Abstract
Background
People with dementia can have feeding and swallowing difficulties (dysphagia). Modification of the consistency of food or fluids, or both, is a common management strategy. However, diet modification can affect quality of life and may lead to dehydration and malnutrition. Evidence on the benefits and risks of modifying food and fluids is mandatory to improve the care of people with dementia and dysphagia.
Objectives
To determine the effectiveness and adverse effects associated with modifying the consistency of food and fluids in improving oral intake and eliminating aspiration in adults with dysphagia and dementia.
Search methods
We searched ALOIS (the Specialised Register of the Cochrane Dementia and Cognitive Improvement Group), the Cochrane Library, MEDLINE via Ovid SP, Embase via Ovid SP, PsycINFO via Ovid SP, CINAHL via EBSCOhost, LILACS via BIREME, ClinicalTrials.gov and the World Health Organization (WHO) Portal on 9 May 2018. We also checked the reference lists of relevant articles to identify any additional studies.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs and cluster‐RCTs published in any language that measured any of the outcomes of interest. We included trials with adults with a clinical diagnosis of dementia with symptoms and signs of dysphagia confirmed on instrumental assessment. We included participants with all types, stages and severities of dementia. Control groups received either no intervention or interventions not involving diet modification or modification to sensory properties of food.
Data collection and analysis
Two review authors independently assessed for inclusion all potential studies identified. Data were extracted independently along with assessment of methodological quality using standard Cochrane methods. We contacted study authors for additional unpublished information.
Main results
No trials on modification of food met the inclusion criteria. We included two studies that examined modification to fluids. Both were part of the same large multicentre trial and included people with dementia and people with or without dementia and Parkinson's disease. Participation in the second trial was determined by results from the first trial. With unpublished data supplied by study authors, we examined data from participants with dementia only. The first study, a cross‐over trial, investigated the immediate effects on aspiration of two viscosities of liquids (nectar thick and honey thick) compared to regular liquids in 351 participants with dementia using videofluoroscopy. Regular liquids with a chin down head posture, as well as regular liquids without any intervention were also compared. The sequence of interventions during videofluoroscopy may have influenced response to intervention. The second study, a parallel designed RCT, compared the effect of nectar and honey thick liquids with a chin down head posture over a three‐month period in a subgroup of 260 participants with dementia. Outcomes were pneumonia and adverse intervention effects. Honey thick liquids, which are more consistent with descriptors for 'spoon thick' or 'extremely thick' liquids, showed a more positive impact on immediate elimination of aspiration during videofluoroscopy, but this consistency showed more adverse effects in the second follow‐up study. During the second three‐month follow‐up trial, there were a greater number of incidents of pneumonia in participants receiving honey thick liquids than those receiving nectar thick liquids or taking regular liquids with a chin down posture. There were no deaths classified as 'definitely related' to the type of fluids prescribed. Neither trial addressed quality of life. Risk of bias for both studies is high. The overall quality of evidence for outcomes in this review is low.
Authors' conclusions
We are uncertain about the immediate and long‐term effects of modifying the consistency of fluid for swallowing difficulties in dementia as too few studies have been completed. There may be differences in outcomes depending on the grade of thickness of fluids and the sequence of interventions trialled in videofluoroscopy for people with dementia. Clinicians should be aware that while thickening fluids may have an immediate positive effect on swallowing, the long‐term impact of thickened fluids on the health of the person with dementia should be considered. Further high‐quality clinical trials are required.
Plain language summary
Modifying the consistency of food and fluids for swallowing difficulties in dementia
Background
Individuals with dementia often present with swallowing difficulties (dysphagia). The consequences can include choking, dehydration, malnutrition, weight loss, pneumonia and death. Modification of food and liquid is a popular management strategy. It is believed that increasing the viscosity of liquids or altering the consistency of food allows individuals a better opportunity to swallow, with a reduced risk of choking or liquids entering the airway. However, there is growing evidence suggesting that this strategy can lead to dehydration, malnutrition, negative psychological/social consequences, and can affect quality of life for the person with dementia.
Review question
We wished to find out if changing the viscosity or consistency of food or fluids, or both, makes swallowing safer and has positive outcomes for people with dementia in terms of respiratory status, nutritional status and quality of life. We wanted to examine if modifying food or fluids, or both, also had any adverse effects for the person with dementia.
Study characteristics
We found two studies, which were both part of the same multicentre trial and included people with dementia and people with or without dementia and Parkinson's disease. We included data on people with dementia only. The first of the two studies looked at the immediate effects of two viscosities of liquids compared to regular thin liquids on aspiration (entry of food or fluid into the lungs) in 351 people with dementia. This study also compared drinking regular thin liquids using a chin down head posture as well as drinking regular thin liquids without any changes to head position; the main outcome was fluid entering the lungs. Using a subgroup of 260 people with dementia from the first study, the second study compared the effect of the same liquid viscosities with a chin down head posture. The effectiveness of these interventions on the incidence of pneumonia and adverse effects of these interventions was examined over a three‐month period.
Study results
Honey thick viscosity liquids, which clinically are similar to descriptions of 'very thick liquids', had a more positive immediate impact on preventing fluid entering the lungs when examined during videofluoroscopy (specialised swallow x‐ray) examination. However, during the three‐month follow‐up period there were a greater number of incidents of pneumonia in the group of people with dementia receiving these honey thick liquids, than those receiving nectar thick liquids and those receiving regular thin liquids with a chin down posture. There were no deaths classified as 'definitely related' to the type of liquids that the person with dementia was receiving.
Conclusion
There were a number of methodological flaws in both studies in this review and these were acknowledged by the authors. While thickening fluids may have an immediate positive effect on swallow function, clinicians should consider the effects of this intervention on the person with dementia in the longer‐term. People with dementia on thickened fluids require long‐term follow‐up. The overall risk of bias of included studies is high. The quality of evidence is low. Further well‐designed research is needed.
Summary of findings
Background
Description of the condition
Dementia is a progressive and largely irreversible clinical syndrome that is characterised by a widespread impairment of mental function. Although many people with dementia retain positive personality traits and personal attributes, as their condition progresses they can experience some or all of memory loss, language impairment, disorientation, changes in personality, difficulties with activities of daily living, self‐neglect, psychiatric symptoms (for example, apathy, depression or psychosis) and out‐of‐character behaviour (Weiner 2009).
According to Prince 2013, it is estimated that 35.6 million people were living with dementia worldwide in 2010, with this number expected to almost double every 20 years, to 65.7 million in 2030 and 115.4 million in 2050. However, the prevalence of dementia based on more recent research is anticipated to be lower than predicted due to limitations of earlier epidemiology studies (Matthews 2013; Satizabal 2016).
Types of dementia include vascular dementia, Lewy body dementia, frontotemporal dementia, mixed type dementia and the most common form, Alzheimer's disease. The various subtypes of dementia have characteristic clinical features and a different course of disease progression, although there is a high degree of overlap between them (Walshe 2014). The course of the illness may be gradual and insidious, as is classically the case in Alzheimer's disease (Cahill 2012). The disease has no single cause or cure, however, increasing age remains by far the single strongest risk factor for dementia (Daviglus 2010; Ferri 2005).
Individuals with dementia often present with feeding difficulties or swallowing difficulties (dysphagia), or both (Langmore 2007; Takizawa 2016). Feeding difficulties can include difficulty self‐feeding, with problems initiating feeding tasks, transferring food into the mouth and maintaining attention to the feeding task (Chang 2011). Dysphagia is defined as a swallowing disorder that involves any one or more of the oral, pharyngeal or oesophageal stages of swallowing. This review concerns dysphagia rather than feeding problems per se.
Dysphagia is always a symptom of an underlying disease or difficulty and can have a multitude of causes, which may be neurological, surgical, mechanical or psychological (Easterling 2008). Individuals with dementia may also have other characteristics that can influence swallowing such as increased age, reduced physical mobility, poor dentition, dependent feeding and use of medications which can affect swallow function (Smith 2009). Dysphagia can manifest through clinical signs, such as leakage of food or liquids on eating and drinking, drooling, coughing or choking during or after eating or drinking, food sticking in the oropharyngeal or oesophageal regions, regurgitation, gastroesophageal reflux and odynophagia (pain on swallowing) (Groher 2010). The consequences of dysphagia for patients with dementia can include dehydration, malnutrition, weight loss, aspiration pneumonia and death (Gräsbeck 2003; Hudson 2000; Langmore 2002). All individuals with dementia can develop dysphagia, most often in the later stages of the illness (Suh 2009).
Description of the intervention
A range of interventions are used in the management of dysphagia in people with dementia (Alagiakrishnan 2013). These can include behavioural strategies (Brush 1998), modification of food consistencies (Logemann 2008), postural manoeuvres (for example, chin down posture) (Robbins 2008), pharmaceutical interventions (Wada 2001), environmental modification (Koss 1998), or enteral feeding (Kuo 2009). Although researchers have investigated the effectiveness of behavioural interventions for dysphagia in acute and subacute stroke (Geeganage 2012), the evidence to support this intervention for individuals with dementia and dysphagia is scant. Postural changes have some benefit and some studies suggest that a simple chin down posture is as effective as diet modification (Logemann 2008). The National Institute for Health and Care Excellence (NICE) guidelines on dementia advises that enteral feeding should only be considered where dysphagia is a transient phenomenon and that enteral feeding should not generally be used with individuals with severe dementia (NICE 2006). Although some researchers suggest that percutaneous endoscopic gastrostomy should be considered in people with moderate ‐ severe dementia, when malnutrition and aspiration are present (Nunes 2016), the consensus remains that oral intake, modified as necessary, should be the main aim of treatment for individuals presenting with oral feeding difficulties (Royal College of Physicians 2010).
Modification of food and liquid is therefore an important management strategy for dysphagia in people with dementia. This, coupled with the increase in research, awareness and prevalence of dysphagia in individuals with dementia, means that modifying the consistency of food and fluids for swallowing difficulties has become topical for speech and language therapists, medical physicians, nurses, members of multidisciplinary care teams, people with dementia themselves, their caregivers and also their families. A recent review on the effects of bolus viscosity on safety and efficacy of swallowing, concluded that increasing bolus viscosity can increase safety of swallowing, but increasing viscosity can also increase the amount of residue in the pharynx and heighten the risk for post‐swallow aspiration (Newman 2016). Newman 2016 did not consider people with dementia specifically, and included all studies of varying methodological quality. Our systematic review is specifically concerned with the effectiveness and safety of modifying the consistency of food and fluids in people with dementia. For the purpose of this review, modification of food and fluids includes any intervention that involved alteration to the consistency of food or liquids given to people with dysphagia resulting from dementia.
