness were compatible with both reductions and small increases in incidence following vaccination with the 25 mm compared with the 16 mm needle (after second dose RD ‐4%, 95% CI ‐10% to 2%; after third dose RD ‐4%, 95% CI ‐11% to 3%).
Individual non‐severe local reactions (swelling, tenderness, redness, and hardness) after meningitis C vaccination
There were no statistically significant differences in the incidence of each individual local reaction outcome between the needle size groups on the day after vaccination with each dose of the MenC vaccine. The RD effect estimates (25 mm versus 16 mm) ranged from ‐6% (95% CI ‐13% to 1%) for redness after the third dose of the vaccine to 1% (95% CI ‐4% to 6%) for tenderness after the second dose.
Needle overpenetration (needle contacting bone)
The precise number of events in each needle size group was not recorded. Diggle 2006 reported that approximately 4000 injections were administered during the trial and that each of the three nurses who performed the vaccination procedures reported hitting bone "less than five times in total."
Serious adverse events after vaccination
Only one infant in the 25 G 25 mm group experienced a systemic reaction requiring overnight hospital admission after the second dose of concurrent DTwP‐Hib and MenC vaccination. No other infants participating in the trial were reported as having experienced a serious adverse event.
2. Comparisons between needles with different gauges and the same length
Comparison 2a: 25 G 25 mm versus 23 G 25 mm needles
Two trials provided data for this comparison (Diggle 2006; Pathak 2007). In both trials, 25 G 25 mm and 23 G 25 mm needles were used to administer vaccines to infants using the WHO injection technique. In Pathak 2007, the first, second, or third dose of a combination vaccine with a whole‐cell pertussis component (DTwP or DTwP‐Hib or DTwP‐Hib‐Hep B vaccine) was administered into the anterolateral thigh of infants aged approximately one to six months. The vaccines administered in Diggle 2006 are described under Comparison 1 (above).
25 G (narrow‐gauge) versus 23 G (wide‐gauge) needles ‐ effect on vaccine immunogenicity
Pathak 2007 did not measure immunogenicity outcomes, therefore the results presented below are derived from the Diggle 2006 trial.
Seroprotection rates
All infants for whom outcome data were available reached antibody titre level thresholds of protection against diphtheria in both the 25 G (157/157) and 23 G (154/154) needle groups. Similarly, the seroprotection rates against tetanus were 100% in both the 25 G (199/199) and 23 G (203/203) groups. Seroprotection rates against Hib disease were 88% (182/206) in the 25 G group and 85% (178/208) in the 23 G group (RD 3%, 95% CI ‐4% to 9%). Seroprotection rates against MenC were 99% (188/189) in the 25 G group and 100% (196/196) in the 23 G group (RD ‐1%, 95% CI ‐2% to 1%).
Based on an MID in seroprotection rates of 10%, we judged the immune response to all four vaccine antigen components (diphtheria, tetanus, Hib, and MenC) to be equivalent in the two needle gauge groups.
We judged the quality of evidence for seroprotection to be moderate, downgrading by one level for indirectness due to the use of substitute endpoints in lieu of patient‐important outcomes.
Geometric mean antibody concentrations and geometric mean antibody titres
The ratio (25 G versus 23 G) of the GMCs of diphtheria antibodies was 0.93 (95% CI 0.76 to 1.14) and of tetanus antibodies was 0.96 (95% CI 0.80 to 1.15). The GMC of Hib antibodies was higher in the 25 G than in the 23 G group, but the lower limit of the CI accompanying the effect estimate did not exclude the absence of any difference between the groups (ratio of GMCs 1.29, 95% CI 0.98 to 1.69). The ratio of the GMT of serogroup C meningococcal glycoconjugate antibodies was 0.93 (95% CI 0.72 to 1.2).
25 G (narrow‐gauge) versus 23 G (wide‐gauge) needles ‐ effects on pain, crying, and other reactogenicity events
Pain
Only one trial reported pain outcomes (Pathak 2007). The group of infants vaccinated with the 25 G (narrow‐gauge) needle had higher mean net pain scores on an MBPS than the group vaccinated with the 23 G (wide‐gauge) needle (6.6, standard deviation (SD) 1.5 with 25 G needle versus 5.9, SD 1.3 with 23 G needle; mean difference (MD) 0.70, 95% CI 0.39 to 1.01). Mean pain scores were also higher in the 25 G group compared with the 23 G group when pain was assessed by a researcher using a 100‐millimetre VAS (67.2, SD 17.5 with 25 G needle versus 59.9, SD 16 with 23 G needle; MD 7.30, 95% CI 3.63 to 10.97). However, mean pain scores were similar in the two groups when pain was assessed by the mothers or guardians of infants using the VAS (52.2, SD 24.8 with 25 G needle versus 50.6, SD 26.3 with 23 G needle; MD 1.60, 95% CI ‐4.0 to 7.2).
Based on an MID of 1 point on the 10‐point MBPS scale (or its equivalent on other scales, i.e. a 10‐millimetre difference on a 100‐millimetre VAS), all reported differences between the groups in this trial may be clinically unimportant.
We judged the quality of evidence to be low, downgrading by one level due to uncertainty over the potential for detection bias and by one level for inconsistency taking into account 1) the difference between the results of the parental and researcher pain assessments using the VAS; and 2) the reporting of vaccination‐related procedural pain in only one trial, thus precluding an assessment of the consistency or inconsistency of results across trials.
Crying
Two trials reported crying outcomes that were measured in different ways. Pathak 2007 measured procedural crying (i.e. crying during and immediately after the vaccination procedure), and Diggle 2006 measured persistent inconsolable crying lasting for four or more hours at any time point after vaccination. We rated the quality of evidence separately for each outcome, as we did not consider that an overall rating for crying was warranted given the disparities in the outcome definitions.
In Pathak 2007, the group of infants vaccinated with the 25 G (narrow‐gauge) needle had a longer mean crying time than the group vaccinated with the 23 G (wide‐gauge) needle (45.4 seconds, SD 27 with 25 G needle versus 37.4 seconds, SD 19.3 with 23 G needle; MD 8, 95% CI 2.86 to 13.14). The risk of an infant still crying at 30, 60, and 90 seconds postvaccination was also higher in the 25 G group than in the 23 G group (RR 1.43, 95% CI 1.18 to 1.73 at 30 seconds; RR 1.34, 95% CI 0.84 to 2.14 at 60 seconds; RR 30.62, 95% CI 1.85 to 507.37 at 90 seconds). We judged the quality of evidence for "procedural crying" to be moderate, downgrading by one level because the outcome was reported in only one trial, thus precluding any evaluation of the consistency or inconsistency of results across trials.
In Diggle 2006, after any dose of the two vaccines administered in the trial, persistent inconsolable crying lasting for four or more hours was reported in 2.2% (5/224) of infants in the 25 G group and in 1.7% (4/235) of infants in the 23 G group (RD 0.5%, 95% CI ‐2% to 3%). We judged the quality of evidence for persistent inconsolable crying to be very low. The rationale for our judgement was identical to that described for this outcome under Comparison 1 above.
Severe local reaction after vaccination with a whole‐cell pertussis‐containing combination vaccine
In Diggle 2006, after administration of the first dose of the vaccine, no infants vaccinated with the 25 G 25 mm needle and only one infant vaccinated with the 23 G 25 mm needle experienced redness and swelling covering more than two‐thirds of the anterolateral thigh necessitating withdrawal from the trial (RD ‐0.4%, 95% CI ‐2% to 1%). No infants in either needle gauge group experienced a severe local reaction after the second and third doses of the vaccine. In Pathak 2007, no infants in either needle gauge group were reported to have experienced a severe local reaction.
We rated the quality of evidence to be high for this outcome. We did not downgrade for imprecision, as there was only one event in the Diggle 2006 trial, and hence the CI for the relative effect translated to clinically small differences in absolute effects. We did not downgrade the evidence for risk of bias despite incomplete blinding of outcome assessment in the trials, as we considered that the extent and clinical severity of these severe reactions reduced the level of subjectivity in outcome assessment.
Severe local reaction after meningitis C vaccination
In Diggle 2006, no infants in either needle gauge group experienced a severe local reaction after MenC vaccination.
Non‐severe local reactions (composite outcome) after vaccination with a whole‐cell pertussis‐containing combination vaccine
Diggle 2006 and Pathak 2007 reported composite local reaction outcomes postvaccination, but the components of the composite differed between the trials. In Diggle 2006, any local reaction was defined as any swelling, tenderness, redness, or hardness. In Pathak 2007, any local reaction was defined as any swelling, tenderness, redness, or restriction of movement.
In Diggle 2006, the incidence of any local reaction on the day after the first, second, and third doses of a DTwP‐Hib vaccine was lower in the group vaccinated with the narrower gauge needle, but the CIs accompanying the RD effect estimates were compatible with both reductions and increases in the incidence of local reactions following vaccination with the 25 G needle compared with the 23 G needle (first dose: RD ‐3%, 95% CI ‐12% to 6%; second dose: RD ‐5%, 95% CI ‐14% to 4%; third dose RD ‐7%, 95% CI ‐16% to 2%).
In Pathak 2007, the incidence of any local reaction on the day after vaccination was also lower in the group vaccinated with the narrower gauge needle, but the effect estimate was accompanied by a wide CI (RR 0.77, 95% CI 0.32 to 1.82). As restriction of movement is not typically included as a component of composite local reaction outcome measures in vaccine clinical trials, we used the composite outcome reported in Diggle 2006 in Table 2.
We judged the quality of evidence to be low for the composite local reaction outcomes reported in Diggle 2006, downgrading for risk of bias due to incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias, and for imprecision due to the width of the CIs accompanying the effect estimates.
Non‐severe local reactions (composite outcome) after meningitis C vaccination
In Diggle 2006, data on the incidence of any local reaction (composite outcome: any swelling, tenderness, redness, or hardness) were not available at 24 hours (day one). We have therefore presented summary effects across all time points measured in the trial.
After each dose of the vaccine, the CIs accompanying the RD point estimates were compatible with both reductions and increases in the rates of local reactions following vaccination with the 25 G needle compared with the 23 G needle (first dose: RD ‐6%, 95% CI ‐15% to 3%; second dose: RD 4%, 95% CI ‐5% to 13%; third dose: RD ‐5%, 95% CI ‐14% to 5%).
Fever, other systemic reactions, and use of paracetamol after vaccination
Both trials reported the incidence of postvaccination fever (Diggle 2006; Pathak 2007). Summary effect data across all time points were not available in Pathak 2007, therefore we have presented the results for fever on day one after vaccination for this trial.
The direction of effect varied between the trials. In Diggle 2006, fever incidence at any time point after any dose of the two vaccines administered in the trial was higher in the group of infants vaccinated with the 25 G needle (26%) compared with the 23 G needle (20%) (RD 6%, 95% CI ‐2% to 13%). By contrast, in Pathak 2007, fever incidence on the day after vaccination was lower in the group vaccinated with the 25 G needle (62%) compared with the 23 G needle (78%) (RD ‐16%, 95% CI ‐34% to 1%). The CIs accompanying the effect estimates in both trials did not rule out the absence of any difference between the needle gauge groups.
The rates of fever were substantially higher in both needle size groups in the Pathak 2007 trial than in the Diggle 2006 trial. The reason for this disparity is unclear, but it may potentially be due to differences in the definitions of fever used in the trials (axillary temperature 38 °C or greater measured using a digital thermometer in Diggle 2006; axillary temperature greater than 37.8 °C measured predominantly with a mercury thermometer in Pathak 2007). The disparity may also be due to differences in the vaccines administered in the trials, differences in the characteristics of the study populations, and differences in the risk of bias between the trials.
There were no statistically significant differences between the needle size groups in the incidence of all other systemic outcomes. The RD effect estimates (25 G versus 23 G) were: ‐4% (95% CI ‐11% to 3%) in Diggle 2006 and ‐6% (95% CI ‐20% to 8%) in Pathak 2007 for paracetamol use; ‐2% (95% CI ‐9% to 5%) for sleepier than usual (Diggle 2006); ‐3% (95% CI ‐10% to 5%) for vomiting more than three times in 24 hours (Diggle 2006); and ‐6% (95% CI ‐15% to 3%) for eating less than usual (Diggle 2006).
We assigned a very low quality of evidence rating for these outcomes. We downgraded for imprecision due to the width of the CIs accompanying the effect estimates for some outcomes and for inconsistency for the outcome of fever, taking into account the variation in results between the trials and our inability to definitively explain the reasons for this disparity. For outcomes reported in a single trial, we downgraded by one level as we were unable to evaluate the consistency or inconsistency of results across trials. We also downgraded by one level for risk of bias due to incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias.
Individual non‐severe local reactions (swelling, tenderness, redness, hardness, restriction of movement) after vaccination with a combination vaccine with a whole‐cell pertussis component
Both Diggle 2006 and Pathak 2007 reported on swelling, tenderness, and redness after vaccination. Diggle 2006 also reported hardness at the injection site, and Pathak 2007 reported postvaccination restriction of movement. There were no statistically significant differences in the incidence of any of these reactions between the 25 G and 23 G needle groups on the day after vaccination with any dose of the vaccines administered in the trials. The RD effect estimates (25 G versus 23 G) in the Diggle 2006 trial ranged from ‐7% (95% CI ‐16% to 1%) for hardness after the third dose of the vaccine to 2% (95% CI ‐3% to 8%) for swelling after the first vaccine dose. In Pathak 2007, the RD effect estimates ranged from ‐13% (95% CI ‐29% to 3%) for tenderness to ‐5% (95% CI ‐17% to 7%) for redness.
Individual non‐severe local reactions (swelling, tenderness, redness, and hardness) after meningitis C vaccination
In Diggle 2006, there were no statistically significant differences in the incidence of each local reaction between the 25 G and 23 G needle groups on the day after vaccination with each dose of the MenC vaccine. The RD effect estimates (25 G versus 23 G) ranged from ‐7% (95% CI ‐14% to 0%) for redness after the third dose of the vaccine to 4% (0% to 7%) for swelling after the second dose of the vaccine.
Needle overpenetration (needle contacting bone)
Diggle 2006 did not report the precise number of events in each needle size group (see entry under this heading in Comparison 1 for additional details). Needle contact with bone was not recorded in Pathak 2007.
Serious adverse events after vaccination
In Diggle 2006, only one infant in the 25 G group experienced a systemic reaction requiring overnight hospital admission after the second dose of concurrent DTwP‐Hib and MenC vaccination. No other infants in the Diggle 2006 or Pathak 2007 trials were reported as having experienced a severe adverse event.
Comparison 2b: 24 G versus 23 G; 24 G versus 22 G; 23 G versus 22 G needles
Only one trial compared the effects of 24 G 25 mm, 23 G 25 mm, and 22 G 25 mm needles (Nirupam 2008). In this trial, the first dose of a DTwP vaccine and the first dose of a Hep B vaccine were administered concurrently into the left (DTwP vaccine) and right (Hep B vaccine) anterolateral thighs of infants aged six to 10 weeks using the WHO injection technique. We did not complete 'Summary of findings' tables for the comparisons between 24 G, 23 G, and 22 G needles, and have not provided in the sections below our rating of the quality of evidence for each individual outcome reported in the trial for each comparison. Overall, we judged the quality of the evidence to be very low for the reactogenicity outcomes reported in the trial. Event rates were low in the trial for several outcomes, and there were only 50 participants in each of the three needle size groups, hence there were wide CIs accompanying many of the effect measures necessitating downgrading evidence quality for imprecision. We also downgraded the evidence quality for incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias. In addition, we downgraded for 'consistency unknown', as all outcomes for these comparisons were reported in only a single trial, thus precluding any evaluation of the consistency of results across trials. We have presented the number of events that occurred in each needle size group or the event rates in each group for all of the reactogenicity outcomes reported in this trial. We have not presented effect sizes for the differences between the groups.
Immunogenicity and pain
The trial did not measure or report immunogenicity outcomes and vaccination‐related procedural pain.
Crying
Only one infant in the 22 G group experienced persistent inconsolable crying for more than three hours postvaccination. Persistent inconsolable crying was not reported in any infants in the 24 G and 23 G groups.
Severe local reaction after vaccination
No infants in any of the needle gauge groups were reported as having experienced severe local reactions (redness and swelling covering more than two‐thirds of the anterolateral thigh) after vaccination.
Fever, other systemic reactions, and use of paracetamol after vaccination
The incidence of postvaccination fever at any time point after concurrent vaccination with the DTwP and Hep B vaccines in the 24 G group was 24% (12/50), in the 23 G group 24.5% (12/49), and in the 22 G group 30% (15/50). Other systemic reactions were infrequently reported in the needle gauge groups. No infants in the 24 G and 22 G groups and only one infant in the 23 G group experienced vomiting postvaccination. Drowsiness postvaccination was reported in only one infant in the 24 G group and in no infants in the 23 G and 22 G groups. Refusal to feed was reported in one infant in the 22 G and 23 G groups and no infants in the 24 G group. Irritability was reported in two infants in the 22 G group and one infant in the 23 G and 24 G groups. The incidence of postvaccination paracetamol use in the 24 G group was 24% (12/50), the 23 G group 22.4% (11/49), and the 22 G group 30% (15/50).
Non‐severe local reactions after vaccination
The trial did not report composite local reaction outcomes. Individual local reaction outcomes (swelling, tenderness, and redness) were reported at six hours and on days one, two, and three after vaccination with the DTwP (left thigh) and Hep B (right thigh) vaccines. We have presented the results at day one postvaccination below.
Swelling, tenderness, and redness after DTwP vaccination
The incidence of swelling on the day after vaccination in the 24 G group was 4% (2/50), the 23 G group 6.3% (8/49), and the 22 G group 14% (7/50). The incidence of tenderness on the day after vaccination was also lower in the 24 G group (2%) compared with the 23 G (6.1%) and 22 G (12%) groups. Redness on the day after vaccination was infrequently reported in all needle gauge groups (24 G (0%), 23 G (4%), 22 G (2%)).
Swelling, tenderness, and redness after hepatitis B vaccination
The incidence of swelling on the day after vaccination in the 24 G group was 0% (0/50), the 23 G group 0% (0/49), and the 22 G group 4% (2/50). The incidence of tenderness on the day after vaccination in the needle size groups was: 0% (24 G), 8.2% (23 G), and 8% (22 G). No redness was reported in any infant in any of the needle gauge groups on the day after Hep B vaccination.
Serious adverse events after vaccination
Only one infant in the 22 G group experienced seizures requiring hospital admission. No other serious adverse events were reported.
3. Comparison between needles with different lengths and different gauges
Two trials provided data for this comparison (Diggle 2000a; Diggle 2006). Both trials compared 23 G 25 mm and 25 G 16 mm needles for administering vaccines using the WHO injection technique with the needle inserted to its full length up to the needle hub. In Diggle 2000a, the third dose of a DTwP‐Hib vaccine was administered into the anterolateral thigh of four‐month‐old infants. The vaccines administered in Diggle 2006 are described under Comparison 1 (above).
23 G 25 mm versus 25 G 16 mm needles – effects on vaccine immunogenicity
Diggle 2000a did not measure immunogenicity outcomes, therefore the results presented below are derived from the Diggle 2006 trial. The numbers of infants in each needle size group for whom immunogenicity data were available are described under Comparisons 1 and2 above.
Seroprotection rates
All infants for whom outcome data were available reached antibody titre level thresholds of protection (seroprotection) against diphtheria, tetanus, and MenC in both the 23 G 25 mm and the 25 G 16 mm groups. Seroprotection rates against Hib disease were 85% (178/208) in the 23 G 25 mm group and 80% (156/194) in the 25 G 16 mm group (RD 5%, 95% CI ‐2% to 13%).
Based on an MID in seroprotection rates of 10%, we judged the immune response to the diphtheria, tetanus, and MenC vaccine antigen components to be equivalent in the two needle size groups. The longer needle may result in a superior immune response to the Hib component of the combined vaccine, but the evidence is inconclusive, as the lower boundary of the CI accompanying the effect estimate is compatible with little or no difference between the needle size groups.
We judged the quality of evidence for seroprotection to be moderate, downgrading by one level for indirectness due to the use of substitute endpoints in lieu of patient‐important outcomes.
Geometric mean antibody concentrations and geometric mean antibody titres
The ratios (23 G 25 mm versus 25 G 16 mm) of the GMCs were: 1.13 (95% CI 0.91 to 1.40) for diphtheria antibodies; 1.01 (95% CI 0.84 to 1.22) for tetanus antibodies; and 1.05 (95% CI 0.78 to 1.42) for Hib antibodies. The GMT of serogroup C meningococcal glycoconjugate antibodies was higher in the group vaccinated with the 23 G 25 mm needle, but the lower limit of the CI accompanying the effect estimate did not exclude the absence of any difference between the needle size groups (ratio of GMTs 1.3, 95% CI 0.99 to 1.70).
23 G 25 mm versus 25 G 16 mm needles – effects on pain, crying, and other reactogenicity events
Pain
Neither of the trials measured vaccination‐related procedural pain (Diggle 2000a; Diggle 2006).
Crying
Diggle 2000a did not measure crying. In Diggle 2006, after any dose of the two vaccines administered in the trial, persistent inconsolable crying lasting for four or more hours was reported in 1.7% (4/235) of infants in the 23 G 25 mm group and in 0.9% (2/223) of infants in the 25 G 16 mm group (RR 1.9, 95% CI 0.35 to 10.26). As the event rates were low in the two needle size groups, the wide CI for the relative effect translated to a small difference in absolute effect (RD 0.8%, 95% CI ‐1% to 3%).
We judged the quality of evidence for persistent inconsolable crying to be very low. The rationale for our judgement is identical to that described for this outcome under Comparison 1 above.
Severe local reaction after DTwP‐Hib vaccination
In Diggle 2000a, no infants in either needle size group experienced a severe local reaction after the third dose of the vaccine.
In Diggle 2006, 10 infants who were vaccinated with the 25 G 16 mm needle experienced redness and swelling covering more than two‐thirds of the anterolateral thigh necessitating withdrawal from the trial and contraindicating further receipt of DTwP‐Hib vaccine. Nine of these infants had a severe reaction after the first dose of the vaccine, and the remaining infant experienced the reaction after the second dose. Only one infant vaccinated with the 23 G 25 mm needle had a severe local reaction, which occurred after the first dose of the vaccine (RD after first dose: ‐4%, 95% CI ‐6% to ‐1%). Based on these data, one additional infant would be prevented from experiencing a severe local reaction after the first dose of a DTwP‐Hib vaccine for every 25 infants vaccinated with the 23 G 25 mm rather than the 25 G 16 mm needle (NNTB 25, 95% CI 17 to 100).
We rated the quality of evidence for this outcome to be moderate. All of the severe reactions occurred in the Diggle 2006 trial, and all but one of these reactions occurred after the first dose of the vaccine. Severe local reactions may be more likely to occur after the first vaccine dose, and this dose was not administered in the Diggle 2000a trial, therefore we were unable to reach a judgement regarding the consistency or inconsistency of results across trials and downgraded the quality of evidence by one level on this basis. We did not downgrade the quality of the evidence for risk of bias despite incomplete blinding of outcome assessment in the trials. We considered that the extent and clinical severity of these severe reactions reduced the level of subjectivity in outcome assessment.
Severe local reaction after meningitis C vaccination
In Diggle 2006, no infants in either needle size group experienced a severe local reaction after MenC vaccination.
Non‐severe local reactions (composite outcome) after DTwP‐Hib vaccination
Diggle 2000a and Diggle 2006 reported composite local reaction outcomes postvaccination, but the components of the composite differed between the trials. In Diggle 2000a, any local reaction was defined as any swelling, tenderness, or redness, whereas in Diggle 2006, hardness was also included as a component of the composite.
In Diggle 2000a, data were not available for the composite outcome on the day after vaccination with the third dose of the vaccine. The incidence of any local reaction across all time points was lower in the group vaccinated with the 23 G 25 mm needle (62%) compared with the 25 G 16 mm group (84%) (RD ‐22%, 95% CI ‐38% to ‐6%; NNTB 5, 95% CI 3 to 17).
In Diggle 2006, the incidence of any local reaction (any swelling, tenderness, redness, or hardness) on the day after vaccination with each dose of the vaccine was consistently lower in the 23 G 25 mm group compared with the 25 G 16 mm group:
-
after first dose: 39% (23 G 25 mm) versus 56% (25 G 16 mm):
RD ‐17% (95% CI ‐26% to ‐8%);
NNTB 6 (95% CI 4 to 13).
-
after second dose: 41% (23 G 25 mm) versus 55% (25 G 16 mm):
RD ‐14% (95% CI ‐23% to ‐4%);
NNTB 8 (95% CI 5 to 25).
-
after third dose: 44% (23 G 25 mm) versus 57% (25 G 16 mm):
RD ‐13% (95% CI ‐22% to ‐3%);
NNTB 8 (95% CI 5 to 34).
We rated the quality of evidence to be moderate for these composite outcomes, downgrading for risk of bias due to incomplete blinding of outcome assessment and the resultant uncertainty over the potential for bias.
Non‐severe local reactions (composite outcome) after meningitis C vaccination
In Diggle 2006, data on the incidence of any local reaction (composite outcome: any swelling, tenderness, redness, or hardness) were not available at 24 hours (day one). We have therefore presented summary effects across all time points measured in the trial.
After each dose of the MenC vaccine, the CIs accompanying the RD point estimates were compatible with both reductions and increases in the rates of local reactions following vaccination with the 23 G 25 mm needle compared with the 25 G 16 mm needle (first dose: RD ‐4%, 95% CI ‐13% to 5%; second dose: RD 1%, 95% CI ‐8% to 10%; third dose: RD 2%, 95% CI ‐7% to 12%).
Fever, other systemic reactions, and use of paracetamol after vaccination
Diggle 2000a did not report these outcomes.
In Diggle 2006, the incidence of postvaccination fever at any time point after concurrent administration of any dose of DTwP‐Hib and MenC vaccines was 20% in the 23 G 25 mm group compared with 18% in the 25 G 16 mm group (RD 2%, 95% CI ‐5% to 10%).
There were no statistically significant differences between the needle size groups in the incidence of all other systemic outcomes. The RD effect estimates (23 G 25 mm versus 25 G 16 mm) were: 2% (95% CI ‐5% to 9%) for paracetamol use; ‐1% (95% CI ‐8% to 6%) for sleepier than usual; 1% (95% CI ‐6% to 8%) for vomiting more than three times in 24 hours; and 6% (95% CI ‐3% to 15%) for eating less than usual.
We assigned a very low quality of evidence rating for these outcomes. The rationale for our judgement is identical to that described for these outcomes under Comparison 1 above.
Individual non‐severe local reactions (swelling, tenderness, redness, and hardness) after the first and second doses of a DTwP‐Hib vaccine
In Diggle 2006, the incidence of each individual local reaction on the day after administration of the first dose of the vaccine was lower in infants vaccinated with the 23 G 25 mm needle compared with the 25 G 16 mm needle. Calculations of the NNTB indicate that the expected number of infants who would need to be vaccinated with the 23 G 25 mm rather than the 25 G 16 mm needle in order to prevent an additional postvaccination local reaction at 24 hours were 10 (95% CI 6 to 25) for swelling; 12 (95% CI 7 to 50) for tenderness; 12 (95% CI 6 to 50) for redness; and 8 (95% CI 5 to 20) for hardness.
Similar results for redness and hardness were observed after the second dose of the vaccine. The CIs accompanying the effect estimates for swelling and tenderness after the second vaccine dose were compatible with both reductions and increases in the rates of these reactions following vaccination with the 23 G 25 mm needle compared with the 25 G 16 mm needle (RD ‐5%, 95% CI ‐12% to 2% for swelling; RD 1%, 95% CI ‐5% to 8% for tenderness).
Swelling, tenderness, redness, and hardness after the third dose of a DTwP‐Hib vaccine
Diggle 2000a and Diggle 2006 reported on swelling, redness, and tenderness at six hours and on days one, two, and three after the third dose of a DTwP‐Hib vaccine. We performed a meta‐analysis (random effects) of the trial data for each outcome at day one postvaccination. Vaccination with a 23 G 25 mm needle was associated with a reduced incidence of swelling (RR 0.58, 95% CI 0.36 to 0.93; I2 = 54%), tenderness (RR 0.63, 95% CI 0.40 to 1.00; I2 = 0%), and redness (RR 0.61, 95% CI 0.36 to 1.01; I2 = 73%) on the day after vaccination compared with the use of a 25 G 16 mm needle. The upper limits of the CIs accompanying the effect estimates for tenderness and redness were compatible with no difference between the groups (Analysis 1.1; see Figure 3). As specified in the review protocol (Beirne 2013), we repeated meta‐analyses using a fixed‐effect model. The effect estimates obtained were similar, although the CIs were narrower (see Appendix 7).
1.1. Analysis.
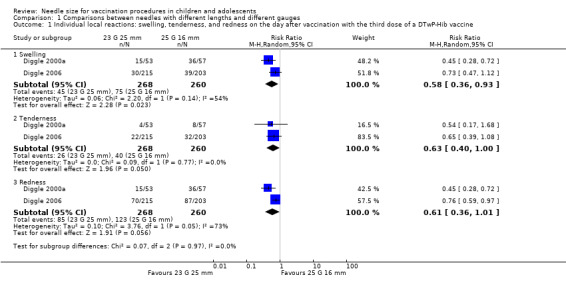
Comparison 1 Comparisons between needles with different lengths and different gauges, Outcome 1 Individual local reactions: swelling, tenderness, and redness on the day after vaccination with the third dose of a DTwP‐Hib vaccine.
3.
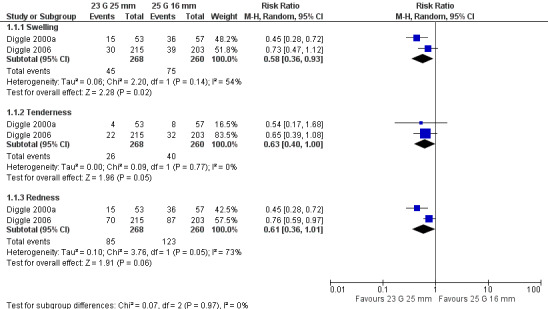
Forest plot of comparison: 3 Comparisons between needles with different lengths and different gauges, outcome: 3.1 Individual local reactions: swelling, tenderness, and redness on the day after vaccination with the third dose of a DTwP‐Hib vaccine.
Diggle 2000a did not report hardness at the injection site after vaccination. In Diggle 2006, the incidence of hardness on the day after vaccination was lower in the group vaccinated with the 23 G 25 mm needle (28%) compared with the 25 G 16 mm needle (37%) (RR 0.76, 95% CI 0.57 to 1.00). The upper limit of the CI accompanying the effect estimate was compatible with no difference between the groups.
Swelling, tenderness, redness, and hardness after meningitis C vaccination
In Diggle 2006, there were no statistically significant differences in the incidence of each individual local reaction outcome between the needle size groups on the day after vaccination with each dose of the MenC vaccine. The RD effect estimates (23 G 25 mm versus 25 G 16 mm) ranged from ‐4% (95% CI ‐9% to 1%) for hardness after the second dose of the vaccine to 3% (95% CI ‐3% to 9%) for redness after the second dose of the vaccine.
