Abstract
Background
This is the third update of a review that was originally published in the Cochrane Library in 2002, Issue 2. People with cancer, their families and carers have a high prevalence of psychological stress, which may be minimised by effective communication and support from their attending healthcare professionals (HCPs). Research suggests communication skills do not reliably improve with experience, therefore, considerable effort is dedicated to courses that may improve communication skills for HCPs involved in cancer care. A variety of communication skills training (CST) courses are in practice. We conducted this review to determine whether CST works and which types of CST, if any, are the most effective.
Objectives
To assess whether communication skills training is effective in changing behaviour of HCPs working in cancer care and in improving HCP well‐being, patient health status and satisfaction.
Search methods
For this update, we searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 4), MEDLINE via Ovid, Embase via Ovid, PsycInfo and CINAHL up to May 2018. In addition, we searched the US National Library of Medicine Clinical Trial Registry and handsearched the reference lists of relevant articles and conference proceedings for additional studies.
Selection criteria
The original review was a narrative review that included randomised controlled trials (RCTs) and controlled before‐and‐after studies. In updated versions, we limited our criteria to RCTs evaluating CST compared with no CST or other CST in HCPs working in cancer care. Primary outcomes were changes in HCP communication skills measured in interactions with real or simulated people with cancer or both, using objective scales. We excluded studies whose focus was communication skills in encounters related to informed consent for research.
Data collection and analysis
Two review authors independently assessed trials and extracted data to a pre‐designed data collection form. We pooled data using the random‐effects method. For continuous data, we used standardised mean differences (SMDs).
Main results
We included 17 RCTs conducted mainly in outpatient settings. Eleven trials compared CST with no CST intervention; three trials compared the effect of a follow‐up CST intervention after initial CST training; two trials compared the effect of CST and patient coaching; and one trial compared two types of CST. The types of CST courses evaluated in these trials were diverse. Study participants included oncologists, residents, other doctors, nurses and a mixed team of HCPs. Overall, 1240 HCPs participated (612 doctors including 151 residents, 532 nurses, and 96 mixed HCPs).
Ten trials contributed data to the meta‐analyses. HCPs in the intervention groups were more likely to use open questions in the post‐intervention interviews than the control group (SMD 0.25, 95% CI 0.02 to 0.48; P = 0.03, I² = 62%; 5 studies, 796 participant interviews; very low‐certainty evidence); more likely to show empathy towards their patients (SMD 0.18, 95% CI 0.05 to 0.32; P = 0.008, I² = 0%; 6 studies, 844 participant interviews; moderate‐certainty evidence), and less likely to give facts only (SMD ‐0.26, 95% CI ‐0.51 to ‐0.01; P = 0.05, I² = 68%; 5 studies, 780 participant interviews; low‐certainty evidence). Evidence suggesting no difference between CST and no CST on eliciting patient concerns and providing appropriate information was of a moderate‐certainty. There was no evidence of differences in the other HCP communication skills, including clarifying and/or summarising information, and negotiation. Doctors and nurses did not perform differently for any HCP outcomes.
There were no differences between the groups with regard to HCP 'burnout' (low‐certainty evidence) nor with regard to patient satisfaction or patient perception of the HCPs communication skills (very low‐certainty evidence). Out of the 17 included RCTs 15 were considered to be at a low risk of overall bias.
Authors' conclusions
Various CST courses appear to be effective in improving HCP communication skills related to supportive skills and to help HCPs to be less likely to give facts only without individualising their responses to the patient's emotions or offering support. We were unable to determine whether the effects of CST are sustained over time, whether consolidation sessions are necessary, and which types of CST programs are most likely to work. We found no evidence to support a beneficial effect of CST on HCP 'burnout', the mental or physical health and satisfaction of people with cancer.
Plain language summary
Do courses aimed at improving the way healthcare professionals communicate with people who have cancer impact on their physical and mental health?
What is the aim of this review? The aim of this Cochrane review was to find out if communication skills training (CST) for healthcare professionals working with people who have cancer has an impact on how healthcare professionals communicate and on the physical and mental health of the patients. What types of studies did we include? We included only randomised trials (RCTs) that evaluated the impact of CST for healthcare professionals (doctors, nurses and other allied health professionals) who work with people with cancer. We included different types of CST and evaluated its impact on healthcare professionals and their patients, through the following reported outcomes: use of open questions, elicited concerns, delivery of appropriate information, empathy demonstration, use of fact contents, healthcare professional 'burnout' and patient anxiety.
What are the main results of the review? We found 17 RCTs comparing CST with no CST. The studies used encounters with real and simulated patients to measure the communication outcomes. The evidence on whether CST leads to an improvement of the use of open questions is very uncertain. However, we did show that CST probably improves healthcare professional empathy and reduces the likelihood of their giving facts only without individualising their responses to the patient's emotions or offering support. . CST probably does not have an effect on the ability of healthcare professionals to elicit concerns or to give appropriate information.
Evidence suggesting that CST might prevent healthcare professional 'burnout' is of low‐certainty and it is very uncertain whether CST has an effect on patient anxiety.
What do they mean? CST probably helps healthcare professionals to empathise more with their patients, and probably improves some aspects of their communication skills. These changes might lead to better patient outcomes; however, evidence on the latter is very uncertain and more research is needed.
Summary of findings
Summary of findings for the main comparison. CST compared to control for healthcare professionals working with people who have cancer.
| Communication skills training for healthcare professionals working with people who have cancer | |||||
| Patient or population: Healthcare professionals working with people who have cancer Intervention: Communication skills training Comparison: No communication skills training | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments |
| Risk with CST | |||||
| Used open questions | SMD 0.25 higher (0.02 higher to 0.48 higher) | — | 796 (5 RCTs) | ⊕⊝⊝⊝ Very low 1 2 3 | It is not clear whether communication skills training leads to an improvement of used open questions, because the certainty of the evidence is very low |
| Elicited concerns | SMD 0.24 higher (0.12 lower to 0.60 higher) | — | 221 (3 RCTs) | ⊕⊕⊕⊝ Moderate 2 | Communication skills training probably increases elicited concerns. |
| Gave appropriate information | SMD 0.08 lower (0.26 lower to 0.10 higher) | — | 489 (4 RCTs) | ⊕⊕⊕⊝ Moderate 3 | Communication skills training probably does not increase the delivery of appropriate information. |
| Showed empathy | SMD 0.18 higher (0.05 higher to 0.32 higher) | — | 844 (6 RCTs) | ⊕⊕⊕⊝ Moderate 2 3 | Communication skills training probably increases empathy demonstration |
| Gave facts only | SMD 0.26 lower (0.51 lower to 0.01 lower) | — | 780 (5 RCTs) | ⊕⊕⊝⊝ Low 2 3 4 | Communication skills training may decrease fact contents, but the certainty of this evidence is low |
| Emotional exhaustion: Maslach Burnout Inventory: | SMD 0.16 lower (0.44 lower to 0.12 higher) | — | 202 (3 RCTs) | ⊕⊕⊝⊝ Low 2 3 | Communication skills training may decrease emotional exhaustion, but the certainty of this evidence is low |
| Patient anxiety | SMD 0.17 higher (0.24 lower to 0.59 higher) | — | 770 (3 RCTs) | ⊕⊝⊝⊝ Very low 2 3 5 | It is not clear whether communication skills training leads to an improvement of patient anxiety, because the certainty of the evidence is very low |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | |||||
| GRADE Working Group grades of evidence High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate‐certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low‐certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low‐certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
1 We downgraded two levels of certainty of evidence due to inconsistency because there are I2 = 74% in simulated patients and 43.4% between real and simulated patients 2 We downgraded one level of certainty of evidence due to imprecision because each end of confidence interval leads a different decision. 3 We downgraded one level of certainty of evidence due to risk of bias because most of trials have varied methodological limitations. 4 We downgraded one level of certainty of evidence due to inconsistency because there are I2 = 69% in simulated patients, I2 = 52% in real patients and 43,4% between real and simulated patients 5 We downgraded one level of certainty of evidence due to inconsistency evaluated with I2 = 72%
Background
This is an updated version of a review that was originally published in the Cochrane Database of Systematic Reviews in 2002 (Fellowes 2002) and updated in 2004 (Moore 2004) and 2013 (Moore 2013). Good communication between healthcare professionals (HCPs) and patients is essential for high‐quality health care. Effective communication benefits the well‐being of patients and HCPs, influencing the rate of patient recovery, effective pain control, adherence to treatment regimens, and psychological functioning (Fallowfield 1990; Gattellari 2001; Stewart 1989; Stewart 1996; Vogel 2009). People with cancer have a high prevalence of psychological stress, and need emotional and social support. Hence, it is important that from the start there is adequate communication about the diagnosis, prognosis and treatment alternatives (Hack 2012). Furthermore, treatment of psychological stress may have a positive effect on quality of life (Girgis 2009).
Conversely, ineffective communication can leave patients feeling anxious, uncertain and generally dissatisfied with their care (Hagerty 2005), and has been linked to a lack of compliance with recommended treatment regimens (Turnberg 1997). Avoiding disclosing cancer as the diagnosis has been linked to higher rates of depression and anxiety and lower use of coping skills (Donovan‐Kicken 2011). Complaints about HCPs made by patients frequently focus, not on a lack of clinical competence per se, but rather on a perceived failure of communication and an inability to adequately convey a sense of care (Moore 2011; Lussier 2005). Communication issues are an important factor in litigation (Levinson 1997).
Ineffective communication is also linked to increased stress, lack of job satisfaction and emotional burnout amongst HCPs (Fallowfield 1995; Ramirez 1995). Self‐awareness, reflection and learning about communication skills may have benefits for health professionals, and prevent burnout.
Most people with cancer prefer a patient‐centred or collaborative approach (Dowsett 2000; Hubbard 2008; Tariman 2010); however, there is a minority who prefer a more task‐centred approach. Furthermore, patient preferences regarding the communication of bad news have been found to be culturally dependent (Fujimori 2009).This makes it imperative that HCPs understand the needs of the individual patient (Dowsett 2000; Sepucha 2010). The type of relationship that occurs in reality can be very different from that preferred by patients and doctors (Tariman 2010; Taylor 2011), and the literature suggests that people with cancer continue to have unmet communication needs (Hack 2005). Taylor 2011 reported that a majority of clinicians liked to include emotional issues during their interviews with people with cancer, however, clinical interviews tend to be predominated by biomedical discussion with only a minimal time dedicated to psychosocial issues (Hack 2012; Vail 2011).
The ability to communicate effectively is a pre‐condition of qualification for most HCPs (ACGME 2009; CanMEDS 2011; GMC 2009). As communication skills do not reliably improve with experience alone (Cantwell 1997), communication skills training (CST) is mandatory in many training programs, therefore, considerable effort and expense is being dedicated to CST.
There has also been increasing interest in the effect of training patients in communication skills prior to their consultation (Kinnersley 2007), including question prompts for people with cancer (Brandes 2015), and it has been suggested that it may be more effective to train both HCPs and patients in communication skills.
Description of the intervention
CST courses or workshops generally focus on communication between HCPs and patients during the formal assessment procedure (interview), and include emphasis on skills for building a relationship, providing structure to the interview, initiating the session, gathering information, explaining, planning and closure (Silverman 2005). Building a relationship may be particularly relevant with people with cancer where promoting a greater disclosure of individual concerns and feelings may enable optimum care. Breaking bad news and shared decision‐making have been other focuses of CST for HCPs involved in cancer care (Fallowfield 2004; Paul 2009).
Most approaches to teaching communication in health care incorporate cognitive, affective and behavioural components, with the general aim of promoting greater self‐awareness in the HCP. CST based on acquiring skills may be more effective than programmes based on attitudes or specific tasks (Kurtz 2005), and is considered to be more effective if experiential. The essential components that facilitate learning have been highlighted in guidelines (Gysels 2004; Stiefel 2010) and include the following.
Systematic delineation and definition of the essential skills (verbal, non‐verbal and paralinguistic). Skills that are effective in communication with people with cancer are defined (e.g. the use of open questions, incorporating a psychosocial assessment, demonstrating empathy). Pitfalls include leading questions, focusing only on the physical and failing to explore the more psychological issues and premature reassurance. However, some claim that the evidence base for this definition of essential skills is still weak (Cegala 2002; Paul 2009).
Observation of learners: through the use of learning techniques such as role‐play, participants are then given the opportunity to practice their communication skills using facilitating behaviours and avoiding blocking behaviours in a 'safe’ environment. Often, role‐playing is aided by the use of simulated patients trained to represent someone with cancer, and who can provide a range of cues and responses to communication in the role‐play, thus providing a safe opportunity for HCPs to practice communication skills without distressing patients (Aspegren 1999; Kruijver 2001; Nestel 2007).
Well‐intentioned, descriptive feedback, which may be verbal or written.
Video or audio‐recordings and review permitting self‐reflection.
Repeated practice.
Active small group or one‐to‐one 'learner‐centred' learning.
Facilitators with training and experience (Bylund 2009).
CST has been delivered in a variety of ways, for example, via sessions integrated into degree or diploma studies (e.g. Wilkinson 1999) or three‐ to five‐day workshops using actors as simulated patients (Fallowfield 1990; Heaven 1995; Razavi 2000). The optimal length for CST is under debate. Gysels 2004 argues that longer courses are more effective. However, in time‐pressured health care, there is an expectation to train effectively in less on‐site time. E‐learning (learning conducted via electronic media, typically on the Internet) and B‐learning (face‐to‐face training and online education) are methods that perhaps should be incorporated in CST. There has been some move towards a unified approach to teaching communication skills for professionals who work within the cancer field (Arraras 2015).
There is a wide variety of models and approaches to trials of CST and interpreting the data is often hampered by poor methodological quality (Fallowfield 2004), and difficulty in recruitment of HCPs (Gyawali 2015). There has been some debate about the current paradigm that supports research and education in this area (Salmon 2017; Silverman 2017) and increasing interest in including the views of patients and professionals in the design of the CST course (Solomon 2017). In 2013, the third version of this Cochrane review concluded, based on 15 randomised controlled trials, that CST courses appear to be effective in improving some types of HCP communication skills particularly related to information gathering and supportive skills, but there was insufficient evidence to determine whether the effects of CST are sustained over time, whether consolidation sessions are necessary, and which types of CST programs are most likely to work (Moore 2011) . Since then, other reviewers have reached the same conclusions in different ways (Barth 2011; Bylund 2010; Kissane 2012). Whilst some have suggested that these positive effects can be maintained over time, others have concluded that a strong evidence base for a significant effect on trial outcomes is lacking (Alvarez 2006), particularly for an effect on patient outcomes (Uitterhoeve 2010).
Why it is important to do this review
There has been much research in this area since the original Cochrane review was published, including several randomised controlled trials (RCTs), which were scant at the time of the original review. Other more recent reviews in the field have included a variety of studies with different study designs, however, none have conducted meta‐analyses of the results from RCTs. By undertaking this systematic review and keeping it up‐to‐date, we aim to critically evaluate all RCTs that have investigated the effectiveness of CST for HCPs working in cancer care, in order to enable evidence‐based teaching and practice in this important and expanding area. Furthermore, we hoped that a review and meta‐analysis of data from such RCTs would provide stronger evidence of any potential benefits that CST may have on HCP behaviour and provide guidance on the optimal methodology and length of training, as well as how to ensure that these newly acquired skills are transferred to the workplace.
Objectives
To assess whether communication skills training (CST) is effective in changing behaviour of healthcare professionals (HCPs) working in cancer care and in improving HCP well‐being, patient health status and satisfaction.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), including cluster‐randomised studies.
Types of participants
Types of healthcare professionals (HCPs): all qualified HCPs (medical, nursing and allied health professionals including residents (i.e. doctors on post‐graduate training courses) within all hospital, hospice and ambulatory care settings, working in cancer care. If a study included other non‐professionals but the percentage of professionals in the sample was more than 60%. If a study also included HCPs working in non‐cancer care and the percentage of HCPs working in cancer care was more than 60%. Training of intermediaries (e.g. interpreters, advocates, self‐help groups) was not considered.
Types of patients: men and women with a diagnosis of cancer, at any stage of treatment. If a study included people with other diagnoses but the people with cancer made up more than 60% of the study sample. We included studies that assessed interviews in both real and simulated patients (for definition see Appendix 1).
Types of encounters: consultations and interviews where the care of people with cancer is the main aim. We excluded trials that studied encounters where the aim was to improve the quality of informed consent or to disclose information for informed patient consent to participate in a RCT.
Types of interventions
We included only studies in which the intervention group had communication skills training (CST) (e.g. study days, teaching pack, distance learning, workshops; and including any mode of training such as audio‐tape feedback, videotape recording of interviews, role‐play, group discussion, didactic teaching), and in which the control group received nothing beyond the usual, or received an alternative training to the intervention group. We included all types and approaches to teaching, any length of training and any focus of communication between professionals and people with cancer within the context of patient care. We excluded studies whose focus was communication skills in encounters related to informed consent for research. This specific type of CST is under discussion as the subject of a separate Cochrane review.
Types of outcome measures
We included outcomes that measured changes in HCP behaviour or skills, other HCP outcomes and patient‐related outcomes at any time after the intervention. We anticipated that many of these outcomes would be measured by validated study‐specific observational rating scales and potentially subject to a high degree of inter‐trial methodological heterogeneity. Studies that only reported outcomes of changes in attitudes/knowledge on the part of the HCPs or patients without examining resulting changes in behaviour of HCPs were excluded from the review, as self‐perceived improvements have been shown to be over‐optimistic (Chant 2002; Dickson 2012).
Primary outcomes
HCP communication skills
Information‐gathering skills, such as open questions, leading questions, facilitation, clarifying and summarising
Discovering the patients perspective such as eliciting concerns
Explaining and planning skills such as giving the appropriate information, checking understanding, and negotiating procedures and future arrangements
Supportive, building relationship skills such as empathy, responding to emotions/psychological utterances; and offering support
Undesirable outcomes, including blocking behaviours such as interruptions and false reassurances, and providing facts only
Secondary outcomes
Other HCP outcomes
Burnout
Patient‐rated outcomes
-
Patient health status
Anxiety level/psychological distress
Quality of life
-
Patient Perception
Perception of HCP's communication skills: clarification, assessment of concerns, information, support, trust
Satisfaction
Outcomes of 'significant other'
-
Perception of significant other
Perception of HCP's communication skills: clarification, assessment of concerns, information, support, trust
Satisfaction
Search methods for identification of studies
Electronic searches
For the original review, the following databases were searched.
CENTRAL (the Cochrane Library, 2001, Issue 3)
MEDLINE (1966 to November 2001)
Embase (1980 to November 2001)
PsycInfo (1887 to November 2001)
CINAHL (1982 to November 2001)
AMED (1985 to October 2001)
SIGLE (Start to March 2002) (Grey literature database held by British Library)
Dissertation Abstracts International (1861 to March 2002)
Evidence‐Based Medical Reviews (1991 to March/April 2001)
For the first and second updates of the review, the search strategy was modified by Jane Hayes (JH), Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers (CGNOC), who extended the searches of CENTRAL, MEDLINE, Embase, PsycInfo and CINAHL to Novemeber 2003 and Febuary 2012. In addition, JH searched the Database of Reviews of Effects (DARE) in the Cochrane Library in September 2011. No language restrictions were applied. (See Appendix 2, Appendix 3, Appendix 4 for search strategies).
For this (third update) review, we used the same search strategies. Jo Platt of the CGNOC extended the searches of CENTRAL, MEDLINE, Embase, PsycInfo and CINAHL to May 2018:
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 4)
MEDLINE via Ovid (February 2012 to May week 4 2018)
Embase via Ovid (February 2012 to 2018 week 22)
PsycInfo (February 2012 to May 2018)
CINAHL (February 2012 to May 2018)
No language restrictions were applied. We searched in Epistemonikos database for studies included in similar reviews.
Searching other resources
We searched the US National Library of Medicine Clinical Trial Registry (ClinicalTrials.gov) and handsearched the reference lists of relevant studies that we identified from the electronic searches and the conference abstracts of the annual International Psycho‐Oncology Society meetings.
Data collection and analysis
Selection of studies
For the original review (Version 1), two of three review authors, Deborah Fellowes (DF), Susie Wilkinson (SW) and Philippa Moore (PM) independently applied inclusion criteria to each identified study. For the first and second update (Versions 2 and 3), two out of three review authors (PM and Solange Rivera Mercado (SRM) or Monica Grez Artigues (MGA)) independently evaluated identified studies for inclusion.
For this third update (Version 4), all articles found by the search strategy were submitted to the tool CollaboratronTM (Epistemonikos Foundation). The title and summary of all articles was reviewed independently by two of the review authors (PM, SRM, Gonzalo Bravo‐Soto (GB) or Camila Olivares (CO)) Disagreements were resolved by discussion between the review authors. We identified potentially eligible studies from the search abstracts and retrieved the full text of the articles if the review criteria were met, or if the abstract contained insufficient information to assess the review criteria.
Data extraction and management
For the original data extraction, two review authors recorded the methodology (including study design, participants, sample size, intervention, length of follow‐up and outcomes), quality and results of the included studies on a standardised data extraction form.
For the updated reviews, we designed a new data extraction form to include some specific outcomes and a 'Risk of bias' assessment. Two of the review authors extracted data independently (PM, SRM, MGA, GB, CO) and resolved any disagreement by discussion. We entered the data into Review Manager software Revman 5.3 (RevMan 2011) and checked for accuracy.
Assessment of risk of bias in included studies
The quality of eligible studies was assessed independently by three review authors (DF, SW, PM) for the original review, and by two of the review authors (PM, SRM, GB, CO) for the updates of the review. For included studies, we assessed the risk of bias as follows.
Selection bias: random sequence generation and allocation concealment
Detection bias: blinding of outcome assessment
Attrition bias: incomplete outcome data
Reporting bias: selective reporting of outcomes
Other possible sources of bias
For further details see Appendix 5.
Measures of treatment effect
Tools for assessing communication were diverse and usually consisted of validated questionnaires and scales. Data for all outcomes were continuous. We had planned to measure the mean difference (MD) between treatment arms, however most trials measured the same outcome using different scales, and so we used the standardised mean difference (SMD) for all meta‐analyses.
Unit of analysis issues
The units of analyses included the HCPs, their patients and significant others, and their encounters/conversations/interviews. Two of the review authors (PM, SRM MG, GB or CO) reviewed unit of analysis issues according to Higgins 2011, and differences were resolved by discussion. These included reports where there were multiple observations for the same outcome, e.g. several interviews involving the same HCP for the same outcome at different time points. When there were multiple time points for observation, we considered the data from the time point closest to the end of intervention as the post‐intervention measurement. This ranged from immediately post‐intervention to three months post‐intervention. We also analysed the longest follow‐up measurement for each study which ranged from two to 36 months.
Dealing with missing data
For included studies, we noted the level of attrition. Studies with greater than 20% attrition were considered at moderate to high risk of bias. For all outcomes, we attempted to carry out analyses on an intention‐to‐treat basis. We did not impute missing outcome data. If data were missing or only imputed data were reported, we attempted to contact trial authors to request the missing data.
Assessment of heterogeneity
We assessed the heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials (the I² statistic) (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). We considered a P value of less than 0.10 and an I² > 50% to represent substantial heterogeneity.
Assessment of reporting biases
We intended to examine funnel plots corresponding to meta‐analysis of the primary outcome to assess the potential for small study effects such as publication bias if a sufficient number of studies were identified, however, there were fewer than 10 studies in all meta‐analyses.
Data synthesis
We used the random‐effects model with inverse variance weighting for all meta‐analyses (DerSimonian 1986) and pooled the SMDs, presenting these results with the corresponding 95% confidence intervals (CIs).
Subgroup analysis and investigation of heterogeneity
To investigate heterogeneity, we carried out subgroup analyses of the primary outcomes according to staff group (e.g. doctors and nurses), patient type (e.g. real or simulated) and type of comparison (e.g. CST versus no‐CST or CST with follow‐up versus CST alone). We had intended to carry out subgroup analyses according to the type of CST e.g. didactic teaching, distance learning, role‐play workshops, however this was not possible due to the wide variety of interventions included. We will attempt subgroup analyses in future versions of this review.
Sensitivity analysis
We performed sensitivity analysis for the primary outcomes to investigate heterogeneity between studies. Three studies compared a CST intervention with no CST after giving preliminary CST to all HCP participants (intervention and control groups). Where any of these three studies contributed to meta‐analyses, we performed sensitivity analyses by excluding these data and compared the results.
Summary of findings
We presented an the overall certainty of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias), but also to external validity such as directness of results (Langendam 2013). We created a 'Summary of findings' table based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and using GRADEpro GDT. We used the GRADE checklist and GRADE Working Group certainty of evidence definitions (Meader 2014). We downgraded the evidence from 'high' certainty by one level for serious (or by two for very serious) concerns for each limitation.
High‐certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate‐certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low‐certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect.
Very low‐certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect
Results
Description of studies
Results of the search
The searches of the original review and the three updates combined retrieved 3969 records, which we subsequently screened by title and abstract. We assessed 250 full‐text papers for inclusion in this review. We excluded 175 of those articles for the reasons we have described in the Characteristics of excluded studies table.
In total, we included 17 trials (56 publications) that fulfilled our criteria (see the Characteristics of included studies table for details); two studies (Fallowfield 2002; Razavi 1993) from the original review (Fellowes 2002)), one additional study (Razavi 2002) from the first update (Moore 2004), and 12 from the second update (Moore 2013) (Butow 2008; Fujimori 2014 (previously Fujimori 2011); Goelz 2011; Heaven 2006; Kruijver 2001; Lienard 2010; Merckaert 2015 (previously Gibon 2011); Razavi 2003; Stewart 2007; Tulsky 2011; van Weert 2011; Wilkinson 2008;). In this third update, we included two new trials (Epstein 2017; Gorniewicz 2017) and six articles with new information of trials already included: Bragard 2010, in: Lienard 2010; Cavinet 2014, in: Razavi 2002; Fujimori 2014 and Fujimori 2014,in: Fujimori 2014; Merckaert 2015 and Leonard 2016, in: Merckaert 2015.
We have summarised the study selection processes in Figure 1; Figure 2; Figure 3
1.
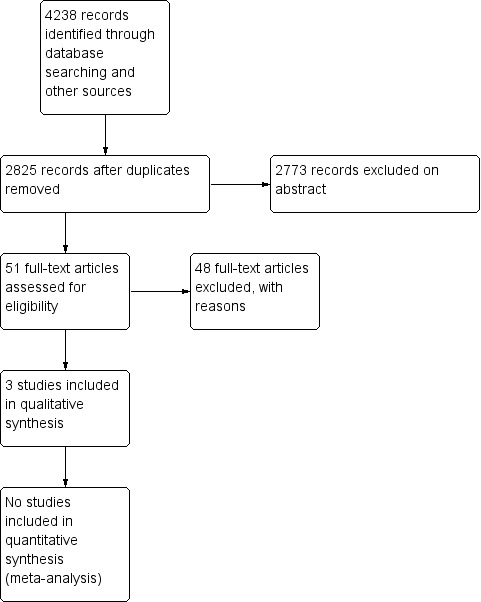
Study flow diagram of original searches (November 2001 and November 2003)
2.
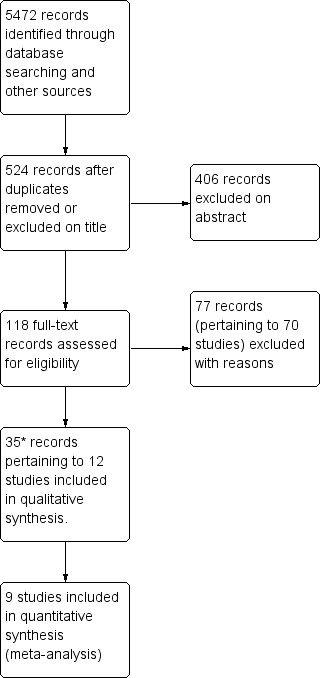
Study flow diagram of updated searches to 28 February 2012.
*Therefore, 15 studies and 44 records in total (updated search results plus original results)
3.
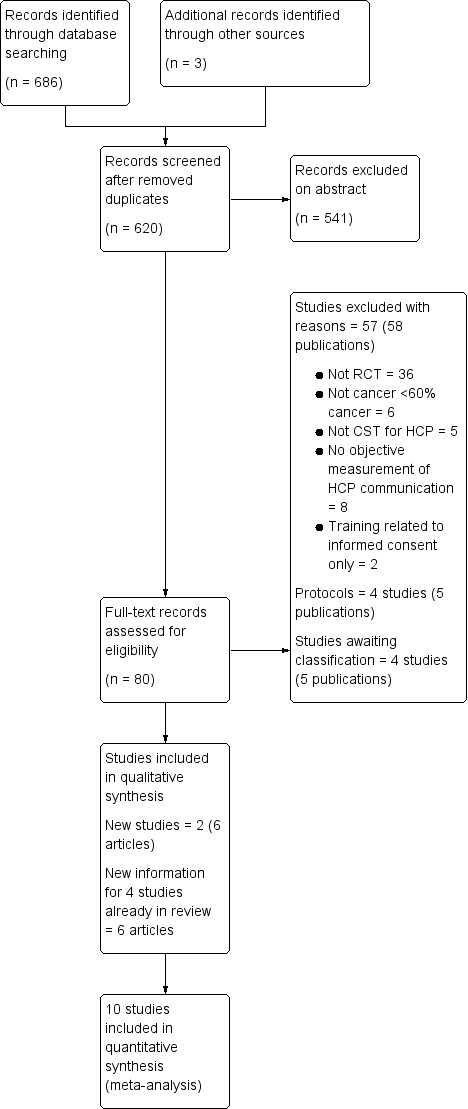
Study flow diagram of updated searches from February 2012 to June 2018
Included studies
We identified 17 trials in total (56 publications). All trials were published in full. We found four protocols of ongoing trials and contacted the authors but received no further information at the time of publication (Berger‐Höger 2015, De Figuereido 2015; Libert 2017; Parker 2016).
Eleven trials (Butow 2008; Fujimori 2014; Goelz 2011; Gorniewicz 2017; Kruijver 2001; Lienard 2010; Merckaert 2015; Razavi 1993; Razavi 2002; van Weert 2011; Wilkinson 2008) investigated the effect of communication skills training (CST) in the intervention group compared with a control group with no intervention.
Two trials (Epstein 2017; van Weert 2011) compared the effect of CST and patient coaching in the intervention group compared with a control group with no intervention.
One trial (Fallowfield 2002) compared two interventions in four comparative groups: CST or no training, and the provision of individual feedback or no feedback.
Three trials assessed the effect of a follow‐up intervention after initial training: six bi‐monthly consolidation workshops of three hours in length (Razavi 2003), four half‐day supervision sessions spread over four weeks (Heaven 2006), and CD‐ROM (Tulsky 2011).
One study compared different durations of CST (Stewart 2007).
Overall, the communication skills of 1249 healthcare professional (HCP) participants were reported in these studies and 2638 real patient encounters and 1605 simulated patient encounters were analysed. People with cancer were from various cancer care settings (63% women; mean age 61 years) and the studies enrolled the following HCPs.
Doctors (10 trials): Butow 2008 = 30 oncologists; Epstein 2017 = 38 oncologists; Fallowfield 2002 = 160 oncologists; Fujimori 2014 = 30 oncologists; Goelz 2011 = 41 mainly haematology/oncology doctors; Gorniewicz 2017 = 38 residents; Lienard 2010 = 113 residents; Razavi 2003 = 63 physicians (62% oncologists); Stewart 2007 = 51 doctors (18 oncologists, 17 family physicians and 16 surgeons); Tulsky 2011 = 48 oncologists.
Nurses (six studies): Heaven 2006 = 61 nurses; Kruijver 2001 = 53 nurses; Razavi 1993 = 72 nurses; Razavi 2002 = 116 nurses; van Weert 2011 = 48 nurses; Wilkinson 2008 = 172 nurses.
Other HCPs: one trial studied the effect of CST on radiotherapy teams, which included a mixed group of 96 doctors, nurses, physicists and secretaries (Merckaert 2015).
The majority of the trials were conducted in Europe, with the exception of Stewart 2007 (Canada), Butow 2008 (Australia); Fujimori 2014 (Japan) and Tulsky 2011; Epstein 2017; Gorniewicz 2017 (USA). The average age of the HCP participants (15 studies) was 38 years and the number of HCPs in the studies ranged from 30 to 172 (mean, 71). Women comprised approximately 36% of participants in the trials involving doctors and approximately 92% of those involving nurses. Their experience working with people with cancer ranged from < two years to 24 years. With regard to previous CST, one study reported that 47% of the participants had received > 50 hours of CST prior to the trial (Heaven 2006); two studies reported that participants had received no previous CST (Goelz 2011; Wilkinson 2008).
Most studies were conducted in the hospital outpatient setting except for two studies that involved professionals working in the community (primary care and hospices) (Heaven 2006; Wilkinson 2008) and four that involved HCPs working in an inpatient setting (Kruijver 2001; Lienard 2010; Razavi 2002; van Weert 2011).
Type of intervention
The objective of most trials was to train the professionals in general communication skills (Fallowfield 2002; Fujimori 2014; Heaven 2006; Merckaert 2015; Razavi 1993; Razavi 2002; Stewart 2007; Wilkinson 2008). Two trials aimed to train professionals specifically to detect and respond to patients emotions (Butow 2008;Tulsky 2011). Four trials trained HCPs in giving bad news (Fujimori 2014; Gorniewicz 2017; Lienard 2010; Razavi 2003). Epstein 2017 and Goelz 2011 trained HCPs in addressing palliative care and the transition to palliative care, respectively. Kruijver 2001 concentrated on CST for nurses' admission interviews, and van Weert 2011 on CST for patient education on chemotherapy. Merckaert 2015 had a component of team communication training.
