Abstract
Epidemiologic studies suggest an increased risk of lung cancer with exposure to welding fumes, but controlled animal studies are needed to support this association. Oropharyngeal aspiration of collected “aged” gas metal arc–stainless steel (GMA–SS) welding fume has been shown by our laboratory to promote lung tumor formation in vivo using a two-stage initiation–promotion model. Our objective in this study was to determine whether inhalation of freshly generated GMA-SS welding fume also acts as a lung tumor promoter in lung tumor-susceptible mice. Male A/J mice received intraperitoneal (IP) injections of corn oil or the chemical initiator 3-methylcholanthrene (MCA; 10 μg/g) and 1 week later were exposed by whole-body inhalation to air or GMA-SS welding aerosols for 4 h/d × 4 d/w × 9 w at a target concentration of 40 mg/m3. Lung nodules were enumerated at 30 weeks post-initiation. GMA-SS fume significantly promoted lung tumor multiplicity in A/J mice initiated with MCA (16.11 ± 1.18) compared to MCA/air-exposed mice (7.93 ± 0.82). Histopathological analysis found that the increased number of lung nodules in the MCA/GMA-SS group were hyperplasias and adenomas, which was consistent with developing lung tumorigenesis. Metal deposition analysis in the lung revealed a lower deposited dose, approximately fivefold compared to our previous aspiration study, still elicited a significant lung tumorigenic response. In conclusion, this study demonstrates that inhaling GMA-SS welding fume promotes lung tumorigenesis in vivo which is consistent with the epidemiologic studies that show welders may be at an increased risk for lung cancer.
Keywords: A/J mouse, Cancer, Stainless steel, Welding, Inhalation
Introduction
Welding, a process of joining metals, is a common industrial practice worldwide. It is estimated that there are nearly 400,000 full-time welding occupations in the USA alone as of 2014 (Bureau of Labor Statistics 2016–2017). Gas metal arc (GMA) welding is a type of electric arc welding where an electric arc is established between the work piece and a consumable wire electrode. High temperatures create a molten pool into which the electrode is continuously fed and the metals fuse together as they cool. This process utilizes an inert shielding gas to protect the weld from oxidative weakening and vaporizes metals, forming metal oxides which react with air to form welding fumes. The welding fumes are a complex mixture of gases and metal oxides that are derived primarily from the electrode. However, the shielding gas, electrode coating, base metal, and paint or other surface coatings may also contribute to the welding fume composition (Antonini 2003).
GMA-stainless steel (SS) welding fumes are a complex mixture composed primarily of metal-rich particulate matter that contains carcinogenic (hexavalent chromium [Cr VI] and nickel [Ni]) and non-carcinogenic (manganese [Mn] and iron [Fe]) metals. A number of well-documented, harmful effects have been associated with welding fume exposure. The most common acute health effect of welding fume inhalation is metal fume fever, characterized by flulike symptoms, cough, and dyspnea, while the most common chronic health effect is bronchitis (Antonini 2003). Lung cancer from exposure to welding fumes is also an area of concern, yet few controlled animal studies have investigated this association. Several epidemiological studies support the hypothesis that exposure to welding fume increases lung cancer risk (Korczynski 2000; Lauritsen and Hansen 1996; Matrat et al. 2016; Steenland 2002). The need for animal studies is critical, as worker exposure is not always well documented. In addition, workers may be exposed to additional occupational agents or confounders (e.g., smoking) that complicate epidemiological studies (Antonini 2014). The International Agency for Research on Cancer (IARC) advisory group on the Monograph priorities for 2008 listed welding fume as a high-priority agent for further evaluation of lung cancer risk in humans. Welding fume is classified as a group 2B carcinogen (possibly carcinogenic to humans) and will be re-evaluated by an IARC working group in 2017 (IARC 2008).
Previous research in our laboratory has shown that GMA-SS welding fume persists in the lung for 1.5 years and triggers mild, chronic inflammation, but docs not initiate tumor formation in lung tumor-susceptible A/J mice (Zeidler-Erdely et al. 2011). In a two-stage initiation-promotion model of lung tumorigenesis, however, GMA-SS fume was a significant lung tumor promoter in A/J mice exposed via oropharyngeal aspiration (Zeidler-Erdely et al. 2013). Given these results, we aimed to determine whether inhalation of GMA-SS welding fume also promotes lung tumors in this susceptible mouse strain. Inhalation is the preferred route for welding fume-related toxicity studies in animals because it closely simulates the occupational exposure with respect to both particle size and surface properties of the fume (e.g., reactivity) and lung particle deposition.
Material and methods
Animals
Male A/J mice, 4–5 weeks of age, were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in an Association for Assessment and Accreditation of Laboratory Animal Care-Accredited, specific pathogen-free, environmentally controlled facility. All mice were free of endogenous viral pathogens, parasites, mycoplasmas, Helicobacter, and CAR bacillus. Mice were housed in groups of five in ventilated cages and provided high-efficiency particulate filtered air under a controlled light cycle (12 h light/12 h dark) at a standard temperature (22–24 °C) and 30–70% relative humidity. Animals were acclimated to the animal facility for 1 week and allowed access to a conventional diet (6% irradiated NIH-31 Diet, Envigo RMS, Inc., Madison, WI) and tap water ad libitum. All procedures were performed using protocols approved by the National Institute for Occupational Safety and Health (NIOSH) Institutional Animal Care and Use Committee.
