Abstract
Background
Treament‐related diarrhoea is one of the most common and troublesome adverse effects related to chemotherapy or radiotherapy in people with cancer. Its reported incidence has been as high as 50% to 80%. Severe treatment‐related diarrhoea can lead to fluid and electrolyte losses and nutritional deficiencies and could adversely affect quality of life (QoL). It is also associated with increased risk of infection in people with neutropenia due to anticancer therapy and often leads to treatment delays, dose reductions, or treatment discontinuation. Probiotics may be effective in preventing or treating chemotherapy‐ or radiotherapy‐induced diarrhoea.
Objectives
To evaluate the clinical effectiveness and side effects of probiotics used alone or combined with other agents for prevention or treatment of chemotherapy‐ or radiotherapy‐related diarrhoea in people with cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 7), MEDLINE (1946 to July week 2, 2017), and Embase (1980 to 2017, week 30). We also searched prospective clinical trial registers and the reference lists of included studies.
Selection criteria
We included randomised controlled trials (RCTs) investigating the effects of probiotics for prevention or treatment of chemotherapy‐ or radiotherapy‐related diarrhoea in people with cancer.
Data collection and analysis
Two review authors independently selected studies, extracted data, and assessed risk of bias. We used random‐effects models for all meta‐analyses. If meta‐analysis was not possible, we summarised the results narratively.
Main results
We included 12 studies involving 1554 participants. Eleven studies were prevention studies, of which seven compared probiotics with placebo (887 participants), one compared two doses of probiotics with each other and with placebo (246 participants), and three compared probiotics with another active agent (216 participants).The remaining study assessed the effectiveness of probiotics compared with placebo for treatment of radiotherapy‐related diarrhoea (205 participants).
For prevention of radiotherapy (with or without chemotherapy)‐induced diarrhoea, review authors identified five heterogeneous placebo‐controlled studies (with 926 participants analysed). Owing to heterogeneity, we could not carry out a meta‐analysis, except for two outcomes. For occurrence of any diarrhoea, risk ratios (RRs) ranged from 0.35 (95% confidence interval (CI) 0.26 to 0.47) to 1.0 (95% CI 0.94 to 1.06) (three studies; low‐certainty evidence). A beneficial effect of probiotics on quality of life could neither be demonstrated nor refuted (two studies; low‐certainty evidence). For occurrence of grade 2 or higher diarrhoea, the pooled RR was 0.75 (95% CI 0.55 to 1.03; four studies; 420 participants; low‐certainty evidence), and for grade 3 or higher diarrhoea, RRs ranged from 0.11 (95% CI 0.06 to 0.23) to 1.24 (95% CI 0.74 to 2.08) (three studies; low‐certainty evidence). For probiotic users, time to rescue medication was 36 hours longer in one study (95% CI 34.7 to 37.3), but another study reported no difference (moderate‐certainty evidence). For the need for rescue medication, the pooled RR was 0.50 (95% CI 0.15 to 1.66; three studies; 194 participants; very low‐certainty evidence). No study reported major differences between groups with respect to adverse effects. Although not mentioned explicitly, no studies reported deaths, except one in which one participant in the probiotics group died of myocardial infarction after three sessions of radiotherapy.
Three placebo‐controlled studies, with 128 analysed participants, addressed prevention of chemotherapy‐induced diarrhoea. For occurrence of any diarrhoea, the pooled RR was 0.59 (95% CI 0.36 to 0.96; two studies; 106 participants; low‐certainty evidence). For all other outcomes, a beneficial effect of probiotics could be neither demonstrated nor refuted (one to two studies; 46 to 106 participants; all low‐certainty evidence). Studies did not address quality of life nor time to rescue medication.
Three studies compared probiotics with another intervention in 213 participants treated with radiotherapy (with or without chemotherapy). One very small study (21 participants) reported less diarrhoea six weeks after treatment when dietary counselling was provided (RR 0.30, 95% CI 0.11 to 0.81; very low‐certainty evidence). In another study (148 participants), grade 3 or 4 diarrhoea occurred less often in the probiotics group than in the control group (guar gum containing nutritional supplement) (odds ratio (OR) 0.38, 95% CI 0.16 to 0.89; low‐certainty evidence), and two studies (63 participants) found less need for rescue medication of probiotics versus another active treatment (RR 0.44, 95% CI 0.22 to 0.86; very low‐certainty evidence). Studies did not address quality of life nor time to rescue medication.
One placebo‐controlled study with 205 participants addressed treatment for radiotherapy‐induced diarrhoea and could not demonstrate or refute a beneficial effect of probiotics on average diarrhoea grade, time to rescue medication for diarrhoea (13 hours longer in the probiotics group; 95% CI ‐0.9 to 26.9 hours), or need for rescue medication (RR 0.74, 95% CI 0.53 to 1.03; moderate‐certainty evidence). This study did not address quality of life.
No studies reported serious adverse events or diarrhoea‐related deaths.
Authors' conclusions
This review presents limited low‐ or very low‐certainty evidence supporting the effects of probiotics for prevention and treatment of diarrhoea related to radiotherapy (with or without chemotherapy) or chemotherapy alone, need for rescue medication, or occurrence of adverse events. All studies were underpowered and heterogeneous. Severe side effects were absent from all studies.
Robust evidence on this topic must be provided by future methodologically well‐designed trials.
Plain language summary
Live micro‐organisms for prevention or treatment of diarrhoea in people with cancer who are treated with chemotherapy or radiotherapy
Background Up to 80% of people treated with chemotherapy or radiotherapy for cancer suffer from diarrhoea ‐ one of the most common and troublesome side effects. Severe diarrhoea can lead to dehydration (fluid and salts loss) and malnutrition from changes to digestion and bowel habits and could adversely affect quality of life. It is also associated with increased risk of infection in people with low white cell blood count related to cancer treatment. Diarrhoea often leads to delays in cancer treatment or the need to lower the dose or even discontinue cancer treatment. Foods containing live bacteria or yeast (probiotics) might have a beneficial effect on the occurrence and severity of diarrhoea.
Aim of the review To evaluate the effects of live micro‐organisms (probiotics) in preventing the occurrence or reducing the severity of diarrhoea in people with cancer who are receiving chemotherapy or radiotherapy.
Main findings Overall, the studies we found do not give a clear answer on whether probiotics reduce the occurrence or severity of diarrhoea, improve quality of life, or reduce the need for other medication. However, an analysis of only well‐performed studies demonstrated a beneficial effect for some outcomes.
With regard to prevention of diarrhoea compared with placebo in participants treated with radiotherapy with or without chemotherapy, we are not able to conclude whether use of probiotics would be beneficial based on the five relevant studies.
For prevention of diarrhoea due to chemotherapy alone, three studies suggested that use of probiotics may not reduce diarrhoea, and one study reported use of less rescue medication for diarrhoea.
Three studies that compared probiotics with another agent for preventing diarrhoea in patients treated with radiotherapy with or without chemotherapy found beneficial effects of probiotics for the occurrence and severity of diarrhoea and the need for rescue medication.
With respect to treatment of diarrhoea due to radiotherapy, we found only one study that did not demonstrate a clear effect of probiotics compared with placebo.
No study reported serious adverse events nor deaths related to diarrhoea.
Certainty of the evidence The quality (certainty) of the evidence in prevention studies was low to very low. For the only study that assessed the effects of probiotics on treatment for diarrhoea, the certainty of the evidence was moderate.
What are the conclusions? Evidence supporting the effects of probiotics in preventing or treating diarrhoea related to cancer treatment is insufficient. However, probiotics appear to be safe, as no studies have found severe side effects.
Summary of findings
Background
Description of the condition
Diarrhoea is one of the most common and troublesome adverse effects related to cancer chemotherapy or pelvic or abdominal radiotherapy (Benson 2004). The incidence of all grades of diarrhoea during chemotherapy and/or radiotherapy has been reported to be as high as 50% to 80% (Sanguineti 2008). Up to one‐third of people experience severe (grade 3 or 4) diarrhoea (Maroun 2007), especially with those regimens that include bolus 5‐fluorouracil (5‐FU) or irinotecan. Severe treatment‐related diarrhoea can lead to fluid and electrolyte losses and nutritional deficiencies from alterations in gastrointestinal transit and digestion, and could adversely affect quality of life (QoL). Diarrhoea is also associated with increased risk of infection in people with treatment‐related neutropenia. Diarrhoea often leads to treatment delays, dose reductions, or treatment discontinuation. Furthermore, a small but significant mortality risk is associated with chemotherapy‐induced diarrhoea. This level of morbidity reveals the need for a more comprehensive assessment of diarrhoea and a more aggressive and systematic treatment approach. This systematic review defines diarrhoea as three or more loose or liquid stools per day.
Description of the intervention
According to the definition currently adopted by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO), probiotics are "live microorganisms which when administered in adequate amounts confer a health benefit on the host" (FAO and WHO 2001). Lactic acid bacteria and bifidobacteria are the most common types of microbes used as probiotics, but certain yeasts and bacilli may also be helpful. Probiotics are commonly consumed as part of fermented foods with specially added active live cultures such as yogurt and soy yogurt, or as dietary supplements. Although probiotics are often used to prevent or treat gastrointestinal conditions such as diarrhoea, probiotics themselves can also produce abdominal side effects, such as bloating and flatulence. Side effects of probiotics usually appear to be mild (Eskesen 2015).
How the intervention might work
Probiotics such as Lactobacillus rhamnosus GG, which is a strain of L rhamnosus isolated in 1983 from the intestinal tract of a healthy human being, are thought to work by stimulating the cell proliferation rate of bowel epithelial cells, thus enhancing repair of mucosa damaged by radiotherapy and/or chemotherapy. Also, these lactobacilli may restore the bacterial equilibrium within the bowel, inhibiting bacterial translocation into the tissues and stimulating the local and systemic immune response to pathogens (Banasaz 2002; Khaled 2003; Mack 2003; Mattar 2001; Vaarala 2003).
Why it is important to do this review
A meta‐analysis of randomised controlled trials (RCTs) revealed that co‐administration of some probiotics such as L rhamnosus GG with standard rehydration therapy reduced the duration of diarrhoea by one day in children younger than five years with acute‐onset diarrhoea (Huang 2002). Some RCTs have also shown that probiotics are of benefit for treatment of antibiotic‐associated diarrhoea and for prevention of nosocomial (hospital‐acquired) diarrhoea in infants (Cremonini 2002; Szajewska 2001). Furthermore, a recent Cochrane review demonstrated that, based on moderate‐certainty evidence, probiotics are both safe and effective for prevention of Clostridium difficile‐associated diarrhoea in adults and children but do not significantly reduce the incidence of C difficile infection compared with placebo or no treatment (Goldenberg 2013). It also has been found that nutritional intervention with the probiotic drink containing Lactobacillus casei DN‐114 001 does not reduce the incidence of radiotherapy‐induced diarrhoea (Giralt 2008). Previous studies have demonstrated that probiotic supplementation is well tolerated and may reduce the frequency of severe diarrhoea and abdominal discomfort related to chemotherapy or radiotherapy (Delia 2007; Osterlund 2007; Salminen 1988; Urbancsek 2001). Some trials have found that probiotic lactic acid‐producing bacteria offer an easy‐to‐use, safe, and feasible approach to protecting people with cancer against the risk of chemotherapy‐ or radiotherapy‐induced diarrhoea (Delia 2007; Osterlund 2007; Urbancsek 2001). Although probiotics are thought to be safe and to have few side effects, people who have intestinal damage, immune problems, or overgrowth of bacteria in the intestines are at risk of having the micro‐organisms leave the gastrointestinal tract and possibly cause multiple organ failure. Moreover, it has been reported that L rhamnosus and L casei may be involved in infections, such as abscesses, meningitis, and septic arthritis (available at www.mayoclinic.org/drugs‐supplements/acidophilus/background/hrb‐20058615).
Probiotics may provide a beneficial effect for people with chemotherapy‐ or radiotherapy‐induced diarrhoea; therefore, it is important to systematically review the current evidence to assess the effects of probiotic therapy on clinically relevant endpoints in people with cancer receiving chemotherapy, radiotherapy, or both. Previous systematic reviews on this topic have yielded conflicting results. One systematic review found no differences between probiotic supplementation and control in preventing or treating radiotherapy‐ or chemotherapy‐induced diarrhoea (Fuccio 2009). Two other recent systematic reviews concluded that probiotics may provide a beneficial effect for prevention of chemotherapy‐ or radiotherapy‐induced diarrhoea (Liu 2017; Wang 2016), and a fourth systematic review explored the effects of probiotics on the severity and frequency of combined antibiotic‐associated and chemotherapy‐associated diarrhoea and found a reduction in severity and frequency and in the requirement for medication (Redman 2014).
Objectives
To evaluate the clinical effectiveness and side effects of probiotics used alone or combined with other agents for prevention or treatment of chemotherapy‐ or radiotherapy‐related diarrhoea in people with cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Adults aged 18 years and over with histologically diagnosed cancer at any stage of disease and receiving chemotherapy or radiotherapy (with or without chemotherapy).
Types of interventions
Probiotics versus any other intervention (observation, usual care, placebo, or other active agents) for prevention and/or treatment of diarrhoea induced by radiotherapy (with or without chemotherapy) or chemotherapy alone;
Probiotics combined with other agents versus the same agents without probiotics for prevention and/or treatment of diarrhoea induced by radiotherapy (with or without chemotherapy) or chemotherapy alone;
One regimen of probiotic administration versus a different regimen of probiotic administration (i.e. different kind of medication, intake, dosage, and timing) for prevention and/or treatment of diarrhoea induced by radiotherapy (with or without chemotherapy) or chemotherapy alone.
We included studies that looked at the following probiotics: lactobacilli (i.e. Lactobacillus acidophilus, Lactobacillus casei, Lactobacillus jensenii), bifidobacteria (i.e. Bifidobacterium longum, Bifidobacterium breve,Bifidobacterium oval, Bifidobacterium thermophilum), and Saccharomyces buollardii. We also included studies that combined the use of probiotics with prebiotics (i.e. agents that induce the growth or activity of micro‐organisms).
Types of outcome measures
Primary outcomes
For prevention studies: proportion of participants with diarrhoea
For treatment studies: reduction in severity of diarrhoea (e.g. according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) (NCI 2013; CTCAE 2010).
Quality of life measured on a scale that is validated through reporting of norms in a peer‐reviewed publication (e.g. EORTC‐QLQ‐C30 (a questionnaire developed to assess the quality of life of people with cancer) or a generic instrument, such as Short Form (SF)‐36)
Secondary outcomes
For prevention studies: severity of diarrhoea (e.g. according to the National Cancer Institute's CTCAE (NCI 2013; CTCAE 2010))
Time to rescue medication for diarrhoea
Proportion of participants requiring rescue medication for diarrhoea
Adverse effects such as sepsis, dysbacteria (microbial imbalance in e.g. the colon or small intestine tested by terminal restriction fragment length polymorphism (T‐RFLP)), hypersensitivity (especially in high‐risk populations such as those that are immunocompromised or have central lines in situ), abscesses, meningitis, and septic arthritis (as reported by the Mayo Clinic and available at http://www.mayoclinic.org/drugs‐supplements/acidophilus/safety/hrb‐20058615)
Mortality related to diarrhoea (if deaths occurred in participants with grade 3 or 4 diarrhoea, we would define them as deaths related to diarrhoea)
We did not define the required time points of outcome measurements in advance, and we extracted the time points at which outcomes were collected as presented by study authors.
We presented 'Summary of findings' tables to report the following outcomes.
Any diarrhoea.
Quality of life.
-
Diarrhoea.
Grade 2 or higher.
Grade 3 or higher.
Grade 4.
Time to rescue medication.
Rescue medication required for diarrhoea.
Adverse events.
Mortality.
Search methods for identification of studies
Electronic searches
We conducted a broad search to ensure maximum recall of the relevant literature. We performed a comprehensive search of different electronic databases using a combination of free text and medical subject heading (MeSH) terms to identify potential studies for inclusion in the review. We applied no restrictions on language.
We searched the following databases.
CENTRAL (2017, Issue 7) (Appendix 1).
MEDLINE (1946 to July week 2, 2017) (Appendix 2).
Embase (1980 to July week 30, 2017) (Appendix 3).
Searching other resources
We searched the following prospective trial registers for controlled trials in progress using the key words 'probiotics' AND 'cancer', and we updated this search on 15 June 2017.
ClinicalTrials.gov (http://clinicaltrials.gov/).
International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en/).
We also screened the reference lists of included studies and used the 'similar articles' feature in PubMed to look for all included studies to identify any additional studies that might have been missed by our search (11 August 2017).
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database and removed duplicates. Two review authors (FvdW, PH) examined the remaining references independently. We excluded studies that clearly did not meet the inclusion criteria (based on screening of titles, abstracts, or both). We obtained the full‐text articles of potentially eligible studies, and both review authors independently assessed whether these studies met the review inclusion criteria. A third review author (RS) arbitrated any differences of opinion. We documented excluded studies and stated reasons for exclusion according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (Excluded studies).
Data extraction and management
Two review authors (FvdW, PH) independently extracted data. One review author (FvdW) entered the results into Review Manager 2014, and the other review author (PH) checked them for correctness. DW assisted in processing studies that were published in Chinese.
For each included study, we collected data on characteristics of participants (age, gender distribution, cancer details (e.g. type, stage, grade, histology, performance status), diarrhoea severity, previous treatment including type of cancer treatment), characteristics of interventions (type, formulation, dose, duration, regimen), outcomes that were addressed, and duration of follow‐up.
For time‐to‐event data (mortality related to diarrhoea, time to rescue medication), we extracted the log of the hazard ratio [log(HR)] and its standard error (SE) from trial reports; if these were not reported, we attempted to estimate the log(HR) and its SE using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events, presence of diarrhoea), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the total number of participants assessed at endpoint to estimate a risk ratio (RR).
For continuous outcomes (e.g. QoL measures), we extracted the final mean value and the standard deviation (SD) of the outcome of interest and the total number of participants assessed in each treatment arm at the end of follow‐up to estimate the mean difference between treatment arms and its SE.
When possible, we extracted all data relevant to an intention‐to‐treat (ITT) analysis, in which participants were analysed in the groups to which they were assigned.
We extracted the time points at which outcomes were collected as presented by study authors and, if necessary, analysed them separately.
Assessment of risk of bias in included studies
Two review authors (FvdW, PH) independently assessed the risk of bias of all included RCTs using the Cochrane tool for assessing risk of bias (Higgins 2011); we resolved differences by discussion or by appeal to a third review author (RS). DW assisted with assessment of studies published in Chinese.
We summarised the results in a 'Risk of bias summary'. We interpreted the results of meta‐analyses in the light of findings of these risk of bias assessments.
Measures of treatment effect
We expressed treatment effects as RRs with 95% confidence intervals (CIs) for dichotomous outcomes. For time‐to‐event data, we used the hazard ratio (HR), if possible. For continuous outcomes, we calculated mean differences (MDs) with 95% CIs. When different instruments or scales were used to assess the same outcome, we calculated standardised mean differences (SMDs).
Unit of analysis issues
For meta‐analyses in which studies were included that compared more than one intervention with the same control intervention, the denominator of the control intervention was halved. There were no further unit of analysis issues in the included studies.
Dealing with missing data
We did not impute missing outcome data for any outcomes.
Assessment of heterogeneity
We assessed heterogeneity according to guidance provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Besides visual inspection of forest plots, we formally tested for statistical heterogeneity using the natural approximate Chi² test, which provides evidence of variation in effect estimates beyond that of chance. Because the Chi² test has low power to assess heterogeneity when an analysis includes a small number of participants or trials, we set the P value conservatively at 0.1.
We quantified heterogeneity by using the I² statistic, which calculates the percentage of variability due to heterogeneity (differences between studies) rather than chance, with I² values of 50% to 90% indicating substantial heterogeneity.
We planned to examine potential sources of clinical heterogeneity by performing subgroup analyses as specified under Subgroup analysis and investigation of heterogeneity. We planned to examine potential sources of methodological heterogeneity by conducting sensitivity analyses, as specified under Sensitivity analysis.
Assessment of reporting biases
We would have used funnel plots to assess the potential for small‐study effects, such as selective publication, if more than 10 included studies were available for a comparison. However, this was not the case for any of our analyses.
Data synthesis
We used a random‐effects model for all meta‐analyses. We separately analysed results for participants treated with radiotherapy (with or without chemotherapy) and those treated with chemotherapy alone. We used Review Manager 2014 for meta‐analysis. If meta‐analysis was not possible, we summarised the results narratively.