Needle overpenetration (needle contacting bone)
Diggle 2006 did not report the precise number of events in each needle size group (see entry under this heading in Comparison 1 for additional details). Diggle 2000a did not report needle contact with bone.
Serious adverse events after vaccination
In Diggle 2000a and Diggle 2006, no infants in either the 23 G 25 mm or the 25 G 16 mm group were reported as having experienced a serious adverse event.
Sensitivity analyses
Apart from repeating meta‐analyses using fixed‐effect and random‐effects models, we did not conduct any of the other sensitivity analyses prespecified in our protocol due to the small number of trials (two) included in the meta‐analyses (see Differences between protocol and review).
During the review process, we made several post hoc decisions with regard to the analysis of data that could have influenced the main findings of the review. For example, we made a post hoc decision to analyse trial data pertaining to local reactions at the 24‐hour time point, or the nearest approximation to this time point (where these data were available from trials). We consider that the time point selection was appropriate, and the rationale for this decision is explained in the Unit of analysis issues section. Nevertheless, as local reaction outcomes were reported at several separate time points (six hours, day one, day two, day three) and across all time points in some trials, we conducted sensitivity analyses to investigate if our overall findings regarding the effects of needle size on local reactions were robust to decisions about time point selection. These sensitivity analyses are reported in Appendix 7. The results of the analyses, particularly for the comparisons between needles of different lengths (25 mm versus 16 mm), illustrate that although the magnitude of the intervention effect varied depending on time point selection, the direction of effect was entirely consistent for all analyses. Furthermore, the differences in effect sizes at different time points were between small and large beneficial effects in favour of the longer needle. Our overall interpretation of the evidence (that 23 G 25 mm needles and 25 G 25 mm needles probably reduce the incidence of local reactions compared with 25 G 16 mm needles) would therefore not have materially altered according to time point selection.
We also made a post hoc decision about the selection of a value for MID in seroprotection rates (10%) between groups, and have explained the rationale for this decision in the Data collection and analysis section. We performed a sensitivity analysis using an MID of 5% (see Appendix 7), and our conclusions about the effects of needle size on DTwP‐Hib vaccine immunogenicity would not have materially altered if we had used the lower MID value.
We also performed a sensitivity analysis to investigate if our interpretation of the evidence pertaining to the effects of needle size on the immune response to the Hib component of the vaccine would have varied depending on the choice of cut‐off threshold level for seroprotection (1.0 µg/mL or greater versus 0.15 µg/mL or greater). Our overall conclusions were robust to threshold selection (see Appendix 7).
Finally, we made a post hoc decision to highlight in the 'Summary of findings' tables the results pertaining to the effects of needle size on a composite local reaction outcome rather than on the individual components of the composite. We conducted sensitivity analyses to investigate if there were disparities between the estimates of intervention effect on the composite outcome and the estimates of intervention effect on individual components of the composite (see Appendix 7). The analyses indicate that there were some variations in the magnitude of the intervention effect on individual components of the composite for all of the main comparisons made in the review. However, the direction of effect was generally consistent across individual components, particularly for the comparisons between the 25 mm and 16 mm needles, and this direction of effect was accurately reflected in the effect size for the composite outcome. We consider that the results of this analysis justify our decision to present the composite outcome in the 'Summary of findings' tables.
Discussion
Summary of main results
Our review included five trials involving 1350 randomised participants. Three of the trials (1135 participants) contributed data to the comparisons between 25 G 25 mm, 23 G 25 mm, and 25 G 16 mm needles. These trials involved infants, predominantly between the ages of two and six months, who were undergoing intramuscular vaccination procedures with combination vaccines containing DTwP antigens with or without other vaccine antigen components including Hib (DTwP‐Hib) and Hep B (DTwP‐Hib‐Hep B). A MenC conjugate vaccine was administered concurrently in one trial. The vaccines were administered in the anterolateral thigh with the skin stretched flat and the needle inserted at a 90° angle through the skin and up to the needle hub in healthy infants (WHO injection technique). We have summarised the principal findings from these trials in Table 1; Table 2; and Table 3.
There is probably little or no difference in immune response between using 25 G 25 mm, 23 G 25 mm, and 25 G 16 mm needles to administer a series of three doses of a DTwP‐Hib vaccine to infants aged two, three, and four months (moderate‐quality evidence). We identified no trials that measured the incidence of vaccine‐preventable diseases, and our conclusions regarding the likely effects of needle size on immune response are based on seroprotection rates to the diphtheria, tetanus, and Hib vaccine antigen components. No trials measured the immune response to the pertussis component of the vaccine.
Using either 25 G 25 mm or 23 G 25 mm needles to administer a DTwP‐Hib vaccine probably leads to fewer severe local reactions (extensive redness and swelling) after the first vaccine dose and fewer non‐severe local reactions (redness, swelling, tenderness, and hardness) at 24 hours after the first, second, and third vaccine doses compared with the use of a 25 G 16 mm needle (moderate‐quality evidence).
Using a wider gauge (23 G) 25 mm needle to administer a DTwP combination vaccine may slightly reduce vaccination‐related procedural pain (low‐quality evidence) and probably leads to a slight reduction in the duration of crying time immediately following vaccination (moderate‐quality evidence) compared with a narrower gauge (25 G) 25 mm needle. However, the effects are probably not large enough to be of any practical or clinical importance to patients, parents, and healthcare providers.
Use of the narrower gauge (25 G) 25 mm needle may result in a small reduction in the incidence of local reactions at 24 hours after DTwP vaccination compared with the wider gauge (23 G) 25 mm needle. The effect estimates are imprecise, and further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate (low‐quality evidence).
The comparative effects of 23 G 25 mm, 25 G 25 mm, and 25 G 16 mm needles on the incidence of fever and persistent inconsolable crying following DTwP vaccination are uncertain due to the very low quality of the evidence. Similarly, there is insufficient evidence to permit any definitive conclusions about the effects, if any, of needle size on other systemic adverse events including drowsiness, loss of appetite, and vomiting.
Only one trial compared the effects of using 23 G 25 mm, 25 G 25 mm, and 25 G 16 mm needles to administer a MenC vaccine (a vaccine that has a better reactogenicity profile than DTwP vaccines) to infants aged two months (first vaccine dose), three months (second dose), and four months (third dose). These needles probably produce a comparable immune response to the MenC vaccine (moderate‐quality evidence). However, the comparative effects of the needles on postvaccination local and systemic reactions are uncertain due to the imprecision of effect estimates. In addition, the MenC vaccination schedule (timing between vaccine doses) used in the trial is no longer recommended, and the applicability of the trial results to contemporary schedules is uncertain. We have discussed this further in the Overall completeness and applicability of evidence section.
One small trial compared the effects of using 38 mm versus 25 mm needles to administer a Hep B vaccine to obese adolescents. Another trial compared the effects of using 22 G 25 mm, 23 G 25 mm, and 24 G 25 mm needles to administer a DTwP vaccine and a Hep B vaccine to infants. The evidence from these trials was of insufficient quality and the effect estimates were insufficiently precise to allow any confident statements to be made about the comparative effects of these needle sizes on vaccine immunogenicity and reactogenicity.
Overall completeness and applicability of evidence
The external validity of the evidence presented is constrained by the small number of trials included in the review and the types of participants, interventions, and outcomes investigated in the trials.
Types of participants
Our review was confined to studies involving children (defined as people aged less than 10 years) and adolescents (defined as people aged 10 to 24 years), therefore no conclusions can be drawn about the effects of using needles of different sizes for vaccination procedures in adults. Only one trial included in the review involved obese adolescents aged 14 to 24 years, but we judged the quality of the evidence for the outcomes measured in the trial to be very low, and hence the effects of using needles of different sizes for vaccination procedures in this population group are uncertain. The remaining four trials involved infants under the age of six months, and the majority of trial participants were aged between six weeks and four months. In addition, most of the trial participants were healthy and generally of normal weight for their age, with the exception of a small proportion (14%) of the participants in one trial, Pathak 2007, who were malnourished (weight categorised as per WHO growth standards) (WHO 2006). There is a paucity of evidence regarding the effects of using needles of different sizes for vaccination procedures in children outside of these age groups, in children who are overweight or obese, and in malnourished children.
Types of interventions
The trials included in our review compared a limited range of needle lengths and gauges, and our findings cannot be extrapolated to needles of other sizes. However, the most robust evidence from our review pertains to the comparative effects of 23 G 25 mm, 25 G 25 mm, and 25 G 16 mm needles, which are the most commonly used needles sizes in clinical practice for intramuscular vaccination procedures in children and adolescents (DoH UK 2012a).
All trials included in our review involved participants undergoing intramuscular vaccination procedures. No conclusions can be drawn about the effects of using needles of different sizes for administering vaccines prescribed for delivery by subcutaneous or intradermal routes.
In the four included trials that involved infants, the vaccines were administered in the anterolateral thigh with the skin stretched flat and the needle inserted at a 90° angle to the skin surface. There is an absence of evidence from RCTs regarding the effects of using needles of different sizes for administering vaccines intramuscularly using other injection techniques, such as the bunching technique commonly used in the USA, and for angles of needle insertion deviating from the perpendicular. Furthermore, no trials evaluated the effects of using needles of different sizes for administering vaccines to children in the deltoid muscle of the upper arm.
In four trials, combination vaccines were administered to trial participants containing DTwP with or without other vaccine antigen components including Hib (DTwP‐Hib) and Hep B (DTwP‐Hib‐Hep B). The whole‐cell pertussis component of such combination vaccines has been shown to be primarily, but not exclusively, responsible for local and systemic reactions occurring after vaccination. This has been demonstrated in studies that have compared reactogenicity event rates after DTwP versus DT vaccination and DTwP versus DTaP (acellular pertussis) vaccination (WHO 2014a). In addition, combinations of DTwP and Hep B with or without Hib do not result in adverse reactions that materially exceed in either frequency or severity those seen with the same DTwP vaccine given alone (Decker 2013). The results of our review are therefore likely to be most applicable in populations and settings where combination DTwP vaccines are used either alone or in combination with other vaccine antigens. In this context, the review findings are of most relevance to low‐ and middle‐income countries, where combination vaccines with whole‐cell pertussis components are the vaccine of choice (Vashishtha 2013; WHO 2013c).
Due to their lower reactogenicity, acellular pertussis vaccines (aP) containing purified, inactivated components of Bordetella pertussis cells have replaced whole‐cell pertussis vaccines in many high‐income countries (Sheridan 2012).* For example, in the UK a combination vaccine (DTaP‐IPV‐Hib‐Hep B) with an aP component is currently (2017 to 2018) included in the routine vaccination schedule (PHE 2017). In the USA, whole‐cell pertussis vaccines are no longer available, and only aP vaccines are currently licensed for use (FDA 2018). Due to the different reactogenicity profiles of vaccines with acellular and whole‐cell pertussis components, it cannot be assumed that similar effect sizes to those reported in our review, particularly in relation to severe and non‐severe local reactions, would be observed in populations and settings where aP‐containing vaccines are predominantly or exclusively used.
Only one trial included in our review involved the administration of a MenC vaccine (Diggle 2006). The vaccine schedule used in the trial (first, second, and third doses of a Men C conjugate vaccine at ages two, three, and four months) is no longer recommended in the UK, where the trial was conducted. It has been replaced by a schedule whereby a Hib‐MenC vaccine dose is given at 12 months of age, and a MenACWY conjugate vaccine dose is given at 14 years of age (PHE 2016). Furthermore, in Diggle 2006, approximately 75% of the infants received Meningitec vaccine. This particular vaccine is not recommended for use in the new vaccination schedule. The applicability of the results presented in our review to the current MenC vaccination schedule is therefore uncertain.**
*NOTE: in July 2014, the WHO issued revised guidance on the choice of pertussis vaccines based on evidence indicating that "licenced aP vaccines have lower initial efficacy, faster waning of immunity and possibly a reduced impact on transmission relative to currently internationally available wP vaccines" (WHO 2014b). The WHO has advised that "countries currently using aP vaccine may continue using this vaccine but should consider the need for additional booster doses and strategies to prevent early childhood mortality in case of resurgence of pertussis" (WHO 2014b). This guidance was reinforced in a subsequent WHO position paper on pertussis vaccines (WHO 2015b).
**NOTE: the current WHO immunisation schedule is that children aged two to 11 months require two doses of a monovalent MenC conjugate vaccine administered at an interval of at least two months, and a booster about one year after (WHO 2017).
Types of outcomes
Our review was compromised by the absence of evidence pertaining to the primary outcomes of interest. We identified no trials that investigated the effect of needle size on the incidence of vaccine‐preventable diseases such as diphtheria, tetanus, pertussis, Haemophilus influenzae type b disease, and hepatitis B. In many countries, vaccination programmes have substantially reduced the incidence of these diseases, thus trials addressing clinical outcomes would require impractically large sample sizes and duration of follow‐up. However, in some settings and for some diseases (e.g. tetanus and Hib) trials reporting clinical outcomes could theoretically be expected. The lack of clinical disease endpoints weakens the conclusions that can be drawn from the small number of trials included in the review (two) that evaluated the effects of needle size on vaccine immunogenicity.
We identified no trials that evaluated the effect of needle length on vaccination‐related procedural pain and procedural crying (crying during and immediately after the vaccination procedure), and we located only one trial that evaluated the effect of needle gauge on these outcomes. The paucity of trials reporting these outcomes is particularly surprising given that the pain associated with vaccination procedures is widely recognised as a source of anxiety and distress for people receiving the vaccine injections, their parents/guardians, and the healthcare providers who administer the injections (Schechter 2007).
How do the results of our review fit into the context of current practice?
National Immunization Technical Advisory Groups in several countries including the UK, Ireland, the USA, and Australia recommend the use of needles 25 mm in length for intramuscular vaccination procedures in the anterolateral thigh of infants under the age of 12 months (see Appendix 1). This recommendation is supported by our review, which found that both severe and non‐severe local reactions probably occur less frequently when 25 mm rather than 16 mm needles are used to administer DTwP combination vaccines to infants. National Immunization Technical Advisory Groups have also made recommendations regarding appropriate needle sizes for vaccination procedures in preterm infants, children over the age of 12 months, and adolescents. We are unable to comment on these recommendations, as the trials included in our review either did not include these population groups, or the quality of evidence was too low to allow any judgements to be made.
Quality of the evidence
Our review included five trials involving 1350 randomised participants. Overall, the quality of evidence was compromised, and our confidence in effect estimates limited, by the use of substitute (surrogate) immunogenicity outcomes in trials, incomplete blinding of outcome assessment, small number of trials, heterogeneity of needle sizes compared in these trials, heterogeneity of vaccines administered, and heterogeneity of outcomes (including definitions of outcomes, methods used to measure outcomes, and the time points for outcome measurement), which generally precluded meta‐analysis. We formally rated the quality of the evidence for outcomes included in 'Summary of findings' tables using the GRADE system. The ratings ranged from very low for some outcomes such as fever and persistent inconsolable crying (indicating considerable uncertainty regarding the estimates of effect) to moderate for other outcomes such as non‐severe local reactions after DTwP‐Hib vaccination (indicating that further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate). We have provided below a synopsis of our decisions with regard to each of the five factors that we considered when determining the quality of the evidence.
1. Risk of bias: in four of the trials included in our review we judged that there was a low risk of selection bias because robust sequence generation and allocation concealment methods were used. In one trial, allocation was not concealed, and we assigned a high risk of bias for this domain (Middleman 2010).
In all five trials, complete blinding of trial participants, their parents or guardians, and personnel was not ensured because colour‐coded needles were used in all trials, and the lengths of the needles differed in three of the trials. Nevertheless, we judged that there was a low risk of performance bias in all trials, and have outlined the rationale for this decision in detail in the Risk of bias in included studies section.
In reaching judgements regarding the risks of detection bias arising from lack of blinding of outcome assessors in trials, we took into account the people assessing the outcome and the subjectivity and objectivity of the outcomes. In the two trials that assessed immunogenicity outcomes using laboratory assays, we considered that there was a low risk of detection bias. Only one trial assessed the effects of needle gauge on vaccination‐related procedural pain and procedural crying. Due to the colour‐coding of the needle hubs, we considered that there was uncertainty over the potential for detection bias for the subjective outcome of pain, and we assigned an unclear risk of bias judgement. However, we considered that crying time (assessed from digital camera recordings) was a more objective outcome, and we considered that there was a low risk of detection bias for this outcome. We reached a similar decision regarding the risk of detection bias in relation to the trials that assessed persistent inconsolable crying.
Other local and systemic reactogenicity outcomes (including redness, swelling, tenderness, and fever) were assessed by parents in a number of trials. Due to lack of blinding or incomplete blinding of parents in all trials that measured these outcomes, we considered that the potential for bias was uncertain, and we assigned an unclear risk of bias judgement. We considered that this uncertainty merited downgrading the quality of evidence for these outcomes.
We deemed attrition bias problematic in only one trial where there was a notable disparity between the number of participants randomised and the number analysed (Middleman 2010). We judged that there was a low risk of attrition bias in the remaining trials because there was either minimal loss to follow‐up, or missing outcome data were balanced in numbers across comparison groups with similar reasons for missing data across groups.
We considered that there was a low risk of reporting bias in one trial where we had access to all of the original trial data and we were confident that we had access to the trial results for all of the prespecified primary and secondary outcomes that were of interest to our review. For the remaining trials, we considered that there was an unclear risk of reporting bias because we did not examine the trial protocols, and it was unclear if the trial reports contained all expected outcomes.
2. Indirectness: in the two trials that evaluated the effect of needle size on vaccine immunogenicity, substitute outcomes were used (seroprotection rates, geometric mean antibody concentrations, or geometric mean antibody titres) instead of patient‐important outcomes (incidence of vaccine‐preventable diseases). We therefore downgraded the quality of evidence by one level.
3. Imprecision: most of the outcomes reported in our review were dichotomous, and decisions on downgrading due to imprecision were based on a consideration of the 95% CIs around relative risk effect estimates. We downgraded for imprecision if the 95% CIs included both 'no effect' and the suggested GRADE threshold for downgrading of a relative risk reduction (RRR) or relative risk increase (RRI) greater than 25%. The only exception to this occurred when event rates were very low for some outcomes and where the 95% CIs around relative effect estimates were very wide, but 95% CIs around absolute effect estimates were narrow. For example, when comparing severe local reactions after vaccination with 23 G 25 mm versus 25 G 25 mm needles, we considered the results from two trials that involved 559 participants. Only one severe local reaction occurred in people vaccinated with the 23 G needle, and none occurred in people vaccinated with the 25 G needle. We did not downgrade the quality of evidence pertaining to this outcome for imprecision.
The results pertaining to most of the outcomes reported in the only trial that compared 22 G, 23 G, and 24 G needles were imprecise (Nirupam 2008). There were only 50 participants in each group in the trial, and event rates were low for most outcomes, inevitably resulting in wide CIs around effect estimates and downgrading for imprecision. Consequently, we were unable to make any confident statements regarding the comparative effects of these needle gauges. Similarly, the only trial that compared 38 mm and 25 mm needles had a small sample size, and the width of the CI around the effect estimate for the seroprotection endpoint in the trial merited downgrading the quality of evidence for imprecision (Middleman 2010).
4. Inconsistency: assessment of the degree of heterogeneity of results across trials was compromised by the small number of studies included in our review. Some outcomes such as procedural pain and crying were reported in only one trial, thus precluding an evaluation of the consistency or inconsistency of results across trials. As explained in the Data collection and analysis section, we decreased the strength of evidence grade for specific outcomes where there was only a single trial evidence base. An exception to this occurred in relation to local reaction outcomes (redness, swelling, tenderness, and hardness) in the Diggle 2006 trial, which were recorded and analysed after each of the three doses of the two vaccines administered in the trial. In this instance, we were able to examine the consistency of effect estimates after each vaccine dose. In the same trial, two substitute (surrogate) measures of immune response were used, and we were able to compare the results for these two substitute endpoints when evaluating the immunogenicity evidence base for consistency.
5. Publication bias: due to the small number of trials included in the review, we did not conduct a formal assessment of the likelihood of publication bias via the construction and examination of funnel plots. Our search for relevant trials was comprehensive and included searches of electronic databases and clinical trial registries, and handsearching of reference lists of relevant narrative and systematic reviews, evidence‐based clinical practice guidelines, key textbooks, and conference proceedings. In addition, we did not impose any language restrictions on our searches. Although we cannot entirely exclude the possibility of publication bias, we consider that there is a low likelihood that we have overlooked relevant trials. We decided not to downgrade the quality of evidence for publication bias.
Potential biases in the review process
The strengths of the review process include the comprehensive search of electronic databases, reference lists, conference proceedings, and clinical trial registries to identify published and unpublished trials. The robustness of the review process was further enhanced by the use of three review authors (PB, SH, SC) to independently extract data from included trials and assess the risk of bias. In addition, all relevant data entered into Review Manager 5 by PB was re‐checked independently by SH and SC (RevMan 2014). We also contacted the authors of all included trials to obtain missing data and for data clarification.
Our review has some potential limitations, particularly in relation to a number of post hoc decisions about the analysis of data from included trials and the presentation of results in 'Summary of findings' tables that we did not prespecify in the review protocol (Beirne 2013). We have discussed the potential biases inherent in these post hoc decisions below. We have also discussed some of the challenges we encountered during the review process when assessing risk of bias and when applying the GRADE system for rating the quality of evidence.
Use of composite local reaction endpoints in 'Summary of findings' tables
In the three 'Summary of findings' tables constructed for this review we made a post hoc decision to present composite local reaction outcomes (any redness, swelling, tenderness, or hardness) rather than individual local reaction outcomes, and the 'illustrative comparative risks' for these composites were based on the data obtained in the largest trial included in the review (Diggle 2006). The use of composite outcomes in clinical trials can be misleading if it is erroneously assumed that the intervention effect reported in the trial applies equally to all components of the composite, whereas in reality the intervention effect may vary across components of the composite that have different clinical importance (Cordoba 2010). Of particular concern is a scenario where the intervention effect on the composite outcome and the intervention effect on one or more individual components of the composite are in different directions. This issue did not arise in relation to the main comparisons made in our review between 25 mm and 16 mm needles (25 G 25 mm versus 25 G 16 mm; 23 G 25 mm versus 25 G 16 mm in infants undergoing DTwP vaccination). We conducted sensitivity analyses to investigate the consistency of the estimates of intervention effects on the composite outcome and on the individual components of the composite in the Diggle 2006 trial. Although there were some variations in the magnitude of intervention effects across individual local reaction outcomes, the direction of effect was remarkably consistent across all outcomes, and this direction of effect was accurately reflected in the composite outcome measure. On balance, we consider that including a composite outcome rather than individual local reaction outcomes in the 'Summary of findings' tables was the most efficient way to summarise the effects of needle size on vaccine reactogenicity without overwhelming the reader with information regarding intervention effects on individual local reaction outcomes.
Time point selection
Another potential source of bias in the review was the selection of the time point at which we analysed trial data relating to the effects of needle size on the composite local reaction outcome in the Diggle 2006 trial. We made a post hoc decision to use 24 hours postvaccination or the nearest approximation to this time point. As explained in the Unit of analysis issues section, we consider that this time point was the most appropriate at which to analyse trial data. Nevertheless, this decision could have biased the inferences drawn in our review regarding the effect of needle size on local reactions if there were systematic differences in the results of analyses conducted at different time points (e.g. six hours, day one, day two, and day three postvaccination). We explored the potential for bias by performing sensitivity analyses to investigate how robust our conclusions were to decisions about time point selection. These sensitivity analyses indicated that although our estimates of the magnitude of the intervention effect would have varied depending on time point selection (particularly for the comparisons between 25 mm and 16 mm needles), the direction of effect was overwhelmingly consistent across time points, and our overall conclusions would not have materially altered according to time point selection. We consider that the results of these sensitivity analyses further enhance confidence in the findings of our review.
Selection of minimum important differences
Our assessment of the effects of needle size on vaccine immunogenicity was based on differences in seroprotection rates between groups, and we used as an MID an RD of 10%. For pain, we selected an MID between groups of 1 point on the 10‐point MBPS scale or its equivalent on other scales. Although these MIDs were not specified a priori in the review protocol, their selection was not arbitrary, and they were identified using approaches suggested by the Agency for Healthcare Research and Quality (AHRQ) (AHRQ 2012). Although there is obviously potential for debate over the precise values of these MIDs, we consider that the methods used to identify them were appropriate, and we have described these methods in the Data collection and analysis section.
Use of the Cochrane 'Risk of bias' tool and application of the GRADE system
Our experiences during the review process were commensurate with those highlighted in a number of studies that have demonstrated poor reliability between review authors when applying the Cochrane 'Risk of bias' tool to assess the risk of bias in RCTs (Hartling 2009; Hartling 2013; Armijo‐Olivo 2014). In our review, the three review authors (PB, SH, SC) who independently assessed the risk of bias of included trials were in full agreement only for the domains of sequence generation and allocation concealment. There were some disagreements between review team members with regard to all other domains, and thus the final judgements presented in this review represent the outcome of a process of discussion and consensus. In order to ensure complete transparency in our review, we have explained the rationale for our decisions both in the Risk of bias in included studies section and in the 'Risk of bias' table for each trial in the Characteristics of included studies table. We have provided more detail in these sections than would typically be presented in many systematic reviews, as we consider that this additional detail will assist readers in understanding the reasons for our judgements.
Similarly, all of the decisions reached in our review regarding the quality of evidence ratings using the GRADE system represent the outcome of a process of discussion and consensus between review team members (PB, SH, SC) rather than unanimous agreement. To ensure transparency in our review, and in accordance with best practice, we have provided detailed footnotes in all 'Summary of findings' tables to explain our reasons for downgrading or not downgrading the quality of the evidence.
We departed from formal guidance on using the GRADE system with regard to the criterion of 'inconsistency' for outcomes reported in a single trial. Exponents of the GRADE approach have suggested that reviewers should not downgrade for inconsistency when there is only one study. For example, Schünemann 2011c has stated that "... The obvious answer is that there is no inconsistency as there is only one study and therefore one would not downgrade the quality of evidence on the basis of only one available study. Some of the reasons for this are that the one study can be a very large study that evaluated all sorts of different populations and provides information and estimates of effect that we can be very confident in. Another reason is to ask where there would be a threshold level for judging inconsistency, whether it be the availability of two studies or three studies. Once again, the bottom line is there is no downgrading for inconsistency when there is only one study." For some of the outcomes reported in single trials in our review we did not deem it appropriate to follow this guidance, as we did not consider that some of the relevant trials could be deemed "very large [studies] that evaluated all sorts of different populations and provides information and estimates of effect that we can be very confident in." We therefore adopted an approach suggested by the AHRQ of downgrading evidence for outcomes with a single trial evidence base on the grounds that consistency is unknown (a full explanation is provided in the Data collection and analysis section) (Owens 2009; Owens 2010). The only exceptions to this approach occurred where 1) trials reported two substitute immunogenicity endpoints, and we were able to consider the consistency of the effects sizes for the two endpoints; and 2) trials reported on local reactions after the first, second, and third doses of a vaccine, and we were able to consider the consistency of the effect sizes after each dose of the vaccine. Although there is obviously potential for debate over the methods we adopted, we consider that our approach has resulted in GRADE ratings that accurately reflect our confidence in the estimates of effect.
Agreements and disagreements with other studies or reviews
During the literature searches, we located six studies evaluating the effects of needle size on vaccine immunogenicity and reactogenicity that did not meet our selection criteria. We also identified a number of systematic reviews and several studies that made recommendations regarding the optimal needle length for intramuscular vaccination procedures based on ultrasound, magnetic resonance imaging (MRI), or computed tomography (CT) measurements of the thickness of subcutaneous fat and muscle at recommended vaccination sites in children and adolescents. We have summarised the results of relevant studies under three separate headings below. The evidence presented has not been systematically reviewed, and a formal assessment of the risk of bias for each study has not been conducted.
1. Agreements and disagreements with the results of excluded studies that evaluated the effects of needle size on vaccine immunogenicity and reactogenicity
We excluded two studies that involved adults (Shaw 1989; Johnsen 1995), one study where insufficient outcome data were available (Ozdemir 2012), one randomised trial where different injection techniques were used in the comparison groups (Cook 2005), and one randomised trial where different syringes were used in the comparison groups (Fateh 2014). We have not discussed the results from these studies because either the study populations were not children or adolescents, the outcome data were considered incomplete, or because the comparison groups intentionally differed with regard to factors other than needle size that could influence vaccine immunogenicity and reactogenicity. The principal results from the remaining two excluded studies are summarised below (Ipp 1989; Jackson 2008).
The participants in Ipp 1989 were healthy children aged 18 months attending private paediatric practices in Toronto, Canada for routine well‐child care. The children were sequentially assigned to receive a combined diphtheria, tetanus, whole‐cell pertussis, and inactivated polio vaccine (DTwP‐IPV) via intramuscular injection into the deltoid muscle with a 25 G 16 mm needle (74 children) or into the anterolateral thigh with either a 25 G 25 mm needle (67 children) or a 25 G 16 mm needle (64 children). The injection technique used in the study was not specified. All local and systemic postvaccination reactions were recorded by parents at four and 24 hours postvaccination. As the injection sites differed in the comparison groups (deltoid in one group, anterolateral thigh in two groups), we only considered the results relating to the vaccination procedures performed in the anterolateral thigh.
The study authors reported no statistically significant differences in fever, prolonged or unusual crying, and pain between the groups vaccinated with 25 G 25 mm and 25 G 16 mm needles. However, these outcomes were defined and measured in different ways to the trials included in our review, and the study results cannot be directly compared with our review findings. The incidence of redness at the vaccination site was lower in children vaccinated with the 25 G 25 mm needle (13.4%) compared with the 25 G 16 mm needle (40.6%) (P < 0.001). The incidence of swelling was also lower in children vaccinated with the longer needle (13.4% with 25 G 25 mm needle versus 32.8% with 25 G 16 mm needle; P = 0.015). These results are commensurate with our review findings of a reduced risk of local reactions associated with the use of 25 G 25 mm needles compared with 25 G 16 mm needles.
The participants in Jackson 2008 were children (median age 4.5 years) participating in a prospective postlicensure assessment of the safety of the fifth consecutive dose of a combined diphtheria, tetanus, and acellular pertussis vaccine (DTaP). The children were vaccinated at a large health maintenance organisation in Washington state by clinical staff "according to their usual practice." The vaccination technique(s) used to administer the vaccine were not described. Of the 1315 children included in the final analysis, 1174 were vaccinated in the upper arm (381 with a 16 mm needle and 793 with a 25 mm needle), and 141 were vaccinated in the thigh (49 with a 16 mm needle and 92 with a 25 mm needle). The gauges of the 16 mm needles were 23 G (0.2% of needles), 25 G (49%), and 26 G (49%) (gauge unknown for 1.8% of the needles). The gauges of the 25 mm needles were 22 G (0.3%), 23 G (8%), and 25 G (91%) (gauge unknown for 0.7% of needles).
