Abstract
Background
Although supervised exercise therapy (SET) provides significant symptomatic benefit for patients with intermittent claudication (IC), it remains an underutilized tool. Widespread implementation of SET is restricted by lack of facilities and funding. Structured home‐based exercise therapy (HBET) with an observation component (e.g., exercise logbooks, pedometers) and just walking advice (WA) are alternatives to SET. This is the second update of a review first published in 2006.
Objectives
The primary objective was to provide an accurate overview of studies evaluating effects of SET programs, HBET programs, and WA on maximal treadmill walking distance or time (MWD/T) for patients with IC. Secondary objectives were to evaluate effects of SET, HBET, and WA on pain‐free treadmill walking distance or time (PFWD/T), quality of life, and self‐reported functional impairment.
Search methods
The Cochrane Vascular Information Specialist searched the Cochrane Vascular Specialised Register (December 16, 2016) and the Cochrane Central Register of Controlled Trials (2016, Issue 11). We searched the reference lists of relevant studies identified through searches for other potential trials. We applied no restriction on language of publication.
Selection criteria
We included parallel‐group randomized controlled trials comparing SET programs with HBET programs and WA in participants with IC. We excluded studies in which control groups did not receive exercise or walking advice (maintained normal physical activity). We also excluded studies comparing exercise with percutaneous transluminal angioplasty, bypass surgery, or drug therapy.
Data collection and analysis
Three review authors (DH, HF, and LG) independently selected trials, extracted data, and assessed trials for risk of bias. Two other review authors (MvdH and JT) confirmed the suitability and methodological quality of trials. For all continuous outcomes, we extracted the number of participants, mean outcome, and standard deviation for each treatment group through the follow‐up period, if available. We extracted Medical Outcomes Study Short Form 36 outcomes to assess quality of life, and Walking Impairment Questionnaire outcomes to assess self‐reported functional impairment. As investigators used different scales to present results of walking distance and time, we standardized reported data to effect sizes to enable calculation of an overall standardized mean difference (SMD). We obtained summary estimates for all outcome measures using a random‐effects model. We assessed the quality of evidence using the GRADE approach.
Main results
For this update, we included seven additional studies, making a total of 21 included studies, which involved a total of 1400 participants: 635 received SET, 320 received HBET, and 445 received WA. In general, SET and HBET programs consisted of three exercise sessions per week. Follow‐up ranged from six weeks to two years. Most trials used a treadmill walking test to investigate effects of exercise therapy on walking capacity. However, two trials assessed only quality of life, functional impairment, and/or walking behavior (i.e., daily steps measured by pedometer). The overall methodological quality of included trials was moderate to good. However, some trials were small with respect to numbers of participants, ranging from 20 to 304.
SET groups showed clear improvement in MWD/T compared with HBET and WA groups, with overall SMDs at three months of 0.37 (95% confidence interval [CI] 0.12 to 0.62; P = 0.004; moderate‐quality evidence) and 0.80 (95% CI 0.53 to 1.07; P < 0.00001; high‐quality evidence), respectively. This translates to differences in increased MWD of approximately 120 and 210 meters in favor of SET groups. Data show improvements for up to six and 12 months, respectively. The HBET group did not show improvement in MWD/T compared with the WA group (SMD 0.30, 95% CI ‐0.45 to 1.05; P = 0.43; moderate‐quality evidence).
Compared with HBET, SET was more beneficial for PFWD/T but had no effect on quality of life parameters nor on self‐reported functional impairment. Compared with WA, SET was more beneficial for PFWD/T and self‐reported functional impairment, as well as for some quality of life parameters (e.g., physical functioning, pain, and physical component summary after 12 months), and HBET had no effect.
Data show no obvious effects on mortality rates. Thirteen of the 1400 participants died, but no deaths were related to exercise therapy. Overall, adherence to SET was approximately 80%, which was similar to that reported with HBET. Only limited adherence data were available for WA groups.
Authors' conclusions
Evidence of moderate and high quality shows that SET provides an important benefit for treadmill‐measured walking distance (MWD and PFWD) compared with HBET and WA, respectively. Although its clinical relevance has not been definitively demonstrated, this benefit translates to increased MWD of 120 and 210 meters after three months in SET groups. These increased walking distances are likely to have a positive impact on the lives of patients with IC. Data provide no clear evidence of a difference between HBET and WA. Trials show no clear differences in quality of life parameters nor in self‐reported functional impairment between SET and HBET. However, evidence is of low and very low quality, respectively. Investigators detected some improvements in quality of life favoring SET over WA, but analyses were limited by small numbers of studies and participants. Future studies should focus on disease‐specific quality of life and other functional outcomes, such as walking behavior and physical activity, as well as on long‐term follow‐up.
Plain language summary
Supervised exercise therapy vs home‐based exercise therapy vs walking advice for patients with leg pain while walking (intermittent claudication)
Background
Intermittent claudication is a cramping leg pain that occurs during walking and is relieved by a short period of rest. It is caused by inadequate blood flow to the muscles of the leg due to atherosclerosis (hardening of the arteries). Exercise therapy provides significant symptomatic benefit for patients with intermittent claudication. Patients are recommended to walk at least three times a week by themselves. However, they can also participate in a formal supervised exercise program that involves walking on a treadmill or complete a structured home‐based exercise program with an observation component (e.g., exercise logbooks, pedometers).
Study characteristics and key results
We included 21 trials in which a total of 1400 participants with intermittent claudication (65% male, mean age 66 years) had been assigned to supervised exercise therapy, home‐based exercise therapy, or walking advice (search last run December 2016). The overall methodological quality of included trials was moderate to good. However, some trials had enrolled only small numbers of participants. Trials lasted from six weeks to two years.
This review shows that patients participating in a supervised exercise program improve their walking ability to a greater extent than those completing a home‐based exercise program or just following walking advice. After three months, the maximal walking distance for participants following the supervised exercise program was 120 or 210 meters farther than the maximal walking distance for those who followed a home‐based exercise program or received walking advice, respectively. To put these increases in context, a US football field is roughly 90 meters (or 100 yards) long. Before participating in the exercise program, the maximal walking distance of participants was 290 meters with a pain‐free walking distance of 140 meters, so this improvement is likely to have a positive impact on their lives. Results of the home‐based exercise program were similar to those reported for walking advice.
Compared with home‐based exercise therapy, supervised exercise therapy was more beneficial for pain‐free walking distance but had no effect on quality of life measures nor on self‐reported functional impairment. Compared with walking advice, supervised exercise therapy was more beneficial for pain‐free walking distance and self‐reported functional impairment, as well as for some quality of life measures (e.g., physical functioning, pain, and physical component summary after 12 months), and home‐based exercise therapy had no effect.
Data show no obvious effects on mortality rates. Thirteen of the 1400 participants died, but no deaths were related to exercise therapy. Overall, adherence to supervised exercise therapy was approximately 80%, which was similar to that reported with home‐based exercise therapy. Only limited adherence data were available for walking advice groups.
Quality of the evidence
Evidence of moderate and high quality shows that supervised exercise therapy improves walking distance (maximal and pain‐free) to a greater extent than home‐based exercise therapy and walking advice, respectively. Trials show no clear differences in quality of life measures nor in self‐reported functional impairment between supervised exercise therapy and home‐based exercise therapy. However, evidence is of low and very low quality, respectively. Investigators detected some improvements in quality of life favoring supervised exercise therapy over walking advice, but analyses were limited by small numbers of studies and participants. More research is needed on disease‐specific quality of life and other functional outcomes, such as walking behavior and physical activity, as well as on long‐term follow‐up.
Adhering to an exercise program is important because it leads to decreased leg pain and improved quality of life, as well as to likely improvement in general physical condition.
Summary of findings
Background
Description of the condition
Peripheral artery disease (PAD) is a chronic arterial occlusive disease caused by progressive atherosclerosis. Several arterial segments can be affected, such as the aorta and iliac, femoral, popliteal, and lower leg arteries. The incidence of PAD increases progressively with age, in particular after age 40. The prevalence of PAD, defined as an ankle‐brachial index (ABI; the ratio of blood pressure in the lower legs to blood pressure in the arms) < 0.90 in either leg, is 0.9% between the ages of 40 and 49, 2.5% between the ages of 50 and 59, 4.7% between the ages of 60 and 69, and 14.5% in those 70 years of age and older (Selvin 2004). In 2010, 202 million people worldwide were coping with PAD (Fowkes 2013). During the preceding decade, the number of affected individuals increased by 13% in high‐income countries and by 29% in low‐income or middle‐income countries. These huge numbers illustrate that PAD has become a global health problem affecting vast numbers of individuals. Risk factors for the development of lower limb PAD are similar to those for coronary artery disease and include smoking, diabetes mellitus, hypertension, and hypercholesterolemia (Gerhard‐Herman 2017).
PAD presentation comprises a spectrum ranging from asymptomatic disease to intermittent claudication (IC), critical limb ischemia (CLI), and finally limb loss. The most common symptom is IC, defined as a cramping leg pain that occurs during walking and is relieved by a short period of rest. Because of this condition, patients have diminished maximal and pain‐free walking capacity. IC restricts activity and mobility and considerably reduces health‐related quality of life (Dumville 2004; McDermott 2001). In addition, IC is closely associated with cardiovascular morbidity and mortality owing to the ongoing generalized atherosclerotic process. Patients with IC have a five‐year all‐cause mortality rate of 10% to 15%, and a 20% chance of a non‐fatal cardiovascular event (Gerhard‐Herman 2017). When IC progresses to CLI, an even higher mortality rate of 25% after one year has been reported (Conte 2015).
The primary treatment goal in patients with IC is to improve ambulatory function and quality of life. Traditionally, randomized controlled trials (RCTs) of therapeutic interventions have used treadmill walking performance as an objective measure reflecting patients’ functional limitations. The graded treadmill test has a large dynamic range that can reproducibly define an individual’s PAD‐limited maximal and pain‐free walking capacity (Brass 2007; Gardner 1991; Labs 1999). Maximal walking distance or time represents a physiological peak performance based on the patient’s limb pathophysiology. When used as a primary endpoint, maximal walking capacity assessed by a graded treadmill test is sensitive to change with a variety of interventions.
Description of the intervention
Because of serious health risks, all patients with IC should receive multi‐component therapy consisting of cardiovascular risk modification, lifestyle coaching, and symptomatic treatment (Conte 2015). Symptomatic treatment options for IC include percutaneous transluminal angioplasty (PTA), bypass surgery, and drug therapy. However, current evidence supports exercise therapy as the primary treatment for improvement of walking capacity and health‐related quality of life in patients with IC (Aboyans 2017; Conte 2015; Gerhard‐Herman 2017; Layden 2012). Erb first suggested this effective treatment (Erb 1898). In 1966, the first RCT of exercise therapy for patients with IC demonstrated obvious improvement in treadmill walking ability (Larsen 1966). In a Cochrane systematic review of RCTs, Leng and later, in the updated version, Lane described significantly improved maximal walking time: mean difference 4.51 minutes (95% confidence interval [CI] 3.11 to 5.92) with overall improvement in walking ability of approximately 50% to 200% associated with exercise compared with usual care or placebo (Leng 2000; Lane 2014). However, the exercise programs included in this meta‐analysis varied widely, ranging from physician‐recommended unsupervised walking in the community to formal supervised exercise programs involving walking on a treadmill.
How the intervention might work
Exercise therapy provides significant symptomatic benefit for patients with IC. However, the exact mechanisms for this improvement remain unclear (Beckitt 2012). Mechanisms of response to exercise therapy have been reviewed previously and include improvement in walking efficiency, induction of vascular angiogenesis, reduced inflammatory activation, increased exercise pain tolerance, reduced endothelial and mitochondrial dysfunction, and metabolic adaptations within skeletal muscle (Conte 2015; Gustafsson 2001; Hamburg 2011; Stewart 2008; Zwierska 2005). Further benefits of exercise therapy include reduction in cardiovascular risk factors such as diabetes mellitus, hypertension, and hypercholesterolemia. Therefore, exercise is implemented in secondary prevention therapies for patients with coronary artery disease (Piepoli 2014; Smith 2011). Given its clear benefits, the importance of exercise therapy is highlighted in contemporary international guidelines (Aboyans 2017; Conte 2015; Gerhard‐Herman 2017; Layden 2012). Exercise programs are usually regular commitments that are run twice or three times per week for a minimum of 30 minutes per session, lasting from six weeks to a year. In daily practice, lack of specific individual guidance and absence of uniform supervision appear to be important barriers to initiation and continuation of exercise therapy (Bartelink 2004).
Why it is important to do this review
Before the original version of this review was released in 2006, prescribed exercise therapy consisted mostly of “go home and walk” advice (walking advice [WA]) received from the physician (e.g., general practitioner, vascular surgeon), sometimes accompanied by a brochure (Bendermacher 2006). After this review was published, more studies compared supervised exercise therapy (SET) with non‐supervised exercise therapy. Although SET programs proved more effective in increasing maximal and pain‐free walking distance or time compared with non‐supervised exercise programs, they remain an underutilized tool. In 2012, an international survey found that only 30% of vascular surgeons had access to SET programs, and members of this group showed significant heterogeneity in the way they implemented these programs (Makris 2012). Widespread implementation of SET is restricted by the combination of an insufficient number of available facilities and issues of reimbursement, awareness, and motivation (Conte 2015; Fokkenrood 2012; Lauret 2012; Makris 2012; Stewart 2002; Stewart 2008). To overcome some of these problems in the Netherlands, a community‐based network for SET was implemented (Lauret 2012). Community‐based SET solves the problems of transportation time and costs for individual patients, as well as the restricted capacity of hospital‐based SET (Bendermacher 2007; Kruidenier 2009). Others have suggested that exercise programs should be initiated in a home‐based environment, thereby diminishing the scope of labor‐intensive supervision (Collins 2011; Regensteiner 1997). Structured home‐based exercise therapy (HBET) with an observation component (e.g., exercise logbooks, pedometers) and specific walking advice may provide an effective alternative, especially when facilities and funding for SET programs are not available (Makris 2012).
Several reviews have assessed the value of different conservative treatment options in IC (Al‐Jundi 2013; Back 2015; Fakhry 2012; Gommans 2014; Lane 2014; Li 2015; Makris 2012; Vemulapalli 2015; Wind 2007). However, to date, few meta‐analyses have compared SET with HBET and WA. Previous versions of this Cochrane review (search last run September 2012) compared SET with non‐supervised exercise therapy that involved both WA and HBET programs (Bendermacher 2006; Fokkenrood 2013). Although these different approaches are likely to vary somewhat in terms of clinical outcomes and cost‐effectiveness (Al‐Jundi 2013), no direct comparisons between HBET and WA were made. For this update, we reviewed effects of these unsupervised exercise interventions separately. With disregard for all financial and organizational aspects, we believe it is important to provide an accurate systematic review of effects of SET programs in relation to non‐supervised exercise programs on walking distance or time, quality of life, and functional impairment for patients with IC.
Objectives
The primary objective was to provide an accurate overview of studies evaluating effects of SET programs, HBET programs, and WA on maximal treadmill walking distance or time (MWD/T) for patients with IC. Secondary objectives were to evaluate effects of SET, HBET, and WA on pain‐free treadmill walking distance or time (PFWD/T), quality of life, and self‐reported functional impairment.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group RCTs comparing SET programs with HBET programs and WA in participants with IC. We included trials irrespective of whether an intention‐to‐treat analysis was carried out.
Types of participants
Trials included adults (18 years and older) with IC (Fontaine II/Rutherford 1 to 3) due to atherosclerotic disease, diagnosed by questionnaire or clinically, who were considered for conservative treatment (Fontaine 1954; Rutherford 1997). We excluded studies of participants with asymptomatic lower limb atherosclerosis identified by testing.
Types of interventions
We included all trials comparing an SET program with an HBET program and/or WA for treatment of patients with IC. We excluded studies in which control groups did not receive exercise or walking advice (maintained normal physical activity). We also excluded studies comparing exercise with PTA, bypass surgery, or drug therapy. We included trials that compared three or more different exercise programs: SET, HBET, and/or WA versus other kinds of programs. From these trials, we considered only participants treated by SET, HBET, and WA.
Supervised exercise therapy
Treatment comprised a formal SET program provided with or without additional walking advice. An SET program had to consist of more than six consecutive weeks of training, with more than 50% of total exercise time spent on walking or training the lower limbs. Training was hospital‐based or community‐based and was provided under the supervision of a physical therapist or other medically trained personnel. Inclusion of trials was not limited by frequency, duration, or intensity of exercise sessions.
Home‐based exercise therapy
An HBET program was defined as structured walking advice supplemented with an observation component (e.g., exercise logbooks, pedometers). Training was actively monitored by medically trained personnel, and participants were prompted by regular contact and exercise support (provided face‐to‐face or by telephone).
Walking advice
WA was defined as “go home and walk” advice provided with or without a predefined exercise scheme. Participants were actively advised to increase physical activity levels by walking. However, no supervision or monitoring was provided.
Types of outcome measures
We included studies only if reported outcome measures were available at baseline and after at least six weeks of follow‐up.
Primary outcomes
Maximal treadmill walking distance or time (MWD/T)
Secondary outcomes
Pain‐free treadmill walking distance or time (PFWD/T)
Quality of life (Medical Outcomes Study Short Form 36)
Self‐reported functional impairment (Walking Impairment Questionnaire)
Mortality
Adherence to exercise program
Search methods for identification of studies
We applied no restriction on language of publication.
Electronic searches
The Cochrane Vascular Information Specialist (CIS) searched the following databases for relevant trials.
Cochrane Vascular Specialised Register (December 16, 2016)
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 11) via the Cochrane Register of Studies Online
See Appendix 1 for details of the search strategy used to search CENTRAL.
The Cochrane Vascular Specialised Register is maintained by the CIS and is constructed through weekly electronic searches of MEDLINE Ovid, Embase Ovid, CINAHL, and AMED, and by handsearching of relevant journals. The full list of databases, journals, and conference proceedings searched, as well as the search strategies used, are presented in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The CIS searched the following trial registries for details of ongoing and unpublished studies.
ClinicalTrials.gov (www.clinicaltrials.gov)
World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch)
International Standard Randomised Controlled Trial Number registry (www.isrctn.com)
Searching other resources
We searched the reference lists of relevant studies identified through searches for other potential trials.
Data collection and analysis
Selection of studies
Three review authors (DH, HF, and LG) independently selected trials for this review. Two other review authors (MvdH and JT) confirmed the suitability of selected trials for inclusion. We resolved disagreements regarding inclusion/exclusion of selected trials through discussion.
Data extraction and management
Three review authors (DH, HF, and LG) independently extracted data using a standard data collection form and entered data into Review Manager 5 software (RevMan 2014). When necessary, we sought additional information from included trials.
For all continuous outcomes (i.e., walking distance or time, quality of life, functional impairment), we extracted the number of participants, mean outcome, and standard deviation for each treatment group through the follow‐up period, if available. We also recorded other details of included trials, for example, country, study setting, inclusion and exclusion criteria, participant characteristics, types of interventions, and numbers of dropouts in each group. We contacted study authors to request missing information regarding their methods.
Assessment of risk of bias in included studies
Three review authors (DH, HF, and LG) assessed trials for risk of bias. Two other review authors (MvdH and JT) confirmed the methodological quality of trials, primarily for adequacy of allocation concealment and follow‐up. For trials that compared exercise programs with walking distance or time as the primary outcome, blinding of participants and personnel was not possible. Therefore, we did not consider this a flaw.
We graded study quality in a table of risk of bias on the basis of a checklist of design components. This checklist comprised random sequence generation, allocation concealment, blinding of outcome assessments, incomplete outcome data, and selective reporting. We achieved consensus through informal discussion. We summarized the adequacy of each category as having "low", "unclear", or "high" risk, according to criteria provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Measures of treatment effect
We standardized reported data to effect sizes to enable calculation of an overall standardized mean difference, or we calculated an overall difference in means. If standard errors had been reported (and study authors did not reply to our request to send unpublished data), we converted these to standard deviations according to instructions provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used a random‐effects model to obtain summary estimates for all outcome measures.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
We contacted study authors to request missing data. For each trial, we extracted the number of participants originally allocated to each treatment group, and we performed an intention‐to‐treat analysis.
Assessment of heterogeneity
We used both a Chi2 test and an I2 statistic to test for heterogeneity between trial results. We used tests of heterogeneity at a significance level of P < 0.10 to examine whether observed variation in trial results was compatible with the variation expected by chance alone. We pooled trial results by meta‐analysis.
Assessment of reporting biases
To prevent language bias, we did not impose a language restriction. In case of sufficient studies (≥ 10) in the largest meta‐analysis, we planned to assess publication bias by using a funnel plot (Higgins 2011).
Data synthesis
If data were available, we performed statistical analyses using Review Manager 5 software (RevMan 2014). As investigators used different scales to present results of walking distance and time (meters, seconds, or minutes), we standardized reported data to effect sizes to enable calculation of an overall standardized mean difference (SMD). In this circumstance, we used weighted means to standardize reported means and standard deviations (SDs) to a uniform scale before they could be combined. Then, we expressed the difference in mean outcome for each study relative to the SD observed in that study: SMD = (mean of group A ‐ mean of group B) / pooled SD (Altman 1991). To interpret the clinical relevance and impact of the intervention effect on walking capacity, we re‐expressed the pooled effect in meters. For this purpose, we multiplied the overall SMD by the SD for meters. We obtained this SD as the pooled SD in all studies that presented the results of walking capacity on the meter scale. If outcome measurements in all studies were based on the same scale (quality of life and functional impairment), we calculated an overall difference in means (mean difference [MD]). For studies with non‐parametric data, we calculated SDs by dividing the interquartile range (IQR) by 1.35, according to instructions provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We obtained summary estimates for all outcome measures using a random‐effects model owing to substantial heterogeneity in inclusion and exclusion criteria, participant characteristics, and types of interventions.
Subgroup analysis and investigation of heterogeneity
In the previous version of this review in 2013, review authors performed subgroup analyses by dividing non‐supervised exercise groups into HBET and WA groups (Fokkenrood 2013). However, they did not perform direct comparisons between HBET and WA. For this update, we performed separate analyses of SET versus HBET, SET versus WA, and HBET versus WA. We investigated no additional subgroups.
Sensitivity analysis
We examined individual study effects on reported results by removing each study one at a time to investigate whether excluding a particular study significantly changed the results. We excluded studies with apparent risk of bias.
"Summary of findings" tables
We constructed "Summary of findings" tables using GRADEproGDT software to present the main review findings (GRADEproGDT 2015). Because we assessed different comparisons, we developed a "Summary of findings" table for each comparison (SET vs HBET, SET vs WA, and HBET vs WA). We judged walking distance or time, quality of life, and functional impairment outcomes at three months' follow‐up as most important and clinically relevant. For quality of life and functional impairment outcomes, we decided to report only summary or combined scores. We used the system developed by the GRADE Working Group to grade the quality of evidence for each outcome as "high", "moderate", "low", or "very low", based on risk of bias, heterogeneity, and precision of effect estimates, and in keeping with guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (Atkins 2004; Higgins 2011).
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies.
Results of the search
See Figure 1.
1.
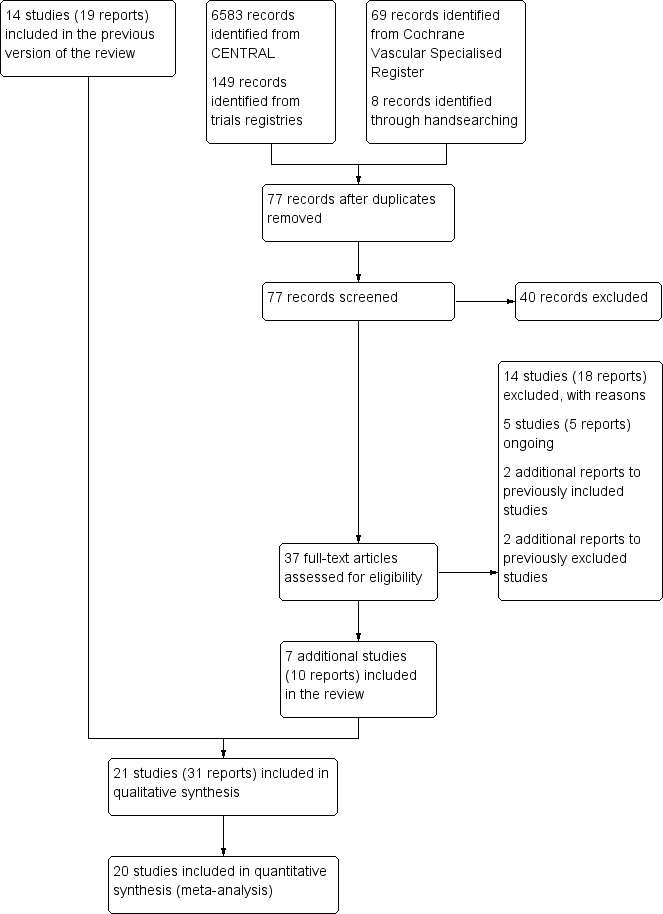
Study flow diagram.
For this update, the electronic searches identified a total of 69 potential articles and the handsearch yielded eight potential articles for inclusion. After title and abstract evaluation, 37 articles on exercise therapy in participants with IC remained for full‐text analysis. We also re‐evaluated the 72 articles documented in the previous review of 2013 (19 included and 53 excluded).
Selection process
We obtained full‐text copies of the 109 articles (37 new and 72 previously documented in the 2013 review) for further assessment. In total, we excluded 73 articles reporting on 55 studies (Characteristics of excluded studies), and we included 31 articles reporting on 21 studies (Characteristics of included studies). In addition, we identified five ongoing trials with potentially useable outcome measures (Characteristics of ongoing studies).
Unpublished data
To avoid publication bias, we contacted 11 authors of included studies to request unpublished data for assessment of primary or secondary outcome measures. Six authors provided additional data (Allen 2010; Collins 2011; Kakkos 2005; Nicolai 2010; Sanderson 2006; Treat‐Jacobson 2009). We were unable to obtain appropriate or adequate data for five studies (Cheetham 2004; Cunningham 2012; Gardner 2011; Gardner 2014; Mays 2015).
Included studies
For this update, we included seven additional studies (Allen 2010; Christman 2003; Cunningham 2012; Gardner 2014; Guidon 2013; Mays 2015; Sandercock 2007), making a total of 21 included studies, which involved a total of 1400 participants: 635 received SET, 320 received HBET, and 445 received WA. We have provided a summary of included studies in the Characteristics of included studies table. Trials were conducted in the USA (n = 11), the UK (n = 6), Australia (n = 1), Ireland (n = 1), the Netherlands (n = 1), and South Africa (n = 1). Three trials were conducted between 2013 and 2015 (Gardner 2014; Guidon 2013; Mays 2015). Four others were conducted from 2003 to 2012 but were not included in previous versions of this review (Allen 2010; Christman 2003; Cunningham 2012; Sandercock 2007).
Eight trials were relatively small, involving fewer than 30 participants (Hodges 2008; Kakkos 2005; Mays 2015; Parr 2009; Regensteiner 1997; Sanderson 2006; Savage 2001; Treat‐Jacobson 2009). Eight others included more than 30 but fewer than 70 participants (Allen 2010; Cheetham 2004; Christman 2003; Cunningham 2012; Guidon 2013; Patterson 1997; Sandercock 2007; Stewart 2008). The five remaining trials were relatively large, involving more than 70 participants (Collins 2011; Gardner 2011; Gardner 2012; Gardner 2014; Nicolai 2010). The largest trial, which was conducted in the Netherlands, consisted of 304 participants (Nicolai 2010).
Six trials compared SET with HBET (Allen 2010; Gardner 2014; Patterson 1997; Regensteiner 1997; Savage 2001; Treat‐Jacobson 2009), nine compared SET with WA (Cheetham 2004; Gardner 2012; Guidon 2013; Hodges 2008; Kakkos 2005; Nicolai 2010; Parr 2009; Sanderson 2006; Stewart 2008), and four compared HBET with WA (Christman 2003; Collins 2011; Cunningham 2012; Mays 2015). The two remaining trials investigated effects of SET, HBET, and WA using a three‐armed study design (Gardner 2011; Sandercock 2007).
Four trials investigated different modes of exercise therapy (e.g., walking, strength training, cycling, arm‐ergometry) (Gardner 2014; Parr 2009; Sanderson 2006; Treat‐Jacobson 2009). Another trial compared exercise with intermittent pneumatic foot and calf compression (Kakkos 2005). For this review, we used data from the walking groups only. One trial that was designed to investigate effects of HBET encouraged participants to walk one day per week with the study exercise instructor and other participants, as available (Collins 2011). The previous review of 2013 included this group as an SET group. For this update, we included this group as an HBET group. Another trial randomized participants to exercise therapy in the form of WA, SET, or SET with daily accelerometer feedback (Nicolai 2010). Because almost 30% of participants reported non‐use of the accelerometer, study authors decided to analyze the SET and SET with feedback groups together. We therefore did the same.
Inclusion criteria
For patients in nine trials to be eligible for inclusion, IC symptoms had to be stable for several months (Allen 2010; Cheetham 2004; Guidon 2013; Kakkos 2005; Mays 2015; Patterson 1997; Regensteiner 1997; Sanderson 2006; Stewart 2008). Other trials did not mention this criterion.
All trials included both males and females, except for one trial that included only male veterans (Regensteiner 1997). The percentage of males in SET, HBET, and WA groups was 66.4% (range 45% to 100%), 60.6% (range 44% to 100%), and 65.2% (range 54% to 89%), respectively. However, one trial did not report the sex of participants (Hodges 2008).
The mean age of participants in SET, HBET, and WA groups was 66.3 (range 57 to 69), 66.5 (range 62 to 70), and 66.6 (range 61 to 70) years, respectively. For the six trials that used age restrictions, patients had to be older than 18 years of age (Treat‐Jacobson 2009), older than 40 years of age (Collins 2011; Mays 2015), between 40 and 75 years of age (Christman 2003), older than 50 years of age (Savage 2001), or between 50 and 75 years of age (Patterson 1997).
A diagnosis of PAD was an essential inclusion criterion in all trials. In one trial, inclusion required a clinical diagnosis of IC (Savage 2001). Two others included patients with IC if the diagnosis of PAD was confirmed on Duplex ultrasonography or angiography (Kakkos 2005; Parr 2009). The 18 remaining trials included patients with IC if they had a low ABI at rest or a decrease in ABI after exercise (Allen 2010; Cheetham 2004; Christman 2003; Collins 2011; Cunningham 2012; Gardner 2011; Gardner 2012; Gardner 2014; Guidon 2013; Hodges 2008; Mays 2015; Nicolai 2010;Patterson 1997; Regensteiner 1997; Sandercock 2007; Sanderson 2006; Stewart 2008; Treat‐Jacobson 2009). Collins 2011 included only patients with a diagnosis of PAD and a diagnosis of diabetes mellitus type 1 or 2. In one trial, some participants had undergone PTA four to six weeks before baseline testing (Mays 2015). For these patients, a normal ABI was not exclusionary.
Exclusion criteria
Exclusion criteria used by included studies were variable. In general, investigators mentioned ischemic rest pain, comorbid illness with limitations in an exercise program, and recent PTA or bypass surgery. One trial excluded patients with diabetes mellitus (Regensteiner 1997), and another mentioned tobacco use as an exclusion criterion (Savage 2001). Kakkos 2005 excluded patients with MWD greater than 300 meters or less than 50 meters, and Nicolai 2010 excluded those with MWD greater than 500 meters. Two studies excluded patients for whom screening treadmill tests were different by more than 25% in terms of MWD (Kakkos 2005; Treat‐Jacobson 2009). Collins 2011 excluded patients with no available phone, and Sanderson 2006 excluded those who lived farther than 50 km from the research venue. Six trials excluded patients who used medications for treatment of IC, such as cilostazol and pentoxifylline (Cheetham 2004; Gardner 2011; Gardner 2012; Gardner 2014; Mays 2015; Savage 2001).
Interventions
Supervised exercise therapy
In 17 trials, investigators treated participants (n = 635) with an SET program. In general, SET programs consisted of three exercise sessions per week for a duration of six weeks (Parr 2009; Sanderson 2006), three months (Allen 2010; Gardner 2011; Gardner 2014; Hodges 2008; Patterson 1997; Regensteiner 1997; Sandercock 2007; Savage 2001; Treat‐Jacobson 2009), six months (Gardner 2012; Kakkos 2005), or 12 months (Nicolai 2010). However, three trials provided SET once a week for six months (Cheetham 2004), or twice a week for three months (Guidon 2013; Stewart 2008). Seven trials instructed participants to undertake further unsupervised exercise at home (Cheetham 2004; Hodges 2008; Kakkos 2005; Nicolai 2010; Sandercock 2007; Sanderson 2006; Treat‐Jacobson 2009). Two others did not provide advice or instructions to participants to perform additional exercise away from the research center (Gardner 2011; Gardner 2012).
Generally, a training session involved walking on a treadmill with varying intensity until moderate or intense pain occurred, and this was followed by a short period of rest. Four trials described an alternative training regimen with walking training as the dominant exercise but with additional exercises for lower limb strengthening or cardiovascular training (Cheetham 2004; Guidon 2013; Parr 2009; Patterson 1997). One trial did not include walking and described exercise as mainly focused on the calf muscle (Stewart 2008). The duration of each SET session varied between 30 and 70 minutes.
Five trials provided participants with an initial SET program of three months and an additional unsupervised follow‐up period of three months (Patterson 1997; Savage 2001; Stewart 2008; Treat‐Jacobson 2009), or nine months (Guidon 2013). Two studies treated participants with an initial six months of SET and an additional six months of unsupervised follow‐up (Cheetham 2004; Kakkos 2005). The remaining trials did not include such follow‐up periods. In one trial, participants attended weekly health education lectures related to PAD (Patterson 1997), and in three others, participants maintained a record of any exercise performed beyond SET (Regensteiner 1997; Sandercock 2007; Treat‐Jacobson 2009).
Home‐based exercise therapy
In 12 trials, investigators treated participants (n = 320) with an HBET program. Investigators designed HBET programs to be similar to SET programs. All trials provided participants with a specific exercise prescription. HBET programs consisted of three exercise sessions per week for a duration of three months (Allen 2010; Christman 2003; Gardner 2011; Gardner 2014; Mays 2015; Patterson 1997; Regensteiner 1997; Sandercock 2007; Treat‐Jacobson 2009), four months (Cunningham 2012), or six months (Collins 2011; Savage 2001). One trial encouraged participants to walk one day per week with the study exercise instructor and other participants, as available (Collins 2011). For a second trial, the intervention comprised two sessions provided by a trainee health psychologist, trained in motivational interviewing techniques, delivered at participants' homes (Cunningham 2012). For a third trial, the investigator met with participants after four and eight weeks to walk in the community setting of their choice (Mays 2015). For all trials, a training session involved walking until moderate or intense pain occurred, and this was followed by a short period of rest. The duration of each HBET session varied between 30 and 50 minutes.
All HBET programs included follow‐up with a healthcare professional, provided face‐to‐face (Christman 2003; Gardner 2011; Gardner 2014; Patterson 1997; Treat‐Jacobson 2009), or by telephone (Allen 2010; Collins 2011; Cunningham 2012; Mays 2015; Regensteiner 1997; Sandercock 2007; Savage 2001). However, the frequency of follow‐up was weekly (Christman 2003; Mays 2015; Patterson 1997; Regensteiner 1997; Sandercock 2007; Treat‐Jacobson 2009), biweekly (Collins 2011; Gardner 2011), triweekly (Allen 2010), monthly (Gardner 2014; Savage 2001), or once per six weeks (Cunningham 2012). In addition, nine trials provided exercise logbooks (Allen 2010; Christman 2003; Gardner 2011; Gardner 2014; Mays 2015; Patterson 1997; Regensteiner 1997; Sandercock 2007; Treat‐Jacobson 2009), and five trials used pedometers (Christman 2003; Collins 2011; Gardner 2011; Gardner 2014; Mays 2015). Five HBET programs included education about PAD (Christman 2003; Collins 2011; Cunningham 2012; Mays 2015; Patterson 1997). Four trials involved participants in psychological interventions/behavior change techniques including goal setting, barrier identification, and problem solving (Christman 2003; Collins 2011; Cunningham 2012; Mays 2015).
One trial provided participants with an initial HBET program of three months, consisting of weekly group education sessions and an individualized exercise prescription, and an additional three months of follow‐up phone calls every two weeks (Christman 2003). Two studies treated participants with an initial three months of HBET and an additional three months of unstructured follow‐up (Patterson 1997; Treat‐Jacobson 2009). The remaining trials did not include such follow‐up periods.
