Abstract
Despite widespread practice, there is very little, high-level evidence supporting the indications for and effectiveness of cardiopulmonary/chest physiotherapy (CPT) in critically ill infants and children. Conversely, most studies highlight the detrimental effects or lack of effect of different manual modalities. Conventional CPT should not be a routine intervention in the pediatric intensive care unit, but can be considered when obstructive secretions are present which impact on lung mechanics and/or gaseous exchange and/or where there is the potential for long-term complications. Techniques such as positioning, early mobilization, and rehabilitation have been shown to be beneficial in adult intensive care patients; however, little attention has been paid to this important area of practice in pediatric intensive care units. This article presents a narrative review of chest physiotherapy in pediatric critical illness, including effects, indications, precautions, and specific treatment modalities and techniques.
Keywords: chest physiotherapy, intensive care, rehabilitation, pediatric, physiotherapy
Introduction
Chest physiotherapy (CPT) is part of the accepted care of intubated children in many pediatric intensive care units (PICU) globally in spite of a limited evidence base, largely because of the risks of obstruction of the small diameter endotracheal (ET) tubes used when ventilating young infants and children.1 2 It is accepted that mucociliary clearance is compromised in intubated patients, owing to a combination of factors including the inability to close the glottis, inadequately humidified inspired gas, airway irritation, and altered sputum rheology from respiratory infectious processes.3 4 Therefore, all intubated and mechanically ventilated infants and children will require ET tube suctioning, but only a small proportion of these children may also benefit from CPT, to mobilize and facilitate secretion removal, and prevent or relieve airway obstruction.5 6
This review describes the current effects, indications for, and modalities of CPT currently used to treat critically ill and injured children being managed in the PICU. Other articles in this edition will discuss comprehensive rehabilitation, of which CPT is a component.
Effects of CPT and indications: The main aim of CPT in pediatric respiratory disease is to assist the removal of obstructive tracheobronchial secretions, thereby reducing airway resistance and improving work of breathing and gaseous exchange; facilitating early weaning from the ventilator; preventing or resolving respiratory complications, reexpanding collapsed lobes; and hastening recovery.7 8 9 10 11 The long-term outcomes of critical pediatric illness or injury are also paramount in terms of preventing or minimizing the complications of critical illness and immobility (e.g., postural deformities, muscle deconditioning), and optimizing functional outcomes after PICU. The precise role of the physiotherapist in different intensive care settings varies according to the country of location, local tradition, staffing levels, training, and expertise.2
The most common physiotherapy modalities applied to ventilated pediatric patients are positioning, mobilization, percussion and vibrations (manual techniques), manual hyperinflation, and ET tube suctioning.2 Conventional CPT usually refers to the manual application of techniques such as percussions and vibrations, usually combined with gravity-assisted positioning (postural drainage). However, the modern approach is appropriately much broader, with attention being paid to the holistic multisystem care of children with complex disease processes. The awareness that all systems are interrelated is essential in planning appropriate treatments for critically ill children; for example, by facilitating trunk rotation to encourage normal developmentally appropriate translational movements, thoracic mobility will also be enhanced, secretions may be mobilized, and ventilation may be optimized. Similarly, if one positions a child to prevent pressure sores or to normalize tone, there will also be effects on the lungs in terms of alteration of ventilation and perfusion and prevention of positional atelectasis and consolidation. Thus, it is the author's opinion that CPT in the PICU should not be applied in isolation, but rather in combination with interventions such as rehabilitation, developmental stimulation, and supportive care. Although manual CPT may be useful in specific circumstances and disease conditions, it may be useless or even harmful in others.10 In the critically ill child particularly, any potential benefits of CPT must therefore be carefully balanced against risk of harm before implementing treatment.
The evidence base for CPT in PICU is extremely limited, with many studies suggesting that CPT may be either useless or frankly harmful for several conditions.1 10 12 13 14 15 16 17 18 19 20 As for adults, CPT and suctioning of ventilated children may affect the respiratory system, cardiovascular system, central nervous system, and metabolic demand.2 12 Numerous complications have been attributed to the combination of CPT and ET tube suctioning in neonates, infants, and children, including hypoxia, increased metabolic demand and oxygen consumption, cardiac arrhythmias, changes in blood pressure, raised intracranial pressure and decreased cerebral oxygenation, gastroesophageal reflux, pneumothoraces, rib fractures and periosteal reactions, atelectasis, and death.9 10 13 14 15 17 18 19 21 22 23 24 25 26
Ventilated children are at risk of ventilator-induced lung injury, ventilator-associated pneumonia, oxygen toxicity, hyperinflation, positional atelectasis and/or consolidation, impaired mucociliary clearance, and decreased functional residual capacity due to loss of laryngeal braking.27 28 Increasing volume and viscosity of secretions as a consequence of the ET tubes (foreign body), inadequate humidification of ventilator gases, and disease processes themselves may lead to airway obstruction, infection, atelectasis, and ultimately chronic lung disease.29 As a result, some physiotherapists consider it necessary to treat all ventilated children in an attempt to reduce the incidence of these sequelae. However, evidence supporting “prophylactic” CPT for intubated children is sparse.
Manual multimodal CPT was shown to be associated with improved tidal volume, respiratory compliance, and alveolar dead space compared with ET tube suctioning alone, in a randomized crossover trial of ventilated children.7 30 However, this was not translated into improved blood gases. The CPT group did show a greater drop in airways resistance, suggesting better secretion clearance than suction alone. Importantly, almost a third of patients in both groups deteriorated following the study intervention, and even in retrospect the authors could not identify reasons for response or lack thereof to therapy. This study was limited by a lack of standardization of intervention.7 30
Considering the lack of evidence supporting the use of prophylactic CPT in ventilated infants and children, as well as the potential complications, it is suggested that respiratory management of ventilated children focus on good general nursing and ventilatory management, including analgesia, regular changes in position and early mobilization, lung protective ventilatory strategies, minimal effective inhaled oxygen levels, adequate humidification, and impeccable hygiene and infection control practices. Physiotherapists should engage in the aforementioned holistic care practices, but conventional manual CPT is not indicated routinely for ventilated children.27 This is supported by Krause and Hoehn1 who state, “In mechanically ventilated children, CPT cannot be regarded as a standard treatment modality. CPT must be considered as the most stimulating and disturbing intensive care procedure in mechanically ventilated patients and should not be administered in children with low cardiopulmonary reserve attributable to increased oxygen consumption and increases in intracranial pressure.”
Considering that the main aim of conventional CPT is to reduce or eliminate the mechanical consequences of obstructive secretions, only children with excessive airway secretions or an inability to clear secretions are likely to potentially benefit from treatment.27 Comprehensive reviews of the literature have concluded that the only pediatric condition for which there is reasonable evidence in support of CPT is for the management of children with cystic fibrosis.27 31 Despite a lack of robust evidence, CPT is likely to be beneficial for the treatment of atelectasis when it is caused by mucus plugging and for the management of children admitted to PICU with neuromuscular disease and respiratory exacerbations.27 31 32 33 34 35 CPT has been shown, at best, to be of minimal to no benefit in acute asthma, bronchiolitis, and respiratory failure without atelectasis.20 25 27 31 36 Two randomized controlled trials of hospitalized children with primary pneumonia have not shown any benefit of CPT in improving clinical outcomes.37 38 However, the study of Lukrafka et al38 may have been underpowered to detect a 2-day increase in hospital length of stay in the intervention group and Paludo et al37 also reported a longer duration of coughing (p = 0.04) and added sounds on auscultation (p = 0.03) in those who received CPT compared with controls.
