Abstract
Background
Stroke is an important cause of death and disability worldwide. Since high blood pressure is an important risk factor for stroke and stroke recurrence, drugs that lower blood pressure might play an important role in secondary stroke prevention.
Objectives
To investigate whether blood pressure‐lowering drugs (BPLDs) started at least 48 hours after the index event are effective for the prevention of recurrent stroke, major vascular events, and dementia in people with stroke or transient ischaemic attack (TIA). Secondary objectives were to identify subgroups of people in which BPLDs are effective, and to investigate the optimum systolic blood pressure target after stroke or TIA for preventing recurrent stroke, major vascular events, and dementia.
Search methods
In August 2017, we searched the Trials Registers of the Cochrane Stroke Group and the Cochrane Hypertension Group, the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8), MEDLINE Ovid (1946 to August 2017), Embase Ovid (1974 to August 2017), ClinicalTrials.gov, the ISRCTN Registry, Stroke Trials Registry, Trials Central, and the World Health Organization (WHO) International Clinical Trials Registry Platform Portal.
Selection criteria
Randomised controlled trials (RCTs) of BPLDs started at least 48 hours after stroke or TIA.
Data collection and analysis
Two review authors independently screened all titles and abstracts, selected eligible trials, extracted the data, assessed risk of bias, and used GRADE to assess the quality of the evidence. If necessary, we contacted the principal investigators or corresponding authors for additional data.
Main results
We included 11 studies involving a total of 38,742 participants: eight studies compared BPLDs versus placebo or no treatment (35,110 participants), and three studies compared different systolic blood pressure targets (3632 participants). The risk of bias varied greatly between included studies. The pooled risk ratio (RR) of BPLDs for recurrent stroke was 0.81 (95% confidence interval (CI) 0.70 to 0.93; 8 RCTs; 35,110 participants; moderate‐quality evidence), for major vascular event 0.90 (95% CI 0.78 to 1.04; 4 RCTs; 28,630 participants; high‐quality evidence), and for dementia 0.88 (95% CI 0.73 to 1.06; 2 RCTs; 6671 participants; high‐quality evidence). We mainly observed a reduced risk of recurrent stroke in the subgroup of participants using an angiotensin‐converting enzyme (ACE) inhibitor or a diuretic (I2 statistic for subgroup differences 72.1%; P = 0.006). The pooled RR of intensive blood pressure‐lowering for recurrent stroke was 0.80 (95% CI 0.63 to 1.00), and for major vascular event 0.58 (95% CI 0.23 to 1.46).
Authors' conclusions
Our results support the use of BPLDs in people with stroke or TIA for reducing the risk of recurrent stroke. Current evidence is primarily derived from trials studying an ACE inhibitor or a diuretic. No definite conclusions can be drawn from current evidence regarding an optimal systolic blood pressure target after stroke or TIA.
Plain language summary
Blood pressure drugs for preventing stroke and cardiovascular diseases in patients with a stroke or transient ischaemic attack (TIA)
Questions
Do blood pressure drugs prevent stroke, other blood vessel diseases, and dementia, in people with a stroke or transient ischaemic attack (TIA)? What blood pressure target is best for preventing stroke, other blood vessel diseases, and dementia, in people with a stroke or TIA?
Background
Stroke, due to blocked or bleeding blood vessels in the brain affects about 14 million people worldwide each year. Stroke survivors are at increased risk of recurrent stroke, other blood vessel diseases, and dementia. High blood pressure is an important risk factor that can increase this risk. Blood pressure‐lowering drugs are known to prevent first ever stroke. However, in stroke survivors lowering the blood pressure too far (using blood pressure drugs) may be harmful especially early after the stroke. Therefore, we reviewed trials that tested blood pressure‐lowering drugs started at least 48 hours after the stroke or TIA.
Study characteristics: this review is up‐to‐date to August 2017. We included 11 trials involving 38,742 participants: eight trials assessed the effect of blood pressure drugs, and three trials compared different blood pressure targets. Ten studies were hospital‐based and one trial was performed in a general practitioner setting. Not all trials contributed information to all outcomes.
Key results: blood pressure drugs lowered the risk of recurrent stroke in patients with a stroke or TIA, whereas there is insufficient evidence to conclude whether they reduce the risk of other blood vessel diseases and dementia. There is also insufficient evidence to conclude which blood pressure target is best for patients with a stroke or TIA.
Quality of the evidence: overall, the quality of the trials in this review was moderate. However, we found similar results in an analysis using only high‐quality trials. More research is needed to investigate whether blood pressure drugs also prevent dementia, and what blood pressure targets are best for patients with a stroke or TIA.
Summary of findings
Summary of findings for the main comparison. Blood pressure‐lowering drugs (BPLDs) compared to placebo or no treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of TIA or stroke.
| Blood pressure‐lowering drugs (BPLDs) compared to placebo or no treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of TIA or stroke | |||||
|
Patient or population: patients with a history of TIA or stroke Settings: in hospital or community Intervention: BPLDs Comparison: placebo or no treatment | |||||
| Outcomes | № of participants (studies) Follow‐up | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with placebo or no treatment | Risk difference with BPLDs | ||||
| Recurrent stroke of any type | 35,110 (8 RCTs) | ⊕⊕⊕⊝ Moderatea | RR 0.81 (0.70 to 0.93) | Study population | |
| 101 per 1000 | 19 fewer per 1000 (30 fewer to 7 fewer) | ||||
| Time to recurrent stroke | 26,889 (3 RCTs) | ⊕⊕⊕⊕ High | HR 0.82 (0.65 to 1.03) | Study population | |
| Not available | Not available | ||||
| Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause) | 28,630 (4 RCTs) | ⊕⊕⊕⊕ High | RR 0.90 (0.78 to 1.04) | Study population | |
| 151 per 1000 | 15 fewer per 1000 (33 fewer to 6 more) | ||||
| Myocardial infarction | 34,747 (6 RCTs) | ⊕⊕⊕⊕ High | RR 0.90 (0.72 to 1.11) | Study population | |
| 21 per 1000 | 2 fewer per 1000 (6 fewer to 2 more) | ||||
| Vascular death | 34,747 (6 RCTs) | ⊕⊕⊕⊕ High | RR 0.85 (0.76 to 0.95) | Study population | |
| 47 per 1.000 | 7 fewer per 1000 (11 fewer to 2 fewer) | ||||
| Death by any cause | 35,110 (8 RCTs) | ⊕⊕⊕⊝ Moderatea | RR 0.98 (0.91 to 1.05) | Study population | |
| 79 per 1000 | 2 fewer per 1000 (7 fewer to 4 more) | ||||
| Dementia | 6671 (2 RCTs) | ⊕⊕⊕⊕ High | RR 0.88 (0.73 to 1.06) | Study population | |
| 67 per 1000 | 8 fewer per 1000 (18 fewer to 4 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BPLDs: blood pressure‐lowering drugs; CI: confidence interval; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded the quality of evidence for the outcomes 'recurrent stroke of any type', and 'death by any cause' because multiple studies with a high or unclear risk of bias were included for these outcomes.
Background
Description of the condition
Stroke is the second most common cause of death and the second leading cause of disability‐adjusted life‐years (DALYs) lost worldwide (GBD 2016). Despite improved prevention and management of stroke in high‐income countries, the numbers of worldwide incident strokes, prevalent stroke survivors, DALYs lost due to stroke, and stroke‐related deaths are still increasing. In 2016, an estimated 13.7 million incident strokes and 5.5 million stroke‐related deaths occurred, and 116 million DALYs were lost due to stroke (GBD 2016). Stroke patients are at high risk of stroke recurrence, with cumulative risks increasing from 3% at 30 days to 40% at 10 years after their first stroke (Mohan 2011). Patients with a transient ischaemic attack (TIA) are also at high risk for stroke recurrence, with reviews reporting a cumulative stroke risk of 3% at 30 days (Valls 2016), and 18% at 10 years (van Wijk 2005). In addition, TIA patients have a cumulative 10‐year mortality risk of 34% and a cumulative 10‐year risk of major vascular event of 36% (van Wijk 2005).
Description of the intervention
Elevated blood pressure is a well‐known and modifiable risk factor for ischaemic and haemorrhagic stroke, as well as for myocardial infarction, major vascular events, and dementia (Lewington 2002; Qiu 2005). There is overwhelming evidence that treatment with blood pressure‐lowering drugs (BPLDs) is effective for the primary prevention of strokes and major vascular events in patients with elevated blood pressure (BPLTTC 2003; Lackland 2014). However, there is still considerable debate on the effectiveness of BPLDs in secondary prevention of stroke (Feldstein 2014). One reason why the effect of BPLDs after a first stroke is doubted, is the possible existence of a J‐ or U‐shaped curve representing the association between blood pressure levels and recurrent stroke (Irie 1993). If such an association indeed exists, lowering blood pressure below certain thresholds could increase the risk of recurrent stroke. Furthermore, there are concerns about a harmful decrease in cerebral perfusion as a result of decreased or disrupted autoregulation in patients with a history of stroke, especially in elderly patients (Birns 2005), which might be associated with an increased risk of dementia.
How the intervention might work
All BPLDs reduce blood pressure, which in turn decreases endothelial dysfunction and thereby the risk of atherosclerosis and small vessel disease. In addition, different BPLD classes have different sites and mechanisms of action, and therefore class‐specific effects.
Why it is important to do this review
Patients with a TIA or stroke are at risk for recurrence, major vascular events, and dementia. High blood pressure is a known and modifiable risk factor for these events.
Objectives
To investigate whether blood pressure‐lowering drugs (BPLDs) started at least 48 hours after the index event are effective for the prevention of recurrent stroke, major vascular events, and dementia in people with stroke or transient ischaemic attack (TIA). Secondary objectives were to identify subgroups of people in which BPLDs are effective, and to investigate the optimum systolic blood pressure target after stroke or TIA for preventing recurrent stroke, major vascular events, and dementia.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) only. We did not limit trials by any concomitant disease, baseline cardiovascular risk, or language. The effect must be estimated either in the main analysis or in a subgroup analysis. The effect must be estimated in an intention‐to‐treat analysis. We did not use individual participant data.
Types of participants
Adult patients with an ischaemic stroke, haemorrhagic stroke or TIA, regardless of presence of elevated blood pressure. We defined haemorrhagic stroke as intracerebral haemorrhage, thus excluding subarachnoid haemorrhage.
Types of interventions
We included trials comparing BPLDs (regardless of type, dosage or administration route) versus placebo or no treatment. We also included trials comparing intensive blood pressure lowering (defined as a blood pressure target < 130/85 mmHg) with standard blood pressure lowering (defined as a blood pressure target < 140 to 160/90 to 100 mmHg). The intervention was started at least 48 hours after the index event. We did not define a maximum time period for treatment initiation. We included trials with a factorial design if the investigators accounted for a possible interaction between the different interventions.
Types of outcome measures
We included trials if they reported any of our outcome measures. All outcome measures are expressed as risk ratios (RRs), except for the secondary outcome measure 'time to recurrent stroke', which is time‐to‐event data. We only took into account composite endpoints (major vascular event and any stroke or death) if they were reported identically.
Primary outcomes
Recurrent stroke of any type (fatal and non‐fatal).
Secondary outcomes
Time to recurrent stroke
Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause)
Myocardial infarction
Vascular death
Death by any cause
Dementia
Ischaemic stroke
Haemorrhagic stroke
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We did not impose any language restrictions and we arranged for the translation of articles where necessary.
Electronic searches
On 29 August 2017, we searched the Trials Registers of the Cochrane Stroke Group, the Cochrane Hypertension Group, the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8) in the Cochrane Library (Appendix 1), MEDLINE Ovid (from 1946 to 29 August 2017) (Appendix 2), and Embase Ovid (from 1974 to 29 August 2017) (Appendix 3).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialists Brenda Thomas and Joshua Cheyne (Appendix 2), and they adapted it for the other databases.