Walking advice
In 15 trials, participants (n = 445) received WA. Generally, investigators encouraged participants to walk more on their own but did not provide specific recommendations regarding an exercise program (Christman 2003; Collins 2011; Cunningham 2012; Gardner 2011; Gardner 2012; Guidon 2013; Hodges 2008; Mays 2015; Parr 2009; Sandercock 2007; Sanderson 2006; Stewart 2008). However, three trials advised participants to walk at least three times weekly to near‐maximal pain (Cheetham 2004; Kakkos 2005; Nicolai 2010). Besides providing WA, five trials treated participants with standard medical therapy (i.e., antiplatelet therapy, lipid‐lowering therapy, modification of other atherosclerotic risk factors) (Cheetham 2004; Cunningham 2012; Kakkos 2005; Nicolai 2010; Sanderson 2006). Two others provided no other risk factor management or lifestyle modification to any study groups (Gardner 2011; Gardner 2012). In two trials, participants received additional written exercise advice or a brochure (Cheetham 2004; Nicolai 2010), and in another, participants viewed an educational video about PAD (Collins 2011).
Outcome measures
Most trials used a treadmill walking test to investigate effects of exercise therapy on walking capacity. However, two trials assessed only quality of life, functional impairment, and/or walking behavior (i.e., daily steps measured by pedometer) (Cunningham 2012; Guidon 2013). Nineteen trials measured MWD/T (Allen 2010; Cheetham 2004; Christman 2003; Collins 2011; Gardner 2011; Gardner 2012; Gardner 2014; Hodges 2008; Kakkos 2005; Mays 2015; Nicolai 2010; Parr 2009; Patterson 1997; Regensteiner 1997; Sandercock 2007; Sanderson 2006; Savage 2001; Stewart 2008; Treat‐Jacobson 2009), 16 trials PFWD/T (Allen 2010; Cheetham 2004; Christman 2003; Collins 2011; Gardner 2011; Gardner 2012; Gardner 2014; Kakkos 2005; Mays 2015; Parr 2009; Patterson 1997; Regensteiner 1997; Sanderson 2006; Savage 2001; Stewart 2008; Treat‐Jacobson 2009), and one trial functional treadmill walking distance or time (FWD/T) (Nicolai 2010). Treadmill tests used varied between trials; three used a fixed protocol (Cheetham 2004; Kakkos 2005; Stewart 2008), and the other 16 used a graded protocol. Ten of these trials used the Gardner‐Skinner protocol, as presented in Gardner 1991 (Allen 2010; Collins 2011; Gardner 2011; Gardner 2012; Gardner 2014; Hodges 2008; Mays 2015; Nicolai 2010; Parr 2009; Savage 2001). Additionally, three trials used a six‐minute walk test (6MWT) to assess walking capacity (Gardner 2012; Gardner 2014; Parr 2009). We calculated data on MWD derived from Cheetham 2004 on the basis of the P value and assumed that SDs of both groups were equal. We extracted data on MWT and PFWT from the accompanying figure in Patterson 1997 and analyzed FWD outcomes from Nicolai 2010 as PFWD outcomes.
Thirteen trials considered participant‐reported outcomes (Cheetham 2004; Collins 2011; Cunningham 2012; Gardner 2011; Gardner 2012; Gardner 2014; Guidon 2013; Kakkos 2005; Mays 2015; Nicolai 2010; Patterson 1997; Regensteiner 1997; Savage 2001). Use of quality of life measures varied among included studies, with some using only generic instruments and others using a combination of both generic and disease‐specific instruments. Ten trials used the Medical Outcomes Study (MOS) Short Form (SF) 36 (Cheetham 2004; Collins 2011; Gardner 2011; Gardner 2014; Guidon 2013; Kakkos 2005; Mays 2015; Nicolai 2010; Patterson 1997; Savage 2001), and one trial the MOS SF 20 (Regensteiner 1997). Nine trials used the Walking Impairment Questionnaire (WIQ) (Collins 2011; Gardner 2011; Gardner 2012; Gardner 2014; Guidon 2013; Kakkos 2005; Mays 2015; Nicolai 2010; Regensteiner 1997). Additionally, three trials used the Intermittent Claudication Questionnaire (Cunningham 2012; Guidon 2013; Kakkos 2005), one trial the Charing Cross Claudication Questionnaire (Cheetham 2004), one trial the Geriatric Depression Score (Collins 2011), one trial the Exercise Behaviors Questionnaire (Collins 2011), one trial the World Health Organization Quality of Life instrument (BREF) (Cunningham 2012), and one trial the Baltimore Activity Scale for Intermittent Claudication (Gardner 2011). We analyzed SF‐20 outcomes from Regensteiner 1997 as SF‐36 outcomes.
Nine trials measured adherence to the SET program by registering attendance at exercise sessions (Cheetham 2004; Gardner 2011; Gardner 2012; Gardner 2014; Kakkos 2005; Parr 2009; Patterson 1997; Stewart 2008; Treat‐Jacobson 2009). Four trials measured adherence in the HBET group by using exercise logbooks (Gardner 2011; Gardner 2014; Mays 2015; Treat‐Jacobson 2009), and one trial in the WA group by using self‐reported compliance (Cheetham 2004).
Cunningham 2012 did not report useable outcome measures for walking distance or time, quality of life, or functional impairment.
Excluded studies
For this update, we excluded 14 additional studies (Castro‐Sanchez 2013; Collins 2007; Collins 2012; Cucato 2013; Delaney 2014; Jakubseviciene 2014; Lee 2007; McDermott 2013; Mika 2013; Parmenter 2013; Pilz 2014; Spafford 2014; Tew 2015; Ventura 1984). We have provided a summary of excluded studies in the Characteristics of excluded studies table. Overall, we excluded 55 studies. Twenty‐four trials compared SET, HBET, or WA with no exercise (Collins 2007; Crowther 2008; Cucato 2013; Fowler 2002; Gardner 2001; Gibellini 2000; Hiatt 1990; Hobbs 2007; Jansen 1991; Langbein 2002; Leon 2005; McDermott 2004; McDermott 2013; Mika 2005; Mika 2011; Schlager 2011; Tew 2009; Tew 2015; Tisi 1997; Tsai 2002; Ventura 1984; Walker 2000; Wood 2006; Zwierska 2005). Seven trials compared exercise with placebo or drug treatment (Arosio 1999; Arosio 2001; Castro‐Sanchez 2013; Ciuffetti 1994; Dahllof 1976; Larsen 1966; Lepantalo 1991). Five trials compared exercise with invasive treatments (Gelin 2001; Greenhalgh 2008; Kruidenier 2011; Murphy 2012; Spronk 2009). Thirteen trials compared different treatment protocols of SET, HBET, or WA (Collins 2012; Delaney 2014; Gardner 2005; Hiatt 1994; Jakubseviciene 2014; Krause 1974; Manfredini 2008; McDermott 2009; Mika 2013; Parmenter 2013; Ritti‐Dias 2010; Spafford 2014; Tebbutt 2011). We excluded six trials because they were not RCTs (Degischer 2002; Fakhry 2011; Lee 2007; Nielsen 1975; Nielsen 1977; Pilz 2014). Review authors had included three of these trials in the original review of 2006 (Degischer 2002; Nielsen 1975; Nielsen 1977). However, we decided that only RCTs would be included in the previous review of 2013 and in the current update.
Ongoing studies
For this update, we found five ongoing trials (ACTRN12616000243415; NCT02075502; NCT02341716; NCT02729090; NCT02879019). We have provided a summary of study protocols in the Characteristics of ongoing studies table.
Risk of bias in included studies
2.
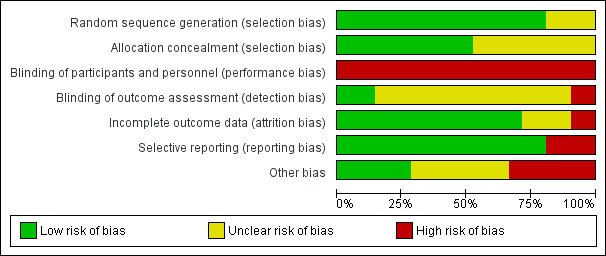
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
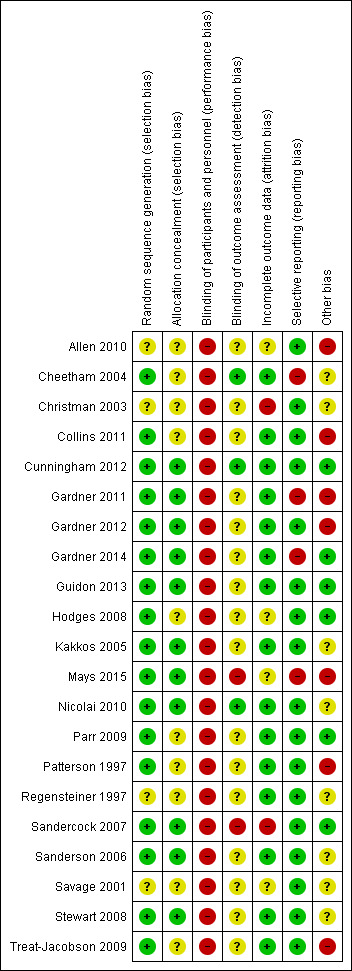
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
We judged 17 studies to have low risk of selection bias owing to adequate generation of a randomized sequence because investigators referred to a random number table (Hodges 2008; Mays 2015; Patterson 1997; Sandercock 2007; Treat‐Jacobson 2009), used a computer random number generator (Cheetham 2004; Collins 2011; Cunningham 2012; Gardner 2011; Gardner 2012; Gardner 2014; Guidon 2013; Kakkos 2005; Nicolai 2010), shuffled envelopes (Sanderson 2006; Stewart 2008), or drew lots (Parr 2009). The four remaining studies provided insufficient information about the sequence generation process to permit a judgement (Allen 2010; Christman 2003; Regensteiner 1997; Savage 2001).
Allocation concealment
We judged 11 studies to have low risk of selection bias owing to adequate concealment of allocations before assignment due to central allocation (Cunningham 2012; Gardner 2011;Gardner 2012; Gardner 2014; Kakkos 2005; Mays 2015; Nicolai 2010; Sandercock 2007), or sealed envelopes (Guidon 2013; Sanderson 2006; Stewart 2008). The ten remaining studies provided insufficient information to permit a judgement (Allen 2010; Cheetham 2004; Christman 2003; Collins 2011; Hodges 2008; Parr 2009; Patterson 1997; Regensteiner 1997; Savage 2001; Treat‐Jacobson 2009).
Blinding
Blinding of participants and personnel
For trials that compared exercise programs with walking distance or time as the primary outcome, blinding of participants and personnel was not possible. For this reason, bias could have been introduced. However, given that all studies experienced the same limitation, we did not consider lack of blinding a flaw.
Blinding of outcome assessment
We judged three studies to have low risk of detection bias because investigators ensured blinding of outcome assessment (Cheetham 2004; Cunningham 2012; Nicolai 2010). We judged two others to have high risk of detection bias because outcome assessment was not blinded (Mays 2015; Sandercock 2007). The 16 remaining studies provided insufficient information to permit a judgement (Allen 2010; Christman 2003; Collins 2011; Gardner 2011; Gardner 2012; Gardner 2014; Guidon 2013; Hodges 2008; Kakkos 2005; Parr 2009; Patterson 1997; Regensteiner 1997; Sanderson 2006; Savage 2001; Stewart 2008; Treat‐Jacobson 2009).
Incomplete outcome data
We judged 15 studies to have low risk of attrition bias because no outcome data were missing (Regensteiner 1997), missing outcome data were balanced in numbers across intervention groups with similar reasons for missing data across groups (Cheetham 2004; Collins 2011; Gardner 2011; Gardner 2012; Guidon 2013; Kakkos 2005; Nicolai 2010; Parr 2009; Patterson 1997; Sanderson 2006; Stewart 2008; Treat‐Jacobson 2009), or because investigators imputed missing data via appropriate methods (Cunningham 2012; Gardner 2014). We judged two others to have high risk of attrition bias due to the quantity, nature, or handling of incomplete outcome data (Christman 2003;Sandercock 2007). For one trial, reasons for missing outcome data were likely to be related to true outcome with imbalance in numbers and reasons (health problems) for missing data across intervention groups (Christman 2003). For the other trial, investigators imputed missing data via potentially inappropriate methods (when data were missing, most recent recorded values were carried forward) (Sandercock 2007). The four remaining studies reported attrition insufficiently to permit a judgement (Allen 2010; Hodges 2008; Mays 2015; Savage 2001). Nine trials reported an intention‐to‐treat analysis (Collins 2011; Cunningham 2012; Gardner 2011; Gardner 2012; Gardner 2014; Kakkos 2005; Mays 2015; Nicolai 2010; Sandercock 2007). Overall, 236 participants (16.9%) were lost to follow‐up (SET: n = 113, 17.8%; HBET: n = 55, 17.2%; WA: n = 68, 15.3%).
Selective reporting
We judged 17 studies to have low risk of reporting bias because published reports included all expected outcomes (Christman 2003; Collins 2011; Cunningham 2012; Gardner 2012; Guidon 2013; Hodges 2008; Parr 2009; Patterson 1997; Regensteiner 1997; Sandercock 2007; Sanderson 2006; Savage 2001; Stewart 2008; Treat‐Jacobson 2009), or because we could obtain missing outcomes from study authors (Allen 2010; Kakkos 2005; Nicolai 2010). Through contact with study authors, we obtained unpublished MWT and PFWT data from one trial (Allen 2010), SF‐36 data from one trial (Nicolai 2010), and WIQ data from two trials (Kakkos 2005; Nicolai 2010). We judged the four remaining studies to have high risk of reporting bias because investigators did not report all outcomes (Cheetham 2004; Gardner 2011; Gardner 2014; Mays 2015). We could not obtain unpublished PFWD data from one trial (Cheetham 2004), SF‐36 data from four trials (Cheetham 2004; Gardner 2011; Gardner 2014; Mays 2015), and WIQ data from one trial (Gardner 2014). We obtained no study protocols of trials.
Other potential sources of bias
We included only eight studies in the largest meta‐analysis (Analysis 1.2). Therefore, we could not detect publication bias by using a funnel plot. However, review authors observed no asymmetrical plots in the previous review of 2013, indicating that publication bias was minimal (Fokkenrood 2013). We judged seven studies to have high risk of participation bias (Allen 2010; Collins 2011; Gardner 2011; Gardner 2012; Mays 2015; Patterson 1997; Treat‐Jacobson 2009). Participants in these trials were volunteers. Therefore, they may represent those more interested in exercise. We judged eight others to have unclear risk of bias for other reasons (Cheetham 2004; Christman 2003; Kakkos 2005; Nicolai 2010; Regensteiner 1997; Sanderson 2006; Savage 2001; Stewart 2008). Four trials reported outcomes in medians and IQRs (Cheetham 2004; Kakkos 2005; Nicolai 2010; Stewart 2008). For these trials, we analyzed medians as means and calculated SDs as described earlier. This could have led to potential bias. We judged the six remaining studies to be free of other sources of bias (Cunningham 2012; Gardner 2014; Guidon 2013; Hodges 2008; Parr 2009; Sandercock 2007).
1.2. Analysis.
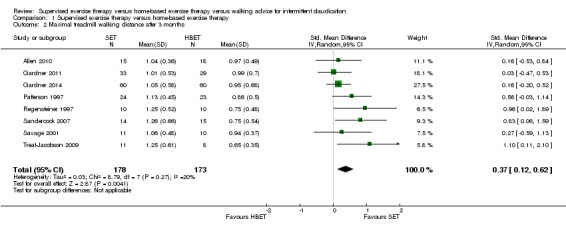
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 2 Maximal treadmill walking distance after 3 months.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Supervised exercise therapy versus home‐based exercise therapy for intermittent claudication.
| Supervised exercise therapy versus home‐based exercise therapy for intermittent claudication | ||||||
| Patient or population: patients with intermittent claudication Setting: community‐based/hospital‐based Intervention: supervised exercise therapy Comparison: home‐based exercise therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with home‐based exercise therapy | Risk with supervised exercise therapy | |||||
| Maximal treadmill walking distance after 3 months | Mean MWD after 3 months with HBET was approximately 590 meters. | Mean MWD after 3 months with SET was SMD 0.37 higher (0.12 higher to 0.62 higher). | ‐ | 351 (8 RCTs) | ⊕⊕⊕⊝ MODERATEa |
This translates to a difference in increased MWD of approximately 120 meters in favor of the SET group (a US football field is roughly 90 meters [or 100 yards] long). |
| Pain‐free treadmill walking distance after 3 months | Mean PFWD after 3 months with HBET was approximately 180 meters. | Mean PFWD after 3 months with SET was SMD 0.51 higher (0.21 higher to 0.81 higher). | ‐ | 322 (7 RCTs) | ⊕⊕⊕⊝ MODERATEa |
This translates to a difference in increased PFWD of approximately 120 meters in favor of the SET group. |
|
Quality of life ‐ physical after 3 months (Short Form 36 physical component summary) Scale: 1 to 100 (higher scores indicate better quality of life) |
Mean SF‐36 PCS after 3 months with HBET was 40. | Mean SF‐36 PCS after 3 months with SET was 0.00 higher (‐4.79 lower to 4.79 higher). | ‐ | 68 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b |
‐ |
|
Quality of life ‐ mental after 3 months (Short Form 36 mental component summary) Scale: 1 to 100 (higher scores indicate better quality of life) |
Mean SF‐36 MCS after 3 months with HBET was 53. | Mean SF‐36 MCS after 3 months with SET was 1.19 higher (‐4.47 lower to 6.86 higher). | ‐ | 68 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b |
‐ |
|
Self‐reported functional impairment after 3 months (Walking Impairment Questionnaire combined) Scale: 1 to 100 (higher scores indicate better walking) |
Mean WIQ combined score after 3 months with HBET was 44. | Mean WIQ combined score after 3 months with SET was MD ‐5.00 lower (‐19.19 lower to 9.19 higher). | ‐ | 62 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc,d |
‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HBET: home‐based exercise therapy; MCS: mental component summary; MD: mean difference; MWD: maximal treadmill walking distance; PCS: physical component summary; PFWD: pain‐free treadmill walking distance; RCT: randomized controlled trial; SET: supervised exercise therapy; SF‐36: Medical Outcomes Study Short Form 36; SMD: standardized mean difference; WIQ: Walking Impairment Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level because results were based on a relatively small sample size. bWe downgraded by one level because of high risk of reporting bias due to unpublished data (Gardner 2011; Gardner 2014). cWe downgraded by two levels because results were based on a relatively small sample size and only one study. dWe downgraded by one level because of high risk of reporting bias due to unpublished data (Gardner 2014).
Summary of findings 2. Supervised exercise therapy versus walking advice for intermittent claudication.
| Supervised exercise therapy versus walking advice for intermittent claudication | ||||||
| Patient or population: patients with intermittent claudication Setting: community‐based/hospital‐based Intervention: supervised exercise therapy Comparison: walking advice | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with walking advice | Risk with supervised exercise therapy | |||||
| Maximal treadmill walking distance after 3 months | Mean MWD after 3 months with WA was approximately 230 meters. | Mean MWD after 3 months with SET was SMD 0.80 higher (0.53 higher to 1.07 higher). | ‐ | 624 (7 RCTs) | ⊕⊕⊕⊕ HIGH |
This translates to a difference in increased MWD of approximately 210 meters in favor of the SET group (a US football field is roughly 90 meters [or 100 yards] long). |
| Pain‐free treadmill walking distance after 3 months | Mean PFWD after 3 months with WA was approximately 190 meters. | Mean PFWD after 3 months with SET was SMD 0.74 higher (0.56 higher to 0.93 higher). | ‐ | 508 (4 RCTs) | ⊕⊕⊕⊕ HIGH |
This translates to a difference in increased PFWD of approximately 140 meters in favor of the SET group. |
|
Quality of life ‐ physical after 3 months (Short Form 36 physical component summary) Scale: 1 to 100 (higher scores indicate better quality of life) |
Mean SF‐36 PCS after 3 months with WA was 39. | Mean SF‐36 PCS after 3 months with SET was 0.47 higher (‐1.74 lower to 2.69 higher). | ‐ | 296 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b |
‐ |
|
Quality of life ‐ mental after 3 months (Short Form 36 mental component summary) Scale: 1 to 100 (higher scores indicate better quality of life) |
Mean SF‐36 MCS after 3 months with WA was 53. | Mean SF‐36 MCS after 3 months with SET was 0.41 higher (‐2.18 lower to 3.00 higher). | ‐ | 296 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b |
‐ |
|
Self‐reported functional impairment after 3 months (Walking Impairment Questionnaire combined) Scale: 1 to 100 (higher scores indicate better walking) |
Mean WIQ combined score after 3 months with WA was 49. | Mean WIQ combined score after 3 months with SET was MD 2.99 higher (‐1.65 lower to 7.63 higher). | ‐ | 483 (4 RCTs) | ⊕⊕⊕⊕ HIGH |
‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MCS: mental component summary; MD: mean difference; MWD: maximal treadmill walking distance; PCS: physical component summary; PFWD: pain‐free treadmill walking distance; RCT: randomized controlled trial; SET: supervised exercise therapy; SF‐36: Medical Outcomes Study Short Form 36; SMD: standardized mean difference; WA: walking advice; WIQ: Walking Impairment Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level because results were based on a relatively small sample size. bWe downgraded by one level because of high risk of reporting bias due to unpublished data (Cheetham 2004; Gardner 2011).
Summary of findings 3. Home‐based exercise therapy versus walking advice for intermittent claudication.
| Home‐based exercise therapy versus walking advice for intermittent claudication | ||||||
| Patient or population: patients with intermittent claudication Setting: community‐based/hospital‐based Intervention: home‐based exercise therapy Comparison: walking advice | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with walking advice | Risk with home‐based exercise therapy | |||||
| Maximal treadmill walking distance after 3 months | Mean MWD after 3 months with WA was approximately 490 meters. | Mean MWD after 3 months with HBET was SMD 0.30 higher (‐0.45 lower to 1.05 higher). | ‐ | 137 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa |
This translates to no clear difference in increased MWD in favor of the HBET group. |
| Pain‐free treadmill walking distance after 3 months | Mean PFWD after 3 months with WA was approximately 190 meters. | Mean PFWD after 3 months with HBET was SMD 0.65 higher (‐0.51 lower to 1.82 higher). | ‐ | 107 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b |
This translates to no clear difference in increased PFWD in favor of the HBET group. |
|
Quality of life ‐ physical after 3 months (Short Form 36 physical component summary) Scale: 1 to 100 (higher scores indicate better quality of life) |
Mean SF‐36 PCS after 3 months with WA was 41. | Mean SF‐36 PCS after 3 months with HBET was 4.50 higher (2.05 higher to 6.95 higher). | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc,d |
‐ |
|
Quality of life ‐ mental after 3 months (Short Form 36 mental component summary) Scale: 1 to 100 (higher scores indicate better quality of life) |
Mean SF‐36 MCS after 3 months with WA was 48. | Mean SF‐36 MCS after 3 months with HBET was 7.10 higher (4.03 higher to 10.17 higher). | ‐ | 20 (1 RCT) | ⊕⊝⊝⊝ VERY LOWc,d |
‐ |
|
Self‐reported functional impairment after 3 months (Walking Impairment Questionnaire combined) Scale: 1 to 100 (higher scores indicate better walking) |
Mean WIQ combined score after 3 months with WA was 46. | Mean WIQ combined score after 3 months with HBET was MD 8.09 higher (‐9.43 lower to 25.60 higher). | ‐ | 79 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa |
‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HBET: home‐based exercise therapy; MCS: mental component summary; MD: mean difference; MWD: maximal treadmill walking distance; PCS: physical component summary; PFWD: pain‐free treadmill walking distance; RCT: randomized controlled trial; SF‐36: Medical Outcomes Study Short Form 36; SMD: standardized mean difference; WA: walking advice; WIQ: Walking Impairment Questionnaire. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level because results were based on a relatively small sample size. bWe downgraded by one level because of heterogeneity in results. cWe downgraded by two levels because results were based on a relatively small sample size and only one study. dWe downgraded by one level because of high risk of reporting bias due to unpublished data (Gardner 2011).
For this update, we calculated an overall standardized mean difference (SMD) for MWD/T and PFWD/T outcomes and an overall difference in means (mean difference [MD]) for SF‐36 and WIQ outcomes.
Supervised exercise therapy versus home‐based exercise therapy
Primary outcome
Maximal treadmill walking distance or time: SET versus HBET
Data on MWD/T after six weeks were available for one trial with a total sample size of 29 participants (Sandercock 2007). Eight trials (n = 351) repeated this outcome after three months (Allen 2010; Gardner 2011; Gardner 2014; Patterson 1997; Regensteiner 1997; Sandercock 2007; Savage 2001; Treat‐Jacobson 2009), and three trials (n = 75) after six months (Patterson 1997; Savage 2001; Treat‐Jacobson 2009).
At six weeks, MWD/T was increased with an overall SMD of 0.93 (95% CI 0.15 to 1.70; P = 0.02; low‐quality evidence) in favor of the SET group. See Analysis 1.1. At three months, the benefit of SET was maintained with an overall SMD of 0.37 (95% CI 0.12 to 0.62; P = 0.004; moderate‐quality evidence). See Analysis 1.2. This translates to a difference in favor of the SET group of approximately 120 meters in increased MWD. At six months, the overall SMD was increased to 0.68 (95% CI 0.07 to 1.30; P = 0.03; moderate‐quality evidence). See Analysis 1.3. Included studies were shown to be homogeneous at three months (I2 = 20%; P = 0.27) and six months (I2 = 36%; P = 0.21).
1.1. Analysis.
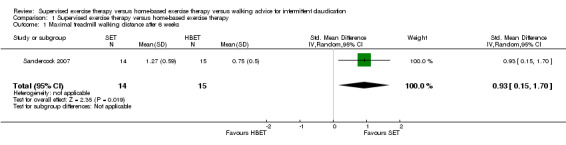
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 1 Maximal treadmill walking distance after 6 weeks.
1.3. Analysis.
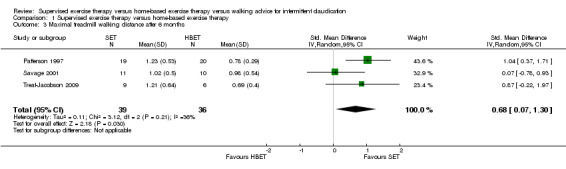
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 3 Maximal treadmill walking distance after 6 months.
Secondary outcomes
Pain‐free treadmill walking distance or time: SET versus HBET
Data on PFWD/T after three months were available for seven trials with a total sample size of 322 participants (Allen 2010; Gardner 2011; Gardner 2014; Patterson 1997; Regensteiner 1997; Savage 2001; Treat‐Jacobson 2009). Three trials (n = 75) repeated this outcome after six months (Patterson 1997; Savage 2001; Treat‐Jacobson 2009).
At three months, PFWD/T was increased with an overall SMD of 0.51 (95% CI 0.21 to 0.81; P = 0.0009; moderate‐quality evidence) in favor of the SET group. See Analysis 1.4. This translates to a difference in favor of the SET group of approximately 120 meters in increased PFWD. At six months, the overall SMD was increased to 1.13 (95% CI 0.63 to 1.63; P < 0.00001; moderate‐quality evidence). See Analysis 1.5. Included studies were shown to be homogeneous at three months (I2 = 35%; P = 0.16) and six months (I2 = 0%; P = 0.58).
1.4. Analysis.
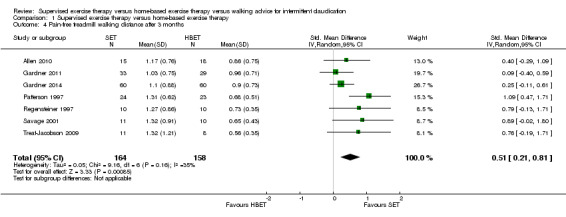
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 4 Pain‐free treadmill walking distance after 3 months.
1.5. Analysis.
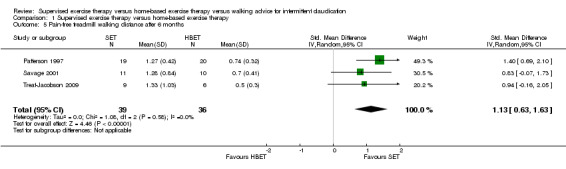
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 5 Pain‐free treadmill walking distance after 6 months.
Quality of life (SF‐36/SF‐20): SET versus HBET
At three months, three trials (n = 130) used the SF‐36 (Gardner 2011; Patterson 1997; Savage 2001), and one trial (n = 20) the SF‐20 (Regensteiner 1997). At six months, two trials (n = 60) used the SF‐36 (Patterson 1997; Savage 2001).
At three months, Gardner 2011, Patterson 1997, Regensteiner 1997, and Savage 2001 reported the following subscales: physical functioning (four studies; Analysis 1.6), role physical (two studies; Analysis 1.7), role emotional (two studies; Analysis 1.8), vitality (two studies; Analysis 1.9), emotional well‐being (three studies; Analysis 1.10), social functioning (three studies; Analysis 1.11), pain (two studies; Analysis 1.12), general health (three studies; Analysis 1.13), physical component summary (two studies; Analysis 1.14), and mental component summary (two studies; Analysis 1.15). Data show no clear differences between SET and HBET groups for any subscale. We judged the quality of evidence for these SF‐36/SF‐20 outcomes at three months to be very low to moderate.
1.6. Analysis.
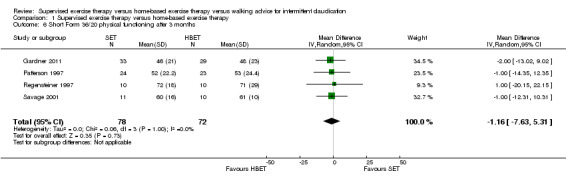
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 6 Short Form 36/20 physical functioning after 3 months.
1.7. Analysis.
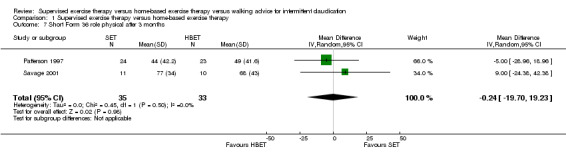
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 7 Short Form 36 role physical after 3 months.
1.8. Analysis.
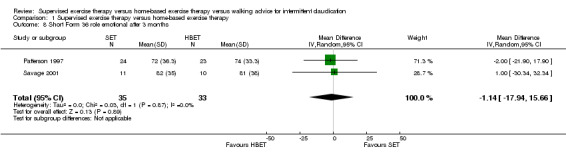
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 8 Short Form 36 role emotional after 3 months.
1.9. Analysis.
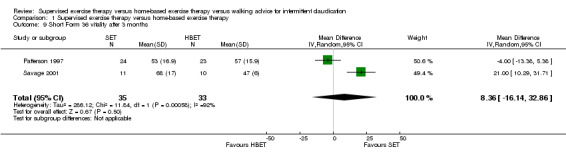
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 9 Short Form 36 vitality after 3 months.
1.10. Analysis.
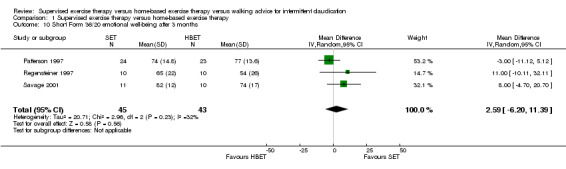
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 10 Short Form 36/20 emotional well‐being after 3 months.
1.11. Analysis.
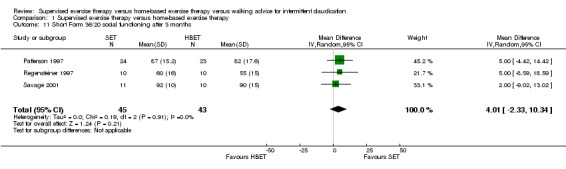
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 11 Short Form 36/20 social functioning after 3 months.
1.12. Analysis.
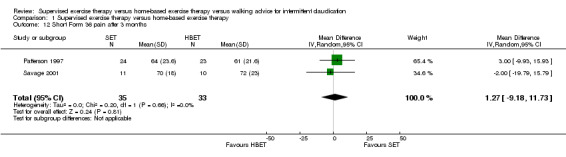
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 12 Short Form 36 pain after 3 months.
1.13. Analysis.
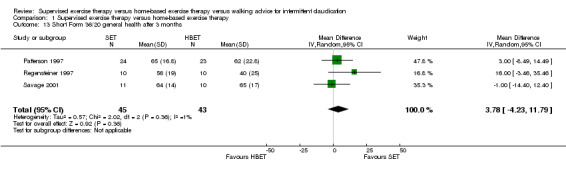
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 13 Short Form 36/20 general health after 3 months.
1.14. Analysis.
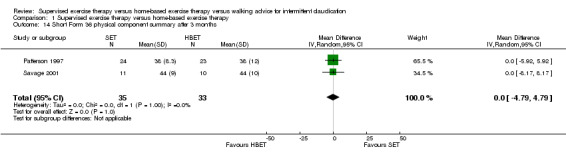
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 14 Short Form 36 physical component summary after 3 months.
1.15. Analysis.
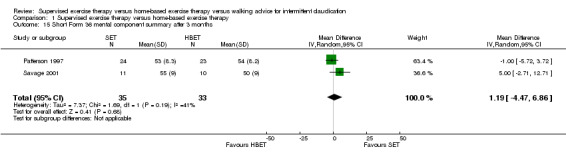
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 15 Short Form 36 mental component summary after 3 months.
At six months, Patterson 1997 and Savage 2001 reported the following subscales: physical functioning (Analysis 1.16), role physical (Analysis 1.17), role emotional (Analysis 1.18), vitality (Analysis 1.19), emotional well‐being (Analysis 1.20), social functioning (Analysis 1.21), pain (Analysis 1.22), general health (Analysis 1.23), physical component summary (Analysis 1.24), and mental component summary (Analysis 1.25). Data show no clear differences between SET and HBET groups for any subscale. We judged the quality of evidence for these SF‐36 outcomes at six months to be moderate.
1.16. Analysis.
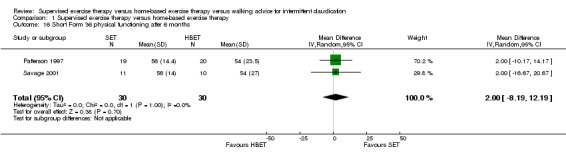
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 16 Short Form 36 physical functioning after 6 months.
1.17. Analysis.
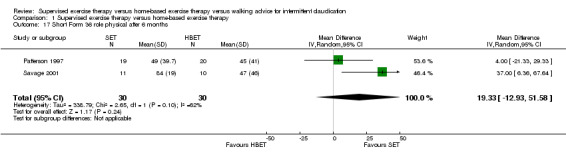
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 17 Short Form 36 role physical after 6 months.
1.18. Analysis.
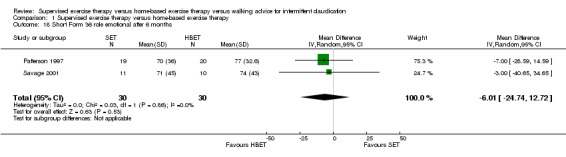
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 18 Short Form 36 role emotional after 6 months.
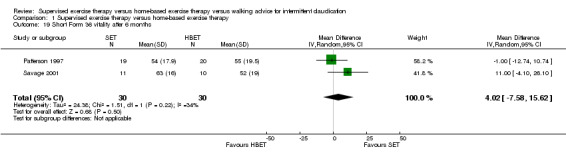
1.19. Analysis.
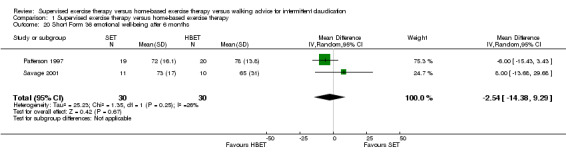
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 19 Short Form 36 vitality after 6 months.
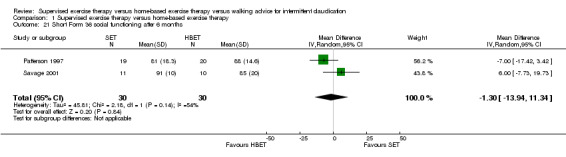
1.20. Analysis.
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 20 Short Form 36 emotional well‐being after 6 months.
1.21. Analysis.
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 21 Short Form 36 social functioning after 6 months.
1.22. Analysis.
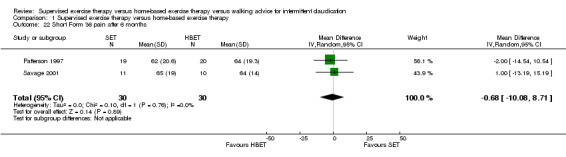
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 22 Short Form 36 pain after 6 months.
1.23. Analysis.
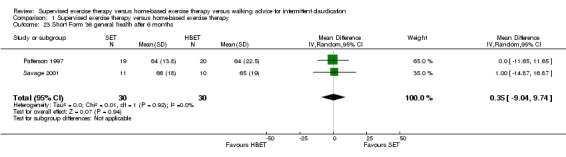
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 23 Short Form 36 general health after 6 months.
1.24. Analysis.
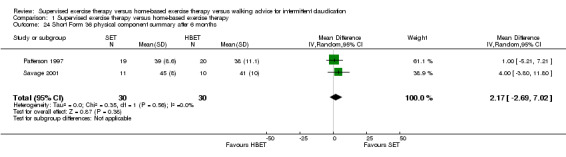
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 24 Short Form 36 physical component summary after 6 months.
