Abstract
Background
Leg ulcers are chronic wounds of the lower leg, caused by poor blood flow, that can take a long time to heal. The pooling of blood in the veins can damage the skin and surrounding tissues, causing an ulcer to form. Venous leg ulcers are associated with impaired quality of life, reduced mobility, pain, stress and loss of dignity. The standard treatment for venous leg ulcers is compression bandages or stockings. Shock wave therapy may aid the healing of these wounds through the promotion of angiogenesis (the formation and development of blood vessels) and reduction of inflammation, though this process is poorly understood at present.
Objectives
To assess the effects of extracorporeal shock wave therapy on the healing and management of venous leg ulceration.
Search methods
In April 2018 we searched the Cochrane Wounds Specialised Register; the Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations); Ovid Embase and EBSCO CINAHL Plus. We also searched clinical trials registries for ongoing and unpublished studies, and scanned reference lists of relevant included studies as well as reviews, meta‐analyses and health technology reports to identify additional studies. We applied no restrictions with respect to language, date of publication or study setting.
Selection criteria
We considered all published and unpublished randomised controlled trials (RCTs) assessing the effectiveness of extracorporeal shock wave therapy in the healing and management of venous leg ulceration.
Data collection and analysis
Two review authors independently performed study selection. We planned that two review authors would also assess the risk of bias of included studies, extract study data and rate the certainty of the evidence using GRADE.
Main results
We found no RCTs that met the inclusion criteria for this review.
Authors' conclusions
We found no RCTs assessing the effectiveness of extracorporeal shock wave therapy in the healing and management of venous leg ulceration. The lack of high‐quality evidence in this area highlights a gap in research and may serve to justify the need for further research and evidence to provide guidance concerning the use of this treatment option for this condition. Future trials should be of clear design and include concomitant use of the current best practice treatment, multilayer compression therapy. Recruitment should aspire to best represent patients seen in clinical practice and patient‐related outcome measures should be included in study design.
Keywords: Humans, Extracorporeal Shockwave Therapy, Extracorporeal Shockwave Therapy/methods, Leg Ulcer, Leg Ulcer/therapy, Varicose Ulcer, Varicose Ulcer/therapy, Wound Healing
Plain language summary
Extracorporeal shock wave therapy for the healing and management of venous leg ulcers
What is the aim of this review?
The aim of this review was to find out whether extracorporeal shock wave therapy (pulses of energy similar to sound waves, transmitted via a pad to the skin) can help to heal venous leg ulcers. Researchers from Cochrane searched for relevant studies (randomised controlled trials) to answer this question but no relevant studies were found.
Key messages
No evidence from randomised controlled trials was available to allow us to evaluate whether extracorporeal shock wave therapy (ESWT) is effective for healing venous leg ulcers. Randomised controlled trials are medical studies where patients are chosen at random to receive different treatments. This type of trial provides the most reliable evidence and there is currently a lack of high‐quality evidence in this area.
What was studied in the review?
Leg ulcers are chronic wounds of the lower leg that can take a long time to heal. Venous leg ulcers are caused by poor blood flow in the legs. Pooling of blood in the veins can damage the skin and surrounding tissues, causing an ulcer to form. Venous leg ulcers are associated with reduced quality of life, reduced mobility, pain, stress and loss of dignity. The standard treatment for venous leg ulcers is compression bandages or stockings.
ESWT was first used to break up kidney stones and gallstones but is now used to treat tendonitis and other joint and muscle conditions. ESWT is also thought to help wounds heal by stimulating circulation, promoting the growth of healthy blood vessels, and by reducing inflammation. This is a new therapy for treating venous leg ulcers.
What are the main results of the review?
We found no randomised controlled trials evaluating the use of ESWT for venous leg ulcers. This highlights a gap in medical evidence which may justify further research into this area.
How up to date is this review?
We searched for studies that had been published up to April 2018.
Background
Description of the condition
Leg ulcers are chronic wounds most commonly described as open lesions of the skin occurring below the knee on the leg or foot, further characterised by healing times of greater than six weeks (SIGN 2010; Van Gent 2010). The causes of leg ulceration are varied and often multifactorial; primary reasons for the development of a leg ulcer include venous insufficiency, arterial insufficiency and diabetes (Mekkes 2003).
Venous ulceration is the most common type of leg ulceration seen in the community. Studies have shown that for people with chronic leg ulcers, 70% to 80% of those ulcers have a venous component (Valencia 2001; Crane 2008). Chronic venous leg ulceration has an estimated prevalence of 1% to 2% of the population in developed countries. Point prevalence for the United Kingdom (UK) is estimated to be between 0.3% and 0.5% (per 1000 population), which increases with age (Reichenberg 2005; Vowden 2009; González‐Consuegra 2011). The natural history of the disease is one of a continuous cycle of healing and breakdown over decades (Smith 2006; Raju 2010).
Venous ulceration is associated with impaired quality of life, reduced mobility, pain, stress and loss of dignity (Persoon 2004; Wilson 2004). Social isolation can be commonplace and is frequently associated with malodorous wounds, swelling and anxiety around exudate levels (fluid seeping from wounds) (Walters 1999; Herber 2007).
Venous ulcers arise as a result of venous valve incompetence and calf muscle pump insufficiency (Palfreyman 1998; Mekkes 2003), which leads to retrograde venous flow, venous hypertension, microcirculatory skin changes and localised tissue damage. Two main mechanisms have been proposed to account for the tissue damage and subsequent ulceration that occurs. The fibrin cuff hypothesis postulates that venous hypertension leads to exudation of fibrin, a protein involved in the clotting of blood, into the surrounding tissues, and leads to the formation of fibrin cuffs around capillaries which impairs gas exchange, leading to tissue damage (Smith 2006). The leucocyte‐ (white blood cell) trapping hypothesis postulates that leucocytes which have become trapped in the microcirculation migrate into surrounding tissues and lead to an inflammatory response with impairment of normal proliferation and skin healing (Saharay 1998; Hahn 1999).
