Abstract
Vitamin D, a free sunshine vitamin available for mankind from nature, is capable to avert many health-related critical circumstances. Vitamin D is no more regarded as a nutrient involved in bone metabolism alone. The presence of vitamin D receptor in a number of tissues implies that vitamin D has various physiological roles apart from calcium and phosphorus metabolism. Low serum vitamin D has been found to be associated with various types of metabolic illness such as obesity, diabetes mellitus, insulin resistance, cardiovascular diseases including hypertension. Various studies reported that vitamin D insufficiency or deficiency in linked with metabolic syndrome risk. This review focuses on various metabolic diseases and its relationship with serum vitamin D status.
Keywords: Vitamin D, Metabolic syndrome, Obesity, Diabetes mellitus, Cardiovascular disease
INTRODUCTION
Metabolic syndrome (MS) has become the most common health challenge worldwide, crossing the barriers of age, sex, and ethnicity. The risk factors associated with MS include hypertension, hyperlipidemia, hyperglycemia, insulin resistance with a strong association with abdominal obesity. Patients with MS have been at an increased risk of developing cardiovascular disease (CVD) and/or type 2 diabetes mellitus (T2DM)1, making it one of the leading causes of early death.
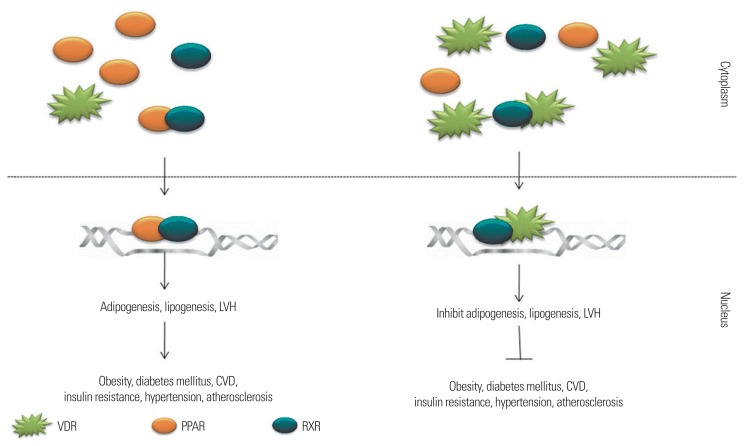
With two decades of research in this field, now various pathways involved in MS are explored and environmental, life style and genetical factors, either in single or combined forms, is responsible for the aggravation of the syndrome.2 Many methods had been proposed in managing and preventing MS, which includes phytotherapy, metabolism intervening drug, diet therapy, physical exercise, hormonal therapy, chemotherapy, etc. Many reviews are available on MS; its prevention and treatment, however, there is still little knowledge on the role of vitamin D and its usefulness in combating MS. Accumulating data suggest that circulating serum vitamin D is inversely related to MS (Fig. 1). This review focuses on the impact of vitamin D on MS.
Figure 1.
The cross-talk between retinoid X receptor (RXR), vitamin D receptor (VDR), and peroxisome proliferator-activated receptor (PPAR). VDR and PPAR share a common binding partner RXR and a common binding site in the DNA, steroid and nuclear hormone receptor binding site. Hence the dominance of expression will be dependent on the availability ratio of VDR and PPAR; both have to compete with one another to get engaged with RXR so that they could get inside the nucleus and could bind at their site of interest. If PPAR concentration is higher than the gene expression of its governance will be dominating the cell, if VDR is higher than the genome will be more accessible for it rather than for PPARs. LVH, left ventricular hypertrophy; CVD, cardiovascular disease.
VITAMIN D AND OBESITY
Obesity can be defined as an abnormal or excessive accumulation of fat in the adipose tissue and the body weigh more than 20% of the recommended weight. The accumulation of body fat may have many reasons in single or combination that includes food addiction, genetic mutation, poor physical activity, endocrine disorder, and poor nutrition status. In past 20 years of investigation to find the relationship between vitamin D and obesity, many clues have been found that reveals strong evidences relating vitamin D deficiency and obesity. An inverse relationship of circulating vitamin D levels and the degree of obesity3, as well as with central adiposity4 is already well documented. 25-Hydroxyvitamin D3 (25(OH)D3)5 in the blood was assessed for vitamin D status and vitamin D deficiency defines as a blood level of 25(OH)D3 level below 20 ng/mL (50 nmol/L).6 Botella-Carretero et al.7 reported that 63% morbidly obese individuals (body mass index [BMI] ≥40 kg/m2) had the MS and the vitamin D deficiency occurred in 50.7% of them. The conclusion was made that vitamin D deficiency was closely related to morbidly obese individuals along with the MS, compared to those who are normal weight individuals. In another study on Canadian normal weight, overweight and obese children and adolescents, it was found that vitamin D deficiency (<40 nmol/L) was in 27% of overweight and obese youth while it was only to 12% in normal weight youth. This study confirmed that obesity was associated serum 25(OH)D concentration in Canadian youth and it was independently associated with vitamin D supplementation and daily milk consumption.8,9 In a study of 21 cohorts (up to 42,024 participants) on the relation of BMI and vitamin D status, it was concluded that higher BMI is associated with lower levels of serum 25(OH)D. A reduction of BMI is expected to prevent vitamin D deficiency.10 A study was conducted on 66 overweight/obese young Spanish women to analyze their vitamin D status in relation to the dietetic and anthropometric differences. It was concluded that risk of vitamin D deficiency is increased in overweight and obese women by excess adiposity than inadequate intake.11 Daniel et al.12 reported a significantly association with vitamin D levels and BMI controlling for different age and ethnic populations. Vitamin D deficiency in obese subjects was appeared in all races, especially, with a higher deficiency incidence in Asians and blacks than in Hispanics and Caucasians.