It is important to note that the child's diagnosis should not form the basis of clinical decision making about whether or not CPT should be performed. Rather, each patient should be clinically assessed to determine whether their individual pathophysiology is potentially amenable to intervention.9 The decision on whether or not CPT may be beneficial for a specific patient should be made on the basis of the presence of an excessive volume and/or retention of pulmonary secretions, and/or lobar or segmental collapse caused by mucus plugging. Furthermore, when weighing up the risks and potential benefits of intervention, one must also take cognizance of whether the specific pulmonary problems are impacting on lung mechanics, gaseous exchange, or have the potential for long-term complications such as bronchiectasis.39 Clearly, the concept of “routine” CPT for children with specific conditions, or for all ventilated children, is inappropriate, outdated, and is a practice which might cause considerable harm with an associated financial and psychosocial cost.1 31 39
Considering the known complications of CPT, relative contraindications and precautions to CPT should include children who are severely ill and/or hemodynamically unstable and those with pulmonary hemorrhage (spontaneous or after surfactant treatment), pulmonary edema, coagulation defects, raised or unstable intracranial pressure, pulmonary hypertension and/or a history of hypertensive crises, and very premature or small for gestational age infants. In certain cases, CPT may be beneficial even in children presenting with one or more of the aforementioned conditions. For example, a child with raised intracranial pressure and acute lung collapse could conceivably benefit from CPT considering that the atelectasis may cause hypoxia and hypercapnia, which could exacerbate intracranial hypertension. By reinflating the collapsed lung with appropriately administered CPT, oxygenation and carbon dioxide elimination could be improved, thereby improving intracranial pressure as well. The physiotherapist working in PICU must be aware of intersystem dynamics and take appropriate precautions if treatment is deemed necessary.
Chest Physiotherapy Modalities
Several CPT modalities are commonly used when treating critically ill infants and children, but very few of these have been rigorously tested in clinical trials.27
Positioning
Therapeutic positioning aims to move secretions from the peripheral to proximal airways by gravity, thereby enhancing mucociliary clearance (postural drainage), increasing lung volumes, reducing the work of breathing, minimizing the work of the heart, and optimizing ventilation–perfusion ratios.2 29
Historically, several postural drainage positions, including inverted head-down positions, were advocated, with no supporting objective evidence. Head-down positioning may, however, increase systemic blood pressure with the potential for intraventricular hemorrhage in neonates, increase gastroesophageal reflux and intracranial pressure, place the diaphragm at mechanical disadvantage, and may increase venous return, thereby increasing the work of the heart.23 40 41 42 43 Conversely, the upright position has been shown to improve end-expiratory lung volumes (keeping the functional residual capacity above closing capacity and therefore preventing airway closure) and oxygenation, and may protect against ventilator-associated pneumonia.44 45 46 47 Considering the lack of supporting evidence and the potential for adverse events, the inverted position should not be used in pediatric practice. In the author's opinion, other positions such as side lying, upright sitting, and prone should rather be used according to the indication, preferably with the head of the bed raised (Fig. 1).
Fig. 1.
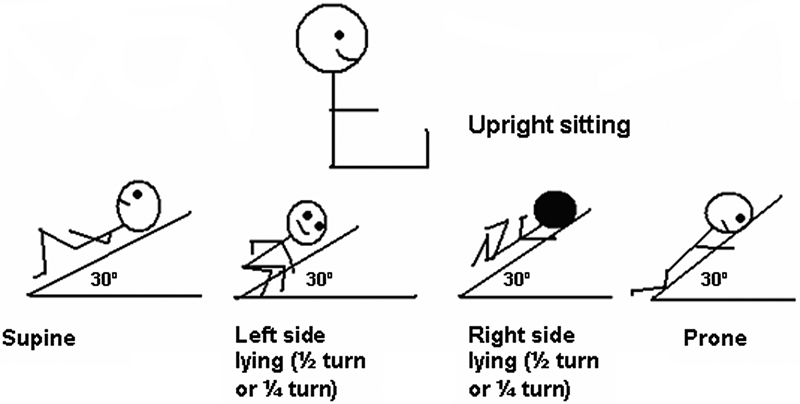
Modified postural drainage positions for pediatric practice.
Despite no proven effect on patient outcome, turning patients from supine to prone dramatically improves oxygenation in mechanically ventilated adults and children with acute lung injury.48 49 50 51 52 53 54 It has been suggested that prone positioning recruits atelectatic dorsal regions of the lung, limits anterior chest wall movement, and reduces the effects of abdominal pressure on the thoracic cavity, thereby promoting more uniform alveolar ventilation—perfusion is redistributed away from the previously dependent lung region and there may be improved ventilation–perfusion matching with a reduction in intrapulmonary shunt.53 55 56
It is well established that spontaneously breathing adults preferentially ventilate their dependent lung regions.57 58 This occurs because of the gravity-related vertical pleural pressure gradient in both the upright and side-lying positions. Dependent lung portions have lower resting volumes and are therefore able to expand more during inspiration, with relatively lower pressures, than the nondependent portions (i.e., they are effectively more compliant). In addition, in the side-lying position, the dome of the lower diaphragm is pushed higher into the chest than the upper diaphragm, increasing the lower diaphragm's contractility and efficiency during spontaneous respiration. Thus, in the awake patient in side lying, the lower lung is normally better ventilated than the upper lung, regardless of the side on which the patient is lying, although there is a tendency toward greater ventilation of the larger right lung.57 58 This adult pattern of ventilation may reverse with anesthetic, muscle relaxants, and positive pressure ventilation which result in a reduced functional residual capacity in both lungs, moving them further down the pressure/volume curve. The dependent lung moves from a steep to a flat part of the curve (requiring higher pressures to attain the same volume changes), and the nondependent part moves from flat to steep (more compliant and requiring less pressure to expand). In addition, if muscle relaxants are used, the curved lower diaphragm confers no advantage because it is no longer contracting; the mediastinum rests on the lower lung impeding expansion. The weight of abdominal contents pushing up on the lung is greatest on the dependent side, compressing the lung, and there is physical compression of the lower lung by the bed.59
Since the 1980s, the pediatric pattern of ventilation was thought to be opposite to that of adults, with preferential ventilation to nondependent lung regions.60 61 However, more recent studies using electrical impedance tomography have shown that there is little difference between adult patterns of ventilation and those of neonates and infants younger than 6 months, both ventilated and spontaneously breathing; spontaneously breathing, healthy, infants and children older than 6 months appear to have a highly variable pattern of ventilation.62 63 64 65 66 67 Importantly, perfusion appears to always be directed to the dependent lung regions, in both children and adults, with the resulting potential of ventilation: perfusion mismatch or correction occurring due to positioning,58 Considering the variability of ventilation distribution in older infants and children, it is therefore suggested that the decision on what position to use clinically should be based on individual response, including an assessment of oxygenation and work of breathing.67 The impact of mechanical ventilation on the distribution of ventilation in children beyond the neonatal age is not yet known.
