Abstract
Background
Healthcare professionals are important contributors to healthcare quality and patient safety, but their performance does not always follow recommended clinical practice. There are many approaches to influencing practice among healthcare professionals. These approaches include audit and feedback, reminders, educational materials, educational outreach visits, educational meetings or conferences, use of local opinion leaders, financial incentives, and organisational interventions. In this review, we evaluated the effectiveness of patient‐mediated interventions. These interventions are aimed at changing the performance of healthcare professionals through interactions with patients, or through information provided by or to patients. Examples of patient‐mediated interventions include 1) patient‐reported health information, 2) patient information, 3) patient education, 4) patient feedback about clinical practice, 5) patient decision aids, 6) patients, or patient representatives, being members of a committee or board, and 7) patient‐led training or education of healthcare professionals.
Objectives
To assess the effectiveness of patient‐mediated interventions on healthcare professionals' performance (adherence to clinical practice guidelines or recommendations for clinical practice).
Search methods
We searched MEDLINE, Ovid in March 2018, Cochrane Central Register of Controlled Trials (CENTRAL) in March 2017, and ClinicalTrials.gov and the International Clinical Trials Registry (ICTRP) in September 2017, and OpenGrey, the Grey Literature Report and Google Scholar in October 2017. We also screened the reference lists of included studies and conducted cited reference searches for all included studies in October 2017.
Selection criteria
Randomised studies comparing patient‐mediated interventions to either usual care or other interventions to improve professional practice.
Data collection and analysis
Two review authors independently assessed studies for inclusion, extracted data and assessed risk of bias. We calculated the risk ratio (RR) for dichotomous outcomes using Mantel‐Haenszel statistics and the random‐effects model. For continuous outcomes, we calculated the mean difference (MD) using inverse variance statistics. Two review authors independently assessed the certainty of the evidence (GRADE).
Main results
We included 25 studies with a total of 12,268 patients. The number of healthcare professionals included in the studies ranged from 12 to 167 where this was reported. The included studies evaluated four types of patient‐mediated interventions: 1) patient‐reported health information interventions (for instance information obtained from patients about patients' own health, concerns or needs before a clinical encounter), 2) patient information interventions (for instance, where patients are informed about, or reminded to attend recommended care), 3) patient education interventions (intended to increase patients' knowledge about their condition and options of care, for instance), and 4) patient decision aids (where the patient is provided with information about treatment options including risks and benefits). For each type of patient‐mediated intervention a separate meta‐analysis was produced.
Patient‐reported health information interventions probably improve healthcare professionals' adherence to recommended clinical practice (moderate‐certainty evidence). We found that for every 100 patients consulted or treated, 26 (95% CI 23 to 30) are in accordance with recommended clinical practice compared to 17 per 100 in the comparison group (no intervention or usual care). We are uncertain about the effect of patient‐reported health information interventions on desirable patient health outcomes and patient satisfaction (very low‐certainty evidence). Undesirable patient health outcomes and adverse events were not reported in the included studies and resource use was poorly reported.
Patient information interventions may improve healthcare professionals' adherence to recommended clinical practice (low‐certainty evidence). We found that for every 100 patients consulted or treated, 32 (95% CI 24 to 42) are in accordance with recommended clinical practice compared to 20 per 100 in the comparison group (no intervention or usual care). Patient information interventions may have little or no effect on desirable patient health outcomes and patient satisfaction (low‐certainty evidence). We are uncertain about the effect of patient information interventions on undesirable patient health outcomes because the certainty of the evidence is very low. Adverse events and resource use were not reported in the included studies.
Patient education interventions probably improve healthcare professionals' adherence to recommended clinical practice (moderate‐certainty evidence). We found that for every 100 patients consulted or treated, 46 (95% CI 39 to 54) are in accordance with recommended clinical practice compared to 35 per 100 in the comparison group (no intervention or usual care). Patient education interventions may slightly increase the number of patients with desirable health outcomes (low‐certainty evidence). Undesirable patient health outcomes, patient satisfaction, adverse events and resource use were not reported in the included studies.
Patient decision aid interventions may have little or no effect on healthcare professionals' adherence to recommended clinical practice (low‐certainty evidence). We found that for every 100 patients consulted or treated, 32 (95% CI 24 to 43) are in accordance with recommended clinical practice compared to 37 per 100 in the comparison group (usual care). Patient health outcomes, patient satisfaction, adverse events and resource use were not reported in the included studies.
Authors' conclusions
We found that two types of patient‐mediated interventions, patient‐reported health information and patient education, probably improve professional practice by increasing healthcare professionals' adherence to recommended clinical practice (moderate‐certainty evidence). We consider the effect to be small to moderate. Other patient‐mediated interventions, such as patient information may also improve professional practice (low‐certainty evidence). Patient decision aids may make little or no difference to the number of healthcare professionals' adhering to recommended clinical practice (low‐certainty evidence).
The impact of these interventions on patient health and satisfaction, adverse events and resource use, is more uncertain mostly due to very low certainty evidence or lack of evidence.
Plain language summary
Patient‐mediated interventions to improve professional practice
What is the aim of the review?
Our aim with this Cochrane review was to assess whether patients can change the performance of healthcare professionals. We collected and analysed all relevant studies to answer this question and found 25 studies.
Key message
This review suggests that patients may change healthcare professionals’ practice though the following three strategies: 1) strategies where patients give healthcare professionals information about themselves; 2) strategies where patients are given healthcare information; and 3) strategies where patients take part in patient education. Patient decision aids may make little or no difference to healthcare professionals’ practice, however, the certainty is low, and these results should be interpreted carefully. We still need more research about the best ways in which patients can change professional practice and about the impact it has on patients’ health.
What was studied in the review?
Many strategies have been tested to see if they can improve healthcare professionals’ practice and make sure that patients receive the best available care. These strategies include sending reminders to healthcare professionals, giving them further education, or giving them financial rewards. These strategies have mostly had only small or moderate effects. Another way of changing what healthcare professionals do is through the patients themselves. These strategies are called 'patient‐mediated interventions'.
What are the main results of the review?
The studies in this review assessed different patient‐mediated strategies compared to usual care or no strategies.
Strategies where patients give information to healthcare professionals
In these studies, patients gave information about their own health, concerns or needs to the doctor. This was usually done by filling in a questionnaire in the waiting area before a consultation. The doctor was then given this information before or at the consultation. The review shows that these strategies:
‐ probably improve the extent to which healthcare professionals follow recommended clinical practice (moderate‐certainty evidence).
We are uncertain about the effect of these strategies on patient health, patient satisfaction and resource use because these outcomes were not measured in the studies or because the certainty of the evidence is very low.
Strategies where information was given to patients
In these studies, patients were given information about recommended care or were reminded to use services, for instance to go for a check‐up. The review shows that these strategies:
‐ may improve the extent to which healthcare professionals follow recommended clinical practice (low‐certainty evidence);
‐ may have little or no effect on patient satisfaction (low‐certainty evidence);
‐ may have little or no effect on some patient health outcomes, such as the number of patients who reach controlled blood pressure (low‐certainty evidence). However, we are uncertain about the effect of these strategies on other patient health outcomes because the certainty of the evidence is very low. We also lack information to draw conclusions about resource use.
Patient education strategies
In these studies, patients took part in patient education such as self‐management programmes, for instance to increase their knowledge about their condition. The review shows that these strategies:
‐ probably improve the extent to which healthcare professionals follow recommended clinical practice (moderate‐certainty evidence);
‐ may slightly improve some patient health outcomes such as the number of patients who reach controlled blood pressure (low‐certainty evidence). However, we are uncertain about the effect of these strategies on other patient health outcomes, patient satisfaction and resource use because these outcomes were not measured in the included studies.
Patient decision aid strategies
In the one study that assessed effect of patient decision aids, patients were given a decision aid consisting of a booklet, personal worksheet, and audiotape to make decisions about their medical management. The review shows that these strategies:
‐ may have little or no effect on the extent to which healthcare professionals follow recommended clinical practice (low‐certainty evidence)
We are uncertain about the effect of these strategies on patient health, patient satisfaction and resource use because these outcomes were not measured in the studies or because the certainty of the evidence is very low.
How up‐to‐date is this review?
We searched for studies up to March 2018 and ongoing studies up to October 2017.
Summary of findings
Background
Description of the condition
Healthcare professionals' performance is not always in line with recommended clinical practices (McGlynn 2003; Runciman 2012; Schuster 1998; Seddon 2001). Reducing the gap between recommended and actual clinical practice is a key element of healthcare quality improvement. Recommended practices are typically formulated in clinical practice guidelines. Clinical practice guidelines have the potential to improve the quality of healthcare and patient outcomes by providing specific recommendations for professional practice (Grol 2003; Schuster 1998; Seddon 2001). Adherence to clinical practice guidelines is thus frequently used as a measure of the quality of healthcare. Various interventions are proposed as means to improve the performance of healthcare professionals, e.g. audit and feedback, reminders, educational material, educational outreach visits, educational meetings or conferences, use of local opinion leaders, financial incentives, organisational interventions, and patient‐mediated interventions.
Description of the intervention
Several definitions of patient‐mediated intervention have been proposed (Grimshaw 2004; Légaré 2014; Robertson 2006). Here we define patient‐mediated interventions according to Légaré 2014: "any intervention aimed at changing the performance of healthcare professionals through interactions with patients, or information provided by or to patients".
Overall, experimental studies of interventions to improve professional practice have yielded small to moderate effects. A Cochrane review shows that audit and feedback probably improves professional practice, but the effectiveness ranges from little or no effect to a substantial effect (Ivers 2012). Reminders, such as computer‐generated reminders delivered on paper to healthcare professionals, probably improve professional practice (Arditi 2017). Printed educational material may also improve professional practice, but the effect seems small, and the certainty of the evidence is low (Giguère 2012). Educational meetings or educational outreach visits may result in modest improvements in professional practice (Forsetlund 2009; O'Brien 2007). Using local opinion leaders may improve professional practice (Flodgren 2011a), as may financial incentives (Flodgren 2011b). Another recent Cochrane review shows that healthcare professionals provided with clinical practice guidelines accompanied by tools developed by guideline producers probably adhere more to clinical guidelines (Flodgren 2016). Organisational interventions, such as provision of pharmaceutical care, medication reviews, and follow‐up visits by a healthcare professional including a pharmacist, nurse or physician, probably make little or no difference to the number of medication errors by primary healthcare professionals that lead to hospital admissions, emergency department visits, or death among adult patients (Khalil 2017).
Direct involvement of patients or their representatives in decision‐making processes is seen both as an ethical imperative, and as a promising approach for quality improvement (Richards 2013). Interventions to promote shared decision‐making (Légaré 2014) and patient‐centred care (Dwamena 2012), including patient‐mediated interventions, have been reviewed elsewhere. Also, the effectiveness of the use of decision aids among people facing treatment or screening decisions has been reviewed elsewhere (Stacey 2017). The focus of the Stacey 2017 Cochrane review was on people's decision‐making processes, behaviour and health, and on outcomes related to health care system cost, use. The studies included in this decision aids review most likely did not address outcomes directly related to changing professional practice and would therefore not be eligible for inclusion in our review.
In this review we focus specifically on the effects of using patient involvement as a means to improving healthcare professionals' performance. This can be done through interactions with patients, or information provided by or to patients. Examples of such interventions include:
patient‐reported health information where patients provide information about their own health, concerns, or needs before a clinical encounter;
patient information where patients are informed about recommended care;
patient education/training/counselling to increase patients' knowledge about their condition;
patient decision aids to ensure that the choices about treatment and management reflect recommended care and the patients' values and preferences;
patient feedback about clinical practice;
patients being members of committees or boards of healthcare organisations;
patient‐led training or education of healthcare professionals.
We have used adherence to clinical practice guidelines and recommendations as a measure for quality of professional practice, as is commonly done, for example in Cochrane reviews of interventions to improve healthcare worker performance (Arditi 2017; Flodgren 2011a; Flodgren 2011b; Flodgren 2016; Forsetlund 2009; Giguère 2012; O'Brien 2007; Tzortziou Brown 2016). It is worth noting that adherence to guidelines is not necessarily what a patient wants. A patient‐mediated intervention could therefore improve professional practice without improving shared decision‐making, and vice versa. Still, it seems reasonable to assume that most recommended clinical practices are in the best interest of the patient, and therefore also in line with the care most patients would want.
The importance of patient involvement at all levels of healthcare services is widely recognised. Patients are, in general, positive to engaging in improving the quality of the care they receive (Schwappach 2010a). Also, patient information materials developed in collaboration with patients is probably more relevant, readable, understandable, and effective in improving knowledge among patients (Nilsen 2006).
On the other hand, concerns have been raised about how patient involvement can affect patients' trust in healthcare professionals and their experience of receiving healthcare (Hrisos 2013; Luszczynska 2007; McGunkin 2006). In addition, patients' comfort level with active involvement may vary considerably, as some might feel that they can appear rude or disrespectful and that this may upset the healthcare professional and, consequently, might compromise their healthcare (Hrisos 2013). Patients may also find it hard to overcome distrust if the independence, agency, or expertise of healthcare professionals is questioned (Plomp 2010).
The patient's socioeconomic status has been shown to correlate with the degree of involvement in treatment decisions (Willems 2005). Patients from higher social classes may get more information from their healthcare professionals because they often communicate more actively (they ask more questions and are more opinionated) and show more affective expressiveness (Willems 2005).
Most healthcare professionals, like patients, welcome patient involvement to improve healthcare safety (Davis 2012a; Davis 2012b; Hrisos 2013; Schwappach 2010b; Schwappach 2011; Schwappach 2013). When patients question or challenge healthcare professionals' practice, however, the healthcare professionals' morale and professional integrity may suffer negative consequences (Hrisos 2013; Schwappach 2010b). Thus, in some situations or cases, the unwanted consequences of patient‐mediated interventions may negatively affect both the patient and the healthcare professional and, thus, the patient‐healthcare professional relationship.
To avoid tensions between healthcare professionals and patients, a conceptual common ground or consensus on how to set treatment and management goals has been recommended (Sugavanam 2013). Collaboration and communication are important factors and communication in the form of discussions may also lead to more reciprocal, trustful relationships and more open information exchanges (Skirbekk 2011).
How the intervention might work
Despite being regarded as a promising approach for improving healthcare systems and and being the focus of research, the theoretical foundation for patient‐mediated interventions seems meagre. Very few, if any, of the studies to evaluate the effectiveness of such interventions have reported use of theory in the development and design of the intervention (Gagliardi 2016; Ng 2017). Still, if healthcare professionals are well‐informed about recommended clinical practices through patients or patients' representatives, or if patients are empowered to ask for appropriate health care, it seems reasonable to believe that this can influence professional practice. Table 5 shows examples of patient‐mediated interventions, how they might influence healthcare workers' behaviour, and possible adverse effects. In Figure 1, we present a summary of various types of patient‐mediated interventions and indicate two mechanisms through which they can improve patient outcomes: directly, and indirectly through improving the care provided by health professionals. This review focuses on the latter mechanism.
1. Examples of patient‐mediated interventions.
| Examples of different types of patient‐mediated interventions | An example | Possible mechanisms of action | How it might have positive effects | How it might have adverse effects |
| Patient‐reported health information about own health/needs/concerns or other relevant outcomes (collecting information from patients and giving it to professionals before, or during a clinical encounter) |
The patient or carer completes a questionnaire or form in the waiting area before a consultation. The doctor is then given this information before or during the consultation. | Information to healthcare professionals from patients → clinical encounter → impact on healthcare professionals' performance | Information from patients about own health/needs/concerns might ensure that professionals get important information that they might otherwise not have received. This information might prompt professionals to improve their practice and provide recommended health care. | This might distract healthcare professionals from focusing on other things or lead to longer consultations without measurable improvements in the quality of care, if the information that is collected turns out not to be important. |
| Patient information where patients are informed about recommended care | The patient is given a brochure with information about cancer screening. | Information to patient from others → clinical encounter → impact on healthcare professionals' performance | Giving recommendations or evidence to patients might lead them to ask for recommended care, and professionals might respond by providing it. | Healthcare professionals might feel threatened by this or disagree with the information given to patients. Patients might become distrustful of the healthcare professionals. |
| Patient education/ training/ counselling to increase patients' knowledge about their condition | The patient signs up for a group‐based self‐management program where she is provided with information about her condition and becomes part of a patient group for sharing of experiences to increase self‐efficacy and coping. | Activation of patient by others → clinical encounter → impact on healthcare professionals' performance | Education/training/counselling to increase patients' knowledge about their condition, which can increasing their self‐efficacy and self‐care skills. This in turn, might encourage patients to get more involved in decisions about their treatment and management and professionals might respond by providing recommended health care. | Healthcare professionals might feel threatened by this or disagree with the patient. It might increase healthcare professionals' burden if they need to spend more time finding answers to patients' questions. Patients might feel more uncomfortable if they have more questions but do not feel comfortable asking them. Patients might not like the answers they are given. This might lead to longer consultations without measurable improvements in the quality of care. |
| Patient feedback about clinical practice (collecting information from patients after an encounter) |
After the patient has used a healthcare service, she might be asked about her experience with the service or doctor. This information is then fed back to the doctors and/or hospital. | Information to healthcare professionals from patients → impact on healthcare professionals' performance | Clinical performance feedback from patients might ensure that professionals get important information that they might otherwise not have received. This information might prompt professionals to improve their practice and provide recommended health care. | This might distract healthcare professionals from focusing on other things or lead to longer consultations without measurable improvements in the quality of care, if the information that is collected turns out not to be important. |
| Patient decision aids to ensure that the choices about treatment and management reflect recommended care and the patients' values and preferences | The patient is provided with information about treatment options including risks and benefits. The patient considers this information, either alone or with a healthcare professional, to reach a decision in accordance with her values and preferences. | Activation of patient by others → clinical encounter → impact on healthcare professionals' performance | Giving recommendations or evidence to patients and encouraging them to engage with their own values and preferences for treatment options might encourage healthcare professionals to provide recommended health care. | Healthcare professionals might feel threatened by this or disagree with the patient. It might increase healthcare professionals' burden if they need to spend more time finding answers to patients' questions. Patients might feel more uncomfortable if they have more questions but do not feel comfortable asking them. Patients might not like the answers they are given. This might lead to longer consultations without measurable improvements in the quality of care. |
| Patients, or patient representatives, being members of a committee or board | A patient representative from a patient organisation is, on behalf of a patient group, part of a hospital board. The board may discuss patient care and make decisions about professional practice within the hospital. | Information to healthcare professionals from patients → committee or board meeting→ impact on healthcare professionals' performance | Patients being part of a prioritisation or agenda deciding process at the health system level might influence professional practice and result in giving patients the recommended health care | Healthcare professionals on the committee or board might feel threatened by this or disagree with the patients' prioritisation or decisions. This might in turn, lead to poor implementation of recommendations or guidelines made within this format. |
| Patient‐led training or education of healthcare professionals | Patients taking part in training of doctors, e.g. to improve communication skills, how to perform physical examinations or the importance of certain clinical procedures. | Information and/or activation of healthcare professionals by patients → impact on healthcare professionals' performance | Patients being part of the education or training of healthcare professional might influence professional practice and result in providing recommended health care | Healthcare professionals might feel threatened by this or disagree with the patient trainer or educator. This might result in non‐adherence to the care recommended in this training or education. |
1.
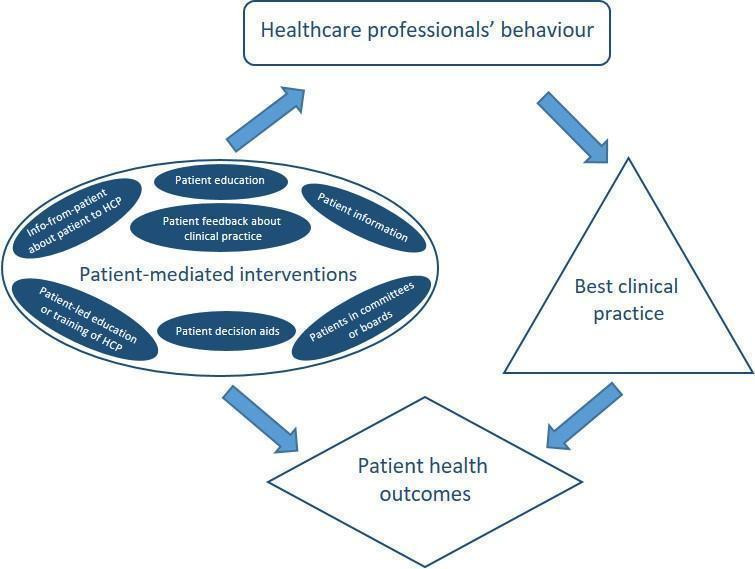
Summary figure of different examples of patient‐mediated interventions and proposal of where within the healthcare system the direct interaction may take place.
Why it is important to do this review
Allthough many systematic reviews exist that have assessed the effect of different patient involvement or patient‐directed interventions, these have mainly focused on patient outcomes, such as satisfaction, well‐being, and health. For example, there are series of Cochrane reviews on patient education/self‐management programs for various conditions, including musculoskeletal‐related conditions (Kroon 2014; Parreira 2017; Poquet 2016), lung‐related conditions (Kelly 2018; Lenferink 2017; McCallum 2017; Peytremann‐Bridevaux 2015; Zwerink 2014), stroke (Fryer 2016), heart‐related conditions (Anderson 2017; Clarkesmith 2017), diabetes type 2 (Attridge 2014; McBain 2016), and cancer‐related conditions (Bennett 2016). The purpose of our review, however, is to assess the effect patients can have on healthcare professionals' performance. Similiarly, there are Cochrane reviews on interventions to promote shared decision‐making (Légaré 2014) and a patient‐centred approach (Dwamena 2012), but these have not focused on the effects on professional practice, i.e. adherence to clinical practice guidelines or recommendations.
Previous systematic reviews have covered patient‐mediated interventions as one of a wide range of interventions aimed at improving professional practice (Davis 1995; Grimshaw 2004; Oxman 1995). Some studies have found mixed effects on professional practice for patient‐mediated interventions (Davis 1995; Oxman 1995), while others have reported moderate to large effects (Grimshaw 2004). The certainty of the evidence in these systematic reviews varies, but is generally low, making it impossible to draw firm conclusions about the effectiveness of these interventions. It is important to do this review as there are, to our knowledge, no recently updated systematic reviews that have assessed the effectiveness of patient‐mediated interventions on healthcare professionals' practice.
Objectives
To assess the effects of patient‐mediated interventions on healthcare professionals' clinical performance (adherence to clinical practice guidelines or recommendations).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised and cluster‐randomised studies comparing a patient‐mediated intervention to no intervention, usual care or other interventions to improve professional practice.
We included full‐text studies, conference abstracts, and unpublished data.
Types of participants
We included practicing healthcare professionals and those in postgraduate training responsible for patient care. We excluded undergraduate students or non‐professional (lay) healthcare workers.
Types of interventions
Types of interventions included
Interventions aimed at changing the performance of healthcare professionals through interactions with patients, or information provided by or to patients, including:
patient‐reported health information where patients provide information about their own health, concerns, or needs before a clinical encounter;
patient information where patients are informed about recommended care;
patient education/training/counselling to increase patients' knowledge about their condition;
patient feedback about clinical practice;
patient decision aids to ensure that the choices about treatment and management reflect recommended care and the patients' values and preferences;
patients being members of committees or boards;
patient‐led training or education of healthcare professionals.
See Table 5 for more detailed information and examples.
We excluded studies where patient‐mediated intervention was a small component in a multi‐component package. We also excluded studies that did not include authentic patients (such as studies including standardised or simulated patients).
Types of comparisons included
We included studies where patient‐mediated interventions were compared with common practice/usual care, or any other intervention to improve professional practice (including comparisons of different types of patient‐mediated interventions).
Types of outcome measures
Primary outcomes
Adherence to recommended clinical practice or clinical practice guidelines by healthcare professionals.
Secondary outcomes
We only included studies that reported relevant primary outcomes. Thus, we extracted secondary outcomes from studies that also reported on adherence to recommended clinical practice or clinical practice guidelines.
-
Patient outcomes
health outcomes
satisfaction with the care they receive
acceptance, confidence in, or satisfaction with the intervention
experiences/perceptions of healthcare professionals' acceptance, confidence in or satisfaction with the intervention
-
Healthcare professional outcomes
satisfaction with the care they provide
acceptance, confidence in or satisfaction with the intervention
We also included data on resource use, adverse events and issues of equity in the included studies.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for primary studies without any language or time limits.
The Cochrane Central Register of Controlled Trials (CENTRAL), part of the Cochrane Library (www.cochranelibrary.com) (searched March 10, 2017)
MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to August 24, 2018, Ovid (searched August 28, 2018 with time limit up to March 10, 2018)
We tested whether or not to search Embase, using the phrase 'patient mediated' in title and abstract. We screened all records that were unique to Embase, found none to be eligible and therefore omitted Embase from our search.
See Appendix 1 for all strategies used, including the MEDLINE strategy, which was peer reviewed using the Peer Review of Electronic Search Strategies (PRESS) checklist (Sampson 2008).
Searching other resources
Grey literature (searched October 2017)
Open Grey (www.opengrey.eu)
Grey Literature Report (www.greylit.org)
Google Scholar (scholar.google.com)
Trial registries (searched September 2017)
International Clinical Trials Registry Platform (ICTRP), Word Health Organization (WHO) (www.who.int/ictrp)
ClinicalTrials.gov, US National Institutes of Health (NIH) (clinicaltrials.gov)
We also:
screened the reference lists of all included studies for relevant studies;
conducted cited reference searches for all included studies using Web of Science, Clarivate Analytics (searched October 2017).
An Information Specialist (MJ) and a review author (MSF) carried out the searches.
Data collection and analysis
Selection of studies
Two review authors (MSF and TKD) screened titles and abstracts independently to assess which studies met the inclusion criteria. We retrieved full‐text copies of all papers that were potentially relevant, including those where the description of the population, intervention, comparison or outcomes was insufficient in the abstract to make a decision about inclusion. Review authors MSF and TKD independently assessed the full‐text copies of the papers for relevance. We resolved any disagreements by discussion and consensus with a third review author (AF). We kept a log of the selection process to complete a PRISMA flow diagram (Moher 2009) using Covidence (Covidence) (see Figure 2). We described studies that initially appeared to meet the inclusion criteria but later were excluded, including the reasons for exclusion, in the Characteristics of included studies table.
2.
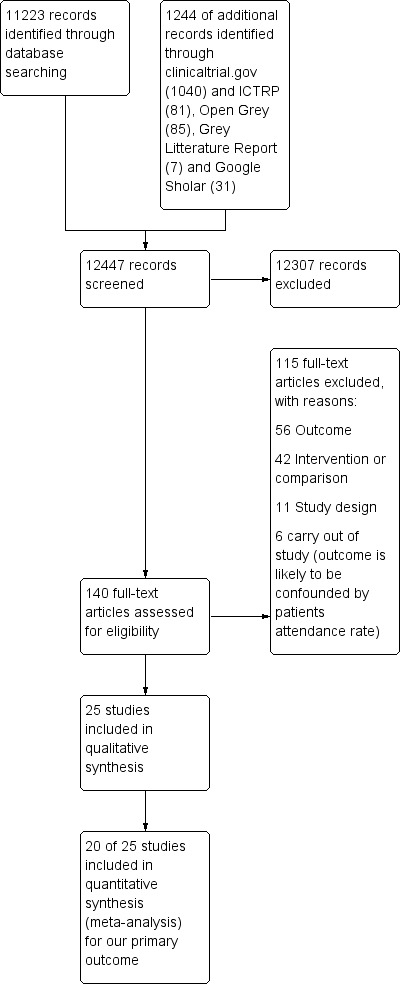
Study flow diagram.
Data extraction and management
Review authors MSF and TKD independently extracted data from each included study using a modified version of the EPOC Data Collection Checklist (EPOC 2017a). We resolved any disagreements by discussion and by consensus. When needed, a third review author (AF) was consulted. Missing or unclear data from a published study were marked clearly on the data collection form. Missing or unclear data were sought from the corresponding author of a published paper.
Assessment of risk of bias in included studies
Review authors MSF and TKD independently assessed the risk of bias in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and in line with the Cochrane Effective Practice and Organisation of Care Group suggested risk of bias criteria (EPOC 2017b). We resolved any discrepancies through discussion.
We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other biases (for cluster‐randomised studies, we judged five additional sources of potential biases under "other biases").
We judged each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and report any deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
For the dichotomous outcomes, we analysed data based on the number of events and the number of people or cases assessed in the intervention and comparison groups. We used these to calculate the risk ratio (RR) with 95% confidence interval (CI). For continuous outcomes, we analysed the data based on the mean, standard deviation (SD) and number of people assessed for both the intervention and comparison groups to calculate mean difference (MD) and 95% CI.
All relevant outcomes reported in the studies were collected along with data on how they were measured (self‐report, medical record, other objective primary or secondary outcome). For all relevant primary and secondary outcomes, we extracted the intervention effect estimates with relevant CIs, and the method of statistical analysis used to calculate it, as reported by the authors of the study. We extracted data from all time points and categorised them into one of three follow‐up time intervals (0 to 3 months, more than 3 months to 12 months, more than 12 months). Studies reporting one outcome in multiple follow‐up intervals were only reported once in our meta‐analyses, with the longest follow‐up. Alos, if a study reported multiple data within one interval, we used the data with the longest follow‐up within that interval.
When the same study reported more than one relevant primary outcome (adherence outcome), we used the primary outcome as defined by the study authors. If a primary outcome was not clearly defined or multiple outcomes were defined as primary or secondary outcomes, we calculated and used the median value from all relevant primary outcomes. When calculating the median from even numbers of outcomes, we chose the outcome with reporting from the most participants. In cases where the number of participants contributing to the outcome was the same, we randomly selected the outcome (flipping coin).
Unit of analysis issues
We found eligible studies with cluster designs (studies in which the unit of allocation is not a person, but a group of people for instance in a clinic). Studies in which comparisons are allocated as groups of people should account for clustering in their analysis. Standard statistical methods assume independence of observation, and for cluster‐design studies the use of these will generally result in artificially small P values and overly narrow CIs for the effect estimates (Ukoumunne 1999), if analysed at the individual level rather than at the cluster level.
We re‐analysed studies with potential unit of analysis errors by using the information on the size number of clusters and the value of the intra‐cluster correlation coefficient (ICC). If no ICC was reported, we used the median ICC value from similar studies found in the University of Edinburgh's Database of ICCs (ABDN 2015). We used the following formula, as suggested by Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): n patients / (1 + ICC (average cluster size ‐1)).