Experimental protocols for animal exposure and welding fume generator
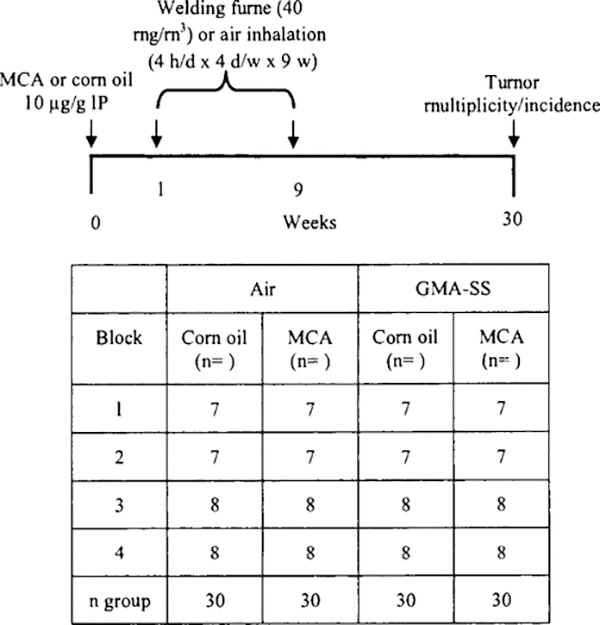
A/J mice, 120 in total, were organized into four groups using a block design for randomization (Fig. 1). On day 1, mice aged 5–6 weeks were intraperitoneally (IP) injected with the chemical initiator, 3-methylcholanthrene (MCA) (Sigma, St. Louis, MO) dissolved in com oil (CO) (Sigma, St. Louis, MO) at a dose of 10 μg/g of body weight or CO alone. MCA was chosen as the initiating agent based on the efficient response of the A/J mouse to this carcinogen in our oropharyngeal aspiration study (Zeidler-Erdely et al. 2013). One week post-initiation, mice were exposed in whole-body inhalation chambers with individual steel mesh cages to aerosols generated during GMA-SS welding or air for 4 h/d and 4 d/w for 9 weeks at a target concentration of 40 mg/m3 (actual 32.3 ± 2.8 mg/m3). The welding wire used was 0.045 in. diameter Lincoln Electric Blue Max MIG 308LSI, and the welding parameters were set to 25 V DC, 300 in. per minute wire feed, 30 L/min of 95% Argon-5% CO2 shielding gas, and a typical welding current of 220 amps.
Fig. 1.
Experimental protocol and block design for two-stage initiation-promotion lung tumorigenesis model in A/J mice. A/J mice, 120 in total, were randomized and separated into four blocks of n = 7 or 8 per exposure group. Mice received intraperitoneal (IP) injections of either MCA or com oil and one week later were exposed to GMA-SS welding fume (40 mg/m3) or air for 4 h/d, 4 d/w, for 9 w. Animal weights were recorded weekly. At 30 weeks, mice were sacrificed for tumor multiplicity and incidence and histopathological studies
The design and construction of the welding fume aerosol generator were previously described (Antonini et al. 2006). This automated robotic welder continuously generated welding fumes by welding beads onto ¼ in. thick plates of mild steel. The resulting fume was carried into a whole-body exposure chamber through a ¾ in. flexible tube by maintaining the chamber at a negative pressure (0.70 in. H20). Particle concentrations within the exposure chamber were continuously monitored with a Data RAM (DR-40000 Thermo Electron Co, Franklin, MA), and gravimetric determinations (37 mm cassettes with 0.45 μ m pore-size Teflon filters) were used to calibrate and verify the Data RAM readings each day. Gas generation, including carbon monoxide (CO), carbon dioxide (CO2), oxygen (O2), and ozone (O3), was continuously monitored. During the welding exposure, O2 levels were maintained above the OSHA minimal acceptable level. O3, CO, and CO2 were below OSHA permissible exposure limits and NIOSH recommended exposure limits (REL) during the entire exposure duration. In the exposure chamber, CO and O3 levels were not significantly higher than background. The exposure system setup was slightly modified from that described previously (Antonini et al. 2006) to reduce the travel time of the particulate fume from the welding torch to the exposure chamber. This was done to ensure delivery of fresher fumes to more closely mimic a worker’s inhalation exposure.
Body weight determination
All mice were weight-matched prior to the inhalation exposure. Mice were weighed weekly throughout the experimental time course and at the 30-week sacrifice.
Whole-lung metal analysis
Male A/J mice were exposed by inhalation to GMA-SS welding aerosols (40 mg/m3) (n = 25) or filtered air (n = 10) for 4 h. Immediately following exposure, whole lungs were excised, trimmed, and lyophilized. The freeze-dried tissue was weighed then acid digested. Inductively coupled argon plasma, atomic emission spectroscopy at NIOSH-Division of Applied Research and Technology (Cincinnati, OH) was used to determine the amount of Cr, Ni, Cu, Fe, and Mn present in the lung according to the draft NIOSH method 8200 modified to accommodate the sample matrix (NIOSH 2003).
Gross lung tumor counts and histopathology
At 30 weeks post-exposure, A/J mice were euthanized with Fatal Plus (100–300 mg/kg IP; 390 mg/ml pentobarbital sodium) (Vortech Pharmaceuticals, Dearborn, MI), weighed, and then the vena cava was cut to exsanguinate the animal. All internal organs were examined for the presence of tumors. The whole lung was then excised. All tumors were clearly defined with no apparent merged tumors. The lungs were inflated and fixed with 10% neutral buffered formalin for 24 h. Tumors were counted and measured 24 h after fixation. Lungs were embedded in paraffin, and then a 5-μm standardized section was cut. Slides were stained with hematoxylin and eosin and interpreted by two separate, contracted, board-certified veterinary pathologists in a blinded fashion for evidence of hyperplasia and neoplasia, inflammation, lymphoid tissue response, and foreign materials by light microscopy. Diagnostic criteria for hyperplastic and neoplastic findings were according to goRENI (http://www.goreni.org/), the standard reference for nomenclature and diagnostic criteria in toxicologic pathology and at the same time the Internet discussion platform for the global initiative “INHAND”—the International Harmonization of Nomenclature and Diagnostic criteria (Keenan et al. 2015; Renne et al. 2009). If abnormal changes were found, severity was scored using the following scale: 1 = minimal, 2 = mild, 3 = moderate, 4 = marked. The final severity score reflects the average of the right and left lung lobe scores and are presented as mean ± standard error. Because bronchiolo-alveolar hyperplasia (BAH) and bronchiolo-alveolar adenomas (BAA) represent a continuum of the proliferative process and there is possible overlap between these diagnoses, the numbers of lesions were combined to compare the tumorigenic potential of each treatment (Renne et al. 2009). However, the gross tumor count at necropsy is more representative of the response because examination of a single histological section per lung underestimates the total number of lesions per lung (Rehm and Ward 1989).
