Abstract
Background
Cryptococcal meningitis is a severe fungal infection that occurs primarily in the setting of advanced immunodeficiency and remains a major cause of HIV‐related deaths worldwide. The best induction therapy to reduce mortality from HIV‐associated cryptococcal meningitis is unclear, particularly in resource‐limited settings where management of drug‐related toxicities associated with more potent antifungal drugs is a challenge.
Objectives
To evaluate the best induction therapy to reduce mortality from HIV‐associated cryptococcal meningitis; to compare side effect profiles of different therapies.
Search methods
We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL, MEDLINE (PubMed), Embase (Ovid), LILACS (BIREME), African Index Medicus, and Index Medicus for the South‐East Asia Region (IMSEAR) from 1 January 1980 to 9 July 2018. We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), ClinicalTrials.gov, and the ISRCTN registry; and abstracts of select conferences published between 1 July 2014 and 9 July 2018.
Selection criteria
We included randomized controlled trials that compared antifungal induction therapies used for the first episode of HIV‐associated cryptococcal meningitis. Comparisons could include different individual or combination therapies, or the same antifungal therapies with differing durations of induction (less than two weeks or two or more weeks, the latter being the current standard of care). We included data regardless of age, geographical region, or drug dosage. We specified no language restriction.
Data collection and analysis
Two review authors independently screened titles and abstracts identified by the search strategy. We obtained the full texts of potentially eligible studies to assess eligibility and extracted data using standardized forms. The main outcomes included mortality at 2 weeks, 10 weeks, and 6 months; mean rate of cerebrospinal fluid fungal clearance in the first two weeks of treatment; and Division of AIDS (DAIDS) grade three or four laboratory events. Using random‐effects models we determined pooled risk ratio (RR) and 95% confidence interval (CI) for dichotomous outcomes and mean differences (MD) and 95% CI for continuous outcomes. For the direct comparison of 10‐week mortality, we assessed the certainty of the evidence using the GRADE approach. We performed a network meta‐analysis using multivariate meta‐regression. We modelled treatment differences (RR and 95% CI) and determined treatment rankings for two‐week and 10‐week mortality outcomes using surface under the cumulative ranking curve (SUCRA). We assessed transitivity by comparing distribution of effect modifiers between studies, local inconsistency through a node‐splitting approach, and global inconsistency using design‐by‐treatment interaction modelling. For the network meta‐analysis, we applied a modified GRADE approach for assessing the certainty of the evidence for 10‐week mortality.
Main results
We included 13 eligible studies that enrolled 2426 participants and compared 21 interventions. All studies were carried out in adults, and all but two studies were conducted in resource‐limited settings, including 11 of 12 studies with 10‐week mortality data.
In the direct pairwise comparisons evaluating 10‐week mortality, one study from four sub‐Saharan African countries contributed data to several key comparisons. At 10 weeks these data showed that those on the regimen of one‐week amphotericin B deoxycholate (AmBd) and flucytosine (5FC) followed by fluconazole (FLU) on days 8 to 14 had lower mortality when compared to (i) two weeks of AmBd and 5FC (RR 0.62, 95% CI 0.42 to 0.93; 228 participants, 1 study), (ii) two weeks of AmBd and FLU (RR 0.58, 95% CI 0.39 to 0.86; 227 participants, 1 study), (iii) one week of AmBd with two weeks of FLU (RR 0.49, 95% CI 0.34 to 0.72; 224 participants, 1 study), and (iv) two weeks of 5FC and FLU (RR 0.68, 95% CI 0.47 to 0.99; 338 participants, 1 study). The evidence for each of these comparisons was of moderate certainty. For other outcomes, this shortened one‐week AmBd and 5FC regimen had similar fungal clearance (MD 0.05 log10 CFU/mL/day, 95% CI ‐0.02 to 0.12; 186 participants, 1 study) as well as lower risk of grade three or four anaemia (RR 0.31, 95% CI 0.16 to 0.60; 228 participants, 1 study) compared to the two‐week regimen of AmBd and 5FC.
For 10‐week mortality, the comparison of two weeks of 5FC and FLU with two weeks of AmBd and 5FC (RR 0.92, 95% CI 0.69 to 1.23; 340 participants, 1 study) or two weeks of AmBd and FLU (RR 0.85, 95% CI 0.64 to 1.13; 339 participants, 1 study) did not show a difference in mortality, with moderate‐certainty evidence for both comparisons.
When two weeks of combination AmBd and 5FC was compared with AmBd alone, pooled data showed lower mortality at 10 weeks (RR 0.66, 95% CI 0.46 to 0.95; 231 participants, 2 studies, moderate‐certainty evidence).
When two weeks of AmBd and FLU was compared to AmBd alone, there was no difference in 10‐week mortality in pooled data (RR 0.94, 95% CI 0.55 to 1.62; 371 participants, 3 studies, low‐certainty evidence).
One week of AmBd and 5FC followed by FLU on days 8 to 14 was the best induction therapy regimen after comparison with 11 other regimens for 10‐week mortality in the network meta‐analysis, with an overall SUCRA ranking of 88%.
Authors' conclusions
In resource‐limited settings, one‐week AmBd‐ and 5FC‐based therapy is probably superior to other regimens for treatment of HIV‐associated cryptococcal meningitis. An all‐oral regimen of two weeks 5FC and FLU may be an alternative in settings where AmBd is unavailable or intravenous therapy cannot be safely administered. We found no mortality benefit of combination two weeks AmBd and FLU compared to AmBd alone. Given the absence of data from studies in children, and limited data from high‐income countries, our findings provide limited guidance for treatment in these patients and settings.
2 April 2019
Up to date
All studies incorporated from most recent search
Updated review: all eligible published studies found in the last search (9 Jul, 2018) were included and three ongoing studies have been identified (see 'Characteristics of ongoing studies' section)
Plain language summary
Treatment for HIV‐associated cryptococcal meningitis
What is the aim of this review?
The aim of this Cochrane Review was to find the best therapy to reduce the risk of death from cryptococcal meningitis in HIV‐positive individuals. The Cochrane review authors analysed data from relevant clinical trials to answer this question and found 13 relevant studies.
Key messages
Shorter initial treatment with one week of combined amphotericin B deoxycholate and flucytosine probably results in lower risk of death than longer treatment with two weeks of combination amphotericin B deoxycholate and flucytosine that has traditionally been recommended in treatment guidelines. The shorter treatment likely results in similar clearance of the infection with less toxicity from the drugs used for treatment. Where amphotericin B deoxycholate cannot be given, two weeks of combined flucytosine with fluconazole is likely a good treatment option. Given the absence of data from studies in children, and limited data from high‐income countries, our findings provide limited guidance for treatment in these patients and settings.
What was studied in this review?
HIV‐associated cryptococcal meningitis is a severe fungal infection of the brain and surrounding membranes that causes about 15% of HIV‐related deaths worldwide. Infection occurs mostly in people with advanced HIV/AIDS and most deaths from cryptococcal meningitis occur in resource‐limited countries. Treatment includes initial antifungal therapy followed by continuation treatment with oral fluconazole. Previous guidelines have recommended two weeks of combination intravenous amphotericin B and oral flucytosine as the best available treatment. However, due to the high cost of treatment and limited availability of these potent antifungal drugs as well as challenges in managing common drug toxicities, resource‐limited countries often use less effective therapies such as oral fluconazole alone.
The review authors compared different antifungal drugs used for initial therapy of HIV‐associated cryptococcal meningitis to determine the best treatment to reduce the risk of death. Several recent clinical trials included in this review studied shorter initial treatment courses or all‐oral treatments for cryptococcal meningitis to reduce drug toxicity and improve affordability in resource‐limited countries where most infections occur.
What are the main results of the review?
The 13 studies included 2426 people and directly compared 21 different therapies. All studies were carried out in adults, and all but two studies were conducted in resource‐limited settings, including 11 of 12 studies with 10‐week mortality data. One recent large study conducted in adults from four countries in Africa contributed to 10 of these comparisons. This study found that one week of combination intravenous amphotericin B deoxycholate and oral flucytosine followed by fluconazole probably resulted in a lower risk of death within 10 weeks than two weeks of combination amphotericin B deoxycholate and flucytosine (moderate‐certainty evidence). The rate of fungal reduction measured in cerebrospinal fluid did not differ between the treatment groups but a shorter duration of amphotericin B deoxycholate and flucytosine was associated with lower risk of life‐threatening toxicities measured through blood testing. These results suggest that shorter one week treatment with amphotericin B deoxycholate and flucytosine is probably better than two weeks of amphotericin B deoxycholate and flucytosine.
In this same study, one week of amphotericin B deoxycholate and flucytosine probably resulted in a lower risk of death than a combination of oral flucytosine and fluconazole (moderate‐certainty evidence). However, risk of death was similar between oral flucytosine and fluconazole and two weeks of amphotericin B deoxycholate and flucytosine (moderate‐certainty evidence). Where intravenous amphotericin B therapy is not available or cannot be safely given to patients, this suggests that combination therapy with oral flucytosine and fluconazole is a good alternative treatment.
Due to the lack of data from studies in children, and limited data from high‐income countries, our findings provide limited guidance for treatment in these patients and settings.
How up‐to‐date is this review?
The review authors initially searched for studies up to 9 July 2018.
Summary of findings
Background
Description of the condition
Cryptococcal meningitis is a severe fungal infection primarily seen in people with compromised cell‐mediated immunity. Most cases occur in the context of advanced HIV disease (defined as cluster of differentiation 4 (CD4) cell count < 200 cells/mm3), with the risk increasing with decreasing CD4 cell count. Rollout of combined antiretroviral therapy (ART) in the 1990s led to a large decline in incident HIV‐associated cryptococcal meningitis in high‐income countries, but a large burden of disease still exists in resource‐limited settings (Dromer 2004; Pyrgos 2013; Rajasingham 2017). This reflects, in part, an enduring high proportion of HIV‐positive individuals who present late for care due to delays in HIV diagnosis and linkage to care (Anderegg 2017). However, a growing proportion of cases (approximately 50%) are now seen in ART‐experienced individuals, reflecting ART default and treatment failure (Beardsley 2016; Rhein 2016; Scriven 2016). In 2014, an estimated 223,100 incident cases and 181,100 deaths occurred globally, and cryptococcal meningitis is estimated to cause up to 15% of HIV‐related deaths (Rajasingham 2017). Approximately 73% of cases are estimated to occur in sub‐Saharan Africa.
Cryptococccal meningitis is caused by environmental yeast species Cryptococcus neoformans and Cryptococcus gattii. C neoformans has a worldwide distribution and causes clinical disease in immunocompromised individuals, whereas C gattii has a more limited geographical distribution and is also associated with disease in immunocompetent hosts (Chen 2000). A typically asymptomatic primary pulmonary infection occurs after inhalation of fungal spores or desiccated yeast. In the absence of an effective immune response to clear or contain infection, yeast can spread to the central nervous system (CNS) to cause a severe meningoencephalitis. Clinical presentation is subacute, and commonly includes fever, headache, neck stiffness, altered mental status, vomiting, and neurological deficits such as cranial nerve palsies (Moosa 1997; Day 2013).
Diagnostic confirmation of cryptococcal meningitis relies on lumbar puncture (LP), which is also an essential part of management to lower raised intracranial pressure. Use of India ink stain to detect yeast in cerebrospinal fluid (CSF) is commonly performed in resource‐limited settings. Testing is cheap, rapid, and requires limited laboratory infrastructure, but has poor sensitivity (Boulware 2014a). Culture, considered the gold standard for diagnosis, is more sensitive but is more expensive, requires additional laboratory infrastructure and expertise, and results are delayed while yeast grow in culture media. Various cryptococcal antigen (CrAg) assays are available that provide rapid results, and CrAg testing of CSF by latex agglutination or, preferably, lateral flow assay is recommended by the World Health Organization (WHO) (WHO 2018). Cryptococcal antigen testing can also be performed on serum or plasma. Although a positive test from a blood sample is not specific for CNS disease, initiation of treatment for cryptococcal meningitis based on a positive test of serum or plasma is recommended if LP cannot be performed or is clinically contraindicated (WHO 2018).
Description of the intervention
Polyenes, azoles, and the pyrimidine analog flucytosine (5FC), are the mainstays of treatment for cryptococcal meningitis. Per WHO guidelines (issued in 2011) and Infectious Disease Society of America guidelines (issued in 2010), the preferred induction therapy for adults includes intravenous (IV) amphotericin deoxycholate (AmBd) (0.7 to 1 mg/kg daily) and oral 5FC (100 mg/kg daily in four divided doses) for a minimum of two weeks, followed by maintenance oral fluconazole (400 to 800 mg daily) for a minimum of eight additional weeks (Perfect 2010; WHO 2011). Liposomal amphotericin (AmB) is less nephrotoxic but more expensive than AmBd, and IV liposomal AmB (3 to 4 mg/kg daily) or IV AmB lipid complex (5 mg/kg daily) may be substituted for AmBd during the induction phase of treatment (Perfect 2010). A phase three clinical trial conducted in Vietnam comparing AmBd 1 mg/kg daily and 5FC 100 mg/kg daily to AmBd 1 mg/kg daily alone found a statistically significant mortality benefit of combination therapy, providing evidence for these recommendations (Day 2013). Treatment guidelines provide tiered recommendations for alternative regimens if first‐line therapy cannot be administered.
Intravenous amphotericin has strong fungicidal activity, and treatment for at least 14 days has been considered first‐line therapy (Perfect 2010; WHO 2011). Major drug‐related adverse effects include nephrotoxicity, hypokalaemia, and anaemia, and therapy should include IV fluid hydration, potassium supplementation, and regular laboratory monitoring for haematological and metabolic toxicities (Bicanic 2015). The relatively high cost of treatment, requirement of IV administration, and limited ability to monitor for drug‐related toxicities in many settings often result in the use of alternative oral antifungal regimens, primarily fluconazole. To reduce the cost of treatment and promote greater use in resource‐limited settings, recent trials have evaluated the efficacy of AmB‐based induction therapy for a duration of less than 14 days (Muzoora 2012; Jarvis 2018; Molloy 2018).
Flucytosine, available orally or intravenously, is recommended in combination with amphotericin as first‐line therapy (Perfect 2010). The most serious drug‐related adverse effect of 5FC is bone marrow suppression. Hepatotoxicity is also a drug‐related toxicity, and patients commonly experience gastrointestinal symptoms (Vermes 2000). These serious adverse effects are uncommon with current dosing recommendations (Loyse 2013a). Flucytosine is practical because of oral availability but is expensive and unavailable in most high‐burden, resource‐limited settings and unregistered throughout Africa (Loyse 2013b).
Fluconazole, an azole drug, is recommended as maintenance therapy for cryptococcal meningitis. However, it is often used for induction therapy in resource‐limited settings due to its low cost (including through a donation programme) and oral formulation. Fluconazole monotherapy is associated with very high mortality compared to amphotericin‐based regimens (Bicanic 2006; Schaars 2006; Rothe 2013). Fluconazole demonstrates weak Cryptococcus spp. killing activity compared to amphotericin, although dose escalation studies show improved fungal killing at high doses without a significant increase in drug‐related side effects (Bicanic 2007; Longley 2008; Milefchik 2008).
Several adjunctive drugs have also been evaluated for the treatment of cryptococcal meningitis in combination with traditional antifungal therapies. These include therapies with immunomodulatory functions such as dexamethasone and interferon‐gamma, acetazolamide to lower intracranial pressure, and sertraline, an antidepressant that also demonstrates antifungal activity against Cryptococcus spp. in in vitro and in animal models (Jarvis 2012; Zhai 2012; Beardsley 2016; Rhein 2016).
In addition to the choice and dose of drug therapy, a number of other factors influence outcomes of treatment for HIV‐associated cryptococcal meningitis. The accumulation of yeast or shed capsular polysaccharides may obstruct CSF reabsorption at arachnoid granulations, leading to elevated intracranial pressure (Loyse 2010). Elevated intracranial pressure is independently associated with mortality, and therapeutic LP after initial diagnostic LP may decrease the risk of death (Bicanic 2009; Meda 2014; Rolfes 2014). Standard electrolyte supplementation and IV fluids with amphotericin therapy may also decrease mortality (Bahr 2014). Furthermore, several randomized controlled trials (RCTs) have demonstrated reduced mortality when ART is delayed for several weeks with either amphotericin‐ or fluconazole‐based regimens, which is possibly explained by early immune reconstitution with ART causing deleterious inflammation in response to living or dead yeast components within the contained CNS space (Makadzange 2010; Bisson 2013; Boulware 2014b).
How the intervention might work
Antifungal therapies with activity against Cryptococcus spp. act by various mechanisms (Table 22). Fluconazole and other azoles inhibit biosynthesis of ergosterol, which is essential in fungal cell membranes; polyenes bind to fungal membrane sterols and disrupt cell membranes; and flucytosine is a pyrimidine analog that blocks fungal ribonucleic acid (RNA) and protein biosynthesis. Among adjunctive treatments, sertraline is fungicidal against Cryptococcus spp. in in vitro and animal studies, although the mechanism of action is unclear. Other adjunctive therapies may improve outcomes through modulation of the immune response or by lowering elevated intracranial pressure.
1. Overview of drugs evaluated for the treatment of HIV‐associated cryptococcal meningitis.
| Class | Mechanism of action | Drug examples for cryptococcal meningitis therapy | Major class side effects |
| Polyenes | Disrupt cell membranes | Amphotericin B (liposomal or non‐liposomal formulations) | Nephrotoxicity, electrolyte abnormalities, anaemia |
| Azoles | Inhibit ergosterol biosynthesis | Fluconazole, voriconazole, itraconazole | Hepatotoxicity, drug‐drug interactions, GI symptoms, rash |
| Pyrimidine analogue | Inhibit fungal RNA and protein biosynthesis | Flucytosine | Bone marrow suppression, hepatotoxicity, GI symptoms |
| Glucocorticoids | Various anti‐inflammatory effects | Dexamethasone | Hyperglycaemia, bleeding, psychiatric effects, secondary hypoaldosteronism, immunosuppression |
| Selective serotonin reuptake inhibitor (SSRI) | 5‐hydroxytryptamine transporter inhibitor, antifungal mechanism of action unclear | Sertraline | Neurocognitive effects, GI symptoms |
| Carbonic anhydrase inhibitor | Reduces intracranial pressure by reducing cerebrospinal fluid production, likely through multiple mechanisms | Acetazolamide | Metabolic acidosis, nephrolithiasis, aplastic anaemia, GI symptoms, paraesthesias |
Abbreviations: GI: gastrointestinal; RNA: ribonucleic acid
Why it is important to do this review
HIV‐associated cryptococcal meningitis is a significant cause of mortality in HIV‐positive individuals, particularly people with advanced HIV disease in resource‐limited settings, and results in up to 15% of HIV‐related deaths (Rajasingham 2017). Although guidelines exist for the antifungal management of cryptococcal meningitis, recommendations are based on limited data from RCTs, and in clinical practice treatment is highly variable due to drug costs, availability, and ability to monitor and manage drug‐related toxicities (Perfect 2010). A Cochrane Review on treatment for HIV‐associated cryptococcal meningitis was published in 2008, but a number of clinical trials comparing new induction regimens as well as several novel therapies have since been published (Sloan 2008).
Evaluation of multiple treatment regimens in predominately small, phase two clinical trials, and a large number of potential independent pairwise comparisons provides a rationale for including a network meta‐analysis (NMA) in a systematic review of these treatment regimens. Furthermore, given the lack of availability or affordability of widely accepted first‐line two‐week AmB and 5FC combination therapy in most resource‐limited settings, which have the overwhelming burden of disease, a ranking of alternative regimens by NMA will support the development of evidence‐based guidance of best treatment options in the context of common resource constraints.
A previous systematic review and NMA on the treatment of HIV‐associated cryptococcal meningitis was published (Campbell 2015). However, the review combined data from RCTs with cohort and other non‐randomized trials, and included re‐treatment cases. Additionally, several RCTs evaluating short‐course induction regimens and novel adjunctive therapies have since been published. This Cochrane Review and NMA therefore incorporates new evidence to inform the best antifungal regimens for the treatment of HIV‐associated cryptococcal meningitis.
Objectives
To evaluate the best induction therapy to reduce mortality from HIV‐associated cryptococcal meningitis
To compare side effect profiles of different therapies
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs that included participants assigned to different induction regimens. We excluded non‐randomized studies.
Types of participants
Inclusion criteria
We included studies limited to HIV‐positive individuals with first episode of cryptococcal meningitis, diagnosed by positive CSF India ink stain, fungal culture, or cryptococcal antigen (CrAg) test. Inclusion was not limited by ART status at enrolment (ART naive or experienced). Studies could be unblinded, single‐blinded, or double‐blinded.
Exclusion criteria
We excluded studies that had re‐treatment cases, as these may be associated with antifungal resistance or may represent partially treated cases (Bicanic 2006). As a serum and plasma CrAg testing is not specific for CNS cryptococcal infection, we excluded any studies that included participants based on a positive blood test or clinical suspicion without microbiological confirmation of CSF. We also excluded cryptococcal antigen screening studies for cryptococcal meningitis prevention. We assessed the amount of and reasons for missing data in studies. We excluded studies with significant (> 20%) loss to follow‐up. We also excluded studies with significant imbalances between groups in clinically relevant parameters (such as baseline severity of disease or CD4 count).
Types of interventions
Interventions
Interventions included the following antifungal drugs or drug classes used for induction therapy: amphotericin B deoxycholate, liposomal/lipid formation amphotericin B, flucytosine, azole drugs, and adjunctive therapies (including but not limited to acetazolamide, dexamethasone, interferon‐gamma, and sertraline). We did not limit study inclusion by duration of induction therapy or by drug dosage.
Comparisons
We considered studies comparing any combination of the following interventions.
AmB deoxycholate for at least two weeks
AmB deoxycholate for less than two weeks
Liposomal/lipid complex AmB for at least two weeks
Liposomal/lipid complex AmB for less than two weeks
Azole drugs
5FC
Adjunctive therapies (for example, sertraline, dexamethasone, acetazolamide, interferon‐gamma (IFNg))
Types of outcome measures
Our primary treatment outcome was mortality. Our secondary outcomes were drug‐related adverse events and rate of fungal clearance. Early fungicidal activity (EFA), or mean rate of fungal clearance in the first two weeks of antifungal therapy, has been shown to correlate with mortality and is frequently reported in phase two studies that are not powered to detect mortality differences and to inform larger studies (Bicanic 2009).
Primary outcomes
-
Mortality, subdivided into:
short term (within two weeks)
medium term (within 10 weeks)
long term (up to six months)
Secondary outcomes
Mean rate of fungal clearance (early fungicidal activity) in the first two weeks of antifungal therapy.
Serious adverse events related to therapy: we described grade three (severe) and grade four (potentially life‐threatening) laboratory events according to Division of AIDS (DAIDS) definitions for main laboratory abnormalities associated with antifungal drugs (DAIDS 2014). We limited comparisons to anaemia, neutropenia, nephrotoxicity, hepatotoxicity (alanine aminotransferase (ALT) elevation), and hypokalaemia.
Search methods for identification of studies
We attempted to identify all potential studies regardless of language or publication status (published, unpublished, in press, and in progress). We restricted searches to studies published since 1 January 1980. We did not initially include search filters for study design.
Electronic searches
We searched the Cochrane Infectious Diseases Group Specialized Register, CENTRAL, MEDLINE (PubMed), Embase (Ovid), LILACS (BIREME), African Index Medicus, and Index Medicus for the South‐East Asia Region (IMSEAR) using the search strategy detailed in Appendix 1, including HIV‐related search terms, search terms for cryptococcal meningitis, and search terms for antifungal and adjunctive therapies. We also searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/search/en/), ClinicalTrials.gov (clinicaltrials.gov), and the ISRCTN registry (www.isrctn.com/) to identify ongoing trials, using ‘HIV', ‘AIDS', ‘cryptococcal meningitis', and ‘antifungal agents' as search terms. We searched from 1 January 1980 up to 9 July 2018 without language restriction.
Searching other resources
We searched for recent studies (1 July 2014 to 9 July 2018) of HIV‐associated cryptococcal meningitis from select conferences:
Conference on Retroviruses and Opportunistic Infections (CROI);
all conferences of the International AIDS Society (IAS);
International Conference on Cryptococcus and Cryptococcosis (ICCC);
Infectious Diseases Society of America (IDSA) IDWeek.
We checked the reference lists of all included studies. We contacted leading researchers to identify any unpublished data.
Data collection and analysis
Selection of studies
We aggregated articles that we had obtained from the electronic database via the search strategy and de‐duplicated in EndNote (EndNote 2013). Two review authors (MWT and AES) independently screened the titles and abstracts of these articles for potential eligibility. Two review authors (MWT and AES) obtained and assessed the full‐texts of potentially eligible articles, and both completed study eligibility forms (Appendix 2). Both review authors evaluated articles for possible duplicated reporting, and one review author (MWT) contacted study authors for additional information as needed. We resolved any discrepancies regarding eligibility of individual studies through discussion or through adjudication by additional review authors (NF and JNJ) when necessary. We documented search results using a flow diagram following PRISMA recommendations, including unique articles retrieved from the initial search, number of titles excluded by review of title and abstract, and number of articles retrieved in full text (Moher 2009). We listed excluded RCTs, with a brief justification for exclusion, in the ‘Characteristics of excluded studies' table. We did not exclude any studies based on language.
Data extraction and management
Two review authors (MWT and AES) independently used a standardized data extraction form to obtain study information, including data outlined in Appendix 3.
Assessment of risk of bias in included studies
We assessed risk of bias with the Cochrane ‘Risk of bias' tool (Higgins 2011). We graded studies as at either ‘low', ‘high', or ‘unclear risk' within seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and bias due to problems not covered elsewhere. We generated a ‘Risk of bias' summary figure for included studies.
Measures of treatment effect
In the analysis of RCTs, we displayed mortality at each mortality endpoint for interventions using forest plots. In each pair‐wise comparison, for dichotomous outcome measures (for example, two‐week mortality) we obtained risk ratios (RRs) with 95% confidence interval (CI). For continuous outcomes (for example, rate of fungal clearance), we calculated pairwise mean differences (MDs) with 95% CI.
Relative ranking
We generated ranking probabilities for each intervention, taking into account each possible rank and cumulative ranking probabilities. We summarized the cumulative ranking probabilities using the surface under the cumulative ranking area (SUCRA) values (Salanti 2011). The SUCRA value represents the probability that a treatment will present the best outcome with no uncertainty and was used to develop a hierarchy of treatments for HIV‐associated cryptococcal meningitis.
Unit of analysis issues
Our unit of analysis was HIV‐positive individuals treated for an initial episode of cryptococcal meningitis.
Cluster‐randomized trials
Cluster‐randomised trials were eligible for inclusion if methods were used that adjusted for clustering of data to analyse effect estimates at the level of the individual. We did not find studies with cluster‐randomized designs.
Cross‐over randomized clinical trials
We excluded any cross‐over randomized clinical trials.
Repeated observations on participants
We assessed individual‐level mortality outcomes for different periods of follow‐up including short‐term (two‐week), medium‐term (up to 10 weeks), and long‐term (up to six months) time frames.
Multiple treatment events
We excluded studies that included re‐treatment cases of HIV‐associated cryptococcal meningitis.
Trials with multiple treatment groups
Cryptococcal meningitis trials may compare more than two treatment groups. We collected data for all comparator arms and analysed multi‐arm trials as multiple separate two‐arm trials in pairwise meta‐analysis. We accounted for the correlation within multi‐arm trials in the NMA (see Data synthesis).
Dealing with missing data
We described missing participant, intervention, and outcome data within included studies. We contacted original investigators to request relevant missing data. For binary outcomes, we performed intention‐to‐treat analyses. We considered participants who were lost to follow‐up as alive after loss to follow‐up. Given the severity of the primary outcome (death) and high risk of bias, we excluded studies that had a significant loss to follow‐up within six months (> 20%). Loss to follow‐up for the 13 studies included in our analysis was very low (median < 1%) and believed to be unlikely to significantly bias results.
