Abstract
Background
The use of systemic immunotherapy targets is emerging as an important treatment option for metastatic urothelial carcinoma, particularly for patients who cannot tolerate or who fail cisplatin‐based chemotherapy. One such target is the inhibition of the checkpoint protein programmed cell death‐1 (PD‐1) receptor and its ligand (PD‐L1) by monoclonal antibodies.
Objectives
To assess the effects of pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy.
Search methods
We performed a Cochrane Rapid Review, limiting our search to published studies in the English language. We searched databases of the medical literature, including the Cochrane Central Register of Controlled Trials and MEDLINE, as well as trial registries including ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP). Our search extended from January 2000 to June 2018.
Selection criteria
We included randomised controlled trials except cross‐over trials and cluster randomised trials. We excluded all other study designs. Participants included had locally advanced or metastatic urothelial carcinoma of the bladder, with disease progression during or following platinum‐containing chemotherapy (synonymous with second‐/third‐/fourth‐line therapy). This review focused on pembrolizumab (synonyms: MK‐3475, lambrolizumab, Keytruda).
Data collection and analysis
Two review authors independently classified and abstracted data from the included study. The certainty of evidence was rated according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
Main results
We identified one randomised controlled trial that included 542 participants, which compared the use of pembrolizumab monotherapy versus chemotherapy for the treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy. Results were reported after a median follow‐up of 14.1 months (range 9.9 to 22.1 months).
Primary outcomes
Pembrolizumab probably reduces the risk of death from any cause (hazard ratio (HR) 0.73, 95% confidence interval (CI) 0.59 to 0.90; moderate certainty evidence). This corresponds to 115 fewer deaths (191 fewer to 38 fewer) per 1000 participants with pembrolizumab at 12 months. We downgraded the certainty of evidence one level for imprecision.
Pembrolizumab may slightly improve quality of life (change from baseline to week 15 assessed with the Core Quality of Life Questionnaire; higher value reflects better quality of life; scale 0 to 100) with a mean difference (MD) of 9.05, 95% CI 4.61 to 13.50; low certainty evidence). We downgraded the certainty of evidence two levels for study limitations and imprecision.
Secondary outcomes
Pembrolizumab may have little or no effect on disease progression (HR 0.98, 95% CI 0.81 to 1.19; low certainty evidence). This corresponds to three fewer patients (42 fewer to 24 more) whose disease progressed per 1000 participants at 12 months. We downgraded the certainty of evidence two levels for study limitations and imprecision.
Pembrolizumab probably improves treatment response (based on complete or partial radiologic response) with a risk ratio (RR) of 1.85, 95% CI 1.24 to 2.77; moderate certainty evidence). This corresponds to 97 more respondents (27 more to 202 more) per 1000 participants with pembrolizumab. We downgraded the certainty of evidence one level for imprecision.
Pembrolizumab may have little or no effect on treatment‐related mortality (RR 0.96, 95% CI 0.24 to 3.79; low certainty evidence). This corresponds to one fewer (12 fewer to 44 more) treatment‐related deaths per 1000 participants with pembrolizumab. We downgraded the certainty of evidence two levels for study limitations and imprecision.
Pembrolizumab may have little or no effect on discontinuations due to adverse events (RR 0.66, 95% CI 0.39 to 1.10). This corresponds to 54 fewer discontinuations per 1000 participants (95% CI 79 fewer to 7 more). We downgraded the certainty of evidence for study limitations and imprecision.
Pembrolizumab may reduce serious adverse events (RR 0.83, 95 CI 0.72 to 0.97; low certainty evidence). This corresponds to 107 fewer serious averse events per 1000 participants (95% CI 19 fewer to 176 fewer). We downgraded two levels for study limitations and imprecision.
Authors' conclusions
The use of pembrolizumab in men with advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy probably improves overall survival when compared with chemotherapy alone. At 12 months follow‐up about 70% of those in the chemotherapy group had died, compared with 59% of those treated with pembrolizumab. We are very uncertain about the effects of pembolizumab on quality of life. Pembolizumab may also improve treatment response rates, and reduce the risk of serious adverse events, but may make little or no difference to discontinuations of treatment due to adverse events. These conclusions are based on a single trial that was sponsored by the producer of pembrolizumab.
Plain language summary
Pembrolizumab versus chemotherapy for treating advanced bladder cancer after recurrence/progression following platinum‐based chemotherapy
Review question
How does pembrolizumab (a newer medicine that works through the body's immune system) compare to chemotherapy in patients with cancer of the inner lining of the urinary system, called urothelial cancer, that has either come back or worsened after treatment?
Background
Medications that target the body's immune system have been used for a long time to treat urothelial cancer. When the cancer has spread to other organs outside the urinary tract, patients are often treated with chemotherapy using medicines called cisplatin or carboplatin (platinum‐containing chemotherapy). However, often the cancer comes back or becomes worse despite treatment. This review considers the evidence for pembrolizumab, which is a member of a new class of medications that work through the immune sytem, and compares it to chemotherapy.
Study characteristics
We considered only randomised controlled trials in this Cochrane Rapid Review, as they offer the most reliable results. This review is current to 20 June 2018.
Key results
We found only one randomised study for our question. Participants included in this trial had metastatic (cancer that has spread to other parts of the body) or advanced cancer that could not be removed by surgery, that had come back or worsened with other chemotherapy.
We found that pembrolizumab probably improves overall survival a little (evidence of moderate certainty). It may improve quality of life slightly (low certainty evidence).
Pembrolizumab may have little or not effect on the time for the cancer to worsen or advance (low certainty evidence). It probably improves treatment response as seen on X‐ray scans such as computer tomography (moderate certainty of evidence).
Pembrolizumab may have little or no effect on deaths resulting from the treatment itself (low certainty evidence) but may result in fewer patients stopping treatment due to unwanted side effects (low certainty evidence). It may also cause less serious side effects.
These conclusions are based on a single trial paid for by the company that makes pembrolizumab.
Certainty of evidence
The certainty of evidence ranged from moderate to very low.
Summary of findings
Summary of findings for the main comparison. Pembrolizumab compared to chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy. A Cochrane Rapid Review.
| Pembrolizumab compared to chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy. A Cochrane Rapid Review | |||||
|
Participants: people with advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy Setting: multicentre; 120 sites in 29 countries Intervention: pembrolizumab Control: chemotherapy (vinflunine or docetaxel or paclitaxel) | |||||
| Outcomes | Number of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with chemotherapy | Risk difference with pembrolizumab | ||||
| Time to death from any cause (here: overall mortality at 12‐month follow‐up) | 542 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | HR 0.73 (0.59 to 0.90) | Study population | |
| 695 per 1000 a | 115 fewer per 1000 (191 fewer to 38 fewer) | ||||
| Quality of life (Change from baseline to week 15) Assessed with: EORTC QLQ‐C30 Scale from 0 to 100 (a higher score represents better quality of life) Follow‐up: from baseline to week 15 | 519 (1 RCT) | ⊕⊕⊝⊝ LOW 2 3 4 | MD 9.05 (4.61 to 13.50) | The mean quality of life (change from baseline to week 15) was ‐8.3 score change b | MD 9.05 score change higher (4.61 higher to 13.50 higher) |
|
Response rate (partial and complete response) Follow‐up: median 14.1 months |
542 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | RR 1.85 (1.24 to 2.77) | Study population | |
| 114 per 1000 | 97 more per 1000 (27 more to 202 more) | ||||
| Treatment‐related mortality Follow‐up: median 14.1 months | 521 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | RR 0.96 (0.24 to 3.79) | Study population | |
| 16 per 1000 | 1 less per 1000 (12 fewer to 44 more) | ||||
| Discontinuation due to adverse event Follow‐up: median 14.1 months | 521 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | RR 0.66 (0.39 to 1.10) | Study population | |
| 110 per 1000 | 37 fewer per 1000 (67 fewer to 11 more) | ||||
|
Serious adverse events (irrespective of attribution to treatment) Follow‐up: median 14.1 months |
521 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | RR 0.83 (0.72 to 0.97) |
Study population | |
| 627 per 1000 | 107 fewer per 1000 (176 fewer to 19 fewer) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). RR 0.66, 95% CI 0.39 to 1.10 CI: confidence interval; HR: hazard ratio; MD: mean difference; RR: risk ratio; RCT: randomised controlled trial; EORTC QLQ‐C30:.European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐C30 | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded for imprecision due to wide confidence intervals.
2 Downgraded for study limitations (performance and detection bias)
3 Additonal concerns about selective reporting bias but not downgraded further.
4 Downgraded for imprecision; 95% CI crosses minimal clinically important difference of 10.
a The baseline risk for death of any cause in the chemotherapy group was assumed to be 69.3% at 12 months as reported by Bellmunt 2017 (at 12 months, the estimated overall survival rate was 43.9% (95% CI 37.8 to 49.9) for participants treated with pembrolizumab and 30.7% (95% CI 25.9 to 36.7) for participants treated with chemotherapy).
b Baseline risk for the chemotherapy group at 15 weeks as reported by Bellmunt 2017 ("From baseline to week 15, scores were stable for pembrolizumab (n = 266) (least squares [LS] mean +0.75 [95% CI –2.34 to +3.83]) but worsened for chemotherapy (n = 254) (LS mean –8.30 [95% CI –11.76 to –4.83]); the difference in LS means between arms was 9.05 (95% CI 4.61‐13.48; nominal 2‐sided P < 0.001)").
Background
Description of the condition
According to 2012 GLOBOCAN data (GLOBOCAN 2012), urothelial carcinoma of the bladder is the ninth most common malignancy worldwide, with nearly 429,000 new cases and 165,000 cancer‐related deaths every year. In addition to the bladder, urothelial carcinoma can affect the renal pelvis, ureters, and the urethra. A diagnosis is typically established by visualisation of the tumour using cross‐sectional imaging or cystoscopy, or both, followed by transurethral resection, which is both diagnostic and therapeutic. Urothelial carcinoma of the bladder is a heterogeneous entity and can vary in presentation from non‐invasive, low‐grade disease to invasive, high‐grade forms that can rapidly progress to early metastasis and death despite aggressive treatment. Invasive urothelial carcinoma of the bladder is usually treated with radical cystectomy and urinary diversion or with radiotherapy and concomitant chemotherapy (EAU 2017; Leitlinienprogramm Onkologie; NCCN Guideline 2017). The surgical therapy can be combined with neoadjuvant or adjuvant chemotherapy (Leitlinienprogramm Onkologie; NCCN Guideline 2017). Metastatic urothelial carcinoma is usually treated with palliative chemotherapy (EAU 2017; Leitlinienprogramm Onkologie; NCCN Guideline 2017). The most effective chemotherapy regimens are combination platinum‐based therapies, such as MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin; Logothetis 1990), or a combination of gemcitabine plus cisplatin or carboplatin (EAU 2017; Leitlinienprogramm Onkologie; NCCN Guideline 2017; von der Maase 2000). Unfortunately, individuals with advanced urothelial carcinoma often have progression or recurrence of urothelial cancer following a first‐line platinum‐containing regimen for metastatic or inoperable locally advanced disease. These individuals are then often managed supportively or with inferior regimens. Vinflunine is often used in Europe as a second‐line chemotherapy regimen and offers a median overall survival of 6.9 months compared to 4.6 months with best supportive care (Bellmunt 2013). The need for novel therapies in this realm is clear.
