Abstract
Background
This is an update of the original Cochrane Review published in Issue 4, 2011.
Attention deficit hyperactivity disorder (ADHD) is the most prevalent of the comorbid psychiatric disorders that complicate tic disorders. Medications commonly used to treat ADHD symptoms include stimulants such as methylphenidate and amphetamine; non‐stimulants, such as atomoxetine; tricyclic antidepressants; and alpha agonists. Alpha agonists are also used as a treatment for tics. Due to the impact of ADHD symptoms on the child with tic disorder, treatment of ADHD is often of greater priority than the medical management of tics. However, for many decades, clinicians have been reluctant to use stimulants to treat children with ADHD and tics for fear of worsening their tics.
Objectives
To assess the effects of pharmacological treatments for ADHD in children with comorbid tic disorders on symptoms of ADHD and tics.
Search methods
In September 2017, we searched CENTRAL, MEDLINE, Embase, and 12 other databases. We also searched two trial registers and contacted experts in the field for any ongoing or unpublished studies.
Selection criteria
We included randomized, double‐blind, controlled trials of any pharmacological treatment for ADHD used specifically in children with comorbid tic disorders. We included both parallel‐group and cross‐over study designs.
Data collection and analysis
We used standard methodological procedures of Cochrane, in that two review authors independently selected studies, extracted data using standardized forms, assessed risk of bias, and graded the overall quality of the evidence by using the GRADE approach.
Main results
We included eight randomized controlled trials (four of which were cross‐over trials) with 510 participants (443 boys, 67 girls) in this review. Participants in these studies were children with both ADHD and a chronic tic disorder. All studies took place in the USA and ranged from three to 22 weeks in duration. Five of the eight studies were funded by charitable organizations or government agencies, or both. One study was funded by the drug manufacturer. The other two studies did not specify the source of funding. Risk of bias of included studies was low for blinding; low or unclear for random sequence generation, allocation concealment, and attrition bias; and low or high for selective outcome reporting. We were unable to combine any of the studies in a meta‐analysis due to important clinical heterogeneity and unit‐of‐analysis issues.
Several of the trials assessed multiple agents. Medications assessed included methylphenidate, clonidine, desipramine, dextroamphetamine, guanfacine, atomoxetine, and deprenyl. There was low‐quality evidence for methylphenidate, atomoxetine, and clonidine, and very low‐quality evidence for desipramine, dextroamphetamine, guanfacine and deprenyl in the treatment of ADHD in children with tics. All studies, with the exception of a study using deprenyl, reported improvement in symptoms of ADHD. Tic symptoms also improved in children treated with guanfacine, desipramine, methylphenidate, clonidine, and a combination of methylphenidate and clonidine. In one study, tics limited further dosage increases of methylphenidate. High‐dose dextroamphetamine appeared to worsen tics in one study, although the length of this study was limited to three weeks. There was appetite suppression or weight loss in association with methylphenidate, dextroamphetamine, atomoxetine, and desipramine. There was insomnia associated with methylphenidate and dextroamphetamine, and sedation associated with clonidine.
Authors' conclusions
Following an updated search of potentially relevant studies, we found no new studies that matched our inclusion criteria and thus our conclusions have not changed.
Methylphenidate, clonidine, guanfacine, desipramine, and atomoxetine appear to reduce ADHD symptoms in children with tics though the quality of the available evidence was low to very low. Although stimulants have not been shown to worsen tics in most people with tic disorders, they may, nonetheless, exacerbate tics in individual cases. In these instances, treatment with alpha agonists or atomoxetine may be an alternative. Although there is evidence that desipramine may improve tics and ADHD in children, safety concerns will likely continue to limit its use in this population.
Keywords: Adolescent; Child; Child, Preschool; Female; Humans; Male; Atomoxetine Hydrochloride; Atomoxetine Hydrochloride/therapeutic use; Attention Deficit Disorder with Hyperactivity; Attention Deficit Disorder with Hyperactivity/drug therapy; Central Nervous System Stimulants; Central Nervous System Stimulants/adverse effects; Central Nervous System Stimulants/therapeutic use; Clonidine; Clonidine/therapeutic use; Desipramine; Desipramine/therapeutic use; Dextroamphetamine; Dextroamphetamine/therapeutic use; Guanfacine; Guanfacine/therapeutic use; Methylphenidate; Methylphenidate/therapeutic use; Randomized Controlled Trials as Topic; Selegiline; Tic Disorders; Tic Disorders/complications; Tic Disorders/drug therapy
Plain language summary
Medications for attention deficit hyperactivity disorder (ADHD) in children with tics
Review question
In children with ADHD and tics, how do medications for ADHD affect symptoms of both disorders?
Background
As many as half of all children with tic disorders (a combination of repetitive motions vocalizations), also have ADHD (issues with hyperactivity, impulsivity and maintaining attention). Symptoms of ADHD are often more disabling for children than their tics. Historically, the reported ability of stimulant medications to worsen tics has limited their use in children who have both a chronic tic disorder (lasting over a year since the first tic onset) and ADHD. To evaluate evidence for this reported phenomenon, we searched for clinical trials of medications for ADHD used specifically in children with tic disorders.
Search date
The evidence is current to September 2017.
Study characteristics
We included eight studies with 510 participants (443 boys, 67 girls) in our review. Participants in these studies were children with both ADHD and a chronic tic disorder. The included studies evaluated several different medications for ADHD, including stimulants (methylphenidate, dextroamphetamine) and non‐stimulants (clonidine, guanfacine, desipramine, atomoxetine, and deprenyl). All studies took place in the USA and ranged from three to 22 weeks in duration.
Study funding sources
Five of the eight studies were funded by charitable organizations or government agencies, or both. One study was funded by the drug manufacturer. The other two studies did not specify the source of funding for the study.
Key results
The trials in this review suggested that several stimulant and non‐stimulant medications may improve ADHD symptoms in children with ADHD and tics. At high doses, dextroamphetamine may initially worsen tics in some children, and dose increases of both dextroamphetamine and methylphenidate may be limited due to tic exacerbation. However, for most children, both tics and ADHD symptoms can improve with use of stimulant medications.
Quality of evidence
There is low‐quality evidence for methylphenidate, atomoxetine, and clonidine, and very low‐quality evidence for desipramine, dextroamphetamine, guanfacine, and deprenyl in the treatment of ADHD in children with tics. The evidence was limited by the small number of trials, small number of participants, and risk of bias of the included studies.
Summary of findings
Summary of findings for the main comparison. Methylphenidate compared with placebo for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders.
| Methylphenidate compared with placebo for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders | ||||
|
Patient or population: children with ADHD and comorbid tic disorders Intervention: methylphenidate Comparison: placebo | ||||
| Outcomes | Effect of treatment | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
ADHD symptom‐related behavior Measured by standardized rating scales: Conners' Abbreviated Teacher Rating Scale, Conners' Abbreviated Parent Rating Scale, IOWA Conners' Teacher Rating Scale, Mothers' Objective Method for Subgrouping, Continuous Performance Task, Conners' Teacher Rating Scale, Conners' Continuous Performance Task |
Tourette's Syndrome Study Group 2002 showed a significant treatment effect using the Conners' Abbreviated Teacher Rating Scale (3.3 points, 98.3% CI −0.2 to 6.8; P = 0.02). | 229 (3 studies) | ⊕⊕⊝⊝ Lowa | – |
| Gadow 2007 showed that all doses (0.1 mg/kg, 0.3 mg/kg, 0.5 mg/kg) of methylphenidate were superior to placebo on all rating scales (Conners' Abbreviated Teacher/Parent Rating Scale, IOWA Conners' Teacher Rating Scale, Mothers' Objective Method for Subgrouping, Continuous Performance Test), with a dose‐dependent effect (F = 24.7; P = 0.001) | ||||
| Castellanos 1997 showed significantly decreased hyperactivity at all doses (15 mg, 25 mg, 45 mg). | ||||
|
Tic severity Measured by standardized rating scales: Yale Global Tic Severity Scale, Tourette Syndrome Severity Scale, Tourette Syndrome Clinical Global Impression Scale, Global Tic Rating Scale, 2‐Minute Tic and Habit Count, Tic Symptom Self‐Report |
Tourette's Syndrome Study Group 2002 found a significant treatment effect using the Yale Global Tic Severity Scale (11.0 points, 98.3% CI 2.1 to 19.8; P = 0.003). | 229 (3 studies) | ⊕⊕⊝⊝ Lowa | – |
| Gadow 2007 found no difference on the Yale Global Tic Severity Scale but found an improvement in tic severity at all doses (0.1 mg/kg, 0.3 mg/kg, 0.5 mg/kg) on the Global Tic Rating Scale completed by teachers (F = 5.33; P = 0.002) | ||||
| Castellanos 1997 found no effect of drug on tic severity for second and third cohorts. Tic severity was significantly greater during week 2 in the first cohort (P < 0.01) | ||||
| ADHD: attention deficit hyperactivity disorder; CI: confidence interval. | ||||
| GRADE Working Group grades of evidence High quality: we are very confidence that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded two levels due to limitations in study design and implementation, and imprecision of results.
Summary of findings 2. Clonidine compared with placebo for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders.
| Clonidine compared with placebo for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders | ||||
|
Patient or population: children with ADHD and comorbid tic disorders Intervention: clonidine Comparison: placebo | ||||
| Outcomes | Effect of treatment | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
ADHD symptom‐related behavior Measured by standardized rating scales: Conners' Abbreviated Teacher Rating Scale, Conners' Abbreviated Parent Rating Scale, IOWA Conners' Teacher Rating Scale, Conners' Continuous Performance Task, Child Behaviour Checklist, Gordon Diagnostic System, Clinical Evaluation of Language Function, Matching Familial Figures Test, Porteus Maze Test, Restricted Academic Test |
Tourette's Syndrome Study Group 2002 found a significant treatment effect using the Conners' Abbreviated Teacher Rating Scale (3.3 points, 98.3% CI −0.2 to 6.8; P = 0.02). | 170 (2 studies) | ⊕⊕⊝⊝ Lowa | – |
| Singer 1995 found no significant difference on any ADHD outcome measures, except the nervous/overactive subscale of the Child Behaviour Checklist (boys aged 6‐11 years). | ||||
|
Tic severity Measured by standardized rating scales: Yale Global Tic Severity Scale, Tourette Syndrome Severity Scale, Global Tic Rating Scale, Tic Symptom Self‐Report, Hopkins Motor/Vocal Scale |
Tourette's Syndrome Study Group 2002 showed a significant treatment effect using the Yale Global Tic Severity Scale (10.9 points, 98.3% CI 2.1 to 19.7; P = 0.003). | 170 (2 studies) | ⊕⊕⊝⊝ Lowa | – |
| Singer 1995 found no significant difference on measures of tic severity. | ||||
| ADHD: attention deficit hyperactivity disorder; CI: confidence interval. | ||||
| GRADE Working Group grades of evidence High quality: we are very confidence that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded two levels due to limitations in study design and implementation, and imprecision of results.
Summary of findings 3. Desipramine compared with placebo for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders.
| Desipramine compared with placebo for attention deficit hyperactivity disorder (ADHD) in children with comorbid tic disorders | ||||
|
Patient or population: children with ADHD and comorbid tic disorders Intervention: desipramine Comparison: placebo | ||||
| Outcomes | Effect of treatment | Number of participants (studies) | Quality of the evidence (GRADE) | Comments |
|
ADHD symptom‐related behavior Measured by standardized rating scales: Child Behaviour Checklist, Gordon Diagnostic System, Clinical Evaluation of Language Function, Matching Familial Figures Test, Porteus Maze Test, Restricted Academic Test, ADHD Rating Scale IV ‐ Parent Version; ADHD Parent Linear Analogue Scale |
Spencer 2002 showed a decrease in scores on the ADHD Rating Scale IV ‐ Parent Version (week 0 = 46 (SD 5.9) points; week 6 = 24 (SD 12) points; P < 0.001). | 75 (2 studies) | ⊕⊝⊝⊝ Very lowa | – |
| Singer 1995 showed that desipramine was superior to placebo on the Parent Linear Analogue Scale for Hyperactivity (desipramine: 32.8 (SD 1.3) points; placebo: 64.4 (SD 0.6) points; P < 0.05). Hyperactivity subscale of the Child Behavior Checklist showed drug effects for males aged 6 to 11 years (desipramine: 68.6 (SD 1.4) points; placebo: 75.8 (SD 1.0) points; P < 0.05). | ||||
|
Tic severity Measured by standardized rating scales: Yale Global Tic Severity Scale, Tourette Syndrome Severity Scale, Hopkins Motor/Vocal Scale; ADHD Parent Linear Analogue Scale |
Spencer 2002 showed a decrease in scores on the Yale Global Tic Severity Scale (week 0 = 63 (SD 18) points; week 6: 43 (SD 23) points; P < 0.001). | 75 (2 studies) | ⊕⊝⊝⊝ Very lowa | – |
| Singer 1995 showed that desipramine was superior to placebo on the Parent Linear Analogue Scale of tic severity (desipramine: 30.0 (SD 0.7) points; placebo: 47.4 SD 1.8 points; P < 0.05). There were no differences on the other measures of tic severity (Tourette Syndrome Severity Scale, Hopkins Motor/vocal scale, Yale Global Tic Severity Scale). | ||||
| ADHD: attention deficit hyperactivity disorder; SD: standard deviation. | ||||
| GRADE Working Group grades of evidence High quality: we are very confidence that the true effect lies close to that of the estimate of the effect. Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||
aDowngraded two levels due to limitations in study design and implementation, and imprecision of results.
Background
Description of the condition
The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, currently recognizes three chronic tic disorders: Tourette syndrome, chronic motor tic disorder, and chronic vocal tic disorder (APA 2013). Tourette syndrome consists of multiple motor tics and one or more vocal tics that have persisted for longer than one year. In both chronic motor tic disorder and chronic vocal disorder, tics must also persist for longer than one year but the spectrum of tics is limited to either motor or vocal subtypes, respectively. Together these disorders affect just over 2% of children (Knight 2012). They fall within a clinical spectrum, with a presumed shared underlying neurobiology.
Chronic tic disorders are frequently complicated by the presence of comorbid psychiatric disorders, of which attention deficit hyperactivity disorder (ADHD) is one of the most prevalent (Martino 2013). The clinical implications of a comorbid diagnosis of ADHD in children with tic disorders are significant. The risk for aggressive and delinquent behavior, and conduct difficulties in children with tic disorders is posed largely by the presence of ADHD (Cavanna 2009), and the greatest independent predictor of psychosocial quality of life in children with tic disorders is ADHD symptom severity (Pringsheim 2007). In contrast, the presence of a tic disorder has limited impact on ADHD outcomes (Spencer 2001).
Rates of association between tic disorders and ADHD are much higher than would be expected due to chance alone. Kurlan 2002 used direct interviews in a community‐based study of school children to determine the prevalence of tic disorders and any comorbid psychopathology. They included 1596 children aged nine to 17 years from 10 New York state school districts over a four‐year period. In this study, 38% of children with tics had a comorbid diagnosis of ADHD. Clinic‐based studies yield even higher rates of comorbid ADHD. In a review of a multi‐site, international database of 3500 people with tic disorders, Freeman 2000 reported that 60% of children with tic disorders also had ADHD, with a range of 33% to 91% among sites reporting more than 50 cases.
The association between tic disorders and ADHD is a compelling one and a number of investigators have proposed that the disorders share a common pathophysiology, reflecting alterations in noradrenergic and dopaminergic transmission inadequately modulating corticostriatal circuits, and thus failing to inhibit intrusive thoughts, sensory input, and motor responses (Steeves 2008). Neurochemical models based on dopaminergic and noradrenergic dysfunction have likewise guided considerations for their treatment.
Description of the intervention
Medications most commonly used to treat ADHD symptoms include the stimulants methylphenidate and amphetamine, followed by non‐stimulants (such as atomoxetine), tricyclic antidepressants, and alpha agonists (Wilens 2006). Due to the impact of ADHD on the child with a tic disorder, treatment for ADHD symptoms is a greater priority than medical treatment for tics. For many decades, clinicians were reluctant to use stimulants to treat symptoms of ADHD in children with tics for fear of worsening the tics. In the 1970s and early 1980s, several case reports and small case series were published of children who experienced the onset or worsening of tics after the use of stimulants for the treatment of ADHD (Golden 1974; Lowe 1982). Despite new evidence that suggests that this relationship was temporal and not causal (Tourette's Syndrome Study Group 2002), package inserts and advertising for US Food and Drug Administration‐approved stimulants for ADHD continue to include warnings against the use of these medications for children with comorbid tic disorders.
