Abstract
Background
This is an update of a Cochrane Review first published in 2001.
Hernias are protrusions of all or part of an organ through the body wall that normally contains it. Groin hernias include inguinal (96%) and femoral (4%) hernias, and are often symptomatic with discomfort. They are extremely common, with an estimated lifetime risk in men of 27%. Occasionally they may present as emergencies with complications such as bowel incarceration, obstruction and strangulation. The definitive treatment of all hernias is surgical repair, inguinal hernia repair being one of the most common surgical procedures performed. Mesh (hernioplasty) and the traditional non‐mesh repairs (herniorrhaphy) are commonly used, with an increasing preference towards mesh repairs in high‐income countries.
Objectives
To evaluate the benefits and harms of different inguinal and femoral hernia repair techniques in adults, specifically comparing closure with mesh versus without mesh. Outcomes include hernia recurrence, complications (including neurovascular or visceral injury, haematoma, seroma, testicular injury, infection, postoperative pain), mortality, duration of operation, postoperative hospital stay and time to return to activities of daily living.
Search methods
We searched the following databases on 9 May 2018: Cochrane Colorectal Cancer Group Specialized Register, Cochrane Central Register of Controlled Trials (Issue 1), Ovid MEDLINE (from 1950), Ovid Embase (from 1974) and Web of Science (from 1900). Furthermore, we checked the WHO International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov for trials. We applied no language or publication restrictions. We also searched the reference lists of included trials and review articles.
Selection criteria
We included randomised controlled trials of mesh compared to non‐mesh inguinal or femoral hernia repairs in adults over the age of 18 years.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Where available, we collected information on adverse effects. We presented dichotomous data as risk ratios, and where possible we calculated the number needed to treat for an additional beneficial outcome (NNTB). We presented continuous data as mean difference. Analysis of missing data was based on intention‐to‐treat principles, and we assessed heterogeneity using an evaluation of clinical and methodological diversity, Chi2 test and I2 statistic. We used GRADE to assess the quality of evidence for each outcome.
Main results
We included 25 studies (6293 participants) in this review. All included studies specified inguinal hernias, and two studies reported that femoral hernias were included.
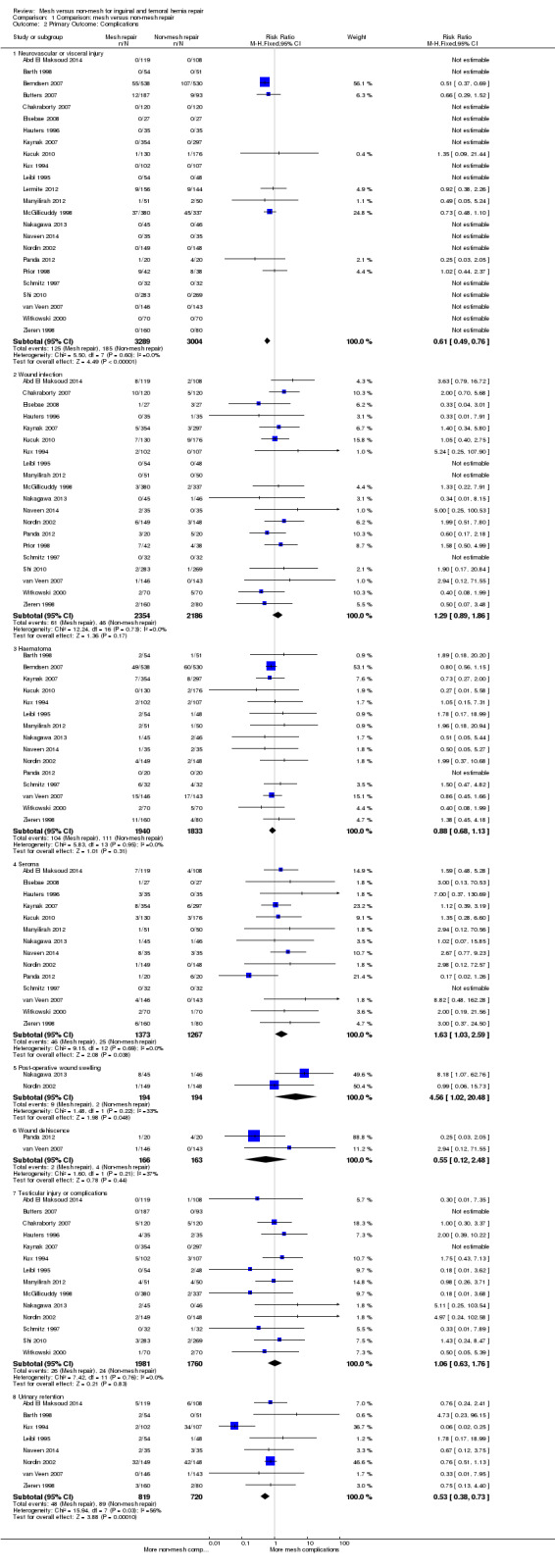
Mesh repair probably reduces the risk of hernia recurrence compared to non‐mesh repair (21 studies, 5575 participants; RR 0.46, 95% CI 0.26 to 0.80, I2 = 44%, moderate‐quality evidence). In absolute numbers, one hernia recurrence was prevented for every 46 mesh repairs compared with non‐mesh repairs. Twenty‐four studies (6293 participants) assessed a wide range of complications with varying follow‐up times. Neurovascular and visceral injuries were more common in non‐mesh repair groups (RR 0.61, 95% CI 0.49 to 0.76, I2 = 0%, NNTB = 22, high‐quality evidence). Wound infection was found slightly more commonly in the mesh group (20 studies, 4540 participants; RR 1.29, 95% CI 0.89 to 1.86, I2 = 0%, NNTB = 200, low‐quality evidence). Mesh repair reduced the risk of haematoma compared to non‐mesh repair (15 studies, 3773 participants; RR 0.88, 95% CI 0.68 to 1.13, I2 = 0%, NNTB = 143, low‐quality evidence). Seromas probably occur more frequently with mesh repair than with non‐mesh repair (14 studies, 2640 participants; RR 1.63, 95% CI 1.03 to 2.59, I2 = 0%, NNTB = 72, moderate‐quality evidence), as does wound swelling (two studies, 388 participants; RR 4.56, 95% CI 1.02 to 20.48, I2 = 33%, NNTB = 72, moderate‐quality evidence). The comparative effect on wound dehiscence is uncertain due to wide confidence intervals (two studies, 329 participants; RR 0.55, 95% CI 0.12 to 2.48, I2 = 37% NNTB = 77, low‐quality evidence). Testicular complications showed nearly equivocal results; they probably occurred slightly more often in the mesh group however the confidence interval around the effect was wide (14 studies, 3741 participants; RR 1.06, 95% CI 0.63 to 1.76, I2 = 0%, NNTB = 2000, low‐quality evidence). Mesh reduced the risk of postoperative urinary retention compared to non‐mesh (eight studies, 1539 participants; RR 0.53, 95% CI 0.38 to 0.73, I2 = 56%, NNTB = 16, moderate‐quality evidence).
Postoperative and chronic pain could not be compared due to variations in measurement methods and follow‐up time (low‐quality evidence).
No deaths occurred during the follow‐up periods reported in the seven studies (2546 participants) reporting this outcome (high‐quality evidence).
The average operating time was longer for non‐mesh repairs by a mean of 4 minutes 22 seconds, despite wide variation across the studies regarding size and direction of effect, thus this result is uncertain (20 studies, 4148 participants; 95% CI ‐6.85 to ‐1.60, I2= 97%, very low‐quality evidence). Hospital stay may be shorter with mesh repair, by 0.6 days (12 studies, 2966 participants; 95% CI ‐0.86 to ‐0.34, I2 = 98%, low‐quality evidence), and participants undergoing mesh repairs may return to normal activities of daily living a mean of 2.87 days sooner than those with non‐mesh repair (10 studies, 3183 participants; 95% CI ‐4.42 to ‐1.32, I2 = 96%, low‐quality evidence), although the results of both these outcomes are also limited by wide variation in the size and direction of effect across the studies.
Authors' conclusions
Mesh and non‐mesh repairs are effective surgical approaches in treating hernias, each demonstrating benefits in different areas. Compared to non‐mesh repairs, mesh repairs probably reduce the rate of hernia recurrence, and reduce visceral or neurovascular injuries, making mesh repair a common repair approach. Mesh repairs may result in a reduced length of hospital stay and time to return to activities of daily living, but these results are uncertain due to variation in the results of the studies. Non‐mesh repair is less likely to cause seroma formation and has been favoured in low‐income countries due to low cost and reduced availability of mesh materials. Risk of bias in the included studies was low to moderate and generally handled well by study authors, with attention to details of allocation, blinding, attrition and reporting.
Plain language summary
Comparing surgical groin hernia repair performed with or without mesh
Review question
This review assessed the difference in outcomes between surgical hernia repair with and without mesh.
Background
Hernias are out‐pouchings of an organ through the body wall that normally contains it; in this review, we refer to the bowel or its surrounding fatty tissues protruding through the abdominal wall in the groin region. This is a very common medical problem, affecting 27 out of every 100 men. These hernias can cause significant discomfort, and can occasionally become so tightly stuck that the blood supply can be cut off (strangulation), requiring emergency surgery. The curative treatment of hernias is surgical repair, which can be closed with sutured techniques (non‐mesh repair) or with a fine mesh to promote tissue growth to strengthen the previously weak area (mesh repair). Mesh repair is becoming increasingly popular in many countries, particularly in conjunction with laparoscopic (key‐hole) surgery.
Search date
We searched a number of databases for studies; this search was last updated on 9 May 2018.
Study characteristics
In this update of a review originally published in 2001, we included a total of 25 studies (with a total of 6293 people) undertaken in a number of different countries. A variety of outcomes were assessed, including return of the hernia after initial repair (hernia recurrence), a variety of complications including pain, duration of surgery, hospital stay and time before going back to normal activities.
Key results
One hernia recurrence is prevented for every 46 mesh repairs performed rather than non‐mesh repairs. Compared to non‐mesh repairs, mesh repairs are more likely to develop collections of fluid next to the surgical wound, but are less likely to result in difficulty urinating following the operation, or injury to nerves, blood vessels or other organs. Postoperative pain could not be clearly compared between studies due to differences in measurement methods and time frames, but overall the studies appeared to indicate that participants who had mesh repairs had less pain. The length of the surgical operation was slightly shorter for mesh repairs. Participants who had a mesh repair were more likely to have a shorter hospital stay and had a shorter average recovery time before returning to their normal activities.
Quality of the evidence
The studies included in this review used good‐quality methods, considered potential factors which could affect the results, and addressed their proposed outcomes clearly. In our assessment of the quality of evidence, we marked down some outcomes to 'moderate' quality, particularly due to variability within results.
Conclusions
Overall, hernia repairs with and without mesh both proved effective in the treatment of hernias, although mesh repairs demonstrated fewer hernia recurrences, a shorter operation time and faster return to normal activities. Non‐mesh repairs are still widely used, often due to the cost and poor availability of the mesh product itself.
Summary of findings
Summary of findings for the main comparison. Mesh compared to non‐mesh repair for inguinal and femoral hernia repair.
| Mesh compared to non‐mesh repair for inguinal and femoral hernia repair | ||||||
| Patient or population: adults undergoing inguinal and femoral hernia repair Setting: multiple hospitals from small to large tertiary centres contributed results Intervention: mesh repair Comparison: non‐mesh repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐mesh repair | Risk with mesh | |||||
| Hernia recurrence | Study population | RR 0.46 (0.26 to 0.80) | 5575 (21 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | In the context of surgical intervention, double blinding is difficult to achieve but most studies at least attempted single blinding. Follow‐up: up to 5 years |
|
| 4 per 100 | 2 per 100 (1 to 3) | |||||
| Surgical complications ‐ neurovascular or visceral injury | Study population | RR 0.61 (0.49 to 0.76) | 6293 (24 RCTs) | ⊕⊕⊕⊕ HIGH | Follow‐up: up to 4.3 years | |
| 6 per 100 | 4 per 100 (3 to 5) |
|||||
| Surgical complications ‐ wound infection | Study population | RR 1.29 (0.89 to 1.86) | 4540 (21 RCTs) | ⊕⊕⊝⊝ LOW 2 | Follow‐up: up to 5 years | |
| 2 per 100 | 3 per 100 (2 to 4) |
|||||
| Surgical complications ‐ wound dehiscence | Study population | RR 0.55 (0.12 to 2.48) | 329 (2 RCTs) | ⊕⊕⊝⊝ LOW 2 | Follow‐up: up to 3 years | |
| 2 per 100 | 1 per 100 (0 to 6) |
|||||
| Mortality (within 30 days post‐surgery) | There were no reported events of mortality within 30 days so this outcome could not be compared. However it can be concluded that both groups have very low rates of postoperative mortality. Follow‐up on mortality: up to 5 years |
|||||
| 0 per 100 | 0 per 100 (0 to 0) | |||||
| Duration of surgery (minutes) |
The mean duration of surgery ranged from 10 to 94 minutes | MD 4.22 minutes lower (6.85 lower to 1.6 lower) | ‐ | 4148 (20 RCTs) | ⊕⊝⊝⊝ VERY LOW 3 | The large degree of heterogeneity is likely to be related to variation in surgeon skill and familiarity with the intervention. |
| Duration of postoperative stay (days) |
The mean duration of postoperative stay ranged from 0.27 to 7.6 days | MD 0.6 days lower (0.86 lower to 0.34 lower) | ‐ | 2966 (12 RCTs) | ⊕⊕⊝⊝ LOW 4 | |
| Time to return to full ADLs (days) | The mean time to return to full ADLs ranged from 2.06 to 26 days | MD 2.87 days lower (4.42 lower to 1.32 lower) | ‐ | 3183 (10 RCTs) | ⊕⊕⊝⊝ LOW 4 | |
| Conversion from laparoscopic to open approach | No studies reported any conversion from laparoscopic to open technique where laparoscopic technique was used for mesh repair. | ‐ | 1680 (5 RCTs) | ⊕⊕⊝⊝ LOW 5 | The only laparoscopic techniques used for comparison were mesh repairs, and none of these studies reported conversion to open repair so this could not be compared. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; ADLs: activities of daily living; RCT: randomised controlled trial; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded one level for inconsistency (moderate heterogeneity)
2 Downgraded two levels for imprecision (wide confidence interval overlapping no effect) and inconsistency (substantial heterogeneity)
3 Downgraded three levels for inconsistency (considerable heterogeneity) and imprecision (wide confidence interval overlapping no effect)
4 Downgraded two levels for inconsistency (considerable heterogeneity)
5 Downgraded two levels for risk of bias and imprecision due to low event rate (not reported)
Background
Description of the condition
A hernia is defined as a protrusion of an organ or part of an organ through the body wall that normally contains it (Brooks 2014a). Abdominal wall hernias are common, with a prevalence in the general population of 4% for those aged over 45 years (Jenkins 2008). Inguinal and femoral hernias are known collectively as groin hernias (Brooks 2014a). Of all groin hernias, 96% are inguinal and 4% are femoral (Rutkow 1993). Men are eight times more likely to develop a groin hernia than women and 20 times more likely to require a groin hernia repair (Brooks 2014a).
It is a very common problem, with an estimated lifetime risk of groin hernia of 27% in men (3% in women) (Fitzgibbons 2015). There is still very limited evidence regarding prevalence, particularly in low‐income countries, but hernia repair is an extremely common general surgical procedure.
Groin hernias may present as a heaviness or discomfort in the groin region, or a visible or palpable bulge. Discomfort is usually most pronounced when intra‐abdominal pressure is increased, for example with heavy lifting, straining or prolonged standing. Risk factors for groin hernias include history of hernia or prior hernia repair, older age, male sex, chronic cough, chronic constipation, abdominal wall injury, smoking and family history of hernia (Brooks 2014a). The current literature seems to support the view that obesity may be a protective factor for groin hernias (Liem 1997; Rosemar 2008; Ruhl 2007).
Inguinal hernias are the most common type of hernia in both genders, accounting for 75% of all abdominal wall hernias, with a lifetime risk of 27% in men and 3% in women (Jenkins 2008). Inguinal hernias are classified as congenital or acquired (Brooks 2014a). Congenital inguinal hernias are caused by a failure of the processus vaginalis (invagination of the parietal peritoneum that precedes the migration and descent of the testes in males) to close. The portion of the processus vaginalis within the inguinal canal is called the 'canal of Nuck' in females and usually obliterates by the eighth foetal month of life (Brooks 2014a). This difference in development, in addition to the protective position of the round ligament, accounts for the far lower rates of inguinal (particularly indirect) hernias in women compared to men. In contrast, acquired hernias are due to the weakening or disruption of the fibromuscular tissues of the abdominal wall, allowing the protrusion of intra‐abdominal contents through the acquired defect (Brooks 2014a). This may be facilitated by inherent connective tissue abnormalities, chronic abdominal wall injury (including any chronic increased intra‐abdominal pressure) and possibly adverse effects of drugs such as glucocorticoids (thinning of skin and weakening of soft tissues) or smoking (Brooks 2014a; Cannon 1981; Sorenson 2002). Acquired hernias can present acutely and may require emergency surgical intervention.
Inguinal hernias are further classified as indirect or direct. Indirect inguinal hernias protrude through the internal inguinal ring, which is the site where the spermatic cord in males and the round ligament in females exits the abdomen (Brooks 2014a). Direct inguinal hernias protrude medial to the inferior epigastric vessels within Hesselbach's triangle (formed by the inguinal ligament inferiorly, the inferior epigastric vessels laterally and the rectus abdominis muscle medially) (Brooks 2014a). Femoral hernias are located inferior to the inguinal ligament and protrude through the femoral ring, medial to the femoral sheath containing the femoral artery and vein. Femoral hernias are acquired; the femoral ring can widen with age or injury (Brooks 2014a). Femoral hernias represent 20% to 31% of repairs in women compared to only 1% in men (Brooks 2014a).
It is important to differentiate femoral hernias from inguinal hernias given that femoral hernias are more likely to strangulate. As women are more likely to have femoral hernias than men, a relatively high proportion of women who present acutely with a symptomatic acquired hernia will require emergency management compared to their male counterparts. (Dahlstrand 2009; Koch 2005; Rosemar 2010). Classically, femoral hernias present as mildly painful non‐reducible groin lumps located inferolateral to the pubic tubercle; inguinal hernias are generally found superolaterally to the pubic tubercle (Whalen 2011). However, femoral hernias tend to move above the inguinal ligament, where they may be mistaken for an inguinal hernia. Differentiation on clinical grounds is notoriously unreliable, and unrelated to the experience of the examining practitioner (Whalen 2011). Ultrasonography, computed tomography or even diagnostic laparoscopy may have a role in further investigation of hernia type or occult hernia (Brooks 2014a; Whalen 2011).
Groin hernias may present as emergencies, with complications such as bowel incarceration and obstruction or strangulation (Brooks 2014a). Incarceration refers to the irreducible trapping of hernia contents within the hernia sac. Reduced venous and lymphatic flow leads to swelling of the incarcerated tissue, which can lead to impediment of arterial supply resulting in ischaemia and necrosis of the hernia contents (strangulation). The overall risk of incarceration and strangulation is low, between 0.3% and 3% per year (Brooks 2014a; Fitzgibbons 2006; Gallegos 1991).
Description of the intervention
The definitive treatment of all hernias is surgical repair, regardless of hernia origin or type. Repair of inguinal hernias is one of the most common general surgical procedures performed (McCormack 2003; Rutkow 1993). Urgent surgical repair is indicated for patients who develop complications. If this is undertaken within approximately four to six hours from onset of symptoms, an emergency surgical repair may prevent loss of bowel from prolonged strangulation (Brooks 2014b). However, for uncomplicated hernias, the optimal timing of repair and aspects of repair technique remain controversial. Currently it is recommended that patients with symptomatic hernias should undergo an elective hernia repair. For patients who are asymptomatic but have risk factors for groin hernia incarceration or strangulation, a hernia repair is generally undertaken as soon as is feasible (Brooks 2014b). For male patients with minimal symptoms, where a 'watchful waiting' approach is taken, the cumulative probability of developing problems such as increasing pain, incarceration or strangulation is 2.8% at three months, 4.5% to 23% at two years, and 31% at four years (Fitzgibbons 2006; Gallegos 1991; O'Dwyer 2006).
The aim of hernia repair surgery is not only to fix the current hernia defect, but also to reduce the risk of recurrence. Recurrence rates for primary hernia repair range from 0.5% to 15% depending on the hernia site, type of repair and clinical circumstances (Brooks 2014b).
Groin hernia repairs can involve the use of a mesh (otherwise known as a hernioplasty) or no mesh (that is, herniorrhaphy). The mesh used in hernia repair is typically made from a synthetic polymer, usually polypropylene, which is inert and does not cause abnormal inflammation. The mesh is lightweight and flexible, and designed to avoid impediment of local structures or positional movement. Meshes may be held in place using partially dissolvable sutures or a fibrin glue (or both), of which the glue may produce a more effective seal (Brooks 2014b). A mesh repair involves covering the hernial defect by placing the mesh on one of the layers of the abdominal wall either using an open approach or a minimal access laparoscopic technique (McCormack 2003). The approach to repair depends on a number of factors in each individual case, including the type of hernial defect, patient factors and the surgeon's preference. With the open approach, the repair is generally anterior to the hernial defect, whereas laparoscopic repair is approached from a posterior aspect. Prosthetic mesh is being increasingly incorporated into hernia surgery (either open or laparoscopic) as a component of tension‐free repair (Brooks 2014b).
Open techniques for inguinal hernia repairs include tension‐free mesh repairs such as the Lichtenstein, plug and patch, and Kugel (preperitoneal) repairs, and non‐mesh primary tissue approximation repairs such as the Shouldice, Bassini and McVay repairs (Brooks 2014b). In the tension‐free mesh repair category, the mesh is placed in front of the transversalis fascia, such as with the Lichtenstein tension‐free hernioplasty, or behind the transversalis fascia in the preperitoneal space, for example, the Kugel procedure (Amid 2005). With the tissue approximation repairs, which do not involve mesh placement, the Shouldice technique is generally the preferred suture‐based repair, which involves a four‐layer reconstruction of the fascia transversalis. Alternatives to the Shouldice technique include the original Bassini method, in which the edges of the defect are simply sewn back together with tension and, less commonly, the McVay method. The McVay method involves reinforcement of the inguinal canal by approximating the transversus abdominis aponeurosis and transversalis fascia to the pectineal ligament, thus restoring the canal floor by bringing together the femoral sheath and the inguinal ligament. It is important to note that the McVay style of repair is also typically used in open femoral hernia repairs, with possible approaches from an infra‐inguinal (Lockwood), trans‐inguinal (Lotheissen) or supra‐inguinal (McEvedy) aspect (Amid 2005).
The two main laparoscopic groin hernia repairs are the totally extraperitoneal (TEP) and transabdominal preperitoneal patch (TAPP) repairs (Bittner 2011), both requiring the use of a mesh. TEP repair is performed by gaining access to the preperitoneal space (that is, the space between the peritoneum and the anterior abdominal wall) using an anterior approach, without ever actually entering the abdomen (Ferzli 1998; McKernan 1993). A TAPP repair, on the other hand, requires the surgeon to enter the peritoneal (abdominal) cavity to access the preperitoneal space. Some of the more significant disadvantages of this approach include potential injury to adjacent organs and, long‐term, adhesions resulting in bowel obstruction (Wake 2005; Vader 1997).
Common early complications of hernia repair surgery include wound seroma or haematoma, urinary retention, bladder injury and superficial wound infection. Complications that may occur later following hernia repair surgery include persistent groin pain and post‐herniorrhaphy neuralgia, testicular complications, deep wound or mesh infection, recurrent hernia and mesh migration or erosion (Brooks 2014c). The incidence of post‐surgical complications is more common following emergent repairs and recurrent hernia repairs (Brooks 2014c).
A Cochrane Review published in 2008 showed that whilst laparoscopic repairs were associated with quicker recovery times and less persistent pain, the procedure itself usually takes longer and has higher rates of bladder and vascular injuries. Hernia recurrence after laparoscopic mesh repair was less common compared to open non‐mesh repair, with the main indicator of recurrence related to the use of a mesh rather than the approach itself (McCormack 2003).
How the intervention might work
Prior to 1958, abdominal wall hernias were closed with primary suture repair. Tension on the weak fascia was thought to be one of the main contributing factors to repair failure. Then, in 1958, Dr Francis Usher published his 'tension‐free' technique using a permanently implanted polypropylene mesh (Read 1999). This led to the Lichtenstein repair some 30 years later, which popularised mesh for hernia repair. The logic that Usher used to explain his use of polypropylene mesh was that the mesh was a material that could be used to close over the hernial defect and provide ongoing reinforcement to the attenuated fascia of the abdominal wall by encouraging growth of connective tissue (scar tissue) around and through the mesh fibres (Doctor 2006). It was expected that the best meshes would be those made of very strong material and able to induce the most fibrosis. Unfortunately, this fibrotic reaction led to pain and movement restriction and it soon became clear that this needed to be reduced. In order to do this, the surface area (and therefore strength) of the mesh had to be reduced. Calculations of intra‐abdominal pressures proved that this would be possible without compromising mesh function. In fact, the tensile strength of a mesh required to withstand the maximum abdominal pressure is only a tenth of that of most meshes (Brown 2010). This realisation led to the concept of lightweight meshes.
