Abstract
Introduction
The 2015 National Institute for Health and Care Excellence guidelines widened the referral criteria for the two-week-wait pathway for suspected lower gastrointestinal cancer. We implemented a straight-to-test protocol to accommodate the anticipated increase in referrals. We evaluated the impact of these changes for relevant pathway metrics and clinical outcomes using a retrospective cohort study with historic controls.
Materials and methods
We analysed data from all patients referred to a teaching hospital via the two-week-wait pathway for suspected lower gastrointestinal cancer under the previous guidelines between 1 March and 31 August 2015 compared with the same period in 2016, when the updated guidelines and straight-to-test protocol had been implemented.
Results
In the 2015 cohort, there were 64 cancer diagnoses from 664 referrals (9.6% pick-up) compared with 58 cancer diagnoses from 954 referrals in the 2016 cohort (6.1% pick-up). Our straight-to-test protocol reduced the median time to cancer diagnosis by 12.5 days (P < 0.001) and reduced the median time to cancer treatment by 7.5 days (P < 0.05) An increased proportion of non-colorectal cancers were diagnosed in 2016 compared with 2015, (37.9% vs 17.2%, P < 0.05) and more adenomas were removed in 2016 compared with 2015 (377 vs 193).
Discussion and conclusion
Our straight-to-test protocol has resulted in a reduction in times to cancer diagnosis and cancer treatment, despite an increase in the number of referrals. The new referral criteria have considerable resource implications, but their implementation did not result in an increase in the total number of cancers diagnosed.
Keywords: Neoplasms, Gastrointestinal neoplasms, Colonoscopy, Diagnosis, Surgery
Introduction
Cancers in solid organs follow a predictable sequence of precursor development, invasion and metastatic spread.1 Critical to the management of many of these cancers is the detection of lesions at an early stage, rendering them susceptible to locally targeted, curative regimens such as surgery and radiotherapy.2
As part of a national initiative to increase early diagnoses of cancer, the first generation of two-week-wait referral pathways were implemented across the UK in response to a Department of Health initiative in 2000. The two-week-wait pathways were designed to accelerate the investigation of patients presenting to their general practitioner with symptoms and signs suggestive of cancer. Colorectal cancer is the fourth most commonly diagnosed cancer in the UK,3 and previous research has correlated shorter times to diagnosis for colorectal cancers with reduced mortality.4,5 While two-week-wait referral pathways have been shown to reduce the times to specialist consultation and diagnosis in colorectal cancer, they have not been shown to reduce times to first treatment, stage at diagnosis or mortality from this disease.6–8 In 2015, the National Institute for Health and Care Excellence (NICE) published updated guidelines that widened the referral criteria for the two-week-wait pathway for suspected lower gastrointestinal cancer,9 with the aims of increasing early diagnoses of cancer and implementing a symptom-focused approach to referral.10 In addition, the NHS England-appointed Cancer Taskforce has detailed new guidelines aimed at having 95% of all patients referred for cancer investigations having a definitive cancer diagnosis, or having cancer ruled out, within 28 days of referral, by 2020.11 These updated guidelines are likely to increase pressure on secondary care to deliver rapid cancer diagnostics.
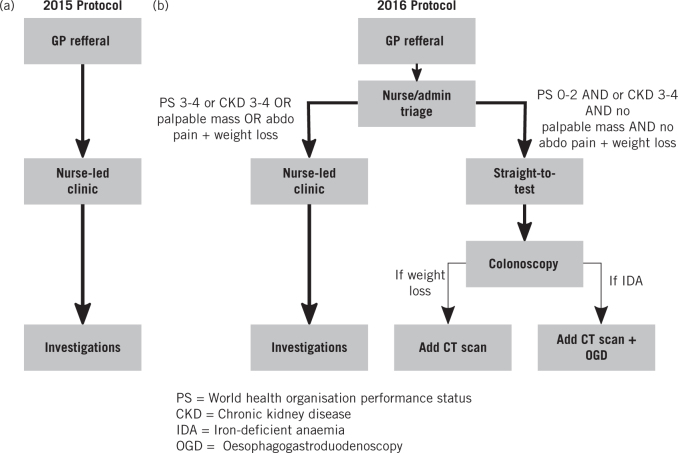
‘Straight-to-test’ protocols have previously been explored by a number of groups as a method of reducing the number of outpatient appointments arising from the two-week-wait pathway for suspected lower gastrointestinal cancer, thus reducing waiting times for diagnosis and treatment.12–15 They require telephone, electronic or paper-based triage of incoming referrals to decide which tests would be most appropriate, and to identify patients for whom prior outpatient assessment in nurse-led clinic is still required. Indeed, work by NHS England has recommended straight-to-test for delivering rapid colorectal cancer diagnoses.11 We implemented a straight-to-test protocol (Fig 1B) for the lower gastrointestinal suspected cancer pathway in a teaching hospital setting, to accommodate the anticipated increase in demand.