Description of the intervention
The use of immunotherapy to treat bladder cancer is well established, particularly the use of intravesical Bacillus Calmette‐Guerin for non‐muscle invasive high‐grade disease (Morales 1976). Systemic immunotherapy targets, such as immune checkpoint receptors and their ligands, have been the focus of several recent clinical trials. For example, the inhibition of the checkpoint protein programmed cell death‐1 (PD‐1) receptor and its ligand (PD‐L1) by monoclonal antibodies (mAb) has elicited effective antitumour responses (Ribas 2015; Sharma 2015). Administered intravenously every two to three weeks, mAbs have shown promising response rates against urothelial carcinoma (Bellmunt 2017; Kim 2015; Plimack 2017; Rosenberg 2016; Sharma 2016). The mAb pembrolizumab targets the PD‐1 receptor, and a randomised controlled trial comparing pembrolizumab with paclitaxel, docetaxel, or vinflunine chemotherapy in individuals in whom urothelial carcinoma had recurred or progressed after platinum‐based chemotherapy has been reported (Bellmunt 2017). Pembrolizumab was associated with longer overall survival and with a lower rate of treatment‐related adverse events than chemotherapy (Bellmunt 2017).
Adverse effects of the intervention
Grade 3 and 4 treatment‐ and immune‐related adverse events were recently reported in 16% and 5%, respectively, of individuals with post‐platinum‐treated advanced urothelial carcinoma using the mAb atezolizumab (Rosenberg 2016). Examples of adverse events included elevated lipase and amylase levels, fatigue, rash, and decreased lymphocyte and neutrophil counts (Rosenberg 2016). Similar adverse events and adverse event rates were reported in a phase Ib study of pembrolizumab in metastatic urothelial carcinoma, with 5 of 29 participants reporting grade 3 or 4 adverse events (Gupta 2015). Treatment‐related deaths due to pneumonitis and thrombocytopenia were reported in a phase I/II study with nivolumab in a similar population (Sharma 2016).
How the intervention might work
Checkpoint proteins such as PD‐1 work to inhibit a host's immune response against a tumour cell by preventing T‐cells from attacking the tumour cells that would otherwise be detected as foreign. Tumour cells, including certain urothelial carcinoma cells, have been shown to express high levels of PD‐1 (Faraj 2015), thereby enabling them to evade a host's normal immune response. Checkpoint inhibitors such as mAbs targeting PD‐1 and its ligand, PD‐L1, can therefore block what would otherwise be an inhibitory effect of T‐cells, in turn "reactivating" a host's immune system against tumour cells (Park 2016).
Why it is important to do this review
Given the paucity of treatment options available for individuals with advanced urothelial carcinoma who had progression or recurrence of urothelial cancer following a first‐line platinum‐containing chemotherapy (e.g. cisplatin, carboplatin) for metastatic or inoperable locally advanced disease, the need for novel therapeutic targets is evident. Pembrolizumab has emerged as a novel immunotherapy option, but to date no systematic review of the available data has been carried out that has carefully evaluated the certainty of evidence using Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology to better inform clinical practice.
Objectives
To assess the effects of pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy.
Methods
Criteria for considering studies for this review
Types of studies
This review is based on a published protocol (Narayan 2017). For details on differences between the protocol and review see 'Differences between protocol and review' section. We included randomised controlled trials except cross‐over trials and cluster randomised trials. We excluded all other study designs.
Types of participants
We included participants with locally advanced (>T2) or metastatic (M1) urothelial carcinoma of the bladder as determined by cross‐sectional imaging or confirmed by biopsy, or both, whose disease progressed during or following platinum‐containing chemotherapy (synonymous with second‐/third‐/fourth‐line therapy). We did not include participants receiving pembrolizumab as first‐line therapy.
Types of interventions
This review focused on pembrolizumab (synonyms: MK‐3475, lambrolizumab, Keytruda). We investigated the following comparisons of experimental intervention versus comparator intervention.
Experimental interventions
Pembrolizumab.
Comparator interventions
Second‐/third‐/fourth‐line chemotherapy.
Comparison
Pembrolizumab versus second‐/third‐/fourth‐line chemotherapy.
Concomitant interventions have to be the same in the experimental and comparator groups to establish fair comparisons. We planned inclusion of all studies comparing pembrolizumab with second‐/third‐/fourth‐line chemotherapy, irrespective of dose, route, frequency or duration.
Types of outcome measures
We predefined the following outcome measures.
Primary outcomes
Time to death from any cause as measured from the time of random sequence generation to time of death irrespective of cause (time‐to‐event outcome).
Quality of life as measured by validated instruments (continuous outcome).
Secondary outcomes
Time to progression as measured from the time of random sequence generation to the time of first confirmed progression, relapse, or death from urothelial carcinoma (time‐to‐event outcome).
Response rate (patients with no complete or partial response), measured as complete response or partial response according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria (categorical outcome; Eisenhauer 2009).
Treatment‐related mortality (dichotomous outcome).
Discontinuation due to adverse events (any grade according to the Common Terminology Criteria for Adverse Events (CTCAE)), measured from the time of random sequence generation to discontinuation of therapy because of an adverse event (dichotomous outcome).
Rate of serious adverse events (grade 3, 4, or 5 according to the CTCAE), such as pruritus, fatigue, diarrhoea, anaemia, constipation, neuropathy, neutropenia, alopecia, hypo‐/hyperthyroidism, pneumonitis, colitis, nephritis, skin reaction, thyroiditis, adrenal insufficiency, myositis, hypophysitis, or cardiovascular events (dichotomous outcome).
If we were unable to retrieve the necessary information to analyse time‐to‐event outcomes, we planned to assess the number of events per total number of included patients for dichotomised outcomes at 6 months and 12 months.
Main outcomes for 'Summary of findings' table
We presented a 'Summary of findings' table reporting the following outcomes listed according to priority. Outcome priority was determined by review authors providing content expertise (FK, PD).
Time to death from any cause (reported as overall mortality at 12 months).
Quality of life.
Response rate (complete or partial response radiographically).
Treatment‐related mortality.
Discontinuations due to adverse events.
Search methods for identification of studies
We conducted a Cochrane Rapid Review. For details on the search strategy see the Appendices section.
Electronic searches
We searched the following sources.
-
Databases of medical literature:
Cochrane Central Register of Controlled Trials (CENTRAL; June 2018);
MEDLINE (via PubMed; January 2000 to June 2018).
Databases of ongoing trials:
ClinicalTrials.gov (www.clinicaltrials.gov/; 2000 to June 2018);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; apps.who.int/trialsearch/; 2000 to June 2018).
This being an expedited, rapid review, we limited our search to published studies and to the use of English as the language of publication. We did not search the databases and web‐sites of institutions, such as pharmaceutical organisations, agencies, and societies. We began the search in 2000 because the underlying mechanism of action of tumour immunotherapy by PD‐L1 blockade was first reported in 2002 (Iwai 2002).
Searching other resources
We checked the reference lists of all identified trials, relevant review articles, and current treatment guidelines for further literature (EAU 2017; Leitlinienprogramm Onkologie), but as this is an expedited review, did not contact experts in the field, drug manufacturers, or regulatory agencies for additional information on unpublished trials.
Data collection and analysis
Selection of studies
We used reference management software to identify and remove potential duplicate records (Endnote 2011). Two review authors (VN, FK) independently scanned the abstract, title, or both, of the remaining records retrieved and investigated all potentially relevant records as full text, mapped records to studies, and classified studies as included studies, excluded studies, studies awaiting classification, or ongoing studies using Covidence software (Covidence). We resolved discrepancies through consensus or consultation with a third review author (PD). We documented reasons for the exclusion of studies that may have reasonably been expected to be included in the review in a 'Characteristics of excluded studies' table. We present an adapted Preferred Reporting Items for Systematic Reviews and Meta‐Analyses ((PRISMA) flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
For studies that fulfilled the inclusion criteria, one review author (FK) extracted key participant and intervention characteristics using a data extraction form based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). A second review author checked data entry (VN). We resolved disagreements by consensus or, when required, by consultation with a third review author (PD).
We extracted the following information.
Study design and number of study centres.
Run‐in period.
Participant inclusion and exclusion criteria.
Participant details, baseline demographics such as visceral/liver metastases, age or ECOG (Eastern Cooperative Oncology Group) performance status.
The number of participants by study/study arm.
Details of relevant experimental and comparator interventions such as dose, route, frequency, and duration.
Definitions of primary and secondary outcomes, and method and timing of outcome measurement.
Study funding sources
Declarations of interest by primary investigators.
We attempted to provide information, including trial identifier, about potentially relevant ongoing studies in the 'Characteristics of ongoing studies' table. We attempted to contact authors of included studies to obtain key missing data when needed.
We extracted outcome data relevant to this review as needed for the calculation of summary statistics and measures of variance. For dichotomous outcomes, we attempted to obtain numbers of events per total number of included participants to enable the population of a 2 x 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we attempted to obtain means and standard deviations or the data necessary to calculate this information. For time‐to‐event outcomes, we attempted to obtain hazard ratios (HRs) with corresponding measures of variance or the data necessary to calculate this information.
Dealing with duplicate and companion publications
For duplicate publications, companion documents, or multiple reports of a primary trial, we maximised the information yield by collating all available data and used the most complete data set aggregated across all known publications. We listed multiple reports of the primary trial as secondary references under the study identifier of the included trial. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Data from clinical trial registers
We extracted data from any included studies published in clinical trial registers.
Assessment of risk of bias in included studies
One review author assessed the risk of bias in each included study (FK), and a second review author checked the data entry (VN). We resolved disagreements by consensus, or by consultation with a third review author (PD).
We assessed the risk of bias in included randomised controlled trials using the Cochrane 'Risk of bias' tool (Higgins 2011b). We assessed the following domains.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other sources of bias.
We judged the study as being at 'low risk', 'high risk', or 'unclear risk' for each domain and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We present a 'Risk of bias' graph to illustrate these findings.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated the risk of bias separately for each outcome (Hróbjartsson 2013), but grouped outcomes as appropriate, as detailed below.
With regard to performance bias, we judged outcomes as being similarly susceptible to performance bias and rated them as one group.
We defined the following endpoints as subjective outcomes in terms of susceptibility to detection bias and rated them as one group.