Lightweight and heavyweight meshes have been used in the repair of hernias. Compared to their heavyweight counterparts, lightweight meshes have large pores (normally 3 mm to 5 mm) and a small surface area. They stimulate a reduced inflammatory reaction and, therefore, have greater elasticity and flexibility (Klinge 2008). They also shrink less and have been shown to cause less pain compared to heavier meshes after Lichtenstein inguinal hernia repairs. Unfortunately, despite these improvements, patients continue to have complications such as recurrence, infection and adhesion formation (Brown 2010). Lightweight mesh may not increase the risk of inguinal hernia recurrence and seems to be associated with the reduced risk of developing chronic pain complications, however, these outcomes continue to be researched in studies with longer follow‐up times (Sajid 2013). Thus, the search for an ideal mesh continued.
The difficulty of finding a single, 'ideal' mesh is acknowledged by the development of composite meshes. These combine more than one material and are the basis of most new mesh designs. The main advantage of the composite meshes is that they can be used in the intraperitoneal space with minimal adhesion formation. Despite the vast selection of brands available, nearly all these meshes continue to use one of the three basic materials: polypropylene, polyester and expanded polytetraflouroethylene (ePTFE). These are used in combination with each other or with a range of additional materials such as titanium, omega 3, poliglecaprone 25 (Monocryl), polyvinylidene difluoride (PVDF) and hyaluronate. However, as might be expected, none of these synthetic materials are without disadvantages (O'Dwyer 2005).
The problems encountered with synthetic materials led to the development of bio‐materials, which are currently the most physiologically based implants. These consist of an acellular collagen matrix derived from human dermis or porcine small intestine submucosa. The matrix allows soft tissue to infiltrate the mesh which eventually becomes integrated into the body by a process of remodelling. Unfortunately, this process also appears to lead to a rapid reduction in their mechanical strength, and concerns regarding this have restricted their use to infected environments (where one would normally use an absorbable synthetic material such as polyglactin 910 (Vicryl)) (Brown 2010).
Why it is important to do this review
Currently, about one million meshes are used per year for hernia repairs globally (Klinge 2002). In 2002, the EU Hernia Trialists Collaboration analysed 58 randomised controlled trials and found that the use of mesh was superior to other techniques; in particular, the meta‐analyses noted fewer recurrences and less postoperative pain with mesh repair compared to all other techniques (EU Hernia Trialists 2002). Despite the favourable results of mesh repair and its adoption as common practice in high‐income countries, it has yet to be integrated as standard practice by all surgeons (Nixon 2009). Non‐mesh repairs are still commonly performed worldwide, particularly in low‐income countries; for example, in some African countries, when surgical treatment is provided (65% to 75% as an emergency procedure rather than elective), fewer than 5% of hernias are repaired using implanted mesh (Yang 2011). This is likely to be related to the increased costs involved in mesh (and also laparoscopic) repair which can be unaffordable in countries where the typical per capita government health expenditure (USD 28 in Ghana, USD 7 in Uganda) is usually less than the price of a single‐use package of commercial mesh (USD 40 to USD 100) (Yang 2011). The biological meshes are available at an even higher cost (Klinge 2008). Research into low‐cost mesh alternatives (such as polyethylene mesh, normally used in mosquito‐netting) and innovative construction of standard commercial meshes are being undertaken and show promise (Lofgren 2016; Yang 2011).
An updated meta‐analysis of the current literature is needed regarding the use of mesh in inguinal and femoral hernia repairs. In clinical practice, laparoscopy is increasingly used as the operative approach of choice. To address this, this review will include an analysis of trials exploring the use of mesh in the context of all operative approaches. The previous Cochrane Review considering mesh versus non‐mesh repairs specified only open repair as an inclusion criterion, and did not include the increasingly favoured laparoscopic approach.
In this meta‐analysis, assessment of the overall quality of the evidence and risk of bias for each outcome will make the available evidence more transparent and allow clinicians to make better informed decisions.
Objectives
To evaluate the benefits and harms of different inguinal and femoral hernia repair techniques in adults, specifically comparing closure with mesh versus without mesh. Outcomes include hernia recurrence, complications (including neurovascular or visceral injury, haematoma, seroma, testicular injury, infection, postoperative pain), mortality, duration of operation, postoperative hospital stay, time to return to activities of daily living and conversion from laparoscopic to open approach.
Methods
Criteria for considering studies for this review
Types of studies
All individual parallel randomised controlled trials (RCTs) and cluster‐RCTs investigating mesh compared to non‐mesh techniques for open or laparoscopic repair of inguinal or femoral hernias were eligible for inclusion.
Types of participants
We included persons aged 18 years or older with a clinically diagnosed inguinal or femoral hernia, or both, where surgical management was indicated (in accordance with the preceding Cochrane Review).
Types of interventions
Concerning inguinal hernias, we accepted any of the following mainstream surgical techniques.
-
Mesh repairs
Open, including the Lichtenstein approach
Laparoscopic, including transabdominal preperitoneal (TAPP) and totally extraperitoneal (TEP) approaches
Any type of commercially marketed non‐absorbable mesh or absorbable biomesh may be used; including 'plug‐and‐patch' kits (that is, absorbable plugs/tacks with (non‐)absorbable patch/mesh).
-
Suture repairs
Tension, including Bassini, McVay and Shouldice approaches
Tension‐free, including Desarda and Guarnieri approaches
Any type of commercially marketed non‐absorbable or absorbable sutures may be used.
Concerning femoral hernias, we accepted any of the following mainstream surgical techniques.
-
Mesh repairs
Open mesh/mesh plug repair
Laparoscopic; including TAPP or TEP approaches
Any type of commercially marketed non‐absorbable mesh or absorbable biomesh may be used; including 'plug‐and‐patch' kits (that is, absorbable plugs/tacks with (non‐)absorbable patch/mesh).
-
Suture repairs
Open McVay suture repair, including Lockwood's infra‐inguinal, Lotheissen's trans‐inguinal and McEvedy's high approaches
Any type of commercially marketed non‐absorbable or absorbable sutures may be used.
Types of outcome measures
Primary outcomes
Recurrence of the same hernia (this excludes formation of a hernia at a new site not previously repaired or reinforced)
-
Surgical complications
Neurovascular or visceral injury
Wound infection at single or multiple sites, including deep and superficial wound infections
Haematoma
Seroma
Postoperative wound swelling
Wound dehiscence
Testicular injury or complications, including testicular swelling and atrophy
Urinary retention postoperatively
Postoperative pain, including acute and chronic pain
Mortality (number of associated deaths within 30 days of the operation during the study trial period)
Secondary outcomes
Duration of surgical operation (minutes)
Duration of postoperative hospital stay (days)
Time required to return to full activities of daily living including work and exercise (days)
Number of operations where conversion from laparoscopic to open approach was required
We will also present a brief narrative account of cost‐effectiveness of repair, as this is considered a clinically significant factor, particularly in low‐income countries, where this plays a large role in access to healthcare (see discussion).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases, with no restrictions on language or date of publication.
Cochrane Central Register of Controlled Trials (CENTRAL Issue 4, May 9th, 2018) (Appendix 1),
Ovid MEDLINE (1950 to May 9th, 2018) (Appendix 2),
Ovid Embase (1974 to May 9th, 2018) (Appendix 3)
Web of Science (1900 to May 9th, 2018) (Appendix 4).
The search was run separately on each database and results were checked against each other.
Searching other resources
We searched relevant clinical trials registers such as the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/) and ClinicalTrials.gov (http://clinicaltrials.gov/) May 9th 2018 for completed and ongoing trials.
In addition to this, we searched reference lists of included trials and review articles, books related to surgical hernia repairs, abstracts from general surgical conferences concerning hernias and mesh repair, and sent written enquiries to the authors of major relevant studies and experts in the field.
Data collection and analysis
Selection of studies
Three review authors (KL, ET, ST) assessed titles and abstracts retrieved from the search to determine their relevance concerning the objectives of this review. We managed disagreements through discussion, a deciding arbiter (MD), or both. We entered all search results into Review Manager 5.3 (RevMan 2014).
Data extraction and management
Two review authors (KL, ET) designed a data extraction sheet for study reports, which was pilot tested using sample studies and revised by the other authors (MD, MVD). Onto this report, two authors (KL, ST) independently extracted and recorded key features of each study including details of the following.
Methods: study design, total duration of study and run in, number of study centres and location, study setting, withdrawals, date of study
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria
Interventions: intervention, comparison, concomitant medications, excluded medications
Outcomes: primary and secondary outcomes specified and collected, time points reported. A brief narrative account of cost effectiveness included in discussion.
Notes: funding for trial, notable conflicts of interest of trial authors
We managed disagreements through discussion, a deciding arbiter (MVD) or both. We entered and presented the data for each included study into a table in Review Manager 5.3 (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (KL, ST) independently analysed each study in conjunction with the Cochrane tool for assessing risk of bias (Higgins 2011) (Appendix 5). This approach uses a domain‐based evaluation that aims to address main potential areas of bias in studies, where a judgement (low, high or unclear risk of bias) is assigned for each of the following domains.
Random sequence generation (low risk if true random sequence generation was described)
Allocation concealment (low risk if sealed, opaque, numbered envelopes were used, or central allocation after registration)
Blinding of participants and personnel (risk of performance bias; low risk of both participant and personnel were blinded to intervention)
Blinding of outcome assessment (risk of detection bias; low risk if both the the assessors were blinded to intervention)
Incomplete or selective outcome data reporting (low risk if more than 80% of those randomly assigned were assessed)
Any other potential sources of bias (e.g. study stopped early because of a data‐dependent process, notable baseline imbalance, surgeon competence or experience).
A high risk of bias indicates that the study design has not met the criteria for a low risk classification as noted above for each of the respective domains. Similarly, an unclear risk of bias denotes that the study has not declared sufficient information regarding their study design to make a judgement. We managed any disagreements through discussion, a deciding arbiter (MVD), or both. We presented our assessment of risk of bias for the included studies in the 'Risk of bias' summary tables and graphs as generated through input into Review Manager 5.3 (RevMan 2014).
Measures of treatment effect
We presented dichotomous (binary) data as a measure of risk by using a risk ratio (RR) with 95% confidence intervals (CIs). Where possible, we calculated the absolute risk reduction (ARR) and number needed to treat to benefit (NNTB) or number needed to treat to harm (NNTH) for comparison against other treatments or non‐treatment.
We presented continuous data as a mean difference (MD) if the same scale was used. Alternatively, a standardised mean difference (SMD) was calculated (that is, an average of the combined standard deviations) in the event that each study used a different scale measuring the same concept. In this case, we assessed the impact of using the highest verses the lowest of the available standard deviations (SDs) on the overall estimate of effect. If SDs were not reported we estimated the SD based on similar studies and used this in the meta‐analysis (Higgins 2011)
The treatment effect was considered statistically significant if the P value was less than 0.05.
Unit of analysis issues
The participant is the unit of analysis in our review. Nonetheless:
if the unit of analysis was not the same as the unit of randomisation, such as in cluster‐randomised trials, we adjusted for clustering by using the guidance given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); and
if there were multiple measurements for the same participant (for example, multiple hernias in the same person), we analysed the data as in a cluster‐randomised trial.
Dealing with missing data
We contacted the trial authors of the original studies if further data or information was required. We performed analyses based on intention‐to‐treat (ITT) principles, whereby the missing data for randomised participants was assumed to be treatment failures in this review. However, this approach of ITT analysis (that is, assuming dropouts as failures) may underestimate the effect of the intervention, therefore we performed both ITT and on‐treatment (that is, non‐ITT) analyses to explore the impact of missing data on the overall outcome (Higgins 2011). Furthermore, for continuous data we assessed the impact of missing data on the overall estimate of effect by imputing missing data in the following ways: best‐case scenario, where the missing data were considered 2 SDs greater in the intervention arm than in the control arm; and worst‐case scenario, where the missing data were considered 2 SDs less than in the control arm.
Assessment of heterogeneity
We assessed the included studies for heterogeneity through three successive steps to determine if they should be pooled with the rest of the included studies or considered separately.
Two review authors (KL, JN) independently analysed the included studies for their heterogeneity, including the extent of clinical diversity (participants, interventions and outcomes), and methodological diversity (study design and risk of bias).
We then assessed the included studies for statistical heterogeneity using the Chi2 test, with a P value of less than 0.10 being statistically significant.
We then calculated the I2 statistic as instructed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), where 0% to 40% is likely to indicate minimal heterogeneity, 30% to 60% may represent moderate heterogeneity, 60% to 90% may represent substantial heterogeneity, and 90% to 100% may represent considerably significant heterogeneity. The importance of the observed value of I2 does depend on the magnitude and direction of the treatment effects, and strength of evidence for heterogeneity (that is, the P value from the Chi2 test or the confidence interval for I2).
Assessment of reporting biases
Given that there was a sufficient number of studies pooled (more than 10), we performed a funnel plot to visually assess the risk of publication bias, where more pronounced asymmetry of the funnel plot may indicate a substantial overestimation of the intervention effect, as recommended in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2011).
Data synthesis
We used the fixed‐effect model in the absence of statistical heterogeneity, according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), where the analysis produced an estimate of the true effect.
In the event of statistical heterogeneity we used the random‐effects model for pooling the study data, using the Mantel‐Haenszel method, according to the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011). We used an I2 of 40% or higher as a cut‐off for heterogeneity, i.e. we used a random effects model if the I2 was 40% or greater. In the event that there was an insufficient number of studies (less than two) to produce an average effect in a random‐effects model, a fixed‐effect model was used.
Where cluster‐RCTs were included, we used the generic inverse variance method (Higgins 2011).
We planned to use the Peto one‐step odds ratio method for meta‐analysis of rare events (event rates below 1%), according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we explored further the treatment effect in specific subgroups, including:
inguinal versus femoral hernia;
direct versus indirect inguinal hernia;
male versus female participants;
participants meeting the American Society of Anaesthesiology (ASA) criteria of 1 to 2 versus participants meeting the ASA criteria of 3 to 4*;
Body Mass Index (BMI) greater than or equal to 30 versus BMI less than 30**;
elective versus emergency surgery;
different types of mesh, for example, biological versus composite synthetics.
*American Society of Anaesthesiology (ASA) physical status classification of perioperative patients: 1 = healthy person; 2 = mild systemic disease; 3 = severe systemic disease; 4 = severe systemic disease that is a constant threat to life (Skalad 1941).
**Body Mass Index (BMI): those greater than or equal to 30 are classified as obese in accordance to WHO 2006.
Sensitivity analysis
If sufficient data were available, we performed the following sensitivity analyses.
In order to determine the impact of risk of bias on the overall effect estimate, we removed high risk of bias studies from the pooled analysis and compared the results. High risk of bias studies were assessed to be 'high risk' in at least one domain (including selection bias).
In order to determine the impact of heterogeneity on the overall estimate of effect, we removed studies that contributed to heterogeneity from the analyses and compared the results.
We used two different methods of pooling to test sensitivity: we pooled all studies together and then removed studies from the meta‐analysis one by one, noting if there was any significant change in the overall results; and simultaneously, we compared the use of a fixed‐effect versus random‐effects model for the pooling analysis as we excluded each study one by one.
GRADE and 'Summary of findings' table
We presented a 'Summary of findings' table including the following outcomes: hernia recurrence, complications, mortality, conversion from laparoscopic to open approach, duration of surgery, duration of postoperative stay and time to return to full activities of daily living. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of evidence as it relates to the studies which contribute data to the meta‐analyses for these outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or up‐grade the quality of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary. Please see Table 2 for more information regarding criteria for quality of evidence grading (according to the GRADE recommendations, Schünemann 2011).
1. Quality of Evidence, GRADE definitions.
| Grade | Definition |
| High | We are very confident that the true effect lies close to that of the estimate of the effect. |
| Moderate | We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low | Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. |
| Very Low | We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. |
Results
Description of studies
Results of the search
We obtained 2006 records (of which 1076 duplicates were removed, leaving 930 records) from our searches of the respective databases, as described in Search methods for identification of studies. As noted in Selection of studies, we subsequently screened the titles and abstracts of 930 studies and retained 37 publications of randomised controlled trials (RCTs) which were considered for inclusion, of which 29 were initially included in this systematic review (see Figure 1 for study flow diagram). Of these 29 reports, we found that some presented results from the same studies at different time points or emphasised a different outcome. Therefore, we have included 25 studies in this review (all data available from each paper was included in our analysis).
1.
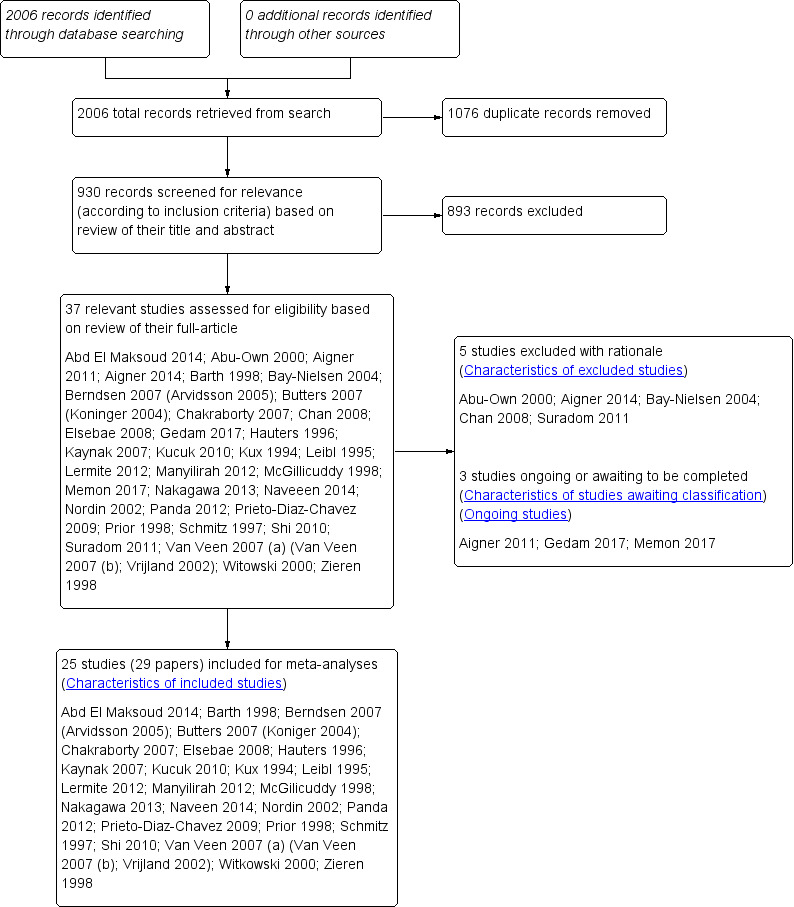
Study flow diagram.
Included studies
Twenty‐five RCTs met the inclusion criteria for this study, reviewing the effectiveness of mesh compared to non‐mesh hernia repairs. A total of 6293 participants (3289 given mesh repair and 3004 given non‐mesh repair) were included in the analysis, from a host of high and low‐income countries including: France, Lebanon, Iceland, Germany, India, Egypt, Belgium, Turkey, Uganda, the USA, Japan, Sweeden, Mexico, the UK, China, Netherlands, and Poland. The most common mesh repair used was Lichtenstein repair (Abd El Maksoud 2014; Barth 1998; Butters 2007; Chakraborty 2007; Elsebae 2008; Kaynak 2007; Kucuk 2010; Kux 1994; Manyilirah 2012; McGillicuddy 1998; Naveen 2014; Nordin 2002; Panda 2012; Prior 1998; van Veen 2007), whilst the most common non‐mesh repair was the Shouldice technique (Barth 1998; Berndsen 2007; Butters 2007; Hauters 1996; Kux 1994; Leibl 1995; Lermite 2012; McGillicuddy 1998; Nordin 2002; Prieto‐Diaz‐Chavez 2009; Schmitz 1997; van Veen 2007; Zieren 1998). Other mesh techniques noted were transabdominal preperitoneal patch (TAPP) repair (Berndsen 2007; Butters 2007; Hauters 1996; Leibl 1995; Zieren 1998), non‐specified 'tension‐free' mesh/plug repair (Lermite 2012; Prieto‐Diaz‐Chavez 2009; Schmitz 1997; Shi 2010), Prolene Hernia System mesh repair (Nakagawa 2013), Shulmann repair (van Veen 2007). Other non‐mesh techniques utilised included modified darn (Abd El Maksoud 2014), Abrahamson's darn (Chakraborty 2007), Bassini (Elsebae 2008; Naveen 2014; Panda 2012; Prior 1998; van Veen 2007; Witkowski 2000), Moloney darn (Kaynak 2007; Kucuk 2010), Desarda (Manyilirah 2012), Marcy (Nakagawa 2013) and McVay repair (van Veen 2007). The number of participants in each study ranged from 40 (Panda 2012), to 1183 (Berndsen 2007). Of the 25 studies, three were published in German (Kux 1994; Leibl 1995; Schmitz 1997), and one in French (Hauters 1996); all others were published in English. The most common outcomes studied included hernia recurrence, complications and postoperative pain. Most studies targeted participants with reducible, unilateral, primary hernias requiring elective repair.
Excluded studies
We excluded five studies; two did not meet the inclusion criteria of being a prospective randomised controlled trial (Bay‐Nielsen 2004; Chan 2008), and one did not meet inclusion criteria of being a randomised controlled trial (Suradom 2011). We excluded the remaining two studies as they compared only mesh versus mesh techniques (Abu‐Own 2000; Aigner 2014).
Risk of bias in included studies
Allocation
In the assessment of the risk of selection bias, for a study to qualify as low‐risk it had to describe an adequate system of random sequence generation, either via computer‐generated software or handling through an independent centre. Conversely, studies deemed high‐risk typically used allocation methods that were more transparent, with blinding risk. Overall, we assessed 13 studies as low‐risk (Abd El Maksoud 2014; Berndsen 2007; Chakraborty 2007; Manyilirah 2012; McGillicuddy 1998; Nakagawa 2013; Naveen 2014; Prieto‐Diaz‐Chavez 2009; Prior 1998; Shi 2010; van Veen 2007; Witkowski 2000; Zieren 1998) and Elsebae 2008 as high risk (participants were given a registration number upon enrolment in the trial and were assigned according to even/odd number), with the remaining studies classified as unclear.
Additionally, allocation concealment methods were assessed; acceptable methods included central randomisation or allocation with the use of sequentially numbered, opaque and sealed envelopes. Overall, we assessed ten studies as low‐risk (Abd El Maksoud 2014; Berndsen 2007; Chakraborty 2007; Hauters 1996; Manyilirah 2012; Nakagawa 2013; Nordin 2002; Prior 1998; van Veen 2007; Witkowski 2000), with the remaining studies classified as unclear.
Blinding
In the assessment of performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), it is recognised that given the nature of the intervention as a surgical procedure, true double‐blinding may be difficult to achieve. But given the subjective nature of certain outcomes — e.g. postoperative pain reported by participants and, to a lesser extent, clinical complications detected in follow‐up — there is potential for bias to occur if participants/personnel and outcome assessors are not blinded. Subsequently, studies assessed as having low risk of performance bias had to describe a sufficient method of blinding the participant; and to be assessed as having low risk of detection bias they had to have adequate blinding of the outcome assessor. When no method of blinding was outlined, we assigned a judgement of unclear risk of bias, and if blinding was unlikely to be achieved given the description of methods, these studies were assessed as high risk of bias.
Six of the included studies were described as low‐risk for performance bias (Barth 1998; Lermite 2012; Manyilirah 2012; Nakagawa 2013; Nordin 2002; Prior 1998) and one was found to be high risk, described as an open label study (Hauters 1996), with the remaining classified as unclear risk given lack of description regarding blinding methods.
Four studies were found to be at high risk of detection bias since the outcomes were assessed by surgeons who had performed the procedure, or were otherwise not blinded at the time of assessment (Abd El Maksoud 2014; Hauters 1996; Nakagawa 2013; Prieto‐Diaz‐Chavez 2009). We assessed five studies as being low risk of detection bias (Manyilirah 2012; Nordin 2002; Panda 2012; Prior 1998; van Veen 2007), and the remaining studies had an unclear risk of bias, as no explicit description of a blinding process could be interpreted from the text.
Incomplete outcome data
In the assessment of attrition bias, a study determined to be low‐risk had to demonstrate no missing outcome data, or if there were incomplete data with loss to follow‐up, it had to account for the missing data or perform an intention‐to‐treat analysis as applicable. Sixteen studies handled loss to follow‐up clearly, with an intention‐to‐treat model, and were determined to be at low risk of attrition bias (Barth 1998; Berndsen 2007; Butters 2007; Chakraborty 2007; Kaynak 2007; Leibl 1995; Lermite 2012; Manyilirah 2012; McGillicuddy 1998; Nakagawa 2013; Naveen 2014; Nordin 2002; Schmitz 1997; van Veen 2007; Witkowski 2000; Zieren 1998). A high‐risk study had to demonstrate missing outcome data or withdrawals/exclusions from the study, that may be directly related to an outcome, however we identified no studies in this risk group. We judged the remaining studies as having unclear risks of attrition bias, often due to a lack of reporting regarding dropouts and loss to follow‐up (Abd El Maksoud 2014; Elsebae 2008; Hauters 1996; Kaynak 2007; Kucuk 2010; Kux 1994; Panda 2012; Prieto‐Diaz‐Chavez 2009; Prior 1998; Shi 2010).
