Abstract
Background
Prolonged treatment with benzodiazepines is common practice despite clinical recommendations of short‐term use. Benzodiazepines are used by approximately 4% of the general population, with increased prevalence in psychiatric populations and the elderly. After long‐term use it is often difficult to discontinue benzodiazepines due to psychological and physiological dependence. This review investigated if pharmacological interventions can facilitate benzodiazepine tapering.
Objectives
To assess the benefits and harms of pharmacological interventions to facilitate discontinuation of chronic benzodiazepine use.
Search methods
We searched the following electronic databases up to October 2017: Cochrane Drugs and Alcohol Group's Specialised Register of Trials, CENTRAL, PubMed, Embase, CINAHL, and ISI Web of Science. We also searched ClinicalTrials.gov, the WHO ICTRP, and ISRCTN registry, and checked the reference lists of included studies for further references to relevant randomised controlled trials.
Selection criteria
We included randomised controlled trials comparing pharmacological treatment versus placebo or no intervention or versus another pharmacological intervention in adults who had been treated with benzodiazepines for at least two months and/or fulfilled criteria for benzodiazepine dependence (any criteria).
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We included 38 trials (involving 2543 participants), but we could only extract data from 35 trials with 2295 participants. Many different interventions were studied, and no single intervention was assessed in more than four trials. We extracted data on 18 different comparisons. The risk of bias was high in all trials but one. Trial Sequential Analysis showed imprecision for all comparisons.
For benzodiazepine discontinuation, we found a potential benefit of valproate at end of intervention (1 study, 27 participants; risk ratio (RR) 2.55, 95% confidence interval (CI) 1.08 to 6.03; very low‐quality evidence) and of tricyclic antidepressants at longest follow‐up (1 study, 47 participants; RR 2.20, 95% CI 1.27 to 3.82; low‐quality evidence).
We found potentially positive effects on benzodiazepine withdrawal symptoms of pregabalin (1 study, 106 participants; mean difference (MD) ‐3.10 points, 95% CI ‐3.51 to ‐2.69; very low‐quality evidence), captodiame (1 study, 81 participants; MD ‐1.00 points, 95% CI ‐1.13 to ‐0.87; very low‐quality evidence), paroxetine (2 studies, 99 participants; MD ‐3.57 points, 95% CI ‐5.34 to ‐1.80; very low‐quality evidence), tricyclic antidepressants (1 study, 38 participants; MD ‐19.78 points, 95% CI ‐20.25 to ‐19.31; very low‐quality evidence), and flumazenil (3 studies, 58 participants; standardised mean difference ‐0.95, 95% CI ‐1.71 to ‐0.19; very low‐quality evidence) at end of intervention. However, the positive effect of paroxetine on benzodiazepine withdrawal symptoms did not persist until longest follow‐up (1 study, 54 participants; MD ‐0.13 points, 95% CI ‐4.03 to 3.77; very low‐quality evidence).
The following pharmacological interventions reduced symptoms of anxiety at end of intervention: carbamazepine (1 study, 36 participants; MD ‐6.00 points, 95% CI ‐9.58 to ‐2.42; very low‐quality evidence), pregabalin (1 study, 106 participants; MD ‐4.80 points, 95% CI ‐5.28 to ‐4.32; very low‐quality evidence), captodiame (1 study, 81 participants; MD ‐5.70 points, 95% CI ‐6.05 to ‐5.35; very low‐quality evidence), paroxetine (2 studies, 99 participants; MD ‐6.75 points, 95% CI ‐9.64 to ‐3.86; very low‐quality evidence), and flumazenil (1 study, 18 participants; MD ‐1.30 points, 95% CI ‐2.28 to ‐0.32; very low‐quality evidence).
Two pharmacological treatments seemed to reduce the proportion of participants that relapsed to benzodiazepine use: valproate (1 study, 27 participants; RR 0.31, 95% CI 0.11 to 0.90; very low‐quality evidence) and cyamemazine (1 study, 124 participants; RR 0.33, 95% CI 0.14 to 0.78; very low‐quality evidence). Alpidem decreased the proportion of participants with benzodiazepine discontinuation (1 study, 25 participants; RR 0.41, 95% CI 0.17 to 0.99; number needed to treat for an additional harmful outcome (NNTH) 2.3 participants; low‐quality evidence) and increased the occurrence of withdrawal syndrome (1 study, 145 participants; RR 4.86, 95% CI 1.12 to 21.14; NNTH 5.9 participants; low‐quality evidence). Likewise, magnesium aspartate decreased the proportion of participants discontinuing benzodiazepines (1 study, 144 participants; RR 0.80, 95% CI 0.66 to 0.96; NNTH 5.8; very low‐quality evidence).
Generally, adverse events were insufficiently reported. Specifically, one of the flumazenil trials was discontinued due to severe panic reactions.
Authors' conclusions
Given the low or very low quality of the evidence for the reported outcomes, and the small number of trials identified with a limited number of participants for each comparison, it is not possible to draw firm conclusions regarding pharmacological interventions to facilitate benzodiazepine discontinuation in chronic benzodiazepine users. Due to poor reporting, adverse events could not be reliably assessed across trials. More randomised controlled trials are required with less risk of systematic errors ('bias') and of random errors ('play of chance') and better and full reporting of patient‐centred and long‐term clinical outcomes. Such trials ought to be conducted independently of industry involvement.
Plain language summary
Medications for discontinuation of long‐term benzodiazepine use
Background
Benzodiazepines are widely prescribed for long‐term use despite recommendations of only short‐term use. It is often difficult to discontinue benzodiazepines after more than a few weeks of treatment due to the development of physical and psychological dependence. This review aimed to assess the effect and safety of medications to facilitate benzodiazepine discontinuation in chronic benzodiazepine users.
Search date
The evidence is current to October 2017.
Study characteristics
We identified 38 randomised controlled trials involving 2543 participants who had either been treated for more than two months with benzodiazepines, or who had been diagnosed with benzodiazepine dependence. We included studies irrespective of whether benzodiazepines were prescribed for anxiety, insomnia, or any other condition.
The average age of participants was around 50 years, and the majority of participants were women in most studies. Twenty‐four trials were conducted in Europe; eight trials in the US or Canada; and six trials in Asia. The trials involved a wide range of medications to facilitate reduction or discontinuation of benzodiazepine use. Fourteen of the 38 included studies were partly funded by the drug manufacturer; nine studies were funded by government agencies; and 15 studies did not state the source of funding. The duration of the trials ranged between 1 and 24 weeks; the average trial duration was 9 weeks.
Key results
We extracted data on 18 different comparisons in a total of 2295 participants. We are uncertain whether valproate and tricyclic antidepressants increase the chance of discontinuing benzodiazepines, and whether benzodiazepine withdrawal symptoms are reduced by pregabalin, captodiame, paroxetine, tricyclic antidepressants, and flumazenil, as we assessed the quality of the evidence as very low. We are uncertain as to whether symptoms of anxiety after withdrawal of benzodiazepines are reduced by carbamazepine, pregabalin, captodiame, paroxetine, and flumazenil, as we assessed the quality of the evidence as very low. The effects of the evaluated medications were too uncertain to inform clinical practice due to risk of bias (systematic errors with overestimation of benefits and underestimation of harms) and risk of chance occurrence (random errors giving any result). Tolerability and safety were poorly reported across the included studies, making it impossible to assess the balance between possible benefits and adverse effects. Consequently, no conclusions can be drawn about the effectiveness of the interventions.
Quality of the evidence
The quality of the evidence was generally low or very low due to the small number of trials including a limited number of participants for each comparison; dissimilar results across studies; poor study design; and pronounced financial involvement of the pharmaceutical industry. Randomised controlled trials are therefore needed without risk of bias and random significant results involving long‐term assessments of participants conducted without involvement of industry.
Summary of findings
Summary of findings for the main comparison. Valproate compared with placebo or no intervention for benzodiazepine discontinuation in chronic benzodiazepine users.
| Valproate compared with placebo or no intervention for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: valproate Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Valproate | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 2.55 (1.08 to 6.03) | 27 (1 study) | ⊕⊝⊝⊝ very low1,2 | The required information size of 1918 participants was not met. | |
| 679 per 1000 | 1000 per 1000 (142 to 1000) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 1.57 (0.80 to 3.09) | 24 (1 study) | ⊕⊝⊝⊝ very low1,2 | ||
| 500 per 1000 | 785 per 1000 (400 to 1000) | |||||
| Benzodiazepine withdrawal symptoms, end of intervention | The mean benzodiazepine withdrawal symptoms in the intervention groups was 0.15 standard deviations lower (0.68 lower to 0.37 higher). | 56 (2 studies) | ⊕⊝⊝⊝ very low3,4 | SMD ‐0.15 (‐0.68 to 0.37). As a rule of thumb, 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. |
||
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | (0 study) | No included study measured this outcome. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio: SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1No details provided regarding random sequence generation, allocation concealment, and blinding, leading to unclear risk of selection bias, performance and detection bias (downgraded one level). 2Required information size not met (downgraded two levels due to serious imprecision: the sample size is far from the required one). 3Unclear risk of selection bias, attrition bias, reporting bias and high risk of performance bias (downgraded one level). 4Required information size not met (downgraded two levels due to serious imprecision).
Summary of findings 2. Carbamazepine compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Carbamazepine compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: carbamazepine Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Carbamazepine | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.33 (0.99 to 1.8) | 147 (3 studies) | ⊕⊕⊝⊝ low1,2 | Trial Sequential Analysis showed that only 7.0% of the required information size (2109) was reached, indicating that insufficient information has been obtained. | |
| 480 per 1000 | 638 per 1000 (475 to 864) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 1.41 (0.86 to 2.29) | 40 (1 study) | ⊕⊝⊝⊝ very low3,4 | ||
| 524 per 1000 | 739 per 1000 (450 to 1000) | |||||
| Benzodiazepine withdrawal symptoms, end of intervention | The mean benzodiazepine withdrawal symptoms in the intervention groups was 1.14 standard deviations lower (2.43 lower to 0.16 higher). | 76 (2 studies) | ⊕⊝⊝⊝ very low1,5,6 | SMD ‐1.14 (‐2.43 to 0.16). As a rule of thumb, 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. |
||
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | (0 study) | No included study measured this outcome. | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio: SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection bias. One study with high risk of attrition, reporting, and other bias (downgraded one level). 2Required information size not met (downgraded one level due to imprecision). 3Unclear risk of selection and attrition bias (downgraded one level). 4Required information size not met, and 95% CI includes both no effect and appreciable benefit (downgraded two levels due to imprecision). 5Required information size not met (downgraded one level for imprecision). 6Significant heterogeneity (downgraded one level for inconsistency).
Summary of findings 3. Lithium compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Lithium compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: lithium Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Lithium | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.05 (0.86 to 1.28) | 230 (1 study) | ⊕⊕⊝⊝ low1,2 | The required information size of 1918 participants was not met. | |
| 617 per 1000 | 648 per 1000 (531 to 790) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection, attrition, and reporting bias (downgraded one level). 2The required information size of 1918 participants was not met (downgraded one level due to imprecision).
Summary of findings 4. Pregabalin compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Pregabalin compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: pregabalin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Pregabalin | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.44 (0.92 to 2.25) | 106 (1 study) | ⊕⊝⊝⊝ very low1,2 | The required information size of 1918 participants was not met. | |
| 360 per 1000 | 518 per 1000 (331 to 810) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine withdrawal symptoms, Physician Withdrawal Checklist (PWCL), end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, PWCL, end of intervention in the intervention group was 3.10 lower (3.51 to 2.69 lower). | ‐ | 106 (1 study) | ⊕⊝⊝⊝ very low1,2 | MD ‐3.10 (‐3.51 to ‐2.69) |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection bias and high risk of attrition and other bias (downgraded two levels). 2Required information size not met (downgraded one level due to imprecision).
Summary of findings 5. Captodiame compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Captodiame compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: captodiame Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Captodiame | |||||
| Benzodiazepine discontinuation, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included studies measured this outcome. |
| Benzodiazepine withdrawal symptoms, Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ), end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, BWSQ, end of intervention in the intervention group was 1.00 lower (1.13 to 0.87 lower). | ‐ | 81 (1 study) | ⊕⊝⊝⊝ very low1,2 | MD ‐1.00 (‐1.13 to ‐0.87) The required information size of 229 participants was not met. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection and reporting bias. High risk of other bias (downgraded one level). 2Required information size not met (downgraded two levels due to imprecision).
Summary of findings 6. Paroxetine compared with placebo or no intervention for benzodiazepine discontinuation in chronic benzodiazepine users.
| Paroxetine compared with placebo or no intervention for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: paroxetine Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or Control | Paroxetine | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.45 (0.88 to 2.39) | 221 (3 studies) | ⊕⊝⊝⊝ very low1,2 | Trial Sequential Analysis showed that only 2.34% of the required information size (9448) was reached, indicating that insufficient information has been obtained. | |
| 504 per 1000 | 731 per 1000 (444 to 1000) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | (0 study) | ‐ | No included study measured this outcome. | |
| Benzodiazepine withdrawal symptoms, BWSQ, end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, BWSQ, end of intervention in the intervention groups was 3.57 lower (5.34 to 1.8 lower). | ‐ | 99 (2 studies) | ⊕⊝⊝⊝ very low3,4 | MD ‐3.57 (‐5.34 to ‐1.8). Trial Sequential Analysis showed that the required information size of 229 participants was not reached. However, the alpha‐spending boundaries for benefit were crossed, indicating that sufficient information was obtained, and the result was not due to random error. |
| Benzodiazepine withdrawal symptoms, BWSQ, longest follow‐up: 6 months | ‐ | The mean benzodiazepine withdrawal symptoms, BWSQ, longest follow‐up: 6 months in the intervention group was 0.13 lower (4.03 lower to 3.77 higher). | ‐ | 54 (1 study) | ⊕⊝⊝⊝ very low5,6 | MD ‐0.13 (‐4.03 to 3.77) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BWSQ: Benzodiazepine Withdrawal Symptom Questionnaire; CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection and attrition bias. High risk of performance, detection, reporting, and other bias (downgraded two levels). 2Required information size not met (downgraded one level due to imprecision). 3Unclear risk of selection bias. High risk of performance, detection, reporting, and other bias (downgraded two levels). 4The required information size was not met (downgraded one level due to imprecision). 5Unclear risk of selection bias. High risk of reporting and other bias (downgraded one level). 6Required information size not met (downgraded two levels due to imprecision).
Summary of findings 7. Tricyclic antidepressants compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Tricyclic antidepressants compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: tricyclic antidepressants Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tricyclic antidepressants | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.82 (0.52 to 1.28) | 105 (2 studies) | ⊕⊝⊝⊝ very low1,2 | Trial Sequential Analysis showed that only 7.82% of the required information size (1343) was reached, indicating that insufficient information has been obtained. | |
| 451 per 1000 | 370 per 1000 (235 to 577) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 2.2 (1.27 to 3.82) | 47 (1 study) | ⊕⊕⊝⊝ low3,4 | ||
| 375 per 1000 | 825 per 1000 (476 to 1000) | |||||
| Benzodiazepine withdrawal symptoms, Physician Withdrawal Checklist, end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms in the intervention group was 19.78lower (20.25 lower to 19.31 lower). | ‐ | 38 (1 study) |
⊕⊝⊝⊝ very low4,5 | MD ‐19.78 (‐20.25 to ‐19.31) |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection bias and high risk of attrition and other bias (downgraded one level). 2Required information size not met (downgraded one level due to imprecision). 3Unclear risk of selection and attrition bias (downgraded one level). 4Required information size not met (downgraded two levels due to imprecision). 5High risk of performance, detection, and reporting bias (downgraded two levels).
Summary of findings 8. Alpidem compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Alpidem compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: alpidem Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Alpidem | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.41 (0.17 to 0.99) | 25 (1 study) | ⊕⊕⊝⊝ low1 | The required information size of 1918 participants was not met. | |
| 750 per 1000 | 308 per 1000 (128 to 743) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Withdrawal syndrome (clinical diagnosis), end of intervention | Study population | RR 4.86 (1.12 to 21.14) | 145 (1 study) | ⊕⊝⊝⊝ low2,3 | ||
| 29 per 1000 | 143 per 1000 (33 to 622) | |||||
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Required information size not met (downgraded two levels due to imprecision). 2Required information size not met (downgraded one level due to imprecision).
3Unclear risk of selection and other bias, high risk of attrition bias (downgraded one level)
Summary of findings 9. Buspirone compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Buspirone compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: buspirone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Buspirone | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.82 (0.49 to 1.37) | 143 (4 studies) | ⊕⊕⊝⊝ low1,2 | Trial Sequential Analysis showed that only 4.23% of the required information size (3381) was reached, indicating that insufficient information has been obtained. | |
| 563 per 1000 | 462 per 1000 (276 to 772) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 0.60 (0.34 to 1.05) | 23 (1 study) | ⊕⊕⊝⊝ low2,3 | ||
| 917 per 1000 | 550 per 1000 (312 to 962) | |||||
| Benzodiazepine withdrawal symptoms, end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, end of intervention in the intervention groups was 4.69 higher (14.47 lower to 23.85 higher). | ‐ | 17 (1 study) | ⊕⊝⊝⊝ very low1,4 | MD 4.69 (‐14.47 to 23.87) |
| Benzodiazepine withdrawal symptoms, longest follow‐up | ‐ | The mean benzodiazepine withdrawal symptoms, longest follow‐up in the intervention groups was 1.34 lower (14.31 lower to 11.63 higher). | ‐ | 15 (1 study) | ⊕⊝⊝⊝ very low3,4 | MD ‐1.34 (‐14.31 to 11.63) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection, performance, and reporting bias. High risk of attrition and other bias (downgraded one level). 2Required information size not met (downgraded one level due to imprecision). 3Unclear risk of selection and reporting bias. High risk of attrition bias (downgraded one level). 4Reguired information size not met (downgraded two levels due to serious imprecision).
Summary of findings 10. Melatonin compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Melatonin compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients (3 studies), outpatients in methadone maintenance treatment (1 study) Intervention: melatonin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Melatonin | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.20 (0.73 to 1.96) | 219 (4 studies) | ⊕⊝⊝⊝ very low1,2 | Trial Sequential Analysis showed that only 6.37% of the required information size (3438) was reached, indicating that insufficient information has been obtained. | |
| 417 per 1000 | 500 per 1000 (304 to 817) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Study population | RR 1.03 (0.47 to 2.27) | 38 (1 study) | ⊕⊝⊝⊝ very low2,3,4 | ||
| 389 per 1000 | 401 per 1000 (183 to 883) | |||||
| Benzodiazpine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection, attrition, and reporting bias. High risk of other bias (downgraded one level). 2Required information size not met, and the 95% CI includes both no effect and appreciable benefit (downgraded two levels due to imprecision). 3Unclear risk of selection and reporting bias (downgraded one level). 4Required information size not met (downgraded two levels due to imprecision).
Summary of findings 11. Flumazenil compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Flumazenil compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients in methadone maintenance treatment (2 studies), outpatients (1 study) Intervention: flumazenil Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Flumazenil | |||||
| Benzodiazepine discontinuation, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | ‐ | The mean benzodiazepine withdrawal symptoms, end of intervention in the intervention groups was 0.95 standard deviations lower (1.71 to 0.19 lower). | ‐ | 58 (3 studies) | ⊕⊝⊝⊝ very low1,2 | SMD ‐0.95 (‐1.71 to ‐0.19) As a rule of thumb, 0.2 represents a small effect, 0.5 a moderate effect, and 0.8 a large effect. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardised mean difference | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection bias and high risk of performance, detection, and other bias (downgraded one level). 2Required information size not met (downgraded two levels due to imprecision).
Summary of findings 12. Progesterone compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Progesterone compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: progesterone Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Progesterone | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.15 (0.52 to 2.54) | 35 (1 study) | ⊕⊝⊝⊝ very low1,2 | The required information size of 1918 participants was not met. | |
| 417 per 1000 | 479 per 1000 (217 to 1000) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection and attrition bias (downgraded one level). 2Required information size not met, and the 95% CI includes both no effect and appreciable benefit (downgraded two levels due to imprecision).
Summary of findings 13. Magnesium aspartate compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Magnesium aspartate compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: magnesium aspartate Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Magnesium aspartate | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.80 (0.66 to 0.96) | 144 (1 study) | ⊕⊝⊝⊝ very low1,2 | The required information size of 1918 participants was not met. | |
| 853 per 1000 | 683 per 1000 (563 to 819) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection, detection, and attrition bias (downgraded one level). 2Required information size not met (downgraded two levels due to imprecision).
Summary of findings 14. Homéogène 46/Sedatif PC (homeopathic drugs) compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users.
| Homéogène 46/Sedatif PC (homeopathic drugs) compared with placebo for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: Homéogène 46/Sedatif PC (homeopathic drugs) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Homéogène 46/Sedatif PC (homeopathic drugs) | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 0.79 (0.36 to 1.7) | 51 (1 study) | ⊕⊝⊝⊝ very low1,2 | The required information size was not met. | |
| 381 per 1000 | 301 per 1000 (137 to 648) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection, attrition, and other bias (downgraded one level). 2Required information size not met, and the 95% CI includes both no effect and appreciable benefit (downgraded two levels due to imprecision).
Summary of findings 15. Carbamazepine compared with tricyclic antidepressant for benzodiazepine discontinuation in chronic benzodiazepine users.
| Carbamazepine compared with tricyclic antidepressant for benzodiazepine discontinuation in chronic benzodiazepine users | ||||||
| Patient or population: adults who withdraw from chronic benzodiazepine use Settings: outpatients Intervention: carbamazepine Comparison: tricyclic antidepressant | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Tricyclic antidepressant | Carbamazepine | |||||
| Benzodiazepine discontinuation, end of intervention | Study population | RR 1.00 (0.78 to 1.29) | 48 (1 study) | ⊕⊕⊝⊝ low1,2 | The required information size was not met. | |
| 833 per 1000 | 833 per 1000 (650 to 1000) | |||||
| Benzodiazepine discontinuation, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, end of intervention | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| Benzodiazepine withdrawal symptoms, longest follow‐up | Not estimable | ‐ | ‐ | (0 study) | ‐ | No included study measured this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Unclear risk of selection, detection, and attrition bias (downgraded one level). 2Required information size not met (downgraded one level due to imprecision).
Background
Description of the condition
Benzodiazepines are widely prescribed, and consumption remains high despite a modest overall decline during the last couple of decades (Islam 2014; Tsimtsiou 2009). A US national survey indicated a prevalence of benzodiazepine consumption of 3.8% among non‐institutionalised adults (Paulose‐Ram 2007), and the prevalence approached 8% in a Dutch survey of elderly people (Sonnenberg 2012). Another US survey reported an increase in the percentage of adults filling a benzodiazepine prescription from 4.1% in 1996 to 5.6% in 2013 (Bachhuber 2016). A survey in a New Zealand psychiatric outpatient setting documented that one‐third of the patients were prescribed benzodiazepines or benzodiazepine‐like drugs, and the majority of prescriptions were long‐standing (Huthwaite 2013).
Benzodiazepines are indicated for short‐term treatment of anxiety and insomnia, but prescriptions are often prolonged due to the development of dependence and a lack of knowledge of non‐pharmacological management of anxiety, insomnia, and similar symptoms (Ashton 2005; Huthwaite 2013; O'Brien 2005). Gradual dose reduction of benzodiazepines is recommended above abrupt discontinuation to minimise withdrawal symptoms, including the risk of withdrawal seizures (Dell'osso 2013). The importance of individual adjustment of withdrawal rate is emphasised in clinical practice guidelines. The individually adjusted withdrawal rate should include consideration of benzodiazepine type and dosage, original reason for prescribing, environmental stressors, and amount of available support (Ashton 2005). The duration of tapering is thus sometimes prolonged for months or years; however, very slow tapering rates do not seem superior to faster tapering regimens (Parr 2009). Withdrawal symptoms may manifest both physically (e.g. flu‐like complaints, muscle cramps) and psychologically (e.g. irritability, insomnia, perceptual changes, anxiety, depersonalisation, derealisation) (Baldwin 2013). Withdrawal symptoms therefore often resemble the symptoms that led to the initial benzodiazepine prescription, erroneously leading patients and caregivers to assume that continued prescription is required. Discontinuation is thus complicated by a mixture of withdrawal symptoms and original symptoms that might reoccur in an exaggerated form (rebound symptoms). Psychological interventions (e.g. relaxation training, psycho‐education) for managing rebound symptoms are superior to gradual dose reduction alone in patients in primary care settings (Parr 2009). Adverse reactions associated with benzodiazepine treatment include cognitive impairment (Barker 2004; Glass 2005), psychomotor impairments with increased risk of falls, Woolcott 2009, and accidents (Smink 2010), daytime sedation (Glass 2005), and increased risk of dementia (Billioti 2012; Gallacher 2012; Wu 2009). Although benzodiazepines initially improve sleep continuity parameters (e.g. sleep latency, total sleep time) (Buscemi 2007a), the drugs decrease the amount of deep sleep (Parrino 1996), thereby exerting a negative effect on the overall sleep architecture. Moreover, development of tolerance to the sedative effects implies that the original dose of the drug has progressively less effect, and higher doses are needed to obtain the desired effect (Vinkers 2012). Another concern associated with prolonged benzodiazepine use is the increased mortality reported in a number of observational studies (Bachhuber 2016; Kripke 1998; Kripke 2012; Mallon 2009; Weich 2014). However, this issue is controversial because of conflicting results (Gisev 2011; Hausken 2007; Jaussent 2013), and the lack of appropriate confounder control in many of the studies showing increased mortality (Kripke 1998; Kripke 2012; Mallon 2009).
The majority of benzodiazepine prescriptions occur in general practice, where the following characteristics are associated with increased risk of long‐term use: psychiatric comorbidity, older age, being less educated, being lonely, and using more avoidant coping behaviour (Zandstra 2004). In the elderly, benzodiazepine prescribing rates are especially high, and in this population prescriptions are associated with female sex, low level of education, low income, chronic physical diseases, functional limitations, cognitive impairment, depression, anxiety, and insomnia (Sonnenberg 2012). In opioid users, the additional use of benzodiazepines is associated with increased risk of adverse reactions and overdose due to the depression of the central nervous system exerted by both types of drugs, in particular in combination with alcohol intake (Jones 2014). Use of opioids is increasing, especially in the US, both as analgesics for people with chronic pain and as illicit drug use (Manchikanti 2008; Manchikanti 2012). The management of benzodiazepine dependence in subpopulations with comorbid substance abuse including opioid use therefore warrants attention.
Description of the intervention
Currently, no drugs are recommended or approved for the management of benzodiazepine dependence or facilitation of withdrawal after long‐term use. Theoretically, a drug can facilitate benzodiazepine discontinuation in several ways: by ameliorating physical withdrawal symptoms (e.g. propranolol to reduce tremor and tachycardia); by reducing psychological craving (i.e. administering non‐benzodiazepine sedating drugs); or by treating underlying insomnia or anxiety symptoms (e.g. melatonin, buspirone, imipramine). Antiepileptics and antidepressants are among the drugs most often evaluated, but with conflicting results (Parr 2009; Voshaar 2006). Abrupt cessation of benzodiazepine treatment followed by administration of flumazenil (a benzodiazepine receptor antagonist) has also been investigated (Gerra 2002), but the feasibility of this approach is limited by the intravenous administration formulation and the need for continuous medical monitoring. An intervention to facilitate benzodiazepine discontinuation can be administered with the aim of 1) benzodiazepine cessation and thereafter discontinuation of the experimental drug, or 2) substituting the ongoing benzodiazepine treatment, that is replacing the benzodiazepine treatment with another temporary or chronic drug with a more favourable adverse reaction profile.
How the intervention might work
A Cochrane Review covering the literature until October 2004 investigated pharmacological interventions for management of benzodiazepine mono‐dependence in outpatient settings (Denis 2006). The conclusion was that gradual taper was preferable to abrupt discontinuation, and that carbamazepine, but not other investigated compounds, might be an effective intervention for gradual benzodiazepine discontinuation. However, the evidence was not strong enough to guide clinical recommendation. A meta‐analysis including both inpatient and outpatient settings reported that augmentation of guided discontinuation programmes with imipramine was more effective than guided discontinuation alone (Voshaar 2006). Another systematic review of approaches to benzodiazepine discontinuation in general practice and outpatient settings published in 2009 did not support substitutive pharmacotherapies to assist benzodiazepine discontinuation (Parr 2009). Psychological interventions have been found to be superior to gradual dose reduction (Parr 2009; Voshaar 2006), and are the topic of another recently published Cochrane Review (Darker 2015).
The pharmacological interventions hitherto investigated have tried to address the pharmacology of benzodiazepines and have thereby theoretically tried to counteract the withdrawal symptoms or to treat re‐emerging insomnia and anxiety. In this respect, carbamazepine has been one of the most promising drugs so far (Denis 2006), but other drugs are accessible such as melatonin to counteract insomnia developed as part of the withdrawal syndrome (Garfinkel 1999), or pregabalin to reduce symptoms of general anxiety emerging or worsening when benzodiazepines are withdrawn (Hadley 2012).
Why it is important to do this review
Long‐term benzodiazepine use is generally inappropriate due to adverse reactions (e.g. impaired psychomotor and cognitive functioning) and the risks of development of dependence and addiction. Distressing adverse reactions often complicate withdrawal attempts, and therefore it is important to evaluate whether any pharmacological intervention may facilitate the withdrawal or discontinuation of benzodiazepines. This could potentially minimise both individual and societal costs associated with the often extensive and prolonged withdrawal regimens. Since the previous reviews were conducted (Denis 2006; Parr 2009; Voshaar 2006), new studies investigating how to facilitate benzodiazepine discontinuation have been published, and a new systematic review was therefore warranted.
Objectives
To assess the benefits and harms of pharmacological interventions to facilitate discontinuation of chronic benzodiazepine use.
Methods
Criteria for considering studies for this review
Types of studies
We included relevant randomised controlled trials irrespective of publication type, publication date, publication language, and publication status. We did not include quasi‐randomised clinical studies and observational studies. In making this decision we are well aware that we achieve more focus on potential benefits and less on potential harms, since rare adverse events that develop only after long‐term exposure are underestimated in randomised controlled trials.
Types of participants
Adult (aged 18 years or older) chronic benzodiazepine users defined as daily use of benzodiazepines for a minimum duration of two months; or people diagnosed with benzodiazepine dependence by any diagnostic criteria (e.g. International Classification of Diseases (ICD)‐10: F13.1 or F13.2). We also included participants with psychiatric or somatic comorbidities. Benzodiazepines in this review included the benzodiazepine‐like compounds (sometimes referred to as Z‐drugs, e.g. zolpidem and zopiclone).
Types of interventions
Experimental intervention
The experimental intervention could be any drug administered to facilitate benzodiazepine withdrawal or to switch from benzodiazepine treatment to another drug. We included interventions conducted in general practice, outpatient settings, and in hospitalised patients.
Control intervention
The control interventions included:
treatment as usual, as defined by the trialists;
placebo;
any active pharmacological comparator.
Co‐interventions
Co‐interventions of any kind were allowed, as long as they were delivered equally in both intervention groups.
Types of outcome measures
We assessed all outcomes at two time points:
end of intervention, as defined by the trialists. This was the primary outcome time point in the review;
longest follow‐up, as defined by the trialists.
Primary outcomes
Benzodiazepine discontinuation (defined as cessation) measured by examining the blood or urine concentration of the participant or by self reported use.
Benzodiazepine withdrawal symptoms as measured by relevant questionnaires.
Serious adverse events, defined as any adverse event that results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, or is a congenital anomaly or birth defect (ICH GCP).
Secondary outcomes
Benzodiazepine mean dose.
Insomnia as measured by any relevant questionnaire.
Anxiety as measured by any relevant questionnaire.
Comorbid substance abuse as measured by self reported use of other drugs or alcohol.
Non‐serious adverse events, defined as any non‐serious undesirable medical event experienced by participants during a clinical trial that does not necessarily have a causal relationship with the intervention (ICH GCP).
Relapse to benzodiazepine use (defined according to the trialists), assessed only at longest follow‐up after end of intervention among the subgroup of participants who discontinued benzodiazepine use at end of intervention.
Discontinuation due to adverse events assessed only at the end of intervention.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following electronic databases:
Cochrane Drugs and Alcohol Group's Specialised Register of Trials (searched on 17 October 2017);
The Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 9);
PubMed (January 1966 to 17 October 2017);
Embase (Embase.com) (January 1974 to 17 October 2017);
CINAHL (Cumulative Index to Nursing and Allied Health Literature) (EbscoHOST) (1982 to 17 October 2017);
Web of Knowledge, Web of Science (1990 to 17 October 2017).