To assess the certainty of the body of evidence for each outcome, we used the GRADE approach as described by the GRADE Working Group and in the Cochrane Handbook for Systematic Reviews of Interventions (Guyatt 2011; Higgins 2011), which takes into account issues related not only to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results. We summarised our judgements in ‘Summary of findings’ tables.
We downgraded the evidence from 'high' certainty by one level for serious (or by two levels for very serious) concerns for each limitation.
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We had planned to conduct subgroup analyses to investigate possible differences between groups. However, because the data required to perform subgroup analyses by age, stage, and length of follow‐up could not be retrieved or did not vary, we were not able to perform these analyses.
Sensitivity analysis
When possible, we performed sensitivity analyses by excluding from the meta‐analysis studies at high risk of bias and studies with more than 10% missing outcome data. See Effects of interventions.
Results
Description of studies
Results of the search
We identified 481 studies by searching the primary electronic databases (65 in CENTRAL, 87 in MEDLINE, 329 in Embase). Of these, 124 were duplicates, leaving 357 abstracts and titles identified as original publications. Of these, 37 studies were eligible for full‐text review, 12 RCTs met the eligibility criteria (see Characteristics of included studies), and one study (published as a conference abstract) presented an interim analysis of an ongoing RCT in which blinding was still maintained. We classified this study under 'Ongoing studies' (see Characteristics of ongoing studies) (Sharma 2013). We classified another study (also presented as a conference abstract) as 'Awaiting assessment', because it is not clear whether participants in the study received chemotherapy or radiotherapy (see Characteristics of studies awaiting classification) (Theodoropoulos 2013). We excluded the remaining 23 studies and provided reasons for their exclusion under Characteristics of excluded studies. We used the 'similar articles' feature of included studies in PubMed and retrieved a total of 1203 records, but no new studies met eligibility criteria. We present the PRISMA study flow chart in Figure 1.
1.
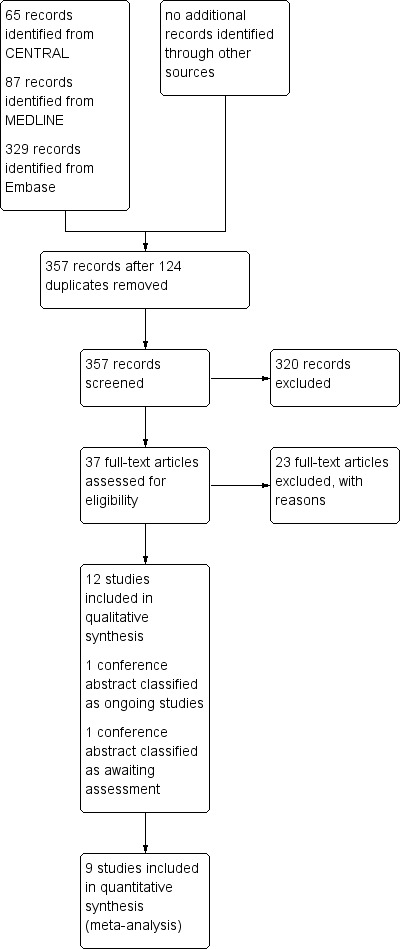
Study flow diagram (search date 24 July 2017).
We searched a prospective trial register (https://clinicaltrials.gov/) using the key words 'probiotics' AND 'cancer' and discovered seven additional relevant registered studies, which we have added to the Characteristics of ongoing studies table.
Included studies
Twelve studies involving 1554 participants met the inclusion criteria (Chen 2014; Chitapanarux 2010; Delia 2007; Demers 2014; Giralt 2008; Liu 2000; Mansouri‐Tehrani 2016; Mego 2015; Osterlund 2007; Salminen 1988; Timko 2010; Urbancsek 2001). Of these, two studies were published in Chinese (Chen 2014; Liu 2000). Eleven were prevention studies, of which seven compared probiotics with placebo (Chen 2014; Chitapanarux 2010; Delia 2007; Giralt 2008; Liu 2000; Mansouri‐Tehrani 2016; Mego 2015), one compared two doses of probiotics with each other and with placebo (Demers 2014), and three compared probiotics with another active agent (Osterlund 2007; Salminen 1988; Timko 2010). The remaining study concerned treatment of radiotherapy‐related diarrhoea and compared probiotics with placebo (see Characteristics of included studies) (Urbancsek 2001).
1. Prevention of diarrhoea
For prevention of diarrhoea, we identified eight placebo‐controlled studies and three studies comparing probiotics versus another active intervention or standard therapy.
1.1. Probiotics versus placebo
1.1.1. Patients treated with radiotherapy (with or without chemotherapy)
Five studies compared probiotics with placebo for prevention of diarrhoea in participants undergoing radiotherapy with or without chemotherapy (Chitapanarux 2010; Delia 2007; Demers 2014; Giralt 2008; Mansouri‐Tehrani 2016) (Table 5). Of these, one study included some participants who had already developed grade 1 diarrhoea at baseline, and another study addressed prevention of grade 2 or higher diarrhoea (Giralt 2008).
1. Probiotics vs placebo for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy).
| Study ID and participants | Intervention(s) | Results |
|
Chitapanarux 2010 Participants undergoing whole pelvis radiotherapy and brachytherapy plus weekly cisplatin 40 mg/m² |
Intervention: 2 × 10⁹ units of Lactobacillus acidophilus plus Bifidobacterium bifidum (equivalent to 2 capsules) 2 times a day before meals (morning and evening), beginning 7 days before the start of radiotherapy and continuing every day during radiotherapy (n = 32) Control: Identical‐appearing placebo on the same schedule (n = 31) |
Proportion of participants with diarrhoea: "During irradiation, diarrhoea occurred in all patients" Quality of life: not assessed Severity of diarrhoea: grades 2/3: 3/32 versus 14/31 (RR 0.21, 95% CI 0.07 to 0.65) Time to rescue medication for diarrhoea: not assessed Proportion of participants requiring rescue medication for diarrhoea: 3/32 versus 10/31 (RR 0.29, 95% CI 0.09 to 0.96) Adverse events: "There were no adverse events attributable to the study drug" Mortality: study authors reported no deaths |
|
Delia 2007 Participants who underwent adjuvant postoperative radiotherapy after surgery for sigmoid, rectal, or cervical cancer |
Intervention: VSL#3, 1 sachet tid (each sachet containing 450 billions/g of viable lyophilised bacteria, including 4 strains of Lactobacillus (L casei, L plantarum, L acidophilus, and L delbrueckii subsp bulgaricus), 3 strains of Bifidobacterium (B longum, B breve, and B infantis), and 1 strain ofStreptococcus salivarius subspthermophilus) from first day of radiotherapy until end of scheduled cycles of radiotherapy (n = 245) Control: VSL#3‐identical‐appearing placebo (n = 245) |
Proportion of participants with diarrhoea: 42/243 versus 119/239 (RR 0.35, 95% CI 0.26 to 0.47) Quality of life: not assessed Severity of diarrhoea: grade 3/4: 8/243 versus 69/239 (RR 0.11, 95% CI 0.06 to 0.23) Time to rescue medication (loperamide) for diarrhoea, mean in hours (SD): 122 (8) versus 86 (6) (MD 36, 95% CI 34.74 to 37.26) Proportion of participants requiring rescue medication for diarrhoea: not reported Adverse events: "No case of bacteremia, sepsis, or septic shock due to the probiotic lactobacilli was reported among the VSL#3 recipients during the treatment period with the probiotic preparation or during the six months beyond active treatment. Likewise, no case of bacteremia, sepsis, or septic shock due to organisms other than the probiotic lactobacilli was recognized during the period of active treatment. We did not recognize any other toxicity reasonably attributable to VSL#3" Mortality: "No tumor‐ or treatment‐related deaths or deaths from other causes were recorded in either group during the period of radiation therapy" NB: One participant in the probiotics group died of myocardial infarction and was excluded from the analyses |
|
Demers 2014 Participants with pelvic cancer who were to receive radiotherapy treatments, with or without chemotherapy |
Intervention group 1: standard dose of double‐strain Bifilact probiotics (Lactobacillus acidophilus LAC‐361 and Bifidobacterium longum BB‐536) twice a day (1.3 billion CFU) (n = 91 randomised, n = 81 analysed) Intervention group 2: high dose of double‐strain Bifilact probiotics (Lactobacillus acidophilus LAC‐361 and Bifidobacterium longum BB‐536) 3 times a day (10 billion CFU) (n = 64 randomised, n = 59 analysed) Control group: placebo (n = 91 randomised, n = 89 analysed) |
Proportion of participants with diarrhoea: Standard 69/81 versus 80/86 (RR 0.92, 95% CI 0.81 to 1.03) High 49/59 versus 80/86 (RR 0.89, 95% CI 0.78 to 1.03) Quality of life (EORTC‐QLQ‐C30): "The wellbeing of participants did change over time. Overall QoL decreased by the end of the treatment, but increased again two weeks post‐treatment (P<0.0001). Probiotic intake did not affect the quality of life of participants in this study" Severity of diarrhoea: Grade 2+: Standard 51/81 versus 68/86 (RR 0.80, 95% CI 0.63 to 1.00) High 39/59 versus 68/86 (RR 0.84, 95% CI 0.66 to 1.06) Grade 3+: Standard 14/81 versus 26/86 (RR 0.57, 95% CI 0.32 to 1.02) High 14/59 versus 26/86 (RR 0.78, 95% CI 0.45 to 1.37) Grade 4: Standard 2/81 versus 9/86 (RR 0.27, 95% CI 0.05 to 1.39) High 4/59 versus 9/86 (RR 0.58, 95% CI 0.17 to 2.04) Time to rescue medication (loperamide) for diarrhoea, mean in days (SD): "There was no significant difference (P = 0.89) among groups for the time until first intake of loperamide. The first capsule of loperamide (Imodium) was taken on days 19.7 (placebo), 20.4 (standard dose), and 20.9 (high‐dose)" Proportion of participants requiring rescue medication for diarrhoea: "The percentage of participants that took loperamide was 42.5% for the placebo group, 30.2% for the standard‐dose group, and 27.4% for the high‐dose group, but this difference was not significant (P = 0.30)" Adverse events: "Other variables analyzed as a part of this study were the number of hospitalizations, the number of treatment interruptions and the reduction of either chemotherapy doses or radiotherapy treatments as a result of severe diarrhoea or abdominal pain. None of these variables differed among the groups after statistical analyses. Intake of Bifilact was well tolerated. No septicemia was recorded although a few cases of neutropenia occurred during treatment" Mortality: study authors reported no deaths |
|
Giralt 2008 Women with endometrial or cervical carcinoma requiring postoperative pelvic radiotherapy with or without concomitant weekly cisplatin The same investigator weekly evaluated all participants and asked all to record the number of bowel movements and stool consistency every day. Evaluation for each participant took up to 6 months |
Intervention: 96 mL 3 times daily of a fermented liquid yogurt containing approximately 10⁸ CFU/g of Lactobacillus casei DN‐114 001, in addition to the standard starters Streptococcus thermophilus and Lactobacillus delbrueckii, subsp bulgaricus (n = 56) Control: same amount of matching placebo, prepared by sterilising the active product with 4 kGy administered for 5 minutes (n = 62) |
Proportion of participants with diarrhoea: not reported Quality of life: QLQ‐C30 global score (change from baseline), mean (SD): 4.28 (11.02) versus 0.58 (10.22) (MD 3.70, 95% CI ‐1.21 to 8.61) Severity of diarrhoea: Grade ≥ 2: 30/44 versus 24/41 (RR 1.16, 95% CI 0.84 to 1.62) Grade ≥ 3: 20/44 versus 15/41 (RR 1.24, 95% CI 0.74 to 2.08) Time to rescue medication for diarrhoea: not assessed Proportion of participants requiring rescue medication for diarrhoea: 16/44 versus 12/41 (RR 1.24, 95% CI 0.67 to 2.30) Adverse events: "No differences were found with regard to the complications reported at 6 months. In >80% of cases, the participants and physicians reported an increase in bowel movements and changes in stool consistency; however, most changes were minimal. A pathologic increase in fecal calprotectin was observed in 1 patient of the 12 analyzed in the active group versus. 3 of the 11 analyzed in the placebo group. The study product was well tolerated, and none of the adverse events reported were considered related" Mortality: study authors reported no deaths |
|
Mansouri‐Tehrani 2016 Participants with pelvic cancer. All participants received conventional radiotherapy (5 fractions weekly for 4 to 5 weeks) |
Intervention group 1: 2 probiotic capsules containing Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus bulgaricus, Bifidobacterium breve, Bifidobacterium longum, and Streptococcus thermophilus per day after consumption of 150 grams of low‐fat yogurt (n = 22) Intervention group 2 (not used in this review): 2 probiotic capsules and 30 grams honey per day after consumption of 150 grams of low‐fat yogurt and 15 grams honey at night (n = 21) Control group: 2 placebo capsules per day after consumption of 150 grams of low‐fat yogurt (n = 24) |
Proportion of participants with diarrhoea: not reported Quality of life: not addressed Severity of diarrhoea: Grade 2 + 3: 7/22 versus 17/24 (RR 0.45, 95% CI 0.23 to 0.87) "Mean diarrhea grade in weeks 4 and 5 was significantly higher in the placebo group than the probiotic [and probiotic plus honey] groups (p = 0.007 and 0.001 for probiotic and p ˂ 0.001 and p = 0.001 for probiotic plus honey in weeks 4 and 5 respectively)" Time to rescue medication for diarrhoea: not addressed Proportion of participants requiring rescue medication for diarrhoea: 2/22 versus 9/24 (RR 0.24, 95% CI 0.06 to 1.00) Adverse events: "During pelvic radiotherapy, three patients (they belonged to probiotic user; with or without honey) complained of upper abdominal pain. The causal link between the complaint and the probiotic was not investigated" Bloating: 19/22 versus 10/24 (RR 2.07, 95% CI 1.26 to 3.42) "The results of the Chi‐square test showed that the number of patients with bloating in the probiotic groups (alone or plus honey) was significantly higher than the placebo group (P=0.002 and 0.021 for the probiotic and the probiotic plus honey groups, respectively)" Mortality: study authors reported no deaths |
CFU: colony‐forming units.
CI: confidence interval.
EORTC‐QLQ‐C30: questionnaire developed to assess the quality of life of patients with cancer, version 3.
MD: mean difference.
QoL: quality of life.
RR: risk ratio.
SD: standard deviation.
The first study compared a probiotic containing live Lactobacillus acidophilus plus Bifidobacterium bifidum (n = 32) versus placebo (n = 31) in 63 participants who were undergoing pelvic radiotherapy concurrent with weekly cisplatin. Researchers found no significant differences between the two groups in terms of participant characteristics or pelvic radiotherapy technique at baseline. The study reported on four outcomes of interest: proportion of participants with diarrhoea, severity of diarrhoea, proportion of participants requiring rescue medication, and adverse events. Trialists evaluated these outcomes weekly during radiotherapy.
The second study evaluated the efficacy of a high‐potency probiotic preparation for prevention of radiotherapy‐induced diarrhoea in people with cancer (Delia 2007). This study involved 490 participants who were randomly assigned to treatment with VSL#3 (n = 245) or identical‐appearing placebo (n = 245). VSL#3 contained Lactobacillus casei, Lactobacillus plantarum, L acidophilus, Lactobacillus delbrueckii subsp bulgaricus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, and Streptococcus salivarius subsp thermophilus. One sachet was given three times a day, starting from the first day of radiotherapy until the end of scheduled cycles of radiotherapy. Researchers found no significant differences between the two groups in patient characteristics at baseline. This study reported on five outcomes of interest: proportion of participants with diarrhoea, severity of diarrhoea, time to rescue medication, mortality caused by diarrhoea, and adverse events. Trialists evaluated these outcomes weekly until one month after completion of radiotherapy.
The third study included three intervention arms: a standard dose (twice a day) and a high dose (three times a day) of double‐strain Bifilact probiotics (Lactobacillus acidophilus LAC‐361 and Bifidobacterium longum BB‐536; Virage Santé, Québec, Canada) and placebo (Demers 2014). This study included 229 participants with pelvic (gynaecological, rectal, or prostate) cancer and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 who were to receive radiotherapy at a minimum of 40 Gy at the pelvic level, with or without chemotherapy. Data show no important baseline differences between groups with respect to type of cancer, age, and gender. The study reported on six outcomes of interest: proportion of participants with diarrhoea, severity of diarrhoea, time to rescue medication, proportion of participants needing rescue medication, quality of life, and adverse events. All participants were followed up to a maximum of 10 weeks.
The fourth study compared a probiotic drink (n = 56) with placebo (n = 62) in participants with gynaecological cancer who were undergoing pelvic radiotherapy (45 to 50 Gy, conventional fractionation) for cervical carcinoma (radiotherapy and weekly cisplatin) or endometrial adenocarcinoma (postoperative radiotherapy) (Giralt 2008). The probiotic drink consisted of liquid yogurt containing Lactobacillus casei DN‐114 001 at 108 colony‐forming units (CFU)/g. Researchers found no significant differences between the two groups in participant characteristics at baseline. This study reported on four outcomes of interest: severity of diarrhoea (graded weekly according to the CTCAE), proportion of people requiring rescue medication, quality of life, and adverse events. All participants were evaluated weekly by the same investigator and were asked to record the number of bowel movements and stool consistency every day. Each participant was evaluated up to six months.
The fifth study compared use of probiotic capsules (n = 22) versus placebo (n = 24) in participants who were undergoing five weekly fractions of conventional pelvic radiotherapy (total dose: 4000 to 5000 cGy (1.8 Gy/d)) for treatment of pelvic cancer (Mansouri‐Tehrani 2016). This review did not discuss a second intervention group that received honey in addition to a probiotic. The probiotic capsules consisted of L casei, L acidophilus, L rhamnosus, L bulgaricus, B breve, B longum, and S thermophilus. This study reported on four outcomes of interest: severity of diarrhoea (graded weekly according to CTCAE), time to rescue medication, proportion of people requiring rescue medication, and adverse events. Researchers evaluated these outcomes weekly during four‐week radiotherapy.
1.1.2. Patients treated with chemotherapy alone
Three studies compared probiotics with placebo for prevention of diarrhoea in participants undergoing chemotherapy (Chen 2014; Liu 2000; Mego 2015) (Table 6).
2. Probiotics vs placebo for prevention of diarrhoea induced by chemotherapy.
| Study ID and participants | Intervention(s) | Results |
|
Chen 2014 Participants with colorectal cancer undergoing intravenous chemotherapy |
Intervention: combined Clostridium butyricum and Bifidobacterium capsule (n = 35) Control: placebo (n = 35) Both interventions (3 capsules, 3 times a day) were administered from 5 days before surgery for colorectal cancer to 7 days after surgery in each group. All participants received intravenous chemotherapy (calcium folinate 300 mg, fluorouracil 500 mg) during surgery |
Proportion of participants with diarrhea: 6/30 versus 12/30 (RR 0.50, 95% CI 0.22 to 1.16) Quality of life: not assessed Severity of diarrhoea: not assessed Time to rescue medication for diarrhoea: not assessed Proportion of participants requiring rescue medication for diarrhoea: not assessed Adverse events: abdominal distension: 18/30 versus 11/30 (RR 1.64, 95% CI 0.94 to 2.85) Systemic inflammatory response syndrome (SIRS): 7/30 versus 9/30 (RR 0.78, 95% CI 0.33 to 1.82) Infection of incisional wound: 3/30 versus 3/30 (RR 1.0, 95% CI 0.22 to 4.56) Pulmonary infection: 2/30 versus 2/30 (RR 1.0, 95% CI 0.15 to 6.64) Urinary tract infection: 2/30 versus 2/30 (RR 1.0, 95% CI 0.15 to 6.64) Duration of fever (days): 4.5 ± 1.0 versus 4.6 ± 1.2 (MD ‐0.10 days, 95% CI ‐0.66 to 0.46) Hypoproteinaemia: 5/30 versus 4/30 (RR 1.25, 95% CI 0.37 to 4.21) Side effects relevant to drug: 0/30 versus 0/30 Mortality: study authors reported no deaths |
|
Liu 2000 Participants with cancer of the lung, stomach, colon, rectum, or breast or with metastatic neck carcinoma who were to receive chemotherapy |
Cross‐over study with 22 participants Intervention: Bifidobacterium combined with chemotherapy Control: chemotherapy alone Bifidobacterium capsule (2 capsules per time, 2 times a day) was taken from 1 day before chemotherapy to the sixth day of chemotherapy in each phase. Length of the washout period in this cross‐over study was about 21 days |
Proportion of participants with diarrhoea: 6/22 versus 10/22 (no paired analysis presented) Quality of life: not assessed Severity of diarrhoea: Grade 2+: 3/22 versus 7/22 (no paired analysis presented) Grade 3+: 1/22 versus 4/22 (no paired analysis presented) Time to rescue medication: not assessed Proportion of participants requiring rescue medication for diarrhoea: not assessed Adverse events: not assessed Mortality: study authors reported no deaths |
|
Mego 2015 Participants with colorectal cancer starting a new line of chemotherapy |
Intervention: probiotic formula Colon Dophilus™ 3*1 capsule per day orally for 12 weeks "Each capsule contained 10*109 CFU of bacteria. Each capsule contained 10 lyophilized probiotic strains including Bifidobacterium breve HA‐129 (25%), Bifidobacterium bifidum HA‐132 HA (20%), Bifidobacterium longum HA‐135 (14.5%), Lactobacillus rhamnosus HA‐111 (8%), Lactobacillus acidophilus HA‐122 (8%), Lactobacillus casei HA‐108 (8%), Lactobacillus plantarum HA‐119 (8%), Streptococcus thermopilus HA‐110 (6%), Lactobacillus brevis HA‐112 (2%), Bifidobacterium infantis HA‐116 (0.5%) (n = 23)" Control: placebo (n = 23). "Each capsule with placebo contained only inactive ingredients without probiotic bacteria, and placebo capsules were prepared by the central pharmacy. The placebo was indistinguishable from the capsule with probiotics in terms of color, appearance, taste, smell, size, shape, and other properties and contained the same additives as probiotic capsule" |
Proportion of participants with diarrhoea: 9/23 versus 14/23 (RR 0.64, 95% CI 0.35 to 1.18) Quality of life: not assessed Severity of diarrhoea: Grade 2+: 4/23 versus 6/23 (RR 0.67, 95% CI 0.22 to 2.05) Grade 3+: 0/23 versus 4/23 (RR 0.11, 95% CI 0.01 to 1.95) Grade 4: 0/23 versus 1/23 (RR 0.33, 95% CI 0.01 to 7.78) Time to rescue medication for diarrhoea: not assessed Proportion of participants requiring rescue medication for diarrhoea: "participants on probiotic arm used less loperamide and diphenoxylate/atropine compared to participants on placebo arm" Adverse events: "We received filled study diaries from 38 (82.6%) of patients. ….. We did not observe any infection caused by probiotic strains used in this study" Mortality: study authors reported no deaths |
CI: confidence interval.
