Abstract
Background
Brain radionecrosis (tissue death caused by radiation) can occur following high‐dose radiotherapy to brain tissue and can have a significant impact on a person's quality of life (QoL) and function. The underlying pathophysiological mechanism remains unclear for this condition, which makes establishing effective treatments challenging.
Objectives
To assess the effectiveness of interventions used for the treatment of brain radionecrosis in adults over 18 years old.
Search methods
In October 2017, we searched the Cochrane Register of Controlled Trials (CENTRAL), MEDLINE, Embase and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) for eligible studies. We also searched unpublished data through Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials for ongoing trials and handsearched relevant conference material.
Selection criteria
We included randomised controlled trials (RCTs) of any intervention directed to treat brain radionecrosis in adults over 18 years old previously treated with radiation therapy to the brain. We anticipated a limited number of RCTs, so we also planned to include all comparative prospective intervention trials and quasi‐randomised trials of interventions for brain radionecrosis in adults as long as these studies had a comparison group that reflects the standard of care (i.e. placebo or corticosteroids). Selection bias was likely to be an issue in all the included non‐randomised studies therefore results are interpreted with caution.
Data collection and analysis
Two review authors (CC, PB) independently extracted data from selected studies and completed a 'Risk of bias' assessment. For dichotomous outcomes, the odds ratio (OR) for the outcome of interest was reported. For continuous outcomes, treatment effect was reported as mean difference (MD) between treatment arms with 95% confidence intervals (CIs).
Main results
Two RCTs and one prospective non‐randomised study evaluating pharmacological interventions met the inclusion criteria for this review. As each study evaluated a different drug or intervention using different endpoints, a meta‐analysis was not possible. There were no trials of non‐pharmacological interventions that met the inclusion criteria.
A very small randomised, double‐blind, placebo‐controlled trial of bevacizumab versus placebo reported that 100% (7/7) of participants on bevacizumab had reduction in brain oedema by at least 25% and reduction in post‐gadolinium enhancement, whereas all those receiving placebo had clinical or radiological worsening or both. This was an encouraging finding but due to the small sample size we did not report a relative effect. The authors also failed to provide adequate details regarding the randomisation and blinding procedures Therefore, the certainty of this evidence is low and a larger RCT adhering to reporting standards is needed.
An open‐label RCT demonstrated a greater reduction in brain oedema (T2 hyperintensity) in the edaravone plus corticosteroid group than in the corticosteroid alone group (MD was 3.03 (95% CI 0.14 to 5.92; low‐certainty evidence due to high risk of bias and imprecision); although the result approached borderline significance, there was no evidence of any important difference in the reduction in post‐gadolinium enhancement between arms (MD = 0.47, 95% CI ‐ 0.80 to 1.74; low‐certainty evidence due to high risk of bias and imprecision).
In the RCT of bevacizumab versus placebo, all seven participants receiving bevacizumab were reported to have neurological improvement, whereas five of seven participants on placebo had neurological worsening (very low‐certainty evidence due to small sample size and concerns over validity of analyses). While no adverse events were noted with placebo, three severe adverse events were noted with bevacizumab, which included aspiration pneumonia, pulmonary embolus and superior sagittal sinus thrombosis. In the RCT of corticosteroids with or without edaravone, the participants who received the combination treatment were noted to have significantly greater clinical improvement than corticosteroids alone based on LENT/SOMA scale (OR = 2.51, 95% CI 1.26 to 5.01; low‐certainty evidence due to open‐label design). No differences in treatment toxicities were observed between arms.
One included prospective non‐randomised study of alpha‐tocopherol (vitamin E) versus no active treatment was found but it did not include any radiological assessment. As only one included study was a double‐blinded randomised controlled trial, the other studies were prone to selection and detection biases.
None of the included studies reported quality of life outcomes or adequately reported details about corticosteroid requirements.
A limited number of prospective studies were identified but subsequently excluded as these studies had a limited number of participants evaluating different pharmacological interventions using variable endpoints.
Authors' conclusions
There is a lack of good certainty evidence to help quantify the risks and benefits of interventions for the treatment of brain radionecrosis after radiotherapy or radiosurgery. In an RCT of 14 patients, bevacizumab showed radiological response which was associated with minimal improvement in cognition or symptom severity. Although it was a randomised trial by design, the small sample size limits the quality of data. A trial of edaravone plus corticosteroids versus corticosteroids alone reported greater reduction in the surrounding oedema with combination treatment but no effect on the enhancing radionecrosis lesion. Due to the open‐label design and wide confidence intervals in the results, the quality of this data was also low. There was no evidence to support any non‐pharmacological interventions for the treatment of radionecrosis. Further prospective randomised studies of pharmacological and non‐pharmacological interventions are needed to generate stronger evidence. Two ongoing RCTs, one evaluating bevacizumab and one evaluating hyperbaric oxygen therapy were identified.
Plain language summary
Interventions for the treatment of brain radionecrosis (radiation‐induced damage) after brain radiation treatment
Background When brain tissue dies due to a reaction to radiotherapy it is called brain radionecrosis. Brain radionecrosis can cause damage to the patient’s ability function. What this looks like depends where the radionecrosis has happened in the brain and it can impact on a patient’s quality of life. There are currently limited available treatments for brain radionecrosis. Patients are commonly given powerful anti‐inflammatory drugs (called corticosteroids) and some patients may require surgery to remove the area of brain that has radionecrosis. More effective treatments for this condition are needed.
Study characteristics In October 2017, we searched a list of literature databases and conference proceedings to identify studies that evaluated treatments for brain radionecrosis. A total of three studies were identified that evaluated drugs of which only two were RCTs and one of these RCTs had only 14 participants. No studies evaluating non‐drug treatments were identified.
Key findings The two drugs compared to corticosteroids alone in this review were bevacizumab (a drug affecting the blood vessels) and edaravone (a powerful antioxidant).
A very small‐sized study reported that bevacizumab improved the appearance of the radionecrosis on magnetic resonance imaging (MRI). This was associated with improvement in neurological symptoms than placebo but also with severe side effects.
Edaravone in combination with corticosteroids improved the appearance of radionecrosis on MRI; this was associated with improvement in the reported symptoms using the LENT/SOMA scale. However, the patient and treating team were aware of the particular treatment the patient was receiving, so the reported symptoms may have been influenced by this.
None of the included studies reported quality of life outcomes or adequately reported details about corticosteroid requirements.
Finally a two arm non‐randomised study of vitamin E versus no active treatment based on patient preference reported improvement in learning and memory, but this study did not report any imaging response. The results may have been influenced as patients chose their study treatment thus introducing other potential biases.
Certainty of the evidence Based on the findings of this review the certainty of the available evidence is low/very low, which limits our ability to help determine the risks and benefits of the evaluated treatments for brain radionecrosis. The studies were at risk of bias due to aspects of their study designs and/or very limited number of participants. There is a great need for higher‐quality evidence with larger multi‐centre randomised control trials of treatments for brain radionecrosis. In our search of the literature for this review, two ongoing RCTs, one evaluating bevacizumab and one evaluating hyperbaric oxygen therapy were identified.
Summary of findings
Background
Description of the condition
Brain radionecrosis (tissue death caused by radiation) is a complication that may follow radiotherapy to all or part of the brain (Blonigen 2010; Giglio 2003). It is most commonly observed following high‐dose radiation treatment for primary or secondary brain tumours. But brain radionecrosis can also develop following high‐dose radiotherapy to non‐central nervous system (CNS) tumours in close approximation to brain tissue, such as nasopharyngeal carcinoma.
The incidence of symptomatic brain radionecrosis is increasing because of the growing use of stereotactic radiosurgery and administration of higher cumulative doses of radiation during initial therapy and with salvage radiation therapy for primary and secondary malignant brain tumours, as well as head and neck cancers. Sterotactic radiosurgery can be thought of as a minimally invasive form of surgical practice using numerous focused radiation beams, or a highly precise radiation treatment using very few fractions (treatment sessions) of radiation. Following radiosurgery, the risk of radionecrosis has been estimated at about 10% at one year, although the risk may be higher with longer follow‐up or following repeated radiation treatments (Blonigen 2010; Mayer 2008; Shaw 2000). The overall risk of radionecrosis following fractionated radiotherapy to the brain or head and neck area is not well estimated in the current era of intensity modulated radiotherapy (IMRT). However, with more focal radiation delivery techniques, the patterns and distribution of brain radionecrosis appear to be more focal than previously observed (Suh 2010).
To date, brain radionecrosis has largely been a clinical diagnosis based on clinical and radiological presentation. Clinically, patients can present with or develop significant neurological deterioration, functional loss, and in some cases death. Conventional imaging has limited capability to reliably differentiate tumour progression from radionecrosis. Stereotactic biopsy is sometimes used to aid diagnosis, but this approach also fails to have 100% sensitivity or specificity. To avoid the need for invasive surgical procedures for diagnosis, which can be associated with additional risks to the patient, efforts have been invested in improving the capabilities of imaging investigations to confirm a radiological diagnosis. This includes the use of quantitative measures from conventional magnetic resonance imaging (MRI) and use of perfusion and diffusion MRI, MR spectroscopy, and positron emission tomography (PET) imaging. Studies suggest that the best sensitivity and specificity can be achieved by using a combination of these imaging measures to diagnose brain radionecrosis (Barajas 2009; Dequesada 2008; Hoefnagels 2009).
Description of the intervention
Initial therapy for symptomatic brain radionecrosis is typically high‐dose corticosteroids (Gonzalez 2007). If symptoms continue to progress despite the initiation of high‐dose corticosteroids, surgical resection may be considered (McPherson 2004).
Pharmacological
We defined pharmacological interventions as a drug given by any route at any therapeutic dose with the intention of reducing the clinical and radiological features of brain radionecrosis. Small single‐institution experimental trials and case series have been reported for pharmacological interventions with anticoagulants, vitamin E and pentoxifylline, and antiangiogenic therapy.
Non‐pharmacological
We defined non‐pharmacological interventions as any non‐drug intervention applied to reduce the clinical and radiological features of brain radionecrosis. Small single‐institution experimental trials and case series have been reported for non‐pharmacological interventions with hyperbaric oxygen therapy and laser‐induced thermal therapy.
