Abstract
Background
Pelvic organ prolapse (POP) is common in women and is frequently associated with stress urinary incontinence (SUI). In many cases however, SUI is present only with the prolapse reduced (occult SUI) or may develop after surgical treatment for prolapse (de novo SUI).
Objectives
To determine the impact on postoperative bladder function of surgery for symptomatic pelvic organ prolapse with or without concomitant or delayed two‐stage continence procedures to treat or prevent stress urinary incontinence.
Search methods
We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE‐In‐Process, ClinicalTrials.gov, WHO ICTRP, handsearching journals and conference proceedings (searched 11 November 2017) and reference lists of relevant articles. We also contacted researchers in the field.
Selection criteria
Randomised controlled trials (RCTs) including surgical operations for POP with or without continence procedures in continent or incontinent women. Our primary outcome was subjective postoperative SUI. Secondary outcomes included recurrent POP on examination, overactive bladder (OAB) symptoms, and voiding dysfunction.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane.
Main results
We included 19 RCTs (2717 women). The quality of the evidence ranged from low to moderate. The main limitations were risk of bias (especially blinding of outcome assessors), indirectness and imprecision associated with low event rates and small samples.
POP surgery in women with SUI
Vaginal repair with vs without concomitant mid‐urethral sling (MUS)
A concomitant MUS probably improves postoperative rates of subjective SUI, as the evaluated clinical effect appears large (risk ratio (RR) 0.30, 95% confidence interval (CI) 0.19 to 0.48; 319 participants, two studies; I² = 28%; moderate‐quality evidence), and probably decreases the need for further continence surgery (RR 0.04, 95% CI 0.00 to 0.74; 134 participants, one study; moderate‐quality evidence). This suggests that if the risk of SUI with POP surgery alone is 39%, the risk with an MUS is between 8% and 19%.
Rates of recurrent POP on examination, OAB, and voiding dysfunction were not reported.
Vaginal repair with concomitant vs delayed MUS
Evidence suggested little or no difference between groups in reporting postoperative SUI (RR 0.41, 95% CI 0.12 to 1.37; 140 participants, one study; moderate‐quality evidence).
Rates of recurrent POP on examination, OAB, and voiding dysfunction and the need for further surgery were not reported.
Abdominal sacrocolpopexy with vs without Burch colposuspension
An additional Burch colposuspension probably has little or no effect on postoperative SUI at one year (RR 1.38, 95% CI 0.74 to 2.60; 47 participants, one study; moderate‐quality evidence), OAB symptoms (RR 0.85, 95% CI 0.61 to 1.18; 33 participants, one study; moderate‐quality evidence), or voiding dysfunction (RR 0.96, 95% CI 0.06 to 14.43; 47 participants, one study; moderate‐quality evidence). Rates of recurrent POP and the need for further surgery were not reported.
POP surgery in women with occult SUI
Vaginal repair with vs without concomitant MUS
MUS probably improves rates of subjective postoperative SUI (RR 0.38, 95% CI 0.26 to 0.55; 369 participants, five studies; I² = 44%; moderate‐quality evidence). This suggests that if the risk with surgery alone is 34%, the risk with a concomitant MUS is between 10% and 22%. Evidence suggests little or no difference between groups in rates of recurrent POP (RR 0.86, 95% CI 0.34 to 2.19; 50 participants, one study; moderate‐quality evidence), OAB symptoms (RR 0.75, 95% CI 0.52 to 1.07; 43 participants, one study; low‐quality evidence), or voiding dysfunction (RR 1.00, 95% CI 0.15 to 6.55; 50 participants, one study; low‐quality evidence). The need for further surgery was not reported.
POP surgery in continent women
Vaginal repair with vs without concomitant MUS
Researchers provided no conclusive evidence of a difference between groups in rates of subjective postoperative SUI (RR 0.69, 95% CI 0.47 to 1.00; 220 participants, one study; moderate‐quality evidence). This suggests that if the risk with surgery alone is 40%, the risk with a concomitant MUS is between 19% and 40%. Rates of recurrent POP, OAB, and voiding dysfunction and the need for further surgery were not reported.
Abdominal sacrocolpopexy with vs without Burch colposuspension
We are uncertain whether there is a difference between groups in rates of subjective postoperative SUI (RR 1.31, 95% CI 0.19 to 9.01; 379 participants, two studies; I² = 90%; low‐quality evidence), as RCTs produced results in different directions with a very wide confidence interval. We are also uncertain whether there is a difference between groups in rates of voiding dysfunction (RR 8.49, 95% CI 0.48 to 151.59; 66 participants, one study; low‐quality evidence) or recurrent POP (RR 0.98, 95% CI 0.74 to 1.30; 250 participants, one study; moderate‐quality evidence. No study reported OAB symptoms and need for further surgery.
Vaginal repair with armed anterior vaginal mesh repair vs anterior native tissue
Anterior armed mesh repair may slightly increase postoperative de novo SUI (RR 1.58, 95% CI 1.05 to 2.37; 905 participants, seven studies; I² = 0%; low‐quality evidence) but may decrease recurrent POP (RR 0.29, 95% CI 0.22 to 0.38; 848 participants, five studies; I² = 0%; low‐quality evidence). There may be little or no difference in rates of voiding dysfunction (RR 1.65, 95% CI 0.22 to 12.10; 125 participants, two studies; I² = 0%; low‐quality evidence). Rates of OAB and the need for further surgery were not reported.
Adverse events were infrequently reported in all studies; cost was not studied in any trial.
Authors' conclusions
In women with POP and SUI (symptomatic or occult), a concurrent MUS probably reduces postoperative SUI and should be discussed in counselling. It might be feasible to postpone the MUS and perform a delayed (two‐stage) continence procedure, if required.
Although an abdominal continence procedure (Burch colposuspension) during abdominal POP surgery in continent women reduced de novo SUI rates in one underpowered trial, another RCT reported conflicting results. Adding an MUS during vaginal POP repair might reduce postoperative development of SUI.
An anterior native tissue repair might be better than use of transobturator mesh for preventing postoperative SUI; however, prolapse recurrence is more common with native tissue repair.
Plain language summary
Surgery for women with pelvic organ prolapse with or without continence procedures
Review question
To assess the outcomes of operations for pelvic organ prolapse (POP) with or without operations to treat or prevent stress urinary incontinence (SUI). Background
Pelvic organ prolapse is a common condition, especially among women who have given birth and who are postmenopausal. It involves the descent of pelvic organs such as the womb (uterus), bladder, bowel, and vagina within and outside of the vaginal opening. It is often associated with urinary leakage on coughing or physical exertion as in sports (termed 'stress urinary incontinence'). However, in some women, the prolapse prevents leakage from the urethra and stress urinary incontinence might be present only with re‐placement of the prolapsed organs in the vagina during vaginal examination (termed 'occult SUI'). Stress urinary incontinence may also develop only after surgical treatment of prolapse (termed 'de novo SUI'). To date, the best treatment for women undergoing surgery for symptomatic pelvic organ prolapse with and without incontinence conditions is not known.
Study characteristics
Cochrane review authors searched different registers for relevant studies and collected, summarised, and analysed appropriate data to help identify the optimal treatment. Data are current to December 2017.
Key results
Reviewers included 19 randomised controlled trials in this review (2717 women), including surgical operations for POP with or without continence procedures in continent or incontinent women. Our primary outcome was subjective postoperative SUI. Secondary outcomes included recurrent POP on examination, overactive bladder (OAB) symptoms, voiding dysfunction, and need for further surgery.
Surgery to treat women with POP and stress urinary incontinence
In two studies of moderate quality, women with stress incontinence benefited from an additional continence procedure (mid‐urethral sling) at the time of vaginal prolapse repair for the outcome of postoperative SUI. The continence procedure might also be postponed for three months after prolapse surgery with similar success rates. In this situation, some women might avoid an additional continence operation.
It remains unclear whether abdominal prolapse repair (sacrocolpopexy or sacrohysteropexy) with an additional abdominal continence procedure (Burch colposuspension) improves urinary leakage after surgery.
Surgery to treat women with POP and occult stress urinary incontinence
Five moderate‐quality studies of women with prolapse and observed urinary leakage during vaginal examination with a reduced prolapse reported benefit from an additional continence procedure (mid‐urethral sling) when undergoing vaginal prolapse surgery.
Surgery to treat continent women with POP
Evidence from one moderate‐quality study was inconclusive as to any benefit of an additional continence procedure (mid‐urethral sling) when women underwent vaginal prolapse surgery.
Whether abdominal prolapse repair (sacrocolpopexy) with an additional abdominal continence procedure (Burch colposuspension) improves urinary leakage after surgery remains unclear, as two low‐quality studies reported conflicting results.
Seven low‐quality studies reported that fewer women had urinary leakage after vaginal native tissue repair compared to women who received a vaginal mesh implant for prolapse. However, vaginal mesh placement reduced the chance of recurrent prolapse.
Quality of the evidence
The quality of the evidence ranged from low to moderate. The main limitations in the quality of the evidence were risk of bias when those assessing the outcome of the surgery were not blinded to the type of surgery, indirectness when a study had a different focus to our review, and imprecision associated with small numbers of women who participated in the trials.
Summary of findings
Background
Pelvic organ prolapse (POP) is common and is seen on examination in 40% to 60% of parous women (Handa 2004; Hendrix 2002). POP is often associated with stress urinary incontinence (SUI): approximately 55% of women with stage 2 POP (prolapse to the hymen ±1 cm) have concurrent SUI, and only 33% of women with stage 4 POP have SUI (Slieker‐ten Hove 2009), probably due to kinking of the urethra when the prolapse advances.
When the prolapse is reduced digitally, with the help of a pessary or speculum during clinical examination, SUI might be demonstrated in up to 68% (Haessler 2005; Reena 2007; Visco 2008). If SUI is present only when the prolapse is reduced in otherwise continent women, this type of SUI is defined as 'occult SUI'. Women with occult SUI are at risk of developing symptomatic SUI after POP surgery (Haessler 2005).
Also, preoperatively continent women with POP and no symptomatic or occult SUI on examination may develop SUI symptoms postoperatively (Haessler 2005). This situation is defined as 'de novo stress urinary incontinence'. De novo SUI might occur after repair of POP because the surgery has unkinked the preoperatively obstructed urethra.
Therefore, this systematic review aims to determine the outcome of surgery with or without concomitant or delayed continence procedures in women with symptomatic pelvic organ prolapse with or without symptomatic or occult SUI on postoperative bladder function .
Description of the condition
Pelvic organ prolapse is the descent of one or more of the pelvic organs (uterus, vagina, bladder, or bowel). Types of prolapse include:
upper vaginal prolapse (i.e. uterus, vaginal vault (after hysterectomy when the top of the vagina drops down));
anterior vaginal wall prolapse (i.e. cystocoele (bladder descends), urethrocoele (urethra descends), paravaginal defect (pelvic fascia defect)); and
posterior vaginal wall prolapse (i.e. enterocoele (small bowel descends), rectocoele (rectum descends), perineal deficiency).
Women with prolapse commonly have a variety of pelvic floor symptoms. Symptoms of prolapse include pelvic heaviness; a bulge, lump, or protrusion coming down from the vagina; a dragging sensation in the vagina; and backache. Symptoms of bladder, bowel, or sexual dysfunction are frequently present. For example, women may need to reduce the prolapse by using their fingers to push the prolapse up to facilitate urinary voiding or defecation. These symptoms may be directly related to the prolapsed organ, for example, poor urinary stream when a cystocoele is present, or obstructed defecation when a rectocoele is present. They may also be independent of the prolapse, for example, symptoms of overactive bladder (OAB) when a cystocoele is present.
Stress urinary incontinence is the "complaint of involuntary loss of urine on effort or physical exertion (e.g. sporting activities), or on sneezing or coughing" (Haylen 2010). It occurs in approximately 50% of postmenopausal women and is often associated with POP. If the prolapse is more advanced, SUI might disappear as the result of kinking of the urethra (Slieker‐ten Hove 2009). However, on examination with the prolapse reduced, SUI can be often be demonstrated (Haessler 2005; Reena 2007; Visco 2008). This is defined as "occult or latent stress incontinence: (new) stress incontinence only observed after the reduction of co‐existent prolapse" (Haylen 2010). In this review, we will consistently use the term 'occult SUI'. To date it is not clear which method is best for reducing POP: neither reduction with speculum nor pessary provided acceptable positive predictive values to identify women who would benefit from a concomitant continence procedure during POP surgery. However, negative predictive values were 92.5% (95% confidence interval (CI) 90.3 to 1.00) and 91.1% (95% CI 88.5 to 99.7), respectively, which shows that women who test negative for occult SUI are at low risk of developing SUI postoperatively (Ellström 2011). If SUI develops after POP surgery in preoperatively continent women without occult SUI, this is termed 'de novo SUI' consistently in our review.
Causes of pelvic organ prolapse and SUI are complex and multi‐factorial. Possible risk factors include pregnancy, childbirth, congenital or acquired connective tissue abnormalities, denervation or weakness of the pelvic floor, ageing, hysterectomy, menopause, and factors associated with chronically raised intra‐abdominal pressure (ICI 2017).
Description of the intervention
Treatment for POP with or without SUI depends on the severity of the prolapse, associated symptoms, the woman's wish and general health, and surgeon preference and capabilities. Options available for treatment include conservative, mechanical, and surgical interventions. Surgical methods to treat anterior, posterior, and apical compartment POP and use of transvaginal mesh are described in conjoint reviews: Maher 2016a; Maher 2016b; Maher 2016c; and Mowat 2018.
Conservative and mechanical interventions have been considered in separate Cochrane reviews: Bugge 2013 and Hagen 2011.
This review considers surgical procedures for women with symptomatic pelvic organ prolapse with or without concomitant SUI or occult SUI. Aims of surgery include restoration of normal vaginal anatomy and restoration or maintenance of normal bladder, bowel, and sexual function.
A wide variety of abdominal, laparoscopic, and vaginal surgical techniques are available for the treatment of individuals with POP and SUI. The Committee for "Pelvic Organ Prolapse Surgery" of the International Consultation on Incontinence published an algorithm for the surgical management of pelvic organ prolapse, taking into account evidence from randomised and non‐randomised trials (Figure 1; ICI 2017).
1.
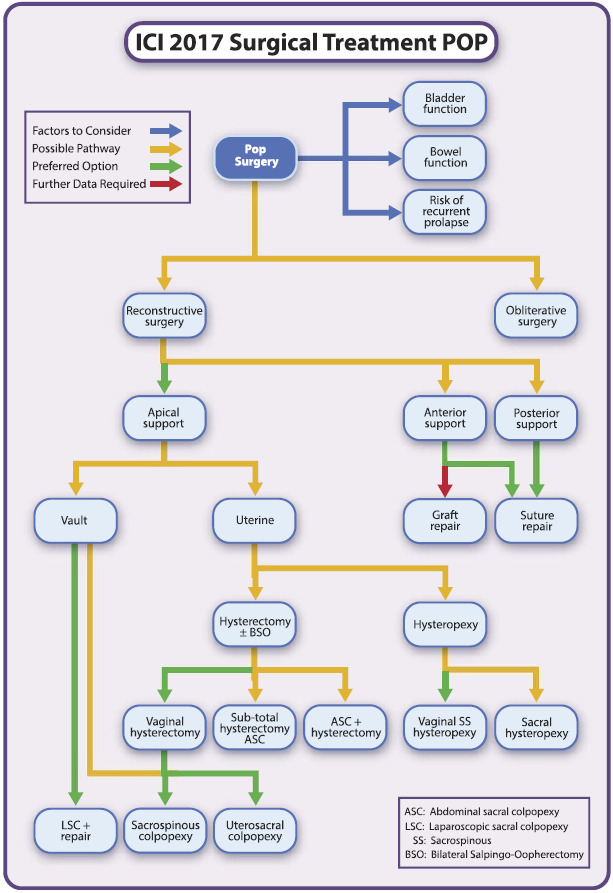
Decision pathway pelvic organ prolapse surgery ‐ published with permission of Wolters Kluwer. Maher CF, Baessler KK, Barber MD, Cheon C, Consten ECJ, Cooper KG, et al. Summary: 2017 International Consultation on Incontinence Evidence‐Based Surgical Pathway for Pelvic Organ Prolapse. Female Pelvic Medicine & Reconstructive Surgery 2018 April 28 [Epub ahead of print]. https://insights.ovid.com/pubmed?pmid=29727373. (Maher 2018)
Two main approaches can be differentiated but can also be combined during surgery (for further description of the procedures, see Appendix 1).
Vaginal approaches to treat POP include hysterectomy, anterior or posterior vaginal wall repair (colporrhaphy), McCall culdoplasty, Manchester repair (amputation of the cervix with uterine suspension to the cardinal ligaments), prespinous and sacrospinous colpopexy, enterocoele ligation, paravaginal repair, the Le Fortes procedure (colpocleisis), and perineal reconstruction.
Abdominal approaches to treat POP include total or subtotal hysterectomy, sacrocolpopexy, sacrohysteropexy or cervicopexy, paravaginal repair, vault suspension and uterosacral ligament plication, enterocoele ligation, and posterior vaginal wall repair. Abdominal surgery can be performed through an open incision or through keyhole incisions via the laparoscope or robot.
A combination of some of these procedures may be employed in the surgical correction of prolapse, as frequently more than one type of prolapse may occur.
Although any restoration of the anterior vaginal wall anatomy by anterior colporrhaphy or suspension of the uterus or the vaginal vault may already reduce SUI symptoms, these procedures are not considered formal continence surgery in this review. We will include in this review the following current standard continence procedures.
Vaginal mid‐urethral sling (MUS) procedures (e.g. tension‐free vaginal tape (TVT), transobturator tape (TOT), single‐incision slings).
Abdominal (open or laparoscopic) colposuspension procedures: Burch colposuspension and its modifications.
Urethral bulking agents.
Although a Burch colposuspension is considered formal continence surgery, it may also restore normal anatomy of the anterior vaginal wall. This is particularly true if a cystocoele is caused by paravaginal defects. Historically, Burch colposuspension and its modifications were also considered POP surgery, whereas anterior colporrhaphy was deemed a continence procedure.
The choice of operation depends on a number of factors, which include the nature, site, and severity of the prolapse; whether additional symptoms are affecting urinary, bowel, or sexual function; the general health of the woman; and surgeon preference and capability.
Procedures to treat or prevent SUI can be performed at the same time or later, depending on preoperative symptoms or demonstration of occult incontinence. Concurrent as well as delayed continence surgery will therefore also be considered in this review.
These issues require extensive counselling of the patient and may include discussions on the need for concomitant hysterectomy, continence surgery, and the use of mesh.
How the intervention might work
The aim of POP surgery is to restore pelvic floor anatomy and function by correcting the support defect or incorporating surrogate structures. This may include:
repair of defects of the endopelvic fascia: anterior and posterior repair (colporrhaphy);
(re)attachment of the uterus or vaginal vault to the uterosacral ligaments: uterosacral ligament fixation;
attachment of the uterus or vaginal vault to the sacrospinous ligament: sacrospinous colpopexy, sacrospinous hysteropexy;
attachment of the uterus, cervix (after subtotal hysterectomy), or vaginal vault to the sacrum with mesh interposition: sacrocolpopexy, sacrocervicopexy, sacrohysteropexy; and
if fascia or ligaments are not available or are deemed insufficient, vaginal mesh might be employed: anterior mesh overlay or inlay and anterior armed mesh (transobturator/obturator fixation with or without apical fixation).
The aim of formal continence surgery at the time of POP repair is to prevent or treat SUI by increasing support to the urethra and the bladder neck (bladder neck elevation during Burch colposuspension) or to support the mid‐urethra (mid‐urethral slings).
As POP surgery might already restore anatomy and function in the anterior compartment, this review will compare different POP operations alone as well as in contemporaneous or delayed combination with formal continence surgery.
These surgical approaches are available to prevent or treat women with symptomatic POP with and without SUI.
POP surgery alone.
POP surgery with concomitant continence surgery.
POP surgery and subsequent delayed continence surgery (two‐stage operation).
Why it is important to do this review
Although a wide variety of surgical treatments are available for POP with or without SUI, the optimal treatment for the individual situation with or without symptomatic SUI or findings on examination like occult SUI has not been established. It is unclear when continence procedures should be performed concomitantly or delayed as a two‐stage POP that includes a continence procedure, and which POP operations might sufficiently support the urethra or bladder neck, thereby treating or preventing postoperative symptomatic SUI in women with preoperative symptomatic or occult SUI and symptomatic POP.
Provided that sufficient numbers of trials of adequate quality have been conducted, the most reliable evidence is likely to come from consideration of randomised controlled trials, and this is the basis for this review. The aim is to help identify optimal practice while highlighting topics that need further research.
Objectives
To determine the impact on postoperative bladder function of surgery for symptomatic pelvic organ prolapse with or without concomitant or delayed two‐stage continence procedures to treat or prevent stress urinary incontinence.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). Studies were required to have a sample size of at least 20 in each group and a follow‐up time of at least six months.
Types of participants
Adult women seeking treatment for symptomatic pelvic organ prolapse with or without symptomatic or occult SUI.
Pelvic organ prolapse (POP) includes:
upper vaginal prolapse (uterine or vaginal vault);
anterior vaginal wall prolapse (cystocoele, urethrocoele, paravaginal defect); and
posterior vaginal wall prolapse (enterocoele, rectocoele, perineal deficiency).
We will include studies of the following groups of women with POP.
Women with stress urinary incontinence.
Women with occult stress urinary incontinence on examination with the prolapse reduced.
Continent women.
Stress urinary incontinence may have been diagnosed or described or excluded by employing standardised or preferably validated questionnaires, a clinical stress test with and without the prolapse reduced, or urodynamic studies.
We will include women with or without previous pelvic floor surgery including operations for POP or incontinence.
Types of interventions
We assessed trials comparing any type of abdominal or vaginal surgery for pelvic organ prolapse with or without concomitant or delayed continence surgery. We did not include comparisons of conservative interventions like pessaries or pelvic floor muscle training.
We included the following surgical operations to correct pelvic organ prolapse.
Abdominal or laparoscopic sacrocolpopexy or sacrohysteropexy.
Vaginal sacrospinous colpopexy or hysteropexy.
Anterior native tissue repair (colporrhaphy).
Anterior repair with mesh placement: armed or as overlay or inlay.
Although any restoration of the anterior vaginal wall anatomy by anterior colporrhaphy or suspension of the uterus or the vaginal vault may already reduce SUI symptoms, we do not consider these procedures formal continence surgery for the purposes of this review. We will include the following current standard continence procedures in the review.
Vaginal mid‐urethral sling (MUS) procedures (e.g. tension‐free vaginal tape, transobturator tape, single‐incision sling).
Abdominal (open or laparoscopic) colposuspension procedures: Burch colposuspension and its modifications.
Urethral bulking agent.
Types of outcome measures
Primary outcomes
-
Women's observations related to stress urinary incontinence (subjective outcome)
Subjective postoperative stress urinary incontinence (de novo, persistent, cured, or improved SUI)
Secondary outcomes
-
Clinicians' observations related to stress urinary incontinence and pelvic organ prolapse (objective outcome)
Objective stress urinary incontinence on examination (positive stress test) or urodynamic studies
Recurrent POP on examination
-
Associated pelvic floor symptoms
Overactive bladder symptoms (de novo, persistent, cured, or improved OAB)
Voiding dysfunction (de novo, persistent, cured, or improved VD)
Pelvic pain
Sexual problems including dyspareunia
Perceived cure of or improvement in prolapse symptoms
Condition‐specific quality of life questionnaires (related to pelvic floor function)
-
Surgical outcome measures
Further continence surgery
-
Complications
Adverse effects (e.g. return to theatre, damage to surrounding viscera, mesh or graft exposure, graft rejection)
-
Economic measures
Costs of interventions or resources
Formal economic evaluations
Search methods for identification of studies
We did not impose any language restrictions or other limits on any of the searches, which we have detailed below.
Electronic searches
This review drew on the search strategy developed for the Cochrane Incontinence Review Group. Relevant trials were identified from the Group's Specialised Register of controlled trials, which is described, along with the Review Group search strategy, under the Group's module in the Cochrane Library. The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, MEDLINE, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and by handsearching of journals and conference proceedings. The Incontinence Group Specialised Register was searched using the Group's own keyword system (all searches were of the keyword field of Reference Manager 12, Thomson Reuters; last search date 30 November 2017). These are the search terms that were used.