In August 2017, we searched for ongoing and unpublished trials in ClinicalTrials.gov (clinicaltrials.gov/), the ISRCTN Registry (www.isrctn.com), Stroke Trials Registry (www.strokecenter.org/trials), Trials Central (www.trialscentral.org), and WHO International Clinical Trials Registry Platform (ICTRP) Portal (Appendix 4).
Searching other resources
We checked the reference lists of all relevant articles reporting studies selected for inclusion in this review retrieved from the above‐mentioned searches. When clarification was needed, we contacted the principal investigators or corresponding authors, or both, of the relevant studies.
Data collection and analysis
Selection of studies
Two review authors (TPZ and either NDK or ER) independently screened all titles, abstracts and keywords of publications identified by the searches to assess their eligibility. We obtained full‐texts of all possibly eligible manuscripts, and two review authors (TPZ and NDK) selected trials based on our inclusion criteria. Disagreements were resolved by discussion and, if necessary, by a third review author (ER). We included a PRISMA flowchart of our study selection (Moher 2009).
Data extraction and management
Two review authors (TPZ and NDK) independently extracted the data. They resolved disagreements by discussion and, if necessary, by involving a third review author (ER). We used data reported in the published sources for analysis in this review. If reported data conflicted between two reports of the same study, we used the data of the most recent report. Where we needed additional data, we attempted to contact the principal investigator(s) or the corresponding author of the study or report. On a standardised data extraction form we recorded:
general information: published/unpublished, title, authors, reference, country, language of publication, year of publication, duplicate publications, sponsor, setting;
trial characteristics/methodology: design, inclusion and exclusion criteria, method of randomisation, sequence generation, allocation concealment, blinding (participants/personnel and outcome assessors) and analysis (intention‐to‐treat, per protocol), duration of follow‐up, presence of selective reporting, other types of bias;
participant characteristics: number of participants randomised to each arm, age, sex, ethnicity, type of index event, presence of elevated blood pressure, level of blood pressure, compliance, duration of follow‐up;
intervention characteristics: type, class and dose of BPLD, timing of treatment initiation, duration of treatment, additional interventions;
outcomes: all specified primary and secondary outcomes, other reported outcomes, number of participants with complete follow‐up and reasons for loss to follow‐up.
Assessment of risk of bias in included studies
To assess risk of bias in each included study, two review authors (TPZ and NDK) independently assessed the methodological quality using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We created a 'Risk of bias' table per study, including a description and a judgement (low, high, or unclear risk of bias) for each of the seven types of possible bias. We considered studies with three or more entries of high or unclear risk of bias as being of low methodological quality. We summarised the risk of bias in a 'Risk of bias' graph and summary.
Measures of treatment effect
We expressed the size of the treatment effect on the dichotomous outcome measures as pooled risk ratios (RRs) with 95% confidence intervals (CIs). We expressed the outcome 'time to recurrent stroke' as pooled log hazard ratio estimate with 95% CIs.
Unit of analysis issues
For each study we considered whether participants were randomised individually or as a group and whether there were multiple observations for the same outcome.
Dealing with missing data
We attempted to collect missing data by contacting (through multiple e‐mails) the principal investigators and corresponding authors of the studies in question.
Assessment of heterogeneity
We calculated Tau2, Chi2, and I2 to explore the between‐study variance. We considered I2 statistic percentages greater than 50% as an indicator of substantial heterogeneity.
Assessment of reporting biases
We did not create funnel plots to assess reporting biases, as the number of included studies per comparison (BPLD versus placebo or no treatment; intensive blood pressure‐lowering versus mild or standard blood pressure‐lowering) was lower than 10.
Data synthesis
We processed data and performed quantitative analyses using Review Manager 5 (Review Manager 2014). We used random‐effects models for all analyses, regardless of the statistical amount of heterogeneity, to take heterogeneous designs and participants into account.
Subgroup analysis and investigation of heterogeneity
We defined the following subgroup analyses to investigate heterogeneous results for the primary outcome 'recurrent stroke of any type' and the secondary outcomes 'major vascular event' and 'dementia'.
Subgroups based on baseline blood pressure, according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7) classification (Chobanian 2003) (normal blood pressure, defined as a systolic blood pressure < 120 mmHg and a diastolic blood pressure < 80 mmHg; prehypertension, defined as a systolic blood pressure 120 to 139 mmHg or a diastolic blood pressure 80 to 89 mmHg; stage 1 hypertension, defined as a systolic blood pressure 140 to 159 mmHg or a diastolic blood pressure 90 to 99 mmHg; stage 2 hypertension, defined as a systolic blood pressure ≥ 160 mmHg or a diastolic blood pressure ≥ 100 mmHg).
Subgroups based on class of BPLD (diuretics, angiotensin‐converting enzyme (ACE) inhibitors, calcium channel blockers, angiotensin II type 1 receptor blockers, adrenergic beta‐antagonists, other).
Subgroups based on self‐reported ethnicity (Blacks, Whites, Hispanics, Asians, others).
Subgroups based on prior history of hypertension (yes versus no).
Subgroups based on age (< 60 years, 60 to 79 years, 80 years or older).
Subgroups based on index stroke event (TIA, ischaemic stroke, haemorrhagic stroke).
Subgroups based on sex (men versus women).
Sensitivity analysis
We planned the following sensitivity analyses.
Methodological quality (0 to 2 entries of unclear or high risk versus 3 or more entries of unclear or high risk).
Time of treatment initiation (within 2 weeks after stroke or TIA versus after 2 weeks following stroke or TIA).
Duration of treatment and follow‐up (shorter versus longer than 1 year).
Level of achieved systolic blood pressure‐lowering in comparison with baseline (> 5 mmHg versus ≤ 5 mmHg).
Level of achieved diastolic blood pressure‐lowering in comparison with baseline (> 3 mmHg versus ≤ 3 mmHg).
Contrast in achieved systolic blood pressure‐lowering between intervention and control group (> 3 mmHg versus ≤ 3 mmHg).
GRADE assessment and 'Summary of findings' table
We used the GRADEpro Gudeline Development Tool to create a 'Summary of findings' table for the comparison of BPLDs versus placebo or no treatment (GRADEpro GDT; Table 1). This table presents the results and the quality of the evidence of the main outcomes, using the GRADE system, which classifies the quality of evidence as high, moderate, low, and very low. The outcomes included are: recurrent stroke of any type, time to recurrent stroke, major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause), myocardial infarction, vascular death, death by any cause, and dementia. The GRADE approach appraises the quality of the body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item assessed. The quality of a body of evidence is based on within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias (Schünemann 2011).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search strategy yielded 23,932 unique results, of which we excluded 23,093 after reading titles and abstracts (Figure 1). We retrieved the remaining 839 records for detailed evaluation. Among these, we identified 13 randomised controlled trials (RCTs) eligible for inclusion.
1.
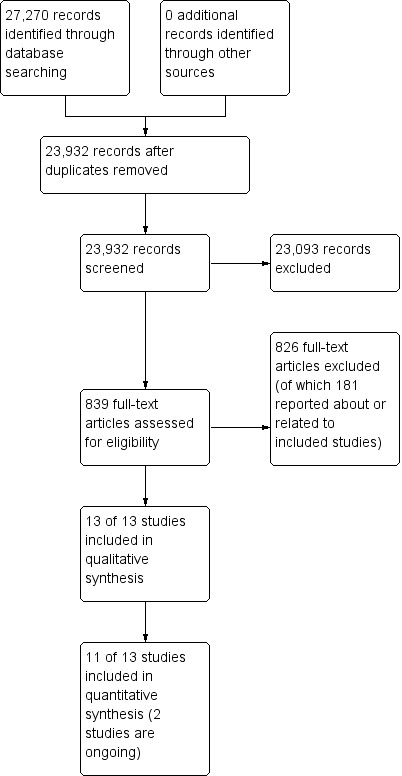
Study flow diagram.
Two of the 13 trials that met our inclusion criteria are ongoing: The NCT01220622 is investigating the effect of nimodipine started within seven days after ischaemic stroke on mortality at six months; the NCT01563731 trial studies the effect of three different systolic blood pressure targets (< 145 to 135, < 135 to 125, and < 125 mmHg) on stroke and cardiovascular events in people with a transient ischaemic attack (TIA) or stroke (Characteristics of ongoing studies).
Included studies
In total, we included 11 studies involving 38,742 participants (Characteristics of included studies); eight studies in the BPLD versus placebo or no treatment comparison, and three studies in the intensive blood pressure‐lowering versus mild or standard blood pressure‐lowering comparison.
Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment
Eight studies (35,110 participants) met the inclusion criteria for our primary and secondary objectives (Carter 1970; Co‐operative Study 1975; Marti Masso 1990; Dutch TIA Trial 1993; PATS 1995; PROGRESS 2001; PRoFESS 2008; TEST 1995), the largest of which included 20,332 participants (PRoFESS 2008).
Type of intervention
Five studies (28,454 participants) compared a single BPLD with placebo (Dutch TIA Trial 1993; PATS 1995; PRoFESS 2008; TEST 1995), or with no treatment (Marti Masso 1990), two trials investigated individually tailored regimens consisting of either one or two BPLDs versus placebo or no treatment (Carter 1970; PROGRESS 2001), and one trial investigated a combined blood pressure‐lowering intervention versus placebo (Co‐operative Study 1975).
Five classes of BPLDs were evaluated; angiotensin‐converting enzyme (ACE) inhibitors (with individually tailored addition of diuretics, PROGRESS 2001), beta‐blockers (Dutch TIA Trial 1993; TEST 1995), angiotensin II receptor antagonists (PRoFESS 2008), diuretics (Co‐operative Study 1975: a combination of two diuretic drugs; PATS 1995), calcium channel blockers (Marti Masso 1990), and an individually tailored regimen of diuretics and centrally acting BPLDs (Carter 1970). Two trials had a 2x2 factorial design (Dutch TIA Trial 1993; PRoFESS 2008), both also comparing different antithrombotic regimens.
Index event
In four studies, the qualifying event could either be a TIA, ischaemic stroke, or haemorrhagic stroke (Co‐operative Study 1975; PATS 1995; PROGRESS 2001; TEST 1995). One of these studies also included people with a subarachnoid haemorrhage (112 of 5665 participants (2.0%), PATS 1995), and one study did not require a specific qualifying event but a prior history of TIA or stroke (PROGRESS 2001). Three studies included people with a TIA or ischaemic stroke (Dutch TIA Trial 1993; Marti Masso 1990; PRoFESS 2008). One study only included people with an ischaemic stroke (Carter 1970). However, this diagnosis was based on a lumbar puncture negative for blood, as computerised tomography (CT) scanners were not yet available to reliably distinguish between ischaemic and haemorrhagic stroke. Six out of the seven studies, including TIA participants, based inclusion on the clinical syndrome only, whereas one study required confirmatory brain imaging (CT or magnetic resonance imaging (MRI), PRoFESS 2008).
Time window for inclusion
Five trials included participants within a limited period after the index event: three weeks (TEST 1995), three months (Dutch TIA Trial 1993; PRoFESS 2008), one year (Co‐operative Study 1975), and five years (PROGRESS 2001). One of these studies recommended participants to be stable two weeks after the index event (PROGRESS 2001), and one used a six‐week run‐in period before participants were randomised (Co‐operative Study 1975). Median time from index event to randomisation was reported in two of these trials; 15 days (PRoFESS 2008), and eight months (PROGRESS 2001). One study reported that 22% of participants were randomised within one week from index event (Dutch TIA Trial 1993). One study did not report data on time between index event and start of treatment (TEST 1995).
Three trials included participants after a minimum time period after the index event; two weeks (Carter 1970), four weeks (PATS 1995), and one year (Marti Masso 1990). Two of these studies did not report median time from index event to intervention, whereas one study reported a median of 31 months (PATS 1995).
Blood pressure at baseline
Increased blood pressure at baseline was not part of our inclusion criteria. Overall mean blood pressure at baseline was not different between participants randomised to blood pressure‐lowering or to placebo or no treatment (Analysis 1.10; Analysis 1.11).