All local and systemic postvaccination reactions were recorded by parents on the evening of vaccination and for the next six days. The primary data analysis conducted by the study authors involved comparisons of the risks of each outcome (across all time points) between 25 mm and 16 mm needles, irrespective of needle gauge. In a multivariate analysis, RRs were adjusted for age, gender, and body mass index.
In the multivariate analyses of children vaccinated in the arm, use of a 25 mm needle versus a 16 mm needle was associated with a reduced incidence of any redness, swelling, and pain at the injection site (redness: 65% with 25 mm needle versus 76% with 16 mm needle; RR 0.87, 95% CI 0.81 to 0.94; swelling: 55% with 25 mm needle versus 67% with 16 mm needle; RR 0.83, 95% CI 0.75 to 0.91; pain: 53% with 25 mm needle versus 61% with 16 mm needle; RR 0.86, 95% CI 0.77 to 0.96).
For vaccinations administered in the thigh, use of the longer needle was associated with a reduced incidence of redness and swelling. However, the CIs accompanying all multivariate adjusted RR effect estimates were compatible with both reductions and increases in the incidence of reactions following vaccination with the 25 mm compared with the 16 mm needle (redness: 40% with 25 mm needle versus 49% with 16 mm needle; RR 0.81, 95% CI 0.56 to 1.16; swelling: 28% with 25 mm needle versus 35% with 16 mm needle; RR 0.90, 0.53 to 1.51; pain: 48% with 25 mm needle versus 49% with 16 mm needle; RR 1.03, 95% CI 0.72 to 1.47). There were also no statistically significant differences in the incidence of fever between needle size groups at either vaccination site (arm or thigh).
Direct comparison between the results of this study and the findings of our review is complicated by several factors including differences in the types of populations, interventions, and outcomes (including outcome definitions). Nevertheless, the overall results pertaining to the effect of needle length on redness and swelling at the injection site were consistent with the findings of our review: the incidence of these reactions was lower in children vaccinated with a 25 mm rather than a 16 mm needle.
2. Agreements and disagreements with the findings of other systematic reviews
Davenport 2003 conducted a systematic review to determine the effect of needle size on the incidence of local reactions following immunisation. Two studies met the selection criteria for the review (Ipp 1989; Diggle 2000a). Diggle 2000a compared 23 G 25 mm versus 25 G 16 mm needles, and Ipp 1989 compared 25 G 25 mm versus 25 G 16 mm needles for administering DTwP combination vaccines into the anterolateral thigh.
Davenport 2003 only extracted data from the two studies for the outcomes of redness and swelling at 24 hours postvaccination and reported that "no other outcomes of local reaction measured in the studies were used, as the data was not comparable" (Davenport 2003). A fixed‐effect meta‐analysis was conducted to pool the results of the two studies. The pooled relative risks (16 mm versus 25 mm) at 24 hours were 2.52 (95% CI 1.70 to 3.72) for redness and 2.31 (95% CI 1.55 to 3.43) for swelling. Davenport 2003 concluded that "use of the 25 mm needle to administer vaccines in infants is favoured by both the individual studies and the meta‐analysis as being significantly less likely to produce redness and swelling within 24 hours."
There are several differences between this review and ours. First, our review is more up‐to‐date and includes additional studies that were not published at the time the Davenport 2003 review was conducted. Second, Davenport 2003 included one study that we excluded from our review because the participants were not randomly allocated to the comparison groups (the results of this study are summarised under heading 1. above) (Ipp 1989). Third, Davenport 2003 combined the results of the two studies despite the differences in the gauges of the longer needles used in the studies (25 G 25 mm needles were used in Ipp 1989; 23 G 25 mm needles were used in Diggle 2000a). In our review, we only combined the results of trials where the lengths and gauges of the needles compared in each trial were the same.
Despite these differences, the overall conclusion reached by Davenport 2003 is compatible with the findings of our review that use of a 25 mm needle for administering childhood vaccines in the anterolateral thigh reduces the risk of local reactions compared with the use of a 16 mm needle.
Taddio 2009b conducted a systematic review to determine the effectiveness of physical interventions and injection techniques for reducing pain during vaccine injection in children and adolescents. The review included randomised and quasi‐randomised trials involving people up to 18 years of age who were undergoing immunisation with a vaccine that required injection in any setting and where pain or distress was measured within five minutes of the vaccination procedure using validated techniques. A total of 19 trials were identified that met the selection criteria. However, no trials were identified that evaluated the effects of needle size on pain. A more recent review of the evidence on interventions for the management of vaccination‐related pain also identified this research gap regarding the effect of needle size on pain and fear (Noel 2015). This is commensurate with the findings of our review that there is a dearth of research evaluating the effects of needle size on the pain experienced during and immediately after vaccination procedures; we found only one trial that investigated the effect of needle gauge on vaccination‐related procedural pain. This paucity of research is equally applicable to vaccination procedures performed on adults. In a systematic review of measures for reducing injection pain during adult immunisation, Hogan 2010 reported that there were no studies evaluating the effect of needle length or needle gauge on acute pain from vaccine injections.
3. Agreements and disagreements with the results of studies measuring the thickness of subcutaneous tissue and muscle at vaccination sites
Several researchers have conducted imaging studies using ultrasound or MRI and CT scans to measure the thickness of subcutaneous fat and muscle at recommended vaccination sites in children and adolescents. These measurements have been used to make recommendations regarding the optimal needle length for administering vaccines intramuscularly. The recommendations emerging from these studies are typically based on the estimated length of needle that is considered sufficient, when used with a particular vaccination technique and with a specific angle of needle insertion, to penetrate the full thickness of subcutaneous tissues and enter muscle but without overpenetrating the muscle thereby risking needle contact with the underlying bone.
In order to compare and contrast the findings of our review with the results of these studies, we examined only the results and recommendations from imaging studies pertaining to populations and vaccination procedures that were similar to those involved in the trials included in our review. In this context, we excluded one study involving neonates (Lo 1992), and one study involving adults (Poland 1997), because no trials included in our review involved these age groups. We also excluded one study that involved early adolescents aged 11 years (mean body mass index percentile 58th) to 15 years (median body mass index percentile 62nd) (Koster 2009). We deemed the participants in this study as not sufficiently similar to the participants in the only trial included in our review that involved obese adolescents aged 14 to 24 years (Middleman 2010).
We identified six studies where imaging methods were used to measure the thickness of subcutaneous tissue and muscle in the anterolateral thigh of infants aged between two and six months (Hick 1989; Chugh 1993; Groswasser 1997; Cook 2002; Lippert 2008; Nakayama 2016). These studies also included recommendations regarding appropriate needle length for vaccination procedures using an injection technique with the needle inserted at a 90° angle to the skin. We have summarised details of the measurements obtained in these studies in Appendix 8. We have also presented below a synopsis of the main recommendations emerging from the studies and have highlighted possible reasons for disparities between the recommendations made in different studies. In addition, we have outlined the reasons why caution should be exercised when comparing the findings of the trials included in our review with the results of these imaging studies.
Hick 1989 conducted ultrasound skin‐to‐muscle measurements and skin‐to‐bone measurements of the anterolateral thigh of 24 infant girls and boys aged four months (16 weeks) at the Mayo Clinic in the USA. Based on these measurements, it was estimated that the muscle would not have been penetrated (and therefore that the vaccine would not have been deposited intramuscularly) in 25% of the study participants if a 16 mm needle was inserted at a 90° angle to the longitudinal axis of the leg. In another study in the USA, Lippert 2008 reviewed CT and MRI scans of 250 infants who had attended a large children's hospital in the midwestern US. Only 12 of the CT and MRI scans reviewed were from infants aged one year or under. Lippert 2008 recommended a 22 or 25 mm needle for intramuscular injections in the anterolateral thigh of infants (see Appendix 8).
In contrast, in a study conducted in India, Chugh 1993 estimated from ultrasound measurements that use of 15 mm needles inserted at a 90° angle would have reached the anterolateral thigh muscle layer in 100% of the study participants aged six to 12 weeks (52 infants); 97% of participants aged 13 to 18 weeks (58 infants); and 100% of participants aged 19 to 24 weeks. The study authors advocated the use of 15 mm needles with the WHO injection technique for vaccination procedures in these age groups and estimated that there was a significant risk of striking bone if a 25 mm needle was used. Similarly, based on ultrasound measurements of the thigh of 40 infants with a median age of 12 weeks in a children's hospital in Belgium, Groswasser 1997 reported that using the WHO injection technique "should allow perfect intramuscular vaccine delivery using 16 mm needles." Groswasser 1997 also cautioned that using 25 mm needles with the WHO technique "could present a real danger of damaging neurovascular structures and bone." In a study conducted in Australia, Cook 2002 performed ultrasound measurements of the anterolateral thigh of 45 infants aged two to six months and concluded that a 16 mm needle used with the WHO injection technique should be sufficient to penetrate muscle and ensure intramuscular vaccine delivery. Similar recommendations were made by Nakayama 2016 in a study involving ultrasound thigh measurements of 154 infants aged two to six months attending three paediatric hospitals in Japan. Based on the measurements, the researchers estimated that a 16 mm needle inserted at a 90° angle would have penetrated to the muscle in all age groups, while a 25 mm needle would have reached bone in infants aged two months (see Appendix 8).
There are several possible explanations for the disparities between some of the recommendations emerging from these imaging studies. First, there may have been differences in the weight of the study participants conducted in different countries. Details of the weights of infants in the two studies conducted in the USA were not reported, but it is possible that they had better nutritional status and higher mean weights than the participants in the studies conducted in India, Belgium, and Japan. Second, the results of these studies may be sensitive to the methods used to obtain the measurements. For example, the angle and positioning of the ultrasound transducer on the anterolateral thigh vaccination site differed in the studies conducted by Hick 1989 and Groswasser 1997, which may explain some of the disparity between the measurements obtained in these studies (see Appendix 8). In addition, pressure from the ultrasound transducer may compress the tissue during measurement, thereby impacting on the readings obtained. This may partially explain the differences in measurements obtained in studies where ultrasound was used compared with non‐contact measurements obtained via CT and MRI scans in the study conducted by Lippert 2008. The results of the Lippert 2008 study were also subject to measurement error inherent in the computer software used to measure the thickness of subcutaneous tissue and muscle from CT and MRI scans. The measurement error was ± one pixel, and the spacing of the pixels was not a standard size for all participants. These potential sources of measurement error in studies are another reason why caution should be exercised when interpreting the results.
Comparison between results of imaging studies and the findings of our review
The results of the two imaging studies conducted in the USA support the contention that a 16 mm needle may not be sufficiently long to consistently penetrate the muscle of the anterolateral thigh of infants under the age of six months undergoing vaccination procedures (Hick 1989; Lippert 2008). This may explain the findings from the two trials included in our review of a decreased rate of local reactions associated with the use of a 25 mm compared with a 16 mm needle for vaccination procedures (Diggle 2000a; Diggle 2006). Use of the shorter needle may result in the deposition of vaccine constituents, including adjuvants, subcutaneously rather than intramuscularly, thereby resulting in an increased rate of local reactions. However, as details of the weights of the study populations in Hick 1989 and Lippert 2008 were not available, we are unable to determine if the infants included in these studies are truly comparable to the participants in Diggle 2000a and Diggle 2006.
The weights of the infants in the Diggle 2000a and Diggle 2006 studies were higher than the weights of infants in the imaging studies conducted in India and Belgium. The warnings by Chugh 1993 and Groswasser 1997 of a significant risk of a 25 mm needle overpenetrating muscle and striking bone may therefore not be applicable to the study populations in Diggle 2000a and Diggle 2006. This is supported by the fact that needle contact with bone was a rare occurrence in Diggle 2006 (see Effects of interventions).
Comparison between the findings of our review and the results of these imaging studies is further complicated by the fact that the study participants may not be representative of populations undergoing vaccination procedures. Only two studies involved infants attending for vaccinations (Chugh 1993; Cook 2002). In the remaining studies, the participants were infants attending a "well child clinic", Hick 1989, and infants attending children's hospitals (Groswasser 1997; Lippert 2008; Nakayama 2016). The hospital participants in these studies may have had systematically different characteristics to healthy infants in the general population, thereby compromising the external validity of the study results.
Finally, it should be noted that the ability of these imaging studies to inform clinical practice is limited because images of the subcutaneous tissue and muscle were not taken while needles of different sizes were actually inserted to confirm the subcutaneous or intramuscular location of the needle tips or their proximity to bone. Such studies can therefore only provide indirect evidence regarding appropriate needle length for intramuscular vaccination procedures. The only reliable way to investigate the effects of using needles of different lengths on vaccine immunogenicity and reactogenicity is to conduct RCTs where actual vaccination procedures are performed and clinically relevant outcomes are measured. In this context, the results of the trials included in our review provide direct evidence that should be used to inform clinical decisions regarding the choice of appropriate needle sizes for vaccination procedures.
Authors' conclusions
Implications for practice.
Our review findings are most applicable to healthy infants between the ages of approximately two and six months undergoing intramuscular vaccination in the anterolateral thigh with combined vaccines containing diphtheria, tetanus, and whole‐cell pertussis antigens (DTwP vaccines) using an injection technique (WHO technique) where the skin is stretched flat and the needle is inserted at a 90° angle through the skin and up to the needle hub:
using either a 25 G 25 mm or a 23 G 25 mm needle for the vaccination procedure probably leads to fewer severe and non‐severe postvaccination local reactions while achieving a comparable immune response to 25 G 16 mm needles (moderate‐quality evidence);
using a wider gauge 23 G 25 mm needle may slightly reduce the pain associated with the vaccination procedure (low‐quality evidence) and probably leads to a slight reduction in the duration of crying time immediately following vaccination (moderate‐quality evidence) compared with a narrower gauge 25 G 25 mm needle. The estimated effects are probably not large enough to be of any practical importance to patients, parents, and healthcare providers;
the narrower gauge 25 G 25 mm needle may result in a small reduction in the incidence of local reactions compared with the 23 G 25 mm needle. We are unable to make confident statements about the precise magnitude of any reduction as the trial estimates are imprecise (low‐quality evidence);
we do not have sufficient evidence to determine if there are any differences between 25 G 25 mm, 23 G 25 mm, and 25 G 16 mm needles in the incidence of systemic adverse events following vaccination including fever, persistent inconsolable crying, drowsiness, loss of appetite, and vomiting.
The main findings of our review were derived from a small number of trials that evaluated the effects of using needles of different sizes to administer combination vaccines with a whole‐cell pertussis (wP) component. The review findings are therefore likely to be most relevant in low‐ and middle‐income countries, where wP vaccines are predominantly used. These vaccines have a different reactogenicity profile and cause more local and systemic reactions than vaccines with an acellular pertussis (aP) component that are used in most high‐income countries. It cannot be assumed that similar results to those reported in our review, particularly in relation to the effects of needle size on local reactions, would be observed in populations and settings where aP vaccines are predominantly or exclusively used.
Implications for research.
Our review included only a small number of randomised controlled trials (RCTs) that evaluated the effects of a limited range of needle sizes for administering vaccines to a restricted number of populations (predominantly infants between the ages of two and six months). As such, our review has identified several areas where additional RCTs are required to inform healthcare decisions regarding the choice of appropriate needle sizes for vaccination procedures in children and adolescents. In formulating our research recommendations, we have considered the types of interventions and populations and the types of outcomes that should be considered in future trials. We have also included some recommendations regarding trial reporting.
Types of populations and interventions
As highlighted in the Background section of this review, there are inconsistencies in the recommendations made by National Immunization Technical Advisory Groups (NITAGs) in different countries regarding the sizes of needles that should be used when administering vaccines to children and adolescents. These variations are indicative of uncertainty regarding the optimal needle sizes that should be used for vaccination procedures in people of specific ages or body masses. Randomised controlled trials are required to address these uncertainties, and we consider that the current recommendations made by NITAGs can act as a useful template to inform the interventions and populations that should be considered in future trials.
For intramuscular vaccination procedures in the anterolateral thigh of infants under the age of 12 months, several NITAGs recommend the use of 25 mm needles with needle gauges ranging from 22 G to 25 G. The trials included in our review provide some evidence that needle gauge may affect vaccination‐related procedural pain and the incidence of local reactions. Additional RCTs are warranted to evaluate the effects of 22 G, 23 G, 24 G, and 25 G 25 mm needles on pain outcomes and other reactogenicity events.
For intramuscular vaccination procedures in the anterolateral thigh of toddlers aged between approximately 12 months and three years, some NITAGs recommend the use of 25 to 32 mm needles with gauges ranging from 22 G to 25 G. Trials should be conducted to identify the optimal needle length and gauge for vaccination procedures in the anterolateral thigh in this population group.
For intramuscular vaccination procedures in the deltoid area of older children and adolescents, NITAG recommendations regarding needle length vary from 16 mm to 25 mm with needle gauges ranging from 22 G to 25 G. Trials should be conducted to identify the optimal needle length and gauge for vaccination procedures in this population group.
Only one trial included in our review involved an obese population. Obesity increases the subcutaneous tissue thickness, and overweight and obese children and adolescents receiving intramuscular injections may require longer needles to ensure that the vaccine is administered into muscle. Given the rising levels of obesity in many countries, trials are required to evaluate the effects of using needles of different sizes for vaccination procedures in overweight and obese individuals.
In all of the trials included in our review, the intramuscular vaccination procedures involved stretching the skin flat before needle insertion. We did not identify any trials where the bunching technique was used to administer vaccines intramuscularly (see Background for a description of this technique). Randomised controlled trials comparing the effects of using needles of different sizes with this vaccination technique in various age groups are warranted. Longer needles are likely to be required to reach the muscle with the bunching rather than the stretching technique, and this should be taken into account by researchers when deciding on the needle sizes to compare in trials.
Our review identified no RCTs that evaluated the effects of using needles of different sizes for administering vaccines intended for delivery via intradermal and subcutaneous routes. For intradermal vaccine administration, needles 10 mm to 20 mm in length with gauge sizes varying from 25 G to 27 G have been recommended. For subcutaneous vaccinations, needles 16 mm to 25 mm in length with gauge sizes ranging from 23 G to 26 G have been recommended. Trials to identify the optimal needle length and gauge for vaccines administered intradermally and subcutaneously are warranted.
The effects of only two aspects of needle geometry (length and gauge) were investigated in the trials included in our review. Trials should be conducted to evaluate the effects, particularly on vaccination‐related procedural pain, of other needle characteristics including needle bevel (e.g. three‐bevel versus five‐bevel needle), needle coating (e.g. silicone versus no silicone), and needle composition (e.g. stainless steel versus chrome nickel steel).
The vaccines used in future trials should be those routinely administered as part of national immunisation schedules. Several of the trials included in our review involved the administration of combination vaccines with reactogenic wP vaccine antigen components; trials involving vaccines with different reactogenicity profiles are required (e.g. acellular pertussis vaccines).
Types of outcomes
The aim of any vaccination procedure should be to attain the maximum immunity with the least possible harm. Trials evaluating the effects of needle size for vaccination procedures should therefore ideally measure both immunogenicity and reactogenicity outcomes.
Immunogenicity outcomes
We identified no trials that reported on the incidence of vaccine‐preventable diseases. Although these endpoints could not reasonably be expected in trials conducted in countries with low disease incidence, in some settings and for some diseases (e.g. tetanus, Haemophilus influenzae type b (Hib)) clinical disease endpoints could potentially be used in trials. Where the use of clinical immunogenicity endpoints is neither practical nor feasible, two substitute immunogenicity outcomes should be used: 1) seroprotection (i.e. the proportion of vaccine recipients who responded in a prescribed manner by reaching predefined threshold levels of protection against disease); and 2) geometric mean antibody concentrations or geometric mean antibody titres. We have summarised generally accepted thresholds of vaccine‐induced correlates and surrogates of protection for selected vaccines in Appendix 3.
Reactogenicity outcomes
We identified only one trial that evaluated the effect of needle size on vaccination‐related procedural pain and procedural crying (crying with immediate onset after vaccination). Future trials should use pain assessment tools with established validity and reliability to measure the pain response to vaccination procedures using needles of differing lengths and gauges. We have listed several of these tools in Appendix 2.
Some of the trials included in our review used different definitions of reactogenicity events, and some adverse events (e.g. fever, swelling, and tenderness) were measured in different ways in different trials. Future trials should use standardised case definitions for adverse events following immunisation (AEFI) to ensure comparability of results across clinical trials and to facilitate meta‐analysis of results from different trials. We suggest that researchers should adopt the case definitions for AEFIs developed by the Brighton Collaboration. Researchers should also consult the detailed guidelines accompanying each case definition, which are designed to facilitate the standardised collection, analysis, and presentation of information about adverse events following immunisation (Brighton Collaboration 2014; see also Beigel 2007; Gidudu 2008; Bonhoeffer 2009).
Trial reporting
Some adverse events (e.g. needle contact with bone) were not reported in trial publications, and we were unable to determine definitively if such events did not occur, or if such events may have occurred but were simply not recorded and reported. We recommend that trial authors should explicitly mention in trial publications any adverse events that did not occur during the trial.
Our review identified several other deficiencies in trial reporting, most notably in relation to the details provided regarding the needles, vaccines, and the vaccination procedures used in trials. At a minimum, we would suggest that trial reports should provide details on:
-
types of needles, including:
needle length and gauge;
colour‐coding of the needle hubs;
needle composition (e.g. surgical‐grade stainless steel, chrome nickel steel);
needle coating (e.g. silicone);
needle bevel (e.g. three‐bevel needle, five‐bevel needle);
needle hub (e.g. luer lock plastic hub, luer lock aluminium hub).
-
types of vaccines, including:
type and formulation of vaccine administered, including details of the biological characteristics of the vaccine (e.g. live attenuated or inactivated component vaccine, pH, and osmolality of the vaccine) and the composition of the vaccine (e.g. presence or absence of adjuvant);
brand name of vaccine, manufacturer details, and vaccine batch number;
volume of vaccine administered.
-
vaccination technique, including:
details of the personnel who administered the vaccination and any training provided;
vaccination site;
position of infant during vaccination procedure;
bunching or stretching of skin and underlying tissues before needle insertion;
angle of needle insertion;
depth of needle insertion (e.g. needle inserted to full depth (i.e. to the needle hub), 2 mm of needle exposed between the skin and needle hub).
What's new
| Date | Event | Description |
|---|---|---|
| 2 February 2018 | New citation required but conclusions have not changed | The updated search identified no new trials that met the selection criteria for the review. |
| 2 February 2018 | New search has been performed | We have updated this review to include the results of a new search on 24 October 2017. |
Acknowledgements
We would like to thank the following people for their invaluable assistance in helping us to complete our review:
Jane Hayes, former Trials Search Co‐ordinator with the Pain, Palliative Care and Supportive Care (PaPaS) Group, for her assistance in devising the search strategy.
Jo Abbott, Information Specialist with the PaPaS group for running the searches and for assisting with our queries in relation to the searching process.
Jessica Thomas, former Managing Editor of the PaPaS group, for her advice, support, and encouragement during the title registration process.
Yvonne Roy, former Assistant Managing Editor of the PaPaS group, for her assistance during the early stages of the review process.
Anna Erskine, Managing Editor, and Christopher Eccleston, Co‐ordinating Editor, of the PaPaS group, for patiently guiding us through the entire review process.
Kerry Harding, Managing Editor of the PaPaS group, for her invaluable support during the review update.
Mairead McIntyre, Elizabeth Royle, and Manal Kassab for their constructive comments on the draft review protocol.
Sheena Derry, Manal Kassab, Derek Ansel, and Vinutha P for their feedback and constructive comments on the draft of the final review. Emma Fisher for her invaluable comments on the updated review.
Garret Cahill, Interlibary Loans in the Boole Library, University College Cork, for his assistance in locating several papers.
A special word of thanks is due to the authors of all trials included or considered for inclusion in our review. All authors were more than generous with their time and went to considerable lengths to furnish detailed responses to our requests for information. Our sincere thanks to: Linda Diggle, Harish Pemde, Nilay Nirupam, Bhavneet Bharti, Amy Middleman, Roberta Anding, and Fuat Canpolat.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Needle size recommendations for administering vaccines via the intramuscular route made by National Immunization Technical Advisory Groups (NITAGs) in 4 countries
| Needle size recommendations made by NITAGs in 4 countries for administering intramuscular vaccine injections at preferred injection sites in children and adolescents | ||||
| Country/NITAG | Age or size of vaccine recipient |
Preferred injection site |
Needle size | |
| Gauge | Length | |||
| UK/JCVI (DoH UK 2012a) |
Preterm or very small infants | A‐L thigh | NS | 16 mm |
| Infants < 1 year | A‐L thigh | 23 G or 25 G | 25 mm | |
| Older children and adults | Deltoid | 23 G or 25 G | 25 mm | |
| Ireland/NIAC (NIAC 2016) |
Infants < 2.5/3 kg | A‐L thigh | NS | 16 mm |
| Birth to < 12 months | A‐L thigh | 23 G to 25 G | 25 mm | |
| 12 to < 36 months | A‐L thigh or deltoid | 23 G to 25 G | 25 mm | |
| 3 years and older1 | Deltoid | 23 G to 25 G | 25 mm | |
| US/ACIP2 (Kroger 2017) |
Neonates (first 28 days of life) | A‐L thigh | 22 G to 25 G | 16 mm3 |
| Infants 1 to 12 months | A‐L thigh | 22 G to 25 G | 25 mm | |
| Toddlers 1 to 2 years | A‐L thigh | 22 G to 25 G | 25 to 32 mm | |
| Children 3 to 18 years | Deltoid | 22 G to 25 G | 16 mm3 to 25 mm | |
| Australia/ATAGI (ATAGI 2016) |
Preterm babies (< 37 weeks' gestation) up to age 2 months; or very small infants | A‐L thigh | 23 G or 25 G4 | 16 mm |
| Infants < 12 months | A‐L thigh | 23 G or 25 G4 | 25 mm | |
| Children ≥ 12 months, adolescents, and adults5 | Deltoid | 23 G or 25 G4 | 25 mm | |
| ACIP: Advisory Committee on Immunization Practices; ATAGI: Australian Technical Advisory Group on Immunisations; JCVI: Joint Committee on Vaccines and Immunisation; NIAC: National Immunisation Advisory Committee. A‐L: anterolateral; NS: gauge not explicitly specified. 1A 40 mm needle is recommended in women > 90 kg and men > 118 kg (gauge not specified). 2The guidance states that "The needle gauge for intramuscular injection is 22‐25 gauge. A decision on needle length and site of injection must be made for each person on the basis of the size of the muscle, the thickness of adipose tissue at the injection site, the volume of the material to be administered, injection technique, and the depth below the muscle surface into which the material is to be injected" (Kroger 2017). 316 mm is deemed to be adequate if the skin is stretched tightly and subcutaneous tissues are not bunched. 4If using a narrow 25 G needle for an intramuscular vaccination, it is recommended that the vaccine is injected slowly over a count of 5 seconds to avoid injection pain and muscle trauma (ATAGI 2016). 5A 23 G or 25 G 38 mm needle is recommended for a very large or obese person. The precise reasons for some of the disparities in needle size recommendations between different countries are unclear. One contributory factor may be the use of different research evidence to inform the recommendations (see Appendix 9). | ||||
Appendix 2. Pain assessment tools with established validity and reliability
For the purposes of this review, we will use the same definition of "established validity and reliability" specified in the protocol for a Cochrane Review of psychological interventions for needle‐related procedural pain (Uman 2005), namely "prior publication in at least one scientific paper from a peer‐reviewed journal." These validated and reliable pain assessment tools will include, but may not be limited to, the tools recommended by the Brighton Collaboration for assessing immunisation site pain (Gidudu 2012), the tools listed in the Royal College of Nursing's clinical practice guideline on the recognition and assessment of pain in children (RCN 2009), and the tools specified in the protocols for Cochrane Reviews that have evaluated the effects of interventions for needle‐related procedural pain or procedural pain (Lander 2002; Uman 2005; Pillai Riddell 2006; Harrison 2010; Kassab 2010; Hogan 2012). The names of these tools are provided in the tables below.
| Table A: Pain assessment tools recommended by the Brighton Collaboration for assessment of acute and delayed pain following immunisation (Gidudu 2012) | ||
| Age | Assessment methods for acute pain following immunisation: assessor and tool | Assessment methods for delayed pain following immunisation: assessor and tool |
| Pre‐verbal child | ||
| ≤ 18 months | Clinician: MBPS Parent: NRS |
Parent: NRS (for pre‐verbal children ≤ 3 years) |
| > 18 months | Clinician: FLACC Parent: NRS |
|
| Verbal child | ||
| ≥ 3 to 6 years | Child: Poker Chip | Child: Poker Chip |
| ≥ 4 years | Child: FPS‐R | Child: FPS‐R |
| ≥ 9 years | Child: NRS | Child: NRS |
| FLACC: Face Legs Activity Crying Consolability scale (Merkel 1997); FPS‐R: Faces Pain Scale Revised (Hicks 2001); MBPS: Modified Behavioural Pain Scale (Taddio 1995); NRS: Numerical Rating Scale (Miró 2009; von Baeyer 2009; Bailey 2010); Poker Chip: Poker Chip tool (Hester 1979). NOTE: the references cited above are those specified by the Brighton Collaboration (Gidudu 2012). | ||
The following tables summarise pain scales described by the Royal College of Nursing as valid and reliable tools for assessing pain intensity in neonates and non‐verbal children with cognitive impairment (Table B) and infants and verbal children without cognitive impairments (Table C) (RCN 2009). References for all scales mentioned in the tables are provided in RCN 2009.
| Table B: Pain scales for assessing pain intensity in neonates and non‐verbal children with cognitive impairment | |||
| Pain scales for neonates | Pain scales for non‐verbal children with cognitive impairment | ||
| Tool name | Features | Tool name | Features |
| COMFORT | OR; T; PM | Face, Legs, Activity, Cry, Consolability (FLACC) | OR |
| CRIES | OR; T; PM | Paediatric Pain Profile (PPP) | OR |
| Neonatal Facial Coding System (NFCS) | OR; T | Non‐Communicating Children's Pain Checklist ‐ Revised (NCCPC‐R) | OR |
| Nepean NICU Pain Assessment Tool (NNICUPAT) | OR; T | NCCPC‐PV (Non‐Communicating Children's Pain Checklist ‐ Post‐operative Version) | OR |
| Neonatal Infant Pain Scale (NIPS) | OR; T | ‐ | |
| Objective Pain Scale (OPS) | OR; T; PM | ||
| Pain Assessment Tool (PAT) | OR; T; PM | ||
| Premature Infant Pain Profile (PIPP) | OR; T; PM | ||
| OR: observer rated; PM: tool includes physiological measures; T: requires training. | |||
| Table C: Pain scales for infants and verbal children | |
| Tool name | Features |
| Alder Hey Triage Pain Scale (AHTPS) | OR; T |
| Cardiac Analgesic Assessment Tool (CAAT) | OR; T |
| Chedoke‐McMaster Paediatric Pain Management Sheet | OR; T; SR |
| Colour Analogue Scale | T; SR |
| Children’s Hospital of Eastern Ontario Pain Scale (CHEOPS) | OR |
| COMFORT | OR; T; PM |
| Derbyshire Children’s Hospital Pain Tool (DCHPT) | OR; T |
| FACES scale (Wong‐Baker) | OR; T |
| FACES scale (a six‐graded faces scale by Tree Takarn) | SR; T |
| Faces Pain Scale (FPS; by Bieri) | SR; T |
| Face, Legs, Arms, Cry, Consolability (FLACC) | OR; T |
| Nursing Assessment of Pain Intensity (NAPI; a modification of CHEOPS) | OR; T |
| OUCHER | SR; T |
| Poker Chip Tool | SR; T |
| Post‐operative Pain Score (POPS) | OR; T |
| Pain Rating Scale | OR; T |
| Sheffield Children’s Hospital Facial Expression Scale | SR; T |
| Toddler Preschool Post‐operative Pain Scale (TPPPS) | OR; T |
| University of Wisconsin Pain Scale | OR; T |
| Visual Analogue Scale (self rated) | SR; T |
| Visual Analogue Scale (observer rated) | OR; T |
| Verbal Rating Scale | SR; T |
| Word Descriptor Scale | SR; T |
| Word Graphic Rating Scale | SR; T |
| OR: observer rated; PM: tool includes physiological measures; SR: self report tool; T: requires training. | |
Tools not mentioned in Tables A to C (above) but that are cited in protocols for Cochrane Reviews that have evaluated the effects of interventions for needle‐related procedural pain or procedural pain include the following:
Douleur Aiguë du Noveau‐né pain scale (DAN) (cited in protocol by Hogan 2012);
Riley Infant Pain Scale (RIPS); Infant Body Coding System (IBCS); E'chelle Bouleur Inconfort Nouveau‐Ne' (EDIN) (cited in protocol by Kassab 2010);
Baby Facial Action Coding System; Maximally Discriminative Facial Movement Coding System; Children's and Infants postoperative Pain Scale; Clinical Scoring System; Modified Postoperative Comfort Score; Liverpool Infant Distress Scale; Neonatal Pain, Agitation and Sedation Scale (N‐PASS); Scale for Use in Newborns (SUN); Pain Assessment in Neonates Scale (PAIN); Bernese Pain Scale (cited in protocol by Pillai Riddell 2006).