The patients' perspective was included in the design (one trial, Gorniewicz 2017) and videos of patients' perspectives were used in the intervention (two trials Gorniewicz 2017; Stewart 2007).
Most trials specified the use of learner‐centred, experiential, adult education methods by experienced facilitators (13 trials: Butow 2008; Epstein 2017; Fallowfield 2002; Fujimori 2014; Goelz 2011; Heaven 2006; Lienard 2010; Merckaert 2015; Razavi 2003; Stewart 2007; Tulsky 2011; van Weert 2011; Wilkinson 2008). Co‐teaching was stated in nine studies (Butow 2008; Epstein 2017; Fujimori 2014; Goelz 2011; Heaven 2006; Kruijver 2001; Merckaert 2015; Razavi 1993; Razavi 2003). CST was taught in small groups (range three to 15 participants) in 12 trials (Butow 2008; Fallowfield 2002; Goelz 2011; Heaven 2006; Kruijver 2001; Lienard 2010; Razavi 1993; Razavi 2002; Razavi 2003; Stewart 2007; van Weert 2011; Wilkinson 2008). All small‐group studies used role‐play, although it was often unclear if the cases used were pre‐defined or true cases of the participants, and if the role‐play was between participants or with simulated patients. Two studies described individual on‐site training (Epstein 2017; Heaven 2006). Real patients were used in the preparation of one intervention (Gorniewicz 2017) and in video‐clips in van Weert 2011, but no trials used real patients during on‐site training.
Most interventions included written material (nine trials); Butow 2008; Fallowfield 2002; Goelz 2011; Kruijver 2001Razavi 1993; Razavi 2002; Razavi 2003; Stewart 2007; Wilkinson 2008), and short didactic lectures (10trials; Butow 2008; Fujimori 2014; Goelz 2011; Kruijver 2001; Lienard 2010; Merckaert 2015; Razavi 1993; Razavi 2002; Razavi 2003; Wilkinson 2008). Eight trials specified the use of role‐modelling (Butow 2008; Gorniewicz 2017; Heaven 2006; Kruijver 2001; Lienard 2010; Stewart 2007; Tulsky 2011; Wilkinson 2008); and 13 trials specified the use of audio or video material (Butow 2008; Epstein 2017; Fallowfield 2002; Fujimori 2014; Goelz 2011; Gorniewicz 2017; Heaven 2006; Kruijver 2001; Razavi 1993; Stewart 2007; Tulsky 2011; van Weert 2011; Wilkinson 2008). Two trials described b‐learning: 1.5 hour video conferences as follow‐up after the CST (Butow 2008), and e‐learning prior to face‐to‐face session (van Weert 2011) Two trials described only e learning (Gorniewicz 2017; Tulsky 2011).
The participants received feedback from their tutors either verbally ;Butow 2008; Epstein 2017;Goelz 2011; Heaven 2006; Kruijver 2001; Lienard 2010; Razavi 1993; Razavi 2002; Razavi 2003; Stewart 2007; Tulsky 2011; Wilkinson 2008), or in writing (Epstein 2017; Fallowfield 2002). In addition, there was feedback from the simulated patients (two trials Butow 2008; Epstein 2017), and from the participants' peers (three trials Goelz 2011; Fujimori 2014; van Weert 2011; No study stated whether the feedback was structured using a checklist.
Duration of intervention
Three trials had very short training: one hour e‐learning Gorniewicz 2017 and short on‐site training with no follow‐up: Epstein 2017 (95 minutes); Stewart 2007 (six hours). Four trials included on‐site training that lasted 24 hours or less with no follow‐up intervention (10 hours: Fujimori 2014; 24 hours: Razavi 1993; 24 hours over three days:Fallowfield 2002 and Wilkinson 2008).
Seven trials included on‐site training of less than 24 hours but with follow‐up sessions, including:
three‐day course followed by four three‐hour weekly sessions with one‐to‐one supervision (Heaven 2006);
1.5‐day course followed by four 1.5‐hour monthly video conferences (Butow 2008);
one day course with a follow‐up meeting at six weeks (van Weert 2011);
19‐hour course followed by six three‐hour consolidation workshops (Razavi 2003);
18‐hour course with a follow‐up meeting at two months (Kruijver 2001);
11‐hour course followed by one‐to‐one coaching at 12 weeks (Goelz 2011);
one‐hour lecture followed by the use of a CD‐ROM for one month (Tulsky 2011).
Three trials had longer on‐site training: 38 hours (Merckaert 2015), 30 hours (Lienard 2010) and 105 hours (Razavi 2002).
Some on‐site training was on consecutive days (Fallowfield 2002: three‐day residential course; Wilkinson 2008: two days; Fujimori 2014); other on‐site training was spread over a longer period of time (Epstein 2017; Lienard 2010; Razavi 1993; Razavi 2002; Razavi 2003), ranging from weekly for three weeks (Razavi 2003) to bimonthly over an eight‐month period (Lienard 2010).
Measurement of Outcomes
Primary Outcomes
Most studies measured outcomes before and after the CST (or no CST). Changes in HCP behaviour were measured in interviews involving simulated and/or real patients as follows:
simulated patients only: six trials (Butow 2008; Fujimori 2014; Goelz 2011; Gorniewicz 2017; Razavi 1993; Stewart 2007);
real patients only: five trials (Epstein 2017; Fallowfield 2002; Tulsky 2011; van Weert 2011; Wilkinson 2008);
real and simulated patients: six trials (Heaven 2006; Kruijver 2001; Lienard 2010; Merckaert 2015; Razavi 2002; Razavi 2003).
One trial measured HCP behaviour in interviews with simulated patients only when real patients were not available, however, the data were analysed together (Wilkinson 2008). Investigators reported on a total of 1739 recordings of simulated patient encounters and 5662 recordings of real patient encounters.
The number of real patient interviews per HCP, assessed at each assessment point, ranged from one (Epstein 2017; Razavi 2002; Razavi 2003) to six (Kruijver 2001). Interviews were mostly assessed using audio‐recording ( Epstein 2017; Heaven 2006; Lienard 2010; Merckaert 2015; Razavi 2002; Razavi 2003; Stewart 2007; Tulsky 2011), or video recording (Butow 2008; Fallowfield 2002; Goelz 2011; Kruijver 2001; Razavi 1993; Razavi 2002; Razavi 2003; van Weert 2011; Wilkinson 2008).
HCP communication skills were evaluated using a variety of scales (see Table 2). Almost every trial used its own unique scale; only three scales were used in more than one study: the Cancer Research Campaign Workshop Evaluation Manual (CRCWEM) (Booth 1991) (Razavi 1993; Razavi 2002; Razavi 2003); and LaComm, a French Communication Analysis Software (LaComm; Gibon 2010) (Merckaert 2015; Lienard 2010), and The Roter interaction analysis system (RIAS) (Fujimori 2014; Kruijver 2001). Most studies mention that their scale had been validated. The scales had an average of 25 variables (range six to 84). Most studies used more than one rater, and the inter‐rater reliability was considered acceptable by the authors and ranged from 0.49 to 0.94.
1. Scales used to measure HCP communication skills.
| Abbreviation | Name of scale | Studies included in review that used scale | Validation reference (if any) |
| APPC | Active Patient Participating Coding | Epstein 2017 | Street 2003; Street 2007 |
| BBN | Breaking Bad News Skills rating form checklist | Gorniewicz 2017 | |
| CGAS | Common Ground Assessment Summary form | Gorniewicz 2017 | Lang 2004 |
| Com‐on | COMmunication challenges in ONcology | Goelz 2011 | Stubenrauch 2012 |
| CRCWEM | Cancer Research Campaign Workshop Evaluation Manual | Razavi 1993; Razavi 2002; Razavi 2003 | Booth 1991 |
| CSRS | Communication Skills Rating Scale | Wilkinson 2008 | Wilkinson 1991 |
| HPSD | Harvard Third Psychosociological Dictionary | Razavi 2002 | |
| LaComm | LaComm | Merckaert 2015; Lienard 2010; Razavi 2002 |
Gibon 2010 http://www.lacomm.be/index.php |
| MIARS | Medical Interview Aural Rating Scale | Heaven 2006 | Heaven 2001 |
| MIPS | Medical Interaction Process System | Fallowfield 2002 | Ford 2000 |
| MRID | Martindale Regressive Imagery Dictionary | Razavi 2002 | |
| PCCM | Patient Centred Communication Measure | Stewart 2007 | Brown 1995 |
| PTCC | Prognostic and Treatment Choices | Epstein 2017 | Back 2003;Shields 2009 |
| QUOTE | Quality of Care through Patient's Eyes | van Weert 2011 | van Weert 2009 |
| RIAS | Roter Interaction Analysis System | Kruijver 2001;Fujimori 2014 |
http://www.riasworks.com/background.html Roter 2002; Ong 1998; Ishikawa 2002 |
| SHARE | Fujimori 2014 | Fujimori 2007 | |
| Verona | Verona VR‐CoDES System | Epstein 2017 | Del Piccolo 2011;Zimmermann 2011 |
All the trials included measurement of outcomes relating to HCPs' supportive/building relationship skills (Table 3). One study measured supportive skills only for HCPs outcomes (Tulsky 2011). Other frequently measured outcomes related to:
2. Types of HCP communication skills *.
| Outcome | Definition | Examples |
| Information gathering skills | ||
| Open questioning techniques | Questions or statements designed to introduce an area of inquiry without unduly shaping or focusing the content of the response | "How are you doing?"; "Tell me how you've been getting on since we last met..." |
| Half‐open questioning techniques | Questions that limit the response to a more precise field | "What makes your headaches better or worse?" |
| Closed questioning technique | Questions for which a specific often one‐word answer such as yes or no is expected, limiting the response to a narrow field set by the questioner | "Do you have nausea?"; "How many days have you had the headaches for?" |
| Eliciting concerns | A combination of open and closed questions to make a precise assessment of the patients perspective | "Tell me more about it from the beginning..."; "What worries you the most?"; "What do you think might be happening?" |
| Clarifying/summarising | Checking out statements that are vague or need amplification and summarising (the deliberate step of making an explicit verbal summary to verify ones understanding of what the patient said) | "Could you explain what you mean by light headed?" "Can I just see if I have got it right? You have had headaches before, but over the last two week you have had a different sort of pain . . . " |
| Explanation and Planning | ||
| Giving appropriate information | The correct amount and type of information (procedural, medical, psychological) to address patient needs and facilitate understanding | ''There are three important things I want to explain today. First I want to tell you what I think is wrong, second what tests we should do, and third what treatment options are available'' |
| Checking understanding | Checking patients understanding by direct questions or asking the patient to restate in own words | "Do you understand what I mean?" |
| Negotiating | Negotiating procedure or future arrangements by taking into account the patient's concerns | ''Do you mind if I examine you today? Would you prefer it if your husband came with you?'' |
| Supportive or relationship building skills | ||
| Acknowledging concerns | Verbalising the thoughts and concerns expressed by the patient, and express acceptance | "I can see that you are worried by all this"; "I sense that you feel uneasy about having to come to see me ‐ that's ok, many people feel that way when they first come here" |
| Showing empathy | Verbalising the feelings and emotions expressed by the patient | ''I can sense how angry you have been feeling about your illness. I can understand that it must be frightening to think the pain will come back'' |
| Reassurance | To reassure appropriately about a potential discomfort or uncertainty without providing false reassurance | ''I will do my best to help you'' |
*Adapted from Silverman 2005 and LaComm.
information gathering e.g. open questions (10 studies: Butow 2008; Fallowfield 2002; Heaven 2006; Kruijver 2001; Lienard 2010; Merckaert 2015; Razavi 1993; Razavi 2002; Razavi 2003; Wilkinson 2008); clarifying or summarising (eight studies: Fallowfield 2002; Goelz 2011; Gorniewicz 2017; Kruijver 2001; Merckaert 2015; Razavi 1993; Razavi 2002; Razavi 2003) ,and eliciting concerns (10 studies: Butow 2008; Epstein 2017; Goelz 2011; Gorniewicz 2017; Heaven 2006; Kruijver 2001; Razavi 1993; Razavi 2002; Razavi 2003; Stewart 2007);
explaining and planning e.g. appropriate information giving (10 studies: (Epstein 2017; Fallowfield 2002; Fujimori 2014; Goelz 2011; Lienard 2010; Razavi 2002; Razavi 2003; Stewart 2007; van Weert 2011: Wilkinson 2008), and negotiating (seven studies: Butow 2008; Heaven 2006; Lienard 2010; Merckaert 2015; Razavi 1993; Razavi 2003; Stewart 2007).
Secondary Outcomes
Other HCP outcomes that were measured in these studies included:
HCP health status (six trials: Butow 2008; Kruijver 2001; Lienard 2010; Razavi 1993; Razavi 2002; Razavi 2003);
HCP perception of the interview (three trials: Fallowfield 2002; Razavi 2002; Razavi 2003);
HCP perception of their behaviour change (nine trials: Fallowfield 2002; Fujimori 2014; Lienard 2010; Merckaert 2015; Kruijver 2001; Razavi 2002; Razavi 2003; Tulsky 2011; Wilkinson 2008);
HCP perception of their attitude change (eight trials: Butow 2008; Fallowfield 2002; Kruijver 2001; Lienard 2010; Razavi 1993; Razavi 2002; Razavi 2003; Wilkinson 2008).
We considered HCP perceptions to be very subjective outcomes and so excluded these from our review.
Patient outcomes were measured in 13 trials, 6166 patients (Butow 2008; Epstein 2017; Fallowfield 2002; Fujimori 2014; Kruijver 2001; Lienard 2010; Merckaert 2015; Razavi 2002; Razavi 2003; Stewart 2007; Tulsky 2011; van Weert 2011; Wilkinson 2008) including:
patients' perception of the interview (11 trials: Epstein 2017; Fallowfield 2002; Fujimori 2014; Gorniewicz 2017: Kruijver 2001; Lienard 2010; Razavi 2002; Razavi 2003; Stewart 2007; Tulsky 2011; Wilkinson 2008);
patients' trust in oncologist (Fujimori 2014; Tulsky 2011)
patient health status (10 trials: Butow 2008; Epstein 2017;Fallowfield 2002; Fujimori 2014; Gorniewicz 2017; Kruijver 2001; Merckaert 2015; Razavi 2003; Stewart 2007; Wilkinson 2008);
objective measure of patients communication (five trials: Kruijver 2001; Lienard 2010; Razavi 2002; Razavi 2003; van Weert 2011).
Two trials measured HCP communication with 'significant others' (Goelz 2011; Razavi 2003); one trial measured the satisfaction of 'significant others' (Razavi 2003).
All secondary outcomes except the objective measurement of patient communication were measured with questionnaires, most of which were developed locally and it was not always stated whether they had been previously validated (see Table 4 and Table 5). The following validated questionnaires were used:
3. Scales used for other HCP outcomes.
| Abbreviation | Name of scale | Studies included in review that used scale | Validation reference (if any) |
| MBI | Masslach Burnout inventory | Butow 2008; Fujimori 2014; Kruijver 2001; Lienard 2010; Razavi 2003 | Schaulell 1993 |
| NSS | Nursing Stress Scale | Razavi 1993; Razavi 2002 | Gray‐Toft 1981 |
| PPSB | Physician Psychosocial Belief questionnaire; | Fallowfield 2002 | Ashworth 1984 |
| SDAQ | Semantic Differential Attitude Questionnaire | Razavi 1993; Razavi 2002 | Silberfarb 1980 |
4. Scales for measuring patient outcomes.
| Abbreviation | Name of scale | Studies included in review that used scale | Validation reference (if any) |
| BSI | Brief Symptom Inventory | Stewart 2007 | Derogatis 1977 |
| CDIS | Cancer Diagnostic Interview Scale | Stewart 2007 | Roberts 1994 |
| EORTC QLQ‐C30: | European Organisation for Research and Treatment of Cancer, Quality of Life Questionnaire‐Core 30 | Butow 2008; Kruijver 2001 | Aaronson 1993; Hjermstad 1995 |
| EORTCR | European Organisation of research and treatments of Cancer Outpatient Satisfaction with Care Questionaire | Merckaert 2015 | Poinsot 2006 |
| FACT | Functional Assessment of Cancer Therapy scale | Epstein 2017 | Cella 1993 |
| GHQ‐12 | General health Questionnaire | Fallowfield 2002; Wilkinson 2008 | Williams 1988 |
| HADS | Hospital Anxiety and Depression Scale | Butow 2008; Fujimori 2014; Merckaert 2015; Razavi 2003 | Julian 2011; Snaith 1986 |
| HCCQ | Health Care Climate Questionairre | Epstein 2017 | Williams 1998 |
| McGill QOL | McGill Quality of Life Questionairre | Epstein 2017 | Cohen 1995 |
| NPIRQ | Netherlands Patient Information Recall Questionaire | van Weert 2011 | Jansen 2008 |
| PEPPI | Perceived Efficacy in Patient.Physician Interactions scale | Epstein 2017 | Maly 1998 |
| PIQ | Perception of Interview Questionnaire | Razavi 2003 | |
| PPPC | Patients perception of patient centeredness | Stewart 2007 | Henbest 1990 |
| PSCQ | Patient Satisfaction with Communication Questionnaire | Fallowfield 2002; Wilkinson 2008 | Ware 1983 |
| PSIAQ | Patient Satisfaction with Interview Assessment Questionnaire | Razavi 2002 | |
| PSQ‐C | Patient Satisfaction Questionnaire (PSQ‐C) | Kruijver 2001 | Blanchard 1986 |
| SCNS | Supportive Care needs survey (Boyes) | Butow 2008 | Sanson‐Fisher 2000 |
| STAI‐S | State Trait Anxiety Inventory‐State | Razavi 2003; Wilkinson 2008 |
Speilberger 1983 http://www.theaaceonline.com/stai.pdf Julian 2011 |
| Single item ( Feel better?) | Stewart 2007 | Henbest 1990 | |
| THC | The Human Connection scale | Epstein 2017 | Mack 2009 |
HCP health status: Maslach Burnout Inventory (MBI) Schaulell 1993 (used by Fujimori 2014; Kruijver 2001; Lienard 2010; Razavi 2003);
Nursing Stress scale Gray‐Toft 1981 (used by Razavi 1993; Razavi 2002);
-
Patients' perception of satisfaction with the interview:
Patients perception of Patient Centredness Henbest 1990 (used by Stewart 2007);
Patient Satisfaction Questionairre (PSQ‐C) Blanchard 1986 (used by Kruijver 2001);
Patient Staisfaction with Communication Questionairre Ware 1983 (used by Fallowfield 2002; Wilkinson 2008);
European Organization for Research and Treatment of Cancer (EORTC) Outpatient Satisfaction with Care Questionairre Poinsot 2006 (used by Merckaert 2015);
Support Care Needs Survey Sanson‐Fisher 2000 (used by Butow 2008);
Perceived Efficacy of patient Physician Interaction (PEPPI) Maly 1998 (used by Epstein 2017);
The human connection scale Mack 2009 (used by Epstein 2017);
Helath Care Climate Questionairre Williams 1998 (used by Epstein 2017).
-
Patient health status:
General Health Questionnaire 12 or GHQ12 Williams 1988 (used by Fallowfield 2002; Wilkinson 2008);
European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire‐30 or QLQ‐C30 Aaronson 1993; Hjermstad 1995 (used by Butow 2008; Kruijver 2001; Razavi 2002);
Hospital Anxiety and Depression Scale (HADS) Snaith 1986; Julian 2011 (used by Butow 2008; Fujimori 2014; Razavi 2003;
Speilberger's State Trait Anxiety Inventory (STAI‐S) Speilberger 1983 (used by Razavi 2003; Wilkinson 2008;
Functional Assessment of Cancer Therapy scale Cella 1993 (used by Epstein 2017);
McGill Quality of Life questionnaire Cohen 1995 (used by Epstein 2017);
Timing of the measurement of outcomes
Most studies measured communication skills prior to the intervention (within one to four weeks) and after a post‐intervention period (between one week and six months). Two studies had a further measurement at 12 and 15 months post‐intervention, respectively (Butow 2008; Fallowfield 2002). Three studies evaluated the effects of follow‐up CST interventions conducted between one and six months after the preliminary CST intervention (Heaven 2006; Razavi 2003; Tulsky 2011). One study measured patient outcomes for three years after intervention (Epstein 2017).
Excluded studies
We excluded 175 studies (183) articles after full‐text assessment because they did not meet our criteria for study design (see the 'Characteristics of excluded studies’ table); 132 of these studies were either not RCTs, or were not intervention studies of communication skills training. We excluded the remaining 43 RCTs for the following reasons:
13 trials not CST for HCPs;
11 CST in HCPs who did not work specifically in cancer care or where < 60% HCOs worked in cancer care;
3 trials CST was aimed at facilitating recruitment of patients to trials;
1 trial CST was only measured in the intervention group not the control group;
15 trials HCP behaviour change was not measured or was self‐assessed.
Risk of bias in included studies
We considered studies to be at a low risk of overall bias if we assessed the individual ’risk of bias’ criteria as ’low risk’ in 3/6 criteria. As a result, we considered 15 of the 17 included RCTs to be at a low risk of overall bias (Characteristics of included studies and Figure 4)
4.
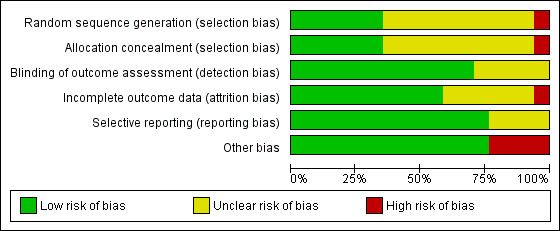
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Randomisation was computer‐generated in four trials (Goelz 2011; Lienard 2010; Tulsky 2011; Wilkinson 2008); by random number tables in two trials (Butow 2008; Stewart 2007); stratified block‐randomisation in one trial (Epstein 2017), and was not described in 10 trials.
Allocation concealment was described in six trials (Butow 2008; Epstein 2017; Razavi 1993; Stewart 2007; Tulsky 2011; Wilkinson 2008), and unclear (not described) in 11 trials.
Blinding
Blinding of participants was not possible in these trials, however, outcome assessment was clearly stated as blinded in 12 of the 17 trials. Most studies pre‐specified their outcomes and reported their pre‐specified primary outcomes. The following studies stated measuring some patient outcomes, however, did not report these results: Epstein 2017; Fallowfield 2002; Razavi 2002 and Razavi 2003.
Incomplete outcome data
Loss to follow‐up in relation to the primary outcomes was unclear in seven trials and considered 'low risk' in 10 trials with attrition rates ranging from 0% to 20%.
Selective reporting
In the majority of studies all pre‐specified outcomes were described. No protocols were available for Gorniewicz 2017 and Merckaert 2015. Merckaert 2015 did not report results of the Hospital Anxiety and Depression Scale (HADS) questionnaire. Epstein 2017 did not report the results of all the patient questionnaires listed in the protocol.
Other potential sources of bias
Four studies reported differences between the study groups in baseline characteristics of the HCPs (Goelz 2011; Merckaert 2015; Tulsky 2011; Wilkinson 2008), or patients (Razavi 2003). In two studies that measured outcomes at several points in time, it was unclear which participant interviews were included in their analyses (Lienard 2010; van Weert 2011).
Effects of interventions
See: Table 1
Communication skills training (CST) compared to no CST
Healthcare professionals (HCP) outcomes
Communication skills
Seven studies (Fujimori 2014; Lienard 2010; Merckaert 2015; Razavi 1993; Razavi 2002; Razavi 2003; Tulsky 2011) contributed data to these meta‐analyses: six of these studies contributed data to the 'simulated patients' subgroup and five contributed data to the 'real patients' subgroup. HCPs in these studies included 263 doctors (four studies (Fujimori 2014; Lienard 2010; Razavi 2003; Tulsky 2011), 188 nurses (Razavi 1993; Razavi 2002), and one mixed group or radiotherapy team of 80 HCPs (Merckaert 2015).
At the post‐intervention assessment, HCPs in the intervention group were more likely than the control group to:
use open questions (standardised mean difference (SMD) 0.25, 95% confidence interval (CI) 0.02 to 0.48; P = 0.03, I² = 62%; 5 studies, 796 participant interviews; very low‐certainty evidence Analysis 1.1;
show empathy (SMD 0.18, 95% CI 0.05 to 0.32; P = 0.008, I² = 0%; 6 studies, 844 participant interviews) moderate‐certainty evidence; Analysis 1.2;
less likely to 'give facts only' without individualising their responses to the patient's emotions or offering support. (SMD ‐0.26, 95% CI ‐0.51 to ‐0.01; P = 0.05, I² = 68%; 5 studies, 780 participant interviews; low‐certainty evidence; Analysis 1.3.
1.1. Analysis.
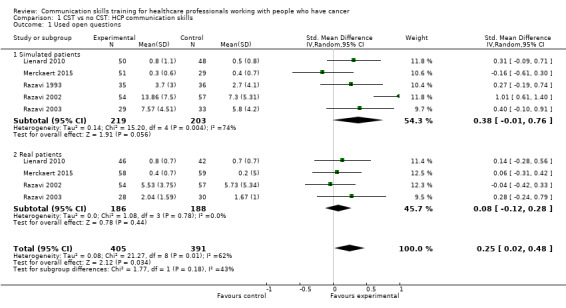
Comparison 1 CST vs no CST: HCP communication skills, Outcome 1 Used open questions.
1.2. Analysis.
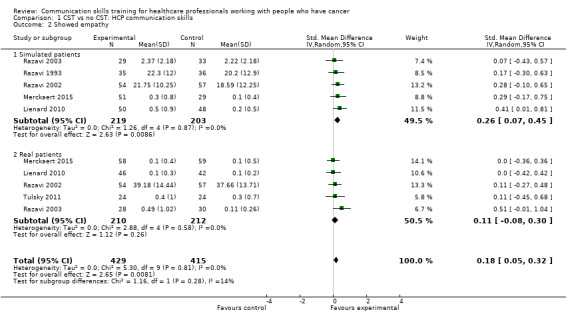
Comparison 1 CST vs no CST: HCP communication skills, Outcome 2 Showed empathy.
1.3. Analysis.
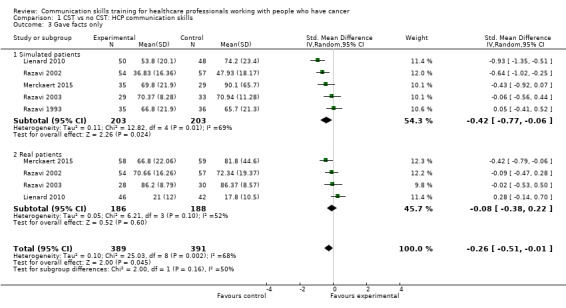
Comparison 1 CST vs no CST: HCP communication skills, Outcome 3 Gave facts only.
There were no differences between CST and no CST on eliciting patient concerns (Analysis 1.4) and providing appropriate information (Analysis 1.5); moderate‐certainty evidence, although eliciting concerns showed a tendency in favour of the CST intervention. No differences were found in the other HCP communication skills, including clarifying and/or summarising information, and negotiation (Analysis 1.6; Analysis 1.7.
1.4. Analysis.
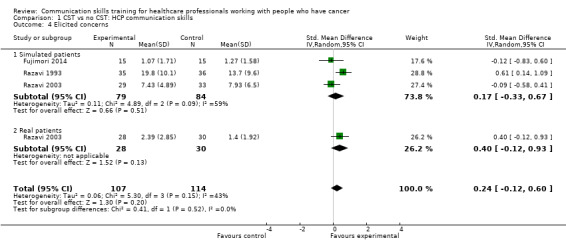
Comparison 1 CST vs no CST: HCP communication skills, Outcome 4 Elicited concerns.
1.5. Analysis.
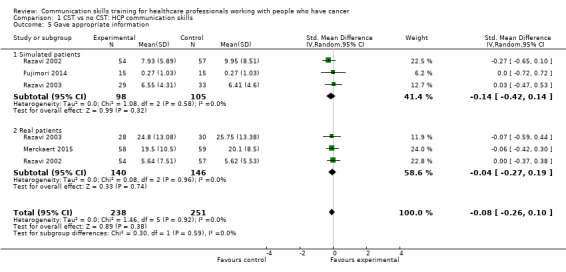
Comparison 1 CST vs no CST: HCP communication skills, Outcome 5 Gave appropriate information.
1.6. Analysis.
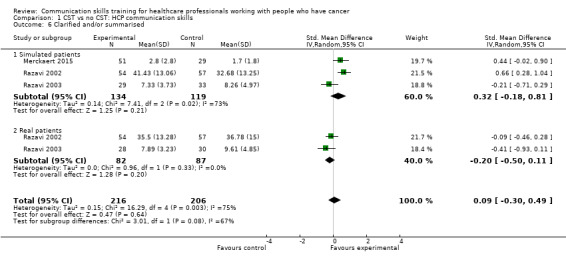
Comparison 1 CST vs no CST: HCP communication skills, Outcome 6 Clarified and/or summarised.
1.7. Analysis.
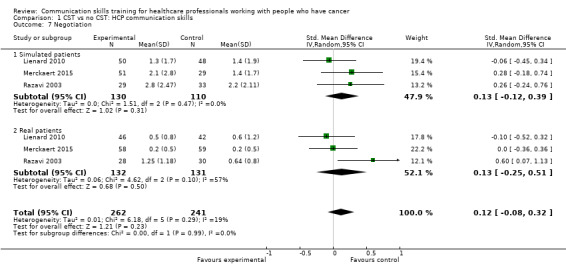
Comparison 1 CST vs no CST: HCP communication skills, Outcome 7 Negotiation.
Other HCP communication skills that were evaluated in some studies but that were either not included in our 'Types of outcome measures', or that gave insufficient data for inclusion in meta‐analyses (e.g. only gave P values), included the following.
Emotional depth: Merckaert 2015 and Kruijver 2001 reported significantly greater emotional depth in the intervention groups compared with the control groups, P = 0.03 and P = 0.05, respectively.
Empathy: Butow 2008 found less empathy in intervention group compared with the control group at six months post‐intervention (P = 0.024).
Checking that the patient understands: Kruijver 2001 reported significantly less checking of patient understanding in the CST group than in the control group; whereas Fallowfield 2002; Fujimori 2014 and Goelz 2011 reported no significant difference between the groups.
Appropriate information: there was less appropriate information giving in the CST groups than the control groups in Kruijver 2001 (P < 0.05), Lienard 2010 (P value < 0.001) and van Weert 2011 (P value < 0.01).
Team orientated focus: Merckaert 2015 reported greater team orientated focus in favour of the intervention group (P = 0.023).
Blocking behaviours: no significant effect of CST was found by Butow 2008 (P = 0.66), Heaven 2006 and Razavi 1993; whereas, Wilkinson 2008 found significantly less blocking behaviour in the intervention group (P = 0.001).
Global score: there was a significant intervention effect on global communication scores in the threes studies that measured this: Wilkinson 2008; (P value < 0.001) Goelz 2011 (P = 0.007) and Gorniewicz 2017 with a composite score (P = 0.001).
Preamble to 'Breaking bad news' (BBN): Fujimori 2014 reported a statistically significant intervention effect (P = <0.001). Gorniewicz 2017 found no effect using a composite score.
Several studies showed significant intervention effects on composite scores of different communication domains.
Epstein 2017 created a composite score of four pre‐specified communication measures (engaging, responding to patient emotions, informing patients about disease prognosis and treatments, balanced framing of decisions). His composite measure of patient‐centred communication showed a significant intervention effect (estimated adjusted intervention effect 0.34 95% CI 0.06 to 0.62; P = 0.02), but none of the four pre‐specified communication measures showed a significant difference between groups.
Fujimori 2014 found that three of four categories formed by 27 specific skills had statistically significant intervention effects (setting supportive environment P = 0.002, considering how to deliver bad news P = 0.001, emotional support P = 0.011). Only seven of the 27 specific skills showed significant intervention effects.
Gorniewicz 2017 found significant intervention effects in three composite scores on BBN: breaking bad news P = 0.004; communication related to patient emotions P = 0.034; 'determines patient readiness' to proceed P = 0.041; and four general communication composite scores: active listening (P = 0.011); addressing feelings (P value < 0.001); closing interview P = 0.002 and global P = 0.001.
van Weert 2011 found a significant intervention effect on one of seven dimensions from 67 specific communication skills: communicating realistic expectations P < 0.01. The dimension rehabilitation improvement was significantly better in the control group P value < 0.01.
Doctors only
Four studies enrolling doctors contributed data to these subgroup analyses (Fujimori 2014; Lienard 2010; Razavi 2003; Tulsky 2011)); the results were consistent with the main findings. At the post‐intervention assessment, doctors in the intervention group were more likely than those in the control group to:
use open questions (SMD 0.27, 95% CI 0.05 to 0.50; P = 0.02, I² = 0%; 2 studies, 306 participant interviews; Analysis 2.1)
show empathy (SMD 0.22, 95% CI 0.01 to 0.43; P = 0.04, I² = 0% 3 studies, 354 participant interviews; Analysis 2.2).
2.1. Analysis.

Comparison 2 CST vs no CST: HCP communication skills: doctors only, Outcome 1 Used open questions.
2.2. Analysis.
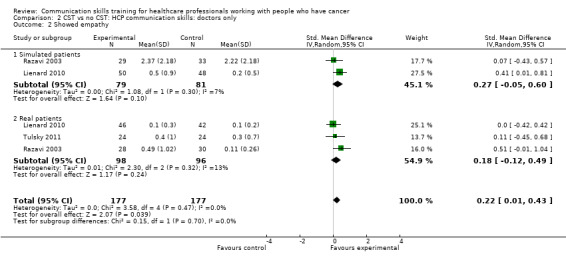
Comparison 2 CST vs no CST: HCP communication skills: doctors only, Outcome 2 Showed empathy.