We searched the databases using MeSH and free‐text terms relating to substance use disorders. We combined the PubMed search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). Detailed search strategies were developed for each database used, accounting for differences in controlled vocabulary and syntax rules. For details see Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6.
We searched the following trials registries on 17 October 2017:
ClinicalTrials.gov (clinicaltrials.gov);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp);
the ISRCTN registry (www.isrctn.com).
Searching other resources
We searched for further relevant trials by screening reference lists of previous review papers and those of all articles selected for inclusion.
Where possible, we contacted the first author of each included study to seek information about further relevant published and unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (LB and BE, or LB and JR) independently screened titles of all studies obtained by the search strategy. After excluding all obviously irrelevant articles, we screened the abstracts of all remaining publications. We obtained all potentially relevant studies in full text, and two review authors independently assessed these studies for inclusion in the review (LB and BE, or LB and JR). During this process, we linked multiple reports of the same trial.
Data extraction and management
Two review authors (LB and BE, or LB and JR) extracted data from the included studies using a standard extraction form. Any disagreements were resolved by consensus between raters (LB and BE, or LB and JR), and if not possible by judgement of authors JL and CG.
We extracted the following data.
General information: publication status, title, authors’ names, source, country, contact address, language of publication, year of publication, duplicate publication.
Trial characteristics: design and setting.
Interventions: type of pharmacological intervention, dose, duration, type of control intervention.
Participants: inclusion and exclusion criteria, number of participants in intervention and control groups, participant demographics such as sex and age, baseline characteristics, and number of participants lost to follow‐up.
Outcomes: please see Types of outcome measures above.
Risk of bias: please see Assessment of risk of bias in included studies below.
Assessment of risk of bias in included studies
We assessed the risk of bias for randomised controlled trials using the criteria recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The recommended approach for assessing risk of bias in studies included in a Cochrane Review is a two‐part tool, addressing seven specific domains, namely sequence generation and allocation concealment (selection bias), blinding of participants and providers (performance bias), blinding of outcome assessor (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias including industry bias (Lundh 2017). The first part of the tool involves describing what was reported to have happened in the trial. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry, in terms of low, high, or unclear risk. To make these judgements we used the criteria indicated by the Cochrane Handbook adapted to the addiction field. See Appendix 7 for details.
We addressed the domains of sequence generation and allocation concealment (avoidance of selection bias) in the tool by a single entry for each study.
Regarding blinding of participants, personnel, and outcome assessor (avoidance of performance bias and detection bias), we planned to consider these items separately for objective outcomes (e.g. urine drug screening) and subjective outcomes (e.g. severity of signs and symptoms of withdrawal, adverse events). However, since all available outcomes were self reported, the dichotomisation into objective and subjective outcomes was not relevant.
We considered incomplete outcome data (avoidance of attrition bias) for all outcomes.
Overall assessment of risk of bias
We classified a trial as at low risk of bias only if all of the bias components described in the above paragraphs were classified as at low risk of bias. If one or more of the bias domains were classified as at unclear or high risk of bias, we classified the trial as at high risk of bias. If we found no trials at low risk of bias or only a very few trials at low risk of bias, we planned to identify a group of trials with lower risk of bias, defined as those having low risk of bias in the following domains: generation of allocation sequence, allocation concealment, and blinding of participants and treatment providers. However, since we classified only one trial as at low risk of bias, and thus the majority of the trials (k = 37; 97%) as at high risk of bias, it was not possible to apply this classification in the current review.
Measures of treatment effect
For dichotomous outcomes, we calculated a risk ratio (RR) with 95% confidence interval (CI), and in case of a significant result based on trials at low risk of bias, we reported the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) as the inverse of the absolute risk difference.
For continuous data, we calculated the mean difference (MD) between groups. We did not calculate effect size measures (standardised mean difference (SMD)) for all outcomes because of the inherent limitations associated with this measure (Higgins 2011). However, if scales of very considerable similarity were used, we could presume there was a small difference in the different measurements, and we calculated the effect size and planned to transform the effect back to the units of one or more of the specific instruments. However, due to marked differences in among‐participant variability, we did not find it relevant to re‐express the SMD using one of the specific measurement instruments.
Unit of analysis issues
The trial participant was the unit of analysis.
1. Cluster trials
No cluster‐randomised trials were included. If one or more cluster‐randomised trials had been included, we would have calculated the ‘design effect’ as described in our protocol (Baandrup 2015).
2. Cross‐over trials
We used data only from the first phase of cross‐over trials.
3. Studies with multiple intervention groups
Where a trial involved more than two intervention groups, we included both when relevant, or included data from the most relevant comparison if it was not appropriate from a clinical point of view to combine the experimental intervention groups into a single group (Higgins 2011).
Dealing with missing data
We tried to contact the first authors of studies to supply any missing data with regard to the defined outcomes. However, many of the included studies were old and the reported author contact details were outdated. It was thus impossible to contact many of the authors, and even the authors of newer studies did not reply to our queries for missing data.
Dichotomous data
For dichotomous outcomes, we did not impute missing values and analysed data as a complete‐case analysis.
Continuous data
If standard deviations (SDs) were not reported, we calculated them, if possible, using other data from the trial. If calculation of the SD was impossible, we imputed SDs from trials with similar characteristics if we considered this to be a valid approach.
Assessment of heterogeneity
We assessed statistical heterogeneity in the trials both by visual inspection of a forest plot and by using a standard Chi2 value with a significance level of P = 0.10. We assessed heterogeneity by use of the I2 statistic. We interpreted an I2 estimate greater than or equal to 50% and a significant Chi2 statistic as evidence of substantial heterogeneity (Higgins 2011). If this was the case, we explored the reasons for heterogeneity. If there was high inconsistency, and a clear reason was found, we planned to present data separately. We only performed a meta‐analysis if a sufficient number of studies were identified and if combining these studies was feasible as judged by clinical and statistical characteristics.
Assessment of reporting biases
We planned to inspect funnel plot symmetry when at least 10 trials were included in the meta‐analysis (Egger 1997; Macaskill 2001), bearing in mind that publication bias does not necessarily cause asymmetry, and that asymmetry may have other causes than publication bias. The inspection by funnel plot was not possible because none of the meta‐analyses included at least 10 trials.
For each included study, we investigated whether a study protocol was available. We searched PubMed, other major reference databases, and the Internet for a study protocol if a web address was not specifically stated in the article. This search could reveal abstracts or presentations relating to the study, and a comparison of outcomes with published outcomes was then possible. For newer studies, we searched for information on predefined outcome measures in trial registries. We had planned to construct a matrix containing recorded outcomes in each study, which then could indicate which studies did not report outcomes reported by the majority of included studies. However, during the process of data extraction and quality assessment, it was very evident which trials were associated with reporting bias, since these trials did not report the most evident outcome, namely some measure of benzodiazepine consumption.
Data synthesis
We divided the analyses according to type of experimental drug and pooling of drugs where a class effect could be expected (i.e. pooling of data from trials investigating drugs with a similar pharmacological profile, if clinical and statistical heterogeneity allowed). We performed meta‐analyses according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using the software Review Manager 5 (RevMan 2014). We further conducted Trial Sequential Analysis using the software available from CTU 2011.
Meta‐analysis
We performed meta‐analyses using a random‐effects model based on expectations of substantial heterogeneity among included trials (Deeks 2011; DeMets 1987; DerSimonian 1986). However, in case we found that one or two trials dominated the reported evidence (i.e. constituted more than 75% of the evidence), and there was substantial heterogeneity, we planned to synthesise data when appropriate and to emphasise results from the fixed‐effect model. However, due to the nature of the extracted data this scenario was not relevant.
Trial Sequential Analysis
We applied Trial Sequential Analysis, CTU 2011; Thorlund 2011b, because cumulative meta‐analyses are at risk of producing random errors due to sparse data and repetitive testing of the accumulating data (Brok 2008; Brok 2009; Thorlund 2009; Thorlund 2010; Wetterslev 2008; Wetterslev 2009). To minimise random errors, we calculated the required information size (i.e. the number of participants needed in a meta‐analysis to detect or reject a certain intervention effect) (Thorlund 2011a; Wetterslev 2008; Wetterslev 2017). The required information size for a meta‐analysis corresponds to the sample size for a single trial (Wetterslev 2017). The required information size takes into account: the event proportion in the control group; the assumption of a plausible risk ratio (RR) reduction, or the RR reduction observed in the included trials with low risk of bias; and the assumed heterogeneity, Turner 2014, or diversity of the meta‐analysis (Wetterslev 2008; Wetterslev 2009; Wetterslev 2017).
Trial Sequential Analysis enables testing for significance each time a new trial is added to the meta‐analysis (Thorlund 2011b; Wetterslev 2008; Wetterslev 2017). We added the trials according to the year of publication, and if more than one trial had been published in a year, we added trials alphabetically according to the last name of the first author. On the basis of the required information size and risk of type I and type II errors, we further constructed trial sequential monitoring boundaries. These boundaries determine the statistical inference one may draw from a meta‐analysis that has not reached the required information size. If the trial sequential boundary is crossed before the required information size is reached, firm evidence may perhaps be established and further trials may turn out to be superfluous. On the other hand, it is probably necessary to continue doing trials in order to detect or reject a certain intervention effect, if the trial sequential boundaries are not crossed.
As default, we originally planned to use a type I error of 5%, and a type II error of 20%. However, to account for multiplicity, we decreased the risk of type I error for the three primary outcomes to 2.5%, and to 1.25% for the seven secondary outcomes (Jakobsen 2014). Furthermore, we decreased the risk of type II error to 10%. For dichotomous outcomes, we had planned to estimate the required information size based on the proportion of participants with an outcome in the control group, a risk ratio of 20% or as suggested by the trials with low risk of bias, a diversity of 30% and 60%, or as suggested by the trials in the meta‐analysis. Specifically, for the primary dichotomised outcome 'benzodiazepine discontinuation', we used a control event proportion of 48% for all analyses, as this was the observed mean for trials assessing this outcome. For continuous outcomes, we estimated the required information size based on the SD observed in the control group of trials with low risk of bias and a minimal relevant difference of 50% of this SD, a diversity of 30% and 60%, or as suggested by the trials in the meta‐analysis. Specifically, for the secondary continuous outcome 'anxiety', assessed with the Hamilton Anxiety Rating Scale (HAM‐A), we used a variance of 103 (corresponding to an SD of 10 points), and a minimal relevant difference of 5 points for all analyses, as this was the highest observed variance in the trials assessing this outcome. Likewise, we used a variance of 20 points (corresponding to an SD of 4.5 points), and a minimal relevant difference of 2.25 points for all analyses of benzodiazepine withdrawal symptoms assessed with the Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ), as this was the highest observed variance in the trials assessing this outcome.
It was only possible to conduct Trial Sequential Analysis for eight outcomes, because fewer than two trials reported the same outcome, using the same instrument. In such situations the accrued information is such a small proportion of the required information size that Trial Sequential Analysis figures become uninterpretable.
Subgroup analysis and investigation of heterogeneity
We had planned to group the results from included studies according to the following methodological or clinical issues.
Trials at low risk of bias compared to trials at high risk of bias, or if we found no trials with low risk of bias, we would compare trials at lower risk of bias to trials at high risk of bias.
Type of benzodiazepine or benzodiazepine‐related drug.
Trials with different types of treatment setting (e.g. general practice compared to outpatient setting compared to inpatient setting).
Trials with different modes of benzodiazepine tapering (e.g. prescheduled or symptom‐guided).
Participants with concurrent psychiatric illness compared to participants without concurrent psychiatric illness.
Trials with different duration of the intervention: short (0 to 2 months), medium (3 to 6 months), and long (> 6 months).
Trials including inpatients compared to trials including outpatients.
Participants with other substance dependence versus participants with only benzodiazepine dependence/chronic use.
However, due to the high number of different pharmacological add‐on agents with few trials per agent, we chose not to perform any of our planned subgroup analyses.
Sensitivity analysis
We had planned to perform a sensitivity analysis to examine the impact of our assumptions regarding missing data. Furthermore, we had planned a sensitivity analysis to investigate the influence of trials with low compliance with study medication compared to trials with high compliance with study medication (compliance as defined by the trialists).
Assumption for lost dichotomous data
We had planned to perform two sensitivity analyses:
’Best‐worst‐case’ scenario: It will be assumed that all participants lost to follow‐up in the experimental group had no outcome, and that all participants lost to follow‐up in the control group had the outcome.
’Worst‐best‐case’ scenario: It will be assumed that all participants lost to follow‐up in the experimental group had the outcome, and that all participants lost to follow‐up in the control group had no outcome.
Assumptions for lost continuous data
Where assumptions had to be made regarding missing SDs (see: Dealing with missing data), we had planned sensitivity analysis to test how prone results were to change when 'completer' data only were compared to the imputed data using the above assumption. If there was a substantial difference, we had planned to report results and discuss them but continue to employ our assumption. Imputation of data turned out to be relevant for only one comparison: alpidem versus placebo (anxiety; Analysis 8.3).
8.3. Analysis.
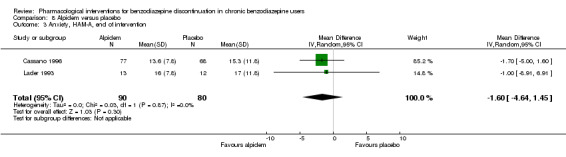
Comparison 8 Alpidem versus placebo, Outcome 3 Anxiety, HAM‐A, end of intervention.
However, due to the high number of different pharmacological add‐on agents with few trials per agent, we chose not to perform any of our planned sensitivity analyses.
'Summary of findings'
We used the GRADE system to assess the quality of the body of evidence and constructed 'Summary of findings' tables for the two primary outcomes on benzodiazepine consumption, employing GRADEpro software (GRADE). We assessed five factors of the study design and implementation of available trials that may downgrade the quality of the evidence, namely: risk of bias; indirectness of evidence (population, intervention, control, outcomes); unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of results; and high probability of publication bias.
Based on this, we defined the levels of evidence as follows (Balshem 2011).
High‐quality evidence when "we are very confident that the true effect lies close to that of the estimate of the effect".
Moderate‐quality evidence when "we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different".
Low‐quality evidence when the following statement applies: "Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect".
Very low‐quality evidence when the following statement applies: "We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect".
Results
Description of studies
See Characteristics of included studies section.
Results of the search
Our search strategy identified 3280 unique records. After removal of duplicates and irrelevant records, the number was reduced to 1239. Of these, we excluded 1174 after screening, and a further 22 after reviewing the full texts (Figure 1).
1.
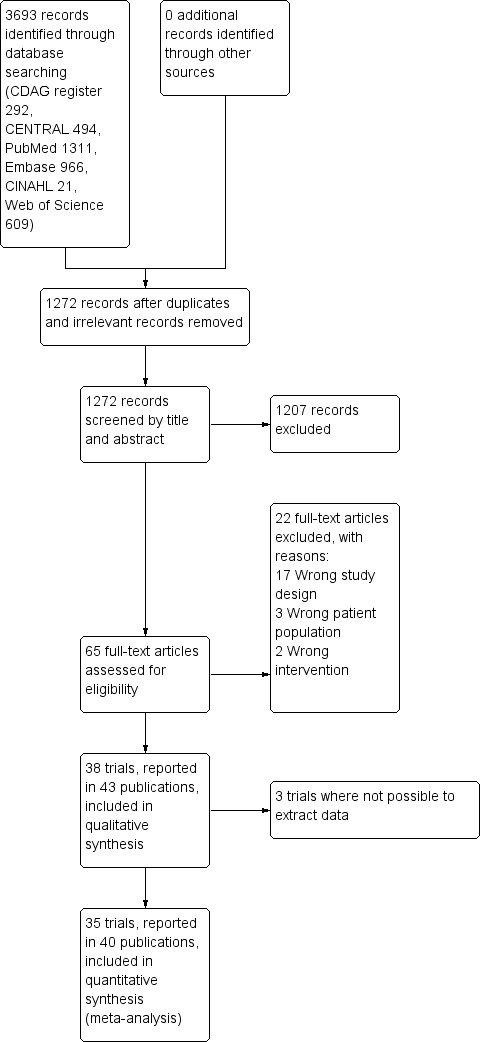
Study flow diagram.
Included studies
We included 43 publications reporting on 38 trials. Due to general poor reporting of the trials, we were not able to extract relevant data from all of the trials, even after attempting to contact the authors where possible. For this reason, we have not extracted data from Romach 1998 (experimental drug: ondansetron), Saul 1989 (experimental drug: atenolol), and Mariani 2016 (experimental drug: gabapentin). As a result, we included 35 trials involving 2295 participants with data in the quantitative meta‐analyses. See Characteristics of included studies.
Most of the trials (n = 35) involved a comparison between an active medication versus placebo or no intervention, while three trials investigated the experimental drug against another active comparator. The experimental drugs investigated were diverse, which limited the pooled analyses that were possible. If clinically relevant, we grouped investigational drugs with a similar mechanism, for example tricyclic antidepressants. To retain the clinical relevance of the meta‐analyses, we did not group medications with dissimilar pharmacological action even though they may belong to the same Anatomical Therapeutic Chemical‐group, for example valproate and carbamazepine trials were not pooled. The interventions investigated and included in the quantitative meta‐analyses were as follows.
Valproate versus placebo, Rickels 1999, or no intervention (Vorma 2011).
Carbamazepine versus placebo (Di Costanzo 1992; Klein 1994; Schweizer 1991).
Lithium versus placebo (Lecrubier 2005).
Pregabalin versus placebo (Hadley 2012).
Captodiame versus placebo (Mercier‐Guyon 2004).
Paroxetine versus placebo, GlaxoSmithKline 2002; Zitman 2001, or no intervention (Nakao 2006).
Tricyclic antidepressants (dosulepin (Tyrer 1996), imipramine (Rickels 2000; Rynn 2003), or trazodone (Zhang 2013)) versus placebo.
Alpidem versus placebo (Cassano 1996; Lader 1993).
Buspirone versus placebo (Ashton 1990; Lader 1987; Morton 1995; Udelman 1990).
Melatonin (short‐acting, Peles 2007; Vissers 2007, and prolonged‐release, Baandrup 2016; Garfinkel 1999) versus placebo.
Flumazenil versus placebo (Gerra 1993; Gerra 2002; Harrison‐Read 1996).
Propranolol versus placebo (Tyrer 1981).
Progesterone versus placebo (Schweizer 1995).
Magnesium aspartate versus placebo (Hantouche 1998).
Homeopathic drugs versus placebo (Cialdella 2001).
Carbamazepine versus tricyclic antidepressant (tianeptine) (Kornowski 2002).
Bromazepam versus cyamemazine (Lemoine 2006).
Zopiclone versus flunitrazepam (Pat‐Horenczyk 1998).
Out of the 38 trials, 24 were single‐centre (Ashton 1990; Baandrup 2016; Cialdella 2001; Di Costanzo 1992; Garfinkel 1999; Gerra 1993; Gerra 2002; Harrison‐Read 1996; Klein 1994; Kornowski 2002; Mariani 2016; Morton 1995; Nakao 2006; Pat‐Horenczyk 1998; Peles 2007; Rickels 1999; Rickels 2000; Romach 1998; Rynn 2003; Schweizer 1991; Schweizer 1995; Tyrer 1996; Vorma 2011; Zhang 2013), and 14 were multicentre (Cassano 1996; GlaxoSmithKline 2002; Hadley 2012; Hantouche 1998; Lader 1987; Lader 1993; Lecrubier 2005; Lemoine 2006; Mercier‐Guyon 2004; Saul 1989; Tyrer 1981; Udelman 1990; Vissers 2007; Zitman 2001).
The majority of the trials were performed in outpatient settings. The three trials investigating intravenous injection of flumazenil were conducted in inpatient settings (Gerra 1993; Gerra 2002; Harrison‐Read 1996), as was a trial with rapid benzodiazepine dose reduction in opioid maintenance patients (Vorma 2011). In Gerra 1993 and Gerra 2002, participants were hospitalised for the duration of the trial (seven and eight days, respectively) whereas participants in Harrison‐Read 1996 were hospitalised as they received a challenge with flumazenil and were thereafter treated as outpatients. Twenty‐four trials were conducted in Europe; eight trials in the US or Canada; and six trials in Asia.
Nine trials reported the source of funding as research grants (Baandrup 2016; Cialdella 2001; Mariani 2016; Peles 2007; Rickels 1999; Rickels 2000; Schweizer 1991; Schweizer 1995; Vorma 2011), and the funding was unclear for 15 trials (Cassano 1996; Di Costanzo 1992; Gerra 1993; Gerra 2002; Hantouche 1998; Kornowski 2002; Lader 1987; Lader 1993; Lecrubier 2005; Nakao 2006; Saul 1989; Tyrer 1981; Tyrer 1996; Vissers 2007; Zhang 2013). Fourteen trials used medications provided by the manufacturing company (Ashton 1990; Garfinkel 1999; GlaxoSmithKline 2002; Hantouche 1998; Harrison‐Read 1996; Klein 1994; Lemoine 2006; Mercier‐Guyon 2004; Morton 1995; Pat‐Horenczyk 1998; Romach 1998; Rynn 2003; Udelman 1990; Zitman 2001), and in all but one of these studies information regarding the degree of involvement of the pharmaceutical company was insufficient.
The trials investigated participants with a varying clinical picture dominated by anxiety. Three trials specifically investigated participants in opioid maintenance treatment (Mariani 2016; Peles 2007; Vorma 2011). In most trials, the majority of participants were women. The mean age was around 50 years (+/‐ 10 years) in most trials, and mean duration of benzodiazepine use was between 5 and 10 years in most trials. In eight trials there was no information at all on baseline characteristics. Eight trials applied abrupt discontinuation of benzodiazepine treatment with follow‐up periods between 1 and 8 weeks, whereas the remainder of the trials applied a gradual benzodiazepine dosage reduction regimen lasting between 2 and 24 weeks.
Trial duration ranged between 1 and 24 weeks, and mean trial duration was 9.4 weeks.
Excluded studies
We excluded 22 studies that were considered potentially relevant and assessed in detail (Figure 1). The reasons for exclusion were: 17 studies had a study design not fulfilling our inclusion criteria; 3 studies had a patient population not fulfilling our inclusion criteria; and 2 studies had interventions not fulfilling our inclusion criteria. For further details see Characteristics of excluded studies section.
Risk of bias in included studies
The overall risk of bias associated with the included studies is summarised in Figure 2 and Figure 3. Please also see Characteristics of included studies table.
2.
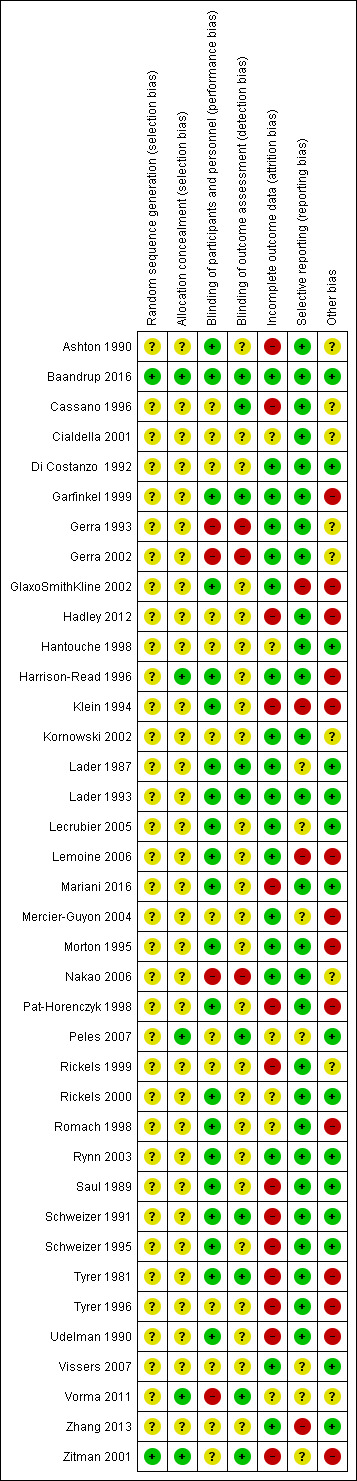
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
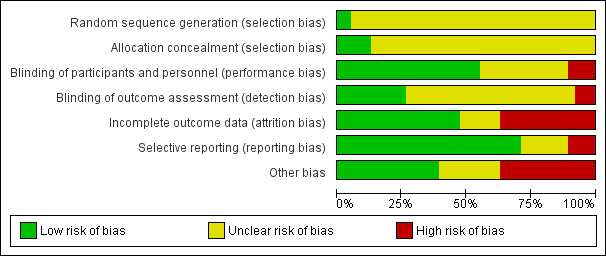
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
We judged only two trials as at low risk of bias because the random sequence was described as computer‐generated (Baandrup 2016; Zitman 2001). For all other trials (n = 36; 95%), information on random sequence generation was not provided and they were therefore judged as at unclear risk of selection bias.
Allocation concealment
We judged five trials as at low risk of bias because they sufficiently described how the randomisation was administered in a way that staff and trial participants could not anticipate to which group the next participant would be randomised. In two trials, randomisation was administered by a third party who paced and distributed the trial medication using numbered medication containers (Baandrup 2016; Zitman 2001); in one trial consecutive container numbers were used (Peles 2007); in one trial allocation was performed by an independent pharmacist (Harrison‐Read 1996); and one trial used sealed envelopes (Vorma 2011). We judged all other studies (n = 33; 87%) as at unclear risk of selection bias due to lack of information on allocation concealment.
Blinding
Performance bias
We evaluated 13 trials as at unclear risk of performance bias due to insufficient description of blinding procedures for participants and personnel (Cassano 1996; Cialdella 2001; Di Costanzo 1992; Hadley 2012; Hantouche 1998; Kornowski 2002; Mercier‐Guyon 2004; Peles 2007; Rickels 1999; Tyrer 1996; Vissers 2007; Zhang 2013; Zitman 2001). We considered more than half of the trials (n = 21; 55%) as at low risk of performance bias due to sufficient blinding procedures of participants and personnel. Four of the included trials were not blinded for participants and personnel and were therefore judged as at high risk of performance bias (Gerra 1993; Gerra 2002; Nakao 2006; Vorma 2011).
Detection bias
The majority of included trials (n = 25; 66%) were associated with unclear risk of detection bias due to insufficient descriptions of what was done to ensure blinding of outcome assessors. Three of the included trials were not blinded for outcome assessors and were therefore judged as at high risk of detection bias (Gerra 1993; Gerra 2002; Nakao 2006). We judged 10 trials as at low risk of detection bias because they provided sufficient information on blinding of outcome assessors (Baandrup 2016; Cassano 1996; Garfinkel 1999; Lader 1987; Lader 1993; Peles 2007; Schweizer 1991; Tyrer 1981; Vorma 2011; Zitman 2001).
Incomplete outcome data
We judged 14 studies (37%) as at high risk of attrition bias due to unacceptably high dropout rates, that is close to 50% (Ashton 1990; Cassano 1996; Hadley 2012; Klein 1994; Lader 1987; Mariani 2016; Pat‐Horenczyk 1998; Saul 1989; Schweizer 1991; Schweizer 1995; Tyrer 1981; Tyrer 1996; Udelman 1990; Zitman 2001). We judged six studies (16%) as at unclear risk of attrition bias due to missing information on flow of participants through the trials (Cialdella 2001; Hantouche 1998; Peles 2007; Rickels 2000; Romach 1998; Vorma 2011). We judged the remaining studies (n = 18; 47%) as at low risk of attrition bias.
Selective reporting
We judged four studies (11%) as at high risk of reporting bias because important outcome measures were not reported, that is benzodiazepine dosage at follow‐up (GlaxoSmithKline 2002; Klein 1994; Zhang 2013), or reporting of an unusual primary outcome (Lemoine 2006). We judged seven studies (18%) as at unclear risk of reporting bias due to insufficient information regarding whether selective reporting was present (Lader 1987; Lecrubier 2005; Mercier‐Guyon 2004; Peles 2007; Vissers 2007; Vorma 2011; Zitman 2001). We judged the remaining studies (n = 27; 71%) as at low risk of reporting bias.
Other potential sources of bias
We judged nine trials (24%) as at unclear risk of other bias mostly due to unclear or lacking description of funding. We rated 14 (37%) of the included trials as at high risk of other bias due to missing information on how the funding pharmaceutical company was involved in designing the trial as well as in analysing and interpreting the results.
Overall risk of bias
According to criteria described above, we could classify only one trial as at low risk of bias (Baandrup 2016), and no other trial qualified as at lower risk of bias. The large majority of the trials were thus at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15
Valproate versus placebo or no intervention
Results for this comparison were mainly from a small trial comparing valproate versus placebo (Rickels 1999). Results showed a beneficial effect of valproate on benzodiazepine discontinuation at end of intervention (Analysis 1.1: 1 study, 27 participants; RR 2.55, 95% CI 1.08 to 6.03; GRADE: very low‐quality evidence) and on benzodiazepine relapse at end of intervention (Analysis 1.2: 1 study, 27 participants; RR 0.31, 95% CI 0.11 to 0.90; GRADE: very low‐quality evidence). However, there was no effect on benzodiazepine discontinuation at longest follow‐up (Analysis 1.3: 1 study, 24 participants; RR 1.57, 95% CI 0.80 to 3.09; GRADE: very low‐quality evidence), benzodiazepine relapse at longest follow‐up (Analysis 1.4: 1 study, 24 participants; RR 0.43, 95% CI 0.13 to 1.39; GRADE: very low‐quality evidence), or symptoms of anxiety at end of intervention (Analysis 1.5: 1 study, 27 participants; MD ‐0.40 points, 95% CI ‐6.47 to 5.67; GRADE: very low‐quality evidence).
1.1. Analysis.
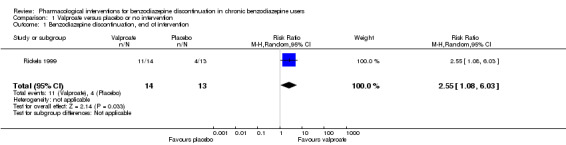
Comparison 1 Valproate versus placebo or no intervention, Outcome 1 Benzodiazepine discontinuation, end of intervention.
1.2. Analysis.
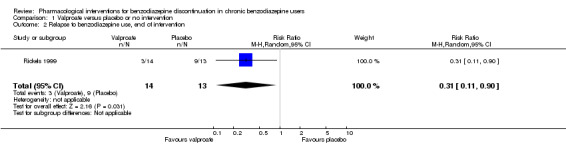
Comparison 1 Valproate versus placebo or no intervention, Outcome 2 Relapse to benzodiazepine use, end of intervention.
1.3. Analysis.
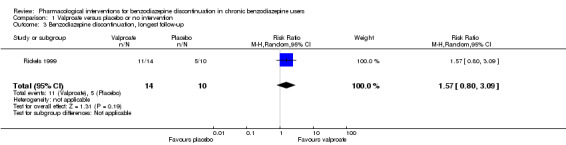
Comparison 1 Valproate versus placebo or no intervention, Outcome 3 Benzodiazepine discontinuation, longest follow‐up.
1.4. Analysis.
Comparison 1 Valproate versus placebo or no intervention, Outcome 4 Relapse to benzodiazepine use, longest follow‐up.
1.5. Analysis.
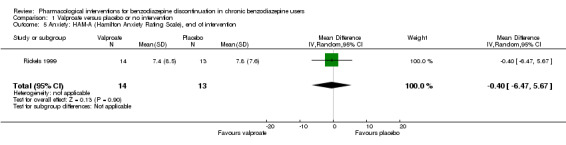
Comparison 1 Valproate versus placebo or no intervention, Outcome 5 Anxiety: HAM‐A (Hamilton Anxiety Rating Scale), end of intervention.
For benzodiazepine discontinuation, we calculated the required information size to be 1918 participants, using a control event proportion of 48%, a relative risk reduction of 20%, type I error of 2.5%, power of 90%, and a diversity of 30%. We could not perform Trial Sequential Analysis as there was only one trial.
For benzodiazepine withdrawal symptoms, it was possible to perform a meta‐analysis using data from Rickels 1999 and data from another small trial investigating benzodiazepine withdrawal in methadone maintenance users, Vorma 2011, comparing valproate versus no intervention. This meta‐analysis indicated no difference between intervention groups (Analysis 1.6: 2 studies, 56 participants; SMD ‐0.15, 95% CI ‐0.68 to 0.37; GRADE: very low‐quality evidence). Due to marked differences in among‐participant variability, we did not re‐express the SMD using one of the specific measurement instruments. Results were similar if analysing placebo or no intervention separately as control group.