Quality of life.
Progression‐free survival.
Response rate (patients with complete or partial response).
Treatment‐related mortality.
Rate of serious adverse events.
We defined the following endpoint as an objective outcome in terms of susceptibility to detection bias.
Overall survival.
We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and grouped outcomes with like judgements when reporting our findings in the 'Risk of bias' tables.
We summarised the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome.
Measures of treatment effect
We expressed dichotomous data as a risk ratio (RR) with 95% confidence interval (CI). For continuous outcomes measured on the same scale, we estimated the intervention effect using the mean difference (MD) with 95% CI. For continuous outcomes measuring the same underlying concept (e.g. health‐related quality of life) but using different measurement scales, we planned to calculate the standardised mean difference (SMD). We expressed time‐to‐event data as HRs with 95% CIs or used an indirect estimation method if HRs were not given (Parmar 1998; Tierney 2007).
Unit of analysis issues
The unit of analysis was the individual participant. If we had identified trials with more than two intervention groups for inclusion in the review, we would have handled these in accordance with guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c).
Dealing with missing data
We planned to obtain missing data from study authors, if feasible, and planned to perform intention‐to‐treat analyses if data were available; we otherwise performed analyses as treated and would have indicated this as a potential source of bias. We investigated attrition rates (e.g. drop‐outs, losses to follow up, and withdrawals) and critically appraised issues of missing data. We did not plan to impute missing data.
Assessment of heterogeneity
We planned to assess heterogeneity; however, we included only one randomised controlled trial and therefore assessment of heterogeneity was not possible.
Assessment of reporting biases
We obtained the study protocol of the included randomised controlled trial to assess for selective outcome reporting. We also searched for completed but not reported trials in trial registers (ClinicalTrials.gov; WHO ICTRP).
Data synthesis
We presented data from the included randomised controlled trial using Review Manager software (Review Manager 2014) in accordance with the guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For dichotomous outcomes we used the Mantel‐Haenszel method; for continuous outcomes we used the inverse variance method; and for time‐to‐event outcomes we used the generic inverse variance method.
For the analyses of individual serious adverse events with very low events rates we used Peto's odds ratio method as suggested by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011d).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to potentially introduce clinical heterogeneity, and carried out the following subgroup analyses to test for subgroup differences in Review Manager (Review Manager 2014).
Performance status (Eastern Cooperative Oncology Group (ECOG) 0 or 1 versus ≥ 2).
Time since last chemotherapy administration (< three months versus ≥ three months).
Degree of pretreatment (second‐ versus third‐ versus fourth‐line or more).
PDL‐1 tumour expression status (positive versus negative).
Sensitivity analysis
We only identified one randomised controlled trial and were therefore not able to perform sensitivity analyses.
'Summary of findings' table
We present the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account five criteria related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity (directness of results; Guyatt 2008). We used the GRADEpro Guideline Development Tool to assess the quality of the evidence, according to the recommendations of the GRADE working group (GRADEpro GDT). Two review authors (FK, PD) independently rated the certainty of evidence for each outcome as 'high', 'moderate', 'low', or 'very low'; we resolved discrepancies by consensus or, when needed, by the arbitration of a third review author (NS). We present a summary of the evidence for the main outcomes in a 'Summary of findings' table; these tables provide key information about the best estimate of the magnitude of the effect in relative terms and present absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in the effect estimates for each outcome (Guyatt 2011; Schünemann 2011). If meta‐analysis was not possible, we planned to present results in a narrative 'Summary of findings' table.
See Types of outcome measures for the outcomes included in the 'Summary of findings' table.
Results
Description of studies
Results of the search
We identified 352 records following our database search, and after screening by title and abstract, evaluated 13 full‐text articles for eligibility. Only one study, reported in six records, ultimately met the inclusion criteria for assessment of the study question (for details see 'Figure 1'). Four of the six records were additionally found by handsearching or screening of reference lists. We did not identify any completed but not reported trials.
1.
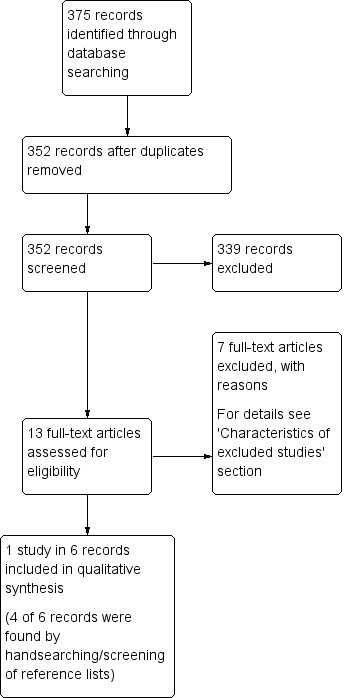
Study flow diagram.
Included studies
We identified one randomised controlled trial (Bellmunt 2017). For details see 'Characteristics of included studies' table, Table 2, and Table 3.
1. Baseline characteristics.
| Intervention(s) and comparator(s) | Duration of follow‐up | Description of participants | Trial period (year to year) | Country | Setting | Ethnic groups (%) | |
| Bellmunt 2017 | I: pembrolizumab | Median 14.1 months (for quality of life: from randomisation to week 15) | Participants with advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy | 2014‐2015 | International | Multicentre | ‐ |
| C: paclitaxel or docetaxel or vinflunine | ‐ |
C: comparator; I: intervention; ‐: not reported.
2. Participant disposition.
| Intervention(s) and comparator(s) | Sample size | N screened/eligible | N randomised | N ITT | N analysed for overall survival, progression‐frees survival, and response rate | N analysed for quality of life | N analysed for treatment‐related mortality, discontinuation due to adverse events, and adverse events | Follow‐upa | |
| Bellmunt 2017 | I1: Pembrolizumab | 542 | 748 | 270 | 270 | 270 | 266 | 266 | Median 14.1 months (9.9‐22.1; for quality of life: from randomisation to week 15) |
| C1: Paclitaxel or docetaxel or vinflunine | 272 | 272 | 272 | 254 | 255 | ||||
| Total: | 542 | 542 | 542 | 520 | 521 | ||||
| Grand total | All interventions | 270 | |||||||
| All comparators | 272 | ||||||||
| All interventions and comparators | 542 | ||||||||
aFollow‐up under randomised conditions until end of trial or, if not available, duration of intervention.
C: comparator; I: intervention; ITT: intention‐to‐treat.
The included trial compared pembrolizumab monotherapy with chemotherapy (paclitaxel or vinflunine or docetaxel) for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy. 748 participants were screened for enrolment in 120 sites in 29 countries (Australia, Austria, Belgium, Canada, Chile, Denmark, France, Germany, Hungary, Ireland, Israel, Italy, Japan, Republic of Korea, Netherlands, New Zealand, Norway, Peru, Poland, Portugal, Puerto Rico, Romania, Singapore, Spain, Sweden, Taiwan, Turkey, United Kingdom, and United States). Between November 2014 and November 2015, 542 participants were randomly assigned in this trial that was sponsored by the producer of pembrolizumab. The median duration of follow‐up was 14.1 months (range 9.9 to 22.1 months). The majority of participants had an ECOG performance status of 0 to 1 (pembrolizumab n = 262/270; chemotherapy n = 264/272) and a visceral disease (pembrolizumab n = 240/270; chemotherapy n = 233/272). Liver metastases were evident in 33.7% to 35.1% of participants (pembrolizumab n = 91/270; chemotherapy n = 95/272).
Excluded studies
For details see 'Characteristics of excluded studies' section.
We excluded seven studies after assessing for eligibility (wrong comparator, n = 1; first‐line therapy, n = 2; comments to other articles/letters, n = 3; wrong intervention, n = 1).
Risk of bias in included studies
For details see 'Characteristics of included studies' with 'Risk of bias' table, 'Table 1' and 'Figure 2' and 'Figure 3'.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
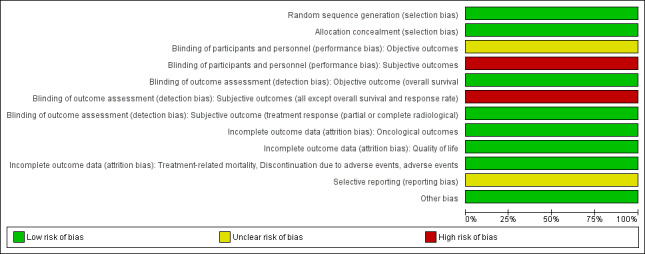
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Random sequence generation and allocation concealment were performed adequately, and we judged this study to be at low risk of selection bias.
Blinding
Performance bias
Participants and personnel were not blinded but we are uncertain whether this could have plausibly effected overall survival; we therefore rated the risk of bias as unclear for this outcome.
All others outcomes (quality of life, time to progression, response rate, discontinuation due to adverse events and serious adverse events) were judged to be potentially susceptible to co‐interventions, thereby making blinding important; we rated the risk of bias as high risk.
Detection bias
Blinding of outcome assessors is not relevant to overall survival; we rated the risk of bias as low. The assessors of radiographical responses were reported to be blinded; we rated the risk of bias as low also.
All others outcomes (quality of life, time to progression, discontinuation due to adverse events and serious adverse events) which involve judgments on the part of the unblinded participants or investigators, or both, we judged to be potentially susceptible to detection bias, thereby making blinding important; we rated the risk of bias as high risk.
Incomplete outcome data
All participants who were randomised were included in the analysis for overall survival, progression‐free survival, and response rate. Attrition was less than 10% in either group for all other outcomes. We judged the risk of attrition bias as low for all outcomes.
Selective reporting
A protocol was available and the reported outcomes and their analyses in the completed study corresponded to how these had been planned. However, quality of life was not listed as a predefined outcome in the ClinicalTrials.gov registry (NCT02256436). We therefore assigned a judgment of unclear risk of reporting bias for the outcome of quality of life.
Other potential sources of bias
No other potential sources of bias were identified.
Effects of interventions
See: Table 1
1. Pembrolizumab versus chemotherapy
1.1 Primary outcomes
1.1.1. Time to death from any cause
Pembrolizumab probably extends time to death from any cause (HR 0.73, 95% CI 0.59 to 0.90; 1 study; 542 participants; median follow‐up 14.1 months; Analysis 1.1; Figure 4; moderate certainty evidence). This corresponds to 695 deaths per 1000 participants with chemotherapy and 115 fewer (191 fewer to 38 fewer) deaths per 1000 participants with pembrolizumab. We downgraded the certainty of the evidence by one level for imprecision (Table 1).
1.1. Analysis.
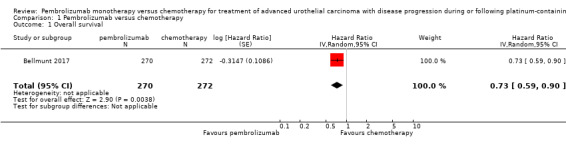
Comparison 1 Pembrolizumab versus chemotherapy, Outcome 1 Overall survival.
