Abstract
Background
Dornase alfa is currently used as a mucolytic to treat pulmonary disease (the major cause of morbidity and mortality) in cystic fibrosis. It reduces mucus viscosity in the lungs, promoting improved clearance of secretions. This is an update of a previously published review.
Objectives
To determine whether the use of dornase alfa in cystic fibrosis is associated with improved mortality and morbidity compared to placebo or other medications that improve airway clearance, and to identify any adverse events associated with its use.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register which comprises references identified from comprehensive electronic database searches, handsearching relevant journals and abstracts from conferences. Date of the most recent search of the Group's Cystic Fibrosis Register: 23 April 2018.
Clinicaltrials.gov and the International Clinical Trials Registry Platform were also searched to identify unpublished or ongoing trials. Date of most recent search: 07 June 2018.
Selection criteria
All randomised and quasi‐randomised controlled trials comparing dornase alfa to placebo, standard therapy or other medications that improve airway clearance.
Data collection and analysis
Authors independently assessed trials against the inclusion criteria; two authors carried out analysis of methodological quality and data extraction. GRADE was used to assess the level of evidence.
Main results
The searches identified 69 trials, of which 19 (2565 participants) met our inclusion criteria. Fifteen trials compared dornase alfa to placebo or no dornase alfa (2447 participants); two compared daily dornase to hypertonic saline (32 participants); one compared daily dornase alfa to hypertonic saline and alternate day dornase alfa (48 participants); one compared dornase alfa to mannitol and the combination of both drugs (38 participants). Trial duration varied from six days to three years.
Dornase alfa compared to placebo or no treatment
Dornase alfa improved forced expiratory volume at one second at one month (four trials, 248 participants), three months (one trial, 320 participants; moderate‐quality evidence), six months (one trial, 647 participants; high‐quality evidence) and two years (one trial, 410 participants). Limited low‐quality evidence showed no difference between groups for changes in quality of life. There was a decrease in pulmonary exacerbations with dornase alfa in trials of up to two years (moderate‐quality evidence). One trial that examined the cost of care, including the cost of dornase alfa, found that the cost savings from dornase alfa offset 18% to 38% of the medication costs.
Dornase alfa: daily versus alternate day
One cross‐over trial (43 children) found no differences between treatment regimens for lung function, quality of life or pulmonary exacerbations (low‐quality evidence).
Dornase alfa compared to other medications that improve airway clearance
Results for these comparisons were mixed. One trial (43 children) showed a greater improvement in forced expiratory volume at one second for dornase alfa compared to hypertonic saline (low‐quality evidence), and one trial (23 participants) reported no difference in lung function between dornase alfa and mannitol or dornase alfa and dornase alfa plus mannitol (low‐quality evidence). One trial (23 participants) found a difference in quality of life favouring dornase alfa when compared to dornase alfa plus mannitol (low‐quality evidence); other comparisons found no difference in this outcome (low‐quality evidence). No trials in any comparison reported any difference between groups in the number of pulmonary exacerbations (low‐quality evidence).
When all comparisons are assessed, dornase alfa did not cause significantly more adverse effects than other treatments, except voice alteration and rash.
Authors' conclusions
There is evidence to show that, compared with placebo, therapy with dornase alfa improves lung function in people with cystic fibrosis in trials lasting from one month to two years. There was a decrease in pulmonary exacerbations in trials of six months or longer. Voice alteration and rash appear to be the only adverse events reported with increased frequency in randomised controlled trials. There is not enough evidence to firmly conclude if dornase alfa is superior to other hyperosmolar agents in improving lung function.
Plain language summary
Dornase alfa, an inhaled drug, for treating lung disease in cystic fibrosis
Review question
We reviewed the evidence about the effect of using inhaled dornase alfa for treating lung disease in people with cystic fibrosis.
Background
Cystic fibrosis is an inherited condition which affects the movement of salt across cells in the body and affects, for example, the sweat glands, airways, pancreas and male reproductive system. Lung disease is the most common cause of death in people with cystic fibrosis and although the average life expectancy has increased over the last 30 years, it is still only 48.5 years in developed countries. People with cystic fibrosis develop chronic lung disease because of thick mucus that builds up in the lungs which causes infections and inflammation. Dornase alfa was developed to thin out this mucus, so it is easier for people to cough it up from their lungs; this in turn should decrease the number of infections and amount of inflammation and prevent chronic lung disease.
Search date
The evidence is current to: 23 April 2018.
Study characteristics
We included 19 trials with 2565 people with cystic fibrosis; 15 trials (2447 people) compared dornase alfa to placebo (a dummy treatment with no active medication) or no dornase alfa treatment; two trials (32 people) compared daily dornase to hypertonic saline; one trial (48 people) compared daily dornase alfa with hypertonic saline and alternate day dornase alfa; and one trial (38 people) compared dornase alfa to mannitol and the combination of both drugs. People from all age groups (infants through to adults) took part in the trials which lasted from six days to three years.
Key results
Dornase alfa compared to placebo or no treatment
We found that dornase alfa improves lung function within one month when compared to a placebo or no treatment and this improvement was also seen in longer trials lasting from six months to two years (eight trials; 1708 participants). There were also fewer exacerbations (flare up of lung inflammation) in these longer trials. One trial found that the cost savings from dornase alfa offset 18% to 38% of the medication costs.
Dornase alfa ‐ daily versus alternate day
One trial (43 children) found no differences between treatment schedules for lung function, quality of life or pulmonary exacerbations.
Dornase alfa compared to other medications that improve airway clearance
The results from trials comparing dornase alfa to hypertonic saline or mannitol were mixed. One trial (43 children) showed a greater improvement in lung function with dornase alfa compared to hypertonic saline and one trial (23 participants) reported no difference in lung function between dornase alfa and mannitol or dornase alfa and dornase alfa plus mannitol. In one trial (23 participants) quality of life scores were better with dornase alfa alone than with dornase alfa plus mannitol; other drug comparisons found no difference between treatments for quality of life. No trials in any comparison of treatments reported any difference between groups in the number of pulmonary exacerbations.
Overall, no serious side effects were reported, with only rash and a change in voice seen more frequently in those people taking dornase alfa. However, it is not definitively clear from the current evidence if dornase alfa is better than other medications such as hypertonic saline or mannitol.
Quality of the evidence
The quality of evidence from the trials comparing dornase alfa to placebo or no treatment was moderate to high for lung function results, but only one trial reported any changes in quality of life so the evidence for this outcome is limited.
Also, there were few trials comparing different treatment schedules of dornase alfa (e.g. once a day versus twice a day) or comparing dornase alfa to other medications which help with clearing secretions, so current evidence from these trials is limited and of low quality.
Summary of findings
Background
Description of the condition
Cystic fibrosis (CF) is the most common life‐limiting autosomal recessive disorder amongst people of Northern European descent, affecting about one in every 2300 births. Pulmonary disease is the major cause of morbidity and mortality in CF (Flume 2007).
People with CF inherit an abnormality in the cystic fibrosis transmembrane regulator protein leading to an abnormal movement of chloride and sodium across the airway epithelium. The reduced secretion of chloride into and the excessive absorption of sodium from the airway surface liquid results in a diminished airway surface liquid layer. Consequently, there is decreased mucociliary and cough clearance of airway secretions. The retained airway secretions allow development of a chronic endobronchial infection and induce an exuberant neutrophilic inflammatory response. The large influx of neutrophils into the airways release proteolytic enzymes and oxidants. When the neutrophils die, large quantities of deoxyribonucleic acid (DNA) are released causing the sputum to be thick and tenacious. The thick secretions lead to mucus plugging of the airways and further cycles of infection and inflammation. There is evidence that the initiation of significant airway damage occurs early with findings of pathogenic bacteria, airway inflammation and imaging changes in infants diagnosed by newborn screening (Sly 2009). The unremitting endobronchial infection and neutrophilic inflammation gradually result in irreversible bronchiectasis and eventual respiratory failure.
Description of the intervention
Dornase alfa (Pulmozyme®) is a highly purified solution of recombinant human deoxyribonuclease (rhDNase); it reduces mucus viscosity in the lungs, promoting improved clearance of secretions. The recommended dose for use in most people with CF is 2.5 mg (in one single‐use ampoule) inhaled once daily using a recommended nebuliser. Dornase alfa is used in conjunction with other standard CF therapies.
How the intervention might work
In the 1950s it was shown that the enzyme; bovine deoxyribonuclease (DNase) reduced the viscosity of sputum taken from people with CF by digesting the airway extracellular DNA released from neutrophils (Lieberman 1968). However, clinical trials of bovine DNase had to be stopped due to adverse effects. In 1990 dornase alfa was produced and since 1992 it has been used as a mucolytic to treat people with CF. In contrast, medications such as hypertonic saline and mannitol are osmotically active and are felt to improve mucociliary clearance by rehydrating the airway surface liquid.
Why it is important to do this review
In 2015, the average cost of dornase alfa per person, per year was CDN 14,300, while the cost of hypertonic saline (Nebusal™ 4 ml 7%) was CDN 880 (Cho E 2015 [pers comm]) and mannitol was CDN 11,374 (NICE 2012). In addition, the treatment burden of people with CF is increasingly being recognized with the average time spent on therapies being 108 minutes per day, with the use of two or more nebulised medications significantly adding to this burden (Sawicki 2009). It is important to understand the clinical benefits of medications in order to weigh the monetary and time costs of these therapies.
This is an update of a previously published review (Jones 2003; Jones 2010; Kearney 1998).
Objectives
To determine whether the use of dornase alfa in cystic fibrosis is associated with improved mortality and morbidity compared to placebo or other medications that improve airway clearance, and to identify any adverse events associated with its use.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised controlled trials (published and unpublished) with either parallel or cross‐over design.
Types of participants
Children and adults, of any age, with CF diagnosed clinically and by sweat or genetic testing. Participants with all stages of lung disease were included.
Types of interventions
Dornase alfa administered at any dose, using any nebuliser, at any frequency and for any duration. We compared dornase alfa to placebo or other medications that are adjuncts to airway clearance (typically hyperosmotic agents such as hypertonic saline or mannitol).
Types of outcome measures
The following outcomes were grouped into those measured at up to one month, three, six and 12 months and annually thereafter.
Primary outcomes
-
Changes in lung function from baseline
forced expiratory volume at one second (FEV1)
forced vital capacity (FVC)
lung clearance index (LCI)
forced expiratory volume at 0.5 seconds (FEV0.5 )
Change from baseline in quality of life (QoL)
Mean number of exacerbations
Secondary outcomes
Number of deaths
Number of days treatment with intravenous (IV) antibiotics
Number of days treatment with oral antibiotics
Number of days in hospital due to respiratory exacerbations
Change in weight from baseline
Number of adverse events such as alteration in voice, haemoptysis, bronchospasm
Cost (including indirect costs of therapy)
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
Relevant trials were identified from the Group's Cystic Fibrosis Trials Register using the term: dornase alfa.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of theCochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cystic Fibrosis and Genetic Disorders Group website.
Date of the most recent search of the Group's register: 23 April 2018.
The trials database Clinicaltrials.gov and the International Clinical Trials Registry Platform was also searched to identify unpublished or ongoing trials using the terms dornase alfa (or dnase or pulmozyme) and cystic fibrosis. Date of most recent search: 07 June 2018.
Data collection and analysis
Selection of studies
From the 2015 update, the lead author (CY) and a colleague (MC or MM) independently selected the trials to be included in the review. There were no disagreements about the selection of included trials, but if there are any such disagreements in the future, we will reach a consensus by discussion.
Data extraction and management
The lead author and a colleague (MC or MM) independently extracted data on lung function (FEV1, FVC, LCI, FEV0.5), QoL, exacerbations, deaths, days of oral and IV antibiotics, number of days in hospital, change in weight, adverse events and cost. There were no disagreements about the extracted data, but if there are any such disagreements in the future, we will reach a consensus by discussion.
In previous versions of this review, all trials that reported data at time points of one month or less were combined in a meta‐analysis (Jones 2003; Kearney 1998). It has since been decided that due to the fact that the trial by Wilmott was conducted over two weeks during an acute exacerbation (in contrast to the other trials which recruited participants with stable disease), it would be more appropriate to exclude the trial from this analysis and to analyse it separately (Wilmott 1996).
Assessment of risk of bias in included studies
The lead author (CY) and a colleague (MM, MC) assessed the risk of bias in the included trials using the Cochrane tool for this as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In particular they recorded details for:
generation of allocation sequence;
concealment of allocation;
blinding;
incomplete outcome data;
selective reporting;
other potential sources of bias.
For each of these items the authors assessed the risk of bias for each trial as high, low or unclear.
Measures of treatment effect
For dichotomous data we used the risk ratio (RR) with 95% confidence intervals (CIs) as a measure of treatment effect, where appropriate. For continuous outcomes, we recorded mean change from baseline for each group and standard deviation (SD) for each group. We calculated a pooled estimate of treatment effect by calculating the mean difference (MD) with 95% CIs or the generic inverse variance as appropriate.
Unit of analysis issues
Where trials measured data longitudinally, we based the analysis on the final time point results. Jones discusses methods for the analysis of aggregate longitudinal data (Jones 2009); however, the information that is required to conduct these type of analyses is not available for the trials in this review. We analysed trials with a cross‐over design according to the methodology recommended by Elbourne (Elbourne 2002). We analysed the lung function data from the Amin trial using the generic inverse variance (GIV) and analysed the dichotomous outcomes as if it were a parallel trial (which is a conservative method) (Amin 2011). We were able to analyse the data from the Suri trial using GIV (Suri 2001), but were only able to analyse the data from the Castile trial and the Minasian trial as if they were parallel trials (conservative method) (Castile 2009, Minasian 2010). We were only able to report the data from the remaining cross‐over trials in narrative form (Adde 2004; Ballmann 2002; Robinson 2000).
Dealing with missing data
The authors requested individual patient data from all trials that are contained within this review. Genentech have not yet agreed to provide data on the trials that they funded, but we remain hopeful that this position may change (Fuchs 1994; Laube 1996; McCoy 1996; Quan 2001; Ramsey 1993; Ranasinha 1993; Robinson 2005; Shah 1995a; Wilmott 1996). We are grateful to Mrs Mary Dodd, Dr Fabíola Adde, Dr. Reshma Amin and Pharmaxis for providing individual patient data (Adde 2004; Amin 2011; Dodd 2000; Minasian 2010). We have included data from three of these trials in this review (Adde 2004; Amin 2011; Dodd 2000; Minasian 2010); however, we were not able to de‐code the raw data from the Dodd trial and therefore have not included these data (Dodd 2000).
Assessment of heterogeneity
We assessed heterogeneity using the I² statistic (Higgins 2003). Although the interpretation of I² depends on the magnitude and direction of the effect as well as the strength of evidence for heterogeneity, we used the following thresholds to assess I²:
0% to 40%: likely not important;
30% to 60%: moderate heterogeneity;
50% to 90%: substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Assessment of reporting biases
Due to the chronic nature of the disease, in many CF trials investigators collect data longitudinally at different time points throughout the course of the trial. In all the included trials, we examined when data were collected during the trial and also which data were reported in the trial publication. For outcomes that included data from more than 10 trials, we planned to create a funnel plot to assess for publication bias.
Data synthesis
When we judged heterogeneity to likely be not important, we performed a fixed‐effect analysis. If heterogeneity between trials was more than moderate (i.e. more than 50% to 60%), we performed a random‐effects analysis.
In previous versions of this review, authors combined all trials which reported data at time points of one month or less in a meta‐analysis (Jones 2003; Kearney 1998). We have since decided that due to the fact that the trial by Wilmott was conducted over two weeks during an acute exacerbation (in contrast to the other trials which recruited participants with stable disease), it would be more appropriate to exclude the trial from this analysis and to analyse it separately (Wilmott 1996).
Summary of findings and quality of the evidence (GRADE)
In a post hoc change, the authors have presented five summary of findings tables; one for each comparison (Summary of findings table 1; Summary of findings table 2; Summary of findings table 3; Summary of findings table 4; Table 5).
Summary of findings 5. Dornase alfa versus dornase alfa and mannitol.
| Dornase alfa compared with dornase alfa and mannitol for cystic fibrosis | ||||||
|
Patient or population: Children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa Comparison: Dornase alfa and Mannitol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dornase alfa and mannitol | Dornase alfa | |||||
|
Mean absolute change in FEV1 (L) at 3 months |
See comment | See comment | MD 0.10 (‐0.06 to 0.25) | up to 231 (1 cross‐over study) |
⊕⊕⊝⊝ low2,3 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
|
Mean absolute change in FVC (L) at 3 months |
See comment | See comment | MD 0.13 (‐0.11 to 0.37) | up to 231 (1 cross‐over study) |
⊕⊕⊝⊝ low2,3 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
|
Change in quality of life ‐ CFQ‐R at 3 months |
See comment | See comment | MD 10.61 (0.27 to 20.95) | up to 231 (1 cross‐over study) |
⊕⊕⊝⊝ low2,3 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
|
Number of people experiencing exacerbations at 3 months |
261 per 1000 | 143 per 1000 (41 to 501) | RR 0.55 (0.16 to 1.92) | up to 231 (1 cross‐over study) |
⊕⊕⊝⊝ low2,3 | RR <1 indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| *Assumed and corresponding risk not calculated lung function and quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study. CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. In the crossover trial, 21 participants completed the dornase alfa arm and 23 participants completed the dornase alfa plus mannitol arm (Minasian 2010).
2. Downgraded once for lack of applicability: Minasian included children only so results are not applicable to adults (Minasian 2010).
3. Downgraded once for high risk of bias due to lack of blinding.
Primary outcomes of changes in lung function from baseline, change in QoL from baseline and number of pulmonary exacerbations are presented in the summary of findings tables at three or six months (or both) (or the nearest reported time point). For clarity in the tables, we chose to report relative changes in FEV1 and FVC as important lung function outcomes.
We determined the quality of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one trial, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if for a serious limitation and by two levels if very serious.
Subgroup analysis and investigation of heterogeneity
We defined the following subgroup analysis a priori to be performed if there were enough trials for inclusion in the analysis:
age group ‐ paediatric (0 to 18 years) versus adult (over 18 years);
disease severity ‐ severe (FEV1 or FVC less than 40% predicted) versus moderate (FEV1 or FVC 40% to 80% predicted) versus mild (FEV1 or FVC over 80% predicted);
dose of medication ‐ once‐daily versus twice‐daily administration.
Sensitivity analysis
In future updates (if possible) we will perform a sensitivity analysis based on the risk of bias of the included trials, including and excluding quasi‐randomised trials.
Results
Description of studies
Results of the search
The searches identified 69 trials, of which 19 trials with a total of 2565 participants met our inclusion criteria. We excluded 50 trials (seeFigure 1).
1.
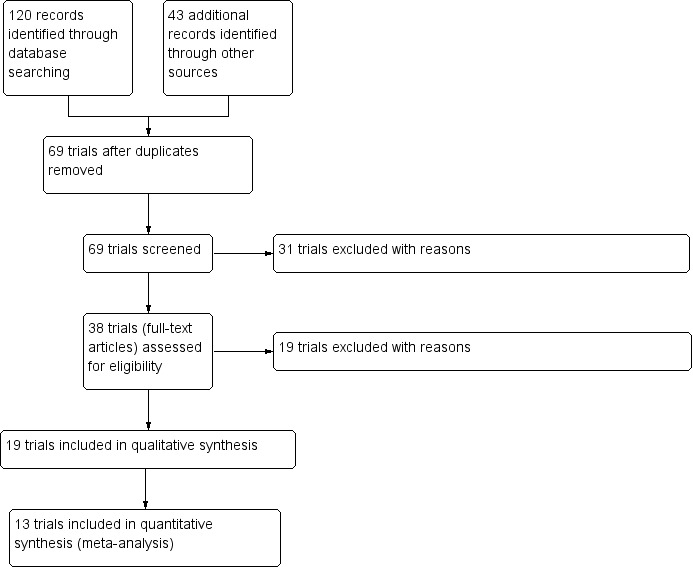
Study flow diagram.
Included studies
We included 19 trials with a total of 2565 participants in the review (Table 6). Three papers analysed the healthcare costs of using dornase alfa (Menzin 1996; Oster 1995; von der Schulenburg 1995) using the data from the included Fuchs trial (Fuchs 1994). Three trials were available in abstract form only (Adde 2004; Castile 2009; Dodd 2000); but the remaining included trials were published as full papers.
1. Summary of included trials.
| Study | Comparison group | Duration of treatment | Frequency of dornase treatment | Study design |
| Amin 2011 | Placebo | 4 weeks | once daily | cross‐over |
| Castile 2009 | Placebo | 6 months | once daily | cross‐over |
| Dodd 2000 | Placebo | 2 weeks | once daily | cross‐over |
| Frederiksen 2006 | No treatment | 1 year | once daily | parallel |
| Fuchs 1994 | Placebo and twice‐daily dornase | 6 months | once or twice daily | parallel |
| Laube 1996 | Placebo | 6 days | twice a day | parallel |
| McCoy 1996 | Placebo | 3 months | once daily | parallel |
| Paul 2004 | No treatment | 3 years | twice a day | parallel |
| Quan 2001 | Placebo | 2 years | once a day | parallel |
| Ramsey 1993 | Placebo | 10 days | twice a day (0.6 mg, 2.5 mg or 10 mg) | parallel |
| Ranasinha 1993 | Placebo | 10 days | twice a day | parallel |
| Robinson 2000 | Placebo | 7 days | once a day | cross‐over |
| Robinson 2005 | Placebo | 1 year | once a day | parallel |
| Shah 1995a | Placebo | 2 weeks | twice a day | parallel |
| Wilmott 1996* | Placebo | 15 days | twice a day | parallel |
| Suri 2001 | Hypertonic saline and alternate day dornase | 3 months | once a day, alternate day | cross‐over |
| Adde 2004 | Hypertonic saline | 4 weeks | once daily | cross‐over |
| Ballmann 2002 | Hypertonic saline | 3 weeks | once daily | cross‐over |
| Minasian 2010 | Mannitol and mannitol plus dornase | 3 months | once daily | cross‐over |
*Trial done during acute exacerbation
Fifteen trials (n = 2447) compared dornase alfa to placebo or no dornase alfa treatment (Amin 2011; Castile 2009; Dodd 2000; Frederiksen 2006; Fuchs 1994; Laube 1996; McCoy 1996; Paul 2004; Quan 2001; Ramsey 1993; Ranasinha 1993; Robinson 2000; Robinson 2005; Shah 1995a; Wilmott 1996). One trial (n = 48) compared daily dornase alfa to hypertonic saline and to alternate day dornase alfa (Suri 2001), and two trials (n = 32) compared dornase alfa to hypertonic saline (Adde 2004; Ballmann 2002). The remaining trial (n = 38) compared dry powder mannitol to dornase alfa and to a combination of both drugs (Minasian 2010).
Dornase alfa versus placebo or no dornase alfa treatment
There were 15 trials (n = 2447) included in this comparison (Amin 2011; Castile 2009; Dodd 2000; Frederiksen 2006; Fuchs 1994; Laube 1996; McCoy 1996; Paul 2004; Quan 2001; Ramsey 1993; Ranasinha 1993; Robinson 2000; Robinson 2005; Shah 1995a; Wilmott 1996).
Trial design
Most of these trials were of parallel design, but we included four trials of cross‐over design (Amin 2011; Castile 2009; Dodd 2000; Robinson 2000). Amin used two four‐week treatment periods with a four‐week washout period (Amin 2011); Castile used six‐month treatment periods with no washout (Castile 2009); Dodd had two‐week treatment periods with a seven‐day washout period (Dodd 2000); and Robinson used seven‐day treatment periods with a two‐week washout (Robinson 2000). The duration of the trials varied from six days (Laube 1996) to three years (Paul 2004) (Table 6). Duration of treatment was less than or equal to one month in eight trials (Amin 2011; Dodd 2000; Laube 1996; Ramsey 1993; Ranasinha 1993; Robinson 2000; Shah 1995a; Wilmott 1996), three months in one trial (McCoy 1996), six months in two trials (Castile 2009; Fuchs 1994), one year in two trials (Frederiksen 2006; Robinson 2005), two years in one trial (Quan 2001) and three years in one trial (Paul 2004).