Selective reporting
In the assessment of reporting bias, a study deemed low‐risk was required to sufficiently report and discuss the primary or secondary outcomes explicitly outlined in their methodology or abstract. We assessed 22 studies as being low‐risk (Abd El Maksoud 2014; Barth 1998; Berndsen 2007; Butters 2007; Elsebae 2008; Kaynak 2007; Kucuk 2010; Kux 1994; Leibl 1995; Lermite 2012; Manyilirah 2012; McGillicuddy 1998; Nakagawa 2013; Naveen 2014; Nordin 2002; Panda 2012; Prieto‐Diaz‐Chavez 2009; Prior 1998; Schmitz 1997; Shi 2010; Witkowski 2000; Zieren 1998). In studies that were assessed as high‐risk, there may have been evidence through earlier versions or protocols, that outcomes initially intended to be analysed, were excluded in newer publications; or if there was suspicion that outcomes that were deemed to be particularly relevant, were being omitted. In one study identified as high‐risk (Chakraborty 2007), it was recognised that the study had been published in a low‐income nation and while the study portrayed one intervention as being more costly than another, cost had not been measured as an outcome, underlying a possible agenda with the potential to introduce bias. A second high‐risk study (van Veen 2007), intended to measure quality of life as an outcome in an earlier publications and protocols (van Veen 2007), but was not discussed in the final publication. Two other studies were at unclear risk of bias (Hauters 1996; Leibl 1995).
We created funnel plots for each outcome involving 10 or more studies, in order to assess for publication bias and consider other contributing factors of asymmetry. None of the studies measuring dichotomous outcomes clearly identified evidence of publication bias, resembling the classic symmetrical inverted funnel (see Figure 2 and Figure 3). The complications were assessed in funnel plots individually and none that included more than 10 studies demonstrated significant asymmetry. However, duration of surgery demonstrated a very wide spread, without any studies in the bottom half of the graph, which is potentially suggestive of publication bias (excluding smaller studies without statistically significant effects). This outcome also had significant heterogeneity which likely contributed to this appearance. The funnel plot for duration of postoperative stay was fairly symmetrical and resembled the classic shape, however the funnel plot for duration to return to full activities of daily living was similarly was 'top‐heavy' in appearance though much less wide‐spread. This is again in the context of a large amount of heterogeneity.
2.
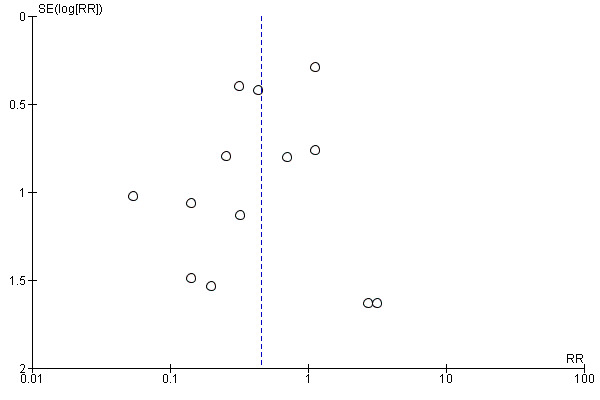
Funnel plot of comparison: mesh vs non‐mesh repair, primary outcome: hernia recurrence.
3.
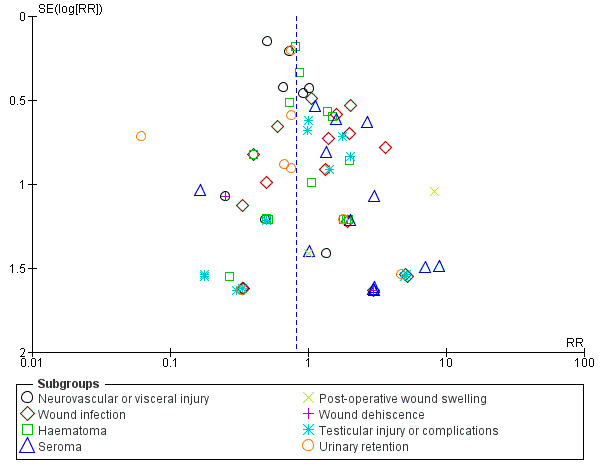
Funnel plot of comparison: mesh vs non‐mesh repair, primary outcome: complications.
Other potential sources of bias
One of the included studies declared a commercial source of funding: Berndsen 2007 declared financial support from Ethicon Endosurgery, Johnson and Johnson companies, however in another publication of this study by Arvidsson and colleagues, the authors went on to clarify that Ethicon did not have any involvement in the design or conduct of the study, or data analysis. Another study, Manyilirah 2012, declared financial support from Makere University and Mulago National Referral and Teaching Hospital. All other papers declared no conflict of interest and did not describe any sources of funding.
Please refer to Figure 4 and Figure 5 for summaries of our 'Risk of bias' analysis of included studies.
4.
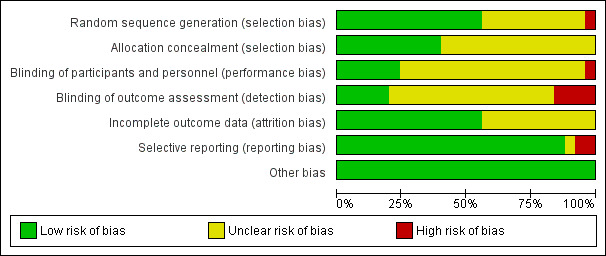
Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
5.
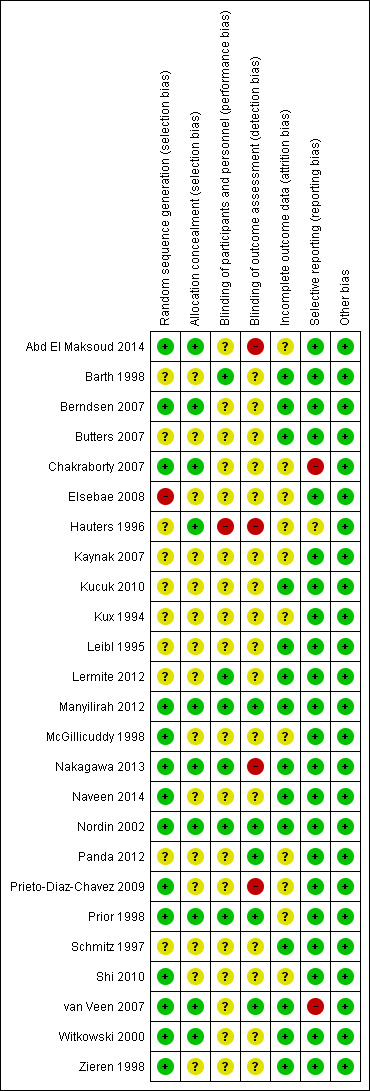
Risk of bias summary: review authors' judgements about each 'Risk of bias' item for each included study.
Effects of interventions
See: Table 1
Primary outcomes
Hernia recurrence
Twenty‐one studies were eligible for evaluation of hernia recurrence as a primary outcome (Abd El Maksoud 2014; Barth 1998; Berndsen 2007; Butters 2007; Chakraborty 2007; Leibl 1995; Elsebae 2008; Kux 1994; Hauters 1996; Kaynak 2007; Kucuk 2010; McGillicuddy 1998; Nakagawa 2013; Naveen 2014; Nordin 2002; Panda 2012; Prior 1998; Shi 2010; van Veen 2007; Witkowski 2000; Zieren 1998). Eight of these studies did not report recurrence in either mesh or non‐mesh group (Barth 1998; Chakraborty 2007; Leibl 1995; Kucuk 2010; Nakagawa 2013; Panda 2012; Prior 1998; Zieren 1998). On review of the remaining studies, in total there were 52/2834 events of hernia recurrence in the mesh repair group, compared to 110/2741 events of hernia recurrence in the non‐mesh repair group. We performed the analysis using a random‐effects model due to there being moderate heterogeneity (I2 = 44%). Meta‐analysis demonstrated a statistically significant difference in recurrence between the mesh and non‐mesh groups, with hernia recurrence occurring more frequently in the non‐mesh group (risk ratio (RR) 0.46, 95% confidence interval (CI) 0.26 to 0.80, moderate‐quality evidence). See Analysis 1.1 and Figure 6. The NNTB is 46; that is, one hernia recurrence was prevented for every 46 mesh repairs performed rather than non‐mesh repairs.
1.1. Analysis.
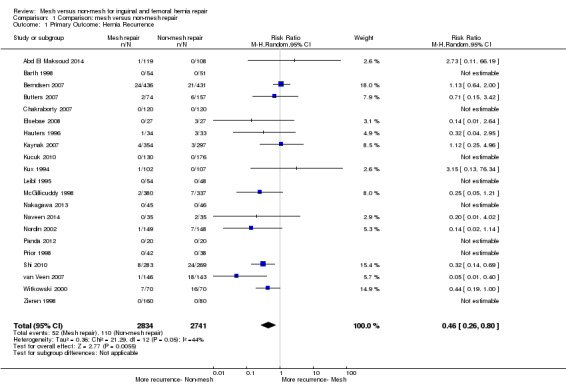
Comparison 1 Comparison: mesh versus non‐mesh repair, Outcome 1 Primary Outcome: Hernia Recurrence.
6.
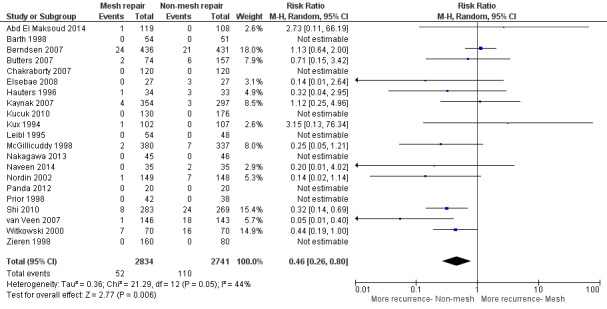
Forest plot of comparison: 1 Comparison: Mesh vs Non‐Mesh repair, outcome: 1.1 Primary Outcome: Hernia Recurrence.
Using the GRADE working group grades of evidence (Schünemann 2011), we downgraded our assessment of the quality of the evidence by one level, to moderate quality, due to inconsistency (moderate heterogeneity); these results are outlined in the Table 1. Included studies did not provide adequate data to enable a subgroup analysis of femoral and inguinal hernias (or did not include femoral hernias).
Sensitivity analysis
We conducted a sensitivity analysis to assess the impact of risk of bias on the overall outcome; this did not significantly change the overall estimate of effect (RR 0.54, 95% CI 0.32 to 0.93). After the studies demonstrating high risk of bias were removed, we deemed the quality of the evidence according to GRADE to have increased from moderate to high due to a reduction in heterogeneity (I2 = 39%). On further investigation we noted that one study, Berndsen 2007, contributed largely to the heterogeneity for this outcome; once this study was removed, the I2 reduced to 7%, although the RR remained consistent (RR 0.38, 95% CI 0.24 to 0.60).
Surgical complications
Twenty‐four studies reported complications as a study outcome (Abd El Maksoud 2014; Barth 1998; Berndsen 2007; Butters 2007; Chakraborty 2007; Elsebae 2008; Hauters 1996; Kaynak 2007; Kucuk 2010; Kux 1994; Leibl 1995; Lermite 2012; Manyilirah 2012; McGillicuddy 1998; Nakagawa 2013; Naveen 2014; Nordin 2002; Panda 2012; Prior 1998; Schmitz 1997; Shi 2010; van Veen 2007; Witkowski 2000; Zieren 1998). Serious operative complications were reported by all papers to be rare. Some of these included deep wound infection, neurovisceral damage and testicular damage/atrophy. Two studies (Lermite 2012; Prior 1998) did not specify which group sustained some specific listed complications, so part of their data was not included as it could not be assigned to a group. Paraesthesia, if assessed as a stand‐alone outcome, was included in the neurovascular injury group. Only one study, Kucuk 2010, addressed mesh rejection as an outcome (reported three events of rejection in the mesh group), and no subgroup analysis could be conducted as there was no available comparison. An overall analysis of complications was not performed in favour of subgroup analysis due to the risk of overlapping totals. Please refer to Figure 7, Analysis 1.2, Table 3 and Table 4 for details of analysis.
7.
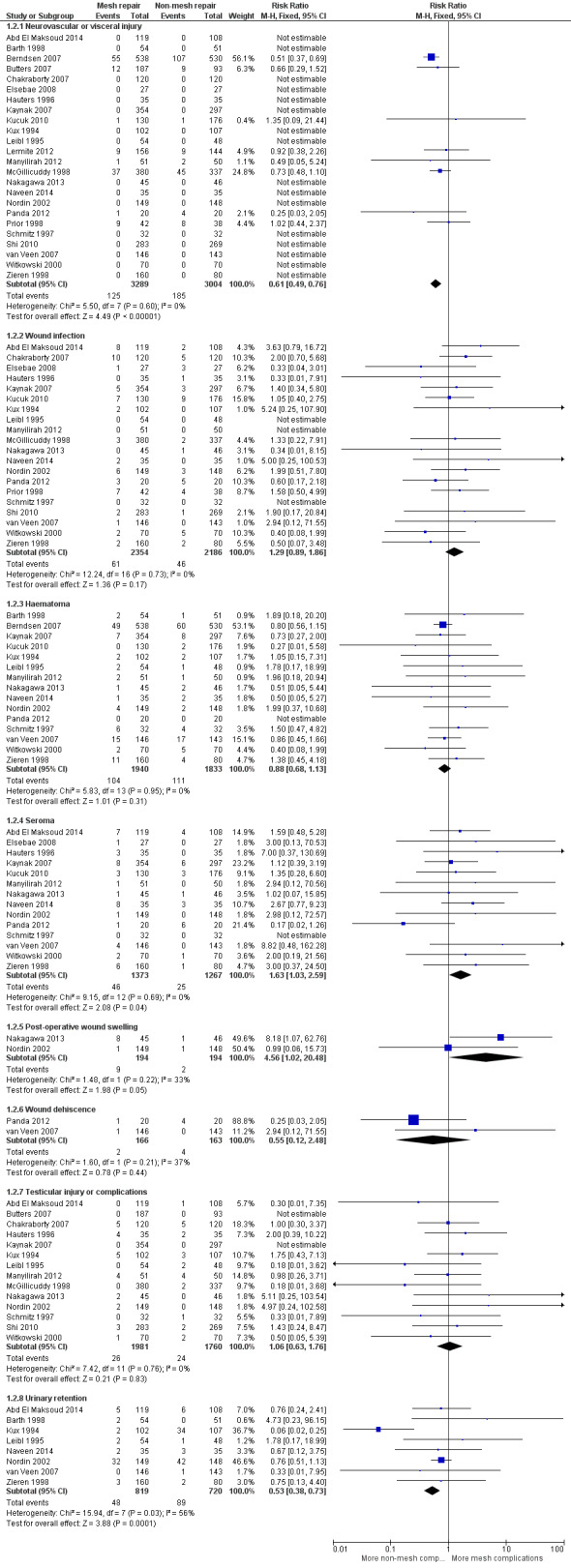
Forest plot of comparison: 1 Comparison 1: Mesh vs Non‐Mesh repair, outcome: 1.2 Primary Outcome: Complications.
1.2. Analysis.
Comparison 1 Comparison: mesh versus non‐mesh repair, Outcome 2 Primary Outcome: Complications.
2. Overview of complications reported in primary studies.
| Study | Group (Total number) | Neurovascular injury (including paraesthesia) or visceral injury | Wound infection | Haematoma | Seroma | Postoperative wound swelling | Wound dehiscence | Testicular injury or complications | Urinary retention | Total complications |
| Abd El Maksoud 2014 | Mesh (119) | Not reported | 8 | Not reported | 7 | Not reported | Not reported | 0 | 5 | 20 |
| Non‐mesh (108) | Not reported | 2 | Not reported | 4 | Not reported | Not reported | 1 | 6 | 13 | |
| Barth 1998 | Mesh (54) | Not reported | Not reported | 2 | Not reported | Not reported | Not reported | Not reported | 2 | 4 |
| Non‐mesh (51) | Not reported | Not reported | 1 | Not reported | Not reported | Not reported | Not reported | 0 | 1 | |
| Berndsen 2007 | Mesh (538) | 55 | Not reported | 49 | Not reported | Not reported | Not reported | Not reported | Not reported | 104 |
| Non‐mesh (530) | 107 | Not reported | 60 | Not reported | Not reported | Not reported | Not reported | Not reported | 167 | |
| Butters 2007 | Mesh (187) | 12 | Not reported | Not reported | Not reported | Not reported | Not reported | 0 | Not reported | 12 |
| Non‐mesh (93) | 9 | Not reported | Not reported | Not reported | Not reported | Not reported | 0 | Not reported | 9 | |
| Chakraborty 2007 | Mesh (120) | 0 | 10 | Not reported | Not reported | Not reported | Not reported | 5 | Not reported | 15 |
| Non‐mesh (120) | 0 | 5 | Not reported | Not reported | Not reported | Not reported | 5 | Not reported | 10 | |
| Elsebae 2008 | Mesh (27) | Not reported | 1 | Not reported | 1 | Not reported | Not reported | Not reported | Not reported | 2 |
| Non‐mesh (27) | Not reported | 3 | Not reported | 0 | Not reported | Not reported | Not reported | Not reported | 3 | |
| Hauters 1996 | Mesh (35) | Not reported | 0 | Not reported | 3 | Not reported | Not reported | 4 | Not reported | 7 |
| Non‐mesh (35) | Not reported | 1 | Not reported | 0 | Not reported | Not reported | 2 | Not reported | 3 | |
| Kaynak 2007 | Mesh (354) | Not reported | 5 | 7 | 8 | Not reported | Not reported | 0 | Not reported | 20 |
| Non‐mesh (297) | Not reported | 3 | 8 | 6 | Not reported | Not reported | 0 | Not reported | 17 | |
| Kucuk 2010 | Mesh (130) | 1 | 7 | 0 | 3 | Not reported | Not reported | Not reported | Not reported | 11 |
| Non‐mesh (176) | 1 | 9 | 2 | 3 | Not reported | Not reported | Not reported | Not reported | 15 | |
| Kux 1994 | Mesh (102) | Not reported | 2 | 2 | Not reported | Not reported | Not reported | 5 | 2 | 11 |
| Non‐mesh (107) | Not reported | 0 | 2 | Not reported | Not reported | Not reported | 3 | 34 | 39 | |
| Leibl 1995 | Mesh (54) | Not reported | 0 | 2 | Not reported | Not reported | Not reported | 0 | 2 | 4 |
| Non‐mesh (48) | Not reported | 0 | 1 | Not reported | Not reported | Not reported | 2 | 1 | 4 | |
| Lermite 2012 | Mesh (156) | 9 | Group not specified | Group not specified | Group not specified | Not reported | Not reported | Not reported | Group not specified | 9 |
| Non‐mesh (144) | 9 | Group not specified | Group not specified | Group not specified | Not reported | Not reported | Not reported | Group not specified | 9 | |
| Manyilirah 2012 | Mesh (51) | 1 | 0 | 2 | 1 | Not reported | Not reported | 4 | Not reported | 8 |
| Non‐mesh (50) | 2 | 0 | 1 | 0 | Not reported | Not reported | 4 | Not reported | 7 | |
| McGillicuddy 1998 | Mesh (380) | 37 | 3 | Not reported | Not reported | Not reported | Not reported | 0 | Not reported | 40 |
| Non‐mesh (337) | 45 | 2 | Not reported | Not reported | Not reported | Not reported | 2 | Not reported | 47 | |
| Nakagawa 2013 | Mesh (45) | Not reported | 0 | 1 | 1 | 8 | Not reported | 2 | Not reported | 12 |
| Non‐mesh (46) | Not reported | 1 | 2 | 1 | 1 | Not reported | 0 | Not reported | 5 | |
| Naveen 2014 | Mesh (35) | Not reported | 2 | 1 | 8 | Not reported | Not reported | Not reported | 2 | 13 |
| Non‐mesh (35) | Not reported | 0 | 2 | 3 | Not reported | Not reported | Not reported | 3 | 8 | |
| Nordin 2002 | Mesh (149) | Not reported | 6 | 4 | 1 | 1 | Not reported | 2 | 32 | 46 |
| Non‐mesh (148) | Not reported | 3 | 2 | 0 | 1 | Not reported | 0 | 42 | 48 | |
| Panda 2012 | Mesh (20) | 1 | 3 | 0 | 1 | Not reported | 0 | Not reported | Not reported | 5 |
| Non‐mesh (20) | 4 | 5 | 0 | 6 | Not reported | 0 | Not reported | Not reported | 15 | |
| Prior 1998 | Mesh (42) | 9 | 7 | Group not specified | Group not specified | Not reported | Not reported | Not reported | Not reported | 16 |
| Non‐mesh (38) | 8 | 4 | Group not specified | Group not specified | Not reported | Not reported | Not reported | Not reported | 12 | |
| Schmitz 1997 | Mesh (32) | Not reported | 0 | 6 | 0 | Not reported | Not reported | 0 | Not reported | 6 |
| Non‐mesh (32) | Not reported | 0 | 4 | 0 | Not reported | Not reported | 1 | Not reported | 5 | |
| Shi 2010 | Mesh (283) | 2 | Not reported | Not reported | Not reported | Not reported | Not reported | 3 | Not reported | 5 |
| Non‐mesh (269) | 1 | Not reported | Not reported | Not reported | Not reported | Not reported | 2 | Not reported | 3 | |
| van Veen 2007 | Mesh (146) | Not reported | 1 | 15 | 4 | Not reported | 1 | Not reported | 0 | 21 |
| Non‐mesh (143) | Not reported | 0 | 17 | 0 | Not reported | 0 | Not reported | 1 | 18 | |
| Witkowski 2000 | Mesh (70) | Not reported | 2 | 2 | 2 | Not reported | Not reported | 1 | Not reported | 7 |
| Non‐mesh (70) | Not reported | 5 | 5 | 1 | Not reported | Not reported | 2 | Not reported | 13 | |
| Zieren 1998 | Mesh (160) | Not reported | 2 | 11 | 6 | Not reported | Not reported | Not reported | 3 | 22 |
| Non‐mesh (80) | Not reported | 2 | 4 | 1 | Not reported | Not reported | Not reported | 2 | 9 |
3. Mesh compared to non‐mesh repair for inguinal and femoral hernia repair, complications subgroups.
| Mesh compared to non‐mesh repair for inguinal and femoral hernia repair; complications subgroups | ||||||
| Patient or population: adults undergoing inguinal and femoral hernia repair Setting: multiple hospitals from small to large tertiary centres contributed results Intervention: mesh Comparison: non‐mesh repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐mesh repair | risk with mesh | |||||
| Complications ‐ neurovascular or visceral injury | Study population | RR 0.61 (0.49 to 0.76) | 6293 (24 RCTs) | ⊕⊕⊕⊕ HIGH | Follow‐up: up to 4.3 years | |
| 6 per 100 | 4 per 100 (3 to 5) | |||||
| Complications ‐ wound infection | Study population | RR 1.29 (0.89 to 1.86) | 4540 (21 RCTs) | ⊕⊕⊝⊝ LOW 1 | Follow‐up: up to 5 years | |
| 2 per 100 | 3 per 100 (2 to 4) | |||||
| Complications ‐ haematoma | Study population | RR 0.88 (0.68 to 1.13) | 3773 (16 RCTs) | ⊕⊕⊝⊝ LOW 1 | Follow‐up: up to 5 years | |
| 6 per 100 | 5 per 100 (4 to 7) | |||||
| Complications ‐ seroma | Study population | RR 1.63 (1.03 to 2.59) | 2640 (14 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | Follow‐up: up to 4 years | |
| 2 per 100 | 3 per 100 (2 to 5) | |||||
| Complications ‐ postoperative wound swelling | Study population | RR 4.56 (1.02 to 20.48) | 388 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 2 | Follow‐up: up to 5 years | |
| 1 per 100 | 5 per 100 (1 to 21) | |||||
| Complications ‐ wound dehiscence | Study population | RR 0.55 (0.12 to 2.48) | 329 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 | Follow‐up: up to 3 years | |
| 2 per 100 | 1 per 100 (0 to 6) | |||||
| Complications ‐ testicular injury or complications | Study population | RR 1.06 (0.63 to 1.76) | 3741 (14 RCTs) | ⊕⊕⊝⊝ LOW 1 | Follow‐up: up to 4 years | |
| 1 per 100 | 1 per 100 (1 to 2) | |||||
| Complications ‐ urinary retention | Study population | RR 0.53 (0.38 to 0.73) | 1539 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 3 | The degree of heterogeneity may be related to differing definitions or measurement of urinary retention Follow‐up: up to 18 months |
|
| 12 per 100 | 7 per 100 (5 to 9) | |||||
| Complications ‐ pain | No clear conclusion could be reached regarding post‐operative and chronic pain in mesh compared to non‐mesh hernia repair, as the studies used different methods and grading scores to determine severity of pain, as well as many different time intervals chosen for analysis. | ‐ | 4999 (22 RCTs) | ⊕⊝⊝⊝ VERY LOW 4 | No meaningful meta‐analysis was able to be performed due to inconsistent methods/lack of comparable endpoints. Follow‐up: up to 5 years |
|
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels for imprecision (wide confidence interval overlapping no effect) and inconsistency (substantial heterogeneity)
2 Downgraded one level for imprecision (wide confidence interval, relatively small population)
3 Downgraded one level for inconsistency (substantial heterogeneity)
4 Downgraded three levels for risk of bias (subjective nature of outcome and various methods of measurement), inconsistency, imprecision and indirectness (various measures, including indirectly with analgesia use)
Neurovascular or visceral injury
There was high‐quality evidence showing that neurovascular and visceral injuries were more common in the non‐mesh repair group (RR 0.61, 95% CI 0.49 to 0.76, I2 = 0%, NNTB = 22). This was assessed in 24 of 25 studies, representing an event rate of 185/3004, compared to 125/3289 in the mesh group.