Overall, poor vitamin D status could be positively associated with obesity, but the relationship between these variables has not been precisely determined. The possible reason could be that obese individuals have insufficient sun exposure and outdoor activities.13 Additionally, vitamin D receptor (VDR) expression in adipose tissue, food intake and exercise levels also seem to be associated to the vitamin D deficiency in obesity.14,15
VITAMIN D AND ADIPOCYTE
Adipogenesis is the process of cellular differentiation during which preadipocytes are transformed mature adipocytes. Hypertrophy and hyperplasticity of adipocytes are the reason for adiposity and latter obesity. Previous studies reported that Cyp27b1 gene encodes the enzyme converting 25(OH)D3 to 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) in adipose tissue of rodent and human.16 Near about two decades ago researchers reported that vitamin D was able to inhibit adipogenesis process. According to this study triglyceride accumulation was decreased by 50% in 3T3-L1 preadipocytes treated with 1,25-D compared to the control.17,18 Many in vitro studies on murine, porcine, chicken and human adipocyte cell lines revealed that vitamin D interferes in the adipocyte differentiation process and thus inhibits adipogenesis. The master regulator of adipogenesis peroxisome proliferator-activated receptor gamma (PPARγ) shares a common heterodimeric binding partner, retinoid X receptor (RXR), with VDR, this makes a situation of rivalry between PPARγ and RXR.19 Increased expression of VDR compared to Pparg decreases the chances of adipogenesis related gene expression and as a result the expansion of mitotic clonal population of adipocytes decreases. In a study on porcine derived preadipocyte, 1,25(OH)2D3 inhibited cell differentiation and suppressed the expression of Pparg mRNA along with the expression of adipocyte marker genes such as Lpl, Pck2, Scd, and Glut4.20 Although previous studies have suggested the inhibitory effect of 1,25(OH)2D3 on preadipocyte differentiation, as a possible reason for the antiadipogenic effect of vitamin D, it could be mediated by the wingless-type MMTV integration site/β-catenin pathway which is implicated in various cellular functions and processes21 and it is a negative regulator of adipogenesis.22 Studies on other types of cell lines also concluded same type of results (Table 1).20,23–33 Mesenchymal cells collected from murine bone marrow when cultured in induction media along with 18×10−12 mol/L of vitamin D showed poor differentiation of bone marrow derived cells into adipocytes.23
Table 1.
Effect of vitamin D3 on adipogenesis of in vitro
| Author (year) | Cell type | Conclusion made |
|---|---|---|
| Basoli et al. (2017)24 | Adipose-derived stem cells | Inhibited adipogenesis: suppression of specific adipogenesis orchestrating genes |
| Chang and Kim (2016)25 | 3T3-L1 | Decreased adipocyte lipid storage: increased NAD-SIRT1 pathway |
| Ricciardi et al. (2015)26 | Immortalized brown fat cell line | Suppressed differentiation: suppression of Pparg |
| Sakuma et al. (2012)27 | 3T3-L1 | Inhibited adipocyte differentiation: suppression of Pparg and Cebpa, Erk and maintenance of WNT/β-catenin pathway |
| Sun and Zemel (2008)28 | 3T3-L1 | Inhibited adipogenesis: liganded nVDR with 1,25(OH)2D |
| Cianferotti and Demay (2007)29 | Murine bone marrow stromal cells | Inhibited adipogenesis: suppression of Dkk1 and Sfrp2 (inhibitors of the canonical WNT signaling pathway) |
| Zhuang et al. (2007)20 | Porcine preadipocyte | Inhibited adipogenesis: suppression of Pparg and interfered with the induction of Rxra |
| Kong and Li (2006)30 | 3T3-L1 | Inhibited adipogenesis: decreased expression of Cebpa and via acting as a Pparg antagonist |
| Duque et al. (2004)23 | Mice bone marrow cells | Inhibited adipogenesis |
| Huang et al. (2002)31 | Rat adipocyte | Inhibits glucose uptake by adipocytes |
| Shi et al. (2002)32 | Human adipocytes | Inhibited adipogenesis: suppression of UCP2 expression |
| Kelly and Gimble (1998)33 | Murine bone marrow stromal cell (BMS2) | Inhibited adipocyte differentiation: decreased late adipocyte gene markers, Ap2 and adipsin |
WNT, wingless-type MMTV integration site; nVDR, nuclear vitamin D receptor; 1,25(OH)2D, 1,25-dihydroxyvitamin D; UCP2, uncoupling protein 2.