Figure 1.
(A) Protocol used in 2015 for the two-week-wait pathway for suspected lower gastrointestinal cancer. (B) The new protocol implemented in 2016 for the two-week-wait pathway for suspected lower gastrointestinal cancer which included a straight-to-test pathway.
We present a service evaluation designed to examine whether the implementation of a straight-to-test protocol, with patients referred under the new guidelines, would lead to a reduced time to cancer diagnosis and cancer treatment when compared with a historic control cohort, where patients were referred under the old guidelines. Furthermore, we aimed to characterise the patient cohorts diagnosed with cancer including the numbers, locations and stages of all cancers diagnosed. We evaluated whether changes in the referral criteria would alter the stage at which cancer is diagnosed and the proportions of non-colorectal cancers diagnosed, owing to the milder, less established symptoms that now meet referral criteria. In addition, we aimed to analyse the referrals in which cancer was excluded to ascertain their overall resource implications. We evaluated these metrics and additional questions using a retrospective cohort study design with historical controls in a teaching hospital setting.
Materials and methods
Straight-to-test pathway
For the 2015 cohort (old guidelines), the two-week-wait pathway for suspected lower gastrointestinal cancer involved assessment of all patients referred in a nurse-led clinic followed by arrangement of appropriate investigations (Fig 1A). For the 2016 cohort (new guidelines), referral forms for all patients were first triaged by the colorectal nursing team. Patients with World Health Organization Performance Status 3 or higher, chronic kidney disease 3–4, palpable masses and/or abdominal pain with weight loss were seen in nurse-led clinic prior to investigations being ordered. All other patients were referred straight to test, with individual tests ordered according to presenting symptoms (Fig 1B).
Data collection
Our study design was a retrospective cohort study with historic controls. In terms of inclusion criteria, we collected data from all patients referred to Addenbrooke’s Hospital via the two-week-wait pathway for suspected lower gastrointestinal cancer under the previous guidelines between 1 March and 31 August 2015 and between 1 March and 31 August 2016, when the updated guidelines and straight-to-test protocol had been implemented. Data were acquired via the hospital electronic record system (Epic) and the colorectal unit database. Measurement bias was minimised as each cohort was analysed on the same electronic database and each analyst was tasked with collecting data from equal proportions of patients from 2015, 2016 (nurse-led clinic) and 2016 (straight-to-test). Data collected included time from referral to histologically confirmed cancer diagnosis and referral to first disease-modifying treatment, total number of patients diagnosed with cancer, tumour location, tumour stage, total number of histologically confirmed adenomas excised at colonoscopy, referral criteria fulfilled, tests performed and appointments cancelled and relevant patient demographics (e.g. age and sex).
In terms of exclusion criteria, incorrectly submitted referrals and data from patients who self-discharged prior to tests or clinical assessment were excluded from the study. The numbers of patients at each stage in the 2015 and 2016 referral pathways are summarised in Supplementary Figure 1.
During both study periods, if a patient chose to refuse any investigation, the risks of self-discharge were explained over the telephone, where possible, before being discharged back to their general practitioner with an invitation for future re-referral.
The adenoma count comprised all adenomas excised in the first colonoscopy/sigmoidoscopy, as well as any that were excised in subsequent investigations performed because of the initial colonoscopy/sigmoidoscopy.
The number of tests performed was ascertained by observing the tests requested and performed following the first nurse-led clinic appointment or following paper-based triage in the straight-to-test protocol. Costings were derived from the National Tariff workbook. Any further investigations requested or performed thereafter were not included in our tests performed dataset.