1.10. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 10 Blood pressure baseline (systolic).
1.11. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 11 Blood pressure baseline (diastolic).
Follow‐up
Median overall follow‐up duration was reported in six studies, and ranged from 12 to 47 months. Seven trials reported (recurrent) stroke as the primary outcome measure (Carter 1970; Co‐operative Study 1975; Marti Masso 1990; PATS 1995; PRoFESS 2008; PROGRESS 2001; TEST 1995), whereas one trial primarily reported a composite endpoint of major vascular events (Dutch TIA Trial 1993).
Intensive versus standard blood pressure‐lowering
Three trials (3632 participants) met the inclusion criteria for our second comparison, intensive blood pressure‐lowering versus mild or standard blood pressure‐lowering (PAST‐BP 2016; PODCAST 2017; SPS3 2013). All three trials compared a lower versus a higher systolic blood pressure target, although with slightly different targets (< 130 mmHg versus 130 to 149 mmHg (SPS3 2013); < 130 mmHg or a reduction of 10 mmHg if systolic blood pressure was between 125 mmHg and 140 mmHg at randomisation versus < 140 mmHg (PAST‐BP 2016), < 125 mmHg versus < 140 mmHg (PODCAST 2017). The qualifying event was TIA or stroke in one trial (PAST‐BP 2016), stroke in one trial (PODCAST 2017), and lacunar stroke only in one trial (SPS3 2013). Participants were randomised between two weeks and seven months after the qualifying event, with a median 62 days in one trial (SPS3 2013), a median 4.5 months in one trial (PODCAST 2017), and unknown median time in one trial (PAST‐BP 2016). Median overall follow‐up was 12 months (PAST‐BP 2016), 24 months (PODCAST 2017), and 44 months (SPS3 2013). One trial had a 2x2 factorial design, also comparing different antithrombotic regimens (SPS3 2013). One trial primarily reported recurrent stroke (SPS3 2013), whereas the other trials primarily reported change in systolic blood pressure (PAST‐BP 2016), and Addenbrooke's Cognitive Examination‐Revised score (PODCAST 2017).
Excluded studies
We excluded three studies after discussing their eligibility with the third review author. We excluded two trials because of the large proportion of participants in the control group in whom BPLDs were started during follow‐up, resulting in a lack of contrast between the two treatment arms (Lithell 2003; Schrader 2003). One trial did not report baseline characteristics for the intention‐to‐treat population, and a small subset of participants did not have an index TIA or stroke (Lee 2015).
Risk of bias in included studies
In all included studies, we assessed the risk of selection bias, performance bias, attrition bias, detection bias and reporting bias. We used the 'Risk of bias' tool in the Cochrane Handbook for Systematic Reviews of Interventions to classify the seven described domains as 'low risk', 'high risk' or 'unclear risk' (Higgins 2011). An overview is given in the 'Risk of bias summary' (Figure 2), and the 'Risk of bias graph' (Figure 3).
2.
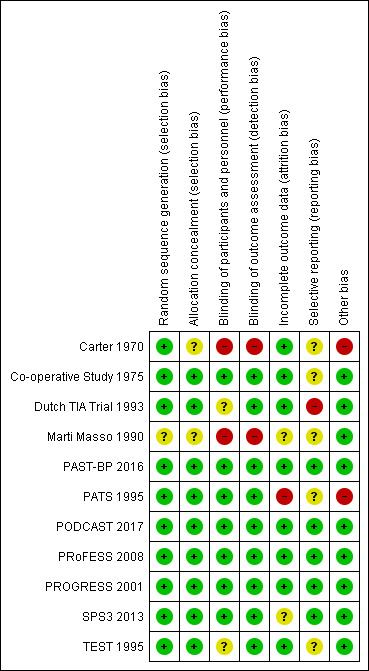
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Two studies did not describe their methods of allocation concealment (Carter 1970; Marti Masso 1990); we assessed these to have an unclear risk of bias. One study did not describe their method of sequence generation (Marti Masso 1990); we assessed this as an unclear risk of bias. We assessed the remaining studies as having a low risk of selection bias.
Blinding
Two studies did not describe their methods of blinding (Carter 1970; Marti Masso 1990); we assessed these to have a high risk of bias. Two studies only reported their blinding of participants and personnel as being "double‐blind" (Dutch TIA Trial 1993; TEST 1995); we assessed these to have an unclear risk of bias. We assessed the remaining studies as having a low risk of performance bias and detection bias.
Incomplete outcome data
All trials were analysed according to the intention‐to‐treat principle. One study used a per protocol analysis for their primary outcome measure (blood pressure reduction), and intention‐to‐treat analyses for the clinical outcome events (PAST‐BP 2016); as we did not use their primary outcome measure, we assessed this study as having a low risk of bias. One study did not address loss to follow‐up (Marti Masso 1990), and one study only reported overall (reasons for) loss to follow‐up (SPS3 2013); we assessed these studies as having an unclear risk of bias. One study reported 28% loss to follow‐up without further explanation (PATS 1995); we assessed this study to be at high risk of bias. We assessed the remaining studies as having low risk of attrition bias.
Selective reporting
One study was designed to report functional outcome at three months, but primarily reported major vascular events instead (Dutch TIA Trial 1993); we assessed this study as having a high risk of bias. Because there was no study protocol available or published, we assessed five studies as having an unclear risk of bias (Carter 1970; Co‐operative Study 1975; Marti Masso 1990; PATS 1995; TEST 1995), whereas the remaining studies were assessed as having a low risk of reporting bias (PAST‐BP 2016; PODCAST 2017; PRoFESS 2008; PROGRESS 2001; SPS3 2013).
Other potential sources of bias
One study was performed by a single‐investigator, and did not report a definition of the primary endpoint (Carter 1970). In another study, both the number of participants per treatment group and the number of outcome events differed between subsequent reports (PATS 1995); based on these findings we assessed these studies as having a high risk of bias. We did not identify any potential sources of bias in the remaining studies.
Effects of interventions
See: Table 1
See Data and analyses.
Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment
See Table 1
Recurrent stroke of any type
Data from eight trials (35,110 participants) were available for our primary analysis (Carter 1970; Co‐operative Study 1975; Dutch TIA Trial 1993; Marti Masso 1990; PATS 1995; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled risk ratio (RR) of BPLDs for recurrent stroke was 0.81 (95% confidence interval (CI) 0.70 to 0.93; moderate‐quality evidence; Analysis 1.1). To explore the substantial heterogeneity (I2 = 61%), we performed subgroup analyses.
1.1. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 1 Recurrent stroke of any type.
Subgroup analyses
Recurrent stroke of any type by baseline systolic blood pressure
Data from three trials (6656 participants) were available (Carter 1970; Co‐operative Study 1975; PROGRESS 2001). The pooled RRs of BPLDs for recurrent stroke were 0.65 (95% CI 0.51 to 0.83) in participants with a baseline systolic blood pressure of 160 mmHg or above, and 0.71 (95% CI 0.57 to 0.89) in those with a baseline systolic blood pressure between 140 and 160 mmHg. The RRs of BPLDs for recurrent stroke were 0.86 (95% CI 0.67 to 1.12) in participants with a baseline systolic blood pressure between 120 and 140 mmHg, and 1.01 (95% CI 0.47 to 2.19) in those with a baseline systolic blood pressure below 120 mmHg (Analysis 2.1). The I2 statistic for subgroup differences was 5.3% (P = 0.37).
2.1. Analysis.
Comparison 2 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (subgroups), Outcome 1 Recurrent stroke of any type by baseline systolic blood pressure (SBP).
Recurrent stroke of any type by intervention
Data from eight trials (35,110 participants) were available (Carter 1970; Co‐operative Study 1975; Marti Masso 1990; Dutch TIA Trial 1993; PATS 1995; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled RRs of BPLDs for recurrent stroke were 0.73 (95% CI 0.64 to 0.84) in participants using ACE inhibitors, 0.72 (95% CI 0.59 to 0.87) in those using diuretics, and 0.94 (95% CI 0.75 to 1.18) in those using beta‐blockers. The RRs of BPLDs for recurrent stroke were 0.95 (95% CI 0.87 to 1.03) in participants using angiotensin receptor blockers, and 0.55 (95% CI 0.18 to 1.67) in those using calcium channel blockers (Analysis 2.2). The I2 statistic for subgroup differences was 72.1% (P = 0.006).
2.2. Analysis.
Comparison 2 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (subgroups), Outcome 2 Recurrent stroke of any type by intervention.
Recurrent stroke of any type by type of index event
Data from one trial (5854 participants) were available (PROGRESS 2001). The RRs of BPLDs for recurrent stroke were 0.76 (95% CI 0.64 to 0.89) in participants with an ischaemic index stroke, 0.59 (95% CI 0.39 to 0.89) in those with an haemorrhagic index stroke, and 0.77 (95% CI 0.50 to 1.18) in those with an index TIA (Analysis 2.3). The I2 statistic for subgroup differences was 0% (P = 0.53).
2.3. Analysis.
Comparison 2 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (subgroups), Outcome 3 Recurrent stroke of any type by type of index event.
Sensitivity analyses
Recurrent stroke of any type, methodological quality
Five trials (29,082 participants) were of high methodological quality (Co‐operative Study 1975; Dutch TIA Trial 1993; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled RR of BPLDs for recurrent stroke was 0.86 (95% CI 0.75 to 1.00; Analysis 3.1).
3.1. Analysis.
Comparison 3 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (sensitivity analyses), Outcome 1 Recurrent stroke of any type, methodological quality.
Recurrent stroke of any type, minimum 5 mmHg systolic blood pressure reduction
Four trials (33,575 participants) achieved a minimum systolic blood pressure reduction of 5 mmHg at the end of the observation period (Dutch TIA Trial 1993; PATS 1995; PROGRESS 2001; PRoFESS 2008). The pooled RR of BPLDs for recurrent stroke was 0.81 (95% CI 0.68 to 0.96; Analysis 3.2).
3.2. Analysis.
Comparison 3 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (sensitivity analyses), Outcome 2 Recurrent stroke of any type, minimum 5 mmHg systolic blood pressure (SBP) reduction.
Recurrent stroke of any type, minimum 3 mmHg diastolic blood pressure reduction
Five trials (34,295 participants) achieved a minimum systolic blood pressure reduction of 3 mmHg at the end of the observation period (Dutch TIA Trial 1993; PATS 1995; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled RR of BPLDs for recurrent stroke was 0.84 (95% CI 0.72 to 0.97; Analysis 3.3).
3.3. Analysis.
Comparison 3 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (sensitivity analyses), Outcome 3 Recurrent stroke of any type, minimum 3 mmHg diastolic blood pressure (DBP) reduction.
Recurrent stroke of any type, excluding patients with subarachnoid haemorrhage as possible index event
One trial not only included participants with an ischaemic of haemorrhagic stroke, but also with subarachnoid haemorrhage (PATS 1995). Recurrent stroke was reported separately for participants with an ischaemic stroke as index event and a haemorrhagic stroke as index event (comprising both intracerebral and subarachnoid haemorrhage). Therefore, we performed this additional sensitivity analysis, excluding the participants from this trial with a haemorrhagic stroke as index event.
Data from eight trials (33,690 participants) were available (Carter 1970; Co‐operative Study 1975; Dutch TIA Trial 1993; Marti Masso 1990; PATS 1995; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled RR of BPLDs for recurrent stroke was 0.81 (95% CI 0.70 to 0.93; Analysis 3.4).
3.4. Analysis.
Comparison 3 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (sensitivity analyses), Outcome 4 Recurrent stroke of any type, excluding patients with subarachnoid haemorrhage as possible index event.
We did not perform subgroup analyses based on self‐reported ethnicity, prior history of hypertension, age, and sex because of insufficient studies reporting estimates for these subgroups. We did not perform sensitivity analyses based on time of treatment initiation, duration of treatment and follow‐up, or the achieved contrast in systolic blood pressure‐lowering between intervention and control. All studies treated and followed participants for at least one year, and studies did not uniformly report time of treatment initiation or the contrast in systolic blood pressure‐lowering at the end of the observation period.