The size of trials varied from 19 participants (Amin 2011) to 968 participants (Fuchs 1994).
Participants
Four trials included adults only (Dodd 2000; Laube 1996; Ranasinha 1993; Robinson 2000). Four trials included children only; one trial enrolled children aged six to 10 years (Quan 2001), two trials enrolled participants aged six to 18 years (Amin 2011; Robinson 2005) and the remaining trial recruited infants with a mean (SD) age of 42 (32) weeks (Castile 2009). Seven trials included mixed adult and paediatric populations. One trial included participants aged one year and over (Frederiksen 2006), four trials included participants aged five years or older (Fuchs 1994; Paul 2004; Shah 1995a; Wilmott 1996), one trial included participants aged seven years or older (McCoy 1996) and a further trial included participants aged eight years or older (Ramsey 1993).
All trials except for one included participants with stable lung disease; only Wilmott looked at the effects of dornase alfa during treatment for a respiratory exacerbation (Wilmott 1996).
Severity of lung disease varied across the trials. Two trials recruited only participants with severe lung disease (FVC less than 40% predicted) (McCoy 1996; Shah 1995a). Five trials studied participants who had mild to moderate disease (FVC greater than 35% to 40% predicted) (Fuchs 1994; Quan 2001; Ramsey 1993; Ranasinha 1993; Wilmott 1996). One trial looked at participants with moderate disease (FVC between 35% and 75% predicted) (Laube 1996). Three trials included participants with mild lung disease, defined as FVC greater than or equal to 85% in one trial (Robinson 2005), or FEV1 greater than 80% in two trials (Amin 2011; Paul 2004). Three trials did not report information on severity of disease (Castile 2009; Dodd 2000; Frederiksen 2006). The participants in the Castile trial were all infants, so this information would not be available and the abstract simply stated that the participants were all clinically well.
Interventions
The dose and frequency of dornase alfa received by participants varied. Six trials used 2.5 mg dornase alfa twice daily in the treatment group (Laube 1996; Paul 2004; Ranasinha 1993; Robinson 2000; Shah 1995a; Wilmott 1996). Seven trials used used 2.5 mg dornase alfa once daily (Amin 2011; Castile 2009; Dodd 2000; Frederiksen 2006; McCoy 1996; Quan 2001; Robinson 2005). Ramsey gave three different doses of dornase alfa as a twice‐daily regimen: 0.6 mg; 2.5 mg; and 10 mg (Ramsey 1993). Fuchs administered a dose of 2.5 mg dornase alfa either once or twice daily (Fuchs 1994).
In two trials the placebo used was normal saline solution (Dodd 2000; Robinson 2005), six trials stated that the placebo used was excipient alone (Fuchs 1994; Laube 1996; Ranasinha 1993; Shah 1995a; Wilmott 1996; Robinson 2000) and five trials stated that a placebo was used but did not give a formal definition (Amin 2011; Castile 2009; McCoy 1996; Quan 2001; Ramsey 1993).
Outcomes
All trials assessed lung function parameters (FEV1 % predicted, FVC % predicted) with one trial examining FEV0.5 in infants. Three trials assessed QoL; however, only one trial used a validated measure (CFQ‐R) (Amin 2011). None of the trials reported respiratory exacerbations expressed as mean number per period of follow up. Adverse events and deaths were reported in eight trials (Amin 2011; Fuchs 1994; McCoy 1996; Quan 2001; Ramsey 1993; Ranasinha 1993; Shah 1995a; Wilmott 1996). One trial reported on the use of IV antibiotics and the days in hospital (McCoy 1996) and one trial reported on weight (Quan 2001).
Dornase alfa versus hyperosmolar agents
Trial design
Four trials are included in this comparison and all of these trials had a cross‐over design (Adde 2004; Ballmann 2002; Minasian 2010; Suri 2001). Adde used two four‐week treatment periods with a two‐week washout period (Adde 2004). Ballmann used two three‐week treatment periods with a three‐week washout period (Ballmann 2002). Both Minasian and Suri employed three treatment periods, each lasting 12 weeks, with a two‐week washout period between each treatment period (Minasian 2010; Suri 2001). Miniasian was the only trial to compare the combination of dornase and mannitol to each of these agents alone (Minasian 2010).
Participants
Two trials enrolled only children; in one trial ages ranged from nine to 17 years (Minasian 2010) and in the second trial they ranged from five to 18 years (Suri 2001). Ballmann did not specify the age of participants for recruitment purposes, but did state that the mean age of included participants was 13.3 years (Ballmann 2002). The remaining trial recruited both adults and children, age range 8.7 years to 25.8 years (Adde 2004).
One trial included participants with moderate lung disease, FEV1 between 40% and 70% predicted (Minasian 2010). A second trial recruited participants with FEV1 over 70% predicted (Suri 2001). The remaining two trials did not report on lung function as a measure of disease severity (Adde 2004; Ballmann 2002), but Ballmann described participants as a 'group of mild to moderately severely ill children' (Ballmann 2002).
Interventions
Three trials compared dornase alfa to hypertonic saline (Adde 2004; Ballmann 2002; Suri 2001). The first trial compared 2.5 mg dornase alfa once daily to 10 ml hypertonic saline (6%) once daily (Adde 2004), while the second trial compared 2.5 mg dornase alfa once daily to 10 ml hypertonic saline (5.8%) once daily (Ballmann 2002). Suri compared dornase alfa 2.5 mg once daily to dornase alfa 2.5 mg on alternate days and also to twice‐daily 5 ml hypertonic saline (7%) (Suri 2001). Minasian ran a three‐arm trial comparing 2.5 mg dornase alfa twice daily to 400 mg mannitol twice daily and to a combination of both agents (again twice daily) (Minasian 2010).
Outcomes assessed
All of the trials looked at improvements in lung function (FEV1 % predicted or L, FVC % predicted or L) (Adde 2004; Ballmann 2002; Minasian 2010; Suri 2001). Two trials reported on QoL; one used a self‐administered quality of well‐being score (Suri 2001) and the second used the CFQ‐R (Minasian 2010). The same two trials reported on pulmonary exacerbations (Minasian 2010; Suri 2001), but only one of these defined what was meant by the term (Suri 2001). Only one trial reported on adverse events (Minasian 2010) and only one trial reported on weight, number of days in hospital and cost (Suri 2001).
Excluded studies
We have excluded 50 trials, details are given in the tables (Characteristics of excluded studies) and the PRISMA diagram (Figure 1).
Eleven trials were excluded due to trial methodology: 10 because they were not clearly RCTs (Diot 2009; Furuya 2001; Hubbard 1992; Mainz 2011; NCT00311506; NCT02722122; NCT02682290; NCT00843817; Shah 1995b; Shah 1995c); and one because it was an 'N‐of‐1' trial design (Weck 1999).
Two trials were excluded as the participants did not have CF (Riethmueller 2006; EUCTR2006‐002098‐30‐NL) and three trials were excluded as the participants were already on dornase alfa at entry to trial (Dab 2000; Genentech 2010; EUCTR2007‐000935‐25‐NL).
We excluded 31 trials on account of the interventions. One did not use dornase alfa as part of the intervention (Laube 2005) and one trial compared mannitol to control (Bilton 2011). A further trial did not randomise participants by dornase alfa use, investigators studied vitamin E in people with CF and presented results stratified by dornase alfa use (Kelijo 2001). One trial studiedin vitro elasticity in CF sputum (King 1997). Three trials assessed interventions to improve adherence to dornase alfa therapy (NCT01025258; NCT01232478; NCT02301377). Four trials compared different nebulisers (Elkins 2006; Johnson 2006; Sawicki 2014; Shah 1997) and three compared the dispensing methods or delivery technique of the drug (Bakker 2010; Hagelberg 2008; Potter 2008); a further six trials were excluded as they looked at the timing of administration (Anderson 2009; Bishop 2011; Fitzgerald 2005; van der Giessen 2007a; van der Giessen 2007b; Wilson 2007). Six trials were excluded because dornase alfa was given intranasally after sinus surgery or for sinusitis (Cimmino 2005; Craig 2013; Lahiri 2012; Mainz 2011; Mainz 2014; NCT01155752). One trial compared sputum characteristics following either dornase alfa or normal saline with airway clearance techniques (Majaesic 1996) and a further trial studied sputum rheology after dornase alfa therapy (Griese 1997). Two trials looked at the utility of using CT scan changes as an outcome measure (Nasr 2001; Robinson 2002). Finally, one trial was excluded because it was designed with the aim of producing an objective means of selecting those people with CF who would benefit most from dornase alfa (Bollert 1999).
One trial was excluded after the authors were contacted to confirm that no outcomes relevant to this review were collected; although this trial looked at infant pulmonary function they only measured FRC and maximal flow at FRC (ten Berge 2003). One trial examining the use of dornase alfa in pre‐school children was terminated without results because of difficulty obtaining reliable lung function data (Freemer 2010).
In one trial all participants received dornase alfa and there was no control intervention (Heijerman 1995).
Risk of bias in included studies
In order to assess the risk of bias, we examined the following: generation of treatment allocation schedule; concealment of treatment allocation schedule; blinding; incomplete outcome data; selective reporting; and other potential sources of bias. Please see the tables for details for each of these for each trial (Characteristics of included studies). A summary of the risk of bias for each trial is presented as a figure (Figure 2).
2.
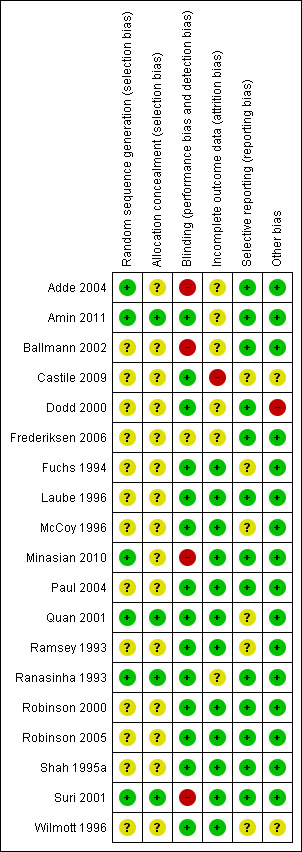
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of allocation sequence
Dornase alfa versus placebo or no dornase alfa treatment
It was clear in only three trials that generation of allocation schedule was adequate and there was a low risk of bias (Amin 2011; Quan 2001; Ranasinha 1993). In the remaining 12 included trials for this comparison, while each trial was described as randomised no details of the randomisation method were stated; therefore, the risk of bias was judged to be unclear (Castile 2009; Dodd 2000; Frederiksen 2006; Fuchs 1994; Laube 1996; McCoy 1996; Paul 2004; Ramsey 1993; Robinson 2000; Robinson 2005; Shah 1995a; Wilmott 1996).
Dornase alfa versus hyperosmolar agents
In three trials in this comparison, it was clear that the generation of allocation schedule had a low risk of bias (Adde 2004; Minasian 2010; Suri 2001). One trial stated that allocation was randomised but did not provide details and was therefore judged as unclear (Ballmann 2002).
Concealment of allocation
Dornase alfa versus placebo or no dornase alfa treatment
The concealment of treatment allocation was adequate, and hence the risk of bias was low, in three trials (Amin 2011; Quan 2001; Ranasinha 1993); the risk of bias was unclear in the remaining 12 trials (Castile 2009; Dodd 2000; Frederiksen 2006; Fuchs 1994; Laube 1996; McCoy 1996; Paul 2004; Ramsey 1993; Robinson 2000; Robinson 2005; Shah 1995a; Wilmott 1996).
Dornase alfa versus hyperosmolar agents
In the Suri trial, an independent trial co‐ordinator concealed the allocation schedule, so the risk of bias was judged to be low (Suri 2001). The details of concealment of treatment allocation schedule, and thus also the risk of bias, was unclear in the remaining trials (Adde 2004; Ballmann 2002; Minasian 2010).
Blinding
Dornase alfa versus placebo or no dornase alfa treatment
Two trials stated that all participants, clinicians and research personnel were blinded to the treatments (Amin 2011; Castile 2009) and 12 trials stated that the design was double blinded and the risk of bias was low in these trials. In one trial no information was provided about the blinding and the risk of bias was therefore unclear (Frederiksen 2006).
Dornase alfa versus hyperosmolar agents
In all four trial reports, it is stated that the trial was not blinded due to the taste of either the hypertonic saline or mannitol (Adde 2004; Ballmann 2002; Minasian 2010; Suri 2001). In one trial, it was stated that the technicians performing the testing were blinded to the intervention (Adde 2004). The four trials reported a mixture of objective outcome measurements (lung function measurement) (Adde 2004; Ballmann 2002) and subjective outcomes determined by the participant (e.g. QoL, adverse events) (Minasian 2010; Suri 2001), therefore risk of bias was deemed high for all four trials.
Incomplete outcome data
Dornase alfa versus placebo or no dornase alfa treatment
We judged 10 trials to have a low risk of bias due to incomplete outcome data. An intention‐to‐treat analysis was performed in seven trials and these were judged to have a low risk of bias (Fuchs 1994; Laube 1996; Paul 2004; Quan 2001; Ramsey 1993; Robinson 2005; Wilmott 1996). In the 2000 trial by Robinson, 15 participants were randomised, but data were only included for 13 participants (no intention‐to‐treat analysis). Two participants withdrew due to pulmonary exacerbations (an a priori protocol violation), one of these was from the placebo group and the other from the dornase alfa group, given that the withdrawals were balanced between treatment groups, there is a low risk of bias (Robinson 2000). In the trials by Shah and McCoy, the risk of bias was considered low since there were few missing data (Shah 1995a; McCoy 1996). An intention‐to‐treat analysis was not possible in the Shah trial where five (out of 70) participants did not complete the 14‐day trial period: one received a heart‐lung transplant; two withdrew consent; and two from the dornase alfa group died. Changes in lung function could therefore not be analysed on an intention‐to‐treat basis; however, adverse events and deaths were analysed on this basis (Shah 1995a). In the McCoy trial, two participants from the dornase alfa group did not have lung function recorded (McCoy 1996). Furthermore, three participants inadvertently received dornase alfa instead of placebo; the lung function data for these participants were analysed on an intention‐to‐treat basis. For analysis of safety data McCoy published results for these participants as if they had been randomised to dornase alfa (McCoy 1996). One of the authors (CA Johnson) has since been contacted and has kindly provided data enabling intention‐to‐treat analysis for the purpose of this review. This did not significantly alter the results.
The risk of bias due to incomplete outcome data is deemed to be unclear in four trials (Amin 2011; Dodd 2000; Frederiksen 2006; Ranasinha 1993). Amin (n = 19) states results were analysed based on the intention‐to‐treat principle; however, only data from 17 participants who provided data for all four trial visits were included (Amin 2011). One participant withdrew after two trial visits because of a pulmonary exacerbation requiring IV antibiotics and a second participant did not have an acceptable LCI during one visit; but it was not clear which treatment these participants had received. It was not clear whether an intention‐to‐treat analysis was performed in the remaining three trials (Dodd 2000; Frederiksen 2006; Ranasinha 1993).
In the trial performed by Castile, follow‐up lung function data were only presented for 19 out of 24 recruited participants and the reasons for dropping out were not clear; therefore, the risk of bias due to incomplete outcome data was considered high (Castile 2009).
Dornase alfa versus hyperosmolar agents
Withdrawals were discussed in detail by Suri and Minasian (seeCharacteristics of included studies), and hence the risk of bias is judged to be low with regards to incomplete outcome data in these two trials (Minasian 2010; Suri 2001). The published data for the Minasian trial only included the 20 participants who completed all three arms of the trial; however, Pharmaxis provided the data analysed by intention‐to‐treat which were used in this review (Minasian 2010). In the remaining two trials, it was not clear whether there had been any withdrawals as these were not discussed (Adde 2004; Ballmann 2002). The risk of bias is therefore judged to be unclear in these two trials.
Selective reporting
Dornase alfa versus placebo or no dornase alfa treatment
Due to the chronic nature of the disease, in many CF trials data are collected longitudinally at different time points throughout the course of the trial. For all the trials we included in this review, we examined when data were collected during the trial and also which data were reported in the trial publication(s). Nine trials reported all time points as well as all outcomes identified in the protocol and we judged these to have a low risk of bias (Amin 2011; Dodd 2000; Frederiksen 2006; Laube 1996, Paul 2004; Ranasinha 1993; Robinson 2000; Robinson 2005; Shah 1995a). Five of the trials reported measuring outcomes at time points which were then not presented in the 'Results' section of the published papers, which may lead to a risk of bias (Fuchs 1994; McCoy 1996; Quan 2001; Ramsey 1993; Wilmott 1996). Castile reported all time points; however, did not report on number of antibiotic days as was intended from the protocol and we judged this to constitute an unclear risk of bias (Castile 2009).
Dornase alfa versus hyperosmolar agent
Outcomes were reported for all time points in all four trials and we judged these to have a low risk of bias (Adde 2004; Ballmann 2002; Suri 2001; Minasian 2010). Miniasian did not report all outcomes as intended in the protocol; however, none of these affected the main outcomes of interest, so the risk of bias was judged as low (Minasian 2010).
Other potential sources of bias
Dornase alfa versus placebo or no dornase alfa treatment
There was an unclear risk of bias for one trial in this comparison where the type of antibiotic used was a potential confounder: eight out of 36 participants in the placebo group received an oral antibiotic versus eight out of the 44 in the treatment group (Wilmott 1996).
Four trials in this group were cross‐over in design with varying washout periods ranging from no washout to four weeks (Amin 2011; Castile 2009; Dodd 2000; Robinson 2000). Data from the Ranasinha trial provides information on the duration of treatment effect with dornase alfa used twice daily (Ranasinha 1993). Participants in that trial were followed weekly after treatment was discontinued and FEV1 and FVC returned to baseline measures 11 to 18 days and 4 to 11 days after treatment discontinuation, respectively. Therefore a washout period of between two and three weeks should be adequate for trials of dornase alfa and we judged two trials to have a low risk of bias despite the cross‐over design (Amin 2011; Robinson 2000). Of the two trials with washout periods less than this, one did not provide any data for this review, so we judged this trial to have an unclear risk of bias (Dodd 2000); and the second did not show a difference between the placebo and dornase alfa group (Castile 2009). It might be expected that a lingering treatment effect of dornase alfa in the Castile trial would lead to a greater decline in lung function in the placebo group and we judged this trial to have a high risk of bias.
We judged the remaining trials in this comparison to have a low risk of bias from any other potential sources as we were unable to identify any.
Dornase alfa versus hyperosmolar agents
All four trials in this group were cross‐over in design, with washout periods ranging from two to three weeks (Adde 2004; Ballmann 2002; Minasian 2010; Suri 2001). The appropriate washout period for dornase alfa is discussed above and data from previous mannitol trials suggest that lung function returns to baseline two weeks after discontinuation of mannitol (Jacques 2008); however, similar data are not available for hypertonic saline. Given that all trials had an appropriate washout period, we do not think this would have led to any bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Dornase alfa versus placebo or no dornase alfa treatment.
| Dornase alfa compared with placebo or no dornase alfa treatment for cystic fibrosis | ||||||
|
Patient or population: Adults and children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa Comparison: Placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no dornase alfa treatment | Dornase alfa | |||||
|
Relative mean percentage change in FEV1 (% predicted) at 3 months |
The relative mean percentage change in FEV1 (% predicted) was 2.10 | The relative mean percentage change in FEV1 (% predicted) was 7.30 higher (4.04 higher to 10.56 higher) |
NA | 320 (1 study)1 | ⊕⊕⊕⊝ moderate2 | |
|
Relative mean percentage change in FEV1 (% predicted) at 6 months |
The relative mean percentage change in FEV1 (% predicted) was 0.00 | The relative mean percentage change in FEV1 (% predicted) was 5.80 higher (3.99 higher to 7.61 higher) |
NA | 647 (1 study)1 | ⊕⊕⊕⊕ high3 | Result presented from once‐daily dornase alfa group. Significant benefit for dornase alfa also present in twice‐daily dornase alfa group |
|
Relative mean percentage change in FVC (% predicted) at 3 months |
The relative mean percentage change in FVC (% predicted) was 7.30 | The relative mean percentage change in FVC (% predicted) was 5.10 higher (1.23 higher to 8.97 higher) |
NA | 318 (1 study)4 | ⊕⊕⊕⊝ moderate2 | |
|
Relative mean percentage change in FVC (% predicted) at 6 months |
See comment | See comment | MD 3.80 (2.62 to 4.98) | 647 (1 study)1 | ⊕⊕⊕⊕ high3 | Mean difference between groups only presented. Result presented from once‐daily dornase alfa group. Significant benefit for dornase alfa also present in twice‐daily dornase alfa group |
|
Change in quality of life ‐ CFQ‐R respiratory at 1 month |
See comment | See comment | MD 0.84 (‐10.74 to 12.42) | 19 (1 cross‐over study)5 |
⊕⊕⊝⊝ low6,7 | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
|
Change in quality of life ‐ CFQ‐R respiratory (parent) at 1 month |
See comment | See comment | MD 9.78 (‐2.58 to 22.14) | 19 (1 cross‐over study)5 |
⊕⊕⊝⊝ low6,7 | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
|
Number of people experiencing exacerbations at up to 2 years |
252 per 1000 | 196 per 1000 (156 to 242) |
RR 0.78 (0.62 to 0.96) |
1157 (3 studies)8 |
⊕⊕⊕⊝ moderate9 | RR <1 indicates an advantage for dornase alfa. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Assumed and corresponding risk not calculated for quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study CI: confidence interval; RR: risk ratio MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Additionally four trials included in analysis at one month showed a significant advantage to dornase alfa over placebo or no dornase alfa treatment (Laube 1996; Ramsey 1993; Ranasinha 1993; Shah 1995a). Three studies not included in pooled analysis showed no difference between groups in relative FEV1(L) (Robinson 2000) and relative FEV1 (% predicted) (Wilmott 1996) or absolute FEV1 (% predicted) (Amin 2011) at one month. At one year, one study showed a significant advantage to dornase alfa over placebo or no dornase alfa treatment (Frederiksen 2006) and one study showed no difference between treatments (Robinson 2005). At one year, one study showed a significant advantage to dornase alfa over placebo or no dornase alfa treatment (Quan 2001) and at three years, one study showed no significant difference between treatments (Paul 2004).
2. Downgraded due to indirectness: participants in McCoy 1996 had severe lung disease (FVC below 40%).
3. No evidence of imprecision, inconsistency, indirectness, publication bias or serious risk of bias.
4. Additionally four trials included in analysis at one month (Laube 1996; Ramsey 1993; Ranasinha 1993; Shah 1995a) showed a significant advantage to dornase alfa over placebo or no dornase alfa treatment. One study not included in pooled analysis showed a significant advantage in relative FVC (L) to dornase alfa over placebo or no dornase alfa treatment (Robinson 2000) and one study showed no significant different in absolute FVC (% predicted) between groups (Amin 2011) at one month. No significant difference was found between groups at one year (Robinson 2005) and at two years (Quan 2001).
5. Additionally, four studies reported quality of life data which could not be included in pooled analysis. Wilmott 1996 showed no difference between groups in CFQ‐R. Ramsey reported that the frequency and magnitude of improvement across all quality of life questions was greater among participants receiving dornase alfa (Ramsey 1993). Ranasinha reported significant improvements in overall well‐being and significant improvements in general well‐being, cough frequency and chest congestion (Ranasinha 1993) and Fuchs reported significant improvements in well‐being score and dyspnoea score on dornase alfa compared to placebo (Fuchs 1994).
6. Downgraded once for lack of applicability: Amin included children only so results are not applicable to adults (Amin 2011).
7. Downgraded once for imprecision: wide confidence intervals around the effect size due to limited sample size of the trial.
8. Additionally, one study reported an age‐adjusted RR of having more than one respiratory exacerbation, but these data were not included in the pooled analysis (McCoy 1996). No significant difference was found between dornase alfa and control.
9. Downgraded once as data from one cross‐over trial was analysed as parallel data (Amin 2011), which is a conservative approach.