References for all of these tools are provided in the protocols by Pillai Riddell 2006; Kassab 2010; and Hogan 2012.
Appendix 3. Quantitative correlates and surrogates of protection after vaccination
| Table A: Thresholds of vaccine‐induced correlates and surrogates of protection for selected vaccines (adapted from Plotkin 2010; Thakur 2012) | ||||||
| Vaccine | Test | Threshold of protection | Serum IgG | Mucosal IgG | Mucosal IgA | T cells |
| Diphtheria | Toxin neutralisation | 0.01 to 0.1 IU/mL | ++ | |||
| Hepatitis A | ELISA | 10 mIU/mL | ++ | |||
| Hepatitis B | ELISA | 10 mIU/mL | ++ | |||
| Hib polysaccharides | ELISA | 1 μg/mL | ++ | + | ||
| Hib conjugate | ELISA | 0.15 μg/mL | ++ | ++ | ||
| Hib polysaccharides | ELISA | 1.0 μg/mL | ++ | ++ | ||
| Japanese encephalitis | Neutralisation | 1:10 titre | ++ | |||
| Measles | Microneutralisation | 120 to 200 mIU/mL | ++ | +(CD8+) | ||
| Meningococcal | Bactericidal | 1/4 (human complement) 1/8 (rabbit complement) |
++ | + | ||
| Pertussis | ELISA (toxin) | 5 units | ||||
| Pneumococcus | ELISA; opsonophagocytosis | 0.20 to 0.35 μg/mL (for children); 1/8 dilution | ||||
| Rubella | Immunoprecipitation | 10 to 15 IU/mL | ++ | |||
| Tetanus | Toxin neutralisation | 0.01 IU/mL | ||||
| Varicella | FAMA gp ELISA | ≥ 1:64 titre; ≥ 5 IU/mL | ++ | +(CD4+) | ||
| Yellow fever | Neutralisation | 0.7 LNI | ++ | |||
| ELISA: enzyme‐linked immunosorbent assay; FAMA: fluorescent antibody‐to‐membrane‐antibody; Hib: Haemophilus influenzae type b; Ig: immunoglobulin; LNI: log neutralisation index. | ||||||
Appendix 4. Glossary of selected terms used within the review
Adjuvant: a vaccine component that is intended to modify or augment the effects of a vaccine by stimulating the immune system to respond more vigorously to the vaccine antigen. Aluminium salts are most often used as adjuvants in contemporary vaccines.
Anaphylaxis: "a sudden and severe allergic reaction, which results in a serious fall in blood pressure and/or respiratory obstruction and may cause unconsciousness and death if not treated immediately" (ATAGI 2013: p 489).
Brachial neuritis: "Pain in the arm, causing persisting weakness of the limb on the side of vaccination" (ATAGI 2013: p 489).
Brighton Collaboration: an international voluntary collaboration that facilitates the development, evaluation, and dissemination of high‐quality information about the safety of human vaccines (Bonhoeffer 2002). The Brighton Collaboration "develops standardized case definitions [for adverse events following immunisation] and guidelines for data collection, analysis and presentation via participation of more than 500 experts from 57 countries from public health, clinical care, academic, regulatory organizations and industry" (Kohl 2005).
Cellulitis: "diffuse and especially subcutaneous inflammation of connective tissue" (Merriam‐Webster 2013).
Correlate of vaccine protection: in this Cochrane Review, a correlate of protection is defined in accordance with the definition proposed by Plotkin 2008b: "A specific immune response to a vaccine that is closely related to protection against infection, disease or other defined end point". Correlates of protective immunity usually entail vaccine‐induced immune responses. Historically these responses have been defined in terms of antibody titres, although current technology also allows consideration of cell‐mediated, mucosal and memory‐based immune responses (Hudgens 2004). Widely accepted immunological correlates of protection exist for certain antigens and consist of "defined humoral antibody responses above which there is a high likelihood of protection in the absence of any host factors that might increase susceptibility to the infectious agent" (EMA 2005).
Febrile: "related to a fever, as in febrile illness and febrile convulsions" (ATAGI 2013: p 491).
Geometric mean: the average of logarithmic values, converted back to the base. It is less sensitive than the arithmetic mean to one or a few extreme values (CDC 2006b). The geometric mean is the measure of choice for variables measured on an exponential or logarithmic scale, such as dilutional titres of assays, and it is a standard statistic used to summarise immunogenicity values. If the observations are titres, the geometric mean titre (GMT) is used. If the observations are concentrations, the geometric mean concentration (GMC) is used (Nauta 2011). Both GMC and GMT are commonly used immune response endpoints in vaccine efficacy trials (Horne 2001).
Geometric mean fold increase (GMFI): refers to the postvaccination antibody level divided by the prevaccination antibody level.
Haematoma: "a mass of usually clotted blood that forms in a tissue, organ, or body space as a result of a broken blood vessel" (Merriam‐Webster 2013).
Hypotonic‐hyporesponsive episode (shock, collapse): "the sudden onset of pallor or cyanosis, limpness (muscle hypotonia), and reduced responsiveness or unresponsiveness occurring after vaccination, where no other cause is evident, such as a vasovagal episode or anaphylaxis. The episode usually occurs 1 to 48 hours after vaccination and resolves spontaneously" (ATAGI 2013: p 491).
Immunobiologic: "Antigenic substances (e.g., vaccines and toxoids) or antibody‐containing preparations (e.g., globulins and antitoxins) from human or animal donors. These products are used for active or passive immunization or therapy. Examples of immunobiologics include antitoxin, immune globulin and hyperimmune globulin, monoclonal antibodies, toxoids, and vaccines" (CDC 2011).
Immunogenicity: the ability of a vaccine to induce a humoral‐mediated or a cell‐mediated (or both) immune response. The ideal endpoint for evaluating the immune response to an administered vaccine is the incidence of the disease the vaccine is designed to prevent. However, commonly used endpoints in vaccine clinical trials include the geometric mean concentration (GMC) or geometric mean titre (GMT) of antibodies elicited by the vaccine and the 'proportion of seroprotected vaccine recipients' (see Glossary entry below for an explanation of this term).
Jet injectors: these are "needle‐free devices that pressurize liquid medication, forcing it through a nozzle orifice into a narrow stream capable of penetrating skin to deliver a drug or vaccine into intradermal, subcutaneous, or intramuscular tissue" (CDC 2011).
Morphological: "of, relating to or concerned with form or structure" (Merriam‐Webster 2013).
Necrosis: "Death of living tissue; specifically: death of a portion of tissue differentially affected by local injury" (Merriam‐Webster 2013).
Needle size: in this Cochrane review, the term 'needle size' is used to refer to two dimensions of needle geometry, namely gauge and length.
National Immunization Technical Advisory Groups (NITAGs): these groups are "Expert advisory committees that provide recommendations to guide a country's national immunization programmes and policies. They consist of independent experts with the technical capacity to evaluate new and existing immunization interventions. The premise of these groups is to facilitate a systematic, transparent process for developing immunization policies by making evidence‐based technical recommendations to the national government" (Bryson 2010). One global survey of these advisory groups reported the existence of NITAGs in 89 countries (Bryson 2010). Details of the NITAGs in different countries can be obtained from the SIVAC initiative's (Supporting National Independent Immunization and Vaccine Advisory Committees) NITAG Resource Center (AMP 2012a; AMP 2012b).
Paraesthesia: "a sensation of pricking, tingling, or creeping on the skin having no objective cause and usually associated with injury or irritation of a sensory nerve or nerve root" (Merriam‐Webster 2013).
Proportion of seroprotected vaccine recipients: this refers to the proportion of vaccine recipients who respond in a prescribed manner to the vaccine administered. This endpoint in vaccine clinical trials is particularly meaningful "if there is a particular threshold level of immune response that is believed to be important. For example, for Haemophilus influenzae type b (Hib), proportion of recipients with a postvaccination concentration of anti‐polyribosyl ribitol phosphate antibody that is ≥0.15µg/mL and ≥1.00µg/mL have been used to evaluate the immune response to the Hib component" (Horne 2001).
Reactogenicity: in accordance with other Cochrane Reviews (e.g. Bar‐On 2012), the term reactogenicity is used in this Cochrane Review to refer to adverse events following the administration of a vaccine. Common reactogenicity events that occur following vaccination include pain, redness, swelling, induration and tenderness at the injection site, local hypersensitivity reactions, and systemic adverse reactions that include fever, malaise, myalgia, irritability, headache, and loss of appetite (DoH UK 2012b).
Serious adverse event: for the purposes of this review, this term refers to any untoward medical occurrence after vaccine administration that at any dose results in death, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity, or is life‐threatening (WHO 2013b).
Substitute immunogenicity endpoint: in this Cochrane Review the term 'substitute endpoint' is a general term that includes both correlates and surrogates of vaccine protection, or 'intermediate endpoints', i.e. immune response quantities that may be measured instead of the clinical endpoint (i.e. disease) of ultimate interest. The term 'substitute endpoint' has been used in recent World Health Organization publications in recognition of the fact that the terms 'correlates' and 'surrogates' of protection are defined and used inconsistently in the international literature (WHO 2013a).
Surrogate of vaccine protection: in this Cochrane Review, a surrogate is defined in accordance with the definition proposed by Plotkin 2008b: "a quantified specific immune response to a vaccine that is not in itself protective but that substitutes for the true (perhaps unknown) correlate."
Syncope (faint): "episode of pallor and unresponsiveness or reduced responsiveness or feeling light‐headed AND occurring while vaccine is being administered or shortly after (usually within 5 minutes) AND bradycardia AND resolution of symptoms with a change in position (supine position or head between knees or limbs elevated)" (ATAGI 2013: p 493).
Vaccine antigen: "the active component of a vaccine is known as the vaccine "antigen". This is a modified or partial form of the virus, bacteria or the toxin that causes the disease against which the vaccine protects. The vaccine antigen is altered from its original form so it no longer causes disease, but it can produce an immune response" (NCIRS 2013: p 1).
Vaccination and immunisation: although the terms 'vaccination' and 'immunisation' are frequently used interchangeably in the international literature, they are not strictly synonymous "because the administration of an immunobiologic cannot be equated automatically with development of adequate immunity" (CDC 2011). In this Cochrane Review, the term 'vaccination' is used to refer to the physical act of administering any vaccine or toxoid. The term 'immunisation' is used to refer to the process of inducing or providing immunity by administering an immunobiological (CDC 2011). The only exception to this occurs in the context of the phrases 'adverse events following immunisation (AEFI)' and 'immunisation schedules', which are established terms that are widely used in the international literature. Within the context of these phrases, the word 'immunisation' should be understood as referring to vaccine administration rather than the process of inducing immunity.
Appendix 5. Search strategies for CENTRAL (the Cochrane Library), MEDLINE and MEDLINE in Progress (Ovid), Embase (Ovid), and CINAHL (EBSCO)
CENTRAL
#1 MeSH descriptor: [Immunization] explode all trees
#2 MeSH descriptor: [Immunization Programs] explode all trees
#3 MeSH descriptor: [Injections] explode all trees
#4 (immuni* or vaccin* or inject*):ti,ab,kw (Word variations have been searched)
#5 #1 or #2 or #3 or #4
#6 needle*:ti,ab,kw (Word variations have been searched)
#7 MeSH descriptor: [Needles] this term only
#8 #6 or #7
#9 #5 and #8
MEDLINE (Ovid)
1 exp Immunization/
2 exp Immunization Programs/
3 exp Injections/
4 (immuni* or vaccin* or inject*).mp.
5 or/1‐4
6 Needles/
7 needle*.mp.
8 or/6‐7
9 5 and 8
10 randomized controlled trial.pt.
11 controlled clinical trial.pt.
12 randomized.ab.
13 placebo.ab.
14 drug therapy.fs.
15 randomly.ab.
16 trial.ab.
17 or/10‐16
18 exp animals/ not humans.sh.
19 17 not 18
20 9 and 19
Embase (Ovid)
1. exp Immunization/
2. exp Immunization Programs/
3. exp Injections/
4. (immuni* or vaccin* or inject*).mp.
5. or/1‐4
6. Needles/
7. needle*.mp.
8. or/6‐7
9. 5 and 8
10. random$.tw.
11. factorial$.tw.
12. crossover$.tw.
13. cross over$.tw.
14. cross‐over$.tw.
15. placebo$.tw.
16. (doubl$ adj blind$).tw.
17. (singl$ adj blind$).tw.
18. assign$.tw.
19. allocat$.tw.
20. volunteer$.tw.
21. Crossover Procedure/
22. double‐blind procedure.tw.
23. Randomized Controlled Trial/
24. Single Blind Procedure/
25. or/10‐24
26. (animal/ or nonhuman/) not human/
27. 25 not 26
28. 9 and 27
CINAHL (EBSCO)
S19 S9 AND S18
S18 S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17
S17 (allocat* random*)
S16 (MH "Quantitative Studies")
S15 (MH "Placebos")
S14 placebo*
S13 (random* allocat*)
S12 (MH "Random Assignment")
S11 (Randomi?ed control* trial*)
S10 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S9 S5 AND S8
S8 S6 OR S7
S7 needle*
S6 (MH "Needles")
S5 (S1 OR S2 OR S3 OR S4)
S4 (immuni* or vaccin* or inject*)
S3 (MH "Injections+")
S2 (MH "Immunization Programs")
S1 (MH "Immunization+")
Appendix 6. Details of the number of records identified through database searching and via other sources for review update
| Databases searched | Number of records identified |
| The Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO) (24 October 2017) | 688 |
| MEDLINE and MEDLINE in Progress via Ovid (November 2014 to 23 October 2017) | 768 |
| Embase via Ovid (November 2014 to 2017 week 43) | 524 |
| CINAHL via EBSCOhost (November 2014 to October 2017) | 17 |
| Other sources | |
| Conference abstracts | 531 |
| ClinicalTrials.gov | 38 |
| WHO International Clinical Trials Registry Platform (ICTRP) | 319 |
| Total | 2885 |
| Total after removal of duplicates | 2151 |
Details of the records identified in the original review are available in Appendix 6 in Beirne 2015.
Appendix 7. Sensitivity analyses
1. Meta‐analyses performed with random‐effects and fixed‐effect models
In the Effects of interventions section of the review, we presented the results of a random‐effects meta‐analysis of the trial data from Diggle 2000a and Diggle 2006 for the outcomes of swelling, tenderness, and redness at 24 hours (day 1) after vaccination with the third dose of a diphtheria, tetanus, whole‐cell pertussis, and Haemophilus influenzae type b (DTwP‐Hib) vaccine. We repeated the analysis using a fixed‐effect model to determine if our overall interpretation of the evidence was robust to decisions about meta‐analysis model. The table below compares the pooled effect measures for each outcome at day 1 postvaccination using both fixed‐effect and random‐effects meta‐analyses.
|
Sensitivity analysis: comparison between the results of random‐effects and fixed‐effect meta‐analyses of the trial data from Diggle 2000a and Diggle 2006 at day 1 post‐vaccination Population: infants aged 4 months undergoing vaccination with the third dose of a DTwP‐Hib vaccine Intervention and comparison: 23 G 25 mm vs 25 G 16 mm needles Outcomes: swelling, tenderness, and redness | ||
| Outcomes and time points |
Risk ratio (95% CI) Random‐effects |
Risk ratio (95% CI) Fixed‐effect |
| Pooled results for swelling at day 1 | 0.58 (0.36 to 0.93) | 0.60 (0.43 to 0.82) |
| Pooled results for tenderness at day 1 | 0.63 (0.40 to 1.00) | 0.63 (0.39 to 1.00) |
| Pooled results for redness at day 1 | 0.61 (0.36 to 1.01) | 0.67 (0.54 to 0.84) |
| CI: confidence interval; DTwP‐Hib: diphtheria, tetanus, whole‐cell pertussis, and Haemophilus influenzae type b. | ||
The point estimates for the pooled risk ratios for each outcome were similar for fixed‐effect and random‐effects meta‐analyses. The only material difference between the analyses was that the non‐significant pooled estimate for redness at day 1 postvaccination with the random‐effects model was rendered statistically significant with the use of the fixed‐effect model. We consider that our overall interpretation of the evidence (that 23 G 25 mm needles probably reduce local reactions compared with 25 G 16 mm needles) was robust to variations in decisions about meta‐analysis model.
2. Meta‐analyses performed with random‐effects and fixed‐effect models and at 4 time points postvaccination (6 hours, day 1, day 2, day 3)
In the Effects of interventions section of the review, we presented the results of a random‐effects meta‐analysis of the trial data from Diggle 2000a and Diggle 2006 for the outcomes of swelling, tenderness, and redness at 24 hours (day 1) after vaccination with the third dose of a DTwP‐Hib vaccine. As explained in the Unit of analysis issues section of the review, we believe that the 24‐hour time point was an appropriate time point at which to present the results of local reaction analyses. Nevertheless, we conducted a sensitivity analysis to determine if our overall interpretation of the evidence was robust to decisions about time point selection and meta‐analysis model. The table below compares the pooled effect measures for each outcome at each time point (6 hours, day 1, day 2, and day 3) using both fixed‐effect and random‐effects meta‐analyses.
|
Sensitivity analysis: comparison between the results of random‐effects and fixed‐effect meta‐analyses of the trial data from (Diggle 2000a; Diggle 2006) at 6 hours and on days 1, 2 and 3 post‐vaccination Population: infants aged 4 months undergoing vaccination with the third dose of a DTwP‐Hib vaccine Intervention and comparison: 23 G 25 mm vs. 25 G 16 mm needles Outcomes: swelling, tenderness, and redness | ||
| Outcomes and time points |
Risk ratio (95% CI) Random‐effects |
Risk ratio (95% CI) Fixed‐effect |
| Pooled results for swelling at 6 hours | 0.55 (0.30 to 1.03) | 0.59 (0.43 to 0.82) |
| Pooled results for swelling at day 1 | 0.58 (0.36 to 0.93) | 0.60 (0.43 to 0.82) |
| Pooled results for swelling at day 2 | 0.44 (0.29 to 0.68) | 0.45 (0.29 to 0.68) |
| Pooled results for swelling at day 3 | 0.32 (0.17 to 0.59) | 0.31 (0.17 to 0.59) |
| Pooled results for tenderness at 6 hours | 0.79 (0.58 to 1.06) | 0.78 (0.58 to 1.05) |
| Pooled results for tenderness at day 1 | 0.63 (0.40 to 1.00) | 0.63 (0.39 to 1.00) |
| Pooled results for tenderness at day 2 | 0.64 (0.22 to 1.87) | 0.65 (0.30 to 1.40) |
| Pooled results for tenderness at day 3 | 0.99 (0.33 to 2.96) | 0.96 (0.33 to 2.83) |
| Pooled results for redness at 6 hours | 0.81 (0.61 to 1.06) | 0.83 (0.69 to 1.01) |
| Pooled results for redness at day 1 | 0.61 (0.36 to 1.01) | 0.67 (0.54 to 0.84) |
| Pooled results for redness at day 2 | 0.50 (0.14 to 1.82) | 0.70 (0.50 to 0.97) |
| Pooled results for redness at day 3 | 0.30 (0.08 to 1.07) | 0.34 (0.18 to 0.64) |
| CI: confidence interval. | ||
In general, the confidence intervals around the effect estimates were narrower with the fixed‐effect than with the random‐effects meta‐analyses, and some non‐statistically significant analyses using the random‐effects model were rendered statistically significant with the use of the fixed‐effect model. Effect sizes were larger for swelling and redness for the analyses at later time points compared with earlier time points. The event rates for tenderness in both needle size groups in the two trials were very low at days two and three, and hence the confidence intervals around the pooled risk ratio estimates were very wide.
Although the magnitude of the effect varied at different time points, the direction of effect was consistent across all time points and indicative of a reduced incidence of local reactions following vaccination with the 23 G 25 mm needle compared with the 25 G 16 mm needle. We therefore consider that our overall conclusion (that 23 G 25 mm needles probably reduce local reactions compared to 25 G 16 mm needles) was reasonably robust to variations in decisions about meta‐analysis model and the time points for analyses.
3. Comparison of effect sizes at different time points (6 hours, day 1, day 2, day 3, and at any time point postvaccination) for the composite outcome in the Diggle 2006 trial
In the Effects of interventions section of the review and in the 'Summary of findings' tables, we presented the results for the effects of needle size on a composite local reaction outcome (any swelling, tenderness, redness, or hardness) at 24 hours (day 1) after vaccination with the first, second, and third doses of a DTwP‐Hib vaccine in the Diggle 2006 trial. As explained in the Unit of analysis issues section of the review, we believe that the 24‐hour time point was an appropriate time point at which to present the results of local reaction analyses. Nevertheless, we conducted a sensitivity analysis to determine if our overall interpretation of the evidence for each of the three main comparisons made in the review was robust to time point selection for this composite outcome.
Comparison 1:
The table below compares the effect measures for comparison 1: 25 G 25 mm versus 25 G 16 mm needles for the composite local reaction outcome at each time point (6 hours, day 1, day 2, and day 3) after each dose of the DTwP‐Hib vaccine in the Diggle 2006 trial.
|
Sensitivity analysis: comparison of risk ratio effect estimates for the composite outcome of 'any local reaction' in the Diggle 2006 trial at various time points Population: infants aged 2 to 4 months undergoing DTwP‐Hib vaccination Interventions: 25 G 25 mm vs 25 G 16 mm needles Outcomes: any local reaction (any swelling, redness, tenderness, or hardness) | |
| Time points and vaccine dose |
Risk ratio (95% CI) |
| 6 hours after first dose | 0.78 (0.68 to 0.90) |
| Day 1 after first dose | 0.64 (0.52 to 0.79) |
| Day 2 after first dose | 0.49 (0.35 to 0.70) |
| Day 3 after first dose | 0.57 (0.34 to 0.95) |
| At any time point after first dose | 0.80 (0.70 to 0.90) |
| 6 hours after second dose | 0.86 (0.72 to 1.03) |
| Day 1 after second dose | 0.67 (0.54 to 0.83) |
| Day 2 after second dose | 0.51 (0.38 to 0.69) |
| Day 3 after second dose | 0.39 (0.24 to 0.64) |
| At any time point after second dose | 0.84 (0.72 to 0.97) |
| 6 hours after third dose | 0.77 (0.64 to 0.93) |
| Day 1 after third dose | 0.65 (0.52 to 0.80) |
| Day 2 after third dose | 0.51 (0.37 to 0.71) |
| Day 3 after third dose | 0.44 (0.26 to 0.73) |
| At any time point after third dose | 0.75 (0.64 to 0.89) |
| CI: confidence interval. | |
The magnitude of the intervention effect varied depending on time point selection, with larger effects sizes for analyses at days 2 and 3 compared with day 1. The effect sizes were smaller for the analyses conducted at 6 hours and across all time points compared with the day 1 analysis. However, the direction of effect was entirely consistent for all analyses irrespective of time point, and the differences in effect sizes at different time points were between small and large beneficial effects in favour of the longer needle. Thus, although our estimates of the magnitude of the intervention effect would have varied depending on time point selection, our overall conclusion that the 25 G 25 mm needle probably reduces the incidence of local reactions would not have materially altered according to time point selection.
Comparison 2:
The table below compares the effect measures for comparison 2: 25 G 25 mm versus 23 G 25 mm needles for the composite local reaction outcome at each time point (6 hours, day 1, day 2, day 3, and at any time point) after each dose of the DTwP‐Hib vaccine in the Diggle 2006 trial.
|
Sensitivity analysis: comparison of risk ratio effect estimates for the composite outcome of 'any local reaction' in the Diggle 2006 trial at various time points Population: infants aged 2 to 4 months undergoing DTwP‐Hib vaccination Interventions: 25 G 25 mm vs 23 G 25 mm needles Outcomes: any local reaction (any swelling, redness, tenderness, or hardness) | |
| Time points and vaccine dose |
Risk ratio (95% CI) |
| 6 hours after first dose | 0.88 (0.76 to 1.02) |
| Day 1 after first dose | 0.92 (0.73 to 1.17) |
| Day 2 after first dose | 0.92 (0.62 to 1.38) |
| Day 3 after first dose | 1.23 (0.66 to 2.29) |
| At any time point after first dose | 0.90 (0.79 to 1.03) |
| 6 hours after second dose | 0.90 (0.76 to 1.08) |
| Day 1 after second dose | 0.89 (0.70 to 1.12) |
| Day 2 after second dose | 0.78 (0.56 to 1.10) |
| Day 3 after second dose | 0.66 (0.38 to 1.14) |
| At any time point after second dose | 0.92 (0.79 to 1.08) |
| 6 hours after third dose | 0.84 (0.70 to 1.02) |
| Day 1 after third dose | 0.84 (0.66 to 1.06) |
| Day 2 after third dose | 0.67 (0.47 to 0.95) |
| Day 3 after third dose | 0.81 (0.46 to 1.43) |
| At any time point after third dose | 0.79 (0.67 to 0.94) |
| CI: confidence interval. | |
The direction of effect was consistently in favour of the narrower gauge needle at all time points, with the exception of the analysis conducted at day 3 after the first vaccine dose. The only material alteration to our assessment of the evidence would have occurred if we had chosen to present the results of analyses across all time points rather than at day 1 postvaccination. Taking into account the width of the confidence intervals around the effect estimates at day 1, we downgraded for imprecision because the confidence intervals included the suggested GRADE threshold for imprecision (a relative risk reduction or relative risk increase of 25%). However, this downgrading would not have occurred for the effect estimates calculated across all time points as the confidence intervals were narrower. Our rating of the quality of evidence would therefore have been raised from 'low' to 'moderate' quality if we had presented the results of analyses across all time points rather than at the 24‐hour time point. The alteration in rating would have resulted in a change in the qualitative emphasis of our conclusions: instead of stating that the 25 G needle may reduce local reactions compared to the 23 G needle, we would have stated that the 25 G needle probably reduces local reactions. Nevertheless, we consider that the more conservative conclusion (may reduce) is reasonable taking into account the fact that the analyses at any time point after the first and second doses of the vaccine were not statistically significant and precluded making confident statements about the precise magnitude of the effect.
Comparison 3:
The table below compares the effect measures for comparison 3: 23 G 25 mm versus 25 G 16 mm needles for the composite local reaction outcome at each time point (6 hours, day 1, day 2, day 3, and at any time point) after each dose of the DTwP‐Hib vaccine in the Diggle 2006 trial.
|
Sensitivity analysis: comparison of risk ratio effect estimates for the composite outcome of 'any local reaction' in the Diggle 2006 trial at various time points Population: infants aged 2 to 4 months undergoing DTwP‐Hib vaccination Interventions: 23 G 25 mm vs 25 G 16 mm needles Outcomes: any local reaction (any swelling, redness, tenderness, or hardness) | |
| Time points and vaccine dose |
Risk ratio (95% CI) |
| 6 hours after first dose | 0.89 (0.78 to 1.00) |
| Day 1 after first dose | 0.69 (0.57 to 0.84) |
| Day 2 after first dose | 0.53 (0.38 to 0.74) |
| Day 3 after first dose | 0.46 (0.27 to 0.80) |
| At any time point after first dose | 0.88 (0.79 to 0.99) |
| 6 hours after second dose | 0.95 (0.81 to 1.12) |
| Day 1 after second dose | 0.76 (0.62 to 0.92) |
| Day 2 after second dose | 0.65 (0.50 to 0.85) |
| Day 3 after second dose | 0.59 (0.39 to 0.90) |
| At any time point after second dose | 0.91 (0.79 to 1.04) |
| 6 hours after third dose | 0.92 (0.78 to 1.09) |
| Day 1 after third dose | 0.77 (0.64 to 0.94) |
| Day 2 after third dose | 0.76 (0.57 to 1.00) |
| Day 3 after third dose | 0.54 (0.34 to 0.86) |
| At any time point after third dose | 0.95 (0.83 to 1.09) |
| CI: confidence interval. | |
The magnitude of the intervention effect varied depending on time point selection. The effect sizes were larger for the analyses at days 2 and 3 compared with day 1. The effect sizes were smaller for the analyses conducted at 6 hours and across all time points compared with the day 1 analysis. In addition, the analyses at six hours and across all time points were not statistically significant. Nevertheless, the direction of effect was entirely consistent for all analyses irrespective of time point, and the differences in effect sizes at different time points were between small and large beneficial effects in favour of the longer needle. In addition, although the analyses at six hours and across all time points were not statistically significant, this would not have resulted in a downgrading of the quality of evidence for imprecision, as the confidence intervals did not include the suggested GRADE threshold for downgrading (a relative risk reduction or relative risk increase of 25%). We also factored into our interpretation of the evidence the meta‐analyses of the results of the Diggle 2006 and Diggle 2000a trial pertaining to individual components of the composite (redness, swelling, and tenderness) after the third dose of a DTwP‐Hib vaccine. The results of these meta‐analyses strengthened the evidence in favour of a reduced rate of local reactions associated with the 23 G 25 mm needle compared with the 25 G 16 mm needle.