There were no differences between the intervention and control groups in the meta‐analyses of the following outcomes: clarifying and summarising, eliciting concerns, giving appropriate information and giving facts only (Analysis 2.3; Analysis 2.4; Analysis 2.5; Analysis 2.6).
2.3. Analysis.
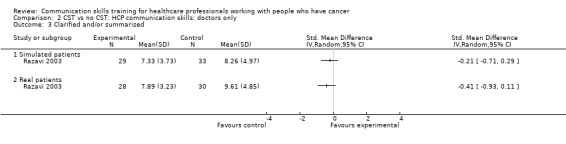
Comparison 2 CST vs no CST: HCP communication skills: doctors only, Outcome 3 Clarified and/or summarised.
2.4. Analysis.
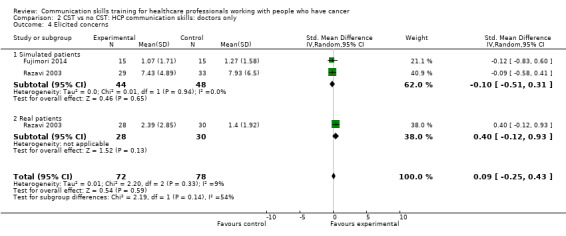
Comparison 2 CST vs no CST: HCP communication skills: doctors only, Outcome 4 Elicited concerns.
2.5. Analysis.
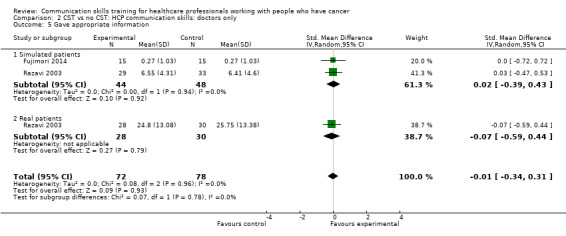
Comparison 2 CST vs no CST: HCP communication skills: doctors only, Outcome 5 Gave appropriate information.
2.6. Analysis.
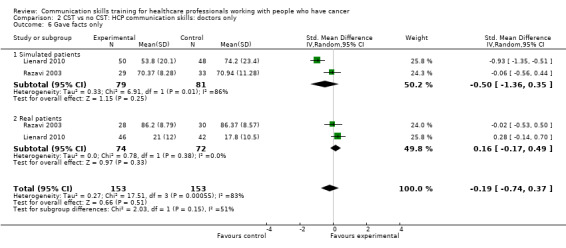
Comparison 2 CST vs no CST: HCP communication skills: doctors only, Outcome 6 Gave facts only.
Nurses only
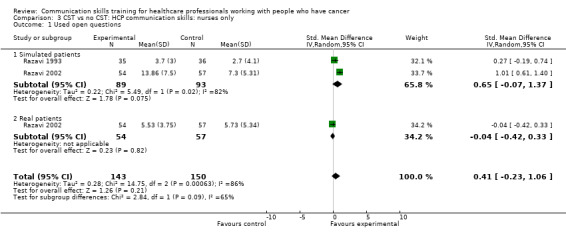
Only two studies contributed data to these subgroup analyses (Razavi 1993; Razavi 2002). At the post‐intervention assessment, there were no differences between the intervention and control groups in any of the meta‐analyses (Analysis 3.1; Analysis 3.4; Analysis 3.5; Analysis 3.6). For two outcomes (clarifying/summarising Analysis 3.2; and eliciting concerns Analysis 3.3), only one study contributed data (Razavi 2002)
3.1. Analysis.
Comparison 3 CST vs no CST: HCP communication skills: nurses only, Outcome 1 Used open questions.
3.4. Analysis.
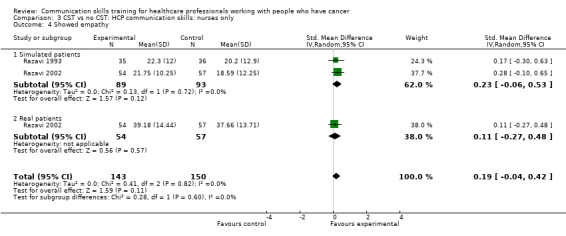
Comparison 3 CST vs no CST: HCP communication skills: nurses only, Outcome 4 Showed empathy.
3.5. Analysis.
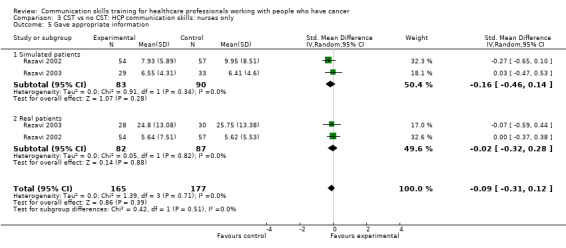
Comparison 3 CST vs no CST: HCP communication skills: nurses only, Outcome 5 Gave appropriate information.
3.6. Analysis.
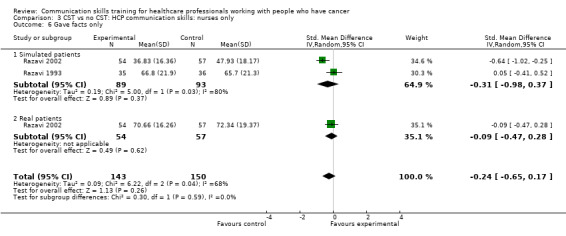
Comparison 3 CST vs no CST: HCP communication skills: nurses only, Outcome 6 Gave facts only.
3.2. Analysis.
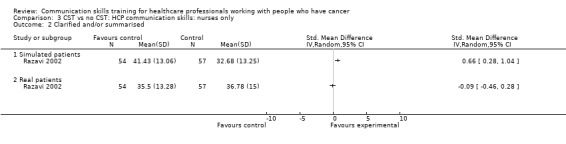
Comparison 3 CST vs no CST: HCP communication skills: nurses only, Outcome 2 Clarified and/or summarised.
3.3. Analysis.

Comparison 3 CST vs no CST: HCP communication skills: nurses only, Outcome 3 Elicited concerns.
Sensitivity analyses
We performed sensitivity analyses of our primary HCP outcomes to exclude studies that evaluated follow‐up interventions, i.e. Razavi 2003 and Tulsky 2011. We noted the following effects:
Analysis 1.1 the use of ’open questions’ no longer showed any difference when one study (Razavi 2003) was removed (4 studies, 676 participant interviews; SMD 0.23; 95% CI ‐0.06 to 0.06; P = 0.69; I² = 71%);
Analysis 1.2: showing 'empathy' continued to show a small difference when two studies (Razavi 2003 and Tulsky 2011)were excluded (4 studies, 676 participant interviews; SMD 0.17 95% CI 0.02 to 0.32; P = 0.74; I² = 0%);
the results of the other primary analyses either remained either very similar to the original analyses, or they contained insufficient studies for meta‐analyses to be performed.
We also performed subgroup analyses to determine whether there were significant differences in primary outcomes between nurses and doctors participating in these trials (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6), however, tests for subgroup differences were not significant.
4.1. Analysis.
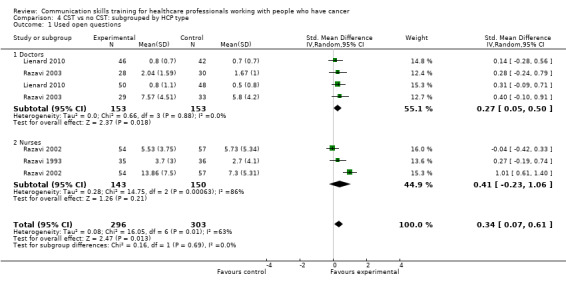
Comparison 4 CST vs no CST: subgrouped by HCP type, Outcome 1 Used open questions.
4.2. Analysis.
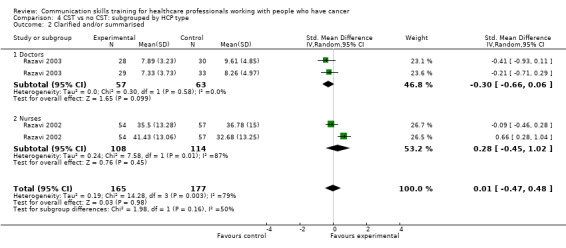
Comparison 4 CST vs no CST: subgrouped by HCP type, Outcome 2 Clarified and/or summarised.
4.3. Analysis.
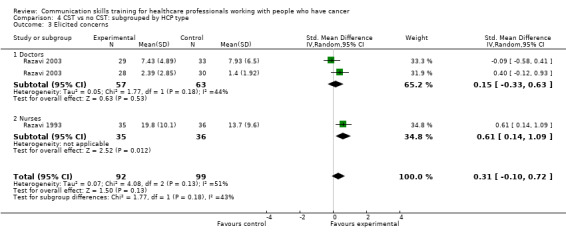
Comparison 4 CST vs no CST: subgrouped by HCP type, Outcome 3 Elicited concerns.
4.4. Analysis.
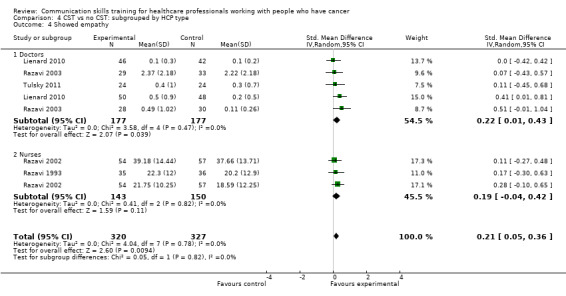
Comparison 4 CST vs no CST: subgrouped by HCP type, Outcome 4 Showed empathy.
4.5. Analysis.
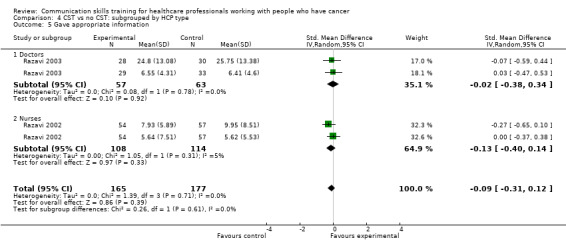
Comparison 4 CST vs no CST: subgrouped by HCP type, Outcome 5 Gave appropriate information.
4.6. Analysis.
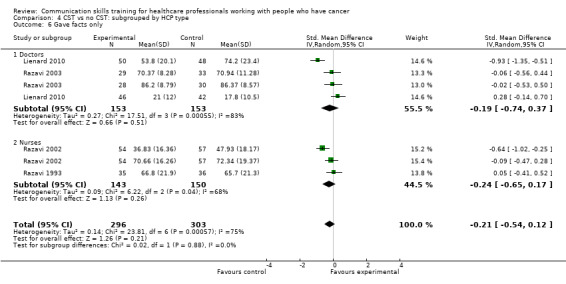
Comparison 4 CST vs no CST: subgrouped by HCP type, Outcome 6 Gave facts only.
Other HCP outcomes
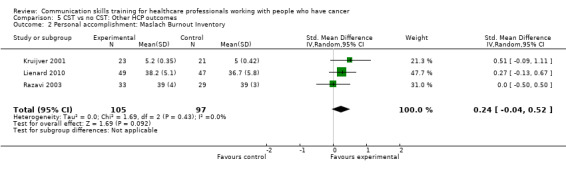
Three studies (Kruijver 2001; Lienard 2010; Razavi 2003) contributed data to meta‐analyses relating to HCP 'burnout'. Kruijver 2001 enrolled nurses and Lienard 2010 and Razavi 2003 enrolled residents (doctors in specialist postgraduate training) and doctors (62% were oncologists), respectively. Burnout was measured using the Maslach Burnout Inventory (MBI). For the outcome 'emotional exhaustion' there was no difference in mean scores between the intervention and control groups (SMD ‐0.16, 95% CI ‐0.44 to 0.12; P = 0.26, I² = 0%; 3 studies, 202 participant interviews: Analysis 5.1). For the outcome 'personal accomplishment' there was no difference between the intervention and control groups (SMD 0.24, 95% CI ‐0.04 to 0.52; P = 0.09, I² = 0%; 3 studies, 202 participant interviews; Analysis 5.2). For the outcome 'depersonalisation' with data from just one study (Lienard 2010), there was no difference between the intervention and control groups (mean difference (MD) 0.50, 95% CI ‐1.50 to 2.50; P = 0.62; 1 study, 96 participant interviews; low‐certainty evidence; Analysis 5.3). Butow 2008 also reported 'burnout' and found no significant effect of CST on this outcome, however did not report these data in a usable form for this meta‐analysis.
5.1. Analysis.
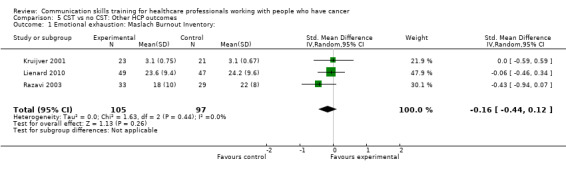
Comparison 5 CST vs no CST: Other HCP outcomes, Outcome 1 Emotional exhaustion: Maslach Burnout Inventory:.
5.2. Analysis.
Comparison 5 CST vs no CST: Other HCP outcomes, Outcome 2 Personal accomplishment: Maslach Burnout Inventory.
5.3. Analysis.
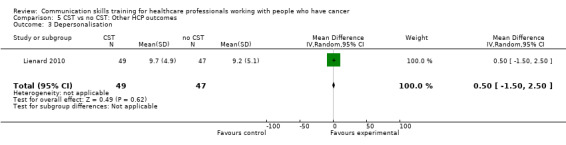
Comparison 5 CST vs no CST: Other HCP outcomes, Outcome 3 Depersonalisation.
Patient outcomes
'Patient anxiety' was measured in two studies (Razavi 2003; Wilkinson 2008) using the Spielberger State of Anxiety Inventory (STAI‐S) and in one study (Fujimori 2014) using the HADS. Anxiety scores decreased in both groups in both studies after all the interviews; in Fujimori 2014 the mean reduction in anxiety scores (pre‐ and post‐interview) was significantly greater in the control group (SMD 0.17; 95% CI ‐0.24 to 0.59; P = 0.41; I² = 78%, 3 studies, 770 participant interviews; very low‐certainty; Analysis 6.2).
6.2. Analysis.
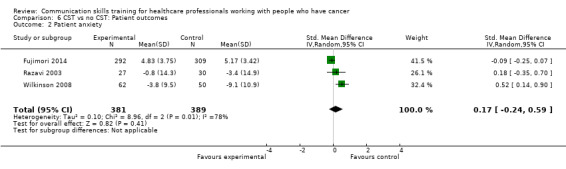
Comparison 6 CST vs no CST: Patient outcomes, Outcome 2 Patient anxiety.
Wilkinson 2008 evaluated patient 'psychiatric morbidity', assessed by the GHQ 12 questionnaire, and found it to be significantly lower in the intervention group than the control group (one study, 127 participant interviews; SMD ‐0.36, 95% CI ‐0.71 to ‐0.01; Analysis 6.1; P = 0.05), however, this study reported significantly greater baseline anxiety in the control group.
6.1. Analysis.

Comparison 6 CST vs no CST: Patient outcomes, Outcome 1 Patient psychiatric morbidity (GHQ 12).
There were no differences in either the outcomes 'patient perception of HCP communication skills' (Razavi 2002; Razavi 2003) (2 studies, 170 participant interviews; SMD ‐0.14; 95% CI ‐0.44 to 0.16; P = 0.37; I² = 0% Analysis 6.3) nor 'patient satisfaction with communication' (Fallowfield 2002; Wilkinson 2008) (2 studies, 429 participant interviews; SMD 0.20; 95% CI ‐0.23 to 0.63; P = 0.36; I² = 74%; very low‐certainty; Analysis 6.4).
6.3. Analysis.
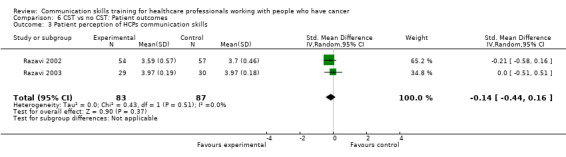
Comparison 6 CST vs no CST: Patient outcomes, Outcome 3 Patient perception of HCPs communication skills.
6.4. Analysis.
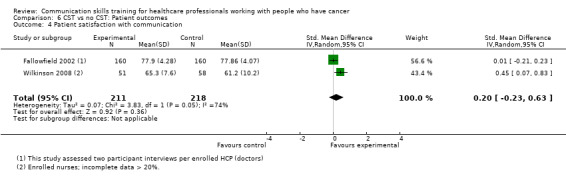
Comparison 6 CST vs no CST: Patient outcomes, Outcome 4 Patient satisfaction with communication.
Patient outcomes that were either not included in our 'Types of outcome measures', or that gave insufficient data for inclusion in meta‐analyses (e.g. only gave P values), included the following.
Patient trust: two studies reported significantly greater patient trust in the intervention group (Fujimori 2014; Tulsky 2011) (P = 0.009 and P = 0.036, respectively).
Quallity of life: Kruijver 2001 found statistically significant improvement in only 1/30 items; and Butow 2008 found no significant differences. Epstein 2017 did not find any statistical difference using a single item scale nor on the McGill Quality of life Questionnaire nor on the Functional Assessment of Cancer Therapy scale.
Anxiety: Butow 2008 reported a reduction in patient anxiety (telephone interviews) one week after the consultation in the intervention group (P = 0.021). This change was not maintained in telephone interviews three months later.
Depression: Butow 2008 found no difference in patient depression (telephone interviews) at one week after the consultation in the intervention group.
Distress: Fujimori 2014 reported that distress scores were 'significantly decreased' in the intervention group compared with the control group.
Satisfaction: Fujimori 2014 reported that distress scores were 'significantly decreased' in the intervention group compared with the control group. Merckaert 2015 reported no significant differences in satisfaction between patients of the intervention group and the control group when considering the whole team, but in relation to the nurses care, there was a higher level of satisfaction (P = 0.028) in the intervention group.
Health care utilisation: Epstein 2017 found no intervention effects in aggressive treatments and hospice use in the last 30 days of life.
Recall of information: van Weert 2011 reported a 'marginally significant' improvement in total patient recall following HCP CST. This was a composite score of six sub‐categories, only two of which has statistical difference between groups in favour of intervention.
Number of questions asked by patients: van Weert 2011 found the number of questions asked by patients and companions increased in the intervention group (P = < 0.05).
'Significant other' outcomes
One study reported no differences in relatives' anxiety or satisfaction between intervention and control groups (Razavi 2003), however the data given were insufficient for meta‐analysis. Goelz 2011 found improvements in some HCP behaviour in relation to relatives in simulated interviews (P < 0.001).
Effect of CST over time
Two trials studied the effect of CST up to one year after the intervention. Butow 2008 reported that clinically significant improvements in doctors communication skills at six months were maintained at 12 months in the group that received CST, however these improvements were not significant. Doctors in the intervention group scored lower on responding to distress than the control group at 12 months.
Fallowfield 2002 evaluated all participants at three months post‐intervention and evaluated the intervention group only at 15 months post‐intervention. For the intervention group doctors, most statistically significant benefits of CST (appropriate questions and responses) displayed at three months were maintained at 15 months, however, there was a drop off in empathy scores (P < 0.001). At 15 months post‐intervention, the investigators also noted a significant improvement in the HCPs' summarising of information for the patients (P = 0.038), and that they interrupted less (P < 0.001) than at the three‐month assessment.
Follow‐up CST compared with no follow‐up CST
Three trials studied the effect of follow‐up interventions (Heaven 2006; Razavi 2003; Tulsky 2011), however, they reported little data that we could use in our meta‐analyses, most of which (Analysis 7.1; Analysis 7.2; Analysis 7.3; Analysis 7.4; Analysis 7.5; Analysis 7.6; Analysis 7.7) contain data from only one study (Razavi 2003). However, meta‐analysis of two studies was possible for the outcome 'empathy'. We found no difference between the intervention and control groups with regard to this outcome (SMD 0.23, 95% CI ‐0.07 to 0.54; P = 0.14; I² = 0%; 2 studies, 168 participant interviews; Analysis 7.4).
7.1. Analysis.
Comparison 7 Follow‐up CST vs no follow‐up CST: HCP communication skills, Outcome 1 Used open questions.
7.2. Analysis.
Comparison 7 Follow‐up CST vs no follow‐up CST: HCP communication skills, Outcome 2 Clarified and/or summarised.
7.3. Analysis.
Comparison 7 Follow‐up CST vs no follow‐up CST: HCP communication skills, Outcome 3 Elicited concerns.
7.4. Analysis.
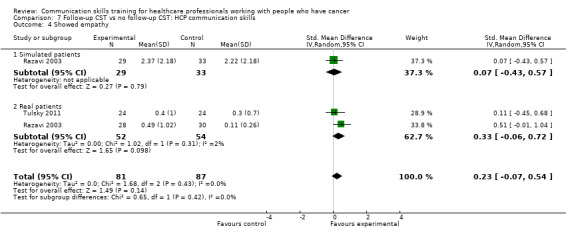
Comparison 7 Follow‐up CST vs no follow‐up CST: HCP communication skills, Outcome 4 Showed empathy.
7.5. Analysis.
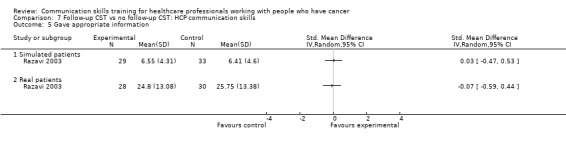
Comparison 7 Follow‐up CST vs no follow‐up CST: HCP communication skills, Outcome 5 Gave appropriate information.
7.6. Analysis.
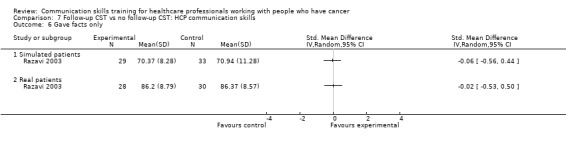
Comparison 7 Follow‐up CST vs no follow‐up CST: HCP communication skills, Outcome 6 Gave facts only.
7.7. Analysis.
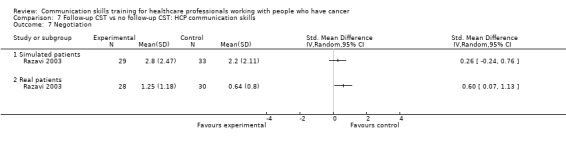
Comparison 7 Follow‐up CST vs no follow‐up CST: HCP communication skills, Outcome 7 Negotiation.
Individually, these studies reported the following.
Razavi 2003 reported some statistically significant improvements in doctors' communication skills after a single 2.5 day CST workshop followed by six, bimonthly, three‐hour consolidation workshops compared with a single 2.5 day CST workshop only. These significant improvements included: open questions in simulated interviews (P = 0.014); checking understanding (P = < 0.01); and empathic statements in real patient interviews (P = 0.009) and in interviews where a relative was present. In addition, patients interviewed by doctors who received the follow‐up CST perceived that their doctor had a better understanding of their disease than patients of doctors who received no follow‐up CST (P = 0.04). The follow‐up CST had no significant effect on patient satisfaction or anxiety levels, except in interviews with relatives, where the patients, but not the relatives, were reported to be more globally satisfied (P = 0.024).
Tulsky 2011 reported a improvement in oncologists communication skills in interviews with real patients after a CST lecture and the use of a follow‐up CD‐ROM, compared with a control group who had received a CST lecture only: 'empathic statements' (P = 0.024) and 'response to empathic opportunity' (P = 0.03) were improved in the intervention group. 'patient trust' also improved (P = 0.036).
Heaven 2006 failed to show any difference in nurses' communication skills in real patient encounters after receiving a three‐day CST course followed by four half‐day supervision sessions spread over four weeks, compared with the three‐day CST course only.
Comparison of different types of CST
One trial with 51 participants (18 oncologists, 17 family physicians and 16 surgeons) compared a six‐hour student‐centred, experiential CST, with a two‐hour small‐group discussion commenced with a video (Stewart 2007). No differences were found between the groups in HCP behaviour outcomes in the post‐intervention simulated interviews, however, in the subgroup analysis of family physicians, those who participated in the six‐hour course showed better scores in offering support (P = 0.02), information sharing (P = 0.05), and exploring and validating whole person issues (P = 0.02 and P = 0.05, respectively) compared with those who participated in the two‐hour course. In the subgroup of surgeons, patient satisfaction and perception of well‐being improved after the six‐hour course (P = 0.02 and P = 0.03, respectively). Overall, there was no significant effect on the patients' psychological distress; however, using a single validated question, more patients "felt better" with HCPs who had undergone the six‐hour training course than with HCP participants of the two‐hour course (P = 0.02).
Feedback compared to no feedback
Only one study reported this comparison (Fallowfield 2002) and found no between HCP communication skills in groups receiving 'feedback' or 'no feedback'.
Discussion
Summary of main results
We performed meta‐analyses of seven healthcare professional (HCP) communication skill outcomes (using open questions, clarifying/summarising, eliciting concerns, showing empathy, giving appropriate information, giving facts only, and negotiating), three 'other' HCP outcomes relating to 'burnout '(emotional exhaustion, depersonalisation, personal accomplishment), and four patient outcomes (psychiatric morbidity, anxiety, perception of HCP communication, satisfaction with HCP communication). Overall, 10 studies contributed data to the meta‐analyses.
HCPs in the intervention groups were more likely to show empathy towards their patients (6 studies, 844 participant interviews; P = 0.008, I² = 0%) and less likely to 'give facts only' without individualising their responses to the patient's emotions or offering support (5 studies, 780 participant interviews; P = 0.05, I² = 68%). We considered these findings to be of a moderate‐ and low‐certainty, respectively (Table 1). They were also more likely to use open questions in the post‐intervention interviews than the control group (five studies, 796 participant interviews; P = 0.03, I² = 62%); however the certainty of evidence was very low. In subgroup analyses according to staff type, these benefits of communication skills training (CST) remained statistically significant when 'doctors only' were included in the meta‐analyses, but not for 'nurses only'; however, doctors and nurses did not perform differently for any HCP outcomes.
There were no differences in the other HCP communication skills. Tests for subgroup differences (between real and simulated patients) were significant.
HCP 'burnout' was assessed post‐intervention in five studies using the Maslach Burnout Inventory (MBI). Three studies could be included in a meta‐analysis: one was conducted in nurses, two trials in doctors (mainly oncologists). There were no differences between the intervention and control groups with regard to 'emotional exhaustion' (202 participant interviews; P = 0.26 I² = 0%); 'personal accomplishment' (202 participant interviews; P = 0.09, I² = 0%) or 'depersonalisation' with data from just one study (96 participants, MD 0.50, 95% CI ‐1.50 to 2.50; P = 0.62). We consider this evidence to be of a low‐certainty.
With regard to patient outcomes, three studies contributed data to the outcome 'patient anxiety'. There were no differences between the intervention and control groups (770 participant interviews; SMD 0.17; 95% CI ‐0.24 to 0.59; P = 0.41; I² = 78%). In a study of 172 nurses, psychiatric morbidity was found to be lower in the intervention group than the control group (SMD ‐0.36, 95% CI ‐0.71 to ‐0.01 P = 0.05). There were no differences in 'patient perception of HCPs communication skills' (two studies, 170 participant interviews; SMD ‐0.14; 95% CI ‐0.44 to 0.16; P = 0.37; I² = 0%), and 'patient satisfaction with communication' (two studies, 429 participant interviews; SMD 0.20; 95% CI ‐0.23 to 0.63; P = 0.36; I² = 74%) in meta‐analyses of these outcomes.
Overall completeness and applicability of evidence
These meta‐analyses offer limited evidence that communications skills training of HCPs working in cancer care has a beneficial effect on some HCP communication skills when assessed up to six months after the training course or workshop. The types of skills that showed improvement in our meta‐analyses were related to information gathering (open questions) and supportive or relationship‐building skills (empathy) and giving facts only. However, in the use of open questions, the certainty of evidence was very low. These benefits probably apply to both doctors and nurses as tests for subgroup differences were not significant.
The types of CST, length of training and time spread were diverse and it was not possible to draw conclusions as to the relative efficacy of the different programs. These results, therefore, are not necessarily applicable to all types of CST. In future versions of this review, it may be desirable to subgroup our results according to intervention type; this was not possible for the current version due to the small number of contributing studies. Furthermore, longer‐term follow‐up is necessary to ascertain whether these skills are retained. In the 17 included studies, the longest follow‐up for HCP communication skills occurred in Butow 2008 and Fallowfield 2002, at 12 and 15 months post‐intervention, respectively. These studies give conflicting results and we were unable to combine these data in a meta‐analysis.
Three trials studied the effects of follow‐up interventions on HCP communication skills and reported some positive effects on the maintenance of behaviour change in clinical practice (Heaven 2006; Razavi 2003; Tulsky 2011), however, the longest follow‐up period was six months, and meta‐analyses including these studies were not possible except for the outcome 'empathy', for which we found no difference. The efficacy of follow‐up CST is inconclusive based on the available evidence.
Few studies reported patient health‐related outcomes and those that did had little usable data. Evidence for a beneficial effect on patients' psychological and physical health is lacking and further research is needed in this regard. All trials were performed in developed countries and, thus, the results may not be widely applicable to less‐developed regions.
Quality of the evidence
We graded the review evidence according to guidelines from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), that supports the GRADE approach, defining the certainty of the body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest. Downgrading of evidence can occur if there are limitations in the design and implementation of available studies, the data are inconsistent or imprecise reflected by wide confidence intervals, the evidence is indirect or there is a high probability of publication bias.
We consider the evidence related to three primary outcomes, 'empathy', 'giving appropriate information' and 'eliciting concerns' to be of moderate‐certainty. (Table 1). We considered the evidence relating to 'open questions' to be of very low‐certainty, downgrading it due to inconsistency, imprecision and risk of bias amongst studies included in the meta‐analysis of this outcome.
The certainty of evidence for secondary outcomes 'emotional exhaustion' and 'patient anxiety' is low and very low respectively. This is due to imprecision, risk of bias and inconsistency.
In general, most included studies displayed considerable heterogeneity in terms of the types of CST, the types of patients (real or simulated), the outcomes assessed, the measurement tools used to evaluate outcomes, and other variables.
Potential biases in the review process
For the protocol and original 2003 review (Fellowes 2002), we defined 'Types of outcomes' simply as 'changes in behaviour or skills measured using objective and validated scales'. However, for the first update, we defined primary and secondary outcomes more clearly. By so doing, we may have introduced bias into the review. In addition, by choosing to extract data and perform meta‐analyses, thereby possibly limiting the review findings to a handful of outcomes, rather than present the data of the 17 studies in a narrative review, we may have introduced bias. Several studies reported other HCP behavioural outcomes (i.e. that were not included in our list of outcomes) and we hope that by presenting these additional data, we have been able to present the wide range of evidence (and certainty of evidence) available to inform opinion.
Some trials reported statistically significant effects (both positive and negative) of various HCP communication outcomes but were limited by the inadequate reporting of data, such that the data could not be used in meta‐analyses. Types of limited reporting included only giving P values, percentages, or means without numbers assessed or standard deviations. The fact that useable data for these outcomes were not available, may have inherently biased the review. For example, three studies (Kruijver 2001; Lienard 2010; van Weert 2011) individually reported statistically significantly less 'appropriate information giving' in their intervention groups than the control groups, suggesting that CST may negatively impact this outcome, but there were no accompanying extractable data to support the reports. Our meta‐analysis of this outcome included data from only three studies and we found no significant difference between the two groups, although the point‐estimate favoured the control group (Analysis 1.5).
In some studies, outcomes consisted of phrases, or aspects of scales that we had not included as outcomes for this review. Almost every trial used its own unique scale with an average of 25 variables (range, six to 84); with only three scales used in more than one study. We used standardised mean differences to adjust for these different scales and random‐effects methods for all meta‐analyses, to minimise potential biases.
Lastly, by including data from the studies of follow‐up interventions (three studies) in our meta‐analyses of 'CST versus no CST', we may have introduced bias into our meta‐analyses. All HCPs in these studies received preliminary CST and then subsequently randomised to receive a follow‐up CST intervention. We performed sensitivity analyses to determine what effect including these studies had on our overall results and reported these findings.
Agreements and disagreements with other studies or reviews
Previous reviews Barth 2011; Paul 2009; Uitterhoeve 2010 have consistently concluded that CST leads to better HCP communication behaviours. Barth 2011 included 13 studies (three non‐randomised) and extracted effect sizes for the outcomes HCP behaviour, HCP attitudes and patient outcomes. It is not clear to us how they combined the several aspects of HCP behaviour into a single effect size as the included studies reported diverse behaviour outcomes, however, they report a low to moderate effect of CST on HCP behaviour. Thus, our findings seem to agree. Barth 2011 also performed subgroup analysis to assess the effect of the duration of the CST course on HCP behaviour and reported a trend toward shorter courses being less successful than longer ones; this finding supports the conclusions of Gysels 2004, but we were unable to corroborate these findings.
Healthcare professional attitude change is a very subjective outcome and, although CST has been reported in other reviews to have a positive effect on this outcome (Barth 2011), we have not included it in our review. Barth 2011 suggests that the inability to show profound results following CST workshops may be due to the high pre‐intervention competencies in the participants taking part in the CST. This is a good point. Most of our included studies were conducted in oncologists and cancer care nurses with experience ranging from two to 24 years.
We agree with the findings of other reviews (Barth 2011; Paul 2009; Uitterhoeve 2010), that CST in HCPs appears to have little effect on patient outcomes, however high‐certainty data for patient outcomes are scarce. The Kissane 2012 review expressed uncertainty as to whether the skills acquired from CST are maintained in the long term; we agree that the long‐term benefits of CST are not clearly established. Our findings support the recommendations for the development of standardised outcome measures for future research in the consensus statement of European experts (Stiefel 2010).