4.

Forest plot of comparison: 1 Pembrolizumab versus chemotherapy, outcome: 1.1 Overall survival.
1.1.2. Quality of life
Quality of life (change from baseline to week 15) was not listed as a predefined outcome in the ClinicalTrials.gov registry (NCT02256436). Quality of life was assessed with the Core Quality of Life Questionnaire (QLQ‐C30). Quality of life scores from baseline to week 15 were stable for pembrolizumab, while they decreased with chemotherapy, but the difference did not quite meet the threshold of a minimal clinically important difference of 10 (MD 9.05, 95% CI 4.61 to 13.50; 1 study; 520 participants; Analysis 1.2; Figure 5; low quality evidence). A high score represents better quality of life on this scale. We downgraded the certainty of evidence two levels for study limitations and imprecision (Table 1).
1.2. Analysis.
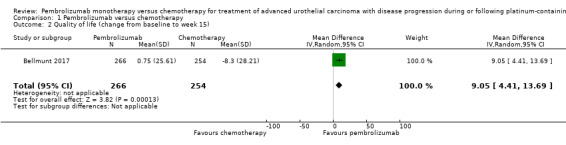
Comparison 1 Pembrolizumab versus chemotherapy, Outcome 2 Quality of life (change from baseline to week 15).
5.
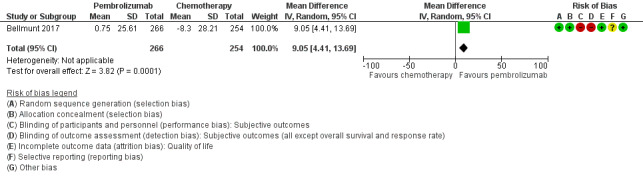
Forest plot of comparison: 1 Pembrolizumab versus chemotherapy, outcome: 1.2 Quality of life (change from baseline to week 15).
1.2. Secondary outcomes
1.2.1. Time to progression
Pembolizumab may have little or no effect on time to progression (HR 0.98, 95% CI 0.81 to 1.19; 1 study; 542 participants; median follow‐up 14.1 months; Analysis 1.3). This corresponds to three fewer (42 fewer to 24 more) progression events per 1000 participants at 12‐month follow‐up. We downgraded the certainty of evidence for imprecision.
1.3. Analysis.

Comparison 1 Pembrolizumab versus chemotherapy, Outcome 3 Progression‐free survival.
1.2.2. Response rate (partial and complete response)
Response rate (partial or complete radiologic response to therapy) was probably improved slightly with pembrolizumab (RR 1.85, 95% CI 1.24 to 2.77; 1 study; 542 participants; median follow‐up 14.1 months; Analysis 1.4; Figure 6; moderate quality evidence). This corresponds to 114 respondents per 1000 participants with chemotherapy and 97 more (27 more to 202 more) respondents per 1000 participants with pembrolizumab. Certainty of evidence was judged to be moderate (downgraded one level for imprecision; Table 1).
1.4. Analysis.

Comparison 1 Pembrolizumab versus chemotherapy, Outcome 4 Response rate (partial and complete response).
6.
Forest plot of comparison: 1 Pembrolizumab versus chemotherapy, outcome: 1.8 Serious adverse events (irrespective of attribution to treatment).
1.2.3. Treatment‐related mortality
Pembrolizumab may have little or no impact on treatment‐related mortality (HR 0.96, 95% CI 0.24 to 3.79; 1 study; 521 participants; median follow‐up 14.1 months; Analysis 1.5). This corresponds to one less treatment‐related death per 1000 participants (95% CI: 12 fewer to 44 more). The certainty of evidence was judged to be low, we downgraded two levels for study limitations and imprecision; Table 1).
1.5. Analysis.

Comparison 1 Pembrolizumab versus chemotherapy, Outcome 5 Treatment‐related mortality.
1.2.4. Discontinuation due to adverse events (any grade)
Pembrolizumab may have little of no effect on discontinuations due to adverse events (RR 0.66, 95% CI 0.39 to 1.10; 1 study; 521 participants; median follow‐up 14.1 months; Analysis 1.6). This corresponds to 54 fewer discontinuations per 1000 participants (95% CI 79 fewer to 7 fewer). We downgraded the certainty of evidence for study limitations and imprecision (Table 1).
1.6. Analysis.
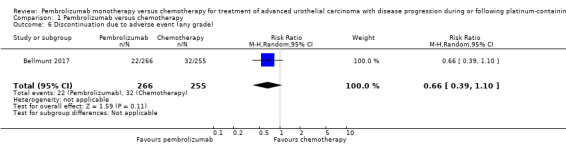
Comparison 1 Pembrolizumab versus chemotherapy, Outcome 6 Discontinuation due to adverse event (any grade).
1.2.5. Serious adverse events (irrespective of attribution to treatment)
We included adverse events data of grade 3, 4, or 5 according to CTCAE regardless of attribution to treatment by the investigator. Pembrolizumab may reduce serious adverse events (RR 0.83, 95 CI 0.72 to 0.97; low certainty evidence Analysis 1.7). This corresponds to 107 fewer serious averse events per 1000 participants (95% CI 19 fewer to 176 fewer). We downgraded two levels for study limitations and imprecision.
1.7. Analysis.
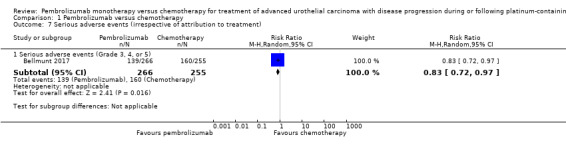
Comparison 1 Pembrolizumab versus chemotherapy, Outcome 7 Serious adverse events (irrespective of attribution to treatment).
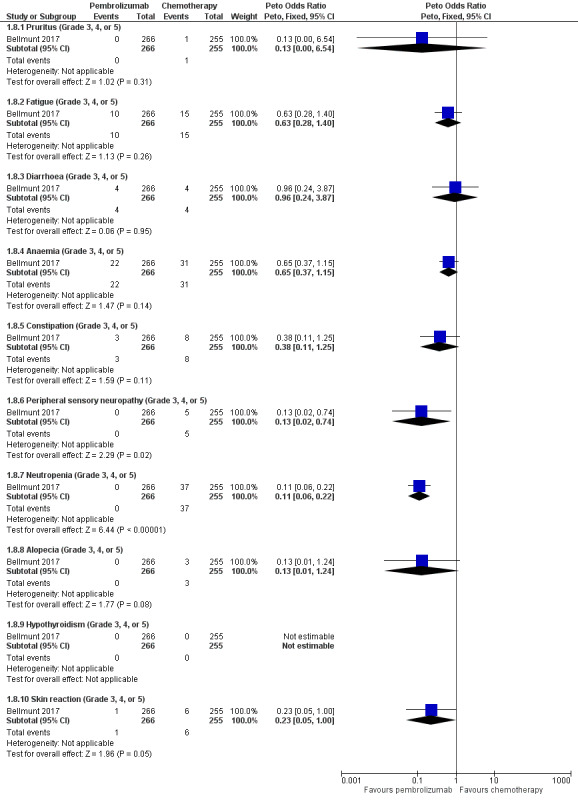
Individual serious adverse events (any adverse events of grade 3, 4, or 5 according to CTCAE)
Pembrolizumab probably decreases the rate of neutropenia (OR 0.11, 95% CI 0.06 to 0.22; 1 study; 521 participants; Analysis 1.8; Figure 6). We are uncertain whether pembrolizumab improves or reduces the rate of pruritus, fatigue, diarrhoea, anaemia, constipation, peripheral sensory neuropathy, alopecia, hypothyroidism, or skin reaction as the certainty of the evidence is limited by imprecision, as well as performance and detection bias.
1.8. Analysis.
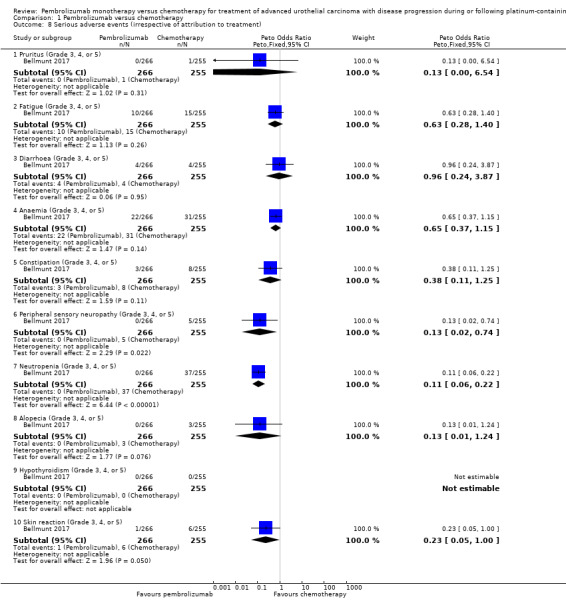
Comparison 1 Pembrolizumab versus chemotherapy, Outcome 8 Serious adverse events (irrespective of attribution to treatment).
We identified no adverse events data regardless of attribution to treatment by the investigators for pneumonitis (only treatment‐related events were reported: pembrolizumab 6/266 versus chemotherapy 0/255), hyperthyroidism (only treatment‐related events were reported: pembrolizumab 0/266 versus chemotherapy 0/255), colitis (only treatment‐related events were reported: pembrolizumab 3/266 vs. chemotherapy 0/255), nephritis (only treatment‐related events were reported: pembrolizumab 2/266 versus chemotherapy 0/255), thyroiditis (only treatment‐related events were reported: pembrolizumab 0/266 versus chemotherapy 0/255), adrenal insufficiency (only treatment‐related events were reported: pembrolizumab 1/266 versus chemotherapy 0/255), and myositis (only treatment‐related events were reported: pembrolizumab 0/266 versus chemotherapy 1/255).
We identified no adverse events data for hypophysitis and cardiovascular events.
Subgroup analyses
Preplanned subgroup analyses
We performed preplanned subgroup analyses with regard to overall survival.
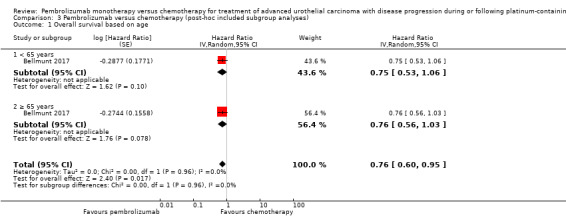
Performance status (ECOG 0 or 1 versus ≥ 2)
For details see Analysis 2.1. Of 542 participants, 526 had an ECOG 0 to 1 (262 in the pembrolizumab group, 264 in the chemotherapy group); and 6 had an ECOG ≥ 2 (2 in the pembrolizumab group, 4 in the chemotherapy group; 10 participants had missing data). We did not find evidence for subgroup differences (ECOG 0 to 1 versus ≥ 2; P = 0.66).