Dealing with missing data
We attempted to contact study authors in order to verify key study characteristics and to obtain missing numerical outcome data where possible. In cases where this was unsuccessful, we have reported the data as 'not reported' and have not attempted to impute the missing values. The potential impact of the missing data is explored in the 'Assessment of risk of bias' section of the review.
Assessment of heterogeneity
By examining study populations, interventions and outcomes, we considered if the studies were similar enough to be pooled in a meta‐analysis. We assessed the degree of statistical heterogeneity by visual examination of the scatter of effect estimates on forest plots and by using the Chi2 and I2 statistics (Higgins 2003).
Assessment of reporting biases
The tendency for inconclusive results to remain unpublished may impact the findings of a systematic review. We attempted to obtain study protocols to assess selective outcome reporting. Another important factor that might introduce biases is the small‐study effects. We planned to use funnel plots to assess small‐study effects for 10 or more studies investigating a particular outcome according to Egger 1997 (for continuous outcomes) and Harbord 2006 (for dichotomous outcomes). A funnel plot was created for the patient information comparison which had 12 studies in the meta‐analysis Figure 3. Even though we did not find clear evidence for a publication bias, we cannot rule out the possibility. Also, we failed to find more studies with few participants and negative effect estimates, and we should therefore be cautious when we interpret that we have little to indicate a potential publication bias in our result.
3.
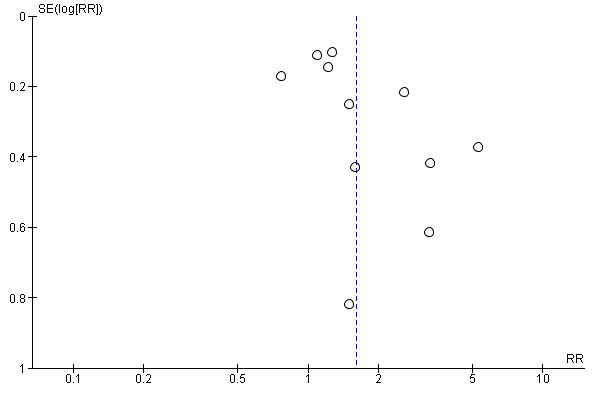
Funnel plot of comparison: 2 Patient information interventions versus comparisons, outcome: 2.1 Adherence to recommended practice.
Data synthesis
We grouped patient‐mediated interventions according to the six categories listed under Types of interventions, and categorised the interventions of the included studies accordingly. We then prepared tables summarising the findings of studies for each type of relevant primary and secondary outcome.
We prepared separate meta‐analyses for each type of intervention and visualised the different types of comparisons in the forest plot.
We carried out the meta‐analyses by using Review Manager 5 (RevMan 2014). We used random‐effects meta‐analysis for combining data, as we anticipated that there may be natural heterogeneity between studies attributable to the variation across similar interventions, populations and implementation strategies. For continuous variables, we used the inverse‐variance method while for dichotomous variables we used the method proposed by Mantel‐Haenszel.
For the included studies with three or more arms, we only extracted data from the two most relevant comparisons for our question.
Summary of findings
We summarised the findings of the different types of patient‐mediated interventions for the following outcomes in 'Summary of findings' tables.
Adherence to recommended clinical practice or clinical practice guidelines by healthcare professionals
Patient health outcomes (desirable and undesirable health outcomes)
Patients' satisfaction with the care they receive
Adverse events
Resource use
Two review authors (MSF and TKD) independently assessed the certainty of the evidence (high, moderate, low, and very low) using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias). We used methods and recommendations described in Section 8.5 and Chapter 12 of Higgins 2011 and the EPOC worksheets (EPOC 2017c), using GRADEpro software (GRADEpro GDT 2015). We resolved disagreements on certainty ratings by discussion and consulted a third review author (AF) when disagreement persisted. Our decisions to down‐ or upgrade are presented in footnotes in the tables. We used plain language statements to report these findings in the review (EPOC 2017d).
Subgroup analysis and investigation of heterogeneity
We assessed heterogeneity between studies by visually inspecting forest plots and, if possible, by performing subgroup analyses (see below). Since the importance of inconsistency depends on several factors, we used the guide to interpret heterogeneity as outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; and 75% to 100% would be considerable heterogeneity.
When the effect estimates varied considerably across studies of similar types of patient‐mediated interventions, we explored whether the following factors could explain the observed variation.
Direction of change required (increase current behaviour, decrease current behaviour, mix, or unclear). Hypothesis: effect on increasing a behaviour is larger than that on decreasing behaviour.
Recipient (physician; other healthcare professionals). Hypothesis: clinical practice is more difficult to change among physicians than among non‐physicians.
Risk of bias (high; unclear; low). Hypothesis: effect sizes are smaller when risk of bias is low.
Baseline clinical performance (continuous measure of healthcare professionals' compliance with recommended clinical practice or clinical guidelines). Hypothesis: when baseline clinical performance is low, effect sizes are larger.
Sensitivity analysis
We did not perform any sensitivity analysis.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies.
Results of the search
We identified a total of 12,247 records from the electronic and supplementary searches (11,003 from electronic database searching and 1244 of additional records identified through clinicaltrial.gov (1040) and ICTRP (81), Open Grey (85), Grey Litterature Report (7) and Google Sholar (31)) Figure 2. Two review authors (MSF and TKD) independently screened 12,247 titles and abstracts and found 12,107 records to be irrelevant and these were directly excluded. Full‐text publications were retrieved for 139 of the 140 potential relevant studies. For one study we only had information presented in an abstract (Caskey 2011). We included 25 studies (Alder 2005; Aragones 2010; Brody 1990; Caskey 2011; Christy 2013; Goldberg 2012; Herman 1995; Jacobson 1999; Kattan 2006; Kenealy 2005; Khan 2011; Kravitz 2012; Krol 2004; Leveille 2009; Mazonson 1996; McAlister 2005; McKinstry 2006; Miaskowski 2004; Mouland 1997; Nagykaldi 2012; Quinn 2008; Thiboutot 2013; Thomas 2003; Turner 1990; Wright 2012). We also identified two ongoing studies (NCT01904656; NCT02686775).
Included studies
The 25 included studies are described in detail in the Characteristics of included studies.
Study design
Fifteen studies were randomised at the individual level. Twelve of these studies had the patient as the unit of randomisation (Alder 2005; Christy 2013; Jacobson 1999; Kattan 2006; Khan 2011; Kravitz 2012; Leveille 2009; McKinstry 2006; Miaskowski 2004; Mouland 1997; Quinn 2008; Thomas 2003), and three had the healthcare professional as the unit (Aragones 2010; Goldberg 2012; Turner 1990). Ten studies were cluster‐randomised studies. Among the cluster‐randomised studies, five had the healthcare professional as the unit of randomisation (Caskey 2011; Kenealy 2005; Krol 2004; Mazonson 1996; Thiboutot 2013), and five had the healthcare practice as the unit of randomisation (Brody 1990; Herman 1995; McAlister 2005; Nagykaldi 2012; Wright 2012). Cluster‐randomisation may lead to misleading findings unless the results are adjusted for clustering effects. The idea is to reduce the size of each trial to its ‘effective sample size’ to prevent artificially small P values. To prevent this 'unit of analysis error' caused by clustering, we re‐analysed the studies included in our meta‐analyses by using the information on the number of clusters and the assumed value of the intra‐cluster correlation coefficient (ICC). We have analysed the impact of clustering effects among all the ten cluster‐randomised studies. For the five studies in which healthcare professionals were the unit of randomisation (Caskey 2011; Kenealy 2005; Krol 2004; Mazonson 1996; Thiboutot 2013), the median ICC among similar studies for our primary outcome was 0.000 (95% CI; 0, 0.142) according to the University of Edinburgh's Database of ICCs (ABDN 2015). The effective sample sizes of these studies were thus the same as reported by the study authors. The effective sample size for the five studies in which the healthcare practice was the unit of randomisation (Brody 1990; Herman 1995; McAlister 2005; Nagykaldi 2012; Wright 2012), the median ICC among similar studies for our primary outcome in the University of Edinburgh's Database of ICCs (ABDN 2015) was 0.076 (95% CI, 0, 0.219). We did not attempt to re‐analyse studies that were not pooled in a meta‐analysis (Brody 1990; Nagykaldi 2012). The effective total sample sizes for the three cluster‐randomised studies included in our meta‐analyses (Herman 1995; McAlister 2005; Wright 2012) were calculated and are listed in Table 6.
2. Descriptive reporting of all relevant primary outcomes from included studies.
| Study | Primary outcomes | Findings |
| Alder 2005 | Antibiotic prescriptions (Recommended clinical practice is less antibiotic prescriptions to children with ear‐nose‐throat infections) |
Author’s quote: “A significant protective effect is demonstrated for the SCT‐based communication intervention (OR = 0.171, p = 0.042)” N= 40 (20 patients in each comparison group). |
| Aragones 2010 | Physician recommendation of colorectal cancer screening (Recommended clinical practice is to increase screening) |
Intervention: 19/31 (61.3%) Comparison: 14/34 (41.2%) Outcome also included in meta‐analysis |
| Brody 1990 | Number of counselling items done by healthcare professional (Desired practice is more counselling of people with mental problems) |
Patient‐reported Intervention: 2.8 (se=1.62), N= 29 Comparison: 2.9 (se=1.41), N= 50 Healthcare professional reported Intervention: 2.8 (se=1.62), N= 29 Comparison: 2.9 (se=1.41), N= 50 ** did not attempt to accounting for clustering because the study was not pooled in a meta‐analysis |
| Caskey 2011 | Pertussis (Tdap) vaccination (Desired practice is to increase vaccination) |
Intervention: 89/687 (13%) Comparison: 76/715 (10.6%) Outcome also included in meta‐analysis ** with accounting for clustering (ICC = 0.000), the effective total sample size remained the same |
| Christy 2013 | 1. Primary care provider write an order for a colorectal cancer screening test 2. Doctor recommended fecal occult blood test (FOBT) 3. Doctor recommended colonoscopy (Desired practice is to increase screening) |
1. Doctor recommendation of FOBT: OR=1.15 (95% CI: 0.81, 1.63), p=0.420 N= 659 (intervention: 319 and comparison: 340) 2. Doctor recommendation of colonoscopy: OR=1.34 (95% CI: 0.93, 1.92), p= 0.114 N= 659 (intervention: 319 and comparison: 340) 3. Authors quote: “PCPs of those who received the computer‐delivered tailored intervention were more likely to write orders for a CRC screening test (OR=1.48; 95% CI=[1.11, 1.96]; p‐value=0.007).” |
| Goldberg 2012 | 1. Correctly identified level of chronic asthma control 2. Correctly identified child’s asthma trajectory 3. Correctly identified level of medication adherence 4. Correctly identified degree of disease burden to the family (Desired practice is more accurate identification of asthma morbidity) |
1. Intervention: 17/40 (43%) Comparison: 7/37 (19%) 2.* Intervention: 29/40 (72%) Comparison: 17/37 (45%) 3. Intervention: 29/40 (72%) Comparison: 18/37 (48%) 4. Intervention: 30/40 (74%) Comparison: 13/37 (35%) * outcome also included in meta‐analysis (median outcome) |
| Herman 1995 | 1. Number of women offered mammogram 2. Number of women offered clinical breast exam 3. Number of women offered mammogram among those not previously having a mammogram 4. Number of women with a documented clinical breast exam among those not previously having a clinical breast exam (Desired practice is to increase preventive services) |
1. Intervention: 28.4%, N=not reported Comparison: 19.4%, N=not reported 2. Intervention:25%, N=not reported Comparison: 17.9%, N=not reported 3. Intervention: 50/159 (31.4%) Comparison: 29/161 (18%) 4.* Intervention: 40/183 (21.9%) **3/13 when adjusted for clustering Comparison: 34/192 (17.9%) **2/13 when adjusted for clustering * outcome also included in meta‐analysis (median outcome of 3 and 4). ** with accounting for clustering (ICC=0.076), the effective total sample size was 39 patients (13 patients to each group, if evenly distributed between 3 arms). |
| Jacobson 1999 | 1. Clinician recommended vaccine 2. Administration of the vaccine at that clinic visit (Desired practice is to increase vaccination) |
1. Intervention: 60/221 (27.1%) Comparison: 13/212 (6.1%) 2.* Intervention: 44/221 (19.9%) Comparison: 8/212 (3.8%) * outcome also included in meta‐analysis (primary outcome defined by study author) |
| Kattan 2006 | Change in medication when indicated by NAEPP guideline recommended practice (Change according to recommended clinical practice) |
Intervention: 105 persons stepped up per 1332 step‐up letters* sent to providers Comparison: 49 persons stepped up per 1117 “non‐sent potential” step‐up letters* sent to providers *identified cases in need of stepping up medication (referred to as step‐up letters that could have been sent 1‐6 times per patient that needed step‐up) |
| Kenealy 2005 | Diabetes screening of eligible patients who visited a family practitioner (Recommended clinical practice is to increase screening of eligible people) |
Intervention: 392/1639 (23.9%) Comparison: 240/1550 (15.5%) Outcome also included in meta‐analysis ** with accounting for clustering (ICC = 0.000), the effective total sample size remained the same |
| Khan 2011 | 1. Diabetes medication prescriptions 2. Hypertension medications (Desired practice is intensification of diabetes therapy) |
1.* Intervention: 51/53 (96.2%) Comparison: 35/47 (74.5%) 2. Intervention: 43/53 (81.1%) Comparison: 30/47 (63.8%) * outcome also included in meta‐analysis (median outcome) |
| Kravitz 2012 | Physician‐directed adjustment in analgesia | Intervention: 75/125 (60%) Comparison: 48/132 (36.4%) Outcome also included in meta‐analysis |
| Krol 2004 | 1. Stopped or reduced PPI dose 2. Stopped prescribed PPI 3. Had increased PPI dose (Desired practice is reduction in PPI medication) |
1.* Intervention: 12/54 (22.2%) Comparison: 3/44 (6.8%) 2. Intervention: 7/54 (13%) Comparison: 2/44 (4.5%) 3. Intervention: 3/54 (5.6%) Comparison: 6/44 (13.6%) * outcome also included in meta‐analysis (primary outcome defined by study author) ** with accounting for clustering (ICC = 0.000), the effective total sample size remained the same |
| Leveille 2009 | Screened condition identified at the index visit (Desired practice is to increase identification of mental problems) |
Intervention: 69/115 (60%) Comparison: 65/118 (55.1%) Outcome also included in meta‐analysis |
| Mazonson 1996 | Recognition of mental health problems (Desired practice is to increase identification of mental problems) |
Intervention: 114/357 (31.9%) Comparison: 40/216 (18.5%) Outcome also included in meta‐analysis ** with accounting for clustering (ICC = 0.000), the effective total sample size remained the same |
| McAlister 2005 | 1. The proportion of patients whose therapy met the ACCP treatment recommendations – at 3 months 2. The proportion of patients whose therapy met the ACCP treatment recommendations – at 12 months |
1. Intervention: 89/219 (40.6%) Comparison: 79/215 (36.7%) 2.* Intervention: 70/219 (32%) Comparison: 80/215 (37.4%) * outcome also included in meta‐analysis (secondary outcome defined by study authors, but we predefined in our protocol that we would choose the outcome with the longest follow‐up as our primary outcome.) ** with accounting for clustering (ICC = 0.076), the effective total sample size was 353 patients (178 patients in intervention group and 175 patients in comparison group). |
| McKinstry 2006 | 1. Proportion of patients prescribed statins according to guideline 2. Proportion of patients prescribed aspirin according to guideline (Recommended clinical practice is adherence to hypertension treatment Guidelines) |
1.* Intervention: 39/134 (29%) Comparison: 54/142 (38%) 2. Intervention: 53/88 (60%) Comparison: 55/95 (58%) * outcome also included in meta‐analysis (median outcome) |
| Miaskowski 2004 | Appropriate analgesic prescription (around the clock plus as needed) | Intervention: 34/92 (37%) Comparison: 26/80 (32.5%) Outcome also included in meta‐analysis |
| Mouland 1997 | 1. No benzodiazepines prescription 2. 50‐90% reduction in benzodiazepines prescriptions 3. 0‐49% reduction in benzodiazepines prescriptions 4. Increase in benzodiazepines prescriptions 5. Average prescriptions of benzodiazepines (defined daily doses) (Recommended clinical practice is less benzodiazepines prescriptions in mental health) |
1.* Intervention: 29/92 (32%) Comparison: 6/63 (10%) 2. Intervention: Approximately 25%, N=92 Comparison: Approximately 22%, N=63 3. Intervention: Approximately 36%, N=92 Comparison: Approximately 47%, N=63 4. Intervention: Approximately 8%, N=92 Comparison: Approximately 20%, N=63 5. Intervention: Before: 24.63 DDD/month (range 5‐80). After: 12.40 DDD/ month (range 0‐70), N=92 Comparison: Before: 29.02 ODD/ month (range 4‐108). After: 22.39 DDD/ month (range 0 ‐ 102), N=63 * outcome also included in meta‐analysis (the only relevant outcome reported dichotomously with complete numbers) |
| Nagykaldi 2012 | 1. Adults provided all recommended preventive services 2. Adults given low dose aspirin, if indicated 3. Adults given Pneumococcal vaccination because of chronic health conditions 4. Adults given Pneumococcal vaccination because of chronic health conditions 5. Children given all recommended immunizations (Desired practice is increased coverage of preventive services) |
1. Intervention: 84.4%, N=not reported Comparison: 67.6%, N=not reported 2. Intervention: 78.6%, N=not reported Comparison: 52.3%, N=not reported 3. Intervention: 82.5%, N=not reported Comparison: 53.9%, N=not reported 4. Intervention: 86.3%, N=not reported Comparison: 44.6%, N=not reported 5. Intervention: 95.5%, N=not reported Comparison: 87.2%, N=not reported ** did not attempt to accounting for clustering because the study was not pooled in a meta‐analysis |
| Quinn 2008 | 1. Medications titrated or changed by their healthcare professional 2. Medication errors identified by their healthcare professional (Desired practice is to follow prescribing guidelines) |
1.* Intervention: 11/13 (84.6%) Comparison: 3/63 (23.1%) 2. Intervention: 7/13 (53.4%) Comparison: 0/13 (0%) * outcome also included in meta‐analysis (median outcome) |
| Thiboutot 2013 | 1. Perform serum creatinine tests 2. Perform urine protein tests 3. Perform serum potassium tests 4. Doctor recommended starting a new blood pressure medication 5. Doctor recommended increasing dose of a blood pressure medication (Desired practice is medication intensification among patients whose blood pressure was not at target) |
1. Intervention: 211/282 (74.8%) Comparison: 156/218 (71.6%) 2.* Intervention: 86/282 (30.5%) Comparison: 58/218 (26.6%) 3. Intervention: 209/282 (74.1%) Comparison: 153/218 (70.2%) 4. Intervention: 21/179 (11.7%) Comparison: 13/149 (8.7%) 5. Intervention: 18/168 (10.7%) Comparison: 13/144 (9%) * outcome also included in meta‐analysis (median outcome) ** with accounting for clustering (ICC = 0.000), the effective total sample size remained the same |
| Thomas 2003 | Primary care physician recommended vaccine (Recommended clinical practice is to increase vaccination) |
Intervention: 64/189 (33.9%) Comparison: 24/182 (13.2%) Outcome also included in meta‐analysis |
| Turner 1990 | 1. Perform pap‐smear 2. Perform breast exam 3. Schedule mammography 4. Stool occult test 5. Give influenza vaccine 6. Give pneumococcal vaccine (Recommended clinical practice is to increase vaccination) |
1. Intervention: 28/94 indicated (29.8%) Comparison: 30/151 indicated (19.9%) 2. Intervention: 44/84 indicated (52.4%) Comparison: 58/118 indicated (49.2%) 3. Intervention: 18/147 indicated (12.2%) Comparison: 25/130 indicated (19.2%) 4.* Intervention: 86/132 indicated (65.2%) Comparison: 91/196 indicated (46.4%) 5. Intervention: 59/86 indicated (68.6%) Comparison: 51/177 indicated (28.8%) 6. Intervention: 19/86 indicated (22.1%) Comparison: 29/118 indicated (24.6%) * outcome also included in meta‐analysis (median outcome) |
| Wright 2012 | 1. Give influenza vaccines 2. Perform mammography 3. Perform pap smears 4. Give pneumococcal vaccine 5. Test bone density 6. Test cholesterol (Recommended clinical practice is to increase vaccination) |
1.* Intervention: 50/227 (22%) Comparison: 40/285 (14%) 2. Intervention: 51/105 (48.6%) Comparison: 28/95 (29.5%) 3. Intervention: 25/61 (41%) Comparison: 7/67 (10.4%) 4. Intervention: 11/86 (12.8%) Comparison: 10/113 (8.9%) 5. Intervention: 2/24 (8.3%) Comparison: 3/132 (2.3%) 6. Intervention: 20/43 (46.5%) Comparison: 14/48 (29.2%) * outcome also included in meta‐analysis (median outcome) ** with accounting for clustering (ICC = 0.076), the effective total sample size was 102 patients (45 patients in intervention group and 57 patients in comparison group). |
Most of the studies had two comparison arms, except for Brody 1990, Herman 1995 and Thomas 2003, which had three arms, and Alder 2005 and Kenealy 2005, which had four arms. We selected and analysed data from two relevant arms per study (see Characteristics of included studies for description).
Population/participants
Patients
The total number of patients included in the studies of this review was 12,268 (the total number of patients would be 16,700 if we had included all comparison arms in the studies). The included sample size varied from 40 participants (Alder 2005) to 3189 (Kenealy 2005). The number of patients contributing to our meta‐analyses for the primary outcome is 8749. Ten studies were on preventive care with a general patient population (Caskey 2011; Nagykaldi 2012; Turner 1990; Wright 2012) or an 'at risk' patient population (Aragones 2010; Christy 2013; Herman 1995; Jacobson 1999; Kenealy 2005; Thomas 2003), of which all except one study (Jacobson 1999) defined risk based on an age‐threshold, often 50 years or older. One study, which was on vaccination, defined 'at risk' as having a chronic condition. The preventive service provided in the studies included cancer screening (Aragones 2010; Christy 2013; Herman 1995), diabetes screening (Kenealy 2005), vaccination (Caskey 2011; Jacobson 1999; Nagykaldi 2012; Thomas 2003), and both vaccination and cancer screening (Turner 1990; Wright 2012). Fifteen studies were on identification, treatment or management of patients with certain conditions such as mental health problems (Brody 1990; Mazonson 1996; Mouland 1997), asthma (Goldberg 2012; Kattan 2006), diabetes (Khan 2011; Quinn 2008), cancer (Kravitz 2012; Miaskowski 2004), hypertension (McKinstry 2006; Thiboutot 2013), heart‐related disease (McAlister 2005), dyspepsia (Krol 2004), and musculoskeletal pain, depression and mobility difficulty (Leveille 2009), and upper respiratory tract symptoms (Alder 2005).
Most studies included adult patients except for three studies (Alder 2005; Goldberg 2012; Kattan 2006) in which the children's mean age varied between three years (Alder 2005) and seven/eight years (Goldberg 2012; Kattan 2006). The total number of children included in our analyses was 1054. In two of these three studies the children were mostly female (Alder 2005; Goldberg 2012). Among the 22 studies with adult patients, 18 studies had a mean patient age of 50 years or more. The mean patient age was below 50 years in three studies (Mazonson 1996; Quinn 2008; Wright 2012), and age was not reported in one study (Caskey 2011). In seventeen of the 22 studies with adult patients over fifty per cent of participants were women. One study recruited only women (Herman 1995), one study did not report on gender (Caskey 2011), and three studies included mostly men (Kenealy 2005; Khan 2011; McAlister 2005). Among the 25 included studies one study recruited only Latino immigrants (Aragones 2010), and another study only African‐Americans (Christy 2013).
Healthcare professionals
All studies involved physicians, but in five studies nurses and physician assistants were also included (Jacobson 1999; Kattan 2006; McKinstry 2006; Nagykaldi 2012; Thomas 2003). The number of healthcare professionals included in the studies was not consistently reported, but for the studies where this information was available the total number ranged from 8 to 167 (see Characteristics of included studies for further details).
Settings
All studies were carried out in the USA apart from five: one in Canada (McAlister 2005), in New Zealand (Kenealy 2005), in Norway (Mouland 1997), in Scotland (McKinstry 2006), and in the Netherlands (Krol 2004). Most studies were conducted in a primary care setting. Three studies were within both specialist and primary care settings (Kattan 2006;Kravitz 2012; Miaskowski 2004), and one study was within specialist care (Goldberg 2012).
Interventions and comparisons
Interventions
We categorised six studies as patient‐reported health information interventions (Brody 1990; Goldberg 2012; Kattan 2006; Kenealy 2005; Mazonson 1996; Quinn 2008). We categorised 13 studies as patient‐information interventions. These included written or electronic reminders, prompts, handouts, posters etc. (Caskey 2011; Herman 1995; Jacobson 1999; Krol 2004; Leveille 2009; McKinstry 2006; Mouland 1997; Turner 1990; Wright 2012) or video or web‐based information (Aragones 2010; Christy 2013; Nagykaldi 2012; Thomas 2003). Five studies were patient‐education interventions (Alder 2005; Khan 2011; Kravitz 2012; Miaskowski 2004; Thiboutot 2013). These varied greatly in content from electronic based education or training (Khan 2011; Thiboutot 2013), to in‐person communication or coaching interventions (Alder 2005; Kravitz 2012), to a multi session nurse‐led patient‐education intervention (Miaskowski 2004). The remaining study was about patient decision aids (McAlister 2005).
We did not identify any studies fulfilling our inclusion criteria that involved other patient‐mediated interventions such as patient feedback about clinical practice, patients being members of committees or boards, or patient‐led training or education of healthcare professionals.
Fourteen studies delivered the intervention at the practice site (Alder 2005; Aragones 2010; Brody 1990; Caskey 2011; Christy 2013; Goldberg 2012; Herman 1995; Jacobson 1999; Kenealy 2005; Khan 2011; Kravitz 2012; Mazonson 1996; Thomas 2003; Turner 1990). The remaining studies delivered the intervention outside the practice, including in the patient’s home, in person (Miaskowski 2004), by telephone (Kattan 2006), electronically (e‐mail or web portal) (Leveille 2009; Nagykaldi 2012; Quinn 2008; Thiboutot 2013; Wright 2012), or by post (Krol 2004; McAlister 2005; McKinstry 2006; Mouland 1997). Among the studies where the intervention was delivered outside the practice, four studies had a "one‐time delivery" of the intervention (Krol 2004; McAlister 2005; McKinstry 2006; Mouland 1997) and seven studies had continuous intervention delivery over three months or less (Kattan 2006; Leveille 2009; Miaskowski 2004; Wright 2012), or over a year (Nagykaldi 2012; Quinn 2008; Thiboutot 2013).
Comparisons
The comparisons were categorised as "no intervention" in 11 studies (Brody 1990; Caskey 2011; Goldberg 2012; Herman 1995; Kattan 2006; Kenealy 2005; Mazonson 1996; Mouland 1997; Nagykaldi 2012; Quinn 2008: Turner 1990) and "usual care" in 14 studies (Alder 2005; Aragones 2010; Christy 2013; Jacobson 1999; Khan 2011; Kravitz 2012; Krol 2004; Leveille 2009; McAlister 2005; McKinstry 2006; Miaskowski 2004; Thiboutot 2013; Thomas 2003; Wright 2012). Among the 11 studies within the "no intervention" comparison category, five studies had a "pure" "no intervention" comparison (Brody 1990; Goldberg 2012; Kattan 2006; Mazonson 1996; Nagykaldi 2012), while in the remaining six, both groups received a non‐patient‐mediated intervention component (Caskey 2011; Herman 1995; Kenealy 2005; Mouland 1997; Quinn 2008;Turner 1990). These non‐patient‐mediated intervention components were typically information or reminders given to healthcare professionals in both groups.
Among the 14 studies within the "usual care" comparison category, two studies were described as having a "usual care" comparison without further description (Aragones 2010; Krol 2004), six studies used a placebo‐like usual care‐comparison, where the comparison group typically received patient information not related to the health condition(s) being studied (Alder 2005; Jacobson 1999; Leveille 2009; Thiboutot 2013; Thomas 2003; Wright 2012) and six studies used a patient information‐like usual care‐comparison, where the comparison group was given minimal patient information about the health condition being studied as part of usual care (Christy 2013; Khan 2011; Kravitz 2012; McAlister 2005; McKinstry 2006; Miaskowski 2004). This was typically untailored or standard information brochures about the health condition being studied and could be given to both the comparison group and patient‐mediated intervention group (Kravitz 2012; McAlister 2005; McKinstry 2006) or to the comparison group only (Christy 2013; Khan 2011;Miaskowski 2004).
Outcomes
Primary outcomes
The primary outcome, adherence to recommended clinical practice, was reported in all 25 studies. The outcomes we defined as primary were defined as primary outcomes in eight studies (Caskey 2011; Goldberg 2012; Jacobson 1999; Kenealy 2005; Krol 2004; Leveille 2009; Mazonson 1996; Wright 2012), and secondary outcomes in eight studies (Aragones 2010; Christy 2013; McAlister 2005; McKinstry 2006; Miaskowski 2004; Quinn 2008; Thiboutot 2013; Thomas 2003). The outcomes were not categorised into primary and secondary outcomes in nine studies (Alder 2005; Brody 1990; Herman 1995; Kattan 2006; Khan 2011; Kravitz 2012; Mouland 1997; Nagykaldi 2012; Turner 1990). All studies except for one (Brody 1990), reported the primary outcome in a dichotomous way.
Secondary outcomes
Secondary outcomes that matched our inclusion criteria were reported in 12 of the 25 included studies (Alder 2005; Brody 1990; Herman 1995; Kattan 2006; Khan 2011; Kravitz 2012; Krol 2004; Leveille 2009; McKinstry 2006; Miaskowski 2004; Quinn 2008; Thiboutot 2013).
Eight of the 12 studies reported patient health outcomes (Brody 1990; Khan 2011; Kravitz 2012; Krol 2004; Leveille 2009; McKinstry 2006; Miaskowski 2004; Thiboutot 2013). Patient satisfaction with the care they received was reported in four studies (Alder 2005; Brody 1990; Leveille 2009; Quinn 2008), and resource use was reported in one study (Kattan 2006).
None of the included studies reported on:
patients' acceptance, confidence in, or satisfaction with the intervention;
patients' experiences / perceptions of healthcare professionals acceptance, confidence in or satisfaction with the intervention;
healthcare professionals' satisfaction with the care they provide;
healthcare professionals' acceptance, confidence in or satisfaction with the intervention;
adverse events;
equity.
For all included outcomes, we narratively report effect estimates as reported by the authors of the study (Table 6; Table 7), and also report how these data were collected (self‐report or medical record) (Characteristics of included studies).