How the intervention might work
There appears to be some commonality in the mechanisms of action of medications used for ADHD that may be related to either direct or indirect modulation of dopamine and norepinephrine neurotransmission. Stimulants block the reuptake of dopamine and norepinephrine into the presynaptic neuron (methylphenidate) or increase the release of these monoamines into the extraneuronal space (amphetamines) (Seiden 1993). Atomoxetine specifically inhibits presynaptic reuptake of norepinephrine, resulting in increased norepinephrine levels in the synapse (Bymaster 2002). The efficacy of tricyclic antidepressants in the treatment of ADHD is likewise thought to be mediated by their action on catecholamine reuptake, particularly on norepinephrine. The alpha‐2 adrenergic agonists appear to alter basal adrenergic tone (Buccafusco 1992). Clonidine stimulates alpha‐2A, ‐2B, and ‐2C receptors, while guanfacine is selective for alpha‐2A receptors. Alpha‐2A and ‐2C receptors are mainly found in the central nervous system, while the alpha‐2B receptors are in vascular smooth muscle. Alpha‐2 agonism inhibits norepinephrine release from the presynaptic neuron, resulting in a reduction of activity in noradrenergic pathways and an attenuation of the sympathetic stress response.
Why it is important to do this review
Given the high frequency of comorbidity of chronic tic disorders and ADHD, the effects of ADHD symptoms on psychosocial quality of life in people with tics, and the concern amongst clinicians about the potential of worsening tics with use of stimulant medications, an up‐to‐date systematic review of pharmacological treatments of ADHD in children with tics is needed. We synthesized the existing evidence for clinicians serving this patient population on the efficacy of these agents for treatment of symptoms of ADHD and their effect on tics. While physicians specializing in this area of practice may already be aware of this literature, children and families affected by ADHD and comorbid tic disorders frequently have concerns about the use of medications and potential worsening of the symptoms of either condition, and often seek advice on these points.
Objectives
To assess the effects of pharmacological treatments for ADHD in children with comorbid tic disorders on symptoms of ADHD and tics.
Methods
Criteria for considering studies for this review
Types of studies
Randomized, double‐blind, controlled trials of any pharmacological treatment for ADHD used specifically in children with comorbid tic disorders. The term double‐blind implies that trial participants, clinicians, and outcome assessors were blinded to treatment allocation. We included both parallel‐group and cross‐over study designs.
Types of participants
Children aged 18 years or younger with a clinical diagnosis of ADHD and a chronic tic disorder (Tourette syndrome, chronic motor tic disorder, or chronic vocal tic disorder). With respect to diagnostic classification systems, acceptable ADHD diagnoses included:
Types of interventions
Any pharmacological treatment for ADHD (stimulant and non‐stimulant), at any dose, taken orally alone or in combination with another drug, compared to placebo.
Types of outcome measures
Primary outcomes
-
ADHD and tic symptom severity measured by validated clinician, teacher, or parent report scales. Specifically, we evaluated:
ADHD symptom‐related behavior in the home setting assessed with, for example, Conners Abbreviated Symptom Questionnaire (ASQ) for Parents (Conners 1990), or ADHD Rating Scale (DuPaul 2016);
ADHD symptom‐related behavior in the school setting assessed with, for example, Conners ASQ for Teachers (Conners 1990); and
tic severity assessed with, for example, Yale Global Tic Severity Scale (TGTSS) (Leckman 1989), Tic Symptom Self‐Report Scale (Leckman 1998), or the Tourette Syndrome Severity Scale (Shapiro 1988).
Secondary outcomes
-
Adverse effects, including:
cardiovascular effects such as changes in heart rate, blood pressure, or the electrocardiogram; and
weight changes.
Search methods for identification of studies
We ran searches for this update in June 2016 and again on 20 September 2017. Search strategies for each database are reported in Appendix 1. Search strategies from the previous version of this review are in Appendix 2.
Electronic searches
For this update, we searched the electronic databases and trial registers listed below. Some new databases have been added since the previous review and others were no longer available to us. These changes are reported in Differences between protocol and review.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 8), in the Cochrane Library, and which includes the Cochrane Developmental, Psychosocial and Learning Problem Specialised Register (searched 20 September 2017).
MEDLINE Ovid (1946 to September week 1 2017).
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid (searched 20 September 2017).
MEDLINE E‐pub Ahead of Print Ovid (searched 20 September 2017).
Embase Ovid (1974 to 19 September 2017).
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 20 September 2017).
PsycINFO Ovid (1806 to September week 2 2017).
Science Citation Index ‐ Expanded Web of Science (SCI; 1970 to 19 September 2017).
Social Sciences Citation Index Web of Science (SSCI; 1970 to 19 September 2017).
Conference Proceedings Citation Index ‐ Science Web of Science (1990 to 19 September 2017).
Conference Proceedings Citation Index ‐ Social Science and Humanities Web of Science (1990 to 19 September 2017).
Cochrane Database of Systemic Reviews (CDSR; 2017, Issue 9) part of the Cochrane Library (searched 20 September 2017).
Database of Abstracts and Reviews of Effects (DARE; 2015, Issue 2. Final Issue) part of the Cochrane Library (searched 6 July 2016).
Epistemonikos (www.epistemonikos.org; searched 20 September 2017).
WorldCat (www.worldcat.org; searched 20 September 2017).
ClinicalTrials.gov (clinicaltrials.gov; searched 20 September 2017).
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en; searched 20 September 2017).
Searching other resources
We contacted the Tourette Syndrome Study Group (based in North America) and other experts in the field, including investigators from all review articles and primary studies identified through searches for this review, to determine if there were any ongoing trials or unpublished results in this area.
Data collection and analysis
Selection of studies
Two review authors (TP and TS) independently assessed titles and abstracts of references retrieved from the searches and selected all potentially relevant studies. Next, they retrieved copies of these articles, which they read in detail and assessed for eligibility (Criteria for considering studies for this review). We resolved any disputes regarding the fulfilment of inclusion criteria by discussion. Review authors were not blinded to the names of the trial authors, institutions, or journals of publication.
We recorded our decisions in a study flow diagram (Moher 2009).
Data extraction and management
Two review authors (TP and TS) independently extracted the following data from each included study and entered it into predesigned summary forms.
Study procedures, including recruitment, diagnosis, medication, dosage, duration, and clinical setting.
Study design (i.e. randomized or quasi‐randomized).
Randomization method.
Method of allocation concealment.
Method of blinding.
Inclusion and exclusion criteria for participants.
Number of participants (total/per group).
Age distribution.
Gender.
Loss of follow‐up.
Premature discontinuation of study, and reasons for discontinuation.
Outcome.
Method of analysis (intention‐to‐treat, per protocol).
Comparability of groups at baseline.
They then compared the extracted data to ensure accuracy. Next, one review author (TP) entered the data into Review Manager 5 (RevMan 5) (Review Manager 2014), and a second review author (TS) checked them. We resolved discrepancies by consensus. As no further studies were included following the July 2016 search, this process was not repeated.
Assessment of risk of bias in included studies
Two review authors (TP and TS) independently assessed the risk of bias in the included studies for each of the seven domains in Appendix 3, and assigned ratings of low risk of bias, high risk of bias, and unclear risk of bias (Higgins 2017). We resolved any discrepancies by consensus.
Measures of treatment effect
Due to significant clinical heterogeneity (differences between studies in the interventions used and how they were administered, and differences in outcomes measured), and incomplete reporting of the results from cross‐over trials, we were unable to conduct a meta‐analysis of study data. We attempted to obtain additional information from authors, but, due to the length of time since many of the trials were performed, this information was either not available or our queries went unanswered. Thus, we have presented the results for studies individually. We recorded the methods that were not pertinent to this version of the review in Appendix 4, for use in an update. See our protocol (Pringsheim 2009).
Unit of analysis issues
Unit‐of‐analysis issues occurred in this review as none of the included cross‐over studies presented paired data for analysis but rather provided the means and standard deviations (SD) for each treatment type. See Appendix 4 and Pringsheim 2009.
Dealing with missing data
We attempted to obtain missing information directly from study authors; however, this did not yield the missing information. We contacted Castellanos regarding additional results from his trial (Castellanos 1997), but, due to the long period which had elapsed since his study was performed, the information was no longer accessible. We also contacted the authors of Tourette's Syndrome Study Group 2002 and Feigin 1996 requesting more information on results but received no replies to our letters. See Appendix 4 and Pringsheim 2009.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the distribution of important participant factors between trials and methodological heterogeneity by comparing trial designs. See also Appendix 4 and Pringsheim 2009.
Assessment of reporting biases
As there was an insufficient number of studies found for each treatment type, we did not create funnel plots to assess publication bias. See Appendix 4 and Pringsheim 2009.
Data synthesis
We did not synthesize results in a meta‐analysis by treatment type because of important clinical heterogeneity and unit‐of‐analysis issues (see the Results section for descriptions of the nature of the heterogeneity encountered). Thus, we have presented the data for each study individually. See Appendix 4 and Pringsheim 2009.
'Summary of findings' table
We created 'Summary of findings' tables using Review Manager 2014, which report the effect estimate for our primary outcomes (ADHD and tic symptom severity) measured at study endpoint, the number of participants, and our ratings for the quality of the evidence. Two review authors (TP and SO) used the GRADE method to assess the quality of the body of evidence for each outcome. While randomized trials are considered high‐quality evidence, we downgraded the level of evidence to moderate or low for all outcomes due to the presence of limitations in the design of available studies, and the overall small number of studies and participants included in studies in this area. This means it is likely that further research could have an important impact on our confidence in the estimate of the effect and may change the estimate.
Subgroup analysis and investigation of heterogeneity
We did not perform a subgroup analysis. See Appendix 4 and Pringsheim 2009.
Sensitivity analysis
We did not perform a sensitivity analysis due to the small number of trials and the inability to conduct a meta‐analysis. See Appendix 4 and Pringsheim 2009.
Results
Description of studies
Results of the search
In the original review, we found 599 citations, of which 21 qualified for further review. Of these, eight met our inclusion criteria (Criteria for considering studies for this review). Of the remaining 13, eight were republications of data already presented in the eight studies included in the review and were listed as secondary publications. The remaining five studies were excluded with reasons (see Excluded studies).
For this update, our search yielded 508 records, once duplicates were removed. We excluded 507 records at title and abstract stage, and retrieved one full‐text report for further inspection. This was subsequently excluded due to lack of double‐blinding (see Excluded studies).
The study review process is summarized in a PRISMA flow diagram (Figure 1).
1.
Study flow diagram illustrating the process for inclusion of studies.
Included studies
See Characteristics of included studies tables.
Study designs
We included eight randomized controlled trials (RCTs) of pharmacological treatments for ADHD in children with comorbid tic disorders (Allen 2005; Castellanos 1997; Feigin 1996; Gadow 2007; Scahill 2001; Singer 1995; Spencer 2002; Tourette's Syndrome Study Group 2002), four of which were cross‐over trials (Castellanos 1997; Feigin 1996; Gadow 2007; Singer 1995).
Location
All studies took place in the USA (Allen 2005; Castellanos 1997; Feigin 1996; Gadow 2007; Scahill 2001; Singer 1995; Spencer 2002; Tourette's Syndrome Study Group 2002).
Participants
The studies included 510 children (443 boys, 67 girls) with diagnoses of ADHD and Tourette syndrome or chronic motor or vocal tic disorder. Sample sizes ranged from 22 in Castellanos 1997 to 148 in Allen 2005.
Interventions
Three trials assessed multiple agents (Castellanos 1997; Singer 1995; Tourette's Syndrome Study Group 2002), and ranged in duration from three (Castellanos 1997) to 22 weeks (Singer 1995).
Castellanos 1997 was a complex placebo‐controlled, cross‐over study, which randomized 22 children into three cohorts, each sequentially receiving for three weeks either placebo or one of three different dosage titrations of methylphenidate (low: 15 mg, medium: 25 mg, high: 45 mg), and one of three different dosage titrations of dextroamphetamine (low: 7.5 mg, medium: 15 mg, high: 22.5 mg).
Singer 1995 was a placebo‐controlled, three‐phase cross‐over study in 34 children, of clonidine, desipramine, and placebo. Each treatment was taken four times daily for six weeks, separated by a one‐week washout period.
Tourette's Syndrome Study Group 2002 was a parallel‐group trial, which randomized 136 children to a flexible dose of methylphenidate (37 children), clonidine (34 children), clonidine plus methylphenidate (33 children), or placebo (32 children) for 16 weeks each (see Table 4).
1. Comparisons.
| Comparisons | Trial(s) |
| Methylphenidate versus placebo |
Castellanos 1997 Gadow 2007 Tourette's Syndrome Study Group 2002 |
| Clonidine versus placebo |
Singer 1995 Tourette's Syndrome Study Group 2002 |
| Methylphenidate plus clonidine versus placebo | Tourette's Syndrome Study Group 2002 |
| Dextroamphetamine versus placebo | Castellanos 1997 |
| Guanfacine versus placebo | Scahill 2001 |
| Atomoxetine versus placebo | Allen 2005 |
| Desipramine versus placebo |
Singer 1995 Spencer 2002 |
| Deprenyl versus placebo | Feigin 1996 |
| Desipramine versus clonidine | Singer 1995 |
The remaining five trials assessed single agents.
Allen 2005 assessed atomoxetine 0.5 mg/kg per day to 1.5 mg/kg per day (76 children), a highly selective, non‐stimulant noradrenergic reuptake inhibitor, versus placebo (72 children) in a parallel‐group trial of 18 weeks' duration with 148 children (131 boys, 17 girls). Participants considered to be clinical non‐responders at week 12 of the study were allowed to withdraw early from the double‐blind study and enter an open‐label study of the drug.
Feigin 1996 assessed the effects of deprenyl, a type B monoamine oxidase inhibitor, compared to placebo in 24 children (21 boys, 3 girls). Children were randomized to treatment with deprenyl 5 mg or matching placebo, given twice daily, for eight weeks and then crossed over to the alternate treatment after a six‐week washout period.
Gadow 2007 assessed methylphenidate in a single agent, cross‐over trial with placebo in which 71 children (57 boys, 14 girls) were randomized to three sequential doses of methylphenidate (0.1 mg/kg, 0.3 mg/kg, and 0.5 mg/kg), given twice daily, seven days a week for two weeks each.
Scahill 2001 assessed guanfacine 1.5 mg to 3 mg per day (an alpha‐2 receptor agonist), divided into three daily doses, versus placebo in a parallel‐group trial of eight weeks' duration. The study randomized 34 children (31 boys, 3 girls) to guanfacine or placebo.
Spencer 2002 compared desipramine versus placebo in 41 children (34 boys, 7 girls) in a parallel‐group trial in which desipramine was titrated weekly up to 3.5 mg/kg per day and given twice daily for six weeks.
Outcome measures
All trials included both ADHD and tic outcomes. Most trials did not specify a primary outcome. The scales chosen to measure ADHD severity varied considerably between studies (Table 5). All studies used the YGTSS for one measure of tic severity. Table 6 lists other measures of tic severity that the studies used. A description of each scales' items and scoring is included in Table 7.
2. Attention deficit hyperactivity disorder symptom severity scales used in this review.
| Scale/measure | Allen 2005 | Castellanos 1997 | Feigin 1996 | Gadow 2007 | Scahill 2001 | Singer 1995 | Spencer 2002 | Tourette's Syndrome Study Group 2002 |
| Conners Abbreviated Teacher Rating Scale | – | – | – | Yes | – | – | – | Yes |
| Conners Abbreviated Parent Rating Scale | – | – | – | Yes | – | – | – | Yes |
| IOWA Conners Teacher Rating Scale | – | – | – | Yes | – | – | – | Yes |
| Mothers' Objective Method for Subgrouping | – | – | – | Yes | – | – | – | – |
| Continuous Performance Task | – | – | – | Yes | Yes | – | – | – |
| ADHD Rating Scale‐IV: Parent Version | Yes | – | – | – | Yes | – | Yes | – |
| Clinical Global Impression Scale – Overall – Severity | Yes | – | – | – | Yes | – | – | – |
| Clinical Global Impression Scale – ADHD/Psychiatric Symptoms | Yes | – | – | – | – | – | – | – |
| ADHD Teacher 39‐Item Conners Rating Scale | – | Yes | – | – | – | – | – | – |
| DuPaul ADHD Scale | – | – | Yes | – | – | – | – | – |
| Parent Conners Questionnaire Hyperactivity Index | – | – | – | – | Yes | – | – | – |
| Child Behaviour Checklist | – | – | – | – | – | Yes | – | – |
| Gordon Diagnostic System | – | – | – | – | – | Yes | – | – |
| Clinical Evaluation of Language Function | – | – | – | – | – | Yes | – | – |
| Matching Familial Figures Test | – | – | – | – | – | Yes | – | – |
| Porteus Maze Test | – | – | – | – | – | Yes | – | – |
| Restricted Academic Test | – | – | – | – | – | Yes | – | – |
| Conners Continuous Performance Task | – | – | – | – | – | – | – | Yes |
ADHD: attention deficit hyperactivity disorder; IOWA: inattention/overactivity with aggression.