1.6. Analysis.
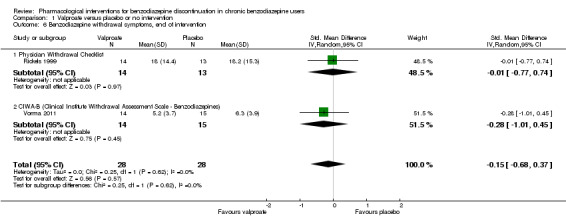
Comparison 1 Valproate versus placebo or no intervention, Outcome 6 Benzodiazepine withdrawal symptoms, end of intervention.
Please see Table 1.
Carbamazepine versus placebo
Results for this comparison stem from three smaller trials (Di Costanzo 1992; Klein 1994; Schweizer 1991), not all of which contribute data to all outcomes. It was possible to perform a meta‐analysis for benzodiazepine discontinuation at end of intervention, where no significant differences between groups were detected (Analysis 2.1: 3 studies, 147 participants; RR 1.33, 95% CI 0.99 to 1.80; GRADE: low quality‐evidence). Trial Sequential Analysis showed that the diversity‐adjusted required information size of 2109 participants was not reached, as the accrued number of participants was only 147 (7.0%), showing that insufficient information has been accrued (Figure 4).
2.1. Analysis.
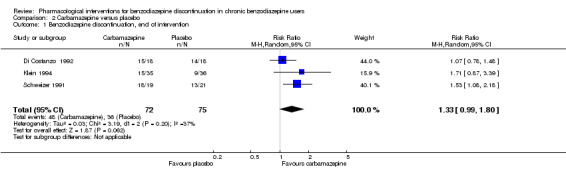
Comparison 2 Carbamazepine versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
4.
Trial Sequential Analysis of comparison: 2 Carbamazepine versus placebo, outcome: 2.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in three trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction (RRR) of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 36% as observed in the trials. The diversity‐adjusted required information size (DARIS) was 2109 participants, and the Trial Sequential Analysis‐adjusted confidence interval is 0.24 to 2.38. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) touches the conventional statistical boundaries, but does not cross the trial sequential monitoring boundaries, and the diversity‐adjusted required information size is not met, showing that insufficient information has been accrued.
For benzodiazepine withdrawal symptoms, results did not favour carbamazepine (Analysis 2.2: 2 studies, 76 participants; SMD ‐1.14, 95% CI ‐2.43 to 0.16; GRADE: very low quality‐evidence). Due to marked differences in among‐participant variability, we did not re‐express the SMD using one of the specific measurement instruments.
2.2. Analysis.
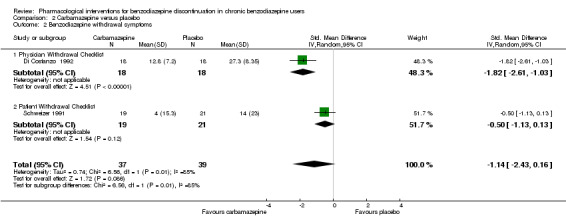
Comparison 2 Carbamazepine versus placebo, Outcome 2 Benzodiazepine withdrawal symptoms.
For the following outcomes, only data from single trials were available, finding no differences between groups regarding benzodiazepine discontinuation at longest follow‐up (Analysis 2.3: 1 study, 40 participants; RR 1.41, 95% CI 0.86 to 2.29; GRADE: very low‐quality evidence), relapse to benzodiazepine use (Analysis 2.4: 1 study, 36 participants; RR 0.33, 95% CI 0.08 to 1.44; GRADE: very low‐quality evidence), and non‐serious adverse events (Analysis 2.6: 1 study, 36 participants; RR 7.00, 95% CI 0.39 to 126.48; GRADE: very low‐quality evidence). Data from a single trial found carbamazepine superior to placebo regarding symptoms of anxiety (Analysis 2.7: 1 study, 36 participants; MD ‐6.00 points, 95% CI ‐9.58 to ‐2.42; GRADE: very low‐quality evidence).
2.3. Analysis.
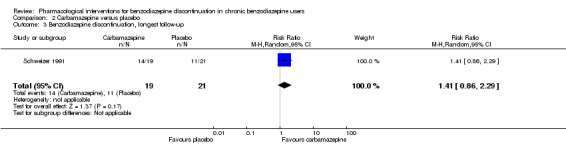
Comparison 2 Carbamazepine versus placebo, Outcome 3 Benzodiazepine discontinuation, longest follow‐up.
2.4. Analysis.
Comparison 2 Carbamazepine versus placebo, Outcome 4 Relapse to benzodiazepine use.
2.6. Analysis.
Comparison 2 Carbamazepine versus placebo, Outcome 6 Non‐serious adverse events.
2.7. Analysis.
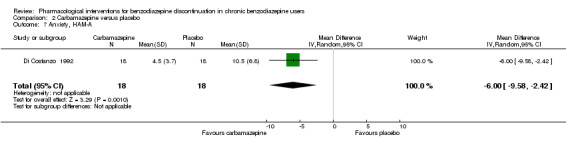
Comparison 2 Carbamazepine versus placebo, Outcome 7 Anxiety, HAM‐A.
When evaluating benzodiazepine withdrawal symptoms, the results were associated with significant heterogeneity. We could identify no obvious reason for this heterogeneity.
Please see Table 2.
Lithium versus placebo
One trial of moderate size investigated lithium versus placebo for benzodiazepine discontinuation and found no difference between groups regarding benzodiazepine discontinuation (Analysis 3.1: 1 study, 230 participants; RR 1.05, 95% CI 0.86 to 1.28; GRADE: low‐quality evidence), non‐serious adverse events (Analysis 3.3: 1 study, 230 participants; RR 1.06, 95% CI 0.75 to 1.49; GRADE: very low‐quality evidence), and discontinuation due to adverse events (Analysis 3.4: 1 study, 230 participants; RR 1.38, 95% CI 0.13 to 15.03; GRADE: very low‐quality evidence) (Lecrubier 2005). Data on other outcomes specified in this review were not available.
3.1. Analysis.
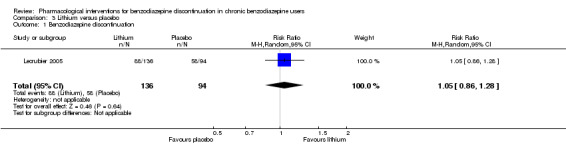
Comparison 3 Lithium versus placebo, Outcome 1 Benzodiazepine discontinuation.
3.3. Analysis.
Comparison 3 Lithium versus placebo, Outcome 3 Non‐serious adverse events.
3.4. Analysis.
Comparison 3 Lithium versus placebo, Outcome 4 Discontinuation due to adverse events.
For benzodiazepine discontinuation, we calculated the required information size to be 1918 participants, using a control event proportion of 48%, a relative risk reduction of 20%, type I error of 2.5%, power of 90%, and a diversity of 30%. We could not perform Trial Sequential Analysis as there was only one trial.
Please see Table 3.
Pregabalin versus placebo
One smaller trial investigated this comparison (Hadley 2012), finding no significant difference between groups regarding benzodiazepine discontinuation (Analysis 4.1: 1 study, 106 participants; RR 1.44, 95% CI 0.92 to 2.25; GRADE: very low‐quality evidence), but finding superior effect of pregabalin regarding benzodiazepine withdrawal symptoms (Analysis 4.2; 1 study, 106 participants; MD ‐3.10 points, 95% CI ‐3.51 to ‐2.69; GRADE: very low‐quality evidence) and symptoms of anxiety (Analysis 4.3: 1 study, 106 participants; MD ‐4.80 points, 95% CI ‐5.28 to ‐4.32; GRADE: very low‐quality evidence). There were no differences between groups for serious and non‐serious adverse events as well as for discontinuation due to side effects (Analysis 4.4; Analysis 4.5; Analysis 4.6).
4.1. Analysis.
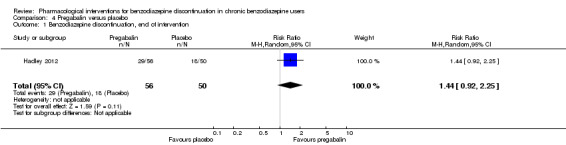
Comparison 4 Pregabalin versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
4.2. Analysis.
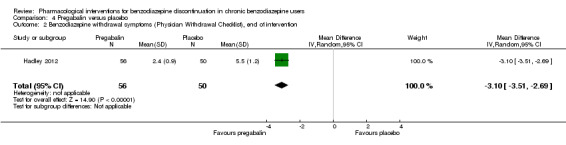
Comparison 4 Pregabalin versus placebo, Outcome 2 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention.
4.3. Analysis.
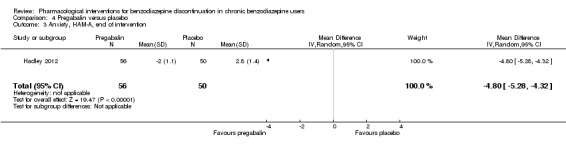
Comparison 4 Pregabalin versus placebo, Outcome 3 Anxiety, HAM‐A, end of intervention.
4.4. Analysis.
Comparison 4 Pregabalin versus placebo, Outcome 4 Serious adverse events.
4.5. Analysis.
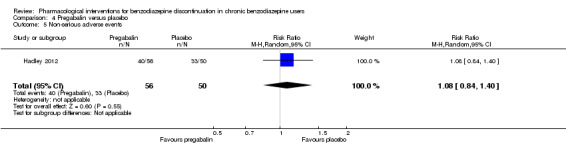
Comparison 4 Pregabalin versus placebo, Outcome 5 Non‐serious adverse events.
4.6. Analysis.
Comparison 4 Pregabalin versus placebo, Outcome 6 Discontinuation due to adverse events.
For benzodiazepine discontinuation, we calculated the required information size to be 1918 participants, using a control event proportion of 48%, a relative risk reduction of 20%, type I error of 2.5%, power of 90%, and a diversity of 30%. We could not perform Trial Sequential Analysis as there was only one trial.
Please see Table 4.
Captodiame versus placebo
One smaller trial compared captodiame (an antihistamine used as a sedative and anxiolytic) with placebo and found that captodiame had a beneficial effect in terms of benzodiazepine withdrawal symptoms (Analysis 5.1: 1 study, 81 participants; MD ‐1.00 points, 95% CI ‐1.13 to ‐0.87; GRADE: very low‐quality evidence) and symptoms of anxiety (Analysis 5.2: 1 study, 81 participants; MD ‐5.70 points, 95% CI ‐6.05 to ‐5.35) (Mercier‐Guyon 2004). Data on other outcomes specified in this review were not available.
5.1. Analysis.
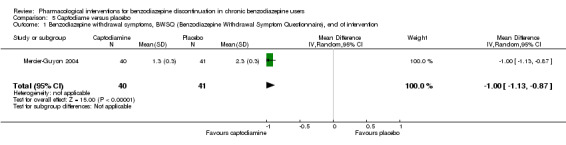
Comparison 5 Captodiame versus placebo, Outcome 1 Benzodiazepine withdrawal symptoms, BWSQ (Benzodiazepine Withdrawal Symptom Questionnaire), end of intervention.
5.2. Analysis.
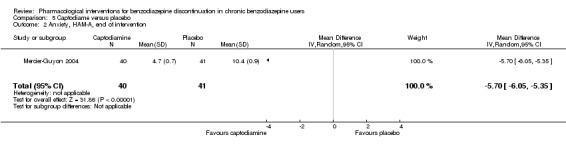
Comparison 5 Captodiame versus placebo, Outcome 2 Anxiety, HAM‐A, end of intervention.
For benzodiazepine withdrawal symptoms assessed with the Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ), we calculated the required information size to be 229 participants, using a variance of 20 points, a minimal relative difference of 2.25 points, type I error of 2.5%, power of 90%, and a diversity of 30%. We could not perform Trial Sequential Analysis as there was only one trial.
Please see Table 5.
Paroxetine versus placebo or no intervention
Three smaller trials evaluated paroxetine to facilitate benzodiazepine discontinuation: two versus placebo, GlaxoSmithKline 2002; Zitman 2001, and one versus no intervention (Nakao 2006). It was possible to perform a meta‐analysis for the following outcomes, where placebo and no intervention were pooled as control group.
Benzodiazepine discontinuation at end of intervention, where no intervention effect could be found (Analysis 6.1: 3 studies, 221 participants; RR 1.45, 95% CI 0.88 to 2.39; GRADE: very low‐quality evidence). Trial Sequential Analysis showed that the diversity‐adjusted required information size of 9448 participants was not reached, as the accrued number of participants was only 210 (2.34%), showing that insufficient information has been obtained (Figure 5). Results for benzodiazepine discontinuation were associated with significant heterogeneity (Analysis 6.1), for which we could not identify any obvious reason.
Benzodiazepine withdrawal symptoms at end of intervention, assessed with BWSQ, indicated a difference in favour of paroxetine (Analysis 6.2: 2 studies, 99 participants; MD ‐3.57 points, 95% CI ‐5.34 to ‐1.80; GRADE: very low‐quality evidence). Trial Sequential Analysis based on a minimal relevant clinical difference of 2.25 points, variance of 20 (empirical data), a type I error of 1.25%, a type II error of 10% (90% power), and diversity of 0% showed that the diversity‐adjusted required information size of 229 participants was not met (Figure 6). However, the cumulative Z‐curve touched the trial sequential monitoring boundaries for benefit, indicating that sufficient information had been obtained, and that the result was not due to random error.
Symptoms of anxiety, assessed with Hamilton Anxiety Rating Scale (HAM‐A), indicated a difference in favour of paroxetine (Analysis 6.3: 2 studies, 99 participants; MD ‐6.75 points, 95% CI ‐9.64 to ‐3.86; GRADE: very low‐quality evidence). We performed Trial Sequential Analysis on anxiety, assessed with HAM‐A, with a minimal relevant clinical difference of 5 points, variance of 103 points, based on a type I error of 1.25%, a type II error of 10% (90% power), and diversity of 0% (Figure 7). The diversity‐adjusted required information size of 236 participants was not met. However, the cumulative Z‐curve crossed the trial sequential monitoring boundaries for benefit, indicating that sufficient information had been obtained, and that the result was not due to random error.
6.1. Analysis.
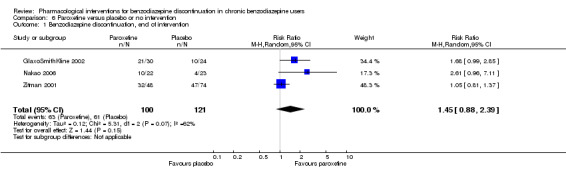
Comparison 6 Paroxetine versus placebo or no intervention, Outcome 1 Benzodiazepine discontinuation, end of intervention.
5.
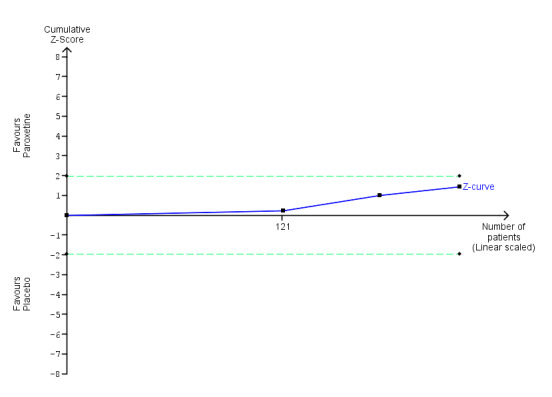
Trial Sequential Analysis of comparison: 6 Paroxetine versus placebo, outcome: 6.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in three trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 86% as observed in the trials. The diversity‐adjusted required information size was 9448 participants, and the Trial Sequential Analysis‐adjusted confidence interval could not be estimated due to lack of information. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) does not cross the conventional statistical boundaries. The trial sequential monitoring boundaries and the diversity‐adjusted required information size are not shown as the accrued number of participants only amounted to 221/9448 (2.34%), showing that insufficient information has been accrued.
6.2. Analysis.
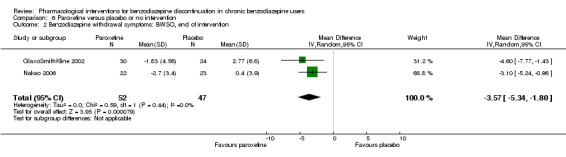
Comparison 6 Paroxetine versus placebo or no intervention, Outcome 2 Benzodiazepine withdrawal symptoms: BWSQ, end of intervention.
6.
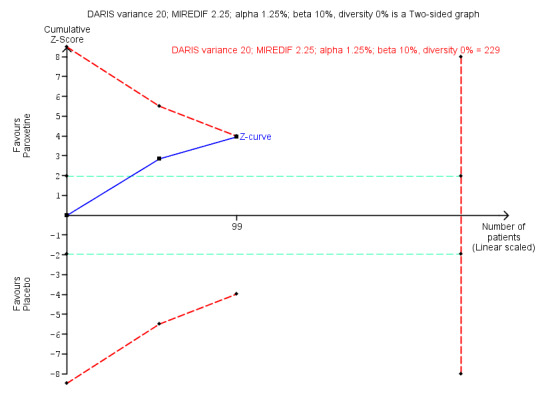
Trial Sequential Analysis of comparison: 6 Paroxetine versus placebo, outcome: 6.2 Benzodiazepine withdrawal symptoms Benzodiazepine Withdrawal Symptom Questionnaire (BWSQ). Trial Sequential Analysis on benzodiazepine withdrawal symptoms assessed with BWSQ assessing a minimal relevant clinical difference (MIREDIF) of 2.25 points, and a variance of 20 points (empirical data), was performed based on a type I error of 1.25%, a type II error of 10% (90% power), and diversity of 0%. The diversity‐adjusted required information size (DARIS) was 229 participants, and the Trial Sequential Analysis‐adjusted confidence interval is ‐7.18 to 0.05. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 0.05. The red inward‐sloping lines represent the trial sequential monitoring boundaries. The cumulative Z‐curve touches the trial sequential monitoring boundaries, indicating that sufficient information was provided.
6.3. Analysis.
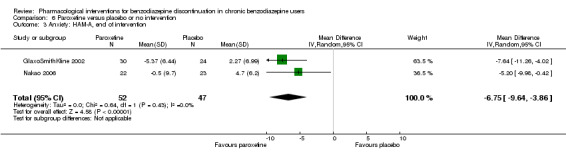
Comparison 6 Paroxetine versus placebo or no intervention, Outcome 3 Anxiety: HAM‐A, end of intervention.
7.
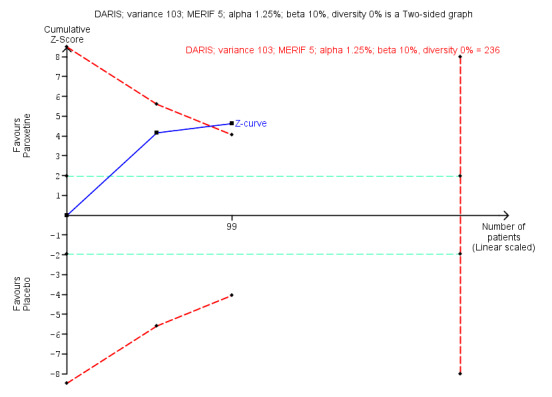
Trial Sequential Analysis of comparison: 6 Paroxetine versus placebo, outcome: 6.3 Anxiety, Hamilton Anxiety Rating Scale (HAM‐A). Trial Sequential Analysis on anxiety evaluated with HAM‐A assessing a minimal relevant clinical difference (MIREDIF) of 5 points, and a variance of 103 points, was performed based on a type I error of 1.25%, a type II error of 10% (90% power), and diversity of 0%. The diversity‐adjusted required information size (DARIS) was 236 participants, and the Trial Sequential Analysis‐adjusted confidence interval is ‐12.72 to ‐0.80. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 0.05. The red inward‐sloping lines represent the trial sequential monitoring boundaries. The cumulative Z‐curve crosses the trial sequential monitoring boundaries, indicating that sufficient information was provided.
Results were similar if analysing placebo or no intervention separately as control group.
One trial evaluated benzodiazepine withdrawal symptoms at longest follow‐up, where no effect of paroxetine could be found (Analysis 6.4: 1 study, 54 participants; MD ‐0.13 points, 95% CI ‐4.03 to 3.77; GRADE: very low‐quality evidence). For non‐serious adverse events, no differences between intervention groups were found (Analysis 6.6).
6.4. Analysis.
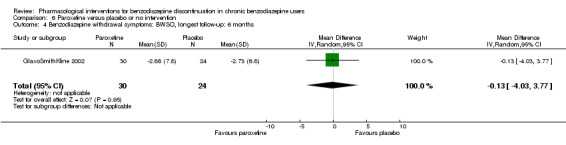
Comparison 6 Paroxetine versus placebo or no intervention, Outcome 4 Benzodiazepine withdrawal symptoms: BWSQ, longest follow‐up: 6 months.
6.6. Analysis.
Comparison 6 Paroxetine versus placebo or no intervention, Outcome 6 Non‐serious adverse events.
Please see Table 6.
Tricyclic antidepressants (dosulepin, imipramine, or trazodone) versus placebo
Four trials, each with a small sample size, investigated tricyclic antidepressants versus placebo (Rickels 2000; Rynn 2003; Tyrer 1996; Zhang 2013). It was possible to perform a meta‐analysis for benzodiazepine discontinuation at end of intervention, where results showed no difference between intervention groups (Analysis 7.1: 2 studies, 105 participants; RR 0.82, 95% CI 0.52 to 1.28; GRADE: very low‐quality evidence). Trial Sequential Analysis showed that the diversity‐adjusted required information size of 1343 participants was not reached, as the accrued number of participants was only 105 (7.81%), indicating that insufficient information has been obtained (Figure 8).
7.1. Analysis.
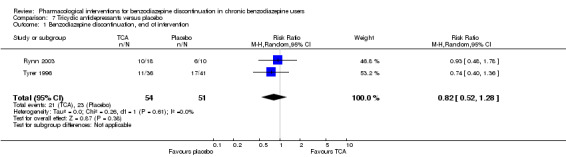
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
8.
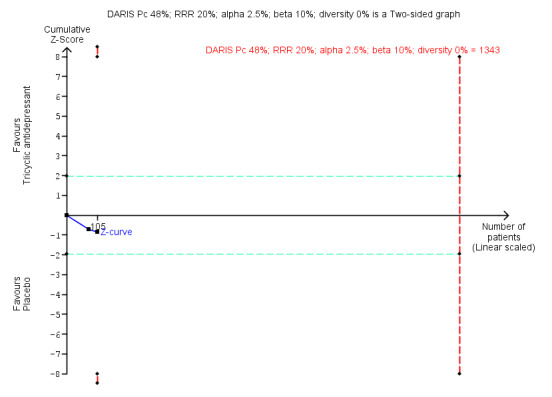
Trial Sequential Analysis of comparison: 7 Tricyclic antidepressants versus placebo, outcome: 7.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in two trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction (RRR) of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 0% as observed in the trials. The diversity‐adjusted required information size (DARIS) was 1343 participants, and the Trial Sequential Analysis‐adjusted confidence interval is 0.20 to 7.55. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) does not cross the conventional statistical boundaries or the trial sequential monitoring boundaries (red dotted lines), and the diversity‐adjusted required information size is not met, showing that insufficient information has been accrued.
Two trials reported symptoms of anxiety (Rynn 2003; Zhang 2013), with no difference between intervention groups (Analysis 7.2: 2 studies, 66 participants; MD ‐10.38 points, 95% CI ‐25.96 to 5.20; GRADE: very low‐quality evidence). These data were associated with significant heterogeneity, the reason for which was that the Chinese study, Zhang 2013, reported results that were far more positive in favour of the experimental drug than the remainder of the included trials.
7.2. Analysis.
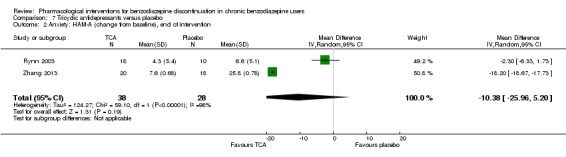
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 2 Anxiety: HAM‐A (change from baseline), end of intervention.
The following outcomes were reported in one trial each: benzodiazepine discontinuation at longest follow‐up, showing a beneficial effect of tricyclic antidepressants (Analysis 7.3: 1 study, 47 participants; RR 2.20, 95% CI 1.27 to 3.82; GRADE: low‐quality evidence); benzodiazepine withdrawal symptoms (Analysis 7.4: 1 study, 38 participants; MD ‐19.78 points, 95% CI ‐20.25 to ‐19.31; GRADE: very low‐quality evidence) with a significant difference between groups in favour of tricyclic antidepressants; relapse to benzodiazepine use, showing no intervention effect (Analysis 7.5: 1 study, 36 participants; RR 2.00, 95% CI 0.73 to 5.47); and discontinuation due to adverse events, also showing no intervention effect (Analysis 7.6: 2 studies, 134 participants; RR 1.16, 95% CI 0.42 to 3.21; GRADE: very low‐quality evidence).
7.3. Analysis.
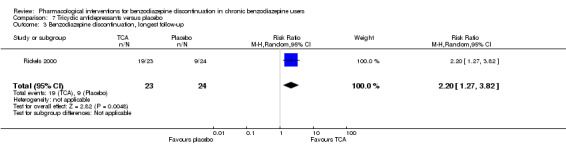
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 3 Benzodiazepine discontinuation, longest follow‐up.
7.4. Analysis.
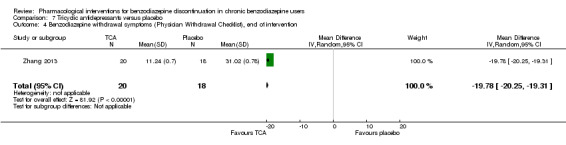
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 4 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention.
7.5. Analysis.
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 5 Relapse to benzodiazepine use, end of intervention.
7.6. Analysis.
Comparison 7 Tricyclic antidepressants versus placebo, Outcome 6 Discontinuation due to adverse events.
Please see Table 7.
Alpidem versus placebo
Two smaller trials compared alpidem (an anxiolytic drug from the imidazopyridine family) with placebo for benzodiazepine discontinuation (Cassano 1996; Lader 1993), showing a disadvantage of alpidem for this outcome at end of intervention (Analysis 8.1: 1 study, 25 participants; RR 0.41, 95% CI 0.17 to 0.99; NNTH 2.3 participants; GRADE: low‐quality evidence). We calculated the required information size to be 1918 participants, using a control event proportion of 48%, a relative risk reduction of 20%, type I error of 2.5%, power of 90%, and a diversity of 30%. Withdrawal syndrome was also analysed, suggesting a disadvantage of alpidem (Analysis 8.2: 1 study, 145 participants; RR 4.86, 95% CI 1.12 to 21.14; NNTH 5.9 participants; GRADE: low‐quality evidence). We calculated the required information size to be 9202 participants, using a control event proportion of 15%, a relative risk reduction of 20%, type I error of 2.5%, power of 90%, and a diversity of 30%. We could not perform Trial Sequential Analysis for either of these outcomes because they were only reported in one trial.
8.1. Analysis.
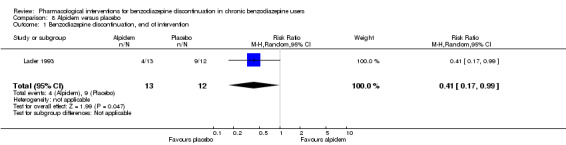
Comparison 8 Alpidem versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
8.2. Analysis.
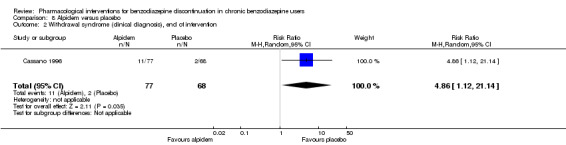
Comparison 8 Alpidem versus placebo, Outcome 2 Withdrawal syndrome (clinical diagnosis), end of intervention.
It was possible to perform a meta‐analysis for symptoms of anxiety, as assessed by HAM‐A, where no intervention effect was found (Analysis 8.3: 2 studies, 170 participants; MD ‐1.60 points, 95% CI ‐4.64 to 1.45; GRADE: low‐quality evidence). In Trial Sequential Analysis using a minimal relevant clinical difference of 5 points, variance of 103 points, type I error of 1.25%, type II error of 10% (90% power), and diversity of 0% (Figure 9), we found that the diversity‐adjusted required information size of 235 participants was not met. However, the cumulative Z‐curve crossed the beta‐spending (futility) boundaries, indicating that an intervention effect, if any, was less than 5 points.
9.
Trial Sequential Analysis of comparison: 8 Alpidem versus placebo, outcome: 8.3 Anxiety, Hamilton Anxiety Rating Scale (HAM‐A). Trial Sequential Analysis on anxiety evaluated with HAM‐A assessing a minimal relevant clinical difference (MIREDIF) of 5 points, and a variance of 103 points (empirical data), was performed based on a type I error of 1.25%, a type II error of 10% (90% power), and diversity of 0%. The diversity‐adjusted required information size (DARIS) was 235 participants, and the Trial Sequential Analysis‐adjusted confidence interval is ‐6.28 to 3.08. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 0.05. The red inward‐sloping lines represent the trial sequential alpha‐spending monitoring boundaries, while the red outward‐sloping lines represent the beta‐spending (futility) boundaries. The cumulative Z‐curve crosses the beta‐spending (futility) boundaries, showing that an intervention effect, if any, is less than 5 points.
One trial reported relapse to benzodiazepine use, suggesting no intervention effect (Analysis 8.4: 1 study, 145 participants; RR 0.33, 95% CI 0.09 to 1.20; GRADE: very low‐quality evidence).
8.4. Analysis.
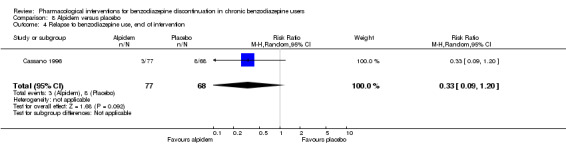
Comparison 8 Alpidem versus placebo, Outcome 4 Relapse to benzodiazepine use, end of intervention.
Please see Table 8.
Buspirone versus placebo
Four trials examined use of buspirone compared with placebo (Ashton 1990; Lader 1987; Morton 1995; Udelman 1990), each with a small sample size. It was possible to perform a meta‐analysis with regard to benzodiazepine discontinuation at end of intervention, finding no difference between intervention groups (Analysis 9.1: 4 studies, 143 participants; RR 0.82, 95% CI 0.49 to 1.37; GRADE: low‐quality evidence). Trial Sequential Analysis showed that the diversity‐adjusted required information size of 3381 participants was not reached, as the accrued number of participants was only 143 (4.23%), showing that insufficient information has been accrued (Figure 10).
9.1. Analysis.
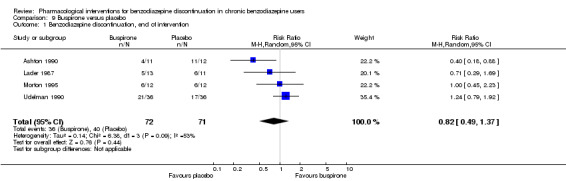
Comparison 9 Buspirone versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
10.
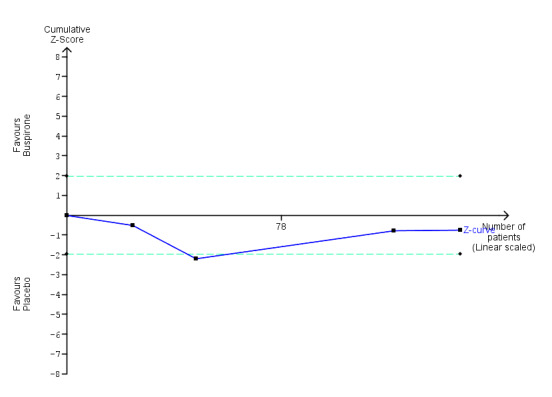
Trial Sequential Analysis of comparison: 9 Buspirone versus placebo, outcome: 9.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in four trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 60% as observed in the trials. The diversity‐adjusted required information size was 3381 participants, and the Trial Sequential Analysis‐adjusted confidence interval could not be estimated due to lack of information. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) does not cross the conventional statistical boundaries. The trial sequential monitoring boundaries and the diversity‐adjusted required information size are not shown, as the accrued number of participants only amounted to 143/3381 (4.23%), showing that insufficient information has been accrued.
There was also no intervention effect for anxiety symptoms at end of intervention (Analysis 9.2: 2 studies, 41 participants; SMD 0.18, 95% CI ‐0.50 to 0.86; GRADE: very low‐quality evidence).