RR: risk ratio.
SIRS: systemic inflammatory response syndrome.
The first study compared combined Clostridium butyricum and Bifidobacterium versus placebo in 70 participants (35 in each group) who underwent surgery for colorectal cancer (Chen 2014). All participants received intravenous chemotherapy during surgery. Both groups were well balanced with respect to tumour location (right or left portion of the colon, or rectum) and tumour stage (stage I to II, 21 versus 23; stage III to IV, 9 versus 7). This study reported on two outcomes of interest: proportion of participants with diarrhoea and adverse events. Study authors did not report the time points of outcome measurement.
The second study was a cross‐over study including 22 participants with cancer who received chemotherapy (Liu 2000). Eight participants had lung cancer, five gastric cancer, four colorectal cancer, four breast cancer, and one metastatic neck cancer. Bifidobacterium (two capsules, two times a day) with chemotherapy was compared with chemotherapy alone and was administered from one day before chemotherapy to the sixth day of chemotherapy. The washout period lasted about 21 days. This study reported on two outcomes of interest: proportion of participants with diarrhoea and severity of diarrhoea. Study authors did not report the time points of outcome measurement.
The third study compared the Colon Dophilus™ 3*1 capsule per day orally for 12 weeks versus placebo in 46 participants (23 in each group) with colorectal cancer who were about to start chemotherapy based on irinotecan, with ECOG performance status 0 to 1 and life expectancy longer than three months (Mego 2015). Study authors described some baseline differences with respect to gender (more males in the probiotic arm), more participants in the probiotics group with colon cancer (69.6% versus 52.2%), and more participants in the placebo group undergoing resection of the primary tumour. This study reported on four outcomes of interest: incidence and severity of diarrhoea, proportion of participants requiring rescue medication, and adverse events. Study authors did not report the time points of outcome measurement.
1.2. Probiotics versus another active intervention or standard therapy
1.2.1. Patients treated with radiotherapy (with or without chemotherapy)
Three studies compared effects of probiotics versus another active intervention in participants undergoing radiotherapy with or without chemotherapy (Osterlund 2007; Timko 2010; Salminen 1988) (Table 7).
3. Probiotics vs active treatment for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy).
| Study ID and participants | Intervention(s) | Results |
|
Osterlund 2007 Participants with Dukes' B or C colorectal cancer or metastatic colorectal cancer who underwent chemotherapy and radiotherapy |
Intervention:Lactobacillus rhamnosus GG (administered orally as gelatin capsules twice daily at a dose of 1 to 2 × 10¹º per day during 24 weeks of adjuvant cancer chemotherapy (n = 98) Control: guar gum containing nutritional supplement (contains 11 g guar gum and 550 kcal or 2300 kJ), administered daily, on cycle days 7 to 14, for 8 days per month (n = 52) All participants received dietary counselling |
Proportion of participants with diarrhoea: not reported Quality of life: not assessed Severity of diarrhoea: grade 3‐4 OR 0.38 (95% CI 0.16 to 0.89) Time to rescue medication for diarrhoea: not assessed Proportion of participants requiring rescue medication for diarrhoea: not assessed Adverse events (Common Toxicity Criteria of the National Cancer Institute of Canada scale version 2): Any adverse event grade 3 or 4: OR 0.77 (95% CI 0.35 to 1.72) Stomatitis grade 3 or 4: OR 0.59 (95% CI 0.26 to 1.35) Neutropenia grade 3 or 4: OR 2.00 (95% CI 0.74 to 4.89) Neutropenic infection grade 3 or 4: OR 2.62 (95% CI 0.53 to 13.00) Hand‐foot syndrome grade 3: 2/97 versus 1/51 (OR: no convergence) Mortality: study authors reported no deaths |
|
Salminen 1988 Participants with carcinoma of the cervix or uterus who were to receive radiotherapy |
Intervention: dietary counselling recommending a low‐fat and low‐residue diet during radiotherapy and a daily dose of at least 2 × 10⁹ live Lactobacillus acidophilus bacteria in the form of a yogurt‐type product (150 mL of a fermented milk test product) and 6.5% lactulose as substrate for the bacteria; 150 mL of the product daily for 5 days before radiotherapy, daily throughout the radiotherapy period including the interval, and then for 10 days after completion of the therapy regimen (n = 12) Control: dietary counselling only recommending a low‐fat and low‐residue diet during radiotherapy (n = 12) |
Proportion of participants with diarrhoea: "All subjects in the control group suffered from diarrhoea during the radiotherapy" During treatment, control time 2: 3/11 versus 8/10 (RR 0.34, 95% CI 0.12 to 0.94) During treatment, control time 3: 2/11 versus 9/10 (RR 0.20, 95% CI 0.06 to 0.72) During treatment, control time 4: 2/11 versus 8/10 (RR 0.23, 95% CI 0.06 to 0.83) Six weeks after treatment: 3/11 versus 9/10 (RR 0.30, 95% CI 0.11 to 0.81) "The incidence of diarrhoea was significantly smaller in the yoghurt group than in the control group (P<0.01)" Quality of life: not assessed Severity of diarrhoea: not assessed Time to rescue medication for diarrhoea: not assessed Proportion of participants requiring rescue medication for diarrhoea: 2/11 versus 6/10 (RR 0.30, 95% CI 0.08 to 1.17) Adverse events: "There were no differences in the incidence of vomiting, nausea, abdominal pain, loss of appetite or weight loss between the groups. However, the yoghurt group experienced more flatulence than the controls" Mortality: study authors reported no deaths |
|
Timko 2010 Participants with cancer who underwent adjuvant postoperative radiotherapy therapy in the abdominal and pelvic region, with or without chemotherapy |
Intervention: probiotic preparation "5"‐strain Dophilus (55% Lactobacillus rhamnosus, 20% Bifidobacterium adolescentis, 5% Lactobacillus acidophilus, 5% Bifidobacterium longum, 15% Enterococcus faecium) with a count of 6 billion active bacteria/capsules at a daily dosage of 2 × 1 capsule (n = 22) Control: Hylak Tropfen Forte preparation (i.e. cell‐free fermentation products of Lactobacillus helveticus and gut symbionts (100 mL containing: 24.95 g Escherichia coli metabolita, 12.5 g Streptococci faecalis metabolita, 12.5 g Lactobacillus acidophilus metabolita, 49.9 g Lactobacillus helveticus metabolita) in doses of 40 drops, 3 times per day (n = 20) |
Proportion of participants with diarrhoea: not assessed Quality of life: not assessed Severity of diarrhoea: not assessed Time to rescue medication for diarrhoea: not assessed Proportion of participants requiring rescue medication for diarrhoea (diphenoxylate): RR 0.50, 95% CI 0.23 to 1.09 Adverse events: Abdominal pain: 25% versus 22% "All these participants were being treated with pelvic radiotherapy with chemotherapy, except for one patient of L‐Group. Chemotherapy thus seemed to result in increased toxicity" "None of the participants discontinued treatment for gastrointestinal toxicity" Mortality: study authors reported no deaths |
CI: confidence interval.
OR: odds ratio.
RR: risk ratio.
The first study used a factorial design and compared two 5‐FU‐based regimens and the effects of Lactobacillus or fibre supplementation on treatment tolerability (Osterlund 2007). A total of 150 participants received the diagnosis of colorectal cancer and were randomly allocated to receive monthly 5‐FU and leucovorin bolus injections or a bimonthly 5‐FU bolus plus continuous infusion for 24 weeks as postoperative adjuvant therapy. Participants also were randomised to receive L rhamnosus GG supplementation (1 to 2 × 10¹⁰ per day) or fibre (11 g guar gum per day) during chemotherapy. All participants received dietary counselling. Researchers reported no differences between the two groups in participant characteristics at baseline. This study reported on three outcomes of interest: proportion of participants with diarrhoea, severity of diarrhoea, and adverse events. Trialists evaluated these outcomes four‐weekly during chemotherapy and radiotherapy and at protocol‐determined intervals (ranging from two to six months) post treatment.
The second study assessed the efficacy of adding live L acidophilus cultures to dietary counselling for prevention of intestinal side effects (Salminen 1988). Twenty‐four female participants with gynaecological malignancies and scheduled for internal and external irradiation of the pelvic area were randomised to the intervention group (150 mL of a fermented milk test product supplying them with at least 2 × 10⁹ live L acidophilus bacteria daily and 6.5% lactulose as substrate for the bacteria and dietary counselling recommending a low‐fat and low‐residue diet during radiotherapy) or to the control group (dietary counselling only). Researchers reported no baseline characteristics. This study reported on three outcomes of interest: proportion of participants with diarrhoea, proportion of participants requiring rescue medication, and adverse events. Trialists evaluated these outcomes during and six weeks after treatment.
The third study assessed the efficacy of a probiotic preparation for prevention of radiotherapy‐induced diarrhoea in people with cancer (Timko 2010). Investigators randomised 42 participants who had undergone adjuvant postoperative radiotherapy after abdominal and pelvic cancer to receive either a probiotic preparation with "5"‐strain Dophilus (twice per day) containing five probiotic cultures (55% L rhamnosus, 20% B adolescentis, 5% L acidophilus, 5% B longum, and 15% Enterococcus faecium) or a preparation with Hylak Tropfen Forte (i.e. cell‐free fermentation products of Lactobacillus helveticus and gut symbionts (100 mL containing 24.95 g Escherichia coli metabolita, 12.5 g Streptococcus faecalis metabolita, 12.5 g L acidophilus metabolita, 49.9 g L helveticus metabolita)) at doses of 40 drops, three times per day. Supplementation started on the first day and lasted until completion of radiotherapy. Study authors stated that there were differences between the two groups regarding gender and primary tumour site at baseline (no further details were reported). In addition, during radiotherapy, 27% of participants treated with probiotics required diphenoxylate treatment compared with 55% treated with Hylak Tropfen Forte, and 9% needed administration of antibiotics compared with 25% in the Hylak group. We excluded these participants from the analyses as investigators could not estimate the ways in which these treatments influenced the composition of intestinal bacterial flora. All participants were treated with pelvic radiotherapy with chemotherapy, except for one from the probiotic group. According to study authors, chemotherapy seemed to have resulted in increased toxicity. This study reported on two outcomes of interest: proportion of participants requiring rescue medication and adverse events. Trialists evaluated these outcomes over one to five weeks during radiotherapy.
1.2.2. Patients treated with chemotherapy alone
We found no studies for this group of patients.
2. Treatment of diarrhoea
For treatment of diarrhoea, we identified one placebo‐controlled study including participants undergoing radiotherapy (Urbancsek 2001). We identified no studies that included participants with diarrhoea who had received chemotherapy alone. Urbancsek 2001 compared the efficacy and tolerability of L rhamnosus (Antibiophilus) versus placebo in 205 participants suffering from mild to moderate diarrhoea induced by radiotherapy (Table 8). Investigators found no differences between the two groups in participant characteristics at baseline. This study reported on four outcomes of interest: severity of diarrhoea, time to rescue medication for diarrhoea, proportion of participants requiring rescue medication, and adverse events. Participants were followed up one week after completion of treatment.
4. Probiotics vs placebo for treatment of diarrhoea induced by radiotherapy.
| Study ID and participants | Intervention(s) | Results |
|
Urbancsek 2001 Participants with cancer who developed diarrhoea within 4 weeks after receiving radiotherapy in the abdominal region |
Intervention: Lactobacillus rhamnosus (Antibiophilus, with each sachet containing 1.5 g of Lactobacillus rhamnosus equivalent to 1.5 × 10⁹ colony‐forming units) 3 times a day (n = 102) Control: identical‐appearing sachets of placebo, each containing 700 mg corn starch, 797 mg microcrystalline cellulose, 1.37 mg iron oxide, 1.13 mg dispersed orange (colouring agent), and 1 mg caramel aroma 3 times a day (n = 103) |
Proportion of participants with diarrhoea: not reported Quality of life: not assessed Severity of diarrhea: average grade rated by investigators using standard scores of 0 for none, 1 for mild, 2 for moderate, and 3 for severe diarrhoea: Study start: 2.0 versus 2.2 Study end: 0.7 versus 1.0 Time (hours) to rescue medication for diarrhoea: 138 (SE = 5) versus 125 (SE= 5) (MD 13, 95% CI ‐0.86 to 26.86) Proportion of participants requiring rescue medication for diarrhoea: RR 0.74, 95% CI 0.53 to 1.03 Adverse events: "Serious adverse events (in GCP terms) were not observed in this study. In both study groups, three participants reported adverse events. In the Antibiophilus1 group, three participants reported gastrointestinal problems (mild to moderate); in the placebo group, two participants reported gastrointestinal events (moderate to severe), and one patient observed a mild labial oedema. All documented events were of a transient nature; in three patients, symptomatic treatment of adverse events was prescribed" Mortality: study authors reported no deaths |
CI: confidence interval.
GCP: Good Clinical Practice.
MD: mean difference.
RR: risk ratio.
Excluded studies
We excluded 23 studies. Twelve studies were not relevant to our review question, seven were not RCTs, and four were not primary studies. We have presented excluded studies and reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
The risk of bias assessment for each study can be found in the 'Risk of bias' tables (see Characteristics of included studies). We have presented a summary of risk of bias assessments in Figure 2. Here, we discuss the overall results of the risk of bias assessments.
2.
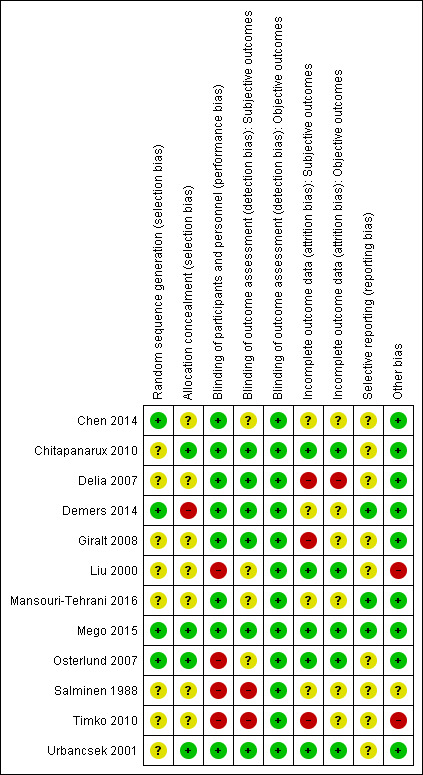
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generally, little information was provided on methods used for randomisation or methods used to maintain concealment of allocation, leading to an unclear risk of bias judgement for random sequence generation and allocation concealment. Four studies scored low risk of bias for random sequence generation (Chen 2014; Demers 2014; Mego 2015; Osterlund 2007). We considered four studies to have low risk of bias for allocation concealment (Chitapanarux 2010; Mego 2015; Osterlund 2007; Urbancsek 2001). One study scored high risk of bias regarding allocation concealment because the investigator, who knew the coding system, assigned participants to the different groups (Demers 2014).
Blinding
Four studies scored a high risk of performance bias for participants and/or personnel, as these studies were non‐blinded (Liu 2000; Osterlund 2007; Salminen 1988; Timko 2010). We considered two studies to have high risk of detection bias (subjective outcomes) (Salminen 1988; Timko 2010). We assessed four studies as having unclear risk of bias owing to insufficient information (Chen 2014; Liu 2000; Mansouri‐Tehrani 2016; Osterlund 2007).
Incomplete outcome data
Three studies scored high risk of attrition bias for subjective outcomes owing to exclusion of participants for intervention‐related reasons ‐ as in Delia 2007 ‐ or a substantial number of dropouts (28% (33/118) in Giralt 2008, and 27% and 55% in the intervention and control groups, respectively, in Timko 2010). Another four studies scored unclear risk owing to insufficient information (Chen 2014; Demers 2014; Mansouri‐Tehrani 2016) or unclear influence of a relatively large number of dropouts in a small study (Salminen 1988). We judged the remaining studies to have low risk of attrition bias for subjective outcomes. For objective outcomes (mortality), we considered one study to have high risk of attrition bias (Delia 2007), and for six studies, the risk of bias was unclear (Chen 2014; Demers 2014; Giralt 2008; Mansouri‐Tehrani 2016; Salminen 1988; Timko 2010).
Selective reporting
In three studies (Demers 2014; Mansouri‐Tehrani 2016; Mego 2015), it was clear that there was no selective reporting of data because original study protocols were available. For the other nine studies, the study protocols were not available, and therefore we judged the risk of reporting bias to be unclear.