Time to recurrent stroke
We transformed tabular data for the Co‐operative Study 1975 into a hazard ratio using spreadsheets developed by Tierney et al (Tierney 2007; Wang 2013).
Data from three trials (26,889 participants) were available (Co‐operative Study 1975; PROGRESS 2001; PRoFESS 2008). The pooled hazard ratio of BPLDs for time to recurrent stroke was 0.82 (95% CI 0.65 to 1.03; high‐quality evidence; Analysis 1.2).
1.2. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 2 Time to recurrent stroke.
Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause)
Data from four trials (28,630 participants) were available (Dutch TIA Trial 1993; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled RR of BPLDs for major vascular event was 0.90 (95% CI 0.78 to 1.04; high‐quality evidence; Analysis 1.3). To explore the substantial heterogeneity (I2 = 75%), we performed a subgroup analysis.
1.3. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 3 Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause.
Subgroup analyses
Major vascular event by intervention
Data from four trials (28,630 participants) were available (Dutch TIA Trial 1993; PROGRESS 2001; PRoFESS 2008; TEST 1995). The RRs of BPLDs for major vascular event were 0.76 (95% CI 0.68 to 0.85) in participants using ACE inhibitors, and 0.94 (95% CI 0.88 to 1.01) in those using angiotensin receptor blockers. The pooled RR of BPLDs for major vascular event was 1.01 (95% CI 0.84 to 1.21) in participants using beta‐blockers (Analysis 2.4).
2.4. Analysis.
Comparison 2 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (subgroups), Outcome 4 Major vascular event by intervention.
We did not perform subgroup analyses based on baseline blood pressure, self‐reported ethnicity, prior history of hypertension, age, index stroke event and sex because of insufficient studies reporting estimates for these subgroups.
Ischaemic stroke
Data from three trials (26,701 participants) were available (Marti Masso 1990; PROGRESS 2001; PRoFESS 2008). The pooled RR of BPLDs for ischaemic stroke was 0.86 (95% CI 0.70 to 1.05; Analysis 1.4).
1.4. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 4 Ischaemic stroke.
Haemorrhagic stroke
Data from two trials (26,437 participants) were available (PROGRESS 2001; PRoFESS 2008). The pooled RR of BPLDs for haemorrhagic stroke was 0.66 (95% CI 0.39 to 1.12; Analysis 1.5).
1.5. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 5 Haemorrhagic stroke.
Myocardial infarction
Data from six trials (34,747 participants) were available (Co‐operative Study 1975; Dutch TIA Trial 1993; PATS 1995; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled RR of BPLDs for myocardial infarction was 0.90 (95% CI 0.72 to 1.11; high‐quality evidence; Analysis 1.6).
1.6. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 6 Myocardial infarction.
Vascular death
Data from six trials (34,747 participants) were available (Co‐operative Study 1975; Dutch TIA Trial 1993; PATS 1995; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled RR of BPLDs for vascular death was 0.85 (95% CI 0.76 to 0.95; high‐quality evidence; Analysis 1.7).
1.7. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 7 Vascular death.
Death by any cause
Data from eight trials (35,110 participants) were available (Carter 1970; Co‐operative Study 1975; Marti Masso 1990; Dutch TIA Trial 1993; PATS 1995; PROGRESS 2001; PRoFESS 2008; TEST 1995). The pooled RR of BPLDs for death by any cause was 0.98 (95% CI 0.91 to 1.05; moderate‐quality evidence; Analysis 1.8).
1.8. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 8 Death by any cause.
Dementia
Data from two trials (6671 participants) were available (PROGRESS 2001; PRoFESS 2008). The pooled RR of BPLDs for dementia was 0.88 (95% CI 0.73 to 1.06; high‐quality evidence; Analysis 1.9).
1.9. Analysis.
Comparison 1 Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment, Outcome 9 Dementia.
We did not perform subgroup analyses based on baseline blood pressure, self‐reported ethnicity, prior history of hypertension, age, index stroke event, and sex, because of insufficient studies reporting estimates for these subgroups.
Intensive versus standard blood pressure‐lowering
One trial classified their outcome event 'intracranial haemorrhage' as either 'intracerebral', 'subdural or epidural' or 'other' (SPS3 2013). We did not count 'subdural or epidural' and 'other' as 'stroke of any type' or 'haemorrhagic stroke'. However, we included these outcome events in the 'time to recurrent stroke' analyses, as only summary estimates (hazard ratio and 95% CI) were reported for these time‐to‐event data.
Data from three trials (3632 participants) were available for recurrent stroke of any type, major vascular event, myocardial infarction, and death by any cause (PAST‐BP 2016; PODCAST 2017; SPS3 2013). The pooled RRs of intensive blood pressure‐lowering were 0.80 (95% CI 0.63 to 1.00) for recurrent stroke (Analysis 4.1), 0.58 (95% CI 0.23 to 1.46) for major vascular event (Analysis 4.3), 0.90 (95% CI 0.58 to 1.38) for myocardial infarction (Analysis 4.6), and 1.08 (95% CI 0.83 to 1.39) for death by any cause (Analysis 4.8).
4.1. Analysis.
Comparison 4 Intensive versus standard blood pressure‐lowering, Outcome 1 Stroke of any type (fatal and non‐fatal).
4.3. Analysis.
Comparison 4 Intensive versus standard blood pressure‐lowering, Outcome 3 Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause.
4.6. Analysis.
Comparison 4 Intensive versus standard blood pressure‐lowering, Outcome 6 Myocardial infarction.
4.8. Analysis.
Comparison 4 Intensive versus standard blood pressure‐lowering, Outcome 8 Death by any cause.
Data from two trials (3549 participants) were available for vascular death (PAST‐BP 2016; SPS3 2013). The pooled RR of intensive blood pressure‐lowering was 0.87 (95% CI 0.56 to 1.35) (Analysis 4.7).
4.7. Analysis.
Comparison 4 Intensive versus standard blood pressure‐lowering, Outcome 7 Vascular death.
Data from two trials (3103 participants) were available for ischaemic and haemorrhagic stroke (PODCAST 2017; SPS3 2013). The pooled RR of intensive blood pressure‐lowering was 0.86 (95% CI 0.67 to 1.09) for ischaemic stroke (Analysis 4.4), and 0.42 (95% CI 0.17 to 1.02) for haemorrhagic stroke (Analysis 4.5).
4.4. Analysis.
Comparison 4 Intensive versus standard blood pressure‐lowering, Outcome 4 Ischaemic stroke.
4.5. Analysis.
Comparison 4 Intensive versus standard blood pressure‐lowering, Outcome 5 Haemorrhagic stroke.
Data from one trial (3020 participants) were available for time to recurrent stroke (SPS3 2013). The hazard ratio of intensive blood pressure‐lowering for time to recurrent stroke was 0.81 (95% CI 0.64 to 1.03; Analysis 4.2).
4.2. Analysis.
Comparison 4 Intensive versus standard blood pressure‐lowering, Outcome 2 Time to recurrent stroke.
Data from one trial (83 participants) were available for dementia (PODCAST 2017). The RR of intensive blood pressure‐lowering for dementia was 5.12 (95% CI 0.25 to 103.48; Analysis 4.9).
Discussion
Summary of main results
Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment
Blood pressure‐lowering drugs (BPLDs) initiated at least 48 hours after stroke or transient ischaemic attack (TIA) reduced the risk of recurrent stroke. We observed substantial heterogeneity, and predefined subgroup analyses were hampered by lack of trials assessing these specific subgroups.
BPLDs also reduced the risk of vascular death but did not reduce time to recurrent stroke, and risk of major vascular event, ischaemic stroke, haemorrhagic stroke, myocardial infarction, death by any cause, or dementia. However, point estimates for all secondary outcome measures were in favour of treatment with BPLDs.
Intensive versus standard blood pressure‐lowering
Intensive blood pressure‐lowering did not reduce the risk of recurrent stroke, time to recurrent stroke, risk of major vascular event, ischaemic stroke, myocardial infarction, vascular death, death by any cause, and dementia, whereas it did reduce the risk of haemorrhagic stroke. However, we observed a favourable trend for intensive blood pressure‐lowering in the outcomes recurrent stroke, time to recurrent stroke, and major vascular events.
Overall completeness and applicability of evidence
Our primary analysis is based on 35,110 participants, and we assessed secondary outcome measures in a large subset of these participants, except for dementia, which was only assessed in a relatively small number of participants (n = 6671).
We included only three studies (3632 participants) comparing different blood pressure targets, and the largest of these studies only included participants with a lacunar stroke as index event. Therefore, many questions regarding the optimal blood pressure target after TIA or stroke remain unanswered.
Quality of the evidence
Overall, the trials included in our main analyses ranged from low to substantial risk of bias. In a sensitivity analysis based on high risk of bias, we excluded three trials. Two of these trials mainly suffered from insufficient blinding and lack of description of their randomisation procedures, whereas the third suffered from significant numbers of loss to follow‐up and differing numbers of outcome events between different reports.
The resulting quality of the evidence, as assessed in the GRADE format, was moderate for our primary outcome measure, 'recurrent stroke of any type'. The quality of evidence was high for most of our secondary outcome measures, except for 'death by any cause', which we also downgraded to moderate.
Potential biases in the review process
We conducted an extensive literature search according to current Cochrane standards, without language restrictions. Therefore, we consider it unlikely that we missed potentially relevant studies.
We did not use individual participant data. Therefore, we did not include participants from trials that investigated BPLDs in a more general population, as none of these trials reported a subgroup analysis of people with a history of TIA or stroke.
We included one trial that also included 2.0% of participants with subarachnoid haemorrhage (PATS 1995). Therefore, we performed an additional sensitivity analysis, in which we excluded patients with subarachnoid haemorrhage as possible index event.
Agreements and disagreements with other studies or reviews
Two similar meta‐analyses on the effect of BPLDs in the secondary prevention of stroke were performed previously (Lakhan 2009; Liu 2009), which also found a positive effect of BPLDs on reducing recurrent stroke (pooled odds ratios 0.71 (95% confidence interval (CI) 0.59 to 0.86) and 0.78 (95% CI 0.68 to 0.90) respectively). Due to differences in inclusion criteria, the previous meta‐analyses included three and two additional studies (respectively) in comparison with our meta‐analysis, which resulted in the slight differences in effect estimates and CIs between all meta‐analyses.
Authors' conclusions
Implications for practice.
Our results support the use of blood pressure‐lowering drugs (BPLDs) in secondary prevention after transient ischaemic attack (TIA) or stroke. Current evidence is primarily derived from trials of treatment with an angiotensin‐converting enzyme (ACE) inhibitor or diuretic. BPLDs appeared to be most effective in people with high baseline blood pressure (> 140 mmHg); however, differences between these subgroups were not statistically significant.
Only limited data comparing different systolic blood pressure targets were available. Therefore, no conclusions can be drawn from current evidence regarding an optimal systolic blood pressure target after TIA or stroke.
Implications for research.
An individual participant data meta‐analysis could further identify specific subgroups of people who are likely to benefit from BPLDs after TIA or stroke. We recommend that future studies assess whether BPLDs lower the risk of dementia in TIA and stroke patients.
Additional large randomised controlled trials (RCTs) are needed to investigate the optimal blood pressure target after TIA or stroke.