VITAMIN D AND THERMOGENESIS
Thermogenesis, the process of heat generation is an essential mechanism in keeping constant body temperature when the surrounding temperature starts dropping. In terms of obesity management thermogenesis is taken as a way for reducing stored fat by subjecting them to β-oxidation that results in the production of adenosine triphosphate as well as heat. In a human study, body weight and fat mass were decreased by low energy diet and vitamin D supplementation34,35 and in mice model, vitamin D protected the mice from diet induced obesity due to increased adipose tissue apoptosis.36 Furthermore, thermogenesis and fat oxidation rates in subsequent meals were significantly increased through vitamin D-rich breakfast intake, suggesting a direct link to metabolism.37 Uncoupling protein 1 is expressed in brown adipose tissue and enhanced thermogenesis reaction, and is regulated by VDR. Human adipocyte uncoupling protein 2 expression is suppressed through the nuclear VDR by the 1,25(OH)2D3 treatment.32 Suppression of 1,25(OH)2D3 and consequent up-regulation of Ucp2 increased thermogenesis in mice fed with high calcium diets. In the study by Wong et al.38, they created a VDR null mutant mice model. Body fat accumulation, triglyceride and cholesterol levels were reduced in the VDR null mice under normal calcium level compared to their wild type counterpart. Further the rate of β-oxidation in the white adipose tissue was higher; the Ucp1, Ucp2, and Ucp3 gene expression was also upregulated in the VDR-null mice comparing to the wild-type mice.
VITAMIN D AND ATHEROSCLEROSIS
Atherosclerosis is the buildup of cholesterol-rich lipids on the arterial wall accompanied with inflammatory responses.39 Vitamin D deficiency and insufficiency are related with increased risk of subclinical atherosclerosis. A positive inverse correlation is seen between atheroscleric plaque formation and level of circulating vitamin D. In Oh et al.’s study40, they found that vitamin D protects against foam cell formation and inhibits macrophage cholesterol uptake in patients with T2DM. When macrophages isolated from diabetic patients were cultured in media with or without adding vitamin D and was loaded with modified low-density lipoprotein cholesterol, 1,25(OH)2D3 inhibited the foam cell formation by reducing uptake of acetylated low-density lipoprotein or oxidized low-density lipoprotein (OxLDL) cholesterol in diabetic patients only. The modified LDL induced foam cells formation in VDR deleted macrophages from diabetic patients. Vitamin D prevented OxLDL-derived cholesterol uptake as well as downregulated the c-Jun N terminal kinase activation, reduced PPAR expression and CD36 expression. In another study by Freedman et al.41, on African Americans, where 340 African-Americans with T2DM were evaluated, they found that 25(OH)D had a positive association with aorta and carotid artery calcified atherosclerotic plaque formation in African-Americans. Since, dark pigmented skin limits the synthesis of vitamin D in a given time of sunlight exposure when compared with the white skin, dark skin individuals need six times longer exposure than white skin to produce sufficiency vitamin D. Despite of low vitamin D and calcium intake, blacks have far less calcium in their arteries. It is reported that more black patients with diabetes mellitus have heart disease than white patients and lower calcified atherosclerotic plaques are associated with a lower risk of heart disease in blacks. However, high vitamin D intake accelerates vascular calcification in animal model.42 This study reported that when rodents were fed with high cholesterol diets along with high dose of vitamin D2 (300,000 IU/kg body weight/day) up to 4 weeks, accelerated calcified plaque formation in the arteries43 was observed, indicating that both excess and deficiency are detrimental.
VITAMIN D AND HYPERTENSION
Vitamin D deficiency and insufficiency have been observed to upregulate the renin-angiotensin-aldosterone system (RAAS), resulting in hypertension. Studies found that both the systolic and diastolic blood pressure are decreased by administering vitamin D in older adults with existing high blood pressure. There is direct link between vitamin D and renin angiotensin system (RAS) as vitamin D acts as a potent endocrine suppressor in renin biosynthesis through regulation of the RAS.44 In a double-blind study, 400 IU of vitamin D3 with 1,000 mg of elemental calcium daily, or placebo were given to 36,282 postmenopausal women, it was observed that the blood pressure changed along with the incidence of hypertension. There was no clinically meaningful effects in systolic blood pressure and diastolic blood pressure between the treatment and comparison groups for a median follow-up time of 7 years.45
However, in another study by Judd et al.46, nine subjects were categorized into three groups, either placebo, vitamin D3 or vitamin D2. Dose of 200,000 IU vitamin D3 (n=3) or matching placebo (n=3) given orally to the individuals weekly, or 0.5 μg calcitriol (n=2) was taken twice every day for 3 weeks. It was observed intake of calcitriol lowered the systolic blood pressure by 9% when compared to the placebo. However after the period of conclusion the systolic blood pressure returned to pretreatment levels 1 week later. A recent study by Forman et al.47 on human subjects supports the inverse correlation of RAS with vitamin D. The study which was conducted on 184 normotensive humans suggested that low plasma 25(OH)D levels may result in upregulation of the RAS in otherwise healthy humans.