Summary of findings 2. Dornase alfa daily versus alternate days.
| Dornase alfa daily compared with dornase alfa on alternate days for cystic fibrosis | ||||||
|
Patient or population: Children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa daily Comparison: Dornase alfa alternate days | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Dornase alfa alternate days | Dornase alfa daily | |||||
|
Mean relative percentage change in FEV1 (L) at 3 months |
See comment | See comment | MD 2.00 (‐5.00 to 9.00) | 43 (1 cross‐over study) |
⊕⊕⊝⊝ low1,2 | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
|
Mean relative percentage in FVC (L) at 3 months |
See comment | See comment | MD 0.03 (‐0.06 to 0.12) | 43 (1 cross‐over study) |
⊕⊕⊝⊝ low1,2 | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
|
Mean relative percentage in quality of life score at 3 months |
See comment | See comment | MD 0.01 (‐0.02 to 0.04) | 43 (1 cross‐over study) |
⊕⊕⊝⊝ low1,2 | Positive MD indicates an advantage for dornase alfa daily. Participants received both interventions in cross‐over design. |
|
Number of pulmonary exacerbations at 3 months |
17 exacerbations | 18 exacerbations | NA (see comment) | 43 (1 cross‐over study) |
⊕⊕⊝⊝ low1,2 | No difference was found in the number of pulmonary exacerbations (no statistical comparison made) |
| *Assumed and corresponding risk not calculated lung function and quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study. CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. Downgraded once for lack of applicability: Suri included children only so results are not applicable to adults (Suri 2001).
2. Downgraded once for high risk of bias due to lack of blinding.
Summary of findings 3. Dornase alfa versus hypertonic saline.
| Dornase alfa compared with hypertonic saline for cystic fibrosis | ||||||
|
Patient or population: Children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa (once daily) Comparison: Hypertonic saline | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Hypertonic Saline | Dornase alfa | |||||
|
Mean relative percentage in FEV1 (L) at 3 months |
See comment | See comment | MD 8.00 (2.00 to 14.00) | up to 431,2 (1 cross‐over study) (see comment) |
⊕⊕⊝⊝ low3,4 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
|
Mean relative percentage in FVC (L) at 3 months |
See comment | See comment | MD 0.08, (‐0.02 to 0.18) | up to 431,2 (1 cross‐over study) |
⊕⊕⊝⊝ low3,4 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
|
Mean relative percentage in quality of life score at 3 months |
See comment | See comment | MD 0.03, (‐0.01 to 0.07) | up to 431,2 (1 cross‐over study) |
⊕⊕⊝⊝ low3,4 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
|
Number of pulmonary exacerbations at 3 months |
15 exacerbations | 17 exacerbations | NA (see comment) | up to 431,2 (1 cross‐over study) |
⊕⊕⊝⊝ low3,4 | No difference was found in the number of pulmonary exacerbations (no statistical comparison made) |
| *Assumed and corresponding risk not calculated lung function and quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study. CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. In the cross‐over trial, 43 participants completed the dornase alfa arm and 40 completed the hypertonic saline arm (Suri 2001).
2. Two additional cross‐over trials compared dornase alfa and hypertonic saline, no significant differences were found between the treatments for % change in FEV1 and other primary outcomes of the review were not recorded in these trials (Adde 2004; Ballmann 2002).
3. Downgraded once for lack of applicability: Suri included children only so results are not applicable to adults (Suri 2001).
4. Downgraded once for high risk of bias due to lack of blinding.
Summary of findings 4. Dornase alfa versus mannitol.
| Dornase alfa compared with mannitol for cystic fibrosis | ||||||
|
Patient or population: Children with cystic fibrosis Settings: Outpatients Intervention: Dornase alfa Comparison: Mannitol | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Mannitol | Dornase Alfa | |||||
|
Mean absolute change in FEV1 (L) at 3 months |
See comment | See comment | MD 0.02 (‐0.11 to 0.16) | up to 231 (1 cross‐over study) |
⊕⊕⊝⊝ low2,3 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
|
Mean absolute change in FVC (L) at 3 months |
See comment | See comment | MD ‐0.02, (‐0.23 to 0.19) | up to 231 (1 cross‐over study) |
⊕⊕⊝⊝ low2,3 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
|
Change in quality of life ‐ CFQ‐R at 3 months |
See comment | See comment | MD 10.61 (0.27 to 20.95) | up to 231 (1 cross‐over study) |
⊕⊕⊝⊝ low2,3 | Positive MD indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| Number of people experiencing exacerbations ‐ at 3 months | 130 per 1000 | 143 per 1000 (33 to 631) |
RR 1.10 (0.25 to 4.84) |
up to 231 (1 cross‐over study) |
⊕⊕⊝⊝ low2,3 | RR <1 indicates an advantage for dornase alfa. Participants received both interventions in cross‐over design. |
| *Assumed and corresponding risk not calculated for lung function and quality of life. Relative effect and 95% CI presented is adjusted for the cross‐over design of the study. CFQ‐R: Cystic Fibrosis Questionnaire ‐ Revised; CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. In the cross‐over trial, 21 participants completed the dornase alfa arm and 23 participants completed the mannitol arm (Minasian 2010).
2. Downgraded once for lack of applicability: Minasian included children only so results are not applicable to adults (Minasian 2010).
3. Downgraded once for high risk of bias due to lack of blinding.
In the summary of findings tables, the quality of the evidence has been graded for pre‐defined outcomes (see above) and definitions of these gradings provided.
Dornase alfa versus placebo or no dornase alfa treatment
Results that are reported in the graphs for Fuchs were from the once‐daily group (results from the twice‐daily group have been reported where possible) (Fuchs 1994). Also, Ramsey investigated three doses of dornase alfa, the results in the graphs are from the treatment group that were randomised to 2.5 mg of dornase alfa. A summary of key findings for this comparison has been presented in a table (Table 1).
Primary outcomes
1. Changes in lung function (FEV1, FVC, LCI, FEV0.5) from baseline
The changes in FEV1, FVC and LCI for Quan and Amin were reported as absolute differences and the results for the other trials were reported as relative differences (seePublished notes) (Amin 2011; Quan 2001). It was not clear if the change in FEV0.5 reported by Castile was an absolute or relative difference (Castile 2009).
Results for FEV1 from the Wilmott and Ranasinha trials were estimated from the graphs that were included in the primary papers (Ranasinha 1993; Wilmott 1996). One trial provided data for outcomes at both three and 12 months; both data sets are included in the analysis (Robinson 2005).
a. Mean percentage change in FEV1 ‐ in participants with stable disease
i. at one month
This outcome was reported in six trials at the one‐month time point (dornase alfa n = 151, control n = 157) (Amin 2011; Laube 1996; Ramsey 1993; Ranasinha 1993; Robinson 2000; Shah 1995a). However, data from two trials were not included in the pooled analysis because one trial reported absolute and not relative changes in FEV1 (Amin 2011) and another trial reported results in litres and not % predicted (Robinson 2000); therefore, the pooled analysis includes a total of 248 participants (dornase alfa n = 121, control n = 127). When analysed, data showed a difference in percentage change in FEV1, MD 9.51% (95% CI 0.67 to 18.35) (Analysis 1.1). Due to the substantial heterogeneity between the four trials in the pooled analysis (I² = 88%), we employed a random‐effects model; we also considered the planned subgroup analyses based on age of participants, disease severity and frequency of dosing (once daily versus twice daily).
1.1. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 1 Relative mean % change in FEV1 (% predicted).
A subgroup analysis could not be undertaken for paediatric versus adult participants because there were no trials including only children and trials with both paediatric and adult participants did not report the data for these groups separately. We were able to undertake a subgroup analysis based on disease severity with three trials including participants with moderate disease (dornase n = 90, control n = 93) (Laube 1996; Ramsey 1993; Ranasinha 1993) and one trial including participants with severe disease (dornase n = 31, control n = 34) (Shah 1995a). Those with moderate disease had significant improvements in FEV1, MD 14.26 (95% CI 10.79 to 17.74), whereas those with severe disease did not, MD ‐2.81 (95% CI ‐8.77 to 3.15). In addition the heterogeneity in the subgroup analysis decreased to I² = 0 suggesting that disease severity accounted for the heterogeneity in the original analysis (Analysis 1.2).
1.2. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 2 Relative mean % change in FEV1 (% predicted) at one month ‐ subgroup analysis by disease severity.
A subgroup analysis based on frequency of drug administration was not possible, because all four trials used dornase twice daily.
There was no absolute difference between groups in FEV1 in the Amin trial, MD 0.08% (95% CI ‐5.59 to 5.74) (Analysis 1.3). There was no significant difference reported in FEV1 (L) between the dornase group (7.5% change) and the placebo group (3.4 % change) (Robinson 2000) (Table 7).
1.3. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 3 Absolute mean % change in FEV1 (% predicted).
2. Robinson 2000 ‐ DNase versus placebo.
| Pre dornase alfa | Post dornase alfa | Pre placebo | Post placebo | |
| FEV1 (L) mean (SD) |
2.63 (0.31) | 2.8 (0.32) | 2.63 (0.32) | 2.70 (0.32) |
| FVC (L) mean (SD) |
4.03 (0.35) | 4.21 (0.35) | 4.12 (0.36) | 4.06 (0.38) |
FEV1: forced expiratory volume at one second FVC: forced vital capacity SD: standard deviation
ii. at three months
This was reported in one trial in which participants had severe lung disease (FVC below 40%) (dornase alfa n = 158, control n = 162) (McCoy 1996). The MD in percentage change in FEV1 was 7.30% (95% CI 4.04 to 10.56) (Analysis 1.1) (moderate‐quality evidence).
iii. at six months
This outcome was reported in one trial at the six‐month time point (dornase alfa n = 322, control n = 325) (Fuchs 1994). The MD in percentage change in FEV1 for the once‐daily treatment group was 5.80% (95% CI 3.99 to 7.61) (high‐quality evidence). For the twice‐daily dosage group mean improvement was 5.60 (95% CI 4.90 to 6.29) (Analysis 1.1).
iv. at one year
Analysable data for this outcome were reported in one trial at the one‐year time point (dornase alfa n = 8, control n = 11) (Robinson 2005). The MD in percentage change in FEV1 was 0.70 (95% CI ‐11.26 to 12.66) (Analysis 1.1). A second trial reported a median increase in FEV1 of 7.3% in the treatment group compared to 0.9% in the placebo group (P < 0.05) (Frederiksen 2006).
v. at two years
One trial reported on this outcome at the two‐year time point (dornase alfa n = 204, control n = 206) (Quan 2001); and showed a MD 3.24% (95% CI 1.03 to 5.45) (Analysis 1.4).
1.4. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 4 Absolute mean % change in FEV1 (% predicted).
vi. at three years
One trial which was designed to assess lung inflammation reported on this outcome at the three‐year time point (dornase alfa n = 46, control n = 39) (Paul 2004). Trialists reported the median rate of decline in FEV1 at ‐1.99% in the dornase group and ‐3.26% in those not receiving dornase; this result was not significantly different (Paul 2004).
b. Mean percentage change in FEV1 ‐ in participants with acute pulmonary exacerbations
i. at one month This outcome was reported in one trial at the one‐month time point (dornase alfa n = 43, control n = 37) (Wilmott 1996). Our analysis showed no difference between groups, MD 1.00 (95% CI ‐13.93 to 15.93) (Analysis 1.5).
1.5. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 5 Relative mean % change in FEV1 (in participants with acute exacerbations).
c. Mean percentage change in FVC ‐ in participants with stable disease
i. at one month
This outcome was reported in six trials at the one‐month time point (dornase alfa n = 151, control n = 157) (Amin 2011; Laube 1996; Ramsey 1993; Ranasinha 1993; Shah 1995a; Robinson 2000). As for the results for FEV1, the data from Amin were not included in the pooled analysis because the trial reported absolute and not relative changes and the data from the Robinson trial were not included because the trial reported FVC in litres and not % predicted (pooled analysis dornase alfa n = 121, control n = 127).
The pooled analysis showed a difference in relative percentage change in FVC, MD 7.52% (95% CI 1.34 to 13.69) (Analysis 1.6). There was substantial heterogeneity between the trials (I² = 69%), therefore a random‐effects model was used.
1.6. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 6 Relative mean % change in FVC (% predicted).
We originally planned to undertake subgroup analyses based on age of participants, disease severity and dose frequency (once‐daily versus twice‐daily dosing). A subgroup analysis with paediatric versus adult participants was not possible because there were no trials including only children. We were able to perform a subgroup analysis on disease severity with three trials including participants with moderate disease (dornase alfa n = 90, control n = 93) (Laube 1996; Ramsey 1993; Ranasinha 1993) and one trial including participants with severe disease (dornase alfa n = 31, control n = 34) (Shah 1995a). Similar to the findings for FEV1, those with moderate disease showed significant improvements in FVC, MD 10.98 (95% CI 7.68 to 14.29), whereas those with severe disease did not, MD ‐4.90 (95% CI ‐15.15 to 5.35). In addition, the heterogeneity in this subgroup analysis decreased to I² = 0% suggesting that disease severity accounted for some of the heterogeneity in the original analysis (Analysis 1.8). A subgroup analysis based on frequency of drug administration was not possible because all four trials administered dornase alfa twice daily.
1.8. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 8 Relative mean % change in FVC at one month ‐ subgroup analysis by disease severity.
Amin reported the absolute difference in FVC, but analysis of the data showed no difference between treatment groups, MD ‐3.61% (95% CI ‐10.02 to 2.80) (Amin 2011) (Analysis 1.9). Robinson reported a significant difference in FVC between the placebo group (‐2.2% change) and the dornase alfa group (5.4% change) (P < 0.02) (Robinson 2000) (Table 7).
1.9. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 9 Absolute mean % change in FVC (% predicted).
ii. at three months
The mean percentage change in FVC was reported in one trial at the three‐month time point (dornase alfa n = 156, control n = 162) (McCoy 1996). Analysis showed a difference between groups, MD 5.10% (95% CI 1.23 to 8.97) (Analysis 1.6) (moderate‐quality evidence).
iii. at six months
One trial of once‐daily and twice‐daily dornase alfa compared to placebo reported on this outcome at the six‐month time point (once‐daily dornase alfa n = 322, twice‐daily dornase alfa n = 321, control n = 325) (Fuchs 1994). In participants receiving once‐daily dornase alfa, FVC improved by MD 3.80 (95% CI 2.62 to 4.98) compared to control (high‐quality evidence); and for those on the twice‐daily regimen by MD 3.00 (95% CI 1.82 to 4.18) compared to control (Analysis 1.7).
1.7. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 7 Relative mean % change in FVC (% predicted).
iv. at one year
This outcome was reported in one trial at the one‐year time point (dornase alfa n = 8, control n = 11) (Robinson 2005). Analysis showed no difference between treatment groups, MD ‐5.70 (95% CI ‐15.87 to 4.47) (Analysis 1.6).
v. at two years
One trial reported the absolute mean difference between the two groups at two years (dornase alfa n = 204, control n = 206), showing MD 0.70 (95% CI ‐1.24 to 2.64) (Quan 2001) (Analysis 1.10).
1.10. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 10 Absolute mean % change in FVC (% predicted).
vi. at three years
One trial, whose primary objective was to assess lung inflammation, reported on the change in FVC at three years (dornase alfa n = 46, control n = 39) (Paul 2004). The trial reported a significant decrease in the annual median decline in FVC in the group not receiving dornase alfa; whereas, the participants receiving dornase alfa did not have a significant decrease in FVC over time.
d. LCI
One trial reported on LCI at one month (dornase alfa n = 17, control n = 17) (Amin 2011). Our analysis produced a non‐significant result, MD ‐0.90 (95% CI ‐1.87 to 0.07) (Analysis 1.11). However, the published paper reports a significant difference in LCI between the groups (P = 0.02) (Amin 2011). This is likely due to the fact the investigators used a model that took participants' baseline lung function into account when analysing the data which we are not able to do when analysing data in RevMan. It should be noted that, contrary to other measures of lung function, a decrease in LCI is beneficial.
1.11. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 11 Absolute mean change in LCI.
e. FEV0.5 z score
Only one cross‐over trial involving 19 infants (dornase alfa n = 19, control n = 19) reported on this outcome at the six‐month time point (Castile 2009). Analysis showed the MD in the FEV0.5 z score was 0.10 (95% CI ‐0.57 to 0.77) (Analysis 1.12).
1.12. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 12 Absolute change in FEV0.5 (z score).
2. Mean percentage change in quality of life score
Many of the trials did not use the same QoL measurements precluding pooling of data. Although Ranasinha and Fuchs described similar measures of quality of life, Ranasinha did not report specific QoL scores (Fuchs 1994; Ranasinha 1993).
Wilmott and Amin reported that the QoL scores they obtained showed no significant difference between the groups, in terms of improvement in cough and congestion, activity limitation, emotional well‐being, fatigue, days of restriction to bed and general health perception (Wilmott 1996) or in either version of the CFQ‐R (Amin 2011) (Analysis 1.13; Analysis 1.14) (low‐quality evidence). Fuchs used a five‐point well‐being score and also evaluated a CF symptom score and dyspnoea scale. There was a significant improvement in the well‐being score and dyspnoea score compared to placebo in the once‐daily dornase alfa group but not in the twice‐daily dornase alfa group; both groups reported an improvement in the CF symptom score (Fuchs 1994). Ranasinha stated that there was a non‐significant improvement in dyspnoea, and overall well‐being and significant improvements in general well‐being, cough frequency and chest congestion (Ranasinha 1993). Ramsey reported that the frequency and magnitude of improvement across all QoL questions was greater among participants receiving dornase alfa (Ramsey 1993).
1.13. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 13 Quality of life ‐ CFQ‐R respiratory.
1.14. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 14 Quality of life ‐ CFQ‐R Parent respiratory.
3. Mean number of respiratory exacerbations
Trials included participants with stable lung disease. None of the included trials reported respiratory exacerbations expressed as a mean number per period of follow up. However, three trials reported either the RR or the number of people experiencing respiratory exacerbations, therefore these data have been included within the review (Amin 2011; Fuchs 1994; Quan 2001). Additionally, one trial reported an age‐adjusted RR of having more than one respiratory exacerbation, but these data were not included in the pooled analysis (McCoy 1996).
The definition of a respiratory (pulmonary) exacerbation varied in the trials. Fuchs defined an exacerbation as the need for parenteral antibiotics because of any four of the following 12 signs or symptoms: change in sputum; new or increased haemoptysis; increased cough; increased dyspnoea; malaise, fatigue or lethargy; temperature above 38°C; anorexia or weight loss; sinus pain or tenderness; change in sinus discharge; change in physical exam of the chest; decrease in pulmonary function by 10% or more from a previously recorded value; or radiographic changes indicative of pulmonary infection (Fuchs 1994). Quan defined an exacerbation as respiratory symptoms requiring IV antibiotics (Quan 2001). The remaining two trials did not include a specific definition for pulmonary exacerbations (Amin 2011; McCoy 1996). The Amin trial planned to withdraw participants who had a pulmonary exacerbation requiring IV antibiotics and one participant was withdrawn for this reason, but it was not reported which treatment group this participant was from (Amin 2011).
We included data for this outcome from trials lasting one month (Amin 2011), six months (Fuchs 1994) and two years (Quan 2001) (dornase alfa n = 575, control n = 576). This yielded a RR of 0.78 (95% CI 0.62 to 0.96) in favour of dornase alfa (Analysis 1.15) (moderate‐quality evidence). In the trial by Fuchs, it was noted that participants aged 17 to 23 years had a higher incidence of exacerbations regardless of treatment group, and the once‐daily group had a higher percentage of participants in this age range; therefore, they calculated an age‐adjusted RR for the once‐daily group at 0.72 (95% CI 0.52 to 0.98) and for the twice‐daily group at 0.63 (95% CI 0.46 to 0.87) (Fuchs 1994).
1.15. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 15 Number of people experiencing exacerbations.
A three‐month trial including participants with severe disease (dornase alfa n = 158, control n = 162) reported the age‐adjusted RR of having more than one respiratory exacerbation during the trial as 0.93 (95%CI 0.69 to 1.21) (McCoy 1996).
Secondary outcomes
1. Mortality
This outcome was reported in seven trials in total (dornase alfa n = 841, control n = 849): in four trials at one month (Laube 1996; Ramsey 1993; Ranasinha 1993; Shah 1995a); in one trial at three months (McCoy 1996); in one trial at six months (Fuchs 1994); and in one trial at two years (Quan 2001). The RR of death was 1.70 (95% CI 0.70 to 4.14) with 12 deaths in the dornase alfa group and seven deaths in the control group (Analysis 1.16). The majority of deaths (17 of 19 deaths) were reported from two trials which enrolled participants with severe lung disease (Shah 1995a; McCoy 1996).
1.16. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 16 Number of deaths.
2. Mean number of days IV antibiotics used
a. at three months
One trial reported on this outcome at three months (dornase alfa n = 158, control n = 162) (McCoy 1996). Analysis showed the difference between the treated and control groups was MD ‐2.96 (95% ‐7.29 to 1.37) (Analysis 1.17).
1.17. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 17 Mean number of days IV antibiotics used.
3. Mean number of days oral antibiotics used
No trial reported on this outcome.
4. Mean number of days of inpatient treatment
a. at three months
One trial reported on this outcome at three months (dornase alfa n = 158, control n = 162) (McCoy 1996). The difference between the groups was not statistically significant, MD 0.93 (95% CI ‐2.19 to 4.05) (Analysis 1.18).
1.18. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 18 Mean number of days inpatient treatment.
b. at six months
One trial reported the mean number of inpatient days at six months (dornase alfa n = 322, control n = 325) (Fuchs 1994). Participants treated with the once‐daily regimen spent 1.3 fewer days in hospital compared to placebo (P = 0.06) and participants receiving twice‐daily dornase alfa spent 1.0 fewer days in hospital compared to placebo (P < 0.05).
5. Mean change in weight from baseline
a. at two years
Only Quan reported on the mean change in weight from baseline at two years (dornase alfa n = 236, control n = 234) (Quan 2001). The weight‐for‐age percentile decreased in both groups from baseline to the end of the trial; the difference between the treatment groups was not statistically significant, MD ‐0.20 (95% CI ‐2.42 to 2.02) (Analysis 1.19).
1.19. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 19 Mean change in weight from baseline.
6. Number of participants experiencing adverse events by end of the trial
a. haemoptysis (blood stained sputum)
This outcome was reported in three trials (dornase alfa n = 393, control = 395) with trial durations of one month (Ranasinha 1993; Shah 1995a) and six months (Fuchs 1994). There was no increased risk of haemoptysis with dornase alfa treatment, RR 0.88 (95% CI 0.50 to 1.55) (Analysis 1.20).
1.20. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 20 Adverse event ‐ haemoptysis (blood‐stained sputum).
b. dyspnoea (shortness of breath)
This outcome was reported in four trials (dornase alfa n = 551, control = 557) with trial durations of one month (Ranasinha 1993; Shah 1995a), three months (McCoy 1996) and six months (Fuchs 1994). There was no increased risk of dyspnoea with dornase alfa treatment, RR 1.00 (95% CI 0.85 to 1.18) (Analysis 1.21).
1.21. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 21 Adverse event ‐ dyspnoea (shortness of breath).
c. pneumothorax
Three trials of participants with stable disease reported on this outcome (dornase alfa n = 393, control = 395) with trial durations of one month (Ranasinha 1993; Shah 1995a) and six months (Fuchs 1994). There was no increased risk of pneumothorax with dornase alfa treatment, RR 0.60 (95% CI 0.08 to 4.50) (Analysis 1.22).
1.22. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 22 Adverse event ‐ pneumothorax.
The trial which enrolled participants with an acute exacerbation also reported this outcome (dornase alfa n = 43, control n = 37) (Wilmott 1996), with one participants in the treatment group having a pneumothorax, RR 2.65 (95% CI 0.10 to 66.96) (Analysis 1.23).