3. Descriptive reporting of all relevant secondary outcomes from included studies.
| Study | Secondary outcomes | Findings |
| Alder 2005 | Patient satisfaction with the care they receive 1. General satisfaction 2. Interpersonal manner 3. Time spent with doctor |
Author’s quote: “Significant associations were observed for General Satisfaction (p = 0.002), Interpersonal Manner (p = 0.010), and Time Spent with Doctor (p = 0.002)”. |
| Aragones 2010 | No relevant secondary outcomes reported | |
| Brody 1990 | Patient health outcomes 1. Patients with a psychological disorder (outcome could not be categorised into our categories because desired direction not provided) 2. Control over stress Patient satisfaction with the care they receive 3. Patient report of rating of amount of time spent counselling (1=no time, 5= >15 minutes) 4. Patient report of rating of satisfaction with physician (scale range not reported, higher score means better) |
1. Intervention: 71%, N= 29 Comparison: 56%, N=50 2. Authors quote: “…52% felt they experienced some increase in their sense of control over stress following the medical visit.” “… 32% of control patients who indicated some beneficial changes in their control over stress. 3. Intervention: 2.9 (se=0.2), N= 29 Comparison: 2.5 (se=0.1), N=50 4. Intervention: 4.7 (se=0.1), N= 29 Comparison: 4.3 (se=0.1), N=50 |
| Caskey 2011 | No relevant secondary outcomes reported | |
| Christy 2013 | No relevant secondary outcomes reported | |
| Goldberg 2012 | No relevant secondary outcomes reported | |
| Herman 1995 | No relevant secondary outcomes reported | |
| Jacobson 1999 | No relevant secondary outcomes reported | |
| Kattan 2006 | Patient health outcomes Maximum symptom days (outcome could not be categorised into our categories) Resource use Intervention cost and cost effectiveness |
Author’s quote: “It took 40 minutes per child to reach the caretaker and make the assessment call, enter the data, and mail the letter. In calculating the costs, we used an hourly wage of $15 for a clerical employee. There were 6 calls per child per year resulting in a cost of $60. We estimated $10 for supplies and informational materials for the PCP. Because some PCPs had 1 child in the study, the cost for these materials on a per child basis was $9.20. The intervention was estimated to cost $69.20 per child over the year. When this cost was added to the cost of health services use for the year by intervention children and compared with the cost of health service use by control children, there was a savings of $337.00 per child in the intervention group. The Monte Carlo simulations, using the observed distributions of symptom days and resource use, showed that the intervention had a 97% chance of being cost saving.” |
| Kenealy 2005 | No relevant secondary outcomes reported | |
| Khan 2011 | Patient health outcomes HbA1c (outcome could not be categorised into our categories) |
Intervention: Before: 9.1 (sd=2.5). After: 7.6 (sd=1.8), N= 53 Comparison: Before: 9.4 (sd=2.7). After: 8.6 (sd=2.5), N=47 |
| Kravitz 2012 | Patient health outcomes 1. Pain severity 2. Pain‐related impairment |
1. Pain severity. Coefficient 0.05 (95% CI ‐0.39, 0.49) p=0.81. Pain severity is the mean of worst and average pain, scaled 0‐10, with 10 representing maximal pain (Intervention group N= 126, comparison group N= 132) 2. Pain‐related impairment. Coefficient ‐0.08 (95% CI ‐0.28, 0.12) p=0.44. Pain impairment is scaled 1‐5, with 5 representing maximal impairment (Intervention group N= 126, comparison group N= 132) |
| Krol 2004 | Patient health outcomes 1. Dyspesia severity is high 2. Mental health (RAND‐36, higher score means a more favourable health state) 3. Vitality (RAND‐36, higher score means a more favourable health state) |
1. Intervention: Before: 29/63. After: 19/59 Comparison: Before 23/50. After: 20/45 2.* Intervention: Before: 23.5, N=63. After: 22.6, N= 59 Comparison: Before: 24, N=50. After: 23.1, N=45 3.* Intervention: Before: 17, N=63. After: 16.5, N= 59 Comparison: Before: 16, N=50. After: 16.4, N=45 * No sd (standard deviation) provided |
| Leveille 2009 | Patient satisfaction with the care they receive (at 1 week) 1. Rate the medical care in visit (on a 1‐10 scale, 10 is best) 2. Doctor definitely showed concern about health/feelings 3. Doctor definitely spent enough time Patient health outcomes (at 3 months) 4. Fair to poor health 5. Pain subscale SF‐36 (moderate‐severe) 6. Average pain rating (on a 1‐10 scale, 10 is most) (outcome could not be categorised into our categories) |
1. Intervention: 9.4 (sd=0.9), N=94 Comparison: 9.1 (sd=1.1), N=92 2. Intervention: 86/94 Comparison: 82/92 3. Intervention: 75/94 Comparison: 68/92 4. Intervention: Before: 19/71. After: 17/71 Comparison: Before: 15/71. After: 13/71 5. Intervention: Before: 40/64. After: 36/64 Comparison: Before: 38/59. After: 35/59 6. Intervention: Before: 4.5 (sd=2.2). After: 3.3 (sd=2.9), N= 64 Comparison: Before: 5.1 (sd=2.0). After: 3.8 (sd=3.1), N=59 |
| Mazonson 1996 | No relevant secondary outcomes reported | |
| McAlister 2005 | No relevant secondary outcomes reported | |
| McKinstry 2006 | Patient health outcomes 1. Blood pressure (controlled, systolic and diastolic) 2. Cholesterol (outcome could not be categorised into our categories) |
1. Intervention: Controlled: Before: 64/148, after: 71/131. Systolic: Before: 147 mmHg (sd=19), N=148, after: 148 mmHg (sd=22), N= 131. Diastolic: after: 84 mmHg (sd=10), after: 80 mmHg (sd=12), N=131 Comparison: Controlled: Before: 69/146, after: 71/130 Systolic: Before: 146 mmHg (sd=19), N=146, after: 148 mmHg mmHg (sd=21), N=130 Diastolic: Before: 82 mmHg (sd=11), N=146, after: 80 mmHg (sd=12), N=130 2. Intervention: Before: 5.4 (sd=1.2), N= 148, after: 5.2 mmol/L (sd=1.0), N=131 Comparison: Before: 5.4 (sd=1.1), N= 146, 5.2 mmol/L (sd=1.1), N=130 |
| Miaskowski 2004 | Patient health outcomes (average pain) |
Author’s quote: “For average pain, a significant group time interaction (P < 0.0001) as well as significant main effects of group (P < 0.026) and time (P < 0.0001) were found. Tests of simple effects within the two groups showed a significant decrease in average pain scores over time in the intervention group (P < 0.0001) but not in the standard care group (P = 0.857).” Self‐report before bedtime for 6 weeks using a descriptive numeric rating scale that ranged from 0 (none) to 10 (excruciating). |
| Mouland 1997 | No relevant secondary outcomes reported | |
| Nagykaldi 2012 | No relevant secondary outcomes reported | |
| Quinn 2008 | Patient health outcomes 1. HbA1c 2. Depression diagnosis (outcome could not be categorised into our categories because desired direction not provided) Patient satisfaction with the care they receive 3. Healthcare provider's diabetes management improved by receipt of blood sugar measurements (patient survey) |
1.* Intervention: Before: 9.51%. After: 7.48%, N=13
Comparison: Before: 9.05%. After: 8.37%, N=13 2. Intervention: 1/13 (9.1%) Comparison: 3/13 (20%) 3. Intervention: 13/13 (100%) Comparison: 5/13 (27.5%) *No sd (standard deviation) provided |
| Thiboutot 2013 | Patient health outcomes (controlled blood pressure) |
Intervention: 201/282 (71.3%) Comparison: 143/218 (65.6%) |
| Thomas 2003 | No relevant secondary outcomes reported | |
| Turner 1990 | No relevant secondary outcomes reported | |
| Wright 2012 | No relevant secondary outcomes reported |
When the same study reported more than one relevant primary outcome (adherence outcome), we used the primary outcome as defined by the study authors. If a primary outcome was not clearly defined (Herman 1995; Khan 2011; Turner 1990), or multiple outcomes were defined as primary (Goldberg 2012; Wright 2012) or secondary outcomes (McKinstry 2006; Thiboutot 2013), we calculated and used the median value from all relevant primary outcomes. When calculating the median from even numbers of outcomes (Goldberg 2012; Herman 1995; Khan 2011; McKinstry 2006; Turner 1990; Wright 2012), we chose the outcome with reporting from the most participants (Herman 1995; McKinstry 2006; Turner 1990; Wright 2012). In cases where the number of participants contributing to the outcome was the same, we randomly selected the outcome (flip of a coin) (Goldberg 2012; Khan 2011).
The time points at which our primary outcomes were measured was within the 0‐3 months interval in most of the studies except from four studies (Krol 2004; McAlister 2005; McKinstry 2006; Mouland 1997), in which our primary outcomes were measured within the 3‐12 months interval.
Excluded studies
We excluded 115 studies, see Characteristics of excluded studies. Fifty‐six studies were excluded on the basis of outcomes and 42 studies on the basis of interventions or comparisons. The remaining studies were excluded on the basis of study design (11 studies) and the way the studies were carried out (no guarantee that a clinical encounter took place and thus the outcome is likely to be confounded by patients' attendance rates) (six studies).
Risk of bias in included studies
The judgments for the risk of bias from the 25 included studies are summarised in Figure 4 and Figure 5. We found 10 studies with adequate randomisation generation (Goldberg 2012; Jacobson 1999; Kattan 2006; Kenealy 2005; Khan 2011; Kravitz 2012; McAlister 2005; McKinstry 2006; Mouland 1997; Thiboutot 2013). Two studies had high risk of allocation bias due to lack of a random sequence generation (Thomas 2003; Turner 1990). Thirteen studies had unclear reporting of the randomisation (Alder 2005; Aragones 2010; Brody 1990; Caskey 2011; Christy 2013; Herman 1995; Krol 2004; Leveille 2009; Mazonson 1996; Miaskowski 2004; Nagykaldi 2012; Quinn 2008; Wright 2012).
4.
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
5.
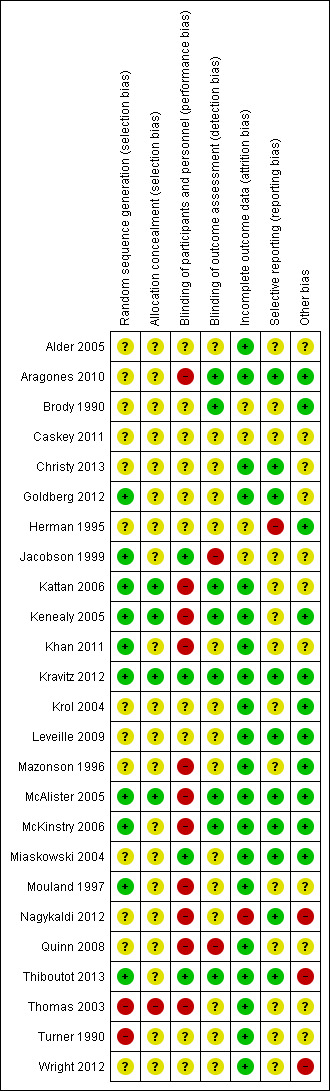
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Ten studies reported adequate randomisation generation (Goldberg 2012; Jacobson 1999; Kattan 2006; Kenealy 2005; Khan 2011; Kravitz 2012; McAlister 2005; McKinstry 2006; Mouland 1997; Thiboutot 2013). Thirteen studies had unclear reporting of the sequence generation (Alder 2005; Aragones 2010; Brody 1990; Caskey 2011; Christy 2013; Herman 1995; Krol 2004; Leveille 2009; Mazonson 1996; Miaskowski 2004; Nagykaldi 2012; Quinn 2008; Wright 2012) and two studies had high risk of bias due to lack of a random sequence generation (Thomas 2003; Turner 1990).
Allocation concealment
We judged allocation concealment to be adequate in four studies (Kattan 2006; Kenealy 2005; Kravitz 2012; McAlister 2005).Twenty studies had unclear reporting of allocation concealment (Alder 2005; Aragones 2010; Brody 1990; Caskey 2011; Christy 2013; Goldberg 2012; Herman 1995; Jacobson 1999; Khan 2011; Krol 2004; Leveille 2009; Mazonson 1996; McKinstry 2006; Miaskowski 2004; Mouland 1997; Nagykaldi 2012; Quinn 2008; Thiboutot 2013; Turner 1990; Wright 2012) and one study had high risk of bias due to lack of adequate allocation concealment.
Blinding
We judged participants and personnel to be blinded in four studies (Jacobson 1999; Kravitz 2012; Miaskowski 2004; Thiboutot 2013) and not blinded in 11 studies (Aragones 2010; Kattan 2006; Kenealy 2005; Khan 2011; Mazonson 1996; McAlister 2005; McKinstry 2006; Mouland 1997; Nagykaldi 2012; Quinn 2008; Thomas 2003). We judged the remaining 10 studies (Alder 2005; Brody 1990; Caskey 2011; Christy 2013; Goldberg 2012; Herman 1995; Krol 2004; Leveille 2009; Turner 1990; Wright 2012) to have unclear risk of bias because these studies did not sufficiently describe participant and personnel blinding.
We judged outcome assessors to be blinded in eight studies (Aragones 2010; Brody 1990; Kattan 2006; Kenealy 2005; Kravitz 2012; McAlister 2005; McKinstry 2006; Thiboutot 2013) and not blinded in two studies (Jacobson 1999; Quinn 2008). We judged the remaining 15 studies (Alder 2005; Caskey 2011; Christy 2013; Goldberg 2012; Herman 1995; Khan 2011; Krol 2004; Leveille 2009; Mazonson 1996; Miaskowski 2004; Mouland 1997; Nagykaldi 2012; Thomas 2003; Turner 1990; Wright 2012) to have unclear risk of bias because these studies did not sufficiently describe blinding of outcome assessors.
Incomplete outcome data
We found no indication of incomplete outcome data in most of the studies (Alder 2005; Aragones 2010; Christy 2013; Goldberg 2012; Kattan 2006; Kenealy 2005; Khan 2011; Kravitz 2012; Krol 2004; Leveille 2009; Mazonson 1996; McAlister 2005; McKinstry 2006; Miaskowski 2004; Mouland 1997; Quinn 2008; Thiboutot 2013; Thomas 2003; Turner 1990; Wright 2012). We judged one study (Nagykaldi 2012) to have high risk of bias and four studies (Brody 1990; Caskey 2011; Herman 1995; Jacobson 1999; ) to have unclear risk of attrition bias.
Selective reporting
We could not decide if there was a risk of selective reporting in more than half of the studies (Alder 2005; Brody 1990; Caskey 2011; Jacobson 1999; Kattan 2006; Kenealy 2005; Khan 2011; Krol 2004; Mazonson 1996; Mouland 1997; Quinn 2008; Thomas 2003; Turner 1990; Wright 2012). We judged one study (Herman 1995) to have high risk of bias and 10 to have of low risk of bias (Aragones 2010; Christy 2013; Goldberg 2012; Kravitz 2012; Leveille 2009; McAlister 2005; McKinstry 2006; Miaskowski 2004; Nagykaldi 2012; Thiboutot 2013).
Other potential sources of bias
We inspected all the studies for potential bias due to baseline imbalance in key characteristics and baseline outcome imbalance. We found high risk of baseline imbalance in key charcteristics in two studies (Alder 2005;Wright 2012). We judged 11 studies to have low risk of bias (Aragones 2010; Brody 1990; Christy 2013; Kenealy 2005; Khan 2011; Leveille 2009; Mazonson 1996; McAlister 2005; McKinstry 2006; Miaskowski 2004; Nagykaldi 2012) and 12 to have unclear risk of baseline imbalance (Caskey 2011; Goldberg 2012; Herman 1995; Jacobson 1999; Kattan 2006; Kravitz 2012; Krol 2004; Mouland 1997; Quinn 2008; Thiboutot 2013; Thomas 2003; Turner 1990). For baseline outcome imbalance, five out of 25 had low risk (Khan 2011; McAlister 2005; McKinstry 2006; Miaskowski 2004; Mouland 1997), while the remaining 20 had unclear risk. Only two (McAlister 2005; McKinstry 2006) of the 25 studies reported the relevant primary outcome at baseline, one reported one of the primary outcomes, but not the one used for the meta‐analysis (Mouland 1997) while three studies reported secondary outcomes at baseline (Khan 2011; McKinstry 2006; Miaskowski 2004).
Ten studies were cluster‐randomised studies (Brody 1990; Caskey 2011; Herman 1995; Kenealy 2005; Krol 2004; Mazonson 1996; McAlister 2005; Nagykaldi 2012; Thiboutot 2013; Wright 2012) and we searched for information about five additional sources of potential biases. There was high risk of bias in three of the ten studies (Nagykaldi 2012; Thiboutot 2013; Wright 2012) and low risk of bias in six studies (Brody 1990; Herman 1995; Kenealy 2005; Krol 2004; Mazonson 1996; McAlister 2005). The remaining study was judged to be unclear (Caskey 2011). The rationale for all the judgements are presented in the table of Risk of bias in included studies.
Among the 15 individual randomised studies (Alder 2005; Aragones 2010; Christy 2013; Goldberg 2012; Jacobson 1999; Kattan 2006; Khan 2011; Kravitz 2012; Leveille 2009; McKinstry 2006; Miaskowski 2004; Mouland 1997; Quinn 2008; Thomas 2003; Turner 1990) we found no indication of other risk of bias in five of these studies (Aragones 2010; Kravitz 2012; Leveille 2009; McKinstry 2006; Miaskowski 2004,) but the remaining ten studies were unclear (Alder 2005; Christy 2013; Goldberg 2012; Jacobson 1999; Kattan 2006; Khan 2011; Mouland 1997; Quinn 2008; Thomas 2003; Turner 1990).
Thus all in all, we found no indication of other risk of bias in 11 studies (Aragones 2010; Brody 1990; Herman 1995; Kenealy 2005; Kravitz 2012; Krol 2004; Leveille 2009; Mazonson 1996; McAlister 2005; McKinstry 2006; Miaskowski 2004), high risk of bias in three studies (Nagykaldi 2012; Thiboutot 2013; Wright 2012), and unclear risk in eleven studies (Alder 2005; Caskey 2011; Christy 2013; Goldberg 2012; Jacobson 1999; Kattan 2006; Khan 2011; Mouland 1997; Quinn 2008; Thomas 2003; Turner 1990).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Patient‐reported health information interventions versus comparisons to improve professional performance.
| Patient‐reported health information interventions versus comparisons to improve professional performance | ||||||
| Patient or population: general patient population, "at risk" patient population and patient population with a specific condition or disease Setting: primary care (mostly) Intervention: patient‐reported health information interventions Comparison: no intervention or usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens? | |
| Risk with comparisons | Risk with patient‐reported health information interventions | |||||
| Adherence to recommended clinical practice (0‐3 months follow‐up) | 17 per 100 | 26 per 100 (23 to 30) | RR 1.59 (1.41 to 1.81) | 3865 (4 RCTsA) | ⊕⊕⊕⊝ MODERATE1 | Patient‐reported health information interventions probably improve healthcare professionals' adherence to recommended clinical practice compared to no intervention or usual care |
| Desirable patient health outcomes (0‐3 months follow‐up) | 32 per 100 | 52 per 100 (38 to 100) | RR 1.62 (0.95 to 2.76) | 79 (1 RCTB) | ⊕⊝⊝⊝ VERY LOW2 3 | We are uncertain about the effect of patient‐reported health information interventions on desirable patient health outcomes because the certainty of the evidence is very low |
| Undesirable patient health outcomes | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on undesirable patient health outcomes |
|
Patient satisfaction Number of satisfied patients (0‐3 months follow‐up) |
38 per 100 | 94 per 100 (49 to 100) | RR 2.45 (1.27 to 4.74) | 26 (1 RCTC) | ⊕⊝⊝⊝ VERY LOW2 3 | We are uncertain about the effect of patient‐reported health information interventions on the number of satisfied patients because the certainty of the evidence is very low |
|
Patient satisfaction The degree of satisfaction (unknown scale, but higher score means higher degree of satisfaction) (0‐3 months follow‐up) |
The mean patient satisfaction score was 4.3 points | The mean patient satisfaction was 0.40 points higher (0.12 higher to 0.68 higher) | ‐ | 79 (1 RCTB) | ⊕⊝⊝⊝ VERY LOW2 4 | We are uncertain about the effect of patient‐reported health information interventions on the degree of patient satisfaction because the certainty of the evidence is very low |
| Adverse events | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on adverse events |
| Resource use (0‐3 months follow‐up) | The findings are narratively presented in Table 3. The researchers in this study reported a total cost of 69.20 US $ per child | We did not judge the certainty of the evidence for this outcome | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio, RCT: randomised trial | ||||||
|
GRADE Working Group grades of evidence
High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low.
Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate.
Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high.
Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
1 Downgraded one level because we judged only 1 of 4 studies to have low risk of bias
2 Downgraded one level because we judged the study to have potential risk of bias
3 Downgraded two levels for imprecision because of very few events (and one small study only)
4 Downgraded two levels for imprecision because of a very small sample size (and one small study only)
A Goldberg 2012; Kenealy 2005; Mazonson 1996; Quinn 2008
B Brody 1990
C Quinn 2008
Summary of findings 2. Patient information interventions versus comparisons to improve professional performance.
| Patient information interventions versus comparisons to improve professional performance | ||||||
| Patient or population: general patient population, "at risk" patient population and patient population with a specific condition or disease Setting: primary care (mostly) Intervention: patient information interventions Comparison: no intervention or usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens? | |
| Risk with comparisons | Risk with patient information interventions | |||||
| Adherence to recommended clinical practice (0‐12 months follow‐up) | 20 per 100 | 32 per 100 (24 to 42) | RR 1.60 (1.20 to 2.13) | 3502 (11 RCTsA) | ⊕⊕⊝⊝ LOW1 2 | Patient information interventions may improve healthcare professionals' adherence to recommended clinical practice compared to no intervention or usual care |
| Desirable patient health outcomes (3‐12 months follow‐up) | 55 per 100 | 54 per 100 (43 to 68) | RR 0.99 (0.79 to 1.24) | 261 (1 RCTB) | ⊕⊕⊝⊝ LOW5 6 | There may be little or no difference in the number of people with desirable health outcomes among people in the patient information intervention group compared to those in the usual care group |
| Undesirable patient health outcomes (0‐12 months follow‐up) | 28 per 100 | 27 per 100 (15 to 48) | RR 0.94 (0.53 to 1.67) | 246 (2 RCTsC) | ⊕⊝⊝⊝ VERY LOW1 3 | We are uncertain about the effect of patient information interventions on undesirable patient outcomes because the certainty of the evidence is very low |
|
Patient satisfaction Number of satisfied patients (0‐3 months follow‐up) |
89 per 100 | 92 per 100 (83 to 100) | RR 1.03 (0.93 to 1.13) | 186 (1 RCTD) | ⊕⊕⊝⊝ LOW5 6 | There may be little or no difference in the number of satisfied patients among those in the patient information intervention group compared to those in the usual care group |
|
Patient satisfaction The degree of satisfaction (on a 1‐10 scale where 10 is highest degree of satisfaction) (0‐3 months follow‐up) |
The mean patient satisfaction score was 9.1 points | The mean patient satisfaction was 0.30 points higher (0.01 higher to 0.59 higher) | ‐ | 186 (1 RCTD) | ⊕⊕⊝⊝ LOW4 5 | There may be little or no difference in the degree of satisfaction among patients in the patient information intervention group compared to those in the usual care group |
| Adverse events | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on adverse events |
| Resource use | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on resource use |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; RCT: randomised trial | ||||||
|
GRADE Working Group grades of evidence
High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low.
Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate.
Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high.
Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
1 Downgraded one level because all the studies were judged to have potential risk of bias,
2 Downgraded one level for inconsistency because of statistical heterogeneity (I2 is 79%)
3 Downgraded two levels for imprecision because of few events and a 95% CI that crosses the line of "no effect"
4 Downgraded one level for imprecision because of small study sample
5 Downgraded one level because we judged the study to have potential risk of bias
6 Downgraded one level for imprecision because of few events
A Aragones 2010; Caskey 2011; Herman 1995; Jacobson 1999; Krol 2004; Leveille 2009; McKinstry 2006; Mouland 1997; Thomas 2003; Turner 1990; Wright 2012
B McKinstry 2006
C Krol 2004; Leveille 2009
D Leveille 2009 (patient satisfaction was assessed using both a dichotomous and a continuous outcome in this study)
Summary of findings 3. Patient education interventions versus comparisons to improve professional performance.
| Patient education interventions versus comparisons to improve professional performance | ||||||
| Patient or population: general patient population, "at risk" patient population and patient population with a specific condition or disease Setting: primary care (mostly) Intervention: patient education interventions Comparison: no intervention or usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens? | |
| Risk with comparisons | Risk with patient education interventions | |||||
| Adherence to recommended clinical practice (0‐3 months follow‐up) | 35 per 100 | 46 per 100 (39 to 54) | RR 1.31 (1.12 to 1.54) | 1029 (4 RCTsA) | ⊕⊕⊕⊝ MODERATE1 | Patient education interventions probably improve healthcare professionals' adherence to recommended clinical practice compared to no intervention or usual care |
| Desirable patient health outcomes (0‐3 months follow‐up) | 66 per 100 | 72 per 100 (63 to 81) | RR 1.09 (0.96 to 1.23) | 500 (1 RCTB) | ⊕⊕⊕⊝ LOW2 3 | Patient education interventions may slightly increase the number of people with desirable health outcomes compared to usual care |
| Undesirable patient health outcomes | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on undesirable patient health outcomes |
|
Patient satisfaction Number of satisfied patients |
Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on patient satisfaction |
|
Patient satisfaction The degree of satisfaction |
Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on patient satisfaction |
| Adverse events | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on adverse events |
| Resource use | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on resource use |
|
GRADE Working Group grades of evidence
High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low.
Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate.
Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high.
Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
1 Downgraded one level because most of the studies were assessed as having potential risk of bias
2 Downgraded one level for imprecision because the 95% CI crosses the line of "no effect"
3 Downgraded one level because the study has potential risk of bias (allocation concealment and other biases related to cluster issues)
A Khan 2011; Kravitz 2012; Miaskowski 2004; Thiboutot 2013
B Thiboutot 2013
Summary of findings 4. Patient decision aid interventions versus comparisons to improve professional performance.
| Patient decision aid interventions versus comparisons to improve professional performance | ||||||
| Patient or population: patient population with a specific condition or disease Setting: primary care Intervention: patient decision aid interventions Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | What happens? | |
| Risk with comparisons | Risk with patient‐reported health information interventions | |||||
| Adherence to recommended clinical practice (12 months follow up) | 37 per 100 | 32 per 100 (24 to 43) | RR 0.86 (0.65 to 1.15) | 353 (1 RCTA) | ⊕⊕⊕⊝ LOW1 2 | There may be little or no difference in the number of healthcare professionals' adhering to recommended clinical practice in the patient decision aid group compared to usual care |
| Desirable patient health outcomes | Not reported | ‐ | ‐ | ‐ | ‐ | The included study did not report on desirable patient health outcomes |
| Undesirable patient health outcomes | Not reported | ‐ | ‐ | ‐ | ‐ | The included study did not report on undesirable patient health outcomes |
|
Patient satisfaction Number of satisfied patients |
Not reported | The included study did not report on patient satisfaction outcomes | ||||
|
Patient satisfaction The degree of satisfaction (unknown scale, but higher score means higher degree of satisfaction) |
Not reported | ‐ | ‐ | ‐ | ‐ | The included study did not report on patient satisfaction outcomes |
| Adverse events | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on adverse events |
| Resource use | Not reported | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on resource use |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
|
GRADE Working Group grades of evidence
High certainty: This research provides a very good indication of the likely effect. The likelihood that the effect will be substantially different** is low.
Moderate certainty: This research provides a good indication of the likely effect. The likelihood that the effect will be substantially different** is moderate.
Low certainty: This research provides some indication of the likely effect. However, the likelihood that it will be substantially different** is high.
Very low certainty: This research does not provide a reliable indication of the likely effect. The likelihood that the effect will be substantially different** is very high. ** Substantially different = a large enough difference that it might affect a decision | ||||||
1 Downgraded one level because the study was assessed as having high risk of performance bias (no blinding of patients or healthcare professionals)
2 Downgraded one level for imprecision because of few events and because the 95% CI crosses the line of "no effect"
A McAlister 2005
See Table 1; Table 2; Table 3, and Table 4 for patient‐mediated interventions versus comparisons. The comparisons were categorised as "no intervention" and "usual care" (see Types of interventions) and these comparisons were merged for analysis and reporting because they appeared quite similar.
Adherence to recommended clinical practice was our primary outcome. We included 20 studies and a total of 8749 patients in our meta‐analyses. Our meta‐analyses show that patient‐reported health information interventions and patient education interventions probably improve professional performance and the two other types of patient‐mediated interventions may improve professional performance (patient information) or may have little or no impact (patient decision aids) (Analysis 1.1; Analysis 2.1; Analysis 3.1; Analysis 4.1).
1.1. Analysis.
Comparison 1 Patient‐reported health information interventions versus comparisons, Outcome 1 Adherence to recommended practice.
2.1. Analysis.
Comparison 2 Patient information interventions versus comparisons, Outcome 1 Adherence to recommended practice.
3.1. Analysis.
Comparison 3 Patient education interventions versus comparisons, Outcome 1 Adherence to recommended practice.
4.1. Analysis.
Comparison 4 Patient decision aids, Outcome 1 Adherence to recommended practice.
Patient‐reported health information interventions
Primary outcome
Adherence to recommended clinical practice
Six studies about patient‐reported health information interventions reported on our primary outcome (Brody 1990; Goldberg 2012; Kattan 2006; Kenealy 2005; Mazonson 1996; Quinn 2008). We included four studies (Goldberg 2012; Kenealy 2005; Mazonson 1996; Quinn 2008) in our meta‐analysis (Analysis 1.1). We report on two studies narratively (Table 6) due to incomplete outcome reporting (Kattan 2006) or because the outcome was reported as a continuous variable (Brody 1990).The effect estimate expressed as risk ratio (RR), is 1.59 (95% confidence interval (CI) 1.41 to 1.81; 4 studies, 3865 patients) (Analysis 1.1).
In absolute numbers: for every 100 patients consulted or treated in the patient‐reported health information group there probably are 26 (95% CI 23 to 30) that are in accordance with recommended clinical practice compared to 17 per 100 in the comparison group (no intervention or usual care). We judged the certainty of the evidence as moderate. We can thus conclude that patient‐reported health information interventions probably improve healthcare professionals' adherence to recommended clinical practice compared to no intervention, usual care, or other interventions.