3. Tic severity symptom scales used in this review.
| Scale/measure | Allen 2005 | Castellanos 1997 | Feigin 1996 | Gadow 2007 | Scahill 2001 | Singer 1995 | Spencer 2002 | Tourette's Syndrome Study Group 2002 |
| Yale Global Tic Severity Scale |
Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Tourette Syndrome Severity Scale |
– | – | – | Yes | – | Yes | – | – |
| Tourette Syndrome Clinical Global Improvement |
Yes | – | – | Yes | – | – | – | – |
| Global Tic Rating Scale |
– | – | – | Yes | – | – | – | Yes |
| 2‐Minute Tic and Habit Count |
– | – | – | Yes | – | – | – | – |
| Tic Symptom Self‐Report | Yes | – | – | – | – | – | – | Yes |
| Goetz Tic Severity Scale |
– | – | Yes | – | – | – | – | – |
| Hopkins Motor/Vocal Scale | – | – | – | – | – | Yes | – | – |
4. Description of scales used in included studies.
| Scale/measure | Number of items | Scoring |
| Conners' Abbreviated Symptoms Questionnaire for Teachers (ASQ) | 10 items pertaining to the child's behavior | Rated on a 4‐point Likert scale, ranging from 0 (not at all), 1 (just a little), 2 (pretty much) to 3 (very much true), with a possible total score ranging from 0 to 30. Higher scores indicate worse symptoms |
| Yale Global Tic Severity Scale (YGTSS) | 5 items on the number, frequency, intensity, complexity, and interference from motor tics, and 5 items on the number, frequency, intensity, complexity, and interference from vocal tics, and 1 item on overall impairment | The Total Motor Tic Score is derived by adding the 5 motor tics items (each item ranges from 0 to 5, total motor tic score ranges from 0 to 25). The Total Vocal Tic Score is derived by adding the 5 phonic tics items (each item ranges from 0 to 5, total vocal tic ranges from 0 to 25). The Total Tic Score is a summation of the Total Motor Tic and Total Vocal Tic Scores. The Overall Impairment Rating is rated on a 51‐point scale anchored by 0 (no impairment) and 50 (severe impairment). Finally, the Global Severity Score is a summation of the Total Motor Tic Score, Total Vocal Tic Score, and Overall Impairment Rating (range 0 to 100). Higher scores indicate worse symptoms. |
| Global Tic Rating Scale | 9 items, with the first 5 referring to the frequency of motor (3 items) and phonic tics (2 items) according to body region, which are summed to produce motor and phonic tic frequency subscores, respectively | All items are rated on a scale from 0 (never) to 3 (very much). Total score ranges from 0 to 27. Higher scores indicate worse symptoms |
| ADHD Rating Scale IV ‐ Parent Version | 18‐item questionnaire. 9 questions each on inattention and hyperactivity‐impulsivity, where the odd‐numbered items represent the inattention subscale, and the even‐numbered items represent the hyperactive/impulsive subscale | Items coded on 4‐point Likert scale using scores 1 (never or rarely), 2 (sometimes), 3 (often), or 4 (very often). Total score ranges from 18 to 52. Raw scores are converted to percentiles. |
| ADHD Parent Linear Analogue Scale | 10‐cm line on which both the parent and physician separately rank symptoms | The ends of each line represent 0 (no symptoms) and 10 (most severe) |
| Child Behaviour Checklist (CBCL) | 113 items across 8 subscales assessing maladaptive behavioral and emotional problems:
|
Items are coded from 0 to 2, scored 0 (not at all), 1 (somewhat true), or 2 (very true). CBCL profile for each category, with scores below the 95th percentile in the normal range, and above the 98th percentile in the clinical range. Higher scores indicate worse symptoms. |
| Conners' Abbreviated Parent Rating Scale | 48 items across 6 subscales:
|
All items are rated on a scale from 0 (never) to 3 (very much). Total score ranges from 0 to 144. Higher scores indicate worse symptoms |
| IOWA Conners' Teacher Rating Scale | 10 items. Consists of 5‐item subscales designed to assess inattention/overactivity and aggression Inattention/overactivity:
Aggression:
|
Scored 0 (not at all), 1 (just a little), 2 (pretty much), or 3 (very much). Total score ranges from 0 to 30. Higher scores indicate worse symptoms |
| Mothers' Objective Method for Subgrouping | Contains 10 (hyperactivity or ADHD, or both) symptoms arranged in a checklist format. Generates a hyperactivity scale score and an aggression scale score | 1 indicates checked and 0 unchecked. Total score ranges from 0 to 10. Higher scores indicate worse symptoms |
| Continuous Performance Task (CPT) | Computer‐administered and scored measure of sustained visual attention and motor response inhibition. The test takes about 15 minutes to administer and yields measures of omissions, commissions, and reaction time. | Omission errors measure inattention, commission errors measure impulsivity |
| Conners' Teacher Rating Scale | 39 items clustered into 5 factors, including conduct problems, daydreaming, inattention, anxious‐fearful, and hyperactive behavior ADHD Teacher 39‐Item |
All items are rated on a scale from 0 (never) to 3 (very much). Total score ranges from 0 to 137. Raw scores for each scale are converted to T scores, incorporating normative adjustments for age and sex, with scores of at least 70 considered clinically elevated |
| Conners' Continuous Performance Task (CPT) | Visual‐motor task. Respondents must rapidly and accurately hit the space bar after every letter presented except the letter 'X'. Several variables may be derived from the Conners' CPT, including errors of omission and commission, mean hit reaction time (RT), mean hit RT standard error. | Omission errors measure inattention, commission errors measure impulsivity |
| Tourette Syndrome Severity Scale | 5‐item scale
|
Higher scores indicates worse symptoms |
| Tourette Syndrome Clinical Global Impression (CGI) Scale | Observer‐rated scale that measures illness severity (CGI‐S), or global improvement (CGI‐I) | 7‐point scale, with the severity of illness scale (CGI‐S) using a range of responses from 1 (normal) to 7 (among the most severely ill people). CGI‐I scores range from 1 (very much improved) to 7 (very much worse) |
| 2‐Minute Tic and Habit Count | The physician counts separately the number of brief, jerky (i.e. tics) and rhythmic (i.e. stereotypic, habit) movements and vocalizations during quiet conversation in an office setting. | Higher scores indicates worse symptoms |
| Tic Symptom Self‐Report | 40‐item checklist containing 20 motor tic items and 20 phonic tic items | 0–3 scale corresponding with absent (score of 0) to very frequent and forceful (score of 3). Total score ranges from 0 to 120. Higher scores indicate worse symptoms |
| Goetz Tic Severity Scale | Videotape protocol involving a 10‐minute film of people placed in front of a video camera in a quiet room. 2 body views are recorded, full frontal body (far) and head and shoulders only (near), under 2 conditions: relaxed with the examiner in the room, and relaxed with the patient alone in the room 5 domains are rated:
|
0–4 scoring format. For all domains, 0 represents normal function without evidence of tic disability. Higher scores indicate worse symptoms |
| Hopkins Motor/Vocal Scale | Consists of a series of linear analog scales (10 cm) on which both the parent and physician separately rank each tic (motor and vocal) symptom, taking into consideration its frequency, intensity, degree of interference, and impairment | The ends of each line represent 0 (no tics) and 10 (most severe). The line can be subdivided roughly into 4 ranges: mild, moderate, moderately severe, and severe |
| Du Paul ADHD Rating Scale | 14 items, assessing separate factors of inattention and hyperactivity‐impulsivity | Rated on a 0 (normal) to 3 (severe) scale, yielding a total score ranging from 0 to 42. Higher scores indicate worse symptoms |
| Parent Conners' Questionnaire Hyperactivity Index | 10‐item rating scale identifying hyperactive children | Each item is rated from 0 to 3 (range 0–30). Higher scores indicate worse symptoms |
ADHD: attention deficit hyperactivity disorder.
Excluded studies
We excluded six studies from this review. See Characteristics of excluded studies tables.
In the original review, we excluded five studies (Howson 2004; Law 1999; Niederhofer 2003; Nolan 1999; Sallee 1994). We excluded:
one study of iofexidine in children with tic disorders and ADHD, as the manuscript was retracted from the journal due to plagiarism and possible fraudulent results (Niederhofer 2003);
one study of methylphenidate in children with ADHD, which excluded children with severe motor or vocal tics and Tourette syndrome from the study and did not report the effects of methylphenidate on ADHD symptoms (Law 1999);
one study that looked at the effects of stimulant withdrawal on tics in children with ADHD (Nolan 1999), and therefore did not meet the inclusion criteria (Criteria for considering studies for this review);
two studies that assessed the effects of pharmacological treatments on cognition and attention but not the primary outcomes of ADHD or tic severity (Howson 2004; Sallee 1994).
In this update, we excluded one study because it did not utilize a double‐blind protocol (Lyon 2010).
Risk of bias in included studies
See also 'Risk of bias' tables, in the Characteristics of included studies tables, and Figure 2.
2.
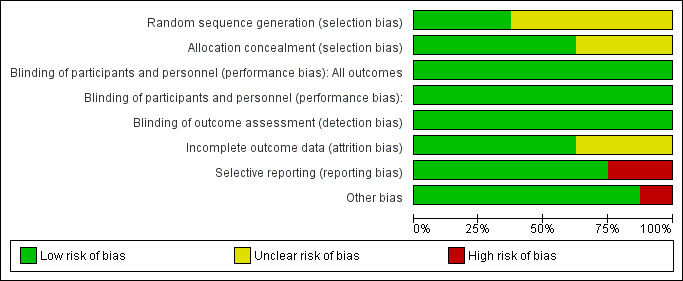
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We judged sequence generation to be at low risk of bias for three studies (Feigin 1996; Spencer 2002; Tourette's Syndrome Study Group 2002). The remaining five studies did not describe sequence generation and we judged these studies at unclear risk of bias on this domain.
Five studies adequately described allocation concealment (Allen 2005; Gadow 2007; Singer 1995; Spencer 2002; Tourette's Syndrome Study Group 2002). The remaining three studies inadequately described allocation concealment, preventing us from making a judgement on whether or not it was adequate.
Blinding
Blinding of participants, clinicians, and outcome assessors were evaluated separately and was a requirement for inclusion. Therefore, all included studies were at low risk of performance and detection bias.
Incomplete outcome data
Three of the eight studies did not adequately addressed incomplete outcome data. Feigin 1996 had a very high dropout of study participants after the first period of the study, especially in the treatment group, and it was unclear if data from participants who dropped out of the study were included in the analysis. Gadow 2007 did not explain how they handled incomplete data sets in the analysis. Castellanos 1997 provided few raw data from study results; the reported only F scores and P values for analysis of variance tests. In addition, all of the above studies, which were cross‐over studies, did not provide paired data for analysis. Rather, each study provided only means and SDs for each treatment type and, with the exception of Castellanos 1997, did not provide original data. The remaining five studies adequately addressed incomplete outcome data.
Selective reporting
Singer 1995 did not provide outcome data for many variables described as collected in the Methods section, only presenting data for those scales showing significant changes. Also, they often reported 'male only' results. Consequently, we judged this study at high risk of reporting bias. Castellanos 1997 was at high risk of bias as very few data were presented on the results of the analysis. We judged the remaining five studies at low risk of reporting bias.
Other potential sources of bias
Allen 2005 had a high rate of early treatment termination at 12 weeks in both treatment groups and thus was at high risk of other bias. None of the other studies appeared to have other potential sources of bias and thus were at low risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3
All treatments, with the exception of deprenyl, were efficacious in treating the symptoms of ADHD. Tic symptoms improved in children treated with methylphenidate, clonidine, methylphenidate plus clonidine, guanfacine and desipramine (see Table 1; Table 2; Table 3). Fear of worsening tics limited dose increases of methylphenidate in one study (Tourette's Syndrome Study Group 2002). High‐dose dextroamphetamine appeared to worsen tics in one study (Castellanos 1997), although the length of this study was only three weeks.
Primary outcomes: attention deficit hyperactivity disorder and tic symptom severity
Methylphenidate
The Tourette's Syndrome Study Group 2002 parallel‐group study randomized children to: clonidine, flexible dose of methylphenidate, clonidine plus methylphenidate, or placebo, for 16 weeks each. The primary outcome was the change from baseline to week 16 in the ADHD Conners ASQ for Teachers; the main secondary outcome was the change from baseline in the YGTSS. On the ASQ, there was a statistically significant treatment effect in comparison to placebo with methylphenidate alone (3.3 points, 98.3% confidence interval (CI) −0.2 to 6.8; P = 0.02, reported in study) and with clonidine plus methylphenidate (6.3 points, 98.3% CI 2.8 to 9.8; P < 0.001). YGTSS scores also improved compared to placebo, with a statistically significant treatment effect observed for methylphenidate alone (11.0 points, 98.3% CI 2.1 to 19.8; P = 0.003), and for methylphenidate plus clonidine (11.0 points, 98.3% CI 2.1 to 19.8; P = 0.003).
The lowest rate of reported adverse effects occurred in the methylphenidate‐only group: 20% of participants treated with methylphenidate reported a worsening of tics as an adverse event compared to 22% receiving placebo (and 26% treated with clonidine alone). Paradoxically, however, tics limited further dosage increases more often for participants assigned to methylphenidate alone (35%) than for participants assigned to methylphenidate plus clonidine (15%), clonidine alone (18%), or placebo (19%).
In the Gadow 2007 cross‐over trial, which randomized children to three different doses of methylphenidate and placebo for two weeks each, the primary outcome was the YGTSS score. Regarding ADHD symptoms, all three doses of methylphenidate were superior to placebo on all rating scales used, including the ASQ. There was a dose‐dependent effect, with methylphenidate 0.5 mg/kg showing superiority to the lower doses of methylphenidate on the ASQ. Mean scores on the ASQ were: 11.6 (SD 6.9) points during placebo treatment; 8.0 (SD 6.0) points with methylphenidate 0.1 mg/kg; 7.3 (SD 5.8) points with methylphenidate 0.3 mg/kg; and 5.7 (SD 5.1) points with methylphenidate 0.5 mg/kg (F = 24.7; P < 0.001).
On the YGTSS, there was no difference in tic severity between treatments with respect to mean total motor, total phonic, impairment, or global severity scores. The teacher ratings on the Global Tic Rating Scale, however, indicated an improvement in tic severity with methylphenidate treatment compared to placebo with all doses (F ratio 5.33; P = 0.002); although one measure, the two‐minute tic/habit count, found an increase in simple motor tics during treatment with methylphenidate 0.3 mg/kg and methylphenidate 0.5 mg/kg compared to placebo (F = 3.96; P = 0.009).
In the Castellanos 1997 cross‐over trial in which children were randomized to three weeks each of methylphenidate, dextroamphetamine, and placebo, methylphenidate significantly decreased hyperactivity at all doses. In the first cohort of 10 participants, analysis of variance of total tic severity showed that tic severity was significantly greater during the second week of methylphenidate treatment (P < 0.01) than during any of the placebo weeks, or during the third week of methylphenidate treatment. In the second and third cohorts of participants, there was no significant main effect of drug on tic severity.
A meta‐analysis of studies evaluating methylphenidate was not possible for several reasons. First, the cross‐over trial by Gadow 2007 presented data as means and SDs for each methylphenidate dosage and placebo, rather than providing a paired analysis. Meta‐analysis of these data, therefore, runs the risk of a unit‐of‐analysis error. Second, the manuscript for Castellanos 1997 only provided F scores from the analysis of variance of tic severity as it related to drug and dosage. No other raw data were provided and, on contacting the study author, these data were no longer available.
Dextroamphetamine
Castellanos 1997 was a placebo‐controlled, cross‐over study of dextroamphetamine, which randomized 20 children into three cohorts. Each child received one of three different dosage titrations of dextroamphetamine to a target dosage of 7.5 mg, 15 mg, or 22.5 mg, twice daily for three weeks. In all cohorts, dextroamphetamine significantly decreased hyperactivity, measured by teachers using the Conners 39‐item Teacher Rating Scale, but there was no significant interaction between drug and dose, indicating that additional improvements in hyperactivity were not observed for higher doses.