The current gold standard in the management of chronic venous leg ulcers revolves around high compression multilayer bandaging (SIGN 2010). Multilayer compression bandaging aims to improve venous return and reduce venous hypertension (Valencia 2001; Etufugh 2007). Elastic multi‐component bandages such as four layer bandaging and comparative two‐layer systems are used. These consist of an initial layer of orthopaedic wool, a crepe bandage, an elastic bandage and an elastic cohesive bandage as the outer layer (Marston 1999). The high pressure is sustained for a considerable time, allowing for a weekly change of dressings. With multilayer compression therapy, healing rates of around 70% at six months have been achieved in specialist clinics. Simple, nonadherent primary wound dressings are currently recommended in conjunction with compression bandaging (SIGN 2010). Other known treatments for this condition include the use of various impregnated primary dressings, hyperbaric oxygen therapy and treatment of underlying venous insufficiency via surgery, endovenous laser (EVLT), radiofrequency (RFA) and sclerotherapy treatments.
Description of the intervention
Extracorporeal shock waves (ECSWs) are low‐energy pulse waves that were first put to clinical use in the treatment of urolithiasis, whereby kidney stones (urinary calcinosis) are broken up by the shock wave energy (Shrivistava 2005). Since then their application has been extended to the treatment of fractured bones with an interrupted healing process (non‐union fractures), tendon injury and osteonecrosis, a condition whereby bone breaks down faster than it can be replenished (Schaden 2007).
More recently, the ability of ECSWs to improve the healing of wounds, ulcers and burns has been assessed. The incidental discovery that shock waves may have an effect upon wound healing was made in 2006 (Schaden 2007; Arnó 2010; Mittermayr 2011); the treatment in this context has remained novel.
Shock waves carry energy, have a short life cycle and are able to travel through a physical medium such as liquid or gas. Shock waves are generated through the transformation of electric energy into mechanical energy. This transformation can occur in one of three ways: electromagnetic generation utilises a strong magnetic field to create a slow, low‐pressure acoustic pulse (sound wave pulse); piezoelectric generation relies upon the rapid contraction and expansion of crystals via the application of a high voltage charge to achieve an acoustic pulse; and electrohydraulic utilises a shock wave pulse released by high voltage electrode water vaporisation (Ogden 2001; Mouzopoulos 2007).
Shock waves are defined by their waveform, number and frequency of impulses, and energy flux density (the rate at which energy is transferred through the physical medium). Standardised, disease‐specific protocols pertaining to the use of shock wave therapy in wound care are lacking (Schaden 2007). In the treatment of wounds, lower flux densities are typically used, providing lower energy levels. Regardless of their characteristics or mode of generation, shock waves can be delivered to a target area either in a focused or dispersed manner through the use of specific applicator units (Mittermayr 2011). All three modes of shock wave generation are found in current clinical practice. Both focused and un‐focused (dispersed) applicator units have been utilised in the delivery of treatment for soft tissue wounds, with typical energy levels of 0.037 mJ/mm2 to 0.1 mJ/mm2 (Schaden 2007; Saggini 2008). The use of shock waves in the treatment of soft tissue wounds is currently rare in the UK, with most use found in central European countries and the US.
How the intervention might work
In humans, ECSWs have been shown to promote the formation and development of blood vessels (angiogenesis) and to reduce inflammation (Wang 2011b).The mechanism of how ECSW therapy may aid wound healing is poorly understood at present, however several animal model studies have shown increased levels of signal proteins (vascular endothelial growth factor (VEGF) and factor HIF‐1alpha) following treatment. These proteins are in part responsible for the restoration of tissue oxygen supply when blood circulation is inadequate (Chen 2004; Nishida 2004; Wang 2004; Ma 2007). This angiogenic process is stimulated by the application of ESCWs and plays an important role in wound healing (Stojadinovic 2008; Mittermayr 2011).
Why it is important to do this review
Venous ulceration is a common, chronic condition resulting in significantly impaired quality of life and substantial burden to all healthcare systems. The use of shock waves in the treatment of venous leg ulcers is a novel therapy; a comprehensive review of all relevant and available randomised controlled trials is required to inform practice.
Objectives
To assess the effects of extracorporeal shock wave therapy on the healing and management of venous leg ulceration.
Methods
Criteria for considering studies for this review
Types of studies
Types of studies considered included randomised controlled trials (RCTs). We made no restrictions on the basis of language, publication status or age range.
Types of participants
We planned to include people over the age of 18 years, from any care setting and socio‐economic background, with active lower limb ulceration of venous aetiology. Guidelines in the UK indicate assessment of ankle brachial indices should be performed to rule out arterial disease, and many diagnostic assessments also include duplex ultrasound imaging to identify venous reflux (SIGN 2010); we planned to accept studies in which a diagnosis of venous ulceration had been made irrespective of whether the ankle brachial indices were reported. We planned to include studies where lower limb venous ulceration was either the focus of the study or was included within a study evaluating a broader range of soft tissue wounds. In the case of the latter, we would have stratified the results according to wound aetiology.
Types of interventions
We planned to include studies evaluating the use of low energy, focused or non‐focused extracorporeal shock waves (ECSWs) in the context of venous ulcer treatment.
Eligible comparators would have included:
ECSW compared with no treatment or sham treatment;
ECSW compared with dressings (with or without compression treatment);
ECSW compared with alternative treatment, for example truncal venous surgery (including endovenous laser treatment, radiofrequency and sclerotherapy), hyperbaric oxygen therapy;
head‐to‐head comparisons of varying types, modes and strengths of ECSW treatment.
Shock waves produced by any of the three accepted methods were considered for inclusion; these comprise electrohydraulic, electromagnetic and piezoelectric principles of shock wave generation. We would have excluded studies examining ECSW use for the treatment of chronic tendinopathies, impaired bone healing function, urinary and biliary clacinosis and myocardial ishchaemia.