Modification of liquids can include changing the consistency to different degrees by adding a thickening agent to the liquid. The consistencies of liquids can range from 'water‐like' liquids to 'pudding‐like' liquids. The consistency of foods can also be altered from a regular texture to 'extensively modified textured food'. The terminology and definitions of different food and fluid consistencies vary both within and between countries. Table 4 and Table 5 provide published national descriptors and unpublished information regarding the terminology and definitions for modified food and fluids used in Australia, Ireland, Japan, New Zealand, Sweden, UK, USA, Denmark, Canada, Spain, the Netherlands and Brazil (Cichero 2013). The viscosity of liquid is measured in centipoise (cPs) or millipascal‐second (mPa) (with 1cP = 1 mPa) and can vary according to thickening agent. According to Garcia 2005, the viscosity of a nectar or honey‐like liquid is highly dependent on the type of thickening product used and the time that it is allowed to thicken. The temperature and type of liquid to be thickened is also a factor in influencing viscosity. Garcia 2005 found that there is also variability in viscosity measurements, even when the same product is used to thicken liquids. Thickeners are traditionally made of modified starch granules that have the capacity to absorb water and increase the viscosity of liquids. They tend to have a grainy texture and are 'starchy' (Matta 2006; Newman 2016). Newer thickeners involve xanthan gum, and unlike modified gum thickeners, these are reported to be more stable and also more palatable (Newman 2016).
1. Fluid consistencies (Cichero 2013).
| Country | |||||
| USA (NDD) National Dysphagia Diet Task Force 2002 |
Thin (1‐50 cPsa) |
Nectar‐like (51‐350 cPs) |
Honey‐like (351‐1750 cPs) |
Spoon thick (> 1750 cPs) |
|
| UK National Patent Safety Agency 2011 |
Thin | Naturally thick fluid | Thickened fluid ‐ stage 1 |
Thickened fluid ‐ stage 2 |
Thickened fluid ‐ stage 3 |
| Australia Atherton 2007 |
Regular | ‐ | Level 150 ‐ mildly thick |
Level 400 ‐ moderately thick |
Level 900 ‐ extremely thick |
| Ireland IASLT/INDI 2009 |
Regular | Grade 1 ‐ very mildly thick |
Grade 2 ‐ mildly thick |
Grade 3 ‐ moderately thick |
Grade 4 ‐ extremely thick |
| Japan Ministry of Health Labour and Welfare 2009 |
Less mildly thick (< 50 mPa.sb) |
Mildly thick (50‐150 mPa.s) |
Moderately thick (150‐300 mPa.s) |
Extremely thick (300‐500 mPa.s) |
Over extremely thick (> 500 mPa.s) |
| Canada | Regular/thin/clear | Nectar/stage 1/level 1/>250 cPs/51‐350 cPs |
Honey/stage 2/level 2/>800 cPs/351c‐1750 cPs/default thick |
Pudding/spoon thick/stage 3/level 3/>2000 cPs/>1750 cPs | |
| Denmark Tolstrup Anderson 2013 |
Normal | Chocolate milk | Syrup | Jelly | |
| Spain | Thin | Medium | Full protection/thick/pudding | ||
| Netherlands | Thin | Thickened | Pudding‐like | ||
| Brazil | Normal or thin | Thicker liquid | Nectar or honey | Paste or creamy (homogenous or heterogenous) |
|
| Sweden Wendin 2010 |
Liquids | Thickened liquids | |||
acPs: centipoise
bmPa.s: millipascal second
Both are units of viscosity: 1cP = 1mPsa
2. Food consistencies (Cichero 2013).
| Country | ||||||
| USA (NDD) National Dysphagia Diet Task Force 2002 |
Regular | Dysphagia advanced (bite sized, < 2.5 cm) |
Dysphagia mechanically altered (0.6 cm) |
Dysphagia pureed | ||
| UK National Patent Safety Agency 2011 |
Texture E ‐ fork mashable dysphagia diet (1.5 cm) |
Texture D ‐ pre‐mashed dysphagia diet (0.2 cm) |
Texture C ‐ thick pureed dysphagia diet |
Texture B ‐ thin pureed dysphagia diet |
||
| Australia Atherton 2007 |
Regular | Texture A ‐ soft (1.5cm) |
Texture B ‐ minced + moist (0.5 cm) |
Texture C ‐ smooth pureed |
Texture D ‐ liquidised |
|
| Ireland IASLT/INDI 2009 |
Texture A ‐ soft |
Texture B ‐ minced and moist |
Texture C ‐ smooth pureed |
Texture D ‐ liquidised |
||
| Japan Ministry of Health Labour and Welfare 2009 |
Level 5 Normal diet |
Level 4 Soft food |
Level 3 (Dysphagia diet) Paste containing meat/fish |
Level 2 (Dysphagia diet) Jelly food with protein (Rough jelly surface) |
Level 1 (Dysphgaia diet) Smooth jelly food with protein, except for meat and fish |
Level 0 (Test food) Smooth jelly food without protein |
| Canada | Easy to chew or regular/general/ dysphagia general |
Chopped or diced/dysphagia soft/dysphagia soft + minced/stage 3/level 3/dental soft/easy to chew with minced meat/cut up |
Advanced minced/minced with finger food/diced/chopped/soft minced | Minced/mashed/modified minced/dysphagia fully totally minced/level 2 mechanical/minced moist/minced meat modified vegetables | Pureed/thin pureed/dysphagia pureed/stage 1/level 1/semi‐pureed | Blended/liquidised |
| Denmark Tolstrup Anderson 2013 |
Normal | Soft | Puree | |||
| Spain | Normal | Easy mastication | Puree | |||
| Netherlands | Normal | Normal with soft meat/fish/chicken ‐ no particulates (e.g. peas, rice) |
Mashed | Puree | ||
| Brazil | Solid | Soft solid or puree | ||||
| Sweden Wendin 2010 |
Regular or cut |
Coarse paté | Timbales | Jellied products | High viscosity fluids |
Low viscosity fluids |
The rationale for altering the consistency of food and liquids is that this can compensate for a swallowing deficit or change the swallow pattern toward the goal of improved swallow function (Groher 2010). Modifying the consistency of food and liquids is one of the most common strategies used in diet modification by speech and language therapists, based on the assumption that in doing so it can prevent aspiration (food or liquid entering the lungs). The International Dysphagia Diet Standardisation Initiative has developed global standardised terminology and definitions for textured modified foods and thickened liquids for individuals of all ages with dysphagia, in all care settings and for all cultures (IDDSI 2016).
For the purposes of this review, modifying food and fluid consistency as an intervention can be provided in any setting and can be delivered by a trained person or a team. Diet can be given orally with the person with dementia self‐feeding or the person can be assisted with his or her eating and drinking by trained carers. Food or liquid consistencies should be delivered by carers trained to modify the diet specifically according to instructions given by a speech and language therapist or other appropriately trained healthcare professionals following assessment.
How the intervention might work
Modifications to food and liquid consistencies are hypothesised to lead to physiological changes in swallowing, including changes in lingual, submental and hyolaryngeal activity and duration of hyolaryngeal closure (Newman 2016; Robbins 2008). Increasing the viscosity of a fluid can lead to a reduced rate of liquid bolus transit and increased sensory awareness (Dantas 1990; Troche 2008). It can also influence opening of the upper oesophageal sphincter. This reduced rate of bolus movement and increase in sensory awareness may enhance the safety and efficiency of swallowing, thus reducing the risk of aspiration or penetration of fluid into the airway. It is believed that increasing the viscosity of the fluid bolus by altering its consistency allows individuals a better opportunity to swallow with a reduced risk of airway compromise.
Similarly, altering the consistency of food is thought to lead to physiological changes, which can reduce an individual's risk of aspiration. Food is often modified according to a patient's oral motor control (Garcia 2010). It is believed that by modifying the consistency of food, oral preparation of the bolus is more efficient. This is thought to improve an individual's ability to swallow the food bolus safely.
Why it is important to do this review
It is recommended that artificial feeding should only be considered if dysphagia is thought to be a transient phenomenon and should not generally be used in people with severe dementia for whom dysphagia or disinclination to eat is a manifestation of disease severity (NICE 2006; Royal College of Physicians 2010). As a result of these guidelines, modified consistency food and liquids are used increasingly with people presenting with dysphagia as a result of dementia.
The belief that altering the consistency of food and fluids can help individuals with dementia and dysphagia swallow more safely and more efficiently is widely held. However, there is evidence that it can have significant psychological and social consequences and can affect quality of life. It may also lead to dehydration and malnutrition, as thickened liquids can be unpalatable and the choice of food that is recommended may be limited (Easterling 2008; Ekberg 2002; Newman 2016). Other studies suggest that drinking very thick liquids (those at the consistency of honey or thicker) may in fact be harmful for older adults with swallowing problems (Robbins 2008).
Evidence on the benefits and risks of modifying the consistency of food and fluids, as well as directions for research is mandatory to improve the care of people with dementia and dysphagia. This information is required to inform decision making by clinicians, multidisciplinary teams, people with dementia, their families and other key stakeholders.
Objectives
To determine the effectiveness and adverse effects associated with modifying the consistency of food and fluids in improving oral intake and eliminating aspiration in adults with oropharyngeal dysphagia and dementia.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised controlled trials (RCTs), quasi‐RCTs and cluster‐RCTs published in any language. We classified relevant RCTs as all trials that involve at least one group receiving modified food or fluid consistency, or both, aimed at improving or eliminating dysphagia, and one group receiving a control treatment or no treatment, with concurrent enrolment and follow‐up of the test and control treated groups, and where treatments to be administered are allocated by a random process, such as the use of a random number table (Lefebvre 2011).
We classified as quasi‐RCTs all trials of similar design, where the method of allocation to the treatment group is known but is not considered strictly random, for example, alternate allocation by day or date of birth or medical record number. Control measures could include for example, placebos, active treatment, no treatment, dosage forms and regimens (Lefebvre 2011).
Types of participants
We included trials with adults with a clinical diagnosis of dementia who have symptoms and signs of difficulty swallowing and in whom aspiration or penetration has been confirmed by a full clinical bedside evaluation, videofluoroscopy or fibreoptic endoscopic examination of swallowing (FEES) using valid reliable measures, where available, such as the Penetration‐Aspiration Scale (Rosenbek 1996). We included trials with participants who suffered any type and severity of dementia. We imposed no limitations regarding the stage of dementia. We included people with dementia in all settings. We included studies with mixed populations, if we could extract data for the people with dementia and dysphagia. We contacted authors of trials for this data if not available in published reports.
Types of interventions
Intervention
Diet modification involving any alteration to the consistency of food or fluids, or both, given to people with dementia and dysphagia
Comparisons
Intervention versus no intervention (i.e. modified consistency food or fluids, or both, versus a normal diet that is not modified in either consistency, volume, taste or temperature or alternative non‐feeding)
Intervention versus other intervention that does not involve diet modification (i.e. modified consistency food or fluids, or both, versus any other intervention that does not involve modification of food or fluids such as compensatory strategies, e.g. chin tuck, head turn)
Intervention versus other intervention that involves modification to sensory properties of food or fluid only (i.e. modified consistency food or fluids, or both, versus a diet that does not modify consistency but involves modification to sensory properties of food or fluids, or both, such as carbonation, temperature etc.)