9.2. Analysis.
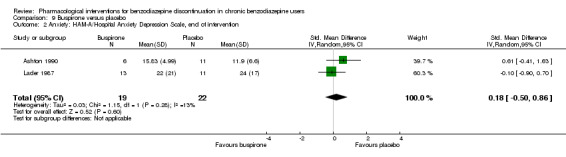
Comparison 9 Buspirone versus placebo, Outcome 2 Anxiety: HAM‐A/Hospital Anxiety Depression Scale, end of intervention.
The following outcomes were reported in only one trial each and with no sign of intervention effect: benzodiazepine withdrawal symptoms at end of intervention (Analysis 9.3: 1 study, 17 participants; MD 4.69 points, 95% CI ‐14.47 to 23.85; GRADE: very low‐quality evidence), benzodiazepine discontinuation at longest follow‐up (Analysis 9.4: 1 study, 23 participants; RR 0.60, 95% CI 0.34 to 1.05; GRADE: low‐quality evidence), benzodiazepine withdrawal symptoms at longest follow‐up (Analysis 9.5: 1 study, 15 participants; MD ‐1.34 points, 95% CI ‐14.31 to 11.63; GRADE: very low‐quality evidence), and symptoms of anxiety at longest follow‐up (Analysis 9.6: 1 study, 12 participants; MD 2.75 points, 95% CI ‐2.83 to 8.33; GRADE: very low‐quality evidence). Due to marked differences in among‐participant variability, we did not re‐express the SMD using one of the specific measurement instruments.
9.3. Analysis.
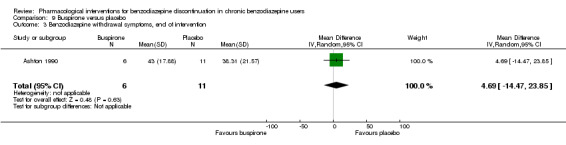
Comparison 9 Buspirone versus placebo, Outcome 3 Benzodiazepine withdrawal symptoms, end of intervention.
9.4. Analysis.
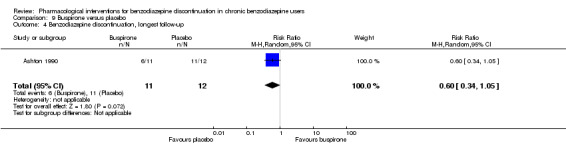
Comparison 9 Buspirone versus placebo, Outcome 4 Benzodiazepine discontinuation, longest follow‐up.
9.5. Analysis.
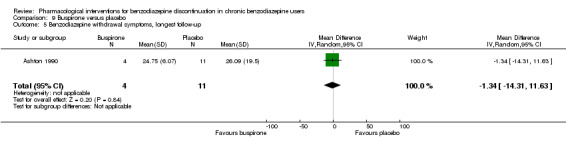
Comparison 9 Buspirone versus placebo, Outcome 5 Benzodiazepine withdrawal symptoms, longest follow‐up.
9.6. Analysis.
Comparison 9 Buspirone versus placebo, Outcome 6 Anxiety, Hospital Anxiety Depression Scale, longest follow‐up.
Results for benzodiazepine discontinuation (Analysis 9.1) were associated with significant heterogeneity, with the most marked differences between Ashton 1990 (more in favour of placebo or no difference between groups) and Udelman 1990 (more in favour of buspirone). We could not identify any obvious reasons for the observed heterogeneity.
Please see Table 9.
Melatonin versus placebo
Four trials of small to moderate sample size investigated melatonin (both short‐acting and prolonged release formulation) versus placebo for benzodiazepine discontinuation at end of intervention (Baandrup 2016; Garfinkel 1999; Peles 2007; Vissers 2007). It was possible to perform a meta‐analysis for benzodiazepine discontinuation at end of intervention, which showed no difference between intervention groups (Analysis 10.1: 4 studies, 219 participants; RR 1.20, 95% CI 0.73 to 1.96; GRADE: very low‐quality evidence). Trial Sequential Analysis showed that the diversity‐adjusted required information size of 3438 participants was not reached, as the accrued number of participants was only 219 (6.37%), showing that insufficient information has been accrued (Figure 11).
10.1. Analysis.
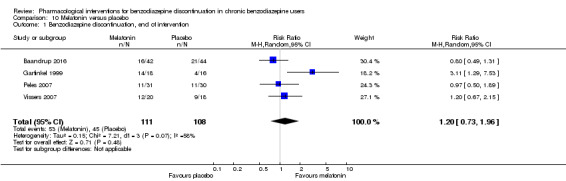
Comparison 10 Melatonin versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
11.
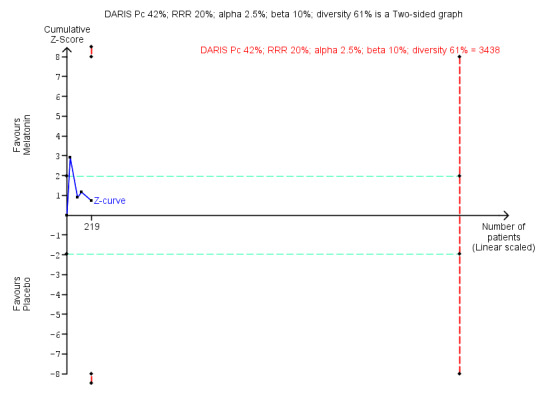
Trial Sequential Analysis of comparison: 10 Melatonin versus placebo, outcome: 10.1 Benzodiazepine discontinuation. Trial Sequential Analysis on benzodiazepine discontinuation in four trials was performed based on the proportion with benzodiazepine discontinuation in the control group set at 48%, a relative risk reduction (RRR) of 20%, a type I error of 2.5%, a type II error of 10% (90% power), and diversity of 61% as observed in the trials. The diversity‐adjusted required information size (DARIS) was 3438 participants, and the Trial Sequential Analysis‐adjusted confidence interval is 0.11 to 6.25. The blue line represents the cumulative Z‐score of the meta‐analysis. The green lines represent the conventional statistical boundaries of P = 5%. The cumulative Z‐curve (blue line) does not cross the conventional statistical boundaries or the trial sequential monitoring boundaries (red dotted lines), and the diversity‐adjusted required information size is not met, showing that insufficient information has been accrued.
We found no intervention effect likewise for insomnia (Analysis 10.2: 3 studies, 150 participants; SMD ‐1.23, 95% CI ‐2.70 to 0.23; GRADE: very low‐quality evidence) or discontinuation due to adverse events (Analysis 10.3: 2 studies, 120 participants; RR 2.10, 95% CI 0.20 to 22.26; GRADE: very low‐quality of evidence). The following outcomes were reported in one trial each: benzodiazepine discontinuation at longest follow‐up, showing no intervention effect (Analysis 10.4: 1 study, 38 participants; RR 1.03, 95% CI 0.47 to 2.27; GRADE: very low‐quality evidence), adverse events, with no intervention effect (Analysis 10.5: 1 study, 86 participants; RR 0.97, 95% CI 0.52 to 1.82; GRADE: very low‐quality evidence), and relapse to benzodiazepine use, also with no indication of intervention effect (Analysis 10.6: 1 study, 38 participants; RR 1.80, 95% CI 0.37 to 8.68; GRADE: very low‐quality evidence).
10.2. Analysis.
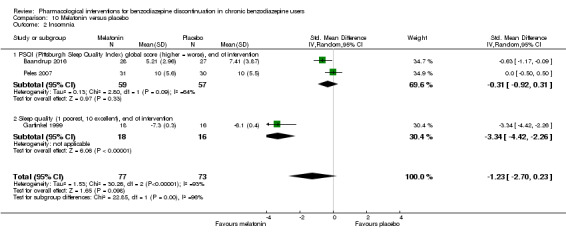
Comparison 10 Melatonin versus placebo, Outcome 2 Insomnia.
10.3. Analysis.
Comparison 10 Melatonin versus placebo, Outcome 3 Discontinuation due to adverse events.
10.4. Analysis.
Comparison 10 Melatonin versus placebo, Outcome 4 Benzodiazepine discontinuation, longest follow‐up.
10.5. Analysis.
Comparison 10 Melatonin versus placebo, Outcome 5 Adverse events.
10.6. Analysis.
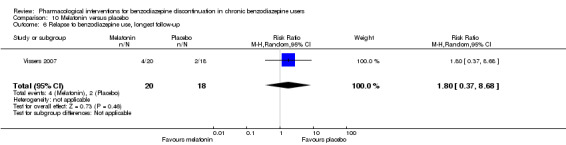
Comparison 10 Melatonin versus placebo, Outcome 6 Relapse to benzodiazepine use, longest follow‐up.
When evaluating insomnia, the results were associated with significant heterogeneity; this was explained by Garfinkel 1999, which showed a markedly significant result in favour of melatonin, whereas the two other the trials included in this meta‐analysis showed no or a much smaller difference between intervention groups (Baandrup 2016; Peles 2007). We could not identify any obvious reason for this observed heterogeneity, other than Garfinkel 1999 being the only one of these studies involved with a pharmaceutical company.
Please see Table 10.
Flumazenil versus placebo
Three small trials examined whether flumazenil can aid in benzodiazepine discontinuation (Gerra 1993; Gerra 2002; Harrison‐Read 1996). It was possible to perform a meta‐analysis for benzodiazepine withdrawal symptoms at end of intervention, which showed a beneficial effect of flumazenil (Analysis 11.1: 3 studies, 58 participants; SMD ‐0.95, 95% CI ‐1.71 to ‐0.19; GRADE: very low‐quality evidence). Due to marked differences in among‐participant variability, we did not re‐express the SMD using one of the specific measurement instruments. We could not calculate the required information size because the trials did not use the same instrument, and results were reported using SMD.
11.1. Analysis.
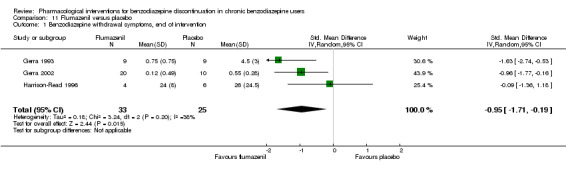
Comparison 11 Flumazenil versus placebo, Outcome 1 Benzodiazepine withdrawal symptoms, end of intervention.
The following outcomes were reported in one trial each: symptoms of anxiety, with results in favour of flumazenil (Analysis 11.2: 1 study, 18 participants; MD ‐1.30 points, 95% CI ‐2.28 to ‐0.32; GRADE: very low‐quality evidence) and benzodiazepine mean dose at end of intervention, with no difference between groups (Analysis 11.3: 1 study, 10 participants; MD ‐3.70 points, 95% CI ‐22.06 to 14.66; GRADE: very low‐quality evidence). As previously noted, one of the flumazenil studies was ended prematurely due to severe withdrawal symptoms elicited during the trial procedure (Harrison‐Read 1996).
11.2. Analysis.
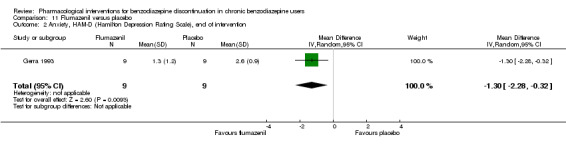
Comparison 11 Flumazenil versus placebo, Outcome 2 Anxiety, HAM‐D (Hamilton Depression Rating Scale), end of intervention.
11.3. Analysis.
Comparison 11 Flumazenil versus placebo, Outcome 3 Benzodiazepine mean dose, end of intervention.
When evaluating benzodiazepine withdrawal symptoms, the results were associated with significant heterogeneity for which no obvious reason could be identified.
Please see Table 11.
Propranolol versus placebo
One small trial evaluated propranolol versus placebo (Tyrer 1981). Only data on relapse to benzodiazepine use at end of intervention were available, showing no effect of the study intervention (Analysis 12.1: 1 study, 40 participants; RR 0.64, 95% CI 0.31 to 1.30; GRADE: very low‐quality evidence).
12.1. Analysis.
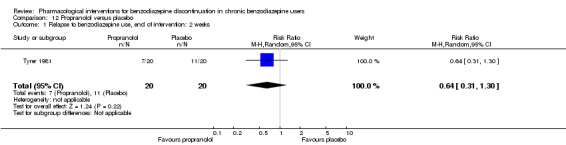
Comparison 12 Propranolol versus placebo, Outcome 1 Relapse to benzodiazepine use, end of intervention: 2 weeks.
Progesterone versus placebo
One small trial evaluated this comparison (Schweizer 1995), reporting no intervention effect on benzodiazepine discontinuation at end of intervention (Analysis 13.1: 1 study, 35 participants; RR 1.15, 95% CI 0.52 to 2.54; GRADE: very low‐quality evidence), and a difference between groups in favour of placebo for non‐serious adverse events (Analysis 13.2: 1 study, 35 participants; RR 3.13, 95% CI 1.15 to 8.54; GRADE: very low‐quality evidence).
13.1. Analysis.
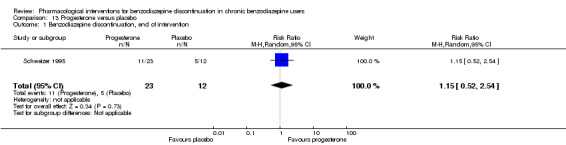
Comparison 13 Progesterone versus placebo, Outcome 1 Benzodiazepine discontinuation, end of intervention.
13.2. Analysis.
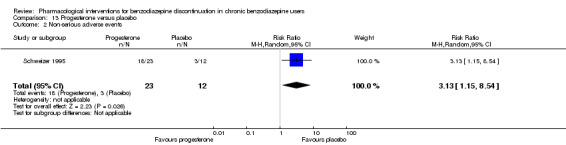
Comparison 13 Progesterone versus placebo, Outcome 2 Non‐serious adverse events.
For benzodiazepine discontinuation, we calculated the required information size to be 1918 participants, using a control event proportion of 48%, a relative risk reduction of 20%, type I error of 2.5%, power of 90%, and a diversity of 30%. We could not perform Trial Sequential Analysis as there was only one trial.
Please see Table 12.
Magnesium aspartate versus placebo
One moderately sized trial compared magnesium aspartate (a mineral supplement) with placebo (Hantouche 1998), and found a beneficial effect of placebo for benzodiazepine discontinuation (Analysis 14.1: 1 study, 144 participants; RR 0.80, 95% CI 0.66 to 0.96; NNTH 5.8; GRADE: very low‐quality evidence), but no difference between groups for symptoms of anxiety (Analysis 14.2: 1 study, 144 participants; MD ‐0.80 points, 95% CI ‐2.73 to 1.13; GRADE: very low‐quality evidence), benzodiazepine relapse (Analysis 14.3: 1 study, 144 participants; RR 0.93, 95% CI 0.46 to 1.87; GRADE: very low‐quality evidence), non‐serious adverse events (Analysis 14.4: 1 study, 144 participants; RR 0.49, 95% CI 0.18 to 1.35; GRADE: very low‐quality evidence), and discontinuation due to adverse events (Analysis 14.5: 1 study, 144 participants; RR 0.40, 95% CI 0.13 to 1.18; GRADE: very low‐quality evidence).
14.1. Analysis.
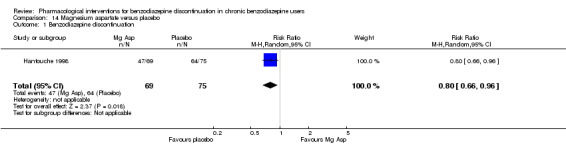
Comparison 14 Magnesium aspartate versus placebo, Outcome 1 Benzodiazepine discontinuation.
14.2. Analysis.
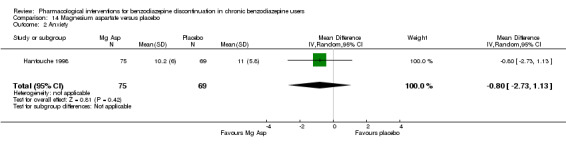
Comparison 14 Magnesium aspartate versus placebo, Outcome 2 Anxiety.
14.3. Analysis.
Comparison 14 Magnesium aspartate versus placebo, Outcome 3 Relapse to benzodiazepine use.
14.4. Analysis.
Comparison 14 Magnesium aspartate versus placebo, Outcome 4 Non‐serious adverse events.
14.5. Analysis.
Comparison 14 Magnesium aspartate versus placebo, Outcome 5 Discontinuation due to adverse events.
For benzodiazepine discontinuation, we calculated the required information size to be 1918 participants, using a control event proportion of 48%, a relative risk reduction of 20%, type I error of 2.5%, power of 90%, and a diversity of 30%. We could not perform Trial Sequential Analysis because there was only one trial.
Please see Table 13.
Homeopathic drugs versus placebo
One small trial compared two homeopathic drugs ("Homéogène 46" and "Sédatif PC") versus placebo (Cialdella 2001), from which it was only possible to extract data on benzodiazepine discontinuation. When combining the homeopathic drugs as one experimental group versus placebo, the results showed no intervention effect (Analysis 15.1: 1 study, 51 participants; RR 0.79, 95% CI 0.36 to 1.70; GRADE: very low‐quality evidence).
15.1. Analysis.
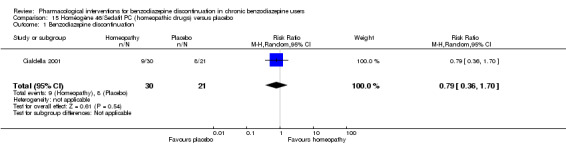
Comparison 15 Homéogène 46/Sedatif PC (homeopathic drugs) versus placebo, Outcome 1 Benzodiazepine discontinuation.
For benzodiazepine discontinuation, we calculated the required information size to be 1918 participants, using a control event proportion of 48%, a relative risk reduction of 20%, type I error of 2.5%, power of 90%, and a diversity of 30%. We could not perform Trial Sequential Analysis as there was only one trial.
Please see Table 14.
Carbamazepine versus tricyclic antidepressant (tianeptine)
Only one trial examined this comparison (Kornowski 2002), finding no additional effect of carbamazepine compared with tricyclic antidepressant for benzodiazepine discontinuation at end of intervention (Analysis 16.1: 1 study, 48 participants; RR 1.00, 95% CI 0.78 to 1.29; GRADE: low‐quality evidence) and relapse to benzodiazepine use (Analysis 16.2: 1 study, 48 participants; RR 1.00, 95% CI 0.28 to 3.54; GRADE: low‐quality evidence). Data on other outcomes specified in this review were not available.
16.1. Analysis.
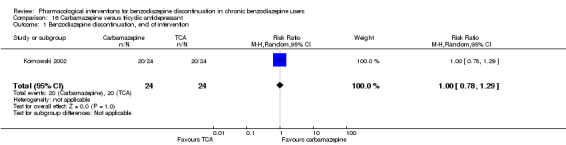
Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 1 Benzodiazepine discontinuation, end of intervention.
16.2. Analysis.
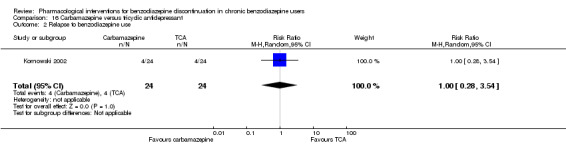
Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 2 Relapse to benzodiazepine use.
Please see Table 15.
Bromazepam versus cyamemazine
One moderately sized trial examined bromazepam versus cyamemazine (a first‐generation antipsychotic drug of the phenothiazine class with anxiolytic efficacy) (Lemoine 2006), reporting a difference in favour of cyamemazine for relapse to benzodiazepine use (Analysis 17.1: 1 study, 124 participants; RR 0.33, 95% CI 0.14 to 0.78; GRADE: very low‐quality evidence), but no difference between groups for symptoms of anxiety (Analysis 17.2: 1 study, 160 participants; MD 0.50 points, 95% CI ‐1.23 to 2.23) or discontinuation due to adverse events (Analysis 17.3: 1 study, 160 participants; RR 2.87, 95% CI 0.79 to 10.44; GRADE: very low‐quality evidence). We identified a difference in favour of bromazepam for non‐serious adverse events (Analysis 17.4: 1 study, 160 participants; RR 1.68, 95% CI 1.01 to 2.78; GRADE: very low‐quality evidence).
17.1. Analysis.
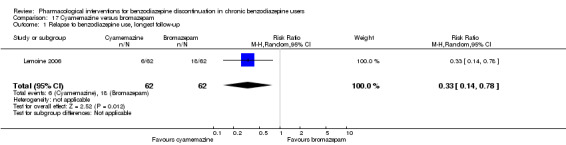
Comparison 17 Cyamemazine versus bromazepam, Outcome 1 Relapse to benzodiazepine use, longest follow‐up.
17.2. Analysis.
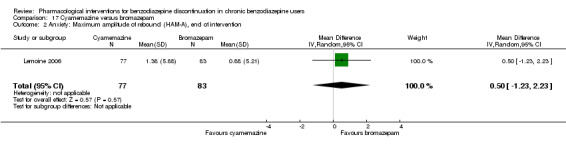
Comparison 17 Cyamemazine versus bromazepam, Outcome 2 Anxiety: Maximum amplitude of rebound (HAM‐A), end of intervention.
17.3. Analysis.
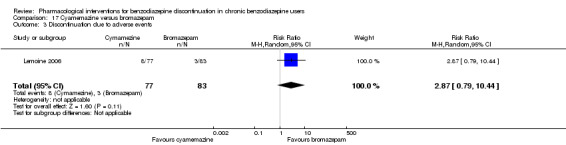
Comparison 17 Cyamemazine versus bromazepam, Outcome 3 Discontinuation due to adverse events.
17.4. Analysis.
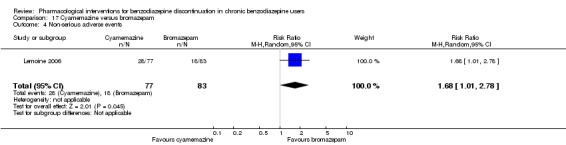
Comparison 17 Cyamemazine versus bromazepam, Outcome 4 Non‐serious adverse events.
Zopiclone versus flunitrazepam
One small trial examined zopiclone (a short‐acting benzodiazepine‐like drug) versus flunitrazepam (a potent intermediate‐acting benzodiazepine, which in many countries is no longer in use due to severe side effects) (Pat‐Horenczyk 1998), finding no indication of intervention effect for relapse to benzodiazepine use (Analysis 18.1: 1 study, 18 participants; RR 1.05, 95% CI 0.23 to 4.78; GRADE: very low‐quality evidence). Data on other outcomes specified in this review were not available.
18.1. Analysis.
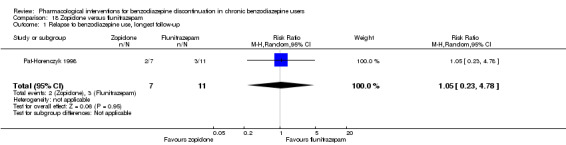
Comparison 18 Zopiclone versus flunitrazepam, Outcome 1 Relapse to benzodiazepine use, longest follow‐up.
Ondansetron, atenolol, and gabapentin
As described above, we were not able to extract data from three trials: Romach 1998 (ondansetron versus placebo), Saul 1989 (atenolol versus placebo), and Mariani 2016 (gabapentin versus placebo). None of these trials reported any effect on applied outcome measures of the respective experimental drug.
Adverse events
In general, adverse events were insufficiently reported, making it difficult to reliably assess the tolerability and safety of the investigated compounds.
Discussion
Summary of main results
We included 38 trials in this review, but were able to extract data from only 35 trials investigating a total of 18 different comparisons for the primary outcome of benzodiazepine discontinuation. Valproate and tricyclic antidepressants seemed to have a potentially positive effect on benzodiazepine discontinuation, whereas withdrawal symptoms seemed to be potentially ameliorated by pregabalin, captodiame, paroxetine, tricyclic antidepressants, and flumazenil. The following pharmacological agents seemed to potentially reduce symptoms of anxiety: carbamazepine, pregabalin, captodiame, paroxetine, and flumazenil. However, flumazenil seemed to be associated with a high risk of precipitating a severe withdrawal syndrome, since one of the trials was prematurely ended due to observation of unacceptable adverse events (severe panic reactions). Alpidem seemed to worsen both the probability of discontinuing benzodiazepines and the intensity of withdrawal symptoms and should not be further investigated for this use. Likewise, magnesium aspartate seemed to decrease the proportion of participants discontinuing benzodiazepines.
Overall completeness and applicability of evidence
A plethora of different drugs have been investigated in a number of small trials, many of which are of questionable quality, poorly reported, and thus difficult to extract data from. The data set is therefore not complete, but nevertheless judged by the review authors to give a full representation of the current body of evidence in this area. A substantial proportion of included trials were initiated and sponsored by the pharmaceutical industry.
Quality of the evidence
We generally rated the quality of the evidence as very low or low, representing small studies of generally poor methodology and poor reporting. Especially as many of the trials were of older date, modern standards of design and reporting were not fulfilled. As a result, the conclusions of this review should be considered tentative at best. Nonetheless, the review provides an overview of the current status of evidence and points to future directions for research on the development of pharmacotherapies for benzodiazepine dependence.
Potential biases in the review process
Some of the authors of this review also authored one of the included trials (Baandrup 2016). Data extraction and risk of bias assessment of this particular trial was done by LB and JR, the latter of whom was not involved in the trial in any way. The trial examined melatonin for benzodiazepine discontinuation. The meta‐analysis showed no benefit of melatonin for any of the reported outcomes, and thus we do not believe that our involvement in one the included trials in any way biased the results.
In our protocol, we planned to only focus our assessments on randomised controlled trials (Baandrup 2015). By doing so, we are well aware of the fact that we put an overemphasis on potential beneficial effects of the assessed interventions and ran the risks of overlooking harms. The reasons for these considerations are that harms are generally not well reported in randomised controlled trials, and that observational studies are usually needed to detect rare‐ and late‐occurring adverse events (Ioannidis 2004; Ioannidis 2009).
We did not search relevant databases of regulatory authorities as this was not planned in our protocol. We could therefore have overlooked relevant trials that have not been published in the usual literature. In all likelihood, such trials are at high risk of showing neutral or negative intervention effects, but for completeness of literature searches such databases of regulatory authorities need to be searched in future updates of this review (Schroll 2015).
Agreements and disagreements with other studies or reviews
A number of other reviews have been published on similar topics. A Cochrane Review investigating pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings in 35 studies pointed to a potential value of carbamazepine, but concluded that larger, controlled studies were needed (Denis 2006). The current review confirms the potential value of carbamazepine, but the body of evidence is too weak to translate this into a clinical recommendation.
Voshaar 2006 found that augmentation of systematic benzodiazepine discontinuation with imipramine was superior to systematic discontinuation alone. This conclusion is in agreement with the current review, although we could only identify a possible beneficial effect of tricyclic antidepressant on benzodiazepine discontinuation at longest follow‐up, based on data from a single trial. Furthermore, we report a potential favour of tricyclic antidepressants regarding benzodiazepine withdrawal symptoms, but this was also based on only one trial with results that generally seemed biased.
In Parr 2009, gradual dose reduction plus substitutive pharmacotherapies was compared with gradual dose reduction alone, restricted to general practice and outpatient settings. An evaluation of benzodiazepine cessation rate in 18 studies found promise for a few substitutive pharmacotherapies (melatonin, paroxetine, trazodone, and valproate), but concluded that the current evidence was insufficient to support their use. Our review, which included several more studies, adds further value to the suggestion of further investigating this topic, potentially involving paroxetine (and/or other selective serotonin reuptake inhibitors (SSRIs)) and valproate.
Darker 2015 examined psychosocial interventions for benzodiazepine withdrawal, finding a short‐term beneficial effect of cognitive behavioural therapy that did not last beyond three months. Motivational interviewing had no effect on benzodiazepine discontinuation. Other promising interventions investigated in single trials included a tailored letter from the general practitioner, standardised interview, and relaxation technique. The evidence base for using non‐pharmacological interventions to facilitate benzodiazepine use is unfortunately thus not much more convincing than the evidence gathered in the current review on pharmacological interventions.
Authors' conclusions
Implications for practice.
The current systematic review with meta‐analysis and Trial Sequential Analysis could not find any pharmaceutical add‐on to facilitate the withdrawal process. Some drugs seem to be associated with beneficial effects, but the quality of the evidence was too low to lead to any clinical recommendations.
Implications for research.
Future research of the potential of pharmacological agents to facilitate benzodiazepine withdrawal should focus on drugs with a potential to benefit the patients according to the results of the current meta‐analysis and limit the different pharmacological interventions evaluated. Such randomised controlled trials should be assessed versus adequate placebo or nocebo to provide proper blinding and be designed according to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (Chan 2015), and reported according to the CONSORT guidelines (Schulz 2011).
Acknowledgements
The authors thank Zuzana Mitrova for conducting the literature searches. The authors thank the following people for their help in data extraction and 'Risk of bias' assessment of the non‐English language reports: Kasia Kalinowska (Kornowski 2002), Xue Jie Song (Zhang 2013), and Ileana Codruta Nielsen (Di Costanzo 1992).