Authors' conclusions
Implications for practice.
Communication skills training for healthcare professionals (HCPs) working in cancer care using learner‐centred, experiential education methods by experienced facilitators, can result in improvements of some communication skills, particularly empathy, and can help HCP to be less likely to give facts only without individualising their responses to the patient's emotions or offering support. Whilst improving these communication skills, CST courses should also aim to ensure appropriate eliciting‐concerns and information‐giving skills in HCP participants. It is unclear whether the skills acquired by HCPs are retained in the long term. In addition, it is unclear what type, duration and intensity of CST is most effective, and whether consolidation workshops may improve the impact of CST. CST appears to have little measurable benefit to the mental or physical health, and satisfaction of people with cancer and does not appear to reduce 'burnout' in HCPs.
Implications for research.
The original version of this review called for further research and the number of randomised trials has since increased dramatically. However the diversity of studies, particularly in the scales used to measured HCP communication skills, continues to limit the conclusions of this updated review. We recommend that randomised controlled (RCTs) use standard validated scales, and that (limited) core study outcomes (both for HCP outcomes and patient outcomes) are identified and pre‐specified. Several validated scales to measure HCP communication now exist (Table 2) but investigators should ensure that their outcomes permit comparability between studies. It may be preferable to use real patients for measurement of HCP communication in studies of CST interventions to ensure clinically meaningful results. Trials should include clear reporting of trial methods and study outcomes, and data should be reported in full e.g. continuous data as means with standard deviations and the number analysed per outcome.
Other important questions remain unanswered:
the optimal length of training/course structure;
the long‐term efficacy of communication skills training;
the role of e‐learning;
compulsory rather than voluntary training;
the role of consolidation courses;
the role of training both HCPs and patients in communication skills.
What's new
| Date | Event | Description |
|---|---|---|
| 23 July 2018 | New citation required but conclusions have not changed | Two additional studies included and 57 excluded. Conclusions unchanged. |
| 23 July 2018 | New search has been performed | Literature searches updated. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 19 February 2013 | New search has been performed | Review updated to include 15 RCTs. |
| 18 February 2013 | New citation required but conclusions have not changed | We found more evidence to show that CSTs may be helpful in improving some HCP outcomes. |
| 6 August 2012 | New search has been performed | Out of 119 potentially eligible records identified by the updated searches, we included 12 additional studies (Butow 2008, Merckaert 2015, Goelz 2011, Heaven 2006, Kruijver 2001, Razavi 2002, Razavi 2003, Stewart 2007, Lienard 2010, Tulsky 2011, Wilkinson 2008; Fujimori 2014) (33 records) and we identified six records relating to the three previously included studies. Therefore, from the updated search, we included 39 new records relating to 15 studies. We excluded 80 newly identified records (70 studies). |
| 24 February 2012 | New search has been performed | Search updated producing 358 records. |
| 9 September 2011 | New search has been performed | Search updated producing 411 records including 37 duplicates. Three potentially eligible studies identified. |
| 21 January 2011 | New search has been performed | Search updated producing 2,508 records including 43 duplicates.Thirty potentially eligible studies/reports identified. |
Acknowledgements
We thank the following people for their assistance.
Clare Jess, Jo Morrison and Gail Quinn of the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers (CGNOC) for their editorial support and Jo Platt of the CGNOC in Bath, UK, who conducted the updated searches.
Susie Wilkinson (SW) and Deborah Fellows (DF) for their contributions as co‐authors of the original published review. SW wrote the original protocol and DF conducted the original search and wrote the first draft of the original review.
Gabriel Rada and Macarena Morel from the team of Cochrane Chile (Centro Evidencia UC, Pontificia Universidad Catolica de Chile) for bibliographic, editorial and statistical advice, and constant support.
Chrysi Leliopoulou and Heather Dickinson for their assistance with the original version of this review.
This project was supported by the National Institute for Health Research, via Cochrane Incentive funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group (Award reference number 17/62/36). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Definition of a simulated patient
A simulated patient (SP) in health education is an individual who is trained to act as a real patient in order to simulate a set of symptoms or problems. An individual SP is typically selected on the basis of gender, body habitus (physical characteristics), previous surgeries, past medical history and sometimes level of education and/or language. She/he is matched to a case requirement and trained to reliably portray (and often to accurately recall) details of what was said and done in a medical encounter. SPs may also be trained to provide accurate, written and objective reports by means of checklists. In addition, SPs can be trained to provide patient‐centred, subjective rating and descriptive evaluation of examinee behaviour. This can provide a basis for constructive verbal or written post‐encounter feedback to the student or doctor by the SP (Adamo 2003).
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Medical Oncology explode all trees #2 MeSH descriptor Oncologic Nursing, this term only #3 MeSH descriptor Neoplasms explode all trees #4 MeSH descriptor Palliative Care, this term only #5 (terminal* or palliat* or cancer* or oncol* or hospice*) #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Education, Medical, Continuing, this term only #8 MeSH descriptor Education, Nursing, Continuing, this term only #9 MeSH descriptor Programmed Instruction as Topic explode all trees #10 (train* or educat* or workshop* or module* or teach* or curricul* or learn*) #11 (#7 OR #8 OR #9 OR #10) #12 MeSH descriptor Communication explode all trees #13 communicat* or interview* #14 (#12 OR #13) #15 evaluat* or assess* or critique* or measure* or outcome* or effect* or change* or result* or trial* or prospective* or followup or follow‐up #16 (#6 AND #11 AND #14 AND #15)
Appendix 3. MEDLINE (Ovid) search strategy
1 exp Medical Oncology/ 2 Oncologic Nursing/ 3 exp Neoplasms/ 4 Palliative Care/ 5 (terminal* or palliat* or cancer* or oncol* or hospice*).mp. 6 1 or 2 or 3 or 4 or 5 7 Education, Medical, Continuing/ 8 Education, Nursing, Continuing/ 9 exp Programmed Instruction as Topic/ 10 (train* or educat* or workshop* or module* or teach* or curricul* or learn*).mp. 11 7 or 8 or 9 or 10 12 exp Communication/ 13 (communicat* or interview*).mp. 14 12 or 13 15 (evaluat* or assess* or critique* or measure* or outcome* or effect* or change* or result* or trial* or prospective* or followup or follow‐up).mp. 16 6 and 11 and 14 and 15 17 randomised controlled trial.pt. 18 controlled clinical trial.pt. 19 randomized.ab. 20 placebo.ab. 21 clinical trials as topic.sh. 22 randomly.ab. 23 trial.ti. 24 17 or 18 or 19 or 20 or 21 or 22 or 23 25 16 and 24
key: mp=title, original title, abstract, name of substance word, subject heading word, unique identifier
Appendix 4. Embase (Ovid) search strategy
1 exp oncology/ 2 exp oncology nursing/ 3 exp neoplasm/ 4 exp palliative therapy/ 5 (terminal* or palliat* or cancer* or oncol* or hospice*).mp. 6 1 or 2 or 3 or 4 or 5 7 exp medical education/ 8 exp nursing education/ 9 exp continuing education/ 10 (train* or educat* or workshop* or module* or teach* or curricul* or learn*).mp. 11 7 or 8 or 9 or 10 12 exp interpersonal communication/ 13 (communicat* or interview*).mp. 14 12 or 13 15 (evaluat* or assess* or critique* or measure* or outcome* or effect* or change* or result* or trial* or prospective* or followup or follow‐up).mp. 16 6 and 11 and 14 and 15 17 crossover procedure/ 18 double blind procedure/ 19 randomised controlled trial/ 20 single blind procedure/ 21 random*.mp. 22 factorial*.mp. 23 (crossover* or cross over* or cross‐over*).mp. 24 placebo*.mp. 25 (doubl* adj blind*).mp. 26 (singl* adj blind*).mp. 27 assign*.mp. 28 allocat*.mp. 29 volunteer*.mp. 30 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31 16 and 30
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer
Appendix 5. Assessment of the Risk of bias for Included studies
We assessed the risk of bias as follows.
(1) Method of randomisation
Methodological quality of randomisation was classified as follows.
Low risk of bias(any truly random process, e.g. random number table;computer random number generator)
High risk of bias (any non‐random process, e.g. odd or even date of birth;hospital or clinic record number) or
Unclear risk of bias (no details provided)
(2) Allocation concealment
Low risk of bias e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes)
High risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth)
Unclear risk of bias
(3) Blinding
In studies of communication skills training, both the participants and the providers of the training would be aware of study arm allocation, therefore it was not possible to assess 'performance bias'. However, the outcome assessment could be blinded, therefore we assessed blinding of outcome assessment (Protection against 'detection bias') as:
Low, high or unclear risk of bias
(4) Incomplete outcome data
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes.
We assessed methods as:
low risk of bias (e.g. less than 20% missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review had been reported);
high risk of bias (where not all the study’s pre‐specified outcomes were reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so could not be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we had about other possible sources of bias. We assessed whether each study was free of other problems that could put it at risk of bias, as follows:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
Appendix 6. Search results for the original review
For the original 2001 review, 48 studies identified by the search were classified as follows.
Included (2)
Excluded (46)
The included studies were Fallowfield 2002 and Razavi 1993.
The 46 excluded studies that had warranted further consideration were excluded for methodological reasons. Most (27) were excluded as they measured changes in attitudes and/or knowledge, rather than skills (Anderson 1982; Baile 1997; Baile 1999; Berman 1983; Bird 1993; Cantwell 1997; Cowan 1997; Craytor 1978; Delvaux 1997; Dixon 2001; Durgahee 1997; Fallowfield 1998; Fallowfield 2001; Ferrell 1998a; Girgis 1997; Gordon 1995; Hainsworth 1996; Hall 1999; Hallenbeck 1999; Hulsman 1997; Linder 1999; Lloyd‐Williams 1996; Parle 1997; Razavi 1991; Smith 1991; Von Gunten 1998; Wong 2001). Many of these studies would also have been excluded for not having a separate control group. A further 14 studies were excluded as they had no separate control group, although they were longitudinal (Booth 1996; Charlton 1993; Faulkner 1984; Faulkner 1992; Glimelius 1995; Heaven 1995; Heaven 1996; Maguire 1996a; Maguire 1996b; Matrone 1990; Razavi 2000; Rutter 1996; Wilkinson 1998; Wilkinson 1999). Five were excluded due to the subjective nature of their evaluation of communication skills (largely based on perceived improvement by the participant) (Andrew 1998; de Rond 2000; Ferrell 1998b; La Monica 1987; Shorr 2000) Only one of this latter group included a separate control group of non‐trained staff (de Rond 2000).
In November 2003, three further studies were added to the review; one was included (Razavi 2002) and the remaining two were excluded (Finset 2003; Libert 2003). Therefore, in total, three studies were included and 48 were excluded.
Data and analyses
Comparison 1. CST vs no CST: HCP communication skills.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Used open questions | 5 | 796 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [0.02, 0.48] |
| 1.1 Simulated patients | 5 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.01, 0.76] |
| 1.2 Real patients | 4 | 374 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.12, 0.28] |
| 2 Showed empathy | 6 | 844 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.05, 0.32] |
| 2.1 Simulated patients | 5 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [0.07, 0.45] |
| 2.2 Real patients | 5 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.08, 0.30] |
| 3 Gave facts only | 5 | 780 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.51, ‐0.01] |
| 3.1 Simulated patients | 5 | 406 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.42 [‐0.77, ‐0.06] |
| 3.2 Real patients | 4 | 374 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.38, 0.22] |
| 4 Elicited concerns | 3 | 221 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.12, 0.60] |
| 4.1 Simulated patients | 3 | 163 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.33, 0.67] |
| 4.2 Real patients | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.12, 0.93] |
| 5 Gave appropriate information | 4 | 489 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.26, 0.10] |
| 5.1 Simulated patients | 3 | 203 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.42, 0.14] |
| 5.2 Real patients | 3 | 286 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.27, 0.19] |
| 6 Clarified and/or summarised | 3 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.30, 0.49] |
| 6.1 Simulated patients | 3 | 253 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.18, 0.81] |
| 6.2 Real patients | 2 | 169 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.50, 0.11] |
| 7 Negotiation | 3 | 503 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.08, 0.32] |
| 7.1 Simulated patients | 3 | 240 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.12, 0.39] |
| 7.2 Real patients | 3 | 263 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.25, 0.51] |
Comparison 2. CST vs no CST: HCP communication skills: doctors only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Used open questions | 2 | 306 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.05, 0.50] |
| 1.1 Simulated patients | 2 | 160 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.03, 0.66] |
| 1.2 Real patients | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.13, 0.52] |
| 2 Showed empathy | 3 | 354 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.01, 0.43] |
| 2.1 Simulated patients | 2 | 160 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.05, 0.60] |
| 2.2 Real patients | 3 | 194 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.12, 0.49] |
| 3 Clarified and/or summarised | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Real patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Elicited concerns | 2 | 150 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.25, 0.43] |
| 4.1 Simulated patients | 2 | 92 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.51, 0.31] |
| 4.2 Real patients | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.12, 0.93] |
| 5 Gave appropriate information | 2 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.34, 0.31] |
| 5.1 Simulated patients | 2 | 92 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.39, 0.43] |
| 5.2 Real patients | 1 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.59, 0.44] |
| 6 Gave facts only | 2 | 306 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.74, 0.37] |
| 6.1 Simulated patients | 2 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.36, 0.35] |
| 6.2 Real patients | 2 | 146 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.17, 0.49] |
Comparison 3. CST vs no CST: HCP communication skills: nurses only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Used open questions | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.23, 1.06] |
| 1.1 Simulated patients | 2 | 182 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.07, 1.37] |
| 1.2 Real patients | 1 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.42, 0.33] |
| 2 Clarified and/or summarised | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Real patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Elicited concerns | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Showed empathy | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.04, 0.42] |
| 4.1 Simulated patients | 2 | 182 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.06, 0.53] |
| 4.2 Real patients | 1 | 111 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.27, 0.48] |
| 5 Gave appropriate information | 2 | 342 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.31, 0.12] |
| 5.1 Simulated patients | 2 | 173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.46, 0.14] |
| 5.2 Real patients | 2 | 169 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.32, 0.28] |
| 6 Gave facts only | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.65, 0.17] |
| 6.1 Simulated patients | 2 | 182 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.98, 0.37] |
| 6.2 Real patients | 1 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.47, 0.28] |
Comparison 4. CST vs no CST: subgrouped by HCP type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Used open questions | 4 | 599 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.07, 0.61] |
| 1.1 Doctors | 2 | 306 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.05, 0.50] |
| 1.2 Nurses | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.23, 1.06] |
| 2 Clarified and/or summarised | 2 | 342 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.47, 0.48] |
| 2.1 Doctors | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.66, 0.06] |
| 2.2 Nurses | 1 | 222 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.45, 1.02] |
| 3 Elicited concerns | 2 | 191 | Std. Mean Difference (IV, Random, 95% CI) | 0.31 [‐0.10, 0.72] |
| 3.1 Doctors | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.33, 0.63] |
| 3.2 Nurses | 1 | 71 | Std. Mean Difference (IV, Random, 95% CI) | 0.61 [0.14, 1.09] |
| 4 Showed empathy | 5 | 647 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [0.05, 0.36] |
| 4.1 Doctors | 3 | 354 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [0.01, 0.43] |
| 4.2 Nurses | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.04, 0.42] |
| 5 Gave appropriate information | 2 | 342 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.31, 0.12] |
| 5.1 Doctors | 1 | 120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.38, 0.34] |
| 5.2 Nurses | 1 | 222 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.40, 0.14] |
| 6 Gave facts only | 4 | 599 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.54, 0.12] |
| 6.1 Doctors | 2 | 306 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.74, 0.37] |
| 6.2 Nurses | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.65, 0.17] |
Comparison 5. CST vs no CST: Other HCP outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Emotional exhaustion: Maslach Burnout Inventory: | 3 | 202 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.44, 0.12] |
| 2 Personal accomplishment: Maslach Burnout Inventory | 3 | 202 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.04, 0.52] |
| 3 Depersonalisation | 1 | 96 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐1.50, 2.50] |
Comparison 6. CST vs no CST: Patient outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient psychiatric morbidity (GHQ 12) | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Patient anxiety | 3 | 770 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.24, 0.59] |
| 3 Patient perception of HCPs communication skills | 2 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.44, 0.16] |
| 4 Patient satisfaction with communication | 2 | 429 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.23, 0.63] |
Comparison 7. Follow‐up CST vs no follow‐up CST: HCP communication skills.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Used open questions | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Real patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clarified and/or summarised | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Real patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Elicited concerns | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Real patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Showed empathy | 2 | 168 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.07, 0.54] |
| 4.1 Simulated patients | 1 | 62 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.43, 0.57] |
| 4.2 Real patients | 2 | 106 | Std. Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.06, 0.72] |
| 5 Gave appropriate information | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Real patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Gave facts only | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Real patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Negotiation | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Simulated patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Real patients | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Butow 2008.
| Methods | RCT | |
| Participants | 30 medical and radiation oncologists from 6 Australian teaching hospitals. Age = 36.5 to 51 years; years of experience = 7.5 to 24.3 years. 343 cancer patients (60% women) answered questionnaires post‐consultation |
|
| Interventions | 1.5 day workshop with 3 to 6 participants, followed by four 1.5 hour video conferences incorporating role‐play of doctor‐generated scenarios. Workshop included DVD modelling ideal behaviour; role‐play and feedback with an SP using standardised cases and from own experience, booklet summarising evidence, video of own role‐play. Emphasis on how to establish a collaborative framework, and how to respond to anxiety, depression, distress and anger | |
| Outcomes | HCP (oncologist) outcomes on video of SP interview at baseline, immediately post‐intervention and 6 months post‐intervention (or equivalent timings for control group)
HCP (oncologist). 'burnout' measured using MBI* Patient outcomes:
|
|
| Notes | There was a trend for training to be successful in increasing some HCP communication skills, however, no changes were statistically significant. Anxiety was reduced in patients interviewed by oncologists from the intervention group one week later (P = 0.021) No other statistically significant differences were found in patient outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised at individual level using random number tables and Excel software |
| Allocation concealment (selection bias) | Low risk | Allocated centrally by research team |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description of total number of HCP measured post‐intervention. Low attrition in HCP and patient questionnaires |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Other bias | Low risk | Baseline characteristics of the two groups were similar |
Epstein 2017.
| Methods | Cluster‐RCT | |
| Participants | 38 medical oncologists from 7 community hospitals and clinics and 3 academic centres in USA; mean age 44 years, 71% male 130 patients with advanced cancer received coaching 383 community‐dwelling adult patients with advanced cancer participated in audio‐recorded consultations (3 to 4 per oncologist prior to intervention and up to 10 per oncologist post‐intervention) and 265 of these patients answered questionnaires. |
|
| Interventions | 2 sessions (total 1.75 hours) of in‐office physician training. Training included video and feedback from SP consultations 1‐hour patient and caregiver coaching sessions plus 3 follow‐up phone calls. Coaching included a question prompt list to help bring their concerns to the attention of the oncologist at their office visits. Both interventions focused on 4 key domains of patient‐centred communication: engaging, responding, informing, balanced framing |
|
| Outcomes | HCP (oncologists) outcome on audio‐recording of real patient interviews at baseline and post ‐intervention
Patient Outcomes:
|
|
| Notes | The composite communication score has an estimated adjusted intervention effect of 0.34 (CI, 0.06 to 0.62, P = 0.02) corresponding to 5.7 additional "engaging" statements (44% increase); 0.6 additional response to emotion (71% increase) 1.4 additional statements regarding prognosis and treatment choices (38% increase). Only 1 of the individual communication components (engaging) was statistically significant. There was no statistically significant difference in the other outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A stratified block‐randomisation scheme is used to assure balanced assignment by clinic site and cancer focus. Oncologists are grouped into a site according to their health centre, clinic, or practice of employment |
| Allocation concealment (selection bias) | Low risk | Only study statisticians were aware of random number sequence and treatment assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Transciptionists, coders and abstractors all blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss (7%/5%) in patients, no loss in doctors |
| Selective reporting (reporting bias) | Unclear risk | Protocol clear, details of all patient questionnaires not reported |
| Other bias | Low risk | None |
Fallowfield 2002.
| Methods | RCT Written feedback followed by course, or course alone, or written feedback alone, or control |
|
| Participants | 160 medical, surgical and radiation oncologists from 34 cancer centres in the UK; 69% men. 640 cancer patients (60% women) participated in the videotaped consultations (2 video‐tapes per oncologist at baseline and 3 months post‐intervention). 1816 cancer patients answered questionnaires. |
|
| Interventions | Cancer Research UK Communication Skills Program. Intensive 3‐day residential course and/or feedback pack | |
| Outcomes | HCP (oncologist) outcomes on video of RP interviews at baseline, and 3 months post‐intervention (or equivalent timings for control group:
Patient outcomes:
|
|
| Notes | CST group had a statistically significant improvement in oncologists' attitudes to psychosocial issues (P = 0.002) and a non‐significant positive effect on patient satisfaction. Follow‐up for 12 months revealed no demonstrable attrition in most of the skills improvement, some new skills, but a decline in expressions of empathy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not fully described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Video raters blinded as far as possible for time point of assessment and group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low risk on HCP behaviour outcomes; 21% attrition for patient outcomes; only intervention group followed up at 12 months |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were described |
| Other bias | Low risk | Baseline characteristics of two groups were similar |
Fujimori 2014.
| Methods | RCT | |
| Participants | 30 oncologists from 2 Japanese hospitals: average age 35.5 (range 30 to 50) 13% female 1181 cancer outpatients answered questionnaires |
|
| Interventions | A 2 day CST workshop (intervention) or 'no CST' (control). Workshop based on SHARE model of Breaking bad news using didactic material and video, 8 hours of role‐play with pre‐designed scenarios and SP in small groups (4 participants, 2 facilitators, 1 SP) | |
| Outcomes | HCP outcomes:
Patient outcomes:
Assessed at baseline, 1 week post‐CST or 2 weeks post‐baseline in controls |
|
| Notes | The intervention group showed more appropriate behaviour/skills over time in 3 of 4 composite scores: setting a supportive environment P = 0.002; considering how to break news P = 0.001; ability to deliver information; providing reassurance P = 0.011 In 3 of 27 categories. The intervention group showed more appropriate skills: greeting the patient cordially; not beginning bad news delivery without preamble; accepting patients expressions of emotions all P = <0.01. HADS depression score was significantly lower (P = 0.027) and Trust score significantly higher (P = 0.009 in patients seen by the oncologists in the intervention group. No difference was found in HADS anxiety score, HADS total score or patient satisfaction |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | Low risk | Only 20% of the invited oncologists responded (although 46% of the female oncologists invited responded) Patients who completed the questionnaires were not selected on a bad news scenario and included patients newly diagnosed and those in follow‐up Baseline characteristics of two groups were similar |
Goelz 2011.
| Methods | RCT conducted from June 2007 ‐ Feb 2009 | |
| Participants | 41 doctors (39 from Department of Haematology/Oncology, one from Gynaecology, one from Surgery) | |
| Interventions | CST in the form of COM‐ON‐p(communication challenges in oncology related to the transition to palliative care training program), including a one hour pre‐assessment with SPs, an 11‐hour training course (main focus practice with SPs using cases of participants) plus a half‐hour individual coaching session two weeks later. The courses were run in groups of 8/9 participants by two experienced facilitators. | |
| Outcomes | HCP (doctors communication skills in video‐recorded SP consultations pre‐intervention and five weeks post‐intervention using COM‐ON‐checklist included:
|
|
| Notes | The average overall estimate of effect favoured the intervention group (P = 0.0007). There was a statistically significant difference between intervention and control group in all sections in favour of the intervention group including: specific palliative communication skills (P value < 0.0026); general communication skills (P value < 0.0078); and involvement of significant others (P value <0.0.0051) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation to two groups in blocks of 8 by an 'external statistician' |
| Allocation concealment (selection bias) | Unclear risk | Allocation by Fax |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported |
| Other bias | High risk | Doctors in the intervention group had significantly more professional (P = 0.02) experience compared with those in the control group |
Gorniewicz 2017.
| Methods | RCT | |
| Participants | 38 family and internal medicine first‐year residents: average age 29.6; 53% male; from 1 university in USA | |
| Interventions | 60‐minute On‐line training in breaking bad news using didactic material, quizzes and videos of semi‐structured interviews with patients who described challenging communication situations, and of interviews between simulated doctors and patients. Five‐step patient‐centred model | |
| Outcomes | HCP (residents) communication skills in video‐recorded SP consultations of colon cancer patient pre‐intervention and 31 days post‐intervention using Breaking bad news (BBN) skills checklist and Common Ground Assessment Summary form(CGA):
|
|
| Notes | Two of five items on BBN scale (BBN P = 0.004, communication related to emotions P = 0.034), and 4 of five items on CGA were significantly higher (P < 0.01) in intervention group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters were blinded to the participant’s level of education, the OSCE interview sequence (baseline or follow‐up), and group status (intervention or control) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Residents evaluated before and after intervention with same simulated patient scenario Study also included students but data were analysed separately |
Heaven 2006.
| Methods | RCT | |
| Participants | 61 UK nurses, all of whom received basic 3‐day CST training prior to randomisation: 68% working in palliative care; mean age 42 years; all but one female; 41% worked in community only, 21% hospital only, 38% hospital and community 449 RP encounters (75% women, mean age 61 years) 122 SP encounters |
|
| Interventions | Four 3‐hour supervision sessions plus feedback on video of interview with RPs. Both intervention and control groups had basic training prior to baseline |
|
| Outcomes | HCP (nurses) communication skills assessed in audio‐recording of SP and RP interviews at 1 and 3 months post‐intervention, rated using MIARS:
|
|
| Notes | Some communication behaviours were enhanced in the intervention group after supervision, including psychological exploration (P = 0.039) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data collectors and judges were blinded to time and group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 84% follow‐up at 3 months |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Baseline characteristics of two groups were similar except the control group had more communication skills training (P = 0.037) |
Kruijver 2001.
| Methods | RCT. | |
| Participants | 53 nurses from 11 wards in 3 Dutch hospitals: mean age was 32 years, 83% women; mean of 5 years' experience in oncology 265 recently diagnosed cancer patients admitted for treatment had their admission interview with the nurse video‐recorded and 258 of these patients answered questionnaires (55% women, mean age 55 years) |
|
| Interventions | Six 3‐hour sessions with 10‐15 participants run by two trainers with experience in clinical patient care. CST included theory, demonstration of skills, and feedback on role‐playing | |
| Outcomes | HCP (nurses) communication skills assessed on video recordings of SP interviews (one month post‐intervention) and 5 RP admission interviews between 1 to 7 months post‐intervention using RIAS*:
Nurses' 'burnout' was measured using MBI Patients outcomes:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 53 participants " randomised at ward level" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Independent rater but blinded status not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 83% to 86% follow‐up; no published data of the RP video‐recordings |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Baseline characteristics of two groups were similar |
Lienard 2010.
| Methods | RCT | |
| Participants | 113 Belgian residents who had been, or were, working with cancer patients 1124 hospitalised patients were audio‐recorded during the clinical round and 731 of these patients answered questionnaires 88 patient encounters analysed (56% women; mean age 55 years) |
|
| Interventions | 40‐hour training programme (17 hours on two‐person interview skills, 10 hours on three‐person interviews, 10 hours on stress management, 3 hours on integration of skills), bimonthly over an 8‐month period. Small groups (maximum 7 participants). Comprised a one‐hour theoretical session, role‐plays of pre‐defined cases, and cases from participants with immediate feedback. | |
| Outcomes | HCP (residents) communication skills were analysed in audio‐tapes of 1 SP encounter pre‐ and post‐intervention or at 8 months (control group) and 1 RP interview during a clinical round post‐intervention or at 8 months (control group) using LaComm*:
Also time spent on the 3 phases of breaking bad news and precision of the delivery of diagnosis. Residents' burnout' was measured pre‐ and post‐intervention using MBI. Residents' physiological arousal was measured during the SP interviews Patient outcomes:
|
|
| Notes | Statistically significant improvement was found in 2 of 12 items of HCP skills with RPs. No effect on empathy or supportive skills in RPs. Significant increase in open questions, empathy, and concise precise diagnoses in SPs, but significant decrease in other information with SP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessor blinded to time assessment and group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Trained residents took part in an average of 25 hours (62%) of a training program (range 8 to 40 hours). 77% follow‐up in RPs; 86% follow‐up in SPs |
| Selective reporting (reporting bias) | Unclear risk | Subgroup analysis of % training attendance |
| Other bias | High risk | Selection of interview for HCP communication analysis unclear. Number of people with cancer : < 40% of patient interviews analysed and numbers are unclear in 'patient satisfaction' outcome |
Merckaert 2015.
| Methods | RCT | |
| Participants | 96 participants of 4 radiotherapy teams comprising secretaries (16%), physicists (7.5%), nurses (49%), doctors (27.5 %) 237 breast cancer patients participated in radiotherapy sessions and answered questionnaires 96 of these patients had their first and last radiotherapy planning sessions audio‐recorded and were included in the analysis. |
|
| Interventions | 38 hours of CST over a 4‐month period; 2 modules: 16h patient orientated communication skills module (12 hours in mono‐disciplinary groups) and 22 hours team‐resource‐orientated communication skills module | |
| Outcomes | HCP communication skills rated using the scale LaComm* on 1 audio‐recording of the first and last radiotherapy planning sessions with a patient with recently operated breast cancer and on audio‐recordings of simulated anxious breast cancer patient (SP) interview at baseline and post‐intervention (or equivalent timings for control group):
Patient outcomes:
|
|
| Notes | With RP the Intervention group has significantly more appropriate behaviour for some communication skill outcomes (First planning session: open and open directive questions P = 0.003; setting information P = 0.010. Last session: checking P = 0.029). With SP the intervention group had significantly more appropriate behaviours/skills: team‐orientated focus (P = 0.023), empathy (P = 0.037) and emotional words (P = 0.030). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Difference in number of participants between different publications All teams completed all assessments. 83% participants completed SP assessment 10% of patients did not complete the patient satisfaction scale |
| Selective reporting (reporting bias) | Unclear risk | No protocol available; HADS results have not yet been published |
| Other bias | High risk | Some differences in baseline characteristics of two groups including work experience in oncology, full‐time occupation, and % of non‐professionals (higher proportion of non HCP (secretaries and physicists) in intervention group (31% versus 10%)). Team size very variable 2 teams (65 participants) in intervention, 2 teams (31 participants) in control. Not all team members agreed to participate in training programme, but all team members were evaluated |
Razavi 1993.
| Methods | RCT | |
| Participants | 72 oncology nurses from 4 hospitals in France and Belgium participated | |
| Interventions | 24‐hour training program taught in 8 weekly, 3‐hour sessions | |
| Outcomes | HCP (nurses) communication skills in first 5 minutes of video‐taped SP interviews, pre‐ and 2 months post‐training, rated using CRCWEM* (Cancer Research Campaign Workshop Evaluation Manual):
Nurses' attitudes (SDAQ*), occupational stress (NSS*) and self‐perception |
|
| Notes | Trained group were assessed as 'more in control of the interview' than the untrained group during the follow‐up interview (P = 0.02). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Twelve participants per institution were "randomly assigned" to two groups |
| Allocation concealment (selection bias) | Low risk | Allocation by sealed envelopes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Video raters blinded for group; questionnaire assessors not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition rates: one dropout, three incomplete data sets out of 72 participants |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Baseline characteristics of two groups were similar |
Razavi 2002.
| Methods | RCT | |
| Participants | 116 oncology nurses from 33 hospitals in Belgium 114 cancer patients during first week of hospitalisation |
|
| Interventions | 105‐hour communications skills workshop with 10 participants, run by psychologist, taught over 3 months for one week per month | |
| Outcomes | HCP (nurses) communication skills in video‐taped SP interviews and audio‐taped RP interviews pre‐ and post‐intervention (or equivalent timings for control group), and three months later rated using CRCWEM* (Cancer Research Campaign Workshop Evaluation Manual), plus dictionaries (HPSD* and MRID*) and LACOMM* and PainComCode:
Nurses' Stress (NSS*) and Attitudes (SDAQ*) Patient outcomes:
|
|
| Notes | Patients interviewed by trained nurses used more emotional words associated with 'distress' than did those seen by untrained nurses (P = 0.005).There was a positive training effect on patient satisfaction (P value < 0.01) In SP interviews, nurses asked more questions about the emotional component of pain and used less inhibitory communication strategies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisation was performed every time there were 20 nurses enrolled |
| Allocation concealment (selection bias) | High risk | Randomisation was performed every time there were 20 nurses enrolled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blinded by time and group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 86% follow‐up for HCP behavioural outcome in RP and SP interviews |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Baseline characteristics were similar in both groups |
Razavi 2003.
| Methods | RCT of follow‐up consolidation sessions after both groups had basic training | |
| Participants | 63 physicians (62% oncologists) from Belgium hospitals, age 43+/‐7, 55% men, with average 14 years of experience in oncology and 43% no prior CST. All had participated in a 19‐hour CST workshop (consisting of two, 8‐hour/day sessions and one, 3‐hour evening session). 59 cancer patients, undergoing a 'breaking news' interview (67% women mean age 58 years). 53 cancer patients (65% women mean age 60 years) in encounters with relatives (48% women mean age 57 years) |
|
| Interventions | Six, 3‐hour per evening, bimonthly, consolidation sessions over three months | |
| Outcomes | HCP (doctors) outcomes:
Patient outcomes:
Significant other outcomes:
|
|
| Notes | There was no effect of consolidation workshops on doctors' ability to detect patient distress | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 72 participants "randomly assigned" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blinded for time (pre/post) and group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 81% follow‐up for SP and RP; and 77% follow‐up for RP interview with significant other |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Baseline characteristics of the two HCP groups were similar. Baseline scores of patient anxiety were markedly higher in patients seen by the control group. |
Stewart 2007.
