Abstract
Introduction
We examine the influence of variations in provision of cardiac surgery in the UK at hospital level on patient outcomes and also to assess whether there is an inequality of access and delivery of healthcare. Cardiothoracic surgery has pioneered the reporting of surgeon-specific outcomes, which other specialties have followed. We set out to identify factors other than the individual surgeon, which can affect outcomes and enable other surgical specialties to adopt a similar model.
Materials and methods
A retrospective analysis of prospectively collected data of patient and hospital level factors between 2013 and 2016 from 16 cardiac surgical units in the UK were analysed through the Society for Cardiothoracic Surgery of Great Britain and Ireland and the Royal College of Surgeons Research Collaborative. Patient demographic data, risks factors, postoperative complications and in-hospital mortality, as well as hospital-level factors such as number of beds and operating theatres, were collected. Correlation between outcome measures was assessed using Pearson’s correlation coefficient. Associations between hospital-level factors and outcomes were assessed using univariable and multivariable regression models.
Results
Of 50,871 patients (60.5% of UK caseload), 25% were older than 75 years and 29% were female. There was considerable variation between units in patient comorbidities, bed distribution and staffing. All hospitals had dedicated cardiothoracic intensive care beds and consultants. Median survival was 97.9% (range 96.3–98.6%). Postoperative complications included re-sternotomy for bleeding (median 4.8%; range 3.5–6.9%) and mediastinitis (0.4%; 0.1–1.0%), transient ischaemic attack/cerebrovascular accident (1.7%; range 0.3–3.0%), haemofiltration (3.7%; range 0.8–6.8%), intra-aortic balloon pump use (3.3%; range 0.4–7.4%), tracheostomy (1.6%; range 1.3–2.6%) and laparotomy (0.3%; range 0.2–0.6%). There was variation in outcomes between hospitals. Univariable analysis showed a small number of positive associations between hospital-level factors and outcomes but none remained significant in multivariable models.
Conclusions
Variations among hospital level factors exists in both delivery of, and outcomes, following cardiac surgery in the UK. However, there was no clear association between these factors and patient outcomes. This negative finding could be explained by differences in outcome definition, differences in risk factors between centres that are not captured by standard risk stratification scores or individual surgeon/team performance.
Keywords: Cardiac Surgery, Outcomes, Perioperative care
Introduction
Variations in healthcare provision exist within the UK.1 This does not refer to variations in access to healthcare but to hospital-level factors. Until now, outcomes of cardiac surgery have been scrutinised based on the results of individual surgeons.2 The term used is surgeon-specific mortality. However, there are other factors which may have a role in defining outcomes following surgery. Outcomes may be influenced by the level and expertise of all individuals and systems involved in delivery of care including competent delivery of the organisation of care, anaesthesia and intensive care.3,4 In addition, surrogates of unmeasured hospital-level factors, such as annual procedural case volume,4,5 opportunities for research and formal training, or subspecialisation, have been used to identify differences in outcome. Variations in any of these factors may partly explain differing outcomes amongst units and are the focus of this study.
The outcomes of cardiac surgery are reported annually in the UK.6 These are reported as unit and surgeon-specific results.7 The Society for Cardiothoracic Surgery in Great Britain and Ireland (SCTS) has been at the forefront of reporting results made available on public portals.8,9 Cardiac surgery has pioneered the reporting of outcomes following surgery and relating it to individual surgeons.10 The influence of other human and organisational factors is less studied. To date, patient demographics, preoperative risks factors and in-hospital mortality after surgery have been reported. However, postoperative complications are collected at hospital level but not reported to the national database.
Our aim was to identify hospital-level factors associated with quality of care. By identifying these, we aim to provide tangible targets for service and delivery improvement and attempt to standardise and provide excellent, high-quality cardiac surgical care, regardless of where the patient undergoes surgery. Furthermore, this study aims to better inform other surgical specialties in reporting outcome data and examining the role of hospital systems and non-surgeon-specific factors. We set out to examine several of these factors and their influence on postoperative mortality and complications.
Materials and methods
Study protocol
This study was endorsed by SCTS, the Royal College of Surgeons of England (RCS) Research Collaborative and the Association for Cardiothoracic Anaesthesia and Critical Care (ACTACC). All adult cardiac surgical units in the UK were invited to participate. Sixteen of 30 units who were contacted volunteered to take part. Representatives from each unit assisted in data collection. Data were also extracted from the National Institute of Cardiovascular Outcome Research (NICOR) National Adult Cardiac Surgery Audit database. From this database, surgeon- and unit-specific mortality were calculated. The data submitted by each unit was checked against that of NICOR. Any discrepancies were checked by the unit representative and the data analyst/manager.
Permission to study the data was granted by the Healthcare Quality Improvement Partnership. National ethical approval has been obtained and permission granted from NICOR.