Appendices
Appendix 1. Search strategy for Cochrane Drugs and Alcohol Group's Specialised Register of Trials
1. ((benzodiazepine* ):xdi) AND (INREGISTER)
2. ((((benzodiazepine* OR chlordiazepoxide OR diazepam OR alprazolam OR lorazepam OR prazepam OR clobazam OR bromazepam OR flurazepam OR triazolam OR clonazepam OR temazepam OR nitrazepam OR lormetazepam OR flunitrazepam OR oxazepam OR zopiclone OR zolpidem OR zaleplone OR eszopiclone) NEAR3 (abuse* OR abusing OR addict* OR chronic OR dependen* OR 'long‐term' OR 'misus* OR overuse)))) AND (INREGISTER)
3. #1 OR #2
4. (((abstinen* OR abstain* OR cessat* OR detox* OR discontinu* OR reduce* OR reducing OR reduct* OR stop* OR taper* OR withdraw* OR substitut*))) AND (INREGISTER)
5. #3 AND #4
Appendix 2. Search strategy for the Cochrane Central Register of Controlled Trials (CENTRAL)
1. MeSH descriptor: [Substance‐Related Disorders] this term only
2. (benzodiazepine* near (abuse* or abusing or addict* or chronic or dependen* or 'long‐term' or 'misus* or overuse)):ti,ab,kw (Word variations have been searched)
3. MeSH descriptor: [Substance Withdrawal Syndrome] this term only
4. #1 or #2 or #3
5. (benzodiazepine* or BZD or chlordiazepoxide or diazepam or alprazolam or lorazepam or prazepam or clobazam or bromazepam or flurazepam or triazolam or clonazepam or temazepam or nitrazepam or lormetazepam or flunitrazepam or oxazepam or zopiclone or zolpidem or zaleplone or eszopiclone):ti,ab,kw (Word variations have been searched)
6. MeSH descriptor: [Benzodiazepines] explode all trees
7. #5 or #6
8. (abstinen* or abstain* or cessat* or detox* or discontinu* or reduce* or reducing or reduct* or stop* or taper* or withdraw* or substitut*):ti,ab,kw (Word variations have been searched)
9. #4 and #7 and #8 in Trials
Appendix 3. PubMed search strategy
1. "Substance‐Related Disorders"[Mesh]
2. abuse*[tiab] OR abusing[tiab] OR addict*[tiab] OR chronic[tiab] OR dependen*[tiab] OR “long‐term”[tiab] OR misus*[tiab] OR overuse[tiab]
3. "Substance Withdrawal Syndrome"[Mesh]
4. #1 OR #2 OR #3
5. "Benzodiazepines"[Mesh]
6. Benzodiazepine*[tiab] OR BZD[tiab] OR chlordiazepoxide[tiab] OR diazepam[tiab] OR alprazolam[tiab] OR lorazepam[tiab] OR prazepam[tiab] OR clobazam[tiab] OR bromazepam[tiab] OR flurazepam[tiab] OR triazolam[tiab] OR clonazepam[tiab] OR temazepam[tiab] OR nitrazepam[tiab] OR lormetazepam[tiab] OR flunitrazepam[tiab] OR oxazepam[tiab] or zopiclone[tiab] OR zolpidem[tiab] OR zaleplone[tiab] OR eszopiclone[tiab]
7. #5 OR #6
8. abstinen*[tiab] OR abstain*[tiab] OR cessat*[tiab] OR detox*[tiab] OR discontinu*[tiab] OR reduce*[tiab] OR reducing[tiab] OR reduct* [tiab] OR stop*[tiab] OR taper*[tiab] OR withdraw*[tiab] OR substitut*[tiab]
9. randomised controlled trial [pt]
10. controlled clinical trial [pt]
11. randomised [tiab]
12. placebo [tiab]
13. clinical trials as topic [mesh: noexp]
14. randomly [tiab]
15. trial [ti]
16. #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
17. animals [mh] NOT humans [mh]
18. #16 NOT #17
19. #4 AND #7 AND #8 AND #18
Appendix 4. Search strategy for Embase
1. substance abuse'/exp OR 'drug dependence'/exp
2. (benzodiazepine* NEAR/6 (abuse* OR abusing OR addict* OR chronic OR dependen* OR 'long‐term' OR 'misus* OR overuse)):ab,ti
3. withdrawal syndrome'/exp
4. #1 OR #2 OR #3
5. benzodiazepine derivative'/exp
6. enzodiazepine*:ab,ti OR BZD:ab,ti OR chlordiazepoxide:ab,ti OR diazepam:ab,ti OR alprazolam:ab,ti OR lorazepam:ab,ti OR prazepam:ab,ti OR clobazam:ab,ti OR bromazepam:ab,ti OR flurazepam:ab,ti OR triazolam:ab,ti OR clonazepam:ab,ti OR temazepam:ab,ti OR nitrazepam:ab,ti OR lormetazepam:ab,ti OR flunitrazepam:ab,ti OR oxazepam:ab,ti OR zopiclone:ab,ti OR zolpidem:ab,ti OR zaleplone:ab,ti OR eszopiclone:ab,ti
7. #5 OR #6
8. abstinen*:ab,ti OR abstain*:ab,ti OR cessat*:ab,ti OR detox*:ab,ti OR discontinu*:ab,ti OR reduce*:ab,ti OR reducing:ab,ti OR reduct* :ab,ti OR stop*:ab,ti OR taper*:ab,ti OR withdraw*:ab,ti OR substitut*:ab,ti
9. #4 AND #7 AND #8
10. 'randomised controlled trial'/exp
11. 'single blind procedure'/exp
12. 'double blind procedure'/exp
13. 'crossover procedure'/exp
14. #10 OR #11 OR #12 OR #13
15. random*:ab,ti
16. placebo*:ab,ti
17. allocat*:ab,ti
18. crossover*:ab,ti
19. 'cross over':ab,ti
20. trial:ti
21. (doubl* NEXT/1 blind*):ab,ti
22. #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21
23. #14 OR #22
24. 'animal'/de
25. 'animal experiment'/de
26. nonhuman'/de
27. #24 OR #25 OR #26
28. 'human'/de
29. #27 AND #28
30. #27 NOT #29
31. #23 NOT #30
32. #9 AND #31
Appendix 5. Search strategy for CINAHL
1. (MH "Substance Use Disorders+")
2. TX (benzodiazepine* N6 (abuse* or abusing or addict* or chronic or dependen* or 'long‐term' or 'misus* or overuse))
3. TI ( (benzodiazepine* N6 (abuse* or abusing or addict* or chronic or dependen* or 'long‐term' or 'misus* or overuse)) ) OR AB ( (benzodiazepine* N6 (abuse* or abusing or addict* or chronic or dependen* or 'long‐term' or 'misus* or overuse)) )
4. (MH "Substance Withdrawal Syndrome+")
5. S1 OR S2 OR S3 OR S4
6. TI ( benzodiazepine* or BZD or chlordiazepoxide or diazepam or alprazolam or lorazepam or prazepam or clobazam or bromazepam or flurazepam or triazolam or clonazepam or temazepam or nitrazepam or lormetazepam or flunitrazepam or oxazepam or zopiclone or zolpidem or zaleplone or eszopiclone ) OR AB ( benzodiazepine* or BZD or chlordiazepoxide or diazepam or alprazolam or lorazepam or prazepam or clobazam or bromazepam or flurazepam or triazolam or clonazepam or temazepam or nitrazepam or lormetazepam or flunitrazepam or oxazepam or zopiclone or zolpidem or zaleplone or eszopiclone )
7. (MH "Antianxiety Agents, Benzodiazepine+")
8. S6 OR S7
9. TX (abstinen* or abstain* or cessat* or detox* or discontinu* or reduce* or reducing or reduct* or stop* or taper* or withdraw* or substitut*)
10. S5 AND S8 AND S9
11. MH "Clinical Trials+"
12. PT Clinical trial
13. TI clinic* N1 trial* or AB clinic* N1 trial*
14. TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* )
15. AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* )
16. TI randomi?ed control* trial* or AB randomi?ed control* trial*
17. MH "Random Assignment"
18. TI random* allocat* or AB random* allocat*
19. MH "Placebos"
20. TI placebo* or AB placebo*
21. MH "Quantitative Studies"
22. S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21
23. S10 AND S22
24. S10 AND S22Exclude MEDLINE records
Appendix 6. Search strategy for Web of Science
1. TS=((benzodiazepine* OR chlordiazepoxide OR diazepam OR alprazolam OR lorazepam OR prazepam OR clobazam OR bromazepam OR flurazepam OR triazolam OR clonazepam OR temazepam OR nitrazepam OR lormetazepam OR flunitrazepam OR oxazepam OR zopiclone OR zolpidem OR zaleplone OR eszopiclone) NEAR/6 (abuse* OR abusing OR addict* OR chronic OR dependen* OR 'long‐term' OR 'misus* OR overuse))
2. TOPIC: (abstinen* OR abstain* OR cessat* OR detox* OR discontinu* OR reduce* OR reducing OR reduct* OR stop* OR taper* OR withdraw* OR substitut*)
3. #2 AND #1
4. TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
5. #4 AND #3
Appendix 7. Criteria for risk of bias
| Item | Judgement | Description |
| 1. Random sequence generation (selection bias) | Low risk | The investigators describe a random component in the sequence generation process such as: random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimisation. |
| Unclear risk | Insufficient information about the sequence generation process to permit judgement of low or high risk. | |
| High risk | The investigators describe a non‐random component in the sequence generation process such as: odd or even date of birth; date (or day) of admission; hospital or clinic record number; alternation; judgement of the clinician; results of a laboratory test or a series of tests; availability of the intervention. | |
| 2. Allocation concealment (selection bias) | Low risk | Investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes. |
| Unclear risk | Insufficient information to permit judgement of low or high risk. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definitive judgement. | |
| High risk | Investigators enrolling participants could possibly foresee assignments because one of the following methods was used: open random allocation schedule (e.g. a list of random numbers); assignment envelopes without appropriate safeguards (e.g. if envelopes were unsealed or nonopaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| 3. Blinding of participants and providers (performance bias) | Low risk | Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. Placebo should be identical to the intervention regarding appearance, colour, solubility, taste, and smell. Or no blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding. |
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| High risk | No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding. Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. |
|
| 4.Blinding of outcome assessor (detection bias) | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| High risk | No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding. OR Blinding of outcome assessment, but it is likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. |
|
| 5. Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias). Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size. Missing data have been imputed using appropriate methods, e.g. multiple imputation. All randomised participants are reported/analysed in the group to which they were allocated by randomisation irrespective of non‐compliance and co‐interventions (intention to treat). |
| Unclear risk | Insufficient information to permit judgement of low or high risk (e.g. number randomised not stated, no reasons for missing data provided; number of dropouts not reported for each group). | |
| High risk | Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups. For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate. For continuous outcome data, plausible effect size (difference in means or standardised difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size. ‘As‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation. |
|
| 6. Selective reporting (reporting bias) | Low risk | The trial protocol is available and all of the trial’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way. The trial protocol is not available, but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon). |
| Unclear risk | Insufficient information to permit judgement of low or high risk. | |
| High risk | Not all of the trial’s prespecified primary outcomes have been reported. One or more primary outcomes are reported using measurements, analysis methods, or subsets of the data (e.g. subscales) that were not prespecified. One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect). One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. The trial report fails to include results for a key outcome that would be expected to have been reported for such a trial. |
|
| 7. Other bias including industry bias | Low risk | The trial appears to be free of other components that could put it at risk of bias. |
| Unclear risk | The trial may or may not be free of other components that could put it at risk of bias. | |
| High risk | There are other factors in the trial that could put it at risk of bias, in particular the risk of industry bias will be evaluated. |
Data and analyses
Comparison 1. Valproate versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [1.08, 6.03] |
| 2 Relapse to benzodiazepine use, end of intervention | 1 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.11, 0.90] |
| 3 Benzodiazepine discontinuation, longest follow‐up | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.80, 3.09] |
| 4 Relapse to benzodiazepine use, longest follow‐up | 1 | 24 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.39] |
| 5 Anxiety: HAM‐A (Hamilton Anxiety Rating Scale), end of intervention | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐6.47, 5.67] |
| 6 Benzodiazepine withdrawal symptoms, end of intervention | 2 | 56 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.68, 0.37] |
| 6.1 Physician Withdrawal Checklist | 1 | 27 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.77, 0.74] |
| 6.2 CIWA‐B (Clinical Institute Withdrawal Assessment Scale ‐ Benzodiazepines) | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐1.01, 0.45] |
| 7 Discontinuation due to adverse events | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Serious adverse events | 1 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.7. Analysis.
Comparison 1 Valproate versus placebo or no intervention, Outcome 7 Discontinuation due to adverse events.
1.8. Analysis.
Comparison 1 Valproate versus placebo or no intervention, Outcome 8 Serious adverse events.
Comparison 2. Carbamazepine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 3 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.99, 1.80] |
| 2 Benzodiazepine withdrawal symptoms | 2 | 76 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐2.43, 0.16] |
| 2.1 Physician Withdrawal Checklist | 1 | 36 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐2.61, ‐1.03] |
| 2.2 Patient Withdrawal Checklist | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.13, 0.13] |
| 3 Benzodiazepine discontinuation, longest follow‐up | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.86, 2.29] |
| 4 Relapse to benzodiazepine use | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.08, 1.44] |
| 5 Serious adverse events | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Non‐serious adverse events | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.39, 126.48] |
| 7 Anxiety, HAM‐A | 1 | 36 | Mean Difference (IV, Random, 95% CI) | ‐6.0 [‐9.58, ‐2.42] |
| 8 Discontinuation due to adverse events | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.5. Analysis.
Comparison 2 Carbamazepine versus placebo, Outcome 5 Serious adverse events.
2.8. Analysis.
Comparison 2 Carbamazepine versus placebo, Outcome 8 Discontinuation due to adverse events.
Comparison 3. Lithium versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.86, 1.28] |
| 2 Serious adverse events | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Non‐serious adverse events | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.49] |
| 4 Discontinuation due to adverse events | 1 | 230 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.13, 15.03] |
3.2. Analysis.
Comparison 3 Lithium versus placebo, Outcome 2 Serious adverse events.
Comparison 4. Pregabalin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.92, 2.25] |
| 2 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐3.1 [‐3.51, ‐2.69] |
| 3 Anxiety, HAM‐A, end of intervention | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐4.8 [‐5.28, ‐4.32] |
| 4 Serious adverse events | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.16, 2.85] |
| 5 Non‐serious adverse events | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.84, 1.40] |
| 6 Discontinuation due to adverse events | 1 | 106 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.31, 2.59] |
Comparison 5. Captodiame versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine withdrawal symptoms, BWSQ (Benzodiazepine Withdrawal Symptom Questionnaire), end of intervention | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.00 [‐1.13, ‐0.87] |
| 2 Anxiety, HAM‐A, end of intervention | 1 | 81 | Mean Difference (IV, Random, 95% CI) | ‐5.7 [‐6.05, ‐5.35] |
| 3 Serious adverse events | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Non‐serious adverse events | 1 | 81 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
5.3. Analysis.
Comparison 5 Captodiame versus placebo, Outcome 3 Serious adverse events.
5.4. Analysis.
Comparison 5 Captodiame versus placebo, Outcome 4 Non‐serious adverse events.
Comparison 6. Paroxetine versus placebo or no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.88, 2.39] |
| 2 Benzodiazepine withdrawal symptoms: BWSQ, end of intervention | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐3.57 [‐5.34, ‐1.80] |
| 3 Anxiety: HAM‐A, end of intervention | 2 | 99 | Mean Difference (IV, Random, 95% CI) | ‐6.75 [‐9.64, ‐3.86] |
| 4 Benzodiazepine withdrawal symptoms: BWSQ, longest follow‐up: 6 months | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐4.03, 3.77] |
| 5 Serious adverse events | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Non‐serious adverse events | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.35, 5.03] |
6.5. Analysis.
Comparison 6 Paroxetine versus placebo or no intervention, Outcome 5 Serious adverse events.
Comparison 7. Tricyclic antidepressants versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.52, 1.28] |
| 2 Anxiety: HAM‐A (change from baseline), end of intervention | 2 | 66 | Mean Difference (IV, Random, 95% CI) | ‐10.38 [‐25.96, 5.20] |
| 3 Benzodiazepine discontinuation, longest follow‐up | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [1.27, 3.82] |
| 4 Benzodiazepine withdrawal symptoms (Physician Withdrawal Checklist), end of intervention | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐19.78 [‐20.25, ‐19.31] |
| 5 Relapse to benzodiazepine use, end of intervention | 1 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.73, 5.47] |
| 6 Discontinuation due to adverse events | 2 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.42, 3.21] |
Comparison 8. Alpidem versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.17, 0.99] |
| 2 Withdrawal syndrome (clinical diagnosis), end of intervention | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 4.86 [1.12, 21.14] |
| 3 Anxiety, HAM‐A, end of intervention | 2 | 170 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐4.64, 1.45] |
| 4 Relapse to benzodiazepine use, end of intervention | 1 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.20] |
| 5 Serious adverse events | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Discontinuation due to adverse events | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.05, 4.46] |
8.5. Analysis.
Comparison 8 Alpidem versus placebo, Outcome 5 Serious adverse events.
8.6. Analysis.
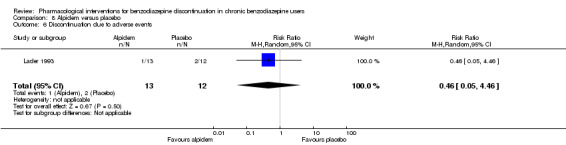
Comparison 8 Alpidem versus placebo, Outcome 6 Discontinuation due to adverse events.
Comparison 9. Buspirone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 4 | 143 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.49, 1.37] |
| 2 Anxiety: HAM‐A/Hospital Anxiety Depression Scale, end of intervention | 2 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.50, 0.86] |
| 3 Benzodiazepine withdrawal symptoms, end of intervention | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 4.69 [‐14.47, 23.85] |
| 4 Benzodiazepine discontinuation, longest follow‐up | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.34, 1.05] |
| 5 Benzodiazepine withdrawal symptoms, longest follow‐up | 1 | 15 | Mean Difference (IV, Random, 95% CI) | ‐1.34 [‐14.31, 11.63] |
| 6 Anxiety, Hospital Anxiety Depression Scale, longest follow‐up | 1 | 12 | Mean Difference (IV, Random, 95% CI) | 2.75 [‐2.83, 8.33] |
| 7 Discontinuation due to adverse events | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.92] |
9.7. Analysis.
Comparison 9 Buspirone versus placebo, Outcome 7 Discontinuation due to adverse events.
Comparison 10. Melatonin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 4 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.73, 1.96] |
| 2 Insomnia | 3 | 150 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.70, 0.23] |
| 2.1 PSQI (Pittsburgh Sleep Quality Index) global score (higher = worse), end of intervention | 2 | 116 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.92, 0.31] |
| 2.2 Sleep quality (1 poorest, 10 excellent), end of intervention | 1 | 34 | Std. Mean Difference (IV, Random, 95% CI) | ‐3.34 [‐4.42, ‐2.26] |
| 3 Discontinuation due to adverse events | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 2.10 [0.20, 22.26] |
| 4 Benzodiazepine discontinuation, longest follow‐up | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.47, 2.27] |
| 5 Adverse events | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.82] |
| 6 Relapse to benzodiazepine use, longest follow‐up | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.8 [0.37, 8.68] |
Comparison 11. Flumazenil versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine withdrawal symptoms, end of intervention | 3 | 58 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.71, ‐0.19] |
| 2 Anxiety, HAM‐D (Hamilton Depression Rating Scale), end of intervention | 1 | 18 | Mean Difference (IV, Random, 95% CI) | ‐1.3 [‐2.28, ‐0.32] |
| 3 Benzodiazepine mean dose, end of intervention | 1 | 10 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐22.06, 14.66] |
Comparison 12. Propranolol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse to benzodiazepine use, end of intervention: 2 weeks | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.31, 1.30] |
Comparison 13. Progesterone versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.52, 2.54] |
| 2 Non‐serious adverse events | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 3.13 [1.15, 8.54] |
Comparison 14. Magnesium aspartate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.66, 0.96] |
| 2 Anxiety | 1 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐2.73, 1.13] |
| 3 Relapse to benzodiazepine use | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.46, 1.87] |
| 4 Non‐serious adverse events | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.18, 1.35] |
| 5 Discontinuation due to adverse events | 1 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.13, 1.18] |
Comparison 15. Homéogène 46/Sedatif PC (homeopathic drugs) versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.36, 1.70] |
Comparison 16. Carbamazepine versus tricyclic antidepressant.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Benzodiazepine discontinuation, end of intervention | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.78, 1.29] |
| 2 Relapse to benzodiazepine use | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.28, 3.54] |
| 3 Serious adverse events | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
16.3. Analysis.
Comparison 16 Carbamazepine versus tricyclic antidepressant, Outcome 3 Serious adverse events.
Comparison 17. Cyamemazine versus bromazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse to benzodiazepine use, longest follow‐up | 1 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.14, 0.78] |
| 2 Anxiety: Maximum amplitude of rebound (HAM‐A), end of intervention | 1 | 160 | Mean Difference (IV, Random, 95% CI) | 0.50 [‐1.23, 2.23] |
| 3 Discontinuation due to adverse events | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 2.87 [0.79, 10.44] |
| 4 Non‐serious adverse events | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [1.01, 2.78] |
Comparison 18. Zopiclone versus flunitrazepam.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Relapse to benzodiazepine use, longest follow‐up | 1 | 18 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.23, 4.78] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ashton 1990.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks Single‐centre |
|
| Participants |
Baseline characteristics Buspirone
Placebo
Inclusion criteria: above 18 years of age, continuous benzodiazepine therapy for a minimum of 6 months, wish to withdraw from benzodiazepines Exclusion criteria: use of other psychotropic medication, abuse of alcohol or drugs, major psychiatric or physical disease Pretreatment group differences: Mean benzodiazepine dosage at baseline was 15.5 mg in buspirone group and 7.5 mg in placebo group. |
|
| Interventions | Benzodiazepine taper schedule: all participants switched to an equivalent dose of diazepam, stable dosage for 4 weeks, then taper with 25% each week for 4 weeks to 0, then 4 weeks without benzodiazepines.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Bristol Myers CNS provided buspirone and placebo tablets and covered laboratory and administrative expenses. Country: UK Setting: Outpatients, participants referred from their GP, rapid benzodiazepine tapering regimen Declarations of interest: Not mentioned Author's name: Ashton CH Institution: Department of Pharmacological Sciences, University of Newcastle upon Tyne, NE2 4HH Email: Address: Department of Pharmacological Sciences, University of Newcastle upon Tyne, NE2 4HH |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly assigned..." Comment: Not further described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: The study was carried out double‐blind using matching placebo tablets. Quote: "double‐blind...either buspirone or matching placebo tablets..." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: No actions to adjust for high dropout (64%) in the intervention group compared with the placebo group (8%). |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available, but no reason to suspect selective outcome reporting |
| Other bias | Unclear risk | Comment: The role of Bristol Myers insufficiently described. |
Baandrup 2016.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 24 weeks Single‐centre |
|
| Participants |
Baseline characteristics Pronlonged‐release melatonin (PRM)
Placebo
Inclusion criteria: Age 18 years or above, an ICD‐10 diagnosis of schizophrenia (F20), schizoaffective disorder (F25), or bipolar disorder (F31). Bipolar patients were required to be euthymic a the time of inclusion. Treatment with antipsychotic drug(s) for at least 3 months before inclusion, treatment with 1 or more benzodiazepine derivatives or benzodiazepine‐related drugs for at least 3 months before inclusion, fertile women: negative pregnancy test at baseline and the use of safe contraceptives (intrauterine devices or hormonal contraception) throughout the trial period, written informed consent. Exclusion criteria: Known aggressive or violent behaviour, mental retardation, pervasive developmental disorder, or dementia, epilepsy, terminal illness, severe somatic comorbidity, or inability to understand Danish, allergy to compounds in the trial medication (melatonin, lactose, starch, gelatine, and talc), hepatic impairment, pregnancy or nursing, lack of informed consent. Pretreatment group differences: None |
|
| Interventions | Benzodiazepine taper schedule: gradual reduction of usual benzodiazepine dosage (including benzodiazepine‐related drugs) at an approximate rate of 10% to 20% every second week.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: The Research Fund of the Mental Health Services of the Capital Region in Denmark financed the trial with a post doc grant and a grant for external randomisation and database management. Country: Denmark Setting: Mainly outpatients Declarations of interest: None Author's name: Baandrup L Institution: Centre for Neuropsychiatric Schizophrenia Research (CNSR) & Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), University of Copenhagen, Mental Health Centre Glostrup, Mental Health Services – Capital Region of Denmark, Glostrup Email: lone.baandrup@regionh.dk Address: Mental Health Centre Glostrup, Mental Health Services – Capital Region of Denmark, DK‐2600 Glostrup |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Central randomisation was performed by the Copenhagen Trial Unit (CTU) with computer‐generated, permuted randomisation allocation sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation sequence and block sizes were kept unknown to the investigator. Allocation ratio was 1:1. The investigator contacted the CTU and provided a personal pin code, participant civil registration number, participant trial identification number, and the value of the stratification variable of benzodiazepine dosage (low (15 mg diazepam equivalents) or high (15 mg diazepam equivalents)) at baseline. Then the randomisation was announced as a trial medication container number and confirmation sent by e‐mail" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Thus, the placebo was matched to the study medication for taste, smell, colour, size and solubility. CTU held the randomisation code and the trial was not unblinded until all data were registered, primary analyses finished and conclusions drawn" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Trial participants, staff, and outcome assessors were blinded to the allocated treatment. We maintained blinding using matching placebo and an independent unit to perform the randomisation and do the packaging and labelling of the trial medication. Both PRM and placebo were encapsulated in lactose‐ containing gelatine capsules to optimise the blinding" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Data complete for the primary outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: Primary outcome, etc. reported in published trial protocol. |
| Other bias | Low risk | Comment: No other apparent source of bias |
Cassano 1996.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks (4 weeks double‐blind followed by 2 weeks single‐blind placebo) Multicentre |
|
| Participants |
Baseline characteristics Alpidem
Placebo
Inclusion criteria: Outpatients with generalised anxiety disorder (GAD; DSM‐III‐R, item 300.02) or adjustment disorder with anxious mood (DSM‐III‐R, item 309.24). Consecutive patients of either sexes, aged between 18 and 60 years, taking non‐hypnotic benzodiazepines for anxiety as continuous course of therapy of at least 1 year duration, at a dose schedule corresponding to 30 mg or less of diazepam per day, were considered eligible. Exclusion criteria: Montgomery–Åsberg Depression Rating Scale was administered to exclude depressed patients (total score > 18). Pretreatment: No significant differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: all benzodiazepines abruptly discontinued at inclusion.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: No information Country: Italy Setting: Outpatients Declarations of interest: Not mentioned Author's name: Cassano GB Institution: Clinica Psichiatrica, University degli Studi di Pisa Email: Address: Clinica Psichiatrica, University degli Studi di Pisa, Ospedale Santa Chiara, Via Roma 67, 56100 Pisa |
|
| Notes | The study lasted 6 weeks: a 4‐week comparative period (phase I) to prevent and treat benzodiazepine withdrawal symptoms (primary aim) was followed by a 2‐week single‐blind period with placebo (phase II) to monitor the occurrence of withdrawal symptoms after abrupt discontinuation of alpidem (secondary aim). 6 weeks was chosen as endpoint because alpidem is a Z‐drug. According to the review protocol, such studies are included if data are available on relevant outcomes AFTER withdrawal of the new benzodiazepine/Z‐drug, in this case after discontinuation of alpidem. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The Italian multicentre (15 centres), double‐blind, randomised (versus placebo), parallel group study" Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The diagnosis of withdrawal symptoms was made by a respected academic expert, in blind conditions, on the basis of the definition in the protocol" Comment: Done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 10 (11.5%) discontinued in the alpidem group, 18 (21%) in the placebo group. |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol published but could not be retrieved. No reason to suspect selective outcome reporting |
| Other bias | Unclear risk | Comment: Source of financing not described. |
Cialdella 2001.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 1 month Single‐centre |
|
| Participants |
Baseline characteristics Homéogène
Sédatif PC
Placebo
Inclusion criteria: At least 18 years of age, at least 3 months use of benzodiazepines at low dosage (max 10 mg/day diazepam equivalents), clinically stable for at least 1 month Exclusion criteria: Severe insomnia, severe psychiatric disorders, alcohol or substance abuse disorder, previous seizures, current use of muscle relaxants, clonidine, or psychotropic drugs. Pretreatment: Higher scores on somatic symptoms in Homéogène group |
|
| Interventions | Benzodiazepines substituted (no taper schedule) with study drug:
Both experimental drugs were homeopathic drugs. |
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Laboratoires Boiron, l'Agence Nationale de Valirisation de la Recherce Country: France Setting: Outpatients Declarations of interest: Not mentioned Author's name: Cialdella P Institution: Service de Pharmacologie Clinique, Faculté RTH Laënnec Email: Address: |
|
| Notes | Homéogène and Sédatif groups were combined as 1 homeopathic drug group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Described as double‐blind, but lacks a description of what have been done to ensure blinding of participants and study personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described. Insufficient information to permit judgement of low or high risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 25% attrition. An ITT approach was used, but distribution of attrition between groups was not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: No obvious selective outcome reporting |
| Other bias | Unclear risk | Comment: Role of funding source not described, both industry and publicly funded. |
Di Costanzo 1992.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 4 weeks Single‐centre |
|
| Participants |
Baseline characteristics Carbamazepine
Placebo
Inclusion criteria: > 60 years of age, GAD, benzodiazepine abuse, minimum duration of benzodiazepine treatment 6 months Exclusion criteria: None described. Pretreatment: No significant pretreatment differences |
|
| Interventions | Benzodiazepine taper schedule: 25% benzodiazepine dose reduction every week
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not reported Country: Italy Setting: Outpatients Declarations of interest: Not mentioned Author's name: Di Constanzo E Institution: Servizio Psichiatrico Email: Address: Viale Spellanzon, 55 31015 Conegliano |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Described as double‐blind, but what has been done to ensure blinding of participants and study personnel is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information to permit judgement of low or high risk |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 4 (26.6%) and 3 (21.4%) participants did not complete benzodiazepine cessation but participated in the study. |
| Selective reporting (reporting bias) | Low risk | Comment: No indication of selective outcome reporting |
| Other bias | Low risk | Comment: No apparent other source of bias |
Garfinkel 1999.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks double‐blind, 6 weeks single‐blind Single‐centre |
|
| Participants |
Baseline characteristics Controlled‐release melatonin
Placebo
Inclusion criteria: People with a daily use of benzodiazepines for more than 6 months, expressed willingness to discontinue the use, living independently Exclusion criteria: Cognitive impairment, liver or renal disorders Pretreatment: No significant differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: participants were encouraged to reduce their usual benzodiazepine therapy dosage 50% during week 2, 75% during weeks 3 and 4, and then to discontinue benzodiazepine therapy completely during weeks 5 and 6. Participants who did not succeed in stopping benzodiazepine therapy during period 1 were encouraged to further reduce benzodiazepine dosage 50%, 75%, and 100% during weeks 8, 9 and 10, 11 and 12, respectively.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Neurim Pharmaceuticals sponsored study medication and study nurse; statistical evaluations performed independently. Country: Israel Setting: Outpatients, living independently Declarations of interest: Not mentioned Authors name: Doron Garfinkel Institution: Department of Neurobiochemistry, Tel Aviv University Email: Navazis@ccsg.tau.ac.il Address: Tel Aviv 69978, Israel |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects were randomised to receive either 2 mg of CRM therapy or a placebo that was identical in appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Collection and entry of all data were completed before revealing the randomisation codes of the study" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All included participants analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: No indications of selective reporting |
| Other bias | High risk | Comment: The trial was partly financed by a company with an interest in given result, the company's role in interpreting the data is not sufficiently described. |
Gerra 1993.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group, stratifies for flunitrazepam/lormetazepam at baseline Blinding: Single Duration: 7 days Single‐centre |
|
| Participants |
Baseline characteristics Not reported Inclusion criteria: 18 to 40 years of age, flunitrazepam abuse at a dose of 10 to 12 mg/day (18 participants) or lormetazepam abuse at a dose of 8 to 10 mg/day (18 participants). All participants met the criteria of the DSM‐III‐R for benzodiazepine withdrawal syndrome. Abuse was defined as use for at least 9 months. Informed consent was obtained from all participants. Excluded criteria: Psychiatric patients were not included in the study. Daily urine samples were taken to rule out the abuse of morphine, methadone, cocaine, amphetamine, barbiturates, cannabis, and ethanol during the study. Pretreatment: Not reported |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: abrupt cessation
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not reported Country: Italy Setting: Inpatients Declarations of interest: Not mentioned Author's name: Gilberto Gerra Institution: University of Parma Email: Address: USL n. 4, Via Guasti S. Cecilia, 3, Parma 43100, Italy |
|
| Notes | Results were reported separately for flunitrazepam and lormetazepam users. To avoid including several comparisons from the same study, we only included results for the lormetazepam users in this meta‐analysis (flunitrazepam is now very seldom used in clinical practice and in many countries is no longer registered for use). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Placebo groups B and D were only treated with saline solution for 7 days." Comment: Only participants were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Study only described as single‐blinded, therefore probably not done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All participants analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: No indication of selective outcome reporting |
| Other bias | Unclear risk | Comment: Insufficient information regarding sponsorship |
Gerra 2002.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Single Duration: 8 days Single‐centre |
|
| Participants |
Baseline characteristics Flumazenil IV
Oxazepam tapering
Placebo
Inclusion criteria: History of benzodiazepine dependence according to DSM‐IV criteria. Exclusion criteria: Severe chronic liver or renal diseases or other chronic physical disorders, recent onset of significant weight loss or gain, endocrinopathies, neurological disorders, immunopathy, in particular HIV disease, a positive family history of cardiovascular disease and hypertension, current abuse of illicit drugs and alcohol Pretreatment: None in reported parameters |
|
| Interventions |
Intervention characteristics All participants received high doses of oxazepam (120 mg/day) during the last week before detoxification (pretreatment week).