In the first cohort of 10 participants, tic severity was significantly greater during the second (15 mg twice daily) and third (22.5 mg twice daily) weeks of dextroamphetamine treatment compared to the weeks on placebo (F = 3.50, 98.3% CI 4 to 36; P = 0.03, reported in study). In the second cohort of six participants, there was no significant main effect of the drug on tic severity. In the third cohort of four participants, there was a trend for tic severity to be greater with dextroamphetamine, but this did not reach statistical significance.
Clonidine
In the Tourette's Syndrome Study Group 2002, in comparison to placebo, there was a statistically significant treatment effect on the ASQ with clonidine alone (3.3 points, 98.3% CI –0.2 to 6.8; P = 0.02), and with clonidine plus methylphenidate (6.3 points, 98.3% CI 2.8 to 9.8; P < 0.0001). YGTSS scores also improved compared to placebo, with a statistically significant treatment effect observed for clonidine alone (10.9 points, 98.3% CI 2.1 to 19.7; P = 0.003), and for clonidine plus methylphenidate (11.0 points, 98.3% CI 2.1 to 19.8; P = 0.003).
Singer 1995 was a three‐arm cross‐over study comparing clonidine 0.05 mg, given four times daily, to placebo and desipramine 25 mg given four times daily. The authors did not define a primary outcome and presented data for only those scales showing significant changes. In this study, clonidine did not show a significant difference compared to either placebo or desipramine on any of the outcome measures of ADHD and tic severity, with the exception of the nervous/overactive subscale of the Child Behavior Checklist in a subgroup of boys aged six to 11 years, in which clonidine was superior to placebo.
We were unable to perform a meta‐analysis of the two studies evaluating clonidine for two reasons. The Tourette's Syndrome Study Group 2002 trial presented data as means and SDs for each treatment type rather than in a paired analysis, creating a risk for a unit‐of‐analysis error. The studies also had significant clinical heterogeneity: duration of treatment of six weeks in Singer 1995 versus 16 weeks for Tourette's Syndrome Study Group 2002. As clinical response times for clonidine can take several months, a difference of 10 weeks of treatment time between studies is likely to be clinically significant.
Guanfacine
Scahill 2001 was an eight‐week, parallel‐group trial of guanfacine versus placebo in 34 children; there was no primary outcome defined. After eight weeks of treatment, guanfacine significantly reduced symptoms of ADHD and tics measured by the ADHD Rating Scale Total Score completed by the teacher (guanfacine: 37.2 (SD 8.4) points at baseline, 23.6 (SD 13.6) points at endpoint; placebo: 34.4 (SD 9.3) points at baseline, 31.7 (SD 11.2) points at endpoint (t = 2.80, df = 32; P < 0.01)), and the YGTSS total tic score (guanfacine: 15.2 (SD 6.6) points at baseline, 10.7 (SD 7.0) points at endpoint; placebo: 15.4 (SD 7.0) points at baseline, 15.4 (SD 5.5) points at endpoint (t = 2.02, df = 30; P = 0.05)).
Atomoxetine
In Allen 2005, a parallel‐group study of atomoxetine in 148 children (0.5 mg/kg/day to 1.5 mg/kg/day), the primary stated objective was to test the hypothesis that atomoxetine does not worsen tics in participants with ADHD and a comorbid tic disorder (i.e. is non‐inferior relative to placebo). The primary efficacy measure was the YGTSS total tic score and secondary assessment measures included the ADHD Rating Scale total score, parent version. With respect to measures of ADHD severity, children in the atomoxetine group had a mean decrease of 10.9 (SD 10.9) points on the ADHD Rating Scale total score compared to a decrease of 4.9 (SD 10.3) points in the placebo group (P = 0.002).
On the primary outcome measure, tic severity measured using the YGTSS, atomoxetine was non‐inferior relative to placebo after 18 weeks of treatment. The lower limit of the one‐sided CI for the difference in mean change between the two treatment groups (placebo and atomoxetine) was 0.27, which, being greater than the prespecified lower limit of –3.7, indicated non‐inferiority. The atomoxetine group showed a greater mean improvement in the YGTSS at the endpoint (–5.5 (SD 6.9) points) compared to placebo (–3.0 (SD 8.7) points), but this difference was not statistically significant (P = 0.06).
Desipramine
Two studies evaluated desipramine in children with ADHD and a chronic tic disorder: the Spencer 2002 parallel‐group study of 41 children, comparing placebo to desipramine titrated weekly up to 3.5 mg/kg/day for six weeks, and the Singer 1995 cross‐over study comparing desipramine to both placebo and clonidine.
Spencer 2002 did not specify a primary outcome, although they assessed ADHD severity using the ADHD rating scale and tic severity with the YGTSS. At week six, compared to baseline, both ADHD symptoms and tic severity significantly improved in children treated with desipramine whereas they did not in children treated with placebo. The ADHD rating scale score decreased from 46 (SD 5.9) points at week zero to 24 (SD 12) points at week six (P < 0.001), and the YGTSS score decreased from 63 (SD 18) points at week zero to 43 (SD 23) points at week six (P < 0.001). There were no changes in measures of anxiety, obsessive‐compulsive behaviors, or depression between desipramine and placebo.
In the study by Singer 1995, with respect to ADHD symptoms, desipramine was superior to placebo (and clonidine) in the Parent Linear Analogue Scale for Hyperactivity (P < 0.05), with a mean score of 32.8 (SD 1.3) points during desipramine treatment compared to 64.4 (SD 0.6) points during placebo treatment (and 51.6 (SD 2.2) during clonidine treatment). The hyperactivity subscale of the Child Behavior Checklist demonstrated a statistically significant drug effect only for boys aged six to 11 years for desipramine compared to both clonidine and placebo treatment (P < 0.05). Hyperactivity subscale scores were 68.6 (SD 1.4) points during desipramine treatment compared to 75.8 (SD 1.0) points during placebo treatment (and 70.7 (SD 1.2) points during clonidine treatment).
With respect to effect on tic severity, desipramine was superior to both placebo and clonidine (P < 0.05) on the Parent Linear Analogue Scale. Mean scores were 30.0 (SD 0.7) points during desipramine treatment compared to 47.4 (SD 1.8) points during placebo treatment (and 41.4 (SD 1.1) points during clonidine treatment). Other measures of tic severity, including the Tourette Syndrome Severity Scale and the YGTSS, did not demonstrate significant differences between treatment groups.
Singer 1995 was a cross‐over trial that presented data as means and SDs for each treatment type, rather than in a paired analysis. We were unable to perform a meta‐analysis of Singer 1995 and Spencer 2002 because of the risk of a unit‐of‐analysis error.
Deprenyl
In the Feigin 1996 cross‐over trial of deprenyl versus placebo, the primary outcome measure for ADHD was the total score on the DuPaul ADHD Scale, and the primary outcome measure for tics was the total score on the YGTSS. The primary analysis revealed no significant improvement on the DuPaul ADHD Scale with deprenyl (mean improvement 1.3 points, 95% CI –2.7 to 5.3; P = 0.50). The YGTSS total score improved by a mean of 9.3 points with deprenyl (95% CI –0.4 to 19.0; P = 0.06), but this was not statistically significant. Nine of the 24 participants dropped out of the study before entering the second treatment period (six who had received deprenyl and three who had received placebo).
Secondary outcomes: adverse effects
Methylphenidate
In Gadow 2007, there were higher levels of somatic symptoms (sleep and appetite problems, headache, stomach upset, dizziness) on the Stimulant Side Effects Checklist during methylphenidate treatment than with placebo (F = 8.1; P < 0.001). Diastolic blood pressure was higher during treatment with methylphenidate 0.5 mg/kg or 0.1 mg/kg compared with placebo; and heart rate was higher during treatment with methylphenidate 0.3 mg/kg and 0.5 mg/kg compared to placebo.
In Castellanos 1997, appetite suppression with transient weight loss occurred in three children during methylphenidate treatment, and initial insomnia occurred in two children with methylphenidate.
Dextroamphetamine
In Castellanos 1997, appetite suppression with transient weight loss occurred in four children on dextroamphetamine. Initial insomnia occurred in 10 children on dextroamphetamine.
Clonidine
In Tourette's Syndrome Study Group 2002, sedation was common in children receiving clonidine, with 48% of the clonidine‐treated participants reporting this adverse effect compared to 14% of participants treated with methylphenidate and 6% with placebo.
In Singer 1995, specific adverse effects were not reported. The authors reported that 15/34 children experienced at least one drug‐related problem during placebo treatment, compared to 28/34 children during clonidine treatment.
Guanfacine
In Scahill 2001, there was no significant difference between the guanfacine and placebo groups in any adverse effects, including laboratory test results, weight, or cardiovascular parameters. One participant in the guanfacine group withdrew at week four of the study due to sedation.
Atomoxetine
In Allen 2005, rates of decreased appetite and nausea were significantly higher in participants treated with atomoxetine than with placebo (decreased appetite: 16% with atomoxetine versus 3% with placebo; P = 0.01; nausea: 16% with atomoxetine versus 1% with placebo; P = 0.002). The atomoxetine group showed a mean decrease of bodyweight at endpoint (–0.9 (SD 1.9) kg) that was significantly different from the weight gain seen in the placebo group (1.6 (SD 2.3) kg). Participants receiving atomoxetine also had a significant increase in heart rate (+8.3 (SD 12.0) beats per minute) compared to the decrease in heart rate seen in the placebo group (–1.2 (SD 12.7) beats per minute). Electrocardiography revealed a decrease in QT interval in the atomoxetine group versus a slight increase in the placebo group.
Desipramine
In Spencer 2002, there were no serious adverse events. Children treated with desipramine had significantly higher rates of appetite suppression (24%) compared to placebo (0%) (P < 0.02). Mild but statistically significant increases in diastolic blood pressure and pulse rate also occurred in the desipramine‐treated participants.
In Singer 1995, specific adverse effects were not reported. The authors stated that 15/34 children reported at least one drug‐related problem during placebo treatment compared to 26/34 children during desipramine treatment.
Deprenyl
In Feigin 1996, adverse events were not reported to have occurred more frequently with treatment with deprenyl than with placebo; however, the authors did not include a description of adverse events by treatment group.
Discussion
Summary of main results
The findings of this review suggest that there are a number of medicines available to treat children with ADHD and comorbid tic disorders. All the agents, with the exception of deprenyl, reported improvements in symptoms of ADHD in children with tic disorders, however, the quality of evidence available was low.
The data from Tourette's Syndrome Study Group 2002 suggest that methylphenidate and clonidine have similar efficacy in treating symptoms of ADHD, and their combination is superior to either treatment alone. These findings may be seen as contrary to clinical experience, which has traditionally proposed that stimulants are more effective in treating ADHD symptoms than alpha agonists (Connor 1999). This unexpected result may have been due to the doses of methylphenidate used in the study which, at 25 mg/day, is lower than doses used in clinical practice for all but very young children. In Singer 1995, desipramine was superior to clonidine for the treatment of ADHD symptoms. However, this trial has limited applicability for two reasons. First, in general, the effects of clonidine may take several months to become apparent and a six‐week trial of this medication would likely be inadequate to evaluate efficacy. Second, desipramine is now used only on rare occasions in children because of concerns about cardiac toxicity (Amitai 2006).
The three studies of methylphenidate suggested that this drug does not worsen tics in the majority of children. It should be noted that the dose of methylphenidate in Tourette's Syndrome Study Group 2002 was on the lower end of what is used in clinical practice (mean 26 mg daily). There has been only one study of dextroamphetamine on tic symptoms. Castellanos 1997 found worsening of tics during the second (15 mg twice daily) and third (22.5 mg twice daily) weeks of dextroamphetamine treatment compared to the weeks on placebo. As treatment was for only three weeks, it is not clear if worsening of tic symptoms would have resolved over time. The dosages used in this study are on the high end of what is used in clinical practice. There was no further clinical benefit for ADHD symptoms with the higher doses of dextroamphetamine compared to the lower dosage used (7.5 mg twice daily), which did not worsen tics. The result from this single study suggested that the lower dosage of dextroamphetamine could be considered when treating children with comorbid ADHD and tics.
Tics do not appear to worsen with alpha agonists, and the majority of studies reported an improvement in tic severity with this class of drug. Tics significantly improved in both trials studying desipramine (Singer 1995; Spencer 2002); however, safety concerns have meant that it is no longer routinely used in children (Riddle 1993). The effect of atomoxetine on tic severity was non‐inferior to placebo (Allen 2005).
Overall completeness and applicability of evidence
Overall, there was a small number of trials assessing pharmacological treatments for ADHD in children with tic disorders. There are some gaps in the current available evidence, with no trials of long‐acting stimulants in children with tic disorders or trials using a number of newer ADHD treatments (see Implications for research). As ADHD and tic disorders frequently occur together, the evidence is applicable clinically. The evidence is limited by the short duration of the majority of trials.
Quality of the evidence
Important methodological limitations reduced the impact of most of the trials included in this review, with the body of evidence considered low for methylphenidate, atomoxetine, and clonidine, and very low for desipramine, dextroamphetamine, guanfacine, and deprenyl. Many of the trials were small, selective outcome reporting was occasionally an issue, and reporting of results from cross‐over trials was generally poor with no studies presenting paired data for analysis.
Potential biases in the review process
We did not identify any potential biases in the review process.
Agreements and disagreements with other studies or reviews
Bloch 2009 conducted a meta‐analysis on the treatment of ADHD in children with comorbid tic disorders. Their study included the same trials as those reported in our review but they double counted one trial as two, mistaking a separate report, Gadow 2007, as an additional trial. They have drawn similar conclusions to our systematic review but elected to perform a meta‐analysis of the data, despite the degree of clinical heterogeneity and the unit‐of‐analysis issues identified above, which, in our view, would preclude such an analysis. Additionally, Cohen 2015 conducted a meta‐analysis of RCTs of psychostimulants for ADHD, focusing specifically on data related to the onset of tics reported in these trials. They concluded that the use of psychostimulants was not associated with new onset of, or increase in, the severity of pre‐existing tics. Their study included 22 trials, none of which are included in our review, and their findings supported our conclusions.
Authors' conclusions
Implications for practice.
Drugs of the stimulant class have generally been thought to provide the most reliable and robust treatment responses for symptoms of ADHD in children with tics. Given the methodological difficulties inherent in comparing effect sizes across studies with divergent inclusion criteria, efficacy measures, and designs, this review can provide no evidence‐based recommendations for choosing between treatment options. Stimulants will likely continue to be considered as first‐line treatment for children with moderate‐to‐severe symptoms of ADHD in children with tic disorders. Although, overall, stimulants have not been shown to worsen tics in most participants with tic disorders, they may still exacerbate tics in individual cases. In these instances, treatment with alpha agonists or atomoxetine could be considered as alternatives. Although there is evidence that desipramine may improve both tics and ADHD in children, safety concerns will likely continue to limit its use.
Implications for research.
This study highlights the need for adherence to minimal common standards of study design and efficacy measures of pharmacological treatments of tics and ADHD for different agents to enable subsequent comparison of results. A variety of newer agents appear to improve symptoms of ADHD and therefore are candidates for double‐blind, placebo‐controlled, head‐to‐head trials in children with tic disorders and ADHD. These agents include: lisdexamfetamine, a long‐acting stimulant prodrug (Biederman 2007); the newer antidepressant bupropion, a dual norepinephrine and dopamine reuptake inhibitor (Conners 1996); the novel non‐stimulant, modafinil, an agent with both dopaminergic and postsynaptic alpha‐1 adrenergic effects (Kahbazi 2009); and nicotine, which enhances noradrenergic and dopaminergic transmission (Potter 2004; Shytle 2002).
Larger, confirmatory studies with the existing agents used for treatment of ADHD would be desirable, including studies of guanfacine and clonidine, as well as studies of the established stimulants in the longer‐acting forms that are now more commonly used, and the long‐acting form of guanfacine. Longer‐term evaluation of dextroamphetamine would also be warranted given ongoing concerns over its ability to exacerbate tics in a small number of participants.
The agents used to treat isolated ADHD may improve ADHD symptoms in participants with comorbid tic disorders. This suggests that the pathophysiology of ADHD may be similar in both groups. Each of the agents reviewed in this study has a different mechanism of action or has preferential effects on different receptor subtypes. A more detailed analysis of the interaction between the specific mechanism of action of each agent and its symptomatic effects on ADHD and tics may provide further insights into the underlying pathophysiology of both conditions.