Wound infection
Wound infection was found more commonly in the mesh group, (in 20 of 25 studies) with an event rate of 61/2354 (compared to 46/2186 in the non‐mesh group), with unconvincing effect size (RR 1.29, 95% CI 0.89 to 1.86, I2 = 0%, NNTB = 200, low‐quality evidence).
Haematoma
Haematomas were found more often in the non‐mesh group (111/1833) than the mesh group (104/1940), in 15 of 25 studies (RR 0.88, 95% CI 0.68 to 1.13, I2 =0%, NNTB = 143, low‐quality evidence).
Seroma
The mesh repair groups demonstrated a higher risk of seroma in 14 of 25 studies, with an event rate of 46/1373 compared to 25/1267 events in the non‐mesh group (RR 1.63, 95% CI 1.03 to 2.59, I2 = 0%, NNTB = 72, moderate‐quality evidence).
Postoperative wound swelling
The mesh repair groups demonstrated a higher risk of postoperative wound swelling: 9/194 events compared to 2/194 events in the non‐mesh group (2 of 25 studies) (RR 4.56, 95% CI 1.02 to 20.48, I2 = 33% , NNTB = 72, moderate‐quality evidence).
Wound dehiscence
Wound dehiscence events were found more often in the non‐mesh group (4/163 compared to 2/166, 2 of 25 studies), but this difference had a wide confidence interval with an unconvincing effect size (RR 0.55, 95% CI 0.12 to 2.48, I2 = 37%, NNTB = 77, low‐quality evidence).
Testicular injury or complications, including testicular swelling and atrophy
Testicular complications showed nearly equivocal results in the 14 of 25 studies that contributed data. However, a slight, non‐statistically significant increase was evident in the mesh group, with an event rate of 26/1981 (compared to 24/1760 in the non‐mesh group) (RR 1.06, CI 0.63 to 1.76, I2 = 0%, NNTB = 2000, low‐quality evidence).
Urinary retention postoperatively
Non‐mesh groups had more postoperative urinary retention (8 of 25 studies): 89/720 events compared to 48/819 events in the mesh group (RR 0.53, 95% CI 0.38 to 0.73, I2 = 56%, NNTB = 16, moderate‐quality evidence).
Postoperative pain, including acute and chronic pain
Regarding postoperative and chronic pain, 12 studies used the visual analogue scale to measure this outcome (Abd El Maksoud 2014; Butters 2007; Berndsen 2007; Shi 2010; Lermite 2012; Manyilirah 2012; Nakagawa 2013; Nordin 2002; Prieto‐Diaz‐Chavez 2009; Prior 1998; Witkowski 2000; Zieren 1998). Four studies quantified pain as a dichotomous outcome (reporting pain as present or not present) (Kucuk 2010; McGillicuddy 1998; Panda 2012; van Veen 2007); others used a numerical rating scale and one study measured pain indirectly through analgesia requirement (Barth 1998; Chakraborty 2007; Naveen 2014). Due to the many different methods of data collection and different time frames for analysis, we were unable to adequately compare these results for meta‐analysis (GRADE rating = very low quality), however, generally the studies indicated that postoperative pain is greater in the groups with non‐mesh repairs.
Sensitivity analysis
Sensitivity analysis of included studies did not demonstrate any significant changes in overall effect for most complications. Notably, none of the studies with high risk of bias contributed to neurovascular/visceral injury analysis (Analysis 1.2.1), so there was no change in estimate. However for both the postoperative wound swelling and wound dehiscence outcomes, we removed one of two studies; this resulted in RR 0.99, 95% CI 0.06 to 15.73 and RR 0.25, 95% CI 0.03 to 2.05, respectively, and a resulting drop in the quality of evidence to very low, according to GRADE criteria. When checking for heterogeneity‐inducing studies in the urinary retention outcome, we noted that removal of one study, Kux 1994 reduced heterogeneity (I2 dropped from 56% to 0%), however the overall result still supported the finding of fewer events in the mesh group (RR 0.79, 95% CI 0.56 to 1.13).
Mortality
Seven studies outlined and discussed mortality as an outcome (Berndsen 2007; Butters 2007; Elsebae 2008; Lermite 2012; McGillicuddy 1998; Nordin 2002; Prior 1998), but none of them reported any deaths. Mortality was not discussed in the remaining 18 included studies.
Secondary outcomes
Duration of surgical operation
Twenty studies measured duration of surgical operation (Abd El Maksoud 2014; Barth 1998; Berndsen 2007; Chakraborty 2007; Schmitz 1997; Leibl 1995; Elsebae 2008; Hauters 1996; Kaynak 2007; Kucuk 2010; Lermite 2012; Manyilirah 2012; Nakagawa 2013; Naveen 2014; Nordin 2002; Panda 2012; Prieto‐Diaz‐Chavez 2009; Prior 1998; Witkowski 2000; Zieren 1998). Of these, three did not provide enough data in their publications to calculate standard deviation (Chakraborty 2007; Nordin 2002; Prior 1998). We were therefore unable to include these studies in the meta‐analysis. Overall, longer durations were noted in the non‐mesh group, with surgery taking a mean of four minutes and 22 seconds longer (with a CI ranging between ‐6.85 to ‐1.6; where negative numerals indicate non‐mesh repair duration being longer). Please refer to Figure 8 for further details. However, there was a large amount of intrinsic heterogeneity between studies, as may be expected (I2 = 97%), so we applied a random‐effects model, and overall this represented very low‐quality evidence. Sensitivity analysis demonstrated no significant difference to the overall effect and heterogeneity remained very high; the mean duration was 3.58 minutes lower in the mesh group, with a larger range, and the GRADE rating remained low.
8.
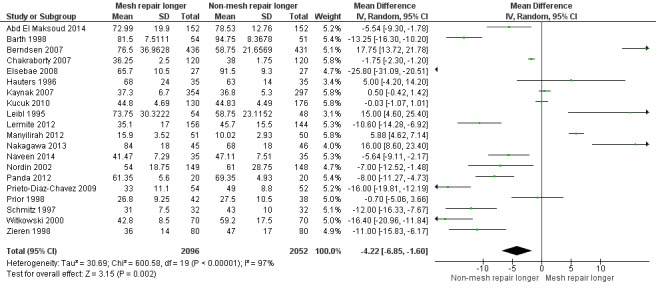
Forest plot of comparison: 1 Outcomes, outcome: 1.10 Duration of surgery.
Duration of postoperative hospital stay
Sixteen studies addressed postoperative stay in hospital as an outcome (Abd El Maksoud 2014; Chakraborty 2007; Elsebae 2008; Kaynak 2007; Kux 1994; Lermite 2012; Nakagawa 2013; Naveen 2014; Nordin 2002; Panda 2012; Prieto‐Diaz‐Chavez 2009; Prior 1998; Shi 2010; van Veen 2007; Witkowski 2000; Zieren 1998). However, four of these studies could not be included in the meta‐analysis, due to inadequate data in which the standard deviations were not reported, and otherwise could not be estimated (Chakraborty 2007; Kux 1994; Nakagawa 2013; Prior 1998).
As there was a large degree of heterogeneity between the studies (I2 = 98%), we used a random‐effects model was for analysis. Additionally, the use of a random‐effects model was seen to be more appropriate; under the fixed‐effect model the result was heavily skewed by a single study with a weighting of 99.4%, whereas under the random‐effects model this study had a weighting of 11.6% (Prieto‐Diaz‐Chavez 2009). The result was statistically significant (P < 0.00001), demonstrating mesh repair as the intervention associated with a shorter postoperative stay by 0.6 days (95% CI ‐0.86 to ‐0.34), though the quality of the evidence according to GRADE was low. Sensitivity analysis did not make a significant difference to the overall effect (the mean difference was 1.04 days sooner, 95% CI ‐1.80 to ‐0.28; and the GRADE rating remained low), with a high degree of heterogeneity remaining (I2 = 98%).
Time to return to activities of daily living
Thirteen studies addressed the time to return to activities of daily living (ADLs) as an outcome (Abd El Maksoud 2014; Barth 1998; Berndsen 2007; Chakraborty 2007; Kaynak 2007; Kux 1994; Leibl 1995; Lermite 2012; Manyilirah 2012; Nordin 2002; Prieto‐Diaz‐Chavez 2009; van Veen 2007; Zieren 1998). However, three studies were not included in the forest plot analysis, due to insufficient data (Chakraborty 2007; Kux 1994; Leibl 1995). The meta‐analysis revealed a mean difference of 2.87 days sooner to return to ADLs in the mesh group (95% CI ‐4.42 to ‐1.32); the quality of the evidence was low. We used a random‐effects model due to there being high heterogeneity (I2 = 96%) and a wide range (2 to 26 days admission). Sensitivity analysis did not make any significant difference to the overall effect (the mean time to return to ADLs was 2.86 days sooner in mesh group, and the evidence was of moderate quality).
Conversion from laparoscopic to open surgery
None of the included studies reported any conversion from laparoscopic to open technique. Only five of the included studies compared a laparoscopic technique (laparoscopic transabdominal preperitoneal (TAPP) repairs) (Berndsen 2007; Butters 2007; Hauters 1996; Leibl 1995; Zieren 1998). All other studies compared an open mesh and non‐mesh technique of hernia repair.
Conversions from non‐mesh to mesh technique were documented in two studies, either due to technical difficulty or because it was deemed necessary for best possible repair by the surgeon (Nordin 2002; van Veen 2007). One of these studies also reported seven conversions from mesh to non‐mesh repair (van Veen 2007). One study confirmed that all participants received their allocated interventions (Manyilirah 2012), but the rest of the studies reviewed did not discuss operation conversions at all.
Subgroup analysis
Hernia type
All included studies specified inguinal hernias; only two studies reported including femoral hernias in their analysis (Prieto‐Diaz‐Chavez 2009; Witkowski 2000). In each, the number of femoral hernias in the mesh group was proportionately half the number in the non‐mesh group (5.4% compared to 10.3% of total participants in Prieto‐Diaz‐Chavez 2009, and 5.5% compared to 11% in Witkowski 2000). The outcomes were discussed with all types of hernia included in each group as a whole, therefore inadequate data were accessible to perform subgroup analysis on inguinal compared to femoral hernias.
Nineteen studies included both direct and indirect hernias in each hernia repair group (Abd El Maksoud 2014; Barth 1998; Berndsen 2007; Chakraborty 2007; Elsebae 2008; Hauters 1996; Kaynak 2007; Kucuk 2010; Leibl 1995; Manyilirah 2012; Nakagawa 2013; Naveen 2014; Nordin 2002; Prior 1998; Schmitz 1997; Shi 2010; van Veen 2007; Witkowski 2000; Zieren 1998). Seventeen of these trials provided ratios in each group (Barth 1998; Chakraborty 2007; Elsebae 2008; Hauters 1996; Kaynak 2007; Kucuk 2010; Leibl 1995; Manyilirah 2012; Nakagawa 2013; Naveen 2014; Nordin 2002; Prior 1998; Schmitz 1997; Shi 2010; van Veen 2007; Witkowski 2000; Zieren 1998). No comment was made in six studies whether hernias were found to be direct or indirect at the time of repair (Butters 2007; Kux 1994; Lermite 2012; McGillicuddy 1998; Panda 2012; Prieto‐Diaz‐Chavez 2009). As results were never reported separately for direct and indirect hernias, we were unable to perform subgroup analysis.
Gender
Eight studies included only male participants, either by inclusion criteria or incidentally (Abd El Maksoud 2014; Berndsen 2007; Butters 2007; Chakraborty 2007; Elsebae 2008; Lermite 2012; McGillicuddy 1998; Nordin 2002). Those that included both genders found women to be the minority in each group, without exception (Hauters 1996; Kaynak 2007; Kucuk 2010; Leibl 1995; Manyilirah 2012; Nakagawa 2013; Prieto‐Diaz‐Chavez 2009; Schmitz 1997; Shi 2010; Witkowski 2000; Zieren 1998). Two studies included both male and female participants but no further detail was provided (Naveen 2014; Panda 2012) and four studies did not report or discuss gender (Barth 1998; Kux 1994; Prior 1998; van Veen 2007). Again, because only general statements were made for each group and not linked to specific outcome results, we could not perform the planned subgroup analysis.
Comparison of comorbidities
American Society of Anaesthesiology (ASA) physical status classification of perioperative participants at baseline was reported (or reported to be measured without providing detail) in five studies (Berndsen 2007; Elsebae 2008; Lermite 2012; Prior 1998; Witkowski 2000).
Thirteen studies reported body mass index (BMI) or participant weight (Abd El Maksoud 2014; Berndsen 2007; Butters 2007; Hauters 1996; Kux 1994; Leibl 1995; Lermite 2012; Manyilirah 2012; McGillicuddy 1998; Nakagawa 2013; Prieto‐Diaz‐Chavez 2009; van Veen 2007; Zieren 1998). Of these, nine studies specifically compared median or mean BMI (Abd El Maksoud 2014; Butters 2007; Hauters 1996; Lermite 2012; Manyilirah 2012; Nakagawa 2013; Prieto‐Diaz‐Chavez 2009; van Veen 2007; Zieren 1998), which was found to be comparable between groups in each study.
Emergent and elective repairs
Only one study clearly selected participants presenting with strangulated inguinal hernias (Elsebae 2008). Another, Panda 2012, included participants with 'obstructed' inguinal hernias, although the clinical circumstances were not discussed in detail and it was deemed to be unclear regarding emergent or elective repair status, so a subgroup analysis could not be performed. All other studies examined hernia repair in an elective setting.
Discussion
Summary of main results
We included 25 studies in this systematic review, with a total of 6293 participants in a diversity of settings in high and low‐income countries. Mesh intervention provided a statistically significant reduction in hernia recurrence, but reliability of this finding was downgraded to moderate quality of evidence due to lack of blinding. This is in accordance with the findings of the EU Hernia Trialists (EU Hernia Trialists 2002). The moderate heterogeneity detected in the analysis of hernia recurrence was attributed, in part, to the variety of mesh versus non‐mesh techniques and open versus laparoscopic techniques used across the selected studies. It may also have been affected by the temporal differences in follow‐up. Additionally, the diversity in operator experience, procedures and anaesthetic management would have contributed to this heterogeneity. Neurovascular and visceral injuries and postoperative urinary retention were less likely in the mesh group. Non‐mesh interventions were found to have a lower rate of seroma and postoperative wound swelling, indicative of a more significant local reaction to the prosthesis. They were found to have slightly lower rates of wound infection and haematoma. Wound infection particularly has been raised as a concern of potentially having an association with mesh repairs over non‐mesh repairs, although the evidence in the literature has remained controversial (Falagas 2005). A number of large studies have shown similar rates of wound infection in both groups (Falagas 2005), as we have found in this review. There may be a number of factors contributing to infection risk, including possible differences in technique or location of repair. Non‐mesh repairs had a slightly higher rate of testicular complications (including a range of severity from postoperative scrotal swelling to testicular atrophy) and wound dehiscence. All of these postoperative complications had a moderate to high GRADE of evidence. Nonetheless, apart from neurovascular and visceral injury and urinary retention, most had quite wide confidence intervals and poor effect size, leading us to conclude that these differences are of minimal clinical significance.
No clear conclusion was reached regarding postoperative and chronic pain in mesh compared to non‐mesh inguinal hernia repair, although most studies favoured mesh repair as having less associated postoperative pain. As pain is a symptom with often multi‐faceted pathophysiology and affected by many personal factors, it is difficult to measure objectively, and a number of different scales and methods of assessment were used in these studies (e.g. pain at rest/with movement, at different intervals postoperatively, with grading scales, with/without the use of analgesia and quantifying pain by analysing analgesic requirement). Moreover, several studies in this review failed to include the number of participants who reported significant pain but instead, provided an average score of pain at certain points in time postoperatively. Multiple studies utilised the Visual Analogue Scale (VAS) (a psychometric response scale used in questionnaires as a measurement instrument for subjective characteristics or attitudes that cannot be directly measured). Even with a common gauge for pain, it was difficult to interpret the data; different authors used different values to denote “significant pain”; whereas some studies identify this to be 200 mm (Berndsen 2007), a more recent study by (Boonstra 2014) recognises that mild pain is represented by values 0.1 cm to 3.8 cm, moderate pain by 3.9 cm to 5.7 cm, and severe pain 5.8 cm to 10 cm. We downgraded our GRADE assessment of the quality of the evidence for this outcome by three levels (very low quality) due to inconsistency and variability in scoring/measurement methods.
No analysis was performed regarding surgery‐related deaths as no events were reported in any studies.
Mesh repairs had a shorter operative time however we downgraded our GRADE assessment by three levels, to very low quality, due to imprecision and a considerable amount of heterogeneity for this outcome. It should be noted that different methods of mesh and non‐mesh surgeries may have contributed to this heterogeneity, including some studies using laparoscopic methods in one or both groups (specifically described in Berndsen 2007; Leibl 1995; Hauters 1996; Nakagawa 2013; Zieren 1998), compared to others who used only open surgical methods. Among these, one study specified only open techniques based on expected optimal management in the setting of emergent surgery for incarcerated hernias (specifically, Elsebae 2008), but generally incarcerated/strangulated hernias met the exclusion criteria for most studies analysed in this review. As laparoscopic methods become more widespread, this may impact on the duration of surgical hernia repairs. Different surgeons had different average operating times; skill and experience of the surgeon will impact largely on the outcome, as well as his/her familiarity with the techniques used. Postoperative hospital stay was reduced in the mesh group by only 0.6 days (moderate GRADE). An additional half‐day of hospital stay is unlikely to be clinically significant, and this result would be very system‐ and clinician‐dependent. Between the studies, the difference in length of stay ranged from 0 (van Veen 2007) to 3.1 days (Shi 2010). And of the 12 studies included in our meta‐analysis of this outcome (Abd El Maksoud 2014; Elsebae 2008; Kaynak 2007; Lermite 2012; Naveen 2014; Nordin 2002; Panda 2012; Prieto‐Diaz‐Chavez 2009; Shi 2010; van Veen 2007; Witkowski 2000; Zieren 1998), only three (Abd El Maksoud 2014; Elsebae 2008; Nordin 2002) found that participants in the mesh repair group had a longer postoperative stay. The source of the significant heterogeneity may be in part attributed to the differences in the patient populations between the studies, i.e. comorbidities, selection of emergent or elective hernia repairs and also differences in postoperative management and hospital policy. A similar high degree of heterogeneity was evident in time to return to ADLs (resulting in downgrading to moderate quality evidence), returning to daily activities a mean of 2.87 days earlier in the mesh repair group.
Overall completeness and applicability of evidence
We conducted a comprehensive electronic search of several databases (see Search methods for identification of studies for details) to obtain the included studies. Completed and ongoing trials were searched for in relevant clinical trials registers such as the World Health Organization International Clinical Trials Registry Platform and ClinicalTrials.gov for completeness. We also reviewed reference lists of included trials and review articles, books related to surgical hernia repairs, abstracts from general surgical conferences concerning hernias and mesh repair. We imposed no restrictions on publication or language, in order to minimise the risk of missing relevant studies. Studies from 15 different countries were gathered (United States, United Kingdom, Uganda, Turkey, The Netherlands, Sweden, Poland, Mexico, Lebanon, Japan, India, Germany, France, Egypt and China), representing different regions and different socioeconomic settings. The commonality of hernias worldwide (Brooks 2014b) was reflected in this review by including robust multi‐centre studies with large cohort sizes from different countries; the consistency of the results of our meta‐analysis, which supports the mainstream use of mesh repairs, are therefore relevant to all patients requiring surgical hernia repair. In saying that, the specific surgical techniques used and emphasis on cost were often informed by the location of the study and economic status. Overall, this is unlikely to have an impact on the applicability of the results, although it should be noted that routine open repairs in a non‐emergent setting may no longer be relevant in many high‐income countries, just as in the resource‐poor setting mesh repair may not always be feasible. The most common mesh repair used was Lichtenstein repair (Abd El Maksoud 2014; Barth 1998; Butters 2007; Chakraborty 2007; Elsebae 2008; Kaynak 2007; Kucuk 2010; Kux 1994; Manyilirah 2012; McGillicuddy 1998; Naveen 2014; Nordin 2002; Panda 2012; Prior 1998; van Veen 2007), whilst the most common non‐mesh repair was the Shouldice technique (Barth 1998; Berndsen 2007; Butters 2007; Hauters 1996; Kux 1994; Leibl 1995; Lermite 2012; McGillicuddy 1998; Nordin 2002; Prieto‐Diaz‐Chavez 2009; Schmitz 1997; van Veen 2007; Zieren 1998), which is consistent with the commonly performed repair techniques outlined in Description of the intervention) and reflective of practice.
Cost of surgery and hospital admission
Of the included studies, six included cost‐effectiveness as an outcome or directly commented on the comparative cost (Barth 1998; Chakraborty 2007; Kaynak 2007; Prieto‐Diaz‐Chavez 2009; Shi 2010; Zieren 1998). A number of others, mainly originating in low‐income countries, included a comment on cost as part of an introductory or discussion statement, generally stating that non‐mesh repairs are more cost‐effective (mainly due to cost of mesh materials) (Abd El Maksoud 2014; Manyilirah 2012; Naveen 2014). Due to the scope of this review and the different currencies used to calculate cost and limited information regarding what was included in cost analysis, we did not perform a meta‐analysis. If only material costs were considered, mesh repairs were more expensive than non‐mesh repairs. One study reported the difference in surgical material cost was INR 250 to 350 for the non‐mesh repairs compared to INR 1900 to 2100 for mesh repairs (Chakraborty 2007). Another reported the average material cost of mesh repair was CNY 6221.3 compared to CNY 4518 (Shi 2010). Another reported material costs that were significantly higher for the laparoscopic mesh procedure (USD 1211) compared to the open mesh (USD 124) and open non‐mesh (USD 69) procedures (Zieren 1998). A further study did not provide details but stated that the cost of the non‐mesh repair was 'significantly less' than that of mesh repair (Kaynak 2007). However, if other factors and costs were considered, mesh repairs may be more cost‐effective. For example, in Barth 1998, it was reported that a mesh was USD 46; but with the longer duration of surgery in a non‐mesh repair (while there was no additional cost for a mesh), the extra operative time on average cost USD 180. One study provided a detailed analysis of cost of mesh compared with non‐mesh repair and concluded that mesh repair overall costs were on average USD 837.66 compared to non‐mesh costs of USD 885.15 (Prieto‐Diaz‐Chavez 2009). In this analysis they considered a number of factors, including operative time, hospital stay, other healthcare costs and disability‐adjusted life years, as well as the material costs of the surgery.
Cost is a clinically significant factor, particularly in low‐income countries, where this plays a large role in access to healthcare. However, cheaper materials, such as mosquito mesh, have been proposed as an affordable alternative in theses areas. If effective, this may nullify the concerns regarding cost for mesh repair. So far results have been promising; a recent study describes low‐cost meshes worth less than USD 1 (compared to USD 125) commercial meshes in Uganda, with very comparable clinical outcomes and complication rates (Lofgren 2016).
Quality of the evidence
Overall, the methodological quality of the included studies was good, considering the nature of the intervention measured. The number of studies contributed a large number of participants to the meta‐analysis and most studies demonstrated clear methods and addressed potential biases, providing results applicable to an international audience where mesh and non‐mesh methods of hernia repair are available.
Whilst the studies demonstrated a good methodological approach, we downgraded our assessment of the quality of evidence contributing to many outcomes (hernia recurrence, wound infection, haematoma, seroma, wound dehiscence, wound swelling, urinary retention, duration of postoperative stay, time to return to ADLs), due to there being wide confidence intervals, heterogeneity and inconsistency. There were no outliers that appeared to significantly skew results. Wound swelling and dehiscence were sparsely reported, with only two studies assessing these outcomes (Nakagawa 2013 and Nordin 2002 for wound swelling, and Panda 2012 and van Veen 2007 for wound dehiscence). In general, the studies addressed their proposed outcomes well. Due to the nature of the intervention, blinding is difficult to achieve; as a result the method/attempt for blinding was not described or was unclear in most studies, with the exception of six studies which at least confirmed single, if not double, blinding (Manyilirah 2012; Nakagawa 2013; Nordin 2002; Panda 2012; Prior 1998; van Veen 2007).
For the outcome neurovascular/visceral injury, we deemed the evidence to be of high quality. For all other complications apart from pain, we deemed the evidence to be of moderate quality. The evidence on postoperative pain was judged to be of very low quality, largely due to inconsistency of measurement methods and interval of follow‐up, and therefore incomparability. Duration of surgery demonstrated significant heterogeneity of results, with an overall finding that non‐mesh repairs take longer than mesh by about four minutes; this heterogeneity is likely to be due to variation in operative experience and skill between surgeons. We accounted for this statistical heterogeneity by using a random‐effects model and downgrading the evidence a further level. Overall sensitivity analysis did not make any significant difference to estimate effects.
Please refer to Table 1 and Table 4 for further detail regarding quality of evidence.
Potential biases in the review process
Throughout the review process, at a number of stages the review authors undertook tasks independently to reduce the risk of potential bias. Following an initial search of the electronic databases, numerous studies and their respective abstracts were reviewed by three study authors independently to include/exclude them, based on the protocol criteria. Similarly the data extraction and assessment of the risk of bias was undertaken independently by two of the study authors, where differences were resolved through discussion. However, several of the studies that were included in the review were in foreign languages, which led to deviation from our initial methodology. Due to costs of translating the full article to English, the data extraction and assessment of bias was not performed by one of the review authors but rather by a translator employed by Cochrane. To avoid bias relating to heterogeneity, we used a random‐effects model when heterogeneity was found to be moderate or greater.
