Abstract
Chronic osteomyelitis with proliferative periostitis is a rare form of osteomyelitis that is characterised by new bone formation with periosteal reaction. It is also traditionally known as Garre’s osteomyelitis. The common sources of infection of the jaw include dental caries associated with periapical periodontitis, periodontitis, fractures and nonodontogenic infections. Chronic osteomyelitis with proliferative periostitis mainly presents in younger patients. Here, we present a case of a 12-year-old patient with chronic osteomyelitis with proliferative periostitis with no definitive infection source such as pericoronitis, caries and periodontitis. Therapeutic measures involved surgical debridement and antibiotics. Disease remission and a normal facial symmetrical morphology were observed at the six-month follow-up.
Keywords: Osteomyelitis, Periostitis, Mandibular, Garre
Introduction
Chronic osteomyelitis with proliferative periostitis is also traditionally known as Garre’s osteomyelitis. Garre’s osteomyelitis of the jaw refers to a particular type of pathological change and clinical manifestation of chronic marginal osteomyelitis, which is also known as proliferative osteomyelitis or low-toxicity sclerosing osteomyelitis. This type of osteomyelitis is more common in younger patients, and the lesions more commonly occur in the mandible. Garre’s osteomyelitis is caused by a low virulence infection and is characterised by chronic non-suppurative osteomyelitis with proliferative osteomyelitis.1 Chronic osteomyelitis with proliferative periostitis is an uncommon disease associated with new bone formation with periosteal reaction.
Sources of infection of the jaw include dental caries associated with periapical periodontitis, periodontitis, fractures and nonodontogenic infections.2 Garre’s osteomyelitis occurs mostly in young patients secondary to odontogenic infections and often involves the unilateral mandibular body.3–5 The premolar and molar regions of the mandible are most often affected.6
The imaging findings in Garre’s osteomyelitis of the jaws are bone hyperplasia and destruction, and some of these findings are similar to the x-ray manifestations of malignant tumours. Garre’s osteomyelitis manifests as periosteal thickening and subperiosteal new bone formation, radiopaque lamination is observed parallel to the surface of the cortical bone. The number of laminations varies from 1 to 12, and radiolucent separation is seen between the newly formed bone and the original cortical bone. Most lesions are localised around the buccal cortex of the teeth from which the periostitis originates. Histological examination showing new bone formation under the periosteal layer is characteristic of the disease. On the surface of the dense bone, the new bone trabeculae are perpendicular to the bone surface and are arranged in parallel and surrounded by osteoblasts.
We present a case of extensive chronic osteomyelitis with proliferative periostitis that resembled osteogenic sarcoma.
Case history
A 12-year-old girl was referred to the Department of Oral and Maxillofacial Surgery, Shandong University Stomatological Hospital (Jinan, China), with a four-month history of swelling of the left mandibular body. An extra-oral examination revealed facial asymmetry (Fig 1). There were no subjective symptoms. The patient had no history of trauma to the left facial area, nor had she been treated with antibiotics. Two cutaneous fistulas had formed, with surrounding skin redness, and increased skin temperature. Microbial cultures were collected, but no bacterial growth was detected by bacterial culture and drug sensitivity test.
Figure 1.
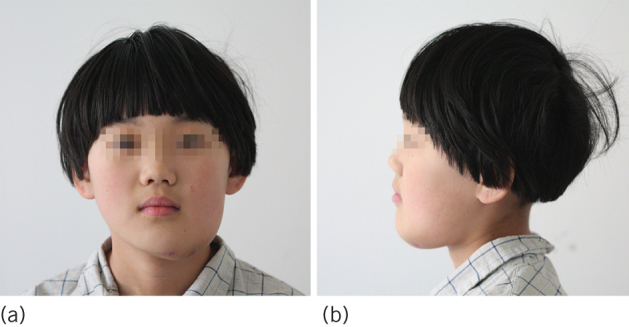
Clinical photographs. (a) Swelling of the left mandibular area from the chin to the mandibular angle. (b) Lateral view.
On intra-oral examination, the patient had full, well-maintained dentition with erupting second molars. The swelling and expansion of vestibular groove and central cusp deformity of the lower second premolar were found, and pulp viability testing of all the lower teeth was normal. There were no dental caries or periodontal lesions, and no intra-oral draining sinus was found.