Types of outcome measures
Primary outcomes
Complete wound healing measured by:
time to complete wound healing;
proportion of index ulcers completely healed;
adverse effects, including participant‐reported pain from intervention (measured using a visual analogue scale, such as a numeric box scale (NBS).
Secondary outcomes
Change in ulcer size (percentage change from baseline)
Quality of life (measured using a standardised generic questionnaire such as EQ‐5D, SF‐36, SF‐12 or SF‐6)
Volume of exudate (utilising subjective measurement, such as low, medium, high)
Daily ulcer pain (measured using a visual analogue scale, such as an NBS)
Ulcer recurrence (defined as a new lesion in the skin where complete healing had occurred)
Treatment cost
Search methods for identification of studies
Electronic searches
We searched the following electronic databases to identify reports of relevant clinical trials:
the Cochrane Wounds Specialised Register (searched 4 April 2018);
the Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 3) in the Cochrane Library (searched 4 April 2018);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 4 April 2018);
Ovid Embase (1974 to 4 April 2018);
EBSCO CINAHL Plus (1937 to 4 April 2018).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 1. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2018). We applied no restrictions with respect to language, date of publication or study setting.
We also searched the following clinical trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov) (searched April 2018);
World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/trialsearch) (searched April 2018).
Search strategies for clinical trial registries can be found in Appendix 2.
Searching other resources
We examined the reference lists of all identified, relevant studies in order to locate further studies not highlighted by the electronic search.
Data collection and analysis
Data collection and analysis were carried out according to methods stated in the published protocol (Cooper 2015), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two review authors (BC, PB) independently assessed studies for potential inclusion. They examined references drawn from initial searches for relevance; studies considered for inclusion were retrieved in full and selected according to the criteria for considering studies for this review described above.
We have included a study flow diagram as recommended by the PRISMA statement (Liberati 2009), to illustrate the results of all searching activity and the process of screening and selecting studies for inclusion in the review.
Data extraction and management
Had we identified eligible studies, two review authors (BC, PB) would have independently used a data extraction sheet to summarise studies. In cases where multiple publications had arisen from a study, we would have identified one publication as the primary reference but all studies would have been maximally data extracted.
We intended to extract the following data:
trial authors;
year of publication;
country where RCT performed;
care setting;
unit of investigation (participant, leg or ulcer);
overall sample size and methods used to estimate statistical power;
participant selection criteria;
number of participants randomised to each treatment arm;
baseline characteristics of participants per treatment arm (gender, age, baseline ulcer area and volume, ulcer duration, prevalence of comorbidities such as diabetes, prevalence of clinically infected wounds or colonised wounds, previous history of ulceration, baseline levels of wound exudate, and participant mobility);
details of the dressing/treatment regimen prescribed for each treatment arm including details of concomitant therapy (for example: compression);
duration of treatment;
duration of follow‐up;
statistical methods utilised in data analysis;
primary and secondary outcomes measured;
primary and secondary outcome data by treatment arm;
adverse effects of treatment (per arm with quantity and type);
withdrawals (per treatment arm with quantity and reason);
source of trial funding.
Assessment of risk of bias in included studies
Had studies met our inclusion criteria, two review authors (PB, BC) would have independently assessed each included study using the Cochrane tool for assessing risk of bias (Higgins 2011). This tool addresses six specific domains: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other potential sources of bias (for this review, baseline comparability of groups for factors such as surface area and duration of ulcer). We planned to classify included RCTs as being at an overall high risk of bias if they were rated as 'high risk' for any one of three key domains: allocation concealment, blinded outcome assessment of healing, and completeness of outcome data. We would have classified RCTs as having an overall low risk of bias if rated as 'low risk' in the three key domains of allocation concealment, blinded outcome assessment of healing, and completeness of outcome data.
We would have made individual assessments of participant blinding and blinding of outcome assessors in included studies. We planned to present our assessment of risk of bias using two 'Risk of bias' summary figures; one which is a summary of bias for each item across all studies, and a second which shows a cross‐tabulation of each trial by all of the 'Risk of bias' items.
Measures of treatment effect
We planned to perform data analysis according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). One review author would have entered quantitative data into Review Manager 5, another would have checked it. We would have analysed the data using RevMan 5 (Review Manager 2014). We planned to present the outcome results for each trial with 95% confidence intervals (CI).
We hoped to report estimates for dichotomous outcomes (e.g. ulcers healed during time period, number of infected ulcers) as risk ratios (RR).
We would have expressed continuous outcomes (such as changes in ulcer area) as mean differences (MD) and overall effect size (with 95% CI calculated) or as standardised mean differences (SMDs) if different methods of measurement had been used in the studies.
We planned to analyse time‐to‐event data utilising survival, time‐to‐event approaches, with adjustment for baseline size if data had been available. We also planned to plot, and if feasible, pool, estimates of hazard ratio and 95% CI as presented in the trial reports using the generic inverse variance method in Review Manager 5. Time‐to‐event data incorrectly reported (as mean and standard deviation, SD) would not have been analysed but instead discussed separately within the review.
Unit of analysis issues
Had studies met our inclusion criteria, we would have recorded whether these studies presented outcomes in relation to a wound, a participant or as multiple wounds on the same participant. We would also have analysed the level at which study randomisation had occurred.
Dealing with missing data
Had we identified any studies which met our inclusion criteria, we would have attempted to contact the trial investigators in cases of missing data. Should trials have reported complete healing outcomes for only those participants who completed the trial (i.e. participants withdrawing and lost to follow‐up were excluded from the analysis), we would have treated the participants who were not included in the analysis as if their wound did not heal. Should trials have reported results for participants who completed the trial without specifying the numbers initially randomised per group, we would have presented only complete case data. For other outcomes the same analysis would have been applied.