2.1. Analysis.
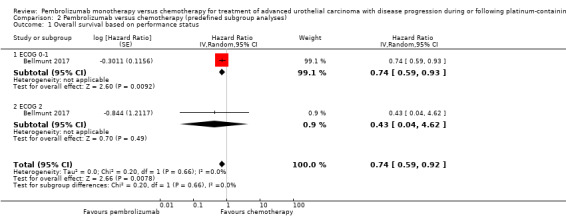
Comparison 2 Pembrolizumab versus chemotherapy (predefined subgroup analyses), Outcome 1 Overall survival based on performance status.
Time since last chemotherapy administration (< three months versus ≥ three months)
For details see Analysis 2.2. Of 542 participants, 207 received chemotherapy for less than three months (103 in the pembrolizumab group, 104 in the chemotherapy group) and 333 received chemotherapy for greater than or equal to three months (166 in the pembrolizumab group, 167 in the chemotherapy group). We did not find evidence for subgroup differences (last chemotherapy administration < three months versus ≥ three months; P = 0.35).
2.2. Analysis.
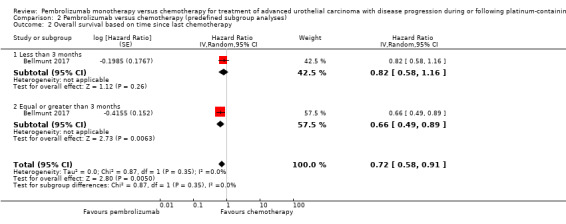
Comparison 2 Pembrolizumab versus chemotherapy (predefined subgroup analyses), Outcome 2 Overall survival based on time since last chemotherapy.
Degree of pretreatment (second‐ versus third‐ versus fourth‐line or more)
For details see Analysis 2.3. Of 542 participants, 84 had adjuvant or neoadjuvant chemotherapy, 340 had one prior treatment for metastatic disease, and 115 had two prior treatments for metastatic disease. We did not find evidence for subgroup differences (degree of pretreatment; P = 0.79).
2.3. Analysis.
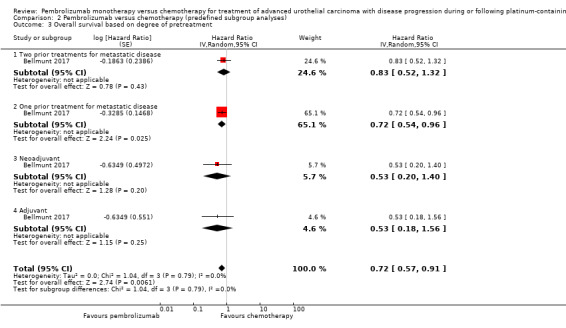
Comparison 2 Pembrolizumab versus chemotherapy (predefined subgroup analyses), Outcome 3 Overall survival based on degree of pretreatment.
PD‐L1 tumour expression status (positive versus negative; 1% cutoff)
For details see Analysis 2.4. Of 298 participants, 184 had a tumour PD‐L1 combined positive score of less than one per cent (negative according to our definition) and 142 of 230 participants had a positive score greater than or equal to one per cent (positive according to our definition). We did not find evidence for subgroup differences (P = 0.11).
2.4. Analysis.

Comparison 2 Pembrolizumab versus chemotherapy (predefined subgroup analyses), Outcome 4 Overall survival based on PD‐L1 tumour expression status.
Post‐hoc included subgroup analyses
We also included further not‐preplanned subgroup analyses (see Analysis 3.1 ‐ Analysis 3.12).
3.1. Analysis.
Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 1 Overall survival based on age.
3.12. Analysis.

Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 12 Overall survival based on investigator's choice of chemotherapy.
Smoking status
For details see Analysis 3.3. Of 542 participants, 67 were current smokers (29 in the pembrolizumab group, 38 in the chemotherapy group), 284 were former smokers (136 in the pembrolizumab group, 148 in the chemotherapy group), and 187 never smoked (104 in the pembrolizumab group, 83 in the chemotherapy group). We found heterogeneity between these two subgroups (I² = 75%). The test for subgroup differences showed a difference between the subgroups (P = 0.02). Overall survival was probably more improved with pembrolizumab in current smokers (HR 0.32, 95% CI 0.15‐0.68) compared to former smokers (HR 0.71, 95% CI 0.52 to 0.97) or participants who had never smoked (HR 1.06, 95% CI 0.72 to 1.55).
3.3. Analysis.

Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 3 Overall survival based on smoking status.
For all other subgroup analyses, the test for subgroup differences showed no difference (P > 0.05; Analysis 3.1; Analysis 3.2; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 3.7; Analysis 3.8; Analysis 3.9; Analysis 3.10; Analysis 3.11; Analysis 3.12).
3.2. Analysis.
Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 2 Overall survival based on sex.
3.4. Analysis.

Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 4 Overall survival based on histologic type.
3.5. Analysis.
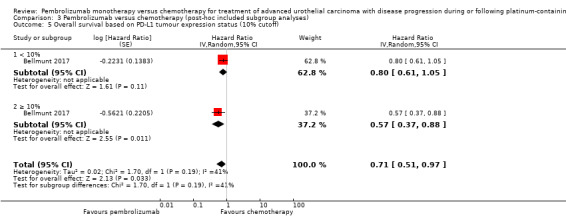
Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 5 Overall survival based on PD‐L1 tumour expression status (10% cutoff).
3.6. Analysis.
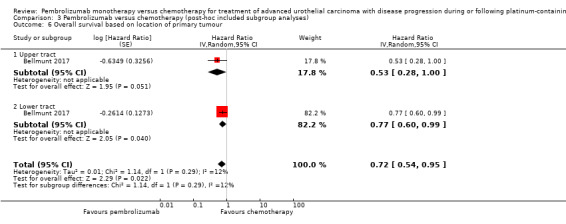
Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 6 Overall survival based on location of primary tumour.
3.7. Analysis.

Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 7 Overall survival based on location of metastases.
3.8. Analysis.
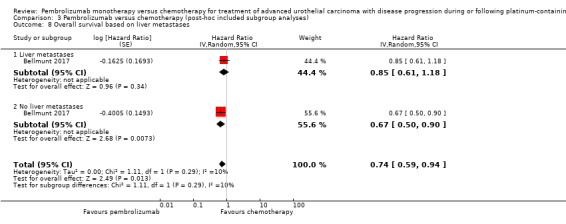
Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 8 Overall survival based on liver metastases.
3.9. Analysis.
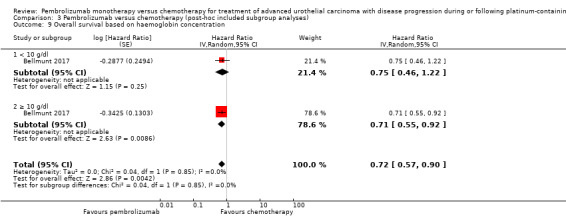
Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 9 Overall survival based on haemoglobin concentration.
3.10. Analysis.
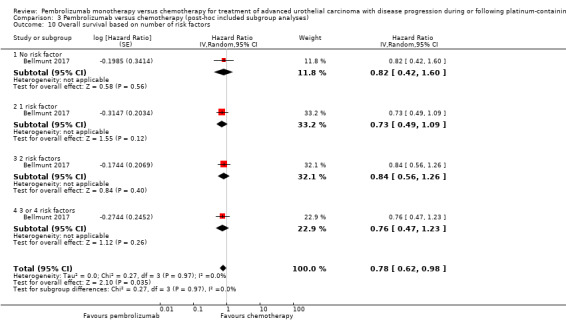
Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 10 Overall survival based on number of risk factors.
3.11. Analysis.

Comparison 3 Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses), Outcome 11 Overall survival based on previous platinum therapy.
Discussion
Summary of main results
We identified one randomised controlled trial with 542 participants that compared pembrolizumab monotherapy with chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy. Pembrolizumab probably extends time to death from any cause (moderate certainty of evidence) and may improve quality of life slightly. It may not impact treatment related mortality, but may reduce discontinuations due to adverse events and serious adverse events.
Overall completeness and applicability of evidence
This Cochrane Rapid Review is based on only one randomised controlled trial (Bellmunt 2017). However, the included study evaluated the drug pembrolizumab in a clinically important population seen in daily clinical practice. The participants and interventions conformed to the review question and the study reported on all predefined outcomes. Our patient important co‐primary outcome quality of life was not listed as a predefined outcome in the ClinicalTrials.gov registry (NCT02256436), but results were presented in abstract form at ASCO GU 2017 conference. No quality of life data were presented in the published manuscript.
The included study analysed only a few relevant adverse events, and the confidence intervals for adverse events are wide, leading to the presumption that current evidence is limited by imprecision. Further research including post‐market phase IV studies appear necessary for the evaluation of safety and rare adverse events.
In a letter to the editor regarding Bellmunt 2017, Liang and Zhu remarked that the prespecified subgroup analysis for geographic region (East Asia versus non–East Asia and European Union versus non–European Union) was not reported, and raised concerns that variations in the geographic regions could affect the response to pembrolizumab (Liang 2017). We were unable to address this concern.
Additionally, no platinum‐containing second‐line chemotherapy (e.g. MVAC or gemcitabine plus cisplatin or carboplatin) was used to compare against pembrolizumab. Pembrolizumab was compared only with vinflunine, docetaxel, or paclitaxel, which may represent inferior regimens with reported minimal or non‐durable response rates. This may cast a more favourable light on the new agent, pembrolizumab.
A detailed review of Kaplan‐Meier curves of pembrolizumab show characteristics that are noteworthy. Treatment response is seen as a parameter of uncertain value in check‐point‐blockade‐inhibition because it is observed that in certain participants there is a short progression initially after therapy start with objective response in the late therapy process and that certain participants will profit from continuing immunotherapy beyond first progression (Hodi 2016). In the case of this study, second‐line chemotherapy appeared to be more superior to pembrolizumab in the first four months. Only afterwards did the survival curves cross and favour pembrolizumab. Additionally, only a certain fraction of participants appeared to benefit from pembrolizumab treatment; however, these individuals saw markedly long survival. There is further research necessary for improved selection of participants that will profit from pembrolizumab therapy.
Quality of the evidence
We rated the certainty of evidence about effects as moderate to low. Reasons for downgrading included concerns over performance and detection bias (due to lack of blinding) and imprecision. For details on certainty of evidence see Table 1.