Data analysis
Data from all patients were collated using Microsoft Excel, then were anonymised and imported into RStudio for statistical analysis and graphical display. Mann-Whitney U-tests were used for comparisons of quantitative data, and chi-squared tests were used for comparisons of categorical data. Differences were considered significant if the P-value was less than 0.05.
Results
Number of referrals and cancer diagnoses
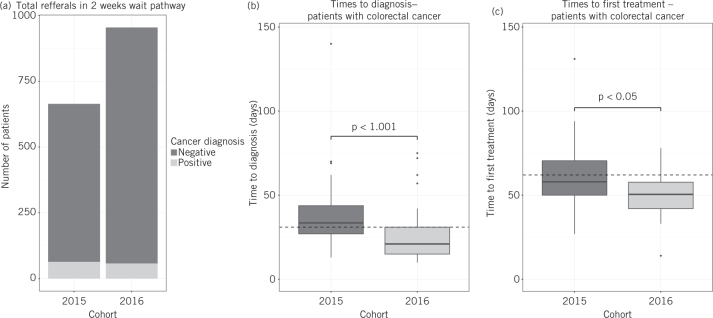
During the study periods, a total of 1618 patients were included (664 in 2015 and 954 in 2016; Fig 2A; Supplementary Fig 1). There were no significant differences in the sex (47% male and 53% female in 2016 and 46% male and 54% female in 2015, chi-squared test) or age (median age 71.2 and 70.6 for 2016, under new guidelines, and 2015, under old guidelines, respectively, Mann-Whitney U-test) of the referred populations. However, the number of cancers diagnosed was comparable: 58 in 2016 and 64 in 2015, representing 6.1% and 9.6% pick-up rates respectively (Fig 2A; Table 1). In 2016, the patients referred to nurse-led clinics were significantly older (median age 69.6 and 75.1 in straight-to-test and nurse-led clinics respectively, P < 0.0001, Mann-Whitney U-test) and had worse performance statuses. This nurse-led clinic cohort in 2016 also had a higher cancer pick-up rate: 9.2% in nurse-led clinics compared with 4.8% in the straight-to-test cohort.
Figure 2.
(A) The total numbers of patients referred on the two-week-wait pathway during the respective time-periods in 2015 and 2016 are displayed. Each cohort is divided into those that received a diagnosis of cancer because of the referral (positive), and those that did not (negative). (B, C) The times to (B) diagnosis and (C) first treatment from the point of receipt of general practitioner referral for patients who were diagnosed with colorectal cancer via the two-week-wait pathway in 2015 relative to 2016 are displayed. Of the patients diagnosed with cancer at any site, 57% of the 2016 cohort (33 patients) were assessed on the straight-to-test pathway and the remaining 43% (25 patients) were assessed on the nurse-led clinic pathway. All referrals in 2015 were assessed by the nurse-led clinic pathway. Statistical comparisons between the 2015 and 2016 cohorts were performed using the Mann-Whitney U-test. The dotted lines represent the respective national targets of 31 days to diagnosis, and 62 days to treatment.
Table 1.
Cancer diagnoses in 2016 and 2015 cohorts. ‘All colorectal cancers’ includes all-stage cancer diagnoses. In 2016 and 2015, the sites of non-colorectal cancers were: anus, ovary, pancreas, haematological and lung. In 2016, the following additional cancers were found: oesophagus, parotid gland, prostate, perianal skin, breast, stomach and metastatic disease of unknown origin. In 2015, a non-colorectal neuroendocrine tumour was also diagnosed. The Dukes’ staging of colorectal cancers was not significantly different in 2016 relative to 2015 (chi-squared test, excluding cases with unknown staging). An increased proportion of cancer diagnoses were non-colorectal in 2016 relative to 2015 (chi-squared test, P < 0.01). Brackets represent percentage of all referrals for that year, except for Dukes’ staging, which represents the percentage of colorectal cancer with full staging information available. The numbers of patients who underwent adenoma resection, as part of the two-week-wait pathway for suspected lower gastrointestinal cancer, classified into low-, intermediate- and high-risk categories as defined by Atkin et al,16 are presented. No significant difference was observed between the proportion of patient risk categories in the two cohorts as assessed by chi-squared test.