Thus, although our estimates of the magnitude of the intervention effect would have varied depending on time point selection, our overall conclusion that the 23 G 25 mm needle probably reduces the incidence of local reactions compared to the 25 G 16 mm needle would not have materially altered according to time point selection.
4. Comparison of effect sizes for the composite outcome in the Diggle 2006 trial with the effect sizes for the individual components of the composite
The use of a composite outcome in the 'Summary of findings' tables for a systematic review inevitably results in a 'loss of information' due to the combination of several separate outcomes into a single outcome measure. Of particular concern is that users of a review may assume that the intervention effect applies equally to all components of the composite, whereas in reality the intervention effect may vary across individual components of the composite that have different clinical importance. In such a scenario the use of a composite outcome could be potentially misleading.
The composite outcome in the Diggle 2006 trial arguably included individual components with differing clinical importance. For example, redness and hardness at the injection site may not be regarded by clinicians, patients (consumers), parents, and policymakers as having the same importance as tenderness or swelling. We therefore conducted sensitivity analyses to investigate if there were disparities between the estimates of intervention effect on the composite outcome (any redness, swelling, tenderness, or hardness) and the estimates of intervention effect on individual components of the composite in this trial. The results of the sensitivity analyses are presented in the tables below. The analyses indicate that there were some variations in the magnitude of the intervention effect on individual components of the composite for all of the main comparisons made in the review. However, the direction of effect was generally consistent across individual components, particularly for the comparisons between the 25 mm and 16 mm needles, and this direction of effect was accurately reflected in the effect size for the composite outcome. On balance, we consider that the decision to present composite rather than individual local reaction outcomes in the 'Summary of findings' tables was appropriate and that it was an efficient way to summarise the effects of needle size on vaccine reactogenicity without overwhelming the reader with information on intervention effects on individual local reaction outcomes.
|
Sensitivity analysis: comparison of risk ratio effect estimates for the composite outcome of 'any local reaction' in the Diggle 2006 trial at day 1 vs effect estimates for the individual components of the composite Population: infants aged 2 to 4 months undergoing DTwP‐Hib vaccination Intervention and comparison: 25 G 25 mm vs 25 G 16 mm needles Outcomes: composite outcome; individual outcomes | |
| Outcome and vaccine dose |
Risk ratio (95% CI) |
| Composite day 1 after first dose | 0.64 (0.52 to 0.79) |
| Swelling day 1 after first dose | 0.59 (0.38 to 0.92) |
| Tenderness day 1 after first dose | 0.61 (0.40 to 0.91) |
| Redness day 1 after first dose | 0.68 (0.47 to 0.98) |
| Hardness day 1 after first dose | 0.56 (0.42 to 0.77) |
| Composite day 1 after second dose | 0.67 (0.54 to 0.83) |
| Swelling day 1 after second dose | 0.66 (0.42 to 1.02) |
| Tenderness day 1 after second dose | 0.70 (0.41 to 1.18) |
| Redness day 1 after second dose | 0.65 (0.48 to 0.88) |
| Hardness day 1 after second dose | 0.53 (0.40 to 0.71) |
| Composite day 1 after third dose | 0.65 (0.52 to 0.80) |
| Swelling day 1 after third dose | 0.40 (0.23 to 0.69) |
| Tenderness day 1 after third dose | 0.76 (0.46 to 1.23) |
| Redness day 1 after third dose | 0.64 (0.49 to 0.84) |
| Hardness day 1 after third dose | 0.55 (0.40 to 0.76) |
| CI: confidence interval. | |
|
Sensitivity analysis: comparison of risk ratio effect estimates for the composite outcome of 'any local reaction' in the Diggle 2006 trial at day 1 vs effect estimates for the individual components of the composite Population: infants aged 2 to 4 months undergoing DTwP‐Hib vaccination Intervention and comparison: 25 G 25 mm vs 23 G 25 mm needles Outcomes: composite outcome; individual outcomes | |
| Outcome and vaccine dose |
Risk ratio (95% CI) |
| Composite day 1 after first dose | 0.92 (0.73 to 1.17) |
| Swelling day 1 after first dose | 1.24 (0.72 to 2.12) |
| Tenderness day 1 after first dose | 1.02 (0.64 to 1.61) |
| Redness day 1 after first dose | 1.08 (0.71 to 1.63) |
| Hardness day 1 after first dose | 0.90 (0.64 to 1.26) |
| Composite day 1 after second dose | 0.89 (0.70 to 1.12) |
| Swelling day 1 after second dose | 0.88 (0.55 to 1.41) |
| Tenderness day 1 after second dose | 0.64 (0.38 to 1.07) |
| Redness day 1 after second dose | 0.86 (0.62 to 1.18) |
| Hardness day 1 after second dose | 0.81 (0.58 to 1.12) |
| Composite day 1 after third dose | 0.84 (0.66 to 1.06) |
| Swelling day 1 after third dose | 0.55 (0.31 to 0.97) |
| Tenderness day 1 after third dose | 1.16 (0.68 to 2.00) |
| Redness day 1 after third dose | 0.85 (0.63 to 1.14) |
| Hardness day 1 after third dose | 0.73 (0.52 to 1.03) |
| CI: confidence interval. | |
5. Choice of minimum important differences for differences in seroprotection rates between needle size groups
For differences in seroprotection rates between needle size groups, we used as a minimum important difference (MID) a risk difference (RD) of 10% based on the recommended non‐inferiority protection rate for vaccines specified by the Committee for Proprietary Medicinal Products (CPMP 1999). However, we accept that this choice of MID is debatable, and a case could reasonably be made for a lower value. For example, we identified a small number of non‐inferiority trials of combination vaccines that have specified a 5% MID in seroprotection rates (e.g. Collins 2004; de Menezes Martins 2008). We therefore conducted a sensitivity analysis to investigate if our interpretation of the evidence from the Diggle 2006 trial pertaining to the effects of needle size on the immune response to the diphtheria, tetanus, whole‐cell pertussis, and Haemophilus influenzae type b (DTwP‐Hib) vaccine would have altered depending on the choice of MID. The tables below indicate that the choice of an MID of 5% rather than 10% would not have resulted in a material alteration to our conclusion that there is probably little or no difference in immune response between 25 G 25 mm, 23 G 25 mm, and 25 G 16 mm needle sizes.
| Sensitivity analysis: effects of needle size on the immune response to the DTwP‐Hib vaccine. Does the interpretation of results from the Diggle 2006 trial vary depending on the choice of MIDs (10% or 5%) between needle size groups? | ||
| Comparison 1: comparisons between needles with different lengths and the same gauges | ||
| Needle sizes compared | MID | Interpretation of results |
| 25 G 25 mm vs 25 G 16 mm |
10% difference in seroprotection rates |
The CIs around the effect estimates indicate that the immune response to the diphtheria and tetanus vaccine antigen components is probably equivalent between the needle size groups. The longer needle may result in a superior immune response to the Hib component of the vaccine (RD 8%, 95% CI 1% to 15%), but the results are inconclusive as the CI crosses the threshold for an important effect (10%), but the lower boundary of the CI is close to the 'null value'. |
| 5% difference | Interpretation same as above | |
| Comparison 2: comparisons between needles with different gauges but with the same length | ||
| 25 G 25 mm vs 23 G 25 mm |
10% difference in seroprotection rates |
The CIs around the effect estimates indicate that the immune response to the diphtheria, tetanus, and Hib vaccine antigen components is probably equivalent between the needle size groups. |
| 5% difference | Interpretation same as above for diphtheria and tetanus. The narrower gauge needle may result in a superior immune response to the Hib component of the vaccine (RD 3%, 95% CI ‐4% to 9%), but the results are inconclusive. | |
| Comparison 3: comparisons between needles with different gauges and different lengths | ||
| 23 G 25 mm vs 25 G 16 mm |
10% difference in seroprotection rates |
The CIs around the effect estimates indicate that the immune response to the diphtheria and tetanus vaccine antigen components is probably equivalent between the needle size groups. The longer needle may result in a superior immune response to the Hib component of the vaccine (RD 5%, 95% CI ‐2% to 13%), but the results are inconclusive. |
| 5% difference | Interpretation same as above | |
| CI: confidence interval; DTwP‐Hib: diphtheria, tetanus, whole‐cell pertussis, and Haemophilus influenzae type b; Hib: Haemophilus influenzae type b; RD: risk difference. | ||
6. Choice of antibody titre level threshold of protection against Haemophilus influenzae type b disease
For seroprotection against Haemophilus influenzae type b (Hib) disease, we chose an antibody titre level threshold of 1.0 µg/mL or greater and presented the results of analyses based on this threshold in the 'Summary of findings' tables and in the Effects of interventions section of the review. However, some population level studies suggest that an antibody concentration of 0.15 µg/mL or greater provides adequate short‐term protection against invasive Hib disease, but that a concentration of 1.0 µg/mL or greater is necessary for long‐term protection (Chandran 2013). We therefore performed a sensitivity analysis to investigate if our interpretation of the evidence pertaining to the effects of needle size on the immune response to the Hib component of the vaccine would have varied depending on the choice of threshold.
| Sensitivity analysis: effects of needle size on vaccine immunogenicity. Does the interpretation of results vary depending on the choice of cut‐off antibody titre level threshold (≥ 1.0 µg/mL or ≥ 0.15 µg/mL) for seroprotection against Haemophilus influenzae type b disease? | |||
| Comparison 1: comparisons between needles with different lengths and the same gauges | |||
| Needle sizes compared | Cut‐off threshold for Hib antibody titre levels |
RD (95% CI) |
Interpretation of results (based on an MID of 10%) |
| 25 G 25 mm vs 25 G 16 mm |
≥ 1.0 µg/mL | 8% (1% to 15%) |
The longer needle may result in a superior immune response to the Hib component of the vaccine, but the results are inconclusive (the CI crosses the threshold for an important effect (10%), but the lower boundary of the CI is close to the ‘null value’). |
| ≥ 0.15 µg/mL | 4% (1% to 8%) |
Immune response probably equivalent between groups | |
| Comparison 2: comparisons between needles with different gauges but with the same length | |||
| 25 G 25 mm vs 23 G 25 mm |
≥ 1.0 µg/mL | 3% (‐4% to 9%) |
Immune response probably equivalent between groups |
| ≥ 0.15 µg/mL | 5% (1% to 9%) |
Immune response probably equivalent between groups | |
| Comparison 3: comparisons between needles with different gauges and different lengths | |||
| 23 G 25 mm vs 25 G 16 mm |
≥ 1.0 µg/mL | 5% (‐2% to 12%) |
The longer needle may result in a superior immune response to the Hib component of the vaccine, but the results are inconclusive. |
| ≥ 0.15 µg/mL | ‐1% (‐6% to 4%) |
Immune response probably equivalent between groups | |
| CI: confidence interval; Hib: Haemophilus influenzae type b; MID: minimum important difference; RD: risk difference. | |||
Using a cut‐off point of 1.0 µg/mL or greater, we were unable to exclude the possibility that the longer needles (25 G 25 mm, 23 G 25 mm) may result in a superior immune response to the Hib component of the DTwP‐Hib vaccine compared with the 25 G 16 mm needle (the confidence intervals for the effect estimates crossed the threshold for an important effect (MID of 10%)). However, the trial results were inconclusive, as the confidence intervals were also compatible with little or no difference between the groups. Had we used a cut‐off point of 0.15 µg/mL or greater and the same MID, we would have concluded that the immune response was equivalent between the needle size groups for all comparisons (1, 2, and 3) because the 95% confidence intervals accompanying all effect estimates excluded the MID value of 10%. The choice of cut‐off point would not have influenced our GRADE rating. Our overall conclusion that there is probably little or no difference in immune response between using 23 G 25 mm, 25 G 25 mm, and 25 G 16 mm needles to administer a series of three doses of a DTwP‐Hib vaccine would thus not have materially altered depending on the choice of cut‐off point for seroprotection against Hib disease.
Appendix 8. Results of 'imaging studies' measuring subcutaneous tissue and muscle thickness at the anterolateral thigh vaccination site
| Results of 6 studies measuring the thickness of subcutaneous tissue and muscle at the anterolateral thigh vaccination site in infants aged 2 to 12 months | ||||||
| Study ID and setting | Measurement method | Study population |
Mean (± SD) weight (kg) or weight percentiles or weight (kg) range |
Mean thickness of SCT (mm ± SD) |
Mean thickness of muscle (mm ± SD) |
Skin‐to‐bone distance (mm ± SD) |
| Hick 1989: infants attending a "well child clinic" in Mayo Clinic, USA | Ultrasound1 | 4 months (n = 24) 13 M, 11 F |
Details not provided | 14 ± 2.4 (M) 13 ± 2.8 (F) |
Details not provided | 32 ± 4.5 (M) 28 ± 4.7 (F) |
| Chugh 1993:infants attending an immunisation clinic at a hospital in New Delhi, India | Ultrasound2 | 6 to 12 weeks (n = 52) |
4.6 ± 1.09 | 10.3 ± 2.3 | 8.3 ± 1.7 | 18.7 ± 3.5 |
| 13 to 18 weeks (n = 58) |
6.06 ± 0.98 | 10.4 ± 2.1 | 11.3 ± 2.8 | 21.7 ± 3.8 | ||
| 19 to 24 weeks (n = 63) |
6.2 ± 0.88 | 9.5 ± 1.9 | 11.2 ± 2.9 | 20.7 ± 3.9 | ||
| Groswasser 1997: people from different departments of the Queen Fabiola University Children’s Hospital, Brussels, Belgium | Ultrasound3 | Median age 12 weeks (range 9 to 27 weeks) (n = 40) |
Between 10th and 50th percentiles of Belgian growth curves | 8 (± 0.3) (R) 8.1 (± 0.3) (L) |
9.2 (± 0.3) (R) 9.3 (± 0.3) (L) |
17.3 (± 0.5) (R) 17.5 (± 2.7) (L) |
| Cook 2002: infants attending for vaccination at a general medical practice in Taree, New South Wales, Australia | Ultrasound4 | 2 months (n = 14) |
5.3 ± 0.7 Mean weight percentile 58% |
8.6 ± 3.0 (range 6 to 15.1) |
10.5 ± 2.4 (range 6.2 to 14.3) |
Not provided |
| 4 months (n = 13) |
7.1 ± 0.9 Mean weight percentile 74% |
9.4 ± 2.0 (range 6.5 to 13.5) |
12.2 ± 2.0 (range 9.6 to 15.3) |
Not provided | ||
| 6 months (n = 18) | 8.3 ± 1.2 Mean weight percentile 73% |
10.2 ± 2.1 (range 6.7 to 13.5) |
14.8 ± 2.0 (range 10.1 to 17.1) |
Not provided | ||
| Lippert 2008: people at a large children's hospital in the midwestern US who had an MRI or CT scan of their normal thigh between the ages of 2 months and 6 years | MRI or CT scan5 | 0 to 12 months (n = 12) |
Details not provided for these infants | 10.9 ± 2.77 (M) 14.6 ± 3.76 (F) |
15.3 ± 2.39 (M) 18.8 ± 3.51 (F) |
Not provided |
| 1 infant age 2 months |
7 | 15 | 22 | |||
| 1 infant age 6 months |
13 | 15 | 28 | |||
| 2 infants age 7 months |
17 13 |
19 18 |
36 31 |
|||
| Nakayama 2016: infants visiting the paediatric departments of 3 general hospitals in Tokyo, Shizuoka, and Osaka in Japan | Ultrasound6 | 2 months (n = 31) |
< 5 kg (n = 3) 5 to < 6 kg (n = 20) 6 to < 7 kg (n = 6) 7 to < 8 kg (n = 2) |
Not provided | Not provided | 23.5* (95% CI 22.35, 25.06) |
| 3 months (n = 33) |
5 to < 6 kg (n = 9) 6 to < 7 kg (n = 18) 7 to < 8 kg (n = 3) 8 to < 9 kg (n = 2) |
Not provided | Not provided | 25.6* (95% CI 24.59, 27.16) |
||
| 4 months (n = 30) |
< 5 kg (n = 2) 6 to < 7 kg (n = 17) 7 to < 8 kg (n = 9) 8 to < 9 kg (n = 2) |
Not provided | Not provided | 26.6* (95% CI 25.35, 28.31) |
||
| 5 months (n = 30) |
5 to < 6 kg (n = 3) 6 to < 7 kg (n = 9) 7 to < 8 kg (n = 12) 8 to < 9 kg (n = 4) 9 to < 10 kg (n = 1) |
Not provided | Not provided | 27.3* (95% CI 26, 29.87) |
||
| 6 months (n = 30) |
6 to < 7 kg (n = 7) 7 to < 8 kg (n = 15) 8 to < 9 kg (n = 7) 9 to < 10 kg (n = 1) |
Not provided | Not provided | 27.8* (95% CI 25.98, 29.87) |
||
| CI: confidence interval; CT: computed tomography; F: female; M: male; MRI: magnetic resonance imaging; SCT: subcutaneous tissue; SD: standard deviation. *Only the 95% CIs were provided in the published report of the study. The means were estimated from the graphs published in the papers using a web‐based plot digitizer (Rohatgi 2017). | ||||||
|
1Hick 1989: measurements were performed using a 10‐millihertz frequency ultrasound with a theoretical axial resolution of 0.5 mm (Diasonics, Inc., Milpitas, CA). Measurements were obtained at a point equidistant from the right anterior iliac crest and the superior border of the right patella, in the mid‐line. The ultrasound transducer was lightly applied to the skin to avoid tissue compression. Skin‐to‐muscle measurements were obtained in longitudinal plane and skin‐to‐bone measurements were obtained in the transverse plane. Two measurements were taken in each plane and the mean calculated. Details of the ultrasound operator(s) and any training or calibration exercises to ensure intra‐ and interoperator consistency were not provided. 2Chugh 1993: measurements were performed using high‐resolution real‐time linear 7.5‐millihertz ultrasound (ALOKA SSD). On the anterolateral aspect of the middle one‐third of the left thigh, the transducer of the ultrasound machine was lightly applied so as to ensure that tissues under the transducer were not compressed. Details of the ultrasound operator(s) and any training or calibration exercises to ensure intra and interoperator consistency were not provided. 3Groswasser 1997: measurements were performed using high‐frequency real‐time ultrasonography (ALOKA 2000 SSD) with a 6‐centimetre‐long 7.5‐hertz transducer. For the quadriceps, the anterolateral aspect of the thigh at the junction of the upper third and lower two‐thirds of the muscle was examined at a 45° angle to the horizontal plane. The transducer was applied lightly to the skin to avoid tissue compression. Two concordant measurements were performed, at a 90° angle both to the skin and to the long axis of the leg or arm; an image take at each point provided an automatic measurement in millimetres of the morphometric parameters. Two operators performed the experiments, each doing approximately half of the measurements. Details of any training or calibration exercises to ensure intra‐ and interoperator consistency were not provided. 4Cook 2002: measurements were performed using a high‐resolution real‐time ultrasonography with a 4‐centimetre footprint and 7‐millihertz linear transducer. Anterolateral thigh measurements were made at the junction of the upper third and lower middle thirds of the muscle mass, with the ultrasound probe applied at 45° to the vertical at right angles to the skin's plane and parallel to the long axis of the leg, with the child gently restrained with his or her pelvis flat on the examination couch. The transducer was applied lightly to the skin to avoid tissue compression. Measurements were made on both thighs and data pooled for analysis due to lack of significant difference between thigh measurements. Details of the ultrasound operator(s) and any training or calibration exercises to ensure intra and interoperator consistency were not provided. 5Lippert 2008: the thickness of the subcutaneous fat and muscle was measured from the CT or MRI scan of the thigh in the middle third of the vastus lateralis (anterlateral thigh area) at a 90° angle to the skin. Picture Archiving and Communications System software (GE Healthcare, Piscataway, NJ) was used to make these measurements to a scale of 1:1 to maximise the accuracy. Details of the person(s) who conducted the measurements and any training or calibration exercises to ensure intra‐ and interoperator consistency were not provided. 6Nakayama 2016: the researchers state that "ultrasonic echograms were performed on the middle of the [....] centro‐lateral thigh using Viamo SSA‐640A with the linear probe PLT‐740AT (Toshiba Medical Systems, Japan), Aplio 400/500 with th linear probe PLT‐704SBT (Toshiba Medical Systems, Japan) and Prosound SSD‐α10 with the linear probe UST‐5411 (Hitachi Aloka‐Medical, Japan)." The skin was stretched flat during measurement. Details of the ultrasound operator(s) and any training or calibration exercises to ensure intra‐ and interoperator consistency were not provided. | ||||||
Appendix 9. Evidence used to support needle size recommendations for administering vaccines intramuscularly made by National Immunization Technical Advisory Groups in 4 countries
| Table A: Evidence used to inform needle size recommendations for intramuscular injections made by National Immunization Technical Advisory Groups (NITAGs) in 4 countries | ||||
| Country/NITAG | Evidence/publications cited to support needle size recommendations | |||
| Systematic reviews | RCTs or CCTs | Ultrasound studies of muscle and subcutaneous fat thickness | Other: e.g. guidelines, textbooks, editorials, opinion pieces, etc. | |
| UK/JCVI (DoH UK 2012a)a |
0 | 2 (Diggle 2000a; Diggle 2006) |
1 (Poland 1997) |
1 textbook (Plotkin 2008a) 1 guideline (VATF 2001) 1 editorial/opinion piece (Zuckerman 2000) |
| Ireland/NIAC (NIAC 2016)b |
0 | ‐ | ‐ | 5 guidelines (CDC 2011; DoH UK 2012a; ATAGI 2013; AAP 2015; AHS 2015) |
| US/ACIP (Kroger 2017)c |
0 | 2 (Ipp 1989; Middleman 2010) |
2 (Groswasser 1997; Poland 1997) |
2 editorials/opinion pieces (Bergeson 1982; Zuckerman 2000) |
| Australia/ATAGI (ATAGI 2016)d |
0 | 3 (Diggle 2000a; Diggle 2006; Ipp 1989) |
3 (Groswasser 1997; Poland 1997; Cook 2006) |
1 guideline (CDC 2011) |
| ACIP: Advisory Committee on Immunization Practices; ATAGI: Australian Technical Advisory Group on Immunisations; CCT: Controlled Clinical Trial; JCVI: Joint Committee on Vaccines and Immunisation; RCT: Randomised Controlled Trial. NIAC: National Immunisation Advisory Committee. aSee the section of the guidance entitled "Choice of needle size" (pp 29‐30). bSee the bibliography of Chapter 2 of the guidance entitled "General Immunisation Procedures". No references are cited in the main text of Chapter 2, therefore it is impossible to state precisely which of the publications listed in the bibliography have been used to support specific needle size recommendations. cSee Section 6 of the guidance entitled "Vaccine administration" (pp 82‐107). dSee Section 2.2.5 of the guidance entitled "Vaccine injection techniques" and Table 2.2.2. | ||||
Data and analyses
Comparison 1. Comparisons between needles with different lengths and different gauges.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Individual local reactions: swelling, tenderness, and redness on the day after vaccination with the third dose of a DTwP‐Hib vaccine | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Swelling | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.36, 0.93] |
| 1.2 Tenderness | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.40, 1.00] |
| 1.3 Redness | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.01] |
Characteristics of studies
Characteristics of included studies [author‐defined order]
Diggle 2000a.
| Methods | Randomised, controlled, parallel‐group trial with 2 groups | |
| Participants |
Trial setting: Buckinghamshire, England. 8 general medical practices (out of 12 general practices approached). 2 practices were in semi‐rural villages, 4 were situated near large housing estates (1 with a high ethnic population), and 2 were in residential areas of the town (described as "up‐market areas") Participants: healthy infants (n = 119)* attending for third primary immunisation due at 16 weeks of age in 1999 and spring 2000 Exclusion criteria: routine exclusion criteria for children receiving primary immunisations, as specified in national guidance at the time the trial was conducted (DoH 1996) Of the 110 infants included in the final data analysis, 58% were male, 66% were 16 to 17 weeks of age at the time of vaccination, 24.5% were 18 to 19 weeks of age, and 9.5% were ≥ 20 weeks of age. The mean (SD) weights were 6.7 kg (0.9) in the 23 G 25 mm group and 6.8 kg (0.9) in the 25 G 16 mm group. |
|
| Interventions |
Needle sizes 23 G 25 mm, blue hub needle (n = 58) 25 G 16 mm, orange hub needle (n = 61) Vaccine(s) administered The third dose of DTwP‐Hib vaccine. The vaccine contained an aluminium adjuvant. At the start of the trial all practices were using a 0.5 mL mix of Pasteur‐Mérieux DTwP‐Hib vaccine. A change in national vaccine supply necessitated a change to a 1.0 mL mix of Evans DTwP and Wyeth Lederle HibTiter In the 23 G 25 mm group, 8/53 (15%) infants who completed the trial received the 0.5 mL vaccine In the 25 G 16 mm group, 8/57 (14%) infants who completed the trial received the 0.5 mL vaccine The 0.5 mL vaccine was presented in a uni‐dose bypass syringe that allowed liquid DTwP vaccine in the upper barrel to reconstitute dried Hib in the lower portion of the syringe. Injection technique 8 practice nurses administered the vaccines. The principal trial author (a paediatric research nurse working with the Oxford Vaccine Group) recruited and trained the practice nurses and instructed them verbally, by demonstration, and in writing to use the standard intramuscular injection technique for infants advocated by the WHO (WHO 1998). Practice nurses administered vaccines into the anterolateral thigh with the skin stretched taut and the needle inserted at a 90° angle to the skin and up to the needle hub. 75% of the infants who completed the trial in the 23 G 25 mm group were vaccinated in the right leg compared with 79% in the 25 G 16 mm group. |
|
| Outcomes |
Local reactions: erythema (redness), swelling, and tenderness at the injection site at 6 hours (on the evening after vaccination) and on the subsequent 3 evenings (day 1, day 2 and day 3)** Composite local reaction: any local reaction (any redness, swelling, or tenderness) at any time point after vaccination |
|
| Methods used to measure the outcomes | Parents were asked to examine their child's leg each evening and to complete a "Local Reaction Diary" on day 0 (6 hours after vaccination) and on days 1, 2, and 3 after vaccination. If a reaction continued after day 3, parents were asked to record the date that the reaction ended. Redness: using a ruler provided with the diary, parents measured and recorded the widest part (in mm) of any visible redness at the injection site. Swelling: using the ruler, parents measured and recorded the widest part (in mm) of any swelling that they could see or feel at the injection site, including any lump/hardness that they could feel beneath the skin. Tenderness: parents gently moved their child's leg and graded tenderness on a 0 to 3 scale: Grade 3 = child cried when their leg was moved Grade 2 = child cried or protested when the injection site was touched Grade 1 = minor reaction when the injection site was touched Grade 0 = no reaction present When analysing the data, the trial authors used a dichotomous classification for each reported outcome (i.e. outcome present/absent) and compared the incidence of each local reaction between the groups at 6 hours and on days 1, 2, and 3 after vaccination. Differences in the size of reaction between the groups were compared using a Chi2 test for trend. |
|
| Missing outcome data | In the 23 G 25 mm group, outcome data were missing for 5 (8.6%) of the randomised trial participants. Parental diaries reporting local reactions were not returned for these 5 infants. In the 25 G 16 mm group, outcome data were missing for 4 (6.5%) of the randomised trial participants. Parental diaries reporting local reactions were not returned for 3 infants, and 1 infant was mistakenly included in the study at the second rather than the third vaccination. Taking into account these reasons for missing outcome data, we judged that the data were likely to be missing at random (i.e. the fact that these data were missing was probably unrelated to actual values of the missing data). |
|
| Funding | The trial was funded by the Smith and Nephew Foundation, London, through a nursing research scholarship award 1998/1999. | |
| Notes | *The original intention was to recruit 250 infants for the trial. However, the trial was stopped early due to problems with the national vaccine supply when there was a nationwide replacement of the whole‐cell pertussis component of the combined vaccine with an acellular component that had a different reactogenicity profile. In accordance with the guidance in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c), we did not include the early stopping of the trial in the 'Risk of bias' assessment. **Immediate postvaccination reactions were also recorded by practice nurses. The stated purpose of recording these reactions was to allow practice nurses to explain to the parent how to measure any reactions; these data were not included in the final analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors describe a random component in the sequence generation process (use of a computer‐generated blocked randomisation scheme stratified by practice). |
| Allocation concealment (selection bias) | Low risk | We judged that participants and investigators enrolling participants could not have foreseen needle size allocations in advance of, or during, enrolment due to the use of sequentially numbered, sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research nurses were not blinded, and blinding of the parents of infants undergoing vaccination was incomplete. However, we judged that no trial outcomes were likely to be influenced by this lack of blinding (see Risk of bias in included studies for an explanation of this judgement). |
| Blinding of outcome assessment (detection bias) Reactogenicity outcomes (other than pain, crying) | Unclear risk | Blinding of the outcome assessors (parents) for reactogenicity outcomes was incomplete, and we considered that there was uncertainty over the potential for bias (see Risk of bias in included studies for an explanation of this judgement). |
| Incomplete outcome data (attrition bias) Reactogenicity outcomes | Low risk | Missing outcome data were reasonably balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | We considered that there was insufficient information to permit a judgement of low risk or high risk (see Risk of bias in included studies for an explanation of this judgement). |
| Other bias | Low risk | This trial appeared to be free of other sources of bias. |
Diggle 2006.
| Methods | Randomised, controlled, parallel‐group, non‐inferiority trial with 3 groups | |
| Participants |
Trial setting: 2 primary care trusts in England: 1. Vale of Aylesbury; 2. Buckinghamshire and North East Oxfordshire. 18 general medical practices (out of 35 general practices approached). The locations of practices ranged from affluent villages to council housing estates, with 2 practices comprising a high proportion of ethnic minority families. Participants: healthy infants (n = 696) attending for routine vaccinations due at 2, 3, and 4 months of age and where the first routine vaccination was due between February 2002 and January 2004 Exclusion criteria: < 37 weeks' gestation, birth weight < 2500 g, had a severe chronic disease, and were undergoing treatment likely to alter the immune response to vaccines or had conditions that could bias the evaluation of an immune response (e.g. congenital or acquired immunodeficiency) Of the randomised infants (n = 696), 52% were male. At the time of the first, second, and third vaccinations infants were aged: first: 7.8 to 11 weeks (mean about 8.8); second: 11.7 to 16 weeks (mean about 13); and third: 15.8 to 21 weeks (mean about 17.3). The mean (SD) weights of the infants at the time of vaccination were: 23 G 25 mm: 5.2 kg (0.7); 25 G 16 mm: 5.3 kg (0.7); 25 G 25 mm: 5.3 kg (0.7), for the first vaccination 23 G 25 mm: 6.0 kg (0.8); 25 G 16 mm: 6.0 kg (0.7); 25 G 25 mm: 6.1 kg (0.8), for the second vaccination 23 G 25 mm: 6.6 kg (0.8); 25 G 16 mm: 6.8 kg (0.8); 25 G 25 mm: 6.8 kg (0.8), for the third vaccination |
|
| Interventions |
Needle sizes Group 1: 23 G 25 mm, blue hub needle (n = 240) Group 2: 25 G 16 mm, orange hub needle (n = 230) Group 3: 25 G 25 mm, orange hub needle (n = 226) Vaccines administered 1. First, second, and third doses (0.5 mL)* of DTwP‐Hib vaccine (ACT‐Hib DTwP; Pasteur‐Mérieux‐MSD, Berkshire). The vaccine contained an aluminium adjuvant. 2. First, second, and third doses (0.5 mL)* of MenC vaccine. 72% of infants received Meningitec (Wyeth Vaccines, Berkshire, UK) at the first and second doses and 76% at the third dose, and the remainder received Menjugate (Chiron Vaccines, Liverpool, UK). Both of the MenC vaccines were conjugated to the CRM197 diphtheria‐based protein, and both contained an aluminium adjuvant. 3. At each dose, all infants received the same brand of live oral polio vaccine (Poliomyelitis Monodose vaccine, GlaxoSmithKline). Injection technique 3 qualified and experienced paediatric nurses, who were appointed specifically for the trial and who were trained by the principal trial author, administered the vaccines either within the general practice setting or at the child's home if parents were unable to attend the general practice. The nurses used the standard intramuscular injection technique for infants advocated by the WHO (WHO 2004). The vaccines were injected into the anterolateral thigh with the skin stretched flat between the thumb and forefinger and the needle inserted at a 90° angle into the skin up to the hub. The DTwP‐Hib vaccine was administered in the right thigh, and the MenC vaccine was given concurrently into the left thigh. |
|
| Outcomes |
Immunogenicity outcomes Vaccine non‐response (immunogenicity failure): the number of participants who failed to reach the following predefined protective antibody concentration thresholds:
Antibody concentrations: geometric mean concentrations of diphtheria, tetanus, and Haemophilus influenzae type B antibodies and geometric mean titres of serogroup C meningococcal glycoconjugate antibodies 28 to 42 days after the third vaccine dose Local reactions: redness, swelling, hardness, and tenderness at both injection sites at 6 hours (on the evening after vaccination) and on the subsequent 3 evenings (day 1, day 2, and day 3) after vaccination with each vaccine dose Severe local reaction: redness and swelling after vaccination covering more than two‐thirds of the anterolateral thigh Composite local reactions: any local reaction (any redness, swelling, hardness, or tenderness) at any time point after vaccination Systemic reactions and use of analgesics:
|
|
| Methods used to measure the outcomes |
Immunogenicity outcomes Following application of a local anaesthetic cream (Ametop; Smith and Nephew Healthcare, Hull, UK), a 5 mL venous blood sample was taken by a research nurse at 28 to 42 days after administration of the third vaccine dose. All venepuncture procedures were carried out in the family home. Analyses were conducted at 3 separate laboratories.