Assessment of heterogeneity
We considered the following potential causes of clinical and methodological heterogeneity: ART status, timing of ART, study settings (resource‐limited country versus high‐income country), inclusion of participants with abnormal mental status (for example, Glasgow coma score less than 15), one or more LP after diagnostic LP (lowering of intracranial pressure), dosing of antifungal therapy, risk of bias (assessed by ‘Risk of bias' tool).
We evaluated statistical heterogeneity using the tau2 and I2 values in both comparison‐specific and common heterogeneity pairwise meta‐analyses (see Data synthesis). We could not evaluate statistical heterogeneity through visual inspection of forest plots, subgrouping, or meta‐regression, as there were few studies contributing to each comparison.
Assessment of transitivity and inconsistency
The validity of NMA relies on the assumption of transitivity – that the sets of trials in each comparison are similar other than the intervention, allowing meaningful indirect comparisons of interventions that were not evaluated in the same study. We evaluated transitivity by assessing the distribution of effect modifiers across studies. We also examined inconsistency, the statistical agreement between direct and indirect estimates.
We evaluated local consistency between direct and indirect comparisons using the node‐splitting and loop‐specific approaches, and global consistency using design‐by‐treatment interaction modelling, which integrates loop inconsistency with consideration of design inconsistency (Dias 2010; Higgins 2012). If we identified inconsistency, we more closely examined the potential effect modifiers in inconsistent loops and conducted a sensitivity analysis excluding studies that may be sources of inconsistency.
Potential effect modifiers included the following.
ART status
Timing of ART
Study settings (resource‐limited country versus high‐income country)
Inclusion of participants with abnormal mental status (for example, Glasgow coma score less than 15)
One or more LP after diagnostic LP (lowering of intracranial pressure)
Dosing of antifungal therapies
Assessment of reporting biases
We planned to assess reporting bias by examining asymmetry in funnel plots of pairwise comparisons that had a sufficient number of studies (at least 10), however there were too few studies for this analysis. We planned to assess reporting bias across the network using comparison‐adjusted funnel plots (Chaimani 2013). As we were not able to identify a meaningful treatment order and there were too few studies per comparison, we did not carry out this analysis.
Data synthesis
We first conducted pairwise meta‐analyses for all direct comparisons using random‐effects models. We conducted these analyses assuming both comparison‐specific heterogeneity (that is, each direct comparison has a separate heterogeneity estimate) and a common heterogeneity across comparisons to determine tau2 and I2 values. We performed pairwise meta‐analyses using the ‘metan' (for the comparison‐specific heterogeneity analysis) and ‘mvmeta' (for the common heterogeneity analysis) commands in Stata (Version 13) (Stata 2013) and tools in Review Manager 5 (RevMan 2014).
We then performed NMA, combining direct outcomes data within studies and indirect data across studies, to compare primary and secondary outcomes from multiple interventions. We performed NMA following the multivariate meta‐regression approach (White 2011; White 2012). We used random‐effects models that account for within‐study correlation of multi‐arm trials (Lu 2006). We modelled treatment differences (log risk ratios for binary outcomes and mean differences for continuous outcomes) and treatment rankings. We performed network meta‐analyses using the ‘network' suite of commands in Stata (Version 13) (White 2015).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis to examine sources of heterogeneity in studies when sufficient data were available, including: ART status, timing of ART, study settings (resource‐limited country versus high‐income country), inclusion of participants with abnormal mental status (for example, Glasgow coma score less than 15), one or more LP after diagnostic LP (lowering of intracranial pressure), dosing of amphotericin, and dosing of azole drug.
These analyses were limited due to the small number of included studies and general lack of heterogeneity in the above factors.
Sensitivity analysis
For studies with missing data or reporting of per‐protocol results on mortality, we planned to perform sensitivity analyses imputing missing data under the best and worst cases (no missing participants experience the event or all missing participants experience the event, respectively), but did not perform these analyses as the amount of missing data was minimal in most included studies. We excluded studies with > 20% missing mortality outcome data within six months.
Presentation of results
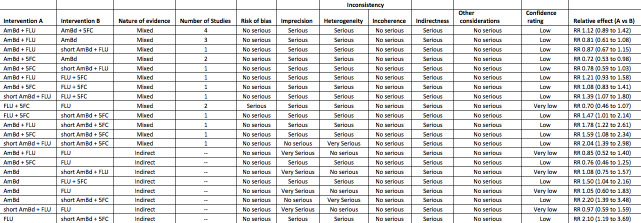
We presented both a pairwise comparison analysis to show pooled effect estimates and certainty of the evidence for all the direct comparisons, and a NMA to demonstrate the relative ranking of each comparison. We reported pairwise estimates of treatment effects in tables (for example, RRs and 95% CI for mortality and culture clearance outcomes and MDs and 95% CI for rate of fungal clearance) from both pairwise meta‐analyses and network meta‐analyses along with a table of probability rankings based on SUCRA. We generated a ‘Summary of findings' table with mortality outcomes by intervention group (absolute risk and risk ratio) along with the number of studies and participants assigned to intervention arm, certainty of the evidence (GRADE), and additional comments as indicated. We also presented a GRADE assessment using the CINeMA tool, which is based on modified approach for NMA (Salanti 2014; CINeMA 2017). We limited GRADE assessment to 10‐week mortality for pairwise comparisons and the NMA. The modified GRADE criteria we used were as follows.
GRADE assessment
Study limitations
Serious limitations: comparison dominated by evidence at unclear risk of bias. Downgraded by one level.
Imprecision
Serious limitations: CI extends into clinically unimportant effects. Downgraded by one level.
Very serious limitations: CI extends into clinically unimportant effects in both directions. Downgraded by two levels.
Inconsistency
Serious limitations: predictive interval for treatment effect includes effects which change interpretation (neither intervention favoured). Downgraded by one level.
Indirectness
Serious limitations: insufficient evidence for the plausibility of the transitivity assumption. Downgraded by one level. In the presence of serious or very serious limitations due to inconsistency, downgraded jointly with inconsistency.
Results
Description of studies
Results of the search
We identified 1086 records of potentially eligible studies from the electronic searches, and five records through additional sources. From these, 13 were included in the review. The study selection process is presented in Figure 1.
1.
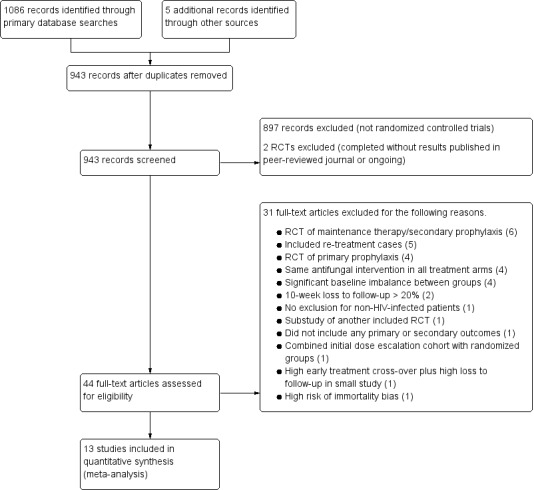
Study flow diagram.
Included studies
Setting
We identified 13 RCTs that met our inclusion criteria. Most (11 of 13) were from resource‐limited settings in sub‐Saharan Africa or Southeast Asia. One study was from North America (van der Horst 1997), and one small study was from the Netherlands and Australia (Leenders 1997). One study was conducted in the USA and Thailand, with 70% of participants recruited from Thailand (Pappas 2009). One study, conducted in Malawi, Zambia, Tanzania, and Cameroon, contributed to 10 pairwise comparisons and was the only study for nine of these comparisons (Molloy 2018).
Participants and study procedures
All studies were carried out in adults. Studies were similar with respect to key characteristics that are known to influence mortality. Early initiation of ART has been associated with higher mortality in HIV‐positive patients with cryptococcal disease due to immune reconstitution inflammatory syndrome (Boulware 2014b; Makadzange 2010). In all studies that specified timing of ART initiation, ART was delayed for a minimum of two weeks. No study excluded people with evidence of altered mental status, for example depressed Glasgow coma scale score, which is associated with worse outcomes (Jarvis 2014). In all but one study (Mayanja‐Kizza 1998), patients were scheduled to receive at least one additional LP after diagnostic LP during induction, an intervention that reduces intracranial pressure and may improve survival (Bicanic 2009;Meda 2014;Rolfes 2014). Use of combined ART at the time of cryptococcal meningitis diagnosis was uncommon in most studies (< 10%); however, the three most recently conducted studies found 32% to 59% of patients with reported ART exposure at the time of enrolment. Loss to follow‐up was uncommon in most studies, ranging from 0 to 16% (median 2%) of participants.
Interventions
All studies evaluating amphotericin B deoxycholate‐based therapy (10 of 13 included studies) used the World Health Organization recommended doses of 0.7 to 1.0 mg/kg/day (WHO 2011). Dose of fluconazole ranged from 200 mg/day to 1200 mg/day. Flucytosine dose was 100 mg/kg/day in four divided doses in all studies that used this drug except one (Mayanja‐Kizza 1998), which used 150 mg/kg/day in three divided doses. Details of the included studies are presented in the Characteristics of included studies tables.
Outcomes
Mortality
Twenty‐one direct comparisons for mortality were made in 13 studies. All studies that reported two‐week mortality also followed participants for a minimum of 10 weeks from enrolment. Four studies reported six‐month mortality.
Early fungicidal activity
Nine included studies provided mean early fungicidal activity (EFA) values that compared 13 interventions.
DAIDS grade three/four laboratory toxicities
Eight of 13 studies reported laboratory toxicities according to the DAIDS classification system: eight reported anaemia; eight reported renal dysfunction; seven reported neutropenia; seven reported hypokalaemia; and seven reported transaminase elevation.
Excluded studies
After de‐duplication reduced the number of studies to 943, we excluded 897 studies that were not RCTs and two studies that were completed without results published in a peer‐reviewed journal. We excluded an additional 31 studies based on full‐text review for reasons described in the Characteristics of excluded studies table, including four studies for significant baseline imbalances between treatment groups and two for > 20% loss to follow‐up.
Risk of bias in included studies
Risk of bias for the 13 included studies is summarized in Figure 2 and Figure 3.
2.
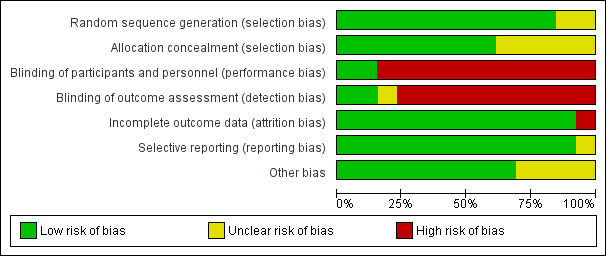
‘Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
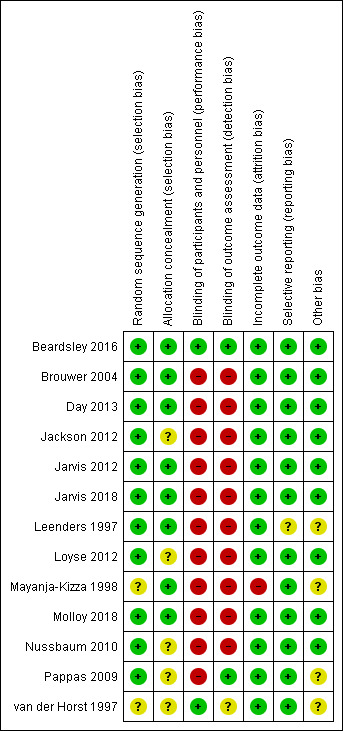
‘Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All trials were randomized, but two studies did not state whether random sequence generation was used and were therefore assessed as at unclear risk of bias (van der Horst 1997; Mayanja‐Kizza 1998). Similarly, five studies were at unclear bias for allocation concealment because the method of allocation concealment was not clearly stated (van der Horst 1997; Pappas 2009; Nussbaum 2010; Jackson 2012; Loyse 2012).
Blinding
Most studies did not blind participants, personnel, and outcome assessors and were at high risk of bias for both domains. Only one study was at low risk of bias for both domains (Beardsley 2016). We did not downgrade GRADE assessments for performance or detection bias for the other included studies, as we judged that measurement of the primary outcome (mortality) was unlikely to be biased due to lack of blinding.
Incomplete outcome data
All but one study was at low risk of attrition bias due to very low loss to follow‐up. One included study was at high risk of bias due to 14% (8/58) loss to follow‐up by six months (Mayanja‐Kizza 1998).
Selective reporting
All but one study was at low risk of reporting bias and reported on all prespecified primary and secondary outcomes. One study was at unclear risk of reporting bias because primary and secondary outcomes were not clearly stated, and the authors reported results on multiple clinical and mycological outcomes (Leenders 1997).
Other potential sources of bias
Four studies had direct support from pharmaceutical manufacturers of study drugs, or authors received research support from manufacturers without the role of the drug companies clearly stated and were therefore assessed as at unclear risk of other potential sources of bias (Leenders 1997; van der Horst 1997; Mayanja‐Kizza 1998; Pappas 2009).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16; Table 17; Table 18; Table 19; Table 20; Table 21
Summary of findings for the main comparison. One week of AmBd + 5FC compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis.
| One week of AmBd + 5FC compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 1 week of AmBd + 5FC Comparison: 2 weeks of AmBd + 5FC | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + 5FC | Risk with 1 week of AmBd + 5FC | ||||
| Mortality: 10 weeks | 383 per 1000 | 237 per 1000 (161 to 356) | RR 0.62 (0.42 to 0.93) | 228 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 2. One week of AmBd + 5FC compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis.
| One week of AmBd + 5FC compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 1 week of AmBd + 5FC Comparison: 2 weeks of AmBd + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + FLU | Risk with 1 week of AmBd + 5FC | ||||
| Mortality: 10 weeks | 412 per 1000 | 239 per 1000 (161 to 355) | RR 0.58 (0.39 to 0.86) | 227 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 3. One week of AmBd + 5FC compared to one week of AmBd + FLU for HIV‐associated cryptococcal meningitis.
| One week of AmBd + 5FC compared to one week of AmBd + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 1 week of AmBd + 5FC Comparison: 1 week of AmBd + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 1 week of AmBd + FLU | Risk with 1 week of AmBd + 5FC | ||||
| Mortality: 10 weeks | 486 per 1000 | 238 per 1000 (165 to 350) | RR 0.49 (0.34 to 0.72) | 224 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 4. One week of AmBd + 5FC compared to two weeks of 5FC + FLU for HIV‐associated cryptococcal meningitis.
| One week of AmBd + 5FC compared to two weeks of 5FC + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: Randomized controlled trial Intervention: 1 week of AmBd + 5 FC Comparison: 2 weeks of 5FC + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of 5FC + FLU | Risk with 1 week of AmBd + 5FC | ||||
| Mortality: 10 weeks | 351 per 1000 | 239 per 1000 (165 to 348) | RR 0.68 (0.47 to 0.99) | 338 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 5. Two weeks of 5FC + FLU compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis.
| Two weeks of 5FC + FLU compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of 5FC + FLU Comparison: 2 weeks of AmBd + 5FC | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + 5FC | Risk with 2 weeks of 5FC + FLU | ||||
| Mortality: 10 weeks | 383 per 1000 | 352 per 1000 (264 to 471) | RR 0.92 (0.69 to 1.23) | 340 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 6. Two weeks of 5FC + FLU compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis.
| Two weeks of 5FC + FLU compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of 5FC + FLU Comparison: 2 weeks of AmBd + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + FLU | Risk with 2 weeks of 5FC + FLU | ||||
| Mortality: 10 weeks | 412 per 1000 | 350 per 1000 (264 to 466) | RR 0.85 (0.64 to 1.13) | 339 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 7. Two weeks of AmBd + 5FC compared to two weeks of AmBd for HIV‐associated cryptococcal meningitis.
| Two weeks of AmBd + 5FC compared to two weeks of AmBd for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of AmBd + 5FC Comparison: 2 weeks of AmBd | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd | Risk with 2 weeks of AmBd + 5FC | ||||
| Mortality: 10 weeks | 409 per 1000 | 270 per 1000 (188 to 388) | RR 0.66 (0.46 to 0.95) | 231 (2 RCTs) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from two studies with few events.
Summary of findings 8. Two weeks of AmBd + FLU compared to two weeks of AmBd for HIV‐associated cryptococcal meningitis.
| Two weeks of AmBd + FLU compared to two weeks of AmBd for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of AmBd + FLU Comparison: 2 weeks of AmBd | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd | Risk with 2 weeks of AmBd + FLU | ||||
| Mortality: 10 weeks | 338 per 1000 | 317 per 1000 (186 to 547) | RR 0.94 (0.55 to 1.62) | 371 (3 RCTs) | ⊕⊕⊝⊝ LOW1,2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for indirectness. Some participants received a lower dose of fluconazole than currently recommended in combination with AmBd. Pappas 2009 excluded from enrolment patients who were not expected to survive two weeks, so the study population may not be representative of general patients with cryptococcal meningitis. 2Downgraded one level for imprecision. Few events with broad CI including appreciable benefit and appreciable harm.
Summary of findings 9. Two weeks of AmBd + 5FC compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis.
| Two weeks of AmBd + 5FC compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of AmBd + 5FC Comparison: 2 weeks of AmBd + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + FLU | Risk with 2 weeks of AmBd + 5FC | ||||
| Mortality: 10 weeks | 355 per 1000 | 320 per 1000 (245 to 423) | RR 0.90 (0.69 to 1.19) | 538 (4 RCTs) | ⊕⊕⊝⊝ LOW1,2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for indirectness. Some participants received a lower dose of fluconazole than is currently recommended in combination with AmBd. A few participants in one study also received a different azole drug, voriconazole. 2Downgraded one level for imprecision. Few overall events.
Summary of findings 10. Two weeks of AmBd + FLU + steroids compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis.
| Two weeks of AmBd + FLU + steroids compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of AmBd + FLU + steroids Comparison: 2 weeks of AmBd + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + FLU | Risk with 2 weeks of AmBd + FLU + steroids | ||||
| Mortality: 10 weeks | 412 per 1000 | 473 per 1000 (383 to 584) | RR 1.15 (0.93 to 1.42) | 450 (1 RCT) | ⊕⊕⊕⊕ HIGH |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
Summary of findings 11. One week of AmBd + FLU compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis.
| One week of AmBd + FLU compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 1 week of AmBd + FLU Comparison: 2 weeks of AmBd + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + FLU | Risk with 1 week of AmBd + FLU | ||||
| Mortality: 10 weeks | 412 per 1000 | 486 per 1000 (363 to 651) | RR 1.18 (0.88 to 1.58) | 225 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 12. One week of AmBd + FLU compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis.
| One week of AmBd + FLU compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 1 week of AmBd + FLU Comparison: 2 weeks of AmBd + 5FC | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + 5FC | Risk with 1 week of AmBd + FLU | ||||
| Mortality: 10 weeks | 383 per 1000 | 486 per 1000 (360 to 658) | RR 1.27 (0.94 to 1.72) | 226 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 13. One week of AmBd + FLU compared to two weeks of 5FC + FLU for HIV‐associated cryptococcal meningitis.
| One week of AmBd + FLU compared to two weeks of 5FC + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 1 week of AmBd + FLU Comparison: 2 weeks of 5FC + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of 5FC + FLU | Risk with 1 week of AmBd + FLU | ||||
| Mortality: 10 weeks | 351 per 1000 | 488 per 1000 (376 to 632) | RR 1.39 (1.07 to 1.80) | 336 (1 RCT) | ⊕⊕⊕⊝ MODERATE1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for imprecision. Data from a single study with few events.
Summary of findings 14. Two weeks of FLU compared to two weeks of 5FC + FLU for HIV‐associated cryptococcal meningitis.
| Two weeks of FLU compared to two weeks of 5FC + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of FLU Comparison: 2 weeks of 5FC + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of 5FC + FLU | Risk with 2 weeks of FLU | ||||
| Mortality: 10 weeks | 392 per 1000 | 573 per 1000 (376 to 875) | RR 1.46 (0.96 to 2.23) | 98 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW1,2,3 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for risk of bias. High loss to follow‐up by 10 weeks. 2Downgraded one level for indirectness. Participants in one study received a lower dose of fluconazole than is recommended and a higher dose of flucytosine. 3Downgraded two levels for imprecision. Data from two small studies with few events.
Summary of findings 15. Two weeks of L‐AmB compared to two weeks of AmBd for HIV‐associated cryptococcal meningitis.
| Two weeks of L‐AmB compared to two weeks of AmBd for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of L‐AmB Comparison: 2 weeks of AmBd | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd | Risk with 2 weeks of L‐AmB | ||||
| Mortality: 10 weeks | 154 per 1000 | 66 per 1000 (6 to 654) | RR 0.43 (0.04 to 4.25) | 28 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1,2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: AmBd: amphotericin B deoxycholate; CI: confidence interval; L‐AmB: liposomal amphotericin B; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for risk of bias. Study sponsored by study drug manufacturer, and role of funder not stated. Mortality also not stated as primary outcome of interest. 2Downgraded two levels for imprecision. Data from single small study with few events and broad CI including appreciable benefit and appreciable harm.
Summary of findings 16. Short‐course L‐AmB + FLU compared to two weeks of L‐AmB + FLU for HIV‐associated cryptococcal meningitis.
| Short‐course L‐AmB + FLU compared to two weeks of L‐AmB + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: short‐course L‐AmB + FLU Comparison: 2 weeks of L‐AmB + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of L‐AmB + FLU | Risk with short‐course L‐AmB + FLU | ||||
| Mortality: 10 weeks | 286 per 1000 | 294 per 1000 (134 to 643) | RR 1.03 (0.47 to 2.25) | 79 (1 RCT) | ⊕⊕⊝⊝ LOW1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: CI: confidence interval; FLU: fluconazole; L‐AmB: liposomal amphotericin B; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded two levels for imprecision. Data from a single small study with few events and broad CI including appreciable benefit and appreciable harm.
Summary of findings 17. Two weeks of AmBd + 5FC + FLU compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis.
| Two weeks of AmBd + 5FC + FLU compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of AmBd + 5FC + FLU Comparison: 2 weeks of AmBd + 5FC | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + 5FC | Risk with 2 weeks of AmBd + 5FC + FLU | ||||
| Mortality: 10 weeks | 67 per 1000 | 187 per 1000 (22 to 1000) | RR 2.81 (0.33 to 24.16) | 31 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for indirectness. Data generated from a single study that used a low dose of fluconazole. 2Downgraded two levels for imprecision. Data from a single small study with few events and broad CI including appreciable benefit and appreciable harm.
Summary of findings 18. Two weeks of AmBd + 5FC + FLU compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis.
| Two weeks of AmBd + 5FC + FLU compared to two weeks of AmBd + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of AmBd + 5FC + FLU Comparison: 2 weeks of AmBd + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + FLU | Risk with 2 weeks of AmBd + 5FC + FLU | ||||
| Mortality: 10 weeks | 438 per 1000 | 188 per 1000 (57 to 599) | RR 0.43 (0.13 to 1.37) | 32 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1,2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for indirectness. Data generated from a single small study that used a lower dose of fluconazole than is currently recommended. 2Downgraded two levels for imprecision. Data generated from a single small study with very few events.
Summary of findings 19. Two weeks of AmBd + 5FC + FLU compared to two weeks of AmBd for HIV‐associated cryptococcal meningitis.
| Two weeks of AmBd + 5FC + FLU compared to two weeks of AmBd for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of AmBd + 5FC + FLU Comparison: 2 weeks of AmBd | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd | Risk with 2 weeks of AmBd + 5FC + FLU | ||||
| Mortality: 10 weeks | 188 per 1000 | 188 per 1000 (45 to 793) | RR 1.00 (0.24 to 4.23) | 32 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1,2 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded one level for indirectness. Data generated from a single study that used a low dose of fluconazole. 2Downgraded two levels for imprecision. Data from a single small study with few events and broad CI including appreciable benefit and appreciable harm.
Summary of findings 20. One week of AmBd + 5FC + FLU compared to one week of AmBd + FLU for HIV‐associated cryptococcal meningitis.
| One week of AmBd + 5FC + FLU compared to one week of AmBd + FLU for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 1 week of AmBd + 5FC + FLU Comparison: 1 week of AmBd + FLU | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 1 week of AmBd + FLU | Risk with 1 week of AmBd + 5FC + FLU | ||||
| Mortality: 10 weeks | 350 per 1000 | 301 per 1000 (122 to 735) | RR 0.86 (0.35 to 2.10) | 40 (1 RCT) | ⊕⊕⊝⊝ LOW1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; FLU: fluconazole; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded two levels for imprecision. Data from a single small study with few events and broad CI including appreciable benefit and appreciable harm.
Summary of findings 21. Two weeks of AmBd + 5FC + IFNg compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis.
| Two weeks of AmBd + 5FC + IFNg compared to two weeks of AmBd + 5FC for HIV‐associated cryptococcal meningitis | |||||
| Patient or population: HIV‐infected individual with first episode of cryptococcal meningitis Setting: randomized controlled trial Intervention: 2 weeks of AmBd + 5FC + IFNg Comparison: 2 weeks of AmBd + 5FC | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with 2 weeks of AmBd + 5FC | Risk with 2 weeks of AmBd + 5FC + IFNg | ||||
| Mortality: 10 weeks | 323 per 1000 | 297 per 1000 (155 to 571) | RR 0.92 (0.48 to 1.77) | 88 (1 RCT) | ⊕⊕⊝⊝ LOW1 |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CI: confidence interval; IFNg: interferon gamma 1b; RCT: randomized controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
1Downgraded two levels for imprecision. Data generated from a single study with few events and broad CI that included appreciable benefit and appreciable harm.
Pairwise comparisons
The results we present below for pairwise comparisons are derived from our analysis assuming comparison‐specific heterogeneity in Review Manager 5. Our analyses in Stata assuming comparison‐specific and common heterogeneity produced similar results (results not shown).
One week of amphotericin B deoxycholate with flucytosine versus alternative regimens
One RCT conducted across nine centres in Malawi, Zambia, Tanzania, and Cameroon provided data for these comparisons (Molloy 2018). This study was designed as a non‐inferiority trial comparing one‐week amphotericin B deoxycholate (AmBd)‐based regimens, two‐week AmBd‐based regimens, and an all‐oral regimen of flucytosine (5FC) and fluconazole (FLU). Overall, 712 HIV‐positive adults with first episode of cryptococcal meningitis were randomized 2:1:1:1:1 to (i) two weeks of 5FC 100 mg/kg/day and FLU 1200 mg/day, (ii) two weeks of AmBd 1 mg/kg/day and FLU 1200 mg/day, (iii) two weeks of AmBd 1 mg/kg/day and 5FC 100 mg/kg/day, (iv) one week of AmBd 1 mg/kg/day and FLU 1200 mg/day followed on days 8 to 14 by FLU 1200 mg/day, or (v) one week of AmBd 1 mg/kg/day and 5FC 100 mg/kg/day followed by FLU 1200 mg/day on days 8 to 14.