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not mentioned Country: Italy Setting: Inpatients Declarations of interest: Not mentioned Author's name: Gilberto Gerra Institution: Addiction Research Center, Ser.T., AUSL, Parma, Italy Email: gerra@polaris.it Address: Gilberto Gerra, Centro Studi Farmacotossicodipendenze, Ser.T., A.U.S.L., Via Spalato 2,43100 Parma, Italy |
|
| Notes | Only the comparison between flumazenil and placebo was considered relevant and included in the meta‐analysis, cf. Cochrane Handbook on multiple comparisons. Rate of relapse NOT reported for the placebo group because: (quote) Long‐term outcome of group C (placebo) patients was not evaluated in comparison with A and B patients because they received low‐dose benzodiazepine treatment for 2 weeks, immediately after the detoxification procedure, for ethical reasons. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described Quote: "The study was single‐blind, randomised and placebo‐controlled." |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "the trial was single‐blind, permitting direct clinical interventions in the case of dramatic withdrawal symptoms" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Though not clearly described, judging from the text it appears that no participants withdrew during the 8‐day intervention trial. |
| Selective reporting (reporting bias) | Low risk | Comment: No reason to suspect selective outcome reporting |
| Other bias | Unclear risk | Comment: Funding not described. |
GlaxoSmithKline 2002.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks Multicentre |
|
| Participants |
Baseline characteristics Paroxetine
Placebo
Inclusion criteria: Participants were males or females aged > 18 years suffering from 1 or more of the following anxiety disorders of non‐severe degree in axis I: panic attack disorder (with or without agoraphobia), GAD, social anxiety/social phobia or mixed anxiety and depression disorder with significant anxiety; people continuously treated with benzodiazepines (any) for at least 6 consecutive months prior to the screening visit at doses between 2 and 8 mg/day of lorazepam or equivalent; a total score ≤ 16 on the HAM‐A and MADRS at screening and baseline. Exclusion criteria: People suffering (or diagnosed within the 6 months prior to screening) from 1 or more of the following conditions: major depressive episode; post‐traumatic stress disorder; obsessive‐compulsive disorder; eating behavioural disorders, people diagnosed with dysthymia or who had suffered from dysthymia in the 6 months prior to screening; people with a concomitant psychotic disorder, or history of psychotic disorder; people having a concomitant bipolar disorder or history of bipolar disorder, or having a cyclothymic disorder, or had suffered from it in the past; people who met DSM‐IV (protocol appendix O) criteria for substance (alcohol or drugs) abuse or dependence, except for benzodiazepine, within 6 months prior to screening; current suicidal or homicidal risk; and people who had electroconvulsive therapy in the 3 months prior to screening. Pretreatment: No significant group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 4‐week open‐label run‐in period during which participants were switched from their original benzodiazepine to an equivalent dosage of chlordemethyldiazepam (between 2 and 8 mg/d). The taper schedule during the treatment phase not described.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: GlaxoSmithKline Country: Italy Setting: Outpatients Declarations of interest: Not mentioned Comments: Unpublished phase III study Author's name: GlaxoSmithKline Institution: Email: Address: Clinical Study Register (www.gskclinicalstudyregister.com) 2002 |
|
| Notes | Change scores extracted, final scores not available. Standard deviation calculated from CI using the following formula: SE = (upper limit – lower limit of CI)/3.92 Standard deviation σ = standard error x √n |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "12‐week double blind, multicentre, randomised, placebo‐controlled, parallel group" Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects were randomised to either paroxetine or placebo and entered the 12‐week double‐blind, randomised treatment phase. Dosage of paroxetine or matched placebo started with..." Comment: Double‐blind and using matched placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 versus 8 participants withdrew, but ITT analysis data extracted for this meta‐analysis. |
| Selective reporting (reporting bias) | High risk | Comment: No protocol available, benzodiazepine dose at follow‐up not described. |
| Other bias | High risk | Comment: Study funded by the study drug manufacturer, no information available on involvement in design, data collection, etc. |
Hadley 2012.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks (6 weeks during tapering and 6 weeks post‐tapering) Multicentre |
|
| Participants |
Baseline characteristics Pregabalin
Placebo
Inclusion criteria: Adult outpatients aged 18 to 65 years were enrolled if they met DSM‐IV criteria for a primary lifetime diagnosis of GAD, and if they were receiving stable treatment with a benzodiazepine in daily doses of 1 to 4 mg/day (in alprazolam dose equivalents) for 8 to 52 weeks. A primary diagnosis of GAD was made, based on predominant clinical presentation, using the module P form of the Mini International Neuropsychiatric Interview (MINI)‐Plus version 5.0.0 (Sheehan et al, 1997). The current diagnosis of GAD could be sub‐threshold due to treatment. Exclusion criteria: (1) women who were pregnant, lactating, or of childbearing potential who were not using a medically approved form of contraception; (2) 17‐item HAM‐D total score > 15; (3) a history of anxiolytic non‐response to benzodiazepines or pregabalin, or hypersensitivity to either class of drug; (4) they met DSM‐IV criteria in the past 6 months of major depressive disorder, dysthymia, social phobia, post‐traumatic stress disorder, body dysmorphic disorder, or eating disorder; (5) met DSM‐IV criteria in the past 5 years of schizophrenia, psychotic disorder, bipolar affective disorder, obsessive–compulsive disorder, substance dependence (excluding nicotine), or in the past year for substance abuse; (6) currently receiving cognitive behavioural therapy for GAD or other anxiety disorder; (7) a history of seizure disorder, except febrile seizures of childhood; (8) a history of neuropathic pain or narrow angle glaucoma; (9) receiving treatment with fluoxetine (in past 5 weeks) or any psychotropic other than benzodiazepines (in past 2 weeks), or electroconvulsive therapy (in past 6 months); (10) positive urine drug screen for amphetamines, barbiturates, ethanol, narcotics, non‐benzodiazepine sedatives and hypnotics, cocaine, phencyclidine, cannabinoids or other illegal or illicit drugs; (11) considered by the investigator to be at risk for suicide or aggressive behaviour; (12) any serious or uncontrolled medical illness in the opinion of the investigator that would render the person unsuitable for the study; or (13) creatinine clearance 60 mL/min. Pretreatment: None |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: switch to equivalent dose alprazolam, 2‐week stabilisation phase before randomisation, 25% reduction per week, permitted up to 6 weeks to complete the alprazolam taper, after maintained 6 weeks on double‐blind treatment, then 1 week taper off study medication
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Funded by Pfizer Country: 20 investigational sites in Spain, Mexico, France, Italy, Costa Rica, the Czech Republic, and Guatemala Setting: Outpatients Declarations of interest: Dr Schweizer was at the time of the writing of the manuscript employee of Paladin Consulting Group Inc., which was a paid consultancy to Pfizer Inc. At the time the study was conducted and the paper was initially drafted, Dr Sallie J Hadley was an employee of Pfizer Inc. and owns stock in Pfizer. Dr Francine S Mandel was a full‐time employee of Pfizer Inc. Dr Edward Schweizer owns stock in Pfizer and has received payments for consulting and/or medical writing services from Alkermes, Bristol‐Myers Squibb, Sumitomo Dainippon Pharma, Eli Lilly, Memory Pharmaceuticals, Neurocrine Biosciences, and Pfizer Inc. Author's name: Sallie J Hadley Institution: Pfizer Inc., New York, NY, USA Email: francine.mandel@pfizer.com Address: Francine S Mandel, Pfizer Inc., 235 East 42nd Street, New York, NY, USA |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients...were randomised on a one‐to‐one basis to 12 weeks of double‐blind treatment with either pregabalin or placebo" Comment: Described as "double‐blind" but what has been done to ensure blinding of participants and study personnel is not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: High attrition rate in both the pregabalin group (46.4%) and the placebo group (62.0%) |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | High risk | Comment: Study funded by Pfizer, no indication of role of funding body in design, data collection, analysis, and interpretation of the data. |
Hantouche 1998.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 3 months Multicentre |
|
| Participants |
Baseline characteristics Magnesium aspartate
Placebo
Inclusion criteria: Outpatients, 18 to 65 years of age, chronic users of lorazepam, alprazolam, or bromazepam (> 6 months, regular dose => 3 mg lorazepam equivalents), benzodiazepines prescribed due to an anxious disorder now in remission defined as score on Hamilton Anxiety < 14 and Raskin‐Depression < 6, no major psychiatric disorder, at least 1 trial of unsuccessful benzodiazepine withdrawal, a wish to discontinue benzodiazepine use Exclusion criteria: Severe hepatic or renal dysfunction, alcohol or substance use disorder, currently trying to discontinue use of tobacco, current psychotherapy, use of other psychotropics within 6 months, treatment with a magnesium salt or calcium within 1 month, regular use of magnesium aspartate during 1 month within the last 6 months Pretreatment: No significant pretreatment group differences |
|
| Interventions | Benzodiazepine taper schedule: co‐administration of benzodiazepine and study drug for 1 month, gradual taper of benzodiazepine during the next month (50% of dosage for 2 weeks, 25% for 2 weeks, then stop), follow‐up during a third month after complete benzodiazepine discontinuation
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not reported Country: France Setting: Outpatients Declarations of interest: Not mentioned Author's name: Hantouche EG Institution: Département de Psychiatrie, Groupe Hospitaliers de la Pitrie‐Salpetriere Email: Address: 47, Boulevard de l'Hopital, 75013 Paris |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Described as double‐blind, what has been done to ensure blinding of participants and study personnel is not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Not described |
| Selective reporting (reporting bias) | Low risk | Comment: No indications of reporting bias |
| Other bias | Low risk | Comment: No apparent other sources of bias |
Harrison‐Read 1996.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 3 weeks Single‐centre |
|
| Participants |
Baseline characteristics Flumazenil IV challenge
Placebo
Inclusion criteria: People were recruited to the study if they had been taking benzodiazepines in usual therapeutic doses (< 30 mg per day of diazepam or equivalent) for 3 months or more, and if they had experienced withdrawal problems on discontinuing medication. Exclusion criteria: (i) regular intake of any other psychotropic medication, (ii) a diagnosis of schizophrenia, epilepsy, or cardiorespiratory disease Pretreatment: No significant pretreatment differences |
|
| Interventions |
Intervention characteristics
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Roche Products Ltd supplied unmarked ampoules of flumazenil and vehicle solution and a grant towards the cost of the project. Country: UK Setting: Outpatients (inpatients when receiving flumazenil challenge) Declarations of interest: Not mentioned Comments: The study was approved by the local ethics committee but, owing to the unexpectedly severe reactions shown in some participants, it was felt to be unethical to continue with the study after 10 participants had been tested using the original protocol. Author's name: Harrison‐Read PE Institution: Academic Unit of Psychiatry, St Charles Hospital, Exmoor Street, London W10 6DZ Email: Address: Academic Unit of Psychiatry, St Charles Hospital, Exmoor Street, London W10 6DZ |
|
| Notes | Study discontinued due to unacceptable adverse effects (marked panic reaction in the 4 participants who received flumazenil), beginning within 30 seconds of the end of the injection. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "High risk and low risk subjects were allocated separately at random to placebo or flumazenil challenge by an independent pharmacist." Comment: Description of how the sequence was generated was insufficient. |
| Allocation concealment (selection bias) | Low risk | Comment: Allocation was done by independent pharmacist. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "This ’challenge test’ was carried out double‐blind, with both subject and experimenter being unaware of the identify of the substance being injected" Comment: Described as double‐blind and using placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Immediately afterwards, the subject began filling in the BWSQ and the MRS, and then repeated these measures at 5, 15, 25, 35, 45 and 60 min post‐injection. Pulse and blood pressure were recorded as before" Comment: Description is insufficient to judge the risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All randomised participants analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: No reason to suspect selective outcome reporting |
| Other bias | High risk | Comment: As the reaction to acute challenge with flumazenil proved to be unexpectedly severe, the study was stopped after only 10 participants had been recruited for the study: 4 were allocated to the flumazenil group and 6 to the placebo group. Despite separately randomising high‐ and low‐risk participants, the early cessation of the study led to unequal distribution between the 2 treatment groups: 1 out of the 4 participants in the flumazenil group and 3 out of the 6 in the placebo group were high‐risk participants. In addition, the study was supported by a company. |
Klein 1994.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: Approximately 5 weeks (dependent on duration of taper phase) Single‐centre |
|
| Participants |
Baseline characteristics Carbamazepine: not available Placebo: not available Inclusion criteria: DSM‐III‐R diagnosis of panic disorder or generalised anxiety disorder Exclusion criteria: 1) Lifetime history of psychotic disorder, 2) Bipolar disorder, 3) Seizure disorder, 4) Severe head trauma, 5) Major depression, 6) Abuse of alcohol or other substances, 7) Obsessive‐compulsive disorder, 8) PTSD, 9) Pregnancy, 10) Active systemic illness with chronic medication Pretreatment: Reported to be non‐significant but not reported for the carbamazepine versus placebo group, only reported for the panic disorder versus the GAD group |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 25% every third day
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Supported by the Upjohn Company Country: Israel Setting: Outpatients Declarations of interest: Not mentioned Author's name: Ehud Klein Institution: Rambam Medical Center and University of Vermont Email: Address: Rambam Medical Center, Rapapport Faculty of Medicine, Technion‐IIT, Bat Galim, Haifa, Israel |
|
| Notes | Baseline characteristics for the carbamazepine versus placebo group were not reported, only for panic disorder group versus generalised anxiety disorder group. Same problem when reporting the results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described in detail, but it is stated that randomisation was stratified by diagnosis and alprazolam daily dosage |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients entered the controlled portion of the study and were randomly assigned, in a double‐blind fashion, to receive either carbamazepine or placebo as adjunctive treatment...In order to maintain blindness of the study throughout the taper period, patients received a fixed number of capsules with a gradually increasing proportion of identical placebo capsules substituting for the alprazolam" Comment: The use of carbamazepine versus placebo (the primary interest for the current review) was double‐blinded with identical placebo. The alprazolam taper was single‐blind, but these data are not considered here. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Very high dropout rates (56% vs 71%), and no ITT analysis performed. |
| Selective reporting (reporting bias) | High risk | Comment: No reporting on benzodiazepine dosage or withdrawal symptoms |
| Other bias | High risk | Comment: Role of supporting company not described |
Kornowski 2002.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 28 days Single‐centre |
|
| Participants |
Baseline characteristics Carbamazepine
Tianeptine
Inclusion criteria: ICD‐10 criteria for benzodiazepine dependence, 18 to 65 years of age Exclusion criteria: Previously treated with 1 or both of the experimental drugs, psychotic symptoms, not treated with other psychotropic drugs until 2 weeks before inclusion, pregnant or nursing, substance abuse, severe somatic illness Pretreatment: No significant pretreatment differences |
|
| Interventions | Benzodiazepines substituted with
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not mentioned Country: Poland Setting: Inpatients Declarations of interest: Not mentioned Comments: No data reported for the outcomes, only overall results from statistical analyses. Author's name: Kornowski J Institution: Psychiatric Hospital in Starogard Gdansk Email: kornowski@dobrynet.pl Address: 83‐200 Starogard Gdansk |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Blinding not described, not possible to judge whether participants and personnel were blinded, also it is not stated if the study was open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 4 (17%) dropped out in each group because they ingested benzodiazepines during the trial(detected by urine screen), but all participants were included in the statistical analyses. |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent reporting bias |
| Other bias | Unclear risk | Comment: No apparent other sources of bias |
Lader 1987.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks Single‐centre |
|
| Participants |
Baseline characteristics Only reported for the total sample: men: 41.7%; age: 39.1 years; years of benzodiazepine use: 8.4 years Inclusion criteria: More than 6 months of benzodiazepine use, physically dependent, no requirements of further benzodiazepine treatment as deemed by mental state assessment Exclusion criteria: Abuse of alcohol or other drugs Pretreatment: Not described |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 2 weeks on unchanged dosage, 2 weeks on halved benzodiazepine dosage, 2 weeks with no benzodiazepines (followed by 2 weeks with placebo in both groups and 2 weeks with no study medication)
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not mentioned Country: UK Setting: Outpatients Declarations of interest: Not mentioned Authors name: Malcolm Lader Institution: Institute of Psychiatry, University of London Email: Address: Institute of Psychiatry, University of London, De Crespigny Park, Denmark Hill, London, SE5 8AF England |
|
| Notes | For reasons that are unclear, results are reported at week 3, i.e. after the first week of benzodiazepine reduction to half. That is, results are not available for week 6, when benzodiazepines have been tapered off. Figure 2 shows the temporal pattern for Hamilton Anxiety Scale (i.e. all time points available graphically) but only for the successful completers (5 buspirone, 6 placebo). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "During the first two weeks of withdrawal (3 and 4), buspirone or placebo was substituted for the benzodiazepine in an initial dosage of 5 mg (one capsule) twice daily, followed by 10 mg (two capsules) twice daily...The study was conducted double‐blind in that neither investigator nor patient knew whether placebo or buspirone was being administered during weeks 2 to 5" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: It is stated that "investigators" were blinded. Judged as done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Successful completers: no attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Comment: No data on benzodiazepine dosage in the 2 groups, but the trial was designed to stop benzodiazepine use, and therefore dose reduction was not considered. However, the choice of using 3 weeks as primary time point does not seem justified. |
| Other bias | Low risk | Comment: No other apparent source of bias |
Lader 1993.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 8 weeks Single‐centre |
|
| Participants |
Baseline characteristics Alpidem (a Z‐drug)
Placebo
Inclusion criteria: Benzodiazepine use for more than 6 months, less than 30 mg/d diazepam equivalents, regarded as dependent (problems on previous attempts to lower the dosage), 18 to 65 years of age, within 20% of normal body weight Exclusion criteria: Major physical or psychiatric illness, drug abusers, women of child‐bearing age unless on adequate contraception Pretreatment: Not described |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 2 weeks on unchanged dosage, 2 weeks on halved benzodiazepine dosage, 2 weeks with no benzodiazepines (followed by 2 weeks with halved dosage study medication and 2 weeks with no study medication)
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not described Country: UK Setting: Outpatients Declarations of interest: Not mentioned Author's name: Lader M Institution: Institute of Psychiatry Email: Address: Institute of Psychiatry, De Crespigny Park, Denmark Hill, London SES 8AF, UK |
|
| Notes | Anxiety and benzodiazepine withdrawal symptoms: the results are only shown in graphic as mean values, SDs not reported. SDs for HAM‐A were therefore imputed from Cassano 1996, which is a similar trial also using alpidem to facilitate benzodiazepine withdrawal. It was not possible to impute SDs for benzodiazepine withdrawal symptoms because withdrawal symptoms in Cassano 1996 were reported as a dichotomised variable, whereas they were reported as a continuous variable in Lader 1993. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The study was conducted double‐blind in that neither investigators nor patients knew whether placebo or alpidem was being administered during weeks 3‐8" Comment: Study described as double‐blinded and using placebo. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: It is stated that "investigators" were blinded. Judged as done. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: High dropout, however only completion could be extracted from the study, and this is not biased by the high dropout rate. |
| Selective reporting (reporting bias) | Low risk | Comment: No indications of selective reporting |
| Other bias | Low risk | Comment: No other apparent bias |
Lecrubier 2005.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 16 weeks Multicentre |
|
| Participants |
Baseline characteristics Lithium
Placebo
Inclusion criteria: Outpatients, 18 to 65 years old, receiving benzodiazepines for at least 6 months at a daily dose ranging from 10 to 40 mg diazepam or equivalent and wishing to withdraw benzodiazepine treatment Exclusion criteria: Anxiety disorder with a score of 15 or above on the HAM‐A, major depressive disorder, social phobia, alcohol or substance abuse according to Mini International Neuropsychiatric Interview, and/or other serious pathology. Tranquilisers including antihistamines, hypnotics, anxiolytics, and lithium salts were not allowed. Pretreatment: No significant pretreatment group differences |
|
| Interventions | Benzodiazepine taper schedule: 4 weeks stable benzodiazepine and lithium versus placebo, 4 weeks benzodiazepine withdrawal ‐ reduction with 50% every week, 8 weeks lithium maintenance
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not described Country: France Setting: Outpatients Declarations of interest: Not mentioned Author's name: Lecrubier Institution: Inserm unité 302, service de psychiatrie AD Email: lecru@ext.jussieu.fr Address: Hôpital Pitié‐Salpêtrière, 17, boulevard de l’hôpital, 75013 Paris, France |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "double‐blind, randomised study" Comment: Not sufficiently described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Lithium gluconate and placebo were dispensed in vials and were indistinguishable in terms of appearance, taste and smell" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 244 participants were randomised: 146 to lithium and 98 to placebo. Only participants entering the benzodiazepine tapering phase were analysed (136 participants allocated to lithium and 94 to placebo), thus attrition rate of 7% and 4%, respectively. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Benzodiazepine dose at endpoint not reported, only participants who succeeded in discontinuing benzodiazepine usage. |
| Other bias | Low risk | Comment: No other apparent source of bias |
Lemoine 2006.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 4 weeks Multicentre |
|
| Participants |
Baseline characteristics Bromazepam
Cyamemazine
Inclusion criteria: Participants were aged 18 to 65 years, treated for anxiety for at least 3 months with benzodiazepines (bromazepam, lorazepam, alprazolam, or oxazepam) at a daily dose of 5 to 20 mg diazepam‐equivalent, and requiring a withdrawal. A < 18 score in the Hamilton Anxiety Rating Scale was required. Exclusion criteria: Female patients were excluded if they were pregnant or likely to become so or if they were breastfeeding. Individuals incapable of completing a questionnaire or of properly giving informed consent were also excluded. In addition, current treatment with any psychotropic drug or any other central nervous system active medication was forbidden. The presence of comorbid depression was also an exclusion criterion. Pretreatment: NS |
|
| Interventions |
Intervention characteristics
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not described. 1 of the authors affiliated with Sanofi‐Aventis. Country: France Setting: Outpatients Declarations of interest: Not mentioned Author's name: Patrick Lemoine Institution: Unite´ Clinique de Psychiatrie Biologique, Bron Email: garayperso@aol.com Address: 46bis rue Gallie´ni, 91360 Villemoisson‐sur‐Orge, France |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both drugs were administered in identical soft gelatin capsules" Comment: Sufficient blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT analysis performed. |
| Selective reporting (reporting bias) | High risk | Comment: Protocol not available, unusual primary outcome (maximum amplitude of anxiety rebound). |
| Other bias | High risk | Comment: Role of Sanofi‐Aventis not described. |
Mariani 2016.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 8 weeks Single‐centre |
|
| Participants |
Baseline characteristics Gabapentin
Placebo
Inclusion criteria: Meeting DSM‐IV criteria for current benzodiazepine abuse or dependence and opioid dependence, and being treated for opioid dependence with methadone, 18 to 65 years of age Exclusion criteria: (1) Any Axis I psychiatric disorder as defined by DSM‐IV‐TR that was unstable or would be disrupted by study medication or by an effort to discontinue benzodiazepines; (2) Acute physiological withdrawal or a history of seizures during alcohol or sedative‐hypnotic withdrawal; (3) Individuals with cocaine dependence as their primary substance use disorder diagnosis; (4) Individuals with unstable physical disorders or impaired kidney function; (5) Prescribed psychotropic medications other than methadone or medications prescribed for pain syndromes that would be disrupted by study medication or by an effort to discontinue benzodiazepines; (6) Anticonvulsants prescribed for pain syndromes; (7) Known sensitivity to gabapentin; (8) Individuals who had exhibited suicidal or homicidal behaviour within the past 2 years or had current active suicidal ideation; (9) Individuals physiologically dependent on any other drugs (excluding nicotine, caffeine, methadone); (10) Individuals currently prescribed gabapentin; and (11) Individuals requiring pharmacological detoxification from benzodiazepines in the past year and are unlikely to be able to tolerate taper off of benzodiazepines Pretreament differences: None reported. |
|
| Interventions |
Intervention characteristics
|
|
| Outcomes | Benzodiazepine mean dose | |
| Identification |
Sponsorship source: Funding for this work was provided by National Institute on Drug Abuse grants K23‐ DA021209 (Mariani), P50‐DA09236 (Kleber), K24‐ DA022412 (Nunes), and K24 029647 (Levin). Country: USA Setting: Methadone maintenance outpatients Declarations of interest: None Authors name: John J Mariani Institution: Division on Substance Abuse, New York State Psychiatric Institute, New York, NY, USA Email: jm2330@columbia.edu Address: New York State Psychiatric Institute, Division on Substance Abuse, 1051 Riverside Drive, Unit 66, New York, NY 10032, USA |
|
| Notes | Data not reported sufficiently, not possible to extract results relevant to this review. The author has not responded to our queries. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation method not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: All capsules were over‐capsulated with riboflavin to ensure compliance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Only 50% were retained in the study. |
| Selective reporting (reporting bias) | Low risk | Comment: Selective outcome reporting not evident. |
| Other bias | Low risk | Comment: No other apparent source of bias |
Mercier‐Guyon 2004.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks (2 weeks taper off, 4 weeks assessment, ends day 45) Multicentre |
|
| Participants |
Baseline characteristics Captodiame
Placebo
Inclusion criteria: Participants aged 25 to 55 years who had been prescribed certain benzodiazepines (lorazepam, bromazepam, alprazolam, oxazepam, or clobazam) in the official recommended dose range for the treatment of an anxiety disorder for at least 6 months, stable benzodiazepine dosage over the 6‐month period. Since alertness was assessed with a driving simulation test, included participants were required to be in possession of a valid driving license for at least 5 years. Exclusion criteria: People with a history of alcohol dependence in the previous 5 years were excluded, as were those consuming excessive quantities of alcohol as defined in the CAGE questionnaire. Proven consumption (either openly declared or detected by urine testing) of illicit psychotropic drugs (opiates, cocaine, cannabis, amphetamines) or of any other sedatives also constituted grounds for exclusion. Additionally, people with severe, unstable, or uncontrolled hepatic, renal, or cardiac insufficiency, with glaucoma or prostate hypertrophy, or with any psychiatric disease other than generalised anxiety disorders were also excluded. Female individuals who were pregnant or breastfeeding were excluded. Pretreatment: No significant group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: half dose first week of experimental treatment, a quarter dose the second week, then discontinuation on day 14
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: This study was funded by Laboratoires Bailly‐Creat, Paris, France, manufacturers of captodiame (Covatine), who financed the honoraria of the participating physicians and the statistical analysis. Country: France Setting: Outpatients Declarations of interest: Not mentioned Comments: Benzodiazepine dose during and after discontinuation not recorded/documented. Authors name: Merzecier‐Guyon C Institution: Centre d’Etudes et de Recherches en Médecine du Trafic, Annecy, France Email: cermtcmg@wanadoo.fr Address: Dr C Mercier‐Guyon, CERMT, BP 132, 74004 ANNECY Cedex, France |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not sufficiently described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "randomised, double‐blind, placebo‐controlled trial" Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All randomised participants analysed. |
| Selective reporting (reporting bias) | Unclear risk | Quote: "we have little information available on real benzodiazepine use during the discontinuation and follow‐up phases, which is the most relevant measure of successful benzodiazepine" Comment: No information due to study design, not interpreted as being left out intentionally |
| Other bias | High risk | Quote: "This study was funded by Laboratoires Bailly‐Creat, Paris, France, manufacturers of captodiamine (Covatine), who financed the honoraria of the participating physicians and the statistical analysis." Comment: No indication of sponsor's influence on study analysis, etc.; interpreted as high risk of bias |
Morton 1995.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 16 weeks Single‐centre |
|
| Participants |
Baseline characteristics Not described Inclusion criteria: Referred to Benzodiazepine Withdrawal Clinic, benzodiazepines had been taken long term (> 6 months) at normal dose (< 30 mg/day of diazepam or equivalent), 18 to 70 years of age, body weight within normal limits Exclusion criteria: Major physical or psychiatric illnesses, drug abuse, women of childbearing age unless taking effective contraceptive measures Pretreatment: Not reported |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 4 weeks buspirone/placebo stabilisation, 6 weeks tapering to zero, 4 weeks of benzodiazepine abstinence, buspirone/placebo halved in dosage and then stopped 2 weeks later
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: This study was supported by a grant from Bristol‐Myers Squibb UK to the Institute of Psychiatry. Country: UK Setting: Outpatients Declarations of interest: Not mentioned Author's name: Morton S Institution: Institute of Psychiatry, University of London Email: Address: Institute of Psychiatry, De Crespigny Park, London SE5 8AFUK |
|
| Notes | Means only given in figures (HAM‐A and benzodiazepine withdrawal symptoms), no SDs reported, not possible to impute in a methodologically valid way. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Half the patients were given buspirone in flexible dosage according to the usual criteria of clinical need, at a minimum of 15 mg/day in divided doses. The others received matching placebo in equivalent flexible dosage, again according to apparent clinical need...The study was conducted double‐blind with reference to whether buspirone or placebo was being administered in weeks 2‐18" Comment: Done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: No risk of bias regarding the main outcome (benzodiazepine cessation), but the secondary outcomes were only analysed for the participants who had discontinued treatment, i.e. half of the participants. |
| Selective reporting (reporting bias) | Low risk | Comment: No reason to suspect selective outcome reporting |
| Other bias | High risk | Comment: Financed by a grant from Bristol‐Myers Squibb, but further information on potential influence on design, etc. not provided. |
Nakao 2006.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel Blinding: None, open‐label Duration: 8 weeks Single‐centre |
|
| Participants |
Baseline characteristics: Not reported Inclusion criteria: The participant selection criteria were as follows: (i) those aged 20 to 70 years; (ii) those whose medical condition was stable and drug regimens unchanged for longer than 3 months; (iii) those who had been prescribed either alprazolam, bromazepam, etizolam, or lorazepam for at least 3 months prior to visiting the clinic; and (iv) those who were able to visit the clinic for an 8‐week intervention (or control) period. Exclusion criteria: DSM‐IV major depression Pretreatment: No baseline characteristics provided. |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 8‐week gradual benzodiazepine discontinuation
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not described Country: Japan Setting: Outpatients Declarations of interest: Not mentioned Author's name: Nakao M Institution: Division of Psychosomatic Medicine, Teikyo University Hospital Email: mnakao@med.teikyo‐u.ac.jp Address: Department of Hygiene and Public Health, Teikyo University School of Medicine, 2‐11‐1 Kaga, Itabashi‐ku, Tokyo, Japan |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: No placebo: open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: Not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All randomised participants analysed. |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol provided, but all relevant outcomes seem to be reported. |
| Other bias | Unclear risk | Comment: No other apparent biases, the funding of the study not described. |
Pat‐Horenczyk 1998.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 4 weeks Single‐centre |
|
| Participants |
Baseline characteristics Zopiclone
Flunitrazepam
Inclusion criteria: Long‐term usage of benzodiazepine hypnotics (range 6 months to 22 years), use of flunitrazepam for at least 3 months with stabilisation at a nightly dosage of 1 mg for at least 1 month before inclusion Exclusion criteria: Other benzodiazepine consumption, use of psychotropic medications Pretreatment: No significant group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: during the first part of the study, participants were either switched gradually to 1) zopiclone (3.75 mg and then 7.5 mg) over a 2‐week period (N = 7), or 2) continued their usual treatment of flunitrazepam 1 mg (N = 11). In the second part of the trial, the hypnotic (either zopiclone or flunitrazepam) was gradually withdrawn according to a 2‐step scheme over 2 weeks. |
|
| Outcomes |
|
|
| Identification |
Sponsorship source: This study was supported by Rhone‐Poulenc Rorer Ltd, France, and the Technion Sleep Medicine Center, Israel Country: Israel Setting: Outpatients Declarations of interest: Not mentioned Authors name: Ruth Pat‐Horenczyk Institution: Technion Sleep Laboratory, Faculty of Medicine Email: Address: Technion Sleep Laboratory, Faculty of Medicine, Gutwirth Building, Technion‐Israel Institute of Technology, Haifa 32000, Israel |
|
| Notes | Data from the benzodiazepine withdrawal questionnaires were not reported, thus only data on benzodiazepine relapse and insomnia could be extracted from this trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind randomised", "ZOP and FLU were encapsulated, and dummy placebo capsules were given to the patients who did not switch to ZOP, so that during the 5‐week period, all patients consumed", "two identical‐looking pills each night." Comment: Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 20/24 participants completed the 5‐week withdrawal programme, however analysis was performed on women only due to uneven gender distribution between groups and high dropout rate among men. All 5 male participants had been randomised to the zopiclone group, 3 of whom dropped out, and it was decided to perform the analyses on women only (n = 18). |
| Selective reporting (reporting bias) | Low risk | Comment: Unlikely |
| Other bias | High risk | Comment: Role of funding source not explicitly described. |
Peles 2007.
