Abstract
Background
This review focuses on non‐dispensing services from pharmacists, i.e. pharmacists in community, primary or ambulatory‐care settings, to non‐hospitalised patients, and is an update of a previously‐published Cochrane Review.
Objectives
To examine the effect of pharmacists' non‐dispensing services on non‐hospitalised patient outcomes.
Search methods
We searched CENTRAL, MEDLINE, Embase, two other databases and two trial registers in March 2015, together with reference checking and contact with study authors to identify additional studies. We included non‐English language publications. We ran top‐up searches in January 2018 and have added potentially eligible studies to 'Studies awaiting classification'.
Selection criteria
Randomised trials of pharmacist services compared with the delivery of usual care or equivalent/similar services with the same objective delivered by other health professionals.
Data collection and analysis
We used standard methodological procedures of Cochrane and the Effective Practice and Organisation of Care Group. Two review authors independently checked studies for inclusion, extracted data and assessed risks of bias. We evaluated the overall certainty of evidence using GRADE.
Main results
We included 116 trials comprising 111 trials (39,729 participants) comparing pharmacist interventions with usual care and five trials (2122 participants) comparing pharmacist services with services from other healthcare professionals. Of the 116 trials, 76 were included in meta‐analyses. The 40 remaining trials were not included in the meta‐analyses because they each reported unique outcome measures which could not be combined. Most trials targeted chronic conditions and were conducted in a range of settings, mostly community pharmacies and hospital outpatient clinics, and were mainly but not exclusively conducted in high‐income countries. Most trials had a low risk of reporting bias and about 25%‐30% were at high risk of bias for performance, detection, and attrition. Selection bias was unclear for about half of the included studies.
Compared with usual care, we are uncertain whether pharmacist services reduce the percentage of patients outside the glycated haemoglobin target range (5 trials, N = 558, odds ratio (OR) 0.29, 95% confidence interval (CI) 0.04 to 2.22; very low‐certainty evidence). Pharmacist services may reduce the percentage of patients whose blood pressure is outside the target range (18 trials, N = 4107, OR 0.40, 95% CI 0.29 to 0.55; low‐certainty evidence) and probably lead to little or no difference in hospital attendance or admissions (14 trials, N = 3631, OR 0.85, 95% CI 0.65 to 1.11; moderate‐certainty evidence). Pharmacist services may make little or no difference to adverse drug effects (3 trials, N = 590, OR 1.65, 95% CI 0.84 to 3.24) and may slightly improve physical functioning (7 trials, N = 1329, mean difference (MD) 5.84, 95% CI 1.21 to 10.48; low‐certainty evidence). Pharmacist services may make little or no difference to mortality (9 trials, N = 1980, OR 0.79, 95% CI 0.56 to 1.12, low‐certaintly evidence).
Of the five studies that compared services delivered by pharmacists with other health professionals, no studies evaluated the impact of the intervention on the percentage of patients outside blood pressure or glycated haemoglobin target range, hospital attendance and admission, adverse drug effects, or physical functioning.
Authors' conclusions
The results demonstrate that pharmacist services have varying effects on patient outcomes compared with usual care. We found no studies comparing services delivered by pharmacists with other healthcare professionals that evaluated the impact of the intervention on the six main outcome measures. The results need to be interpreted cautiously because there was major heterogeneity in study populations, types of interventions delivered and reported outcomes.There was considerable heterogeneity within many of the meta‐analyses, as well as considerable variation in the risks of bias.
Plain language summary
Can services delivered by pharmacists improve patient health?
What is the aim of this review?
To test whether services provided by pharmacists improve patient health. We identified 116 studies to answer this question.
Key messages
Some services provided by pharmacists can have positive effects on patient health, including improved management of blood pressure and physical function. The pharmacist services did not reduce hospital visits or admissions. Services delivered by pharmacists produced similar effects on patient health compared with services delivered by other healthcare professionals.
What was studied in the review?
Pharmacists deliver a wide range of services to patients. We need to know which pharmacist services are effective in helping patients to improve their health. This review included studies of pharmacist services for a wide range of conditions including high blood pressure and diabetes. The review measured the effect of these services on benefits (improved health outcomes) as well as harms (unplanned hospital admissions, adverse drug effects).
What are the main results of the review?
We found 116 relevant studies which involved 41,851 participants. Studies were conducted in 25 countries with the USA, UK, Canada and Australia contributing most studies. Many were conducted in community pharmacies (chemist shops) and hospital outpatient clinics. The studies compared services delivered by pharmacists with either usual care or with care delivered by other health professionals. The studies were of overall high quality, although some had problems because they did not include all the relevant information needed to assess quality.
Of the 111 studies that compared pharmacist services with usual care, 47 studies reported the most important outcomes. Compared with usual care, pharmacist services may reduce the percentage of patients whose blood pressure is outside the target range. It is uncertain whether services delivered by pharmacists reduce the number of patients with glycated haemoglobin levels outside the target range, because the certainty of the evidence is very low. Pharmacist services may make little or no difference to hospital attendance or admissions or to adverse drug effects or to death rates. Pharmacist services may slightly improve physical functioning.
We found no studies comparing services delivered by pharmacists with other healthcare professionals that evaluated the impact of the intervention on the six main outcome measures.
How up‐to‐date is this review?
We searched for studies that had been published up to March 2015. We ran top‐up searches in January 2018 and have added potentially eligible studies to 'Studies awaiting classification'.
Summary of findings
Summary of findings for the main comparison. Pharmacists' non‐dispensing roles targeting non‐hospitalised patients compared with the delivery of no comparable service for health problem or population.
| Pharmacists' non‐dispensing roles targeting non‐hospitalised patients compared with the delivery of no comparable service for health problem or population | |||||
| Patient or population: Health problem or population Setting: Outpatient settings Intervention: Pharmacist services targeting patients Comparison: Delivery of no comparable service | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Risk with the delivery of no comparable service | Risk with Pharmacist services targeting patients | ||||
| % outside blood pressure range | Study population | OR 0.40 (0.29 to 0.55) | 4107 (18 randomised trials) | ⊕⊕⊝⊝a,b,c,d LOW | |
| 550 per 1000 | 328 per 1000 (261 to 402) | ||||
| % outside HbA1c range | Study population | OR 0.29 (0.04 to 2.22) | 558 (5 randomised trials) | ⊕⊝⊝⊝b,d,e,f VERY LOW | |
| 782 per 1000 | 509 per 1000 (125 to 888) | ||||
| Hospital attendance/admission | Study population | OR 0.85 (0.65 to 1.11) | 3631 (14 randomised trials) | ⊕⊕⊕⊝b MODERATE | |
| 214 per 1000 | 188 per 1000 (150 to 232) | ||||
| Adverse drug effects | Study population | OR 1.65 (0.84 to 3.24) | 590 (3 randomised trials) | ⊕⊕⊝⊝b,g LOW | |
| 139 per 1000 | 211 per 1000 (120 to 344) | ||||
| SF‐36 Physical Functioning | The mean SF‐36 Physical Functioning was 53.2 | MD 5.84 higher (1.21 higher to 10.48 higher) | ‐ | 1329 (7 randomised trials) | ⊕⊕⊝⊝b,g LOW |
| Mortality | Study population | ||||
| 137 per 1000 | 111 per 1000 (81 to 150) | OR 0.79 (0.56, 1.12) | 1980 (9 randomised trials) | ⊕⊕⊝⊝b,g LOW | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; MD: Mean difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aWe downgraded the evidence by one level because of serious inconsistency. bWe downgraded the evidence by one level because of serious indirectness of evidence. cWe downgraded the evidence by one level because of suspected publication bias. dWe upgraded the evidence by one level because of the magnitude of the effect. eWe downgraded the evidence by two levels because of very serious inconsistency. fWe downgraded the evidence by two levels because of very serious imprecision. gWe downgraded the evidence by one level because of serious imprecision.
Background
The roles of pharmacists in patient care have expanded from the traditional tasks of dispensing medications and providing basic medication counselling to working with other health professionals and the public. This has led to greater involvement of pharmacists across full health systems including in community pharmacies, general medical practices and hospitals. Recent systematic reviews have identified benefits of pharmacist‐provided services in terms of patient outcomes and have included the effect of pharmacists in low‐income countries (Pande 2013), targeting patients with specific conditions (Greer 2016; Koshman 2008) and risk factors (Altowaijri 2013; Charrois 2012) at specific stages in their journey of care (Mekonnen 2016; Walsh 2016) and with specific services (Hatah 2014; Jokanovic 2017). This systematic review focuses on services provided by pharmacists to non‐hospitalised patients, i.e. individuals living in community or ambulatory‐care settings, with any clinical condition. The previous version of this review (Nkansah 2010) included interventions to influence patient outcome and healthcare professional behaviour. Due to the high numbers of new eligible studies that were identified for this update, the review was split and this current version includes only trials which report the effect of pharmacist interventions on patient outcome.
Description of the condition
We cover a wide range of health conditions in this review, including chronic diseases, e.g. hypertension, diabetes, asthma. In addition, the patient populations varied, e.g. hospital outpatients, people living in the community.
Description of the intervention
A range of single or combined interventions (Michie 2014) can be delivered by pharmacists to improve patient outcomes. These can include medication reviews to assess the safety and effectiveness of current medication regimens and to identify medicines which need to be stopped or treatment which should be started. Pharmacists can provide educational interventions to improve patients' knowledge of the medicines, and persuasive techniques to encourage them to use their medications effectively. Pharmacist‐led interventions can also train and enable patients to administer their medication to optimise their health outcomes.
How the intervention might work
Different interventions can achieve their effect by different mechanisms of action. For example, education‐based interventions (Michie 2014) could provide patients with the knowledge they need to use their medicines effectively and thereby achieve improved health outcomes, e.g. lowered blood pressure, improved glycated haemoglobin management. During medication reviews, pharmacists could identify medicines which are likely to cause harm which could then be stopped, thereby reducing adverse events arising including unscheduled hospital admissions.
Why it is important to do this review
This systematic review focuses on non‐dispensing services provided by pharmacists to non‐hospitalised patients. Health systems in many countries struggle to meet patients' healthcare needs. Innovative services are therefore needed to increase capacity and optimise patient outcomes. Pharmacists are society's experts on medicines and medicines are the most commonly‐used therapeutic intervention. The optimal use of medicines should enhance patient outcome and minimise medicine‐related harm. It was important to undertake this review because large numbers of trials have been conducted to explore the effect of pharmacist services on the health outcomes of non‐hospitalised patients and these data needed to be synthesised to derive evidence of their effectiveness compared with usual care, as well as compared with similar services delivered by other health professionals. This is an update of previous versions of this review (Bero 1995; Beney 2000; Nkansah 2010).
Objectives
To examine the effect of pharmacists' non‐dispensing services on non‐hospitalised patient outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised trials. Both patient‐randomised and cluster‐randomised trials were eligible for inclusion. We did not restrict by language or publication status.
Types of participants
Any individual who received services from outpatient pharmacists. Pharmacists included community pharmacists, pharmacists working in other primary care settings, e.g. general medical practices, as well as pharmacists who provide services to hospital outpatients. We included studies where pharmacists delivered services to outpatients in a clinic attached to a hospital or a day hospital. We excluded studies involving services to hospital inpatients or residential care facilities. We included studies if the patients were recruited as inpatients or at discharge, but where the intervention was conducted in an outpatient setting. Any health condition could be included. We included study participants of any age.
Types of interventions
The types of interventions we included were any services delivered by pharmacists other than drug compounding or dispensing. We included interventions if they sought to improve patient health through the use or cessation of medication. We included multidisciplinary interventions if either (a) the multidisciplinary team was led by a pharmacist or (b) most (> 50%) of the intervention was delivered by pharmacists. This latter criterion excluded interventions where the pharmacist played only a minor role in the intervention.
We excluded some intervention types that have recently been addressed in Cochrane and other systematic reviews (e.g. Sinclair 2004), and all health promotion interventions, as well as interventions which were solely focused on medication adherence and automated care programmes.
We made two types of comparison:
Pharmacist services targeting non‐hospitalised patients compared with the delivery of no comparable service for the health problem or population.
Pharmacist services targeting non‐hospitalised patients compared with services delivered by other health professionals for the health problem or population.
Types of outcome measures
We included a broad range of outcome measures associated with health, service utilisation and healthcare‐related harm. We selected commonly‐used objective outcomes to facilitate comparison and meta‐analysis. Outcome measure selection was informed by guidelines and discussion with clinicians with expertise in specific conditions. For example, we sought national or international guidelines to identify the clinical outcomes most frequently used in disease management. Where no clear evidence was available to inform our decision‐making process, we consulted one or more clinicians to determine the most meaningful outcome measures used in everyday practice. For completeness, we have included trials which fulfilled the above inclusion criteria but which did not present data on the outcome measures of interest.
Main outcome measures
We evaluate six main outcome measures in this review: percentage outside blood pressure range as defined by the study authors; percentage outside glycated haemoglobin (HbA1c) range as defined by the study authors; hospital attendance/admission; adverse drug effects; SF‐36 physical functioning (Ware 1989); and mortality. We present these outcomes in Table 1.
Other outcome measures
We also include other frequently‐reported outcome measures in meta‐analyses when available e.g. systolic and diastolic blood pressure, glycated haemoglobin.
Search methods for identification of studies
Previous versions of this review involved both automated searches based on key terms and manual searches of relevant journals and conference abstracts. In this update, we included all studies included in previous versions that met the revised inclusion criteria, as well as all studies identified from a new electronic database search.
Electronic searches
We conducted systematic searches in the following databases to March 2015, without language restrictions:
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, issue 2) via Ovid;
Cochrane Database of Abstracts of Reviews of Effects (DARE; 2015, issue 2) via Ovid;
Cochrane Health Technology Assessment database (HTA; 2015, issue 2) via Ovid;
Cochrane NHS Economic Evaluations Database (NHSEED; 2015, issue 2) via Ovid;
MEDLINE (Ovid) (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations) (1946 to 2015)
Embase (Ovid) (1974 to 2015)
CINAHL (EBSCO) (1981 to 2015)
ProQuest Dissertations & Theses Global (including UK & Ireland) (1861 to 2015)
We present search strategies in Appendix 1. We translated non‐English publications prior to data extraction. We ran top‐up searches in 2018 and added potentially eligible studies to 'Studies awaiting classification'.
Searching other resources
We also searched:
ongoing or unpublished trials in the International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/), and in ClinicalTrials.gov, US National Institutes of Health (NIH) (clinicaltrials.gov/).
We followed Cochrane recommendations for additional search methods by:
Reviewing reference lists of all included studies and relevant systematic reviews.
Contacting authors of relevant studies/reviews to clarify reported published information (as described above) and to seek unpublished results/data.
Data collection and analysis
Selection of studies
Two review authors (MdBa, CS) independently assessed trials for inclusion in the review. We screened the titles/abstracts to eliminate obviously irrelevant studies. We retrieved the full text of each potentially relevant article and combined multiple reports on the same study. We assessed the full‐text articles against the inclusion criteria. If the two primary assessors did not reach agreement through discussion, we consulted a third study author (MCW). We reassessed studies included in the previous version of this review for continued eligibility for inclusion in the update.
Data extraction and management
Review author pairs (MdBa, CS and PR, AW) independently extracted data from all newly‐identified studies. We extracted data using a modified version of the EPOC Data Extraction Checklist (EPOC 2017a). To streamline the data collection process, we built a data entry database using the Epi Info platform (Epi Info 2010) available for reference/use on figshare.com research repository (De Barra 2016). We contacted study authors for additional material if necessary. A third assessor (MCW) resolved any discrepancies.
We re‐assessed studies included in the previous version of this review for continued eligibility for inclusion in the update. We extracted additional data from studies included in the previous review that met the eligibility criteria for this update. We captured details on the content, format and delivery of the intervention. For newly‐identified studies, where necessary we contacted study authors. We also extracted data for the clinical condition targeted, the number of participants and their demographics, outcome measures, setting and country. We also retrieved the type and number of pharmacists involved.
Assessment of risk of bias in included studies
Two review authors (MdBa, CS) independently assessed the risks of bias of all studies eligible for the review, using the Cochrane 'Risk of bias' tool (Chapter 8, Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook)) (Higgins 2011). We resolved discrepancies by discussion. We scored performance bias as low risk if the personnel delivering the intervention were blind to allocation, or if it was unlikely that intervention delivery systematically differed from the described methods due to knowledge of allocation. We scored detection bias as low risk if the assessor was blind to the participant's condition or if the outcome involved little or no subjective estimation of true outcome level (e.g. low density lipoprotein (LDL) measures or hospitalisations). Where the assessor was not blinded and the outcome assessment involved subjective estimation (e.g. quality‐of‐life measures, manual sphygmomanometer, 'falls' where these were undefined), we scored risk of detection bias as high. We assessed attrition bias using the holistic approach to judging recommended in Section 8.13 of the Cochrane Handbook (Higgins 2011). Studies with differential attrition bias < 10% were low risk if total attrition was < 80% and the causes for missing data appeared similar across study arms. Studies that reported intention‐to‐treat analyses were scored low risk. We describe the 'Risk of bias' characteristics for included studies in the Characteristics of included studies table.
Measures of treatment effect
Where data were reported at multiple time points, we used data reported at 12 months (or the closest time point to 12 months).
Continuous outcomes
We extracted a combination of baseline and final‐score data for continuous outcomes. We included final‐score data if available, with the mean difference (MD) in final scores used as the measure of treatment effect. If only data from change scores were available, we used these in the meta‐analyses.
Binary outcomes
For binary outcomes, we used the odds ratio (OR) as the measure of treatment effect. We framed the outcomes so that an event was negative rather than positive, so that ORs less than one always favour the pharmacist group.
Overall effect size
We calculated a standardised effect size for each study (see 'Main outcome measures').
For continuous outcomes, we calculated the standardised mean difference (SMD) (also known as Hedges' g) to represent the difference between groups on a standardised scale. For binary outcomes, we calculated the log odds ratio, using the method recommended in the Cochrane Handbook 9.4.6 to convert this to an SMD by multiplying it by 0.5513 (Chinn 2000; Higgins 2011). We transformed effect sizes if necessary so that values less than zero always favour the pharmacist group.
Although we used a mixture of final scores and change scores for continuous outcomes, following the advice of the Cochrane Handbook 9.4.5.2 we did not do this for the SMD outcome. If a study only reported change scores for the planned outcome, then we chose a different outcome if possible, or we dropped the study from the SMD analysis.
We could not calculate effect sizes for every study. For example, this situation arose if no useable quantitative data were available or if only medians were available.
Meta‐analysis outcomes
We undertook meta‐analyses of the six main outcome measures. We included these six outcomes in the GRADE assessment. We present a full list of all outcomes in Appendix 2.
Unit of analysis issues
We include both patient‐randomised and cluster‐randomised trials in this review. We used the guidance in the Cochrane Handbook 6.4.4 when incorporating cluster‐randomised trials in the meta‐analyses (Higgins 2011). We reduced the effective sample sizes of cluster‐randomised trials by dividing by the design effect, 1 + (M‐1)*ICC, where M is the average cluster size in the intervention arm and ICC is the intraclass correlation coefficient. As no trial in the review reported ICCs, we used an estimated ICC of 0.06 based on De Vera 2014, that had identified reported ICCs in trials of pharmacist interventions.
Dealing with missing data
If trials reported means without standard deviations (SDs), we used a variety of approaches to estimate standard deviations, including their derivation from 95% confidence intervals (CIs) and from reported standard errors. If no measure of variability was available, we imputed standard deviations using the average standard deviation of the other trials within the review. We did this for four outcomes: systolic blood pressure; diastolic blood pressure; SF‐36 Physical Functioning; and Asthma Control Questionnaire (ACQ). For some binary outcomes, we estimated numerators and denominators from reported percentages. For one trial, (Bernsten 2001), we estimated denominators using dropout rates which had been reported on a country‐by‐country basis. We imputed standard deviations for the following outcomes measures (n = number of trials): systolic blood pressure (13); diastolic blood pressure (10); SF‐36 (3); and glycated haemoglobin change (1). We estimated numerators from reported denominators and percentages for seven studies.
Assessment of heterogeneity
We examined heterogeneity using Chi2 tests, and used the I2 statistic to quantify the effect of heterogeneity on the results; I2 > 50% reflects 'substantial' heterogeneity and > 75% 'considerable heterogeneity (Cochrane Handbook 9.5.2 (Higgins 2011)).
Assessment of reporting biases
We assessed the presence of publication bias by visual inspection of funnel plots (by NWS) for each meta‐analysis.
Data synthesis
We conducted standard meta‐analyses for all outcomes which had been reported by at least two trials. We chose a random‐effects model because of the expected between‐study heterogeneity. For continuous outcomes, we pooled only trials reporting the same outcome using the same units, although there was often variation in the types of intervention assessed. We pooled mean differences using the inverse variance approach (Higgins 2011). Three outcomes (blood glucose, total cholesterol and LDL cholesterol) were reported using a mixture of units (mmol/l or mg/dl), so we used conversion formulae (Diabetes UK; Rugge 2011) to convert these to mmol/l. We included a mixture of trials that reported final scores as well as studies that reported change from baseline.
We combined binary data using the Mantel‐Haenszel approach. For some binary outcomes, we pooled trials where the exact definitions varied: e.g. the proportion outside a stated range for blood pressure or glycated haemoglobin, with the specific range sometimes varying between trials. We also included an outcome for hospital attendance/admission which included hospital admission, re‐hospitalisation or emergency admission, depending on the trial.
For three‐arm trials, we created two groups (intervention versus control) using appropriate pooling formulae. For some trials, we pooled two intervention arms, and for others two control arms. In some cases, this resulted in a composite arm of two rather different intervention groups, although both met the review inclusion criteria. There were no trials with four or more arms.
Summary of findings
We assessed the certainty of the evidence using the GRADE approach, i.e. the five GRADE considerations (trial limitations, consistency of effect, imprecision, indirectness and publication bias) (Guyatt 2008). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook (Higgins 2011) and the EPOC worksheets (EPOC 2017b). One review author (NWS) assessed the certainty of the evidence and a second author (MCW) then reviewed and confirmed these assessments. We created two 'Summary of findings' tables for the main intervention comparisons and included the following important outcomes:
Percentage outside target blood pressure range
Percentage outside target glycated haemoglobin range
Hospital attendance/admission
Adverse drug effects
SF‐36 Physical Functioning
Mortality
Subgroup analysis and investigation of heterogeneity
We had planned no subgroup analyses a priori, and performed none. We assessed heterogeneity using the I2 statistic (see above).
Sensitivity analysis
We had planned no sensitivity analyses a priori, and performed none.
Results
Description of studies
Results of the search
We retrieved 1903 records after de‐duplication from the electronic searches and excluded 1657 citations based upon a screen of the title and abstract. We reviewed the full text of 246 records and identified 116 for inclusion in this review (Table 2), 87 of which we identified for this update (Figure 1). One three‐arm trial (Hay 2006) could be included in both comparisons. The top‐up searches, conducted in January 2018, identified 2277 citations after de‐duplication, of which 331 were classified as > 90% chance of being a randomised trial by the classifier (EPOC 2017a). Of these, we added 95 to Studies awaiting classification.
1. Included studies (N = 116) and outcome measures presented in meta‐analyses.
| Author/Year | Clinical condition | Outcome measures used for meta‐analyses |
| Adibe 2013a | Diabetes (Type 2) | ‐ |
| Adler 2004 | Major depression and/or dysthymia | ‐ |
| Albsoul‐Younes 2011 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Ali 2012 | Diabetes (Type 2) | Fasting blood glucose (mmol/l) |
| Amariles 2012 | Cardiovascular disease | Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg); Total cholesterol (mmol/L) |
| Andres 2007 | Diabetes (Type 2) | HbA1c (%) |
| Armour 2007 | Asthma | ‐ |
| Barbanel 2003‐ | Asthma | ‐ |
| Bernsten 2001 | Older Patients (aged > 65) | Hospital attendance/admission |
| Blalock 2010 | At‐risk patients (Older patients (aged > 65) receiving medication that increases their risk of falling) | ‐ |
| Bogden 1998 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Bond 2000 | Repeat prescribing | Hospital attendance/admission; Adverse drug effects |
| Borenstein 2003a | Hypertension | % outside blood pressure range |
| Bosnic‐Anticevich 2010 | Asthma/Chronic Obstructive Pulmonary Disease (COPD) | ‐ |
| Boyd 2013 | Non‐adherence in chronic conditions | ‐ |
| Brook 2003 | Depression | ‐ |
| Bruhn 2013 | Pain (Chronic) | ‐ |
| Capoccia 2004 | Depression | ‐ |
| Castejon 2013 | Diabetes | % outside HbA1c range; HbA1c (%) |
| Carter 2008 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Charrois 2006 | Asthma | Hospital attendance/admission |
| Chisholm 2002 | Transplant patients (renal with focus on BP control) | Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Choe 2005 | Diabetes (Type 2) | HbA1c (%) |
| Chrischilles 2014 | Adults with disability | Adverse drug effects |
| Clifford 2005 | Diabetes (Type 2) (vascular risk factors) | HbA1c (%) |
| Cody 1998 | Health Related Quality of Life (Short Form Survey 36) | ‐ |
| Cordina 2001 | Asthma | ‐ |
| De Castro 2006 | Hypertension | Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Di Donato 2014 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Doucette 2009 | Diabetes | HbA1c (%); Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg); LDL Cholesterol (mmol/L) |
| Edwards 2014 | Chemotherapy | ‐ |
| Farsaei 2011 | Diabetes (Type 2) | % outside HbA1c range; HbA1c (%); Fasting blood glucose (mmol/l) |
| Faulkner 2000 | Hypercholesterolaemic patients receiving combination drug therapy | Total cholesterol (mmol/L); LDL Cholesterol (mmol/L) |
| Finley 2003 | Depression | ‐ |
| Garção 2002 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| García‐Cárdenas 2013 | Asthma | ‐ |
| Gattis 1999a | Heart failure | Mortality |
| González‐Martin 2003 | Asthma | Forced expiratory volume (FEV1) |
| Goodyer 1995 | Heart failure | ‐ |
| Green 2008 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Hammad 2011 | Metabolic syndrome | Fasting blood glucose (mmol/l); Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Hawes 2013 | Patients at risk of rehospitalisation | Hospital attendance/admission |
| Hawkins 1979 | Hypertension and Diabetes | Diastolic blood pressure (mmHg) (Comparison 2) |
| Hay 2006 | Knee pain | ‐ |
| Hendrie 2014 | Type 2 Diabetes | ‐ |
| Hirsch 2014 | Blood pressure | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Ho 2013 | Acute Coronary Syndrome | % outside blood pressure range;Hospital attendance/admission; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg); LDL Cholesterol (mmol/L); Mortality |
| Holland 2005 | Multiple conditions | ‐ |
| Hunt 2008 | Hypertension | % outside blood pressure range; SF‐36 physical functioning; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Jaber 1996 | Diabetes | Fasting blood glucose (mmol/l) |
| Jackson 2004 | Anticoagulation (Warfarin) | Hospital attendance/admission; Mortality |
| Jahangard‐Rafsanjani 2014 | Diabetes | HbA1c (%); Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Jarab 2012 | Chronic Obstructive Pulmonary Disease | Hospital attendance/admission; Forced expiratory volume (FEV1) |
| Khdour 2009 | Chronic Obstructive Pulmonary Disease | Forced expiratory volume (FEV1) |
| Krass 2007 | Diabetes | HbA1c (%); Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Kritikos 2007 | Asthma | ‐ |
| Krska 2001 | Multiple conditions | ‐ |
| Lai 2013 | Osteoporosis (postmenopausal) | ‐ |
| Lee 2006 | Elderly with coronary risk factors | Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Lenaghan 2007 | Multiple conditions | Mortality |
| Lenander 2014 | Polypharmacy (> 5 medications) | ‐ |
| Li 2014 | Chronic Obstructive Pulmonary Disease | ‐ |
| Lopez 2006 | Heart failure | Hospital attendance/admission; Mortality |
| Losada‐Camacho 2014 | Epilepsy | ‐ |
| Magid 2013 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Mahwi 2013 | Diabetes (Type 2) | HbA1c (%); Fasting blood glucose (mmol/l) |
| Malone 2001 | At‐risk patients (high risk of drug related problems (DRPs)) | Total cholesterol (mmol/L) |
| Margolis 2013 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg); |
| Marques 2013 | Depression | ‐ |
| Marra 2012 | Osteoarthritis (Knee) | ‐ |
| Mazroui 2009 | Type 2 diabetes | SF‐36 physical functioning; HbA1c (%); Fasting blood glucose (mmol/l); Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg);Total cholesterol (mmol/L) |
| McAlister 2014 | Cerebrovascular Accident (BP/lipid levels after stroke) | Systolic blood pressure (mmHg) (Comparison 2) |
| Mehos 2000 | Hypertension | SF‐36 physical functioning; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Mehuys 2008 | Asthma | Hospital attendance/admission; Peak Flow (%) |
| Milos 2013 | Multiple conditions | ‐ |
| Murray 2007 | Heart failure | ‐ |
| Naunton 2003 | Multiple conditions | Hospital attendance/admission; Mortality |
| Obreli‐Neto 2015 | Older patients (with diabetes and hypertension) | % outside blood pressure range;% outside HbA1c range; HbA1c (%); Fasting blood glucose (mmol/l); Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg); LDL Cholesterol (mmol/L) |
| Okamoto 2001 | Hypertension | Systolic blood pressure (mmHg) (Comparison 2); Diastolic blood pressure (mmHg) (Comparison 2) |
| Olesen 2014 | Polypharmacy (older patients) | Hospital attendance/admission; Mortality |
| Park 1996 | Hypertension | Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Paulos 2005 | Dyslipidaemia | Total cholesterol (mmol/L) |
| Peterson 2004 | Dyslipidaemia | Total cholesterol (mmol/L) |
| Reid 2005 | Hypertension | % outside blood pressure range |
| Rickles 2005 | Depression | ‐ |
| Rothman 2005 | Diabetes (Type 2) | Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Rubio‐Valera 2012 | Depression | ‐ |
| Sadik 2005 | Heart failure | SF‐36 physical functioning |
| Salazar‐Ospina 2017 | Bipolar Diseases | ‐ |
| Samtia 2013 | Diabetes (Type 2) | HbA1c (%); Fasting blood glucose (mmol/l) |
| Sarkadi 2004 | Diabetes (Type 2) | HbA1c (%) |
| Schneider 1982 | Hypertension and Congestive Heart Failure | ‐ |
| Schneiderhan 2014 | Metabolic Syndrome | % outside HbA1c range |
| Sellors 2003 | Multiple conditions | SF‐36 physical functioning |
| Sidel 1990 | Multiple conditions | ‐ |
| Silveira 2014 | HIV | ‐ |
| Simpson 2011 | Diabetes (Type 2) | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg);Total cholesterol (mmol/L); LDL Cholesterol (mmol/L) |
| Solomon 1998 | Chronic Obstructive Pulmonary Disease | Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg); Dyspnoea |
| Sookaneknun 2004 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Stewart 2014 | Hypertension (primary) | Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Suppapitiporn 2005 | Type 2 diabetes | HbA1c (%) |
| Tang 2014 | Epilepsy | ‐ |
| Tannenbaum 2014 | Benzodiazepine users | ‐ |
| Taveira 2011 | Cardiovascular risk | % outside HbA1c range; HbA1c (%); Systolic blood pressure (mmHg); LDL Cholesterol (mmol/L) |
| Taveira 2014 | Cardiovascular risk | ‐ |
| Taylor 2003 | Multiple conditions | SF‐36 physical functioning |
| Tommelein 2013 | Chronic Obstructive Pulmonary Disease | Dyspnoea |
| Tsuyuki 2002 | Cardiacovascular risk; atherosclerotic disease or diabetes | ‐ |
| Tsuyuki 2015 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Verret 2012 | Anticoagulant patients/stroke risk | Hospital attendance/admission; Adverse drug effects; Mortality |
| Vivian 2002 | Hypertension | % outside blood pressure range; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Volume 2001 | Polypharmacy (older patients > 3 medications) | ‐ |
| Wal 2013 | Hypertension | SF‐36 physical functioning; Diastolic blood pressure (mmHg); Systolic blood pressure (mmHg) |
| Weinberger 2002 | Chronic Obstructive Pulmonary Disease | Hospital attendance/admission; Peak Flow (%) |
| Wu 2006 | Various | Mortality |
| Zermansky 2001 | Multiple conditions | Hospital attendance/admission |
1.
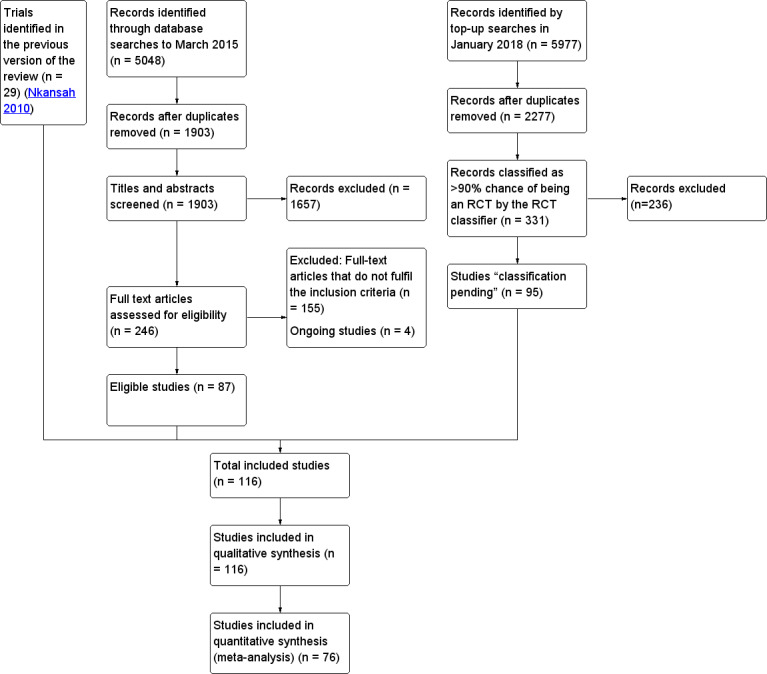
Study flow diagram.
Included studies
Participants
Trials were conducted in 24 countries with the USA (42), UK (13), Canada (11) and Australia (10) contributing most of the studies (n = 76 (66%)). Studies were also included from Spain (5), Brazil (4), Jordan (3) and Sweden (3), with two studies each from Belgium, Chile, China, Colombia, India, Iran, Thailand and the United Arab Emirates (UAE). Single studies were included from Denmark, Hong Kong, Iraq, Malaysia, Malta, the Netherlands, Nigeria, and Portugal. In addition, one study was multi‐centred with countries participating across Europe. The total number of randomised participants was 41,851; this ranged from 21 to 6000 participants per trial (median = 198). A wide range of clinical conditions and medicine‐related behaviours were targeted (Appendix 2), including hypertension (27), diabetes (20), asthma and/or chronic obstructive pulmonary disease (COPD) (14), depression (7), cardiovascular disease (5), heart failure (5), and cholesterol/lipid management (4). In addition, some studies targeted specific patient populations, e.g. those with multiple conditions (receiving multiple medicines) (9), general medicines management (including managing potential risk/harm) (10), older participants (4). Few studies included pain management (2), epilepsy (2) or metabolic syndrome (2), and single studies targeted HIV, cancer, arthritis, bipolar disease and osteoporosis.
Interventions
The studies were conducted in a range of settings. The most common settings in which the pharmacists delivered their interventions were community pharmacies and primary care practices or clinics, hospital outpatient clinics and specialist clinics. Other settings included the patient's home including telephone follow‐up, as well as community settings. The categorisation of the delivery setting was problematic due to the variation of terminology used across studies and countries. Fifty‐one studies involved one participating site, 61 involved multiple sites, and for four studies the number of participating sites was unclear.
The average duration of intervention (i.e. first interaction to last interaction) was 7.4 months (standard deviation: 5.6) and involved an average of 5.6 (standard deviation: 5.6) healthcare provider‐patient interactions, including phone calls. Face‐to‐face interaction between the pharmacist and the patient was involved in 108 studies and was combined with telephone contact in 36 studies, or with printed materials in 45 studies. Many studies used combinations of interactions. In general, the interventions were poorly described with non‐specific definitions and vague descriptions, and lacked detail.
Most interventions targeted one of two of the following types of behaviour:
1. Suboptimal prescribing targeted by medication reviews, home monitoring to derive better data for future prescriptions, rationalisation of prescriptions, identification and resolution of medicine discrepancies, as well as contact with prescribers to modify prescriptions.
2. Suboptimal use of prescribed medication targeted by interventions to improve medicine use through a variety of methods including education, synchronisation of medicine refills, provision of compliance devices and patient follow‐up.
For study details see the Characteristics of included studies table.
Outcomes
Of the 116 trials, 76 were included in meta‐analyses. The 40 remaining trials were not included in the meta‐analyses because they each reported unique outcome measures which could not be combined. In total, 73 trials were eligible for the comparison of pharmacist‐led service and usual care, and three for pharmacist‐led service with other healthcare professional.
Excluded studies
We eliminated 152 studies. The main reasons for exclusion were that the interventions were not delivered predominantly by a pharmacist or that they targeted hospitalised patients. Three studies were excluded for specific reasons, as presented in the Characteristics of excluded studies.
Risk of bias in included studies
We present the results of the 'Risk of bias' assessment in Figure 2 and Figure 3. Thirteen studies (11.2%) had no identifiable biases (Green 2008; Malone 2001Margolis 2013; McAlister 2014; Olesen 2014; Peterson 2004; Rothman 2005; Sarkadi 2004; Simpson 2011; Stewart 2014; Tannenbaum 2014; Tommelein 2013; Wu 2006).
2.
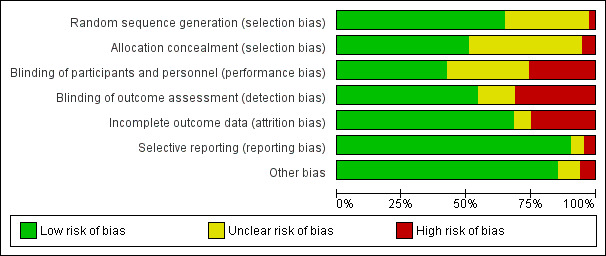
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We determined the risk of selection bias associated with random sequence generation to be low in 75 trials, high in three trials and unclear in 38 trials. We determined that risk of selection bias due to allocation concealment was low in 59 trials, high in six trials and unclear in 51 trials.
Blinding
We determined that risk of performance bias due to blinding of participants was low in 49 trials, high in 30 trials and unclear in 37 trials. We determined that risk of detection bias due to blinding of personnel was low in 62 trials, high in 36 trials and unclear in 18 trials.
Incomplete outcome data
We determined that risk of attrition bias was low in 79 trials, high in 29 trials and unclear in 8 trials.
Selective reporting
We determined that risk of bias was low for 'incomplete reporting of data' in 105 trials, high in five trials and unclear in six trials.
Other potential sources of bias
We assessed the risk of specific biases as 'unclear' in many trials, due to incomplete reporting.
Effects of interventions
See: Table 1
Comparison 1: Pharmacist services targeting patients versus usual care
Seventy‐three trials compared pharmacist services targeting patients versus usual care for which useable data were available that could be included in one or more meta‐analyses. We performed meta‐analyses for 15 outcomes. Trials could be included in more than one meta‐analysis if they presented relevant data. For most meta‐analyses there was no clear evidence of funnel plot asymmetry, although only a few included more than 10 trials.
Percentage outside blood pressure range
Eighteen trials (4107 participants) evaluated whether blood pressure fell outside a specified range (Analysis 1.1). These trials used a mixture of systolic and diastolic blood pressure and a variety of target ranges, but we used systolic blood pressure in our analysis if both were reported. The results indicate that those in the pharmacist groups may be less likely to have blood pressure outside the target range (OR 0.40, 95% CI 0.29 to 0.55, low‐certainty evidence; I2 = 81%). The asymmetric pattern shown in the funnel plot for this meta‐analysis could be an indication of publication bias.
1.1. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 1 % outside blood pressure range.
Percentage outside glycated haemoglobin range
We are uncertain whether pharmacist services improve the percentage of patients outside the glycated haemoglobin target range (5 trials, N = 558, OR 0.29, 95% CI 0.04 to 2.22, very low‐certainty evidence, I2 = 92%) (Analysis 1.2).
1.2. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 2 % outside HbA1c range.
Hospital attendance/admission
Pharmacist services probably lead to little or no difference in hospital attendance or admissions (14 trials, N = 3631, OR 0.85, 95% CI 0.65 to 1.11, moderate‐certainty evidence, I2 = 44%) (Analysis 1.3).