What's new
| Date | Event | Description |
|---|---|---|
| 20 September 2017 | New search has been performed | Updated following a new search conducted in July 2016 and September 2017. |
| 29 September 2016 | New citation required but conclusions have not changed | Following review of the abstracts for the new citations obtained, which was conducted by TP and SO, it was determined that none of the new citations matched inclusion criteria and thus the conclusions remain unchanged. Formatting was updated to match new review criteria. |
Acknowledgements
Tamara Pringsheim acknowledges the Departments of Clinical Neurosciences and Pediatrics at the University of Calgary for their support; Cochrane Developmental, Psychosocial and Learning Problems (CDPLP) for their assistance; and the external reviewers of the manuscript.
Appendices
Appendix 1. Search strategies from 2009 onwards
Cochrane Central Register of Controlled Trials (CENTRAL)
CENTRAL (2017, Issue 8), searched 20 September 2017 (21 records) CENTRAL (2016, Issue 6), searched 6 July 2016 (47 records)
#1[mh ^"attention deficit and disruptive behavior disorders"] #2[mh " attention deficit disorder with hyperactivity"] #3[mh "conduct disorder"] #4(ADHD or ADDH or ADHS or "AD/HD" or HKD or TDAH) #5((attention* or behav*) near/3 (defic* or dysfunc* or disorder*)) #6((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)) #7(impulsiv* or inattentiv* or inattention*) #8[mh hyperkinesis] #9(hyperkin* or hyper next kin*) #10(minimal* near/3 brain near/3 (disorder* or dysfunct* or damage*)) #11(hyperactiv* or hyper next activ*) #12{or #1‐#11} #13[mh Tics] #14[mh "tic disorders"] #15[mh "tourette syndrome"] #16(tic or tics) #17Tourette* #18(habit* near/3 (spasm* or chorea*)) #19{or #13‐#18} #20#12 and #19 #21[mh infant] #22[mh child] #23[mh adolescent] #24(child* or boy* or girl* or infant* or baby or babies or teen* or adolescen* or toddler* or pre‐school* or preschool* or schoolchild*) #25{or #21‐#24} #26#20 and #25 Publication Year from 2009 to 2016, in Trials #27#20 and #25 Publication Year from 2016 to 2017, in Trials
MEDLINE Ovid
MEDLINE (1946 to September week 1 2017) (43 records) MEDLINE (1946 to June week 4 2016) (10 records)
1 "attention deficit and disruptive behavior disorders"/ 2 attention deficit disorder with hyperactivity/ 3 conduct disorder/ 4 ADHD.tw,kw. 5 ADDH.tw,kw. 6 ADHS.tw,kw. 7 ("AD/HD" or HKD).tw,kw. 8 TDAH.tw,kw. 9 ((attention$ or behav$) adj3 (defic$ or dysfunc$ or disorder$)).tw,kw. 10 ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw,kw. 11 (impulsiv$ or inattentiv$ or inattention$).tw,kw. 12 hyperkinesis/ 13 (hyperkin$ or hyper‐kin$).tw,kw. 14 (minimal adj3 brain adj3 (disorder$ or dysfunct$ or damage$)).tw,kw. 15 (hyperactiv$ or hyper‐activ$).tw,kw. 16 or/1‐15 17 tics/ 18 tic disorders/ 19 tourette syndrome/ 20 (tic or tics).tw,kw. 21 Tourette$.tw,kw. 22 (habit$ adj3 (spasm$ or chorea$)).tw,kw. 23 or/17‐22 24 infant/ 25 exp child/ 26 adolescent/ 27 (child$ or boy$ or girl$ or infant$ or baby or babies or teen$ or adolescen$ or toddler$ or pre‐school$ or preschool$ or schoolchild$).tw,kw. 28 or/24‐27 29 exp Antidepressive Agents/ 30 antidepress$.tw,kw. 31 Clonidine/ 32 desipramine/ 33 exp Dextroamphetamine/ 34 Guanfacine/ 35 exp Methylphenidate/ 36 atomoxetine.mp. 37 catapres$.mp. 38 clonidine.mp. 39 concerta.mp. 40 desipramine.mp. 41 dexamfetamine.mp. 42 dexedrine.mp. 43 dextroamphetamine.mp. 44 dixarit.mp. 45 equasym.mp. 46 guanfacine.mp. 47 medikinet.mp. 48 methylphenidat$.mp. 49 ritalin$.mp. 50 strattera.mp. 51 or/29‐50 52 16 and 23 and 28 and 51 53 limit 52 to yr="2009 ‐Current" 54 limit 52 to ed="20160623 ‐ 20170918"
MEDLINE In‐Process and Other Non‐Indexed Citations Ovid
MEDLINE In‐Process and Other Non‐Indexed Citations (19 September 2017) (18 records) MEDLINE In‐Process and Other Non‐Indexed Citations (1 July 2016) (14 records)
1 ADHD.tw,kw. 2 ADDH.tw,kw. 3 ADHS.tw,kw. 4 ("AD/HD" or HKD).tw,kw. 5 TDAH.tw,kw. 6 ((attention$ or behav$) adj3 (defic$ or dysfunc$ or disorder$)).tw,kw. 7 ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw,kw. 8 (impulsiv$ or inattentiv$ or inattention$).tw,kw. 9 (hyperkin$ or hyper‐kin$).tw,kw. 10 (minimal adj3 brain adj3 (disorder$ or dysfunct$ or damage$)).tw,kw. 11 (hyperactiv$ or hyper‐activ$).tw,kw. 12 (tic or tics).tw,kw. 13 Tourette$.tw,kw. 14 (habit$ adj3 (spasm$ or chorea$)).tw,kw. 15 (child$ or boy$ or girl$ or infant$ or baby or babies or teen$ or adolescen$ or toddler$ or pre‐school$ or preschool$ or schoolchild$).tw,kw. 16 (antidepress$ or anti‐depress$).tw,kw. 17 atomoxetine.tw,kw. 18 catapres$.tw,kw 19 clonidine.tw,kw. 20 concerta.tw,kw. 21 desipramine.tw,kw. 22 dexamfetamine.tw,kw. 23 dexedrine.tw,kw. 24 dextroamphetamine.tw,kw. 25 dixarit.tw,kw. 26 equasym.tw,kw. 27 guanfacine.tw,kw. 28 medikinet.tw,kw. 29 methylphenidat$.tw,kw. 30 ritalin$.tw,kw. 31 strattera.tw,kw. 32 or/1‐11 33 or/12‐14 34 32 and 33 35 15 and 32 and 33 36 or/17‐31 37 35 and 36
MEDLINE Epub Ahead of Print Ovid
MEDLINE Epub Ahead of Print (19 September 2017) (1 record) MEDLINE Epub Ahead of Print (1 July 2016) (4 records)
1 ADHD.tw,kw. 2 ADDH.tw,kw. 3 ADHS.tw,kw. 4 ("AD/HD" or HKD).tw,kw. 5 TDAH.tw,kw. 6 ((attention$ or behav$) adj3 (defic$ or dysfunc$ or disorder$)).tw,kw. 7 ((disrupt$ adj3 disorder$) or (disrupt$ adj3 behav$) or (defian$ adj3 disorder$) or (defian$ adj3 behav$)).tw,kw. 8 (impulsiv$ or inattentiv$ or inattention$).tw,kw. 9 (hyperkin$ or hyper‐kin$).tw,kw. 10 (minimal adj3 brain adj3 (disorder$ or dysfunct$ or damage$)).tw,kw. 11 (hyperactiv$ or hyper‐activ$).tw,kw. 12 (tic or tics).tw,kw. 13 Tourette$.tw,kw. 14 (habit$ adj3 (spasm$ or chorea$)).tw,kw. 15 (child$ or boy$ or girl$ or infant$ or baby or babies or teen$ or adolescen$ or toddler$ or pre‐school$ or preschool$ or schoolchild$).tw,kw. 16 (antidepress$ or anti‐depress$).tw,kw. 17 atomoxetine.tw,kw. 18 catapres$.tw,kw 19 clonidine.tw,kw. 20 concerta.tw,kw. 21 desipramine.tw,kw. 22 dexamfetamine.tw,kw. 23 dexedrine.tw,kw. 24 dextroamphetamine.tw,kw. 25 dixarit.tw,kw. 26 equasym.tw,kw. 27 guanfacine.tw,kw. 28 medikinet.tw,kw. 29 methylphenidat$.tw,kw. 30 ritalin$.tw,kw. 31 strattera.tw,kw. 32 or/1‐11 33 or/12‐14 34 32 and 33 35 15 and 32 and 33 36 or/17‐31 37 35 and 3
Embase Ovid
Embase (1974 to 19 September 2017) (21 records) Embase (1974 to 2016 week 27) (142 records)
1 attention deficit disorder/ 2 hyperactivity/ 3 conduct disorder/ 4 ADHD.tw. 5 ADDH.tw. 6 ADHS.tw. 7 "AD/HD".tw. 8 "add".tw. 9 (attention$ adj3 (defic$ or dysfunc$ or disorder$)).tw. 10 (behav$ adj3 (dysfunc$ or disorder$)).tw. 11 disruptiv$.tw. 12 (minimal adj3 brain adj3 (disorder$ or dysfunct$ or damage$)).tw. 13 (impulsiv$ or inattentiv$ or inattention$).tw. 14 disruptiv$.tw. 15 (overactiv$ or over‐activ$).tw. 16 hyperkinesis/ 17 (hyperactiv$ or hyper‐activ$).tw. 18 (hyperkin$ or hyper‐kin$ or hkd).tw. 19 or/1‐18 20 tic/ 21 gilles de la tourette syndrome/ 22 (tic or tics)tw,kw. 23 Tourette$.tw,kw. 24 (habit$ adj3 (spasm$ or chorea$)).tw,kw. 25 or/20‐24 26 19 and 25 27 exp child/ 28 adolescence/ 29 (child$ or boy$ or girl$ or infant$ or baby or babies or teen$ or adolescen$ or toddler$ or pre‐school$ or preschool$ or schoolchild$).tw,kw. 30 or/27‐29 31 26 and 30 32 exp antidepressant agent/ 33 (antidepress$ or anti‐depress$).tw,kw. 34 atomoxetine/ 35 clonidine/ 36 desipramine/ 37 dexamphetamine/ 38 methylphenidate/ 39 atomoxetine.mp. 40 catapres$.mp. 41 clonidine.mp. 42 concerta.mp. 43 desipramine.mp. 44 dexamfetamine.mp. 45 dexedrine.mp. 46 dextroamphetamine.mp. 47 dixarit.mp. 48 equasym.mp. 49 medikinet.mp. 50 methylphenidat$.mp. 51 ritalin$.mp. 52 strattera.mp. 53 or/32‐52 54 31 and 53 55 Randomized controlled trial/ 56 controlled clinical trial/ 57 Single blind procedure/ 58 Double blind procedure/ 59 triple blind procedure/ 60 Crossover procedure/ 61 (crossover or cross‐over).tw. 62 ((singl$ or doubl$ or tripl$ or trebl$) adj1 (blind$ or mask$)).tw. 63 Placebo/ 64 placebo.tw. 65 prospective.tw. 66 factorial$.tw. 67 random$.tw. 68 assign$.ab. 69 allocat$.tw. 70 volunteer$.ab. 71 or/55‐70 72 54 and 71 73 limit 72 to yr="2009 ‐Current" 74 limit 72 to yr="2016 ‐Current"
CINAHL EbscoHOST
CINAHL (searched 20 September 2017) 13 records CINAHL (searched 5 July 2016) 64 records
S1 (MH "Attention Deficit Hyperactivity Disorder") S2 ADHD S3 ADDH S4 ADDH S5 ADHS S6 "AD/HD" S7 ((attention* or behav*) n3 (defic* or dysfunc* or disorder*)) S8 ((disrupt* N3 disorder*) or (disrupt* N3 behav*) or (defian* N3 disorder*) or (defian* N3 behav*)) S9 (impulsiv* or inattentiv* or inattention*) S10 (MH "Hyperkinesis") S11 hyperkine* S12 (minimal N3 brain N3 (disorder* or dysfunct* or damage*)) S13 hyperactiv* S14 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 S15 (MH "Tic") S16 (MH "Tourette Syndrome") S17 (tic or tics) S18 Tourette* S19 (habit* N3 (spasm* or chorea*)) S20 S15 OR S16 OR S17 OR S18 OR S19 S21 S14 AND S20 S22 (MH "Child+") S23 (MH "Adolescence") OR (MH "Young Adult") S24 (child* or boy* or girl* or infant* or baby or babies or teen* or adolescen* or toddler* or pre‐school* or preschool* or schoolchild*) S25 S22 OR S23 OR S24 S26 (MH "Clinical Trials+") S27 MH random assignment S28 (MH "Meta Analysis") S29 (MH "Crossover Design") S30 (MH "Quantitative Studies") S31 PT randomized controlled trial S32 PT Clinical trial S33 trial* S34 ("follow‐up study" or "follow‐up research") S35 (prospectiv* study or prospectiv* research) S36 placebo* S37 (MH "Program Evaluation") S38 (MH "Treatment Outcomes") S39 TI(single N2 mask* or single N2 blind*) OR AB(single N2 mask* or single N2 blind*) S40 TI((doubl* N2 mask*) or (doubl* N2 blind*)) OR AB((doubl* N2 mask*) or (doubl* N2 blind*)) S41 TI ((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*)) OR AB((tripl* N2 mask*) or (tripl* N2 blind*)) or ((trebl* N2 mask*) or (trebl* N2 blind*) S42 random* S43 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 S44 S21 AND S43 S45 EM 2009‐ S46 S44 AND S45 S47 EM 20160701‐ S48 S44 AND S47
PsycINFO Ovid
PsycINFO (1806 to September Week 2 2017) 9 records PsycINFO (1806 to June Week 5 2016) 63 records
1 exp attention deficit disorder/ 2 exp Behavior Problems/ 3 Impulsiveness/ 4 hyperkinesis/ 5 adhd.tw. 6 addh.tw. 7 adhs.tw. 8 "ad/hd".tw. 9 TDAH.tw. 10 hyperactiv$.tw. 11 hyper‐activ$.tw. 12 overactiv$.tw. 13 over‐activ$.tw. 14 hyperkin$.tw. 15 hyper‐kin$.tw. 16 hkd.tw. 17 (minimal adj3 brain$ adj3 (damag$ or disorder$ or dysfunc$)).tw. 18 (attention$ adj3 (deficit$ or disorder$ or dysfunc$)).tw. 19 (behav$ adj3 (dysfunc$ or disorder$)).tw. 20 disruptiv$.tw. 21 (impulsiv$ or inattentiv$ or inattentiv$).tw. 22 or/1‐21 23 tics/ 24 Tourette Syndrome/ 25 (tic or tics).tw. 26 tourette$.tw. 27 (habit$ adj3 (spasm$ or chorea$)).tw. 28 or/24‐27 29 22 and 28 30 exp antidepressant drugs/ 31 (antidepress$ or anti‐depress$).tw. 32 ATOMOXETINE/ 33 clonidine/ 34 desipramine/ 35 dextroamphetamine/ 36 methylphenidate/ 37 atomoxetine.mp. 38 catapres$.mp. 39 clonidine.mp. 40 concerta.mp. 41 desipramine.mp. 42 dexamfetamine.mp. 43 dexedrine.mp. 44 dextroamphetamine.mp. 45 dixarit.mp. 46 equasym.mp. 47 Guanfacine.mp. 48 medikinet.mp. 49 methylphenidat$.mp. 50 ritalin$.mp. 51 strattera.mp. 52 or/30‐51 53 29 and 52 54 limit 53 to yr="2009 ‐Current" 55 limit 53 to yr="2016 ‐Current"
Science Citation Index ‐ Expanded (SCI‐EXPANDED) and Social Sciences Citation Index (SSCI) (Web of Science)
SCI and SSCI (1970 to 19 September 2017) (10 records) SCI and SSCI (1970 to 5 July 2016) (225 records)
19#17 AND #16 AND #15 AND #12 Indexes=SCI‐EXPANDED, SSCI Timespan=2016‐2017 # 18#17 AND #16 AND #15 AND #12 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 17TS=(child* or boy* or girl* or infant* or baby or babies or teen* or adolescen* or toddler* or pre‐school* or preschool* or schoolchild*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 16TS=(random* or trial* or control* or blind* or allocat* or assign* or group* or prospective or placebo* ) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 15#14 OR #13 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 14TS=(antidepress* OR anti‐depress*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 13TS=(atomoxetine OR clonidine OR concerta OR desipramine OR dexamfetamine OR dexedrine OR dextroamphetamine OR dixarit OR equasym OR guanfacine OR medikinet OR methylphenidat* OR ritalin OR strattera ) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 12#11 AND #8 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 11#10 OR #9 Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 10TS=(habit* near/3 (spasm* or chorea*)) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 9TS=(tic OR tics OR Tourette*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 8#7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 7TS=(hyperactiv* or hyper‐activ*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 6TS=(minimal* near/3 brain near/3 (disorder* or dysfunct* or damage*)) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 5TS=(hyperkin* or hyper‐kin*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 4TS=(impulsiv* or inattentiv* or inattention*) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 3TS=((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 2TS=((attention* or behav*) near/3 (defic* or dysfunc* or disorder*)) Indexes=SCI‐EXPANDED, SSCI Timespan=All years # 1TS= (ADHD or ADDH or ADHS or "AD/HD" or HKD or TDAH) Indexes=SCI‐EXPANDED, SSCI Timespan=All years
Conference Proceedings Citation Index‐ Science (CPCI‐S) and Conference Proceedings Citation Index‐ Social Science & Humanities (CPCI‐SSH) Web of Science
CPCI‐S and CPCI‐SSH (1990 to 19 September 2017) (0 records) CPCI‐S and CPCI‐SSH (1990 to 5 July 2016) (16 records)
19(#17 AND #16 AND #15 AND #12) Indexes=CPCI‐S, CPCI‐SSH Timespan=2016‐2017 [Final search line September 2017 ] # 18#17 AND #16 AND #15 AND #12 [Final search line July 2016] Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 17TS=(child* or boy* or girl* or infant* or baby or babies or teen* or adolescen* or toddler* or pre‐school* or preschool* or schoolchild*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 16TS=(random* or trial* or control* or blind* or allocat* or assign* or group* or prospective or placebo* ) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 15#14 OR #13 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 14TS=(antidepress* OR anti‐depress*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 13TS=(atomoxetine OR clonidine OR concerta OR desipramine OR dexamfetamine OR dexedrine OR dextroamphetamine OR dixarit OR equasym OR guanfacine OR medikinet OR methylphenidate OR ritalin OR strattera ) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 12#11 AND #8 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 11#10 OR #9 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 10TS=(habit* near/3 (spasm* or chorea*)) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 9TS=(tic OR tics OR Tourette*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 8#7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 7TS=(hyperactiv* or hyper‐activ*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 6TS=(minimal* near/3 brain near/3 (disorder* or dysfunct* or damage*)) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 5TS=(hyperkin* or hyper‐kin*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 4TS=(impulsiv* or inattentiv* or inattention*) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 3TS=((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years # 2TS=((attention* or behav*) near/3 (defic* or dysfunc* or disorder*)) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years #1 TS= (ADHD or ADDH or ADHS or "AD/HD" or HKD or TDAH) Indexes=CPCI‐S, CPCI‐SSH Timespan=All years
Cochrane Database of Systematic Reviews (CDSR) part of the Cochrane Library
CDSR (2017, Issue 9), searched 20 September 2017 (0 records) CDSR (2016, Issue 7), searched 6 July 2016 (2 records)
#1[mh ^"attention deficit and disruptive behavior disorders"] #2[mh " attention deficit disorder with hyperactivity"] #3[mh "conduct disorder"] #4(ADHD or ADDH or ADHS or "AD/HD" or HKD or TDAH):ti,ab,kw #5((attention* or behav*) near/3 (defic* or dysfunc* or disorder*)):ti,ab,kw #6((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)):ti,ab,kw #7(impulsiv* or inattentiv* or inattention*):ti,ab,kw #8[mh hyperkinesis] #9(hyperkin* or hyper next kin*):ti,ab,kw #10(minimal* near/3 brain near/3 (disorder* or dysfunct* or damage*)):ti,ab,kw #11(hyperactiv* or hyper next activ*):ti,ab,kw #12{or #1‐#11} #13[mh Tics] #14[mh "tic disorders"] #15[mh "tourette syndrome"] #16(tic or tics):ti,ab,kw #17Tourette*:ti,ab,kw #18(habit* near/3 (spasm* or chorea*)):ti,ab,kw #19{or #13‐#18} #20#12 and #19 #21[mh infant] #22[mh child] #23[mh adolescent] #24(child* or boy* or girl* or infant* or baby or babies or teen* or adolescen* or toddler* or pre‐school* or preschool* or schoolchild*):ti,ab,kw #25{or #21‐#24} #26#20 and #25 Publication Year from 2009 to 2016, in Cochrane Reviews (Reviews and Protocols) #27#20 and #25 Online Publication Date from Jun 2016 to Sep 2017, in Cochrane Reviews (Reviews and Protocols)
Database of Abstracts of Reviews of Effect (DARE) part of the Cochrane Library
DARE (2015, Issue 2), searched 6 July 2016 (1 record). This was the final issue of DARE, and so was not searched in 2017.