VITAMIN D AND CVD
CVD always finds company with metabolic diseases. Recent research highlighted the importance of vitamin D deficiency in several CVD conditions. Vitamin D deficiency has been positively correlated with poor cardiac contractility48, increased myocardial collagen content49, and cardiac tissue maturation.50 VDRs have a broad tissue distribution that includes vascular smooth muscle, endothelium, and cardiomyocytes.51 It has been well documented that the occurrence of CVD related mortality has a strong association with environmental factors such as geographic latitude, altitude, season, and the place of residence (urban or rural).52–54
Interestingly, all these factors have a strong relationship with the UVB exposure and therefore the serum vitamin D level.55 Therefore, it is no doubt that vitamin D has a critical role in maintaining cardiovascular health. A wide range of mechanisms may play role in vitamin D dependent cardiovascular health maintenance that include suppression of the RAAS56, inhibition of growth of vascular smooth muscle cells57,58, the suppression of vascular calcification59, the upregulation of anti-inflammatory cytokines by suppressing proinflammatory cytokines60, and prevention of secondary hyperparathyroidism61, and other beneficial effects on cardiovascular risk factors.62 Wang et al.56 did a study on 1,739 subjects (mean age, 59 years; 55% women; all white) without previous CVD history. Their serum vitamin D level was assessed marked deficiency if found below <15 ng/mL and <10 ng/mL. Near about 28% of the subjects showed vitamin D status <15 ng/mL and about 9% had <10 ng/mL. With a span of 5.4 years 120 subjects encounter the first cardiovascular event. Supporting to vitamin D deficiency and occurrence of CVD event, one study reported that 60,000 IU of monthly oral vitamin D supplementation for 16 weeks improved the vascular endothelial function significantly in 45 African-American adults.63 A study by Vimaleswaran et al.64 suggested that with increase in lowers the risk of development of CVD, showing a causal relationship. The VDR appears to be widely distributed, including in cardiovascular tissue. However, the current trials and evidence are not enough to identify the cause between vitamin D deficiency and CVD related deaths.65
VITAMIN D AND INSULIN RESISTANCE AND DIABETES MELLITUS
The insulin deregulation and diabetes mellitus go hand in hand. People with increase BMI in younger age suffers from diabetic complication in middle age. Insulin resistance is the most common cause for non-insulin dependent diabetic mellitus (NIDDM). Insulin resistance increases the risk for other disease in an individual. People with insulin resistance are more prone to suffer with NIDDM66, CVD67–69 and kidney disease.70,71 Accumulating evidence suggests that decrease in insulin secretion in both humans as well as animal models has a strong correlation with vitamin D deficiency.72 It has been reported by several studies that the lower level of vitamin D triggers the development of insulin resistance and thus NIDDM by deregulating the insulin sensitivity or β-cell function, or both.72–75
A two decade old report by Taylor and Wise76 stated that, in three separate cases on British Asians subjects with vitamin D deficiency and NIDDM; replacement of vitamin D led to an increase in insulin resistance along with deterioration of glycemic control in all the three cases. However, another report by Schwalfenberg77 showed a different picture. In his report, he did vitamin D replacement in two elderly female subjects of 63 and 71 years old suffering from diabetics and were dependent in exogenous insulin. They were diagnosed with vitamin D insufficiency (25 nmol/L and 34 nmol/L, respectively). Vitamin D supplementation was given with a dose of 3,000 IU/day and 2,000 IU/day, respectively. After 6 months and 9 months for each subject, their vitamin D level was increased to 140 nmol/L and 107 nmol/L, respectively. The glycosylated hemoglobin level dropped from 8.4% to 7.4% and 13.3% to 12.2%, respectively. This showed that vitamin D sufficiency improves glucose tolerance. In addition, the study by Pinelli et al.78 on 542 Arab Americans showed that about 75% were in vitamin D insufficiency (5 to <20 ng/mL) condition while 24% were suffering from hypovitaminosis D (20 to <40 ng/mL) condition. Men with glucose intolerance were having lower vitamin D level comparing to normoglycemic individual. No such relation was found in women participants. The homeostatic model assessment index of insulin resistance, triglycerides, fasting plasma glucose was negatively correlated with serum 25(OH)D level.71 In a recent study by He et al.79, insulin sensitivity and T2DM prevention were improved in subjects with T2DM when the supplement dose was >2,000 IU/day (P=0.047).
VITAMIN D SUPPLEMENTATION: NECESSITY AND SAFETY
Vitamin D supplementation dosage is still an issue of controversy. In 2010, the Food and Nutrition Board of the Institute of Medicine, US recommended the dietary reference intake (DRI) allowance of vitamin D to be 400 IU/day (10 mcg/day) for infants and 600 IU/day (15 mcg/day) for children and adult male and female subjects up to age 70, whereas the vitamin D intake for males and females aged over 70 the adequate intake level is set 600 IU (15 mcg/day).80 This DRI allowance of vitamin D recommendations were based on the calcium metabolism by vitamin D and effective elimination and/or prevention of rickets. The definition for vitamin D insufficiency or deficiency was pronounced as circulating vitamin D level 52 to 72 nmol/L81 and <50 nmol/L, respectively.51,82–84
The vitamin D toxicity is a matter of concern, but the intoxication caused by vitamin D is very rare, and can caused by ingestion of high dosage deliberately because the vitamin D synthesis resulting from the exposure to sunlight, and those obtained through fortified foods do not sum up to make large amounts of vitamin D.