1.23. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 23 Adverse event ‐ pneumothorax (in participants with acute exacerbations).
d. voice alteration
Seven trials of participants with stable disease reported on this outcome (dornase alfa n = 831, control n = 839) with durations of one month (Ramsey 1993; Ranasinha 1993; Shah 1995a), three months (McCoy 1996), six months (Fuchs 1994) and two years (Quan 2001). Participants were more likely to experience voice alteration with dornase alfa compared to placebo, RR 1.69 (95% CI 1.20 to 2.39) and this was seen more commonly in trials lasting between one and three months, but not in longer trials (Analysis 1.24). In the trial that compared once‐daily to twice‐daily use of dornase alfa over six months, there was no difference in voice alteration between the two groups, RR 1.34 (95% 0.64 to 2.78) (Fuchs 1994) (Analysis 1.25).
1.24. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 24 Adverse event ‐ voice alteration.
1.25. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 25 Adverse event ‐ voice alteration (1x versus 2x daily treatment).
The trial in people with an acute exacerbation also reported on this outcome (dornase alfa n = 43, control n = 37) (Wilmott 1996), but found no statistically significant difference between the treatment or control groups, RR 2.58 (95% CI 0.55 to 12.03) (Analysis 1.26).
1.26. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 26 Adverse event ‐ voice alteration (in participants with acute exacerbations).
e. rash
Occurence of a rash was reported in two trials (dornase alfa n = 558, control n = 559) of six months (Fuchs 1994) and two years duration (Quan 2001). There was an increased risk of rash in participants taking dornase alfa, RR 2.40 (95% CI 1.16 to 4.99) (Analysis 1.27).
1.27. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 27 Adverse event ‐ rash.
f. other adverse events
A number of other adverse events were documented and are presented in the analysis; in no case was there an increased risk in participants treated with dornase alfa: three trials reported chest pain (Analysis 1.28); one trial reported cough (Analysis 1.29); one trial reported increased sputum production (Analysis 1.30); one trial reported dry throat (Analysis 1.31); six trials reported pharyngitis (Analysis 1.32); three trials reported laryngitis (Analysis 1.33); two trials reported conjunctivitis (Analysis 1.34); three trials reported wheeze (Analysis 1.35); and one trial reported facial oedema (Analysis 1.36).
1.28. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 28 Adverse event ‐ chest pain.
1.29. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 29 Adverse event ‐ cough (new or increased).
1.30. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 30 Adverse event ‐ increased sputum production.
1.31. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 31 Adverse event ‐ dry throat.
1.32. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 32 Adverse event ‐ pharyngitis.
1.33. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 33 Adverse event ‐ laryngitis.
1.34. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 34 Adverse event ‐ conjunctivitis.
1.35. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 35 Adverse event ‐ wheeze.
1.36. Analysis.
Comparison 1 Dornase alfa versus placebo, Outcome 36 Adverse event ‐ facial oedema.
7. Cost of treatment
Three papers examined the cost of health care for participants involved in the Fuchs trial, which lasted for 20 weeks (Fuchs 1994).
The report by Oster prospectively documented how participants used health care and then, using secondary data sources, estimated the cost of hospitalisation and outpatient antibiotic treatment for participants in the trial. This information was then used to compare the cost of all respiratory tract infection‐related health care (including non‐protocol defined respiratory tract infections) between the two treatment groups and the control group. The authors estimated that the mean total cost of respiratory tract infection‐related care was USD 6443, USD 4761 and USD 5628 for the placebo, once‐daily and twice‐daily dosage regimens respectively. This cost included all outpatient antibiotic therapy, as well as estimates of cost for any inpatient care. The estimates did not include the cost of dornase alfa itself, as this was not marketed at the time of the trial. Once dornase alfa was marketed, at a cost of USD 27 per ampoule, they were able to estimate that the reduced cost of respiratory tract infection‐related care would offset between 18.3% and 37.5% of the cost of therapy itself (Oster 1995).
The report by von der Schulenburg used the same data from the Fuchs trial, but used health insurance costs to estimate what would have been the costs of healthcare treatment in German CF centres for participants receiving once daily dornase alfa versus those participants receiving placebo (von der Schulenburg 1995). The total cost for the health care of participants, if they had been treated in a German CF centre, was DM 5879 (USD 3551) for the group receiving once‐daily dornase alfa versus DM 7849 (USD 4742) for the placebo group. This included the cost of inpatient admissions, outpatient appointments and investigations. The cost of all antibiotics used was DM 2954 (USD 1784) per participant in the treated group versus DM 4213 (USD 2545) in the placebo group. The estimates did not include the cost of dornase alfa itself, as this was not marketed at the time of the trial.
Similarly, the Menzin report analysed data from the Fuchs trial to estimate the reduction in cost of respiratory tract infection‐related care (excluding the cost of dornase alfa itself) in the UK, France, Italy and Germany (Fuchs 1994; Menzin 1996). Variations in medical practice in these countries led to a range of cost reductions from GBP 434 (USD 700) in the UK to a maximum of FF 13,872 (USD 2100) in France. The estimates did not include the cost of dornase alfa itself, as this was not marketed at the time of the trial.
Dornase alfa daily versus alternate days
One cross‐over trial compared the use of once‐daily dornase alfa to alternate‐day use over two separate three‐month treatment periods (daily use n = 43, alternate day use n = 43) (Suri 2001). A summary of key findings for this comparison has been presented in a table (Table 2).
Primary outcomes
1. Changes in lung function (FEV1, FVC) from baseline
Changes in FEV1 and FVC were expressed as relative % change (seePublished notes). There was no difference found between the two groups in FEV1, MD 2.00 (95% CI ‐5.00 to 9.00) (Analysis 2.1) or FVC, MD 0.03 (95% CI ‐0.06 to 0.12) (Analysis 2.2) (both low‐quality evidence).
2.1. Analysis.
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 1 Mean % change in FEV1.
2.2. Analysis.
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 2 Mean % change in FVC.
2. Mean percentage change in quality of life score
There was no difference found between the two groups in QoL score, MD 0.01 (95% CI ‐0.02 to 0.04) (Analysis 2.3) (low‐quality evidence).
2.3. Analysis.
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 3 Mean % change in quality of life score.
3. Number of respiratory exacerbations
There was no difference found between the two groups in the number of participants experiencing one or more pulmonary exacerbations (18 in the once‐daily group and 17 in the alternate‐day group) (low‐quality evidence).
Secondary outcomes
1. Mortality
The trial did not measure this outcome.
2. Mean number of days IV antibiotics used
The trial did not measure this outcome.
3. Mean number of days oral antibiotics used
The trial did not measure this outcome.
4. Mean number of days inpatient treatment
There was no difference found between the two groups in the number of days of inpatient treatment, MD ‐0.93 (95%CI ‐3.24 to 1.38) (Analysis 2.4).
2.4. Analysis.
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 4 Mean number of days inpatient treatment.
5. Mean change in weight from baseline
There was no difference found between the two groups in the change in weight from baseline, MD ‐0.09 kg (95% CI ‐0.73 to 0.55) (Analysis 2.5).
2.5. Analysis.
Comparison 2 Dornase alfa once daily versus dornase alfa on alternate days, Outcome 5 Mean change in weight (kg) from baseline.
6. Number of participants experiencing adverse events by end of trial
The trial did not measure this outcome.
7. Cost of treatment
The Suri trial also examined the cost of therapy including intervention and non‐intervention drugs, hospital and community care (Suri 2001). The cost of daily dornase alfa over the 12‐week treatment period was GBP 1749 and the cost of alternate day dornase alfa was GBP 857. Total costs were on average GBP 513.00 (95% CI ‐546.00 to 1510.00) higher in the daily use group.
Dornase alfa versus hyperosmolar agents (hypertonic saline or mannitol)
Comparator medications for improving mucus clearance which were included in this review were hypertonic saline (HS) in three trials and mannitol in one trial. For HS, one trial used 5 ml of 7% HS twice daily (Suri 2001), a second trial used 10 ml of 5.8% HS once daily (Ballmann 2002) and the third trial used 10 ml of 6% HS twice daily (Adde 2004). These doses of HS were compared with once‐daily dornase alfa (Adde 2004; Ballmann 2002; Suri 2001). Minasian compared twice‐daily 400 mg mannitol to twice‐daily 2.5 mg dornase alfa (Minasian 2010). A summary of key findings for these comparisons have been presented in the tables (Table 3; Table 4; Table 5).
Primary outcomes
1. Changes in lung function (FEV1 and FVC) from baseline
Four trials lasting three weeks (Ballmann 2002), four weeks (Adde 2004) and three months (Minasian 2010; Suri 2001) reported on changes in lung function. Data from the Suri and Minasian trials were not pooled because Suri reported lung function in % predicted; whereas, Minasian reported lung function in litres (Minasian 2010; Suri 2001). Data from Ballman and Adde could not be pooled because only group means and SDs were provided. Two trials reported the relative change in lung function (Minasian 2010; Suri 2001), but the remaining two trials did not specify whether data were for absolute or relative changes (Adde 2004; Ballmann 2002).
a. mean percentage change or change in L in FEV1
At the time point of up to one month, one trial (dornase alfa n = 14, HS n = 14) reported the mean (SD) increase in FEV1 was 7.7% (14%) with HS versus 9.3% (11.7%) with dornase alfa (no significant difference between groups) (Ballmann 2002). In this trial, the number of participants that had at least a 10% increase in FEV1 from baseline was four in the dornase alfa group, and four in the HS group, with two participants improving with either treatment (Ballmann 2002). At the same time point, a second trial (dornase alfa n = 18, HS n = 18) reported that FEV1 did not significantly change after treatment with either HS or dornase alfa (Adde 2004) (Table 8).
3. Adde 2004 ‐ DNase versus hypertonic saline results.
| Pre‐hypertonic saline | Post hypertonic saline | Pre dornase alfa | Post dornase alfa | P value | |
| FEV1 (% predicted) mean (SD) |
47 (18) | 46 (18) | 49 (15) | 50 (21) | NS |
FEV1: forced expiratory volume at one second NS: non‐significant SD: standard deviation
At three months, Suri (dornase alfa n = 43, HS n = 40) reported an advantage for daily dornase alfa over HS, MD 8.00% (95% CI 2.00% to 14.00%) (Suri 2001) (Analysis 3.1) (low‐quality evidence). Within the trial there were varying individual responses to dornase alfa and HS, with 50% of participants experiencing a 10% improvement in FEV1 with dornase alfa and 35% having a 10% improvement in FEV1 with HS.
3.1. Analysis.
Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 1 Mean % change in FEV1.
The trial comparing dornase alfa and mannitol (dornase alfa n = 21, mannitol n = 23) did not report a significant difference between the two interventions for FEV1 MD 0.02 L (95% CI ‐0.11 to 0.16) (Minasian 2010) (Analysis 4.1) (low‐quality evidence).
4.1. Analysis.
Comparison 4 Dornase alfa versus mannitol, Outcome 1 Mean absolute change in FEV1 (L).
b. mean percentage change or change in L in FVC
Two trials did not report on the change in FVC (Adde 2004; Ballmann 2002). At three months, Suri (dornase alfa n = 43, HS n = 40) reported that the difference between the once‐daily treatment group and the HS treatment group was 0.08% (95% CI ‐0.02 to 0.18) (Suri 2001) (Analysis 3.2) (low‐quality evidence).
3.2. Analysis.
Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 2 Mean % change in FVC.
The trial comparing dornase alfa and mannitol (dornase alfa n = 21, mannitol n = 23) reported no difference in change in FVC between groups, MD ‐0.02 L (95% CI ‐0.23 to 0.19) (Minasian 2010) (Analysis 4.2) (low‐quality evidence).
4.2. Analysis.
Comparison 4 Dornase alfa versus mannitol, Outcome 2 Mean absolute change in FVC (L).
2. Mean percentage change in quality of life score
Two trials measured QoL, but used different tools precluding pooling of results (Minasian 2010; Suri 2001). Suri reported that the difference between the once‐daily dornase alfa and HS was MD 0.03% (95% CI ‐0.01% to 0.07%) (Suri 2001) (Analysis 3.3) (low‐quality evidence). Miniasian used the CFQ‐R to assess QoL and expressed this as the absolute change from baseline; investigators did not find a difference between the mannitol and dornase alfa groups, MD 4.1 (95% CI ‐6.40 to 14.6) (Minasian 2010) (Analysis 4.3) (low‐quality evidence).
3.3. Analysis.
Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 3 Mean % change in quality of life score.
4.3. Analysis.
Comparison 4 Dornase alfa versus mannitol, Outcome 3 Quality of life ‐ CFQ‐R.
3. Number of respiratory exacerbations
Suri measured the mean number of respiratory exacerbations reported these as not being statistically significant (Suri 2001). The absolute number of people who experienced one or more exacerbations whilst taking HS was 15 and for the once‐daily dornase alfa participants it was 18 (Suri 2001) (low‐quality evidence). Miniasian reported on exacerbations which required IV antibiotics in terms of absolute numbers per participant (Minasian 2010). Investigators did not find a difference between the two groups, RR 1.10 (95% CI 0.25 to 4.84) (Analysis 4.4) (low‐quality evidence).
4.4. Analysis.
Comparison 4 Dornase alfa versus mannitol, Outcome 4 Number of people experiencing exacerbations.
Secondary outcomes
1. Mortality
There were no deaths reported in any of the trials (Adde 2004; Ballmann 2002; Minasian 2010; Suri 2001).
2. Mean number of days IV antibiotics used
No trials looked at this outcome.
3. Mean number of days oral antibiotics used
No trials looked at this outcome.
4. Mean number of days inpatient treatment
One trial reported on this outcome and found no difference between the once‐daily dornase alfa and the HS groups, MD ‐0.40 (95% CI ‐2.32 to 1.52) (Suri 2001) (Analysis 3.4).
3.4. Analysis.
Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 4 Mean number of days inpatient treatment.
5. Mean change in weight from baseline
Again, only one trial reported on this outcome (Suri 2001). There was no difference found between the once‐daily dornase alfa and the HS groups, MD ‐0.42 (95% CI ‐1.04 to 0.2) (Analysis 3.5).
3.5. Analysis.
Comparison 3 Dornase alfa daily versus hypertonic saline, Outcome 5 Mean change in weight (kg) from baseline.
6. Number of participants experiencing adverse events by end of trial
Two trial reported adverse events (Minasian 2010; Suri 2001).
Suri reported no significant difference in the number of adverse effects between the different groups (Suri 2001). In the HS group, with the initial dose three participants (6%) experienced significant bronchospasm (a greater than 15% decrease in FEV1 despite initial treatment with bronchodilators) requiring withdrawal from the trial. A further five participants reported a salty taste, but this was not severe enough for them to drop out of the trial. It was found that HS tended to make the participants cough during administration. The 10 most frequent adverse events were increased cough, coryza, throat infection, allergic reaction to antibiotic, wheeze, breathlessness, haemoptysis, chest pain, eye irritation and oral thrush (Suri 2001).
Minasian reported the following side effects were not more common in either treatment group (Analysis 4.5): cough, RR 0.08 (95% CI 0.01 to 1.40); ear infection, RR 0.36 (95% CI 0.02 to 8.47); musculoskeletal pain, RR 0.36 (95% CI 0.02 to 8.47); or pharyngitis, RR 0.36 (95% CI 0.02 to 8.47). However, nine out of 38 (24%) participants screened had significant bronchoconstriction (at least a 15% decrease in FEV1) with the mannitol challenge, even when pre‐treated with bronchodilators and were not included in the trial (Minasian 2010).
4.5. Analysis.
Comparison 4 Dornase alfa versus mannitol, Outcome 5 Adverse events at 3 months.
7. Cost of treatment
Only Suri investigated the mean cost difference between daily dornase alfa and HS at 12 weeks. The drug cost per day was reported to be GBP 0.38 (USD 0.59) for HS and GBP 20.39 (USD 31.85) for once‐daily dornase alfa. The average total cost of an occupied bed per day ranged from GBP 280 (USD 438) to GBP 397 (USD 620). Over the 12‐week treatment period the mean drug cost of daily dornase alfa was GBP 1755 (USD 2741) compared with GBP 37 (USD 58) for HS. The difference in the total health service cost between daily dornase alfa and HS was calculated, MD GBP 1409.00 (95% CI 440.00 to 2318.00) (MD USD 2200 (95% CI 687 to 3620)) (Suri 2001).
*USD equivalent not reported in paper but estimated based on conversion of GBP 1 to USD 1.56.
Dornase versus a combination of dornase and a hyperosmolar agent
Only one trial compared mannitol (400 mg twice daily) with a combination of mannitol (400 mg twice daily) and dornase alfa (2.5 mg twice daily) (Minasian 2010).
1. Changes in lung function (FEV1, FVC ) from baseline
There was no difference between the two groups in either FEV1, MD 0.10 L (95% CI ‐0.06 to 0.25) (Analysis 5.1) or FVC, MD 0.13 L (95% CI ‐0.11 to 0.37) (Analysis 5.2) (both low‐quality evidence).
5.1. Analysis.
Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 1 Mean absolute change in FEV1 (L).
5.2. Analysis.
Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 2 Mean absolute change in FVC (L).
2. Mean percentage change in quality of life score
The change in QoL using the CFQ‐R was just in favour of dornase alfa, MD 10.61 (95% CI 0.27 to 20.95) (Analysis 5.3) (low‐quality evidence).
5.3. Analysis.
Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 3 Quality of life ‐ CFQ‐R.
3. Number of respiratory exacerbations
There was no difference found between the two groups in the number of participants experiencing pulmonary exacerbations, RR 0.55 (95% CI 0.16 to 1.92) (Analysis 5.4) (low‐quality evidence).
5.4. Analysis.
Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 4 Number of people experiencing exacerbations.
Secondary outcomes
1. Mortality
The trial did not measure this outcome.
2. Mean number of days IV antibiotics used
The trial did not measure this outcome.
3. Mean number of days oral antibiotics used
The trial did not measure this outcome.
4. Mean number of days inpatient treatment
The trial did not measure this outcome.
5. Mean change in weight from baseline
The trial did not measure this outcome.
6. Number of participants experiencing adverse events by end of trial
There was no difference found between the two groups in the rates of adverse events of: cough, RR 0.22 (95% CI 0.01 to 4.30); headache, RR 0.36 (95% CI 0.02 to 8.47); nausea, RR 0.36 (95% CI 0.02 to 8.47); or rash, RR 0.36 (95% CO 0.02 to 8.47) (Analysis 5.5).
5.5. Analysis.
Comparison 5 Dornase alfa versus dornase alfa and mannitol, Outcome 5 Adverse events at 3 months.
7. Cost of treatment
The trial did not measure this outcome.
Discussion
Summary of main results
Dornase versus placebo
Dornase alfa improved lung function in trials of up to one month duration compared to placebo, mean difference (MD) in forced expiratory volume at one second (FEV1) per cent (%) predicted 9.51% (95% confidence interval (CI) 0.67 to 18.35). This overall improvement was due to the improvement in participants with moderate disease severity, as demonstrated by a subgroup analysis of this group which showed an improvement, MD 14.26% (95% CI 10.79 to 17.74), compared to the group with severe disease which did not show any improvement, MD ‐2.81% (95% CI ‐8.77 to 3.15). Unfortunately there was only one trial which included participants with severe disease. We were not able to include participants with mild disease in the pooled result; however, one small trial including only participants with mild disease showed no change in FEV1, MD 0.08% (95% CI ‐5.59 to 5.74) (Amin 2011). The Amin trial also looked at lung clearance index (LCI) and identified a decrease in LCI of ‐0.90 (95% CI ‐1.87 to 0.07) in the dornase alfa group compared to placebo. This decrease was significant when baseline lung function was taken into account, which emphasizes the importance of using more sensitive measures of lung function in people with mild lung disease. There were fewer trials of longer duration, but FEV1 was significantly better in the dornase alfa group in trials ranging from three months to two years. This included trials involving participants with severe disease (McCoy 1996) as well as mild to moderate disease (Fuchs 1994; Quan 2001). It was not possible to perform a subgroup analysis comparing trials using once‐daily versus twice‐daily dornase alfa, but the single large trial which compared these two interventions directly, did not find a difference in FEV1 between the groups (Fuchs 1994). Interestingly, one small trial that directly compared once‐daily versus alternate‐day dornase alfa also did not find a difference in FEV1 (Suri 2001).
Dornase alfa also decreased the number of participants experiencing pulmonary exacerbations, which is an important outcome measure in cystic fibrosis (CF). We calculated the risk ratio (RR) of a pulmonary exacerbation as 0.78 (95% CI 0.62 to 0.96) in participants receiving dornase alfa compared to control. Quality of life improved in some trials and was unchanged in others. Dornase alfa was well‐tolerated and other than voice alteration, RR 1.69 (95% CI 1.2 to 2.39), and rash, RR 2.4 (95% CI 1.16 to 4.99), side effects were not more common than in the control group.
There have not been new trials examining the cost effectiveness of dornase alfa, but as concluded with earlier versions of this review, the healthcare costs of people treated with dornase alfa are lower (Oster 1995; von der Schulenburg 1995). However, this saving only offsets between 18.3% to 37.5% of the cost of dornase alfa (Oster 1995). Given that the cost of dornase alfa has not decreased since 2010, these cost estimates are still relevant. One difficulty in interpreting the cost effectiveness of dornase alfa is that the cost benefits of improving lung function over the long term are difficult to model.
Dornase versus hyperosmolar agents
Mucolytic and hyperosmolar agents are the most common groups of medications that help with mucous clearance. Unfortunately there are few high‐quality trials comparing these two types of medication and none of the results could be pooled in this review because of differences in how outcomes were reported.
Trials of one month or less did not find a significant difference in FEV1 between hypertonic saline (HS) and dornase alfa (Adde 2004; Ballmann 2002); whereas a three‐month trial reported an improvement with dornase compared to HS, MD 8.00% (95% CI 2.00% to 14.00%) (Suri 2001). The only trial comparing dornase alfa to mannitol did not find a difference in FEV1 between the two interventions, MD 0.02 L (95% CI ‐0.11 to 0.16); neither did this trial find a difference in FEV1 when mannitol combined with dornase alfa was compared to dornase alfa alone, MD 0.10 L (95% CI ‐0.06 to 0.25) (Minasian 2010).
The two trials reporting on the number of participants experiencing exacerbations found no difference between treatment groups (Minasian 2010; Suri 2001). Quality of life improved in some trials, but was unchanged in others.
Adverse events were not significantly different between the groups receiving dornase alfa and hyperosmolar agents, although 6% and 24% of potential participants experienced bronchoconstriction with the initial doses of HS and mannitol respectively, and were excluded from the trials.
Given that the cost of dornase alfa is 10 times that of HS, it is not surprising that the difference in the total health service cost was GBP 1409 (95% CI GBP 440 to GBP 2318) higher for the daily dornase alfa group compared to the HS group (Suri 2001). However, this trial was only three months in duration and differences in the numbers of exacerbations were not significant, which would be expected to affect health service costs.
Overall completeness and applicability of evidence
The objectives of this review were to determine if there was an improvement in morbidity or mortality with the use of dornase alfa, to identify any adverse events associated with the use of dornase alfa and to determine the efficacy of dornase alfa compared with other medications for improving airway clearance.
There is evidence to support the short‐term benefit of dornase alfa in improving lung function; however, other outcomes such as the frequency of pulmonary exacerbations require trials of longer duration. The trial by Fuchs used data from a CF registry to determine that a trial lasting 48 weeks was needed to assess pulmonary exacerbations and only two of this review's included trials reporting on exacerbations as an outcome were of sufficient duration (Fuchs 1994; Quan 2001). Given the improvement in prognosis for people with CF, it is difficult to detect differences in mortality unless trials include participants with severe disease and are long enough in duration. Only two trials included participants with severe disease making it difficult to reach firm conclusions on the effect of dornase alfa on mortality (McCoy 1996; Shah 1995a).
Dornase alfa is approved for use as a once‐daily medication in most countries. Different dose frequency regimens of dornase alfa were used in this review; ranging from alternate‐day use to twice‐daily use; only two trials compared these regimens directly (Fuchs 1994; Suri 2001). In the trial comparing once‐daily to twice‐daily dornase alfa, there was a similar improvement in lung function between the groups, although only the twice‐daily group showed a significant decrease in the number of participants experiencing an exacerbation. It is not clear from the current evidence if an alternate‐day regimen would be equally efficacious as this has only been studied in one small trial of three months duration.