| Cohort | ||
| 2015 | 2016 | |
| Total referrals | 664 | 954 |
| Cancer diagnoses | 64 (9.6%) | 58 (6.1%) |
| Colorectal cancer: | ||
| All | 53 (8.0%) | 36 (3.8%) |
| Dukes’ A or B | 25 (47.2%) | 11 (33.3%) |
| Dukes’ C or D | 28 (52.8%) | 22 (66.7%) |
| Unknown stage | 0 | 3 |
| Non-colorectal cancers | 11 (1.7%) | 22 (2.3%) |
| Adenoma: | ||
| Low-risk | 91 (67.9%) | 161 (69.7%) |
| Intermediate risk | 33 (24.6%) | 49 (21.2%) |
| High risk | 10 (7.5%) | 21 (9.1%) |
| Total | 134 | 231 |
The locations of the cancers detected were significantly different between the two cohorts, with proportionately more non-colorectal cancers in 2016 than 2015 (37.9% vs 17.2%, respectively, P < 0.01, chi-squared test; Table 1). There was no significant difference in the proportion of colorectal cancers at Dukes’ stage A or B compared with Dukes’ stage C or D at diagnosis (33.3% and 66.7% for 2016 vs 47.2% and 52.8% for 2015, Dukes’ A/B and Dukes’ C/D, respectively, chi-squared test; Table 1).
Time to diagnosis and treatment of colorectal cancer
A total of 36 and 53 colorectal cancer patients were diagnosed via the two-week-wait referral pathway, in the 2016 and 2015 study periods, respectively (Table 1). The median time to diagnosis of colorectal cancer was reduced to 21 days in 2016 from 33.5 days in 2015 (P < 0.001, Mann-Whitney U-test, Fig 2B). Furthermore, the median time to treatment initiation was reduced to 50.5 days in 2016 from 58 days in 2015 (P < 0.05, Mann-Whitney U-test, Fig 2C).
Investigations performed
The investigations performed in 2016 (954 patients) and 2015 (664 patients) are listed in Supplementary Table 1. On a per patient basis, proportionately more colonoscopies were performed in 2016 than in 2015 (P < 0.00001, chi-squared test; Supplementary Table 1) and proportionately less computed tomography (CT) colonography, oesophagogastroduodenoscopy and magnetic resonance imaging (P < 0.00001, P < 0.05, P < 0.001 respectively, chi-squared test; Supplementary Table 1). Proportions of patients for whom CT and flexible sigmoidoscopy were performed were not significantly different between the two cohorts (chi-squared test; Supplementary table 1). To our knowledge, no adverse events or admissions occurred following tests performed because of straight-to-test referrals.
More patients had adenomas excised in 2016 than in 2015 (231 vs 134 patients, respectively; Table 1). There was no significant difference in the proportion of low-, intermediate- or high-risk adenomas, as defined by Atkin et al (Table 1).16 More adenomas were removed in 2016 when compared with the 2015 pathway (377 and 193 adenomas, respectively) but the number of adenomas per patient was comparable between the two cohorts (0.50 in 2016 and 0.45 adenomas in 2015).
Referral criteria fulfilled
The positive predictive value of each referral criterion for the 2016 and 2015 cohorts, assessed across all referrals in each time period, was calculated (Supplementary Table 2). The positive predictive values ranged from 3.5% to 19.1% in the 2016 cohort and from 4.3% to 21.0% in the 2015 cohort.
The referral criteria fulfilled were recorded when a non-colorectal cancer was diagnosed in the 2016 and 2015 cohorts (Supplementary Table 2). No single criterion accounted for the non-colorectal cancers diagnosed in either cohort.
Cost analysis
Using the latest NHS costing tariff, the total cost of providing tests for the two-week-wait pathway for the 2016 cohort was £417,875 compared with £281,784 in 2015 (Table 2): a £136,091 difference. These costs equated to £438.04 on tests per referral in 2016 and £424.37 on tests per referral in 2015. In our 2016 cohort, 391 fewer nurse-led clinic appointments were required compared with the 2015 cohort (273 in 2016, 664 in 2015). Using the consultant-led clinic tariff (£143 per appointment) as a maximum estimate of nurse-led clinic appointments, the total cost of nurse-led clinics in 2016 was £39,039 compared with £94,952 in 2015: a £55,913 difference.