The major side-effect resulting from vitamin D toxicity is increased calcium deposition in the body leading to hypercalcemia. Mild asymptomatic hypercalcemia has been reported after supplementation of 1,400–4,000 IUs of vitamin D leading to increase in serum vitamin D level between 197 and 255 nmol/L in children.85 Severe hypercalcemia is caused by exposure to large oral vitamin D doses of up to 60,000 IU in infants.86,87 Whereas, in healthy adults in a clinical setting it was found that supplementation even up to 50,000 IUs of vitamin D2 every alternative week (equivalent to approximately 3,300 IU daily) for a period of 6 years, helped in maintaining the 25(OH)D concentrations at a range of 100–150 nmol/L, without promoting any vitamin D toxicity.88
In agreement with other studies, Ekwaru et al.89 reported that 25(OH)D concentration was significantly increased up to 150 nmol/L in Canadian adults oral supplemented with 20,000 IUs of vitamin D3 daily. Several data considered, hypercalcemia from vitamin D toxicity is also rare, but a dangerous state for the organism and should receive adequate and sensible treatment.
CONCLUSION
While considering the importance of vitamin D beyond bone health, further clinical trials are required before making claims about the efficiency of vitamin D in prevention of chronic disease. The upper limit of safe consumption for vitamin D according to the Institute of Medicine in the United States has been set to 4,000 IU.80 Before accepting the failure of vitamin D in preventing or curing any disease, dosage level must be considered. The dosage of vitamin D for studies investigating the effect vitamin D against different chronic diseases should consider the body composition and genetic background of the subjects.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Wu Y, Ding Y, Tanaka Y, Zhang W. Risk factors contributing to type 2 diabetes and recent advances in the treatment and prevention. Int J Med Sci. 2014;11:1185–200. doi: 10.7150/ijms.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Gorman C, Lucas R, Taylor B. Environmental risk factors for multiple sclerosis: a review with a focus on molecular mechanisms. Int J Mol Sci. 2012;13:11718–52. doi: 10.3390/ijms130911718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu S, Song Y, Ford ES, Manson JE, Buring JE, Ridker PM. Dietary calcium, vitamin D, and the prevalence of metabolic syndrome in middle-aged and older U.S. women. Diabetes Care. 2005;28:2926–32. doi: 10.2337/diacare.28.12.2926. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Ajani UA, McGuire LC, Liu S. Concentrations of serum vitamin D and the metabolic syndrome among U.S. adults. Diabetes Care. 2005;28:1228–30. doi: 10.2337/diacare.28.5.1228. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF. Vitamin D: evolutionary, physiological and health perspectives. Curr Drug Targets. 2011;12:4–18. doi: 10.2174/138945011793591635. [DOI] [PubMed] [Google Scholar]
- 7.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–80. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Greene-Finestone LS, Garriguet D, Brooks S, Langlois K, Whiting SJ. Overweight and obesity are associated with lower vitamin D status in Canadian children and adolescents. Paediatr Child Health. 2017;22:438–44. doi: 10.1093/pch/pxx116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saneei P, Salehi-Abargouei A, Esmaillzadeh A. Serum 25-hydroxy vitamin D levels in relation to body mass index: a systematic review and meta-analysis. Obes Rev. 2013;14:393–404. doi: 10.1111/obr.12016. [DOI] [PubMed] [Google Scholar]
- 10.Vimaleswaran KS, Berry DJ, Lu C, Tikkanen E, Pilz S, Hiraki LT, et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts. PLoS Med. 2013;10:e1001383. doi: 10.1371/journal.pmed.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Rodríguez E, Navia B, López-Sobaler AM, Ortega RM. Vitamin D in overweight/obese women and its relationship with dietetic and anthropometric variables. Obesity (Silver Spring) 2009;17:778–82. doi: 10.1038/oby.2008.649. [DOI] [PubMed] [Google Scholar]
- 12.Daniel D, Hardigan P, Bray N, Penzell D, Savu C. The incidence of vitamin D deficiency in the obese: a retrospective chart review. J Community Hosp Intern Med Perspect. 2015;5:26069. doi: 10.3402/jchimp.v5.26069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16:341–9. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 14.Wong KE, Kong J, Zhang W, Szeto FL, Ye H, Deb DK, et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J Biol Chem. 2011;286:33804–10. doi: 10.1074/jbc.M111.257568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML. Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics. 2009;124:e362–70. doi: 10.1542/peds.2009-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK, et al. 1Alpha,25-dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112:122–6. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida Y, Taniguchi H, Baba S. Possible involvement of 1 alpha,25-dihydroxyvitamin D3 in proliferation and differentiation of 3T3-L1 cells. Biochem Biophys Res Commun. 1988;151:1122–7. doi: 10.1016/S0006-291X(88)80482-0. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Hiragun A. Demonstration of 1 alpha,25-dihydroxyvitamin D3 receptor-like molecule in ST 13 and 3T3 L1 preadipocytes and its inhibitory effects on preadipocyte differentiation. J Cell Physiol. 1988;135:545–50. doi: 10.1002/jcp.1041350326. [DOI] [PubMed] [Google Scholar]