| Methods | RCT | |
| Participants | 51 doctors (18 oncologists, 17 family doctors and 16 surgeons) from 3 towns in Canada. 102 cancer patients who attended outpatient clinics of oncologists and surgeons. |
|
| Interventions | 6‐hour intensive CST course including literature, physicians and patients perspectives, video modelling poor and better behaviour, role‐play, video and feedback with SP using standardised cases. Emphasis on exploring patients perspectives. Control group received the standard 2‐hour small group discussion triggered by video of interview between physician and breast cancer standardised patient. |
|
| Outcomes | HCP (doctors) communication skills in video‐taped SP interviews at baseline and after intervention. Rated using PCCM*:
Patient outcomes (measured only for surgeons and oncologists in both groups):
|
|
| Notes | Training had a positive impact on patients' satisfaction (P = 0.03) and "feeling better" (P = 0.02). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Randomized done by project co‐ordinator |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters and patients were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% for HCP behaviour outcome; 44.3% patient response rate to patient questionnaire |
| Selective reporting (reporting bias) | Low risk | Subgroup analysis (family physicians) on selected outcomes |
| Other bias | Low risk | Baseline characteristics were similar in both groups of HCP |
Tulsky 2011.
| Methods | Single‐blind RCT; stratified by site, gender and oncologist speciality. | |
| Participants | 48 oncologists (medical, gynaecological and radiation), all of whom received a one‐hour lecture on communication skills. 264 patients with advanced cancer (65% women mean age 60 years). |
|
| Interventions | Computerised intervention (interactive CD‐ROM) organised in five 15‐minute modules and included principles of effective communication, recognising and responding to empathic opportunities, conveying prognosis and answering difficult questions. Included tailored feedback from oncologists' own recorded conversations. | |
| Outcomes | HCP (oncologists) outcomes:
Patient outcomes (measured one week after the encounter by telephone survey):
|
|
| Notes | CST aimed to influence a limited number of skills. Median time of training program = 64 minutes (58 to 99). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced randomisation in a 1:1 ratio by site, sex and speciality. Statistician performed minimisation method of randomisation to ensure balanced groups |
| Allocation concealment (selection bias) | Low risk | Statistician revealed the randomisation results only to project co‐ordinator and principal investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Single‐blind. Patients were blinded to their oncologists' group allocation, as were the two audio‐coders |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/24 used CD‐ROM in intervention, but all included in evaluation. 4/264 encounters could not be assessed due to technical problems. Overall missing data < 20% |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Baseline characteristics comparable except for fewer Caucasian doctors in the intervention group (76% vs 92%). Unclear if scales/questionnaires used were validated |
van Weert 2011.
| Methods | Cluster‐RCT conducted in inpatients | |
| Participants | 58 hospital nurses providing patient education about chemotherapy. 210 older cancer patients receiving chemotherapy (35% women, mean age 72 years). |
|
| Interventions | Individualised web‐based video feedback; a 1‐day CST conducted in groups of 6‐11 nurses focusing on patient education about chemotherapy; observation and feedback of colleagues interviews; and a half‐day follow‐up session and booklet. | |
| Outcomes | HCP (nurses) outcomes:
Patient outcomes:
|
|
| Notes | Improvements in "discussing patient expectations", checking, and some affective responses. Patients asked more questions in intervention group. Limited effect on patient recall (marginally significant improvement on overall recall) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent observers of videos were blinded to group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description of the number of nurses who participated in the videos analysed post‐treatment |
| Selective reporting (reporting bias) | Low risk | All 7 dimensions of communication reported |
| Other bias | Low risk | Baseline characteristics comparable |
Wilkinson 2008.
| Methods | Multi‐centre RCT | |
| Participants | 172 nurses (94% women) from hospices (60%) and community (30%) in UK. 312 cancer patients (85% women, mean age 32 years) |
|
| Interventions | A 3‐day course for max. 12 participants run by 2 co‐facilitators. Course included literature, nurses perspectives, video‐modelling ideal behaviour, audio‐recording with RPs and role‐play with SPs using standardised and participant cases, both with feedback. Emphasis on exploring nurses individual difficulties. | |
| Outcomes | HCP (nurses) outcomes (coded from audio‐tapes of RP admittance interviews and 12 weeks' post‐intervention; rated using CSRS*):
Patient outcomes:
|
|
| Notes | Tendency to improve patient satisfaction and general health. No statistical difference in mean change of patients' anxiety. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer‐generated numbers |
| Allocation concealment (selection bias) | Low risk | Statistician performed randomisation before the study commenced and kept the results in sealed envelopes in the central research department |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Raters blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 90% follow‐up but missing data stated |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes described |
| Other bias | High risk | Higher professional grades in control group |
Abbreviations:
CI: confidence interval;CST: communication skills training; EORTC: European Organization for Research and Treatment of Cancer; HADS: Hospital Anxiety and Depression Scale; HCP: healthcare professional; MBI: Maslach Burnout Inventory; QOL: quality of life; RIAS: Roter interaction analysis system ; RCT: randomised controlled trial; RP: real patient;SP: simulated patient;
* See Table 5; Table 2; and Table 4 for key to scale abbreviations.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ades 2001 | Not an RCT. No CST. |
| Alexander 2006 | Not an RCT. Case‐control study of a course to improve residents' communication skills with patients at end of life. |
| Anderson 1982 | Not an RCT. No controls, post‐CST course measurement only. |
| Andrew 1998 | Not an RCT. Qualitative study of CST in palliative care. |
| Archer 2004 | Not an RCT, no objective behavioural outcome measurement. |
| Arranz 2005 | Not an RCT. Post‐intervention assessment counselling course for nurses. No objective measurements of skills. |
| Arrighi 2010 | Not an RCT. Exploratory study conducted in patients not HCPs. |
| Aubin‐Auger 2014 | Not patients with cancer |
| Back 2005 | Not an RCT. Questionaire survey of bereaved relatives. |
| Back 2007 | Not an RCT. Pre‐post cohort study of a 4‐day residential workshop in oncology fellows with objective measurements of HCP behaviour. |
| Baile 1997 | Not an RCT. Pre‐post cohort study of a 3‐day CST. Only subjective measurement of behavioural change reported. |
| Baile 1999 | Not an RCT. Pre‐post cohort study of a 2.5‐day CST. Only subjective measurement of behavioural change reported. |
| Beach 2014 | Not an RCT |
| Berman 1983 | Not an RCT. Post‐study subjective measurement of behavioural change of an annual seminar for interns on caring for dying patients. |
| Bernacki 2013 | No objective HCP behavioural change measured |
| Bernacki 2015 | No objective HCP behavioural change measured |
| Bernard 2010 | Not an RCT. Case‐control study of a course for medical oncologists and nurses with pre‐post measurements of HCP skills and defence mechanisms. |
| Bernhard 2012 | Not an RCT |
| Bibila 2014 | Not an RCT |
| Bird 1993 | Not an RCT. Post‐study subjective measurement of behavioural change of 2.5‐day residential workshop. |
| Blödt 2016 | No objective HCP behavioural change measured |
| Booth 1996 | Not an RCT. Pre‐post cohort study of a 6‐session CST course for hospice nurses measuring HCP skills in audio‐taped interviews. |
| Brown 1999 | An RCT of CST in ambulatory care, not in cancer care. No objective measure of HCP skills. |
| Brown 2007 | Not an RCT. A course of communication skills training for oncologists involved in conducting clinical trials in oncology. Training was aimed at improving patient understanding and acceptance of clinical trials. |
| Brown 2011 | Not an RCT. Study of oncologists communication during interviews when recruiting patients for Phase 1 trials. |
| Brown 2012 | HCP communication skills were not assessed. |
| Burgess 2008 | Not an RCT. |
| Butow 2015 | CST was aimed at facilitating recruitment of patients to trials |
| Bylund 2010 | Not an RCT. Pre‐post study of CST course for oncologists. |
| Bylund 2011a | Not an RCT. Description of implementation of CST curriculum and impressions of participants. |
| Bylund 2011b | Not an RCT. Before‐after assessment of a non‐controlled study of CST for patients. |
| Cantwell 1997 | Not an RCT. A qualitative study of junior doctors opinion of undergraduate communication skills in relation to patients with cancer. |
| Caps 2010 | Not an RCT. |
| Chandawarkar 2011 | Not an RCT. Pre‐post assessment using simulated patients of CST for surgical residents. |
| Charlton 1993 | Not an RCT. |
| Clark 2009 | An RCT of patients with cancer receiving patient‐centred care or usual care. |
| Claxton 2011 | An RCT of email education for residents about palliative care. Not specifically communication skills. No objective measurement of skills reported. |
| Clayton 2012 | Not an RCT |
| Connolly 2010 | Not an RCT. Post course measurement of "Sage and Thyme" communication course. No objective measurement of skills reported. |
| Cooley 2014 | Not CST |
| Cort 2009 | RCT studying the effect of a course on cognitive behaviour therapy. No objective measurement of behavioural change reported. |
| Cowan 1997 | Not an RCT. Measured changes in attitudes/knowledge, not behaviour. No separate control group. |
| Craytor 1978 | Not an RCT. Measured changes in attitudes/knowledge, not behaviour. No separate control group. |
| Crit 2006 | Not an RCT. |
| Curtis 2013 | CST in HCPs < 60% of whom did not work specifically in cancer care |
| Das Gracas 2016 | Not CST |
| de Bie 2011 | Not CST. Trial involved training patients to reduce anxiety prior to colonoscopy. |
| de Rond 2000 | Quasi‐RCT on training nurses about pain management. Only subjective measurement of behavioural change. |
| Del Vento 2009 | Not an RCT. Qualitative study of how experienced doctors give good and bad news. |
| Delvaux 1997 | Not an RCT. Psychological training programme. |
| Ditton‐Phare 2016 | Not an RCT |
| Dixon 2001 | Not an RCT. Pre‐post study of 12 week distance education for nurses working in breast cancer care. Only subjective measurement of behavioural change reported. |
| Downar 2017 | CST in HCPs who did not work specifically in cancer care |
| Durgahee 1997 | Not an RCT. 5 years experience of reflection through story‐telling for students of palliative care. |
| Epner 2014 | Not an RCT |
| Fallowfield 1998 | Not an RCT. Cohort of 178 senior oncologists who assisted 1.5‐ or 3‐day CST. Only subjective measurement of behavioural change reported. |
| Fallowfield 2001 | Not an RCT. Cohort of 129 nurses. Only subjective measurement of behavioural change reported. |
| Fallowfield 2012 | Not an RCT. |
| Faulkner 1984 | Not an RCT. Cohort of 8 nurses working in cancer who assisted CST. |
| Faulkner 1992 | Not an RCT. Evaluation of training programmes for communication skills in palliative care. |
| Favre 2007 | Not an RCT. Pre‐post defence mechanism assessment of CST for oncologists. |
| Ferrell 1998a | Not an RCT. Pre‐post assessment of HOPE course for HCP in palliative care. Only subjective measurement of behavioural change reported. |
| Ferrell 1998b | Not an RCT. Pre‐post assessment of HOPE course for HCP in palliative care. Only subjective measurement of behavioural change reported. |
| File 2014 | Not an RCT |
| Fineberg 2005 | Not an RCT. Quasi‐experimental design with pre‐post assessment of a course on family communication in palliative care for interdisciplinary students. Only subjective measurement of behavioural change reported. |
| Finset 2003 | Not an RCT. Pre‐post assessment of CST for HCP. Only subjective measurement of behavioural change reported. |
| Fujimori 2003 | Not an RCT.Post course assessment of CST for oncologists. Only subjective measurement of behavioural change reported. |
| Fukui 2008 | RCT of CST for nurses in cancer care. No objective measurement of behavioural change reported. Patient outcomes were only measured in the intervention group, not in the control group. |
| Gerhart 2016 | Not an RCT |
| Gibon 2017 | Not an RCT |
| Girgis 1997 | Not an RCT. Measured change in attitude/knowledge not skills, not behaviour. |
| Glimelius 1995 | Not an RCT. |
| Golding 2016 | Not an RCT |
| Gordon 1995 | Not an RCT. Post course assessment of 2.5‐day or 5‐day course of CST. Only subjective measurement of behavioural change reported. |
| Gorniewicz 2017a | CST in HCPs < 60% of whom worked specifically in cancer care |
| Gouveia 2017 | No objective HCP behavioural change measured |
| Griffiths 2015 | Not an RCT |
| Gulbrandsen 2013 | CST in HCPs who did not work specifically in cancer care |
| Gutheil 2005 | Not an RCT. Patients not HCPs trained in communication skills. |
| Hainsworth 1996 | RCT of a course for nurses on death education. Not specifically for nurses working in cancer care. Only subjective measurement of behavioural change reported. |
| Hall 1999 | Not an RCT |
| Hallenbeck 1999 | Not an RCT. A questionnaire of interns before and after their rotation in palliative care. |
| Hallford 2011 | Not CST |
| Hamilton 2014 | No objective HCP behavioural change measured |
| Heaven 1995 | Not an RCT. A 10‐week CST for hospice nurses with assessment of ability to elicit patient concerns. |
| Heaven 1996 | Not an RCT. A 10‐week CST for hospice nurses with assessment of abiltity to elicit patient concerns. |
| Hellbom 2001 | Not an RCT. Post course assessment of a 4‐session CST course. Only subjective measurement of behavioural change reported. |
| Hendricks‐Ferguson 2015 | Not an RCT |
| Henoch 2013 | No objective HCP behavioural change measured |
| Hietanen 2007 | Not an RCT. Case‐control study of a course on communication skills training for physicians involved in conducting clinical trials in oncology. Training was aimed at improving patient understanding and acceptance of clinical trials. |
| Hill 2013 | Not an RCT |
| Hillen 2014 | Not CST |
| Hoffman 2002 | Not an RCT. Description of CST course for oncology residents and their views about the course. |
| Hulsman 1997 | Not an RCT. Pre‐post assessment of a computer‐assisted CST for doctors in cancer care. Only subjective measurement of behavioural change reported. |
| Hulsman 2002 | Not an RCT. Pre‐post assessment of a computer assisted CST for doctors in cancer care using videotapes of real patient encounter. |
| Hundley 2008 | An RCT of a course of delivering bad news. Only subjective measurement of behavioural change reported. |
| Jefford 2011 | Not an RCT. Patients received care package. |
| Johnson 2012 | Not an RCT |
| Johnson 2013 | Not an RCT |
| Jors 2016 | Not an RCT |
| Ju 2014 | Not an RCT |
| Ke 2008 | An RCT of 50‐minute CST lecture for nurses. Only subjective measurement of behavioural change reported. |
| Keir 2013 | Not an RCT |
| Kelley 2012 | Not an RCT |
| Kinnane 2011 | Not an RCT. Study conducted in volunteers not HCPs. |
| Kortes‐Miller 2016 | Not an RCT |
| Kruse 2003 | Not an RCT. Pre‐post assessment of a comparison between 6‐hour and 24‐hour CST programs. |
| Kubota 2012 | Not an RCT |
| Kubota 2015 | No objective HCP behavioural change measured |
| La Monica 1987 | Not an RCT. Study of 4‐week session on responding to empathy. |
| Ladouceur 2003 | Not an RCT. Post course assessment of course of breaking bad news. Only subjective measurement of behavioural change reported. |
| Larbig 2009 | Not an RCT. On‐line counselling for patients. |
| Lenzi 2011 | Not an RCT. Pre‐ and post‐assessment of a 3‐day CST workshop in a cohort of 57 Italian oncologists. Only subjective measurement of behavioural change reported. |
| Libert 2003 | Not an RCT. A cohort of physicians were assessed with regard to their communication skills. |
| Libert 2016 | Not an RCT |
| Linder 1999 | Measured change in attitude/knowledge not skills, not behaviour. |
| Liu 2007 | Not an RCT. Quasi‐experimental study of CST in nurses. |
| Lloyd‐Williams 1996 | Not an RCT. Measured change in attitude/knowledge not skills, not behaviour. |
| Loiselle 2011 | Not an RCT. |
| Macauley 2011 | Not an RCT. |
| Madhavan 2011 | Not an RCT. |
| Maguire 1996a | Not an RCT. Pre‐post assessment of a 3‐5‐day course on key communication skills for HCP in cancer care. Measurement with simulated and real patient encounters. |
| Maguire 1996b | Not an RCT. Similar to Maguire 1996a. |
| Martinez 2009 | Not an RCT, a survey of patient satisfaction with communication/information. |
| Matrone 1990 | Not an RCT. |
| Medendorp 2017 | Not an RCT |
| Melo 2011 | Not an RCT. A case‐control study of a course on communication, spiritual advice and death for HCP. Only subjective measurements of behaviour change and measurement of burnout. |
| Meystre 2013 | Not an RCT |
| Morita 2014 | No objective HCP behavioural change measured |
| Nellis 2016 | Not an RCT |
| O'Connor 2011 | Not an RCT. A survey of focus groups including pharmacists, nurses and doctors. |
| Parle 1997 | Not an RCT. Post‐course assessment of a 3‐day workshop on difficult situations. Only subjective assessment of behavioural change reported. |
| Patel 2016 | Not an RCT |
| Pekmezaris 2011 | Not an RCT. Pre‐post assessment of a course for residents about end of life care. Only subjective measurement of behavioural change are reported. |
| Pelayo 2011 | RCT of on‐line course on palliative care. Only subjective measurement of behavioural change reported. |
| Pieterse 2006 | Not an RCT. Pre‐ and post‐test study of CST for genetic counsellors. |
| Rangachari 2014 | Not an RCT |
| Rask 2009 | RCT of a 33‐hour CST course for nurses. No objective measurement of behaviour change reported. Assessment of patient perception of HCP's skills. |
| Razavi 1991 | Not an RCT. Study of a brief psychological training for HCP working with terminal cancer patients. Only subjective measurements of behaviour and attitude change reported. |
| Razavi 2000 | Not an RCT. Study comparing different simulated patients to measure behavioural change after CST. |
| Razavi 2009 | Not an RCT, a summary of research. |
| Rose 2008 | Not an RCT. A review of psycho‐oncology interventions for patients with cancer. |
| Rosenbloom 2007 | RCT of an intervention for patients with cancer comparing nurse assessment of quality of life compared to normal care. |
| Roter 1995 | RCT of CST for primary care physicians. Study not primarily related to cancer care. Assessment using audio‐tapes of encounters with distressed and non‐distressed real and simulated patients. |
| Rush 2015 | Not an RCT |
| Rushton 2006 | Not an RCT |
| Rutter 1996 | Not an RCT |
| Schenker 2015 | Not an RCT |
| Schmitz 2016 | CST in HCPs who did not work specifically in cancer care |
| Schulman‐Green 2003 | Not an RCT. Qualitative study of how HCP learn about caring for the dying. |
| Shannon 2011 | Not an RCT. Post‐assessment study of a brief CST for nurses. Only subjective assessment of change reported. |
| Shields 2010 | An RCT of coaching for survivors of breast cancer |
| Shipman 2008 | Not an RCT |
| Shorr 2000 | Not an RCT. Cohort study of invention to help HCP discuss end of life issues with patients, not specifically limited to cancer care. |
| Singy 2012 | Not an RCT |
| Smith 1991 | Not an RCT. Case‐controlled study of a 1‐month CST for residents. Only subjective measurement of change reported. |
| Smith 2010 | An RCT of an intervention comparing a pain/communication session to normal care for patients with cancer. |
| Song 2013 | Not an RCT |
| Street 2010 | RCT of CST training (tailored education‐coaching) for patients with cancer. |
| Szmuilowicz 2010 | An RCT of CST in HCPs who did not work specifically in cancer care. |
| Tannen 2014 | Not an RCT. Summary of the first update of this review |
| Timmermans 2006 | Not an RCT. Pre‐post study of CST training for radiation oncologists. Assessment of oncologists and patient communication in audiotapes of real patient encounters. |
| Ullrich 2011 | Not an RCT. Pre‐post quasi‐RCT of CST for speech therapists. Only subjective measurement of change were reported. |
| Vadaparampil 2016 | Not an RCT |
| Von Gunten 1998 | Not an RCT. Measured change in attitude/knowledge not skills, not behaviour. |
| Walter 2016 | Not an RCT |
| Wetzel 2011 | RCT of training in stress management for surgeons, not communication training. Not limited to cancer care. |
| Wilkinson 1998 | Not an RCT. Cohort study with pre‐post assessment of 26‐hour CST (including knowledge, attitude and skills training) for nurses. Audiotaped patient encounters measured behavioural change. |
| Wilkinson 1999 | Not an RCT. Long‐term follow‐up of cohort study. |
| Wilkinson 2003 | Not an RCT. Cohort study with pre‐post assessment of 3‐day CST for nurses. Audiotaped patient encounters measured behavioural change. |
| Wittenberg 2016 | Not RCT |
| Wong 2001 | Not an RCT. Post‐assessment of a course on death education for nurses. Only subjective measurement of changes reported. |
| Wuensch 2011 | RCT of communication skills training for physicians involved in conducting clinical trials in oncology. Training was aimed at improving patient understanding and acceptance of clinical trials. |
| Wuensch 2014 | CST was aimed at facilitating recruitment of patients to trials |
| Yildirim 2013 | Not an RCT |
CST: communication skills training;HCP: healthcare professional; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Brock 2017.
| Methods | RCT pilot study |
| Participants | Thirty‐five paediatric doctors in post‐graduate training in cardiology, critical care, haematology/oncology, and neonatology at two institutions |
| Interventions | Intervention group participants participated in a two‐day program over three months (three simulations and videotaped PC panel). Control group participants received written education designed to be similar in content and time |
| Outcomes | External reviewers rated simulation‐group encounters on nine communication domains. |
| Notes | Nonsustained improvement in four domains: relationship building (P = 0.01), opening discussion (P = 0.03), gathering information (P = 0.01), and communicating accurate information (P = 0.04) |
Coltoff 2018.
| Methods | RCT |
| Participants | solid tumour oncologists from four hospitals |
| Interventions | Interventon group participated in an interactive 2‐hour training session and then four coaching sessions in their actual practice environment |
| Outcomes | Communication skills were coded from recordings of goal of care interviews before and after the intervention |
| Notes | The intervention group used significantly more communication skills during the post‐intervention interviews |
Henselmans 2018.
| Methods | RCT with four parallel arms |
| Participants | Oncologists randomised to intervention or usual care; Patients with metastatic or unresectable cancer with a median life expectancy <12 months randomised to intervention or usual care |
| Interventions | The oncologist training consists of a reader, two group sessions (3.5 hour including modelling videos and role‐play), a booster feedback session (1 hour) and a consultation room tool. The patient communication aid consists of a home‐sent question prompt list and a value clarification exercise to prepare patients for Shared Decision Making (SDM) in the consultation |
| Outcomes | The primary outcome is observed SDM in audio‐recorded consultations with real and standardised patients. SDM will be measured using two instruments: the validated Observing Patient Involvement (OPTION) tool and 4SDM, a new tool. Secondary outcomes include patient and oncologist evaluation of communication and decision‐making, the decision made, quality of life, potential adverse outcomes such as anxiety and hopelessness, and consultation duration immediately after the consultation and at 3 and 6 months |
| Notes | Protocol |
Scholl 2018.
| Methods | Stepped wedge cluster‐randomised trial |
| Participants | HCPs (Physicians and Nurses) from three clinical teams from one university cancer centre in Germany |
| Interventions | The HCPs will receive SDM training and individual coaching. The patients will receive a patient activation strategy and patient information material and decision aids, The outcome evaluation will consist of four measurement points. The primary outcome is adoption of SDM, measured by the 9‐item Shared Decision Making Questionnaire. A range of other implementation outcomes will be assessed (i.e. acceptability, readiness for implementing change, appropriateness, penetration). The implementation process will be evaluated using stakeholder interviews and field notes. This will allow adapting interventions if necessary |
| Outcomes | The primary outcome will measure adoption of SDM in audio‐taped interviews, measured by the 9‐item Shared Decision Making Questionnaire |
| Notes | Protocol |
HCP: healthcare professionals; RCT: randomised controlled trial; SDM: shared decision making
Characteristics of ongoing studies [ordered by study ID]
Berger‐Höger 2015.
| Trial name or title | Informed shared decision‐making supported by decision coaches for women with ductal carcinoma in situ: study protocol for a cluster randomised controlled trial |
| Methods | Multi‐centred cluster RCT CST for doctors and nurses focusing on shared decision‐making together with the use of decision aids for patients |
| Participants | Doctors and nurses from 16 breast cancer centres; breast cancer patients |
| Interventions | CST for doctors (2 hours) and nurses (4 days) focusing on shared decision‐making together with the use of decision aids for patients |
| Outcomes | Patients’ involvement in shared decision‐making as assessed by theMAPPIN‐Odyad (Multifocal approach to the ‘sharing’ in shared decision‐making: observer instrument dyad). Secondary endpoints include the sub‐measures of the MAPPIN‐inventory (MAPPIN‐Onurse, MAPPIN‐Ophysician, MAPPIN‐Opatient, MAPPIN‐Qnurse, MAPPIN‐Qpatient and MAPPIN‐Qphysician), informed choice, decisional conflict and the duration of encounters |
| Starting date | Currently, the recruitment of breast care centres is ongoing. Patients will be recruited from July 2015 until March 2016 |
| Contact information | Birte.Berger‐Hoeger@uni‐hamburg.de University of Hamburg, MIN‐Faculty, Unit of Health Sciences and Education, Martin‐Luther‐King‐Platz 6, D‐20146 Hamburg, Germany. 2mediStatistica Neuenrade, Lambertusweg 1b, D‐58809 Neuenrade, Germany. |
| Notes | Protocol |
De Figuereido 2015.
| Trial name or title | ComOn Coaching: Study protocol of a randomised controlled trial to assess the effect of a varied number of coaching sessions on transfer into clinical practice following communication skills training |
| Methods | RCT |
| Participants | Physicians of two German medical centres will participate in a workshop for communication skills |
| Interventions | One coaching session compared to 4 coaching sessions after workshop for communication skills |
| Outcomes | Primary Outcome measures of changes in HCP communication using video‐recordings of real patients at three time points Secondary outcomes: HCP self evaluation of communication, evaluations of the consultation by HCP and patients |
| Starting date | Unclear |
| Contact information | marcelo.de.figueiredo@uniklinik‐freiburg.de Department of Psychosomatic Medicine and Psychotherapy, Freiburg University Medical Center, Hauptstr. 8, D‐79104, Freiburg, Germany |
| Notes | Protocol |
Libert 2017.
| Trial name or title | Communication about uncertainty and hope: A randomised controlled trial assessing the efficacy of a communication skills training program for physicians caring for cancer patients |
| Methods | RCT |
| Participants | Physician participants will be randomly assigned in groups to a 30‐hour CST program (experimental group) or to a waiting list (control group). |
| Interventions | The training program will include learner‐centred, skills focused, practice‐oriented techniques |
| Outcomes | HCP communication will be measured in simulated patient encounters at baseline and after CST or after 4 months for control group. Outcomes include communicational, psychological and physiological measures |
| Starting date | Unclear |
| Contact information | yves.libert@bordet.be; livia.peternelj@bordet.be Université Libre de Bruxelles, Faculté des Sciences Psychologiques et de l’Éducation, Av. F. Roosevelt, 50 (CP 191), 1050 Brussels, Belgium |
| Notes | Protocol |
Parker 2016.
| Trial name or title | Protocol for a cluster randomised trial of a communication skills intervention for physicians to facilitate survivorship transition in patients with lymphoma |
| Methods | Multicentred RCT |
| Participants | Physicians Patients who have achieved complete remission after completion of first‐line therapy |
| Interventions | CST in survivorship planning consultation skills compared to training in wellness rehabilitation |
| Outcomes | Primary outcome: HCP communication skills measured in RP encounters Other outcomes: Patient knowledge and adherence to plan; perceptions of the doctor‐patient relationship, decreased cancer worry and depression, QOL, satisfaction of care, usage of health system |
| Starting date | Unclear |
| Contact information | Dr Patricia Parker; Parkerp@mskcc.org |
| Notes | Protocol |
CST: communication skills training; HCP: healthcare professionals; QOL: quality of life; RCT: randomised controlled trial; RP: real patient
Differences between protocol and review
The original review was a narrative review that included two studies (Fallowfield 2002; Razavi 1993). Whereas the protocol and original review included pre‐test/post‐test study designs in the Types of studies for consideration, for the updated review we included only randomised controlled trials (RCTs).
For the protocol and original version of the review, we defined Types of outcome measures as follows: 'Outcomes were changes in behaviour or skills measured using objective and validated scales.' For the 2013 update, we attempted to specify Types of outcome measures more clearly, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
In this update we have included text in Data extraction and management section related to the development of the 'Summary of findings' table.
Contributions of authors
PM, SR, GB, CO sifted and screened the retrieved titles/abstracts. At least two review authors (PM, SR, GB, CO) classified the studies and extracted data from included studies. The original review was written by Deborah Fellowes (DF). PM and TL wrote the first draft of the first update. PM wrote the first draft of the second update. All five current review authors read and agreed the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Cochrane Incentive Scheme 2017 award. Reference number 17/62/36
Declarations of interest
Philippa M Moore: none known
Solange Rivera: none known
Gonzalo A Bravo‐Soto: none known
Camila Olivares: none known
Theresa A Lawrie: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Butow 2008 {published data only}
- Butow P, Cockburn J, Girgis A, Bowman D, Schofield P, D'Este C, et al. Increasing oncologists' skills in eliciting and responding to emotional cues: evaluation of a communication skills training program. Psycho‐Oncology 2008;17(3):209‐18. [PUBMED: 17575560] [DOI] [PubMed] [Google Scholar]
- Girgis A, Cockburn J, Butow P, Bowman D, Schofield P, Stojanovski E, et al. Improving patient emotional functioning and psychological morbidity: Evaluation of a consultation skills training program for oncologists. Patient Education and Counseling 2009;77(3):456‐62. [PUBMED: 19819660] [DOI] [PubMed] [Google Scholar]
Epstein 2017 {published data only}
- Epstein RM, Duberstein PR, Fenton JJ, Fiscella K, Hoerger M, Tancredi DJ, et al. Effect of a patient‐centered communication intervention on oncologist‐patient communication, quality of life and health care utilization in advanced cancer: the VOICE randomized clinical trial. Jama Oncology 2017;3(1):92‐100. [DOI: 10.1001/jamaoncol.2016.4373; PUBMED: 27612178] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton JJ, Kravitz RL, Duberstein P, Tancredi DJ, Xing G, Epstein R. A cluster randomized trial of a patient‐centered communication intervention in advanced cancer: The Values and Options In Cancer Care (VOICE) study. Journal of Clinical Oncology 2016;34(26):suppl 2‐2. [Google Scholar]
- Hoerger M, Epstein RM, Winters PC, Fiscella K, Duberstein PR, Gramling R, et al. Values and options in cancer care (VOICE): study design and rationale for a patient‐centered communication and decision‐making intervention for physicians, patients with advanced cancer, and their caregivers. BMC Cancer 2013;13:188. [DOI: 10.1186/1471-2407-13-188; PUBMED: 23570278] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenbach RA, Brandes K, Fiscella K, Kravitz RL, Butow PN, Walczak A, et al. Promoting end‐of‐life discussions in advanced cancer: effects of patient coaching and question prompt lists. Journal of Clinical Oncology 2017;35(8):842‐51. [DOI: 10.1200/JCO.2016.68.5651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fallowfield 2002 {published data only}
- Fallowfield L, Jenkins V, Farewell V, Saul J, Duffy A, Eves R. Efficacy of a Cancer Research UK communication skills training model for oncologists: a randomised controlled study. Lancet 2002;359(9307):650‐6. [DOI: 10.1016/S0140-6736(02)07810-8] [DOI] [PubMed] [Google Scholar]
- Fallowfield L, Jenkins V, Farewell V, Solis TI. Enduring impact of communication skills training: results of a 12‐month follow‐up. British Journal of Cancer 2003;89(8):1445‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins V, Fallowfield L. Can communication skills training alter physicians' beliefs and behavior in clinics?. Journal of Clinical Oncology 2002;20(3):765‐9. [DOI: 10.1200/JCO.2002.20.3.765] [DOI] [PubMed] [Google Scholar]
- Shilling V, Jenkins V, Fallowfield L. Factors affecting patient and clinician satisfaction with the clinical consultation: can communication skills training for clinicians improve satisfaction?. Psycho‐Oncology 2003;12(6):599‐611. [DOI: 10.1002/pon.731] [DOI] [PubMed] [Google Scholar]
Fujimori 2014 {published data only}
- Fujimori M, Shirai Y, Asai M, Akizuki N, Katsumata N, Kubota K, et al. Development and preliminary evaluation of communication skills training program for oncologists based on patient preferences for communicating bad news. Palliative & Supportive Care 2014;12(5):379‐86. [DOI] [PubMed] [Google Scholar]
- Fujimori M, Shirai Y, Asai M, Kubota K, Katsumata N, Uchitomi Y. Effect of communication skills training program for oncologists based on patient preferences for communication when receiving bad news: a randomized controlled trial. Journal of Clinical Oncology 2014;32(20):2166‐72. [DOI: 10.1200/JCO.2013.51.2756] [DOI] [PubMed] [Google Scholar]
- Fujimori MS. Effect of communication skills training program for oncologists based on the patient preferences for communicating bad news: A randomized control trial. Psycho‐Oncology Conference: 13th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Antalya Turkey. Conference Start: 20111016 Conference End: 20111020. 2011:Conference Publication:[var.pagings].