1.3. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 3 Hospital attendance/admission.
Adverse drug effects
Pharmacist services may make little or no difference to adverse drug effects (3 trials, N = 590, OR 1.65, 95% CI 0.84 to 3.24, low‐certainty evidence, I2 = 52%) (Analysis 1.4).
1.4. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 4 Adverse drug effects.
SF‐36 physical functioning
Pharmacist services may slightly improve physical functioning (measured by the SF‐36) (7 trials, N = 1329, MD 5.84, 95% CI 1.21 to 10.48, low‐certainty evidence, I2 = 84%) (Analysis 1.5).
1.5. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 5 SF‐36 Physical Functioning.
Mortality
Pharmacist services may make little or no difference to mortality (9 trials, N = 1980, OR 0.79, 95% CI 0.56 to 1.12, low‐certaintly of evidence, I2 = 13%) (Analysis 1.6).
1.6. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 6 Mortality.
Other outcomes
Other effects for HbA1c
Mean HbA1c was 0.77 units lower for those receiving a pharmacist intervention (15 trials, N = 2298, MD −0.77, 95% CI −0.97 to −0.58, I2 = 77%) (Analysis 1.7). Patients in the pharmacist groups tended to have lower fasting blood glucose than those in control groups (8 trials, N = 1349, MD −1.17 mmol/l, 95% CI −1.71 to −0.63, I2 = 74%) (Analysis 1.8).
1.7. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 7 HbA1c (%).
1.8. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 8 Fasting blood glucose (mmol/l).
Continuous measures of blood pressure
Thirty‐one trials (N = 5939) and 32 trials (N = 6003) were included in the meta‐analyses of diastolic and systolic blood pressure, respectively. On average, there was evidence that pharmacist interventions reduced diastolic blood pressure by −3.50 points (95% CI −5.44 to −1.56) and systolic blood pressure by −5.96 points (95% CI −7.35 to −4.57) compared with usual care (Analysis 1.9; Analysis 1.10). In both analyses, there was evidence of statistical heterogeneity (I2 = 94% and 74%, respectively).
1.9. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 9 Diastolic blood pressure (mmHg).
1.10. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 10 Systolic blood pressure (mmHg).
Lipids
Overall, patients in the pharmacist groups tended to have lower total cholesterol (7 trials, N = 1592, MD −0.35 mmol/l, 95% CI −0.56 to −0.13, I2 = 77%) (Analysis 1.11). There was little or no difference for LDL cholesterol (6 trials, N = 854, MD −0.14 mmol/l, 95% CI −0.30 to 0.02, I2 = 56%) (Analysis 1.12).
1.11. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 11 Total cholesterol (mmol/l).
1.12. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 12 LDL Cholesterol (mmol/l).
Respiratory function
A small number of trials were included in the meta‐analyses for each of three respiratory outcomes: FEV1 (3 trials, N = 291), peak flow (2 trials, N = 460) and dyspnoea (2 trials, N = 820). There was no evidence of an effect of the pharmacist intervention on any of these outcomes: FEV1: MD 0.11, 95% CI −0.01 to 0.23, I2 = 0%; Analysis 1.13; Peak flow: MD: 3.36, 95% CI −0.36 to 7.09, I2 = 0%; Analysis 1.14; Dyspnoea: OR 0.90, 95% CI 0.68 to 1.20, I2 = 0%; Analysis 1.15.
1.13. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 13 FEV1.
1.14. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 14 Peak Flow (%).
1.15. Analysis.
Comparison 1 Pharmacist services targeted at patients versus the delivery of no comparable service, Outcome 15 Dyspnoea.
Comparison 2: Pharmacist services targeting patients versus other healthcare professionals
Five trials compared pharmacist services targeting patients versus care provided by healthcare professionals, for which useable data were available that could be included in one or more meta‐analyses. We performed meta‐analyses for two outcomes and calculated an overall standardised effect size for five trials included in the meta‐analysis.
Percentage outside blood pressure range
We did not find any studies comparing pharmacists' non‐dispensing roles targeting non‐hospitalised patients with other healthcare professionals that reported on the percentage outside blood pressure range.
Percentage outside glycolated haemoglobin range
We did not find any studies comparing pharmacists' non‐dispensing roles targeting non‐hospitalised patients with other healthcare professionals that reported on percentage outside glycolated haemoglobin range.
Hospital attendance/admission
We did not find any studies comparing pharmacists' non‐dispensing roles targeting non‐hospitalised patients with other healthcare professionals that reported on hospital attendance/admission.
Adverse drug effects
We did not find any studies comparing pharmacists' non‐dispensing roles targeting non‐hospitalised patients with other healthcare professionals that reported on adverse drug effects.
SF‐36 physical functioning
We did not find any studies comparing pharmacists' non‐dispensing roles targeting non‐hospitalised patients with other healthcare professionals that reported on SF‐36 physical functioning.
Mortality
We did not find any studies comparing pharmacists' non‐dispensing roles targeting non‐hospitalised patients with other healthcare professionals that reported on mortality.
Other outcome measures
Compared with other healthcare professionals, pharmacist services were not associated with differences in systolic blood pressure (3 trials, N = 1238, MD 1.31, 95% CI −6.22 to 8.84, I2 = 94%) (Analysis 2.1) and diastolic blood pressure (2 trials, N = 959, MD −1.36, 95% CI −4.30 to 1.59, I2 = 86%) (Analysis 2.2).
2.1. Analysis.
Comparison 2 Pharmacist services targeted at patients versus services delivered by other health professionals, Outcome 1 Systolic blood pressure (mmHg).
2.2. Analysis.
Comparison 2 Pharmacist services targeted at patients versus services delivered by other health professionals, Outcome 2 Diastolic blood pressure (mmHg).
Discussion
Summary of main results
We included 116 randomised trials in this review, most of which (n = 111) compared pharmacist services with usual care, with the remaining five comparing pharmacist services with those delivered by other health professionals.
Compared with usual care, we are uncertain whether pharmacist services improved the percentage of patients outside the glycolated haemoglobin target range (very low‐certainty evidence). Pharmacist services may make little or no difference to hospital attendance or readmission (moderate‐certainty evidence) or to adverse drug effects (low‐certainty evidence). Pharmacist services may, however,reduce the percentage of patients whose blood pressure is outside the target range (low‐certainty evidence) and may also slightly improve physical functioning (low‐certainty evidence).
We did not find any trials comparing pharmacists' non‐dispensing roles with services delivered by other health professionals that assessed the percentage of patients outside blood pressure or glycolate haemoglobin target range, hospital attendance and admission, adverse drug effects, physical functioning or mortality.
In addition to the main outcomes discussed above and reported in the Table 1, we also include secondary outcome measures. We did not assess these secondary outcomes using GRADE for certainty of evidence. Compared with usual care, pharmacist services achieved reductions in systolic and diastolic blood pressure of −5.96 mmHg and −3.50 mmHg, respectively. A reduction in systolic blood pressure of 5 mmHg is associated with a 34% reduction in stroke and 21% reduction in ischaemic heart disease (Law 2003), and as such, the results also suggest that these effects are clinically relevant. Furthermore, compared with usual care, pharmacist services achieved reductions in glycolated haemoglobin, fasting blood glucose and total cholesterol. Conversely, pharmacist services made little or no difference to low density lipoprotein levels or respiratory function, compared with usual care.
Most trials were conducted in anglophone high‐income countries, and results should therefore be interpreted with caution for their relevance to lower‐income countries. The aim of many trials was to achieve improved control of hypertension and blood glucose, which could have led to falls, postural hypotension and hypoglycaemia; these potential harms were not assessed. This review therefore does not comment on the potential harms of the pharmacist services evaluated by the included trials.
Overall completeness and applicability of evidence
We searched multiple sources of data to identify eligible trials, performing duplicate, independent data extraction for all components. Evidence of potential publication bias was demonstrated in Analysis 1.1 (% outside blood pressure range). The original review used a mainly narrative approach and only three small meta‐analyses were possible. The larger number of trials in this update allow a wider range of quantitative meta‐analyses. We calculated effect sizes for many of the included trials, enabling standard meta‐analyses to be conducted.
As expected, we detected substantial heterogeneity in most of the meta‐analyses undertaken, possibly due to variation in interventions tested and definitions used. Using GRADE, we downgraded all outcomes to moderate certainty due to high risks of bias, with some outcomes being further downgraded due to high levels of heterogeneity.
The pharmacist services were poorly described and thus limit the ability to replicate these interventions for future trials or for service delivery. The use of checklists for reporting interventions, such as Template for Intervention Description and Replication (TiDieR) (Hoffman 2014) should enhance completeness of reporting and replicability of future service evaluations. There was little or no discussion of the mechanisms of action by which the pharmacist services were hypothesised to improve patient outcomes. The Behaviour Change Technique Taxonomy and Behaviour Change Wheel (Michie 2014) have been used to categorise the active ingredients or behaviour change techniques (BCTs) of interventions and to identify interventions likely to achieve the desired behavioural goal. The use of taxonomies and frameworks for developing and evaluating interventions could provide clarity about the anticipated or intended mechanisms of action of pharmacist interventions. The effectiveness of pharmacist interventions could be diminished if their recommendations on prescribed medicines need to be actioned by a third party, e.g. a doctor. In some countries, however, pharmacists are able to prescribe and to directly effect any changes in prescribed medicines to enhance patient outcomes. Few trials in this review included or reported whether the participating pharmacist(s) were qualified prescribers.
Certainty of the evidence
With the trials included in the analysis of pharmacist interventions compared with usual care, the certainty of the evidence is very low or low for most of the outcomes. This is mainly explained by major heterogeneity in study populations, types of interventions delivered and reported outcomes. Three trials were included in the meta‐analyses of pharmacist interventions compared with interventions delivered by healthcare professionals, with very low certainty of the evidence. Evidence is limited on whether pharmacist‐led services achieve equivalent patient outcomes compared with other healthcare professional provision.
Potential biases in the review process
The extensive searches performed by the EPOC team are likely to have identified most or all relevant trials. Duplicate, independent screening and data extraction processes minimised bias and reduced error, although incomplete descriptions of study procedures and interventions complicated this task. Publication biases and strategic selection of outcomes may also have led to an inflation of the estimated effect size.
Agreements and disagreements with other studies or reviews
The results of this systematic review generally concur with those of other reviews of pharmacist services conducted in different settings or with different health conditions or patient populations, which report mixed evidence of the benefit of pharmacy interventions (Altowaijri 2013; Charrois 2012; Greer 2016; Hatah 2014; Jokanovic 2017; Koshman 2008; Mekonnen 2016; Pande 2013; Walsh 2016). An earlier Cochrane Review (Glynn 2010) of interventions to improve hypertension suggested that pharmacist‐led interventions showed promising results. In this updated review, patients who received pharmacist‐led services were less likely to have blood pressure outside the target range compared with patients receiving usual care.
Authors' conclusions
Implications for practice.
The results need to be interpreted cautiously because there was major heterogeneity in study populations, types of interventions delivered and reported outcomes.There was considerable heterogeneity within many of the meta‐analyses as well as considerable variation in the risks of bias.
This review demonstrates that pharmacist services have varying effects on patient outcomes compared with usual care. Some services appear to have little effect whilst others have the potential to improve important outcomes on a scale which is clinically important.
There was little or no difference between the effectiveness of interventions that were pharmacist‐led compared with the same intervention being delivered by other healthcare professionals. This is an important finding in terms of role substitution, with particular implications for costs. For example, if pharmacists can achieve similar effects compared with doctors, service delivery by the former is likely to cost less than the latter. However, we did not examine costs and resources required for delivering interventions, so the cost effectiveness of these services remains to be established.
Implications for research.
The development of future pharmacist services should be informed by existing knowledge about effective intervention design and development. Further research is required to help identify which components of an intervention are more effective and under what conditions. We also need a deeper understanding of why certain interventions but not others are effective in some clinical domains, and why certain interventions only work in some populations or settings but not in others. These factors may explain the high heterogeneity often observed in this review.
There is a need for better alignment between health priorities and the clinical topics and behaviours selected and targeted by pharmacist‐led services. Whilst most of the included trials targeted non‐communicable diseases, thereby reflecting the global burden of disease, a number of conditions identified as future priorities were under‐represented in this review (WHO 2011), e.g. HIV, Alzheimer’s Disease, mental health conditions, and cancer.
There is now an abundance of research evaluating pharmacist effectiveness. Future trials should better describe research methods as well as intervention and comparator interventions delivered, in order to enhance the certainty of the evidence and the replicability of interventions. The potential harms of these services should also be explored. High‐quality economic evaluations of these services should assist policy‐makers in deciding on investing in these additional pharmacy services.
Feedback
Is there now a magnitude of evidence of no or little benefit?, 18 September 2018
Summary
The comment received by Dr. Evan Ackermann asked: "Is there now a magnitude of evidence of no or little benefit from "expanded pharmacy services" that would support health funders not supporting these services any further? With over 20 years of research on pharmacists expanded roles, it is time to draw a close to this type of research?"
Reply
On behalf of the review team, Professor Margaret Watson responded to the above: "On the contrary, the evidence presented in this review indicates that pharmacist services can achieve meaningful improvements with some but not all important patient outcomes. Future research should explore which elements and combination of elements of these services are driving these effects."
Contributors
Dr. Evan Ackermann (comment author), Chair, Royal Australian College of General Practitioners (RACGP) Expert Committee ‐ Quality Care
Professor Margaret (Mags) C. Watson (resonse author),Professor of Health Services Research, University of Bath, UK
What's new
| Date | Event | Description |
|---|---|---|
| 2 December 2018 | Amended | Minor amendment to incorporate feedback received September 18, 2018 and the review authors response. |
History
Review first published: Issue 9, 2018
| Date | Event | Description |
|---|---|---|
| 4 April 2018 | Feedback has been incorporated | The feedback and queries from reviewers has been addressed and the review updated. |
| 21 March 2018 | Amended | The review was updated to address peer reviewers' comments and suggestions and now contains 116 studies. |
| 21 March 2018 | Amended | The title for the review was amended |
| 21 November 2017 | New search has been performed | This is an update of a review last published in 2010, which in now split into two separate reviews. This review focuses specifically on effects on patient outcomes and includes a selected range of outcomes. |
| 7 November 2017 | New citation required and conclusions have changed | We introduced changes to comply with current Cochrane methodological standards, including GRADE and the 'Summary of findings' table. This review now includes 116 studies. We have added several additional meta‐analyses for a range of outcomes, which demonstrate that pharmacist services have varying effects on patient outcomes compared with usual care. There was little or no difference between the effectiveness of interventions that were pharmacist‐led compared with the same intervention being delivered by other healthcare professionals. |
| 18 November 2016 | Amended | text updated and validation report items addressed |
| 1 December 2010 | Amended | Conflict of interest modified. |
| 16 June 2010 | New citation required but conclusions have not changed | New search, criteria for included studies changed to only include RCTs, new authors |
| 16 June 2010 | New search has been performed | Reconciled old and new studies |
| 21 August 2008 | Amended | Converted to new review format. |
| 18 January 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We are very grateful to the Chief Scientist Office, Scottish Government, for funding this review (CZH/4/1041). The authors wish to thank the members of Cochrane Effective Practice and Organisation of Care (EPOC) Group who supported this review, particularly Ms Tamara Rader and Mr Paul Miller for conducting the searches, and Ms Julia Worswick for her continued and good‐natured assistance throughout the update. We are very grateful to Dr Imran Omar for providing additional technical support. We thank Ms Caroline Burnett, Ms Andrea Fraser, Mrs Bev Smith and Ms Lynn McKenzie for their administrative and clerical support of this review.
We thank the referees whose comments improved the reporting and interpretation of this review. These include:
External referees: Yoon K Loke; Newton Opiyo; Internal editor: Carmel Hughes; Statistical editor: Sofia Massa; Contact editor: Gillian Leng; Managing editor: Daniela Gonçalves‐Bradley
We also thank National Institute for Health Research, via Cochrane Infrastructure funding to the EPOC Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies
Medline (OVID)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) <1946 to Present>
Search Date: March 2, 2015
1. Pharmacists/ or Pharmacists' Aides/ (11431)
2. Pharmaceutical Services/ (4317)
3. pharmacist?.ti,ab. (20403)
4. ((pharmaceutical or pharmacotherapy or pharmacotherapies or pharmacotherapeutic or prescribing or prescriber? or dosing or dosage) adj2 (advice or care or management or recommendation? or service or services)).ti,ab. (4872)
5. (pharmacist? adj2 (managed or comanag$ or co‐manag$ or case manag$)).ti,ab. (357)
6. Drug Information Services/ (3640)
7. ((drug or prescription?) adj2 (information adj2 (service or services or advice or recommendat$ or education$))).ti,ab. (367)
8. drug educator?.ti,ab. (5)
9. or/1‐8 (32751)
10. Outpatient Clinics, Hospital/ or Pain Clinics/ or Outpatients/ (24657)
11. (outpatient? or out‐patient?).ti. or ((outpatient? or out‐patient?) adj2 (care or clinic? or drug therapy or management or pharmaceutical or prescription? or visit?)).ab. (54319)
12. Ambulatory care/ or exp Ambulatory Care Facilities/ (78057)
13. (ambulatory or outpatient? or out‐patient?).ti. (49572)
14. ((ambulatory or outpatient? or out‐patient?) adj2 (care or facility or facilities or patient? or clinic?)).ab. (51236)
15. Home Care Agencies/ or Hospitals, Community/ (11643)
16. (home care or patient? home? or homecare or community hospital?).ti,ab. (26408)
17. (community adj3 (health$ adj (centre or centres or center? or clinic?))).ti,ab. (4932)
18. exp Community Health Services/ (500019)
19. Community Health Nursing/ (18483)
20. (community adj2 (care or healthcare or health care or patient? care or (health$ adj2 service?))).ti,ab. (11488)
21. (community adj3 (health$ adj (centre or centres or center? or clinic? or unit or units))).ti,ab. (4968)
22. exp Primary Prevention/ or Patient Education as Topic/ (184041)
23. ((immuni?ation? or vaccination?) adj2 (clinic or clinics or service or services)).ti,ab. (1301)
24. (mobile adj (clinic? or healthcare or care)).ti,ab. (448)
25. (((early intervention or preventive or preventative or prevention) adj2 service?) or anonymous testing).ti,ab. (6926)
26. ((consumer or patient?) adj2 education$).ti,ab. (16496)
27. Self Care/ or Blood Glucose Self‐Monitoring/ or Self Administration/ (37116)
28. (self care or self manag$ or self administration).ti,ab. (26649)
29. or/10‐28 (805009)
30. Physicians, Primary Care/ or General Practitioners/ or Physicians, Family/ (18329)
31. General practice/ or Family Practice/ or Primary Care Nursing/ (64545)
32. ((general or family) adj3 (practice? or practitioner? or Physician? or doctor?)).ti,ab. (96029)
33. Primary health care/ (55449)
34. (primary adj2 (care or healthcare)).ti,ab. (90606)
35. or/30‐34 (217858)
36. Patient Compliance/ or Medication Adherence/ (55541)
37. Patient Care/ or Patient Care Management/ or Patient‐Centered care/ (21135)
38. Disease Management/ or Case Management/ (20765)
39. professional‐patient relations/ (22010)
40. "Continuity of Patient Care"/ (14812)
41. or/36‐40 (129007)
42. clinical clerkship/ or education, medical, continuing/ or education, nursing, continuing/ (45982)
43. (continuing adj2 (doctor? or medical or nurse or nursing or nurses or physician? or practitioner? or family physician? or GP) adj2 education$).ti,ab. (4983)
44. (detailing or detailer?).ti,ab. (3988)
45. or/42‐43 (47805)
46. 9 and 29 (7830)
47. 9 and 35 (2895)
48. 9 and 41 (2851)
49. 9 and 45 (329)
50. (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti. (936569)
51. exp animals/ not humans.sh. (3987626)
52. 50 not 51 (863695)
53. clinical trial/ or multicenter study/ (619543)
54. random$.ti,ab. or controlled.ti. (793429)
55. (control adj2 (group or groups or patient? or cohort?)).ti,ab. (354151)
56. evaluation studies as topic/ (119788)
57. (comparative study or evaluation studies or "research support American recovery and reinvestment act" or research support NIH extramural or research support NIH intramural or research support non us govt or research support us govt non phs or research support us govt phs).pt. (8454230)
58. (evaluation or change or effect or effectiveness).ti. or (quality adj2 improv$).ti,ab. or impact?.ti,ab. or patient outcomes.ti,ab. (1779157)
59. ((or/53‐55) or ((or/56‐57) and 58)) not 51 (1798844)
60. (or/46‐49) and 52 (1218)
61. ((or/46‐49) and 59) not 60 (1393)
62. remove duplicates from 60 (1196
63. remove duplicates from 61 (1374)
Embase (OVID)
Embase Classic+Embase <1947 to 2015 February 27>
Search Date: March 2, 2015
1. *Pharmacist/ 17634
2. pharmacist?.ti,ab. 44766
3. ((pharmaceutical or pharmacotherapy or pharmacotherapies or pharmacotherapeutic or prescribing or prescriber? or dosing or dosage) adj2 (advice or care or management or recommendation? or service or services)).ti,ab. 8765
4. (pharmacist? adj2 (managed or comanag$ or co‐manag$ or case manag$)).ti,ab. 638
5. ((drug or prescription?) adj2 (information adj2 (service or services or advice or recommendat$ or education$))).ti,ab. 557
6. drug educator?.ti,ab. 15
7. or/1‐6 56864
8. *outpatient department/ or *outpatient/ or *outpatient care/ or *ambulatory care/ 37977
9. (outpatient? or out‐patient?).ti. or ((outpatient? or out‐patient?) adj2 (care or clinic? or drug therapy or management or pharmaceutical or prescription? or visit?)).ab. 82015
10. *ambulatory care/ 11976
11. (ambulatory or outpatient? or out‐patient?).ti. 65057
12. ((ambulatory or outpatient? or out‐patient?) adj2 (care or facility or facilities or patient? or clinic?)).ab. 81470
13. *community hospital/ or *community mental health center/ 6877
14. *community health nursing/ or *community psychiatric nursing/ or *community care/ or *community mental health/ or *community medicine/ 42037
15. *home care/ or *home health agency/ or *home mental health care/ or *home rehabilitation/ or *home respiratory care/ or *visiting nursing service/ 28185
16. (home care or patient? home? or homecare or community hospital?).ti,ab. 33559
17. (community adj3 (health$ adj (centre or centres or center? or clinic?))).ti,ab. 6125
18. *community health nursing/ or *community psychiatric nursing/ or *community care/ or *community mental health/ or *community medicine/ 42037
19. (community adj2 (care or healthcare or health care or patient? care or (health$ adj2 service?))).ti,ab. 14526
20. (community adj3 (health$ adj (centre or centres or center? or clinic? or unit or units))).ti,ab. 6170
21. *primary prevention/ or *patient education/ 30335
22. exp *vaccination/ or *immunization/ 87274
23. ((immuni?ation? or vaccination?) adj2 (clinic or clinics or service or services)).ti,ab. 1482
24. (mobile adj (clinic? or healthcare or care)).ti,ab. 504
25. (((early intervention or preventive or preventative or prevention) adj2 service?) or anonymous testing).ti,ab. 8454
26. ((consumer or patient?) adj2 education$).ti,ab. 23686
27. *self care/ or *self help/ or *self monitoring/ 17507
28. (self care or self manag$ or self administration).ti,ab. 36132
29. *home health agency/ 26
30. *community program/ 469
31. or/8‐30 441848
32. *primary medical care/ or *primary health care/ or family medicine/ 49741
33. *general practice/ or *general practitioner/ 54750
34. ((general or family) adj3 (practice? or practitioner? or Physician? or doctor?)).ti,ab. 124919
35. (primary adj2 (care or healthcare)).ti,ab. 114224
36. or/32‐35 250478
37. *patient compliance/ 18355
38. *patient care/ or *patient care planning/ 56343
39. *case management/ or *disease management/ 7750
40. *patient assessment/ 723
41. *medical assessment/ or *"evaluation and follow up"/ 1880
42. *eye care/ or *foot care/ or *blood glucose monitoring/ 4815
43. or/37‐42 88885
44. *continuing education/ or *residency education/ 17150
45. (continuing adj2 (doctor? or medical or nurse or nursing or nurses or physician? or practitioner? or family physician? or GP) adj2 education$).ti,ab. 6436
46. (detailing or detailer?).ti,ab. 5388
47. or/44‐45 22694
48. clinical trial/ or multicenter study/ 889647
49. random$.ti,ab. or controlled.ti. 1022116
50. (control adj2 (group or groups or patient? or cohort?)).ti,ab. 495154
51. multicenter study/ 115967
52. 7 and 31 6253
53. (7 and 36) not 52 3652
54. (7 and 43) not (or/52‐53) 1330
55. (7 and 47) not (or/52‐54) 302
56. (random$ or placebo$ or double‐blind$).tw. 1072053
57. multicenter study/ or controlled clinical trial/ or clinical trial/ or controlled study/ or randomized controlled trial/ 5045905
58. exp animals/ or exp Invertebrates/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 21731421
59. human/ or normal human/ or human cell/ 15790114
60. 58 and 59 15743053
61. 58 not 60 5988368
62. (or/56‐57) not 61 3761811
63. 52 and 62 1228
64. 53 and 62 904
65. 54 and 62 187
66. 55 and 62 42
67. or/63‐67 2361
68. remove duplicates from 67 2333
The Cochrane Library (OVID)
Search Date: March 4, 2015
1 non‐dispensing.ti,ab. (18)
2 (pharmacist? adj2 (physician? or doctor?)).ti. (45)
3 (evaluation and pharmacist?).ti. (36)
4 (pharmacist? adj2 (care or case manag$ or comanag$ or co‐manag$ or delivered or directed or disease manag$ or educator? or led or managed or outreach or prescriber? or prescribing)).ti,ab. (401)
5 ((community pharmacy or community pharmacies) adj4 (patient ? care or case manag$ or comanag$ or co‐manag$ or delivered or directed or disease manag$ or educator? or led or managed or outreach or prescriber? or prescribing)).ti,ab. (24)
6 (pharmacist? adj2 (advice or consultation? or consultant? or counsel$ or initiated or intervention? or participation)).ti,ab. (491)
7 (pharmacist? adj3 (role or roles) adj5 (change or changing or changes or new or increas$)).ti,ab. (5)
8 (pharmacy and care).ti. (40)
9 or/1‐8 [Keyword] (832)
10 (community adj2 (pharmacist? or pharmacy)).ti,ab. (347)
11 (pharmacist? adj2 (managed or comanag$ or co‐manag$ or case manag$)).ti,ab. (55)
12 ((pharmaceutical or pharmacotherapy or pharmacotherapies or pharmacotherapeutic or prescribing or prescriber? or dosing or dosage) adj2 (advice or care or management or recommendation? or service or services)).ti,ab. (657)
13 (pharmacist? adj2 (managed or comanag$ or co‐manag$ or case manag$)).ti,ab. (55)
14 ((drug or prescription?) adj2 (information adj2 (service or services or advice or recommendat$ or education$))).ti,ab. (10)
15 drug educator?.ti,ab. (1)
16 (outpatient? or out‐patient?).ti. or ((outpatient? or out‐patient?) adj2 (care or clinic? or drug therapy or management or pharmaceutical or prescription? or visit?)).ab. (175808)
17 (ambulatory or outpatient? or out‐patient?).ti. (166725)
18 ((ambulatory or outpatient? or out‐patient?) adj2 (care or facility or facilities or patient? or clinic?)).ab. (368619)
19 (home care or patient? home? or homecare or community hospital?).ti,ab. (1892)
20 (community adj3 (health$ adj (centre or centres or center? or clinic?))).ti,ab. (389)
21 (community adj2 (care or healthcare or health care or patient? care or (health$ adj2 service?))).ti,ab. (1061)
22 (community adj3 (health$ adj (centre or centres or center? or clinic? or unit or units))).ti,ab. (393)
23 ((immuni?ation? or vaccination?) adj2 (clinic or clinics or service or services)).ti,ab. (85)
24 (mobile adj (clinic? or healthcare or care)).ti,ab. (20)
25 (((early intervention or preventive or preventative or prevention) adj2 service?) or anonymous testing).ti,ab. (547)
26 ((consumer or patient?) adj2 education$).ti,ab. (2220)
27 (self care or self manag$ or self administration).ti,ab. (3865)
28 ((general or family) adj3 (practice? or practitioner? or Physician? or doctor?)).ti,ab. (7580)
29 (primary adj2 (care or healthcare)).ti,ab. (9565)
30 (continuing adj2 (doctor? or medical or nurse or nursing or nurses or physician? or practitioner? or family physician? or GP) adj2 education$).ti,ab. (238)
31 (detailing or detailer?).ti,ab. (283)
32 10 or 11 or 12 or 14 (1004)
33 32 not 9 (677)
34 pharmacist?.ti. and (or/16‐31) (447)
35 34 or 33 or 9 (1570)
36 from 35 keep 1‐21 [CDSR] (21)
37 from 35 keep 22‐45 [ACP] (24)
38 from 35 keep 46‐99 [DARE] (54)
39 from 35 keep 100‐1479 [CENTRAL] (1380)
40 from 35 keep 100‐1479 [CENTRAL] (1380)
41 from 35 keep 1480‐1496 [MTH] (17)
42 from 35 keep 1497‐1503 [HTA] (7)
43 from 35 keep 1504‐1570 [NHS EED] (67)
Cinahl (EBSCO)
Search Date: March 1, 2015
S29 S19 AND S28 (291)
S28 S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 (144,381)
S27 TI controlled AND TI ( trial or trials or study or experiment* or intervention ) (16,915)
S26 AB ( (multicent* n2 design*) or (multicent* n2 study) or (multicent* n2 studies) or (multicent* n2 trial*) ) or AB ( (multi‐cent* n2 design*) or (multi‐cent* n2 study) or (multi‐cent* n2 studies) or (multi‐cent* n2 trial*) ) (6,262)
S25 TI multicentre or multicenter or multi‐centre or multi‐center (4,202)
S24 TI ( cluster N2 trial* or cluster N2 study or cluster N2 group or cluster N2 groups or cluster N2 cohort or cluster N2 design or cluster N2 experiment* ) OR AB ( cluster N2 trial* or cluster N2 study or cluster N2 group or cluster N2 groups or cluster N2 cohort or cluster N2 design or cluster N2 experiment* ) (1,569) S23 TI ( control group or control groups OR control* experiment* or control* design or controlled study ) OR AB ( control group OR control groups or control* cohort* or controlled experiment* controlled design or controlled study) (47,039)
S22 TI random* or AB random* (102,748)
S21 TI ( “clinical study” or “clinical studies” ) or AB ( “clinical study” or “clinical studies” ) (6,586)
S20 (MM "Clinical Trials+") (7,876)
S19 S16 OR S18 (3,048)
S18 S7 AND s17 (1,849)
S17 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 (448,860)
S16 S1 OR S2 OR S3 OR S4 OR S5 OR S6 (2,069)
S15 (MH "Patient Care") OR (MH "Continuity of Patient Care") OR (MH "Multidisciplinary Care Team") OR (MH "Disease Management") (40,058)
S14 TI ( detailing or detailer* or outreach ) OR AB ( detailing or detailer* or outreach ) (4,338)
S13 (MH "Education, Medical, Continuing") OR (MH "Education, Nursing, Continuing") (12,240)
S12 (MH "Primary Health Care") OR (MH "Physicians, Family") (33,768)
S11 (MH "Community Mental Health Services+") OR (MH "Drug Information Services+") OR (MH "Family Planning+") OR (MH "Home Health Care") OR (MH "Maternal Health Services") OR (MH "Preventive Health Care") OR (MH "Diagnostic Services+") OR (MH "Health Education+") OR (MH "Postnatal Care+") OR (MH "Community Health Nursing+") (160,946)
S10 (MH "Community Health Centers") (2,458)
S9 (MH "Outpatients") OR (MH "Outpatient Service") OR (MH "Ambulatory Care Facilities+") (38,658)
S8 TI ( (role or outpatient? or community or out‐patient? or ambulatory) ) OR AB ( (role or outpatient? or community or out‐patient? or ambulatory) ) (227,897)
S7 (MH "Pharmacists") OR TI Pharmacist* (4,841)
S6 TI (pharmacist* n2 role*) OR AB ( ((pharmacist* n2 role*) N3 (chang* or new or increas*)) ) (182)
S5 TI ( (pharmacist* n2 advice) or (pharmacist* n2 consult*) or (pharmacist* n2 counsel*) or (pharmacist* n2 initiated) or (pharmacist* n2 intervention) ) OR AB ( (pharmacist* n2 advice) or (pharmacist* n2 consult*) or (pharmacist* n2 counsel*) or (pharmacist* n2 initiated) or (pharmacist* n2 intervention) ) (418)
S4 TI community pharma* (400)
S3 AB (pharmacist* n2 evaluation) or (pharmacist* n2 managed) or (pharmacist* n2 care) or (pharmacist* n2 comanag$) or (pharmacist* n2 manag*) or (pharmacist* n2 delivered) or (pharmacist* n2 directed) or (pharmacist* n2 educator*) or (pharmacist* n2 led) or (pharmacist* n2 outreach) or (pharmacist* n2 prescrib*) (465)
S2 TI ( evaluation or managed or care or comanag$ or management or delivered or directed or educator* or led or outreach or prescrib* ) AND TI pharmacist* (594)
S1 TI non‐dispensing OR AB non‐dispensing OR TI ( (pharmacist* n2 physician*) OR (pharmacist* n2 doctor*) ) OR AB ( (pharmacist* n2 physician*) OR (pharmacist* n2 doctor*) )
ProQuest Dissertations & Theses
(TI(pharmacy OR pharmacist) AND TI(community OR outpatient OR multidisciplinary OR delivery))
ClinicalTrials.gov
WHO International Clinical Trials Registry Platform (ICTRP)
Search terms:
Community pharmacy
Community Pharmacist
Outpatient pharmacy
Outpatient pharmacist
Out‐patient pharmacy
Out‐patient pharmacist
Appendix 2. Outcome Measures by Clinical Condition
COPD: 1. Forced expiratory volume (FEV1) 2. Forced vital capacity 3. MRC Dyspnoea Score (or other validated COPD (chronic obstructive pulmonary disease) score) 4. BMI 5. Saturated oxygen (if severe disease) 6. Symptom control might be measured with some or all of the following:Breathlessness, Exacerbation frequency, Exercise tolerance
Depression 1. BDI 2. BAI 3. Patient satisfaction
Diabetes 1. Blood glucose 2. HbA1c mmol/mol 3. HbA1c % 4. Diabetes Quality of life
Hypertension 1. Systolic
2. Diastolic
Asthma 1. Validated asthma tool 2. Lung function: measured as FEV1 or PEF 3. Number of exacerbations 4. No daytime symptoms 5. No nighttime wakening
Polypharmacy
1. Adherence 2. Number of hospitalisations 3. Mortality 4. Drug related problems
5. Self rated health
6. Number of drugs
Posthospitalization care transitions 1. Hospital admissions 2. Emergency room attendance 3. Resolution of medicine discrepancies 4. Health care use (contacts and hospital care)
Bipolar disorder 1. Number of hospitalisations 2. Number of emergency consultations 3. Number of unscheduled outpatient visits
HIV 1. Adherence 2. Depressoin 3. Alcohol consumption
Mental illness 1. Metabolic risk 2. % Taking antipsychotics 3. Number of metabolic syndrome risk parameters
Anticoagulation 1. Therapeutic INR (anticoagulation) achieved 2. Bleeding 3. Hospital readmission due to anticoagulation problem.