How the intervention might work
Although the underlying pathophysiology of radionecrosis is still unclear, it has been suggested that high‐dose radiation results in disruption of the blood‐brain barrier, which leads to vasogenic oedema (Schultheiss 1995). Accumulation of vasogenic oedema can lead to vascular compromise, which can ultimately result in tissue hypoxia (decrease in tissue oxygen levels), which induces release of proteins that stimulate blood vessel growth and leakiness, including vascular endothelial growth factor (VEGF), causing further potentiation of peritumoral vasogenic oedema (swelling around the tumour) (Nordal 2004; Plateel 1995).
Pharmacological
Pharmacological interventions have been aimed at stopping and reversing this proposed VEGF‐driven pathological cascade. For instance, corticosteroids reduce the vasogenic oedema and in turn can help prevent vascular compromise and hypoxia, which perpetuate the pathological cascade. The antiangiogenic agent, bevacizumab, is a monoclonal antibody that binds VEGF and has shown promising results in early clinical reports (Gonzalez 2007; Wong 2008).
Non‐pharmacological
Non‐pharmacological interventions may also impact this proposed VEGF‐driven pathological cascade. Surgical resection immediately relieves the mass effect that is leading to vascular compromise, which is perpetuating the pathological process (Truong 2006). Hyperbaric oxygen has the potential to reverse tissue hypoxia to directly stop any further hypoxic tissue necrosis and decrease the release of the VEGF that is perpetuating the pathological process (Leber 1998; Ohguri 2007).
Why it is important to do this review
As a result of a lack of information from any larger trials or a systematic review to guide the management of brain radionecrosis, there is significant heterogeneity in the treatment of brain radionecrosis across institutions. This systematic review provides an up‐to‐date evaluation of available scientific information and clinical trial evidence to guide the current management of brain radiation necrosis.
Objectives
Primary outcome
To assess the effectiveness of interventions used for the treatment of brain radionecrosis in adults over 18 years old.
Secondary outcomes
To assess the safety of interventions used for the treatment of brain radionecrosis in adults by evaluating treatment‐related adverse events.
To assess the effectiveness of interventions used for the treatment of brain radionecrosis in adults based on clinical improvement based on physician‐reported or patient‐reported symptoms and functional status.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any intervention for the management of brain radionecrosis in adults previously treated with radiation therapy to the brain. Due to the limited number of RCTs, we also included all comparative prospective intervention trials of any intervention for brain radionecrosis in adults. Quasi‐RCTS were eligible for inclusion if identified. For non‐pharmacological interventions, we additionally included one non‐randomised study of any treatment intended for the treatment of brain radionecrosis as long as the studies had an acceptable comparison group (e.g. standard of care therapy such as corticosteroids). An included non‐RCT was prone to the risk of selection bias so it was given little weight in the review and we acknowledge its limitations in the results and discussion.
Types of participants
We included studies that evaluated adults over 18 years old previously treated with radiosurgery or fractionated radiotherapy to the brain or head and neck region with a diagnosis of brain radionecrosis based on clinical and radiological criteria. Pathological confirmation was not required, as tissue confirmation is not frequently acquired in clinical practice and often does not provide a definitive diagnosis due to the presence of necrosis and tumour in the pathological specimen.
Clinical and radiological diagnosis of brain radionecrosis was defined as "a growing enhancing lesion with associated oedema in the region of prior high‐dose radiation in the presence of low suspicion of active tumour, lack of tumour involvement within the brain (e.g.head and neck cancer), or advanced imaging evidence to suggest absence of active tumour".
Types of interventions
Pharmacological interventions
For pharmacological interventions, we investigated the efficacy and effectiveness of any dose of agent given by any route for the purpose of treating brain radionecrosis. Antioxidant agents such as vitamin E and antiangiogenic agents such as bevacizumab were included. To improve the clinical relevance of this review, we included studies that compared the efficacy and effectiveness of these agents against standard clinical care, which typically includes corticosteroid therapy.
Non‐pharmacological Interventions
For non‐pharmacological interventions, we included any treatment given with the aim of treating brain radionecrosis to improve symptoms and prevent progression of the process. These treatments were likely to include surgery and hyperbaric oxygen therapy. For these studies, we reported efficacy and effectiveness as well as toxicity. These interventions were considered against outcomes reported for standard care, typically corticosteroid therapy.
Types of outcome measures
Primary outcomes
The primary outcome was radiological response, defined as any reduction in contrast‐enhancing lesions or oedema (i.e. T2‐weighted hyperintensity on MRI or hypodensity on computed tomography (CT)).
Secondary outcomes
Clinical improvement, defined as documented physician‐reported or patient‐reported improvement in neurological status, symptoms, or functional status such as neurocognitive function.
Corticosteroid requirements, reported as the ability of patients to decrease their corticosteroid dose or to stop corticosteroids completely.
Treatment‐related severe adverse events, including death, haemorrhage, haematological toxicity, pulmonary toxicity, cardiac toxicity, gastrointestinal (GI) toxicity, and infection. Mild and moderate adverse events were not considered within this review.
Quality of life (QoL), using scales such as the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI‐BT) and the European Organization for Research and Treatment of Cancer core QoL questionnaire (EORTC QLQ‐C30).
We presented 'Summary of findings tables' Table 1;Table 2 reporting the following outcomes listed in order of priority.
Summary of findings for the main comparison. Bevacizumab compared to placebo for the treatment of brain radionecrosis after radiotherapy or radiosurgery.
| Bevacizumab compared to placebo for the treatment of brain radionecrosis after radiotherapy or radiosurgery | ||||
| Patient or population: people previously treated with radiosurgery or fractionated radiotherapy to the brain or head and neck region with a diagnosis of brain radionecrosis based on clinical and radiological criteria Setting: hospital Intervention: bevacizumab Comparison: placebo | ||||
| Outcomes | Summary | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) |
| Reduction in oedema (T2 hyperintensity) | Very small sample size so results were reported descriptively. At 6 weeks, all seven (100%) participants on bevacizumab had a reduction in brain oedema by at least 25%, whereas 0/7 participants receiving placebo had this reduction. All participants in the placebo arm had clinical and/or radiological progression. | Not reported due to sparse data | 14 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 |
| Reduction in post‐gadolinium enhancement | Very small sample size so results were reported descriptively. Very small sample size so results were reported descriptively. At 6 weeks, all seven (100%) participants on bevacizumab had a reduction in post‐gadolinium enhancement, whereas 0/7 participants receiving placebo had this reduction. All participants in the placebo arm had clinical and/or radiological progression. | Not reported due to sparse data | 14 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 |
| Reduction in neurological/clinical symptoms/severity Follow‐up: range 6 weeks to 12 weeks | 7 or 7 who received bevacizumab had stable or reduced clinical symptoms versus only 2 of 7 who received placebo. 5 of 7 patients had worsening neurological symptoms at 3.1 to 8.8 weeks after the first dose of placebo. Since there appeared to be no differences in neurocognitive test results in the trial versus cross‐over bevacizumab treated participant combined, all bevacizumab participants were pooled into one group. While the validity of this is questionable, no significant differences in neurocognitive function changes or symptom severity were observed with bevacizumab treatment compared with placebo in any of the analyses |
Not reported due to sparse data and validity concerns | 14 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 3 |
| Severe adverse events | No Severe adverse events in 7/7 placebo patients. 3/11 patients who received bevacizumab had severe adverse events (although this overlapped with cross‐over patients). | 14 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | |
| Quality of life | None of the included studies reported quality of life outcomes or adequately reported details about corticosteroid requirements | |||
| Corticosteroid requirements | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1 Downgraded due to small sample size
2 Lack of information regarding the randomisation and blinding procedures and overall high risk of bias
3 Concerns over validity of analyses
Summary of findings 2. Edaravone plus corticosteroids compared to corticosteroids for the treatment of brain radionecrosis after radiotherapy or radiosurgery.
| Edaravone plus corticosteroids compared to corticosteroids for the treatment of brain radionecrosis after radiotherapy or radiosurgery | ||||
| Patient or population: people previously treated with radiosurgery or fractionated radiotherapy to the brain or head and neck region with a diagnosis of brain radionecrosis based on clinical and radiological criteria Setting: hospital Intervention: edaravone + corticosteroids Comparison: corticosteroids | ||||
| Outcomes | Summary | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) |
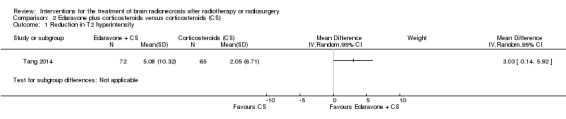
| Reduction in oedema (T2 hyperintensity) | At 3 months after completing study treatment, greater reduction in brain oedema (5.08 ± 10.32 cm2) was noted in the edaravone + corticosteroid group (edaravone 30 mg orally twice daily for 14 days plus conventional methylprednisolone 500 mg IV x 3 days followed by tapering prednisone orally) than in the corticosteroid alone group (2.05 ± 6.71 cm2) although the result approached borderline significance (P = 0.04) | MD = 3.03, 95% CI 0.14 to 5.92 | 137 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 |
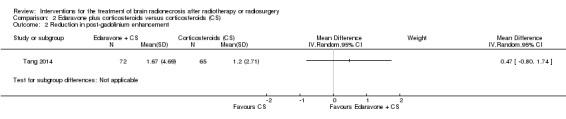
| Reduction in post‐gadolinium enhancement | At 3 months after completing study treatment, there was no evidence of any important difference in the reduction in post‐gadolinium enhancement between arms (1.67 ± 4.69 cm2; control group, 1.20 ± 2.71 cm2) | MD = 0.47, 95% CI ‐0.80 to 1.74 | 137 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 |
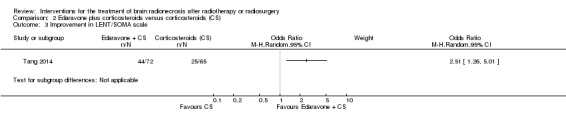
| Improvement in LENT/SOMA | At 3 months after completing study treatment, participants who received the combination treatment were noted to have significantly greater clinical improvement than corticosteroids alone measured using the Late Effects Normal Tissue Task Force‐Subjective, Objective, Management, Analytic (LENT‐SOMA) scale and defining improvement as a reduction in the LENT‐SOMA scale of > 1. | OR = 2.51, 95% CI 1.26 to 5.01 | 137 (1 RCT) | ⊕⊝⊝⊝ LOW 1 2 3 |
| Change in neurocognitive function | Not reported | |||
| Severe adverse events | No differences in treatment toxicities were observed between arms and no severe adverse events were reported. | Not reported | 137 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 |
| Quality of life | None of the included studies reported quality of life outcomes or adequately reported details about corticosteroid requirements | |||
| Corticosteroid requirements | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||
1 Open‐label trial
2 At overall high risk of bias and/or imprecision in estimate
3 Inadequate blinding giving high risk of detection bias
Radiological response
Treatment‐related severe adverse events
QoL
Corticosteroid requirements
Search methods for identification of studies
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL, 2017, Issue 10, MEDLINE (1946 to October week 1 2017), Embase (1980 to 2017 week 41), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1982 to October 2017). We have listed the MEDLINE, Embase and CENTRAL search strategies in Appendix 1, Appendix 2 and Appendix 3.