Types of outcome measures
Primary outcomes
Aspiration of food or fluids, or both, as rated on objective instrumental swallowing assessment (e.g. videofluoroscopy, fibreoptic endoscopic examination of swallowing (FEES) safety)
Nutritional status, as measured by change in weight, change in grip strength, change in calorific intake, change in standardised and validated screening tool, such as the Mini Nutritional Screening Tool (Guigoz 1996), or the Malnutrition Universal Screening Tool (Stratton 2004); change in number of hospitalisations for rehydration
Respiratory status, defined by clinical assessment that may include a chest x‐ray, change in incidence of aspiration‐related pneumonia
Adverse events associated with diet modification, including hospitalisation, psychological effects, aspiration pneumonia, malnutrition, dehydration and death
Secondary outcomes
Non‐compliance with dietary modifications, such as person with dementia's refusal to take modified food or fluid, or both
Change in quality of life, as measured by person with dementia or carer report, validated quality of life measures, validated psychosocial impact measures
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialized Register on 09 May 2018. We used the search terms: diet*, food*, liquid*, fluid*, solid*, feed*, eat*, meal*, swallow*.
ALOIS is maintained by the Information Specialists of the Cochrane Dementia and Cognitive Improvement Group and contains dementia and cognitive improvement studies identified as follows.
Monthly searches of a number of major healthcare databases: MEDLINE, Embase, CINAHL, PsycINFO and LILACS.
Monthly searches of a number of trial registers: metaRegister of Controlled Trials; Umin Japan Trial Register; World Health Organization Clinical Trials Registry Platform portal (which covers ClinicalTrials.gov (www.clinicaltrials.gov); ISRCTN (apps.who.int/trialsearch); Chinese Clinical Trials Register (http://www.chictr.org.cn/abouten.aspx); German clinical trials register (https://www.drks.de/drks_web/) ; Iranian Registry of Clinical Trials (http://www.irct.ir) and the Netherlands National Trials Register (http://www.trialregister.nl/trialreg/index.asp), plus others).
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library.
Monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
We conducted additional separate searches in many of the above sources to ensure that the most up‐to‐date results were retrieved. The sources searched and the search strategies used for the retrieval of reports of trials can be seen in Appendix 1.
Searching other resources
We reviewed reference lists from all included studies to identify other relevant trials. We handsearched published abstracts of conference proceedings from both the Dysphagia Research Society and the European Society of Swallowing Disorders (both published in the journal Dysphagia). We reviewed ProQuest Dissertations & Theses for relevant dissertation abstracts and we contacted authors for data on trials, as relevant.
Data collection and analysis
We used the methods for this study in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
We merged the search results using EndNote reference management software and removed duplicate records of the same report. Two review authors (EF and MW) independently read through titles, abstracts and key words identified from the literature search. Search results were categorised as either 'relevant', 'potentially relevant' or 'not relevant'. If it was unclear from titles and abstracts whether a study should be included, copies of these trial reports were obtained for further analysis. We resolved any disagreement on selection of studies by consensus discussion.
We retrieved full texts of relevant and potentially relevant reports. Two review authors (EF, MW) examined all final full texts of relevant reports for compliance with eligibility criteria. When the eligibility of the study was in question, we contacted the authors of the report for additional information. The review team were not blinded to information about study authors, institutions, journal of publication or results. There were no disagreements regarding the selection of studies. Only two trials were eligible for inclusion in this review.
Data extraction and management
Two review authors (EF and MW) independently extracted the data from each study using a specifically devised data extraction form. We resolved disagreements by discussion. We entered the data in Review Manager 5 software (Review Manager 2014). When information regarding any of the data was unclear we contacted the trial authors for further information. EF and MW independently rated the quality of evidence for each outcome using the GRADE approach (Ryan 2016).
Assessment of risk of bias in included studies
Two review authors (EF and MW) independently assessed the risk of bias for each study using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions and Cochrane's tool for assessing methodological quality and risk of bias (Higgins 2011). Where characteristics of the trials were unclear, we contacted the authors of the studies to obtain further information.
We assessed the risk of bias in each domain and categorised them as follows.
Low risk of bias: plausible bias that is unlikely to seriously alter the results (categorised as 'low' in the 'Risk of bias' table).
High risk of bias: plausible bias that seriously weakens confidence in the results (categorised as 'high' in the 'Risk of bias' table).
Unclear risk of bias: plausible bias that raises some doubts about the results, or inadequate information with which to make a decision (categorised as 'unclear' in the 'Risk of bias' table).
Measures of treatment effect
Included outcomes were either dichotomous or continuous. We used risk ratio (RR) and 95% confidence interval (CI) for the analysis of dichotomous outcomes, and mean difference (MD) or standardised mean difference (SMD) and 95% CI for continuous outcomes.
Unit of analysis issues
The unit of analysis is the individual with dementia. Included trials were either a parallel design or cross‐over design. We ensured that the number of measurements in the analysis matched the number of individuals that were randomised to the intervention.
Dealing with missing data
The review authors contacted trial authors to supply missing data from included studies. We included in the analyses all participants with dementia randomised to each group and analysed all participants with dementia in the group to which they were allocated.
Assessment of heterogeneity
As there were only two trials eligible for inclusion and both were part of the same larger study, examining different outcomes and pooling of the results was not appropriate.
Assessment of reporting biases
We aimed to minimise reporting biases through a comprehensive search for studies, inclusion of unpublished studies and use of trial registries. We aimed to reduce or eliminate reporting biases by considering the following: publication bias, time lag bias, duplicate publication bias, location bias, citation bias, language bias or outcome reporting bias. This was not possible given that there were only two individual trials eligible for inclusion.
Data synthesis
Both included studies were too disparate in terms of outcomes to allow for pooling of results in a meta‐analysis. Therefore, we described the results of the trials in terms of a narrative summary.
Subgroup analysis and investigation of heterogeneity
We did not perform a subgroup analysis (or meta‐regression) as there were insufficient data available.
Sensitivity analysis
We did not complete a sensitivity analysis in this review.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We retrieved 1117 records through database searching. We obtained 655 records after removing duplicates. We did not identify any ongoing studies and no studies are awaiting classification. A detailed description of results from the search can be seen in Figure 1.
1.
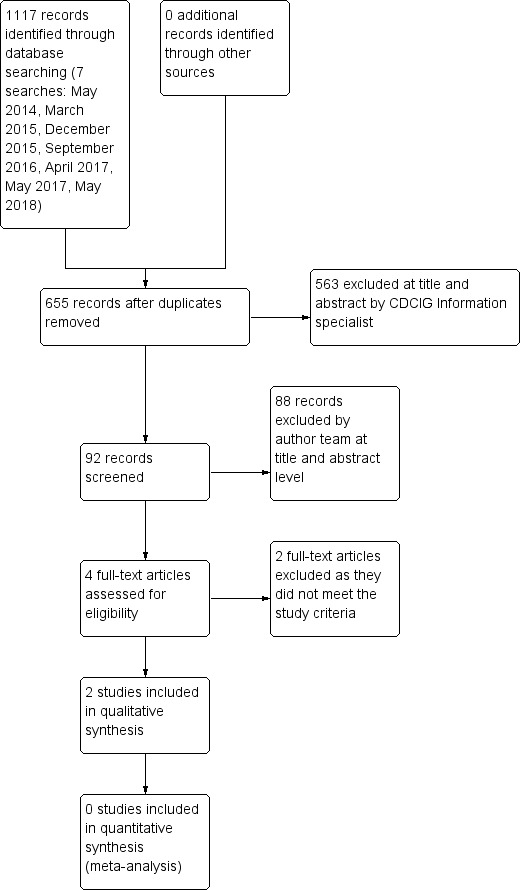
Study flow diagram.
Included studies
Two studies met the inclusion criteria (Logemann 2008; Robbins 2008). Both studies were part of the same large multicentre trial. Study participants in Robbins 2008 were a subgroup of participants in Logemann 2008. Both studies were reported in English. The trials included people with dementia, people with dementia and Parkinson's disease, and people with Parkinson's disease only. Logemann 2008 investigated the immediate effects of thickened liquids (nectar and honey thick liquids) compared with a chin down head posture and regular thin liquids as well as regular thin liquids without any postural adjustment on aspiration of fluids. Robbins 2008 examined the effectiveness of thickened liquids (nectar and honey thick liquids) compared with a chin down posture on the cumulative incidence of pneumonia in participants over a three‐month period. A chin down or chin tuck head posture involves touching the chin to the front of the neck. It is hypothesised to reduce the distance between the base of the tongue and the posterior pharyngeal wall, reduce the distance between the epiglottis and the posterior pharyngeal wall and reduce the space also between the base of the epiglottis and the arytenoid (Logemann 2008; Welch 1993). This intervention has been found to be effective in people who aspirate thin liquids as a result of stroke, acquired brain injury and head and neck cancer (Shanahan 1993).
Settings
Both studies were conducted in the USA and involved the same research teams. The research was funded by a number of agencies including the National Institute on Deafness and Other Communication Disorders (https://www.nidcd.nih.gov), National Institutes of Health (https://www.nih.gov), Novartis and E‐Z‐EM (https://www.novartis.com).
Design
Both studies were randomised controlled trials (RCTs). Logemann 2008 used a cross‐over design comparing aspiration on regular thin liquids with chin down head posture, nectar thick and honey thick liquids. Robbins 2008 used a parallel design and compared the effectiveness of drinking regular thin liquids using a chin down head posture and two consistencies of thickened liquid (nectar and honey thick), taken in a neutral head position, on the development of pneumonia, diagnosed by radiography or by the presence of respiratory indicators.
Sample sizes
The multicentre trial assessed participants in 47 acute care hospitals and 79 subacute residential facilities (Logemann 2008; Robbins 2008). Logemann 2008 included 711 patients; some of these had dementia, some had Parkinson's disease with no dementia, and some had Parkinson's disease and dementia. For the purposes of this review, we excluded the participants with dementia associated with Parkinson's disease from the analysis, as we hypothesised that this group may present with a different type of dysphagia because of the Parkinson's disease. We also excluded the participants without dementia from the analysis. Forty‐nine per cent of this entire cohort (351/711 people) in Logemann 2008 had some form of dementia and did not have Parkinson's disease. Robbins 2008 included 515 patients with dementia or Parkinson's disease, or both, in their follow‐up study. Fifty per cent of this cohort (260/515 people) had some form of dementia and did not have Parkinson's disease.
Participants
Data on the people with dementia were not available in the published reports. We contacted authors and obtained unpublished data from the authors on participants with a diagnosis of dementia only. Participants included in this review were people with dementia who were observed to aspirate 3 mL of thin liquids from a spoon or when drinking from a cup without any intervention (i.e. modification of fluid consistency or use of chin down posture) during videofluoroscopy.
From the unpublished data provided by the authors, Logemann 2008 examined 351 participants with dementia (male: 204, female: 147; age range: 50 to 95 years; mean age: 81.26 years; SD: 7.54 years). Robbins 2008 examined 260 participants with dementia (male: 155, female: 105; age range: 51 to 95 years; mean age: 81.18 years; SD: 8.18 years).
In Logemann 2008, 109 participants had Alzheimer's disease, 109 had single or multistroke type dementia, and 133 people had other unclassified types of dementia. In Robbins 2008, 88 participants had Alzheimer's disease, 75 had single or multistroke dementia, and 97 had other unclassified types of dementia. See Characteristics of included studies for details.
Participants in the Logemann 2008 trial who exhibited no aspiration on any of the three interventions, or who aspirated on each of the three interventions but wished to continue with oral intake, were eligible for participation in the Robbins 2008 study.