Study design
General. We performed a retrospective analysis of prospectively collected data from April 2013 to March 2016 of adult patients undergoing cardiac surgery at UK NHS hospitals. Data on structure, process and outcomes were collected for this period using a survey of providers. Mortality was defined as in-hospital death. Clinical data, including patient demographics and comorbidity were collected from NICOR. Postoperative complications were reported by the unit representative only, since these data are not routinely reported to NICOR. Other variables such as intensive care provision were collected from the National Cardiac Benchmarking Collaborative and Intensive Care National Audit and Research Centre (ICNARC). Data on teaching, research and training were collected from the Specialty Advisory Committee at RCS.
Patient related and demographic factors
Gender, age, pulmonary disease, extracardiac arteriopathy, neurological dysfunction, previous cardiac surgery, creatinine, active endocarditis, critical preoperative state (patient being ventilated before surgery), left ventricular function, recent myocardial infarction and pulmonary hypertension were collected.
Operative data
The following surgery types were recorded: isolated coronary artery bypass graft surgery, isolated aortic valve replacement, isolated mitral valve surgery, surgery on the thoracic aorta and any of the combinations of these and redo operations. In addition, the availability of left- and right-ventricular assist device and extracorporeal membrane oxygenator was noted.
Staff
Numbers of consultants (substantive and locum), national trainees and non-national trainees (non-training grades, research fellows), core trainees, foundation year, surgical nurse practitioners and any other category of staff were collected. All consultants were reported to be full time. The numbers of fully qualified, trainee and agency perfusion staff were also collected.
The numbers of cardiac surgical beds and mixed beds (cardiac plus thoracic or other specialties) and the number of nurses per occupied bed were recorded.
The numbers of research fellows and whether they were registered for a higher degree was recorded.
Provisions for intensive care
Whether the unit had a dedicated cardiothoracic intensive care unit or was part of a general intensive care unit was noted. Number of intensive care beds allocated to cardiac surgery, number of nurses per intensive care bed and percentage bed occupancy (based on the number of nights spent on intensive care) were collected. Also, whether the unit had dedicated intensive care specialist consultants or was mainly run by cardiac surgeons was recorded.
Patient outcomes
Mortality was defined as in-hospital death after first operation. Risk adjustment was undertaken using an adaptation of the logistic EuroSCORE model.11 The following postoperative complications were recorded: re-sternotomy for bleeding, re-sternotomy for mediastinitis, new transient ischaemic attack (TIA) or cerebrovascular accident (CVA), new requirement for haemofiltration or dialysis, use of intra-aortic balloon pump (IABP), tracheostomy and laparotomy.
Data collection
A dedicated research fellow and research administrator were the central point for data collection. Each unit representative submitted data to these individuals. In addition, NICOR, ICNARC and RCS data were checked against submitted data and any discrepancies addressed.
Statistical analysis
Survey responses were summarised by the number and percentage of units for binary responses and the median, interquartile range (IQR) and range across units for continuous responses. Variation in outcome measures across units was presented as funnel plots (outcome plotted against number of cases) with funnel lines representing the 95% and 99.8% ranges of the distribution (corresponding to 2 and 3 standard deviations, SD). Correlation between outcome measures was assessed using Pearson’s correlation coefficient (R).
For outcomes available from at least 80% of responding units, ordinary least squares linear regression models were fitted at the hospital level relating each outcome to the following factors:
volume (linear – number of cases per year during the time period)
number of beds on surgical wards (linear – per 1000 cases/year)
number of patients per nurse on surgical wards (linear – per additional patient/nurse)
number of critical care beds (linear – per 1000 cases/year)
number of critical care consultants (linear – per 1000 cases/year)
number of critical care trainees/clinical fellows (linear – per 1000 cases/year)
research active unit (yes/no – defined by the presence of staff/students registered for higher degrees).
These factors were selected following review of descriptive survey responses (and prior to any modelling of outcomes) to reflect factors that varied across units and had a plausible relationship with outcomes. For each fitted regression model, the assumption of heteroscedasticity was tested with the Breusch–Pagan test. Univariable models were initially fitted for each outcome on each factor. Factors that were available from at least 75% of hospitals were then combined in a multivariable model for each outcome. Multicollinearity was assessed with variance inflation factors and covariates were removed from the multivariable models until all remaining covariates had variance inflation factors less than 20.
Role of the funding source
The study was sponsored by SCTS, RCS Research Collaborative and ACTACC and all were jointly involved in the study design. Design, collection, analysis and interpretation of data were performed by representatives from each group. Funding sources had no role in the writing of the manuscript or the decision to submit it for publication. The corresponding author had full access to data in the study and was responsible for the decision to submit for publication.
Patient involvement
Four patient representatives from the SCTS and ACTACC were involved at the outset of the project and in initial planning and design. It became apparent that patients were unaware of the role of other team members involved in delivering cardiac surgical care, apart from the surgeon, and that they were under the impression that outcome was purely determined by the surgeon’s individual skills.