Potential biases in the review process
This Cochrane Rapid Review provides an overview of current evidence in a limited time frame and therefore uses streamlined systematic review methods for providing available evidence with shorter turnaround time. Nevertheless, this rapid review was performed with a broad search strategy in multiple biomedical databases and the evaluation of the literature and data extraction were performed by two independent review authors. While it is theoretically possible that additional studies may have been conducted but not yet published, or that additional studies may not have been identified, this is unlikely.
Agreements and disagreements with other studies or reviews
A systematic review published by Wang and colleagues assessed immune‐related adverse events and included 46 studies representing 12,808 oncologic patients (including melanomas, Hodgkin lymphomas, urothelial carcinomas, breast cancers, non‐small cell lung cancers, renal cell carcinomas, colorectal cancers, and others; Wang 2017). They evaluated different immune‐checkpoint inhibitors including nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab, and BMS‐936559. The authors found that in patients treated with PD‐1 signalling inhibitors, the overall incidence of immune‐related adverse events was 26.82% (I2 = 92.80) in any grade and 6.10% (I2 = 52.00) in severe grades (Wang 2017). However, interpretation of the data is limited because of missing control group evaluation data and high heterogeneity.
A systematic review published by Rijinder et al. evaluated immune checkpoint inhibitors in urological cancers (Rijnders 2017). They similarly identified one study of pembrolizumab in second‐line treatment for urothelial carcinoma and concluded that this therapy may be safe and confers a survival benefit in advanced urothelial carcinoma (Rijnders 2017). However, there is no 'Risk of bias' assessment or grading of certainty of evidence provided.
The guideline of the European Association of Urology recommends that vinflunine should be offered to patients progressing after platinum‐based combination chemotherapy for metastatic disease. However, vinflunine has not been approved by the FDA for this indication in the United States (EAU 2017). There is also a statement that it may be a reasonable strategy to re‐challenge former cisplatin‐sensitive patients if progression occurs at least six to twelve months after first‐line cisplatin‐based combination chemotherapy (EAU 2017). The bladder cancer guideline of the National Comprehensive Cancer Network recommends that consideration of checkpoint inhibitors must be integrated into therapeutic planning for all patients with locally advanced and metastatic disease and both guidelines recommend an enrolment of participants treated with immunotherapy in clinical trials (EAU 2017; NCCN Guideline 2017).
Also, the Institute for Quality and Efficiency in Healthcare (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen), a German agency that is responsible for assessing the quality and efficacy of medical treatments, concluded that there is evidence of a considerable additional benefit with pembrolizumab (IQWiG 2017).
Authors' conclusions
Implications for practice.
Pembrolizumab monotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy compared to chemotherapy probably improves overall survival to a small degree and may also improve quality of life although we are uncertain about this. It may also offer benefits in terms of response rate and rates of serious adverse events. In the setting of limited therapeutic alternatives, there appears to be a role for this agent in the therapeutic armamentarium.
Implications for research.
This review identified only one randomised controlled trial to contribute to its findings, and conclusions are limited primarily by imprecision and performance or detection biases. More rigorous trials are necessary in the future. In particular, future studies should place greater emphasis on quality of life assessment.
Only a subset of participants appear to benefit from pembrolizumab treatment. There is an urgent need for research to identify such patients prospectively in the future.
Furthermore, phase IV post‐marketing studies should be conducted for the evaluation of long‐term drug safety and assessment of rare yet potentially serious adverse events.
Notes
Parts of the methods section are based on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by Cochrane Urology.
Acknowledgements
The authors thank the Cochrane Urology staff members and the peer‐reviewers Maximilian Burger, Mark Klein, and Angelika Borkowetz for their support.
Appendices
Appendix 1. Search strategies
| Search terms for the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) |
| Pembrolizumab, MK‐3475, Keytruda |
| Search strategy for the Cochrane Library |
| #1 Pembrolizumab #2 MK‐3475 #3 Keytruda #4 Lambrolizumab #5 #1 or #2 or #3 or #4 #6 MeSH descriptor: [Urinary Bladder Neoplasms] explode all trees #7 ((bladder or urothelial) near/3 (cancer* or carcinoma* or neoplas* or tumo?r* or malignan* or adenocarcinoma* or mass*)):ti,ab,kw #8 #6 or #7 #9 #8 and #5 |
Appendix 2. MEDLINE search strategy
| # | Search Statement |
| 1 | pembrolizumab.mp. |
| 2 | keytruda.mp. |
| 3 | (MK adj2 "3475").tw. |
| 4 | mk‐3475.mp. |
| 5 | lambrolizumab.mp. |
| 6 | 1 or 2 or 3 or 4 or 5 |
| 7 | exp Urinary Bladder Neoplasms/ |
| 8 | exp Urothelium/ |
| 9 | transitional cell carcinoma.mp. or exp Carcinoma, Transitional Cell/ |
| 10 | exp Neoplasms/ |
| 11 | ((bladder or urotheli* or uninary or transitional) adj4 (carcinoma* or cancer* or neoplas* or adenocarcinoma* or mass or malignan*)).mp. |
| 12 | 7 or 9 or 11 |
| 13 | 6 and 12 |
| 14 | urotheli*.mp. |
| 15 | 6 and 10 and 14 |
| 16 | 13 or 15 |
| 17 | 6 and 9 |
| 18 | 16 or 17 |
| 19 | (("tcc" or transitional) adj3 cell adj3 carcinoma*).mp. |
| 20 | 6 and 19 |
| 21 | 18 or 20 |
| 22 | 7 or 10 |
| 23 | 6 and 8 and 22 |
| 24 | 21 or 23 |
Data and analyses
Comparison 1. Pembrolizumab versus chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | 542 | Hazard Ratio (Random, 95% CI) | 0.73 [0.59, 0.90] |
| 2 Quality of life (change from baseline to week 15) | 1 | 520 | Mean Difference (IV, Random, 95% CI) | 9.05 [4.41, 13.69] |
| 3 Progression‐free survival | 1 | 542 | Hazard Ratio (Random, 95% CI) | 0.98 [0.81, 1.19] |
| 4 Response rate (partial and complete response) | 1 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.24, 2.77] |
| 5 Treatment‐related mortality | 1 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.24, 3.79] |
| 6 Discontinuation due to adverse event (any grade) | 1 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.10] |
| 7 Serious adverse events (irrespective of attribution to treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Serious adverse events (Grade 3, 4, or 5) | 1 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.72, 0.97] |
| 8 Serious adverse events (irrespective of attribution to treatment) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 Pruritus (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.54] |
| 8.2 Fatigue (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.63 [0.28, 1.40] |
| 8.3 Diarrhoea (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.24, 3.87] |
| 8.4 Anaemia (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.37, 1.15] |
| 8.5 Constipation (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.11, 1.25] |
| 8.6 Peripheral sensory neuropathy (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.02, 0.74] |
| 8.7 Neutropenia (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.06, 0.22] |
| 8.8 Alopecia (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 1.24] |
| 8.9 Hypothyroidism (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.10 Skin reaction (Grade 3, 4, or 5) | 1 | 521 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.00] |
Comparison 2. Pembrolizumab versus chemotherapy (predefined subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival based on performance status | 1 | Hazard Ratio (Random, 95% CI) | 0.74 [0.59, 0.92] | |
| 1.1 ECOG 0‐1 | 1 | Hazard Ratio (Random, 95% CI) | 0.74 [0.59, 0.93] | |
| 1.2 ECOG 2 | 1 | Hazard Ratio (Random, 95% CI) | 0.43 [0.04, 4.62] | |
| 2 Overall survival based on time since last chemotherapy | 1 | Hazard Ratio (Random, 95% CI) | 0.72 [0.58, 0.91] | |
| 2.1 Less than 3 months | 1 | Hazard Ratio (Random, 95% CI) | 0.82 [0.58, 1.16] | |
| 2.2 Equal or greater than 3 months | 1 | Hazard Ratio (Random, 95% CI) | 0.66 [0.49, 0.89] | |
| 3 Overall survival based on degree of pretreatment | 1 | Hazard Ratio (Random, 95% CI) | 0.72 [0.57, 0.91] | |
| 3.1 Two prior treatments for metastatic disease | 1 | Hazard Ratio (Random, 95% CI) | 0.83 [0.52, 1.32] | |
| 3.2 One prior treatment for metastatic disease | 1 | Hazard Ratio (Random, 95% CI) | 0.72 [0.54, 0.96] | |
| 3.3 Neoadjuvant | 1 | Hazard Ratio (Random, 95% CI) | 0.53 [0.20, 1.40] | |
| 3.4 Adjuvant | 1 | Hazard Ratio (Random, 95% CI) | 0.53 [0.18, 1.56] | |
| 4 Overall survival based on PD‐L1 tumour expression status | 1 | Hazard Ratio (Random, 95% CI) | 0.75 [0.51, 1.08] | |
| 4.1 PD‐L1 positive (>1% cut off) | 1 | Hazard Ratio (Random, 95% CI) | 0.61 [0.43, 0.87] | |
| 4.2 PD‐L1 negative (<1% cut off) | 1 | Hazard Ratio (Random, 95% CI) | 0.89 [0.66, 1.20] |
Comparison 3. Pembrolizumab versus chemotherapy (post‐hoc included subgroup analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival based on age | 1 | Hazard Ratio (Random, 95% CI) | 0.76 [0.60, 0.95] | |
| 1.1 < 65 years | 1 | Hazard Ratio (Random, 95% CI) | 0.75 [0.53, 1.06] | |
| 1.2 ≥ 65 years | 1 | Hazard Ratio (Random, 95% CI) | 0.76 [0.56, 1.03] | |
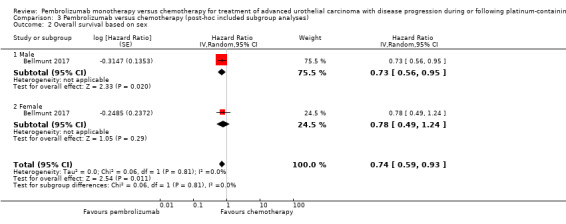
| 2 Overall survival based on sex | 1 | Hazard Ratio (Random, 95% CI) | 0.74 [0.59, 0.93] | |
| 2.1 Male | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.56, 0.95] | |
| 2.2 Female | 1 | Hazard Ratio (Random, 95% CI) | 0.78 [0.49, 1.24] | |
| 3 Overall survival based on smoking status | 1 | Hazard Ratio (Random, 95% CI) | 0.68 [0.41, 1.15] | |
| 3.1 Current | 1 | Hazard Ratio (Random, 95% CI) | 0.32 [0.15, 0.68] | |
| 3.2 Former | 1 | Hazard Ratio (Random, 95% CI) | 0.71 [0.52, 0.97] | |
| 3.3 Never | 1 | Hazard Ratio (Random, 95% CI) | 1.06 [0.72, 1.56] | |
| 4 Overall survival based on histologic type | 1 | Hazard Ratio (Random, 95% CI) | 0.72 [0.54, 0.97] | |