Mortality
At 10 weeks, participants in the group receiving one week of AmBd and 5FC followed by FLU on days 8 to 14 had lower mortality than participants randomized to other induction therapies. At 10 weeks, there was a 38% reduced risk of death (risk ratio (RR) 0.62, 95% CI 0.42 to 0.93; 228 participants, 1 study, Analysis 1.1, Table 1: subgroup 2; moderate‐certainty evidence) compared to two weeks of AmBd and 5FC; a 42% reduced risk of death (RR 0.58, 95% CI 0.39 to 0.86; 227 participants, 1 study, Analysis 2.1, Table 2: subgroup 2; moderate‐certainty evidence) compared to two weeks of AmBd and FLU; a 51% reduced risk of death (RR 0.49, 95% CI 0.34 to 0.72; 224 participants, 1 study, Analysis 3.1, Table 3: subgroup 2; moderate‐certainty evidence) compared to one week of AmB and FLU followed by FLU on days 8 to 14; and a 32% reduced risk of death (RR 0.68, 95% CI 0.47 to 0.99; 338 participants, 1 study, Analysis 4.1, Table 4: subgroup 2; moderate‐certainty evidence) compared to two weeks of 5FC and FLU.
1.1. Analysis.
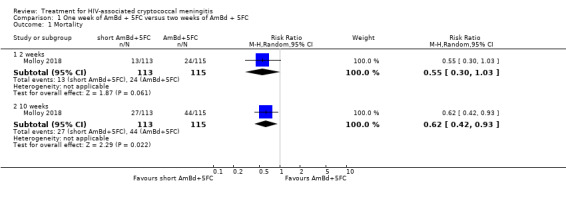
Comparison 1 One week of AmBd + 5FC versus two weeks of AmBd + 5FC, Outcome 1 Mortality.
2.1. Analysis.
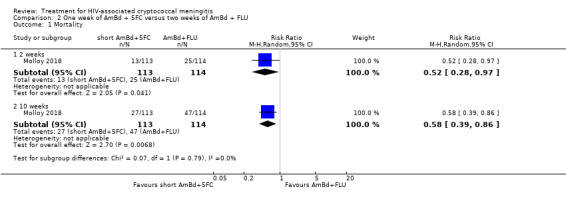
Comparison 2 One week of AmBd + 5FC versus two weeks of AmBd + FLU, Outcome 1 Mortality.
3.1. Analysis.
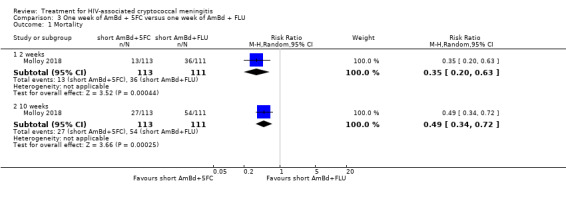
Comparison 3 One week of AmBd + 5FC versus one week of AmBd + FLU, Outcome 1 Mortality.
4.1. Analysis.
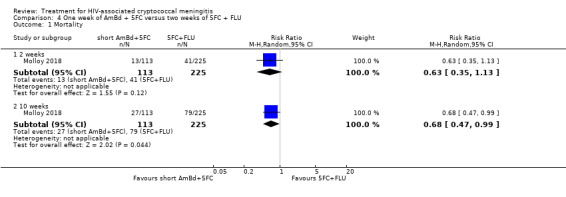
Comparison 4 One week of AmBd + 5FC versus two weeks of 5FC + FLU, Outcome 1 Mortality.
All data were generated from a single trial with few overall events (< 200). No direct comparisons were made to findings from high‐income settings, where mortality with two weeks of AmBd‐based induction regimens is observed to be lower (Saag 1991; van der Horst 1997).
Early fungicidal activity
One week of AmBd and 5FC followed by FLU on days 8 to 14 did not result in significantly lower EFA compared to two weeks of AmBd and 5FC (186 participants, 1 study, Analysis 1.2). The mean rate of fungal clearance was higher with one week of AmBd and 5FC followed by FLU on days 8 to 14 compared to two weeks of AmBd and FLU (mean difference (MD) ‐0.07 log10 CFU/mL/day, 95% CI ‐0.14 to 0.00; 192 participants, 1 study, Analysis 2.2); one week of AmBd and FLU followed by FLU on days 8 to 14 (MD ‐0.08 log10 CFU/mL/day, 95% CI ‐0.15 to ‐0.01; 179 participants, 1 study, Analysis 3.2); and two weeks of 5FC and FLU (MD ‐0.18 log10 CFU/mL/day, 95% CI ‐0.24 to ‐0.12; 280 participants, 1 study, Analysis 4.2).
1.2. Analysis.
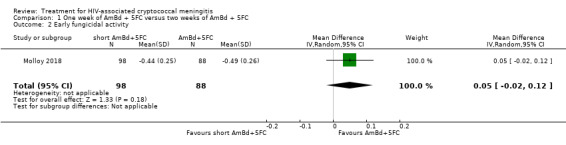
Comparison 1 One week of AmBd + 5FC versus two weeks of AmBd + 5FC, Outcome 2 Early fungicidal activity.
2.2. Analysis.
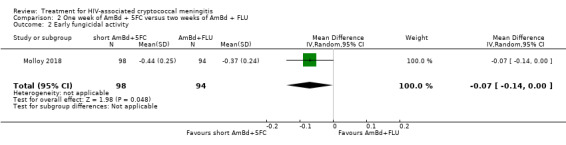
Comparison 2 One week of AmBd + 5FC versus two weeks of AmBd + FLU, Outcome 2 Early fungicidal activity.
3.2. Analysis.
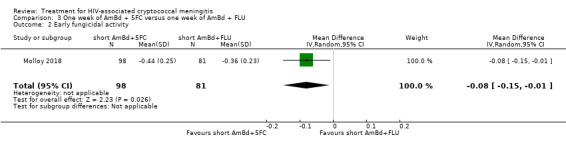
Comparison 3 One week of AmBd + 5FC versus one week of AmBd + FLU, Outcome 2 Early fungicidal activity.
4.2. Analysis.
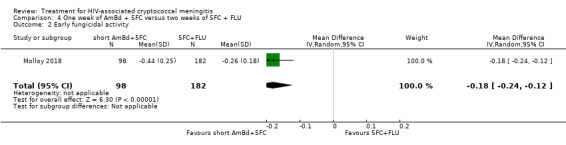
Comparison 4 One week of AmBd + 5FC versus two weeks of 5FC + FLU, Outcome 2 Early fungicidal activity.
DAIDS grade three/four events
Compared to two weeks of AmBd and 5FC, a one‐week regimen of AmBd and 5FC was associated with a lower risk of grade three or four anaemia (RR 0.31, 95% CI 0.16 to 0.60; 228 participants, 1 study, Analysis 1.3: subgroup 1). Compared to two weeks of AmBd and FLU, a one‐week regimen of AmBd and 5FC was associated with a lower risk of grade three or four anaemia (RR 0.36, 95% CI 0.18 to 0.71; 227 participants, 1 study, Analysis 2.3: subgroup 1) and renal toxicity (RR 0.29, 95% CI 0.10 to 0.85; 227 participants, 1 study, Analysis 2.3: subgroup 2). Compared to one week of AmBd and FLU, a one‐week regimen of AmBd and 5FC was associated with a lower risk of grade three or four anaemia (RR 0.41, 95% CI 0.21 to 0.82; 224 participants, 1 study, Analysis 3.3: subgroup 1). Compared to two weeks of 5FC and FLU, a one‐week regimen of AmBd and 5FC was associated with a higher risk of grade three or four hypokalaemia (RR 5.97, 95% CI 1.65 to 21.63; 338 participants, 1 study, Analysis 4.3: subgroup 4), but there was no difference with respect to other toxicities.
1.3. Analysis.
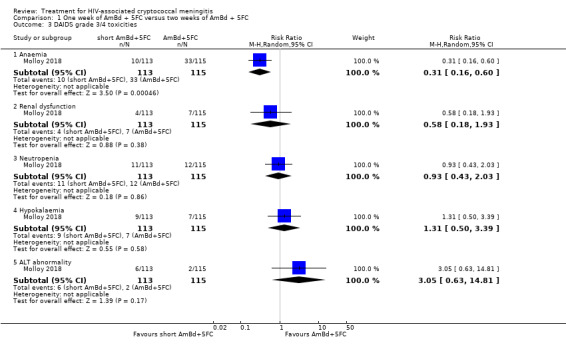
Comparison 1 One week of AmBd + 5FC versus two weeks of AmBd + 5FC, Outcome 3 DAIDS grade 3/4 toxicities.
2.3. Analysis.
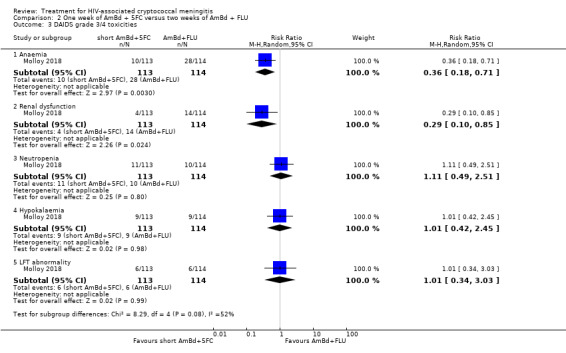
Comparison 2 One week of AmBd + 5FC versus two weeks of AmBd + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
3.3. Analysis.
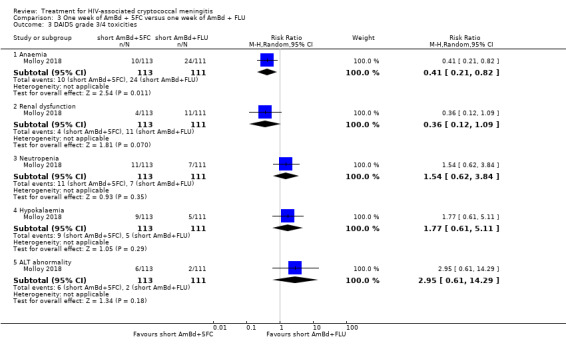
Comparison 3 One week of AmBd + 5FC versus one week of AmBd + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
4.3. Analysis.
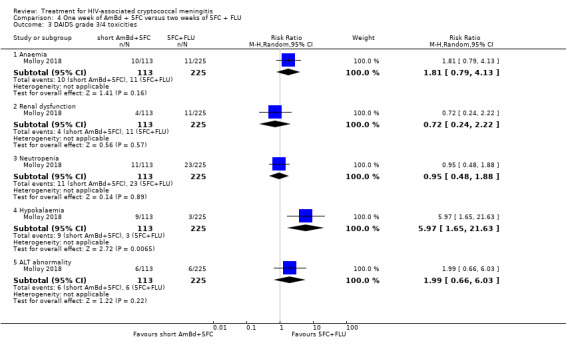
Comparison 4 One week of AmBd + 5FC versus two weeks of 5FC + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
Two weeks of oral flucytosine and fluconazole versus alternative regimens
One RCT from nine centres in Malawi, Zambia, Tanzania, and Cameroon compared two weeks of an all‐oral regimen of 5FC 100 mg/kg/day and FLU 1200 mg/day with several AmBd‐based induction regimens (Molloy 2018). This phase three study was designed as a non‐inferiority trial.
Mortality
At two weeks, there was no significant difference in mortality in participants randomized to two weeks of 5FC and FLU compared to participants randomized to two weeks of AmBd 1 mg/kg/day and 5FC 100 mg/kg/day (340 participants, 1 study, Analysis 5.1: subgroup 1) or two weeks of AmBd 1 mg/kg/day and FLU 1200 mg/day (339 participants, 1 study, Analysis 6.1: subgroup 1). By 10 weeks, there was no difference in mortality in the 5FC and FLU group compared to the group receiving two weeks of AmBd and 5FC (340 participants, 1 study, Analysis 5.1, Table 5: subgroup 2; moderate‐certainty evidence) or the group receiving two weeks of AmBd and FLU (339 participants, 1 study, Analysis 6.1, Table 6: subgroup 2; moderate‐certainty evidence).
5.1. Analysis.
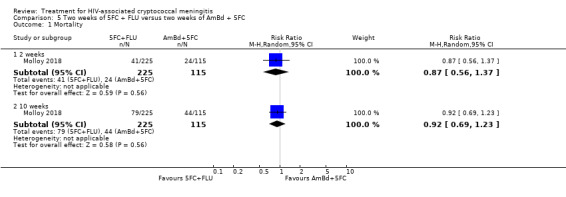
Comparison 5 Two weeks of 5FC + FLU versus two weeks of AmBd + 5FC, Outcome 1 Mortality.
6.1. Analysis.
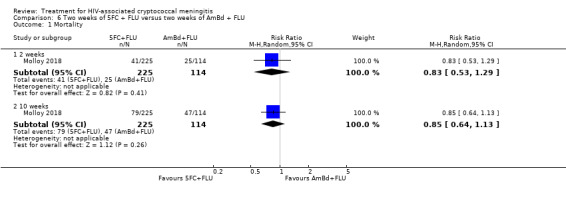
Comparison 6 Two weeks of 5FC + FLU versus two weeks of AmBd + FLU, Outcome 1 Mortality.
Early fungicidal activity
The mean rate of fungal clearance was lower with two weeks of 5FC and FLU than with two weeks of AmBd and 5FC (MD 0.23 log10 CFU/mL/day, 95% CI 0.17 to 0.29; 270 participants, 1 study, Analysis 5.2) as well as with two weeks of AmBd and FLU (MD 0.11 log10 CFU/mL/day, 95% CI 0.05 to 0.17; 276 participants, 1 study, Analysis 6.2).
5.2. Analysis.
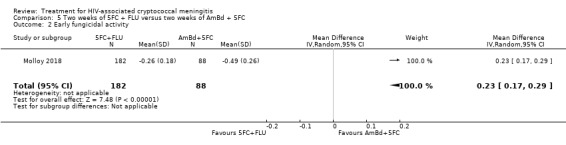
Comparison 5 Two weeks of 5FC + FLU versus two weeks of AmBd + 5FC, Outcome 2 Early fungicidal activity.
6.2. Analysis.
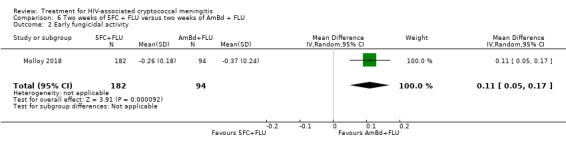
Comparison 6 Two weeks of 5FC + FLU versus two weeks of AmBd + FLU, Outcome 2 Early fungicidal activity.
DAIDS grade three/four events
The risk of DAIDS grade three or four anaemia (RR 0.17, 95% CI 0.09 to 0.32; 340 participants, 1 study, Analysis 5.3: subgroup 1) and hypokalaemia (RR 0.22, 95% CI 0.06 to 0.83; 340 participants, 1 study, Analysis 5.3: subgroup 4) was lower in the 5FC and FLU arm compared with the arm receiving two weeks of AmBd and 5FC. The risk of DAIDS anaemia (RR 0.20, 95% CI 0.10 to 0.39; 339 participants, 1 study, Analysis 6.3: subgroup 1) and hypokalaemia (RR 0.17, 95% CI 0.05 to 0.61; 339 participants, 1 study, Analysis 6.3: subgroup 4) was lower in the 5FC and FLU arm compared with the arm receiving two weeks of AmBd and FLU. The risk of renal toxicity (RR 0.40, 95% CI 0.19 to 0.85; 339 participants, 1 study, Analysis 6.3: subgroup 2) was also lower with 5FC and FLU compared to those receiving two weeks of AmBd and FLU.
5.3. Analysis.
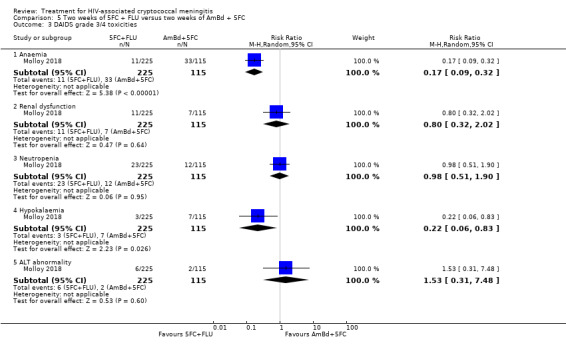
Comparison 5 Two weeks of 5FC + FLU versus two weeks of AmBd + 5FC, Outcome 3 DAIDS grade 3/4 toxicities.
6.3. Analysis.
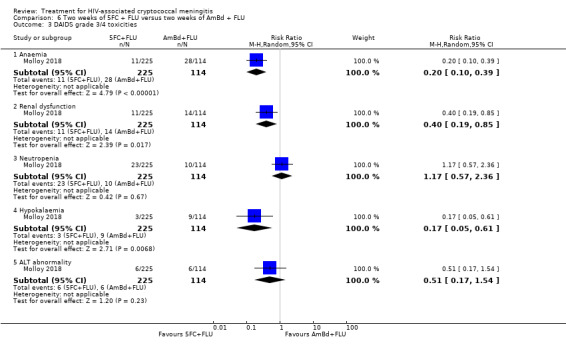
Comparison 6 Two weeks of 5FC + FLU versus two weeks of AmBd + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
Two weeks of amphotericin B deoxycholate and flucytosine versus two weeks of amphotericin B deoxycholate
Three studies compared induction with two weeks of AmBd and 5FC versus two or more weeks of AmBd alone. These included a small trial from Thailand (Brouwer 2004), a large trial from Vietnam (Day 2013), and a large trial from the USA (van der Horst 1997). The trial from the USA reported similar and very low mortality by two weeks in both groups (6%). After two weeks, investigators randomized participants who were clinically stable or improving to fluconazole or itraconazole consolidation. Due to risk of bias related to exclusion of participants who were not stable or improving, we did not evaluate outcomes beyond two weeks for this study. Amphotericin B deoxycholate alone was continued for four weeks in the study from Vietnam, but for two weeks in the other studies.
Mortality
At two weeks, there was a non‐significant reduction in mortality in participants who received combination AmBd and 5FC compared to AmBd alone (612 participants, 3 studies, Analysis 7.1: subgroup 1; Figure 4) but a significant mortality reduction at 10 weeks (RR 0.66, 95% CI 0.46 to 0.95; 231 participants, 2 studies, Analysis 7.1, Table 7: subgroup 2; Figure 4; moderate‐certainty evidence). The study from Vietnam followed participants up to six months and found a persistent survival benefit with combined AmBd and 5FC (RR 0.64, 95% CI 0.46 to 0.88; 199 participants, 1 study, Analysis 7.1: subgroup 3).
7.1. Analysis.
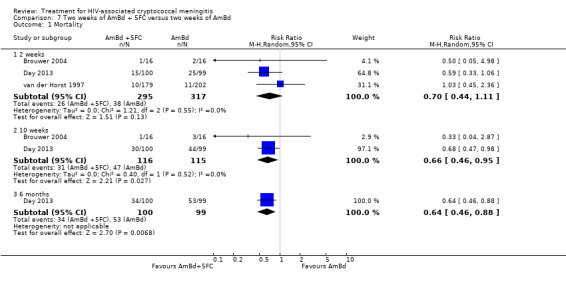
Comparison 7 Two weeks of AmBd + 5FC versus two weeks of AmBd, Outcome 1 Mortality.
4.
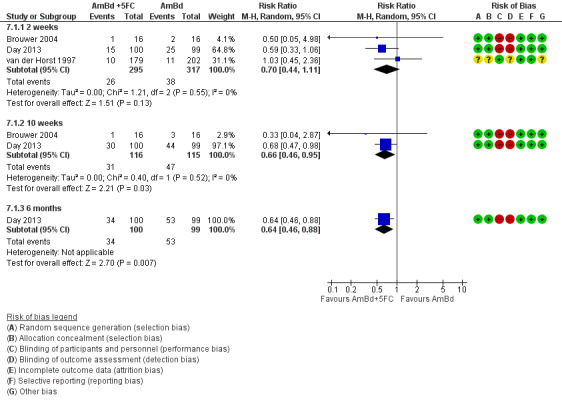
Forest plot of comparison: 7 Two weeks of AmBd + 5FC versus two weeks of AmBd, outcome: 7.1 Mortality.
Early fungicidal activity
Pooled data showed an improved rate of fungal clearance with combined AmBd and 5FC compared to AmBd alone (MD ‐0.15 log10 CFU/mL/day, 95% CI ‐0.26 to ‐0.04; 225 participants, 2 studies, Analysis 7.2; Figure 5).
7.2. Analysis.
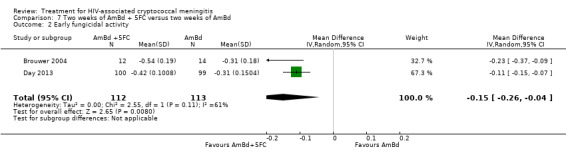
Comparison 7 Two weeks of AmBd + 5FC versus two weeks of AmBd, Outcome 2 Early fungicidal activity.
5.
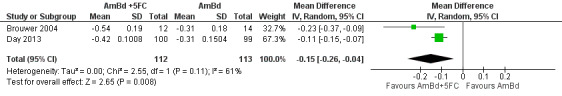
Forest plot of comparison: 7 Two weeks of AmBd + 5FC versus two weeks of AmBd, outcome: 7.2 Early fungicidal activity.
DAIDS grade three/four events
In one study that evaluated DAIDS toxicity (Day 2013), neutropenia was more common with combined AmBd and 5FC than with AmBd alone (RR 4.46, 95% CI 0.99 to 20.10; 199 participants, 1 study, Analysis 7.3: subgroup 3), but the risk of other toxicities did not differ between groups. The study combined elevated transaminases as a safety outcome but did not report abnormal alanine aminotransferase individually. In the AmBd and 5FC group, 6/100 experienced transaminitis versus 11/99 in the AmBd group.
7.3. Analysis.
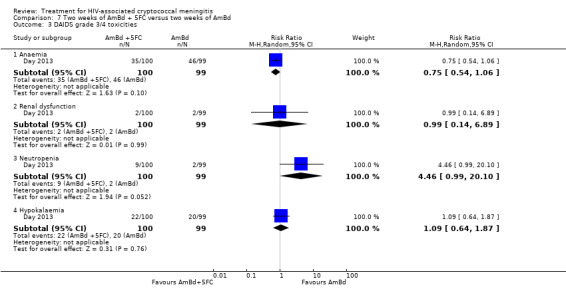
Comparison 7 Two weeks of AmBd + 5FC versus two weeks of AmBd, Outcome 3 DAIDS grade 3/4 toxicities.
Two weeks of amphotericin B deoxycholate and fluconazole versus two weeks of amphotericin B deoxycholate
Three studies compared two weeks of AmBd and FLU with at least two weeks of AmBd alone, including a small study from Thailand (Brouwer 2004), a large study from Vietnam (Day 2013), and a small study from the USA and Thailand (Pappas 2009). The study from Thailand used a low dose of FLU (400 mg/day) combined with AmBd, and the study from the USA and Thailand had one intervention with a low dose (400 mg/day) and one with a higher dose (800 mg/day) of FLU with AmBd. The study from Vietnam administered FLU at 800 mg/day. Amphotericin B deoxycholate alone was continued for four weeks in the Day 2013 study, but for two weeks in the other studies.
Mortality
No significant difference in mortality was observed at two weeks (371 participants, 3 studies, Analysis 8.1: subgroup 1; Figure 6) or 10 weeks (371 participants, 3 studies, Analysis 8.1, Table 8: subgroup 2; Figure 6; low‐certainty evidence) follow‐up. A single study found no significant improvement in survival with combined therapy compared to AmBd alone (198 participants, 1 study, Analysis 8.1: subgroup 3) with follow‐up to six months (Day 2013). Considering only participants who received AmBd with high‐dose FLU (the currently recommended dose of 800 mg/day or higher) versus AmBd alone, again no significant difference in mortality was observed at two weeks, 10 weeks, or six months.
8.1. Analysis.
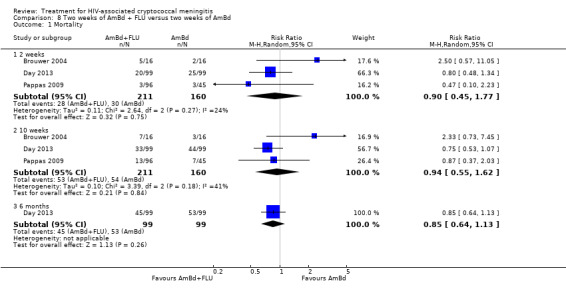
Comparison 8 Two weeks of AmBd + FLU versus two weeks of AmBd, Outcome 1 Mortality.
6.
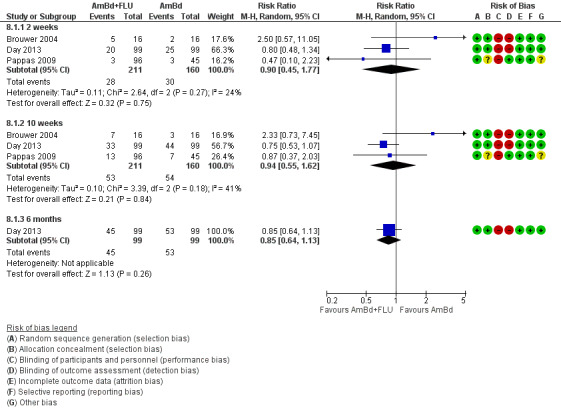
Forest plot of comparison: 8 Two weeks of AmBd + FLU versus two weeks of AmBd, outcome: 8.1 Mortality.
Early fungicidal activity
Based on pooled data from two studies, rate of fungal clearance did not differ between AmBd with FLU and AmBd arms (223 participants, 2 studies, Analysis 8.2; Figure 7).
8.2. Analysis.
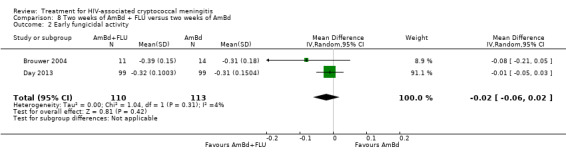
Comparison 8 Two weeks of AmBd + FLU versus two weeks of AmBd, Outcome 2 Early fungicidal activity.
7.
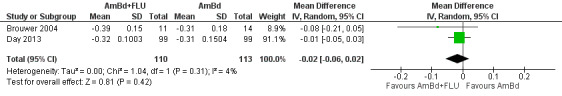
Forest plot of comparison: 8 Two weeks of AmBd + FLU versus two weeks of AmBd, outcome: 8.2 Early fungicidal activity.
DAIDS grade three/four events
Based on one study (Day 2013), risk of anaemia was lower with combined AmBd and FLU compared to AmBd alone (RR 0.63, 95% CI 0.43 to 0.91; 198 participants, 1 study, Analysis 8.3). However, participants randomized to AmBd alone received a longer dose of the drug compared to the combination AmBd and FLU arm (four weeks versus two weeks), which is a plausible explanation for this finding. The risk of other toxicities did not differ between groups. The study reported elevated transaminases but did not report abnormal alanine aminotransferase individually. In the AmBd and FLU group, 14/99 experienced transaminitis versus 11/99 in the AmBd group.
8.3. Analysis.
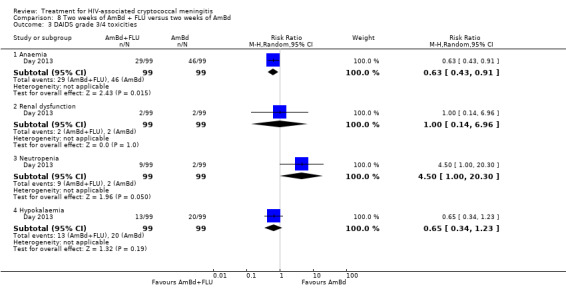
Comparison 8 Two weeks of AmBd + FLU versus two weeks of AmBd, Outcome 3 DAIDS grade 3/4 toxicities.
Two weeks of amphotericin B deoxycholate and flucytosine versus two weeks of amphotericin B deoxycholate and fluconazole
Four RCTs compared two weeks of AmBd and 5FC with two weeks of AmBd and FLU, including one small study from Thailand (Brouwer 2004), one large study from Vietnam (Day 2013), one small study from South Africa (Loyse 2012), and one large study from nine centres in Malawi, Zambia, Tanzania, and Cameroon (Molloy 2018). The South African study randomized participants to receive FLU 800 mg/day (22 participants), FLU 1200 mg/day (23 participants), or voriconazole 600 mg/day (13 participants) combined with AmBd, with azole therapies combined in the analysis. The Vietnam and multisite studies used FLU doses of 800 mg/day and 1200 mg/day, respectively. The Thailand study randomized some participants to low‐dose FLU (400 mg/day).