Interventions
In Logemann 2008's cross‐over design, participants received three interventions in a randomly assigned order within the same session.
Interventions included:
chin down head posture while consuming thin liquids;
nectar thick liquids in a neutral head position with no postural adjustment;
honey thick liquids in a neutral head position with no postural adjustment.
Viscosity was measured in centipoise (cPs) for these liquid categories: thin (1–50 cPs), nectar thick (51–350 cPs), and honey thick (351–1750 cPs). The thin, nectar thick, and honey thick liquids were commercially produced by E‐Z‐EM Corporation (Lake Success, NY; Varibar) specifically for the study to allow for standardisation of viscosity. The viscosity of the Varibar thin liquid was 15 cPs; nectar thick liquid was 300 cPs and honey thick liquid was 3000 cPs. Varibar thin liquid is a barium sulfate liquid (40% weight/volume after reconstitution) for oral administration. Varibar nectar thick and honey thick products contain xanthan gum and modified corn starch.
In Robbins 2008's parallel design, participants were initially randomly assigned to one of two interventions: (1) chin down head posture while consuming regular thin liquids; or (2) thickened fluids in a neutral head position, with further randomisation of participants in the thickened liquid group to (a) nectar thick liquids in a neutral head position or (b) honey thick liquids in a neutral head position.
Nectar and honey thick viscosity liquids were made for participants using Resource Thicken Up (Nestle Healthcare Nutrition, Minneapolis, Minnesota). This is a starch‐based thickening agent made from modified corn starch. It is important to note that the viscosity of 3000 cPs is considered much thicker than honey in many countries and the description may be misleading (see Table 4). It was expected that all liquids would be provided to participants consistent with the intervention to which the participant was randomly assigned. Initially, all participants were monitored at all meals by caregivers and study staff for compliance with the assigned intervention. During the course of the study, to lessen the burden on caregivers, mealtime monitoring was reduced to randomised sets of three meals per week across the three interventions.
Outcomes
The outcome of the Logemann 2008 study was successful immediate elimination of aspiration. Outcomes of interest investigated in Robbins 2008 included respiratory status, adverse effects (including hospitalisations and death) and compliance with interventions. Neither trial addressed quality of life. We excluded data from Logemann 2008 assessing participant preference for different interventions as the outcome data were obtained only on participants with a diagnosis of Parkinson's Disease and participant preference was not measured for participants with a diagnosis of dementia.
Excluded studies
After screening, we excluded 88 studies; see Characteristics of excluded studies. We excluded studies as they did not measure outcomes of interest for this review or were not RCTs, quasi‐RCTs or cluster‐RCTs. We did not identify any ongoing studies and no studies are awaiting classification.
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3 for details regarding risk of bias in included studies. We considered that there was a high risk of bias overall. There were problems with the design of both included studies and this is discussed in Brandt 2006, a report by the authors on the challenges in conducting both clinical trials. Additional information on risk of bias in Robbins 2008 and Logemann 2008, was provided in this article.
2.
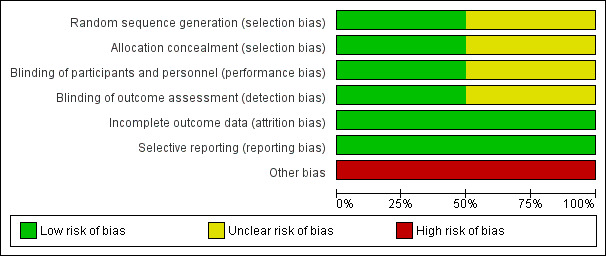
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
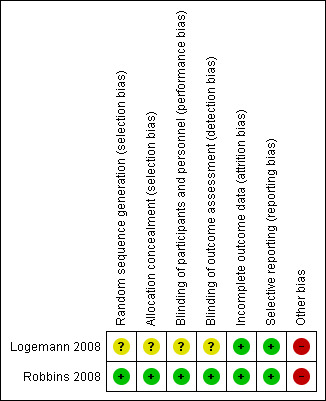
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Logemann 2008 did not provide information on concealment approaches. After demonstrating aspiration during a clinical videofluoroscopy, participants were randomised in the radiographic suite to a randomised sequence of three interventions (chin down head posture, nectar thick liquids and honey thick liquids). Participants received all three interventions. It is unclear if selection bias exists for Logemann 2008 as there is little information on randomisation methods and two participants were given all three interventions but not in a randomly assigned order. It is not clear if these participants had dementia. We therefore rated selection bias as unclear for Logemann 2008.
In Robbins 2008, randomisation sequences for primary assignment (chin down head posture versus thickened fluids) were described in detail and developed by a statistician at the statistical and data centre. The sequences were stratified by participant age and diagnosis and included randomly assigned block sizes of 32, 40 and 48 within each of the four strata. If a participant was assigned to a thickened liquid, a second randomisation was done to assign the participant to either the 'nectar thick' liquids group or 'honey thick' liquids group with equal probability. We rated the risk of selection bias in this study as low.
Blinding
In the videofluoroscopy suite in Logemann 2008, participants and personnel could not be blinded as to the intervention given the behavioural nature of the intervention for the chin down posture and the nature of modified fluids (Brandt 2006). Videofluoroscopy exams and outcome data were scored by experienced raters (Brandt 2006). Blinding of the outcome assessors was not possible in terms of awareness of the intervention provided (Brandt 2006), but there is no information on inter or intrarater reliability of the raters. There is also no reference to the standardisation of the videofluoroscopy equipment, and it is suggested that the pulse rate of the videofluoroscopy equipment can affect judgements of swallowing impairment (Bonilha 2013). We therefore rated the risk of performance and detection bias as unclear for Logemann 2008.
In Robbins 2008, neither the participants nor direct caregivers were blinded to the intervention assignment due to the positional nature of the chin down intervention and the different viscosities of the thickened liquid interventions (Brandt 2006). However, the review authors judge that the outcome measurements are not likely to be influenced by the lack of blinding. Outcomes in Robbins 2008 required objective documentation of a pneumonia event. Speech and language therapists, other healthcare professionals involved in the study, the study chair, the principal investigator of the Communication Sciences and Disorders Research Group (CSDRG) and staff at the co‐ordinating centre with day‐to‐day responsibility for working with the sites to resolve data queries and missing forms were blinded to any outcome reporting or analyses during the trial (Brandt 2006). We therefore rated the risk of bias for Robbins 2008 as low.
Incomplete outcome data
In Logemann 2008, the rates of aspiration during each intervention were provided. In Robbins 2008, information was provided regarding participant follow‐up and reasons for incomplete data were provided. Authors supplied all study data on request. We rated the risk of attrition bias for both studies as low.
Selective reporting
All analysed outcomes are included in the report for Logemann 2008 and Robbins 2008. Although the study protocols are not available, we judged the risk of reporting bias to be low.
Other potential sources of bias
The same participants were recruited to Logemann 2008 and Robbins 2008. In Logemann 2008's cross‐over trial, the interventions were delivered at the same point in time, however, there was a high risk for fatigue during the study, which would impact participants' response to intervention. There is no reference to the washout period between interventions in this study. Thicker consistencies may result in residue remaining in the oropharynx after a swallow. This residue may increase the likelihood of aspiration in subsequent swallows. Therefore, the response to the intervention may be affected by the earlier intervention delivered in the sequence. For statistical analysis, Logemann 2008 used a multivariable model that controlled for age, gender, and order in which the interventions were presented. They found that participants were significantly more likely to aspirate on honey thick liquids when this consistency was presented last in the sequence of interventions than when this consistency was presented first in the sequence (odds ratio (OR) 1.54, 95% confidence interval (CI) 1.17 to 2.01) (Logemann 2008).
In Robbins 2008, there was also a risk of bias in the delivery of the intervention, i.e. modification of the diet. There is a possibility that liquids provided to participants were not at the exact level of thickness or viscosity recommended and may have differed from the viscosity delivered in Logemann 2008, despite using the same descriptors of nectar thick and honey thick. Standardised recipes matching the viscosities of the barium products were developed for a wide variety of thickened beverages, but there is limited evidence that these viscosities were tightly controlled. There is no reference to the procedures in place to ensure that all liquids were thickened correctly to the specified consistency or viscosity. The authors also acknowledge that adherence to prescribed interventions was a problem. Meal monitoring was reduced to randomised sets of three meals per week across the three interventions and it is difficult to ascertain if the participants complied with the intervention outside of these times. In addition, the viscosity of honey thick liquids (3000 cPs) used in Logemann 2008 and Robbins 2008 were thicker than the descriptions used in the Dysphagia Diet Food Texture Descriptors (National Dysphagia Diet Task Force 2002). See Characteristics of included studies.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Nectar thick liquids (300 cPs) compared with regular liquids 15cPs.
| Nectar thick fluids (300 cPs) compared with regular fluids (15 cPs) to eliminate aspiration | |||
|
Patient or population: adults with dementia and dysphagia Settings: acute and subacute facilities in USA Intervention: nectar thick fluids (300 cPs) in neutral head position Comparison: regular non‐modified fluids (15 cPs) in neutral head position | |||
| Outcomes | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Elimination of aspiration on videofluoroscopy | 351 participants (1 RCT) | ⊕⊕⊝⊝ lowa | In Logemann 2008, all 351 people with dementia aspirated on regular fluids on videofluoroscopy, and so were eligible for the study, but 20% did not aspirate again on any of the interventions in the study. Nectar thick liquids (300 cPs) eliminated aspiration for 110/351 (31%) with 241/351 (69%) aspirating on this consistency. Although dementia subtypes are crudely categorised within the studies, there were some trends reported in this study. 64/109 (58.7%) people with Alzheimer's disease aspirated on nectar thick liquids with no postural adjustment in comparison to 80/109 (73.4%) people with single or multistroke type dementia and 97/133 (72.9%) people with other types of dementia. However, fatigue and the sequence of the interventions in the study may have influenced the results. The quality of the evidence is therefore graded as low. |
| GRADE Working Group grades of evidence
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate. RCT: randomised controlled trial | |||
aWe downgraded the quality of evidence by two levels for serious concerns about risk of bias and imprecision.
Summary of findings 2. Honey thick liquids (3000 cPs) compared with regular liquids (15cPs).
| Honey thick fluids (3000 cPs) compared with regular fluids (15 cPs) to eliminate aspiration | |||
|
Patient or population: adults with dementia and dysphagia Settings: acute and subacute facilities in USA Intervention: honey thick fluids (3000 cPs) in neutral head position Comparison: regular non‐modified fluids (15 cPs) in neutral head position | |||
| Outcomes | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Elimination of aspiration on videofluoroscopy | 351 participants (1 RCT) | ⊕⊕⊝⊝ lowa | In Logemann 2008, honey thick liquids (3000 cPs) eliminated aspiration for 42% (146/351) with 58% (205/351) aspirating on this consistency. Honey consistency fluids were more effective in reducing the frequency of aspiration than nectar consistency (P < 0.001). However, the authors note that participants were significantly more likely to aspirate on honey thick fluids when the intervention was presented last in the sequence than when the honey thick fluid intervention was presented first in the sequence (OR 1.54, 95% CI 1.17 to 2.01) (Logemann 2008). On honey thick fluids, 55/109 (50.5%) people with Alzheimer's disease aspirated on this consistency while 67/109 (61.5%) people with single or multistroke type dementia and 83/133 (62.4%) people with other types of dementia aspirated on honey thick fluids. Fatigue and the sequence of the interventions in the study may have influenced the results. The quality of the evidence is therefore graded as low. |
| GRADE Working Group grades of evidence
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate. RCT: randomised controlled trial | |||
aWe downgraded the quality of evidence by two levels for serious concerns about risk of bias and imprecision.