1.25. Analysis.
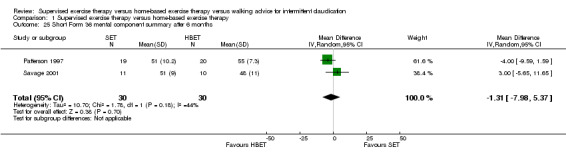
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 25 Short Form 36 mental component summary after 6 months.
No statistical heterogeneity was evident in quality of life analyses, except in the vitality subscale at three months (I2 = 92%; P = 0.0006).
Self‐reported functional impairment (WIQ): SET versus HBET
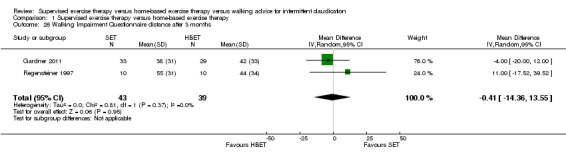
Two trials (n = 82) used the WIQ after three months (Gardner 2011; Regensteiner 1997). Both trials presented results for the distance and speed domains. Only Gardner 2011 reported the stair and combined domains. No domains showed improvement with SET compared with HBET. See Analysis 1.26, Analysis 1.27, Analysis 1.28, and Analysis 1.29. We judged the quality of evidence for these WIQ outcomes at three months to be very low to moderate. No statistical heterogeneity was evident in functional impairment analyses.
1.26. Analysis.
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 26 Walking Impairment Questionnaire distance after 3 months.
1.27. Analysis.
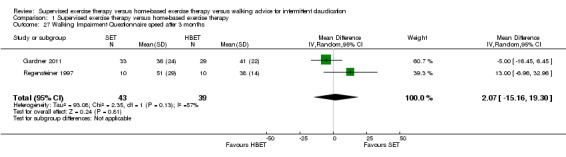
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 27 Walking Impairment Questionnaire speed after 3 months.
1.28. Analysis.
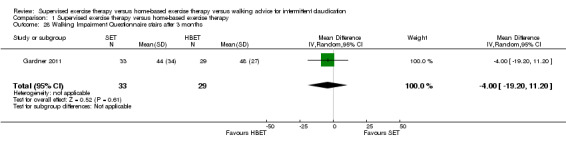
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 28 Walking Impairment Questionnaire stairs after 3 months.
1.29. Analysis.
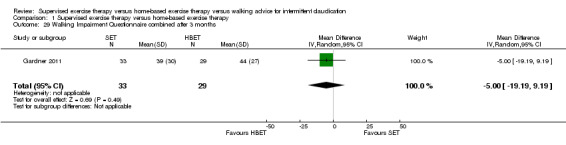
Comparison 1 Supervised exercise therapy versus home‐based exercise therapy, Outcome 29 Walking Impairment Questionnaire combined after 3 months.
Supervised exercise therapy versus walking advice
Primary outcome
Maximal treadmill walking distance or time: SET versus WA
Data on MWD/T after six weeks were available for six trials with a total sample size of 261 participants (Gardner 2012; Hodges 2008; Kakkos 2005; Parr 2009; Sandercock 2007; Sanderson 2006). Seven trials (n = 624) repeated this outcome after three months (Cheetham 2004; Gardner 2011; Gardner 2012; Hodges 2008; Nicolai 2010; Sandercock 2007; Stewart 2008), five trials (n = 483) after six months (Cheetham 2004; Gardner 2012; Kakkos 2005; Nicolai 2010; Stewart 2008), two trials (n = 308) after nine months (Cheetham 2004; Nicolai 2010), and three trials (n = 321) after 12 months (Cheetham 2004; Kakkos 2005; Nicolai 2010).
At six weeks, MWD/T was increased with an overall SMD of 0.62 (95% CI 0.27 to 0.98; P = 0.0006; moderate‐quality evidence) in favor of the SET group. See Analysis 2.1. At three months, the overall SMD was increased to 0.80 (95% CI 0.53 to 1.07; P < 0.00001; high‐quality evidence). See Analysis 2.2. This translates to a difference in favor of the SET group of approximately 210 meters in increased MWD. At six, nine, and 12 months, the benefit of SET was maintained with overall SMDs of 0.75 (95% CI 0.44 to 1.05; P < 0.00001; high‐quality evidence), 0.73 (95% CI ‐0.17 to 1.64; P = 0.11; moderate‐quality evidence), and 0.72 (95% CI 0.18 to 1.26; P = 0.009; moderate‐quality evidence), respectively. See Analysis 2.3, Analysis 2.4, and Analysis 2.5.
2.1. Analysis.
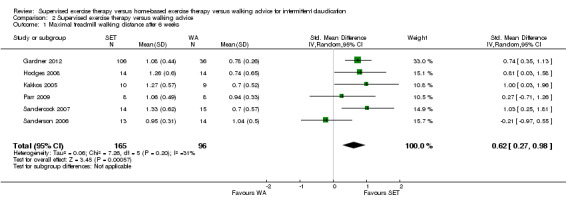
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 1 Maximal treadmill walking distance after 6 weeks.
2.2. Analysis.
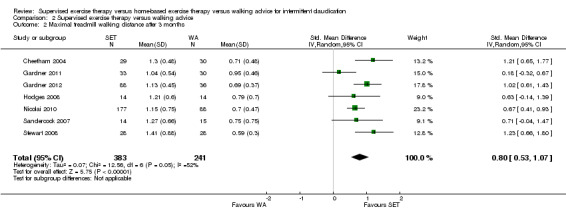
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 2 Maximal treadmill walking distance after 3 months.
2.3. Analysis.
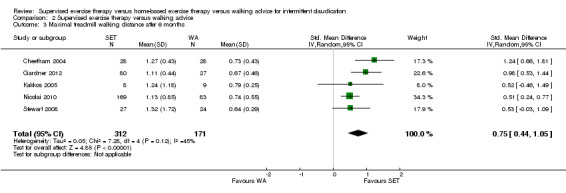
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 3 Maximal treadmill walking distance after 6 months.
2.4. Analysis.
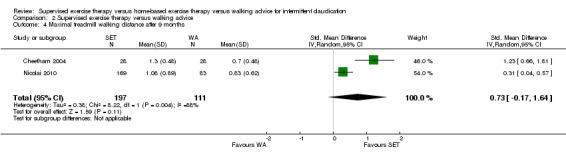
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 4 Maximal treadmill walking distance after 9 months.
2.5. Analysis.
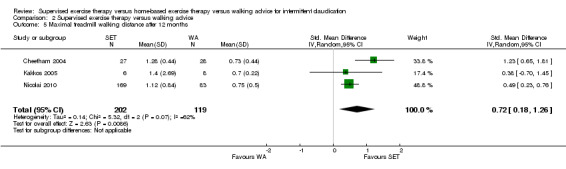
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 5 Maximal treadmill walking distance after 12 months.
At six weeks, included studies were shown to be homogeneous with I2 of 31% (P = 0.20). At three months, moderate heterogeneity was evident with I2 of 52% (P = 0.05). This heterogeneity is largely due to individual study effects of Gardner 2011. When we performed a sensitivity analysis by excluding this trial, we achieved statistical homogeneity (I2 = 20%; P = 0.28), resulting in an increased overall SMD of 0.89 (95% CI 0.67 to 1.11; P < 0.00001). At six months, included studies were shown to be homogeneous with I2 of 45% (P = 0.12). At nine and 12 months, substantial heterogeneity was evident with I2 of 88% (P = 0.004) and 62% (P = 0.07), respectively. Heterogeneity at 12 months is largely due to individual study effects of Cheetham 2004. When we performed a sensitivity analysis by excluding this trial, we achieved statistical homogeneity (I2 = 0%; P = 0.83), resulting in a decreased overall SMD of 0.49 (95% CI 0.23 to 0.75; P = 0.0002).
Secondary outcomes
Pain‐free treadmill walking distance or time: SET versus WA
Data on PFWD/T after six weeks were available for four trials with a total sample size of 204 participants (Gardner 2012; Kakkos 2005; Parr 2009; Sanderson 2006). Four trials (n = 508) repeated this outcome after three months (Gardner 2011; Gardner 2012; Nicolai 2010; Stewart 2008), four trials (n = 427) after six months (Gardner 2012; Kakkos 2005; Nicolai 2010; Stewart 2008), one trial (n = 252) after nine months (Nicolai 2010), and two trials (n = 266) after 12 months (Kakkos 2005; Nicolai 2010).
At six weeks, PFWD/T was increased with an overall SMD of 0.47 (95% CI 0.16 to 0.77; P = 0.003; moderate‐quality evidence) in favor of the SET group. See Analysis 2.6. At three months, the overall SMD was increased to 0.74 (95% CI 0.56 to 0.93; P < 0.00001; high‐quality evidence). See Analysis 2.7. This translates to a difference in favor of the SET group of approximately 140 meters in increased PFWD. At six, nine, and 12 months, the benefit of SET was maintained with overall SMDs of 0.60 (95% CI 0.39 to 0.82; P < 0.00001; high‐quality evidence), 0.39 (95% CI 0.12 to 0.65; P = 0.004; low‐quality evidence), and 0.47 (95% CI 0.21 to 0.73; P = 0.0004; moderate‐quality evidence), respectively. See Analysis 2.8, Analysis 2.9, and Analysis 2.10. Included studies were shown to be homogeneous at six weeks (I2 = 0%; P = 0.64), three months (I2 = 0%; P = 0.89), six months (I2 = 4%; P = 0.37), and 12 months (I2 = 0%; P = 0.67).
2.6. Analysis.
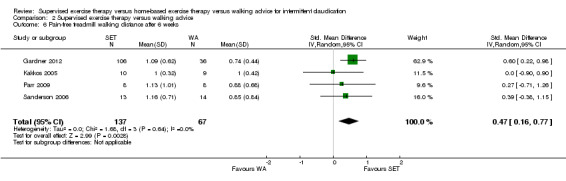
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 6 Pain‐free treadmill walking distance after 6 weeks.
2.7. Analysis.
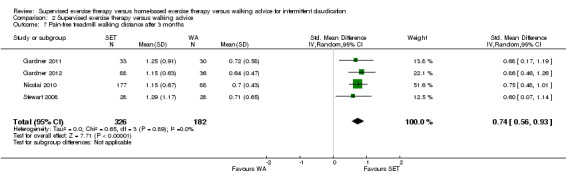
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 7 Pain‐free treadmill walking distance after 3 months.
2.8. Analysis.
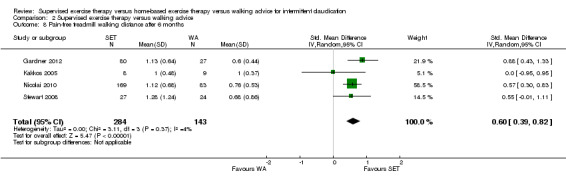
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 8 Pain‐free treadmill walking distance after 6 months.
2.9. Analysis.
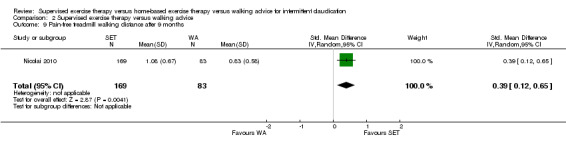
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 9 Pain‐free treadmill walking distance after 9 months.
2.10. Analysis.
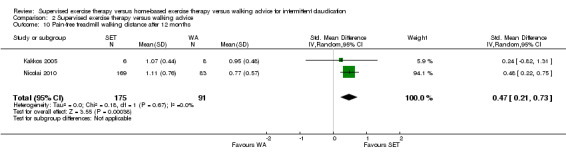
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 10 Pain‐free treadmill walking distance after 12 months.
Quality of life (SF‐36): SET versus WA
One trial (n = 19) used the SF‐36 after six weeks (Kakkos 2005), three trials (n = 359) after three months (Gardner 2011; Guidon 2013; Nicolai 2010), two trials (n = 296) after six months (Kakkos 2005; Nicolai 2010), one trial (n = 252) after nine months (Nicolai 2010), and three trials (n = 295) after 12 months (Guidon 2013; Kakkos 2005; Nicolai 2010).
At six weeks, Kakkos 2005 reported the following subscales: physical functioning (Analysis 2.11), role physical (Analysis 2.12), role emotional (Analysis 2.13), vitality (Analysis 2.14), emotional well‐being (Analysis 2.15), social functioning (Analysis 2.16), pain (Analysis 2.17), general health (Analysis 2.18), physical component summary (Analysis 2.19), and mental component summary (Analysis 2.20). Data show no clear differences between SET and WA groups for any subscale, except for role physical, for which data show a difference in favor of WA (MD ‐50.00, 95% CI ‐75.95 to ‐24.05; P = 0.0002). We judged the quality of evidence for these SF‐36 outcomes at six weeks to be low.
2.11. Analysis.
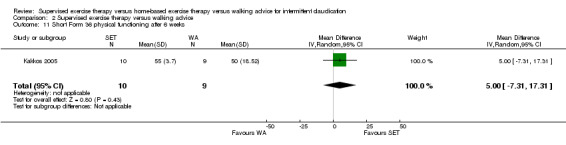
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 11 Short Form 36 physical functioning after 6 weeks.
2.12. Analysis.
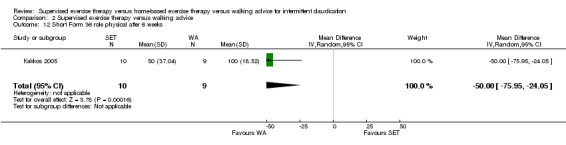
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 12 Short Form 36 role physical after 6 weeks.
2.13. Analysis.
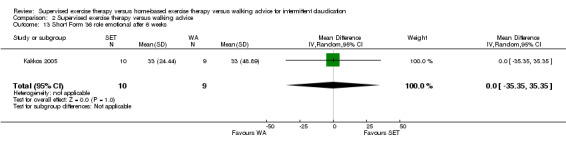
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 13 Short Form 36 role emotional after 6 weeks.
2.14. Analysis.
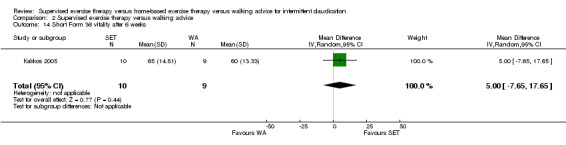
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 14 Short Form 36 vitality after 6 weeks.
2.15. Analysis.
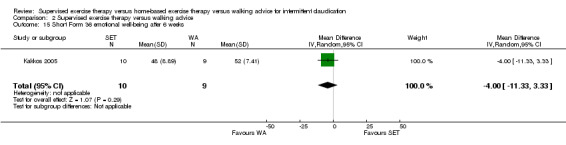
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 15 Short Form 36 emotional well‐being after 6 weeks.
2.16. Analysis.
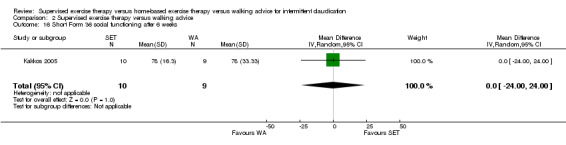
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 16 Short Form 36 social functioning after 6 weeks.
2.17. Analysis.
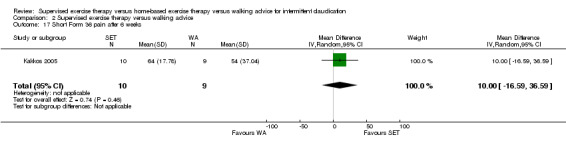
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 17 Short Form 36 pain after 6 weeks.
2.18. Analysis.
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 18 Short Form 36 general health after 6 weeks.
2.19. Analysis.
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 19 Short Form 36 physical component summary after 6 weeks.
2.20. Analysis.
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 20 Short Form 36 mental component summary after 6 weeks.
At three months, Gardner 2011, Guidon 2013, and Nicolai 2010 reported the following subscales: physical functioning (three studies; Analysis 2.21), role physical (two studies; Analysis 2.22), role emotional (two studies; Analysis 2.23), vitality (two studies; Analysis 2.24), emotional well‐being (two studies; Analysis 2.25), social functioning (two studies; Analysis 2.26), pain (two studies; Analysis 2.27), general health (two studies; Analysis 2.28), physical component summary (two studies; Analysis 2.29), and mental component summary (two studies; Analysis 2.30). Data show no clear differences between SET and WA groups for any subscale. We judged the quality of evidence for these SF‐36 outcomes at three months to be low to moderate.
2.21. Analysis.
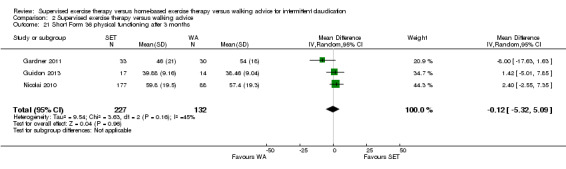
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 21 Short Form 36 physical functioning after 3 months.
2.22. Analysis.
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 22 Short Form 36 role physical after 3 months.
2.23. Analysis.
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 23 Short Form 36 role emotional after 3 months.
2.24. Analysis.
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 24 Short Form 36 vitality after 3 months.
2.25. Analysis.
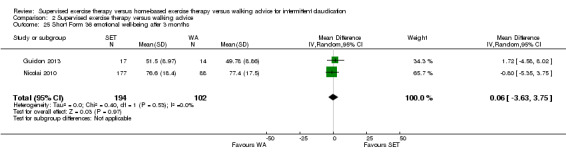
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 25 Short Form 36 emotional well‐being after 3 months.
2.26. Analysis.
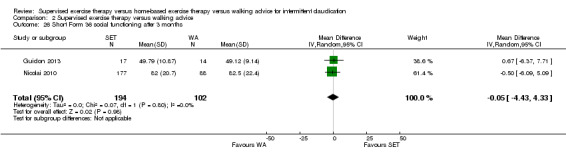
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 26 Short Form 36 social functioning after 3 months.
2.27. Analysis.
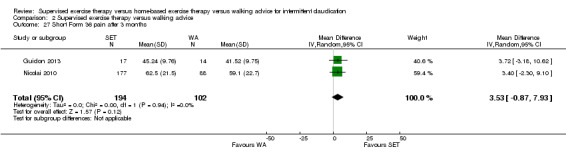
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 27 Short Form 36 pain after 3 months.
2.28. Analysis.
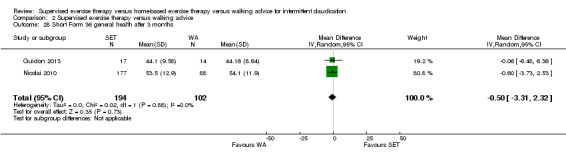
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 28 Short Form 36 general health after 3 months.
2.29. Analysis.
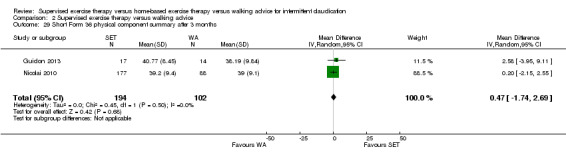
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 29 Short Form 36 physical component summary after 3 months.
2.30. Analysis.
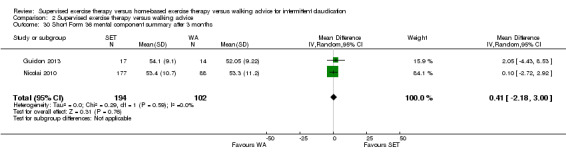
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 30 Short Form 36 mental component summary after 3 months.
At six months, Kakkos 2005 and Nicolai 2010 reported the following subscales: physical functioning (Analysis 2.31), role physical (Analysis 2.32), role emotional (Analysis 2.33), vitality (Analysis 2.34), emotional well‐being (Analysis 2.35), social functioning (Analysis 2.36), pain (Analysis 2.37), general health (Analysis 2.38), physical component summary (Analysis 2.39), and mental component summary (Analysis 2.40). Data show no clear differences between SET and WA groups for any subscale. We judged the quality of evidence for these SF‐36 outcomes at six months to be low to moderate.
2.31. Analysis.
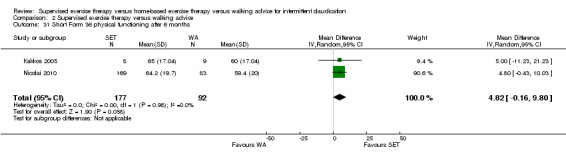
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 31 Short Form 36 physical functioning after 6 months.
2.32. Analysis.
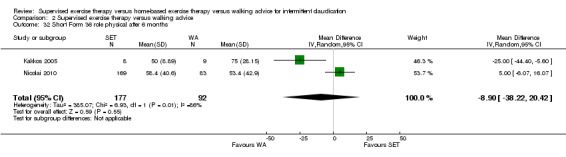
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 32 Short Form 36 role physical after 6 months.
2.33. Analysis.
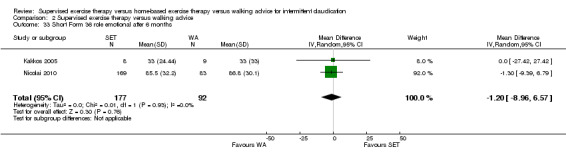
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 33 Short Form 36 role emotional after 6 months.
2.34. Analysis.
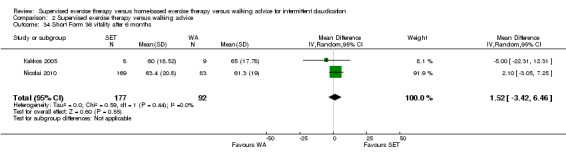
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 34 Short Form 36 vitality after 6 months.
2.35. Analysis.
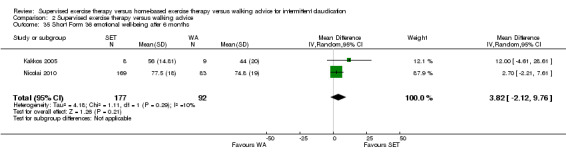
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 35 Short Form 36 emotional well‐being after 6 months.
2.36. Analysis.
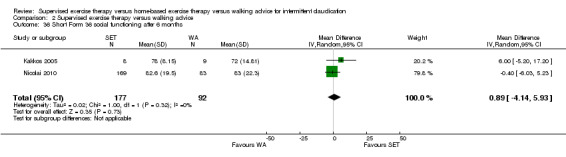
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 36 Short Form 36 social functioning after 6 months.
2.37. Analysis.
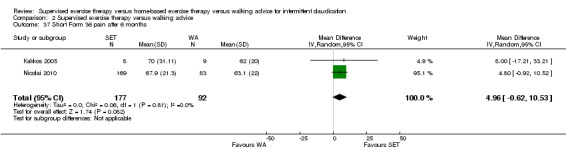
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 37 Short Form 36 pain after 6 months.
2.38. Analysis.
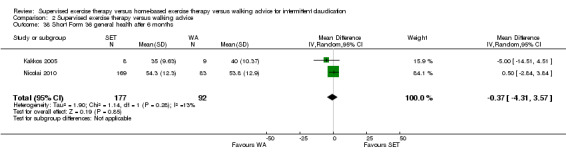
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 38 Short Form 36 general health after 6 months.
2.39. Analysis.
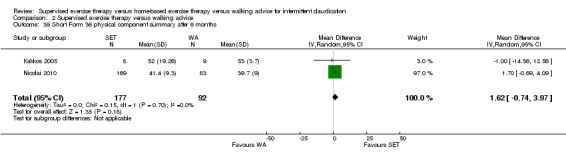
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 39 Short Form 36 physical component summary after 6 months.
2.40. Analysis.
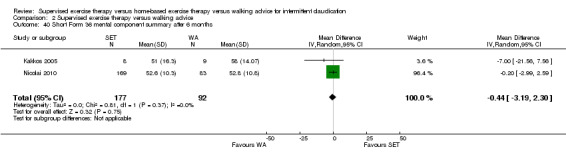
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 40 Short Form 36 mental component summary after 6 months.
At nine months, Nicolai 2010 reported the following subscales: physical functioning (Analysis 2.41), role physical (Analysis 2.42), role emotional (Analysis 2.43), vitality (Analysis 2.44), emotional well‐being (Analysis 2.45), social functioning (Analysis 2.46), pain (Analysis 2.47), general health (Analysis 2.48), physical component summary (Analysis 2.49), and mental component summary (Analysis 2.50). Data show benefit of SET over WA for physical functioning (MD 8.40, 95% CI 2.91 to 13.89; P = 0.003), pain (MD 7.90, 95% CI 1.26 to 14.54; P = 0.02), and physical component summary (MD 3.00, 95% CI 0.51 to 5.49; P = 0.02), and no clear differences in any other subscale. We judged the quality of evidence for these SF‐36 outcomes at nine months to be low.
2.41. Analysis.
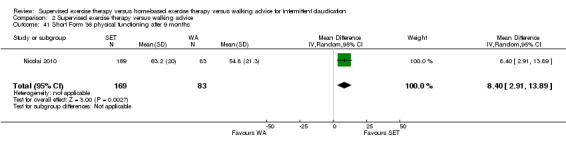
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 41 Short Form 36 physical functioning after 9 months.
2.42. Analysis.
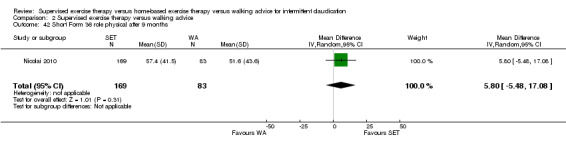
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 42 Short Form 36 role physical after 9 months.
2.43. Analysis.
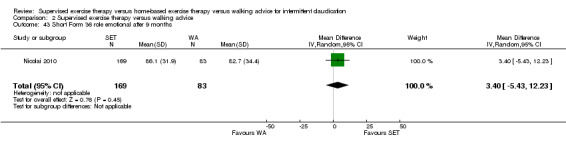
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 43 Short Form 36 role emotional after 9 months.
2.44. Analysis.
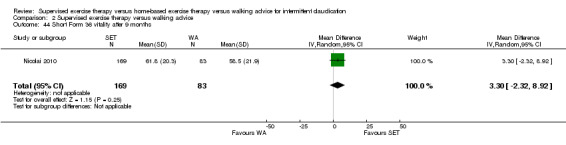
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 44 Short Form 36 vitality after 9 months.
2.45. Analysis.
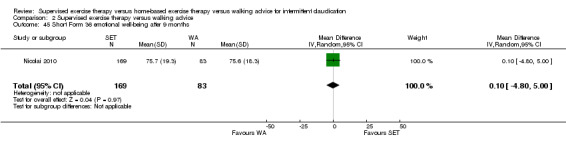
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 45 Short Form 36 emotional well‐being after 9 months.
2.46. Analysis.
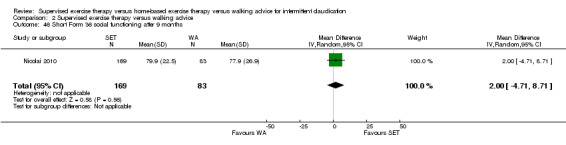
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 46 Short Form 36 social functioning after 9 months.
2.47. Analysis.
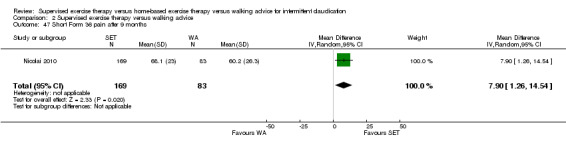
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 47 Short Form 36 pain after 9 months.
2.48. Analysis.
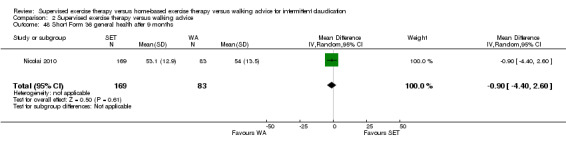
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 48 Short Form 36 general health after 9 months.
2.49. Analysis.
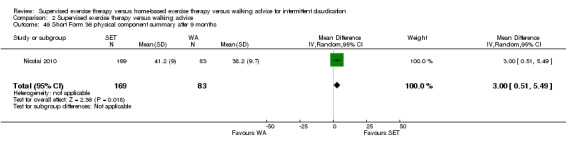
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 49 Short Form 36 physical component summary after 9 months.
2.50. Analysis.
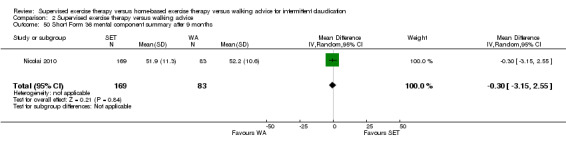
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 50 Short Form 36 mental component summary after 9 months.
At 12 months, Guidon 2013, Kakkos 2005, and Nicolai 2010 reported the following subscales: physical functioning (Analysis 2.51), role physical (Analysis 2.52), role emotional (Analysis 2.53), vitality (Analysis 2.54), emotional well‐being (Analysis 2.55), social functioning (Analysis 2.56), pain (Analysis 2.57), general health (Analysis 2.58), physical component summary (Analysis 2.59), and mental component summary (Analysis 2.60). Data show benefit of SET over WA for physical functioning (MD 5.59, 95% CI 1.09 to 10.08; P = 0.01), pain (MD 7.65, 95% CI 3.15 to 12.15; P = 0.0009), and physical component summary (MD 2.76, 95% CI 0.43 to 5.09; P = 0.02), and no clear differences in any other subscale. We judged the quality of evidence for these SF‐36 outcomes at 12 months to be moderate.
2.51. Analysis.
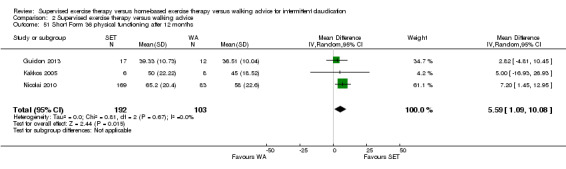
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 51 Short Form 36 physical functioning after 12 months.
2.52. Analysis.
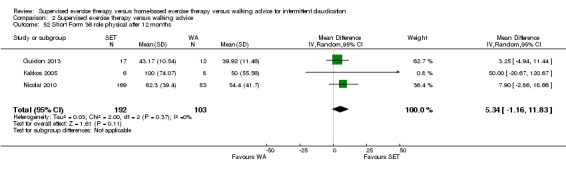
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 52 Short Form 36 role physical after 12 months.
2.53. Analysis.
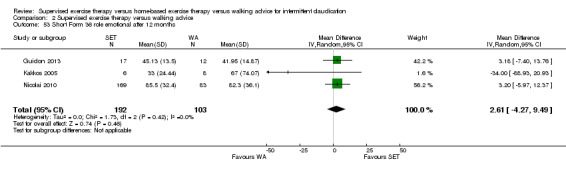
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 53 Short Form 36 role emotional after 12 months.
2.54. Analysis.
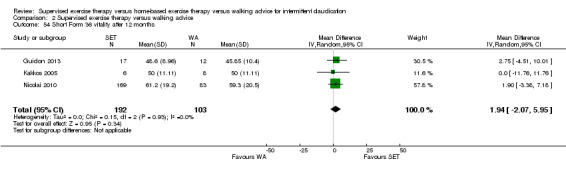
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 54 Short Form 36 vitality after 12 months.
2.55. Analysis.
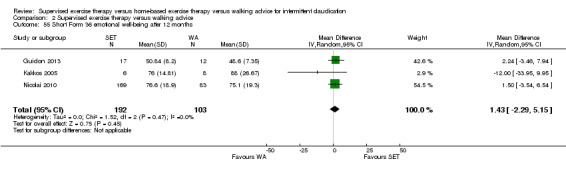
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 55 Short Form 36 emotional well‐being after 12 months.
2.56. Analysis.
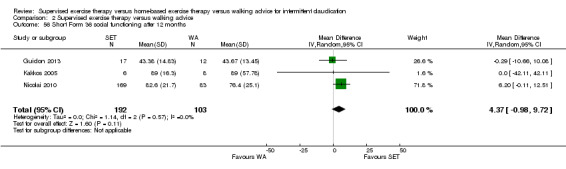
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 56 Short Form 36 social functioning after 12 months.
2.57. Analysis.
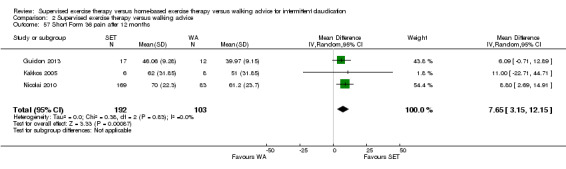
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 57 Short Form 36 pain after 12 months.
2.58. Analysis.
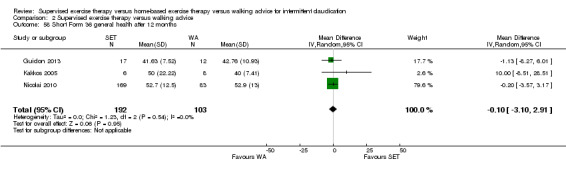
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 58 Short Form 36 general health after 12 months.
2.59. Analysis.
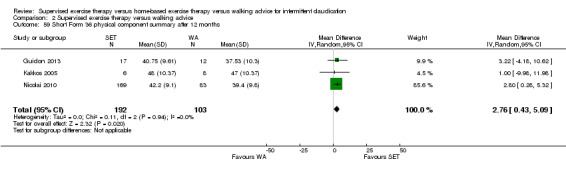
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 59 Short Form 36 physical component summary after 12 months.
2.60. Analysis.
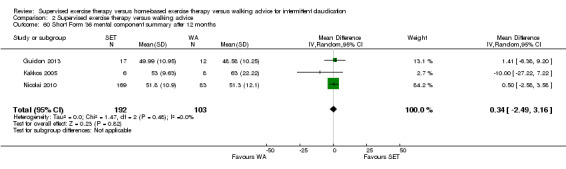
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 60 Short Form 36 mental component summary after 12 months.
No statistical heterogeneity was evident in quality of life analyses, except in the role physical subscale at six months (I2 = 86%; P = 0.008).
Self‐reported functional impairment (WIQ): SET versus WA
Two trials (n = 161) used the WIQ after six weeks (Gardner 2012; Kakkos 2005), four trials (n = 483) after three months (Gardner 2011; Gardner 2012; Guidon 2013; Nicolai 2010), three trials (n = 376) after six months (Gardner 2012; Kakkos 2005; Nicolai 2010), one trial (n = 252) after nine months (Nicolai 2010), and three trials (n = 295) after 12 months (Guidon 2013; Kakkos 2005; Nicolai 2010). All domains (distance, speed, stairs, and combined) were available for each time point.
At six weeks and three months, no domains showed improvement with SET compared with WA. See Analysis 2.61, Analysis 2.62, Analysis 2.63, Analysis 2.64, Analysis 2.65, Analysis 2.66, Analysis 2.67, and Analysis 2.68. We judged the quality of evidence for these WIQ outcomes at six weeks to be low to moderate, and at three months to be high.
2.61. Analysis.
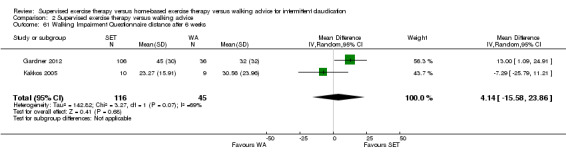
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 61 Walking Impairment Questionnaire distance after 6 weeks.
2.62. Analysis.
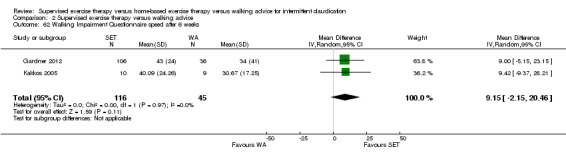
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 62 Walking Impairment Questionnaire speed after 6 weeks.
2.63. Analysis.
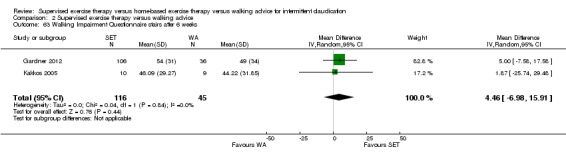
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 63 Walking Impairment Questionnaire stairs after 6 weeks.
2.64. Analysis.
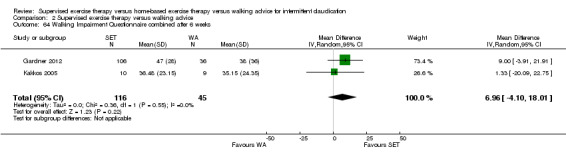
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 64 Walking Impairment Questionnaire combined after 6 weeks.
2.65. Analysis.
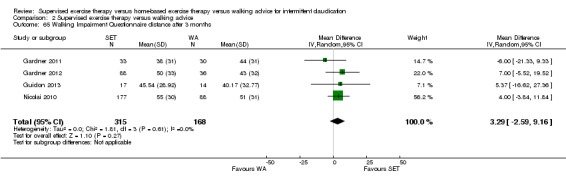
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 65 Walking Impairment Questionnaire distance after 3 months.