We identified all relevant articles on PubMed, and used the "Related articles" feature to carry out a further search for newly published articles. We applied no language restrictions in our searches.
Searching other resources
Unpublished and grey literature
We searched Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials for ongoing trials.
If through these searches we identified ongoing trials that have not been published, we contacted the principal investigators to request relevant data. We approached the major co‐operative trial groups active in this area. We searched conference proceedings and abstracts through ZETOC (http://zetoc.mimas.ac.uk). We searched theses and dissertations through WorldCat (http://firstsearch.oclc.org).
Handsearching
We handsearched the reference lists of included studies, key textbooks, and previous systematic reviews. We also handsearched journals and conference materials from the past year in the following sources.
Annual Meeting of the European Association of Neuro‐Oncology (EANO)
Annual Congress of the European Society for Radiotherapy and Oncology (ESTRO)
Annual Meeting of the World Federation of Neuro‐Oncology (WFNO)
Annual Meeting of the American Society of Clinical Oncology (ASCO)
Annual Meeting of the American Society for Therapeutic Radiation Oncology (ASTRO)
Annual Meeting of the American Society of Neuro‐Oncology (SNO)
Bienniel Congress of the International Stereotactic Radiosurgery Society (ISRS)
Biennial Meeting of the Leksell Gamma Knife Society (LGKS)
Annual Meeting of the Multinational Association of Supportive Care in Cancer (MASCC)
Other resources
We made efforts for personal communication with authors of relevant trials and experts at major hospitals performing clinical trials to identify further data that may or may not have been published. We included papers in all languages and carried out translations, if necessary.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database, Endnote. All duplicate references were removed, and two review authors (CC, PB) independently examined the remaining references. The review authors were not blinded to study authors or to affiliations of the studies. We excluded studies that clearly did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Two review authors (CC, PB) independently assessed the eligibility of retrieved papers. Review authors resolved any disagreements by discussion and documented reasons for exclusion.
Data extraction and management
For included trials, data were abstracted as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (CC, PB) abstracted data onto a data abstraction form designed for the review.
This form included the following data.
Article details (author, year of publication, journal citation, country, and language)
Intervention (characteristics and duration)
Study design and methodology (including inclusion and exclusion criteria, assignment process, and timing of measurements)
Population demographics and total number involved; details of the health status of participants, including tumour histology, prior treatment details, and performance status
Outcome measures (radiological response, clinical improvement, corticosteroid requirements, QoL, and adverse events)
Risk of bias
We collated and entered data into RevMan 5 2014.
When possible, all data extracted were based on an intention‐to‐treat (ITT) analysis, such that participants were analysed in the groups to which they were assigned. The time points at which outcomes were collected and reported were noted.
For dichotomous variables, we recorded the outcome of interest and the number of participants in each treatment arm assessed at available study time points and number who experienced the outcome of interest.
For continuous outcomes, we recorded the final value and standard deviation (SD) of the outcome of interest and the number of participants in each treatment arm assessed at the end of follow‐up, and we used these values to estimate the mean difference (MD) between treatment arms and its standard error (SE). We noted time points at which outcomes were collected and reported.
Assessment of risk of bias in included studies
We used Cochrane's tool for assessing the risk of bias in included studies (Higgins 2011a). This included assessment of:
selection bias: random sequence generation and allocation concealment;
performance bias: blinding of participants and personnel (patients and treatment providers);
detection bias: blinding of outcome assessment;
attrition bias: incomplete outcome data (i.e. if at least 80% of patients were assessed for the primary outcome);
reporting bias: selective reporting of outcomes; and
other possible sources of bias.
We judged all bias criteria and reported them as having low, high, or unclear risk of bias. We classified the risk of bias as unclear when insufficient information was provided, or when there was uncertainty over the potential for bias. All differences in 'Risk of bias' assessment between the two review authors (CC, PB) were resolved by discussion.
Measures of treatment effect
Dichotomous data
For dichotomous outcomes (e.g. reduction in lesion volume or not, clinical improvement or not), we recorded for each study the number of participants who experienced the outcome of interest following treatment at an early time point (within four months of treatment) and at last follow‐up. The odds ratio (OR) with 95% confidence interval (CI) for the outcome of interest was reported.
Continuous data
For continuous outcomes (e.g. lesion volume, QoL measures), we expressed treatment effect as MD between treatment arms with 95% CIs, when appropriate; we planned to use standardised mean difference (SMD) method if necessary.
Unit of analysis issues
Two review authors (CC, PB) reviewed unit of analysis issues according to information provided in the Cochrane Handbook for Systematic Reviews of Interventions and resolved differences by discussion (Higgins 2011). These included any reports describing individuals receiving more than one intervention (e.g. the cross‐over trial, simultaneous treatment of methods for each individual) or multiple observations for the same outcome (e.g. repeated measurements, recurring events).
Dealing with missing data
We did not impute missing outcome data for any outcome. If data for the primary outcome were missing, we attempted to contact trial authors to request data on outcomes among participants who were assessed.
We included details of missing data in the narrative summary and 'Risk of bias' table, alongside an assessment of the extent to which missing data could have altered the results of the review.
Data synthesis
As pertinent studies with similar participants, interventions, and outcomes were not identified, we were unable to pool data in meta‐analyses using RevMan 5 2014 as we had planned. Consequently results of included studies are reported narratively outlining results of single study analyses and it was not necessary to assess heterogeneity, reporting biases (e.g. funnel plots to assess potential for small‐study effects such as publication bias) or conduct subgroup or sensitivity analyses.
Results
Description of studies
Results of the search
A total of 3488 studies were identified through CENTRAL, MEDLINE and Embase using the outlined search strategy. The search strategy resulted in 1744 publications once duplicates were removed. Additional search strategies did not yield any further prospective studies on the treatment of brain radionecrosis.
Filtering through the titles and abstracts of the 1744 publications, which included a number of relevant reports addressing the treatment of brain radionecrosis but only seven articles retrieved in full text were potentially eligible. Of these three studies were included in the narrative synthesis (Figure 1). We also identified two ongoing trials and a trial awaiting classification (see below).
1.
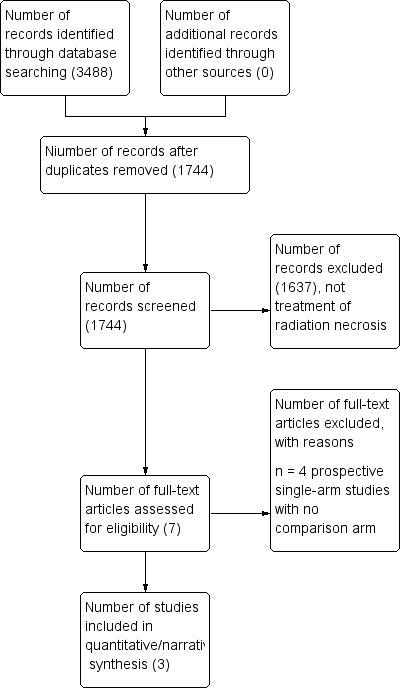
Study flow diagram.
Included studies
A total of three prospective studies investigating treatment for brain radionecrosis were included: two randomised controlled trials (RCTs) (one double‐blind, placebo‐controlled and the other an open‐label trial), and one non‐randomised study. Detailed information on the studies included in this review are summarised in the Characteristics of included studies section.
Pharmacological
Defining pharmacological interventions as a drug given by any route at any therapeutic dose with the intention of reducing the clinical and radiological features of brain radionecrosis, we identified three prospective trials comparing three different agents against a control arm.
Two prospective randomised trials of pharmacological interventions were identified. A randomised, double‐blind, placebo‐controlled trial of bevacizumab 7.5 mg/kg every three weeks x two cycles versus placebo every three weeks x two cycles in adults treated with radiotherapy for brain or head and neck neoplasm completed at least ten months prior to study entry, who developed a radiological diagnosis of brain radionecrosis based on magnetic resonance imaging (MRI) criteria. Patients were allowed to be taking corticosteroids prior to study participation, but they were required to be taking a stable dose for at least one week prior to receiving study treatment. The primary endpoint was radiological response, defined as at least a 25% reduction in brain oedema at six weeks of treatment compared with pre‐treatment. Brain oedema was measured as the volume of hyperintensity on T2‐FLAIR MR images (Levin 2011).
The second randomised trial was an open‐label trial of patients treated with methylprednisolone 500 mg intravenously x three days followed by prednisone orally on a tapering schedule over 30 days, as tolerated, with or without the addition of edaravone 30 mg orally twice daily for 14 days. Eligible patients were adults (> 18 years old) treated with radiotherapy at least six months prior to study enrolment who had radiographic evidence of radionecrosis based on MRI features. This trial also defined response as at least 25% reduction in volume of T2‐hyperintensity and the primary endpoint was evaluated at three months following the start of treatment (Tang 2014).
One prospective non‐randomised study allowed patients to choose between vitamin E 1000 international units (IU) twice daily for one year or no active treatment. Eligible patients were adults treated with radiotherapy for nasopharyngeal carcinoma with no evidence of recurrence for at least five years who have developed radiological evidence of unilateral or bilateral temporal lobe necrosis without mental impairment. Unlike the two randomised studies, serial imaging was not evaluated in this study. Patients were assessed at baseline and at one year using a battery of in‐house and more widely utilised neuropsychological tests including the Cantonese version of the Mini‐Mental Status Examination (CMMSE), Hong Kong List Learning Test (HKLLT), Visual Reproduction subtest of the Wechsler Memory Scale III (WMS‐III VR), Category Fluency Test (CFT) and computerized Cognitive Flexibility Test (Chan 2004).