Summary of findings 3. Regular fluids (15cPs) and chin down head posture compared with regular fluids (15cPs) and neutral head position.
| Regular fluids (15 cPs) and chin down head posture compared with regular fluids (15 cPs) and neutral headposition to eliminate aspiration | |||
|
Patient or population: adults with dementia and dysphagia Settings: acute and subacute facilities in USA Intervention: regular fluids (15 cPs) and chin down head posture Comparison: regular fluids (15 cPs) in neutral head position | |||
| Outcomes | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Elimination of aspiration on videofluoroscopy | 351 participants (1 RCT) | ⊕⊕⊝⊝ lowa | Aspiration was eliminated for 26% (90/351) of participants with chin down head posture on regular fluids (15 cPs) when compared to regular fluids and neutral head position. 74% (261/351) of participants aspirated on regular fluids with chin down head posture. 69/109 (63.3%) people with Alzheimer's disease aspirated on regular fluids when using a chin down head posture; 89/109 (81.7%) people with single or multistroke type dementia aspirated on regular fluids when using a chin down posture and 103/133 (77.4%) people with other types of dementia aspirated on this consistency when using a chin down posture. Fatigue and the sequence of the interventions in the study may have influenced the results. The quality of the evidence is therefore graded as low. |
| GRADE Working Group grades of evidence
High quality: further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: we are very uncertain about the estimate. RCT: randomised controlled trial | |||
aWe downgraded the quality of evidence by two levels for serious concerns about risk of bias and imprecision.
See Table 1, Table 2, Table 3.
We compared the following interventions.
-
Intervention (modified fluids ‐ nectar thick and honey thick) versus no intervention (regular fluids)
All participants had aspirated on normal thin fluids (15 cPs) without any intervention, and so were eligible for the Logemann 2008 and Robbins 2008 studies. Interventions using fluids thickened to nectar (300 cPs) and honey (3000 cPs) consistency could be compared to no intervention in Logemann 2008. Both grades of fluid thickness (nectar thick versus honey thick) could also be compared with each other.
-
Intervention (modified fluids ‐ nectar thick and honey thick) versus other intervention that does not involve diet modification (chin down posture and regular fluids)
Diet modification with two grades of fluid thickness were compared with no modification to fluid consistency swallowed using a chin down head posture in Logemann 2008 and Robbins 2008.
The GRADE rating for quality of evidence was 'low' for all comparisons (Ryan 2016), i.e. nectar thick liquids (300 cPs) compared with regular liquids (15 cPs), honey thick liquids (3000 cPs) compared with regular fluids (15 cPs), and regular fluids (15 cPs) and chin down head posture compared with regular fluids (15 cPs) and neutral head position to eliminate aspiration. Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Primary outcomes
Logemann 2008 looked at the immediate effects of these interventions on the elimination of aspiration. Robbins 2008 conducted a three‐month follow‐up study on participants from Logemann 2008. Participants who did equally well (all conditions eliminated aspiration) or equally poorly (no conditions eliminated aspiration) but wished to continue oral intake, despite being warned about risk for pneumonia were randomly assigned to one of two interventions (thin liquid (15 cPs) swallowed in chin down posture) and thickened liquids with further randomisation within the thickened liquid group to nectar thick liquid (300 cPs) swallowed in neutral head position and honey thick liquid (3000 cPs) swallowed in neutral head position.
Logemann 2008 examined aspiration of fluids rated on objective instrumental swallowing assessment (videofluoroscopy). Robbins 2008 looked at the development of pneumonia diagnosed by chest x‐ray or by the presence of three respiratory indicators. Robbins 2008 provided the authors with unpublished data on adverse events judged to be associated with the intervention for people with dementia.
We were unable to pool data from the included studies because each study investigated different outcomes of interest. We therefore report on individual study results for the primary and secondary outcomes.
1. Efficacy of modified fluids (nectar and honey thick) versus no intervention
Aspiration
Overall, modification of fluids eliminated aspiration for some people with dementia with a statistically significant difference between effects of the grades of fluid consistency in eliminating aspiration. There was low‐quality evidence for this outcome (Ryan 2016).
In Logemann 2008, all 351 people with dementia aspirated on regular fluids on videofluoroscopy, and so were eligible for the study. Aspiration was eliminated for 26% on chin down head posture. Nectar thick liquids (300 cPs) eliminated aspiration for 110/351 (31%) with 241/351 (69%) aspirating on this consistency. Honey thick liquids (3000 cPs) eliminated aspiration for 146/351 (42%) with 205/351 (58%) of people with dementia who aspirated on regular fluids aspirating on honey thick fluids. Honey thick liquid was more effective in reducing the frequency of aspiration than nectar thick consistency (P < 0.001). However, the authors note that participants were significantly more likely to aspirate on honey thick liquids when the intervention was presented last in the sequence than when the honey thick liquid intervention was presented first in the sequence (OR 1.54, 95% CI 1.17 to 2.01) (Logemann 2008).
Overall, 69/351 (20%) people with dementia did not aspirate on any of the three interventions. Fourteen per cent (49/351) aspirated on one of the three interventions; 41/351 (12% ) aspirated on two of the three interventions; 192/351 (55%) aspirated on all three interventions (risk difference (RD) ‐0.67, 95% CI ‐0.71 to ‐0.63).
Athough dementia subtypes are crudely categorised within the studies, there were some trends reported in the data for Logemann 2008. On nectar thick liquids with no postural adjustment 64/109 (58.7%) people with Alzheimer's disease aspirated in comparison to 80/109 (73.4%) people with single or multistroke type dementia and 97/133 (72.9%) people with other types of dementia. On honey thick liquids, 55/109 (50.5%) people with Alzheimer's disease aspirated on this consistency, while 67/109 (61.5%) people with single or multistroke type dementia and 83/133 (62.4%) people with other types of dementia aspirated on honey thick liquids.
Nutritional status, respiratory status and adverse events were not recorded.
2. Efficacy of modified fluids (nectar and honey thick) versus chin down head posture and unmodified fluids
Aspiration
In Logemann 2008, 261/351 (74%) aspirated on normal unmodified fluids in chin down head posture while 241/351 (69%) aspirated on nectar thick liquid in a neutral head position and 205/351 (58%) aspirated on a honey thick liquid in a neutral head position. In pair‐wise comparisons, there was a stronger statistically significant difference between chin down head posture and honey thick liquids in eliminating aspiration (P < 0.001) than between chin down head posture and nectar thick liquids (P < 0.05). Chin down head posture significantly reduced the occurrence of aspiration on regular thin fluids when compared with regular thin fluids using a normal head posture (RD ‐0.73, 95% CI ‐0.77 to ‐0.69). Fourteen per cent (49/351) aspirated on one of the three interventions; 41/351 (12%) aspirated on two of the three interventions. Sixty‐nine out of 351 (20%) people with dementia did not aspirate on any of the interventions and 192/351 (55%) aspirated on all three interventions. Both of these groups were eligible for the follow‐up Robbins 2008 study (see below). There was low‐quality evidence for this outcome (Ryan 2016).
Of the participants with dementia, there were again trends according to the subtype of dementia. Sixty‐nine out of 109 (63.3%) people with Alzheimer's disease aspirated on normal fluids when using a chin down head posture; 89/109 (81.7%) people with single or multistroke type dementia aspirated on normal fluids when using a chin down posture and 103/133 (77.4%) people with other types of dementia aspirated on this consistency when using a chin down posture.
Nutritional status
In Robbins 2008, 260 people with dementia were eligible to participate from the Logemann 2008 study. Two hundred and sixty of the 351 (74%) people with dementia participated in this arm of the trial. These were randomised to chin down posture and thickened liquids. Participants randomised to thickened liquids were further randomised to nectar or honey consistencies. Over half, (131/260) (50.4%) were randomly assigned to normal thin liquids (15 cPs) using the chin down head posture intervention. Sixty‐six of the 260 participants (26%) were assigned to the nectar thick liquids (300 cPs) in a neutral head position intervention and 63/260 (24%) were assigned to the honey thick liquids (3000 cPs) in a neutral head position intervention. Nutritional status was not formally evaluated. However, the authors provided unpublished data on weight loss for participants with dementia for the study period and no weight loss was recorded for all participants, despite the fact that there were hospitalisations for malnutrition and dehydration. Five participants with dementia presented with dehydration during the study. Three recorded as "possibly related to study intervention"; and two "probably related to the study intervention". Both of these participants were on thickened fluids ‐ one on nectar and one on honey thick fluids. Further information on the impact of the intervention on nutritional status is described further under 'Hospitalisations' below.
Respiratory status
In Robbins 2008, definite pneumonia was diagnosed by chest radiography or by the presence of three or more respiratory indicators. These were temperature > 38 degrees celsius, rales, ronchi on chest auscultation, sputum gram stain showing substantial leukocytes or sputum culture showing a respiratory pathogen or suspected pneumonia with at least two of the four signs of definite pneumonia listed above. The respiratory status of the participants prior to entering the study was unknown, although participants were excluded if they had pneumonia within six weeks of enrolment. Data on improvement of respiratory function pre‐ and post‐interventions was not obtained. Adverse effects of the intervention on respiratory function is reported in the next section below.
Adverse events
Events of interest were those associated with diet modification including pneumonia, hospitalisation, psychological effects and death. The data on these adverse events were supplied by the authors and are primarily unpublished data. There was low‐quality evidence for this outcome (Ryan 2016).
Respiratory function
There were 56 incidents of pneumonia reported for 49 participants with dementia. Nineteen of the 56 (34%) incidents were reported in people assigned to regular thin liquids with chin down head posture, representing 14.5% of this intervention group. Twelve of the 56 (21%) incidents were reported in people assigned to the nectar thick liquids in the neutral head position intervention, representing 16% of this group and 20 of the 56 (36%) incidents were in the intervention group receiving honey thick liquids intervention, representing 32% of this intervention group.
Of these pneumonias: 19/56 (34%) were deemed "probable aspiration pneumonia"; 19/56 (34%) "possible aspiration pneumonia"; 18/56 (32%) "not aspiration pneumonia". Incidents of "probable aspiration pneumonia" on six occasions (32%) involved participants who were using chin down posture and normal thin liquids, representing 5% of this intervention group. Five incidents of probable aspiration (26%) involved participants receiving nectar thick liquids, representing 8% of this intervention group. Eight incidents (42%) involved participants who were receiving honey thick liquids, representing 13% of this intervention group.
Hospitalisations
Robbins 2008 reports 72 incidents of hospitalisations for 56 participants with a diagnosis of dementia over a three‐month period. Twenty‐nine of the 56 (52%) participants were using chin head down posture with thin liquids, representing 22% of this intervention group. Fifteen of the 56 participants (27%) were receiving nectar thick liquids, representing 24% of this intervention group. Twelve (21%) were receiving honey thick liquids, representing 19% of this intervention group.