Mobilization
The complications of immobility in critical illness are well known in adults, and are likely to be similar in children.68 Rehabilitation in PICU is being addressed in other articles in this issue, and therefore is included only briefly in this review. Mobilization techniques should be selected according to the patients' stability, age, developmental level, and general condition. A range of activities are included in the general term “mobilization,” such as active limb exercises, rolling or turning in bed, sitting in bed or out of bed on a chair, standing, and walking (with or without assistance).2 The aims of mobilization include improving thoracic mobility; increasing lung volume; assisting secretion clearance; improving exercise tolerance, muscle strength, and cardiovascular fitness; preventing postural deformities; improving bone ossification and bladder and bowel function; and providing psychological benefits.2 69 70 In adults, early mobilization in ICU has been shown to be safe and feasible.70 71 72 This has not been well studied in the pediatric population, and clinical practice is likely to vary in this regard. One multicenter Canadian study reported that less than 10% of critically ill children received mobilization therapy.73 There is clearly an urgent need for rigorous, prospective trials on the safety and efficacy of mobilization in the PICU specifically.
Chest Manipulations/Manual CPT
Percussion and vibrations are CPT techniques, performed manually or mechanically, which are widely used to assist with removal of secretions from the lungs. It is thought that the application of manipulations to the chest wall transmits mechanical energy into the airways where thixotropic pulmonary secretions are liquefied, and can then be cleared by positioning, cough, or suctioning.2 74
Manual vibration, with a combination of compression and oscillation, has been shown to increase expiratory flow rate via increased intrapleural pressure in a small study of healthy adults and in ventilated children.74 75 76 Manual percussion has been associated with cardiac arrhythmia and a drop in pulmonary compliance in critically ill adults and both percussion and vibrations have been shown to cause or exacerbate bronchospasm.2 77 An animal study reported that the application of manual techniques was associated with the development of atelectasis.19 The use of percussion or any external vibration method is still not supported by scientific evidence,1 2 35 77 78 and clinical trials are needed in the pediatric age group to determine their efficacy in different contexts.
Manual Hyperinflation
Physiotherapists working in adult intensive care units often use manual hyperinflation techniques in conjunction with other manipulations to expand the lung and loosen secretions, and in some centers the technique is commonly used for critically ill children and infants as well.79 80 81 82 Manual hyperinflation usually consists of a series of deep manual inflations (ideally to a predetermined set pressure or volume) with brief inspiratory holds, followed by a rapid release of the bag to enhance expiratory flow.2 Manual hyperinflation aims to prevent or treat lung collapse, improve oxygenation and compliance, and promote secretion clearance.2
Several concerns exist regarding the use of manual hyperinflation, particularly in the context of PICU care. Manual hyperinflation and manual ventilation, generally, often deliver 100% cold, dry oxygen, by means of devices providing variable, often unmeasured pressures and unknown tidal volumes, frequently without maintaining positive end-expiratory pressure.83 There are conflicting reports on the efficacy of manual hyperinflation in adults, with some reporting improvements in atelectasis, lung compliance, and gas exchange and others reporting no change.2 79 84 85 86 Increased intracranial pressure and significant cardiovascular complications during manual hyperinflation have been reported in adult studies.79
With the application of positive pressure to the lungs, there is the risk of over distension of normal alveoli.2 35 This may be of particular concern in critically ill infants and children given their propensity for baro and volutrauma. Only three studies relating to manual hyperinflation in children were identified for inclusion in a systematic review: two observational studies and one randomized crossover trial.76 82 87 The crossover trial did not analyze different CPT modalities separately and therefore no conclusions can be made regarding the effects of manual hyperinflation itself.7 Therefore, there is insufficient evidence regarding the safety or efficacy of manual hyperinflation in critically ill infants and children and there are reasons for concern for implementing this modality in this population, as outlined further later.
Peak inspiratory pressure is only a proxy for inspired tidal volume. Even if peak inspiratory pressure is measured and controlled, one cannot directly extrapolate tidal volume, which depends on several variables including respiratory compliance (which changes even as the lungs expand during a normal breath).83 The role of “volutrauma” in lung injury is well described, with limitation of inspired tidal volume an essential component of lung protective ventilation strategies in both adults and children.88 89 90 A large tidal volume can cause or exacerbate lung damage regardless of the pressure applied, particularly when the lungs are fragile and immature, with low lung compliance.83 Considering the lack of evidence supporting manual hyperventilation in critically ill infants and children, and the potential for harm, this practice should not be considered an acceptable component of standard CPT in PICU practice.
Breathing Exercises
Several different breathing exercises are sometimes used in the PICU, including deep breathing exercises, positive expiratory pressure (PEP) therapy, localized breathing exercises, active cycle of breathing technique, oscillatory PEP, and autogenic drainage. Evidence supporting the use of these techniques is largely extrapolated from studies on children with cystic fibrosis.91 It has been suggested that deep breathing exercises may be the safest, cheapest, and most effective way of keeping the lungs expanded and secretions moving.92 Breathing exercises are difficult to perform in ventilated children, and therefore are not applied often in the PICU context, but may be useful in the older, nonventilated, cooperative child in the PICU.81
Endotracheal Suctioning
After mobilizing secretions using different CPT modalities, secretions need to be removed from intubated children by ET suctioning. Recommendations and clinical guidelines for ET suctioning have been published previously, but supporting evidence remains weak5 35 93 94 95 96 97 98 99 100 and ET suctioning practices still vary widely among critical care practitioners in different centers.101
ET suctioning is necessary to prevent and remove airway obstruction, but it is not a benign procedure.96 Adverse effects of ET suctioning in all patient groups include hypoxia, pneumothorax, mucosal trauma, atelectasis, loss of ciliary function, bradycardia and other arrhythmias, increases in systemic blood pressure, raised intracranial pressure, and pain.3 93 102 103 104 105 106 107 108 109 110 111 112 113 114 115 116 117 118 119 120 121 122 123 124
Special care should be taken when suctioning patients who have raised intracranial pressure and pulmonary hypertension, as these could be exacerbated by ET suctioning and coughing.116 118 125 Patients with pulmonary edema and pulmonary hemorrhage should only be suctioned when absolutely necessary.126 127 To prevent or reduce the severity of ET-suction–associated complications, care must be taken in using appropriate suction technique (including appropriate selection of catheter size and suction pressure), suctioning only when indicated in the presence of obstructive secretions, limiting the depth of insertion of the suction catheter, preoxygenating, and not instilling saline routinely.96 There is no clear benefit of using closed versus open suction systems.96 128 129 130 131
Conclusion
CPT and ET suctioning should not be performed routinely in the PICU. Considering the lack of evidence supporting CPT and the potential for serious adverse consequences, care should be taken in determining the need for intervention, taking into account the child's age, condition, and the presence of contraindications or precautions, on the basis of comprehensive individual clinical assessment. Modalities used should be carefully selected and applied for each patient to minimize or prevent complications. Rigorous, randomized, controlled clinical trials of sufficient size are urgently needed to develop evidence-based practice guidelines for CPT in critically ill infants and children, and to examine the impact of different modalities on clinically relevant patient outcome measures.