#1[mh ^"attention deficit and disruptive behavior disorders"] #2[mh " attention deficit disorder with hyperactivity"] #3[mh "conduct disorder"] #4(ADHD or ADDH or ADHS or "AD/HD" or HKD or TDAH):ti,ab,kw #5((attention* or behav*) near/3 (defic* or dysfunc* or disorder*)):ti,ab,kw #6((disrupt* near/3 disorder*) or (disrupt* near/3 behav*) or (defian* near/3 disorder*) or (defian* near/3 behav*)):ti,ab,kw #7(impulsiv* or inattentiv* or inattention*):ti,ab,kw #8[mh hyperkinesis] #9(hyperkin* or hyper next kin*):ti,ab,kw #10(minimal* near/3 brain near/3 (disorder* or dysfunct* or damage*)):ti,ab,kw #11(hyperactiv* or hyper next activ*):ti,ab,kw #12{or #1‐#11} #13[mh Tics] #14[mh "tic disorders"] #15[mh "tourette syndrome"] #16(tic or tics):ti,ab,kw #17Tourette*:ti,ab,kw #18(habit* near/3 (spasm* or chorea*)):ti,ab,kw #19{or #13‐#18} #20#12 and #19 #21[mh infant] #22[mh child] #23[mh adolescent] #24(child* or boy* or girl* or infant* or baby or babies or teen* or adolescen* or toddler* or pre‐school* or preschool* or schoolchild*):ti,ab,kw #25{or #21‐#24} #26#20 and #25, in Other Reviews
Epistemonikos
Searched 20 September 2017. Limited to systematic reviews added to database between 6 July 2016 and 20 September 2017 (0 records) Searched 6 July 2016. Limited to systematic reviews (14 records)
(title:(adhd OR hyperactiv* OR attention deficit) OR abstract:(adhd OR hyperactiv* OR attention deficit)) AND (title:(tic* OR Tourette*) OR abstract:(tic* OR Tourette*))
WorldCat
Searched 20 September 2017 (0 records) Searched 6 July 2016 (1 record)
kw:(adhd OR hyperactiv* OR attention deficit*) AND kw:(TIC* OR tourette*) AND kw:(atomoxetine OR clonidine OR concerta OR desipramine OR dexamfetamine OR dexedrine OR dextroamphetamine OR dixarit OR equasym OR guanfacine OR medikinet OR methylphenidate OR ritalin OR strattera )
ClinicalTrials.gov
clinicaltrials.gov
Searched 20 September 2017. Limited to records first received from 1 July 2016 to 20 September 2017 (3 records) Searched 6 July 2016 (31 records)
(adhd AND tic) OR (adhd AND tourette) OR (attention deficit AND tic) OR (attention deficit AND tourette) | Interventional Studies
WHO ICTRP
www.who.int/trialsearch/default.aspx
Searched 20 September 2017. Limited to records first received from 1 July 2016 to 20 September 2017 (2 records) Searched 6 July 2016 (9 records)
Basic search: adhd AND tic OR adhd AND tourette OR attention deficit AND tic OR attention deficit AND tourette
Appendix 2. Search strategies up to 2009
The Cochrane Library
2009, Issue 4.
#1MeSH descriptor Tic Disorders explode all trees #2MeSH descriptor Tics explode all trees #3(tic or tics) #4(tourette*) #5(habit* near/3 spasm*) or (habit* near/3 chorea*) #6(#1 OR #2 OR #3 OR #4 OR #5) #7MeSH descriptor Adolescent explode all trees #8(child* or adolescen* or boy* or girl* or infant* or toddler* or pre‐school* or pre school* or schoolchild*) #9MeSH descriptor Attention Deficit Disorder with Hyperactivity, this term only #10(adhd) #11(addh) #12adhs #13(hyperactiv*) #14hyperkin* #15(attention deficit*) #16(brain dysfunction) #17(#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16) #18(#7 OR #8) #19MeSH descriptor Methylphenidate, this term only #20(methylphenidate or ritalin* or concerta or equasym or medikinet) #21MeSH descriptor Dextroamphetamine, this term only #22(dexamfetamine or dextroamphetamine or dexedrine or atomoxetine or strattera) #23MeSH descriptor Clonidine, this term only #24(clonidine or dixarit or catapres* or guanfacine) #25MeSH descriptor Guanfacine, this term only #26(#19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) #27(#6 AND #17 AND #18 AND #26)
MEDLINE
1950 to July 2009.
1 tic disorders/ or tourette syndrome/ 2 Tics/ 3 (tic or tics).tw. 4 tourette$.tw. 5 (habit$ adj3 (spasm$ or chorea$)).tw. 6 or/1‐5 7 adolescent/ or child/ or child, preschool/ or infant/ 8 (child$ or boy$ or girl$ or infant$ or baby or babies or teen$ or adolescen$ or toddler$ or pre‐school$ or pre school$ or schoolchild$).tw. 9 or/7‐8 10 Attention Deficit Disorder with Hyperactivity/ 11 adhd.tw. 12 addh.tw. 13 adhs.tw. 14 hyperactiv$.tw. 15 hyperkin$.tw. 16 attention deficit$.tw. 17 brain dysfunction.tw. 18 or/10‐17 19 Methylphenidate/ 20 methylphenidate.tw. 21 ritalin$.tw. 22 concerta.tw. 23 equasym.tw. 24 medikinet.tw. 25 Dextroamphetamine/ 26 dexamfetamine.tw. 27 dextroamphetamine.tw. 28 dexedrine.tw. 29 atomoxetine.tw. 30 strattera.tw. 31 Clonidine/ 32 clonidine.tw. 33 dixarit.tw. 34 catapres$.tw. 35 desipramine/ 36 exp Antidepressive Agents/ 37 antidepress$.tw. 38 or/19‐37 39 6 and 9 and 18 and 38
Embase
1980 to July 2009
1 tic disorders/ or tourette syndrome/ 2 Tics/ 3 (tic or tics).tw. 4 tourette$.tw. 5 (habit$ adj3 (spasm$ or chorea$)).tw. 6 or/1‐5 7 adolescent/ or child/ or child, preschool/ or infant/ 8 (child$ or boy$ or girl$ or infant$ or baby or babies or teen$ or adolescen$ or toddler$ or pre‐school$ or pre school$ or schoolchild$).tw. 9 or/7‐8 10 Attention Deficit Disorder with Hyperactivity/ 11 adhd.tw. 12 addh.tw. 13 adhs.tw. 14 hyperactiv$.tw. 15 hyperkin$.tw. 16 attention deficit$.tw. 17 brain dysfunction.tw. 18 or/10‐17 19 Methylphenidate/ 20 methylphenidate.tw. 21 ritalin$.tw. 22 concerta.tw. 23 equasym.tw. 24 medikinet.tw. 25 Dextroamphetamine/ 26 dexamfetamine.tw. 27 dextroamphetamine.tw. 28 dexedrine.tw. 29 atomoxetine.tw. 30 strattera.tw. 31 Clonidine/ 32 clonidine.tw. 33 dixarit.tw. 34 catapres$.tw. 35 guanfacine.tw. 36 Guanfacine/ 37 or/19‐36 38 random$.tw. 39 factorial$.tw. 40 crossover$.tw. 41 cross over$.tw. 42 cross‐over$.tw. 43 placebo$.tw. 44 (doubl$ adj blind$).tw. 45 (singl$ adj blind$).tw. 46 assign$.tw. 47 allocat$.tw. 48 volunteer$.tw. 49 Crossover Procedure/ 50 Double Blind Procedure/ 51 Randomized Controlled Trial/ 52 Single Blind Procedure/ 53 or/38‐52 54 6 and 53 and 18 and 37 and 9
CINAHL (Cumulative Index to Nursing and Allied Health Literature)
1982 to July 2009.
S29 (S28 and S27 and S12 and S6) S28 S16 or S15 or S14 or S13 S27 S26 or S25 or S24 or S23 or S22 or S21 or S20 or S19 or S18 or S17 S26 clonidine or dixarit or catapres* or guanfacine S25 (MH "Clonidine") S24 atomoxetine or strattera S23 dexamfetamine or dextroamphetamine or dexedrine S22 (MH "Dextroamphetamine") S21 medikinet S20 equasym S19 concerta S18 methylphenidate or ritalin S17 (MH "Methylphenidate") S16 attention deficit* or brain dysfunction S15 hyperactiv* or hyperkin* S14 adhd or addh or adhs S13 (MH "Attention Deficit Hyperactivity Disorder") S12 S11 or S10 or S9 or S8 or S7 S11 child* or boy* or girl* or infant* or baby or babies or teen* or adolescen* or toddler* or pre‐school* or pre school* or schoolchild* S10 (MH "Child, Preschool") S9 (MH "Infant") S8 (MH "Child") S7 (MH "Adolescence") S6 S5 or S4 or S3 or S2 or S1 S5 habit* n3 chorea* S4 habit* n3 spasm* S3 tourette* S2 tic or tics S1 (MH "Tic+")
PsycINFO
1806 to July week 4 2009.
1 randomi$.tw. 2 singl$.tw. 3 doubl$.tw. 4 trebl$.tw. 5 tripl$.tw. 6 blind$.tw. 7 mask$.tw. 8 (or/2‐5) adj3 (or/6‐7) 9 clin$.tw. 10 trial$.tw. 11 (clin$ adj3 trial$).tw. 12 placebo$.tw. 13 exp PLACEBO/ 14 crossover.tw. 15 exp Treatment Effectiveness Evaluation/ 16 exp Mental Health Program Evaluation/ 17 random$.tw. 18 assign$.tw. 19 allocate$.tw. 20 (random$ adj3 (assign$ or allocate$)).tw. 21 20 or 16 or 15 or 14 or 13 or 12 or 11 or 8 or 1 22 tourette syndrome/ 23 Tics/ 24 (tic or tics).tw. 25 tourette$.tw. 26 (habit$ adj3 (spasm$ or chorea$)).tw. 27 or/22‐26 28 (child$ or boy$ or girl$ or infant$ or baby or babies or teen$ or adolescen$ or toddler$ or pre‐school$ or pre school$ or schoolchild$).tw. 29 Attention Deficit Disorder with Hyperactivity/ 30 adhd.tw. 31 addh.tw. 32 adhs.tw. 33 hyperactiv$.tw. 34 hyperkin$.tw. 35 attention deficit$.tw. 36 brain dysfunction.tw. 37 or/29‐36 38 Methylphenidate/ 39 methylphenidate.tw. 40 ritalin$.tw. 41 concerta.tw. 42 equasym.tw. 43 medikinet.tw. 44 Dextroamphetamine/ 45 dexamfetamine.tw. 46 dextroamphetamine.tw. 47 dexedrine.tw. 48 atomoxetine.tw. 49 strattera.tw. 50 Clonidine/ 51 clonidine.tw. 52 dixarit.tw. 53 catapres$.tw. 54 guanfacine.tw. 55 Guanfacine/ 56 or/38‐55 57 56 and 27 and 28 and 37 58 21 and 57
Dissertation Abstracts
methylphenidate AND tic OR tics OR tourettes ritalin AND tic OR tics OR tourettes concerta AND tic OR tics OR tourettes equasym AND tic OR tics OR tourettes medikinet AND tic OR tics OR tourettes dexamfetamine AND tic OR tics OR tourettes dextroamphetamine AND tic OR tics OR tourettes dexedrine AND tic OR tics OR tourettes atomoxetine AND tic OR tics OR tourettes strattera AND tic OR tics OR tourettes clonidine AND tic OR tics OR tourettes dixarit AND tic OR tics OR tourettes catapres AND tic OR tics OR tourettes guanfacine AND tic OR tics OR tourettes
Appendix 3. Criteria for assigning 'Risk of bias' judgements
Sequence generation
Description: the method used to generate the allocation sequence was described in detail so as to assess whether it should have produced comparable groups.
Review authors' judgment: was the allocation concealment sequence adequately generated?
Allocation concealment
Description: the method used to conceal allocation sequence was described in sufficient detail to assess whether intervention schedules could have been foreseen in advance of, or during, recruitment.