The two studies not included in the meta‐analysis reported findings in favour of the patient‐reported health information intervention (Kattan 2006) or no effect (Brody 1990) ‐ see Table 6.
Secondary outcomes
Patient outcomes
Desirable patient health outcomes
One study (Brody 1990), reported on desirable health outcomes dichotomously (increase in control over stress) for patient‐reported health information interventions. The result for this outcome is presented in Analysis 1.2. The relative effect estimate, RR, is 1.62 (95% CI 0.95 to 2.76; 1 study, 79 patients). We judged the certainty of the evidence as very low. We are thus uncertain about the effect of patient‐reported health information interventions on desirable patient health outcomes because the certainty of the evidence is very low.
1.2. Analysis.
Comparison 1 Patient‐reported health information interventions versus comparisons, Outcome 2 Desirable patient health outcomes (increased control over stress).
Undesirable patient health outcomes
None of the included studies reported on this outcome.
Patient satisfaction
One study (Quinn 2008), reported on patient satisfaction dichotomously for patient‐reported health information interventions and is presented in Analysis 1.3. The relative effect estimate, RR, is 2.45 (95% CI 1.27 to 4.74; 1 study, 26 patients). We judged the certainty of the evidence as very low. We are thus uncertain about the effect of patient‐reported health information interventions on the number of satisfied patients because the certainty of the evidence is very low.
1.3. Analysis.
Comparison 1 Patient‐reported health information interventions versus comparisons, Outcome 3 Patient satisfaction (with care). Number of satisfied patients.
Another study (Brody 1990) reported on patient satisfaction continuously for patient‐reported health information interventions and is presented in Analysis 1.4. Our summary shows that the mean difference (MD) in the degree of satisfaction is 0.40 points higher (95% CI 0.12 to 0.68 higher; 1 study, 79 patients). We judged the certainty of the evidence as very low. We are thus uncertain about the effect of patient‐reported health information interventions on the degree of patient satisfaction because the certainty of the evidence is very low.
1.4. Analysis.
Comparison 1 Patient‐reported health information interventions versus comparisons, Outcome 4 Patient satisfaction (with healthcare professional). The degree of satisfaction.
Other patient outcomes
None of the included studies reported on other patient outcomes (patients' acceptance, confidence in, or satisfaction with the intervention; patients' experiences / perceptions of healthcare professionals acceptance, confidence in or satisfaction with the intervention).
Healthcare professional outcomes
None of the included studies reported on any healthcare professional outcomes.
Resource use
One study reported on cost‐effectiveness (Kattan 2006), and is narratively presented in Table 7. The researchers in this study reported a total cost of 69.20 US $ per child per year. When this cost was added to the cost of healthcare services use for the year by intervention children and compared with the cost of healthcare service use by children in the comparison group, there was a saving of $337.00 per child in the intervention group. The researchers reported that the intervention had a 97% chance of being cost saving. We did not judge the certainty of the evidence for this outcome.
Adverse events
None of the included studies reported on this outcome.
Equity
None of the included studies reported on this outcome.
Patient information interventions
Primary outcome
Adherence to recommended clinical practice
Thirteen studies about patient information interventions (Aragones 2010; Caskey 2011; Christy 2013; Herman 1995; Jacobson 1999; Krol 2004; Leveille 2009; McKinstry 2006; Mouland 1997; Nagykaldi 2012; Thomas 2003; Turner 1990; Wright 2012) reported on our primary outcome. Eleven studies (Aragones 2010; Christy 2013; Herman 1995; Jacobson 1999; Krol 2004; Leveille 2009; McKinstry 2006; Mouland 1997; Thomas 2003; Turner 1990; Wright 2012) were included in our meta‐analysis (Analysis 2.1) and two studies(Caskey 2011; Nagykaldi 2012) narratively (Table 6) due to incomplete outcome reporting. The effect estimate expressed as RR, is 1.60 (95% CI 1.20 to 2.13; 11 studies, 3502 patients) (Analysis 2.1).
In absolute numbers: for every 100 patients consulted or treated in the patient information group there may be 32 (95% CI 24 to 42) that are in accordance with recommended clinical practice compared to 20 per 100 in the comparison group (no intervention or usual care). We judged the certainty of the evidence as low. We can thus conclude that patient information interventions may improve healthcare professionals' adherence to recommended clinical practice compared to no intervention, usual care, or other interventions.
The two studies not included in the meta‐analysis (Caskey 2011; Nagykaldi 2012) reported findings in favour of the patient information intervention intervention ‐ see Table 6.
There was statistical heterogeneity (I2 = 79%) for the pooled primary outcome for patient information interventions (see Analysis 2.1). The planned subgroup analyses of explanatory factors (risk of bias, direction of change required, type of recipient, and baseline clinical performance) were carried out for two of the predetermined factors; risk of bias (see Analysis 2.2 ) and the direction of change required (see Analysis 2.3). Since the target group (recipients) in all the studies were physicians, 'type of recipient' could not explain the observed statistical heterogeneity. The baseline clinical performance was generally poorly reported so we decided not to carry out a subgroup analysis for this variable either. The two subgroup analyses we carried out did not provide any explanation for the observed statistical heterogeneity.
2.2. Analysis.
Comparison 2 Patient information interventions versus comparisons, Outcome 2 Adherence to recommended practice. Risk of bias.
2.3. Analysis.
Comparison 2 Patient information interventions versus comparisons, Outcome 3 Adherence to recommended practice. Direction of behaviour.
Secondary outcomes
Patient outcomes
Desirable patient health outcomes
One study (McKinstry 2006) reported on desirable health outcomes (controlled blood pressure) for patient information interventions. The result for this outcome is presented in Analysis 2.4. The relative effect estimate, RR, is 0.99 (95% CI 0.79 to 1.24; 1 study, 261 patients). We judged the certainty of the evidence as low. We can thus conclude that there may be little or no difference in the number of people with desirable health outcomes among people in the patient information intervention group compared to those in the comparison group (usual care).
2.4. Analysis.
Comparison 2 Patient information interventions versus comparisons, Outcome 4 Desirable patient health outcomes (controlled blood pressure).
Undesirable patient health outcomes
Two studies (Krol 2004; Leveille 2009) reported on undesirable health outcomes (high dyspepsia severity or fair to poor health) for patient information interventions. The result is presented in Analysis 2.5. The relative effect estimate, RR, is 0.94 (95% CI 0.53 to 1.67; 2 studies, 246 patients). We judged the certainty of the evidence as very low. We are thus uncertain about the effect of patient information interventions on undesirable patient outcomes because the certainty of the evidence is very low.
2.5. Analysis.
Comparison 2 Patient information interventions versus comparisons, Outcome 5 Undesirable patient health outcomes (dyspepsia severity is high, fair to poor health).
Patient satisfaction
One study (Leveille 2009) report on patient satisfaction dichotomously for patient information interventions and is presented in Analysis 2.6. The relative effect estimate, RR, is 1.03 (95% CI 0.93 to 1.13; 1 study, 186 patients). We judged the certainty of the evidence as low. We can thus conclude that there may be little or no difference in the number of satisfied patients among those in the patient information intervention group compared to those in the comparison group (usual care).
2.6. Analysis.
Comparison 2 Patient information interventions versus comparisons, Outcome 6 Patient satisfaction (with healthcare professional). Number of satisfied patients.
The same study (Leveille 2009) reported on patient satisfaction continuously for patient information interventions and is presented in Analysis 2.7. Our summary shows that the in the degree of satisfaction is 0.30 points higher (95% CI 0.01 to 0.59 higher; 1 study, 186 patients) on a scale from one to ten (in which ten is best). We judged the certainty of the evidence as low. We can thus conclude that there may be little or no difference in the degree of satisfaction among patients in the patient information intervention group compared to those in the comparison group (usual care).
2.7. Analysis.
Comparison 2 Patient information interventions versus comparisons, Outcome 7 Patient satisfaction (with care). The degree of satisfaction.
Other patient outcomes
None of the included studies reported on other patient outcomes (patients' acceptance, confidence in, or satisfaction with the intervention; patients' experiences/perceptions of healthcare professionals acceptance, confidence in or satisfaction with the intervention).
Healthcare professional outcomes
None of the included studies reported on any healthcare professional outcomes.
Resource use
None of the included studies reported on this outcome.
Adverse events
None of the included studies reported on this outcome.
Equity
None of the included studies reported on this outcome.
Patient education interventions
Primary outcome
Adherence to recommended clinical practice
Five studies about patient education interventions reported on our primary outcome (Alder 2005; Khan 2011; Kravitz 2012; Miaskowski 2004; Thiboutot 2013). Four studies (Khan 2011; Kravitz 2012; Miaskowski 2004; Thiboutot 2013) were included in our meta‐analysis (Analysis 3.1) and one study (Alder 2005) was reported descriptively (Table 6) due to incomplete outcome reporting. The effect estimate expressed as RR, is 1.31 (95% CI 1.12 to 1.54; 4 studies, 1029 patients) (Analysis 3.1) .
In absolute numbers: for every 100 patients consulted or treated in the patient education group there may be 46 (95% CI 39 to 54) that are in accordance with recommended clinical practice compared to 35 per 100 in the comparison group (no intervention or usual care). We judged the certainty of the evidence as moderate. Thus we can conclude that patient education interventions probably improve healthcare professionals' adherence to recommended clinical practice compared no intervention or usual care.
The study not included in the meta‐analysis (Alder 2005) reported findings in favour of the patient education intervention and is summarised in Table 6.
Secondary outcomes
Patient outcomes
Desirable patient health outcomes
One study (Thiboutot 2013) reported on desirable health outcomes (controlled blood pressure) for patient education interventions. The result for this outcome is presented in Analysis 3.2. The relative effect estimate, RR, is 1.09 (95% CI 0.96 to 1.23; 1 study, 500 patients). We judged the certainty of the evidence as low. We can thus conclude that patient education interventions may slightly increase the number of people with desirable health outcomes compared to usual care.
3.2. Analysis.
Comparison 3 Patient education interventions versus comparisons, Outcome 2 Desirable patient health outcomes (controlled blood pressure).
Undesirable patient health outcomes
None of the included studies reported on this outcome.
Patient satisfaction
None of the included studies reported on this outcome.
Other patient outcomes
None of the included studies reported on other patient outcomes (patients' acceptance, confidence in, or satisfaction with the intervention; patients' experiences/perceptions of healthcare professionals acceptance, confidence in or satisfaction with the intervention).
Healthcare professional outcomes
None of the included studies reported on any healthcare professional outcomes.
Resource use
None of the included studies reported on this outcome.
Adverse events
None of the included studies reported on this outcome.
Equity
None of the included studies reported on this outcome.
Patient decision aid interventions
Primary outcome
Adherence to recommended clinical practice
One study about patient decision aid interventions reported on our primary outcome (McAlister 2005). The result for this outcome is presented in Analysis 4.1. The effect estimate expressed as RR, is 0.86 (95% CI 0.65 to 1.15; 1 study, 353 patients).
In absolute numbers: for every 100 patients consulted or treated in the patient education group there may be 32 (95% CI 24 to 43) that are in accordance with recommended clinical practice compared to 37 per 100 in the comparison group (usual care). We judged the certainty of the evidence as low. Thus patient decision aid interventions may make little or no difference to healthcare professionals' adherence to recommended clinical practice compared to usual care.
Discussion
Summary of main results
We included 25 studies assessing a range of patient‐mediated interventions to improve professional practice, compared to no intervention or usual care. The patient‐mediated interventions in the included studies all fell within the predefined categories in the review protocol and are shown in Table 5. The interventions in the included studies were categorised as patient‐reported health information, patient information, patient education, or patient decision aids and are presented as separate analyses (Analysis 1.1; Analysis 2.1; Analysis 3.1; Analysis 4.1). Most of the studies were carried out in a primary care setting, and about half of the studies focused on the identification, treatment or management of common long‐term conditions (such as diabetes, asthma or depression) while the other half focused on preventive care (such as cancer screening or vaccination).
We found that patient‐reported health information interventions and patient education interventions probably improve professional performance compared to no intervention or usual care (moderate certainty of the evidence). Other patient‐mediated interventions, such as patient information, may also improve professional practice (low certainty of the evidence). Patient decision aids may have little or no impact on professional performance compared to usual care (low certainty of the evidence).
The impacts of these four types of patient‐mediated intervention on health and satisfaction outcomes among patients varies.
The effects of patient‐mediated interventions on the remaining predefined secondary outcomes (healthcare professionals' satisfaction with the care they provide, resource use, patients' acceptance, confidence in, or satisfaction with the intervention, patients' experiences/perceptions of healthcare professionals acceptance, confidence in or satisfaction with the intervention, healthcare professionals' acceptance, confidence in or satisfaction with the intervention, adverse events, and equity) were either not reported or were poorly reported. We therefore cannot conclude regarding these effects.
Overall completeness and applicability of evidence
We did not find any studies that had tested the effect of the other types of patient‐mediated interventions that we had pre‐defined, including patient feedback about clinical practice, patient‐led training of healthcare professionals, or having patients as members of committees or boards.
The majority of the studies were carried out in USA (20 of 25 studies), which may limit the applicability of the findings to other settings. Also, most studies aimed at improving professional practice among physicians, usually in a primary care setting and the applicability to other types of health care providers and other care settings is unclear.
Improved professional practice should translate into improvements in patient outcomes. The combination of low‐certainty evidence for many professional practice‐outcomes and the scarcity of data on patient health outcomes hindered us from drawing any inferences on the association between the two.
Certainty of the evidence
We used the GRADE approach to assess the certainty of the evidence. The certainty of the evidence was judged to be moderate and low for our primary outcome, adherence to recommended clinical practice; very low to low for patient health outcomes; and very low to low for patient satisfaction outcomes. See Table 1; Table 2; Table 3; and Table 4 for GRADE judgements.
Potential biases in the review process
Due to wide variation in the terms and definitions used in this field of research, we performed comprehensive literature searches that covered as many of the potentially relevant terms as possible. These searches identified a very large number of primary studies (over 12, 000) which we assessed in order to identify the 25 included studies. Given the comprehensive nature of the searches that we used, we are fairly confident that the risk that we have missed important relevant published studies is low. The decision to merge 'no intervention' and 'usual care' comparisons is based on our interpretation of the comparison group descriptions in the studies. These descriptions varied greatly and made the grouping challenging. However, we are fairly confident that the two comparisons are sufficiently similar to be merged. Two review authors independently screened potentially eligible studies for inclusion and assessed risk of bias in the included studies. None of the review authors had any conflicts of interest.
Agreements and disagreements with other studies or reviews
The effect size for the primary outcome is considered small to moderate, and is in agreement with findings of previous systematic reviews assessing the effects of different interventions to improve professional practice. Audit and feedback probably improves professional practice, but the effectiveness ranges from little or no effect to a substantial effect (Ivers 2012). Reminders, such as computer‐generated reminders delivered on paper to healthcare professionals, probably improve professional practice (Arditi 2017). Printed educational material may also improve professional practice, but the effect seems small, and the certainty of the evidence is low (Giguère 2012). Educational meetings or educational outreach visits may result in modest improvements in professional practice (Forsetlund 2009; O'Brien 2007). Using local opinion leaders may improve professional practice (Flodgren 2011a), as may financial incentives (Flodgren 2011b). Another recent Cochrane review shows that clinical practice guidelines accompanied by tools intended to improve the use of the guideline probably improve adherence to clinical practice (Flodgren 2016). Organisational interventions, such as provision of pharmaceutical care, medication reviews, follow‐up visits by a healthcare professional including a pharmacist, nurse or physician, probably make little or no difference in medication errors by primary healthcare professionals in adult patients that lead to hospital admissions, emergency department visits, and death (Khalil 2017).
Authors' conclusions
Implications for practice.
Our findings show that some patient‐mediated interventions are relevant approaches to improving professional practice.
We are moderately certain about the positive effects that patient‐reported health information and patient education can have on professional practice. Thus, it seems reasonable to conclude that these types of patient mediated interventions can contribute to improving the quality of health care services.
However, we cannot be certain that all types of patient‐mediate interventions are relevant due to lack of relevant research for several types of interventions such as patient feedback about clinical practice, patients being members of committees or boards, or patient‐led training or education of healthcare professionals. We also know too little about the effects on patients' acceptance, confidence in, or satisfaction with the intervention; patients' experiences / perceptions of healthcare professionals' acceptance, confidence in or satisfaction with the intervention; healthcare professionals' satisfaction with the care they provide; healthcare professionals' acceptance, confidence in or satisfaction with the intervention; adverse events; and equity.
Implications for research.
Patient‐mediated interventions can be defined in various ways, and a common taxonomy or understanding of the term is lacking (Ng 2017). Consequently, categorising various types of patient‐mediated interventions can be challenging ‐ as we experienced when we prepared this review. For instance, to draw a clear line between patient information and patient education interventions has not been straight forward and is, to a large extent, limited to our interpretation of their definitions. The field would likely benefit from having a common framework for defining and classifying patient‐mediated interventions. As with many other behavioural change interventions, the interventions in this field are sometimes based on explicit theoretical approaches, but often they are not (Gagliardi 2016; Ng 2017). The importance of basing interventions on theory is contested (Oxman 2005), but a clearer understanding of the mechanisms through which patient‐mediated interventions may work would likely be helpful.
In addition to the challenge of categorising different types of patient‐mediated interventions, we also had difficulties with the categorisation of comparisons. Terms like "usual care", "standard care", "common practice", "enhanced usual care", "no intervention" etc. are often used, but these are not necessarily self‐explanatory: Usual care can vary tremendously across time and study setting. This, and the fact that many studies do not describe what "usual care" entailed, makes it hard to assess how similar the comparison groups were in the different studies. In future studies more emphasis should be put in carefully describing both the intervention under study and the conditions that applied to the comparison group.
There are several systematic reviews on, for instance, patient education that have reported on relevant patient health outcomes (Anderson 2017; Attridge 2014; Bennett 2016; Clarkesmith 2017; Fryer 2016; Kelly 2018; Kroon 2014; Lenferink 2017; McBain 2016; McCallum 2017; Parreira 2017; Peytremann‐Bridevaux 2015; Poquet 2016; Zwerink 2014). These do not, however, provide answers about impacts on professional practice. It would be of great interest to assess if a patient education intervention that meets this review's definition of a 'patient‐mediated' intervention would have the same effect on patient health as a patient education intervention not defined as "patient‐mediated intervention". Where interventions have an added focus on healthcare professionals' performance, does this lead to important gains in patient health? The effects on patient health reported in the studies included in this review can thus more likely provide answers regarding the linkage, if any, between health outcomes and clinical performance more than studies that do not measure clinical performance simultaneously.
From our findings, little can be said about the resource use and cost‐effectiveness of these types of interventions, as these outcomes were not usually assessed. Also, we know little about the relative effect of patient‐mediated interventions compared to other approaches directed at healthcare professionals, such as audit and feedback, reminders, education etc., as we did not identify any studies that compared these interventions.
We did not find any studies reporting on patients' trust in healthcare professionals. We therefore need more studies that compare patients´ trust levels after different patient‐mediated interventions to enable us to draw conclusions about these effects. In future studies it would be of great interest to compare how patient‐mediated interventions affect the communicative common ground between a patient and a healthcare professional.
What's new
| Date | Event | Description |
|---|---|---|
| 14 September 2018 | Amended | Additional error in abstract corrected. |
| 13 September 2018 | Amended | Minor error in abstract corrected. |
Acknowledgements
We thank Mette Haaland‐Øverby from Norwegian National Advisory Unit on Learning and Mastery in Health for her consumer input on the protocol. A great thank you to the Norwegian Cancer Society for pointing out the importance of conducting this review and for guidance and co‐operation throughout the process. We would like to thank Elizabeth J Paulsen from the Cochrane Effective Practice and Organisation of Care (EPOC) Group, for her support and expertise in editing and submitting the review. We are also very grateful to the EPOC Editor Simon Lewin and peer‐reviewers for providing insightful comments and suggestions to improve the review.
The Norwegian Satellite of the EPOC Group receives funding from the Norwegian Agency for Development Cooperation (Norad), via the Norwegian Institute of Public Health to support review authors in the production of their reviews.
Appendices
Appendix 1. Search strategies
CENTRAL, Cochrane Library (searched 10.03.2017)
| ID | Search | Hits |
| #1 | "patient mediated":ti,ab,kw | 11 |
| #2 | MeSH descriptor: [Patient Participation] this term only | 1111 |
| #3 | MeSH descriptor: [Patient Education as Topic] this term only | 7967 |
| #4 | MeSH descriptor: [Feedback] this term only | 1131 |
| #5 | MeSH descriptor: [Reminder Systems] this term only | 792 |
| #6 | MeSH descriptor: [Self Care] this term only | 3714 |
| #7 | #2 or #3 or #4 or #5 or #6 | 12898 |
| #8 | MeSH descriptor: [Professional Practice] this term only | 128 |
| #9 | MeSH descriptor: [Family Practice] this term only | 2190 |
| #10 | MeSH descriptor: [General Practice] this term only | 355 |
| #11 | MeSH descriptor: [Quality of Health Care] this term only | 1061 |
| #12 | MeSH descriptor: [Quality Improvement] this term only | 447 |
| #13 | MeSH descriptor: [Guideline Adherence] this term only | 972 |
| #14 | MeSH descriptor: [Practice Patterns, Physicians'] this term only | 1270 |
| #15 | MeSH descriptor: [Practice Patterns, Nurses'] this term only | 106 |
| #16 | MeSH descriptor: [Practice Patterns, Dentists'] this term only | 20 |
| #17 | MeSH descriptor: [Physician‐Patient Relations] this term only | 1271 |
| #18 | MeSH descriptor: [Nurse‐Patient Relations] this term only | 355 |
| #19 | MeSH descriptor: [Dentist‐Patient Relations] this term only | 61 |
| #20 | #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 | 7140 |
| #21 | #7 and #20 | 1272 |
| #22 | ("patient directed intervention" or "patient directed interventions" or intervention* directed near/2 patient*):ti,ab,kw | 90 |
| #23 | "patient education":ti,ab,kw | 10133 |
| #24 | (patient* near/3 (education* next program* or "tailored education")):ti,ab,kw | 561 |
| #25 | ("self care intervention" or "self care interventions"):ti,ab,kw | 68 |
| #26 | "self management" next (intervention* or program*):ti,ab,kw | 1019 |
| #27 | patient* near/1 activat*:ti,ab,kw | 287 |
| #28 | patient* next (guideline* or recommendation*):ti,ab,kw | 40 |
| #29 | patient* next (leaflet* or pamphlet* or booklet* or instruction* or information or questionnaire):ti,ab,kw | 1234 |
| #30 | patient*:ti,ab,kw and motivational next interview*:ti,ab,kw | 941 |
| #31 | ((patient* near/3 challenge*) near/3 (care or treatment* or practice or physician* or practitioner* or doctor*)):ti,ab,kw | 38 |
| #32 | (patient* near/3 (raise* next concern* or raise* next issue* or ask* next question*)):ti,ab,kw | 38 |
| #33 | (patient* near/3 remind*):ti,ab,kw | 354 |
| #34 | (patient* and (remind* near/3 telephone or remind* near/3 phone or remind* near/3 letter or remind* near/3 mail or remind* near/3 email)):ti,ab,kw | 373 |
| #35 | (patient* near/3 feedback):ti,ab,kw | 600 |
| #36 | (physician* or doctor*) near/3 feedback:ti,ab,kw | 179 |
| #37 | "patient reported information":ti,ab,kw | 8 |
| #38 | ("patient reported questionnaire" or "patient reported questionnaires"):ti,ab,kw | 32 |
| #39 | patient next profile*:ti,ab,kw | 130 |
| #40 | (("patient involvement" or "patient participation") and quality):ti,ab,kw | 515 |
| #41 | #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 | 14877 |
| #42 | (physician* or doctor* or practitioner* or provider* or consultation or "family practice" or "general practice" or "clinical practice" or "professional practice" or "primary care" or "primary health care" or "primary healthcare" or "secondary care" or "secondary health care" or "secondary healthcare" or hospital*):ti,ab,kw | 143300 |
| #43 | (reduc* or enhanc* or improv* or (change near/6 practice) or "change performance" or "change behavior" or "change behaviour" or increas* or decreas*):ti,ab,kw | 554640 |
| #44 | #41 and #42 and #43 | 5703 |
| #45 | #1 or #21 or #44 in Trials | 5990 |
MEDLINE and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to August 24, 2018, Ovid (searched 28.08.2018)
| # | Searches | Results |
| 1 | patient mediated.ti,ab,kf. | 61 |
| 2 | Patient Participation/ | 22861 |
| 3 | Patient Education as Topic/ | 80213 |
| 4 | Patient Reported Outcome Measures/ | 1943 |
| 5 | Feedback/ | 28584 |
| 6 | Reminder Systems/ | 3114 |
| 7 | Self Care/ | 30687 |
| 8 | or/2‐7 | 155439 |
| 9 | Professional Practice/ | 16265 |
| 10 | Family Practice/ | 64024 |
| 11 | General Practice/ | 11707 |
| 12 | "Quality of Health Care"/ | 67356 |
| 13 | Quality Improvement/ | 17750 |
| 14 | Guideline Adherence/ | 28614 |
| 15 | Practice Patterns, Physicians'/ | 52980 |
| 16 | Practice Patterns, Nurses'/ | 2169 |
| 17 | Practice Patterns, Dentists'/ | 2106 |
| 18 | Physician‐Patient Relations/ | 68359 |
| 19 | Nurse‐Patient Relations/ | 34113 |
| 20 | Dentist‐Patient Relations/ | 8027 |
| 21 | or/9‐20 | 337326 |
| 22 | (reduc* or enhanc* or improv* or (change adj6 practice) or change performance or change behavior or change behaviour or increas* or decreas*).ti,ab,kf. | 8975775 |
| 23 | 8 and 21 and 22 | 8145 |
| 24 | (patient directed intervention? or (intervention? directed adj2 patient?)).ti,ab,kf. | 72 |
| 25 | patient education.ti,ab,kf. | 16486 |
| 26 | (patient* adj3 (education* program* or tailored education)).ti,ab,kf. | 1811 |
| 27 | self care intervention?.ti,ab,kf. | 172 |
| 28 | (self management adj (intervention? or program*)).ti,ab,kf. | 2184 |
| 29 | (patient* adj1 activat*).ti,ab,kf. | 1743 |
| 30 | (patient* adj (guideline? or recommendation?)).ti,ab,kf. | 729 |
| 31 | (patient* adj (leaflet? or pamphlet? or booklet? or instruction? or information or questionnaire?)).ti,ab,kf. | 10898 |
| 32 | (patient* and motivational interview*).ti,ab,kf. | 1440 |
| 33 | (patient* adj3 challenge* adj3 (care or treatment? or practice or physician* or practitioner? or doctor*)).ti,ab,kf. | 982 |
| 34 | (patient* adj3 (raise* concern? or raise* issue? or ask* question?)).ti,ab,kf. | 515 |
| 35 | (patient* adj3 remind*).ti,ab,kf. | 1040 |
| 36 | ((patient* and remind*) adj3 (telephone or phone or letter or mail or email)).ti,ab,kf. | 860 |
| 37 | (patient* adj3 feedback).ti,ab,kf. | 2574 |
| 38 | ((physician* or doctor?) adj3 feedback).ti,ab,kf. | 687 |
| 39 | patient reported information.ti,ab,kf. | 75 |
| 40 | patient reported questionnaire?.ti,ab,kf. | 172 |
| 41 | patient profile?.ti,ab,kf. | 1869 |
| 42 | ((patient involvement or patient participation) and quality).ti,ab,kf. | 984 |
| 43 | or/24‐42 | 42686 |
| 44 | (physician* or doctor* or practitioner* or provider* or consultation or family practice or general practice or clinical practice or professional practice or primary care or primary health care or primary healthcare or secondary care or secondary health care or secondary healthcare or hospital*).ti,ab,kf. | 1918261 |
| 45 | (reduc* or enhanc* or improv* or (change adj6 practice) or change performance or change behavior or change behaviour or increas* or decreas*).ti,ab,kf. | 8975775 |
| 46 | 43 and 44 and 45 | 13491 |
| 47 | 23 or 46 | 20461 |
| 48 | Randomized Controlled Trial.pt. | 467427 |
| 49 | Controlled Clinical Trial.pt. | 92607 |
| 50 | pragmatic clinical trial.pt. | 851 |
| 51 | (randomis* or randomiz* or randomly).ti,ab. | 786868 |
| 52 | (trial or groups).ti,ab. | 2231237 |
| 53 | or/48‐52 | 2702126 |
| 54 | exp Animals/ | 21744614 |
| 55 | Humans/ | 17254039 |
| 56 | 54 not (54 and 55) | 4490575 |
| 57 | review.pt. | 2420138 |
| 58 | meta analysis.pt. | 91815 |
| 59 | news.pt. | 191071 |
| 60 | comment.pt. | 731439 |
| 61 | editorial.pt. | 466391 |
| 62 | cochrane database of systematic reviews.jn. | 13773 |
| 63 | comment on.cm. | 731434 |
| 64 | (systematic review or literature review).ti. | 116961 |
| 65 | or/56‐64 | 7991812 |
| 66 | 53 not 65 | 2067813 |
| 67 | 1 or (47 and 66) | 5491 |
| 68 | remove duplicates from 67 | 5478 |
| 69 | limit 68 to ed=20170310‐20180310 | 422 |
| 70 | 68 not (1$ or 2$).ed. | 428 |
| 71 | 70 and (201703* or 201704* or 201705* or 201706* or 201707* or 201708* or 201709* or 201710* or 201711* or 201712* or 201801* or 201802* or 201803*).dt. | 0 |
| 72 | 69 or 71 | 422 |
OpenGrey
Patient* AND (doctor OR doctors OR physician* OR practitioner* OR nurse*) AND (guideline* OR procedure* or recommendation* or practice*)
Grey Literature Report
"patient involvement"
Google Scholar
1. allintitle:patient involvement, physician
2. allintitle: patient involvement, practitioner
3. allintitle: patient involvement, doctor
ClinicalTrials.gov
1. Intervention/treatment: Behavioral AND Outcomes: Recommended OR evidence based OR clinical practice OR guideline
2. Intervention/treatment: Behavioral AND Outcomes: Doctor OR physician OR provider OR resident OR practitioner
3. Intervention/treatment: Patient‐mediated
ICTRP
1. Intervention: Behavioural AND (doctor OR physician OR provider OR resident OR practitioner)
2. Intervention: patient‐mediated
Data and analyses
Comparison 1. Patient‐reported health information interventions versus comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to recommended practice | 4 | 3865 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.41, 1.81] |
| 2 Desirable patient health outcomes (increased control over stress) | 1 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.95, 2.76] |
| 3 Patient satisfaction (with care). Number of satisfied patients | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 2.45 [1.27, 4.74] |
| 4 Patient satisfaction (with healthcare professional). The degree of satisfaction | 1 | 79 | Mean Difference (IV, Random, 95% CI) | 0.40 [0.12, 0.68] |
Comparison 2. Patient information interventions versus comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to recommended practice | 11 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.20, 2.13] |
| 2 Adherence to recommended practice. Risk of bias | 11 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.20, 2.13] |
| 2.1 Low risk | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Unclear risk | 10 | 3131 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.12, 1.95] |
| 2.3 High risk | 1 | 371 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [1.68, 3.92] |
| 3 Adherence to recommended practice. Direction of behaviour | 11 | 3502 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [1.20, 2.13] |
| 3.1 Increasing a certain behaviour | 9 | 3249 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.10, 1.94] |
| 3.2 Reducing a certain behaviour | 2 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 3.29 [1.67, 6.48] |
| 4 Desirable patient health outcomes (controlled blood pressure) | 1 | 261 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.79, 1.24] |
| 5 Undesirable patient health outcomes (dyspepsia severity is high, fair to poor health) | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.53, 1.67] |
| 6 Patient satisfaction (with healthcare professional). Number of satisfied patients | 1 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.93, 1.13] |
| 7 Patient satisfaction (with care). The degree of satisfaction | 1 | 186 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.01, 0.59] |
Comparison 3. Patient education interventions versus comparisons.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to recommended practice | 4 | 1029 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.12, 1.54] |
| 2 Desirable patient health outcomes (controlled blood pressure) | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.96, 1.23] |
Comparison 4. Patient decision aids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to recommended practice | 1 | 353 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.65, 1.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alder 2005.