Agreements and disagreements with other studies or reviews
Through our review of other literature relating to the topic of mesh versus non‐mesh repair of hernias, we noted a fairly consistent opinion that mesh repair should be preferential to other non‐mesh techniques.
The European Hernia Society (EHS) guidelines provide a grade A recommendation that all adult male patients with inguinal hernia should be operated on using a mesh technique, either with open Lichtenstein or endoscopic inguinal hernia techniques (Simons 2009). Similarly the Danish Surgical Society, informed by the Daniesh Hernia database, states that in any male patient with a groin hernia a mesh repair should be undertaken, with no recommendation for non‐mesh Shouldice, Bassini or McVay techniques (Rosenberg 2011).
The EHS justify their recommendations based on Grade IA evidence of reduced hernia recurrence with mesh repair (EU Hernia Trialists Collaboration 2000; EU Hernia Trialists Collaboration 2002). In the previous version of our Cochrane Review, performed in 2001, the reduced risk of recurrence with mesh was measured to be 50% to 75% (Scott 2001). Also, a more recent multi‐centre trial reports a low cumulative recurrence rate within five years, with totally extraperitoneal (TEP) and Lichtenstein techniques ranging from 1.2% to 3.5% across the treatment groups (Eklund 2009). Subsequently, considering the outcome of hernia recurrence, it appears well‐accepted that a mesh technique is superior to that of non‐mesh; this is equally reflected in our latest analyses (risk ratio 0.46).
When considering the other outcomes of complications or postoperative pain, the advantage of a mesh repair is equivocal. When directly comparing mesh to non‐mesh techniques, the EHS measured the incidence of complications to be similar between treatment groups (EU Hernia Trialists Collaboration 2000; Simons 2009). However, with endoscopic repairs as a subgroup of the mesh group, the EHS has demonstrated with Grade IIB evidence that endoscopic repairs have been associated with higher rates of rare but serious complications such as visceral and vascular injury (Simons 2009). Amongst the included studies of this review, while the recorded rate of complications was 4% to 5% between mesh and non‐mesh — which is comparable — the burden of complications was distributed differently, with greater risk of seroma in the mesh group and more neurovascular or visceral injury in non‐mesh group. Furthermore, the EHS guidelines showed with Grade IIB evidence that mesh repair reduces the risk of chronic pain (Simons 2009); however, in our review we could not find any statistically significant difference between mesh and non‐mesh with regards to postoperative or chronic pain.
Authors' conclusions
Implications for practice.
This review found that mesh inguinal hernia repairs may be associated with better outcomes than non‐mesh repairs in the elective setting (including for outcomes of recurrence, surgical complications, duration of surgery, postoperative stay, time to return to activities of daily living), however non‐mesh repairs remain a viable alternative depending on health service circumstances. The average effect on operative time, hospital stay and return to daily activities is uncertain due to wide variation between the results of the studies. Risk of bias in the included studies was low to moderate and generally handled well by study authors, with attention given to details of allocation, blinding, attrition and reporting.
The additional cost of a mesh in surgical repair (whilst possibly nullified by the shorter operative times and associated costs in the high‐income country) remains a significant and sometimes prohibitive cost in low‐income countries. There was not enough information to provide a conclusion regarding postoperative pain and mortality outcomes.
Implications for research.
A number of gaps exist in the evidence available to date. As demonstrated in the analysis above, there were no statistically significant differences between mesh and non‐mesh repairs for complications including haematoma, wound dehiscence, wound infection, and testicular complications. Additional studies examining these outcomes would help differentiate the two interventions in this respect.
Assessment of postoperative pain was inconsistent amongst included studies and made meaningful analysis impossible. This was primarily due to the plethora of pain assessment techniques used in clinical practice worldwide. Selection of an international standard for pain assessment postoperatively for hernia repair would eliminate this barrier to analysis.
Whilst the included studies were largely sound in their methodology, the various inconsistencies and sources of bias outlined above limited the quality of the subsequent analysis. The inability to blind all investigators — specifically the surgical team — creates a consistent, significant source of bias in these studies. Due to the nature of the intervention, double‐blinding is an unrealistic expectation; however some modifications could be made to attempt to safeguard the blinding process, including having blinded assessors for postoperative reviews and ensuring that all participants at least are blinded to the intervention. Follow‐up time, randomisation techniques, outcome reporting, and complication reporting are all sources of inconsistency between trials. Future studies should focus on rigorous methodological implementation, and extensive reporting of outcomes and complications to improve the quality and comparability of studies. Additionally, studies are inevitably inconsistent when it comes to quantification of qualitative outcomes and assessment of their clinical significance. Identification of the most suitable quantification technique by comparison to previous studies is recommended.
This review does not have the scope to analyse cost reliably, as an economic modelling review would, and costs of the different hernia repairs — including further investigation into cheaper materials — would be useful.
What's new
| Date | Event | Description |
|---|---|---|
| 23 August 2018 | Amended | New searches performed and inserted in this amended and updated review, now complying with the MECIR standards. |
Acknowledgements
This review has been published previously in the Cochrane Library (Scott 2001). We would like to acknowledge the work of the previous authors: Scott N, Go PM, Graham P, McCormack K, Ross S, Grant A.
Claudia Bollig (Cochrane Deutschland) and Amélie Yavchitz (Cochrane France) assisted by providing German and French translation of studies to enable data extraction.
Henning Keinke Andersen from Cochrane Colorectal Cancer Group editorial office and editors provided support, guidance and evaluation of this review.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Hernia, Inguinal] explode all trees #2 MeSH descriptor: [Hernia, Femoral] explode all trees #3 ((Inguina* or femoral or groin*) and herni*):kiik,ab,kw #4 (#1 or #2 or #3) #5 MeSH descriptor: [Laparoscopy] explode all trees #6 MeSH descriptor: [Herniorrhaphy] explode all trees #7 MeSH descriptor: [Sutures] explode all trees #8 ((laparascop* or open or hernia*) and (repair or surg* or intervent* or operat* or approach* or technique*)):ti,ab,kw #9 (herniorrhaph* or hernioplast* or sutur* or tension* or Lichtenstein* or transabdominal preperitoneal or TAPP or totally extraperitoneal or TEP or Bassini* or McVay* or Shouldice* or Desarda* or Guarnieri* or Lockwood* or Lotheissen* or McEvedy*):ti,ab,kw #10 (#5 or #6 or #7 or #8 or #9) #11 MeSH descriptor: [Surgical Mesh] explode all trees #12 (mesh* or plug*):ti,ab,kw #13 (#11 or #12) #14 (#4 and #10 and #13)
Appendix 2. Ovid MEDLINE search strategy
1. exp Hernia, Inguinal/ 2. exp Hernia, Femoral/ 3. ((Inguina* or femoral or groin*) and herni*).mp. 4. 1 or 2 or 3 5. exp Laparoscopy/ 6. exp Herniorrhaphy/ 7. exp Sutures/ 8. ((laparascop* or open or hernia*) and (repair or surg* or intervent* or operat* or approach* or technique*)).mp. 9. (herniorrhaph* or hernioplast* or sutur* or tension* or Lichtenstein* or transabdominal preperitoneal or TAPP or totally extraperitoneal or TEP or Bassini* or McVay* or Shouldice* or Desarda* or Guarnieri* or Lockwood* or Lotheissen* or McEvedy*).mp. 10. 5 or 6 or 7 or 8 or 9 11. exp Surgical Mesh/ 12. (mesh* or plug*).mp. 13. 11 or 12 14. 4 and 10 and 13 15. randomized controlled trial.pt. 16. controlled clinical trial.pt. 17. randomized.ab. 18. placebo.ab. 19. clinical trial.sh. 20. randomly.ab. 21. trial.ti. 22. 15 or 16 or 17 or 18 or 19 or 20 or 21 23. humans.sh. 24. 22 and 23 25. 14 and 24
Appendix 3. Ovid Embase search strategy
1. exp inguinal hernia/ 2. exp femoral hernia/ 3. ((Inguina* or femoral or groin*) and herni*).mp. 4. 1 or 2 or 3 5. exp laparoscopy/ 6. exp herniorrhaphy/ 7. exp suture/ 8. ((laparascop* or open or hernia*) and (repair or surg* or intervent* or operat* or approach* or technique*)).mp. 9. (herniorrhaph* or hernioplast* or sutur* or tension* or Lichtenstein* or transabdominal preperitoneal or TAPP or totally extraperitoneal or TEP or Bassini* or McVay* or Shouldice* or Desarda* or Guarnieri* or Lockwood* or Lotheissen* or McEvedy*).mp. 10. 5 or 6 or 7 or 8 or 9 11. exp surgical mesh/ 12. (mesh* or plug*).mp 13. 11 or 12 14. 4 and 10 and 13 15. CROSSOVER PROCEDURE.sh. 16. DOUBLE‐BLIND PROCEDURE.sh. 17. SINGLE‐BLIND PROCEDURE.sh. 18. (crossover* or cross over*).ti,ab. 19. placebo*.ti,ab. 20. (doubl* adj blind*).ti,ab. 21. allocat*.ti,ab. 22. trial.ti. 23. RANDOMIZED CONTROLLED TRIAL.sh. 24. random*.ti,ab. 25. 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26. 14 and 25
Appendix 4. Science Citation Index‐Expanded/Conference Proceedings Citation Index‐Science
#1 TI=(((Inguina* or femoral or groin*) and herni*)) #2 TOPIC: (((laparascop* or open or hernia*) and (repair or surg* or intervent* or operat* or approach* or technique*))) #3 TOPIC: ((herniorrhaph* or hernioplast* or sutur* or tension* or Lichtenstein* or transabdominal preperitoneal or TAPP or totally extraperitoneal or TEP or Bassini* or McVay* or Shouldice* or Desarda* or Guarnieri* or Lockwood* or Lotheissen* or McEvedy*)) #4 (#3 OR #2) #5 TI=((mesh* or plug*)) #6 (#5 AND #4 AND #1) #7 TOPIC: (((controlled trial or controlled clinical trial or placebo or clinical trial or random* or trial or cct or rct))) #8 (#7 AND #6)
Appendix 5. Criteria for judging risk of bias using the Cochrane 'Risk of bias' assessment tool
|
RANDOM SEQUENCE GENERATION Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence. | |
| Criteria for a judgement of ‘Low risk’ of bias. | The investigators describe a random component in the sequence generation process such as: · referring to a random number table; · using a computer random number generator; · coin tossing; · shuffling cards or envelopes; · throwing dice; · drawing of lots; · minimisation*. *Minimisation may be implemented without a random element, and this is considered to be equivalent to being random. |
| Criteria for the judgement of ‘High risk’ of bias. | The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, for example: · sequence generated by odd or even date of birth; · sequence generated by some rule based on date (or day) of admission; · sequence generated by some rule based on hospital or clinic record number. · other non‐random approaches happen much less frequently than the systematic approaches mentioned above and tend to be obvious. They usually involve judgement or some method of non‐random categorisation of participants, for example: · allocation by judgement of the clinician; · allocation by preference of the participant; · allocation based on the results of a laboratory test or a series of tests; · allocation by availability of the intervention. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
|
ALLOCATION CONCEALMENT Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: · central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); · sequentially numbered drug containers of identical appearance; · sequentially numbered, opaque, sealed envelopes. |
| Criteria for the judgement of ‘High risk’ of bias. | Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on: · using an open random allocation schedule (e.g. a list of random numbers); · assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); · alternation or rotation; · date of birth; · case record number; · any other explicitly unconcealed procedure. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed. |
|
BLINDING OF PARTICIPANTS AND PERSONNEL Performance bias due to knowledge of the allocated interventions by participants and personnel during the study. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following: · no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; · blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following: · no blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; · blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following: · insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; · the study did not address this outcome. |
|
BLINDING OF OUTCOME ASSESSMENT Detection bias due to knowledge of the allocated interventions by outcome assessors. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following: · no blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; · blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following: · no blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; · blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following: · insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; · the study did not address this outcome. |
|
INCOMPLETE OUTCOME DATA Attrition bias due to amount, nature or handling of incomplete outcome data. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any one of the following: · no missing outcome data; · reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); · missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; · for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; · for continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; · missing data have been imputed using appropriate methods. |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following: · reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; · for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; · for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; · ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; · potentially inappropriate application of simple imputation. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Any one of the following: · insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number randomised not stated, no reasons for missing data provided); · the study did not address this outcome. |
|
SELECTIVE REPORTING Reporting bias due to selective outcome reporting. | |
| Criteria for a judgement of ‘Low risk’ of bias. | Any of the following: · the study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; · the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| Criteria for the judgement of ‘High risk’ of bias. | Any one of the following: · not all of the study’s pre‐specified primary outcomes have been reported; · one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. sub‐scales) that were not pre‐specified; · one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); · one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; · the study report fails to include results for a key outcome that would be expected to have been reported for such a study. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. It is likely that the majority of studies will fall into this category. |
|
OTHER BIAS Bias due to problems not covered elsewhere in the table. | |
| Criteria for a judgement of ‘Low risk’ of bias. | The study appears to be free of other sources of bias. |
| Criteria for the judgement of ‘High risk’ of bias. | There is at least one important risk of bias. For example, the study: · had a potential source of bias related to the specific study design used; or · has been claimed to have been fraudulent; or · had some other problem. |
| Criteria for the judgement of ‘Unclear risk’ of bias. | There may be a risk of bias, but there is either: · insufficient information to assess whether an important risk of bias exists; or · insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Comparison: mesh versus non‐mesh repair.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary Outcome: Hernia Recurrence | 21 | 5575 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.26, 0.80] |
| 2 Primary Outcome: Complications | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Neurovascular or visceral injury | 24 | 6293 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.49, 0.76] |
| 2.2 Wound infection | 20 | 4540 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.89, 1.86] |
| 2.3 Haematoma | 15 | 3773 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.68, 1.13] |
| 2.4 Seroma | 14 | 2640 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.03, 2.59] |
| 2.5 Post‐operative wound swelling | 2 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.56 [1.02, 20.48] |
| 2.6 Wound dehiscence | 2 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.12, 2.48] |
| 2.7 Testicular injury or complications | 14 | 3741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.63, 1.76] |
| 2.8 Urinary retention | 8 | 1539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.38, 0.73] |
| 3 Primary outcome: Mortality, 30 days post‐operation | 7 | 2546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Duration of surgical operation | 20 | 4148 | Mean Difference (IV, Random, 95% CI) | ‐4.22 [‐6.85, ‐1.60] |
| 5 Duration of Postoperative Stay | 12 | 2966 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐0.86, ‐0.34] |
| 6 Time to return to full ADLs | 10 | 3183 | Mean Difference (IV, Random, 95% CI) | ‐2.87 [‐4.42, ‐1.32] |
1.3. Analysis.
Comparison 1 Comparison: mesh versus non‐mesh repair, Outcome 3 Primary outcome: Mortality, 30 days post‐operation.
1.4. Analysis.
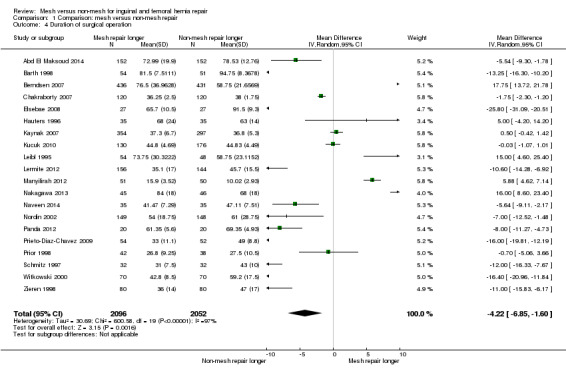
Comparison 1 Comparison: mesh versus non‐mesh repair, Outcome 4 Duration of surgical operation.
1.5. Analysis.
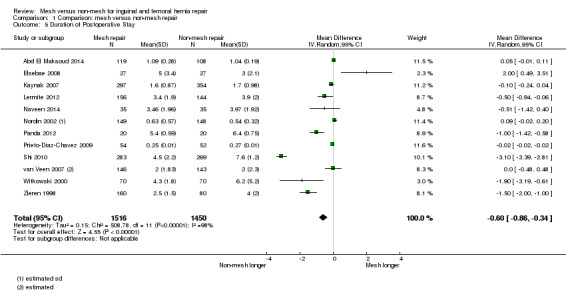
Comparison 1 Comparison: mesh versus non‐mesh repair, Outcome 5 Duration of Postoperative Stay.
1.6. Analysis.
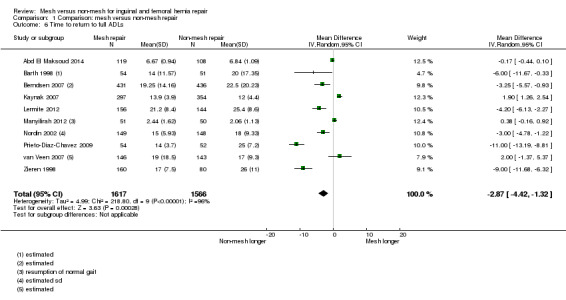
Comparison 1 Comparison: mesh versus non‐mesh repair, Outcome 6 Time to return to full ADLs.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abd El Maksoud 2014.
| Methods | Two hundred and twenty‐seven male participants were randomly allocated into two groups: group I included 108 participants (mean age 37.47 ± 11.97 years) who had a Modified Darn Repair (MDR) and group II that included 119 participants (mean age 37.44 ± 11.93 years) who were subjected to Lichtenstein Procedure (LP). The participants were followed up at 3 months (group I had 92/108, group II had 103/119), 6 months (group I had 88/108, group II had 90/119) and 12 months (group I had 79/108, group II had 82/119) | |
| Participants | Males with primary inguinal hernia (direct or indirect) who were admitted to General Surgery Department, Alexandria Main University Hospital (Egypt) for February 2008 till October 2009. Eligibility criteria included male participants between 18 and 60 years with primary inguinal hernia classified as Gilbert III or IV. | |
| Interventions | For participants with direct inguinal hernia, plication of the fascia transversalis was done first using 2‐O absorbable polyglycolic acid. For participants with indirect inguinal hernia, after excision of the sac and closure of the layers of the spermatic cord, the deep inguinal ring was narrowed by two medial sutures using 2‐O monofilament polypropylene. Modified darn repair The procedure started by taking the first stitch on the pubic tubercle using O polypropylene. The thread was passed through the inguinal ligament and the internal oblique muscle fascia medially (whitish fibres at the insertion of the arching fibres of internal oblique) and the internal oblique fibres laterally in a continuous tension‐free manner. Suturing was proceeded from medial to lateral in a crisscross pattern with 1 cm interval between each suture. On reaching the cord at the deep ring, care was taken to pass the sutures around the cord with no pressure, and then, the sutures are ligated 2–3 cm lateral to the deep ring. Security sutures (2‐O monofilament polypropylene) were taken in a continuous manner starting at the pubic tubercle and passing through the edges of the repair at the inguinal ligament and the internal oblique muscle fascia and through the loops of the previous sutures. Lichtenstein procedure The procedure was done as described by Kingsnorth et al. using a 6 x 11 cm polypropylene mesh. A dose of ceftriaxone (1 g intravenous) was given to all participants just before induction of anaesthesia. |
|
| Outcomes |
Primary end points
Secondary end points
Data were collected and analysed using SPSS 17.0. Student’s t test was used for independent samples to compare chronic postoperative pain (modified visual analogue scale) at 6, 24h, 3, 6, 12 month at rest and 24h, 3, 6, 12 month with movement between the groups. The primary analysis was based on the comparison after 6, 24h, 3, 6, 12 month. The significance level of the t test was set to 0.025 (Bonferroni correction) to keep the overall significance level of 0.05. |
|
| Notes |
Results Recurrence was encountered in only one case after LP. Visual Analogue Scale showed significant more early and late postoperative pain after LP compared to MDR. The operative time for LP (72.99 ± 19.90 min) was significantly shorter compared to MDR (78.53 ± 12.76 min). Both MDR and LP showed no significant differences as regards hospital stay (1.04 ± 0.19 days vs. 1.09 ± 0.28 days), time to return to domestic activity (1.18 ± 0.43 days vs. 1.15 ± 0.36 days), time to return to work activity (6.84 ± 1.09 vs. 6.67 ± 0.94 days), early and late postoperative complications. Conclusions After 1‐year follow‐up, MDR as a tension‐free repair seems comparable to LP with less postoperative pain. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Regardless the hernia was direct or indirect, participants were randomly assigned to two groups. A total number of 240 envelopes were divided into 120 modified darn repair and 120 Lichtenstein Procedure. Envelopes were completely sealed and shuffled. An operative nurse with no clinical involvement in the trial and who was blinded to the procedure chose one envelope just before the surgery and informed the surgeon which procedure was to be done. All participants who were randomised were operated on. |
| Allocation concealment (selection bias) | Low risk | The operative nurse who selected the envelopes in the randomisation process was blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The only blinding was of the operative nurse who selected the envelopes in the randomisation process. Surgeons were not blinded, but were unaware of which procedure would be performed until directly prior to the surgery. It was unclear if the participants were completely blinded or not. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No attempt at blinding was made. Follow‐up was performed by the surgeon in their outpatient clinic. Operative time was measured by the operative nurse at the time of operation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants were followed up after 3, 6 and 12 months. After 12 months, 79 participants in group I and 82 participants in group II presented for follow‐up. Reasons for attrition were not reported beyond 'lost contact'. |
| Selective reporting (reporting bias) | Low risk | All outcomes were fairly reported and discussed in all participants that were able to be followed‐up. |
| Other bias | Low risk | No other significant biases were noted. |
Barth 1998.
| Methods | One hundred and five (105) adult participants were randomised to undergo either a mesh or Shouldice inguinal hernia repair. Five (5) staff surgeons from Hitchcock Medical Center and one (1) staff surgeon from Cheshire Medical Center performed the procedures. The intention was to perform all herniorrhaphies on an outpatient basis; any compelling reasons for admission were documented before operation. A preoperative questionnaire documented employment status and labor intensity. One gram of intravenous cefazolin was used for infection prophylaxis. After operation all participants were given a prescription for 30 tablets of the oral narcotic Percocet (5 mg oxycodone plus 325 mg acetaminophen). If a patient had a previous adverse reaction to Percocet, an analgesic equivalent, Tylenol 3 (30 mg codeine plus 325 mg acetaminophen), was prescribed. participants were asked not take nonsteroidal antiinflammatory drugs. participants were given verbal and written instructions stating that it was permissible to perform any physical activities that comfort allowed. At discharge all participants were given a diary form on which they made daily entries for the number of narcotics taken, activity level, and date of return to work. Pain levels while at rest and walking were also recorded daily on a pain scale that ranged from 0 to 5 (0 = none, 1 = very mild, 2 = mild, 3 = moderate, 4 = severe, and 5 = very severe). Participants were scheduled for a short‐term follow‐up visit 7 days after the operation. At this visit the wound was examined and participants climbed up and down a flight of stairs and recorded their pain with this activity, according to the same pain scale of 0 to 5. Also at this visit, participants were evaluated with two Dartmouth COOP (Cooperative functional assessment) charts. Each chart contained a question on physical fitness or daily activity level, followed by five response categories; each category was linked to a drawing representing that health state. Previous work shows that this simple outcome measure is equivalent to much longer health‐status questionnaires and is a valid assessment tool in the context of clinical trials. Participants were also examined 4 weeks after the operation. |
|
| Participants | Participants 18 years of age or older who were seen by one of these surgeons on an elective basis with a primary inguinal hernia were eligible for this study. Participants with bilateral inguinal hernias were eligible if the repairs were staged. Participants currently taking or previously addicted to narcotics were excluded. | |
| Interventions |
Shouldice repair This was performed as described by Glassow et al, except that 2‐0 Prolene (Ethicon, Inc.) sutures were used instead of steel wires. Lichtenstein mesh repair This was performed as described by Lichtenstein et al., except that 2‐0 Prolene was used instead of 3‐0. The skin was closed with a subcuticular suture. All repairs were performed with either local anaesthetic plus intravenous sedation or a general anaesthetic with a postoperative block, according to the patient's preference. For repairs performed with a local anaesthetic plus intravenous sedation, the patient was given 10 mL of a 1:1 mixture of 1% lidocaine and 0.5% bupivacaine to block the ilioinguinal nerve at the level of the anterior superior iliac spine and 10 mL at the site of the incision to anaesthetize the skin. Additional local anaesthetic was given as needed. Midazolam was used in 1 mg increments for sedation, and fentanyl was used in 25 to 50 microgram increments for analgesia. For repairs performed with a general anaesthetic, thiopental or propofol was used for induction, and vecuronium or succinylcholine was used as a muscle relaxant. Isoflurane, enflurane, or nitrous oxide was used for maintenance. Fentanyl was permitted in a dose of up to 3 micrograms per kilogram. At the end of the procedure, 20 ml 0.5% bupivacaine was injected along the incision and adjacent to the ilioinguinal nerve. The volume of intravenous fluid administered was limited to a maximum of 1 L for all cases. |
|
| Outcomes | Postoperative pain, narcotic use, and time to resumption of usual activities and employment were recorded. Participants were blinded to the type of repair received until all data were collected. | |
| Notes |
Results There was no difference between the herniorrhaphy methods with respect to postoperative pain, duration of narcotic use, and time to resumption of usual activity and employment. Recovery was rapid for both groups of participants. By 3 days after operation, 50% of participants rated their pain as very mild or less and no longer required narcotic analgesics. Participants in both groups returned to usual activity and work by a median of 9 days after operation. Conclusions Both methods can be used to repair inguinal hernias with local anaesthetics in an outpatient setting with minimal morbidity. Despite the "tension‐free" design of the mesh repair; short‐term outcomes of mesh and Shouldice repairs of inguinal hernias do not differ. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed immediately before operation and was stratified according to the staff surgeon, so each staff surgeon would perform an equal number of repairs according to each technique. Exact method for randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | It is unclear if there was any allocation concealment, the randomisation process was poorly reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants were blinded to the type of repair they underwent until the completion of the study (4 weeks after operation). It is not clear if any investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants were blinded for the duration of the study (but were made aware of which procedure they received at the conclusion of the study). Surgeons were not blinded. The study did not report who conducted the follow‐up assessments, consequently it is not clear whether they were blinded to the procedure that they participants had received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. Study adequately accounted for 2 participants randomly assigned to Shouldice repair, but who received mesh repairs. Intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | Two participants, with large direct hernias, were randomised to the Shouldice repair but, in the opinion of the staff surgeon, required mesh for adequate repair of the inguinal floor. These two participants were analysed by intention‐to‐treat in the Shouldice group. All other outcomes were fairly reported and discussed in all participants that were able to be followed‐up. |
| Other bias | Low risk | No other significant biases were noted. |
Berndsen 2007.