Anti‐psychotics / metabolic syndrome
1. Number of metabolic syndrome risk parameters Osteoporosis 1. Satisfaction 2. Knowledge
Data and analyses
Comparison 1. Pharmacist services targeted at patients versus the delivery of no comparable service.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % outside blood pressure range | 18 | 4107 | Odds Ratio (M‐H, Random, 95% CI) | 0.40 [0.29, 0.55] |
| 2 % outside HbA1c range | 5 | 558 | Odds Ratio (M‐H, Random, 95% CI) | 0.29 [0.04, 2.22] |
| 3 Hospital attendance/admission | 14 | 3631 | Odds Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.11] |
| 4 Adverse drug effects | 3 | 590 | Odds Ratio (M‐H, Random, 95% CI) | 1.65 [0.84, 3.24] |
| 5 SF‐36 Physical Functioning | 7 | 1329 | Mean Difference (IV, Random, 95% CI) | 5.84 [1.21, 10.48] |
| 6 Mortality | 9 | 1980 | Odds Ratio (M‐H, Random, 95% CI) | 0.79 [0.56, 1.12] |
| 7 HbA1c (%) | 15 | 2298 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐0.97, ‐0.58] |
| 8 Fasting blood glucose (mmol/l) | 8 | 1349 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐1.71, ‐0.63] |
| 9 Diastolic blood pressure (mmHg) | 31 | 5939 | Mean Difference (IV, Random, 95% CI) | ‐3.50 [‐5.44, ‐1.56] |
| 10 Systolic blood pressure (mmHg) | 32 | 6003 | Mean Difference (IV, Random, 95% CI) | ‐5.96 [‐7.35, ‐4.57] |
| 11 Total cholesterol (mmol/l) | 7 | 1592 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.56, ‐0.13] |
| 12 LDL Cholesterol (mmol/l) | 6 | 854 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.30, 0.02] |
| 13 FEV1 | 3 | 291 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.01, 0.23] |
| 14 Peak Flow (%) | 2 | 460 | Mean Difference (IV, Random, 95% CI) | 3.36 [‐0.36, 7.09] |
| 15 Dyspnoea | 2 | 820 | Odds Ratio (M‐H, Random, 95% CI) | 0.90 [0.68, 1.20] |
Comparison 2. Pharmacist services targeted at patients versus services delivered by other health professionals.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Systolic blood pressure (mmHg) | 3 | 1238 | Mean Difference (IV, Random, 95% CI) | 1.31 [‐6.22, 8.84] |
| 2 Diastolic blood pressure (mmHg) | 2 | 959 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐4.30, 1.59] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adibe 2013a.
| Methods | Randomised trial | |
| Participants | 220 patients with diabetes (intervention 110; control 110) 2 urban tertiary teaching hospitals Nigeria Year of study: Not stated. |
|
| Interventions | In the pharmaceutical care (PC) group, pharmacists set priorities for patient care, assessed educational needs, identified drug‐related problems, developed a PC plan in collaboration with the patient and the doctor, implemented, monitored and reviewed the plan. Nurses organised patients, conducted point‐of‐care testing, counselled patients,and reinforced the information given to the patients during training sections. Physicians provided the visitation/appointment schedule for the patients, prescribed laboratory tests, and implemented changes in medications. 4 sessions of 90 to 120 minutes Duration 12 months |
|
| Outcomes | Health‐related Quality of Life (HRQoL) | |
| Notes | Funding source: Science and Technology Education Post Basic (STEP‐B) through the University of Nigeria. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised to groups by using online “random sequence generator” |
| Allocation concealment (selection bias) | Low risk | Quote "Allocation was also sorted through online “random sequence generator” which was set in a 2‐column format: the first column was priori designated to the intervention group (55 patients) and the second column to the control group (55 patients)" (per hospital 220 total). |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | No mention of participant blinding |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Assessors were not blinded and a self‐report outcome for HRQoL used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Attrition bias or loss during follow‐up was also a serious threat but was avoided by using an intention‐to‐treat design." Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | None |
Adler 2004.
| Methods | Randomised trial | |
| Participants | 533 patients with depression and/or dysthymia (intervention: 268; control: 265) . 9 Eastern Massachusetts primary care practices USA Year of study: Not stated. |
|
| Interventions | Pharmacists assessed a range of variables; medication history, medication regimen for drug issues, drug efficacy and toxicity, education about depression including symptoms and antidepressants, encouraged anti‐depressant therapy and maintained strong therapeutic communication with patients. This was tailored towards the patient's needs in accordance with depression guidelines. Pharmacists spent 70 minutes per patient across a 6‐month period; minimal intervention was to be 9 appointments over 18 months. | |
| Outcomes | Modified Beck Depression Inventory (mBDI) at 6 months | |
| Notes | Funding source: National Institute of Mental Health under grant RO1 MH56214 Conflict of interest: None stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a "computerised coin‐flip" built into the screener |
| Allocation concealment (selection bias) | Low risk | Randomisation is post‐enrolment |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Outcome are self‐reported and no blinding of personnel or participants |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Non‐blinded patients acted as their own assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Albsoul‐Younes 2011.
| Methods | Randomised trial | |
| Participants | 253 hypertension patients (intervention: 131; control: 122) General hospital Amman, Jordan Year of study: March to November 2009 |
|
| Interventions | Patients met with a pharmacist for 20 ‐ 30 minutes before seeing their physician each month for 6 months. Pharmacists took information on medication history, encouraged compliance, adherence to pharmacological and non‐pharmacological therapy and responded to questions. They also educated the patients about healthy lifestyle using education materials and self‐monitoring of BP. Recommendations were offered to the physician, with notes about cost‐effective drug choices. | |
| Outcomes | Reduction in systolic blood pressure (SBP) at 6 months; Reaching goal BP (SBP < 140 mmHg, diastolic BP < 90mmHg; for diabetic patients it was SBP < 130 mmHg, diastolic BP < 80 mmHg) | |
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by 'coin tossing' |
| Allocation concealment (selection bias) | Low risk | Randomisation is post‐enrolment |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Quote: "Patients were not informed of their study allocation, neither were the physicians, nor the nursing team" but the personnel were aware. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Personnel and possibly patients were aware of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate 97%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Ali 2012.
| Methods | Randomised trial | |
| Participants | 46 participants with type 2 diabetes (intervention 23; control 23) 2 branches of a pharmacy chain in Hertfordshire United Kingdom Year of study: February 2008 and July 2009. |
|
| Interventions | Intervention group received a pharmaceutical care package with regular monitoring and consultations with the community pharmacist for 12 months. Pharmacists carried out a targeted medicine use review (if required) and lifestyle modification counselling with a referral to a general practitioner or other healthcare professional where appropriate. Patients were seen by the pharmacist every month for the first 2 months, and then every 3 months a total of 6 appointments. Duration 12 months |
|
| Outcomes | HbA1C Blood glucose Diabetes Quality of Life |
|
| Notes | Funding source: UK Department of Health. Equipment from Merek Sharp and Dohme Conflict of interest: No party had involvement in the design, conduct or analysis or preparation of the manuscript. However, Professor Robinson from Merck Sharp and Dohme Ltd helped in the analysis and manuscript preparation but received no consulting fee. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted by a computer‐generated randomised list held by the researcher at the School of Pharmacy, eliminating the potential influence of pharmacists on the randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No relevant information provided |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Differences in implementation of the intervention are legitimate parts of the intervention. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Assessors (participants/self‐report) were not blind to intervention but HbA1c is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants missing. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All main outcomes reported |
| Other bias | Low risk | None |
Amariles 2012.
| Methods | Randomised trial | |
| Participants | 714 patients with cardiovascular disease or who were at risk (intervention: 356; control: 358) Multi‐site across 13 Spanish regions. 60 community pharmacies invited and 40 pharmacists performed assessments, suggesting that 40 of the 60 pharmacies participated. Spain Year of study: September 2006 to June2007. |
|
| Interventions | Intervention reviewed drug and clinical records, assessing health problems with current drug therapy, aim for drug therapy outcomes, and educate about cardiovascular risk, prevention and relevance to patient. There were 5 flexible appointments across 32 weeks. | |
| Outcomes | Diastolic blood pressure at 8 months Systolic blood pressure at 8 months Total cholesterol at 8 months in mg per dL |
|
| Notes | Funding source: Funded in part by Roche Diagnostilcs. Emilio García‐Jiménez employed by Stada Laboratory. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each pharmacy entered into the study when the pharmacist submitted by fax or email the record of the first patient who fulfilled the inclusion criteria. Once the study’s coordinator verified the fulfilment of the inclusion criteria, he randomly assigned 1 of the mentioned 50 groups to the pharmacy, providing it with a sequence of 20 codes (ONE or ZERO) that determined which patient was assigned to the intervention group or the control group." Unclear if the study co‐ordinator knew the participants allocation to control or intervention before he decided if they met criteria. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Quote: "Due to the nature of the intervention, participant blinding was not possible. There was no “placebo” treatment, and after randomization, patients were informed of their group assignments." |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | BP is measured by pharmacist aware of allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate 90%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Andres 2007.
| Methods | Randomised trial | |
| Participants | 112 participants with type 2 diabetes (intervention 58; control 56) 144 community pharmacies in the province of Pontevedra Spain 26 pharmacists Year of study: February 2003 to March 2004. |
|
| Interventions | Drug knowledge was assessed by pharmacists using the "Dáder" method (a process for pharmacist follow‐up of patients who are receiving medication). Compliance with medication was assessed using a modified Morisky‐Green questionnaire. Every 3 months Duration 12 months |
|
| Outcomes | HbA1C | |
| Notes | Published in Spanish Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information of randomisation procedure provided |
| Allocation concealment (selection bias) | Unclear risk | No relevant information found |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | In this complex intervention, the personnel are unlikely to have been blinded; implications for performance bias are unclear |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | HBA1c is unlikely to be biased by outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low dropout rate. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All main results reported |
| Other bias | Low risk | None |
Armour 2007.
| Methods | Randomised trial | |
| Participants | 396 asthma patients (intervention 191; control 205) Recruited from 50 pharmacies New South Wales, Queensland and Victoria, Australia. Year of study: November 2004 to July 2005 |
|
| Interventions | Pharmacy Asthma Care Program intervention included targeted counselling and asthma education, medication and lifestyle issues, review of inhaler technique, drug‐related problems, goal setting and review, and possible GP referral. This was developed through 3 visits across a 6‐month period, plus an optional visit at 3 months. | |
| Outcomes | Forced Expiratory Volume (FEV1) at 6 months Mean change in FEV1 from baseline Asthma severity at 6 months |
|
| Notes | Funding source: Australian Department of Health and Ageing as part of the Third Community Pharmacy Agreement. Conflict of interest: None stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Quote: "Pharmacists were not informed as to group allocation; both groups were informed that they were providing an asthma care service involving spirometry. Pharmacies were asked to recruit up to 10 subjects from their customers." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Quote: "Pharmacists were not informed as to group allocation; both groups were informed that they were providing an asthma care service involving spirometry." |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Participants were unblinded and this may have influenced measurement of FEV. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall competion rate 91%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Barbanel 2003.
| Methods | Randomised trial | |
| Participants | 24 patients with asthma (12 intervention group, 12 control group) Community pharmacy in Tower Hamlets, East London United Kingdom Year of study: Not stated. |
|
| Interventions | Pharmacists reviewed inhaler technique, provided personal education on a variety of asthma‐related topics and followed up with patients with weekly telephone calls, vs usual care. Length of intervention ‐ 45 to 60 minutes initial education session and weekly telephone calls Number of interventions ‐ 12 during 3 months | |
| Outcomes | Improvement in asthma symptoms based on North of England asthma symptom scale | |
| Notes | Funding source: Not specified Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were then randomised using sealed envelopes to intervention or control groups". Unclear how random sequence generated |
| Allocation concealment (selection bias) | Low risk | Patients were randomised using sealed envelopes to intervention or control groups. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel unblinded but all differences likely to be legitimate parts of intervention |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Participants were not blinded. Main outcome was subjective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Only 1 outcome measured, which is reported |
| Other bias | Low risk | None |
Bernsten 2001.
| Methods | Cluster‐randomised trial | |
| Participants | 2454 general/elderly patients (Intervention 1290; Control 1164) (86 control sites and 104 intervention sites) Community pharmacies in Denmark, Germany, The Netherlands, Northern Ireland (co‐ordinating centre), Portugal, Republic of Ireland and Sweden Year of study: Not stated. |
|
| Interventions | Community pharmacists provided pharmaceutical care to patients in the intervention group including patient assessment, identification of actual and potential drug‐related problems (e.g. poor compliance, poor knowledge, adverse drug reactions). Data sources included (i) the patient (by informal questioning); (ii) the patient’s general practitioner (GP); and (iii) pharmacy‐held records. Pharmacy interventions included: (i) educating the patient about drug regimen and medical condition(s); (ii) implementing compliance‐improving strategies such as drug reminder charts; and (iii) rationalising and simplifying drug regimens in collaboration with the patient’s GP. Continuous process Duration 18 months |
|
| Outcomes | Hospitalisations over past 18 months | |
| Notes | Funding source: European Commission, under the BIOMED 2 programme for medical research, funded the coordination of this multicentre study Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Half of the recruited sites were randomly assigned as control sites and half as intervention sites and, where possible, control and intervention sites were matched as closely as possible according to size (e.g. total number of patients served), situation (e.g. city centre vs village) and type (e.g. owned by a single proprietor vs part of a national chain). |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Not stated but unlikely due to intervention pharmacist training |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Hospitalisations are an objective measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% but, large changes in sample size due to some arms only running for 6 or 12 months. |
| Selective reporting (reporting bias) | Unclear risk | Major results are reported. Unclear why some results presented by country and some averaged across all |
| Other bias | Unclear risk | Intervention was not the same across all countries Quote: "Each country adapted the manual, translating and modifying sections where appropriate, according to differing national practices." |
Blalock 2010.
| Methods | Randomised trial | |
| Participants | 186 elderly participants (intervention 93; control 93). 100 community pharmacies from the same chain, located in North Carolina USA Year of study: Not stated. |
|
| Interventions | Quote "Intervention was a face‐to‐face medication consultation conducted by a community pharmacy resident. The pharmacist reviewed the patient’s medications and identified potential problems in their drug therapy. Special attention was given to medications that have been found to increase the risk of falling, with an emphasis on Central Nervous System (CNS)‐active medications using structured algorithms. Control group received no medication consultation. Participants in both groups received a packet containing 2 brochures on the prevention of falls developed by the Centers for Disease Control and Prevention (What You Can Do to Prevent Falls and Check for Safety: A Home Fall Prevention Checklist for Older Adults)." 1 45‐minute meeting Duration 12 months |
|
| Outcomes | Number of falls | |
| Notes | Funding source: National Center for Injury Prevention and Control at the Centers for Disease Control and Prevention (R49 CE000196). Conflict of interest: The authors wish to acknowledge Joseph T. Hanlon, PharmD, and Cathleen S. Colón‐Emeric, MD, for their assistance with the development and refinement of the algorithms used in this study. The authors have indicated that they have no other conflicts of interest regarding the content of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Random assignments will be based on a list of random numbers generated using statistics software package" |
| Allocation concealment (selection bias) | Low risk | Quote "620 envelopes will be prepared such that each envelope includes a card on which either 'Experimental Group' or 'Control Group' is written. The envelopes will be sealed and arranged sequentially, by the list of random numbers." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Protocol states that participants were blinded but pharmacists were not. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote "To monitor data quality, all data collection instruments will be reviewed by a research assistant immediately upon their return by study participants. In cases where participants have missed items or provided incomplete, illegible, or ambiguous information, the research assistant will follow‐up with the participant by telephone to obtain the needed information. The research assistants will be blinded to participants' experimental group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High attrition rate but reported as intention‐to‐treat analysis. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Bogden 1998.
| Methods | Randomised trial | |
| Participants | 95 hypertensive patients (intervention 49; control 46). Single hospital outpatient clinic USA Year of study: Not stated. |
|
| Interventions | Both control and intervention arms included strategy and treatment planning with a physician. Intervention patients also received recommendations from a pharmacist for half an hour before each physician visit. 3 visits over 6‐month period | |
| Outcomes | Diastolic Blood Pressure (DBP) at 6 months Systolic Blood Pressure at 6 months % of patients who achieved target blood pressure goals of less than 140 mm Hg for systolic blood pressure and less than 90 mm Hg for diastolic blood pressure in the control and intervention groups |
|
| Notes | Funding source: Queen's medical Centre, Honolulu. research Centres in MinoritiesInstituions Aard(P20 RR11091) from the National Institutes of Health. Conflict of interest: Not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomised by odd/even last digits of social security number |
| Allocation concealment (selection bias) | Low risk | Due to randomisation type no influence of allocation |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Participants were not told to which group they were allocated but would most likely know due to the study procedures. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | BP measured by blinded nurses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall competion rate 92%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Bond 2000.
| Methods | Randomised trial (by medical practice) | |
| Participants | 3074 patients on repeat medications (intervention 1614; control 1460)
Health professionals (delivering intervention): 62
Practices: 19 University‐affiliated setting Medical practices in Grampian, United Kingdom Unit of analysis mismatch corrected (randomised by practice, analysed by patient; analysis accounted for clustering effect) Year of study: 1995 ‐ unclear. |
|
| Interventions | Pharmacist dispensed repeat prescriptions following a protocol to check whether items were required, or patients were experiencing side effects or drug interactions, vs usual care Length of the intervention: not clear Number of interventions: 12 during 12 months | |
| Outcomes | Death rate Adverse drug reactions Hospital admissions |
|
| Notes | Funding source: Grampian Health Board Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised to either the control or intervention group using random‐number tables |
| Allocation concealment (selection bias) | Low risk | Random‐number tables were used. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel unblinded, but there appears to be little potential for bias in implementation of repeat prescriptions. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Unblinded and may or may not have influenced assessment of outcome variables (adverse drug problems) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of missing patients. Large between group attrition >40%. |
| Selective reporting (reporting bias) | Low risk | Main outcomes were reported |
| Other bias | Low risk | None |
Borenstein 2003a.
| Methods | Randomised trial | |
| Participants | 197 hypertensive patients (intervention; 98 control) 2 main offices of one medical practice of general internists and internal medicine sub‐specialists affiliated with a large community hospital USA Year of study: 1996 to 1998 |
|
| Interventions | The intervention was made up of visits by pharmacist who assessed adherence to antihypertensive drugs, side effects, patient habits in accordance with guidelines as well as education about lifestyle modifications. Also follow‐up visits with physicians for treatment plans. On average there were 8 provider interactions over a 12‐month period. | |
| Outcomes | Systolic blood pressure at 12 months Number achieving blood pressure goals at 12 months |
|
| Notes | Funding source: Not specificed. Conflict of interest: Not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear what method of randomisation was used |
| Allocation concealment (selection bias) | Unclear risk | Unclear if patients or personnel were aware of allocation during recruitment |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | BP measurement has low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Unclear regarding method of BP measurement and whether assessor was blind to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 99/635 and 98/637 completed. Between group attrition < 10% but overall attrition >80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Bosnic‐Anticevich 2010.
| Methods | Randomised trial | |
| Participants | 52 patients with either asthma or chronic obstructive pulmonary disease (intervention 26; control 26) 8 community pharmacies Sydney, Australia Year of study: Not stated. |
|
| Interventions | Intervention was given written, verbal and demonstrated instructions on how to use an inhalation device. This education occurred once and was assessed monthly. | |
| Outcomes | Number achieving full‐technique score (8/8) at 4 months | |
| Notes | Funding source: Not specified. Conflict of interest: None stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by means of computer generated random group allocation, prior to study commencement." |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random group allocation, prior to study start |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Participants were blinded to allocation, but the experimenter was not. This may have led to differences besides those specified in the protocol. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Subjective outcome, with researchers measuring and conducting analysis not appearing to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% however, High (˜20%) overall attrition which was related to perception of value |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | High risk | Very small sample size |
Boyd 2013.
| Methods | Randomised trial | |
| Participants | 500 patients starting a new medicine for asthma/chronic obstructive pulmonary disease, type 2 diabetes, hypertension or antiplatelet/anticoagulant treatment (interventioon 250; control 250) Community pharmacy United Kingdom (England) Year of study: Not stated. |
|
| Interventions | Patients randomised to the intervention arm received the New Medicines Service (NMS). The NMS includes patient engagement, intervention and follow‐up. | |
| Outcomes | Unclear. Medication adherence is one of the outcomes for analysis. | |
| Notes | No useable quantitative data Funding source: Department of Health Policy Research Program. Conflict of interest: Not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients randomised 1:1 into 1 of the 2 study arms stratified by drug/disease group within each pharmacy, using the statistical software |
| Allocation concealment (selection bias) | Low risk | Quote: "sequentially numbered tamper‐proof opaque sealed envelopes containing details of allocation group" used. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Unclear until exact methods and outcomes published |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Main outcome: self‐reported adherence |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Results not yet reported |
| Selective reporting (reporting bias) | Unclear risk | Results not yet reported |
| Other bias | Unclear risk | Results not yet reported |
Brook 2003.
| Methods | Randomised trial | |
| Participants | Patients with depression: 135 (intervention 64; control 71)
Health professional (delivering intervention): 19
Practice: not clear Community pharmacy The Netherlands Year of study: April 2000 to April 2001. |
|
| Interventions | Pharmacist coaching patients and take‐home video, vs usual care Length of the intervention: not clear Number of interventions: 3 during 6 months | |
| Outcomes | Disease control assessed by self‐rating 90‐item (Hopkins) Symptom Checklist (SCL‐90) | |
| Notes | Required 75 patients in arm to detect 13% difference in depression at significance level of 0.05. No useable quantitative data. Funding source: Organon unconditionally sponsors International Health Foundation. The study received an unconditional grant from GlaxoSmithKline Conflict of interest: The study was carried out without interference of either of the companies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred at patient level and with a 1:1 ratio, using block randomisation to ensure equal numbers of intervention and control patients by pharmacy. |
| Allocation concealment (selection bias) | Low risk | Randomisation used "block randomization". The whole sample was randomised before delivery to the pharmacies. These forms were precoded and delivered in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Quote: "Neither patients, nor pharmacists were blinded for group assignment" Unclear if this influenced intervention. Same pharmacists delivered both arms, therefore potential for contamination |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Subjective outcome in an unblinded trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | 1 outcome, appropriately reported |
| Other bias | Low risk | None |
Bruhn 2013.
| Methods | Randomised trial | |
| Participants | 193 participants with chronic pain (intervention (1) 70: intervention (2) 63: control 60) 6 general practices United Kingdom Year of study: March 201 to not stated. |
|
| Interventions | The intervention was pharmacist medication review with and without prescribing. Control patients received usual care. Patients attended a face‐to‐face consultation with the pharmacist at which a pharmaceutical care plan was agreed. The plan included medical history, current conditions; known allergies and adverse drug reactions; relevant laboratory results; pain‐related medications prescribed in the previous 10 years; current pain‐related prescription medications; current symptoms; lifestyle issues, including units of alcohol consumed each week; recommendations for changes to medication (if any); whether non‐pharmaceutical treatments had been considered; and any other relevant issues. In the prescribing arm, prescriptions for medicines were issued by the pharmacist. Patients were followed up either by phone or face‐to‐face, at each pharmacist’s discretion. |
|
| Outcomes | Chronic Pain Grade intensity | |
| Notes | Funding source: Medical Research Council (grant ID: 85356). Conflict of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | All participating pharmacists took part in a 2‐day course updating them about pain management. As part of the training, participants defined and agreed the treatment algorithm they would all use. |
| Allocation concealment (selection bias) | Low risk | Patients returning completed questionnaires were randomised by the researcher using a telephone randomisation service with a random number allocation which ensured allocation concealment. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel were necessarily unblinded, but this is unlikely to bias the results. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Largely self‐report and, as patients are unblinded, susceptible to bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition >10%. |
| Selective reporting (reporting bias) | Low risk | All main results reported |
| Other bias | Low risk | None |
Capoccia 2004.
| Methods | Randomised trial | |
| Participants | Patients with depression: 74 (intervention 41; control 33)
Health professional (delivering intervention): 2
Practice: 1 University‐affiliated teaching clinic Outpatient clinic in USA Year of study: Not stated. |
|
| Interventions | Pharmacist collaborating with primary care physicians (PCPs) to provide patient education, antidepressant therapy adjustment, monitoring of adherence and adverse drug reactions and prevention of relapse, vs usual care Length of the intervention: 15 minutes Number of interventions: 13 during 12 months | |
| Outcomes | Disease control using 20‐item Hopkins Symptom Checklist (SCL‐20) | |
| Notes | Not all patients completed 13 sessions
55 patients in each arm required to detect a difference of 28% in clinical improvement rates at 0.05 significance level. Funding source: Aetna Quality of Care Foundation Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | High risk | Not explicitly mentioned in paper |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Unblinded, but likely that all personnel actions fall within protocol directions |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Assessors (participants/self‐report) unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate 91%. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported. |
| Other bias | Unclear risk | Quote: "Data collection was conducted via telephone interviews and thus subject to recall bias." |
Carter 2008.
| Methods | Randomised trial | |
| Participants | 243 hypertension patients (intervention 127; control 116) 5 primary care clinics (intervention 2; control 3). Iowa, USA Year of study: January 2004 to October 2006. |
|
| Interventions | Intervention to address suboptimal medication regimens and poor medication adherence; through strategy planning, adherence aids, and home monitoring. Encouraged to attend 4 clinic meetings on top of baseline interview over 8‐month period, with optional additional visits or phone support | |
| Outcomes | Systolic and diastolic blood pressure (BP) at 4 and 9 months | |
| Notes | Funding source: National Heart, Lung, and Blood Institute (HL069801). Dr Carter supported by the Center for Research in Implementation in Innovative Strategies in Practice (CRIISP), Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (HFP 04–149). Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of clinics was performed using a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No information relevant to concealment of allocation provided. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Neither participants nor personnel were blinded. This may have led to extra intervention changes. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "Two different research nurses were dedicated to patients in either control sites or intervention sites to minimize contamination.", "Individual data elements were double‐entered into a database by a blinded data management team that included data technicians, the data manager, and the biostatistician" and "The 24‐hour results were used as a blinded objective outcome and were not made available to either the patient’s physician or the clinical pharmacist until the patient completed the trial". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Typical/planned BP measures reported |
| Other bias | Low risk | None identified |
Castejon 2013.
| Methods | Randomised trial | |
| Participants | 84 participants with diabetes and their support person (number allocated to each group not stated) Community organisation for under‐served Latinos Florida, USA Year of study: January 2010 to November 2010. |
|
| Interventions | 2 pharmacist‐led counselling sessions on medication, nutrition, exercise, and self‐care to promote behaviour change every 2 weeks for 6 weeks and a follow‐up clinical screening 3 months later Session included the Pharmacist Assessment and Reinforcement of Diabetes Self‐management (PARDS) (1) A 90‐minute focused discussion group (FDG) on type 2 diabetes knowledge, beliefs, and barriers and motivators to clinical and self‐management; (2) a video What is Diabetes (3) training in self‐monitoring of blood glucose |
|
| Outcomes | HbA1C | |
| Notes | Funding source: Centers for Medicare & Medicaid Services Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Unblinded assessors |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | HBA1C, unlikely to be biased |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition > 10%. High attrition rate overall. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | None |
Charrois 2006.
| Methods | Randomised trial | |
| Participants | 71 participants with high‐risk asthma (intervention 37; control 34) Community pharmacies in 2 remote rural communities Alberta, Canada Year of study: Not stated. |
|
| Interventions | Intervention patients received education on asthma (medications, inhaler technique, written asthma education materials and development of action plan), Optimisation of drug therapy and assessment of adherence with formal onward referral as needed to respiratory therapist or physician Follow‐up at 2 weeks by telephone call and at 1, 2, 4 and 6 months Duration: 6 months |
|
| Outcomes | Number of hospitalisations, Asthma Control Questionaire | |
| Notes | Funding source: Canadian Institutes of Health Research, Institute of Health Economics, University Hospital Foundation, and ASTHMA Study (Alberta Strategy to Help Manage Asthma) Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patient is randomised by an internet randomisation service through the Epidemiology Coordinating and Research (EPICORE) Centre, University of Alberta. |
| Allocation concealment (selection bias) | Low risk | As 2 sites did not have internet access, sealed envelopes are provided for randomisation. To help ensure balance, randomisation was done in blocks of 6 and stratified by site. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Performance of usual care may have been influenced by intervention. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Hospitalisation is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition > 10%, however, intention‐to‐treat analysis seems to have been undertaken. |
| Selective reporting (reporting bias) | Low risk | Main outcome measures reported |
| Other bias | Unclear risk | Contamination of the usual‐care group may have occurred, as the caregivers involved in the study were not blinded. As part of the study implementation, we met with all local physicians. |
Chisholm 2002.
| Methods | Randomised trial | |
| Participants | 26 participants with renal transplants (intervention 14; control 12) Tertiary teaching hospital clinics USA Year of study: Not stated. |
|
| Interventions | Intervention patients received input from a clinical pharmacist including medication review focused on controlling blood pressure, and (potential/actual) medication‐related problems. Recommendations for change communicated to nephrologists. For patients more than 8 months post‐transplant, there were pharmacist‐led monthly telephone follow‐ups. Duration 12 months |
|
| Outcomes | Systolic Blood Pressure, compliance rate | |
| Notes | Funding source: Carlos and Marguerite Mason Trust Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. No description, although "prospectively randomised" was stated |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Unblinded, but with objective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | The clinic nurse was blinded as to which patients were in the intervention or control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 dropouts. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Choe 2005.
| Methods | Randomised trial | |
| Participants | Patients: 80 (intervention 41; control 39) with diabetes
Professional (delivering intervention): unclear
Practices: 1 University‐affiliated internal medicine clinic Michigan, USA Year of study: Not stated. |
|
| Interventions | Pharmacist evaluated/modified therapy, educated on diabetes management and complications, performed screening processes and telephone follow‐up, vs usual care. Pharmacist discussed therapeutic recommendations with the primary care physicians, vs usual care Length of intervention: 1 hour Number of interventions: unclear number in 12 months, with another 12 months of follow‐up | |
| Outcomes | HbA1c | |
| Notes | Follow‐up for HbA1c measurement was 13.6 months for intervention group and 14.9 months for control group. Funding source: University of Michigan College of Pharmacy Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation within each stratum was simple: because the study was small, randomisation was done by hand, drawing numbers from a container that included “0” for the control group or “1” for the intervention group. |
| Allocation concealment (selection bias) | Unclear risk | Unit of randomisation by patient; drew numbers (0 or 1) from a container |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Unblinded complex intervention. No interaction in control group |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Unblinded trial, but main outcomes are unlikely to be biased due to objective outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Few outcomes, all reported |
| Other bias | Low risk | None |
Chrischilles 2014.
| Methods | Randomised trial | |
| Participants | 294 participants with acute coronary syndrome (intervention (1) 97; intervention (2) 100; control 97 A community health facility, a community hospital, and a local Arc (a national community‐based organization advocating for and serving people with intellectual and developmental disabilities) Iowa, USA Year of study: Not stated. |
|
| Interventions | Intervention was self‐management/health promotion workshops led by a trained facilitator and pharmacist‐led medication management compared with a 3rd arm (usual care). The intervention programme consisted of 8 weekly 2‐hour workshops. For the purpose of this review, we included only the self‐management/health promotion workshops led by a trained facilitator and pharmacist‐led medication management. | |
| Outcomes | Mean symptoms | |
| Notes | Funding source: This publication was supported by Grant Number 5R01DD000107 from The Centers for Disease Control and Prevention Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 3 people were randomised at a time using sealed envelopes that contained the assignment order that had been randomly pre‐assigned by computer. The envelopes were prepared by an individual not involved in the interventions or data collection. |
| Allocation concealment (selection bias) | Low risk | 3 people were randomised at a time using sealed envelopes that contained the assignment order that had been randomly pre‐assigned by computer. The envelopes were prepared by an individual not involved in the interventions or data collection. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Unblinded and allocation may have influenced the subjective outcome, mean symptoms. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Unblinded and subjective outcome of mean symptoms reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition > 25%, however, complete data available for 96% participants. |
| Selective reporting (reporting bias) | Low risk | Major results reported. Some post hoc analysis |
| Other bias | Low risk | None |
Clifford 2005.
| Methods | Randomised trial | |
| Participants | Patients: 180 (intervention 92; control 88)
Professional (delivering intervention): unclear
Practices: 1 University‐affiliated internal medicine clinic Australia Year of study: February 2001 to November 2002 |
|
| Interventions | Pharmacist assessed patients' drug regimen and clinical parameters, developed therapeutic plan, provided patient education about diet, exercise, compliance and home‐glucose monitoring, and forwarded patient information (medication lists, laboratory results, goals) to primary care pharmacists, vs usual care. Length of intervention: 5 to 30 minutes (average 15 minutes) Number of interventions: 8 in 12 months (face‐to‐face meetings at baseline, 6, and 12 months; 6‐weekly intervals by phone) | |
| Outcomes | HbA1c Fasting plasma glucose, blood pressure, serum lipids, urinary albumin‐to‐creatinine ratio |
|
| Notes | Funding source: The Raine Foundation, University of Western Australia, funded the FDS. R.M.C. was the recipient of a National Health and Medical Research Council of Australia PhD scholarship. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A subset of patients was randomised to the intervention or usual care by consecutive allocation |
| Allocation concealment (selection bias) | High risk | Quote: "randomised...by consecutive allocation" |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel were not blinded but all differences in behaviour between control and intervention arm appear to be legitimate parts of the intervention. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Assessors unblinded, but the main outcome does not allow for significant detection bias. HbA1c is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall competion rate >90% |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | None |
Cody 1998.
| Methods | Randomised trial (by patient) Similar control site: NOT CLEAR | |
| Participants | Community pharmacies of the Kaiser Permanente (number per group unclear)
Patients: 6000
Pharmacies: 9 USA Year of study: January 1993 to February 1995. |
|
| Interventions | Comparison of 3 models Control model: usual care before 1992 in California California state model (1992) which requires outpatient pharmacist to counsel all patients who receive new or changed prescription about directions for use, the importance of compliance, proper storage, and relevant precautions and warnings Kaiser Permanente (KP) model that focuses on a more comprehensive pharmacist consultation and other elements of pharmaceutical care on selected high‐risk patients Duration: 23 months | |
| Outcomes | Quality of life (SF‐36) | |
| Notes | Funding source: Kaiser Permanente Medical Care Program Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "random assignment study" |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly described; appears to have been performed by a central randomised scheme/computer system |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Large complex intervention with non‐blinded personnel |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Mailed survey, assessor is participant: A non‐blinded study with subjective outcome ‐ HRQoL |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall attrition rate > 50%. |
| Selective reporting (reporting bias) | Low risk | Few outcomes, all reported |
| Other bias | Low risk | None |
Cordina 2001.
| Methods | Randomised trial | |
| Participants | 152 participants with asthma (intervention 86; control 66) Community pharmacies Malta Year of study: Not stated. |
|
| Interventions | Intervention patients received community pharmacy‐led verbal counselling, an educational video, an information leaflet, and subsequent monitoring with reinforcement; The education included pathology, avoidance of triggers, use of inhaled drugs and peak flow meters, inhaler technique (verbal, written and video materials). Monitoring included patient‐completed diary cards of peak expiratory flow (PEF) (morning and evening) and symptoms. Community pharmacists reviewed monthly when the patients collected their asthma drugs. Pharmacists received information on the patient’s best peak flow value, smoking history, comorbidities, drug allergies, and prescribed drugs. There was referral to the asthma clinic as needed. Recommendations for treatment changes were made to the patient’s physician. Duration: 12 months. |
|
| Outcomes | SF‐36 Living with Asthma Questionnaire (LWAQ) PEF |
|
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States "random" but no mention of method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | The pharmacist at each site was invited to participate in the study and was informed of the allocation of control or intervention status. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Quote: "Given the nature of the intervention, patients, providers, and the case manager were not blinded to the intervention." |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Unblinded assessors: SF‐36 and LWAQ are high risk as they are subjective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% however, high attrition rates across groups. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
De Castro 2006.
| Methods | Randomised trial | |
| Participants | 71 hypertensive patients (intervention 34; control:37) Specialist clinic Porto Alegre, Brazil Year of study: Not stated. |
|
| Interventions | Intervention designed Dader method; obtain pharmacotherapeutic history, identify and challenge problems, and lifestyle changes to treat hypertension. Control received similar cognitive tests but focused only on drug‐related problems. 24‐week programme | |
| Outcomes | Diastolic and systolic blood pressure (BP) at 4 months 24‐hour systolic BP at 24 weeks |
|
| Notes | Funding source: FAPERGS, FIPE‐HCPA, CNPq Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation was done in blocks of 8 patients each and stratified by gender through a computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | The random allocation was done in blocks of 8 patients each and stratified by gender through a computer‐generated sequence. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Mentions double‐blinding, but unclear if this was successful. Patient was blinded. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Di Donato 2014.
| Methods | Randomised trial | |
| Participants | 302 participants with hypertension (number per group not stated) Community pharmacy chain stores USA Year of study: January 2012 to June 2012 |
|
| Interventions | Pharmacists synchronised all medication (re)fills, including antihypertensive medication(s), prior to the date when the next refill was due and pharmacists checked for any medication changes. At the point of refill pharmacists measured patient blood pressure. Duration: 4 months |
|
| Outcomes | Systolic blood pressure Diastolic blood pressure % within target blood pressure |
|
| Notes | Funding source: The Red Cross Pharmacy Residency Program is funded by a Community Pharmacy Residency Expansion Project grant from the National Association of Chain Drug Stores Foundation. This study was supported by HoMedics, Inc. through product donation. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were enrolled at six retail locations and randomized by research staff into three groups based on enrollment order: control, medication synchronization, or education". This may be less effective than true random allocation. |
| Allocation concealment (selection bias) | High risk | Quote: "Patients were enrolled at six retail locations and randomized by research staff into three groups based on enrollment order: control, medication synchronization, or education. Randomization occurred at the patient level, and within each pharmacy. " Investigators could foresee assignment: |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Most of the outcomes were objective and should be immune to strong bias. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Used an electronic blood pressure measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Statistical analyses were conducted by a 'per protocol' approach (i.e. patients lost to follow‐up were excluded). Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | None |
Doucette 2009.
| Methods | Randomised trial | |
| Participants | 78 diabetic patients (intervention 36; control 42) 7 community pharmacies Iowa, USA Year of study: Not stated. |
|
| Interventions | Discussions regarding medication, clinical goals, self‐care and recommendations for future medication, across 4 quarterly visits | |
| Outcomes | Systolic and diastolic blood pressure change scores Low density lipoprotein cholesterol (LDL‐C) (mg/dL) (change from baseline) HbA1C (%) (change from baseline) |
|
| Notes | Funding source: Community Pharmacy Foundation. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar dropout in both groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Edwards 2014.
| Methods | Randomised trial | |
| Participants | 200 participants with cancer (intervention 100; control 100) Cancer Centre Newfoundland, Canada Year of study: Not stated. |
|
| Interventions | The intervention patients received a visit from the seamless care pharmacist (SCP) prior to the initiation of chemotherapy. The visit included medication history reconciliation. The SCP checked medication against established regimen protocols, including a drug interaction check, recalculation of the dose, and verification of pertinent laboratory values. The patient’s hospital pharmacist, oncology nurse, and attending physician received copies of the report. The SCP counselled the patient on their treatment, identified and resolved any drug‐related problems. Patients were followed up by phone 2 days post‐chemotherapy to identify/resolve drug‐related problems. Duration: unclear |
|
| Outcomes | ||
| Notes | Control group outcomes not presented No useable quantitative data Funding source: Funded through unrestricted research grants from Pfizer, Amgen, and Roche. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised 1:1 to the intervention group or the control group in the clinical trials department using a random‐number generator. |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Unclear |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | High risk | No clear statement of outcomes |
| Other bias | High risk | Outcomes not presented. Length of study not stated |
Farsaei 2011.
| Methods | Randomised trial | |
| Participants | 174 patients with type 2 diabetes (intervention 87; control 87) Isfahan Endocrine & Metabolism Research Center (IEMRC) outpatient clinic Iran Year of study: April 2008 to January 2009 |
|
| Interventions | The intervention group received 2 pharmacist‐delivered educational sessions. The sessions included oral anti‐hyperglycaemic medications, adherence, self‐care management, diabetes diary log and pill box usage. Patient's glycaemic control in the intervention group was followed for 3 months through either telephone or face‐to‐face interviews with the pharmacist. A questionnaire containing patient demographics and lab results (HbA1c and fasting blood glucose) was filled by the pharmacist for each patient in the intervention group and advice was given according to her/his concerns about diabetes control. Patients were phoned or seen weekly for 3 months. Duration 3 months |
|
| Outcomes | % achieving target HbA1c Mean HbA1c Fasting blood glucose |
|
| Notes | Funding source: This study was funded from Isfahan University of Medical Sciences. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients were randomly selected among eligible patients who met inclusion‐exclusion criteria and then allocated into two groups: intervention and control.” |
| Allocation concealment (selection bias) | Unclear risk | No mention of concealment |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel were not blinded, but different staff educated control and intervention participants. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Non‐blinded assessment, but bias unlikely to influence HBA1c |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 59 of 87 intervention completed the trial, 86 of 87 control. Between group attrition > 30%. |
| Selective reporting (reporting bias) | Low risk | All main outcomes reported |
| Other bias | Low risk | None |
Faulkner 2000.
| Methods | Randomised trial | |
| Participants | 30 participants with congestive heart disease (CHD) (intervention 15; control 15) Patients were recruited from a hospital coronary care unit (but setting for intervention was domiciliary) USA Year of study: Not stated. |
|
| Interventions | Intervention patients were phoned weekly. Emphasis was placed on the importance of therapy in reducing the risk of recurrent cardiac events. Patients were questioned about when and where prescriptions were filled, how they paid for their prescriptions, potential side effects, overall well‐being, and specific reasons for noncompliance when applicable. Duration: 12 weeks |
|
| Outcomes | Total cholesterol Low density lipoprotein (LDL) High density lipoprotein (HDL) Triglycerides |
|
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised to telephone contact or no telephone contact using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation concealed. Patients were randomised to telephone contact or no telephone contact using a computer‐generated list of random numbers. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Potentially unblinded but objective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Unclear if blinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% completion rate. |
| Selective reporting (reporting bias) | Unclear risk | Main outcomes reported |
| Other bias | Low risk | None |
Finley 2003.
| Methods | Randomised trial | |
| Participants | 125 patients with depression (intervention 75; control 50)
Professional (delivering intervention): 2
Practice: 1 Outpatient clinic in Kaiser Permanente Medical Center San Rafael, USA Year of study: Not stated. |
|
| Interventions | Pharmacist managed medication regimens, conducted in‐clinic and telephone follow‐ups, and educated patients about medications and disease state, vs usual care. Length of the intervention: 30‐minute initial clinic visit, "brief" second and third clinic visits, 5‐ to 10‐minute telephone calls Number of interventions: 3 clinic visits + 5 telephone follow‐ups during 6 months | |
| Outcomes | Brief Inventory for Depressive Symptoms (BIDS) score % patients with ≥ 50% reduction in BIDS score % patients achieving remission (BIDS score < 9) % patients with reduction in Work and Social Disability Scale (WSDS) score | |
| Notes | Pharmacists met weekly with a psychiatrist ("psychiatric mentor") to present new patients and provide updates on other patients; the psychiatrist was also available for consultations as needed.