Other potential sources of bias
One study showed indications of other bias due to possible imbalances in baseline characteristics (Timko 2010). We considered another study to have high risk of bias because investigators ignored the cross‐over design in the analyses and in the presentation of results (Liu 2000). In another study, the risk of other bias was unclear because study authors provided insufficient information and no table of baseline characteristics (Salminen 1988). We judged the remaining studies to have low risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Probiotics compared with placebo for prevention of diarrhoea in participants with cancer treated with radiotherapy (with or without chemotherapy).
| Probiotics compared with placebo for prevention of diarrhoea in patients with cancer treated with radiotherapy (with or without chemotherapy) | ||||||
| Patient or population: participants with cancer treated with radiotherapy (with or without chemotherapy) Setting: secondary care Intervention: probiotics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with probiotics | |||||
| Any diarrhoea | RRs ranged from 0.35 (95% CI 0.26 to 0.47) to 1.0 (95% CI 0.94 to 1.06) | ‐ | 771 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| Quality of life | Mean quality of life was 0 | MD 3.7 higher (1.21 lower to 8.61 higher) | ‐ | 72 (1 RCT) | ⊕⊕⊝⊝ LOWc,d | A second study in 226 participants reported that probiotic intake did not affect QoL |
| Diarrhoea grade 2 or higher | Study population | RR 0.75 (0.55 to 1.03) | 420 (4 RCTs) | ⊕⊕⊝⊝ LOWd,e | ||
| 676 per 1000 | 507 per 1000 (372 to 696) | |||||
| Diarrhoea grade 3 or higher | RRs ranged from 0.11 (95% CI 0.06 to 0.23) to 1.24 (95% CI 0.74 to 2.08) | ‐ | 793 (3 RCTs) | ⊕⊕⊝⊝ LOWb,f | ||
| Diarrhoea grade 4 | RRs of standard (81 participants) and high doses (59 participants) of probiotics versus placebo (86 participants) were 0.24 (95% CI 0.05 to 1.06) and 0.65 (95% CI 0.21 to 2.01), respectively | ‐ | 226 (1 RCT) | ⊕⊝⊝⊝ VERY LOWg,h | ||
| Time to rescue medication | Mean time to rescue medication was 0 hours | MD 36 hours higher (34.7 higher to 37.3 higher) | ‐ | 482 (1 RCT) | ⊕⊕⊕⊝ MODERATEc | A second study in 226 participants reported no differences between groups |
| Requiring rescue medication for diarrhoea | Study population | RR 0.50 (0.15 to 1.66) | 194 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWd,i | A fourth study in 226 participants reported less use of rescue medication in the probiotics group | |
| 323 per 1000 | 161 per 1000 (48 to 536) | |||||
| Adverse events | No study reported major differences between groups. In one study (46 participants), bloating occurred more often in the probiotic group: RR 2.07, 95% CI 1.26 to 3.42 | ‐ | 902 (5 RCTs) | ⊕⊕⊝⊝ LOWj,k | ||
| Mortality | Although not mentioned explicitly, no studies reported any deaths, except one study, in which 1 participant in the probiotics group died of myocardial infarction after 3 sessions of radiotherapy | ‐ | 902 (5 RCTs) | ⊕⊕⊝⊝ LOWk | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OIS: optimal information size; QoL: quality of life; RCT: randomised controlled trial; RR: risk ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOne study at high risk of selection bias; one study at high risk of attrition bias; one study at unclear risk of bias.
bMajor indications of heterogeneity between studies.
cHigh risk of attrition bias.
dWide CI that includes no effect; OIS not reached.
eOne study at high risk of selection bias and one study at high risk of attrition bias, both leaving those out, changes the effect to the null. Remaining two studies, however, at unclear risk of bias; therefore downgrading by one level.
fOne study at high risk of selection bias; two studies at high risk of attrition bias.
gHigh risk of selection bias.
hWide CIs; OIS not reached.
iOne study at high risk of attrition bias, but no downgrading because of influence to the null. Downgrading by one level because of unclear risk of bias in another study.
jStudies addressed many different adverse events.
kOIS not reached.
Summary of findings 2. Probiotics compared with placebo for prevention of diarrhoea in participants with cancer treated with chemotherapy.
| Probiotics compared with placebo for prevention of diarrhoea in participants with cancer treated with chemotherapy | ||||||
| Patient or population: participants with cancer treated with chemotherapy Setting: secondary care Intervention: probiotics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with probiotics | |||||
| Any diarrhoea | Study population | RR 0.59 (0.36 to 0.96) | 106 (2 RCTs) | ⊕⊕⊝⊝ LOWa | In another cross‐over study, 6 of 22 suffered from diarrhoea during the probiotic period compared with 10 of 22 during the placebo period (no paired analysis presented) | |
| 491 per 1000 | 289 per 1000 (177 to 471) | |||||
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Diarrhoea grade 2 or higher | Study population | RR 0.67 (0.22 to 2.05) | 46 (1 RCT) | ⊕⊕⊝⊝ LOWb,c | In another cross‐over study, 3 of 22 suffered from grade 2 diarrhoea or higher during the probiotic period compared with 7 of 22 during the placebo period (no paired analysis presented) | |
| 261 per 1000 | 175 per 1000 (57 to 535) | |||||
| Diarrhoea grade 3 or higher | Study population | RR 0.11 (0.01 to 1.95) | 46 (1 RCT) | ⊕⊕⊝⊝ LOWb,c | In another cross‐over study, 1 of 22 suffered from grade 3 diarrhoea or higher during the probiotic period compared with 4 of 22 during the placebo period (no paired analysis presented) | |
| 174 per 1000 | 19 per 1000 (2 to 339) | |||||
| Diarrhoea grade 4 | Study population | RR 0.33 (0.01 to 7.78) | 46 (1 RCT) | ⊕⊕⊝⊝ LOWb,c | ||
| 43 per 1000 | 14 per 1000 (0 to 338) | |||||
| Time to rescue medication ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Requiring rescue medication for diarrhoea | Results not quantified. "Participants on probiotic arm used less loperamide and diphenoxylate/atropine compared to participants on placebo arm" | ‐ | 46 (1 RCT) | ⊕⊕⊝⊝ LOWc | ||
| Adverse events | Results not quantified. No differences between groups reported | ‐ | 106 (2 RCTs) | ⊕⊕⊝⊝ LOWc | ||
| Mortality | Although not mentioned explicitly, no studies reported any deaths | ‐ | 128 (3 RCTs) | ⊕⊕⊝⊝ LOWc | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOIS not reached. CI includes irrelevant benefit.
bWide CI that includes both benefit and harm.
cOIS not reached.
Summary of findings 3. Probiotics compared with other active treatment for prevention of diarrhoea in participants with cancer treated with radiotherapy (with or without chemotherapy).
| Probiotics compared with other active treatment for prevention of diarrhoea in participants with cancer treated with radiotherapy (with or without chemotherapy) | ||||||
| Patient or population: participants with cancer treated with radiotherapy (with or without chemotherapy) Setting: secondary care Intervention: probiotics Comparison: other active treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with other active treatment | Risk with probiotics | |||||
| Any diarrhoea | Study population | RR 0.30 (0.11 to 0.81) | 21 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 900 per 1000 | 270 per 1000 (99 to 729) | |||||
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Severity of diarrhoea: grade 3 or higher | Study population | OR 0.38 (0.16 to 0.89) | 148 (1 RCT) | ⊕⊕⊝⊝ LOWb,c | Based on an analysis that addressed the factorial design of this study | |
| 373 per 1000 | 184 per 1000 (87 to 346) | |||||
| Time to rescue medication ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Requiring rescue medication for diarrhoea | Study population | RR 0.44 (0.22 to 0.86) | 63 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 567 per 1000 | 249 per 1000 (125 to 487) | |||||
| Adverse events | No differences between groups in all studies | ‐ | 211 (3 RCTs) |
⊕⊝⊝⊝ VERY LOWa,b,c | ||
| Mortality | Although not mentioned explicitly, no studies reported any deaths | ‐ | 211 (3 RCTs) |
⊕⊕⊝⊝ LOWc,d | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OIS: optimal information size; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aHigh risk of detection bias.
bHigh risk of performance bias.
cVery small study/studies. OIS not reached.
dHigh risk of bias in all studies. Not downgraded for mortality.
Summary of findings 4. Probiotics compared with placebo for treatment of diarrhoea due to radiotherapy (with or without chemotherapy) in participants with cancer.
| Probiotics compared with placebo for treatment of diarrhoea due to radiotherapy (with or without chemotherapy) in participants with cancer | ||||||
| Patient or population: participants with cancer treated with radiotherapy (with or without chemotherapy) Setting: secondary care Intervention: probiotics Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with probiotics | |||||
| Reduction in severity of diarrhoea | "The average diarrhoea grade (rated by the investigators using standard scores ranging from 0 for no diarrhoea to 3 for severe diarrhoea) was 0.7 for the Antibiophilus group and 1.0 for the placebo group at the end of the study (no significant difference between the two groups)" | ‐ | 205 (1 RCT) |
⊕⊕⊕⊝ MODERATEa,b | ||
| Quality of life ‐ not measured | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Time to rescue medication (in hours) | Mean time to rescue medication (in hours) was 0 | MD 13 higher (0.86 lower to 26.86 higher) | ‐ | 205 (1 RCT) | ⊕⊕⊕⊝ MODERATEa,b | |
| Requiring rescue medication for diarrhoea | Study population | RR 0.74 (0.53 to 1.03) | 205 (1 RCT) | ⊕⊕⊕⊝ MODERATEa,b | ||
| 476 per 1000 | 352 per 1000 (252 to 490) | |||||
| Adverse events | "Serious adverse events were not observed. In the Antibiophilus group, three participants reported mild to moderate gastrointestinal problems; in the placebo group two participants reported moderate to severe gastrointestinal events, and one patient observed a mild labial oedema. All documented events were of a transient nature; in three patients, symptomatic treatment of adverse events was prescribed" | ‐ | 205 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ||
| Mortality | Although not mentioned explicitly, no studies reported any deaths | ‐ | 205 (1 RCT) | ⊕⊕⊕⊝ MODERATEa | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; OIS: optimal information size; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aOne study in 205 participants; OIS not reached.
bCI includes both benefit and harm.
We present results separately for prevention (section 1) and treatment (section 2) of diarrhoea. We discriminated between placebo‐controlled studies and studies comparing probiotics with another treatment (subsections 1 and 2, respectively). Finally, because the impact of radiotherapy is considered the most important difference, we presented and analysed studies separately for participants treated with radiotherapy (with or without chemotherapy) and with chemotherapy alone.
1. Prevention of diarrhoea
1.1. Probiotics versus placebo
1.1.1. Participants treated with radiotherapy (with or without chemotherapy)
Five studies with 905 analysed participants compared probiotics with placebo for prevention of radiotherapy (with or without chemotherapy)‐related diarrhoea (Chitapanarux 2010; Delia 2007; Demers 2014; Giralt 2008; Mansouri‐Tehrani 2016).
Proportion of participants with diarrhoea
Three studies addressed this outcome. Owing to heterogeneity, we did not pool results. One study found that the occurrence of diarrhoea of any grade was significantly reduced with probiotics compared with placebo in 482 participants treated with radiotherapy alone (risk ratio (RR) 0.35, 95% confidence interval (CI) 0.26 to 0.47) (Delia 2007). We observed no significant differences between the two groups among participants treated with chemoradiotherapy in the studies of Chitapanarux 2010 (RR 1.0, 95% CI 0.94 to 1.06; 63 participants) and Demers 2014 (two study arms: RR 0.92, 95% CI 0.82 to 1.02 and RR 0.89, 95% CI 0.78 to 1.02 for standard (167 participants) and high doses (145 participants) of the probiotic, respectively) (Analysis 1.1).
1.1. Analysis.
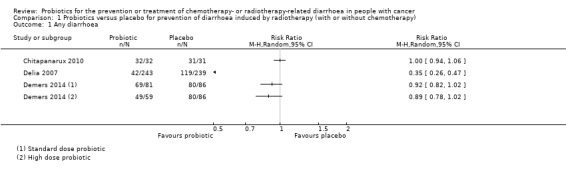
Comparison 1 Probiotics versus placebo for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy), Outcome 1 Any diarrhoea.
Quality of life
Two studies including participants treated with chemoradiotherapy assessed quality of life by using the EORTC‐QLQ‐C30 (version 3) instrument (Table 5). The first study (226 participants) reported: "The well‐being of patients did change over time. Overall QOL decreased by the end of the treatment, but increased again two weeks post‐treatment (P < 0.0001). Probiotic intake did not affect the quality of life of patients in this study" (Demers 2014). In the second study (72 participants), QLQ‐C30 global scores showed mild improvement compared with baseline in both groups but revealed no significant differences between groups (mean difference (MD) 3.7, 95% CI ‐1.2 to 8.6) (Giralt 2008).
Severity of diarrhoea
Four studies addressed the occurrence of diarrhoea grade 2 or higher. Among participants treated with radiotherapy (with or without chemotherapy), the pooled RR for the occurrence of grade 2 or higher diarrhoea with probiotics versus placebo was 0.75 (95% CI 0.55 to 1.03; four studies; 420 participants) (Analysis 1.2) (Chitapanarux 2010; Demers 2014; Giralt 2008; Mansouri‐Tehrani 2016). One study compared standard‐dose and high‐dose probiotics versus placebo and included both comparisons separately in the meta‐analysis (Demers 2014). We ignored the high value for I² (72%) because visual inspection of the forest plot showed sufficient overlap of CIs for the three studies with largest weights, whereas two smaller studies were shown not to influence the results.
1.2. Analysis.
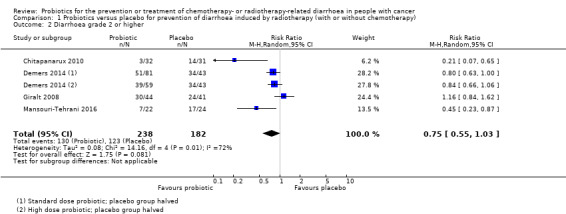
Comparison 1 Probiotics versus placebo for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy), Outcome 2 Diarrhoea grade 2 or higher.
Three studies addressed the occurrence of diarrhoea grade 3 or higher; of these, we included intervention groups from one study receiving standard‐dose and high‐dose probiotics separately (Demers 2014). In 793 participants treated with radiotherapy (with or without chemotherapy), RRs for grade 3 or higher diarrhoea with probiotics versus placebo were heterogeneous (I² = 91%) and ranged from 0.11 (95% CI 0.06 to 0.23) to 1.24 (95% CI 0.74 to 2.08) (Analysis 1.3; Table 1) (Delia 2007; Demers 2014; Giralt 2008).
1.3. Analysis.
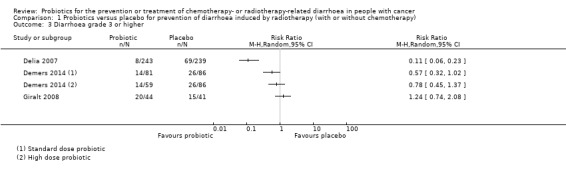
Comparison 1 Probiotics versus placebo for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy), Outcome 3 Diarrhoea grade 3 or higher.
One study reported the occurrence of diarrhoea grade 4 in participants treated with both radiotherapy and chemotherapy. The RRs of standard‐dose (81 participants) and high‐dose (59 participants) probiotics versus placebo (86 participants) were 0.24 (95% CI 0.05 to 1.06) and 0.65 (95% CI 0.21 to 2.01), respectively (Table 5) (Demers 2014).
One study including participants receiving pelvic radiotherapy reported a significantly higher mean diarrhoea grade during weeks 4 and 5 in the placebo group (24 participants) compared with the probiotic group (22 participants; P = 0.007 and P = 0.001, respectively) (Mansouri‐Tehrani 2016).
Time to rescue medication for diarrhoea
Two studies in which participants were treated with radiotherapy (with or without chemotherapy) addressed this outcome (Delia 2007; Demers 2014). In one study (482 participants), the mean time to rescue medication was 36 hours longer in the probiotics group than in the placebo group (95% CI 34.7 to 37.3 hours) (Delia 2007). The second study (226 participants) reported: "no difference (P = 0.89) among the groups for the time until the first intake of loperamide" and "The first capsule of loperamide (Imodium) was taken on day 19.7 (placebo), 20.4 (standard dose), and 20.9 (high‐dose)" (Table 5) (Demers 2014).
Proportion of participants requiring rescue medication for diarrhoea
Four studies addressed this outcome, of which three could be pooled (Chitapanarux 2010; Giralt 2008; Mansouri‐Tehrani 2016). Among participants treated with radiotherapy (with or without chemotherapy), the pooled RR for the need for rescue medication for diarrhoea of probiotics versus placebo was 0.50 (95% CI 0.15 to 1.66; three studies; 194 participants) (Analysis 1.4). Again, we ignored the high value for I² (74%) because visual inspection of the forest plot revealed sufficient overlap of CIs from the three studies. The fourth study (226 participants) did not quantify results but reported less use of rescue medication in the probiotics group (Table 5) (Demers 2014).
1.4. Analysis.
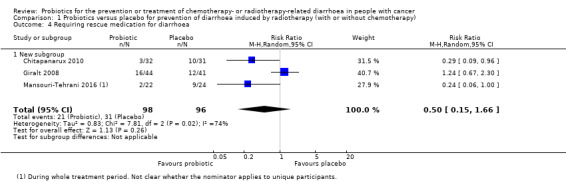
Comparison 1 Probiotics versus placebo for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy), Outcome 4 Requiring rescue medication for diarrhoea.
Adverse events
Five studies addressed this outcome narratively (Table 5) (Chitapanarux 2010; Delia 2007; Demers 2014; Giralt 2008; Mansouri‐Tehrani 2016). One study including 63 participants reported no adverse events attributable to the study drug (Chitapanarux 2010). The second study (482 participants) reported that no case of bacteraemia, sepsis, or septic shock due to the probiotic lactobacilli was reported during the treatment period with the probiotic preparation or during the six months beyond active treatment (Delia 2007). In addition, no case of bacteraemia, sepsis, or septic shock due to organisms other than the probiotic lactobacilli was recognised during the period of active treatment. The third study reported no differences between groups with respect to number of hospitalisations, number of treatment interruptions, and reduction in either chemotherapy doses or radiotherapy treatments (226 participants) (Demers 2014). Researchers also reported that intake of the probiotic was well tolerated, and that no septicaemia was recorded, "although a few cases of neutropenia occurred during treatment". The fourth study (85 participants) found no differences in reported complications at six months between treatment groups (Giralt 2008). Study authors stated that the probiotic was well tolerated, and that none of the adverse events reported were considered related to the probiotic. The fifth study reported that during pelvic radiotherapy, three participants belonging to a probiotic group (with or without honey) complained of upper abdominal pain, but that the causal link with probiotic use was not investigated (46 participants) (Mansouri‐Tehrani 2016). Bloating occurred more often in the probiotic group (19/22 versus 10/24 participants; RR 2.07, 95% CI 1.26 to 3.42).
Mortality
Not all studies mentioned this explicitly, but no studies reported any deaths (Table 5), except in one study, one participant in the probiotics group died of myocardial infarction after three sessions of radiotherapy; this participant was excluded from the analyses (Delia 2007).
1.1.2. Participants treated with chemotherapy alone
Three studies with 138 participants compared probiotics versus placebo for prevention of diarrhoea after treatment with chemotherapy (without radiotherapy) (Chen 2014; Liu 2000; Mego 2015).
Proportion of participants with diarrhoea
Three studies addressed this outcome. Based on two studies (Chen 2014; Mego 2015), the pooled RR for any diarrhoea of probiotics compared with placebo was 0.59 (95% CI 0.36 to 0.96; 106 participants) in favour of probiotics (Analysis 2.1). The third study was a cross‐over study that did not present a paired analysis of the data (Liu 2000). During the probiotic treatment period, six of the 22 participants suffered from any grade of diarrhoea, whereas during the placebo period, 10 participants had diarrhoea (Table 6).
2.1. Analysis.
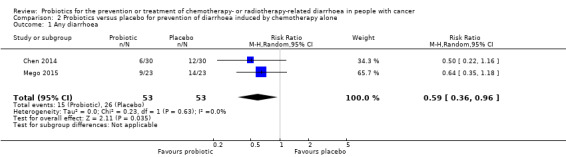
Comparison 2 Probiotics versus placebo for prevention of diarrhoea induced by chemotherapy alone, Outcome 1 Any diarrhoea.
Quality of life
No study addressed this outcome.
Severity of diarrhoea
Two studies addressed the occurrence of diarrhoea grade 2 or higher (Liu 2000; Mego 2015). In one study, the RR of probiotics versus placebo was 0.67 (95% CI 0.22 to 2.05; one study; 46 participants) (Mego 2015). In the other (cross‐over) study (Liu 2000), three of the 22 participants suffered from grade 2 or higher diarrhoea during the probiotic treatment period compared with seven in the placebo period (Table 6).
Two studies addressed the occurrence of diarrhoea grade 3 or higher (Liu 2000; Mego 2015). In one study, the RR for grade 3 or higher diarrhoea for probiotics versus placebo was 0.11 (95% CI 0.01 to 1.95; 46 participants) (Mego 2015). In the other (cross‐over) study (Liu 2000), one of the 22 participants suffered from grade 3 or higher diarrhoea during the probiotic treatment period compared with four in the placebo period (Table 6).
One study reported the occurrence of diarrhoea grade 4. The RR of probiotics versus placebo was 0.33 (95% CI 0.01 to 7.78; 46 participants) (Mego 2015) (Table 6).
Time to rescue medication for diarrhoea
No study addressed this outcome.
Proportion of participants requiring rescue medication for diarrhoea
One study addressed this outcome in 46 participants (Mego 2015). This study did not quantify the results but reported less use of rescue medication in the probiotics group (Table 6).
Adverse events
Two studies addressed this outcome (Table 6). In the first study (60 participants analysed), the occurrence of various adverse events was similar in both treatment groups, and no differences between groups were found for abdominal distension, systemic inflammatory response syndrome (SIRS), infection of the incisional wound, pulmonary infection, urinary tract infection, duration of fever, and hypoproteinaemia. Researchers observed no side effects relevant to drug use (Chen 2014). The other study (46 participants) reported only that based on study diaries, investigators observed no infections caused by probiotic strains (Mego 2015).
Mortality
Not all studies mentioned this explicitly, but no studies reported any deaths (Table 6).