Assessment of heterogeneity
We intended to consider clinical heterogeneity (where trials appear different in terms of participant characteristics, intervention type, duration and outcome type) and statistical heterogeneity. We planned to assess statistical heterogeneity using the Chi2 test (P values less than 0.10 would have been considered to indicate significant heterogeneity) in conjunction with the I2 statistic (Higgins 2003). The I2 statistic estimates the percentage of total variation across trials due to heterogeneity rather than variation due to chance. We would have categorised heterogeneity as follows: I2 values of 40% or less would have indicated a low level of heterogeneity, and values of 75% or above would have represented very high heterogeneity.
Assessment of reporting biases
If possible, we would have used funnel plots to assess reporting bias if a minimum of 10 studies were available for the meta‐analysis of a primary outcome (Sterne 2011).
Data synthesis
We would have combined details of included studies in a narrative review according to type of comparator, possibly by location/type of wound and then by outcomes by time period. We would have considered clinical and methodological heterogeneity and undertaken pooling when studies appeared appropriately similar in terms of wound type, intervention type, duration of follow‐up and outcome type.
We were unable to pre specify the amount of clinical, methodological and statistical heterogeneity included studies but it might have been extensive. Thus, we anticipated using a random‐effects approach for meta‐analysis. Conducting meta‐analysis with a fixed‐effect model in the presence of even minor heterogeneity may provide overly narrow confidence intervals. We would only have used a fixed‐effect approach when clinical and methodological heterogeneity was assessed to be minimal, and the assumption that a single underlying treatment effect is being estimated held. We would have used Chi2 and I2 to quantify heterogeneity but the results of this would not have been used to guide choice of model for meta‐analysis. We would have exercised caution when meta‐analysed data were at risk of small study effects, because a random‐effects model may be unsuitable. In this case, or where there were other reasons to question the selection of a fixed‐effect or random‐effects model, we would have assessed the impact of the approach using sensitivity analyses to compare results from alternate models (Thompson 1999).
We would have presented data using forest plots where possible. For dichotomous outcomes we would have presented the summary estimate as a risk ratio (RR) with 95% CI. Where continuous outcomes were measured in the same way across studies, we planned to present a pooled mean difference (MD) with 95% CI; we planned to pool standardised mean difference (SMD) estimates where studies measured the same outcome using different methods. For time‐to‐event data, we planned to plot (and, if appropriate, pool) estimates of hazard ratios and 95% CIs as presented in the study reports using the generic inverse variance method in Review Manager 5. Where time to healing was analysed as a continuous measure but it was not clear if all wounds healed, we would have documented the use of the outcome in the study but would not have summarised the data in any meta‐analysis.
We would have obtained pooled estimates of treatment effect using Review Manager 5.
'Summary of findings' tables
We had planned to present the main results of the review in 'Summary of findings' tables. Had we identified eligible studies, these tables would have presented key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schünemann 2011a). The 'Summary of findings' tables would also have included an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schünemann 2011b).
We had planned to present the following outcomes in the 'Summary of findings' tables:
time to complete wound healing;
proportion of index ulcers completely healed over a six month period;
adverse effects, including participant‐reported pain from intervention (measured using a visual analogue scale, such as a numeric box scale (NBS).
Subgroup analysis and investigation of heterogeneity
Had we identified studies for inclusion in the review, we would have considered potential sources of heterogeneity and made every effort made to extract sufficient, compatible data to undertake subgroup analysis of individuals. Subgroups may have included demographic divisions, variations in type of shock wave treatment and differing durations of follow‐up.
Sensitivity analysis
We had planned to undertake sensitivity analyses to explore the influence of risk of bias on effect size. We would also have assessed the influence of removing from meta‐analyses, studies classed as having an overall high risk of bias. These analyses would have included only studies that were assessed as having a low risk of bias in all key domains, namely allocation concealment, blinded outcome assessment of healing, and completeness of outcome data for the estimates of treatment effect.
Results
Description of studies
Results of the search
See Figure 1.
1.
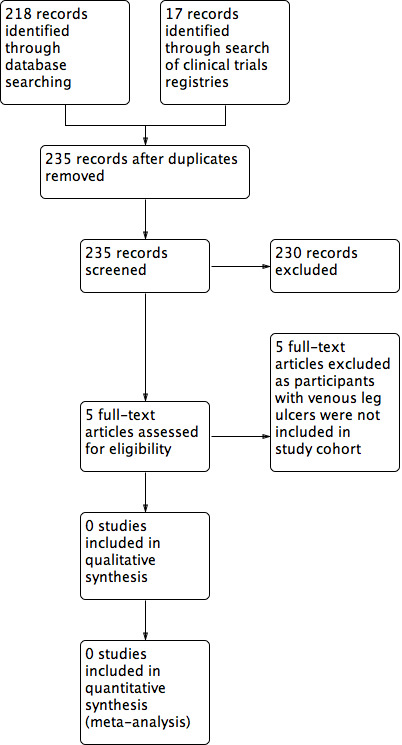
Study flow diagram.
We found and assessed 218 titles and abstracts in electronic format through searches of the Cochrane Wounds Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE, Ovid Embase, and EBSCO CINAHL Plus. In addition, we identified a further 17 titles and abstracts through searches of clinical trial registries (ClinicalTrials.gov and WHO International Clinical Trials Registry Platform). Of these 235 records, we excluded 230 after initial review; we excluded the remaining five studies after reviewing the full text, and concluded that no studies met our inclusion criteria.
Included studies
We found no eligible studies.
Excluded studies
We excluded five randomised controlled trials (RCTs) as we deemed them irrelevant to the focus of this review; these studies are described in the Characteristics of excluded studies section. Of the five excluded studies, all met the inclusion criteria for relevant intervention being studied, but they did not include venous ulceration as a condition within the study.
Risk of bias in included studies
As we identified no eligible studies, it was not possible to assess risk of bias.
Effects of interventions
We found no eligible studies for inclusion.