Results
Of the 30 NHS adult cardiac surgical units in UK, 16 agreed to participate. From April 2013 to March 2016, a total of 50,871 patients underwent cardiac surgery in the 16 units (Table 1), representing 60.5% of all cardiac surgery undertaken in the UK during that period. There was more than a threefold variation in the case volume across units (from 1861 to 5983). The proportion of patients aged over 75 years varied from 15% to 34% and the proportion with pulmonary disease varied from 6% to 20%. Survey results on staffing and theatre and ward capacity are presented in Tables 2 and 3.
Table 1.
Variation in preoperative characteristics across cardiac surgery units.
| Characteristic | Units reporting | Overall | Variation across units | ||
| Median | IQR | Range | |||
| Patients (n) | 16 | 50,871 | 2639 | 2193, 3580 | 1861, 5983 |
| Age > 75 years (%) | 15 | 25 | 25 | 23, 27 | 15, 34 |
| Female (%) | 15 | 28 | 29 | 26, 30 | 24, 31 |
| Pulmonary disease (%)a | 15 | 14 | 15 | 13, 17 | 6, 20 |
| Neurological dysfunction (%)b | 15 | 3.6 | 3.5 | 2.7, 4.0 | 1.0, 6.5 |
| Creatinine > 0.2 mg/dL (%)c | 15 | 1.7 | 1.5 | 0.8, 2.2 | 0.8, 4.5 |
| Poor left-ventricular function (%)d | 15 | 4.7 | 4.9 | 2.9, 5.8 | 1.3, 9.0 |
| Critical preoperative state (%)e | 15 | 2.5 | 2.5 | 1.7, 3.2 | 1.4, 4.8 |
a Defined as long-term use of bronchodilators or steroids for lung disease.
b Defined as previous stroke.
c Measured preoperatively.
d Defined as left-ventricular ejection fraction of ≤ 30%.
e Defined as ventricular arrhythmia or aborted sudden death, preoperative cardiac massage, preoperative ventilation before anaesthetic room, preoperative inotropes or intra-aortic balloon pump, preoperative acute renal failure (anuria or oliguria < 10 ml/hour)
IQR, interquartile range.
Table 2.
Variation in staffing across cardiac surgery units and provisions for mechanical circulatory support.
| Surgical staffing | Units reporting | Overalla | Variation across units | ||
| Median | IQR | Range | |||
| Consultants (n): | 16 | 142 | 7 | 6.5, 12 | 5, 17 |
| Substantive (n) | 16 | 140.6 | 7 | 6, 12.5 | 4, 17 |
| Locum (n) | 16 | 7.3 | 0.2 | 0, 1 | 0, 1 |
| Trainees (n): | 16 | 233.9 | 13.5 | 11.0, 17.5 | 5, 27 |
| NTN | 16 | 62 | 3.5 | 3, 5 | 1, 9 |
| Non-NTN | 16 | 99.3 | 6.5 | 4, 7 | 0, 14 |
| Core trainee | 16 | 41.6 | 2 | 1, 3.5 | 1, 7 |
| Foundation year | 16 | 34 | 2 | 0, 3 | 0, 9 |
| Surgical nurse practitioners (n) | 16 | 59.4 | 3 | 2, 4.5 | 1, 11.6 |
| Perfusionist staffing (n): | |||||
| Perfusionists | 14 | 141.5 | 8 | 7.9, 10.6 | 6, 23 |
| Fully qualified | 14 | 125.5 | 7 | 6.5, 9 | 5, 21 |
| Trainee | 14 | 16.9 | 1 | 1, 1.6 | 0, 2 |
| Permanent | 13 | 118.5 | 8 | 7, 10 | 4, 18 |
| Agency | 13 | 4.2 | 0 | 0, 1 | 0, 1.2 |
| Use of assist device (yes/no) | 16 | 62.5% | n/a | ||
| LVAD and RVAD (yes/no) | 15 | 53.3% | n/a | ||
| ECMO (yes/no) | 15 | 73.3% | n/a | ||
a The number reported is the average over the three years of the study.
ECMO, extracorporeal membrane oxygenation; IQR, interquartile range; LVAD, left-ventricular assist device; NTN, national training number; RVAD, right-ventricular assist device.
Table 3.
Variation in theatre and ward capacity across cardiac surgery units.
| Capacity | Units reporting | Overalla | Variation across units | ||
| Median | IQR | Range | |||
| Cardiothoracic theatres (n): | 16 | 68 | 4 | 3, 5 | 2, 7 |
| Cardiac | 16 | 32 | 2 | 0, 3.5 | 0, 5 |
| Thoracic | 16 | 11 | 0 | 0, 1.5 | 0, 2 |
| Mixed | 16 | 25 | 1 | 0, 3 | 0, 4 |
| Surgical beds (n): | 16 | 699.6 | 43 | 30, 56 | 20, 74 |
| Cardiac | 8 | 244.6 | 32 | 23.5, 36.8 | 20, 40 |
| Thoracic | 8 | 179 | 23 | 16.5, 25 | 16, 34 |
| Mixed | 8 | 276 | 30 | 26.5, 43.5 | 20, 56 |
| Critical care beds allocated to cardiothoracic surgery (n)b | 16 | 314 | 17 | 16, 26 | 10, 32 |
| Bed occupancy (%)c | 9 | 84 | 81, 85 | 75, 100 | |
aThe number reported is the average over the three years of the study.
b Includes both level 3 (intensive care) and level 2 (high dependency) beds.
c Based on number of nights spent on critical care unit.