| Methods |
Study design: Randomised controlled trial Study grouping: Cross‐over Blinding: Double Duration: 6 weeks Single‐centre |
|
| Participants |
Baseline characteristics Not reported for each group because of cross‐over design. Most (70%) of the 80 study participants were male. The mean age during study was 42.6 years, and the mean duration in MMT was 4.4 years. Almost half (48.8%) of the participants had other drug abuse in addition to benzodiazepines in the month prior to study entry. Specifically, 25 had positive urine for opiates, 12 for cocaine, 14 for cannabis, and 5 for amphetamines. With respect to lifetime psychiatric diagnosis, 9 participants (11.3%) had 1 of the psychotic disorders, 18 (22.5%) had an affective disorder, 8 (10%) had an adjustment disorder, 2 (2.5%) had an organic brain disorder, 38 (47.5%) had no DSM‐IV Axis I diagnosis (but all 38 had a DSM‐IV Axis II personality disorder), and 5 (6.3%) had no DSM‐IV Axis I or Axis II psychiatric diagnosis. Inclusion criteria: All patients who were admitted to the MMT clinic between July 1993 and July 2004 were eligible for inclusion in the study. This MMT clinic receives patients who meet DSM‐IV criteria for opioid dependence and report self administration of illicit heroin for 1 year or more. Exclusion criteria: None Pretreatment: No significant group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: run‐in phase: taper, until reaching 6 mg/day clonazepam or equivalent. Week 1 through 6: 0.5 mg/week dose reduction
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: The study was supported (in part) by a grant from The Israel Anti Drug Authority. Country: Israel Setting: Outpatients in methadone maintenance treatment Declarations of interest: Not mentioned Author's name: Einat Peles Institution: Adelson Clinic, Tel Aviv Sourasky Medical Center Email: einatp@tasmc.health.gov.il Address: Adelson Clinic, Tel Aviv Sourasky Medical Center, 1 Henrietta Szold Street, Tel Aviv 64924, Israel |
|
| Notes | Only data from the first period (first 6 weeks) of this cross‐over trial was included. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not sufficiently described |
| Allocation concealment (selection bias) | Low risk | Quote: "consecutive container numbers" Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "codes for melatonin first/placebo first were known only to the pharmacist" Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The codes for melatonin first/placebo first were known only to the pharmacist who prepared the sequence in a random manner and identified it to us only at the end of the study" Comment: Only pharmacist knew the code ‐ done. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "40 patients who started on melatonin and 40 patients who started on placebo. Sixty‐one patients (31 from the ‘melatonin‐first’ group and 30 from the ‘placebo‐first’ group) completed phase one (6 weeks). Forty‐four patients completed all 13 weeks of the study, with no differences between groups (60% of the 40 ‘melatonin‐first’ group and 50% of the 40 ‘placebo‐first’ group (P = 0.5)." Comment: Unclear how the high dropout after 6 weeks affected the results |
| Selective reporting (reporting bias) | Unclear risk | Comment: The division of benzodiazepine continuers/discontinuers in the analysis seems to blur the effect of the study medication in itself. |
| Other bias | Low risk | Comment: No other apparent source of bias |
Rickels 1999.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 13 weeks Single‐centre |
|
| Participants |
Baseline characteristics No group difference. Only combined baseline characteristics reported:
Inclusion criteria: Age range of 21 to 70 years, had to have been on continuous daily treatment with diazepam, lorazepam, or alprazolam for a minimum of 1 year, and needed to be able to provide written informed consent Exclusion criteria: A screening medical history, physical examination, ECG, blood count, blood chemistry, urine analysis, and urine drug screens were performed to confirm each patient’s study eligibility. Pretreatment: No significant group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: gradual taper was initiated at approximately 25% reduction per week, with participants on lower therapeutic benzodiazepine doses possibly being tapered slightly faster, and participants on higher therapeutic doses being tapered slightly slower, but not longer than 6 weeks. After the taper was completed, participants were seen weekly for at least 5 weeks in order to determine their ability to stay off their benzodiazepine. During that time, participants continued to receive their double‐blind study medication. Study medication was discontinued at 5 weeks' post‐taper completion. From 5 to 12 weeks post‐taper, participants left the program and returned to their private physician for doctor’s choice management.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: This study was supported by USPHS Research Grant MHO8957. Country: USA Setting: Outpatients Declarations of interest: The study was supported by a US Public Health Service research grant Author's name: Rickels K Institution: Mood and Anxiety Disorders Section, Department of Psychiatry, University of Pennsylvania Email: Address: University Science Center, 3600 Market Street, Suite 803, Philadelphia, PA 19104‐2649, USA |
|
| Notes | We selected only the valproate vs placebo comparison for this meta‐analysis because we did not consider it relevant to combine the experimental intervention groups into a single group (cf. Cochrane Handbook 16.5.4 on how to include multiple groups from 1 study). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "randomly assigned under double blind conditions to study drug or placebo." Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 15 participants, 5 trazodone (12.2%), 5 valproate (26.3%), and 5 placebo (27.7%), dropped out during the pretreatment phase. The 15 dropouts were compared on a variety of demographic and illness variables with the 63 participants who entered taper, and no significant differences were present. |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | Unclear risk | Comment: Groups were not of equal size. No argument is provided. |
Rickels 2000.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 11 to 13 weeks Single‐centre |
|
| Participants |
Baseline characteristics No significant differences between the groups; only data for the combined participant group reported:
Inclusion criteria: Participants were required to have a diagnosis of generalised anxiety disorder according to DSM‐III‐R, to be at least 21 years old, and to have been taking diazepam, lorazepam, or alprazolam in therapeutic doses continuously for the past 12 months. Exclusion criteria: Panic disorder diagnosis Pretreatment: No significant group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 4‐week stabilisation phase, 4‐ to 6‐week taper phase: 25% reduction per week, 5‐week benzodiazepine‐free phase, the experimental drug continued for the first 3 weeks of the benzodiazepine‐free phase
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Supported by NIMH grant MH‐08957. Dr Greenblatt was supported by NIMH grant MH‐34223 and grant DA‐05258 from the National Institute on Drug Abuse. The medications used were provided by Bristol‐Myers Squibb CNS Group, Wallingford, CT. Country: USA Setting: Outpatients Declarations of interest: Not mentioned Authors name: Karl Rickels Institution: Mood and Anxiety Disorders Section, Department of Psychiatry, University of Pennsylvania, Philadelphia Email: krickels@mail.med.upenn.edu Address: University Science Center, 3600 Market St., Suite 803, Philadelphia, PA 19104 |
|
| Notes | Adverse events not reported appropriately for a meta‐analysis. We selected only the imipramine versus placebo comparison for this meta‐analysis because we did not consider it relevant to combine the experimental intervention groups into a single group (cf. Cochrane Handbook 16.5.4 on how to include multiple groups from 1 study). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Medication was prepared double blind in identical capsules containing either 5 mg buspirone, 25 mg imipramine, or placebo" Comment: Sufficiently done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Not clearly described |
| Selective reporting (reporting bias) | Low risk | Comment: Probably not, relevant outcome measures |
| Other bias | Low risk | Comment: No other sources of bias evident. |
Romach 1998.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 weeks Single‐centre |
|
| Participants |
Baseline characteristics Ondansetron
Placebo
Inclusion criteria: DSM‐III‐R criteria for benzodiazepine dependence, a desire to discontinue use of benzodiazepines, daily use of alprazolam or lorazepam for > 3 months Exclusion criteria: Dosage of lorazepam > 8 mg/day or alprazolam > 5 mg/day, psychoactive substance use disorder (other than benzodiazepines), using other prescribed psychotropic medications (other benzodiazepine, antidepressants, antipsychotics, or anticonvulsants), psychosis, moderate to severe major depression, significant cognitive impairment, or suicidal ideation. Serious medical illness, pregnancy, liver enzymes elevated more than 3 times the upper limit, past history of head trauma Pretreatment: More anxiety patients in the placebo group |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: participants set their benzodiazepine tapering goals weekly with a study team member; the overall goal was benzodiazepine discontinuation within the treatment period (6 weeks).
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: The study was supported in part by Glaxo Wellcome Canada. Country: Canada Setting: Outpatients Declarations of interest: Not mentioned Author's name: Myroslav Romach Institution: Departments of Pharmacology, Medicine, and Psychiatry and Faculty of Pharmacy, University of Toronto and Addiction Research Foundation, Toronto, Ontario, Canada Email: Address: Women's College Hospital, Department of Psychiatry, 76 Grenville St., Toronto, Ontario, Canada M5S 1B2 |
|
| Notes | Not possible to extract results from this study due to poor reporting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Described as double‐blind and identical‐appearing capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not specifically described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Out of 108 participants, only 11 (10%) participants dropped out. However, it is unclear if dropout was balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: No protocol available, but no reason to suspect selective outcome reporting. |
| Other bias | High risk | Comment: Role of medicinal company as funding source not sufficiently described. |
Rynn 2003.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 13 weeks Single‐centre |
|
| Participants |
Baseline characteristics No significant differences between groups; only data for the combined participant group reported:
Inclusion criteria: A diagnosis of panic disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Revised Third Edition, at least 21 years old, and have been taking diazepam, lorazepam, or alprazolam in therapeutic doses continuously for at least the past 12 months (5 mg diazepam was considered equivalent to 1 mg lorazepam and 0.5 mg alprazolam). Individuals must also have expressed a desire to stop benzodiazepine intake. Exclusion criteria: Other psychotropic medication than benzodiazepines Pretreatment: No significant group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: after being kept on a stable benzodiazepine dose for 2 to 4 weeks within the therapeutic range during the screening period, participants were assigned to double‐blind treatment during which time their benzodiazepine intake was not altered. This pretreatment lasted 4 weeks, after which participants were tapered from their benzodiazepine dose over a 6‐week period. Benzodiazepine intake was reduced at the rate of 25% per week. Taper was followed by a 5‐week benzodiazepine‐free phase designed to prospectively assess the participant's clinical status in the initial period while being off benzodiazepines. Double‐blind study treatment was continued for the first 3 weeks of this phase. For the last 2 weeks, placebo was substituted for imipramine and buspirone.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: This research was supported by NIMH grant MH‐08957. Bristol‐Myers Squibb CNS Group (Wallingford, CT) provided all double‐blinded medications. Country: USA Setting: Outpatients Declarations of interest: Not mentioned Authors name: Moira Rynn Institution: Mood and Anxiety Disorders Section, Department of Psychiatry, University of Pennsylvania, Suite 670, 3535 Market Street, Philadelphia, PA 19104‐3309 Email: mrynn2@mail.med.upenn.edu Address: Mood and Anxiety Disorders Section, Department of Psychiatry, University of Pennsylvania, Suite 670, 3535 Market Street, Philadelphia, PA 19104‐3309 |
|
| Notes | Adverse events not appropriately described. We included only the imipramine‐placebo comparison in the meta‐analysis because we did not consider it relevant to combine the experimental intervention groups into a single group (cf. Cochrane Handbook 16.5.4 on how to include multiple groups from 1 study). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Medication was prepared double blind in identical capsules" Comment: Done |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Double‐blind identical capsules ‐ doneNot described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Of 52 patients randomised to 3 treatment conditions, 12 patients did not complete the pretreatment phase and 40 patients entered taper. The 2 patient groups did not differ in any baseline demographic or clinical measures with 1 exception. Dropouts had lower BZ doses at baseline, ex‐ pressed in diazepam equivalents, than patients entering taper (12.1 ± 7.7 versus 25.7 ± 19.5; F = 5.52; df = 1.50; P < 0.02). Dropouts also did not differ from taper patients in treatment assignment ( 2 = 0.69; df = 2; P = NS) or type of BZ at baseline ( 2 = 1.43; df = 2; P = NS). The main reason for dropping out of the program during the pre taper phase were adverse events (2 buspirone, 2 imipramine, and 1 placebo)." Comment: Acceptable and no difference between groups |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not available but no obvious selective outcome reporting |
| Other bias | Low risk | Comment: Role of BMS only to provide double‐blinded study medication |
Saul 1989.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 18 weeks Multicentre |
|
| Participants |
Baseline characteristics Atenolol
Placebo
Inclusion criteria: 18 to 60 years old, daily use of benzodiazepines for at least 8 weeks, not more than 15 mg of diazepam Exclusion criteria: Cerebrovascular or generalised vascular disease, heart block, thyrotoxicosis, premenstrual tension or other trigger of cyclical anxiety and depression, pregnancy, antihypertensive therapy or any drug likely to affect anxiety, and those for whom diazepam would be an unsuitable rescue Pretreatment: None reported. |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: follow‐up visits at 4‐week intervals, participants should have stopped taking benzodiazepines by their 4th visit
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not described Country: UK Setting: Outpatients Declarations of interest: Not mentioned Authors name: Saul PA Institution: General Practitioners, Stuart Clinical Research Group Email: Address: P. A. Saul, 555 Chorley Old Road, Bolton, Lancashire, BL2 6AF, UK |
|
| Notes | None of the results were reported with mean and SD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: Described as double‐blind and matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: High dropout rate: 59 out of 121 withdrew (48.7%) |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | Low risk | Comment: Apparently no other bias |
Schweizer 1991.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Blinding Duration: 8 to 10 weeks Single‐centre |
|
| Participants |
Baseline characteristics No significant differences between the intervention groups, therefore combined group reported:
Inclusion criteria: 18 years or older and receiving a daily dose of benzodiazepine continuously for at least the past year. Individuals were entered directly into the study if they were taking diazepam, alprazolam, or lorazepam in a dose of 40 mg or less diazepam equivalents (5 mg of diazepam = 0.5 mg of alprazolam = 1.0 mg of lorazepam). 6 individuals who were receiving a different benzodiazepine were switched to diazepam in an equivalent dose and stabilised for 3 weeks before entry into the study. Exclusion criteria: A history in the past year of alcohol or substance abuse or dependence, any acute or unstable medical condition, or not practicing adequate contraception Pretreatment: No significant group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 1 to 2 weeks pretreatment, benzodiazepine taper was initiated at a rate of 25% per week and completed over 4 weeks. Once the taper phase was completed and the participant had discontinued benzodiazepine intake, treatment with carbamazepine or placebo was continued for 2 to 4 weeks, then discontinued abruptly.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: This investigation was supported by Public Health Service research grant MH‐08957, Washington, DC. Country: USA Setting: Outpatients Declarations of interest: Not mentioned Author's name: Edward Schweizer Institution: Psychopharmacology Research Unit, Department of Psychiatry, University of Pennsylvania School of Medicine, Philadelphia Email: Address: 203 Piersol Bldg, Hospital of the University of Pennsylvania, 3400 Spruce St, Philadelphia, PA 19104 |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "random and double‐blind fashion", "Carbamazepine was provided in capsules that were identical to the placebo" Comment: Done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Carbamazepine levels were obtained in 12 of 19 patients, with the treating psychiatrist kept blind to the results." Comment: Judged as done since treating psychiatrist was kept blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: Total dropout rate 15/55 (27%), 8 (30%) in the carbamazepine group and 7 (25%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Comment: No obvious selective outcome reporting |
| Other bias | Low risk | Comment: No other obvious sources of bias |
Schweizer 1995.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 10 weeks Single‐centre |
|
| Participants |
Baseline characteristics Not reported Inclusion criteria: At least 18 years of age and taking diazepam, lorazepam, or alprazolam on a continuous daily basis for at least 1 year Exclusion criteria: Individuals were excluded from the study if they had a history of alcohol or substance abuse or dependence in the past year, or if they had any acute or unstable medical condition. Men could be of any age, while women had to be at least 2 years' postmenopause, or to have undergone an ovariectomy. Pretreatment: Unknown |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 2 to 3 weeks pretreatment with experimental drug, taper at the rate of 25% per week, after completion of taper experimental drug was continued for 4 weeks, then abruptly discontinued
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: This study was supported by USPHS Research Grant MHO‐8957. Country: USA Setting: Outpatients Declarations of interest: Not mentioned Author's name: Edward Schweizer Institution: University Science Center, Suite 803, 3600 Market Street, Philadelphia, PA 19104‐2649, USA Email: Address: University Science Center, Suite 803, 3600 Market Street, Philadelphia, PA 19104‐2649, USA |
|
| Notes | Benzodiazepine withdrawal symptoms and anxiety not reported appropriately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "under random, double‐blind conditions", "either micronized oral progesterone in 300 mg capsules or matched placebo" Comment: Described as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 8 (27%) participants in progesterone group versus 1 (8%) participant in placebo group dropped out during the pretreatment phase (due to sedation as side effect). |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | Low risk | Comment: No apparent other bias |
Tyrer 1981.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 2 weeks Multicentre |
|
| Participants |
Baseline characteristics Not reported Inclusion criteria: Monotherapy with diazepam or lorazepam, medication use for at least 4 months regularly, and were thought not to require continued prescription Exclusion criteria: Drugs other than benzodiazepines Pretreatment: Not reported |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: abrupt cessation
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not stated Country: UK Setting: General practice and outpatient psychiatric clinics Declarations of interest: Not mentioned Authors name: Peter Tyrer Institution: Mapperley Hospital, Nottingham, and Poisons Unit, New Cross Hospital, London Email: Address: Mapperley Hospital, Porchester Road, Nottingham NG3 6AA, UK |
|
| Notes | Figure 1 reports withdrawal symptoms in each group, only mean score not SD, and no other measures from which the SD can be calculated. Since this was the only identified study using propranolol, and since the scale for withdrawal symptoms was not a validated scale used in other included studies, it was not possible to safely impute values for SD. Otherwise only dropout rate was reported for each group (55% in placebo group and 36% in propranolol group) = patients returning to same benzodiazepine treatment as previously. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "If patients agreed to enter this double‐blind study their benzodiazepines were stopped and replaced by propranolol or placebo tablets of identical appearance" Comment: Done |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: Daily self ratings, no third party involved in outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 40 patients entering the study 18 (45%) dropped out during the two week period and took their benzodiazepine drugs again." Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: No obvious selective outcome reporting |
| Other bias | High risk | Quote: "I.C.I. Pharmaceuticals Division for providing the propranolol and placebo tablets." Comment: Role, if any, in the analyses not described. |
Tyrer 1996.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks Single‐centre |
|
| Participants |
Baseline characteristics Dosulepin
Placebo
Inclusion criteria: Use of benzodiazepines for at least 6 months and had tried unsuccessfully to reduce or stop benzodiazepines due to apparent withdrawal symptoms, no other medication, written consent Exclusion criteria: Hypertension or the psychiatric diagnoses of major depressive disorder, a psychotic disorder, or melancholia Pretreatment: Not reported |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: reduction of the initial dosage by 20% every 2 weeks with the intention of stopping benzodiazepines entirely at week 8
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Research Department of Boots Drug Company funded the study. Country: UK Setting: Outpatients Declarations of interest: Not mentioned Authors name: Peter Tyrer Institution: St Charles Hospital, London Email: Address: St Charles Hospital, London WlO 6DZ |
|
| Notes | For benzodiazepine withdrawal symptoms and anxiety, means were available from graphs only, but SDs were not reported. It was not possible to calculate SD from the presented data. Adverse events insufficiently reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Randomisation process not described. |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "under cover of randomly allocated dothiepin or placebo tablets...administered using double‐blind procedure." Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "During the 14 weeks 45 patients (21 (51%) allocated to dothiepin and 24 (52%) to placebo) withdrew from the study." Comment: High attrition rate |
| Selective reporting (reporting bias) | Low risk | Comment: No apparent selective outcome reporting |
| Other bias | High risk | Comment: Funded by a drug company. Role of funding source not described. |
Udelman 1990.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 6 to 12 weeks depending on the alprazolam starting dose Multicentre |
|
| Participants |
Baseline characteristics Buspirone
Placebo
Inclusion criteria: 18 to 70 years of age, primary clinical anxiety, alprazolam pharmacotherapy for at least 3 months 0.75 to 3 mg daily, good physical health Exclusion criteria: Significant or uncontrolled organic disease, epilepsy or seizures, nursing/pregnant/not using contraceptive measures, substance use disorder, primary depression, panic disorder, psychosis, severe behaviour disorder, organic mental disorders, serious psychosomatic disorders, hypersensitivity to study drug, other drugs (psychotropics, beta‐blockers, carbamazepine, clonazepam) within 1 month before start of the study Pretreatment: No significant pretreatment group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: 2 weeks of concurrent treatment (study drug + stable benzodiazepine dosage), from the third week tapering of alprazolam 0.5 mg/day each week until the total daily dose was 1.5 mg, after which the rate of tapering was 0.25 mg/day each week; completion of the tapering process was to take from 2 to 8 weeks depending on the alprazolam starting dose. At completion, participants were to continue receiving the study drugs (buspirone or placebo) for an additional 2 weeks.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Bristol‐Myers Squibb Country: USA Setting: Outpatients Declarations of interest: Not mentioned Authors name: Harold D Udelman Institution: Biomedical Stress Research Foundation, Phoenix, Arizona, USA Email: Not available Address: Biomedical Stress Research Foundation, 45 East Born Road, Phoenix, Arizona 85012 |
|
| Notes | Means only given in figures (HAM‐A and benzodiazepine withdrawal symptoms), no SDs reported, not possible to impute in a methodologically valid way. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Patients meeting the selection criteria were randomly assigned to one of two groups to receive either buspirone or placebo...Both buspirone and placebo (indistinguishable in physical appearance) were to be administered at a fixed dose..." Comment: Placebo was described as indistinguishable in physical appearance. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not sufficiently described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: High attrition rate in buspirone group (42%) and in placebo group (53%) |
| Selective reporting (reporting bias) | Low risk | Comment: Protocol not available, but no indication of selective outcome reporting. |
| Other bias | High risk | Comment: Role of funding pharmaceutical company not described. |
Vissers 2007.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 16 weeks (10 weeks to taper off and 6 weeks post‐taper) 9 general practices |
|
| Participants |
Baseline characteristics Melatonin
Placebo
Inclusion criteria: Adult patients who used benzodiazepines as a sleeping medication for more than 3 months (defined as long‐term use) at a minimum of 3 days per week Exclusion criteria: Use of more than 1 benzodiazepine at the same time, use of another type of sleep medication, use of stimulants and alcohol misuse (according to individual's GP), serious mental/somatic disease, or unfit to participate Pretreatment: No significant pretreatment differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: the benzodiazepine dose was converted to an equivalent dose of diazepam, which was stabilised for 2 weeks and then further converted every 2 weeks to 75%, 50%, 25%, 12.5%, and 0% of the original dose.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not described Country: The Netherlands Setting: Outpatients in GP Declarations of interest: Not mentioned Authors name: Vissers FHJA Institution: Department of General Practice, Maastricht University Email: harry.crebolder@hag.unimaas.nl Address: Department of General Practice, Maastricht University, P.O. Box 616, Maastricht 6200 MD, the Netherlands |
|
| Notes | Insomnia: the Sleep Wake Experience List: mean and SD not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not sufficiently described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not sufficiently described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The patients, their GPs and the principal investigator were blinded for the study medication." Comment: What has been done to ensure blinding of participants and study personnel is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Insufficient information to judge the risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: All randomised participants were analysed. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Benzodiazepine withdrawal symptoms ambiguously reported. |
| Other bias | Low risk | Comment: No other apparent biases, funding not reported |
Vorma 2011.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: None ‐ open‐label Duration: 3 weeks of inpatient treatment Single‐centre |
|
| Participants |
Baseline characteristics Valproate
Control
Inclusion criteria: DSM‐IV criteria for opioid dependence and benzodiazepine dependence Exclusion criteria: Pregnancy, active medical illnesses or severe mental disorders, history of convulsions, or unable to speak Finnish Pretreatment: At baseline, the median diazepam‐equivalent dose was 60 mg daily (range 20 to 160 mg) in the valproate group and 30 mg (range 8 to 75 mg) in the control group. |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: reduction with 10 mg diazepam equivalents daily until 40 mg per day, after which reductions were 5 mg daily
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: The study was supported by Annual EVO Financing (special government subsidies) from the Department of Psychiatry, Helsinki University Central Hospital. No support was provided by any pharmaceutical company. Country: Finland Setting: Opioid maintenance treatment, inpatient setting, very rapid benzodiazepine‐tapering regimen Declarations of interest: None Author's name: Helena Vorma Institution: Helsinki University Central Hospital, Department of Psychiatry Email: vorma@hus.fin Address: Helsinki University Hospital, Department of Psychiatry, P.O. Box 590, FI‐00029 HUS, Finland |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Low risk | Quote: "To prevent unequal treatment group sizes, we used block randomisation in blocks of six subjects. Sealed envelopes were used to keep the randomisation sequence unknown. The study was carried out as an open trial, with all outcome ratings assessed blindly to prevent detection bias." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Not done, open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: All outcome assessments were done blindly. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Another 8 subjects discontinued participation in CIWA‐B ratings, but stayed in treatment." Comment: Not described why and from which group, accounted for by LOCF, but difficult to judge if this might give rise to any kind of bias |
| Selective reporting (reporting bias) | Unclear risk | Comment: No protocol available; despite the rapid benzodiazepine taper regimen, it is remarkable that there is no indication of the benzodiazepine‐tapering success |
| Other bias | Unclear risk | Comment: Big difference in benzodiazepine dose between groups at baseline (valproate 60 mg/day, control group 30 mg/day) |
Zhang 2013.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Unknown Duration: 3 months Single‐centre |
|
| Participants |
Baseline characteristics Trazodone
Placebo
Inclusion criteria: Benzodiazepine dependence syndrome (Criteria of Mental Disorders in China, Third Edition), insomnia Exclusion criteria: Abuse of alcohol or other psychoactive drugs, other mental disorders, serious somatic illness, allergic to study medication, suicidal risk, pregnancy, breastfeeding, lack of consent Pretreatment: No significant pretreatment group differences |
|
| Interventions | Benzodiazepine taper schedule: benzodiazepine reduced to half dosage, when participant has had stable sleep for 5 days, then dosage is halved again and so forth.
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: Not reported Country: China Setting: Outpatients Declarations of interest: Not mentioned Author's name: Zhang Hong‐Ju Institution: He'nan Provincial People's Hospital, Zhengzhou Email: hongju_z@yahoo.com.cn Address: Department of Neurology, He'nan Provincial People's Hospital, Zhengzhou 450003, He'nan, China |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: Not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: Not described as blinded or open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: Not described as blinded or open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Attrition: 2 of 38 (5%) participants |
| Selective reporting (reporting bias) | High risk | Comment: No data on use of benzodiazepine at follow‐up |
| Other bias | Low risk | Comment: No other apparent sources of bias |
Zitman 2001.
| Methods |
Study design: Randomised controlled trial Study grouping: Parallel group Blinding: Double Duration: 12 weeks Multicentre |
|
| Participants |
Baseline characteristics Paroxetine
Placebo
Inclusion criteria: Benzodiazepine use for at least 3 months, a diagnosis of major depressive disorder (DSM‐III‐R), at least 18 years of age, written informed consent Exclusion criteria: Depression caused by organic factors, psychosis, schizophrenia, pregnancy, lactation, childbearing potential with a lack of adequate contraception, severe concomitant medical conditions, history of seizure disorders, use of other psychotropic medication during the 3 months prior to screening, clinically significant abnormalities in haematology or clinical chemistry, misuse of alcohol or illicit drugs, excessive use of benzodiazepines (more than 3 times the maximal dose), current suicidal risk Pretreatment: No significant pretreatment group differences |
|
| Interventions |
Intervention characteristics Benzodiazepine taper schedule: weeks 1 to 4: transfer to diazepamweeks 5 to 10: constant dose; weeks 11 to 12: 25% reduction per week; weeks 13 to 14: 12.5% reduction in 4 steps to 0
|
|
| Outcomes |
|
|
| Identification |
Sponsorship source: One of the authors was employed by SmithKline Beecham, which also funded the study. Country: The Netherlands Setting: Outpatients Declarations of interest: Not mentioned Authors name: Frans Zitman Institution: Department of Psychiatry, Leiden and UMC Stat Radbond, Nijmegen Email: f.g.zitman@lumc.nl Address: Department of Psychiatry, Leiden, BIP, P.O. Box 9600, 2300 RC Leiden |
|
| Notes | Anxiety: Not sufficiently reported Withdrawal symptoms: Data not reported for placebo vs paroxetine, but for success vs no‐success groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A randomisation list (1‐330) in blocks of six was obtained by using the blocks of six was obtained by using the random number generator of SPSS/PC+ (SPSS, 1997)." Comment: Done |
| Allocation concealment (selection bias) | Low risk | Quote: "Based on this list, study medication (paroxetine, placebo) was blister packed and wrapped by Genfarma, The Netherlands. Blocks were sequentially distributed to GPs. Unused blocks were reallocated. The list was kept by the Medical Adviser on Safety of the medical department Adviser on Safety of the medical department of SmithKline Beecham, The Netherlands." Comment: Done |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Patients were randomised to 20 mg of paroxetine or placebo in a 1:2 double‐blind fashion" Comment: What was done to ensure blinding of participants and personnel in terms of matching paroxetine/placebo is not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After the database was closed and basic descriptive analyses were done, the actual codes were added to the database" Comment: Done |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 50% completed the programme in the paroxetine group and 43% in the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: Some outcome data not reported for randomisation groups, but for success and no‐success group instead. |
| Other bias | High risk | Comment: Role of funding pharmaceutical company not described. |
AEs: adverse events BWSQ: Benzodiazepine Withdrawal Symptom Questionnaire CI: confidence interval CIWA‐B: Clinical Institute Withdrawal Assessment Scale ‐ Benzodiazepines DSM‐III‐R: Diagnostic and Statistical Manual of Mental Disorders, Revised 3rd Edition DSM‐IV‐TR: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision ECG: electrocardiogram GAD: generalised anxiety disorder GP: general practitioner HADS‐A Hospital Anxiety and Depression Scale ‐ Anxiety subscale HAM‐A: Hamilton Anxiety Rating Scale HAM‐D: Hamilton Depression Rating Scale ICD‐10: International Statistical Classification of Diseases and Related Health Problems, 10th revision ITT: intention‐to‐treat IV: intravenous LOCF: last observation carried forward MADRS: Montgomery–Åsberg Depression Rating Scale MMT: methadone maintenance treatment NIMH: National Institute of Mental Health NS: not specified PSQI: Pittsburgh Sleep Quality Index PTSD: post‐traumatic stress disorder PWC: Physician Withdrawal Checklist SAEs: serious adverse events SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allain 1998 | Wrong patient population: duration of benzodiazepine use above 1 month and no mention of dependence |
| Avedisova 2007 | Wrong study design: not randomised |
| Bobes 2012 | Wrong study design: uncontrolled, observational study |
| Bourgeois 2014 | Wrong study design: uncontrolled, observational study |
| Cantopher 1990 | Wrong study design: co‐intervention not delivered equally in both intervention groups |
| Cohen‐Mansfield 1999 | Wrong study design: observational study |
| Declerck 1999 | Wrong intervention: switch to benzodiazepine‐like drug without discontinuation |
| Emara 2009 | Wrong study design: not randomised |
| Garcia‐Borreguero 1992 | Wrong study design: not randomised |
| Hallstrom 1988 | Wrong study design: co‐intervention not delivered equally in both intervention groups |
| Isaka 2009 | Wrong patient population: not chronic benzodiazepine users |
| Lahteenmaki 2014 | Wrong patient population: not chronic benzodiazepine users |
| Lemoine 1997 | Wrong study design: co‐intervention not delivered equally in both intervention groups |
| Lopatko 2006 | Wrong study design: not randomised |
| Nakajima 2007 | Wrong study design: observational study |
| Petrovic 2002 | Wrong study design: the study investigated gradual benzodiazepine withdrawal versus abrupt discontinuation |
| Rocco 1992 | Wrong study design: not randomised |
| Rubio 2009 | Wrong study design: observational study |
| Saxon 1997 | Wrong study design: study not designed to evaluate benzodiazepine discontinuation |
| Shapiro 1995 | Wrong intervention: switch to benzodiazepine‐like drug without discontinuation |
| Vescovi 1987 | Wrong study design: study not designed to evaluate benzodiazepine discontinuation |
| Weizman 2003 | Wrong study design: not randomised |
Differences between protocol and review
JR was added as a review author because of considerable contribution to data extraction and quality assessment.
Many of the included studies were of older date, and it was therefore not possible to track and contact every first author as stated in the protocol. We contacted those authors with available and updated contact information, by email. However, many of the reported email addresses were outdated as well, and requests were returned due to unknown recipient.
Due to the poor quality of the data, we did not perform any subgroup or sensitivity analyses. However, in the single case where imputation of standard deviations was applied (Analysis 8.3) (Lader 1993), we checked that results remained substantially unchanged when excluding this trial from the analysis.
Benzodiazepine withdrawal in opioid maintenance users was mentioned as a point of focus in the protocol. However, we could only include data from two smaller studies in this review where opioid maintenance users were tapered from usual benzodiazepine use: Peles 2007 investigating melatonin and Vorma 2011 investigating valproate. Mariani 2016 also included this group of patients in a trial investigating gabapentin, but it was not possible to extract data from this trial. Due to the paucity of data, we could not draw any conclusions regarding opioid maintenance patients discontinuing benzodiazepines. However, this is an important focus for future research since there are indications that benzodiazepine use is particularly problematic in opioid maintenance users, with an increased risk of toxic overdose and death when the substances are used together (Webster 2011). Active use of benzodiazepines have been found to be present in 17% of deaths involving opioid analgesics in the US (Warner 2009). The US in particular has witnessed a rapidly increasing number of patients chronically treated with opioids (Manchikanti 2012; Skolnick 2018).
Contributions of authors
All authors contributed to the review concept and design. LB and BE or LB and JR assessed studies for inclusion, risk of bias, and data extraction. LB drafted the manuscript, which was reviewed, corrected, and then accepted by all authors.
JL and CG were responsible for the planning of statistical procedures. JL performed the Trial Sequential Analyses. BG provided advice during study design, data collection and data interpretation.
Sources of support
Internal sources
-
Mental health services ‐ Capital Region of Denmark, Denmark.
Postdoctoral grant to LB
External sources
No sources of support supplied
Declarations of interest
LB is the sponsor‐investigator of one of the studies included in this review (Baandrup 2016). A review author independent of this trial acted as the second review author, thus ensuring unbiased data extraction and 'Risk of bias' assessment.
BE has received lecture fees from Bristol‐Myers Squibb, Otsuka Pharma Scandinavia AB, and Eli Lilly and Company and is part of the Advisory Board of Eli Lilly Danmark A/S, Janssen‐Cilag A/S, and Takeda Pharmaceutical Company Ltd.