| Methods |
Study design: randomised trial. Number of study arms: 4. Unit of randomisation: patient (parent). Study period: Aug – Dec 2000. Measurement points of outcomes: post intervention. Analysis method: not reported. |
|
| Participants |
Setting Healthcare setting: primary care (2 primary care clinics). Country: USA. Patients Inclusion/exclusion criteria: parents of children aged 1 to 10, with complaints of ear pain, sore throat, cough, congestion and/or fever that had not received antibiotic therapy during the previous two weeks. Numbers of patients: 40 (in study n = 80 with 4 arms). In intervention: 20. In comparison: 20. (In arm 3 n = 20 and in arm 4 n = 20). Characteristics of patients (children):
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: totals not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: a combination of a communication promotion intervention and antibiotic information intervention. Antibiotic information was provided first, then, once the parent had been encouraged to use antibiotics for his or her child only when necessary, the researcher transitioned to the communication intervention. Patient‐mediated intervention category: patient education. Comparison: usual care (placebo‐like). Child nutrition was the focus of the comparison. (The study had a third and fourth arm not addressed here consisting of the patient‐mediated intervention without the communication component and patient‐mediated intervention without the antibiotic information component, respectively). |
|
| Outcomes |
Relevant primary outcomes Antibiotic prescriptions Measurement: not reported, but most likely patient‐reported (parent). Unit of measurement: odds ratio (OR), absolute numbers not reported. Relevant secondary outcomes General satisfaction Interpersonal manner Time spent with doctor Measurement: patient‐reported (parents). Unit of measurement: P values for group differences, absolute numbers not reported. * Primary outcome in study: not reported. |
|
| Notes | We attempted to contact the first author. No reply received. Findings are descriptively reported, and not included in meta‐analysis. Funding: not reported. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few participants were lost (one participant in the control condition is not included in analysis). |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Aragones 2010.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: healthcare professional (1 patient per healthcare professional). Study period: Jul 2006 – May 2007. Measurement points of outcomes: 3 months post intervention. Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: primary care clinic (of a large teaching hospital). Country: USA. Participants Inclusion/exclusion criteria: Latino immigrant Spanish‐speaking patients, 50 years or older, who used the primary care facility as their regular source of care for at least the previous two years. Exclusion: those with current CRC screening, with gastrointestinal symptoms, a personal history of cancer, a family history of CRC, who had a visit with a physician with a patient already in the study, and those who did not consent to participate. Numbers of participants: 65. In intervention: 31. In comparison: 34. Characteristics of participants:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 65. In intervention: 31. In comparison: 34. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: patients were shown a Spanish language colorectal cancer educational video on a portable personal digital video device while they waited for their visits. They were also given a brochure summarising the video and a one‐page reminder to hand to their physician. Patient‐mediated intervention category: patient information. Comparison: usual care. No more information provided. |
|
| Outcomes |
Relevant primary outcomes Physician recommendation of screening Measurement: medical record. Unit of measurement: absolute numbers. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: CRC screening completion. Secondary outcomes were physician recommendation of any CRC screening test recommended in the guidelines and patient adherence to physician CRC screening recommendation. |
|
| Notes |
Funding: Centers for Disease Control and Prevention (CDC). Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk". Author's quote: "Randomization was performed by computer before patient recruitment." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Author's quote: "Intervention patients were also given a one‐page reminder to hand to their physicians notifying them of 1) their patients’ eligibility for CRC screening, and 2) their patients’ receipt of CRC education." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were reviewed by a research assistant not involved in patient recruitment and blind to the randomisation assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for. |
| Selective reporting (reporting bias) | Low risk | Protocol is not accounted for, but found on clinicaltrials.gov (NCT00836303). No obvious deviations found. |
| Other bias | Low risk | No indication of other biases. |
Brody 1990.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 3 (4 arms, but two control arms were lumped together and analysed as one group). Unit of randomisation: practice. Study period: Mar – Jul 1988. Measurement points of outcomes: post intervention (immediately after the medical visit). Analysis method: not reported. |
|
| Participants |
Setting Healthcare setting: primary care (4 medical clinics). Country: USA. Patients Inclusion/exclusion criteria: patients who scored above 3 or more in 12‐item version of the General Health Questionnaire (GHQ). Numbers of patients: 79 (in study n = 103 with 3 arms). In intervention: 29. In comparison: 50 (from two control arms). (In arm 3 n = 24). Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: physicians received information about their patient's mental health problem prior to seeing that patient. Patient‐mediated intervention category: patient‐reported health information about own health/needs/concerns. Comparison: no intervention. Two of the four clinics served as controls, since the residents in these clinics were not exposed to either of these two interventions. These two clinics differed from each other, however, in the leveI of the residents' awareness of this study. Residents in one of these clinics were asked to complete a questionnaire after seeing each study patient. The residents in the other clinic were not asked to complete these questionnaires and were, therefore, less likely to be cognizant of this study. (The study had a third arm not addressed here consisting of the patient‐mediated intervention plus a counselling protocol for healthcare professionals). |
|
| Outcomes |
Relevant primary outcomes Counselling items by healthcare professional Measurement: two separate reports: patient‐reported and healthcare professionals reported. Unit of measurement: average numbers of counselling items (means +/‐ SD). Relevant secondary outcomes Patients with a psychological disorder Measurement: self‐report healthcare professional (physician). Control over stress Measurement: self‐report by patient. * Primary outcome in study: not reported. |
|
| Notes |
Funding: the Robert Wood Johnson Foundation, Princeton, NJ, and the Henry J. Kaiser Family Foundation, Meulo Park, California. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The post‐visit patient questionnaire was administered by a second research assistant who was also blinded to the study's hypothesis and the patients intervention group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ (e.g. number randomised not stated, no reasons for missing data provided).The number of patients for each group and each outcome is uncertain as they do not provide enough information. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Low risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias.
|
Caskey 2011.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 2. Unit of randomisation: healthcare professional. Study period: Dec 2009 – May 2010. Measurement points of outcomes: not reported. Analysis method: not reported. |
|
| Participants |
Setting Healthcare setting: general internal medicine clinic. Country: USA. Patients Inclusion/exclusion criteria: not reported. Numbers of patients: 1402. In intervention: 687. In comparison: 715. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 12. In intervention: 6. In comparison: 6. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: exam‐room education posters. Patient‐mediated intervention category: patient information. Comparison: no intervention (placebo‐like). All physicians received a clinical reminder in the medical record for vaccination at the beginning of intervention period. |
|
| Outcomes |
Relevant primary outcomes Pertussis (Tdap) vaccination Measurement: medical record. Unit of measurement: absolute numbers. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: only one outcome reported. |
|
| Notes | Abstract only. We attempted to contact the first author. No reply received. Funding: not reported. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias
|
Christy 2013.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: 2008 ‐ 2010. Measurement points of outcomes: post intervention (1 week after). Analysis method: per protocol (reported by study authors). |
|
| Participants |
Setting Healthcare setting: primary care clinics (11 clinics). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: self‐identified as black or African‐American, 51–80 years, English‐speaking, and currently non‐adherent to CRC screening guidelines. Exclusion: personal history of CRC or adenomatous polyps requiring surveillance colonoscopy; medical condition precluding CRC screening; cognitive, speech, or hearing impairment; and current adherence to CRC screening guidelines. Numbers of patients: 817. In intervention: 407. In comparison: 410. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: primary care provider (physician). Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 164. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: a clinic‐based, computer‐delivered tailored interactive program about colorectal cancer screening. Patient‐mediated intervention category: patient information. Comparison: usual care (patient information‐like). Non‐tailored brochure about colorectal cancer screening provided at the clinic. |
|
| Outcomes |
Relevant primary outcomes Primary care provider write an order for a colorectal cancer screening test Measurement: medical records. Unit of measurement: relative numbers, odds ratio. Doctor recommended fecal occult blood test (FOBT) Doctor recommended colonoscopy Measurement: patient‐reported. Unit of measurement: relative numbers, odds ratio. They were reported as predictors for another outcome "self‐reported screening discussion with primary care provider". Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: colorectal cancer screening test discussion. |
|
| Notes |
Funding: funded by the National Cancer Institute at the National Institutes of Health. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few lost to follow‐up, evenly distributed. Per protocol analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol is not accounted for, but found on clinicaltrials.gov (NCT00672828). No obvious deviations found. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
Goldberg 2012.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: healthcare professional. Study period: April 2011 ‐ May 2012. Measurement points of outcomes: post intervention (immediately after the medical visit post intervention). Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: hospital (children's hospital). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: 1) child aged 1–17 years presenting with a chief complaint consistent with an asthma exacerbation, such as wheeze or trouble breathing, 2) the child had a history of asthma by parent report, and 3) the visit was believed to be consistent with an asthma exacerbation by the treating attending. Exclusion: patients were excluded if the treating physician was not part of the study, the child had a major pulmonary or cardiac comorbid illness, the child’s parent was non‐English speaking, or if the child was triaged to the med‐trauma bay for severe respiratory distress. Numbers of patients: 77 children. In intervention: 40. In comparison: 37. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 17. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: parents of children with asthma filled out a questionnaire (PACCI‐ED) that measures five domains of asthma health: 1) current control, 2) trajectory, 3) risk, 4) medication adherence and 5) burden. The physicians allocated to this intervention group received the PACCI‐ED filled out by parents and were told what it is used for and that they could use it to complete the clinician assessment form. Patient‐mediated intervention category: patient‐reported health information about own health/needs/concerns. Comparison: no intervention. Physicians in this group had no known exposure to the PACCI‐ED before or during the study. They completed the questions on the clinician assessment form also. |
|
| Outcomes |
Relevant primary outcomes Correctly identified level of chronic asthma control Correctly identified child’s asthma trajectory Correctly identified level of medication adherence Correctly identified degree of disease burden to the family Measurement: clinician assessment form. Unit of measurement: per cent. Relevant secondary outcomes Not relevant outcomes reported. * Primary outcome in study: all four outcomes. |
|
| Notes |
Funding: Rhode Island Hospital (described in protocol). Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A block randomisation scheme with block sizes of 4 was used to randomise physicians prior to beginning the study. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. The physicians were, however, not aware of the study hypothesis. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT‐analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol is not accounted for, but found on clinicaltrials.gov (NCT00836303). No obvious deviations found. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. Unit of randomisation was the healthcare professionals and we do not know their characteristics. |
Herman 1995.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 3. Unit of randomisation: practice (3 practices in total). Study period: Oct 1989 ‐ Mar 1990. Measurement points of outcomes: 3 months post intervention. Analysis method: not reported. |
|
| Participants |
Setting Healthcare setting: public hospital (3 practices). Country: USA. Patients Inclusion/exclusion criteria: all women older than 65 years attending the ambulatory medical clinic were included. Numbers of patients: Total: 839 randomised to 3 arms (not reported the totals for the two arms relevant here). In intervention: not reported (provided only for subgroups of women). In comparison: not reported (provided only for subgroups of women). (In arm 3: n = not reported (provided only for subgroups of women)). Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 45 (n = 66 for all 3 arms). In intervention: 22. In comparison: 23. (In arm 3 n = 21) Characteristics of healthcare professionals:
|
|
| Interventions |
Description of intervention patient‐mediated: in the clinic assigned to intervention, educational materials were given to the patient by the nurse at each clinic visit. The nurses used the "What Every Woman Should Know About Mammography" pamphlet, as well as an additional sheet outlining the specific importance of mammography for the older woman. Patient‐mediated intervention category: patient information. Comparison: no intervention (placeo‐like). A monograph with breast screening recommendations and a lecture on preventive services was also provided bimonthly as part of an ambulatory services lecture series. (The study had a third arm not addressed here consisting of the patient‐mediated intervention plus a prevention team). |
|
| Outcomes |
Relevant primary outcomes Number of women offered mammogram Number of women offered clinical breast exam Measurement: medical records. Unit of measurement: per cent of women. Number of women offered clinical breast examination among those not previously having a clinical breast exam Number of women offered mammography among those not previously having a mammography Measurement: medical records. Unit of measurement: absolute numbers of women without previous clinical breast examination or mammography. Relevant secondary outcomes No relevant reported. * Primary outcome in study: not reported. |
|
| Notes |
Funding: the Case Western Reserve University Teaching Nursing Home Program. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. "The three group practices were assigned randomly to one of three study arms". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Trained research assistant performed outcome assessment, but unclear if blinded or not. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: “839 older women were seen in one of the three practices during the 6‐month intervention period. Thirty one patients were excluded because of dementia or severe illness and five medical records could not be located. Final analysis included 803 women seen by the physician, nurse practitioner, or by the nurse for either a medication refill or education visit”. |
| Selective reporting (reporting bias) | High risk | Protocol not accounted for or found in clinicaltrials.gov. Relevant outcomes were reported for a subgroup of women. Incomplete reporting to make an analysis of the total sample. |
| Other bias | Low risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias:
|
Jacobson 1999.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: May ‐ June 1998. Measurement points of outcomes: post intervention (immediately after the medical visit). Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: hospital (ambulatory care clinic in a public teaching hospital). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: visits to follow management of hypertension, diabetes, heart failure, or other chronic medical problems. Exclusion: Patients not meeting these inclusion criteria, in addition to those with chart‐documented receipt of the vaccine within the past 5 years, walk‐in visits, first visits, medication‐refill visits in which patients did not see their primary care providers, blind patients, patients with clinically documented dementia, and non English‐ speaking patients were excluded. Numbers of patients: 433. In intervention: 221. In comparison: 212. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians (house officers) (n = 148), physician assistants (n = 2) and nurse practitioners (n = 6). Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 156. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: one‐page, low‐literacy (below fifth‐grade level) educational handout encouraging patients to "ask your doctor about the pneumonia shot". Patient‐mediated intervention category: patient information. Comparison: usual care (placebo‐like).1‐page, low‐literacy educational handout to patients conveying information about nutrition. |
|
| Outcomes |
Relevant primary outcomes Clinician recommended vaccine Measurement: patient‐reported. Unit of measurement: absolute numbers. Administration of the vaccine at that clinic visit Measurement: medical record. Unit of measurement: absolute numbers. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: administration of the vaccine at that clinic visit and discussion about the vaccine. |
|
| Notes |
Funding: the National Vaccine Program, Centers for Disease Control and Prevention and the Georgia Emerging lnfections Program, Atlanta, Ga. Also funded by lndigent Care Trust Funds from the State of Georgia to the Office of Health Promotion and Disease Prevention at Grady Health Systems. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation (block size = 1). |
| Allocation concealment (selection bias) | Unclear risk | The first patient enrolled each morning in each clinic section was systematically assigned to the intervention group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Clinicians and patients were not informed of the nature of the study. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of staff members. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Fifty‐eight of 221 patients in the intervention group and 57 of 212 patients in the comparison group had protocol violations or incomplete data collection. High attrition rate (over 20%), but they have performed an ITT‐analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
Kattan 2006.
| Methods |
Study design: randomised trial. Number of study arms: 2 ( 2 x 2 factorial design). Unit of randomisation: patient. Study period: Oct 1998 – Aug 2000. Measurement points of outcomes: post intervention (months 4–14 while the intervention was running. Outcome time point reported as "during the intervention year"). Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: inpatient units of hospitals, emergency departments (EDs), and community paediatric clinics. Country: USA. Patients Inclusion/exclusion criteria: eligibility was limited to residents of census tracts in which 20% or more of households had incomes below the federal poverty level except in Seattle, where patients could be enrolled if they met Medicaid eligibility. Other inclusion criteria included a history of 1 or more hospitalisation or 2 unscheduled visits for asthma in the previous 6 months and a positive allergy skin test to 1 or more of 11 indoor allergens. Children were excluded if they made 2 or more visits to an asthma specialist or asthma clinic in the previous 6 months or if they had any other serious chronic illness. Numbers of patients: 937 children. In intervention: 471. In comparison: 466. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians, nurse practitioners, physician's assistants. Inclusion/exclusion criteria: Numbers of healthcare professionals: total number not reported. In intervention: 435 healthcare professionals. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: computer‐generated letters based on information collected from the child’s carer through bi‐monthly telephone calls (CATI calls) conducted by the centralised service for all the study sites. The letter to the physician caring for that child summarised the child’s asthma symptoms, health service use, and medication use with a corresponding recommendation to step up or step down medications (in accordance with guidelines). Patient‐mediated intervention category: patient‐reported health information about own health/needs/concerns. Comparison: no intervention. computer‐generated letters were not sent to the healthcare professionals of children in the comparison group. For this group, the information from the CATI calls was used to determine what recommendation would have been generated had the child been in the intervention group. |
|
| Outcomes |
Relevant primary outcomes Change in medication when indicated (by guidelines) Measurement: patient‐reported. Changes in medications were determined from the CATI call after a scheduled visit. Step up in medications was defined as an increase from no antiinflammatory use to any anti‐inflammatory use or from occasional to daily anti‐inflammatory use. Unit of measurement: the number of patients with changed medication from the amount of step‐up letters sent to physicians. Relevant secondary outcomes Symptoms because of asthma per 2 weeks
Measurement: patient‐reported. Determined from the CATI call after a scheduled visit. Unit of measurement: continuous. How many times or on a scale. * Primary outcome in study: not reported. |
|
| Notes | We attempted to contact the first author. No reply received. Funding: National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, and the National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services, and the National Center for Research Resources. Conflict of interest: Dr Steinbach has received lecture fees from GlaxoSmithKline and consulting fees from Aventis; Dr Gruchalla is a member of the GlaxoSmithKline Allergy Fellowship Grant review committee; Dr Morgan has received consulting fees from Genentech; and Dr O’Connor is GlaxoSmithKline‐Data Safety and Monitoring Board chair and Astellas Pharma‐Data Safety and Monitoring Board chair. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Group assignments were randomly pre‐assigned to study identification numbers by the coordinating centre using a random number generator with a uniform distribution and blocks of size 8 and 12 within the site". |
| Allocation concealment (selection bias) | Low risk | Quote: "Group assignments were supplied to sites in opaque envelopes and labelled with sequential study identification numbers, which were opened by the site interviewers on determination of the child’s eligibility". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither study staff nor participants were blinded to group assignment. Although study staff and participants were aware of group assignments, they were not aware of the content of the letter sent to PCP. Unclear if physicians were attempted blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The interviewers were blinded to study group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up was minimal and equally distributed. ITT‐analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
Kenealy 2005.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 4. Unit of randomisation: healthcare professional. Study period: 2 months. Measurement points of outcomes: post intervention (outcomes were measured during the two months the study was running). Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: primary care (family practices). Country: New Zealand. Patients Inclusion/exclusion criteria: 50 years or older, no known diabetes, no glucose test within the last three years visiting a healthcare professional. Numbers of patients: 3189 (n = 5628 with 4 arms). In intervention: 1639. In comparison: 1550. (In arm 3 n = 983 and in arm 4 n = 1456). Characteristics of patients:
Healthcare professionals Type of healthcare professionals: family practitioners. Inclusion/exclusion criteria: family practitioners were eligible for the study if they: 1) used a specific patient management computer software, 2) recorded their medical consultation notes on the computer within their consultations, 3) had received laboratory glucose results electronically for at least 1 year, 4) saw at least 10 individual patients aged 50 years or older per month, and 5) worked in the Auckland region. Numbers of healthcare professionals: 55 (n = 107 for all 4 arms) and 33 family practices (n = 66 for all 4 arms) randomised. In intervention: 27 family practitioners and 16 practices. In comparison: 28 family practitioners and 17 practices. (in arm 3: n = 24 family practitioners and 16 practices. In arm 4: n = 28 family practitioners and 17 practices). Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: for healthcare professionals allocated to this intervention their patients filled out a diabetes risk self‐assessment form and gave the filled out form to the healthcare professional (family practitioner) before the consultation. The form, which was adapted from the American Diabetes Association contained information asked patients about their age, ethnicity, weight (body mass index), whether they had a near family members with diabetes, whether they were a woman who had a baby weighing more than 4 kg at birth, and exercise habits. Patient‐mediated intervention category: patient‐reported health information about own health/needs/concerns. Comparison: no intervention (placebo‐like). All healthcare professionals, before group assignment, were visited by research staff to inform about the study, and to provide uniform education on diabetes screening and on how to use both the computer reminder and the patient form (PROMs). A copy of a recent article on diabetes screening and a laminated card summarising the same information was also given each healthcare professional. (The study had a third and fourth arm not addressed here consisting of reminder to healthcare professional and patient‐mediated intervention plus a reminder to healthcare professional, respectively). |
|
| Outcomes |
Relevant primary outcomes Diabetes screening of eligible patients who visited a family practitioner (according to guideline recommendations). Measurement: a visit was defined by the presence of an invoice during the study period. A patient was considered ‘‘screened’’ if they had a laboratory glucose test result in the computer during the study. Unit of measurement: absolute numbers. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: diabetes screening of eligible patients who visited a family practitioner (according to guideline recommendations). |
|
| Notes | First author Dr Timothy Kenealy was contacted and provided requested information. Funding: Health Research Council of New Zealand and Auckland Faculty of the Royal New Zealand College of General Practitioners. Conflict of interest: None disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For the first randomisation, an independent person used Excel to assign a random number between 0 and 1 to each of the 398 FPs. A prior decision was made to invite FPs assigned random numbers 0 to 0.5. An independent person used Excel to generate random numbers in blocks of 8. For the second randomisation, practices were stratified according to number of doctors (solo, 2 to 4 doctors, 5 or more doctors), to protect the intervention groups from gross discrepancies in practice size". |
| Allocation concealment (selection bias) | Low risk | An independent person placed the names of intervention groups in sealed and consecutively numbered envelopes. Thus no indication of selection bias for this cluster‐randomised study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither healthcare professionals nor patients were blinded to intervention delivery. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment via extracted computer records, outcome objective low possibility of assessment bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate among the recruited and randomised healthcare professionals. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol was not published. |
| Other bias | Low risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias:
|
Khan 2011.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: Feb 2007 ‐ Jun 2008. Measurement points of outcomes: 2 months post intervention. Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: clinic (urban diabetes self‐management clinic that serves uninsured patients). Country: USA. Patients Inclusion/exclusion criteria:Iinclusion: 18 years or older, verbal fluency in English, and responsibility for their own diabetes self‐management. Numbers of patients: 129. In intervention: 67. In comparison: 62. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: patients were given waiting room‐administered, low‐literacy, computer multimedia diabetes education program. Patient‐mediated intervention category: patient education. Comparison: usual care (patient information‐like). Patients in this group read an educational brochure. In addition, a short diabetes crossword puzzle based on the brochure was distributed. |
|
| Outcomes |
Relevant primary outcomes Diabetes medication prescribed Antihypertensive medications prescribed Measurement: patient self‐report, routinely verified by clinic physicians. Unit of measurement: absolute numbers. Relevant secondary outcomes HbA1c Measurement: objective measurements by use of phlebotomy at first visit and 3 months later. Unit of measurement: absolute numbers. * Primary outcome in study: not reported. |
|
| Notes |
Funding: the Agency for Healthcare Research and Quality. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author's quote: "Random allocation took place by the research assistant pulling a card out of a box, with each card indicating group assignment (computer multimedia program vs. comparison)." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants not blinded because of the nature of the study, but physicians were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 15 in comparison group and 14 in the intervention group were lost to follow up. ITT‐analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
Kravitz 2012.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: Oct 2006 ‐? Measurement points of outcomes: post intervention for primary outcome and 2, 6 and 12 weeks post intervention for secondary outcomes. Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: medical centre (3 health systems and 1 private practice). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: patients eligible for enrolment in the study included all cognitively intact, English speaking adults obtaining care (active treatment or surveillance) from participating oncologists for selected solid tumours and who reported more than minimal cancer related pain. More than minimal pain was defined as a score of 4 or greater (on a scale of 0‐10). Exclusion: Major surgical procedure scheduled within six weeks, enrolled in hospice, followed by pain management service, already contacted for study, difficulty thinking or expressing herself, unable to receive and/or complete mailed enrolment materials. Numbers of patients: 258 In intervention: 126 In comparison: 132 Characteristics of patients:
Healthcare professionals Type of healthcare professionals: general practitioner. Inclusion/exclusion criteria: Inclusion: medical, radiation, and (after March 2008) gynaecological oncologists (including both staff physicians and clinical fellows) were deemed eligible if they saw patients at one of the participating sites and were in clinical practice at least 20% time (i.e. at least 1 full day per week). Numbers of healthcare professionals: 49 in total. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Intervention: Description of patient‐mediated intervention: patients received tailored education and coaching (TEC) in a private space just before the index visit by a health educator (lay individuals who had undertaken 30‐40 hours of study‐specific training). Patient‐mediated intervention category: patient education (coaching). Comparison: usual care (patient information‐like). Patients in this group received enhanced usual care where health educator verbally reviewed selected aspects of a National Cancer Institute booklet on pain control. |
|
| Outcomes |
Relevant primary outcomes Physician‐directed adjustment in analgesia (new type of or dose/amount adjustment of existing) Measurement: patient‐reported. Unit of measurement: absolute numbers. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: not reported. |
|
| Notes |
Funding: the American Cancer Society and the National Institute of Mental Health. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author's quote: "computer generated, stratified, blocked‐randomization scheme to assure balanced assignment within physicians and encoded three‐digit treatment assignment sequences to preserve concealment". |
| Allocation concealment (selection bias) | Low risk | See comment above. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Author's quote: "To preserve blinding, treatment assignment (0/1) was encoded as a set of 3‐digit numbers maintained by the study statistician. The encoded sequences were printed on two adhesive labels, one affixed to the patient's Enrollment Interview form and another to the Tracking Sheet in each patient's Case Report File (CRF)". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See comment above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In total, 7 were lost to follow‐up, 3 in comparison group and 4 in intervention group. ITT‐ analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol accounted for and exists on clinicaltrials.gov (NCT00283166). No obvious deviations found. |
| Other bias | Low risk | No indication of other biases. |
Krol 2004.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 2. Unit of randomisation: healthcare professional. Study period: 2001. Measurement points of outcomes: 12 and 20 weeks post intervention. Analysis method: not reported. |
|
| Participants |
Setting Healthcare setting: primary care (general practice). Country: the Netherlands. Patients Inclusion/exclusion criteria: Inclusion: patients who had been using proton pump inhibitors (PPIs) on prescription (from their general practitioner) for at least 12 weeks. Exclusion: younger than 18 years, not able to fill in a questionnaire in the Dutch language, serious disease, oesophagitis grade C or D Numbers of patients: 160 randomised. In intervention: 88. In comparison: 72. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: general practitioner. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 20 in total. In intervention: 11. In comparison: 9. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: a simple information leaflet was sent to patients by the GPs in the intervention group. The leaflet gave information about updated recommendations made to GPs about the clinical management of dyspepsia and emphasised the importance of reducing inappropriate use of PPIs. Suggestions were made to reduce or stop using PPIs and advice was given on how to reduce the use and when to seek help from the GP for this. Patients made their own decisions about whether to visit the GP or not. GPs received instruction in a brief visit from a researcher and a flowchart based on the content of the updated Dutch guideline. Patient‐mediated intervention category: patient information. Comparison: usual care. No more information provided. |
|
| Outcomes |
Relevant primary outcomes Stopped or reduced PPI dose Stopped prescribed PPI Had increased PPI dose Measurement: medical record. Unit of measurement: absolute numbers. Relevant secondary outcomes Patient outcomes Dyspesia severity high Measurement: medical record. Unit of measurement: absolute numbers. Mental health Vitality Measurement: patient‐reported. Unit of measurement: mean. * Primary outcome in study: stopped or reduced PPI dose. |
|
| Notes |
Funding: not reported. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Author's quote: "The GPs were allocated at random to either the experimental group or the control group by an independent statistician". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Relatively high attrition rate, but evenly distributed with explanations. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Low risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias:
|
Leveille 2009.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: Aug 2005 ‐ Aug 2006. Measurement points of outcomes: post intervention (in the medical visit) for primary outcome and 1 week and 3 months after the medical visit (post intervention) for secondary outcomes. Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: hospitals and primary care (2 hospital‐based practices and 2 community‐based affiliated practices). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: patients were eligible to participate if they were aged 20 years or older and screened positive for any of our 3 target conditions: chronic musculoskeletal pain, mobility difficulty, or depression. Exclusion: patients currently receiving care for their chronic condition from a specialist physician or therapist. Numbers of patients: 241. In intervention: 121. In comparison: 120. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: primary care practitioners. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: patients received a standardised PatientSite message from the nurse e‐coach that provided a brief description of the screened condition(s) and general tips on how to communicate more effectively with one's primary care practitioner. Patient‐mediated intervention category: patient information. Comparison: usual care (placebo‐like). Patients received a general message through PatientSite containing URL links to US Government web sites with general health information (home pages for the US Department of Health and Human Services and the Centers for Disease Control and Prevention) (placebo). Primary care practitioners immediately were sent PatientSite messages notifying them of the conditions for which their patients screened positive, regardless of group assignment. |
|
| Outcomes |
Relevant primary outcomes Screened condition identified in the index visit Measurement: medical record. Unit of measurement: absolute numbers. Relevant secondary outcomes Rate the medical care in visit Measurement: patient‐reported. Unit of measurement: on 0‐10 scale (best = 10), mean ± SD. Doctor definitely showed concern about health/feelings. Doctor definitely spent enough time. Measurement: patient‐reported. Unit of measurement: absolute numbers of patients reporting the outcome occurring/happening. Pain subscale SF‐36 (moderate‐severe) Measurement: patient‐reported. Unit of measurement: absolute number of patients reporting the outcome occurring/happening. Average pain rating (0‐10, 10 is most) Measurement: patient‐reported. Unit of measurement: on 0‐10 scale (worst/most = 10), mean ± SD. * Primary outcome in study: detection and treatment of the target conditions and symptom burden related to these conditions. |
|
| Notes |
Funding: The Robert Wood Johnson Foundation (RWJF) Health e‐Technologies Initiative. Conflict of interest: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Author's quote: "Patients who screened positive and were eligible for the study were automatically randomized to the control or intervention groups stratified by provider". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information about the sequence generation process to permit judgement of 'Low risk' or 'High risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Described in detailed flow chart and equal lost to follow‐up in both arms. ITT‐analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol not accounted for, but found at clinicaltrial.gov (NCT00130416). No serious protocol deviations. |
| Other bias | Low risk | No indication of other biases. |
Mazonson 1996.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 2. Unit of randomisation: healthcare professional. Study period: about 1 year. Measurement points of outcomes: post intervention. Analysis method: not reported. |
|
| Participants |
Setting Healthcare setting: primary care (Health Maintenance Organisation (HMO)). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: 21‐65 years and symptoms of anxiety and/or depression on Hopkins Symptom Checklist (SCL‐90) above ‘threshold’ on two occasions. Exclusion: previously diagnosed mental health condition or received treatment in the past 6 months. Numbers of patients: 618. In intervention (patient‐mediated intervention): 389. In comparison: 229. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: Primary care physicians. Inclusion/exclusion criteria: Not reported. Numbers of healthcare professionals: 75 healthcare professionals, representing 23 practices. In intervention (patient‐mediated intervention): 40. In comparison: 35. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: the intervention was designed to provide patient self‐reported information on anxiety and depression symptoms and disorders to primary care physicians. Patients filled out the forms and a mental health patient profile was created that summarised and given to the treating physician. Along with the patient profile created, the physicians were offered additional support and information from the study researchers in a 1 hour face‐to face meeting. Follow‐up information in the patient profiles was provided to the physician at 11 weeks and 5 months. The patients were not aware of their scores. Patient‐mediated intervention category: patient‐reported health information about own health/needs/concerns. Comparison: no intervention. No feedback of PROMs scores to physicians or patients. The patient profiles were provided to the comparison physicians after the study. |
|
| Outcomes |
Relevant primary outcomes Recognition of mental health problems (any chart notation or description related to anxiety, stress, depression, or other mental health condition) Measurement: medical records. Unit of measurement: absolute numbers. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: chart notation of anxiety, depression, or other mental health diagnoses or symptoms, referral to mental health specialists, prescription of psychotropic medications, hospitalisation, and office visits. |
|
| Notes |
Funding: the Upjohn Company. Conflict of interest: one author was a former employee at the company that funded the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No indication of attempting to blind the participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Most physicians in both groups stayed on and all the patient was accounted for. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Low risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias:
|
McAlister 2005.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 2. Unit of randomisation: practice. Study period: not reported. Measurement points of outcomes: 3 months and 12 months post intervention. Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: primary care (102 primary care practices). Country: Canada. Patients Inclusion/exclusion criteria: all adult patients with nonvalvular atrial fibrillation (diagnosed by their physician and confirmed by electrocardiogram) who were not living in an institution and had no other indication for or a contraindication to warfarin or ASA were identified in participating practices. Numbers of patients: 446. In intervention: 228. In comparison: 218. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals: not reported.