Statistical comparisons and analysis
Statistical analyses were performed using JMP version 12. Factorial analysis of variance (ANOVA) was utilized on continuous variables to make comparisons between the treatment groups. For some variables, a log transformation was performed on the data to reduce heterogeneous variance and meet the assumptions of an ANOVA. Gross tumor counts and histopathology counts from sections were analyzed similarly. Tumor incidence (presence or absence of tumors) was analyzed using a Chi-square test in SAS ‘Proc Freq,’ while tumor multiplicity (number of tumors per lung) was analyzed using Poisson regression in SAS ‘Proc Genmod’. In cases where overdispersion existed, a negative binomial regression was performed. Analyses were performed independently on CO and MCA treated animals, and only utilized data from those animals surviving to the 30-week time point. For all analyses, a p value of <0.05 was set as the criteria for significance.
Results
Welding fume characteristics
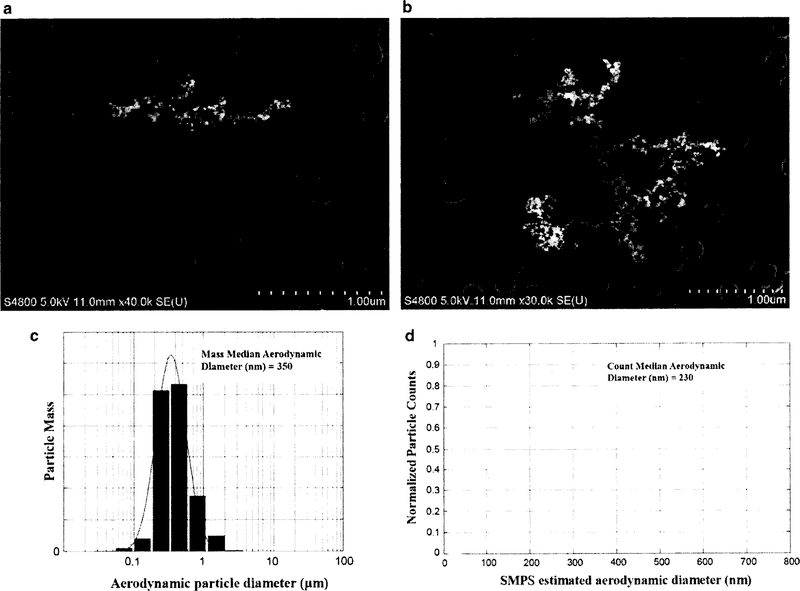
A summary of the characteristics of GMA-SS welding fume and images of the fume are presented in Fig. 2. Particle mass size distribution was measured for this newer configuration using a Micro-Orifice Uniform Deposit Impactor (MOUDI, model 110; MSP corp., Shoreview, Minn.) with additional Nano-MOUDI stages (MSP model 115). The mass median aerodynamic diameter (MMAD) was 350 nm. The count-based particle size distributions of the particles were measured using a Scanning Mobility Particle Sizer (SMPS model 3936, TSI Inc, Shoreview, MN). The SMPS estimated the count median aerodynamic diameter to be 230 nm. Particle imaging was achieved with a scanning electron microscope (SEM; JEOL 6400, JEOL Inc). Inspection of physical characteristics of the particles showed many small nano-spheres (10–50 nm) linked together into long chain-like structures often with several branches. Elemental analysis of the welding particles was previously carried out (Zeidler-Erdely et al. 2013). The overall particulate fume was composed of 57% Fe, 20.2% Cr, 13.8% Mn, 8.8% Ni, 0.2% copper (Cu) and trace amounts of silicon (Si), aluminum (Al), and vanadium (V). Hexavalent chromium (Cr(VI)) levels were also measured and determined to be 2929 ppm. GMA-SS welding fume is largely water insoluble with a soluble/insoluble ratio of 0.006 (Antonini et al. 1999).
Fig. 2.
GMA-SS welding fume characteristics, a, b are SEM images depicting small nano-spheres (10–50 nm) linked together in long chain-like structures often with several branches. The bottom panels show the particle size distribution of the generated welding fume in terms of the mass median aerodynamic diameter (MMAD) (c) and estimated count median aerodynamic diameter (d)
Lung metal deposition after GMA-SS welding fume inhalation
Table 1 shows the lung metal deposition in A/J mice after 4 h of inhalation of GMA-SS welding fume. The most abundant metal measured in the lung was Fe followed by Cr, Mn, Ni, and Cu, as predicted from the previous characterization of this fume (Antonini et al. 2006). The metal analysis by wt% of the whole lungs (60% Fe, 17% Cr, 14% Mn, 8% Ni, and 0.8% Cu) agrees with the wt% of collected fume.
Table 1.
Lung metal deposition in A/J mice after stainless steel welding fume inhalation for 4 h at a target concentration of 40 mg/m3
| Exposure | Cr (μg/lung) | Cu (μg/lung) | Fe (μg/lung) | Mn (μg/lung) | Ni (μg/lung) |
|---|---|---|---|---|---|
| Air | 0.02 ± 0.01 | 0.22 ± 0.03 | 8.97 ± 0.36 | 0.02 ± 0.00 | 0.01 ± 0.00 |
| GMA-SS | 1.65 ± 0.05 | 0.32 ± 0.01 | 15.12 ± 0.39 | 1.48 ± 0.04 | 0.82 ± 0.02 |
Freeze-dried whole-lung tissue was analyzed for aluminum (Al), chromium (Cr), copper (Cu), iron (Fe), manganese (Mn), nickel (Ni), titanium (Ti), and zinc (Zn) by Inductively Coupled Plasma-Atomic Emission Spectroscopy. Samples were prepared according to draft NIOSH Analytical Method 8200 for bulk tissue samples. Trace amounts of Al, Ti, and Zn were found. In cases in which no result was measured, the limit of quantification (LOQ) was used in calculating the average deposition. Values are mean ± standard error of the mean (n = 10 air; n = 25 GMA-SS); GMA-SS—gas metal arc-stainless steel welding fume
The analysis of the metals shows a cumulative increase of ~10.1 μg of total GMA-SS fume deposited in the lung from a single 4-h exposure. The alveolar deposition in the mice was equated to the human by the equations below using the previous threshold limit value-time weighted average (TLV-TWA) of 5 mg/m3 for total welding fume and the PEL of 5 μg/m3 for Cr(VI). Previously, we estimated that 70% of the total dose reached the alveolar space (10.1 μg/d × 0.70 = 7.07 μg/d) (Erdely et al. 2011a; Raabe et al. 1988). The mice were exposed for 36 days (9 w at 4 d/w) for an approximate total alveolar deposition of 254.5 μg.