2.66. Analysis.
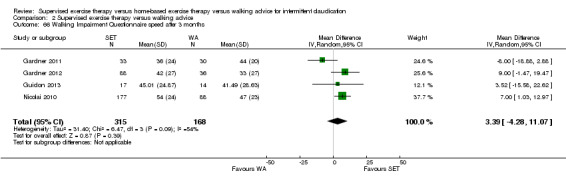
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 66 Walking Impairment Questionnaire speed after 3 months.
2.67. Analysis.
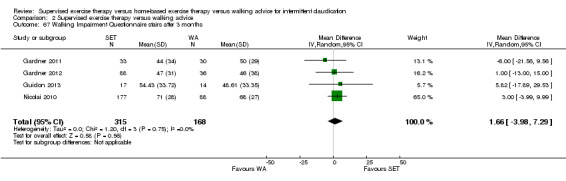
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 67 Walking Impairment Questionnaire stairs after 3 months.
2.68. Analysis.
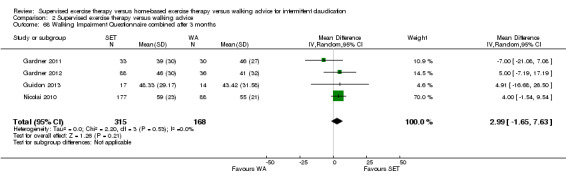
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 68 Walking Impairment Questionnaire combined after 3 months.
At six months, distance (MD 9.17, 95% CI 2.81 to 15.53; P = 0.005), stair (MD 6.51, 95% CI 0.07 to 12.95; P = 0.05), and combined scores (MD 5.99, 95% CI 0.56 to 11.42; P = 0.03) showed clear improvement with SET. See Analysis 2.69, Analysis 2.71, and Analysis 2.72, respectively. The speed domain also indicated benefit of SET compared with WA (MD 6.51, 95% CI 0.07 to 12.95; P = 0.05). See Analysis 2.70. We judged the quality of evidence for these WIQ outcomes at six months to be moderate.
2.69. Analysis.
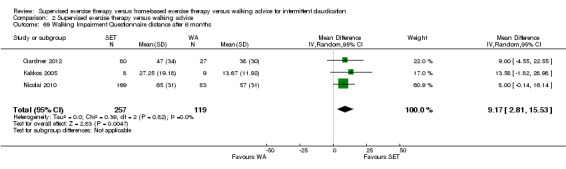
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 69 Walking Impairment Questionnaire distance after 6 months.
2.71. Analysis.
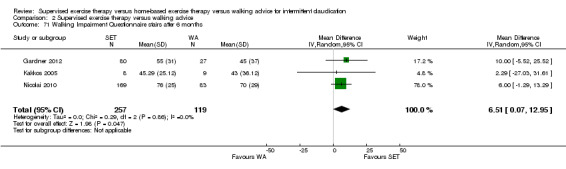
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 71 Walking Impairment Questionnaire stairs after 6 months.
2.72. Analysis.
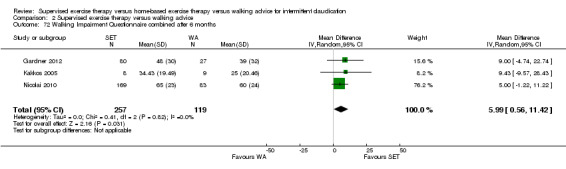
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 72 Walking Impairment Questionnaire combined after 6 months.
2.70. Analysis.
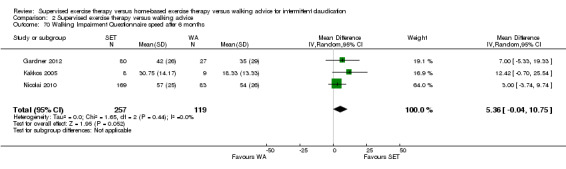
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 70 Walking Impairment Questionnaire speed after 6 months.
At nine months, distance (MD 10.00, 95% CI 1.50 to 18.50; P = 0.02), speed (MD 12.00, 95% CI 6.38 to 17.62; P < 0.0001), and combined scores (MD 10.00, 95% CI 4.04 to 15.96; P = 0.001) showed clear improvement with SET. See Analysis 2.73, Analysis 2.74, and Analysis 2.76, respectively. The stair domain also indicated benefit of SET compared with WA (MD 5.00, 95% CI ‐2.37 to 12.37; P = 0.18). See Analysis 2.75. We judged the quality of evidence for these WIQ outcomes at nine months to be low.
2.73. Analysis.
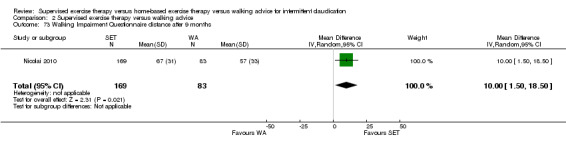
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 73 Walking Impairment Questionnaire distance after 9 months.
2.74. Analysis.
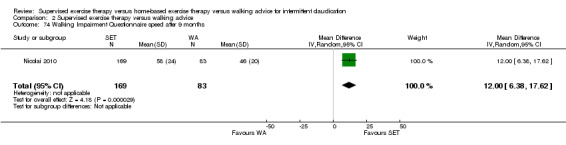
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 74 Walking Impairment Questionnaire speed after 9 months.
2.76. Analysis.
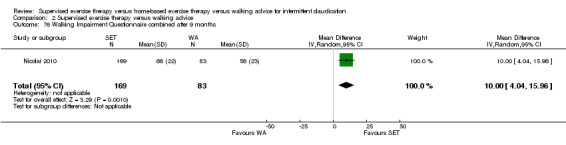
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 76 Walking Impairment Questionnaire combined after 9 months.
2.75. Analysis.
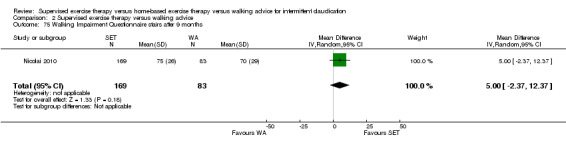
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 75 Walking Impairment Questionnaire stairs after 9 months.
At 12 months, distance (MD 10.84, 95% CI 4.81 to 16.86; P = 0.0004), speed (MD 9.32, 95% CI 3.64 to 15.00; P = 0.001), and combined scores (MD 8.76, 95% CI 2.78 to 14.74; P = 0.004) showed clear improvement with SET. See Analysis 2.77, Analysis 2.78, and Analysis 2.80, respectively. The stair domain also indicated benefit of SET compared with WA (MD 6.48, 95% CI ‐0.61 to 13.58; P = 0.07). See Analysis 2.79. We judged the quality of evidence for these WIQ outcomes at 12 months to be moderate.
2.77. Analysis.
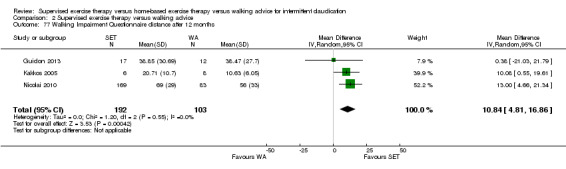
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 77 Walking Impairment Questionnaire distance after 12 months.
2.78. Analysis.
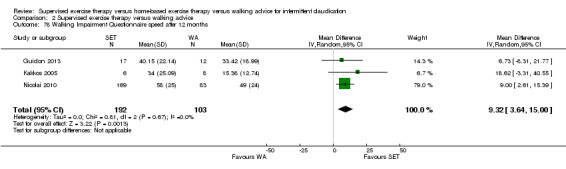
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 78 Walking Impairment Questionnaire speed after 12 months.
2.80. Analysis.
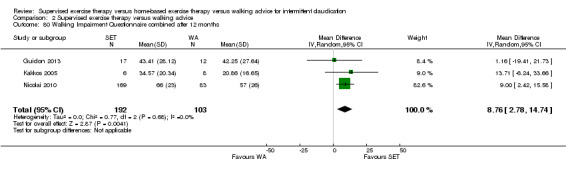
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 80 Walking Impairment Questionnaire combined after 12 months.
2.79. Analysis.
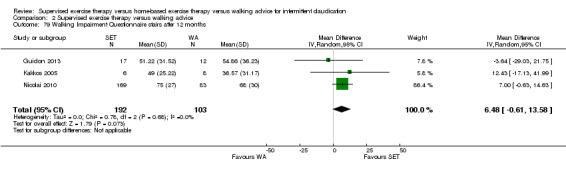
Comparison 2 Supervised exercise therapy versus walking advice, Outcome 79 Walking Impairment Questionnaire stairs after 12 months.
Substantial heterogeneity was evident in the distance domain at six weeks (I2 = 69%; P = 0.07) and in the speed domain at three months (I2 = 54%; P = 0.09). When we performed a sensitivity analysis of the speed domain at three months by excluding Gardner 2011, we achieved statistical homogeneity (I2 = 0%; P = 0.88), resulting in clear improvement with SET (MD 7.22, 95% CI 2.22 to 12.22; P = 0.005).
Home‐based exercise therapy versus walking advice
Primary outcome
Maximal treadmill walking distance or time: HBET versus WA
Data on MWD/T after six weeks were available for one trial with a total sample size of 30 participants (Sandercock 2007). Four trials (n = 137) repeated this outcome after three months (Christman 2003; Gardner 2011; Mays 2015; Sandercock 2007), and two trials (n = 148) after six months (Christman 2003; Collins 2011).
At six weeks and at three and six months, data show no clear difference in increased MWD/T between groups with overall SMDs of 0.16 (95% CI ‐0.56 to 0.87; P = 0.67; low‐quality evidence), 0.30 (95% CI ‐0.45 to 1.05; P = 0.43; moderate‐quality evidence), and ‐0.24 (95% CI ‐0.57 to 0.08; P = 0.14; moderate‐quality evidence), respectively. See Analysis 3.1, Analysis 3.2, and Analysis 3.3.
3.1. Analysis.
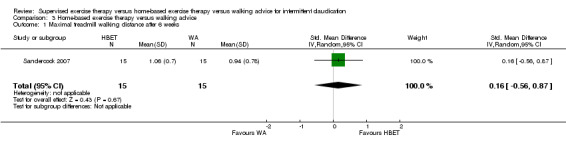
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 1 Maximal treadmill walking distance after 6 weeks.
3.2. Analysis.
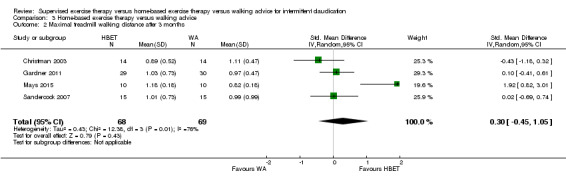
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 2 Maximal treadmill walking distance after 3 months.
3.3. Analysis.
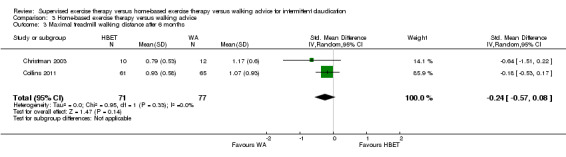
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 3 Maximal treadmill walking distance after 6 months.
At three months, substantial heterogeneity was evident with I2 of 76% (P = 0.006). This heterogeneity is largely due to individual study effects of Mays 2015. When we performed a sensitivity analysis by excluding this trial, we achieved statistical homogeneity (I2 = 0%; P = 0.51). However, this did not significantly change the results (SMD ‐0.05, 95% CI ‐0.41 to 0.32; P = 0.80). At six months, included studies were shown to be homogeneous with I2 of 0% (P = 0.33).
Secondary outcomes
Pain‐free treadmill walking distance or time: HBET versus WA
Data on PFWD/T after three months were available for three trials with a total sample size of 107 participants (Christman 2003; Gardner 2011; Mays 2015). Two trials (n = 148) repeated this outcome after six months (Christman 2003; Collins 2011).
At three and six months, data show no clear difference in increased PFWD/T between groups with overall SMDs of 0.65 (95% CI ‐0.51 to 1.82; P = 0.27; low‐quality evidence) and ‐0.08 (95% CI ‐0.41 to 0.24; P = 0.62; moderate‐quality evidence), respectively. See Analysis 3.4 and Analysis 3.5.
3.4. Analysis.
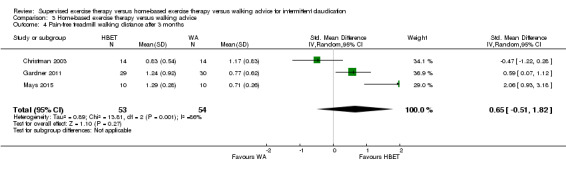
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 4 Pain‐free treadmill walking distance after 3 months.
3.5. Analysis.
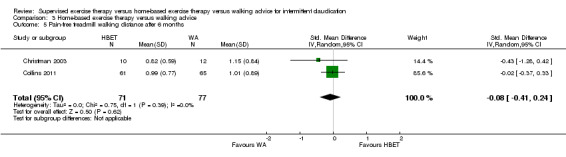
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 5 Pain‐free treadmill walking distance after 6 months.
At three months, substantial heterogeneity was evident with I2 of 86% (P = 0.001). We performed a sensitivity analysis by removing each study one at a time noting no significant change in results. At six months, included studies were shown to be homogeneous with I2 of 0% (P = 0.39).
Quality of life (SF‐36): HBET versus WA
Two trials (n = 79) used the SF‐36 after three months (Gardner 2011; Mays 2015), and one trial (n = 126) after six months (Collins 2011).
At three months, Gardner 2011 and Mays 2015 reported the following subscales: physical functioning (one study; Analysis 3.6), physical component summary (one study; Analysis 3.7), and mental component summary (one study; Analysis 3.8). Data show a difference in favor of HBET for physical component summary (MD 4.50, 95% CI 2.05 to 6.95; P = 0.0003) and mental component summary (MD 7.10, 95% CI 4.03 to 10.17; P < 0.00001). No clear difference for physical functioning was evident. We judged the quality of evidence for these SF‐36 outcomes at three months to be very low.
3.6. Analysis.
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 6 Short Form 36 physical functioning after 3 months.
3.7. Analysis.
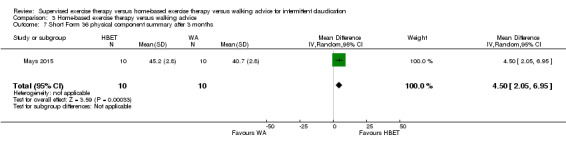
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 7 Short Form 36 physical component summary after 3 months.
3.8. Analysis.
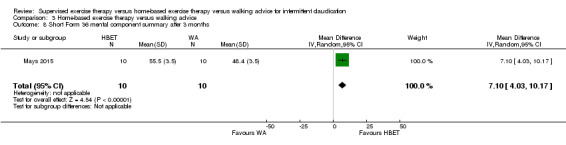
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 8 Short Form 36 mental component summary after 3 months.
At six months, Collins 2011 reported the following subscales: physical functioning (Analysis 3.9), role physical (Analysis 3.10), role emotional (Analysis 3.11), vitality (Analysis 3.12), emotional well‐being (Analysis 3.13), social functioning (Analysis 3.14), pain (Analysis 3.15), general health (Analysis 3.16), physical component summary (Analysis 3.17), and mental component summary (Analysis 3.18). Data show no clear differences between HBET and WA groups for any subscale. We judged the quality of evidence for these SF‐36 outcomes at six months to be low.
3.9. Analysis.
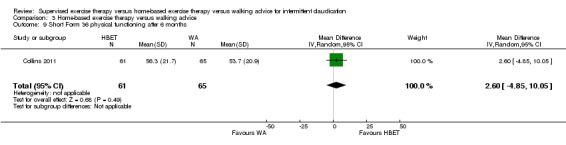
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 9 Short Form 36 physical functioning after 6 months.
3.10. Analysis.
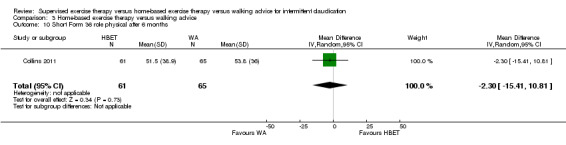
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 10 Short Form 36 role physical after 6 months.
3.11. Analysis.
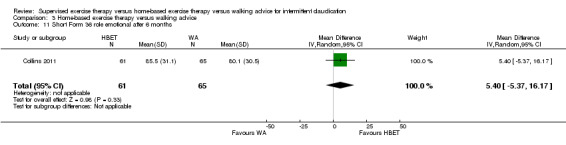
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 11 Short Form 36 role emotional after 6 months.
3.12. Analysis.
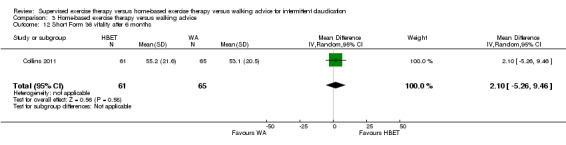
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 12 Short Form 36 vitality after 6 months.
3.13. Analysis.
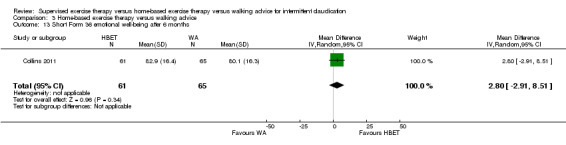
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 13 Short Form 36 emotional well‐being after 6 months.
3.14. Analysis.
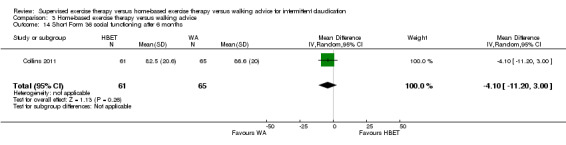
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 14 Short Form 36 social functioning after 6 months.
3.15. Analysis.
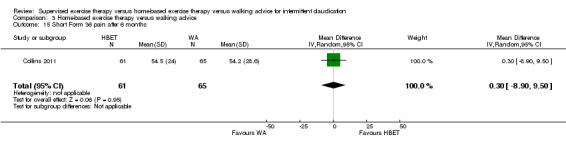
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 15 Short Form 36 pain after 6 months.
3.16. Analysis.
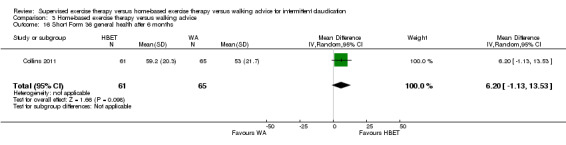
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 16 Short Form 36 general health after 6 months.
3.17. Analysis.
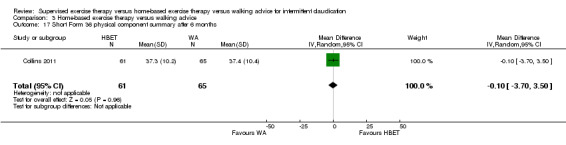
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 17 Short Form 36 physical component summary after 6 months.
3.18. Analysis.
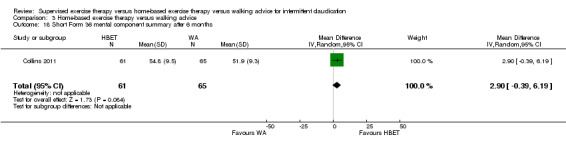
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 18 Short Form 36 mental component summary after 6 months.
Self‐reported functional impairment (WIQ): HBET versus WA
Two trials (n = 79) used the WIQ after three months (Gardner 2011; Mays 2015), and one trial (n = 126) after six months (Collins 2011). All domains (distance, speed, stairs, and combined) were available for each time point. No domains showed improvement with HBET compared with WA. See Analysis 3.19, Analysis 3.20, Analysis 3.21, Analysis 3.22, Analysis 3.23, Analysis 3.24, Analysis 3.25, and Analysis 3.26. We judged the quality of evidence for these WIQ outcomes at three months to be moderate, and at six months to be low.
3.19. Analysis.
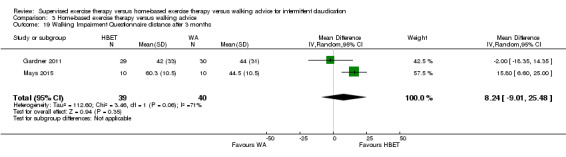
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 19 Walking Impairment Questionnaire distance after 3 months.
3.20. Analysis.
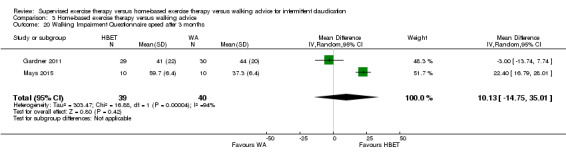
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 20 Walking Impairment Questionnaire speed after 3 months.
3.21. Analysis.
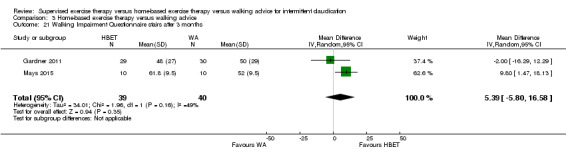
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 21 Walking Impairment Questionnaire stairs after 3 months.
3.22. Analysis.
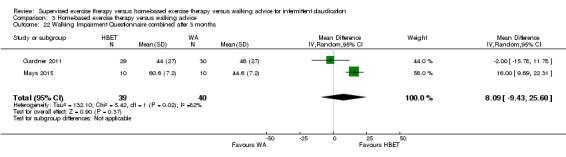
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 22 Walking Impairment Questionnaire combined after 3 months.
3.23. Analysis.
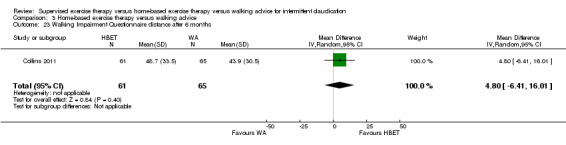
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 23 Walking Impairment Questionnaire distance after 6 months.
3.24. Analysis.
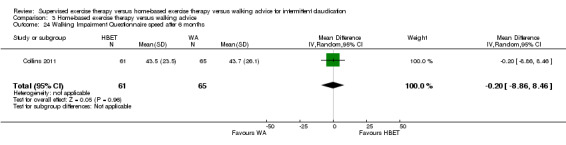
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 24 Walking Impairment Questionnaire speed after 6 months.
3.25. Analysis.
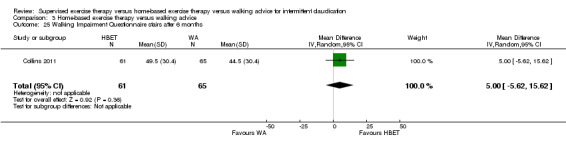
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 25 Walking Impairment Questionnaire stairs after 6 months.
3.26. Analysis.
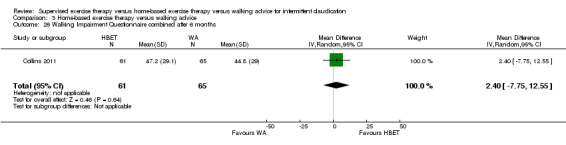
Comparison 3 Home‐based exercise therapy versus walking advice, Outcome 26 Walking Impairment Questionnaire combined after 6 months.
At three months, substantial heterogeneity was evident in distance (I2 = 71%; P = 0.06), speed (I2 = 94%; P < 0.0001), and combined scores (I2 = 82%; P = 0.02).
Mortality
In five of the 21 trials, a total of 13 participants died during the course of the study (Cheetham 2004; Kakkos 2005; Nicolai 2010; Patterson 1997; Stewart 2008). Three of these deaths were attributed to a vascular event. In Cheetham 2004, two participants died; one from recurrent pancreatitis, and one from pneumonia (one in each group). In Kakkos 2005, one participant randomized to SET, who had stopped physiotherapy because of bladder cancer, developed acute leg ischemia following his cancer operation and subsequently died. In Nicolai 2010, four participants in the SET group died (complication lower extremity bypass surgery, n = 1; lung carcinoma, n = 1; ruptured abdominal aortic aneurysm, n = 1; pancreatic cancer, n = 1), and three participants in the WA group (coronary artery disease, n = 2; renal cell carcinoma, n = 1). In Stewart 2008, one participant in the SET group had a fatal stroke. Patterson 1997 reported two deaths in the SET group but did not mention the cause of death. The remaining trials reported no deaths.
Adherence to exercise program
Ten of the 21 trials studied adherence to the exercise program (Cheetham 2004; Gardner 2011; Gardner 2012; Gardner 2014; Kakkos 2005; Mays 2015; Parr 2009; Patterson 1997; Stewart 2008; Treat‐Jacobson 2009). Cheetham 2004 asked participants at six months whether they walked "less than three times", "three times", or "more than three times" a week. More than twice as many people in the SET group as in the WA group claimed to be walking more than three times a week. In Gardner 2011 and Gardner 2014, participants were given a step activity monitor and were instructed to wear it during each exercise session. Additionally, they received an exercise logbook in which to record their walking sessions. Adherence to SET and HBET was similar (SET: 82% to 85%; HBET: 81% to 83%). In Gardner 2012, adherence to SET was 74% during the entire study. However, exercise adherence progressively declined from the first two months (86%) to the final two months (63%). Unfortunately, no adherence data were available for groups that received WA at baseline only (Gardner 2011; Gardner 2012). Kakkos 2005, Parr 2009, Patterson 1997, and Stewart 2008 noted attendance of the SET group only (attendance rates: 60%, 89%, 88%, and 79%, respectively), whereas Mays 2015 noted compliance of the HBET group only (compliance rate: 82%). In Treat‐Jacobson 2009, 73% of participants from the SET group completed all 36 exercise sessions and 97% completed at least 75% of prescribed training sessions. Conversely, 75% of HBET group participants reported that they participated in outside exercise at least three days per week.
Discussion
Summary of main results
Primary outcome
See Table 1, Table 2, and Table 3.
We included in this review data from 21 randomized controlled trials (RCTs), enrolling a total of 1400 participants. Clear differences in improvement in maximal treadmill walking distance or time (MWD/T) consistently favored supervised exercise therapy (SET) compared with home‐based exercise therapy (HBET) and walking advice (WA). Data show no clear differences between HBET and WA. Heterogeneity was present in the SET versus WA analysis at three and 12 months, as well as in the HBET versus WA analysis at three months. This heterogeneity is likely a result of individual study effects (Cheetham 2004; Gardner 2011; Mays 2015). Indeed, removing these trials from the analyses resulted in absence of statistical heterogeneity and continued differences in MWD/T favoring SET.
In addition to chance alone, several factors may have contributed to observed heterogeneity in SET versus WA analyses. First, in Gardner 2011, participants in the SET group more often were female and more often had diabetes mellitus compared with those in other trials. Women with intermittent claudication (IC) and diabetes mellitus represent a vulnerable subgroup of patients who respond poorly to a program of exercise rehabilitation (Gardner 2011). This provides a possible explanation for smaller improvements reported with SET compared with WA at three months. Second, in Cheetham 2004, participants in the SET group were five years younger than those in the WA group. This could have resulted in greater differences between SET and WA at 12 months.
Furthermore, several factors in Mays 2015 may have contributed to outlying positive results of HBET compared with WA at three months. First, some participants had undergone percutaneous transluminal angioplasty (PTA) before baseline testing. However, data show no significant interaction between response and subgroups of participants (those with PTA vs those without PTA). Second, participants in the HBET group received in‐hospital exercise therapy on a treadmill for the first two weeks, thereby increasing the intensity of supervision compared with that provided in other trials. Third, this trial used data at 14 weeks' follow‐up in the three‐month analysis, whereas other trials used data at 12 weeks' follow‐up.
Reported data on MWD/T were standardized to allow calculation of the difference in increase between the three treatment groups. When standardized data were translated back to walking distances, summary estimates of MWD/T showed differences in increases of approximately 120 and 210 meters favoring SET over HBET and WA at three months, respectively. To put these increases in context, a US football field is roughly 90 meters (or 100 yards) long. These differences were maintained after six months in the SET versus HBET analysis, and after 12 months in the SET versus WA analysis.
Secondary outcomes
In line with MWD/T, pain‐free treadmill walking distance or time (PFWD/T) showed a greater increase with SET than with HBET and WA, with differences in increases of approximately 120 and 140 meters favoring SET over HBET and WA at three months, respectively. These differences were maintained after six months in the SET versus HBET analysis, and after 12 months in the SET versus WA analysis. Again, data show no clear differences between HBET and WA. However, substantial heterogeneity was evident in the HBET versus WA analysis at three months. Sensitivity analysis performed by removing each study one at a time did not significantly change the results. We have failed to identify a plausible explanation for this inconsistency.
At three and six months, quality of life (Medical Outcomes Study Short Form [SF] 36]) outcomes were not different between SET programs and non‐supervised exercise programs. However, physical functioning, pain, and physical component summary after 12 months showed clear improvement with SET compared with WA. Although most other subscales suggested possible benefit of SET, these changes were not clear. This may be explained by an underpowered analysis. Moreover, quality of life analyses between HBET and WA were limited by the small number of included studies. Overall, we obtained complete SF‐36 data from four studies (n = 364) at three months' follow‐up (Guidon 2013; Nicolai 2010; Patterson 1997; Savage 2001), from five studies (n = 455) at six months' follow‐up (Collins 2011; Kakkos 2005; Nicolai 2010; Patterson 1997; Savage 2001), and from three studies (n = 295) at 12 months' follow‐up (Guidon 2013; Kakkos 2005; Nicolai 2010). Four other studies also recorded SF‐36 outcomes, but we were not able to obtain the raw data (Cheetham 2004; Gardner 2011; Gardner 2014; Mays 2015). This could have led to potential reporting bias. Furthermore, a paucity of disease‐specific participant‐reported outcomes prevented meta‐analysis of disease‐specific quality of life. Thus, caution is recommended in interpreting these findings.
Self‐reported functional impairment (Walking Impairment Questionnaire [WIQ]) outcomes were increased by SET compared with WA. At six and 12 months, two domains as well as combined scores showed clear improvement with SET, and the other domain suggested possible benefit. However, data show no clear differences in SET versus HBET and HBET versus WA analyses. In line with the SF‐36 analyses, some of the WIQ analyses may be underpowered, in particular those between SET and HBET.
Data show no obvious effects on mortality rates. Thirteen of the 1400 participants died, but no deaths were related to exercise therapy. Overall, adherence to SET was approximately 80%, which was similar to that reported with HBET. Only limited adherence data were available for WA groups. On the basis of trial results, nothing can be suggested about the influence of methods used for measuring adherence on effects of exercise therapy.
Overall completeness and applicability of evidence
Participants
Both inclusion and exclusion criteria were variable across studies. As most people with IC are elderly, comorbidities are common. However, investigators excluded many patients with pre‐existing medical conditions because exercise was deemed not practical or safe. Perceived uncertainties regarding safety may also contribute to underuse of SET in daily practice. However, the Gommans 2015 review reported an exceedingly low all‐cause complication rate (only eight adverse events per 82,725 patient‐hours of SET).
Interventions
All trials were performed in a hospital‐based setting, except for one trial that was performed in a community‐based setting (Nicolai 2010). Kruidenier 2009 concluded that community‐based SET was as effective as SET provided through a hospital‐based approach, so it seems unlikely that this potential factor of heterogeneity limits the applicability of trial results. In four trials, walking training was complemented with exercises for lower limb strengthening or cardiovascular training (Cheetham 2004; Guidon 2013; Parr 2009; Patterson 1997), and in another, SET included calf muscle exercises without walking (Stewart 2008). Data provide no indications that these SET sessions were less stringent than those provided in other trials. In a Cochrane review, Lauret 2014 compared the effectiveness of different modes of exercise therapy. Review authors concluded that data provide no clear evidence of differences between supervised walking exercise and alternative exercise modes in improving MWD and PFWD. However, the sample sizes of included studies were very small. Thus, more research is needed to allow meaningful comparisons. Seven trials had a partially supervised follow‐up period (Cheetham 2004; Guidon 2013; Kakkos 2005; Patterson 1997; Savage 2001; Stewart 2008; Treat‐Jacobson 2009). When sensitivity analyses were performed by removing these trials, differences in MWD/T favoring SET were unchanged. This suggests that SET with a partially supervised follow‐up period has a prolonged positive effect on walking capacity and could be as effective as SET with a fully supervised follow‐up period.
HBET programs were considerably heterogeneous, ranging from specific walking advice and use of exercise logbooks to programs combining psychological interventions and behavior change techniques (Al‐Jundi 2013). However, in many cases, intervention components were poorly described and unjustified. The aggregated sample size of the HBET group was considerably smaller than that of the other two treatment groups. These facts may have contributed to an indistinct effect assessment and may also explain the absence of a difference between HBET and WA groups.
WA varied from simple walking advice to a more specific exercise prescription. However, no supervision or monitoring was provided.
Outcome measures
All trials used a treadmill walking test to investigate the effectiveness of exercise therapy, except for two trials that did not assess walking capacity (Cunningham 2012; Guidon 2013). For over 30 years, MWD/T performed on a graded treadmill test has been the "gold standard" for estimating the walking capacity of patients with IC. The treadmill test has a sound physiological basis, has received broad acceptance in clinical practice, and has well‐established test characteristics and the ability to safely and robustly quantify changes associated with efficacious interventions (Hiatt 2014). Nonetheless, treadmill testing may be problematic in clinical trials that compare SET with non‐supervised exercise therapy, as participants in the SET group will be more familiar with treadmill walking, possibly leading to a disproportionate apparent benefit. Moreover, single MWD/T assessment may not properly reflect walking impairment in patients with IC because substantial variability has been noted between parameters (Fokkenrood 2015a). In contrast, evidence on patients with IC demonstrates that walking performance measured by the six‐minute walk test (6MWT) may better represent daily physical functioning and quality of life, and may better predict risk for mortality and mobility loss (McDermott 2014). However, a major limitation of the 6MWT is that it is limited to a relatively short duration, which induces a ceiling effect, potentially leading to underestimation of walking capacity in patients with IC with mild to moderate limitations. Unfortunately, included studies rarely reported 6MWT results, preventing meta‐analysis. Community‐based global positioning system (GPS) measurement has been recently proposed as a potentially innovative way to assess outdoor walking distance and speed in patients with IC, opening new perspectives in the study of walking capacity (Le Faucheur 2008; Le Faucheur 2015). Future studies should compare the graded treadmill test, 6MWT, and community‐based GPS procedure to determine the best functional outcome measure in peripheral artery disease (PAD).
The discrepancy between quality of life and walking distances may be due to lack of disease‐specific quality of life assessment in most studies (Vemulapalli 2015). Generic quality of life tools, such as the SF‐36 and EuroQol, typically do not address the emotional and psychosocial impact of disease‐specific physical limitations, and thus may not adequately assess therapy‐related improvements in quality of life. Conversely, validated disease‐specific tools, such as the Vascular Quality of Life Questionnaire, Peripheral Artery Questionnaire, and Peripheral Artery Disease Quality of Life Questionnaire, address not only limitations in walking capacity and activities of daily living, but also the emotional and psychosocial impact of these limitations (Morgan 2001; Spertus 2004; Treat‐Jacobson 2012). Therefore, both generic and disease‐specific quality of life questionnaires are recommended as endpoints for future studies.
Additionally, functional impairment questionnaires, such as the WIQ, provide data about patient‐perceived walking performance. It is interesting to note that lower baseline WIQ stair‐climbing scores and greater declines in WIQ stair‐climbing, distance, and speed scores were associated with higher all‐cause and cardiovascular mortality in patients with PAD (Jain 2012; Jain 2013). Furthermore, previous work demonstrated that self‐reported outdoor walking speed is likely to be strongly associated with overall health and mortality risk (McDermott 2016). Thus, poor WIQ scores may identify a subset of patients with poorer overall cardiovascular status and greater mortality risk. Consequently, interventions that improve WIQ scores, such as SET programs, may reduce mortality in people with PAD. Further study is needed to determine whether SET programs are associated with survival benefit.
Applicability
To determine the clinical relevance of the ability to walk 120 and 210 meters farther than HBET and WA groups, respectively, one should realize that the mean MWD at baseline is 290 meters with an even shorter mean PFWD of 140 meters. Hence, this improvement is likely to help with independence. In addition to improving walking ability, exercise therapy is effective in preventing cardiovascular events, and this can fulfill an important role in cardiovascular risk management for patients with IC (Horton 2009; Stewart 2002). Results of our review reveal improved treadmill walking performance in favor of SET. However, treadmill walking is an artificial form of walking that does not necessarily correspond to walking ability in daily life (Gommans 2016). A recent study demonstrated that patients who are able to walk farther will not always use this capacity to walk more often, longer, or with greater intensity (Fokkenrood 2015b). This finding suggests that solely focusing on improvement in walking capacity has limited value in optimizing SET treatment efficacy. Future research should therefore focus on optimization of SET programs other than for increasing walking capacity alone. In line with growing interest in potential associations between sedentary activity and its impact on cardiovascular risk reduction and mortality, walking behavior and physical activity measured by accelerometer may be incorporated as outcome parameters in future studies comparing different treatment modalities for PAD.
Quality of the evidence
The overall methodological quality of included trials was moderate to good. However, some trials were small with respect to numbers of participants, ranging from 20 to 304. We noted two important limitations in the quality of evidence. First, the nature of the interventions made blinding of participants and personnel effectively impossible. Second, participation bias may have influenced the results, as enrollment in a study motivated participants to walk (Collins 2011).