Numbers of primary care practices: 102. In intervention: 50. In comparison: 52. |
|
| Interventions |
Description of patient‐mediated intervention: patients received a decision aid consisting of a booklet and audiotape that are designed to be self‐administered by patients at home. Patient‐mediated intervention category: patient decision aid. Comparison: usual care (patient information‐like). All potential trial participants attended a group tutorial session before enrolment thus being provided with information about nonvalvular atrial fibrillation) |
|
| Outcomes |
Relevant primary outcomes The proportion of patients whose therapy met the ACCP treatment recommendations Measurement: assessed by telephone follow‐up with patients and review of their medical, pharmacy and laboratory records. Unit of measurement: absolute numbers. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: short‐time effect on proportion of patients whose therapy met the ACCP treatment recommendations. Secondary outcome was the long‐time effect on proportion of patients whose therapy met the ACCP treatment recommendations. |
|
| Notes |
Funding: the Canadian Stroke Network, the Alberta Heritage Foundation for Medical Research (AHFMR), and the University Hospital Foundation, Edmonton. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Author's quote: "Randomization was done centrally to preserve allocation concealment using a computer generated sequence". |
| Allocation concealment (selection bias) | Low risk | See comment above.Thus no indication of selection bias for this cluster‐randomised study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and providers were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients are accounted for. ITT‐analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol referred to (ISRCTN14429643). No serious protocol deviations. |
| Other bias | Low risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias:
|
McKinstry 2006.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: 1 year starting 2002. Measurement points of outcomes: 1 year after intervention. Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: primary care (family practice). Country: Scotland. Patients Inclusion/exclusion criteria: people older than 18 years who had at least one systolic blood pressure recorded > 150 mmHg. Numbers of patients: 294. In intervention: 146. In comparison: 148. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians and nurses. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals: not reported.
|
|
| Interventions |
Description of patient‐mediated intervention: patients were sent: 1) a standard information booklet from the British Hypertension Society (BHS), 2) a detailed guideline, and 3) a record card derived from the Lothian Hypertension Guideline which gave general information about blood pressure, but also provided the patient with clear guidelines as to how their blood pressure should be managed by medical and nursing staff, and a clear exhortation to question their care if the guideline was not being adhered to. The intervention was limited to the distribution of the guideline. Clinical staff members in the practice were fully informed of its content and were told to make use of it if patients took it with them to consultations. However, there was no follow‐up mailing or telephone intervention to reinforce its use. Patient‐mediated intervention category: patient information. Comparison: usual care (patient information‐like). Patients were sent a standard information booklet from the British Hypertension Society (BHS) by post. |
|
| Outcomes |
Relevant primary outcomes: Proportion of patients prescribed statins according to guideline Proportion of patients prescribed aspirin according to guideline Measurement: medical record. Unit of measurement: per cent correct prescriptions. Relevant secondary outcomes Blood pressure (controlled, less than 150/90 mmHg) Cholesterol Measurement: medical record. Unit of measurement: per cent correct prescriptions. Anxiety Depression Measurement: patient self‐report. Unit of measurement: means. * Primary outcome in study: average systolic blood pressure. Secondary outcomes: proportion of patients with blood pressure < 150 mmHg systolic and < 90 mmHg diastolic, average cholesterol, proportion of patients prescribed statins and aspirin according to guideline, hospital anxiety and depression score. |
|
| Notes |
Funding: Chief Scientist Office of the Scottish Executive. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | They used computerised random number generation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients were not blinded. At the time of taking the blood pressure the nurses were blind to the status of the patients (a few patients did, however, reveal which group they were in). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The research nurse, blind to patient randomisation, examined participants prescribing records for evidence of aspirin and statin use. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Losses to follow‐up were minimal and equally distributed. ITT‐analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol not accounted for, but found at clinicaltrial.gov (NCT00148434). No obvious protocol deviations. |
| Other bias | Low risk | No indication of any other biases. |
Miaskowski 2004.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: not reported. Measurement points of outcomes: post intervention (measured over the 6 weeks the study took place and after the intervention). Analysis method: Not reported. |
|
| Participants |
Setting Healthcare setting: hospital (a university‐based cancer centre, two community‐based oncology practices, one health maintenance organisation, one outpatient radiation therapy centre, one veteran’s affairs facility, and one military hospital). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: adult oncology outpatients (18 years or older) who were able to read, write, and understand English. All patients had Karnofsky performance scores of 50 or more, average pain intensity. scores of 2.5 or more, and radiographic evidence of bone metastasis. Numbers of patients: 174. In intervention: 93. In comparison: 81. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: PRO‐SELF group patients were seen by specially trained intervention nurses and received a psychoeducational intervention, were taught how to use a pillbox, and were given written instructions on how to communicate with their physician about unrelieved pain and the need for changes in their analgesic prescriptions. Patients were coached during two follow‐up home visits and three phone calls on how to improve their cancer pain management. Patient‐mediated intervention category: patient education. Comparison: usual care (patient information‐like). Patients in the standard care arm were seen by a research nurse three times and were called three times by phone between the home visits. Patients in the standard care group received the patient version of the Cancer Pain Guideline published by the Agency for Health Care Policy and Research (AHCPR) and were seen by a research nurse in their homes at weeks 1, 3, and 6. Telephone interviews were conducted at weeks 2, 4, and 5. The focus of the visits and phone calls was on monitoring patients’ level of adherence with completing the diary. |
|
| Outcomes |
Relevant primary outcomes Appropriate analgesic prescription (around‐the‐clock (ATC) + as‐needed (PRN)) Measurement: type of opioid prescription (no opioid, only PRN opioid, only ATC opioid, both ATC + PRN opioid. Unit of measurement: Percent. Total dose of opioid analgesics prescribed pr patient per 24 hours Measurement: not reported. Unit of measurement: changes, from baseline, in total dose of opioid analgesics (mg of morphine) prescribed on a 24‐hour basis. Relevant secondary outcomes Patient outcomes Different pain intensity measurements: Average pain Worst pain Least pain Measurement: patient self‐report before bedtime for 6 weeks using a descriptive numeric rating scale that ranged from 0 (none) to 10 (excruciating). Unit of measurement: 1‐10 score, mean. * Primary outcome in study: pain intensity. The secondary outcomes were opioid analgesic intake and appropriate analgesic prescription. |
|
| Notes |
Funding: the National Cancer Institute. Additional funding from Janssen Pharmaceutica and Purdue Pharma LP. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both patients and clinicians at the study sites were blinded to the patient’s group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Author's quote: "Thirty‐eight patients (i.e. 22 in the PRO‐SELF group and 16 in the standard care group) did not complete the entire study for a variety of reasons, including increased severity of illness or intervening cancer treatments that required hospitalization (n 28; 16 in the PRO‐SELF group and 12 in the standard care group) and death (n 10; six in the PRO‐SELF group and four in the standard care group)." |
| Selective reporting (reporting bias) | Low risk | Protocol is not accounted for, but found on clinicaltrials.gov (NCT00708019). No obvious protocol deviations. |
| Other bias | Low risk | No indication of any other biases. |
Mouland 1997.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: 1994 ‐ 1995. Measurement points of outcomes: 4‐12 months post intervention (average 6 months). Analysis method: not reported. |
|
| Participants |
Setting Healthcare setting: primary care (4 primary care practices). Country: Norway. Patients Inclusion/exclusion criteria: Inclusion: assumed daily use of benzodiazepine for at least 3 months of 0.2 or more DDD (daily defined dose). Exclusion: chronic psychosis, severe personal disorders, serious somatic illness, alcohol or drug abuse or daily use of analgesia with codeine. Numbers of patients: 169. In intervention: 100. In comparison: 69. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: general practitioner. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 8. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: patients were sent a letter arguing for reduction of daily benzodiazepine intake, or cessation of the drug. Patient‐mediated intervention category: patient information. Comparison: no intervention (placebo‐like). No letters sent to patients. All clinicians participating in the trial was provided with information about reducing benzodiazepine use. |
|
| Outcomes |
Relevant primary outcomes No benzodiazepines prescription 50‐90% reduction in benzodiazepines prescriptions 0‐49% reduction in benzodiazepines prescriptions Increase in benzodiazepines prescriptions Measurement: medical record. Unit of measurement: per cent. Average prescription of benzodiazepines in a 6‐month period Measurement: medical record. Unit of measurement: DDD (daily defined dose). Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: not reported. |
|
| Notes | Funding: not reported. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The allocation of patients was decided on the basis of the birth date, but the randomisation of which dates was performed by toss a coin. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Patients and healthcare providers knew if the patients received a letter. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 patients were lost to follow‐up and reasons are death, institutionalised or moved to another physician; 8 in letter group and 6 in comparison group. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
Nagykaldi 2012.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 2. Unit of randomisation: practice. Study period: 1 year. Measurement points of outcomes: post intervention (1 year after intervention started). Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: primary care (8 Physicians Resource/Research Network clinicians practices). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: patients that had been seen at least twice by the enrolled physician in the last year, were either children (less than 6 years old) or between 40 and 75 years old (women) or 50 and 75 years old (men), could understand and respond in English, and had a basic level of computer skills, and understand/respond to web content phrased at 6th grade level. Numbers of patients: 560. In intervention: not reported, but we assume 280 enrolled. No information about the group distribution after lost to follow‐up. In comparison: not reported, but we assume 280 enrolled. No information about the group distribution after lost to follow‐up. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians, nurse practitioners and physician assistants. Inclusion/exclusion criteria: Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: patients were offered access to use Wellness Portal—a novel, web‐based patient portal that focuses on wellness, prevention, and longitudinal health assistance. They were also encouraged to print their wellness plan and discuss the plan with their physician at their next office visit. Patient‐mediated intervention category: patient information (patient portal). Comparison: no intervention. Patients in these practices were not given access to the portal and they did not receive personalised recommendations or a wellness plan. |
|
| Outcomes |
Relevant primary outcomes Adults provided all recommended preventive services Adults given low dose aspirin, if indicated Adults given Pneumococcal vaccination because of chronic health conditions Adults given Pneumococcal vaccination because of chronic health conditions Children given all recommended immunisations Measurement: patient‐reported via the portal web and Medical records of patients (paper and electronic) were reviewed in the practice to determine the number and type of selected preventive services received before and during the 12‐month study period. Unit of measurement: percent. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: not reported. |
|
| Notes | We attempted to contact the first author. No reply received. Funding: not reported. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Author's quote: "Pairs of clinician practices were matched on location and practice type (urban, suburban, or rural and solo, small, or midsize) and then randomized within pairs to intervention and control arms”. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Author's quote: "outcome evaluations were completed without an explicit knowledge of group affiliations". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost to follow‐up reported and 31.5%. It is unclear if any groups had higher attrition than others. ITT‐analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol not accounted for, but found at clinicaltrial.gov (NCT01520662). No obvious protocol deviations. |
| Other bias | High risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias:
|
Quinn 2008.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: patient. Study period: 2006 (3‐month study). Measurement points of outcomes: post intervention. Analysis method: not reported. |
|
| Participants |
Setting Healthcare setting: primary care (one community endocrinology and two community primary care practices). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: patients 18–70 years old who had a diagnosis of type 2 diabetes for at least 6 months. Study patients were required to have an A1c of 7.5% or more and to have been on a stable diabetes therapeutic regimen for 3 months prior to study enrolment. Numbers of patients: 30. In intervention: 15. In comparison: 15. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals: not reported.
|
|
| Interventions |
Description of patient‐mediated intervention: the intervention group received cell phone based software that provided real‐time feedback on patients’ blood glucose levels, displayed patients’ medication regimens, incorporated hypo‐ and hyperglycaemia treatment algorithms, and requested additional data needed to evaluate diabetes management. Patient data captured and transferred to secure servers were analysed by proprietary statistical algorithms. The system sent computer‐generated logbooks (with suggested treatment plans) to intervention patients’ healthcare provider (physician) Patient‐mediated intervention category: patient‐reported health information about own health/needs/concerns. Comparison: no intervention (placebo‐like). Patients randomised to this group received blood glucose (BG) monitors and adequate BG testing strips and lancets for the duration of the study. They were asked to fax or call in their BG logbooks every 2 weeks to their healthcare provider (physician) until their BG levels were stabilised in the target ranges or until their healthcare provider (physician) changed testing frequency. |
|
| Outcomes |
Relevant primary outcomes Medications titrated or changed by their healthcare provider Medication errors identified by their healthcare provider Measurement: medical record. Unit of measurement: absolute numbers. Relevant secondary outcomes HbA1c Measurement: medical record. Unit of measurement: percent. Depression diagnosis Measurement: medical record. Unit of measurement: absolute numbers. Provider diabetes management improved by receipt of blood sugar measurements Measurement: patient self‐report. Unit of measurement: absolute numbers. * Primary outcome in study: HbA1c. Secondary outcomes were on health care provider (HCP) adherence to prescribing guidelines and HCPs’ adoption of the technology. |
|
| Notes |
Funding: LifeScan, Inc. and Nokia, Inc. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 30 randomised and 26 analysed. Author's quote: "Characteristics for drop‐out subjects were not different from the remaining study subjects". |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
Thiboutot 2013.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 2. Unit of randomisation: healthcare professional. Study period: 1 year. Measurement points of outcomes: post intervention (at end of study (1 year)). Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: primary care practices. Country: USA. Patients Inclusion/exclusion criteria: Inclusion: Age 21 years or older, fluent in English, at least 2 high blood pressure readings in the previous 12 months (130/80 mmHg or higher for patients with diabetes or chronic kidney disease, 140/90 mmHg or higher for patients without), and their physician was participating in the study. Exclusion: Receiving care from another physician for hypertension treatment (e.g. cardiologist), hospitalised for a psychiatric disorder in the past 3 years, participating in another clinical research study, pregnant or planned to become pregnant in the next 12 months, planning on moving out of the area in the next 12 months, no personal access to the Internet at home or at work, and no personal email account. Numbers of patients: 500. In intervention: 282. In comparison: 218. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: primary care physicians. Inclusion/exclusion criteria: physicians that were board‐certified in internal medicine or family practice, did not have specialty training in nephrology or cardiology, were clinically active (at least 50% of their time spent providing direct primary care), were not planning to retire in the next two years, listed as retired, part‐time or inactive. Numbers of healthcare professionals: 54. In intervention: 27. In comparison: 27. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: patients received a Web‐based intervention for 12 months, which included: 1) Web‐based hypertension feedback based on the individual patient’s self‐report of health variables decision rules, and tailored feedback based on recommendations from JNC 7, 2) a “pocket chart” that patients could print and take to their doctor visits to help them record their blood pressure that could later be entered into the website, and 3) automated reminders that tracked the dates of upcoming visits with their PCP to remind patients to use the website before physician visits. Patients were expected to use the website at least once each month and received reminder emails if 30 days had elapsed since the last time they used the website. Patient‐mediated intervention category: patient education. Comparison: usual care (placebo‐like). Patients received the same components of the intervention as intervention condition patients (e.g. Web‐based personalised feedback, pocket chart, email reminders), but the website focused on preventive services that were not related to hypertension care (e.g. mammography screening, tetanus immunisations) and were recommended by the USPSTF (placebo). |
|
| Outcomes |
Relevant primary outcomes Hypertension screening tests(creatinine, urine protein, serum potassium) Measurement: medical records. Unit of measurement: absolute numbers. Doctor recommended starting a new blood pressure medication Measurement: patient self‐report. Unit of measurement: absolute numbers. Doctor recommended increasing dose of a blood pressure medication Measurement: patient self‐report. Unit of measurement: absolute numbers. Relevant secondary outcomes Patient outcomes: Controlled blood pressure Measurement: medical records. Unit of measurement: absolute numbers. * Primary outcome in study: change in blood pressure and change in the percentage of patients in each group with controlled blood pressure. Secondary outcomes were hypertension screening tests, lifestyle counselling, and medication intensification. |
|
| Notes |
Funding: the National Heart, Lung and Blood Institute. The user interface development was done by Digital Alternatives under contract by authors. To ensure fidelity to the use of the intervention, patients in both conditions were eligible to receive US $5 for each month they used the website, for a potential total of US $60. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The primary care physicians were enrolled and randomised into 1 of 2 conditions by selecting an envelope containing a document with one of the two conditions assigned (intervention or comparison condition) from a stack of sealed envelopes. A statistician generated the order of the envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To minimise the potential for unblinding physicians, all recruitment letters and discussions with physicians stated that the overall goal of the study was to improve primary and secondary prevention for patients with hypertension. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | To reduce the chances that staff would treat patients differently, particularly while assessing outcomes, staff were blinded to the condition of the provider. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rate. ITT‐analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol is not accounted for, but found on clinicaltrials.gov (NCT00377208). No obvious deviations found. |
| Other bias | High risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias:
|
Thomas 2003.
| Methods |
Study design: randomised trial. Number of study arms: 3. Unit of randomisation: patient. Study period: Aug – Sept 1998 patient charts were screened for eligibility as patients presented for their clinic visits. No more information provided. Measurement points of outcomes: post intervention (immediately after clinic visit). Analysis method: ITT (reported by study authors). |
|
| Participants |
Setting Healthcare setting: public hospital. Country: USA. Patients Inclusion/exclusion criteria: Inclusion: At least one of the targeted vaccine indications (age > 65 years, heart or lung disease, or diabetes) and were not previously vaccinated. Exclusion: deafness, blindness, language barriers, chart‐documented dementia, and ineligible clinic visits (such as walk‐in visits, first‐time visits, and medication refill visits in which patients did not see a provider). Numbers of patients: 371 (in study n = 558 with 3 arms). In intervention: 189. In comparison: 182. (In arm 3 n = 187). Characteristics of patients:
Healthcare professionals Type of healthcare professionals: primary care physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: not reported. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: Patients saw a videotape and received a intervention brochure about the pneumococcal vaccine. The brochure presented minimal information about the vaccine and prompted the patient to ask his/her doctor about the pneumonia shot today. Patient‐mediated intervention category: patient information. Comparison: usual care (placebo‐like). Patients received a brochure about nutrition. (The study had a third arm not addressed here consisting of the patient‐mediated intervention with a control brochure (nutrition) in stead of pneumococcal vaccine brochure). |
|
| Outcomes |
Relevant primary outcomes Primary care physician recommended vaccine Measurement: patient self‐report. Unit of measurement: absolute numbers. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: discussion of vaccine and patients receiving vaccine. Secondary outcomes were patient read brochure, patient showed brochure to primary care physician, and primary care physician recommended vaccine. |
|
| Notes |
Funding: National Vaccine Program and the CDC Emerging Infections Program. Also supported in part by Indigent Care Trust Funds from the State of Georgia to the Office of Health Promotion and Disease Prevention at Grady Health System. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Author's quote: "For the randomization, each eligible patient was sequentially assigned to the VB, V, or C groups by the study staff; thus, the first and then every third eligible patient was assigned to the VB group, every third eligible patient following a VB patient was assigned to the V group, and every third eligible patient following a V patient was assigned to the C group". |
| Allocation concealment (selection bias) | High risk | See comment above. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT‐analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
Turner 1990.
| Methods |
Study design: randomised trial. Number of study arms: 2. Unit of randomisation: healthcare professional. Study period: Sept 1987 – May 1988 Measurement points of outcomes: post intervention, but not reported exactly time point. Analysis method: Not reported. |
|
| Participants |
Setting Healthcare setting: outpatient centre. Country: USA. Patients Inclusion/exclusion criteria: not reported. Numbers of patients: 423. In intervention: 177. In comparison: 246. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 24. In intervention: 12. In comparison: 12. Characteristics of healthcare professionals:
|
|
| Interventions |
Description of patient‐mediated intervention: patients received a health maintenance prompt card from a clinic receptionist and instructed to keep this card, bring it to all future appointments, and show it to the physician. No attempt was made to educate about health maintenance. Patient‐mediated intervention category: Patient information. Comparison: no intervention (placebo‐like). Patients did not receive prompt cards. A computer‐prompting system was instituted to remind all residents to perform a list of preventive measures when indicated. |
|
| Outcomes |
Relevant primary outcomes Pap‐smear Breast exam Mammography scheduled Stool occult test Influenza vaccine Pneumococcal vaccine Measurement: medical record. Unit of measurement: absolute numbers of indicated. Relevant secondary outcomes No relevant outcomes reported. * Primary outcome in study: not reported. |
|
| Notes |
Funding: North Carolina United Way. Conflict of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Author's quote: "The groups were randomized into control and experimental groups based on their assigned clinic day". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
Wright 2012.
| Methods |
Study design: cluster‐randomised trial. Number of study arms: 2. Unit of randomisation: practices. Study period: Sept 2005 – Mar 2007. Measurement points of outcomes: 60 days post intervention. Analysis method: ITT and on‐treatment (reported by study authors). |
|
| Participants |
Setting Healthcare setting: primary care (11 primary care practices). Country: USA. Patients Inclusion/exclusion criteria: Inclusion: To participate in this study, patients had to have an active Patient gateway account and a primary care provider assigned in the Longitudinal Medical Record (LMR). Numbers of patients: 3979 eligible to start with, 856 eligible to receive reminders (indications). In intervention: 396. In comparison: 460. Characteristics of patients:
Healthcare professionals Type of healthcare professionals: physicians. Inclusion/exclusion criteria: not reported. Numbers of healthcare professionals: 167. In intervention: not reported. In comparison: not reported. Characteristics of healthcare professionals:
Practices Numbers of practices: 11. In intervention: 7. In comparison: 4. |
|
| Interventions |
Description of patient‐mediated intervention: patients with an active Patient gateway account in the intervention arm could receive any of six types of health maintenance reminders as indicated: bone density testing, cholesterol testing, influenza vaccination, mammography, Pap smear and pneumococcal vaccination. Information was transmitted to the LMR, through which the patient’s PCP could review eJournals and order screenings. Providers received reminders when a patient was due for a health maintenance procedure. Patient‐mediated intervention category: patient information. Comparison: usual care (placebo‐like). Patients in the active control arm were invited to complete eJournals that allowed them to review and modify medication and allergy lists and diabetes management information (placebo). Providers received reminders when a patient was due for a health maintenance procedure. The primary difference between the arms was the content of the modules patients reviewed after opening an eJournal. |
|
| Outcomes |
Relevant primary outcomes Influenza vaccines Mammography Pap smears Pneumovax Bone density Cholesterol Measurement: not reported, but most likely medical record. Unit of measurement: absolute numbers of those indicated. Relevant secondary outcomes No relevant outcomes reported. * Primary outcomes in study: adherence to guideline‐based care recommendations (all outcomes here within). |
|
| Notes |
Funding: AHRQ. Conflict of interest: none disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author's quote: "Randomization was carried out by the study statistician who had no further role in the project". |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information about the allocation procedure. Cluster‐randomised study and thus increased risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants are accounted for. ITT‐analysis. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of 'Low risk' or 'High risk'. Protocol not accounted for or found in clinicaltrials.gov. |
| Other bias | High risk | This is a cluster‐randomised trial and thus we have judged additional sources of potential bias.
|
ASA: acetylsalicylic acid CEC: colorectal cancer CI: confidence interval ICC: intra‐cluster correlation coefficient ITT: intention‐to‐treat SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2014 | No relevant professional performance outcomes reported |
| Alexander 2011 | Not patient‐mediated intervention(s) |
| Altiner 2007 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Amble 2015 | No relevant professional performance outcomes reported |
| Anderson 2004 | No relevant professional performance outcomes reported |
| Ansari 2003 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Atherton‐Naji 2001 | The relevant professional performance outcomes are not reported with recommended or desired direction |
| Barr 2001 | No relevant professional performance outcomes reported |
| Basch 1999 | No relevant professional performance outcomes reported |
| Becker 1989 | No relevant professional performance outcomes reported |
| Bessette 2011 | The relevant professional performance outcomes is likely to be confounded by patients attendance rates |
| Bickman 2011 | No relevant professional performance outcomes reported |
| Bird 1990 | No relevant professional performance outcomes reported |
| Bloomfield 2005 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Branch 1999 | No relevant professional performance outcomes reported (skills rather than performance) |
| Brinkman 2007 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Brodey 2005 | No relevant professional performance outcomes |
| Burack 1994 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Burack 1996 | No relevant professional performance outcomes reported |
| Burack 1998 | No relevant professional performance outcomes reported |
| Burack 2003 | No relevant professional performance outcomes reported |
| Campbell 1997 | No relevant professional performance outcomes reported |
| Chang 2012 | Not a RCT |
| Chodosh 2015 | No relevant professional performance outcomes reported |
| Chou 2011 | No relevant professional performance outcomes reported |
| Clementz 1990 | No relevant professional performance outcomes reported |
| Clever 2006 | Not a RCT |
| Clover 1992 | No relevant professional performance outcomes reported |
| Cohen‐Cline 2014 | No relevant professional performance outcomes reported |
| Cooper 2011 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Cooper 2013 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Corson 2011 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Costanza 2007 | No relevant professional performance outcomes reported |
| Datto 2003 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Deeb 1988 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Dietrich 2013 | No relevant professional performance outcomes reported |
| Dolan 2002 | No relevant professional performance outcomes reported |
| Early 2015 | No relevant professional performance outcomes reported |
| Echeverry 2003 | Not a RCT |
| Feder 1999 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Finlay 1999 | No relevant professional performance outcomes reported |
| Fisher 2011 | No relevant professional performance outcomes reported |
| Fleisher 1999 | No relevant professional performance outcomes reported |
| Flottorp 2002 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Fluckiger 2012 | No relevant professional performance outcomes reported |
| Fortuna 2014 | No relevant professional performance outcomes reported |
| Förberg 2017 | Not patient‐mediated intervention(s) |
| Gabbay 2012 | Not patient‐mediated intervention(s) |
| Galliher 2010 | No relevant professional performance outcomes reported. Author contacted. No reply received. |
| Garcia 2013 | Not patient‐mediated intervention(s) |
| Garcia 2015 | Not patient‐mediated intervention(s) |
| Gersch 2014 | No relevant professional performance outcomes reported |
| Ghadieh 2015 | No relevant professional performance outcomes reported |
| Ginson 2000 | Not patient‐mediated intervention(s) |
| Gooding 2012 | Not a RCT |
| Grace 2005 | Not a RCT |
| Greco 2001 | No relevant professional performance outcomes reported |
| Haskard 2008 | No relevant professional performance outcomes reported |
| Hornberger 1997 | No relevant professional performance outcomes reported |
| Jager 2017 | The patient‐mediated intervention is one of two or more components in an intervention package. It is not the main component |
| Katz 2011 | Comparison of two similar patient‐mediated interventions (differ by one intervention component) |
| Kinugasa 2014 | Not a RCT |
| Kravitz 2005 | Not patient‐mediated intervention(s) |
| Lafata 2007 | The relevant professional performance outcomes is likely to be confounded by patients attendance rates |
| Lawton 2017 | No relevant professional performance outcomes reported |
| Levy 2013 | No relevant professional performance outcomes reported |
| Linder 2009 | Not patient‐mediated intervention(s) |
| Little 2004 | No relevant professional performance outcomes reported. Author contacted and reply received |
| Liu 2016 | Not patient‐mediated intervention(s) |
| Lynch 2004 | No relevant professional performance outcomes reported |
| Manfredi 1998 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Marshall 2016 | Not patient‐mediated intervention(s) |
| Marteau 2010 | No relevant professional performance outcomes reported |
| Menon 2011 | The relevant professional performance outcomes is likely to be confounded by patients attendance rates |
| Michalopoulou 2010 | Not a RCT |
| Mitchell 2005 | Not patient‐mediated intervention(s) |
| Mohler 1995 | No relevant professional performance outcomes reported |
| Myers 2007 | No relevant professional performance outcomes reported |
| Myers 2008 | No relevant professional performance outcomes reported |
| Myers 2011 | No relevant professional performance outcomes reported |
| O'Connor 2009 | The relevant professional performance outcomes is likely to be confounded by patients attendance rates |
| Olsson 2012 | No relevant professional performance outcomes reported |
| Ornstein 1991 | The relevant professional performance outcomes is likely to be confounded by patients attendance rates |
| Osborn 2010 | Not patient‐mediated intervention(s) |
| Osman 1994 | The relevant professional performance outcomes are not reported with recommended or desired direction |
| Osman 2002 | The relevant professional performance outcomes are not reported with recommended or desired direction |
| Persell 2008 | No relevant professional performance outcomes reported |
| Porter 2006 | Not a RCT |
| Raisch 1999 | Not a RCT |
| Reinders 2010 | Not a RCT |
| Rise 2012 | No relevant professional performance outcomes reported |
| Robling 2012 | Not patient‐mediated intervention(s) |
| Roland 1989 | No relevant professional performance outcomes reported |
| Rosenthal 2005 | Not patient‐mediated intervention(s) |
| Rosser 1991 | The relevant professional performance outcomes is likely to be confounded by patients attendance rates |
| Rubenstein 1995 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Sherrard 2015 | No relevant professional performance outcomes reported |
| Simon 2012 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Smeele 1999 | Not patient‐mediated intervention(s) |
| Smit 2005 | The patient‐mediated intervention is one of two or more components in an intervention package. It is not the main component. |
| Solomon 2007 | The patient‐mediated intervention is one of two or more components in an intervention package. It is not the main component |
| Sonnichsen 2010 | The patient‐mediated intervention is one of two or more components in an intervention package. It is not the main component |
| Spahr 2006 | Not a RCT |
| Spaic 2013 | The patient‐mediated intervention is one of two or more components in an intervention package. It is not the main component |
| Thapar 2002 | No relevant professional performance outcomes reported |
| Valanis 2002 | No relevant professional performance outcomes reported |
| Vallès 2002 | Not patient‐mediated intervention(s) |
| Vallès 2003 | Not patient‐mediated intervention(s) |
| Vickrey 2006 | The patient‐mediated intervention is one of two or more components in an intervention package. It is not the main component |
| Vingerhoets 2001 | No relevant professional performance outcomes reported |
| Wasson 1999 | The patient‐mediated intervention is one of two or more components in an intervention package directed at providers |
| Wensing 2003 | No relevant professional performance outcomes |
| Wilson 1993 | No relevant professional performance outcomes |
| Wynia 2010 | Not patient‐mediated intervention(s) |
| Zermansky 2001 | Not patient‐mediated intervention(s) |
RCT: randomised trial
Characteristics of ongoing studies [ordered by study ID]
NCT01904656.