What's new
| Date | Event | Description |
|---|---|---|
| 2 August 2018 | Amended | Amendment to the 'Main results' section in the Abstract. |
Acknowledgements
We thank Brenda Thomas and Joshua Cheyne from the Cochrane Stroke Group for their help and advice in our search process, Hazel Fraser from Cochrane Stroke for her continuing support in general, and Nancy Thornton for commenting and improving our Plain Language Summary.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2016, Issue 6: searched July 2016);
1 [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"]
#2 (stroke or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or isch*emi* next attack* or tia or tias):ti,ab
#3 ((brain* or cerebr* or cerebell* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or MCA) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus* or hypox* or vasospasm)):ti,ab
#4 ((brain* or cerebr* or cerebell* or intracerebral or intracran* or intraventricular or infratentorial or supratentorial or basal next gangli*) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab
#5 #1 or #2 or #3 or #4
#6 [mh "antihypertensive agents"] or [mh "vasodilator agents"] or [mh "adrenergic agonists"] or [mh diuretics] or [mh thiazides] or [mh "sodium chloride symporter inhibitors"] or [mh "sodium potassium chloride symporter inhibitors"]
#7 [mh "angiotensin‐converting enzyme inhibitors"] or [mh "angiotensin II type 1 receptor blockers"] or [mh "calcium channel blockers"] or [mh "adrenergic beta‐antagonists"] or [mh "adrenergic alpha antagonists"]
#8 [mh enalapril] or [mh ^losartan] or [mh hydralazine]
#9 [mh ^Hypertension/AE,DE,DT,PC] or [mh ^"Blood Pressure"/DE,PD]
#10 (antihypertens* or anti‐hypertens*):ti,ab
#11 (("Blood pressure" or hypertens*) near/5 (lower* or reduc* or decreas*)):ti,ab
#12 (angiotensin near/3 convert* near/3 enzyme near/3 (inhibit* or antagonist* or block*)):ti,ab
#13 (((ace or renin) near/3 inhibit*) or ACEI):ti,ab
#14 (angiotensin near/3 receptor* near/3 (inhibit* or antagonist* or block*)):ti,ab
#15 (calcium near/2 (inhibit* or antagonist* or block*)):ti,ab
#16 (adrenergic near/3 beta* near/3 (inhibit* or antagonist* or block*)):ti,ab
#17 (adrenergic near/3 alpha* near/3 (inhibit* or antagonist* or block*)):ti,ab
#18 ((loop or ceiling) next diuretic*):ti,ab
#19 (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide*):ti,ab 5009
#20 (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide or "s‐1520" or s1520 or "se‐1520" or se1520):ti,ab
#21 (alacepril or altiopril or benazepril or captopril or ceronapril or cilazapril or delapril or enalapril or fosinopril or idapril or imidapril or lisinopril or moexipril or moveltipril or pentopril or perindopril or quinapril or ramipril or spirapril or temocapril or trandolapril or zofenopril or aliskiren or remikiren):ti,ab
#22 ("KT3‐671" or candesartan or eprosartan or irbesartan or losartan or olmesartan or tasosartan or telmisartan or valsartan):ti,ab
#23 (amlodipine or amrinone or bencyclane or bepridil or cinnarizine or conotoxins or diltiazem or felodipine or fendiline or flunarizine or gallopamil or isradipine or lidoflazine or "magnesium sulphate" or mibefradil or nicardipine or nifedipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or verapamil or "omega‐agatoxin iva" or "omega‐conotoxin gvia" or "omega‐conotoxins"):ti,ab
#24 (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or "methylpropionic acid" or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or "methyl dopa" or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or "alpha methyl dopa"):ti,ab
#25 (reserpine or serpentina or rauwolfia or serpasil):ti,ab
#26 (clonidine or adesipress or arkamin or caprysin or catapres* or catasan or chlofazolin or chlophazolin or clinidine or clofelin* or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin* or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or "m‐5041t" or normopresan or paracefan or "st‐155" or "st 155" or "tesno timelets"):ti,ab
#27 (hydralazin* or hydrallazin* or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or "1‐hydrazinophthalazine" or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat):ti,ab
#28 (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol):ti,ab
#29 (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin):ti,ab
#30 {or #6‐#29}
#31 #5 and #30
Appendix 2. MEDLINE search strategy
MEDLINE (1946 to July 2016) in Ovid;
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/
2. (stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or isch?emi$ attack$ or tia$1).tw.
3. ((brain$ or cerebr$ or cerebell$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or intraventricular or infratentorial or supratentorial or basal gangli$) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. 1 or 2 or 3 or 4
6. exp antihypertensive agents/ or exp vasodilator agents/ or exp adrenergic agonists/ or exp diuretics/ or exp thiazides/ or exp sodium chloride symporter inhibitors/ or exp sodium potassium chloride symporter inhibitors/
7. exp angiotensin‐converting enzyme inhibitors/ or exp angiotensin II type 1 receptor blockers/ or exp calcium channel blockers/ or exp adrenergic beta‐antagonists/ or exp adrenergic alpha antagonists/
8. exp enalapril/ or losartan/ or exp hydralazine/
9. Hypertension/ae, de, dt, pc or Blood Pressure/de, pd
10. (antihypertens$ or anti‐hypertens$).tw.
11. ((Blood pressure or hypertens$) adj5 (lower$ or reduc$ or decreas$)).tw.
12. (angiotensin adj3 convert$ adj3 enzyme adj3 (inhibit$ or antagonist? or block$)).tw.
13. (((ace or renin) adj3 inhibit$) or ACEI).tw.
14. (angiotensin adj3 receptor? adj3 (inhibit$ or antagonist? or block$)).tw.
15. (calcium adj2 (inhibit$ or antagonist? or block$)).tw.
16. (adrenergic adj3 beta$ adj3 (inhibit$ or antagonist? or block$)).tw.
17. (adrenergic adj3 alpha$ adj3 (inhibit$ or antagonist? or block$)).tw.
18. ((loop or ceiling) adj diuretic?).tw.
19. (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).mp.
20. (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide or s‐1520 or s1520 or se‐1520 or se1520).mp.
21. (alacepril or altiopril or benazepril or captopril or ceronapril or cilazapril or delapril or enalapril or fosinopril or idapril or imidapril or lisinopril or moexipril or moveltipril or pentopril or perindopril or quinapril or ramipril or spirapril or temocapril or trandolapril or zofenopril or aliskiren or remikiren).mp.
22. (KT3‐671 or candesartan or eprosartan or irbesartan or losartan or olmesartan or tasosartan or telmisartan or valsartan).mp.
23. (amlodipine or amrinone or bencyclane or bepridil or cinnarizine or conotoxins or diltiazem or felodipine or fendiline or flunarizine or gallopamil or isradipine or lidoflazine or magnesium sulfate or mibefradil or nicardipine or nifedipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or verapamil or omega‐agatoxin iva or omega‐conotoxin gvia or omega‐conotoxins).mp.
24. (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp.
25. (reserpine or serpentina or rauwolfia or serpasil).mp.
26. (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp.
27. (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).mp.
28. (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).mp.
29. (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).mp.
30. or/6‐29
31. Randomized Controlled Trials as topic/
32. random allocation/
33. Controlled Clinical Trials as topic/
34. control groups/
35. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
36. Clinical Trials Data Monitoring Committees/
37. double‐blind method/
38. single‐blind method/
39. Placebos/
40. placebo effect/
41. Therapies, Investigational/
42. Drug Evaluation/
43. Research Design/
44. randomized controlled trial.pt.
45. controlled clinical trial.pt.
46. clinical trial.pt.
47. random$.tw.
48. random$.tw.
49. (clinical$ adj5 trial$).tw.
50. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
51. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
52. placebo$.tw.
53. or/31‐52
54. 5 and 30 and 53
55. exp animals/ not humans.sh.
56. 54 not 55
Appendix 3. Embase search strategy
Embase (1980 to July 2016) in Ovid;
1. cerebrovascular disease/ or basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke/
2. stroke patient/ or stroke unit/
3. (stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or isch?emi$ attack$ or tia$1).tw.
4. ((brain$ or cerebr$ or cerebell$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or MCA) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypox$ or vasospasm)).tw.
5. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or intraventricular or infratentorial or supratentorial or basal gangli$) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
6. 1 or 2 or 3 or 4 or 5
7. exp antihypertensive agent/ or antihypertensive activity/ or antihypertensive therapy/ or exp vasodilator agent/ or exp adrenergic receptor stimulating agent/ or exp diuretic agent/
8. exp dipeptidyl carboxypeptidase inhibitor/ or exp angiotensin receptor antagonist/ or exp calcium channel blocking agent/ or exp beta adrenergic receptor blocking agent/ or exp alpha adrenergic receptor blocking agent/
9. losartan/ or hydralazine/
10. hypertension/ae, dt, pc or exp blood pressure/pd
11. (antihypertens$ or anti‐hypertens$).tw.
12. (hypotensive adj3 (agent$ or drug or drugs)).tw.
13. ((Blood pressure or hypertens$) adj5 (lower$ or reduc$ or decreas$)).tw.
14. (angiotensin adj3 convert$ adj3 enzyme adj3 (inhibit$ or antagonist? or block$)).tw.
15. (((ace or renin) adj3 inhibit$) or ACEI).tw.
16. (angiotensin adj3 receptor? adj3 (inhibit$ or antagonist? or block$)).tw.
17. (calcium adj2 (inhibit$ or antagonist? or block$)).tw.
18. (adrenergic adj3 beta$ adj3 (inhibit$ or antagonist? or block$)).tw.
19. (adrenergic adj3 alpha$ adj3 (inhibit$ or antagonist? or block$)).tw.
20. ((loop or ceiling) adj diuretic?).tw.
21. (amiloride or benzothiadiazine or bendroflumethiazide or bumetanide or chlorothiazide or cyclopenthiazide or furosemide or hydrochlorothiazide or hydroflumethiazide or methyclothiazide or metolazone or polythiazide or trichlormethiazide or veratide or thiazide?).mp.
22. (chlorthalidone or chlortalidone or phthalamudine or chlorphthalidolone or oxodoline or thalitone or hygroton or indapamide or metindamide or s‐1520 or s1520 or se‐1520 or se1520).mp.
23. (alacepril or altiopril or benazepril or captopril or ceronapril or cilazapril or delapril or enalapril or fosinopril or idapril or imidapril or lisinopril or moexipril or moveltipril or pentopril or perindopril or quinapril or ramipril or spirapril or temocapril or trandolapril or zofenopril or aliskiren or remikiren).mp.
24. (KT3‐671 or candesartan or eprosartan or irbesartan or losartan or olmesartan or tasosartan or telmisartan or valsartan).mp.
25. (amlodipine or amrinone or bencyclane or bepridil or cinnarizine or conotoxins or diltiazem or felodipine or fendiline or flunarizine or gallopamil or isradipine or lidoflazine or magnesium sulfate or mibefradil or nicardipine or nifedipine or nimodipine or nisoldipine or nitrendipine or perhexiline or prenylamine or verapamil or omega‐agatoxin iva or omega‐conotoxin gvia or omega‐conotoxins).mp.
26. (methyldopa or alphamethyldopa or amodopa or dopamet or dopegyt or dopegit or dopegite or emdopa or hyperpax or hyperpaxa or methylpropionic acid or dopergit or meldopa or methyldopate or medopa or medomet or sembrina or aldomet or aldometil or aldomin or hydopa or methyldihydroxyphenylalanine or methyl dopa or mulfasin or presinol or presolisin or sedometil or sembrina or taquinil or dihydroxyphenylalanine or methylphenylalanine or methylalanine or alpha methyl dopa).mp.
27. (reserpine or serpentina or rauwolfia or serpasil).mp.
28. (clonidine or adesipress or arkamin or caprysin or catapres$ or catasan or chlofazolin or chlophazolin or clinidine or clofelin$ or clofenil or clomidine or clondine or clonistada or clonnirit or clophelin$ or dichlorophenylaminoimidazoline or dixarit or duraclon or gemiton or haemiton or hemiton or imidazoline or isoglaucon or klofelin or klofenil or m‐5041t or normopresan or paracefan or st‐155 or st 155 or tesno timelets).mp.
29. (hydralazin$ or hydrallazin$ or hydralizine or hydrazinophtalazine or hydrazinophthalazine or hydrazinophtalizine or dralzine or hydralacin or hydrolazine or hypophthalin or hypoftalin or hydrazinophthalazine or idralazina or 1‐hydrazinophthalazine or apressin or nepresol or apressoline or apresoline or apresolin or alphapress or alazine or idralazina or lopress or plethorit or praeparat).mp.