Goelz 2011 {published data only}
- Goeltz TW. The transition from curative to palliative care in oncology‐a special challenge requires special communication skills training. Evaluation of a specific and individualized training concept‐a RCT. Psycho‐Oncology Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625. 2009:Conference Publication:[var.pagings].
- Goelz T, Wuensch A, Stubenrauch S, Ihorst G, Figueiredo M, Bertz H, et al. Specific training program improves oncologists' palliative care communication skills in a randomized controlled trial. Journal of Clinical Oncology 2011;29(25):3402‐7. [DOI: 10.1200/JCO.2010.31.6372] [DOI] [PubMed] [Google Scholar]
- Goelz TW. Psycho‐Oncology; Conference(var.pagings). Conference Publication, 2010; Vol. 19(Suppl 2):S1‐S313.
Gorniewicz 2017 {published data only}
- Bishop TW, Gorniewicz J, Floyd M, Tudiver F, Odom A, Zoppi K. Innovative patient‐centered skills training addressing challenging issues in cancer communications: Using patient's stories that teach. International Journal of Psychiatry in Medicine 2016;51(4):357‐66. [DOI: 10.1177/0091217416659272] [DOI] [PubMed] [Google Scholar]
- Gorniewicz J, Floyd M, Krishnan K, Bishop TW, Tudiver F, Lang F. Breaking bad news to patients with cancer: A randomized control trial of a brief communication skills training module incorporating the stories and preferences of actual patients. Patient Education and Counseling 2017;100(4):655‐66. [DOI: 10.1016/j.pec.2016.11.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heaven 2006 {published data only}
- Heaven C, Clegg J, Maguire P. Transfer of communication skills training from workshop to workplace: the impact of clinical supervision. Patient Education and Counselling 2006;60(3):313‐25. [DOI: 10.1016/j.pec.2005.08.008] [DOI] [PubMed] [Google Scholar]
Kruijver 2001 {published and unpublished data}
- Kruijver IPM, Kerkstra A, Kerssens JJ, Bensing JM, Wiel HBM, Wind RM, et al. Communication between nurses and admitted cancer patients: the impact of a communication training program on nurse and patient outcomes. Chapter 5: Kruijver thesis 145‐66.
- Kruijver IPM, Kerkstra A, Kerssens JJ, Holtkamp CCM, Bensing JM, Wiel HBM. Communication between nurses and simulated patients with cancer: evaluation of a communication training programme (including commentary by Heaven C and Payne S). European Journal of Oncology Nursing 2001;5(3):140‐53. [DOI] [PubMed] [Google Scholar]
Lienard 2010 {published data only}
- Bragard I, Etienne AM, Libert Y, Merckaert I, Lienard A, Meunier J, et al. Efficacy of a communication and stress management training on residents' stress to communicate, self‐efficacy and burnout level: A randomized controlled study [conference abstract]. Psycho‐Oncology: the 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria, 21‐25 June 2009. Conference publication, 2009:S252. [DOI: 10.1002/pon] [DOI]
- Bragard I, Etienne AM, Merckaert I, Libert Y, Razavi D. Efficacy of a communication and stress management training on medical residents' self‐efficacy, stress to communicate and burnout: a randomized controlled study. Journal of Health Psychology 2010;15(7):1075‐81. [DOI: 10.1177/1359105310361992.] [DOI] [PubMed] [Google Scholar]
- Damas AM. Impact of a communication skills training program on residents' bad news disclosure in a simulated consultation: A randomised study. Psycho‐Oncology Conference abstract: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS, Vienna, Austria. 2009; Vol. 18:S269.
- Gibon AS, Merckaert I, Libert Y, Lie A, Etienne AM, Reynaert C, et al. The efficacy of a communication skills training program on successive sequences of breaking bad news simulated consultation: A randomized controlled study. Psycho‐Oncology Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625. 2009; Vol. 18:s9. [DOI: 10.1002/pon.1594] [DOI]
- Hasoppe J, Merckaert I, Libert Y, Bragard I, Etienne AM, Reynaert C, et al. Communication skills training: A study of residents' psychological and physiological variables which facilitate or inhibit the learning of assessment.. Psycho‐Oncology Conference abstract: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS, Vienna, Austria. 2009; Vol. 18:s269. [DOI: 10.1002/pon.1594] [DOI]
- Lienard A, Merckaert I, Libert Y, Bragard I, Delvaux N, Etienne M, et al. Is it possible to improve residents breaking bad news skills? A randomised study assessing the efficacy of a communication skills training program. British Journal of Cancer 2010;103(2):171‐7. [DOI: 10.1038/sj.bjc.6605749] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienard A, Merckaert I, Libert Y, Bragard I, Delvaux N, Etienne M, et al. Transfer of communication skills to the workplace during clinical rounds: impact of a program for residents. PLoS One 2010;5(8):e12426. [DOI: 10.1371/journal.pone.0012426] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienard AM, Merckaert I, Libert Y, Bragard I, Delvaux N, Etienne AM, et al. Is it possible to improve residents breaking bad news skills? A randomised study assessing the efficacy of a communication skills training program. Psycho‐Oncology Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625. 2009; Vol. 18:s277. [DOI: 10.1002/pon.1594] [DOI]
- Lienard AM, Merckaert I, Libert Y, Bragard I, Delvaux N, Etienne AM, et al. Transfer of communication skills training to workplace: Impact of a program for residents. Psycho‐Oncology Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625. 2009; Vol. 18:s76. [DOI: 10.1002/pon.1594] [DOI]
- Merckaert I, Liénard A, Libert Y, Bragard I, Delvaux N, Etienne AM, et al. Is it possible to improve the breaking bad news skills of residents when a relative is present? A randomised study. British Journal of Cancer 2013;109(10):2507‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier J, Merckaert I, Libert Y, Delvaux N, Etienne AM, Liénard A, et al. The effect of communication skills training on residents' physiological arousal in a breaking bad news simulated task.. Patient Education and Counseling 2013;93(1):40‐7. [DOI: 10.1016/j.pec.2013.04.020] [DOI] [PubMed] [Google Scholar]
Merckaert 2015 {published data only}
- Gibon AS, Merckaert I, Lienard A, Libert Y, Marchal S, Etienne AM, et al. Is it possible to improve radiotherapy team communication skills? A randomized study assessing the efficacy of a training program in the context of an encounter with a simulated anxious patient called Mrs Leblanc. Psycho‐Oncology. 2011; Vol. 13th World Congress of the International Psycho‐Oncology Society 16/01/2011 ‐20/01/2011, Antalya, Turkey:102. [DOI: 10.1002/pon.2077] [DOI]
- Gibon AS, Merckaert I, Liénard A, Libert Y, Delvaux N, Marchal S, et al. Is it possible to improve radiotherapy team members' communication skills? A randomised study assessing the efficacy of a 38‐h communication skills training program. Radiation Oncology (London, England) 2013;109(1):170‐7. [DOI: 10.1016/j.radonc.2013.08.019] [DOI] [PubMed] [Google Scholar]
- Liénard A, Delevallez F, Razavi D, Gibon AS, Libert Y, Delvaux N, et al. Is it possible to improve communication around radiotherapy delivery: A randomized study to assess the efficacy of team training?. Radiotherapy and Oncology 2016;119(2):361‐7. [DOI] [PubMed] [Google Scholar]
- Merckaert I, Delevallez F, Gibon AS, Liénard A, Libert Y, Delvaux N, et al. Transfer of communication skills to the workplace: impact of a 38‐hour communication skills training program designed for radiotherapy teams. Journal of Clinical Oncology 2015;33(8):901‐9. [DOI: 10.1200/JCO.2014.57.3287] [DOI] [PubMed] [Google Scholar]
Razavi 1993 {published data only}
- Delvaux N, Razavi D, Marchal S, Bredart A, Farvacques C, Paesmans M. The effects of a 24hr psychological training programme on attitudes, communication skills and occupational stress in oncology: a randomised study. Quality of Life Research 1993;2:55‐6. [DOI] [PubMed] [Google Scholar]
- Razavi D, Delvaux N, Marchal S, Bredart A, Farvacques C, Paesmans M. The effects of a 24‐h psychological training program on attitudes, communication skills and occupational stress in oncology: a randomised study. European Journal of Cancer 1993;29A(13):1858‐63. [DOI] [PubMed] [Google Scholar]
Razavi 2002 {published data only}
- Canivet D, Delvaux N, Gibon AN, Slachmuylder JL, Razavi D. Impact of a communication skills training on nurses communication efficiency: A qualitative analysis of an encounter with Mrs Gudule, a simulated cancer patient anxious about taking morphine. Psycho‐Oncology Conference: 13th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Antalya Turkey. Conference Start: 20111016 Conference End: 20111020. 2011; Vol. 20:107. [DOI: 10.1002/pon.2078] [DOI]
- Canivet D, Delvaux N, Gibon AS, Brancart C, Slachmuylder JL, Razavi D. Improving communication in cancer pain management nursing: a randomised controlled study assessing the efficacy of a communication skills training program. Support Care Cancer 2014;22(12):3311‐20. [DOI] [PubMed] [Google Scholar]
- Delvaux N, Razavi D, Marchal S, Bredart A, Farvacques C, Slachmuylder JL. Effects of a 105 hours psychological training program on attitudes, communication skills and occupational stress in oncology: a randomised study. British Journal of Cancer 2004;90(1):106‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon AS, Durieux JF, Merckaert I, Delvaux N, Farvacques C, Libert Y, et al. Development of the LaComm, a French medical communication analysis software: A Study assessing its sensitivity to change. Psycho‐Oncology. 2010; Vol. 19(Suppl 2):S133. [DOI: 10.1002/pon.1776] [DOI] [PubMed]
- Razavi D, Delvaux N, Marchal S, Durieux J‐F, Farvacques C, Dubus L, et al. Does training increase the use of more emotionally laden words by nurses when talking with cancer patients? A randomised study. British Journal of Cancer 2002;87(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Razavi 2003 {published data only}
- Bragard I, Libert Y, Etienne AM, Merckaert I, Delvaux N, Marchal S, et al. Insight on variables leading to burnout in cancer physicians. Journal of Cancer Education: the official journal of the American Association for Cancer 2010;25(1):109‐15. [DOI] [PubMed] [Google Scholar]
- Delvaux N, Merckaert I, Marchal S, Libert Y, Conradt S, Boniver J, et al. Physicians' communication with a cancer patient and a relative: a randomised study assessing the efficacy of consolidation workshops. Cancer 2005;103(11):2397‐411. [DOI] [PubMed] [Google Scholar]
- Lienard A, Merckaert I, Libert Y, Delvaux N, Marchal S, Boniver J, et al. Factors that influence cancer patients' and relatives' anxiety following a three‐person medical consultation: impact of a communication skills training program for physicians. Psycho‐Oncology 2008;17(5):488‐96. [DOI: 10.1002/pon.1262] [DOI] [PubMed] [Google Scholar]
- Lienard A, Merckaert I, Libert Y, Delvaux N, Marchal S, Boniver J, et al. Factors that influence cancer patients' anxiety following a medical consultation: impact of a communication skills training programme for physicians. Annals of Oncology: the official journal of the European Society for Medical Oncology / ESMO 2006;17(9):1450‐8. [PUBMED: 16801333] [DOI] [PubMed] [Google Scholar]
- Merckaert I, Libert Y, Delvaux N, Marchal S, Boniver J, Etienne AM, et al. Factors influencing physicians' detection of cancer patients' and relatives' distress: can a communication skills training program improve physicians' detection?. Psycho‐Oncology 2008;17(3):260‐9. [DOI: 10.1002/pon.1233] [DOI] [PubMed] [Google Scholar]
- Merckaert I, Libert Y, Delvaux N, Marchal S, Boniver J, Etienne AM, et al. Factors that influence physicians' detection of distress in patients with cancer: can a communication skills training program improve physicians' detection?. Cancer 2005;104(2):411‐21. [DOI: 10.1002/cncr.21172] [DOI] [PubMed] [Google Scholar]
- Razavi D, Merckaert I, Marchal S, Libert Y, Conradt S, Boniver J, et al. How to optimise physicians' communication skills in cancer care: results of a randomised study assessing the usefulness of post‐training consolidation workshops. Journal of Clinical Oncology : official journal of the American Society of Clinical Oncology 2003;21(16):3141‐9. [PUBMED: 12915605] [DOI] [PubMed] [Google Scholar]
Stewart 2007 {published data only}
- Stewart M, Brown JB, Hammerton J, Donner A, Gavin A, Holliday RL, et al. Improving communication between doctors and breast cancer patients. Annals of Family Medicine 2007;5(5):387‐94. [DOI: 10.1370/afm.721] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tulsky 2011 {published data only}
- Koropchak CM, Pollak KI, Arnold RM, Alexander SC, Skinner CS, Olsen MK, et al. Studying communication in oncologist‐patient encounters: the SCOPE Trial. Palliative Medicine 2006;20(8):813‐9. [DOI: 10.1177/0269216306070657] [DOI] [PubMed] [Google Scholar]
- Rodriguez KL, Bayliss N, Alexander SC, Jeffreys AS, Olsen MK, Pollak KI, et al. How oncologists and their patients with advanced cancer communicate about health‐related quality of life. Psycho‐Oncology 2010;19(5):490‐9. [DOI: 10.1002/pon.1579] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner CS, Pollak KI, Farrell D, Olsen MK, Jeffreys AS, Tulsky JA. Use of and reactions to a tailored CD‐ROM designed to enhance oncologist‐patient communication: The SCOPE trial intervention. Patient Education and Counseling 2009;77(1):90‐6. [DOI: 10.1016/j.pec.2009.02.010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsky JA, Arnold RM, Alexander SC, Olsen MK, Jeffreys AS, Rodriguez KL, et al. Enhancing communication between oncologists and patients with a computer‐based training program: a randomized controlled trial. Annals of Internal Medicine Nov 2011;155(9):593‐601. [DOI: 10.7326/0003-4819-155-9-201111010-00007] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Weert 2011 {published data only}
- Weert JC, Jansen J, Spreeuwenberg PM, Dulmen S, Bensing JM. Effects of communication skills training and a Question Prompt Sheet to improve communication with older cancer patients: a randomized controlled trial. Critical Reviews in Oncology‐Hematology 2011;80(1):145‐59. [DOI: 10.1016/j.critrevonc.2010.10.010] [DOI] [PubMed] [Google Scholar]
Wilkinson 2008 {published data only}
- Wilkinson S, Perry R, Blanchard K, Linsell L. Effectiveness of a three‐day communication skills course in changing nurses' communication skills with cancer/palliative care patients: a randomised controlled trial. Palliative Medicine 2008;22(4):365‐75. [DOI: 10.1177/0269216308090770] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ades 2001 {published data only}
- Ades T, Gansler T, Eyre H. Communicating with patients about quality of life issues. CA: A Cancer Journal for Clinicians 2001;51(4):211‐2. [PUBMED: 11577487] [DOI] [PubMed] [Google Scholar]
Alexander 2006 {published data only}
- Alexander SC, Keitz SA, Sloane R, Tulsky JA. A controlled trial of a short course to improve residents' communication with patients at the end of life. Academic Medicine 2006;81(11):1008‐12. [DOI: 10.1097/01.ACM.0000242580.83851.ad] [DOI] [PubMed] [Google Scholar]
Anderson 1982 {published data only}
- Anderson JL. Evaluation of a practical approach to teaching about communication with terminal cancer patients. Medical Education 1982;16(4):202‐7. [DOI: 10.1111/j.1365-2923.1982.tb01249.x] [DOI] [PubMed] [Google Scholar]
Andrew 1998 {published data only}
- Andrew M. The dilemmas of experiential teaching: toward an interpretive approach. Journal of Continuing Education in Nursing 1998;29(2):73‐8. [PUBMED: 9582775] [DOI] [PubMed] [Google Scholar]
Archer 2004 {published data only}
- Archer M, Daniel R, Riley J, Wynburne J. Empowering people with cancer. Expert Review of Anticancer Therapy 2004;4(3 Suppl. 1):S51‐7. [PUBMED: 15230685] [DOI] [PubMed] [Google Scholar]
Arranz 2005 {published data only}
- Arranz P, Ulla SM, Ramos JL, Rincón C, López‐Fando T. Evaluation of a counselling training program for nursing staff. Patient Education & Counseling 2005;56(2):233‐9. [DOI: 10.1016/j.pec.2004.02.017] [DOI] [PubMed] [Google Scholar]
Arrighi 2010 {published data only}
- Arrighi E, Jovell AJ, Navarro MD. Therapeutic value in oncology. Patients', caregivers' and professionals' perspective [El valor terapeutico en oncologia. La perspectiva de pacientes, familiares y profesionales]. Psicooncologia 2010;2‐3:363‐74. [DOI: 10.5209/rev_PSIC.2010.v7.n2.15900] [DOI] [Google Scholar]
Aubin‐Auger 2014 {published data only}
- Aubin‐Auger I, Laouénan C, Bel J, Mercier A, Baruch D, Lebeau JP, et al. Efficacy of communication skills training on colorectal cancer screening by GPs: a cluster randomised controlled trial. European Journal of Cancer Care 2016;25(1):18‐26. [DOI: 10.1111/ecc.12310; PUBMED: 25851842] [DOI] [PubMed] [Google Scholar]
Back 2005 {published data only}
- Back AL. How should physicians communicate the transition to palliative care?. Nature Clinical Practice Oncology 2005;2(3):136‐7. [DOI: 10.1038/ncponc0100] [DOI] [PubMed] [Google Scholar]
Back 2007 {published data only}
- Back AL, Arnold RM, Baile WF, Fryer‐Edwards KA, Alexander SC, Barley GE, et al. Efficacy of communication skills training for giving bad news and discussing transitions to palliative care. Archives of Internal Medicine 2007;167(5):453‐60. [DOI: 10.1001/archinte.167.5.453] [DOI] [PubMed] [Google Scholar]
Baile 1997 {published data only}
- Baile WF, Lenzi R, Kudelka AP, Maguire P, Novack D, Goldstein M, et al. Improving physician‐patient communication in cancer care: outcome of a workshop for oncologists. Journal of Cancer Education 1997;12(3):166‐73. [DOI: 10.1080/08858199709528481] [DOI] [PubMed] [Google Scholar]
Baile 1999 {published data only}
- Baile WF, Kudelka AP, Beale EA, Glober GA, Myers EG, Greisinger AJ, et al. Communication skills training in oncology. Description and preliminary outcomes of workshops on breaking bad news and managing patient reactions to illness. Cancer 1999;86(5):887‐97. [PUBMED: 10463990] [PubMed] [Google Scholar]
Beach 2014 {published data only}
- Beach WA, Buller MK, Dozier DM, Buller DB, Gutzmer K. The Conversations About Cancer (CAC) project: assessing feasibility and audience impacts from viewing. The Cancer Play. Health Communication 2014;29(5):462‐72. [DOI: 10.1080/10410236.2013.767874; PUBMED: 24098921] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berman 1983 {published data only}
- Berman S, Villarreal S. Use of a seminar as an aid in helping interns care for dying children and their families. Clinical Pediatrics 1983;22(3):175‐9. [DOI: 10.1177/000992288302200302] [DOI] [PubMed] [Google Scholar]
Bernacki 2013 {published data only}
- Bernacki RE, Block SD. Serious illness communications checklist. Virtual Mentor 2013;15(12):1045‐9. [DOI: 10.1001/virtualmentor.2013.15.12.stas1-1312] [DOI] [PubMed] [Google Scholar]
Bernacki 2015 {published data only}
- Bernacki R, Hutchings M, Vick J, Smith G, Paladino J, Lipsitz S, et al. Development of the Serious Illness Care Program: a randomised controlled trial of a palliative care communication intervention. BMJ Open 2015;5(10):e009032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernard 2010 {published data only}
- Bernard M, Roten Y, Despland JN, Stiefel F. Communication skills training and clinicians' defences in oncology: an exploratory, controlled study. Psycho‐Oncology 2010;19(2):209‐15. [DOI: 10.1002/pon.1558] [DOI] [PubMed] [Google Scholar]
Bernhard 2012 {published data only}
- Bernhard J, Butow P, Aldridge J, Juraskova I, Ribi K, Brown R. Communication about standard treatment options and clinical trials: can we teach doctors new skills to improve patient outcomes?. Psycho‐Oncology 2012;21(12):1265‐74. [DOI: 10.1002/pon.2044; PUBMED: 23208837] [DOI] [PubMed] [Google Scholar]
Bibila 2014 {published data only}
- Bibila S, Rabiee F. Training the powerful: Issues that emerged during the evaluation of a communication skills training programme for senior cancer care professionals. European Journal of Cancer Care 2014;23(4):531‐44. [DOI: 10.1111/ecc.12167; PUBMED: 24373021] [DOI] [PubMed] [Google Scholar]
Bird 1993 {published data only}
- Bird J, Hall A, Maguire P, Heavy P. Workshops for consultants on the teaching of communication skills. Medical Education 1993 1993;27(2):181‐5. [DOI: 10.1111/j.1365-2923.1993.tb00250.x] [DOI] [PubMed] [Google Scholar]
Blödt 2016 {published data only}
- Blödt S, Mittring N, Schützler L, Fischer F, Holmberg C, Horneber M, et al. A consultation training program for physicians for communication about complementary medicine with breast cancer patients: a prospective, multi‐centred, cluster‐randomized, mixed‐method pilot study. BMC Cancer 2016;16(1):843. [PUBMED: 27809814] [DOI] [PMC free article] [PubMed] [Google Scholar]
Booth 1996 {published data only}
- Booth K, Maguire PM, Butterworth T, Hillier VF. Perceived professional support and the use of blocking behaviours by hospice nurses. Journal of Advanced Nursing 1996;24(3):522‐7. [PUBMED: 8876412] [DOI] [PubMed] [Google Scholar]
Brown 1999 {published data only}
- Brown JB, Boles M, Mullooly JP, Levinson W. Effect of clinician communication skills training on patient satisfaction. A randomized, controlled trial. Annals of Internal Medicine 1999;131(11):822‐9. [PUBMED: 10610626] [DOI] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Brown RF, Butow PN, Boyle F, Tattersall MH. Seeking informed consent to cancer clinical trials; evaluating the efficacy of doctor communication skills training. Psycho‐Oncology 2007;16(6):507‐16. [DOI: 10.1002/pon.1095] [DOI] [PubMed] [Google Scholar]
Brown 2011 {published data only}
- Brown R, Bylund CL, Siminoff LA, Slovin SF. Seeking informed consent to Phase I cancer clinical trials: identifying oncologists' communication strategies. Psycho‐Oncology 2011;20(4):361‐8. [DOI: 10.1002/pon.1748] [DOI] [PubMed] [Google Scholar]
Brown 2012 {published data only}
- Bernhard J, Butow P, Aldridge J, Juraskova I, Ribi K, Brown R. Communication about standard treatment options and clinical trials: can we teach doctors new skills to improve patient outcomes?. Psycho‐Oncology 2012;21(12):1265‐74. [DOI: 10.1002/pon.2044; PUBMED: 23208837] [DOI] [PubMed] [Google Scholar]
- Bernhard J, Butow P, Aldridge J, Ribi K, Juraskova I, Brown R. An international randomized trial of skills training for oncologists concerning communication about standard treatment options and clinical trials with patients with early breast cancer. Psycho‐Oncology Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625. 2009; Vol. 18:s76. [DOI: 10.1002/pon.1594] [DOI]
- Brown R, Butow H, Wilson‐Genderson M, Bernhard J, Ribi K, Juraskova I. Meeting breast cancer patients' decision‐making preferences in oncology consultations: Impact on decision specific outcomes. Psycho‐Oncology Conference: 13th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Antalya Turkey. Conference Start: 20111016 Conference End: 20111020. 2011; Vol. 20:61. [DOI: 10.1002/pon.2077] [DOI]
- Brown R, Butow P, Wilson‐Genderson M, Bernhard J, Ribi K, Juraskova I. Meeting the decision‐making preferences of patients with breast cancer in oncology consultations: impact on decision‐related outcomes. Journal of Clinical Oncology 2012;30(8):857‐62. [DOI: 10.1200/JCO.2011.37.7952] [DOI] [PubMed] [Google Scholar]
Burgess 2008 {published data only}
- Burgess CC, Bish AM, Hunter HS, Salkovskis P, Michell M, Whelehan P, et al. Promoting early presentation of breast cancer: Development of a psycho‐educational intervention. Chronic Illness 2008;4(1):13‐27. [DOI: 10.1177/1742395307084404] [DOI] [PubMed] [Google Scholar]
Butow 2015 {published data only}
- Butow P, Brown R, Aldridge J, Juraskova I, Zoller P, Boyle F, et al. Can consultation skills training change doctors' behaviour to increase involvement of patients in making decisions about standard treatment and clinical trials: A randomised controlled trial. Health Expect 2015;18(6):2570‐83. [DOI: 10.1111/hex.12229; PUBMED: 24975503] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bylund 2010 {published data only}
- Bylund CL, Brown R, Gueguen JA, Diamond C, Bianculli J, Kissane DW. The implementation and assessment of a comprehensive communication skills training curriculum for oncologists. Psycho‐Oncology 2010;19(6):583‐93. [DOI: 10.1002/pon.1585] [DOI] [PubMed] [Google Scholar]
Bylund 2011a {published data only}
- Bylund CL, Brown RF, Bialer PA, Levin TT, Lubrano di Ciccone B, Kissane DW, et al. Developing and implementing an advanced communication training program in oncology at a comprehensive cancer center. Journal of Cancer Education 2011;26(4):604‐11. [DOI: 10.1007/s13187-011-0226-y] [DOI] [PubMed] [Google Scholar]
Bylund 2011b {published data only}
- Bylund CL, Goytia J, D'Agostino A, Bulone L, Horner J, Li Y, et al. Evaluation of a pilot communication skills training intervention for minority cancer patients. Journal of Pyschosocial Oncology 2011;29(4):347‐59. [PUBMED: 21966720] [PMC free article] [PubMed] [Google Scholar]
Cantwell 1997 {published data only}
- Cantwell BM, Ramirez AJ. Doctor‐patient communication: a study of junior house officers. Medical Education 1997;31(1):17‐21. [PUBMED: 9231119] [DOI] [PubMed] [Google Scholar]
Caps 2010 {published data only}
- Caps E, Libert Y, Marchal S, Bragard I, Etienne AM, Reynaert CH, et al. Optimizing team members communication skills in radiation therapy: content and feasibility of a Belgian Inter‐university curriculum. Psycho‐Oncology; conference. 2010; Vol. 19 (Suppl 2):S268.