Mortality
Two weeks of AmBd and 5FC had a non‐significant survival benefit compared to two weeks of AmBd and FLU at two weeks (538 participants, 4 studies, Analysis 9.1: subgroup 1; Figure 8); at 10 weeks (538 participants, 4 studies, Analysis 9.1, Table 9: subgroup 2; Figure 8; low‐certainty evidence); and at six months (199 participants, 1 study, Analysis 9.1: subgroup 3; Figure 8).
9.1. Analysis.
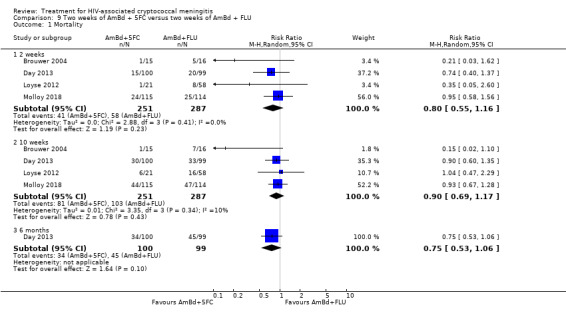
Comparison 9 Two weeks of AmBd + 5FC versus two weeks of AmBd + FLU, Outcome 1 Mortality.
8.
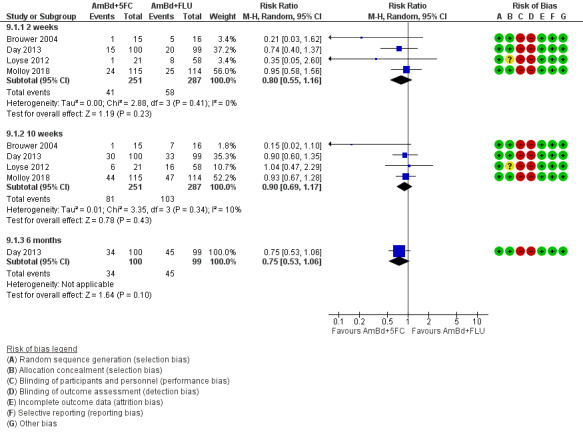
Forest plot of comparison: 9 Two weeks of AmBd + 5FC versus two weeks of AmBd + FLU, outcome: 9.1 Mortality.
Early fungicidal activity
The pooled mean rate of fungal clearance was better with two weeks of AmBd and 5FC than with two weeks of AmBd and FLU (MD ‐0.09 log10 CFU/mL/day, 95% CI ‐0.14 to ‐0.05; 474 participants, 4 studies, Analysis 9.2; Figure 9).
9.2. Analysis.
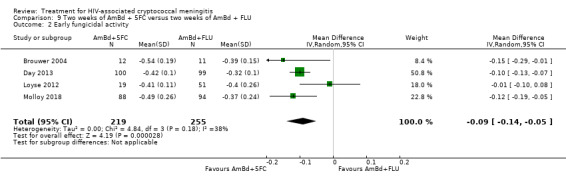
Comparison 9 Two weeks of AmBd + 5FC versus two weeks of AmBd + FLU, Outcome 2 Early fungicidal activity.
9.
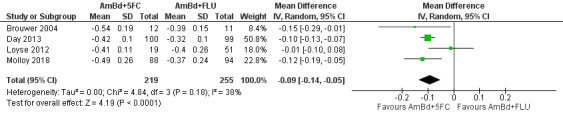
Forest plot of comparison: 9 Two weeks of AmBd + 5FC versus two weeks of AmBd + FLU, outcome: 9.2 Early fungicidal activity.
DAIDS grade three/four events
Based on combined data from three studies, we found no difference in DAIDS grade three or four laboratory events (507 participants, 3 studies, Analysis 9.3).
9.3. Analysis.
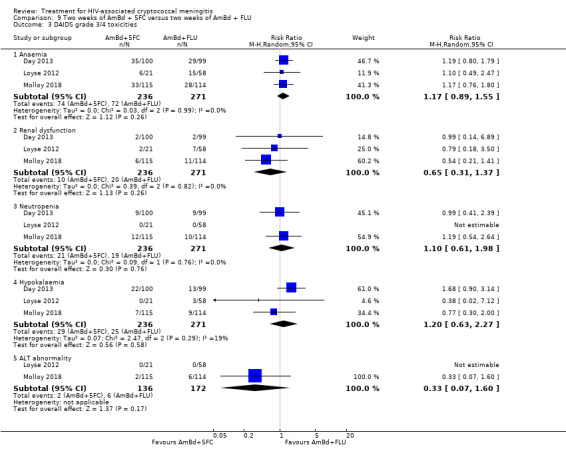
Comparison 9 Two weeks of AmBd + 5FC versus two weeks of AmBd + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
Adjunctive steroid therapy
One large study, a double‐blinded RCT from Vietnam, Thailand, Indonesia, Laos, Uganda, and Malawi, randomized 451 participants 1:1 to receive two weeks of AmBd and FLU with adjunctive dexamethasone or two weeks of AmBd and FLU only (Beardsley 2016).
The investigators planned enrolment of 880 participants. However, interim analysis from the data and safety monitoring board recommended study termination due to findings of harm associated with dexamethasone use across key outcomes, including rate of fungal clearance, risk of adverse events, and patient disability.
Mortality
Investigators found non‐significant increases in mortality in the dexamethasone arm by 10 weeks (450 participants, 1 study, Analysis 10.1, Table 10: subgroup 2; high‐certainty evidence) and by six months (450 participants, 1 study, Analysis 10.1: subgroup 3).
10.1. Analysis.
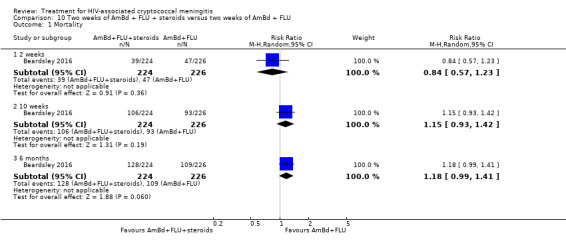
Comparison 10 Two weeks of AmBd + FLU + steroids versus two weeks of AmBd + FLU, Outcome 1 Mortality.
Early fungicidal activity
The mean rate of fungal clearance was lower in the dexamethasone arm than in the placebo arm (MD 0.10 log10 CFU/mL/day, 95% CI 0.06 to 0.14; 450 participants, 1 study, Analysis 10.2).
10.2. Analysis.
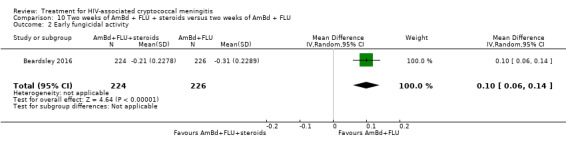
Comparison 10 Two weeks of AmBd + FLU + steroids versus two weeks of AmBd + FLU, Outcome 2 Early fungicidal activity.
DAIDS grade three/four events
The risk of grade three or four renal dysfunction was higher in the dexamethasone arm than in the placebo arm (RR 1.59, 95% CI 1.18 to 2.16; 450 participants, 1 study, Analysis 10.3: subgroup 2), and the risk of hypokalaemia was lower in the dexamethasone arm (RR 0.83, 95% CI 0.69 to 0.98; 450 participants, 1 study, Analysis 10.3: subgroup 4).
10.3. Analysis.
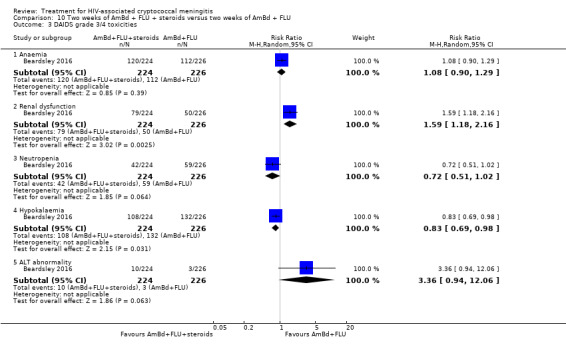
Comparison 10 Two weeks of AmBd + FLU + steroids versus two weeks of AmBd + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
One week of amphotericin B deoxycholate and fluconazole versus alternative regimens
A large study from nine centres in Malawi, Zambia, Tanzania, and Cameroon compared one week of AmBd 1 mg/kg/day and FLU 1200 mg/day followed by FLU 1200 mg/day on days 8 to 14 to three additional interventions (Molloy 2018): (i) two weeks of AmBd 1 mg/kg/day and 5FC 100 mg/kg/day; (ii) two weeks of AmBd 1 mg/kg/day and FLU 1200 mg/day; and (iii) two weeks of 5FC 100 mg/kg/day and FLU 1200 mg/day.
Mortality
One week of AmBd and FLU was associated with a non‐significant increase in mortality at two and 10 weeks compared to two weeks of AmBd and FLU (225 participants, 1 study, Analysis 11.1, Table 11: subgroup 1 and 2; moderate‐certainty evidence); mortality was significantly increased compared to two weeks of AmBd and 5FC at two weeks (RR 1.55, 95% CI 1.00 to 2.43; 226 participants, 1 study; Analysis 12.1: subgroup 1) but not at 10 weeks (226 participants, 1 study, Analysis 12.1, Table 12: subgroup 2; moderate‐certainty evidence). One week of AmBd and FLU was also associated with a significant increase in mortality at two and 10 weeks compared to two weeks of 5FC and FLU (two weeks: RR 1.78, 95% CI 1.21 to 2.62; 10 weeks: RR 1.39, 95% CI 1.07 to 1.80; 336 participants, 1 study, Analysis 13.1, Table 13: subgroups 1 and 2; moderate‐certainty evidence).
11.1. Analysis.
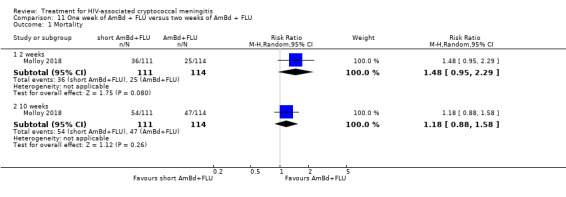
Comparison 11 One week of AmBd + FLU versus two weeks of AmBd + FLU, Outcome 1 Mortality.
12.1. Analysis.
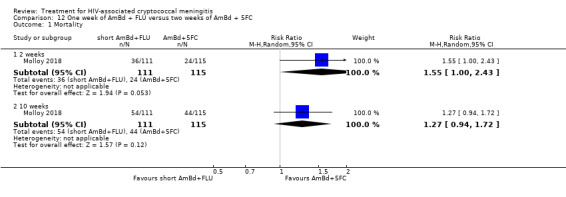
Comparison 12 One week of AmBd + FLU versus two weeks of AmBd + 5FC, Outcome 1 Mortality.
13.1. Analysis.
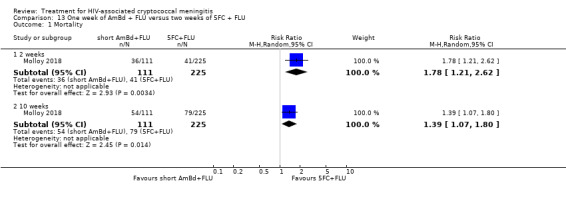
Comparison 13 One week of AmBd + FLU versus two weeks of 5FC + FLU, Outcome 1 Mortality.
Early fungicidal activity
There was no difference in rate of fungal clearance comparing one week of AmBd and FLU to two weeks of AmBd and FLU (175 participants, 1 study, Analysis 11.2). One week of AmBd and FLU was associated with lower mean fungal clearance rate compared to two weeks of AmBd and 5FC (MD 0.13 log10 CFU/mL/day, 95% CI 0.06 to 0.20; 169 participants, 1 study, Analysis 12.2) and a higher mean fungal clearance compared to two weeks of 5FC and FLU (MD ‐0.10 log10 CFU/mL/day, 95% CI ‐0.16 to ‐0.04; 263 participants, 1 study, Analysis 13.2).
11.2. Analysis.
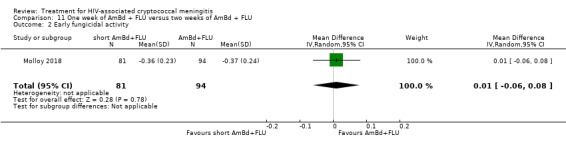
Comparison 11 One week of AmBd + FLU versus two weeks of AmBd + FLU, Outcome 2 Early fungicidal activity.
12.2. Analysis.
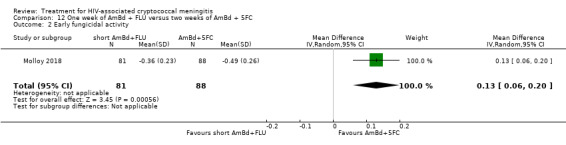
Comparison 12 One week of AmBd + FLU versus two weeks of AmBd + 5FC, Outcome 2 Early fungicidal activity.
13.2. Analysis.
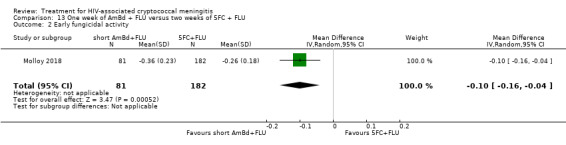
Comparison 13 One week of AmBd + FLU versus two weeks of 5FC + FLU, Outcome 2 Early fungicidal activity.
DAIDS grade three/four events
One week of AmBd and FLU was associated with no difference in grade three or four toxicities compared to two weeks of AmBd and 5FC (225 participants, 1 study, Analysis 11.3) or AmBd and FLU (226 participants, 1 study, Analysis 12.3). The risk of anaemia was higher with one week of AmBd and FLU compared to two weeks of 5FC and FLU (RR 4.42, 95% CI 2.25 to 8.70; 336 participants, 1 study, Analysis 13.3: subgroup 1), but no difference was found for other laboratory adverse events.
11.3. Analysis.
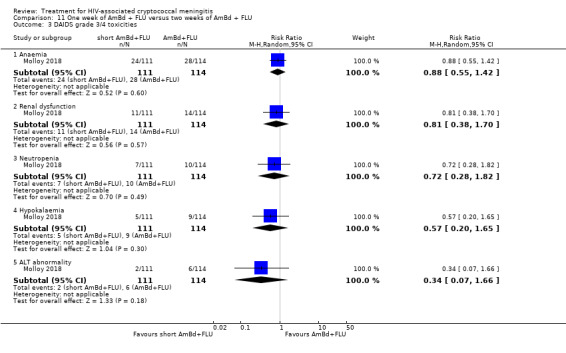
Comparison 11 One week of AmBd + FLU versus two weeks of AmBd + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
12.3. Analysis.
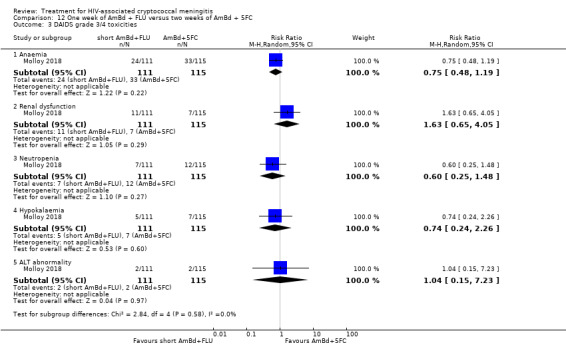
Comparison 12 One week of AmBd + FLU versus two weeks of AmBd + 5FC, Outcome 3 DAIDS grade 3/4 toxicities.
13.3. Analysis.
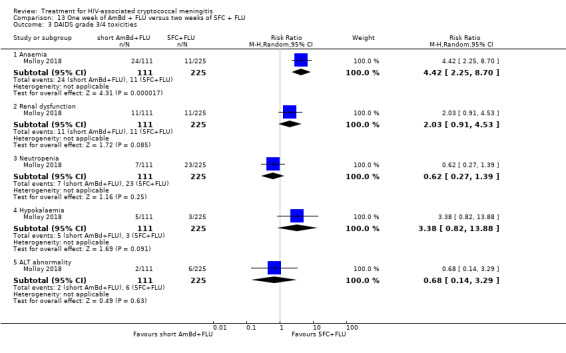
Comparison 13 One week of AmBd + FLU versus two weeks of 5FC + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
Fluconazole monotherapy
Two small trials compared two weeks of 5FC and FLU with FLU monotherapy (Mayanja‐Kizza 1998; Nussbaum 2010). One small study at a single centre in Malawi compared two weeks of 5FC 100 mg/kg/day in four divided doses and FLU 1200 mg/day to two weeks of FLU 1200 mg/day (Nussbaum 2010). A second study from a single centre in Uganda compared two weeks of 5FC 150 mg/kg/day in three divided doses and FLU 200 mg/day for two months with FLU 200 mg/day for two months, a suboptimal FLU dose (Mayanja‐Kizza 1998; Bicanic 2007; Longley 2008). A high proportion of participants were lost to follow‐up in the Uganda study (8/58) by 10 weeks.
Mortality
Mortality was higher with FLU monotherapy compared to combination 5FC and FLU at two weeks (RR 3.04, 95% CI 1.31 to 7.06; 98 participants, 2 studies, Analysis 14.1: subgroup 1) and 10 weeks (RR 1.46, 95% CI 0.96 to 2.23; 98 participants, 2 studies, Analysis 14.1, Table 14: subgroup 2; very low‐certainty evidence). Six‐month mortality estimates were not available, as follow‐up did not extend beyond 10 weeks.
14.1. Analysis.
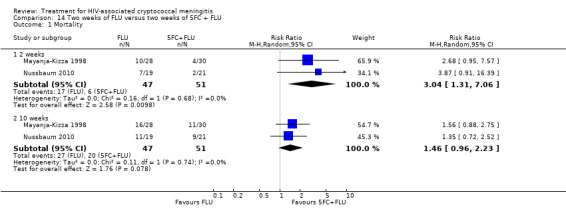
Comparison 14 Two weeks of FLU versus two weeks of 5FC + FLU, Outcome 1 Mortality.
Early fungicidal activity
One study measured EFA (Nussbaum 2010), and showed a significantly lower mean rate of fungal clearance with FLU monotherapy compared to combination FLU and 5FC (MD 0.17 log10 CFU/mL/day, 95% CI 0.08 to 0.26; 37 participants, 1 study, Analysis 14.2).
14.2. Analysis.
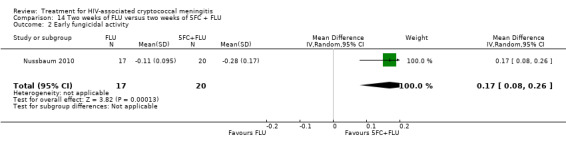
Comparison 14 Two weeks of FLU versus two weeks of 5FC + FLU, Outcome 2 Early fungicidal activity.
DAIDS grade three/four events
One small study found no significant difference in grade three or four events between groups (40 participants, 1 study, Analysis 14.3) (Nussbaum 2010).
14.3. Analysis.
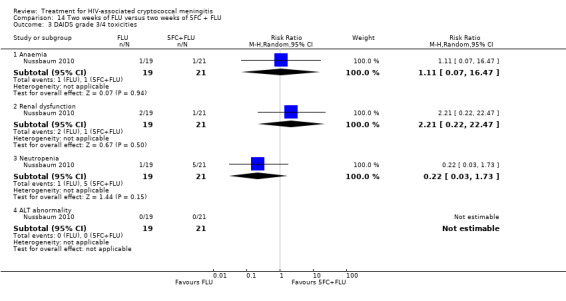
Comparison 14 Two weeks of FLU versus two weeks of 5FC + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
Liposomal amphotericin B regimens
Two studies evaluated induction therapy with liposomal AmB (L‐AmB) (Leenders 1997; Jarvis 2018). One small study from the Netherlands and Australia randomized 28 participants to three weeks of L‐AmB 4 mg/kg/day or three weeks of AmBd 0.7 mg/kg/day followed by FLU 400 mg/day consolidation (Leenders 1997).
One recently completed trial in Botswana and Tanzania randomized participants to two weeks of L‐AmB 3 mg/kg/day or one of three short courses of high‐dose L‐AmB, a single dose of L‐AmB 10 mg/kg on day one, L‐AmB 10 mg/kg on day one and 5 mg/kg on day three, or L‐AmB 10 mg/kg on day one and 5 mg/kg on days three and seven (Jarvis 2018). All participants received FLU 1200 mg/day for two weeks followed by 800 mg/day consolidation. This phase two study was powered to detect a non‐inferior EFA with short course versus two weeks of L‐AmB and was discontinued early when interim analysis found it had reached the prespecified non‐inferiority margin.
Mortality
No deaths were reported within two weeks in the study comparing L‐AmB to AmBd (Leenders 1997). Mortality up to 10 weeks and at six months was low in both arms, with no significant difference between groups (28 participants, 1 study, Analysis 15.1, Table 15: subgroups 2 and 3; very low‐certainty evidence).
15.1. Analysis.
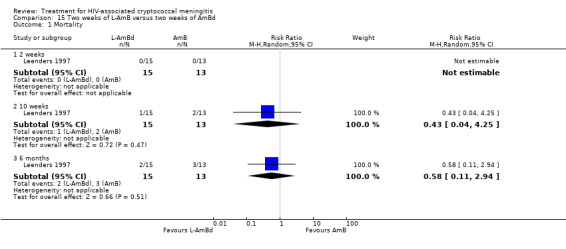
Comparison 15 Two weeks of L‐AmB versus two weeks of AmBd, Outcome 1 Mortality.
In the study comparing two weeks of L‐AmB and FLU with short‐course L‐AmB with FLU, mortality up to two weeks and 10 weeks (79 participants, 1 study, Analysis 16.1, Table 16: subgroups 1 and 2; low‐certainty evidence) did not differ between groups. A larger phase three study is underway that will compare a single 10 mg/kg dose of L‐AmB and two weeks of 5FC 100 mg/kg/day and FLU 1200 mg/day with one week of AmBd 1 mg/kg/day and 5FC 100 mg/kg/day followed by FLU 1200 mg/day on days 8 to 14.
16.1. Analysis.
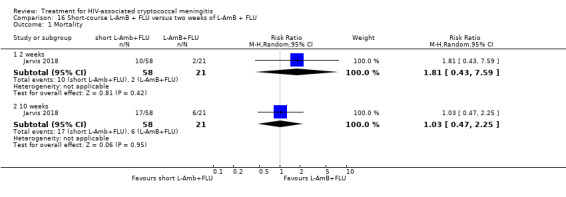
Comparison 16 Short‐course L‐AmB + FLU versus two weeks of L‐AmB + FLU, Outcome 1 Mortality.
Early fungicidal activity
The Leenders 1997 study did not compare EFA between treatment regimens. A faster mean rate of fungal clearance was observed in participants who received short‐course L‐AmB with FLU compared to those who received two weeks of L‐AmB with FLU in the Jarvis 2018 study (MD ‐0.10 log10 CFU/mL/day, 95% CI ‐0.21 to 0.01; 67 participants, 1 study, Analysis 16.2).
16.2. Analysis.
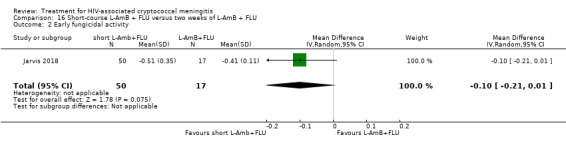
Comparison 16 Short‐course L‐AmB + FLU versus two weeks of L‐AmB + FLU, Outcome 2 Early fungicidal activity.
DAIDS grade three/four events
The Leenders 1997 study did not report DAIDS grade three or four laboratory events. The risk of hypokalaemia was lower in participants who received short‐course L‐AmB and FLU compared to those who received two weeks of L‐AmB and FLU in the Jarvis 2018 study (RR 0.07, 95% CI 0.01 to 0.58; 79 participants, 1 study, Analysis 16.3: subgroup 4).
16.3. Analysis.
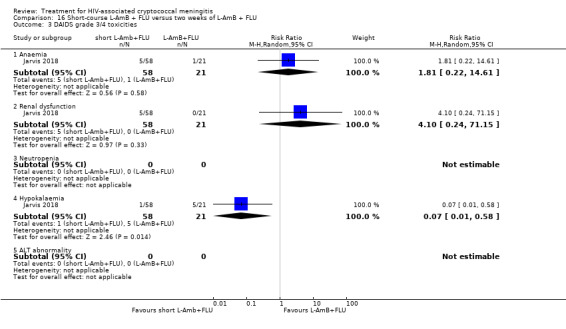
Comparison 16 Short‐course L‐AmB + FLU versus two weeks of L‐AmB + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
Amphotericin B deoxycholate with flucytosine and fluconazole
One small study conducted at a single centre in Thailand compared a three‐drug induction regimen of two weeks of AmBd 0.7 mg/kg/day, 5FC 100 mg/kg/day, and FLU 400 mg/kg/day with: (i) two weeks of AmBd 0.7 mg/kg/day and 5FC 100 mg/kg/day, (ii) two weeks of AmBd 0.7 mg/kg/day and FLU 400 mg/day, and (iii) two weeks of AmBd 0.7 mg/kg/day (Brouwer 2004).
One small study conducted at a single centre in Malawi compared a three‐drug induction regimen of one week of AmBd 1 mg/kg/day with two weeks of 5FC 100 mg/kg/day and FLU 1200 mg/day, and one week of AmBd 1 mg/kg/day with two weeks of FLU 1200 mg/day (Jackson 2012). Both regimens were followed by FLU 800 mg/day consolidation.
Mortality
In the Brouwer 2004 study, mortality was very low at two and 10 weeks in all arms with no significant difference in survival observed between two weeks of AmBd and 5FC and FLU compared to two weeks of AmBd and 5FC (31 participants, 1 study, Analysis 17.1, Table 17: subgroups 1 and 2; low‐certainty evidence), two weeks of AmBd with FLU (32 participants, 1 study, Analysis 18.1, Table 18: subgroups 1 and 2; very low‐certainty evidence), and two weeks of AmBd alone (32 participants, 1 study, Analysis 19.1, Table 19: subgroups 1 and 2; very low‐certainty evidence).
17.1. Analysis.
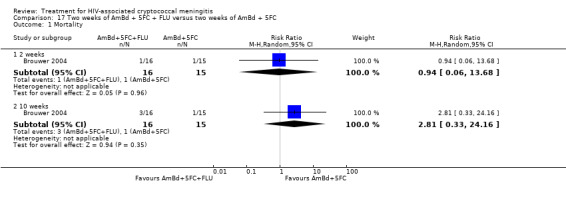
Comparison 17 Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd + 5FC, Outcome 1 Mortality.
18.1. Analysis.
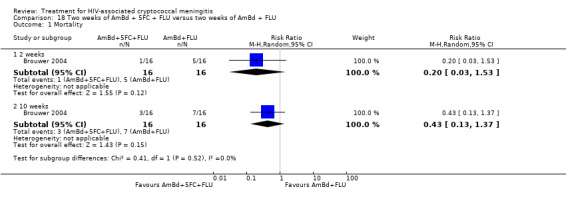
Comparison 18 Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd + FLU, Outcome 1 Mortality.
19.1. Analysis.
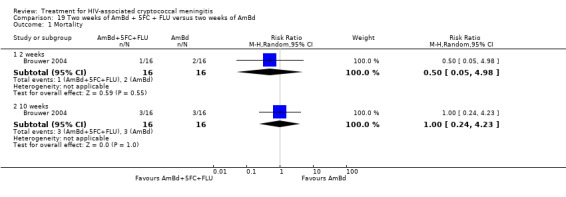
Comparison 19 Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd, Outcome 1 Mortality.