Despite these limitations, trials consistently reported greater improvements in MWD/T and PFWD/T in favor of SET compared with HBET and WA. This body of evidence allows robust conclusions regarding these outcomes. However, less solid conclusions can be drawn regarding quality of life and functional impairment because of the paucity of available evidence.
When assessing SET versus HBET at three months, we judged the quality of evidence to be moderate for MWD/T and PFWD/T owing to a relatively small sample size (< 400 participants). See Table 1. We judged the quality of evidence for quality of life to be low owing to a small sample size and high risk of reporting bias due to unpublished data (Gardner 2011; Gardner 2014). We judged the quality of evidence for functional impairment to be very low owing to high risk of inaccuracy (sample size and only one study) and reporting bias (Gardner 2014).
When assessing SET versus WA at three months, we judged the quality of evidence to be high for MWD/T, PFWD/T, and functional impairment. See Table 2. We judged the quality of evidence for quality of life to be low owing to a relatively small sample size and high risk of reporting bias due to unpublished data (Cheetham 2004; Gardner 2011).
When assessing HBET versus WA at three months, we judged the quality of evidence to be moderate for MWD/T and functional impairment owing to a relatively small sample size. See Table 3. We judged the quality of evidence to be low for PFWD/T owing to a small sample size and heterogeneity in results. We judged the quality of evidence for quality of life to be very low owing to high risk of inaccuracy (sample size and only one study) and reporting bias due to unpublished data (Gardner 2011).
Potential biases in the review process
For this update, we tried to minimize heterogeneity in the non‐supervised exercise group by performing separate analyses of SET versus HBET, SET versus WA, and HBET versus WA. However, we noted some heterogeneity in the intervention groups. Furthermore, we calculated overall standardized mean differences (SMDs) to reduce potential heterogeneity caused by differences in the treadmill protocols. To reduce heterogeneity in participant‐reported outcomes, we used only SF‐36 and WIQ data. Because of differences between included studies and daily practice regarding inclusion and exclusion criteria, such as comorbidities, generalization of the results of this meta‐analysis may be a topic of discussion. Unfortunately, not all outcome data were available for analysis. This potentially introduced reporting bias to our review. We did not conduct a formal analysis of publication bias by using a funnel plot owing to the limited number of studies included in the largest meta‐analysis. However, the previous review of 2013 suggested that publication bias was not a matter of importance (Fokkenrood 2013).
Agreements and disagreements with other studies or reviews
Our findings are in line with those reported in previous versions of this review (Bendermacher 2006; Fokkenrood 2013), as well as with findings of several other systematic reviews. Lane 2014 suggested that exercise therapy should play an important part in the care of selected patients with IC, to improve walking times and distances. Effects were demonstrated following three months of SET, although some programs lasted longer than one year. Wind 2007 and Vemulapalli 2015 concluded that SET was more effective than non‐supervised exercise therapy in improving MWD and PFWD for patients with IC. However, data show no differences in generic quality of life (SF‐36) nor in self‐reported functional impairment (WIQ) (Vemulapalli 2015). Fakhry 2012 compared SET with non‐interventional observation to identify the most important exercise components resulting in an optimal training protocol. SET was effective in improving MWD and PFWD for patients with IC. However, none of the predefined exercise components including intensity, duration, or content of the program were independently associated with significant improvements in MWD or PFWD.
Several other systematic reviews have investigated effects of structured HBET programs in patients with IC (Al‐Jundi 2013; Back 2015; Li 2015; Makris 2012). Based on low quality of evidence, these reviews concluded that HBET programs may improve MWD/T, PFWD/T, and quality of life when compared with baseline, or in comparison with usual care/observation control. However, improvements attained with HBET programs may be inferior to those evoked by SET.
The Gommans 2014 meta‐analysis studied the effect of supervision on walking capacity in patients with IC by categorizing RCTs according to type of support: no exercise, WA, HBET, and SET. The intensity of supervision was directly related to improved MWD and PFWD. SET was superior to other conservative treatment regimens with respect to improvement in walking distance at all follow‐up times. However, the difference between HBET and SET at six months' follow‐up was not significant.
Several plausible mechanisms might explain the beneficial results of SET over non‐supervised exercise programs. The workload for treadmill walking as performed during SET is generally greater than the workload for level ground walking at "normal" pace as performed during non‐supervised exercise therapy (Degischer 2002). Although it is very difficult to objectify the intensity of training, it is generally assumed that home training cannot be considered to be performed with the same energy expenditure as required for training under supervision (Nielsen 1975). A higher workload will lead to a larger positive effect on the general physical condition of the patient, possibly as a result of increased cardiovascular stress, providing a better stimulus for exercise‐induced adaptations (Hamburg 2011). Furthermore, direct supervision offers additional encouragement and motivation to patients, possibly resulting in a higher adherence rate, which can be explained in part by the Hawthorne effect, as mentioned by Wind 2007. The Hawthorne effect describes the fact that awareness of being under observation can alter the way a patient behaves, or can positively influence the outcome. In this review, adherence to SET was similar to that reported with HBET. However, adherence in the supervised setting is effortlessly measurable during the session in contrast to adherence in the home setting.
Authors' conclusions
Implications for practice.
Evidence of moderate and high quality shows that SET provides an important benefit for treadmill‐measured walking distance (MWD and PFWD) compared with HBET and WA, respectively. Although its clinical relevance has not been definitively demonstrated, this benefit translates to increased MWD of 120 and 210 meters after three months in SET groups. These increased walking distances are likely to have a positive impact on the lives of patients with IC. Data provide no clear evidence of a difference between HBET and WA. Trials show no clear differences in quality of life parameters nor in self‐reported functional impairment between SET and HBET. However, evidence is of low and very low quality, respectively. Investigators detected some improvements in quality of life favoring SET over WA, but analyses were limited by small numbers of studies and participants.
Our results are consistent with international guidelines recommending SET as first‐line treatment for patients with IC (Aboyans 2017; Conte 2015; Gerhard‐Herman 2017; Layden 2012). Nevertheless, most patients with IC do not participate in SET. Availability of SET programs varies widely throughout the world. Moreover, medical insurance typically does not pay for SET services. Recently, the Centers for Medicare & Medicaid Services in the USA determined that evidence is sufficient to cover SET for beneficiaries with IC for treatment of symptomatic PAD (CMS Decision Memo 2017). Professionals in the vascular field are obliged to make SET available for all patients with IC.
Implications for research.
Robust evidence shows effects of exercise therapy on walking capacity parameters in IC. However, studies assessing the impact of exercise programs on walking behavior, physical activity, and costs, as well as their long‐term effects on cardiovascular risk factors, morbidity, and mortality, are lacking. Future studies should focus on disease‐specific quality of life and patient expectations and satisfaction. Furthermore, research is needed to explore ways to optimize exercise program components (e.g., frequency, duration, and intensity of exercise sessions; different modes of exercise therapy; endurance training vs interval training; implementation of lifestyle interventions). Future research should include a variety of functional outcome measures (i.e., treadmill test, 6MWT, GPS tracking, WIQ, generic and disease‐specific quality of life), as well as people who have comorbidities, as these are more representative of the PAD population.
An SET program appears to be the preferred treatment option, and ongoing program modifications could lead to a more viable exercise schedule for patients with IC. Future research should focus on identifying an optimal mixture of SET and HBET programs. It is interesting to note that monitoring options could be extended by incorporating eHealth and mHealth technologies (Fokkenrood 2012; Makris 2012). Modern smartphones contain GPS functions, are widely used, and often are carried throughout the day. A dedicated application on a smartphone may be a valid alternative for measuring walking behavior over prolonged periods. Such technologies may improve adherence and consequently treatment effectiveness.
What's new
| Date | Event | Description |
|---|---|---|
| 3 October 2017 | New citation required but conclusions have not changed | Three new authors joined the review team. Searches were rerun. Seven additional studies were included and 14 additional studies were excluded. Five ongoing studies were identified. Review text was updated to reflect current Cochrane standards. "Risk of bias" tables were completed and "Summary of findings" tables were added. Conclusions were not changed. |
| 3 October 2017 | New search has been performed | Searches were rerun. Seven additional studies were included and 14 additional studies were excluded. Five ongoing studies were identified. |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 26 September 2008 | Amended | Review was converted to new review format. |
| 14 November 2006 | Amended | Review was edited to amend CDSR citations. |
Acknowledgements
We acknowledge the support provided by Dr. Marlene Stewart, Managing Editor of Cochrane Vascular; Dr. Cathryn Broderick, Assistant Managing Editor of Cochrane Vascular; and Dr. Karen Welch, Cochrane Vascular Information Specialist, in updating this review.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MESH DESCRIPTOR Arteriosclerosis | 868 |
| #2 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #3 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 71 |
| #4 | MESH DESCRIPTOR Atherosclerosis | 619 |
| #5 | MESH DESCRIPTOR Arterial Occlusive Diseases | 724 |
| #6 | MESH DESCRIPTOR Intermittent Claudication | 712 |
| #7 | MESH DESCRIPTOR Ischemia | 789 |
| #8 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2201 |
| #9 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 9009 |
| #10 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 7829 |
| #11 | (peripheral near3 dis*):TI,AB,KY | 3327 |
| #12 | (claudic* or IC):TI,AB,KY | 3005 |
| #13 | (isch* or CLI):TI,AB,KY | 23402 |
| #14 | arteriopathic or leriche*:TI,AB,KY | 60 |
| #15 | dysvascular*:TI,AB,KY | 10 |
| #16 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 94 |
| #17 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 138 |
| #18 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 76 |
| #19 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1107 |
| #20 | MESH DESCRIPTOR Iliac Artery | 144 |
| #21 | MESH DESCRIPTOR Popliteal Artery | 278 |
| #22 | MESH DESCRIPTOR Femoral Artery | 810 |
| #23 | MESH DESCRIPTOR Tibial Arteries | 33 |
| #24 | (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 1143 |
| #25 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 or #24 | 43291 |
| #26 | MESH DESCRIPTOR Exercise EXPLODE ALL TREES | 16348 |
| #27 | MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES | 8492 |
| #28 | MESH DESCRIPTOR Physical Exertion EXPLODE ALL TREES | 3536 |
| #29 | MESH DESCRIPTOR Sports EXPLODE ALL TREES | 11660 |
| #30 | MESH DESCRIPTOR Exercise Movement Techniques EXPLODE ALL TREES | 1324 |
| #31 | MESH DESCRIPTOR Locomotion EXPLODE ALL TREES | 5174 |
| #32 | MESH DESCRIPTOR Fitness Centers EXPLODE ALL TREES | 33 |
| #33 | (physical near3 (exertion or endurance or therap* or conditioning or activit* or fitness)):TI,AB,KY | 24168 |
| #34 | exercis*:TI,AB,KY | 50196 |
| #35 | (fitness near3 (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*)):TI,AB,KY | 1056 |
| #36 | ((training or conditioning) near3 (circuit or intervention* or protocol* or program* or activit* or regim*)):TI,AB,KY | 8569 |
| #37 | (walk* or run* or treadmill or aerobic or swim* or danc*):TI,AB,KY | 31310 |
| #38 | kinesiotherap*:TI,AB,KY | 1533 |
| #39 | ((endurance or aerobic or cardio*) near3 (fitness or train* or intervention* or protoco* or program* or therap* or activit* or regim*)):TI,AB,KY | 10031 |
| #40 | #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 | 84997 |
| #41 | #25 AND #40 | 6583 |
| #42 | * NOT SR‐PVD:CC AND 17/10/2012 TO 31/10/2015:DL | 210312 |
| #43 | #41 AND #42 | 1338 |
| #44 | (coronary or heart or hypercholest* or stroke):TI | 49602 |
| #45 | #43 NOT #44 | 899 |
| #46 | (obesity or hyperlipid* or dyslipid*):TI | 4819 |
| #47 | #45 NOT #46 | 884 |
| #48 | MESH DESCRIPTOR Arteriosclerosis | 868 |
| #49 | MESH DESCRIPTOR Arteriolosclerosis EXPLODE ALL TREES | 0 |
| #50 | MESH DESCRIPTOR Arteriosclerosis Obliterans | 71 |
| #51 | MESH DESCRIPTOR Atherosclerosis | 619 |
| #52 | MESH DESCRIPTOR Arterial Occlusive Diseases | 724 |
| #53 | MESH DESCRIPTOR Intermittent Claudication | 712 |
| #54 | MESH DESCRIPTOR Ischemia | 789 |
| #55 | MESH DESCRIPTOR Peripheral Vascular Diseases EXPLODE ALL TREES | 2201 |
| #56 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD ):TI,AB,KY | 9009 |
| #57 | ((arter* or vascular or vein* or veno* or peripher*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 7829 |
| #58 | (peripheral near3 dis*):TI,AB,KY | 3327 |
| #59 | (claudic* or IC):TI,AB,KY | 3005 |
| #60 | (isch* or CLI):TI,AB,KY | 23402 |
| #61 | arteriopathic or leriche*:TI,AB,KY | 60 |
| #62 | dysvascular*:TI,AB,KY | 10 |
| #63 | (leg near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 94 |
| #64 | (limb near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 138 |
| #65 | ((lower near3 extrem*) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) ):TI,AB,KY | 76 |
| #66 | MESH DESCRIPTOR Leg EXPLODE ALL TREES WITH QUALIFIERS BS | 1107 |
| #67 | MESH DESCRIPTOR Iliac Artery | 144 |
| #68 | MESH DESCRIPTOR Popliteal Artery | 278 |
| #69 | MESH DESCRIPTOR Femoral Artery | 810 |
| #70 | MESH DESCRIPTOR Tibial Arteries | 33 |
| #71 | (((femor* or iliac or popliteal or fempop* or crural or poplite* or infrapopliteal or inguinal or femdist* or inguinal or infrainquinal or tibial) near3 (occlus* or reocclus* or re‐occlus* or steno* or restenos* or obstruct* or lesio* or block* or harden* or stiffen* or obliter*) )):TI,AB,KY | 1143 |
| #72 | #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 or #71 | 43291 |
| #73 | MESH DESCRIPTOR Exercise EXPLODE ALL TREES | 16348 |
| #74 | MESH DESCRIPTOR Exercise Therapy EXPLODE ALL TREES | 8492 |
| #75 | MESH DESCRIPTOR Physical Exertion EXPLODE ALL TREES | 3536 |
| #76 | MESH DESCRIPTOR Sports EXPLODE ALL TREES | 11660 |
| #77 | MESH DESCRIPTOR Exercise Movement Techniques EXPLODE ALL TREES | 1324 |
| #78 | MESH DESCRIPTOR Locomotion EXPLODE ALL TREES | 5174 |
| #79 | MESH DESCRIPTOR Fitness Centers EXPLODE ALL TREES | 33 |
| #80 | (physical near3 (exertion or endurance or therap* or conditioning or activit* or fitness)):TI,AB,KY | 24168 |
| #81 | exercis*:TI,AB,KY | 50196 |
| #82 | (fitness near3 (train* or intervention* or protocol* or program* or therap* or activit* or regim* or centre* or center*)):TI,AB,KY | 1056 |
| #83 | ((training or conditioning) near3 (circuit or intervention* or protocol* or program* or activit* or regim*)):TI,AB,KY | 8569 |
| #84 | (walk* or run* or treadmill or aerobic or swim* or danc*):TI,AB,KY | 31310 |
| #85 | kinesiotherap*:TI,AB,KY | 1533 |
| #86 | ((endurance or aerobic or cardio*) near3 (fitness or train* or intervention* or protoco* or program* or therap* or activit* or regim*)):TI,AB,KY | 10031 |
| #87 | #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 | 84997 |
| #88 | #72 AND #87 | 6583 |
Appendix 2. Trials registries searches
ClinicalTrials.gov
65 studies found for: intermittent claudication AND exercise
World Health Organization International Clinical Trials Registry Platform
62 records for 61 trials found for: intermittent claudication AND exercise
International Standard Randomised Controlled Trial Number registry
22 results found for: intermittent claudication AND exercise
Data and analyses
Comparison 1. Supervised exercise therapy versus home‐based exercise therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximal treadmill walking distance after 6 weeks | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | 0.93 [0.15, 1.70] |
| 2 Maximal treadmill walking distance after 3 months | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [0.12, 0.62] |
| 3 Maximal treadmill walking distance after 6 months | 3 | 75 | Std. Mean Difference (IV, Random, 95% CI) | 0.68 [0.07, 1.30] |
| 4 Pain‐free treadmill walking distance after 3 months | 7 | 322 | Std. Mean Difference (IV, Random, 95% CI) | 0.51 [0.21, 0.81] |
| 5 Pain‐free treadmill walking distance after 6 months | 3 | 75 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.63, 1.63] |
| 6 Short Form 36/20 physical functioning after 3 months | 4 | 150 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐7.63, 5.31] |
| 7 Short Form 36 role physical after 3 months | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐19.70, 19.23] |
| 8 Short Form 36 role emotional after 3 months | 2 | 68 | Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐17.94, 15.66] |
| 9 Short Form 36 vitality after 3 months | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 8.36 [‐16.14, 32.86] |
| 10 Short Form 36/20 emotional well‐being after 3 months | 3 | 88 | Mean Difference (IV, Random, 95% CI) | 2.59 [‐6.20, 11.39] |
| 11 Short Form 36/20 social functioning after 3 months | 3 | 88 | Mean Difference (IV, Random, 95% CI) | 4.01 [‐2.33, 10.34] |
| 12 Short Form 36 pain after 3 months | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 1.27 [‐9.18, 11.73] |
| 13 Short Form 36/20 general health after 3 months | 3 | 88 | Mean Difference (IV, Random, 95% CI) | 3.78 [‐4.23, 11.79] |
| 14 Short Form 36 physical component summary after 3 months | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐4.79, 4.79] |
| 15 Short Form 36 mental component summary after 3 months | 2 | 68 | Mean Difference (IV, Random, 95% CI) | 1.19 [‐4.47, 6.86] |
| 16 Short Form 36 physical functioning after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐8.19, 12.19] |
| 17 Short Form 36 role physical after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 19.33 [‐12.93, 51.58] |
| 18 Short Form 36 role emotional after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐6.01 [‐24.74, 12.72] |
| 19 Short Form 36 vitality after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 4.02 [‐7.58, 15.62] |
| 20 Short Form 36 emotional well‐being after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐14.38, 9.29] |
| 21 Short Form 36 social functioning after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐13.94, 11.34] |
| 22 Short Form 36 pain after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐10.08, 8.71] |
| 23 Short Form 36 general health after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐9.04, 9.74] |
| 24 Short Form 36 physical component summary after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | 2.17 [‐2.69, 7.02] |
| 25 Short Form 36 mental component summary after 6 months | 2 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐7.98, 5.37] |
| 26 Walking Impairment Questionnaire distance after 3 months | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐14.36, 13.55] |
| 27 Walking Impairment Questionnaire speed after 3 months | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 2.07 [‐15.16, 19.30] |
| 28 Walking Impairment Questionnaire stairs after 3 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐19.20, 11.20] |
| 29 Walking Impairment Questionnaire combined after 3 months | 1 | 62 | Mean Difference (IV, Random, 95% CI) | ‐5.0 [‐19.19, 9.19] |
Comparison 2. Supervised exercise therapy versus walking advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximal treadmill walking distance after 6 weeks | 6 | 261 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [0.27, 0.98] |
| 2 Maximal treadmill walking distance after 3 months | 7 | 624 | Std. Mean Difference (IV, Random, 95% CI) | 0.80 [0.53, 1.07] |
| 3 Maximal treadmill walking distance after 6 months | 5 | 483 | Std. Mean Difference (IV, Random, 95% CI) | 0.75 [0.44, 1.05] |
| 4 Maximal treadmill walking distance after 9 months | 2 | 308 | Std. Mean Difference (IV, Random, 95% CI) | 0.73 [‐0.17, 1.64] |
| 5 Maximal treadmill walking distance after 12 months | 3 | 321 | Std. Mean Difference (IV, Random, 95% CI) | 0.72 [0.18, 1.26] |
| 6 Pain‐free treadmill walking distance after 6 weeks | 4 | 204 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.16, 0.77] |
| 7 Pain‐free treadmill walking distance after 3 months | 4 | 508 | Std. Mean Difference (IV, Random, 95% CI) | 0.74 [0.56, 0.93] |
| 8 Pain‐free treadmill walking distance after 6 months | 4 | 427 | Std. Mean Difference (IV, Random, 95% CI) | 0.60 [0.39, 0.82] |
| 9 Pain‐free treadmill walking distance after 9 months | 1 | 252 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [0.12, 0.65] |
| 10 Pain‐free treadmill walking distance after 12 months | 2 | 266 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [0.21, 0.73] |
| 11 Short Form 36 physical functioning after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐7.31, 17.31] |
| 12 Short Form 36 role physical after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐50.00 [‐75.95, ‐24.05] |
| 13 Short Form 36 role emotional after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐35.35, 35.35] |
| 14 Short Form 36 vitality after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐7.65, 17.65] |
| 15 Short Form 36 emotional well‐being after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐11.33, 3.33] |
| 16 Short Form 36 social functioning after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐24.00, 24.00] |
| 17 Short Form 36 pain after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 10.0 [‐16.59, 36.59] |
| 18 Short Form 36 general health after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐18.00, 18.00] |
| 19 Short Form 36 physical component summary after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐7.22, 9.22] |
| 20 Short Form 36 mental component summary after 6 weeks | 1 | 19 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐11.99, 9.99] |
| 21 Short Form 36 physical functioning after 3 months | 3 | 359 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐5.32, 5.09] |
| 22 Short Form 36 role physical after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐8.44, 9.30] |
| 23 Short Form 36 role emotional after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐3.68, 8.49] |
| 24 Short Form 36 vitality after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐4.42, 6.63] |
| 25 Short Form 36 emotional well‐being after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐3.63, 3.75] |
| 26 Short Form 36 social functioning after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐4.43, 4.33] |
| 27 Short Form 36 pain after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | 3.53 [‐0.87, 7.93] |
| 28 Short Form 36 general health after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐3.31, 2.32] |
| 29 Short Form 36 physical component summary after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐1.74, 2.69] |
| 30 Short Form 36 mental component summary after 3 months | 2 | 296 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐2.18, 3.00] |
| 31 Short Form 36 physical functioning after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | 4.82 [‐0.16, 9.80] |
| 32 Short Form 36 role physical after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | ‐8.90 [‐38.22, 20.42] |
| 33 Short Form 36 role emotional after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐8.96, 6.57] |
| 34 Short Form 36 vitality after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | 1.52 [‐3.42, 6.46] |
| 35 Short Form 36 emotional well‐being after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | 3.82 [‐2.12, 9.76] |
| 36 Short Form 36 social functioning after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐4.14, 5.93] |
| 37 Short Form 36 pain after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | 4.96 [‐0.62, 10.53] |
| 38 Short Form 36 general health after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐4.31, 3.57] |
| 39 Short Form 36 physical component summary after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | 1.62 [‐0.74, 3.97] |
| 40 Short Form 36 mental component summary after 6 months | 2 | 269 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐3.19, 2.30] |
| 41 Short Form 36 physical functioning after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 8.40 [2.91, 13.89] |
| 42 Short Form 36 role physical after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 5.80 [‐5.48, 17.08] |
| 43 Short Form 36 role emotional after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐5.43, 12.23] |
| 44 Short Form 36 vitality after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 3.30 [‐2.32, 8.92] |
| 45 Short Form 36 emotional well‐being after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐4.80, 5.00] |
| 46 Short Form 36 social functioning after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐4.71, 8.71] |
| 47 Short Form 36 pain after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 7.90 [1.26, 14.54] |
| 48 Short Form 36 general health after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐4.40, 2.60] |
| 49 Short Form 36 physical component summary after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 3.0 [0.51, 5.49] |
| 50 Short Form 36 mental component summary after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐3.15, 2.55] |
| 51 Short Form 36 physical functioning after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 5.59 [1.09, 10.08] |
| 52 Short Form 36 role physical after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 5.34 [‐1.16, 11.83] |
| 53 Short Form 36 role emotional after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 2.61 [‐4.27, 9.49] |
| 54 Short Form 36 vitality after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 1.94 [‐2.07, 5.95] |
| 55 Short Form 36 emotional well‐being after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 1.43 [‐2.29, 5.15] |
| 56 Short Form 36 social functioning after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 4.37 [‐0.98, 9.72] |
| 57 Short Form 36 pain after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 7.65 [3.15, 12.15] |
| 58 Short Form 36 general health after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐3.10, 2.91] |
| 59 Short Form 36 physical component summary after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 2.76 [0.43, 5.09] |
| 60 Short Form 36 mental component summary after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 0.34 [‐2.49, 3.16] |
| 61 Walking Impairment Questionnaire distance after 6 weeks | 2 | 161 | Mean Difference (IV, Random, 95% CI) | 4.14 [‐15.58, 23.86] |
| 62 Walking Impairment Questionnaire speed after 6 weeks | 2 | 161 | Mean Difference (IV, Random, 95% CI) | 9.15 [‐2.15, 20.46] |
| 63 Walking Impairment Questionnaire stairs after 6 weeks | 2 | 161 | Mean Difference (IV, Random, 95% CI) | 4.46 [‐6.98, 15.91] |
| 64 Walking Impairment Questionnaire combined after 6 weeks | 2 | 161 | Mean Difference (IV, Random, 95% CI) | 6.96 [‐4.10, 18.01] |
| 65 Walking Impairment Questionnaire distance after 3 months | 4 | 483 | Mean Difference (IV, Random, 95% CI) | 3.29 [‐2.59, 9.16] |
| 66 Walking Impairment Questionnaire speed after 3 months | 4 | 483 | Mean Difference (IV, Random, 95% CI) | 3.39 [‐4.28, 11.07] |
| 67 Walking Impairment Questionnaire stairs after 3 months | 4 | 483 | Mean Difference (IV, Random, 95% CI) | 1.66 [‐3.98, 7.29] |
| 68 Walking Impairment Questionnaire combined after 3 months | 4 | 483 | Mean Difference (IV, Random, 95% CI) | 2.99 [‐1.65, 7.63] |
| 69 Walking Impairment Questionnaire distance after 6 months | 3 | 376 | Mean Difference (IV, Random, 95% CI) | 9.17 [2.81, 15.53] |
| 70 Walking Impairment Questionnaire speed after 6 months | 3 | 376 | Mean Difference (IV, Random, 95% CI) | 5.36 [‐0.04, 10.75] |
| 71 Walking Impairment Questionnaire stairs after 6 months | 3 | 376 | Mean Difference (IV, Random, 95% CI) | 6.51 [0.07, 12.95] |
| 72 Walking Impairment Questionnaire combined after 6 months | 3 | 376 | Mean Difference (IV, Random, 95% CI) | 5.99 [0.56, 11.42] |
| 73 Walking Impairment Questionnaire distance after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 10.0 [1.50, 18.50] |
| 74 Walking Impairment Questionnaire speed after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 12.0 [6.38, 17.62] |
| 75 Walking Impairment Questionnaire stairs after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐2.37, 12.37] |
| 76 Walking Impairment Questionnaire combined after 9 months | 1 | 252 | Mean Difference (IV, Random, 95% CI) | 10.0 [4.04, 15.96] |
| 77 Walking Impairment Questionnaire distance after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 10.84 [4.81, 16.86] |
| 78 Walking Impairment Questionnaire speed after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 9.32 [3.64, 15.00] |
| 79 Walking Impairment Questionnaire stairs after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 6.48 [‐0.61, 13.58] |
| 80 Walking Impairment Questionnaire combined after 12 months | 3 | 295 | Mean Difference (IV, Random, 95% CI) | 8.76 [2.78, 14.74] |
Comparison 3. Home‐based exercise therapy versus walking advice.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maximal treadmill walking distance after 6 weeks | 1 | 30 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.56, 0.87] |
| 2 Maximal treadmill walking distance after 3 months | 4 | 137 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.45, 1.05] |
| 3 Maximal treadmill walking distance after 6 months | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.57, 0.08] |
| 4 Pain‐free treadmill walking distance after 3 months | 3 | 107 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.51, 1.82] |
| 5 Pain‐free treadmill walking distance after 6 months | 2 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.41, 0.24] |
| 6 Short Form 36 physical functioning after 3 months | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐16.56, 4.56] |
| 7 Short Form 36 physical component summary after 3 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 4.5 [2.05, 6.95] |
| 8 Short Form 36 mental component summary after 3 months | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 7.10 [4.03, 10.17] |
| 9 Short Form 36 physical functioning after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 2.60 [‐4.85, 10.05] |
| 10 Short Form 36 role physical after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | ‐2.30 [‐15.41, 10.81] |
| 11 Short Form 36 role emotional after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 5.40 [‐5.37, 16.17] |
| 12 Short Form 36 vitality after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐5.26, 9.46] |
| 13 Short Form 36 emotional well‐being after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐2.91, 8.51] |
| 14 Short Form 36 social functioning after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | ‐4.10 [‐11.20, 3.00] |
| 15 Short Form 36 pain after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐8.90, 9.50] |
| 16 Short Form 36 general health after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 6.20 [‐1.13, 13.53] |
| 17 Short Form 36 physical component summary after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐3.70, 3.50] |
| 18 Short Form 36 mental component summary after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 2.90 [‐0.39, 6.19] |
| 19 Walking Impairment Questionnaire distance after 3 months | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 8.24 [‐9.01, 25.48] |
| 20 Walking Impairment Questionnaire speed after 3 months | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 10.13 [‐14.75, 35.01] |
| 21 Walking Impairment Questionnaire stairs after 3 months | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 5.39 [‐5.80, 16.58] |
| 22 Walking Impairment Questionnaire combined after 3 months | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 8.09 [‐9.43, 25.60] |
| 23 Walking Impairment Questionnaire distance after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐6.41, 16.01] |
| 24 Walking Impairment Questionnaire speed after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐8.86, 8.46] |
| 25 Walking Impairment Questionnaire stairs after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 5.0 [‐5.62, 15.62] |
| 26 Walking Impairment Questionnaire combined after 6 months | 1 | 126 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐7.75, 12.55] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 2010.
| Methods | Study design: RCT Method of randomization: NA |
|
| Participants | Country: USA Setting: clinics and community No. of participants: Baseline: SET: n = 15; HBET: n = 18 3 months: SET: n = 15; HBET: n = 18 Age, years (SD): SET: 67.9 (10.1); HBET: 66.7 (11.9) Sex, % male: SET: 67; HBET: 44 PAD diagnosed by: ABI Inclusion criteria: stable IC for > 3 months and ABI < 0.9 at rest Exclusion criteria: past medical history of gangrene; impending limb loss or osteomyelitis; lower extremity vascular surgery, angioplasty, or lumbar sympathectomy within 3 months of enrollment; severe peripheral neuropathy; any condition other than PAD that limits walking; unstable angina; history of significant left main disease or 3‐vessel coronary artery disease or recent myocardial infarction; chest pain during treadmill exercise that appears before onset of claudication; > 3 mm ST depression during exercise |
|
| Interventions | SET: exercise training, 3 times per week for 3 months; supervised by trained exercise physiologist HBET: walking, 3 times per week for 30 minutes; keeping careful notes regarding activity and called once every 3 weeks to answer any exercise‐related questions Duration: 3 months Follow‐up period: 3 months |
|
| Outcomes | Treadmill test: Gardner protocol, which maintains 2 mph with 2% grade increase every 2 minutes Outcomes: gas exchange analysis, claudication onset time (COT), peak walking time (PWT), blood pressure, arterial vasoreactivity measures, nitric oxide metabolite measures Participant‐reported outcomes: rating of perceived exertion Adherence: NA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition to permit judgement |
| Selective reporting (reporting bias) | Low risk | COT and PWT outcomes reported incompletely; missing outcomes could be obtained from trial authors |
| Other bias | High risk | Participants recruited from the community Funding: National Heart, Lung, and Blood Institute, National Institutes of Health, and Office of Research on Women's Health grants |
Cheetham 2004.
| Methods | Study design: RCT Method of randomization: computer randomized |
|
| Participants | Country: UK Setting: regional vascular center No. of participants: Baseline: SET: n = 29; WA: n = 30 6 months: SET: n = 28; WA: n = 28 12 months: SET: n = 27; WA: n = 28 Age, years (SD): SET: 65; WA: 70 Sex, % male: 73 PAD diagnosed by: ABI Inclusion criteria: resting ABI < 0.9 or positive response to validated stress test; PAD, confirmed by Duplex scans of affected leg(s); positive response to Edinburgh Claudication Questionnaire; minimum 6‐month period of stable symptoms of mild to moderate IC Exclusion criteria: not fulfilling all inclusion criteria; severe IC deemed to warrant radiological or surgical intervention; critical ischemia; significant comorbidity preventing participation in exercise program; vascular or endovascular intervention within previous 2 years; having received pharmacological agents aimed at improving symptoms within previous 6 months |
|
| Interventions | SET: once‐weekly 45‐minute supervised exercise and motivation class for 6‐month period (walking circuit and seven 2‐minute exercise stations aimed at lower limb strengthening); verbal and written exercise advice; best medical treatment WA: verbal and written exercise advice; best medical treatment Duration: 6 months Follow‐up period: 12 months |
|
| Outcomes | Treadmill test: fixed‐load treadmill at 3.5 km/h with 12% gradient Outcomes: initial claudication distance (ICD), absolute claudication distance (ACD), resting ABI Participant‐reported outcomes: SF‐36, Charing Cross Claudication Questionnaire Adherence: self‐reported compliance |
|
| Notes | SDs of ACD were calculated on the basis of P value; it was assumed that SDs of both groups were equal. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis not described; 4 of 59 participants lost to follow‐up (SET: n = 2; WA: n = 2) |
| Selective reporting (reporting bias) | High risk | No ICD and not all SF‐36 outcomes (physical functioning subscale only) reported; physical functioning subscale outcomes reported incompletely, so they could not be entered into meta‐analysis; missing outcomes could not be obtained from trial authors |
| Other bias | Unclear risk | Medians of ACD reported; SDs calculated on the basis of P value; it was assumed that SDs of both groups were equal |
Christman 2003.
| Methods | Study design: RCT Method of randomization: NA |
|
| Participants | Country: USA Setting: several large vascular surgery offices No. of participants: Baseline: HBET: n = 21; WA: n = 17 3 months: HBET: n = 14; WA: n = 14 6 months: HBET: n = 10; WA: n = 12 Age, years (SD): HBET: 66.14 (4.91); WA: 67.69 (2.94) Sex, % male: HBET: 71.4; WA: 68.8 PAD diagnosed by: ABI Inclusion criteria: ABI < 0.9 and/or decrease in ankle pressure ≥ 15 mmHg after standard exercise protocol; between ages of 40 and 75 with arterial claudication symptoms, not exercising; in precontemplation, contemplation, or preparation stage of change for exercise and smoking Exclusion criteria: lack of interest or inability to walk on treadmill, cardiologist did not want participation in unsupervised exercise program, already in action or maintenance stages of exercise; not meeting hemodynamic criteria or ischemic rest pain or tissue loss; inability to tolerate exercise as result of comorbid illness such as arthritis or chronic obstructive pulmonary disease |
|
| Interventions | HBET: 12‐week education intervention consisting of one 1‐hour class per week and personalized home‐based exercise prescription (3 times/week); smoking cessation manual; exercise diary; contacted by telephone every 2 weeks for encouragement WA: admonition to begin exercising and quit smoking with no additional follow‐up Duration: 3 months Follow‐up period: 6 months |
|
| Outcomes | Treadmill test: graded, progressive treadmill exercise test initiated at 1 mph with grade of 5%, increasing in speed and grade at 5‐minute intervals through 4 stages to 2.5 mph at 10% grade Outcomes: claudication pain time, maximal walking time, smoking cessation rate Participant‐reported outcomes: stage of change for exercise and smoking cessation, decisional balance for exercise and smoking, exercise self‐efficacy Adherence: NA |
|
| Notes | Dissertation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for missing outcome data likely to be related to true outcome, with imbalance in numbers and reasons (health problems) for missing data across intervention groups ITT analysis not described; 16 of 38 participants lost to follow‐up (HBET: n = 11; WA: n = 5) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Unclear risk | Dissertation Funding: National Institute of Nursing Research grant, Society of Vascular Nursing grant, and Sigma Theta Tau grant |
Collins 2011.