| 4.1 Transitional cell | 1 | Hazard Ratio (Random, 95% CI) | 0.80 [0.62, 1.03] | |
| 4.2 Mixed | 1 | Hazard Ratio (Random, 95% CI) | 0.58 [0.37, 0.91] | |
| 5 Overall survival based on PD‐L1 tumour expression status (10% cutoff) | 1 | Hazard Ratio (Random, 95% CI) | 0.71 [0.51, 0.97] | |
| 5.1 < 10% | 1 | Hazard Ratio (Random, 95% CI) | 0.80 [0.61, 1.05] | |
| 5.2 ≥ 10% | 1 | Hazard Ratio (Random, 95% CI) | 0.57 [0.37, 0.88] | |
| 6 Overall survival based on location of primary tumour | 1 | Hazard Ratio (Random, 95% CI) | 0.72 [0.54, 0.95] | |
| 6.1 Upper tract | 1 | Hazard Ratio (Random, 95% CI) | 0.53 [0.28, 1.00] | |
| 6.2 Lower tract | 1 | Hazard Ratio (Random, 95% CI) | 0.77 [0.60, 0.99] | |
| 7 Overall survival based on location of metastases | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.59, 0.91] | |
| 7.1 Lymph node only | 1 | Hazard Ratio (Random, 95% CI) | 0.46 [0.18, 1.18] | |
| 7.2 Visceral disease | 1 | Hazard Ratio (Random, 95% CI) | 0.75 [0.60, 0.94] | |
| 8 Overall survival based on liver metastases | 1 | Hazard Ratio (Random, 95% CI) | 0.74 [0.59, 0.94] | |
| 8.1 Liver metastases | 1 | Hazard Ratio (Random, 95% CI) | 0.85 [0.61, 1.18] | |
| 8.2 No liver metastases | 1 | Hazard Ratio (Random, 95% CI) | 0.67 [0.50, 0.90] | |
| 9 Overall survival based on haemoglobin concentration | 1 | Hazard Ratio (Random, 95% CI) | 0.72 [0.57, 0.90] | |
| 9.1 < 10 g/dl | 1 | Hazard Ratio (Random, 95% CI) | 0.75 [0.46, 1.22] | |
| 9.2 ≥ 10 g/dl | 1 | Hazard Ratio (Random, 95% CI) | 0.71 [0.55, 0.92] | |
| 10 Overall survival based on number of risk factors | 1 | Hazard Ratio (Random, 95% CI) | 0.78 [0.62, 0.98] | |
| 10.1 No risk factor | 1 | Hazard Ratio (Random, 95% CI) | 0.82 [0.42, 1.60] | |
| 10.2 1 risk factor | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.49, 1.09] | |
| 10.3 2 risk factors | 1 | Hazard Ratio (Random, 95% CI) | 0.84 [0.56, 1.26] | |
| 10.4 3 or 4 risk factors | 1 | Hazard Ratio (Random, 95% CI) | 0.76 [0.47, 1.23] | |
| 11 Overall survival based on previous platinum therapy | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.58, 0.92] | |
| 11.1 Cisplatin | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.56, 0.95] | |
| 11.2 Carboplatin | 1 | Hazard Ratio (Random, 95% CI) | 0.74 [0.47, 1.17] | |
| 12 Overall survival based on investigator's choice of chemotherapy | 1 | Hazard Ratio (Random, 95% CI) | 0.73 [0.61, 0.88] | |
| 12.1 Paclitaxel | 1 | Hazard Ratio (Random, 95% CI) | 0.76 [0.55, 1.05] | |
| 12.2 Docetaxel | 1 | Hazard Ratio (Random, 95% CI) | 0.76 [0.55, 1.05] | |
| 12.3 Vinflunine | 1 | Hazard Ratio (Random, 95% CI) | 0.69 [0.51, 0.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bellmunt 2017.
| Methods | Parallel RCT Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Characteristics:
|
|
| Interventions |
Number of study centres: 748 patients were screened for enrolment in 120 sites in 29 countries (Australia, Austria, Belgium, Canada, Chile, Denmark, France, Germany, Hungary, Ireland, Israel, Italy, Japan, Republic of Korea, Netherlands, New Zealand, Norway, Peru, Poland, Portugal, Puerto Rico, Romania, Singapore, Spain, Sweden, Taiwan, Turkey, United Kingdom, United States). Run‐in period: 11/2014‐11/2015. Extension period: no. Intervention: pembrolizumab 200 mg IV on Day 1 Q3W; 270 randomised patients, 266 patients received treatment. Comparison: paclitaxel 175 mg/m2 IV or docetaxel 75 mg/m2 IV or vinflunine 320 mg/m2 IV, on Day 1 Q3W, 272 randomised patients, 255 received treatment, 84 patients received docetaxel, 84 received paclitaxel, and 87 received vinflunine. Other co‐interventions for both groups: no. |
|
| Outcomes |
Primary outcome measures Overall survival:
Progression‐free survival:
Secondary outcome measures Objective response rate:
Progression‐free survival per modified RECIST 1.1:
Objective response reaction per modified RECIST 1.1:
Adverse event (time frame: up to 31 months):
Discontinuation of study drug due to an adverse event:
Quality of life:
|
|
| Funding sources | Supported by Merck (Kenilworth, NJ) | |
| Declarations of interest |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "...central randomisation as described in the protocol". Comment: Randomization was performed adequately. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Centrally...interactive voice response system/integrated web response system". Comment: allocation concealment was performed adequately. |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk |
Quote from publication: "...there was no blinding". Comment: overall survival was measured and reported. It might be conceivable that even objective outcomes are influenced by lack of blinding. We finally judge that there is an unclear risk of bias. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: "...there was no blinding". Comment: progression‐free survival, treatment‐related mortality, discontinuation due to adverse events, and adverse events were measured and reported (quality of life was not listed as a predefined outcome in ClinicalTrials.gov registry (NCT02256436) and was not reported in the full text publication, but results were presented in abstract form at ASCO GU 2017 conference (Bellmunt 2017)). We judge that subjective outcomes are likely to be influenced by lack of blinding leading to high risk of bias. For response rate, we are uncertain if lack of blinding of participants and personnel might have influenced results, and therefore judged that there is unclear risk of bias for this outcome. |
| Blinding of outcome assessment (detection bias) Objective outcome (overall survival | Low risk |
Quote from publication: no applicable quote. Comment: Not likely that outcome assessment for overall survival would be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes (all except overall survival and response rate) | High risk |
Quote from publication: no applicable quote. Comment: We judged that the assessment of these subjective outcomes is likely to be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcome (treatment response (partial or complete radiological) | Low risk |
Quote from publication: "Imaging data were centrally reviewed, central imaging vendor were blinded to the subject treatment, imaging results were blinded to the clinical study team". Comment: Adequate assurance of blinding |
| Incomplete outcome data (attrition bias) Oncological outcomes | Low risk | All patients randomised were included in the analysis for overall survival, progression‐free survival, and response rate. |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | Missing outcome data are less than 10% in both groups (randomised: 270 pembrolizumab, 272 chemotherapy; in evaluation: 266 pembrolizumab, 253 chemotherapy). We judge that this number of withdrawals is not enough to have a clinically relevant effect. |
| Incomplete outcome data (attrition bias) Treatment‐related mortality, Discontinuation due to adverse events, adverse events | Low risk | Missing outcome data are less than 10% in both groups (randomised: 270 pembrolizumab, 272 chemotherapy; in evaluation: 255 pembrolizumab, 255 chemotherapy). We judge that this number of withdrawals is not enough to have a clinically relevant effect. |
| Selective reporting (reporting bias) | Unclear risk | Quality of life was not listed as a predefined outcome in the ClinicalTrials.gov registry (NCT02256436) |
| Other bias | Low risk | No other potential bias identified. |
ASCO GU: Genitourinary Cancers Symposium of the American Society of Clinical Oncology; CNS: central nervous system; ECOG: Eastern Cooperative Oncology Group; HIV: human immunodeficiency virus; IV: intravenous; mAb: monoclonal antibody; NCT: ClinicalTrials.gov identifier; PD‐1: programmed cell death protein 1; PD‐L1: programmed cell death protein 1 ligand; PSA: prostatic‐specific antigen; Q3W: every three weeks; RCT: randomised controlled trial; RECIST: Response Evaluation Criteria in Solid Tumors.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acerta 2017 | Wrong comparator. |
| Alva 2016 | First‐line therapy. |
| Guo 2017 | Comment to another article. |
| Matthew 2015 | Wrong intervention. |
| Mitchell 2017 | Comment to another article. |
| Powles 2017 | First‐line therapy. |
| Venniyoor 2017 | Comment to another article. |
Differences between protocol and review
This review is based on a published protocol (Narayan 2017).
We renamed the time‐to‐event outcomes of overall survival and progression‐free survival to time‐to‐death of any cause and time to progression, respectively, to better reflect the relevant events and aid in the interpretation of the hazard ratios, in particular for the absolute effect size estimates.
We also performed a post hoc subgroup analysis for smoking status (which did not suggest a subgroup effect).
We have added the outcome of serious adverse events to the 'Summary of findings' table, given its importance to patients and clinical decision‐making.
Contributions of authors
VN drafted the first version; performed literature screening, data extraction, and 'Risk of bias' assessment; and revised/reviewed the final version.
AK provided content expertise and revised/reviewed the final version.
PD provided content expertise; performed literature screening, data extraction, 'Risk of bias' assessment, and interpretation of results including certainty of evidence rating; provided methodological oversight; and revised/reviewed the final version.
NS provided methodological support and reviewed the final version.
MR provided content expertise and revised/reviewed the final version.
CB developed the search strategy and performed the search.
NP provided content expertise and revised/reviewed the protocol.
ECH reviewed and revised the 'Risk of bias' assessment, and contributed to the certainty of evidence ratings.
JHJ provided methodological support.
GG provided methodological support and revised/reviewed the final version.
FK coordinated the work; performed literature screening, data extraction, 'Risk of bias' assessment, and interpretation of results; provided content expertise; and oversaw all aspects of the protocol and rapid review development.
Sources of support
Internal sources
-
University of Erlangen, Germany.
Salary support for Frank Kunath
-
Minneapolis VAMC, Minneapolis, MN, USA.
Salary support for Philipp Dahm
External sources
No sources of support supplied
Declarations of interest
VN: none.
AK: none.
PD: none.
NS: none.
MR: none.
CB: none.
NP: none.
JHJ: none.
ECH: none
GG: none.
FK: none.