({design.cct*} OR {design.rct*}) AND ({topic.prolapse*}) AND ({intvent.surg*})
Trials included in the Incontinence Group Specialised Register are also contained in CENTRAL.
Searching other resources
We handsearched conference proceedings of the annual meetings of relevant societies (i.e. International Urogynecologic Association (IUGA), International Continence Society (ICS), and American Urogynecologic Society (AUGS)), searched the reference lists of relevant articles, and contacted researchers in the field.
Data collection and analysis
Selection of studies
We assessed titles and abstracts of all possibly eligible studies. Two review authors (KB and CS) independently assessed the full report of each study likely to be eligible, using our inclusion criteria. Review authors agreed on whether or not to include the study based on the inclusion criteria for the review.
We have listed excluded studies with reasons for their exclusion in the Characteristics of excluded studies table.
Data extraction and management
At least two review authors (from KB, CS, and CFM) independently extracted and compared data to ensure accuracy. We resolved discrepancies by discussion or by referral to a third party. When trial data were not reported adequately, we attempted to acquire the necessary information from authors in the trial list.
We corresponded with study investigators to ask for further data on methods and/or results, as required.
Assessment of risk of bias in included studies
Two review authors (KB and CS) independently evaluated the included studies for risk of bias using the Cochrane risk of bias assessment tool to assess selection (random sequence generation and allocation concealment), performance (blinding of participants and personnel), detection (blinding of outcome assessors), attrition (incomplete outcome data), reporting (selective reporting), and other bias (Higgins 2011). We assigned judgements as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8.5) (Higgins 2011a). We resolved disagreements by discussion. We fully described all judgements and presented them in the conclusions in the 'Risk of bias' tables, which we incorporated into our interpretation of review findings by performing sensitivity analyses.
Measures of treatment effect
For categorical and dichotomous data, we used the numbers of events in control and intervention groups of each study to calculate a risk ratio (RR). For continuous variables, we calculated mean differences (MDs) between treatment groups. If similar outcomes were reported on different scales, we calculated standardised mean differences (SMDs). We reversed the direction of effect of individual studies, if required, to ensure consistency across trials.
We assessed whether estimates calculated in the review for individual studies were compatible in each case with estimates reported in the study publications.
Unit of analysis issues
Analysis was performed per woman randomised.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis as far as possible (i.e. including all randomised participants in analysis, in the groups to which they were randomised), using Review Manager software (RevMan 2014). We attempted to obtain missing data from the original trialists. When we could not obtain these, we analysed only available data.
If studies reported sufficient detail to calculate mean differences but no information on associated standard deviation (SD), we assumed the outcome to have a standard deviation equal to the highest SD from other studies within the same analysis.
Assessment of heterogeneity
We considered whether clinical and methodological characteristics of included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by measuring I². We regarded an I² measurement greater than 50% as indicating substantial heterogeneity (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by staying alert for duplication of data. If we included ten or more studies in an analysis, we planned to use a funnel plot to explore the possibility of small‐study effects (i.e. a tendency for estimates of the intervention effect to be more beneficial in smaller studies).
Data synthesis
We combined trials only if the interventions were similar enough based on clinical criteria.
We processed included trial data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We undertook meta‐analyses to synthesise trial data, when appropriate. We used a fixed‐effect model for calculations of summary estimates and their 95% confidence intervals (CIs), except where heterogeneity indicated a change to a random‐effects model.
We made the following comparisons.
Vaginal POP surgery with versus without concomitant continence surgery.
Vaginal POP surgery with concomitant versus delayed continence surgery.
Abdominal POP surgery with versus without concomitant continence surgery.
Abdominal POP surgery with one type of concomitant continence procedure versus another type.
One type of POP surgery versus another type of POP surgery.
We conducted separate analyses for different population groups, as follows.
Women with stress urinary incontinence.
Women with occult stress urinary incontinence.
Urinary continent women.
Subgroup analysis and investigation of heterogeneity
When we suspected important heterogeneity from visual inspection of the results, and when the Chi² test for heterogeneity (at 10%) or the I² statistic (I² > 50%) indicated substantial heterogeneity (I² > 50%) (Higgins 2003), we explored possible explanations through subgroup analyses such as clinical or methodological differences between trials. We took any statistical heterogeneity into account when interpreting the results, especially if we noted any variation in the direction of effect.
Sensitivity analysis
We planned to conduct sensitivity analyses for the primary outcomes to determine whether the conclusions were robust to arbitrary decisions made regarding eligibility and analysis. These analyses would have included consideration of whether review conclusions would have differed if:
eligibility had been restricted to studies without high risk of bias (defined as studies that we rated as at low risk of bias with respect to sequence generation and allocation concealment, and that we did not rate as at high risk of bias in any of the domains assessed); or
a random‐effects model had been adopted.
Overall quality of the body of evidence: 'Summary of findings' table
We prepared 'Summary of findings' tables using GRADEpro and Cochrane methods (GRADEproGDT 2015; Higgins 2011). These tables evaluated the overall quality of the body of evidence for main review outcomes (subjective postoperative stress urinary incontinence, recurrent POP on examination, overactive bladder, voiding dysfunction, and need for further surgery) for the main review comparison (POP surgery with vs without a concomitant continence procedure). We prepared a separate 'Summary of findings' table for each population group of interest. We assessed the quality of the evidence using the following GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Two review authors (KB and CS) independently made judgements about evidence quality (high, moderate, low, or very low) and resolved disagreements by discussion. We justified, documented, and incorporated judgements into reporting of results for each outcome.
Results
Description of studies
Results of the search
We assessed full reports of 95 potentially eligible studies.
We have shown the flow of studies through the assessment process in the PRISMA flowchart (Figure 2).
2.
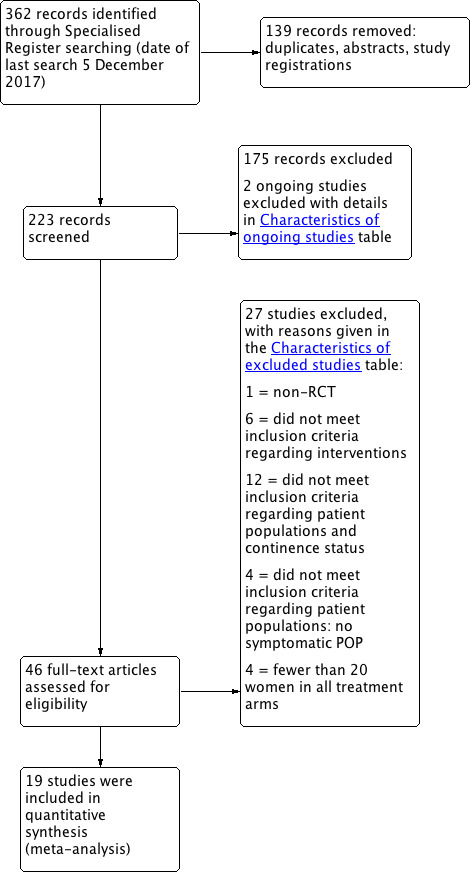
PRISMA study flow diagram.
Included studies
We included 19 studies reporting on 2717 randomised women.
We included the following.
Five studies that assessed continence issues in continent women (Altman 2011; Brubaker 2006; Costantini 2007; Sivaslioglu 2008; Turgal 2013). Two studies are ancillary reports to Altman 2011, and they provide additional information (Ek 2010; Ek 2011). Brubaker 2006 and Costantini 2007 published extended follow‐up at two years and several additional reports, which are clearly marked and assess the same patients.
Four further studies provided separate data on de novo stress urinary incontinence at 3, 12, 24, or 36 months, although trials included women with and without SUI (Hiltunen 2007; Iglesia 2010; Rudnicki 2014; Withagen 2011). Hiltunen 2007 and Rudnicki 2014 also published longer‐term (three years) follow‐up data.
Five studies analysed postoperative SUI in women with POP and occult SUI who did or did not receive an additional mid‐urethral sling (Fuentes 2011; Meschia 2004; Schierlitz 2014; van der Ploeg 2016 (CUPIDO II); Wei 2011). Three trials used a retropubic mid‐urethral sling; Fuentes 2011 used a transobturator tape. van der Ploeg 2016 (CUPIDO II) used both retropubic and transobturator slings. Fuentes 2011 published only an abstract. Schierlitz 2014 published four‐year follow‐up data (Walsh et al).
Five studies assessed continence issues in preoperatively stress urinary incontinent women with POP (Borstad 2010; Colombo 2000; Costantini 2008; Trabuco 2014; van der Ploeg 2015 (CUPIDO I)). Trabuco 2014 also reported two‐year outcomes (Trabuco et al 2016), and Costantini 2008 provided five‐year data.
Design and setting
All studies used computer‐generated randomisation lists and none were quasi‐randomised trials, although Fuentes 2011 did not comment on this. Most studies concealed allocation in opaque envelopes that were opened at the time of surgery. Only one study did not conceal the allocation and used an open list (Colombo 2000), and some studies did not mention concealment strategies (Fuentes 2011; Sivaslioglu 2008; Turgal 2013; Wei 2011; Withagen 2011).
Trials were performed in nine countries (USA, Italy, Netherlands, Finland, Sweden, Denmark, Norway, Turkey, Australia).
Some studies specifically mentioned the setting as a secondary referral centre (Colombo 2000), others as a tertiary referral centre (Brubaker 2006; Costantini 2007; Costantini 2008). Iglesia 2010 emphasised that fellowship‐trained urogynaecologists performed the surgeries. All studies were performed in a dedicated urogynaecological or urological setting.
Twelve trials were multi‐centre studies (Altman 2011; Borstad 2010; Brubaker 2006; Hiltunen 2007; Iglesia 2010; Rudnicki 2014; Schierlitz 2014; Sivaslioglu 2008; van der Ploeg 2015 (CUPIDO I); van der Ploeg 2016 (CUPIDO II); Wei 2011; Withagen 2011).
Only four trials used a double‐blind design (blinding of participants and assessors) (Brubaker 2006; Iglesia 2010; Wei 2011; Withagen 2011), and three trials reported a single‐blind approach (Altman 2011; Costantini 2007; Costantini 2008). Wei 2011 also used sham dressings.
Participants
All studies included continent or incontinent women with symptomatic pelvic organ prolapse quantification (POPQ) stage 2 or higher and Baden‐Walker grade 2 or higher, respectively. Researchers randomised 2717 women and followed up on 2429 of them. Most participants were postmenopausal; no studies focused on premenopausal, elderly, or obese women.
Interventions
Although a great variety of surgeries can be performed for POP, these studies evaluated only the Burch colposuspension or mid‐urethral slings for SUI.
Three studies compared sacrocolpopexy with or without Burch colposuspension (Brubaker 2006; Costantini 2007; Costantini 2008), and one study compared MUS and Burch colposuspension at the time of sacrocolpopexy (Trabuco 2014).
Seven studies compared anterior native tissue repair versus vaginal mesh augmented surgery: two self‐tailored mesh (Hiltunen 2007; Sivaslioglu 2008), three Prolift (Altman 2011; Iglesia 2010; Withagen 2011), one Sofradim (Turgal 2013), and one Avaulta (Rudnicki 2014).
Six studies compared vaginal POP surgery with and without mid‐urethral slings: Meschia 2004,Schierlitz 2014, and Wei 2011 retropubic TVT, Fuentes 2011 one transobturator sling, and both van der Ploeg 2015 (CUPIDO I) and van der Ploeg 2016 (CUPIDO II) retropubic or transobturator slings.
One study compared vaginal POP surgery with concomitant versus delayed retropubic TVT (Borstad 2010).
One study compared Burch colposuspension versus anterior repair (Colombo 2000).
Outcomes
All studies assessed subjective bladder function outcomes. Not all studies reported on POP outcomes. Most trialists employed the POPQ, and only one trial used the Baden‐Walker halfway system (Colombo 2000).
All included trials described stress urinary incontinence symptoms. Some studies also reported results of cough stress tests and urodynamic studies. Researchers infrequently described symptoms of overactive bladder or voiding dysfunction.
All but four trials ‐ Colombo 2000,Meschia 2004,Borstad 2010, and Hiltunen 2007 ‐ used various validated questionnaires to assess bladder, bowel, prolapse, and sexual symptoms. Lack of validated quality of life questionnaires in their native language was one reason (Hiltunen 2007).
Length of follow‐up was 12 months or exceeded 12 months in most trials (Altman 2011; Brubaker 2006; Colombo 2000; Costantini 2007; Costantini 2008; Hiltunen 2007; Iglesia 2010; Meschia 2004; Rudnicki 2014; Schierlitz 2014; Sivaslioglu 2008; van der Ploeg 2015 (CUPIDO I); van der Ploeg 2016 (CUPIDO II); Wei 2011; Withagen 2011). Two trials reported on continence outcomes after six months (Fuentes 2011; Trabuco 2014). Subsequently, Trabuco 2014 published a two‐year follow‐up. Owing to the study design comparing vaginal POP surgery with concomitant versus delayed mid‐urethral sling placement, Borstad 2010 presented results at three months.
Excluded studies
We excluded 27 studies, mainly because the patient populations did not meet our inclusion criteria of women with symptomatic POP with OR without SUI. Many of these studies explored different surgeries for POP but did not include continence outcomes in their study aims and did not assess continent or incontinent women, resulting in different patient populations. Some studies performed POP surgery as an adjunct in asymptomatic women; patient‐centred outcomes cannot be assessed in asymptomatic patients, and interpretation of subjective outcomes is impossible. Furthermore, some studies did not include the minimum of 20 participants in each group.
We have provided full details in the Characteristics of excluded studies tables.
Risk of bias in included studies
Allocation
Sequence generation
We found that all but three studies adequately described the sequence generation process (Fuentes 2011; Trabuco 2014; Turgal 2013). Fuentes 2011 provided an abstract with limited information.
Allocation concealment
Seven trials ensured secure concealment of the randomisation process (Altman 2011; Borstad 2010; Brubaker 2006; Hiltunen 2007; Iglesia 2010; Rudnicki 2014; van der Ploeg 2015 (CUPIDO I)); 11 trials indicated that this was unclear (Costantini 2007; Costantini 2008; Fuentes 2011; Meschia 2004; Schierlitz 2014; Sivaslioglu 2008; Trabuco 2014; Turgal 2013; van der Ploeg 2016 (CUPIDO II); Wei 2011; Withagen 2011). Colombo 2000 used an open list, which is considered inadequate, so we assessed this study as having high risk.
Blinding
Women and surgeons could not be blinded to the procedure when different surgical routes or incisions were compared (Colombo 2000), although Wei 2011 and Altman 2011 used sham incisions. Iglesia 2010 and Trabuco 2014 applied sham dressings for trocar incisions. Eight trials blinded patients and postoperative reviewers (Altman 2011; Brubaker 2006; Costantini 2007; Costantini 2008; Iglesia 2010; Trabuco 2014; Wei 2011; Withagen 2011).
We rated four studies as having low risk of performance bias (Altman 2011; Brubaker 2006; Iglesia 2010; Wei 2011), seven as having unclear risk (Colombo 2000; Costantini 2007; Fuentes 2011; Meschia 2004; Sivaslioglu 2008; Trabuco 2014; Turgal 2013), and eight as high risk (Borstad 2010; Costantini 2007; Hiltunen 2007; Rudnicki 2014; Schierlitz 2014; van der Ploeg 2015 (CUPIDO I); van der Ploeg 2016 (CUPIDO II); Withagen 2011).
We rated seven studies as having low risk of detection bias (Altman 2011; Brubaker 2006; Costantini 2007; Costantini 2008; Iglesia 2010; Trabuco 2014; Wei 2011), four unclear risk (Colombo 2000; Fuentes 2011; Meschia 2004; van der Ploeg 2015 (CUPIDO I) and eight high risk (Borstad 2010; Hiltunen 2007; Rudnicki 2014; Schierlitz 2014; Sivaslioglu 2008; Turgal 2013; van der Ploeg 2016 (CUPIDO II); Withagen 2011)
We have summarised these findings in Figure 3 and Figure 4.
3.
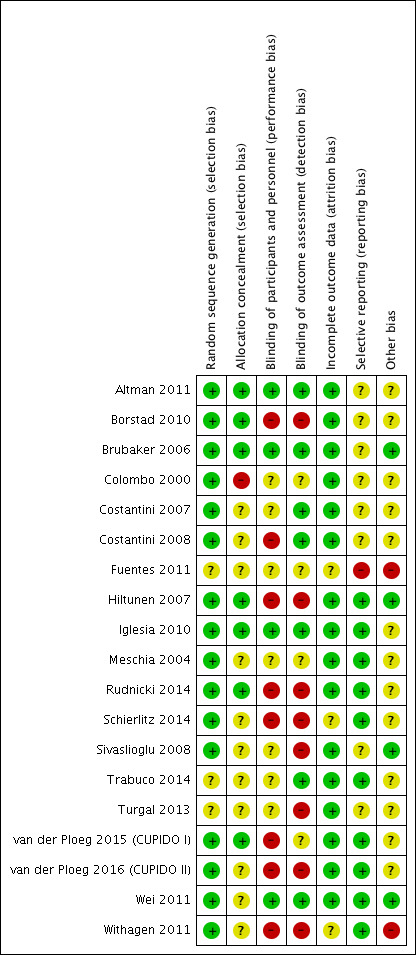
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
4.
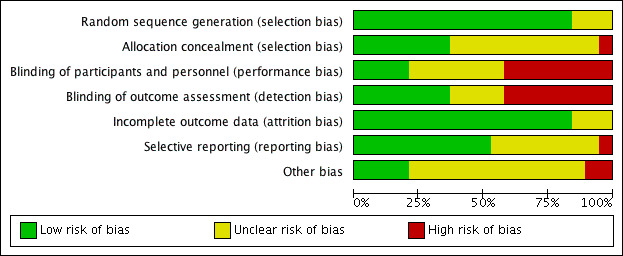
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Incomplete outcome data
Loss to follow‐up was a variable problem, ranging from zero in Meschia 2004 to 25% in Schierlitz 2014.
We rated 16 studies as having low risk of attrition bias (Altman 2011; Borstad 2010; Brubaker 2006; Colombo 2000; Costantini 2007; Costantini 2008; Hiltunen 2007; Iglesia 2010; Meschia 2004; Rudnicki 2014; Sivaslioglu 2008; Trabuco 2014; Turgal 2013; van der Ploeg 2015 (CUPIDO I); van der Ploeg 2016 (CUPIDO II); Wei 2011), and three as having unclear risk (Fuentes 2011; Schierlitz 2014; Withagen 2011).
Selective reporting
Ancillary reports were available for two trials (Altman 2011; Brubaker 2006), and longer‐term follow‐up for seven trials (Brubaker 2006; Costantini 2007; Hiltunen 2007; Iglesia 2010; Rudnicki 2014; Schierlitz 2014; Trabuco 2014). Researchers reported most of the prespecified outcome measures with an emphasis on subjective patient‐related outcomes. Many studies were first published as conference abstracts (e.g. International Urogynecological Association, International Continence Society); later, full manuscripts became available.
We rated 10 studies as having low risk of selective reporting (Hiltunen 2007; Iglesia 2010; Meschia 2004; Rudnicki 2014; Schierlitz 2014; Trabuco 2014; van der Ploeg 2015 (CUPIDO I); van der Ploeg 2016 (CUPIDO II); Wei 2011; Withagen 2011), eight as having unclear risk (Altman 2011; Borstad 2010; Brubaker 2006; Colombo 2000; Costantini 2007; Costantini 2008; Sivaslioglu 2008; Turgal 2013), and one as having high risk (Fuentes 2011).
Other potential sources of bias
All trials reported baseline descriptive characteristics. Withagen 2011 noted important differences between groups.
We rated four studies as having low risk of other bias (Brubaker 2006; Hiltunen 2007; Sivaslioglu 2008; Wei 2011), 13 unclear risk (Altman 2011; Borstad 2010; Colombo 2000; Costantini 2007; Costantini 2008; Iglesia 2010; Meschia 2004; Rudnicki 2014; Schierlitz 2014; Trabuco 2014; Turgal 2013; van der Ploeg 2015 (CUPIDO I); van der Ploeg 2016 (CUPIDO II), and two high risk (Fuentes 2011; Withagen 2011).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. POP surgery with concomitant continence procedure compared to no concomitant continence procedure in women with POP and SUI.
| POP surgery with concomitant continence procedure compared to without concomitant continence procedure in women with POP and SUI | |||||||
| Patient or population: women with POP and SUI Setting: hospital Intervention: POP surgery with continence procedure Comparison: POP surgery without continence procedure | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with POP surgery without continence procedure | Risk with POP surgery with continence procedure | ||||||
| Vaginal POP surgery with vs without MUS Follow‐up: 12 months | Subjective postoperative SUI | 394 per 1000 | 118 per 1000 (75 to 189) | RR 0.30 (0.19 to 0.48) | 319 (2 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Recurrent POP on examination | No data available | ||||||
| Overactive bladder symptoms (cured/improved) | No data available | ||||||
| Voiding dysfunction | No data available | ||||||
| Vaginal POP surgery with vs without MUS Follow‐up: mean 12 months | Further continence surgery | 169 per 1000 | 7 per 1000 (0 to 125) | RR 0.04 (0.00 to 0.74) | 134 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| Vaginal POP surgery with concomitant vs delayed continence surgery: additional concomitant MUS vs delayed MUS Follow‐up: mean 12 months | Subjective postoperative SUI | 113 per 1000 | 46 per 1000 (14 to 155) | RR 0.41 (0.12 to 1.37) | 140 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| Recurrent POP on examination | No data available | ||||||
| Overactive bladder symptoms (cured/improved) | No data available | ||||||
| Voiding dysfunction | No data available | ||||||
| Further continence surgery | No data available | ||||||
| Abdominal POP surgery with vs without concomitant continence surgery: additional Burch colposuspension vs sacrocolpopexy alone: 1‐year FU | Subjective postoperative SUI | 391 per 1000 | 540 per 1000 (290 to 1000) | RR 1.38 (0.74 to 2.60) | 47 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| Recurrent POP on examination | No data available | ||||||
| Overactive bladder symptoms (cured/improved) | 882 per 1000 | 750 per 1000 (538 to 1000) | RR 0.85 (0.61 to 1.18) | 33 (1 RCT) | ⊕⊕⊕⊝ Moderatea | ||
| Voiding dysfunction difficulties | 43 per 1000 | 42 per 1000 (3 to 627) | RR 0.96 (0.06 to 14.43) | 47 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| Further continence surgery | No data available | ||||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FU: follow‐up; MUS: mid‐urethral sling; POP: pelvic organ prolapse; RCT: randomised controlled trial; RR: risk ratio; SUI: stress urinary incontinence; | |||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for serious risk of bias ‐ no blinding of patients or assessors.
bDowngraded one level for serious imprecision with very low event rate and very wide confidence intervals, which cross line of no effect.
Summary of findings 2. Vaginal POP surgery with concomitant continence procedure compared to no concomitant continence procedure in women with POP and occult SUI.
| Vaginal POP surgery with concomitant continence procedure compared to no concomitant continence procedure in women with POP and occult SUI | |||||||
| Patient or population: women with POP and occult SUI Setting: hospital Intervention: vaginal POP surgery with continence procedure Comparison: vaginal POP surgery without continence procedure | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with vaginal POP surgery without concomitant continence procedure | Risk with vaginal POP surgery with concomitant continence procedure | ||||||
| Vaginal POP surgery with or without concomitant continence surgery: additional MUS vs vaginal repair alone | Subjective postoperative SUI | 397 per 1000 | 151 per 1000 (103 to 218) | RR 0.38 (0.26 to 0.55) | 369 (5 RCTs) | ⊕⊕⊕⊝ Moderatea | |
| Recurrent POP on examination | 280 per 1000 | 241 per 1000 (95 to 613) | RR 0.86 (0.34 to 2.19) | 50 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| Overactive bladder symptoms (cured/improved) | 870 per 1000 | 652 per 1000 (452 to 930) | RR 0.75 (0.52 to 1.07) | 43 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| Voiding dysfunction | 80 per 1000 | 18 per 1000 (5 to 64) |
RR 1.00 (0.15 to 6.55) | 50 (1 RCT) |
⊕⊕⊝⊝ Lowa,b | ||
| Further continence surgery | No data available | ||||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FU: follow‐up; MUS: mid‐urethral sling; POP: pelvic organ prolapse; RR: risk ratio; SUI: stress urinary incontinence. | |||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for serious risk of bias ‐ no blinding of patients or assessors, or insufficient information on blinding.
bDowngraded one level for serious imprecision with low event rate and wide CI crossing the line of no effect.