Study was powered to detect compliance outcomes only. Funding source: Sidney Garfield Memorial Fund Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to the collaborative care model or back to usual care in a 3:2 ratio" |
| Allocation concealment (selection bias) | Low risk | Used "sealed envelopes", no mention of whether envelopes were opaque. After the patients completed a brief survey to assess baseline depression severity (Brief Inventory for Depressive Symptoms (BIDS)) and functional impairment (Work and Social Disability Scale (WSDS)), the investigators opened a sealed envelope that determined study group assignment (intervention vs usual care) |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Providers were aware of intervention, but all differences between control and intervention arm are integral to the intervention. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Assessors (participants/self‐report) were unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rates. Between group attrition >20%. 79% of intervention and 50% of control participants returned the survey. |
| Selective reporting (reporting bias) | Low risk | All results reported |
| Other bias | Unclear risk | Potential for seasonality due to 6 months only |
García‐Cárdenas 2013.
| Methods | Randomised trial | |
| Participants | 65 pharmacices and 373 patients with asthma (intervention 208; control 165) Community pharmacies Spain Year of study: November 2010 to June 2011. |
|
| Interventions | Patients visited pharmacy at least 3 times according to need. The pharmacists recorded patient demographic details, and assessed asthma control, medication adherence and inhaler technique. Patients were educated using verbal instructions, physical demonstration and written information about inhaler use. Adherence was explored with the Beliefs about Medicines Questionnaire and Health Beliefs Model Duration: 6 months |
|
| Outcomes | % patients achieving correct inhaler technique, Asthma Control Questionnaire | |
| Notes | Funding source: The study was funded by the AstraZeneca Foundation, who did not interfere with the study design, collection statistical analysis, interpretation of the data and writing of the manuscript, nor in the decision to submit this manuscript for publication Conflict of interest:The study was funded by the AstraZeneca Foundation, who did not interfere with the study design, collection statistical analysis, interpretation of the data and writing of the manuscript, nor in the decision to submit this manuscript for publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Pharmacies were the unit of randomisation and were assigned by an independent researcher after they agreed to participate in the study to either intervention (IG) or control group (CG) using a computer‐generated list of random numbers with ratio 1:1. |
| Allocation concealment (selection bias) | Low risk | Pharmacies were the unit of randomisation and were assigned by an independent researcher after they agreed to participate in the study to either intervention (IG) or control group (CG) using a computer‐generated list of random numbers with ratio 1:1. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Quote: "Given the nature of the intervention pharmacists or patients could not be blinded." Outcomes are at high risk of bias. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Mostly self‐measured or measured by the pharmacists. Opportunity for bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Garção 2002.
| Methods | Randomised trial | |
| Participants | 100 hypertensive patients (intervention: 50; control: 50) 1 community pharmacy Maxial, Portugal Year of study: April 2000 to September 2000. |
|
| Interventions | Individualised intervention based on health promotion by pharmacist Monthly visits for 6 months | |
| Outcomes | Systolic and diastolic blood pressure (BP) at 9 months BP in target range at 6 months |
|
| Notes | Funding source: Not specified Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No randomisation technique described |
| Allocation concealment (selection bias) | Unclear risk | Allocation not described |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Uncear if blinded or consequences of non‐blinding |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Study pharmacist was not blinded and took all measures. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Gattis 1999a.
| Methods | Randomised trial | |
| Participants | 181 patients with heart failure or left ventricular dysfunction (intervention 90; control 91). General cardiology faculty clinic Durham, North Carolina, USA Year of study: October 1996 to July 1997. |
|
| Interventions | Pharmacists for intervention patients offered therapeutic recommendations to their attending physician and discussed changes in to drug therapy with patients. 3 follow‐up phone calls to talk through issues with drug therapy, answer questions and identify clinical events. All 4 interactions over 6 months | |
| Outcomes | All‐cause mortality and non‐fatal heart failure | |
| Notes | Funding source: American Society of Health Systems Pharmacists Research and Education Foundation, Duke Clinical Research Institute Conflict of interest: Not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Allocation occurred after randomisation |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | No blinding; unclear if this influenced delivery or other factors |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Non‐blinding unlikely to affect all‐cause mortality or heart failure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition rate unclear |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Endpoint was not the same for all participants, median of 6 months. Unclear how this would affect the results |
González‐Martin 2003.
| Methods | Randomised trial | |
| Participants | 21 patients with asthma (intervention 11; control 10)
Professional (delivering intervention): not clear
Practice: 1 Outpatient paediatric clinic affiliated with Catholic University Chile Year of study: Not stated. |
|
| Interventions | Pharmacist educated patients on medication therapy and inhaler use using asthma explanatory booklet and prescribed medications brochure, vs usual care. Length of the intervention: 30 minutes Number of interventions: 3 during 9 weeks | |
| Outcomes | Paediatric asthma quality of life questionnaire (PAQLQ) score: emotions, activities, symptoms domains Spirometry testing: Forced Vital Capacity (FVC), Forced Expiratory Volume (FEV1) | |
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "the child was assigned at random to one of the two groups of the study" |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Same unblinded personnel administered both intervention and control arms |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | Low risk | Few outcomes, all reported |
| Other bias | Low risk | None |
Goodyer 1995.
| Methods | Randomised trial | |
| Participants | 100 patients > 70 years (intervention 50;control 50) Outpatient clinics of the Medicine for Elderly Department at Charing Cross Hospital United Kingdom Year of study: Not stated. |
|
| Interventions | Verbal counselling on the correct use of medication + medication calendar and information leaflets Length of intervention: 3 domiciliary visits over a 6‐ to 12‐week period | |
| Outcomes | Compliance (pill count) defined as the % of the number that should have been consumed Patient knowledge Exercise test (distance in 6 minutes and distance until breathless) Clinical assessment Nottingham Health Profile Breathlessness when performing different activities | |
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated to intervention or control groups" |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described explicitly No information provided |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Personnel were not blinded. Unclear if this caused bias |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "clinical assessments [were] carried out by a physicians blinded to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Green 2008.
| Methods | Randomised 3‐armed trial | |
| Participants | 778 participants with hypertension: (Intervention (1) 202; intervention (2) 209; control 207 Setting is 10 primary care medical centres USA Year of study: Not stated. |
|
| Interventions | In the 2 intervention groups patients also received a self‐management support intervention (home blood pressure monitor and training and a web‐based service) in addition to usual care. In one of the intervention groups, a clinical pharmacist provided care management support by a single telephone call and subsequently the internet which provided a template for BP monitoring, current medication, a patient‐selected lifestyle goal, recommended medication changes and follow‐up plan. Communication thereafter was 2‐weekly by the web. Duration: 12 months |
|
| Outcomes | Systolic blood pressure (BP) Diastolic BP Quality of Life |
|
| Notes | Funding source: This research was funded by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health (NIH): Grant R01‐HL075263; Electronic Communications and Blood Pressure Monitoring (e‐BP). Conflict of interest: Dr Ralston received grant funding from Sanofi‐Aventis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Within these two groups, we randomly assign sequential blocks of three, six, or nine to the three intervention groups. Each study coordinator at a given centre is provided packets of nine envelopes from each of the two systolic blood pressure groups and told to take the first envelope from the top of the given blood pressure group to balance intervention assignment within centre and blood pressure groups". |
| Allocation concealment (selection bias) | Low risk | Quote: "Within these two groups, we randomly assign sequential blocks of three, six, or nine to the three intervention groups. Each study coordinator at a given centre is provided packets of nine envelopes from each of the two systolic blood pressure groups and told to take the first envelope from the top of the given blood pressure group to balance intervention assignment within centre and blood pressure groups". |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Main outcomes are objective. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "Recorded blood pressure taken by research assistant blinded to subject’s intervention group". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition overall. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All major results reported |
| Other bias | Low risk | None |
Hammad 2011.
| Methods | Randomised trial | |
| Participants | 199 patients with metabolic syndrome (intervention 112; control 90) 6 family medicine clinics at 1 university hospital Amman, Jordan Year of study: March 2009 to September 2009. |
|
| Interventions | Met with both pharmacist and physician. Pharmacists provided medication counselling, answered questions on self‐monitoring, lifestyle choices, compliance with drug therapy. Education materials were distributed discussing metabolic syndrome and increased risks. Monthly visits across 3 months | |
| Outcomes | Systolic and diastolic blood pressure at 6 months Fasting blood glucose (mg/dL) |
|
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin‐toss method |
| Allocation concealment (selection bias) | Unclear risk | Unclear if the recruiter knew the allocation status of the participant during the consent process |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Unblinded participants and personnel |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Blood pressure was measured monthly by assistant nurses who were blinded to the patient’s study arm assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate >80% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Hawes 2013.
| Methods | Randomised trial | |
| Participants | 61 participants (intervention 24; control 37) Academic medical centre USA Year of study: October 2009 to April 2011 |
|
| Interventions | Intervention group received a care transitions clinic visit with a clinical pharmacist 72 hours post‐discharge. The visit included medication history, identifying and resolving medication discrepancies, creating a current medication list and counselling on medication use. Discrepancies between the Best Possible Medication Discharge List (BPMDL) and the discharge summary were identified and characterised. | |
| Outcomes | Number of re‐hospitalisations | |
| Notes | Funding source: Funding from the American College of Clinical Pharmacy Ambulatory Care Practice and Research Network was used to provide compensation in the form of a $15 gift card from a large retail store to subjects for study participation. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | During the first year of the study, 30 patients were enrolled and a random number generator was used for randomisation. Because of unequal allocation of patients to the study arms, block randomisation with a block size of 4 was used for the second year of the study, during which 31 patients were enrolled. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient Information |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Potential for bias (non‐blinded) |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Seems unlikely. Rehospitalisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Unclear risk | Baseline data not shown in full |
Hawkins 1979.
| Methods | Randomised trial | |
| Participants | 1148 diabetic or hypertensive patients (or both) (intervention 574; control 574).
Episodes of care: 12,918
Professionals (delivering intervention): 2
Practices: 1 Outpatient primary care clinic Texas, USA Year of study: March 1976 to August 1978. |
|
| Interventions | Pharmacist management of drug therapy (physician not involved) vs usual care (physician only) Pharmacists prescribed drugs and modified drug therapy as needed. Length of intervention: 29 months | |
| Outcomes | Kept appointment rate Follow‐up clinic visits Hospital admissions Emergency Department visits Compliance Mean blood pressure Blood sugar level % of patients with decreased blood pressure % of patients with decreased blood sugar levels | |
| Notes | Intervention was delivered by pharmacists who were assisted by trainees. Funding source: DHEW public health service grant Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were assigned randomly into three groups" |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described explicitly No information provided |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel (pharmacists and doctors) were aware of allocation but all differences in implementation of the intervention are a legitimate part of the intervention. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Assessors were not blinded and the outcome blood pressure was assumed to be measured manually |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition > 10%. High overall attrition. Quote: "control groups experienced a significantly greater patient dropout rate and total attrition" 60.8% vs 48.8% completed |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Hay 2006.
| Methods | Randomised 3‐armed trial | |
| Participants | 325 patients with knee pain (enhanced pharmacy intervention 108; community physiotherapy intervention 109; control 108) 15 general practices North Staffordshire, England Year of study: May 2001 to March 2004. |
|
| Interventions | All participants were given a leaflet on knee osteoarthritis about self‐help and exercises. Enhanced pharmacy intervention aimed to optimise pharmacological pain control through drug therapy and reinforce self‐help messages (6 sessions over 10 weeks). Community physiotherapy intervention, which was exercises led by musculoskeletal community physiotherapists (3 ‐ 6 sessions over 10 weeks). Control was just written information (initial visit and 1 phone call 1 week later). | |
| Outcomes | WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) pain score at 12 months | |
| Notes | Funding source: Arthritis Research Campaign, North Staffordshire Primary Care Research Consortium, and the Department of Health National Co‐ordinating Centre for Research Capacity Development. NEF funded by a primary care career scientist award from the Department of Health and NHS R&D. Conflict of interest:None stated. The sponsors of the study had no role in the study design, data collection,data analysis,data interpretation,or writing of the report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number generator |
| Allocation concealment (selection bias) | Low risk | Quote: "We assigned each participant a unique study number, which corresponded with that on a sealed opaque envelope that contained information about participants’ allocated treatment and was issued to the participant by the study nurse." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | By necessity, participants and the health professionals delivering the interventions were not blind to allocation. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Study nurses and researchers who collected, entered, and analysed data were unaware of treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate >80% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Hendrie 2014.
| Methods | Randomised trial | |
| Participants | 245 participants with type 2 diabetes (intervention 119; control 126) 8 metropolitan community pharmacies Perth, Western Australia Year of study: May 2003‐ not stated |
|
| Interventions | Patients in the intervention group received a pharmacist‐led Diabetes Management Education Program (DMEP) Responses to the Diabetes Patient Assessment Questionnaire (DPAQ) were entered into a pharmaceutical care software programme. Based on computerised feedback, the developed personal treatment targets for the patient provided patient education materials.The pharmacist followed up with patients at 1, 3 and 6 months, to review and monitor progress, and support adherence. Duration: 6 months |
|
| Outcomes | SF‐36 | |
| Notes | Funding source: Not specified Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "We paired them based on geographical location and the socioeconomic status of the population served, and then randomly selected one pharmacy in each pair to be in the intervention (DMEP protocol) group, with the other assigned to the control (standard care) group" Randomisation technique not specified |
| Allocation concealment (selection bias) | Unclear risk | No relevant information provided |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel were not blinded, but differences in behaviour are legitimate parts of the protocol. Separate personnel for intervention and control groups |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | In the self‐report outcomes, participants (assessors) were not blinded to outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Quote: "Thirteen intervention group patients (18.6%) and 17 control group patients (18.9%) dropped out of the study for various reasons." |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned are reported |
| Other bias | Low risk | None |
Hirsch 2014.
| Methods | Randomised trial | |
| Participants | 667 participants with hypertension (intervention 339; control 328) University general internal medicine clinic California USA Year of study: July 2010 to June 2012. |
|
| Interventions | Quote "Intervention patients received 4 x 30‐minute pharmacist visits (baseline, 3, 6, and 9 months). The pharmacist assessed the patient’s knowledge of hypertension, current treatment and treatment goals, self‐monitoring behavior, medical and medication history, and current medications. The pharmacist helped the patient to set individual BP goals, reviewed and/or ordered laboratory tests, made adjustments to the antihypertensive‐medication regimen. Each visit was documented. During subsequent visits, the pharmacist reviewed progress laboratory values, adherence, and self‐monitoring behavior and continued to make changes to the antihypertensive‐medication regimen as needed. A physician was always present in the practice and available for consultation as needed." Duration: 9 months |
|
| Outcomes | % achieving target blood pressure Systolic blood pressure (BP) Diastolic BP |
|
| Notes | Funding source:This research was funded by National Institutes of Health (NIH)/National Heart, Lung and Blood Institute grant no. 1RC2HL101811‐01 and by NIH grant nos. UL RR031980 and UL1TR000100. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients were randomly assigned, by a computer‐generated random sequence, to either the intervention group or the usual‐care group. |
| Allocation concealment (selection bias) | High risk | Intervention group participants were randomised before being invited to participate. Control participants were not contacted as no additional care/measurement took place. Many intervention participants declined to participate, creating significant potential for bias. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Unlikely to affect, objective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Quote: "In the PharmD‐PCP MTM [intervention] group, the pharmacist measured the blood pressure (BP) at the beginning of each study visit, as was standard practice for all internal medicine clinic patients, whereas the nursing staff measured BP in the usual‐care patients." Systematic differences in measurement likely to create detection bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition > 30%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | High risk | Additional inclusion criteria were applied to the intervention group after randomisation. Quote: "An additional inclusion criterion of having had a clinic visit in the 6‐month period before screening was applied to ensure that data from only patients who continued to receive primary care pharmacist care for at least 9 months after the index visit were included." |
Ho 2013.
| Methods | Multi‐centre randomised trial | |
| Participants | 253 participants with acute coronary syndrome (ACS) (intervention 129; control 124) 4 Veterans Affairs (VA) medical centres USA Year of study: July 2010 to March 2013. |
|
| Interventions | The intervention comprised four components: 1. Quote "Medication reconciliation: Within 7 to 10 days of hospital discharge, a pharmacist met/phoned patients to address medication problems or adverse effects and reconcile differences in medications between the pre‐hospital and post‐discharge regimens.The pharmacist also provided patients with a pill box for those who did not have one and instructed the patient on how to fill the pill box. 1 month later, the pharmacist called the patient to assess any interim new medications as well as adverse effects to medications and/or adherence issues, and synchronised refill dates of cardiac medications. The pharmacist answered any other questions related to medications, emphasising the importance of continuing to take medications as prescribed. 2. Patient Education: At 1 week and 1 month post‐discharge visit and thereafter by automated voice messages and telephone calls a pharmacist provided education about their medicines when requested by the patient. 3. Collaborative Care: The pharmacist notified the patient’s primary care clinician and/or cardiologist (if the patient had one) that the patient was enrolled in the adherence intervention by having them co‐sign the pharmacists’ initial enrolment note in the computerised medical record. 4. VoiceMessaging: The voice messaging system contacted patients regularly with medication reminders (monthly) and medication refill reminders (timed to refill due dates)" Duration: 12 months |
|
| Outcomes | % achieving target blood pressure Systolic blood pressure (BP) Diastolic BP Mean Low Density Lipoprotein cholesterol |
|
| Notes | Funding source: This study was funded by a Veterans Health Administration Health Service Research & Development (HSR&D) Investigator Initiated Award (grant IIR 08‐302). Dr Bosworth was supported by a senior career scientist award (Research Career Scientist Award VA HSR&D 08‐027). Conflict of interest: The funding agency had no role in design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible patients with ACS were randomised using blocked randomisation stratified by study site in a 1:1 ratio to intervention or usual care. |
| Allocation concealment (selection bias) | Low risk | The allocation sequence was concealed until a patient consented to participate and was generated centrally using the graphical user interface implemented for the study. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | The allocation sequence was concealed until a patient consented to participate and was generated centrally using the graphical user interface implemented for the study. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "3 BP measurements were taken in standard fashion by someone blinded to study group assignment". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis was performed. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Holland 2005.
| Methods | Randomised trial | |
| Participants | 872 elderly patients (intervention 437; control 435) 4 general hospitals and 6 community hospitals Norfolk and Suffolk, UK Year of study: October 2000 to December 2002. |
|
| Interventions | Pharmacists made home visits to talk with patient and carers through self‐medication, drug adherence, symptoms of drug reactions. This was reinforced by a second visit between 6 and 8 weeks later. | |
| Outcomes | Euroqol (EQ)‐5D at 6 months Total number of emergency hospital readmissions in 6 months |
|
| Notes | Funding source: :Research costs were funded by a project grant from NHS Eastern Region R&D and the Academic Pharmacy Practice Unit of the University of East Anglia. RH was funded by the MRC as a research fellow during this study. Excess treatment costs were funded by Norfolk Health Authority, Norfolk SocNorfolk Health Authority, contributed some funding towards this study. Conflict of interest: AL works for a primary care trust, which pays for healthcare services and is interested in interventions to reduce unnecessary readmissions to hospital.The trust’s predecessor part funded this study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used third party telephone randomisation based on a computer generated sequence in blocks of varying length." |
| Allocation concealment (selection bias) | Low risk | Quote: "We used third party telephone randomisation based on a computer generated sequence in blocks of varying length." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Participants were told after randomisation the group to which they had been allocated. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. overall completion rate >80% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Hunt 2008.
| Methods | Randomised trial | |
| Participants | 463 hypertensive patients (intervention 230; control 233). 9 community‐based primary care clinics from primary care research network Oregon, USA Year of study: Not stated. |
|
| Interventions | Intervention comprised physician‐pharmacist collaboration following hypertension management guidelines. Pharmacists reviewed medication, lifestyle habits, assessed vital signs and reactions, provided education, identification of barriers to adherence and provided a regimen. Average of 7.2 total visits between pharmacists and physicians | |
| Outcomes | Systolic and diastolic blood pressure (BP) at 12 months SF‐36 (physical functioning) at 12 months BP in range |
|
| Notes | Funding source: Boehringer Ingelheim funded the cost of the educational mailings and the conduction of the study. Conflict of interest: All data collection, analysis, and reporting were conducted by the study investigators and the Providence research staff. The investigators report no other conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using a computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Using a computer‐generated random sequence |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Participant blinding was not possible. Knowledge of allocation may have influenced behaviour. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Blood pressure was assessed by registered nurses blinded to participants’ randomisation allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% but overall attrition rate >40% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Jaber 1996.
| Methods | Randomised trial | |
| Participants | Urban African‐American patients with diabetes: 39 (intervention 17; control 22)
Health professionals: 1
Practices: 1 University‐affiliated general medicine outpatient clinic Michigan, USA Year of study: Not stated. |
|
| Interventions | Pharmacist provided diabetes education, medication counselling, instructions on dietary regulation, exercise and home glucose monitoring, and evaluation and adjustment of drug regimen, vs usual care. Length of intervention: 4 months | |
| Outcomes | Quality of life Fasting plasma glucose |
|
| Notes | Funding source: Diabetes Research and Education Foundation and Upjohn Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were assigned to an intervention or control group in a randomized, parallel design fashion and followed over a 4‐month period". Unclear how randomisation took place |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described explicitly. No information provided |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel were not blinded but all expected differences in behaviour are part of the intervention. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Unclear if assessors were blind to allocation. Primary outcomes were objective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition > 10%. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Jackson 2004.
| Methods | Randomised trial | |
| Participants | Patients: 128 (intervention 60; control 68)
Health professional (delivering intervention): 1
Practice: 1 Home‐based follow‐up of patients discharged from Royal Hobart acute care teaching hospital in Tasmania, Australia Year of study: Not stated. |
|
| Interventions | Pharmacist conducted home visit to test international normalised ratio (INR) and educate patients about anticoagulant therapy using printed educational materials. Pharmacist informed physicians about patients' INR, recommended dosage adjustments and implemented therapy changes, vs usual care. Length of the intervention: 24 minutes Number of interventions: 4 during 90 days | |
| Outcomes | Therapeutic INR on day 8 after discharge Total, major, and minor bleeding complications within 90 days of discharge | |
| Notes | Funding source: National Institute of Clinical Studies (NICS) and the Royal Hobart Hospital Research Foundation. Roche Diagnostics Pty Ltd (Australia) contributed INR monitors and test strips. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients who provided informed consent were allocated to either an intervention (home monitoring; HM) or control (usual care; UC) group, using a computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Patients were home‐based; allocation was probably adequately concealed. All general practitioners were sent a personalised information letter when their patient was discharged, indicating the group that the patient was enrolled in and what follow‐up they would receive. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Personnel were aware of allocation and this may have influenced treatment in ways not specified by protocol. In particular, GPs caring from UC participants have altered treatment. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Theraputic INR; unclear in terms of objectivity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition overall. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Jahangard‐Rafsanjani 2014.
| Methods | Randomised trial | |
| Participants | 101 participants with diabetes (intervention 51: control 50) Community pharmacy Iran Year of study: Not stated |
|
| Interventions | Intervention group received a Pharmacist–Delivered Diabetes Support Program comprising 5 monthly visits with a telephone call between visits to reinforce treatment adherence and resolve any therapy‐related problems. Education was delivered on diet management, physical activity, and diabetes complications. At the recruitment visit, patients were provided with a blood glucose self‐monitoring device and the required test strips were supplied for 1 month. Patients were trained how to use the device and were requested to document blood glucose levels every other day in a rotating schedule (fasting, post‐prandial, before lunch, before sleep). Each patient was provided with a special logbook and educational pamphlets for the diabetes medications. At each follow‐up visit, medication‐related problems, self‐care issues, and the logbook were discussed with the patient. Duration: 5 months |
|
| Outcomes | HbA1c Systolic blood pressure (BP) Diastolic BP |
|
| Notes | Funding source: Deputy of Research, Tehran University of Medical Sciences. (Project ID: 90‐04‐156‐16161) Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated based on a block randomisation algorithm (1:1 allocation ratio; block size: 4), and 2 authors who were not involved in the recruitment process had access to the randomisation list. |
| Allocation concealment (selection bias) | Low risk | Randomisation sequence was generated based on a block randomisation algorithm (1:1 allocation ratio; block size: 4), and 2 authors who were not involved in the recruitment process had access to the randomisation list. The community pharmacist requested an allocation order using telephone calls after a patient signed the informed consent form. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Unclear if measurement of primary outcomes was blinded HbA1c is an objective outcome. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Unclear if measurement of primary outcomes was blinded HbA1c is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Jarab 2012.
| Methods | Randomised trial | |
| Participants | 133 Chronic Obstructive Pulmonary Disease (COPD) patients (intervention 66; control 67) 1 hospital outpatient clinic, Royal Medical Services Hospital Jordan Year of study: January 2011 to July 2011. |
|
| Interventions | Patients were educated about COPD and management of symptoms. They were assessed for medication use, given an educational booklet with simple exercises. Motivational interviewing was used to improve adherence to prescribed treatment. This intervention was given once and assessed over 6 months. | |
| Outcomes | Forced Expiratory Volume (FEV1) at 6 months Hospital admissions for acute exacerbation during 6 months follow‐up |
|
| Notes | Funding source: Alzaytoonah University of Jordan Conflict of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study participants were randomly assigned to intervention and control groups by a minimisation technique using statistical software. |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Unblinded assessment of most outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall competion rate >80% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Khdour 2009.
| Methods | Randomised trial | |
| Participants | 173 participants with Chronic Obstructive Pulmonary Disease (COPD) (intervention 86: control 87) All participants recruited from an outpatient COPD clinic Northern Ireland, UK Year of study: October 2006 to May 2008. |
|
| Interventions | An individualised face‐to‐face intervention for each COPD patient delivered by the clinical pharmacist focusing on their prescribed medication, adherence, inhaler technique and symptom management. Patient understanding of indications and doses of each medicine, inhaler use were checked and advice was provided on simple exercises for patients to do at home (booklet also provided) and smoking cessation if relevant. A customised action plan for acute exacerbations was developed for each patient. At each 6‐monthly outpatient clinic visit patients received reinforcement of the education on COPD and its treatment from the clinical pharmacist. In addition, follow‐up telephone calls by the clinical pharmacist to reinforce the education and motivate the patients to achieve their goals were made at 3 and 9 months, i.e. between outpatient clinic appointments. Duration: 12 months. |
|
| Outcomes | Health‐related quality of life (HRQoL) St George’s Respiratory Questionnaire (SGRQ) Forced Expiratory Volume (FEV1) |
|
| Notes | Funding source: Chest Heart and Stroke (N. Ireland) for financial support. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out using minimisation method |
| Allocation concealment (selection bias) | Unclear risk | Probably centrally allocated but a little unclear |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Quote: "For operational reasons, the researcher could not be blinded to the group to which the patient belonged" |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Quote: "Patients who had difficulty self‐completing questionnaires, e.g. forgot reading glasses, had the questionnaires read to them. If this occurred, a strict protocol was followed, i.e. the questions were read to the patients and their answers sought without any interpretation ". All of these outcome variables might be influenced by the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | High risk | Lots of variables measured, not all reported. Quote: "In addition to data collected by questionnaire, patients’ charts and computerised hospital records were consulted to obtain information on: emergency department visits within the last year, hospital admissions within the last year, FEV1, medication and medication regimen, body weight and other concomitant illness." |
| Other bias | Low risk | None |
Krass 2007.
| Methods | Randomised trial | |
| Participants | 335 diabetic patients (intervention 176; control 159) 56 pharmacies (intervention 28; control 28) 4 regions of Australia Year of study: March 2004 to September 2004. |
|
| Interventions | Educated about self‐monitoring and given meter for blood glucose, adherence support, medication review, self‐management and lifestyle. Individual goal‐setting and homework sheets to be completed by next visit 5 visits over 6 months |
|
| Outcomes | Diastolic and systolic blood pressure HbA1C |
|
| Notes | Funding source: The Pharmacy Diabetes Care Program was funded by the Australian Government Department of Health and Ageing as part of the Third Community Pharmacy Agreement. Precision Link software from Abbott Diagnostics supported training and individual pharmacists in this study Conflict of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States using Excel but does not say how |
| Allocation concealment (selection bias) | Unclear risk | Unclear how allocation concealment was conducted |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | No blinding. Unclear if it may have influenced performance |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | HbA1c unlikely to be biased by non‐blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate >80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Kritikos 2007.
| Methods | Randomised trial | |
| Participants | 48 participants with asthma (intervention (1) 16; intervention (2) 16; control 16) 6 community pharmacies Sydney, Australia Year of study: January 2005 to July 2005 |
|
| Interventions | Pharmacists delivered a single interactive Asthma Education Programme of 150 minutes to small groups of participants (5 – 8), focusing on asthma management, asthma medication, inhaler use. Relevant written information was also provided. Detailed programme guidelines, (which included the use of an educational resource kit Talk in A Box provided by the Asthma Foundation of New South Wales), were prepared to guide pharmacists through each session and enable standardised delivery of the programme. Duration: Single session |
|
| Outcomes | Proportion with severe asthma, asthma quality of life | |
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Three pharmacies were randomly selected"; no more info on randomisation and "subjects were not randomly selected". |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation concealed |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | No overlap in intervention delivery staff |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Asthma severity is subjective and unclear about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the trial. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Krska 2001.
| Methods | Randomised trial | |
| Participants | 381 elderly patients (intervention 168; control 164; numbers were only given for those that completed the study) Number of participating practices unclear Grampian, Scotland Year of study: Not stated. |
|
| Interventions | Pharmacists interviewed patients in their homes for medication use, use of health and social services and to distribute prescribed medicines and a care plan; listing care issues, output, planned actions and pharmacist input. 2 interviews over 3 months. |
|
| Outcomes | HbA1c SF‐36 |
|
| Notes | Funding source: Conflict of interest: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Following stratification by number of drugs, number of cardiovascular drugs and the presence of a non‐steroidal anti‐inflammatory drug other than low‐dose aspirin on the repeat prescription, patients were allocated randomly to intervention or control." Therefore unclear about the actual method of randomisation for each participant. Only states method for practice. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | No blinding and self‐reported outcome |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | No blinding and self‐reported outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Lai 2013.
| Methods | Randomised trial | |
| Participants | 198 participants with osteoporosis (Intervention 98; control 100) A tertiary hospital osteoporosis clinic Malaysia Year of study: September 2005 to February 2009 |
|
| Interventions | Participants in the intervention group received a ‘‘pharmaceutical care package’’ which included a one‐to‐one, individualised medication review, education on osteoporosis, risk factors, lifestyle modifications, goals of therapy, side effects and the importance of adherence, at months 0 (baseline), 3, 6 and 12, with monthly follow‐ups by telephone calls in between for the first 6 months, then every 3 months up to month 12. Materials included a booklet and a personalised osteoporosis medication regimen. Duration: 12 months |
|
| Outcomes | Quality of Life Questionnaire of the European Foundation for Osteoporosis | |
| Notes | Funding source: This project was funded by the Postgraduate Research Fund P0110/2006B, University of Malaya and the Endocrine Research fund, University of Malaya Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Therefore, participants were first divided into whether they were on alendronate or risedronate, then randomly allocated to the intervention group using the random digits table (98) while the rest were allocated to the control group. |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | No blinding of participants. Some potential for bias |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Quality of life is subjective and therefore categorised as high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Lee 2006.
| Methods | Randomised trial | |
| Participants | 159 elderly patients (intervention 83; control 76) 1 general hospital Washington, USA Year of study: June 2004 to August 2006. |
|
| Interventions | Medication education, time‐specific medication packs. Meet with pharmacists every 2 months over a 6‐month period |
|
| Outcomes | Diastolic and systolic blood pressure at 14 months Low density lipoprotein mg/dL |
|
| Notes | Funding source: This study was partially funded by a competitive junior investigator grant award from the American Society of Health‐System Pharmacists Research and Education Foundation, managed under the auspices of the TRUE Research Foundation. Conflict of interest: Dr Taylor reported receiving research grant and honoraria from Kos Pharmaceuticals, honoraria from Pfizer Pharmaceuticals, Wyeth Pharmaceuticals, and Merck KgA, and a consulting agreement with Alinea Pharmaceuticals.Drs Lee and Grace reported no financial disclosures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation was concealed to both patients and the study personnel who enrolled participants by central control of the randomization sequence." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Unblinded measures of blood pressure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low and similar dropouts |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Lenaghan 2007.
| Methods | Randomised trial | |
| Participants | 136 elderly patients (intervention 69; control 67). 1 community pharmacist and patients from 1 general practice Norfolk, England Year of study: Not stated. |
|
| Interventions | 2 home visits by a community pharmacist discussing drug interactions, education of medicines, removal of out‐of‐date drugs and assessment of need for adherence aid. Visits were arranged to include the carer of the elderly patient. Pharmacists discussed any issues with the general practitioner for possible changes to medication prescription. | |
| Outcomes | Euroqol (EQ)‐5D Hospital admissions All‐cause mortality |
|
| Notes | Funding source: The main author’s post was funded by NHS Executive Eastern Region research funding. Conflict of interest: The medication review intervention was funded by Holt Medical Practicewho hosted the research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomisation was carried out by a third party, and was stratified by whether the patient lived alone." |
| Allocation concealment (selection bias) | Unclear risk | Unclear if the person enrolling the participant was aware of allocation |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Knowing they were in the intervention group may have resulted in behaviour change. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Hospital readmissions, deaths etc. not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% however overall completion rate <80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Lenander 2014.
| Methods | Randomised trial | |
| Participants | 209 participants with drug‐related problems (intervention 107: control 102) Primary care centre Stockholm, Sweden Year of study: September 2004 to not stated. |
|
| Interventions | Intervention group received a medication review performed by a certified geriatrics pharmacist, involving a standardised semi‐structured questionnaire that allowed patient interaction. Computerised patient records were checked for prescriptions, drug indications, and plans for evaluation. Drugs and dosages were evaluated to correlate with renal function, good practice and the drug formulary. A patient‐centred technique was used, focusing on the patients’ answers to assess understanding of and concordance with drug treatment. The patients were also asked about prescribers other than their GP, and use of non‐prescription and herbal drugs. Concluding pharmaceutical advice was given to patients and entered into the computerised patient record. Duration: single session |
|
| Outcomes | Total drug‐related problems Number of drugs Healthcare use: hospitalisations |
|
| Notes | Funding source: The trial was funded by Stockholm County Council, the Stockholm Drug and Therapeutics Committee, and Apoteket AB Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how randomisation occurred |
| Allocation concealment (selection bias) | Low risk | Seems to have happened before any non‐standardised patient contact (a letter) |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | No interaction with pharmacist in control group |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Data were analysed by an independent certified geriatrics pharmacist, blinded to patient group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10%. however, high attrition (>30%) overall |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Li 2014.
| Methods | Randomised trial | |
| Participants | 117 participants with Chronic Obstructive Pulmonary Disease (COPD) (intervention 58: control 59) Participants recruited from University hospital China Year of study: February 2012 to January 2014 |
|
| Interventions | Intervention group patients received pharmacist‐led individualised education sessions (20 ‐ 30 minutes each session, 5 ‐ 6 sessions) on effective use of respiratory devices, pathophysiology of the disease, interpretation of medical testing and rationale for medication. Medication management records evaluated each participant’s preferences and analysed possible barriers to medication adherence. Telephone calls (4 ‐ 5 sessions) were made at the midpoint between clinic visits. During telephone counselling, the pharmacist asked about the patient’s treatment effects, clarified any misconceptions, explained the nature of any side effects and reminded patients of their next clinical appointment. Duration: 6 months |
|
| Outcomes | Health‐related quality of life (HRQoL) | |
| Notes | Funding source: Not specified Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The pharmacists were blinded to the randomisation codes, which were computer‐generated and sealed in envelopes labelled with consecutive numbers. |
| Allocation concealment (selection bias) | Low risk | The pharmacists were blinded to the randomisation codes, which were computer‐generated and sealed in envelopes labelled with consecutive numbers. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Unblinded and with subjective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Low risk of bias in detection: surveys completed by participant |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10%, however, high attrition overall (˜30% lost at 1‐year follow‐up). |
| Selective reporting (reporting bias) | Low risk | All major outcomes reported |
| Other bias | Low risk | None |
Lopez 2006.
| Methods | Randomised trial | |
| Participants | 134 participants with heart failure (intervention 70: control 64) Patients recruited from 2 hospitals Spain Year of study: September 2000 to not stated. |
|
| Interventions | Intervention group received a pharmacist‐led programme comprising a face‐to‐face visit at discharge and a follow‐up phone call. At discharge information tailored to the patient was provided on the main characteristics of heart failure (pathogenesis and symptoms), diet and drug therapy. Verbal communication was complemented by written materials. Monthly during the first 6 months of follow‐up, and subsequently, every 2 months, a telephone call was made to the patient's home to reinforce the information provided. Duration: 1 year |
|
| Outcomes | Number of hospital readmissions, EuroQol | |
| Notes | Funding source: This study (PI00/0665) was co‐financed with a grant from the Health Research Fund (Fondo de Investigación Sanitaria, FIS) and the European Regional Development Fund (ERDF) Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomized to one of the two groups through a randomisation software. |
| Allocation concealment (selection bias) | Low risk | Neither the physician nor the nurse responsible for the patient knew the allocation until the educational intervention, the day of discharge. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Personnel not blinded |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Assessors unblinded. Number of hospital readmissions is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition >20%. High attrition overall (>40%). |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Losada‐Camacho 2014.
| Methods | Randomised trial | |
| Participants | 182 participants with epilepsy (intervention 70: control 74) Outpatient with epilepsy and a referral centre Colombia Year of study: June 2010 to September 2012. |
|
| Interventions | Intervention group received a pharmaceutical care programme consisting of 1. Monthly or bi‐monthly interviews including medication review; treatment adherence (importance of regular use, and provision of adherence aids e.g. a medication record, a pill box, an alarm clock as a reminder of when medications should be taken); registration of seizures and possible triggers based on a patient's completed seizure journal); therapeutic drug monitoring in accordance with the guidelines of the International League Against Epilepsy. Importance of lifestyle was emphasised. A guide for patients with epilepsy was sent by e‐mail so that it could be discussed at face‐to‐face interviews and specific brochures were delivered according to the needs of each patient. 2. Monthly lectures on: Epilepsy in women, Quality of life and epilepsy, Pharmacological and non‐pharmacological treatment in epilepsy, Contraception, Fertility, Pregnancy and childbirth, Sleep hygiene, Breastfeeding and home care, Menopause and bone health and how to improve memory. Duration: 6 months |
|
| Outcomes | Quality of life in epilepsy inventory‐31 scores | |
| Notes | Funding source: This study was funded by a competitive investigator grant award from the Universidad Nacional de Colombia (Colombia) ‐ Research Division of Bogotá (ref: 202010011419 Quipu Code) Conflict of interest: The Universidad Nacional de Colombia had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data or in the preparation, review or approval of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation sequence was generated by ballot papers drawn from an urn without the principal investigator and the co‐ordinator knowing the results in advance. |
| Allocation concealment (selection bias) | Low risk | The concealment was performed by placing the ballot papers in individual, opaque, sealed envelopes, numbered sequentially, which were handled exclusively by the study co‐ordinator. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Quote: "Although the study was not blinded, it was explained to the patients that due to the large number of patients, all could not be served at the same time and therefore the study was conducted in two stages whose sequence was decided randomly, so they could begin the process of pharmaceutical care immediately, or do it six months after the second questionnaire session. In this way the effect of knowing the group assigned was avoided and those in the control group were rewarded for their participation in the study programme by receiving PC after answering the questionnaires the second time. The study was blind to the neurologists. They were informed that the RCT was taking place in the institution but did not know which patients were participating in the trial. Due to the study’s design, the principal investigator was not blinded to the patients’ allocation." |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "Although the study was not blinded, it was explained to the patients that due to the large number of patients, all could not be served at the same time and therefore the study was conducted in two stages whose sequence was decided randomly, so they could begin the process of pharmaceutical care immediately, or do it six months after the second questionnaire session. In this way the effect of knowing the group assigned was avoided and those in the control group were rewarded for their participation in the study programme by receiving PC after answering the questionnaires the second time. The study was blind to the neurologists. They were informed that the RCT was taking place in the institution but did not know which patients were participating in the trial. Due to the study’s design, the principal investigator was not blinded to the patients’ allocation." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% however overall attrition > 20%. |
| Selective reporting (reporting bias) | High risk | Multiple outcomes |
| Other bias | Low risk | None |
Magid 2013.
| Methods | Randomised trial | |
| Participants | 348 hypertensive patients (intervention 175; control 173) 10 primary care clinics Colorado, USA Year of study: Not stated. |
|
| Interventions | Both groups were given education materials for managing high blood pressure. Intervention group also received a home blood pressure (BP) cuff and training of use. They were required to upload their BP 3 times a week for pharmacist review who would make medication adjustments, review adherence and flag high reports. They would communicate this by phone or e‐mail. | |
| Outcomes | Diastolic and systolic BP Achievement of BP goal at 6 months |
|
| Notes | Funding source: Funded in part by the American Heart Association. Conflict of interest: None stated. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Heart Association. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random allocation sequence was computer‐generated using stratified randomisation with an allocation ratio of 1:1. |
| Allocation concealment (selection bias) | Low risk | Quote: "The sequence was concealed from the patient until the baseline visit." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | BP measurement has low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "Patients in both groups returned for a clinic visit at 6 months, at which time they had their BP taken by a research assistant blinded to study group assignment using the same standardized protocol that was used at the baseline visit." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall attrition rate >80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Mahwi 2013.
| Methods | Randomised trial | |
| Participants | 130 participants (intervention 65; control 65) Diabetic Centre Sulaimany, Iraq Year of study: September 2010 to January 2011. |
|
| Interventions | Pharmaceutical care. The intervention group was followed up for 3 visits. The interval between each visit ranged from 5 to 6 weeks with continuous weekly telephone calls for the follow‐up. Duration: 15 ‐ 18 weeks Number of Interventions: 3 visits, every 5 ‐ 6 weeks |
|
| Outcomes | Fasting plasma glucose (FPG) HbA1c |
|
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this study, patients were divided into two groups by simple randomization technique" Unclear how this actually happened |
| Allocation concealment (selection bias) | Unclear risk | Quote: "In this study, patients were divided into two groups by simple randomization technique" Unclear if selection bias an issue |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Not stated but objective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Not stated but objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Malone 2001.