In the Jackson 2012 study, no difference in mortality was observed between groups within two weeks or 10 weeks (40 participants, 1 study, Analysis 20.1, Table 20: subgroups 1 and 2; low‐certainty evidence).
20.1. Analysis.
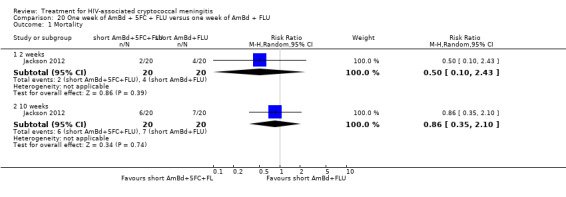
Comparison 20 One week of AmBd + 5FC + FLU versus one week of AmBd + FLU, Outcome 1 Mortality.
Early fungicidal activity
In the Brouwer 2004 study, mean rate of fungal clearance was lower in the AmBd and 5FC and FLU group compared to the AmBd and 5FC group (MD 0.16 log10 CFU/mL/day, 95% CI 0.03 to 0.29; 27 participants, 1 study, Analysis 17.2). No difference was observed in mean EFA between the AmBd and 5FC and FLU group and either the AmBd with FLU (26 participants, 1 study, Analysis 18.2) or AmBd alone group (29 participants, 1 study, Analysis 19.2).
17.2. Analysis.
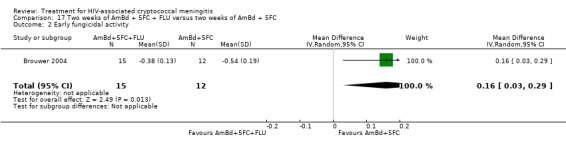
Comparison 17 Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd + 5FC, Outcome 2 Early fungicidal activity.
18.2. Analysis.
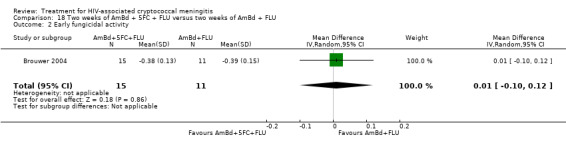
Comparison 18 Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd + FLU, Outcome 2 Early fungicidal activity.
19.2. Analysis.
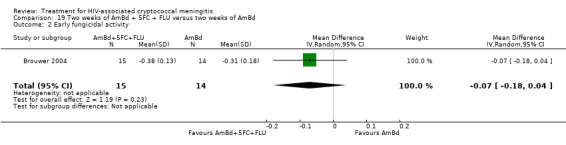
Comparison 19 Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd, Outcome 2 Early fungicidal activity.
In the Jackson 2012 study, mean rate of fungal clearance was greater in the one week of AmBd and 5FC and FLU group compared to the one week of AmBd and FLU group (MD ‐0.12 log10 CFU/mL/day, 95% CI ‐0.23 to ‐0.01; 37 participants, 1 study, Analysis 20.2).
20.2. Analysis.
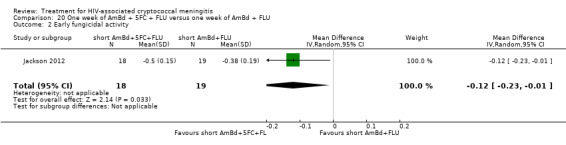
Comparison 20 One week of AmBd + 5FC + FLU versus one week of AmBd + FLU, Outcome 2 Early fungicidal activity.
DAIDS grade three/four events
The Brouwer 2004 study did not report DAIDS grade three or four events. The risk of events did not differ between the one week of AmBd and 5FC and FLU group and the one week of AmBd and FLU group in the Jackson 2012 study (40 participants, 1 study, Analysis 20.3).
20.3. Analysis.
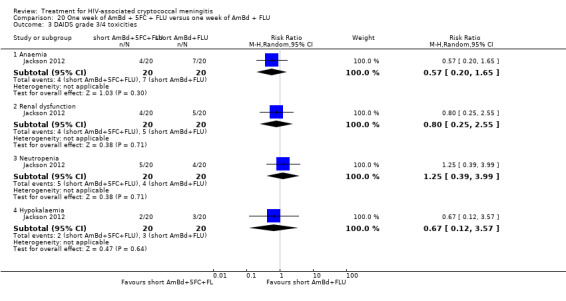
Comparison 20 One week of AmBd + 5FC + FLU versus one week of AmBd + FLU, Outcome 3 DAIDS grade 3/4 toxicities.
Adjunctive interferon‐gamma therapy
One small study from South Africa evaluated adjunctive interferon gamma 1b (IFNg) with two weeks of AmBd 1 mg/kg/day and 5FC 100 mg/kg/day compared to two weeks of AmBd 1 mg/kg/day and 5FC 100 mg/kg/day without IFNg (Jarvis 2012). The study included two IFNg groups: one group received IFNg 100 µg subcutaneously on days 1 and 3, and one group received IFNg 100 µg subcutaneously on days 1, 3, 5, 8, 10, and 12. The study was powered to evaluate improvement in EFA associated with adjunctive IFNg rather than mortality benefit with IFNg.
Mortality
No difference was observed in mortality between groups at two and 10 weeks (88 participants, 1 study, Analysis 21.1, Table 21: subgroups 1 and 2; low‐certainty evidence).
21.1. Analysis.
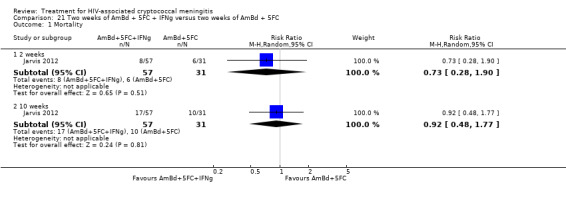
Comparison 21 Two weeks of AmBd + 5FC + IFNg versus two weeks of AmBd + 5FC, Outcome 1 Mortality.
Early fungicidal activity
The mean EFA was greater for participants in the adjunctive IFNg groups (MD ‐0.15 log10 CFU/mL/day, 95% CI ‐0.24 to ‐0.06; 88 participants, 1 study, Analysis 21.2).
21.2. Analysis.
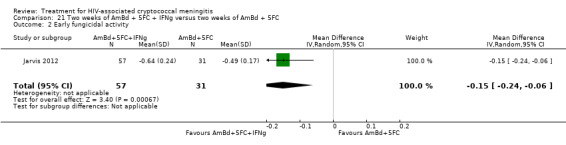
Comparison 21 Two weeks of AmBd + 5FC + IFNg versus two weeks of AmBd + 5FC, Outcome 2 Early fungicidal activity.
DAIDS grade three/four events
No difference was observed between groups in DAIDS grade three or four events (88 participants, 1 study, Analysis 21.3).
21.3. Analysis.
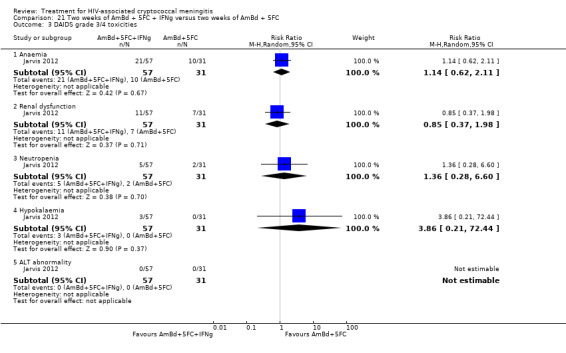
Comparison 21 Two weeks of AmBd + 5FC + IFNg versus two weeks of AmBd + 5FC, Outcome 3 DAIDS grade 3/4 toxicities.
GRADE assessment for pairwise comparisons
We conducted GRADE assessment for 10‐week mortality for all comparisons.
Risk of bias
We downgraded few comparisons for risk of bias. We downgraded one comparison (two weeks of FLU versus two weeks of 5FC and FLU) due to high loss to follow‐up in one of two studies included in the comparison. We also downgraded a second comparison (two weeks of AmBd versus two weeks of L‐AmB) for risk of bias. The single study for this comparison was sponsored by a study drug manufacturer, with the role of the sponsor not stated. Additionally, mortality was not stated as a primary outcome of interest.
Imprecision
We downgraded all but one comparison for imprecision due to insufficient participants and events (fewer than 200 observed deaths by 10 weeks).
Inconsistency
Due to the limited number of studies for most comparisons, there was limited assessment of heterogeneity. Of those comparisons for which heterogeneity could be assessed, no studies demonstrated statistical heterogeneity.
Indirectness
We downgraded six comparisons for using lower doses of fluconazole than recommended in current treatment guidelines (two weeks of AmBd and FLU versus two weeks of AmBd; two weeks of AmBd and 5FC versus two weeks of AmBd and FLU; two weeks of FLU versus two weeks of 5FC and FLU; two weeks of AmBd and 5FC and FLU versus two weeks of AmBd and 5FC; two weeks of AmBd and 5FC and FLU versus two weeks of AmBd and FLU; and two weeks of AmBd and 5FC versus two weeks of AmBd).
Publication bias
We did not detect publication bias.
Ten‐week mortality network meta‐analysis
Overview
A network plot for 10‐week mortality is shown in Figure 10. The nodes (circles) represent the treatment combinations, while the edges (lines) represent direct comparisons between treatment combinations. The size of the node is proportional to the number of participants randomized to the treatment combination, and the width of the edge is proportional to the number of trials comparing the treatment combinations. We excluded a pair of treatment combinations (two weeks of L‐AmB with FLU and short‐course L‐AmB with FLU from Jarvis 2018) from the network meta‐analyses, as these interventions were unconnected to the rest of the network.
10.
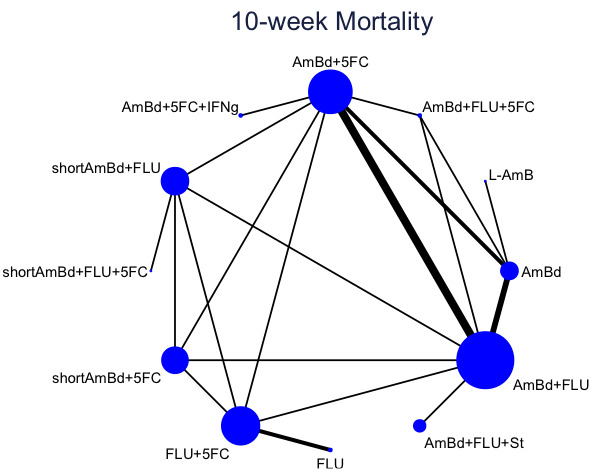
Network plot for 10‐week mortality.
Ten‐week mortality was reported for 12 treatment combinations in 11 trials involving 1961 participants (Leenders 1997; Mayanja‐Kizza 1998; Brouwer 2004; Pappas 2009; Nussbaum 2010; Jackson 2012; Jarvis 2012; Loyse 2012; Day 2013; Beardsley 2016; Molloy 2018). Trials assessed 20 direct comparisons with 16 comparisons (80%) based on one trial (Molloy 2018). The most common comparators in the network were two weeks of AmBd with FLU and AmBd with 5FC, with both examined in six trials (55%). Eight trials (73%) were two‐arm studies, one (9%) was a three‐arm trial, one (9%) was a four‐arm trial, and one (9%) was a five‐arm trial. Due to the limited number of studies and participants contributing to each node, the results of the NMA and associated SUCRA rankings should be interpreted with caution.
Relative effects of treatment combinations derived from network meta‐analysis
The league table shows the relative effects of each treatment combination derived from NMA (Figure 11). Treatment effects derived from NMA were in general similar to those derived from pairwise meta‐analysis.
11.
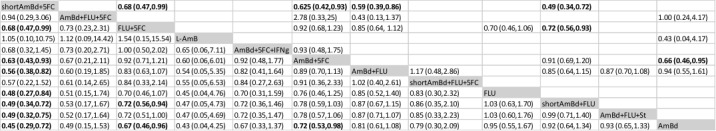
Ten‐week mortality treatment effect estimates. Values in the table are risk ratios (RRs) with 95% confidence intervals. Bolded values represent statistically significant effects. The bottom half of the table represents network meta‐analysis‐derived treatment effects, while the top half represents results from direct comparisons alone. For network meta‐analysis‐derived effects, RR < 1 favours the treatment combination in the column. For effects derived from pairwise meta‐analysis, RR < 1 favours the treatment combination in the row. Treatment combinations are ordered by surface under the cumulative ranking curve (SUCRA) rankings.
Most treatment effects had low precision for the 10‐week mortality estimates. Using two weeks of AmBd and FLU as reference, the RR (95% CI) of 10‐week mortality of each treatment combination in order of SUCRA ranking is: one week of AmBd and 5FC 0.56 (0.38 to 0.82), two weeks of AmBd and 5FC and FLU 0.60 (0.19 to 1.85), two weeks of 5FC and FLU 0.83 (0.63 to 1.07), two weeks of L‐AmB 0.54 (0.05 to 5.35), two weeks of AmBd and 5FC and IFNg 0.82 (0.41 to 1.64), two weeks of AmBd and 5FC 0.89 (0.70 to 1.13), one week of AmBd and 5FC and FLU 0.98 (0.38 to 2.50), FLU 1.18 (0.72 to 1.94), one week of AmBd and FLU 1.14 (0.87 to 1.50), AmBd and FLU and steroids 1.15 (0.93 to 1.42), and AmBd 1.24 (0.92 to 1.65) (Figure 12).
12.
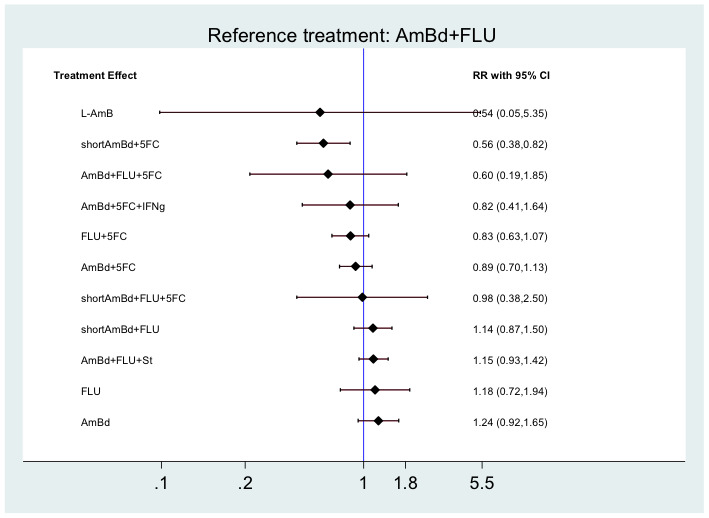
Ten‐week mortality risk for each treatment combination compared to reference of two weeks of AmBd and FLU.
SUCRA rankings
The top three ranked treatment combinations (with SUCRA values) at 10 weeks were one week of AmBd and 5FC (88%), two weeks of AmBd and 5FC and FLU (74%), and two weeks of 5FC and FLU (67%), while the bottom three ranked treatment combinations were one week of AmBd and two weeks of FLU (26%), two weeks of AmBd and FLU and steroids (26%), and two weeks of AmBd (19%) (Figure 13). Of the top ranked treatment combinations, both one week of AmBd and 5FC and two weeks of 5FC and FLU show significant and clinically meaningful reductions in 10‐week mortality risk compared to the bottom ranked treatment combinations. The cumulative ranking probabilities for all treatment combinations are presented in SUCRA plots below.
13.
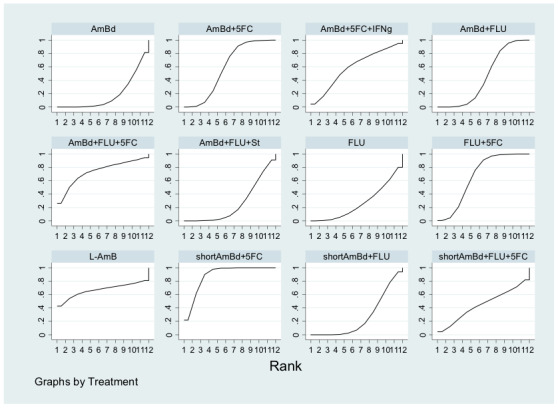
Cumulative ranking probabilities for each treatment combination for 10‐week mortality. The SUCRA value represents the surface underneath the cumulative ranking curve and is the probabilities for each treatment combination to be among the n‐best options. The larger the SUCRA value, the higher the ranking probability for the treatment combination in the network. The SUCRA values for each treatment combination are as follows: AmBd (19%), AmBd + 5FC (59%), AmBd + 5FC + IFNg (59%), AmBd + FLU (45%), AmBd + FLU + 5FC (74%), AmBd + FLU + St (26%), FLU (27%), FLU + 5FC (67%), L‐Amb (67%), shortAmBd + 5FC (88%), shortAmBd + FLU (26%), shortAmBd + FLU + 5FC (45%).
Although the AmBd and 5FC and FLU node ranked highly in the NMA, the direct evidence for this node consisted of a single study that included only 16 participants in one arm of one study, Brouwer 2004, with low mortality in all treatment groups and no significant difference in 10‐week mortality by direct, pairwise comparisons. This result should be interpreted with caution due to the very high likelihood of bias. Similarly, the L‐AmB node included 15 participants from one study in a high‐income setting, Leenders 1997, where mortality was low in both the three weeks of L‐AmB and three weeks of AmBd arms with no significant difference in mortality between arms within 10 weeks in the direct comparison.
Evaluation of transitivity and inconsistency
We did not find evidence that the assumption of transitivity was violated. The distribution of prespecified potential effect modifiers, including ART status, timing of ART, inclusion of participants with abnormal mental status, therapeutic lumbar puncture, dosing of AmBd, and dosing of FLU, were generally similar across comparisons. We examined study setting as a potential source of intransitivity in a subgroup analysis excluding studies conducted in high‐income countries for 10‐week mortality. The findings were similar to the primary analyses. However, our ability to thoroughly examine transitivity was limited by the small number of studies included per comparison, and therefore we cannot be entirely confident that the assumption of transitivity is plausible in our networks.
We found no evidence of inconsistency for any outcome using the design‐by‐treatment interaction modelling, node‐splitting, and loop‐specific approaches. However, due to the limited amount of data available we are not able to draw firm conclusions regarding the presence of inconsistency in our networks.
Modified GRADE for network meta‐analysis
The certainty of the evidence for all comparisons using modified GRADE for NMA was low or very low. Assessment for key comparisons is shown in Figure 14.
14.
Modified GRADE for network meta‐analysis
Discussion
Summary of main results
This review included 13 studies of 2426 participants comparing 21 interventions used for induction therapy for first episode of HIV‐associated cryptococcal meningitis. Based on moderate‐certainty evidence from one study, mortality within 10 weeks was lower with shortened one week of AmBd and 5FC compared to two weeks of AmBd and 5FC, which was previously considered the gold standard, as well as compared to oral 5FC and FLU. Based on moderate‐certainty evidence, no difference in mortality was observed with two weeks of oral 5FC and FLU compared to two weeks of AmBd and 5FC. It is unclear if the addition of FLU to AmBd improves mortality given the low certainty of the evidence contributing to this comparison. Few studies provided data on FLU monotherapy. Low‐certainty evidence suggests that FLU monotherapy may be inferior to the combination of 5FC and FLU, and there were no direct comparisons with AmBd‐based regimens.
Results from the 10‐week NMA complemented results from the pairwise meta‐analyses. Pairwise meta‐analyses alone allowed us to examine 21 treatment combination comparisons for 10‐week mortality, and with NMA we were able to estimate the relative effects for an additional 46 comparisons. The NMA supports the role of a short‐course treatment combination of one week of AmBd and 5FC as an initial option in resource‐limited settings due to its high ranking in the network for mortality outcomes in the context of its statistically and clinically significant effects compared to some other combinations. All‐oral induction therapy with 5FC and FLU also performed well in the NMA. Two weeks of AmBd and 5FC and FLU performed well in the 10‐week NMA. However, this treatment arm included only 16 participants from a single study in which mortality was very low in all four treatment arms. The SUCRA ranking for this treatment is highly likely to be biased and should be interpreted cautiously.
With respect to early fungicidal activity, we found no difference in the rate of CSF fungal clearance with short‐course AmBd and 5FC compared to two weeks of AmBd and 5FC. Our findings support improved fungal clearance of regimens with 5FC in addition to AmBd in comparison to induction regimens containing AmBd with FLU or AmBd alone. Amphotericin B deoxycholate‐based regimens had greater fungal clearance than all‐oral induction therapy with 5FC and FLU.
With respect to DAIDS grade three or four toxicities, traditional two‐week AmBd‐based therapy was associated with higher occurrence of AmBd‐related toxicities compared to short‐course therapy. All‐oral 5FC and FLU therapy was associated with less toxicity (including anaemia, hypokalaemia, and renal dysfunction) compared to several AmBd‐containing regimens.
Regarding emerging adjunctive therapies, there is strong evidence that adjunctive steroid therapy is harmful and should not be used in clinical practice. One study found that adjunctive IFNg used in combination with two weeks of AmBd and 5FC resulted in a faster rate of CSF fungal clearance compared to AmBd and 5FC alone, but this study was not adequately powered to assess differences in mortality associated with the use of adjunctive IFNg.
Overall completeness and applicability of evidence
The evidence provided in this review offers important and timely insights into the best management to reduce mortality from HIV‐associated cryptococcal meningitis in resource‐limited settings where the large majority of disease occurs (Rajasingham 2017). In resource‐limited settings, moderate‐certainty evidence from one study supports the use of one week of AmBd with 5FC followed by high‐dose fluconazole, with improved mortality up to 10 weeks, similar CSF fungal clearance, and reduced treatment toxicity (Molloy 2018). These findings are consistent with non‐RCT data from animal studies demonstrating that near maximal antifungal activity with AmBd therapy can be achieved within three days (Livermore 2014). The findings in this review are widely applicable to the treatment of HIV‐associated cryptococcal meningitis in resource‐limited settings.
This review has several important limitations, as follows.
First, 11 out of 13 studies were conducted in resource‐limited settings, and therefore our findings provide limited guidance for management of HIV‐associated cryptococcal meningitis in high‐income settings where mortality is significantly lower with traditional AmB‐based induction therapy (Saag 1991; van der Horst 1997). No short‐course (less than two weeks of AmB) or all‐oral (5FC and FLU) regimen was evaluated in high‐income country settings, although there is no reason to believe that these regimens would be inferior in these settings. Furthermore, in high‐income settings more expensive but less toxic liposomal AmB is commonly used instead of AmBd, and frequency of and ability to manage treatment‐related toxicities differs between high‐income and resource‐limited settings.
Second, we did not find any RCTs on treatment of HIV‐associated cryptococcal meningitis in children, for whom therapy is guided by RCT evidence in adult populations. Nevertheless, there is no reason to consider that these regimens would be inferior for the treatment of cryptococcal meningitis in children.
Third, relatively few studies overall met the inclusion criteria for this review, which was a limitation for both pairwise meta‐analysis and NMA. Importantly, we excluded observational studies as well as studies with high loss to follow‐up (> 20%) or significant baseline imbalances between comparator groups. We also excluded trials with any non‐HIV‐related cases of cryptococcal meningitis or re‐treatment cases, as these cases may be clinically distinct from HIV‐associated disease with respect to patient comorbidities, Cryptococcus spp. drug resistance, and disease severity, among other factors. Few deaths were observed per treatment arm, and the certainty of evidence for most pairwise comparisons was downgraded for imprecision due to few events (< 200).
Fourth, our review provides limited insight into emerging adjunctive therapies being evaluated for the treatment of HIV‐associated cryptococcal meningitis. Interferon gamma 1b was associated with improved early fungicidal activity with AmBd and 5FC in one phase two RCT, but this study was underpowered to compare differences in mortality up to 10 weeks (Jarvis 2012).
Fifth, the SUCRA rankings should be interpreted cautiously, especially when they are not accompanied by statistically or clinically meaningful effects. Due to the imprecision of treatment effects on mortality throughout the network, the rankings presented should not be considered definitive.
Finally, although commonly used in resource‐limited settings due to low cost and oral administration, oral FLU was compared with other regimens in few included studies. However, observational studies and data from RCTs not meeting the inclusion criteria for this review have consistently found very high mortality associated with the use of FLU monotherapy (Makadzange 2010; Rothe 2013).
Certainty of the evidence
For pairwise comparisons, most comparisons had moderate‐ or low‐certainty evidence. We downgraded the evidence for all but one comparison for imprecision due to few participants and events within 10 weeks (< 200). We also downgraded the evidence for several comparisons with therapies containing FLU for indirectness due to evaluation of regimens containing doses of FLU lower than currently recommended (< 800 mg/day).
For the NMA, the certainty of the evidence was low or very low, due primarily to imprecision, heterogeneity (inconsistency), and indirectness of comparisons.
Potential biases in the review process
We did not identify any major biases in the review process. One author of this Cochrane Review (JNJ) was a principal investigator or co‐investigator in several trials, but did not participate in data extraction or interpretation of results for these studies. We further limited bias during the review process by conducting an extensive search using a wide range of search terms and databases. Two review authors reviewed the search outputs, evaluated eligibility, and extracted data independently. Changes made to the study protocol after publication and after the review process began are detailed in the Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
Several influential studies in this review were recently completed and not included in previous reviews of best treatment for HIV‐associated cryptococcal meningitis (Beardsley 2016; Jarvis 2018; Molloy 2018). A previous NMA did not find evidence for mortality benefit with two weeks of AmBd and 5FC compared to AmBd alone (Campbell 2015). This review had important methodological differences, such as inclusion of both RCTs and observational studies and inclusion of studies with re‐treatment cases.
Authors' conclusions
Implications for practice.
This review has important implications for clinical practice in resource‐limited settings. We found reduced 10‐week mortality with shortened amphotericin B deoxycholate (AmBd) and flucytosine (5FC) induction therapy compared to the current gold standard of two weeks of AmBd and 5FC, based on moderate‐certainty evidence. Moderate‐certainty evidence also indicated that an all‐oral regimen of two weeks of 5FC and fluconazole may be a good alternative to two weeks of AmBd‐based therapy, with no difference in mortality observed between arms. This all‐oral regimen may be a good treatment option in settings where intravenous therapy cannot be safely administered or AmBd is not available. There is a need to expand access to 5FC in resource‐limited settings where HIV‐associated cryptococcal meningitis is most common.
Implications for research.
Our review highlights that limited RCT evidence exists comparing treatment regimens for HIV‐associated cryptococcal meningitis. There is a lack of data on short‐course regimens for the treatment of HIV‐associated cryptococcal meningitis in high‐income settings, where the lower mortality and low burden of disease compared to resource‐limited settings, as well as the positive findings from low‐income settings, will likely preclude such studies. Emerging evidence, including from ongoing phase three studies, will provide further evidence on the efficacy of adjunctive therapies as well as short‐course liposomal amphotericin regimens. Finally, there is very limited evidence supporting treatment choice for children, and as trials are unlikely, results can be extrapolated from adults, albeit downgraded to a lower certainty of evidence.
What's new
| Date | Event | Description |
|---|---|---|
| 24 July 2018 | New search has been performed | This is an update of a review last published in 2011 (Sloan 2011). The review author team updated the protocol extensively, and differences are highlighted in the ‘Differences between protocol and review' section. |
| 24 July 2018 | New citation required and conclusions have changed | We included 13 eligible studies that enrolled 2426 participants and compared 21 interventions. Using random‐effects models we determined pooled risk ratio (RR) and 95% confidence interval (CI) for dichotomous outcomes and mean differences (MD) and 95% CI for continuous outcomes. We performed a network meta‐analysis using multivariate meta‐regression. We modelled treatment differences (RR and 95% CI) and determined treatment rankings for two‐week and 10‐week mortality outcomes using surface under the cumulative ranking curve (SUCRA). We assessed the certainty of the evidence using the GRADE approach. |
Acknowledgements
The Academic Editor of this review update was Professor George Rutherford.