BG is leader of a Lundbeck Foundation Centre of Excellence for Clinical Intervention and Neuropsychiatric Schizophrenia Research, CINS. CINS is independent of H. Lundbeck A/S. The grant was awarded based on international scientific review. BG is part of the study group behind the clinical trial stated by LB in her declaration of interest.
JL, CG, and JR have no known conflicts of interest.
New
References
References to studies included in this review
Ashton 1990 {published data only}
- Ashton CH, Rawlins MD, Tyrer SP. A double‐blind placebo‐controlled study of buspirone in diazepam withdrawal in chronic benzodiazepine users. British Journal of Psychiatry 1990;157:232‐8. [DOI] [PubMed] [Google Scholar]
Baandrup 2016 {published data only}
- Baandrup L, Fagerlund B, Glenthoj B. Neurocognitive performance, subjective well‐being, and psychosocial functioning after benzodiazepine withdrawal in patients with schizophrenia or bipolar disorder: a randomized clinical trial of add‐on melatonin versus placebo. European Archives of Psychiatry and Clinical Neuroscience 2017;267(2):163‐71. [PUBMED: 27400927] [DOI] [PubMed] [Google Scholar]
- Baandrup L, Glenthoj BY, Jennum PJ. Objective and subjective sleep quality: melatonin versus placebo add‐on treatment in patients with schizophrenia or bipolar disorder withdrawing from long‐term benzodiazepine use. Psychiatry Research 2016;240:163‐9. [PUBMED: 27107670] [DOI] [PubMed] [Google Scholar]
- Baandrup L, Lindschou J, Winkel P, Gluud C, Glenthoj BY. Prolonged‐release melatonin versus placebo for benzodiazepine discontinuation in patients with schizophrenia or bipolar disorder: a randomised, placebo‐controlled, blinded trial. World Journal of Biological Psychiatry 2016;17(7):514‐24. [PUBMED: 26086792] [DOI] [PubMed] [Google Scholar]
Cassano 1996 {published data only}
- Cassano GB, Petracca A, Borghi C, Chiroli S, Didoni G, Garreau M. A randomized, double‐blind study of alpidem vs placebo in the prevention and treatment of benzodiazepine withdrawal syndrome. European Psychiatry 1996;11(2):93‐9. [DOI] [PubMed] [Google Scholar]
Cialdella 2001 {published data only}
- Cialdella P, Boissel JP, Belon P, the ASTRHO group. Homeopathic specialties as a substitute for benzodiazepines: a double‐blind vs. placebo study. Thérapie 2001;56:397‐402. [PubMed] [Google Scholar]
Di Costanzo 1992 {published data only}
- Costanzo E, Rovea A. The prophylaxis of benzodiazepine withdrawal syndrome in the elderly: the effectiveness of carbamazepine. A double‐blind study vs. placebo. Minerva Psichiatrica 1992;33:301‐4. [PubMed] [Google Scholar]
Garfinkel 1999 {published data only}
- Garfinkel D, Zisapel N, Wainstein J, Laudon M. Facilitation of benzodiazepine discontinuation by melatonin: a new clinical approach. Archives of Internal Medicine 1999;159(20):2456‐60. [DOI] [PubMed] [Google Scholar]
Gerra 1993 {published data only}
- Gerra G, Marcato A, Caccavari R, Fertonani‐Affini G, Fontanesi B, Zaimovic A, et al. Effectiveness of flumazenil (Ro 15‐1788) in the treatment of benzodiazepine withdrawal. Current Therapeutic Research ‐ Clinical and Experimental 1993;54(5):580‐7. [Google Scholar]
Gerra 2002 {published data only}
- Gerra G, Zaimovic A, Giusti F, Moi G, Brewer C. Intravenous flumazenil versus oxazepam tapering in the treatment of benzodiazepine withdrawal: a randomized, placebo‐controlled study. Addiction Biology 2002;7(4):385‐95. [DOI] [PubMed] [Google Scholar]
GlaxoSmithKline 2002 {unpublished data only}
- GlaxoSmithKline. Clinical comparison of paroxetine and placebo on the symptoms emerging during the taper phase of a chronic benzodiazepine treatment, in patients suffering from a variety of anxiety disorders. GSK ‐ Clinical Study Register (www.gsk‐clinicalstudyregister.com) 2002.
Hadley 2012 {published data only}
- Hadley SJ, Mandel FS, Schweizer E. Switching from long‐term benzodiazepine therapy to pregabalin in patients with generalized anxiety disorder: a double‐blind, placebo‐controlled trial. Journal of Psychopharmacology 2012;26(4):461‐70. [DOI: 10.1177/0269881111405360] [DOI] [PubMed] [Google Scholar]
- Szczypa P, Hadley SJ, Donevan S, Mandel FS, Leon T. P.4.a.016 Switching from long‐term benzodiazepine therapy to pregabalin in patients with generalised anxiety disorder (GAD). European Neuropsychopharmacology 2009;19:S594‐5. [DOI: 10.1016/S0924-977X(09)70952-6] [DOI] [Google Scholar]
Hantouche 1998 {published data only}
- Hantouche EG, Guelfi JD, Comet D. Discontinuation of long‐term benzodiazepine use: double‐blind controlled study of α‐β L‐aspartate magnesium versus placebo in 144 chronic users of benzodiazepines [α‐β L‐aspartate de magnésium dans l'arrêt de la consommation chronique des benzodiazépines: étude contrôlée en double aveugle versus placebo]. L'encéphale 1998;XXIV:469‐79. [PubMed] [Google Scholar]
- Hantouche EG, Jacob L, Comet D, Guelfi JD. Discontinuation of long‐term benzodiazepine use: predictive model of success in a double‐blind, controlled‐study. 150th Annual Meeting of the American Psychiatric Association, 1997 May 17‐22; San Diego, (CA). 1997.
Harrison‐Read 1996 {published data only}
- Harrison‐Read PE, Tyrer P, Lawson C, Lack S, Fernandes C, File SE. Flumazenil‐precipitated panic and dysphoria in patients dependent on benzodiazepines: a possible aid to abstinence. Journal of Psychopharmacology 1996;10(2):89‐97. [DOI] [PubMed] [Google Scholar]
Klein 1994 {published data only}
- Klein E, Colin V, Stolk J, Lenox RH. Alprazolam withdrawal in patients with panic disorder and generalized anxiety disorder: vulnerability and effect of carbamazepine. American Journal of Psychiatry 1994;151(12):1760‐6. [DOI] [PubMed] [Google Scholar]
Kornowski 2002 {published data only}
- Kornowski J. The comparison between tianeptine and carbamazepine in benzodiazepines withdrawal syndrome. Psychiatria Polska 2002;6(Suppl):311‐8. [PubMed] [Google Scholar]
Lader 1987 {published data only}
- Lader M, Olajide D. A comparison of buspirone and placebo in relieving benzodiazepine withdrawal symptoms. Journal of Clinical Psychopharmacology 1987;7(1):11‐5. [PubMed] [Google Scholar]
Lader 1993 {published data only}
- Lader M, Farr I, Morton S. A comparison of alpidem and placebo in relieving benzodiazepine withdrawal symptoms. International Clinical Psychopharmacology 1993;8(1):31‐6. [DOI] [PubMed] [Google Scholar]
Lecrubier 2005 {published data only}
- Lecrubier Y, Fessard N. Benzodiazepine discontinuation in chronic users: a double‐blind trial of lithium gluconate vs placebo [Arrêt des benzodiazépines chez des consommateurs chronique: un essai en double insu du gluconate de lithium vs placebo]. Annales Médico Phychologiques 2005;163:24‐9. [Google Scholar]
Lemoine 2006 {published data only}
- Lemoine P, Kermadi I, Garcia‐Acosta S, Garay RP, Dib M. Double‐blind, comparative study of cyamemazine vs. bromazepam in the benzodiazepine withdrawal syndrome. Progress in Neuro‐Psychopharmacology & Biological Psychiatry 2006;30(1):131‐7. [DOI] [PubMed] [Google Scholar]
Mariani 2016 {published data only}
- Mariani JJ, Malcolm RJ, Mamczur AK, Choi JC, Brady R, Nunes E, et al. Pilot trial of gabapentin for the treatment of benzodiazepine abuse or dependence in methadone maintenance patients. American Journal of Drug and Alcohol Abuse 2016;42(3):333‐40. [PUBMED: 26962719] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mercier‐Guyon 2004 {published data only}
- Mercier‐Guyon C, Chabannes JP, Saviuc P. The role of captodiamine in the withdrawal from long‐term benzodiazepine treatment. Current Medical Research and Opinion 2004;20(9):1347‐55. [DOI: 10.1185/030079904125004457] [DOI] [PubMed] [Google Scholar]
Morton 1995 {published data only}
- Morton S, Lader M. Buspirone treatment as an aid to benzodiazepine withdrawal. Journal of Psychopharmacology 1995;9(4):331‐5. [DOI] [PubMed] [Google Scholar]
Nakao 2006 {published data only}
- Nakao M, Takeuchi T, Nomura K, Teramoto T, Yano E. Clinical application of paroxetine for chronic benzodiazepine users at an internal medicine clinic. Therapeutic Research 2006;27(5):859‐67. [DOI] [PubMed] [Google Scholar]
- Nakao M, Takeuchi T, Nomura K, Teramoto T, Yano E. Clinical application of paroxetine for tapering benzodiazepine use in non‐major‐depressive outpatients visiting an internal medicine clinic. Psychiatry and Clinical Neurosciences 2006;60(5):605‐10. [DOI] [PubMed] [Google Scholar]
Pat‐Horenczyk 1998 {published data only}
- Pat‐Horenczyk R, Hacohen D, Herer P, Lavie P. The effects of substituting zopiclone in withdrawal from chronic use of benzodiazepine hypnotics. Psychopharmacology 1998;140(4):450‐7. [DOI: 10.1007/s002130050789] [DOI] [PubMed] [Google Scholar]
Peles 2007 {published data only}
- Peles E, Hetzroni T, Bar‐Hamburger R, Adelson M, Schreiber S. Melatonin for perceived sleep disturbances associated with benzodiazepine withdrawal among patients in methadone maintenance treatment: a double‐blind randomized clinical trial. Addiction 2007;102(12):1947‐53. [DOI] [PubMed] [Google Scholar]
Rickels 1999 {published data only}
- Rickels K, Schweizer E, Garcia Espana F, Case, G, DeMartinis N, Greenblatt D. Trazodone and valproate in patients discontinuing long‐term benzodiazepine therapy: effects on withdrawal symptoms and taper outcome. Psychopharmacology 1999;141(1):1‐5. [DOI] [PubMed] [Google Scholar]
Rickels 2000 {published data only}
- Rickels K, DeMartinis N, Garcia‐Espana F, Greenblatt DJ, Mandos LA, Rynn M. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long‐term benzodiazepine therapy. American Journal of Psychiatry 2000;157(12):1973‐9. [DOI] [PubMed] [Google Scholar]
Romach 1998 {published data only}
- Romach MK, Kaplan HL, Busto UE, Somer G, Sellers EM. A controlled trial of ondansetron, a 5‐HT3 antagonist, in benzodiazepine discontinuation. Journal of Clinical Psychopharmacology 1998;18(2):121‐31. [DOI] [PubMed] [Google Scholar]
Rynn 2003 {published data only}
- Rynn M, Garcia‐Espana F, Greenblatt DJ, Mandos LA, Schweizer E, Rickels K. Imipramine and buspirone in patients with panic disorder who are discontinuing long‐term benzodiazepine therapy. Journal of Clinical Psychopharmacology 2003;23(5):505‐8. [DOI] [PubMed] [Google Scholar]
Saul 1989 {published data only}
- Saul PA, Korlipara K, Presley P. A randomised, multicentre, double‐blind, comparison of atenolol and placebo in the control of benzodiazepine withdrawal symptoms. Acta Therapeutica 1989;15(2):117‐23. [Google Scholar]
Schweizer 1991 {published data only}
- Schweizer E, Rickels, K, Case WG, Greenblatt DJ. Carbamazepine treatment in patients discontinuing long‐term benzodiazepine therapy. Effects on withdrawal severity and outcome. Archives of General Psychiatry 1991;48(5):448‐52. [DOI] [PubMed] [Google Scholar]
Schweizer 1995 {published data only}
- Schweizer E, Case WG, Garcia‐Espana F, Greenblatt DJ, Rickels K. Progesterone co‐administration in patients discontinuing long‐term benzodiazepine therapy: effects on withdrawal severity and taper outcome. Psychopharmacology 1995;117(4):424‐9. [DOI] [PubMed] [Google Scholar]
Tyrer 1981 {published data only}
- Tyrer P, Rutherford D, Huggett T. Benzodiazepine withdrawal symptoms and propranolol. Lancet 1981;1(8219):520‐2. [DOI] [PubMed] [Google Scholar]
Tyrer 1996 {published data only}
- Tyrer P, Ferguson B, Hallstrom C, Michie M, Tyrer S, Cooper S, et al. A controlled trial of dothiepin and placebo in treating benzodiazepine withdrawal symptoms. British Journal of Psychiatry 1996;168(4):457‐61. [DOI] [PubMed] [Google Scholar]
Udelman 1990 {published data only}
- Udelman HD, Udelman DL. Concurrent use of buspirone in anxious patients during withdrawal from alprazolam therapy. Journal of Clinical Psychiatry 1990;51 Suppl:46‐50. [PubMed] [Google Scholar]
Vissers 2007 {published data only}
- Vissers FH, Knipschild PG, Crebolder HF. Is melatonin helpful in stopping the long‐term use of hypnotics? A discontinuation trial. Pharmacy World & Science 2007;29(6):641‐6. [DOI: 10.1007/s11096-007-9118-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vorma 2011 {published data only}
- Vorma H, Katila H. Effect of valproate on benzodiazepine withdrawal severity in opioid‐dependent subjects: a pilot study. Heroin Addiction and Related Clinical Problems 2011;13(1):15‐20. [Google Scholar]
Zhang 2013 {published data only}
- Zhang H, Jiang X, Ma M, Zhang J. A control study on treatment for benzodiazepine dependence with trazodone. Chinese Journal of Contemporary Neurology and Neurosurgery 2013;13(5):411‐5. [Google Scholar]
Zitman 2001 {published data only}
- Zitman FG, Couvee JE. Chronic benzodiazepine use in general practice patients with depression: an evaluation of controlled treatment and taper‐off: report on behalf of the Dutch Chronic Benzodiazepine Working Group. British Journal of Psychiatry 2001;178:317‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allain 1998 {published data only}
- Allain H, Coz F, Borderies P, Schuck S, Giclais B, Patat A, et al. Use of zolpidem 10 mg as a benzodiazepine substitute in 84 patients with insomnia. Human Psychopharmacology 1998;13(8):551‐9. [Google Scholar]
Avedisova 2007 {published data only}
- Avedisova AS, Iastrebov DV. Use of anxiolytic atarax as a substitutive drug for benzodiazepine tranquilizers. Zhurnal Nevrologii i psikhiatrii imeni S.S. Korsakova 2007;107(3):37‐41. [PubMed] [Google Scholar]
Bobes 2012 {published data only}
- Bobes J, Rubio G, Teran A, Cervera G, Lopez‐Gomez V, Vilardaga I, et al. Pregabalin for the discontinuation of long‐term benzodiazepines use: an assessment of its effectiveness in daily clinical practice. European Psychiatry 2012;27(4):301‐7. [DOI] [PubMed] [Google Scholar]
Bourgeois 2014 {published data only}
- Bourgeois J, Elseviers MM, Bortel L, Petrovic M, Vander Stichele RH. Feasibility of discontinuing chronic benzodiazepine use in nursing home residents: a pilot study. European Journal of Clinical Pharmacology 2014;17(10):1251‐60. [DOI] [PubMed] [Google Scholar]
Cantopher 1990 {published data only}
- Cantopher T, Olivieri S, Cleave N, Edwards JG. Chronic benzodiazepine dependence. A comparative study of abrupt withdrawal under propranolol cover versus gradual withdrawal. British Journal of Psychiatry 1990;156:406‐11. [DOI] [PubMed] [Google Scholar]
Cohen‐Mansfield 1999 {published data only}
- Cohen‐Mansfield J, Lipson S, Werner P, Billig N, Taylor L, Woosley R. Withdrawal of haloperidol, thioridazine, and lorazepam in the nursing home: a controlled, double‐blind study. Archives of Internal Medicine 1999;159(15):1733‐40. [DOI] [PubMed] [Google Scholar]
Declerck 1999 {published data only}
- Declerck A, Smits M. Zolpidem, a valuable alternative to benzodiazepine hypnotics for chronic insomnia?. Journal of International Medical Research 1999;27(6):253‐63. [DOI] [PubMed] [Google Scholar]
Emara 2009 {published data only}
- Emara A, Elgharabawy R. Evaluation of escitalopram in the treatment of benzodiazepines withdrawal syndrome. Basic and Clinical Pharmacology and Toxicology 2009;105(Suppl 1):66. [Google Scholar]
Garcia‐Borreguero 1992 {published data only}
- Garcia‐Borreguero D, Bronisch T, Apelt S, Yassouridis J, Emrich HM. Treatment of benzodiazepine withdrawal symptoms with carbamazepine. Pharmacopsychiatry 1992;25:100. [DOI] [PubMed] [Google Scholar]
Hallstrom 1988 {published data only}
- Hallstrom C, Crouch G, Robson M, Shine P. The treatment of tranquilizer dependence by propranolol. Postgraduate Medical Journal 1988;64 Suppl 2:40‐4. [PubMed] [Google Scholar]
Isaka 2009 {published data only}
- Isaka M. Withdrawal symptoms of benzodiazepines in panic disorder patients' pharmacotherapy. Journal of the Osaka City Medical Center 2009;58(1‐2):11‐20. [Google Scholar]
Lahteenmaki 2014 {published data only}
- Lahteenmaki R, Puustinen J, Vahlberg T, Lyles A, Neuvonen PJ, Partinen M, et al. Melatonin for sedative withdrawal in older patients with primary insomnia: a randomized double‐blind placebo‐controlled trial. British Journal of Clinical Pharmacology 2014;77(6):975‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lemoine 1997 {published data only}
- Lemoine P, Touchon J, Billardon M. Comparison of 6 different methods for lorazepam withdrawal. A controlled study, hydroxyzine versus placebo. Encephale 1997;23(4):290‐9. [PubMed] [Google Scholar]
Lopatko 2006 {published data only}
- Lopatko O, Morefield K, Danz C, Davies J, Ali R, White JM. Reducing benzodiazepine consumption in opioid maintenance therapy patients: A controlled clinical trial. Proceedings of the 68th Annual Scientific Meeting of the College on Problems of Drug Dependence. 2006:20.
Nakajima 2007 {published data only}
- Nakajima S, Uchida H, Suzuki T, Tomita M, Tsunoda K, Kitta M, et al. An open‐label trial of discontinuing benzodiazepines in patients with chronic schizophrenia. Journal of Clinical Psychopharmacology 2007;27(4):401‐3. [DOI] [PubMed] [Google Scholar]
Petrovic 2002 {published data only}
- Petrovic M, Pevernagie D, Mariman A, Maele G, Afschrift M. Fast withdrawal from benzodiazepines in geriatric inpatients: a randomised double‐blind, placebo‐controlled trial. European Journal of Clinical Pharmacology 2002;57:759‐64. [DOI] [PubMed] [Google Scholar]
Rocco 1992 {published data only}
- Rocco PL, Giavedoni A, Pacella G. Withdrawal from benzodiazepines in a hospital setting: an open trial with buspirone. Current Therapeutic Research ‐ Clinical and Experimental 1992;52(3):386‐9. [Google Scholar]
Rubio 2009 {published data only}
- Rubio G, Bobes J, Cervera G, Teran A, Perez M, Lopez‐Gomez V, et al. Benzodiazepines use withdrawal tapering gradually with pregabalin: findings from the medical practice. European Neuropsychopharmacology 2009;19:S652. [Google Scholar]
Saxon 1997 {published data only}
- Saxon L, Hjemdahl P, Hiltunen AJ, Borg S. Effects of flumazenil in the treatment of benzodiazepine withdrawal ‐ a double‐blind pilot study. Psychopharmacology 1997;131(2):153‐60. [DOI] [PubMed] [Google Scholar]
Shapiro 1995 {published data only}
- Shapiro CM, Sherman D, Peck DF. Withdrawal from benzodiazepines by initially switching to zopiclone. European Psychiatry 1995;10:S145‐51. [DOI] [PubMed] [Google Scholar]
Vescovi 1987 {published data only}
- Vescovi PP, Gerra G, Ippolito L. Nicotinic acid effectiveness in the treatment of benzodiazepine withdrawal. Current Therapeutic Research ‐ Clinical and Experimental 1987;41(6):1017‐21. [Google Scholar]
Weizman 2003 {published data only}
- Weizman T, Gelkopf M, Melamed Y, Adelson M, Bleich A. Treatment of benzodiazepine dependence in methadone maintenance treatment patients: a comparison of two therapeutic modalities and the role of psychiatric comorbidity. Australian and New Zealand Journal of Psychiatry 2003;37(4):458‐63. [DOI] [PubMed] [Google Scholar]
Additional references
Ashton 2005
- Ashton H. The diagnosis and management of benzodiazepine dependence. Current Opinion in Psychiatry 2005;18(3):249‐55. [PUBMED: 16639148] [DOI] [PubMed] [Google Scholar]
Baandrup 2015
- Baandrup L, Ebdrup BH, Lindschou J, Gluud C, Glenthøj BY. Pharmacological interventions for benzodiazepine discontinuation in chronic benzodiazepine users. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011481] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bachhuber 2016
- Bachhuber MA, Hennessy S, Cunningham CO, Starrels JL. Increasing benzodiazepine prescriptions and overdose mortality in the United States, 1996‐2013. American Journal of Public Health 2016;106(4):686‐8. [PUBMED: 26890165] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldwin 2013
- Baldwin DS, Aitchison K, Bateson A, Curran HV, Davies S, Leonard B, et al. Benzodiazepines: risks and benefits. A reconsideration. Journal of Psychopharmacology 2013;27(11):967‐71. [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Barker 2004
- Barker MJ, Greenwood KM, Jackson M, Crowe SF. Cognitive effects of long‐term benzodiazepine use: a meta‐analysis. CNS Drugs 2004;18(1):37‐48. [DOI] [PubMed] [Google Scholar]
Billioti 2012
- Billioti de Gage S, Begaud B, Bazin F, Verdoux H, Dartigues JF, Peres K, et al. Benzodiazepine use and risk of dementia: prospective population based study. BMJ 2012;345(27):e6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Buscemi 2007a
- Buscemi N, Vandermeer B, Friesen C, Bialy L, Tubman M, Ospina M, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: a meta‐analysis of RCTs. Journal of General Internal Medicine 2007;22(9):1335‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2015
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krle A‐Jeric K, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Revista Panamericana de Salud Publica(Pan American Journal of Public Health) 2015;38(6):506‐14. [PUBMED: 27440100] [PMC free article] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. ctu.dk/tsa/ (accessed 25 March 2017).
Darker 2015
- Darker CD, Sweeney BP, Barry JM, Farrell MF, Donnelly‐Swift E. Psychosocial interventions for benzodiazepine harmful use, abuse or dependence. The Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD009652.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dell'osso 2013
- Dell'osso B, Lader M. Do benzodiazepines still deserve a major role in the treatment of psychiatric disorders? A critical reappraisal. European Psychiatry: the Journal of the Association of European Psychiatrists 2013;28(1):7‐20. [PUBMED: 22521806] [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
Denis 2006
- Denis C, Fatseas M, Lavie E, Auriacombe M. Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD005194.pub2] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallacher 2012
- Gallacher J, Elwood P, Pickering J, Bayer A, Fish M, Ben‐Shlomo Y. Benzodiazepine use and risk of dementia: evidence from the Caerphilly Prospective Study (CaPS). Journal of Epidemiology and Community Health 2012;66(10):869‐73. [DOI] [PubMed] [Google Scholar]
Gisev 2011
- Gisev N, Hartikainen S, Chen TF, Korhonen M, Bell JS. Mortality associated with benzodiazepines and benzodiazepine‐related drugs among community‐dwelling older people in Finland: a population‐based retrospective cohort study. Canadian Journal of Psychiatry 2011;56(6):377‐81. [DOI] [PubMed] [Google Scholar]
Glass 2005
- Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits. BMJ (Clinical Research Ed.) 2005;331(7526):1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE
- ims.cochrane.org/revman/gradepro.
Hausken 2007
- Hausken AM, Skurtveit S, Tverdal A. Use of anxiolytic or hypnotic drugs and total mortality in a general middle‐aged population. Pharmacoepidemiology and Drug Safety 2007;16(8):913‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huthwaite 2013
- Huthwaite MA, Andersson V, Stanley J, Romans SE. Hypnosedative prescribing in outpatient psychiatry. International Clinical Psychopharmacology 2013;28(4):157‐63. [DOI] [PubMed] [Google Scholar]
ICH GCP
- International Council for Harmonisation (ICH) Topic E 6 (R1) Guideline for Good Clinical Practice. ichgcp.net/pdf/ich‐gcp‐en.pdf (accessed prior to 10 February 2018).
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] [DOI] [PubMed] [Google Scholar]
Ioannidis 2009
Islam 2014
- Islam MM, Conigrave KM, Day CA, Nguyen Y, Haber PS. Twenty‐year trends in benzodiazepine dispensing in the Australian population. Internal Medicine Journal 2014;44(1):57‐64. [DOI] [PubMed] [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [PUBMED: 25416419] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaussent 2013
- Jaussent I, Ancelin ML, Berr C, Peres K, Scali J, Besset A, et al. Hypnotics and mortality in an elderly general population: a 12‐year prospective study. BMC Medicine 2013;11:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2014
- Jones CM, Paulozzi LJ, Mack KA. Alcohol involvement in opioid pain reliever and benzodiazepine drug abuse‐related emergency department visits and drug‐related deaths ‐ United States, 2010. MMWR. Morbidity and Mortality Weekly Report 2014;63(40):881‐5. [PUBMED: 25299603] [PMC free article] [PubMed] [Google Scholar]
Kripke 1998
- Kripke DF, Klauber MR, Wingard DL, Fell RL, Assmus JD, Garfinkel L. Mortality hazard associated with prescription hypnotics. Biological Psychiatry 1998;43(9):687‐93. [DOI] [PubMed] [Google Scholar]
Kripke 2012
- Kripke DF, Langer RD, Kline LE. Hypnotics' association with mortality or cancer: a matched cohort study. BMJ Open 2012;2(1):e000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lundh 2017
- Lundh A, Lexchin J, Mintzes B, Schroll JB, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.MR000033.pub3; PUBMED: 28207928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Mallon 2009
- Mallon L, Broman JE, Hetta J. Is usage of hypnotics associated with mortality?. Sleep Medicine 2009;10(3):279‐86. [DOI] [PubMed] [Google Scholar]
Manchikanti 2008
- Manchikanti L, Singh A. Therapeutic opioids: a ten‐year perspective on the complexities and complications of the escalating use, abuse, and nonmedical use of opioids. Pain Physician 2008;11(2 Suppl):S63‐88. [PUBMED: 18443641] [PubMed] [Google Scholar]
Manchikanti 2012
- Manchikanti L, Helm S Jr, Fellows B, Janata JW, Pampati V, Grider JS, et al. Opioid epidemic in the United States. Pain Physician 2012;15(3 Suppl):ES9‐38. [PUBMED: 22786464] [PubMed] [Google Scholar]
O'Brien 2005
- O'Brien CP. Benzodiazepine use, abuse, and dependence. Journal of Clinical Psychiatry 2005;66 Suppl 2:28‐33. [PubMed] [Google Scholar]
Parr 2009
- Parr JM, Kavanagh DJ, Cahill L, Mitchell G, McD Young R. Effectiveness of current treatment approaches for benzodiazepine discontinuation: a meta‐analysis. Addiction 2009;104(1):13‐24. [DOI] [PubMed] [Google Scholar]
Parrino 1996
- Parrino L, Terzano MG. Polysomnographic effects of hypnotic drugs. A review. Psychopharmacology 1996;126(1):1‐16. [DOI] [PubMed] [Google Scholar]
Paulose‐Ram 2007
- Paulose‐Ram R, Safran MA, Jonas BS, Gu Q, Orwig D. Trends in psychotropic medication use among U.S. adults. Pharmacoepidemiology and Drug Safety 2007;16(5):560‐70. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schroll 2015
- Schroll JB, Bero L. Regulatory agencies hold the key to improving Cochrane Reviews of drugs [editorial]. Cochrane Database of Systematic Reviews 2015; Vol. 4. [DOI: 10.1002/14651858.ED000098; PUBMED: 25904511] [DOI] [PMC free article] [PubMed]
Schulz 2011
- Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery (London, England) 2011;9(8):672‐7. [PUBMED: 22019563] [DOI] [PubMed] [Google Scholar]
Skolnick 2018
- Skolnick P. The opioid epidemic: crisis and solutions. Annual Review of Pharmacology and Toxicology 2018;58:143‐59. [PUBMED: 28968188] [DOI] [PubMed] [Google Scholar]
Smink 2010
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, Gier JJ. The relationship between benzodiazepine use and traffic accidents: a systematic literature review. CNS Drugs 2010;24(8):639‐53. [DOI] [PubMed] [Google Scholar]
Sonnenberg 2012
- Sonnenberg CM, Bierman EJ, Deeg DJ, Comijs HC, Tilburg W, Beekman AT. Ten‐year trends in benzodiazepine use in the Dutch population. Social Psychiatry and Psychiatric Epidemiology 2012;47(2):293‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011a
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis ‐ a simulation study. PLoS ONE 2011;6(10):e25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011b
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf (accessed 19 March 2014).
Tsimtsiou 2009
- Tsimtsiou Z, Ashworth M, Jones R. Variations in anxiolytic and hypnotic prescribing by GPs: a cross‐sectional analysis using data from the UK Quality and Outcomes Framework. British Journal of General Practice 2009;59(563):e191‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2014
- Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between‐study heterogeneity and simple methods for their application in Bayesian meta‐analysis. Statistics in Medicine 2014;34(6):984‐98. [PUBMED: 25475839] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vinkers 2012
- Vinkers CH, Olivier B. Mechanisms underlying tolerance after long‐term benzodiazepine use: a future for subtype‐selective GABA(A) receptor modulators?. Advances in Pharmacological Sciences 2012;2012:416864. [PUBMED: 22536226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Voshaar 2006
- Voshaar RC, Couvee JE, Balkom AJ, Mulder PG, Zitman FG. Strategies for discontinuing long‐term benzodiazepine use: meta‐analysis. British Journal of Psychiatry 2006;189:213‐20. [DOI] [PubMed] [Google Scholar]
Warner 2009
- Warner M, Chen LH, Makuc DM. Increase in fatal poisonings involving opioid analgesics in the United States, 1999‐2006. NCHS Data Brief 2009, (22):1‐8. [PUBMED: 19796521] [PubMed] [Google Scholar]
Webster 2011
- Webster LR, Cochella S, Dasgupta N, Fakata KL, Fine PG, Fishman SM, et al. An analysis of the root causes for opioid‐related overdose deaths in the United States. Pain Medicine (Malden, Mass.) 2011;12 Suppl 2:S26‐35. [PUBMED: 21668754] [DOI] [PubMed] [Google Scholar]
Weich 2014
- Weich S, Pearce HL, Croft P, Singh S, Crome I, Bashford J, et al. Effect of anxiolytic and hypnotic drug prescriptions on mortality hazards: retrospective cohort study. BMJ (Clinical Research Ed.) 2014;348:g1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [PUBMED: 28264661] [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolcott 2009
- Woolcott JC, Richardson KJ, Wiens MO, Patel B, Marin J, Khan KM, et al. Meta‐analysis of the impact of 9 medication classes on falls in elderly persons. Archives of Internal Medicine 2009;169(21):1952‐60. [DOI] [PubMed] [Google Scholar]
Wu 2009
- Wu CS, Wang SC, Chang IS, Lin KM. The association between dementia and long‐term use of benzodiazepine in the elderly: nested case‐control study using claims data. American Journal of Geriatric Psychiatry 2009;17(7):614‐20. [DOI] [PubMed] [Google Scholar]
Zandstra 2004
- Zandstra SM, Rijswijk E, Rijnders CA, Lisdonk EH, Bor JH, Weel C, et al. Long‐term benzodiazepine users in family practice: differences from short‐term users in mental health, coping behaviour and psychological characteristics. Family Practice 2004;21(3):266‐9. [DOI] [PubMed] [Google Scholar]