IQR, interquartile range.
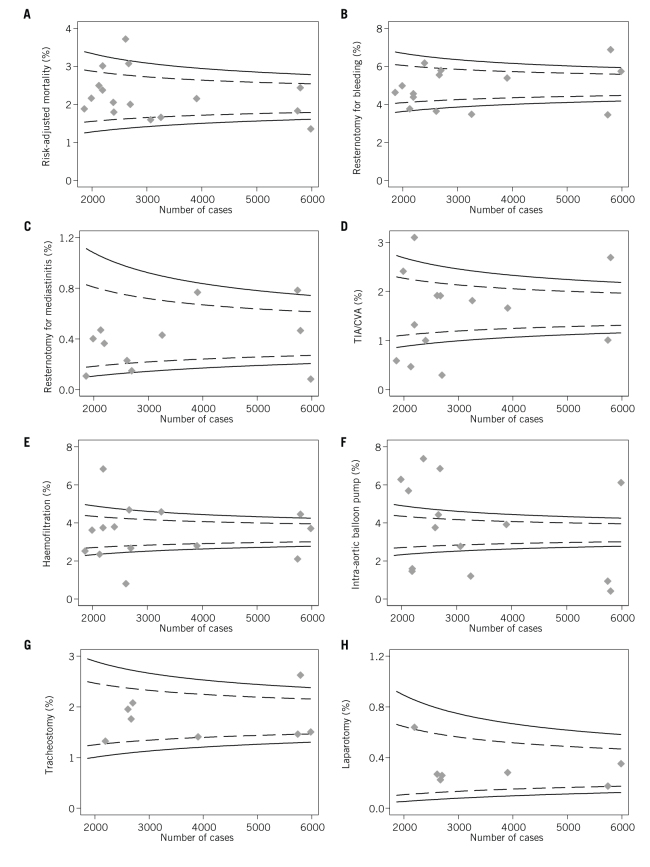
There was considerable variation in the outcomes (Table 4 and Fig 1) including risk-adjusted survival, re-sternotomy for bleeding, re-sternotomy for mediastinitis, TIA/CVA, postoperative haemofiltration, use of intra-aortic balloon pump, tracheostomy and laparotomy. Most outcomes showed more variation across units than would be expected by chance alone, indicated by points lying outside of the funnel lines. Some of the outcomes were defined by the actual intervention for example initiating haemofiltration after surgery or re-sternotomy to control bleeding. It was not possible to elicit whether these decisions made were by individual doctors or were driven by unit protocols.
Table 4.
Variation in risk-adjusted survival and postoperative complications across cardiac surgery units.
| Outcome measure | Units reporting | Overall | Variation across units | ||
| Median | IQR | Range | |||
| Risk-adjusted mortality (%) | 16 | 2.1 | 2.1 | 1.8, 2.5 | 1.4, 3.7 |
| Re-sternotomy for bleeding (%) | 14 | 5.0 | 4.8 | 3.7, 5.7 | 3.5, 6.9 |
| Re-sternotomy for mediastinitis (%) | 11 | 0.4 | 0.4 | 0.2, 0.8 | 0.1, 1.0 |
| TIA/CVA (%) | 13 | 1.6 | 1.7 | 1.0, 1.9 | 0.3, 3.0 |
| Haemofiltration (%) | 14 | 3.5 | 3.7 | 2.5, 4.5 | 0.8, 6.8 |
| IABP use (%) | 14 | 3.2 | 3.3 | 1.5, 5.6 | 0.4, 7.4 |
| Tracheostomy (%) | 8 | 1.8 | 1.6 | 1.4, 2.0 | 1.3, 2.6 |
| Laparotomy (%) | 7 | 0.3 | 0.3 | 0.2, 0.4 | 0.2, 0.6 |
CVA, cerebrovascular accident; IABP, intra-aortic balloon pump; IQR, interquartile range; TIA, transient ischemic attack.
Figure 1.
Funnel plots of variation across units in: (A) risk-adjusted survival (n = 16); (B) re-sternotomy for bleeding (n = 14); (C) re-sternotomy for mediastinitis (n = 11); (D) transient ischaemic attack/cerebrovascular accident (TIA/CVA) (n = 14); (E) haemofiltration (n = 14); (F) intra-aortic balloon pump (IABP) use (n = 14); (G) tracheostomy (n = 8); (H) laparotomy (n = 7). The dashed and solid funnel lines represent 95% and 99.8% control limits, respectively.