| Methods | Randomised trial | |
| Participants | Patients at high risk for medication‐related problems (≥ 3 of following criteria: (1) > 5 medications, (2) > 12 doses a day, (3) > 3 chronic medical conditions, (4) > 4 changes to medication regimen over past year, (5) taking < 80% of medications based on pharmacy refill records, (6) taking medication requiring therapeutic monitoring
Patients: 1054 (intervention 523; control 531)
Health professional (delivering intervention): 78
Practice: 9 Ambulatory care clinics in Veterans Affairs Medical Centers USA Year of study: Not stated. |
|
| Interventions | Pharmacist reviewed medical records, performed physical assessment and laboratory tests to assess appropriateness of medication therapy, modified therapy as necessary, educated patients, and made referrals to other health professionals, vs usual care Length of the intervention: > 15 minutes for > 73% of patient contacts Number of interventions: mean of 3.5 during 12 months | |
| Outcomes | Cholesterol Health‐related quality of life using SF‐36 questionnaire |
|
| Notes | Funding source: Pharmacia & Upjohn, Inc, under the direction of the VA/Pharmacia & Upjohn Steering Committee. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Potential subjects for the study were identified and randomised by the central co‐ordinating centre at the University of Colorado Health Sciences Center. |
| Allocation concealment (selection bias) | Low risk | Quote: "randomised by a central coordinating centre" |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Unblinded, but participants saw different personnel. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Unblinded, but lipid level measurement is an objective outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | None |
Margolis 2013.
| Methods | Randomised trial | |
| Participants | 450 participants with hypertension (intervention 228: control 222) 16 primary care clinics in an integrated health system Minneapolis‐St. Paul, Minnesota, USA Year of study: March 2009 to not stated. |
|
| Interventions | Pharmacist telemonitoring intervention with remote BP measurement. Intervention patients received home monitors that store and transmit blood pressure (BP) data to a secure website through a modem. Pharmacists met with patients for 1 hour during which they reviewed the patient's relevant history, covered general points about hypertension, instructed them on using the home BP telemonitor system and the individualised home BP goal (i.e. < 135/85 mmHg or < 125/75 mmHg for patients with diabetes or kidney disease). 20 patients were instructed to transmit at least 6 BP measurements weekly (3 morning and evening). During the first 6 months of intervention, patients and pharmacists spoke every 2 weeks by phone until BP control was sustained for 6 weeks, then frequency was reduced to monthly. During intervention months 7 ‐ 12, phone visits were every 2 months. During telephone calls, pharmacists emphasised lifestyle changes and medication adherence. They assessed and adjusted antihypertensive drug therapy based on an algorithm using the percentage of home BP readings meeting the goal. Pharmacists communicated with patients' primary care teams through the electronic medical record following each visit. Duration: 12 months intervention, 18 months follow‐up |
|
| Outcomes | Systolic BP Diastolic BP |
|
| Notes | Study is cluster‐randomised by clinic, but all data after that is at patient level. Funding source: Grant received from the National Heart, Lung, and Blood Institute (R01HL090965). Conflict of interest: The sponsor had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The 16 primary care clinics were randomised to either the usual care (n = 8) or intervention (n = 8) arms. Clinics were blocked by size and clinic‐level baseline BP control in 2008 in order to balance those factors across study arms. Patients were linked to their primary care clinic by self‐report and were assigned to the intervention based on which clinic they attended, resulting in 228 patients assigned to TI and 222 patients assigned to UC. |
| Allocation concealment (selection bias) | Low risk | All consenting patients and primary care providers were blinded to the study design and intervention assignment of the clinics, although each patient and their primary care provider were informed of their treatment assignment after randomisation. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Objective outcome measures. also, Quote: "Research clinic coordinators were not blinded to clinics’ treatment assignments, but were trained to treat patients in both study arms identically". |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "Research clinic coordinators were not blinded to clinics’ treatment assignments, but were trained to treat patients in both study arms identically." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Quote: "To account for missing data on continuous outcomes we used maximum likelihood based ignorable methods that yield valid inference when the outcome data are missing at random. We conducted sensitivity analyses adjusting for race and hypertension treatment, which showed some imbalance by study group" |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Marques 2013.
| Methods | Randomised trial | |
| Participants | 58 participants with depression (intervention 31: control 27) Outpatient clinic of Alzira Velano Hospital University of Alfenas, Brazil Year of study: April 2010 to January 2012. |
|
| Interventions | Patient Education using Dáder method Intervention group patients were visited approximately every 30 days; the intervals between visits could be shorter according to the patient’s needs. These patients were given verbal and written information about the treatment, and educational lectures about disease and treatment; interventions with the psychiatrist were performed as needed. Frequency: monthly Duration: 3 months |
|
| Outcomes | Beck depression Inventory (BDI) Becks Anxiety Inventory (BAI) |
|
| Notes | Funding source: Not specified Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to the Dáder Method, the patients in the intervention group were visited approximately every 30 days; the intervals between visits could be shorter according to the patient’s needs. These patients were given verbal and written information about the treatment and educational lectures about disease and treatment; interventions with the psychiatrist were performed as needed. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Unblinded pharmacists conducted the intervention and control arm interaction: bias possible |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Participants unblinded completed self‐report measures. Bias is likely. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | High risk | Only 3 months, seasonality, also numbers differ between table and flow chart |
Marra 2012.
| Methods | Randomised trial | |
| Participants | 139 participants with osteoarthritis (OA): (intervention 73; control 66) Community pharmacies Metropolitan area of Vancouver, Canada Year of study: Not stated. |
|
| Interventions | Pharmacist‐led or educator‐led educational intervention Quote "Intervention patients received one‐on‐one consultation with a pharmacist. Pharmacists offered education, medication review, referral to a physiotherapist and a guided exercise program. We provided education regarding counselling on the symptoms and other aspects of knee OA. Patients were given the opportunity to participate in an Arthritis Self Management Program. Each patient received personalised education from the physiotherapist for a personalised regimen. Patients were told to avoid exercise during active symptom flares. Walking aids were recommended when necessary. At the end of weeks three and six, the patients were reassessed by the physiotherapist and the participant’s exercise recommendations were adjusted as needed. Patients in the intervention group were recommended to attend at least two physiotherapist‐guided exercise sessions per month for a total of 12 sessions." Duration: 6 months |
|
| Outcomes | WOMAC (Western Ontario and McMaster Universities Osteoarthritis Index) | |
| Notes | Funding source: This study was funded by a pilot grant from the Canadian Institutes of Health Research/Canadian Arthritis Network New Emerging Team Grant (Tooling Up for Early Osteoarthritis) and by peer‐reviewed operating grants from the Michael Smith Foundation for Health Research and the Canadian Arthritis Network. Dr. Marra is a Health Services Scholar, supported by the Michael Smith Foundation for Medical Research, and is a Government of Canada Research Chair in Pharmaceutical Outcomes. Dr. Cibere is supported by a JW McConnell Family Foundation Scholar Award and a CIHR Clinical Scientist Award. Dr. Tsuyuki is supported by the Merck Frosst/Aventis Chair in Patient Health Management at the University of Alberta. Dr. Khan is a New Investigator at the Canadian Institutes of Health Research Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | To randomise the pharmacies, values from a uniform (0,1) distribution were generated by the study statistician. Pharmacies were randomized to provide either the intervention (21 pharmacies) or usual care (21 pharmacies). |
| Allocation concealment (selection bias) | Unclear risk | Pharmacy‐level randomisation most important here. Unclear |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Unblinded. Participants were informed whether they were to receive the intervention or usual care after they provided consent. Subjective outcomes subject to bias |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Unblinded. Participants were informed whether they were to receive the intervention or usual care after they provided consent. Subjective outcomes subject to bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All analyses were conducted using intention‐to‐treat principles. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported. 1 primary outcome |
| Other bias | High risk | Baseline differences |
Mazroui 2009.
| Methods | Randomised trial | |
| Participants | 240 diabetic patients (intervention 120; control 120) 1 hospital outpatient clinic United Arab Emirates Year of study: Not stated. |
|
| Interventions | Intervention patients were educated on their illness and medication needs, risk of complications, side effects and storage, healthy lifestyle, and self‐monitoring. They were also given a reinforcing leaflet of this information. 1 initial intervention contact with follow‐up assessments every 4 months for 1 year. | |
| Outcomes | All measured at 12 months Diastolic and systolic blood pressure (BP) Fasting blood glucose mg/dL HbA1c Serum total cholesterol SF‐36 (physical functioning) |
|
| Notes | Funding source: Not stated Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After recruitment, patients were randomly assigned to one of two groups: intervention group or control group." |
| Allocation concealment (selection bias) | Low risk | Allocation occurred after randomisation: Quote: "After recruitment, patients were randomly assigned to one of two groups: intervention group or control group." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Much of the interaction with non‐blinded personnel |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | No evidence of blinding and several subjective measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate >80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
McAlister 2014.
| Methods | Randomised trial | |
| Participants | 279 patients > 18 years who had an ischaemic stroke or transient ischaemic attack confirmed by a stroke specialist at 1 of the 3 stroke prevention clinics (intervention 139: control 136)
Edmonton, Alberta, Canada Hypertension and cholesterol Year of study: 2009 to 2012. |
|
| Interventions | Intervention patients received intensive pharmacist‐led case management, consisting of monthly follow‐up visits with the study pharmacist for 6 months, independent of planned follow‐up with the clinic or family physician. At each visit, the study pharmacist monitored the patient's BP and lipid levels and initiated and/or titrated antihypertensive and/or hypolipidaemic therapy as appropriate. The study pharmacist followed treatment algorithms consistent with Canadian national guidelines. The pharmacist emphasised medication and lifestyle adherence with patients and their caregivers, using the cardiovascular risk profile as an educational aid. The pharmacist also sent a fax to the primary care physician after each visit outlining the status of that patient's atherosclerosis risk factors and any therapy adjustments made. Duration: 6 months |
|
| Outcomes | Systolic blood pressure Low density lipoprotein |
|
| Notes | Funding source: Finlay McAlister and Sumit Majumdar received salary support awards from Alberta Innovates Health Solutions. Finlay McAlister held the University of Alberta Chair in Cardiovascular Outcomes Research. Sumit Majumdar held the Patient Health Management Chair at the University of Alberta. Project‐specific funding for this trial was provided by the Heart and Stroke Foundation of Alberta, the Alberta Heritage Foundation for Medical Research, and Knowledge Translation Canada. Conflict of interest: None of the funders had a role in the design of the study nor in the conduct, analysis, interpretation or reporting of the study, nor access to the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomisation will be done centrally by computer‐generated random numbers, and a secure internet‐based allocation method that ensures allocation concealment" |
| Allocation concealment (selection bias) | Low risk | Quote "Randomisation will be done centrally by computer generated random numbers, and a secure internet‐based allocation method that ensures allocation concealment. As this study is unblinded, variable sized blocked randomisation will also be used to preserve allocation concealment." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | All objective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "with blinded ascertainment of outcome" Quote: "all outcomes were collected in an independent and blinded manner by observers who were masked to baseline measurements and group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. Between group attrition = 10%. |
| Selective reporting (reporting bias) | Low risk | Major results reported as planned |
| Other bias | Low risk | None |
Mehos 2000.
| Methods | Randomised trial | |
| Participants | Patients with stage 1 or 2 hypertension: 41 (intervention 20; control 21)
Health professionals (delivering intervention): not clear
Practices: 1 Family medicine residency training clinic Colorado, USA Year of study: Not stated. |
|
| Interventions | Patients received blood pressure monitors, blood pressure diaries and telephone contacts by pharmacist to evaluate blood pressure and response to therapy, vs usual care without blood pressure self‐monitoring. Pharmacist informed primary care health professionals of patients' blood pressure results and provided therapy recommendations, vs usual care. Length of intervention: 30 minutes (initial visit) Number of interventions: initial visits and phone call follow‐ups over 6 months | |
| Outcomes | Systolic, diastolic, and mean arterial blood pressure | |
| Notes | Funding source: Supported by the 1998–1999 Bristol‐Myers Squibb Pharmacy Practice Hypertension Program grant from the American Association of Colleges of Pharmacy Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized using a deck of cards and enrolled in either the intervention or control group" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomized using a deck of cards". Unclear how this concealed allocation |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Participants were unblinded. BP measurement has low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | BP has low risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | BP and SF‐36, both reported |
| Other bias | Low risk | None |
Mehuys 2008.
| Methods | Randomised trial | |
| Participants | 201 asthma patients (intervention 107: control 94) Recruited consecutively in 66 randomly‐selected pharmacies Flanders, Belgium Year of study: January 2006 to October 2006. |
|
| Interventions | Intervention patients received a protocol defined intervention at the start of the study and at 1‐ and 3‐month follow‐up. Session 1 consisted of personal education from the pharmacist about: correct use of the inhaler device; understanding asthma; symptoms, triggers and early warnings; understanding asthma medication and difference between controller and reliever medication, and smoking cessation (if relevant). At sessions 2 and 3 the pharmacist advice was based on the patient’s asthma score: If score was < 15 (‘‘uncontrolled’’ asthma): immediate referral to general practitioner or respiratory specialist. If score was 15 ‐ 19 (‘‘insufficiently controlled’’ asthma): review inhalation technique and check controller medication adherence. If score > 20 (‘‘well‐controlled’’ asthma): no specific advice was needed. Control group patients received usual pharmacist care. Frequency: sessions at 0, 1 and 3 months Duration: 3 months |
|
| Outcomes | Asthma Control Test score Nights with awakenings Peak expiratory flow (PEF) morning and evening |
|
| Notes | Both control and intervention group involved pharmacy care. Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence of allocation to control or intervention group was predetermined by the investigators based on a randomisation table generated with SPSS 14.0 software. |
| Allocation concealment (selection bias) | Low risk | Serially‐numbered, closed envelopes were made for each participating pharmacy. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Diary data: high risk: Quote: "treatment recording (i) nocturnal awakenings due to asthma, (ii) the number of inhalations of rescue medication (during the day or night), and (iii) the best of 3 measurements of peak expiratory flow (PEF) made with a Mini‐Wright Standard Peak Flow Meter in the morning and evening before medication. PEF data are expressed as the percentage of maximum predicted value based on patient’s sex,age, and height." |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Self‐measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10%, however, overall attrition 25%. Reasons for dropout were personal reasons (15), withdrawal from study of the pharmacist (2), relocation (2), lost to follow‐up (27) and other reasons (5). |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Milos 2013.
| Methods | Randomised trial | |
| Participants | 374 elderly patients (intervention 185; control 189) ≥ 75 years, and living in nursing homes or the community 4 pharmacists with at least 4 years' experience of performing medication reviews Skåne County, Sweden Year of study: September 2011 to February 2012. |
|
| Interventions | Pharmacists conducted a medication review for patients based on electronic medical records without interaction. Recommendations were sent to the patient's physician by team rounds, written contact, personal contact or phone. | |
| Outcomes | Drug‐related problems Number of patients with potentially inappropriate medications Number of patients with unplanned admissions All‐cause mortality |
|
| Notes | Funding source: The study was conducted with government funding for projects involving improvement of drug therapy in the elderly. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed using a random‐number generator and stratified only for geographic area. |
| Allocation concealment (selection bias) | Low risk | After inclusion, the pharmacist used closed, non‐transparent envelopes to randomise the patient to 1 of 2 groups: control or intervention. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Unclear from information provided |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Drug‐related outcomes (number of drugs, drug‐related problems, etc.) unlikely to be biased. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None identified |
Murray 2007.
| Methods | Randomised trial | |
| Participants | 314 participants with heart failure (intervention 122: control 192) University‐affiliated, inner‐city, ambulatory care practice Indiana University Medical Group, Indianapolis, USA Year of study: February 2001 to June 2004 |
|
| Interventions | Patient education and medication distribution. When medications were dispensed, the pharmacist provided patient‐centred verbal instructions and written materials about the medications by using a previously‐tested schema for instruction. Each medication category was assigned an icon (for example, the icon for ACE (angiotensin converting enzyme) inhibitors was a red ace of hearts). The same icon appeared on the container label and lid and on the written patient instructions. Written instructions were aimed at patients with low health literacy and contained an easy‐to‐follow timeline to remind patients when to take their medications. The pharmacist monitored patients’ medication use, healthcare encounters, body weight, and other relevant data by using a study database. Information about patients was communicated as needed to clinic nurses and primary care physicians. Frequency: every 2 months Duration: 9 months |
|
| Outcomes | Mean Emergency Department visits Mean hospital admissions |
|
| Notes | Funding source: Grant Support: In part by National Institutes of Health grants R01 AG19105 and R01 HL 69399 (Dr. Murray, principal investigator) and AG01799 (Dr. Brater, principal investigator; Dr. Murray, co‐principal investigator). Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "We randomly assigned patients, without blocking or stratification, to receive the pharmacy intervention or usual care by using a univariate discrete distribution using pseudo‐random number generation." |
| Allocation concealment (selection bias) | Low risk | Interviewers contacted a centralised data manager at the end of each interview to determine the patient’s study assignment, which was otherwise concealed. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Quote: "[Usual care participants] received their prescription services from pharmacists who rotated through the study pharmacy. These pharmacists had not received the specialized training provided by the interdisciplinary team to the intervention pharmacist and did not have access to the patient‐centered study materials." |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote:"We assessed interviewer blinding by using a computerised closeout protocol at the end of each interview that required interviewers to guess whether each patient was in the intervention or usual care group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | High risk | Health‐related quality of life (HRQoL) and disease‐specific outcomes not reported |
| Other bias | High risk | Quote: "during the busiest times, patients in the intervention and usual care groups may have been in the pharmacy at the same time." |
Naunton 2003.
| Methods | Randomised trial | |
| Participants | 136 elderly patients (intervention 57; control 64) 15 were excluded after randomisation. Patients were recruited from the Royal Hobart Hospital (the only major public hospital in the southern region of Tasmania) a 400‐bed acute care teaching hospital. Visits performed by 1 pharmacist. Southern Tasmania, Australia Year of study: November 2000 to ˜ May 2001. |
|
| Interventions | Patients were visited in their homes 5 days after discharge from hospital. The study pharmacist checked medication adherence and offered additional supports if this was not met. They also offered education about medication, management, compliance; they also discussed queries and improved liaison with health services. A letter was composed with the patient to present to their doctor. Duration: 13 months with 90‐day follow‐up. |
|
| Outcomes | Number of patients with unplanned readmissions All‐cause mortality |
|
| Notes | Funding source: Abbott Australasia Pharmacy Research Grant, through SHPA Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to either an intervention or control group by the study pharmacist (MN) responsible for conducting the home visits, using a computer‐ generated list of random numbers." |
| Allocation concealment (selection bias) | High risk | Allocation by study pharmacist |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Non‐blinded and some potential for bias in interactions |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Unplanned readmissions, deaths etc. not likely to be biased |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Overall completion rate <80%. Attition rate per group not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Obreli‐Neto 2015.
| Methods | Randomised trial | |
| Participants | 200 participants with hypertension or diabetes (intervention 100: control 100). Primary Health Care Unit (PHCU) Salto Grande, Sao Paulo state, Brazil Year of study: October 2006 to October 2009. |
|
| Interventions | Intervention patients received pharmaceutical care in addition to usual care. The pharmaceutical care intervention consisted of individual follow‐ups according to the Pharmacotherapy Workup and educational group activities. The Pharmacotherapy Workup was performed by 4 trained pharmacists. During the Pharmacotherapy Workup, interventions were provided which aimed to improve compliance with the pharmacotherapy. Pharmaceutical care included the assessment of non‐compliance, discussions about the role of medication, suggestions to physicians regarding new drug regimens and the preparation of special packages to provide a visual reminder that a medication was taken. The pharmaceutical care programme was developed individually according to the needs of patients. Educational group activities were also organised once every 6 months, with groups of 20 patients. During these activities, adherence, the dangers of self‐medication, and the correct storage of medicines were discussed. Frequency: every 6 months Duration: 36 months |
|
| Outcomes | Systolic blood pressure (BP) Diastolic BP Fasting glucose HbA1c |
|
| Notes | Funding source: No separate funding was obtained for this study Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequences (100 patients each in the intervention and control groups) |
| Allocation concealment (selection bias) | Low risk | Computer‐generated allocation using medical record numbers |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | All objective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | All objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | Low risk | All major results reported |
| Other bias | Low risk | None |
Okamoto 2001.
| Methods | Randomised trial | |
| Participants | 330 patients with hypertension (intervention group 164; control group 166) Health professional (delivering intervention): 1 Practice: not clear Hypertension and general medicine clinics within a managed care facility USA Year of study: Not stated. |
|
| Interventions | Hypertension care provided by pharmacist or general practitioner Pharmacist managed treatment of patients with hypertension and obtained consent from physicians for therapy changes vs usual care Length of the intervention: not clear Number of interventions: 5 during 6 months | |
| Outcomes | BP – systolic BP ‐ diastolic Health‐related quality of life using SF‐36 |
|
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "If eligible, patients were randomly assigned to one of two groups." |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly described |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | BP measurement has low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Unblinded study, but this seems unlikely to influence an automated BP measure |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | None |
Olesen 2014.
| Methods | Randomised trial | |
| Participants | 630 participants ‐ elderly patients (intervention 315: control 315) 9 pharmacists Aarhus, Denmark Year of study: Not stated. |
|
| Interventions | Intervention‐group patients received a home visit by a pharmacist at the beginning of the project. The pharmacist examined the medicines list to consider possible side effects, interactions, and administration, then simplified the regimen, informed the patients about medication, listened to questions concerning medication, provided information leaflets, and motivated adherence. Participating pharmacists must have had some practical experience or courses in Medication Review. No further training or standardisation was arranged. At 3, 6 and 9 months the same pharmacists telephoned the patients to inquire about the patients’ condition and changes in the medicine, uncover problems and answer questions. Pharmacists could consult the project physician if required. If the physician agreed with the pharmacists concerns, the pharmacist contact the general practitioner. There were no standardised criteria for severity of medication problems. Frequency: Baseline home visit. 3,6,9 months telephone review |
|
| Outcomes | Number of hospitalisations | |
| Notes | Funding source: This study was supported by the Danish Ministry of Health and the Association of Danish Pharmacies. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A total of 945 envelopes (315 per patient subgroup) was prepared with each containing a study inclusion code. At the first home visit by a project nurse, patients were asked to select one envelope. |
| Allocation concealment (selection bias) | Low risk | A total of 945 envelopes (315 per patient subgroup) was prepared with each containing a study inclusion code. At the first home visit by a project nurse, patients were asked to select one envelope. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | It was impossible to conceal the identity of patients in the pharmaceutical care group since the procedures were complex and involved the pharmacists and nurses. However, hospitalisations were deemed to be an objective measure. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Objective outcomes collected from electronic records, hence unlikely to be biased. Probably blinded assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None |
Park 1996.
| Methods | Randomised trial | |
| Participants | 64 patients with hypertension (intervention 32: control 32) Health professionals (delivering intervention): 2 pharmacy residents Practices: 2 (not studied at the same time) 2 sites of a chain pharmacy Chicago, USA Year of study: Ocotober 1993 to May 1994. |
|
| Interventions | Oral and written education about hypertension, its treatments and risk factors to the patients and recommendation to the physician if necessary Length of the intervention: 15 to 30 minutes Frequency of the intervention: 4 in 4 months | |
| Outcomes | Blood Pressure Compliance (pill count) Health Status Questionnaire (HSQ) Hypertension/lipid Form | |
| Notes | The intervention group and control group were different at baseline (in their systolic blood pressure) but the authors did not provide the significance level of this difference. Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients meeting these criteria were randomly assigned to either a control of a study group during the initial screening visit" |
| Allocation concealment (selection bias) | Unclear risk | Allocation procedure not described explicitly Unclear how randomisation occurred or if it was adequately concealed |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | BP measurement has low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | BP measured manually by assessors aware of the participant's allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | Low risk | All major results reported |
| Other bias | High risk | Quote: "Patients populations varied between the two sites" |
Paulos 2005.
| Methods | Randomised trial | |
| Participants | 42 patients with hyperlipidaemia (intervention group 23; control group 19)
Health professional (delivering intervention): 1
Practice: 1 Community pharmacy Chile Year of study: Not stated. |
|
| Interventions | Pharmacist measured total blood cholesterol and triglyceride levels and educated patients on cardiovascular disease, risk factors and appropriate medication use, vs usual care. Length of the intervention: 20 to 25 minutes Number of interventions: 5 during 4 months | |
| Outcomes | Total cholesterol levels Triglyceride levels % of patients with decrease in total cholesterol levels % of patients with decrease in triglyceride levels | |
| Notes | Funding source: Roche Diagnostics, Santiago, Chile, provided support by providing Accutrend GCT device and strips. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The patients admitted to the trial were randomly divided into a control group and an intervention group" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation and allocation process were not described. No clear information |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Personnel were not blinded, same pharmacists delivered both arms. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | The main outcome (cholesterol) is objectively measured. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Original sample size unclear |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes reported (smoking) that seem unrelated to intervention |
| Other bias | Low risk | None |
Peterson 2004.
| Methods | Randomised trial | |
| Participants | 94 patients with cardiovascular disease discharged from the hospital on statin therapy (intervention 46; control 48)
Health professional (delivering intervention): 1
Practice: 1 Acute care teaching hospital (Royal Hobart Hospital) Tasmania, Australia Year of study: April 2001 to October 2001. |
|
| Interventions | Pharmacist conducted home visits to perform cholesterol measurements, assess medication regimen and educate patients about lipid‐lowering drug therapy and dietary and life‐style modifications, vs usual care. Length of the intervention: not clear Number of interventions: 6 during 6 months | |
| Outcomes | Cholesterol level at follow‐up (6 months) | |
| Notes | Funding source: Community Pharmacy Practice Research Grant, through the Guild/Government (Community Pharmacy) Agreement and administered by the Commonwealth Department of Health and Aged Care. Roche Diagnostics Australia provided equipment. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients who provided written, informed consent were allocated to either the intervention or control group, using a computer‐generated list of random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "Computer‐generated list of random numbers". "Patients who provided written, informed consent were allocated to either the intervention or control group, using a computer‐generated list of random numbers". This appears to be centralised allocation. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Personnel were aware of allocation but it is difficult to see how this might have directly influenced intervention, beyond protocol. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Assessors may have been aware of allocation, but this is unlikely to have influenced outcome measurement (a machine read‐off). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Reid 2005.
| Methods | Randomised Trial | |
| Participants | 532 patients with hypertension (intervention 266: control 266) Hypertension Management Clinic United Kingdom Year of study: Augusut 2001 to May 2002. |
|
| Interventions | Implementation of a Hypertension Management Clinic using a treatment protocol based on guidelines. The new Sheffield table was used to estimate cardiovascular risk in patients treated with anti‐hypertensive medication because of its applicability to this patient group. The pharmacist discussed all changes to prescribed medication with the patient and their general practitioner (GP), prior to alteration. Dose titration was undertaken by the pharmacist without GP consultation. Details of the consultation including lifestyle modification advice were documented in the patient records. Changes in medication were entered on the practice computer system and prescriptions were signed by a GP. Blood samples required to monitor treatment or evaluate cardiovascular risk were taken by the pharmacist or nursing staff and patients requiring an electrocardiogram were referred to nursing staff. Patients were allocated 15‐minute appointments and attended the clinic at intervals of 2 weeks to 3 months depending on BP control. | |
| Outcomes | % patients achieving target | |
| Notes | Funding source: Lothian Primary Care Development Fund Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised sequentially, prior to study inception, by the pharmacist into two groups." |
| Allocation concealment (selection bias) | Unclear risk | Randomised before contact. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Unclear if patients were blinded. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Unclear if assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% however, large overall attrition. Quote: "Group 1 (n = 92) [intervention] Of 266 patients identified, 73 were excluded. A total of 193 patients were invited to attend the clinic of whom 92 (47.7%) attended. Group 2 (n = 68) [control] Of 266 patients identified, 107 were excluded. A total of 159 patients were invited to attend the clinic of whom 68 (42.8%) attended". Unclear whether these patients received the same offer |
| Selective reporting (reporting bias) | Low risk | Most key results presented |
| Other bias | Low risk | None |
Rickles 2005.
| Methods | Randomised trial | |
| Participants | 63 patients presenting with new antidepressant prescriptions (intervention 31; control 32)
Health professional (delivering intervention): 14
Practice: 8 Community pharmacies within a large managed care organization Wisconsin, USA Year of study: October 2001 to September 2002. |
|
| Interventions | Pharmacist provided monthly telephone‐based education on antidepressant use and goal of therapy and monitoring of adverse effects and adherence, vs usual care. Length of the intervention: 19, 12, and 11 minutes for first, second, and third phone call, respectively Number of interventions: 3 during 3 months |
|
| Outcomes | > 50% improvement in depression symptoms measured with Beck Depression Inventory‐II (BDI‐II) | |
| Notes | Past use of psychiatric medications was different between groups at baseline.
Study was powered to detect compliance outcomes only. Funding source: Sonderegger Research Center and predoctoral National Research Service Award through the National Institute of Mental Health. Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "When a patient was enrolled from that site, the researcher would randomly select a number out of the envelope" |
| Allocation concealment (selection bias) | Low risk | Assignment sealed in an envelope; envelope not reported as "opaque". Experimenters had no knowledge of forthcoming allocations. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Experimenters were unblinded but given that control participants received no intervention (phone call) bias is unlikely. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Participants were unblinded and this may have influenced self‐reported responses. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | Low risk | All major outcome reported |
| Other bias | Unclear risk | Despite randomisation, intervention patients were more likely than usual‐care patients to have a history of psychotropic medication use. |
Rothman 2005.
| Methods | Randomised trial | |
| Participants | 217 patients with type 2 diabetes (intervention 112, control 105) North Carolina, USA Year of study: February 2001 to April 2003. |
|
| Interventions | The intervention included intensive educational sessions, evidence‐based algorithms, and proactive management of clinical parameters. | |
| Outcomes | Systolic blood pressure (BP) Diastolic BP |
|
| Notes | Funding source: Robert Wood Johnson Clinical Scholars Program, the University of North Carolina Program on Health Outcomes, the University of North Carolina Division of General Internal Medicine, University of North Carolina Hospital Performance Improvement Department, University of North Carolina Pharmacy, the Vanderbilt Center for Health Services Research, and the Vanderbilt Diabetes Research and Training Center Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned patients to the intervention or control group using a random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Assignment was contained in sealed envelopes that were opened by the study co‐ordinator. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Not blinded but outcomes are objective |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Not blinded but outcomes are objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | Low risk | All relevant reported at 12 months and baseline |
| Other bias | Low risk | Baseline differences Quote: "The intervention patients were slightly older than the control patients (P=0.05) and more likely to be African American (P=0.10)." "We tried to limit this concern by performing adjusted analyses, and these findings were similar to those from our unadjusted findings" |
Rubio‐Valera 2012.
| Methods | Randomised trial | |
| Participants | 179 participants with depression (intervention 87: control 92) 13 pharmacies (34 pharmacists) Gavó, a city situated in the province of Barcelona, Spain Year of study: OCtober 2008 to not stated. |
|
| Interventions | The intervention consisted of a series of educational interventions focused on improving patients' knowledge of antidepressant medication, including the importance of compliance. Moreover, in patients with a sceptical attitude towards medication, the intervention aimed to reduce stigma, reassure the patient about possible side effects, and stress the importance of following GPs' advice. Number of Interventions: initial visit plus single (?) follow‐up Number of follow‐ups unclear |
|
| Outcomes | Mean severity of depression Health‐related Quality of Life |
|
| Notes | Funding source: Carlos III Health Institute Grant Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was generated at the patient level by a computerized random‐number generator following a permuted block design." |
| Allocation concealment (selection bias) | Low risk | Quote: "To assure the concealment of allocation, every GP receives a set of 10 sequentially numbered, opaque, sealed envelopes containing patient assignment. Envelopes were generated by an external investigator and details of the series are unknown to any of the GPs or pharmacists in the study." |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Unblinded participants and subjective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "Blinding of participants and pharmacists is not possible because of the type of intervention. However, the assessment visits and data analysis are conducted by independent and blinded evaluators" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition >20% Quote: "Only 87 (95%) and 64 (74%) in the control and intervention group, respectively, received the intervention as allocated and were included in the PP analysis." |
| Selective reporting (reporting bias) | Low risk | All major results reported |
| Other bias | Low risk | None |
Sadik 2005.
| Methods | Randomised Trial | |
| Participants | 221 patients with heart failure (intervention 109; control 112)
Health professional (delivering intervention): 1
Practice: 1 Outpatient clinic in Al‐Ain Hospital Al‐Ain, United Arab Emirates Year of study: Not stated. |
|
| Interventions | Pharmacist providing patient education about heart failure medications and disease management during clinic follow‐up visits, printed booklet on heart failure, symptom monitoring diary card. Pharmacist discussed drug therapy with patients' physicians, vs usual care Length of the intervention: not clear Number of interventions: 5 during 12 months | |
| Outcomes | Quote "At the 3‐monthly outpatient clinics, both groups of patients were assessed as per initial baseline assessments as follows: 2‐min walk test (including time to walk 25 and 50 m), BP, body weight, pulse, FVC, quality of life questionnaires (MLHF questionnaire and the SF36), questionnaire on symptoms and knowledge of, and compliance with, prescribed medication and lifestyle advice. Medication knowledge was scored as a percentage value relating to the number of correct answers given to questions on name of prescribed medications, daily dosage, strength, purpose of each medication and significant side effects. A score of <50% was deemed to be poor knowledge. In relation to compliance with prescribed medications, patient self‐report on missing doses or taking extra doses of their medication, without medical advice to do so, was considered noncompliance. Regarding compliance with lifestyle advice, questions on the following were asked to each patient: dietary modification and sodium restriction, limitation of or abstinence from alcohol, restricted fluid intake, not sleeping flat, taking mild to moderate exercise and smoking cessation (if appropriate). Each parameter was awarded one mark." | |
| Notes | Patients were recruited from the hospital ward and hospital outpatient clinic; Intervention took place in hospital outpatient clinic. Funding source: Not specified Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation carried out using minimisation method |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly described |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Personnel were not blinded to allocation. Unclear if/how this may have biased results |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Baseline measurements were performed by a research pharmacist with the exception of the 2‐minute walk test and theFVC test, which were performed by nursing staff or a pharmacy technician. They were blinded to the group to which individual patients had been assigned and received training on test administration. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Quote: "Two patients in each group died during the study; in addition, three patients withdrew from the intervention group and six from the control group during the study" |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Salazar‐Ospina 2017.
| Methods | Randomised trial | |
| Participants | 92 patients (intervention group 43; control 49) Psychiatric clinic La Ceja, Antioquia, Colombia Year of study: November 2011 to June 2014. |
|
| Interventions | Patients assigned to the intervention group received usual care, verbal and written counselling about bipolar disease, and pharmaceutical care for 1 year from a specially‐trained pharmacist using the Dader Method. | |
| Outcomes | Number of hospitalisations, emergency service consultations, unscheduled outpatient visits, and clinical evaluation of symptomatology | |
| Notes | Funding source: This research was financed in part by Humax Pharmaceutical S.A., providing the PhD student with a salary and the written material used in this work Conflict of interest: Salazar‐Ospina received funding from Credito Beca Francisco José de Caldas Scholarship for Doctoral Programs (528). González‐Avendaño is an employee of Humax Pharmaceutical. The other authors reported nothing to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Participants were randomized to intervention or control groups in sequential order, and they were followed for 12 months" |
| Allocation concealment (selection bias) | High risk | Given the allocation method, it is probable that staff knew to which group the (potential) participant would be allocated. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | The staff and patients understood allocation so blinding may not have been achieved. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Biased assessment unlikely as outcome measure was hospitalisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | Main outcomes specified. |
| Other bias | Low risk | None |
Samtia 2013.
| Methods | Randomised trial | |
| Participants | 348 participants with diabetes (intervention 178: control 170) Selected diabetes clinics Southern Punjab (Nishter Hospital Multan and DHQ Hospital Layyah),India Year of study: March 2011 to not stated. |
|
| Interventions | Patient education Intervention group patients received predefined specialised care. The components of care were: education of disease including short‐ and long‐term complications; medication adherence and its effects on glycaemic control; education about timing of medication use in relation to food; education about dietary restrictions; education about sensory changes including foot examination; the role of exercise in achieving glycaemic control; the role of self‐monitoring of blood glucose to achieve glycaemic control; education about control of HbA1c values and fasting blood glucose; and smoking cessation. If relevant. Frequency: every 4 weeks Duration: 5 months |
|
| Outcomes | Fasting blood glucose HbA1c |
|
| Notes | Funding source: Not specified Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear how randomisation performed Quote:"Patients were randomly assigned into control (n=170) and intervention groups (n=178)." |
| Allocation concealment (selection bias) | Unclear risk | Unclear if allocation concealed |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | No blinding, but the intervention knowledge seems unlikely to affect objective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | No blinding, but the intervention knowledge seems unlikely to affect objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% Quote: "Almost all the patients included completed the study (control group: 168/170 and intervention group: 174/178)." |
| Selective reporting (reporting bias) | Low risk | Main outcomes present Note that before‐and‐after results reported rather than control versus intervention or "difference in the difference". |
| Other bias | Low risk | None |
Sarkadi 2004.
| Methods | Randomised trial | |
| Participants | 64 patients with diabetes mellitus Type II (intervention 33; control 31)
Health professional (delivering intervention): unclear
Practice: unclear Community pharmacies in Sweden Year of study: Not stated. |
|
| Interventions | Pharmacist led an educational programme using a video, a dice game and a booklet on diabetes management to promote dietary modifications, exercise and blood glucose control and referred patients to health professionals in cases of unsatisfactory glucose control, vs no intervention. Length of the intervention: unclear Number of interventions: 3 during 1 year; 1 year follow‐up after intervention completion | |
| Outcomes | HbA1c at 12 months (end of study) HbA1c at 24 months (follow‐up) | |
| Notes | Pharmacist‐led educational group had assistance from a diabetes nurse specialist on the first 2 occasions; patients were self‐referred to the programme. Funding source: Swedish Foundation for Health‐care Sciences and Allergy Research Grant No. V2000 225, the National Corporation of Swedish Pharmacies, and Uppsala University. Funding for the first author, Anna Sarkadi from the Knut and Alice Wallenberg Foundation in Stockholm, Sweden, grant nr. KAW 2001.0303. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "For those participants eligible for randomisation, the informed consent sheet and the questionnaire were put into an unmarked envelope, one for each participant. The identical envelopes were then put into a box. Each time 20 complete sets of participant items were collected, randomisation was performed. An assistant mixed the envelopes in the box, took them out one at a time, and randomly placed them into two piles. A third person, acting as a witness, pointed out which pile should be allocated to the intervention group and which pile to the control group." Appropiate randomisation procedure |
| Allocation concealment (selection bias) | Low risk | Quote: "An assistant mixed envelopes in a box, took them out one at a time, and randomly placed them into two piles. A third person, acting as a witness, pointed out which pile should be allocated to the intervention group and which pile to the control group" |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | No‐one was blinded, but HBA1c unlikely to be biased |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | No‐one was blinded, but HBA1c unlikely to be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | Low risk | 1 main outcome reported |
| Other bias | Low risk | None |
Schneider 1982.
| Methods | Randomised trial | |
| Participants | 40 patients with essential hypertension and congestive heart failure (intervention 20; control 20)
Health professional (delivering intervention): 1
Practice: 1 Outpatient medicine clinic University Hospital Clinic, Ohio State University, USA Year of study: Not stated. |
|
| Interventions | Pharmaceutical care Pharmacist examined and evaluated patients during a clinic visit Pharmacist communicated findings and suggestions to physician, vs usual care Length of intervention: 12 months |
|
| Outcomes | Systolic and diastolic blood pressure % target blood pressure achieved |
|
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to a study or a control group". |
| Allocation concealment (selection bias) | Unclear risk | Not explicitly described |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | The personnel (doctors and pharmacists) were not necessarily unblinded and this may have influenced protocol implementation. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | BP mostly objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data from all 40 patients presented. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in Methods appear in Results |
| Other bias | Low risk | None |
Schneiderhan 2014.