30. (acebutolol or adimolol or afurolol or alprenolol or amosulalol or arotinolol or atenolol or befunolol or betaxolol or bevantolol or bisoprolol or bopindolol or bornaprolol or brefonalol or bucindolol or bucumolol or bufetolol or bufuralol or bunitrolol or bunolol or bupranolol or butofilolol or butoxamine or carazolol or carteolol or carvedilol or celiprolol or cetamolol or chlortalidone cloranolol or cyanoiodopindolol or cyanopindolol or deacetylmetipranolol or diacetolol or dihydroalprenolol or dilevalol or epanolol or esmolol or exaprolol or falintolol or flestolol or flusoxolol or hydroxybenzylpinodolol or hydroxycarteolol or hydroxymetoprolol or indenolol or iodocyanopindolol or iodopindolol or iprocrolol or isoxaprolol or labetalol or landiolol or levobunolol or levomoprolol or medroxalol or mepindolol or methylthiopropranolol or metipranolol or metoprolol or moprolol or nadolol or oxprenolol or penbutolol or pindolol or nadolol or nebivolol or nifenalol or nipradilol or oxprenolol or pafenolol or pamatolol or penbutolol or pindolol or practolol or primidolol or prizidilol or procinolol or pronetalol or propranolol or proxodolol or ridazolol or salcardolol or soquinolol or sotalol or spirendolol or talinolol or tertatolol or tienoxolol or tilisolol or timolol or tolamolol or toliprolol or tribendilol or xibenolol).mp.
31. (alfuzosin or bunazosin or doxazosin or metazosin or neldazosin or prazosin or silodosin or tamsulosin or terazosin or tiodazosin or trimazosin).mp.
32. or/7‐31
33. Randomized Controlled Trial/
34. Randomization/
35. Controlled Study/
36. control group/
37. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
38. Double Blind Procedure/
39. Single Blind Procedure/ or triple blind procedure/
40. placebo/
41. trial.ti.
42. "types of study"/
43. random$.tw.
44. (controlled adj5 (trial$ or stud$)).tw.
45. (clinical$ adj5 trial$).tw.
46. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
47. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
48. placebo$.tw.
49. or/33‐48
50. 6 and 32 and 49
51. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
52. 50 not 51
Appendix 4. Trial registers search strategy
The basic search strategy we used for all trial registers was (cerebrovascular OR cerebral OR stroke OR transient ischemic attack OR TIA) AND (antihypertensive OR blood pressure), adapted for each trial register based on their specific search functionality.
Data and analyses
Comparison 1. Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent stroke of any type | 8 | 35110 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.70, 0.93] |
| 2 Time to recurrent stroke | 3 | 26889 | Hazard Ratio (Random, 95% CI) | 0.82 [0.65, 1.03] |
| 3 Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause | 4 | 28630 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.04] |
| 4 Ischaemic stroke | 3 | 26701 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.70, 1.05] |
| 5 Haemorrhagic stroke | 2 | 26437 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.12] |
| 6 Myocardial infarction | 6 | 34747 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.72, 1.11] |
| 7 Vascular death | 6 | 34747 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.76, 0.95] |
| 8 Death by any cause | 8 | 35110 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.91, 1.05] |
| 9 Dementia | 2 | 6671 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.06] |
| 10 Blood pressure baseline (systolic) | 5 | 34295 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.39, 0.37] |
| 11 Blood pressure baseline (diastolic) | 5 | 34295 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.22, 0.24] |
Comparison 2. Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent stroke of any type by baseline systolic blood pressure (SBP) | 3 | 6656 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.64, 0.83] |
| 1.1 SBP < 120 mmHg | 1 | 350 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.47, 2.19] |
| 1.2 SBP 120 ‐ 139 mmHg | 1 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.67, 1.12] |
| 1.3 SBP 140 ‐ 159 mmHg | 2 | 2565 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.57, 0.89] |
| 1.4 SBP > 160 mmHg | 3 | 1954 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.51, 0.83] |
| 2 Recurrent stroke of any type by intervention | 8 | 35110 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.70, 0.93] |
| 2.1 ACE inhibitors | 1 | 6105 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.64, 0.84] |
| 2.2 Angiotensin receptor antagonists | 1 | 20332 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.87, 1.03] |
| 2.3 Beta‐blockers | 2 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.75, 1.18] |
| 2.4 Calcium channel blockers | 1 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.18, 1.67] |
| 2.5 Diuretics | 3 | 6216 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.59, 0.87] |
| 3 Recurrent stroke of any type by type of index event | 1 | 5854 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.64, 0.85] |
| 3.1 TIA | 1 | 981 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.50, 1.18] |
| 3.2 Ischaemic stroke | 1 | 4262 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.64, 0.89] |
| 3.3 Intracerebral haemorrhage | 1 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.39, 0.89] |
| 3.4 Combined Ischaemic stroke and Intracerebral haemorrhage | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Major vascular event by intervention | 4 | 28630 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.78, 1.04] |
| 4.1 ACE inhibitors | 1 | 6105 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.68, 0.85] |
| 4.2 Angiotensin receptor antagonists | 1 | 20332 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.88, 1.01] |
| 4.3 Beta‐blockers | 2 | 2193 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.84, 1.21] |
Comparison 3. Blood pressure‐lowering drugs (BPLDs) versus placebo or no treatment (sensitivity analyses).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Recurrent stroke of any type, methodological quality | 5 | 29082 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.75, 1.00] |
| 2 Recurrent stroke of any type, minimum 5 mmHg systolic blood pressure (SBP) reduction | 4 | 33575 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.68, 0.96] |
| 3 Recurrent stroke of any type, minimum 3 mmHg diastolic blood pressure (DBP) reduction | 5 | 34295 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.72, 0.97] |
| 4 Recurrent stroke of any type, excluding patients with subarachnoid haemorrhage as possible index event | 8 | 33690 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.70, 0.93] |
Comparison 4. Intensive versus standard blood pressure‐lowering.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Stroke of any type (fatal and non‐fatal) | 3 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.00] |
| 2 Time to recurrent stroke | 1 | 3020 | Hazard Ratio (Random, 95% CI) | 0.81 [0.64, 1.03] |
| 3 Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause | 3 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.23, 1.46] |
| 4 Ischaemic stroke | 2 | 3103 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.67, 1.09] |
| 5 Haemorrhagic stroke | 2 | 3103 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.17, 1.02] |
| 6 Myocardial infarction | 3 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.58, 1.38] |
| 7 Vascular death | 2 | 3549 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.35] |
| 8 Death by any cause | 3 | 3632 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.83, 1.39] |
| 9 Dementia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carter 1970.
| Methods | RCT | |
| Participants | Total number of participants randomised: 99 Setting: hospital Participants: hypertensive survivors below the age of 80 of ischaemic type major strokes Mean age: 64 years Sex: 57% men Country: UK |
|
| Interventions | Tailored individually
|
|
| Outcomes | Recurrent stroke of any type Death by any cause |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were placed at random into treated or control groups." |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding described, and outcome possibly influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding described, and outcome possibly influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of the 99 patients in the trial, 2 have been lost to follow‐up." Comment: missing outcome data balanced, and the proportion of missing outcomes compared with the observed event risk is unlikely to induce a clinically relevant impact on the effect estimate |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol published |
| Other bias | High risk | Comment: no endpoint definition given, single investigator study |
Co‐operative Study 1975.
| Methods | RCT | |
| Participants | Total number of participants randomised: 452 Setting: hospital Participants: hypertensive patients with a stroke or TIA in the previous year Mean age: 59 years Sex: 59% men Country: USA |
|
| Interventions | Deserdipine (0.5 mg once a day) and methyclothiazide (5 mg twice a day) | |
| Outcomes | Recurrent stroke of any type Time to recurrent stroke Myocardial infarction Vascular death Death by any cause |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The biostatistical section was responsible for assignment of patients to drug or placebo regimens." |
| Allocation concealment (selection bias) | Low risk | Quote: "The biostatistical section was responsible for (...), distribution of medication by mail to the individual clinics." Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the doctor nor the patient was aware of whether placebo or drug had been supplied." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A stroke endpoint (...) also was confirmed by a majority of a committee consisting of two members outside of the study and the Central Registry neurologist." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: although the number of participants lost to follow‐up was high, it was balanced between the groups in numbers and reasons |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol published |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Dutch TIA Trial 1993.
| Methods | RCT | |
| Participants | Total number of participants randomised: 1473 Setting: hospital Participants: patients with a TIA or non‐disabling stroke less than 3 months before Mean age: unknown (52% of participants > 65 years) Sex: 64% men Country: the Netherlands |
|
| Interventions | Atenolol (50 mg once a day) | |
| Outcomes | Recurrent stroke of any type Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause) Vascular death Death by any cause |
|
| Notes | Also compared two antithrombotic regimens in a 2x2 factorial design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "... use of random permuted blocks" |
| Allocation concealment (selection bias) | Low risk | Quote: "Blinded randomization codes were distributed by telephone." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: method of blinding not described other than being double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All outcome events were independently classified by at least three members of the Auditing Committee for Outcome Events, without knowledge of treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "No patient was lost to follow‐up." |
| Selective reporting (reporting bias) | High risk | Comment: in the protocol manuscript, the primary outcome was defined as the modified Rankin Scale, but this outcome is not reported |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
Marti Masso 1990.
| Methods | RCT | |
| Participants | Total number of participants randomised: 264 Setting: hospital Participants: patients with TIA or minor stroke in the previous 12 months Mean age: 61 years Sex: 71% men Country: Spain |
|
| Interventions | Nicardipine (20 mg three times a day) | |
| Outcomes | Recurrent stroke of any type Ischaemic stroke Death by any cause |
|
| Notes | Distribution between intervention and control suggests a randomisation ratio of 2:1, which remains unexplained | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding described, and outcome possibly influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "At three, six and 12 months the patients were asked about new symptoms of ischaemic episodes." Comment: no blinding described, and outcome possibly influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the study did not address this outcome |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol published |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
PAST‐BP 2016.
| Methods | Primary care‐based, pragmatic RCT | |
| Participants | Total number of participants randomised: 529 Setting: general practitioner Participants: patients with a past history of stroke or TIA Mean age: 72 years Sex: 59% men Country: UK |
|
| Interventions | Intensive versus guideline blood pressure‐lowering (target systolic blood pressure < 130 mmHg or a target reduction of 10 mmHg if blood pressure was between 125‐140 versus target systolic blood pressure < 140 mmHg) | |
| Outcomes | Recurrent stroke of any type Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause) Myocardial infarction Death by any cause |
|
| Notes | The time period between prior stroke or TIA and enrolment or intervention is unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: not specifically mentioned, but probably adequately done because of the use of minimisation |
| Allocation concealment (selection bias) | Low risk | Quote: "The central study team at the University of Birmingham randomized patients (...). The research nurse ascertained treatment allocation either by telephone or online" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: no blinding of participants and personnel, but outcomes are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Key secondary events (stroke; myocardial infarction; fatal coronary heart disease and other cardiovascular death) will be reviewed by independent clinicians blinded to treatment to ensure unbiased coding of these events." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All patients were followed up for clinical events and deaths." |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol published; all prespecified outcomes reported |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
PATS 1995.
| Methods | RCT | |
| Participants | Total number of participants randomised: 5665 Setting: hospital Participants: patients with a history of TIA or stroke Mean age: 60 years Sex: 72% men Country: China |
|
| Interventions | Indapamide (2.5 mg once a day) | |
| Outcomes | Recurrent stroke of any type Myocardial infarction Vascular death Death by any cause |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The Coordinating Center at the Fu Wai hospital generated a randomization list for each center." |
| Allocation concealment (selection bias) | Low risk | Quote: "The sealed envelope system was used to randomize the participants." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "(...) or a pill of matching placebo" Comment: probably adequately done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An Endpoint Committee, blinded to the randomization of patients, reviewed the clinical records and adjudicated all primary and secondary end points." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: high proportion of incomplete outcome data, such that it could have influenced effect estimates |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol published |
| Other bias | High risk | Comment: multiple reports with different numbers of participants and outcome events |
PODCAST 2017.