Chandawarkar 2011 {published data only}
- Chandawarkar RY, Ruscher KA, Krajewski A, Garg M, Pfeiffer C, Singh R, et al. Pretraining and posttraining assessment of residents' performance in the fourth accreditation council for graduate medical education competency: Patient communication skills. Archives of Surgery 2011;146(8):916‐21. [DOI: 10.1001/archsurg.2011.167] [DOI] [PubMed] [Google Scholar]
Charlton 1993 {published data only}
- Charlton R. Education about death and dying at Otago University Medical School. New Zealand Medical Journal 1993;106(966):447‐9. [PUBMED: 8233175] [PubMed] [Google Scholar]
Clark 2009 {published data only}
- Clark P. Reducing psychological distress in the in‐patient oncology treatment setting using FLEX Care: An intervention study. Psycho‐Oncology Conference: 6th Annual Conference of the American Psychosocial Oncology Society, APOS Charlotte, NC United States. Conference Start: 20090205 Conference End: 20090208. 2009; Vol. 18:S4. [DOI: 10.1002/pon.1526] [DOI]
Claxton 2011 {published data only}
- Claxton R, Marks S, Buranosky R, Rosielle D, Arnold RM. The educational impact of weekly e‐mailed fast facts and concepts. Journal of Palliative Medicine 2011;14(4):475‐81. [DOI: 10.1089/jpm.2010.0418] [DOI] [PubMed] [Google Scholar]
Clayton 2012 {published data only}
- Clayton JM, Adler JL, O'Callaghan A, Martin P, Hynson J, Butow PN, et al. Intensive communication skills teaching for specialist training in palliative medicine: development and evaluation of an experiential workshop. Journal of Palliative Medicine 2012;15(5):585‐91. [DOI: 10.1089/jpm.2011.0292; PUBMED: 22433021] [DOI] [PubMed] [Google Scholar]
Connolly 2010 {published data only}
- Connolly M, Perryman J, McKenna Y, Orford J, Thomson L, Shuttleworth J, et al. SAGE & THYME: a model for training health and social care professionals in patient‐focussed support. Patient Education and Counseling 2010;79(1):87‐93. [DOI: 10.1016/j.pec.2009.06.004] [DOI] [PubMed] [Google Scholar]
Cooley 2014 {published data only}
- Cooley ME, Blonquist T, Catalano P, Lobach D, Braun I, Halpenny B, et al. Point‐of‐care clinical decision support for cancer symptom management: Results of a group randomised trial. Journal of Clinical Oncology 2014;32(31 suppl):1‐1. [DOI: 10.1200/jco.2014.32.31_suppl.1] [DOI] [Google Scholar]
Cort 2009 {published data only}
- Cort E, Moorey S, Hotopf M, Kapari M, Monroe B, Hansford P, et al. Palliative care nurses' experiences of training in cognitive behaviour therapy and taking part in a randomised controlled trial. International Journal of Palliative Nursing 2009;15(6):290‐8. [DOI: 10.12968/ijpn.2009.15.6.42988] [DOI] [PubMed] [Google Scholar]
Cowan 1997 {published data only}
- Cowan DH, Laidlaw JC, Russell ML. A strategy to improve communication between health care professionals and people living with cancer: II. Follow‐up of a workshop on the teaching and assessment of communication skills in Canadian Medical Schools. Journal of Cancer Education 1997;12(3):161‐5. [DOI: 10.1080/08858199709528480] [DOI] [PubMed] [Google Scholar]
Craytor 1978 {published data only}
- Craytor JK, Brown JK, Morrow GR. Assessing learning needs of nurses who care for persons with cancer. Cancer Nursing 1978;1(3):211‐20. [PUBMED: 248304] [PubMed] [Google Scholar]
Crit 2006 {published data only}
- No authors listed. The critical need to communicate effectively with the children of cancer patients. Journal of Supportive Oncology 2006;4(2):75‐6. [PUBMED: 16499125] [PubMed] [Google Scholar]
Curtis 2013 {published data only}
- Curtis JR, Back AL, Ford DW, Downey L, Shannon SE, Doorenbos AZ, et al. Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: A randomized trial. JAMA 2013;310(21):2271‐81. [DOI: 10.1001/jama.2013.282081; PUBMED: 24302090] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall J, Back L, Ford W, Downey L, Shannon E, Doorenbos Z, et al. Effect of communication skills training for residents and nurse practitioners on quality of communication with patients with serious illness: A randomized trial: Correction. JAMA 2014;311(13):1360. [PUBMED: 24302090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Das Gracas 2016 {published data only}
- Das Graças Silva Matsubara M, Domenico EBL. Virtual learning environment in continuing education for nursing in oncology: an experimental study. Journal of Cancer Education 2016;31(4):804‐10. [DOI: 10.1007/s13187-015-0889-x; PUBMED: 26224242] [DOI] [PubMed] [Google Scholar]
de Bie 2011 {published data only}
- Bie RP, Massuger LF, Lenselink CH, Derksen YH, Prins JB, Bekkers RL. The role of individually targeted information to reduce anxiety before colposcopy: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 2011;118(8):945‐50. [DOI: 10.1111/j.1471-0528.2011.02996.x] [DOI] [PubMed] [Google Scholar]
Delvaux 1997 {published data only}
- Delvaux N, Razavi D. Psychological training for health‐care professionals in oncology. A way to improve communication skills. Annals of the New York Acadamy of Science 1997;809:336‐49. [PUBMED: 9103585] [DOI] [PubMed] [Google Scholar]
Del Vento 2009 {published data only}
- Vento A, Bavelas J, Healing S, MacLean G, Kirk P. An experimental investigation of the dilemma of delivering bad news. Patient Education and Counselling 2009;77(3):443‐9. [DOI: 10.1016/j.pec.2009.09.014] [DOI] [PubMed] [Google Scholar]
de Rond 2000 {published data only}
- Rond MEJ, Wit R, Dam FS, Muller MJ. A pain monitoring program for nurses: effects on communication, assessment and documentation of patients' pain. Journal of Pain & Symptom Management 2000;20(6):424‐39. [DOI: ] [DOI] [PubMed] [Google Scholar]
Ditton‐Phare 2016 {published data only}
- Ditton‐Phare P, Sandhu H, Kelly B, Kissane D, Loughland C. Pilot evaluation of a communication skills training program for psychiatry residents using standardized patient assessment. Academic Psychiatry 2016;40(5):768‐75. [DOI: 10.1007/s40596-016-0560-9; PUBMED: 27137767] [DOI] [PubMed] [Google Scholar]
Dixon 2001 {published data only}
- Dixon H, Hordern A, Borland R. The Breast Cancer Distance Education Program: development and evaluation of a course for specialist breast cancer nurses. Cancer Nursing 2001;24(1):44‐52. [DOI] [PubMed] [Google Scholar]
Downar 2017 {published data only}
- Downar J, McNaughton N, Abdelhalim T, Wong N, Lapointe‐Shaw L, Seccareccia D, et al. Standardized patient simulation versus didactic teaching alone for improving residents' communication skills when discussing goals of care and resuscitation: a randomised controlled trial. Palliative Medicine 2017; Vol. 31, issue 2:130‐9. [DOI: 10.1177/0269216316652278] [DOI] [PubMed]
Durgahee 1997 {published data only}
- Durgahee T. Reflective practice: nursing ethics through story telling. Nursing Ethics: an International Journal for Health Care Professionals 1997;4(2):135‐46. [DOI: 10.1177/096973309700400205] [DOI] [PubMed] [Google Scholar]
Epner 2014 {published data only}
- Epner DE, Baile WF. Difficult conversations: Teaching medical oncology trainees communication skills one hour at a time. Academic Medicine 2014;89(4):578‐84. [DOI: 10.1097/ACM.0000000000000177; PUBMED: 24556763] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fallowfield 1998 {published data only}
- Fallowfield L, Lipkin M, Hall A. Teaching senior oncologists communication skills: results from phase I of a comprehensive longitudinal program in the United Kingdom. Journal of Clinical Oncology 1998;16(5):1961‐8. [DOI: 10.1200/JCO.1998.16.5.1961] [DOI] [PubMed] [Google Scholar]
Fallowfield 2001 {published data only}
- Fallowfield L, Saul J, Gilligan B. Teaching senior nurses how to teach communication skills in oncology. Cancer Nursing 2001;24(3):185‐91. [PUBMED: 11409062] [PubMed] [Google Scholar]
Fallowfield 2012 {published data only}
- Fallowfield LJ, Solis‐Trapala I, Jenkins VA. Evaluation of an educational program to improve communication with patients about early‐phase trial participation. Oncologist 2012;17(3):377‐83. [DOI: 10.1634/theoncologist.2011-0271; PUBMED: 22382459] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faulkner 1984 {published data only}
- Faulkner A, Maguire P. Teaching ward nurses to monitor cancer patients. Clinical Oncology 1984;10(4):383‐9. [PUBMED: 6509820] [PubMed] [Google Scholar]
Faulkner 1992 {published data only}
- Faulkner A. The evaluation of training programmes for communication skills in palliative care. Journal of Cancer Care 1992;1(2):75‐8. [Google Scholar]
Favre 2007 {published data only}
- Favre N, Despland JN, Roten Y, Drapeau M, Bernard M, Stiefel F. Psychodynamic aspects of communication skills training: a pilot study. Supportive Care in Cancer 2007;15(3):333‐7. [DOI: 10.1007/s00520-006-0150-6] [DOI] [PubMed] [Google Scholar]
Ferrell 1998a {published data only}
- Ferrell BR, Virani R, Grant M. HOPE. Home Care Outreach for Palliative Care Education. Cancer Practice 1998;6(2):79‐85. [PUBMED: 9573907] [DOI] [PubMed] [Google Scholar]
Ferrell 1998b {published data only}
- Ferrell BR, Virani R, Grant M. Improving end‐of‐life care education in home care. Journal of Palliative Medicine 1998;1(1):11‐9. [DOI: 10.1089/jpm.1998.1.11] [DOI] [PubMed] [Google Scholar]
File 2014 {published data only}
- File W, Bylund CL, Kesselheim J, Leonard D, Leavey P. Do pediatric hematology/oncology (PHO) fellows receive communication training?. Pediatric Blood & Cancer 2014;61(3):502‐6. [DOI: 10.1002/pbc.24742; PUBMED: 24039096] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fineberg 2005 {published data only}
- Fineberg IC. Preparing professionals for family conferences in palliative care: evaluation results of an interdisciplinary approach. Journal of Palliative Medicine 2005;8(4):857‐66. [DOI: 10.1089/jpm.2005.8.857] [DOI] [PubMed] [Google Scholar]
Finset 2003 {published data only}
- Finset A, Ekeberg O, Eide H, Aspegren K. Long term benefits of communication skills training for cancer doctors. Psycho‐Oncology 2003;12(7):686‐93. [DOI: 10.1002/pon.691] [DOI] [PubMed] [Google Scholar]
Fujimori 2003 {published data only}
- Fujimori M, Oba A, Koike M, Okamura M, Akizuki N, Kamiya M, et al. Communication skills training for Japanese oncologists on how to break bad news. Journal of Cancer Education 2003;18(4):194‐201. [DOI: 10.1207/s15430154jce1804_6] [DOI] [PubMed] [Google Scholar]
Fukui 2008 {published data only}
- Fukui S, Ogawa K, Ohtsuka M, Fukui N. Effect of communication skills training on nurses' detection of patients' distress and related factors after cancer diagnosis: a randomized study. Psycho‐Oncology 2009;18(11):1156‐64. [DOI: 10.1002/pon.1429] [DOI] [PubMed] [Google Scholar]
- Fukui S, Ogawa K, Ohtsuka M, Fukui N, Fukui S, Ogawa K, et al. A randomized study assessing the efficacy of communication skill training on patients' psychologic distress and coping: nurses' communication with patients just after being diagnosed with cancer. Cancer 2008;113(6):1462‐70. [DOI] [PubMed] [Google Scholar]
Gerhart 2016 {published data only}
- Gerhart J, O’Mahony S, Abrams I, Grosse J, Greene M, Levy M. A pilot test of a mindfulness‐based communication training to enhance resilience in palliative care professionals. Journal of Contextual Behavioral Science 2016;5:89‐96. [DOI: 10.1016/j.jcbs.2016.04.003] [DOI] [Google Scholar]
Gibon 2017 {published data only}
- Gibon A‐S, Durieux JF, Merckaert I, Delvaux N, Farvacques C, Libert Y, et al. Development of the LaComm 1.0, A French medical communication analysis software: a study assessing its sensitivity to change. Patient Education and Counseling 2017;100(2):297‐304. [DOI: 10.1016/j.pec.2016.08.005] [DOI] [PubMed] [Google Scholar]
Girgis 1997 {published data only}
- Girgis A, Sanson‐Fisher RW, McCarthy WH. Communicating with patients: surgeons' perceptions of their skills and need for training. Australia and New Zealand Journal of Surgery 1997;67(11):775‐80. [PUBMED: 9396993] [DOI] [PubMed] [Google Scholar]
Glimelius 1995 {published data only}
- Glimelius B, Birgegard G, Hoffman K, Kvale G, Sjödén PO. Information to and communication with cancer patients: Improvements and psychosocial correlates in a comprehensive care program for patients and their relatives. Patient Education and Counselling 1995;25(2):171‐82. [PUBMED: 7659630] [DOI] [PubMed] [Google Scholar]
Golding 2016 {published data only}
- Golding E, Lampkin A, Larach MW, Klima L. Effect of a communication skills intervention on resident comfort and knowledge of discussing goals of care and advance care planning. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology 2016;34(26):50. [Google Scholar]
Gordon 1995 {published data only}
- Gordon GH, Rust K. Evaluating a faculty development course on medical interviewing. In: Lipkin M, Putnam SM, Lazare A editor(s). The Medical Interview. New York, NY, USA: Springer‐Verlag, 1995:436‐47. [DOI: 10.1007/978-1-4612-2488-4_37] [DOI] [Google Scholar]
Gorniewicz 2017a {published data only}
- Gorniewicz J, Floyd M, Krishnan K, Bishop TW, Tudiver F, Lang F. Breaking bad news to patients with cancer: A randomized control trial of a brief communication skills training module incorporating the stories and preferences of actual patients. Patient Education and Counseling 2017; Vol. 100, issue 4:655‐66. [DOI: 10.1016/j.pec.2016.11.008; PUBMED: 27876220] [DOI] [PMC free article] [PubMed]
Gouveia 2017 {published data only}
- Gouveia L, Janvier A, Dupuis F, Duval M, Sultan S. Comparing two types of perspective taking as strategies for detecting distress amongst parents of children with cancer: a randomised trial. PLoS One 2017; Vol. 12, issue 4:e0175342. [DOI: 10.1371/journal.pone.0175342; PUBMED: 28384315] [DOI] [PMC free article] [PubMed]
Griffiths 2015 {published data only}
- Griffiths J, Wilson C, Ewing G, Connolly M, Grande G. Improving communication with palliative care cancer patients at home ‐ A pilot study of SAGE & THYME communication skills model. Eur J Oncol Nurs 2015;19(5):465‐72. [DOI: 10.1016/j.ejon.2015.02.005; PUBMED: 25782722] [DOI] [PubMed] [Google Scholar]
Gulbrandsen 2013 {published data only}
- Gulbrandsen P, Jensen BF, Finset A, Blanch‐Hartigan D. Long‐term effect of communication training on the relationship between physicians' self‐efficacy and performance. Patient Education and Counseling 2013;91(2):180‐5. [DOI: 10.1016/j.pec.2012.11.015; PUBMED: 23414658] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gutheil 2005 {published data only}
- Gutheil IA, Heyman JC. Communication between older people and their health care agents: results of an intervention. Health & Social Work 2005;30(2):107‐16. [DOI: 10.1093/hsw/30.2.107] [DOI] [PubMed] [Google Scholar]
Hainsworth 1996 {published data only}
- Hainsworth DS. The effect of death education on attitudes of hospital nurses toward care of the dying. Oncology Nursing Forum 1996;23(6):963‐7. [PUBMED: 8829166] [PubMed] [Google Scholar]
Hall 1999 {published data only}
- Hall P, Weaver L, Hupe D, Seely JF. Community‐based palliative care education: Can it improve care of the terminally ill?. Academic Medicine 1999;74(Suppl 10):S105‐7. [DOI] [PubMed] [Google Scholar]
Hallenbeck 1999 {published data only}
- Hallenbeck JL, Bergen MR. A medical resident inpatient hospice rotation: experiences with dying and subsequent changes in attitudes and knowledge. Journal of Palliative Medicine 1999;2(2):197‐208. [DOI: 10.1089/jpm.1999.2.197] [DOI] [PubMed] [Google Scholar]
Hallford 2011 {published data only}
- Hallford DJ, McCabe MP, Mellor D, Davison TE, Goldhammer DL, George K, et al. Intervention for depression among palliative care patients and their families: A study protocol for evaluation of a training program for professional carestaff. BMC Palliative Care 2011;10:11. [DOI: 10.1186/1472-684X-10-11; PUBMED: 21668988] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hamilton 2014 {published data only}
- Hamilton G, Ortega R, Hochstetler V, Pierson K, Lin P, Lowes S. Teaching communication skills to hospice teams: comparing the effectiveness of a communication skills laboratory with in‐person, second life, and phone role‐playing. American Journal of Hospice & Palliative Care 2014;31(6):611‐8. [DOI: 10.1177/1049909113504481; PUBMED: 24037542] [DOI] [PubMed] [Google Scholar]
Heaven 1995 {published data only}
- Heaven CM, Maguire GP. Training hospice nurses to elicit patient concerns. Psycho‐Oncology. Conference proceedings: British Psychosocial Oncology Group 11th Annual Conference. 1995; Vol. 4, issue 2:89.
Heaven 1996 {published data only}
- Heaven CM, Maguire P. Training hospice nurses to elicit patient concerns. Journal of Advanced Nursing 1996;23(2):280‐6. [PUBMED: 8708240] [DOI] [PubMed] [Google Scholar]
Hellbom 2001 {published data only}
- Hellbom M, Brandberg Y, Kurland J, Arving C, Thalén‐Lindström A, Glimelius B, et al. Assessment and treatment of psychosocial problems in cancer patients: an exploratory study of a course for nurses. Patient Education and Counseling 2001;45(2):101‐6. [PUBMED: 11687322] [DOI] [PubMed] [Google Scholar]
Hendricks‐Ferguson 2015 {published data only}
- Hendricks‐Ferguson VL, Kane JR, Pradhan KR, Shih CS, Gauvain KM, Baker JN, et al. Evaluation of physician and nurse dyad training procedures to deliver a palliative and end‐of‐life communication intervention to parents of children with a brain tumor. Journal of Pediatric Oncology Nursing 2015;32(5):337‐47. [DOI: 10.1177/1043454214563410; PUBMED: 25623029] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henoch 2013 {published data only}
- Henoch I, Danielson E, Strang S, Browall M, Melin‐Johansson C. Training intervention for health care staff in the provision of existential support to patients with cancer: A randomized, controlled study. Journal of Pain and Symptom Management 2013;46(6):785‐94. [10.1016/j.jpainsymman.2013.01.013; PUBMED: 23764108] [DOI] [PubMed] [Google Scholar]
Hietanen 2007 {published data only}
- Hietanen PS, Aro AR, Holli KA, Schreck M, Peura A, Joensuu HT. A short communication course for physicians improves the quality of patient information in a clinical trial. Acta Oncologica 2007;46(1):42‐8. [DOI: 10.1080/02841860600849067] [DOI] [PubMed] [Google Scholar]
Hill 2013 {published data only}
- Hill A. Compassionate communication training with cancer patients and caregivers: Empathy, self‐compassion, and well‐being. Alliant International University. San Francisco: Alliant International University, California School of Professional Psychology, 2012. [Google Scholar]
Hillen 2014 {published data only}
- Hillen MA, Haes HC, Stalpers LJ, Klinkenbijl JH, Eddes EH, Butow PN, et al. How can communication by oncologists enhance patients' trust? An experimental study. Annals of Oncology 2014;25(4):896‐901. [DOI: 10.1093/annonc/mdu027; PUBMED: 24615411] [DOI] [PubMed] [Google Scholar]
Hoffman 2002 {published data only}
- Hoffman M, Steinberg M. Development and implementation of a curriculum in communication skills and psycho‐oncology for medical oncology fellows. Journal of Cancer Education 2002;17(4):196‐200. [DOI: 10.1080/08858190209528837] [DOI] [PubMed] [Google Scholar]
Hulsman 1997 {published data only}
- Hulsman RL, Ros WJ, Janssen M, Winnubst JA. Interact Cancer. The development and evaluation of a computer‐assisted course on communication skills for medical specialists in oncology. Patient Education & Counseling 1997;30(2):129‐41. [PUBMED: 9128615] [DOI] [PubMed] [Google Scholar]
Hulsman 2002 {published data only}
- Hulsman RL, Ros WJ, Winnubst JA, Bensing JM. The effectiveness of a computer‐assisted instruction programme on communication skills of medical specialists in oncology. Medical Education 2002;36(2):125‐34. [DOI: ] [DOI] [PubMed] [Google Scholar]
Hundley 2008 {published data only}
- Hundley G. The effectiveness of "delivering unfavourable news to patients diagnosed with cancer" training program for oncologists in Uzbekistan. Dissertation Abstracts International Section A: Humanities and Social Sciences. Orlando, Florida: University of Central Florida, 2008; Vol. 2156, issue 69:6.
- Hundley G. The effectiveness of "delivering unfavourable news to patients diagnosed with cancer" training program for oncologists in Uzbekistan. PhD dissertation 2008; Vol. UMI Number: 3319247:iii‐15.
Jefford 2011 {published data only}
- Jefford M, Lotfi‐Jam K, Baravelli C, Grogan S, Rogers M, Krishnasamy M, et al. Development and pilot testing of a nurse‐led posttreatment support package for bowel cancer survivors. Cancer Nursing 2011;34(3):E1‐10. [DOI: 10.1097/NCC.0b013e3181f22f02] [DOI] [PubMed] [Google Scholar]
Johnson 2012 {published data only}
- Johnson D, Coulter P, Niemeyer B, Sandstedt E, Paffel A, Dorsey B, et al. Strengthening ambulatory palliative care support: the Advanced Illness Coordinated Care Program (P6). Journal of Pain and Symptom Management 2012;43(2):317‐8. [DOI: 10.1016/j.jpainsymman.2011.12.008] [DOI] [Google Scholar]
Johnson 2013 {published data only}
- Johnson LA, Gorman C, Morse R, Firth M, Rushbrooke S. Does communication skills training make a difference to patients' experiences of consultations in oncology and palliative care services?. European Journal of Cancer Care 2013;22(2):202‐9. [DOI: 10.1111/ecc.12014; PUBMED: 23121207] [DOI] [PubMed] [Google Scholar]
Jors 2016 {published data only}
- Jors K, Seibel K, Bardenheuer H, Buchheidt D, Mayer‐Steinacker R, Viehrig M, et al. Education in end‐of‐life care: What Do Experienced Professionals Find Important?. Journal of Cancer Education 2016;31(2):272‐8. [DOI: ; PUBMED: 25773135.J] [DOI] [PubMed] [Google Scholar]
Ju 2014 {published data only}
- Ju M, Berman AT, Hwang WT, Lamarra D, Baffic C, Suneja G, et al. Assessing interpersonal and communication skills in radiation oncology residents: A pilot standardized patient program. International Journal of Radiation Oncology, Biology, Physics 2014;88(5):1129‐35. [DOI: 10.1016/j.ijrobp.2014.01.007; PUBMED: 24661666] [DOI] [PubMed] [Google Scholar]
Ke 2008 {published data only}
- Ke LS, Chiu TY, Hu WY, Lo SS, Ke LS, Chiu TY, et al. Effects of educational intervention on nurses' knowledge, attitudes, and behavioral intentions toward supplying artificial nutrition and hydration to terminal cancer patients. Supportive Care in Cancer 2008;16(11):1265‐72. [DOI: 10.1007/s00520-008-0426-0] [DOI] [PubMed] [Google Scholar]
Keir 2013 {published data only}
- Keir A, Wilkinson D. Communication skills training in paediatrics. Journal of Paediatrics and Child Health 2013;49:624–8. [DOI: 10.1111/jpc.12216] [DOI] [PubMed] [Google Scholar]
Kelley 2012 {published data only}
- Kelley AS, Back AL, Arnold RM, Goldberg GR, Lim BB, Litrivis E, et al. Geritalk: Communication skills training for geriatric and palliative medicine fellows. Journal of the American Geriatric Society 2012;60(2):332‐7. [DOI: 10.1111/j.1532-5415.2011.03787.x.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinnane 2011 {published data only}
- Kinnane NA, Waters T, Aranda S. Evaluation of a pilot 'peer support' training programme for volunteers in a hospital‐based cancer information and support centre. Supportive Care in Cancer 2011;19(1):81‐90. [DOI: 10.1007/s00520-009-0791-3] [DOI] [PubMed] [Google Scholar]
Kortes‐Miller 2016 {published data only}
- Kortes‐Miller K, Jones‐Bonofiglio K, Hendrickson S, Kelley ML. Dying with Carolyn: using simulation to improve communication skills of unregulated care providers working in long‐term care. Journal of Applied Gerontology 2016;35(12):1259‐78. [DOI] [PubMed] [Google Scholar]
Kruse 2003 {published data only}
- Kruse J, Schmitz N, Woller W, Clar B, Meyer E, Grinschgl A, et al. [Effects of a psychosocial education programme to improve doctor‐patient interaction with cancer patients]. Zeitschrift für Psychosomatische Medizin und Psychotherapie 2003;49(3):232‐45. [PUBMED: 12964130] [DOI] [PubMed] [Google Scholar]
Kubota 2012 {published data only}
- Kubota K. Current status and future direction of communication skill training program. Annals of Oncology. 10th Annual Meeting of the Japanese‐Society‐of‐Medical‐Oncology (JSMO). 2012; Vol. 23.
Kubota 2015 {published data only}
- Kubota Y, Okuyama T, Uchida M, Umezawa S, Nakaguchi T, Sugano K, et al. Effectiveness of a psycho‐oncology training program for oncology nurses: A randomized controlled trial. Psycho‐Oncology 2015;25(6):712‐8. [DOI: 10.1002/pon.4000] [DOI] [PubMed] [Google Scholar]
Ladouceur 2003 {published data only}
- Ladouceur R, Goulet F, Gagnon R, Boulé R, Girard G, Jacques A, et al. Breaking bad news: impact of a continuing medical education workshop. Journal of Palliative Care 2003;19(4):238‐45. [PubMed] [Google Scholar]
La Monica 1987 {published data only}
- Monica EL, Madea AR, Oberst MT. Empathy and nursing care outcomes. Scholarly Inquiry for Nursing Practice 1987;1(3):197‐213. [PubMed] [Google Scholar]
Larbig 2009 {published data only}
- Larbig WD, David N, Prudlo U, Schlenker P. Psychosocial online counselling in breast cancer patients. Psycho‐Oncology Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625. 2009; Vol. 18:S233. [DOI: 10.1002/pon.1594] [DOI]
Lenzi 2011 {published data only}
- Lenzi R, Baile WF, Costantini A, Grassi L, Parker PA. Communication training in oncology: results of intensive communication workshops for Italian oncologists. European Journal of Cancer Care 2011;20(2):196‐203. [DOI: 10.1111/j.1365-2354.2010.01189.x] [DOI] [PubMed] [Google Scholar]
Libert 2003 {published data only}
- Libert Y, Janne P, Razavi D, Merckaert I, Scalliet P, Delvaux N, et al. Impact of medical specialists' locus of control on communication skills in oncological interviews. British Journal of Cancer 2003;88(4):502‐9. [DOI: 10.1038/sj.bjc.6600966] [DOI] [PMC free article] [PubMed] [Google Scholar]
Libert 2016 {published data only}
- Libert Y, Canivet D, Menard C, Achte L, Farvacques C, Merckaert I, et al. Predictors of physicians’ satisfaction with their management of uncertainty during a decision‐making encounter with a simulated advanced stage cancer patient. Patient Education and Counseling 2016;99(7):1121‐9. [DOI: 10.1016/j.pec.2016.01.008] [DOI] [PubMed] [Google Scholar]
Linder 1999 {published data only}
- Linder JF, Blais J, Enders SR, Melberg SE, Meyers FJ. Palliative education: a didactic and experiential approach to teaching end‐of‐life care. Journal of Cancer Education 1999;14(3):154‐60. [DOI: 10.1080/08858199909528607] [DOI] [PubMed] [Google Scholar]
Liu 2007 {published data only}
- Liu J, Mok E, Wong T, Xue L, Xu B. Evaluation of an integrated communication skills training program for nurses in cancer care in Beijing, China. Nursing Research 2007;56(3):202‐9. [DOI: 10.1097/01.NNR.0000270030.82736.8c] [DOI] [PubMed] [Google Scholar]
Lloyd‐Williams 1996 {published data only}
- Lloyd‐Williams M, Lloyd‐Williams F. Communication skills: the House Officer's perception. Journal of Cancer Care 1996;5(4):151‐3. [Google Scholar]
Loiselle 2011 {published data only}
- Loiselle CG. Training the next generation of researchers in psychosocial oncology: Research priorities and future directions. Psycho‐Oncology: Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625.. 2009; Vol. 18:S138. [DOI: 10.1002/pon.1594] [DOI]
Macauley 2011 {published data only}
- Macauley R, Billings JA. Teaching small groups in palliative care. Journal of Palliative Medicine 2011;14(1):91‐5. [DOI: 10.1089/jpm.2010.0215] [DOI] [PubMed] [Google Scholar]
Madhavan 2011 {published data only}
- Madhavan S, Sanders AE, Chou WYS, Shuster A, Boone KW, Dente MA, et al. Pediatric palliative care and eHealth: Opportunities for patient‐centered care. American Journal of Preventative Medicine 2011;5 (Suppl 2):S208‐216. [DOI: 10.1016/j.amepre.2011.01.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maguire 1996a {published data only}
- Maguire P, Faulkner A, Booth K, Elliott C, Hillier V. Helping cancer patients disclose their concerns. European Journal of Cancer 1996;32A(1):78‐81. [PUBMED: 8695247] [DOI] [PubMed] [Google Scholar]
Maguire 1996b {published data only}
- Maguire P, Booth K, Elliott C, Jones B. Helping health professionals involved in cancer care acquire key interviewing skills‐‐the impact of workshops. European Journal of Cancer 1996;32A(9):1486‐9. [PUBMED: 8911106] [DOI] [PubMed] [Google Scholar]
Martinez 2009 {published data only}
- Martinez LS, Schwartz JS, Freres D, Fraze T, Hornik RC. Patient‐clinician information engagement increases treatment decision satisfaction among cancer patients through feeling of being informed. Patient Education and Counseling 2009;77(3):384‐90. [DOI: 10.1016/j.pec.2009.09.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matrone 1990 {published data only}
- Matrone JS. The effect of a staff development program on nursing case management competencies and patient outcomes in the acute care setting. [PhD thesis] 1990; Vol. Boston College, USA.