| Methods | Randomised trial | |
| Participants | 121 participants (intervention 61: control 60) Metabolic syndrome/psychotic 3 community mental health clinic setting Minnesota, USA Year of study: February 2012 to January 2014 |
|
| Interventions | Pharmacist comprehensive medication management not described | |
| Outcomes | Taking antipsychotic medicines | |
| Notes | Funding source: Founded by Medica Foundation, Minneaplois, Minnesota and Peters Institute of Pharmaceutical Care, College of Pharmacy, University of Minnesota, Minneapolis Conflict of interest: Dr Scheniiderhan has received honoraria from the American Society of Health System Pharmacists. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a block randomization schedule was used to ensure balanced treatment assignment of subjects recruited at each site" |
| Allocation concealment (selection bias) | Low risk | Quote: "a block randomization schedule was used to ensure balanced treatment assignment of subjects recruited at each site" A centralised call‐in system was used to inform the investigators of the participant’s random group assignment |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Intervention unclear |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Unclear who collected data; blinding unclear |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10%, however, overall attrition rate >20%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Sellors 2003.
| Methods | Randomised trial | |
| Participants | 889 elderly patients (intervention 431; control:458) 48 physicians (intervention 24; control 24) Ontario, Canada. Year of study: August 1999 to ˜ July 2000 |
|
| Interventions | Structured medication assessment by pharmacist with patient, which assessed needs, drug‐related problems and course of action. This was discussed with the physician, who then indicate their recommendation intentions and plan. 5 months later physician‐pharmacist discussion of what recommendations have been implemented. 4 months later pharmacist phoned patient to discuss drug therapy. | |
| Outcomes | SF‐36 (physical functioning) at 12 months | |
| Notes | Funding source: Funding was provided by the Health Transition Fund, Health Canada, and in kind support from the Department of Family Medicine, McMaster University, and the Centre for Evaluation of Medicines, St. Joseph’s Healthcare, Hamilton, Ont. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The pair of physicians in each postal code area were randomly allocated, in a concealed fashion, to the intervention or control group, using a central telephone randomisation procedure based on computer‐generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted by a research team member who was blinded to the practices’ identities. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Neither family physicians nor their patients were blinded to their allocation group. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Unblinded and self‐reported SF36 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Sidel 1990.
| Methods | Randomised trial | |
| Participants | 284 elderly patients (intervention 141; control 143) who were Medicare recipients living in the study area 1 pharmacist Norwood, New York City, USA Year of study: Not stated. |
|
| Interventions | Patient‐specific packet containing information on prescription and medication, home‐visit explained this packet, could contact physicians if wanted, counselled patient about drug use, encouraged adherence and checked for out‐of‐date medicine. At least 2 visits by pharmacist across 6 x 1‐month periods, with additional phone contact as necessary. | |
| Outcomes | Total Ambulatory Care visits past 3 months (change scores) at 36 months | |
| Notes | Funding source: National Institute on Aging (P01AG03424 and R0 lAG08125) Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assigned by randomised tables |
| Allocation concealment (selection bias) | Low risk | Separate people enrolled and randomised participants. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Little information about blinding or probable consequences |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Little information about blinding or probable consequences |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% however, overall high attrition >20% |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Silveira 2014.
| Methods | Randomised trial | |
| Participants | 332 participants receiving care for HIV infection at the Service for Specialized Assistance in HIV (SAEH) (intervention 166: control 166) School of Medicine, in Pelotas, southern Brazil Year of study: Not stated. |
|
| Interventions | Pharmaceutical care using the Dáder method. Quote "Intervention patients received structured counselling on their prescription regimens, at the time of initial drug dispensing and at monthly refill visits. The key elements of pharmaceutical care were: reviewing the prescription with the patient; reviewing a card on which medications were colour‐coded to facilitate recognition and reduce confusion that might arise from complicated drug names; reviewing the schedule, length, and date of the next appointment; reviewing the patient’s understanding of the prescription by asking him/her to describe it for the pharmacist; and giving patients verbal information on the expected side effects of their medications. Patients were instructed to call the pharmacist if side effects occurred. After the counselling session, the pharmacist verified that all components of the intervention had been delivered." Duration: 1 year |
|
| Outcomes | Proportion of patients reporting adherence to ART. Proportion of patients with undetectable viral load | |
| Notes | No extractable data. Funding source: The University of California San Francisco and grants by the US National Institutes of Health (NIH): Fogarty International Center (FIC) D43TW005799; National Institute for Mental Health (NIMH) P30MH062246, R25MH064712; and the FIC AIDS International Training and Research Program (AITRP) D43TW000003. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consenting participants were randomised using a random‐number table. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Non‐blinded randomised controlled trial. Unsure of effect on outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Self‐reported main outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10% |
| Selective reporting (reporting bias) | Low risk | Main outcomes reported |
| Other bias | Low risk | None |
Simpson 2011.
| Methods | Randomised trial | |
| Participants | 260 participants with diabetes (intervention 131: control 129). Primary care clinics in Edmonton, Canada Year of study: February 2006 to January 2009. |
|
| Interventions | The intervention programme began with an in‐person visit with a study pharmacist to identify all prescription, nonprescription, complementary, and alternative medications. Pharmacists measured the patient’s height, weight, heart rate, and blood pressure. Blood pressure was measured according to the Canadian Hypertension Education Program recommendations using an automated machine. Pharmacists then formulated guideline‐concordant recommendations to optimise medication management of blood pressure and other cardiovascular risk factors. These recommendations were discussed with the primary care physician who was responsible for authorising medication changes. The pharmacist then worked independently with the patient to implement these changes. Frequency: Once at beginning of year Duration: 1 year |
|
| Outcomes | HbA1c Systolic BP Diastolic BP United Kingdom Prospective Diabetes Study (UKPDS) Risk Engine Score |
|
| Notes | Funding source: Canadian Diabetes Association, the Institute of Health Economics, and the Alberta Heritage Foundation for Medical Research. Conflict of interest:None of the agencies were involved in the design and conduct of the study; collection, management, and interpretation of the data; and preparation, review, or approval of the manuscript. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A central randomization service provided computer generated random sequences stratified by the primary care clinic for treatment allocation." |
| Allocation concealment (selection bias) | Low risk | Quote: "Pharmacists, analysts, and investigators were unaware of the block size and allocation sequence to preserve allocation concealment" |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Unblinded participants, but little cause for concern here due to objective outcomes |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Quote: "a randomized controlled trial with blinded ascertainment of outcomes" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. Between group attrition < 10% Missing data were replaced by carrying the last observation forward. |
| Selective reporting (reporting bias) | Low risk | Main outcomes clearly specified and reported |
| Other bias | Low risk | None |
Solomon 1998.
| Methods | Randomised trial | |
| Participants | Patients with hypertension and/or chronic obstructive pulmonary disease (COPD) ‐ hypertension arm 133 (intervention 63; control 70); COPD arm 98 (intervention 43; control 55)
Health professionals: not clear
Practices: 11 Outpatient clinics at 10 Veterans Administration Medical Centers and 1 university hospital in USA Year of study: Not stated. |
|
| Interventions | Pharmacist‐provided clinical pharmaceutical care services vs usual care Pharmaceutical care services included clinical management of hypertension and COPD by standardised patient assessment activities, pharmacists' involvement with the healthcare team, collaboration with physicians to develop patient‐specific plan, patient education on hypertension and COPD, counselling to address patients' questions or concerns, and regular patient assessments and care. Length of intervention: approximately 60 minutes for initial visits, 30 minutes for follow‐up visits Number of interventions: monthly visits over 6 months |
|
| Outcomes | Blood pressure (hypertension arm) Borg Scale (COPD arm) | |
| Notes | Intention‐to‐treat analysis not done (number of patients reported is number of patients analysed; number of patients randomised not clear). Funding source: Novartis Pharmaceuticals corperation, East Hanover, N.J. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "study assistants assigned the patients using a table of random numbers". |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation concealment |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Unblinded personnel, potential for bias |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Blood pressure measurement and interview may have been conducted by an experimenter who was not blinded to patient allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Report describes "evaluable patients". Unclear how many recruited into trial |
| Selective reporting (reporting bias) | Low risk | All main results reported. Post hoc tests labelled as such |
| Other bias | Low risk | None |
Sookaneknun 2004.
| Methods | Randomised trial | |
| Participants | 235 patients with hypertension (intervention 118; control 117)
Health professionals: not clear
Practices: 3 University‐affiliated community pharmacy and 2 primary care units in Thailand (Mahasarakham, Takonyarng village, Kharmrieng village) Year of study: Ocotober 2002 to July 2003. |
|
| Interventions | Pharmacist provided monthly consultation and blood pressure monitoring, vs usual care Pharmacist made medication regimen change recommendations to physicians after identifying drug‐related problems Length of the intervention: 30 to 50 minutes Number of interventions: 6 (monthly) during 6 months | |
| Outcomes | Blood pressure | |
| Notes | Funding source: Research grant from Chiang Mai University, Thailand Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "A simple randomization technique was used to assign the patients to a treatment group and a control group." Unclear how randomisation occurred |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | BP measurement has low risk of performance bias. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | BP measured manually by assessors aware of the participant's allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many completed the trial |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Stewart 2014.
| Methods | Randomised trial | |
| Participants | 60 pharmacies, 395 patients with hypertension (intervention 207: control 188) Pharmacies from metropolitan, regional and remote areas in three Australian states (Victoria, Western Australia and Tasmania) were contacted by telephone and informed about the project. Year of study: July 2009 to January 2010. |
|
| Interventions | Pharmacist care Patients in the Pharmacist Care Group received a package of interventions from the pharmacist for enhancing their antihypertensive medication adherence, which includes: a home blood pressure (BP) monitor with the capacity to store and download BP readings to be used for discussion at 3‐ and 6‐month follow‐ups; training by the pharmacist on self‐monitoring of BP, motivational interviewing and education by the pharmacist to help patients improve their medication adherence and achieve target BP; pharmacist‐initiated home medicines review, dose administration aid and/or patient medication profile, where necessary; medication use review to identify and resolve possible medication‐related hypertension (e. g. due to non‐steroidal anti‐inflammatory drugs, cold preparations, complementary medicines, etc); referral to a general practitioner when needed (e.g. very high blood pressure); and refill reminders (by either text, telephone or mail) from their pharmacist at a chosen number of days before their antihypertensive medication dispensing is due. |
|
| Outcomes | Systolic BP Diastolic BP |
|
| Notes | Randomisation: 60 pharmacies recruited and randomised ‐ 30 pharmacist care and 30 in control group. Five either withdrew or were withdrawn (1 intervention, 4 countrol). Funding source: Australian Government Department of Health and Ageing (as part of the Fourth Community Pharmacy Agreement through the Fourth Community Pharmacy Agreement Research & Development Grants Program managed by the Pharmacy Guild of Australia). Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out at a central location using the sealed opaque envelope technique. |
| Allocation concealment (selection bias) | Low risk | The randomisation process was carried out by 1 of the investigators using the ‘sealed envelope technique’. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Blinding unclear Low risk for BP |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Low risk for BP and all other measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis performed. Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All main results reported.
Many subgroup analyses reported in the Results but not in the Methods. These subgroup data were not analysed in our meta‐analyses. |
| Other bias | Low risk | None |
Suppapitiporn 2005.
| Methods | Randomised trial | |
| Participants | 360 diabetic patients (intervention 180; control 180) King Chulalongkorn Hospital Bangkok, Thailand Year of study: January to Dcember 2004. |
|
| Interventions | All participants received diabetic drug counselling by a pharmacist; 1) counselling alone; 2) diabetic booklet; 3) specialised medication containers; 4) diabetes education, booklet, medication containers. Interventions were received at the initial visit and at 6‐month assessment follow‐ups. |
|
| Outcomes | HbA1c at 6 months | |
| Notes | Funding source: Not specified Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "a simple randomisation was performed". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Not stated, but medical records were used to get outcomes so unlikely to be biased. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Actual completion rate unknown. |
| Selective reporting (reporting bias) | High risk | Quote: "patient records used to obtain patients response to intervention". |
| Other bias | Low risk | None identified |
Tang 2014.
| Methods | Randomised trial | |
| Participants | 124 participants with epilepsy (intervention 59: control 65) Patients with epilepsy who were treated at the outpatient clinic of Neurology Huashan Hospital, University of Fudan, Shanghai, China Year of study: Not stated. |
|
| Interventions | Education and behavioural intervention Intervention patients were educated by a pharmacist according to the guidelines of the American Society of Health‐System Pharmacists about pharmacist‐conducted patient education and counselling. Patients received monthly calls from the pharmacist and were instructed about their medications and asked to adhere to their anti‐epileptic medication. There was also a behavioural intervention based on cue‐dose training therapy. The medication schedule used in this programme was presented in the form of a table that illustrated the daily medication therapy of patients with pictures of anti‐epileptic medication, and it provided patients with cues to take their medications. Frequency: Monthly phone calls, initial education session, persistent cues Duration: 6 months |
|
| Outcomes | Seizure control (50% reduction from baseline), Quality of life | |
| Notes | Funding source: Not specified Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A list of 300 random numbers between 0 and 9 was generated using a statistical package. The patients were numbered according to the order in which they were recruited. Patients who had received an even randomly‐generated number were assigned to group I, and patients who received odd numbers were assigned to group II. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Unblinded study with substantial potential bias |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Seizure change: low risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Tannenbaum 2014.
| Methods | Randomised trial | |
| Participants | 303 elderly patients on benzodiazepines (intervention 148: control 155) The study included 30 community pharmacies (cluster units) Montreal, Canada. Year of study: 2010 to 2012. |
|
| Interventions | Written educational material to facilitate benzodiazepine withdrawal The patient empowerment intervention consisted of an 8‐page booklet based on social constructivist learning and self‐efficacy theory. The intervention comprised a self‐assessment component about the risks of benzodiazepine use, presentation of the evidence for benzodiazepine‐induced harms, knowledge statements designed to create cognitive dissonance about the safety of benzodiazepine use, education about drug interactions, peer champion stories to augment self‐efficacy, suggestions for equally or more effective therapeutic substitutes for insomnia or anxiety or both, and stepwise tapering recommendations. The intervention asked participants to discuss the de‐prescribing recommendations with their physician or pharmacist or both. The intervention was personalised according to the participant’s pharmacy profile to include the name of the specific benzodiazepine the participant was taking. The intervention was mailed to the intervention group within 1 week of group allocation while the usual care (wait list) group received the educational tool 6 months following group allocation. Duration: 6 months |
|
| Outcomes | Discontinuation of benzodiazepine use | |
| Notes | Funding source: Canadian Institutes of Health Research Conflict of interest: Mr Martin received a bursary from the Michel Saucier Endowed Chair in Pharmacology,Health,and Aging of the Faculty of Pharmacy of the Universitéde Montréal, and Drs Tannenbaum and Ahmed were clinician scientists funded by the Fonds de Recherche en Santé de Quebec. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A statistician, blinded to pharmacy and cluster size, generated a random allocation sequence using computer‐generated random digit numbers. |
| Allocation concealment (selection bias) | Low risk | Up until the point of randomisation, neither the research assistant, the cluster representative (the pharmacist), nor the client knew the allocation of the clusters. After randomisation, only the research assistant was aware of treatment allocation. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Pharmacists and participants were not informed, and remained unaware of the fact that there was another group in the study; nor were they informed of the procedures for the other arm. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | 1 investigator and 1 research nurse, blinded to group allocation, independently assessed outcomes according to a prespecified protocol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Taveira 2011.
| Methods | Randomised trial | |
| Participants | 88 participants with diabetes (intervention 44: control 44) Eligible patients were identified by a combination of review of the Providence VAMC electronic medical record system and referral by primary care providers. USA Year of study: December 2006 to not stated. |
|
| Interventions | A multidisciplinary education and pharmacist‐led intensive behavioural and pharmacological group intervention. Intervention patients attended 4 once‐weekly sessions of 2 hours, followed by 5 monthly booster sessions with approximately 4 to 6 participants in each session. Each session consisted of 2 parts: i) education and ii) behavioural and pharmacological interventions for hypertension, hyperlipidaemia, hyperglycaemia and tobacco use. The education portion included interactive lectures provided by a nurse, nutritionist, or the clinical pharmacists who were certified in diabetes education. Each session focused on 1 or 2 self‐care behaviours, such as goal setting, to promote health and problem‐solving for daily living or integration of psychosocial adjustment to daily life. At each session, food logs were reviewed by the pharmacist and participants were reminded of their nutrition goals. Participants prepared healthy food choices during these sessions and were advised of the availability of nutrition programmes. The pharmacological and behavioural intervention was conducted by a clinical pharmacist certified in diabetes education who performed a group assessment to determine the degree to which patients felt they could manage the daily aspects of diabetes care through discussion and use of the Perceived Competence for Diabetes Scale. Each participant was provided with a cardiovascular risk report card containing medical history, current medications, vital signs, and laboratory test results. Medications for blood pressure, cholesterol, diabetes, and tobacco cessation were initiated or titrated based on previously established treatment algorithms. Each group member was provided with individualised homework for medication changes and a behaviour change goal, such as exercise recommendations, dietary modifications, and blood glucose or blood pressure monitoring. A clinical pharmacist used theory‐based counselling and reinforcement to change outcome expectations and to increase behaviours that would improve diabetes self‐care behaviours such as increasing physical activity and healthy eating. Demonstration and coaching to increase self‐efficacy for self‐care skills, such as monitoring of blood glucose and logging daily dietary intake, were also performed. Number of Interventions: 4 once‐weekly sessions of 2 hours, followed by 5 monthly booster sessions held in a classroom with approximately 4 to 6 participants in each session. |
|
| Outcomes | HbA1C Systolic BP Low density lipoprotein‐cholesterol (LDL‐C) |
|
| Notes | Funding source: American College of Clinical Pharmacy Astra‐Zeneca Health Outcomes Research Award (Dr. Taveira), American Society of Health System Pharmacists and Education Foundation Federal Services Research Grant Program (Dr. Cohen), and VA HSR&D Merit Review Award IAB 06‐269 (Drs. Taveira, Cohen, and Wu). Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to the intervention arm or standard care arm using simple coin toss randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No relevant information |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Unclear if participants were blinded |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Unclear if assessors were blinded, but HbA1C is an objective measure. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Unclear risk | Most outcomes were reported |
| Other bias | Low risk | None |
Taveira 2014.
| Methods | Randomised trial | |
| Participants | 200 patients at cardiovascular risk (group intervention 72; individual intervention 73; control 55) 1 primary care clinic USA Year of study: October 2003 to December 2006. |
|
| Interventions | Group medical intervention: 4 visits of 120 minutes held every 3 months for 12 months. Patients were encouraged to bring social support, educated about healthy lifestyles, behavioural and pharmacological interventions for hyperglycaemia, hypertension and dyslipidaemia. Provided with individualised cardiovascular risk report card which was updated at each session. Individualised homework given for medication changes, goals and self‐monitoring and phone contact as needed. Individual intervention: 30‐minute visits once every 3 months for 12 months. Assessment of medication adherence, blood pressure, vital signs with reference to nutritionist or therapist as necessary. |
|
| Outcomes | Failure to maintain guideline goals was defined as an HbA1c > 7% (> 53 mmol/mol) Outcomes presented as differences in failure rates rather than end point scores |
|
| Notes | Funding source: Supported by Merck and Co. Inc. Disease Management Grant Program, Providence VA Medical Center, University of Rhode Island College of Pharmacy. Conflict of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Unblinded personnel and patients may have influenced behaviour. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | HBA1c unlikely to be biased |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Taylor 2003.
| Methods | Randomised trial | |
| Participants | 81 patients enrolled; 69 high‐risk patients reported (intervention 33; control 36). 3 community‐based family medicine clinics affiliated with the University of Alabama School of Medicine—Tuscaloosa Alabama, USA Year of study: December 1998 to not stated. |
|
| Interventions | Intervention received usual care alongside pharmacotherapeutic interventions by a pharmacists. Meeting with pharmacist 20 minutes before physician; identifying and preventing problems related to drug therapy. Pharmacist made recommendations to physicians and provided drug and disease information. Written materials and devices to improve compliance were provided. | |
| Outcomes | SF‐36 (physical functioning) at 12 months | |
| Notes | Funding source: ASHP Research and Education Foundation Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated Quote: "Patients were randomly assigned to a control group or an intervention group". |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Patients and personnel not blinded and potential for performance bias exists |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | SF‐36 with no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Tommelein 2013.
| Methods | Randomised trial | |
| Participants | 734 participants with chronic obstructive pulmonary disease (COPD) (intervention 371: control 363) 170 community pharmacies Belgium Year of study: December 2010 to not stated. |
|
| Interventions | Patients in the intervention group received a 2‐session intervention; 1 session at the start of the study and 1 at 1 month. All interventions were given during one‐to‐one counselling sessions. To support interventions, pharmacists were provided with information leaflets on COPD, demonstration inhaler units and a list of practical solutions to specific nonadherent behaviour. Session 1 at baseline included structured patient education (verbal and written form) about COPD pathophysiology, medication dose and Inhalation technique. The importance of treatment adherence, possible side effects, self‐management (e.g. lifestyle advice) and smoking cessation were covered. The follow up session at 1 month covered the same topics and discussed changes to adherence. Duration: 15 ‐ 25 minutes |
|
| Outcomes | Medical Research Council Dyspnoea Score, Euroqol (EQ)‐5D utility score (scale ‐0.18 to 1) | |
| Notes | No extractable outcomes except for EQ‐5D. Funding source: Ghent University, Liège University and GlaxoSmithKline (protocol number of the grant 114684). Conflict of interest: Dr Brusselle reportedtohavereceivedagrantfromGlaxoSmithKline;is a member of the board for AstraZeneca, BoehringerIngelheim, GlaxoSmithKline and Novartis; has received payment for lectures at AstraZeneca, BoehringerIngelheim, Chiesi, GlaxoSmithKline, MerckSharp&Dohme, Novartis, Pfizer and UCB. Dr Remon reported to have received grants from IOF fund, FWO Vlaanderen and IWT; has received royalties from Tibotec/Biovail. Dr Van Bortel reported that he has been a consultant at the Drug Research Unit Maastricht; is employed by the Ghent University; has received royalties concerning educational pharmacological books; has received payment for travel accommodations concerning expenses unrelated to the trial from Daiichi‐Sankyo and Servier. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central Web‐based randomisation system, created by an independent investigator. As the intervention was educational, blinding of pharmacists was not possible. |
| Allocation concealment (selection bias) | Low risk | To conceal assignment, pharmacists performed allocation through a central Web‐based randomisation system, created by an independent investigator. As the intervention was educational, blinding of pharmacists was not possible. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Patients were not told the study group to which they were assigned. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Low risk: participant‐completed measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Tsuyuki 2002.
| Methods | Randomised trial | |
| Participants | 675 cardiovascular risk patients (intervention 344; control 331) 54 community pharmacies Alberta and Saskatchewan, Canada. Year of study: 1998 to 2000. |
|
| Interventions | Pharmacists interviewed patients to determine modifiable cardiovascular risk factors and give education using a brochure. Pharmacists sent recommendations to physicians and encouraged patients to make an appointment. During 5 follow‐up sessions either by phone or in person over 16 week period, further education and suggestions were provided, as well as checking adherence and whether patients had seen their physician. | |
| Outcomes | The primary end point was a composite measure representing improvement in the process of cholesterol risk management. It consisted of measurement of a complete fasting cholesterol panel by the primary care physician or prescription of a new cholesterol‐lowering medication or an increase in dosage of a cholesterol‐lowering medication. As a composite end point, only the first event attained in the cluster was counted. | |
| Notes | Funding source: Supported by unrestricted grants from the University Hospital Foundation (Edmonton), Merck Frosst Canada Ltd, The Alberta College of Pharmacists (Edmonton), and the Institute of Economics (Edmonton) Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted via a computer‐ generated sequence using block randomization (block size of 4) with stratification by study center (pharmacy)". |
| Allocation concealment (selection bias) | Low risk | Computer‐generated block randomisation |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Non‐blinded personnel and patients may have behaved differently on account of trial allocation |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Objective outcome measures |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Tsuyuki 2015.
| Methods | Randomised trial | |
| Participants | 248 hypertensive patients (intervention 181; control 67) 23 pharmacies Alberta, Canada Year of study: July 2009 to May 2013. |
|
| Interventions | Patients received enhanced pharmacist care, guided by national hypertension guidelines. This included assessment, counselling about cardiovascular risk and blood pressure control, review of medications, drug therapy changes, lifestyle advice and written information about hypertension. The patient's general practitioner was aware of any changes to medication and assessment results. Follow‐up occurred monthly until target BP was achieved for 2 visits, and then every 3 months for study period | |
| Outcomes | Systolic and diastolic BP % achieving target BP |
|
| Notes | Funding source: RxACTION was supported by grants from the Canadian Institutes of Health Research, Alberta Innovates–Health Solutions, Merck, the Canadian Foundation for Pharmacy, and the Cardiovascular Health and Stroke Strategic Clinical Network of Alberta Health Services. The study was further supported by ManthaMed through the in‐kind provision of BpTRU devices. Dr Houle received funding as a graduate student from the Canadian Institutes of Health Research, the Interdisciplinary Chronic Disease Collaboration (funded by Alberta Innovates–Health Solutions), and Hypertension Canada. Dr McAlister was supported by a salary award from Alberta Innovates–Health Solutions and the University of Alberta Chair in Cardiovascular Outcomes Research. Conflict of interest: Dr Tsuyuki has received research funds for investigator‐initiated trials from AstraZeneca, Sanofi, and Merck and has provided consulting for PharmaSmart International and Boehringer Ingelheim. The other authors report no conflicts. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted at the level of the patient and was performed by a centralised secure website to ensure concealment. |
| Allocation concealment (selection bias) | Low risk | Randomisation was conducted at the level of the patient and was performed by a centralised secure website to ensure concealment. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Because of the nature of the intervention, blinding was not possible. Possibility that knowledge of allocation could alter participant or personnel behaviour |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | All BP measurements performed by the pharmacist were made with an automated device which takes 6 readings, discarding the first and taking the average of the remainder. Home measurements were performed with an automated home BP monitor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Unequal number of participants in control (n = 67), intervention (n = 181), although intervention group was split in 2, but outcomes reported as a whole. |
Verret 2012.
| Methods | Randomised trial | |
| Participants | 114 participants (intervention 58: control 56) Specialised anticoagulation clinic of the Montreal Heart Institute Montreal, Canada Year of study: November 2009 to May 2010. |
|
| Interventions | Self‐management of anticoagulation control versus standard care as control Instruction on self‐management Patients randomised to the self‐management group immediately received training on the use of an automated device and the self‐management algorithm developed by the investigators. This included instructions on the frequency of International Normalised Ratio (INR) monitoring, specific recommendations on what to do in the case of high or low INR, how and when to communicate with the pharmacists in the self‐management programme, how to use the device, and the patient’s responsibility in the programme. The session concluded with clinical scenarios, during which patients had to apply their knowledge. They returned 1 week later to validate their use of the device and the algorithm. Patients who had difficulty using the device or algorithm at the second visit were invited to an additional second or third visit. If difficulties persisted, the patient was not allowed to undergo self‐management. On a weekly basis, patients in the self‐management group monitored their INR and adjusted their dose of warfarin according to the algorithm. Through a voicemail message, patients were required to communicate their INR result and any adjustment performed. The patient was contacted if no telephone call was received on the expected day, or if an error in management occurred. If the INR was outside the algorithm limits, the dose was adjusted by the pharmacist. Number of Interventions: 2 ‐ 3 visits over 2 ‐ 3 weeks, then weekly telephone communication Duration: 4 months |
|
| Outcomes | Adverse events, Quality of Life (QoL) ‐ general treatment satisfaction | |
| Notes | Funding source: Dr. de Denus was supported by the Fonds de la Recherche en Sante du Quebec and the Universite de Montreal Beaulieu‐Saucier Chair in Pharmacogenomics. The Coaguchek XS devices and CoaguChek XS PT test strips were provided by Roche Diagnostics Canada. Conflict of interest: Dr. de Denus was supported by the Fonds de la Recherche en Sante du Quebec and the Universite de Montre al Beaulieu‐Saucier Chair in Pharmacogenomics. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was generated by using permuted random blocks of sizes 4 and 6. This list was generated by the Montreal Heart Institute Co‐ordinating Center Biostatistics Department using statistical software. |
| Allocation concealment (selection bias) | Low risk | Patients were then randomised to continue their management at the anticoagulation clinic (control group) or to switch to self‐management (self‐management group). Patients randomised to the control group received no further training. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Intervention group received training on use of a device that the control group did not receive. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | No objective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Vivian 2002.
| Methods | Randomised trial | |
| Participants | 56 hypertensive patients (group numbers not stated) The study was conducted at the Veterans Affairs Medical Center Philadelphia, Pennsylvania, USA Year of study: Not stated. |
|
| Interventions | Pharmacist‐managed hypertension clinic Patients in the intervention group were scheduled to see the clinical pharmacist once a month at the pharmacist‐managed hypertension clinic. A prescribing pharmacist made appropriate drug therapy changes (in both drug selection and dosage) for blood pressure control in accordance with guidelines. The pharmacist did not make any changes in their patients’ other drugs that may adversely affect blood pressure. Drug counselling, consisting of a discussion about side effects, recommended lifestyle changes, and an assessment of compliance, was provided at each visit. Number of Interventions: 1 a month Duration: 6 months |
|
| Outcomes | Systolic BP Diastolic BP Health‐related Quality of Life |
|
| Notes | Funding source: Christian R. and Mary F. Lindback Foundation. Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to either the intervention group or the control group" |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | BP objective. Satisfaction possibly biased |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Unclear risk | Quote: "Measurements were obtained by a clinical pharmacist using an auscultatory sphygmomanometer." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | Main results presented |
| Other bias | Low risk | None |
Volume 2001.
| Methods | Randomised trial | |
| Participants | 363 elderly participants (group numbers not stated) Ambulatory elderly (≥ 65 years of age) patients who were concurrently using 3+ medications according to pharmacy profile. 16 community pharmacies Alberta, Canada Year of study: June 1997 to not stated. |
|
| Interventions | Pharmaceutical care Treatment pharmacists were enrolled in an intensive education programme designed to give them the necessary skill sets to provide care to study patients. Treatment pharmacists used an initial interview and frequent follow‐up communication with the patient and other caregivers. In addition, pharmaceutical care interventions were often due to an in‐depth review of the information collected by establishing a therapeutic relationship with the patient as opposed to being triggered by the receipt of a prescription, as was the case in the control pharmacies. The frequency, number and duration of interventions was unclear. Duration of study: 16 months. |
|
| Outcomes | None available | |
| Notes | Funding source: Hoechst Marion Roussel provided an unconditional grant Conflict of interest: None stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study statistician did not know the identity of the pharmacies and randomly assigned pharmacies from 6 of the 8 pairs to either the treatment or the control group. |
| Allocation concealment (selection bias) | Low risk | The study statistician did not know the identity of the pharmacies and randomly assigned pharmacies from 6 of the 8 pairs to either the treatment or the control group. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Unclear risk | Quote: "Treatment pharmacists were enrolled in an intensive education program designed to give them the necessary skills..." Personnel were not blinded. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | High risk | Quote: "It was not possible to blind patients to the intervention" and adherence to medication regimens and patient satisfaction were measured with "self‐report measures". Hence unblinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only 5 of 8 intervention pharmacists and 7 of 8 control pharmacists provided data. Reasons for lack of data provision included lack of owner commitment. |
| Selective reporting (reporting bias) | Unclear risk | Outcomes unavailable |
| Other bias | Unclear risk | Unclear |
Wal 2013.
| Methods | Randomised trial | |
| Participants | 142 hypertensive patients (intervention 72: control 70) The study was conducted in the outpatient unit of the medicine department in Lakshmi Pat Singhania. Institute of Cardiology Kanpur, India. Year of study: July 2010 to August 2011 |
|
| Interventions | Pharmaceutical care Intervention group patients received pharmaceutical care including written, validated health education material. Patients were counselled on the names, indications, adverse effects and specific administration instruction for their antihypertensive medications. Physical activity or exercise performed by patients was assessed by interviewing the patients. A study‐specific patient counselling documentation form was used. Blood pressure readings were noted in the data collection form at baseline and first and second follow‐up. Potential problems were also discussed with physicians and documented. The control group did not receive any pharmaceutical care. Number and frequency of interventions unclear. Duration: 13 months |
|
| Outcomes | Systolic BP Diastolic BP Quality of Life (SF‐36) |
|
| Notes | Funding source: Supported by intervention cardiologists and Medical Superintendent of LPS institute of Cardiology Kanpu Conflict of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Enrolled patients were randomised by the block randomisation method into 2 groups, control and intervention. |
| Allocation concealment (selection bias) | Unclear risk | Unclear if concealment occurred |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | BP is an objective measure |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | BP is an objective measure |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10% however, high overall attrition. 54/72 in intervention group and 48/70 in control group completed the trial. |
| Selective reporting (reporting bias) | Low risk | Main results reported |
| Other bias | Low risk | None |
Weinberger 2002.
| Methods | Randomised trial by practice: 36 drugstores divided into 12 clusters of 3 geographically‐proximal drugstores | |
| Participants | 1113 patients with chronic obstructive pulmonary disease (COPD) and asthma
Asthma ‐ 660 (pharmaceutical care programme 262, peak flow monitoring control 233, usual care control 165)
COPD ‐ 453 (pharmaceutical care programme 185, peak flow monitoring control 130, usual care control 138)
Health professional (delivering intervention): Unclear
Practice: 36 Community pharmacies Indianapolis, USA Year of study:July 1998 to not stated. |
|
| Interventions | Pharmaceutical care: patients received peak flow monitor + instructions for use, written educational materials, and monthly telephone calls from research personnel to collect Peak Expiratory Flow Rate (PEFR) results; pharmacist assessed PEFR results and other relevant medical information (medications, refill history, Emergency Department visits and hospitalisations) and implemented pharmaceutical care activities) vs Peak flow monitoring: patients received peak flow monitors and instructions for use and monthly telephone calls from research personnel to collect peak flow PEFR results (results were not seen by the pharmacist) vs Usual care: patients did not receive peak flow monitors but received monthly follow‐up phone calls from research personnel. Number of interventions: mean 19.4 in asthma, 22.4 in COPD patients over 12 months |
|
| Outcomes | PEFR (combined for asthma and COPD patients) at 12 months Health‐related quality of life (HRQOL) for asthma patients at 12 months HRQOL for COPD patients at 12 months | |
| Notes | Funding source: Department of Veterans Affairs Conflict of interest: Newell and Collins were employed by CVS throughout the project |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a random number chart" |
| Allocation concealment (selection bias) | Low risk | Not stated but unlikely due to nature of intervention |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Both baseline and follow‐up interviewers blind |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Both baseline and follow‐up interviewers blind for PEFR |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Between group attrition < 10%, however, high attrition overall |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Wu 2006.
| Methods | Randomised trial | |
| Participants | 442 participants (general medicine patients) (intervention 219: control 223) Specialist medical clinics of the Prince of Wales Hospital Hong Kong (catchment population of 1.2 million) Year of study: Not stated. |
|
| Interventions | Telephone intervention Intervention group patients received a 10‐ to 15‐minute telephone call from a pharmacist between clinic visits throughout the study period. The pharmacist asked about the patient’s treatment regimens; clarified any misconceptions; explained the nature of any side effects; reminded patients of their next clinic appointment; reinforced the importance of treatment compliance and discussed relevant aspects of self‐care, such as diet, exercise, and self‐monitoring. Due to frequent changes of attending doctors, information was not fed back to the clinic staff, although patients were encouraged to report all side effects, self‐initiated changes in regimen, or concerns to their doctors at their next visit. Control group patients received no interventions. Number of Interventions: 10 ‐ 15 minutes, every 2 to 4 months Duration: 2 years |
|
| Outcomes | Mortality | |
| Notes | Funding source: Hong Kong Government Health Care and Promotion Fund (HSRC/HCPF grant 226103) and MSD international grant. Conflict of interest: :JCNC and PCYT are investigators in clinical trials and research programmes sponsored by MSD. JCNC is also a member of the MSD Worldwide Diabetes Advisory Board. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | At the enrolment visit, eligible patients were reassessed for compliance. The pharmacist was blinded to the randomisation codes, which were computer‐generated by a statistician and sealed in envelopes labelled with consecutive numbers. The envelopes were opened by the clinic nurse in an ascending manner, and patients were allocated to the intervention or control group. |
| Allocation concealment (selection bias) | Low risk | At the enrolment visit, eligible patients were reassessed for compliance. The pharmacist was blinded to the randomisation codes, which were computer‐generated by a statistician and sealed in envelopes labelled with consecutive numbers. The envelopes were opened by the clinic nurse in an ascending manner, and patients were allocated to the intervention or control group. |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | Low risk | Quote: "blinding was not possible because the intervention was complex and caregivers were involved. Personnel were not blinded, but with this telephone intervention it is unlikely that knowledge of allocation undermined protocol delivery. |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Deaths: objective outcome |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | None |
Zermansky 2001.
| Methods | Randomised trial | |
| Participants | 1188 elderly patients (intervention 608; control 580) 4 general practices 1 pharmacist Leeds, United Kingdom Year of study: June 1999 to June 2000. |
|
| Interventions | Patients had 1 consultation with pharmacists to identify drugs, assess adherence, identify issues. Review active medical problems. Pharmacists could offer minor changes to treatment or could refer to general practitioner if recommendations were more major. | |
| Outcomes | Number of repeat prescriptions Hospital admissions at 12 months |
|
| Notes | Funding source: NHS Research and Development National Coordinating Centre for Health Technology Assessment. Conflict of interest: None declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Those who consented were randomised to an intervention group (clinical review by pharmacist) or control group (normal care) by computer‐generated random numbers." |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers |
| Blinding of participants and personnel (performance bias) All Outcomes/Outcome 1 | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) All Outcomes/Outcome 1 | Low risk | Changes to prescriptions seems unlikely to be biased. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Between group attrition < 10%. Overall completion rate > 80%. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | None identified |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bayraktar‐Ekincioglu 2013 | Insufficient information provided |
| Gangwar 2014 | Insufficient information provided |
| Varma 1999 | Included hospitalised and non‐hospitalised patients; data not presented separately |
Characteristics of studies awaiting assessment [ordered by study ID]
Aguiar 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Al Hamarneh 2018.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Al‐Tameemi 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Aljumah 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Almomani 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Anderegg 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Avery 2012.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Basger 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Basheti 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Batta 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Boudreau 2002.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Butt 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Cani 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Cantrill 2010.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Carter 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Choi 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Chow 2014.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Chow 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Chow 2015a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Clyne 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Cooney 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
De Azevedo 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Dischinger 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Elhatab 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Erku 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Erku 2017a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Garcia 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Geurts 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Goldfien 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Grainger‐Rousseau 1996.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Haag 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hedegaard 2014.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hedegaard 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hedegaard 2015a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Hedegaard 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Houle 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Iqbal 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Isetts 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
ISRCTN10671625 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Kandasamy 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Korcegez 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Lainscak 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Lalonde 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Lim 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Loganadan 2012.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Lowrie 2012.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Lyons 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Manfrin 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Mansell 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Margolis 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Marra 2011.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Marra 2011a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Martin 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Martin 2017a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Mateti 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
McNamara 2011.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Mendes 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Mikuls 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Nguyen 2011.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Obarcanin 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Obarcanin 2015a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ojieabu 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ojieabu 2017a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Okada 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Olivera 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Omran 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Periasamy 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pevnick 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Pistja 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Renuga 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Rubio‐Valera 2009.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Scala 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Schmiedel 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Schneiderhan 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Shao 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Siaw 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Smith 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Souter 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tahaineh 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tan 2011.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tierney 2005.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tsuyuki 2015a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tsuyuki 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tsuyuki 2016a.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tsuyuki 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Tuttle 2018.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Ummavathy 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Van Der Meer 2016.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Verret 2011.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Vinluan 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Wishah 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Wongpakaran 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Yang 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Yang 2017.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Zhao 2015.
| Methods | Not yet assessed |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
Da Silva 2012.
| Trial name or title | da Silva 2012 |
| Methods | Randomised trial. Impact of pharmaceutical care on the quality of life of patients with Chagas disease and heart failure |
| Participants | 88 adult patients with Chagas heart disease complicated by heart failure Conducted at the Evandro Chagas Clinical Research Institute (IPEC), Rio de Janeiro, Brazil |
| Interventions | Quote "All patients from both groups will take part in medical consultations every month. After each medical consultation, a pharmacist blinded to the patient’s assignment will interview all patients, to identify compliance to treatment and any drug‐related problems (DRPs). After this, all patients will interact with the clinical pharmacist. Those randomised to the control group will receive all prescription medications, while those patients randomised to the intervention group will not only receive all prescription medications but will also undergo pharmaceutical care, to solve DRPs, confirm, and reinforce their compliance to the medical prescription. Whenever the pharmacist identifies a DRP in the intervention group, s/he will interact with the physician, to solve the DRP. All patients will take part in a pharmaceutical consultation at the end of the follow‐up, to identify DRPs, complete quality‐of‐life questionnaires, and perform six‐minute‐walk tests." |
| Outcomes | Quality of Life ‐ evaluated using the 36‐item short‐form (SF‐36) and the Minnesota Living with HF Questionnaire (MLHFQ) |
| Starting date | December 2012 |
| Contact information | Evandro Chagas Clinical Research Institute, Oswaldo Cruz Foundation, Av. Brasil 4365, Rio de Janeiro, RJ 21040‐900, Brazil |
| Notes | Results not yet published |
Forster 2015.
| Trial name or title | Forster 2015 |
| Methods | Randomised trial. Effectiveness of a computerized drug‐monitoring programme to detect and prevent adverse drug events and medication non‐adherence in outpatient ambulatory care: study protocol of a randomized controlled trial |
| Participants | 2200 adult ambulatory patients in the province of Québec, Canada, who have been prescribed an incident medication for the management or prevention of a chronic health condition |
| Interventions | Quote "The use of the ISTOP‐ADE system, which consists of an interactive voice response system (IVRS) paired with pharmacist support. The IVRS will call patients at 3 and 17 days post‐prescription to determine if they are experiencing any problems and connect them with a pharmacist when required or desired by the patient." |
| Outcomes | Medication persistence at 180 days |
| Starting date | October 2015 |
| Contact information | Clinical Epidemiology Program, Ottawa Hospital Research Institute, 1053 Carling Avenue, Ottawa, ON K1Y 4E9, Canada |
| Notes | Results not yet published |
Kuhmmer 2015.
| Trial name or title | Kuhmmer 2015 |
| Methods | Randomised trial |
| Participants | Participants with hypertension and diabetes Recruited from a public emergency department, Southern Brazil |
| Interventions | Quote "Immediately post‐discharge, intervention group received a structured 30‐minute adherence‐focused intervention including: discussion on hypertension and/or diabetes, risk of complications, prescribed drug therapy, correct use of medications and proper dosage, possible adverse effects, route of administration, schedule of administration, correct storage and any necessary lifestyle modifications. Printed educational material, with information on hypertension and/ or diabetes medications, including suggested lifestyle interventions (for example, reduce salt and sugar intake, practice regular physical activity, smoking cessation, reducing alcohol consumption, monitor stress levels in day‐to‐day and reduce weight and keep it within the normal range) was handed to patients" |
| Outcomes | Not applicable |
| Starting date | Unknown |
| Contact information | |
| Notes | Results not yet published |
Porteous 2013.
| Trial name or title | Porteous 2013 |
| Methods | Randomised trial |
| Participants | Participants with allergic rhinitis Community pharmacies in NHS Grampian and NHS Greater Glasgow & Clyde, United Kingdom |
| Interventions | Community pharmacy‐delivered goal‐focused approach The intervention was developed to enhance replicability of the intervention by applying a reliable and valid taxonomy of behaviour change techniques (BCTs). The core BCTs identified in the intervention are captured by 4 of the taxonomy’s 16 clusters: Goals and planning (specific BCTs: goal‐setting (outcome); goal‐setting (behaviour); problem‐solving; action‐planning), Natural consequences (specific BCT: information about health consequences), Regulation (specific BCT: pharmacological support), and Feedback and Monitoring (specific BCTs: self‐monitoring of behaviour; self‐monitoring of outcome(s) of behaviour). The BCTs were operationalised in the Help for Hay Fever intervention. Community pharmacy staff were trained. 1 pharmacist and at least 1 pharmacy assistant from each of the 6 intervention pharmacies attended a 3‐hour training workshop. The workshop included training in self‐management theory, the use of goal‐setting as a behaviour change technique, participant recruitment (including taking consent) and a role‐play scenario. |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Results not yet published. Protocol paper only |
Differences between protocol and review
This review was originally part of a broader review evaluating the effectiveness of outpatient pharmacists' non‐dispensing roles on patient outcomes and prescribing patterns, first published under the title: Expanding the roles of outpatient pharmacists: effects on health services utilisation, costs, and patient outcomes in Issue 2, 2000 of the Cochrane Library (Bero 1995, Beney 2000, Nkansah 2010). As more data became available, the broader review was split, with this current version focusing solely upon the effect of pharmacists' non‐dispensing services on non‐hospitalised patient outcomes.