| Methods | One‐thousand one‐hundred and eighty‐three participants (1183) were randomised in a multi‐centre trial with the primary aim of comparing recurrence rates after laparoscopic transabdominal preperitoneal patch (TAPP)/mesh repair (586) and Shouldice/suture (597) repair. In the Berndsen 2007 study 431 participants from the Shouldice group and 436 from TAPP/mesh group successfully returned for follow‐up at 5 years. In the Arvidsson 2005 study, 454 participants from the TAPP/mesh group and 466 participants from the Shouldice group were followed‐up at 5 years. The participants were clinically examined after 1 and 5 years, and answered questionnaires 2 and 3 years postoperation. | |
| Participants | Male participants between 30 and 70 years with unilateral primary inguinal hernia were eligible for randomisation. Seven surgical centres in Sweden participated in the study: 2 university hospitals and 5 regional/county hospitals. Participants with a history of multiple abdominal operations or concomitant disease contraindicating general anaesthesia were excluded, as also were participants regarded as unable to participate in postoperative follow‐up because of drug abuse, psychiatric disorders, or language difficulties. | |
| Interventions | The TAPP procedure was used to facilitate a mesh repair, and modified Shouldice procedure was used to facilitate a suture repair. TAPP procedure In the TAPP procedure a peritoneal pocket was created and the hernia sac was either dissected and inverted (direct and short indirect sacs) or divided (long indirect sacs). The Cooper’s ligament and pubic bone were exposed as was the spermatic cord for a distance of at least 4 cm from the internal ring. A 7 cm x 12 cm polypropylene mesh (Prolene; Ethicon, Somerville, NJ, USA) was inserted and fixed by use of an endoscopic stapler (EMS Multifeed Staplegun; Ethicon). Care was taken not to apply staples below the inguinal ligament. Modified Shouldice procedure In the modified Shouldice procedure the cremaster muscle was transected at the internal ring. Indirect sacs were dissected to the internal ring, ligated, and extirpated. The posterior inguinal wall was opened from the internal ring to the pubic tubercle. A three‐layer repair was performed using a running 2‐0 polypropylene suture. A relaxation incision was optional. No prophylactic antibiotics were used in either approaches. |
|
| Outcomes |
In the 2007 study
Measured at 5 years following procedure, calculated as a median time (with the range given) amongst the group In the 2005 study
|
|
| Notes |
In the 2007 study Of 1,068 operated participants, 867 were eligible for analysis after 5 years (81.2%). The percentage of participants experiencing discomfort of any kind were 8.5% in the TAPP group and 11.4% (P = 0.156) in the Shouldice group. Although discomfort was usually mild, it was severe for 0.2% in the TAPP group and 0.7% in the Shouldice group. Severe pain the first postoperative week was a risk factor for late discomfort in the Shouldice group (odds ratio 2.25, P = 0.022) but not in the TAPP group. No other risk factor for late discomfort was found. Overall, there was no difference between late discomfort at five‐year follow‐up after laparoscopic TAPP and Shouldice repair. In the 2005 study Recurrences were evenly distributed between groups throughout the follow‐up period. The cumulative recurrence rate after 5 years was 6.6 per cent in the TAPP group and 6.7 per cent in the Shouldice group. Postoperative pain was a risk factor for recurrence after Shouldice operation but not after TAPP repair. There was a correlation between a low surgeon’s performance score and recurrence. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation and allocation of participants into either TAPP/mesh or Shouldice/suture repair groups, that ensured equal baseline demographics. Noted that out of the original 1183 intended for randomisation, only 1068 proceeded to be operated on; dropouts during randomisation were accounted for and openly disclosed as randomisation error, patient refusal of operation, patient moving to another area of residence, emergency operations that had to be done before the study, and other miscellaneous reasons. |
| Allocation concealment (selection bias) | Low risk | Randomisation and allocation was done so within the confidence of the computer programme and its operator who was impartial towards the participants and had nothing further to do with the study. Allocations were delivered via a confidentially sealed and opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no information in the published journal of the study regarding attempts at blinding either the participants or personnel assessing outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information in the published journal of the study regarding attempts at blinding either the participants or personnel assessing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Majority of participants were successfully able to be followed‐up by the 5th year. In the 2007 study 431 out of the 538 operated on in the TAPP/mesh group, and 436 out of the 530 from the Shouldice/suture group (> 80%). In the 2005 study 454 from TAPP/mesh and 466 successfully returned for follow‐up at 5 years. There is no clear explanation for this discrepancy in data. Generally those that failed to follow‐up were unable to attend the follow‐up appointment; it is nonetheless noted that 20 people died within the 5 years follow‐up period, and it is not noted whether or not this is directly attributable to the procedure. |
| Selective reporting (reporting bias) | Low risk | All outcomes were fairly reported and discussed in all participants that were able to be followed‐up. |
| Other bias | Low risk | Funding noted from Ethicon Endosurgery and Johnson & Johnson company |
Butters 2007.
| Methods | Two‐hundred and eighty (280) men with a primary hernia were randomised prospectively to undergo Shouldice/suture (93), open Lichtenstein/mesh (93), or laparoscopic TAPP/mesh (94) repair. Participants were examined after 12 and 52 months to assess hernia recurrence, nerve damage, testicular atrophy and patient satisfaction. | |
| Participants | Any adult male (defined as greater than the age of 18) that had a primary unilateral inguinal hernia was eligible for this study. | |
| Interventions | The laparoscopic TAPP and open Lichestein procedure uses mesh in their repair, and the Shouldice procedure utilises sutures.
All participants were operated under general anaesthesia and received one prophylactic dose of amoxicillin/clavulanic‐acid preoperatively. |
|
| Outcomes | Follow‐up at 52 months, assessing:
Follow‐up at 12 months, assessing:
|
|
| Notes | This study found that hernia recurrence occurred in six participants after Shouldice suture repair, and in one patient each after Lichtenstein and TAPP mesh repairs at the 52 month endpoint. Furthermore, all recurrences after tension‐free mesh repairs were diagnosed within the first year after surgery. Nerve injuries were significantly more frequent after open Shouldice and Lichtenstein repairs, with greatest patient satisfaction after laparoscopic TAPP repair. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In a time interval of 12 months, 280 male participants with inguinal hernias were selected and randomly assigned to three different groups. However, it is noted that the study authors did not disclose how they achieved randomisation (for example, if it was through computer generation or otherwise). |
| Allocation concealment (selection bias) | Unclear risk | The results of the randomisation were delivered via sealed opaque envelopes to the participants. However, it is not clarified whether or not the study authors were involved in the allocation process or made privy to the participant details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no information in the published journal of the study regarding attempts at blinding either the participants or personnel assessing outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information in the published journal of the study regarding attempts at blinding either the participants or personnel assessing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants from the study were accounted for. A total of 49 participants were lost to follow‐up at the 52 month mark, which included 14 deaths that may not be reasonably attributable to the intervention. |
| Selective reporting (reporting bias) | Low risk | All outcomes were fairly reported and discussed in all participants that were able to be followed‐up. |
| Other bias | Low risk | No other significant biases were noted. |
Chakraborty 2007.
| Methods | Two‐hundred and forty (240) participants were randomised to either receive a 'darn' suture repair (120) or have a 'mesh' inserted (120). Participants were reviewed during their postoperative period (up to 1 month) and at 1, 3, 6, 9, 12, and 18 months upon discharge. | |
| Participants | Any male who had an unilateral inguinal hernia, which were nonobstructive, reducible, and either primary or recurrent. Those who presented with bilateral inguinal hernias, or complications including obstruction, strangulation or significant irreducibility were excluded form this study. | |
| Interventions |
"Darn" group In the "darn" group, participants received a 3‐layered darn repair using 1‐0 prolene sutures over a 2‐0 prolene bed. "Mesh" group In the "mesh" group, participants received a prolene mesh secured with 2‐0 prolene sutures. All participants received general and local anaesthesia, and a prophylactic dose of cefuroxime briefly preoperatively. |
|
| Outcomes |
Peri‐post‐operative follow‐up within 1 month
Follow‐up at 1, 3, 6, 9, 12 and 18 months (note: only total results at the 18 month endpoint published)
|
|
| Notes | In summary, this study concluded that mesh repair is on par (that is, no better or worse) when compared with the traditional darn suture repair; both groups recorded no recurrences to date, and postoperative complications were minimal in both arms. This study was conducted in India, a low‐income nation, and whilst the study authors empathised with the contemporary view that mesh technique is increasing in popularity, it remains costly, especially for those in low‐income nations seeking early treatment; whereby, a well constructed darn suture is an equally effective and less costly treatment option for inguinal hernias according to this study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported the use of a closed‐box system where names where names were randomly drawn out in sealed envelopes; of which there were equal number of envelopes for either groups. An impartial person unrelated to the study was employed to draw the participants out of the box and to ensure that baseline demographics of participants in both groups were equally matched prior to confirming the allocations. |
| Allocation concealment (selection bias) | Low risk | Selected based on standardised inclusion (and exclusion) criteria as described in the participants section above; participants were examined by three experienced impartial clinicians which were unrelated to the study or its authors. Allocations were delivered by a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There was no information in the published journal of the study regarding attempts at blinding either the participants or personnel assessing outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information in the published journal of the study regarding attempts at blinding either the participants or personnel assessing outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is no mention if any participants had failed to follow‐up, nor is there any confirmation that there was a zero attrition rate. The discussion of the results is based on the assumption that no one has dropped out over the 18 months. |
| Selective reporting (reporting bias) | High risk | This study may have an agenda to portray mesh and non‐mesh techniques on par with another due to cost concerns in a low‐income nation. Interestingly, the study has failed to include cost as one of its outcome measures and there are concerns regarding sufficient follow‐up regarding its participants. |
| Other bias | Low risk | No other significant biases were noted. |
Elsebae 2008.
| Methods | In the period from May 2004 to December 2006, 54 participants were submitted to emergency operation because of strangulated inguinal hernia. The participants were randomised into two groups (27 participants in each group) | |
| Participants | 54 participants 18 years of age or older, who were submitted to emergency operation because of strangulated inguinal hernia in the period from May 2004 to December 2006. Participants with a recurrent hernia, participants with preoperative peritonitis, inflammatory hernia and/or associated other hernias or intraabdominal masses or ascites were excluded from the study. | |
| Interventions |
Group A Open tension‐free anterior repair utilising a monofilament polypropylene mesh according to Lichtenstein ‘‘tension‐free’’ technique Group B Bassini technique |
|
| Outcomes | Assessment of the primary outcome included surgical complications and hospital stay and secondary outcome was the recurrence of hernia. | |
| Notes | This study concludes that the use of Lichtenstein ‘‘tension‐free’’ technique in emergency treatment of strangulated inguinal hernia is safe, effective with an acceptably low rate of postoperative complications and without recurrence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | The sequence generation process was described as follows; participants were divided into two groups A and B (27 participants in each group) depending on whether the patient’s registration number in the study, was odd or even respectively. |
| Allocation concealment (selection bias) | Unclear risk | No information in published study regarding method of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in published study regarding attempts at blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information in published study regarding attempts at blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information in published study regarding whether all participants were followed up/attempts of following up incomplete data |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | No other significant biases were noted. |
Hauters 1996.
| Methods | This study compared a transabdominal preperitoneal (mesh) repair and Shouldice repair, with 70 participants randomly allocated to 2 groups. | |
| Participants | 20yrs, primary unilateral hernia without any history of major abdominal surgery | |
| Interventions | Randomisation in 2‐arm mesh or Shouldice repair | |
| Outcomes | Pain, hospital long of stay, morbidity, return to home activities and to work, recurrence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Allocation by envelope without precision of “sealed or opaque envelope” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Not described |
| Other bias | Low risk | Not described |
Kaynak 2007.
| Methods | This study compared two tension‐free techniques of inguinal hernia repair: the Moloney darn repair (MDR) and Lichtenstein mesh hernioplasty (LMH). The subjects of this study were 651 participants from a total 732 who underwent open inguinal herniorrhaphy clinic between January 2000 and January 2006. Physical examinations were done on postoperative days (PODs) 1, 7, and 30, and then every 6 months thereafter. |
|
| Participants | 651 participants who underwent open repair of an inguinal hernia at the 4th General Surgery Department, Goztepe Training and Research Hospital in Turkey between January 2000 and January 2006. | |
| Interventions |
Moloney darn repair (MDR) Two continuous rows of no. 1 mono‐ filament non‐absorbable polypropylene sutures were inserted between the conjoint tendon and the iliopubic tract, with the first row including the fascia transversalis. The darn was created without tension and incorporated muscle tissue and the transversalis fascia. An internal inguinal ring was always made, as defined in the original method. Lichtenstein mesh hernioplasty (LMH) LMH was performed by placing polypropylene mesh on the fascia transversalis between the iliopubic tract and conjoint area. All participants received antibiotics preoperatively, as a single dose of first‐generation cephalosporin. |
|
| Outcomes | Analgesic requirement in the first 24 hours, operative time, hospital stay, early postoperative complications, time until return to work, and recurrence, between participants who underwent MDR (group A) and participants who underwent LMH (group B). | |
| Notes | This study concludes that both MDR and LMH resulted in rapid recovery and low recurrence rates; however, the advantage of the MDR lies in the fact that it does not require mesh, so it is much less expensive. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to one of the two groups in consecutive groups of five. Unclear method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information in published study regarding method of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in published study regarding attempts at blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information in published study regarding attempts at blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | As 31 (4.5%) could not be followed up, only 651 participants were included in this study. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | No other significant biases were noted. |
Kucuk 2010.
| Methods | A herniorrhaphy procedure was performed in a total of 306 participants between January 2003 and December 2008. The duration of operations and complication and recurrent rates were compared between the two groups. The data were collected postoperatively after the 1st week, 1st, 3rd, 6th, and 12th month, and 2nd and 3rd year, or at any time which the participants needed admission due to any problems. |
|
| Participants | 306 participants with inguinal hernia. Participants had inguinal hernia as a primary disease and recurrent hernia and incarcerated hernias were not included. | |
| Interventions |
Moloney darn Darn method was performed by suturing between the inguinal ligament and fascia of the internal oblique muscle fascia by using O monofilament polyprolene suture. The Wrst suture began at the medial site from the pubic tubercle and continued to the site of the internal inguinal ring. After placing the first suture, a second suture was done 1 cm forward and was continued between the inguinal ligament and the internal oblique muscle fascia. The sensory nerves were preserved in all cases with gentle tissue handling, gentle dissection, meticulous haemostasis, and avoidance of extensive thermal injury. Lichtenstein procedure A 7.5 x 15 cm polypropylene mesh in Group II. The mesh was positioned on the inguinal floor between the inguinal ligament and the internal oblique muscle fascia. The meshes were provided by the institution and originated from different companies. |
|
| Outcomes | Early complications: haematoma formation, seroma collection, and wound infection. Late complications: chronic pain, loss of sensation at the operation site, and the rejection of mesh. |
|
| Notes | This study concluded that the darn repair method is simple, safe, and has similar recurrence rates when compared to the Lichtenstein method in inguinal hernia participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study states that participants were randomly chosen. No information in published study regarding method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information in published study regarding method of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in published study regarding attempts at blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information in published study regarding attempts at blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No information in published study regarding whether all participants were followed up/attempts of following up incomplete data |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | No other significant biases were noted. |
Kux 1994.
| Methods | 209 people with primary inguinal hernias were randomly allocated to Shouldice repair (107 people) or Lichtenstein patch (102 people) | |
| Participants |
Inclusion criteria Age: ≥ 60 years, hernia type classification according to Schumpelick: II or III. Exclusion criteria Recurrent inguinal hernia and femoral hernia, in general, were not treated with the Lichtenstein‐patch, only participants with an assumed weak fascia transversalis and an assumed increased risk for recurrence were included. |
|
| Interventions | Shouldice repair or Lichtenstein patch techniques were described. The local anaesthesia was direct infiltration of 1% xylocaine without epinephrine. |
|
| Outcomes | Recurrence, local anaesthetic use/painkillers, disability at work, duration of hospitalisation, pain, postoperative complications, relapse 2.5 year follow‐up |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No further details were mentioned apart from stating that the participants were randomly allocated to one of the two groups. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned, no loss to follow‐up identifiable. |
| Selective reporting (reporting bias) | Low risk | All outcomes discussed |
| Other bias | Low risk | No other risk of biases found |
Leibl 1995.
| Methods | Prospective randomised trial During the period May‐December 1993, 102 participants were allocated according to a randomisation plan to the Shouldice procedure (group A) or the laparoscopic procedure (group B). |
|
| Participants | 102 participants with unilateral primary inguinal hernias Inclusion criteria Unilateral inguinal hernia (not reaching the scrotum). Exclusion criteria Participants after midline lower abdominal incision as a possible obstacle to a laparoscopic lower abdominal incision, participants with an increased risk of operation according to American Society of Anaesthesiology (ASA; > 2) |
|
| Interventions | Both procedures were performed under general anaesthesia (standardised ethrane‐narcosis). Shouldice procedure (48 people) The Shouldice‐reparation was conducted according to a technique reported by Schumpelick. A stat dose of antibiotics was provided for prophylaxis of infections due to implantation of extraneous material. Laparoscopic TAPP procedure (54 people) The laparoscopic intervention was always done in Trendelenberg position for a better exposure of the inguinal region. After siting of the pneumoperitoneum, a 10 mm 30°‐angled optic was inserted via infra‐umbilical access. On both sides of the navel, lateral of the rectus‐edge, a 12 mm‐operation port was inserted each.The operator was placed at the contralateral side, the camera was placed opposite. The preparation was started with a curved peritoneal incision above the hernia localization, which reached from the plica umbilicalis medialis to the spina iliaca. The incised peritoneum was removed from the inguinal region. The indirect hernia sac was always completely isolated from cremaster. After exact anatomic preparation, a 12 x 10 cm polypropylene mesh was inserted subperitoneal with driving under the cremaster, so a tension‐free reconstruction of the inguinal back wall with a complete cover of Hesselbach’s triangle was achieved. The inner inguinal ring was reconstructed and the mesh position was saved with 8‐10 titan clips. Afterwards, the closure of the peritoneal incision was done through a continuous suture. |
|
| Outcomes | Postoperative complications (e.g. hematoma), spontaneous micturition, use of painkillers, mobilisation, convalescence (especially duration of disability of work) and recurrence rate. | |
| Notes | Test of statistical significance was done with the Wilcoxon‐test for unpaired samples and with Chi2 test | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were divided into groups “according to a randomisation plan” |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not directly mentioned; In the discussion section, the authors say that the non‐calculable bias of the subjective pain assessment and the prior‐informed expectations of the participants, especially towards the gentle minimal‐invasive method should not be underestimated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Median follow‐up: 16 months (13‐21): One drop out in the laparoscopic group due to death (myocardial infarct) |
| Selective reporting (reporting bias) | Low risk | Outcomes discussed. |
| Other bias | Low risk | Not described |
Lermite 2012.
| Methods | 300 participants were randomised; 144 assigned to the Shouldice group and 156 to the Mesh Plug group. A prospective, randomised comparator trial in three hospitals was performed between August 2001 and March 2006. | |
| Participants | Male participants (19‐75 years old) with unilateral inguinal hernias. Exclusionary criteria included bilateral and recurrent hernias. All of the participants had to be available for 3 year follow up. | |
| Interventions | Mesh Plug and Shouldice repair | |
| Outcomes | Intensity of post operative pain, quality of life | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind randomisation occurred with regard to surgical technique. Nurse assessed postoperative pain three times a day with a visual analogue scale |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Limitations of the intervention studied prevent good blinding of the outcome assessment, however is less likely given blinding of assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 lost to follow up between post operative day 8 ‐ 45, representing one from each group. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | No other significant biases were noted. |
Manyilirah 2012.
| Methods | A total of 101 participants (51 in the Lichtenstein arm and 50 in the Desarda arm) were enrolled into this single‐centre double‐blind randomised controlled trial. The outcome measures were evaluated at 1–2 hours, 3, 7 and 14 days. | |
| Participants | 101 black African participants aged 18 years and above who presented with a primary, reducible inguinal or inguino‐scrotal hernia and consented to participate were enrolled consecutively into the study. Participants with giant inguino‐scrotal hernias, obstructive uropathy or chronic obstructive pulmonary disease, and impaired mental state were excluded from the study. | |
| Interventions | Non‐mesh (Desarda) and mesh (Lichtenstein) method | |
| Outcomes | The primary outcome variables were: pain score (VAS on scale of 0–10) and time taken to return to normal gait (days). The secondary outcome variables were: operative time (min) and intra‐operative complications. |
|
| Notes | The results of the study showed that the effectiveness of the Desarda technique with respect to influencing the early clinical outcomes of hernia repair is similar to that of the Lichtenstein method. However, the operator in this study showed that the Desarda repair requires significantly shorter operative time. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated simple random sequence, the participants were assigned to either Desarda or Lichtenstein treatment arms. Accuracy of randomisation was analysed using the v2 Contingency Table Test |
| Allocation concealment (selection bias) | Low risk | The allocations were concealed in sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐centre, double‐blind randomised controlled trial (only the outcomes assessor and participants were blinded), carried out at Mulago Hospital, the teaching hospital for Makerere University School of Medicine. Evaluation of effectiveness of blinding was also analysed using percentage agreements. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 101 participants were enrolled into the study. All participants received their allocated intervention. While two participants (3.9%) in the mesh (Lichtenstein) arm were lost to follow‐up, only one participant (2.0%) in the non‐mesh (Desarda) arm did not complete the follow‐up. However, the difference in the loss to follow‐up between the study arms was not statistically significant (v2 = 0.323, P = 0.570) |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | Declared financial support from Makere University and Mulago National Referral and Teaching Hospital |
McGillicuddy 1998.
| Methods | All male participants with inguinal hernias who presented to the Lansing Hernia Center between January 1, 1990, and December 31, 1995 were included in the study. All participants were informed of ongoing study and asked by author to participate. Follow‐up was performed at 1, 4 and 52 weeks. |
|
| Participants | 672 men with inguinal hernias, age 20 to 19 years, seen at the hernia centre | |
| Interventions | Slightly modified Shouldice and Lichtenstein repairs | |
| Outcomes | Recurrence rates, symptoms including patient satisfaction, and infections | |
| Notes | Both types of hernia repair are comparable an effective, but long‐term results favour the Lichtenstein technique for reducing recurrence is, ease of technical mastery, and applications to the outpatient setting by use of a local anaesthetic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated using the coin toss method. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Author reports 99.9% at 1 week, 99.9% at 1 month, 97.9% at 1 year, 82.4% at 2 years, 67.3% at 3 years and 64.5% at 4 years. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | No other significant biases were noted. |
Nakagawa 2013.
| Methods | Ninety‐one (91) adults between the ages of 49 and 80 years were randomised to undergo a Marcy repair (46) or Prolene Hernia System (PHS) (mesh) repair (45). Participants were evaluated and followed up postoperatively, at one week, one month, six months and then every six months up to three years postoperatively. They were assessed for recurrence, pain and infection as well as secondary endpoints including seroma/haematoma formation, testicular symptoms and objective inflammatory markers. | |
| Participants | Adults between the ages of 49 and 80 years, with a finding of a type I‐1 or I‐2 hernia (based on the Japanese Hernia Society Classification) at the time of surgery were eligible for this study. | |
| Interventions | Following initial dissection, the size of the hernia orifice defect was measured to confirm an I‐1 or I‐2 hernia and coexisting type II (direct) hernia was excluded. The participant was then allocated a repair group and one of the following was performed. Marcy repair The hernia sac was ligated after sufficient dissection and then the transverse fascia was sutured to tighten the inguinal ring. PHS repair The preperitoneal cavity was dissected, underlay patch inserted and spread and connector placed in internal inguinal ring. The patch was anchored to firm fascia on the anterior surface of the pubis with two sutures and to transversalis fascia, internal oblique fascia and inguinal ligament with a further six or seven sutures. All participants were given prophylactic antibiotic therapy (ampicillin) and pre‐anaesthetic buprenorphine and atropine. |
|
| Outcomes | The following outcomes were assessed postoperatively, at 1 week, 1 month, 6 months and every 6 months up to 3 years postoperatively.