Factored for human dose using previous welding fume TLV of 5 mg/m3
Fume concentration × min volume × exposure duration × deposition efficiency) = deposited human dose 5 mg/m3 × (20 L/min)(10−3 m3/L) × (8 h/day) (60 min/h) × 0.16 = 7.7 mg deposited per 8 h day in humans Human equivalent dose to mouse by alveolar surface area (SA) (Stone et al. 1992)
Factored for human dose using Cr(VI) PEL of 5 μg/m3
Cr(VI) concentration × min volume × exposure duration × deposition efficiency) = deposited human dose 5 μg/m3 × (20 L/min)(10−3 m3/L) × (8 h/day) (60 min/h) × 0.16 = 7.7 μg deposited per 8 h day in humans In the GMA-SS fume, Cr(VI) was 0.29% of the total welding fume (Keane et al. 2009; Zeidler-Erdely et al. 2013). Using our alveolar deposition dose of 7.07 μg roughly 0.0205 μg (7.07 × 0.0029) would be Cr(VI). Human equivalent dose to mouse by SA:
Morbidity and mortality
A timeline of the experimental protocol for the two-stage (initiation-promotion) carcinogenesis model is shown in Fig. 1. Initial body weights at week 0 [mean ± standard error (SE)] were 18.56 ± 0.35, 19.1 ± 0.39, 18.25 ± 0.31, and 19.05 ± 0.36 for the CO/air, CO/GMA-SS, MCA/air, and MCA/GMA-SS groups, respectively. Body weights increased steadily and were not changed due to exposure from week 0 to 30 with 10.26 ± 0.58, 9.46 ± 0.56, 11.38 ± 0.65, 9.78 ± 0.61 for the CO/air, CO/GMA-SS, MCA/air, and MCA/GMA-SS groups, respectively. Morbidity and mortality throughout the study were low (~ 5%), and no abnormalities, such as other tumor types besides lung, were found at the terminal sacrifice at 30 weeks. In total, six mice died during the course of the study and were not included in the final analysis of the data. Necropsy determined that all six mice died from morbidities that included enlarged heart or cause of death otherwise undetermined.
Gross tumor multiplicity and incidence
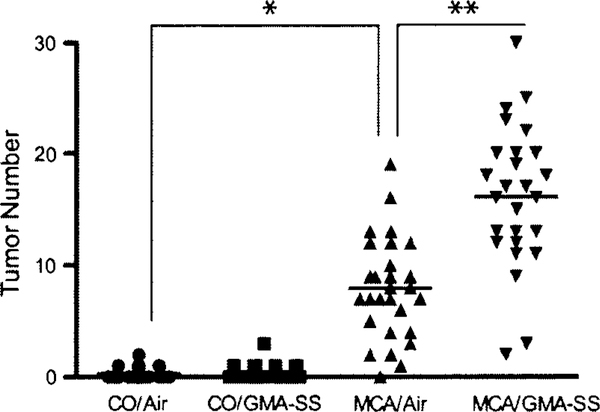
GMA-SS welding fume significantly promoted lung tumors in the A/J mouse 30 weeks after initiation with MCA. The grossly observed tumor multiplicity (average tumor number/mouse lung ± SE) for all groups is shown in Fig. 3. There was no effect of welding fume alone on tumor multiplicity (CO/air, 0.32 ± 0.10; CO/GMA-SS, 0.45 ± 0.13; p = 0.44). In animals initiated with MCA, tumor multiplicity was 7.93 ± 0.82 and 16.11 ± 1.18 for air and GMA-SS, respectively (p < 0.0001). Average tumor incidence (% of tumor-bearing mice) was 29% in CO/air and 38% in CO/GMA-SS-exposed animals. Reports in the literature indicate the background tumor frequency in A/J mice between 43 and 53 weeks to be 31–40% (Curtin et al. 2004; Groch et al. 1997). The mice in this study were 35 or 36 weeks old upon sacrifice, indicating the observed tumor incidence is consistent with the literature. As expected, tumor incidence was >96% in all MCA-initiated groups (n = 29 for MCA/air and n = 28 for MCA/GMA-SS groups) which confirmed successful experimental administration as well as its carcinogenic effectiveness in A/J mice. Total and average tumor number per treatment group across each of the individual lung regions is described in Table 2. MCA/GMA-SS-exposed mice had significantly greater lung tumor multiplicity in every lung region compared to MCA/air (p < 0.009). There was no difference between CO/air and CO/GMA-SS groups (p = 0.44).
Fig. 3.
Lung tumor multiplicity upon gross examination in A/J mice promoted with air or GMA-SS welding fume. At 30 weeks, MCA initiation followed by GMA-SS welding fume exposure increased lung tumor multiplicity (average tumor number/mouse lung) significantly above MCA/air-exposed animals (7.93 ± 0.82 and 16.11 ± 1.18, respectively). *p < 0.0001—compared to CO/air, **p < 0.0001—compared to MCA/air
Table 2.