Review authors' judgment: was allocation adequately concealed?
Blinding of participants and personnel
Description: the method used to blind participants and personnel from knowledge of which intervention a participant received was described.
Review authors' judgment: was knowledge of the allocated intervention adequately prevented during the study?
Blinding of outcome assessment
Description: the method used to blind outcome assessors from knowledge of which intervention a participant received was described.
Review authors' judgment: was knowledge of the allocated intervention adequately prevented during the study?
Incomplete outcome data
Description: if studies did not report intention‐to‐treat analyses, we attempted to obtain missing data by contacting the study authors. We extracted and reported data on exclusions, as well the numbers involved (compared with total randomized) and the reasons for exclusion, when reported or obtained from study investigators.
Review authors' judgment: were incomplete data dealt with adequately by the reviewers?
Selective outcome reporting
Description: attempts were made to assess the possibility of selective outcome reporting by investigators. This was done by checking study protocols when available through trial registries and comparing the outcomes listed in the protocol with the published report. We made comparisons between outcomes listed in the Methods section of the manuscript and those listed in the Results section.
Review authors' judgment: are reports of the study free of suggestion of selective outcome reporting?
Other sources of bias
Description: other concerns about bias not addressed in the domains above, including:
design‐specific risk of bias (i.e. washout adequacy in cross‐over trials);
early stopping;
baseline imbalance;
inappropriate administration of a cointervention; and
used an insensitive instrument to measure outcomes.
Review authors' judgement: was the study apparently free of other problems that could put it at a high risk of bias?
Appendix 4. Methods described in protocol for meta‐analysis
Measures of treatment effect
Binary outcome data
We will use risk ratio (RR) estimations with 95% confidence intervals (CIs) for binary outcomes.
Continuous outcome data
We will analyze data on continuous outcomes using either mean differences (MDs), or standardized mean differences (SMDs) if continuous outcomes are measured with similar but not identical instruments across studies. All analyses will include all participants in the treatment groups to which they were allocated, if data permit.
Unit of analysis issues
Cluster‐randomized trials
We do not anticipate finding any cluster‐randomized trials.
Cross‐over trials
When conducting a meta‐analysis combining the results of cross‐over trials, we will use the inverse variance methods recommended by Elbourne 2002. Where data presented from a cross‐over trial are restricted (and more information is not available from the original investigators), we will use the presented data within the first phase only, up to the point of cross‐over.
Dealing with missing data
We will attempt to obtain missing information from study authors directly. If we are unable to obtain the missing information, we will impute the missing data with replacement values, and treat these as if they were observed.
Assessment of heterogeneity
We will assess statistical heterogeneity by examining the I2 statistic, a quantity which describes approximately the proportion of variation in point estimates that is due to heterogeneity rather than sampling error. In addition, we will use a Chi2 test of homogeneity, to determine the strength of evidence that heterogeneity is genuine.
Assessment of reporting biases
We will draw funnel plots (effect size versus standard error) to assess publication bias if we find sufficient studies. Asymmetry of the plots may indicate publication bias, although they may also represent a true relationship between trial size and effect size. If such a relationship is identified, we will further examine the clinical diversity of the studies as a possible explanation (Egger 1997).
Data synthesis
We will synthesize results using a meta‐analysis by treatment type if there is no important clinical heterogeneity. For example, if studies have a very different study design or methodology, or if trial participants are very different between studies, a meta‐analysis may not be appropriate. We will use both the random‐effects and fixed‐effect models. When we report the results of the random‐effects model, we will also report tau2.
Subgroup analysis and investigation of heterogeneity
We will analyze trials in subgroups based on treatment type, for example, methylphenidate, atomoxetine.
Sensitivity analysis
We will conduct sensitivity analyses to assess the impact of risk of bias on our results.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2005.
| Methods | Double‐blind, parallel‐group study | |
| Participants |
Country: USA Inclusion criteria: children aged 7‐17 years, meeting APA 2000 criteria for ADHD and TS or chronic motor tic disorder Mean age: 11.2 (SD 2.5) years Sample size: 148 (placebo = 72, atomoxetine = 76) Sex: 131 boys, 17 girls |
|
| Interventions |
Intervention: atomoxetine Dose: 0.5‐1.5 mg/kg per day Control: placebo Administration: administered in a divided dose, once in the morning and once in the late afternoon, for 18 weeks under double‐blind conditions |
|
| Outcomes |
|
|
| Notes |
Study start and end dates: no information Funding: funded by Eli Lilly and Company Author affiliations:
Conflicts of interest:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no details provided |
| Allocation concealment (selection bias) | Low risk | Comment: randomization carried at visit 2 by a computerized interactive voice response system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: incomplete outcome data addressed |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes included |
| Other bias | High risk | Comment: high rate of early treatment termination in both treatment groups at 12 weeks |
Castellanos 1997.
| Methods | Double‐blind, placebo‐controlled, cross‐over study of methylphenidate and dextroamphetamine | |
| Participants |
Country: USA Inclusion criteria: children aged 6‐13 years, meeting APA 1987 criteria for ADHD and Tourette syndrome Mean age: 9.4 (SD 2.0) years Sample size: 22 Sex: all boys |
|
| Interventions |
Intervention: methylphenidate and dextroamphetamine, each at 3 possible doses Dose: methylphenidate: 15 mg (low), 25 mg (medium) and 45 mg (high); dextroamphetamine: 7.5 mg (low), 15 mg (medium) and 22.5 mg (high) Control: placebo Administration: doses were given twice daily at breakfast and lunch for a 1‐week period for each sequence. 1 group of 12 boys was given drug dosages in a low, medium, and high sequence for 1 week each. 1 group of 6 boys was given drug dosages in a low, medium, and medium sequence for 1 week each. 1 group of 4 boys was given drug dosages in a low, high, and high sequence for 1 week each. |
|
| Outcomes |
|
|
| Notes |
Study start and end dates: no information Funding: no information Author affiliations:
Conflicts of interests: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: little raw data provided |
| Selective reporting (reporting bias) | High risk | Comment: did not include all expected outcomes. No measure of ADHD inattentive symptoms or parent report of ADHD symptoms included |
| Other bias | Low risk | Comment: no other bias apparent |
Feigin 1996.
| Methods | Randomized, double‐blind, placebo‐controlled, cross‐over study | |
| Participants |
Country: USA Inclusion criteria: children aged 7‐16 years, meeting APA 1987 criteria for Tourette syndrome and ADHD Mean age: 12 years Sample size: 24 Sex: 21 boys, 3 girls |
|
| Interventions |
Intervention: deprenyl Dose: 5 mg twice daily Control: placebo Administration: 2 × 8‐week treatment periods separated by 6‐week washout period. Participants given deprenyl or placebo for 8 weeks, followed by cross‐over to the alternate treatment after a washout period of 6 weeks |
|
| Outcomes |
|
|
| Notes |
Study start and end dates: no information Funding: no information Author affiliations:
Conflicts of interest: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomization plan generated by a Fortran program |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: not clear if those who dropped out of the study had their data included in the analysis. Very high dropout rate after first period, especially in treatment group |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes included |
| Other bias | Low risk | Comment: no other bias apparent |
Gadow 2007.
| Methods | Double‐blind, cross‐over study | |
| Participants |
Country: USA Inclusion criteria: children aged 6‐12 years, meeting APA 1987 or APA 2000 criteria for ADHD and chronic motor tic disorder or TS Mean age: 8.95 (± 1.4) years Sample size: 71 Sex: 57 boys, 14 girls |
|
| Interventions |
Intervention: methylphenidate Dose: 0.1 mg/kg, 0.3 mg/kg, 0.5 mg/kg Control: placebo Administration: participants received placebo and 3 doses of methylphenidate (0.1 mg/kg, 0.3 mg/kg, and 0.5 mg/kg) for 2 weeks each. Medication was administered twice daily in the aforementioned dose, 3.5 hours apart, 7 days per week for 2 weeks |
|
| Outcomes |
|
|
| Notes |
Study start and end dates: no information Funding: funded, in part, by a research grant from the Tourette Syndrome Association and United States Public Health Service grant number MH 45358 from the NIMH. Author affiliations: all of the authors are with the Department of Psychiatry and Behavioral Science, State University of New York, Stony Brook, NY Conflicts of interests: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: dose schedules assigned on a random basis |
| Allocation concealment (selection bias) | Low risk | Comment: details provided in paper referenced in the methods section of study manuscript |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: did not explain how incomplete data sets were handled |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes included |
| Other bias | Low risk | Comment: no other bias apparent |
Scahill 2001.
| Methods | Randomized, placebo‐controlled, parallel‐group study of guanfacine versus placebo | |
| Participants |
Country: USA Inclusion criteria: children aged 7‐15 years, meeting APA 2000 criteria for ADHD and a chronic tic disorder Mean age: 10.4 (SD 2.0) years Sample size: 34 (placebo = 17, guanfacine = 17) Sex: 31 boys, 3 girls |
|
| Interventions |
Intervention: guanfacine Dose: 1.5‐3 mg per day Control: placebo Administration: divided into 3 daily doses, for 8 weeks |
|
| Outcomes |
|
|
| Notes |
Study start and end dates: no information Funding: funded, in part, by grant number MO1‐RR‐06022, from the Children's Clinical Research Center; Mental Health Research Center grant number MH‐30929; and grant from the Tourette Syndrome Association Author affiliations: authors from the Yale Child Study Center, New Haven, CT Conflicts of interests: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: incomplete outcome data addressed |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes included |
| Other bias | Low risk | Comment: no other bias apparent |
Singer 1995.
| Methods | Randomized, placebo‐controlled, cross‐over study of clonidine and desipramine | |
| Participants |
Inclusion criteria: children aged 7‐14 years, meeting APA 1980 and APA 1987 criteria for TS and ADHD Mean age: 10.6 years Sample size: 34 Sex: 31 boys and 3 girls |
|
| Interventions |
Country: USA Intervention: clonidine, desipramine Dose: clonidine 0.05 mg, desipramine 25 mg Control: placebo Administration: given 4 times daily for 6 weeks. 1‐week washout period between treatments |
|
| Outcomes |
|
|
| Notes |
Study start and end dates: no information Funding: funded by grants from the Tourette Syndrome Association and the United States Public Health Service (grant numbers NS 27327 and HD 25806) Author affiliations: authors from the Departments of Neurology and Pediatrics at Johns Hopkins University School of Medicine, Baltimore, MD Conflicts of interest: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not described |
| Allocation concealment (selection bias) | Low risk | Comment: medications, prepared by the Johns Hopkins Hospital pharmacy, provided as uniform‐appearing capsules in numbered containers |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: incomplete outcome data addressed |
| Selective reporting (reporting bias) | High risk | Comment: not all expected outcomes included. Outcome data not provided for many variables. Often reported 'male only' results |
| Other bias | Low risk | Comment: no other bias apparent |
Spencer 2002.
| Methods | Double‐blind, parallel‐group trial of desipramine versus placebo | |
| Participants |
Country: USA Inclusion criteria: children aged 5‐17 years, with APA 2000 diagnosis of ADHD and TS or chronic motor tic disorder Mean age: not provided for overall sample; desipramine: 10.6 (SD 2.4) years, placebo: 11.3 (SD 3.0) years Sample size: 41 (placebo = 20, desipramine = 21) Sex: 34 boys, 7 girls |
|
| Interventions |
Intervention: desipramine Dose: titrated weekly up to 3.5 mg/kg Control: placebo Administration: given twice daily (maximum dose split into two doses) for 6 weeks |
|
| Outcomes |
|
|
| Notes |
Study start and end dates: no information Funding: funded partly by the Tourette Sydrome Association and grant number R29 MH57511 from the NIMH, Bethesda, MD Author affiliations:
Conflicts of interest: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Pharmacy randomized participants using separate balanced randomization" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization codes were kept in sealed envelopes in the medical records. Medication was given in identical appearing 25 mg capsules." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: incomplete outcome data addressed |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes included |
| Other bias | Low risk | Comment: no other bias apparent |
Tourette's Syndrome Study Group 2002.
| Methods | Randomized, controlled, parallel‐group study of clonidine, methylphenidate, clonidine plus methylphenidate or placebo | |
| Participants |
Country: USA Inclusion criteria: children aged 7‐14 years, meeting DSM‐IV criteria for ADHD and a chronic tic disorder Mean age: not provided for overall sample; placebo: 9.7 (SD 1.8) years, methylphenidate: 10.7 (SD 2.0) years, clonidine: 9.7 (SD 1.8) years, clonidine plus methylphenidate: 10.6 (SD 1.9) years Sample size: 136 (placebo = 32, methylphenidate = 37, clonidine = 34, clonidine plus methylphenidate = 33) Sex: 85% boys |
|
| Interventions |
Intervention: clonidine, methylphenidate Dose: flexible. Mean dose:
Control: placebo Administration: 2‐3 times per day for 16 weeks |
|
| Outcomes |
|
|
| Notes |
Study start and end dates: no information Funding:
Author affiliations: no information Conflicts of interest: no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomly assigned by central co‐ordinating center. Computer‐generated randomization plan |
| Allocation concealment (selection bias) | Low risk | Comment: central co‐ordinating center |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: double blinded |
| Blinding of participants and personnel (performance bias) | Low risk | Comment: double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: double blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: incomplete outcome data addressed |
| Selective reporting (reporting bias) | Low risk | Comment: all expected outcomes included |
| Other bias | Low risk | Comment: no other bias apparent |
ADHD: attention deficit hyperactivity disorder; APA: American Psychiatric Association; CGI: Clinical Global Impression; DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; IOWA: inattention/overactivity with aggression; NIMH: National Institute of Mental Health; NINDS: National Institute of Neurological Disorders and Stroke; SD: standard deviation; TS: Tourette syndrome.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Howson 2004 | Primary outcome to assess immediate effect of a single transdermal dose of nicotine on tics and objective indices of sustained attention. Participants did not have to have ADHD to participate in study |
| Law 1999 | Study excluded participants with Tourette syndrome |
| Lyon 2010 | Study did not use double‐blind procedures |
| Niederhofer 2003 | Study retracted from journal due to suspicion of fraudulent results and plagiarism |
| Nolan 1999 | Study looked at the effect of stimulant withdrawal on tics rather than the effect of the medication on ADHD and tic symptom severity |
| Sallee 1994 | Study on the effect of pimozide on cognition. Not all participants had ADHD |
ADHD: attention deficit hyperactivity disorder.
Differences between protocol and review
-
Authors
Sydney Osland was added as an author to update the review.
-
We added two MEDLINE segments, which are update daily, to make our search as up‐to‐date as possible (MEDLINE In‐Process and Other Non‐Indexed Citations and MEDLINE EPub Ahead of Print).
BIOSIS Previews was not searched for this update as it was no longer available to the review team or editorial base. Instead, we searched four databases from Web of Science (Science Citation Index ‐ Expanded Web of Science, Social Sciences Citation Index Web of Science, Conference Proceedings Citation Index ‐ Science Web of Science, and Conference Proceedings Citation Index ‐ Social Science and Humanities Web of Science) for all years.
Dissertation Express was replaced by a theses search using WorldCat because it allows more complex search strings and does not limit the number of hits that are returned.
The Current Controlled Trials meta‐Register service (mRCT) was under review at the time the searches were run and was replaced with ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en).
We also searched sources of systematic reviews to check relevant reference lists (Cochrane Database of Systematic Reviews (CDSR), Database of Abstracts of Effect (DARE), and Epistemonikos).
-
We included a list of methods that were described in the protocol, Pringsheim 2009, but not used in the review in Appendix 4.
-
Assessment of risk of bias in included studies
We evaluated blinding of participants and personnel separately to blinding of outcome assessment due the importance of 'triple blinding;' that is, when outcome assessors, participants, and personnel are unaware of treatment assignment.
-
The updated review included the addition of a study flow diagram (Figure 1), due to the new methodological requirements for Cochrane Reviews (Lefebvre 2011).
Contributions of authors
SO: reviewed abstracts for inclusion in this update of the review and updated the review to meet current methodological standards.
TS: selected which trials to include, extracted data from trials, interpreted the analysis, edited the final review, and will keep the review up‐to‐date.
TP: drafted the protocol, developed the search strategy (with guidance from Margaret Anderson, CDPLP), selected which trials to include, extracted data from trials, entered data into Review Manager 2014, carried out the analysis, interpreted the analysis, drafted the final review, and will keep the review up‐to‐date.
Sources of support
Internal sources
-
Department of Clinical Neurosciences, University of Calgary, Canada.
Employer for SO and TP
External sources
None, Other.