We tried to use a consistent strategy to deal with the large variety of outcomes reported in the studies. Where multiple outcomes were reported we created a hierarchy of outcomes, both within each outcome category and when choosing a representative outcome for the overall analysis. We applied the Cochrane 'Risk of bias' tool rather than the EPOC 'Risk of bias' tool. To comply with current Methodological Expectations of Cochrane Intervention Reviews (MECIR) standards, we introduced GRADE and added 'Summary of findings' tables for the main comparisons.
MdBa, CS, NWS, MdBr, CM, AJW, PR, MJ and MCW are all new authors with this review.
Contributions of authors
Study concept and design: All authors.
Development of search strategy: Cochrane EPOC.
Searching for studies: MdBa, CS.
Study selection: MdBa, CS, MCW.
Data extraction: MdBa, CS, AJW, PR
Data analysis: All authors.
Drafting the manuscript: All authors.
Critically revising manuscript for important intellectual content and providing final approval of the version to be published: All authors.
MCW is the guarantor for this review.
Sources of support
Internal sources
University of California, San Francisco, USA.
University of Aberdeen, UK.
University of Bath, UK.
External sources
This review update was funded by a grant from the Scottish Government, Chief Scientist Office., UK.
-
Health Foundation, UK, Other.
M Watson was funded by a Health Foundation Improvement Science Fellowship during the preparation of this review.
Declarations of interest
MdBa No known conflict of interest.
CS No known conflict of interest.
NWS No known conflict of interest.
MJ No known conflict of interest.
MdBr No known conflict of interest.
NN No known conflict of interest.
CM No known conflict of interest.
CB No known conflict of interest. Co‐author of Bond 2000; Bruhn 2013; not involved in the data extraction or 'Risk of bias' assessment of these trials.
AJW No known conflict of interest.
PR No known conflict of interest.
MCW No known conflict of interest. Co‐author of Bruhn 2013: not involved in the data extraction or 'Risk of bias' assessment of this trials.
Joint first author
Joint first author
Edited (no change to conclusions)
References
References to studies included in this review
Adibe 2013a {published data only}
- Adibe MO, Ukwe CV, Aguwa CN. The impact of pharmaceutical care intervention on the quality of life of Nigerian patients receiving treatment for Type 2 diabetes. Value in Health Regional Issues 2013;2(2):240‐7. [DOI] [PubMed] [Google Scholar]
Adler 2004 {published data only}
- Adler DA, Bungay KM, Wilson IB, Pei Y, Supran S, Peckham E, et al. The impact of a pharmacist intervention on 6‐month outcomes in depressed primary care patients. General Hospital Psychiatry 2004;26(3):199‐209. [DOI] [PubMed] [Google Scholar]
Albsoul‐Younes 2011 {published data only}
- Albsoul‐Younes AM, Hammad EA, Yasein NA, Tahaineh LM. Pharmacist‐physician collaboration improves blood pressure control. Saudi Medical Journal 2011;32(3):288‐92. [PubMed] [Google Scholar]
Ali 2012 {published data only}
- Ali M, Schifano F, Robinson P, Phillips G, Doherty L, Melnick P, et al. Impact of community pharmacy diabetes monitoring and education programme on diabetes management: a randomised controlled study. Diabetic Medicine 2012;29:e326–e333. [DOI] [PubMed] [Google Scholar]
Amariles 2012 {published data only}
- Amariles P, Sabater‐Hernández D, García‐Jiménez E, Rodríguez‐Chamorro MA, Prats‐Más R, Marín‐Magán F, et al. Effectiveness of Dader Method for pharmaceutical care on control of blood pressure and total cholesterol in outpatients with cardiovascular disease or cardiovascular risk: EMDADER‐CV randomized controlled trial. Journal of Managed Care Pharmacy 2012;18(4):311‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Andres 2007 {published data only}
- Andres Rodriguez NF, Fornos Perez J, Andres Iglesias JC. Assessment of knowledge/compliance in a drug therapy follow‐up program involving type 2 diabetic patients in community pharmacy: a randomized study [Valoracion del conocimiento/cumplimiento en un programa de seguimiento farmacoterapeutico en diabeticos tipo 2 en farmacia comunitaria: Estudio aleatorizado]. Pharmaceutical Care España 2007;9(1):2‐9. [Google Scholar]
Armour 2007 {published data only}
- Armour C, Bosnic‐Anticevich S, Brillant M, Burton D, Emmerton L, Krass I, et al. Pharmacy Asthma Care Program (PACP) improves outcomes for patients in the community. Thorax 2007;62(6):496‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbanel 2003 {published data only}
- Barbanel D, Eldridge S, Griffiths C. Can a self‐management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax 2003;58(10):851‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernsten 2001 {published data only}
- Bernsten C, Bjorkman I, Caramona M, Crealey G, Frokjaer B, Grundberger E, et al. Improving the well‐being of elderly patients via community pharmacy‐based provision of pharmaceutical care: a multicentre study in seven European countries. Drugs & Aging 2001;18(1):63‐77. [DOI] [PubMed] [Google Scholar]
Blalock 2010 {published data only}
- Blalock SJ, Casteel C, Roth MT, Ferreri S, Demby KB, Shankar V. Impact of enhanced pharmacologic care on the prevention of falls: a randomized controlled trial. American Journal of Geriatric Pharmacotherapy 2010;8(5):428‐40. [DOI] [PubMed] [Google Scholar]
Bogden 1998 {published data only}
- Bogden PE, Abbott RD, Williamson P, Onopa JK, Koontz LM. Comparing standard care with a physician and pharmacist team approach for uncontrolled hypertension. Journal of General Internal Medicine 1998;13(11):740‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bond 2000 {published data only}
- Bond C, Matheson C, Williams S, Williams P, Donnan P. Repeat prescribing: a role for community pharmacists in controlling and monitoring repeat prescriptions. British Journal of General Practice 2000;50(453):271‐5. [PMC free article] [PubMed] [Google Scholar]
Borenstein 2003a {published data only}
- Borenstein JE, Graber G, Saltiel E, Wallace J, Ryu S, Jackson A, et al. Physician‐pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 2003;23(2):209‐16. [DOI] [PubMed] [Google Scholar]
Bosnic‐Anticevich 2010 {published data only}
- Bosnic‐Anticevich SZ, Sinha H, So S, Reddel HK. Metered‐dose inhaler technique: the effect of two educational interventions delivered in community pharmacy over time. Journal of Asthma 2010;47(3):251‐6. [DOI] [PubMed] [Google Scholar]
Boyd 2013 {published data only}
- Boyd M, Waring J, Barber N, Mehta R, Chuter A, Avery AJ, et al. Protocol for the new medicine service study: a randomized controlled trial and economic evaluation with qualitative appraisal comparing the effectiveness and cost effectiveness of the new medicine service in community pharmacies in England. Trials 2013;14(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brook 2003 {published data only}
- Brook OH, Hout HP, Nieuwenhuysea H, Haan M. Effects of coaching by community pharmacists on psychological symptoms of antidepressant users: a randomised controlled trial. European Neuropsychopharmacology 2003;13(5):347‐54. [DOI] [PubMed] [Google Scholar]
Bruhn 2013 {published data only}
- Bruhn H, Bond CM, Elliott AM, Hannaford PC, Lee AJ, McNamee P, et al. Pharmacist‐led management of chronic pain in primary care: results from a randomised controlled exploratory trial.. BMJ Open 2013;3(4):e002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Capoccia 2004 {published data only}
- Boudreau DM, Capoccia KL, Sullivan SD, Blough DK, Ellsworth AJ, Clark DL, et al. Collaborative care model to improve outcomes in major depression. Annals of Pharmacotherapy 2002;36(4):585‐91. [DOI] [PubMed] [Google Scholar]
- Capoccia KL, Boudreau DM, Blough DK, Ellsworth AJ, Clark DR, Stevens NG, et al. Randomized trial of pharmacist interventions to improve depression care and outcomes in primary care. American Journal of Health‐System Pharmacy 2004;61(4):364‐72. [DOI] [PubMed] [Google Scholar]
Carter 2008 {published data only}
- Carter BL, Bergus GR, Dawson JD, Farris KB, Doucette WR, Chrischilles EA, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. Journal of Clinical Hypertension 2008;10(4):260‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castejon 2013 {published data only}
- Castejon AM, Calderon JL, Perez A, Millar C, McLaughlin‐Middlekauff J, Sangasubana N, et al. A community‐based pilot study of a diabetes pharmacist intervention in Latinos: Impact on weight and hemaglobin A1c. Journal of Health Care for the Poor and Underserved 2013;24(4):48‐60. [DOI] [PubMed] [Google Scholar]
Charrois 2006 {published data only}
- Charrois LT, Newman CS, Senthilselvan A, Tsuyuki TR. Improving asthma control in the rural setting: The BREATHE (Better Respiratory Education and Asthma Treatment in Hinton and Edson) study. Canadian Pharmacists Journal 2006;139(4):44‐50. [Google Scholar]
Chisholm 2002 {published data only}
- Chisholm MA, Mulloy LL, Jagadeesan M, Martin BC, DiPiro JT. Effect of clinical pharmacy services on the blood pressure of African‐American renal transplant patients. Ethnicity and Disease 2002;12(3):392‐7. [PubMed] [Google Scholar]
Choe 2005 {published data only}
- Choe HM, Mitrovich S, Dubay D, Hayward RA, Krein SL, Vijan S. Proactive case management of high‐risk patients with type 2 diabetes mellitus by a clinical pharmacist: a randomised controlled trial. American Journal of Managed Care 2005;11(4):253‐60. [PubMed] [Google Scholar]
Chrischilles 2014 {published data only}
- Chrischilles E, Doucette W, Farris K, Lindgren S, Gryzlak B, Rubenstein L, et al. Medication therapy management and complex patients with disability: a randomized controlled trial. Annals of Pharmacotherapy 2014;48(2):158‐67. [DOI] [PubMed] [Google Scholar]
Clifford 2005 {published data only}
- Clifford RM, Davis WA, Batty KT, Davis TM. Effect of a pharmaceutical care program on vascular risk factors in type 2 diabetes: The Fremantle Diabetes Study. Diabetes Care 2005;28(4):771‐6. [DOI] [PubMed] [Google Scholar]
Cody 1998 {published data only}
- Cody M, McCombs JS, Parker JP. The Kaiser Permanente/USC Patient Consultation Study: change in quality of life. University of Southern California. American Journal of Health‐System Pharmacy 1998;55(24):2615‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- McCombs JS, Liu G, Shi J, Feng W, Cody M, Parker JP, et al. The Kaiser Permanente/USC Patient Consultation Study: change in use and cost of health care services. American Journal of Health‐System Pharmacy 1998;55(23):2485‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cordina 2001 {published data only}
- Cordina M, McElnay JC, Hughes CM. Assessment of a community pharmacy‐based program for patients with asthma. Pharmacotherapy 2001;21(10):1196‐203. [DOI] [PubMed] [Google Scholar]
De Castro 2006 {published data only}
- Castro MS, Fuchs FD, Santos MC, Maximiliano P, Gus M, Moreira LB, et al. Pharmaceutical care program for patients with uncontrolled hypertension. Report of a double‐blind clinical trial with ambulatory blood pressure monitoring. American Journal of Hypertension 2006;19(5):528‐33. [DOI] [PubMed] [Google Scholar]
Di Donato 2014 {published data only}
- DiDonato KL, Vetter KR, Liu Y, May JR, Hartwig DM. Examining the effect of a medication synchronization or an education program on health outcomes of hypertensive patients in a community pharmacy setting. Innovations in Pharmacy 2014;5(3):15. [Google Scholar]
Doucette 2009 {published data only}
- Doucette WR, Witry MJ, Farris KB, McDonough RP. Community pharmacist‐provided extended diabetes care. Annals of Pharmacotherapy 2009;43(5):882‐9. [DOI] [PubMed] [Google Scholar]
Edwards 2014 {published data only}
- Edwards SJ, Abbott R, Edwards J, LeBlanc M, Dranitsaris G, Donnan J, et al. Outcomes assessment of a pharmacist‐directed seamless care program in an ambulatory oncology clinic. Journal of Pharmacy Practice 2014;27(1):46‐52. [DOI] [PubMed] [Google Scholar]
Farsaei 2011 {published data only}
- Farsaei S, Sabzghabaee AM, Zargarzadeh AH, Amini M. Effect of pharmacist‐led patient education on glycemic control of type 2 diabetics: a randomized controlled trial. Journal of Research in Medical Sciences 2010;15(6):317‐23. [PMC free article] [PubMed] [Google Scholar]
Faulkner 2000 {published data only}
- Faulkner MA, Wadibia EC, Lucas BD, Hilleman DE. Impact of pharmacy counseling on compliance and effectiveness of combination lipid‐lowering therapy in patients undergoing coronary artery revascularization: a randomized, controlled trial. Pharmacotherapy 2000;20(4):410‐6. [DOI] [PubMed] [Google Scholar]
Finley 2003 {published data only}
- Finley PR, Rens HR, Pont JT, Gess SL, Louie C, Bull SA, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy 2003;23(9):1175‐85. [DOI] [PubMed] [Google Scholar]
Garção 2002 {published data only}
- Garção JA, Cabrita J. Evaluation of a pharmaceutical care program for hypertensive patients in rural Portugal. Journal of the American Pharmaceutical Association (Washington, D.C., 1996) 2002;42(6):858‐64. [DOI] [PubMed] [Google Scholar]
García‐Cárdenas 2013 {published data only}
- García‐Cárdenas V, Sabater‐Hernández D, Kenny P, Martínez‐Martínez F, Faus MJ, Benrimoj SI. Effect of a pharmacist intervention on asthma control: a cluster randomised trial. Respiratory Medicine 2013;107(9):1346‐55. [DOI] [PubMed] [Google Scholar]
Gattis 1999a {published data only}
- Gattis WA, Hasselblad V, Whellan DJ, O'Connor CM. Reduction in heart failure events by the addition of a clinical pharmacist to the heart failure management team: results of the Pharmacist in Heart Failure Assessment Recommendation and Monitoring (PHARM) Study. Archives of Internal Medicine 1999;159(16):1939‐45. [DOI] [PubMed] [Google Scholar]
González‐Martin 2003 {published data only}
- González‐Martin G, Joo I, Sánchez I. Evaluation of the impact of a pharmaceutical care program in children with asthma. Patient Education and Counselling 2003;49(1):13‐8. [DOI] [PubMed] [Google Scholar]
Goodyer 1995 {published data only}
- Goodyer LI, Miskelly F, Milligan P. Does encouraging good compliance improve patients' clinical condition in heart failure?. British Journal of Clinical Practice 1995;49(4):173‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Green 2008 {published data only}
- Green BB, Anderson ML, Ralston JD, Catz SL, Cook AJ. Blood pressure 1 year after completion of web‐based pharmacist care. JAMA Internal Medicine 2013;173(13):1250‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, et al. Effectiveness of home blood pressure monitoring, web communication, and pharmacist care on hypertension control: The e‐BP randomized controlled trial. JAMA 2008;299(24):2857–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hammad 2011 {published data only}
- Hammad EA, Yasein N, Tahaineh L, Albsoul‐Younes AM. A randomized controlled trial to assess pharmacist‐physician collaborative practice in the management of metabolic syndrome in a university medical clinic in Jordan. Journal of Managed Care Pharmacy 2011;17(4):295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawes 2013 {published data only}
- Hawes EM, Maxwell WD, White SF, Mangun J, Lin FC. Impact of an outpatient pharmacist intervention on medication discrepancies and health care resource utilization in posthospitalization care transitions. Journal of Primary Care & Community Health 2014;5(1):14‐8. [DOI] [PubMed] [Google Scholar]
Hawkins 1979 {published data only}
- Hawkins DW, Fiedler FP, Douglas HL, Eschbach RC. Evaluation of a clinical pharmacist in caring for hypertensive and diabetic patients. American Journal of Hospital Pharmacy 1979;36(10):1321‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Hay 2006 {published data only}
- Hay EM, Foster NE, Thomas E, Peat G, Phelan M, Yates HE, et al. Effectiveness of community physiotherapy and enhanced pharmacy review for knee pain in people aged over 55 presenting to primary care: pragmatic randomised trial. BMJ (Clinical research ed.) 2006;333(7576):995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hendrie 2014 {published data only}
- Hendrie D, Miller TR, Woodman RJ, Hoti K, Hughes J. Cost‐effectiveness of reducing glycaemic episodes through community pharmacy management of patients with type 2 diabetes mellitus. Journal of Primary Prevention 2014;35(6):439‐49. [DOI] [PubMed] [Google Scholar]
Hirsch 2014 {published data only}
- Hirsch JD, Steers N, Adler DS, Kuo GM, Morello CM, Lang M, et al. Primary care‐based, pharmacist‐physician collaborative medication‐therapy management of hypertension: a randomized, pragmatic trial. Clinical Therapeutics 2014;36(9):1244‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2013 {published data only}
- Ho PM, Lambert‐Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, et al. Multifaceted Intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Internal Medicine 2013;174(2):186‐93. [DOI] [PubMed] [Google Scholar]
Holland 2005 {published data only}
- Holland R, Lenaghan E, Harvey I, Smith R, Shepstone L, Lipp A, et al. Does home based medication review keep older people out of hospital? The HOMER randomised controlled trial. BMJ (Clinical research ed.) 2005;330(7486):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 2008 {published data only}
- Hunt JS, Siemienczuk J, Pape G, Rozenfeld Y, MacKay J, LeBlanc BH, et al. A randomized controlled trial of team‐based care: impact of physician‐pharmacist collaboration on uncontrolled hypertension. Journal of General Internal Medicine 2008;23(12):1966‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaber 1996 {published data only}
- Jaber LA, Halapy H, Fernet M, Tummalapalli S, Diwakaran H. Evaluation of a pharmaceutical care model on diabetes management. Annals of Pharmacotherapy 1996;30(3):238‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jackson 2004 {published data only}
- Jackson SL, Peterson GM, Vial JH, Jupe DM. Improving the outcomes of anticoagulation: an evaluation of home follow‐up of warfarin initiation. Journal of Internal Medicine 2004;256(2):137‐44. [DOI] [PubMed] [Google Scholar]
Jahangard‐Rafsanjani 2014 {published data only}
- Jahangard‐Rafsanjani Z, Sarayani A, Nosrati M, Saadat N, Rashidian A, Hadjibabaie M, et al. Effect of a community pharmacist‐delivered diabetes support program for patients receiving specialty medical care: a randomized controlled trial. Diabetes Educator 2015;41(1):127‐35. [DOI] [PubMed] [Google Scholar]
Jarab 2012 {published data only}
- Jarab AS, AlQudah SG, Khdour M, Shamssain M, Mukattash TL. Impact of pharmaceutical care on health outcomes in patients with COPD. International Journal of Clinical Pharmacy 2012;34(1):53‐62. [DOI] [PubMed] [Google Scholar]
Khdour 2009 {published data only}
- Khdour MR, Kidney JC, Smyth BM, McElnay JC. Clinical pharmacy‐led disease and medicine management programme for patients with COPD. British Journal of Clinical Pharmacology 2009;68(4):588–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krass 2007 {published data only}
- Krass I, Armour CL, Mitchell B, Brillant M, Dienaar R, Hughes J, et al. The Pharmacy Diabetes Care Program: assessment of a community pharmacy diabetes service model in Australia. Diabetic Medicine 2007;24(6):677‐83. [DOI] [PubMed] [Google Scholar]
Kritikos 2007 {published data only}
- Kritikos V, Armour CL, Bosnic‐Anticevich SZ. Interactive small‐group asthma education in the community pharmacy setting: a pilot study. Journal of Asthma 2007;44(1):57‐64. [DOI] [PubMed] [Google Scholar]
Krska 2001 {published data only}
- Krska J, Cromarty JA, Arris F, Jamieson D, Hansford D, Duffus PR, et al. Pharmacist‐led medication review in patients over 65: a randomized, controlled trial in primary care. Age and Ageing 2001;30(3):205‐11. [DOI] [PubMed] [Google Scholar]
Lai 2013 {published data only}
- Lai PS, Chua SS, Chan SP. Impact of pharmaceutical care on knowledge, quality of life and satisfaction of postmenopausal women with osteoporosis. International Journal of Clinical Pharmacy 2013;35(4):629‐37. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee JK, Grace KA, Taylor AJ. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low‐density lipoprotein cholesterol: a randomized controlled trial. JAMA 2006;296(21):2563‐71. [DOI] [PubMed] [Google Scholar]
Lenaghan 2007 {published data only}
- Lenaghan E, Holland R, Brooks A. Home‐based medication review in a high risk elderly population in primary care‐‐the POLYMED randomised controlled trial. Age and Ageing 2007;36(3):292‐7. [DOI] [PubMed] [Google Scholar]
Lenander 2014 {published data only}
- Lenander C, Elfsson B, Danielsson B, Midlov P, Hasselstrom J. Effects of a pharmacist‐led structured medication review in primary care on drug‐related problems and hospital admission rates: a randomized controlled trial. Scandinavian Journal of Primary Health Care 2014;32(4):180‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2014 {published data only}
- Li W, Xinyun Y, Jie L, Lianghui L, Hongying L, Zeguang Z. Effect of pharmaceutical care on medication adherence and hospital admission in patients with chronic obstructive pulmonary disease (COPD): a randomized controlled study. Journal of Thoracic Disease 2014;6(6):656‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2006 {published data only}
- Lopez CC, Falces SC, Cubi QD, Arnau BA, Ylla BM, Muro PN, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow‐up in patients with heart failure. Farmacia Hospitalaria 2006;30:328‐42. [DOI] [PubMed] [Google Scholar]
Losada‐Camacho 2014 {published data only}
- Losada‐Camacho M, Guerrero‐Pabon MF, Garcia‐Delgado P, Martínez‐Martínez F. Impact of a pharmaceutical care programme on health‐related quality of life among women with epilepsy: a randomised controlled trial (IPHIWWE study). Health and Quality of Life Outcomes 2014;12(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magid 2013 {published data only}
- Magid DJ, Olson KL, Billups SJ, Wagner NM, Lyons EE, Kroner BA. A pharmacist‐led, American Heart Association Heart360 Web‐enabled home blood pressure monitoring program. Circulation. Cardiovascular Quality and Outcomes 2013;6(2):157‐63. [DOI] [PubMed] [Google Scholar]
Mahwi 2013 {published data only}
- Mahwi TO, Obied KA. Role of the pharmaceutical care in the management of patients with type 2 diabetes mellitus. International Journal of Pharmaceutical Sciences and Research 2013;4(4):1363‐9. [Google Scholar]
Malone 2001 {published data only}
- Ellis SL, Carter BL, Malone DC, Billups SJ, Okano GJ, Valuck RJ, et al. Clinical and economic impact of ambulatory care clinical pharmacists in management of dyslipidemia in older adults: The IMPROVE study. Impact of Managed Pharmaceutical Care on Resource Utilization and Outcomes in Veterans Affairs Medical Centers. Pharmacotherapy 2000;20(12):1508‐16. [DOI] [PubMed] [Google Scholar]
- Malone DC, Carter BL, Billups SJ, Valuck RJ, Barnette DJ, Sintek CD, et al. Can clinical pharmacists affect SF‐36 scores in veterans at high risk for medication‐related problems?. Medical Care 2001;39(2):113‐22. [DOI] [PubMed] [Google Scholar]
Margolis 2013 {published data only}
- Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, et al. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA 2013;310(1):46‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marques 2013 {published data only}
- Marques LA, Galduroz JC, Fernandes MR, Oliveira CC, Beijo LA, Noto AR. Assessment of the effectiveness of pharmacotherapy follow‐up in patients treated for depression. Journal of Managed Care Pharmacy 2013;19(3):218‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marra 2012 {published data only}
- Marra CA, Cibere J, Grubisic M, Grindrod KA, Gastonguay L, Thomas JM, et al. Pharmacist‐initiated intervention trial in osteoarthritis: a multidisciplinary intervention for knee osteoarthritis. Arthritis Care & Research 2012;64(12):1837–45. [DOI] [PubMed] [Google Scholar]
- Marra CA, Tsuyuki RT, Soon JA, Gastonguay L, Oteng B, Cibere J, et al. Design of a randomized trial of a multidisciplinary intervention for knee osteoarthritis: Pharmacist initiated Intervention Trial in Osteoarthritis (PhIT‐OA). Canadian Pharmacists' Journal 2008;141(1):33‐8. [Google Scholar]
Mazroui 2009 {published data only}
- Al Mazroui NR, Kamal MM, Ghabash NM, Yacout TA, Kole PL, McElnay JC. Influence of pharmaceutical care on health outcomes in patients with Type 2 diabetes mellitus. British Journal of Clinical Pharmacology 2009;67(5):547‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
McAlister 2014 {published data only}
- McAlister FA, Majumdar SR, Padwal RS, Fradette M, Thompson A, Buck B, et al. Case management for blood pressure and lipid level control after minor stroke: PREVENTION randomized controlled trial. Canadian Medical Association Journal 2014;186(8):577‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehos 2000 {published data only}
- Mehos BM, Saseen JJ, MacLaughlin EJ. Effect of pharmacist intervention and initiation of home blood pressure monitoring in patients with uncontrolled hypertension. Pharmacotherapy 2000;20(11):1384‐9. [DOI] [PubMed] [Google Scholar]
Mehuys 2008 {published data only}
- Mehuys E, Bortel L, Bolle L, Tongelen I, Annemans L, Remon JP, et al. Effectiveness of a pharmacist intervention for asthma control improvement: randomised controlled trial. European Respiratory Journal 2008;31(4):790‐9. [DOI] [PubMed] [Google Scholar]
Milos 2013 {published data only}
- Milos V, Rekman E, Bondesson Å, Eriksson T, Jakobsson U, Westerlund T, et al. Improving the quality of pharmacotherapy in elderly primary care patients through medication reviews: a randomised controlled study. Drugs & Aging 2013;30(4):235‐46. [DOI] [PubMed] [Google Scholar]
Murray 2007 {published data only}
- Murray DM, Young J, Hoke S, Tu W, Weiner M, Morrow D, et al. Pharmacist intervention to improve medication adherence in heart failure. Annals of Internal Medicine 2007;146(10):714‐25. [DOI] [PubMed] [Google Scholar]
Naunton 2003 {published data only}
- Naunton M, Peterson GM. Evaluation of home‐based follow‐up of high‐risk elderly patients discharged from hospital. Journal of Pharmacy Practice and Research 2003;33(3):176‐82. [Google Scholar]
Obreli‐Neto 2015 {published data only}
- Obreli‐Neto PR, Marusic S, Guidoni CM, Baldoni AO, Renovato RD, Pilger D, et al. Economic evaluation of a pharmaceutical care program for elderly diabetic and hypertensive patients in primary health care: a 36‐month randomized controlled clinical trial. Journal of Managed Care & Specialty Pharmacy 2015;21(1):66‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Okamoto 2001 {published data only}
- Okamoto MP, Nakahiro RK. Pharmacoeconomic evaluation of a pharmacist‐managed hypertension clinic. Pharmacotherapy 2001;21(11):1337‐44. [DOI] [PubMed] [Google Scholar]
Olesen 2014 {published data only}
- Olesen C, Harbig P, Buus KM, Barat I, Damsgaard EM. Impact of pharmaceutical care on adherence, hospitalisations and mortality in elderly patients. International Journal of Clinical Pharmacy 2014;36(1):163‐71. [DOI] [PubMed] [Google Scholar]
Park 1996 {published data only}
- Park JJ, Kelly P, Carter BL, Burgess PP. Comprehensive pharmaceutical care in the chain setting. Journal of the American Pharmaceutical Association 1996;NS36(7):443‐51. [DOI] [PubMed] [Google Scholar]
Paulos 2005 {published data only}
- Paulos CP, Nygren CE, Celedon C, Carcamo CA. Impact of a pharmaceutical care program in a community pharmacy on patients with dyslipidemia. Annals of Pharmacotherapy 2005;39(5):939‐43. [DOI] [PubMed] [Google Scholar]
Peterson 2004 {published data only}
- Peterson GM, Fitzmaurice KD, Naunton M, Vial JH, Stewart K, Krum H. Impact of pharmacist‐conducted home visits on the outcomes of lipid‐lowering drug therapy. Journal of Clinical Pharmacy and Therapeutics 2004;29(1):23‐30. [DOI] [PubMed] [Google Scholar]
Reid 2005 {published data only}
- Reid F, Murray P, Storrie M. Implementation of a pharmacist‐led clinic for hypertensive patients in primary care‐a pilot study. Pharmacy World and Science 2005;27(3):202‐7. [DOI] [PubMed] [Google Scholar]
Rickles 2005 {published data only}
- Rickles NM, Svarstad BL, Statz‐Paynter JL, Taylor LV, Kobak KA. Pharmacist telemonitoring of antidepressant use: effects on pharmacist‐patient collaboration. Journal of the American Pharmacists' Association 2005;45(3):344‐53. [DOI] [PubMed] [Google Scholar]
Rothman 2005 {published data only}
- Rothman RL, Malone R, Bryant B, Shintani AK, Crigler B, Dewalt DA, et al. A randomized trial of a primary care‐based disease management program to improve cardiovascular risk factors and glycated hemoglobin levels in patients with diabetes. American Journal of Medicine 2005;118(3):276‐84. [DOI] [PubMed] [Google Scholar]
Rubio‐Valera 2012 {published data only}
- Rubio‐Valera M, March PM, Fernández A, Peñarrubia‐María MT, Travé P, López Del Hoyo Y, et al. Evaluation of a pharmacist intervention on patients initiating pharmacological treatment for depression: a randomized controlled superiority trial. European Neuropsychopharmacology 2013;23(9):1057‐66. [DOI] [PubMed] [Google Scholar]
Sadik 2005 {published data only}
- Sadik A, Yousif M, McElnay JC. Pharmaceutical care of patients with heart failure. British Journal of Clinical Pharmacology 2005;60(2):183‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salazar‐Ospina 2017 {published data only}
- Salazar‐Ospina A, Amariles P, Hincapié‐García JA, González‐Avendaño S, Benjumea DM, Faus MJ. Effectiveness of the Dader method for pharmaceutical care on patients with bipolar I disorder: results from the EMDADER‐TAB Study. Journal of Managed Care & Specialty Pharmacy 2017;23(1):74‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Samtia 2013 {published data only}
- Samtia AM, Rasool MF, Ranjha NM, Usman F, Javed I. A multifactorial intervention to enhance adherence to medications and disease‐related knowledge in type 2 diabetic patients in southern Punjab, Pakistan. Tropical Journal of Pharmaceutical Research 2013;12(5):851‐6. [Google Scholar]
Sarkadi 2004 {published data only}
- Sarkadi A, Rosenqvist U. Experience‐based group education in Type 2 diabetes: a randomised controlled trial. Patient Education and Counseling 2004;53(3):291‐8. [DOI] [PubMed] [Google Scholar]
Schneider 1982 {published data only}
- Schneider PJ, Larrimer JN, Visconti JA, Miller WA. Role effectiveness of a pharmacist in the maintenance of patients with hypertension and congestive heart failure. Contemporary Pharmacy Practice 1982;5(2):74‐9. [PubMed] [Google Scholar]
Schneiderhan 2014 {published data only}
- Schneiderhan ME, Shuster SM, Davey CS. Twelve‐month prospective randomized study of pharmacists utilizing point‐of‐care testing for metabolic syndrome and related conditions in subjects prescribed antipsychotics. Primary Care Companion to CNS Disorders 2014;16(5):1669. [DOI: 10.4088/PCC.14m01669] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sellors 2003 {published data only}
- Sellors J, Kaczorowski J, Sellors C, Dolovich L, Woodward C, Willan A, et al. A randomized controlled trial of a pharmacist consultation program for family physicians and their elderly patients. Canadian Medical Association Journal 2003;169(1):17‐22. [PMC free article] [PubMed] [Google Scholar]
Sidel 1990 {published data only}
- Sidel VW, Beizer JL, Lisi‐Fazio D, Kleinmann K, Wenston J, Thomas C, et al. Controlled study of the impact of educational home visits by pharmacists to high‐risk older patients. Journal of Community Health 1990;15(3):163‐74. [DOI] [PubMed] [Google Scholar]
Silveira 2014 {published data only}
- Silveira MP, Guttier MC, Page K, Moreira LB. Randomized controlled trial to evaluate the impact of pharmaceutical care on therapeutic success in HIV‐infected patients in southern Brazil. AIDS & Behavior 2014;18(1):75‐84. [DOI] [PubMed] [Google Scholar]
Simpson 2011 {published data only}
- Simpson SH, Majumdar SR, Tsuyuki RT, Lewanczuk RZ, Spooner R, Johnson JA. Effect of adding pharmacists to primary care teams on blood pressure control in patients with type 2 diabetes. Diabetes Care 2011;34(1):20‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solomon 1998 {published data only}
- Solomon DK, Portner TS, Bass GE, Gourley DR, Gourley GA, Holt JM, et al. Clinical and economic outcomes in the hypertension and COPD arms of a multicenter outcomes study. Journal of the American Pharmacists' Association 1998;38(5):574‐85. [DOI] [PubMed] [Google Scholar]
Sookaneknun 2004 {published data only}
- Sookaneknun P, Richards RM, Sanguansermsri J, Teerasut C. Pharmacist involvement in primary care improves hypertensive patient clinical outcomes. Annals of Pharmacotherapy 2004;38(12):2023‐8. [DOI] [PubMed] [Google Scholar]
Stewart 2014 {published data only}
- Stewart K, George J, Namara KP, Jackson SL, Peterson GM, Bereznicki LR, et al. A multifaceted pharmacist intervention to improve antihypertensive adherence: A cluster‐randomized, controlled trial (HAPPy trial). Journal of Clinical Pharmacy and Therapeutics 2014;39(5):527‐34. [DOI] [PubMed] [Google Scholar]
Suppapitiporn 2005 {published data only}
- Suppapitiporn S, Chindavijak B, Onsanit S. Effect of diabetes drug counseling by pharmacist, diabetic disease booklet and special medication containers on glycemic control of type 2 diabetes mellitus: a randomized controlled trial. Journal of the Medical Association of Thailand = Chotmaihet thangphaet 2005;88 Suppl 4:S134‐41. [PubMed] [Google Scholar]
Tang 2014 {published data only}
- Tang F, Zhu G, Jiao Z, Ma C, Chen N, Wang B. The effects of medication education and behavioral intervention on Chinese patients with epilepsy. Epilepsy and Behavior 2014;37:157‐164. [DOI] [PubMed] [Google Scholar]
Tannenbaum 2014 {published data only}
- Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: The EMPOWER cluster randomized trial. JAMA Internal Medicine 2014;174(6):890‐8. [DOI] [PubMed] [Google Scholar]
Taveira 2011 {published data only}
- Taveira TH, Dooley AG, Cohen LB, Khatana SA, Wu WC. Pharmacist‐led group medical appointments for the management of type 2 diabetes with comorbid depression in older adults. Annals of Pharmacotherapy 2011;45(11):1346‐55. [DOI] [PubMed] [Google Scholar]
Taveira 2014 {published data only}
- Taveira TH, Wu WC. Interventions to maintain cardiac risk control after discharge from a cardiovascular risk reduction clinic: a randomized controlled trial. Diabetes Research & Clinical Practice 2014;105(3):327‐35. [DOI] [PubMed] [Google Scholar]
Taylor 2003 {published data only}
- Taylor CT, Byrd DC, Krueger K. Improving primary care in rural Alabama with a pharmacy initiative. American Journal of Health‐System Pharmacy 2003;60(11):1123‐9. [DOI] [PubMed] [Google Scholar]
Tommelein 2013 {published data only}
- Tommelein E, Mehuys E, Hees T, Adriaens E, Bortel L, Christiaens T, et al. Effectiveness of pharmaceutical care for patients with chronic obstructive pulmonary disease (PHARMACOP): A randomized controlled trial. British Journal of Clinical Pharmacology 2014;77(5):756‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuyuki 2002 {published data only}
- Tsuyuki RT, Johnson JA, Teo KK, Simpson SH, Ackman ML, Biggs RS, et al. A randomized trial of the effect of community pharmacist intervention on cholesterol risk management: the Study of Cardiovascular Risk Intervention by Pharmacists (SCRIP). Archives of Internal Medicine 2002;162(10):1149‐55. [DOI] [PubMed] [Google Scholar]
Tsuyuki 2015 {published data only}
- Tsuyuki RT, Houle SK, Charrois TL, Kolber MR, Rosenthal MM, Lewanczuk R, et al. for the RxACTION Investigators. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: The Alberta Clinical Trial in Optimizing Hypertension (RxACTION). Circulation 2015;132(2):93‐100. [DOI] [PubMed] [Google Scholar]
Verret 2012 {published data only}
- Verret L, Justine C, Rozon A, Saudrais‐Janecek S, St‐Onge A, Nguyen A, et al. Impact of a pharmacist‐led warfarin self‐management program on quality of life and anticoagulation control: a randomized trial. Pharmacotherapy 2012;32(10):871‐9. [DOI] [PubMed] [Google Scholar]
Vivian 2002 {published data only}
- Vivian EM. Improving blood pressure control in a pharmacist‐managed hypertension clinic. Pharmacotherapy 2002;22(12):1533‐40. [DOI] [PubMed] [Google Scholar]
Volume 2001 {published data only}
- Volume CI, Farris KB, Kassam R, Cox CE, Cave A. Pharmaceutical care research and education project: patient outcomes. Journal of the American Pharmacists' Association 2001;41(3):411‐20. [DOI] [PubMed] [Google Scholar]
Wal 2013 {published data only}
- Wal P, Wal A, Bhandari A, Pandey U, Rai AK. Pharmacist involvement in the patient care improves outcome in hypertension patients. Journal of Research in Pharmacy Practice 2013;2(3):123‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberger 2002 {published data only}
- Weinberger M, Murray MD, Marrero DG, Brewer N, Lykens M, Harris LE, et al. Effectiveness of pharmacist care for patients with reactive airways disease: A randomized controlled trial. JAMA 2002;288(13):1594‐602. [DOI] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu JY, Leung WY, Chang S, Lee B, Zee B, Tong PC, et al. Effectiveness of telephone counselling by a pharmacist in reducing mortality in patients receiving polypharmacy: randomised controlled trial. BMJ 2006;333(7567):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zermansky 2001 {published data only}
- Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ (Clinical research ed.) 2001;323(7325):1340‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bayraktar‐Ekincioglu 2013 {published data only}
- Bayraktar‐Ekincioglu A, Hudson S. Design and evaluation of monitoring programme for methotrexate users in the arthritic population ‐ a study at the primary and the secondary care interface. Turkish Journal of Pharmaceutical Sciences 2013;10(1):125‐36. [Google Scholar]
Gangwar 2014 {published data only}