1.2. Probiotics versus another active intervention or standard therapy
1.2.1. Participants treated with radiotherapy (with or without chemotherapy)
Three studies with 216 participants compared the effects of probiotics versus another active intervention in participants treated with radiotherapy with or without chemotherapy (Osterlund 2007; Timko 2010; Salminen 1988) (Table 7).
Proportion of participants with diarrhoea
One study including 24 participants (21 participants for analysed) reported this outcome (Salminen 1988). Trialists found differences between the two groups for the proportion of participants with diarrhoea in favour of probiotics at all three follow‐up measurements during treatment (exact timing was not reported by study authors) and six weeks after treatment: during treatment, RR 0.34, 95% CI 0.12 to 0.94; RR 0.20, 95% CI 0.06 to 0.72; and RR 0.23, 95% CI 0.06 to 0.83, respectively; six weeks after treatment, RR 0.30, 95% CI 0.11 to 0.81.
Quality of life
No study reported on quality of life.
Severity of diarrhoea
One study including 148 participants reported this outcome (with the odds ratio as the measure of treatment effect) (Osterlund 2007). Compared with those in the control group (guar gum containing nutritional supplement), fewer participants in the Lactobacillus group had grade 3 to 4 diarrhoea (odds ratio (OR) 0.38, 95% CI 0.16 to 0.89).
Time to rescue medication for diarrhoea
No study reported time to rescue medication for diarrhoea.
Proportion of participants requiring rescue medication for diarrhoea
Two studies reported this outcome (Salminen 1988; Timko 2010). The pooled RR for the need for rescue medication of probiotics versus other active treatment was 0.44 (95% CI 0.22 to 0.86; 63 participants) (Analysis 3.1).
3.1. Analysis.
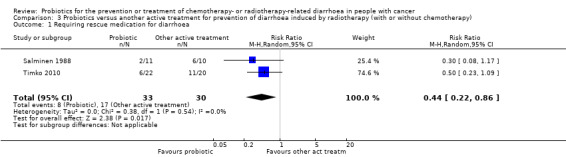
Comparison 3 Probiotics versus another active treatment for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy), Outcome 1 Requiring rescue medication for diarrhoea.
Adverse events
All three studies reported this outcome. In the first study, which reported the odds ratio as the measure of treatment effect (Osterlund 2007), study authors reported no significant differences for any of the studied adverse events amongst 148 participants.
Any adverse event grade 3 to 4: OR 0.77 (95% CI 0.35 to 1.72).
Stomatitis grade 3 to 4: OR 0.59 (95% CI 0.26 to 1.35).
Neutropenia grade 3 to 4: OR 2.00 (95% CI 0.74 to 4.89).
Neutropenic infection grade 3 to 4: OR 2.62 (95% CI 0.53 to 13.00).
Hand‐foot syndrome grade 3: 2/97 versus 1/51 (OR: no convergence).
The second study including 21 participants reported no differences in the incidence of vomiting, nausea, abdominal pain, loss of appetite, or weight loss between the two groups, but the probiotics group experienced more flatulence than was reported by the dietary counselling group (Salminen 1988).
The third study (42 participants) stated that abdominal pain was reported by 25% of participants in the probiotic group and 22% of those in the Hylak group (Timko 2010).
Mortality
Not all studies mentioned this explicitly, but no studies reported any deaths (Table 7).
1.2.2. Participants treated with chemotherapy alone
We found no studies for this group of participants.
2. Treatment of diarrhoea
2.1. Probiotics versus placebo
2.1.1. Participants treated with radiotherapy (with or without chemotherapy)
One study compared the efficacy and tolerability of Lactobacillus rhamnosus (Antibiophilus) versus placebo in 205 participants with mild to moderate diarrhoea induced by radiotherapy (Table 8) (Urbancsek 2001).
Reduction in severity of diarrhoea
The average diarrhoea grade (rated by investigators using standard scores ranging from 0 for no diarrhoea to 3 for severe diarrhoea) was 0.7 for the Antibiophilus group and 1.0 for the placebo group at the end of the study (no significant differences between the two groups). Patients' self‐ratings with regard to diarrhoea grade and faeces consistency showed a difference in treatment‐by‐time interaction (P < 0.001), but it is unclear how this result should be interpreted.
Quality of life
This study did not report on quality of life.
Time to rescue medication for diarrhoea
Time to rescue medication for diarrhoea (derived from patients' diaries) was longer in the probiotics group than in the placebo group (MD 13 hours, 95% CI ‐0.86 to 26.86; 205 participants).
Proportion of participants requiring rescue medication for diarrhoea
The proportion of participants requiring rescue medication for diarrhoea showed no significant differences between groups (RR 0.74, 95% CI 0.53 to 1.03; 205 participants).
Adverse events
Study authors reported that they observed no serious adverse events and "In the Antibiophilus group, three participants reported mild to moderate gastrointestinal problems; in the placebo group, two participants reported moderate to severe gastrointestinal events, and one patient observed a mild labial oedema. All documented events were of a transient nature; in three patients, symptomatic treatment of adverse events was prescribed".
Mortality
Although not mentioned explicitly, no study reported any deaths (Table 8).
2.1.2. Participants treated with chemotherapy alone
We found no studies for this group of participants.
2.2. Probiotics versus another active intervention or standard therapy
2.2.1. Participants treated with radiotherapy (with or without chemotherapy)
We found no studies for this group of participants.
2.2.2. Participants treated with chemotherapy alone
We found no studies for this group of participants.
Sensitivity analyses
When we excluded studies at high risk of bias from the analyses, one study was left in Analysis 1.1, and the RR for the occurrence of diarrhoea for probiotics versus placebo became 1.00 (95% CI 0.94 to 1.06). In Analysis 1.2, the RR for diarrhoea grade 2 or higher changed from 0.75 (95% CI 0.55 to 1.03) to 0.35 (95% CI 0.17 to 0.73), and for Analysis 1.4, the RR for the need for rescue medication changed from 0.50 (95% CI 0.15 to 1.66) to 0.27 (95% CI 0.11 to 0.67). In the latter two cases, two studies at unclear risk of bias remained in the meta‐analysis. For all other analyses, we could not perform sensitivity analyses because all studies that were included in the (meta‐)analysis were at high or low risk of bias.
Only one study had more than 10% missing outcome data (Chen 2014). The RR for any diarrhoea changed from 0.59 (95% CI 0.36 to 0.96) to 0.64 (95% CI 0.35 to 1.18; one study; Analysis 2.1).
Discussion
In this review, we summarised available evidence from randomised controlled trials (RCTs) assessing the effects of probiotics for prevention or treatment of radiotherapy (with or without chemotherapy)‐ or chemotherapy‐related diarrhoea. We included 12 studies involving 1554 participants. Eleven were prevention studies; seven of these compared probiotics with placebo (887 participants), one compared two doses of probiotics with each other and with placebo (246 participants), and three compared probiotics with another active agent (216 participants). The remaining study examined treatment for radiotherapy‐related diarrhoea and compared probiotics versus placebo (205 participants).
Summary of main results
For prevention of radiotherapy‐induced diarrhoea (with or without chemotherapy), we identified five studies including 905 participants (Table 1). Researchers could neither demonstrate nor refute a beneficial effect of probiotics compared with placebo on occurrence of diarrhoea, quality of life, severity of diarrhoea, or the proportion of participants requiring rescue medication (low to very low certainty of evidence). However, sensitivity analyses that omitted studies at high risk of bias revealed a beneficial effect for the occurrence of grade 2 or higher diarrhoea and the proportion of participants requiring rescue medication but showed no effect on the occurrence of any diarrhoea. In one study, time to rescue medication was on average 36 hours longer for probiotic users (95% confidence interval (CI) 34.7 to 37.3), but another study reported no difference (moderate certainty of evidence). No studies reported serious adverse events or diarrhoea‐related deaths (low certainty of evidence).
For prevention of chemotherapy‐induced diarrhoea, researchers described a beneficial effect of probiotics compared with placebo for occurrence of any diarrhoea (risk ratio (RR) 0.59, 95% CI 0.36 to 0.96; two RCTs; 106 participants), which was confirmed in a third cross‐over study including 22 participants (low certainty of evidence) (Table 2). For severity of diarrhoea and the need for rescue medication, trialists could neither demonstrate nor refute a difference in effect (low certainty of evidence; one RCT; 46 participants). No studies reported serious adverse events or diarrhoea‐related deaths (low certainty of evidence). No studies reported on quality of life nor time to rescue medication.
The three studies comparing probiotics versus another active agent in participants treated with radiotherapy (with or without chemotherapy) found differences between the two groups in favour of probiotics for the proportion of participants with diarrhoea six weeks after treatment (very low certainty of evidence; RR 0.30, 95% CI 0.11 to 0.81; one RCT; 21 participants), the occurrence of grade 3 or 4 diarrhoea (low certainty of evidence; odds ratio (OR) 0.38, 95% CI 0.16 to 0.89; one RCT; 148 participants), and the proportion of participants requiring rescue medication (very low certainty of evidence; RR 0.44, 95% CI 0.22 to 0.86; two RCTs; 63 participants) (Table 3). No studies reported differences in the occurrence of serious adverse events (very low certainty of evidence), and no studies reported any deaths (low certainty of evidence). Researchers did not examine quality of life nor time to rescue medication.
The remaining study examined treatment for radiotherapy‐related diarrhoea (Table 4). This study compared probiotics versus placebo in 205 participants. Study authors could not demonstrate nor refute a beneficial effect of probiotics on average diarrhoea grade, time to rescue medication for diarrhoea (13 hours longer in the probiotics group; 95% CI ‐0.9 to 26.9 hours), or need for rescue medication (RR 0.74, 95% CI 0.53 to 1.03) (moderate certainty of evidence). They reported no difference in the occurrence of serious adverse events (moderate certainty of evidence) and no diarrhoea‐related deaths. These researchers did not examine quality of life.
Overall completeness and applicability of evidence
For prevention of radiotherapy‐induced diarrhoea (with or without chemotherapy), five placebo‐controlled studies with 902 participants are currently available, but the number of participants included in the three studies evaluating prevention of chemotherapy‐induced diarrhoea is limited. Relevance to participants of some beneficial effects, such as delayed requirement for rescue medication by 36 hours, is questionable. Although some analyses suggest benefit from preventative probiotics, these limitations preclude any firm recommendations in favour of preventative probiotics. In addition, the effects of probiotics are strain‐specific (Rijkers 2011). Therefore, these results cannot be extrapolated to other strains of probiotics.
Comparisons of probiotics versus other active treatments are scarce and were performed in studies with few participants and providing evidence of low to very low certainty. Although for some outcomes, trialists reported a beneficial effect of probiotics compared with an alternative treatment, these studies do not permit firm conclusions regarding the choice between available treatment options.
For treatment of diarrhoea, we identified only one study. This study investigated treatment for mild diarrhoea, and the results allow firm conclusions regarding a beneficial effect. This study did not examine quality of life.
Quality of the evidence
The 'Summary of findings' tables for each comparison show that review authors downgraded the certainty (quality) of evidence for most outcomes using GRADE. Except for the only study that addressed treatment for radiotherapy‐induced diarrhoea (moderate certainty of evidence for all outcomes), the certainty of evidence was low to very low for most of the outcomes of prevention studies. We downgraded the certainty of evidence mainly for imprecision (e.g. wide 95% CIs that included both beneficial and harmful effects, optimal information size (OIS) not reached) and various types of study limitations. This implies that uncertainty about the effectiveness of probiotics remains.
Potential biases in the review process
We performed a comprehensive search of several electronic databases; however we did not search for conference abstracts. Therefore, it is possible that we missed some unpublished trials or data. In an attempt to overcome this, we searched prospective trial registries. In addition, we performed study selection, data extraction, and risk of bias assessment in duplicate to prevent bias in the review process.
For studies that were retrieved, we could not obtain from publications all the data required to make judgements for all risk of bias items.
Agreements and disagreements with other studies or reviews
We found four similar systematic reviews that evaluated effects of prevention or treatment of diarrhoea or both for participants undergoing radiotherapy or chemotherapy. Authors of the oldest review found no differences between probiotics and control for prevention of diarrhoea or for treatment of radiotherapy‐ or chemotherapy‐induced diarrhoea (Fuccio 2009). A second systematic review assessed the efficacy and safety of probiotics in people with cancer (Redman 2014). Review authors concluded that probiotics may reduce the severity and frequency of diarrhoea (both antibiotic‐associated and chemotherapy‐associated diarrhoea) and the requirement for antidiarrhoeal medication for patients with cancer. The third review reported an overall beneficial effect of probiotics for prevention of chemoradiotherapy‐induced diarrhoea, especially grade 2 or higher (Wang 2016). The most recent review addressed the effects of probiotics for prevention of radiotherapy‐induced diarrhoea (Liu 2017); review authors concluded that probiotics may be beneficial for preventing radiotherapy‐induced diarrhoea. However, it is not clear what definition trial authors used for the outcome diarrhoea. Review authors included one extra, small study (apparently a conference abstract) that we did not identify, but they made no reference to this study in their report. In our review, we included one study ‐ Mansouri‐Tehrani 2016 ‐ that was not (yet) identified by Liu 2017. Finally, we were more stringent in our risk of bias assessments and in refraining from pooling for some outcomes in the presence of heterogeneity.
Authors' conclusions
Implications for practice.
Prevention of diarrhoea
Overall, available evidence regarding the use of probiotics to prevent radiotherapy‐ or chemotherapy‐induced diarrhoea remains inconclusive. Randomised controlled trials (RCTs) could not deliver proof of clear benefit, as studies were underpowered or were at risk of bias. Moreover, for some outcomes, heterogeneity between studies was considerable and benefits, if any, were small.
However, for prevention of diarrhoea in patients receiving radiotherapy to the pelvis (without chemotherapy), investigators in a well‐powered RCT have compared probiotics versus placebo. This study suggests that use of probiotics may decrease the incidence and severity of diarrhoea, and a recent small study has confirmed these results (TCTR20170314001). As in the other studies, these two studies observed no severe side effects of probiotics. Although the first study had high risk of bias and the second study unclear risk of bias, based on these results, preventative probiotics could be considered for people undergoing radiotherapy, as associated adverse events are limited.
For prevention of chemotherapy‐induced diarrhoea, results suggest that probiotics may prevent diarrhoea. However, no firm conclusions can be drawn because the included studies were very small, and only one was judged to be at low risk of bias.
Treatment of diarrhoea
Available evidence shows that the benefit of probiotics for treatment of radiotherapy‐induced diarrhoea could be neither demonstrated nor refuted. For chemotherapy‐induced diarrhoea, we found no evidence.
Implications for research.
For prevention of chemotherapy‐ or radiotherapy‐related diarrhoea, current evidence is of low to very low certainty. Future studies should be designed to minimise potential biases that have been inherent in previous studies and should ensure sufficient power by calculating a sample size in advance that also addresses the rate of loss to follow‐up.
Currently available evidence is insufficient for judgement of the efficacy of probiotics for treatment of radiotherapy‐induced diarrhoea. Future researchers may wish to focus on mild to moderate diarrhoea and probiotics as an add‐on to more active treatment, and new studies should examine treatment for chemotherapy‐induced diarrhoea.
All studies should adhere to CONSORT reporting guidelines to enable assessment of the design and conduct of studies, and consideration should be given to agreement on important core outcome sets, as different studies use different measures, introducing heterogeneity, which prevents meta‐analysis. Agreed important core outcomes should be measured in a standardised way to allow pooling of results.
Acknowledgements
We would like to thank Cochrane Gynaecological, Neuro‐oncology, and Orphan Cancer Group for advice and support provided, in particular Clare Jess, Managing Editor, and Jo Platt, Information Specialist. We would also like to thank the peer reviewers for their constructive comments: Mario Crucian, Kathie Godfrey, Karen Moody, Ben Carter, Jennifer Cohen, Andy Bryant, Ruth Payne and Arno Mank.
The original protocol for this review was designed and written by Ting Wang, Lili Luo, Taixiang Wu, and Qinghua Zhou, and was published in December 2010. We are much indebted to the original review author team for preparation of the protocol.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology, and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. The Cochrane Library (CENTRAL) search strategy
#1 MeSH descriptor Neoplasms explode all trees #2 neoplasm* or cancer* or tumor* or tumour* or malignan* or carcinom* #3 (#1 OR #2) #4 MeSH descriptor Diarrhea explode all trees #5 diarrh* #6 antidiarrh* #7 anti‐diarrh* #8 (#4 OR #5 OR #6 OR #7) #9 MeSH descriptor Probiotics explode all trees #10 probiotic* #11 MeSH descriptor Prebiotics explode all trees #12 prebiotic* #13 MeSH descriptor Lactobacillus explode all trees #14 lactobacillus #15 MeSH descriptor Bifidobacterium explode all trees #16 bifidobacterium #17 saccharomyces boulardii #18 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17) #19 (#3 AND #8 AND #18)
Appendix 2. MEDLINE (Ovid) search strategy
1 exp Neoplasms/ 2 (neoplasm* or cancer* or tumor* or tumour* or malignan* or carcinom*).mp. 3 1 or 2 4 exp Diarrhea/ 5 diarrh*.mp. 6 antidiarrh*.mp. 7 anti‐diarrh*.mp. 8 6 or 4 or 7 or 5 9 exp Probiotics/ 10 probiotic*.mp. 11 Prebiotics/ 12 prebiotic*.mp. 13 exp Lactobacillus/ 14 lactobacillus.mp. 15 Bifidobacterium/ 16 bifidobacterium.mp. 17 saccharomyces boulardii.mp. 18 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19 3 and 8 and 18 21 controlled clinical trial.pt. 22 randomized.ab. 23 placebo.ab. 24 drug therapy.fs. 25 randomly.ab. 26 trial.ab. 27 groups.ab. 28 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29 19 and 28
key: mp=title, original title, abstract, name of substance word, subject heading word, unique identifier; pt=publication type; ab=abstract; fs=floating subheading
Appendix 3. Embase (Ovid) search strategy
1 exp neoplasm/ 2 (neoplasm* or cancer* or tumor* or tumour* or malignan* or carcinom*).mp. 3 1 or 2 4 exp diarrhea/ 5 diarrh*.mp. 6 antidiarrh*.mp. 7 anti‐diarrh*.mp. 8 4 or 5 or 6 or 7 9 exp probiotic agent/ 10 probiotic*.mp. 11 prebiotic agent/ 12 prebiotic*.mp. 13 Lactobacillus/ 14 lactobacillus*.mp. 15 exp Bifidobacterium/ 16 bifidobacterium.mp. 17 saccharomyces boulardii.mp. 18 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19 3 and 8 and 18 20 crossover procedure/ 21 double‐blind procedure/ 22 randomized controlled trial/ 23 single‐blind procedure/ 24 random*.mp. 25 factorial*.mp. 26 (crossover* or cross over* or cross‐over*).mp. 27 placebo*.mp. 28 (double* adj blind*).mp. 29 (singl* adj blind*).mp. 30 assign*.mp. 31 allocat*.mp. 32 volunteer*.mp. 33 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 34 19 and 33 35 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 36 34 not 35
key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer
Data and analyses
Comparison 1. Probiotics versus placebo for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any diarrhoea | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Diarrhoea grade 2 or higher | 4 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.55, 1.03] |
| 3 Diarrhoea grade 3 or higher | 3 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Requiring rescue medication for diarrhoea | 3 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.15, 1.66] |
| 4.1 New subgroup | 3 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.15, 1.66] |
Comparison 2. Probiotics versus placebo for prevention of diarrhoea induced by chemotherapy alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any diarrhoea | 2 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.36, 0.96] |
Comparison 3. Probiotics versus another active treatment for prevention of diarrhoea induced by radiotherapy (with or without chemotherapy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Requiring rescue medication for diarrhoea | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.22, 0.86] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2014.
| Methods | Study design: randomised controlled trial Duration of the study, recruitment: 2006 to 2010 Country: China |
|
| Participants | Participants with colorectal cancer: age, mean ± SD: intervention group: 60.3 ± 17.2, control group: 59.8 ± 18.7 | |
| Interventions | Intervention (n = 35): combined Clostridium butyricum and Bifidobacterium capsule: 3 capsules, 3 times a day, administered from 5 days before to 7 days after surgery Control (n = 35): placebo All participants received intravenous chemotherapy (calcium folinate 300 mg, fluorouracil 500 mg) during surgery |
|
| Outcomes | Proportions of diarrhoea, all‐cause mortality, several other clinical outcomes (first postoperative exhaust time, first defecation time, incidence of abdominal distension, incidence of systematic inflammatory response syndrome (SIRS), time of intraperitoneal catheter drain, incidence of infection of incisional wound, incidence of pulmonary infection, incidence of urinary tract infection, time of fever, incidence of hypoproteinaemia, length of consumption of antibiotics, length of hospital stay, side effects relevant to drug), biochemical indices | |
| Notes | Article written in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was generated through a random number table |
| Allocation concealment (selection bias) | Unclear risk | No relevant information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled trial with similar packaging of capsules |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Insufficient information for judgement |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Five participants in each group were excluded because of metastasis or non‐adherence to treatment strategy. Reasons for dropout were not specified per group |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Five participants in each group were excluded because of metastasis or non‐adherence to treatment strategy. Reasons for dropout were not specified per group |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available, but all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Chitapanarux 2010.