Until such evidence is available, “… those involved in the management of pediatric respiratory disorders should avoid the unnecessary distress to both the child and family of useless treatment and the potentially serious consequences of inappropriate intervention.”10
References
- 1.Krause M F, Hoehn T. Chest physiotherapy in mechanically ventilated children: a review. Crit Care Med. 2000;28(5):1648–1651. doi: 10.1097/00003246-200005000-00067. [DOI] [PubMed] [Google Scholar]
- 2.Stiller K. Physiotherapy in intensive care: towards an evidence-based practice. Chest. 2000;118(6):1801–1813. doi: 10.1378/chest.118.6.1801. [DOI] [PubMed] [Google Scholar]
- 3.Bailey C, Kattwinkel J, Teja K, Buckley T. Shallow versus deep endotracheal suctioning in young rabbits: pathologic effects on the tracheobronchial wall. Pediatrics. 1988;82(5):746–751. [PubMed] [Google Scholar]
- 4.Fisher B J, Carlo W A, Doershuk C F. Philadelphia, PA: Lea & Febiger; 1990. Fetus, newborn, child, adolescent. In: Scarpelli EM, ed. Pulmonary Physiology. 2nd ed; pp. 422–428. [Google Scholar]
- 5.Young J. To help or hinder. Endotracheal suction and the intubated neonate. J Neonatal Nurs. 1995;1:23–28. [Google Scholar]
- 6.Guglielminotti J, Alzieu M, Maury E, Guidet B, Offenstadt G. Bedside detection of retained tracheobronchial secretions in patients receiving mechanical ventilation: is it time for tracheal suctioning? Chest. 2000;118(4):1095–1099. doi: 10.1378/chest.118.4.1095. [DOI] [PubMed] [Google Scholar]
- 7.Main E, Castle R, Newham D, Stocks J. Respiratory physiotherapy vs. suction: the effects on respiratory function in ventilated infants and children. Intensive Care Med. 2004;30(6):1144–1151. doi: 10.1007/s00134-004-2262-0. [DOI] [PubMed] [Google Scholar]
- 8.Ntoumenopoulos G. Questioning chest physiotherapy. Chest. 1997;112(1):292–293. doi: 10.1378/chest.112.1.292-a. [DOI] [PubMed] [Google Scholar]
- 9.Oberwaldner B. Physiotherapy for airway clearance in paediatrics. Eur Respir J. 2000;15(1):196–204. doi: 10.1183/09031936.00.15119600. [DOI] [PubMed] [Google Scholar]
- 10.Wallis C, Prasad A. Who needs chest physiotherapy? Moving from anecdote to evidence. Arch Dis Child. 1999;80(4):393–397. doi: 10.1136/adc.80.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciesla N D. Chest physical therapy for patients in the intensive care unit. Phys Ther. 1996;76(6):609–625. doi: 10.1093/ptj/76.6.609. [DOI] [PubMed] [Google Scholar]
- 12.Weissman C, Kemper M, Damask M C, Askanazi J, Hyman A I, Kinney J M. Effect of routine intensive care interactions on metabolic rate. Chest. 1984;86(6):815–818. doi: 10.1378/chest.86.6.815. [DOI] [PubMed] [Google Scholar]
- 13.Chalumeau M, Foix-L'Helias L, Scheinmann P, Zuani P, Gendrel D, Ducou-le-Pointe H. Rib fractures after chest physiotherapy for bronchiolitis or pneumonia in infants. Pediatr Radiol. 2002;32(9):644–647. doi: 10.1007/s00247-002-0755-y. [DOI] [PubMed] [Google Scholar]
- 14.Chanelière C, Moreux N, Pracros J P, Bellon G, Reix P. Rib fractures after chest physiotherapy: a report of 2 cases [in French] Arch Pediatr. 2006;13(11):1410–1412. doi: 10.1016/j.arcped.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 15.Harding J E, Miles F K, Becroft D M, Allen B C, Knight D B. Chest physiotherapy may be associated with brain damage in extremely premature infants. J Pediatr. 1998;132(3, Pt 1):440–444. doi: 10.1016/s0022-3476(98)70017-4. [DOI] [PubMed] [Google Scholar]
- 16.Button B M, Heine R G, Catto-Smith A G, Phelan P D, Olinsky A. Postural drainage and gastro-oesophageal reflux in infants with cystic fibrosis. Arch Dis Child. 1997;76(2):148–150. doi: 10.1136/adc.76.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Button B M, Heine R G, Catto-Smith A G, Phelan P D, Olinsky A. Chest physiotherapy, gastro-oesophageal reflux, and arousal in infants with cystic fibrosis. Arch Dis Child. 2004;89(5):435–439. doi: 10.1136/adc.2003.033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reines H D, Sade R M, Bradford B F, Marshall J. Chest physiotherapy fails to prevent postoperative atelectasis in children after cardiac surgery. Ann Surg. 1982;195(4):451–455. doi: 10.1097/00000658-198204000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zidulka A, Chrome J F, Wight D W, Burnett S, Bonnier L, Fraser R. Clapping or percussion causes atelectasis in dogs and influences gas exchange. J Appl Physiol (1985) 1989;66(6):2833–2838. doi: 10.1152/jappl.1989.66.6.2833. [DOI] [PubMed] [Google Scholar]
- 20.Roqué i Figuls M, Giné-Garriga M, Granados Rugeles C, Perrotta C. Chest physiotherapy for acute bronchiolitis in paediatric patients between 0 and 24 months old. Cochrane Database Syst Rev. 2012;2:CD004873. doi: 10.1002/14651858.CD004873.pub4. [DOI] [PubMed] [Google Scholar]
- 21.Fox W W, Schwartz J G, Shaffer T H. Pulmonary physiotherapy in neonates: physiologic changes and respiratory management. J Pediatr. 1978;92(6):977–981. doi: 10.1016/s0022-3476(78)80381-3. [DOI] [PubMed] [Google Scholar]
- 22.Gray P H Flenady V J Blackwell L Potential risks of chest physiotherapy in preterm infants J Pediatr 19991351131, author reply 132 [DOI] [PubMed] [Google Scholar]
- 23.Vandenplas Y, Diericx A, Blecker U, Lanciers S, Deneyer M. Esophageal pH monitoring data during chest physiotherapy. J Pediatr Gastroenterol Nutr. 1991;13(1):23–26. doi: 10.1097/00005176-199107000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Argent A C, Morrow B M. What does chest physiotherapy do to sick infants and children? Intensive Care Med. 2004;30(6):1014–1016. doi: 10.1007/s00134-004-2216-6. [DOI] [PubMed] [Google Scholar]
- 25.Asher M I, Douglas C, Airy M, Andrews D, Trenholme A. Effects of chest physical therapy on lung function in children recovering from acute severe asthma. Pediatr Pulmonol. 1990;9(3):146–151. doi: 10.1002/ppul.1950090305. [DOI] [PubMed] [Google Scholar]
- 26.Raval D, Yeh T F, Mora A, Cuevas D, Pyati S, Pildes R S. Chest physiotherapy in preterm infants with RDS in the first 24 hours of life. J Perinatol. 1987;7(4):301–304. [PubMed] [Google Scholar]