Additionally, the peripheral white blood count, neutrophil count and levels of C‐reactive protein (CRP) and fibrinogen were assessed pre‐operatively and on days 3 and 7 postoperatively as an objective evaluation of inflammatory response to the surgery. |
|
| Notes | The operative time was significantly longer in PHS repair compared to Marcy repair, but no difference was seen in postoperative use of analgesics. No hernia recurrences occurred in the follow‐up period for either group. About two‐thirds of participants in both groups complained of pain at least once during the follow‐up but no differences were noted between the groups. Infeciton only occurred in one participant who had undergone a Marcy repair. Wound swelling was more common with PHS repair than Marcy repair, and occurred within one week of surgery in both groups. The mean CRP level on postop day 3 was significantly higher in the PHS group than the Marcy group, but had decreased to the same level by postoperative day 7. Two participants in the PHS repair group reported testicular symptoms but this is not expanded upon. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At the time of surgery, participants who met the inclusion criteria and had provided written informed consent pre‐operatively were randomly allocated to a Marcy repair or PHS repair. Randomisation was performed using a computer‐generated random number table and office personnel in the Surgery Outpatient Department at Hiatsuka city were tasked with separating envelopes in the order of enrolment. Any change in the procedure after allocation was made by the attending surgeon if deemed necessary on an intention‐to‐treat basis. |
| Allocation concealment (selection bias) | Low risk | The attending surgeon telephoned the outpatient department from the operating theatre during surgery in order to request that an envelope be opened containing a random group allocation, and confirmed the procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although a true double‐blind study cannot be performed in the setting of surgical intervention, a single‐blinded study was achieved. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No attempt was made to blind the assessors who performed the follow‐up outcome assessment; postoperative follow‐up was performed by the surgeon in charge of the study who knew the allocated surgical procedure. Primary endpoints were assessed by history and physical examination. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were lost to follow‐up, however one patient in the Marcy repair group was subsequently excluded for liver cirrhosis that was recognised postoperatively and one in the PHS group due to a combined hernia being identified after allocation. Intention‐to‐treat analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed, although little detail was provided about testicular complications/symptoms. |
| Other bias | Low risk | No other significant biases were noted. |
Naveen 2014.
| Methods | 70 participants admitted in the surgical wards of Sri Adichunchanagiri Hospital and Research Center, BG Nagara, India, and diagnosed to have an inguinal hernia, were included in the study. They were randomly allocated to undergo a Modified Bassini's repair (MBR) (35) or a Lichtenstein Mesh Repair (LMR) (35). | |
| Participants | Any patient with an inguinal hernia (irrespective of type, direct and indirect) admitted at the time of enrolment was included apart from the following exclusion criteria: congential inguinal hernia, pregnant women, participants with recurrent/bilateral/complicated inguinal hernia and participants with coagulopathy or on anticoagulant therapy. | |
| Interventions | A classical incision was used for all participants; 2.5cm above and parallel to the medial three‐fifths of the inguinal ligament. Then participants underwent either of the following. Modified Bassini's repair Sac was separated from cord structures and dealt with according to the type of hernia, conjoint tendon was sutured onto the inguinal ligament Lichtenstein repair After dissection of the sac, polypropylene mesh was placed on the defect and fixed to the inguinal ligament below and to the conjoint tendon with sutures All participants were given pre‐operative antibiotic prophylaxis with cefotaxime and both groups were only administered spinal anaesthesia with diclofenac for postoperative analgesia. Sutures were removed 7 days postoperatively. |
|
| Outcomes | Participants were evaluated postoperatively for the following outcomes.
Participants were followed up once a month for the first 3 months, once every 3 months thereafter in the first year and 6 months thereafter to a total of 18 months, assessed for recurrence and overall well‐being. |
|
| Notes | Mean duration of surgery in the LMR group was significantly less than the MBR group. Pain on postoperative day 7 was significantly more in the LMR group compare to the MBR group, but no statistically significant difference was noted at day 30. 2.9% of participants undergoing LMR and 5.7% of participants undergoing MBR developed haematomas, and 22.9% of LMR compared to 8.6% of MBR participants developed seromas. 2 participants undergoing LMR repair developed postoperative wound infection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors report that randomisation was performed via computer‐generated sequences from SAS software. |
| Allocation concealment (selection bias) | Unclear risk | No information was offered regarding allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was offered regarding blinding of participants/personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was offered regarding blinding of participants/personnel during outcome follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two participants in each group were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | None identified. |
Nordin 2002.
| Methods | 300 men with primary inguinal hernia were randomised to either a Shouldice repair (148) or a Lichtenstein hernia repair (149). Outcomes were followed up at 8 weeks postoperatively. | |
| Participants | Men between the ages of 25 and 75 years with a clinically manifest unilateral primary inguinal hernia. Exclusion criteria included irreducibility, femoral hernia, coagulopathy/anticoagulant therapy and participants unfit for anaesthesia. | |
| Interventions | In a pretrial training programme, the five participating general surgeons were taught to perform Shouldice and Lichtenstein hernia repairs in a standard manner.
Surgery was performed under regional or general anaesthesia in accordance with the patient's preference or the anaesthetist's opinion, and all participants were injected with local anaesthetic at the end of the operation. Prophylactic antibiotics were not used. |
|
| Outcomes | At 8 weeks postoperatively:
At one year postoperatively, an assessment of recovery and pain was repeated. Participants with persisting discomfort or incomplete recovery were examined clinically. Three years postoperatively, an independent assessor examined the participants with respect to:
|
|
| Notes | The duration of surgery was uniformly less in the Lichtenstein technique compared to the Shouldice method. No statistically significant differences were noted between the groups regarding postoperative complications. Infections were superficial, apart from one patient in the mesh group who developed a deep scrotal infection with resulting testicular atrophy. One further patient, also in the mesh group, developed testicular atrophy following postoperative orchitis. The duration of hospital stay did not differ between the two groups. Two participants in the Shouldice group had persistent moderate pain classified as neuralgia due to radiation along an inguinal nerve. There were no other significant differences in pain between the two groups at any time in follow‐up. At follow‐up between 36 and 77 months, seven recurrent hernias were found in the Shouldice group compared to one in the Lichtenstein group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation was performed in blocks (ten participants per group). |
| Allocation concealment (selection bias) | Low risk | Just prior to the skin incision, one of the consecutively numbered sealed envelopes was opened, data were recorded and transferred onto a database. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded regarding allocation and personnel were blinded until the moments before surgery. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The three‐year follow‐up clinical examination was performed by a surgeon who was not informed about the surgical technique used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation, 3 men were excluded; one being found to have no hernia, and two decided to opt out of the study. Of the remaining 297 participants, 13 were lost to follow‐up by 3 years. During the study four participants died from causes unrelated to their hernia surgery. In three patients, the surgery was converted from a Shouldice to Lichtenstein technique due to being technically difficult. Participants were analysed by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed in participants that were able to be followed‐up. |
| Other bias | Low risk | No other significant biases were noted. |
Panda 2012.
| Methods | Forty (40) adults with obstructed inguinal hernia were prospectively randomised to undergo either a modified Bassini tissue repair or a Lichtenstein repair with polypropylene mesh. Participants were followed‐up over a two year period. | |
| Participants | Adults between the ages of 20 and 65 with obstructed inguinal hernias. Participants with preoperative peritonitis, gut perforation or resection/anastomosis performed or with severe comorbidities were excluded. | |
| Interventions | Participants underwent:
No further information was offered regarding procedure technique. Materials used were listed, including suture type and mesh. All participants were given antibiotic prophylaxis with ceftriaxone. All operations were out‐of‐hours surgery between 8pm and 8am performed by senior registrar‐level surgeons. |
|
| Outcomes | Participants were followed up postoperatively and again at 2 years, assessing the following.
|
|
| Notes | Mean operating time was significantly less in mesh repair compared to tissue repair. More early complications were noted in the tissue repair group, including a significant increase in seroma formation. Average postoperative hospital stay was reduced in the mesh repair group. The two‐year follow‐up showed no recurrence in either group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided apart from an assertion that the participants were randomised |
| Allocation concealment (selection bias) | Unclear risk | No information provided apart from an assertion that the participants were randomised |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Limited information provided apart from an assertion that the study is single‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | It was noted that the surgical team was not aware of the inclusion in the study during operation and follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided about loss‐to‐follow up or intention to treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | None identified. |
Prieto‐Diaz‐Chavez 2009.
| Methods | One hundred and six (106) participants with an inguinal hernia were randomised into an open‐tension (Shouldice) (52) or mesh‐plug tension‐free (MPTF) (54) hernia repair and were followed up at a two‐year interval. | |
| Participants | Participants between the ages of 18 and 65 years scheduled for a unilateral, primary or first‐recurrence inguinal hernia repair procedure at the General Hospital of Colima/Mexican Institute of Social Security, Colima, Mexico. Participants with irreducible hernias, morbid obesity, severe comorbidities, history of substance abuse, pregnancy, bilateral or more than one recurrence of hernias were excluded. | |
| Interventions | Open‐tension (Shouldice) or mesh‐plug tension‐free (MPTF) | |
| Outcomes | Outcomes included the following.
Follow‐up assessment was performed at 7 days, 8 weeks, 12 and 24 months. |
|
| Notes | Surgery time, time to full recovery, pain VAS, use of analgesia, off‐work time and hospital stay were all significantly lower for the MPTF repair. Disability adjusted life years were reduced 56% with the MPTF repair, which represented a total savings of USD 12656.60 with this procedure compared to Shouldice repair. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned numbers according to a computer‐generated random‐number table to receive one of the two techniques. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment technique is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The appropriate hernioplasty group was known at the operating room, and all participating personnel as well as the investigator performing result analysis were blinded to the assigned procedure. The blinding technique is not discussed in any further detail. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Follow‐up was performed by the study surgeon. As a result, we must assume that the assessor was aware of the allocated surgery for each participant. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information was provided regarding participant loss‐to‐follow up or intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | None identified. |
Prior 1998.
| Methods | Eighty (80) participants with an inguinal hernia were randomised to undergo a modified Bassini repair (38) or a Lichtenstein repair (42) with follow‐up postoperatively and at 7 weeks. | |
| Participants | Adults over 18 years of age with an inguinal hernia. Those who presented emergently, or with recurrent herniae were excluded. | |
| Interventions | Participants underwent either:
No further detail was provided regarding surgical technique. Antibiotic prophylaxis was provided to the Lichtenstein group but none given to the Bassini group, in a reflection of common practice. All participants were given opiate analgesia. |
|
| Outcomes | Postoperative follow‐up was performed at a mean of 7 weeks, assessing outcomes including the following.
|
|
| Notes | Duration of surgery was affected significantly by seniority/experience of surgical staff, but there was no statistically significant difference in time between the two groups of hernia repair. Mean hospital stay was similar in both groups. Bassini repair participants experienced significantly more postoperative pain than those in the Lichtenstein group. In general, postoperative complications were similar in both groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Equal numbers of envelopes containing a piece of paper on which was written 'Bassini' or 'Lichtenstein' were created. |
| Allocation concealment (selection bias) | Low risk | Envelopes were selected just prior to the operating session by a person not involved in the trial. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants in the study were blinded. For obvious reasons, the personnel/surgical team delivering care could not be blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The surgeon performing the postoperative assessment was not informed of the treatment group of the participants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 15 participants were lost to follow‐up. No further detail was provided, including whether intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | All outcomes were reported and discussed. |
| Other bias | Low risk | None identified |
Schmitz 1997.
| Methods | Prospective randomised study with 64 participants included, comparing mesh and non‐mesh inguinal hernia repairs | |
| Participants | All 94 participants with primary inguinal hernia were operated on, of which, according to statistical calculations and inclusion criteria, 64 were included in the study. Inclusion criteria Male and female, age: 17‐75 years, informed consent (comment: participants were told about both surgical methods), primary hernia. Exclusion criteria Acute incarceration, immune suppression, coagulation disorders, relapse, hepatosis, diabetes mellitus, malignant tumour |
|
| Interventions |
Group A Tension‐free surgical treatment (n = 32) Group B Shouldice herniorrhaphy (n = 32): as per the original publication All participants were operated on with endotracheal anaesthesia. Four surgeons with long lasting Shouldice operation experience took part in the study. Mean operation duration was 43 ± 10 min in Shouldice group and 31 ± 7.5 in TF‐group |
|
| Outcomes | Surgical early complications (including hematomas, infections, seromas and testicular swelling) and wound healing were assessed till the day of discharge on the 6th postoperative day. Postoperative pain was assessed using a VAS and quantifying the amount of paracetamol taken by individual participants. | |
| Notes | A 2 day earlier release of pain was suspected using TF‐method in comparison to Shouldice method, so a population size of n = 32 participants/group was calculated using t‐tests for unpaired samples for comparison of means (assumed standard deviation: 2 days) to calculate a level of significance P < 0.05 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised by 'letter' (no details described, not described if envelopes were used) immediately before operation |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Classification of hernia type and wound status postoperative (hematoma, infections, seroma, testicle swelling) is reported for all 64 participants. No attrition reported. |
| Selective reporting (reporting bias) | Low risk | Different categories of wound status (hematoma, infections, seroma, testicle swelling) are reported for the period of day 1‐6. Acetaminophen use and pain on lying, walking and sitting up were reported as diagrams, no exact numbers reported (picture 1‐4) |
| Other bias | Low risk | No other sources of bias found |
Shi 2010.
| Methods | A total of 552 participants with inguinal hernia who were subjected to surgical treatment in the Affiliated Provincial Hospital of Shandong were randomly divided into the following two groups: the Bassini group (n = 269) and the tension‐free mesh group (n = 283). Cases were followed for 36–60 months, one week, one month, three months, and six months on an outpatient basis, as well as after six months with a telephone follow‐up. Those who died of unrelated diseases during the follow‐up period were included in the non‐recurrence group. | |
| Participants | Participants identified with a clinical evidence of a reducible mass in the inguinal area, with B‐ultrasound suggesting communication of the mass with the abdominal cavity. | |
| Interventions |
|
|
| Outcomes | Outcomes measured postoperatively at one week, one month on an outpatient basis; then six months subsequently with telephone‐follow up.
Other outcomes reported
|
|
| Notes | Tension‐free mesh hernioplasty is indicated for most inguinal hernia participants due to the low recurrence rate, rapid recovery time, and treatment success, but the traditional Bassini procedure has lower cost and other beneficial effects and is still suitable for some participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by drawing of lots. |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment process described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants or personnel was described in the article. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding of outcome assessors was described in the article. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study states that cases were followed up for 36‐60 months; it does not account for this variable period of follow‐up other than to establish that "those who died of unrelated diseases during the follow‐up period were included in the non‐recurrence group." Out of the 552 cases, there are no numbers representative of the cases that completed the study; of the number of those lost to follow‐up, cases deceased or excluded for other reasons. Overall insufficient reporting regarding attrition |
| Selective reporting (reporting bias) | Low risk | The initial study protocol was not available, but the published report includes all outcomes introduced in the methods. |
| Other bias | Low risk | None identified |
van Veen 2007.
| Methods | Between September 1993 and January 1996, 300 participants scheduled for repair of unilateral primary inguinal hernia were randomised to either non‐mesh (n = 150) or mesh repair (n =150). Six hospitals participated in the study. Participants were followed up at 1 week and 1, 6, 12, 18, 24 and 36 months in an outpatient setting. In the van Veen 2007 study, 153 participants were available for long‐term 10‐year follow‐up. | |
| Participants | Included participants over the age of 18 with primary unilateral inguinal hernia. Participants with bilateral hernias were excluded. | |
| Interventions |
Non‐mesh repair (n = 150) According to the surgeon's method of choice, provided that 2‐0 polypropylene sutures were to be used Mesh repair (n = 150) According to strict protocol as described by Lichtenstein and Shulman using polypropylene prosthetic mesh of 7.5 cm x 15 cm |
|
| Outcomes | Outcomes measured:
|
|
| Notes | Mesh inguinal hernia repair was associated with a lower recurrence rate than non‐mesh repair. No differences were found in complication rate, postoperative pain and quality of life, and mesh repair proved to be cost‐effective. 10‐year follow‐up study provides evidence that mesh repair of inguinal hernia is equal to non‐mesh repair with respect to long term chronic pain. But based on the present long‐term findings, it is concluded that non‐mesh primary inguinal hernia repair should be completely abandoned in adults if recurrence rates are to be reduced. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was achieved by calling an independent randomisation centre, where computer‐generated lists were available, stratified by hospital. |
| Allocation concealment (selection bias) | Low risk | Use of centralised randomisation service |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding of participants or personnel was described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors performing physical examination to determine recurrence or complications were blinded to the intervention received. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data was addressed; reasons for withdrawal and exclusion were reported. It was recognised by the study investigators that 10‐year follow‐up was difficult to obtain. |
| Selective reporting (reporting bias) | High risk | The definition of postoperative pain is considered as a dichotomous outcome and positive if it meets the criteria of "persistent pain and discomfort interfering with daily activity". In the earlier 2002 version of the study, quality of life was measured as an outcome, and other variations of pain i.e. paroxysmal pain and groin numbness were considered. Neither of these outcomes were discussed or reported in the 2007 publication. |
| Other bias | Low risk | None identified |
Witkowski 2000.
| Methods | Between 1995 and 1998, 140 participants were referred for inguinal repair. Randomised group allocation was performed with participants assigned to either a plug group (n = 70) or mesh plug group (n = 70). Participants were discharged home as their comfort allowed. The first follow‐up examination was at 4 weeks and the second at 1‐4 years after surgery. | |
| Participants | Participants with inguinal hernias. Excluded participants with age <14, pregnancy, presence of local or diffuse infection (i.e. skin, lung, sepsis), or refusal of random group allocation. | |
| Interventions |
Mesh‐plug repair (n = 70) Performed as described by Rutkow using a cone‐shaped mesh rolled up during the operation from polypropylene mesh Bassini repair (n = 70) Performed with non‐absorbable, monofilament, interrupted sutures 2‐0 In both groups, the skin was closed with non‐absorbable continuous 4‐0 sutures. |
|
| Outcomes | Outcomes measured:
|
|
| Notes | The study revealed the advantages of mesh‐plug procedure over the Bassini operation; shorter operating time, less postoperative pain, faster return to daily activity, lower recurrence rate and greater patient satisfaction. Postoperative complication rates and long‐term discomfort did not differ statistically between the two groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a blind envelope system. |
| Allocation concealment (selection bias) | Low risk | Blind envelope system was utilised for random allocation. They were kept sealed, until broken in the anaesthetic room before surgery. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding process of participants or personnel was described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding process of outcome assessors was described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 5 drop‐outs from the Bassini group and none from the mesh‐plug group. They were adequately accounted for as being lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All the specified primary and secondary outcomes were addressed. |
| Other bias | Low risk | None identified. |
Zieren 1998.
| Methods | In a prospective randomised study, early postoperative results of laparoscopic transabdominal preperitoneal (TAPP) repair (n = 80), open plug and patch (PP) repair (n = 80) and Shouldice's operation (n = 80) were compared. Patient self‐assessment regarding pain was evaluated 3 times a day for 2 months postoperatively. Outpatient physical examinations were performed on the 7th and 14th postoperative day, then after 3 months and every 6 months thereafter (mean follow‐up = 25 months). | |
| Participants | Participants to be operated on for primary inguinal hernia. The study excluded participants with contraindications for general anaesthesia, cardiac insufficiency (NYHA III or IV), age under 18 years, coagulation disorders and incarceration of the inguinal hernia. | |
| Interventions |
Laparoscopic TAPP repair (n = 80) Performed using a 12x10cm Prolene (polypropylene) mesh Open PP repair (n = 80) Performed with techniques described by Rutkow and Robbins. A 5x5 cm Prolene mesh plug to be inserted behind the internal ring and secured to its margin with only one suture. An additional 10x5 cm onlay patch, placed on the fascia transversalis with an aperture for the spermatic cord and fixed by one suture near to the pubic tubercle. Both arms of the slit were sutured together, thereby functioning as a pseudo‐internal ring. Shouldice's operation (n = 80) Transversalis fascia was doubled with a running monofilament non‐absorbable suture in two layers, taking small and closely placed bites of tissue. A second continuous suture started at the internal ring fixing the transversus muscle and the oblique muscle to the inguinal ligament. All of the operations were performed by five different surgical residents with one of two surgeons with special experience in hernia surgery present at every operation to guarantee standardised technique. |
|
| Outcomes |
|
|
| Notes | Based on the results of the study, both tension‐free methods were superior to the Shouldice operation concerning postoperative pain and analgesia requirements. In comparison with the laparoscopic procedure, PP can be performed under local anaesthetic and is less expensive regarding material costs in the operation room. PP is a promising method of inguinal hernia repair in adults, because it is less expensive, can be performed under local anaesthesia and offers a high comfort level for the patient. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Preoperative stratification according to age, gender and type of hernia followed by random allocation by computer‐randomisation |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment process was described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No blinding process for participants or personnel was described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding process for outcome assessors was described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The study reported results for all 240 cases, so there were no incomplete data. |
| Selective reporting (reporting bias) | Low risk | All the outcomes were analysed and reported. |
| Other bias | Low risk | None identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abu‐Own 2000 | This is a randomised controlled trial comparing outcomes for participants receiving a Lichtenstein patch repair and a mesh plug repair. It did not compare these outcomes to a non‐mesh technique and hence does not meet the inclusion criteria for this study. |
| Aigner 2014 | This is a prospective, randomised controlled trial comparing outcomes for participants receiving a plug and patch open repair with totally extraperitoneal inguinal hernia repair. It did not compare these outcomes to a non‐mesh technique and hence does not meet the inclusion criteria for this study. |
| Bay‐Nielsen 2004 | This is a post‐hoc retrospective analysis of chronic pain after open mesh versus sutured repair of indirect inguinal hernia repair in young males. The study randomly selected equal numbers of participants who had already received open mesh vs sutured repair of indirect inguinal hernias from the Danish and Swedish Hernia Dabatase Collaboration, and posted a questionnaire for the participants to complete. From the 2612 participants that responded (response rate 80.9%), the study concluded that chronic pain is common after primary inguinal hernia repair in young males, and that there is no difference in the pain associated with open mesh and non‐mesh repair. This study was excluded because it did not meet the inclusion criteria of being a prospective randomised controlled trial. |
| Chan 2008 | This is a non‐randomised prospective study comparing the long‐term outcomes of a non‐mesh suture/tissue‐based complete groin repair and the preperitoneal mesh repair techniques. Two hundred fifty‐six participants were enrolled, with 225 completing 5 years of follow up. Median age was 55 years, and hernias on the right side were more common (63.1%). Concurrent inguinal hernias were found in 115 participants (51%), and 41 (18.2%) had a previous inguinal hernia repair. A complete groin repair was performed in 120 participants and a preperitoneal mesh repair in 78. The remaining had an infrainguinal mesh repair. The overall recurrence rate was 3.1%, with a median time to recurrence of 12 months. There was no significant difference between mesh and suture repairs. Chronic postoperative pain was experienced by 20 participants (8.9%). The study concluded that femoral hernias can be repaired electively with a tissue‐based or a preperitoneal mesh technique, with durable long term results. Mesh repair is indicated for recurrent femoral hernias, inguinofemoral hernias, pre vascular hernias, association with concurrent direct hernias, and, if tension is anticipated, with complete groin repair. Infrainguinal mesh repair is used only when there has been a successful previous inguinal hernia repair. This study was excluded because it did not meet the inclusion criteria of being a randomised prospective controlled trial. |
| Suradom 2011 | This is a non‐randomised prospective study. Its objective is to compare the effectiveness of umbrella made‐mesh plugs compared to other methods of herniorrhaphy. 194 participants were recruited in the study; males aged 16‐86 with a primary diagnosis of an indirect inguinal hernia. Assigned to two periods of elective surgery. First period was between 2003 and 2005, with assignment to either Bassini repair (n = 58) or Bassini repair with umbrella made‐mesh plug (n = 42). The second period was between 2005 and 2008, with assignment to either Lichtenstein repair (n = 40) or umbrella made mesh plug with a patching tail (n = 54). Outcomes measured included mean operating time, duration of hospital stay, complications and recurrence with follow‐up over 2 years. Conclusion that usage of umbrella made mesh plugs was a safe method of groin herniorrhaphy. This study was excluded as it did not meet inclusion criteria of being a randomised controlled trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Aigner 2011.
| Methods | Participants were randomised to undergo open tension‐free plug and patch (PP) and totally extraperitoneal patch (TEP) hernioplasty from 2005 to 2009. Pain assessment was conducted by using the numerical rating scale (NRS) and the McGill Pain Questionnaire preoperatively, 6, 12 and 24 months postoperatively. All participants obtained same analgesics and documented pain in a NRS based 4‐week‐diary. |
| Participants | Of the 162 male participants with unilateral inguinal hernia; 79 underwent TEP and 83 PP |
| Interventions | Open tension‐free plug and patch and TEP hernioplasty |
| Outcomes | Mean follow‐up was 2.9 ± 1.2 years. One recurrent hernia was observed in the TEP group. Mean preoperative NRS score was 2 and 2, 0.3 and 0.4 at 6 months, 0.1 and 0.3 at 12 months, 0.2 and 0.1 at 24 months postoperatively in the PP and TEP groups respectively (P > 0.05). Data from the 4‐week pain diaries revealed no significant difference in pain intensity in the first two weeks postoperatively (VAS 2‐4, P > 0.05). Participants in the PP group required more additional analgesics on day four and five postoperatively (P˜0.037 and 0.005, respectively). The data demonstrate no significant differences concerning postoperative pain between tension‐free PP and TEP hernia repair. |
| Notes | Abstract only |
Gedam 2017.
| Methods | Comparative study, not clear if randomised |
| Participants | Participants with inguinal hernia. 187 cases were allocated into 2 groups. Desarda (D Group) had 92 and Lichtenstein (L Group) had 95 participants. |
| Interventions | Comparison of the tissue based Desarda technique with standard Lichtenstein repair in treatment of primary inguinal hernia. Desarda technique (no mesh) and standard Lichtenstein repair (mesh) |
| Outcomes | Primary outcome factor was early (< 1 year) recurrence of inguinal hernia. Secondary outcome factors included operative time measured from skin incision to skin closure. Postoperative pain scores were assessed on day 1, 3, 7, 30 and 90 using Visual analogue scale. Time taken to return to basic and home activities was calculated. Cord oedema, groin discomfort, seroma, fever, surgical site infections, chronic pain, etc. were evaluated as postoperative complications. |
| Notes | Conclusion: the results of inguinal hernia treatment with the Desarda technique are similar to the results after standard Lichtenstein operations. |
Memon 2017.
| Methods | Prospective randomised control trial (RCT). |
| Participants | Ninety‐two male participants from 20‐60 years of age reported for direct or indirect inguinal hernia with open Mesh/Lichtenstein or darn repair, in emergency or electively, from January 2014 to December 2015. |
| Interventions | Comparison of Lichtenstein repair and tension‐free Darn repair |
| Outcomes | The primary end point was to compare the surgical site infection, length of hospital stay and hernia recurrence with different techniques. |
| Notes | Conclusion: Lichtenstein is more promising in comparison to Darn repair in terms of recurrence in inguinal hernia. |
Differences between protocol and review
Hernia recurrence data were collected and analysed in accordance with the previously published protocol. Duration of follow‐up and follow‐up intervals varied considerably across studies, which largely prevented subgroup analysis. For some planned subgroup analyses (such as comparing outcomes for inguinal versus femoral hernia repairs) there were not enough available data to compare. The primary and secondary outcomes were organised differently compared to the protocol, with sub‐analysis of complications and mortality analysed as a primary rather than secondary outcomes. This was changed in order to prioritise outcomes that were more clinically relevant. In addition, a narrative account of comparative repair costs was completed as this was identified as a significant factor in clinical practice. Minor changes were made for methods of data collection, extraction and analysis, including use of risk ratios rather than odds ratios. Initially, in Measures of treatment effect, we included the condition of using rate ratios for data reported as rates. However, as none of our prespecified outcomes were reported as rates and we did not use this technique in pooling, this point was removed. Initially with data synthesis, if studies demonstrated significant heterogeneity we were not planning to pool them in meta‐analysis. However, the outcomes that demonstrated significant heterogeneity, such as duration of surgery or postoperative stay (not unexpected considering the type of outcome), could still be clearly compared, and we therefore pooled them for meta‐analysis.