- 19.Wood RJ. Vitamin D and adipogenesis: new molecular insights. Nutr Rev. 2008;66:40–6. doi: 10.1111/j.1753-4887.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhuang H, Lin Y, Yang G. Effects of 1,25-dihydroxyvitamin D3 on proliferation and differentiation of porcine preadipocyte in vitro. Chem Biol Interact. 2007;170:114–23. doi: 10.1016/j.cbi.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Veeman MT, Axelrod JD, Moon RT. A second canon: functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/S1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 22.Wodarz A, Nusse R. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol. 1998;14:59–88. doi: 10.1146/annurev.cellbio.14.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Duque G, Macoritto M, Kremer R. 1,25(OH)2D3 inhibits bone marrow adipogenesis in senescence accelerated mice (SAM-P/6) by decreasing the expression of peroxisome proliferator-activated receptor gamma 2 (PPARgamma2) Exp Gerontol. 2004;39:333–8. doi: 10.1016/j.exger.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Basoli V, Santaniello S, Cruciani S, Ginesu GC, Cossu ML, Delitala AP, et al. Melatonin and vitamin D interfere with the adipogenic fate of adipose-derived stem cells. Int J Mol Sci. 2017;18:E981. doi: 10.3390/ijms18050981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang E, Kim Y. Vitamin D decreases adipocyte lipid storage and increases NAD-SIRT1 pathway in 3T3-L1 adipocytes. Nutrition. 2016;32:702–8. doi: 10.1016/j.nut.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Ricciardi CJ, Bae J, Esposito D, Komarnytsky S, Hu P, Chen J, et al. 1,25-Dihydroxyvitamin D3/vitamin D receptor suppresses brown adipocyte differentiation and mitochondrial respiration. Eur J Nutr. 2015;54:1001–12. doi: 10.1007/s00394-014-0778-9. [DOI] [PubMed] [Google Scholar]
- 27.Sakuma S, Fujisawa J, Sumida M, Tanigawa M, Inoda R, Sujihera T, et al. The involvement of mitogen-activated protein kinases in the 1α,25-dihydroxy-cholecalciferol-induced inhibition of adipocyte differentiation in vitro. J Nutr Sci Vitaminol (Tokyo) 2012;58:1–8. doi: 10.3177/jnsv.58.1. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Zemel MB. 1Alpha, 25-dihydroxyvitamin D and corticosteroid regulate adipocyte nuclear vitamin D receptor. Int J Obes (Lond) 2008;32:1305–11. doi: 10.1038/ijo.2008.59. [DOI] [PubMed] [Google Scholar]
- 29.Cianferotti L, Demay MB. VDR-mediated inhibition of DKK1 and SFRP2 suppresses adipogenic differentiation of murine bone marrow stromal cells. J Cell Biochem. 2007;101:80–8. doi: 10.1002/jcb.21151. [DOI] [PubMed] [Google Scholar]
- 30.Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290:E916–24. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Ishizuka T, Miura A, Kajita K, Ishizawa M, Kimura M, et al. Effect of 1 alpha,25-dihydroxy vitamin D3 and vitamin E on insulin-induced glucose uptake in rat adipocytes. Diabetes Res Clin Pract. 2002;55:175–83. doi: 10.1016/S0168-8227(01)00324-2. [DOI] [PubMed] [Google Scholar]
- 32.Shi H, Norman AW, Okamura WH, Sen A, Zemel MB. 1Alpha,25-dihydroxyvitamin D3 inhibits uncoupling protein 2 expression in human adipocytes. FASEB J. 2002;16:1808–10. doi: 10.1096/fj.02-0255fje. [DOI] [PubMed] [Google Scholar]
- 33.Kelly KA, Gimble JM. 1,25-Dihydroxy vitamin D3 inhibits adipocyte differentiation and gene expression in murine bone marrow stromal cell clones and primary cultures. Endocrinology. 1998;139:2622–8. doi: 10.1210/endo.139.5.5970. [DOI] [PubMed] [Google Scholar]
- 34.Raederstorff D. Antioxidant activity of olive polyphenols in humans: a review. Int J Vitam Nutr Res. 2009;79:152–65. doi: 10.1024/0300-9831.79.3.152. [DOI] [PubMed] [Google Scholar]
- 35.Zhu W, Cai D, Wang Y, Lin N, Hu Q, Qi Y, et al. Calcium plus vitamin D3 supplementation facilitated fat loss in overweight and obese college students with very-low calcium consumption: a randomized controlled trial. Nutr J. 2013;12:8. doi: 10.1186/1475-2891-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sergeev IN, Song Q. High vitamin D and calcium intakes reduce diet-induced obesity in mice by increasing adipose tissue apoptosis. Mol Nutr Food Res. 2014;58:1342–8. doi: 10.1002/mnfr.201300503. [DOI] [PubMed] [Google Scholar]
- 37.Ping-Delfos WC, Soares M. Diet induced thermogenesis, fat oxidation and food intake following sequential meals: influence of calcium and vitamin D. Clin Nutr. 2011;30:376–83. doi: 10.1016/j.clnu.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Wong KE, Szeto FL, Zhang W, Ye H, Kong J, Zhang Z, et al. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab. 2009;296:E820–8. doi: 10.1152/ajpendo.90763.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muscogiuri G, Annweiler C, Duval G, Karras S, Tirabassi G, Salvio G, et al. Vitamin D and cardiovascular disease: from atherosclerosis to myocardial infarction and stroke. Int J Cardiol. 2017;230:577–84. doi: 10.1016/j.ijcard.2016.12.053. [DOI] [PubMed] [Google Scholar]