- 27.Schechter M S Airway clearance applications in infants and children Respir Care 200752101382–1390., discussion 1390–1391 [PubMed] [Google Scholar]
- 28.Morrow B M, Argent A C, Jeena P M, Green R J. Guideline for the diagnosis, prevention and treatment of paediatric ventilator-associated pneumonia. S Afr Med J. 2009;99(4, Pt 2):255–267. [PubMed] [Google Scholar]
- 29.Clini E, Ambrosino N. Early physiotherapy in the respiratory intensive care unit. Respir Med. 2005;99(9):1096–1104. doi: 10.1016/j.rmed.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 30.Main E, Stocks J. The influence of physiotherapy and suction on respiratory deadspace in ventilated children. Intensive Care Med. 2004;30(6):1152–1159. doi: 10.1007/s00134-004-2261-1. [DOI] [PubMed] [Google Scholar]
- 31.Walsh B K Hood K Merritt G Pediatric airway maintenance and clearance in the acute care setting: how to stay out of trouble Respir Care 20115691424–1440., discussion 1440–1444 [DOI] [PubMed] [Google Scholar]
- 32.Peroni D G, Boner A L. Atelectasis: mechanisms, diagnosis and management. Paediatr Respir Rev. 2000;1(3):274–278. doi: 10.1053/prrv.2000.0059. [DOI] [PubMed] [Google Scholar]
- 33.Bilan N, Galehgolab B A, Shoaran M. Medical treatment of lung collapse in children. Pak J Biol Sci. 2009;12(5):467–469. doi: 10.3923/pjbs.2009.467.469. [DOI] [PubMed] [Google Scholar]
- 34.Galvis A G, Reyes G, Nelson W B. Bedside management of lung collapse in children on mechanical ventilation: saline lavage—simulated cough technique proves simple, effective. Pediatr Pulmonol. 1994;17(5):326–330. doi: 10.1002/ppul.1950170510. [DOI] [PubMed] [Google Scholar]
- 35.Branson R D Secretion management in the mechanically ventilated patient Respir Care 200752101328–1342., discussion 1342–1347 [PubMed] [Google Scholar]
- 36.Hondras M A, Linde K, Jones A P. Manual therapy for asthma. Cochrane Database Syst Rev. 2005;(2):CD001002. doi: 10.1002/14651858.CD001002.pub2. [DOI] [PubMed] [Google Scholar]
- 37.Paludo C, Zhang L, Lincho C S, Lemos D V, Real G G, Bergamin J A. Chest physical therapy for children hospitalised with acute pneumonia: a randomised controlled trial. Thorax. 2008;63(9):791–794. doi: 10.1136/thx.2007.088195. [DOI] [PubMed] [Google Scholar]
- 38.Lukrafka J L, Fuchs S C, Fischer G B, Flores J A, Fachel J M, Castro-Rodriguez J A. Chest physiotherapy in paediatric patients hospitalised with community-acquired pneumonia: a randomised clinical trial. Arch Dis Child. 2012;97(11):967–971. doi: 10.1136/archdischild-2012-302279. [DOI] [PubMed] [Google Scholar]
- 39.De Boeck K, Vermeulen F, Vreys M, Moens M, Proesmans M. Airway clearance techniques to treat acute respiratory disorders in previously healthy children: where is the evidence? Eur J Pediatr. 2008;167(6):607–612. doi: 10.1007/s00431-008-0689-y. [DOI] [PubMed] [Google Scholar]
- 40.Crane L D, Zombek M, Krauss A N, Auld P AM. 1173 Comparison of chest physiotherapy techniques in infants with HMD. Pediatr Res. 1978;12:559. [Google Scholar]
- 41.Button B M, Heine R G, Catto-Smith A G. et al. Chest physiotherapy in infants with cystic fibrosis: to tip or not? A five-year study. Pediatr Pulmonol. 2003;35(3):208–213. doi: 10.1002/ppul.10227. [DOI] [PubMed] [Google Scholar]
- 42.Emery J R, Peabody J L. Head position affects intracranial pressure in newborn infants. J Pediatr. 1983;103(6):950–953. doi: 10.1016/s0022-3476(83)80728-8. [DOI] [PubMed] [Google Scholar]
- 43.Vivian-Beresford A, King C, MaCauley H. Neonatal post-extubation complications: the preventative role of physiotherapy. Physiother Can. 1987;39:184–190. [Google Scholar]
- 44.Stark A R, Waggener T B, Frantz I D III, Cohlan B A, Feldman H A, Kosch P C. Effect on ventilation of change to the upright posture in newborn infants. J Appl Physiol. 1984;56(1):64–71. doi: 10.1152/jappl.1984.56.1.64. [DOI] [PubMed] [Google Scholar]
- 45.Dellagrammaticas H D, Kapetanakis J, Papadimitriou M, Kourakis G. Effect of body tilting on physiological functions in stable very low birthweight neonates. Arch Dis Child. 1991;66(4 Spec No):429–432. doi: 10.1136/adc.66.4_spec_no.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drakulovic M B, Torres A, Bauer T T, Nicolas J M, Nogué S, Ferrer M. Supine body position as a risk factor for nosocomial pneumonia in mechanically ventilated patients: a randomised trial. Lancet. 1999;354(9193):1851–1858. doi: 10.1016/S0140-6736(98)12251-1. [DOI] [PubMed] [Google Scholar]
- 47.Nunn J F. Oxford: Butterworth-Heinemann Ltd; 1993. Nunn's Applied Respiratory Physiology. 4th ed. [Google Scholar]
- 48.Curley M A, Hibberd P L, Fineman L D. et al. Effect of prone positioning on clinical outcomes in children with acute lung injury: a randomized controlled trial. JAMA. 2005;294(2):229–237. doi: 10.1001/jama.294.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casado-Flores J, Martínez de Azagra A, Ruiz-López M J, Ruiz M, Serrano A. Pediatric ARDS: effect of supine-prone postural changes on oxygenation. Intensive Care Med. 2002;28(12):1792–1796. doi: 10.1007/s00134-002-1527-8. [DOI] [PubMed] [Google Scholar]
- 50.Dupont H, Mentec H, Cheval C, Moine P, Fierobe L, Timsit J F. Short-term effect of inhaled nitric oxide and prone positioning on gas exchange in patients with severe acute respiratory distress syndrome. Crit Care Med. 2000;28(2):304–308. doi: 10.1097/00003246-200002000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Jolliet P, Bulpa P, Chevrolet J C. Effects of the prone position on gas exchange and hemodynamics in severe acute respiratory distress syndrome. Crit Care Med. 1998;26(12):1977–1985. doi: 10.1097/00003246-199812000-00023. [DOI] [PubMed] [Google Scholar]
- 52.Kornecki A, Frndova H, Coates A L, Shemie S D. 4A randomized trial of prolonged prone positioning in children with acute respiratory failure. Chest. 2001;119(1):211–218. doi: 10.1378/chest.119.1.211. [DOI] [PubMed] [Google Scholar]