Table 2.
A breakdown of costings for testing done on the two-week-wait pathway for suspected lower gastrointestinal cancer using the unit costings determined by the NHS National tariff. This analysis excludes any biopsy costs which were found to be comparable between the two cohorts (Table 1).
| Unit cost (£) | 2015 cohort (n) | Total cost (£) | 2016 cohort (n) | Total cost (£) | |
| Colonoscopy | 403 | 475 | 191,425 | 795 | 320,385 |
| CT | 100 | 213 | 21,300 | 316 | 31,600 |
| OGD | 341 | 115 | 39,215 | 132 | 45,012 |
| CT colonography | 196 | 104 | 20,384 | 54 | 10,584 |
| Flexible sigmoidoscopy | 310 | 20 | 6,200 | 29 | 8,990 |
| MRI | 163 | 20 | 3,260 | 8 | 1,304 |
| Total cost of tests | 281,784 | 417,875 | |||
| Cost of tests per referral | 424.37 | 438.02 |
CT, computed tomography; MRI, magnetic resonance imaging; OGD, oesophagogastroduodenoscopy.
Discussion and conclusion
We have demonstrated that implementation of a straight-to-test protocol under the updated NICE guidelines led to reduced time to diagnosis and reduced time to treatment of colorectal cancers across the entire referred population, despite a 43.7% increase in referral load (Fig 2A–C). These findings corroborate previous data on feasibility of straight-to-test for suspected lower gastrointestinal cancer,15 and time savings that straight-to-test protocols can help to provide.12 They also corroborate a recent study comparing patients referred straight-to-test on the two-week-wait pathway for suspected lower gastrointestinal cancer under the new NICE guidelines with those who attended nurse-led clinics.13 Crucially, our inclusion of the 2015 cohort as a control group enabled demonstration of reduced times to diagnosis and treatment, under the new referral criteria, across the entire referred population. In our straight-to-test protocol, patients that met the nurse-led clinic criteria for assessment prior to investigation were significantly older and had a higher cancer pick-up rate, in-line with previous studies.
We examined the impact of the new referral criteria on numbers and types of cancers diagnosed. The new referral criteria reduced the overall positive predictive value of individual referrals from 9.6% to 6.1% for the diagnosis of cancer, and from 8.0% to 3.8% for the diagnosis of colorectal cancer. These figures were comparable with, although slightly higher than, the respective targets set by NICE of 5% prior to guideline changes and 3% following guideline changes. This reduced predictive value did not equate to more cancers or colorectal cancers being diagnosed, nor did it change the proportion of early stage cancers at diagnosis (Table 1). We cannot exclude the possibility that the historic cohort reflected an unusually high cancer pick-up rate for the region concerned, and future studies with longer sampling periods and in other regions would be required to confirm that these findings are generalisable within the UK. While no more colorectal cancers were diagnosed under the new criteria, we did detect more cancers at non-colorectal sites (Table 1). These diagnoses could not be ascribed to individual referral criteria (Supplementary Table 2), but may result from their symptom-oriented design.9,10 The previous criteria were, by contrast, largely based on epidemiological studies of colorectal cancer.10 The detection of non-colorectal cancers may reflect an absolute increase in the number of CT scans performed due to the increased number of referrals and the addition of ‘weight loss’, in combination with rectal bleeding or abdominal pain, as a reason for referral (Fig 1; Table 2).