- 40.Oh J, Weng S, Felton SK, Bhandare S, Riek A, Butler B, et al. 1,25(OH)2 vitamin D inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–98. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freedman BI, Wagenknecht LE, Hairston KG, Bowden DW, Carr JJ, Hightower RC, et al. Vitamin D, adiposity, and calcified atherosclerotic plaque in African-Americans. J Clin Endocrinol Metab. 2010;95:1076–83. doi: 10.1210/jc.2009-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang YH, Jin JS, Yi DW, Son SM. Bone morphogenetic protein-7 inhibits vascular calcification induced by high vitamin D in mice. Tohoku J Exp Med. 2010;221:299–307. doi: 10.1620/tjem.221.299. [DOI] [PubMed] [Google Scholar]
- 43.Tang FT, Chen SR, Wu XQ, Wang TQ, Chen JW, Li J, et al. Hypercholesterolemia accelerates vascular calcification induced by excessive vitamin D via oxidative stress. Calcif Tissue Int. 2006;79:326–39. doi: 10.1007/s00223-006-0004-8. [DOI] [PubMed] [Google Scholar]
- 44.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin-angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;89–90:387–92. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Margolis KL, Ray RM, Van Horn L, Manson JE, Allison MA, Black HR, et al. Effect of calcium and vitamin D supplementation on blood pressure: the Women’s Health Initiative Randomized Trial. Hypertension. 2008;52:847–55. doi: 10.1161/HYPERTENSIONAHA.108.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Judd SE, Raiser SN, Kumari M, Tangpricha V. 1,25-Dihydroxyvitamin D3 reduces systolic blood pressure in hypertensive adults: a pilot feasibility study. J Steroid Biochem Mol Biol. 2010;121:445–7. doi: 10.1016/j.jsbmb.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Forman JP, Williams JS, Fisher ND. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension. 2010;55:1283–8. doi: 10.1161/HYPERTENSIONAHA.109.148619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function. III. Effects on physical and morphological properties. Am J Physiol. 1990;258(1 Pt 1):E134–42. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 49.Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. Effect of paricalcitol on left ventricular mass and function in CKD: the OPERA trial. J Am Soc Nephrol. 2014;25:175–86. doi: 10.1681/ASN.2013010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weishaar RE, Simpson RU. The involvement of the endocrine system in regulating cardiovascular function: emphasis on vitamin D3. Endocr Rev. 1989;10:351–65. doi: 10.1210/edrv-10-3-351. [DOI] [PubMed] [Google Scholar]
- 51.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–73. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 52.Faeh D, Gutzwiller F, Bopp M Swiss National Cohort Study Group. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation. 2009;120:495–501. doi: 10.1161/CIRCULATIONAHA.108.819250. [DOI] [PubMed] [Google Scholar]
- 53.Fabsitz R, Feinleib M. Geographic patterns in county mortality rates from cardiovascular diseases. Am J Epidemiol. 1980;111:315–28. doi: 10.1093/oxfordjournals.aje.a112903. [DOI] [PubMed] [Google Scholar]
- 54.Regidor E, Reques L, Giráldez-García C, Miqueleiz E, Santos JM, Martínez D, et al. The association of geographic coordinates with mortality in people with lower and higher education and with mortality inequalities in Spain. PLoS One. 2015;10:e0133765. doi: 10.1371/journal.pone.0133765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr. 2005;94:483–92. doi: 10.1079/BJN20051544. [DOI] [PubMed] [Google Scholar]
- 56.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13(6 Pt 2):954–9. doi: 10.1161/01.HYP.13.6.954. [DOI] [PubMed] [Google Scholar]
- 58.Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, et al. 25-Hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–71. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 59.Kim JK, Park MJ, Song YR, Kim HJ, Kim SG. Vitamin D: a possible modifying factor linking obesity to vascular calcification in hemodialysis patients. Nutr Metab (Lond) 2017;14:27. doi: 10.1186/s12986-017-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Banerjee A, Khemka VK, Roy D, Dhar A, Sinha Roy TK, Biswas A, et al. Role of pro-inflammatory cytokines and vitamin d in probable Alzheimer’s disease with depression. Aging Dis. 2017;8:267–76. doi: 10.14336/AD.2016.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jean G, Vanel T, Terrat JC, Chazot C. Prevention of secondary hyperparathyroidism in hemodialysis patients: the key role of native vitamin D supplementation. Hemodial Int. 2010;14:486–91. doi: 10.1111/j.1542-4758.2010.00472.x. [DOI] [PubMed] [Google Scholar]
- 62.Al-Dujaili EA, Munir N, Iniesta RR. Effect of vitamin D supplementation on cardiovascular disease risk factors and exercise performance in healthy participants: a randomized placebo-controlled preliminary study. Ther Adv Endocrinol Metab. 2016;7:153–65. doi: 10.1177/2042018816653357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harris RA, Pedersen-White J, Guo DH, Stallmann-Jorgensen IS, Keeton D, Huang Y, et al. Vitamin D3 supplementation for 16 weeks improves flow-mediated dilation in overweight African-American adults. Am J Hypertens. 2011;24:557–62. doi: 10.1038/ajh.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, et al. LifeLines Cohort Study investigators. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2:719–29. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schöttker B, Brenner H. Vitamin D as a resilience factor, helpful for survival of potentially fatal conditions: a hypothesis emerging from recent findings of the ESTHER cohort study and the CHANCES consortium. Nutrients. 2015;7:3264–78. doi: 10.3390/nu7053264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ozougwu JC, Obimba KC, Belonwu CD, Unakalamba CB. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. J Physiol Pathophysiol. 2013;4:46–57. doi: 10.5897/JPAP2013.0001. [DOI] [Google Scholar]