More data are needed comparing dornase to hyperosmolar agents before definitive conclusions can be reached.
Quality of the evidence
Most trials were judged to have a low risk of performance, detection, reporting and attrition bias. Many of the included trials did not have enough information in the publication to determine if there was a risk of selection bias. This reduces the strength of evidence available. Also, the pooled results for lung function from the shorter trials showed considerable heterogeneity and although this may be explained by the subgroup analysis by disease severity; this heterogeneity reduces the strength of evidence in favour of using dornase alfa.
According to the GRADE approach, the quality of the evidence in the trials which compared dornase alfa to placebo or no dornase alfa treatment was judged to be moderate to high quality for lung function outcomes and exacerbations. The quality of the evidence for quality of life was limited for this comparison and therefore judged to be low. The quality of the evidence for dornase alfa compared to other controls (HS, mannitol or daily dornase alfa compared to alternate days) was limited and from open label trials and therefore judged to be low.
Potential biases in the review process
For this review, we searched all relevant sources for potential trials and the inclusion of hand‐searching abstracts from the North American and European Cystic Fibrosis Conferences increases the likelihood that all relevant trials have been identified.
Agreements and disagreements with other studies or reviews
No other systematic reviews have been identified which compare the use of dornase alfa to placebo or control in people with CF. The Cochrane review of HS for people with CF included trials comparing dornase alfa to HS and identified the same trials as were included in this review (Wark 2009). The authors of that review concluded that HS should be recommended for use in CF, but not in preference to dornase alfa given that there was insufficient evidence of superiority and less evidence for long‐term benefit in lung function.
Authors' conclusions
Implications for practice.
Therapy with dornase alfa is associated with an improvement in lung function in short‐term trials as well as longer trials lasting up to two years. Although there was no significant difference between groups in a trial lasting three years, lung function was not the primary outcome within this trial which was therefore not powered to detect differences in lung function. There was a reduction in the risk of infective exacerbations using a once‐daily regimen, risk ratio (RR) 0.78 (95% confidence interval (CI) 0.62 to 0.96). Not all people with cystic fibrosis (CF) increase their lung function with dornase alfa, but the effects on lung function are seen in within one month; therefore, if dornase alfa is started for this indication, a one‐month trial should detect improvements in lung function. It should be noted that improvements in lung function did not predict which individuals experienced a decrease in exacerbations with dornase alfa in the single trial that examined this (Quan 2001); thus, a longer trial may be needed to assess this outcome in people with CF.
The effect of dornase alfa on mortality is inconclusive due to trials of short duration.
Dornase alfa is a well‐tolerated therapy with only voice alteration and rash being reported with increased frequency in groups treated with dornase alfa.
Data from comparative trials of dornase alfa and hyperosmolar agents, suggests that dornase alfa is superior to hypertonic saline in improving lung function, but there was no reported difference in the time to or frequency of pulmonary exacerbations. However, the longest trial to assess this was three months in duration, which is likely not long enough to detect differences in pulmonary exacerbations. There was no differences detected between dornase alfa and mannitol; and in the first trial to assess a combination of dornase with a hyperosmolar agent compared to either agent alone, there was no improvements noted with the combination of medications.
Implications for research.
There is a paucity of data looking at the efficacy of dornase alfa in children under the age of six years. Given the early development of lung disease in children with CF and the increased availability of lung function testing in children under six years of age, further trials should be undertaken in this age group. There is also a need for trials of a longer duration to determine if dornase alfa is superior to hyperosmolar agents, and if there is additive benefit of using both dornase alfa and hyperosmolar agents. Given that these different regimens have implications for cost as well as treatment burden, further data will be important to determine the optimal regimen.
What's new
| Date | Event | Description |
|---|---|---|
| 31 July 2018 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register identified seven new references potentially eligible for inclusion in the review. One reference has been added to the already included study (Quan 2001). One reference has been added to the already excluded study (Mainz 2014). On closer inspection, it became clear that the abstract by Middleton et al previously listed under an excluded study (Fitzgerald 2005) is an additional reference to a published full paper identified and excluded at this search (Bishop 2011). Both the Middleton abstract and the full paper are now listed under the excluded study ID (Bishop 2011). One further new study with three references has been excluded (van der Giessen 2007b) as has one study with a single reference (Kelijo 2001). We undertook additional searches of ClinicalTrials.gov and the International Clinical Trials Registry Platform. Of the 18 trials identified on ClincalTrials.gov, seven trials were already included in this review (Amin 2011; Castile 2009; Freemer 2010; Lahiri 2012; Mainz 2014; Minasian 2010; Sawicki 2014). The remaining 11 trials were excluded (Bilton 2011; Mainz 2011; NCT00311506; NCT00434278; NCT00843817; NCT01025258; NCT01155752; NCT01232478; NCT02301377; NCT02682290; NCT02722122). Of the five trials identified on the International Clinical Trials Registry Platform, one was a duplicate of a trial identified on ClinicalTrials.gov (NCT02722122) and a further two trials were already included in this review (Diot 2009; Minasian 2010). The remaining two trials were excluded (EUCTR2006‐002098‐30‐NL; EUCTR2007‐000935‐25‐NL). |
| 31 July 2018 | New citation required but conclusions have not changed | No new data have been added to the review, therefore our conclusions remain the same. Sarah Nolan and Mark Chilvers have stepped down from the author team. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 4, 1998
| Date | Event | Description |
|---|---|---|
| 10 March 2016 | New citation required but conclusions have not changed | Ashley Jones and Colin Wallis have stepped down from the review and there are four new authors Dr Connie Yang, Dr Mark Chilvers, Dr Mark Montgomery and Sarah Nolan The new data added to the review have not changed the conclusions. |
| 10 March 2016 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified 17 new references to 10 separate trials which were potentially eligible for inclusion in this review. One of these is an additional reference to a trial previously listed as 'Awaiting classification' and which has now been included (Minasian 2010). Two new trials, with a total of four new references have been included (Amin 2011; Castile 2009); six new trials with 11 references have been excluded (Anderson 2009; Bakker 2010; Mainz 2014; Sawicki 2014; Shah 1997; Diot 2009). A further reference was added to an already excluded trial (van der Giessen 2007a). One trial (two references) which was previously excluded on the grounds that no relevant outcomes were reported, has now been included and some lung function data presented (Robinson 2000). A further reference was previously listed as a separate excluded trial has been re‐classified as an additional reference to a trial that was included in the previous review (Robinson 2005). One trial that was previously listed as 'Awaiting assessment' has now been excluded (Cimmino 2005). |
| 30 November 2009 | New citation required but conclusions have not changed | Catherine Kearney is no longer part of the review team. |
| 30 November 2009 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified 14 references which were potentially eligible for inclusion in the review. Of these one was an additional reference to an already included study (Suri 2001); two were additional references to already excluded studies (Fitzgerald 2005; van der Giessen 2007a); and eleven were references to new studies (Frederiksen 2006 (2 references); Hagelberg 2008; Mainz 2011 (2 references); Minasian 2010a; Potter 2008; Riethmueller 2006; van der Giessen 2007b (2 references); Wilson 2007); We included one new study (Frederiksen 2006) and a further study has been added to 'Studies awaiting classification' (Minasian 2010a). The remaining six studies were excluded (Wilson 2007; Mainz 2011; van der Giessen 2007b; Hagelberg 2008; Potter 2008; Riethmueller 2006). |
| 12 August 2008 | Amended | Converted to new review format. |
| 20 February 2008 | New search has been performed | The search of the Cystic Fibrosis Trials Register identified seven new references for this review. Two of these (Griese 2005; Ratjen 2005) are additional references to an already included study (Paul 2004). Two new studies were excluded as they did not compare Dornase alpha to another intervention (Laube 2005; van der Giessen 2007a). A further two new studies were excluded as they were comparisons of different types of nebuliser (Elkins 2006; Johnson 2006). The final new study has been listed as 'Awaiting assessment' until the authors are able to obtain further details from the primary investigators (Cimmino 2005). One study, previously listed as 'Awaiting assessment' has now been moved to 'Excluded studies' (ten Berge 2003). |
| 20 February 2008 | Amended | The term 'recombinant human deoxyribonuclease' has been replace throughout the review (including in the title) with the approved name for this drug 'Dornase alpha'. A new plain language summary has been written in line with latest guidance from The Cochrane Collaboration. |
| 19 May 2006 | New search has been performed | The search of the Cystic Fibrosis Trials Register identified two new references for this review. One of these (Ratjen 2005) is an additional reference to an already included study (Paul 2004). The other (Graseman 2004) is an additional reference to another included study (Quan 2001). Neither new references have added any new data to this review. |
| 23 February 2005 | Amended | In previous versions of this review all trials that reported data at one month were combined in a meta‐analysis (Jones 2003; Kearney 1998). It has since been decided that due to the fact that the trial by Wilmott was conducted over two weeks during an acute exacerbation (in contrast to the other trials which recruited participants with stable disease), it would be more appropriate to exclude the trial from this analysis and to analyse it separately (Wilmott 1996). This has change has been made in this update. Quan 2001 The treatment effect is reported as the absolute difference: Difference = FEV1% predicted at end of treatment ‐ FEV1 % predicted at baseline. Other studies reported the relative difference : (FEV1 during treatment ‐ FEV1 at baseline) / FEV1 at baseline |
| 23 February 2005 | New search has been performed | The search of the Cystic Fibrosis Trials Register identified new trials eligible for inclusion in the review. Two trials have been included in this update (Adde 2004; Paul 2004); a further trial has now been added to 'Studies awaiting assessment' (ten Berge 2003). |
| 25 February 2004 | Feedback has been incorporated | A 'Comment and Criticism' entitled: "Reporting of FEV1 and FVC" (and the response from the reviewers) was attached to this review on Issue 1, 2004. This is archived at the following site and can be accessed via inserting this unique number ‐ CD001127: http://www.update‐software.com/comcritusers/ |
| 23 May 2003 | New citation required and conclusions have changed | Title change from 'DNase for cystic fibrosis'.
The lead author is now Mr Ashley Jones. Five new studies have been included (Dodd 2000; Quan 2001; Suri 2001; Ballmann 2002; Robinson 2002) ‐ including the results from a two‐year placebo controlled trial. Two trials have now been included that compare rhDNase to other mucolytics. |
| 13 January 2003 | Feedback has been incorporated | A 'Comment and Criticism' entitled: "Olsen O, Herxheimer A, April 1999" (and the response from the reviewers) was attached to this review on Issue 1, 2003. This is archived at: the following site and can be accessed via inserting this unique number ‐ CD001127: http://www.update‐software.com/comcritusers/ |
Notes
Absolute difference = (post intervention value) ‐ (pre intervention value)
Relative difference = [(post intervention value) ‐ (pre intervention value)] /( pre intervention value)
Acknowledgements
We thank Tracey Remmington for independently extracting data and assessing trial quality for an earlier version of this review. We would also like to thank Mr Ashley Jones, Dr Colin Wallis and Dr Catherine Kearney for their contributions to earlier versions of this review (1998 ‐ 2012). Also thanks to Dr Mark Chilvers for his contribution to updates of the review published between 2012 and 2016, and to Dr. Sarah Nevitt (nee Nolan) for her contributions and statistical advice for the updated review published in 2016.
This systematic review has been made possible due to researchers providing their data and helpfully answering our queries. These include:
Dr H Fuchs and Dr C Johnson, Genentech, California, USA;
Prof Wilmott, Children's Hospital Medical Centre, Cincinnati, USA;
Dr P Shah, Chelsea and Westminster Hospital, London, UK;
Prof Zach, Uni Klinik fur Kinder‐und Jugendheilkunde, Germany;
W Greiner, North German Centre for Health Research, Germany;
Prof M Hodson, Royal Brompton Hospital, London, UK;
Dr Brinckswirth, St. Bartholomew's and The Royal London School of Medicine and Dentistry, London, UK;
Dr M Aitken, University of Washington Medical Centre, Washington, USA;
Mrs Mary Dodd, University Hospital of South Manchester, UK;
Dr Fabíola V. Adde, School of Medicine, Universidade de São Paulo, Brazil;
Dr. R Amin, Hospital for Sick Children, Toronto, CA;
Contacts at Pharmaxis who kindly supplied the additional data for the analyses: Frazer Chidwick, Kristen Morgan, Joanna Leadbetter and Brett Charlton.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Dornase alfa versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relative mean % change in FEV1 (% predicted) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 At 1 month | 4 | 248 | Mean Difference (IV, Random, 95% CI) | 9.51 [0.67, 18.35] |
| 1.2 At 3 months | 1 | 320 | Mean Difference (IV, Random, 95% CI) | 7.30 [4.04, 10.56] |
| 1.3 At 6 months | 1 | 647 | Mean Difference (IV, Random, 95% CI) | 5.8 [3.99, 7.61] |
| 1.4 At 12 months | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐11.26, 12.66] |
| 2 Relative mean % change in FEV1 (% predicted) at one month ‐ subgroup analysis by disease severity | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moderate | 3 | 183 | Mean Difference (IV, Fixed, 95% CI) | 14.26 [10.79, 17.74] |
| 2.2 Severe | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐2.81 [‐8.77, 3.15] |
| 3 Absolute mean % change in FEV1 (% predicted) | 1 | Mean difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1 month | 1 | Mean difference (Fixed, 95% CI) | 0.08 [‐5.59, 5.74] | |
| 4 Absolute mean % change in FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 2 years | 1 | 410 | Mean Difference (IV, Fixed, 95% CI) | 3.24 [1.03, 5.45] |
| 5 Relative mean % change in FEV1 (in participants with acute exacerbations) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to 1 month | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐13.93, 15.93] |
| 6 Relative mean % change in FVC (% predicted) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 At 1 month | 4 | 248 | Mean Difference (IV, Random, 95% CI) | 7.52 [1.34, 13.69] |
| 6.2 At 3 months | 1 | 318 | Mean Difference (IV, Random, 95% CI) | 5.10 [1.23, 8.97] |
| 6.3 At 12 months | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐15.87, 4.47] |
| 7 Relative mean % change in FVC (% predicted) | 1 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 7.1 At 6 months (once daily) | 1 | 2 | Mean Difference (Random, 95% CI) | 3.80 [2.62, 4.98] |
| 7.2 At 6 months (twice daily) | 1 | 2 | Mean Difference (Random, 95% CI) | 3.00 [1.82, 4.18] |
| 8 Relative mean % change in FVC at one month ‐ subgroup analysis by disease severity | 4 | 248 | Mean Difference (IV, Fixed, 95% CI) | 9.49 [6.34, 12.63] |
| 8.1 Moderate | 3 | 183 | Mean Difference (IV, Fixed, 95% CI) | 10.98 [7.68, 14.29] |
| 8.2 Severe | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐4.90 [‐15.15, 5.35] |
| 9 Absolute mean % change in FVC (% predicted) | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 9.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | ‐3.61 [‐10.02, 2.80] | |
| 10 Absolute mean % change in FVC (% predicted) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 At 2 years | 1 | 410 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐1.24, 2.64] |
| 11 Absolute mean change in LCI | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 11.1 At 1 month | 1 | 34 | Mean Difference (Fixed, 95% CI) | ‐0.9 [‐1.87, 0.07] |
| 12 Absolute change in FEV0.5 (z score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 12.1 At 6 months | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [‐0.57, 0.77] |
| 13 Quality of life ‐ CFQ‐R respiratory | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 13.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | 0.84 [‐10.74, 12.42] | |
| 14 Quality of life ‐ CFQ‐R Parent respiratory | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 14.1 At 1 month | 1 | Mean Difference (Fixed, 95% CI) | 9.78 [‐2.58, 22.14] | |
| 15 Number of people experiencing exacerbations | 3 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.62, 0.96] |
| 16 Number of deaths | 7 | 1690 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.70, 4.14] |
| 16.1 At 1 month | 4 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 100.53] |
| 16.2 At 3 months | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.56, 4.22] |
| 16.3 At 6 months | 1 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.06, 16.07] |
| 16.4 At 2 years | 1 | 470 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Mean number of days IV antibiotics used | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 17.1 At 3 months | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | ‐2.96 [‐7.29, 1.37] |
| 18 Mean number of days inpatient treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 At 3 months | 1 | 320 | Mean Difference (IV, Fixed, 95% CI) | 0.93 [‐2.19, 4.05] |
| 19 Mean change in weight from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 19.1 At 2 years | 1 | 470 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.42, 2.02] |
| 20 Adverse event ‐ haemoptysis (blood‐stained sputum) | 3 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.50, 1.55] |
| 21 Adverse event ‐ dyspnoea (shortness of breath) | 4 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.85, 1.18] |
| 22 Adverse event ‐ pneumothorax | 3 | 788 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.08, 4.50] |
| 23 Adverse event ‐ pneumothorax (in participants with acute exacerbations) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.11, 61.75] |
| 24 Adverse event ‐ voice alteration | 6 | 1670 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.20, 2.39] |
| 25 Adverse event ‐ voice alteration (1x versus 2x daily treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26 Adverse event ‐ voice alteration (in participants with acute exacerbations) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.58 [0.55, 12.03] |
| 27 Adverse event ‐ rash | 2 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [1.16, 4.99] |
| 28 Adverse event ‐ chest pain | 3 | 1151 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.59, 1.70] |
| 29 Adverse event ‐ cough (new or increased) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30 Adverse event ‐ increased sputum production | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31 Adverse event ‐ dry throat | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32 Adverse event ‐ pharyngitis | 6 | 1612 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.91, 1.46] |
| 33 Adverse event ‐ laryngitis | 3 | 1187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.68, 3.68] |
| 34 Adverse event ‐ conjunctivitis | 2 | 1117 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.50, 3.13] |
| 35 Adverse event ‐ wheeze | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.15, 2.41] |
| 36 Adverse event ‐ facial oedema | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.62 [0.40, 143.52] |
Comparison 2. Dornase alfa once daily versus dornase alfa on alternate days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean % change in FEV1 | 1 | Mean difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | Mean difference (Fixed, 95% CI) | 2.0 [‐3.00, 9.00] | |
| 2 Mean % change in FVC | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.06, 0.12] | |
| 3 Mean % change in quality of life score | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.01 [‐0.02, 0.04] | |
| 4 Mean number of days inpatient treatment | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.93 [‐3.24, 1.38] | |
| 5 Mean change in weight (kg) from baseline | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.09 [‐0.73, 0.55] |
Comparison 3. Dornase alfa daily versus hypertonic saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean % change in FEV1 | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 8.0 [2.00, 14.00] | |
| 2 Mean % change in FVC | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.08 [‐0.02, 0.18] | |
| 3 Mean % change in quality of life score | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 3.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | 0.03 [‐0.01, 0.07] | |
| 4 Mean number of days inpatient treatment | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 4.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.4 [‐2.32, 1.52] | |
| 5 Mean change in weight (kg) from baseline | 1 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 5.1 At 3 months | 1 | Mean Difference (Fixed, 95% CI) | ‐0.42 [‐1.04, 0.20] |
Comparison 4. Dornase alfa versus mannitol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean absolute change in FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.11, 0.16] |
| 2 Mean absolute change in FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.23, 0.19] |
| 3 Quality of life ‐ CFQ‐R | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 3 months | 1 | 56 | Mean Difference (IV, Fixed, 95% CI) | 4.1 [‐6.40, 14.60] |
| 4 Number of people experiencing exacerbations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 3 months | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.25, 4.84] |
| 5 Adverse events at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cough | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 1.40] |
| 5.2 Ear infection | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.3 Musculoskeletal pain | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.4 Pharyngitis | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
Comparison 5. Dornase alfa versus dornase alfa and mannitol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean absolute change in FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.06, 0.25] |
| 2 Mean absolute change in FVC (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At 3 months | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.11, 0.37] |
| 3 Quality of life ‐ CFQ‐R | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 3 months | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 10.61 [0.27, 20.95] |
| 4 Number of people experiencing exacerbations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Adverse events at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Cough | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.01, 4.30] |
| 5.2 Headache | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.3 Nausea | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
| 5.4 Rash | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adde 2004.
| Methods | Open randomised trial. Cross‐over design. Duration: 4 weeks for each treatment arm with a 2‐week washout period. |
|
| Participants | 18 participants (13 female). Age range 8.7 ‐ 25.8 years. |
|
| Interventions | Treatment: 2.5 mg rhDNase once daily. Control: 10 ml 6% HS once daily. |
|
| Outcomes | Included in this review: FEV1 (% predicted), FVC (% predicted). Not included in review: symptoms score, semi quantitative sputum cultures, in vitro studies of mucociliary transport, cough clearance, acceptance of treatment by participants. |
|
| Notes | Details from abstract as well as obtained from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised and information from authors indicates random numbers table used with sequence of treatments kept in the pharmacy in numbered envelopes. |
| Allocation concealment (selection bias) | Unclear risk | Information from authors not clear if investigators were involved in the randomisation. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded due to the taste of HS, although technician who performed pulmonary function was blinded and only objective measures were in the included outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear if all randomised participants completed both treatments or if there were any withdrawals. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | Cross‐over design with washout period of 2 weeks which should be adequate for lung function to return to baseline. |
Amin 2011.
| Methods | Randomised, placebo‐controlled trial. Cross‐over design. Duration: 4 weeks of treatment followed by a 4‐week washout before switching to alternate treatment. Single centre. |
|
| Participants | 19 randomised, 17 participants (11 females, 8 males) completed. Age 6 ‐ 18 years old; mean (SD) age 10.3 (3.4) years. |
|
| Interventions | Treatment: nebulised rhDNase 2.5 mg administered once daily via the PARI LC1 Star® nebuliser. Control: placebo administered once daily via the PARI LC1 Star® nebuliser. |
|
| Outcomes | Included in this review: LCI, FEV1 (% predicted, z score), FVC (% predicted, z score), CFQ‐R respiratory and parent respiratory domain, adverse events, exacerbations. Not included in this review: FEF25‐75. | |
| Notes | Visits occurred at 0, 4, 8 and 12 weeks after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed computer‐generated randomisation. |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by a research pharmacist not otherwise involved in the trial. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All participants (solutions indistinguishable from each other), clinicians and outcome assessors blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported that data analysed according to the intention‐to‐treat principle, however, data only reported on 17 who completed trial compared to the 19 that were randomised. Missing data from 2 participants: the LCI results of 1 participant failed to meet the quality control criteria for 1 of the 4 trial visits; 1 other participant dropped out of the trial after 2 visits because of a pulmonary exacerbation requiring IV antibiotics (protocol identified reason for withdrawal from trial), but not clear what treatment the participant had completed before withdrawal. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Cross‐over design with washout period of 4 weeks which should be adequate for lung function to return to baseline. |
Ballmann 2002.
| Methods | Open pilot trial. Cross‐over design. Duration: 2 treatment periods of 3 weeks, with a 3‐week washout period. Participants were assessed before and after each period. |
|
| Participants | 14 participants (mean age 13.3 years) with mild to moderate pulmonary involvement. Withdrawals were not discussed within the paper. | |
| Interventions | Treatment: 2 puffs salbutamol via a spacer prior to nebulisation of 2.5 mg dornase alfa once daily. Control: 2 puffs salbutamol via a spacer prior to nebulisation of 10 ml 5.85% HS once daily. |
|
| Outcomes | Change from baseline for FEV1 (% predicted), not clear if relative or absolute change. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not clear. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded, due to the taste of the hypertonic saline. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No discussion of whether ITT analysis performed. Withdrawals were not discussed within the paper. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | Cross‐over design with washout period of 3 weeks which should be adequate for lung function to return to baseline. |
Castile 2009.
| Methods | Randomised double‐blind placebo‐controlled trial. Cross‐over design. Duration: 6 months for each treatment arm, but no washout period stated. |
|
| Participants | 24 infants, clinically well at time of entry into trial. Not stated how many in each group.