Thirty‐one incidents of hospitalisations were associated with nutrition and hydration. Reasons for hospitalisations reported were dehydration (15 hospitalisations), loss of appetite (6 hospitalisations) and reduced food intake (10 hospitalisations). Of the 15 incidents of hospitalisations for dehydration, 7/15 (47%) hospitalisations were associated with participants using a chin down posture, 7/15 (47%) hospitalisations were associated with participants receiving nectar thick liquids, and 1/15 (6%) hospitalisation was associated with a participant receiving honey thick liquids.
Of the six hospitalisations for loss of appetite, 4/6 (67%) hospitalisations were associated with participants using a chin down posture with no modification to fluids and 2/6 (33%) were associated with participants on nectar thick liquids.
There were 10 hospitalisations for reduced food intake. Of the 10 hospitalisations, 6/10 (60%) were associated with participants using a chin down posture with no modifications to fluids, 3/10 (30%) were associated with participants receiving nectar thick liquids, and 1/10 (10%) were associated with participants receiving honey thick liquids.
Psychological effects
Psychological effects and quality of life were not examined as outcomes in participants with dementia.
Other adverse events
Eight of the 260 participants with dementia (3%) had urinary tract infections that the authors reported as "possibly related to the study intervention". Three of these eight participants were using chin down posture with regular thin liquids, representing 2% of this intervention group; five were on thickened liquids; two were receiving nectar thick liquids, representing 3% of this intervention group; and three were receiving honey thick liquids, representing 5% of this intervention group. One participant presented with a fever. This participant was receiving nectar thick liquids. The authors again reported this adverse event as "possibly related to the study intervention".
Deaths
In Robbins 2008, 41 participants with a diagnosis of dementia died during this study. Of these 41 participants, 16/41 (39%) deaths were recorded as "definitely not related to the intervention", 15/41 (37%) as "probably not related"; 9/41 (22%) were "unable to judge if related, and 1/41 (2%) was recorded as "probably related" to the intervention. There were no deaths classified as 'Definitely related' to the study procedure (i.e. the type of liquids the participant was receiving).
Twenty‐two (54%) participants who died were using a chin down head posture with thin liquids, representing 17% of this group. The deaths of 11/22 (50%) using chin down head posture were recorded as "definitely not related to the intervention"; 5/22 (23%) were recorded as "probably not related to the intervention"; 6/22 (27%) were recorded as "unable to judge if related to the intervention". No death was reported as 'probably related or definitely related to the intervention or to the study procedure' (i.e. the type of liquids that the participant was receiving).
Nine of the 41 participants (22%) who died were receiving nectar thick liquids, representing 14% of this group. Two of the nine (22%) deaths were recorded as "definitely not related to the intervention", five (56%) were recorded as "probably not related to the intervention", and two (22%) were recorded as "unable to judge if related to the intervention". No death was recorded as 'probably related to the intervention' or 'definitely related to the intervention'. None of these deaths were reported as 'definitely' related to the study procedure (i.e. the type of liquids the participant was receiving).
Ten of the 41 participants (24%) who died were receiving honey thick liquids, representing 16% of this group. Three of the 10 (30%) deaths were recorded as "definitely not related to the intervention". Five (50%) were recorded as "probably not related to the intervention", one (10%) was recorded as "unable to judge if related to the intervention", and one (10%) as "probably related to the intervention". No death was recorded as 'definitely related to the intervention'. None of these deaths were reported as 'definitely' related to the study procedure (i.e. the type of liquids the participant was receiving).
3. Efficacy of Modified Fluids (nectar and honey thick) versus other intervention that involves modification to sensory properties of food or fluid only
This comparison was not included in the studies eligible for this review.
Secondary outcomes
Non‐compliance with dietary modifications
Robbins 2008 measured participant compliance with each intervention monthly, over a three‐month period. Compliance was described as greater than 50% adherence to the intervention. The following are compliance rates for each intervention for participants with dementia.
Chin down posture: month 1 = 20/32 (63%); month 2 = 16/26 (62%); month 3 = 13/23 (57%).
Nectar thick liquids: month 1 = 26/32 (81%); month 2 = 18/25 (72%); month 3 = 16/22 (73%).
Honey thick liquids: month 1 = 33/35 (94%); month 2 = 24/27 (89%); month 3 = 21/23 (91%).
The percentages for compliance were highest for the honey thick liquids and lowest for the chin down head posture. Information on factors influencing compliance, such as severity of dementia requiring more recall of instructions for chin down head posture or dependence on others for meal preparation is unavailable. There was low‐quality evidence for this outcome (Ryan 2016).
Both studies were terminated early. The Data and Safety Monitoring Committee recommended discontinuing enrolment on the basis of a futility analysis, suggesting that enrolling additional participants would not change the findings (Robbins 2008).
Discussion
Summary of main results
This review examined the effectiveness of modifying the consistency of food and fluids in improving oral intake and eliminating aspiration in adults with dysphagia and dementia. We aimed to identify if any adverse effects were associated with modifying food and fluids. We did not retrieve any trials that involved modifying food; this highlights a gap in the research, as many people with dementia may be eating a pureed or soft consistency diet that may impact the outcomes specified here. The two included studies were part of the same large multicentre trial and included people with dementia and people with or without dementia and Parkinson's disease. Both examined modifying the viscosity of fluids. People with dementia comprised 49% of the study group in Logemann 2008 and 74% in Robbins 2008. While there were methodological flaws associated with both studies (and acknowledged by the authors), the findings from each study have relevance to the design of further research in the area and for consideration in clinical practice.
The methodology in both studies differed. Logemann 2008 examined the immediate effects of modification of fluids in the context of a clinical instrumental assessment environment, while Robbins 2008 assessed the longer‐term effects on people with dementia. One could argue that the Logemann 2008 study should be excluded on the basis that it provides information on the elimination of aspiration through diet modification at a specific snapshot in time; it was a cross‐over study and a washout period between interventions is not described. As dementia and dysphagia are both progressive, we planned to only include cross‐over trials in the review if the data from the first intervention period were reported, and we planned to use this trial data. However, we did change this in retrospect (see Differences between protocol and review). While Logemann 2008 was a cross‐over trial that randomised the order of interventions, data from the first intervention period are not available; the authors statistically analysed the order effects of the interventions on outcomes in people with dementia. We included the study as we believe that the findings are valuable in addressing the objectives of the review. Evaluation of the efficacy of diet modification in eliminating aspiration is carried out typically in these clinical settings using instrumental assessment. It is typically assumed that the positive effects of diet modification in eliminating aspiration during videofluoroscopy are reflected in the longer‐term outcomes for patients, but the findings from both trials challenge this assumption.
Logemann 2008 found that the most successful intervention for immediately eliminating thin liquid aspiration was honey thick liquid (3000 cPs) followed by nectar thick liquid (300 cPs), and then thin liquids using a chin down head posture. Robbins 2008, in a three‐month follow‐up study, found that participants assigned to a thickened liquids intervention had greater incidence of dehydration, urinary tract infection, and fever in comparison to participants using the chin down head posture and regular fluids. This suggests the need to consider the long‐term effects of diet modification with prolonged follow‐up of people with dementia who are on modified diets. Other modifications to fluids may show promise in future studies e.g. carbonation and altering temperature (Bulow 2003; Michou 2012; Sdravou 2012). These interventions need to be explored further with people with dementia.
People with dementia and dysphagia may perform differently on thickened fluids compared to other populations. People with dementia did perform differently to other clinical groups in both trials. In Logemann 2008, people with dementia, when compared with people with Parkinson's disease and no dementia, had a greater risk of aspiration on chin down head posture than on modified fluids, suggesting that this intervention may be different according to population and that interventions that are effective in some patient groups may not apply to people with dementia.
The consistency of fluid is important. While people with dementia in Logemann 2008 aspirated more frequently on nectar thick consistency than on honey thick liquid consistency, when compared with people with Parkinson's disease and no dementia, their risk of aspiration on nectar thick liquids was marginally lower than on honey thick liquids. The safety of thickened fluids for people with dementia may be highly individual therefore, and the degree of viscosity of fluids is important for reducing the risk of aspiration. In a clinical setting there is variability in thickening fluids (Newman 2016).
The products used to thicken fluids may influence outcome of interventions. Robbins 2008 used a starch‐based thickener (Resource Thicken Up, Nestlé Healthcare Nutrition) which may not have matched the consistencies used during the videofluoroscopy examinations where a product with a reasonably controlled level of viscosity (Varibar) was used. The use of a starch‐based thickener also means viscosities used may be more unstable (Garcia 2005). Starch‐based thickeners may not dissolve well in some liquids, they can appear 'cloudy', may taste 'starchy,' and may continue to thicken over time. Bolus rheology is complex and can be influenced by a variety of factors e.g. temperature and saliva (Hanson 2012). It is also suggested that fluids thickened with modified starch may be associated with increased pharyngeal residue in some populations (Vilardell 2016).
The person with dementia's response to the intervention may be influenced by the sequencing of the intervention in videofluoroscopy, as in Logemann 2008, where fatigue or residue from earlier swallows are factors influencing outcome. It is established that higher viscosity liquids tend to cause greater residue in the pharynx (Hind 2012). Videofluoroscopy also provides a single snap shot of swallow function at a specific point in time. Sixty‐nine people with dementia (20%) who aspirated, and so were eligible for the Logemann 2008 trial, did not aspirate again on any consistency during the videofluoroscopy study.
Severity and type of dementia may be important. In Logemann 2008, people with severe dementia exhibited least effectiveness on all interventions. The subtype of dementia may need to be explored. There were some differences in response to intervention according to the subtype of dementia. Almost two‐thirds (69/109; 63.3%) of people with Alzheimer's disease aspirated on regular fluids when using a chin down head posture; 89/109 (81.7%) people with single or multistroke type dementia aspirated on normal fluids when using a chin down posture and 103/133 (77.4%) people with other types of dementia aspirated on this consistency when using a chin down head posture. This suggests that further studies should consider the type of dementia in selecting participants for trials. While numbers are small and need to be considered in the context of other variables, it is known that dysphagia varies according to the subtype of dementia (Walshe 2014).
Brandt 2006 provides direction for the design and implementation of clinical trials in this area. They cite economic challenges, a constantly changing health environment and institutions new to research in general and clinical trials in particular, as the main difficulties in the conduct of these trials.
Overall completeness and applicability of evidence
There is insufficient evidence to allow definitive statements to be made on the effectiveness of modifying the consistency of fluids for swallowing difficulties in dementia. Both included trials suggest that thickening fluids in the long‐term may be associated with adverse events for some people with dementia.
However, it is not possible to reach any further conclusions based on only two trials with inherent methodological flaws.
Quality of the evidence
The authors used GRADE to rate the quality of evidence for each outcome (Ryan 2016). The overall rating of evidence for outcomes in this review using GRADE is low. We downgraded the quality of the evidence because of serious concern about risk of bias and imprecision.
Potential biases in the review process
The authors are not aware of any potential biases in the review process.
Agreements and disagreements with other studies or reviews
We are not aware of any other systematic reviews on this topic.
Authors' conclusions
Implications for practice.