A panoramic radiograph showed an unerupted third molar on the left mandible and a radiolucent appearance of the mandibular body. A sclerotic appearance was observed over the left mandibular body. This feature was observed in detail with cone-beam computed tomography (CBCT; Figures 2 and 3). Attenuation of bone marrow in the left mandibular body was increased. There were osteolytic and sclerotic bone lesions in the left mandibular body. Periosteal reaction with new bone formation was observed in the medial and lateral portions of the mandibular body. Osteolytic changes were also observed inside the new bone formation.
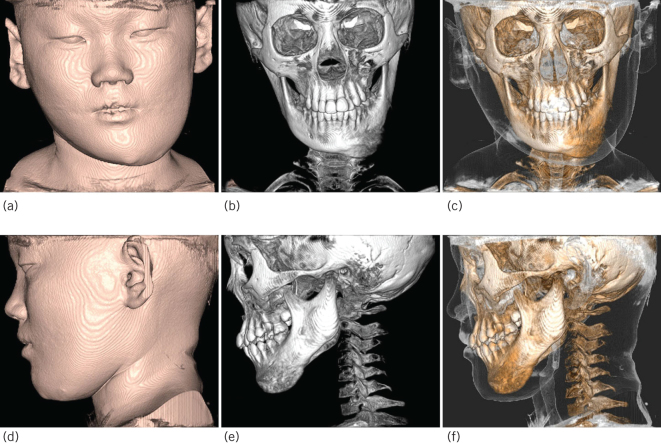
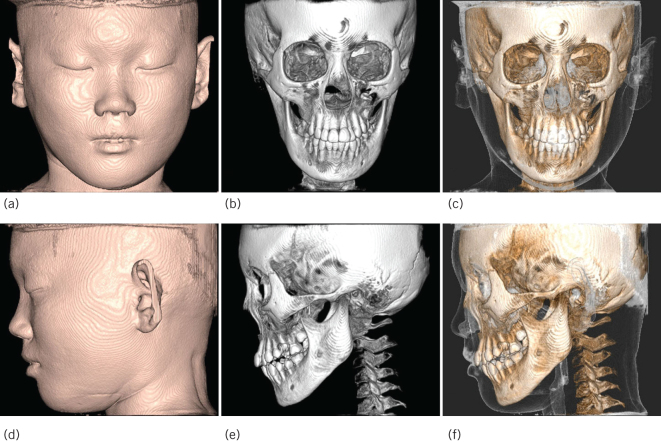
Figure 2.
The lesion of cone-beam computed tomography three-dimensional reconstruction images. Frontal view image: (a) Soft tissue image. (b) Bone image. (c) Bone + Soft tissue image. Lateral view image: (d) Soft tissue image. (e) Bone image. (f) Bone + Soft tissue image.
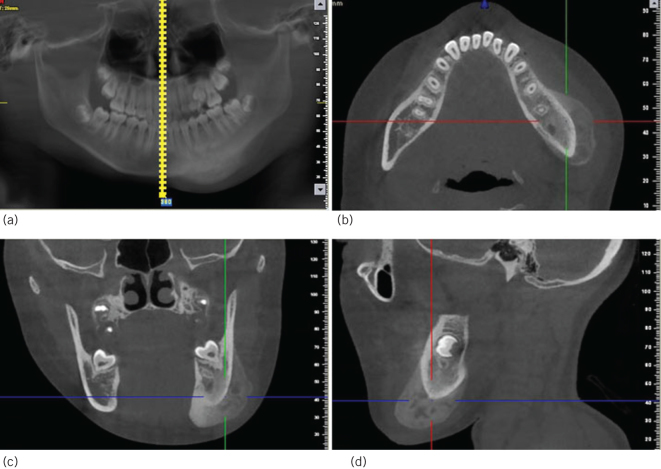
Figure 3.
Cone beam computed tomography images. Osteolytic and sclerotic lesions. (a) Panoramic image. (b) Cross section image. (c) Coronal section image. (d) Sagittal plane image.