Contributions of authors
All of the authors contributed to the drafting of the review. Professor Mieke van Driel provided methodological advice and guidance and Dr Manvinder Dhillon provided content advice throughout the review process.
Sources of support
Internal sources
Bond University, Australia.
University of Queensland, Australia.
Monash University, Australia.
Queensland Health, Australia.
Griffith University, Australia.
University of Sydney, Australia.
External sources
No sources of support supplied
Declarations of interest
None of the authors have any conflicts of interest to declare.
New
References
References to studies included in this review
Abd El Maksoud 2014 {published data only}
- Abd El Maksoud W, Abd El Salam M, Ahmed HH. Comparative study between Lictenstein procedure and modified darn repair in treating primary inguinal hernia: a prospective randomised controlled trial. Hernia 2014;18(2):231‐6. [DOI] [PubMed] [Google Scholar]
Barth 1998 {published data only}
- Barth RJ, Burchard KW, Tosteson A, Sutton JE, Colacchio TA. Short‐term outcome after mesh or Shouldice herniorrhaphy: A randomized, prospective study. Surgery 1998;123:121‐6. [PubMed] [Google Scholar]
Berndsen 2007 {published data only}
- Arvidsson D, Berndsen FH, Larsson LG, Leijonmarck CE, Rimback G, et al. Randomized clinical trial comparing 5‐year recurrence rate after laparoscopic versus Shouldice repair of primary inguinal hernia. British Journal of Surgery 2005;92:1085‐91. [DOI] [PubMed] [Google Scholar]
- Berndsen FH, Petersson U, Arvidsson D, Leijonmarck CE, Rudberg C, Smedberg S, et al. Discomfort five years after laparoscopic and Shouldice inguinal hernia repair: a randomised trial with 867 patients. Hernia 2007;11:307‐13. [DOI] [PubMed] [Google Scholar]
Butters 2007 {published data only}
- Butters M, Redecke J, Koninger J. Long‐term results of a randomised clinical trial of Shouldice, Lichtenstein and transabdominal preperitoneal hernia repairs. British Journal of Surgery 2007;94:562‐5. [DOI] [PubMed] [Google Scholar]
- Koninger J, Redecke J, Butters M. Chronic pain after hernia repair: a randomised trial comparing Shouldice, Lichtenstein and TAPP. Langenbecks Archives of Surgery 2004;389:361‐5. [DOI] [PubMed] [Google Scholar]
Chakraborty 2007 {published data only}
- Chakraborty S, Mukherjee A, Bhattacharya M. Tension‐free inguinal hernia repair comparing darn with mesh: a prospective randomised controlled clinical trial. Indian Journal of Surgery 2007;69(2):52‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elsebae 2008 {published data only}
- Elsebae M, Nasr M, Said M. Tension‐free repair versus Bassini technique for strangulated inguinal hernia: A controlled randomized study. International Journal of Surgery 2008;6:302‐5. [DOI] [PubMed] [Google Scholar]
Hauters 1996 {published data only}
- Hauters Ph, Meunier D, Urgyan S, Jouret J, Janssen P, NYS JM. Controlled prospective study comparing laparoscopic and Shouldice in the treatment of unilateral inguinal hernia [Etude prospective controlee comparant laparoscopie et Shouldice dans le traitement de la hernie inguinale unilaterale]. Annales de Chirurgie 1996;50(9):776‐81. [PubMed] [Google Scholar]
Kaynak 2007 {published data only}
- Kaynak B, Celik F, Guner A, Guler K, Kaya M, Celik M. Moloney darn repair versus Lichtenstein mesh hernioplasty for open inguinal hernia repair. Surgery Today 2007;37:958–60. [DOI] [PubMed] [Google Scholar]
Kucuk 2010 {published data only}
- Kucuk H, Sikar H, Kurt N, Uzun H, Eser M, Tutal F, et al. Lichtenstein or darn procedure in inguinal hernia repair: a prospective randomized comparative study. Hernia 2010;14:357–60. [DOI] [PubMed] [Google Scholar]
Kux 1994 {published data only}
- Kux M, Fuchsjager N, Feichter A. Lichtenstein patch versus Shouldice technique in primary inguinal hernias with high recurrence risk. [Lichtenstein‐Patch versus Shouldice‐Technik bei primaren leistenhernien mit hoher rezidivgefahrdung]. Chirurg 1994;65:59‐62. [PubMed] [Google Scholar]
Leibl 1995 {published data only}
- Leibl B, Daubler P, Schwarz J, Ulrich M, Bittner R. Standardised laparoscopic hernia repair vs. Shouldice repair: results of a randomised comparative study [Standardisierte laparoskopische hernioplastik vs shouldice‐reparation: ergebnisse einer randomisierten vergleichsstudie]. Chirurg 1995;66:895‐8. [PubMed] [Google Scholar]
Lermite 2012 {published data only}
- Lermite E, Arnaud JP. Prospective randomized study comparing quality of life after Shouldice or mesh plug repair for inguinal hernia: short term results. Surgical Technology International XXII 2012;22:101‐6. [PubMed] [Google Scholar]
Manyilirah 2012 {published data only}
- Manyilirah W, Kijjambu S, Upoki A, Kiryabwire J. Comparison of non‐mesh (Desarda) and mesh (Lichtenstein) methods for inguinal hernia repair among black African patients: a short‐term double‐blind RCT. Hernia 2011;16:133–44. [DOI] [PubMed] [Google Scholar]
McGillicuddy 1998 {published data only}
- McGillicuddy JE. Prospective randomized comparison of the Shouldice and Lichtenstein hernia repair procedures. Archieves of Surgery 1998;133:974‐8. [DOI] [PubMed] [Google Scholar]
Nakagawa 2013 {published data only}
- Nakagawa M, Nagase T, Akatsu T, Imai S, Fujimura N, Asagoe T, et al. A randomized prospective trial comparing clinical outcomes 3 years after surgery by Marcy repair and Prolene Hernia System repair for adult indirect inguinal hernia. Surgery Today 2013;43:1109‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naveen 2014 {published data only}
- Naveen N, Srinath R. A comparative study between Modified Bassini's Repair and Lichtenstein Mesh Repair (LMR) of inguinal hernias in rural population. Journal of clinical and diagnostic research 2014;8(2):88‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nordin 2002 {published data only}
- Nordin P, Bartelmess P, Jansson C, Svensson C, Edlund G. Randomized trial of Lichtenstein versus Shouldice hernia repair in general surgical practice. British Journal of Surgery 2002;89:45‐9. [DOI] [PubMed] [Google Scholar]
Panda 2012 {published data only}
- Panda N, Ghoshal P, Das S, Das R. Lichtenstein's mesh versus Bassini tissue repair technique for obstructed inguinal hernia: a controlled randomized study. European Surgery 2012;44:314‐8. [Google Scholar]
Prieto‐Diaz‐Chavez 2009 {published data only}
- Prieto‐Diaz‐Chavez E, Medina‐Chavez JL, Anaya‐Prado R. A cost‐effective analysis of tension‐free versus Shouldice inguinal hernia repair: a randomised double‐blind clinical trial. Hernia 2009;13:233‐8. [DOI] [PubMed] [Google Scholar]
Prior 1998 {published data only}
- Prior MJ, Williams EV, Shukla HS, Phillips S, Vig S, Lewis M. Prospective randomized controlled trial comparing Lichtenstein with modified Bassini repair of inguinal hernia. Journal of the Royal College of Surgeons of Edinburgh 1998;43:82‐6. [PubMed] [Google Scholar]
Schmitz 1997 {published data only}
- Schmitz R, Treckmann J, Shah S, Schneider K. The tension free technique in open inguinal hernia repair: a prospective randomised study for post‐operative pain perception [Die „Tension‐free‐Technik“ bei offener Leistenhernienreparation: Eine prospektive, randomisierte Studie zur postoperativen Schmerzperception]. Chirurg 1997;68:259‐63. [DOI] [PubMed] [Google Scholar]
Shi 2010 {published data only}
- Shi Y, Su Z, Li L, Liu H, Jing C. Comparing the effects of Bassini versus tension‐free hernioplasty: 3 years’ follow up. Frontiers of Medicine in China 2010;4(4):463‐8. [DOI] [PubMed] [Google Scholar]
van Veen 2007 {published data only}
- Vrijland WW, Tol MP, Luijendik W, Hop WCJ, Busschbach JJV, Lange DCD, et al. Randomized clinical trial of non‐mesh versus mesh repair of primary inguinal hernia. British Journal of Surgery 2002;89:293‐7. [DOI] [PubMed] [Google Scholar]
- Veen R, Wijsmuller A, Vrijland W, Hop W, Lange J, Jeekel J. Long‐term follow‐up of a randomised clinical trial of non‐mesh versus mesh repair of primary inguinal hernia. Surgery 2007;94:506‐10. [DOI] [PubMed] [Google Scholar]
- Veen R, Wijsmuller A, Vrijland W, Hop W, Lange J, Jeekel J. Randomised clinical trial of mesh versus non‐mesh primary inguinal hernia repair: Long term chronic pain at 10 years. Surgery 2007;142:695‐8. [DOI] [PubMed] [Google Scholar]
Witkowski 2000 {published data only}
- Witkowski P, Pirski MI, Adamonis W, Smietanksi M, Draczkowski T, Sledzinski Z. Mesh plug versus Bassini operation: a randomized prospective study. Hernia 2000;4:305‐10. [Google Scholar]
Zieren 1998 {published data only}
- Zieren J, Zieren H, Jacobi CA, Wenger FA, Muller JM. Prospective randomized study comparing laparoscopic and open tension‐free inguinal hernia repair with Shouldice’s operation. American Journal of Surgery 1998;175(4):330‐3. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abu‐Own 2000 {published data only}
- Abu‐Own A, Onwudike M, Haque KA, Barker SG. Primary inguinal hernia repair utilizing the 'mesh plug' technique. Ambulatory Surgery 2000;8:31‐5. [Google Scholar]
Aigner 2014 {published data only}
- Aigner F, Augustin F, Kaufmann C, Schlager A, Ulmer H, et al. Prospective, randomized‐controlled trial comparing postoperative pain after plug and patch open repair with totally extraperitoneal inguinal hernia repair. Hernia 2014;18:237‐42. [DOI] [PubMed] [Google Scholar]
Bay‐Nielsen 2004 {published data only}
- Bay‐Nielsen M, Nilsson E, Nordin P, Kehlet H. Chronic pain after open mesh and sutured repair of indirect inguinal hernia in young males. British Journal of Surgery 2003;91:1372‐6. [DOI] [PubMed] [Google Scholar]
Chan 2008 {published data only}
- Chan G, Chan CK. Longterm results of a prospective study of 225 femoral hernia repairs: Indications for tissue and mesh repair. Journal of the American Collegel of Surgeons 2008;207:360‐7. [DOI] [PubMed] [Google Scholar]
Suradom 2011 {published data only}
- Suradom C, Palaphun J. The usage of two umbrella made‐mesh plugs in herniorrhaphy: comparative study with Bassini and Lichtenstein method.. Journal of the Medical Association of Thailand (Chotmaihet thangphaet) 2011;94(11):1373‐9. [PubMed] [Google Scholar]
References to studies awaiting assessment
Aigner 2011 {published data only}
- Aigner F, Augustin F, Kaufmann C, Schlager A, Ulmer H, Pratschke J, et al. Prospective, randomised‐controlled trial comparing postoperative pain after open and minimal invasive inguinal hernia repair (Abstract). European Surgery 2011;43:39. [DOI] [PubMed] [Google Scholar]
Gedam 2017 {published data only}
- Gedam BS, Bansod PY, Kale VB, Shah Y, Akhtar M. A comparative study of Desarda's technique with Lichtenstein mesh repair in treatment of inguinal hernia: a prospective cohort study. International Journal of Surgery 2017;39:150‐155. [DOI] [PubMed] [Google Scholar]
Memon 2017 {published data only}
- Memon GA, Shah SKA, Habib ur R. An experience with mesh versus darn repair in inguinal hernias. Pakistan Journal of Medical Sciences 2017;33:699‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Amid 2005
- Amid PK. Groin hernia repair: open techniques. World Journal of Surgery 2005;28(8):1046‐51. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bittner 2011
- Bittner R, Arregui ME, Bisgaard T, Dudai M, Ferzli GS, Fitzgibbons RJ, et al. Guidelines for laparoscopic (TAPP) and endoscopic (TEP) treatment of inguinal hernia [International Endohernia Society (IEHS)]. Journal of Endoscopic Surgery 2011;25(9):2773‐843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boonstra 2014
- Boonstra AM, Schiphorst Preuper HR, Balk GA, Stewart RE. Cut‐off points for mild, moderate, and severe pain on the visual analogue scale for pain in patients with chronic musculoskeletal pain. Pain 2014;155(12):2545‐50. [DOI] [PubMed] [Google Scholar]
Brooks 2014a
- Brooks DC, Obeid A, Hawn M. Classification, clinical features and diagnosis of inguinal and femoral hernias in adults. Up To Date 2014.
Brooks 2014b
- Brooks DC. Overview of treatment for inguinal and femoral hernia in adults. Up To Date 2014.
Brooks 2014c
- Brooks DC. Overview of complications of inguinal and femoral hernia repair. Up To Date 2014.
Brown 2010
- Brown CN, Finch JG. Which mesh for hernia repair?. Annals of the Royal College of Surgeons, England 2010;92(4):272‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cannon 1981
- Cannon DJ, Read RC. Metastatic emphysema: a mechanism for acquiring inguinal herniation. Annals of Surgery 1981;194(3):270‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahlstrand 2009
- Dahlstrand U, Wollert S, Nordin P, Sandblom G, Gunnarsson U. Emergency femoral hernia repair: a study based on a national register. Annals of Surgery 2009;249(4):672. [DOI] [PubMed] [Google Scholar]
Doctor 2006
- Doctor HG. Evaluation of various prosthetic materials and newer meshes for hernia repairs. Journal of Minimal Access Surgery 2006;2(3):110‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eklund 2009
- Eklund AS, Montgomery AK, Rasmussen IC, Sandbue RP, Bergkvist LA, Rudberg CR. Low recurrence rate after laparoscopic (TEP) and open (Lichtenstein) inguinal hernia repair: a randomized, multicentre trial with 5‐year follow‐up. Annals of Surgery 2009;249(1):33‐8. [DOI] [PubMed] [Google Scholar]
EU Hernia Trialists 2002
- The EU Hernia Trialists Collaboration. Repair of groin hernia with synthetic mesh: meta‐analysis of RCT. Annals of Surgery 2002;235:322‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
EU Hernia Trialists Collaboration 2000
- EU Hernia Trialists Collaboration 2000. Mesh compared with non‐mesh methods of open groin hernia repair: systematic review of randomized controlled trials. British Journal of Surgery 2000;87:854‐9. [DOI] [PubMed] [Google Scholar]
EU Hernia Trialists Collaboration 2002
- EU Hernia Trialists Collaboration. Open mesh versus non‐mesh repair of groin hernia meta‐analysis of randomized trials leased on individual patient data. Hernia 2002;6:130‐6. [DOI] [PubMed] [Google Scholar]
Falagas 2005
- Falagas ME, Kasiakou SK. Mesh‐related infections after hernia repair surgery. Clinical microbiology and infection 2005;11(1):3‐8. [DOI] [PubMed] [Google Scholar]
Ferzli 1998
- Ferzli G, Sayad P, Huie F, Hallak A, Usal H. Endoscopic extraperitoneal herniorrhaphy. A 5‐year experience. Journal of Endoscopic Surgery 1998;12(11):1311. [DOI] [PubMed] [Google Scholar]
Fitzgibbons 2006
- Fitzgibbons RJ Jr, Giobbie‐Hurder A, Gibbs JO, Dunlop DD, Reda DJ, et al. Watchful waiting vs repair of inguinal hernia in minimally symptomatic men: a randomized clinical trial. JAMA 2006;295(3):285. [DOI] [PubMed] [Google Scholar]
Fitzgibbons 2015
- Fitzgibbons RJ, Forse RA. Groin hernias in adults. New England Journal of Medicine 2015;372:756‐63. [DOI] [PubMed] [Google Scholar]
Gallegos 1991
- Gallegos NC, Dawson J, Jarvis M, Hobsley M. Risk of strangulation in groin hernias. British Journal of Surgery 1991;78(10):1171. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group. GRADEpro GDT. McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jenkins 2008
- Jenkins JT, O'Dwyer PJ. Inguinal hernias. BMJ 2008;336:269‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klinge 2002
- Klinge U, Klosterhalfen B, Birkenhauer V, Junge K, Conze J, Schumpelick V. Impact of polymer pore size on the interface scar formation in a rat model. Journal of the Surgical Resident 2002;103:208‐14. [DOI] [PubMed] [Google Scholar]
Klinge 2008
- Klinge U. Mesh for hernia repair. British Journal of Surgery 2008;95:539‐40. [DOI] [PubMed] [Google Scholar]
Koch 2005
- Koch A, Edwards A, Haapaniemi S, Nordin P, Kald A. Prospective evaluation of 6895 groin hernia repairs in women. British Journal of Surgery 2005;92(12):1553. [DOI] [PubMed] [Google Scholar]
Liem 1997
- Liem MS, Graaf Y, Zwart RC, Geurts I, Vroonhoven TJ. Risk factors for inguinal hernia in women: a case‐control study. The Coala Trial Group. American Journal of Epidemiology 1997;146(9):721‐6. [DOI] [PubMed] [Google Scholar]
Lofgren 2016
- Löfgren J, Nordin P, Ibingira C, Matovu A, Galiwango E, Wladis A. A randomized trial of low‐cost mesh in groin hernia repair. New England Journal of Medicine 2016;374:146‐53. [DOI] [PubMed] [Google Scholar]
McCormack 2003
- McCormack K, Scott N, Go PM, Ross SJ, Grant A, Collaboration the EU Hernia Trialists. Laparoscopic techniques versus open techniques for inguinal hernia repair. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001785] [DOI] [PMC free article] [PubMed] [Google Scholar]
McKernan 1993
- McKernan JB, Laws H. Laparoscopic repair of inguinal hernias using a totally extraperitoneal prosthetic approach. Journal of Endoscopic Surgery 1993;7(1):26. [DOI] [PubMed] [Google Scholar]
Nixon 2009
- Nixon SJ, Jawaid H. Recurrence after inguinal hernia repair at ten years by open darn, open mesh and TEP – no advantage with mesh. The Surgeon 2009;7:71‐4. [DOI] [PubMed] [Google Scholar]
O'Dwyer 2005
- O'Dwyer, Kingsworth AN, Molloy RG, Small PK, Lammers B, Horeyseck G. Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. British Journal of Surgery 2005;92:166‐70. [DOI] [PubMed] [Google Scholar]
O'Dwyer 2006
- O'Dwyer PJ, Norrie J, Alani A, Walker A, Duffy F, Horgan P. Observation or operation for patients with an asymptomatic inguinal hernia: a randomized clinical trial. Annals of Surgery 2006;244(2):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Read 1999
- Read RC. Francis C. Usher, herniologist of the twentieth century. Hernia 1999;3(3):167‐71. [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosemar 2008
- Rosemar A, Angeras U, Rosengren A. Body mass index and groin hernia: a 34‐year follow‐up study in Swedish men. Annals of Surgery 2008;247(6):1064. [DOI] [PubMed] [Google Scholar]
Rosemar 2010
- Rosemar A, Angeras U, Rosengren A, Nordin P. Effect of body mass index on groin hernia surgery. Annals of Surgery 2010;252(2):397‐401. [DOI] [PubMed] [Google Scholar]
Rosenberg 2011
- Rosenberg J, Bisgaard T, Kehlet H, Wara P, Asmussen T, Juul P, et al. Danish Hernia Database recommendations for the management of inguinal and femoral hernia in adults. Danish Medical Bulletin 2011;58(2):42‐3. [PubMed] [Google Scholar]
Ruhl 2007
- Ruhl CE, Everhart JE. Risk factors for inguinal hernia among adults in the US population. American Journal of Epidemiology 2007;165(10):1154‐61. [DOI] [PubMed] [Google Scholar]
Rutkow 1993
- Rutkow IM, Robbins AW. Demographic, classificatory, and socioeconomic aspects of hernia repair in the United States. Surgical Clinics of North America 1993;73(3):413. [DOI] [PubMed] [Google Scholar]
Sajid 2013
- Sajid MS, Leaver C, Baig M, Sains P. Lightweight versus Heavyweight mesh for open repair of inguinal hernia. Cochrane Database of Systematic Reviews February 2013, Issue 2. [CENTRAL: 10.1002/14651858.CD009475] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al editor(s). Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Simons 2009
- Simons MP, Aufenacker T, Bay‐Nielsen M, Bouillot JL, Campanelli G, Conze J, et al. European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia 2009;13(4):343‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skalad 1941
- Saklad M. Grading of patients for surgical procedures. Anesthesiology 1941;2:281‐4. [Google Scholar]
Sorenson 2002
- Sørensen LT, Friis E, Jørgensen T, Vennits B, Andersen BR, Rasmussen GI, et al. Smoking is a risk factor for recurrence of groin hernia. World Journal of Surgery 2002;26(4):397‐400. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D, editor(s). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Vader 1997
- Vader VL, Vogt DM, Zucker KA, Thilstead JP, Curet MJ. Adhesion formation in laparoscopic inguinal hernia repair. Journal of Endoscopic Surgery 1997;11(8):825. [DOI] [PubMed] [Google Scholar]
Wake 2005
- Wake BL, McCormack K, Fraser C, Vale L, Perez J, Grant A. Transabdominal pre‐peritoneal (TAPP) vs totally extraperitoneal (TEP) laparoscopic techniques for inguinal hernia repair. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004703.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whalen 2011
- Whalen HR, Kidd GA, O'Dwyer PJ. Femoral hernias. BMJ 2011;343:d7668. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organisation. BMI Classification. Global Database on Body Mass Index. 2006 (Last retrieved July 27, 2012).
Yang 2011
- Yang J, Papandria D, Rhee D, Perry H, Abdullah F. Low‐cost mesh for inguinal hernia repair in resource‐limited settings. Hernia 2011;15:485‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Scott 2001
- Scott N, Go PM, Graham P, McCormack K, Ross SJ, Grant AM. Open mesh versus non‐mesh for groin hernia repair. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD002197] [DOI] [PubMed] [Google Scholar]