Clinicians should be aware that while thickening fluids may have an immediate effect on the swallow function during videofluoroscopy, people with dementia on thickened fluids require long‐term follow‐up, with monitoring for adverse events such as respiratory events, malnutrition and dehydration. It is important that the risks, benefits and lack of evidence to support the use of thickened liquids are discussed with patients and family members. Clinicians also need to be aware that the efficacy of thickened fluids may be influenced by the type of dementia, although this requires further investigation.
Implications for research.
The findings from this review have significant implications for the conduct of future trials in this area.
Cluster‐randomised controlled trials (RCTs) may play a role here. Weijenberg 2013 argues for matched cluster‐randomised, single‐blinded, multicentre study designs for examining interventions such as this in people with dementia residing in care homes. Heterogeneity through careful design can be reduced, and the intervention can be delivered in the patient's care environment. A key strength is the direct clinical applicability of the results.The advantages of a cluster‐RCT are that larger numbers of participants can be recruited over a smaller number of research sites. These research sites would be specifically trained in the intervention, thus reducing some of the problems encountered in the Robbins 2008 study, as discussed by Brandt 2006. Cluster‐RCTs may also eliminate the potential for contamination, where participants are allocated to different interventions within one setting and care staff may treat the control group differently because of exposure to interventions during training. Control sites can provide usual care. Studies should compare different food and fluid interventions using clinically relevant outcomes e.g. pneumonia, nutritional status, etc.
The population of people with dementia need to be clearly defined with consideration to the stage and type of dementia. Dementia should be staged using a recognised and validated tool. In Robbins 2008, subtypes of dementia were crudely defined and did not permit further analysis. However, preliminary data suggests that subtypes and severity may be important factors in influencing outcomes.
It is important that people with dementia are involved at the early stages of the disease, to obtain information on quality of life and diet preference. Previous research suggests that thickening liquids can impact on quality of life (Hao Wang 2016; Swan 2015).
Where possible, instrumental assessments using valid reliable outcome measures must be used to determine the nature and severity of dysphagia. Aspiration should be rated using a validated scale, such as the Penetration Aspiration scale (Rosenbek 1996).
The intervention (thickened fluids) should be clearly defined. Specifically, the rheology of fluids needs to be standardised, and those fluids that are found to eliminate aspiration on videofluoroscopy should be matched closely at mealtime and in longer‐term follow‐up studies. The barium used in Logemann 2008 was a commercial product (Varibar). This may not have been the correct viscosity as it may have been thicker than regular thin liquids e.g. water (1 cP at 20° Celsius), and the fact that the viscosity of honey thick liquids (3000 cPs) used in this study were thicker than the descriptions used in national diet descriptors (National Dysphagia Diet Task Force 2002). There was no information provided regarding flow characteristics and shear rate (a quantified measure of the speed and flow of a liquid) in either study. It is now widely accepted that information on the viscosity of liquids alone is insufficient (Steele 2015). Future research studies should provide sufficient detail on these bolus characteristics to allow for replication and translation to clinical practice. No information was provided in Robbins 2008 regarding the preparation of liquids (e.g. temperature) and challenges in achieving a consistent viscosity are well documented in starch‐based thickeners (Payne 2011). Further research should address this.
Consideration should be given to the type of thickener used (xanthan gum versus corn starch) and the efficacy of these thickening agents for people with dementia must be considered. Different levels of viscosities should be trialled, with subgroup analysis of the efficacy of different viscosities on swallowing outcomes.
A comparison intervention group should include a 'usual care' group, where individuals with dementia have chosen not to have thickened fluids. In Robbins 2008 a control group was not included.
Other forms of diet modification should be examined. These should include use of carbonation, varying fluid temperatures, etc. The mealtime environment must also be considered in designing clinical trails, as high levels of distraction can increase the risk of aspiration and choking.
The assessment procedure used in research protocols should mimic typical swallowing behaviour. In Logemann 2008, participants were instructed to take the thickened fluids from a spoon, hold in their mouths and then swallow it on command. This method of swallowing also does not mirror typical swallowing behaviour. People with more severe dementia may find this instruction difficult and are more likely to swallow the liquid immediately or hold the bolus in the mouth for a prolonged period without swallowing. Cross‐over trials should ensure that a washout period is built into the intervention study, with control for patient fatigue if multiple swallow trials are used.
The outcomes of the study that relate to respiratory function should be objectively measured. In Robbins 2008, aspiration as a contributing factor to the incidents of pneumonia was not clearly diagnosed, and therefore the findings are difficult to interpret reliably. In Robbins 2008, 41 participants with a diagnosis of dementia died during this study. There were no deaths classified as 'definitely related' to the study procedure (i.e. the type of liquids the participant was receiving). Participants assigned to thickened liquids had greater compliance with this intervention than those assigned to normal thin liquids with chin down head posture. It is possible that memory difficulties associated with dementia may have impacted on participants' consistent use of a chin down head posture.
Adherence to interventions must be closely monitored for reliability of findings.
Outcomes examined should include respiratory function, nutritional status, quality of life, and participant satisfaction, in addition to elimination of aspiration and adverse events. Outcome measures should include a validated measure of nutrition and objective measures of respiratory function, detection of aspiration‐related events and confirmation of the presence or absence of aspiration‐related pneumonia.
Follow‐up of participants should be extended beyond three months to at least two years so that the effectiveness of the intervention is evident. Participant follow‐up should include three‐, six‐, nine‐, 12‐ and 18‐month time points.
All clinical trials should be reported according to CONSORT guidelines (Schulz 2010).
Acknowledgements
As part of the pre‐publication process, information specialists Anna Noer‐Storr and Candida Fenton provided support in developing our search strategy.
Appendices
Appendix 1. Sources searched and search strategies
| Source | Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) ALL DATES [Latest search: 09 May 2018] |
Diet* OR food* OR liquid* OR fluid* OR solid* OR feed* OR eat* OR meal* OR swallow* | May 2014: 240 March 2015: 0 December 2015: 0 September 2016: 0 May 2017: 0 May 2018: 0 |
| 2. MEDLINE In‐process and other non‐indexed citations and MEDLINE 1946‐present (Ovid SP) [Latest search: 09 May 2018] |
1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. exp Deglutition Disorders/ 22. dysphagia.ti,ab. 23. swallow*.ti,ab. 24. ((cough* or chok*) adj6 (eat* or food or meal* or drink*)).ti,ab. 25. "food sticking".ti,ab. 26. regurgitat*.ti,ab. 27. odynophagia.ti,ab. 28. drool*.ti,ab. 29. ("weight loss" or (los* adj3 weight)).ti,ab. 30. (nutri* adj3 deficien*).ti,ab. 31. oesophagitis.ti,ab. 32. "peptic stricture".ti,ab. 33. or/21‐32 34. 20 and 33 35. Diet/ 36. diet*.ti,ab. 37. (fluid* or liquid* or drink*).ti,ab. 38. (food* or solid* or meal* or consistency).ti,ab. 39. or/35‐38 40. 34 and 39 41. randomized controlled trial.pt. 42. controlled clinical trial.pt. 43. randomized.ab. 44. placebo.ab. 45. randomly.ab. 46. trial.ab. 47. groups.ab. 48. or/41‐47 49. 40 and 48 50. 2014*.ed. 51. 50 and 49 |
May 2014: 3 March 2015: 5 December 2015: 6 September 2016: 58 May 2017: 9 May 2018: 9 |
| 3. EMBASE 1974‐2017 May 01 (Ovid SP) [Latest search: 09 May 2018] |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. dysphagia/ 25. dysphagia.ti,ab. 26. swallow*.ti,ab. 27. ((cough* or chok*) adj6 (eat* or food or meal* or drink*)).ti,ab. 28. "food sticking".ti,ab. 29. regurgitat*.ti,ab. 30. odynophagia.ti,ab. 31. drool*.ti,ab. 32. ("weight loss" or (los* adj3 weight)).ti,ab. 33. (nutri* adj3 deficien*).ti,ab. 34. oesophagitis.ti,ab. 35. "peptic stricture".ti,ab. 36. or/24‐35 37. 23 and 36 38. randomized controlled trial/ 39. controlled clinical trial/ 40. randomly.ab. 41. placebo.ab. 42. trial.ab. 43. "single‐blind*".ti,ab. 44. "double‐blind*".ti,ab. 45. or/38‐44 46. 37 and 45 47. 2014*.em. 48. 46 and 47 |
May 2014: 12 March 2015: 28 December 2015: 36 September 2016: 11 May 2017: 41 May 2018: 103 |
| 4. PSYCINFO 1806‐April week 4 2017 (Ovid SP) [Latest search: 09 May 2018] |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. exp Dysphagia/ or exp Swallowing/ 26. dysphagia.ti,ab. 27. swallow*.ti,ab. 28. ((cough* or chok*) adj6 (eat* or food or meal* or drink*)).ti,ab. 29. "food sticking".ti,ab. 30. regurgitat*.ti,ab. 31. odynophagia.ti,ab. 32. drool*.ti,ab. 33. ("weight loss" or (los* adj3 weight)).ti,ab. 34. (nutri* adj3 deficien*).ti,ab. 35. oesophagitis.ti,ab. 36. "peptic stricture".ti,ab. 37. or/25‐36 38. 24 and 37 39. trial.ab. 40. "single‐blind*".ti,ab. 41. randomly.ab. 42. "double‐blind*".ti,ab. 43. exp Clinical Trials/ 44. rct.ti,ab. 45. or/39‐44 46. 38 and 45 |
May 2014: 1 March 2015: 3 December 2015: 7 September 2016: 10 May 2017: 4 May 2018: 4 |
| 5. CINAHL (EBSCOhost) [Latest search: 09 May 2018] |
S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 (MH "Swallowing Therapy") OR (MH "Deglutition Disorders") S21 TX dysphagia S22 TX swallow* S23 TX (cough* or chok*) n6 (eat* or food or meal* or drink*) S24 TX "food sticking" S25 TX regurgitat* S26 TX odynophagia S27 TX drool* S28 TX "weight loss" or (los* n3 weight) S29 TX nutri* n3 deficien* S30 TX oesophagitis S31 TX "peptic stricture" S32 S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 S33 S19 AND S32 S34 MH "Clinical Trials" S35 TX trial S36 TX "single‐blind*" S37 TX "double‐blind*" S38 TX "treatment as usual" S39 TX randomly S40 S34 OR S35 OR S36 OR S37 OR S38 OR S39 S41 S33 AND S40 S42 EM 2014 S43 S41 AND S42 |
May 2014: 1 March 2015: 11 December 2015: 15 September 2016: 21 May 2017: 14 May 2018: 21 |