Total and average (in parenthesis) tumor number across individual lung lobes following GMA-SS welding fume inhalation exposure 30 weeks post-initiation with MCA or corn oil
| n | Left | Apical | Cardiac | Diaphragmatic | Azygos | |
|---|---|---|---|---|---|---|
| Corn oil/air | 28 | 3(0.11 ±0.06) | 3(0.11 ±0.06) | 0 | 3 (0.11 ±0.06) | 0 |
| Corn oil/GMA-SS | 29 | 5 (0.17 ±0.07) | 1 (0.03 ± 0.03) | 0 | 5 (0.17 ±0.09) | 2 (0.07 ± 0.05) |
| MCA/air | 29 | 78 (2.69 ± 0.39)* | 30(1.03 ±0.20)* | 25 (0.86 ±0.15)* | 67 (2.31 ±0.36)* | 30(1.03 ±0.25)* |
| MCA/GMA-SS | 28 | 150 (5.35 ± 0.54)** | 68 (2.43 ±0.42)** | 63 (2.25 ±0.35)** | 110(3.93 ±0.37)** | 60 (2.14 ±0.32)** |
GMA-SS gas metal arc-stainless steel, MCA 3-methylcholanthrene
p < 0.0001—compared to Corn oil/air
p < 0.009—compared to MCA/air
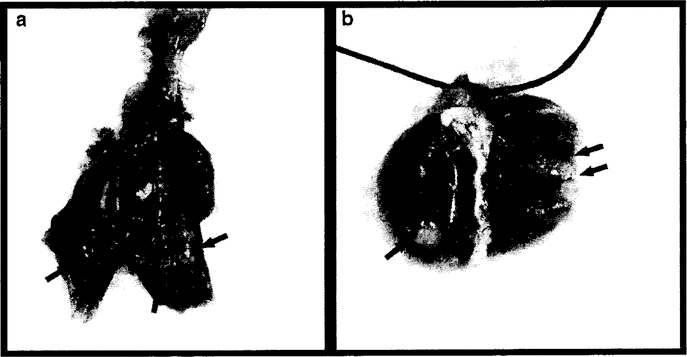
Gross lung morphology from a GMA-SS-exposed mouse initiated with MCA is shown in Fig. 4. Welding fume deposition was visible in exposed mouse lungs and appeared black-brown in color. Tumors appeared white in color and opaque on initial gross exam and became more well-defined after fixation which aided enumeration. At 30 weeks, tumors were between −0.5 mm and ~3 mm, with most tumors ~1 mm.
Fig. 4.
Gross images of lung tumors promoted by GMA-SS welding fume 30 weeks after initiation with MCA. a Shows lung tumor morphology before fixation and b represents tumors 24 h post-fixation. Asterisks indicate areas of welding fume deposition and arrows indicate lung tumors. Most tumors were ~1 mm in diameter
Histopathological evaluation of lung lesions, inflammation, and welding fume presence
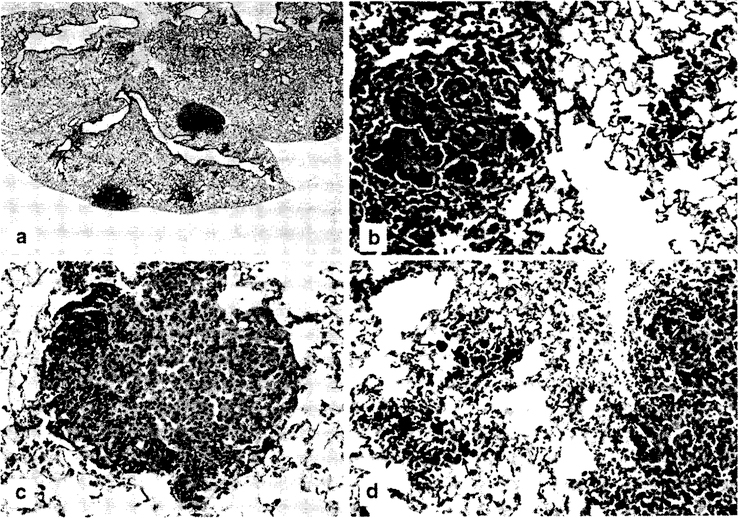
CO/air animals had no lymphoid infiltrate or foreign material (brown-black pigment, i.e., welding fume) and hyperplasia was unremarkable (0.09 ± 0.05). There were 5 total lesions reported in the CO/air group. The CO/GMA-SS-exposed group had minimal, but significant, lymphoid infiltrates (0.22 ± 0.063; p < 0.03) and foreign material (1.86 ± 0.07; p < 0.03) compared to CO/air. There were 2 total hyperplastic lesions reported in this group. Welding fume, indicated by black-brown foreign material presence in the lungs, was found in both GMA-SS-exposed groups (CO and MCA) in every lung section. Significant hyperplasia (1.20 ± 0.11; p < 0.03) and increased total preneoplastic/neoplastic lesions (BAH and BAA) were reported in the MCA/GMA-SS animals compared to MCA/air animals (114 versus 70; p < 0.03). Histopathological assessment of the lungs from a separate, second board-certified veterinary pathologist confirmed these findings (Table 3). The total number of proliferative lesions for MCA/GMA-SS-exposed mice was 153 compared to 90 for MCA/air. Increased perivascular mononuclear infiltrate in the MCA/GMA-SS group consisting of small aggregates or cuffs of lymphocytes, sometimes mixed with a few plasma cells, adjacent to or around multiple scattered vessels was also reported. This infiltrate was not observed in any of the MCA/air lungs. Because there were no significant findings in the CO groups besides evidence of welding fume and minimal lymphoid infiltrates in GMA-SS-exposed mice, a second evaluation was not deemed necessary. Figure 5a demonstrates a BAA and two areas of BAH. Figure 5b shows the adenoma in panel a at 20× magnification adjacent to areas of welding fume deposition. Panels c and d demonstrate a BAA at 40× and BAH at 20×, respectively. The BAA were more compact nodules composed of enlarged type II cells that obscured the normal tissue morphology. These benign neoplasms had a smooth margin that frequently caused compression of surrounding tissue. The cells sometimes had prominent nucleoli and frequently formed radiating proliferations around blood vessels. BAH were round to irregular in shape with increased numbers of Type II cells lining alveolar septae. The cells were of normal size and normal tissue architecture was retained. The margins of these lesions were irregular and did not cause compression of surrounding tissues. In addition, the number of adenomas in the MCA/GMA-SS group was significantly greater than in the MCA/air group (p < 0.05; Table 3) (McConnell et al. 1986).