Age: mean (SD) 42 (32) weeks. Gender distribution not stated. |
|
| Interventions | Treatment: nebulised rhDNase 2.5 mg once daily. Control: placebo once daily. |
|
| Outcomes | Included in this trial: changes in infant PFTs (% predicted and z scores for change in FEV0.5) Not included in this review: FEF25‐75, RV/TLC, change in CT score, change in air trapping, antibiotic treatment days. |
|
| Notes | Only data for 19 infants for LFTs and 21 infants for CT scans. Data only available from abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no details given |
| Allocation concealment (selection bias) | Unclear risk | Not stated in abstract. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Investigators and parents blinded to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow up lung function data for only 19 of 24 recruited and CT scan data for only 21 of 24 recruited infants were reported. Not clear which groups infants dropped out from. |
| Selective reporting (reporting bias) | Unclear risk | Antibiotic treatment days not reported. |
| Other bias | Unclear risk | Cross‐over design with no stated washout period (abstract only). |
Dodd 2000.
| Methods | Randomised, double‐blind placebo‐controlled trial. Cross‐over design. Duration: 2 treatment periods of 14‐days with a 7‐day wash out period between each period. Measurements were taken at the beginning and end of each treatment period. |
|
| Participants | 23 participants randomised. Age: (mean) 27.5 years. Withdrawals were not discussed within the paper. Disease severity was not discussed. |
|
| Interventions | Treatment: 2.5 mg rhDNase once daily. Control: 2.5 ml 0.9% saline once daily. |
|
| Outcomes | FEV1. | |
| Notes | Raw data provided; however no data legend therefore unable to analyse, FEV1 not reported in abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No discussion of whether ITT analysis performed. Withdrawals were not discussed within the paper. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | High risk | Cross‐over trial with 7‐day washout period, which is not long enough for lung function to return to baseline; however data from this trial were not available for analysis in this review. |
Frederiksen 2006.
| Methods | Randomised controlled trial. Parallel design. Duration: 1 year. |
|
| Participants | 72 CF participants. Age: range 1.1 ‐ 24.8 years. Gender split: 34 males, 38 females. Exclusion criteria: chronic lung infection, or treatment with rhDNase in previous 2 months. 2 participants excluded, 1 from treatment group, 1 as had been randomised twice (both times to no treatment group). |
|
| Interventions | Treatment: aerosolised rhDNase 2.5 mg once daily. Control: no rhDNase treatment. |
|
| Outcomes | FEV1. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but process not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Nothing stated in paper. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants excluded. One participant was included twice (both times in the untreated group), one from the treated group because he did not take the inhalations for more than 5 months, but it did not state why he discontinued treatment |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Fuchs 1994.
| Methods | Randomised, double‐blind trial. Parallel design with 3 arms. Duration: 24 weeks. Measurements were taken on days 7, 14 and every 14 days thereafter. |
|
| Participants | 968 participants randomised, diagnosed CF on genotype, sweat test or clinically. Age: over 5 years. More participants aged 17 ‐ 23 years were in the once daily rhDNase arm. Disease status: FVC > 40 % predicted and clinically stable. 25 people withdrew from the trial, 8 in the placebo group and once‐daily group and 9 in the twice‐daily group. |
|
| Interventions | Treatment 1: nebulised rhDNase 2.5 mg once daily (n = 322). Treatment 2: nebulised rhDNase 2.5 mg twice daily (n = 321). Control: placebo (n = 325). |
|
| Outcomes | Outcomes included in this review: mean % change in FVC and FEV1, number of participants needing IV antibiotics for at least 1 chest exacerbation (protocol defined), mean number of days IV antibiotics used, mean number of days as an inpatient, number of deaths and number experiencing an adverse event. Not included in this review: CF symptom score, dyspnoea score. Cost of treatment is reported by von der Schulenberg (1995), Oster (1995) and Menzin (1996). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT principle was used. 25 participants withdrew from the trial, 8 in the placebo group and once‐daily group and 9 in the twice‐daily group. |
| Selective reporting (reporting bias) | Unclear risk | Measurements were taken on days 7, 14 and every 14 days thereafter. The published trial reported the end of trial results only. |
| Other bias | Low risk | None identified. |
Laube 1996.
| Methods | Randomised, double‐blind trial. Parallel design. Duration: 6 days. |
|
| Participants | 20 adults with stable stage CF, FVC 35% ‐ 75% predicted and non‐smokers. Age: over 18 years. The published paper stated that there were no withdrawals. |
|
| Interventions | Treatment: 2.5 mg nebulised rhDNase twice daily (n = 10). Control: placebo twice daily (n = 10). |
|
| Outcomes | Included in this review: mean change in % predicted FVC and FEV1. Not included: aerosol distribution homogeneity, changes in mucociliary clearance and changes in cough frequency. |
|
| Notes | Measurements were taken on day 6 only and reported in the paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis was used in this trial. The published paper stated that there were no withdrawals. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
McCoy 1996.
| Methods | Randomised, double‐blind trial. Parallel design. Duration: 12 weeks. Measurements were taken on days 8, 15, 29, 57 and 85. |
|
| Participants | 320 participants with CF diagnosed clinically, by genotype or sweat test. Age: range 7 to 57 years. Disease status: FVC < 40 % predicted. Baseline lung function in the treatment group was lower than that of the control group, P < 0.05. 40 participants withdrew from the trial (see details below). |
|
| Interventions | Treatment: nebulised rhDNase 2.5 mg once daily (n = 158). Control: placebo once daily (n = 162). |
|
| Outcomes | Included in this review: mean change in % predicted FVC and FEV1, number of deaths and number experiencing adverse event, relative risk of one or more respiratory exacerbation. Not included in this review: mean number of days IV antibiotics used, mean number of days as an inpatient and mean dyspnoea score. |
|
| Notes | Mean number of days IV antibiotics used, mean number of days as an inpatient and mean dyspnoea score were said not to differ significantly. In this trial 3 participants allocated to receive placebo, actually received rhDNase. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT principle used. 2 participants from the rhDNase arm of the trial did not have lung function recorded. 3 participants inadvertently received rhDNase instead of placebo (the results for these participants for lung function and respiratory exacerbations were analysed on an ITT basis, for safety data the results for these participants were published as if they had been randomised to rhDNase). 40 participants withdrew from the trial, 5 due to adverse events, 10 withdrew consent, 1 did not comply with the trial protocol, 15 died, 2 were unavailable for follow up and 7 stopped for a medical procedure. |
| Selective reporting (reporting bias) | Unclear risk | Measurements were taken on days 8, 15, 29, 57 and 85. The 85‐day mean was reported in the paper. |
| Other bias | Low risk | None identified. |
Minasian 2010.
| Methods | Randomised open‐label trial. Cross‐over design with 3 arms. Total duration 42 weeks; each arm lasted 12 weeks with a 2‐week washout period between treatment blocks where all mucolytics were stopped. Primary endpoint measured at beginning and end of each treatment block. |
|
| Participants | 38 children with CF. Age: range 9 ‐ 17 years (mean age 13 years). |
|
| Interventions | Treatment 1: 2.5 mg nebulised rhDNase twice daily (n = 21). Treatment 2: combination of 2.5 mg nebulised rhDNase and 400 mg dry powder mannitol via Osmohaler twice daily (n = 23). Control: 400 mg dry powder mannitol via Osmohaler twice daily (n = 23). |
|
| Outcomes | Included in this review: FEV1 (L),,FVC (L), pulmonary exacerbations, CFQ‐R respiratory and parent respiratory domain, adverse events Not included in this review: FEF25‐75, sputum microbiology, exercise tolerance, lung inflammation, cost‐effectiveness. |
|
| Notes | Pulmonary exacerbation, adverse events and quality of life data not published, although data provided by Pharmaxis. 8 drop outs due to side effects, and these 8 were not included in the final analysis. Outcomes that were part of the original protocol that were not included in any of the provided data included markers of lung inflammation and cost‐effectiveness data. Prior to randomisation 9 out of 38 participants had significant bronchoconstriction to a mannitol challenge and were not randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as randomised, but details of randomisation process not discussed in paper. Dr Minasian provided additional information ‐ participants were allocated a unique randomisation number and treatment schedule with equal probability for assignment to treatment sequences. Randomisation was carried out in balanced blocks with separate schedules created for each of the 2 recruiting centres. |
| Allocation concealment (selection bias) | Unclear risk | Method not clear. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label ‐ not blinded. Outcomes included subjective measures such as quality of life and adverse events therefore risk of bias considered high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 8 withdrawals in total. 21 participants received rhDNase, 23 participants received mannitol and 23 participants received both. Data analysed per protocol on 20 participants who completed all 3 treatments. |
| Selective reporting (reporting bias) | Low risk | Published data only reported FEV1, FVC and FEF25‐75 but unpublished data provided for remainder of outcomes (except exercise tolerance, cost‐effectiveness, lung inflammation). |
| Other bias | Low risk | Cross‐over design with washout period of 2 weeks which should be adequate for lung function to return to baseline. |
Paul 2004.
| Methods | Randomised controlled trial. Parallel design. Duration: 3 years; participants were evaluated clinically every 3 months. |
|
| Participants | 85 participants randomised. Age: range 5 ‐ 37 years. Disease status: normal lung function (FEV1 > 80% predicted) and clinically stable. |
|
| Interventions | Treatment: rhDNase 2.5 mg twice daily (n = 46). Control: no rhDNase (n = 39). |
|
| Outcomes | Used in this review: FEV1, FVC. Not used in this review: FEF25‐75, inflammatory markers (IL‐8) and microbiology from alveolar lavage samples. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was based on ITT. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Quan 2001.
| Methods | Randomised, double‐blind parallel placebo controlled trial. Duration: 96 weeks. Measurements taken at week 4, 12 and every 12 weeks thereafter. Multicentre: 49 CF centres. |
|
| Participants | 474 children randomised, 410 completed the trial. 60 participants withdrew from the trial, 472 (out of 474) had follow‐up data. The ITT population was 470. Age: range 6 ‐ 10 years (mean age 8.4 years). Disease status: FVC > 85% predicted. |
|
| Interventions | Treatment: 2.5 mg rhDNase once daily (n = 239). Control: placebo once daily (n = 235). |
|
| Outcomes | Pulmonary function (FEV1, FVC) and exacerbations, deaths, adverse events, change in weight for age. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer, stratifying by centre using a permuted block design. |
| Allocation concealment (selection bias) | Low risk | Carried out by a pharmacy. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT approach was used. 60 participants withdrew from the trial, 472 (out of 474) had follow‐up data. The ITT population was 470. |
| Selective reporting (reporting bias) | Unclear risk | Measurements taken at week 4, 12 and every 12 weeks thereafter. The end of trial results were reported. |
| Other bias | Low risk | None identified. |
Ramsey 1993.
| Methods | Randomised, double‐blind trial. Parallel design with 4 arms. Duration: 10 days. Participants were followed up for a further 32 days. |
|
| Participants | 181 participants diagnosed with CF by genotype or sweat test. Age: range 8 to 65 years. Disease status: stable stage CF, FVC ≥ 40% of predicted. Data collected on all participants at end of trial. The paper stated that there were no withdrawals. |
|
| Interventions | Treatment 1: rhDNase 0.6 mg twice daily (n = 45). Treatment 2: rhDNase 2.5 mg twice daily (n = 44). Treatment 3: rhDNase 10 mg twice daily (n = 44). Control: placebo twice daily (n = 48). |
|
| Outcomes | Outcomes included in this review: mean % change in FVC and FEV1, number of deaths and number experiencing adverse event. Not included in this review; airway reactivity to rhDNase, mean rank change in quality of life score and the mean change in dyspnoea score. |
|
| Notes | Measurements taken on days 1, 3, 6, 10, with follow‐up data on days 14, 21, 28 and 42. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysed on an ITT basis. The paper stated that there were no withdrawals. |
| Selective reporting (reporting bias) | Unclear risk | Measurements taken on days 1, 3, 6, 10 with follow‐up data on days 14, 21, 28 and 42. Data were reported in the paper on days 3, 10, 21 and 42. |
| Other bias | Low risk | None identified. |
Ranasinha 1993.
| Methods | Randomised, double‐blind, safety and efficacy trial. Parallel design Duration: 10 days with follow up to 42 days. Measurements taken at days 3, 6 and 10. |
|
| Participants | 71 adults with CF diagnosed by genotype, sweat test. Disease status: stable disease and FVC > 40% predicted. |
|
| Interventions | Treatment: nebulised rhDNase 2.5 mg twice daily (n = 36). Control: placebo twice daily (n = 35). |
|
| Outcomes | Included in this review: relative mean change in % predicted FVC and FEV1 with baseline data calculated from the average of the day ‐3 and day 1 data and the treatment data calculated based on the average of the day 3, 6 and 10 data; number of deaths; and number experiencing an adverse event. Not included in this review: mean number of days of antibiotics used as only recorded at end of 42‐day follow‐up period. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned a carton number based on a randomisation list with a permuted block design, which was generated by Genentech. |
| Allocation concealment (selection bias) | Low risk | Unidentifiable cartons of active drug and placebo were numbered and provided to the pharmacist for dispensing. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT was not discussed. |
| Selective reporting (reporting bias) | Low risk | Measurements taken at days 3,6 and 10 (during treatment) then at day 14, 21, 28 and 42 following treatment. All were included. |
| Other bias | Low risk | None identified. |
Robinson 2000.
| Methods | Randomised double‐blind placebo‐controlled trial. Cross‐over design. Duration: 7 days of treatment for each intervention with 2‐week wash‐out in between. Single centre. |
|
| Participants | 15 participants randomised who were rhDNase naive. Age: 18.5 to 38.1 years old. Gender split: 9 males, 4 females. Disease status: clinically stable, mild to severe lung disease (FEV1 27.2% to 103.2% of predicted). |
|
| Interventions | Treatment: rhDNase 2.5 mg administered once daily by PARI LC Plus® nebuliser. Control: placebo administered once daily by PARI LC Plus® nebuliser. |
|
| Outcomes | Used in review: FEV1 (L), FVC (L). Not used in review: mucociliary clearance, cough clearance, FEF25‐75 (L/s). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described, although both medications were iso‐ismolar and given via the same nebuliser. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Stated as double‐blind but method not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not ITT. 15 participants randomised and data for 13 participants ‐ 2 participants withdrew because of respiratory exacerbations requiring IV antibiotics (1 from placebo group, 1 from rhDNase group). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | Cross‐over design with washout period of 2 weeks which should be adequate for lung function to return to baseline. |
Robinson 2005.
| Methods | Randomised double‐blind, placebo‐controlled trial. Parallel design. Duration: 1 year. Participants evaluated at 3 months and 1 year. |
|
| Participants | 25 children randomised. Age: range 6 ‐ 18 years old. Disease status: normal or mildly reduced lung function (FVC ≥ 85%, FEV1 > ˜70%). There were 4 withdrawals, all were for non‐trial drug‐related reasons. |
|
| Interventions | Treatment: rhDNase 2.5 mg once daily. Control: normal saline aerosol once daily. |
|
| Outcomes | Included in this review: FEV1(% predicted), FVC (% predicted). Not included in this review: FEF25‐75, high resolution CT scores, composite score including high resolution CT and PFT data. |
|
| Notes | Measurements were taken at 3 and 12 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blinded (investigators, participants blinded to treatments until trial end). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis used. 4 withdrawals, all were for non‐trial drug‐related reasons. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Shah 1995a.
| Methods | Randomised double‐blind trial. Parallel design. Duration: 14 days, with 6‐month open follow up. ITT was not discussed. |
|
| Participants | 70 participants with CF diagnosed by sweat test or genotype. Age: 5 years or over. Disease status: severe lung disease (FVC < 40% predicted). Specified 5 dropouts (2 died, 2 withdrew consent, 1 had a heart lung transplant). |
|
| Interventions | Treatment: 2.5 mg nebulised rhDNase twice daily (n = 35). Control: placebo twice daily (n = 35). |
|
| Outcomes | Included in review: mean change in % predicted FVC and FEV1; number of deaths; number experiencing an adverse event. Not included in the review; dyspnoea score; and quality of life score as data not provided. Reported as not significant. |
|
| Notes | 6‐month open‐ended phase not included in review as no control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised but no method was described. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT not possible for some outcomes. 5 out of 70 participants did not complete the 14‐day trial period, 1 received a heart‐lung transplant, 2 withdrew consent and 2 from the dornase alfa treated group died. Changes in lung function could therefore not be analysed on an ITT basis, but adverse events and deaths were analysed on this basis. |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | None identified. |
Suri 2001.
| Methods | Open randomised controlled trial. Cross‐over design. Duration: 3 treatment periods of 12 weeks with a 2‐week wash out period between each period. Measurements were taken at the start and end of each 12‐week period. |
|
| Participants | 48 children randomised, 45 completed first treatment period, 44 completed the second treatment period and 40 completed the third treatment period. Age: range 7.3 ‐ 17 years. |
|
| Interventions | Treatment 1: 2.5 mg rhDNase once daily. Treatment 2: alternate day 2.5 mg rhDNase. Treatment 3: 5 mL 7% HS twice daily. |
|
| Outcomes | Primary outcome was FEV1; secondary outcomes were FVC, number of pulmonary exacerbations, weight gain, quality of life, exercise tolerance and the total costs of hospital and community care. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was used. Randomisation carried out by telephone to an independent trials co‐ordinating unit, and stratified by hospital and balanced after each group of 12 children. |
| Allocation concealment (selection bias) | Low risk | Independent trials co‐ordinator. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded, due to the taste of the HS. Outcomes included subjective measures including quality of life therefore risk of bias considered high. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 48 children randomised, 45 completed 1st treatment period, 44 completed the 2nd treatment period and 40 completed the 3rd treatment period. Data analysed according to ITT principle |
| Selective reporting (reporting bias) | Low risk | None identified. |
| Other bias | Low risk | Cross‐over design with washout period of 2 weeks which should be adequate for lung function to return to baseline. |
Wilmott 1996.
| Methods | Randomised double‐blind trial. Parallel design. Duration: 15 days during a respiratory exacerbation. Measurements taken on days 1, 8 and 15. |
|
| Participants | 80 participants admitted to hospital for at least 1 night for treatment of a chest exacerbation (protocol defined) with FVC > 35% predicted. CF was diagnosed on genotype, sweat test. Age: over 5 years. No withdrawals mentioned in the paper. |
|
| Interventions | Treatment: nebulised rhDNase 2.5 mg twice daily (n = 43) Control: nebulised placebo twice daily (n = 37). |
|
| Outcomes | Mean change in % predicted FVC and FEV1, number of deaths and number experiencing an adverse event, quality of life score and dyspnoea score. | |
| Notes | Potential confounder was type of antibiotic used: 8 of 36 placebo participants received an oral antibiotic versus 8 out of the 44 in the treatment group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. |
| Allocation concealment (selection bias) | Unclear risk | Method unclear. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, no further details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT. No withdrawals mentioned in the paper. |
| Selective reporting (reporting bias) | Unclear risk | Measurements taken on days 1, 8 and 15, no reported results, graph shown in paper. |
| Other bias | Unclear risk | Potential confounder was type of antibiotic used: 8 of 36 placebo participants received an oral antibiotic versus 8 out of the 44 in the treatment group. |
<: less than >: greater than % predicted: percent predicted CF: cystic fibrosis CFQ‐R: CF questionnaire‐revised CI: confidence interval CT: computer tomography FEF25‐75: forced expiratory flow at 25 to 75% of the FVC FEV1: forced expiratory volume at one second FVC: forced vital capacity HS: hypertonic saline ITT: intention‐to‐treat IV: intravenous LCI: lung clearance index PFT: pulmonary function test rhDNase: recombinant human deoxyribonuclease RV: residual volume SD: standard deviation TLC: total lung capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2009 | Trial of timing of rhDNase inhalation in relation to physio (rhDNase dose does not differ between treatment arms). |
| Bakker 2010 | Trial comparing deposition of rhDNase by different methods of breathing and drug delivery; volume of rhDNase the same in all treatment arms. |
| Bilton 2011 | Trial of mannitol (not rhDNase). |
| Bishop 2011 | Comparison of timing of rhDNase delivery in relation to physio (rhDNase dose does not differ between treatment arms). |
| Bollert 1999 | This trial was designed with the aim of producing an objective means of selecting those people with CF who would benefit most from dornase alfa. The trial was a cross‐over design. Outcomes such as lung function, symptom scores, oximetry and exercise test response were measured and then scored on a weighted points system which could not be analysed according to our protocol. 3 participants had completed 2 assessment periods; 1 was classed as a responder, having scored 18 or more points out of a total of 27. |
| Cimmino 2005 | Trial of post‐sinus surgery administering rhDNase intranasally. |
| Craig 2013 | rhDNase delivered nasally for sinusitis in people with CF. |
| Dab 2000 | Participants currently on rhDNase at entry to trial. |
| Diot 2009 | Not an RCT. |
| Elkins 2006 | This is a comparison of two different types of nebuliser. |
| EUCTR2006‐002098‐30‐NL | Participants did not have CF. |
| EUCTR2007‐000935‐25‐NL | Participants on rhDNase at entry to trial. |
| Fitzgerald 2005 | Comparison of timing of rhDNase delivery in relation to physio (rhDNase dose does not differ between treatment arms). |
| Freemer 2010 | Trial terminated as unable to measure pre‐school lung function data. |
| Furuya 2001 | Not an RCT. |
| Genentech 2010 | Participants were already on rhDNase and trial was designed as a withdrawal trial. |
| Griese 1997 | This trial examined the effects of rhDNase on sputum rheology as compared to physiological saline over at least 4 months and did not include relevant clinical outcomes. |
| Hagelberg 2008 | Comparison of dispensing methods of rhDNase. |
| Heijerman 1995 | No comparator treatment; all participants received rhDNase. |
| Hubbard 1992 | This cross‐over trial in 16 adults was not clearly stated to be randomised. 2 of the investigators knew whether participants were allocated to receive placebo or treatment first. |
| Johnson 2006 | This is a comparison of 2 different types of nebuliser. |
| Kelijo 2001 | Participants were randomised to vitamin E therapy or placebo not randomised by rhDNase use; this paper presented results of the vitamin E trial by rhDNase use. |
| King 1997 | This trial examines the effects of rhDNase and hypertonic saline on sputum rheology in vitro and therefore not relevant to this review. |
| Lahiri 2012 | rhDNase delivered nasally for sinusitis in CF. |
| Laube 2005 | This does not use rhDNase versus another intervention; comparison of aerosol distribution with or without positive expiratory pressure. |
| Mainz 2011 | Pilot study for 2014 Mainz trial. Nasal inhalation for rhinosinusitis not airway clearance. |
| Mainz 2014 | Nasal inhalation for rhinosinusitis not airway clearance. |
| Majaesic 1996 | This cross‐over trial of 8 people with CF aged 6 to 18 years compared the viscosity of sputum cleared by CCP as compared to HFCC. The participants were randomised to receive either rhDNase or normal saline prior to either CCP or HFCC. |
| Nasr 2001 | Trial using CT scans to measure clinical response to rhDNase and establish how to measure effects of rhDNase not effects themselves. |
| NCT00311506 | Observational study looking at 6‐minute walk test in people with CF and advanced lung disease. |
| NCT00434278 | RCT of effect of rhDNase withdrawal on exercise tolerance in people with CF; all participants on rhDNase at start of trial. Terminated for administrative reasons, no safety concerns. |
| NCT00843817 | Not an RCT; trial examining the biodistribution of serine proteases in CF sputum. |
| NCT01025258 | Trial of interventions to improve adherence, not a trial comparing rhDNase to another group. |
| NCT01155752 | Trial of rhDNase for sinusitis, but was withdrawn from registry before enrolment due to lack of funding. |
| NCT01232478 | Trial of interventions to improve adherence, not a trial comparing rhDNase to another group. |
| NCT02301377 | Trial of interventions to improve adherence, not a trial comparing rhDNase to another group. |
| NCT02682290 | Not an RCT; study of rheologic properties of mucous, before and after analysis after administration of DNase, no relevant comparator. |
| NCT02722122 | Not an RCT; no comparison group, all participants received AIR rhDNase. |
| Potter 2008 | Comparison of 2 delivery techniques. |
| Riethmueller 2006 | Not people with CF. |
| Robinson 2002 | Trial of quantitative HRCT air trapping analysis in people with CF with mild lung disease during a rhDNase intervention; aim was to establish how to measure effects of rhDNase not measuring effects themselves. |
| Sawicki 2014 | Trial comparing administration of rhDNase via two different nebulisers. |
| Shah 1995b | Not an RCT; review of rhDNase use. |
| Shah 1995c | 6‐month trial of rhDNase in stable CF; an open‐label extension to a phase II trial where there was no re‐randomisation and all participants received rhDNase. |
| Shah 1997 | Trial comparing administration of rhDNase via two different nebulisers. |
| ten Berge 2003 | Authors contacted and trial does not report on any outcome relevant to this review. |
| van der Giessen 2007a | Comparison of timing of rhDNase delivery in relation to physio (rhDNase dose does not differ between treatment arms). |
| van der Giessen 2007b | Comparison of timing of rhDNase delivery, morning versus evening (rhDNase dose does not differ between treatment arms). |
| Weck 1999 | N‐of‐1 trial design. |
| Wilson 2007 | The comparison in this trial was between the timing of rhDNase administration, which is the subject of a different review. |
ACT: airway clearance techniques CCP: conventional chest physiotherapy CF: cystic fibrosis HFCC: high frequency chest compressions HRCT: high‐resolution computer tomography RCT: randomised controlled trial rhDNase: dornase alfa
Differences between protocol and review
Update 2016
Two outcome measures have been added to the primary outcome of changes in lung function: lung clearance index and forced expiratory volume at 0.5 seconds (FEV0.5 ). Lung clearance index has the potential to detect onset of patchy respiratory involvement in CF in mild or early lung disease. FEV0.5 is a more valid measure in young children because of short expiratory times.