- 53.Pelosi P, Tubiolo D, Mascheroni D. et al. Effects of the prone position on respiratory mechanics and gas exchange during acute lung injury. Am J Respir Crit Care Med. 1998;157(2):387–393. doi: 10.1164/ajrccm.157.2.97-04023. [DOI] [PubMed] [Google Scholar]
- 54.Pelosi P, Brazzi L, Gattinoni L. Prone position in acute respiratory distress syndrome. Eur Respir J. 2002;20(4):1017–1028. doi: 10.1183/09031936.02.00401702. [DOI] [PubMed] [Google Scholar]
- 55.Matthews B D, Noviski N. Management of oxygenation in pediatric acute hypoxemic respiratory failure. Pediatr Pulmonol. 2001;32(6):459–470. doi: 10.1002/ppul.1159. [DOI] [PubMed] [Google Scholar]
- 56.Marraro G A. Innovative practices of ventilatory support with pediatric patients. Pediatr Crit Care Med. 2003;4(1):8–20. doi: 10.1097/00130478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 57.Riedel T, Richards T, Schibler A. The value of electrical impedance tomography in assessing the effect of body position and positive airway pressures on regional lung ventilation in spontaneously breathing subjects. Intensive Care Med. 2005;31(11):1522–1528. doi: 10.1007/s00134-005-2734-x. [DOI] [PubMed] [Google Scholar]
- 58.Bhuyan U, Peters A M, Gordon I, Davies H, Helms P. Effects of posture on the distribution of pulmonary ventilation and perfusion in children and adults. Thorax. 1989;44(6):480–484. doi: 10.1136/thx.44.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benumof J L, Alfery D D. Philadelphia, PA: Churchill Livingstone; 2000. Anesthesia for Thoracic Surgery. [Google Scholar]
- 60.Davies H, Helms P, Gordon I. Effect of posture on regional ventilation in children. Pediatr Pulmonol. 1992;12(4):227–232. doi: 10.1002/ppul.1950120406. [DOI] [PubMed] [Google Scholar]
- 61.Heaf D P, Helms P, Gordon I, Turner H M. Postural effects on gas exchange in infants. N Engl J Med. 1983;308(25):1505–1508. doi: 10.1056/NEJM198306233082505. [DOI] [PubMed] [Google Scholar]
- 62.Senol G, Kirakli C, Halilçolar H. In vitro antibacterial activities of oral care products against ventilator-associated pneumonia pathogens. Am J Infect Control. 2007;35(8):531–535. doi: 10.1016/j.ajic.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 63.Li Z, Kosorok M R, Farrell P M. et al. Longitudinal development of mucoid Pseudomonas aeruginosa infection and lung disease progression in children with cystic fibrosis. JAMA. 2005;293(5):581–588. doi: 10.1001/jama.293.5.581. [DOI] [PubMed] [Google Scholar]
- 64.Ren C L, Brucker J L, Rovitelli A K, Bordeaux K A. Changes in lung function measured by spirometry and the forced oscillation technique in cystic fibrosis patients undergoing treatment for respiratory tract exacerbation. Pediatr Pulmonol. 2006;41(4):345–349. doi: 10.1002/ppul.20390. [DOI] [PubMed] [Google Scholar]
- 65.Pham T M, Yuill M, Dakin C, Schibler A. Regional ventilation distribution in the first 6 months of life. Eur Respir J. 2011;37(4):919–924. doi: 10.1183/09031936.00034310. [DOI] [PubMed] [Google Scholar]
- 66.Hough J L, Johnston L, Brauer S, Woodgate P, Schibler A. Effect of body position on ventilation distribution in ventilated preterm infants. Pediatr Crit Care Med. 2013;14(2):171–177. doi: 10.1097/PCC.0b013e31826e708a. [DOI] [PubMed] [Google Scholar]
- 67.Lupton-Smith A R, Argent A C, Rimensberger P C, Morrow B M. Challenging a paradigm: positional changes in ventilation distribution are highly variable in healthy infants and children. Pediatr Pulmonol. 2014;49(8):764–771. doi: 10.1002/ppul.22893. [DOI] [PubMed] [Google Scholar]
- 68.Desai S V, Law T J, Needham D M. Long-term complications of critical care. Crit Care Med. 2011;39(2):371–379. doi: 10.1097/CCM.0b013e3181fd66e5. [DOI] [PubMed] [Google Scholar]
- 69.Zafiropoulos B, Alison J A, McCarren B. Physiological responses to the early mobilisation of the intubated, ventilated abdominal surgery patient. Aust J Physiother. 2004;50(2):95–100. doi: 10.1016/s0004-9514(14)60101-x. [DOI] [PubMed] [Google Scholar]
- 70.Bailey P, Thomsen G E, Spuhler V J. et al. Early activity is feasible and safe in respiratory failure patients. Crit Care Med. 2007;35(1):139–145. doi: 10.1097/01.CCM.0000251130.69568.87. [DOI] [PubMed] [Google Scholar]
- 71.Li Z, Peng X, Zhu B, Zhang Y, Xi X. Active mobilization for mechanically ventilated patients: a systematic review. Arch Phys Med Rehabil. 2013;94(3):551–561. doi: 10.1016/j.apmr.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 72.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit Care Med. 2013;41(6):1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 73.Choong K, Foster G, Fraser D D. et al. Acute rehabilitation practices in critically ill children: a multicenter study. Pediatr Crit Care Med. 2014;15(6):e270–e279. doi: 10.1097/PCC.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 74.McCarren B, Alison J A, Herbert R D. Vibration and its effect on the respiratory system. Aust J Physiother. 2006;52(1):39–43. doi: 10.1016/s0004-9514(06)70060-5. [DOI] [PubMed] [Google Scholar]
- 75.McCarren B, Alison J A, Herbert R D. Manual vibration increases expiratory flow rate via increased intrapleural pressure in healthy adults: an experimental study. Aust J Physiother. 2006;52(4):267–271. doi: 10.1016/s0004-9514(06)70006-x. [DOI] [PubMed] [Google Scholar]
- 76.Gregson R K, Shannon H, Stocks J, Cole T J, Peters M J, Main E. The unique contribution of manual chest compression-vibrations to airflow during physiotherapy in sedated, fully ventilated children. Pediatr Crit Care Med. 2012;13(2):e97–e102. doi: 10.1097/PCC.0b013e3182230f5a. [DOI] [PubMed] [Google Scholar]
- 77.Kirilloff L H, Owens G R, Rogers R M, Mazzocco M C. Does chest physical therapy work? Chest. 1985;88(3):436–444. doi: 10.1378/chest.88.3.436. [DOI] [PubMed] [Google Scholar]
- 78.van der Schans C P, Postma D S, Koëter G H, Rubin B K. Physiotherapy and bronchial mucus transport. Eur Respir J. 1999;13(6):1477–1486. doi: 10.1183/09031936.99.13614879. [DOI] [PubMed] [Google Scholar]