| Methods | Study design: RCT Method of randomization: permutated blocks with randomized block sizes |
|
| Participants | Country: USA Setting: clinics and communities; referred by physicians or self‐referred from flyers distributed at health fairs, community centers, and churches; media advertisements; word of mouth; postcards No. of participants: Baseline: HBET: n = 72; WA: n = 73 6 months: HBET: n = 61; WA: n = 65 Age, years (SD): HBET: 66.2 (10.2); WA: 66.8 (10.1) Sex, % male: HBET: 65; WA: 73 PAD diagnosed by: ABI Inclusion criteria: resting or postexercise ABI < 0.90, toe‐brachial index < 0.7, or prior surgery for PAD with continued exertional leg symptoms not including joint pain; men and women aged 40 years and older with diagnosis of PAD; diagnosis of diabetes mellitus type 1 or 2; leg symptoms at enrollment Exclusion criteria: no intention to start exercising in next 6 months; no available phone, foot or lower leg amputation, critical leg ischemia, or lower extremity revascularization within 6 months before enrollment; myocardial infarction within preceding 3 months; evidence of significant coronary ischemia at low workload; systolic blood pressure > 180 mmHg or diastolic pressure > 110 mmHg; diagnosis of life‐threatening malignancy within previous year; exercise tolerance limited by leg pain of non‐vascular origin or other factors |
|
| Interventions | HBET: 7‐minute educational video about PAD and strategies for disease and risk factor management (aerobic activity); one‐on‐one interaction with research coordinator at baseline; walking training (3 days per week) and weekly group walking classes with instructor; biweekly telephone calls for 6 months WA: 7‐minute educational video about PAD and strategies for disease and risk factor management (aerobic activity); twice‐monthly phone calls with research coordinator Duration: 6 months Follow‐up period: 6 months |
|
| Outcomes | Treadmill test: Gardner‐Skinner graded exercise treadmill test Outcomes: maximum pain distance, onset of pain distance Participant‐reported outcomes: WIQ, SF‐36, Geriatric Depression Score, self‐efficacy, Exercise Behaviors Questionnaire Adherence: NA |
|
| Notes | People with diabetes only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome date balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis described; 19 of 145 participants lost to follow‐up (HBET: n = 11; WA: n = 8) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | High risk | Patients self‐referred from flyers distributed at health fairs, community centers, and churches; media advertisements; word of mouth; postcards People with diabetes only Six months' follow‐up: SDs of SF‐36 and WIQ imputed with baseline SDs; means and SDs of PCS and MCS of SF‐36 calculated Funding: American Diabetes Association |
Cunningham 2012.
| Methods | Study design: RCT Method of randomization: research randomizer |
|
| Participants | Country: UK Setting: single acute health board No. of participants: Baseline: HBET: n = 28; WA: n = 30 4 months: HBET: n = 28; WA: n = 30 12 months: HBET: n = 28; WA: n = 30 2 years: HBET: n = 28; WA: n = 30 Age, years (SD): HBET: 66.3 (6.3); WA: 64.5 (10.2) Sex, % male: HBET: 64; WA: 70 PAD diagnosed by: ABI Inclusion criteria: newly diagnosed IC in 1 or both legs; arterial disease confirmed by combination of Duplex ultrasonography and magnetic resonance angiography Exclusion criteria: inability to give informed consent, or medically inadvisable to increase walking owing to comorbidity |
|
| Interventions | HBET: two 1‐hour sessions, 1 week apart, delivered in participants' homes; in session 1, therapist elicited participants' beliefs about illness and about walking; in session 2, therapist worked with participants to draw up individualized walking action plans, based on recommendation of walking for at least a half‐hour 3 times per week, and walking to near‐maximal pain; 5 home visits; usual care (behavior change advice (including general advice to increase walking), information sheet about PAD, and consultations with vascular surgeon after recruitment); antiplatelet and lipid‐lowering therapy; telephone calls after 6 and 12 weeks WA: 4 home visits; non‐walking‐related conversation in attempt to control for potentially confounding effects of attention/social contact; usual care (including general advice to increase walking); antiplatelet and lipid‐lowering therapy; telephone calls after 6 and 12 weeks Duration: 2 weeks Follow‐up period: 2 years |
|
| Outcomes | Treadmill test: NA Outcomes: change in daily walking using a pedometer; decision on treatment (surgery/angioplasty vs conservative treatment) Participant‐reported outcomes: participant perception of PFWD by self‐report; disease‐specific quality of life with Intermittent Claudication Questionnaire; general quality of life with World Health Organization Quality of Life instrument (BREF) Adherence: NA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; missing data imputed via appropriate methods ITT analysis described; 7 of 58 participants lost to follow‐up (HBET: n = 4; WA: n = 3) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Study appears to be free of other sources of bias Funding: University of Stirling |
Gardner 2011.
| Methods | Study design: RCT Method of randomization: offsite random number program with blocking |
|
| Participants | Country: USA Setting: vascular clinic referrals and newspaper advertisements No. of participants: Baseline: SET: n = 40; HBET: n = 40; WA: n = 39 3 months: SET: n = 33; HBET: n = 29; WA: n = 30 Age, years (SD): SET: 66 (12); HBET: 65 (11); WA: 65 (10) Sex, % male: SET: 45; HBET: 45; WA: 54 PAD diagnosed by: ABI Inclusion criteria: history of any type of exertional leg pain, ambulation during graded treadmill test limited by leg pain consistent with IC, and ABI < 0.90 at rest or ABI < 0.73 after exercise Exclusion criteria: absence of PAD, inability to obtain ABI measure because of non‐compressible vessels, asymptomatic PAD determined from medical history and verified during graded treadmill test, use of cilostazol and pentoxifylline initiated within 3 months before investigation, exercise tolerance limited by factors other than leg pain, and active cancer, renal disease, or liver disease |
|
| Interventions | SET: 12 weeks of supervised, intermittent treadmill walking for 3 days/week at speed of 2 mph; participants wore step activity monitor during each exercise session HBET: 12 weeks of intermittent walking to near‐maximal claudication pain for 3 days/week at self‐selected pace; participants wore step activity monitor during each exercise session and received exercise logbook; during brief 15‐minute meetings, participants discussed their progress with exercise physiologist, were given feedback, and were given new instructions WA: encouragement to walk more but no specific recommendations about exercise program Duration: 3 months Follow‐up period: 3 months |
|
| Outcomes | Treadmill test: Gardner maximal treadmill test Outcomes: claudication onset time, peak walking time, peak oxygen uptake, walking economy, fractional utilization, ambulatory activity Participant‐reported outcomes: WIQ, Baltimore Activity Scale for Intermittent Claudication, SF‐36 Adherence: exercise sessions completed, total volume of exercise |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis described; 27 of 119 participants lost to follow‐up (SET: n = 7; HBET: n = 11; WA: n = 9) |
| Selective reporting (reporting bias) | High risk | Not all SF‐36 outcomes (physical functioning subscale only) reported; missing outcomes could not be obtained from trial authors |
| Other bias | High risk | Participants recruited by newspaper advertisements Funding: National Institute on Aging, Oklahoma Center for Advancement of Science and Technology grant, and Oklahoma University Health Sciences Center General Clinical Research Center grant |
Gardner 2012.
| Methods | Study design: RCT Method of randomization: random number program with blocking |
|
| Participants | Country: USA Setting: vascular clinic and newspaper and radio advertisements No. of participants: Baseline: SET: n = 106; WA: n = 36 6 weeks: SET: n = 106; WA: n = 36 3 months: SET: n = 88; WA: n = 36 6 months: SET: n = 80; WA: n = 27 Age, years (SD): SET: 68 (8); WA: 68 (8) Sex, % male: SET: 86; WA: 83 PAD diagnosed by: ABI Inclusion criteria: history of claudication, ambulation during graded treadmill test limited by claudication, ABI < 0.90 at rest or 20% decrease in ABI after exercise Exclusion criteria: absence of PAD; asymptomatic PAD determined from medical history and verified during graded treadmill test; rest pain PAD; inability to obtain ABI measure due to non‐compressible vessels; use of cilostazol and pentoxifylline < 3 months of investigation; lower extremity revascularization < 3 months before investigation; exercise tolerance limited by any disease process other than PAD; uncontrolled hypertension, uncontrolled diabetes, active cancer, renal insufficiency, or abnormal liver function; non‐compliance with baseline testing |
|
| Interventions | SET: 6 months of supervised, intermittent treadmill walking to near‐maximal claudication pain 3 days per week WA: encouragement to walk more but no specific recommendations regarding exercise program Duration: 6 months Follow‐up period: 6 months |
|
| Outcomes | Treadmill test: progressive, graded treadmill protocol (walking speed of 2 mph beginning at 0% grade, which increased by 2% every 2 minutes; Gardner‐Skinner protocol) Outcomes: claudication onset time, peak walking time, peak oxygen uptake, ABI, ischemic window, pain‐free and total distance walked during 6‐minute walk test, physical activity, calf blood flow under resting, reactive hyperemic, and maximal hyperemic conditions Participant‐reported outcomes: WIQ Adherence: exercise sessions completed, total exercise time, total distance walked |
|
| Notes | Data at 2 months' follow‐up were used in 6‐week analysis; data at 4 months' follow‐up were used in 3‐month analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis described; 35 of 142 participants lost to follow‐up (SET: n = 26; WA: n = 9) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | High risk | Patients recruited from newspaper and radio advertisements Data at 2 months' follow‐up used in 6‐week analysis; data at 4 months' follow‐up used in 3‐month analysis Funding: National Institute on Aging grants, Claude D. Pepper Older Americans Independence Center grant, and Geriatric, Research, Education, and Clinical Center grant |
Gardner 2014.
| Methods | Study design: RCT Method of randomization: off‐site random number program with blocking |
|
| Participants | Country: USA Setting: vascular labs and vascular clinics No. of participants: Baseline: SET: n = 60; HBET: n = 60 3 months: SET: n = 60; HBET: n = 60 Age, years (SD): SET: 65 (11); HBET: 67 (10) Sex, % male: SET: 48; HBET: 52 PAD diagnosed by: ABI Inclusion criteria: history of ambulatory leg pain; ambulatory leg pain confirmed by treadmill exercise; ABI < 0.90 at rest or < 0.73 after exercise Exclusion criteria: absence of PAD; non‐compressible vessels; asymptomatic PAD; use of medications indicated for treatment of claudication initiated within 3 months before investigation; exercise limited by other diseases or conditions; active cancer; end‐stage renal disease defined as Stage V chronic kidney disease; abnormal liver function; failure to complete baseline run‐in phase within 3 weeks |
|
| Interventions | SET: 3 months of intermittent walking to mild to moderate claudication pain 3 days per week at speed of 2 mph and at grade equal to 40% of highest workload achieved during baseline maximal treadmill test; participants wore step activity monitor during each exercise session HBET: 3 months of intermittent walking to mild to moderate claudication pain 3 days per week at self‐selected pace; participants wore step activity monitor during each exercise session and returned monitor and logbook to research staff; during brief 15‐minute meetings, monitoring data were downloaded, results were reviewed, and feedback was provided for upcoming month of training Duration: 3 months Follow‐up period: 3 months |
|
| Outcomes | Treadmill test: graded maximal treadmill test (Gardner‐Skinner protocol) Outcomes: claudication onset time, peak walking time, ABI, ischemic window, calf muscle StO2, walking economy and fractional utilization during submaximal walking economy test, total walking distance during 6‐minute walk test, ambulatory activity, diastolic pulse contour analysis Participant‐reported outcomes: WIQ, SF‐36 Adherence: exercise sessions completed, total volume of exercise |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; missing data imputed via appropriate methods ITT analysis described; 15 of 120 participants lost to follow‐up (SET: n = 8; HBET: n = 7) |
| Selective reporting (reporting bias) | High risk | Not all SF‐36 outcomes (physical functioning subscale only) reported; WIQ and physical functioning subscale outcomes reported incompletely, so they could not be entered into meta‐analysis; missing outcomes could not be obtained from trial authors. |
| Other bias | Low risk | Study appears to be free of other sources of bias Funding: National Institute of Aging grant, Oklahoma Center for Advancement of Science and Technology grant, and OUHSC General Clinical Research Center grant |
Guidon 2013.
| Methods | Study design: RCT Method of randomization: computer‐generated random sealed envelope method |
|
| Participants | Country: Ireland Setting: non‐invasive vascular laboratory No. of participants: Baseline: SET: n = 28; WA: n = 16 3 months: SET: n = 17; WA: n = 14 12 months: SET: n = 17; WA: n = 12 Age, years (SD): SET: 67.0 (8.6); WA: 67.1 (7.5) Sex, % male: SET: 68; WA: 75 PAD diagnosed by: ABI Inclusion criteria: Fontaine Stage II diagnosed by history of leg pain on exercise relieved by rest, classified by presence/absence of pulse/s, site of pain, ABI < 0.9 at rest and/or decrease in ankle pressure by ≥ 15 mmHg after exercise, stable disease for 3 months and residing within geographical catchment area of hospital Exclusion criteria: Fontaine Stage I, III, and IV; coexisting clinical condition that precluded participation in exercise program, including unstable cardiorespiratory disease, neurological/orthopedic limitation to exercise, poorly controlled hypertension, active major medical problem including but not limited to cancer, renal/liver disease, dementia, poorly controlled diabetes mellitus; abdominal aortic aneurysm; myocardial infarction within previous 6 months; acute onset or within first months of onset of claudication, and revascularization procedure/surgery within previous 6 months |
|
| Interventions | SET: twice‐weekly SET programme for 12 weeks (walking and other aerobic exercise modalities using range of exercise equipment) WA: advice regarding exercise and smoking cessation Duration: 3 months Follow‐up period: 12 months |
|
| Outcomes | Treadmill test: NA Outcomes: NA Participant‐reported outcomes: WIQ, Intermittent Claudication Questionnaire, SF‐36 Adherence: NA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis not described; 15 of 44 participants lost to follow‐up (SET: n = 11; WA: n = 4) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Study appears to be free of other sources of bias Funding: Royal College of Surgeons in Ireland Research Committee grant |
Hodges 2008.
| Methods | Study design: RCT Method of randomization: computerized random numbers table |
|
| Participants | Country: UK Setting: vascular outpatient clinic No. of participants: Baseline: SET: n = 14; WA: n = 14 6 weeks: SET: n = 14; WA: n = 14 3 months: SET: n = 14; WA: n = 14 Age, years (SD): 68 (8) Sex, % male: NA PAD diagnosed by: ABI Inclusion criteria: PAD (ABI < 0.9 at rest) and symptomatic IC (Edinburgh Walking Questionnaire) Exclusion criteria: inability to complete familiarization test, poorly controlled hypertension, poorly controlled diabetes, severe coronary artery disease, valvular heart disease, and debilitating pulmonary disease |
|
| Interventions | SET: 12 weeks of supervised exercise twice weekly; treadmill walking until Stage III or IV on PAD pain scale; further exercise session at home WA: walking as often as possible, but no exercise regimen to follow Duration: 3 months Follow‐up period: 3 months |
|
| Outcomes | Treadmill test: graded progressive treadmill exercise test with initial speed of 3.2 km/h and 0% gradient for 2 minutes; gradient increased by 2% every 2 minutes, and speed remaining constant (Gardner‐Skinner protocol) Outcomes: maximal walking time, peak oxygen uptake, maximal heart rate, respiratory exchange ratio, rate of perceived exertion, pain, mean arterial pressure, cardiac output, cardiac power output Participant‐reported outcomes: NA Adherence: NA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Referring to random number table |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition to permit judgement |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Kakkos 2005.
| Methods | Study design: RCT Method of randomization: blind, block "telephone" randomization procedure by means of computer |
|
| Participants | Country: UK Setting: vascular outpatient clinics No. of participants: Baseline: SET: n = 12; WA: n = 9 6 weeks: SET: n = 10; WA: n = 9 6 months: SET: n = 8; WA: n = 9 12 months: SET: n = 6; WA: n = 8 Age, years (SD): SET: 69 (11.8); WA: 66 (10.5) Sex, % male: SET: 92; WA: 89 PAD diagnosed by: Duplex ultrasonography or angiography Inclusion criteria: stable IC for > 6 months (San Diego claudication questionnaire) due to superficial femoral artery occlusion > 6 cm in length on ultrasonography and/or angiography Exclusion criteria: duration of symptoms < 6 months, previous angioplasty or arterial surgery to symptomatic leg, myocardial infarction within previous 6 months, inability to manage treadmill examination or training and any psychiatric illness or other reason making follow‐up difficult, ischemic rest pain, gangrene or ischemic ulceration, inability to attend SET program or severe peripheral neuropathy, ABI at enrollment > 0.9 or non‐compressible calf arteries precluding ABI measurement, iliac occlusions or stenoses amenable to surgery or angioplasty, femoral artery occlusion < 6 cm as shown on Duplex, suitable for angioplasty and limited exercise capacity caused by symptoms of angina, congestive heart failure, chronic obstructive pulmonary disease, disease of spinal column, venous disease, neurological disease, mental illness or arthritis; maximum claudication distance > 300 meters or < 50 meters; screening treadmill tests different by > 25% in terms of absolute claudication distance |
|
| Interventions | SET: advice to attend SET program 3 times per week for 6 months and exercise daily by walking as much as possible to near‐maximal pain; advice to stop smoking, antiplatelet therapy, and lipid‐lowering agents WA: advice to exercise daily by walking as much as possible to near‐maximal pain; advice to stop smoking, antiplatelet therapy, and lipid‐lowering agents Duration: 6 months Follow‐up period: 12 months |
|
| Outcomes | Treadmill test: constant load treadmill test (10% gradient, 3.5 km/h) Outcomes: ABI, initial claudication distance (ICD), absolute claudication distance (ACD), calf arterial inflow Participant‐reported outcomes: SF‐36, WIQ, intermittent claudication questionnaire Adherence: attendance rate of SET classes |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis described; 7 of 21 participants lost to follow‐up (SET: n = 6; WA: n = 1) |
| Selective reporting (reporting bias) | Low risk | WIQ outcomes reported incompletely; missing outcomes could be obtained from trial authors |
| Other bias | Unclear risk | Medians and IQRs of ICD, ACD, and SF‐36 reported; SDs calculated by dividing IQRs by 1.35 |
Mays 2015.
| Methods | Study design: RCT Method of randomization: computer‐generated random allocation scheme |
|
| Participants | Country: USA Setting: vascular and internal medicine clinics and hospital/university‐wide email and newsletter recruitment announcements No. of participants: Baseline: HBET: n = 10; WA: n = 10 3 months: HBET: n = 10; WA: n = 10 Age, years (SD): HBET: 67.6 (11.8); WA: 63.1 (6.7) Sex, % male: HBET: 80; WA: 80 PAD diagnosed by: ABI Inclusion criteria: ABI < 0.90; > 40 years of age, peripheral endovascular therapy 4 to 6 weeks before baseline testing or presented with stable IC symptoms and no previous revascularization within 4‐ to 6‐week window Exclusion criteria: lower extremity amputation(s) that interfered with walking on treadmill, critical limb ischemia, PAD of non‐atherosclerotic nature, primarily limited in walking by comorbidities other than IC, severe cardiac ischemia as documented on non‐invasive testing, previous myocardial infarction, transient ischemic attack or stroke 3 months before screening, treated with pentoxifylline or cilostazol for IC |
|
| Interventions | HBET: in‐hospital exercise training on treadmill for initial 2 weeks; participants then completed 12 weeks of community‐based walking exercise training (3 days/week); participants wore piezoelectric activity monitor during exercise sessions and recorded details about each session in provided walking exercise log; investigators called participants weekly with specific prompting; operational coaching model provided training guidance and help in addressing local barriers to exercise training in community; local walking environment was evaluated with audit tool WA: verbal advice to exercise but no other formal training Duration: 14 weeks Follow‐up period: 14 weeks |
|
| Outcomes | Treadmill test: Gardner protocol with participants walking until maximal claudication pain or other exercise‐induced factor Outcomes: peak oxygen consumption, peak walking time, claudication onset time, Short Physical Performance Battery, 4‐meter walking velocity test Participant‐reported outcomes: WIQ, SF‐36 Adherence: exercise sessions completed |
|
| Notes | 3 of 10 participants in HBET group and 4 of 10 participants in WA group had undergone peripheral endovascular therapy 4 to 6 weeks before baseline testing; no significant interaction was found between response and subgroups of participants. Data at 14 weeks' follow‐up were used in 3‐month analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Referring to random number table |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition to permit judgement ITT analysis described; 5 of 25 participants lost to follow‐up owing to lack of evaluable data post randomization |
| Selective reporting (reporting bias) | High risk | Not all SF‐36 outcomes (PCS and MCS only) reported; PCS and MCS outcomes reported incompletely; missing outcomes could not be obtained from trial authors |
| Other bias | High risk | Patients recruited through hospital/university‐wide email and newsletter recruitment announcements Data at 14 weeks' follow‐up used in 3‐month analysis Three months' follow‐up SDs of SF‐36 imputed with baseline SDs Funding: National Institutes of Health/National Center for Research Resources Colorado Clinical Translational Science Institute grant; National Institute of Health/National Heart, Lung, and Blood Institute National Research Service Awards grants |
Nicolai 2010.
| Methods | Study design: RCT Method of randomization: computer‐generated block randomization list stratified by center |
|
| Participants | Country: Netherlands Setting: outpatient vascular surgery clinics No. of participants: Baseline: SET: n = 202; WA: n = 102 3 months: SET: n = 177; WA: n = 88 6 months: SET: n = 169; WA: n = 83 9 months: SET: n = 169; WA: n = 83 12 months: SET: n = 169; WA: n = 83 Age, years (SD): SET: 65.9 (9.7); WA: 66.9 (8.6) Sex, % male: SET: 66.8; WA: 55.9 PAD diagnosed by: ABI Inclusion criteria: ABI < 0.9; Stage II PAD according to Fontaine and absolute claudication distance (ACD) < 500 meters as assessed with standardized treadmill test Exclusion criteria: prior SET program for IC, previous peripheral vascular intervention, insufficient command of Dutch language, serious cardiopulmonary limitations, previous lower limb amputation, psychiatric instability, and any other serious comorbidity that might hinder physical training |
|
| Interventions | SET: supervised program by local community‐based physical therapists with frequency of 2 to 3 sessions of 30 minutes weekly; verbal walking advice and brochure; cardiovascular risk management, cholesterol‐lowering medication, antiplatelet therapy, advice to stop smoking, and modification of other atherosclerotic risk factors; 93 of 202 participants received accelerometer to provide daily feedback on physical activity WA: verbal walking advice and brochure; cardiovascular risk management, cholesterol‐lowering medication, antiplatelet therapy, advice to stop smoking, and modification of other atherosclerotic risk factors Duration: 12 months Follow‐up period: 12 months |
|
| Outcomes | Treadmill test: standardized progressive treadmill test with constant speed of 3.2 km/h, starting with 0% inclination, increasing every 2 minutes by 2% (Gardner‐Skinner protocol) Outcomes: ACD, functional claudication distance (FCD) Participant‐reported outcomes: WIQ, SF‐36 Adherence: NA |
|
| Notes | SET and SET with feedback groups were combined into single group. FWD outcomes were analyzed as PFWD outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer random number generator |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis described; 52 of 304 participants lost to follow‐up (SET: n = 33; WA: n = 19) |
| Selective reporting (reporting bias) | Low risk | WIQ and SF‐36 outcomes reported incompletely; missing outcomes could be obtained from trial authors |
| Other bias | Unclear risk | SET and SET with feedback groups combined into single group FWD outcomes analyzed as PFWD outcomes Medians and IQRs of ACD and FCD reported; SDs calculated by dividing IQRs by 1.35 Funding: Netherlands Organisation for Health Research and Development grant |
Parr 2009.
| Methods | Study design: RCT Method of randomization: randomization decided by participants drawing intervention group name |
|
| Participants | Country: South Africa Setting: department of vascular surgery No. of participants: Baseline: SET: n = 10; WA: n = 10 6 weeks: SET: n = 8; WA: n = 8 Age, years (SD): SET: 57 (14); WA: 62 (10) Sex, % male: SET: 63; WA: 63 PAD diagnosed by: Duplex ultrasonography Inclusion criteria: medical history of PAD with IC Exclusion criteria: rest pain, exercise tolerance limited by medical conditions other than PAD, or significant chronic obstructive pulmonary disease |
|
| Interventions | SET: structured exercise rehabilitation 3 times per week for 45‐minute period for 6 weeks (walking on treadmill and circuit training program) WA: advice to walk as much as possible at home Duration: 6 weeks Follow‐up period: 6 weeks |
|
| Outcomes | Treadmill test: graded treadmill exercise test; speed held constant at 3.2 km/h; gradient increased by 2% every 2 minutes (Gardner‐Skinner protocol) Outcomes: ABI, heart rate, brachial blood pressure, PFWD, MWD, ventilation, VO2, respiratory exchange ratio, PFWD and MWD in 6‐minute walk test, strength testing, body mass, fat % Participant‐reported outcomes: perceived pain Adherence: compliance with training sessions |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing of lots |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis not described; 4 of 20 participants lost to follow‐up (SET: n = 2; WA: n = 2) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Patterson 1997.
| Methods | Study design: RCT Method of randomization: randomization within 3 strata by computer matrix |
|
| Participants | Country: USA Setting: physician referral and newspaper advertisements No. of participants: Baseline: SET: n = 27; HBET: n = 28 3 months: SET: n = 24; HBET: n = 23 6 months: SET: n = 19; HBET: n = 20 Age, years (SD): SET: 67.9 (7.5); HBET: 70.3 (8.6) Sex, % male: SET: 59.3; HBET: 46.4 PAD diagnosed by: ABI Inclusion criteria: resting ABI < 0.9 and decrease in ankle pressure ≥ 15 mmHg after standard exercise protocol; between ages of 50 and 75 with arterial claudication symptoms longer than 3 months' duration; meeting hemodynamic criteria Exclusion criteria: not meeting hemodynamic criteria for ischemic rest pain or tissue loss; inability to participate in exercise program due to limitations of comorbid illness; exercise‐related ischemia |
|
| Interventions | SET: 12‐week SET program consisting of three 1‐hour sessions per week of treadmill and aerobic exercise; weekly lectures related to peripheral vascular disease HBET: weekly exercise instruction (minimum of 3 times per week); weekly lectures related to peripheral vascular disease; weekly exercise logs maintained by participants and reviewed with study nurses; individual exercise counseling and review of home protocol by study nurse Duration: 3 months Follow‐up period: 6 months |
|
| Outcomes | Treadmill test: graded progressive maximal treadmill exercise test initiated at 1 mph with grade of 5%, increasing in speed and grade at 5‐minute intervals through 4 stages to 2.5 mph at 10% grade Outcomes: claudication pain time (CPT), maximal walking time (MWT), resting ABI and recovery times to self‐reported pain relief and to return to baseline ABI Participant‐reported outcomes: SF‐36 Adherence: attendance at exercise sessions |
|
| Notes | CPT and MWT were extracted from figure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Referring to random number table |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis not described; 16 of 55 participants lost to follow‐up (SET: n = 8; HBET: n = 8) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | High risk | Participants recruited by newspaper advertisements CPT and MWT extracted from figure Funding: American Heart Association grant |
Regensteiner 1997.
| Methods | Study design: RCT Method of randomization: NA |
|
| Participants | Country: USA Setting: vascular clinic No. of participants: Baseline: SET: n = 10; HBET: n = 10 3 months: SET: n = 10; HBET: n = 10 Age, years (SD): SET: 65 (7); HBET: 64 (7) Sex, % male: NA PAD diagnosed by: ABI Inclusion criteria: disabling IC; claudication symptoms stable over 3‐month period before enrollment; PAD (ABI < 0.94 at rest that decreased to < 0.73 after exercise) Exclusion criteria: leg pain at rest, ischemic ulceration, or gangrene; inability to walk on treadmill at speed ≥ 2 mph, or exercise capacity limited by symptoms of angina, congestive heart failure, chronic obstructive pulmonary disease, or arthritis; diabetes; vascular surgery or angioplasty within previous year |
|
| Interventions | SET: 12 weeks, 3 times per week, hospital‐based, supervised treadmill walking exercise program HBET: 12 weeks, home‐based program of walking (≥ 3 times/week); detailed exercise prescription; supervising nurse called participants every week to record number of walking sessions and walking time per session, and to give support Duration: 3 months Follow‐up period: 3 months |
|
| Outcomes | Treadmill test: graded treadmill protocol at initial workload of 2 mph, 0% grade for 3 minutes; subsequent stages increased by 3.5% in grade every 3 minutes to maximal claudication pain Outcomes: peak walking time, pain‐free walking time, peak oxygen consumption, resting ABI, peak heart rate, peak respiratory exchange ratio Participant‐reported outcomes: WIQ, SF‐20 Adherence: NA |
|
| Notes | SF‐20 outcomes were analyzed as SF‐36 outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Unclear risk | SF‐20 outcomes analyzed as SF‐36 outcomes Funding: Denver Veterans Administration Hospital grant |
Sandercock 2007.
| Methods | Study design: RCT Method of randomization: random number tables |
|
| Participants | Country: UK Setting: vascular outpatient clinic No. of participants: Baseline: SET: n = 14; HBET: n = 15; WA: n = 15 6 weeks: SET: n = 14; HBET: n = 15; WA: n = 15 3 months: SET: n = 14; HBET: n = 15; WA: n = 15 Age, years (SD): SET: 66 (8); HBET: 62 (14); WA: 67 (6) Sex, % male: SET: 71; HBET: 80; WA: 67 PAD diagnosed by: ABI Inclusion criteria: ABI < 0.94 at rest; symptomatic IC during walking Exclusion criteria: inability to perform familiarization test, poorly controlled hypertension, poorly controlled diabetes, severe coronary artery disease, valvular heart disease, limb ischemia, and debilitating pulmonary disease |
|
| Interventions | SET: twice a week, 30 minutes of treadmill walking; exercise diary; instructed to undertake 1 additional 30‐minute walking session per week HBET: exercise diary; instructed to undertake three 30‐minute walking sessions per week at rating of perceived effort (RPE) of 12 to 14; contacted weekly by telephone and given support and encouragement in adhering to protocol WA: verbal information regarding safety and efficacy of walking exercise but no specific instructions regarding exercise duration, intensity, or frequency Duration: 3 months Follow‐up period: 3 months |
|
| Outcomes | Treadmill test: graded treadmill test; initial speed of 2 miles/h for 2 minutes; gradient then increased by 2% every 2 minutes until test termination Outcomes: maximal walking time, peak oxygen uptake, respiratory exchange ratio, heart rate variability measures Participant‐reported outcomes: RPE, pain rating Adherence: NA |
|
| Notes | When data were missing, most recent recorded values were carried forward. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Referring to random number table |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data imputed via potentially inappropriate methods; when data were missing, most recent recorded values were carried forward ITT analysis described; 3 of 44 participants lost to follow‐up (SET: n = 2; HBET: n = 1; WA: n = 0) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Sanderson 2006.
| Methods | Study design: RCT Method of randomization: closed envelope system |
|
| Participants | Country: Australia Setting: NA No. of participants: Baseline: SET: n = 14; WA: n = 14 6 weeks: SET: n = 13; WA: n = 14 Age, years (SD): SET: 62 (6); WA: 61 (10) Sex, % male: SET: 62; WA: 57 PAD diagnosed by: ABI Inclusion criteria: reduced ABI (< 0.9) in ≥ 1 limb and documented history of IC Exclusion criteria: living > 50 km from research venue, not responding to invitation to participate, inablity to participate for personal reasons, ineligible because of reduced cardiac function or unstable angina, rest pain, recent surgery or cardiovascular event, other medical conditions for which exercise testing and training were contraindicated; not primarily limited by claudication, ischemic ECG changes, or uncontrolled hypertension |
|
| Interventions | SET: 3 supervised treadmill training sessions per week each for period of 6 weeks; standard cardiovascular risk factor modification WA: advice concerning need to stop smoking and to exercise; standard cardiovascular risk factor modification Duration: 6 weeks Follow‐up period: 6 weeks |
|
| Outcomes | Treadmill test: maximal graded walking test on motorized treadmill at constant speed of 2.7 km/h; gradient set at 0% for first 5 minutes of test, then increased by 2% every 3 minutes until participant failed to sustain task Outcomes: PFWT, MWT, pain‐free cycling time and total time spent cycling during maximal graded cycle test, heart rate and pulmonary gas exchange data, minute ventilation, oxygen consumption, carbon dioxide production, ABI Participant‐reported outcomes: NA Adherence: NA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis not described; 1 of 28 participants lost to follow‐up (SET: n = 1; WA: n = 0) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Unclear risk | Setting is unclear Funding: National Heart Foundation grant |
Savage 2001.
| Methods | Study design: RCT Method of randomization: NA |
|
| Participants | Country: USA Setting: clinical practice of 3 vascular surgeons No. of participants: Baseline: SET: n = 11; HBET: n = 10 3 months: SET: n = 11; HBET: n = 10 6 months: SET: n = 11; HBET: n = 10 Age, years (SD): SET: 66.4 (9.1); HBET: 66.1 (8.9) Sex, % male: SET: 73; HBET: 70 PAD diagnosed by: clinical diagnosis of IC Inclusion criteria: older than 50 years of age with clinical diagnosis of IC; grade I or category 1, 2, or 3 claudication according to Society for Vascular Surgery/International Society for Cardiovascular Surgery's standardized reporting system Exclusion criteria: unstable cardiopulmonary disease, severe lower extremity arthritis, tobacco use, weight > 40 kg above ideal, renal insufficiency, use of beta‐blocking drugs, use of pentoxifylline or cilostazol within 8 weeks of entry to study, functioning lower extremity bypass, or severe cognitive impairment |
|
| Interventions | SET: structured SET program; 3 exercise sessions per week for 12 weeks; exclusive treadmill walking; at end of 12 weeks, participants in SET group transitioned to HBET program for additional 12 weeks HBET: exercise at least 3 times weekly, specifically walking to point of intense pain, resting, then continuing; participants contacted briefly by telephone once per month by registered nurse to discuss program Duration: 3 months Follow‐up period: 6 months |
|
| Outcomes | Treadmill test: symptom‐limited electrocardiographically monitored treadmill test; constant walking speed of 2 miles per hour beginning at 0% grade and increasing grade by 2% every 2 minutes until ACD (Gardner‐Skinner protocol) Outcomes: absolute claudication distance, initial claudication distance, peak oxygen consumption, ABI Participant‐reported outcomes: SF‐36 Adherence: NA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation process to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition to permit judgement |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Unclear risk | Means and SDs of PCS and MCS of SF‐36 calculated Funding: General Clinical Research Center, University of Vermont College of Medicine grant, Medical Research Council of Canada grant, and American Heart Association grant |
Stewart 2008.
| Methods | Study design: RCT Method of randomization: sealed envelopes |
|
| Participants | Country: UK Setting: hospital vascular surgery clinic No. of participants: Baseline: SET: n = 30; WA: n = 30 3 months: SET: n = 28; WA: n = 28 6 months: SET: n = 27; WA: n = 24 Age, years (SD): SET: 68 (7.73); WA: 68 (8.87) Sex, % male: SET: 67; WA: 73 PAD diagnosed by: ABI Inclusion criteria: calf or buttock claudication limiting exercise and ABI ≤ 0.90 in affected leg Exclusion criteria: comorbidity that limited exercise, symptoms of recent onset or recent revascularization; wide variation in symptom severity on different days or recent periods of symptom improvement, history of recent myocardial infarction |
|
| Interventions | SET: 3‐month‐long, twice‐weekly circuit‐based exercise program supervised in hospital physiotherapy gymnasium; after 3 months, participants continued with unsupervised exercise for 3 additional months WA: exercise advice alone Duration: 3 months Follow‐up period: 6 months |
|
| Outcomes | Treadmill test: constant‐load treadmill test (2.5 km/h, incline of 10 degrees) Outcomes: PFWD, MWD, ABI, heart rate, blood pressure, lactate change with walking Participant‐reported outcomes: NA Adherence: attendance at classes |
|
| Notes | Treadmill walking were not included; exercises were mainly focused on calf muscle. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Shuffling envelopes |
| Allocation concealment (selection bias) | Low risk | Central allocation; sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis not described; 9 of 60 participants lost to follow‐up (SET: n = 3; WA: n = 6) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | Unclear risk | Treadmill walking not included; exercises mainly focused on calf muscle Medians and IQRs of PFWD and MWD reported; SDs calculated by dividing IQRs by 1.35 |
Treat‐Jacobson 2009.