There was no clear pattern between the different measures and outlier status by individual units, with seven of the nine units being both a ‘positive’ 3SD outlier for at least one measure and a ‘negative’ 3SD outlier for at least one other. Correspondingly, the correlation between measures was generally low with the highest degree of correlation being between TIA/CVA, haemofiltration and laparotomy (R = 0.62 to 0.77; Table 5).
Table 5.
Correlation between outcome measures.
| Risk-adjusted mortality | Re-sternotomy for bleeding | Re-sternotomy for mediastinitis | TIA/CVA | Haemofiltration | IABP use | Tracheostomy | |
| Re-sternotomy for bleeding | R = −0.191 P = 0.51 n = 14 |
||||||
| Re-sternotomy for mediastinitis | R = −0.006 P = 0.99 n = 11 |
R = −0.211 P = 0.53 n = 11 |
|||||
| TIA/CVA | R = 0.450 P = 0.12 n = 13 |
R = 0.133 P = 0.67 n = 13 |
R = 0.157 P = 0.66 n = 10 |
||||
| Haemofiltration | R = −0.022 P = 0.94 n = 14 |
R = 0.282 P = 0.33 n = 14 |
R = −0.010 P = 0.98 n = 11 |
R = 0.618
P = 0.024 n = 13 |
|||
| IABP use | R = −0.145 P = 0.62 n = 14 |
R = 0.325 P = 0.28 n = 13 |
R = −0.504 P = 0.14 n = 10 |
R = −0.476 P = 0.12 n = 12 |
R = −0.273 P = 0.37 n = 13 |
||
| Tracheostomy | R = 0.175 P = 0.68 n = 8 |
R = 0.556 P = 0.15 n = 8 |
R = −0.251 P = 0.59 n = 7 |
R = 0.012 P = 0.98 n = 7 |
R = −0.143 P = 0.74 n = 8 |
R = −0.107 P = 0.80 n = 8 |
|
| Laparotomy | R = 0.193 P = 0.68 n = 7 |
R = 0.000 P = 1.00 n = 7 |
R = −0.268 P = 0.61 n = 6 |
R = 0.758 P = 0.081 n = 6 |
R = 0.766
P = 0.045 n = 7 |
R = −0.229 P = 0.622 n = 7 |
R = −0.469 P = =0.29 n = 7 |
Bold text indicates statistically significant correlations.
CVA, cerebrovascular accident; IABP, intra-aortic balloon pump; TIA, transient ischemic attack.
On univariable analysis, the only significant associations between prespecified factors and outcomes were increasing provision of ward beds was associated with an increased rate of both TIA/CVA and haemofiltration; increasing number of patients per nurse on the ward was associated with an increased rate of haemofiltration; and increasing number of critical care trainees/fellows was associated with decreased survival (Table 6).
Table 6.
Results of the regression analyses.
| Outcome/factors | Univariable | Multivariable | ||||
| (n) | Coefficient (95% CI) | P-value | (n) | Coefficient (95% CI) | P-value | |
| Risk-adjusted mortality: | ||||||
| Volumea | 16 | −0.43 (−1.14, 0.28) | 0.22 | 12 | −0.51 (−2.58, 1.57) | 0.57 |
| Ward bedsb | 16 | 0.23 (−0.05, 0.51) | 0.10 | 12 | 0.07 (−0.43, 0.56) | 0.76 |
| Patients per nursec | 9 | −0.17 (−0.45, 0.11) | 0.19 | – | - | – |
| Critical care bedsd | 16 | −0.01 (−0.21, 0.15) | 0.73 | 12 | −0.05 (−0.25, 0.16) | 0.58 |
| Critical care consultantse | 12 | 0.05 (−0.04, 0.14) | 0.24 | – | - | – |
| Critical care traineese | 12 | 0.08 (0.01, 0.15) | 0.038 | 12 | 0.07 (−0.08, 0.21) | 0.31 |
| Research active unitf | 16 | 0.11 (−0.60, 0.81) | 0.75 | 12 | 0.02 (−2.00, 2.04) | 0.98 |
| Resternotomy for bleeding: | ||||||
| Volumea | 14 | 0.56 (−0.76, 1.87) | 0.92 | 12 | 0.56 (−2.26, 3.38) | 0.65 |
| Ward bedsb | 14 | 0.06 (−0.50, 0.63) | 0.81 | 12 | 0.32 (−0.35, 0.99) | 0.29 |
| Patients per nursec | 7 | 0.16 (−0.25, 0.57) | 0.35 | – | - | – |
| Critical care bedsd | 14 | 0.03 (−0.11, 0.17) | 0.64 | 12 | −0.01 (−0.29, 0.26) | 0.92 |
| Critical care consultantse | 11 | −0.05 (−0.24, 0.13) | 0.54 | – | - | - |
| Critical care traineese | 12 | −0.09 (−0.23, 0.04) | 0.15 | 12 | −0.17 (−0.36, 0.03) | 0.084 |