| Trial name or title | CBPR Strategies to Increase colorectal cancer screening in Ohio Appalachia |
| Methods | RCT |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Intervention: "Get Behind your health". Patients are exposed to the "Get Behind Your Health!" media campaign intervention comprising 3 phases: the media campaign, the medical chart reminder, and a combination of media campaign and chart reminder. Comparison: patients are exposed to a Healthy Eating "Peaches!"‐ media campaign intervention comprising 3 phases: the media campaign, the medical chart reminder, and a combination of media campaign and chart reminder. Patients also undergo telephone interviews during years 2‐4. |
| Outcomes | Primary Outcomes Rates of colorectal cancer screening‐within‐guidelines |
| Starting date | September 2009 |
| Contact information | Principal Investigator: Electra Paskett, Ohio State University |
| Notes | Status September 2017: Ongoing, but not recruiting participants. |
NCT02686775.
| Trial name or title | The PACO Project: a clinical study of a PAtient COach program in vulnerable lung cancer Patients (PACO) |
| Methods | RCT |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Intervention:patient coach: 5 face‐to‐face sessions of approximately 1‐2 hours duration and 3 phone calls from inclusion to one month after end of first line treatment. Deviations from this schedule might depend on the treatment modules and on the wishes and needs of the patient. Several patients will continue directly into palliative care and the coach will thus support this transition. Comparison: standard care |
| Outcomes | Primary Outcomes Receipt of first‐line treatment according to clinical guidelines |
| Starting date | January 2016 |
| Contact information | Principal Investigator: Susanne O Dalton, Danish Cancer Society Research Center, sanne@cancer.dk |
| Notes | Status September 2017: Currently recruiting participants |
CBPR: Community‐Based Participatory Research CRC: colorectal cancer RCT:randomised trial
Contributions of authors
MSF led the work with and wrote the protocol, performed some of the searches, screened studies for inclusion, extracted data, assessed risk of bias, assessed certainty of the evidence (GRADE), and drafted the review.
TKD assisted with the protocol, screened studies for inclusion, extracted data, assessed risk of bias, assessed certainty of the evidence (GRADE), and commented on drafts of the review.
AF assisted with the protocol, assisted with screening of studies for inclusion, and commented on drafts of the review.
MJ designed and carried out most of the searches.
SF provided general advices on the protocol and commented on drafts of the review.
HS provided general advices on the protocol and commented on drafts of the review.
Sources of support
Internal sources
Department for Evidence Synthesis, Norwegian Institute of Public Health, Oslo, Norway.
Norwegian National Advisory Unit on Learning and Mastery in Health, Oslo, Norway.
External sources
No sources of support supplied
Declarations of interest
Marita S Fønhus: none known.
Therese K Dalsbø: none known.
Marit Johansen: none known.
Atle Fretheim: none known.
Helge Skirbekk: none known.
Signe A Flottorp: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Alder 2005 {published data only}
- Alder SC, Trunnell EP, White GL, Lyon JLJ, Reading JP, Magill MK. Reducing parental demand for antibiotics by promoting communication skills. American Journal of Health Education 2005;363(3):132‐9. [Google Scholar]
Aragones 2010 {published data only}
- Aragones A, Schwartz MD, Shah NR, Gany FM. A randomized controlled trial of a multilevel intervention to increase colorectal cancer screening among Latino immigrants in a primary care facility. Joutnal of General Interal Medicine 2010;25(6):564‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brody 1990 {published data only}
- Brody DS, Lerman CE, Wolfson HG, Caputo GC. Improvement in physicians' counseling of patients with mental health problems. Archives of Internal Medicine 1990;150(5):993‐8. [PubMed] [Google Scholar]
Caskey 2011 {published data only}
- Caskey R, Weiner S, Gerber B. Exam‐room based education to influence vaccination behavior among veteran patients in a primary care setting. Journal of General Internal Medicine 2011;26:S271. [Google Scholar]
Christy 2013 {published data only}
- Christy SM, Perkins SM, Tong Y, Krier C, Champion VL, Skinner CS, et al. Promoting colorectal cancer screening discussion: a randomized controlled trial. American Journal of Preventive Medicine 2013;44(4):325‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goldberg 2012 {published data only}
- Goldberg EM, Laskowski‐Kos U, Wu D, Gutierrez J, Bilderback A, Okelo S, et al. Can the pediatric asthma control and communication instrument (PACCI) be used in the ED to improve clinicians' assessment of asthma control?. Academic Emergency Medicine. Conference: 2012 Annual Meeting of the Society for Academic Emergency Medicine. Vol. Conference Publication: (var.pagings): 19, issue s1:S348.
Herman 1995 {published data only}
- Herman CJ, Speroff T, Cebul RD. Improving compliance with breast cancer screening in older women. Results of a randomized controlled trial. Archives of Internal Medicine 1995;155(7):717‐22. [PubMed] [Google Scholar]
Jacobson 1999 {published data only}
- Jacobson TA, Thomas DM, Morton FJ, Offutt G, Shevlin J, Ray S. Use of a low‐literacy patient education tool to enhance pneumococcal vaccination rates. A randomized controlled trial. Jama 1999;282(7):646‐50. [DOI] [PubMed] [Google Scholar]
Kattan 2006 {published data only}
- Kattan M, Crain EF, Steinbach S, Visness CM, Walter M, Stout JW, et al. A randomized clinical trial of clinician feedback to improve quality of care for inner‐city children with asthma. Pediatrics 2006;117(6):e1095‐103. [DOI] [PubMed] [Google Scholar]
Kenealy 2005 {published and unpublished data}
- Kenealy T, Arroll B, Petrie KJ. Patients and computers as reminders to screen for diabetes in family practice. Randomized‐controlled trial. Journal of General Internal Medicine 2005;20(10):916‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2011 {published data only}
- Khan MA, Shah S, Grudzien A, Onyejekwe N, Banskota P, Karim S, et al. A diabetes education multimedia program in the waiting room setting. Diabetes Therapy Research, Treatment and Education of Diabetes and Related Disorders 2011;2(3):178‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kravitz 2012 {published data only}
- Kravitz RL, Tancredi DJ, Jerant A, Saito N, Street RL, Grennan T, et al. Influence of patient coaching on analgesic treatment adjustment: secondary analysis of a randomized controlled trial. J Pain Symptom Manage 2012;43(5):874‐84. [DOI] [PubMed] [Google Scholar]
Krol 2004 {published data only}
- Krol N, Wensing M, Haaijer‐Ruskamp F, Muris JW, Numans ME, Schattenberg G, et al. Patient‐directed strategy to reduce prescribing for patients with dyspepsia in general practice: a randomized trial. Alimentary Pharmacology & Therapeutics 2004;19(8):917‐22. [DOI] [PubMed] [Google Scholar]
Leveille 2009 {published data only}
- Leveille SG, Huang A, Tsai SB, Allen M, Weingart SN, Iezzoni LI. Health coaching via an internet portal for primary care patients with chronic conditions: a randomized controlled trial. Medical Care 2009;47(1):41‐7. [DOI] [PubMed] [Google Scholar]
Mazonson 1996 {published data only}
- Mazonson PD, Mathias SD, Fifer SK, Buesching DP, Malek P, Patrick DL. The mental health patient profile: does it change primary care physicians' practice patterns?. Journalof the American Board of Family Practice/ American Board of Family Practice 1996;9(5):336–45. [PubMed] [Google Scholar]
McAlister 2005 {published data only}
- McAlister FA, Man‐Son‐Hing M, Straus SE, Ghali WA, Anderson D, Majumdar SR, et al. Impact of a patient decision aid on care among patients with nonvalvular atrial fibrillation: a cluster randomized trial. CMAJ : Canadian Medical Association journal 2005;173(5):496‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKinstry 2006 {published data only}
- McKinstry B, Hanley J, Heaney D, McCloughan L, Elton R, Webb DJ. Impact on hypertension control of a patient‐held guideline: a randomised controlled trial. British Journal of General Practice 2006;56(532):842‐7. [PMC free article] [PubMed] [Google Scholar]
Miaskowski 2004 {published data only}
- Miaskowski C, Dodd M, West C, Schumacher K, Paul SM, Tripathy D, et al. Randomized clinical trial of the effectiveness of a self‐care intervention to improve cancer pain management. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology 2004;22(9):1713‐20. [DOI] [PubMed] [Google Scholar]
Mouland 1997 {published data only}
- Mouland G. A letter to benzodiazepine users‐‐an efficient way to reduce the prescription. Tidsskrift for den Norske lægeforening 1997;117(21):3097‐100. [PubMed] [Google Scholar]
Nagykaldi 2012 {published data only}
- Nagykaldi Z, Aspy CB, Chou A, Mold JW. Impact of a Wellness Portal on the delivery of patient‐centered preventive care. Journal of the American Board of Family Medicine 2012;25(2):158‐67. [DOI] [PubMed] [Google Scholar]
Quinn 2008 {published data only}
- Quinn CC, Clough SS, Minor JM, Lender D, Okafor MC, Gruber‐Baldini A. WellDoc mobile diabetes management randomized controlled trial: change in clinical and behavioral outcomes and patient and physician satisfaction. Diabetes Technology & Therapeutics 2008;10(3):160‐8. [DOI] [PubMed] [Google Scholar]
Thiboutot 2013 {published data only}
- Thiboutot J, Sciamanna C, Falkner B, Kephart D, Stuckey H, Adelman AM, et al. Effects of a web‐based patient activation intervention to overcome clinical inertia on bloodpressure control: cluster randomized controlled trial.. Journal of Medical Internet Research 2013;15(9):e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2003 {published data only}
- Thomas DM, Ray SM, Morton FJ, Drew JS, Offutt G, Whitney CG, et al. Patient education strategies to improve pneumococcal vaccination rates: randomized trial. Journal of Investigative Medicine 2003;51(3):141‐8. [DOI] [PubMed] [Google Scholar]
Turner 1990 {published data only}
- Turner RC, Waivers LE, O'Brien K. The effect of patient‐carried reminder cards on the performance of health maintenance measures. Archives of Internal Medicine 1990;150(3):645‐7. [PubMed] [Google Scholar]
Wright 2012 {published data only}
- Wright A, Poon EG, Wald J, Feblowitz J, Pang JE, Schnipper JL, et al. Randomized controlled trial of health maintenance reminders provided directly to patients through an electronic PHR. Journal of General Internal Medicine 2012;27(1):85‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2014 {published data only}
- Adams WG, Phillips BD, Bacic JD, Walsh KE, Shanahan CW, Paasche‐Orlow MK. Automated conversation system before pediatric primary care visits: a randomized trial. Pediatrics 2014;134(3):e691‐99. [DOI] [PubMed] [Google Scholar]
Alexander 2011 {published data only}
- Alexander KP, Wang TY, Li S, Lytle BL, Slattery LE, Calhoun S, et al. Randomized trial of targeted performance feedback to facilitate quality improvement in acutemyocardial infarction care. Circulation Cardiovascular Quaityl Outcomes 2011;4(1):129‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Altiner 2007 {published data only}
- Altiner A, Brockmann S, Sielk M, Wilm S, Wegscheider K, Abholz HH. Reducing antibiotic prescriptions for acute cough by motivating GPs to change their attitudes to communication and empowering patients: a cluster‐randomized intervention study. Journal of Antimicrobial Chemotherapy 2007;60(3):638‐44. [DOI] [PubMed] [Google Scholar]
Amble 2015 {published data only}
- Amble I, Gude T, Stubdal S, Andersen BJ, Wampold BE. The effect of implementing the Outcome Questionnaire‐45.2 feedback system in Norway: A multisite randomized clinical trial in a naturalistic setting. Psychotherapy Research 2015;25(6):669‐77. [DOI] [PubMed] [Google Scholar]
Anderson 2004 {published data only}
- Anderson KO, Mendoza TR, Payne R, Valero V, Palos GR, Nazario A, et al. Pain education for underserved minority cancer patients: a randomized controlled trial. Journal of Clinical Oncology 2004;22(24):4918‐25. [DOI] [PubMed] [Google Scholar]
Ansari 2003 {published data only}
- Ansari M, Shlipak MG, Heidenreich PA, Ostaeyen D, Pohl EC, Browner WS, et al. Improving guideline adherence: a randomized trial evaluating strategies to increase beta‐blocker use in heart failure. Circulation 2003;107(22):2799‐804. [DOI] [PubMed] [Google Scholar]
Atherton‐Naji 2001 {published data only}
- Atherton‐Naji A, Hamilton R, Riddle W, Naji S. Improving adherence to antidepressant drug treatment in primary care: A feasibility study for a randomized controlled trial of education intervention. Primary Care Psychiatry 2001;7(2):61‐7. [Google Scholar]
Barr 2001 {published data only}
- Barr JK, Franks AL, Lee NC, Antonucci DM, Rifkind S, Schachter M. A randomized intervention to improve ongoing participation in mammography. American Journal of Managed Care 2001;7(9):887‐94. [PubMed] [Google Scholar]
Basch 1999 {published data only}
- Basch CE, Walker EA, Howard CJ, Shamoon H, Zybert P. The effect of health education on the rate of ophthalmic examinations among African Americans with diabetes mellitus. American Journal of Public Health 1999;89(12):1878‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 1989 {published data only}
- Becker D M, Gomez E B, Kaiser D L, Yoshihasi A, Hodge R H Jr. Improving preventive care at a medical clinic: how can the patient help?. American Journal of Preventive Medicine 1989;5(6):353‐9. [PubMed] [Google Scholar]
Bessette 2011 {published data only}
- Bessette L, Davison KS, Jean S, Roy S, Ste‐Marie LG, Brown JP. The impact of two educational interventions on osteoporosis diagnosis and treatment after fragility fracture:a population‐based randomized controlled trial. Osteoporos International 2011;22(12):2963‐72. [DOI] [PubMed] [Google Scholar]
Bickman 2011 {published data only}
- Bickman L, Kelley SD, Breda C, Andrade AR, Riemer M. Effects of routine feedback to clinicians on mental health outcomes of youths: results of a randomized trial. Psychiatric Services 2011;62(12):1423‐9. [DOI] [PubMed] [Google Scholar]
Bird 1990 {published data only}
- Bird JA, McPhee SJ, Jenkins C, Fordham D. Three strategies to promote cancer screening. How feasible is wide‐scale implementation?. Medical Care 1990;28(11):1005‐12. [DOI] [PubMed] [Google Scholar]
Bloomfield 2005 {published data only}
- Bloomfield HE, Nelson DB, Ryn M, Neil BJ, Koets NJ, Basile JN, et al. A trial of education, prompts, and opinion leaders to improve prescription of lipid modifying therapy by primary care physicians for patients with ischemic heart disease. Quality & Safety in Health Care 2005;14(4):258‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branch 1999 {published data only}
- Branch VK, Graves G, Hanczyc M, Lipsky PE. The utility of trained arthritis patient educators in the evaluation and improvement of musculoskeletal examination skills of physicians in training. Arthritis Care and Research : the official journal of the Arthritis Health Professions Association 1999;12(1):61‐9. [DOI] [PubMed] [Google Scholar]
Brinkman 2007 {published data only}
- Brinkman WB, Geraghty SR, Lanphear BP, Khoury JC, Gonzalez del Rey JA, Dewitt TG, et al. Effect of multisource feedback on resident communication skills and professionalism: a randomized controlled trial. Archives of Pediatrics & Adolescent Medicine 2007;161(1):44‐9. [DOI] [PubMed] [Google Scholar]
Brodey 2005 {published data only}
- Brodey BB, Cuffel B, McCulloch J, Tani S, Maruish M, Brodey I, et al. The acceptability and effectiveness of patient‐reported assessments and feedback in a managed behavioral healthcare setting. American Journal of Managed Care 2005;11(12):774‐80. [PubMed] [Google Scholar]
Burack 1994 {published data only}
- Burack RC, Gimotty PA, George J, Stengle W, Warbasse L, Moncrease A. Promoting screening mammography in inner‐city settings: a randomized controlled trial of computerized reminders as a component of a program to facilitate mammography. Medical Care 1994;32(6):609‐24. [DOI] [PubMed] [Google Scholar]
Burack 1996 {published data only}
- Burack RC, Gimotty PA, George J, Simon MS, Dews P, Moncrease A. The effect of patient and physician reminders on use of screening mammography in a health maintenance organization. Results of a randomized controlled trial. Cancer 1996;78(8):1708‐21. [PubMed] [Google Scholar]
Burack 1998 {published data only}
- Burack RC, Gimotty PA, George J, McBride S, Moncrease A, Simon MS, Dews P, Coombs J. How reminders given to patients and physicians affected pap smear use in a health maintenance organization: results of a randomized controlled trial. Cancer 1998;82(12):2391‐400. [DOI] [PubMed] [Google Scholar]
Burack 2003 {published data only}
- Burack RC, Gimotty PA, Simon M, Moncrease A, Dews P. The effect of adding Pap smear information to a mammography reminder system in an HMO: results of randomized controlled trial. Preventive Medicine 2003;36(5):547‐54. [DOI] [PubMed] [Google Scholar]
Campbell 1997 {published data only}
- Campbell E, Peterkin D, Abbott R, Rogers J. Encouraging underscreened women to have cervical cancer screening: the effectiveness of a computer strategy. Preventive medicine 1997;26(6):801‐7. [DOI] [PubMed] [Google Scholar]
Chang 2012 {published data only}
- Chang TE, Jing Y, Yeung AS, Brenneman SK, Kalsekar I, Hebden T, et al. Effect of communicating depression severity on physician prescribing patterns: findings from the Clinical Outcomes in MEasurement‐based Treatment (COMET) trial. General Hospital Psychiatry 2012;34(2):105‐12. [DOI] [PubMed] [Google Scholar]
Chodosh 2015 {published data only}
- Chodosh J, Colaiaco BA, Connor KI, Cope DW, Liu H, Ganz DA, et al. Dementia care management in an underserved community: the comparative effectiveness of two different approaches. Journal of Aging & Health 2015;27(5):864‐93. [DOI] [PubMed] [Google Scholar]
Chou 2011 {published data only}
- Chou PL, Lin CC. A pain education programme to improve patient satisfaction with cancer pain management: a randomised control trial. Journal of Clinical Nursing 2011;20(13‐4):1858‐69. [DOI] [PubMed] [Google Scholar]
Clementz 1990 {published data only}
- Clementz GL, Aldag JC, Gladfelter TT, Barclay AM, Brooks HF. A randomized study of cancer screening in a family practice setting using a recall model. Journal of Family Practice 1990;30(5):537‐41. [PubMed] [Google Scholar]
Clever 2006 {published data only}
- Clever SL, Ford D E, Rubenstein LV, Rost KM, Meredith L‐S, Sherbourne CD, et al. Primary care patients' involvement in decision‐making is associated with improvement in depression. Medical Care 2006;44(5):398‐405. [DOI] [PubMed] [Google Scholar]
Clover 1992 {published data only}
- Clover KA, Redman S, Forbes JF, Sanson‐Fisher RW, Dickinson JA. Promotion of attendance for mammographic screening through general practice: a randomised trial of two strategies. Medical Journal of Australia 1992;156(2):91‐4. [PubMed] [Google Scholar]
Cohen‐Cline 2014 {published data only}
- Cohen‐Cline H, Wernli KJ, Bradford SC, Boles‐Hall M, Grossman DC. Use of interactive voice response to improve colorectal cancer screening. Medical Care 2014;52(6):496‐9. [DOI] [PubMed] [Google Scholar]
Cooper 2011 {published data only}
- Cooper LA, Roter DL, Carson K A, Bone LR, Larson S M, Miller ER 3rd, et al. A randomized trial to improve patient‐centered care and hypertension control in underserved primary care patients. Journal of General Internal Medicine 2011;26(11):1297‐304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2013 {published data only}
- Cooper LA, Ghods Dinoso BK, Ford DE, Roter D L, Primm AB, Larson SM, et al. Comparative effectiveness of standard versus patient‐centered collaborative care interventions for depression among African Americans in primary care settings: the BRIDGE Study. Health Services Research 2013;48(1):150‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corson 2011 {published data only}
- Corson K, Doak MN, Denneson L, Crutchfield M, Soleck G, Dickinson KC, et al. Primary care clinician adherence to guidelines for the management of chronic musculoskeletal pain: results from the study of the effectiveness of a collaborative approach to pain. Pain Medicine 2011;12(10):1490‐501. [DOI] [PubMed] [Google Scholar]
Costanza 2007 {published data only}
- Costanza ME, Luckmann R, Stoddard AM, White MJ, Stark JR, Avrunin JS, et al. Using tailored telephone counseling to accelerate the adoption of colorectal cancer screening. Cancer Detection & Prevention 2007;31(3):191‐8. [DOI] [PubMed] [Google Scholar]
Datto 2003 {published data only}
- Datto CJ, Thompson R, Horowitz D, Disbot M, Oslin DW. The pilot study of a telephone disease management program for depression. General Hospital Psychiatry 2003;25(3):169‐77. [DOI] [PubMed] [Google Scholar]
Deeb 1988 {published data only}
- Deeb LC, Pettijohn FP, Shirah J K, Freeman G. Interventions among primary‐care practitioners to improve care for preventable complications of diabetes. Diabetes Care 1988;11(3):275‐80. [DOI] [PubMed] [Google Scholar]
Dietrich 2013 {published data only}
- Dietrich AJ, Tobin JN, Robinson CM, Cassells A, Greene MA, Dunn VH, et al. Telephone outreach to increase colon cancer screening in medicaid managed care organizations: a randomized controlled trial. Annals of Family Medicine 2013;11(4):335‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dolan 2002 {published data only}
- Dolan JG, Frisina S. Randomized controlled trial of a patient decision aid for colorectal cancer screening. Medical Decision Making : an international journal of the Society for Medical Decision Making 2002;22(2):125‐39. [DOI] [PubMed] [Google Scholar]
Early 2015 {published data only}
- Early F, Everden AJ, O'Brien CM, Fagan PL, Fuld JP. Patient agenda setting in respiratory outpatients: A randomized controlled trial. Chronic Respiratory Disease 2015;12(4):347‐56. [DOI] [PubMed] [Google Scholar]
Echeverry 2003 {published data only}
- Echeverry DM, Dike MR, Washington C, Davidson MB. The impact of using a low‐literacy patient education tool on process measures of diabetes care in a minority population. Journal of the National Medical Association 2003;95(11):1074‐81. [PMC free article] [PubMed] [Google Scholar]
Feder 1999 {published data only}
- Feder G, Griffiths C, Eldridge S, Spence M. Effect of postal prompts to patients and general practitioners on the quality of primary care after a coronary event (POST): randomised controlled trial. BMJ (Clinical research ed.) 1999;318(7197):1522‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finlay 1999 {published data only}
- Finlay IG, Wyatt P. Randomised cross‐over study of patient‐held records in oncology and palliative care. Lancet (London, England) 1999;353(9152):558‐9. [DOI] [PubMed] [Google Scholar]
Fisher 2011 {published data only}
- Fischer HH, Eisert SL, Durfee MJ, Moore SL, Steele AW, McCullen K, et al. The impact of tailored diabetes registry report cards on measures of disease control: a nested randomized trial. BMC Medical Informatics & Decision Making 2011;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleisher 1999 {published data only}
- Fleisher LA, Mark L, Lam J, Pearlman A, Fisher Q, Snyder DS, et al. Disseminating information using an anesthesiology consultant report: impact on patient perceptions of quality of care. Journal of Clinical anesthesia 1999;11(5):380‐5. [DOI] [PubMed] [Google Scholar]
Flottorp 2002 {published data only}
- Flottorp S, Oxman A D, Havelsrud K, Treweek S, Herrin J. Cluster randomised controlled trial of tailored interventions to improve the management of urinary tract infections in women and sore throat. BMJ 2002;325(7360):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fluckiger 2012 {published data only}
- Fluckiger C, Re AC, Wampold BE, Znoj H, Caspar F, Jorg U. Valuing clients' perspective and the effects on the therapeutic alliance: a randomized controlled study of an adjunctive instruction. Journal of Counseling Psychology 2012;59(1):18‐26. [DOI] [PubMed] [Google Scholar]
Förberg 2017 {published data only}
- Förberg U, Unbeck M, Wallin L, Johansson E, Petzold M, Ygge BM, et al. Effects of computer reminders on complications of peripheral venous catheters and nurses' adherence to a guideline in paediatric care‐‐a cluster randomised study. Implementation Science 2017;11:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortuna 2014 {published data only}
- Fortuna RJ, Idris A, Winters P, Humiston SG, Scofield S, Hendren S, et al. Get screened: a randomized trial of the incremental benefits of reminders, recall, and outreach on cancer screening. Journal of General Internal Medicine 2014;29(1):90‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gabbay 2012 {published data only}
- Gabbay RA, Añel‐Tiangco RM, Dellasega C, Mauger DT, Adelman A, Horn DHA. Diabetes Nurse Case Management and Motivational Interviewingfor Change (DYNAMIC): Results of a 2‐year randomized controlled pragmatic trial. Journal of Diabetes 2013;5(3):349‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Galliher 2010 {published data only}
- Galliher JM, Post DM, Weiss BD, Dickinson LM, Manning BK, Staton EW, et al. Patients' question‐asking behavior during primary care visits: a report from the AAFP National Research Network. Annals of Family Medicine 2010;8(2):151‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2013 {published data only}
- Garcia BH, Giverhaug T, Utnes J, Smabrekke L. A follow‐up program for patients with established coronary heart disease led by a clinical pharmacist: A randomized controlled trial. International Journal of Clinical Pharmacy 2013;35(6):1255‐6. [Google Scholar]
Garcia 2015 {published data only}
- Garcia BH, Giverhaug T, Hogli J U, Skjold F, Smabrekke L. A pharmacist‐led follow‐up program for patients with established coronary heart disease in North Norway ‐ a randomized controlled trial. Pharmacy Practice 2015;13(2):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gersch 2014 {published data only}
- Gersch C, Ebel M. [Both patients and physicians benefit from internet‐based communication (APIKAP study)]. MMW Fortschritte der Medizin 2014;156 Suppl 2:31‐8. [PubMed] [Google Scholar]
Ghadieh 2015 {published data only}
- Ghadieh AS, Hamadeh GN, Mahmassani DM, Lakkis NA. The effect of various types of patients' reminders on the uptake of pneumococcal vaccine in adults: A randomized controlled trial. Vaccine 2015;33(43):5868‐72. [DOI] [PubMed] [Google Scholar]
Ginson 2000 {published data only}
- Ginson SH, Malmberg C, French DJ. Impact on vaccination rates of a pharmacist‐initiated influenza and pneumococcal vaccination program. Canadian Journal of Hospital Pharmacy 2000;53(4):270‐5. [Google Scholar]
Gooding 2012 {published data only}
- Gooding HC, Blood EA, Sharma N. An educational intervention to increase internists' confidence with and provision of preventive services to adolescents and young adults. Teaching & Learning in Medicine 2012;24(4):321‐6. [DOI] [PubMed] [Google Scholar]
Grace 2005 {published data only}
- Grace SL, Evindar A, Brooks D, Jaglal S, Abramson BL, Nolan R. Increasing patient‐initiation of cardiac rehabilitation referral in female percutaneous coronary intervention patients. Canadian Journal of Cardiovascular Nursing 2005;15(1):23‐7. [PMC free article] [PubMed] [Google Scholar]
Greco 2001 {published data only}
- Greco M, Brownlea A, McGovern J. Impact of patient feedback on the interpersonal skills of general practice registrars: results of a longitudinal study. Medical Eeducation 2001;35(8):748‐56. [DOI] [PubMed] [Google Scholar]
Haskard 2008 {published data only}
- Haskard KB, Williams SL, DiMatteo MR, Rosenthal R, White MK, Goldstein MG. Physician and patient communication training in primary care: effects on participation and satisfaction.[Erratum appears in Health Psychol. 2009 Mar;28(2):263]. Health Psychology 2008;27(5):513‐22. [DOI] [PubMed] [Google Scholar]
Hornberger 1997 {published data only}
- Hornberger J, Thom D, MaCurdy T. Effects of a self‐administered previsit questionnaire to enhance awareness of patients' concerns in primary care. Journal of General Internal Medicine 1997;12(10):597‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jager 2017 {published data only}
- Jager C, Freund T, Steinhauser J, Stock C, Krisam J, Kaufmann‐Kolle P, et al. Impact of a tailored program on the implementation of evidence‐based recommendations for multimorbid patients with polypharmacy in primary care practices‐results of a cluster‐randomized controlled trial. Implementation Science 2017;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2011 {published data only}
- Katz ML, Fisher JL, Fleming K, Paskett ED. Patient activation increases colorectal cancer screening rates among low‐income minority patients. Cancer Epidemiology, Biomarkers & Prevention 2012;21(1):45‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinugasa 2014 {published data only}
- Kinugasa Y, Kato M, Sugihara S, Yanagihara K, Yamada K, Hirai M, et al. Multidisciplinary intensive education in the hospital improves outcomes for hospitalized heart failure patients in a Japanese rural setting. BMC Health Services Research 2014;14:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kravitz 2005 {published data only}
- Kravitz RL, Epstein RM, Feldman MD, Franz CE, Azari R, Wilkes MS, et al. Influence of patients' requests for direct‐to‐consumer advertised antidepressants: a randomized controlled trial.[Erratum appears in JAMA. 2005 Nov 16;294(19):2436]. JAMA 2005;293(16):1995‐2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lafata 2007 {published data only}
- Lafata JE, Kolk D, Peterson EL, McCarthy BD, Weiss TW, Chen Y‐T, Muma BK. Improving osteoporosis screening: results from a randomized cluster trial. Society of General Internal Medicine 2007;22(3):346‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawton 2017 {published data only}
- Lawton R, O'Hara J K, Sheard L, Armitage G, Cocks K, Buckley H, et al. Can patient involvement improve patient safety? A cluster randomised control trial of the Patient Reporting and Action for a Safe Environment (PRASE) intervention. BMJ Quality & Safety 2017;03:03. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levy 2013 {published data only}