We thank Tihana Bicanic, Anna Chaimani, Paul Garner, and Angela Loyse for providing valuable feedback on the protocol; Vittoria Lutje and Joy Oliver for their assistance with development of the review search; and Anne‐Marie Stephani and Deirdre Walshe with assistance as former and current Cochrane Infectious Diseases Group (CIDG) Managing Editors.
Ingrid Eshun‐Wilson is supported by the Effective Health Care Research Consortium. This Consortium and the CIDG editorial base is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
Appendices
Appendix 1. Search strategy
PubMed
| No. | Query |
| #4 | Search (#1 AND #2 AND #3) |
| #3 | Search (Antifungal agents[mh] OR azole*[tiab] OR fluconazole[tiab] OR amphotericin[tiab] OR flucytosine[tiab] OR sertraline[tiab] OR dexamethasone[tiab] OR voriconazole[tiab] OR acetazolamide[tiab] OR diflucan[tiab] OR itraconazole[tiab] OR rifampin[tiab] OR 5‐FC[tiab]) |
| #2 | Search ("Meningitis, Cryptococcal"[Mesh] OR cryptococcal meningitis[tiab] OR cryptococcal meningitis[tiab] OR cryptococcal meningitides[tiab] OR cerebral cryptococcosis[tiab] OR cerebral cryptococcoses[tiab] OR toruloma*[tiab] OR cryptococcus neoforman[mh] OR cryptococcus neoforman[tiab] OR ((cryptococcal[tiab] OR cryptococcal[tiab] OR cyptococcosis[tiab] OR cryptococcoses[tiab] OR Cryptococcus[tiab]) AND (meningitis[tiab])) |
| #1 | Search (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) |
Embase
| No. | Query |
| #11 | #1 AND #7 AND #8 AND #9 AND [1980‐2017]/py |
| #10 | #1 AND #7 AND #8 AND #9 |
| #9 | ‘antifungal agent'/syn OR azole*:ab,ti OR fluconazole:ab,ti OR amphotericin:ab,ti OR flucytosine:ab,ti OR sertraline:ab,ti OR dexamethasone:ab,ti OR voriconazole:ab,ti OR acetazolamide:ab,ti OR diflucan:ab,ti OR itraconazole:ab,ti OR rifampin:ab,ti OR ‘5‐fc':ab,ti |
| #8 | ‘cryptococcal meningitis'/de OR ‘cryptococcal meningitis':ab,ti OR ‘cryptococcus meningitis':ab,ti OR ‘cryptococcal meningitis':ab,ti OR ‘cryptococcal meningitides':ab,ti OR ‘cerebral cryptococcosis':ab,ti OR ‘cerebral cryptococcoses':ab,ti OR toruloma*:ab,ti OR ‘cryptococcus neoforman':ab,ti OR (cryptococcal:ab,ti OR cryptococcal:ab,ti OR cryptococcosis:ab,ti OR cryptococcoses:ab,ti AND meningitis:ab,ti) |
| #7 | #2 NOT #6 |
| #6 | #3 NOT #5 |
| #5 | #3 AND #4 |
| #4 | ‘human'/de OR ‘normal human'/de OR ‘human cell'/de |
| #3 | ‘animal'/de OR ‘animal experiment'/de OR ‘invertebrate'/de OR ‘animal tissue'/de OR ‘animal cell'/de OR ‘nonhuman'/de |
| #2 | ‘randomised controlled trial'/de OR ‘randomised controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR ‘crossover procedure'/de OR ‘crossover procedure' OR ‘double‐blind procedure'/de OR ‘double‐blind procedure' OR ‘single‐blind procedure'/de OR ‘single‐blind procedure' OR (doubl* NEAR/3 blind*):ab,ti OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR (cross NEXT/1 over*):ab,ti |
| #1 | ‘human immunodeficiency virus infection'/exp OR ‘human immunodeficiency virus infection' OR ‘human immunodeficiency virus'/exp OR ‘human immunodeficiency virus' OR ‘human immunodeficiency virus':ab,ti OR ‘human immuno+deficiency virus':ab,ti OR ‘human immunedeficiency virus':ab,ti OR ‘human immune+deficiency virus':ab,ti OR hiv:ab,ti OR ‘hiv‐1':ab,ti OR ‘hiv‐2':ab,ti OR ‘acquired immunodeficiency syndrome':ab,ti OR ‘acquired immuno+deficiency syndrome':ab,ti OR ‘acquired immunedeficiency syndrome':ab,ti OR ‘acquired immune+deficiency syndrome':ab,ti |
CENTRAL
| ID | Search |
| #1 | MeSH descriptor: [HIV Infections] explode all trees |
| #2 | MeSH descriptor: [HIV] explode all trees |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only |
| #6 | #1 or #2 or #3 or #4 or #5 |
| #7 | [mh "meningitis, cryptococcal"] or "cryptococcal meningitis":ti,ab,kw or "cryptococcal meningitis":ti,ab,kw or "cryptococcal meningitides":ti,ab,kw or "cerebral cryptococcosis":ti,ab,kw or "cerebral cryptococcoses":ti,ab,kw or toruloma*:ti,ab,kw or [mh "cryptococcus neoforman"] or "cryptococcus neoforman":ti,ab,kw or ((cryptococcal:ti,ab,kw or cryptococcal:ti,ab,kw or cyptococcosis:ti,ab,kw or cryptococcoses:ti,ab,kw or Cryptococcus:ti,ab,kw) and meningitis:ti,ab,kw) (Word variations have been searched) |
| #8 | [mh "antifungal agents"] or azole*:ti,ab,kw or fluconazole:ti,ab,kw or amphotericin:ti,ab,kw or flucytosine:ti,ab,kw or sertraline:ti,ab,kw or dexamethasone:ti,ab,kw or voriconazole:ti,ab,kw or acetazolamide:ti,ab,kw or diflucan:ti,ab,kw or itraconazole:ti,ab,kw or rifampin:ti,ab,kw or "5‐FC":ti,ab,kw (Word variations have been searched) |
| #9 | #6 and #7 and #8 in Trials |
LILACS, IMSEAR, African Medicus Index
cryptococc$ [Words] and meningitis or brain or menin$ [Words] and randomised or trial or controlled or placebo or double‐blind$ or single‐blind$ [Words]
Appendix 2. Sample study eligibility form
| Review title | Treatment of HIV‐associated cryptococcal meningitis | ||
| Reviewer ID | |||
| Study ID (first author surname and year of publication) | |||
| Study characteristics | Review inclusion criteria | Yes/No/Unclear |
Location in text (pg & ¶/fig/table) |
| 1. Type of study | Randomized trial | ||
| Non‐randomized trial | |||
| Cohort study | |||
| Quasi‐experimental study | |||
| Other design (specify): | |||
| 2. Participants | HIV‐infected with initial episode of cryptococcal meningitis? | ||
| 3. Diagnosis | Diagnosis confirmed by CSF India ink stain, fungal culture, or CrAg testing? | ||
| 4. Types of intervention | Two or more treatments compared? | ||
| 5. Types of outcome measures | Included mortality, safety, or microbiological outcome(s)? | ||
| 6. Decision: | (For inclusion, must answer YES for RCT for item 1 and YES for items 2‐6) | ||
| 7. Reason for exclusion | |||
| 8. Notes: | |||
Appendix 3. Data extraction form
| Methods | Study design: Randomization method: Blinding: |
| Participants | Country/countries: Inclusion criteria: Exclusion criteria: Number of participants: Number dropped out: Age: Gender: Number on ART: Baseline CD4 count: Baseline Glasgow coma scale (GCS) score or altered mental status: |
| Intervention |
For each intervention group Specific intervention
General to all intervention groups Protocol for LP after diagnostic LP: Toxicity monitoring: ART started after initiation of treatment for CM: Timing of ART initiation: |
| Outcomes | Intention‐to‐treat or per‐protocol outcomes: Mortality
Early fungicidal activity: Serious adverse events: Missing participant data: |
| Notes | Years study conducted: Sources of funding: Conflicts of interest: Potentially relevant studies in references: |
Data and analyses
Comparison 1. One week of AmBd + 5FC versus two weeks of AmBd + 5FC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.30, 1.03] |
| 1.2 10 weeks | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.42, 0.93] |
| 2 Early fungicidal activity | 1 | 186 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.02, 0.12] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.60] |
| 3.2 Renal dysfunction | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.18, 1.93] |
| 3.3 Neutropenia | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.43, 2.03] |
| 3.4 Hypokalaemia | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.50, 3.39] |
| 3.5 ALT abnormality | 1 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 3.05 [0.63, 14.81] |
Comparison 2. One week of AmBd + 5FC versus two weeks of AmBd + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.28, 0.97] |
| 1.2 10 weeks | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.39, 0.86] |
| 2 Early fungicidal activity | 1 | 192 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.14, ‐0.00] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.18, 0.71] |
| 3.2 Renal dysfunction | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.10, 0.85] |
| 3.3 Neutropenia | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.49, 2.51] |
| 3.4 Hypokalaemia | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.42, 2.45] |
| 3.5 LFT abnormality | 1 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.34, 3.03] |
Comparison 3. One week of AmBd + 5FC versus one week of AmBd + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.20, 0.63] |
| 1.2 10 weeks | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.34, 0.72] |
| 2 Early fungicidal activity | 1 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.15, ‐0.01] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.82] |
| 3.2 Renal dysfunction | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.09] |
| 3.3 Neutropenia | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.62, 3.84] |
| 3.4 Hypokalaemia | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.61, 5.11] |
| 3.5 ALT abnormality | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.61, 14.29] |
Comparison 4. One week of AmBd + 5FC versus two weeks of 5FC + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.35, 1.13] |
| 1.2 10 weeks | 1 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.47, 0.99] |
| 2 Early fungicidal activity | 1 | 280 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.24, ‐0.12] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.79, 4.13] |
| 3.2 Renal dysfunction | 1 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.24, 2.22] |
| 3.3 Neutropenia | 1 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.48, 1.88] |
| 3.4 Hypokalaemia | 1 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 5.97 [1.65, 21.63] |
| 3.5 ALT abnormality | 1 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [0.66, 6.03] |
Comparison 5. Two weeks of 5FC + FLU versus two weeks of AmBd + 5FC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.56, 1.37] |
| 1.2 10 weeks | 1 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.69, 1.23] |
| 2 Early fungicidal activity | 1 | 270 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.17, 0.29] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.09, 0.32] |
| 3.2 Renal dysfunction | 1 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.32, 2.02] |
| 3.3 Neutropenia | 1 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.51, 1.90] |
| 3.4 Hypokalaemia | 1 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.06, 0.83] |
| 3.5 ALT abnormality | 1 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.31, 7.48] |
Comparison 6. Two weeks of 5FC + FLU versus two weeks of AmBd + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.53, 1.29] |
| 1.2 10 weeks | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 2 Early fungicidal activity | 1 | 276 | Mean Difference (IV, Random, 95% CI) | 0.11 [0.05, 0.17] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.10, 0.39] |
| 3.2 Renal dysfunction | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.19, 0.85] |
| 3.3 Neutropenia | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.57, 2.36] |
| 3.4 Hypokalaemia | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.61] |
| 3.5 ALT abnormality | 1 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.17, 1.54] |
Comparison 7. Two weeks of AmBd + 5FC versus two weeks of AmBd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 3 | 612 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.44, 1.11] |
| 1.2 10 weeks | 2 | 231 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.95] |
| 1.3 6 months | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.46, 0.88] |
| 2 Early fungicidal activity | 2 | 225 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.26, ‐0.04] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.54, 1.06] |
| 3.2 Renal dysfunction | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.14, 6.89] |
| 3.3 Neutropenia | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 4.46 [0.99, 20.10] |
| 3.4 Hypokalaemia | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.64, 1.87] |
Comparison 8. Two weeks of AmBd + FLU versus two weeks of AmBd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.45, 1.77] |
| 1.2 10 weeks | 3 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.55, 1.62] |
| 1.3 6 months | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.13] |
| 2 Early fungicidal activity | 2 | 223 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.06, 0.02] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 3.2 Renal dysfunction | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.14, 6.96] |
| 3.3 Neutropenia | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 4.5 [1.00, 20.30] |
| 3.4 Hypokalaemia | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.34, 1.23] |
Comparison 9. Two weeks of AmBd + 5FC versus two weeks of AmBd + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 4 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.16] |
| 1.2 10 weeks | 4 | 538 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.69, 1.17] |
| 1.3 6 months | 1 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.53, 1.06] |
| 2 Early fungicidal activity | 4 | 474 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.14, ‐0.05] |
| 3 DAIDS grade 3/4 toxicities | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.89, 1.55] |
| 3.2 Renal dysfunction | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.31, 1.37] |
| 3.3 Neutropenia | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.61, 1.98] |
| 3.4 Hypokalaemia | 3 | 507 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.63, 2.27] |
| 3.5 ALT abnormality | 2 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.07, 1.60] |
Comparison 10. Two weeks of AmBd + FLU + steroids versus two weeks of AmBd + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.23] |
| 1.2 10 weeks | 1 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.93, 1.42] |
| 1.3 6 months | 1 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.99, 1.41] |
| 2 Early fungicidal activity | 1 | 450 | Mean Difference (IV, Random, 95% CI) | 0.1 [0.06, 0.14] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.90, 1.29] |
| 3.2 Renal dysfunction | 1 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.18, 2.16] |
| 3.3 Neutropenia | 1 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.02] |
| 3.4 Hypokalaemia | 1 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.69, 0.98] |
| 3.5 ALT abnormality | 1 | 450 | Risk Ratio (M‐H, Random, 95% CI) | 3.36 [0.94, 12.06] |
Comparison 11. One week of AmBd + FLU versus two weeks of AmBd + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.95, 2.29] |
| 1.2 10 weeks | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.88, 1.58] |
| 2 Early fungicidal activity | 1 | 175 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.06, 0.08] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.55, 1.42] |
| 3.2 Renal dysfunction | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.38, 1.70] |
| 3.3 Neutropenia | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.28, 1.82] |
| 3.4 Hypokalaemia | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.20, 1.65] |
| 3.5 ALT abnormality | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.07, 1.66] |
Comparison 12. One week of AmBd + FLU versus two weeks of AmBd + 5FC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.00, 2.43] |
| 1.2 10 weeks | 1 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.94, 1.72] |
| 2 Early fungicidal activity | 1 | 169 | Mean Difference (IV, Random, 95% CI) | 0.13 [0.06, 0.20] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.19] |
| 3.2 Renal dysfunction | 1 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.65, 4.05] |
| 3.3 Neutropenia | 1 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.25, 1.48] |
| 3.4 Hypokalaemia | 1 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.24, 2.26] |
| 3.5 ALT abnormality | 1 | 226 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.15, 7.23] |
Comparison 13. One week of AmBd + FLU versus two weeks of 5FC + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.21, 2.62] |
| 1.2 10 weeks | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.07, 1.80] |
| 2 Early fungicidal activity | 1 | 263 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.04] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 4.42 [2.25, 8.70] |
| 3.2 Renal dysfunction | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.91, 4.53] |
| 3.3 Neutropenia | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.39] |
| 3.4 Hypokalaemia | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 3.38 [0.82, 13.88] |
| 3.5 ALT abnormality | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.14, 3.29] |
Comparison 14. Two weeks of FLU versus two weeks of 5FC + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 3.04 [1.31, 7.06] |
| 1.2 10 weeks | 2 | 98 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.96, 2.23] |
| 2 Early fungicidal activity | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.08, 0.26] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.07, 16.47] |
| 3.2 Renal dysfunction | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.21 [0.22, 22.47] |
| 3.3 Neutropenia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.73] |
| 3.4 ALT abnormality | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. Two weeks of L‐AmB versus two weeks of AmBd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 10 weeks | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.04, 4.25] |
| 1.3 6 months | 1 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.11, 2.94] |
Comparison 16. Short‐course L‐AmB + FLU versus two weeks of L‐AmB + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.43, 7.59] |
| 1.2 10 weeks | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.47, 2.25] |
| 2 Early fungicidal activity | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.21, 0.01] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.22, 14.61] |
| 3.2 Renal dysfunction | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 4.10 [0.24, 71.15] |
| 3.3 Neutropenia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Hypokalaemia | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.58] |
| 3.5 ALT abnormality | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd + 5FC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 13.68] |
| 1.2 10 weeks | 1 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.33, 24.16] |
| 2 Early fungicidal activity | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.16 [0.03, 0.29] |
Comparison 18. Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.03, 1.53] |
| 1.2 10 weeks | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.37] |
| 2 Early fungicidal activity | 1 | 26 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.10, 0.12] |
Comparison 19. Two weeks of AmBd + 5FC + FLU versus two weeks of AmBd.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.05, 4.98] |
| 1.2 10 weeks | 1 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.24, 4.23] |
| 2 Early fungicidal activity | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.04] |
Comparison 20. One week of AmBd + 5FC + FLU versus one week of AmBd + FLU.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.5 [0.10, 2.43] |
| 1.2 10 weeks | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.35, 2.10] |
| 2 Early fungicidal activity | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.23, ‐0.01] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.20, 1.65] |
| 3.2 Renal dysfunction | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.8 [0.25, 2.55] |
| 3.3 Neutropenia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.39, 3.99] |
| 3.4 Hypokalaemia | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.12, 3.57] |
Comparison 21. Two weeks of AmBd + 5FC + IFNg versus two weeks of AmBd + 5FC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 2 weeks | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.28, 1.90] |
| 1.2 10 weeks | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.48, 1.77] |
| 2 Early fungicidal activity | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.24, ‐0.06] |
| 3 DAIDS grade 3/4 toxicities | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Anaemia | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.62, 2.11] |
| 3.2 Renal dysfunction | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.37, 1.98] |
| 3.3 Neutropenia | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [0.28, 6.60] |
| 3.4 Hypokalaemia | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.21, 72.44] |
| 3.5 ALT abnormality | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beardsley 2016.
| Methods |
Study type: blinded RCT Setting: 13 hospitals in Vietnam, Thailand, Indonesia, Laos, Uganda, and Malawi |
|
| Participants |
Inclusion criteria: HIV infected; ≥ 18 years of age; diagnosed with cryptococcal meningitis by CSF India ink or CrAg or blood or CSF culture Exclusion criteria: pregnant; more than 7 days of anticryptococcal therapy; renal failure; receiving glucocorticoids or glucocorticoids indicated for a pre‐existing condition; gastrointestinal bleeding Number randomized: 451 (1 excluded after randomization due to administrative error) Age: median 35 years Gender: 62% male CD4 T‐cell count: median 18 cells/μL (AmBd and fluconazole with dexamethasone); 20 cells/μL (AmBd and fluconazole with placebo) Baseline ART: 40% with prior ART use Baseline GCS/AMS: GCS < 15 in 19% of participants |
|
| Interventions |
Intervention
Consolidation: Fluconazole 800 mg/day for 8 weeks then 200 mg/day maintenance dose Postdiagnosis ART: median time to ART 46 days (AmBd and fluconazole with dexamethasone); 42 days (AmBd and fluconazole with placebo) Lumbar puncture schedule: scheduled on days 1, 3, 7, and 14 and performed as clinically indicated Laboratory monitoring: haemoglobin, white blood cells, platelet count days 1, 7, and 14; Na, K, urea, Cr days 1, 3, 7, 11, and 14 |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: Daily while hospitalized and scheduled assessments on days 21, 28, 42, 80, and 182 |
|
| Notes |
Date of study: 2013 to 2014 (study stopped early after data and safety monitoring committee determined dexamethasone therapy was causing harm across key outcomes) Funding: UK Department for International Development; The Wellcome Trust (UK); Medical Research Council (UK) Joint Global Health Trials Scheme Declaration of conflict of interest by authors: None reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomization with variable block size of 4 and 6 with computer‐generated sequence, stratified by study site |
| Allocation concealment (selection bias) | Low risk | Computer‐generated randomization accessible only to central pharmacists |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and clinicians blinded to intervention group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6‐month outcome available for all participants. |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Low risk | None noted. |
Brouwer 2004.
| Methods |
Study type: Unblinded RCT Setting: 1 hospital in Thailand |
|
| Participants |
Inclusion criteria: HIV infected; diagnosed by CSF India ink or CrAg test Exclusion criteria: pregnant; previous cryptococcal meningitis; ALT > 5 times upper limit of normal; ANC < 500 x 106/L; platelet count < 50 x 106/L; previous serious reaction to study drugs Number randomized: 64 Age: median 33 years Gender: 60% male CD4 T‐cell count: median 9 cells/μL Baseline ART: no Baseline GCS/AMS: GCS < 15 or seizures in 19% of participants |
|
| Interventions |
Intervention
Consolidation: fluconazole 400 mg/day for 8 weeks, then 200 mg/day maintenance dose Postdiagnosis ART: no participants started on ART within 10 weeks of follow‐up Lumbar puncture schedule: scheduled on days 3, 7, and 14 and as clinically indicated Laboratory monitoring: not specified |
|
| Outcomes |
Primary outcome
Secondary outcome
Outcome assessment schedule: daily while hospitalized and scheduled assessments on days 21, 28, 42, 80, and 182 |
|
| Notes |
Date of study: 2002 Funding: Wellcome Trust; Lancet international fellowship; St George’s Hospital; Netherlands Foundation for the Advancement of Tropical Medicine Declaration of conflict of interest by authors: None reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1:1:1 randomization with blocks of 16 with pre‐study generation of sealed envelopes prepared by an independent person |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes prepared by independent person |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes unblinded by assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes up to 10 weeks for all participants |
| Selective reporting (reporting bias) | Low risk | Authors reported all primary and secondary outcomes. |
| Other bias | Low risk | None noted. |
Day 2013.
| Methods |
Study type: unblinded RCT Setting: 1 hospital in Vietnam |
|
| Participants |
Inclusion criteria: HIV infected; > 14 years of age; positive CSF India ink or CrAg, CSF or blood culture, or blood CrAg with titre > 1:10 Exclusion criteria: pregnant; previous cryptococcosis; antifungal therapy for more than 3 days; renal or liver failure; receiving rifampicin; did not provide written informed consent Number randomized: 299 Age: median 28 years (AmBd); 28 years (AmBd and flucytosine); 27 years (AmBd and fluconazole) Gender: 82% male (AmBd); 80% male (AmBd and flucytosine); 85% male (AmBd and fluconazole) CD4 T‐cell count: median 18 cells/μL (AmBd); 17 cells/μL (AmBd and flucytosine); 14 cells/μL (AmBd and fluconazole) Baseline ART: 3% on ART at baseline Baseline GCS/AMS: GCS < 15 in 28% of participants |
|
| Interventions |
Intervention
Consolidation: fluconazole 400 mg/day up to 8 weeks, then 200 mg/day maintenance dose Postdiagnosis ART: ART initiation at discretion of physicians at follow‐up (89/299 of participants started ART within 6 months and 8/299 within 2 weeks of randomization) Lumbar puncture schedule: scheduled weekly for 1 month and as clinically indicated Laboratory monitoring: weekly CBC, urea, electrolytes, LFTs |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: Daily while hospitalized, then monthly until 6 months |
|
| Notes |
Date of study: 2004 to 2010 Funding: Wellcome Trust and British Infection Society Declaration of conflict of interest by authors: None reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1:1 randomization with computer‐generated sequence in blocks of 9 |
| Allocation concealment (selection bias) | Low risk | Randomization list held securely at trial centre |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes unblinded by assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status at 6 months missing in few (7/299) participants |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Low risk | None noted; pharmaceutical companies supplied reduced study drug but had no role in design or interpretation of study. |
Jackson 2012.
| Methods |
Study type: unblinded RCT Setting: 1 referral hospital in Malawi |
|
| Participants |
Inclusion criteria: HIV infected; ART‐naive; diagnosed by CSF India ink confirmed by CrAg test or culture or by CSF CrAg test Exclusion criteria: pregnant or breastfeeding; previous cryptococcal meningitis; ALT > 1200 IU/L; ANC < 500 x 106/L; platelets < 50 x 106/L; contraindication to any study medication Number randomized: 43 (3 excluded after randomization for false‐positive India ink) Age: median 35 years Gender: 65% male CD4 T‐cell count: median 41 cells/μL Baseline ART: none (excluded if prior ART) Baseline GCS/AMS: GCS < 15 in 25% of participants |
|
| Interventions |
Intervention 1: AmBd 1.0 mg/kg/day for 1 week and fluconazole 1200 mg/day for 2 weeks 2: AmBd 1.0 mg/kg/day for 1 week and fluconazole 1200 mg/day and flucytosine 100 mg/kg/day for 2 weeks Consolidation: fluconazole 800 mg/day for 2 weeks, then 400 mg/day for 8 weeks, then 200 mg/day maintenance dose Postdiagnosis ART: ART initiated at 4 weeks. Lumbar puncture schedule: scheduled on days 1, 3, 7, and 14 and as clinically indicated Laboratory monitoring: CBC, ALT, AST, K, Cr 3 times per week for first 2 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: daily while hospitalized, then scheduled for visit at 4 weeks to initiate ART, then followed until 10 weeks |
|
| Notes |
Date of study: 2009 to 2010 Funding: Medical Research Council (UK); Department for International Development in Malawi (UK) Declaration of conflict of interest by authors: none reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomization stratified by GCS < 15 using random computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes unblinded by assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status known for most participants (39/40) at 10 weeks |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Low risk | None noted. |
Jarvis 2012.
| Methods |
Study type: unblinded RCT Setting: 1 hospital in South Africa |
|
| Participants |
Inclusion criteria: HIV infected; ART‐naive; ≥ 21 years of age; positive CSF India ink or CrAg test Exclusion criteria: pregnant or breastfeeding; previous cryptococcal meningitis; ALT > 200 IU/mL; ANC < 500 x 106 cells/L; platelets < 50 x 106 cells/L; previous serious reaction to study drugs or contraindication to study drugs Number randomized: 90 (2 excluded after randomization due to prior episode of cryptococcal meningitis and low platelets) Age: median 32 years Gender: 44% male CD4 T‐cell count: median 27 cells/μL Baseline ART: none (excluded if prior ART) Baseline GCS/AMS: GCS < 15 in 37% of participants |
|
| Interventions |
Intervention
Consolidation: fluconazole 400 mg/day up to 8 weeks, then 200 mg/day maintenance dose Postdiagnosis ART: protocol for ART initiation between 2 and 4 weeks Lumbar puncture schedule: scheduled days 1, 3, 7, and 14 and as clinically indicated Laboratory monitoring: alternate‐day renal function and electrolyte testing and twice‐weekly CBC and LFTs during first 2 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: daily while hospitalized, then up to 1 year after enrolment |
|
| Notes |
Date of study: 2007 to 2010 Funding: Wellcome Trust Declaration of conflict of interest by authors: none reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1:1 randomization with random computer‐generated lists with block sizes of 8, stratified by GCS of 15 or GCS < 15 |
| Allocation concealment (selection bias) | Low risk | Numbers maintained in sealed envelopes prepared by independent individuals. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes unblinded by assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status at 10 weeks complete in all groups |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Low risk | None noted. |
Jarvis 2018.