Summary of findings 3. Vaginal or abdominal POP surgery with concomitant continence procedure compared to no concomitant continence procedure in continent women with POP.
| Vaginal or abdominal POP surgery with concomitant continence procedure compared to no concomitant continence procedure in continent women with POP | |||||||
| Patient or population: continent women with POP Setting: hospital Intervention: vaginal or abdominal POP surgery with concomitant continence procedure Comparison: vaginal or abdominal POP surgery without concomitant continence procedure | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with vaginal or abdominal POP surgery without concomitant continence procedure | Risk with vaginal or abdominal POP surgery with concomitant continence procedure | ||||||
| Vaginal POP surgery with or without concomitant continence surgery: additional MUS vs vaginal repair alone | Subjective postoperative SUI | 407 per 1000 | 281 per 1000 (191 to 407) | RR 0.69 (0.47 to 1.00) | 220 (1 RCT) | ⊕⊕⊕⊝ Moderatea | |
| Recurrent POP on examination | No data available | ||||||
| Overactive bladder symptoms (cured/improved) | No data available | ||||||
| Voiding dysfunction difficulties | No data available | ||||||
| Further continence surgery | No data available | ||||||
| Abdominal POP surgery with or without concomitant continence surgery: additional Burch colposuspension vs sacrocolpopexy alone | Subjective postoperative SUI/de novo SUI 1‐year FU | 347 per 1000 | 455 per 1000 (66 to 1000) | RR 1.31 (0.19 to 9.01) | 379 (2 RCTs) | ⊕⊕⊝⊝ Lowb, c | |
| Recurrent POP on examination | 436 per 1000 | 427 per 1000 (323 to 567) | RR 0.98 (0.74 to 1.30) | 250 (1 RCT) | ⊕⊕⊕⊝ Moderatec | ||
| Overactive bladder symptoms (cured/improved) | No data available | ||||||
| Voiding dysfunction difficulties | 0 per 1000 | 0 per 1000 (0 to 0) | RR 8.49 (0.48 to 151.59) | 66 (1 RCT) | ⊕⊕⊝⊝ Lowd | ||
| Further continence surgery | No data available | ||||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FU: follow‐up; MUS: mid‐urethral sling; POP: pelvic organ prolapse; RCT: randomised controlled trial; RR: risk ratio; SUI: stress urinary incontinence. | |||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for serious indirectness ‐ women without SUI symptoms were included; some of them had occult SUI but results were presented separately.
bDowngraded one level ‐ studies showed diverging results with a high grade of heterogeneity.
cDowngraded one level due to imprecision ‐ wide confidence interval.
dDowngraded two levels for very serious imprecision ‐ very wide confidence interval.
Summary of findings 4. Vaginal POP surgery with armed mesh compared to anterior native tissue repair for continent women with POP.
| Vaginal POP surgery with armed mesh compared to anterior native tissue repair for continent women with POP | |||||||
| Patient or population: continent women with POP Setting: hospital Intervention: vaginal POP surgery with armed mesh Comparison: anterior native tissue repair | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | ||
| Risk with anterior native tissue repair | Risk with vaginal POP surgery with armed mesh | ||||||
| One type of POP surgery vs another: armed anterior mesh vs anterior native tissue repair | Subjective postoperative SUI | 73 per 1000 | 115 per 1000 (76 to 172) | RR 1.58 (1.05 to 2.37) | 905 (7 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Recurrent POP on examination | 475 per 1000 | 138 per 1000 (104 to 180) | RR 0.29 (0.22 to 0.38) | 848 (5 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| Overactive bladder symptoms (cured/improved) | No data available | ‐ | |||||
| Voiding dysfunction difficulties | 16 per 1000 | 27 per 1000 (4 to 195) | RR 1.65 (0.22 to 12.10) | 125 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| Further continence surgery | No data available | ||||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; POP: pelvic organ prolapse; RCT: randomised controlled trial; RR: risk ratio; SUI: stress urinary incontinence. | |||||||
| GRADE Working Group grades of evidence. High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded one level for serious risk of bias ‐ no blinding of outcome assessors.
bDowngraded one level for indirectness ‐ primary outcome was POP.
1 COMPARISONS OF SURGERY TO TREAT WOMEN WITH PELVIC ORGAN PROLAPSE AND SYMPTOMATIC STRESS URINARY INCONTINENCE
1.1 Vaginal POP surgery with vs without concomitant continence surgery
1.1.1 Additional mid‐urethral sling vs vaginal repair alone
Primary outcome
1.1.1.1 Women's observations: subjective postoperative stress urinary incontinence
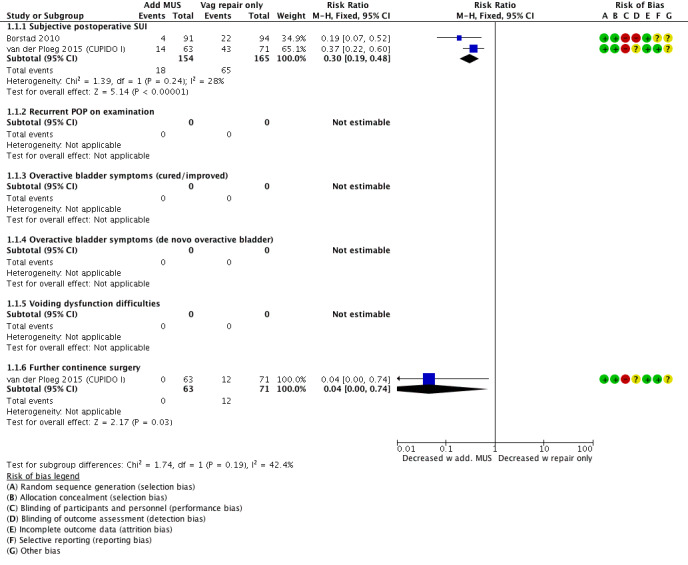
Fewer women reported postoperative stress urinary incontinence following concomitant mid‐urethral sling compared with vaginal repair alone; therefore a concomitant MUS probably improves postoperative rates of subjective SUI, as the evaluated clinical effect appears large (risk ratio (RR) 0.30, 95% confidence interval (CI) 0.19 to 0.48; 319 participants, two studies; I² = 28%; moderate‐quality evidence; Analysis 1.1;Figure 5Borstad 2010; van der Ploeg 2015 (CUPIDO I)). This suggests that if the risk of SUI with POP surgery alone is 39%, the risk with an MUS is between 8% and 19%.
1.1. Analysis.
Comparison 1 Comparisons of surgery in women with POP and SUI, Outcome 1 Vaginal POP surgery with vs without concomitant continence surgery: additional MUS vs vaginal repair alone.
5.
Forest plot of comparison: 1 Comparisons of surgery in women with POP and SUI, outcome: 1.1 Vaginal POP surgery with vs without concomitant continence surgery: additional MUS vs vaginal repair alone.
As Borstad 2010 was assessed as high risk in blinding patients (which was not possible given the trial design) while also blinding assessors, we performed the analysis without this study (RR 0.37, 95% CI 0.22 to 0.60; 134 participants, one study; van der Ploeg 2015 (CUPIDO I)), which did not markedly change the result. Also, employing a random‐effects model resulted in minimal changes.
Secondary outcomes
1.1.1.2 Clinician's observations: POP on examination or objective stress urinary incontinence
No data were available.
1.1.1.3 Associated pelvic floor symptoms
No data were available.
1.1.1.4 Surgical outcome measures
Further continence surgery
Further continence surgery was less likely in the group that had additional MUS; therefore a concomitant MUS may decrease the need for further continence surgery (RR 0.04, 95% CI 0.00 to 0.74; 134 participants, one study; Analysis 1.1; van der Ploeg 2015 (CUPIDO I)).
1.1.1.5 Complications
No data were available.
1.1.1.6 Economic measures
No data were available.
1.2 Vaginal POP surgery with concomitant vs delayed continence surgery
1.2.1 Vaginal POP surgery with concomitant vs delayed MUS
Primary outcome
1.2.1.1 Women's observations: subjective postoperative stress urinary incontinence
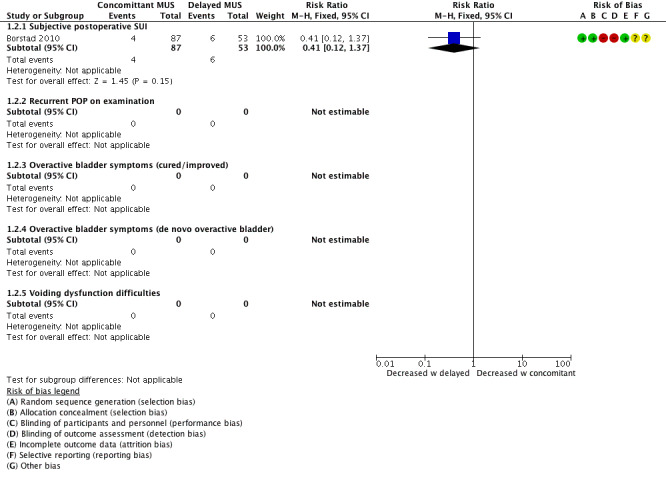
There appeared to be little or no difference between groups in reporting postoperative SUI (RR 0.41, 95% CI 0.12 to 1.37; 140 women, one study; Analysis 1.2;Figure 6; Borstad 2010). This suggests that if the risk of postoperative SUI with delayed MUS is 11%, then the risk with concomitant MUS would be between 1% and 16%, which is considered a clinically negligible effect.
1.2. Analysis.
Comparison 1 Comparisons of surgery in women with POP and SUI, Outcome 2 Vaginal POP surgery with concomitant vs delayed continence surgery: additional concomitant MUS vs delayed MUS.
6.
Forest plot of comparison: 1 Comparisons of surgery in women with POP and SUI, outcome: 1.2 Vaginal POP surgery with concomitant vs delayed continence surgery: additional concomitant MUS vs delayed MUS.
Secondary outcomes
1.2.1.2 Clinician's observations: POP on examination or objective stress urinary incontinence
No data were available.
1.2.1.3 Associated pelvic floor symptoms
No data were available.
1.2.1.4 Surgical outcome measures
No data were available.
1.2.1.5 Complications
No data were available.
1.2.1.6 Economic measures
No data were available.
1.3 Abdominal POP surgery with vs without concomitant continence surgery
1.3.1 Abdominal sacrocolpopexy with additional Burch colposuspension vs sacrocolpopexy alone
Primary outcome
1.3.1.1 Women's observations: subjective postoperative stress urinary incontinence
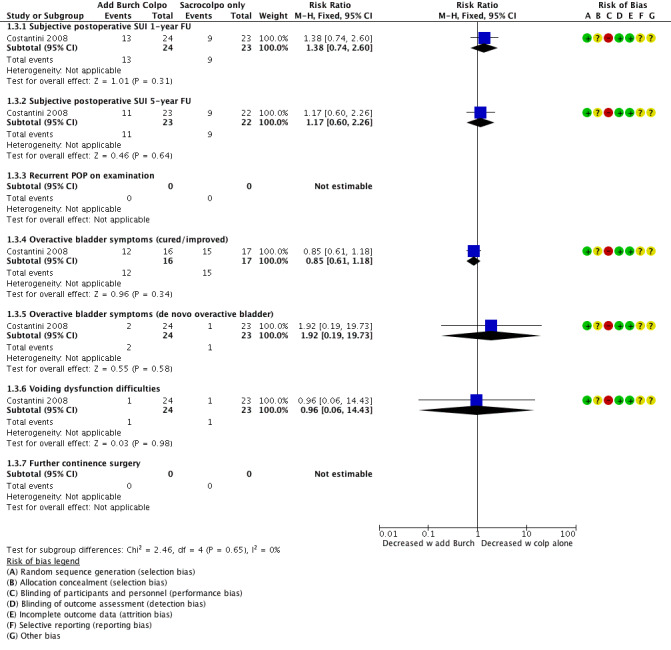
An additional Burch colposuspension may have little or no effect on postoperative SUI at one‐year follow‐up (RR 1.38, 95% CI 0.74 to 2.60; 47 women; Costantini 2008), or at five‐year follow‐up (RR 1.17, 95% CI 0.60 to 2.26; 45 women, one study; Analysis 1.3; Figure 7; Costantini 2008). This suggests that if the risk of postoperative SUI without additional Burch colposuspension is 39%, the risk with Burch colposuspension would be between 29% and 100%.
1.3. Analysis.
Comparison 1 Comparisons of surgery in women with POP and SUI, Outcome 3 Abdominal POP surgery with vs without concomitant continence surgery: additional Burch colpo vs sacrocolpopexy alone.
7.
Forest plot of comparison: 1 Comparisons of surgery in women with POP and SUI, outcome: 1.3 Abdominal POP surgery with vs without concomitant continence surgery: additional Burch colpo vs sacrocolpopexy alone.
Secondary outcomes
1.3.1.2 Clinician's observations: POP on examination or objective stress urinary incontinence
No data were available.
1.3.1.3 Associated pelvic floor symptoms
Overactive bladder symptoms
An additional Burch colposuspension may have little or no effect on the number of women with cured or improved symptoms (RR 0.85, 95% CI 0.61 to 1.18; 33 participants, one study) nor on the number of women with de novo overactive bladder (RR 1.92, 95% CI 0.19 to 19.73; 47 participants, one study; Analysis 1.3).
1.3.1.4 Surgical outcome measures
No data were available.
1.3.1.5 Complications
No data were available.
1.3.1.6 Economic measures
No data were available.
1.4 Abdominal POP surgery with different concomitant continence procedures
1.4.1 Abdominal sacrocolpopexy with MUS vs Burch colposuspension
Primary outcome
1.4.1.1 Women's observations: subjective postoperative stress urinary incontinence
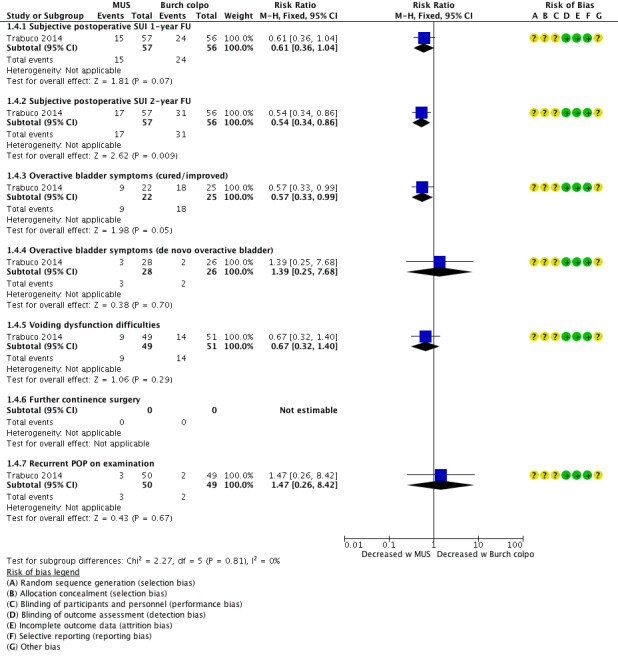
There was probably little or no difference in postoperative subjective SUI between groups at one‐year follow‐up (RR 0.61, 95% CI 0.36 to 1.04; 113 women, 1 study; Analysis 1.4;Figure 8; Trabuco 2014).
1.4. Analysis.
Comparison 1 Comparisons of surgery in women with POP and SUI, Outcome 4 Abdominal POP surgery with different concomitant continence procedures: additional MUS vs Burch colpo at sacral colpopexy.
8.
Forest plot of comparison: 1 Comparisons of surgery in women with POP and SUI, outcome: 1.4 Abdominal POP surgery with different concomitant continence procedures: additional MUS vs Burch colpo at sacral colpopexy.
However, at two years postoperatively, an additional MUS probably reduced postoperative SUI compared with a concomitant Burch colposuspension (RR 0.57, 95% CI 0.33 to 0.99; 113 women, one study; Analysis 1.4).
Secondary outcomes
1.4.1.2 Clinician's observations: POP on examination
Additional MUS or Burch colposuspension may have little or no effect on postoperative POP on examination (RR 1.47, 95% CI 0.26 to 8.42; 99 women; Analysis 1.4; Trabuco 2014).
1.4.1.3 Associated pelvic floor symptoms
Overactive bladder symptoms
Fewer women in the MUS group reported cured or improved symptoms; therefore the MUS in addition to a sacrocolpopexy may slightly reduce postoperative OAB symptoms (RR 0.57, 95% CI 0.33 to 0.99; 47 participants, one study; Analysis 1.4).
There was probably little or no difference in the number of women with de novo overactive bladder (RR 1.39, 95% CI 0.25 to 7.68; 54 participants, one study; Analysis 1.4).
Voiding dysfunction
There was probably little or no effect of MUS or Burch colposuspension on postoperative voiding dysfunction (RR 0.67, 95% CI 0.32 to 1.40; 100 participants, one study; Analysis 1.4).
1.4.1.4 Surgical outcome measures
No data were available.
1.4.1.5 Complications
No data were available.
1.4.1.6 Economic measures
No data were available.
1.5 One type of POP/continence surgery vs another type of POP surgery
1.5.1 Abdominal continence surgery (Burch colposuspension) vs vaginal POP surgery (anterior repair)
One small trial compared Burch colposuspension versus anterior colporrhaphy to treat women with SUI and cystocoele (Colombo 2000). Although a Burch colposuspension is primarily considered as continence surgery, we include it here as it also addresses anterior vaginal wall prolapse, especially if a cystocoele is caused by paravaginal support defects.
Primary outcome
1.5.1.1 Women's observations: subjective postoperative stress urinary incontinence
Fewer women reported postoperative stress urinary incontinence following Burch colposuspension compared with anterior colporrhaphy; therefore a Burch colposuspension may improve postoperative SUI rates (RR 0.29, 95% CI 0.12 to 0.71; 68 women, one study; Analysis 1.5, Figure 9; Colombo 2000).
1.5. Analysis.
Comparison 1 Comparisons of surgery in women with POP and SUI, Outcome 5 Abdominal continence surgery vs vaginal POP surgery: Burch colpo vs anterior repair.
9.
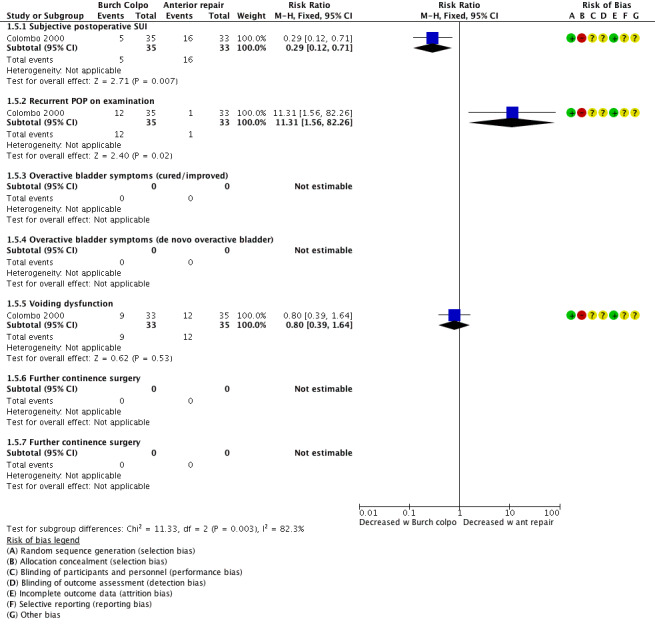
Forest plot of comparison: 1 Comparisons of surgery in women with POP and SUI, outcome: 1.5 Abdominal continence surgery vs vaginal POP surgery: Burch colpo vs anterior repair.
Secondary outcomes
1.5.1.2 Clinician's observations: POP on examination
Women who underwent Burch colposuspension were more likely to have recurrent POP on examination than women who had anterior colporrhaphy; therefore an anterior native tissue repair may improve postoperative POP (RR 11.31, 95% CI 1.56 to 82.26; 68 women, one study; Analysis 1.5; Colombo 2000).
1.5.1.3 Associated pelvic floor symptoms
Voiding dysfunction
Burch colposuspension or anterior colporrhaphy may have little or no effect on postoperative voiding function (RR 0.80, 95% CI 0.39 to 1.64; 68 participants, one study; Analysis 1.5; Colombo 2000).
1.5.1.4 Surgical outcome measures
No data were available.
1.5.1.5 Complications
No data were available.
1.5.1.6 Economic measures
No data were available.
2. COMPARISONS OF SURGERY TO TREAT WOMEN WITH POP AND OCCULT SUI
All studies in this population compared POP surgery with a concomitant continence procedure versus POP surgery alone.
2.1 Vaginal POP surgery with vs without concomitant continence surgery
2.1.1 Vaginal POP surgery with an additional MUS vs vaginal repair alone
Primary outcome
2.1.1.1 Women's observations: subjective postoperative stress urinary incontinence
Rates of subjective postoperative SUI were lower in the group receiving a concurrent sub‐urethral sling; therefore a concomitant MUS probably improves postoperative subjective SUI rates (RR 0.38, 95% CI 0.26 to 0.55; 369 participants, five studies; I² = 44%, moderate‐quality evidence; Analysis 2.1;Figure 10). This suggests that if the risk with surgery alone is 34%, the risk with a concomitant MUS is between 10% and 22%.
2.1. Analysis.
Comparison 2 Comparisons of surgery in women with POP and occult SUI, Outcome 1 Vaginal POP surgery with or without concomitant continence surgery: additional MUS vs vaginal repair alone.
10.
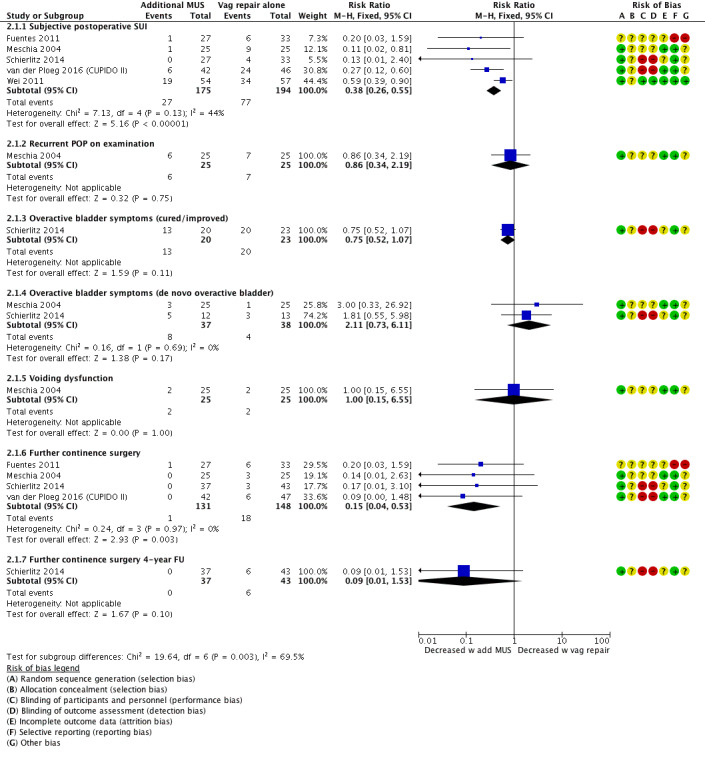
Forest plot of comparison: 2 Comparisons of surgery in women with POP and occult SUI, outcome: 2.1 Vaginal POP surgery with or without concomitant continence surgery: additional MUS vs vaginal repair alone.
As all studies were assessed as having unclear risk of allocation concealment, we could not perform the prespecified sensitivity analysis as all studies would have to be excluded. The random‐effects model showed a marginal difference from the fixed‐effect model (RR 0.33, 95% CI 0.17 to 0.66). We conclude that despite only moderate‐quality evidence, an additional concomitant MUS leads to a large clinical effect and benefit.
Secondary outcomes
2.1.1.2 Clinician's observations: POP on examination
Recurrent POP on examination was not different between groups in one study, implying that there may be little or no difference in postoperative POP (RR 0.86, 95% CI 0.34 to 2.19; 50 participants, one study; low‐quality evidence; Analysis 2.1).