Table 3.
Severity scores for abnormal morphological findings and numbers of adenomas and hyperplastic lesions observed in lung sections of A/J mice exposed to GMA-SS welding fume by inhalation at 30 weeks post-initiation with MCA
| Lymphoid Infiltrates* | Foreign material* | Hyperplasia Severity* | Hyperplasia | Adenoma | Total lesions | |
|---|---|---|---|---|---|---|
| MCA/air | – | 0.03 ± 0.03 | 1.41 ± 0.19 | 70 | 20 | 90 |
| MCA/GMA-SS | 0.34 ± 0.07** | 1.5 ± 0.08** | 1.86 ±0.19 | 119‡ | 34^ | 153‡ |
GMA-SS gas metal arc-stainless steel, MCA 3-methylcholanthrene
Severity scores are the averages of the left and right lung lobes and are presented as mean ± standard error. Lymphoid infiltrates represents perivascular and peribronchiolar mononuclear cells. Foreign material refers to the presence of brown pigment in the lungs. Severity was scored as 1 = minimal, 2 = mild, 3 = moderate, 4 = marked
– Indicates no findings
p < 0.0002—compared to MCA/air
p < 0.004—compared to MCA/air
p < 0.05—compared to MCA/air
Fig. 5.
Photomicrographs of lung tissue from MCA/GMA-SS exposed mice, a A bronchiolo-alveolar adenoma and two areas of bronchiolo-alveolar hyperplasia in the lung at 2× magnification, b Demonstrates the adenoma in a at 20× magnification, c Shows a bronchiolo-alveolar adenoma at 40× magnification. Cells are enlarged and forming a compact mass that obscures normal architecture and causes compression of adjacent tissue, d Shows a bronchiolo-alveolar hyperplasia in the lung at 20× magnification. Arrows depict brown pigment (welding fume particles) within alveolar macrophages. H&E stain
Discussion
This study was the first to find that inhalation of GMA-SS welding fume can promote lung tumorigenesis in vivo. Tumor multiplicity increased twofold after initiation with the chemical initiator MCA. Histopathology analysis confirmed the gross findings and showed a significant increase in lung adenomas and combined adenomas and hyperplastic lesions in welding fume-exposed animals. In addition, welding fume exposure increased inflammatory infiltrates. Interestingly, the tumorigenic potential of the inhaled welding fume was achieved at a significantly lower total-deposited dose compared to our previous oropharyngeal aspiration exposure in mice (Zeidler-Erdely et al. 2013). The results of this study further support the epidemiological findings of an association between lung cancer and welding.
The measured lung deposition following welding fume exposure in this study after a single (4 h) exposure was 10.1 μg, or a cumulative dose of 254.5 μg in the lung alveolar region (see calculation in results). This dose represents approximately two times the former TLV-TWA of 5 mg/m3 for 8 h/d, a level commonly exceeded in the workplace (Korczynski 2000). This exposure in terms of the TLV is equivalent to 14 weeks (36 days of exposure × 2 = 72 days) of constant exposure to GMA-SS fume for 8 h/d. If a welder was exposed to GMA-SS fume for 5% (½0) of their working time at the maximum concentration, then the exposure would be 280 weeks (14 weeks × 20), or 5.6 years. Therefore, the deposited dose in our study is occupationally relevant because epidemiologic research has demonstrated a 70% increase in the risk of lung cancer among workers who welded for at least 5% or more of their working time (Matrat et al. 2016).
Our laboratory previously demonstrated that at 78 weeks post-oropharyngeal aspiration, lung tumor incidence in A/J mice approached significance (p = 0.057; n = 16) and a trend for increased tumor multiplicity was found after exposure to GMA-SS welding PM alone (Zeidler-Erdely et al. 2008). In a follow-up study, GMA-SS welding fume delivered via oropharyngeal aspiration significantly increased tumor multiplicity at 30 weeks post-initiation using a two-stage (initiation-promotion) model with the chemical initiator MCA (Zeidler-Erdely et al. 2013). These preliminary studies suggest that GMA-SS fume may be a weak carcinogen in A/J mice; however, oropharyngeal aspiration is considered to be less relevant to a real-world exposure because it delivers a bolus exposure to the lung, potentially overestimating the hazard. Inhalation studies are an important next step as they closely resemble the occupational route of exposure in welders and avoid this potential bolus effect. Inflammation is well known to be a hallmark of cancer, and epidemiologic studies have indicated nearly a quarter of human cancers are associated with inflammation (Colotta et al. 2009; Punturieri et al. 2009). Our laboratory demonstrated that inhalation and oropharyngeal aspiration induce different inflammatory responses in A/J mice, suggesting these exposure methods may also differ in their ability to promote lung tumor formation (Zeidler-Erdely et al. 2008, 2011). Inhalation of GMA-SS welding fume causes a delayed rise in PMN compared to aspiration and also induces a more complex cytokine profile. A potential explanation for the differing inflammatory responses between oropharyngeal and inhalation exposure is the dose rate at which fume is deposited (Baisch et al. 2014; Bonner et al. 2013). Former studies have indicated that the dose rate is an important determinant of the acute inflammatory response in the respiratory tract. Baisch et al. demonstrated a higher inflammatory response in F-344 rats following intratracheal instillation of TiO2 compared with an equivalent dose delivered via inhalation. Our former aspiration protocol delivered five bolus doses of 340 μg or 680 μg (1.7 or 3.4 mg cumulative) of GMA-SS welding PM once a week for 5 weeks (Zeidler-Erdely et al. 2013). In this study, inhalation exposure deposited ~10 μg per day (or 360 μg estimated total) over 36 days. Despite this much lower calculated total lung burden and dose rate, we found a significant lung tumor promotion for inhalation of welding fume as was also observed with high dose rate delivery oropharyngeal aspiration in our previous study. The objective of the present study was to identify if GMA-SS fume at a reasonable exposure level is a tumor promoter. Limitations must be noted when comparing lung tumor multiplicity rates from this and our previous study published in 2013. Indeed, the present study reports results for only a single total inhaled dose, limiting its usefulness in determining thresholds and dose-response effects. Also, the particle deposition patterns may differ (e.g., upper airways) between the inhalation and oropharyngeal aspiration exposure routes.