| Methods | Study design: parallel‐group RCT Duration of the study: 2007 to 2009 Median overall treatment time: 48 versus 51 days Country: Thailand |
|
| Participants | Participants aged ≥ 18 and ≤ 65 years old, with FIGO stage IIB to IIIB squamous cell carcinoma of the cervix, who were planned to receive standard treatment for locally advanced cervical cancer of external beam whole pelvis radiotherapy and brachytherapy plus weekly cisplatin 40 mg/m², with ECOG performance status 0 to 1 and negative anti‐HIV status Stage of cervical cancer, n (%):
ECOG performance status, n (%):
Median age, years: 47 versus 52 Sex: female "Age, stage of disease, performance status, and whole pelvis radiotherapy technique did not show any difference between the two groups" |
|
| Interventions | Intervention (n = 32): 2 × 10⁹ units of Lactobacillus acidophilus plus Bifidobacterium bifidum (equivalent to 2 capsules) 2 times a day before meals (morning and evening), beginning 7 days before the start of radiotherapy and continuing every day during radiotherapy Control (n = 31): identical‐appearing placebo administered on the same schedule All participants were scheduled for external pelvic radiotherapy at a dose of 200 cGy per fraction, 5 fractions per week. All participants received weekly cisplatin 40 mg/m² for 6 weeks during radiotherapy |
|
| Outcomes | Occurrence of diarrhoea (graded weekly according to the Common Toxicity Criteria (CTC) system), need for antidiarrhoeal medication, stool consistency, white and red blood cell count in stool | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised study, yet method of randomisation not described. |
| Allocation concealment (selection bias) | Low risk | "Pre‐packaged (blinded) study medication differing solely in the patient numbers on the medication package was provided by the sponsor" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was described to be 'double‐blind': neither the patient nor the treating physician knew if the patient was on study drug or placebo |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Participants were blinded to treatment allocation: low risk for assessment of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | All 63 participants were eligible and assessable |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | All 63 participants were assessed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available, but all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Delia 2007.
| Methods | Study design: parallel‐group RCT Duration of the study: 1995 to 2005 Follow‐up: "The study subjects were followed up weekly during the scheduled cycle of radiation therapy and then 1 month after completion of radiation therapy" Country: Italy |
|
| Participants | Participants received adjuvant postoperative radiotherapy after surgery for sigmoid, rectal, or cervical cancers and had no contraindication for probiotic or antibiotic therapy or radiotherapy Median age, years: not reported Sex: not reported "The randomization was balanced between treatment groups in terms of sex, age, nodal involvement, tumor grade and size, local invasion at operation, invasion of contiguous structures at histology, and postoperative complications" |
|
| Interventions | Intervention (n = 245): VSL#3 (VSL Pharmaceuticals, Fort Lauderdale, MD) Control (n = 245): VSL#3‐identical‐appearing placebo Radiotharapy: total X‐ray dose between 60 and 70 Gy |
|
| Outcomes | Incidence and severity of radiotherapy‐induced diarrhoea, time from start of the study to use of loperamide as rescue medication, daily number of bowel movements | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised study, yet method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Double‐blind, placebo‐controlled trial: low risk for assessment of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Double‐blind, placebo‐controlled trial: low risk for assessment of objective outcomes (mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | Intervention group (n = 2 dropouts): 1 participant withdrew his consent after the first session of radiotherapy, and 1 died of myocardial infarction after 3 sessions of radiotherapy; both participants were excluded from analysis of results Control group (n = 6 dropouts): 6 participants were withdrawn after a few sessions of radiotherapy owing to the occurrence of severe diarrhoea resistant to loperamide and the usual standard of care; these participants were excluded from analysis of results. No intention to treat |
| Incomplete outcome data (attrition bias) Objective outcomes | High risk | Intervention group (n = 2 dropouts): 1 participant withdrew his consent after the first session of radiotherapy, and one died of myocardial infarction after 3 sessions of radiotherapy; both participants were excluded from analysis of results Control group (n = 6 dropouts): 6 participants were withdrawn after a few sessions of radiotherapy owing to the occurrence of severe diarrhoea resistant to loperamide and the usual standard of care; these participants were excluded from analysis of results. No intention to treat |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available, but all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Demers 2014.
| Methods | Study design: placebo‐controlled RCT, 3 parallel groups Duration of the study, recruitment: 2006 to 2010 Follow‐up: maximum duration of follow‐up was 10 weeks Country: Canada |
|
| Participants | Participants (≥ 18 years old) with pelvic (gynaecological, rectal, or prostate) cancer and ECOG performance status of 0 or 1 who were to receive radiotherapy treatments at a minimum of 40 Gy at the pelvic level, with or without chemotherapy
Exclusion criteria: previous radiotherapy treatment in the pelvic or abdominal region, medical history of gastrointestinal disorders, pregnancy, breastfeeding, neutropenia, probiotic intolerance Participant characteristics (standard dose versus higher dose versus placebo), n (%):
Mean age, years: 61.4 versus 62.0 versus 60.6 Male sex, n (%): 58 (72) versus 39 (66) versus 56 (63) "The data reveal that the participants were well distributed among groups and protocol fidelity exceeded 90% in each of the three groups" |
|
| Interventions | Intervention 1 (n = 91 randomised, n = 81 analysed): standard dose of double‐strain Bifilact probiotics (Lactobacillus acidophilus LAC‐361 and Bifidobacterium longum BB‐536) twice a day (1.3 billion CFU) Intervention 2 (n = 64 randomised, n = 59 analysed): high dose of double‐strain Bifilact probiotics (Lactobacillus acidophilus LAC‐361 and Bifidobacterium longum BB‐536) 3 times a day (10 billion CFU) Control group: placebo (n = 91 randomised, n = 86 analysed) |
|
| Outcomes | Proportion of participants with diarrhoea, quality of life (EORTC‐QLQ‐C30), need for antidiarrhoeal medication, number of bowel movements, abdominal pain, stool consistency, compliance, number of hospitalisations, number of treatment interruptions, and reduction in chemotherapy doses or radiotherapy treatments as a result of severe diarrhoea or abdominal pain | |
| Notes | Trial registration: NCT01839721; number analysed in the control group (86) based on Table 2 of the study report | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were block‐randomised by the research nurse according to the random list generated by blocks of 2, 4,or 6 patients, according to random permutations [...] another random block using higher probiotics dosage to the randomization was added with preservation of the double blind. New random lists were generated for each stratum with a 3:1:1 ratio (higher dose, standard dose, placebo) to compensate for the late start of the higher dose group" |
| Allocation concealment (selection bias) | High risk | "All the bottles had a similar appearance; they were all identified by the commercial brand Bifilact. Also the group, either A, B or C, was circled on that bottle, depending on whether that bottle belonged to the placebo group, standard dose group, or high dose group. Only the nurse knew the coding system, the nurse also assigned the patient to a group, according to the randomization list" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The two registered dieticians and caregivers were blinded to these processes to preserve the double blind" Participants were blinded as well |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | "The two registered dieticians and caregivers were blinded to these processes to preserve the double blind" Participants were blinded as well |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | n = 17 dropped out before first radiotherapy (standard‐dose group n = 10, high‐dose group n = 5, placebo group n = 2) and were excluded from the analysis. Not clear whether this may have influenced the results n = 7 discontinued intervention (standard‐dose group n = 1, high‐dose group n = 3, placebo group n = 3); however all were analysed in the group to which they were randomised |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Survival status of 17 excluded participants was unknown |
| Selective reporting (reporting bias) | Low risk | Outcomes reported in publication correspond to outcomes prespecified in study protocol |
| Other bias | Low risk | No indication of other bias |
Giralt 2008.
| Methods | Study design: placebo‐controlled parallel‐group RCT Duration of the study, recruitment: November 2002 to December 2005 Follow‐up: 6 months Country: Spain |
|
| Participants | Female gender, age ≥ 18 years, good performance status (ECOG functional status < 2), and diagnosis of endometrial adenocarcinoma requiring postoperative pelvic RT or advanced cervical squamous cell carcinoma treated with pelvic RT and concomitant weekly cisplatin Primary tumour site:
ECOG performance status:
Mean age, years (SD): 60.9 (11.8) versus 59.3 (12.8) Sex: all female "Both groups were well matched according to standard variables at baseline" |
|
| Interventions | Intervention (n = 44): 96 mL 3 times daily of a fermented liquid yogurt containing approximately 10⁸ CFU/g of Lactobacillus casei DN‐114 001, in addition to the standard starters Streptococcus thermophilus and Lactobacillus delbrueckii, subsp bulgaricus Control (n = 41): same amount of matching placebo, prepared by sterilising the active product with 4 kGy administered for 5 minutes Total radiation dose was 45 to 50 Gy, conventional fractionation. Participants with cervical cancer received a weekly intravenous dose of cisplatin 40 mg/m² during external beam RT. Antiemetic treatment was provided with 5‐HT₃ blockers to maintain oral tolerability, as required |
|
| Outcomes | Reduction of the incidence of diarrhoea, defined by a Common Toxicity Criteria grade ≥ 2 or the need for loperamide | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done in blocks and was stratified by tumour type |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Double‐blind, placebo‐controlled trial: low risk for assessment of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | 118 participants were randomly allocated to the active product or placebo. Subsequent review determined that 33 of the total number of participants were ineligible and were excluded from the study. Of these 33 participants, 17 withdrew prematurely for personal reasons, 11 were excluded for protocol violations, and 5 were excluded for lack of compliance. The remaining 85 participants constituted the study group. Of these 85 participants, 44 received the active product, and 41 placebo |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Survival status of excluded participants not reported |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available, but all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Liu 2000.
| Methods | Study design: randomised cross‐over trial Duration of the study, recruitment: no information Country: China |
|
| Participants | 22 participants with cancer (8 with lung cancer, 5 with gastric cancer, 4 with colorectal cancer, 4 with breast cancer, 1 with neck metastatic carcinoma) 13 males and 9 females Age, median (range): 59 (35 to 73) years |
|
| Interventions | Intervention (n = 11): Bifidobacterium combined with chemotherapy Control (n = 11): chemotherapy alone Bifidobacterium capsule (2 capsules per time, 2 times a day) was taken from 1 day before chemotherapy to the sixth day of chemotherapy in each phase. Length of the washout period was about 21 days |
|
| Outcomes | Severity of diarrhoea | |
| Notes | Article written in Chinese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | During the control phase, no treatment was provided |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | No loss to follow‐up |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available; however all outcomes prespecified in the methods section were reported in the results section |
| Other bias | High risk | Cross‐over design ignored in the analyses |
Mansouri‐Tehrani 2016.
| Methods | Study design: placebo‐controlled RCT with 3 parallel groups Duration of the study, recruitment: October 2012 to May 2013 Follow‐up: 5 weeks Country: Iran |
|
| Participants | 78 participants between 20 and 85 years of age with diagnosis of pelvic cancer (colorectal, prostate, endometrial, bladder, ovarian, cervical, bone sarcoma) who were about to receive radiotherapy (11 participants dropped out) Exclusion criteria: opioid usage, antimicrobial treatment, presence of any acute or chronic gastrointestinal condition associated with diarrhoea for ≥ 1 month before inclusion Mean age ± SD: 63.7 ± 15.1 versus 57.9 ± 17.5 versus 64.2 ± 11.7 years Male/female: 14/8 versus 8/13 versus 17/7 Cancer site, n:
|
|
| Interventions | Probiotics: ‘LactoCareOD’ (Zist Takhmir Company, Tehran, Iran) containing Lactobacillus casei 1.5 × 10⁹ CFU, Lactobacillus acidophilus 1.5 × 10¹º CFU, Lactobacillus rhamnosus 3.5 × 10⁹ CFU, Lactobacillus bulgaricus 2.5 × 10⁸ CFU, Bifidobacterium breve 1 × 10¹º CFU, Bifidobacterium longum 5 × 10⁸ CFU, and Streptococcus thermophilus 1.5 × 10⁸ CFU per 500 mg Intervention group 1 (n = 22): 2 probiotic capsules per day after consumption of 150 grams of low‐fat yogurt Intervention group 2 (n = 21): 2 probiotic capsules and 30 grams honey per day after consumption of 150 grams of low‐fat yogurt and 15 grams of honey at night Placebo group (n = 24): 2 placebo capsules per day after consumption of 150 grams of low‐fat yogurt All participants received conventional radiotherapy for 4 to 5 weeks (total dose from 4000 to 5000 cGy (1.8 Gy/d)) |
|
| Outcomes | Severity of diarrhoea according to the Common Toxicity Criteria of the National Cancer Institute, stool consistency according to an adapted Bristol Scale, daily number of bowel movements, need for antidiarrhoeal medication, bloating | |
| Notes | Trial registration: IRCT2015030421338N1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A randomized, placebo‐controlled study was performed"/"Simple randomization was used to allocate patients to three groups" |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No details provided, but group 1 received probiotics and group 3 (control group) received similar medication with placebo capsules |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | "The patients evaluated for the daily number of bowel movement (defecation), diarrhea grade, stool consistency score, the need for antidiarrheal medication and bloating weekly by one person" Not sure whether this person was unaware of the treatment group |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Double‐blind, placebo‐controlled trial: low risk for assessment of objective outcomes (mortality) |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | "Among 78 patients involved in this study, 11 patients were excluded for failure to follow up" Reasons for dropout and intervention group of dropouts not presented |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | "Among 78 patients involved in this study, 11 patients were excluded for failure to follow up" Reasons for dropout and intervention group of dropouts not presented |
| Selective reporting (reporting bias) | Low risk | Outcomes presented according to trial registration form (IRCT2015030421338N1); laboratory outcomes reported in another paper |
| Other bias | Low risk | No indications of other bias |
Mego 2015.
| Methods | Study design: placebo‐controlled RCT, conducted at 6 cancer centres Duration of the study, recruitment: between January 2011 and December 2013 Follow‐up: not reported Country: Slovakia |
|
| Participants | Inclusion criteria: age ≥ 18 years, histologically proven colorectal cancer, starting new line of chemotherapy based on irinotecan, ECOG performance status 0 or 1, life expectancy > 3 months; no psychological, familial, sociological or geographical condition potentially hampering compliance with study protocol and follow‐up schedule Exclusion criteria: impossible to take oral medication, active infection treated by antibiotic therapy, ileostomy, hypersensitivity to study drug, any concurrent malignancy other than non‐melanoma skin cancer, other cancer in past 5 years, serious concomitant systemic disorders or diseases incompatible with the study (at the discretion of investigator) Median age (range): 62 (45 to 75) versus 64 (42 to 81) years Male gender, n (%): 14 (60.9) versus 12 (52.2) Primary tumour site, n (%):
Karnofsky performance status, n (%):
Type of chemotherapy, n (%):
Biological therapy, n (%):
|
|
| Interventions | Intervention (n = 23): probiotic formula Colon Dophilus™ (produced by Harmoniom International, Inc., Mirabel, Canada) 3*1 capsule per day orally for 12 weeks
"Each capsule contained 10*10⁹ CFU of bacteria. Each capsule contained 10 lyophilized probiotic strains including Bifidobacterium breve HA‐129 (25%), Bifidobacterium bifidum HA‐132 HA (20%), Bifidobacterium longum HA‐135 (14.5%), Lactobacillus rhamnosus HA‐111 (8%), Lactobacillus acidophilus HA‐122 (8%), Lactobacillus casei HA‐108 (8%), Lactobacillus plantarum HA‐119 (8%), Streptococcus thermopilus HA‐110 (6%), Lactobacillus brevis HA‐112 (2%), Bifidobacterium infantis HA‐116 (0.5%)" Control (n = 23): placebo Participants received full supportive care during irinotecan‐based chemotherapy including antidiarrhoeal drugs (loperamide, diphenoxylate/atropine) treatment, antiemetics, and analgesics when appropriate as a standard of care |
|
| Outcomes | Grade 3 or 4 toxicity or SAE‐related toxicity according to NCI Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE) Any grade gastrointestinal symptoms (enteritis, colitis, constipation, abdominal distension, bloating, flatulence, gastritis, dyspepsia, nausea, vomiting) according to NCI Common Terminology Criteria for Adverse Events Version 4.0 (CTCAE) |
|
| Notes | Trial registration: NCT01410955 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were centrally randomised in ratio 1:1" "Participants were allocated to one of the treatment groups (probiotic or placebo) based on preformed randomization table" "Random allocation sequence was generated using random number table. Participants were stratified according to center, treatment with cetuximab, and irinotecan regimen (weekly versus every 2 to 3 weeks)" |
| Allocation concealment (selection bias) | Low risk | "After signing of informed consent, each patient received study number and investigator called to randomization center" "Investigator received the identification number of containers for randomised patient, and patient received corresponding containers" "Patients, investigators and statisticians were blinded to treatment allocation. All containers with probiotics/placebo looked the same and were sequentially numbered" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, investigators and statisticians were blinded to treatment allocation. All containers with probiotics/placebo looked the same and were sequentially numbered" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Participants were blinded |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | No loss to follow‐up |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the prospective trial register seem to have been presented (although it is not clear whether the 2‐year time frame was applied) |
| Other bias | Low risk | "The study was prematurely terminated due to slow accrual, when only 49 of 220 planned participants were accrued" |
Osterlund 2007.
| Methods | Study design: open‐label, prospective, randomised, single‐institution, 2 × 3 factorial design study Duration of the study, recruitment: November 1997 to August 2001 Follow‐up: 4‐weekly during chemotherapy and radiotherapy and at protocol‐determined intervals (ranging from 2 to 6 months) post treatment Country: Finland |
|
| Participants | Study participants had either Dukes’ B or C colorectal cancer (n = 126) or metastatic colorectal cancer that had been rendered free from all overt metastases by surgery (Dukes’ D; n = 24) Site, n (%)
Dukes’ stage, n (%)
*Patients were rendered free from all macroscopic cancer by surgery Median age, years (range): 60 (31 to 75) Sex (M/F): 51/47 versus 25/27 "The treatment arms were balanced with gender, the WHO performance status, primary tumour site, Dukes’ stage, and radiation therapy given" |
|
| Interventions | Intervention (n = 98):Lactobacillus rhamnosus GG (administered orally as gelatin capsules twice daily at a dose of 1 to 2 × 10¹º per day during 24 weeks of adjuvant cancer chemotherapy Control (n = 52): guar gum containing nutritional supplement (contains 11 g guar gum and 550 kcal or 2300 kJ), administered daily, on cycle days 7 to 14, for 8 days per month Chemotherapy: monthly 5‐FU and leucovorin bolus injections (the Mayo regimen) or a bimonthly 5‐FU bolus plus continuous infusion (the simplified de Gramont regimen) for 24 weeks as postoperative adjuvant therapy. Pelvic radiotherapy for rectal cancer was administered to a total cumulative dose of 50.4 Gy in 1.8‐Gy daily fractions over 5.5 weeks (except for participants who underwent abdominoperineal resection, when the dose was limited to 45 Gy). All participants received dietary counselling |
|
| Outcomes | Frequency of grade 3 and 4 diarrhoea; treatment‐related adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to study treatments was performed by a computerised minimisation technique and 1 out of 6 chances. Participants were randomly allocated at a 1:1 ratio to receive the simplified de Gramont regimen or the Mayo regimen as adjuvant chemotherapy. Participants were also randomly assigned to receive or not receive at a 2:1 ratio Lactobacillus rhamnosus GG and at a 1:2 ratio fibre‐containing nutritional support (guar gum) |
| Allocation concealment (selection bias) | Low risk | "The allocation group was concealed until interventions had been assigned" Yet, not described how |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The study was not placebo‐controlled nor blinded to administration of the dietary supplements" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | "The study was not placebo‐controlled nor blinded to administration of the dietary supplements, which may or may not have influenced assessment of subjective outcomes such as adverse effects" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | None of the participants were lost to follow‐up: study was analysed according to the intention‐to‐treat principle. Sixteen (11%) subjects did not complete the scheduled 6 months of adjuvant chemotherapy because of adverse events (n = 7, 6 of whom received bolus 5‐FU), cancer recurrence (n = 5), or concomitant disease (n = 4). Two participants (both in the continuous 5‐FU group) who did not receive any of the study treatments owing to postoperative complications were not included in safety or efficacy analyses, leaving 148 participants for inclusion in these analyses |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | No participants were lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | "Outcome was analysed as defined in the study protocol" No study protocol was available, but all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
Salminen 1988.