Discussion
Summary of main results
Despite an extensive search of the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase, EBSCO CINAHL, the World Health Organization (WHO) International Clinical Trials Registry and ClinicalTrials.gov, we did not find any studies that met the inclusion criteria for this review. We excluded studies where the intervention met inclusion criteria but the subject of the study did not include ulceration of venous aetiology.
Overall completeness and applicability of evidence
We found no randomised controlled trials (RCTs) assessing the effectiveness of extracorporeal shock wave therapy in the healing and management of venous leg ulceration.
Quality of the evidence
We found no studies conducted to address our objectives; therefore, we were unable to assess the quality of the evidence.
Potential biases in the review process
We found no studies relevant for inclusion in this review. We performed a comprehensive search of the literature, and performed study selection in accordance with recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). In addition to our primary search, we also searched clinical trial registries (ClinicalTrials.gov and WHO International Clinical Trials Registry Platform) and again, we found no relevant studies for inclusion in this review. We considered the evidence available; however it is possible that there may be unpublished data that we were unable to access. There is limited potential for publication bias.
Agreements and disagreements with other studies or reviews
Several published literature reviews regard shock wave therapy for wound healing as safe, with potential for further investigation (Qureshi 2011; Mittermayr 2012). However, few of the incorporated studies include venous ulceration and reviews rely upon non‐randomised trials or case series reports, rather than randomised controlled trials.
Favourable outcomes are reported in several non‐randomised studies of shock wave therapy, most notably in: 'Shock wave therapy for acute and chronic soft tissue wounds: a feasibility study' and 'Extracorporeal shock wave therapy for the management of chronic ulcers in the lower extremities' (Schaden 2007; Saggini 2008). Both studies include venous ulceration. Whilst this type of evidence may be of some use in decision making, it lacks the rigour of a randomised controlled trial. Randomised controlled trials which examine extracorporeal shock wave therapy for generalised soft tissue wounds exist, but none met the inclusion criteria for this review because they did not specifically identify venous insufficiency as wound aetiology.
Authors' conclusions
Implications for practice.
We found no randomised controlled trials (RCTs) to determine the effectiveness of extracorporeal shock wave therapy in the healing and management of venous leg ulceration. There is an absence of high‐quality research regarding the effects of this treatment option for this condition.
Implications for research.
We found no RCTs assessing the effectiveness of extracorporeal shock wave therapy in the healing and management of venous leg ulceration. The role of shock wave therapy as a primary treatment or adjuvant to best practice in routine care remains unclear and requires further assessment. Non‐randomised studies report generally positive outcomes and an absence of adverse events, which suggests that an RCT focusing on venous ulceration alone would be justifiable. Poor reporting of study methodology is detrimental to the validity of the existing knowledge base. Prospective studies focusing on the treatment of venous ulceration should be of clear technique and design; this would ideally include concomitant use of the current best practice treatment, multilayer compression therapy. Recruitment should aspire to best represent patients seen in clinical practice. Patient‐related outcome measures (PROMs), specifically quality of life assessment, are underreported and should be included in study design. Cost analysis should also be considered.
Acknowledgements
We acknowledge the contribution of Julie Brittenden, who conceived the review question and advised on the review and its development.
We also acknowledge the contribution of peer referees Susan O’Meara, Amanda Briant, Giovanni Casazza, Madhu Periasamy and Jane Nadel, who commented on the protocol, and Rebeca Illescas‐Montes, Zipporah Iheozor‐Ejiofor and Janet Gunderson who commented on the review. We would like to thank Megan Prictor for copy‐editing the protocol and Jessica Sharp for copy‐editing the review.
Appendices
Appendix 1. Search strategies
Cochrane Wounds Specialised Register
1 MESH DESCRIPTOR Ultrasonic Surgical Procedures EXPLODE ALL AND INREGISTER 2 MESH DESCRIPTOR Ultrasonic Therapy EXPLODE ALL AND INREGISTER 3 MESH DESCRIPTOR Ultrasonics EXPLODE ALL AND INREGISTER 4 MESH DESCRIPTOR Ultrasonic Waves EXPLODE ALL AND INREGISTER 5 MESH DESCRIPTOR High‐Energy Shock Waves EXPLODE ALL AND INREGISTER 6 MESH DESCRIPTOR Sound AND INREGISTER 7 (shockwave or (shock* near4 wave*)) AND INREGISTER 8 (ultraso*) AND INREGISTER 9 (lithotrip*) AND INREGISTER 10 ESWT AND INREGISTER 11 ECST AND INREGISTER 12 ECSW AND INREGISTER 13 ECSL AND INREGISTER 14 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 15 MESH DESCRIPTOR Leg Ulcer EXPLODE ALL AND INREGISTER 16 ((varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or "ulcus cruris" or (ulcer* next cruris)) AND INREGISTER 17 #15 OR #16 18 #14 AND #17
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Ultrasonic Surgical Procedures] explode all trees #2 MeSH descriptor: [Ultrasonic Therapy] explode all trees #3 MeSH descriptor: [Ultrasonics] explode all trees #4 MeSH descriptor: [Ultrasonic Waves] explode all trees #5 MeSH descriptor: [High‐Energy Shock Waves] explode all trees #6 MeSH descriptor: [Sound] this term only #7 (shockwave or (shock* near/4 wave*)):ti,ab,kw #8 (ultraso*):ti,ab,kw #9 (lithotrip*):ti,ab,kw #10 (ESWT):ti,ab,kw #11 (ECST):ti,ab,kw #12 (ECSW):ti,ab,kw #13 (ESWL):ti,ab,kw #14 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 #15 MeSH descriptor: [Leg Ulcer] explode all trees #16 ((varicose next ulcer*) or (venous next ulcer*) or (leg next ulcer*) or (stasis next ulcer*) or (crural next ulcer*) or "ulcus cruris" or (ulcer* next cruris)):ti,ab,kw #17 #15 or #16 #18 #14 and #17
Ovid MEDLINE