| Methods |
Study type: unblinded RCT Setting: 1 referral hospital in Botswana and 2 in Tanzania |
|
| Participants |
Inclusion criteria: ≥ 18 years of age; positive CSF India ink or cryptococcal antigen Exclusion criteria: pregnant or breastfeeding; prior cryptococcal meningitis or more than 48 hours of antifungal treatment; previous serious reaction to study drugs Number randomized: 81 (1 participant excluded after randomization due to prior episode of cryptococcal meningitis) Age: median 38 years Gender: 54% male CD4 T‐cell count: median 32 cells/μL Baseline ART: 32% on ART at enrolment Baseline GCS/AMS: 28% with GCS < 15 |
|
| Interventions |
Intervention
Consolidation: fluconazole 800 mg/day for 8 weeks then 200 mg/day maintenance Postdiagnosis ART: initiated at 4 to 6 weeks in ART‐naive participants Lumbar puncture schedule: scheduled at days 3, 7, 14, and 21; additional therapeutic LP if CSF opening pressure > 30 cm H2O or symptoms of raised intracranial pressure Laboratory monitoring: alternate day renal function and electrolyte assessment and twice‐weekly CBC and ALT for first 2 weeks |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: clinical monitoring daily for first 2 weeks or until discharge (whichever was later); clinic follow‐up visits weeks 3, 4, 6, and 10 |
|
| Notes |
Date of study: 2014 to 2016 Funding: Gilead investigator‐initiated award Declaration of conflict of interest by authors: none reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by means of random computer‐generated lists with allocation ratio of 1:1 and block sizes of 8; stratified by GCS < 15 and ART status |
| Allocation concealment (selection bias) | Low risk | Randomization lists were accessible to an independent statistician who prepared sealed envelopes that were sent to study sites |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants. |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Low risk | Research funded by a pharmaceutical company through an investigator‐initiated grant; sponsors played no part in the design, conduct, data analysis, or publication. |
Leenders 1997.
| Methods |
Study type: unblinded RCT Setting: academic hospitals in the Netherlands and Australia |
|
| Participants |
Inclusion criteria: HIV infected; ≥ 18 years of age; positive CSF India ink or CrAg with confirmation by positive CSF culture or CSF CrAg with positive blood culture Exclusion criteria: previous cryptococcal meningitis; Cr > 250 μmol/L Number randomized: 30 (2 excluded after randomization including comatose patient without written informed consent from family and patient with negative CSF culture) Age: median 41 years (AmBd); 40 years (liposomal AmB) Gender: not specified CD4 T‐cell count: median 35 cells/μL (AmBd); 35 cells/μL (liposomal AmB) Baseline ART: 85% (AmBd); 47% (liposomal AmB) Baseline GCS/AMS: altered mental status 15% (AmBd); 40% (liposomal AmB) |
|
| Interventions |
Intervention
Consolidation: fluconazole 400 mg/day up to 10 weeks, then 200 mg/day maintenance dose Postdiagnosis ART: not specified Lumbar puncture schedule: scheduled days 7, 14, and 21, after 10 weeks, and as clinically indicated Laboratory monitoring: twice‐weekly BUN, Cr, and K; weekly CBC and LFTs |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: daily for first 3 weeks, then at least every 2 weeks until 10 weeks, with further follow‐up until 6 months |
|
| Notes |
Date of study: 1992 to 1995 Funding: NeXstar Pharmaceuticals Declaration of conflict of interest by authors: industry‐sponsored (role of pharmaceutical company, which manufactured study drug AmBisome, not specified) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomization stratified by clinical site; performed by means of sealed envelopes generated centrally at Dutch and Australian co‐ordination sites |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes maintained separately at study co‐ordinating centres |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes unblinded by assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Little loss to follow‐up in (1 of 25 participants) |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary outcomes not specific, reported on multiple clinical and mycological outcomes |
| Other bias | Unclear risk | Sponsored by drug manufacturer, involvement in study not clearly stated |
Loyse 2012.
| Methods |
Study type: unblinded RCT Setting: 2 hospitals in South Africa |
|
| Participants |
Inclusion criteria: HIV infected; ART‐naive; ≥ 18 years of age; positive CSF India ink confirmed by culture Exclusion criteria: pregnant or breastfeeding; previous cryptococcal meningitis; ALT > 200 IU/mL; ANC < 500 x 106 cells/L; platelets < 50 x 106 cells/L; previous serious reaction to study drugs or contraindication to study drugs Number randomized: 80 (1 excluded after randomization for prior history of cryptococcal meningitis) Age: median 34 years Gender: 49% male CD4 T‐cell count: median 37 cells/μL Baseline ART: none (excluded if prior ART) Baseline GCS/AMS: GCS < 15 in 15% of participants |
|
| Interventions |
Intervention
Consolidation: fluconazole 400 mg/day up to 8 weeks, then 200 mg/day maintenance dose Postdiagnosis ART: protocol for ART initiation > 2 weeks after starting antifungal therapy Lumbar puncture schedule: scheduled days 3, 7, and 14 and as clinically indicated Laboratory monitoring: alternate‐day renal function, twice‐weekly CBC and LFTs |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: daily while hospitalized, started on ART at 2 weeks, and followed up to 6 months from enrolment |
|
| Notes |
Date of study: 2006 to 2008 Funding: Wellcome Trust; Medical Research Council (UK) Declaration of conflict of interest by authors: none reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1:1:1 randomization using computer‐generated sequence, stratified by baseline altered mental status |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes unblinded by assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status at 10 weeks in most (75/79) participants |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Low risk | None noted. |
Mayanja‐Kizza 1998.
| Methods |
Study type: unblinded RCT Setting: 1 hospital in Uganda |
|
| Participants |
Inclusion criteria: HIV infected; ≥ 16 years of age; positive CSF India ink or CrAg Exclusion criteria: pregnant; antifungal therapy in the previous month; comatose Number randomized: 58 Age: median 34 years Gender: 43% male CD4 T‐cell count: mean 77 cells/μL Baseline ART: no Baseline GCS/AMS: excluded if comatose; 52% of participants noted to have normal sensorium (no stupor or somnolence) |
|
| Interventions |
Intervention
Consolidation: fluconazole 200 mg 3 times weekly Postdiagnosis ART: no Lumbar puncture schedule: scheduled after 2 months and 6 months Laboratory monitoring: laboratory monitoring schedule not specified |
|
| Outcomes |
Primary outcome
Secondary outcome
Outcome assessment schedule: Participants assessed by primary medicine service while hospitalized, with clinic follow‐up at 2, 4, 8, and 10 weeks, then monthly up to 3 years. |
|
| Notes |
Date of study: 1994 Funding: Pfizer; Japanese Ministry of Education, Science, and Culture Declaration of conflict of interest by authors: Not specified; role of pharmaceutical sponsor not specified |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 1:1 randomization; method of randomization not specified |
| Allocation concealment (selection bias) | Low risk | Used sealed envelopes for allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes unblinded by assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition of 8/58 participants by 6 months |
| Selective reporting (reporting bias) | Low risk | Authors reported on primary and secondary outcome measures. |
| Other bias | Unclear risk | Sponsored by pharmaceutical company; role of company in conduct of study not specified |
Molloy 2018.
| Methods |
Study type: unblinded RCT Setting: 9 hospitals in Malawi, Zambia, Tanzania, and Cameroon |
|
| Participants |
Inclusion criteria: ≥ 18 years of age; positive CSF India ink or cryptococcal antigen Exclusion criteria: pregnant or breastfeeding; prior cryptococcal meningitis; > 1 dose of AmBd or > 1 treatment dose (1200 mg) or > 7 low doses (200 mg) of fluconazole in the 2 weeks before screening; previous adverse reaction to study drugs; taking contraindicated concomitant drugs; ALT > 5 times upper limit of normal or ANC < 500 x 106/L included as late exclusion criteria after screening; patients with elevated creatinine > 220 µmol/L the day after enrolment despite hydration were withdrawn from the study Number randomized: 721 (43 excluded from all analyses because of predefined late exclusion criteria (30), immediately withdrew consent (3), cryptococcal meningitis negative (7), or prior cryptococcal meningitis (3)) Age: median 36 years (fluconazole and flucytosine), 38 years (combined 1‐week AmBd arms), 37 years (combined 2‐week AmBd arms) Gender: 53% male (fluconazole and flucytosine), 61% (combined 1‐week AmBd arms), 58% (combined 2‐week AmBd arms) CD4 T‐cell count: median 25 cells/μL (fluconazole and flucytosine), 26 cells/μL (combined 1‐week AmBd arms), 26 cells/μL (combined 2‐week AmBd arms) Baseline ART: 59% were taking or had previously taken ART overall Baseline GCS/AMS: 24% with GCS < 15 (fluconazole and flucytosine), 20% (combined 1‐week AmBd arms), 28% (combined 2‐week AmBd arms) |
|
| Interventions |
Intervention
Consolidation: fluconazole 800 mg/day for 4 weeks then 400 mg/day for 4 weeks then 200 mg/day thereafter Postdiagnosis ART: initiated at 4 weeks in ART‐naive participants or participants who had discontinued therapy Lumbar puncture schedule: scheduled at days 7 and 14; additional daily therapeutic LPs for participants with high CSF pressure until pressure was controlled Laboratory monitoring: laboratory blood tests monitored regularly during the first 2 weeks. |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: participants were followed daily in hospital and up to 10 weeks. |
|
| Notes |
Date of study: 2013 to 2016 Funding: Medical Research Council (UK); France REcherche Nord & Sud Sida‐HIV et Hépatites (France) Declaration of conflict of interest by authors: none reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization in 2:1:1:1:1 ratio (2 for fluconazole and flucytosine arm) using computer‐generated randomization with block sizes of 18, 24, and 30 at each site |
| Allocation concealment (selection bias) | Low risk | Trial pharmacist and clinician responsible for randomising participants by taking sealed envelopes containing randomized treatment sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low loss to follow‐up (4/678) |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Low risk | No other bias |
Nussbaum 2010.
| Methods |
Study type: unblinded RCT Setting: 1 referral hospital in Malawi |
|
| Participants |
Inclusion criteria: HIV infected; ART‐naive; diagnosed by CSF India ink or CrAg test
Exclusion criteria: pregnant or breastfeeding; previous cryptococcal meningitis; ALT > 200 IU/mL; ANC < 500 x 106/L; platelets < 50 x 106/L; contraindication to any study medication Number randomized: 43 (3 excluded after randomization for breastfeeding, false diagnosis due to sampling error, and positive CSF CrAg but negative confirmatory culture) Age: median 36 years Gender: 66% male CD4 T‐cell count: median 21 cells/μL Baseline ART: none (excluded if prior ART) Baseline GCS/AMS: GCS < 15 in 39% of participants |
|
| Interventions |
Intervention
Consolidation: fluconazole 800 mg/day for 2 weeks, then 400 mg/day for 8 weeks, then 200 mg/day maintenance dose Postdiagnosis ART: ART initiated at 4 weeks. Lumbar puncture schedule: scheduled on days 1, 3, 7, and 14 and as clinically indicated Laboratory monitoring: at least 3 blood counts per week and AST, ALT, and chemistries once weekly for 2 weeks, with ALT and AST repeated at weeks 4, 6, and 10 |
|
| Outcomes |
Primary outcome
Secondary outcomes
Outcome assessment schedule: daily while hospitalized, then scheduled for visit at 4 weeks to initiate ART, then followed until 10 weeks |
|
| Notes |
Date of study: 2008 (study terminated early when data safety monitoring committee found primary endpoint was reached in interim analysis) Funding: Medical Research Council (UK); UNC Center for AIDS Research Declaration of conflict of interest by authors: none reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1 randomization stratified by GCS < 15 using random computer‐generated list with block size of 8 |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes unblinded by assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Vital status known for most participants (39/40) at 10 weeks |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Low risk | None noted. |
Pappas 2009.
| Methods |
Study type: unblinded RCT Setting: 8 hospitals in the USA and 5 hospitals in Thailand |
|
| Participants |
Inclusion criteria: ≥ 13 years of age; positive CSF culture Exclusion criteria: pregnant or breastfeeding; prior cryptococcal meningitis; concomitant CNS illness that would interfere with assessment of response; creatinine clearance < 50 mL/min; ALT > 5 x ULN; coma; anticipated survival < 14 days; previous antifungal therapy > 3 days; untreated active tuberculosis Number randomized: 143 (8 excluded for negative CSF culture, withdrawal of consent before treatment, poor renal function, prior episode of cryptococcosis, or receipt of substantial antifungal therapy or rifampicin before study enrolment) Age: mean 37 years (AmBd); 36 years (AmBd and fluconazole 400 mg); 36 years (AmBd and fluconazole 800 mg) Gender: 65% male (AmBd); 65% male (AmBd and fluconazole 400 mg); 64% male (AmBd and fluconazole 800 mg) CD4 T‐cell count: median 18 cells/μL (AmBd); 17 cells/μL (AmBd and fluconazole 400 mg/day); 15 cells/μL (AmBd and fluconazole 800 mg/day) Baseline ART: 8% of participants on ART at baseline Baseline GCS/AMS: excluded if in coma |
|
| Interventions |
Intervention
Consolidation: fluconazole 400 mg/day for 8 weeks in intervention 1 and intervention 2; fluconazole 800 mg/day for 8 weeks in intervention 3 Postdiagnosis ART: participants on baseline ART continued; investigators discouraged initiation of ART before 42 days; at day 42, 17% (AmBd), 4% (AmBd and fluconazole 400 mg/day), and 22% (AmBd and fluconazole 800 mg/day) had initiated ART. Lumbar puncture schedule: encouraged aggressive management of raised intracranial pressure with LP; scheduled on days 14 and, if still positive, repeated on days 42 or 70 Laboratory monitoring: monitoring schedule not specified |
|
| Outcomes |
Primary outcomes
Secondary outcomes: none Outcome assessment schedule: participants evaluated regularly while hospitalized, then followed through 100 days. |
|
| Notes |
Date of study: 2005 to 2007 Funding: National Institutes of Health (USA); fluconazole donated by Pfizer Declaration of conflict of interest by authors: Authors declared research support from several pharmaceutical companies including manufacturer of study drug. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 1:1:1 randomization via adaptive randomization system stratified by country and CSF opening pressures (≤ 250 mm versus > 250 mm) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded data review committee adjudicated study data related to clinical and safety outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants. |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary clinical and safety outcomes. |
| Other bias | Unclear risk | Several authors received research support from pharmaceutical companies, including manufacturer of study drug. |
van der Horst 1997.
| Methods |
Study type: 2‐step, double‐blinded RCT Setting: Multiple hospitals in the USA |
|
| Participants |
Inclusion criteria: ≥ 13 years of age; positive CSF culture Exclusion criteria: pregnant or breastfeeding; prior cryptococcal meningitis or more than 1 mg/kg amphotericin B or 1200 mg fluconazole, itraconazole, or ketoconazole for current episode; taking medications that affected metabolism or decreased absorption of itraconazole; receiving ≥ 50 mg/day hydrocortisone; history of acute hepatitis or moderate‐to‐severe haematologic, renal, or hepatic dysfunction; comatose; unable to take medications by mouth or nasogastric tube; had a history of other opportunistic infections requiring treatment during the first 2 weeks Number randomized: 408 randomized in step 1 randomization to AmBd and flucytosine or AmBd (27 ineligible due to negative CSF culture (19), receiving corticosteroids or rifampicin (4), prior cryptococcal disease (2), HIV‐negative (1), or pregnant (1)); 306 randomized in step 2 to fluconazole or itraconazole maintenance of stable or improved in step 1, receipt minimal dose of 7.5 mg/kg AmBd, ability to take oral medications, hepatic enzymes less than 10 times ULN and bilirubin less than 2 times ULN Age: median 37 years Gender: 87% male (AmBd and flucytosine); 91% male (AmBd) CD4 T‐cell count: median 20 cells/μL (AmBd and flucytosine); 18 cells/μL (AmBd) Baseline ART: 27% (AmBd and flucytosine); 26% (AmBd) Baseline GCS/AMS: 89% with normal mental status |
|
| Interventions |
Step 1 induction
Step 2 consolidation
Postdiagnosis ART: not specified Lumbar puncture schedule: scheduled lumbar punctures at weeks 2, 4, and 10 Laboratory monitoring: monitoring twice weekly for 2 weeks; weekly for next 2 weeks; then every 2 weeks until 10 weeks |
|
| Outcomes |
Primary outcomes
Secondary outcome
Outcome assessment schedule: clinical monitoring twice weekly for 2 weeks; weekly for next 2 weeks; then every 2 weeks until 10 weeks |
|
| Notes |
Date of study: 1991 to 1994 Funding: National Institute of Allergy and Infectious Diseases, AIDS Clinical Trial Group, National Center for Research Services, and Janssen Research Foundation; drugs provided by Bristol‐Myers Squibb (AmBd), Roche Laboratories (flucytosine), Roerig‐Pfizer (fluconazole), and Janssen Research Foundation (itraconazole) Declaration of conflict of interest by authors: research supported by several pharmaceutical companies as well as public sources |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not specified; stratified by altered mental status and institution in step 1 and baseline mental status and therapy received in step 2 |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported for all participants. |
| Selective reporting (reporting bias) | Low risk | Authors reported on all primary and secondary outcomes. |
| Other bias | Unclear risk | Research funded in part by pharmaceutical companies, including manufacturer of study drug. |
Abbreviations: ALT: alanine aminotransferase; AmBd: amphotericin B deoxycholate; AMS: altered mental status; ANC: absolute neutrophil count; ART: antiretroviral therapy; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CBC: complete blood count; CD4: cluster of differentiation 4; CNS: central nervous system; CrAg: cryptococcal antigen; Cr: creatinine; CSF: cerebrospinal fluid; DAIDS: Division of AIDS; GCS: Glasgow coma scale; K: potassium; LFT: liver function test; LP: lumbar puncture; Na: sodium; RCT: randomized controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bicanic 2008 | Compared 2 interventions of the same combination antifungal drugs, 2 weeks of AmBd 0.7 mg/kg/day or AmBd 1 mg/kg/day both combined with flucytosine 100 mg/kg/day |
| Bisson 2013 | Small trial evaluating early (within 7 days) versus delayed (after 4 weeks) ART initiation following diagnosis of cryptococcal meningitis; all participants received standard induction treatment of AmBd 0.7 mg/kg/day for 2 weeks |
| Boulware 2014b | RCT comparing early versus delayed ART initiation in participants treated with AmBd and fluconazole; patients were enrolled 1 week after initiating therapy, before which an estimated 12% of patients had already died |
| Bozzette 1991 | RCT with participants randomized to maintenance therapy |
| Brouwer 2007 | Subanalysis of included RCT, Brouwer 2004, comparing participants treated with IV versus oral formulations of flucytosine |
| Chariyalertsak 2002 | RCT with participants randomized to consolidation therapy, not induction therapy |
| Chetchotisakd 2004 | RCT of primary prophylaxis against cryptococcal meningitis, not induction therapy |
| Chotmongkol 1997 | RCT of consolidation therapy with itraconazole versus no therapy after CSF culture clearance |
| Chotmongkol 2005 | Small study of 40 participants in which participants in study arms were not well matched for severity of disease (45% in AmBd plus rifampicin arm versus 0% in AmBd arm had positive Cryptococcus blood cultures at baseline); important baseline characteristics including CD4 T‐cell count were not described |
| de Gans 1992 | Small study of 25 participants randomized to itraconazole versus AmBd and flucytosine for induction therapy where 2/14 participants in itraconazole group crossed over within 4 hours and an additional 2 participants were lost to follow‐up |
| Hamill 2010 | Included re‐treatment cases of cryptococcal meningitis |
| Jadhav 2010 | Excluded patients who had not received a minimum of 8 doses of liposomal amphotericin treatment, introducing immortality bias |
| Joly 1996a | Included patients with relapsed cryptococcal meningitis |
| Joly 1996b | Included patients with relapsed cryptococcal meningitis |
| Larsen 1990 | Small study of 20 participants comparing fluconazole to AmBd and flucytosine, which was excluded for several reasons: did not do intention‐to‐treat analysis; excluded significant number of participants (6/26) from analysis after enrolment; enrolled non‐HIV‐positive patient; final comparison groups poorly matched on CD4 count |
| Makadzange 2010 | Evaluated timing of ART (within 72 hours of cryptococcal meningitis diagnosis or after 10 weeks); all participants received fluconazole induction therapy; a large proportion of patients died before enrolment, introducing a risk of immortality bias |
| Mfinanga 2015 | RCT with participants randomized to intervention including cryptococcal antigen screening for prevention of cryptococcal meningitis |
| Mootsikapun 2003 | RCT with participants randomized to consolidation therapy, not induction therapy |
| Newton 2002 | Small RCT of adjunctive acetazolamide that did not limit inclusion to patients with HIV‐associated cryptococcal meningitis |
| Pappas 2004 | Included patients with relapsed cryptococcal meningitis |
| Powderly 1992 | RCT of secondary prophylaxis for prevention of recurrent cryptococcal meningitis |
| Powderly 1995 | RCT of primary prophylaxis for fungal infections |
| Rhein 2016 | Initially was a dose escalation study of adjunctive sertraline as therapy for cryptococcal meningitis; investigators compared outcomes to historic controls |
| Saag 1991 | RCT that included patients with relapsed cryptococcal meningitis |
| Saag 1999 | RCT of consolidation therapy for cryptococcal meningitis |
| Sharkey 1996 | Small RCT comparing AmBd with different doses of liposomal amphotericin B in which CD4 count and other baseline characteristics were not well matched between groups |
| Tansuphaswadikul 2006 | Small RCT comparing 1‐week and 2‐week induction therapy with AmBd with very high loss to follow‐up (33%) by 10 weeks, which could bias mortality results |
| Techapornroong 2007 | Small RCT comparing daily and alternate‐day AmBd induction therapy with very high loss to follow‐up (25%) by 3 months, which could bias mortality results |
| Vaidhya 2015 | RCT comparing AmBd with AmBd and fluconazole that did not include any primary or secondary outcomes (mortality, early fungicidal activity, or adverse events) |
| Vibhagool 2003 | RCT of secondary prophylaxis for cryptococcal meningitis |
| Villanueva‐Lozano 2018 | Small RCT of 12 participants comparing amphotericin and fluconazole with adjunctive sertraline and amphotericin and fluconazole with placebo that had significant imbalance in CD4 count between treatment arms; also excluded one patient from analysis for not receiving per‐protocol treatment |
Abbreviations: AmBd: amphotericin B deoxycholate; ART: antiretroviral therapy; CD4: cluster of differentiation 4; CSF: cerebrospinal fluid; IV: intravenous; RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
ISRCTN72509687.
| Trial name or title | High dose AMBISOME on a fluconazole backbone for cryptococcal meningitis induction therapy in sub‐Saharan Africa |
| Methods | Phase 3 non‐inferiority RCT from 6 sites in South Africa, Botswana, Zimbabwe, Malawi, and Uganda with planned enrolment of 850 participants |
| Participants | Age ≥ 18 years with first episode of HIV‐associated cryptococcal meningitis |
| Interventions | (i) Single dose of 10 mg/kg IV liposomal AmB with 2 weeks 5FC 100 mg/kg/day and FLU 1200 mg/day (ii) 1 week of AmBd 1 mg/kg/day and 5FC 100 mg/kg/day followed by FLU 1200 mg/day days 8 to 14 |
| Outcomes | Primary: 10‐week mortality |
| Starting date | 2017 |
| Contact information | joseph.jarvis@lshtm.ac.uk |
| Notes | Original protocol was to compare short‐course L‐AmB and 2 weeks of AmBd with FLU as a second drug for both regimens; however, protocol was modified after results from Molloy 2018 study became available showing mortality benefit with 1 week of AmBd and 5FC followed by FLU days 8 to 14. |
NCT00885703.
| Trial name or title | High‐dose fluconazole for the treatment of cryptococcal meningitis in HIV‐positive individuals |
| Methods | Phase 1/2 dose‐finding RCT of high‐dose fluconazole for HIV‐associated cryptococcal meningitis with or without AmBd 0.7 to 1.0 mg/kg/day |
| Participants | Age ≥ 16 years with HIV‐associated cryptococcal meningitis |
| Interventions | Multistage study of high‐dose FLU with or without AmBd |
| Outcomes | Primary: discontinuation of study‐provided FLU or AmBd; qualitative and quantitative CSF culture results at entry, 2 weeks, and through 24 weeks; survival up to 24 weeks |
| Starting date | 2010 |
| Contact information | umeshlalloo@gmail.com |
| Notes | Completed study with results not published in peer‐reviewed journal but presented at Conference on Retroviruses and Opportunistic Infections 2018 |
NCT01802385.
| Trial name or title | Adjunctive sertraline for the treatment of HIV‐associated cryptococcal meningitis (ASTRO‐CM) |
| Methods | Phase 3 RCT |
| Participants | ≥ 18 years of age with HIV‐associated cryptococcal meningitis |
| Interventions | 2 weeks of AmBd 0.7 to 1.0 mg/kg/day and FLU 800 to 1200 mg/day plus sertraline 400 mg/day for 2 weeks then sertraline 200 mg/day for 12 weeks then tapered over 3 weeks versus 2 weeks of AmBd 0.7 to 1.0 mg/kg/day and FLU 800 to 1200 mg/day |
| Outcomes | Primary: 18‐week survival |
| Starting date | 2015 |
| Contact information | david.meya@gmail.com |
| Notes | Study terminated during interim monitoring for futility, results not published in peer‐reviewed journal but presented at Conference on Retroviruses and Opportunistic Infections 2018. |
Abbreviations: 5FC: flucytosine; AmBd: amphotericin B deoxycholate; CSF: cerebrospinal fluid; FLU: fluconazole; IV: intravenous; L‐AmB: liposomal amphotericin; RCT: randomized controlled trial
Differences between protocol and review
This is an update of a published Cochrane review (Sloan 2011). The new review author team extensively revised the protocol, which is available on the CIDG website at cidg.cochrane.org/our‐reviews under the subheading ‘Related content'.
Differences between the revised protocol (10 August 2017) and review update
Types of studies
The protocol stated that, in addition to comparing different treatment regimens, we would also make comparisons between different doses of a single agent or the same induction therapy with different timing of antiretroviral therapy. However, as timing of ART initiation for HIV‐associated cryptococcal meningitis is a distinct clinical question, we excluded studies on timing of ART from our review (Makadzange 2010; Bisson 2013; Boulware 2014b). Additionally, we excluded one RCT that compared different doses of amphotericin B deoxycholate (0.7 mg/kg/day or 1 mg/kg/day) with flucytosine, as this RCT was not subject to analysis in either pairwise comparison or NMA (Bicanic 2008).
Types of participants
The protocol stated that studies with a significant amount of missing outcomes data would either be excluded or presented with sensitivity analyses. Out of concern that participants lost to follow‐up were not missing at random and were more likely to have died compared to participants with complete follow‐up, in the review we instead excluded any study with missing outcomes data within six months on more than 20% of participants.
Methods
Secondary outcomes
For our secondary outcome of serious adverse events, the protocol did not provide the specific laboratory toxicities we would compare between induction regimens. In the review, we limited these to common toxicities from antifungal therapies used for the treatment of cryptococcal meningitis, including anaemia, renal dysfunction, neutropenia, hypokalaemia, and liver function test abnormalities (specifically alanine aminotransferase).
Dealing with missing data
The protocol stated that we would perform sensitivity analyses for studies with significant missingness of outcomes data. Because we believed that missingness of outcomes data was unlikely to be missing at random, we excluded any study with missing outcomes data within six months on more than 20% of participants
Presentation of results
The protocol stated that we would perform GRADE assessments for pairwise meta‐analysis and modified GRADE assessment for the network meta‐analysis but did not state at which mortality time point (2 weeks, 10 weeks, or 6 months) assessments would be made. In the review, we limited GRADE assessment to 10‐week mortality, as we believed this was the most clinically relevant time point, and all included studies followed participants at both two‐week and 10‐week mortality endpoints (with 10‐week mortality for one study excluded due to secondary randomization after two weeks) (van der Horst 1997). Few studies followed participants up to six months, so we did not perform GRADE assessment at this time point or six‐month network meta‐analysis due to the limited number of network comparisons. Additionally, although not explicitly stated in the protocol, in the review we downgraded for imprecision studies with fewer than 200 deaths overall in pairwise comparisons. Most studies were at high risk of performance and detection bias according to the Cochrane ‘Risk of bias' assessment. However, we decided not to downgrade studies in the GRADE risk of bias domain for performance and detection bias, as we believed that our primary outcome of interest, death, was unlikely to be influenced by performance or detection bias.
Contributions of authors
Mark W Tenforde contributed to study design and protocol writing, performed literature reviews and data extraction, data analysis and interpretation, and wrote the first draft of the manuscript.