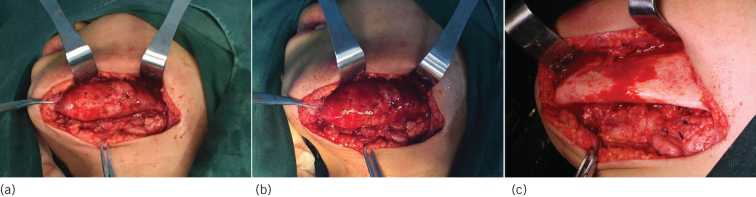
During surgery under general anaesthesia, an incision was made at the lower aspect of the mandible, and the lesion was found located deep within the body of the mandible; from the chin to the mandibular angle, there was diffuse swelling and bulging both buccally and lingually, but the margins of the lesion were clear, and the mandibular ramus and condyle were not invaded (Fig 4A). Insect-like projections were observed in the posterior part of the lesion. A large amount of granulation tissue and purulent discharge were visible, and part of the periosteum contained reactive hyperplasia (Fig 4B). Based on preoperative surgical planning, the new bone formation overlying the cortical bone of the mandibular body was identified, and the swelling was resected with the use of an osteotome and a powered surgical apparatus; decortication was performed until a boundary of normal tissue was reached. Abnormal bone around the mental nerve was also resected, so the mental nerve could be preserved (Fig 4C) and the resected specimens were sent for biopsy.
Figure 4.
Surgical intervention, the swelling: (a) preoperatively; (b) intraoperatively; and (c) postoperatively.
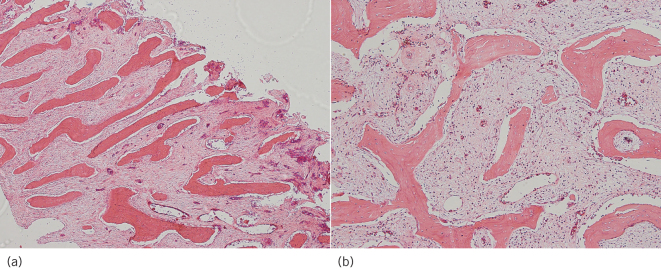
Histological examination showed new bone formation with periosteal reactivity. On the surface of the dense bone, the new bone trabeculae were perpendicular to the bone surface and were arranged in parallel and surrounded by osteoblasts (Fig 5). The intertrabecular bone space was filled with fibrous connective tissue with scattered lymphocytes and plasma cell infiltrates. Based on the pathological findings, the final diagnosis of the mandibular lesion was chronic osteomyelitis with proliferative periostitis.
Figure 5.
Histological examination. (a) Bony trabeculae of the reactive bone arranged perpendicular to the bone surface (haematoxylin and eosin staining, ×40). (b) Dense fibrous stroma with chronic inflammation in the intertrabecular space (haematoxylin and eosin staining, ×100).
Seven days after surgery, the patient did not experience any particular discomfort. The sutures were removed seven days after surgery, and the patient was discharged ten days later. One month after surgery, the left mandibular body showed normal contour and the swelling and infection had resolved. On CBCT imaging performed at a six-month follow-up, the new bone formation and sclerotic changes in the left mandibular body had disappeared; additionally, the patient had recovered normal facial symmetry (Fig 6).
Figure 6.
At six-month follow-up. Radiographical image showing symmetrical face contour. Frontal view image: (a) Soft tissue image. (b) Bone image. (c) Bone + Soft tissue image. Lateral view image: (d) Soft tissue image. (e) Bone image. (f) Bone + Soft tissue image.
Discussion
Most cases of Garre’s osteomyelitis are caused by periodontitis and pericoronitis of the mandibular molars,7 and often involve the unilateral mandible; cases involving the entire mandible are very rare. Eversole et al summarised key characteristics for the differential diagnosis of Garre’s osteomyelitis:8
facial asymmetry caused by local bone swelling
histopathological findings confirming a benign periosteal fibrous lesion
lesion occurring secondary to infection, trauma, or other stimuli
the ability to completely or partially reshape the bone after removing the excess bone.
For this type of lesion, imaging and bone biopsy should be performed to exclude other types of diseases, such as infantile cortical hyperplasia, Ewing’s sarcoma, osteogenic sarcoma, cherubism and histiocytosis. Although this disease has been reported frequently in the literature, the causes of this disease and the reasons for its recurrence are poorly understood. The rate of occurrence of a positive bacterial culture is low, the course of the disease is long and the effect of medical treatment is limited. Therefore, the final treatment is not clear. Surgical removal of diseased teeth, endodontic treatment and systemic antibiotics are widely used for the treatment of this disease.9–10 For limited lesions, good therapeutic effects and a favourable prognosis are usually achieved.