1 exp Ultrasonic Surgical Procedures/ 2 exp Ultrasonic Therapy/ 3 Ultrasonics/ 4 exp Ultrasonic Waves/ 5 High‐Energy Shock Waves/ 6 Sound/ 7 (shockwave or (shock* adj4 wave*)).tw. 8 ultraso*.tw. 9 lithotrip*.tw. 10 ESWT.tw. 11 ECST.tw. 12 ECSW.tw. 13 ESWL.tw. 14 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 15 exp Leg Ulcer/ 16 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris).tw. 17 15 or 16 18 14 and 17 19 randomised controlled trial.pt. 20 controlled clinical trial.pt. 21 randomi?ed.ab. 22 placebo.ab. 23 clinical trials as topic.sh. 24 randomly.ab. 25 trial.ti. 26 or/19‐25 27 exp animals/ not humans.sh. 28 26 not 27 29 18 and 28
Ovid Embase
1 exp ultrasound surgery/ 2 exp ultrasound therapy/ 3 shock wave therapy/ 4 ultrasound/ 5 sound/ 6 (shockwave or (shock* adj4 wave*)).tw. 7 ultraso*.tw. 8 lithotrip*.tw. 9 ESWT.tw. 10 ECST.tw. 11 ECSW.tw. 12 ESWL.tw. 13 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 14 exp leg ulcer/ 15 (varicose ulcer* or venous ulcer* or leg ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris).tw. 16 14 or 15 17 13 and 16 18 Randomized controlled trials/ 19 Single‐Blind Method/ 20 Double‐Blind Method/ 21 Crossover Procedure/ 22 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 23 (doubl* adj blind*).ti,ab. 24 (singl* adj blind*).ti,ab. 25 or/18‐24 26 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 27 human/ or human cell/ 28 and/26‐27 29 26 not 28 30 25 not 29 31 17 and 30
EBSCO CINAHL Plus
S30 S16 AND S29 S29 S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 S28 TI allocat* random* or AB allocat* random* S27 MH "Quantitative Studies" S26 TI placebo* or AB placebo* S25 MH "Placebos" S24 TI random* allocat* or AB random* allocat* S23 MH "Random Assignment" S22 TI randomi?ed control* trial* or AB randomi?ed control* trial* S21 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S20 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S19 TI clinic* N1 trial* or AB clinic* N1 trial* S18 PT Clinical trial S17 MH "Clinical Trials+" S16 S12 AND S15 S15 S13 OR S14 S14 TI ( (varicose ulcer* or venous ulcer* or leg* ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris ) OR AB ( (varicose ulcer* or venous ulcer* or leg* ulcer* or stasis ulcer* or crural ulcer* or ulcus cruris or ulcer cruris ) S13 (MH "Leg Ulcer+") S12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 S11 TI ESWL OR AB ESWL S10 TI ECSW OR AB ECSW S9 TI ECST OR AB ECST S8 TI ESWT OR AB ESWT S7 TI lithotrip* OR AB lithotrip* S6 TI ultraso* OR AB ultraso* S5 TI ( (shockwave or (shock* N4 wave*)) ) OR AB ( (shockwave or (shock* N4 wave*)) ) S4 (MH "Sound") S3 (MH "Ultrasonics+") S2 (MH "Ultrasonic Therapy") S1 (MH "Ultrasonic Surgical Procedures+")
Appendix 2. Search of clinical trials registries
The following clinical trials registries were searched:
ClinicalTrials.gov
WHO International Clinical Trials Registry Platform
The search term 'shockwave AND ulcer' was utilised.
Appendix 3. Cochrane tool for assessing risk of bias
1. Was the allocation sequence randomly generated?
Low risk of bias
The investigators describe a random component in the sequence generation process such as: referring to a random number table; using a computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
High risk of bias
The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: sequence generated by odd or even date of birth; sequence generated by some rule based on date (or day) of admission; sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process provided to permit a judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially‐numbered drug containers of identical appearance; sequentially‐numbered, opaque, sealed envelopes.
High risk of bias
Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: use of an open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. envelopes were unsealed, non‐opaque, or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. This is usually the case if the method of concealment is not described, or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding ‐ was knowledge of the allocated interventions adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, but outcome assessment was blinded and the non‐blinding of others unlikely to introduce bias.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others likely to introduce bias.
Unclear
Either of the following.
Insufficient information to permit judgement of low or high risk of bias.
The study did not address this outcome.
4. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data are unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data are balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is not enough to have a clinically relevant impact on the observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data are likely to be related to the true outcome, with either an imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with the observed event risk is enough to induce clinically relevant bias in the intervention effect estimate.
For continuous outcome data, a plausible effect size (difference in means or standardised difference in means) among missing outcomes is enough to induce a clinically relevant bias in the observed effect size.
'As‐treated' analysis done with a substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Either of the following.
Insufficient reporting of attrition/exclusions to permit a judgement of low or high risk of bias (e.g. number randomised not stated, no reasons for missing data provided).
The study did not address this outcome.
5. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Either of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes is/are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes was/were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review is/are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information provided to permit a judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
6. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Larkin 2010 | Participants with venous leg ulcers were not included in the study cohort (intervention meets inclusion criteria). |
| Moretti 2009 | Participants with venous leg ulcers were not included in the study cohort (intervention meets inclusion criteria). |
| Omar 2014 | Participants with venous leg ulcers were not included in the study cohort (intervention meets inclusion criteria). |
| Wang 2009 | Participants with venous leg ulcers were not included in the study cohort (intervention meets inclusion criteria). |
| Wang 2011a | Participants with venous leg ulcers were not included in the study cohort (intervention meets inclusion criteria). |
Contributions of authors
Ben Cooper: conceived, designed and co‐ordinated the review; produced the first draft of the review; contributed to writing and editing the review; wrote to study authors/experts/companies; approved the final review prior to submission; and is a guarantor of the review.