Medendorp 2017 {published data only}
- Medendorp NM, Visser LNC, Hillen MA, Haes JCJM, Smets EMA. How oncologists' communication improves (analogue) patients' recall of information. A randomised video‐vignettes study. Patient Education and Counseling 2017;100(7):1338‐44. [DOI: 10.1016/j.pec.2017.02.012] [DOI] [PubMed] [Google Scholar]
Melo 2011 {published data only}
- Melo CG, Oliver D. Can addressing death anxiety reduce health care workers' burnout and improve patient care?. Journal of Palliative Care 2011;27(4):287‐95. [PUBMED: 22372283] [PubMed] [Google Scholar]
Meystre 2013 {published data only}
- Meystre C, Bourquin C, Despland JN, Stiefel F, Roten Y. Working alliance in communication skills training for oncology clinicians: A controlled trial. Patient Counseling and Health Education 2013;90(2):233‐8. [DOI: 10.1016/j.pec.2012.10.013] [DOI] [PubMed] [Google Scholar]
Morita 2014 {published data only}
- Morita T, Tamura K, Kusajima E, Sakai S, Kawa M, Imura C, et al. Nurse education program on meaninglessness in terminally III cancer patients: A randomized controlled study of a novel two‐day workshop. Journal of Palliative Medicine 2014;17(12):1298‐305. [DOI: 10.1089/jpm.2013.0559.] [DOI] [PubMed] [Google Scholar]
Nellis 2016 {published data only}
- Nellis ME, Howell JD, Ching K, Bylund C. The use of simulation to improve resident communication and personal experience at end‐of‐life care. Journal of Pediatric Intensive Care 2016;6(2):91–7. [DOI: 10.1055/s-0036-1584684] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Connor 2011 {published data only}
- O'Connor M, Fisher C, French L, Halkett G, Jiwa M, Hughes J. Exploring the community pharmacist's role in palliative care: Focusing on the person not just the prescription. Patient Education and Counseling 2011;83(3):458‐64. [DOI: 10.1016/j.pec.2011.04.037] [DOI] [PubMed] [Google Scholar]
Parle 1997 {published data only}
- Parle M, Maguire P, Heaven C. The development of a training model to improve health professionals' skills, self‐efficacy and outcome expectancies when communicating with cancer patients. Social Science & Medicine 1997;44(2):231‐40. [PUBMED: 9015875] [DOI] [PubMed] [Google Scholar]
Patel 2016 {published data only}
- Patel RV, Golding E, Lampkin A, Larach MW, Klima L. Effect of a communication skills intervention on resident comfort and knowledge of discussing goals of care and advance care planning. Journal of Clinical Oncology 2016;34(26_suppl):50‐50. [DOI: 10.1200/jco.2016.34.26_suppl.50] [DOI] [Google Scholar]
Pekmezaris 2011 {published data only}
- Pekmezaris R, Walia R, Nouryan C, Katinas L, Zeitoun N, Alano G, et al. The Impact of an end‐of‐life communication skills intervention on physicians‐in‐training. Gerontology & Geriatrics Education 2011;32(2):152‐64. [DOI: 10.1080/02701960.2011.572051] [DOI] [PubMed] [Google Scholar]
Pelayo 2011 {published data only}
- Pelayo M, Cebrian D, Areosa A, Agra Y, Izquierdo JV, Buendia F. Effects of online palliative care training on knowledge, attitude and satisfaction of primary care physicians. BMC Family Practice 2011;12:37. [DOI: 10.1186/1471-2296-12-37] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pieterse 2006 {published data only}
- Pieterse AH, Dulmen AM, Beemer FA, Ausems MG, Bensing JM. Tailoring communication in cancer genetic counseling through individual video‐supported feedback: a controlled pretest‐post test design. Patient Education and Counseling 2006;60(3):326‐35. [DOI: 10.1016/j.pec.2005.06.009] [DOI] [PubMed] [Google Scholar]
Rangachari 2014 {published data only}
- Rangachari D, Donehower RC. Across the cancer continuum: A communication skills curriculum for oncology fellows. Journal of Clinical Oncology 2014;32 (suppl)(15):1. [DOI: 10.1200/jco.2014.32.15_suppl.e20556] [DOI] [Google Scholar]
Rask 2009 {published data only}
- Rask MT, Jensen ML, Andersen J, Zachariae R. Effects of an intervention aimed at improving nurse‐patient communication in an oncology outpatient clinic. Cancer Nursing 2009;32(1):E1‐11. [DOI: 10.1097/01.NCC.0000343365.13871.12.] [DOI] [PubMed] [Google Scholar]
Razavi 1991 {published data only}
- Razavi D, Delvaux N, Farvacques C, Robaye E. Brief psychological training for health professionals dealing with cancer patients: a one‐year assessment. General Hospital Psychiatry 1991;13:253‐60. [DOI] [PubMed] [Google Scholar]
Razavi 2000 {published data only}
- Razavi D, Delvaux N, Marchal S, Cock M, Farvacques C, Slachmuylder JL. Testing health care professionals' communication skills: the usefulness of highly emotional standardized role‐playing sessions with simulators. Psycho‐Oncology 2000;9(4):293‐302. [DOI: ] [DOI] [PubMed] [Google Scholar]
Razavi 2009 {published data only}
- Razavi D. Physicians, team work and the cancer patient: The acquisition of communication skills as a promoter of care quality and as a professional stress antidote. Psycho‐Oncology Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625. 2009; Vol. 18:S6. [DOI: 10.1002/pon.1594] [DOI]
Rose 2008 {published data only}
- Rose JH, Radziewicz R, Bowmans KF, O'Toole EE. A coping and communication support intervention tailored to older patients diagnosed with late‐stage cancer. Clinical Interventions in Aging 2008;3(1):77–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenbloom 2007 {published data only}
- Rosenbloom SK, Victorson DE, Hahn EA, Peterman AH, Cella D. Assessment is not enough: a randomised controlled trial of the effects of HRQL assessment on quality of life and satisfaction in oncology clinical practice. Psycho‐Oncology 2007;16(12):1069‐79. [DOI] [PubMed] [Google Scholar]
Roter 1995 {published data only}
- Roter DL, Hall JA, Kern DE, Barker LR, Cole KA, Roca RP. Improving physicians' interviewing skills and reducing patients' emotional distress. A randomized clinical trial. Archives of Internal Medicine 1995;155(17):1877‐84. [DOI: 10.1001/archinte.1995.00430170071009] [DOI] [PubMed] [Google Scholar]
Rush 2015 {published data only}
- Rush CL, Darling M, Elliott MG, Febus‐Sampayo I, Kuo C, Munoz J, et al. Engaging Latina cancer survivors, their caregivers, and community partners in a randomized controlled trial: Nueva Vida intervention. Quality of Life Research 2015;24(5):1107‐18. [DOI: 10.1007/s11136-014-0847-9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rushton 2006 {published data only}
- Rushton CH, Reder E, Hall B, Comello K, Sellers DE, Hutton N. Interdisciplinary interventions to improve pediatric palliative care and reduce health care professional suffering. Journal of Palliative Medicine 2006;9(4):922‐33. [DOI: 10.1089/jpm.2006.9.922] [DOI] [PubMed] [Google Scholar]
Rutter 1996 {published data only}
- Rutter DR, Iconomou G, Quine L. Doctor‐patient communication and outcome in cancer patients: An intervention. Psychology & Health 1996;12(1):57‐71. [Google Scholar]
Schenker 2015 {published data only}
- Schenker Y, White D, Rosenzweig M, Chu E, Moore C, Ellis P, et al. Care management by oncology nurses to address palliative care needs: A pilot trial to assess feasibility, acceptability, and perceived effectiveness of the CONNECT intervention. Journal of Palliative Medicine 2015;18(3):232‐40. [DOI: 10.1089/jpm.2014.0325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitz 2016 {published data only}
- Schmitz CC, Braman JP, Turner N, Heller S, Radosevich DM, Yan Y, et al. Learning by (video) example: a randomized study of communication skills training for end‐of‐life and error disclosure family care conferences. American Journal of Surgery 2016; Vol. 212, issue 5:996‐1004. [DOI: 10.1016/j.amjsurg.2016.02.023; PUBMED: 27474496] [DOI] [PubMed]
Schulman‐Green 2003 {published data only}
- Schulman‐Green D. How do physicians learn to provide palliative care?. Journal of Palliative Care 2003;19(4):246‐52. [PubMed] [Google Scholar]
Shannon 2011 {published data only}
- Shannon SE, Long‐Sutehall T, Coombs M. Conversations in end‐of‐life care: Communication tools for critical care practitioners. Nursing in Critical Care 2011;16(3):124‐30. [DOI: 10.1111/j.1478-5153.2011.00456.x] [DOI] [PubMed] [Google Scholar]
Shields 2010 {published data only}
- Shields CG, Ziner KW, Bourff SA, Schilling K, Zhao Q, Monahan P, et al. An intervention to improve communication between breast cancer survivors and their physicians. Journal of Psychosocial Oncology 2010;28(6):610‐29. [DOI: 10.1080/07347332.2010.516811] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shipman 2008 {published data only}
- Shipman C, Burt J, Ream E, Beynon T, Richardson A, Addington‐Hall J. Improving district nurses' confidence and knowledge in the principles and practice of palliative care. Journal of Advanced Nursing 2008;63(5):494‐505. [DOI: 10.1111/j.1365-2648.2008.04729.x] [DOI] [PubMed] [Google Scholar]
Shorr 2000 {published data only}
- Shorr AF, Niven AS, Katz DE, Parker JM, Eliasson AH. Regulatory and educational initiatives fail to promote discussions regarding end‐of‐life care. Journal of Pain & Symptom Management 2000;19(3):168‐73. [DOI] [PubMed] [Google Scholar]
Singy 2012 {published data only}
- Singy P, Bourquin C, Sulstarova B, Stiefel F. The impact of communication skills training in oncology: A linguistic analysis. Journal of Cancer Education 2012;27(3):404‐8. [DOI: 10.1007/s13187-012-0385-5] [DOI] [PubMed] [Google Scholar]
Smith 1991 {published data only}
- Smith RC, Osborn G, Hoppe RB, Lyles JS, Egeren LV, Henry R, et al. Efficacy of a one‐month training block in psychosocial medicine for residents: a controlled study. Journal of General Internal Medicine 1991;6(6):535‐43. [DOI: 10.1007/BF02598223] [DOI] [PubMed] [Google Scholar]
Smith 2010 {published data only}
- Smith MY, DuHamel KN, Egert J, Winkel G. Impact of a brief intervention on patient communication and barriers to pain management: results from a randomised controlled trial. Patient Education and Counseling 2010;81(1):79‐86. [DOI: 10.1016/j.pec.2009.11.02] [DOI] [PubMed] [Google Scholar]
Song 2013 {published data only}
- Song L, Bensen JT, Zimmer C, Sleath B, Blackard B, Fontham E, et al. Patient‐health care provider communication among patients with newly diagnosed prostate cancer: Findings from a population‐based survey. Patient Education and Counseling 2013;91(1):79‐84. [DOI: 10.1016/j.pec.2012.12.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Street 2010 {published data only}
- Street RL, Slee C, Kalauokalani DK, Dean DE, Tancredi DJ, Kravitz RL. Improving physician‐patient communication about cancer pain with a tailored education‐coaching intervention. Patient Education and Counseling 2010;80(1):42‐7. [DOI: 10.1016/j.pec.2009.10.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Szmuilowicz 2010 {published data only}
- Szmuilowicz E, el‐Jawahri A, Chiappetta L, Kamdar M, Block S. Improving residents' end‐of‐life communication skills with a short retreat: a randomized controlled trial. Journal of Palliative Medicine 2010;13(4):439‐52. [DOI: 10.1089/jpm.2009.0262] [DOI] [PubMed] [Google Scholar]
Tannen 2014 {published data only}
- Tannen A. Communication skills training for health professionals working with people who have cancer. Cancer Nursing 2014;37(6):476‐7. [DOI: 10.1097/NCC.0000000000000210] [DOI] [PubMed] [Google Scholar]
Timmermans 2006 {published data only}
- Timmermans LM, Maazen RW, Leer JW, Kraaimaat FW. Palliative or curative treatment intent affects communication in radiation therapy consultations. Psycho‐Oncology 2006;15(8):713‐25. [DOI: 10.1002/pon.1008] [DOI] [PubMed] [Google Scholar]
- Timmermans LM, Maazen RW, Spaendonck KP, Leer JW, Kraaimaat FW. Enhancing patient participation by training radiation oncologists. Patient Education and Counseling 2006;63(1‐2):55‐63. [DOI: 10.1016/j.pec.2005.08.010] [DOI] [PubMed] [Google Scholar]
Ullrich 2011 {published data only}
- Ullrich P, Wollbruck D, Danker H, Singer S. Evaluation of psycho‐social training for speech therapists in oncology. Impact on general communication skills and empathy. A qualitative pilot study. Journal of Cancer Education 2011;26(2):294‐300. [DOI: 10.1007/s13187-010-0148-0] [DOI] [PubMed] [Google Scholar]
Vadaparampil 2016 {published data only}
- Vadaparampil ST, Gwede CK, Meade C, Kelvin J, Reich RR, Reinecke J, et al. ENRICH: a promising oncology nurse training program to implement ASCO clinical practice guidelines on fertility for AYA cancer patients. Patient Education and Counseling 2016; Vol. 99, issue 11:1907‐10. [DOI: 10.1016/j.pec.2016.05.013.] [DOI] [PMC free article] [PubMed]
Von Gunten 1998 {published data only}
- Gunten CF, Martinez J, Neely KJ, Twaddle M, Preodor M. Clinical experience in hospice and palliative medicine for clinicians in practice. Journal of Palliative Medicine 1998;1(3):249‐55. [DOI: 10.1089/jpm.1998.1.249] [DOI] [PubMed] [Google Scholar]
Walter 2016 {published data only}
- Walter J, Shah P, Odeniyi F, Madrigal V, Feudtner C. Teaching paediatric intensive care physicians communication skills: The enduring effects. Journal of Pain and Symptom Management 2016;51(2):431‐2. [DOI: 10.1016/j.jpainsymman.2015.12.048] [DOI] [Google Scholar]
Wetzel 2011 {published data only}
- Wetzel CM, George A, Hanna GB, Athanasiou T, Black SA, Kneebone RL, et al. Stress management training for surgeons‐a randomized, controlled, intervention study. Annals of Surgery 2011;253(3):488‐94. [DOI: 10.1097/SLA.0b013e318209a594] [DOI] [PubMed] [Google Scholar]
Wilkinson 1998 {published data only}
- Wilkinson S, Roberts A, Aldridge J. Nurse‐patient communication in palliative care: an evaluation of a communication skills programme. Palliative Medicine 1998;12(1):13‐22. [DOI: 10.1191/026921698675034697] [DOI] [PubMed] [Google Scholar]
Wilkinson 1999 {published data only}
- Wilkinson S, Bailey K, Aldridge J, Roberts A. A longitudinal evaluation of a communication skills programme. Palliative Medicine 1999;13(4):341‐8. [DOI: 10.1191/026921699672159169] [DOI] [PubMed] [Google Scholar]
Wilkinson 2003 {published data only}
- Wilkinson SM, Leliopoulou C, Gambles M, Roberts A. Can intensive three‐day programmes improve nurses' communication skills in cancer care?. Psycho‐Oncology 2003;12(8):747‐59. [DOI: 10.1002/pon.698] [DOI] [PubMed] [Google Scholar]
Wittenberg 2016 {published data only}
- Wittenberg E, Ferrell B, Goldsmith J, Ragan SL, Paice J. Assessment of a statewide palliative care team training course: COMFORT Communication for Palliative Care Teams. Journal of Palliative Medicine 2016;19(7):746‐52. [DOI: 10.1089/jpm.2015.0552; PUBMED: 27285175] [DOI] [PubMed] [Google Scholar]
Wong 2001 {published data only}
- Wong FK, Lee WM, Mok E. Educating nurses to care for the dying in Hong Kong: a problem‐based learning approach. Cancer Nursing 2001;24(2):112‐21. [DOI] [PubMed] [Google Scholar]
Wuensch 2011 {published data only}
- Wuensch A, Goelz T, Bertz H, Wirsching M, Fritzsche K. Disclosing information about randomised controlled trials in oncology: training concept and evaluation of an individualised communication skills training for physicians COM‐ON‐rct. European Journal of Cancer Care 2011;20(5):570‐6. [DOI] [PubMed] [Google Scholar]
- Wuensch A, Goelz T, Ihorst G, Terris DD, Bertz H, Bengel J, et al. Effects of individualised communication skills training on physicians' discussion of clinical trials in oncology: results from a randomised controlled trial. Unpublished manuscript 2011. [DOI] [PMC free article] [PubMed]
- Wuensch AG, Goelz T, Bertz H, Fritzsche K. Individualised communication skills training for randomised clinical trials and therapy options in oncology. Training concept and results of a RCT. Psycho‐Oncology Conference: 11th World Congress of Psycho‐Oncology of the International Psycho‐Oncology Society, IPOS Vienna Austria. Conference Start: 20090621 Conference End: 20090625. 2009; Vol. 18:S61. [DOI: 10.1002/pon.1594] [DOI]
Wuensch 2014 {published data only}
- Wuensch A, DeFigueiredo M, Golz T, Ihorst G, Bertz H, Fritzsche K. Individualised communication skills training for randomised clinical trials in oncology. Final results of a RCT. Oncology Research and Treatment 2014;37:27‐8. [Google Scholar]
Yildirim 2013 {published data only}
- Yildirim NK, Terakye G, Ozbas AA. Communication skills of nurses caring for oncology patients: A multicentric study. Psycho‐Oncology 2013;22:130. [Google Scholar]
References to studies awaiting assessment
Brock 2017 {published data only}
- Brock KE, Cohen HJ, Sourkes BM, Good JJ, Halamek LP. Training Pediatric Fellows in Palliative Care: a pilot comparison of simulation training and didactic education. Journal of Palliative Medicine 2017;20(10):1074‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Coltoff 2018 {published data only}
- Coltoff A, Lindenberger E, Kelley A, Gelfman L, Morrison L, Bickell N, et al. Efficacy of a novel communication skills intervention for practicing oOncologists. Journal of Pain and Symptom Management 2018;55(2):675‐6. [Google Scholar]
- Smith C, Annadurai V, Adelson K, Pintova S, Gonsky J, Egorova N, et al. Goals of care conversations in advanced cancer: patient perceptions versus reality. Journal of Pain and Symptom Management 2018;55(2):604‐5. [Google Scholar]
Henselmans 2018 {published data only}
- Henselmans I, Smets EMA, Haes JCJM, Dijkgraaf MGW, Vos FY, Laarhoven HWM. A randomised controlled trial of a skills training for oncologists and a communication aid for patients to stimulate shared decision making about palliative systemic treatment (CHOICE): study protocol. BMC Cancer 2018;18(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scholl 2018 {published data only}
- Scholl I, Hahlweg P, Lindig A, Bokemeyer C, Coym A, Hanken H, et al. Evaluation of a program for routine implementation of shared decision‐making in cancer care: study protocol of astepped wedge cluster randomised trial. Implementation Science 2018;13(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Berger‐Höger 2015 {published data only}
- Berger‐Höger B, Liethmann K, Mühlhauser I, Haastert B, Steckelberg A. Informed shared decision‐making supported by decision coaches for women with ductal carcinoma in situ: study protocol for a cluster randomised controlled trial. Trials 2015;16(1):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Figuereido 2015 {published data only}
- Figueiredo MN, Rodolph B, Bylund CL, Goelz T, Heußner P, Sattel H, et al. ComOn Coaching: Study protocol of a randomized controlled trial to assess the effect of a varied number of coaching sessions on transfer into clinical practice following communication skills training. BMC Cancer 2015;15(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo MN, Rudolph B, Bylund CL, Goelz T, Heußner P, Sattel H, et al. Erratum to: ComOn Coaching: study protocol of a randomised controlled trial to assess the effect of a varied number of coaching sessions on transfer into clinical practice following communication skills training. BMC Cancer 2015;15:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Libert 2017 {published data only}
- Libert Y, Peternelj L, Bragard I, Liénard A, Merckaert I, Reynaert C, et al. Communication about uncertainty and hope: A randomised controlled trial assessing the efficacy of a communication skills training program for physicians caring for cancer patients. BMC Cancer 2017;17(1):476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2016 {published data only}
- Parker PA, Banerjee SC, Matasar MJ, Bylund CL, Franco K, Li Y, et al. Protocol for a cluster randomised trial of a communication skills intervention for physicians to facilitate survivorship transition in patients with lymphoma. BMJ 2016;6:e011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: A Quality‐of‐Life Instrument for Use in International Clinical Trials in Oncology. Journal of the National Cancer Institute 1993;85(5):365‐76. [DOI] [PubMed] [Google Scholar]
ACGME 2009
- ACGME (Accreditiation Council for Graduate Medical Education). Common Program Requirements. ACGME 2009. Available from: http://www.acgme.org/acWebsite/dutyHours/dh_dutyhoursCommonPR07012007.pdf.
Adamo 2003
- Adamo G. Simulated and standardized patients in OSCEs: achievements and challenges 1992‐2003. Medical Teacher 2003;25(3):262‐70. [DOI] [PubMed] [Google Scholar]
Alvarez 2006
- Alvarez MP, Agra Y. Systematic review of educational interventions in palliative care for primary care physicians. Palliative Medicine 2006;20(7):673‐83. [DOI] [PubMed] [Google Scholar]
Arraras 2015
- Arraras JI, Kuljanic K, Sztankay M, Wintner LM, Costantini A, Chie WC, et al. Initial phases in the development of a European Organisation for Research and Treatment of Cancer communication‐specific module. Psycho‐Oncology 2015;24(2):236‐40. [DOI] [PubMed] [Google Scholar]
Ashworth 1984
- Ashworth CD, Williamson P, Montano D. A scale to measure physician beliefs about psychosocial aspects of patient care. Social Science & Medicine 1984;19(11):1235‐8. [DOI] [PubMed] [Google Scholar]
Aspegren 1999
- Aspegren K. BEME guide No. 2: Teaching and learning communication skills in medicine: a review with quality grading of articles. Medical Teaching 1999;21(6):563‐70. [DOI] [PubMed] [Google Scholar]
Back 2003
- Back AL, Arnold RM, Quill TE. Hope for the best, and prepare for the worst. Annals of Internal Medicine 2003;138(5):439‐43. [DOI] [PubMed] [Google Scholar]
Barth 2011
- Barth JL. Efficacy of communication skills training courses in oncology: A systematic review and meta‐analysis. Annals of Oncology 2011;22(5):1030‐40. [DOI] [PubMed] [Google Scholar]
Blanchard 1986
- Blanchard CG, Ruckdeschel JC, Fletcher BA, Blanchard EB. The impact of oncologists' behaviors on patient satisfaction with morning rounds. Cancer 1986;58(2):387‐93. [DOI] [PubMed] [Google Scholar]
Booth 1991
- Booth K, Maguire P. Development of a rating system to assess interaction between cancer patients and health professionals. Report to Cancer Research Campaign 1991:37.
Brandes 2015
- Brandes K, Linn AJ, Butow PN, Weert JC. The characteristics and effectiveness of Question Prompt List interventions in oncology: a systematic review of the literature. Psycho‐Oncology 2015;24(3):245‐52. [DOI] [PubMed] [Google Scholar]
Brown 1995
- Brown J Stewart M Tessier S. Assessing Communiction between Patients and doctors: A Manual for scoring Patient Centered Communication. Centre for studies in Family Medicine and Thames Valley Family Practice Research Unit 1995; Vol. Working paper Series:95‐102.
Bylund 2009
- Bylund CL, Brown RF, Lubrano di CB, Diamond C, Eddington J, Kissane DW. Assessing facilitator competence in a comprehensive communication skills training programme. Medical Education 2009;43(4):342‐9. [DOI] [PubMed] [Google Scholar]
CanMEDS 2011
- Royal College of Physicians and Surgeons of Canada. CanMEDS: better standards, better physicians, better care. Available at: http://www.royalcollege.ca/public/resources/aboutcanmeds 2011.
Cegala 2002
- Cegala DJ, Lenzmeier Broz S. Physician communication skills training: a review of theoretical backgrounds, objectives and skills. Medical Education 2002;36(11):1004‐16. [DOI] [PubMed] [Google Scholar]
Cella 1993
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of CancerTherapy scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11(3):570‐9. [DOI] [PubMed] [Google Scholar]
Chant 2002
- Chant S, Jenkinson T, Randle J, Russell G. Communication skills: some problems in nursing education and practice. Journal of Clinical Nursing 2002;11(1):12‐21. [DOI] [PubMed] [Google Scholar]
Cohen 1995
- Cohen SR, Mount BM, Strobel MG, Bui F. The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliative Medicine 1995;9(3):207‐19. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
Del Piccolo 2011
- Piccolo L, Haes H, Heaven C, Jansen J, Verheul W, Bensing J, et al. Development of the Verona coding definitions of emotional sequences to code health providers'responses (VR‐CoDES‐P) to patient cues and concerns. Patient Education and Counseling 2011;82(2):149‐55. [DOI] [PubMed] [Google Scholar]
Derogatis 1977
- Derogatis I. Administration, Scoring and Procedures Manual for the Revised Version of Other Instruments of the Psychopathology Rating Scale Series. Baltimore: Baltimore Md: Clinical Psychometrics Research Unit, John Hopkins Univsersity of Medicine, 1977. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(Pt A):177‐88. [DOI] [PubMed] [Google Scholar]
Dickson 2012
- Dickson RP, Engelberg RA, Back AL, Ford DW, Curtis JR. Internal medicine trainee self‐assessments of end‐of‐life communication skills do not predict assessments of patients, families, or clinician‐evaluators. Journal of Palliative Medicine 2012;15(4):418‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donovan‐Kicken 2011
- Donovan‐Kicken E, Caughlin JP. Breast cancer patients' topic avoidance and psychological distress: the mediating role of coping. Journal of Health Psychology 2011;16(4):596‐606. [DOI] [PubMed] [Google Scholar]
Dowsett 2000
- Dowsett SM, Saul JL, Butow PN, Dunn SM, Boyer MJ, Findlow R, et al. Communication styles in the cancer consultation: preferences for a patient‐centred approach. Psycho‐Oncology 2000;9(2):147‐56. [DOI] [PubMed] [Google Scholar]
Fallowfield 1990
- Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ 1990;301(6752):575‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fallowfield 1995
- Fallowfield, L. Communication skills for oncologists. Clinical Medicine 1995;5(1):99‐103. [Google Scholar]
Fallowfield 2004
- Fallowfield L, Jenkins V. Communicating sad, bad, and difficult news in medicine. Lancet 2004;363(9405):24. [DOI] [PubMed] [Google Scholar]
Ford 2000
- Ford S, Hall A, Ratcliffe D, Fallowfield L. The Medical Interaction Process System (MIPS): an instrument for analysing interviews of oncologists and patients with cancer. Social Science & Medicine 2000;50(4):553‐66. [DOI] [PubMed] [Google Scholar]
Fujimori 2007
- Fujimori M, Akechi T, Morita T, Inagaki M, Akizuki N, Sakano Y, et al. Preferences of cancer patients regarding the disclosure of bad news. Psycho‐Oncology 2007;16(6):573‐81. [DOI] [PubMed] [Google Scholar]
Fujimori 2009
- Fujimori M, Uchitomi Y. Preferences of cancer patients regarding communication of bad news: a systematic literature review. Japanese Journal of Clinical Oncology 2009;39(4):201‐16. [DOI] [PubMed] [Google Scholar]
Gattellari 2001
- Gattellari M, Butow PN, Tattersall MH. Sharing decisions in cancer care. Social Science & Medicine 2001;52(12):1865‐78. [DOI] [PubMed] [Google Scholar]
Gibon 2010
- Gibon AS, Durieux JF. Development of the Lacomm, a French medical communication analysis software: A study assessing its sensitivity to change. Psycho‐Oncology. http://www.lacomm.be/index.php, 2010; Vol. 19(Suppl.2):Oral Abstracts S133.
Girgis 2009
- Girgis A, Breen S, Stacey F, Lecathelinais C. Impact of two supportive care interventions on anxiety, depression, quality of life, and unmet needs in patients with non localized breast and colorectal cancers. Journal of Clinical Oncology 2009;27(36):6180‐90. [DOI] [PubMed] [Google Scholar]
GMC 2009
- General Medical Council. Tomorrow's Doctors. Available from: http://www.gmc‐uk.org/education/undergraduate/tomorrows_doctors_2009.asp.
Gray‐Toft 1981
- Gray‐Toft P, Anderson J. The Nursing Stress Scale: Development of an instrument. Journal of Behavioral Assessment 1981;3(1):11‐23. [Google Scholar]
Gyawali 2015
- Gyawali B, Tsukuura H, Honda K, Shimokata T, Ando Y. Some questions on the randomised controlled trial of communication skills training for oncologists. Journal of Clinical Oncology 2015;33(2):22. [DOI] [PubMed] [Google Scholar]
Gysels 2004
- Gysels M, Richardson A, Higginson IJ. Communication training for health professionals who care for patients with cancer: a systematic review of effectiveness (Provisional abstract). Supportive Care in Cancer 2004;12(10):692‐700. [DOI] [PubMed] [Google Scholar]
Hack 2005
- Hack TF, Degner LF, Parker PA. The communication goals and needs of cancer patients: a review. Psycho‐Oncology 2005;14(10):831‐45. [DOI] [PubMed] [Google Scholar]
Hack 2012
- Hack TF, Ruether JD, Pickles T, Bultz BD, Chateau D, Degner LF. Behind closed doors II: systematic analysis of prostate cancer patients' primary treatment consultations with radiation oncologists and predictors of satisfaction with communication. Psycho‐Oncology 2012;21(8):807‐17. [DOI: 10.1002/pon.1984] [DOI] [PubMed] [Google Scholar]
Hagerty 2005
- Hagerty RG, Butow PN, Ellis PM, Dimitry S, Tattersall MH. Communicating prognosis in cancer care: a systematic review of the literature. Annals of Oncology 2005;16(7):1005‐53. [DOI] [PubMed] [Google Scholar]
Heaven 2001
- Heaven C. The role of clinical supervision in communication skills. Vol. PhD thesis, Manchester: University of Manchester, 2001. [Google Scholar]
Henbest 1990
- Henbest RJ, Stewart M. Patient‐centredness in the consultation. 2: Does it really make a difference?. Family Practice 1990;7(1):28‐33. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org, 2011. [Google Scholar]
Hjermstad 1995
- Hjermstad MJ, Fossa SD, Bjordal K, Kaasa S. Test/retest study of the European Organization for Research and Treatment of Cancer Core Quality‐of‐Life Questionnaire. Journal of Clinical Oncology 1995;13(5):1249‐54. [DOI] [PubMed] [Google Scholar]
Hubbard 2008
- Hubbard G, Kidd L, Donaghy E. Preferences for involvement in treatment decision making of patients with cancer: a review of the literature. European Journal of Oncology Nursing 2008;12(4):299‐318. [DOI] [PubMed] [Google Scholar]
Ishikawa 2002
- Ishikawa H, Takayama T, Yamazaki Y, Seki Y, Katsumata N, Aoki Y. The interaction between physician and patient communication behaviors in Japanese cancer consultations and the influence of personal and consultation characteristics. Patient Education and Counseling 2002;46(4):277‐85. [DOI] [PubMed] [Google Scholar]
Jansen 2008
- Jansen J, Weert J, Meulen N, Dulmen S, Heeren T, Bensing J. Recall in older cancer patients: measuring memory for medical information. Gerontologist 2008;48(2):149‐57. [DOI] [PubMed] [Google Scholar]
Julian 2011
- Julian LJ. Measures of anxiety: State‐Trait Anxiety Inventory (STAI), Beck Anxiety Inventory (BAI), and Hospital Anxiety and Depression Scale‐Anxiety (HADS‐A). Arthritis Care Research 2011;63(Suppl 11):S467‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kinnersley 2007
- Kinnersley P, Edwards A, Hood K, Cadbury N, Ryan R, Prout H, et al. Interventions before consultations for helping patients address their information needs. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004565.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kissane 2012
- Kissane DW, Bylund CL, Banerjee SC, Bialer PA, Levin TT, Maloney EK, et al. Communication skills training for oncology professionals. Journal of Clinical Oncology 2012;30(11):1242‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurtz 2005
- Kurtz S, J Silverman, Draper J. Teaching and Learning Communication Skills in Medicine. Radcliffe Medical Press, 2005. [Google Scholar]
LaComm
- La Comm, A French Communication Analysis Software. http://www.lacomm.be/Ressources/documents/htmlPosterVenise1.php Accessed 21/09/2012:1‐3.
Lang 2004
- Lang F, McCord R, Harvill L, Anderson DS. Communication assessment using the common ground instrument: psychometric properties.. Family Medicine 2004;36(3):189‐98. [PubMed] [Google Scholar]
Langendam 2013
- Langendam MW, Akl EA, Dahm P, Glasziou P, Guyatt G, Schunemann HJ. Assessing and presenting summaries of evidence in Cochrane Reviews. Systematic Reviews 2013;23(2):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levinson 1997
- Levinson W, Roter DL, Mulhooly JP, Dull V, Frankel R. Physician‐patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA: the Journal of the American Medical Association 1997;277(7):553‐9. [DOI] [PubMed] [Google Scholar]
Lussier 2005
- Lussier MT, Richard C. Doctor‐patient communication: complaints and legal actions. Canadian Family Physician 2005;51:37‐9. [PMC free article] [PubMed] [Google Scholar]
Mack 2009
- Mack JW, Block SD, Nilsson M, Wright A, Trice E, Friedlander R, et al. Measuring therapeutic alliance between oncologists and patients with advanced cancer: the Human Connection Scale. Cancer 2009;115(14):3302‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maly 1998
- Maly RC, Frank JC, Marshall GN, DiMatteo MR, Reuben DB. Perceived efficacy in patient‐physician interactions (PEPPI): validation of an instrument in older persons. Journal of the American Geriatrics Society 1998;46(7):889‐94. [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2011
- Moore P, Vargas A, Nunez S, Macchiavello S. [A study of hospital complaints and the role of the doctor‐patient communication]. Revista Medica de Chile 2011;139(7):880‐5. [PubMed] [Google Scholar]
Nestel 2007
- Nestel D, Tierney T. Role‐play for medical students learning about communication: guidelines for maximising benefits. BMC Medical Education 2007;7:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ong 1998
- Ong LM, Visser MR, Kruyver IP, Bensing JM, Brink‐Muinen, Stouthard JM, et al. The Roter Interaction Analysis System (RIAS) in oncological consultations: psychometric properties. Psycho‐Oncology 1998;7(5):387‐401. [DOI] [PubMed] [Google Scholar]
Paul 2009
- Paul CL, Clinton‐McHarg T, Sanson‐Fisher RW, Douglas H, Webb G. Are we there yet? The state of the evidence base for guidelines on breaking bad news to cancer patients. European Journal of Cancer 2009;45(17):2960‐6. [DOI] [PubMed] [Google Scholar]
Poinsot 2006
- Poinsot R, Altmeyer A, Conroy T, Savignoni A, Asselain B, Léonard I, et al. Multisite validation study of questionnaire assessing out‐patient satisfaction with care questionnaire in ambulatory chemotherapy or radiotherapy treatment. Bulletin du Cancer 2006;93(3):315‐27. [PubMed] [Google Scholar]
Ramirez 1995
- Ramirez AJ, Graham J, Richards MA, Cull A, Gregory WM, Leaning MS, et al. Burnout and psychiatric disorder among cancer clinicians. British Journal of Cancer 1995;71(6):1263‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1994
- Roberts CS, Cox CE, Reintgen DS, Baile WF, Gibertini M. Influence of physician communication on newly diagnosed breast patients' psychologic adjustment and decision‐making. Cancer 1994;74(1 Suppl):336‐41. [DOI] [PubMed] [Google Scholar]
Roter 2002
- Roter D, Larson S. The Roter interaction analysis system (RIAS): utility and flexibility for analysis of medical interactions. Patient Education and Counselling 2002;46(4):243‐51. [DOI] [PubMed] [Google Scholar]
Salmon 2017
- Salmon P, Young B. A new paradigm for clinical communication: critical review of literature in cancer care. Medical Education 2017;51(3):258‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanson‐Fisher 2000
- Sanson‐Fisher R, Grigis A, Boyes A, Bonevski B, Burton L, Cook P. Supportive Care Review Group. The unmet supportive care needs of patients with cancer. Cancer 2000;88(1):226‐37. [DOI] [PubMed] [Google Scholar]
Schaulell 1993
- Schaulell WB, van Dierendonk. The construct validity of two burnout measures. Journal of Organizational Behaviour 1993;14(7):631‐47. [Google Scholar]
Sepucha 2010
- Sepucha K, Ozanne EM. How to define and measure concordance between patients' preferences and medical treatments: A systematic review of approaches and recommendations for standardization. Patient Education and Counselling 2010;78(1):12‐23. [DOI] [PubMed] [Google Scholar]
Shields 2009
- Shields CG, Coker CJ, Poulsen SS, Doyle JM, Fiscella K, Epstein RM, et al. Patient‐centered communication and prognosis discussions with cancer patients. Patient Education and Counseling 2009;77(3):437‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silberfarb 1980
- Silberfarb PM, Levine PM. Psychosocial aspects of neoplastic disease. III. Group support for the oncology nurse. General Hospital Psychiatry 1980;2(3):192‐7. [DOI] [PubMed] [Google Scholar]
Silverman 2005
- Silverman J, Kurtz S, Draper J. Skills for Communicating with Patients. 2nd Edition. Oxford: Radcliffe Medical Press, 2005. [Google Scholar]
Silverman 2017
- Silverman J, Weel‐Baumgarten E, Butow P, Fallowfield L, Bylund C, Deveugele M, et al. A new paradigm or a mis‐representation of current communication research and teaching?. Medical Education 2017;51(12):1289‐90. [DOI] [PubMed] [Google Scholar]
Snaith 1986
- Snaith RP, Zigmond AS. The hospital anxiety and depression scale. BMJ (Clinical Research and Education) 1986;292(6516):344. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 2017
- Solomon R, Smith C, Kallio J, Fenollosa A, Benerofe B, Jones L, et al. Speaking Up: How patient and physician voices shaped a trial to improve goals‐of‐care discussions. Patient 2017;10(4):489‐501. [DOI] [PubMed] [Google Scholar]
Speilberger 1983
- Spielberger CD, Gorssuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc, 1983. [Google Scholar]
Stewart 1989
- Stewart MA, Brown JB, Weston WW. Patient‐centred interviewing part III: Five provocative questions. Canadian Family Physician 1989;35:159‐61. [PMC free article] [PubMed] [Google Scholar]
Stewart 1996
- Stewart MA. Effective physician‐patient communication and health outcomes: a review. Canadian Medical Association Journal 1996;152(9):1423‐33. [PMC free article] [PubMed] [Google Scholar]
Stiefel 2010
- Stiefel F, Barth J, Bensing J, Fallowfield L, Jost L, Razavi D, et al. Communication skills training in oncology: a position paper based on a consensus meeting among European experts in 2009. Annals of Oncology 2010;21(2):204‐7. [DOI] [PubMed] [Google Scholar]
Street 2003
- Street RL Jr, Krupat E, Bell RA, Kravitz RL, Haidet P. Beliefs about control in the physician‐patient relationship: effect on communication in medical encounters. Journal of the American Geriatrics Society 2003;18(8):609‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Street 2007
- Street RL Jr, Gordon H, Haidet P. Physicians' communication and perceptions of patients: is it how they look, how they talk, or is it just the doctor?. Social Science & Medicine 2007;65(3):586‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stubenrauch 2012
- Stubenrauch S, Schneid EM, Wunsch A, Helmes A, Bertz H, Fritzsche K, et al. Development and evaluation of a checklist assessing communication skills of oncologists: the COM‐ON‐Checklist. Journal of Evaluation in Clinical Practice 2012;18(2):225‐30. [DOI] [PubMed] [Google Scholar]
Tariman 2010
- Tariman JD, Berry DL, Cochrane B, Doorenbos A, Schepp K. Preferred and actual participation roles during health care decision making in persons with cancer: a systematic review. Annals of Oncology 2010;21(6):1145‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2011
- Taylor J. The moral aesthetics of simulated suffering in standardized patient performances. Culture, Medicine and Psychiatry 2011;35(2):134‐62. [DOI] [PubMed] [Google Scholar]
Turnberg 1997
- Turnberg L. Improving Communication etween Doctors and Patients. Royal College of Physicians 1997. [PMC free article] [PubMed]
Uitterhoeve 2010
- Uitterhoeve RJ, Bensing JM, Grol RP, Demulder PHM, Achterberg T. The effect of communication skills training on patient outcomes in cancer care: a systematic review of the literature. European Journal of Cancer Care 2010;19(4):442‐57. [DOI] [PubMed] [Google Scholar]
Vail 2011
- Vail L, Sandhu H, Fisher J, Cooke H, Dale J, Barnett M. Hospital consultants breaking bad news with simulated patients: an analysis of communication using the Roter Interaction Analysis System. Patient Education and Counselling 2011;83(2):185‐94. [DOI] [PubMed] [Google Scholar]
van Weert 2009
- Weert JC, Jansen J, Bruijn GJ, Noordman J, van DS, Bensing JM. QUOTEchemo: a patient‐centred instrument to measure quality of communication preceding chemotherapy treatment through the patient's eyes. European Journal of Cancer 2009;45(17):2967‐76. [DOI] [PubMed] [Google Scholar]
Vogel 2009
- Vogel BA, Leonhart R, Helmes AW. Communication matters: the impact of communication and participation in decision making on breast cancer patients' depression and quality of life. Patient Education and Counselling 2009;77(3):391‐7. [DOI] [PubMed] [Google Scholar]
Ware 1983
- Ware JE Jr, Snyder MK, Wright WR, Davies AR. Defining and measuring patient satisfaction with medical care. Evaluation and Program Planning 1983;6(3‐4):247‐63. [DOI] [PubMed] [Google Scholar]
Wilkinson 1991
- Wilkinson S. Factors influencing nurses’ verbal communication with cancer patients. Vol. PhD Thesis, Manchester: University of Manchester, 1991. [Google Scholar]
Williams 1988
- Williams P. A Users’ guide to the general health questionnaire. Windsor, Berks: NFER‐Nelson, 1988. [Google Scholar]
Williams 1998
- Williams GC, Rodin GC, Ryan RM, Grolnick WS, Deci EL. Autonomous regulation and long‐term medication adherence in adult outpatients. Health Psychology 1998;17(3):269‐76. [DOI] [PubMed] [Google Scholar]
Zimmermann 2011
- Zimmermann C, Piccolo L, Bensing J, Bergvik S, Haes H, Eide H, et al. Coding patient emotional cues and concerns in medical consultations: the Verona coding definitions of emotional sequences (VR‐CoDES). Patient Education and Counseling 2011;82(2):141‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Fellowes 2002
- Fellowes D, Wilkinson SSM, Moore PM. Communication skills training for health care professionals working with cancer patients, their families and/or carers. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD003751] [DOI] [PubMed] [Google Scholar]
Moore 2004
- Moore PM, Wilkinson SSM, Rivera Mercado S. Communication skills training for health care professionals working with cancer patients, their families and/or carers. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003751.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2013
- Moore PM, Rivera Mercado S, Grez Artigues M, Lawrie TA. Communication skills training for healthcare professionals working with people who have cancer. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD003751.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