| Research active unitf | 14 | 0.94 (−0.30, 2.18) | 0.12 | 12 | 1.25 (−1.49, 3.99) | 0.31 |
| TIA/CVA: | ||||||
| Volumea | 13 | 0.35 (−0.91, 1.62) | 0.55 | 11 | 1.78 (−0.58, 4.14) | 0.11 |
| Ward bedsb | 13 | 0.47 (0.11, 0.83) | 0.015 | 11 | 0.50 (−0.06, 1.06) | 0.069 |
| Patients per nursec | 7 | 0.14 (−0.26, 0.54) | 0.41 | – | - | – |
| Critical care bedsd | 13 | −0.00 (−0.12, 0.11) | 0.94 | 11 | 0.08 (−0.16, 0.31) | 0.45 |
| Critical care consultantse | 10 | 0.06 (−0.16, 0.28) | 0.56 | – | - | – |
| Critical care traineese | 11 | 0.05 (−0.07, 0.17) | 0.37 | 0.05 (−0.12, 0.21) | 0.48 | |
| Research active unitf | 13 | 0.23 (−0.88, 1.35) | 0.66 | 11 | −0.99 (−3.37, 1.38) | 0.33 |
| Haemofiltration: | ||||||
| Volumea | 14 | −0.07 (−1.89, 1.76) | 0.94 | 12 | 0.89 (−3.65, 5.43) | 0.65 |
| Ward bedsb | 14 | 0.80 (0.24, 1.37) | 0.009 | 12 | 0.99 (−0.09, 2.07) | 0.067 |
| Patients per nursec | 7 | 0.60 (0.36, 0.84) | 0.001 | – | - | – |
| Critical care bedsd | 13 | 0.03 (−0.15, 0.21) | 0.74 | 12 | −0.03 (−0.48, 0.41) | 0.87 |
| Critical care consultantse | 11 | 0.06 (−0.18, 0.31) | 0.58 | – | - | – |
| Critical care traineese | 12 | 0.08 (−0.12, 0.27) | 0.39 | 12 | −0.03 (−0.35, 0.29) | 0.82 |
| Research active unitf | 14 | 0.73 (−1.05, 2.51) | 0.39 | 12 | 0.25 (−4.17, 4.66) | 0.90 |
| IABP use: | ||||||
| Volumea | 14 | −1.32 (−4.12, 1.48) | 0.32 | 12 | −0.85 (−9.03, 7.33) | 0.81 |
| Ward bedsb | 14 | −0.16 (−1.33, 1.00) | 0.77 | 12 | −0.60 (−2.54, 1.35) | 0.48 |
| Patients per nursec | 7 | −0.16 (−1.26, 0.95) | 0.73 | – | – | – |
| Critical care bedsd | 14 | 0.13 (−0.11, 0.37) | 0.26 | 12 | 0.22 (−0.58, 1.02) | 0.53 |
| Critical care consultantse | 11 | −0.10 (−0.48, 0.29) | 0.58 | – | – | – |
| Critical care traineese | 12 | −0.11 (−0.42, 0.19) | 0.43 | 12 | −0.12 (−0.69, 0.44) | 0.61 |
| Research active unitf | 14 | 0.93 (−1.75, 3.62) | 0.46 | 12 | 0.39 (−7.56, 8.33) | 0.12 |
a Per 1000 cases/year.
b Per additional 10 beds/1000 cases/year.
c Per additional patient/nurse.
d Per additional bed/1000 cases/year.
e per additional staff member/1000 cases/year.
f yes vs no,
CI, confidence interval; CVA, cerebrovascular accident; IABP, intra-aortic balloon pump; N, number of units; TIA, transient ischaemic attack.
Number of patients per nurse on the ward was not included in multivariable models because 18% of the values were missing. Variance inflation factors for the remaining predictors suggested multicollinearity was present. On further investigation, this was found to be due to strong correlation between number of critical care beds and number of critical care consultants. Number of critical care consultants was therefore excluded from the multivariable model.
No factors remained significant in the resulting multivariable models (Table 6). Tests for lack of heteroscedasticity were non-significant for all models and all variance inflation factors were less than five in the final models. Model R2 values for the multivariable models ranged from 0.34 (intra-aortic balloon pump use) to 0.69 (TIA/CVA).
Discussion
In this national survey, linked with high-quality, prospectively collected patient data, we identified significant variation between providers in risk-adjusted survival and other outcomes following cardiac surgery in the UK. There were also variations in staffing and other provisions for perioperative care. However, in multivariable modelling, we were not able to establish any significant associations between provision and outcomes.