| Methods | Study design: RCT Method of randomization: simple randomization tables |
|
| Participants | Country: USA Setting: clinical referral and media advertisements No. of participants: Baseline: SET: n = 13; HBET: n = 8 3 months: SET: n = 11; HBET: n = 8 6 months: SET: n = 9; HBET: n = 6 Age, years (SD): SET: 64 (11.7); HBET: 70 (7.8) Sex, % male: SET: 64; HBET: 88 PAD diagnosed by: ABI Inclusion criteria: age > 18 years and lifestyle‐limiting claudication; ABI < 0.90 and/or decrement in ABI > 10% following symptom‐limited treadmill exercise test; ability to walk at rate of 2.0 mph on treadmill; able and willing to participate in 12‐week SET program; fasting glucose levels within acceptable range for exercise training in patients with diabetes mellitus Exclusion criteria: uncontrolled hypertension; ischemic rest leg pain and/or leg/foot ulceration, or impending gangrene; exercise capacity limited by health problems other than claudication such as angina pectoris, severe arthritis, marked dyspnea on exertion; recent myocardial infarction or unstable coronary heart disease; coronary or lower extremity revascularization procedure within previous 3 months; MWDs determined with graded exercise tests varied > 25% |
|
| Interventions | SET: treadmill walking in exercise laboratory 3 times per week for 12 weeks, for total of 36 sessions HBET: specific, standardized, written walking instructions and daily exercise record (≥ 3 times per week); weekly visits in exercise laboratory for review of exercise records Duration: 3 months Follow‐up: 6 months |
|
| Outcomes | Treadmill test: symptom‐limited, graded, cardiopulmonary treadmill exercise test; walking on treadmill at speed of 2 mph starting at 0% grade; increased 3.5% every 3 minutes until 10.5% grade was obtained, at which time speed was increased by 0.5 mph every 3 minutes, while grade maintained at 10.5% Outcomes: PFWD, MWD, ABI, blood pressure, heart rate, VO2 peak uptake, upper limb peak exercise capacity via arm‐ergometry exercise test Participant‐reported outcomes: NA Adherence: exercise sessions completed, any exercise performed beyond SET |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Referring to random number table |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding; inherent to study design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups ITT analysis not described; 6 of 21 participants lost to follow‐up (SET: n = 4; HBET: n = 2) |
| Selective reporting (reporting bias) | Low risk | Published reports included all expected outcomes |
| Other bias | High risk | Study participants recruited through media advertisements Funding: Scientist Development grant, American Heart Association Northland Affiliate |
ABI: ankle‐brachial index; ACD: absolute claudication distance; COT: claudication onset time; CPT: claudication pain time; ECG: electrocardiogram; FCD: functional claudication distance; FWD/T: functional treadmill walking distance or time; HBET: home‐based exercise therapy; IC: intermittent claudication; ICD: initial claudication distance; IQR: interquartile range; ITT: intention‐to‐treat; MCS: mental component summary; MWD/T: maximal treadmill walking distance or time; NA: not available; PAD: peripheral artery disease; PCS: physical component summary; PFWD/T: pain‐free treadmill walking distance or time; PWT: peak walking time; RCT: randomized controlled trial; RPE: rating of perceived effort; SD: standard deviation; SET: supervised exercise therapy; SF‐20: Medical Outcomes Study Short Form 20; SF‐36: Medical Outcomes Study Short Form 36; StO2: tissue (muscle) oxygen saturation; VO2: maximum amount of oxygen consumed during exercise; WA: walking advice; WIQ: Walking Impairment Questionnaire.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arosio 1999 | This trial compared exercise with iloprost |
| Arosio 2001 | This trial compared exercise with iloprost |
| Castro‐Sanchez 2013 | This trial compared 3 physical therapy modalities with placebo |
| Ciuffetti 1994 | This trial compared exercise with pentoxifylline |
| Collins 2007 | This trial compared HBET with no exercise |
| Collins 2012 | This trial compared walking with poles with traditional walking |
| Crowther 2008 | This trial compared standard medical therapy (SMT) plus SET, SMT alone (no exercise), and non‐PAD control |
| Cucato 2013 | This trial compared walking training with stretching classes |
| Dahllof 1976 | This trial compared exercise with placebo |
| Degischer 2002 | This trial compared SET plus clopidogrel, SET alone, and WA; this was not an RCT; it was included in the 2006 version of this review |
| Delaney 2014 | This trial compared combined treadmill and lower limb resistance SET with treadmill‐only SET |
| Fakhry 2011 | This trial compared SET with HBET; this was not an RCT |
| Fowler 2002 | This trial compared "stop smoking and keep walking" with "usual care" (no exercise) |
| Gardner 2001 | This trial compared SET with no exercise |
| Gardner 2005 | This trial compared low‐intensity exercise rehabilitation with high‐intensity exercise rehabilitation |
| Gelin 2001 | This trial compared SET, surgical intervention, and no treatment |
| Gibellini 2000 | This trial compared SET with no exercise |
| Greenhalgh 2008 | This trial compared PTA with no PTA against a background of SET and best medical therapy |
| Hiatt 1990 | This trial compared SET with no exercise |
| Hiatt 1994 | This trial compared treadmill walking exercise, strength training, and no exercise |
| Hobbs 2007 | This trial compared best medical therapy (BMT) plus SET plus cilostazol, BMT plus SET, BMT plus cilostazol, and BMT alone (no exercise) |
| Jakubseviciene 2014 | This trial compared SET plus HBET with SET alone (different treatment protocols) in patients post lower limb bypass surgery |
| Jansen 1991 | This trial compared SET with no exercise |
| Krause 1974 | This trial compared SET with SET (different treatment protocols) |
| Kruidenier 2011 | This trial compared percutaneous vascular intervention (PVI) plus SET with PVI alone |
| Langbein 2002 | This trial compared polestriding exercise plus vitamin E, polestriding exercise plus placebo, no exercise plus vitamin E, and no exercise plus placebo |
| Larsen 1966 | This trial compared exercise with placebo |
| Lee 2007 | This trial compared conservative medical therapy (CMT) plus SET with CMT alone (WA); this was not an RCT |
| Leon 2005 | This trial compared HBET with no exercise in apparently healthy middle‐aged men |
| Lepantalo 1991 | This trial compared SET plus placebo with SET alone |
| Manfredini 2008 | This trial compared HBET with HBET (different treatment protocols); this is not an RCT |
| McDermott 2004 | This trial compared SET with no exercise in patients with PAD but no symptoms of IC |
| McDermott 2009 | This trial compared treadmill exercise with resistance training in patients with PAD with or without IC |
| McDermott 2013 | This trial compared HBET with no exercise in patients with PAD with or without IC |
| Mika 2005 | This trial compared SET with no exercise |
| Mika 2011 | This trial compared SET with no exercise |
| Mika 2013 | This trial compared pain‐free treadmill training with moderate treadmill training |
| Murphy 2012 | This trial compared optimal medical care (OMC) plus SET, OMC plus stent revascularization, and OMC alone |
| Nielsen 1975 | This trial compared SET with WA; this was not an RCT; it was included in the 2006 version of this review |
| Nielsen 1977 | This trial compared SET with WA; this was not an RCT; it was included in the 2006 version of this review |
| Parmenter 2013 | This trial compared high‐intensity progressive resistance training, low‐intensity non‐progressive resistance training, and WA |
| Pilz 2014 | This trial compared 6‐month SET with 12‐month SET combining endurance and strength training; this was not an RCT |
| Ritti‐Dias 2010 | This trial compared strength training with walking training |
| Schlager 2011 | This trial compared best medical treatment (BMT) plus SET with BMT alone (no exercise) |
| Spafford 2014 | This trial compared Nordic pole HBET with standard HBET |
| Spronk 2009 | This trial compared SET with endovascular revascularization |
| Tebbutt 2011 | This trial compared standard care (SC) plus a plantar flexion device with SC alone (WA) |
| Tew 2009 | This trial compared arm‐crank exercise with no exercise |
| Tew 2015 | This trial compared HBET with no exercise |
| Tisi 1997 | This trial compared SET with no exercise |
| Tsai 2002 | This trial compared SET with no exercise |
| Ventura 1984 | This trial compared HBET with no exercise |
| Walker 2000 | This trial compared upper limb exercise, lower limb exercise, and control with no training; participants in the control group were not randomized but were recruited on an ad hoc basis in parallel with the main trial |
| Wood 2006 | This trial compared SET with no exercise |
| Zwierska 2005 | This trial compared upper limb aerobic exercise, lower limb aerobic exercise, and no exercise |
BMT: best medical therapy; CMT: conservative medical therapy; HBET: home‐based exercise therapy; IC: intermittent claudication; OMC: optimal medical care; PAD: peripheral artery disease; PTA: percutaneous transluminal angioplasty; PVI: percutaneous vascular intervention; RCT: randomized controlled trial; SC: standard care; SET: supervised exercise therapy; SMT: standard medical therapy; WA: walking advice.
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000243415.
| Trial name or title | Comparison of the effects of supervised treadmill walking training and supervised walking with poles on functional capabilities in patients with intermittent claudication |
| Methods |
Study type: Interventional Study design: Randomized controlled trial |
| Participants | 90 patients with intermittent claudication Inclusion criteria: ‐Atherosclerosis of lower extremities evaluated according to Fontaine classification as degree IIA and IIB ‐Ankle‐brachial index < 0.9 ‐No systemic contraindications to undertake the proposed forms of exercise ‐Written consent to participate in a clinical trial Exclusion criteria: ‐Inability to walk on a treadmill at a speed of 2 mph ‐Recently completed vascular treatments (< 6 months) ‐Changes in pharmacological treatment (< 6 months) ‐Symptomatic coronary artery disease ‐Exertional dyspnea ‐Resting blood pressure > 160/100 mmHg ‐Resting tachycardia > 100/min ‐Thrombophlebitis ‐Arterial embolism ‐Active cancer ‐Exercise‐induced asthma ‐Cardiorespiratory failure (NYHA III) |
| Interventions | 12‐week supervised treadmill walking training 3 times a week vs 12‐week supervised walking training with poles 3 times a week |
| Outcomes |
Primary outcome measures: ‐Maximal walking distance (treadmill test, Gardner protocol) ‐Metabolic cost of walking determined by oxygen consumption measure (graded treadmill walking test with simultaneous breath‐by‐breath VO2 measurements) ‐Vascular endothelial function ‐ method of determining degree of dilatation of the brachial artery ‐ FMD flow‐mediated dilatation Secondary outcome measures: ‐Assessment of functional walking ability (WIQ [Walking Impairment Questionnaire]) ‐Pain‐free walking distance (treadmill test, Gardner protocol) |
| Starting date | April 2013 |
| Contact information | Miss Ewelina Rosloniec Akademia Wychowania Fizycznego w Krakowie Zawodzie 1a/18, 31‐232 Krakow, Poland +48 518494666 ewelinarosloniec@gmail.com |
| Notes |
NCT02075502.
| Trial name or title | Community walking exercise for patients with peripheral artery disease (GAIT) |
| Methods |
Study type: ‐Interventional Study design: ‐Allocation: randomized ‐Intervention model: parallel assignment ‐Masking: single blind (investigator) ‐Primary purpose: treatment |
| Participants | 134 patients with peripheral artery disease (PAD) Inclusion criteria: ‐Men and women with diagnosis of atherosclerotic PAD ‐≥ 40 years of age ‐Abnormal ankle‐brachial index (ABI) ≤ 0.90 ‐ABI > 0.90 and < 1.00, post‐exercise ABI drop ≥ 15% compared with resting ABI ‐Patients receiving lower extremity exercise therapy (ET) or peripheral open intervention ‐Patients not receiving lower extremity ET or peripheral open intervention but present with stable claudication and an abnormal ABI Exclusion criteria: ‐Lower extremity amputation(s), including a toe amputation, which interfere(s) with walking on the treadmill ‐Individuals with critical limb ischemia defined by ischemic rest pain or ischemic ulcers/gangrene on lower extremities ‐PAD of non‐atherosclerotic nature (e.g., fibromuscular dysplasia, irradiation, endofibrosis) ‐Coronary artery bypass grafts or major surgical procedures within 6 months before screening ‐Individuals whose walking exercise is primarily limited by symptoms of chronic obstructive pulmonary disease, angina, or heart failure ‐Individuals who are unable to walk on the treadmill at a speed ≥ 2 mph for ≥ 1 minute ‐Individuals who have had a myocardial infarction within 3 months before screening ‐Individuals who demonstrate symptoms consistent with acute coronary syndrome ‐Individuals who exhibit ischemia as documented on the 12‐lead electrocardiogram including horizontal or down‐sloping ST‐segment depression ≥ 0.5 mm at rest and > 1 mm with exercise in 2 contiguous leads, relative to the PR‐segment (ST‐segment measured 0.08 seconds after the J point, ST‐segment elevation ≥ 1 mm) ‐Individuals who have had a transient ischemic attack or stroke 3 months before screening ‐Individuals with left bundle branch block or sustained ventricular tachycardia (> 0 seconds) during screening ‐Individuals with uncontrolled hypertension (≥ 180 systolic or ≥ 100 diastolic resting blood pressure) during screening ‐Treatment with pentoxifylline or cilostazol for claudication 4 weeks before screening; patients can be reconsidered for study inclusion following a 1‐month washout period from these medications ‐Electrolyte abnormalities (e.g., potassium < 3.3 mmol/L) ‐Pregnancy, fertility without protection against pregnancy (for women of childbearing potential, a serum pregnancy test will be performed at screening) ‐Incarcerated individuals ‐Individuals acutely impaired by alcohol or other illicit drugs ‐Poorly controlled diabetes defined as glycated hemoglobin > 12% ‐Severely anemic patients (Hgb < 11 g/dL for women and < 10 g/dL for men) ‐For patients who have not received peripheral revascularization, ABI > 0.90 ‐For patients with equivocal resting ABIs (0.91 to 0.99), a drop < 15% in the postexercise ABI ‐For individuals with non‐compressible vessels (ABI > 1.39) who have a toe‐brachial index (TBI) > 0.70 ‐Inability to speak English ‐Other clinically significant disease that is, in the opinion of the study team, not stabilized or may otherwise confound results of the study |
| Interventions | Community‐based walking exercise program with detailed training, monitoring, and coaching (TMC) exercise components vs standard of care (exercise advice) |
| Outcomes |
Primary outcome measure: ‐Change in peak walking time (PWT) Secondary outcome measures: ‐Change in claudication onset time (COT) ‐Change in patient‐reported outcomes ‐Change in peak oxygen uptake ‐Change in functional ability ‐Evaluation of total volume of activity ‐Evaluation of exercise adherence |
| Starting date | February 2014 |
| Contact information | Ryan J. Mays, PhD, MPH, MS 406‐327‐1731; ryan.mays@providence.org |
| Notes |
NCT02341716.
| Trial name or title | Hospital‐ and home‐based Supervised exercise versus UNsupervised walk advice For patients with InTermittent Claudication (SUNFIT) |
| Methods |
Study type: ‐Interventional Study design: ‐Allocation: randomized ‐Intervention model: parallel assignment ‐Masking: single blind (outcomes assessor) ‐Primary purpose: treatment |
| Participants | 165 patients with intermittent claudication Inclusion criteria: ‐Intermittent claudication in 1 or both legs with a typical history and ankle‐brachial index (ABI) ≤ 0.90 and/or ≥ 30% postexercise reduction in ABI ‐Symptom duration > 6 months ‐Intermittent claudication is the walk‐limiting condition Exclusion criteria: ‐Invasive treatment for intermittent claudication performed within 3 months ‐Invasive treatment for intermittent claudication considered necessary within 12 months ‐Inability to understand Swedish, answer questionnaires, or perform walk test |
| Interventions | Hospital‐based (SET) supervised exercise program vs home‐based (HET) supervised exercise program vs unsupervised walk advice (WA) |
| Outcomes |
Primary outcome measures: ‐Six‐minute‐walk‐test walking distance (6MWD) ‐Generic health‐related quality of life (SF‐36) Secondary outcome measures: ‐Disease‐specific health‐related quality of life (VascuQoL) ‐Walking impairment (Walking Impairment Questionnaire [WIQ]) ‐Physical activity ‐Compliance with exercise therapy ‐HbA1c and serum lipids ‐Ankle‐brachial index (ABI) ‐Patient‐specified goals with treatment (PSFS) ‐Cardiovascular events ‐Musculoskeletal events ‐Vascular surgical revascularization ‐Disease‐specific health‐related quality of life (VascuQoL) ‐Walking impairment (Walking Impairment Questionnaire [WIQ]) ‐Physical activity ‐Compliance to exercise therapy ‐Patient‐specified goals with treatment (PSFS) |
| Starting date | September 2014 |
| Contact information | Lennart Jivegård, MD, Lecturer; +46313427486; lennart.jivegard@vgregion.se Joakim Nordanstig, MD, PhD; +46313421000; joakim.nordanstig@vgregion.se |
| Notes |
NCT02729090.
| Trial name or title | Effects of exercise in the functional capacity, central artery and rigidity ankle brachial index |
| Methods |
Study type: ‐Interventional Study design: ‐Allocation: randomized ‐Intervention model: parallel assignment ‐Masking: single blind (investigator) ‐Primary purpose: treatment |
| Participants | 70 patients with peripheral arterial disease (PAD) Inclusion criteria: ‐Diagnosis of PAD and intermittent claudication (IC) ‐Stage I or II of Fontaine ‐Intermittent claudication symptoms for ≥ 3 months with ankle‐brachial index (ABI) at rest ≤ 0.90 in 1 or 2 legs Exclusion criteria: ‐Ischemia criticism in 1 of the lower limbs ‐Moderate or severe ulcers on 1 of the lower limbs ‐Orthopedic issues that prevent practicing the exercises or conducting evaluations ‐Participation in other studies with rehabilitation ‐Decompensated hypertension ‐Decompensated diabetes ‐Cardiovascular events < 3 months ‐Severe lung disease |
| Interventions | Combined aerobic strength training vs aerobic only training |
| Outcomes |
Primary outcome measure: ‐Cardiopulmonary test: 6‐minute walk test Secondary outcome measures: ‐Pulse wave velocity ‐One maximum repetition test ‐Ankle‐brachial index ‐Edinburgh Claudication Questionnaire |
| Starting date | May 2015 |
| Contact information | Eduardo L. Garcia, MD; 0555133597634; rceduardogarcia@gmail.com Rosane M. Nery, PhD; 0555133597634; rosane.nery@gmail.com |
| Notes |
NCT02879019.
| Trial name or title | Walking Training in Peripheral Artery Disease (GrEnADa Sub‐study) (GrEnADa) |
| Methods |
Study type: ‐Interventional Study design: ‐Allocation: randomized ‐Intervention model: parallel assignment ‐Masking: single blind (outcomes assessor) ‐Primary purpose: treatment |
| Participants | 34 women with peripheral artery disease (PAD) Inclusion criteria: ‐Ankle‐brachial index (ABI) ≤ 0.9 in 1 or 2 legs ‐Fontaine Stage II of PAD ‐Body mass index < 35 kg/m2 ‐Resting systolic blood pressure (BP) < 160 mmHg and diastolic BP < 105 mmHg ‐Ability to walk ≥ 2 minutes at 3.2 km/h ‐Ability to undertake an incremental treadmill test ‐Decrease ≥ 15% in ABI after a maximal treadmill test ‐Not currently engaging in any regular exercise program Exclusion criteria: ‐Exercise‐induced signs of myocardial ischemia or complex ventricular arrhythmias ‐Cardiovascular autonomic neuropathy ‐Use of beta‐blocker, non‐dihydropyridine calcium antagonists, or insulin and hormone replacement therapy |
| Interventions | Supervised walking training vs stretching exercise |
| Outcomes |
Primary outcome measures: ‐Change in walking capacity at 12 weeks' follow‐up ‐Change in functional capacity at 12 weeks' follow‐up ‐Change in heart rate pain threshold at 12 weeks' follow‐up Secondary outcome measures: ‐Change in ankle‐brachial index decrease at 12 weeks' follow‐up ‐Change in ischemic window at 12 weeks' follow‐up ‐Change in autonomic modulation at 12 weeks' follow‐up ‐Change in cardiac output at 12 weeks' follow‐up ‐Change in vascular function at 12 weeks' follow‐up |
| Starting date | January 2017 |
| Contact information | Veronique Cornelissen, PhD; 003216329152; veronique.cornelissen@kuleuven.be |
| Notes |
6MWD: six‐minute walking distance; ABI: ankle‐brachial index; BP: blood pressure; COT: claudication onset time; ET: exercise therapy; FMD: flow‐mediated dilatation; HbA1c: glycated hemoglobin; HET: home exercise therapy; Hgb: hemoglobin; IC: intermittent claudication; NYHA: New York Heart Association; PAD: peripheral artery disease; PSFS: patient‐specified goals with treatment; PWT: peak walking time; SET: supervised exercise therapy; SF‐36: Medical Outcomes Study Short Form 36; TBI: toe‐brachial index; TMC: training, monitoring, and coaching; VascuQoL: Vascular Quality of Life Questionnaire; VO2: maximum amount of oxygen consumed during exercise; WA: walking advice; WIQ: Walking Impairment Questionnaire.
Differences between protocol and review
The protocol of this review can be obtained from Bendermacher 2005.
We assessed the methodological quality of trials using the risk of bias method provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we completed "Risk of bias" tables. We assessed the quality of evidence using the GRADE approach (Atkins 2004), and we added "Summary of findings" tables.
The 2006 and 2013 reviews compared SET with non‐supervised exercise therapy. However, "non‐supervised exercise therapy" is a vague term, as this could be promoted by basic advice to walk more (i.e., WA) through to complex, multi‐component behavior change programs (i.e., HBET). For this update, we reviewed effects of HBET and WA separately.
Because of the larger number of eligible studies, we decided that only RCTs would be included in the previous review of 2013 and in the current update. We excluded three studies that had been included in the original review of 2006 (Degischer 2002; Nielsen 1975; Nielsen 1977).
The Rutherford classification is increasingly used to define PAD (Rutherford 1997). Therefore, we decided to add this classification to the inclusion criteria.
Because of the extensive quality of life assessment, we removed the secondary outcome "functional status". We added the secondary outcome "self‐reported functional impairment".
We rephrased the secondary outcome "compliance" to "adherence to exercise program" to avoid misunderstanding of the intended variable.
Contributions of authors
David Hageman: selected trials, assessed trial quality, extracted data, performed data analysis, and wrote the text of this update. Hugo JP Fokkenrood: selected trials, assessed trial quality, extracted data, and revised the text. Lindy NM Gommans: selected trials, assessed trial quality, extracted data, and revised the text. Marijn ML van den Houten: confirmed the suitability and quality of trials and revised the text. Joep AW Teijink: confirmed the suitability and quality of trials and revised the text.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding to Cochrane Vascular (13/89/23). The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Declarations of interest
David Hageman: none known. Hugo JP Fokkenrood: none known. Lindy NM Gommans: none known. Marijn ML van den Houten: none known. Joep AW Teijink: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Allen 2010 {published and unpublished data}
- Allen JD, Stabler T, Kenjale A, Ham KL, Robbins JL, Duscha BD, et al. Plasma nitrite flux predicts exercise performance in peripheral arterial disease after 3 months of exercise training. Free Radical Biology & Medicine 2010;49(6):1138‐44. [PUBMED: 20620208] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duscha BD, Robbins JL, Jones WS, Kraus WE, Lye RJ, Sanders JM, et al. Angiogenesis in skeletal muscle precede improvements in peak oxygen uptake in peripheral artery disease patients. Arteriosclerosis, Thrombosis, and Vascular Biology 2011;31(11):2742‐8. [PUBMED: 21868709] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheetham 2004 {published data only (unpublished sought but not used)}
- Cheetham DR, Burgess L, Ellis M, Williams A, Greenhalgh RM, Davies AH. Does supervised exercise offer adjuvant benefit over exercise advice alone for the treatment of intermittent claudication? A randomised trial. European Journal of Vascular and Endovascular Surgery 2004;27(1):17‐23. [PUBMED: 14652832] [DOI] [PubMed] [Google Scholar]
Christman 2003 {published data only}
- Christman SK. The impact of a nurse implemented, theory‐based exercise adoption and smoking cessation program on functional status, stage of change, decisional balance, and self‐efficacy with claudicants. Intervention to slow progression of peripheral arterial disease (Dissertation). Ohio State University, 2003. [Google Scholar]
Collins 2011 {published and unpublished data}
- Collins TC, Lunos S, Carlson T, Henderson K, Lightbourne M, Nelson B, et al. Effects of a home‐based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care 2011;34(10):2174‐9. [PUBMED: 21873560] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cunningham 2012 {published data only (unpublished sought but not used)}
- Cunningham MA, Swanson V, Holdsworth RJ, O'Carroll RE. Late effects of a brief psychological intervention in patients with intermittent claudication in a randomized clinical trial. British Journal of Surgery 2013;100(6):756‐60. [PUBMED: 23468185] [DOI] [PubMed] [Google Scholar]
- Cunningham MA, Swanson V, O'Carroll RE, Holdsworth RJ. Randomized clinical trial of a brief psychological intervention to increase walking in patients with intermittent claudication. British Journal of Surgery 2012;99(1):49‐56. [PUBMED: 22038532] [DOI] [PubMed] [Google Scholar]
Gardner 2011 {published data only (unpublished sought but not used)}
- Gardner AW, Parker DE, Montgomery PS, Blevins SM. Diabetic women are poor responders to exercise rehabilitation in the treatment of claudication. Journal of Vascular Surgery 2014;59(4):1036‐43. [PUBMED: 24246541] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner AW, Parker DE, Montgomery PS, Scott KJ, Blevins SM. Efficacy of quantified home‐based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation 2011;123(5):491‐8. [PUBMED: 21262997] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2012 {published data only}
- Gardner AW, Montgomery PS, Parker DE. Optimal exercise program length for patients with claudication. Journal of Vascular Surgery 2012;55(5):1346‐54. [PUBMED: 22459748] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardner 2014 {published data only (unpublished sought but not used)}
- Gardner AW, Parker DE, Montgomery PS, Blevins SM. Step‐monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. Journal of the American Heart Association 2014;3(5):e001107. [PUBMED: 25237048] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauer K, Exaire JE, Stoner JA, Saucedo JF, Montgomery PS, Gardner AW. Effect of exercise training on clot strength in patients with peripheral artery disease and intermittent claudication: An ancillary study. SAGE Open Medicine 2015;3:2050312115575938. [PUBMED: 26770772] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guidon 2013 {published data only}
- Guidon M, McGee H. One‐year effect of a supervised exercise programme on functional capacity and quality of life in peripheral arterial disease. Disability and Rehabilitation 2013;35(5):397‐404. [PUBMED: 22804715] [DOI] [PubMed] [Google Scholar]
Hodges 2008 {published data only}
- Hodges LD, Sandercock GR, Das SK, Brodie DA. Randomized controlled trial of supervised exercise to evaluate changes in cardiac function in patients with peripheral atherosclerotic disease. Clinical Physiology and Functional Imaging 2008;28(1):32‐7. [PUBMED: 18005078] [DOI] [PubMed] [Google Scholar]
Kakkos 2005 {published and unpublished data}
- Kakkos SK, Geroulakos G, Nicolaides AN. Improvement of the walking ability in intermittent claudication due to superficial femoral artery occlusion with supervised exercise and pneumatic foot and calf compression: a randomised controlled trial. European Journal of Vascular and Endovascular Surgery 2005;30(2):164‐75. [PUBMED: 15890545] [DOI] [PubMed] [Google Scholar]
Mays 2015 {published data only (unpublished sought but not used)}
- Mays RJ, Hiatt WR, Casserly IP, Rogers RK, Main DS, Kohrt WM, et al. Community‐based walking exercise for peripheral artery disease: an exploratory pilot study. Vascular Medicine (London, England) 2015;20(4):339‐47. [PUBMED: 25755148] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nicolai 2010 {published and unpublished data}
- Gommans LN, Scheltinga MR, Sambeek MR, Maas AH, Bendermacher BL, Teijink JA. Gender differences following supervised exercise therapy in patients with intermittent claudication. Journal of Vascular Surgery 2015;62(3):681‐8. [PUBMED: 26304482] [DOI] [PubMed] [Google Scholar]
- Nicolai SP, Hendriks EJ, Prins MH, Teijink JA. Optimizing supervised exercise therapy for patients with intermittent claudication. Journal of Vascular Surgery 2010;52(5):1226‐33. [PUBMED: 20692797] [DOI] [PubMed] [Google Scholar]
- Nicolai SP, Prins MH, Teijink JA. Supervised exercise therapy is more effective than walking advice in patients with intermittent claudication; a randomized multicenter study [Gesuperviseerde looptherapie effectiever dan loopadvies bij patiënten met claudicatio intermittens; gerandomiseerde multicentrische studie]. Nederlands Tijdschrift voor Geneeskunde 2011;155:A2643. [Google Scholar]
- Nicolai SP, Teijink JA, Prins MH. Multicenter randomized clinical trial of supervised exercise therapy with or without feedback versus walking advice for intermittent claudication. Journal of Vascular Surgery 2010;52(2):348‐55. [PUBMED: 20478681] [DOI] [PubMed] [Google Scholar]
- Asselt AD, Nicolai SP, Joore MA, Prins MH, Teijink JA. Cost‐effectiveness of exercise therapy in patients with intermittent claudication: supervised exercise therapy versus a 'go home and walk' advice. European Journal of Vascular and Endovascular Surgery 2011;41(1):97‐103. [PUBMED: 21159527] [DOI] [PubMed] [Google Scholar]
Parr 2009 {published data only}
- Parr BM, Noakes TD, Derman EW. Peripheral arterial disease and intermittent claudication: efficacy of short‐term upper body strength training, dynamic exercise training, and advice to exercise at home. South African Medical Journal = Suid‐Afrikaanse tydskrif vir geneeskunde 2009;99(11):800‐4. [PUBMED: 20218480] [PubMed] [Google Scholar]
Patterson 1997 {published data only (unpublished sought but not used)}
- Patterson RB, Pinto B, Marcus B, Colucci A, Braun T, Roberts M. Value of a supervised exercise program for the therapy of arterial claudication. Journal of Vascular Surgery 1997;25(2):312‐8; discussion 318‐9. [PUBMED: 9052565] [DOI] [PubMed] [Google Scholar]
- Pinto BM, Marcus BH, Patterson RB, Roberts M, Colucci A, Braun C. On‐site versus home exercise programs: psychological benefits for individuals with arterial claudication. Journal of Aging and Physical Activity 1997;5:311‐28. [Google Scholar]
Regensteiner 1997 {published data only (unpublished sought but not used)}
- Regensteiner JG, Meyer TJ, Krupski WC, Cranford LS, Hiatt WR. Hospital vs home‐based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology 1997;48(4):291‐300. [PUBMED: 9112877] [DOI] [PubMed] [Google Scholar]
Sandercock 2007 {published data only}
- Sandercock GR, Hodges LD, Das SK, Brodie DA. The impact of short term supervised and home‐based walking programmes on heart rate variability in patients with peripheral arterial disease. Journal of Sports Science & Medicine 2007;6(4):471‐6. [PUBMED: 24149480] [PMC free article] [PubMed] [Google Scholar]
Sanderson 2006 {published and unpublished data}
- Sanderson B, Askew C, Stewart I, Walker P, Gibbs H, Green S. Short‐term effects of cycle and treadmill training on exercise tolerance in peripheral arterial disease. Journal of Vascular Surgery 2006;44(1):119‐27. [PUBMED: 16828435] [DOI] [PubMed] [Google Scholar]
Savage 2001 {published data only}
- Savage P, Ricci MA, Lynn M, Gardner A, Knight S, Brochu M, et al. Effects of home versus supervised exercise for patients with intermittent claudication. Journal of Cardiopulmonary Rehabilitation 2001;21(3):152‐7. [PUBMED: 11409225] [DOI] [PubMed] [Google Scholar]
Stewart 2008 {published data only}
- Stewart AH, Smith FC, Baird RN, Lamont PM. Local versus systemic mechanisms underlying supervised exercise training for intermittent claudication. Vascular and Endovascular Surgery 2008;42(4):314‐20. [PUBMED: 18319355] [DOI] [PubMed] [Google Scholar]
Treat‐Jacobson 2009 {published and unpublished data}
- Bronas UG, Treat‐Jacobson D, Leon AS. Comparison of the effect of upper body‐ergometry aerobic training vs treadmill training on central cardiorespiratory improvement and walking distance in patients with claudication. Journal of Vascular Surgery 2011;53(6):1557‐64. [PUBMED: 21515017] [DOI] [PubMed] [Google Scholar]
- Treat‐Jacobson D, Bronas UG, Leon AS. Efficacy of arm‐ergometry versus treadmill exercise training to improve walking distance in patients with claudication. Vascular Medicine (London, England) 2009;14(3):203‐13. [PUBMED: 19651669] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arosio 1999 {published data only}
- Arosio E, Cuzzolin L, Marchi S, Minuz P, Degan M, Crivellente F, et al. Increased endogenous nitric oxide production induced by physical exercise in peripheral arterial occlusive disease patients. Life Sciences 1999;65(26):2815‐22. [PUBMED: 10622270] [DOI] [PubMed] [Google Scholar]
Arosio 2001 {published data only}
- Arosio E, Minuz P, Prior M, Zuliani V, Gaino S, Marchi S, et al. Vascular adhesion molecule‐1 and markers of platelet function before and after a treatment with iloprost or a supervised physical exercise program in patients with peripheral arterial disease. Life Sciences 2001;69(4):421‐33. [PUBMED: 11459433] [DOI] [PubMed] [Google Scholar]
Castro‐Sanchez 2013 {published data only}
- Castro‐Sanchez AM, Mataran‐Penarrocha GA, Feriche‐Fernandez‐Castanys B, Fernandez‐Sola C, Sanchez‐Labraca N, Moreno‐Lorenzo C. A program of 3 physical therapy modalities improves peripheral arterial disease in diabetes type 2 patients: a randomized controlled trial. Journal of Cardiovascular Nursing 2013;28(1):74‐82. [PUBMED: 22222177] [DOI] [PubMed] [Google Scholar]
Ciuffetti 1994 {published data only}
- Ciuffetti G, Paltriccia R, Lombardini R, Lupattelli G, Pasqualini L, Mannarino E. Treating peripheral arterial occlusive disease: pentoxifylline vs exercise. International Angiology 1994;13(1):33‐9. [PUBMED: 7915754] [PubMed] [Google Scholar]
Collins 2007 {published data only}
- Collins TC, Johnson SL, Souchek J. Unsupervised walking therapy and atherosclerotic risk‐factor management for patients with peripheral arterial disease: a pilot trial. Annals of Behavioral Medicine 2007;33(3):318‐24. [PUBMED: 17600459] [DOI] [PubMed] [Google Scholar]
Collins 2012 {published data only}
- Collins EG, McBurney C, Butler J, Jelinek C, O'Connell S, Fritschi C, et al. The effects of walking or walking‐with‐poles training on tissue oxygenation in patients with peripheral arterial disease. International Journal of Vascular Medicine 2012;2012:985025. [PUBMED: 23050152] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins EG, O'Connell S, McBurney C, Jelinek C, Butler J, Reda D, et al. Comparison of walking with poles and traditional walking for peripheral arterial disease rehabilitation. Journal of Cardiopulmonary Rehabilitation and Prevention 2012;32(4):210‐8. [PUBMED: 22595894] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2008 {published data only}
- Crowther RG, Leicht AS, Spinks WL, Sangla K, Quigley F, Golledge J. Effects of a 6‐month exercise program pilot study on walking economy, peak physiological characteristics, and walking performance in patients with peripheral arterial disease. Vascular Health and Risk Management 2012;8:225‐32. [PUBMED: 22566743] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RG, Spinks WL, Leicht AS, Sangla K, Quigley F, Golledge J. Effects of a long‐term exercise program on lower limb mobility, physiological responses, walking performance, and physical activity levels in patients with peripheral arterial disease. Journal of Vascular Surgery 2008;47(2):303‐9. [PUBMED: 18241753] [DOI] [PubMed] [Google Scholar]
- Leicht AS, Crowther RG, Golledge J. Influence of peripheral arterial disease and supervised walking on heart rate variability. Journal of Vascular Surgery 2011;54(5):1352‐9. [PUBMED: 21784603] [DOI] [PubMed] [Google Scholar]
Cucato 2013 {published data only}