- 79.Patman S, Jenkins S, Stiller K. Manual hyperinflation—effects on respiratory parameters. Physiother Res Int. 2000;5(3):157–171. doi: 10.1002/pri.196. [DOI] [PubMed] [Google Scholar]
- 80.McCarren B, Chow C M. Manual hyperinflation: a description of the technique. Aust J Physiother. 1996;42(3):203–208. doi: 10.1016/s0004-9514(14)60387-1. [DOI] [PubMed] [Google Scholar]
- 81.McCord J, Krull N, Kraiker J. et al. Cardiopulmonary physical therapy practice in the paediatric intensive care unit. Physiother Can. 2013;65(4):374–377. doi: 10.3138/ptc.2012-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.de Godoy V C, Zanetti N M, Johnston C. Manual hyperinflation in airway clearance in pediatric patients: a systematic review. Rev Bras Ter Intensiva. 2013;25(3):258–262. doi: 10.5935/0103-507X.20130043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.O'Donnell C P, Davis P G, Morley C J. Resuscitation of premature infants: what are we doing wrong and can we do better? Biol Neonate. 2003;84(1):76–82. doi: 10.1159/000071008. [DOI] [PubMed] [Google Scholar]
- 84.Choi J S, Jones A Y. Effects of manual hyperinflation and suctioning in respiratory mechanics in mechanically ventilated patients with ventilator-associated pneumonia. Aust J Physiother. 2005;51(1):25–30. doi: 10.1016/s0004-9514(05)70050-7. [DOI] [PubMed] [Google Scholar]
- 85.Stiller K, Geake T, Taylor J, Grant R, Hall B. Acute lobar atelectasis. A comparison of two chest physiotherapy regimens. Chest. 1990;98(6):1336–1340. doi: 10.1378/chest.98.6.1336. [DOI] [PubMed] [Google Scholar]
- 86.Barker M, Adams S. An evaluation of a single chest physiotherapy treatment on mechanically ventilated patients with acute lung injury. Physiother Res Int. 2002;7(3):157–169. doi: 10.1002/pri.252. [DOI] [PubMed] [Google Scholar]
- 87.Gregson R K, Stocks J, Petley G W. et al. Simultaneous measurement of force and respiratory profiles during chest physiotherapy in ventilated children. Physiol Meas. 2007;28(9):1017–1028. doi: 10.1088/0967-3334/28/9/004. [DOI] [PubMed] [Google Scholar]
- 88.Brochard L, Roudot-Thoraval F, Roupie E. et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158(6):1831–1838. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 89.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157(1):294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 90.Carpenter T. Novel approaches in conventional mechanical ventilation for paediatric acute lung injury. Paediatr Respir Rev. 2004;5(3):231–237. doi: 10.1016/j.prrv.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 91.Bradley J M, Moran F M, Elborn J S. Evidence for physical therapies (airway clearance and physical training) in cystic fibrosis: an overview of five Cochrane systematic reviews. Respir Med. 2006;100(2):191–201. doi: 10.1016/j.rmed.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 92.de Boeck C, Zinman R. Cough versus chest physiotherapy. A comparison of the acute effects on pulmonary function in patients with cystic fibrosis. Am Rev Respir Dis. 1984;129(1):182–184. doi: 10.1164/arrd.1984.129.1.182. [DOI] [PubMed] [Google Scholar]
- 93.Boothroyd A E, Murthy B V, Darbyshire A, Petros A J. Endotracheal suctioning causes right upper lobe collapse in intubated children. Acta Paediatr. 1996;85(12):1422–1425. doi: 10.1111/j.1651-2227.1996.tb13946.x. [DOI] [PubMed] [Google Scholar]
- 94.American Association for Respiratory Care . AARC clinical practice guideline. Endotracheal suctioning of mechanically ventilated adults and children with artificial airways. Respir Care. 1993;38(5):500–504. [PubMed] [Google Scholar]
- 95.Runton N. Suctioning artificial airways in children: appropriate technique. Pediatr Nurs. 1992;18(2):115–118. [PubMed] [Google Scholar]
- 96.Morrow B M, Argent A C. A comprehensive review of pediatric endotracheal suctioning: Effects, indications, and clinical practice. Pediatr Crit Care Med. 2008;9(5):465–477. doi: 10.1097/PCC.0b013e31818499cc. [DOI] [PubMed] [Google Scholar]
- 97.Hodge D. Endotracheal suctioning and the infant: a nursing care protocol to decrease complications. Neonatal Netw. 1991;9(5):7–15. [PubMed] [Google Scholar]
- 98.Young C S. Recommended guide lines for suction. Physiotherapy. 1984;70(3):106–108. [PubMed] [Google Scholar]
- 99.Tolles C L, Stone K S. National survey of neonatal endotracheal suctioning practices. Neonatal Netw. 1990;9(2):7–14. [PubMed] [Google Scholar]
- 100.Copnell B, Fergusson D. Endotracheal suctioning: time-worn ritual or timely intervention? Am J Crit Care. 1995;4(2):100–105. [PubMed] [Google Scholar]
- 101.Kelleher S, Andrews T. An observational study on the open-system endotracheal suctioning practices of critical care nurses. J Clin Nurs. 2008;17(3):360–369. doi: 10.1111/j.1365-2702.2007.01990.x. [DOI] [PubMed] [Google Scholar]
- 102.Kerem E, Yatsiv I, Goitein K J. Effect of endotracheal suctioning on arterial blood gases in children. Intensive Care Med. 1990;16(2):95–99. doi: 10.1007/BF02575301. [DOI] [PubMed] [Google Scholar]
- 103.Singh N C, Kissoon N, Frewen T, Tiffin N. Physiological responses to endotracheal and oral suctioning in paediatric patients: the influence of endotracheal tube sizes and suction pressures. Clin Intensive Care. 1991;2(6):345–350. [PubMed] [Google Scholar]
- 104.Loubser M D, Mahoney P J, Milligan D W. Hazards of routine endotracheal suction in the neonatal unit. Lancet. 1989;1(8652):1444–1445. doi: 10.1016/s0140-6736(89)90144-x. [DOI] [PubMed] [Google Scholar]
- 105.Anderson K D, Chandra R. Pneumothorax secondary to perforation of sequential bronchi by suction catheters. J Pediatr Surg. 1976;11(5):687–693. doi: 10.1016/0022-3468(76)90091-9. [DOI] [PubMed] [Google Scholar]
- 106.Kuzenski B M. Effect of negative pressure on tracheobronchial trauma. Nurs Res. 1978;27(4):260–263. [PubMed] [Google Scholar]
- 107.Nagaraj H S, Shott R, Fellows R, Yacoub U. Recurrent lobar atelectasis due to acquired bronchial stenosis in neonates. J Pediatr Surg. 1980;15(4):411–415. doi: 10.1016/s0022-3468(80)80745-7. [DOI] [PubMed] [Google Scholar]