The outcome 'Mean number of deaths' has been moved from 'Primary outcomes' to 'Secondary outcomes', since current Cochrane policy is to limit the number of primary outcomes to three.
In a post hoc change, in line with Cochrane guidance, the authors have presented five summary of findings tables; one for each comparison including the primary outcomes of the review at the three or six months follow up, or both.
Contributions of authors
Original review
Dr Kearney and Dr Wallis screened, appraised and abstracted data.
Dr Kearney sought additional information from authors. Data entry for the original review was performed by Dr Kearney and interpreted by Dr Kearney, Dr Wallis, Prof Ashby and with advice from the Cochrane Cystic Fibrosis and Genetic Disorders Group.
The review was conceived by the Cochrane Cystic Fibrosis and Genetic Disorders Group and designed by Dr Kearney.
May 2003
Change of lead reviewer from Dr Catherine Kearney to Mr Ashley Jones. Mr Ashley Jones and a colleague, Miss Tracey Remmington, carried out additional screening.
Mr Ashley Jones completed data entry.
October 2009
Dr Catherine Kearney has stepped down from the review team.
March 2016
Change of lead reviewer from Mr Ashley Jones, who has stepped down from the review, to Dr Connie Yang. Dr Connie Yang now acts as guarantor for the review. Dr Mark Chilvers, Dr Mark Montgomery and Sarah Nolan are now co‐authors on the review.
September 2018
Dr Connie Yang led the update with Dr Mark Montgomery working with her to assess the search results for study selection.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Dr Connie Yang has received support from Novartis to attend the Killarney Cystic Fibrosis Meeting in 2014 and the Pediatric Advisory Board.
Dr. Montgomery has no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adde 2004 {published data only}
- Adde FV, Borges KTL, Hatanaka ACF, Nakaie CMA, Cardieri JMA, Oliveira RC, et al. Hypertonic saline X recombinant human DNase: a randomised cross‐over study in 18 cystic fibrosis patients [abstract]. Journal of Cystic Fibrosis 2004;3(Suppl 1):S66. [Google Scholar]
Amin 2011 {published data only}
- Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, et al. The effect of dornase alfa on ventilation in homogeneity in patients with cystic fibrosis. European Respiratory Journal 2011;37(4):806‐12. [CFGD Register: BD163b] [DOI] [PubMed] [Google Scholar]
- Amin R, Subbarao P, Lou W, Jabar A, Balkovec S, Jensen R, et al. The effect of dornase alfa on ventilation in homogeneity in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 2010;45(Suppl 33):361, Abstract no: 396. [CFGD Register: BD163a] [Google Scholar]
Ballmann 2002 {published data only}
- Ballmann M, Hardt H. Hypertonic saline and recombinant human DNase: a randomised cross‐over pilot study in patients with cystic fibrosis. Journal of Cystic Fibrosis 2002;1(1):35‐7. [DOI] [PubMed] [Google Scholar]
- Ballmann M, Hardt H. Hypertonic saline and recombinant human DNase: a randomised cross‐over pilot study in patients with cystic fibrosis [abstract]. 22nd European Cystic Fibrosis Conference; 1998 June 13‐19; Berlin, Germany. 1998:80. [DOI] [PubMed]
Castile 2009 {published data only}
- Castile R, Mueller G, Long F, Flucke R, Baker B, Clifford B. Effects of nebulized recombinant human deoxyribonuclease (dornase alfa) in infants with CF evaluated using infant pulmonary function testing and high resolution computerized tomographic imaging of the chest [abstract]. Pediatric Pulmonology 2009;32(Suppl 32):357, Abstract no: 414. [CFGD Register: BD161a] [Google Scholar]
- NCT00179998. Efficacy of Pulmozyme in infants and young children with cystic fibrosis. http://clinicaltrials.gov/show/NCT00179998 2005. [CFGD Register: BD161b; CRS: 5500100000011130]
Dodd 2000 {published data only}
- Dodd ME, Moorcroft AJ, Haworth CS, Francis S, Miles, J, Clayton N, et al. The effect of rhDNase on exercise performance and gas trapping in adults with cystic fibrosis: a randomised controlled trial [abstract]. Proceedings of the 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm. 2000:147.
Frederiksen 2006 {published data only}
- Frederiksen B, Koch C, Hoiby N, Pressler T, Hansen A. Effect of aerosolised or rhDNase (Pulmozyme®) on pulmonary infections in CF: an open randomised study (abstract). Pediatric Pulmonology 2000;Suppl 20:246. [Google Scholar]
- Frederiksen B, Pressler T, Hansen A, Koch C, Hoiby N. Effect of aerosolized rhDNase (Pulmozyme) on pulmonary colonization in patients with cystic fibrosis. Acta Paediatrica 2006;65(9):1070‐4. [DOI] [PubMed] [Google Scholar]
Fuchs 1994 {published data only}
- Eisenberg J. Clinical development of rhDNase in the United States [Developpement clinique de la rhDNase aux Etats‐Unis]. Archives de Pediatrie 1995;2(7):674‐8. [DOI] [PubMed] [Google Scholar]
- Fuchs HJ, Borowitz D, Christiansen D, Morris E, Nash M, Ramsey B, et al. Aerosolised recombinant human DNase reduces pulmonary exacerbations and improves pulmonary function in patients with cystic fibrosis [abstract]. Proceedings of the 36th Annual Conference on Chest Disease; 1993. 1993.
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. New England Journal of Medicine 1994;331(10):637‐42. [DOI] [PubMed] [Google Scholar]
- Menzin J, Oster G, Davies L, Drummond MF, Greiner W, Lucioni C, et al. A multinational economic evaluation of rhDNase in the treatment of cystic fibrosis. International Journal of Technology Assessment in Healthcare 1996;12(1):52‐61. [DOI] [PubMed] [Google Scholar]
- Oster G, Huse D, Lacey MJ, Regan MM, Fuchs HJ. Effects of recombinant human DNase therapy on healthcare use and costs in patients with cystic fibrosis. Annals of Pharmacotherapy 1995;29(5):459‐64. [DOI] [PubMed] [Google Scholar]
- Ramsey B for the Pulmozyme (rhDNase) Study Group. A summary of the results of the phase III multicenter clinical trial: aerosol administration of recombinant human DNase reduces the risk of respiratory tract infections and improves pulmonary function in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 1993;Suppl 9:152‐3. [Google Scholar]
- Schulenburg JMG, Greiner W, Hardt H. Socioeconomic evaluation of the influence of rhDNase on the costs of treating respiratory tract infections in patients with cystic fibrosis [Sozioökonomische Evaluation des Einflusses von rhDNase auf die Kosten der Behandlung von Infektionen der Atemwege bei Patienten mit zysticher fibrose]. Medizinische Klinik 1995;90(4):220‐4. [PubMed] [Google Scholar]
Laube 1996 {published data only}
- Laube BL, Auci RM, Shield DE, Christiansen DH, Lucas MK, Fuchs HJ, et al. Effect of rhDNase on airflow obstruction and mucociliary clearance in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1996;153(2):752‐60. [DOI] [PubMed] [Google Scholar]
- Laube BL, Auci RM, Shields DE, Christiansen D, Fuchs HJ, Rosenstein BJ. A randomized, placebo‐controlled trial of the effect of recombinant human DNase (rhDNase) on the deposition homogeneity and mucociliary clearance of radioaerosol in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 1993;Suppl 9:155‐6. [Google Scholar]
McCoy 1996 {published data only}
- McCoy K for the Pulmozyme Severe Lung Disease Study Group. rhDNase is well tolerated and effective in cystic fibrosis patients with severe obstructive ling disease [abstract]. Proceedings of the 20th European Cystic Fibrosis Conference; 1995 June 18‐21; Brussels, Belgium. 1995:L41.
- McCoy K, Hamilton S, Johnson CAC, for the Pulmozyme study group. Effects of 12‐week administration of dornase alfa in patients with advanced cystic fibrosis lung disease. Chest 1996;110(4):889‐95. [DOI] [PubMed] [Google Scholar]
Minasian 2010 {published data only}
- Minasian C, Wallis C, Metcalfe C, Bush A. Comparison of inhaled mannitol, daily rhDNase and a combination of both in children with cystic fibrosis: a randomised trial. Thorax 2010;65(1):51‐6. [CFGD Register: BD133b] [DOI] [PubMed] [Google Scholar]
- Minasian CC, Wallis C, Metcalfe C, Bush A. A crossover comparative study of inhaled mannitol, alone and in combination with daily rhDNase, in children with cystic fibrosis [abstract]. Pediatric Pulmonology 2008;43(Suppl 31):301. [CFGD Register: BD133a] [Google Scholar]
Paul 2004 {published data only}
- Griese M, Essl R, Schmidt R, Ballmann M, Paul K, Rietschel E, et al. Sequential analysis of surfactant, lung function and inflammation in cystic fibrosis patients. Respiratory Research 2005;6:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung A, Shute JK, Chen CIU, Ballmann M, Griese M, Ratjen F, et al. Influence of long‐term inhaled rhDNase on free and total IL‐8 concentration in BAL fluid in cystic fibrosis [abstract]. Proceedings of the 12th European Respiratory Society Annual Congress; 2002 Sep 14‐18; Stockholm. 2002:P3285.
- Paul K, Ballmann M, Griese M, Rietschel E, Chen C, Schink T, et al. Effect of rhDNase on endobronchial inflammation in CF patients with mild lung disease: results of multi‐center beat study [abstract]. Pediatric Pulmonology 2002;Suppl 24:310‐11. [Google Scholar]
- Paul K, Rietschel E, Ballmann M, Griese M, Worlitzsch D, Shute J, et al. Effect of treatment with dornase alpha on airway inflammation in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2004;169(6):719‐25. [DOI] [PubMed] [Google Scholar]
- Paul KP, Ballmann M, Dorig G, Griese M, Kleinau I, Ratjen F, et al. Airway inflammation in CF patients with good pulmonary function ‐ baseline data from the multicenter "Beat" study [abstract]. Pediatric Pulmonology 1998;Suppl 17:383‐4. [Google Scholar]
- Paul KP, Rietschel E, Ballmann M, Griese M, Chen CI, Schink T, et al. Longitudinal Evaluation of Airway Inflammation in Stable Cystic Fibrosis (CF) Patients by Bronchoalveolar Lavage (BAL) and Influence rhDNase [abstract]. American Thoracic Society International Conference; 2003 May 16‐21; Seattle, USA.. 2003:Poster E42.
- Ratjen F, Griese M, Ballmann M, Rietschel E, Paul K, Beat Study Group. Dornase alpha in pediatric CF patients with normal lung function ‐ effects on airway inflammation [abstract]. Pediatric Pulmonology 2005;40(Suppl 28):143. [Google Scholar]
- Ratjen F, Paul K, Rietschel E, Nikolaizik W. Treatment with rhDNase reduces DNA levels in BAL fluid of CF patients with mild lung disease [abstract]. Pediatric Pulmonology 2002;Suppl 24:310. [DOI] [PubMed] [Google Scholar]
- Ratjen F, Paul K, Koningsbruggen S, Breitenstein S, Rietschel E, Nikolaizik W. DNA concentrations in BAL fluid of cystic fibrosis patients with early lung disease: Influence of treatment with dornase alpha. Pediatric Pulmonology 2005;39(1):1‐4. [DOI] [PubMed] [Google Scholar]
Quan 2001 {published data only}
- Brody A, Molina PL, Klein JS, Campbell JD, Millard SP, Quan J. High‐resolution CT is more sensitive to longitudinal decline in lung status in young children with CF than pulmonary function tests [abstract]. Pediatric Pulmonology 2003;Suppl 25:318. [CFGD Register: BD98d] [Google Scholar]
- Grasemann H, Lax H, Treseler JW, Colin AA. Dornase alpha and exhaled NO in cystic fibrosis. Pediatric Pulmonology 2004;38(5):379‐85. [CFGD Register: BD150] [DOI] [PubMed] [Google Scholar]
- Konstan MW, Wohl ME, McKenzie S, Sy J, Quan JM, Tiddens HA. A randomized, placebo‐controlled trial of two years' treatment with dornase alfa (Pulmozyme®) in cystic fibrosis patients aged 6‐10 years with early lung disease. Pediatric Pulmonology 2000;Suppl 20:299‐300. [CFGD Register: BD98a] [Google Scholar]
- Quan JM, Tiddens HAWM, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ, et al. A two‐year randomized, placebo controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. Journal of Pediatrics 2001;139(6):813‐20. [CFGD Register: BD98b] [DOI] [PubMed] [Google Scholar]
- Robinson PJ. Dornase alfa in early cystic fibrosis lung disease. Pediatric Pulmonology 2002;34(3):237‐41. [CFGD Register: BD98c] [DOI] [PubMed] [Google Scholar]
- Sanders DB, Li Z, Brody AS. Chest computed tomography predicts the frequency of pulmonary exacerbations in children with cystic fibrosis. Annals of the American Thoracic Society 2015;12(1):64‐9. [CFGD Register: BD98e] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsey 1993 {published data only}
- Ramsey BW, Astley SJ, Aitken ML, Burke W, Colin AA, Dorkin HL, et al. Efficacy and safety of short‐term administration of aerosolized recombinant human deoxyribonuclease in patients with cystic fibrosis. American Review of Respiratory Disease 1993;148(1):145‐51. [DOI] [PubMed] [Google Scholar]
Ranasinha 1993 {published data only}
- Ranasinha C, Assoufi B, Shak S, Christiansen D, Fuchs HJ, Empey D, et al. Efficacy and safety of short‐term administration of aerosolised recombinant human DNase I in adults with stable stage cystic fibrosis. Lancet 1993;342(8865):199‐202. [DOI] [PubMed] [Google Scholar]
- Ranasinha C, Empey D, Geddes D, Fuchs H, Hodson ME. A phase 2 double‐blind placebo controlled study of the pulmonary function and the safety of aerosolised recombinant human DNase in adults with stable stage cystic fibrosis [abstract]. Proceedings of the 11th International Cystic Fibrosis Congress; 1992. 1992:LBS5.
- Shah PL, Scott SF, Knight RA, Marriott C, Ransinha C, Hodson ME. In vivo effects of recombinant human DNase I on sputum in patients with cystic fibrosis. Thorax 1996;51(2):119‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 2000 {published data only}
- Hemming AL, Robinson M, Moriarty C, Bautovich GJ, Bye PTP. Effect of short course of rhDNase on mucociliary clearance in patients with cystic fibrosis [abstract]. Pediatric Pulmonology 1997;Suppl 14:273. [DOI] [PubMed] [Google Scholar]
- Robinson M, Hemming AL, Moriarty B, Eberl S, Bye PTP. Effect of a short course of rhDNase on cough and mucociliary clearance in patients with cystic fibrosis. Pediatric Pulmonology 2000;30(1):16‐24. [CFGD Register: BD76b] [DOI] [PubMed] [Google Scholar]
Robinson 2005 {published data only}
- Robinson T, Leung AN, Northway WH, Blankenberg FG, Chan F, Bloch DA, et al. Composite CT/PFT score: an outcome measure which markedly improves sensitivity to change in early cystic fibrosis lung disease [abstract]. Pediatric Pulmonology 2002;Suppl 24:298. [CFGD Register: BD106a] [Google Scholar]
- Robinson TE, Goris ML, Zhu HJ, Chen X, Bhise P, Sheikh F, et al. Dornase alfa reduces air trapping in children with mild cystic fibrosis lung disease: a quantitative analysis. Chest 2005;128(4):2327‐35. [CFGD Register: BD106c] [DOI] [PubMed] [Google Scholar]
- Robinson TE, Leung AN, Northway WH, Blankenberg FG, Chan FP, Bloch DA, et al. Composite spirometric‐computed tomography outcome measure in early cystic fibrosis lung disease. American Journal of Respiratory and Critical Care Medicine 2003;168(5):588‐93. [CFGD Register: BD106b] [DOI] [PubMed] [Google Scholar]
Shah 1995a {published data only}
- Hodson M. Multicenter study of rhDNase in cystic fibrosis with severe pulmonary involvement [Étude multicentrique de la rhDNase dans les mucoviscidoses avec atteinte pulmonaire grave]. Archives de Pediatrie 1995;2(7):679‐81. [CFGD Register: BD42c] [DOI] [PubMed] [Google Scholar]
- Shah PI, Bush A, Canny GJ, Colin AA, Fuchs HJ, Geddes DM, et al. Recombinant human DNase I in cystic fibrosis patients with severe pulmonary disease: a short‐term, double‐blind study followed by six months open‐label treatment. European Respiratory Journal 1995;8(6):954‐8. [CFGD Register: BD42b] [PubMed] [Google Scholar]
- Shah PL, Dewar A, Hodson ME. Scanning electron microscopy of cystic fibrosis sputum [abstract]. European Respiratory Journal 1995;8(Suppl 19):574S. [CFGD Register: BD38e] [Google Scholar]
- Shah PL, Scott SF, Hodson ME (on behalf of the multicentre study group). Report on a multicentre study using aerosolised recombinant human DNase I in the treatment of cystic fibrosis patients with severe pulmonary disease [abstract]. Pediatric Pulmonology 1993;Suppl 9:157‐8. [CFGD Register: BD42a] [Google Scholar]
- Shah PL, Scott SF, Knight RA, Marriott C, Hodson ME. The effects of recombinant human DNase I on sputum DNA content [abstract]. European Respiratory Journal 1995;8(Suppl 19):574S. [CFGD Register: BD38d] [Google Scholar]
Suri 2001 {published data only}
- Grieve R, Thompson S, Normand C, Suri R, Bush A, Wallis C. A cost‐effectiveness analysis of rhDNase in children with cystic fibrosis. International Journal of Technology Assessment in Health Care 2003;19(1):71‐9. [DOI] [PubMed] [Google Scholar]
- Suri R, Grieve R, Normand C, Metcalfe C, Thompson S, Wallis C, et al. Effects of hypertonic saline, alternate day and daily rhDNase on healthcare use, costs and outcomes in children with cystic fibrosis. Thorax 2002;57(10):841‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri R, Grieve R, Normand C, Metcalfe C, Thompson S, Wallis C, et al. Effects of hypertonic saline, alternate day and daily rhDNase on healthcare use, costs and outcomes in children with cystic fibrosis [abstract]. Thorax 2001;56(Suppl 3):iii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri R, Marshall LJ, Wallis C, Metcalfe C, Bush A, Shute JK. Effects of recombinant human DNase and hypertonic saline on airway inflammation in children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2002;166(3):352‐5. [DOI] [PubMed] [Google Scholar]
- Suri R, Marshall LJ, Wallis C, Metcalfe C, Thompson S, Bush A, et al. Effects of rhDNase and hypertonic saline on airway inflammation in children with cystic fibrosis [abstract]. Pediatric Pulmonology 2001;Suppl 22:281. [Google Scholar]
- Suri R, Metcalfe C, Lees B, Flather M, Normand C, Thompson S, et al. A cross‐over comparative study of hypertonic saline alternate day and daily rhDNase in children with Cystic Fibrosis [abstract]. Thorax 2000;55:A75. [Google Scholar]
- Suri R, Metcalfe C, Lees B, Grieve R, Flather M, Normand C, et al. Comparison of hypertonic saline and alternate‐day or daily recombinant human deoxyribonuclease in children with cystic fibrosis: a randomised trial. Lancet 2001;358(9290):1316‐21. [DOI] [PubMed] [Google Scholar]
- Suri R, Metcalfe C, Wallis C, Bush A. Assessing the usefulness of outcomes measured in a cystic fibrosis treatment trial. Respiratory Medicine 2007;101(2):254‐60. [DOI] [PubMed] [Google Scholar]
- Suri R, Metcalfe C, Wallis C, Bush A. Predicting response to rhDNase and hypertonic saline in children with cystic fibrosis. Pediatric Pulmonology 2004;37:305‐10. [DOI] [PubMed] [Google Scholar]
- Suri R, Wallis C, Bush A. In vivo use of hypertonic saline in CF [abstract]. Pediatric Pulmonology 2000;Suppl 20:125‐6. [Google Scholar]
- Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al. A comparative study of hypertonic saline, daily and alternate‐day rhDNase in children with cystic fibrosis. Health Technology Assessment (HMSO) 2002; Vol. 6, issue No.34. [PubMed]
Wilmott 1996 {published data only}
- Wilmott R and the DNase Multicenter Study Group and Genentech staff. A phase II, double blind, multicenter study of the safety and efficacy of aerosolized recombinant human DNase I in hospitalized patients with CF experiencing acute pulmonary exacerbations [abstract]. Pediatric Pulmonology 1993;Suppl 9:154. [Google Scholar]
- Wilmott RW, Amin RS, Colin AA, Vault A, Dozor AJ, Eigen H, et al. Aerosolized recombinant human DNase in hospitalized cystic fibrosis patients with acute pulmonary exacerbations. American Journal of Respiratory and Critical Care Medicine 1996;153(6 Pt 1):1914‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2009 {published data only}
- Anderson P, Morton J. Evaluation of two different timings of Pulmozyme nebulisation in relation to chest physiotherapy in children with cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2009;8 Suppl 2:S74, Abstract no: 299. [CFGD Register: BD162] [Google Scholar]
Bakker 2010 {published data only}
- Bakker EM, Volpi S, Salonini E, Mullinger B, Kroneberg P, Hop WCJ, et al. Efficacy of peripheral deposition of inhaled rhDNase in CF patients during a respiratory tract infection [abstract]. Journal of Cystic Fibrosis 2010;9 Supplement 1:S62, Abstract no: 239. [CFGD Register: BD155a] [Google Scholar]
- Bakker M, Volpi S, Salonini E, Wiel E, Merkus P, Sintnicolaas C, et al. Peripheral versus central deposition of inhaled rhdnase in children with cystic fibrosis [abstract]. Pediatric Pulmonology 2010;45(S33):306, Abstract no: 244. [CFGD Register: BD155b] [Google Scholar]
- Beukel‐Bakker M, Volpi S, Salonini E, Wiel‐Kooij EC, Sintnicolaas C, Hop WC, et al. Small airways response to dornase alfa improves using controlled inhalation: a randomized controlled trial in cystic fibrosis patients [abstract]. Journal of Cystic Fibrosis 2011;10 Supplement 1:S19, Abstract no: 75. [CFGD Register: BD155c] [Google Scholar]
Bilton 2011 {published data only}
- Bilton D, Robinson P, Cooper P, Gallagher CG, Kolbe J, Fox H, Jaques A, Charlton B, CF301 Study Investigators. Inhaled dry powder mannitol in cystic fibrosis: an efficacy and safety study. [NCT00446680]. European Respiratory Journal 2011;38(5):1071‐80. [DOI] [PubMed] [Google Scholar]
Bishop 2011 {published data only}
- Bishop JR, Erskine OJ, Middleton PG. Timing of dornase alpha inhalation does not affect the efficacy of an airway clearance regimen in adults with cystic fibrosis: a randomised crossover trial. Journal of physiotherapy 2011;57(4):223‐9. [CFGD Register: BD252a] [DOI] [PubMed] [Google Scholar]
- Middleton PG, Bishop J. Dornase alpha and physiotherapy ‐ which should be first? A randomised double‐blind, placebo‐controlled trial in CF adults [abstract]. Pediatric Pulmonology 2001;Suppl 22:310. [CFGD Register: BD252b] [Google Scholar]
Bollert 1999 {published data only}
- Bollert FG, Paton JY, Marshall TG, Calvert J, Greening AP, Innes JA. Recombinant DNase in cystic fibrosis: a protocol for targeted introduction through n‐of‐1 trials. Scottish Cystic Fibrosis Group.. European Respiratory Journal 1999;13(1):107‐13. [DOI] [PubMed] [Google Scholar]
- Bollert FGE, McArthur DA, Greening AP, Innes JA on behalf of the Scottish Cystic Fibrosis Group. Targeted introduction of DNase in Scotland [abstract]. Pediatric Pulmonology 1995;Suppl 12:204. [Google Scholar]
Cimmino 2005 {published data only}
- Cimmino M, Nardone M, Cavaliere M, Plantulli A, Sepe A, Esposito V, et al. Dornase alfa as postoperative therapy in cystic fibrosis sinonasal disease. Archives of Otolaryngology ‐ Head and Neck Surgery 2005;131(12):1097‐101. [DOI] [PubMed] [Google Scholar]
Craig 2013 {published data only}
- Craig T. Pulmozyme in cystic fibrosis with sinusitis. clinicaltrials.gov/ct2/show/NCT01155752 (accessed 14 April 2015).