The combination of no more cancers being diagnosed, no more early colorectal cancers being diagnosed, and an increased proportion of non-colorectal cancers diagnosed under these criteria are informative regarding future policy. The data do not support further reductions in time to diagnosis following referral on clinical grounds alone, nor indeed further widening of clinical referral criteria, as viable methods to increase early diagnoses of colorectal cancer. In this respect, our data are reminiscent of previous comparisons of two-week-wait compared with non-two-week-wait referral pathways which demonstrated no effect on cancer stage at diagnosis and did not demonstrate effects on survival.6–8 Solutions to the problem of early detection in primary care may require adoption of emergent technologies that provide information that is independent of but complementary to the clinical picture. Faecal immunochemical testing or even circulating tumour DNA may be invaluable to this end, and have superior sensitivity/specificity profiles to most clinical criteria.2,17,18 The site-agnostic approach of circulating tumour DNA may be of benefit, given the tendency of the new criteria to pick up cancers across different organ systems (Table 1). Likewise, the overlap of symptomatology (e.g. weight loss, anaemia) and investigations (e.g. CT chest abdomen pelvis) across cancer sites indicates potential time- and cost-savings from integrating referral guidelines for multiple cancers sites with common pathways.19
In terms of the criteria themselves, most referrals in both cohorts were for change in bowel habit, iron deficiency anaemia and rectal bleeding (Supplementary Table 2). The high positive predictive values in both cohorts of iron deficiency anaemia (16.3% in 2016 and 20.1% in 2015) and the low positive predictive values for both change in bowel habit (4.5% in 2016 vs 5.2% in 2015) and rectal bleeding (3.5% in 2016 vs 5.2% in 2015) were comparable to data obtained from previous studies (Supplementary Table 2).18 The lower positive predictive values of rectal bleeding in 2016 may reflect the less restrictive referral criteria: inclusion of people between 50–60 years of age and those with symptoms of less than six weeks’ duration, may have led to proportionately more referrals with non-malignant causes of bleeding such as haemorrhoids. However, studies with greater numbers of patients across multiple regions would be required to confirm the positive predictive values of the new compared with old referral criteria. Longer-term studies may also reveal more patients referred with positive occult blood tests, as general practitioners increase their familiarity with the updated guidelines. Nevertheless, these data provide the first evaluation of the performance of the new referral criteria in the setting of general practitioner referrals to secondary care and may identify areas for future refinement in guideline setting.
More cancellations were observed in the period following the introduction of the straight-to-test protocol (Supplementary Fig 1). The increased cancellations may reflect the lack of reassurance that a specialist consultation may otherwise have provided in an outpatient setting compared with a telephone conversation, or insufficient information available at the level of primary care. An explanation for this could include less consideration of patient preference when deciding upon investigation modality.20 Novel applications of personalised cancer screening, with consideration of patient choice, history and background, may be one way to reduce the number of cancellations to avoid missing potential cancer diagnoses.21 In addition, in our region, general practitioner education programmes are now being trialled with the aim of reducing patient cancellations following straight-to-test referrals.
In terms of investigations, more tests were performed in 2016. Discrepancies in the proportions of tests performed were observed between the two cohorts: this likely resulted from the proforma-based triage system introduced in 2016 (Fig 1B) coupled with widening of the referral criteria. One consequence of performing a greater total number of colonoscopies in 2016 was that more patients had adenomas excised (Table 1; Supplementary Table 1). Neither the number of adenomas excised per patient referred for colonoscopy nor the proportion of patients at high risk differed significantly between the cohorts (Table 1 and Supplementary table 1). Excision of adenomas may represent a relative protection of these patients from colorectal cancer in the future.22 However, this potential benefit must be considered alongside the substantial resource implications of the increased referral load (Tables 1 and 2). More colonoscopy appointments were required, including commencement of weekend lists, but no additional staff were required to meet this demand. Our cost analysis shows comparable per-referral test cost, suggesting that the increase in cost of testing is due to increased referral burden rather than any increased cost associated with the straight-to-test pathway (Table 2). Our implementation of the straight-to-test pathway did free up a total of 391 nurse-led clinic appointments over the 2016 time period, which led to a significant cost saving. However, the patients seen in nurse-led clinics via the straight-to-test pathway were of higher acuity, thus requiring longer consultation time, and much of the time saved due to fewer nurse-led clinic appointments was used to administer the straight-to-test pathway. In summary, the real cost-savings of the straight-to-test protocol were likely to be negligible in contrast to the extra expenditure on investigations brought about by the new referral guidelines.
In conclusion, our straight-to-test protocol reduced times to diagnosis and to treatment despite an increase in referral load. There were no events leading to significant harm because of the tests performed on patients sent straight to test. Future amendments in primary and secondary care may be required to safeguard against increased cancellations. The new criteria had considerable resource implications that were not mitigated by the introduction of the straight-to-test protocol. They did not identify more cancers than before and did not alter the proportions of early diagnoses, but may have altered the types of cancers diagnosed. Taken together, our analyses may guide future policy decisions on increasing early detection of colorectal and other cancers.