It was not possible to allocate serum into the appropriate number of aliquots required to go to the 3 separate laboratories. Priority for the serum analyses were therefore assigned in the order of Hib, tetanus (for the local laboratory), MenC (for the Health Protection Agency laboratory), and diphtheria Ags (for the US laboratory). Fewer serum samples were therefore analysed for diphtheria antibody concentrations. No analysis of the immune response to the polio vaccine was conducted, as the vaccine was administered orally. No analysis of the immune response to the pertussis component of the combined vaccine was conducted due to the absence of a clear correlate of pertussis protection. Local reactions Parents were asked to examine the injection sites on their child's legs (both right and left) each evening and to complete a "Reaction Diary" on day 0 (6 hours after vaccination) and on the subsequent 3 evenings (day 1, day 2, and day 3) after vaccination. If a reaction continued after day 3, parents were asked to record the date that the reaction ended. At the first vaccination visit, the research nurses instructed parents on how to complete the reaction diary, and the demonstration was reinforced by written instructions in the diary. If the nurse had concerns regarding parental understanding of the diary card, the nurse contacted the parent by telephone on the first day postvaccination to offer further clarification if necessary. The methods used to measure redness, swelling, and tenderness were identical to the methods specified in the table above for Diggle 2000a. Hardness: using a ruler provided with the diary, parents measured and recorded the widest part (in mm) of any hardness or thickening of the skin at the injection sites. For the purposes of data analysis, a dichotomous classification was used for each reported outcome (i.e. outcome present/absent). Systemic reactions and use of analgesics Parents were asked to answer the questions listed below and to record general reactions in a diary at 6 hours (on the evening after the vaccination) and on the subsequent 3 evenings (day 1, day 2, and day 3). If a reaction continued after day 3, parents were asked to record the date that the reaction ended.
Parents were also asked to record:
|
|
| Missing outcome data |
Immunogenicity outcomes: of the 696 infants recruited to the study, a serum sample was obtained from 614 (88%) infants. 2 samples were excluded from the immunogenicity endpoints as the infants had been enrolled in error (infants had a birth weight < 2.5 kg). In 120 (20%) of the samples taken, there was insufficient serum to perform immunogenicity analyses for all 4 Ags. Serological analyses were prioritised in the order Hib, tetanus, MenC, and diphtheria Ags; fewer samples were analysed for diphtheria due to smaller amounts of serum obtained. The numbers of samples not analysed for each Ag in each needle group are presented below.
The reasons for missing immunogenicity outcome data were similar across groups (see notes above regarding serum aliquot assignment). On this basis, we judged that these data were likely to be 'missing at random' (i.e. the fact that they were missing was probably unrelated to actual values of the missing data). Reactogenicity outcomes: the proportions of randomised participants analysed for reactogenicity in the needle size groups after each dose of the vaccines were as follows.
During the trial, 11 infants experienced redness and swelling covering more than two‐thirds of the anterolateral thigh, contraindicating receipt of further whole‐cell pertussis‐containing vaccine and therefore necessitating their withdrawal from the trial. 10 of the infants were vaccinated using the narrow, short (25 G 16 mm) needle and 1 using the wide, long (23 G 25 mm) needle. We did not treat the data for these 11 infants as missing outcome data, and analysed and reported severe local reactions separately from other reactogenicity outcomes in the Effects of interventions section. |
|
| Funding | Funding for the trial was provided from 2 sources:
The principal trial author described the funding arrangements as follows: "Funding for the study (£137,000) was awarded through a competitive grant scheme administered by the NHS executive South East Region and the study budget was formally administered through the University of Oxford Department of Paediatrics [....] Additional support, in the form of an unrestricted grant, was provided by Becton Dickinson, manufacturer of injection needles. As each of the three needles were produced by this manufacturer, this funding source did not compromise the integrity of the trial. Neither of the funding sources had any role in study design and conduct; collection, management, analysis, or interpretation of the data; or in preparation, review or approval of the publication manuscript" (Diggle 2006). |
|
| Notes | *Information on the volume of vaccine administered at each dose was obtained from Summary of Product Characteristics for each vaccine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors describe a random component in the sequence generation process (use of a computer‐generated randomisation scheme stratified by general practice with random variable block sizes). |
| Allocation concealment (selection bias) | Low risk | We judged that participants and investigators enrolling participants could not have foreseen needle size allocations in advance of, or during, enrolment due to the use of sequentially numbered, sealed, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research nurses were not blinded, and blinding of the parents of infants undergoing vaccination was incomplete. However, we judged that no trial outcomes were likely to be influenced by this lack of blinding (see Risk of bias in included studies for an explanation of this judgement). |
| Blinding of outcome assessment (detection bias) Immunogenicity outcomes | Low risk | We considered that blinding of outcome assessment was ensured and that it was unlikely that the blinding could have been broken. Blood samples were analysed by laboratory staff who were blinded to needle size allocation. |
| Blinding of outcome assessment (detection bias) Crying | Low risk | Blinding of the outcome assessors (parents) was incomplete. However, we considered it unlikely that parental assessment and reporting of persistent inconsolable crying for ≥ 4 hours would be influenced by knowledge of needle size. |
| Blinding of outcome assessment (detection bias) Reactogenicity outcomes (other than pain, crying) | Unclear risk | Blinding of the outcome assessors (parents) was incomplete, and we considered that there was uncertainty over the potential for bias (see Risk of bias in included studies for an explanation of this judgement). |
| Incomplete outcome data (attrition bias) Immunogenicity outcomes | Low risk | Missing immunogenicity outcome data were reasonably balanced in numbers across the study groups, with similar reasons for missing data across groups. |
| Incomplete outcome data (attrition bias) Reactogenicity outcomes | Low risk | Missing reactogenicity outcome data were balanced in numbers across the needle size groups for all vaccine doses and at all time points. There were similar reasons for missing data across groups, with the exception of trial withdrawals due to severe local reactions (see Risk of bias in included studies for an explanation of this judgement). |
| Selective reporting (reporting bias) | Low risk | Although we did not examine the trial protocol, the principal trial author gave us access to all of the relevant original trial data. We were confident that we had access to the results for all of the prespecified primary and secondary trial outcomes that were of interest to our review. |
| Other bias | Low risk | This trial appeared to be free of other sources of bias. |
Pathak 2007.
| Methods | Randomised, controlled, parallel‐group trial with 2 groups | |
| Participants |
Trial setting: Chandigarh, India. The vaccination room of a tertiary paediatric hospital (the Advanced Pediatrics Center, Postgraduate Institute of Medical Education and Research) Participants: infants (n = 320) up to 24 weeks of age attending for routine primary vaccines in January and February 2007 Exclusion criteria: preterm (gestational age < 37 weeks), chronic or acute illnesses Of the randomised participants (n = 320), 59% were male, 43% were aged ≤ 6 weeks at the time of vaccination, 29% were 7 to 12 weeks, and 28% were 13 to 24 weeks. In the 25 G 25 mm group (n = 161): 17.4% were classified as malnourished based on WHO 2006 growth chart (11.9% Grade I malnutrition; 4.3% Grade II; 1.2% Grade III) In the 23 G 25 mm group (n = 159): 11.2% were classified as malnourished based on WHO 2006 growth chart (6.3% Grade I malnutrition; 4.9% Grade II; 0% Grade III) The mean weights (SD) of the 155 infants who were enrolled and randomised in February 2007 were 4.81 kg (1.05) in the 25 G 25 mm group (n = 82) and 5.13 kg (1.15) in the 23 G 25 mm group (n = 73).* |
|
| Interventions |
Needle sizes 23 G 25 mm needle (n = 159) 25 G 25 mm needle (n = 161) The needle hubs were colour coded (manufacturer: Becton, Dickinson and Company). Vaccines administered Infants received either the first, second, or third doses (0.5 mL) of either:
In the 23 G 25 mm group (n = 159):
In the 25 G 25 mm group (n = 161):
Injection technique Trained staff nurses administered the vaccines using the standard intramuscular injection technique for infants advocated by the WHO. Vaccines were administered into the anterolateral thigh, with the skin stretched flat between the thumb and forefinger, the needle inserted at a 90° angle and pushed down into the muscle. It is unclear if the needle was inserted to its full length up to the hub for all infants; we were informed by 1 of the trial authors that if an infant was malnourished, it is possible that the staff nurses may not have inserted the full length of the needle in order to avoid injury to bone. |
|
| Outcomes |
Pain: measured using the MBPS and a VAS Crying: total crying duration (seconds). The proportions of infants still crying at 30, 60, and 90 seconds after vaccination Local reactions: redness, swelling, tenderness, and restriction of movement on days 1, 2, and 3 after vaccination Composite local reaction: any local reaction (any redness, swelling, tenderness, or restricted movement) on day 1 after vaccination Systemic reactions and medication use: fever and medication use on days 1, 2, and 3 after vaccination |
|
| Methods used to measure the outcomes |
Pain assessment using the MBPS: assessments were conducted by 2 researchers (a nurse and a doctor). Infant behaviour was assessed at baseline using the MBPS around 5 seconds to 1 minute before the vaccination procedure (information provided by trial author). The vaccination procedure was recorded using a digital camera (Nikon Coolpix 7900) 5 seconds before the vaccine was administered and continued until the baby stopped crying. The recordings were reviewed to score postvaccination behaviour of infants using the MBPS. The researchers who conducted the baseline and postvaccination assessments were reportedly blinded to needle gauge (information provided by trial author). Net pain scores were calculated by subtracting prevaccination scores from postvaccination scores. Pain assessment using the VAS: postvaccination pain was also scored by each infant's mother or guardian and by a researcher (nurse) using a VAS. The nurse was blinded to needle gauge (information provided by trial author). The VAS consisted of a straight, hatched horizontal line drawn on paper 100 mm in length and divided into 10 equal parts. The researcher (nurse) explained to each infant's mother that, on a scale of 0 to 100, 0 indicated no pain and 100 indicated maximum possible pain; mothers/guardians were asked to rate their infant's pain accordingly (information provided by trial author). Crying duration: the total crying time (time to cessation of crying) in seconds was measured by a researcher from the video recordings described above. Local reactions, systemic reactions, and medication use: these outcomes were only measured for the 155 infants who were enrolled and randomised in February 2007. A researcher (nurse) made daily telephone calls to parents on the 3 days following vaccination. Parents were asked to report on the presence of redness, swelling, tenderness, restriction of movement, fever, and the use of paracetamol. Study nurses instructed parents on how to report these outcomes. Fever was defined as an axillary temperature of > 37.8 °C measured predominantly using a mercury thermometer, although a few parents were reported to have used a digital thermometer. Parents were asked to take the infant's temperature morning and evening and at any time when parents considered that the infant might be febrile (information provided by trial author). Parents were asked to report any visible redness at the injection site and their subjective perception of any restriction of movement experienced by their infant. Parents were told to report tenderness if their infant cried when the injection site was touched. For swelling, a graded scale (mild, moderate, or severe reaction) was initially used, but the trial authors reported that parents had "difficulty in objectively measuring the swelling as well as induration. Therefore we adopted a binary strategy (presence or absence) of reporting all local and systemic reactions with clustering of mild, moderate and severe reaction in a single group." |
|
| Missing outcome data | Data were available for all of the 320 participants who were enrolled and randomised in January and February 2007 for the outcomes of pain and crying duration. Data on other reactogenicity outcomes were only available for 100/155 (64.5%) participants enrolled and randomised in February 2007. In the 23 G 25 mm group, 26/73 (35.6%) were lost to follow‐up, compared with 29/82 (35.4%) in the 25 G 25 mm group. In both groups, all participants who were lost to follow‐up did not have mobile or land‐line phones and could not be contacted to ascertain outcomes. Taking into account the reason for missing outcome data, we judged that the data were likely to be missing at random (i.e. the fact that these data were missing was probably unrelated to the actual values of the missing data). |
|
| Funding | The trial was not funded and was conducted as a thesis for an Master in Science degree in Nursing. | |
| Notes | *Details on mean weights were not available for the infants enrolled and randomised in February 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors describe a random component in the sequence generation process (use of a computer‐generated randomisation scheme). |
| Allocation concealment (selection bias) | Low risk | We judged that participants and investigators enrolling participants could not have foreseen needle size allocations in advance of, or during, enrolment due to the use of sequentially numbered, sealed, opaque envelopes to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The trained staff nurses who administered the injections were not blinded (needle hubs were colour coded). We deemed blinding of the parents of infants undergoing vaccination to be incomplete. However, we judged that the trial outcomes were not likely to be influenced by lack of blinding (see Risk of bias in included studies for an explanation of this judgement). |
| Blinding of outcome assessment (detection bias) Pain | Unclear risk | We considered that there was some uncertainty over the potential for bias (see Risk of bias in included studies for an explanation of this judgement). |
| Blinding of outcome assessment (detection bias) Crying | Low risk | Crying time was assessed from digital camera recordings by a researcher who was reportedly blinded to needle size (see Risk of bias in included studies for an explanation of this judgement). |
| Blinding of outcome assessment (detection bias) Reactogenicity outcomes (other than pain, crying) | Unclear risk | Blinding of the outcome assessors (parents) was incomplete, and we considered that there was uncertainty over the potential for bias (see Risk of bias in included studies for an explanation of this judgement). |
| Incomplete outcome data (attrition bias) Reactogenicity outcomes | Low risk | There were no missing outcome data for pain and crying duration. For other reactogenicity outcomes, missing data were reasonably balanced in numbers across intervention groups, with similar reasons for missing data across groups. |
| Selective reporting (reporting bias) | Unclear risk | We considered that there was insufficient information to permit a judgement of low or high risk of bias (see Risk of bias in included studies for an explanation of this judgement). |
| Other bias | Low risk | This trial appeared to be free of other sources of bias. |
Nirupam 2008.
| Methods | Randomised, controlled, parallel‐group trial with 3 groups | |
| Participants |
Trial setting: New Delhi, India. A child health promotion centre in a tertiary paediatric hospital Participants: infants (n = 150) aged 6 to 10 weeks attending between February 2008 and March 2009 for the first doses of a DTwP vaccine and a hepatitis B vaccine Exclusion criteria: weight < 2.5 kg, progressive neurological disorders, major congenital anomalies, "any illness and any skin disorder" 51% of the infants were male. The infants weighed 3.5 to 6.5 kg and were 50 to 66 cm in length. In the 22 G 25 mm, 23 G 25 mm, and 24 G 25 mm groups the mean weights (SD) were 4.8 kg (0.6) in 22 G 25 mm, 4.75 kg (0.48) in 23 G 25 mm, and 4.8 kg (0.49) in 24 G 25 mm, and mean lengths (SD) were 57 cm (2.9) 22 G 25 mm, 56.3 cm (2.3) in 23 G 25 mm, and 55.8 cm (2.1) in 24 G 25 mm. |
|
| Interventions |
Needle sizes 22 G 25 mm (n = 50) 23 G 25 mm (n = 50) 24 G 25 mm (n = 50) The needle hubs were colour coded (manufacturer: Becton, Dickinson and Company; surgical grade stainless steel, regular bevel, regular wall type needles). Vaccines administered The first dose of DTwP (Triple Antigen, Serum Institute of India Ltd) and the first dose of a hepatitis B vaccine (GeneVac‐B, Serum Institute of India Ltd) GeneVac‐B consists of purified surface Ag of HBV obtained by culturing genetically engineered Hansenula polymorpha yeast cells expressing the surface Ag gene of the virus. There is no material of human or animal origin. Each paediatric dose of 0.5 mL contains 10 μg of surface Ag adsorbed on ≤ 1.25 mg of aluminium hydroxide, with ≤ 0.01% thimerosal added as a preservative.* Injection technique 2 nurses assisted with the vaccination procedure. 1 nurse injected the vaccine while the other held the child's lower limb steady. The vaccines were administered using the standard intramuscular injection technique for infants advocated by the WHO. The DTwP vaccine was administered in the left and hepatitis B vaccines in the right anterolateral aspects of the thigh with the skin stretched flat between the thumb and forefinger and the needle inserted at a 90° angle into the skin up to the hub. |
|
| Outcomes |
Crying: persistent inconsolable crying Local reactions: redness, swelling, and tenderness at the injection site at 6 hours and on the following 3 evenings (days 1, 2, and 3) after vaccination Systemic reactions and medication use: fever, vomiting, drowsiness, irritability and refusal to feed, seizures. Use of analgesics (paracetamol), domperidone, or promethazine for vomiting |
|
| Methods used to measure the outcomes | Outcomes were recorded by parents using a diary card. Parents were contacted by telephone daily by a doctor (paediatric resident) "to ensure proper observations and entries." The doctor making the phone calls was blinded to needle gauge. The infants were examined in the hospital on day 4 after vaccination and the entries in the diary card were verified by a researcher (1 of the trial authors) who was blinded to the needle group Fever: defined as an axillary temperature > 37.4 °C as measured with a digital thermometer. Parents were asked to take the infants temperature at 6 p.m. each evening and at any time when parents considered that the infant might be febrile (information provided by trial author). Persistent inconsolable crying: defined as persistent crying for > 3 hours Vomiting: defined as regurgitating a large amount of ingested milk 30 minutes after ingestion Drowsiness: graded by parents on a 1 to 4 scale: 1 = normal sleep duration; 2 = more sleepy than usual in terms of duration of sleep, but arouses on own; 3 = more sleep duration but needs to be aroused; 4 = not arousable from sleep Irritability: graded by parents on a 1 to 4 scale: 1 = none; 2 = easily consolable; 3 = requiring increased attention but consolable; 4 = inconsolable Refusal to feed: recorded as either 'Yes' or 'No' Redness: parents were provided with a measuring tape and were asked to measure and record the widest part of any visible redness at the injection site at 6 p.m. each evening. Parents were instructed in the measurement technique "with the use of pictorial representation and visual cues" (information provided by trial authors). Swelling: parents used the measuring tape to record the circumferential diameter of both thighs at the site of maximum swelling at 6 p.m. each evening. This measurement was compared with the baseline measurement taken just prior to vaccination. Parents were instructed in the measurement technique "with the use of pictorial representation and visual cues" (information provided by trial authors). Any swelling was defined as an increase in thigh circumference measurement of 5 to 20 mm from baseline; significant swelling was defined as an increase in thigh circumference measurement of > 20 mm from baseline. Tenderness: parents touched the vaccination site and graded tenderness on a 1 to 4 scale: 1 = no effect on infants activity on touching the site; 2 = makes non‐specific grimace when vaccination site was touched; 3 = withdrew their legs on touching; 4 = cried when vaccination site was touched. Each thigh was tested separately when the child was comfortable and awake prior to touching the site. Parents were instructed that it should be a "gentle press" and that too much force should not be applied at the vaccination site. When analysing the trial data, the trial authors used a dichotomous classification for each reported outcome (i.e. outcome present/absent) and compared the incidence of each local reaction between the groups at 6 hours and on days 1, 2, and 3 after vaccination. |
|
| Missing outcome data | Only 1 trial participant was lost to follow‐up (in the 23 G 25 mm group). The family was reported as "having moved out of the city for personal reasons." | |
| Funding | The trial authors state that "this study was not funded by any agency and the authors did not take any grant from any agency." | |
| Notes | *Details obtained from Shivananda 2006 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors describe a random component in the sequence generation process (use of a computer‐generated randomisation sequence). |
| Allocation concealment (selection bias) | Low risk | We judged that participants and investigators enrolling participants could not have foreseen needle size allocations in advance of, or during, enrolment due to the use of sequentially numbered, sealed, opaque envelopes to conceal allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The nurses who administered the injections were not blinded (needle hubs were colour coded). We deemed blinding of the parents of infants undergoing vaccination as incomplete. However, we judged that the trial outcomes were not likely to be influenced by lack of blinding (see Risk of bias in included studies for an explanation of this judgement). |
| Blinding of outcome assessment (detection bias) Crying | Low risk | We deemed blinding of the outcome assessors (parents) as incomplete. However, we considered it unlikely that parental assessment and reporting of persistent inconsolable crying for > 3 hours would be influenced by knowledge of needle size. |
| Blinding of outcome assessment (detection bias) Reactogenicity outcomes (other than pain, crying) | Unclear risk | Parents recorded local reactions in a diary, and the diary entries were "verified" by a researcher blinded to needle gauge. As the needle hubs were colour coded and as parents were present during the vaccination procedure, we considered that there was uncertainty over the potential for detection bias for these outcomes (see Risk of bias in included studies for an explanation of this judgement). |
| Incomplete outcome data (attrition bias) Reactogenicity outcomes | Low risk | Outcome data were missing for only 1 trial participant. |
| Selective reporting (reporting bias) | Unclear risk | We considered that there was insufficient information to permit a judgement of low risk or high risk (see Risk of bias in included studies for an explanation of this judgement). |
| Other bias | Low risk | This trial appeared to be free of other sources of bias. |
Middleman 2010.
| Methods | Randomised, controlled, parallel‐group trial with 2 groups | |
| Participants |
Trial setting: Houston, TX. Participants enrolled between December 2001 and October 2004 at city clinics, a health fair, and a high school. Vaccinations performed either at a clinic site or in participants' homes if they were unable to attend the clinic. Participants: obese adolescents (n = 65) aged 14 to 24 years, weighing > 90 kg (females) and > 120 kg (males) who reported that they had never received a hepatitis B vaccination series. Exclusion criteria: immune system illness, chronic disease, long‐term steroid use, pregnant, planned significant weight loss Of the 24 adolescents included in the final data analysis 22 were female; age at enrolment (range 14.1 to 24.7 years); BMI (range 31.1 to 49.5 kg/m2); deltoid skinfold thickness (range 32.7 to 49.3 mm); triceps skinfold thickness (range 32 to 50 mm). The trial participants were described as "predominantly Hispanic" and as of low socio‐economic status. |
|
| Interventions |
Needle sizes 38 mm (n = 36) 25 mm (n = 29) The needles had the same gauge, but the precise gauge number is unknown (personal communication with trial authors). It is not known if the hubs of the needles were colour coded. Vaccine(s) administered 3 doses of a recombinant hepatitis B vaccine (Engerix B, GlaxoSmithKline Biologicals, Rixensart, Belgium) administered on a 0 (first dose), 1 month (second dose), 4 month (third dose) schedule. Participants who were younger than 19 years received 0.5 mL of the vaccine, and those aged 19 years or over received 1.0 mL. 5 participants (out of the 14 analysed) in the 38 mm group and 2 participants (out of the 10 analysed) in the 25 mm group received the vaccine series with a 0.5 mL dose. Each 0.5 mL dose of Engerix B contains 10 µg of hepatitis B surface Ag adsorbed on 0.25 mg aluminium as aluminium hydroxide. Each 1 mL dose contains 20 µg of hepatitis B surface Ag adsorbed on 0.5 mg aluminium as aluminium hydroxide. Engerix B is formulated without preservatives and contains trace amounts of thimerosal (< 1.0 µg mercury), sodium chloride (9 mg/mL), and phosphate buffers (disodium phosphate dihydrate, 0.98 mg/mL; sodium dihydrogen phosphate dihydrate, 0.71 mg/mL).* The median time from the first to the third vaccinations was 136 days (range 119 to 177) in the 25 mm (1 inch) group and 130 days (range 123 to 156) in the 38 mm (1½ inch) group. Injection technique The principal trial author (a faculty paediatrician (adolescent medicine subspeciality)) and a medical student (trained by the principal trial author) administered the vaccine injections using the same standardised protocol. Injections were given at a 90° angle to the deltoid muscle, leaving 2 to 3 mm of needle visible between the arm and the needle hub. The skin was stretched before needle insertion (personal communication with trial authors). |
|
| Outcomes |
Immunogenicity outcomes Vaccine non‐response (immunogenicity failure): the number of participants who received all 3 doses of the vaccine but who failed to reach anti‐HBs titre levels ≥ 1.5 mIU/mL. NOTE: in our review we used the cut‐off threshold value of ≥ 10 mIU/mL cited in Appendix 3. Using this threshold value had no impact on the trial results pertaining to seroprotection. Antibody concentrations: antibody titres to hepatitis B surface Ag (anti‐HBs) 2 months after the third vaccination |
|
| Methods used to measure the outcomes | Blood was obtained at baseline and 2 months after the third vaccination. The time from third vaccination to titre assessment was 64 days (range 57 to 72) in the 25 mm needle group and 66 days (range 59 to 76) in the 38 mm needle group. Presence of antibody to hepatitis B surface Ag (anti‐HBs) was determined at baseline (to rule out previous immunisation) and after completion of the vaccination series using the AUSAB kit (Abbott Laboratories, North Chicago, IL). This kit uses a solid‐phase ELISA technique to detect anti‐HBs levels in serum or plasma. Testing was carried out in a laboratory at the Baylor College of Medicine by a laboratory technician who was blinded to group allocation (personal communication with trial authors). | |
| Missing outcome data | In the 25 mm needle group, 19/29 (65.5%) randomised participants were not included in the final data analysis. 10 were withdrawn for positive baseline anti‐HBs (evidence of previous immunisation), 8 moved or did not response to follow‐up communication, and 1 was a vaccine non‐responder (and received a fourth dose of the vaccine as per the study protocol). In the 38 mm needle group, 22/36 (61%) randomised participants were not included in the final data analysis. 7 were withdrawn for positive baseline anti‐HBs, 1 was a vaccine non‐responder, and the remainder either moved, did not respond to follow‐up communication, or voluntarily withdrew from the trial (personal communication with trial author). |
|
| Funding | Funding for the trial was provided from the following sources:
GlaxoSmithKline donated all doses of the Engerix vaccine used in the trial. |
|
| Notes | *Details regarding the formulation of the vaccine were obtained from a 2001 Summary of Product Characteristics for Engerix B (manufactured by SmithKline Beecham Biologicals (now known as GlaxoSmithKline), Rixensart, Belgium) (SKB 2001). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors describe a random component in the sequence generation process (use of a random numbers table). |
| Allocation concealment (selection bias) | High risk | No allocation concealment procedure was used in the trial (personal communication with trial authors). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | There was no blinding of the trial participants and personnel. However, we judged that the trial outcomes were not likely to be influenced by lack of blinding (see Risk of bias in included studies for an explanation of this judgement). |
| Blinding of outcome assessment (detection bias) Immunogenicity outcomes | Low risk | The laboratory technician who analysed the blood samples was unaware of what needle group (25 mm or 38 mm) the samples were from (personal communication with trial authors). |
| Incomplete outcome data (attrition bias) Immunogenicity outcomes | Unclear risk | Missing outcome data were balanced in numbers across the 2 needle size groups with similar reasons for missing data across groups. However, there was a notable disparity between the number of participants randomised (n = 65) and analysed (n = 24), and we considered that there was some uncertainty over the potential for bias. |
| Selective reporting (reporting bias) | Unclear risk | We considered that there was insufficient information to permit a judgement of low risk or high risk (see Risk of bias in included studies for an explanation of this judgement). |
| Other bias | Low risk | There was an imbalance in the ages of the trial participants in the 2 study groups, which resulted in differences between the groups in the dose of the vaccine administered. However, we judged that this was unlikely to have materially influenced the trial results (see Risk of bias in included studies for an explanation of this judgement). |
Ag: antigen anti‐HBs: hepatitis B surface antibodies BMI: body mass index DTwP: diphtheria, tetanus and whole‐cell pertussis DTwP‐Hib: diphtheria, tetanus, whole‐cell pertussis, and Haemophilus influenzae type b DTwP‐Hib‐Hep B: diphtheria, tetanus, whole‐cell pertussis, Haemophilus influenzae type b, and hepatitis B ELISA: enzyme‐linked immunosorbent assay HBV: hepatitis B virus MBPS: Modified Behavioural Pain Scale MenC: meningococcal C NHS: National Health Service (UK) SBA: serum bactericidal activity SD: standard deviation VAS: visual analogue scale WHO: World Health Organization
Characteristics of excluded studies [ordered by year of study]
| Study | Reason for exclusion |
|---|---|
| Ipp 1989 | Not a randomised controlled trial. The trial participants were assigned sequentially to the study groups. Additional notes: children aged 18 months (n = 246) who were receiving a combined diphtheria, tetanus, whole‐cell pertussis, and inactivated polio vaccine (DTwP‐IPV) were assigned sequentially to 1 of 3 groups: intramuscular injection into the deltoid region with a 25 G 16 mm needle; intramuscular injection into the anterolateral thigh with a 25 G 25 mm needle; intramuscular injection into the anterolateral thigh with a 25 G 16 mm needle. |
| Shaw 1989 | Trial participants were not children and adolescents. The mean ages in years of the participants in the 3 comparison groups of the trial were 37.4 (SD 11.0), 35.5 (SD 9.9), and 38.4 (SD 11.7). Additional notes: a parallel‐group randomised trial with 3 groups involving healthy adult healthcare workers. Participants (n = 634) were randomly allocated to receive a plasma‐derived hepatitis B vaccine (Heptavax‐B, MSD) administered with: 1) a 25 mm needle into the deltoid region of the upper arm; 2) a 25 mm needle into the upper lateral quadrant of the buttock; 3) a 50 mm needle into the buttock.* *Administering hepatitis B vaccine into the buttock is no longer recommended for infants, children, adolescents, and adults. The Centers for Disease Control and Prevention have recommended that: "hepatitis B vaccine administered by any route or site other than intramuscularly in the deltoid muscle should not be counted as valid and should be repeated unless serologic testing indicates that an adequate response has been achieved" (CDC 2005; CDC 2006a). |
| Johnsen 1995 | Not a randomised controlled trial and did not involve children or adolescents as defined in this review Additional notes: in 1992, approximately 1600 employees of the Massachusetts Department of Correction (DOC) and 200 healthcare workers in 21 DOC correctional facilities received 3 doses of a hepatitis B vaccine (Engerix‐B) administered into the deltoid muscle. It was subsequently discovered that the vaccines had been administered with needles of different lengths (16, 25, and 38 mm) in different facilities. 225 staff had blood drawn for hepatitis B surface antibody titre determinations, and the immune response was analysed according to needle length. Facilities were classified based on the shortest needle used when more than 1 needle length had been used at a facility. The facility from where blood had been drawn could be identified for only 174 individuals. |
| Cook 2005 | Different injection techniques were used in the comparison groups. Additional notes: children aged 2, 4, 6, and 18 months (n = 375) were randomly allocated to receive an acellular pertussis‐containing and Haemophilus influenzae type b vaccine administered intramuscularly into the anterolateral thigh using 3 injection techniques: 1) the Australian technique with a 23 G 25 mm needle; 2) the US technique with a 23 G 25 mm needle; or 3) the WHO technique with a 25 G 16 mm needle. The trial authors describe these techniques as follows.