New
References
References to studies included in this review
Bellmunt 2017 {published data only}
- Bellmunt J, Sonpavde G, Wit R, Choueiri TK, Siefker‐Radtke AO, Plimack ER, et al. KEYNOTE‐045: randomized phase 3 trial of pembrolizumab (MK‐3475) versus paclitaxel, docetaxel, or vinflunine for previously treated metastatic urothelial cancer. Journal of Clinical Oncology 2015 [Epub ahead of print]; Vol. 33, issue 15 Suppl. [DOI: 10.1200/jco.2015.33.15_suppl.tps4571] [DOI]
- Bellmunt J, Wit R, Vaughn DJ, Fradet Y, Lee J‐L, Fong L, et al. Pembrolizumab as second‐line therapy for advanced urothelial carcinoma. New England Journal of Medicine 2017;376(11):1015‐26. [DOI: 10.1056/NEJMoa1613683] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit R, Bajorin DF, Bellmunt J, Fradet Y, Lee J‐L, Fong L. Health‐related quality of life (HRQoL) of pembrolizumab (pembro) vs chemotherapy (chemo) for previously treated advanced urothelial cancer (UC) in KEYNOTE‐045. Journal of Clinical Oncology 2017;35(15 Suppl):4530. [DOI: 10.1200/JCO.2017.35.15_suppl.4530] [DOI] [Google Scholar]
- Merck Sharp, Dohme Corp. A study of pembrolizumab (MK‐3475) versus paclitaxel, docetaxel, or vinflunine for participants with advanced urothelial cancer (MK‐3475‐045/KEYNOTE‐045). clinicaltrials.gov/ct2/show/NCT02256436 (accessed 1 November 2017).
- Quinn D, Bellmunt J, Wit R, Vaughn DJ, Fradet Y, Lee JL, et al. Keynote‐045: open‐label, phase 3 study of pembrolizumab versus investigator's choice of paclitaxel, docetaxel, or vinflunine for previously treated advanced urothelial cancer. Asia‐Pacific Journal of Clinical Oncology 2017; Vol. 13, issue Suppl S1:35.
- Vaughn DJ, Bellmunt J, Fradet Y, Lee JL, Fong L, Vogelzang NJ, et al. Health‐Related Quality‐of‐Life Analysis From KEYNOTE‐045: A Phase III Study of Pembrolizumab Versus Chemotherapy for Previously Treated Advanced Urothelial Cancer. Journal of Clinical Oncology 2018;36(16):1579‐1587. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Acerta 2017 {published data only}
- Acerta Pharma BV, Merck Sharp, Dohme Corp. Study of the combination of ACP‐196 and pembrolizumab in subjects with platinum resistant urothelial bladder cancer. clinicaltrials.gov/ct2/show/NCT02351739 (accessed 1 November 2017).
Alva 2016 {published data only}
- Alva A, Gschwendt J, Loriot Y, Bellmunt J, Feng D, Poehlein C, et al. KEYNOTE‐361: randomized phase III study of pembrolizumab with or without chemotherapy versus chemotherapy alone in advanced urothelial carcinoma. Journal for ImmunoTherapy of Cancer 2016; Vol. 4, issue Suppl 1.
Guo 2017 {published data only}
- Guo T, Feng C. Pembrolizumab for advanced urothelial carcinoma. New England Journal of Medicine 2017;376(23):2303‐4. [DOI] [PubMed] [Google Scholar]
Matthew 2015 {published data only}
- Galsky M, Hoosier Cancer Research Network and Merck Sharp & Dohme Corp. Testing the PD‐1 inhibitor pembrolizumab as maintenance therapy after initial chemotherapy in metastatic bladder cancer. clinicaltrials.gov/ct2/show/NCT02500121 accessed 1 November 2017.
Mitchell 2017 {published data only}
- Mitchell F. Pembrolizumab as second‐line treatment for urothelial cancer. Lancet Oncology 2017;18(4):e197. [DOI: 10.1016/S1470-2045(17)30156-0] [DOI] [PubMed] [Google Scholar]
Powles 2017 {published data only}
- Powles T, Gschwend JE, Loriot Y, Bellmunt J, Geczi L, Vulsteke C, et al. Phase 3 KEYNOTE‐361 trial: pembrolizumab (pembro) with or without chemotherapy versus chemotherapy alone in advanced urothelial cancer. Journal of Clinical Oncology 2017 [Epub ahead of print]; Vol. 35, issue 15 Suppl. [DOI: 10.1200/JCO.2017.35.15_suppl.TPS4590] [DOI]
Venniyoor 2017 {published data only}
- Venniyoor A. Pembrolizumab for advanced urothelial carcinoma. New England Journal of Medicine 2017;376(23):2302‐3. [DOI: 10.1056/NEJMc1704612] [DOI] [PubMed] [Google Scholar]
Additional references
Bellmunt 2013
- Bellmunt J, Fougeray R, Rosenberg JE, Maase H, Schutz FA, Salhi Y, et al. Long‐term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum‐based chemotherapy. Annals of Oncology 2013;24(6):1466‐72. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 28 August 2017. Melbourne, Australia: Veritas Health Innovation, 2017.
EAU 2017
- Witjes JA, Compérat E, Cowan NC, Gakis G, Hernández V, Lebret T, et al. EAU guidelines on muscle‐invasive and metastatic bladder cancer. uroweb.org/guideline/bladder‐cancer‐muscle‐invasive‐and‐metastatic/ (accessed 1 October 2017).
Eisenhauer 2009
Endnote 2011 [Computer program]
- Thomson Reuters. EndNote X5. Version X5. Thomson Reuters, 2011.
Faraj 2015
- Faraj SF, Munari E, Guner G, Taube J, Anders R, Hicks J, et al. Assessment of tumoral PD‐L1 expression and intratumoral CD8+ T cells in urothelial carcinoma. Urology 2015;85(3):703.e1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
GLOBOCAN 2012
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013;Available from: http://globocan.iarc.fr:accessed 10/2017. [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 1 February 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gupta 2015
- Gupta S, O'Donnell P, Plimack ER, Berger R, Montgomery B, Heath K, et al. Mp68‐11 a Phase 1B Study of Pembrolizumab (Pembro; Mk‐3475) for Advanced Urothelial Cancer. The Journal of Urology 2015;193(4):e861‐2. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical Research Ed.) 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011d
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hodi 2016
- Hodi FS, Hwu WJ, Kefford R, Weber JS, Daud A, Hamid O, et al. Evaluation of immune‐related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. Journal of Clinical Oncology 2016;34(13):1510‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
IQWiG 2017
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). Pembrolizumab (urothelial carcinoma) [Pembrolizumab (Urothelkarzinom)]. Institute for Quality and Efficiency in Health Care 2017; Vol. Dossierbewertung A17‐46, issue Version 1.0:3‐9.
Iwai 2002
- Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD‐L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD‐L1 blockade. Proceedings of the National Academy of Sciences of the United States of America 2002;99(19):12293‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015
- Kim JW, Bellmunt J, Powles T, Loriot Y, Vogelzang MJ, Zambrano CC, et al. Clinical activity, safety, and biomarkers of MPDL3280A in metastatic urothelial bladder cancer: Additional analysis from phase IA study. Journal of Clinical Oncology 2015;33(7 Suppl):297. [Google Scholar]
Leitlinienprogramm Onkologie
- Leitlinienprogramm Onkologie, Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF. S3 ‐ Guideline for early detection, diagnosis, therapy and aftercare for bladder cancer [S3 ‐ Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Harnblasenkarzinoms]. leitlinienprogramm‐onkologie.de/Harnblasenkarzinom.92.0.html (accessed 1 October 2017).
Liang 2017
- Liang F, Zhu J. Pembrolizumab for advanced urothelial carcinoma. New England Journal of Medicine 2017;376(23):2302. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Logothetis 1990
- Logothetis CJ, Dexeus FH, Finn L, Sella A, Amato RJ, Ayala AG, et al. A prospective randomized trial comparing MVAC and CISCA chemotherapy for patients with metastatic urothelial tumors. Journal of Clinical Oncology 1990;8(6):1050‐5. [DOI] [PubMed] [Google Scholar]
Morales 1976
- Morales A, Eidinger D, Bruce AW. Intracavitary Bacillus Calmette‐Guerin in the treatment of superficial bladder tumors. The Journal of Urology 1976;116(2):180‐3. [DOI] [PubMed] [Google Scholar]
NCCN Guideline 2017
- Spiess PE, Agarwal N, Bangs R, Boorjian SA, Buyyounouski MK, Clark PE, et al. Bladder Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2017;15(10):1240‐67. [DOI] [PubMed] [Google Scholar]
Park 2016
- Park JC, Hahn NM. Emerging role of immunotherapy in urothelial carcinoma—Future directions and novel therapies. Urologic Oncology: Seminars and Original Investigations 2016;34(12):566‐76. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [PUBMED: 9921604] [DOI] [PubMed] [Google Scholar]
Plimack 2017
- Plimack ER, Bellmunt J, Gupta S, Berger R, Chow LQ, Juco J. Safety and activity of pembrolizumab in patients with locally advanced or metastatic urothelial cancer (KEYNOTE‐012): a non‐randomised, open‐label, phase 1b study. Lancet Oncology 2017;18(2):212‐20. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ribas 2015
- Ribas A. Releasing the brakes on cancer immunotherapy. New England Journal of Medicine 2015;373(16):1490–2. [DOI] [PubMed] [Google Scholar]
Rijnders 2017
- Rijnders M, Wit R, Boormans JL, Lolkema MP, Veldt AA. Systematic review of immune checkpoint inhibition in urological cancers. European Urology 2017;72(3):411‐23. [DOI] [PubMed] [Google Scholar]
Rosenberg 2016
- Rosenberg JE, Hoffman‐Censits J, Powles T, Heijden MS, Balar AV, Necchi A, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: a single‐arm, multicentre, phase 2 trial. The Lancet 2016;387(10031):1909‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Sharma 2015
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348(6230):56‐61. [DOI] [PubMed] [Google Scholar]
Sharma 2016
- Sharma P, Callahan MK, Bono P, Kim J, Spiliopoulou P, Calvo E, et al. Nivolumab monotherapy in recurrent metastatic urothelial carcinoma (CheckMate 032): a multicentre, open‐label, two‐stage, multi‐arm, phase 1/2 trial. The Lancet Oncology 2016;17(11):1590‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [PUBMED: 17555582] [DOI] [PMC free article] [PubMed] [Google Scholar]
von der Maase 2000
- Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. Journal of Clinical Oncology 2000;18(17):3068‐77. [DOI] [PubMed] [Google Scholar]
Wang 2017
- Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune‐Related Adverse Events Associated with Anti‐PD‐1/PD‐L1 Treatment for Malignancies: A Meta‐Analysis. Frontiers in Pharmacology 2017;8:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Narayan 2017
- Narayan V, Dahm P, Skoetz N, Risk MC, Bonfiorno C, Patel N, et al. Pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum‐containing chemotherapy. A Cochrane Rapid Review. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD012838] [DOI] [PMC free article] [PubMed] [Google Scholar]