Adrienne E Shapiro contributed to study design and protocol writing, performed literature reviews and data extraction, and provided input on the manuscript.
Benjamin Rouse contributed to study design and protocol writing, performed data analysis for the network meta‐analysis, and provided input on the manuscript.
Joseph N Jarvis provided data interpretation and collection and provided input on the manuscript.
Tianjing Li contributed to study design, supervised data analysis for the network meta‐analysis, and provided input on the manuscript.
Ingrid Eshun‐Wilson contributed to study design, data interpretation, and provided input on the manuscript.
Nathan Ford contributed to study design and protocol writing, data and interpretation, and and provided input on the manuscript.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Grant: 5242
Declarations of interest
Mark W Tenforde declares no relevant conflicts of interest. Adrienne E Shapiro has received salary compensation from the University of Washington, as a trainee postdoctoral fellow in Infectious Diseases. A portion of the salary derives from an NIH T32 training grant. Benjamin Rouse declares no relevant conflicts of interest. Joseph N Jarvis has been an investigator in several clinical trials of therapies for HIV‐associated cryptococcal meningitis. He has previously received grant support through his institution from Gilead Sciences Europe (investigator initiated award for the Ambition Phase II Trial) Tianjing Li declares no relevant conflicts of interest. Ingrid Eshun‐Wilson declares no relevant conflicts of interest. Nathan Ford declares no relevant conflicts of interest.
Unchanged
References
References to studies included in this review
Beardsley 2016 {published data only}
- Beardsley J, Wolbers M, Kibengo FM, Ggayi AB, Kamali A, Cuc NT, et al. Adjunctive dexamethasone in HIV‐associated cryptococcal meningitis. New England Journal of Medicine 2016;374(6):542‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brouwer 2004 {published data only}
- Brouwer AE, Rajanuwong A, Chierakul W, Griffin GE, Larsen RA, White NJ, et al. Combination antifungal therapies for HIV‐associated cryptococcal meningitis: a randomised trial. Lancet 2004;363(9423):1764‐7. [DOI] [PubMed] [Google Scholar]
Day 2013 {published data only}
- Day JN, Chau TT, Wolbers M, Mai PP, Dung NT, Mai NH, et al. Combination antifungal therapy for cryptococcal meningitis. New England Journal of Medicine 2013;368(14):1291‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2012 {published data only}
- Jackson AT, Nussbaum JC, Phulusa J, Namarika D, Chikasema M, Kanyemba C, et al. A phase II randomized controlled trial adding oral flucytosine to high‐dose fluconazole, with short‐course amphotericin B, for cryptococcal meningitis. AIDS 2012;26(11):1363‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarvis 2012 {published data only}
- Jarvis JN, Meintjes G, Rebe K, Williams GN, Bicanic T, Williams A, et al. Adjunctive interferon‐γ immunotherapy for the treatment of HIV‐associated cryptococcal meningitis: a randomized controlled trial. AIDS 2012;26(9):1105‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarvis 2018 {published data only}
- Jarvis JN, Leeme TB, Molefi M, Chofle AA, Bidwell G, Tsholo K, et al. Short course high‐dose liposomal amphotericin B for HIV‐associated cryptococcal meningitis: a phase‐II randomised controlled trial. Clinical Infectious Diseases 2018 June 26 [Epub ahead of print]. [DOI: 10.1093/cid/ciy515] [DOI] [PMC free article] [PubMed]
Leenders 1997 {published data only}
- Leenders AC, Reiss P, Portegies P, Clezy K, Hop WC, Hoy J, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS‐associated cryptococcal meningitis. AIDS 1997;11(12):1463‐71. [DOI] [PubMed] [Google Scholar]
Loyse 2012 {published data only}
- Loyse A, Wainwright H, Jarvis JN, Bicanic T, Rebe K, Meintjes G, et al. Histopathology of the arachnoid granulations and brain in HIV‐associated cryptococcal meningitis: correlation with cerebrospinal fluid pressure. AIDS 2010;24(3):405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mayanja‐Kizza 1998 {published data only}
- Mayanja‐Kizza H, Oishi K, Mitarai S, Yamashita H, Nalongo K, Watanabe K, et al. Combination therapy with fluconazole and flucytosine for cryptococcal meningitis in Ugandan patients with AIDS. Clinical Infectious Diseases 1998;26(6):1362‐6. [DOI] [PubMed] [Google Scholar]
Molloy 2018 {published data only}
- Molloy S, Kanyama C, Heyderman R, Loyse A, Kouanfack C, Chanda D, et al. Antifungal combinations for treatment of cryptococcal meningitis in Africa. New England Journal of Medicine 2018;378(11):1004‐17. [DOI] [PubMed] [Google Scholar]
Nussbaum 2010 {published data only}
- Nussbaum JC, Jackson A, Namarika D, Phulusa J, Kenala J, Kanyemba C, et al. Combination flucytosine and high‐dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi. Clinical Infectious Diseases 2010;50(3):338‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pappas 2009 {published data only}
- Pappas PG, Chetchotisakd P, Larsen RA, Manosuthi W, Morris MI, Anekthananon T. A phase II randomized trial of amphotericin B alone or combined with fluconazole in the treatment of HIV‐associated cryptococcal meningitis. Clinical Infectious Diseases 2009;48(12):1775‐83. [DOI] [PubMed] [Google Scholar]
van der Horst 1997 {published data only}
- Horst CM, Saag MS, Cloud GA, Hamill RJ, Graybill JR, Sobel JD, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. National Institute of Allergy and Infectious Diseases Mycoses Study Group and AIDS Clinical Trials Group. New England Journal of Medicine 1997;337(1):15‐21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bicanic 2008 {published data only}
- Bicanic T, Wood R, Meintjes G, Rebe K, Brouwer A, Loyse A, et al. High‐dose amphotericin B with flucytosine for the treatment of cryptococcal meningitis in HIV‐infected patients: a randomized trial. Clinical Infectious Diseases 2008;47(1):123‐30. [DOI] [PubMed] [Google Scholar]
Bisson 2013 {published data only}
- Bisson GP, Molefi M, Bellamy S, Thakur R, Steenhoff A, Tamuhla N, et al. Early versus delayed antiretroviral therapy and cerebrospinal fluid fungal clearance in adults with HIV and cryptococcal meningitis. Clinical Infectious Diseases 2013;56(8):1165‐73. [DOI] [PubMed] [Google Scholar]
Boulware 2014b {published data only}
- Boulware DR, Meya DB, Muzoora C, Rolfes MA, Huppler Hullsiek K, Musubire A, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. New England Journal of Medicine 2014;370(26):2487‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bozzette 1991 {published data only}
- Bozzette SA, Larsen RA, Chiu J, Leal MAE, Jacobsen J, Rothman P, et al. A placebo‐controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. New England Journal of Medicine 1991;324(9):580‐4. [DOI] [PubMed] [Google Scholar]
Brouwer 2007 {published data only}
- Brouwer AE, Kan HJM, Johnson E, Rajanuwong A, Teparrukkul P, Wuthiekanun V, et al. Oral versus intravenous flucytosine in patients with human immunodeficiency virus‐associated cryptococcal meningitis. Antimicrobial Agents and Chemotherapy 2007;51(3):1038‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chariyalertsak 2002 {published data only}
- Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clinical Infectious Diseases 2002;32(2):277‐84. [DOI] [PubMed] [Google Scholar]
Chetchotisakd 2004 {published data only}
- Chetchotisakd P, Sungkanuparph S, Thinkhamrop B, Mootsikapun P, Boonyaprawit P. A multicentre, randomized, double‐blind, placebo‐controlled trial of primary cryptococcal meningitis prophylaxis in HIV‐infected patients with severe immune deficiency. HIV Medicine 2004;5(3):140‐3. [DOI] [PubMed] [Google Scholar]
Chotmongkol 1997 {published data only}
- Chotmongkol V, Sukeepaisarncharoen W, Thavornpitak Y. Comparison of amphotericin B, flucytosine and itraconazole with amphotericin B and flucytosine in the treatment of cryptococcal meningitis in AIDS. Journal of the Medical Association of Thailand 1997;80(7):416‐25. [PubMed] [Google Scholar]
Chotmongkol 2005 {published data only}
- Chotmongkol V, Arayawichanont A, Sawanyawisuth K, Thavornpitak Y. Initial treatment of cryptococcal meningitis in AIDS. Southeast Asian Journal of Tropical Medicine and Public Health 2005;36(1):170‐3. [PubMed] [Google Scholar]
de Gans 1992 {published data only}
- Gans J, Portegies P, Tiessens G, Schattenkerk JKME, Boxtel CJ, Ketel RJ, et al. Itraconazole compared with amphotericin B plus flucytosine in AIDS patients with cryptococcal meningitis. AIDS 1992;6(2):185‐90. [DOI] [PubMed] [Google Scholar]
Hamill 2010 {published data only}
- Hamill RJ, Sobel JD, El‐Sadr W, Johnson PC, Graybill JR, Javaly K, et al. Comparison of 2 doses of liposomal amphotericin B and conventional amphotericin B deoxycholate for treatment of AIDS‐associated acute cryptococcal meningitis: a randomized, double‐blind clinical trial of efficacy and safety. Clinical Infectious Diseases 2010;51(2):225‐32. [DOI] [PubMed] [Google Scholar]
Jadhav 2010 {published data only}
- Jadhav MP, Bamba A, Shinde VM, Gogtay N, Kshirsagar NA, Bichile LS, et al. Liposomal amphotericin B (Fungisome) for the treatment of cryptococcal meningitis in HIV/AIDS patients in India: a multicentric, randomized controlled trial. Journal of Postgraduate Medicine 2010;56(2):71‐5. [DOI] [PubMed] [Google Scholar]
Joly 1996a {published data only}
- Joly V, Aubry P, Ndayiragide A, Carriere I, Kawa E, Mlika‐Cabanne N, et al. Randomized comparison of amphotericin B deoxycholate dissolved in dextrose or Intralipid for the treatment of AIDS‐associated cryptococcal meningitis. Clinical Infectious Diseases 1996;23(3):556‐62. [DOI] [PubMed] [Google Scholar]
Joly 1996b {published data only}
- Joly V, Geoffray C, Reynes J, Goujard C, Méchali D, Maslo C, et al. Amphotericin B in a lipid emulsion for the treatment of cryptococcal meningitis in AIDS patients. Journal of Antimicrobial Chemotherapy 1996;38(1):117‐26. [DOI] [PubMed] [Google Scholar]
Larsen 1990 {published data only}
- Larsen RA, Leal MAE, Chan LS. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS. A randomized trial. Annals of Internal Medicine 1990;113(3):183‐7. [DOI] [PubMed] [Google Scholar]
Makadzange 2010 {published data only}
- Makadzange AT, Ndhlovu CE, Takarinda K, Reid M, Kurangwa M, Gona P, et al. Early versus delayed initiation of antiretroviral therapy for concurrent HIV infection and cryptococcal meningitis in sub‐saharan Africa. Clinical Infectious Diseases 2010;50(11):1532‐8. [DOI] [PubMed] [Google Scholar]
Mfinanga 2015 {published data only}
- Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community‐based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open‐label, randomised controlled trial. Lancet 2015;385(9983):2173‐82. [DOI] [PubMed] [Google Scholar]
Mootsikapun 2003 {published data only}
- Mootsikapun P, Chetchotisakd P, Anunnatsiri S, Choksawadphinyo K. The efficacy of fluconazole 600 mg/day versus itraconazole 600 mg/day as consolidation therapy of cryptococcal meningitis in AIDS patients. Journal of the Medical Association of Thailand 2003;86(4):293‐8. [PubMed] [Google Scholar]
Newton 2002 {published data only}
- Newton PN, Thai le H, Tip NQ, Short JM, Chierakul W, Rajanuwong A, et al. A randomized, double‐blind, placebo‐controlled trial of acetazolamide for the treatment of elevated intracranial pressure in cryptococcal meningitis. Clinical Infectious Diseases 2002;36(5):769‐72. [DOI] [PubMed] [Google Scholar]
Pappas 2004 {published data only}
- Pappas PG, Bustamante B, Ticona E, Hamill RJ, Johnson PC, Reboli A. Recombinant interferon‐gamma 1b as adjunctive therapy for AIDS‐related acute cryptococcal meningitis. Journal of Infectious Diseases 2004;189(12):2185‐91. [DOI] [PubMed] [Google Scholar]
Powderly 1992 {published data only}
- Powderly WG, Saag MS, Cloud GA, Robinson P, Meyer RD, Jacobson JM, et al. A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome. The NIAID AIDS Clinical Trials Group and Mycoses Study Group. New England Journal of Medicine 1992;326(12):783‐8. [DOI] [PubMed] [Google Scholar]
Powderly 1995 {published data only}
- Powderly WG, Finkelstein D, Feinberg J, Frame P, He W, Horst C, et al. A randomized trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. New England Journal of Medicine 1995;332(11):700‐5. [DOI] [PubMed] [Google Scholar]
Rhein 2016 {published data only}
- Rhein J, Morawski BM, Hullsiek KH, Nabeta HW, Kiggundu R, Tugume L, et al. Efficacy of adjunctive sertraline for the treatment of HIV‐associated cryptococcal meningitis: an open‐label dose‐ranging study. Lancet Infectious Diseases 2016;16(7):809‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saag 1991 {published data only}
- Saag MS, Powderly WG, Cloud GA, Robinson P, Grieco MH, Sharkey PK, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS‐associated cryptococcal meningitis. The NIAID Mycoses Study Group and the AIDS Clinical Trials Group. New England Journal of Medicine 1992;326(2):83‐9. [DOI] [PubMed] [Google Scholar]
Saag 1999 {published data only}
- Saag MS, Cloud GA, Graybill JR, Sobel JD, Tuazon CU, Johnson PC, et al. A comparison of itraconazole versus fluconazole as maintenance therapy for AIDS‐associated cryptococcal meningitis. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clinical Infectious Diseases 1999;28(2):291‐6. [DOI] [PubMed] [Google Scholar]
Sharkey 1996 {published data only}
- Sharkey PK, Graybill JR, Johnson ES, Hausrath SG, Pollard RB, Kolokathis A. Amphotericin B lipid complex compared with amphotericin B in the treatment of cryptococcal meningitis in patients with AIDS. Clinical Infectious Diseases 1996;22(2):315‐21. [PubMed] [Google Scholar]
Tansuphaswadikul 2006 {published data only}
- Tansuphaswadikul S, Maek‐a‐Nantawat W, Phonrat B, Boonpokbn L, Mctm AG, Pitisuttithum P. Comparison of one week with two week regimens of amphotericin B both followed by fluconazole in the treatment of cryptococcal meningitis among AIDS patients. Journal of the Medical Association of Thailand 2006;89(10):1677‐85. [PubMed] [Google Scholar]
Techapornroong 2007 {published data only}
- Techapornroong M, Suankratay C. Alternate‐day versus once‐daily administration of amphotericin B in the treatment of cryptococcal meningitis: a randomized controlled trial. Scandinavian Journal of Infectious Diseases 2007;39(10):896‐901. [DOI] [PubMed] [Google Scholar]
Vaidhya 2015 {published data only}
- Vaidhya SA, Gupta BB, Jha RK, Kumar R. Combination versus monotherapy for the treatment of HIV associated cryptococcal meningitis. Journal of Clinical and Diagnostic Research 2015;9(2):OC14‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vibhagool 2003 {published data only}
- Vibhagool A, Sungkanuparph S, Mootsikapun P, Chetchotisakd P, Tansuphaswaswadikul S, Bowonwatanuwong C. Discontinuation of secondary prophylaxis for cryptococcal meningitis in human immunodeficiency virus‐infected patients treated with highly active antiretroviral therapy: a prospective, multicenter, randomized study. Clinical Infectious Diseases 2003;36(10):1329‐31. [DOI] [PubMed] [Google Scholar]
Villanueva‐Lozano 2018 {published data only}
- Villanueva‐Lozano H, Trevino‐Rangel RJ, Gonzalez GM, Hernandez‐Rodriguez PA, Camacho‐Ortiz A, Castillo‐Reyna L, et al. Clinical evaluation of the antifungal effect of sertraline in the treatment of cryptococcal meningitis in HIV patients: a single Mexican center experience. Infection 2018;46(1):25‐30. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN72509687 {unpublished data only}
- ISRCTN72509687. High dose AMBISOME on a fluconazole backbone for cryptococcal meningitis induction therapy in sub‐Saharan Africa. www.isrctn.com/ISRCTN72509687 (first received 23 June 2017).
NCT00885703 {unpublished data only}
- NCT00885703. High‐dose fluconazole for the treatment of cryptococcal meningitis in HIV‐infected individuals (HiFLAC) [A phase I/II dose‐finding study of high‐dose fluconazole treatment in AIDS‐associated cryptococcal meningitis]. clinicaltrials.gov/ct2/show/NCT00885703 (first received 22 April 2009).
NCT01802385 {unpublished data only}
- NCT01802385. Adjunctive sertraline for the treatment of HIV‐associated cryptococcal meningitis (ASTRO‐CM). clinicaltrials.gov/ct2/show/NCT01802385 (first received 1 March 2013).
Additional references
Anderegg 2017
- Anderegg N, Kirk O on behalf of IeDEA‐Global Adults and COHERE. Immunodeficiency at the start of combination antiretroviral therapy in low‐, middle‐ and high‐income countries. 9th International AIDS Society Conference on HIV Science; 2017 July 23‐26; Paris, France. 2017.
Bahr 2014
- Bahr NC, Rolfes MA, Musubire A, Nabeta H, Williams DA, Rhein J, et al. Standardized electrolyte supplementation and fluid management improves survival during amphotericin therapy for cryptococcal meningitis in resource‐limited settings. Open Forum Infectious Diseases 2014;1(2):ofu070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bicanic 2006
- Bicanic T, Harrison T, Niepieklo A, Dyakopu N, Meintjes G. Symptomatic relapse of HIV‐associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clinical Infectious Diseases 2006;43(8):1069‐73. [DOI] [PubMed] [Google Scholar]
Bicanic 2007
- Bicanic T, Meintjes G, Wood R, Hayes M, Rebe K, Bekker LG, et al. Fungal burden, early fungicidal activity, and outcome in cryptococcal meningitis in antiretroviral‐naive or antiretroviral‐experienced patients treated with amphotericin B or fluconazole. Clinical Infectious Diseases 2007;45(1):76‐80. [DOI] [PubMed] [Google Scholar]
Bicanic 2009
- Bicanic T, Brouwer AE, Meintjes G, Rebe K, Limmathurotsakul D, Chierakul W, et al. Relationship of cerebrospinal fluid pressure, fungal burden and outcome in patients with cryptococcal meningitis undergoing serial lumbar punctures. AIDS 2009;23(6):701‐6. [DOI] [PubMed] [Google Scholar]
Bicanic 2015
- Bicanic T, Bottomley C, Loyse A, Brouwer AE, Muzoora C, Taseera K, et al. Toxicity of amphotericin B deoxycholate‐based induction therapy in patients with HIV‐associated cryptococcal meningitis. Antimicrobial Agents and Chemotherapy 2015;59(12):7224‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulware 2014a
- Boulware DR, Rolfes MA, Rajasingham R, Hohenberg M, Qin Z, Taseera K, et al. Multisite validation of cryptococcal antigen lateral flow assay and quantification by laser thermal contrast. Emerging Infectious Diseases 2014;20(1):45‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2015
- Campbell JI, Kanters S, Bennett JE, Thorlund K, Tsai AC, Mills EJ, et al. Comparative effectiveness of induction therapy for human immunodeficiency virus‐associated cryptococcal meningitis: a network meta‐analysis. Open Forum Infectious Diseases 2015;2(1):ofv010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JPT, Mayridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS ONE 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2000
- Chen S, Sorrell T, Nimmo G, Speed B, Currie B, Ellis D, et al. Epidemiology and host‐ and variety‐dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Australasian Cryptococcal Study Group. Clinical Infectious Diseases 2000;31(2):499‐508. [DOI] [PubMed] [Google Scholar]
CINeMA 2017
- CINeMA. Confidence in Network Meta‐Analysis [Software]. Institute of Social and Preventive Medicine, University of Bern 2017. Available from cinema.ispm.ch.
DAIDS 2014
- U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS. Division of AIDS (DAIDS) table for grading the severity of adult and pediatric adverse events, Version 2.0. rsc.tech‐res.com/docs/default‐source/safety/daids_ae_grading_table_v2_nov2014.pdf 2014 (accessed prior to 10 June 2018).
Dias 2010
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. [DOI] [PubMed] [Google Scholar]
Dromer 2004
- Dromer F, Mathoulin‐Pélissier S, Fontanet A, Ronin O, Dupont B, Lortholary O, et al. Epidemiology of HIV‐associated cryptococcosis in France (1985‐2001): comparison of the pre‐ and post‐HAART eras. AIDS 2004;18(3):555‐62. [DOI] [PubMed] [Google Scholar]
EndNote 2013 [Computer program]
- Clavirate Analytics. EndNote. Version Endnote X7 for Mac. Clavirate Analytics, July 2013.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
- Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta‐analysis: concepts and models for multi‐arm studies. Research Synthesis Methods 2012;3(2):98‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jarvis 2014
- Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, et al. Determinants of mortality in a combined cohort of 501 patients with HIV‐associated cryptococcal meningitis: implications for improving outcomes. Clinical Infectious Diseases 2014;58(5):736‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Livermore 2014
- Livermore J, Howard SJ, Sharp AD, Goodwin J, Gregson L, Felton T, et al. Efficacy of an abbreviated induction regimen of amphotericin B deoxycholate for cryptococcal meningoencephalitis: 3 days of therapy is equivalent to 14 days. mBio 2014;5(1):e00725‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Longley 2008
- Longley N, Muzoora C, Taseera K, Mwesigye J, Rwebembera J, Chakera A, et al. Dose response effect of high‐dose fluconazole for HIV‐associated cryptococcal meningitis in southwestern Uganda. Clinical Infectious Diseases 2008;47(12):1556‐61. [DOI] [PubMed] [Google Scholar]
Loyse 2010
- Loyse A, Wainwright H, Jarvis JN, Bicanic T, Rebe K, Meintjes G, et al. Histopathology of the arachnoid granulations and brain in HIV‐associated cryptococcal meningitis: correlation with cerebrospinal fluid pressure. AIDS 2010;24(3):405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loyse 2013a
- Loyse A, Dromer F, Day J, Lortholary O, Harrison TS. Flucytosine and cryptococcosis: time to urgently address the worldwide accessibility of a 50‐year‐old antifungal. Journal of Antimicrobial Chemotherapy 2013;68(11):2435‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loyse 2013b
- Loyse A, Thangaraj H, Easterbrook P, Ford N, Roy M, Chiller T, et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource‐poor countries. Lancet Infectious Diseases 2013;13(7):629‐37. [DOI] [PubMed] [Google Scholar]
Lu 2006
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101:447‐59. [Google Scholar]
Meda 2014
- Meda J, Kalluvya S, Downs JA, Chofle AA, Seni J, Kidenya B, et al. Cryptococcal meningitis management in Tanzania with strict schedule of serial lumber punctures using intravenous tubing sets: an operational research study. Journal of Acquired Immune Deficiency Syndromes 2014;66(2):e31‐6. [DOI] [PubMed] [Google Scholar]
Milefchik 2008
- Milefchik E, Leal MA, Haubrich R, Bozzette SA, Tilles JG, Leedom JM, et al. Fluconazole alone or combined with flucytosine for the treatment of AIDS‐associated cryptococcal meningitis. Medical Mycology 2008;46(4):393‐5. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moosa 1997
- Moosa MY, Coovadia YM. Cryptococcal meningitis in Durban, South Africa: a comparison of clinical features, laboratory findings, and outcome for human immunodeficiency virus (HIV)‐positive and HIV‐negative patients. Clinical Infectious Diseases 1997;24(2):131‐4. [DOI] [PubMed] [Google Scholar]
Muzoora 2012
- Muzoora CK, Kabanda T, Ortu G, Ssentamu J, Hearn P, Mwesigye J, et al. Short course amphotericin B with high dose fluconazole for HIV‐associated cryptococcal meningitis. Journal of Infection 2012;64(1):76‐81. [DOI] [PubMed] [Google Scholar]
Perfect 2010
- Perfect JR, Dismukes WE, Dromer F, Goldman DL, Graybill JR, Hamill RJ, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clinical Infectious Diseases 2010;50(3):291‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pyrgos 2013
- Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR. Epidemiology of cryptococcal meningitis in the US: 1997‐2009. PLoS ONE 2013;8(2):e56269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajasingham 2017
- Rajasingham R, Smith RM, Bark BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV‐associated cryptococcal meningitis: an updated analysis. Lancet Infectious Diseases 2017;17(8):873‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rolfes 2014
- Rolfes MA, Hullsiek KH, Rhein J, Nabeta HW, Taseera K, Schutz C, et al. The effect of therapeutic lumbar punctures on acute mortality from cryptococcal meningitis. Clinical Infectious Diseases 2014;59(11):1607‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rothe 2013
- Rothe C, Sloan DJ, Goodson P, Chikafa J, Mukaka M, Denis B, et al. A prospective longitudinal study of the clinical outcomes of cryptococcal meningitis following treatment induction with 800 mg oral fluconazole in Blantyre, Malawi. PLoS ONE 2013;8(6):e67311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta‐analysis. PLoS ONE 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaars 2006
- Schaars CF, Meintjes GA, Morroni C, Post FA, Maartens G. Outcome of AIDS‐associated cryptococcal meningitis initially treated with 200 mg/day or 400 mg/day of fluconazole. BMC Infectious Diseases 2006;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scriven 2016
- Scriven JE, Lalloo DG, Meintjes G. Changing epidemiology of HIV‐associated cryptococcosis in sub‐Saharan Africa. Lancet Infectious Diseases 2016;16(8):891‐2. [DOI] [PubMed] [Google Scholar]
Sloan 2008
- Sloan D, Dlamini S, Paul N, Dedicoat M. Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource‐limited settings. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD005647.pub2] [DOI] [PubMed] [Google Scholar]
Stata 2013 [Computer program]
- StataCorp. Stata. Version 13.0. StataCorp, June 2013.
Vermes 2000
- Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. Journal of Antimicrobial Chemotherapy 2000;46(2):171‐9. [DOI] [PubMed] [Google Scholar]
White 2011
- White IR. Multivariate random‐effects meta‐regression: updates to mvmeta. Stata Journal 2011;11(2):255‐70. [Google Scholar]
White 2012
- White IR, Barrett JK, Jackson D, Higgins JPT. Consistency and inconsistency in network meta‐analysis: model estimation using multivariate meta‐regression. Research Synthesis Methods 2012;3(2):111‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2015
- White IR. Network meta‐analysis. Stata Journal 2015;15(4):951‐85. [Google Scholar]
WHO 2011
- World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV‐infected adults, adolescents and children. www.who.int/hiv/pub/cryptococcal_disease2011/en/ (accessed 19 June 2018). [PubMed]
WHO 2018
- World Health Organization. Guidelines for the diagnosis, prevention and management of cryptococcal disease in HIV‐infected adults, adolescents and children. www.who.int/hiv/pub/guidelines/cryptococcal‐disease/en/ (accessed 19 June 2018). [PubMed]
Zhai 2012
- Zhai B, Wu C, Wang L, Sachs MS, Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrobial Agents and Chemotherapy 2012;56(7):3758‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Sloan 2006
- Sloan D, Dlamini S, Paul N, Dedicoat M. Treatment of acute cryptococcal meningitis in HIV‐infected adults in resource‐limited settings. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005647] [DOI] [PubMed] [Google Scholar]
Sloan 2011
- Sloan D, Dlamini S, Paul N, Dedicoat M. Treatment of acute cryptococcal meningitis in HIV infected adults, with an emphasis on resource‐limited settings. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD005647.pub2] [DOI] [PubMed] [Google Scholar]