| Methods | Prospective, open‐label blinded endpoint, phase IV RCT | |
| Participants | Total number of participants randomised: 83 Setting: hospital Participants: patients with ischaemic stroke or intracerebral haemorrhage in the past 3 to 7 months Mean age: 74 years Sex: 77% men Country: UK |
|
| Interventions | Intensive versus guideline blood pressure‐lowering (target systolic blood pressure < 125 mmHg versus < 140 mmHg) | |
| Outcomes | Recurrent stroke of any type Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause) Ischaemic stroke Haemorrhagic stroke Myocardial infarction Death by any cause Dementia |
|
| Notes | Stopped early because of slow recruitment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All participants (...) will be randomized centrally using a secure Internet site in real‐time." |
| Allocation concealment (selection bias) | Low risk | Quote: "This approach ensures concealment of allocation, (...)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: no blinding of participants and personnel, but outcomes are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "(...) outcome assessment will be assessed blinded to treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol published; all outcomes relevant to our review were reported |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
PRoFESS 2008.
| Methods | RCT | |
| Participants | Total number of participants randomised: 20,332 Setting: hospital Participants: patients with ischaemic stroke in the previous 90 days Mean age: 66 years Sex: 64% men Country: 35 countries (all major continents) |
|
| Interventions | Telmisartan (80 mg once a day) | |
| Outcomes | Recurrent stroke of any type Time to recurrent stroke Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause) Ischaemic stroke Haemorrhagic stroke Myocardial infarction Vascular death Death by any cause |
|
| Notes | Also compared two antithrombotic regimens in a 2x2 factorial design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: not specifically mentioned, but probably adequately done because of use of stratification |
| Allocation concealment (selection bias) | Low risk | Quote: "(...) with the use of a central telephone system." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The medication was ‘double dummy’ meaning that each patient received each medication, either active or matching placebo, so that all patients received identically appearing medication kits." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An Adjudication and Assessment Committee independent of the Steering Committee and sponsor verifies all primary and key secondary outcomes blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 125 patients (51 in the telmisartan group and 74 in the placebo group) (0.6%) were lost to follow up." Comment: proportion of missing outcome not likely to induce clinically relevant bias in intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol published; all prespecified outcomes reported |
| Other bias | Low risk | Comment: industry sponsored, but no concerns |
PROGRESS 2001.
| Methods | RCT | |
| Participants | Total number of participants randomised: 6105 Setting: hospital Participants: patients with TIA or stroke in the previous 5 years Mean age: 64 years Sex: 70% men Country: Australia, Belgium, China, France, Japan, Italy, New Zealand, Sweden and UK |
|
| Interventions | Perindopril (4 mg once a day) ± indapamide (2.5 mg once a day, Japan: 2 mg once a day) | |
| Outcomes | Recurrent stroke of any type Time to recurrent stroke Major vascular event Ischaemic stroke Haemorrhagic stroke Myocardial infarction (composite of non‐fatal myocardial infarction and fatal coronary) Vascular death Death by any cause Dementia |
|
| Notes | Index event was prior history of stroke or TIA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A minimization algorithm stratifies treatment allocation..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation to active treatment or to placebo was performed by fax from the collaborating clinical centres to the study randomization centre in Auckland." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients assigned placebo received placebo tablets identical in appearance to perindopril." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An endpoint adjudication committee reviewed source documentation for all individuals (...)" Comment: probably done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data balanced, and the proportion not enough to have a clinically relevant impact on the intervention effect estimate |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol published; all prespecified outcomes reported |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
SPS3 2013.
| Methods | RCT | |
| Participants | Total number of participants randomised: 3020 Setting: hospital Participants: patients with a lacunar stroke in the previous 180 days Mean age: 63 years Sex: 63% men Country: North America, Latin America, and Spain |
|
| Interventions | Systolic blood pressure target < 130 mmHg versus target 130‐149 mmHg | |
| Outcomes | Recurrent stroke of any type Time to recurrent stroke Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause) Ischaemic stroke Haemorrhagic stroke Myocardial infarction Vascular death Death by any cause Dementia |
|
| Notes | Also compared two antithrombotic regimens in a 2x2 factorial design | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The schedule was computer generated with a permuted‐block design (variable block size)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation assignments (...) are stored in each clinical centre’s electronic data entry system, and are protected from preview." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: no blinding of participants and personnel, but outcomes are not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All possible outcome and safety events are reviewed by the blinded Events Adjudication Committee (...) who are not otherwise involved in SPS3." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: number and reasons of loss to follow‐up reported, but not per treatment group |
| Selective reporting (reporting bias) | Low risk | Comment: study protocol published; all prespecified outcomes reported |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
TEST 1995.
| Methods | RCT | |
| Participants | Total number of participants randomised: 720 Setting: hospital Participants: patients with TIA or stroke in previous 3 weeks Mean age: 70 years Sex: 60% men Country: Sweden |
|
| Interventions | Atenolol (50 mg once a day) | |
| Outcomes | Recurrent stroke of any type Major vascular event (composite of non‐fatal stroke, non‐fatal myocardial infarction, or death from any vascular cause) Myocardial infarction Vascular death Death by any cause |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated random scheme using a random permuted block design (...)" |
| Allocation concealment (selection bias) | Low risk | Quote: "A computer‐generated random scheme using a random permuted block design (...)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: method of blinding not described other than being double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "An independent End Point Committee reviewed the fatal end‐points..." Comment: probably done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol published |
| Other bias | Low risk | Comment: the study appears to be free of other sources of bias |
RCT: randomised controlled trial TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Lee 2015 | Not all participants had a TIA or stroke as index event |
| Lithell 2003 | No comparison with placebo or no BPLDs (as BPLDs were started in a large proportion of participants in the control group during follow‐up) |
| Schrader 2003 | No comparison with placebo or no BPLDs (as BPLDs were started in a large proportion of participants in the control group during follow‐up) |
BPLDs: blood pressure‐lowering drugs TIA: transient ischaemic attack
Characteristics of ongoing studies [ordered by study ID]
NCT01220622.
| Trial name or title | Efficacy and safety study of nimodipine to prevent mild cognitive impairment after acute ischemic strokes (NICE) |
| Methods | Double‐blind, placebo‐controlled, phase IV randomised controlled trial |
| Participants | Participants aged 30–80 with ischaemic stroke ≤ 7 days |
| Interventions | Nimodipine (90 mg/day) or placebo (90 mg/day) |
| Outcomes | Death by any cause Dementia |
| Starting date | November 2010 |
| Contact information | yongjunwang111@yahoo.com.cn; happyft@sina.com; minnie_wxm@hotmail.com |
| Notes | Status: unknown, results expected 2013 Size: 656 participants (23 Chinese centres) |
NCT01563731.
| Trial name or title | Optimal blood pressure and cholesterol targets for preventing recurrent stroke in hypertensives (ESH‐CHL‐SHOT) |
| Methods | Prospective randomised open‐label blinded endpoint trial, with a 3x2 factorial design |
| Participants | Participants aged > 65 years with stroke or TIA 1‐6 months prior to randomisation |
| Interventions |
|
| Outcomes | Stroke Time to recurrent stroke Major vascular event Ischaemic stroke Haemorrhagic stroke Vascular death Death by any cause Dementia |
| Starting date | April 2013 |
| Contact information | alberto.zanchetti@auxologico.it; g.bilo@auxologico.it |
| Notes | Status: recruiting Size: 7500 planned |
mg: milligram mmHg: millimetres of mercury mmol/L: millimoles per litre TIA: transient ischaemic attack
Differences between protocol and review
Outcomes
We did not include the outcome 'heart failure' because of the difficulties in uniformly defining the condition, and a lack of studies assessing this outcome. Furthermore, we added 'death by any cause' as an outcome measure instead of 'any stroke or death', as total mortality was usually assessed, whereas a composite with stroke was not.
Publication bias
We did not produce a funnel plot to assess publication bias, as we included less than 10 studies per comparison.
Contributions of authors
Draft the protocol: MD Vergouwen, RJ de Haan, M Vermeulen, YB Roos
Develop the search strategy: MD Vergouwen, TP Zonneveld, M Vermeulen, YB Roos, E Richard
Search for trials: TP Zonneveld
Obtain copies of trials: TP Zonneveld, ND Kruyt
Select trials for inclusion: TP Zonneveld, ND Kruyt, E Richard
Extract data: TP Zonneveld, ND Kruyt, E Richard
Enter data into Review Manager: TP Zonneveld
Carry out the analysis: TP Zonneveld, ND Kruyt, PJ Nederkoorn, YB Roos, E Richard
Interpret the analysis: TP Zonneveld, ND Kruyt, PJ Nederkoorn, MD Vergouwen, RJ de Haan, YB Roos, E Richard
Draft the final review: TP Zonneveld, ND Kruyt, PJ Nederkoorn, MD Vergouwen, RJ de Haan, YB Roos, E Richard
Update the review: TP Zonneveld, ND Kruyt, E Richard
Declarations of interest
Thomas P Zonneveld: none known. Edo Richard: none known. Mervyn DI Vergouwen: none known. Paul J Nederkoorn: none known. Rob de Haan: none known. Yvo BW Roos: none known. Nyika D Kruyt: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Carter 1970 {published data only}
- Carter AB. Hypotensive therapy in stroke survivors. Lancet 1970;1:485‐9. [DOI] [PubMed] [Google Scholar]
Co‐operative Study 1975 {published data only}
- Bauer RB. Effect of antihypertensive treatment on stroke recurrence: results of the hypertension‐stroke cooperative study. Cerebral Vascular Diseases: Transactions of the Ninth Princeton Conference. 1975:137‐54.
- Hoobler SW. Antihypertensive therapy for stroke patients. Hypertension and Stroke Control in the Community: Proceedings of a WHO Meeting, March 1974. 1976.