Paul Bachoo: conceived, designed and co‐ordinated the review; advised on the review; and approved the final review prior to submission.
Contributions of editorial base
Joan Webster (Editor): edited the protocol; advised on methodology, interpretation and content and approved the final protocol prior to submission.
Tanya Walsh (Editor) edited the review; advised on methodology, interpretation and review content and approved the final review prior to submission.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process; advised on content; edited the review.
Naomi Shaw (Information Specialist): designed the search strategy and ran the searches and edited the search methods section.
Ursula Gonthier (Editorial Assistant): edited the Plain Language Summary and reference sections.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure to the Cochrane Wounds Review Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Declarations of interest
Ben Cooper: none known. Paul Bachoo: none known.
New
References
References to studies excluded from this review
Larkin 2010 {published data only}
- Larkin AM, Duport S, Clinton M, Hardy M, Andrews K. Randomized control of extracorporeal shock wave therapy versus placebo for chronic decubitus ulceration. Clinical Rehabilitation 2010;24(3):222‐9. [DOI] [PubMed] [Google Scholar]
Moretti 2009 {published data only}
- Moretti B, Notarnicola A, Maggio G, Moretti L, Pascone M, Tafuri S, et al. The management of neuropathic ulcers of the foot in diabetes by shock wave therapy. BMC Musculoskeletal Disorders 2009;10:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Omar 2014 {published data only}
- Omar MT, Alghadir A, Al‐Wahhabi KK, Al‐Askar AB. Efficacy of shock wave therapy on chronic diabetic foot ulcer: a single‐blinded randomized controlled clinical trial. Diabetes Research and Clinical Practice 2014;106(3):548‐54. [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang C‐J, Kuo Y‐R, Wu R‐W, Liu R‐T, Hsu C‐S, Wang F‐S, et al. Extracorporeal shockwave treatment for chronic diabetic foot. Journal of Surgical Research 2009;152(1):96‐103. [DOI] [PubMed] [Google Scholar]
Wang 2011a {published data only}
- Wang C‐J, Wu R‐W, Yang Y‐J. Treatment of diabetic foot ulcers: a comparative study of extracorporeal shockwave therapy and hyperbaric oxygen therapy. Diabetes Research and Clinical Practice 2011;92(2):187‐93. [DOI] [PubMed] [Google Scholar]
Additional references
Arnó 2010
- Arnó A, García O, Hernán I, Sancho J, Acosta A, Barret JP. Extracorporeal shock waves, a new non‐surgical method to treat severe burns. Burns 2010;36(6):844‐9. [DOI] [PubMed] [Google Scholar]
Chen 2004
- Chen YJ, Wurtz T, Wang CJ, Kuo YR, Yang KD, Huang HC, et al. Recruitment of mesenchymal stem cells and expression of TGF‐β1 and VEGF in the early stage of shock wave‐promoted bone regeneration of segmental defect in rats. Journal of Orthopaedic Research 2004;22(3):526‐34. [DOI] [PubMed] [Google Scholar]
Crane 2008
- Crane JS, Cheshire NJ. Chronic ulceration of the leg. Surgery 2008;26:13‐6. [Google Scholar]
Etufugh 2007
- Etufugh CN, Phillips TJ. Venous ulcers. Clinics in Dermatology 2007;25(1):121‐30. [DOI] [PubMed] [Google Scholar]
González‐Consuegra 2011
- González‐Consuegra RV, Verdú J. Quality of life in people with venous leg ulcers: an integrative review. Journal of Advanced Nursing 2011;67(5):926‐44. [DOI] [PubMed] [Google Scholar]
Hahn 1999
- Hahn TL, Unthank JL, Lalka SG. Increased hindlimb leukocyte concentration in a chronic rodent model of venous hypertension. Journal of Surgical Research 1999;81(1):38‐41. [DOI] [PubMed] [Google Scholar]
Herber 2007
- Herber OR, Schnepp W, Rieger MA. A systematic review on the impact of leg ulceration on patients' quality of life. Health and Quality of Life Outcomes 2007;5(4):44‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J, on behalf of the Cochrane Information Retrieval Methods Group. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of Internal Medicine 2009;151(4):65‐94. [DOI] [PubMed] [Google Scholar]
Ma 2007
- Ma HZ, Zeng BF, Li XL. Upregulation of VEGF in subchondral bone of necrotic femoral heads in rabbits with use of extracorporeal shock waves. Calcified Tissue International 2007;81(2):124‐31. [DOI] [PubMed] [Google Scholar]
Marston 1999
- Marston WA, Carlin RE, Passman MA, Farber MA, Keagy BA. Healing rates and cost efficacy of outpatient compression treatment for leg ulcers associated with venous insufficiency. Journal of Vascular Surgery 1999;30(3):491‐8. [DOI] [PubMed] [Google Scholar]
Mekkes 2003
- Mekkes JR, Loots MA, Wal AC, Bos JD. Causes, investigation and treatment of leg ulceration. British Journal of Dermatology 2003;148(3):388‐401. [DOI] [PubMed] [Google Scholar]
Mittermayr 2011
- Mittermayr R, Hartinger J, Layr M, Smolen D, Schaden W, Redl H. Extracorporeal Shock Wave Therapy (ESWT) minimizes ischaemic tissue necrosis irrespective of application time and promotes tissue revascularization by stimulating angiogenesis. Annals of Surgery 2011;253:1024‐32. [DOI] [PubMed] [Google Scholar]
Mittermayr 2012
- Mittermayr R, Antonic V, Hartinger J, Kaufmann H, Redl H, Téot L, et al. Extracorporeal shock wave therapy (ESWT) for wound healing: technology, mechanisms, and clinical efficacy. Wound Repair and Regeneration 2012;20(4):456‐65. [DOI] [PubMed] [Google Scholar]