2.1.1.3 Associated pelvic floor symptoms
Overactive bladder symptoms
There is probably little or no difference between groups in rates of cured or improved overactive bladder (RR 0.75, 95% CI 0.52 to 1.07; 43 participants, one study; low‐quality evidence; Analysis 2.1) or in the number of women with de novo overactive bladder (RR 2.11, 95% CI 0.73 to 6.11; 75 participants, two studies; I² = 0%, moderate‐quality evidence; Analysis 2.1).
Voiding dysfunction
Additional MUS may have little or no effect on postoperative voiding function (RR 1.00, 95% CI 0.15 to 6.55; 50 participants, one study; low‐quality evidence; Analysis 2.1).
2.1.1.4 Surgical outcome measures
Rates of further continence surgery were lower in the group receiving additional MUS; therefore the additional MUS probably reduces the need for further continence surgery (RR 0.15, 95% CI 0.04 to 0.53; 279 participants, four studies; I² = 0%; moderate‐quality evidence; Analysis 2.1). At four‐year follow‐up in one single study, the additional MUS may have had little or no effect on further continence surgery, although the clinical effect was of moderate size, at 15% difference (RR 0.09, 95% CI 0.01 to 1.53; 80 participants, one study; Analysis 2.1).
2.1.1.5 Complications
No data were available.
2.1.1.6 Economic measures
No data were available.
3. COMPARISONS OF SURGERY IN CONTINENT WOMEN WITH POP
3.1 Vaginal POP surgery with vs without concomitant continence surgery
3.1.1 Additional MUS vs vaginal repair only
Primary outcome
3.1.1.1 Women's observations: subjective postoperative stress urinary incontinence
Prophylactic MUS may have little or no effect on reducing postoperative de novo SUI based on low clinical treatment effect of 11% (RR 0.69, 95% CI 0.47 to 1.00; 220 participants, one study; moderate‐quality evidence; Analysis 3.1;Figure 11). This suggests that if the risk with surgery alone is 40%, the risk with a concomitant MUS is between 19% and 40%.
3.1. Analysis.
Comparison 3 Comparisons of surgery in continent women with POP, Outcome 1 Vaginal POP surgery with or without concomitant continence surgery: additional MUS vs vaginal repair alone.
11.
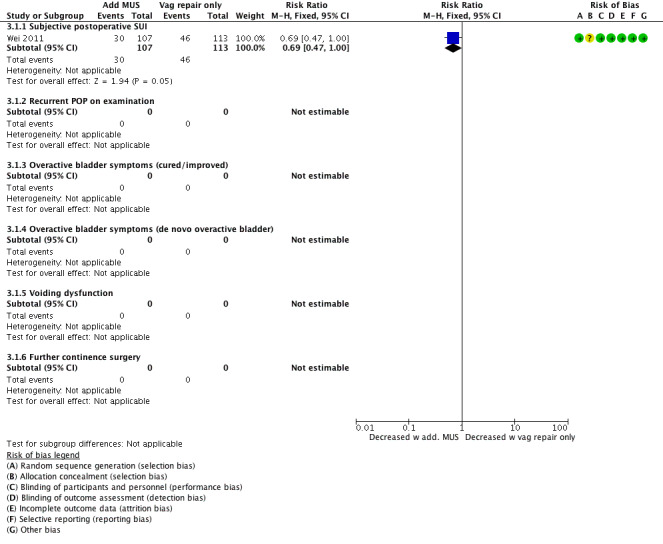
Forest plot of comparison: 3 Comparisons of surgery in continent women with POP, outcome: 3.1 Vaginal POP surgery with or without concomitant continence surgery: additional MUS vs vaginal repair alone.
Secondary outcomes
No data were available on any of our secondary outcomes.
3.2 Abdominal POP surgery with vs without a concomitant continence procedure
3.2.1 Abdominal sacrocolpopexy with additional Burch colposuspension vs sacrocolpopexy alone
Primary outcome
3.2.1.1 Women's observations: subjective postoperative stress urinary incontinence
Additional Burch colposuspension at the time of sacrocolpopexy probably has little or no effect on subjective postoperative SUI at one‐year follow‐up (RR 1.31, 95% CI 0.19 to 9.01; 379 participants, two studies; I² = 90%; low‐quality evidence; Analysis 3.2;Figure 12) and at least two‐year follow‐up (RR 0.96, 95% CI 0.35 to 2.62; 364 participants, two studies; I² = 75%; moderate‐quality evidence; Analysis 3.2). Because of the high I² value, we used a random‐effects model for analysis. Study results were divergent, and one study was at moderate risk of bias. As prespecified, the sensitivity analysis without Costantini 2007 showed that the additional Burch colposuspension reduces postoperative rates of de novo SUI (RR 0.53, 95% CI 0.37 to 0.76; 313 participants, one study; moderate‐quality evidence).
3.2. Analysis.
Comparison 3 Comparisons of surgery in continent women with POP, Outcome 2 Abdominal POP surgery with or without concomitant continence surgery: additional Burch colpo vs sacral colpopexy alone.
12.
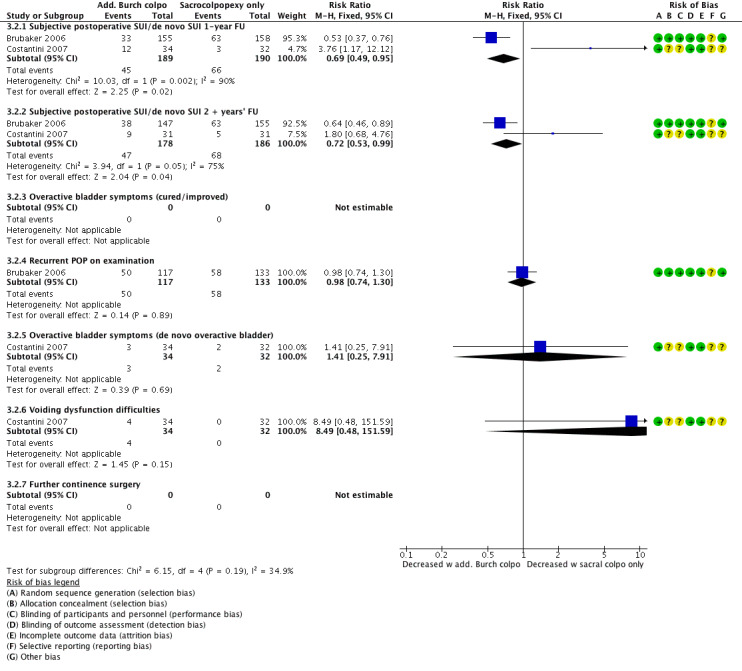
Forest plot of comparison: 3 Comparisons of surgery in continent women with POP, outcome: 3.2 Abdominal POP surgery with or without concomitant continence surgery: additional Burch colpo vs sacral colpopexy alone.
Secondary outcomes
3.2.1.2 Clinician's observations: POP on examination
There was little or no difference in recurrent POP on examination in one trial (RR 0.98, 95% CI 0.74 to 1.30; 250 participants, one study; moderate‐quality evidence; Analysis 3.2).
3.2.1.3 Associated pelvic floor symptoms
Overactive bladder symptoms
There was little or no effect on postoperative rates of de novo overactive bladder (RR 1.41, 95% CI 0.25 to 7.91; 66 participants, one study; moderate‐quality evidence; Analysis 3.2).
Voiding dysfunction
There was little or no effect on postoperative voiding dysfunction (RR 8.49, 95% CI 0.48 to 151.59; 66 participants, one study; low‐quality evidence; Analysis 3.2).
Pelvic‐floor related quality of life measures
There was little or no difference between groups in symptoms measured using the UDI (mean difference (MD) ‐10.70, 95% CI ‐20.56 to ‐0.84; 194 participants, one study; I² = 0%; moderate‐quality evidence; Analysis 3.3) or the PISQ questionnaire (MD ‐0.10, 95% CI ‐1.58 to 1.38; 194 participants, one study; moderate‐quality evidence; Analysis 3.3;Figure 13).
3.3. Analysis.
Comparison 3 Comparisons of surgery in continent women with POP, Outcome 3 Additional Burch colpo vs sacrocolpopexy alone: QoL data.
13.
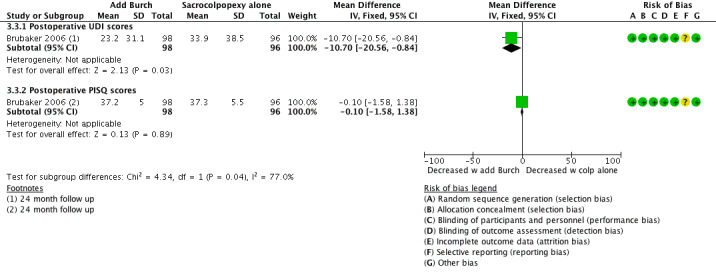
Forest plot of comparison: 3 Comparisons of surgery in continent women with POP, outcome: 3.3 Additional Burch colpo vs sacrocolpopexy alone: QoL data.
3.2.1.4 Surgical outcome measures
No data were available.
3.2.1.5 Complications
No data were available.
3.2.1.6 Economic measures
No data were available.
3.3 One type of POP surgery vs another type of POP surgery
3.3.1 Armed anterior vaginal mesh repair vs anterior native tissue repair
Primary outcome
3.3.1.1 Women's observations: subjective postoperative stress urinary incontinence
Evidence suggests that SUI develops more frequently after anterior vaginal mesh than after anterior repair, implying that anterior mesh repair probably increases postoperative de novo SUI (RR 1.58, 95% CI 1.05 to 2.37; 905 participants, seven studies; I² = 0%; low‐quality evidence; Analysis 3.4;Figure 14). At two‐ to three‐year follow‐up of two studies, this result was maintained (RR 1.88, 95% CI 1.01 to 3.49; 289 participants, two studies; low‐quality evidence; Analysis 3.4).
3.4. Analysis.
Comparison 3 Comparisons of surgery in continent women with POP, Outcome 4 One type of POP surgery vs another: armed anterior mesh vs anterior native repair.
14.
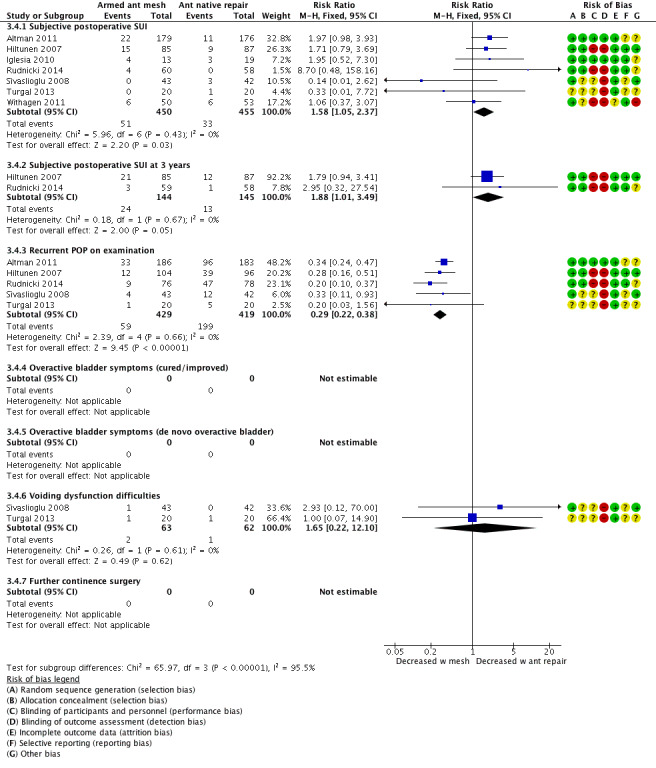
Forest plot of comparison: 3 Comparisons of surgery in continent women with POP, outcome: 3.4 One type of POP surgery vs another: armed anterior mesh vs anterior native repair.
We conducted a prespecified sensitivity analysis to test this result by removing studies that were not at low risk with respect to sequence generation and allocation concealment, and were at high risk in any domain. In this analysis, two studies remained (RR 1.96, 95% CI 1.06 to 3.64; 387 participants, two studies; I² = 0%), but the direction of effect remained the same.
We also tested the effect estimate using a random‐effects model and found that changes were minimal. From this, we conclude that an anterior native tissue repair probably reduces postoperative SUI rates; however, the clinical size of the effect was small at only 4% at one‐year follow‐up and 8% at three‐year follow‐up.
Secondary outcomes
3.3.1.2 Clinician's observations: recurrent POP on examination
Armed mesh implants probably reduce recurrent POP on examination (RR 0.29, 95% CI 0.22 to 0.38; 848 participants, five studies; I² = 0%; low‐quality evidence; Analysis 3.4). We conducted the sensitivity analysis as described in 3.3.1.1, with only one remaining study (RR 0.34, 95% CI 0.24 to 0.47; 369 participants, one study), and the direction of effect remained the same.
3.3.1.3 Associated pelvic floor symptoms
Voiding dysfunction difficulties
There may be little or no difference between groups regarding postoperative voiding dysfunction (RR 1.65, 95% CI 0.22 to 12.10; 125 participants, two studies; I² = 0%; low‐quality evidence; Analysis 3.4).
3.3.1.4 Surgical outcome measures
No data were available.
3.3.1.5 Complications
No data were available.
3.3.1.6 Economic measures
No data were available.
Discussion
Summary of main results
Surgery to treat women with pelvic organ prolapse (POP) and symptomatic stress urinary incontinence
Few trials assessed the outcome of pelvic organ prolapse (POP) surgery with or without concomitant continence surgery in women with POP and stress urinary incontinence (SUI), and conclusions are based on rather small studies.
An additional concomitant mid‐urethral sling procedure at the time of vaginal POP repair significantly reduced postoperative SUI in two studies. Mid‐urethral sling insertion might also be delayed three months after surgery (two‐stage POP‐continence surgery), resulting in similar SUI rates, but some women declined the subsequent delayed continence operation.
One trial did not demonstrate any benefit of an additional Burch colposuspension at the time of abdominal sacrocolpopexy or hysteropexy. When a concomitant continence procedure was planned, in one trial a mid‐urethral sling achieved better results than a Burch colposuspension in women undergoing sacrocolpopexy.
A Burch colposuspension was superior to an anterior repair with regard to SUI and is now considered a continence procedure with limited effect on anterior vaginal wall prolapse, whereas an anterior colporrhaphy is predominantly an operation to treat anterior vaginal wall prolapse.
Surgery to treat women with POP and occult stress urinary incontinence
Women with POP and occult SUI might benefit from a concurrent mid‐urethral sling during vaginal POP surgery.
Surgery to treat continent women with POP
In continent women with symptomatic POP, an anterior vaginal repair proved better than anterior armed mesh regarding de novo SUI postoperatively. However, anterior vaginal mesh placement reduced recurrent POP significantly.
An additional suburethral tape during vaginal POP surgery does not necessarily prevent postoperative SUI. Similarly, during abdominal sacrocolpopexy, an additional Burch colposuspension might not reduce de novo SUI.
Overall completeness and applicability of evidence
We included randomised controlled trials that included continent or stress urinary incontinent women with symptomatic POP and addressed continence issues among their aims. Our defined comparisons followed clinical needs discussed when counselling women: which POP operation should be performed, and should a prophylactic or therapeutic continence procedure be performed in women with POP and SUI or occult SUI or no SUI. Unfortunately, many studies did not include continent or incontinent patient populations and had to be excluded. In contrast, many included studies focused on continence issues and did not present prolapse outcomes.
Quality of the evidence
The quality of the evidence ranged from low to moderate according to GRADE assessment. The main limitations in the quality of the evidence were risk of bias, indirectness when a study had a different focus on outcome measures than our review, and imprecision associated with low event rates and small samples. Whereas blinding of participants and staff was not feasible in many trials, we considered non‐blinding of outcome assessors as high risk and specifically downgraded those studies.
Generally, the validity of these studies seems to have improved, with more trials conforming to CONSORT statements and using validated patient‐centred outcome measures and questionnaires. However, owing to different definitions and inclusion criteria, few meta‐analyses could be performed.
Potential biases in the review process
We are not aware of any biases in the review process. All review authors have been co‐authors on associated reviews within the group in the past. Regarding publication bias, we took specific care to ensure that all ancillary reports on the same patient populations, as well as publication of long‐term results, were estimated as supplementary material. As the literature search included all studies on any POP surgery, we excluded numerous studies based on patient populations, interventions, or comparisons not meeting our inclusion criteria. This might be considered a potential risk for reporting bias. As the aim of this review was to determine effects of POP surgery in clearly distinguishable preoperatively continent or stress urinary incontinent women with POP, we had to exclude studies that failed to include these populations.
Agreements and disagreements with other studies or reviews
Our main results are in concordance with those of a systematic review of randomised controlled trials conducted by Matsuoka (Matsuoka 2015). However, this review analysed all continence procedures together (Burch colposuspension and MUS), which appears difficult from a clinical point of view. Typically, during a vaginal POP repair, a concomitant mid‐urethral sling would be performed (or not), rather than an abdominal Burch colposuspension.
Authors' conclusions
Implications for practice.
In women with POP and SUI (symptomatic or occult), a concurrent MUS probably reduces postoperative SUI and should be discussed during counselling. It might be feasible to postpone the MUS and perform a two‐stage continence procedure.
Although an abdominal continence procedure (Burch colposuspension) during abdominal POP surgery in continent women reduced de novo SUI rates in one underpowered trial, another RCT reported conflicting results. Adding an MUS during vaginal POP repair might slightly reduce the postoperative development of SUI.
An anterior native tissue repair might be better than transobturator mesh for preventing postoperative SUI; however, prolapse recurrence is more common with native tissue repair.
Implications for research.
Apart from emphasising the need for improved methods and reporting of results, further studies should address the concurrent treatment of SUI in women with POP in clearly distinguishable populations of continent or stress urinary incontinent women or women with occult SUI on examination. Future research should also assess the impact of different POP surgeries on bladder function among patient populations with or without SUI.
What's new
| Date | Event | Description |
|---|---|---|
| 26 April 2018 | New citation required and conclusions have changed | The addition of 5 new trials has led to a change to the conclusions of this review. |
| 26 April 2018 | New search has been performed | Comparison of interventions for management of stress urinary incontinence was formerly part of the 2013 Cochrane review titled "Surgical management of pelvic organ prolapse in women". We now present this as a separate review. Five new trials are included that were not included in the previous review: Rudnicki 2014; Trabuco 2014; Turgal 2013; van der Ploeg 2015 (CUPIDO I); van der Ploeg 2016 (CUPIDO II). |
History
Review first published: Issue 8, 2018
| Date | Event | Description |
|---|---|---|
| 29 January 2013 | New citation required but conclusions have not changed | Review updated incorporating 16 new trials |
| 29 January 2013 | New search has been performed | Review updated incorporating 16 new trials |
| 14 April 2010 | Amended | Changed citation; added conflicts |
| 17 November 2009 | New citation required but conclusions have not changed | Full reports of 59 potentially eligible studies were assessed; for this update, 23 new eligible studies were assessed (Al‐Nazer 2007a; Ali 2006a; Allahdin 2008; Barber 2006; Biller 2008; Borstad 2008; Braun 2007a; Carramao 2008a; Constantini 2008; de Tayrac 2008; Dietz 2008a; Glavind 2007; Guerette 2006a; Lim 2007a; Meschia 2007a; Natale 2007; Natale 2009; Nguyen 2008; Nieminen 2008; Pantazis 2008a; Schierlitz 2007a; Segal 2007; Sivaslioglu 2008). Overall, 17 studies were excluded from the review, six during this update (Barber 2006; Biller 2008; Carramao 2008a; Glavind 2007; Meschia 2007a; Segal 2007): full details are given in the "Characteristics of excluded studies" table. In this, the second update, 18 new trials were added (Al‐Nazer 2007; Ali 2006; Allahdin 2008; Borstad 2008; Braun 2007a; Constantini 2007; Constantini 2008; de Tayrac 2008; Dietz 2008a; Guerette 2006; Lim 2007; Natale 2007; Natale 2009; Nguyen 2008; Nieminen 2008; Pantazis 2008; Schierlitz 2007; Sivaslioglu 2008) and 3 previously included studies were updated (Brubaker 2008; Meschia 2007; Roovers 2004). |
| 9 February 2009 | New search has been performed | New search February 2009 |
| 10 October 2008 | Amended | Converted to new review format |
| 17 April 2007 | New citation required and conclusions have changed | Substantive Update, Issue 3, 2007. 22 RCTs (8 new included trials). Findings are still insufficient to provide robust evidence to support current and new practice (such as whether to perform a concurrent continence operation, or to use mesh or grafts). |
Acknowledgements
We acknowledge the work of Elisabeth J Adams and Suzanne Hagen as co authors on the original review, and Charis Glazener as co‐author on the original POP reviews and update.
The authors of the 2018 update would like to thank Sheila Wallace, Information Specialist for the Cochrane Incontinence Review Group, for designing the search strategy and running the searches for this review.
Appendices
Appendix 1. Types of operations
Sacral colpopexy
Aim To correct upper genital tract prolapse
Indication Usually reserved for recurrent prolapse of the upper vagina (recurrent cystocoele, vault, or enterocoele) or massive vaginal eversion
Surgical technique
Usually performed under general anaesthesia
Performed through an incision on the lower abdomen or keyhole
The bladder and rectum are freed from the vagina and permanent mesh supports the front and back wall of the vagina
This mesh is secured to the sacrum (upper tailbone)
Peritoneum (lining of the abdominal cavity) is closed over the mesh
Other repairs are performed as required at the same time including paravaginal repair, perineoplasty, colposuspension, or rectopexy
Bowel preparation is required before surgery
McCall culdoplasty
Indications
Vault prolapse or an enterocoele
Often performed at the time of vaginal hysterectomy to prevent future prolapse
Surgical technique
After the uterus is removed at the time of hysterectomy, the uterosacral ligaments are identified and incorporated into the closure of the peritoneum and upper vagina using 1 to 2 sutures
An anterior or posterior vaginal repair is often performed at the same time
Sacrospinous fixation
Aim This surgery offers support to the upper vagina, minimising risk of recurrent prolapse at this site. The advantage of this surgery is that vaginal length is maintained.
Indication Upper vaginal prolapse (uterine or vault prolapse, enterocoeles)
This procedure can be used in reconstructive vaginal surgery in which increased vaginal length is required.
Procedure
The procedure can be performed under regional or general anaesthesia
A routine posterior vaginal incision is made and is extended to the top of the vagina
Through sharp dissection, the vagina is freed from the underlying rectovaginal fascia and rectum until the pelvic floor (puborectalis) muscle is seen
Through sharp and blunt dissection, the sacrospinous ligament running from the ischial spine to the sacral bone is palpated and identified
Two sutures are placed through the strong ligament and are secured to the top of the vagina. This results in increased support to the upper vagina. There is no shortening of the vagina
Other fascial defects in the vagina are repaired and the vaginal skin is closed
Anterior vaginal repair (colporrhaphy)
Indication
Prolapse of the bladder or urethra
Sometimes used to treat urinary stress incontinence
Surgical technique
The procedure can be performed under regional or general anaesthesia
The vagina overlying the bladder and urethra is incised in the midline
Dissection in a plane directly below the vagina allows the damaged fascia supporting the bladder and urethra to be exposed
The fascia is plicated in the midline using delayed absorbable or permanent sutures
Sometimes excessive vaginal skin is removed
The vaginal skin is then closed
Other sites of prolapse are then repaired as required
Posterior vaginal repair and perineoplasty
Indications Treatment of rectocoele (rectum bulges or herniates forward into the vagina) and defects of the perineum (area separating entrance of the vagina and anus)
Aim Correct defects in the rectovaginal fascia separating rectum and vagina while allowing bowel function to be maintained or corrected without interfering with sexual function
Surgical technique
An incision is made on the posterior wall of the vagina starting at the entrance and finishing at the top of the vagina
Dissecting the vagina and rectovaginal fascia from the vagina until the pelvic floor muscles (puborectalis) are located
Defects in the fascia are corrected by centrally plicating the fascia using delayed absorption sutures
The perineal defects are repaired by placing deep sutures into the perineal muscles to build up the perineal body
The overlying vaginal and vulval skin is then closed
A pack is usually placed into the vagina and a catheter into the bladder at the end of surgery
Anterior or posterior vaginal repair, or both (colporrhaphy)
Indications
Anterior repair: treatment for prolapse of bladder (bladder bulges forward into the vagina; cystocoele) or urethra.