| Methods | Study design: 2‐group parallel RCT Duration of the study: not reported; follow‐up until 6 weeks after completion of treatment Country: Finland |
|
| Participants | Participants with the diagnosis of cervix or uterus carcinoma were included in this study Age: 40 to 75 years Sex: female |
|
| Interventions | Intervention (n = 11): both dietary counselling and a daily dose of ≥ 2 × 10⁹ live Lactobacillus acidophilus bacteria in the form of a yogurt‐type product 150 mL of the product daily for 5 days before radiotherapy, daily throughout the radiotherapy period including the interval, and then for 10 days after completion of the therapy regimen Control (n = 10): dietary counselling only Sum of internal and external radiation was 8000 cGy for the tumour and 5000 cGy for the pelvic area |
|
| Outcomes | Frequency and severity of radiotherapy‐induced diarrhoea, intestinal side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised study, yet method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding: probably not performed |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No information on blinding: probably not performed. High risk of detection bias for subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Two participants were excluded from the control group because of changes in the radiotherapy regimen. One participant from the test group was excluded because she had no pause during radiotherapy. All other participants in the test group tolerated the yogurt treatment well and completed the prescribed treatment regimen Owing to the relatively large numbers of dropouts in this small study, we scored 'unclear' |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Owing to the relatively large numbers of dropouts in this small study, we scored 'unclear' |
| Selective reporting (reporting bias) | Unclear risk | Study was published in 1988, and no study protocol is available. Yet, all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Unclear risk | No baseline characteristics are reported in Table 1. As treatment groups are small, baseline differences could have been present by chance |
Timko 2010.
| Methods | Study design: randomised parallel‐group non‐placebo‐controlled trial Duration of the study, recruitment: June 2005 to March 2006 Country: Slovakia |
|
| Participants | Oncology participants underwent adjuvant postoperative radiotherapy in the abdominal and pelvic region, with or without chemotherapy. Absence of gastrointestinal disorders Median age, years (range): 62 (34 to 82) versus 67 (43 to 83) Sex (male/female): 12/10 versus 16/4 |
|
| Interventions | Intervention (n = 22): L‐Group ‐ Probiotic preparation "5"‐strain Dophilus (55% Lactobacillus rhamnosus, 20% Bifidobacterium adolescentis, 5% Lactobacillus acidophilus, 5% Bifidobacterium longum, 15% Enterococcus faecium) with a count of 6 billion active bacteria/capsule at a daily dosage of 2 × 1 capsule Control (n = 20): H‐Group ‐ Hylak Tropfen Forte preparation (i.e. cell‐free fermentation products of Lactobacillus helveticus and gut symbionts (100 mL containing 24.95 g Escherichia coli metabolita, 12.5 g Streptococcus faecalis metabolita, 12.5 g Lactobacillus acidophilus metabolita, 49.9 g Lactobacillus helveticus metabolita)) at a dose of 40 drops, 3 times per day Radiation total cumulative dose of 50 Gy (2 Gy/d). High‐risk patients (e.g. patients with prostate cancer) received dosage of 6567 Gy (2 Gy/d) |
|
| Outcomes | Incidence and severity of radiotherapy‐induced diarrhoea | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised study, yet method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No information on blinding: probably not performed; no placebo had been used |
| Blinding of outcome assessment (detection bias) Subjective outcomes | High risk | No information on blinding and no placebo: probably not performed. High risk of detection bias for subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | High risk | During RT, 27% of participants in L‐Group required diphenoxylate treatment compared with 55% in H‐Group, and 9% in L‐Group needed administration of antibiotics compared with 25% in H‐Group. As we could not estimate the way in which these treatments influenced the composition of intestinal bacterial flora, we excluded these participants from our comparisons |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Survival status of excluded participants was unknown |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available, but all outcomes prespecified in the methods section were reported in the results section |
| Other bias | High risk | Treatment arms were not balanced by gender and primary tumour site |
Urbancsek 2001.
| Methods | Study design: randomised, double‐blind, parallel‐group design Duration of the study: August 1996 to end of June 1998 Country: Hungary |
|
| Participants | People with cancer in the age range 19 to 75 years developing diarrhoea within 4 weeks after receiving radiotherapy (median cumulative radiation dose 50 Gy per patient) in the abdominal region, patients with clinical evidence of severe diarrhoea‐induced dehydration, and patients with bloody diarrhoea were not eligible Mean age, years (range): 59 (28 to 81) versus 60 (33 to 86) Sex, % (male/female): 25/75 versus 26/76 |
|
| Interventions | Intervention (n = 102): Lactobacillus rhamnosus (Antibiophilus, each sachet containing 1.5 g of Lactobacillus rhamnosus equivalent to 1.5 × 10⁹ CFU) 3 times a day Control (n = 103): identical‐appearing sachets of placebo, each containing 700 mg corn starch, 797 mg microcrystalline cellulose, 1.37 mg iron oxide, 1.13 mg dispersed orange (colouring agent), and 1 mg caramel aroma, 3 times a day |
|
| Outcomes | Time to and frequency of rescue medication per participant. Documentation of any possible adverse reactions was provided on a volunteer basis. Secondary efficacy endpoints included average number of daily bowel movements, diarrhoea grading, and faeces consistency ratings | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised study, yet method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Pre‐packaged (blinded) study medication differing solely in patient numbers on the medication package was provided to investigators by the sponsor |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was described to be 'double‐blind': neither the patient nor the treating physician knew if the patient was receiving study drug or placebo |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Participants were blinded to treatment allocation: low risk for assessment of subjective outcomes |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | No information; however it is unlikely that assessment of objective outcomes (mortality) would have been influenced |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Intention‐to‐treat analysis was performed |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Intention‐to‐treat analysis was performed |
| Selective reporting (reporting bias) | Unclear risk | No study protocol is available, but all outcomes prespecified in the methods section were reported in the results section |
| Other bias | Low risk | No indications of other bias |
5‐FU: 5‐fluorouracil 5‐HT₃: 5‐hydroxytryptamine receptor CFU: colony‐forming units CTC: Common Toxicity Criteria CTCAE: Common Terminology Criteria for Adverse Events ECOG: Eastern Cooperative Oncology Group EORTC‐QLQ‐C30: questionnaire developed to assess the quality of life of patients with cancer FIGO: International Federation of Gynaecology and Obstetrics NCI: National Cancer Institute RCT: randomised controlled trial RT: radiotherapy SAE: serious adverse event SD: standard deviation SIRS: systemic inflammatory response syndrome WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amital 2003 | Editorial |
| Aso 1995 | Study about prophylaxis for recurrence of superficial bladder cancer |
| Delia 2002 | No RCT |
| Delia 2002a | Letter to the editor |
| Dugas 1999 | No RCT |
| Fuccio 2012 | Irrelevant to our review question; no RCT |
| Fuccio 2013 | Letter to the editor |
| Garcia‐Peris 2016 | Comparison of prebiotics versus placebo |
| Horowitz 2003 | No RCT |
| Lacouture 2016 | Combined intervention of probiotic and topical alclometasone that was not randomly allocated |
| Liu 2011 | Participants receiving preoperative chemotherapy or radiotherapy were excluded |
| Liu 2013 | Participants receiving preoperative chemotherapy or radiotherapy were excluded |
| Marteau 2001 | Irrelevant to our review question |
| Marteu 2001 | Narrative review |
| McFarland 1994 | Population irrelevant to our review question |
| Mettler 1973 | No RCT |
| Narayan 2010 | Not cancer‐related diarrhoea; no RCT |
| Ohigashi 2011 | No RCT |
| Okawa 1989 | Outcomes irrelevant to our review question |
| Osterlund 2004 | Comparison not of interest |
| Sasidharan 2016 | Prebiotics |
| Visich 2010 | No RCT |
| Zheng 2006 | Children |
RCT: randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
Theodoropoulos 2013.
| Methods | Single‐centre double‐blind RCT |
| Participants | Participants underwent colectomy for cancer No mention of participants undergoing chemotherapy or radiotherapy |
| Interventions | Synbiotics (combination of prebiotics and probiotics) (n = 38) Placebo (n = 35) Administration at the day participants were able to tolerate PO liquid diet and for 15 days thereafter |
| Outcomes | Primary endpoints: gastrointestinal function‐related quality of life at 1, 3, and 6 months postoperatively (using validated questionnaire GIQLI (Gastrointestinal Quality of Life Index)) Secondary endpoints: assessment of functional bowel disorders (diarrhoea, constipation) based on respective domains of the validated instrument EORTC‐QLQ‐C30 |
| Notes | Clintrial.Gov trial ID NCT01479907 No mention of participants undergoing chemotherapy or radiotherapy Trial may be excluded in a future update |
EORTC‐QLQ‐C30: questionnaire developed to assess the quality of life of patients with cancer, version 3.
GIQLI: Gastrointestinal Quality of Life Index.
PO: by mouth.
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
EUCTR2015‐000868‐34‐DE.
| Trial name or title | Study for investigating the effects of a probiotic on diarrhea caused by chemotherapy in patients with gastric, colon, and rectum cancer |
| Methods | Allocation: randomised controlled trial Control: placebo Study endpoint classification: efficacy study Intervention model: parallel Number of arms: 2 Masking: double‐blind (masked roles: subject, investigator, outcomes assessor) Primary purpose: prevention Study phase: 3 |
| Participants | Gastric or colorectal cancer, for which treatment with 5‐fluorouracil and 1 further chemotherapeutic remedy (irinotecan or a platinum‐based chemotherapeutic remedy) is planned Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention group: Mutaflor Suspension. Oral suspension Placebo group: oral suspension |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | ClinicalTrials.gov Identifier: EUCTR2015‐000868‐34‐DE Study start date: 9 April 2015 |
| Contact information | Contact: Clinical Trials Information info@zkes‐gmbh.de |
| Notes |
NCT00197873.
| Trial name or title | Randomised, double‐blind, placebo‐controlled, cross‐over phase II study on the effects of Lactobacillus rhamnosus GG supplementation in patients on 1st line XELOXA treatment for metastatic colorectal cancer |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: cross‐over assignment Masking: double‐blind (subject, caregiver, investigator, outcomes assessor) Primary purpose: prevention |
| Participants | Estimated enrolment: 84 Ages eligible for study: ≥ 18 years (adult, senior) Genders eligible for study: both Accepts healthy volunteers: no Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Lactobacillus rhamnosus supplementation: Lactophilus supplementation is administered during chemotherapy Placebo is administered during chemotherapy |
| Outcomes | Primary outcome measures: effect on treatment‐related grade 2 to 4 diarrhoea [Time Frame: 18 weeks] [Designated as safety issue: No] Secondary outcome measures: effect on treatment‐related toxicity other than diarrhoea [Time Frame: 18 weeks] [Designated as safety issue: No] Association between supplementation and response [Time Frame: 18 weeks] [Designated as safety issue: No]
|
| Starting date | ClinicalTrials.gov Identifier: NCT00197873 Study start date: September 2005 Estimated primary completion date: October 2016 |
| Contact information | Heikki Joensuu, Professor, Helsinki University |
| Notes |
NCT01790035.
| Trial name or title | A phase I and randomised controlled phase II trial of the probiotic LGG for prevention of side effects in patients undergoing chemoradiation for gastrointestinal cancer |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (subject, investigator) Primary purpose: treatment |
| Participants | Estimated enrolment: 120 Ages eligible for study: ≥ 18 years (adult, senior) Genders eligible for study: both Accepts healthy volunteers: no Inclusion criteria:
Exclusion criteria:
|
| Interventions | Intervention: LGG Comparator: placebo |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | ClinicalTrials.gov Identifier: NCT01790035 Study start date: August 2014 Estimated primary completion date: October 2021 |
| Contact information | Matthew Ciorba, MD; 314‐362‐9054; mciorba@wustl.edu |
| Notes |
NCT02169388.
| Trial name or title | Effects of gut microflora on the immune and nutritional status of CRC patients after chemotherapy |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: double‐blind (subject, caregiver, investigator) Primary purpose: supportive care |
| Participants | Estimated enrolment: 30 Ages eligible for study: 18 to 80 years (adult, senior) Genders eligible for study: both Accepts healthy volunteers: no Inclusion criteria:
Exclusion criteria:
|
| Interventions | Experimental: probiotic (microbial composition using probiotic, 3 capsules/times, 2 times/d for 4 weeks) Placebo comparator: placebo (Microbiota modulation using placebo, 3 capsules/times, 2 times/d for 4 weeks) |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | ClinicalTrials.gov Identifier: NCT02169388 Study start date: June 2014 Estimated primary completion date: January 2015 |
| Contact information | Contact: Yanqing Li, MD, PhD; 86‐531‐82169236 ext 82169508; liyanqing@sdu.edu.cn |
| Notes |
NCT02351089.
| Trial name or title | Probiotics in radiation‐treated gynecologic cancer |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐blind (subject, caregiver, investigator, outcomes assessor) Primary purpose: prevention |
| Participants | Estimated enrolment: 200 Ages eligible for study: ≥ 18 years (adult, senior) Genders eligible for study: female Accepts healthy volunteers: no Inclusion criteria:
Exclusion criteria:
|
| Interventions | Probiotic low dose: capsules containing probiotic powder and corn starch Probiotic high dose: capsules containing probiotic powder and corn starch Placebo: capsules containing corn starch |
| Outcomes | Primary outcome measures: change in incidence of loose/watery stools [Time Frame: Baseline and 10 weeks later] [Designated as safety issue: No] |
| Starting date | ClinicalTrials.gov Identifier: NCT02351089 Study start date: February 2015 Estimated primary completion date: December 2016 (final data collection date for primary outcome measure) |
| Contact information | Contact: Maria Bjurberg, MD, PhD; maria.bjurberg@skane.se |
| Notes |
NCT02819960.
| Trial name or title | Prevention of irinotecan‐induced diarrhea by probiotics |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: double‐blind (participant, care provider) Primary purpose: prevention |
| Participants | Estimated enrolment: 100 Ages eligible for study: ≥ 18 years (adult, senior) Genders eligible for study: female Accepts healthy volunteers: no Inclusion criteria:
Exclusion criteria:
|
| Interventions | Probiotic group: probiotic formula PROBIO‐FIX INUM will be administered at a dose of 3 × 1 cps per day orally for 6 weeks. No premedication or patient monitoring after administration of probiotic formula is required. Probiotic formula may be taken after meals or snacks to reduce stomach upset. Swallow the capsule, or in case of problems with swallowing, capsule can be opened and content mixed with small amount of food. Food must not be hot Placebo group: maltodextrin will be used for placebo group and will be administered at a the same dose as active formula (3 × 1 cps per day orally for 6 weeks) |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | ClinicalTrials.gov Identifier: NCT02819960 Study start date: March 2016 Estimated primary completion date: March 2018 (final data collection date for primary outcome measure) |
| Contact information | Contact: Michal Mego, MD misomego@gmail.com |
| Notes |
Sharma 2013.
| Trial name or title | A phase II/III, randomised, double‐blind, placebo‐controlled study to investigate the efficacy of a probiotic VSL#3 on chemotherapy‐induced diarrhea in people with cancer receiving fluoropyrimidines and/or irinotecan (interim analysis) Clinical Trial Registry number: CTRI/2009/091/001042 |
| Methods | Randomised, parallel‐group, placebo‐controlled trial Country: India |
| Participants | Participants ≥ 18 years with histologically confirmed diagnosis of cancer, treated with fluoropyrimodines and/or irinotecan‐based chemotherapy; ECOG ≤ 2 Participants with recurrent disease must have completed last chemotherapy 4 weeks before enrolment in the study |
| Interventions | VSL#3 sachets; 1 sachet bid for 12 to 16 weeks. Each sachet contains 900 billion CFU Placebo sachets; 1 sachet bid for 16 weeks |
| Outcomes | Primary outcome: incidence and duration of grade 3 and 4 diarrhoea caused by fluoropyrimidines and/or irinotecan Secondary outcomes:
Time points: at all chemotherapy cycles, that is, cycles 1, 2, and 3 of chemotherapy and at the follow‐up visit (i.e. 2 weeks after third cycle of chemotherapy) |
| Starting date | 27/7/2010 |
| Contact information | Dr Atul Sharma; atul1@hotmail.com |
| Notes | Conference abstract (limited reporting of interim analysis without unblinding) |
TCTR20170314001.
| Trial name or title | Effect of probiotics for the prevention of acute radiation‐induced diarrhea among cervical cancer patients |
| Methods | Allocation: randomised controlled trial Control: placebo Study endpoint classification: efficacy study Intervention model: parallel Number of arms: 2 Masking: double‐blind (masked roles: subject, investigator, outcomes assessor) Primary purpose: prevention Study phase: 2 |
| Participants | Radiotherapy‐induced diarrhoea |
| Interventions | Probiotic group: probiotic group was given 1 capsule 3 times daily (each capsule contains functional yogurt 300 mg containing 1.75 billion lyophilised live Lactobacillus acidophilus LA‐5 plus Bifidobacterium animalis subsp lactis BB‐12), beginning from the first day of radiotherapy, continuing every day until the end of radiotherapy Placebo group: placebo group received placebo capsules (containing starch of equal weight as the study drug), which had identical colour and size as the study drug. Treatment schedule was the same as that of the study group |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | ClinicalTrials.gov Identifier: TCTR20170314001 Study start date: 2 May 2016 Primary completion date: 29 November 2016 |
| Contact information | Contact: Ye Htut Linn, MB, BS, MMedSc dryehtutlinn@gmail.com |
| Notes |
5‐FU: 5‐fluorouracil AUC: area under the curve BIA: bioelectrical impedance analysis BMI: body mass index CFU: colony‐forming units CNS: central nervous system CRC: colorectal cancer CRP: C‐reactive protein CTC: Common Toxicity Criteria CTCAE: Common Terminology Criteria for Adverse Events DSMC: Data and Safety Monitoring Committee ECOG PS: Eastern Cooperative Oncology Group Performance Status EGFR: epidermal growth factor receptor FACIT‐D: Functional Assessment of Chronic Illness Therapy for Patients With Diarrhoea GCP: Guideline for Good Clinical Practice IBS: irritable bowel syndrome ICH: International Conference on Harmonisation IEC: independent ethics committee IMMP: no formal explanation was provided; IMMP are a series of new investigational drugs including IMP321, IMP731, IMP701, etc. produced by Immuptep limited IV: intravenous NCI: National Cancer Institute NSAIDs: non‐steroidal anti‐inflammatory drugs NYHA: New York Heart Association PCoA: Bray‐Curtis distance‐based primary co‐ordination analysis rDNA: ribosomal DNA RT: radiotherapy SF‐12: 12‐item Short Form Health Survey TPN: total parenteral nutrition ULN: upper limit of normal WBC: white blood cell
Differences between protocol and review
The current review author team prepared this review based on a protocol that was written by another group of review authors. During the review process, editors and peer referees provided quite a few suggestions for amendments. This resulted in post hoc amendments to the methods section and to the presentation of results.
Based on advice to reduce the number of primary outcomes, we moved the following primary outcomes, which were listed in the protocol, to secondary outcomes: severity of diarrhoea (for prevention studies), time to rescue medication, proportion of participants requiring rescue medication, and mortality related to diarrhoea.
With respect to types of interventions, because radiotherapy (with or without chemotherapy) and chemotherapy have major different effects on the occurrence of diarrhoea, we decided to perform all analyses for these two intervention types separately. Consequently, subgroup analysis according to type of intervention was no longer applicable. We did not perform other predefined subgroup analyses for age, stage, and length of follow‐up, as data could not be retrieved or did not vary.
Contributions of authors
Amending the protocol, selecting studies, assessing risk of bias, extracting data, performing analysis, applying GRADE criteria, and writing the draft review: Dang Wei, Pauline Heus, Fleur van de Wetering, and Rob Scholten.
Commenting on draft protocol and review, and providing content for the discussion and implications sections: Geertjan van Tienhoven and Leen Verleye.
Sources of support
Internal sources
None, Other.
External sources
-
The Belgian Health Care Knowledge Centre (KCE), Belgium.