- 108.Choong K, Chatrkaw P, Frndova H, Cox P N. Comparison of loss in lung volume with open versus in-line catheter endotracheal suctioning. Pediatr Crit Care Med. 2003;4(1):69–73. doi: 10.1097/00130478-200301000-00014. [DOI] [PubMed] [Google Scholar]
- 109.Morrow B, Futter M, Argent A. Effect of endotracheal suction on lung dynamics in mechanically-ventilated paediatric patients. Aust J Physiother. 2006;52(2):121–126. doi: 10.1016/s0004-9514(06)70047-2. [DOI] [PubMed] [Google Scholar]
- 110.Ehrhart I C, Hofman W F, Loveland S R. Effects of endotracheal suction versus apnea during interruption of intermittent or continuous positive pressure ventilation. Crit Care Med. 1981;9(6):464–468. doi: 10.1097/00003246-198106000-00006. [DOI] [PubMed] [Google Scholar]
- 111.Hoellering A B, Copnell B, Dargaville P A, Mills J F, Morley C J, Tingay D G. Lung volume and cardiorespiratory changes during open and closed endotracheal suction in ventilated newborn infants. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F436–F441. doi: 10.1136/adc.2007.132076. [DOI] [PubMed] [Google Scholar]
- 112.Kohlhauser C, Bernert G, Hermon M, Popow C, Seidl R, Pollak A. Effects of endotracheal suctioning in high-frequency oscillatory and conventionally ventilated low birth weight neonates on cerebral hemodynamics observed by near infrared spectroscopy (NIRS) Pediatr Pulmonol. 2000;29(4):270–275. doi: 10.1002/(sici)1099-0496(200004)29:4<270::aid-ppul6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 113.Simbruner G, Coradello H, Fodor M, Havelec L, Lubec G, Pollak A. Effect of tracheal suction on oxygenation, circulation, and lung mechanics in newborn infants. Arch Dis Child. 1981;56(5):326–330. doi: 10.1136/adc.56.5.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zmora E, Merritt T A. Use of side-hole endotracheal tube adapter for tracheal aspiration. A controlled study. Am J Dis Child. 1980;134(3):250–254. doi: 10.1001/archpedi.1980.02130150008003. [DOI] [PubMed] [Google Scholar]
- 115.Cabal L, Devaskar S, Siassi B. et al. New endotracheal tube adaptor reducing cardiopulmonary effects of suctioning. Crit Care Med. 1979;7(12):552–555. doi: 10.1097/00003246-197912000-00009. [DOI] [PubMed] [Google Scholar]
- 116.Fanconi S, Duc G. Intratracheal suctioning in sick preterm infants: prevention of intracranial hypertension and cerebral hypoperfusion by muscle paralysis. Pediatrics. 1987;79(4):538–543. [PubMed] [Google Scholar]
- 117.Tume L, Jinks A. Endotracheal suctioning in children with severe traumatic brain injury: a literature review. Nurs Crit Care. 2008;13(5):232–240. doi: 10.1111/j.1478-5153.2008.00285.x. [DOI] [PubMed] [Google Scholar]
- 118.Kerr M E, Rudy E B, Weber B B. et al. Effect of short-duration hyperventilation during endotracheal suctioning on intracranial pressure in severe head-injured adults. Nurs Res. 1997;46(4):195–201. doi: 10.1097/00006199-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 119.Kerr M E, Sereika S M, Orndoff P. et al. Effect of neuromuscular blockers and opiates on the cerebrovascular response to endotracheal suctioning in adults with severe head injuries. Am J Crit Care. 1998;7(3):205–217. [PubMed] [Google Scholar]
- 120.Evans J C, Vogelpohl D G, Bourguignon C M, Morcott C S. Pain behaviors in LBW infants accompany some “nonpainful” caregiving procedures. Neonatal Netw. 1997;16(3):33–40. [PubMed] [Google Scholar]
- 121.Cignacco E, Hamers J P, van Lingen R A. et al. Pain relief in ventilated preterms during endotracheal suctioning: a randomized controlled trial. Swiss Med Wkly. 2008;138(43–44):635–645. doi: 10.4414/smw.2008.12288. [DOI] [PubMed] [Google Scholar]
- 122.Puntillo K A. Dimensions of procedural pain and its analgesic management in critically ill surgical patients. Am J Crit Care. 1994;3(2):116–122. [PubMed] [Google Scholar]
- 123.Payen J F, Bru O, Bosson J L. et al. Assessing pain in critically ill sedated patients by using a behavioral pain scale. Crit Care Med. 2001;29(12):2258–2263. doi: 10.1097/00003246-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 124.Van de Leur J P, Zwaveling J H, Loef B G, Van der Schans C P. Patient recollection of airway suctioning in the ICU: routine versus a minimally invasive procedure. Intensive Care Med. 2003;29(3):433–436. doi: 10.1007/s00134-003-1640-3. [DOI] [PubMed] [Google Scholar]
- 125.Durand M, Sangha B, Cabal L A, Hoppenbrouwers T, Hodgman J E. Cardiopulmonary and intracranial pressure changes related to endotracheal suctioning in preterm infants. Crit Care Med. 1989;17(6):506–510. doi: 10.1097/00003246-198906000-00004. [DOI] [PubMed] [Google Scholar]
- 126.Pang W W, Chang D P, Lin C H, Huang M H. Negative pressure pulmonary oedema induced by direct suctioning of endotracheal tube adapter. Can J Anaesth. 1998;45(8):785–788. doi: 10.1007/BF03012150. [DOI] [PubMed] [Google Scholar]
- 127.Demers R R. Complications of endotracheal suctioning procedures. Respir Care. 1982;27:453–457. [Google Scholar]
- 128.Copnell B, Tingay D G, Kiraly N J. et al. A comparison of the effectiveness of open and closed endotracheal suction. Intensive Care Med. 2007;33(9):1655–1662. doi: 10.1007/s00134-007-0635-x. [DOI] [PubMed] [Google Scholar]
- 129.Cordero L, Sananes M, Ayers L W. Comparison of a closed (Trach Care MAC) with an open endotracheal suction system in small premature infants. J Perinatol. 2000;20(3):151–156. doi: 10.1038/sj.jp.7200330. [DOI] [PubMed] [Google Scholar]
- 130.Morrow B M, Mowzer R, Pitcher R, Argent A C. Investigation into the effect of closed-system suctioning on the frequency of pediatric ventilator-associated pneumonia in a developing country. Pediatr Crit Care Med. 2012;13(1):e25–e32. doi: 10.1097/PCC.0b013e31820ac0a2. [DOI] [PubMed] [Google Scholar]
- 131.Morrow B M. Closed-system suctioning: why is the debate still open? Indian J Med Sci. 2007;61(4):177–178. [PubMed] [Google Scholar]