Dab 2000 {published data only}
- Dab A, Malfroot D, Baran EM, App M, Coffiner M, Nagy AM. Randomized multicentric double blind study of safety and efficacy of Nacystelyn DPI versus placebo in rhDNase treated cystic fibrosis patients [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3):A72. [Google Scholar]
- Malfroot A, Dab I, Baran D, App EM, Coffiner M, Nagy AM. Randomised multicentric double blind study of tolerability and efficacy of a DPI Nacystelyn versus placebo in cystic fibrosis patients treated by rhDNase for at least 3 months [abstract]. Proceedings of the 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:146.
- Malfroot A, Dab I, Baran D, App EM, Coffiner M, Nagy AM. Randomized multicentric double blind study of tolerability and efficacy of a DPI Nacystelyn versus placebo in cystic fibrosis patients treated by rhDNase for at least 3 months [abstract]. Pediatric Pulmonology 1999;Suppl 19:244. [Google Scholar]
Diot 2009 {published data only}
- Diot P. RhDNase and Biodistribution of PMN Serine Proteases in Cystic Fibrosis. https://clinicaltrials.gov/ct2/show/NCT00843817 accessed April 15, 2015.
Elkins 2006 {published data only}
- Elkins MR, Bye PTP. Comparison of Pari LC‐Star and ‐Plus nebulisers delivering 2.5mg recombinant human deoxyribonuclease (rhDNase) [abstract]. Journal of Cystic Fibrosis 2006;5 Suppl:S42. [Google Scholar]
EUCTR2006‐002098‐30‐NL {published data only}
- EUCTR2006‐002098‐30‐NL. Efficacy of inhaled rhDNase in mechanically ventilated pediatric patients with an atelectasis ‐ rhDNase in ventilated pediatric patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006‐002098‐30‐NL (first received 01 June 2006).
EUCTR2007‐000935‐25‐NL {published data only}
- EUCTR2007‐000935‐25‐NL. Peripheral targeting of inhaled rhDNase in stable CF patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2007‐000935‐25‐NL (first received 02 April 2007).
Fitzgerald 2005 {published data only}
- Fitzgerald DA, Hilton J, Jepson B, Smith L. A crossover, randomized, controlled trial of dornase alfa before versus after physiotherapy in cystic fibrosis. Pediatrics 2005;116(4):e549‐54. [CFGD Register: BD102b] [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Hilton J, Smith L, Jepson B. Is dornase alfa (pulmozyme) more effective before or after physiotherapy? A cross‐over, randomised placebo controlled trial [abstract]. Pediatric Pulmonology 2001;Suppl 22:309‐10. [CFGD Register: BD102a] [Google Scholar]
Freemer 2010 {published data only}
- Genentech Inc. A study of Pulmozyme® (dornase alpha) in 3‐ to 5‐year‐old patients with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT00680316 (accessed 14 April 2015). [clinicaltrials.gov: NCT00680316]
Furuya 2001 {published data only}
- Furuya ME, Lezana‐Fernandez JL, Vargas MH, Hernandez‐Sierra JF, Ramirez‐Figueroa JL. Efficacy of human recombinant DNase in pediatric patients with cystic fibrosis. Archives of Medical Research 2001;32(1):30‐4. [DOI] [PubMed] [Google Scholar]
Genentech 2010 {published data only}
- Genentech Inc. A Trial of Pulmozyme Withdrawal on Exercise Tolerance in Cystic Fibrosis Subjects with Severe Lung Disease (TOPIC). https://clinicaltrials.gov/ct2/show/results/NCT00434278 accessed April 15, 2015.
Griese 1997 {published data only}
- Griese M, App EM, Derouix A, Burkert A, Schams A. Recombinant human DNase (rhDNase) influences phospholipid composition, surface activity, rheology and consecutively clearance indices of cystic fibrosis sputum. Pulmonary Pharmacology and Therapeutics 1997;10(1):21‐7. [DOI] [PubMed] [Google Scholar]
Hagelberg 2008 {published data only}
- Hagelberg M, Dooley MJ, Poole SG, Leung D, Bailey M, Finlayson F, et al. Direct dispensing of dornase alpha improves adherence and lung function in cystic fibrosis [abstract]. Journal of Cystic Fibrosis 2008;7(Suppl 2):S27. [Google Scholar]
Heijerman 1995 {published data only}
- Heijerman HGM, Rossem RN, Bakker W. Effect of rhDNase on lung function and quality of life in adult cystic fibrosis patients. The Netherlands Journal of Medicine 1995;46(6):293‐7. [CFGD Register: BD43] [DOI] [PubMed] [Google Scholar]
Hubbard 1992 {published data only}
- Hubbard RC, McElvaney NG, Birrer P, Shak S, Robinson WW, Jolley C, et al. A preliminary study of aerosolized recombinant human deoxyribonuclease I in the treatment of cystic fibrosis. New England Journal of Medicine 1992;326(12):812‐5. [DOI] [PubMed] [Google Scholar]
Johnson 2006 {published data only}
- Johnson JC, Waldrep JC, Dhand R. Aerosol delivery of recombinant human DNase 1. Comparison of a vibrating mesh nebulizer with a jet nebulizer [abstract]. American Thoracic Society International Conference; 2006 May 19‐24; California, USA. 2006:A82.
Kelijo 2001 {published data only}
- Kelijo DJ, Giroir B, Jialal I. Circulating tumor necrosis factor‐alpha and IL‐6 levels in patients with cystic fibrosis and mild lung disease: effects of Dnase and alpa‐tocopherol therapy. Unpublished article 2001, issue Obtained 2014. [CFGD Register: GN87b]
King 1997 {published data only}
- King M, Dasgupta B, Tomkiewicz RP, Brown NE, Pulmonary Research Group. Rheology of cystic fibrosis sputum after in vitro treatment with hypertonic saline alone and in combination with recombinant human deoxyribonuclease I. American Journal of Respiratory and Critical Care Medicine 1997;156(1):173‐7. [DOI] [PubMed] [Google Scholar]
Lahiri 2012 {published data only}
- Lahiri T. Nasally Delivered Pulmozyme for Sinusitis in Cystic Fibrosis. https://clinicaltrials.gov/ct2/show/NCT00416182?term=nct00416182&rank=1 accessed 14 April 2015.
- Lahiri T, Herrington H, Diehl S, Landrigan G. The effect of intranasal dornase alfa on chronic sinusitis in patients with cystic fibrosis: a pilot study.. Pediatric Pulmonology 2012;S35:354. [Google Scholar]
Laube 2005 {published data only}
- Laube BL, Geller DE, Lin TC, Dalby RN, Diener‐West M, Zeitlin PL. Positive expiratory pressure changes aerosol distribution in patients with cystic fibrosis. Respiratory Care 2005;50(11):1438‐44. [PubMed] [Google Scholar]
- Laube BL, Lin T, Geller D, Dalby R, Zeitlin P. Positive expiratory pressure alters aerosol distribution in CF [abstract]. Pediatric Pulmonology 2000;Suppl 20:247. [Google Scholar]
Mainz 2011 {published data only}
- Mainz J, Mentzel H, Scheider G, Riethmuller J, Schiller I, Ritschel C, et al. Double‐blind, placebo‐controlled pilot trial on sinonasal inhalation of dornase alfa in CF. Pediatric Pulmonology 2008;43(Suppl 31):305. [CFGD Register: BD131b] [Google Scholar]
- Mainz J, Mentzel HJ, Schneider G, Riethmuller J, Schiller I, Ritschel C, et al. Sinu‐nasal inhalation of Dornase alfa in CF. Results of a double‐blind placebo‐controlled pilot trial. Journal of Cystic Fibrosis 2008;7(Suppl 2):S27. [CFGD Register: BD131a] [Google Scholar]
- Mainz JG, Schiller I, Ritschel, Mentzel HJ, Riethmuller J, Koitschev A, et al. Sinonasal inhalation of dornase alfa in CF: A double‐blind placebo‐controlled cross‐over pilot trial. Auris, Nasus, Larynx 2011;38(2):220‐7. [DOI] [PubMed] [Google Scholar]
Mainz 2014 {published data only}
- Mainz JG, Schien C, Schiller I, Schadlich K, Koitschev A, Koitschev C, et al. Sinonasal inhalation of dornase alfa administered by vibrating aerosol to cystic fibrosis patients: a double‐blind placebo‐controlled cross‐over trial. Journal of Cystic Fibrosis 2014;13(4):461‐70. [CFGD Register: BD154b] [DOI] [PubMed] [Google Scholar]
- Mainz JG, Schiller I, Koitschev A, Koitschev C, Riethmuller J, Wiedemann B, et al. Sinonasal inhalation of dornase alfa reduces rhinosinusitis symptoms in CF. Results of a DBPC‐cross‐over‐study [abstract]. Journal of Cystic Fibrosis 2010;9(Suppl 1):S23. [Abstract no.: 88; CFGD Register: BD154a] [Google Scholar]
Majaesic 1996 {published data only}
- Majaesic CM, Montgomery M, Jones R, King M, et al. Reduction in sputum viscosity using high frequency chest compressions (HFCC) compared to conventional chest physiotherapy (CCP) [abstract]. Pediatric Pulmonology 1996;Suppl 13:308. [Google Scholar]
Nasr 2001 {published data only}
- Nasr SZ, Kuhns LR, Brown RW, Hurwitz ME, Sanders GM, Strouse PJ. Use of computerized tomography and chest‐rays in evaluating efficacy of aerosolized recombinant human DNase in cystic fibrosis patients younger than 5 years : A preliminary study. Pediatric Pulmology 2001;31(5):377‐82. [DOI] [PubMed] [Google Scholar]
- Nasr SZ, Kuhns LR, Brown RW, Hurwitz RW, Sanders GM, Stouse PJ. Aerolized recombinant human DNase in cystic fibrosis patients younger than 5 years of age [abstract]. Pediatric Pulmonology 1999;Suppl 19:278. [DOI] [PubMed] [Google Scholar]
NCT00311506 {published data only}
- NCT00311506. A study to determine the variability of a 6‐minute walk test in cystic fibrosis subjects with advanced lung disease. clinicaltrials.gov/ct2/show/NCT00311506 (first received 06 April 2006).
NCT00434278 {published data only}
- NCT00434278. A trial of pulmozyme withdrawal on exercise tolerance in cystic fibrosis subjects with severe lung disease (TOPIC). clinicaltrials.gov/ct2/show/NCT00434278 (first received 13 February 2007).
NCT00843817 {published data only}
- NCT00843817. RhDNase and biodistribution of PMN serine proteases in cystic fibrosis sputum (BioDNase). clinicaltrials.gov/ct2/show/NCT00843817 (first received 13 February 2009).
NCT01025258 {published data only}
- NCT01025258. Improving adherence and clinical outcome in cystic fibrosis (CF) patients. clinicaltrials.gov/ct2/show/NCT01025258 (first received 03 December 2009).
NCT01155752 {published data only}
- NCT01155752. Pulmozyme in cystic fibrosis with sinusitis. clinicaltrials.gov/ct2/show/NCT01155752 (first received 02 July 2010).
NCT01232478 {published data only}
- NCT01232478. I change adherence and raise expectations (iCARE). clinicaltrials.gov/ct2/show/NCT01232478 (first received 02 November 2010).
NCT02301377 {published data only}
- NCT02301377. Exploring novel interventions to improve adherence in children with cystic fibrosis. clinicaltrials.gov/ct2/show/NCT02301377 (first received 25 November 2014).
NCT02682290 {published data only}
- NCT02682290. Assessment of rheological parameters of human sputum (RHEOMUCO). clinicaltrials.gov/ct2/show/NCT02682290 (first received 15 February 2016).
NCT02722122 {published data only}
- NCT02722122. Study to evaluate the safety,tolerability, pharmacokinetics and exploratory efficacy parameters of AIR DNase™ in patients with cystic fibrosis previously treated with Pulmozyme®. clinicaltrials.gov/ct2/show/NCT02722122 (first received 29 March 2016).
Potter 2008 {published data only}
- Potter RW, Hurren TJ, Nickerson C, Hatley RH. Comparison of the delivery characteristics of Dornase alfa from the I‐NEB AAD system and the sidestream jet nebulizer [abstract]. Pediatric Pulmonology 2008;43(Suppl 31):361. [Google Scholar]
Riethmueller 2006 {published data only}
- Riethmueller J, Borth‐Bruhns T, Kumpf M, Vonthein R, Wiskirchen J, Stern M, et al. Recombinant human deoxyribonuclease shortens ventilation time in young, mechanically ventilated children. Pediatric Pulmonology 2006;41(1):61‐6. [DOI] [PubMed] [Google Scholar]
Robinson 2002 {published data only}
- Robinson T, Goris ML, Bhise P, Sathi A, Zhu JH, Moss RB. Quantitative HRCT air trapping analysis in CF subjects with mild lung disease during a pulmozyme intervention study [abstract]. Pediatric Pulmonology 2002;Suppl 24:298. [MU 94] [Google Scholar]
Sawicki 2014 {published data only}
- Konstan MW, Chou W, Trzaskoma B, Xu Y. A study to evaluate the comparable efficacy and safety of Pulmozyme® delivered by the Erapid™ nebulizer system [abstract]. Pediatric Pulmonology 2013;48 Suppl 36:452, Abstract no: 668. [CENTRAL: 999883; CFGD Register: BD219c; CRS: 5500127000000005] [Google Scholar]
- Sawicki GS, Chou W, Raimundo K, Trzaskoma B, Konstan MW. Randomized trial of efficacy and safety of dornase alfa delivered by eRapid nebulizer in cystic fibrosis patients. Journal of Cystic Fibrosis 2015;14(6):777‐83. [CFGD Register: BD219d] [DOI] [PubMed] [Google Scholar]
- Sawicki GS, Konstan M, Chou W, Trzaskoma B, Raimundo K. Impact of dornase alfa delivered by the eRapid™ vs. Pari LC® plus jet nebulizer system on cystic fibrosis‐related quality of life ‐ results from the IMPART Study [abstract]. Pediatric Pulmonology 2014;49 Suppl 38:445‐446, Abstract no: 621. [CENTRAL: 1012526; CFGD Register: BD219a; CRS: 5500131000000180] [Google Scholar]
- Sawicki GS, Konston M, Chou W, Trzaskoma B, Raimundo K. Treatment preferences and satisfaction with dornase alfa delivered by the eRapid™ vs. Pari LC® plus jet nebulizer system to patients with cystic fibrosis ‐ results from the IMPART Study [abstract]. Pediatric Pulmonology 2014;49 Suppl 38:444, Abstract no: 617. [CENTRAL: 1012527; CFGD Register: BD219b; CRS: 5500131000000182] [Google Scholar]
Shah 1995b {published data only}
- Shah PL, Scott SF, Geddes DM, Hodson ME. Two years of experience with recombinant human DNase I in the treatment of pulmonary disease in cystic fibrosis. Respiratory Medicine 1995;89(7):499‐502. [CFGD Register: BD132] [DOI] [PubMed] [Google Scholar]
Shah 1995c {published data only}
- Shah PL, Scott SF, Fuchs HJ, Geddes DM, Hodson ME. Medium term treatment of stable stage cystic fibrosis with recombinant human DNase I. Thorax 1995;50(4):333‐8. [CFGD Register: BD127] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 1997 {published data only}
- Shah PL, Scott SF, Geddes DM, Conway S, Carr S, Wallis C, et al. An evaluation of two aerosol delivery systems for rhDNase [abstract]. Israel Journal of Medical Sciences 1996;32(Suppl):S222. [CFGD Register: DT11a] [DOI] [PubMed] [Google Scholar]
- Shah PL, Scott SF, Geddes DM, Conway S, Watson A, Nazir T, et al. An evaluation of two aerosol delivery systems for rhDNase. European Respiratory Journal 1997;10(6):1261‐6. [CFGD Register: DT11b] [DOI] [PubMed] [Google Scholar]
ten Berge 2003 {published data only}
- Berge M, Wiel E, Tiddens HAWM, Merkus PJFM, Hop WCJ, Jongste JC. DNase in stable cystic fibrosis infants: a pilot study. Journal of Cystic Fibrosis 2003;2(4):183‐8. [DOI] [PubMed] [Google Scholar]
van der Giessen 2007a {published data only}
- Giessen L. Does the timing of inhaled dornase alfa matter?. Journal of Cystic Fibrosis 2009;8(Suppl 1):S6‐9. [CFGD Register: BD116c // BD126c] [DOI] [PubMed] [Google Scholar]
- Giessen LJ, Gosselink R, Jongste JC, Hop WCJ, Tiddens HAWM. Timing of nebulisation of rhDNase and airway clearance techniques (ACT) in children with Cystic Fibrosis. Journal of Cystic Fibrosis 2005;4 Suppl:S97. [CFGD Register: BD116b] [Google Scholar]
- Giessen LJ, Jongste JC, Gosselink R, Hop WC, Tiddens HA. RhDNase before airway clearance therapy improves airway patency in children with CF. Pediatric Pulmonology 2007;42(7):624‐30. [CFGD Register: BD116b] [DOI] [PubMed] [Google Scholar]
van der Giessen 2007b {published data only}
- Giessen L. Does the timing of inhaled dornase alfa matter?. Journal of Cystic Fibrosis 2009;8(Suppl 1):S6‐9. [CFGD Register: BD116c // BD126c] [DOI] [PubMed] [Google Scholar]
- Giessen LJ, Gosselink R, Hop W, Tiddens H. RhDNase before or after going to sleep in children with cystic fibrosis?. Journal of Cystic Fibrosis 2007;6 Suppl 1:S68 Poster 274. [CFGD Register: BD126a] [Google Scholar]
- Giessen LJ, Gosselink R, Hop WC, Tiddens HA. Recombinant human DNase nebulisation in children with cystic fibrosis: before bedtime or after waking up?. European Respiratory Journal 2007;30(4):763‐8. [CFGD Register: BD126b] [DOI] [PubMed] [Google Scholar]
- Giessen LJJ, Bakker M, Hop WCJ, Tiddens HAWM. Nocturnal saturation in children with cystic fibrosis. Proceedings of the European Respiratory Society Annual Congress; 2006 sep 2‐6; Munich, Germany. 2006. [Abstract no.: P4106; CFGD Register: BD126d]
Weck 1999 {published data only}
- Weck MB, Retsch‐Bogart GZ, Scott CS. Efficacy of DNase in individual children using the N‐of‐1 study design [abstract]. Pediatric Pulmonology 1999;Suppl 19:285. [Google Scholar]
Wilson 2007 {published data only}
- Wilson CJ, Robbins LJ, Murphy JM, Chang AB. Is a longer time interval between recombinant human deoxyribonuclease (dornase alfa) and chest physiotherapy better? A multi‐center, randomized crossover trial. Pediatric Pulmonology 2007;42(12):1110‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Cho E 2015 [pers comm]
- Cho E. Cost of medications [personal communication]. Email to: C Yang 15 April 2015.
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Flume 2007
- Flume PA, O'Sullivan BP, Robinson KA, Goss CH, Mogayzel PJ, Willey‐Courand DB, et al. Cystic Fibrosis Pulmonary Guidelines: Chronic Medications for Maintenance of Lung Health. American Journal of Respiratory and Critical Care Medicine 2007;176(10):957‐69. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jacques 2008
- Jaques A, Daviskas E, Turton JA, McKay K, Cooper P, Stirling RG, et al. Inhaled mannitol improves lung function in cystic fibrosis. Chest 2008;133(6):1388‐96. [DOI] [PubMed] [Google Scholar]
Jones 2009
- Jones AP, Riley RD, Williamson PR, Whitehead A. Meta‐analysis of individual patient data versus aggregate data from longitudinal clinical trials. Clinical Trials 2009;6(1):16‐27. [DOI] [PubMed] [Google Scholar]
Lieberman 1968
- Lierberman J. Dornase aerosol effect on sputum viscosity in cases of cystic fibrosis. Journal of the American Medical Association 1968;205:312‐3. [PubMed] [Google Scholar]
Menzin 1996
- Menzin J, Oster G, Davies L, Drummond MF, Greiner W, Lucioni C, et al. A multi national economic evaluation of rhDNase in the treatment of cystic fibrosis. International Journal of Technology Assessment in Healthcare 1996;12(1):52‐61. [DOI] [PubMed] [Google Scholar]
NICE 2012
- National Institute for Health and Clinical Excellence. Mannitol dry powder for inhalation for treating cystic fibrosis. www.nice.org.uk/guidance/ta266/documents/cystic‐fibrosis‐mannitol‐final‐appraisal‐determinaton3 Oct 2012:1‐62.
Oster 1995
- Oster G, Huse D, Lacey MJ, Regan MM, Fuchs HJ. Effects of recombinant human DNase therapy on healthcare use and costs in patients with cystic fibrosis. Annals of Pharmacotherapy 1995;29(5):459‐64. [DOI] [PubMed] [Google Scholar]
Sawicki 2009
- Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self‐management. Journal of Cystic Fibrosis 2009;8(2):91‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sly 2009
- Sly P, Brennan S, Gangell C, Klerk N, Murray C, Mott L, et al. Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. American Journal of Respiratory and Critical Care Medicine 2009;180(2):146‐52. [DOI] [PubMed] [Google Scholar]
von der Schulenburg 1995
- Schulenburg JMG, Greiner W, Hardt H. Socioeconomic evaluation of the influence of rhDNase on the costs of treating respiratory tract infections in patients with cystic fibrosis [Soziookonomische Evaluation des Einflusses von rhDNase auf die Kosten der Behandlung von Infektionen der Atemwege bei Patienten mit zystischer Fibrose]. Medizinische Klinik 1995;90(4):220‐4. [PubMed] [Google Scholar]
Wark 2009
- Wark P, McDonald V. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001506.pub3] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jones 2003
- Jones AP, Wallis CE, Kearney CE. Recombinant human deoxyribonuclease for cystic fibrosis. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD001127] [DOI] [PubMed] [Google Scholar]
Jones 2010
- Jones AP, Wallis C. Dornase alfa for cystic fibrosis. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD001127] [DOI] [PubMed] [Google Scholar]
Kearney 1998
- Kearney CE, Wallis CE. Deoxyribonuclease for cystic fibrosis. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD001127] [DOI] [PubMed] [Google Scholar]