Mouzopoulos 2007
- Mouzopoulos G, Stamatakos M, Mouzopoulos D, Tzurbakis M. Extracorporeal shock wave treatment for shoulder calcific tendonitis: a systematic review. Skeletal Radiology 2007;36.9:803‐11. [DOI] [PubMed] [Google Scholar]
Nishida 2004
- Nishida T, Shimokawa H, Oi K, Tatewaki H, Uwatoku T, Abe K, et al. Extracorporeal cardiac shock wave therapy markedly ameliorates ischemia‐induced myocardial dysfunction in pigs in vivo. Circulation 2004;110(19):3055‐61. [DOI] [PubMed] [Google Scholar]
Ogden 2001
- Ogden JA, Tóth‐Kischkat A, Schultheiss R. Principles of shock wave therapy. Clinical Orthopaedics and Related Research 2001;387:8‐17. [DOI] [PubMed] [Google Scholar]
Palfreyman 1998
- Palfreyman SJ, Lochiel R, Michaels JA. A systematic review of compression therapy for venous leg ulcers. Vascular Medicine 1998;3(4):301‐13. [DOI] [PubMed] [Google Scholar]
Persoon 2004
- Persoon A, Heinen MM, Vleuten CJ, Rooij MJ, Kerkhof PC, Achterberg T. Leg ulcers: a review of their impact on daily life. Journal of Clinical Nursing 2004;13(3):341‐54. [DOI] [PubMed] [Google Scholar]
Qureshi 2011
- Qureshi A, Ross K, Ogawa R, Orgill D. Shock wave therapy in wound healing. Plastic and Reconstructive Surgery 2011;128:721‐7e. [DOI] [PubMed] [Google Scholar]
Raju 2010
- Raju S. Correcting venous insufficiency improves healing of venous ulcers. Disease‐a‐Month 2010;56(11):653‐7. [DOI] [PubMed] [Google Scholar]
Reichenberg 2005
- Reichenberg J, Davis M. Venous ulcers. Seminars in Cutaneous Medicine and Surgery 2005;24(4):216‐26. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saggini 2008
- Saggini R, Figus A, Troccola A, Cocco V, Saggini A, Scuderi N. Extracorporeal Shock Wave Therapy for management of chronic ulcers in the lower extremities. Ultrasound in Medicine and Biology 2008;34(8):1261‐71. [DOI] [PubMed] [Google Scholar]
Saharay 1998
- Saharay M, Shields DA, Georgiannos SN, Porter JB, Scurr JH, Coleridge Smith PD. Endothelial activation in patients with chronic venous disease. European Journal of Vascular and Endovascular Surgery 1998;15(4):342‐9. [DOI] [PubMed] [Google Scholar]
Schaden 2007
- Schaden W, Thiele R, Kölpl C, Pusch M, Nissan A, Attinger CE, et al. Shock wave therapy for the treatment of acute and chronic soft tissue wounds: a feasibility study. Journal of Surgical Research 2007;143.1:1‐12. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Visit GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shrivistava 2005
- Shrivastava SK. Shock wave treatment in medicine. Journal of Biosciences 2005;30(2):269‐75. [DOI] [PubMed] [Google Scholar]
SIGN 2010
- Scottish Intercollegiate Guidelines Network (SIGN). Management of chronic venous leg ulcers: a national clinical guideline. SIGN guideline 120. August 2010. www.sign.ac.uk/pdf/sign120.pdf (accessed 30 May 2017).
SIGN 2018
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/search‐filters.html (accessed 8 June 2018).
Smith 2006
- Smith PC. The causes of skin damage and leg ulceration in chronic venous disease. International Journal of Lower Extremity Wounds 2006;5(3):160‐8. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stojadinovic 2008
- Stojadinovic A, Elster EA, Anam K, Tadaki D, Amare M, Zins S, et al. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis 2008;11(4):369‐80. [DOI] [PubMed] [Google Scholar]
Thompson 1999
- Thompson SG, Sharp SJ. Explaining heterogeneity in meta‐analysis: a comparison of methods. Statistics in Medicine 1999;18:2693‐708. [DOI] [PubMed] [Google Scholar]
Valencia 2001
- Valencia IC, Falabella A, Kirsner RS, Eaglstein WH. Chronic venous insufficiency and venous leg ulceration. Journal of the American Academy of Dermatology 2001;44(3):401‐24. [DOI] [PubMed] [Google Scholar]
Van Gent 2010
- Gent WB, Wilschut ED, Wittens C. Management of venous ulcer disease. BMJ 2010;341:1092‐6. [DOI] [PubMed] [Google Scholar]
Vowden 2009
- Vowden KR, Vowden P. The prevalence, management and outcome for patients with lower limb ulceration identified in a wound care survey within one English health care district. Journal of Tissue Viability 2009;18(1):13‐9. [DOI] [PubMed] [Google Scholar]
Walters 1999
- Walters SJ, Morrell CJ, Dixon S. Measuring health‐related quality of life in patients with venous leg ulcers. Quality of Life Research 1999;8(4):327‐36. [DOI] [PubMed] [Google Scholar]
Wang 2004
- Wang F, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, et al. Ras induction of superoxide activates ERK‐dependent angiogenic transcription factor HIF‐1α and VEGF‐a expression in shock wave‐stimulated osteoblasts. Journal of Biological Chemistry 2004;279(11):10331‐7. [DOI] [PubMed] [Google Scholar]
Wang 2011b
- Wang CJ, Yang YJ, Huang CC. The effects of shockwave on systemic concentrations of nitric oxide level, angiogenesis and osteogenesis factors in hip necrosis. Rheumatology International 2011;31(7):871‐7. [DOI] [PubMed] [Google Scholar]
Wilson 2004
- Wilson AB. Quality of life and leg ulceration from the patient's perspective. British Journal of Nursing 2004;13(11):17. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cooper 2015
- Cooper B, Bachoo P, Brittenden J. Extracorporeal shock wave therapy for the healing and management of venous leg ulcers. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD011842] [DOI] [PMC free article] [PubMed] [Google Scholar]