Posterior repair: correction of bowel prolapse (rectum bulges forward into the vagina; rectocoele).
Vault repair: treat prolapse of upper vagina.
Depending on the side of the defect, the repair can be anterior, posterior, vault, or total. The repair is achieved by the placement of permanent mesh that may result in a stronger repair.
Surgical technique
The procedure can be performed under regional or general anaesthesia. Anterior vaginal repair
Midline incision to the vagina overlying the bladder and urethra
Dissection in a plane directly below the vagina and lateral of the bladder allows the damaged fascia supporting the bladder to be exposed
The fascia is plicated in the midline using sutures
Mesh can be used to reinforce the repair and can be used as an inlay, or anchored through the obturator foramen and exiting through small incisions at both sides of the upper inner thigh
The vaginal skin is closed
Posterior and vault repair
An incision is made to the posterior wall of the vagina
Dissection below the vagina identifies the rectovaginal fascia and opens the space between the rectum and the pelvic floor muscle to the sacrospinous ligaments
Defects in the fascia are corrected by centrally plicating the fascia using sutures
Mesh can be used to reinforce the repair and can be used as an inlay or anchored bilaterally to the pelvic side wall and exiting through a small incision approximately 3 cm lateral and down from the anus
The vaginal skin is then closed
Vaginal paravaginal repair
Aim: the objective of this surgery is to reattach detached lateral vaginal fascia to its normal point of insertion on the lateral side wall. This firm area of attachment is termed the white line or arcus tendineus fascia pelvis.
Indication The repair of anterior wall prolapse due to defects of the lateral supporting tissues.
Procedure The procedure can be performed under regional or general anaesthesia
Routine anterior repair The sharp dissection of the vagina from the bladder fascia continues laterally until the pelvic side wall can be identified.
Permanent or delayed absorbable sutures are placed from the lateral vagina to the firm pelvic side wall tissue (white line or arcus tendineus fascia pelvis). Three to four sutures are placed on each side.
A routine anterior repair with midline plication of the fascia, trimming of excess vaginal skin as required, and closure of the vaginal skin.
Data and analyses
Comparison 1. Comparisons of surgery in women with POP and SUI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal POP surgery with vs without concomitant continence surgery: additional MUS vs vaginal repair alone | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Subjective postoperative SUI | 2 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.19, 0.48] |
| 1.2 Recurrent POP on examination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Overactive bladder symptoms (cured/improved) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Overactive bladder symptoms (de novo overactive bladder) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Voiding dysfunction difficulties | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Further continence surgery | 1 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.74] |
| 2 Vaginal POP surgery with concomitant vs delayed continence surgery: additional concomitant MUS vs delayed MUS | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Subjective postoperative SUI | 1 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.12, 1.37] |
| 2.2 Recurrent POP on examination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Overactive bladder symptoms (cured/improved) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Overactive bladder symptoms (de novo overactive bladder) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Voiding dysfunction difficulties | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Abdominal POP surgery with vs without concomitant continence surgery: additional Burch colpo vs sacrocolpopexy alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Subjective postoperative SUI 1‐year FU | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.74, 2.60] |
| 3.2 Subjective postoperative SUI 5‐year FU | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.60, 2.26] |
| 3.3 Recurrent POP on examination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.4 Overactive bladder symptoms (cured/improved) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 3.5 Overactive bladder symptoms (de novo overactive bladder) | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.19, 19.73] |
| 3.6 Voiding dysfunction difficulties | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.06, 14.43] |
| 3.7 Further continence surgery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Abdominal POP surgery with different concomitant continence procedures: additional MUS vs Burch colpo at sacral colpopexy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Subjective postoperative SUI 1‐year FU | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.36, 1.04] |
| 4.2 Subjective postoperative SUI 2‐year FU | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.34, 0.86] |
| 4.3 Overactive bladder symptoms (cured/improved) | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 0.99] |
| 4.4 Overactive bladder symptoms (de novo overactive bladder) | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.25, 7.68] |
| 4.5 Voiding dysfunction difficulties | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.32, 1.40] |
| 4.6 Further continence surgery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Recurrent POP on examination | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.26, 8.42] |
| 5 Abdominal continence surgery vs vaginal POP surgery: Burch colpo vs anterior repair | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Subjective postoperative SUI | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.12, 0.71] |
| 5.2 Recurrent POP on examination | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.31 [1.56, 82.26] |
| 5.3 Overactive bladder symptoms (cured/improved) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 Overactive bladder symptoms (de novo overactive bladder) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.5 Voiding dysfunction | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.39, 1.64] |
| 5.6 Further continence surgery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.7 Further continence surgery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Comparisons of surgery in women with POP and occult SUI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal POP surgery with or without concomitant continence surgery: additional MUS vs vaginal repair alone | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Subjective postoperative SUI | 5 | 369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.26, 0.55] |
| 1.2 Recurrent POP on examination | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.34, 2.19] |
| 1.3 Overactive bladder symptoms (cured/improved) | 1 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.52, 1.07] |
| 1.4 Overactive bladder symptoms (de novo overactive bladder) | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.73, 6.11] |
| 1.5 Voiding dysfunction | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.55] |
| 1.6 Further continence surgery | 4 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.04, 0.53] |
| 1.7 Further continence surgery 4‐year FU | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.53] |
Comparison 3. Comparisons of surgery in continent women with POP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal POP surgery with or without concomitant continence surgery: additional MUS vs vaginal repair alone | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Subjective postoperative SUI | 1 | 220 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.47, 1.00] |
| 1.2 Recurrent POP on examination | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Overactive bladder symptoms (cured/improved) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Overactive bladder symptoms (de novo overactive bladder) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Voiding dysfunction | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Further continence surgery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Abdominal POP surgery with or without concomitant continence surgery: additional Burch colpo vs sacral colpopexy alone | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Subjective postoperative SUI/de novo SUI 1‐year FU | 2 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.49, 0.95] |
| 2.2 Subjective postoperative SUI/de novo SUI 2 + years' FU | 2 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.53, 0.99] |
| 2.3 Overactive bladder symptoms (cured/improved) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Recurrent POP on examination | 1 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.74, 1.30] |
| 2.5 Overactive bladder symptoms (de novo overactive bladder) | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.25, 7.91] |
| 2.6 Voiding dysfunction difficulties | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.49 [0.48, 151.59] |
| 2.7 Further continence surgery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Additional Burch colpo vs sacrocolpopexy alone: QoL data | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Postoperative UDI scores | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐10.7 [‐20.56, ‐0.84] |
| 3.2 Postoperative PISQ scores | 1 | 194 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.58, 1.38] |
| 4 One type of POP surgery vs another: armed anterior mesh vs anterior native repair | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Subjective postoperative SUI | 7 | 905 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.05, 2.37] |
| 4.2 Subjective postoperative SUI at 3 years | 2 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.01, 3.49] |
| 4.3 Recurrent POP on examination | 5 | 848 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.22, 0.38] |
| 4.4 Overactive bladder symptoms (cured/improved) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Overactive bladder symptoms (de novo overactive bladder) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.6 Voiding dysfunction difficulties | 2 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.22, 12.10] |
| 4.7 Further continence surgery | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altman 2011.
| Methods | Trial design: multi‐centre, parallel‐group, randomised controlled trial involving 58 surgeons at 53 centres | |
| Participants | Number of participants randomised: 410 (transvaginal mesh = 206, colporrhaphy = 204) Number of participants analysed: 389 (transvaginal mesh = 200, colporrhaphy = 189) Mean age (mean ± SD): transvaginal mesh = 64.3 ± 9.8, colporrhaphy = 65.1 ± 9.8 Inclusion criteria: > 18 years, ≥ stage 2 symptomatic cystocoele POPQ Exclusion criteria: previous cancer of any pelvic organ, systemic glucocorticoid treatment, insulin‐treated diabetes, an inability to participate or to provide consent, need for concomitant surgery Setting: hospitals throughout Sweden, Norway, Finland, and Denmark Timing: December 2007 to December 2008 |
|
| Interventions | Intervention: Gynecare transvaginal anterior mesh (Prolift), absorbable sutures, excessive vaginal trimming discouraged, catheter care discretion surgeon (191 underwent surgery as assigned) Comparison: anterior colporrhaphy slow absorption monofilament thread, sham skin markings, excessive trimming vagina discouraged (182 underwent surgery as assigned) Follow‐up at 2 and 12 months |
|
| Outcomes |
Primary outcome: a composite measure defined as POPQ stage 0 or 1 of the anterior vaginal wall (i.e. point Ba of the anterior vaginal wall positioned more than 1 cm above the hymen) + the answer ”no” to the question on vaginal bulging (item 16 of the Urogenidal Distress Inventory (UDI)) Secondary outcomes: individual components of the primary outcome (Ba < ‐1 on POPQ, Q16 on UDI‐ve, surgical complications, adverse events, patient‐reported UDI (compared to baseline at 2 months and 1 year post surgery), sexual function as measured on the PISQ‐12 questionnaire (compared to baseline at 1 year post surgery) |
|
| Notes | Intention‐to‐treat analysis: stated in the protocol that ITT will be used as well as per‐protocol analysis Sample size calculation: yes Trial registration: ClinicalTrials.gov NCT00566917 Funding: funded by grants from the Swedish Society of Medicine, the Karolinska Institutet Research Foundations; regional agreement on clinical research between the Stockholm County Council, the Karolinska Institutet, and Ethicon Conflicts of interest: statement in text of manuscript asserting that although Ethicon co‐sponsored the trial, the manufacturer did not provide the products used and had no involvement in data collection and analysis or in the decision to submit the results for publication. Author financial disclosures are available from the New England Journal of Medicine website as supplementary material; however this does not include other members of the Nordic Transvaginal Mesh Group who were reviewers of surgery |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisations |
| Allocation concealment (selection bias) | Low risk | Secure concealment with remote computer |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to surgical intervention through the use of sham skin markings and were not aware of their group assignment until 1‐year follow‐up had been completed |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | When possible, postoperative examination was performed by a gynaecologist other than the operating surgeon Reviewers: surgeon 1/3, non‐surgeon 2/3 Participant‐completed questionnaires Statistical analysis was conducted by an independent statistician blinded to group assignment until data analysis for primary outcome has been completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patient flow accounted for completely in both groups Women who underwent surgery as per group assignment: transvaginal mesh: 191, colporrhaphy: 182 Loss to follow‐up: 21 participants (6% overall), transvaginal mesh: 14 (7%), colporrhaphy: 7 (4%) Analysed at one year: transvaginal mesh: 186, colporrhaphy: 182 |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes are reported on |
| Other bias | Unclear risk | Groups appear balanced at baseline |
Borstad 2010.
| Methods | Trial design: multi‐centre, parallel‐group randomised controlled trial (7 centres) | |
| Participants | Number of participants randomised: 194 (TVT concomitantly with prolapse repair 95, TVT 3 months after prolapse repair 99) Number of participants analysed: 140 (TVT concomitantly with prolapse repair 87, TVT 3 months after prolapse repair 53) Mean age (mean (range)): TVT concomitantly with prolapse repair 57.2 (31 to 89), TVT 3 months after prolapse repair 59.9 (38 to 85) Inclusion criteria: non‐consecutive women awaiting prolapse surgery with symptomatic and objective (provocation 300 mL) SUI or occult SUI ( SUI with pessary in position) Exclusion criteria: not specified Setting: regional hospitals and University clinics, Norway Timing: 2002 to 2006 |
|
| Interventions | Intervention: TVT performed at the same time as prolapse repair surgery Comparison: TVT performed 3 months after prolapse repair surgery if still clinically indicated (of 99 participants randomised to this group, 53 underwent TVT 3 months post prolapse repair) Follow‐up at 12 months |
|
| Outcomes |
Primary outcome/s: cure of SUI at 12‐month follow‐up, defined as no symptoms of SUI and no visible leakage during coughing in the lithotomy position Secondary outcome/s: reduction in POPQ score |
|
| Notes | Intention‐to‐treat analysis: yes; also "on‐treatment" analysis Sample size calculation: yes ‐ calculated to require 71 participants in each group for 80% power to detect a 20% difference in primary outcome Trial registration: ClinicalTrials.gov NCT00308009 Funding: no details provided of trial funding Conflicts of interest: study authors state no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed centrally by the Department of Epidemiology and Biostatistics, Centre for Clinical Research, Oslo University Hospital; no further information was provided on the method used to generate random sequence. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes, opened consecutively |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and trial personnel were aware of group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Preoperative and postoperative assessors were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up at 1 year: 4 participants at 1 year (3%), TVT concomitantly 4 (4%), TVT after 3 months 0 (0%) |
| Selective reporting (reporting bias) | Unclear risk | Outcomes stated are reported on. Complications are reported but not prespecified. |
| Other bias | Unclear risk | 75% of TVT concomitant group had co‐morbidities, compared to 89% of delayed TVT group, whereas 9% of this group had previous prolapse or incontinence surgery compared to 3% of the TVT concomitant group. |
Brubaker 2006.
| Methods | Trial design: multi‐centre, parallel‐group randomised controlled trial (7 sites) | |
| Participants | Number of participants randomised: 322 (Burch colposuspension 157, control group 165) Number of participants analysed: 322 (Burch colposuspension 157, control group 165) underwent 3‐month follow‐up and were included in the primary analysis, 305 (Burch group 152, control group 153) underwent 1‐year follow‐up, 302 completed some or all of the 2‐year follow‐up (Burch group = 147, control group = 155) and are included in the secondary analyses Mean age (mean ± SD): Burch group = 62.4 ± 9.7, control group = 60.3 ± 10.6 Inclusion criteria: POPQ stage 2 to 4 prolapse (Aa must be ‐1 or worse) and stress continent based on responses of ‘never’ or ‘rarely’ to 6 of the 9 SUI questions of MESA. Despite these criteria, preoperatively 19.2% of participants had SUI defined by PFDI, 10% had bothersome stress urinary incontinence (PFDI Questionnaire), and 39% had a positive stress test with or without prolapse reduction before intervention. From Table 2 of the 3‐month data it appears that these participants were equally distributed between groups. Exclusion criteria: immobile urethrovesical junction, pregnancy, anticipated move away after surgery Setting: hospitals and University medical centres throughout USA Timing: March 2002 to February 2005 |
|
| Interventions | Intervention: abdominal sacrocolpopexy with Burch colposuspension Comparison: abdominal sacrocolpopexy without Burch colposuspension Follow‐up: 3 months, 1 year, and 2 years postoperatively |
|
| Outcomes |
Primary outcome/s: stress incontinence and urge symptoms 3 months after surgery (defined as symptoms (a "yes" response on any of 3 questions on the PFDI stress incontinence questionnaire regarding leakage with coughing, sneezing, laughing, or other physical exertion), stress incontinence during standardised stress testing (coughing at maximal bladder capacity or 300 mL (whichever is less) in supine or standing position), or any treatment for stress incontinence after the study surgery Secondary outcome/s: quality of life measures, serious adverse events |
|
| Notes | Standardised surgery for colposuspension: not standardised paravaginal repair or sacrocolpopexy (17% biological grafts, 43% Mersilene, and 39% polypropylene and minimal use of PFTE (Gore‐tex) (6%) Although surgery was standardised for colposuspension, neither paravaginal repair nor sacrocolpopexy was standardised, with variation in suture type and graft materials used: 17% biological grafts, 43% Mersilene, 39% polypropylene, 6% Gore‐tex. No data on further performed surgeries are provided in the publication. Study terminated after 322 women had been randomised because of significant differences in UI outcomes Results not reported separately according to whether concomitant hysterectomy performed Women remained in allocated groups for analysis (ITT), but analysis was based on endpoint data actually available. Further data were made available in a new report depending upon status of occult stress incontinence (Visco 2008). The prolapse reduction during preoperative stress testing was performed via 5 different methods (swab, manual, speculum, pessary, or forceps), with each woman undergoing 2 types of prolapse reduction. Data from all prolapse reductions (2 for each participant) were reported as a total at 3 months only. Visco concluded that none of the techniques to demonstrate occult urinary incontinence could predict which women would become incontinent or not with or without concomitant continence surgery, although women who did have occult incontinence were more likely to be incontinent afterwards regardless of randomised allocation. Data from all prolapse reductions (2 for each patient) were reported as a total, and in analysing the postintervention continence status of women who did and did not have occult stress incontinence preoperatively, the decision was made to halve the reported total numbers for the analysis. Stress continence at baseline was defined based on responses of ‘never’ or ‘rarely’ to 6 of the 9 SUI questions on the MESA Questionnaire (medical, epidemiological, and social aspects of aging questionnaire). Preoperatively, 19% of participants had SUI defined by the PFDI (Pelvic Floor Distress Inventory), 10% had bothersome stress urinary incontinence according to the PFDI, and 39% had a positive stress test with or without prolapse reduction before surgery. Different and complicated definitions were used to categorise stress continence before and after the interventions, making it more difficult to be classified as stress continent after interventions than before interventions (see included studies tables). 39% classified as stress continent before surgery would have been classified as stress incontinent based on the post‐intervention definition. Use of imputation in the 2‐year results by the study authors is to be applauded. The process utilised ensures that in women undergoing further continence surgery, their continence status before the second intervention or after the surgical intervention outcomes, whichever is worse, is included in the final outcome data. Intention‐to‐treat analysis: yes Sample size calculation: yes, 480 women were required to detect a 10% difference in stress incontinence between the 2 groups Trial registration: no details of trial registration, but protocol is available (Brubaker 2003) Funding: funded by grants from the National Insitiutes of Health ‐ National Institute of Child Health and Human Development Conflicts of interest: Dr Brubaker has received research support and a research consulting fee from Pfizer (New York, NY) and research support from Allergan Inc (Irvine, CA). Dr Richter has received research support and consultant fees from Pfizer. Dr Visco is a paid surgical proctor and consultant for product/procedure development for Intuitive Surgical (Sunnyvale, CA). The other study authors had no potential conflicts to disclose. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisations |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes opened at the time of surgery after anaesthetic was administered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded to group allocation for a minimum of 3 months, and the intention was to maintain this blinding for 2 years. At 2‐year follow‐up, 38% had been unblinded and were aware of their treatment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers and examiners were blinded. Surgeons were unaware of urodynamic findings including urodynamic stress incontinence or occult stress incontinence with or without the prolapse reduced. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No substantial losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Most prespecified outcomes are reported. The protocol states that direct medical cost data will be obtained, but these do not appear to have been reported as yet.. |
| Other bias | Low risk | Groups were comparable at baseline on age, race, ethnic group, marital status, education, parity, method of delivery, distribution of women with positive stress test, OAB, prior hysterectomy, continence, and prolapse surgery. |
Colombo 2000.
| Methods | Trial design: single‐centre, parallel‐group randomised controlled trial | |
| Participants | Number of participants randomised: 71 (Burch group 37, anterior colporrhaphy 34) Number of participants analysed: 68 (Burch group 35, anterior colporrhaphy 33) Mean age (mean ± SD): Burch group = 54.9 ± 8.6, anterior colporrhaphy group = 55.7 ± 10.3 Inclusion criteria: USI, cystocoele > 2 or 3, swab test > 30% Exclusion criteria: detrusor overactivity, previous pelvic floor surgery, high risk for abdominal operation Setting: Department of Obstetrics and Gynecology, University of Milan, Italy Timing: October 1981 to November 1986 |
|
| Interventions | Intervention: Burch group: total abdominal hysterectomy and vault to uterosacral ligament, Moschcowitz, Burch with 3‐4 Ethibond (n = 35) Comparison: anterior colporrhaphy: vaginal hysterectomy, pouch of Douglas obliteration and anchoring of vaginal cuff to uterosacral ligament, catgut plication (n = 33) Follow‐up: 3 months and 6 months postoperatively; thereafter annually for 15 years |
|
| Outcomes |
Primary outcomes: long‐term subjective (no incontinence episodes by history) and objective (negative stress test result) cure rates Secondary outcomes: incidence of prolapse recurrence, vaginal length, dyspareunia |
|
| Notes | Intention‐to‐treat analysis: no Sample size calculation: not stated Trial registration: not stated Funding: not stated. Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table |
| Allocation concealment (selection bias) | High risk | Inadequate: open list |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided of blinding of trial participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss to follow‐up Postoperative: Burch group = 1, anterior colporrhaphy group = 2 10‐year follow‐up: Burch group = 2, anterior colporrhaphy group = 1 15‐year follow‐up: Burch group = 9, anterior colporrhaphy group = 8 |
| Selective reporting (reporting bias) | Unclear risk | Prespecified outcomes are reported. |
| Other bias | Unclear risk | Groups appear balanced at baseline. |
Costantini 2007.
| Methods | Trial design: single‐centre randomised controlled trial | |
| Participants | Number of participants randomised: 66 (group A 34, group B 32) Number of participants analysed: 66 Mean age (mean ± SD): group A = 63 ± 9, group B = 61 ± 8 Inclusion criteria: continent women (women with negative stress test before and after prolapse reduction, no preoperative symptoms of urinary incontinence, negative symptom questionnaire, and no leakage during urodynamics) with 'severe' uterovaginal and vault prolapse (not clearly defined) Exclusion criteria: not stated Setting: University of Perugia, Itali Timing: January 2000 to December 2004 |
|
| Interventions | Intervention: group A sacrocolpopexy + Burch colposuspension (n = 34) Comparison: group B sacrocolpopexy alone (n = 32) |
|
| Outcomes | Primary outcome/s: changes in continence status, anatomical outcome of prolapse repair Secondary outcome/s: changes in subjective symptoms, quality of life measured by IIQ‐7 and UDI‐6 |
|
| Notes | Urinary incontinence was clinically classified "on the basis of the ICS definition and graded on the Ingelman Sunderberg scale". Intention‐to‐treat analysis: All randomised participants were analysed. Sample size calculation: yes, 66 participants calculated to provide 80% to 85% power to detect a 25% to 30% difference in proportion of postoperative incontinence between groups Trial registration: no Funding: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | No details provided of method used to conceal group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial personnel who performed the surgery were not blinded. No details of blinding of participants were provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/34 group A and 2/32 group B participants lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Preoperative UDI scores were given, but no postoperative UDI scores were available. |
| Other bias | Unclear risk | Primary continence assessments were based on a non‐defined stress test and symptoms from the UDI Questionnaire. |
Costantini 2008.
| Methods | Trial design: single‐site RCT | |
| Participants | Number of participants randomised: 47 (group A n = 24, group B n = 23) Number of participants analysed: 47 Mean age (mean ± SD): group A = 60 ± 10, group B = 61 ± 13 Inclusion criteria: women age 18 to 75, POP > stage 2 (BW and POPQ), urinary incontinence defined by ICS Exclusion criteria: uterine fibroids, uterine/cervical malignancy, active PID, allergy to synthetic graft/suture materials, pregnancy/lactation, significant illness, inability to provide informed consent or comply with study protocol Setting: Urology Department, University of Perugia, Italy Timing: January 2002 to June 2006 |
|
| Interventions | Intervention: group A ‐ sacrocolpopexy + Burch 14, sacrohysteropexy + Burch 10 (n = 24) Comparison: group B ‐ sacrocolpopexy 17, sacrohysteropexy 6, no colposuspension (n = 23) Preoperatively incontinence defined by urodynamics: 13 USI, 30 mixed, 4 occult (incontinence with coughing or Valsalva manoeuvre with the prolapse reduced). Distribution of patients with prolapse and incontinence preoperatively between groups is unclear. |
|
| Outcomes |
Primary outcome/s: change in incontinence rate measured by combination of bladder diary, number of pads and stress test without clear definition, anatomical outcome of prolapse as measured by B7W and POPQ Secondary outcome/s: changes in subjective symptoms and quality of life measured by questionnaires, postoperative satisfaction as measured by VAS |
|
| Notes | CONSORT statement: yes Intention‐to‐treat analysis: All participants randomised were analysed. Sample size calculation: yes, 47 participants calculated to provided 80% power to detect up to 30% difference in postoperative conditions between the 2 groups Trial registration: yes (post hoc) Clinicaltrials.gov NCT00576004 Funding: not stated. Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No details were provided of the method used to conceal group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial personnel who performed the operations were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes appear to have been reported. |
| Other bias | Unclear risk | Distribution of POP between groups not clear: 24 uterovaginal, 13 vault, 8 cystocoele, 2 cystocoele and rectocoele Methodological problems with this paper include lack of clear and equal distribution of prolapse grading and incontinence between groups preoperatively, inconsistency of preoperative and postoperative incontinence classifications (urodynamics preoperatively and symptoms postoperatively), and lack of definition of success of prolapse grading and data related to perioperative parameters and complications. |
Fuentes 2011.