Interestingly, “fresh” GMA-SS welding fume as delivered via inhalation is more reactive than “aged” welding fume which is used for oropharyngeal aspiration studies (Antonini et al. 1998). Aged fume has shown to be less inflammatory because it generates significantly less reactive oxygen species than freshly generated welding fume that is used for inhalation studies or generated in the workplace. As such, welding fume is 6–9 times more potent when delivered by inhalation rather than oropharyngeal aspiration (Erdely et al. 2011b; Zeidler-Erdely et al. 2008, 2011). Although the metals are deposited in a lower amount with inhalation, their increased potency may make them more available for DNA damaging effects. Genomic mutations and instability are known enablers of carcinogenesis (Hanahan and Weinberg 2011). Cancer cells often increase their rates of mutation in order to acquire the hallmarks of inducing angiogenesis, resisting cell death, sustaining proliferative signaling, enabling replicative immortality, evading growth suppressors, and activating invasion and metastasis. The heightened free radicals and oxidative stress that occurs with welding fume inhalation exposure independent of dosing rate may also potentially contribute to tumorigenicity (Valavanidis et al. 2013). Therefore, it is likely that the amount of carcinogenic metals in the welding fume may not be the only factor contributing to the formation of lung cancer.
At 30 weeks post-initiation, adenomas and proliferative bronchiolo-alveolar epithelial lesions were the most commonly observed microscopic lung pathologies. This finding is consistent with the literature and our previous observations in A/J mice of this age (Gunning et al. 1991; Zeidler-Erdely et al. 2008). In humans, lung cancers are more diverse than in mice and adenocarcinoma is the most common diagnosis. The adenomas in A/J mice are relevant to the production of adenocarcinomas in humans as these lung adenomas are often the direct precursor to lung adenocarcinomas. Likewise, human and A/J mouse tumors both often arise in the context of atypical hyperplasia in the periphery of the lung (Belinsky et al. 1992; Foley et al. 1991; Westra 2000; Westra et al. 1996). Thus, the A/J mouse is a very useful and relevant model to study welding fume toxicity and lung tumorigenesis.
In conclusion, the current research suggests that SS welding fume may serve as a promoter of chemically initiated lung tumors in the A/J mouse model. Future studies will be directed at investigating additional types of welding fumes. Nearly 90% of welding processes use mild steel (MS), while 10% or less use SS (Bureau of Labor Statistics 2016–2017). However, SS welding is still widely utilized as it offers increased protection from corrosion and rusting to which other metals are susceptible (Keane et al. 2009). Unlike SS electrodes, MS electrodes are comprised mainly of the non-carcinogenic metals Fe and various concentrations of Mn. Interestingly, both MS and SS fumes have been linked epidemiologically to lung cancer in welders. Future studies will focus on MS inhalation using a two-stage initiation-promotion model in A/J mice as reported here.
Footnotes
Compliance with ethical standards
Conflict of interest The authors declare that they have no conflict of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
Human and animal rights All animal studies have been approved by the NIOSH Animal Care and Use Committee (ACUC) and all applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
References
- Antonini JM (2003) Health effects of welding. Crit Rev Toxicol 33:61–103. doi: 10.1080/713611032 [DOI] [PubMed] [Google Scholar]
- Antonini JM (2014) Health effects associated with welding, pp 49–70 doi: 10.1016/6978-0-08-096532-1.00807-4 [DOI]
- Antonini JM, Clarke RW, Krishna Murthy GG, Sreekanthan P, Jenkins N, Eagar TW, Brain JD (1998) Freshly generated stainless steel welding fume induces greater lung inflammation in rats as compared to aged fume. Toxicol Lett 98:77–86 [DOI] [PubMed] [Google Scholar]
- Antonini JM, Lawryk NJ, Murthy GG, Brain JD (1999) Effect of welding fume solubility on lung macrophage viability and function in vitro. J Toxicol Environ Health Part A 58:343–363 [DOI] [PubMed] [Google Scholar]
- Antonini JM et al. (2006) Design, construction, and characterization of a novel robotic welding fume generator and inhalation exposure system for laboratory animals. J Occup Environ Hyg 3:194–203. doi: 10.1080/15459620600584352 (quiz D145) [DOI] [PubMed] [Google Scholar]
- Baisch BL, Corson NM, Wade-Mercer P, Gelein R, Kennell AJ, Oberdorster G, Elder A (2014) Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: the effect of dose rate on acute respiratory tract inflammation. Part Fibre Toxicol 11:5. doi: 10.1186/1743-8977-11-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky SA, Devereux TR, Foley JF, Maronpot RR, Anderson MW (1992) Role of the alveolar type II cell in the development and progression of pulmonary tumors induced by 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone in the A/J mouse. Cancer Res 52:3164–3173 [PubMed] [Google Scholar]
- Bonner JC et al. (2013) Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health Perspect 121:676–682. doi: 10.1289/ehp.1205693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau of Labor Statistics USDoL (2016–2017) Welders, cutters, solderers, and brazers. http://www.bls.gov/ooh/produetion/welders-cutters-solderers-and-brazers.htm