Acknowledgements
We would like to thank the University of Cambridge School of Clinical Medicine and the Cambridge Colorectal Unit.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; (5): 646–674. [DOI] [PubMed] [Google Scholar]
- 2.Cohen JD, Li L, Wang Y et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018; (6378): 926–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Research UK Cancer incidence for common cancers. www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/common-cancers-compared#heading-Zero (cited February 2019).
- 4.Tørring ML, Frydenberg M, Hansen RP et al. Time to diagnosis and mortality in colorectal cancer: a cohort study in primary care. Br J Cancer 2011; (6): 934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neal RD, Tharmanathan P, France B et al. Is increased time to diagnosis and treatment in symptomatic cancer associated with poorer outcomes? Systematic review. Br J Cancer 2015; : S92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flashman K, O’Leary DP, Senapati A, Thompson MR. The Department of Health’s ‘two week standard’ for bowel cancer: is it working? Gut 2004; (3): 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanna SJ, Muneer A, Khalil KH. The two-week-wait for suspected cancer: time for a rethink? Int J Clin Pract 2005; (11): 1,334–1,339. [DOI] [PubMed] [Google Scholar]
- 8.Thornton L, Reader H, Stojkovic S et al. Has the ‘fast-track’ referral system affected the route of presentation and/or clinical outcomes in patients with colorectal cancer? World J Surg Oncol 2016; (1): 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence Suspected Cancer: Recognition and Referral. NICE Guideline NG12 London: NICE; 2015. (last updated 2017). [PubMed] [Google Scholar]
- 10.National Institute for Health and Care Excellence Helping GPs make an early diagnosis of cancer. 2015. www.nice.org.uk/news/feature/helping-gps-make-an-early-diagnosis-of-cancer (cited February 2019).
- 11.NHS England. Implementing a Timed Colorectal Cancer Diagnostic Pathway: A Handbook for Local Health and Care Systems. London: NHS England; 2018. [Google Scholar]
- 12.Hemingway DM, Jameson J, Kelly MJ et al. Straight to test: introduction of a city-wide protocol driven investigation of suspected colorectal cancer. Colorectal Dis 2006; (4): 289–295. [DOI] [PubMed] [Google Scholar]
- 13.Banerjea A, Voll J, Chowdhury A et al. Straight-to-test colonoscopy for 2-week-wait referrals improves time to diagnosis of colorectal cancer and is feasible in a high-volume unit. Colorectal Dis 2017; (9): 819–826. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee S, Fountain G, Stalker M et al. The ‘straight to test’ initiative reduces both diagnostic and treatment waiting times for colorectal cancer: outcomes after 2 years. Colorectal Dis 2010; (10): 250–254. [DOI] [PubMed] [Google Scholar]
- 15.Beggs AD, Bhate RD, Irukulla S et al. Straight to colonoscopy: the ideal patient pathway for the 2-week suspected cancer referrals? Ann R Coll Surg Engl 2011; (2): 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkin WS, Saunders BP. Surveillance guidelines after removal of colorectal adenomatous polyps. Gut 2002; (Suppl 5): V6–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L-L, Li Y-M. Current noninvasive tests for colorectal cancer screening: An overview of colorectal cancer screening tests. World J Gastrointest Oncol 2016; (11): 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jellema P, van der Windt DAWM, Bruinvels DJ et al. Value of symptoms and additional diagnostic tests for colorectal cancer in primary care: systematic review and meta-analysis. BMJ 2010; (3): c1269–c1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicholson BD, Oke J, Friedemann Smith C et al. The Suspected CANcer (SCAN) pathway: protocol for evaluating a new standard of care for patients with non-specific symptoms of cancer. BMJ Open 2018; (1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Inadomi JM, Vijan S, Janz NK et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012; (7): 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selby K, Bartlett-Esquilant G, Cornuz J. Personalized cancer screening: helping primary care rise to the challenge. Public Health Rev 2018; (1): 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkin W, Wooldrage K, Parkin DM et al. Long-term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet 2017; (10076): 1,299–1,311. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.