- Gangwar SS, Monisha N, Nachiya J, Narasingarao K, Parimalakrishnan S, Singh SP. Impact of medication and psychological behaviour assessment by community pharmacists in type 2 diabetes mellitus patients after hospital stay. African Health Sciences 2014;14(3):539‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Varma 1999 {published data only}
- Varma S, McElnay JC, Hughes CM, Passmore AP, Varma M. Pharmaceutical care of patients with congestive heart failure: interventions and outcomes. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy 1999;19(7):860‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Aguiar 2016 {published data only}
- Aguiar PM, Silva CH, Chiann C, Dórea EL, Lyra DP Jr, Stopitis S. Pharmacist‐physician collaborative care model for patients with uncontrolled type 2 diabetes in Brazil: results from a randomized controlled trial. Journal of Evaluation in Clinical Practice 2016;24(1):22‐30. [DOI] [PubMed] [Google Scholar]
Al Hamarneh 2018 {published data only}
- Al Hamarneh YN, Tsuyuki RT, Jones CA, Manns B, Tonelli M, Scott‐Douglass N, et al. Effectiveness of pharmacist interventions on cardiovascular risk in patients with CKD: a subgroup analysis of the randomized controlled REACH Trial. American Journal of Kidney Diseases 2018;71(1):42‐51. [DOI] [PubMed] [Google Scholar]
Aljumah 2016 {published data only}
- Aljumah K. Impact of pharmacist intervention using shared decision making on adherence and measurable depressed patient outcomes. Value in Health 2016;19:A19. [Google Scholar]
Almomani 2017 {published data only}
- Almomani BA, Mayyas RK, Ekteish FA, Ayoub AM, Ababneh MA, Alzoubi SA. The effectiveness of clinical pharmacist's intervention in improving asthma care in children and adolescents: randomized controlled study in Jordan. Patient Education & Counseling 2017;100(4):728‐35. [DOI] [PubMed] [Google Scholar]
Al‐Tameemi 2017 {published data only}
- Al‐Tameemi D, Al‐Tukmagi H. The application of pharmaceutical care program on patients with dyslipidemia in Iraqi community pharmacy. International Journal of Pharmaceutical Sciences Review and Research 2017;47(1):45‐51. [Google Scholar]
Anderegg 2016 {published data only}
- Anderegg MD, Gums TH, Uribe L, Coffey CS, James PA, Carter BL. Physician‐pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension 2016;68(5):1314‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Avery 2012 {published data only}
- Avery AJ, Rodgers S, Cantrill JA, Armstrong S, Cresswell K, Eden M, et al. A pharmacist‐led information technology intervention for medication errors (PINCER): a multicentre, cluster randomised, controlled trial and cost‐effectiveness analysis. Lancet 2012;379(9823):1310‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basger 2015 {published data only}
- Basger BJ, Moles RJ, Chen TF. Impact of an enhanced pharmacy discharge service on prescribing appropriateness criteria: a randomised controlled trial. International Journal of Clinical Pharmacy 2015;37(6):1194‐205. [DOI] [PubMed] [Google Scholar]
Basheti 2016 {published data only}
- Basheti IA, Tadros OK, Aburuz S. Value of a community‐based medication management review service in Jordan: a prospective randomized controlled study. Pharmacotherapy 2016;36(10):1075‐86. [DOI] [PubMed] [Google Scholar]
Batta 2017 {published data only}
- Batta RA, Kasabri V, Akour A, Hyassat D, Albsoul‐Younes A. Impact of clinical pharmacists intervention on management of hyperglycemia in pregnancy in Jordan. International Journal of Clinical Pharmacy 2017;40(1):48‐55. [DOI] [PubMed] [Google Scholar]
Boudreau 2002 {published data only}
- Boudreau DM, Capoccia KL, Sullivan SD, Blough DK, Ellsworth AJ, Clark DL, et al. Collaborative care model to improve outcomes in major depression. Annals of Pharmacotherapy 2002;36(4):585‐91. [DOI] [PubMed] [Google Scholar]
Butt 2016 {published data only}
- Butt M, Mhd Ali A, Bakry MM, Mustafa N. Impact of a pharmacist led diabetes mellitus intervention on HbA1c, medication adherence and quality of life: A randomised controlled study. Saudi Pharmaceutical Journal 2016;24(1):40‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cani 2015 {published data only}
- Cani CG, Lopes Lda S, Queiroz M, Nery M. Improvement in medication adherence and self‐management of diabetes with a clinical pharmacy program: a randomized controlled trial in patients with type 2 diabetes undergoing insulin therapy at a teaching hospital. Clinics (Sao Paulo, Brazil) 2015;70(2):102‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantrill 2010 {published data only}
- Cantrill J. The PINCER trial: A cluster randomised trial comparing the effectiveness of a pharmacist‐led IT‐based intervention with simple feedback in reducing rates of clinically important errors in medicines management in general practices. International Journal of Pharmacy Practice 2010;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carter 2015 {published data only}
- Carter BL, Coffey CS, Ardery G, Uribe L, Ecklund D, James P, et al. Cluster‐randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circulation. Cardiovascular Quality & Outcomes 2015;8(3):235‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2017 {published data only}
- Choi B, Lee S, Jung J, Suh D. Impact of patient education on medication on health outcomes and adherence in patients with asthma. Allergy: European Journal of Allergy and Clinical Immunology. Conference: 36th annual congress of the European Academy of Allergy and Clinical Immunology, EAACI 2017. Finland 2017;72:380‐1. [Google Scholar]
Chow 2014 {published data only}
- Chow EP, Hassali A. Medication counseling beyond institutional: Impact of pharmacist‐led home medication review in type 2 diabetes patients. Value in Health 2014;17(7):A746. [DOI] [PubMed] [Google Scholar]
Chow 2015 {published data only}
- Chow EP, Hassali MA, Saleem F, Aljadhey H. Effects of pharmacist‐led patient education on diabetes‐related knowledge and medication adherence: A home‐based study. Health Education Journal 2015;75(4):421‐33. [Google Scholar]
Chow 2015a {published data only}
- Chow EP, Hassali MA, Saleem F, Kumar R. Assessment of pharmacist‐led home‐based educational intervention among type 2 diabetes patients in the state of Penang, Malaysia. Value in Health 2015;18(7):A866. [Google Scholar]
Clyne 2015 {published data only}
- Clyne B, Smith SM, Hughes CM, Boland F, Bradley MC, Cooper JA, et al. Effectiveness of a multifaceted intervention for potentially inappropriate prescribing in older patients in primary care: A cluster‐randomized controlled trial (OPTI‐SCRIPT Study). Annals of Family Medicine 2015;13(6):545‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooney 2015 {published data only}
- Cooney D, Moon H, Liu Y, Miller RT, Perzynski A, Watts B, et al. A pharmacist based intervention to improve the care of patients with CKD: a pragmatic, randomized, controlled trial. BMC Nephrology 2015;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Azevedo 2017 {published data only}
- Azevedo MG, Pedrosa RS, Aoqui CM, Martins RR, Nagashima Junior T. Effectiveness of home pharmaceutical interventions in metabolic syndrome: A randomized controlled trial. Brazilian Journal of Pharmaceutical Sciences 2017;53(2):e16089. [Google Scholar]
Dischinger 2015 {published data only}
- Dischinger HR, Cheng E, Mann AD, Grueber TM, Hawk S, Davis LA, et al. Decisional support to prevent adverse drug reactions of long latency: pilot randomized controlled intervention for glucocorticoid‐induced diabetes. Journal of Evaluation in Clinical Practice 2015;21(4):614‐9. [DOI] [PubMed] [Google Scholar]
Elhatab 2016 {published data only}
- Elhatab N, Silcock J, Graham A. Knowledge, attitudes and self‐care activities among patients with type II diabetes. European Journal of Hospital Pharmacy 2016;23:A162. [Google Scholar]
Erku 2017 {published data only}
- Erku DA, Ayele AA, Mekuria AB, Belachew SA, Hailemeskel B, Tegegn HG. The impact of pharmacist‐led medication therapy management on medication adherence in patients with type 2 diabetes mellitus: a randomized controlled study. Pharmacy Practice 2017;15(3):1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erku 2017a {published data only}
- Erku DA, Belachew SA, Tegegn HG, Ayele AA. The impact of pharmacist‐led medication therapy management on medication adherence in patients with type 2 diabetes mellitus: A randomized controlled study. Value in Health 2017;20 (9):A402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2015 {published data only}
- Garcia BH, Giverhaug T, Hogli JU, Skjold F, Smabrekke L. A pharmacist‐led follow‐up program for patients with established coronary heart disease in North Norway ‐ a randomized controlled trial. Pharmacy Practice 2015;13(2):575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geurts 2015 {published data only}
- Geurts MM, Stewart RE, Brouwers JR, Graeff PA, Gier JJ. Patient beliefs about medicines and quality of life after a clinical medication review and follow‐up by a pharmaceutical care plan: A study in elderly polypharmacy patients with a cardiovascular disorder. Journal of Pharmaceutical Health Services Research 2015;6(4):171‐6. [Google Scholar]
Goldfien 2017 {published data only}
- Goldfien R, Pressman A, Jacobson A, Ng M, Avins A. A pharmacist‐staffed, virtual gout management clinic for achieving target serum uric acid levels: a randomized clinical trial. Permanente Journal 2017;20(3):18‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grainger‐Rousseau 1996 {published data only}
- Grainger‐Rousseau TJ, McElnay JC. A model for community pharmacist involvement with general practitioners in the management of asthma patients. Journal of Applied Therapeutics 1996;1:145‐61. [Google Scholar]
Haag 2016 {published data only}
- Haag JD, Davis AZ, Hoel RW, Armon JJ, Odell LJ, Dierkhising RA, et al. Impact of pharmacist‐provided medication therapy management on healthcare quality and utilization in recently discharged elderly patients. American Health & Drug Benefits 2016;9(5):259‐68. [PMC free article] [PubMed] [Google Scholar]
Hedegaard 2014 {published data only}
- Hedegaard U, Kjeldsen LJ, Pottegard A, Bak S, Hallas J. Multifaceted intervention including motivational interviewing to support medication adherence after stroke/transient ischemic attack: a randomized trial. Cerebrovascular Diseases Extra 2014;4(3):221‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedegaard 2015 {published data only}
- Hedegaard U, Kjeldsen LJ, Pottegard A, Henriksen JE, Lambrechtsen J, Hangaard J, et al. Improving medication adherence in patients with hypertension: A randomized trial. American Journal of Medicine 2015;128(12):1351‐61. [DOI] [PubMed] [Google Scholar]
Hedegaard 2015a {published data only}
- Hedegaard U, Kjeldsen LJ, Pottegard A, Hallas J. A multifaceted pharmacist intervention to support medication adherence after stroke and transient ischemic attack. International Journal of Clinical Pharmacy 2015;37(1):187. [Google Scholar]
Hedegaard 2016 {published data only}
- Hedegaard U, Kjeldsen LJ, Pottegard A, Henriksen JE, Lambrechtsen J, Hangaard J. Improving medication adherence in patients with hypertension: A randomised controlled trial. International Journal of Clinical Pharmacy 2016;38(6):470‐1. [Google Scholar]
Houle 2016 {published data only}
- Houle SK, Charrois TL, McAlister FA, Kolber MR, Rosenthal MM, Lewanczuk R, et al. Pay‐for‐performance remuneration for pharmacist prescribers' management of hypertension: A substudy of the RxACTION trial. Canadian Pharmacists Journal 2016;149(6):345‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iqbal 2015 {published data only}
- Iqbal MS, Iqbal MZ, Iqbal MW, Nasir S, Bahari MB. Pharmacist‐led interventions to improve health‐related quality of life of pulmonary tuberculosis patients in Pakistan: An insight from a randomized controlled non‐clinical trial. Value in Health 2015;18(3):A27. [Google Scholar]
Isetts 2016 {published data only}
- Isetts BJ, Buffington DE, Carter BL, Smith M, Polgreen LA, James PA. Evaluation of pharmacists' work in a physician‐pharmacist collaborative model for the management of hypertension. Pharmacotherapy:The Journal of Human Pharmacology & Drug Therapy 2016;36(4):374‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN10671625 2017 {published data only}
- ISRCTN10671625. Role of pharmacist in diabetes management at community pharmacy. http://www.isrctn.com/ISRCTN10671625 2017; Vol. https://doi.org/10.1186/ISRCTN10671625:https://doi.org/10.1186/ISRCTN10671625.
Kandasamy 2016 {published data only}
- Kandasamy K, Natarajan A, Sebastian J, Konakalla M, Sam R, Rajagopal SS, et al. Impact of pharmacist intervention in screening and education on blood pressure in a rural area in Southern India. Asian Journal of Pharmaceutical and Clinical Research 2016;9(3):339‐43. [Google Scholar]
Korcegez 2017 {published data only}
- Korcegez EI, Sancar M, Demirkan K. Effect of a pharmacist‐led program on improving outcomes in patients with Type 2 diabetes mellitus from Northern Cyprus: A randomized controlled trial. Journal of Managed Care & Specialty Pharmacy 2017;23(5):573‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lainscak 2016 {published data only}
- Lainscak M, Roblek T, Deticek A, Leskovar B, Horvat M, Belic A, et al. Clinical‐pharmacist intervention reduces clinically relevant drug‐drug interactions in patients with heart failure: A randomized, double‐blind, controlled trial. European Journal of Heart Failure 2016;18:187. [DOI] [PubMed] [Google Scholar]
Lalonde 2017 {published data only}
- Lalonde L, Quintana‐Barcena P, Lord A, Bell R, Clement V, Daigneault AM, et al. Community pharmacist training‐and‐communication network and drug‐related problems in patients With CKD: A multicenter, cluster‐randomized, controlled trial. American Journal of Kidney Diseases 2017;70(3):386‐96. [DOI] [PubMed] [Google Scholar]
Lim 2016 {published data only}
- Lim PC, Lim K, Embee ZC, Hassali MA, Thiagarajan A, Khan TM. Study investigating the impact of pharmacist involvement on the outcomes of diabetes medication therapy adherence program Malaysia. Pakistan Journal of Pharmaceutical Sciences 2016;29(2):595‐601. [PubMed] [Google Scholar]
Loganadan 2012 {published data only}
- Loganadan NK, Lim KY, Nur NM, Ariffin F. Cost‐effectiveness of pharmacist managed medication therapy adherence clinic (MTAC) on type 2 diabetes patients in a Tertiary Hospital in Malaysia. Pharmacotherapy 2012;32:e270. [Google Scholar]
Lowrie 2012 {published data only}
- Lowrie R. Pharmacist intervention in primary care for patients with left ventricular systolic dysfunction. European Heart Journal 2012;33(3):141. [DOI] [PubMed] [Google Scholar]
Lyons 2016 {published data only}
- Lyons I, Barber N, Raynor DK, Wei L. The Medicines Advice Service Evaluation (MASE): a randomised controlled trial of a pharmacist‐led telephone based intervention designed to improve medication adherence. BMJ Quality & Safety 2016;25(10):759‐69. [DOI] [PubMed] [Google Scholar]
Manfrin 2017 {published data only}
- Manfrin A, Tinelli M, Thomas T, Krska J. A cluster randomised control trial to evaluate the effectiveness and cost‐effectiveness of the Italian medicines use review (I‐MUR) for asthma patients. BMC Health Services Research 2017;17(1):300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mansell 2016 {published data only}
- Mansell K, Evans C, Tran D, Sevany S. The association between self‐monitoring of blood glucose, hemoglobin A1C and testing patterns in community pharmacies: Results of a pilot study. Canadian Pharmacists Journal 2016;149(1):28‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Margolis 2015 {published data only}
- Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Maciosek MV, Nyboer RA, et al. A successful multifaceted trial to improve hypertension control in primary care: Why did it work?. Journal of General Internal Medicine 2015;30(11):1665‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marra 2011 {published data only}
- Marra C, Grindrod K, Grubisic M, Gastonguay L, Cibere J, Colley L. The pharmacist initiated intervention trial in osteoarthritis (PhIT‐OA): Clinical outcomes. Journal of Rheumatology 2011;38:1139. [Google Scholar]
Marra 2011a {published data only}
- Marra C, Grindrod K, Grubisic M, Kopec J, Esdaile J, Gastonguay L, et al. The pharmacist‐initiated intervention trial in osteoarthritis (PHIT‐OA): Clinical outcomes (155) and cost‐effectiveness analysis (168). Reumatologia Clinica Suplementos 2011;7:9. [Google Scholar]
Martin 2017 {published data only}
- Martin P, Tannenbaum C, Tamblyn R, Benedetti A, Ahmed S. D‐PRESCRIBE: a randomized cluster controlled trial to reduce inappropriate prescriptions in seniors. Canadian Pharmacists Journal. Conference: Canadian pharmacists association conference, CPha 2017. Canada 2017;150:S14. [Google Scholar]
Martin 2017a {published data only}
- Martin P, Tannenbaum C, Tamblyn R, Benedetti A, Ahmed S. D‐prescribe overtakes empower in patient‐centered deprescribing of benzodiazepines: Preliminary results from a pragmatic cluster‐randomized community‐based trial in Canada. Journal of the American Geriatrics Society 2017;65:S1‐2. [Google Scholar]
Mateti 2016 {published data only}
- Mateti UV, Ummer J, Kodangala S. Impact of clinical pharmacist counselling and education on quality of life in patients with acute coronary syndrome. Indian Journal of Pharmaceutical Education and Research 2016;50(3):360‐7. [Google Scholar]
McNamara 2011 {published data only}
- McNamara K, Stewart K, George J, Jackson S, Peterson G, Hughes J. Efficacy of a pharmacist‐managed intervention for improved blood pressure control in patients with elevated cardiovascular disease risk: Subgroup analysis of the HAPPY RCT. Heart Lung and Circulation 2011;20(Suppl 2):S4. [Google Scholar]
Mendes 2016 {published data only}
- Mendes AE, Lombardi NF, Andrzejevski VS, Frandoloso G, Correr CJ, Carvalho M. Medication reconciliation at patient admission: a randomized controlled trial. Pharmacy Practice 2016;14(1):656. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mikuls 2017 {published data only}
- Mikuls TR, Cheetham TC, Levy GD, Rashid N, Low K, Coburn BW, et al. Improving gout outcomes: the randomized evaluation of an ambulatory care pharmacist‐led intervention to optimize urate lowering pathways (Ramp‐up) study. Arthritis and rheumatology. Conference: American College of Rheumatology. Association of Rheumatology Health Professionals Annual Scientific Meeting, ACR/ARHP 2017. United states 2017;69:http://acrabstracts.org/abstract/improving‐gout‐outcomes‐the‐randomized‐evaluation‐of‐an‐ambulatory‐care‐pharmacist‐led‐intervention‐to‐optimize‐urate‐lowering‐pathways‐ramp‐up‐study/. [Google Scholar]
Nguyen 2011 {published data only}
- Nguyen VH, Poon J, Tokuda L, Sayers J, Wallis RA, Dergalust S. Pharmacist telephone interventions improve adherence to stroke preventive medications and reduce stroke risk factors: A randomized controlled trial. Stroke 2011;42:e244. [Google Scholar]
Obarcanin 2015 {published data only}
- Obarcanin E, Kruger M, Muller P, Nemitz V, Schwender H, Hasanbegovic S, et al. Pharmaceutical care of adolescents with diabetes mellitus type 1: the DIADEMA study, a randomized controlled trial. International Journal of Clinical Pharmacy 2015;37(5):790‐8. [DOI] [PubMed] [Google Scholar]
Obarcanin 2015a {published data only}
- Obarcanin E, Kruger M, Muller P, Nemitz V, Schwender H, Hasanbegovic S. Pharmaceutical care of adolescents with diabetes mellitus type 1: the DIADEMA study, a randomized controlled trial. International Journal of Clinical Pharmacy 2015;37(5):790‐8. [DOI] [PubMed] [Google Scholar]
Ojieabu 2017 {published data only}
- Ojieabu WA, Bello SI, Saka AS. Evaluation of pharmacist's educational and counseling impact on patients' clinical outcomes in a diabetic setting. Value in Health 2017;20 (5):A315. [Google Scholar]
Ojieabu 2017a {published data only}
- Ojieabu WA, Saka SA, Ojieabu CE. Evaluation of pharmacist's educational and counseling impact on patients clinical outcomes in a diabetic setting. Value in Health 2017;20 (9):A483. [Google Scholar]
Okada 2017 {published data only}
- Okada H, Onda M, Shoji M, Sakane N, Nakagawa Y, Sozu T, et al. Effects of lifestyle advice provided by pharmacists on blood pressure: The COMmunity Pharmacists ASSist for Blood Pressure (COMPASS‐BP) randomized trial. Bioscience Trends 2017;11(6):632‐9. [DOI] [PubMed] [Google Scholar]
Olivera 2016 {published data only}
- Olivera CM, Vianna EO, Bonizio RC, Menezes MB, Ferraz E, Cetlin AA. Asthma self‐management model: randomized controlled trial. Health Education Research 2016;31(5):639‐52. [DOI] [PubMed] [Google Scholar]
Omran 2015 {published data only}
- Omran D, Majumdar SR, Johnson JA, Tsuyuki RT, Lewanczuk RZ, Guirguis LM, et al. Pharmacists on primary care teams: Effect on antihypertensive medication management in patients with type 2 diabetes. Journal of the American Pharmacists Association: JAPhA 2015;55(3):265‐8. [DOI] [PubMed] [Google Scholar]
Periasamy 2017 {published data only}
- Periasamy U, Mohd S, Rampal L, Fadhilah SI, Akhtari‐Zavare M, Mahmud R. Effect of chemotherapy counseling by pharmacists on quality of life and psychological outcomes of oncology patients in Malaysia: a randomized control trial. Health & Quality of Life Outcomes 2017;15(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pevnick 2017 {published data only}
- Pevnick JM, Nguyen C, Jackevicius CA, Palmer KA, Shane R, Cook‐Wiens G, et al. Improving admission medication reconciliation with pharmacists or pharmacy technicians in the emergency department: a randomised controlled trial. BMJ Quality & Safety 2017;October(7):512‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pistja 2015 {published data only}
- Pistja E, Themeli A. Pharmacists, valuable members of the hypertension management team. European Journal of Cardiovascular Nursing 2015;14:84‐5. [Google Scholar]
Renuga 2016 {published data only}
- Renuga E, Ramakrishnan SR, Vanitha Rani N, Thennarasu P, Kannan G. Impact of continuous patient counselling on knowledge, attitude, and practices and medication adherence of diabetic patients attending outpatient pharmacy services. Asian Journal of Pharmaceutical and Clinical Research 2016;9(1):345‐50. [Google Scholar]
Rubio‐Valera 2009 {published data only}
- Rubio‐Valera M, Serrano‐Blanco A, Trave P, Pearrubia‐Maria MT, Ruiz M, Pujol MM. Community pharmacist intervention in depressed primary care patients (PRODEFAR study): Randomized controlled trial protocol. BMC Public Health 2009;9:https://doi.org/10.1186/1471‐2458‐9‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scala 2017 {published data only}
- Scala D, Menditto E, Caruso G, Monetti VM, Orlando V, Guerriero F, et al. Are you more concerned about or relieved by medicines? An explorative randomized study of the impact of telephone counseling by pharmacists on patients' beliefs regarding medicines and blood pressure control. Patient Education & Counseling 2017;101(4):679‐86. [DOI] [PubMed] [Google Scholar]
Schmiedel 2015 {published data only}
- Schmiedel K, Mayr A, Fiesler C, Schlager H, Friedland K. Effects of the lifestyle intervention program GLICEMIA in people at risk for type 2 diabetes: a cluster‐randomized controlled trial. Diabetes Care 2015;38(5):937‐9. [DOI] [PubMed] [Google Scholar]
Schneiderhan 2015 {published data only}
- Schneiderhan ME. A 12‐month randomized analyses of pharmacist comprehensive medication management services in community mental health. Journal of Pharmacy Practice 2015;28 (3):318. [Google Scholar]
Shao 2017 {published data only}
- Shao H, Chen G, Zhu C, Chen Y, Liu Y, He Y, et al. Effect of pharmaceutical care on clinical outcomes of outpatients with type 2 diabetes mellitus. Patient Preference & Adherence 2017;11:897‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Siaw 2017 {published data only}
- Siaw MY, Ko Y, Malone DC, Tsou KY, Lew YJ, Foo D, et al. Impact of pharmacist‐involved collaborative care on the clinical, humanistic and cost outcomes of high‐risk patients with type 2 diabetes (IMPACT): a randomized controlled trial. Journal of Clinical Pharmacy & Therapeutics 2017;42(4):475‐82. [DOI] [PubMed] [Google Scholar]
Smith 2017 {published data only}
- Smith JR, Hillman L, Drawz PE. Pharmacist‐based antihypertensive medication review and assignment of morning versus evening dosing of once‐daily antihypertensive medications: A pilot study to assess feasibility and efficacy in chronic kidney disease patients. Clinical & Experimental Hypertension (New York) 2017;https://doi.org/10.1080/10641963.2017.1411493:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Souter 2017 {published data only}
- Souter C, Kinnear A, Kinnear M, Mead G. A pilot study to assess the practicality, acceptability and feasibility of a randomised controlled trial to evaluate the impact of a pharmacist complex intervention on patients with stroke in their own homes. European Journal of Hospital Pharmacy 2017;24:101‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tahaineh 2017 {published data only}
- Tahaineh LM, Khasawneh AH. A randomised control trial to evaluate the clinical pharmacist's role in managing iron deficiency anaemia patients. International Journal of Pharmacy Practice 2017;26(1):55‐62. [DOI] [PubMed] [Google Scholar]
Tan 2011 {published data only}
- Tan PS. Pharmacoeconomic evaluation of a pharmacist‐managed diabetes clinic. Value in Health 2011;14:A97. [Google Scholar]
Tierney 2005 {published data only}
- Tierney WM, Overhage JM, Murray MD, Harris LE, Zhou XH, Eckert GJ, et al. Can computer‐generated evidence‐based care suggestions enhance evidence‐based management of asthma and chronic obstructive pulmonary disease? A randomized, controlled trial. Health Services Research 2005;40(2):477‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuyuki 2015a {published data only}
- Tsuyuki RT, Houle SK, Charrois TL, Kolber MR, Rosenthal MM, Lewanczuk R, et al. Randomized trial of the effect of pharmacist prescribing on improving blood pressure in the community: The Alberta clinical trial in optimizing hypertension (RxACTION). Circulation 2015;132(2):93‐100. [DOI] [PubMed] [Google Scholar]
Tsuyuki 2016 {published data only}
- Tsuyuki RT, Rosenthal M, Pearson GJ. A randomized trial of a community‐based approach to dyslipidemia management: Pharmacist prescribing to achieve cholesterol targets (RxACT Study). Canadian Pharmacists Journal 2016;149(5):283‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsuyuki 2016a {published data only}
- Tsuyuki RT, Al Hamarneh YN, Jones CA, Hemmelgarn BR. The effectiveness of pharmacist interventions on cardiovascular risk: The multicenter randomized controlled RxEACH trial. Journal of the American College of Cardiology 2016;67(24):2846‐54. [DOI] [PubMed] [Google Scholar]
Tsuyuki 2017 {published data only}
- Tsuyuki R, Hassa I, Jones C, Hemmelgarn B, Al Hamarneh Y. Blood pressure reduction by prescribing pharmacists‐ Insights from the multicentre randomized RxEACH study. Canadian Pharmacists Journal. Conference: Canadian Pharmacists' Association Conference, CPhA 2017. Canada 2017;150:S29. [Google Scholar]
Tuttle 2018 {published data only}
- Tuttle KR, Alicic RZ, Short RA, Neumiller JJ, Gates BJ, Daratha KB, et al. Medication therapy management after hospitalization in CKD: A randomized clinical trial. Clinical Journal of The American Society of Nephrology: CJASN 2018;13(2):231‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ummavathy 2015 {published data only}
- Ummavathy P, Sherina MS, Rampal L, Siti Irma Fadhilah I. Outcome of chemotherapy counseling by pharmacists on psychological effects and self esteem among oncology patients in a Government Hospital in Malaysia. Medical Journal of Malaysia 2015;70(3):131‐41. [PubMed] [Google Scholar]
Van Der Meer 2016 {published data only}
- Meer HG, Wouters H, Pras N, Taxis K. Reducing patients' cumulative exposure to anticholinergic and sedative medication with medication reviews: A randomized controlled trial. Pharmacoepidemiology and Drug Safety 2016;25(S3):266. [Google Scholar]
Verret 2011 {published data only}
- Verret L, Couturier J, Rozon A, Saudrais‐Janecek S, St‐Onge A, Nguyen A, et al. Patient self‐management of warfarin in a pharmacist‐led program versus management by a specialized anticoagulation clinic: A randomized trial. Journal of Thrombosis and Thrombolysis 2011;31(3):383‐4. [Google Scholar]
Vinluan 2015 {published data only}
- Vinluan CM, Wittman D, Morisky D. Effect of pharmacist discharge counselling on medication adherence in elderly heart failure patients: A pilot study. Journal of Pharmaceutical Health Services Research 2015;6(2):103‐10. [Google Scholar]
Wishah 2015 {published data only}
- Wishah RA, Al‐Khawaldeh OA, Albsoul AM. Impact of pharmaceutical care interventions on glycemic control and other health‐related clinical outcomes in patients with type 2 diabetes: randomized controlled trial. Diabetes & Metabolic Syndrome 2015;9(4):271‐6. [DOI] [PubMed] [Google Scholar]
Wongpakaran 2017 {published data only}
- Wongpakaran R, Suansanae T, Tan‐Khum T, Kraivichian C, Ongarjsakulman R, Suthisisang C. Impact of providing psychiatry specialty pharmacist intervention on reducing drug‐related problems among children with autism spectrum disorder related to disruptive behavioural symptoms: A prospective randomized open‐label study. Journal of Clinical Pharmacy & Therapeutics 2017;42(3):329‐36. [DOI] [PubMed] [Google Scholar]
Yang 2015 {published data only}
- Yang YS, Wu YC, Lu YL, Kornelius E, Lin YT, Chen YJ, et al. Adherence to self‐care behavior and glycemic effects using structured education. Journal of Diabetes Investigation 2015;6(6):662‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2017 {published data only}
- Yang R, Carter BL, Gums TH, Gryzlak BM, Xu Y, Levy BT. Selection bias and subject refusal in a cluster‐randomized controlled trial. BMC Medical Research Methodology 2017;17:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhao 2015 {published data only}
- Zhao SJ, Zhao HW, Du S, Qin YH. The impact of clinical pharmacist support on patients receiving multi‐drug therapy for coronary heart disease in China. Indian Journal of Pharmaceutical Sciences 2015;77(3):306‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Da Silva 2012 {published data only}
- Silva GM, Chambela MC, Sousa AS, Sangenis LH, Xavier SS, Costa AR, et al. Impact of pharmaceutical care on the quality of life of patients with Chagas disease and heart failure: randomized clinical trial. Trials 2012;13(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forster 2015 {published data only}
- Forster AJ, Erlanger TE, Jennings A, Auger C, Buckeridge D, Walraven C, et al. Effectiveness of a computerized drug‐monitoring program to detect and prevent adverse drug events and medication non‐adherence in outpatient ambulatory care: study protocol of a randomized controlled trial. Trials 2015;16(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuhmmer 2015 {published data only}
- Kuhmmer R, Lima KM, Ribeiro RA, Hammes LS, Bastos GA, Souza MC, et al. Effectiveness of pharmaceutical care at discharge in the emergency department: study protocol of a randomized controlled trial. Trials 2015;16(60):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Porteous 2013 {published data only}
- Porteous T, Wyke S, Smith S, Bond C, Francis J, Lee AJ, et al. Help for hay fever, a goal‐focused intervention for people with intermittent allergic rhinitis, delivered in Scottish community pharmacies: study protocol for a pilot cluster randomized controlled trial. Trials 2013;14(1):217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Altowaijri 2013
- Altowaijri A, Phillips CJ, Fitzsimmons D. A systematic review of the clinical and economic effectiveness of clinical pharmacist intervention in secondary prevention of cardiovascular disease. Journal of Managed Care Pharmacy 2013;19(5):408‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charrois 2012
- Charrois TL, Zolezzi M, Koshman SL, Pearson G, Makowsky M, Durec T, et al. A systematic review of the evidence for pharmacist care of patients with dyslipidemia. Pharmacotherapy 2012;32(3):222‐33. [DOI] [PubMed] [Google Scholar]
Chinn 2000
- Chinn S. A simple method for converting an odds ratio to effect size for use in meta‐analysis. Statistics in Medicine 2000;19(22):3127‐3131. [DOI] [PubMed] [Google Scholar]
De Barra 2016
- Barra M, Scott C, Watson P. Cochrane Review Epi Info Data Collection Tool. Figshare. dx.doi.org/10.6084/m9.figshare.3497807.v1 (accessed 30 June 2018).
De Vera 2014
- Vera MA, Sadatsafavi M, Tsao NW, Lynd LD, Gastonquay L, Lester R, et al. Empowering pharmacists in asthma management through interactive SMS (EmPhAsIS): Study protocol for a randomized controlled trial. Trials 2014;15:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diabetes UK
- Diabetes UK. Blood Sugar Converter. http://www.diabetes.co.uk/blood‐sugar‐converter.html.
Epi Info 2010 [Computer program]
- Dean AG, Arner TG, Sunki GG, Friedman R, Lantinga M, Sangam S, et al. Epi Info™, a database and statistics program for public health professionals. CDC. Atlanta: CDC, 2011.
EPOC 2017a
- Cochrane Effective Practice, Organisation of Care (EPOC). Data collection form. EPOC Resources for review authors, 2017. Available at epoc.cochrane.org/epoc‐specific‐resources‐review‐authors (accessed 30 June 2018).
EPOC 2017b
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors, 2017. Available at: epoc.cochrane.org/resources/epoc‐resources‐review‐authors.
Glynn 2010
- Glynn L, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005182.pub4] [DOI] [PubMed] [Google Scholar]
Greer 2016
- Greer N, Bolduc J, Geurkink E, Rector T, Olson K, Koeller E, et al. Pharmacist‐led chronic disease management: a systematic review of effectiveness and harms compared with usual care. Annals of Internal Medicine 2016 [Epub ahead of print];165:30‐40. [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, Grade Working Group. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatah 2014
- Hatah E, Braund R, Tordoff J, Duffull SB. A systematic review and meta‐analysis of pharmacist‐led fee‐for‐services medication review. British Journal of Clinical Pharmacology 2014;77(1):102‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hoffman 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: Template for intervention description and replication (TIDieR) checklist and guide. British Medical Journal 2014;348:1687. [DOI] [PubMed] [Google Scholar]
Jokanovic 2017
- Jokanovic N, Tan EC, Sudhakaran S, Kirkpatrick CM, Dooley MJ, Ryan‐Atwood TE, et al. Pharmacist‐led medication review in community settings: An overview of systematic reviews. Research in Social and Administrative Pharmacy 2017;13(4):661‐85. [DOI] [PubMed] [Google Scholar]
Koshman 2008
- Koshman SL, Charrois TL, Simpson SH. Pharmacist care of patients with heart failure: a systematic review of randomized trials. Archives of Internal Medicine 2008;168(7):687‐94. [DOI] [PubMed] [Google Scholar]
Law 2003
- Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke. A new preventative strategy. Health Technology Assessment 2003;7(31):n/a. [DOI] [PubMed] [Google Scholar]
Mekonnen 2016
- Mekonnen AB, McLachlan AJ, Brien JE. Effectiveness of pharmacist‐led medication reconciliation programmes on clinical outcomes at hospital transitions: a systematic review and meta‐analysis. BMJ Open 2016;6:e010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2014
- Michie S, Atkins L, West R. The Behaviour Change Wheel. A Guide to Designing Interventions. 1st Edition. London: Silverback Publishing, 2014. [Google Scholar]
Pande 2013
- Pande S, Hiller JE, Nkansah N, Bero L. The effect of pharmacist‐provided non‐dispensing services on patient outcomes, health service utilisation and costs in low‐ and middle‐income countries. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010398] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rugge 2011
- Rugge B, Balshem H, Sehgal R, Relevo R, Gorman P, Helfand M. Screening and treatment of subclinical hypothyroidism or hyperthyroidism. Comparative Effectiveness Reviews. Vol. 24, Agency for Healthcare Research and Quality (US), 2011:Report No.: 11(12)‐EHC033‐EF. [PubMed] [Google Scholar]
Sinclair 2004
- Sinclair HK, Bond CM, Stead LF. Community pharmacy personnel interventions for smoking cessation. Cochrane Database of Systematic Reviews 2004, Issue 2004. [DOI: 10.1002/14651858.CD003698.pub2] [DOI] [PubMed] [Google Scholar]
Walsh 2016
- Walsh KA, O'Riordan D, Kearney PM, Timmons S, Byrne S. Improving the appropriateness of prescribing in older patients: a systematic review and meta‐analysis of pharmacists’ interventions in secondary care. Age and Ageing 2016;45(2):201‐9. [DOI] [PubMed] [Google Scholar]
Ware 1989
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey: Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center, 1989. [Google Scholar]
WHO 2011
- World Health Organisation. WHO Top 20 Projections of Mortality and Causes of Death by 2030 (2011). www.who.int/healthinfo/global_burden_disease/projections/en/ (accessed 30 June 2018).
References to other published versions of this review
Beney 2000
- Beney J, Bero L, Bond CM. Expanding the roles of outpatient pharmacists: effects on health services utilisation, costs, and patient outcomes. Cochrane Database of Systematic Reviews 2000, Issue DOI: 10.1002/14651858.CD000336. [DOI: 10.1002/14651858.CD000336] [DOI] [PubMed] [Google Scholar]
Bero 1995
- Bero LA, Mays NB, Barjesteh K, Bond C. The effect of expanding pharmacists' roles on health services utilisation, costs, and patient outcomes. Cochrane Database of Systematic Reviews 1995, Issue 2. [DOI: 10.1002/14651858.CD000336] [DOI] [PubMed] [Google Scholar]
Nkansah 2010
- Nkansah N, Mostovetsky O, Yu C, Cheng T, Beney J, Bond CM, Bero L. Effect of outpatient pharmacists' non‐dispensing roles on patient outcomes and prescribing patterns.. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD000336.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