This lack of association could be due to differences in demographics which are not captured in the risks stratification models like EuroSCORE, some of these risks factors are frailty and poor mobility; differences in definitions of outcomes and also individual surgeon’s skills. Furthermore, social deprivation has been shown to be associated with outcomes after cardiac surgery in the UK.12
Strengths and weaknesses of the study
This is the first study, to our knowledge, that set out to examine data about a range of outcomes other than mortality on a unit-specific basis in comparison with a range of inputs. The size of the study (over 50,000 cases identified from more than 50% of the adult cardiac surgical units in the UK) has enabled us to make confident findings about the predicted rates of various complications and outcomes. Having identified rates of these outcomes, this will enable individual units and surgeons compare performance.
There are, however, limitations to our study. Despite including more than 50% of all providers in the UK, the 16 units that participated provided only limited power to identify associations between hospital-level factors and patient outcomes. Some of the requested data items were incomplete, such as patients per nurse, which limited the ability to include these items in multivariable models. Furthermore, there is inherent lack of standardisation in the criteria for interventions such as re-sternotomy for bleeding and renal replacement therapy, which may contribute to between unit variation and limit their utility as outcome measures.
Possible explanations and implications for clinicians and policymakers
Since publication of surgeon-specific and centre-specific mortality was mandated for cardiac surgical practice in the UK in 2002,13–15 the focus has been on surgical performance as the driver of mortality variation.6 It is claimed that this process of publication has improved risk adjusted mortality,9,16 but variation in outcomes remains. Multiple other specialties and staffing groups are involved in care delivery. Parallel attempts have been made to look at these various groups such as anaesthetists and their influence on mortality and length of stay, which mostly point to the variation in the patient’s own comorbidity as the most important determining factor.9 There are significant variations in the models with which post-cardiac surgical critical care is delivered making comparison difficult. The importance of this has been recognised and benchmarking systems have been explored.17–19
Risk-adjusted mortality in adult cardiac surgery in the UK has reduced by 25% in the last 10 years.6,7,10,20 The contemporary in-hospital and operative mortality are similarly low in the United States, ranging from 1.0 to 5.1%.21 Mortality having declined despite the increasing risk profile of the patient population, there is an increasing need to minimise complications and improve quality of life following surgery. Our study sought to assess data relating to complications after surgery by examining the structures and processes in peri- and postoperative care.
Centre volume has been identified as a cause of variation in the UK and the United States for specific procedures and pathologies, although this has been challenged in other studies.22–24 In the UK, however, cardiac surgery is centralised in only 30 specialist units and, consequently, despite a threefold variation in volume across the units in the current study, it is likely that all units were above the threshold of cases required for any volume effect to be apparent. New methodologies to investigate the quality of perioperative care and the resulting variations in outcome have been identified as an important topic for research as we move forward from surgeon-specific mortality.24,25
Every unit was an outlier for at least one outcome. Surprisingly, there was limited correlation between these outcome measures. In particular, there was no significant correlation between risk-adjusted survival and other outcome measures. This may be explained by variation in criteria for the interventions; for example, whereas early re-sternotomy for bleeding and early renal replacement therapy signify complications, they may be examples of good practice and may promote survival. Where mortality is recognised as the consequence of failure to rescue, it would be wrong to stigmatise units on the basis of what may be enthusiastic rescue policies.
Although we have shown variations in outcomes, our attempts to identify factors associated with these variations were less productive and some of the potential associations identified in univariable analysis are counterintuitive. The most likely explanation for these counterintuitive and clinically implausible findings is chance alone. Given the number of relationships examined, if all outcomes and predictors were independent, we would expect to have observed three statistically significant results due to chance alone. Multivariable analysis did not identify any individual factors significantly associated with outcomes. A study involving a much larger number of centres/providers would be needed to resolve the complexities of the multiple interdependent factors involved in delivery of perioperative care. However, even with all the cardiac surgical units in the UK included, the challenge may remain too great, and expanding to other countries would introduce further complexities from variation between healthcare systems as well as between hospitals.
Conclusion
We have identified variations in outcomes following cardiac surgery, in addition to risk-adjusted mortality. There is considerable variation in staffing and other provisions for perioperative care, however there was no clear association with patient outcomes. These findings may indicate that there are variations in definitions of outcomes, differences in demographics between centres that are not captured in the risk stratification scores or possibly be related to the individual surgeon. There is a need to standardise and analyse postoperative complications and quality of life and the role of both the surgeon and unit in these outcomes. To achieve lower rates of complications and improved quality of life, the optimum structures and processes need to be identified. Unless these are in place, a meaningful analysis of postoperative outcomes will not be possible.