Non‐pharmacological
Defining non‐pharmacological interventions as any non‐drug intervention employed with the aim of reducing the clinical and radiological features of brain radionecrosis, we did not identify any prospective studies meeting the eligibility criteria for this review.
Ongoing studies
Two ongoing trials of treatment for brain radiation necrosis were identified. The first study entitled 'Adverse radiation effects after Gamma Knife Radio Surgery and Hyperbaric Oxygen Therapy (GKSHBO)' is a single‐arm Italian study evaluating the impact of hyperbaric oxygen therapy (HBO) on clinical improvement and reduction of oedema documented by MRI in patients aged 10 to 75 years with cerebral radiation necrosis following gamma knife radiosurgery (GKS). The study recently opened in March 2016 and the target enrolment is 65 patients.
The second study entitled 'Corticosteroids plus bevacizumab versus corticosteroids plus placebo (BeSt) for radionecrosis after radiosurgery for brain metastases' is a multi‐centred randomised phase II trial of bevacizumab and steroids versus steroids and placebo in adult patients who are on corticosteroids for symptomatic radionecrosis following radiosurgery for brain metastases. The primary endpoint is an improvement in patient‐reported symptoms measured using the MD Anderson Symptom Inventory‐Brain Tumor (MDASI‐BT). The study recently opened April 2016 and the target enrolment is 130 patients, 65 patients per arm.
Excluded studies
We excluded four studies (Furuse 2016; Rao 2014; Wang 2012; Yonezawa 2014) because they were single arm studies with no comparison arm.
Two prospective single‐arm studies of bevacizumab that evaluated nine patients (Yonezawa 2014) and 17 patients (Wang 2012) were excluded due to a lack of a comparison arm that reflects standard care treatment. The study by Yonezawa and colleagues reported a 65% mean reduction in oedema, measured as the hyperintense volume on T2‐FLAIR MR images, and 80% mean reduction in the volume of the enhancing lesion on T1‐weighted MR images, as well as an improvement in KPS in seven of nine patients (77.8%) (Yonezawa 2014) Similarly, Wang and colleagues reported 48.8% mean reduction in hyperintense volume on T2‐FLAIR, 54.9% mean reduction in enhancing volume on the gadolinium‐enhanced T1‐weighted MR images and improvement in KPS in 16 of 17 patients (Wang 2012).
One prospective single‐arm study evaluating the effect of laser‐induced thermal therapy in patients with growing enhancing lesions that were previously treated with SRS, who have KPS > 70 but are poor candidates for re‐irradiation was excluded (Rao 2014). The other excluded study (Furuse 2016) was a single‐arm study of bevacizumab for patients with surgically untreatable, symptomatic brain radionecrosis.
Risk of bias in included studies
The studies included in this review were assessed using the Cochrane 'Risk of bias' tool (Higgins 2011). Any discrepancies in the 'Risk of bias' scores between the two review authors (CC, PD) were resolved by discussion. If there was a risk of unclear bias, attempts were made to contact study authors. A summary of the risks of bias is provided in Figure 2.
2.
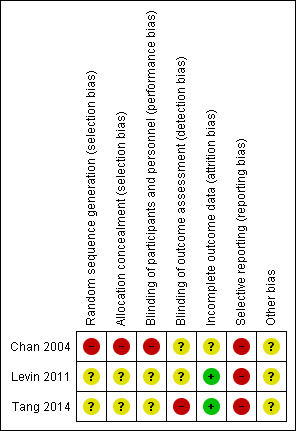
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two trials were RCTs but as details of the randomisation process for these trials were not available, the risk of allocation bias is unclear (Levin 2011, Tang 2014). One study at high risk of allocation bias was a pharmacological intervention study that allowed patient choice to the treatment group or control group (Chan 2004).
Blinding
One of the trials was described as a double‐blinded randomised trial but as the blinding process was not clearly described, the risk of performance or detection bias remains unclear (Levin 2011). Similarly, blinding of personnel, participants and outcome assessors was unclear in Tang 2014. One trial (Chan 2004) was considered at high risk for performance bias because the participants and personnel were not blinded to the intervention and it was also unclear whether or not the outcome assessor was blinded (unclear risk of detection bias).
Incomplete outcome data
One study was at low risk of attrition bias because all participants had complete follow‐up and were assessed for the primary outcome measure (Levin 2011). A second study had a total of 17 participants who withdrew from the study prior to the measurement of the primary outcome and an additional 11 participants lost to follow‐up (Tang 2014). However, since this equated to just 18% attrition, the trial was still considered as being at low risk of attrition bias based on our predefined criteria for low risk of attrition of at least 80% assessment of the primary outcome measure. The third study had an unclear risk of attrition bias because the total number of patients at the end of one year and the time point for the primary endpoint assessment, was not clearly stated (Chan 2004).
Selective reporting
Outcomes were incompletely and/or inadequately reported so selective reporting was adjudged as being at high risk of bias in all three studies.
Other potential sources of bias
There was insufficient information to permit judgement as to whether any other biases may be present in all three studies. Chan 2004 (n=29) and Levin 2011 (n=14) were very small studies but this is not a bias as such and it just means the studies were vastly underpowered and is reflected in the confidence in the estimates. If meta‐analyses had been possible then this would have added power and the precision in effect estimates.
Effects of interventions
The study interventions, outcomes and comparisons were not sufficiently similar to pool data. Three pharmacological studies met the inclusion criteria for this review. Due to differences across the three pharmacological studies, including the mechanism of action of the drugs and evaluated outcomes, the results were reviewed separately. No non‐pharmacological studies met the inclusion criteria for this review.
Pharmacological outcomes
Bevacizumab versus placebo
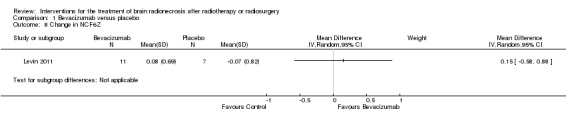
A very small and inadequately powered randomised, double‐blind, placebo‐controlled trial (Levin 2011) of bevacizumab (7.5 mg/kg every three weeks x two cycles) versus placebo reported that 100% (7/7) of participants on bevacizumab had reduction in brain oedema (T2 hyperintense volume) by at least 25% and reduction in post‐gadolinium enhancement, whereas all those receiving placebo had clinical and/or radiological progression (five participants in placebo arm experienced progressive clinical symptoms while two patients had radiological progression without progressive symptoms). This was an encouraging finding but due to the small sample size we did not report a relative effect. The authors also failed to provide adequate details regarding the randomisation and blinding procedures. Therefore the quality of this evidence is low for these outcomes.
All seven participants receiving bevacizumab were reported to have neurological improvement, whereas five of seven participants on placebo had neurological worsening and just two observed an improvement (low‐certainty evidence due to small sample size). Since there appeared to be no differences in neurocognitive test results in the trial versus cross‐over bevacizumab‐treated participants combined, all bevacizumab participants were pooled into one group. While the validity of this is questionable, no significant differences in neurocognitive function changes or symptom severity were observed with bevacizumab treatment compared with placebo in any of the analyses (Analysis 1.1, Analysis 1.2, Analysis 1.3, Analysis 1.4, Analysis 1.5, Analysis 1.6, Analysis 1.8). All seven participants receiving bevacizumab were reported to have neurological improvement, whereas five of seven participants on placebo had neurological worsening (very low‐certainty evidence due to small‐sample size and concerns over validity of the analyses).
1.1. Analysis.
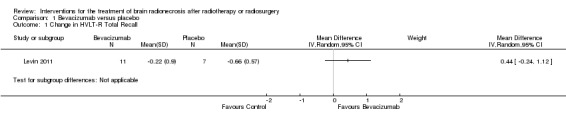
Comparison 1 Bevacizumab versus placebo, Outcome 1 Change in HVLT‐R Total Recall.
1.2. Analysis.
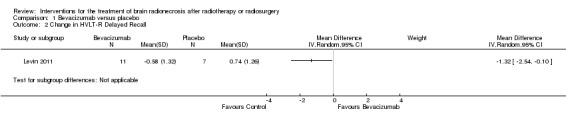
Comparison 1 Bevacizumab versus placebo, Outcome 2 Change in HVLT‐R Delayed Recall.
1.3. Analysis.
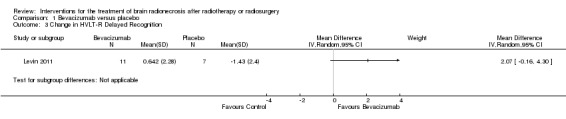
Comparison 1 Bevacizumab versus placebo, Outcome 3 Change in HVLT‐R Delayed Recognition.
1.4. Analysis.
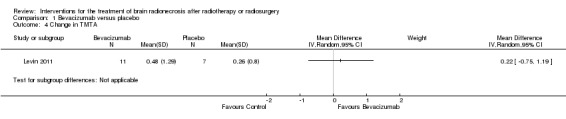
Comparison 1 Bevacizumab versus placebo, Outcome 4 Change in TMTA.
1.5. Analysis.
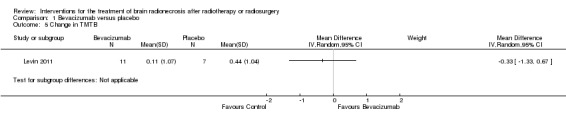
Comparison 1 Bevacizumab versus placebo, Outcome 5 Change in TMTB.
1.6. Analysis.
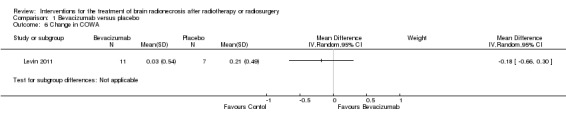
Comparison 1 Bevacizumab versus placebo, Outcome 6 Change in COWA.
1.8. Analysis.
Comparison 1 Bevacizumab versus placebo, Outcome 8 Change in NCF6Z.
No adverse events were reported in the placebo arm, although three severe adverse events were noted with bevacizumab which included aspiration pneumonia, pulmonary embolus and superior sagittal sinus thrombosis.