| Methods | Trial design: parallel‐group randomised controlled trial | |
| Participants | Number of participants randomised: 60 (group A POP surgery + TVTo = 27, POP surgery alone = 33) Number of participants analysed: 60 Mean age: not stated Inclusion criteria: women with occult urinary stress incontinence defined as symptomatically continent women with urodynamic stress incontinence Exclusion criteria: not stated Setting: Perez Carreno Hospital, Caracas, Venezuela Timing: February 2008 to December 2010 |
|
| Interventions | Intervention: any POP surgery including Prolift/vag repairs/colpocleisis with TVTo (n = 27) Comparison: any POP surgery including Prolift/vag repairs/colpocleisis without TVTo (n = 33) Median FU: 20 months |
|
| Outcomes |
Primary outcome/s: need for subsequent anti‐incontinence surgery Secondary outcome/s: urodynamics testing, 1‐hour pad test, 3‐day bladder diary, UDI 6 SF, IIQ 7 SF, PISQ, and visual analogue scale (VAS) score |
|
| Notes | Intention‐to‐treat analysis: All women randomised are analysed. Sample size calculation: no details Trial registration: no details Funding: no details Conflicts of interest: no details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear loss to follow‐up. Abstract states that further women were recruited to the study to allow for participants who were deceased, lost to follow‐up, or withdrawn. |
| Selective reporting (reporting bias) | High risk | Conference abstract only; unable to determine if outcomes reported were all those specified for the trial |
| Other bias | High risk | Conference abstract only |
Hiltunen 2007.
| Methods | Trial design: multi‐centre randomised controlled trial (5 centres) | |
| Participants | Number of participants randomised: 202 (POP repair with mesh 105, POP repair without mesh 97)
Number of participants analysed: 200 (1 withdrawal from mesh group, 1 loss to follow‐up in no mesh group) Mean age (mean ± SD): mesh group = 66 ± 9, no mesh group = 65 ± 9 Inclusion criteria: postmenopausal women with symptomatic anterior vaginal wall prolapse to the hymen or beyond Exclusion criteria: apical defect indicating vaginal fixation or stress urinary incontinence necessitating surgery or main symptomatic prolapse component in the posterior vaginal wall. Also patients with gynaecological tumour or malignancy calling for laparotomy or laparoscopy and those with untreated vaginal infection Setting: 5 hospitals throughout Finland Timing: April 2003 to May 2005 |
|
| Interventions | Intervention: anterior colporrhaphy (AC) + self‐tailored (from a 6 × 11‐cm mesh patch), 4 armed low‐weight polypropylene mesh (n = 104) Comparison: AC using a 0 or 2/0 multi‐filament suture (n = 96) Type of mesh: non‐absorbable monofilament polypropylene (Parietene light, Sofradim, France) Sutures for AC: absorbable 0 or 2/0 multi‐filament suture Concomitant surgery: vaginal hysterectomy, posterior repair, culdoplasty as required, no concomitant continence surgeries performed Follow‐up for 24 months |
|
| Outcomes |
Primary outcome/s: recurrence of anterior vaginal wall prolapse reaching stage 2 by the POPQ system Secondary outcome/s: perioperative and postoperative complications, symptom resolution, post voidal urine residual volume Objective failure Symptomatic prolapse Awareness of bulge at 1 year Awareness of bulge at 2 years Further prolapse surgery Further continence surgery Operating time (minutes) Blood loss (mL) Stress incontinence de novo Mesh erosion Mesh exposure Further surgery for mesh exposure Sexual function |
|
| Notes | Two inconsistencies between 1‐year and 2‐year data. Reduction in mesh exposures from 17% at 1 year to 8% at 2 years is difficult to explain. Furthermore, the percentage of patients having undergone previous prolapse surgery at 1 year was 27% in the AC group and 18% in the mesh group, and the 2‐year report quotes 20% and 14%, respectively. There is also a further discrepancy. At 1 year, de novo SUI was 9/96 as compared to 15/104, and at 3 years the reported rate was lower at 5/96 vs 7/104 rate. Even if some of these underwent continence surgery, they should still be recorded as having de novo stress urinary incontinence. CONSORT statement: yes Intention‐to‐treat analysis: No. 1 withdrawal from mesh group, 1 loss to follow‐up in no mesh group Sample size calculation: yes, 202 participants calculated to allow for 15% dropout and to provide 80% power to detect a 20% difference in primary outcomes between groups Trial registration: Clinicaltrials.gov NCT00420225 Funding: supported by a grant from the Medical Research Funds of the Central Hospital of South Ostrobothnia, Seinäjoki, and Tampere University Hospital, Tampere, Finland. Conflicts of interest: study authors had no potential conflicts of interest to disclose. 3‐year follow up published (Nieminen et al) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisations |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding as to the operative technique was not used. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 202 randomised, 1 withdrawal, and 1 loss to follow‐up. 200 analysed |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes appear reported. |
| Other bias | Low risk | No significant differences in baseline demographics, prior hysterectomy, or prolapse surgeries between the 2 groups |
Iglesia 2010.
| Methods | Trial design: multi‐centre randomised controlled trial involving 6 surgeons at 3 sites | |
| Participants | Number of participants randomised: 65 (mesh group 32, no mesh group 33) Number of participants analysed: 65 Mean age: (mean ± SD): mesh group = 64.4 ± 10.8, no mesh group = 63.5 ± 8.9 Inclusion criteria: ≥ 21 years, grade 2 to 4 (POPQ) uterovaginal or vaginal prolapse and agreed to undergo vaginal surgery, available for 12 months' review, can complete questionnaires Exclusion criteria: multiple medical contraindications, short vagina, uterus > 12 weeks in size, desire future fertility, postpartum Setting: 3 University hospitals in USA Timing: January 2007 to August 2009 |
|
| Interventions | Intervention: anterior Prolift or total vaginal mesh (Prolift) if point C or D on POPQ ≥ 3. No T incisions were performed and hysterectomy was performed if uterus was present (n = 32). Comparison: anterior colporrhaphy with uterosacral colpopexy with polytetrafluoroethylene sutures or sacrospinous colpopexy with Goretex sutures and hysterectomy performed if uterus was present (n = 33) |
|
| Outcomes |
Primary outcome/s: objective failure rate at 1 year (any stage 2 or greater prolapse) Secondary outcome/s: subjective failure, reoperation for prolapse, surgery for mesh exposure, de novo dyspareunia, de novo SUI, responses to a range of quality of life questionnaires, postoperative complications, long‐term complications, serious adverse events |
|
| Notes | Intention‐to‐treat analysis: Manuscript states that 1 participant assigned to the mesh group did not receive mesh and was analysed as a member of the no‐mesh arm. This participant was analysed in the mesh group for 3‐year follow‐up. Sample size calculation: yes, calculated to require 90 participants (45 per arm) to provide 80% power to detect a 20% difference in primary outcome Trial registration: yes. Clinicaltrials.gov NCT00475540 Funding: supported by a grant from the AUGS Foundation and the MedStar Health Research Institute Intramural Grant Program. Prolift mesh kits used in this trial were donated by the mesh manufacturer (Ethicon Women Health and Urology, Somerville, New Jersey, USA). Conflicts of interest: study authors did not report any potential conflicts of interest. The ethics committee stopped the study before completion owing to predetermined stopping criteria of mesh erosion rate >15% being reached, with 65 of the desired sample size of 90 having undergone interventions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisations |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were opened in the operating theatre after participant had received anaesthesia. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial co‐ordinator at each site and participants were blinded to treatment by use of sham dressings. Trial personnel not blinded (e.g. operating theatre staff, inpatient and office personnel) were instructed to not disclose treatment assignment to participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Three‐ and 12‐month follow‐up examinations were conducted by evaluator blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up 3 months postoperatively 27/32 in mesh group and 33/33 in no mesh group underwent 1‐year follow‐up. 25/33 in mesh group and 26/32 in no mesh group underwent 3‐year follow‐up. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes appear reported on. |
| Other bias | Unclear risk | Before surgery, all demographic details were similar between the 2 groups, except group B had lower POPDI‐6 score than group A. The ethics committee stopped the study before completion owing to predetermined stopping criteria of mesh erosion rate > 15% being reached, with 65 of the desired sample size of 90 having undergone interventions. |
Meschia 2004.
| Methods | Trial design: randomised controlled trial | |
| Participants | Number of participants randomised: 50 (25 per arm) Number of participants analysed: 50 Mean age (mean ± SD): 65 ± 8 Inclusion criteria: severe symptomatic genital prolapse and occult stress urinary incontinence Exclusion criteria: age > 70 years, BMI > 30 kg/m², diabetes, previous pelvic or continence surgery, symptoms of SUI, detrusor overactivity, cotton‐swab test > 30 degrees Setting: Department of Obstetrics & Gynecology, Urogynecology Unit, University of Milan, Italy. Timing: February 2000 to June 2001 |
|
| Interventions | Intervention: vaginal prolapse repair and TVT (with Prolene tape) (n = 25) Comparison: vaginal prolapse repair and urethrovesical plication (with 2‐0 permanent‐braided polyester sutures) (n = 25) All women also had vaginal hysterectomy, McCall culdoplasty, and cystocoele repair. Cystocoele (anterior repair) with 2‐0 delayed absorbable sutures (polydioxanone) No sacrospinous ligament fixation performed Rectocoele repair: A: 20/25, B: 23/25 |
|
| Outcomes |
Primary outcome/s: occurrence of de novo stress urinary incontinence after operation Secondary outcome/s: rate of prolapse recurrence for each vaginal site, anatomical outcomes, urodynamic assessment Subjective prolapse symptoms, failure rate Objective failure (overall) Objective failure (anterior) Objective failure (posterior) Objective failure (apex) Further prolapse surgery Further continence surgery SUI subjective SUI objective OAB de novo (new) Voiding dysfunction Recurrent UTIs Adverse effects (bladder perforation, retropubic haematoma) Perioperative outcomes Operation time (minutes) Blood loss (mL) Hb change Days in hospital Time to spontaneous voiding (days) |
|
| Notes | Intention‐to‐treat analysis: all women randomised were analysed as per group allocation. Sample size calculation: yes, 50 participants (25 per arm) was calculated to provide 80% power to detect a 30% to 40% difference in primary outcomes between groups. Trial registration: no details Funding: no details Conflict of interest: no details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Sequentially labelled, sealed envelopes with numbers assigned from a computer‐generated random number list. Unclear if opaque |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomised appear to be analysed. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes appear to be reported. |
| Other bias | Unclear risk | Groups comparable at baseline |
Rudnicki 2014.
| Methods | Trial design: multi‐centre, parallel‐group randomised controlled trial involving 6 centres in Denmark, Norway, Sweden, and Finland | |
| Participants | Number of participants randomised: 161 (transvaginal mesh 79, anterior colporrhaphy 82) Number of participants analysed: 154 (transvaginal mesh 76, colporrhaphy 78) Mean age (mean ± SD): transvaginal mesh = 64.9 ± 6.4, colporrhaphy = 64.7 ± 6.6 Inclusion criteria: ≥ 55 years, ≥ stage 2 anterior vaginal wall prolapse POPQ Exclusion criteria: previous major pelvic surgery, with the exception of a hysterectomy for reasons other than genital prolapse, previous vaginal surgery, or hysterectomy for POP; concomitant prolapse of the uterus, or an enterocoele of stage 1 or higher; previous incontinence sling surgery performed through the obturator membrane; current treatment with corticosteroids; history of genital or abdominal cancer Setting: hospitals in Sweden, Norway, Finland, and Denmark Timing: April 2008 to December 2010 |
|
| Interventions | Intervention: four‐arm transobturator vaginal anterior mesh (Avaulta); the central section is coated with an absorbable hydrophilic film of porcine collagen. Vaginal pack for ≥ 6 hours (79 underwent surgery as assigned) Comparison: anterior colporrhaphy, fascia plicated using intermittent 2–0 absorbable sutures, excessive trimming of vagina (all 82 underwent surgery as assigned) Follow‐up at 3, 12, and 13 months |
|
| Outcomes |
Primary outcome: recurrent anterior prolapse (POPQ stage > 1) Secondary outcomes: quality of life, symptoms, and complications (frequency of erosions, postoperative infections, and dyspareunia). Questionnaires: PFIQ‐7, PFDI‐20, PISQ‐12, UIQ‐7, CRAIQ‐7, POPIQ‐7, POPDI‐6, CRADI‐8, UDI‐6 |
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: yes, to detect a difference of 20% in recurrence rate (defined as ≥ stage 2 cystocoele at 12‐month follow‐up) between the 2 groups. Accordingly, 112 participants had to be randomised. In anticipation of a dropout rate of 15%, the number of participants was increased to 130. Trial registration: ClinicalTrials.gov (NCT00627549): http://clinicaltrials.gov/ct2/show/NCT00774215 Funding: no funding by industry. Funded by Region Sealand Health research fund Conflicts of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block design stratified by centre |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of FU for 3/79 in mesh group and 4/82 in anterior repair group. All accounted for |
| Selective reporting (reporting bias) | Low risk | Outcomes stated are reported on. |
| Other bias | Unclear risk | No significant differences in baseline demographics |
Schierlitz 2014.
| Methods | Trial design: multi‐centre randomised controlled trial (2 sites) | |
| Participants | Number of participants randomised: 80 (no TVT group 43, TVT group 37) Number of participants analysed: at 6 months: no TVT group 39, TVT group 35 Mean age (mean ± SD): no TVT group = 66 ± 9.1, TVT group = 67 ± 10.9 Inclusion criteria: symptomatically continent women with urodynamically demonstrable stress incontinence with or without reduction of prolapse (POPQ ≥ stage 3) Exclusion criteria: contraindications to pelvic surgery such as pelvic infection, fistula, congenital or neurogenic bladder disorder, malignancy, or being medically unfit Setting: 2 tertiary hospitals, Australia Timing: May/June 2003 to August/September 2009 |
|
| Interventions | Intervention: non‐standardised vaginal prolapse surgery with TVT (n = 37) Comparison: non‐standardised vaginal prolapse surgery without TVT (n = 43) No women had bladder neck plications 6 months minimum review, n = 60 at 24 months |
|
| Outcomes |
Primary outcome/s: need for subsequent anti‐incontinence surgery due to symptomatic SUI after 6 months Secondary outcome/s: subjective cure rates, intraoperative and postoperative complications, voiding function, urgency, urge urinary incontinence (UUI) symptoms, change in quality of life as assessed by UDI‐6 and IIq‐7, overall satisfaction with prolapse repair |
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: sample size required calculated at 62 participants (31 per group) based on 90% power to detect a reduction from 50% to 10% in SUI after prolapse repair Trial registration: Australian New Zealand Clinical Trials Registry ACTRN: 12611000844943 Funding: no details Conflicts of interest: study authors report no conflicts of interest Occult SUI was defined as symptomatically continent women with urodynamically demonstrable stress incontinence with or without reduction of the prolapse (POPQ ≥ stage 3) Study authors calculated that a clinician would have to insert 1 TVT sling unnecessarily to prevent 1 woman from needing a sling postoperatively. 2‐year follow‐up; published as an abstract (Walsh et al 2017) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | No details provided of method used to conceal allocation to treatment groups |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of patients |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Some women declined postoperative urodynamic studies as they were asymptomatic, but subjective SUI is main outcome measure. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes are reported. |
| Other bias | Unclear risk | Non‐standardised surgery was performed. |
Sivaslioglu 2008.
| Methods | Trial design: multi‐centre RCT (2 sites) | |
| Participants | Number of participants randomised: 90 Number of participants analysed: 85 Mean age: (mean ± SD): mesh group = 57.7 ± 9.4, site‐specific group = 50.1 ± 9.9 Inclusion criteria: primary cystocoele Exclusion criteria: stress urinary incontinence, concomitant rectocoele or enterocoele or recurrent cystocoele Setting: urogynaecology clinics of Ankara Etlik Maternity and Women’s Health Teaching Hospital Timing: January 2006 to January 2007 |
|
| Interventions | Intervention: self‐styled 4‐armed polypropylene (Parietene, Sofradim, France) mesh, no anterior repair (n = 43) Comparison: site‐specific Polyglactin 910 anterior repair (n = 42) Concomitant surgery not standardised, management of concomitant apical prolapse not specified in either group Follow‐up: mean 12 months (range 8 to 16) |
|
| Outcomes |
Primary outcome/s: objective failure (≥ stage 2 POPQ) Secondary outcome/s: PQoL score postop (mean ± SD), further prolapse surgery, SUI, dyspareunia de novo, mesh erosion |
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: 45 in each arm required Trial registration: not stated Funding: not stated Conflicts of interest: not stated CONSORT statement: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded reviewers performed objective assessment of patient‐completed questionnaires. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 90 women randomised and 5 lost to follow‐up balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | No further validated or structured reporting of secondary outcome findings apart from PQoL score |
| Other bias | Low risk | No funding and no COI |
Trabuco 2014.
| Methods | Trial design: parallel randomised controlled multi‐centre superiority trial | |
| Participants | Number of participants randomised: 113 Number of participants analysed: 104 Mean age: 56 years Inclusion criteria: symptomatic ≥ stage 2 apical or anterior vaginal wall prolapse, opted for an abdominal prolapse repair. Women with a uterus were eligible to participate. Exclusion criteria: known or suspected disease that affects bladder function (e.g. multiple sclerosis, Parkinson disease); pregnancy; desired fertility; urethral diverticulum; history of radical pelvic surgery or pelvic radiation therapy; current chemotherapy or radiation therapy for malignancy Setting: urogynaecology clinics at Mayo Clinic in Rochester, Minnesota, and University of Missouri, Kansas City, Missouri Timing: June 2009 to August 2013 |
|
| Interventions | A: SCP with MUS (n = 53) B: SCP with Burch colposuspension (n = 57) |
|
| Outcomes |
Primary outcome/s: overall continence and stress‐specific continence Secondary outcome/s: patient satisfaction, voiding dysfunction, elevated post void residual, apical or anterior prolapse failure, de novo or resolution of urgency Incontinence,and incontinence severity No differences in age, BMI, history of POP surgeries, POP stage, continence severity. Six‐month review: 104 patients Objective continence: A, 35/53; B, 28/51 Stress‐specific continence: A, 43/53; B, 32/51 De novo UUI: A, 3/28; B, 2/26 Satisfaction rate (answered somewhat or completely: A, 50/53; B, 37/51 Patient perception of improvement (10/10 VAS): A, 38/53; B, 26/51 Report successful operation for SUI (10/10 VAS): A, 38/53; B, 24/51 No difference in mesh exposure No difference in rate of complications |
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: 46 women per group required based on a 2‐sided × 2 test with a type I error level of .05. Assuming a 20% dropout rate, the plan was to recruit 115 trial participants. Trial registration: NCT00934999 Funding: Mayo Clinic Center for Clinical and Transitional Science grant number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health Conflicts of interest: none 2‐year follow‐up: published as an abstract |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not clear |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 113 randomised. 104 followed up at 6 months; MUS (53), Burch (51) |
| Selective reporting (reporting bias) | Low risk | Full report of primary and secondary outcome findings |
| Other bias | Unclear risk | 2‐year follow‐up: reported only as an abstract |
Turgal 2013.
| Methods | Trial design: computer‐randomised prospective trial | |
| Participants | Number of participants randomised: 40 Number of participants analysed: 40 Inclusion criteria: stage 2 or 3 cystocoele according to POPQ Exclusion criteria: urinary incontinence, previous gynaecological operation, concomitant rectocoele or enterocoele, recurrent cystocoele Setting: Urogynecology Clinic of Etlik Zubeyde Hanim Maternity and Women’s Health Teaching and Research Hospital Timing: June 2006 to February 2007 |
|
| Interventions | Intervention: anterior vaginal mesh: polypropylene mesh (Sofradim) through the obturator foramen (n = 20) Comparison: anterior colporrhaphy (n = 20) |
|
| Outcomes |
Primary outcome/s: anatomical (POPQ) and functional effectiveness Secondary outcome/s: urinary and faecal incontinence, pelvic pain Success rate De novo SUI Preop frequency Postop frequency Preop urgency Postop urgency Assessed at 6 weeks, 6 months, 1 year |
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: not stated Trial registration: not stated Funding: not stated Conflicts of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocated by a computer programme |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not stated |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding; surgeon performed assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Prespecifed variables reported on |
| Selective reporting (reporting bias) | Unclear risk | Not detected |
| Other bias | Unclear risk | Unclear |
van der Ploeg 2015 (CUPIDO I).
| Methods | Trial design: multi‐centre RCT (14 centres) | |
| Participants | Number of participants randomised: 138 Number of participants analysed: 134 Inclusion criteria: POP ≥ stage 2, scheduled for transvaginal prolapse surgery with co‐existing SUI Exclusion criteria: occult SUI, post voiding residual ≥ 300 mL, isolated prolapse of posterior compartment, previous urinary incontinence surgery, recent prolapse surgery Setting: multi‐centre Timing: 2007 to 2009 according to trial registration |
|
| Interventions | Intervention: vaginal prolapse repair with MUS (n = 63) Comparison: vaginal prolapse repair without MUS (n = 71) |
|
| Outcomes |
Primary outcome/s: absence of urinary incontinence and SUI 12 months after index surgery and additional treatment for SUI and overactive bladder (OAB) in the first postoperative year Secondary outcome/s: bothersome SUI, objective SUI, a composite endpoint |
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: sample size calculation was based on a 1‐sided test. Accounting for 10% loss to follow‐up, 63 participants per group were needed to detect a 20% decrease in subjective SUI (30% vs 10%) with 80% power and a 1‐sided significance level of 5%. Trial registration: NTR1197 Funding: Academic Medical Center (AMC)Department of Gynaecology Conflicts of interest: Jan‐Paul W.R. Roovers: medical consultant for American Medical Systems (AMS). C. Huub van der Vaart: medical consultant for BARD Medical |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was created by a central computer random number generator using blocks of 4 and stratified for centre and the leading edge of the POP in a 1:1 ratio for the 2 comparison groups. |
| Allocation concealment (selection bias) | Low risk | Sequence list was concealed from investigators and those groups including participants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Unclear risk | No clarification |
van der Ploeg 2016 (CUPIDO II).
| Methods | Trial design: multi‐centre RCT ‐ 13 teaching hospitals | |
| Participants | Number of participants randomised: 225 Number of participants analysed: MUS group 42, no MUS group 47, control group 136 Inclusion criteria: women undergoing vaginal prolapse surgery for ≥ stage 2 POP with preoperative occult stress urinary incontinence Exclusion criteria: women with post voiding residuals > 300 mL, previous incontinence surgery, recent prolapse surgery, unable to give informed consent, recently pregnant or wished to become pregnant, systemic disease that could influence bladder function (e.g. multiple sclerosis; Parkinson’s disease), underwent or were scheduled for chemotherapy or radiotherapy, continent women Setting: 13 centres across The Netherlands Timing: 2007 to 2009 according to trial register |
|
| Interventions | Intervention: vaginal prolapse surgery with MUS (n = 42) Comparison: vaginal prolapse surgery without MUS (n = 47) Control group: POP alone without objective SUI (n = 136) |
|
| Outcomes |
Primary outcome/s: absence of urinary incontinence and SUI 12 months after index surgery and additional treatment for SUI and overactive bladder (OAB) in the first postoperative year Secondary outcome/s: bothersome SUI, objective SUI, a composite endpoint |
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: based on 1‐sided test. For 80% power to detect a 15% difference in subjective SUI (5% SUI in the MUS group vs 20% SUI in the control group) and accounting for 10% loss to follow‐up, 80 women per group were needed. Trial registration: NTR1070 Funding: Academic Medical Center (AMC)Department of Gynaecology Conflicts of interest: Jan‐Paul W.R. Roovers: medical consultant for American Medical Systems (AMS). C. Huub van der Vaart: medical consultant for BARD Medical |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central computer random‐number generator using blocks of 4 and stratified for centre and the leading edge of the POP. After obtaining written informed consent from participants, we used a central password‐protected web‐based application for randomisations and patient data entry. |
| Allocation concealment (selection bias) | Unclear risk | Researchers state that the sequence list was concealed from investigators and participants but also state that participants and outcome assessors were not blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 225 were randomised and 222 were analysed. |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes were reported. |
| Other bias | Unclear risk | Unclear |
Wei 2011.