- Levy BT, Xu Y, Daly JM, Ely JW. A randomized controlled trial to improve colon cancer screening in rural family medicine: an Iowa Research Network (IRENE) study. Journal of the American Board of Family Medicine: JABFM 2013;26(5):486‐97. [DOI] [PubMed] [Google Scholar]
Linder 2009 {published data only}
- Linder JA, Rigotti NA, Schneider LI, Kelley JH, Brawarsky P, Haas J S. An electronic health record‐based intervention to improve tobacco treatment in primary care: a cluster‐randomized controlled trial. Archives of Internal Medicine 2009;169(8):781‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2004 {published data only}
- Little P, Dorward M, Warner G, Moore M, Stephens K, Senior J, et al. Randomised controlled trial of effect of leaflets to empower patients in consultations in primary care. BMJ (Clinical research ed.) 2004;328(7437):10.1136/bmj.37999.716157.44. [DOI: 10.1136/bmj.37999.716157.44] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2016 {published data only}
- Liu C, Zhang X, Wang X, Zhang X, Wan J, Zhong F. Does public reporting influence antibiotic and injection prescribing to all patients? A cluster‐randomized matched‐pair trial in China. Medicine 2016;95(26):e3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lynch 2004 {published data only}
- Lynch FL, Whitlock EP, Valanis BG, Smith SK. Cost‐effectiveness of a tailored intervention to increase screening in HMO women overdue for Pap test and mammography services. Preventive Medicine 2004;38(4):403‐11. [DOI] [PubMed] [Google Scholar]
Manfredi 1998 {published data only}
- Manfredi C, Czaja R, Freels S, Trubitt M, Warnecke R, Lacey L. Prescribe for health. Improving cancer screening in physician practices serving low‐income and minority populations. Archives of Family Medicine 1998;7(4):329‐37. [DOI] [PubMed] [Google Scholar]
Marshall 2016 {published data only}
- Marshall JK, Mbah OM, Ford JG, Phelan‐Emrick D, Ahmed S, Bone L, et al. Effect of patient navigation on breast cancer screening among African American Medicare beneficiaries: a randomized controlled trial. Journal of General Internal Medicine 2016;31(1):68‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marteau 2010 {published data only}
- Marteau TM, Mann E, Prevost AT, Vasconcelos JC, Kellar I, Sanderson S, et al. Impact of an informed choice invitation on uptake of screening for diabetes in primary care (DICISION): randomised trial. BMJ (Clinical research ed.) 2010;340:c2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menon 2011 {published data only}
- Menon U, Belue R, Wahab S, Rugen K, Kinney AY, Maramaldi P, Wujcik D, Szalacha LA. A randomized trial comparing the effect of two phone‐based interventions on colorectal cancer screening adherence. Annals of Behavioral Medicine 2011;42(3):294‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michalopoulou 2010 {published data only}
- Michalopoulou G, Falzarano P, Arfken C, Rosenberg D. Implementing Ask Me 3 to improve African American patient satisfaction and perceptions of physician cultural competency. Journal of Cultural Diversity 2010;17(2):62‐7. [PubMed] [Google Scholar]
Mitchell 2005 {published data only}
- Mitchell E, Sullivan F, Grimshaw JM, Donnan PT, Watt G. Improving management of hypertension in general practice: a randomised controlled trial of feedback derived from electronic patient data. British Journal of General Practice 2005;55(511):94‐101. [PMC free article] [PubMed] [Google Scholar]
Mohler 1995 {published data only}
- Mohler PJ. Enhancing compliance with screening mammography recommendations: a clinical trial in a primary care office. Family Medicine 1995;27(2):117‐21. [PubMed] [Google Scholar]
Myers 2007 {published data only}
- Myers RE, Sifri R, Hyslop T, Rosenthal M, Vernon SW, Cocroft J, et al. A randomized controlled trial of the impact of targeted and tailored interventions on colorectal cancer screening. Cancer 2007;110(9):2083‐91. [DOI] [PubMed] [Google Scholar]
Myers 2008 {published data only}
- Myers RE, Hyslop T, Sifri R, Bittner‐Fagan H, Katurakes NC, Cocroft J, et al. Tailored navigation in colorectal cancer screening. Medical Care 2008;46(9 Suppl 1):S123‐31. [DOI] [PubMed] [Google Scholar]
Myers 2011 {published data only}
- Myers RE, Daskalakis C, Kunkel EJ, Cocroft J R, Riggio JM, Capkin M, et al. Mediated decision support in prostate cancer screening: a randomized controlled trial of decision counseling. Patient Education & Counseling 2011;83(2):240‐6. [DOI] [PubMed] [Google Scholar]
O'Connor 2009 {published data only}
- O'Connor PJ, Sperl‐Hillen J, Johnson PE, Rush WA, Crain AL. Customized feedback to patients and providers failed to improve safety or quality of diabetescare: a randomized trial. Diabetes Care 2009;32:1158‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olsson 2012 {published data only}
- Olsson IN, Runnamo R, Engfeldt P. Drug treatment in the elderly: an intervention in primary care to enhance prescription quality and quality of life. Scandinavian Journal of Primary Health Care 2012;30(1):3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ornstein 1991 {published data only}
- Ornstein SM, Garr DR, Jenkins RG, Rust PF, Arnon A. Computer‐generated physician and patient reminders. Tools to improve population adherence to selected preventive services. Journal of Family Practice 1991;32(1):82‐90. [PubMed] [Google Scholar]
Osborn 2010 {published data only}
- Osborn DP, Nazareth I, Wright CA, King MB. Impact of a nurse‐led intervention to improve screening for cardiovascular risk factors in people with severe mental illnesses. Phase‐two cluster randomised feasibility trial of community mental health teams. BMC Health Services Research 2010;10:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osman 1994 {published data only}
- Osman LM, Abdalla MI, Beattie JA, Ross SJ, Russell IT, Friend JA, et al. Reducing hospital admission through computer supported education for asthma patients. Grampian Asthma Study of Integrated Care (GRASSIC). BMJ (Clinical research ed.) 1994;308(6928):568‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Osman 2002 {published data only}
- Osman LM, Calder C, Godden D, Friend JA, McKenzie L, Legge J, Douglas JG. A randomised trial of self‐management planning for adult patients admitted to hospital with acute asthma. Thorax 2002;57(10):869‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Persell 2008 {published data only}
- Persell SD, Denecke‐Dattalo TA, Dunham DP, Baker DW. Patient‐directed intervention versus clinician reminders alone to improve aspirin use in diabetes: a cluster randomized trial. Joint Commission Journal on Quality & Patient Safety 2008;34(2):98‐105. [DOI] [PubMed] [Google Scholar]
Porter 2006 {published data only}
- Porter SC, Forbes P, Feldman HA, Goldmann DA. Impact of patient‐centered decision support on quality of asthma care in the emergency department. Pediatrics 2006;117(1):e33‐42. [DOI] [PubMed] [Google Scholar]
Raisch 1999 {published data only}
- Raisch DW, Sleath BL. Using feedback letters to influence the use of antiulcer agents in a Medicaid program. Journal of General Internal Medicine 1999;14(3):145‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reinders 2010 {published data only}
- Reinders ME, Blankenstein AH, Horst HE, Knol DL, Schoonheim PL, Marwijk HW. Does patient feedback improve the consultation skills of general practice trainees? A controlled trial. Medical Education 2010;44(2):156‐64. [DOI] [PubMed] [Google Scholar]
Rise 2012 {published data only}
- Rise MB, Eriksen L, Grimstad H, Steinsbekk A. The short‐term effect on alliance and satisfaction of using patient feedback scales in mental health out‐patient treatment. A randomised controlled trial. BMC Health Services Research 2012;12:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robling 2012 {published data only}
- Robling M, McNamara R, Bennert K, Butler CC, Channon S, Cohen D, et al. The effect of the Talking Diabetes consulting skills intervention on glycaemic control and quality of life in children with type 1 diabetes: cluster randomised controlled trial (DEPICTED study). BMJ 2012;344:e2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roland 1989 {published data only}
- Roland M, Dixon M. Randomized controlled trial of an educational booklet for patients presenting with back pain in general practice. Journal of the Royal College of General Practitioners 1989;39(323):244‐6. [PMC free article] [PubMed] [Google Scholar]
Rosenthal 2005 {published data only}
- Rosenthal MS, Lannon CM, Stuart JM, Brown L, Miller WC, Margolis PA. A randomized trial of practice‐based education to improve delivery systems for anticipatory guidance. Archives of Pediatrics & Adolescent Medicine 2005;159(5):456‐63. [DOI] [PubMed] [Google Scholar]
Rosser 1991 {published data only}
- Rosser WW, McDowell I, Newell C. Use of reminders for preventive procedures in family medicine. Canadian Medical Association journal 1991;145(7):807‐14. [PMC free article] [PubMed] [Google Scholar]
Rubenstein 1995 {published data only}
- Rubenstein LV, McCoy JM, Cope DW, Barrett PA, Hirsch SH, Messer KS, et al. Improving patient quality of life with feedback to physicians about functional status. Journal of General Internal Medicine 1995;10(11):607‐14. [DOI] [PubMed] [Google Scholar]
Sherrard 2015 {published data only}
- Sherrard H, Duchesne L, Wells G, Kearns SA, Struthers C. Using interactive voice response to improve disease management and compliance with acute coronary syndrome best practice guidelines: A randomized controlled trial. Canadian Journal of Cardiovascular Nursing 2015;25(1):10‐5. [PubMed] [Google Scholar]
Simon 2012 {published data only}
- Simon W, Lambert MJ, Harris MW, Busath G, Vazquez A. Providing patient progress information and clinical support tools to therapists: effects on patients at risk of treatment failure. Psychotherapy Research 2012;22(6):638‐47. [DOI] [PubMed] [Google Scholar]
Smeele 1999 {published data only}
- Smeele IJ, Grol RP, Schayck CP, Bosch WJ, Hoogen HJ, Muris JW. Can small group education and peer review improve care for patients with asthma/chronic obstructive pulmonary disease?. Quality in Health Care 1999;8(2):92‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smit 2005 {published data only}
- Smit A, Tiemens BG, Ormel J, Kluiter H, Jenner JA, Meer K, et al. Enhanced treatment for depression in primary care: First year results on compliance, self‐efficacy, the use of antidepressants and contacts with the primary care physician. Primary Care & Community Psychiatry 2005;10(2):39‐49. [Google Scholar]
Solomon 2007 {published data only}
- Solomon DH, Polinski JM, Stedman M, Truppo C, Breiner L, Egan C, et al. Improving care of patients at‐risk for osteoporosis: a randomized controlled trial. Journal of General Internal Medicine 2007;22(3):362‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sonnichsen 2010 {published data only}
- Sonnichsen AC, Winkler H, Flamm M, Panisch S, Kowatsch P, Klima G, et al. The effectiveness of the Austrian disease management programme for type 2 diabetes: a cluster‐randomised controlled trial. BMC Family Practice 2010;11:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Spahr 2006 {published data only}
- Spahr CD, Flugstad NA, Brousseau DC. The impact of a brief expectation survey on parental satisfaction in the pediatric emergency department. Academic Emergency Medicine 2006;13(12):1280‐7. [DOI] [PubMed] [Google Scholar]
Spaic 2013 {published data only}
- Spaic T, Mahon J L, Hramiak I, Byers N, Evans K, Robinson T, et al. Multicentre randomized controlled trial of structured transition on diabetes care management compared to standard diabetes care in adolescents and young adults with type 1 diabetes (Transition Trial). BMC Pediatrics 2013;13:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thapar 2002 {published data only}
- Thapar A, Jacoby A, Richens A, Russell I, Roberts C, Porter E, et al. A pragmatic randomised controlled trial of a prompt and reminder card in the care of people with epilepsy. British Journal of General Practice : the journal of the Royal College of General Practitioner 2002;52(475):93‐8. [PMC free article] [PubMed] [Google Scholar]
Valanis 2002 {published data only}
- Valanis BG, Glasgow RE, Mullooly J, Vogt TM, Whitlock EP, Boles SM, et al. Screening HMO women overdue for both mammograms and pap tests. Preventive Medicine 2002;34(1):40‐50. [DOI] [PubMed] [Google Scholar]
Vallès 2002 {published data only}
- Vallès JA, Barreiro M, Cereza G, Ferro JJ, Martínez MJ, Cucurull E, et al. [Acceptance of generic prescribing in general practice: effect of patient education and reference prices]. Gaceta Sanitaria / S.E.S.P.A.S 2002;16(6):505‐10. [DOI] [PubMed] [Google Scholar]
Vallès 2003 {published data only}
- Vallès JA, Barreiro M, Cereza G, Ferro JJ, Martínez MJ, Escribà JM, et al. A prospective multicenter study of the effect of patient education on acceptability of generic prescribing in general practice. Health policy (Amsterdam, Netherlands) 2003;65(3):269‐75. [DOI] [PubMed] [Google Scholar]
Vickrey 2006 {published data only}
- Vickrey BG, Mittman BS, Connor KI, Pearson ML, Della Penna RD, Ganiats TG, et al. The effect of a disease management intervention on quality and outcomes of dementia care: a randomized, controlled trial.[Summary for patients in Ann Intern Med. 2006 Nov 21;145(10):I31; PMID: 17116913]. Annals of Internal Medicine 2006;145(10):713‐26. [DOI] [PubMed] [Google Scholar]
Vingerhoets 2001 {published data only}
- Vingerhoets E, Wensing M, Grol R. Feedback of patients' evaluations of general practice care: a randomised trial. Quality in Health care : QHC 2001;10(4):224‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasson 1999 {published data only}
- Wasson JH, Stukel TA, Weiss JE, Hays RD, Jette AM, Nelson EC. A randomized trial of the use of patient self‐assessment data to improve community practices. Effective Clinical Practice 1999;2(1):1‐10. [PubMed] [Google Scholar]
Wensing 2003 {published data only}
- Wensing M, Vingerhoets E, Grol R. Feedback based on patient evaluations: a tool for quality improvement?. Patient Education and Counseling 2003;51(2):149‐53. [DOI] [PubMed] [Google Scholar]
Wilson 1993 {published data only}
- Wilson SR, Scamagas P, German DF, Hughes GW, Lulla S, Coss S, et al. A controlled trial of two forms of self‐management education for adults with asthma. American Journal of Medicine 1993;94(6):564‐76. [DOI] [PubMed] [Google Scholar]
Wynia 2010 {published data only}
- Wynia K, Annema C, Nissen H, Keyser J, Middel B. Design of a Randomised Controlled Trial (RCT) on the effectiveness of a Dutch patient advocacy case management intervention among severely disabled Multiple Sclerosis patients. BMC Health Services Research 2010;10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zermansky 2001 {published data only}
- Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ (Clinical research ed.) 2001;323(7325):1340‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01904656 {published data only}
- NCT01904656. CBPR Strategies to Increase colorectal cancer screening in Ohio Appalachia. https://clinicaltrials.gov/show/NCT01904656 (date accessed 18th of Sept. 2017).
NCT02686775 {published data only}
- NCT02686775. The PACO Project: A clinical study of a PAtient COach program in vulnerable lung cancer patients (PACO). https://clinicaltrials.gov/show/NCT02686775 (date accessed 18th of Sept. 2017).
Additional references
ABDN 2015
- University of Aberdeen. Research tools. Database of intra‐correlation coefficients (ICCs). www.abdn.ac.uk/hsru/research/research‐tools/ (accessed prior to 7 December 2016).
Anderson 2017
- Anderson L, Brown JPR, Clark AM, Dalal H, Rossau HK, Bridges C, et al. Patient education in the management of coronary heart disease. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD008895.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arditi 2017
- Arditi C, Rège‐Walther M, Durieux P, Burnand B. Computer‐generated reminders delivered on paper to healthcare professionals; effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD001175.pub4] [DOI] [PubMed] [Google Scholar]
Attridge 2014
- Attridge M, Creamer J, Ramsden M, Cannings‐John R, Hawthorne K. Culturally appropriate health education for people in ethnic minority groups with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006424.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2016
- Bennett S, Pigott A, Beller EM, Haines T, Meredith P, Delaney C. Educational interventions for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD008144.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarkesmith 2017
- Clarkesmith DE, Pattison HM, Khaing PH, Lane DA. Educational and behavioural interventions for anticoagulant therapy in patients with atrial fibrillation. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD008600.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence
- Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. Available at www.covidence.org.
Davis 1995
- Davis DA, Thomson MA, Oxman AD, Haynes RB. Changing physician performance. A systematic review of the effect of continuing medical education strategies. JAMA 1995;274(9):700‐5. [DOI] [PubMed] [Google Scholar]
Davis 2012a
- Davis RE, Sevdalis N, Vincent CA. Patient involvement in patient safety: the health‐care professionals' perspective. Journal of Patient Safety 2012;8(4):182‐8. [DOI] [PubMed] [Google Scholar]
Davis 2012b
- Davis RE, Pinto A, Sevdalis N, Vincent C, Massey R, Darzi A. Patients' and health care professionals' attitudes towards the PINK patient safety video. Journal of Evaluation in Clinical Practice 2012;18(4):848‐53. [DOI] [PubMed] [Google Scholar]
Dwamena 2012
- Dwamena F, Holmes‐Rovner M, Gaulden CM, Jorgenson S, Sadigh G, Sikorskii A, et al. Interventions for providers to promote a patient‐centred approach in clinical consultations. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD003267.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2017a
- Cochrane Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC Resources for review authors, 2017. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2017b
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors, 2017. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2017c
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC resources for review authors, 2017. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2017d
- Cochrane Effective Practice, Organisation of Care (EPOC). Reporting the effects of an intervention in EPOC reviews. EPOC Resources for review authors, 2017. Available at: http://epoc.cochrane.org/resources/epoc‐resources‐review‐authors.
Flodgren 2011a
- Flodgren G, Parmelli E, Doumit G, Gattellari M, O’Brien MA, Grimshaw J, et al. Local opinion leaders: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD000125.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flodgren 2011b
- Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD009255] [DOI] [PMC free article] [PubMed] [Google Scholar]
Flodgren 2016
- Flodgren G, Hall AM, Goulding L, Eccles MP, Grimshaw JM, Leng GC, et al. Tools developed and disseminated by guideline producers to promote the uptake of their guidelines. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD010669.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsetlund 2009
- Forsetlund L, Bjørndal A, Rashidian A, Jamtvedt G, O'Brien MA, Wolf FM, et al. Continuing education meetings and workshops: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD003030.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fryer 2016
- Fryer CE, Luker JA, McDonnell MN, Hillier SL. Self management programmes for quality of life in people with stroke. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD010442.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gagliardi 2016
- Gagliardi AR, Légaré F, Brouwers MC, Webster F, Badley E, Straus S. Patient‐mediated knowledge translation (PKT) interventions for clinical encounters: a systematic review. Implement Science 2016;11(26):1‐13. [doi: 10.1186/s13012‐016‐0389‐3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giguère 2012
- Giguère A, Légaré F, Grimshaw J, Turcotte S, Fiander M, Grudniewicz A, et al. Printed educational materials: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD004398.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro Guideline Development Tool. Version accessed prior to 7 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Grimshaw 2004
- Grimshaw JM, Thomas RE, Maclennan G, Fraser C, Ramsay CR, Vale L, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technology Assessment 2004;8:iii‐72. [DOI] [PubMed] [Google Scholar]
Grol 2003
- Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients' care. Lancet 2003;362(9391):1225‐30. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta ‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:344‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hrisos 2013
- Hrisos S, Thomson R. Seeing it from both sides: do approaches to involving patients in improving their safety risk damaging the trust between patients and healthcare professionals? An interview study. PLOS One 2013;8(11):e80759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivers 2012
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard‐Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD000259.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2018
- Kelly C, Grundy S, Lynes D, Evans DJW, Gudur S, Milan SJ, et al. Self‐management for bronchiectasis. Cochrane Database of Systematic Reviews 2018, Issue 2. [DOI: 10.1002/14651858.CD012528.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalil 2017
- Khalil H, Bell B, Chambers H, Sheikh A, Avery AJ. Professional, structural and organisational interventions in primary care for reducing medication errors. Cochrane Database of Systematic Reviews 2017, Issue 10. [DOI: 10.1002/14651858.CD003942.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroon 2014
- Kroon FPB, Burg LRA, Buchbinder R, Osborne RH, Johnston RV, Pitt V. Self‐management education programmes for osteoarthritis. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD008963.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lenferink 2017
- Lenferink A, Brusse‐Keizer M, Valk PDLPM, Frith PA, Zwerink M, Monninkhof EM, vet al. Self‐management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD011682.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luszczynska 2007
- Luszczynska A, Gunson KSE. Predictors of asking medical personnel about handwashing: The moderating role of patients’ age and MRSA infection status. Patient Education and Counseling 2007;68(1):79‐85. [DOI] [PubMed] [Google Scholar]
Légaré 2014
- Légaré F, Stacey D, Turcotte S, Cossi MJ, Kryworuchko J, Graham ID, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006732.pub3] [DOI] [PubMed] [Google Scholar]
McBain 2016
- McBain H, Mulligan K, Haddad M, Flood C, Jones J, Simpson A. Self management interventions for type 2 diabetes in adult people with severe mental illness. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011361.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCallum 2017
- McCallum GB, Morris PS, Brown N, Chang AB. Culture‐specific programs for children and adults from minority groups who have asthma. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD006580.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGlynn 2003
- McGlynn EA, Asch SM, Adams J, Keesey J, Hicks J, DeCristofaro A, et al. The quality of health care delivered to adults in the United States. New England Journal of Medicine 2003;348(26):2635‐45. [DOI] [PubMed] [Google Scholar]
McGunkin 2006
- McGunkin M, Waterman R, Shubin A. Consumer attitudes about health care‐acquired infections and hand hygiene. American Journal of Medical Quality 2006;21(5):342‐6. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006‐12. [DOI] [PubMed] [Google Scholar]
Ng 2017
- Ng JY, Gagliardi AR. The design of behavioural interventions labelled as patient‐mediated: A scoping review. Health Expectations 2017;00:1‐12. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nilsen 2006
- Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004563.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Brien 2007
- O'Brien MA, Rogers S, Jamtvedt G, Oxman AD, Odgaard‐Jensen J, Kristoffersen DT, et al. Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000409.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oxman 1995
- Oxman AD, Thomson MA, Davis DA, Haynes RB. No magic bullets: a systematic review of 102 trials of interventions to improve professional practice. CMAJ: Canadian Medical Association Journal 1995;153(10):1423‐31. [PMC free article] [PubMed] [Google Scholar]
Oxman 2005
- Oxman AD, Fretheim A, Flottorp S. The OFF theory of research utilization. Journal of Clinical Epidemiology 2005;58(2):113‐6. [DOI] [PubMed] [Google Scholar]
Parreira 2017
- Parreira P, Heymans MW, Tulder MW, Esmail R, Koes BW, Poquet N, et al. Back Schools for chronic non‐specific low back pain. Cochrane Database of Systematic Reviews 2017, Issue 8. [DOI: 10.1002/14651858.CD011674.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peytremann‐Bridevaux 2015
- Peytremann‐Bridevaux I, Arditi C, Gex G, Bridevaux PO, Burnand B. Chronic disease management programmes for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD007988.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Plomp 2010
- Plomp HN, Ballast N. Trust and vulnerability in doctor–patient relations in occupational health. Occupational Medicine 2010;60(4):261‐9. [DOI] [PubMed] [Google Scholar]
Poquet 2016
- Poquet N, Lin CWC, Heymans MW, Tulder MW, Esmail R, Koes BW, et al. Back schools for acute and subacute non‐specific low‐back pain. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD008325.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Richards 2013
- Richards T, Montori VM, Godlee F, Lapsley P, Paul D. Let the patient revolution begin. Patients can improve healthcare: it's time to take partnership seriously. BMJ 2013;346:f2614. [DOI] [PubMed] [Google Scholar]
Robertson 2006
- Robertson R, Jochelson K. Interventions that change clinician behaviour: mapping the literature. NICE report, 2006. www.nice.org.uk/Media/Default/About/what‐we‐do/Into‐practice/Support‐for‐service‐improvement‐and‐audit/Kings‐Fund‐literature‐review.pdf (accessed prior to 7 December 2016).
Runciman 2012
- Runciman WB, Hunt TD, Hannaford NA, Hibbert PD, Westbrook JI, Coiera EW, et al. CareTrack: assessing the appropriateness of health care delivery in Australia. Medical Journal of Australia 2012;197(2):100‐5. [DOI] [PubMed] [Google Scholar]
Sampson 2008
- Sampson M, McGowan J, Lefebvre C, Moher D, Grimshaw J. PRESS: Peer Review of Electronic Search Strategies. Ottawa: Agency for Drugs and Technologies in Health, 2008. [Google Scholar]
Schuster 1998
- Schuster MA, McGlynn EA, Brook RH. How good is the quality of health care in the United States?. Milbank Quarterly 1998;76(4):517‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwappach 2010a
- Schwappach DLB. Engaging patients as viligilant partners in safety. A systematic review. Medical Care Research and Review 2010;67(2):119‐48. [DOI] [PubMed] [Google Scholar]
Schwappach 2010b
- Schwappach DLB, Hochreutener MA, Wernli M. Oncology nurses' perceptions about involving patients in the prevention of chemotherapy administration errors. Oncology Nursing Forum 2010;37(2):E84‐91. [DOI] [PubMed] [Google Scholar]
Schwappach 2011
- Schwappach DLB, Frank O, Kopperberg J, Müller B, Wasserfallen JB. Patients' and healthcare workers' perceptions of a patient safety advisory. International Journal for Quality in Health Care 2011;23(6):713‐20. [DOI] [PubMed] [Google Scholar]
Schwappach 2013
- Schwappach DLB, Frank O, Davis RE. A vignette study to examine health care professionals' attitudes towards patient involvement in error prevention. Journal of Evaluation in Clinical Practice 2013;19(5):840‐8. [DOI] [PubMed] [Google Scholar]
Seddon 2001
- Seddon ME, Marshall MN, Campbell SM, Roland MO. Systematic review of studies of quality of clinical care in general practice in the UK, Australia and New Zealand. Quality in Health Care 2001;10(3):152‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skirbekk 2011
- Skirbekk H, Middelthon AL, Hjortdahl P, Finset A. Mandates of trust in the doctor‐patient relationship. Qualitative Health Research 2011;21(9):1182‐90. [DOI] [PubMed] [Google Scholar]
Stacey 2017
- Stacey D, Légaré F, Lewis K, Barry MJ, Bennett CL, Eden KB, Holmes‐Rovner M, Llewellyn‐Thomas H, Lyddiatt A, Thomson R, Trevena L. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD001431.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugavanam 2013
- Sugavanam T, Mead G, Bulley C, Donaghy M, Wijck F. The effects and experiences of goal setting in stroke rehabilitation ‐ a systematic review. Disability Rehabilitation 2013;35(3):177‐90. [DOI] [PubMed] [Google Scholar]
Tzortziou Brown 2016
- Tzortziou Brown V, Underwood M, Mohamed N, Westwood O, Morrissey D. Professional interventions for general practitioners on the management of musculoskeletal conditions. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD007495.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JA, Burney PG. Methods for evaluating area‐wide and organisation‐based interventions in health and health care: a systematic review. Health Technology Assessment 1999;3(5):iii‐92. [PubMed] [Google Scholar]
Willems 2005
- Willems S, Maesschalck S, Deveugele M, Derese A, Maeseneer J. Socio‐economic status of the patient and doctor‐patient communication:does it make a difference?. Patient Education and Counseling 2005;56(2):139‐46. [DOI] [PubMed] [Google Scholar]
Zwerink 2014
- Zwerink M, Brusse‐Keizer M, Valk PDLPM, Zielhuis GA, Monninkhof EM, Palen J, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD002990.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Fønhus 2016
- Fønhus MS, Dalsbø TK, Johansen M, Fretheim A, Skirbekk H, Flottorp S. Patient‐mediated interventions to improve professional practice. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD012472] [DOI] [PMC free article] [PubMed] [Google Scholar]