Edaravone plus corticosteroids versus corticosteroids alone
An open‐label RCT (Tang 2014) demonstrated a greater reduction in brain oedema in the edaravone plus corticosteroid group (edaravone 30 mg orally twice daily for 14 days plus conventional methylprednisolone 500 mg intravenously x three days followed by tapering prednisone orally) than in the corticosteroid alone group (mean difference (MD) was 3.03 (95%CI 0.14 to 5.92), although the result approached borderline significance (P = 0.04) (Analysis 2.1). There was no evidence of any important difference in the reduction in post‐gadolinium enhancement between arms (MD = 0.47, 95% CI ‐0.80 to 1.74; low‐certainty evidence due to high risk of bias and imprecision) (Analysis 2.2).
2.1. Analysis.
Comparison 2 Edaravone plus corticosteroids versus corticosteroids (CS), Outcome 1 Reduction in T2 hyperintensity.
2.2. Analysis.
Comparison 2 Edaravone plus corticosteroids versus corticosteroids (CS), Outcome 2 Reduction in post‐gadolinium enhancement.
In the RCT of corticosteroids with or without edaravone, the participants who received the combination treatment were noted to have significantly greater clinical improvement than corticosteroids alone measured using the Late Effects Normal Tissue Task Force‐Subjective, Objective, Management, Analytic (LENT‐SOMA) scale (OR = 2.51, 95%CI 1.26 to 5.01; low‐certainty evidence due to the open‐label design) (Analysis 2.3). No differences in treatment toxicities were observed between arms and no severe adverse events were reported.
2.3. Analysis.
Comparison 2 Edaravone plus corticosteroids versus corticosteroids (CS), Outcome 3 Improvement in LENT/SOMA scale.
Vitamin E versus no active treatment
The prospective non‐randomised study (Chan 2004) of Vitamin E 1000 IU twice daily for one year versus no active treatment focused on neurocognitive evaluation without reported serial imaging evaluation. Evaluating cognitive function in patients at baseline and after one year of treatment, a 5.3% improvement in global cognitive function on CMMSE was seen in patients who received vitamin E compared with no improvement in the control group (P = 0.007). Assessment of verbal learning using Hong Kong List Learning Test (HKLLT) demonstrated that the treatment group had a 27.2% improvement at one year versus no improvement in the control group. Similarly, improvements were seen for visual memory and recall for the group treated with vitamin E. There was no difference in attention, language or executive function between the two groups at baseline or at one year (Chan 2004). Corticosteroid requirements and adverse events to treatment were not reported in this study.
Discussion
Summary of main results
Pharmacological
This review evaluated the effect of any pharmacological or non‐pharmacological intervention in patients with brain radionecrosis on both radiological response, clinical response and cognitive improvement. Additional secondary endpoints including dexamethasone requirements and quality of life (QoL) measures were planned for this review but there were limited data available for these endpoints within the studies included in this review. A total of 180 participants were included in the review and eligible for analysis, with one RCT contributing 137 of the 180 participants, another RCT just 14 and one non‐randomised two‐arm study evaluated 29 for the effect of pharmacological interventions.
The two randomised studies evaluating pharmacological interventions used different drug agents, different comparison arms and different time points for response evaluation, therefore a meta‐analysis was not completed. But both drugs, edaravone and bevacizumab, were shown to result in a greater radiological response compared with their respective comparison arms of either corticosteroids or placebo, respectively. Levin 2011 reported a 100% radiological reduction in oedema at six weeks after starting treatment in all patients treated with bevacizumab versus clinical or radiological progression in all patients in the placebo arm. Despite this dramatic difference in response, neurocognitive function and symptom severity were largely similar between treatment arms. Tang 2014 reported radiological response, defined as reduction in oedema, in 55.6% of patients treated with edaravone plus corticosteroids versus 35.4% of patients treated with corticosteroids alone. Using late effects in normal tissues subjective, objective, management and analytic scales (LENT/SOMA), patients treated with edaravone reported significant improvement in their symptoms; however, the open‐label design of this study may have impacted the results of this patient‐reported outcome.
Neurocognitive function was evaluated in two pharmacological studies. A non‐randomised study of vitamin E versus no active treatment found that after one year of treatment, patients who received vitamin E had improvement in global cognitive function on the Cantonese version of the Mini‐Mental Status Examination (CMMSE) as well as improvement in verbal learning on the Hong Kong List Learning Tes (tHKLLT) (Chan 2004). In the randomised study of bevacizumab versus placebo, patients receiving bevacizumab also had greater improvement on learning trials and also demonstrated greater improvement in the delayed recognition trial but worsened performance on delayed free recall trial.
Non‐pharmacological
No eligible studies of non‐pharmacological interventions met the eligibility criteria for this review.
Overall completeness and applicability of evidence
Based on this review, although 1744 studies were retrieved through the established search criteria, after excluding reports that were unrelated to brain radionecrosis, retrospective studies, case reports or single‐arm studies, there were limited studies that prospectively evaluated the efficacy of interventions for the treatment of brain radionecrosis. A total of three prospective 2‐arm studies evaluating pharmacological interventions were identified, each evaluating a different drug agent and different endpoints. Therefore a meta‐analysis was not completed for the pharmacological interventions. There were no studies of non‐pharmacological interventions that met inclusion criteria for this review.
Of the trials included in this review, only one study that was a double‐blinded, randomised controlled study was at low risk for any biases; however, this study had only 14 patients, of which only five patients were receiving dexamethasone at the time of study registration. A comparison of outcomes against the current standard treatment of corticosteroids would be particularly useful when considering endpoints such as patient‐reported symptoms or QoL, as corticosteroids are associated with a number of side effects (Giglio 2003). An ongoing study entitled 'Corticosteroids plus bevacizumab versus corticosteroids plus placebo (BeSt) for radionecrosis after radiosurgery for brain metastases' will help evaluate this clinical scenario as it is a multi‐centred randomised phase II trial of bevacizumab and steroids versus steroids and placebo in patients who are on corticosteroids for symptomatic radionecrosis following radiosurgery for brain metastases. The primary endpoint is an improvement in patient‐reported symptoms measured using the MD Anderson Symptom Inventory‐Brain Tumor (MDASI‐BT), which will reflect the effect of bevacizumab on radionecrosis symptoms as well as its associated toxicities compared with the benefit and toxicities associated with corticosteroids (A221208).
Quality of the evidence
The quality of the available evidence is low to very low for all outcomes due to the limited number of prospective studies and fact only single study analyses were possible, the small number of participants in these studies, variability in the endpoints evaluated and all were at an overall high risk of bias. The trial of Levin 2011 was reported as a double‐blind randomised control trial but the evidence was downgraded one level for all outcomes due to the small size of 14 patients (seven patients per arm) and also downgraded one level due to lack of information regarding the randomisation and blinding procedures. All outcomes of the second RCT were downgraded one level as it was open‐label and further downgraded due to concerns regarding detection bias and inadequate blinding in the trial (Tang 2014). One two‐arm non‐randomised study was at risk of selection bias and performance bias because participants were able to choose the treatment arm (Chan 2004) and thus the quality of the evidence for all reported outcomes was very low ('Summary of findings' table therefore not completed since this is not an RCT and in fact we do not want to emphasise study results in a main section of the review). The quality of evidence for treatment‐related severe adverse events was low for bevacizumab due to the small size of the trial (Levin 2011), and also low for the edaravone plus corticosteroid trial due to the open‐label design of the trial that may have impacted the selection of participants who received edaravone (Tang 2014). None of the included studies reported quality of life outcomes or adequately reported details about corticosteroid requirements so quality of the evidence for these outcomes was very low.
Based on the overall low to very low‐certainty of evidence, further research is very likely to have an important impact on our confidence in the estimate of effect of each treatment.
Potential biases in the review process
An extensive search through the listed databases including published studies and conference proceedings, as well as publications listed in the references of review articles was completed for this review, which minimised the risk of missing any eligible studies. We were unable to acquire additional information beyond the published data for the included studies, which limited our ability to report all studies as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Agreements and disagreements with other studies or reviews
Corticosteroids
For patients with symptomatic brain radionecrosis, corticosteroids are typically the first‐line treatment, as they effectively reduce symptoms associated with brain oedema and also inhibit the pro‐inflammatory processes involved in radionecrosis (Giglio 2003). However, withdrawal of corticosteroids may result in a rebound of the oedema and related symptoms and prolonged use of corticosteroids can be associated with significant toxicity including steroid myopathy, iatrogenic Cushing's syndrome and glucose intolerance (Giglio 2003).
Bevacizumab
There have been several recent reviews addressing the use of bevacizumab for brain radionecrosis (Tye 2014; Lubelski 2013) that included data from both retrospective and prospective studies. Lubelski 2013 reported on 30 patients included in seven studies of bevacizumab for patients with brain radionecrosis following treatment for high‐grade glioma. Similar to this review, all patients demonstrated a radiographic response on T1 and T2‐weighted magnetic resonance imaging (MRI). Out of the 23 patients for which clinical data were reported, 16 (70%) showed an improvement in this study (Lubelski 2013). A subsequent broader review that included 71 patients treated with bevacizumab for brain radionecrosis after treatment of any brain tumour across 16 studies reported radiographic improvement in 97% of patients and improvement in performance status in 79% of patients. (Tye 2014) Therefore, these prior reviews are in agreement with the current systematic review that the radiographic response rate to bevacizumab is high for patients with brain radionecrosis and this response may be associated with symptomatic or functional improvement.
Pentoxifylline and vitamin E
Pentoxifylline (PTX) is a methylxanthine derivative that decreases blood viscosity thereby increasing blood circulation and tissue oxygenation. Vitamin E (or tocopherol) acts as a free‐radical scavenger. In a small retrospective study of 11 patients with brain radionecrosis following radiotherapy for brain metastases, meningioma and arteriovenous malformations (AVM), the combination of PTX and vitamin E resulted in radiological improvement in all but one patient, who was eventually confirmed to have tumour recurrence (Williamson 2008). Although the prospective trial included in the current systematic review evaluated the cognitive impact of vitamin E alone and reported greater improvement on cognitive testing at one year of treatment, this prospective trial did not report on radiological response.
Hyperbaric oxygen (HBO) therapy
There are limited small retrospective case reports and case studies reporting the outcomes of HBO therapy for brain radionecrosis. Pasquier 2004 reported limited retrospective reports of HBO therapy for brain radionecrosis as part of a larger review of HBO therapy for radiation injury to all body sites. These retrospective reports suggested promising responses to HBO therapy to some patients, however no prospective data are available to‐date. A trial entitled "Adverse radiation effects after Gamma Knife radio surgery and Hyperbaric Oxygen Therapy (GKSHBO)" was activated in March 2016. This is a single‐arm study evaluating the impact of (HBO on clinical improvement and reduction of oedema in patients who develop cerebral radiation necrosis following gamma knife radiosurgery (GKS) (NCT02714465).