- Hypertension‐Stroke Cooperative Study Group. Effect of antihypertensive treatment on stroke recurrence. Journal of the American Medical Association 1974;229:409‐18. [DOI] [PubMed] [Google Scholar]
Dutch TIA Trial 1993 {published data only (unpublished sought but not used)}
- The Dutch TIA Study Group. The Dutch TIA trial: protective effects of low‐dose aspirin and atenolol in patients with transient ischemic attacks or nondisabling stroke. Stroke 1988;19:512‐7. [DOI] [PubMed] [Google Scholar]
- The Dutch TIA Trial Study Group. A comparison of two doses of aspirin (30 mg vs 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. New England Journal of Medicine 1991;325:1261‐6. [DOI] [PubMed] [Google Scholar]
- The Dutch TIA Trial Study Group. Trial of secondary prevention with atenolol after transient ischemic attack or nondisabling ischemic stroke. Stroke 1993;24:543‐8. [DOI] [PubMed] [Google Scholar]
Marti Masso 1990 {published data only}
- Marti Masso JF, Lozano R. Nicardipine in the prevention of cerebral infarction. Clinical Therapeutics 1990;12:344‐51. [PubMed] [Google Scholar]
PAST‐BP 2016 {published data only (unpublished sought but not used)}
- Fletcher K, Mant J, McManus R, Campbell S, Betts J, Taylor C, et al. Protocol for Past BP: a randomised controlled trial of different blood pressure targets for people with a history of stroke or transient ischaemic attack (TIA) in primary care. BMC Cardiovascular Disorders 2010;10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mant J, McManus RJ, Roalfe A, Fletcher K, Taylor CJ, Martin U, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST‐BP (Prevention After Stroke‐Blood Pressure) randomised controlled trial. BMJ 2016;352:i708. [DOI] [PMC free article] [PubMed] [Google Scholar]
PATS 1995 {published data only (unpublished sought but not used)}
- Liu L, Gong L, Guang Wang J. Blood pressure lowering in patients with cerebrovascular disease: results of the randomized Post‐stroke Antihypertensive Treatment Study (PATS). Journal of the American College of Cardiology 1998;31 (Suppl 2A):211A. [Google Scholar]
- Liu L, Wang Z, Gong L, Zhang Y, Thijs L, Staessen JA, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertension Research 2009;32:1032‐40. [DOI] [PubMed] [Google Scholar]
- PATS Collaborating Group. Post‐stroke antihypertensive treatment study. A preliminary result. Chinese Medical Journal 1995;108:710‐7. [PubMed] [Google Scholar]
PODCAST 2017 {published data only}
- Blackburn DJ, Krishnan K, Fox L, Ballard C, Burns A, Ford GA, et al. Prevention Of Decline in Cognition After Stroke Trial (PODCAST): a study protocol for a factorial randomised controlled trial of intensive versus guideline lowering of blood pressure and lipids. Trials 2013;14:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
PRoFESS 2008 {published data only (unpublished sought but not used)}
- Bath PM, Martin RH, Palesch Y, Cotton D, Yusuf S, Sacco R, et al. Effect of telmisartan on functional outcome, recurrence, and blood pressure in patients with acute mild ischemic stroke: a PRoFESS subgroup analysis. Stroke 2009;40:3541‐6. [DOI] [PubMed] [Google Scholar]
- Diener HC, Sacco R, Yusuf S, Steering Committee, PRoFESS Study Group. Rationale, design and baseline data of a randomized, double‐blind, controlled trial comparing two antithrombotic regimens (a fixed‐dose combination of extended‐release dipyridamole plus ASA with clopidogrel) and telmisartan versus placebo in patients with strokes: the Prevention Regimen for Effectively Avoiding Second Strokes Trial (PRoFESS). Cerebrovascular Diseases 2007;23:368‐80. [DOI] [PubMed] [Google Scholar]
- Diener HC, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Effects of aspirin plus extended‐release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double‐blind, active and placebo‐controlled study. Lancet Neurology 2008;7:875‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf S, Diener HC, Sacco RL, Cotton D, Ounpuu S, Lawton WA, et al. Telmisartan to prevent recurrent stroke and cardiovascular events. New England Journal of Medicine 2008;359:1225‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
PROGRESS 2001 {published data only}
- Arima H, Anderson C, Omae T, Liu L, Tzourio C, Woodward M, et al. Perindopril‐based blood pressure lowering reduces major vascular events in Asian and Western participants with cerebrovascular disease: the PROGRESS trial. Journal of Hypertension 2010;28:395‐400. [DOI] [PubMed] [Google Scholar]
- Arima H, Chalmers J, Woodward M, Anderson C, Rodgers A, Davis S, et al. Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. Journal of Hypertension 2006;24:1201‐8. [DOI] [PubMed] [Google Scholar]
- Arima H, Tzourio C, Anderson C, Woodward M, Bousser MG, MacMahon S, et al. Effects of perindopril‐based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke 2010;41:394‐6. [DOI] [PubMed] [Google Scholar]
- Chapman N, Huxley R, Anderson C, Bousser MG, Chalmers J, Colman S, et al. Effects of a perindopril‐based blood pressure‐lowering regimen on the risk of recurrent stroke according to stroke subtype and medical history: the PROGRESS Trial. Stroke 2004;35:116‐21. [DOI] [PubMed] [Google Scholar]
- Neal B, MacMahon S. The PROGRESS study: rationale and design. Journal of Hypertension 1995;13:1869‐73. [PubMed] [Google Scholar]
- Ninomiya T, Donnan G, Anderson N, Bladin C, Chambers B, Gordon G, et al. Effects of the end point adjudication process on the results of the Perindopril Protection Against Recurrent Stroke Study (PROGRESS). Stroke 2009;40:2111‐5. [DOI] [PubMed] [Google Scholar]
- PROGRESS Collaborative Group. Randomised trial of a perindopril‐based blood‐pressure‐lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet 2001;358:1033‐41. [DOI] [PubMed] [Google Scholar]
- PROGRESS Management Committee. PROGRESS: Perindopril pROtection aGainst REcurrent Stroke Study: status in March 1997. Journal of Human Hypertension 1998;12:627‐9. [DOI] [PubMed] [Google Scholar]
- Progress Management Committee. PROGRESS ‐ Perindopril Protection Against Recurrent Stroke Study: characteristics of the study population at baseline. Journal of Hypertension 1999;17:1647‐55. [PubMed] [Google Scholar]
- Rodgers A, Chapman N, Woodward M, Liu LS, Colman S, Lee A, et al. Perindopril‐based blood pressure lowering in individuals with cerebrovascular disease: consistency of benefits by age, sex and region. Journal of Hypertension 2004;22:653‐9. [DOI] [PubMed] [Google Scholar]
- Tzourio C, Anderson C, Chapman N, Woodward M, Neal B, MacMahon S, et al. Effects of blood pressure lowering with perindopril and indapamide therapy on dementia and cognitive decline in patients with cerebrovascular disease. Archives of Internal Medicine 2003;163:1069‐75. [DOI] [PubMed] [Google Scholar]
SPS3 2013 {published data only}
- Benavente OR, White CL, Pearce L, Pergola P, Roldan A, Benavente MF, et al. The Secondary Prevention of Small Subcortical Strokes (SPS3) study. International Journal of Stroke 2011;6:164‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The SPS3 Study Group. Blood‐pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013;382:507‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CL, Szychowski JM, Roldan A, Benavente MF, Pretell EJ, Brutto OH, et al. Clinical features and racial/ethnic differences among the 3020 participants in the Secondary Prevention of Small Subcortical Strokes (SPS3) trial. Journal of Stroke and Cerebrovascular Diseases 2013;22:764‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
TEST 1995 {published data only}
- Eriksson S, Olofsson BO, Wester PO, for the TEST study group. Atenolol in secondary prevention after stroke. Cerebrovascular Diseases 1995;5:21‐5. [Google Scholar]
References to studies excluded from this review
Lee 2015 {published data only}
- Lee JSW, Chui PY, Ma HM, Auyeung TW, Kng C, Law T, et al. Does low dose angiotensin converting enzyme inhibitor prevent pneumonia in older people with neurologic dysphagia ‐ a randomized placebo‐controlled trial. Journal of the American Medical Directors Association 2015;16(8):702‐7. [DOI] [PubMed] [Google Scholar]
Lithell 2003 {published data only}
- Lithell H, Hansson L, Skoog I, Elmfeldt D, Hofman A, Olofsson B, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double‐blind intervention trial. Journal of Hypertension 2003;21(5):875‐86. [DOI] [PubMed] [Google Scholar]
Schrader 2003 {published data only}
- Schrader J, Lüders S, Kulschewski A, Berger J, Zidek W, Treib J, et al. Acute Candesartan Cilexetil Therapy in Stroke Survivors Study Group. The ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke 2003;34(7):1699‐703. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01220622 {published data only}
- NCT01220622. Efficacy and safety study of nimodipine to prevent mild cognitive impairment after acute ischemic strokes. https://ClinicalTrials.gov/show/NCT01220622 (first received 14 October 14 2010).
- Wang P, Wang Y, Feng T, Zhao X, Zhou Y, Wang Y, et al. Rationale and design of a double‐blind, placebo‐controlled, randomized trial to evaluate the safety and efficacy of nimodipine in preventing cognitive impairment in ischemic cerebrovascular events (NICE). BMC Neurology 2012;12:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01563731 {published data only}
- NCT01563731. Optimal blood pressure and cholesterol targets for preventing recurrent stroke in hypertensives. https://ClinicalTrials.gov/show/NCT01563731 (first received 27 March 2012).
- Zanchetti A, Liu L, Mancia G, Parati G, Grassi G, Stramba‐Badiale M, et al. Blood pressure and LDL‐cholesterol targets for prevention of recurrent strokes and cognitive decline in the hypertensive patient: design of the European Society of Hypertension‐Chinese Hypertension League Stroke in Hypertension Optimal Treatment randomized trial. Journal of Hypertension 2014;32(9):1888‐97. [DOI] [PubMed] [Google Scholar]
- Zanchetti A, Liu L, Mancia G, Parati G, Grassi G, Stramba‐Badiale M, et al. ESH‐CHL‐SHOT trial investigators. Continuation of the ESH‐CHL‐SHOT trial after publication of the SPRINT: Rationale for further study on blood pressure targets of antihypertensive treatment after stroke. Journal of Hypertension 2016;34:393‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Birns 2005
- Birns J, Markus H, Kalra L. Blood pressure reduction for vascular risk: is there a price to be paid?. Stroke 2005;36:1308‐13. [DOI] [PubMed] [Google Scholar]
BPLTTC 2003
- Turnbull F, Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood‐pressure‐lowering regimens on major cardiovascular events results of prospectively‐designed overviews of randomised trials. Lancet 2003;362(9395):1527‐35. [DOI] [PubMed] [Google Scholar]
Chobanian 2003
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560‐72. [DOI] [PubMed] [Google Scholar]
Feldstein 2014
- Feldstein CA. Lowering blood pressure to prevent stroke recurrence: a systematic review of long‐term randomized trials. Journal of the American Society of Hypertension 2014;8(7):503‐13. [DOI] [PubMed] [Google Scholar]
GBD 2016
- GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability‐adjusted life‐years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390(10100):1260‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Irie 1993
- Irie K, Yamaguchi T, Minematsu K, Omae T. The J‐curve phenomenon in stroke recurrence. Stroke 1993;24:1844‐9. [DOI] [PubMed] [Google Scholar]
Lackland 2014
- Lackland DT, Roccella EJ, Deutsch AF, Fornage M, George MG, Howard G, et al. Factors influencing the decline in stroke mortality: a statement from the American Heart Association/American Stroke Association. Stroke 2014;45(1):315‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakhan 2009
- Lakhan SE, Sapko TS. Blood pressure lowering treatment for preventing stroke recurrence: a systematic review and meta‐analysis. International Archives of Medicine 2009;2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewington 2002
- Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration. Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903‐13. [DOI] [PubMed] [Google Scholar]
Liu 2009
- Liu L, Wang Z, Gong L, Zhang Y, Thijs L, Staessen JA, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertension Research 2009;32:1032‐40. [DOI] [PubMed] [Google Scholar]
Mohan 2011
- Mohan KM, Wolfe CDA, Rudd AG, Heuschmann PU, Kolominsky‐Rabas PL, Grieve AP. Risk and cumulative risk of stroke recurrence: a systematic review and meta‐analysis. Stroke 2011;42(5):1489‐94. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher, D, Liberati A Fau ‐ Tetzlaff, Jennifer, Tetzlaff J Fau ‐ Altman, Douglas G, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Med, P. LoS 2009. [PMC free article] [PubMed]
Qiu 2005
- Qiu C, Winblad B, Fratiglioni L. The age‐dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology 2005;4:487‐99. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valls 2016
- Valls J, Peiro‐Chamarro M, Cambray S, Molina‐Seguin J, Benabdelhak I, Purroy F. A current estimation of the early risk of stroke after transient ischemic attack: a systematic review and meta‐analysis of recent intervention studies. Cerebrovascular Diseases 2016;43:90‐8. [DOI] [PubMed] [Google Scholar]
van Wijk 2005
- Wijk I, Kappelle LJ, Gijn J, Koudstaal PJ, Franke CL, Vermeulen M, et al. Long‐term survival and vascular event risk after transient ischaemic attack or minor ischaemic stroke: a cohort study. Lancet 2005;365:2098‐104. [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang Yu, Zeng Tingting. Response to: Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2013;14:391. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Vergouwen 2009
- Vergouwen MD, Haan R, Gool WA, Vermeulen M, Roos YB. Blood‐pressure‐lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attacks. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007858] [DOI] [PMC free article] [PubMed] [Google Scholar]