- 67.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–8. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reaven G, Abbasi F, McLaughlin T. Obesity, insulin resistance, and cardiovascular disease. Recent Prog Horm Res. 2004;59:207–23. doi: 10.1210/rp.59.1.207. [DOI] [PubMed] [Google Scholar]
- 69.Apperloo EM, Pena MJ, de Zeeuw D, Denig P, Heerspink HJ. Individual variability in response to renin angiotensin aldosterone system inhibition predicts cardiovascular outcome in patients with type 2 diabetes: a primary care cohort study. Diabetes Obes Metab. 2018;20:1377–83. doi: 10.1111/dom.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarafidis PA, Bakris GL. Renin-angiotensin blockade and kidney disease. Lancet. 2008;372:511–2. doi: 10.1016/S0140-6736(08)61212-X. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi H, Tokudome G, Hara Y, Sugano N, Endo S, Suetsugu Y, et al. Insulin resistance is a risk factor for the progression of chronic kidney disease. Clin Nephrol. 2009;71:643–51. doi: 10.5414/CNP71643. [DOI] [PubMed] [Google Scholar]
- 72.Martin T, Campbell RK. Vitamin D and diabetes. Diabetes Spectr. 2011;24:113–8. doi: 10.2337/diaspect.24.2.113. [DOI] [Google Scholar]
- 73.Gagnon C, Daly RM, Carpentier A, Lu ZX, Shore-Lorenti C, Sikaris K, et al. Effects of combined calcium and vitamin D supplementation on insulin secretion, insulin sensitivity and β-cell function in multi-ethnic vitamin D-deficient adults at risk for type 2 diabetes: a pilot randomized, placebo-controlled trial. PLoS One. 2014;9:e109607. doi: 10.1371/journal.pone.0109607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao Y, Wu X, Fu Q, Li Y, Yang T, Tang W. The relationship between serum 25-hydroxy vitamin D and insulin sensitivity and β-cell function in newly diagnosed type 2 diabetes. J Diabetes Res. 2015;2015 doi: 10.1155/2015/636891. 636891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park S, Kim DS, Kang S. Vitamin D deficiency impairs glucose-stimulated insulin secretion and increases insulin resistance by reducing PPAR-γ expression in nonobese Type 2 diabetic rats. J Nutr Biochem. 2016;27:257–65. doi: 10.1016/j.jnutbio.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 76.Taylor AV, Wise PH. Vitamin D replacement in Asians with diabetes may increase insulin resistance. Postgrad Med J. 1998;74:365–6. doi: 10.1136/pgmj.74.872.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwalfenberg G. Vitamin D and diabetes: improvement of glycemic control with vitamin D3 repletion. Can Fam Physician. 2008;54:864–6. [PMC free article] [PubMed] [Google Scholar]
- 78.Pinelli NR, Jaber LA, Brown MB, Herman WH. Serum 25-hydroxy vitamin D and insulin resistance, metabolic syndrome, and glucose intolerance among Arab Americans. Diabetes Care. 2010;33:1373–5. doi: 10.2337/dc09-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.He S, Yu S, Zhou Z, Wang C, Wu Y, Li W. Effect of vitamin D supplementation on fasting plasma glucose, insulin resistance and prevention of type 2 diabetes mellitus in non-diabetics: a systematic review and meta-analysis. Biomed Rep. 2018;8:475–84. doi: 10.3892/br.2018.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington (DC): The National Academies Press; 2010. [PubMed] [Google Scholar]
- 81.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 82.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 83.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/S0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 84.Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 85.Vanstone MB, Oberfield SE, Shader L, Ardeshirpour L, Carpenter TO. Hypercalcemia in children receiving pharmacologic doses of vitamin D. Pediatrics. 2012;129:e1060–3. doi: 10.1542/peds.2011-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cesur Y, Caksen H, Gündem A, Kirimi E, Odabaş D. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J Pediatr Endocrinol Metab. 2003;16:1105–9. doi: 10.1515/JPEM.2003.16.8.1105. [DOI] [PubMed] [Google Scholar]
- 87.Zeghoud F, Ben-Mekhbi H, Djeghri N, Garabédian M. Vitamin D prophylaxis during infancy: comparison of the long-term effects of three intermittent doses (15, 5, or 2.5 mg) on 25-hydroxyvitamin D concentrations. Am J Clin Nutr. 1994;60:393–6. doi: 10.1093/ajcn/60.3.393. [DOI] [PubMed] [Google Scholar]
- 88.Pietras SM, Obayan BK, Cai MH, Holick MF. Vitamin D2 treatment for vitamin D deficiency and insufficiency for up to 6 years. Arch Intern Med. 2009;169:1806–8. doi: 10.1001/archinternmed.2009.361. [DOI] [PubMed] [Google Scholar]
- 89.Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014;9:e111265. doi: 10.1371/journal.pone.0111265. [DOI] [PMC free article] [PubMed] [Google Scholar]