Surgery
Although surgery is frequently used in clinical practice to address progressive resectable radionecrosis lesions, no prospective trials of surgical resection for brain radionecrosis were identified in this review. A retrospective series of 24 adult patients who underwent craniotomy and resection of contrast‐enhancing lesions in the temporal lobes (16 unilateral; 8 bilateral) following radiotherapy for nasopharyngeal carcinoma reported a reduction in the extent of brain oedema observed on either computed tomography (CT) or MRI in the 15 patients who had serial imaging. Only one patient required a repeat resection for recurrent necrosis (Wong 2010). In patients who were treated with radiosurgery, a retrospective series of 15 patients treated with surgical resection for radionecrosis reported improvement in brain oedema resulting in either a partial or complete taper off corticosteroids as well as symptom improvement in the majority of patients (Telera 2013).
Laser‐induced thermal therapy (LITT)
Only one single‐arm study of a LITT, a non‐pharmacological intervention, was identified in the search, but this study did not meet eligibility criteria. This single‐arm study of LITT reported promising local control of 75.8% (13 of 15 lesions), and dramatic reductions in lesion volume to less than 10% of the pre‐treated volume in seven of the treated lesions (Rao 2014). However, as this was a single‐arm study with a limited number of patients, further prospective investigation is required to compare the effect of this treatment against current management approaches.
Authors' conclusions
Implications for practice.
There is limited evidence ranging from low to very low‐certainty for each outcome at present, which makes it difficult to draw firm conclusions on the benefits and risks of interventions for the treatment of radiation necrosis. Based on this review, bevacizumab show promising results of improving radiographic oedema and post‐gadolinium enhancement with associated symptomatic improvement. However, this was based on a very small double‐blinded randomised controlled trial of 14 patients in total, which introduces a high risk of bias due to the small sample size despite the high‐quality trial design. Edaravone in combination with corticosteroids also resulted in greater reduction in radiographic oedema than corticosteroids alone but had no impact on the reduction in the enhancing lesion. Again, greater symptomatic improvement was observed with the addition of edaravone to corticosteroids but this was a secondary endpoint of the study. However due to the open‐labelled randomised study design, there is high risk of selection bias. The final prospective study of pharmacological intervention was a non‐randomised study of vitamin E and although this demonstrated improvement in cognitive function based on a battery of tests administered by blinded observers, this study was at high risk for selection bias and performance bias.
Despite a broad search, no non‐pharmacological intervention study was identified meeting the requirements of this review. Therefore, there is currently no prospective evidence to support the use of any non‐pharmacological intervention for the treatment of brain radionecrosis.
Implications for research.
There is a great need for further prospective randomised controlled trials (RCTs) in the treatment of brain radionecrosis. This review identified one trial of bevacizumab and steroids versus placebo and steroids for symptomatic brain radionecrosis that was evaluating patient‐reported symptoms using the MD Anderson Symptom Inventory‐Brain Tumor (MDASI‐BT) as the primary endpoint (A221208), and one prospective trial of hyperbaric oxygen (HBO) therapy for brain radionecrosis following gamma knife radiosurgery (GKS) that was evaluating clinical improvement using the Rankin score as a primary endpoint (NCT02714465). Additional studies evaluating the clinical impact of treatment rather than radiological improvement will provide clinically meaningful evidence.
Acknowledgements
We are grateful for the educational grant funding provided by Society of Neuro‐Oncology, USA to support this review.
With gratitude to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group for its contributions, support and guidance throughout the editorial process. Thanks also Joanne Platt, Information Manager, for designing the search strategy.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to thank all our external peer reviewers (May Tsao, Normand Laperriere, Susan Chang, Helen Bulbeck).
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE Ovid 1 exp Brain/ 2 exp Brain Neoplasms/ 3 brain*.mp. 4 1 or 2 or 3 5 rt.fs. 6 exp Radiotherapy/ 7 Radiosurgery/ 8 (radiotherapy* or radiosurgery).mp. 9 5 or 6 or 7 or 8 10 exp Necrosis/ 11 (necrosis or radionecrosis).mp. 12 Radiation Injuries/ 13 10 or 11 or 12 14 4 and 9 and 13
key:
[mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Brain] explode all trees #2 MeSH descriptor: [Brain Neoplasms] explode all trees #3 brain* #4 #1 or #2 or #3 #5 Any MeSH descriptor with qualifier(s): [Radiotherapy ‐ RT] #6 MeSH descriptor: [Radiotherapy] explode all trees #7 MeSH descriptor: [Radiosurgery] this term only #8 radiotherap* or radiosurgery #9 #5 or #6 or #7 or #8 #10 MeSH descriptor: [Necrosis] explode all trees #11 necrosis or radionecrosis #12 MeSH descriptor: [Radiation Injuries] this term only #13 #10 or #11 or #12 #14 #4 and #9 and #13
Appendix 3. Embase search strategy
1 exp brain/ 2 exp brain tumour/ 3 brain*.mp. 4 1 or 2 or 3 5 rt.fs. 6 exp radiotherapy/ 7 exp radiosurgery/ 8 (radiotherap* or radiosurgery).mp. 9 5 or 6 or 7 or 8 10 exp necrosis/ 11 (necrosis or radionecrosis).mp. 12 exp radiation injury/ 13 10 or 11 or 12 14 4 and 9 and 13
key:
[mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
Data and analyses
Comparison 1. Bevacizumab versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in HVLT‐R Total Recall | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Change in HVLT‐R Delayed Recall | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Change in HVLT‐R Delayed Recognition | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Change in TMTA | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5 Change in TMTB | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6 Change in COWA | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7 Change in MDASI‐Severity | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8 Change in NCF6Z | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
1.7. Analysis.
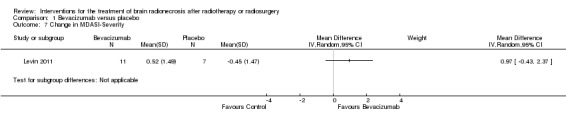
Comparison 1 Bevacizumab versus placebo, Outcome 7 Change in MDASI‐Severity.
Comparison 2. Edaravone plus corticosteroids versus corticosteroids (CS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in T2 hyperintensity | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2 Reduction in post‐gadolinium enhancement | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Improvement in LENT/SOMA scale | 1 | Odds Ratio (M‐H, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chan 2004.
| Methods | 2‐arm, non‐randomised study | |
| Participants | Patients treated with radiotherapy for nasopharyngeal carcinoma with no evidence of recurrence for at least 5 years who have developed radiological evidence of unilateral or bilateral temporal lobe necrosis without mental impairment | |
| Interventions |
Treatment Group: vitamin E 1000 IU twice daily for 1 year Control Group: no active treatment The following neuropsychological tests administered at baseline and at 1 year: Cantonese version of the Mini‐Mental Status Examination (CMMSE), computerised reaction time attention test (developed by a co‐author), Hong Kong List Learning Test (HKLLT), Visual Reproduction subtest of the Wechsler memory Scale III (WMS‐III VR), Category Fluency Test (CFT), and Cognitive Flexibility Test (developed by a co‐author). Patients also completed a questionnaire to rate their opinion on their cognitive ability. |
|
| Outcomes | Total of 29 patients enrolled: 19 patients in the vitamin E treatment group versus 10 patients in the control group Primary endpoint Radiological response: no radiological response was reported. Secondary endpoint Clinical improvement: a significant improvement in global cognitive function was reported for patients who received vitamin E. On CMMSE, the score improved by 5.4% in the treatment group versus the control group (P = 0.007). On the verbal learning assessment using KHLLT, the treatment group had a 27.2% improvement at 1 year versus no improvement in the control group. Similarly, improvements were seen for visual memory and recall for the group treated with vitamin E. There was no difference in attention, language or executive function between the two groups at baseline or at 1 year. Corticosteroid requirement: not reported Toxicities: no AE's reported Quality of Life: none reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐randomised study that allowed patient to choose their treatment allocation |
| Allocation concealment (selection bias) | High risk | The treatment arm was based on patient and physician choice introducing a high risk of selection bias. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The patients and treating physicians were not blinded to the treatment arm. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The risk of bias in the primary outcome measure of neurocognitive function is likely low as the manuscript reports that the examiners administering the neuropsychological tests were blinded to the medication received, but the blinding procedure was not described. Patient self‐evaluation on cognitive function is highly biased as they were not blinded to their treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The total number of patients assessed at 1 year is not stated and therefore the attrition in the primary outcomes measure is unclear. |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis |
| Other bias | Unclear risk | Insufficient information to permit judgement as to whether any other biases may be present |
Levin 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial | |
| Participants | Adult patients treated with radiotherapy for brain or head and neck neoplasm completed at least 10 months prior to study entry, who have a radiological diagnosis of brain radionecrosis based on MRI criteria. Patients were allowed to be taking corticosteroids prior to study participation but they were required to be taking a stable dose for at least 1 week prior to receiving study treatment. | |
| Interventions |
Bevacizumab: 7.5 mg/kg every 3 weeks x 2 cycles Placebo: every 3 weeks x 2 cycles |
|
| Outcomes | Total of 14 patients enrolled: 7 patients in the bevacizumab arm versus 7 patients in the placebo control group Primary endpoint Radiological response: The relative risk (RR) for radiological response was significantly greater with bevacizumab versus placebo with the RR 15.00 (95% CI 1.02 to 220.92). The change in oedema (T2 FLAIR) volume from baseline to 6 weeks where 25% reduction constituted response. There was 100% response for bevacizumab versus progression +14% with placebo (P value = 0.001). Median T1gad volume change was a reduction by 63% for bevacizumab arm versus increase by 17% for placebo (P value = 0.006). Secondary endpoints Clinical improvement: clinically, 5 of 7 patients on placebo had worsening neurologic signs/symptoms from 3.1 to 8.8 weeks after randomisation versus improvement by 6 weeks in all patients receiving bevacizumab. Since there appeared to be no differences in neurocognitive test results in the trial versus cross‐over bevacizumab‐treated participants combined, all bevacizumab participants were pooled into one group. Neurocognitive tests showed that mean NCF6Z score decreased from baseline to 6 weeks by 0.07 with placebo but increased by 0.08 with bevacizumab treatment such that the MD for the bevacizumab arm was MD 0.44 higher (95% CI 0.24 higher to 1.12 higher). Bevacizumab patients had greater improvement than placebo in learning trials and delayed recognition trial on HVLT‐R but bevacizumab patients had worse performance on delayed free recall trial of memory measure (HVLT‐R delayed recall). Patients reported greater interference in their everyday activities from their symptoms (MDASI‐Interference) with bevacizumab treatment than with placebo. Corticosteroid requirement: of the 5 (38%) patients who were on dexamethasone at study registration, 1 patient had dose reduction by 12 weeks and the other 4 tolerated a slow taper. Toxicities: no patients receiving placebo experienced any AE's, whereas 6 of 11 patients who received bevacizumab as initial or cross‐over treatment experienced AE's. Three of these AE's were severe: 1 aspiration pneumonia, 1 pulmonary embolus secondary to deep vein thrombosis, and 1 superior sagittal sinus thrombosis. Quality of Life: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Although this is reported as a double‐blind, randomised study, the method of allocation to the particular study arm and blinding was not described therefore the risk of allocation concealment is unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The blinding procedure for the study participants and key study personnel was not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although a double‐blind design is reported, the blinding procedure for the study participants and key study personnel was not described. Due to the small size of the study, any deficits in the blinding process for even a small number of patients potentially introduces a substantial impact on the study findings. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was complete follow‐up data of all patients for the primary outcome measure or interest. |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis |
| Other bias | Unclear risk | Insufficient information to permit judgement as to whether any other biases may be present |
Tang 2014.