References
- 1.Appleby J, Lyscom T, Raleigh V et al. . Variations in Health Care: The Good, the Bad and the Inexplicable. London: King’s Fund; 2011. [Google Scholar]
- 2.Papachristofi O, Klein A, Sharples L. Evaluation of the effects of multiple providers in complex surgical interventions. Stat Med 2016; (28): 5,222–5,246. [DOI] [PubMed] [Google Scholar]
- 3.Papachristofi O, Sharples LD, Mackay J. et al. The contribution of the anaesthetist to risk-adjusted mortality after cardiac surgery. Anaesthesia 2016; (2): 138–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birkmeyer JD, Stukel TA, Siewers A. et al. Surgeon volume and operative mortality in the United States. N Engl J Med 2003; (22): 2,117–2,127. [DOI] [PubMed] [Google Scholar]
- 5.Birkmeyer JD, Siewers A. et al. Hospital volume and surgical mortality in the United States. N Engl J Med 2002; (15): 1,128–1,137. [DOI] [PubMed] [Google Scholar]
- 6.Society fo. Cardiothoracic Surgery in Great Britain and Ireland. Blue Book Online. http://bluebook.scts.org (cited February 2019).
- 7.Society fo. Cardiothoracic Surgery in Great Britain and Ireland. Outcomes. https://scts.org/outcomes (cited February 2019).
- 8.Bridgewater B, Hickey GL, Cooper G et al. . Publishing cardiac surgery mortality rates: lessons for other specialties. BMJ 2013; : f1139. [DOI] [PubMed] [Google Scholar]
- 9.Williams MP, Modgil V, Drake MJ, Keeley F. The effect of consultant outcome publication on surgeon behaviour: a systematic review and narrative synthesis. Ann R Coll Surg Engl 2018; (6): 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bridgewater B, Keogh B, Kinsman R, Walton P. Sixth National Adult Cardiac Surgical Database Report: Demonstrating Quality. London: Society for Cardiothoracic Surgery in Great Britain and Ireland; 2008. [Google Scholar]
- 11.Society for Cardiothoracic Surgery in Great Britain and Ireland UK Cardiothoracic Units and Outcomes. http://scts.org/hospitals/cardiac (cited February 2019).
- 12.Barnard J, Grant SW, Hickey GL, Bridgewater B. Is social deprivation an independent predictor of outcomes following cardiac surgery? An analysis of 240 221 patients from a national registry. BMJ Open 2015; : e008287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Department o. Health. Learning from Bristol: the Department of Health’s Response to the Report of the Public Inquiry into Children’s Heart Surgery at the Bristol Royal Infirmary 1984–1995. London: Department of Health; 2002. [Google Scholar]
- 14.Bridgewater B, Keogh B. Surgical ‘league tables’. Heart 2008; (7): 936–942. [DOI] [PubMed] [Google Scholar]
- 15.Keogh B, Spiegelhalter D, Bailey A et al. . The legacy of Bristol: public disclosure of individual surgeons’ results. BMJ 2004; (7463): 450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bridgewater B, Grayson AD, Brooks N et al. . Has the publication of cardiac surgery outcome data been associated with changes in practice in northwest England: an analysis of 25,730 patients undergoing CABG surgery under 30 surgeons over eight years. Heart 2007; (6): 744–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doerr F, Badreldin AM, Heldwein M. et al. A comparative study of four intensive care outcome prediction models in cardiac surgery patients. J Cardiothorac Surg 2011; (1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulson TG, Bailey M, Reid C. et al. Acute risk change (ARC) identifies outlier institutions in perioperative cardiac surgical care when the standardized mortality ratio cannot. Absalom AR, editor. Br J Anaesth 2016; (2): 164–171. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher N. Climate change in cardiothoracic intensive care. Anaesthesia 2016; (12): 1,395–1,398. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins DP, Cooper G. Publicly available outcome data for individual surgeons: lessons from cardiac surgery. Eur Urol 2017; (3): 309–310. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs JP, Shahian DM, Prager R. et al. The Society of Thoracic Surgeons National Database 2016 annual report. Ann Thorac Surg 2016; (6): 1,790–1,797. [DOI] [PubMed] [Google Scholar]
- 22.Bottle A, Mariscalco G, Shaw M. et al. Unwarranted variation in the quality of care for patients with diseases of the thoracic aorta. J Am Heart Assoc 2017; (3): e004913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gammie JS, O’Brien SM, Griffith BP et al. . Influence of hospital procedural volume on care process and mortality for patients undergoing elective surgery for mitral regurgitation. Circulation 2007; (7): 881–887. [DOI] [PubMed] [Google Scholar]
- 24.Coulson TG, Mullany DV, Reid C. et al. Measuring the quality of perioperative care in cardiac surgery. Eur Heart J Qual Care Clin Outcomes 2017; (1): 11–19. [DOI] [PubMed] [Google Scholar]
- 25.Likosky DS, Wallace AS, Prager R. et al. Sources of variation in hospital-level infection rates after coronary artery bypass grafting: an analysis of the Society of Thoracic Surgeons Adult Heart Surgery Database. Ann Thorac Surg 2015; (5): 1,570–1,576. [DOI] [PMC free article] [PubMed] [Google Scholar]