|
| Jackson 2008 | Not a randomised controlled trial Additional notes: Children (n = 1498; median age 4.5 years) participating in a postlicensure assessment of the safety of the fifth consecutive dose of a combined diphtheria, tetanus, and acellular pertussis vaccine (DTaP) were vaccinated at a large health maintenance organisation in Washington state by clinical staff "according to their usual practice." Of the 1315 children included in the final analysis, 1174 were vaccinated in the upper arm (381 with a 16 mm needle; 793 with a 25 mm needle), and 141 were vaccinated in the thigh (49 with a 16 mm needle and 92 with a 25 mm needle). |
| Ozdemir 2012 | Precise methods used to allocate participants to study groups could not be definitively determined, and insufficient details were available regarding outcome data. Additional notes: full‐term, healthy macrosomic infants (n = 65) with a birth weight > 4000 g born at a maternity teaching hospital in Turkey between February and April 2011 were vaccinated with 3 doses (0.5 mL) of a recombinant hepatitis B vaccine administered on a 0 (first dose, shortly after birth), 1 month (second dose), 6 month (third dose) schedule. For the first dose of the vaccine, either a 26 G 25 mm needle (n = 32) or a 26 G 16 mm needle (n = 33) was used to administer the vaccine. All subsequent vaccinations (at 1 and 6 months) in both groups were administered using a 26 G 25 mm needle. All infants were vaccinated at birth (first dose) by the same nurse in the hospital. All vaccinations at 1 and 6 months (i.e. second and third doses) were performed by the same nurse at an outpatient clinic. Vaccines were administered into the quadriceps muscle of the anterolateral thigh using the 'pinching technique' described by Groswasser 1997 which "requires bunching the thigh muscle at the injection site to increase muscle mass and to minimize the chance of striking bone." The needle was inserted at a 90° angle to the skin and up to the needle hub (i.e. no needle visible between the skin and hub). |
| Fateh 2014 | Different types of syringe were used in the comparison groups. Additional notes: the trial participants were children (n = 1000) attending 4 primary healthcare centres for either the first dose (due at age 2 months), second (due at age 4 months), third (due at 6 months), fourth (booster 1, due at 18 months), or fifth (booster 2, due at 6 years) doses of a DTwP vaccine. The children were randomly allocated to be vaccinated with either:
The trial authors do not describe the injection technique used to administer the vaccine. The first to the fourth doses of the vaccine were injected into the thigh. The fifth dose was injected into the deltoid. |
DTwP: diphtheria, tetanus, and whole‐cell pertussis SD: standard deviation WHO: World Health Organization
Differences between protocol and review
In the review protocol, we did not specify what measures of treatment effect we would use in instances were continuous data were summarised using geometric means. In accordance with guidance proposed by the Cochrane Infectious Diseases Group (Donegan 2010), we reported geometric mean ratios as effect measures and included this information in the Measures of treatment effect section.
In the review protocol, we included a table in Appendix 3 outlining thresholds of vaccine‐induced correlates and surrogates of protection for selected vaccines. We omitted a threshold for protection against meningitis C based on rabbit complement. We have rectified this omission in the review (see Appendix 3).
We included some outcomes in the 'Summary of findings' tables that were not prespecified in the protocol. We have explained our rationale for the entries in the 'Summary of findings' tables in the Data collection and analysis section, under the heading 'Summary of findings' tables. In the Effects of interventions section of the review we have presented the results for each outcome in the same order as they appear in the 'Summary of findings' tables.
In the review protocol, we did not explain how we would apply the GRADE system to assess the quality of evidence. In the review, we have explained the methods used in the Data collection and analysis section under the heading Methods used to assess the quality of the evidence for outcomes included in 'Summary of finding' tables.
In the review protocol, we did not identify appropriate minimum important differences (MIDs) for the review outcomes. In the review, we have explained our selection of MIDs for specific outcomes in the Data collection and analysis section under the heading Identification and definitions of minimum important differences.
In the review protocol, we did not specify a specific time point at which we would analyse trial data pertaining to local reaction outcomes. In the review, we selected a 24‐hour time point (or the nearest approximation to this time point) and have explained the reasons for this decision in the Unit of analysis issues section.
An additional review team member was recruited (SC) who was not involved in the protocol stage of the review. The tasks fulfilled by all members of the review team are described in the Contributions of authors.
The original text in the protocol describing the types of interventions that would be considered in the review was edited for the final review to enhance clarity and coherence. There was no alteration to the prespecified selection criteria.
We conducted several sensitivity analyses that were not prespecified in our protocol. These analyses and the rationale for the analyses are presented in Appendix 7.
During the review process, we were unable to implement all of the methods outlined in the protocol. In accordance with the advice specified in the Cochrane Handbook for Systematic Reviews of Interventions, we have outlined (below) the methods that were not implemented, and this will serve as a protocol for future updates of the review.
Imputing missing data
In our review protocol (Beirne 2013), we described the methods that we would use in our review to impute missing data in instances where missing outcomes could not reasonably be assumed to be missing at random. In our review, we did not deem it necessary or appropriate to employ any imputation methods to deal with missing data (see Dealing with missing data and the entries for attrition bias in the 'Risk of bias' tables for each included trial). In future updates of this review, if there are instances where missing outcome data cannot be assumed to be missing at random and where the nature of the outcome renders it reasonable to do so, we will impute the missing data with replacement values and conduct sensitivity analyses to investigate how sensitive results are to changes in assumptions regarding the replacement values. We will use both best‐case and worst‐case imputation scenarios for dichotomous outcome data. For continuous outcome data, we will consider using the last observation carried forward (LOCF) approach if the nature of the outcome renders it reasonable to do so and if individual participant data are available from trial authors. In the Discussion section of future review updates, we will discuss the potential impact of missing data and our analysis strategies for dealing with missing data on the findings of the review.
Meta‐analyses of continuous data
In our review protocol (Beirne 2013), we described the methods that we would use to perform meta‐analyses of continuous data. We did not perform any such meta‐analyses due to the small number of trials that reported continuous outcomes. If additional trials reporting continuous outcomes are included in future updates of the review, we will use the mean difference as the summary statistic in meta‐analyses of continuous data when outcome measurements in trials are all made on the same scale. We will pool mean differences using the random‐effects inverse variance method. In instances where the included trials assess the same continuous outcome (e.g. pain) but do so in a variety of ways (e.g. using different pain scales), we will use the standardised mean difference (SMD) as the summary statistic in meta‐analyses and pool SMDs using the random‐effects inverse variance method.
Subgroup analyses
We did not conduct the subgroup analyses prespecified in the review protocol (Beirne 2013), as there was an insufficient number of trials included in our review. In future updates of this review, if sufficient trials are available and if there is evidence of statistical heterogeneity, we will investigate the following characteristics of trials for their possible influence on the magnitude of the intervention effect:
participant characteristics: age, weight (kilograms) or body mass index (BMI), gender;
vaccine characteristics: type of vaccine, formulation of vaccine (including vaccine viscosity);
site of vaccine administration: deltoid, anterolateral thigh, other;
co‐interventions administered during trial: e.g. multiple vaccines administered to trial participants;
technique of vaccine administration: 'bunching' or 'stretching' of skin before needle insertion, angle of needle insertion;
person administering the vaccine: doctor, nurse, other healthcare professional.
Sensitivity analyses
In our review protocol (Beirne 2013), we planned to conduct the following sensitivity analyses to investigate if our conclusions were robust to decisions made during the review process:
in instances where missing outcome data have been imputed with replacement values and included in a meta‐analysis, we planned to repeat our analyses using different assumptions about the replacement values (see Dealing with missing data);
we planned to repeat meta‐analyses including and excluding trials that were judged to have unclear or inadequate allocation concealment;
we planned to repeat meta‐analyses including and excluding trials that were judged to have unclear or inadequate blinding of outcome assessors.
We did not undertake these analyses due to the small number of trials (two) included in the meta‐analyses performed in our review. We will conduct these sensitivity analyses if sufficient trials are available in future updates of our review. However, some of these analyses will not be required if authors of future trials implement appropriate allocation concealment methods and adopt strategies to ensure blinding of outcome assessors.
Contributions of authors
PB co‐ordinated the review team, searched for trials, and screened titles and abstracts of retrieved records for the original and updated review. He also entered citations into Review Manager 5, selected trials for inclusion that met the prespecified selection criteria, developed and piloted the data extraction form, wrote to authors of papers for additional information, assessed the risk of bias in included trials, extracted trial data using the data extraction form, entered data into Review Manager 5, decided which analyses to conduct in consultation with the review team statistician (TF) and all other members of the review team, interpreted the analysis, and drafted the final review. He will co‐ordinate future review updates.
SH searched for trials and screened titles and abstracts of retrieved records for the original and updated review. She also selected trials for inclusion that met the prespecified selection criteria, piloted the data extraction form, assessed the risk of bias in included trials, extracted trial data using the data extraction form, checked all data entered into Review Manager 5 by PB, approved the analyses to be conducted in consultation with other members of the review team, assisted with interpreting the analysis, and assisted with editing and proofreading of the final review.
SC piloted the data extraction form, assessed the risk of bias in included trials, extracted trial data using the data extraction form, checked all data entered into Review Manager 5 by PB, approved the analyses to be conducted in consultation with other members of the review team, assisted with interpreting the analysis, and assisted with editing and proofreading of the final review.
FS searched for trials and screened titles and abstracts of retrieved records for the original review. She also selected trials for inclusion that met the prespecified selection criteria, and assisted with editing and proofreading of the final review.
TF provided statistical advice with regard to data analyses. He assisted with interpreting analyses and with the drafting of aspects of the final review that required statistical input.
FML assisted with interpreting analyses by providing a clinical perspective, assisted with editing and proofreading of the final review, and obtained information on needle sizes supplied with vaccines included in routine immunisation schedules in Ireland.
Sources of support
Internal sources
-
University College Cork (UCC), Ireland.
All review authors are employees of UCC and receive support from the University in the form of a salary.
External sources
No sources of support supplied
Declarations of interest
PB: none known.
SH: none known.
SC: none known.
FS: none known.
TF: none known.
FML: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Diggle 2000a {published data only}
- Diggle L, Deeks J. Effect of needle length on incidence of local reactions to routine immunisation in infants aged four months: randomised controlled trial. BMJ 2000;321:931‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle LE. A randomised controlled trial of different needle lengths on the incidence of local reactions, when administering the combined injection of Diphtheria/Pertussis/Tetanus (DPT) and Haemophilus Influenzae type b (Hib) to infants at 4‐months of age [MSc thesis]. London: The Royal College of Nursing Institute, 1999. [Google Scholar]
Diggle 2006 {published data only}
- Diggle L, Deeks J, Pollard AJ. Effect of needle size on immunogenicity and reactogenicity of vaccines in infants: randomised controlled trial. BMJ 2006; Vol. 333, issue 7568:571. [PUBMED: 16891328] [DOI] [PMC free article] [PubMed]
- Diggle LE. The effect of needle size on the immunogenicity and reactogenicity of vaccines in infancy: a randomised controlled trial [PhD thesis]. Oxford: University of Oxford, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Middleman 2010 {published data only}
- Middleman AB, Anding R, Tung C. Effect of needle length when immunizing obese adolescents with hepatitis B vaccine. Pediatrics 2010;125(3):508‐12. [PUBMED: 20142295] [DOI] [PubMed] [Google Scholar]
- Middleman AB, Tung C, Anding R. Does needle length affect titer level achieved after hepatitis B immunization among overweight youth?. Journal of Adolescent Health 2006;38(2):101. [DOI: 10.1016/j.jadohealth.2005.11.132] [DOI] [Google Scholar]
Nirupam 2008 {unpublished data only}
- Nirupam N, Pemde HK, Dutta AK. A double blind randomized controlled trial to optimize needle gauge for vaccination in infants (as supplied 23 April 2014). Data on file.
Pathak 2007 {published data only}
- Bharti B, Grewal A, Kalia R, Pathak P. Vaccine related reactogenicity for primary immunization: a randomized controlled trial of 23 (wider) vs. 25 (narrower) gauge needles with same lengths. Indian Journal of Pediatrics 2010;77(11):1241‐6. [PUBMED: 20821281] [DOI] [PubMed] [Google Scholar]
- Pathak P, Kalia R, Bharti B. Effect of needle gauge on perception of pain intensity among infants receiving D.P.T. vaccination. Nursing and Midwifery Research Journal 2007;3(4):172‐8. [Google Scholar]
References to studies excluded from this review
Cook 2005 {published data only}
- Cook IF, Murtagh J. Optimal technique for intramuscular injection of infants and toddlers: a randomised trial. Medical Journal of Australia 2005;183(2):60‐3. [DOI] [PubMed] [Google Scholar]
Fateh 2014 {published data only}
- Fateh M, Emamian MH, Zahraei SM, Fotouhi A. Syringe‐type and needle gauge have no role in adverse events following DTwP immunization: a multicenter trial. Pediatric Infectious Disease Journal 2014;33(9):239‐46. [DOI] [PubMed] [Google Scholar]
Ipp 1989 {published data only}
- Ipp MM, Gold R, Goldbach M, Maresky DC, Saunders N, Greenberg S, et al. Adverse reactions to diphtheria, tetanus, pertussis‐polio vaccination at 18 months of age: effect of injection site and needle length. Pediatrics 1989;83:679‐82. [PubMed] [Google Scholar]
Jackson 2008 {published data only}
- Jackson LA, Starkovich P, Dunstan M, Yu O, Nelson J, Dunn J, et al. Prospective assessment of the effect of needle length and injection site on the risk of local reactions to the fifth diphtheria‐tetanus‐acellular pertussis vaccination. Pediatrics 2008;121:646‐52. [DOI: 10.1542/peds.2007-1653] [DOI] [PubMed] [Google Scholar]
Johnsen 1995 {published data only}
- Johnsen C. Evaluation of needle length and hepatitis B vaccine response in correctional personnel in Massachusetts. Journal of Correctional Health Care 1995;2(1):39‐45. [Google Scholar]
Ozdemir 2012 {published data only}
- Ozdemir R, Canpolat FE, Yurttutan S, Oncel MY, Erdeve O, Dilmen U. Effect of needle length for response to hepatitis B vaccine in macrosomic neonates: a prospective randomized study. Vaccine 2012;30(21):3155‐8. [PUBMED: 22446632] [DOI] [PubMed] [Google Scholar]
Shaw 1989 {published data only}
- Shaw FE, Guess HA, Roets JM, Mohr FE, Coleman PJ, Mandel EJ, et al. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 1989;7:425‐30. [DOI] [PubMed] [Google Scholar]
Additional references
AAP 2015
- American Academy of Pediatrics. Red Book: Report of the Committee on Infectious Diseases. 30th Edition. Elk Grove Village, IL: American Academy of Pediatrics, 2015. [Google Scholar]
Ahn 2002
- Ahn W, Bahk J, Lim Y. The "gauge" system for the medical use. Anesthesia and Analgesia 2002;95:1119‐28. [DOI] [PubMed] [Google Scholar]
AHRQ 2012
- Treadwell J, Uhl S, Tipton K, Singh S, Santaguida L, Sun X, et al. Assessing equivalence and noninferiority. Methods research report (prepared by the EPC Workgroup under Contract No. 290‐2007‐10063). Rockville (MD): Agency for Healthcare Research and Quality; 2012 June. AHRQ Publication No. 12‐EHC045‐EF. [PubMed]
AHS 2015
- Alberta Health Services. Standard for the administration of immunizations. www.albertahealthservices.ca/assets/info/hp/cdc/if‐hp‐cdc‐ipsm‐standard‐administration‐immunization‐06‐100.pdf (accessed 25 January 2018).
Ajana 2008
- Ajana F, Sana C, Caulin E. Are there differences in immunogenicity and safety of vaccines according to the injection method? [Existe‐t‐il des différences d'immunogénicité et de tolérance des vaccins en fonction du mode d'injection?]. Médecine et Maladies Infectieuses 2008;38:648‐57. [DOI] [PubMed] [Google Scholar]
Ambuel 1992
- Ambuel B, Hamlett KW, Marx CM, Blumer JL. Assessing distress in pediatric intensive care environments: the COMFORT scale. Journal of Pediatric Psychology 1992;17:95‐109. [DOI] [PubMed] [Google Scholar]
AMP 2012a
- Agence de Médecine Préventive. SIVAC ‐ Supporting National Independent Immunization and Vaccine Advisory Committees. Agence de Médecine Préventive 2012. www.sivacinitiative.org/about‐sivac (accessed 10 May 2012).
AMP 2012b
- Agence de Médecine Préventive. NITAG Resource Center. Agence de Médecine Préventive 2012. www.nitag‐resource.org/en/presentation/nitag‐resource‐center‐article‐28.php (accessed 10 May 2012).
Andrews 1999
- Andrews K, Fitzgerald M. Cutaneous flexion reflex in human neonates: a quantitative study of threshold and stimulus‐response characteristics after single and repeated stimuli. Developmental Medicine and Child Neurology 1999;41:696‐703. [DOI] [PubMed] [Google Scholar]
Arendt‐Nielsen 2006
- Arendt‐Nielsen L, Egekvist H, Bjerring P. Pain following controlled cutaneous insertion of needles with different diameters. Somatosensory and Motor Research 2006;23(1/2):37‐43. [DOI] [PubMed] [Google Scholar]
Armijo‐Olivo 2014
- Armijo‐Olivo S, Ospina M, Costa BR, Egger M, Saltaji H, Fuentes J, et al. Poor reliability between Cochrane reviewers and blinded external reviewers when applying the Cochrane risk of bias tool in physical therapy trials. PLoS ONE 2014;9(5):e96920. [DOI: 10.1371/journal.pone.0096920] [DOI] [PMC free article] [PubMed] [Google Scholar]
ATAGI 2009
- Australian Technical Advisory Group on Immunisation of the Australian Government Department of Health and Ageing. Australian Immunisation Handbook, 9th Edition. Australian Immunisation Handbook. 9th Edition. Canberra: Australian Government Publications, 2008 [updated July 2009]. [Google Scholar]
ATAGI 2013
- Australian Technical Advisory Group on Immunisation of the Australian Government Department of Health and Ageing. Australian Immunisation Handbook. 10th Edition. Canberra: Australian Government Publications, 2013. [Google Scholar]
ATAGI 2016
- Australian Technical Advisory Group on Immunisation of the Australian Government Department of Health and Ageing. The Australian Immunisation Handbook 10th edition. Section 2.2 Administration of vaccines. www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10‐home˜handbook10part2˜handbook10‐2‐2 (accessed 23 January 2018).
Atkinson 2008
- Atkinson WL, Kroger AL, Pickering LK. Section 1: General aspects of vaccination, Part 7: General immunization practices. In: Plotkin S, Orenstein W, Offit P editor(s). Vaccines. 5th Edition. Philadelphia: WB Saunders Company, 2008. [Google Scholar]
Bailey 2010
- Bailey B, Daoust R, Doyon‐Trottier E, Dauphin‐Pierre S, Gravel J. Validation and properties of the verbal numeric scale in children with acute pain. Pain 2010;149:216‐21. [DOI] [PubMed] [Google Scholar]
Bar‐On 2012
- Bar‐On ES, Goldberg E, Hellmann S, Leibovici L. Combined DTP‐HBV‐HIB vaccine versus separately administered DTP‐HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD005530.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barret 2010
- Barret AS, Ryan A, Breslin A, Cullen L, Murray A, Grogan J, et al. Pertussis outbreak in northwest Ireland, January ‐ June 2010. Eurosurveillance 2010;15(35):19654. [www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19654] [PubMed] [Google Scholar]
Beigel 2007
- Beigel J, Kohl KS, Khuri‐Bulos N, Bravo L, Nell P, Marcy SM, et al. Rash including mucosal involvement: case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine 2007;25:5697‐706. [DOI] [PubMed] [Google Scholar]
Beirne 2013
- Beirne PV, Shiely F, Hennessy S, Fitzgerald T, MacLeod F. Needle size for vaccination procedures in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010720] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergeson 1982
- Bergeson PS, Singer SA, Kaplan AM. Intramuscular injections in children. Pediatrics 1982;70:944‐8. [PubMed] [Google Scholar]
Bonhoeffer 2002
- Bonhoeffer J, Kohl K, Chen R, Duclos P, Heijbel H, Heininger U, et al. The Brighton Collaboration: addressing the need for standardized case definitions of adverse events following immunization (AEFI). Vaccine 2002;21:298‐302. [DOI] [PubMed] [Google Scholar]
Bonhoeffer 2004
- Bonhoeffer J, Vermeer P, Halperin S, Kemep A, Music S, Shindman J, et al. Persistent crying in infants and children as an adverse event following immunization: case definition and guidelines for data collection, analysis and presentation. Vaccine 2004;22:586‐91. [DOI] [PubMed] [Google Scholar]
Bonhoeffer 2009
- Bonhoeffer J, Bentsi‐Enchill A, Chen RT, Fisher MC, Gold MS, Hartman K, et al. The Brighton Collaboration Methods Working Group. Guidelines for collection, analysis and presentation of vaccine safety data in pre‐ and post‐licensure clinical studies. Vaccine 2009;27:2282‐8. [DOI] [PubMed] [Google Scholar]
Brighton Collaboration 2014
- Brighton Collaboration. Case definitions: standardized case definitions for global use. brightoncollaboration.org/public/what‐we‐do/setting‐standards/case‐definitions.html (accessed 31 August 2014).
Bryson 2010
- Bryson M, Duclos P, Jolly A, Cakmak N. A global look at National Immunization Technical Advisory Groups. Vaccine 2010;28S:A13‐7. [DOI] [PubMed] [Google Scholar]
CDC 2003
- Centers for Disease Control and Prevention. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS) ‐ United States, 1991‐2001. Morbidity and Mortality Weekly Report 2003;52:1‐24. [PubMed] [Google Scholar]
CDC 2005
- Centers for Disease Control and Prevention. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States. Recommendations of the Advisory Committee on Immunization Practices (ACIP). Part 1: Immunization of infants, children and adolescents. Morbidity and Mortality Weekly Report 2005;54(RR‐16):1‐32. [PubMed] [Google Scholar]
CDC 2006a
- Centers for Disease Control and Prevention. A comprehensive immunization strategy to eliminate transmission of Hepatitis B virus infection in the United States. Morbidity and Mortality Weekly Report 2006;55(RR‐16):1‐33. [PubMed] [Google Scholar]
CDC 2006b
- Centers for Disease Control and Prevention. Principles of Epidemiology in Public Health Practice. An Introduction to Applied Epidemiology and Biostatistics. 3rd Edition. Atlanta, GA: Centers for Disease Control and Prevention, 2006. [Google Scholar]
CDC 2011
- Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morbidity and Mortality Weekly Report 2011;60(2):1‐61. [PubMed] [Google Scholar]
Chandran 2013
- Chandran A, Watt JP, Santosham M. Haemophilus influenzae vaccines. In: Plotkin SA, Orenstein WA, Offit PA editor(s). Vaccines. 6th Edition. Philadelphia, PA: Elsevier Saunders, 2013. [Google Scholar]
Chugh 1993
- Chugh K, Chawla E, Aggarwal BB. Optimum needle length for DPT inoculation of Indian infants. Indian Journal of Pediatrics 1993;60:435‐40. [DOI] [PubMed] [Google Scholar]
Collins 2004
- Collins CL, Salt P, McCarthy N, Chantler T, Lane L, Hemme F, et al. Immunogenicity and safety of a low‐dose diphtheria, tetanus and acellular pertussis combination vaccine with either inactivated or oral polio vaccine as a pre‐school booster in UK children. Vaccine 2004;22:4262‐9. [DOI] [PubMed] [Google Scholar]
Cook 2001
- Cook IF, Murtagh J. Paediatric vaccination practice in a division of general practice. Australian Family Physician 2001;30(12):1185‐9. [PubMed] [Google Scholar]
Cook 2002
- Cook IF, Murtagh J. Needle length required for intramuscular vaccination of infants and toddlers ‐ an ultrasonographic study. Australian Family Physician 2002;31(3):295‐7. [PubMed] [Google Scholar]
Cook 2006
- Cook IF, Williamson M, Pond D. Definition of needle length required for intramuscular deltoid injection in elderly adults: an ultrasonographic study. Vaccine 2006;24:937‐40. [DOI] [PubMed] [Google Scholar]
Cordoba 2010
- Cordoba G, Schwartz L, Woloshin S, Bae H, Gotzsche P. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ 2010;341:c3920. [DOI: 10.1136/bmj.c3920] [DOI] [PMC free article] [PubMed] [Google Scholar]
CPMP 1999
- Committee for Proprietary Medicinal Products. Note for guidance on clinical evaluation of new vaccines. London: The European Agency for the Evaluation of Medicinal Products ‐ Human Medicines Evaluation Unit; 19 May 1999. Report No.: CPMP/EWP/463/97.
Curtis 2012
- Curtis S, Wingert A, Ali S. The Cochrane Library and procedural pain in children: an overview of reviews. Evidence‐Based Child Health 2012;7:1363‐99. [Google Scholar]
Czarnecki 2011
- Czarnecki M, Turner H, Collins P, Doellman D, Wrona S, Reynolds J. Procedural pain management: a position statement with clinical practice recommendations. Pain Management Nursing 2011;12(2):95‐111. [DOI] [PubMed] [Google Scholar]
Daltrey 2000
- Daltrey IR, Kissin MW. Randomized clinical trial of the effect of needle gauge and local anaesthetic on the pain of breast fine‐needle aspiration cytology. British Journal of Surgery 2000;87:777‐9. [DOI] [PubMed] [Google Scholar]
Davenport 2003
- Davenport JM. A systematic review to ascertain whether the standard needle is more effective than a longer or wider needle in reducing the incidence of local reaction in children receiving primary immunization. Journal of Advanced Nursing 2003;46:66‐77. [DOI] [PubMed] [Google Scholar]
de Menezes Martins 2008
- Menezes Martins R, Camacho LAB, Marcovistz R, Noronha TG, Lourdes Sousa Maia M, dos Santos EM, et al. Immunogenicity, reactogenicity and consistency of production of a Brazilian combined vaccine against diphtheria, tetanus, pertussis and Haemophilus influenzae type b. Memórias do Instituto Oswaldo Cruz 2008;103(7):711‐8. [DOI] [PubMed] [Google Scholar]
Decker 2013
- Decker MD, Edwards KM, Bogaerts HH. Combination vaccines. In: Plotkin SA, Orenstein WA, Offit PA editor(s). Vaccines. 6th Edition. Philadelphia, PA: Elsevier Saunders, 2013. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DHHS 2013
- US Department of Health and Human Services, Office of Population Affairs. What is adolescence?. www.hhs.gov/opa/familylife/tech_assistance/etraining/adolescent_brain/Overview/what_is_adolescence/#2 (accessed 19 June 2013).
Diekema 2012
- Diekema DS. Improving childhood vaccination rates. New England Journal of Medicine 2012;366(5):391‐3. [DOI] [PubMed] [Google Scholar]
Diggle 2007
- Diggle L, Richards S. Best practice when immunising children. Primary Health Care 2007;17(7):41‐6. [Google Scholar]
DoH 1996
- Department of Health. Immunisation Against Infectious Diseases. London: Her Majesty's Stationery Office, 1996. [Google Scholar]
DoH UK 2012a
- Department of Health UK. Chapter 4: Immunisation procedures (updated June 2012). In: Salisbury D, Ramsay M, Noakes K editor(s). Immunisation Against Infectious Disease (The Green Book). London: The Stationery Office, 2006:25‐34. [Google Scholar]
DoH UK 2012b
- Department of Health UK. Chapter 8: Vaccine safety and the management of adverse events following immunisation (updated August 2012). In: Salisbury D, Ramsay M, Noakes K editor(s). Immunisation Against Infectious Disease (The Green Book). London: The Stationery Office, 2006:53‐66. [Google Scholar]
Donegan 2010
- Donegan S, Cochrane Infectious Diseases Group. Data collection and analysis: content checklist for intervention reviews. February 2010. cidg.cochrane.org/resources‐review‐authors (accessed 1 March 2014).