The Belgian Health Care Knowledge Centre (KCE) commissioned and supported a series of systematic reviews for the guideline "Supportive treatment for cancer. Part 2: prevention and treatment of adverse events related to chemotherapy and/or radiotherapy for adults with cancer". This protocol was one of the research questions for this guideline.
Declarations of interest
Dang Wei: none known. Pauline Heus: none known. Fleur T van de Wetering: none known. Geertjan van Tienhoven: none known. Leen Verleye: none known. Rob JPM Scholten: none known.
New
References
References to studies included in this review
Chen 2014 {published data only}
- Chen H, Xia Y, Shi C, Liang Y, Yang Y, Qin H. Effects of perioperative probiotics administration on patients with colorectal cancer [Chinese]. Chinese Journal of Clinical Nutrition 2014;22(2):74‐81. [Google Scholar]
Chitapanarux 2010 {published data only}
- Chitapanarux I, Chitapanarux T, Traisathit P, Kudumpee S, Tharavichitkul E, Lorvidhaya V. Randomized controlled trial of live Lactobacillus acidophilus plus Bifidobacterium bifidum in prophylaxis of diarrhea during radiotherapy in cervical cancer patients. Radiation Oncology (London, England) 2010;5:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Delia 2007 {published data only}
- Delia P, Sansotta G, Donato V, Frosina P, Messina G, Renzis C, et al. Use of probiotics for prevention of radiation‐induced diarrhea. World Journal of Gastroenterology 2007;13(6):912‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demers 2014 {published data only}
- Demers M, Dagnault A, Desjardins J. A randomized double‐blind controlled trial: impact of probiotics on diarrhea in patients treated with pelvic radiation. Clinical Nutrition 2014;33(5):761‐7. [DOI: 10.1016/j.clnu.2013.10.015] [DOI] [PubMed] [Google Scholar]
- Germain I, Desjardins J, Demers M, Dagnault A. Phase III study: impact of probiotics on diarrhea in patients treated with pelvic radiation. International Journal of Radiation Oncology Biology Physics 2011;Conference:S667‐8. [Google Scholar]
Giralt 2008 {published data only}
- Giralt J, Regadera JP, Verges R, Romero J, Fuente I, Biete A, et al. Effects of probiotic Lactobacillus casei DN‐114 001 in prevention of radiation‐induced diarrhea: results from multicenter, randomized, placebo‐controlled nutritional trial. International Journal of Radiation Oncology, Biology, Physics 2008;71(4):1213‐9. [DOI] [PubMed] [Google Scholar]
Liu 2000 {published data only}
- Liu JW, Zhou T, Zhang J, Chen YM, Cai X. Prophylayxis of chemotherapy‐induced diarrhea by bifidobacterium agent. Chinese Journal of Microecology 2000;12(3):178‐9. [Google Scholar]
Mansouri‐Tehrani 2016 {published data only}
- Mansouri‐Tehrani HS, Khorasgani MR, Roayaei M. Effects of probiotics with or without honey on radiation‐induced diarrhea. International Journal of Radiation Research 2016;14(3):205‐13. [Google Scholar]
Mego 2015 {published data only}
- Mego M, Chovanec J, Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, et al. Prevention of irinotecan‐induced diarrhea by probiotics: randomized double‐blind, placebo‐controlled phase III study. Journal of Clinical Oncology 2014;32(15_suppl (May 20, 2014)):9611. [Google Scholar]
- Mego M, Chovanec J, Vochyanova‐Andrezalova I, Konkolovsky P, Mikulova M, Reckova M, et al. Prevention of irinotecan induced diarrhea by probiotics: a randomized double blind, placebo controlled pilot study. Complementary Therapies in Medicine 2015;23(3):356‐62. [DOI: ] [DOI] [PubMed] [Google Scholar]
Osterlund 2007 {published data only}
- Osterlund P, Ruotsalainen T, Korpela R, Saxelin M, Ollus A, Valta P, et al. Lactobacillus supplementation for diarrhoea related to chemotherapy of colorectal cancer: a randomised study. British Journal of Cancer 2007;97(8):1028‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salminen 1988 {published data only}
- Salminen E, Elomaa I, Minkkinen J, Vapaatalo H, Salminen S. Preservation of intestinal integrity during radiotherapy using live Lactobacillus acidophilus cultures. Clinical Radiology 1988;39(4):435‐7. [DOI] [PubMed] [Google Scholar]
Timko 2010 {published data only}
- Timko J. Probiotics as prevention of radiation‐induced diarrhoea. Journal of Radiotherapy in Practice 2010;9(4):201‐8. [Google Scholar]
Urbancsek 2001 {published data only}
- Urbancsek H, Kazar T, Mezes I, Neumann K. Results of a double‐blind, randomized study to evaluate the efficacy and safety of Antibiophilus in patients with radiation‐induced diarrhoea. European Journal of Gastroenterology & Hepatology 2001;13(4):391‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amital 2003 {published data only}
- Amital HG. Intelligent nutrition: health‐promoting mechanisms of probiotics. Israel Medical Association Journal 2003;5(11):812‐3. [PubMed] [Google Scholar]
Aso 1995 {published data only}
- Aso YA. Preventive effect of a Lactobacillus casei preparation on the recurrence of superficial bladder cancer in a double‐blind trial. European Urology 1995;27(2):104‐9. [DOI] [PubMed] [Google Scholar]
Delia 2002 {published data only}
- Delia P, Sansotta G, Donato V, Messina G, Frosina P, Pergolizzi S, et al. Prevention of radiation‐induced diarrhea with the use of VSL#3, a new high‐potency probiotic preparation. American Journal of Gastroenterology 2002;97(8):2150‐2. [DOI] [PubMed] [Google Scholar]
Delia 2002a {published data only}
- Delia P, Sansotta G, Donato V, Messina G, Frosina P, Pergolizzi S, et al. Prophylaxis of diarrhoea in patients submitted to radiotherapeutic treatment on pelvic district: personal experience. Digestive & Liver Disease 2002;34(Suppl 2):S84‐6. [DOI] [PubMed] [Google Scholar]
Dugas 1999 {published data only}
- Dugas BM. Immunity and probiotics. Immunology Today 1999;20(9):387‐90. [DOI] [PubMed] [Google Scholar]
Fuccio 2012 {published data only}
- Fuccio LG. Management of intestinal complications in patients with pelvic radiation disease. Clinical Gastroenterology and Hepatology 2012;10(12):1326‐34. [DOI] [PubMed] [Google Scholar]
Fuccio 2013 {published data only}
- Fuccio L, Guido A. Probiotics supplementation for the prevention of gastrointestinal radiation‐induced side effects: the time is now. American Journal of Gastroenterology 2013;108:277. [DOI] [PubMed] [Google Scholar]
Garcia‐Peris 2016 {published data only}
- Garcia‐Peris P, Velasco C, Hernandez M, Lozano MA, Paron L, Cuerda C, et al. Effect of inulin and fructo‐oligosaccharide on the prevention of acute radiation enteritis in patients with gynecological cancer and impact on quality‐of‐life: a randomized, double‐blind, placebo‐controlled trial. European Journal of Clinical Nutrition 2016;70(2):170‐4. [DOI] [PubMed] [Google Scholar]
Horowitz 2003 {published data only}
- Horowitz S. Promoting gut health with probiotics: living medicines for treating digestive disorders. Alternative and Complementary Therapies 2003;9(5):219‐24. [Google Scholar]
Lacouture 2016 {published data only}
- Lacouture ME, Keefe DM, Sonis S, Jatoi A, Gernhardt D, Wang T, et al. A phase II study (ARCHER 1042) to evaluate prophylactic treatment of dacomitinib‐induced dermatologic and gastrointestinal adverse events in advanced non‐small‐cell lung cancer. Annals of Oncology 2016;27(9):1712‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu Z, Qin H, Yang Z, Xia Y, Liu W, Yang J, et al. Randomised clinical trial: the effects of perioperative probiotic treatment on barrier function and post‐operative infectious complications in colorectal cancer surgery ‐ a double‐blind study. Alimentary Pharmacology & Therapeutics 2011;33(1):50‐63. [DOI] [PubMed] [Google Scholar]
Liu 2013 {published data only}
- Liu Z‐H, Huang M‐J, Zhang X‐W, Wang L, Huang N‐Q, Peng H, et al. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double‐center and double‐blind randomized clinical trial. American Journal of Clinical Nutrition 2013;97:117‐26. [DOI] [PubMed] [Google Scholar]
Marteau 2001 {published data only}
- Marteau PRD. Protection from gastrointestinal diseases with the use of probiotics. American Journal of Clinical Nutrition 2001;73(2 Suppl):430S‐6S. [DOI] [PubMed] [Google Scholar]
Marteu 2001 {published data only}
- Marteu P. Prebiotics and probiotics for gastrointestinal health. Clinical Nutrition 2001;20(Suppl 1):41‐5. [Google Scholar]
McFarland 1994 {published data only}
- McFarland LVS. A randomized placebo‐controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. Journal of the American Medical Association 1994;271(24):1913‐8. [PubMed] [Google Scholar]
Mettler 1973 {published data only}
- Mettler L, Romeyke A, Brieler G, Mettler L, Romeyke A, Brieler G. [Effects of para‐ and post‐irradiation abnormal bacterial flora and intestinal irradiation reaction through Bacterium bifidus substitution therapy]. [German]. Strahlentherapie 1973;145(5):588‐99. [PubMed] [Google Scholar]
Narayan 2010 {published data only}
- Narayan SSJ. Probiotics: current trends in the treatment of diarrhea. Hong Kong Medical Journal 2010;16(3):213‐8. [PubMed] [Google Scholar]
Ohigashi 2011 {published data only}
- Ohigashi S, Hoshino Y, Ohde S, Onodera H, Ohigashi S, Hoshino Yo, et al. Functional outcome, quality of life, and efficacy of probiotics in postoperative patients with colorectal cancer. Surgery Today 2011;41(9):1200‐6. [DOI] [PubMed] [Google Scholar]
Okawa 1989 {published data only}
- Okawa TK. Phase II randomized clinical trial of LC9018 concurrently used with radiation in the treatment of carcinoma of the uterine cervix. Its effect on tumor reduction and histology. Cancer 1989;64(9):1769‐76. [DOI] [PubMed] [Google Scholar]
Osterlund 2004 {published data only}
- Osterlund P, Ruotsalainen T, Peuhkuri K, Korpela R, Ollus A, Ikonen M, et al. Lactose intolerance associated with adjuvant 5‐fluorouracil‐based chemotherapy for colorectal cancer. Clinical Gastroenterology and Hepatology 2004;2(8):696‐703. [DOI] [PubMed] [Google Scholar]
Sasidharan 2016 {published data only}
- Sasidharan BK, Viswanathan PN, Prasanna S, Ramadass B, Pugazhendhi S, Ramakrishna BS. Does daily intake of resistant starch reduce the acute bowel symptoms in pelvic radiotherapy? RCT. Radiotherapy and Oncology. 2016; Vol. 2016 Annual Conference of the European Society for Radiotherapy and Oncology, ESTRO 35. Italy. 119:S56‐7.
Visich 2010 {published data only}
- Visich KL, Yeo TP, Visich KL, Yeo TP. The prophylactic use of probiotics in the prevention of radiation therapy‐induced diarrhea. Clinical Journal of Oncology Nursing 2010;14(4):467‐73. [DOI] [PubMed] [Google Scholar]
Zheng 2006 {published data only}
- Zheng SS. Nutritional support of pediatric patients with cancer consuming an enteral formula with fructooligosaccharides. Nutrition Research 2006;26(4):154‐62. [Google Scholar]
References to studies awaiting assessment
Theodoropoulos 2013 {published data only}
- Theodoropoulos G, Memos N, Peitsidou K, Marinatou A, Zografos G. Effects of synbiotics on gastrointestinal function after colectomy for cancer: results from a prospective randomized trial (NCT01479907). Diseases of the Colon and Rectum 2013;Conference: Annual Meeting of the American Society of Colon and Rectal Surgeons, ACSRS 2013 Phoenix, AZ United States. Conference Start: 20130427 Conference End: 20130501. Conference Publication::e296. [Google Scholar]
References to ongoing studies
EUCTR2015‐000868‐34‐DE {unpublished data only}
- Clinical Trials Information. Study for investigating the effects of a probiotic on diarrhea caused by chemotherapy in patients with gastric, colon and rectum cancer. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2015‐000868‐34 (accessed 15 June 2017).
NCT00197873 {published data only}
- Heikki J. Randomized, double blind, placebo controlled, cross‐over phase II study on the effects of Lactobacillus rhamnosus GG supplementation in patients on 1st line XELOXA treatment for metastatic colorectal cancer. https://clinicaltrials.gov/ct2/show/NCT00197873?cond=cancer&intr=probiotics&rank=12 (accessed 27 August 2016).
NCT01790035 {published data only}
- Matthew C. A phase I and randomized controlled phase II trial of the probiotic LGG for prevention of side effects in patients undergoing chemoradiation for gastrointestinal cancer. https://clinicaltrials.gov/ct2/show/NCT01790035?cond=cancer&intr=probiotics&rank=16 (accessed 27 August 2016).
NCT02169388 {published data only}
- Yanqing LI. Effects of gut microflora on the immune and nutritional status of CRC patients after chemotherapy. https://clinicaltrials.gov/ct2/show/NCT02169388?cond=cancer&intr=probiotics&rank=2 (accessed 27 August 2016).
NCT02351089 {published data only}
- Bjurberg M. Probiotics in radiation‐treated gynecologic cancer. https://clinicaltrials.gov/ct2/show/NCT02351089?cond=cancer&intr=probiotics&rank=5 (accessed 27 August 2016).
NCT02819960 {unpublished data only}
- Mego M. Prevention of irinotecan‐induced diarrhea by probiotics. https://www.clinicaltrials.gov/ct2/show/NCT02819960?term=%27probiotics%27+AND+%27cancer%27&rank=12 (accessed 15 June 2017).
Sharma 2013 {published data only}
- Sharma A. A clinical trial to study the efficacy of probiotic VSL#3 on chemotherapy‐induced diarrhoea in cancer patients recieving fluoropyrimidines and/or irinotecan. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=1173 (accessed 15 June 2017).
- Sharma A, Chaudhary SP, Sreenivas V, Prakash S, Raina V, Shukla NK. A phase II/III, randomized, double‐blind, placebo‐controlled study to investigate the efficacy of a probiotic VSL#3 on chemotherapy‐induced diarrhoea in cancer patients receiving fluoropyrimidines and/or irinotecan (interim analysis). European Journal of Cancer 2013;Conference:European Cancer Congress 2013, ECC 2013 Amsterdam Netherlands. [Google Scholar]
TCTR20170314001 {unpublished data only}
Additional references
Banasaz 2002
- Banasaz M, Norin E, Holma R, Midtvedt T. Increased enterocyte production in gnotobiotic rats mono‐associated with Lactobacillus rhamnosus GG. Applied and Environmental Microbiology 2002;68:3031‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benson 2004
- Benson AB 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA Jr, et al. Recommended guidelines for the treatment of cancer treatment‐induced diarrhea. Journal of Clinical Oncology 2004;22:2918‐26. [DOI] [PubMed] [Google Scholar]
Cremonini 2002
- Cremonini F, Caro S, Nista EC, Bartolozzi F, Capelli G, Gasbarrini G, et al. Meta‐analysis: the effect of probiotic administration on antibiotic‐associated diarrhoea. Alimentary Pharmacology and Therapeutics 2002;16:1461‐7. [DOI] [PubMed] [Google Scholar]
CTCAE 2010
- US Department of Health and Human Services, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0. NIH, May 28, 2009 (v4.03: June 14, 2010). [Google Scholar]
Eskesen 2015
- Eskesen D, Jespersen L, Michelsen B, Whorwell PJ, Muller‐Lissner S, Morberg CM. Effect of the probiotic strain Bifidobacterium animalis subsp lactis, BB‐12(R), on defecation frequency in healthy subjects with low defecation frequency and abdominal discomfort: a randomised, double‐blind, placebo‐controlled, parallel‐group trial. British Journal of Nutrition 2015;114(10):1638‐46. [PUBMED: 26382580] [DOI] [PMC free article] [PubMed] [Google Scholar]
FAO and WHO 2001
- Food and Agriculture Organization of the United Nations and the World Health Organization. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria. Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria. 2001. Available from http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf; accessed accessed prior to 24 July 2018.
Fuccio 2009
- Fuccio L, Guido A, Eusebi LH, Laterza L, Grilli D, Cennamo V, et al. Effects of probiotics for the prevention and treatment of radiation‐induced diarrhea. Journal of Clinical Gastroenterology 2009 Jul;43(6):506‐13. [DOI] [PubMed] [Google Scholar]
Goldenberg 2013
- Goldenberg JZ, Ma SSY, Saxton JD, Martzen MR, Vandvik PO, Thorlund K, et al. Probiotics for the prevention of Clostridium difficile‐associated diarrhea in adults and children. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD006095.pub3] [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huang 2002
- Huang JS, Bousvaros A, Lee JW, Diaz A, Davidson EJ. Efficacy of probiotic use in acute diarrhoea in children: a meta‐analysis. Digestive Diseases and Sciences 2002;47:2625‐34. [DOI] [PubMed] [Google Scholar]
Khaled 2003
- Khaled Z, Guandalini S, Hendrickson BA. Filtered supernatant of Lactobacillus GG (LGG) and VSL#3 to prevent Crohn's disease (CD) mucosa adherent coli‐induced degradation of tight junctions (TJ) associated proteins occludin and Zo‐1. Journal of Pediatric Gastroenterology and Nutrition 2003;36:520. [Google Scholar]
Liu 2017
- Liu M‐M, Li S‐T, Shu Y, Zhan H‐Q. Probiotics for prevention of radiation‐induced diarrhea: a meta‐analysis of randomized controlled trials. PLoS ONE 2017;12(6):e0178870. https://doi.org/10.1371/journal.pone.0178870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mack 2003
- Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 2003;52:827‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maroun 2007
- Maroun JA, Anthony LB, Blais N, Burkes R, Dowden SD, Dranitsaris G, et al. Prevention and management of chemotherapy‐induced diarrhoea in patients with colorectal cancer: a consensus statement by the Canadian Working Group on chemotherapy‐induced diarrhoea. Current Oncology 2007;14:13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mattar 2001
- Mattar AF, Drongowski RA, Coran AG, Harmon CM. Effect of probiotics on enterocyte bacterial translocation in vitro. Pediatric Surgery International 2001;17:265‐8. [DOI] [PubMed] [Google Scholar]
NCI 2013
- National Cancer Institute. Diarrhea. Gastrointestinal Complications (PDQ®). http://www.cancer.gov/cancertopics/pdq/supportivecare/gastrointestinalcomplications/HealthProfessional/page5; accessed prior to 24 July 2018.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Redman 2014
- Redman MG, Ward EJ, Phillips RS. The efficacy and safety of probiotics in people with cancer: a systematic review. Annals of Oncology 2014;25(10):1919‐29. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rijkers 2011
- Rijkers GT, Vos WM, Brummer RJ, Morelli L, Corthier G, Marteau P. Health benefits and health claims of probiotics: bridging science and marketing. British Journal of Nutrition 2011;106(9):1291‐6. [PUBMED: 21861940] [DOI] [PubMed] [Google Scholar]
Sanguineti 2008
- Sanguineti G, Endres EJ, Parker BC, Bicquart C, Little M, Chen G, et al. Acute toxicity of whole‐pelvis IMRT in 87 patients with localized prostate cancer. Acta Oncologica 2008;47:301‐10. [DOI] [PubMed] [Google Scholar]
Szajewska 2001
- Szajewska H, Kotowska M, Mrukowicz JZ. Efficacy of Lactobacillus GG in prevention of nosocomial diarrhoea in infants. Journal of Pediatrics 2001;138:361‐5. [DOI] [PubMed] [Google Scholar]
Vaarala 2003
- Vaarala O. Immunological effects of probiotics with special reference to lactobacilli. Clinical and Experimental Allergy 2003;33:1634‐40. [DOI] [PubMed] [Google Scholar]
Wang 2016
- Wang YH, Yao N, Wei KK, Jiang L, Hanif S, Wang ZX, Pei CX. The efficacy and safety of probiotics for prevention of chemoradiotherapy‐induced diarrhea in people with abdominal and pelvic cancer: a systematic review and meta‐analysis. European Journal of Clinical Nutrition 2016;Jun 22:Epub ahead of print. [10.1038/ejcn.2016.102] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
van de Wetering FT 2013
- Wetering FT, Heus P, Verleye L, Tienhoven G, Scholten RJPM. Probiotics for the prevention or treatment of chemotherapy or radiotherapy related diarrhoea in cancer patients. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD008831.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