| Methods | Trial design: multi‐centre (7 clinical sites), randomised, single‐blind, sham‐controlled, surgical intervention trial | |
| Participants | Number of women randomised: 337 Number of women analysed: 327 Inclusion criteria: vaginal prolapse surgery for symptomatic stage 2 anterior compartment prolapse, negative response to 3 questions from PFDI related to stress incontinence Exclusion criteria: prior sling placement, prior urethral surgery or radiation, planing pregnancy, 2 or more hospitalisations in the prior year Setting: multi‐centre trial Timing: Enrollment began in May 2007, and follow‐up was completed in January 2011. |
|
| Interventions | Intervention: vaginal prolapse surgery with TVT (n = 165) Comparison: vaginal prolapse surgery without TVT (n = 172) Follow‐up at 12 months |
|
| Outcomes |
Primary outcome/s: urinary incontinence (stress, urge, or mixed) at 3 months, defined as a positive cough stress test, bothersome incontinence symptoms, treatment for urinary incontinence; and urinary incontinence (stress, urge, or mixed) at 12 months, regardless of whether interim treatment for incontinence had been provided Secondary outcome/s: Medical Outcomes Study 36‐Item Short‐Form Health Survey, Pelvic Floor Distress Inventory, Pelvic Floor Impact Questionnaire, Incontinence Severity Index, Pelvic Organ Prolapse/Urinary Incontinence Sexual Functioning Questionnaire Short Form, visual analogue pain scale adapted for suprapubic pain; severe adverse events |
|
| Notes | Intention‐to‐treat analysis: yes Sample size calculation: 150 participants per group would provide the study with 80% power to detect a 15% between‐group difference in the primary 3‐month endpoint on the basis of a 2‐sample test of proportions, with a 2‐sided significance level of 5% Trial registration: NCT00460434 Funding: grants from Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Health Office of Research on Women's Health Conflicts of interest: OPUS trial: A significant weakness of the evaluation is that definitions for inclusion as stress continent (‐ve answer to 3 PFDI questions related to sui) were less stringent than the definition of UI positive, as outcome includes +ve stress test, questions related to stress or urge incontinence, or treatment for any incontinence. Actually as 108 (group A, 57; group B, 54) women had +ve prolapse reduction stress test before intervention, they would have been deemed positive stress incontinent post intervention and were ‐ve stress incontinent preoperatively on the criteria defined. Women who declined to undergo randomisations were offered the opportunity to participate in a patient‐preference cohort in which the decision for a sling was left up to the patient and her surgeon. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block design stratified by surgeon and type of prolapse surgery |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham dressings. Participants in the randomised cohort, interviewers, and co‐ordinators were unaware of study group assignments, and operative notes and surgical consent forms did not reveal the study group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcomes were questionnaires assessed by blinded reviewers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to treat and failure of review counted as failure, minimal loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | Low risk | Grants from Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Institute of Health Office of Research on Women's Health |
Withagen 2011.
| Methods | Trial design: multi‐centre randomised controlled trial (13 centres, 22 surgeons) | |
| Participants | Number of participants randomised: 194 Number of participants analysed: 190 Inclusion criteria: recurrent ≥ stage 2 anterior and or posterior wall prolapse Exclusion criteria: pregnancy, future pregnancy, prior vaginal mesh repair, a compromised immune system or any other condition that would compromise healing, previous pelvic irradiation or cancer, blood coagulation disorders, renal failure, upper urinary tract obstruction, renal failure and upper urinary tract obstruction, presence of large ovarian cysts or myomas Setting: 13 centres in The Netherlands Timing: June 2006 to July 2008 |
|
| Interventions | Intervention: anterior transobturator mesh (Prolift, n = 95) comparison: vaginal anterior colporrhaphy (n = 99) |
|
| Outcomes |
Primary outcome/s: anatomic failure in any of the treated vaginal compartments, defined as POPQ ≥ stage 2 Secondary outcome/s: blood loss, length of hospitalisations, complications and subjective improvement (Patient Global Impression of Improvement), change in bother and quality of life measured by Urogenital Distress Inventory, Defecatory Distress Inventory, and Incontinence Impact Questionnaire scores |
|
| Notes | Intention‐to‐treat analysis: not stated Sample size calculation: based on the assumption of an estimated overall failure rate of 30% in the conventional surgery group (cure rate of 70%) and 13% in the tension‐free vaginal mesh group (cure rate of 87%). Based on a 2‐tailed hypothesis test with type I error of 5% and 80% power, 88 patients in each group would be required to detect a significant difference ≥ 17%. Anticipating a 10% dropout rate, we planned to enrol 194 patients. Trial registration: NCT00372190 Funding: University‐administered research funds Conflicts of interest: Drs. Milani and den Boon have a consultancy agreement with Ethicon Women’s Health & Urology. Drs. Withagen and Milani are on the Speaker’s Bureau of Ethicon Women’s Health and Urology. Drs. Withagen and Vierhout received an unrestricted educational grant from Ethicon Women’s Health & Urology. Dr. Vervest has received payment from Ethicon Women’s Health & Urology for lectures. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded reviewers: patient‐completed questionnaires |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Minimal loss for follow‐up but incomplete assessment (e.g. questionnaire vs exam) |
| Selective reporting (reporting bias) | Low risk | Prespecified outcomes reported |
| Other bias | High risk | University research fund: all study authors reported financial support from Ethicon Company manufacturing product being evaluated by non‐blinded reviewers. Preoperatively, group A is significantly different from mesh group B as demonstrated by greater degree of prolapse at Ap, Bp, and GH, having significantly higher number with ≥ stage 2 apical compartment prolapse among those in Table I undergoing prior apical surgery, 36% (16/45) in the non‐mesh group versus 18% (10/56) in the mesh group (P = 0.04, odds ratio (OR) 2.54); finally prior sacrocolpopexy was 3 times as frequent in the mesh group. |
BMI: body mass index.
Hb: haemoglobin.
ICS: International Continence Society.
IVS: intravaginal slingplasty.
MUCP: maximum urethral catheter pressure.
OAB: overactive bladder.
PDS: polydioxanone surgical suture (PDS).
PFDI: Pelvic Floor Distress Inventory.
PFIQ: Pelvic Floor Impact Questionnaire.
PGI‐I: Patient Global Impression of Improvement.
PISQ: Pelvic organ prolapse/urinary Incontinence Sexual Questionnaire.
POP: pelvic organ prolapse.
POPQ: pelvic organ prolapse quantification (according to ICS).
P‐QoL: Prolapse Quality of Life Questionnaire.
QoL: quality of life.
RCT: randomised controlled trial.
SUI: stress urinary incontinence (symptom diagnosis).
TVT: tension‐free vaginal tape.
UDI: Urogenital Distress Inventory.
UI: urinary incontinence.
UTI: urinary tract infection.
VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allahdin 2008 | No incontinence issues; different patient population |
| Barber 2006 | Barber compared 2 independent population cohorts. Arm 1 was the pessary group, in which women were randomly allocated between 2 pessary types, and arm two underwent a surgical intervention. As patients were not randomly allocated between pessary and surgery groups, this paper failed to meet the criterion of a randomised controlled trial and was excluded. |
| Bergman 1989 | RCT on anterior colporrhaphy, Pereyra or Burch colposuspension, no data on pelvic organ prolapse given, different patient populations |
| Biller 2008 | Biller and colleagues evaluated inclusion and exclusion of anal purse string suture to minimise contamination during prolapse surgery. This study was excluded from the review as it failed to evaluate pelvic organ prolapse surgical procedures. |
| Boccasanta 2004 | RCT on 2 transanal stapled techniques for outlet obstruction. Outlet obstruction caused not only by rectocoeles but also by descending perineum and intussusception, different patient populations. Prolapse data not presented |
| Carramao 2008a | Camarro and colleagues presented results for 15 women in the hysterectomy group and 16 in the hysteropexy group. This paper was excluded owing to the poor sample size and lack of data regarding continence outcomes, quality of life, and complications. |
| Choe 2000 | RCT on mesh vs vaginal wall sling for stress incontinence. Not all women had pelvic organ prolapse before the operation. |
| Colombo 1996b | RCT on Burch colposuspension and paravaginal defect repair for stress incontinence. No report on treatment of associated anterior vaginal wall prolapse |
| Cruikshank 1999 | RCT on 3 operations for prevention of enterocoele. Study does not include treatment of prolapse. |
| Debodinance 1993 | Comparison of 2 different procedures for stress incontinence and prolapse on examination but no symptomatic pelvic organ prolapse included |
| Del Roy 2010 | Del Roy compared in a single‐centre RCT anterior colporrhaphy vs NAZCA TC™, macroporous polypropylene mesh, in surgical treatment to greater (grade III and IV) anterior vaginal prolapse. 78 women were included in this study. This study was excluded from this review owing to different patient populations and paucity of data regarding distribution of patients within the 2 procedures. |
| Di Palumbo 2003 | RCT with unclear operations and comparisons. No clear definition of success or failure |
| Duggan 2010 | Duggan and Barry assessed short‐term results in an RCT comparing traditional colporrhaphy (n = 16) and mesh repair (n = 19) for anterior compartment prolapse. Because of a predefined decision that papers with fewer than 20 in each treatment group would not be included in the review, the manuscript was excluded. |
| Glazener 2009 | Study did not include continence outcomes in its aims. No separate analysis of incontinence outcomes |
| Hviid 2010 | Study authors did not include continence outcomes in their aims. |
| Lamblin 2014 | Study included women with mixed continence status at baseline and did not provide data separately; therefore different patient populations are included. |
| Lundarelli 2009 | Lundarelli compared polypropylene mesh vs site‐specific repair in the treatment of stage 3 or 4 or recurrent prolapse of the anterior vaginal wall prolapse. This study was excluded from the review, as the sample size of 16 in each group was less than our predetermined group minimum of 20. Furthermore, a mixed continence status was noted at baseline. |
| Menefee 2011 | Study authors did not include continence outcomes in their aims; therefore they reported on different patient populations. |
| Minassian 2014 | Study authors included women with mixed continence status at baseline and did not provide data separately. Some patients in both groups received suburethral tapes; therefore different study populations were reported. |
| Natale 2009 | Study authors did not include continent OR incontinent women in their study. |
| Pantazis 2011 | Study authors included women with mixed continence status at baseline, thus assessing different patient populations. They did not report continence outcomes. |
| Quadri 1985 | Conference abstracts with unclear patient populations, numbers, and definitions, and with limited prolapse data. |
| Rane 2004 | RCT of 3 different operations (vaginal sacrospinous fixation (SSF), posterior intravaginal slingplasty (IVS), sacrocolpopexy (SCP) (abdominal or laparoscopic)) with MRI findings presented. Study authors did not study required patient population. |
| Roovers 2004 | Study authors did not include continent OR incontinent women and did not report on incontinence outcomes based on baseline continence status. |
| Svabik 2014 | Svabik compared sacrospinous fixation and Prolift mesh but did not include incontinent OR continent women in study aims. |
| Tincello 2009 | Tincello reported a pilot randomised patient preference study comparing colposuspension or TVT for urinary incontinence at the time of anterior repair for prolapse. Thirty‐one women were recruited; however only 4 or 2 in each arm randomised. Owing to a predefined decision that papers with fewer than 20 in each treatment group would not be included in the review, the manuscript was excluded. |
| Zargham 2013 | Zargham included women with SUI as the primary complaint, with no symptomatic POP. |
IVS: intravaginal slingplasty.
MRI: magnetic resonance imaging.
POP: pelvic organ prolapse.
RCT: randomised controlled trial.
SCP: sacrocolpopexy.
SSF: sacrospinous fixation.
SUI: stress urinary incontinence.
TVT: tension‐free vaginal tape.
Characteristics of ongoing studies [ordered by study ID]
NCT01095692.
| Trial name or title | ATHENA |
| Methods | RCT |
| Participants | Women with occult UI |
| Interventions | POP + SUI surgery vs POP surgery alone |
| Outcomes |
Primary: To compare the postoperative prevalence of stress incontinence in patients with or without TOT implant during a pelvic organ prolapse surgery [Time Frame: 6 months] Secondary:
|
| Starting date | July 2010 |
| Contact information | A Cortesse: ariane.cortesse@sls.aphp.fr, Assistance Publique, Hôpitaux de Paris. |
| Notes | clinical trials.gov; NCT01095692 |
NCT01802281.
| Trial name or title | SUPeR |
| Methods | RCT |
| Participants | Symptomatic POP |
| Interventions | Native tissue repair with vaginal hysterectomy and suture apical suspension vs uterine conservation with mesh hysteropexy |
| Outcomes | Composite primary outcome of success defined as no prolapse symptoms, no objective prolapse beyond the hymen, and no retreatment of prolapse, with a minimum of 36 months' post surgery follow‐up using survival analyses |
| Starting date | April 2013 |
| Contact information | Charles W Nager, MD. University of California at San Diego, UCSD Women's Pelvic Medicine Center |
| Notes | NCT01802281 |
POP: pelvic organ prolapse.
RCT: randomised controlled trial.
SUI: stress urinary incontinence.
TOT: transobturator tape.
UI: urinary incontinence.
Differences between protocol and review
2013: there were no changes from the protocol. The protocol was written with available studies and clinical needs in mind.
2018: a comparison of surgical interventions for management of continence outcomes was formerly part of the 2013 Cochrane review "Surgical management of pelvic organ prolapse in women". We now present this as a separate review.
Contributions of authors
All review authors contributed to writing the protocol. Three review authors (K Baessler, C Christmann‐Schmid, C Maher) assessed the relevance and eligibility of studies for inclusion in the 2018 review. They then assessed the quality of included studies; independently extracted data from trial reports, interpreted the results, and contributed to the writing of the draft version of the review. All review authors read and approved the review.
Sources of support
Internal sources
-
Cochrane, UK.
Cochrane Review Support Programme: pelvic organ prolapse reviews
External sources
-
National Institute for Health Research (NIHR), Other.
This project was supported by the NIHR, via Cochrane Infrastructure funding to the Cochrane Incontinence Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS, or the Department of Health.
Declarations of interest
KB, JB, CC, TC, NH, and CM have no conflicts of interest to declare.
New
References
References to studies included in this review
Altman 2011 {published data only}
- Altman D, Väyrynen T, Engh ME, Axelsen S, Falconer C, for the Nordic Transvaginal Mesh Group. Anterior colporrhaphy versus transvaginal mesh for pelvic‐organ prolapse. New England Journal of Medicine 2011;364(19):1826‐36. [41463] [DOI] [PubMed] [Google Scholar]
- Ek M, Altman D, Elmér C, Gunnarsson J, Falconer C, Tegerstedt G. Clinical efficacy of a trocar guided mesh kit for the repair of anterior lateral defects (Abstract number 556). 41st Annual Meeting of the International Continence Society (ICS), 2011 Aug 29 to Sept 2, Glasgow, Scotland. 2011, issue 2011. [CRSREF: 3226049]. [Abstract number 556]
- Ek M, Tegerstedt G, Falconer C, Kjaeldgaard A, Rezapour M, Rudnicki M, et al. Urodynamic assessment of anterior vaginal wall surgery: a randomized comparison between colporrhaphy and transvaginal mesh. Neurourology and Urodynamics 2010;29:527‐31. [39589] [DOI] [PubMed] [Google Scholar]
Borstad 2010 {published data only}
- Borstad E, Abdelnoor M, Mogimi K, Sandved M, Majida M, Western K, et al. Surgery for concomitant pelvic organ prolapse and urinary stress incontinence. A multicenter prospective randomized trial to compare the results of an incontinence procedure performed at the time of prolapse repair or 3 months after (Abstract number 120). Neurourology and Urodynamics 2008;27(7):713. [29653] [Google Scholar]
- Borstad E, Abdelnoor M, Staff AC, Kulseng‐Hanssen S. Surgical strategies for women with pelvic organ prolapse and urinary stress incontinence. International Urogynecology Journal and Pelvic Floor Dysfunction 2010;21(2):179‐86. [39362] [DOI] [PubMed] [Google Scholar]
- Borstad E, Kulseng‐Hanssen S, Moghimi K, Sandved M, Majida M, Western K, et al. An incontinence procedure performed at the time of prolapse repair might be unnecessary surgery (Abstract number 35). Neurourology and Urodynamics 2006;25(6):551‐2. [26618] [Google Scholar]
Brubaker 2006 {published data only}
- Brubaker L, Cundiff G, Fine P, Nygaard I, Richter H, Visco A, et al. A randomized trial of colpopexy and urinary reduction efforts (CARE): design and methods. Controlled Clinical Trials 2003;24(5):629‐42. [DOI] [PubMed] [Google Scholar]
- Brubaker L, Cundiff G, Fine P, Nygaard I, Richter H, Visco A, et al. Abdominal sacrocolpopexy with Burch colposuspension to reduce urinary stress incontinence. New England Journal of Medicine 2006;354(15):1557‐66. [DOI] [PubMed] [Google Scholar]
- Brubaker L, Nygaard I, Richter HE, Visco A, Weber AM, Cundiff GW, et al. Two‐year outcomes after sacrocolpopexy with and without Burch to prevent stress urinary incontinence. Obstetrics and Gynecology 2008;112(1):49‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure LA, Brown MB. A likelihood approach to analyzing clinical trial data when treatments favor different outcomes. Contemporary Clinical Trials 2006;27(4):340‐52. [DOI] [PubMed] [Google Scholar]
- Visco AG, Brubaker L, Nygaard I, Richter HE, Cundiff G, Fine P, et al. Pelvic Floor Disorders Network. The role of preoperative urodynamic testing in stress‐continent women undergoing sacrocolpopexy. International Urogynecology Journal 2008;19(5):607‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colombo 2000 {published data only}
- Colombo M, Vitobello D, Proietti F, Milani R. Randomised comparison of Burch colposuspension versus anterior colporrhaphy in women with stress urinary incontinence and anterior vaginal wall prolapse. British Journal of Obstetrics and Gynaecology 2000;107(4):544‐51. [DOI] [PubMed] [Google Scholar]
Costantini 2007 {published data only}
- Costantini E, Lazzeri M, Bini V, Zingaro M, Zucchi A, Porena M. Pelvic organ prolapse repair with and without prophylactic concomitant Burch colposuspension in continent women: a randomized, controlled trial with 8‐year follow up. Journal of Urology 2011;185(6):2236‐40. [DOI] [PubMed] [Google Scholar]
- Costantini E, Zucchi A, Giannantoni A, Mearini L, Bini V, Porena M. Must colposuspension be associated with sacropexy to prevent postoperative urinary incontinence?. European Urology 2007;51(3):788‐94. [DOI] [PubMed] [Google Scholar]
Costantini 2008 {published data only}
- Costantini E, Lazzeri M, Bini V, Zingaro M, Frumenzio E, Porena M. Pelvic organ prolapse repair with and without concomitant Burch colposuspension in incontinent women: a randomised controlled trial with at least 5‐year followup. Obstetrics and Gynecology International 2012;2012:967923. [DOI: 10.1155/2012/967923] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini E, Lazzeri M, Bini V, Zingaro M, Zucchi A, Porena M. Burch colposuspension does not provide any additional benefit to pelvic organ prolapse repair in patients with urinary incontinence: a randomized surgical trial [see comment]. Journal of Urology 2008;180(3):1007‐12. [DOI] [PubMed] [Google Scholar]
Fuentes 2011 {published data only}
- Fuentes AE. A prospective randomised controlled trial comparing vaginal prolapse repair with and without tension‐free vaginal tape transobturator tape (TVTO) in women with severe genital prolapse and occult stress incontinence: long term follow up. International Urogynecology Journal 2011;22(Suppl 1)(Abstract 059):S60‐1. [Google Scholar]
Hiltunen 2007 {published data only}
- Hiltunen R, Nieminen K, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Low‐weight polypropylene mesh for anterior vaginal wall prolapse: a randomized controlled trial. Obstetrics and Gynecology 2007;110(2 pt 2):455‐62. [DOI] [PubMed] [Google Scholar]
- Nieminen K, Hiltunen R, Heiskanen E, Takala T, Niemi K, Merikari M, et al. Symptom resolution and sexual function after anterior vaginal wall repair with or without polypropylene mesh. International Urogynecology Journal. 2008;19(12):1611‐6. [DOI] [PubMed] [Google Scholar]
- Nieminen K, Hiltunen R, Takala T, Heiskanen E, Merikari M, Niemi K, et al. Outcomes after anterior vaginal wall repair with mesh: a randomized, controlled trial with a 3 year follow‐up. American Journal of Obstetrics and Gynecology 2010;203(3):235.e1‐8. [40020] [DOI] [PubMed] [Google Scholar]
Iglesia 2010 {published data only}
- Gutman RE, Nosti PA, Sokol AI, Sokol ER, Peterson JL, Wang H, Iglesia CB. Three‐year outcomes of vaginal mesh for prolapse: a randomized controlled trial. Obstetrics & Gynecology 2013;122(4):770‐77. [DOI] [PubMed] [Google Scholar]
- Iglesia CB, Sokol AI, Sokol ER, Kudish BI. Vaginal mesh for prolapse: a randomized controlled trial. Obstetrics & Gynecology 2010;116(2 Pt 1):293‐303. [39891] [DOI] [PubMed] [Google Scholar]
- Sokol AI, Iglesia CB, Kudish BI, Gutman RE, Shveiky D, Bercik R, et al. One‐year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. American Journal of Obstetrics and Gynecology 2012;206(1):86.e1‐9. [DOI: 10.1016/j.ajog.2011.08.003; 42158] [DOI] [PubMed] [Google Scholar]
Meschia 2004 {published data only}
- Meschia M, Buonaguidi A, Amicarelli F, Pifarotti P, Gattei U, Stoppelli S. A randomized prospective comparison of TVT and endopelvic fascia plication in the treatment of occult stress urinary incontinence in patients with severe genital prolapse (Abstract). International Urogynecology Journal and Pelvic Floor Dysfunction 2001;12 Suppl 3:10. [15457] [Google Scholar]
- Meschia M, Pifarotti P, Gattei U, Ronchetti A, Stoppelli S, Lampugnani F. TVT and prolapse repair for treatment of occult stress urinary incontinence. International Continence Society (ICS) 32nd Annual Meeting; 2002 Aug 28‐30; Heidelberg, Germany. 2002:198‐9. [299]
- Meschia M, Pifarotti P, Spennacchio M, Buonaguidi A, Gattei U, Somigliana E. A randomized comparison of tension‐free vaginal tape and endopelvic fascia plication in women with genital prolapse and occult stress urinary incontinence. American Journal of Obstetrics and Gynecology 2004;190(3):609‐13. [17213] [DOI] [PubMed] [Google Scholar]
- Meschia M, Spennacchio F, Amicarelli P, Pifarotti P, Cavoretto S, Stoppelli S. A randomized prospective comparison of TVT and endopelvic fascia plication in the treatment of occult stress urinary incontinence in patients with genital prolapse: preliminary data. Neurourology and Urodynamics 2001;20(4):423‐4. [Google Scholar]
Rudnicki 2014 {published data only}
- Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. A 3‐year follow‐up after anterior colporrhaphy compared with collagen‐coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 2016;123(1):136‐42. [DOI] [PubMed] [Google Scholar]