| Methods | Open‐label, randomised controlled trial | |
| Participants | Adult patients (> 18 years old) treated with radiotherapy at least 6 months prior to study enrolment who have developed radiographic evidence of radionecrosis based on MRI features | |
| Interventions |
Edaravone: 30 mg given orally twice daily for 14 days and methylprednisolone 500 mg IV x 3 days followed by prednisone orally on a tapering schedule over 30 days, as tolerated. Methylprednisolone: 500 mg IV x 3 days followed by prednisone orally on a tapering schedule over 30 days, as tolerated. |
|
| Outcomes | Total of 154 patients enrolled, of which 137 patients were eligible for analysis: 72 patients in the edaravone plus corticosteroid treatment group versus 65 patients in the corticosteroid group Primary endpoint Radiological response: patients who received edaravone had a greater radiological response with a mean difference (MD) of 3.03 (95% CI 0.14 to 5.92) higher than those who did not receive edaravone. This was observed as a greater reduction in brain oedema by 5.08 + 10.32 cm2 was observed in the edaravone plus corticosteroid group versus 2.05 + 6.71 cm2 in the corticosteroid group (P value = 0.04). Reduction in enhancing lesion volume was also notable in both groups but not significantly greater with the addition of edaravone to corticosteroids compared with corticosteroids alone (P = 0.47). Secondary endpoints Clinical improvement: on LENT/SOMA, patients had a significantly better response with edaravone plus corticosteroids versus corticosteroids (P value = 0.006) such that the RR was 1.59 (95% CI 1.11 to 2.27) Corticosteroid requirement: corticosteroid requirements were not reported. Toxicities: AEs included insomnia, hyperglycaemia, liver dysfunction and epistaxis, but no difference in AEs was observed between treatment arms. Quality of Life: none reported |
|
| Notes | 1:1 randomisation, N = 154 patients enrolled (77 patients per arm). During the study 17 patients withdrew, 5 in the edaravone group and 12 in the control group of which 5 withdrew because they requested edaravone). A total of 11 patients were lost to follow‐up. The toxicities were mild on edaravone and not significantly greater compared with corticosteroids. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | The method of study arm allocation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’; |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | For the primary endpoint of the study of radiological oedema response (primary outcome of the study), the study repots that the neuroradiologists reporting response were blinded to group assignment, but the blinding procedure was not described. For the LENT/SOMA assessment, there is high risk as patients were aware of their treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 17 patients who withdrew from the study and another 11 patients who were lost to follow‐up, summing up to 18% attrition. This leaves at least 80% of enrolled patients to be assessed, therefore meeting the predefined criteria of low risk. |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis |
| Other bias | Unclear risk | Insufficient information to permit judgement as to whether any other biases may be present |
AE: adverse effects CCMSE: Cantonese version of the Mini‐Mental Status Examination CI: confidence interval HKLLT: Hong Kong List Learning TestHVLT‐R: Hopkins Verbal Learning Test Revised IU: international unit IV: intravenous LENT/SOMA: late effects in normal tissues subjective, objective, management and analytic scales MD: mean difference MDASI‐BT: M.D. Anderson Symptom Inventory Brain Tumor Module MRI: magnetic resonance imaging RR: risk ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Furuse 2016 | Single‐arm study of bevacizumab for patients with surgically untreatable, symptomatic brain radionecrosis |
| Rao 2014 | Single‐arm study of laser‐induced thermal therapy for patients with radiological progression that may represent radionecrosis |
| Wang 2012 | Single‐arm study of 17 patients treated with bevacizumab for cerebral radiation necrosis that was poorly controlled with corticosteroids |
| Yonezawa 2014 | Single‐arm study of 9 patients who received bevacizumab 5 mg/kg x 6 cycles |
Characteristics of studies awaiting assessment [ordered by study ID]
Wang 2016.
| Methods | A prospective, randomised, controlled phase II study |
| Participants | People with progressive temporal lobe necrosis |
| Interventions | Patients with progressive TLN were randomly assigned to either the control or the study group in a 1:1 ratio. The control group received corticosteroids with gradually reduced dosage. The study group received NGF with corticosteroids. NGF was dissolved in 2 mL normal saline and injected intramuscularly at 18μg/time, once a day for 2 months. |
| Outcomes | Twenty‐eight cases were enrolled into this study. The objective evaluation showed that the response rate (RR) in the study group was higher than the control group. The ratio was 10 versus 2 (P = 0.006), and 12 versus 3 ( P = 0.002) at 3 to 4 months and 6 to 8 months after intervention, respectively. The subjective evaluation demonstrated both groups were effective in controlling the necrosis‐related symptoms in the first 6 months after treatment. But NGF was more effective than corticosteroids at 9 months (13 versus 4, P = 0.001). The only observed side effect was mild pain at the injection site in 3 patients in the study group. |
| Notes |
NGF:nerve growth factor TLN: temporal lobe necrosis
Characteristics of ongoing studies [ordered by study ID]
A221208.
| Trial name or title | Randomized phase II study: corticosteroids plus bevacizumab versus corticosteroids plus placebo (BeSt) for radionecrosis after radiosurgery for brain metastases |
| Methods | Double‐blinded, randomised, placebo‐controlled trial |
| Participants | Patients with a diagnosis of brain radionecrosis following radiosurgery for brain metastases who remain symptomatic after at least 7 days of corticosteroid therapy. Diagnosis of radionecrosis is based on the presentation of clinical symptoms between 3 and 24 months following radiosurgery along with radiological features including a lesion quotient of < 0.3 and rCBV < 1.5 and PSR > 76%. |
| Interventions |
Bevacizumab: 10 mg/kg IV days 1 and 15 x 4 cycles and corticosteroids (dose adjusted at the discretion of the treating physician) Placebo and corticosteroids: (dose adjusted at the discretion of the treating physician) |
| Outcomes |
Primary objective: to investigate whether the addition of bevacizumab to standard corticosteroid therapy results in greater improvement in symptoms, measured using MD Anderson Symptom Inventory‐Brain Tumor (MDASI‐BT), compared with standard therapy Secondary objectives: (1) to evaluate adverse effects associated with corticosteroids versus bevacizumab; (2) to compare health‐related QoL (HR‐QOL), Dexamethasone Symptoms Questionnaire‐Chronic (DSQ‐C) and interference score from the MDASI‐BT; (3) to compare intracranial progression‐free survival and time to maximum radiographic response between treatment arms; (4) to compare the dose and duration of corticosteroids required between treatment arms Correlative objectives: (1) to explore serum/urine biomarkers that predict for treatment response; (2) to explore early imaging biomarkers that predict for treatment response |
| Starting date | April 2016 |
| Contact information | Caroline Chung, MD phone: 1‐713‐745‐5422 email: cchung3@mdanderson.org |
| Notes |
NCT02714465.
| Trial name or title | Treatment of adverse radiation effects after gamma knife radiosurgery (GKS) and hyperbaric oxygen therapy (HBO) |
| Methods | single‐arm trial |
| Participants |
Inclusion criteria: age 10 to 75, clinical and instrumental signs of radiation necrosis following prior radiosurgery, Italian‐speaking, able to carry out the study interventions Exclusion criteria: enrolled in another clinical trial, life expectancy < 6 months, Rankin Scale > 5, taking contraindicated medications (disulfiram, doxorubicin, cisplatin, mafenide acetate, bleomycin), medical comorbid contraindications to HBO: emphysema, asthma, epilepsy, claustrophobia, Paul Bert Sydrome, Lorrain Smith Syndrome |
| Interventions | 24 sessions of hyperbaric oxygen therapy followed by a break of 10‐15 days for MRI reassessment. If the lesion has regressed, treatment will be suspended, otherwise the patients will continue up to a maximum of 40 sessions in total. |
| Outcomes |
Primary Outcomes: Clinical improvement between days 2 to 25 and Rankin Scale Secondary Outcomes: (1) reduction in the extent of oedema documented by MRI at 1‐3 months following treatment; (2) measurement of complications from hyperbaric oxygen therapy and their severity |
| Starting date | March 2016 |
| Contact information | Simonetta Passarani, MD phone: +39 02 6444 ext 4637 email: simonetta.passarani@ospedaleniguarda.it |
| Notes |
HBO: hyperbaric oxygen IV: intravenous MRI: magnetic resonance imaging rCBV: relative cerebral blood volume
Differences between protocol and review
All of the steps planned in the protocol were completed, if the appropriate data were available. Due to the heterogeneity in the limited number of included studies, meta‐analysis was not completed and data were reported separately for each study, as each study was evaluating a different intervention.