Declarations of interest
SO works as a Research Associate at the Clinical Neurosciences Department, Cumming School of Medicine, University of Calgary, on projects focused on movement disorders, ADHD, obsessive compulsive disorder (OCD) and other associated conditions.
TS: none known.
TP's institute receives grants from Shire Canada. TP is involved in one of these grants to develop a continuing medical education program for physicians on the management of aggression in youth and attention deficit hyperactivity disorder (ADHD). TP's institution holds the funds for this project and approves all expenditure.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Allen 2005 {published data only}
- Allen AJ, Kurlan RM, Gilbert DL, Coffey BJ, Linder SL, Lewis DW, et al. Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 2005;65(12):1941‐9. [DOI: 10.1212/01.wnl.0000188869.58300.a7; PUBMED: 16380617] [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Salle FR, Gilbert DL, Dunn DW, McCracken JT, Coffey BJ, et al. Atomoxetine treatment of ADHD in children with comorbid Tourette syndrome. Journal of Attention Disorders 2008;11(4):470‐81. [DOI: 10.1177/1087054707306109; PUBMED: 17934184] [DOI] [PubMed] [Google Scholar]
Castellanos 1997 {published data only}
- Castellanos FX, Giedd JN, Elia J, Marsh WL, Ritchie GF, Hamburger SD, et al. Controlled stimulant treatment of ADHD and comorbid Tourette's syndrome: effects of stimulant and dose. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(5):589‐96. [DOI: 10.1097/00004583-199705000-00008; PUBMED: 9136492] [DOI] [PubMed] [Google Scholar]
Feigin 1996 {published data only}
- Feigin A, Kurlan R, McDermott MP, Beach J, Dimitsopulos T, Brower CA, et al. A controlled trial of deprenyl in children with Tourette's syndrome and attention deficit hyperactivity disorder. Neurology 1996;46(4):965‐8. [PUBMED: 8780073] [DOI] [PubMed] [Google Scholar]
Gadow 2007 {published data only}
- Gadow KD, Nolan EE, Sprafkin J, Sverd J. School observations of children with attention deficit hyperactivity disorder and comorbid tic disorder: effects of methylphenidate treatment. Journal of Developmental and Behavioural Pediatrics 1995;16(3):167‐76. [PUBMED: 7560119] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE, Sverd J. Methylphenidate in hyperactive boys with comorbid tic disorder: II. Short‐term behavioral effects in school settings. Journal of the American Academy of Child and Adolescent Psychiatry 1992;31(3):462‐71. [DOI: 10.1097/00004583-199205000-00012; PUBMED: 1592778] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Scheider J. Methylphenidate in children with oppositional defiant disorder and both comorbid chronic multiple tic disorder and ADHD. Journal of Child Neurology 2008;23(9):981‐90. [DOI: 10.1177/0883073808315412; PUBMED: 18474932] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Nolan EE, Sverd J, Sprafkin J, Schwartz J. Anxiety and depression symptoms and response to methylphenidate in children with attention deficit hyperactivity disorder and tic disorder. Journal of Clinical Psychopharmacology 2002;22(3):267‐74. [PUBMED: 12006897] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sverd J, Nolan EE, Sprafkin J, Schneider J. Immediate‐release methylphenidate for ADHD in children with comorbid chronic multiple tic disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2007;46(7):840‐8. [DOI: 10.1097/chi.0b013e31805c0860; PUBMED: 17581448] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sverd J, Sprafkin J, Nolan EE, Ezor SN. Efficacy of methylphenidate for attention deficit hyperactivity disorder in children with tic disorder. Archives of General Psychiatry 1995;52(6):444‐55. [PUBMED: 7771914] [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sverd J, Sprafkin J, Nolan EE, Grossman S. Long‐term methylphenidate therapy in children with comorbid attention deficit hyperactivity disorder and chronic multiple tic disorder. Archives of General Psychiatry 1999;56(4):330‐6. [PUBMED: 10197827] [DOI] [PubMed] [Google Scholar]
- Nolan EE, Gadow KD. Children with ADHD and tic disorder and their classmates: behavioural normalization with methylphenidate. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36(5):597‐604. [DOI: 10.1097/00004583-199705000-00009; PUBMED: 9136493] [DOI] [PubMed] [Google Scholar]
Scahill 2001 {published data only}
- Scahill L, Chappell PB, Kim YS, Schultz RT, Katsovich L, Shepherd E, et al. A placebo‐controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. American Journal of Psychiatry 2001;158(7):1067‐74. [DOI: 10.1176/appi.ajp.158.7.1067; PUBMED: 11431228] [DOI] [PubMed] [Google Scholar]
Singer 1995 {published data only}
- Singer HS, Brown J, Quaskey S, Rosenberg LA, Mellits ED, Denckla MB. The treatment of attention deficit hyperactivity disorder in Tourette's syndrome: a double blind placebo‐controlled study with clonidine and desipramine. Pediatrics 1995;95(1):74‐81. [PUBMED: 7770313] [PubMed] [Google Scholar]
Spencer 2002 {published data only}
- Spencer T, Biedermann J, Coffey B, Geller D, Crawford M, Bearman SK, et al. A double‐blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention‐deficit/hyperactivity disorder. Archives of General Psychiatry 2002;59(7):649‐56. [PUBMED: 12090818] [DOI] [PubMed] [Google Scholar]
Tourette's Syndrome Study Group 2002 {published data only}
- Tourette's Syndrome Study Group. Treatment of ADHD in children with tics: a randomized controlled trial. Neurology 2002;58(4):527‐36. [PUBMED: 11865128] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Howson 2004 {published data only}
- Howson AL, Batth S, Ilivitsky V, Boisjoli A, Jaworski M, Mahoney C, et al. Clinical and attentional effects of acute nicotine treatment in Tourette's syndrome. European Psychiatry 2004;19(2):102‐12. [PUBMED: 15132126] [DOI] [PubMed] [Google Scholar]
Law 1999 {published data only}
- Law SF, Schachar RJ. Do typical clinical doses of methylphenidate cause tics in children treated for attention‐deficit hyperactivity disorder?. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(8):944‐51. [DOI: 10.1097/00004583-199908000-00009; PUBMED: 10434485] [DOI] [PubMed] [Google Scholar]
Lyon 2010 {published data only}
- Lyon GJ, Samar SM, Conelea C, Trujillo MR, Lipinski CM, Bauer CC, et al. Testing tic suppression: comparing the effects of dexmethylphenidate to no medication in children and adolescents with attention‐deficit/hyperactivity disorder and Tourette's disorder. Journal of Child and Adolescent Psychopharmacology 2010;20(4):283‐9. [DOI: 10.1089/cap.2010.0032; PMC2958463; PUBMED: 20807066] [DOI] [PMC free article] [PubMed] [Google Scholar]
Niederhofer 2003 {published data only}
- Niederhofer H, Staffen W, Mair A. A placebo controlled study of lofexidine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Journal of Psychopharmacology 2003;17(1):113‐9. [PUBMED: 12680748] [DOI] [PubMed] [Google Scholar]
Nolan 1999 {published data only}
- Nolan EE, Gadow KD, Sprafkin J. Stimulant medication withdrawal during long‐term therapy in children with comorbid attention‐deficit hyperactivity disorder and chronic multiple tic disorder. Pediatrics 1999;103(4 Pt 1):730‐7. [PUBMED: 10103294] [DOI] [PubMed] [Google Scholar]
Sallee 1994 {published data only}
- Sallee FR, Sethuraman G, Rock C. Effects of pimozide on cognition in children with Tourette syndrome: interaction with comorbid attention deficit hyperactivity disorder. Acta Psychiatrica Scandinavica 1994;90(1):4‐9. [PUBMED: 7976448] [DOI] [PubMed] [Google Scholar]
Additional references
Amitai 2006
- Amitai Y, Frischer H. Excess fatality from desipramine in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45(1):54‐60. [DOI: 10.1097/01.chi.0000184931.26176.4a; PUBMED: 16327581] [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington (DC): American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington (DC): American Psychiatric Association, 1987. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Biederman 2007
- Biederman J, Boellner SW, Childress A, Lopez FA, Krishnan S, Zhang Y. Lisdexamfetamine dimesylate and mixed amphetamine salts extended‐release in children with ADHD: a double‐blind, placebo‐controlled, crossover analog classroom study. Biological Psychiatry 2007;62(9):970‐6. [DOI: 10.1016/j.biopsych.2007.04.015; PUBMED: 17631866] [DOI] [PubMed] [Google Scholar]
Bloch 2009
- Bloch MH, Panza KE, Landeros‐Weisenberger A, Leckman JF. Meta‐analysis: treatment of attention‐deficit/hyperactivity disorder in children with comorbid tic disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2009;48(9):884‐93. [DOI: 10.1097/CHI.0b013e3181b26e9f; PMC3943246; PUBMED: 19625978] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buccafusco 1992
- Buccafusco JJ. Neuropharmacologic and behavioural actions of clonidine: interactions with central neurotransmitters. International Review of Neurobiology 1992;33:55‐107. [PUBMED: 1350577] [DOI] [PubMed] [Google Scholar]
Bymaster 2002
- Bymaster FP, Katner JS, Nelso DL, Hemrick‐Luecke SK, Threlkeld PG, Heiligenstein JH, et al. Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002;27(5):699‐711. [DOI: 10.1016/S0893-133X(02)00346-9; PUBMED: 12431845] [DOI] [PubMed] [Google Scholar]
Cavanna 2009
- Cavanna A, Servo S, Monaco F, Robertson M. The behavioral spectrum of Gilles de la Tourette Syndrome. Journal of Neuropsychiatry and Clinical Neurosciences 2009;21(1):13‐23. [DOI] [PubMed] [Google Scholar]
Cohen 2015
- Cohen SC, Mulqueen JM, Ferracioli‐Oda E, Stuckelman ZD, Coughlin CG, Leckman JF, et al. Meta‐analysis: risk of tics associated with psychostimulant use in randomized, placebo‐controlled trials. Journal of the American Academy of Child and Adolescent Psychiatry 2015;54(9):728‐36. [DOI: 10.1016/j.jaac.2015.06.011; PUBMED: 26299294] [DOI] [PubMed] [Google Scholar]
Conners 1990
- Conners CK. Conners' Abbreviated Symptom Questionnaire. Multi‐Health Systems 1990.
Conners 1996
- Conners CK, Casat CD, Gualtieri CT, Weller E, Reader M, Reiss A, et al. Buproprion hydrochloride in attention deficit disorder with hyperactivity. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(10):1314‐21. [DOI: 10.1097/00004583-199610000-00018; PUBMED: 8885585] [DOI] [PubMed] [Google Scholar]
Connor 1999
- Connor DF, Fletcher KE, Swanson JM. A meta‐analysis of clonidine for symptoms of attention‐deficit hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1999;38(12):1551‐9. [DOI: 10.1097/00004583-199912000-00017; PUBMED: 10596256] [DOI] [PubMed] [Google Scholar]
DuPaul 2016
- DuPaul G, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale‐5 for children and adolescents: checklists, norms and clinical interpretation. Guildford Publications, 2016. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [PUBMED: 11914310] [DOI] [PubMed] [Google Scholar]
Freeman 2000
- Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Developmental Medicine and Child Neurology 2000;42(7):436‐47. [PUBMED: 10972415] [DOI] [PubMed] [Google Scholar]
Golden 1974
- Golden GS. Gilles de la Tourette's syndrome following methylphenidate administration. Developmental Medicine and Child Neurology 1974;16(1):76‐8. [PUBMED: 4521612] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
Kahbazi 2009
- Kahbazi M, Ghoreishi A, Rahiminejad F, Mohammadi MR, Kamalipour A, Akhondzadeh S. A randomized double blind and placebo controlled trial of modafanil in children and adolescents with attention deficit and hyperactivity disorder. Psychiatry Research 2009;168(3):234‐7. [DOI: 10.1016/j.psychres.2008.06.024; PUBMED: 19439364] [DOI] [PubMed] [Google Scholar]
Knight 2012
- Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta‐analysis. Pediatric Neurology 2012;47(2):77‐90. [DOI] [PubMed] [Google Scholar]
Kurlan 2002
- Kurlan R, Como PG, Miller B, Palumbo D, Deeley C, Andresen EM, et al. The behavioural spectrum of tic disorders: a community‐based study. Neurology 2002;59(3):414‐20. [PUBMED: 12177376] [DOI] [PubMed] [Google Scholar]
Leckman 1989
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician‐rated scale of tic severity. Journal of the American Academy of Child and Adolescent Psychiatry 1989;28(4):566‐73. [DOI: 10.1097/00004583-198907000-00015; PUBMED: 2768151] [DOI] [PubMed] [Google Scholar]
Leckman 1998
- Leckman JF, Cohen DJ. Tourette's Syndrome ‐ Tics, Obsessions, Compulsions: Developmental Psychopathology and Clinical Care. New York (NY): John Wiley & Sons, 1998. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lowe 1982
- Lowe TL, Cohen DJ, Detlor M, Kremenitzer MW, Shaywitz BA. Stimulant medications precipitate Tourette's syndrome. JAMA 1982;247(12):1729‐31. [PUBMED: 6950128] [PubMed] [Google Scholar]
Martino 2013
- Martino D, Mink J. Tic disorders. Continuum 2013;19(5):1287‐311. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097; PMC2707599; PUBMED: 19621072] [DOI] [PMC free article] [PubMed] [Google Scholar]
Potter 2004
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioural inhibition in adolescents with attention‐deficit/hyperactivity disorder. Psychopharmacology 2004;176(2):182‐94. [DOI: 10.1007/s00213-004-1874-y; PUBMED: 15083253] [DOI] [PubMed] [Google Scholar]
Pringsheim 2007
- Pringsheim T, Lang A, Kurlan R, Pearce M, Sandor P. Health related quality of life in children with TS. Neurology 2007;68(12 Suppl 1):A294. [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riddle 1993
- Riddle MA, Geller B, Ryan N. Another sudden death in a child treated with desipramine. Journal of the American Academy of Child and Adoelscent Psychiatry 1993;32(4):792‐7. [DOI: 10.1097/00004583-199307000-00013; PUBMED: 8340300] [DOI] [PubMed] [Google Scholar]
Seiden 1993
- Seiden LS, Sabol KE, Ricaurte GA. Amphetamine: effects on catecholamine systems and behavior. Annual Review of Pharmacology and Toxicology 1993;33:639‐77. [DOI: 10.1146/annurev.pa.33.040193.003231; PUBMED: 8494354] [DOI] [PubMed] [Google Scholar]
Shapiro 1988
- Shapiro AK, Shapiro ES, Young JG, Feinberg TE. Gilles de la Tourette Syndrome. New York (NY): Raven Press, 1988. [Google Scholar]
Shytle 2002
- Shytle RD, Silver AA, Wilkinson BJ, Sanberg PR. A pilot controlled trial of transdermal nicotine in the treatment of attention deficit hyperactivity disorder. World Journal of Biological Psychiatry 2002;3(3):150‐5. [PUBMED: 12478880] [DOI] [PubMed] [Google Scholar]
Spencer 2001
- Spencer TJ, Biederman J, Faraone S, Mick E, Coffey B, Geller D, et al. Impact of tic disorders on ADHD outcome across the life cycle: findings from a large group of adults with and without ADHD. American Journal of Psychiatry 2001;158(4):611‐7. [DOI: 10.1176/appi.ajp.158.4.611; PUBMED: 11282697] [DOI] [PubMed] [Google Scholar]
Steeves 2008
- Steeves TDL, Fox SH. Neurological basis of serotonin‐dopamine antagonists in the treatment of Gilles de la Tourette syndrome. In: Giovann G, Matteo V, Esposito E editor(s). Progress in Brain Research. Vol. 172, London (UK): Elsevier, 2008:495‐513. [DOI] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. ICD‐10: International Statistical Classification of Diseases and Related Health Problems: 10th Revision. Geneva: World Health Organization, 2005. [Google Scholar]
Wilens 2006
- Wilens TE. Mechanism of action of agents used in attention‐deficit/hyperactivity disorder. Journal of Clinical Psychiatry 2006;67(Suppl 8):32‐8. [PUBMED: 16961428] [PubMed] [Google Scholar]
References to other published versions of this review
Pringsheim 2009
- Pringsheim T, Steeves T. Pharmacological treatment for attention deficit hyperactivity disorder in children with co‐morbid tic disorders. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007990] [DOI] [PubMed] [Google Scholar]
Pringsheim 2011
- Pringsheim T, Steeves T. Pharmacological treatment for Attention Deficit Hyperactivity Disorder (ADHD) in children with comorbid tic disorders. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD007990.pub2] [DOI] [PubMed] [Google Scholar]