- Rudnicki M, Laurikainen E, Pogosean R, Kinne I, Jakobsson U, Teleman P. Anterior colporrhaphy compared with collagen‐coated transvaginal mesh for anterior vaginal wall prolapse: a randomised controlled trial. British Journal of Obstetrics and Gynaecology 2014;121(1):102‐10. [DOI] [PubMed] [Google Scholar]
Schierlitz 2014 {published data only}
- Schierlitz L, Dwyer P, Rosamilia A, Souza A, Murray C, Thomas E, et al. Pelvic organ prolapse surgery with and without tension‐free vaginal tape in women with occult or asymptomatic urodynamic stress incontinence: a randomised controlled trial. International Urogynecology Journal 2014;25:33‐40. [DOI] [PubMed] [Google Scholar]
- Walsh CE, Dwyer PL, Rosamilia A, Murray C, Thomas EA, Hiscock R, et al. A randomised controlled trial comparing pelvic organ prolapse surgery with and without tension‐free vaginal tape in women with occult or asymptomatic urodynamic stress incontinence: 6 year follow‐up. International Urogynecology Journal and Pelvic Floor Dysfunction 2016;27(1 Suppl 1):S57‐8. [DOI] [PubMed] [Google Scholar]
Sivaslioglu 2008 {published data only}
- Sivaslioglu AA, Unlubilgin E, Dolen I. A randomized comparison of polypropylene mesh surgery with site‐specific surgery in the treatment of cystocoele. International Urogynecology Journal. 2008;19(4):467‐71. [DOI] [PubMed] [Google Scholar]
Trabuco 2014 {unpublished data only}
- Trabuco EC, Klingele CJ, Blandon R, Occhino JA, McGree ME, Weaver AL, et al. A randomized comparison of incontinence procedures performed concomitantly with abdominal sacral colpopexy: the Burch versus mid‐urethral sling trial. International Urogynecology Journal 2014;25(Suppl 1):S1‐2. [Google Scholar]
- Trabuco EC, Klingele CJ, Occhino J, Blandon RE, McGree ME, Weaver A, et al. Treatment success of Burch and midurethral sling 2 years following combined procedure with sacrocolpopexy. International Urogynecology Journal and Pelvic Floor Dysfunction 2016;27(1 Suppl):S46. [Google Scholar]
Turgal 2013 {published data only}
- Turgal M, Sivaslioglu A, Yildiz A, Dolen I. Anatomical and functional assessment of anterior colporrhaphy versus polypropylene mesh surgery in cystocele treatment. European Journal of Obstetrics and Gynecology and Reproductive Biology 2013;170(2):555‐8. [DOI] [PubMed] [Google Scholar]
van der Ploeg 2015 (CUPIDO I) {published data only}
- Roovers JPWR, Ploeg M. Concomitant surgery and urodynamic investigation in genital prolapse and stress incontinence. A diagnostic study including outcome evaluation. CUPIDO 1: vaginal prolapse repair and mid urethral sling procedure in women with genital prolapse and predominant stress urinary incontinence. Netherlands Trial Register. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1197 2009. [34193]
- Ploeg J, Rengerink K, Steen A, Leeuwen H, Stekelenburg J, Bongers M, et al. on behalf of the Dutch Urogynaecology Consortium. Transvaginal prolapse repair with or without the addition of a midurethral sling in women with genital prolapse and stress urinary incontinence: a randomised trial. British Journal of Obstetrics & Gynaecology 2015;122:1022‐30. [DOI] [PubMed] [Google Scholar]
- Steen A, Ploeg M, Dijkgraaf MG, V, Roovers JP. Protocol for the CUPIDO trials; multicenter randomized controlled trials to assess the value of combining prolapse surgery and incontinence surgery in patients with genital prolapse and evident stress incontinence (CUPIDO I) and in patients with genital prolapse and occult stress incontinence (CUPIDO II). BMC Women's Health 2010;10:16. [39877] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Ploeg 2016 (CUPIDO II) {published data only}
- Ploeg J, Rengerink K, Steen A, Leeuwen H, Haart C, Roovers JP, on behalf of the Dutch Urogynaecology Consortium. Vaginal prolapse repair with or without a midurethral sling in women with genital prolapse and occult stress urinary incontinence: a randomized trial. International Urogynecology Journal 2016;27(7):1029‐38. [DOI] [PubMed] [Google Scholar]
- Steen A, Ploeg M, Dijkgraaf MG, Vaart H, Roovers JP. Protocol for the CUPIDO trials; multicenter randomized controlled trials to assess the value of combining prolapse surgery and incontinence surgery in patients with genital prolapse and evident stress incontinence (CUPIDO I) and in patients with genital prolapse and occult stress incontinence (CUPIDO II). BMC Women's Health 2010;10:16. [39877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2011 {published data only}
- Kenton K. The value of the preoperative prolapse reduction stress test in women without stress incontinence symptoms undergoing vaginal prolapse surgery with or without a TVT: result from the OPUS trial (Abstract 50). Neurourology and Urodynamics 2011;30(6):870‐1. [42171] [Google Scholar]
- Wei J. A mid urethral sling prevents incontinence among women undergoing vaginal prolapse repair ‐ the OPUS trial (Abstract number 5). Neurourology and Urodynamics 2011;30(6):809‐10. [42165] [Google Scholar]
- Wei J, Nygaard I, Richter H, Brown M, Barber M, Xiao Xu, et al. Outcomes following vaginal prolapse repair and mid urethral sling (OPUS) trial ‐ design and methods. Clinical Trials 2009;6(2):162‐71. [31120] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Nygaard I, Richter H, Nager C, Barber MD, Kenton K. A midurethral sling to reduce incontinence after vaginal prolapse repair. New England Journal of Medicine 2012;366(25):2358‐67. [45050] [DOI] [PMC free article] [PubMed] [Google Scholar]
Withagen 2011 {published and unpublished data}
- Milani AL, Withagen MI, The HS, Nedelcu‐Van der Wijk I, Vierhout ME. Sexual function following trocar‐guided mesh or vaginal native tissue repair in recurrent prolapse: a randomized controlled trial. Journal of Sexual Medicine 2011;8(10):2944‐53. [42064] [DOI] [PubMed] [Google Scholar]
- Withagen MI, Milani AL, Boon Den J, Vervest HA, Vierhout ME. Tension free vaginal mesh compared to conventional vaginal prolapse surgery in recurrent prolapse; a randomized controlled trial (Abstract number 090). International Urogynaecology Journal 2009;20 Suppl 2:S153‐4. [39885] [Google Scholar]
- Withagen MI, Milani AL, Boon J, Vervest HA, Vierhout ME. Trocar‐guided mesh compared with conventional vaginal repair in recurrent prolapse: a randomized controlled trial. Obstetrics and Gynecology 2011;117(2 Pt 1):242‐50. [40881] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allahdin 2008 {published data only}
- Allahdin S, Glazener C, Bain C. A randomised controlled trial evaluating the use of polyglactin mesh, polydioxanone and polyglactin sutures for pelvic organ prolapse surgery. Journal of Obstetrics and Gynaecology 2008;28(4):427‐31. [DOI] [PubMed] [Google Scholar]
- Madhuvrata P, Glazener C, Boachie C, Allahdin S, Bain C. A randomised controlled trial evaluating the use of polyglactin (Vicryl) mesh, polydioxanone (PDS) or polyglactin (Vicryl) sutures for pelvic organ prolapse surgery: outcomes at 2 years. Journal of Obstetrics and Gynaecology 2011;31(5):429‐35. [DOI] [PubMed] [Google Scholar]
Barber 2006 {published data only}
- Barber MD, Walters MD, Cundiff GW, PESSRI Trial Group. Responsiveness of the Pelvic Floor Distress Inventory (PFDI) and Pelvic Floor Impact Questionnaire (PFIQ) in women undergoing vaginal surgery and pessary treatment for pelvic organ prolapse. American Journal of Obstetrics and Gynecology 2006;194(5):1492‐8. [DOI] [PubMed] [Google Scholar]
Bergman 1989 {published data only}
- Bergman A, Koonings PP, Ballard CA. Primary stress urinary incontinence and pelvic relaxation. American Journal of Obstetrics and Gynecology 1989;161(1):97‐101. [DOI] [PubMed] [Google Scholar]
Biller 2008 {published data only}
- Biller DH, Guerette NL, Bena JF, Davila GW. A prospective, randomized controlled trial of the use of an anal purse‐string suture to decrease contamination during pelvic reconstructive surgery. International Urogynecology Journal. 2008;19(1):59‐63. [DOI] [PubMed] [Google Scholar]
Boccasanta 2004 {published data only}
- Boccasanta P, Venturi M, Salamina G, Cesana BM, Bernasconi F, Roviaro G. New trends in the surgical treatment of outlet obstruction: clinical and functional results of two novel transanal stapled techniques from a randomised controlled trial. International Journal of Colorectal Disease 2004;19:359‐69. [DOI] [PubMed] [Google Scholar]
Carramao 2008a {published data only}
- Carramao S, Auge AP, Pacetta AM, Duarte E, Ayrosa P, Lemos NL, et al. A randomized comparison of two vaginal procedures for the treatment of uterine prolapse using polypropylene mesh: hysteropexy versus hysterectomy. Revista do Colegio Brasileiro de Cirurgioes 2009;36(1):65‐72. [DOI] [PubMed] [Google Scholar]
- Carramao S, Lopes E, Auge A, Lemos N, Lunardelli J, Aoki T. A randomized comparison of two vaginal surgery for pelvic organ prolapse: hysterectomy with vaginal sacrospinous ligament fixation versus hysteropexy with repair of pelvic floor using mesh (Abstract number 93). International Urogynecology Journal 2008;19(Suppl 1):96. [Google Scholar]
Choe 2000 {published data only}
- Choe JM, Ogan K, Battino B. Antimicrobial mesh versus vaginal wall sling: a comparative outcomes analysis. Journal of Urology 2000;163(6):1829‐34. [PubMed] [Google Scholar]
Colombo 1996b {published data only}
- Colombo M, Milani R, Vitobello D, Maggioni A. A randomized comparison of Burch colposuspension and abdominal paravaginal defect repair for female stress urinary incontinence. American Journal of Obstetrics and Gynecology 1996;175(1):78‐84. [DOI] [PubMed] [Google Scholar]
Cruikshank 1999 {published data only}
- Cruikshank SH, Kovac SR. Randomized comparison of three surgical methods used at the time of vaginal hysterectomy to prevent posterior enterocele. American Journal of Obstetrics and Gynecology 1999;180(4):859‐65. [DOI] [PubMed] [Google Scholar]
Debodinance 1993 {published data only}
- Debodinance P. Comparison of the Bologna and Ingelman‐Sundberg procedures for stress incontinence associated with genital prolapse: ten‐year follow‐up of a prospective randomized study. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 2000;29(2):148‐53. [PubMed] [Google Scholar]
- Debodinance P, Querleu D. Comparison of the Bologna and Ingelman‐Sundberg procedures for stress incontinence associated with genital prolapse: prospective randomized study. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1993;52(1):35‐40. [DOI] [PubMed] [Google Scholar]
Del Roy 2010 {published data only}
- Roy C. A randomized controlled trial study, to compare colporrhaphy versus NAZCA TC™, Macroporous polypropylene mesh, in surgical treatment to greater anterior vaginal prolapse. Joint Meeting of the International Continence Society (ICS) and the International Urogynecological Association; 2010 Aug 23‐27, Toronto, Canada. 2010. [Abstract number 667]
Di Palumbo 2003 {published data only}
- Palumbo VS. Four‐corner bladder and urethral retropubic suspension versus anterior colporrhaphy in the correction of stress urinary incontinence and urethrocystocele 3‐4. Randomized clinical trial. Urogynaecologia International Journal 2003;17(2):57‐68. [Google Scholar]
Duggan 2010 {published data only}
- Duggan P, Barry C. Anterior compartment prolapse: short term results and quality of life in women randomised to mesh or traditional repair. Joint Meeting of the International Continence Society (ICS) and the International Urogynecological Association; 2010 Aug 23‐27, Toronto, Canada. 2010. [Abstract number 687]
Glazener 2009 {published data only}
- Glazener CMA. Clinical and cost‐effectiveness of surgical options for the management of anterior and/or posterior vaginal wall prolapse: two randomised controlled trials within a comprehensive cohort study (PROSPECT), 2009. www.controlled‐trials.com/ISRCTN60695184 (accessed 13 April 2010).
Hviid 2010 {published data only}
- Hviid U, Hviid TV, Rudnicki M. Porcine skin collagen implants for anterior vaginal wall prolapse: a randomised prospective controlled study. International Urogynecology Journal 2010;21(5):529‐34. [39449] [DOI] [PubMed] [Google Scholar]
Lamblin 2014 {published data only}
- Lamblin G, Van‐Nieuwenhuyse A, Chabert P, Lebail‐Carval K, Moret S, Mellier G. A randomized controlled trial comparing anatomical and functional outcome between vaginal colposuspension and transvaginal mesh. International Urogynecology Journal 2014;25:961–70. [DOI: 10.1007/s00192-014-2344-7] [DOI] [PubMed] [Google Scholar]
Lundarelli 2009 {published data only}
- Lunardelli JL, Auge AP, Lemos NL, Silva Carramao S, Oliveira AL, Duarte E, et al. Polypropylene mesh vs. Site‐specific repair in the treatment of anterior vaginal wall prolapse: preliminary results of a randomized clinical trial. Revista do Colegio Brasileiro de Cirurgioes 2009;36(3):210‐6. [PubMed] [Google Scholar]
Menefee 2011 {published data only}
- Dyer K, Nguyen J, Lukacz E, Simsiman A, Luber K, Menefee S. The Optimal Anterior Repair Study (OARS): a triple arm randomized double blinded clinical trial of standard colporrhaphy, porcine dermis or polypropylene mesh augmented anterior vaginal wall repair (Abstract number 252). Neurourology and Urodynamics 2009;28(7):894‐5. [39346] [Google Scholar]
- Dyer K, Nguyen J, Simsiman A, Lukacz E, Luber K, Menefee S. The Optimal Anterior Repair Study (OARS): a triple arm randomized double blinded clinical trial of standard colporrhaphy versus vaginal paravaginal repair with porcine dermis graft or polypropylene mesh (Abstract number 281). Neurourology and Urodynamics 2010;29(6):1207‐8. [40164] [Google Scholar]
- Menefee SA, Dyer KY, Lukacz ES, Simsiman AJ, Luber KM, Nguyen JN. Colporrhaphy compared with mesh or graft‐reinforced vaginal paravaginal repair for anterior vaginal wall prolapse: a randomized controlled trial. Obstetrics and Gynecology 2011;118(6):1337‐44. [42866] [DOI] [PubMed] [Google Scholar]
Minassian 2014 {published data only}
Natale 2009 {published data only}
- Cervigni M, Natale F, Weir J, Galante L, Panei M, Agostini M, et al. Prospective randomized trial of two new materials for the correction of anterior compartment prolapse: Pelvicol and Prolene Soft (Abstract). Neurourology and Urodynamics 2005;24(5/6):585‐6. [Google Scholar]
- Natale F, Penna C, Padoa A, Agostini M, Simone E, Cervigni M. A prospective, randomized, controlled study comparing Gynemesh(R), a synthetic mesh, and Pelvicol(R), a biologic graft, in the surgical treatment of recurrent cystocele. International Urogynecology Journal 2009;20(1):75‐81. [DOI] [PubMed] [Google Scholar]
Pantazis 2011 {published data only}
- Freeman RM, Pantazis K, Thomson A, Frapell J, Bombieri L, Moran P, et al. A randomised controlled trial of abdominal versus laparoscopic sacrocolpopexy for the treatment of post‐hysterectomy vaginal vault prolapse: LAS study [epub ahead of print]. International Urogynecology Journal 2013; Vol. 24, issue 3:377‐84. [DOI: 10.1007/s00192-012-1885-x; 46279] [DOI] [PubMed]
- Pantazis K, Freeman R, Thomson A, Frappell J, Bombieri L, Moran P, et al. Open and laparoscopic sacrocolpopexy demonstrate clinical equivalence: one year results from the LAS Trial, an RCT comparing the two approaches for treating post hysterectomy vault prolapse (Abstract number 131). Neurourology and Urodynamics 2011;30(6):986‐7. [42187] [Google Scholar]
- Pantazis K, Freeman R, Thomson A, Frappell J, Bombieri L, Waterfield M. Results from the LAS trial, an RCT comparing open abdominal to laparoscopic sacrocolpopexy for the treatment of post hysterectomy vault prolapse (Abstract number 120). International Urogynecology Journal 2008;19 Suppl 1:101‐2. [29178] [Google Scholar]
Quadri 1985 {published data only}
- Quadri G, Scalambrino S, Boisio N, Marchesin R, Milani R. Randomized surgery for incontinence and prolapse: retropubic colposuspension vs anterior repair (abstract). Archives of Gynecology 1985;237(Suppl):402. [8019] [Google Scholar]
Rane 2004 {published data only}
- Rane A, Lim YN, Withey G, Muller R. Magnetic resonance imaging findings following three different vaginal vault prolapse repair procedures: a randomised study. Australian & New Zealand Journal of Obstetrics & Gynaecology 2004;44(2):135‐9. [DOI] [PubMed] [Google Scholar]
Roovers 2004 {published and unpublished data}
- Roovers J, Bleijenberg E, Schagen van Leeuwen J, Scholten P, Vaart H. Long term follow‐up of a randomized controlled trial comparing abdominal and vaginal surgical correction of uterine prolapse (Abstract number 88). International Urogynecology Journal 2008;19 Suppl 1:91‐2. [Google Scholar]
- Roovers JPWR, Bom JG, Vaart CH, Schagen van Leeuwen JH, Scholten PC, Heintz APM. A randomized comparison of post‐operative pain, quality of life, and physical performance during the first six weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourology and Urodynamics 2005;24:334‐40. [DOI] [PubMed] [Google Scholar]
- Roovers JPWR, Vaart CH, Bom JG, Schagen van Leeuwen JH, Scholten PC, Heintz APM. A randomized controlled trial comparing abdominal and vaginal prolapse surgery of patients with descensus uteri grade II ‐ IV (Abstract). International Urogynaecology Journal 2001;12 Suppl 3:S109. [16341] [Google Scholar]
- Roovers JPWR, Vaart CH, Bom JG, Leeuwen JHS, Scholten PC, Heintz APM. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. British Journal of Obstetrics and Gynaecology 2004;111(1):50‐6. [DOI] [PubMed] [Google Scholar]
Svabik 2014 {published data only}
- Svabik K, Martan A, Masata J, El‐Haddad R, Hubka P. Comparison of vaginal mesh repair with sacrospinous vaginal colpopexy in the management of vaginal vault prolapse after hysterectomy in patients with levator aniavulsion: a randomized controlled trial. Ultrasound in Obstetrics and Gynecology 2014;43(4):365–71. [DOI] [PubMed] [Google Scholar]
Tincello 2009 {published data only}
- Tincello DG, Kenyon S, Slack M, Toozs‐Hobson P, Mayne C, Jones D, et al. Colposuspension or TVT with anterior repair for urinary incontinence and prolapse: results of and lessons from a pilot randomised patient‐preference study (CARPET 1). British Journal of Obstetrics and Gynaecology 2009;116(13):1809‐14. [DOI] [PubMed] [Google Scholar]
- Tincello DG, Mayne CJ, Toozs‐Hobson P, Slack M. Randomised controlled trial of colposuspension versus anterior repair plus TVT for urodynamic stress incontinence with anterior vaginal prolapse: proposal (Abstract). International Continence Society, 11th Annual Scientific Meeting; 2004 Mar 18‐19; Bournemouth, United Kingdom. 2004:46. [17170]
Zargham 2013 {published data only}
- Zargham M, Alizadeh F, Tadayyon F, Khorrami MH, Nouri‐Mahdavi K, Gharaati MR, et al. Concomitant surgical correction of severe stress urinary incontinence and anterior vaginal wall prolapse by anterior vaginal wall wrap: 18 months outcomes. Journal of Research in Medical Sciences 2013;18(7):588‐93. [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT01095692 {published data only}
- NCT01095692. Evaluating the necessity of TOT implantation in women with pelvic organ prolapse and occult stress urinary incontinence (ATHENA). www.ClinicalTrials.gov 2011:http://clinicaltrials.gov/ct2/show/ NCT01095692 (accessed 19 April 2011). [41350]
NCT01802281 {published data only}
- SUPeR. Ongoing study April 2013.
Additional references
Bugge 2013
- Bugge C, Adams EJ, Gopinath D, Reid F. Pessaries (mechanical devices) for pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD004010.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ek 2010
- Ek M, Tegerstedt G, Falconer C, Kjaeldgaard A, Rezapour M, Rudnicki M, et al. Urodynamic assessment of anterior vaginal wall surgery: a randomized comparison between colporraphy and transvaginal mesh. Neurourology and Urodynamics 2010;29:527‐31. [39589] [DOI] [PubMed] [Google Scholar]
Ek 2011
- Ek M, Altman D, Elmér C, Gunnarsson J, Falconer C, Tegerstedt G. Clinical efficacy of a trocar guided mesh kit for the repair of anterior lateral defects (Abstract number 556). 41st Annual Meeting of the International Continence Society (ICS), 2011 Aug 29 to Sept 2, Glasgow, Scotland. 2011.
Ellström 2011
- Ellström Engh AM, Ekeryd A, Magnusson A, Olsson I, Otterlind L, Tobiasson G. Can de novo stress incontinence after anterior wall repair be predicted?. Acta Obstetricia et Gynecologica Scandinavica 2011;90(5):488‐493. [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- McMaster University. GRADEpro GDT 2015. Version accessed 1 February 2018. Hamilton (ON): McMaster University, 2015. Available at gradepro.org.
Haessler 2005
- Haessler AL, Lin LL, Ho MH, Betson LH, Bhatia NN. Reevaluating occult incontinence. Current Opinion in Obstetrics and Gynecology 2005;17(5):535‐40. [DOI] [PubMed] [Google Scholar]
Hagen 2011
- Hagen S, Stark D. Conservative prevention and management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003882.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Handa 2004
- Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. American Journal of Obstetrics and Gynecology 2004;190(1):27‐32. [DOI] [PubMed] [Google Scholar]
Haylen 2010
- Haylen BT, Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourology and Urodynamics 2010;29(1):4‐20. [DOI] [PubMed] [Google Scholar]
Hendrix 2002
- Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. American Journal of Obstetrics and Gynecology 2002;186(6):1160‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8. Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICI 2017
- Maher CF, et al. Pelvic organ prolapse surgery. In: Abrams P, Cardozo L, Wagg A, Wein A editor(s). Incontinence. 6th Edition. Bristol: International Continence Society, 2017. [ISBN: 978‐0956960733] [Google Scholar]
Maher 2018
- Maher CF, Baessler KK, Barber MD, Cheon C, Consten ECJ, Cooper KG, et al. Summary: 2017 International Consultation on Incontinence Evidence‐Based Surgical Pathway for Pelvic Organ Prolapse. Female Pelvic Medicine & Reconstructive Surgery 2018 April 28 [Epub ahead of print]. [DOI: 10.1097/SPV.0000000000000591] [DOI] [PubMed]
Matsuoka 2015
- Matsuoka PK, Pacetta AM, Baracat EC, Haddad JM. Should prophylactic anti‐incontinence procedures be performed at the time of prolapse repair? Systematic review. International Urogynecology Journal 2015;26(2):187‐93. [DOI] [PubMed] [Google Scholar]
Reena 2007
- Reena C, Kekre AN, Kekre N. Occult stress incontinence in women with pelvic organ prolapse. International Journal of Gynecology & Obstetrics 2007;97(1):31‐4. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Slieker‐ten Hove 2009
- Slieker‐ten Hove MC, Pool‐Goudzwaard AL, Eijkemans MJ, Steegers‐Theunissen RP, Burger CW, Vierhout ME. The prevalence of pelvic organ prolapse symptoms and signs and their relation with bladder and bowel disorders in a general female population. International Urogynecology Journal and Pelvic Floor Dysfunction 2009;20(9):1037‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visco 2008
- Visco AG, Brubaker L, Nygaard I, Richter HE, Cundiff G, Fine P, et al. The role of preoperative urodynamic testing in stress‐continent women undergoing sacrocolpopexy: the Colpopexy and Urinary Reduction Efforts (CARE) randomized surgical trial. International Urogynecology Journal. 2008;19(5):607‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Maher 2004
- Maher C, Baessler K, Glazener CMA, Adams EJ, Hagen S. Surgical management of pelvic organ prolapse in women. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004014.pub2] [DOI] [PubMed] [Google Scholar]
Maher 2016a
- Maher C, Feiner B, Baessler K, Christmann‐Schmid C, Haya N, Marjoribanks J. Transvaginal mesh or grafts compared with native tissue repair for vaginal prolapse. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012079] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maher 2016b
- Maher C, Feiner B, Baessler K, Christmann‐Schmid C, Haya N, Brown J. Surgery for women with apical vaginal prolapse. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD012376] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maher 2016c
- Maher C, Feiner B, Baessler K, Christmann‐Schmid C, Haya N, Brown J. Surgery for women with anterior compartment prolapse. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD004014.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mowat 2018
- Mowat A, Maher D, Baessler K, Christmann‐Schmid C, Haya N, Maher C. Surgery for women with posterior compartment prolapse. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD012975] [DOI] [PMC free article] [PubMed] [Google Scholar]