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A (2009) Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 30:1073–1081. doi: 10.1093/carcin/bgp127 [DOI] [PubMed] [Google Scholar]
- Curtin GM, Higuchi MA, Ayres PH, Swauger JE, Mosberg AT (2004) Lung tumorigenicity in AJ and rasH2 transgenic mice following mainstream tobacco smoke inhalation. Toxicol Sci 81:26–34. doi: 10.1093/toxsci/kfh175 [DOI] [PubMed] [Google Scholar]
- Erdely A et al. (2011a) Inhalation exposure of gas-metal arc stainless steel welding fume increased atherosclerotic lesions in apolipo-protein E knockout mice. Toxicol Lett 204:12–16. doi: 10.1016/j.toxlet.2011.03.030 [DOI] [PubMed] [Google Scholar]
- Erdely A, Salmen-Muniz R, Liston A, Hulderman T, Zeidler-Erdely PC, Antonini JM, Simeonova PP (2011b) Relationship between pulmonary and systemic markers of exposure to multiple types of welding particulate matter. Toxicology 287:153–159. doi: 10.1016/j.tox.2011.06.008 [DOI] [PubMed] [Google Scholar]
- Foley JF, Anderson MW, Stoner GD, Gaul BW, Hardisty JF, Maronpot RR (1991) Proliferative lesions of the mouse lung: progression studies in strain A mice. Exp Lung Res 17:157–168 [DOI] [PubMed] [Google Scholar]
- Groch KM, Khan MA, Brooks AL, Saffer JD (1997) Lung cancer response following inhaled radon in the A/J and C57BL/6 J mouse. Int J Radiat Biol 71:301–308 [DOI] [PubMed] [Google Scholar]
- Gunning WT, Castonguay A, Goldblatt PJ, Stoner GD (1991) Strain A/J mouse lung adenoma growth patterns vary when induced by different carcinogens. Toxicol Pathol 19:168–175 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. doi: 10.1016/j.ccll.2011.02.013 [DOI] [PubMed] [Google Scholar]
- IARC (2008) Report of the advisory group to recommend priorities for IARC Monographs during 2010–2014, 08/001 edn
- Keane M, Stone S, Chen B, Slaven J, Schwegler-Berry D, Antonini J (2009) Hexavalent chromium content in stainless steel welding fumes is dependent on the welding process and shield gas type. J Environ Monit JEM 11:418–124. doi: 10.1039/b814063d [DOI] [PubMed] [Google Scholar]
- Keenan CM et al. (2015) International harmonization of nomenclature and diagnostic criteria (INHAND): progress to date and future plans. Toxicol Pathol 43:730–732. doi: 10.1177/0192623314560031 [DOI] [PubMed] [Google Scholar]
- Korczynski RE (2000) Occupational health concerns in the welding industry. Appl Occup Environ Hyg 15:936–945. doi: 10.1080/104732200750051175 [DOI] [PubMed] [Google Scholar]
- Lauritsen JM, Hansen KS (1996) Lung cancer mortality in stainless steel and mild steel welders: a nested case-referent study. Am J Ind Med 30:383–391. doi: [DOI] [PubMed] [Google Scholar]
- Matrat M et al. (2016) Welding, a risk factor of lung cancer: the ICARE study. Occup Environ Med 73:254–261. doi: 10.1136/oemed-2015-102964 [DOI] [PubMed] [Google Scholar]
- McConnell EE, Solleveld HA, Swenberg JA, Boorman GA (1986) Guidelines for combining neoplasms for evaluation of rodent carcinogenesis studies. J Natl Cancer Inst 76:283–289 [PubMed] [Google Scholar]
- Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM (2009) Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst 101:554–559. doi: 10.1093/jnci/djp023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raabe OG, Al-Bayati MA, Teague SV, Rasolt A (1988) Regional deposition of inhaled monodisperse coarse and fine aerosol particles in small laboratory animals. Ann Occup Hyg 32:53–63 [Google Scholar]
- Rehm S, Ward JM (1989) Quantitative analysis of alveolar type II cell tumors in mice by whole lung serial and step sections. Toxicol Pathol 17:737–742 [DOI] [PubMed] [Google Scholar]
- Renne R et al. (2009) Proliferative and nonproliferative lesions of the rat and mouse respiratory tract. Toxicol Pathol 37:5s–73s. doi: 10.1177/0192623309353423 [DOI] [PubMed] [Google Scholar]
- Steenland K (2002) Ten-year update on mortality among mild-steel welders. Scand J Work Environ Health 28:163–167 [DOI] [PubMed] [Google Scholar]
- Stone KC, Mercer RR, Gehr P, Stockstill B, Crapo JD (1992) Allometric relationships of cell numbers and size in the mammalian lung. Am J Respir Cell Mol Biol 6:235–243. doi: 10.1165/ajrcmb/6.2.235 [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis K, Loridas S (2013) Pulmonary oxidative stress, inflammation and cancer: respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int J Environ Res Public Health 10:3886–3907. doi: 10.3390/ijerph10093886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra WH (2000) Early glandular neoplasia of the lung. Respir Res 1:163–169. doi: 10.1186/rr28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westra WH, Baas IO, Hruban RH, Askin FB, Wilson K, Offerhaus GJ, Slebos RJ (1996) K-ras oncogene activation in atypical alveolar hyperplasias of the human lung. Cancer Res 56:2224–2228 [PubMed] [Google Scholar]
- Zeidler-Erdely PC et al. (2008) Pulmonary inflammation and tumor induction in lung tumor susceptible A/J and resistant C57BL/6 J mice exposed to welding fume. Part Fibre Toxicol 5:12. doi: 10.1186/1743-8977-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler-Erdely PC et al. (2011) Short-term inhalation of stainless steel welding fume causes sustained lung toxicity but no tumorigenesis in lung tumor susceptible A/J mice. Inhal Toxicol 23:112–120. doi: 10.3109/08958378.2010.548838 [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Meighan TG, Erdely A, Battelli LA, Kashon ML, Keane M, Antonini JM (2013) Lung tumor promotion by chromium-containing welding particulate matter in a mouse model. Part Fibre Toxicol 10:45. doi: 10.1186/1743-8977-10-45 [DOI] [PMC free article] [PubMed] [Google Scholar]