In the case of secondary chronic osteomyelitis with proliferative periostitis, management involves surgical debridement in combination with a course of systemic antibiotics.11 In the present case, the patient underwent surgical debridement and saucerisation in combination with antibiotic therapy. One month after surgery, the left mandibular body showed normal contour, and the swelling and infection had resolved. On CBCT imaging performed at a six-month follow-up, the new bone formation and sclerotic lesion in the left mandibular body had disappeared; in addition, the patient had recovered normal facial symmetry.
Conclusions
This article has the following characteristics: the patient had a lesion of unknown aetiology that was not associated with caries, a chronic periodontal abscess or a history of trauma; these findings suggested that there was an atypical or imperceptible slight infection, making the diagnosis difficult from the beginning. Owing to the wide range of lesions, simple clinical judgment is prone to errors. Auxiliary examinations including CBCT and histopathology confirmed the diagnosis and prevented a misdiagnosis. Treatment involving the complete removal of the lesion, including the surrounding hyperplastic bone and granulation tissue, combined with the use of adequate antibiotics, achieved a better therapeutic effect.
In conclusion, this case demonstrates that the aetiology of chronic osteomyelitis with proliferative periostitis is unknown. In this patient, extensive new bone formation with periosteal reaction was seen from the chin to the mandibular angle. Sclerotic and osteolytic bony changes inside the new bone formation were observed. Based on the clinical examination and radiographic findings, a preliminary diagnosis of chronic osteomyelitis with proliferative periostitis was made. Surgical debridement in combination with the use of systemic antibiotics was effective in treating chronic osteomyelitis with proliferative periostitis.
Acknowledgements
The patient and her parents agreed to her clinical photographs being published in this paper.
References
- 1.Suei Y, Taguchi A, Tanimoto K. Diagnosis and classification of mandibular osteomyelitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2005; (2): 207–214. [DOI] [PubMed] [Google Scholar]
- 2.Neville BW, Damm DD, Allen CM. Oral and Maxillofacial Pathology. 2nd ed Philadelphia: Saunders; 2002. [Google Scholar]
- 3.Nortje CJ, Wood RE, Grotepass F. Periostitis ossificans versus Garre osteomyelitis. Part II: radiologic analysis of 93 cases in the jaws. Oral Surg Oral Med Oral Pathol 1988; (2): 249–260. [DOI] [PubMed] [Google Scholar]
- 4.Spinnato G, Agnihotri N, Ziccardi VB. Garre osteomyelitis in a patient with chromosome 22q11.2 syndrome: a case report. J Oral Maxillofacial Surg 2011; (6): e75–e77. [DOI] [PubMed] [Google Scholar]
- 5.Betts NJ, Abaza NA, Kazemi A. An expansile bony lesion of the posterior mandible in a 12-year-old girl. J Oral Maxillofacial Surg 1996; (2): 203–209. [DOI] [PubMed] [Google Scholar]
- 6.Zand V, Lotfi M, Vosoughhosseini S. Proliferative periostitis: a case report. J Endod 2008; : 481–483. [DOI] [PubMed] [Google Scholar]
- 7.Nakano H, Miki T, Aota K et al. Garre osteomyelitis of the mandible caused by an infected wisdom tooth. Int J Oral Sci 2014; (4): 496–500. [Google Scholar]
- 8.Eversole LR, Leider AS, Corwin J et al. Proliferative periostitis of Garre: its differentiation from other neoperiostoses. J Oral Surg 1979; (10): 725–731. [PubMed] [Google Scholar]
- 9.Van den Bossche LH, Demeulemeester JD, Bossuyt MH. Periodontal infection leading to periostitis ossificans (Garre osteomyelitis) of the mandible. Report of a case. J Periodontol 1993; (1): 60–62. [DOI] [PubMed] [Google Scholar]
- 10.Choung JW. Rotation technique of reduction malar plasty. J Craniofac Surg 2015; (1): 238–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Merkesteyn JP, Groot RH, van de. Akker HP et al. Treatment of chronic suppurative osteomyelitis of the mandible. Int J Oral Maxillofac Surg 1997; : 450–454. [DOI] [PubMed] [Google Scholar]