Data synthesis
As pertinent studies with similar participants, interventions, and outcomes were not identified, we were unable to pool data in meta‐analyses using RevMan 5 2014 as we had planned. Consequently, results of included studies are reported narratively outlining results of single study analyses and it was not necessary to assess heterogeneity, reporting biases (e.g. funnel plots to assess potential for small‐study effects such as publication bias) or conduct subgroup or sensitivity analyses. We had specified the following.
Assessment of heterogeneity
We would have attempted to assess heterogeneity between studies by visual inspection of forest plots and by a formal statistical test of the significance of the heterogeneity (Deeks 2001).
Assessment of reporting biases
Two review authors (CC, PB) would have reviewed and recorded any potential reporting biases. We had planned to examine funnel plots if there were enough trials for a meta‐analysis in order to assess the potential for small‐study effects such as publication bias.
Subgroup analysis and investigation of heterogeneity
We planned analyses of following subgroups.
Radiosurgery versus fractionated radiotherapy
Pathologically‐confirmed radionecrosis versus clinical‐radiological diagnosis alone
Tumor histology for which the initial radiotherapy was provided: brain metastases, primary central nervous system (CNS) tumours, other (likely head and neck cancer)
Sensitivity analysis
In keeping with the information provided in Higgins 2011, the following factors were considered possible sources of heterogeneity across studies: variation in study quality (high or low levels of risk of bias), use of different classes of agents, and dosing or scheduling differences. We had anticipated that additional types of sensitivity analyses may have been identified during the conduct of the review.
Contributions of authors
Draft of the review: all review authors.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Society of Neuro‐Oncology, USA.
Educational grant funding
Declarations of interest
Caroline Chung: none. Andrew Bryant: none Paul Brown: none.
New
References
References to studies included in this review
Chan 2004 {published data only}
- Chan A, Cheung MC, Law SC, Chan JH. Phase II study of alpha‐tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer January 15, 2004;100(2):398‐404. [DOI] [PubMed] [Google Scholar]
Levin 2011 {published data only}
- Levin V, Bidaut L, Hou P, Kumar A, Wefel J, Bekele B, et al. Randomized double‐blind placebo‐controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. International Journal of Radiation Oncology, Biology, Physics 2011;79(5):1487‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2014 {published data only}
- Tang Y, Rong X, Hu W, Li G, Yang X, Yang J, et al. Effect of edaravone on radiation‐induced brain necrosis in patients with nasopharyngeal carcinoma after radiotherapy: a randomized controlled trial. Journal of Neuro‐oncology 21 August 2014;120(2):441‐7. [DOI: 10.1007/s11060-014-1573-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Furuse 2016 {published data only}
- Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arawaka Y, Shinoda J, et al. A prospective, multicentre, single‐arm clinical trial of bevacizumab for patients wtih surgically untreatable, symptomatic brain radiation necrosis. Neuro‐Oncology Practice December 2016;3(4):272‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rao 2014 {published data only}
- Rao MS, Hargreaves EL, Khan AJ, Haffty BG, Danish SF. Magnetic resonance‐guided laser ablation improves local control for postradiosurgery recurrenceand/or radiation necrosis. Neurosurgery 2014;74(6):658‐67. [DOI] [PubMed] [Google Scholar]
Wang 2012 {published data only}
- Wang Y, Pan L, Sheng X, Mao Y, Wang E, Zhang N, Dai J. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. European Journal of Medical Research 2012;17:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yonezawa 2014 {published data only}
- Yonezawa S, Miwa K, Shinoda J, Nomura Y, Asano Y, Nakayama N, et al. Bevacizumab treatment leads to observable morphological and metabolic changes in brain radiation necrosis. Journal of Neuro‐oncology 1 May 2014;119(1):101‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Wang 2016 {published and unpublished data}
- Wang XS, Ying HM, He XY, Zhou ZR, Wu YR, Hu CS. Treatment of cerebral radiation necrosis with nerve growth factor: A prospective, randomized, controlled phase II study. Radiotherapy and Oncology 2016;120(1):69‐75. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
A221208 {published data only}
- Randomized phase II study: corticosteroids plus bevacizumab versus corticosteroids plus placebo (BeSt) for radionecrosis after radiosurgery for brain metastases. Ongoing study April 2016.
NCT02714465 {published data only}
- Treatment of adverse radiation effects after gamma knife radiosurgery (GKS) and hyperbaric oxygen therapy (HBO). Ongoing study March 2016.
Additional references
Barajas 2009
- Barajas R, Chang J, Sneed P, Segal M, McDermott M, Cha S. Distinguishing recurrent intra‐axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility‐weighted contrast‐enhanced perfusion MR imaging. AJNR American Journal of Neuroradiology 2009;30(2):367‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blonigen 2010
- Blonigen B, Steinmetz R, Levin L, Lamba M, Warnick R, Breneman J. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. International Journal of Radiation Oncology, Biology, Physics 2010;77(4):996‐1001. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323(7305):157‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dequesada 2008
- Dequesada I, Quisling R, Yachnis A, Friedman W. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic‐pathological study. Neurosurgery 2008;63(5):898‐903. [DOI] [PubMed] [Google Scholar]
Giglio 2003
- Giglio P, Gilbert M. Cerebral radiation necrosis. Neurologist 2003;9(4):180‐8. [DOI] [PubMed] [Google Scholar]
Gonzalez 2007
- Gonzalez J, Kumar A, Conrad C, Levin V. Effect of bevacizumab on radiation necrosis of the brain. International Journal of Radiation Oncology, Biology, Physics 2007;67(2):323‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Chichester: John Wiley & Sons, 2011.
Higgins 2011a
- Higgins JP, Altman DG. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoefnagels 2009
- Hoefnagels F, Lagerwaard F, Sanchez E, Haasbeek C, Knol D, Slotman B, et al. Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. Journal of Neurology 2009;256(6):878‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leber 1998
- Leber K, Eder H, Kovac H, Anegg U, Pendl G. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotactic & Functional Neurosurgery 1998;70(Suppl 1):229‐36. [DOI] [PubMed] [Google Scholar]
Lubelski 2013
- Lubelski D, Abdullah KG, Weil RJ, Marko NF. Bevacizumab for radiation necrosis following treatment of high grade glioma: a systematic review of the literature. Journal of Neuro‐oncology 2013;115(3):317‐22. [DOI] [PubMed] [Google Scholar]
Mayer 2008
- Mayer R, Sminia P. Reirradiation tolerance of the human brain. International Journal of Oncology, Biology, Physics 2008;70(5):1350‐60. [DOI] [PubMed] [Google Scholar]
McPherson 2004
- McPherson C, Warnick R. Results of contemporary surgical management of RN using frameless stereotaxis and intraoperative magnetic resonance imaging. Journal of Neuro‐oncology 2004;68:41‐7. [DOI] [PubMed] [Google Scholar]
Nordal 2004
- Nordal R, Nagy A, Pintilie M, Wong C. Hypoxia and hypoxia‐inducible factor‐1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clinical Cancer Research 2004;10(10):3342‐53. [DOI] [PubMed] [Google Scholar]
Ohguri 2007
- Ohguri T, Imada H. Effect of prophylactic hyperbaric oxygen treatment for radiation‐induced brain injury after stereotactic radiosurgery of brain metastases. International Journal of Radiation Oncology, Biology, Physics 2007;67(1):249‐55. [DOI] [PubMed] [Google Scholar]
Pasquier 2004
- Pasquier D, Hoelscher T, Schmutz J, Schmutz J, Dische S, Mathieu D, et al. Hyperbaric oxygen therapy in the treatment of radio‐induced lesions in normal tissues: a literature review. Radiotherapy and Oncology 2004;72(1):1‐13. [DOI] [PubMed] [Google Scholar]
Plateel 1995
- Plateel M, Dehouck MP. Hypoxia increases the susceptibility to oxidant stress and the permeability of the blood‐brain barrier endothelial cell monolayer. Journal of Neurochemistry 1995;65(5):2138‐45. [DOI] [PubMed] [Google Scholar]
RevMan 5 2014 [Computer program]
- Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schultheiss 1995
- Schultheiss T, Kun L, Ang K. Radiation response of the central nervous system. International Journal of Radiation Oncology, Biology, Physics 1995;31(5):1093‐112. [DOI] [PubMed] [Google Scholar]
Shaw 2000
- Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90‐05. International Journal of Radiation Oncology, Biology, Physics 2000;47(2):291‐8. [DOI] [PubMed] [Google Scholar]
Suh 2010
- Suh JH. Stereotactic radiosurgery for the management of brain metastases. New England Journal of Medicine 2010;362:1119‐27. [DOI] [PubMed] [Google Scholar]
Telera 2013
- Telera S, Fabi A, Pace A, Vidiri A, Anelli V, Carapella CM, et al. Radionecrosis induced by stereotactic radiosurgery of brain metastases: results of surgery and outcome of disease. Journal of Neuro‐oncology 2013;113(2):313‐25. [DOI] [PubMed] [Google Scholar]
Truong 2006
- Truong M, Clair E, Donahue B, Rush S, Miller D, Formenti S, et al. Results of surgical resection for progression of brain metastases previously treated by gamma knife radiosurgery. Neurosurgery 2006;59(1):86‐97. [DOI] [PubMed] [Google Scholar]
Tye 2014
- Tye K, Engelhard HH, Slavin KV, Nicholas MK, Chmura SJ, Kwok Y, et al. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. Journal of Neuro‐oncology 2014;117(2):321‐7. [DOI] [PubMed] [Google Scholar]
Williamson 2008
- Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotactic Functional Neurosurgery 2008;86(6):359‐66. [DOI] [PubMed] [Google Scholar]
Wong 2008
- Wong ET, Huberman M, Lu X, Mahadevan A. Bevacizumab reverses cerebral radiation necrosis. Journal of Clinical Oncology 2008;26(34):5649‐50. [DOI] [PubMed] [Google Scholar]
Wong 2010
- Wong ST, Loo KT, Yam KY, Hung WM, Fok KF, Yuen SC, et al. Results of excision of cerebral radionecrosis: experience in patients treated with radiation therapy for nasopharyngeal carcinoma. Journal of Neuro‐oncology 2010;113(2):293‐300. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chung 2015
- Chung C, Brown PD. Interventions for the treatment of brain radionecrosis after radiotherapy or radiosurgery. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011492] [DOI] [PMC free article] [PubMed] [Google Scholar]