EMA 2005
- European Medicines Agency, Committee for Human Medicinal Products (CHMP). Note for Guidance on the Clinical Evaluation of Vaccines. London: European Medicines Agency, 2005. [Google Scholar]
EMA 2013
- European Medicines Agency. Infanrix hexa: CHMP assessment report for paediatric use studies submitted according to Article 46 of the Regulation (EC) No. 1901/2006, 2013. www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Assessment_Report_‐_Variation/human/000296/WC500144787.pdf (accessed 27 May 2015). [EMA/149931/2013]
FDA 2018
- US Food, Drug Administration. Vaccines licensed for use in the United States. www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm093833.htm (accessed 22 January 2016).
Flynn 1994
- Flynn PM, Shenep JL, Mao L, Crawford R, Williams BF, Williams BG. Influence of needle gauge in Mantoux skin testing. Chest 1994;106(5):1463‐5. [DOI] [PubMed] [Google Scholar]
Gidudu 2008
- Gidudu J, Kohl KS, Halperin S, Hammer SJ, Heath PT, Hennig R, et al. Brighton Collaboration Local Reactions Working Group for a Local Reaction at or Near Injection Site. A local reaction at or near injection site: case definition and guidelines for collection, analysis and presentation of immunization safety data. Vaccine 2008;26:6800‐13. [DOI] [PubMed] [Google Scholar]
Gidudu 2012
- Gidudu JF, Walco GA, Taddio A, Zempsky WT, Halperin SA, Calugar A, et al. Immunization site pain: case definition and guidelines for collection, analysis, and presentation of immunization safety data. Vaccine 2012;30:4558‐77. [DOI] [PubMed] [Google Scholar]
Gill 2007
- Gill HS, Prausnitz MR. Does needle size matter?. Journal of Diabetes Science and Technology 2007;1(5):725‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011 [Computer program]
- The GRADE Working Group. GRADEprofiler. Version 3.6. GRADE Working Group, 2011.
Groswasser 1997
- Groswasser J, Kahn A, Bouche B, Hanquinet S, Perlmuter N, Hessel L. Needle length and injection technique for efficient intramuscular vaccine delivery in infants and children evaluated through an ultrasonographic determination of subcutaneous and muscle layer thickness. Pediatrics 1997;100:400‐3. [DOI] [PubMed] [Google Scholar]
Guerra 2009
- Guerra FA, Blatter MM, Greenberg DP, Pichichero M, Noriega FR. Safety and immunogenicity of a pentavalent vaccine compared with separate administration of licensed equivalent vaccines in US infants and toddlers and persistence of antibodies before a preschool booster dose: a randomized clinical trial. Pediatrics 2009;123(1):301‐12. [DOI] [PubMed] [Google Scholar]
Guyatt 2013
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing Summary of Findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI] [PubMed] [Google Scholar]
Hamilton 1995
- Hamilton JG. Needle phobia: a neglected diagnosis. Journal of Family Practice 1995;41:169‐75. [PubMed] [Google Scholar]
Harrison 2010
- Harrison D, Yamada J, Adams‐Webber T, Ohlsson A, Beyene J, Stevens B. Sweet‐tasting solutions for needle‐related procedural pain in children aged one to 16 years. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008408] [DOI] [Google Scholar]
Harrison 2015
- Harrison D, Yamada J, Adams‐Webber T, Ohlsson A, Beyene J, Stevens B. Sweet tasting solutions for reduction of needle‐related procedural pain in children aged one to 16 years. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD008408.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartling 2009
- Hartling L, Ospina M, Liang Y, Dryden DM, Hooton N, Seida K, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ 2009;334:b4012. [DOI: 10.1136/bmj.b4012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartling 2013
- Hartling L, Hamm MP, Milne A, Vandermeer B, Santaguida PL, Ansari M, et al. Testing the Risk of Bias tool showed low reliability between individual reviewers and across consensus assessments of reviewer pairs. Journal of Clinical Epidemiology 2013;66:973‐81. [DOI] [PubMed] [Google Scholar]
Hester 1979
- Hester NKO. The preoperational child's reaction to immunization. Nursing Research 1979;28:250‐5. [PubMed] [Google Scholar]
Hick 1989
- Hick JF, Charboneau JW, Brakke DM, Goergen B. Optimum needle length for Diphtheria‐Tetanus‐Pertussis inoculation of infants. Pediatrics 1989;84:136. [PubMed] [Google Scholar]
Hicks 2001
- Hicks CL, Baeyer CL, Spafford PA, Korlaar I, Goodenough B. The Faces Pain Scale ‐ Revised: toward a common metric in pediatric pain management. Pain 2001;93:173‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Higgins 2011c
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Hirsch 2010
- Hirsch LJ, Gibney MA, Albanese J, Qu S, Kassler‐Taub K, Klaff LJ, et al. Comparative glycemic control, safety and patient ratings for a new 4mm x 32G insulin pen needle in adults with diabetes. Current Medical Research and Opinion 2010;26(6):1531‐41. [DOI: 10.1185/03007995.2010.482499] [DOI] [PubMed] [Google Scholar]
Hogan 2010
- Hogan M, Kikuta A, Taddio A. A systematic review of measures for reducing injection pain during adult immunization. Vaccine 2010;28:1514‐21. [DOI] [PubMed] [Google Scholar]
Hogan 2012
- Hogan ME, Shah VS, Smith RW, Yiu A, Taddio A. Glucose for the management of procedural pain in neonates. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009721] [DOI] [Google Scholar]
Horne 2001
- Horne AD, Lachenbruch PA, Getson PR, Hsu HS. Analysis of studies to evaluate immune response to combination vaccines. Clinical Infectious Diseases 2001;33(Suppl 4):S306‐11. [DOI] [PubMed] [Google Scholar]
HPSC 2012
- Health Protection Surveillance Centre. Measles outbreak in West Cork ‐ update 8th June 2012. www.hpsc.ie/hpsc/News/MainBody,1342,en.html (accessed 12 June 2012).
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:e1119. [DOI: 10.1136/bmj.e1119; PUBMED: 2237 1859] [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI: 10.1503/cmaj.120744] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hudgens 2004
- Hudgens MG, Gilbert PB, Self SG. Endpoints in vaccine trials. Statistical Methods in Medical Research 2004;13:1‐26. [DOI] [PubMed] [Google Scholar]
IASP 2004
- International Association for the Study of Pain (IASP) Task Force on Taxonomy. Classification of Chronic Pain. 2nd Edition. Seattle: IASP Press, 2004. [Google Scholar]
IOM 2013
- Institute of Medicine. The Childhood Immunization Schedule and Safety: Stakeholder Concerns, Scientific Evidence, and Future Studies. Washington, DC: National Academies Press, 2013. [PubMed] [Google Scholar]
Ipp 2004
- Ipp M, Cohen E, Goldbach M, Macarthur C. Effect of choice of measles‐mumps‐rubella vaccine on immediate vaccination pain in infants. Archives of Pediatric and Adolescent Medicine 2004;158:323‐6. [DOI] [PubMed] [Google Scholar]
Iserson 1987
- Iserson KV. The origins of the gauge system for medical equipment. Journal of Emergency Medicine 1987;5(1):45‐8. [DOI] [PubMed] [Google Scholar]
ISO 1992
- International Organization for Standardization. ISO 6009: Hypodermic needles for single use ‐ colour coding for identification. Geneva: International Organization for Standardization; 1992 December. Report No.: UDC 615.473.2:62‐777.6.
ISO 1993
- International Organization for Standardization. ISO 7864: Sterile hypodermic needles for single use. Geneva: International Organization for Standardization; 1993 May. Report No.: UDC 615.473.2.014.45.
ISO 2001
- International Organization for Standardization. ISO 9626: Stainless steel tubing for the manufacture of medical devices, Amendment 1.. Geneva: International Organization for Standardization; 2001 June. Report No.: ICS 11.040.20.
Johnston 1996
- Johnston C, Stevens B. Experience in a neonatal intensive care unit affects pain response. Pediatrics 1996;98:925‐30. [PubMed] [Google Scholar]
Kaplan 2004
- Kaplan PS. Adolescence. Boston, MA: Houghton Mifflin, 2004. [Google Scholar]
Kassab 2010
- Kassab M, Foster JP, Foureur M, Fowler C. Sweet‐tasting solutions for needle‐related procedural pain in infants one month to one year of age. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD008411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassab 2011
- Kassab M, Fowler C, Foureur M. Managing immunisation pain in infants. Australian Journal of Child and Family Health Nursing 2011;8(3):4‐9. [Google Scholar]
Kassab 2012
- Kassab M, Foster JP, Foureur M, Fowler C. Sweet‐tasting solutions for needle‐related procedural pain in infants one month to one year of age. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008411.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kiersey 2016
- Kiersey C. The cold chain and vaccine storage. www.hse.ie/eng/health/Immunisation/hcpinfo/conference/kerry166.pdf (accessed 24 January 2018).
Kim 2012
- Kim Y, Prausnitz MR. Enabling skin vaccination using new delivery technologies. Current Topics in Microbiology and Immunology 2012;351:77‐112. [DOI: 10.1007/82_2011_123] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kmietowicz 2012
- Kmietowicz Z. Pertussis cases rise 10‐fold among older children and adults in England and Wales. BMJ 2012;345:e5008. [DOI: 10.1136/bmj.e5008] [DOI] [PubMed] [Google Scholar]
Kohl 2005
- Kohl KS, Bonhoeffer J, Braun MM, Chen RT, Duclos P, Heijbel H, et al. The Brighton Collaboration: creating a global standard for case‐definitions (and guidelines) for adverse events following immunization. In: Henriksen K, Battles JB, Marks ES editor(s). Advances in Patient Safety: From Research to Implementation. Vol. 2: Concepts and Methodology, Rockville, MD: Agency for Healthcare Research and Quality, 2005. [PubMed] [Google Scholar]
Kosalaraksa 2011
- Kosalaraksa P, Thisyakorn U, Benjaponpitak S, Chokephaibulkit K, Santos‐Lima E. Immunogenicity and safety study of a new DTaP‐IPV‐Hep B‐PRP‐T combined vaccine compared to a licensed DTaP‐IPV‐HepB//PRP‐T comparator, both concomitantly administered with a 7‐valent pneumococcal conjugate vaccine at 2, 4, and 6 months of age in Thai infants. International Journal of Infectious Diseases 2011;15(4):e249‐56. [DOI] [PubMed] [Google Scholar]
Koster 2009
- Koster MP, Stellato N, Kohn N, Rubin LG. Needle length for immunization of early adolescents as determined by ultrasound. Pediatrics 2009;124(2):667‐72. [DOI] [PubMed] [Google Scholar]
Kreugel 2007
- Kreugel G, Beijer HJM, Kerstens MN, ter Maaten JC, Sluiter WJ, Boot BS. Influence of needle size for subcutaneous insulin administration on metabolic control and patient acceptance. European Diabetes Nursing 2007;4(2):51‐5. [Google Scholar]
Kroger 2013
- Kroger AT, Atkinson WL, Pickering LK. Section 1: general aspects of vaccination, Part 8: general immunization practices. In: Plotkin S, Orenstein W, Offit P editor(s). Vaccines. 6th Edition. Philadelphia: WB Saunders Company, 2013. [Google Scholar]
Kroger 2017
- Kroger AT, Duchin J, Vázquez M. General best practice guidelines for immunization. Best practice guidance of the Advisory Committee on Immunization Practices (ACIP). www.cdc.gov/vaccines/hcp/acip‐recs/general‐recs/downloads/general‐recs.pdf (accessed 23 January 2018).
Lander 2002
- Lander JA, Weltman BJ. Topical anaesthetics (EMLA and EMETOP creams) for reduction of pain during needle insertion in children. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD004236] [DOI] [Google Scholar]
Lange 2005
- Lange S, Freitag G. Choice of delta: requirements and reality ‐ results of a systematic review. Biometrical Journal 2005;47(1):12‐27. [DOI: 10.1002/bimj.200410085] [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59‐66. [PubMed] [Google Scholar]
Lippert 2008
- Lippert WC, Wall EJ. Optimal intramuscular needle‐penetration depth. Pediatrics 2008;122(3):556‐63. [DOI: 10.1542/peds.2008-0374] [DOI] [PubMed] [Google Scholar]
Lo 1992
- Lo Y, Lu C, Chen L, Huang L, Jong Y. Quantitative measurement of muscle and subcutaneous fat thickness in newborn by real‐time ultrasonography: a useful method for site and depth evaluation in vaccination. Kaohsiung Journal of Medical Science 1992;8:75‐81. [PubMed] [Google Scholar]
Luthy 2009
- Luthy KE, Beckstrand RL, Peterson NE. Parental hesitation as a factor in delayed childhood immunization. Journal of Pediatric Health Care 2009;23(6):388‐93. [DOI] [PubMed] [Google Scholar]
McGrath 1985
- McGrath PJ, Johnson G, Goodman JT. CHEOPS: a behavioural scale for rating postoperative pain in children. Advances in Pain Research and Therapy. Proceedings of the Fourth World Congress on Pain. York: Raven Press, 1985; Vol. 9:395‐402.
McKinstry 2004
- McKinstry B, Trainor D. Getting evidence into practice: using direct research evidence to change medical and nursing culture. Education for Primary Care 2004;15:73‐5. [Google Scholar]
Merck 2007
- Merck, Co. Inc. GARDASIL [Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant]. Description. 2007. www.whale.to/vaccine/gardasil_pi.pdf (accessed 29 January 2013).
Merkel 1997
- Merkel SI, Voepel‐Lewis T, Shayevitz JR, Malviya S. The FLACC: a behavioural scale for scoring postoperative pain in young children. Pediatric Nursing 1997;23:293‐7. [PubMed] [Google Scholar]
Merriam‐Webster 2013
- Merriam‐Webster. Merriam‐Webster Medical Dictionary. www.merriam‐webster.com/medical/ (accessed 23 June 2013).
Miró 2009
- Miró J, Castarlenas E, Huguet A. Evidence for the use of a numerical rating scale to assess the intensity of pediatric pain. European Journal of Pain 2009;13:1089‐95. [DOI] [PubMed] [Google Scholar]
MoH NZ 2017
- Ministry of Health, New Zealand. Immunisation Handbook 2017. Wellington: Ministry of Health, 2017. [Google Scholar]
Nakayama 2016
- Nakayama T, Kohdera U, Fujino M, Tanaka T, Yatabe K, Hashiguchi T, et al. Appropriate needle lengths determined using ultrasonic echograms for intramuscular injections in Japanese infants. Open Journal of Pediatrics 2016;6:163‐70. [Google Scholar]
Nauta 2011
- Nauta J. Statistics in Clinical Vaccine Trials. Heidelberg: Springer, 2011. [Google Scholar]
NCIRS 2013
- National Centre for Immunisation Research and Surveillance. NCIRS Fact Sheet: Vaccine Components. Westmead (New South Wales): National Centre for Immunisation Research and Surveillance; 2013 May.
NHMRC 2000
- National Health and Medical Research Council. The Australian Immunisation Handbook. 7th Edition. Canberra: National Health and Medical Research Council, 2000. [Google Scholar]
NHMRC 2003
- National Health and Medical Research Council. The Australian Immunisation Handbook. 8th Edition. Canberra: National Health and Medical Research Council, 2003. [Google Scholar]
NIAC 2011
- National Immunisation Advisory Committee. Immunisation Guidelines for Ireland. Dublin: Royal College of Physicians of Ireland, 2008 [online only update September 2011]. [ISBN 978‐0‐9559351‐0‐7] [Google Scholar]
NIAC 2016
- National Immunisation Advisory Committee. Immunisation Guidelines for Ireland: General Immunisation Procedures. www.hse.ie/eng/health/immunisation/hcpinfo/guidelines/chapter2.pdf (accessed 23 January 2018).
Noel 2015
- Noel M, Taddio A, McMurtry M, Chambers CT, Pillai Riddell R, Shah V. HELPinKids&Adults knowledge synthesis of the management of vaccination pain and high levels of needle fear: limitations of the evidence and recommendations for future research. Clinical Journal of Pain 2015;31(10S):S124‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Owens 2009
- Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, Bass EB. Grading the strength of a body of evidence when comparing medical interventions. Agency for Healthcare Research and Quality: Methods Guide for Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality, July 2009. [Google Scholar]
Owens 2010
- Owens DK, Lohr KN, Atkins D, Treadwell JR, Reston JT, Bass EB, et al. AHRQ Series Paper 5: Grading the strength of a body of evidence when comparing medical interventions ‐ Agency for Healthcare Research and Quality and the Effective Health‐Care Program. Journal of Clinical Epidemiology 2010;63:513‐23. [DOI] [PubMed] [Google Scholar]
Palmon 1998
- Palmon SC, Lloyd AT, Kirsch JR. The effect of needle gauge and lidocaine pH on pain during intradermal injection. Anesthesia and Analgesia 1998;86:379‐81. [DOI] [PubMed] [Google Scholar]
Petousis‐Harris 2008
- Petousis‐Harris H. Vaccine injection technique and reactogenicity ‐ evidence for practice. Vaccine 2008;26:6299‐304. [DOI] [PubMed] [Google Scholar]
PHE 2016
- Public Health England. MenC vaccination schedule: planned changes from July 2016. London: Public Health England; 2016 March. Report No.: PHE 2015‐755. [PHE publications gateway number: 2015‐755]
PHE 2017
- Public Health England. The routine immunisation schedule ‐ from Autumn 2017. www.gov.uk/government/uploads/system/uploads/attachment_data/file/633693/Complete_imm_schedule_2017.pdf (accessed 22 January 2018).
Pillai Riddell 2006
- Pillai Riddell RR, Uman LS, Gerwitz A, Stevens B. Nonpharmacological interventions for needle‐related procedural pain and post‐operative pain in neonates and infants. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD006275] [DOI] [Google Scholar]
Pillai Riddell 2013
- Pillai Riddell R, Flora D, Stevens S, Stevens B, Cohen L, Greenberg S, et al. Variability in infant acute pain responding meaningfully obscured by averaging pain responses. Pain 2013;154:714‐21. [DOI] [PubMed] [Google Scholar]
Plotkin 2008a
- Plotkin SA, Orenstein WA. Vaccines. 5th Edition. Philadelphia: WB Saunders Company, 2008. [Google Scholar]
Plotkin 2008b
- Plotkin SA. Correlates of vaccine‐induced immunity. Clinical Infectious Diseases 2008;47:401‐9. [DOI] [PubMed] [Google Scholar]
Plotkin 2010
- Plotkin SA. Correlates of protection induced by vaccination. Clinical and Vaccine Immunology 2010;17(7):1055‐65. [DOI: 10.1128/CVI.00131-10] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plotkin 2013
- Plotkin DA, Orenstein WA, Offit PA (editors). Vaccines. 6th Edition. Philadelphia, PA: Elsevier Saunders, 2013. [Google Scholar]
Poland 1997
- Poland GA, Borrud A, Jacobson RM, McDermott K, Wollan PC, Brakke D, et al. Determination of deltoid fat pad thickness: implications for needle length in adult immunization. JAMA 1997;277:1709‐11. [PubMed] [Google Scholar]
Porter 1999
- Porter L, Grunau E, Anand J. Long‐term effects of pain in infants. Journal of Developmental and Behavioural Pediatrics 1999;20:253‐6. [DOI] [PubMed] [Google Scholar]
Pöll 1999
- Pöll JS. The story of the gauge. Anaesthesia 1999;54:575‐81. [DOI] [PubMed] [Google Scholar]
RCN 2001
- Royal College of Nursing. UK Guidance on Best Practice in Vaccine Administration. London: Shire Hall Communications, 2001. [Google Scholar]
RCN 2009
- Royal College of Nursing. The Recognition and Assessment of Acute Pain in Children (Clinical Practice Guideline) ‐ Update of Full Guideline. London: Royal College of Nursing, 2009. [Google Scholar]
RCPCH 2002
- Royal College of Paediatrics and Child Health. Position Statement on Injection Technique. London: Royal College of Paediatrics and Child Health, 2002. [ISBN 1‐900954‐61‐3] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roehr 2010
- Roehr B. Eight babies die in Californian whooping cough outbreak. BMJ 2010;341:421. [DOI] [PubMed] [Google Scholar]
Rohatgi 2017 [Computer program]
- Rohatgi A. WebPlotDigitizer ‐ Web Based Plot Digitizer. Version 4.1. Austin: Ankit Rohatgi, 2017 (accessed 22 January 2018).
Schechter 2007
- Schechter NL, Zempsky WT, Cohen LL, McGrath P, McMurty MC, Bright N. Pain reduction during pediatric immunizations: evidence‐based review and recommendations. Pediatrics 2007;119:e1184‐98. [DOI] [PubMed] [Google Scholar]
Schechter 2010
- Schechter NL, Bernstein BA, Zempsky WT, Bright NS, Willard AK. Educational outreach to reduce immunization pain in office settings. Pediatrics 2010;126(6):1514‐21. [DOI: 10.1542/peds.2010-1597] [DOI] [PubMed] [Google Scholar]
Schwartz 2004
- Schwartz S, Hassman D, Shelmet J, Sievers R, Weinstein R, Liang J, et al. A multicenter, open‐label, randomized, two‐period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge x 6mm needle versus a 29 Gauge x 12.7mm needle in obese patients with diabetes mellitus. Clinical Therapeutics 2004;26(10):1663‐78. [DOI: 10.1016/j.clinthera.2004.10.007] [DOI] [PubMed] [Google Scholar]
Schünemann 2005
- Schünemann HJ, Guyatt GH. Commentary: goodbye M(C)ID! Hello MID, where do you come from?. Health Services Research 2005;40(2):593‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2009
- Schünemann H, Brożek J, Guyatt G, Oxman A (editors). GRADE handbook for grading quality of evidence and strength of recommendation. Updated March 2009. Available at www.guidelinedevelopment.org/handbook.
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Schünemann 2011c
- Schünemann H, Santesso N. Introductory courses for GRADE and summary of findings tables: inconsistency, 2011. cebgrade.mcmaster.ca/Inconsistency/index.html (accessed 30 August 2014).
Sheridan 2012
- Sheridan SL, Ware RS, Grimwood K, Lambert S. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA 2012;308(5):454‐6. [DOI] [PubMed] [Google Scholar]
Shivananda 2006
- Shivananda VS, Srikanth BS, Mohan M, Kulkarni PS. Comparison of two hepatitis B vaccines (Gene Vac‐B and Engerix‐B) in healthy infants in India. Clinical and Vaccine Immunology 2006;13(6):661‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
SKB 2001
- SmithKline Beecham. Engerix‐B. Hepatitis B Vaccine (Recombinant). Prescribing information. Philadelphia (PA): SmithKline Beecham; 2001. Report No.: EB: L33.
Skiveren 2010
- Skiveren J, Larsen HN, Kjaerby E, Larsen R. The influence of needle size on pain perception in patients treated with botulinum toxin A injections for axillary hyperhidrosis. Acta Dermato Venereologica 2010;91(1):72‐4. [DOI: 10.2340/00015555-0991] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at handbook.cochrane.org.
Taddio 1995
- Taddio A, Nulman I, Koren B, Stevens B, Koren G. A revised measure of acute pain in infants. Journal of Pain and Symptom Management 1995;10:456‐63. [DOI] [PubMed] [Google Scholar]
Taddio 1997
- Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet 1997;349:599‐603. [DOI] [PubMed] [Google Scholar]
Taddio 2002
- Taddio A, Shah V, Gilbert‐MacLeod C, Katz J. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA 2002;288:857‐61. [DOI] [PubMed] [Google Scholar]
Taddio 2009a
- Taddio A, Chambers CT, Halperin SA, Ipp M, Lockett D, Rieder MJ, et al. Inadequate pain management during routine childhood immunizations: the nerve of it. Clinical Therapeutics 2009;31(Suppl B):S152‐67. [DOI] [PubMed] [Google Scholar]
Taddio 2009b
- Taddio A, Ilersich A, Ipp M, Shah V. Physical interventions and injection techniques for reducing injection pain during routine childhood immunizations: systematic review of randomized controlled trials and quasi‐randomized controlled trials. Clinical Therapeutics 2009;31(Suppl B):S48‐76. [DOI] [PubMed] [Google Scholar]
Taddio 2010
- Taddio A, Appleton M, Bortolussi R, Chambers C, Dubey V, Halperin S, et al. Reducing the pain of childhood vaccination: an evidence‐based clinical practice guideline summary. Canadian Medical Association Journal 2010;182(18):1989‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taddio 2012
- Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, et al. Survey of the prevalence of immunization non‐compliance due to needle fears in children and adults. Vaccine 2012;30:4807‐12. [DOI] [PubMed] [Google Scholar]
Thakur 2012
- Thakur A, Pedersen LE, Jungersen G. Immune markers and correlates of protection for vaccine induced immune responses. Vaccine 2012;30:4907‐20. [DOI] [PubMed] [Google Scholar]
Thierry‐Carstensen 2012
- Thierry‐Carstensen B, Jordan K, Uhlving H, Dalby T, Sorensen C, Jensen A, et al. A randomised, double‐blind, non‐inferiority clinical trial on the safety and immunogenicity of a tetanus, diphtheria and monocomponent acellular pertussis (TdaP) vaccine in comparison to a tetanus and diphtheria (Td) vaccine when given as booster vaccinations to healthy adults. Vaccine 2012;30:5464‐71. [DOI] [PubMed] [Google Scholar]
Ueffing 2012
- Ueffing E, Tugwell P, Welch V, Petticrew M, Kristjansson E, for the Campbell and Cochrane Equity Methods Group. Equity checklist for systematic review authors, 2012. www.equity.cochrane.org/files/equitychecklist2011.pdf (accessed 27 June 2013).
Uman 2005
- Uman LS, Chambers CT, McGrath PJ, Kisely S. Psychological interventions for needle‐related procedural pain and distress in children and adolescents. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD005179] [DOI] [PubMed] [Google Scholar]
Uman 2013
- Uman LS, Chambers CT, McGrath PJ, Kisely SR. Psychological interventions for needle‐related procedural pain and distress in children and adolescents. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD005179.pub3] [DOI] [PubMed] [Google Scholar]
Vashishtha 2013
- Vashishtha VM, Bansal CP, Gupta SG. Pertussis vaccines: position paper of Indian Academy of Pediatrics (IAP). Indian Pediatrics 2013;50:1001‐9. [DOI] [PubMed] [Google Scholar]
VATF 2001
- Vaccine Administration Task Force. UK Guidance on Best Practice in Vaccine Administration. London: Shire Hall Communications, 2001. [Google Scholar]
von Baeyer 2006
- Baeyer CL. Children's self‐reports of pain intensity: scale selection, limitations and interpretation. Pain Research Management 2006;11(3):157‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
von Baeyer 2009
- Baeyer CL, Spagrud LJ, McCormick JC, Choo E, Neville K, Connelly MA. Three new datasets supporting use of the Numerical rating Scale (NRS‐11) for children's self‐reports of pain intensity. Pain 2009;143:223‐7. [DOI] [PubMed] [Google Scholar]
Watson 2001
- Watson M. Needle gauge is more important than needle length. BMJ 2001;322:492. [PubMed] [Google Scholar]
WHO 1998
- World Health Organization. Module 8, During a session: giving immunisations. Immunisation in Practice. Geneva: World Health Organization, 1998. [Google Scholar]
WHO 2004
- World Health Organization. Immunization in Practice: a Practical Resource Guide for Healthcare Workers [2004 update]. Geneva: World Health Organization, 2004. [Google Scholar]
WHO 2006
- WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatrica 2006;Suppl 450:76‐85. [DOI] [PubMed] [Google Scholar]
WHO 2009
- World Health Organization, UNICEF, World Bank. State of the World's Vaccines and Immunization. 3rd Edition. Geneva: World Health Organization, 2009. [Google Scholar]
WHO 2010
- World Health Organization. Rabies vaccines: WHO position paper. Weekly Epidemiological Record 2010;85:309‐20. [Google Scholar]
WHO 2011
- World Health Organization. Measles outbreaks and importation of wild poliovirus and response. WHO [Europe] Epidemiological Brief 2011;15:1‐8. [Google Scholar]
WHO 2013a
- Djomo PN, Thomas SL, Fine PEM. Correlates of Vaccine‐Induced Protection: Methods and Implications. Geneva: Initiative for Vaccine Research (IVR) of the Department of Immunization, Vaccines and Biologicals, World Health Organization, May 2013. [Google Scholar]
WHO 2013b
- World Health Organization. Vaccine Safety Basics ‐ Learning Manual. Geneva: World Health Organization, 2013. [Google Scholar]
WHO 2013c
- World Health Organization. Pertussis. www.who.int/biologicals/vaccines/pertussis/en/ (accessed 22 August 2014).
WHO 2014a
- World Health Organization. Information sheet: observed rate of vaccine reactions ‐ diphtheria, pertussis, tetanus vaccines, 2014. www.who.int/vaccine_safety/initiative/tools/DTP_vaccine_rates_information_sheet.pdf (accessed 31 July 2014).
WHO 2014b
- World Health Organization. Revised guidance on the choice of pertussis vaccines: July 2014. Weekly Epidemiological Record 2014;30(89):337‐40. [PubMed] [Google Scholar]
WHO 2015a
- World Health Organization. WHO guideline on the use of safety‐engineered syringes for intramuscular, intradermal and subcutaneous injections in health‐care settings, 2015. www.who.int/injection_safety/global‐campaign/injection‐safety_guideline.pdf (accessed 27 February 2015). [WHO/HIS/SDS/2015.5] [PubMed]
WHO 2015b
- World Health Organization. Pertussis vaccines: WHO position paper ‐ August 2015. Weekly Epidemiological Record 2015;35(90):433‐60. [Google Scholar]
WHO 2017
- World Health Organization. Summary of WHO position papers ‐ recommendations for routine immunization (updated March 2017). www.who.int/immunization/policy/Immunization_routine_table1.pdf?ua=1 (accessed 22 January 2018).
Wise 2013
- Wise J. Measles outbreak hits northeast England. BMJ 2013;346:f662. [DOI: 10.1136/bmj.f662] [DOI] [PubMed] [Google Scholar]
Woodin 1995
- Woodin K, Rodewald L, Humiston S, Carges M, Schaffer S, Szilagyi P. Physician and parent opinions: are children becoming pincushions from immunizations?. Archives of Pediatric and Adolescent Medicine 1995;149:845‐9. [DOI] [PubMed] [Google Scholar]
Wright 2009
- Wright S, Yelland M, Heathcote K, Ng S. Fear of needles: nature and prevalence in general practice. Australian Family Physician 2009;38(3):172‐6. [PubMed] [Google Scholar]
Young 2005
- Young KD. Pediatric procedural pain. Annals of Emergency Medicine 2005;45:160‐71. [DOI] [PubMed] [Google Scholar]
Zimmerman 2006
- Zimmerman RK. Size of the needle for infant vaccination. BMJ 2006;333:563‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zuckerman 2000
- Zuckerman JN. The importance of injecting vaccines into muscle. BMJ 2000;321:1237‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Beirne 2015
- Beirne PV, Hennessy S, Cadogan SL, Shiely F, Fitzgerald T, MacLeod F. Needle size for vaccination procedures in children and adolescents. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD010720.pub2] [DOI] [PubMed] [Google Scholar]