- Cucato GG, Chehuen Mda R, Costa LA, Ritti‐Dias RM, Wolosker N, Saxton JM, et al. Exercise prescription using the heart of claudication pain onset in patients with intermittent claudication. Clinics (Sao Paulo, Brazil) 2013;68(7):974‐8. [PUBMED: 23917662] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dahllof 1976 {published data only}
- Dahllof AG, Holm J, Schersten T, Sivertsson R. Peripheral arterial insufficiency, effect of physical training on walking tolerance, calf blood flow, and blood flow resistance. Scandinavian Journal of Rehabilitation Medicine 1976;8(1):UNKNOWN. [PUBMED: 935836] [PubMed] [Google Scholar]
Degischer 2002 {published data only}
- Degischer S, Labs KH, Hochstrasser J, Aschwanden M, Tschoepl M, Jaeger KA. Physical training for intermittent claudication: a comparison of structured rehabilitation versus home‐based training. Vascular Medicine (London, England) 2002;7(2):109‐15. [PUBMED: 12402991] [DOI] [PubMed] [Google Scholar]
Delaney 2014 {published data only}
- Delaney CL, Miller MD, Chataway TK, Spark JI. A randomised controlled trial of supervised exercise regimens and their impact on walking performance, skeletal muscle mass and calpain activity in patients with intermittent claudication. European Journal of Vascular and Endovascular Surgery 2014;47(3):304‐10. [PUBMED: 24445084] [DOI] [PubMed] [Google Scholar]
Fakhry 2011 {published data only}
- Fakhry F, Spronk S, Ridder M, Hoed PT, Hunink MG. Long‐term effects of structured home‐based exercise program on functional capacity and quality of life in patients with intermittent claudication. Archives of Physical Medicine and Rehabilitation 2011;92(7):1066‐73. [PUBMED: 21704786] [DOI] [PubMed] [Google Scholar]
Fowler 2002 {published data only}
- Fowler B, Jamrozik K, Norman P, Allen Y, Wilkinson E. Improving maximum walking distance in early peripheral arterial disease: randomised controlled trial. Australian Journal of Physiotherapy 2002;48(4):269‐75. [PUBMED: 12443521] [DOI] [PubMed] [Google Scholar]
Gardner 2001 {published data only}
- Gardner AW, Katzel LI, Sorkin JD, Bradham DD, Hochberg MC, Flinn WR, et al. Exercise rehabilitation improves functional outcomes and peripheral circulation in patients with intermittent claudication: a randomized controlled trial. Journal of the American Geriatrics Society 2001;49(6):755‐62. [PUBMED: 11454114] [DOI] [PubMed] [Google Scholar]
- Gardner AW, Katzel LI, Sorkin JD, Goldberg AP. Effects of long‐term exercise rehabilitation on claudication distances in patients with peripheral arterial disease: a randomized controlled trial. Journal of Cardiopulmonary Rehabilitation 2002;22(3):192‐8. [PUBMED: 12042688] [DOI] [PubMed] [Google Scholar]
Gardner 2005 {published data only}
- Gardner AW, Montgomery PS, Flinn WR, Katzel LI. The effect of exercise intensity on the response to exercise rehabilitation in patients with intermittent claudication. Journal of Vascular Surgery 2005;42(4):702‐9. [PUBMED: 16242558] [DOI] [PubMed] [Google Scholar]
Gelin 2001 {published data only}
- Gelin J, Jivegard L, Taft C, Karlsson J, Sullivan M, Dahllof AG, et al. Treatment efficacy of intermittent claudication by surgical intervention, supervised physical exercise training compared to no treatment in unselected randomised patients I: one year results of functional and physiological improvements. European Journal of Vascular and Endovascular Surgery 2001;22(2):107‐13. [PUBMED: 11472042] [DOI] [PubMed] [Google Scholar]
- Taft C, Karlsson J, Gelin J, Jivegard L, Sandstrom R, Arfvidsson B, et al. Treatment efficacy of intermittent claudication by invasive therapy, supervised physical exercise training compared to no treatment in unselected randomised patients II: one‐year results of health‐related quality of life. European Journal of Vascular and Endovascular Surgery 2001;22(2):114‐23. [PUBMED: 11472043] [DOI] [PubMed] [Google Scholar]
- Taft C, Sullivan M, Lundholm K, Karlsson J, Gelin J, Jivegard L. Predictors of treatment outcome in intermittent claudication. European Journal of Vascular and Endovascular Surgery 2004;27(1):24‐32. [PUBMED: 14652833] [DOI] [PubMed] [Google Scholar]
Gibellini 2000 {published data only}
- Gibellini R, Fanello M, Bardile AF, Salerno M, Aloi T. Exercise training in intermittent claudication. International Angiology 2000;19(1):8‐13. [PUBMED: 10853679] [PubMed] [Google Scholar]
Greenhalgh 2008 {published data only}
- Greenhalgh RM, Belch JJ, Brown LC, Gaines PA, Gao L, Reise JA, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial disease. European Journal of Vascular and Endovascular Surgery 2008;36(6):680‐8. [PUBMED: 19022184] [DOI] [PubMed] [Google Scholar]
Hiatt 1990 {published data only}
- Hiatt WR, Regensteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation 1990;81(2):602‐9. [PUBMED: 2404633] [DOI] [PubMed] [Google Scholar]
Hiatt 1994 {published data only}
- Hiatt WR, Wolfel EE, Meier RH, Regensteiner JG. Superiority of treadmill walking exercise versus strength training for patients with peripheral arterial disease. Implications for the mechanism of the training response. Circulation 1994;90(4):1866‐74. [PUBMED: 7923674] [DOI] [PubMed] [Google Scholar]
Hobbs 2007 {published data only}
- Hobbs SD, Marshall T, Fegan C, Adam DJ, Bradbury AW. The effect of supervised exercise and cilostazol on coagulation and fibrinolysis in intermittent claudication: a randomized controlled trial. Journal of Vascular Surgery 2007;45(1):65‐70; discussion 70. [PUBMED: 17210383] [DOI] [PubMed] [Google Scholar]
Jakubseviciene 2014 {published data only}
- Jakubseviciene E, Vasiliauskas D, Velicka L, Kubilius R, Milinaviciene E, Vencloviene J. Effectiveness of a new exercise program after lower limb arterial blood flow surgery in patients with peripheral arterial disease: a randomized clinical trial. International Journal of Environmental Research and Public Health 2014;11(8):7961‐76. [PUBMED: 25105547] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jansen 1991 {published data only}
- Jansen T, Weiss T, Amendt K, Hsu E, Hubsch‐Muller C, Diehm C. Effect of a 2‐year ambulatory vascular sports program on walking distance in claudication patients ‐ a controlled study [Einfluss eines ambulanten zweijahrigen Gefasssportprogrammes auf die Gehstreckenentwicklung bei Claudicatio‐Patienten‐‐eine kontrollierte Studie]. VASA. Supplementum 1991;33:175. [PUBMED: 1788664] [PubMed] [Google Scholar]
Krause 1974 {published data only}
- Krause D, Dittmar K. Results of physical therapy of peripheral arterial circulatory disorders (author's transl) [Ergebnisse bei der physikalischen Therapie peripherer arterieller Durchblutungsstorungen]. Munchener Medizinische Wochenschrift (1950) 1974;116(8):385‐8. [PUBMED: 4208097] [PubMed] [Google Scholar]
Kruidenier 2011 {published data only}
- Kruidenier LM, Nicolai SP, Rouwet EV, Peters RJ, Prins MH, Teijink JA. Additional supervised exercise therapy after a percutaneous vascular intervention for peripheral arterial disease: a randomized clinical trial. Journal of Vascular and Interventional Radiology 2011;22(7):961‐8. [PUBMED: 21571547] [DOI] [PubMed] [Google Scholar]
Langbein 2002 {published data only}
- Collins EG, Langbein WE, Orebaugh C, Bammert C, Hanson K, Reda D, et al. Cardiovascular training effect associated with polestriding exercise in patients with peripheral arterial disease. Journal of Cardiovascular Nursing 2005;20(3):177‐85. [PUBMED: 15870588] [DOI] [PubMed] [Google Scholar]
- Collins EG, Langbein WE, Orebaugh C, Bammert C, Hanson K, Reda D, et al. PoleStriding exercise and vitamin E for management of peripheral vascular disease. Medicine and Science in Sports and Exercise 2003;35(3):384‐93. [PUBMED: 12618567] [DOI] [PubMed] [Google Scholar]
- Langbein WE, Collins EG, Orebaugh C, Maloney C, Williams KJ, Littooy FN, et al. Increasing exercise tolerance of persons limited by claudication pain using polestriding. Journal of Vascular Surgery 2002;35(5):887‐93. [PUBMED: 12021703] [DOI] [PubMed] [Google Scholar]
Larsen 1966 {published data only}
- Larsen OA, Lassen NA. Effect of daily muscular exercise in patients with intermittent claudication. Lancet (London, England) 1966;2(7473):1093‐6. [PUBMED: 4162526] [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee HL, Mehta T, Ray B, Heng MS, McCollum PT, Chetter IC. A non‐randomised controlled trial of the clinical and cost effectiveness of a supervised exercise programme for claudication. European Journal of Vascular and Endovascular Surgery 2007;33(2):202‐7. [PUBMED: 17142065] [DOI] [PubMed] [Google Scholar]
Leon 2005 {published data only}
- Leon AS. Does long‐term aerobic exercise slow progression of atherosclerosis?. Clinical Journal of Sport Medicine 2005;15(4):285‐6. [PUBMED: 16003047] [DOI] [PubMed] [Google Scholar]
Lepantalo 1991 {published data only}
- Lepantalo M, Kalajo K, Lilius G. Does drug treatment have a placebo effect on programmed training in intermittent claudication? A randomized clinical trial. Clinical Rehabilitation 1991;5(1):65‐9. [Google Scholar]
Manfredini 2008 {published data only}
- Manfredini F, Malagoni AM, Mascoli F, Mandini S, Taddia MC, Basaglia N, et al. Training rather than walking: the test in ‐train out program for home‐based rehabilitation in peripheral arteriopathy. Circulation Journal 2008;72(6):946‐52. [PUBMED: 18503221] [DOI] [PubMed] [Google Scholar]
McDermott 2004 {published data only}
- McDermott MM, Tiukinhoy S, Greenland P, Liu K, Pearce WH, Guralnik JM, et al. A pilot exercise intervention to improve lower extremity functioning in peripheral arterial disease unaccompanied by intermittent claudication. Journal of Cardiopulmonary Rehabilitation 2004;24(3):187‐96. [PUBMED: 15235301] [DOI] [PubMed] [Google Scholar]
McDermott 2009 {published data only}
- McDermott MM, Ades P, Guralnik JM, Dyer A, Ferrucci L, Liu K, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA 2009;301(2):165‐74. [PUBMED: 19141764] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDermott 2013 {published data only}
- McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Liu K, Spring B, et al. Unsupervised exercise and mobility loss in peripheral artery disease: a randomized controlled trial. Journal of the American Heart Association 2015;4(5):e001659. [PUBMED: 25994445] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Guralnik JM, Criqui MH, Ferrucci L, Zhao L, Liu K, et al. Home‐based walking exercise in peripheral artery disease: 12‐month follow‐up of the GOALS randomized trial. Journal of the American Heart Association 2014;3(3):e000711. [PUBMED: 24850615] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Liu K, Guralnik JM, Criqui MH, Spring B, Tian L, et al. Home‐based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA 2013;310(1):57‐65. [PUBMED: 23821089] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Spring B, Domanchuk K, Tao H, Tian L, Zhao L, et al. A group‐mediated, home‐based physical activity intervention for patients with peripheral artery disease: effects on social and psychological function. Journal of Translational Medicine 2014;12:29. [PUBMED: 24467875] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mika 2005 {published data only}
- Mika P, Spodaryk K, Cencora A, Mika A. Red blood cell deformability in patients with claudication after pain‐free treadmill training. Clinical Journal of Sport Medicine 2006;16(4):335‐40. [PUBMED: 16858218] [DOI] [PubMed] [Google Scholar]
- Mika P, Spodaryk K, Cencora A, Unnithan VB, Mika A. Experimental model of pain‐free treadmill training in patients with claudication. American Journal of Physical Medicine & Rehabilitation 2005;84(10):756‐62. [PUBMED: 16205431] [DOI] [PubMed] [Google Scholar]
Mika 2011 {published data only}
- Mika P, Wilk B, Mika A, Marchewka A, Nizankowski R. The effect of pain‐free treadmill training on fibrinogen, haematocrit, and lipid profile in patients with claudication. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(5):754‐60. [PUBMED: 21450630] [DOI] [PubMed] [Google Scholar]
Mika 2013 {published data only}
- Mika P, Konik A, Januszek R, Petriczek T, Mika A, Nowobilski R, et al. Comparison of two treadmill training programs on walking ability and endothelial function in intermittent claudication. International Journal of Cardiology 2013;168(2):838‐42. [PUBMED: 23117015] [DOI] [PubMed] [Google Scholar]
Murphy 2012 {published data only}
- Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER 3rd, Cohen DJ, Reynolds MR, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. Journal of the American College of Cardiology 2015;65(10):999‐1009. [PUBMED: 25766947] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TP, Cutlip DE, Regensteiner JG, Mohler ER, Cohen DJ, Reynolds MR, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six‐month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation 2012;125(1):130‐9. [PUBMED: 22090168] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 1975 {published data only}
- Nielsen SL, Gyntelberg F, Larsen B, Lassen NA. Hospital versus home training, a clinical trial. Aktuelle Probleme in der Angiologie 1975;30:121‐6. [Google Scholar]
Nielsen 1977 {published data only}
- Nielsen SL, Larsen B, Prahl M, Jensen CT, Jensen BE, Wenkens V. [Hospital training compared with home training in patients with intermittent claudication] [Hospitalstraening contra hjemmetraening af patienter med claudicatio intermittens]. Ugeskrift for laeger 1977;139(46):2733‐6. [PUBMED: 595156] [PubMed] [Google Scholar]
Parmenter 2013 {published data only}
- Parmenter BJ, Raymond J, Dinnen P, Lusby RJ, Fiatarone Singh MA. High‐intensity progressive resistance training improves flat‐ground walking in older adults with symptomatic peripheral arterial disease. Journal of the American Geriatrics Society 2013;61(11):1964‐70. [PUBMED: 24219197] [DOI] [PubMed] [Google Scholar]
Pilz 2014 {published data only}
- Pilz M, Kandioler‐Honetz E, Wenkstetten‐Holub A, Doerrscheidt W, Mueller R, Kurz RW. Evaluation of 6‐ and 12‐month supervised exercise training on strength and endurance parameters in patients with peripheral arterial disease. Wiener klinische Wochenschrift 2014;126(11‐12):383‐9. [PUBMED: 24825596] [DOI] [PubMed] [Google Scholar]
Ritti‐Dias 2010 {published data only}
- Grizzo Cucato G, Moraes Forjaz CL, Kanegusuku H, Rocha Chehuen M, Riani Costa LA, Wolosker N, et al. Effects of walking and strength training on resting and exercise cardiovascular responses in patients with intermittent claudication. VASA. Zeitschrift fur Gefasskrankheiten 2011;40(5):390‐7. [PUBMED: 21948782] [DOI] [PubMed] [Google Scholar]
- Meneses AL, Lima GH, Forjaz CL, Lima AH, Silva GQ, Cucato GG, et al. Impact of a supervised strength training or walking training over a subsequent unsupervised therapy period on walking capacity in patients with claudication. Journal of Vascular Nursing 2011;29(2):81‐6. [PUBMED: 21558030] [DOI] [PubMed] [Google Scholar]
- Ritti‐Dias RM, Wolosker N, Moraes Forjaz CL, Carvalho CR, Cucato GG, Leao PP, et al. Strength training increases walking tolerance in intermittent claudication patients: randomized trial. Journal of Vascular Surgery 2010;51(1):89‐95. [PUBMED: 19837534] [DOI] [PubMed] [Google Scholar]
Schlager 2011 {published data only}
- Schlager O, Giurgea A, Schuhfried O, Seidinger D, Hammer A, Groger M, et al. Exercise training increases endothelial progenitor cells and decreases asymmetric dimethylarginine in peripheral arterial disease: a randomized controlled trial. Atherosclerosis 2011;217(1):240‐8. [PUBMED: 21481871] [DOI] [PubMed] [Google Scholar]
- Schlager O, Hammer A, Giurgea A, Schuhfried O, Fialka‐Moser V, Gschwandtner M, et al. Impact of exercise training on inflammation and platelet activation in patients with intermittent claudication. Swiss Medical Weekly 2012;142:w13623. [PUBMED: 22893497] [DOI] [PubMed] [Google Scholar]
Spafford 2014 {published data only}
- Spafford C, Oakley C, Beard JD. Randomized clinical trial comparing Nordic pole walking and a standard home exercise programme in patients with intermittent claudication. British Journal of Surgery 2014;101(7):760‐7. [PUBMED: 24760745] [DOI] [PubMed] [Google Scholar]
Spronk 2009 {published data only}
- Spronk S, Bosch JL, Hoed PT, Veen HF, Pattynama PM, Hunink MG. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospital‐based exercise training ‐ randomized controlled trial. Radiology 2009;250(2):586‐95. [PUBMED: 19188327] [DOI] [PubMed] [Google Scholar]
Tebbutt 2011 {published data only}
- Tebbutt N, Robinson L, Todhunter J, Jonker L. A plantar flexion device exercise programme for patients with peripheral arterial disease: a randomised prospective feasibility study. Physiotherapy 2011;97(3):244‐9. [PUBMED: 21820543] [DOI] [PubMed] [Google Scholar]
Tew 2009 {published data only}
- Tew G, Nawaz S, Zwierska I, Saxton JM. Limb‐specific and cross‐transfer effects of arm‐crank exercise training in patients with symptomatic peripheral arterial disease. Clinical Science 2009;117(12):405‐13. [PUBMED: 19388883] [DOI] [PubMed] [Google Scholar]
Tew 2015 {published data only}
- Tew GA, Humphreys L, Crank H, Hewitt C, Nawaz S, Al‐Jundi W, et al. The development and pilot randomised controlled trial of a group education programme for promoting walking in people with intermittent claudication. Vascular Medicine (London, England) 2015;20(4):348‐57. [PUBMED: 25858012] [DOI] [PubMed] [Google Scholar]
Tisi 1997 {published data only}
- Tisi PV, Hulse M, Chulakadabba A, Gosling P, Shearman CP. Exercise training for intermittent claudication: does it adversely affect biochemical markers of the exercise‐induced inflammatory response?. European Journal of Vascular and Endovascular Surgery 1997;14(5):344‐50. [PUBMED: 9413374] [DOI] [PubMed] [Google Scholar]
Tsai 2002 {published data only}
- Tsai JC, Chan P, Wang CH, Jeng C, Hsieh MH, Kao PF, et al. The effects of exercise training on walking function and perception of health status in elderly patients with peripheral arterial occlusive disease. Journal of Internal Medicine 2002;252(5):448‐55. [PUBMED: 12528763] [DOI] [PubMed] [Google Scholar]
Ventura 1984 {published data only}
- Ventura MR, Young DE, Feldman MJ, Pastore P, Pikula S, Yates MA. Effectiveness of health promotion interventions. Nursing Research 1984;33(3):162‐7. [PUBMED: 6563534] [PubMed] [Google Scholar]
Walker 2000 {published data only}
- Nawaz S, Walker RD, Wilkinson CH, Saxton JM, Pockley AG, Wood RF. The inflammatory response to upper and lower limb exercise and the effects of exercise training in patients with claudication. Journal of Vascular Surgery 2001;33(2):392‐9. [PUBMED: 11174795] [DOI] [PubMed] [Google Scholar]
- Walker RD, Nawaz S, Wilkinson CH, Saxton JM, Pockley AG, Wood RF. Influence of upper‐ and lower‐limb exercise training on cardiovascular function and walking distances in patients with intermittent claudication. Journal of Vascular Surgery 2000;31(4):662‐9. [PUBMED: 10753273] [DOI] [PubMed] [Google Scholar]
Wood 2006 {published data only}
- Wood RE, Sanderson BE, Askew CD, Walker PJ, Green S, Stewart IB. Effect of training on the response of plasma vascular endothelial growth factor to exercise in patients with peripheral arterial disease. Clinical Science (London, England : 1979) 2006;111(6):401‐9. [PUBMED: 16928196] [DOI] [PubMed] [Google Scholar]
Zwierska 2005 {published data only}
- Saxton JM, Zwierska I, Blagojevic M, Choksy SA, Nawaz S, Pockley AG. Upper‐ versus lower‐limb aerobic exercise training on health‐related quality of life in patients with symptomatic peripheral arterial disease. Journal of Vascular Surgery 2011;53(5):1265‐73. [PUBMED: 21215558] [DOI] [PubMed] [Google Scholar]
- Zwierska I, Walker RD, Choksy SA, Male JS, Pockley AG, Saxton JM. Upper‐ vs lower‐limb aerobic exercise rehabilitation in patients with symptomatic peripheral arterial disease: a randomized controlled trial. Journal of Vascular Surgery 2005;42(6):1122‐30. [PUBMED: 16376202] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12616000243415 {published data only}
- ACTRN12616000243415. Comparison of the effects of supervised treadmill walking training and supervised walking with poles on functional capabilities in patients with intermittent claudication. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12616000243415 (first received February 22, 2016).
NCT02075502 {published data only}
- NCT02075502. Community walking exercise for patients with peripheral artery disease (GAIT). clinicaltrials.gov/ct2/show/NCT02075502 (first received February 13, 2014).
NCT02341716 {published data only}
- NCT02341716. Hospital‐ and home‐based Supervised exercise versus UNsupervised walk advice For patients with InTermittent claudication (SUNFIT). clinicaltrials.gov/ct2/show/NCT02341716 (first received January 12, 2015).
NCT02729090 {published data only}
- NCT02729090. Effects of exercise in the functional capacity, central artery and rigidity ankle brachial index. clinicaltrials.gov/ct2/show/NCT02729090 (first received February 18, 2016).
NCT02879019 {published data only}
- NCT02879019. Walking training in peripheral artery disease (GrEnADa Sub‐study) (GrEnADa). clinicaltrials.gov/ct2/show/NCT02879019 (first received August 11, 2016).
Additional references
Aboyans 2017
- Authors/Task Force Members, Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). European Journal of Vascular and Endovascular Surgery 2017; Vol. Aug 26. pii: S1078‐5884(17)30454‐9. [DOI: 10.1016/j.ejvs.2017.07.018; PUBMED: 28851596] [DOI] [PubMed]
Al‐Jundi 2013
- Al‐Jundi W, Madbak K, Beard JD, Nawaz S, Tew GA. Systematic review of home‐based exercise programmes for individuals with intermittent claudication. European Journal of Vascular and Endovascular Surgery 2013;46(6):690‐706. [PUBMED: 24076079] [DOI] [PubMed] [Google Scholar]
Altman 1991
- Altman DG. Clinical trials. Practical Statistics for Medical Research. London: Chapman & Hall, 1991:440‐76. [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ (Clinical research ed.) 2004;328(7454):1490. [PUBMED: 15205295] [DOI] [PMC free article] [PubMed] [Google Scholar]
Back 2015
- Back M, Jivegard L, Johansson A, Nordanstig J, Svanberg T, Adania UW, et al. Home‐based supervised exercise versus hospital‐based supervised exercise or unsupervised walk advice as treatment for intermittent claudication: a systematic review. Journal of Rehabilitation Medicine 2015;47(9):801‐8. [PUBMED: 26435098] [DOI] [PubMed] [Google Scholar]
Bartelink 2004
- Bartelink ML, Stoffers HE, Biesheuvel CJ, Hoes AW. Walking exercise in patients with intermittent claudication. Experience in routine clinical practice. British Journal of General Practice 2004;54(500):196‐200. [PUBMED: 15006125] [PMC free article] [PubMed] [Google Scholar]
Beckitt 2012
- Beckitt TA, Day J, Morgan M, Lamont PM. Calf muscle oxygen saturation and the effects of supervised exercise training for intermittent claudication. Journal of Vascular Surgery 2012;56(2):470‐5. [PUBMED: 22503174] [DOI] [PubMed] [Google Scholar]
Bendermacher 2007
- Bendermacher BL, Willigendael EM, Nicolai SP, Kruidenier LM, Welten RJ, Hendriks E, et al. Supervised exercise therapy for intermittent claudication in a community‐based setting is as effective as clinic‐based. Journal of Vascular Surgery 2007;45(6):1192‐6. [PUBMED: 17543684] [DOI] [PubMed] [Google Scholar]
Brass 2007
- Brass EP, Jiao J, Hiatt W. Optimal assessment of baseline treadmill walking performance in claudication clinical trials. Vascular Medicine (London, England) 2007;12(2):97‐103. [PUBMED: 17615797] [DOI] [PubMed] [Google Scholar]
CMS Decision Memo 2017
- Decision Memo for Supervised Exercise Therapy (SET) for Symptomatic Peripheral Artery Disease (PAD) (CAG‐00449N). https://www.cms.gov/medicare‐coverage‐database/details/nca‐decision‐memo.aspx?NCAId=287&NcaName=Supervised+Exercise+Therapy+(SET)+for+Symptomatic+Peripheral+Artery+Disease+(PAD)&ExpandComments=y&CommentPeriod=0&bc=gCAAAAAACAAAAA%3d%3d&%20https://www.cms.gov/medicare‐coverage‐database/shared/handlers/highwire.ashx?url=https://www.cms.gov/medicare‐coverage‐database/details/nca‐proposed‐decision‐memo.aspx@@@NCAId$$$287&session=1cin1p45wtawuf3p0uukf4fn&kq=873007742%20(Accessed%20on%20May%2031,%202017). (accessed January 17, 2018).
Conte 2015
- Conte MS, Pomposelli FB, Clair DG, Geraghty PJ, McKinsey JF, Mills JL, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. Journal of Vascular Surgery 2015;61(3 Suppl):2S‐41S. [PUBMED: 25638515] [DOI] [PubMed] [Google Scholar]
Dumville 2004
- Dumville JC, Lee AJ, Smith FB, Fowkes FG. The health‐related quality of life of people with peripheral arterial disease in the community: the Edinburgh Artery Study. British Journal of General Practice 2004;54(508):826‐31. [PUBMED: 15527608] [PMC free article] [PubMed] [Google Scholar]
Erb 1898
- Erb W. About intermittent walking and nerve disturbances due to vascular disease [Uber das "intermitterende Hinken" und andere neröse Storungen in Folge von Gefasserkrankungen]. Deutsche Zeitschrift für Nervenheilkunde 1898;13:1‐76. [Google Scholar]
Fakhry 2012
- Fakhry F, Luijtgaarden KM, Bax L, Hoed PT, Hunink MG, Rouwet EV, et al. Supervised walking therapy in patients with intermittent claudication. Journal of Vascular Surgery 2012;56(4):1132‐42. [PUBMED: 23026425] [DOI] [PubMed] [Google Scholar]
Fokkenrood 2012
- Fokkenrood HJ, Lauret GJ, Scheltinga MR, Spreeuwenberg C, Bie RA, Teijink JA. Multidisciplinary treatment for peripheral arterial occlusive disease and the role of eHealth and mHealth. Journal of Multidisciplinary Healthcare 2012;5:257‐63. [PUBMED: 23093906] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fokkenrood 2015a
- Fokkenrood HJ, Houten MM, Houterman S, Breek JC, Scheltinga MR, Teijink JA. Agreements and discrepancies between the estimated walking distance, nongraded and graded treadmill testing, and outside walking in patients with intermittent claudication. Annals of Vascular Surgery 2015;29(6):1218‐24. [PUBMED: 26004956] [DOI] [PubMed] [Google Scholar]
Fokkenrood 2015b
- Fokkenrood HJ, Lauret GJ, Verhofstad N, Bendermacher BL, Scheltinga MR, Teijink JA. The effect of supervised exercise therapy on physical activity and ambulatory activities in patients with intermittent claudication. European Journal of Vascular and Endovascular Surgery 2015;49(2):184‐91. [PUBMED: 25496986] [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;21(5‐6):499‐533. [PUBMED: 14366554] [PubMed] [Google Scholar]
Fowkes 2013
- Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet (London, England) 2013;382(9901):1329‐40. [PUBMED: 23915883] [DOI] [PubMed] [Google Scholar]
Gardner 1991
- Gardner AW, Skinner JS, Cantwell BW, Smith LK. Progressive vs single‐stage treadmill tests for evaluation of claudication. Medicine and Science in Sports and Exercise 1991;23(4):402‐8. [PUBMED: 2056896] [PubMed] [Google Scholar]
Gerhard‐Herman 2017
- Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135(12):e726‐79. [PUBMED: 27840333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gommans 2014
- Gommans LN, Saarloos R, Scheltinga MR, Houterman S, Bie RA, Fokkenrood HJ, et al. Editor's choice ‐ The effect of supervision on walking distance in patients with intermittent claudication: a meta‐analysis. European Journal of Vascular and Endovascular Surgery 2014;48(2):169‐84. [PUBMED: 24928167] [DOI] [PubMed] [Google Scholar]
Gommans 2015
- Gommans LN, Fokkenrood HJ, Dalen HC, Scheltinga MR, Teijink JA, Peters RJ. Safety of supervised exercise therapy in patients with intermittent claudication. Journal of Vascular Surgery 2015;61(2):512‐8.e2. [PUBMED: 25441008] [DOI] [PubMed] [Google Scholar]
Gommans 2016
- Gommans LN, Hageman D, Jansen I, Gee R, Lummel RC, Verhofstad N, et al. Minimal correlation between physical exercise capacity and daily activity in patients with intermittent claudication. Journal of Vascular Surgery 2016;63(4):983‐9. [PUBMED: 26806522] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- Version accessed 2017. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.. GRADEproGDT: GRADEpro Guideline Development Tool. Version accessed 2017. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015., Available from www.gradepro.org.
Gustafsson 2001
- Gustafsson T, Kraus WE. Exercise‐induced angiogenesis‐related growth and transcription factors in skeletal muscle, and their modification in muscle pathology. Frontiers in Bioscience 2001;6:D75‐89. [PUBMED: 11145922] [DOI] [PubMed] [Google Scholar]
Hamburg 2011
- Hamburg NM, Balady GJ. Exercise rehabilitation in peripheral artery disease: functional impact and mechanisms of benefits. Circulation 2011;123(1):87‐97. [PUBMED: 21200015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hiatt 2014
- Hiatt WR, Rogers RK, Brass EP. The treadmill is a better functional test than the 6‐minute walk test in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130(1):69‐78. [PUBMED: 24982118] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from http://handbook.cochrane.org.
Horton 2009
- Horton ES. Effects of lifestyle changes to reduce risks of diabetes and associated cardiovascular risks: results from large scale efficacy trials. Obesity 2009;17 Suppl 3:S43‐8. [PUBMED: 19927146] [DOI] [PubMed] [Google Scholar]
Jain 2012
- Jain A, Liu K, Ferrucci L, Criqui MH, Tian L, Guralnik JM, et al. The Walking Impairment Questionnaire stair‐climbing score predicts mortality in men and women with peripheral arterial disease. Journal of Vascular Surgery 2012;55(6):1662‐73.e2. [PUBMED: 22608041] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jain 2013
- Jain A, Liu K, Ferrucci L, Criqui MH, Tian L, Guralnik JM, et al. Declining walking impairment questionnaire scores are associated with subsequent increased mortality in peripheral artery disease. Journal of the American College of Cardiology 2013;61(17):1820‐9. [PUBMED: 23500321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kruidenier 2009
- Kruidenier LM, Nicolai SP, Hendriks EJ, Bollen EC, Prins MH, Teijink JA. Supervised exercise therapy for intermittent claudication in daily practice. Journal of Vascular Surgery 2009;49(2):363‐70. [PUBMED: 19028059] [DOI] [PubMed] [Google Scholar]
Labs 1999
- Labs KH, Nehler MR, Roessner M, Jaeger KA, Hiatt WR. Reliability of treadmill testing in peripheral arterial disease: a comparison of a constant load with a graded load treadmill protocol. Vascular Medicine (London, England) 1999;4(4):239‐46. [PUBMED: 10613628] [DOI] [PubMed] [Google Scholar]
Lane 2014
- Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD000990.pub3] [DOI] [PubMed] [Google Scholar]
Lauret 2012
- Lauret GJ, Gijsbers HJ, Hendriks EJ, Bartelink ML, Bie RA, Teijink JA. The ClaudicatioNet concept: design of a national integrated care network providing active and healthy aging for patients with intermittent claudication. Vascular Health and Risk Management 2012;8:495‐503. [PUBMED: 22942648] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauret 2014
- Lauret GJ, Fakhry F, Fokkenrood HJ, Hunink MG, Teijink JA, Spronk S. Modes of exercise training for intermittent claudication. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009638.pub2] [DOI] [PubMed] [Google Scholar]
Layden 2012
- Layden J, Michaels J, Bermingham S, Higgins B. Diagnosis and management of lower limb peripheral arterial disease: summary of NICE guidance. BMJ (Clinical research ed.) 2012;345:e4947. [PUBMED: 22875949] [DOI] [PubMed] [Google Scholar]
Le Faucheur 2008
- Faucheur A, Abraham P, Jaquinandi V, Bouye P, Saumet JL, Noury‐Desvaux B. Measurement of walking distance and speed in patients with peripheral arterial disease: a novel method using a global positioning system. Circulation 2008;117(7):897‐904. [PUBMED: 18250268] [DOI] [PubMed] [Google Scholar]
Le Faucheur 2015
- Faucheur A, Mullenheim PY, Mahe G. Letter by Le Faucheur et al regarding articles, "Six‐minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease" and "The treadmill is a better functional test than the 6‐minute walk test in therapeutic trials of patients with peripheral artery disease". Circulation 2015; Vol. 131, issue 15:e406. [PUBMED: 25869007] [DOI] [PubMed]
Leng 2000
- Leng GC, Fowler B, Ernst E. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000990] [DOI] [PubMed] [Google Scholar]
Li 2015
- Li Y, Li Z, Chang G, Wang M, Wu R, Wang S, et al. Effect of structured home‐based exercise on walking ability in patients with peripheral arterial disease: a meta‐analysis. Annals of Vascular Surgery 2015;29(3):597‐606. [PUBMED: 25449991] [DOI] [PubMed] [Google Scholar]
Makris 2012
- Makris GC, Lattimer CR, Lavida A, Geroulakos G. Availability of supervised exercise programs and the role of structured home‐based exercise in peripheral arterial disease. European Journal of Vascular and Endovascular Surgery 2012;44(6):569‐75; discussion 576. [PUBMED: 23034408] [DOI] [PubMed] [Google Scholar]
McDermott 2001
- McDermott MM, Greenland P, Liu K, Guralnik JM, Criqui MH, Dolan NC, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA 2001;286(13):1599‐606. [PUBMED: 11585483] [DOI] [PubMed] [Google Scholar]
McDermott 2014
- McDermott MM, Guralnik JM, Criqui MH, Liu K, Kibbe MR, Ferrucci L. Six‐minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation 2014;130(1):61‐8. [PUBMED: 24982117] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDermott 2016
- McDermott MM, Guralnik JM, Ferrucci L, Tian L, Kibbe MR, Greenland P, et al. Community walking speed, sedentary or lying down time, and mortality in peripheral artery disease. Vascular Medicine (London, England) 2016;21(2):120‐9. [PUBMED: 26873873] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2001
- Morgan MB, Crayford T, Murrin B, Fraser SC. Developing the Vascular Quality of Life Questionnaire: a new disease‐specific quality of life measure for use in lower limb ischemia. Journal of Vascular Surgery 2001;33(4):679‐87. [PUBMED: 11296317] [DOI] [PubMed] [Google Scholar]
Piepoli 2014
- Piepoli MF, Corra U, Adamopoulos S, Benzer W, Bjarnason‐Wehrens B, Cupples M, et al. Secondary prevention in the clinical management of patients with cardiovascular diseases. Core components, standards and outcome measures for referral and delivery: a policy statement from the cardiac rehabilitation section of the European Association for Cardiovascular Prevention & Rehabilitation. Endorsed by the Committee for Practice Guidelines of the European Society of Cardiology. European Journal of Preventive Cardiology 2014;21(6):664‐81. [PUBMED: 22718797] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rutherford 1997
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. Journal of Vascular Surgery 1997;26(3):517‐38. [PUBMED: 9308598] [DOI] [PubMed] [Google Scholar]
Selvin 2004
- Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999‐2000. Circulation 2004;110(6):738‐43. [PUBMED: 15262830] [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF Secondary Prevention and Risk Reduction Therapy for Patients with Coronary and other Atherosclerotic Vascular Disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation 2011;124(22):2458‐73. [PUBMED: 22052934] [DOI] [PubMed] [Google Scholar]
Spertus 2004
- Spertus J, Jones P, Poler S, Rocha‐Singh K. The peripheral artery questionnaire: a new disease‐specific health status measure for patients with peripheral arterial disease. American Heart Journal 2004;147(2):301‐8. [PUBMED: 14760329] [DOI] [PubMed] [Google Scholar]
Stewart 2002
- Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. New England Journal of Medicine 2002;347(24):1941‐51. [PUBMED: 12477945] [DOI] [PubMed] [Google Scholar]
Treat‐Jacobson 2012
- Treat‐Jacobson D, Lindquist RA, Witt DR, Kirk LN, Schorr EN, Bronas UG, et al. The PADQOL: development and validation of a PAD‐specific quality of life questionnaire. Vascular Medicine (London, England) 2012;17(6):405‐15. [PUBMED: 23184901] [DOI] [PubMed] [Google Scholar]
Vemulapalli 2015
- Vemulapalli S, Dolor RJ, Hasselblad V, Schmit K, Banks A, Heidenfelder B, et al. Supervised vs unsupervised exercise for intermittent claudication: a systematic review and meta‐analysis. American Heart Journal 2015;169(6):924‐37.e3. [PUBMED: 26027632] [DOI] [PubMed] [Google Scholar]
Wind 2007
- Wind J, Koelemay MJ. Exercise therapy and the additional effect of supervision on exercise therapy in patients with intermittent claudication. Systematic review of randomised controlled trials. European Journal of Vascular and Endovascular Surgery 2007;34(1):1‐9. [PUBMED: 17329131] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Bendermacher 2005
- Bendermacher BLW, Willigendael EM, Teijink JAW, Prins MH. Supervised exercise therapy versus non‐supervised exercise therapy for intermittent claudication. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005263] [DOI] [PubMed] [Google Scholar]
Bendermacher 2006
- Bendermacher BL, Willigendael EM, Teijink JA, Prins MH. Supervised exercise therapy versus non‐supervised exercise therapy for intermittent claudication. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005263.pub2] [DOI] [PubMed] [Google Scholar]
Fokkenrood 2013
- Fokkenrood HJ, Bendermacher BL, Lauret GJ, Willigendael EM, Prins MH, Teijink JA. Supervised exercise therapy versus non‐supervised exercise therapy for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD005263.pub3] [DOI] [PubMed] [Google Scholar]