| 6. ISI Web of Science [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports]; BIOSIS Previews [Latest search: 09 May 2018] |
TOPIC: (dement* OR alzheimer* OR "vascular cognitive impairment" OR "lew* bod*" OR CADASIL OR "cognit* impair*") AND TOPIC: (swallow* OR dysphagia OR "food sticking" OR regurgitat* OR "food consistency" OR "fluid consistency") AND TOPIC: (randomly OR placebo OR groups OR trial OR RCT OR randomized OR randomised OR "double‐blind*" OR "single‐blind*" OR CCT OR "cross‐over" OR crossover) Timespan: Year to date. Search language=English |
May 2014: 24 March 2015: 20 December 2015: 25 September 2016: 53 May 2017: 48 May 2018: 48 |
| 7. LILACS (BIREME) [Latest search: 09 May 2018] |
dementia OR demencia OR demência OR alzheimer OR alzheimers OR alzheimer’s [Words] and swallow OR swallowing OR eating OR drinking OR dysphagia [Words] | May 2014: 0 March 2015: 0 December 2015: 1 September 2016: 3 May 2017: 4 May 2018: 2 |
| 8. CENTRAL (CRSO) [Latest search: 09 May 2018] |
#1 MESH DESCRIPTOR dementia EXPLODE ALL TREES #2 dement*:TI,AB,KY #3 alzheimer*:TI,AB,KY #4 #1 OR #2 OR #3 #5 MESH DESCRIPTOR Deglutition Disorders EXPLODE ALL TREES #6 dysphagia:TI,AB,KY #7 swallow*:TI,AB,KY #8 ("food sticking"):TI,AB,KY #9 regurgitat*:TI,AB,KY #10 odynophagia:TI,AB,KY #11 drool*:TI,AB,KY #12 (nutri* adj3 deficien*):TI,AB,KY #13 ("weight loss" or (los* adj3 weight)):TI,AB,KY #14 oesophagitis:TI,AB,KY #15 ("peptic stricture"):TI,AB,KY #16 ((cough* or chok*) adj6 (eat* or food or meal* or drink*)):TI,AB,KY #17 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 #18 #4 AND #17 |
May 2014: 47 March 2015: 0 December 2015: 15 September 2016: 11 May 2017: 14 May 2018: 73 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) [Latest search: 09 May 2018] |
(dysphagia OR swallowing OR deglutition OR regurgitate OR regurgitation OR oesophagitis OR swallow) AND (dementia OR alzheimer’s OR Alzheimer) | May 2014: 8 March 2015: 0 December 2015: 1 September 2016: 3 May 2017: 2 May 2018: 9 |
| 10. ICTRP Search Portal (apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] [Latest search: 09 May 2018] |
(dysphagia OR swallowing OR deglutition OR regurgitate OR regurgitation OR oesophagitis OR swallow) AND (dementia OR alzheimer’s OR Alzheimer) | May 2014: 2 March 2015: 0 December 2015: 1 September 2016: 1 May 2017: 0 May 2018: 2 |
| TOTAL before de‐duplication | May 2014: 338 March 2015: 67 December 2015: 107 September 2016: 198 May 2017: 136 May 2018: 271 |
|
| TOTAL after de‐duplication and first‐assessment | May 2014: 29 March 2015: 0 December 2015: 6 September 2016: 24 May 2017: 23 May 2018: 109 |
|
Appendix 2. Data Extraction Form
Modifying the consistency of food and fluids for swallowing difficulties in dementia
Study ID: ____________________ Lead Author: ___________________ Reviewer Initials: ___________________ Date of Review: _____________________
General study information
| First Author | Year | Journal/Conference Proceeding etc | Country | Language | Single/Multicentre Trial | Study Duration |
| |
Study eligibility
| RCT/CCT | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes/No/Unclear |
Yes/No/Unclear |
Yes/No/Unclear |
Yes/No/Unclear |
| Do not proceed if any of the above answers are “No” If study to be included in “Excluded Studies” section of the review, record below the information to be inserted into “Table of excluded studies” |
| |
Participant and trial characteristics
| Intervention group | Comparison group 1 | Comparison group 2 | |
| Participants | N= | N= | N= |
| Age (mean, median, range, SD): | Mean: Median: Range: SD: |
Mean: Median: Range: SD: |
Mean: Median: Range: SD: |
| Gender of participants: | Male N= Female N= Both N= Not clear |
Male N: Female N: Both N: Not clear |
Male N: Female N: Both N: Not clear |
| Type of dementia within groups: |
1. N= 2. N= 3. N= 4. N= 5. N= |
1. N= 2. N= 3. N= 4. N= 5. N= |
1. N= 2. N= 3. N= 4. N= 5. N= |
| Severity of dementia: | Mild N= Moderate N= Severe N= |
Mild N= Moderate N= Severe N= |
Mild N= Moderate N= Severe N= |
| Co‐morbidities within exclusion criteria |
Trial characteristics
| Treatment group | Comparison group 1 | Comparison group 2 | |
| Interventions 1. Modified fluids 2. Modified food |
|||
| How was participant eligibility defined? | |
||
| Type of thickener used if relevant? | |
||
| Grade of viscosity | |
||
| Type of texture | |
||
| Time frames considered | |
||
| Trial design | |
Methodological quality
|
Selection bias: · Sequence generation · Allocation concealment |
Adequate/Inadequate/Unclear Adequate/Inadequate/Unclear |
Adequate/Inadequate/Unclear Adequate/Inadequate/Unclear |
Adequate/Inadequate/Unclear Adequate/Inadequate/Unclear |
|||
|
Performance bias: · Blinding of participants · Blinding of other personnel |
Yes/No/Unclear Yes/No/Unclear |
Yes/No/Unclear Yes/No/Unclear |
Yes/No/Unclear Yes/No/Unclear |
|||
|
Detection bias: · Use of outcome measures apparent · Blinding of outcome assessors |
Yes/No/Unclear Yes/No/Unclear |
Yes/No/Unclear Yes/No/Unclear |
Yes/No/Unclear Yes/No/Unclear |
|||
|
Reporting bias · Time lag to publication · Language · Duplicate publication · Citation reporting · Outcome reporting |
Yes/No/Unclear ___________________ Yes/No/Unclear Yes/No/Unclear Yes/No/Unclear |
Yes/No/Unclear ___________________ Yes/No/Unclear Yes/No/Unclear Yes/No/Unclear |
Yes/No/Unclear ___________________ Yes/No/Unclear Yes/No/Unclear Yes/No/Unclear |
|||
|
Attrition bias: · Incomplete outcome data · Reasons specified |
Yes/No/Unclear Yes/No/Unclear |
Yes/No/Unclear Yes/No/Unclear |
Yes/No/Unclear Yes/No/Unclear |
|||
| Intention‐to‐treat | All participants entering trial | 15% of fewer included | More than 15% included | Not analysed as “intention to treat” | Unclear | Withdrawals described Yes No |
Data extraction
| Treatment group | Comparison group 1 | Comparison group 2 | |
| Reduction or elimination of aspiration or laryngeal penetration on food or fluids, or both, as rated on objective assessment (videofluoroscopy, fibreoptic endoscopic examination of swallowing (FEES) safety (Yes/No). | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear |
| Change to nutritional status as measured by increase in weight, prevention of weight loss, increase in grip strength, increase in calorific intake, change in standardized and validated screening tool such as The Mini Nutritional Screening Tool (Guigoz 1996), the Malnutrition Universal Screening Tool (Stratton 2004), reduction in number of hospitalisations for rehydration (Yes/No). | Improved/ deteriorated/ unchanged/ not reported | Improved/ deteriorated/ unchanged/ not reported | Improved/ deteriorated/ unchanged/ not reported |
| Change to respiratory status defined by clinical assessment that may include a chest x‐ray, decreased incidence of aspiration‐related pneumonia (Yes/No). | Reduction/No reduction/ Increase/ Not clear |
Reduction/No reduction/ Increase/ Not clear |
Reduction/No reduction/ Increase/ Not clear |
| Adverse events associated with diet modification including hospitalisation, psychological effects, aspiration pneumonia, malnutrition, dehydration and death (Yes/No). | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear |
| Non compliance with dietary modifications. | Yes/No/Unclear | Yes/No/Unclear | Yes/No/Unclear |
| Change in quality of life as measured by patient/carer report, validated quality of life measures, validated psychosocial impact measures. | Improved/ deteriorated/ unchanged/ not reported | Improved/ deteriorated/ unchanged/ not reported | Improved/ deteriorated/ unchanged/ not reported |
| Other information which you feel is relevant to the results |
| |
| Overall quality score (GRADE rating) | |
| · High · Moderate · Low · Very low |
High: randomised trial/double un graded Intervention studies. Further research is very unlikely to change our confidence in the estimate of effect |
| Moderate: downgraded randomised trials/upgraded observational studies. Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate | |
| Low: double‐downgraded randomised trials/observational studies. Low quality ‐ further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate | |
| Very low: triple‐downgraded randomised trials/downgraded observational studies/case series/case reports. Any estimate of effect is very uncertain. | |
Review author comments:
Signed: _____________________________
Date: ____________________________
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Logemann 2008.
| Methods | Multicentre trial Randomised, controlled, cross‐over design |
|
| Participants | The study was conducted in USA. Enrolment was from 20 May 1998 to September 16 2005. 711 participants were recruited from 47 acute care hospitals and 79 subacute residential facilities including nursing homes. 351/711 had dementia. All were over 50 years old with a physician‐identified diagnosis of dementia. Stage and severity of dementia was measured using the Bedford Alzheimer Nursing Severity Scale (Volicer 1994). Participants with dementia are the focus of the analysis. Inclusion criteria
Exclusion criteria
Gender: 204/351 male; 147/351 female Age range: 50‐95 years; mean age: 81.26 years; SD: 7.54 years Type of dementia: 109/351 participants had Alzheimer's disease; 109/351 had single or multistroke dementia; and 133/711 were identified as "other" types of dementia Race: white not of hispanic origin = 279/351; black not of hispanic origin = 43/351; hispanic = 10/351; Asian or Pacific Islander = 18/351; American Indian/Alaskan Native = 0; Other = 1 Feeding method: 31/351 participants had a gastrostomy tube; 320/351 did not have a gastrostomy tube Educational level: 5/351 = no formal education; 83/351 = some grammar school (grades 1‐8); 74/351 = some high school (grades 9‐12); 90/351 = high school graduate or equivalent; 62/351 = one or more years of college; 22/351 = one or more years of graduate education; missing data = 15 |
|
| Interventions | Having confirmed that the person was aspirating on one or more trial swallows of thin liquid on videofluoroscopy, participants were randomised in the radiographic suite to receive all three interventions (randomly assigned order) during videofluoroscopy Intervention 1 Nectar thick liquids (300 cPs) with no postural adjustment Experimental: normal head posture with nectar thick liquids (3 swallows of 3 mL from spoon and 3 self‐regulated swallows performed as separate swallows from 8 oz cup filled with 6 oz regular liquid (15 cPs) Control: normal head posture. Regular thin liquids (15 cPs) (3 swallows of 3 mL from spoon and 3 self‐regulated swallows from 8 oz cup filled with 6 oz liquid 15 cPs) Intervention 2 Honey thick liquids (3000 cPs) with no postural adjustment Experimental: normal head posture with honey thick liquids (3 swallows of 3 mL from spoon and 3 self‐regulated swallows performed as separate swallows from 8 oz cup filled with 6 oz regular thin liquid (15 cPs) Control: normal head posture. Regular thin liquids (15 cPs) (3 swallows of 3 mL from spoon and 3 self‐regulated swallows from 8 oz cup filled with 6 oz liquid) Intervention 3 Chin down posture while consuming regular thin liquids (15 cPs). Chin down posture was defined as touching the chin to the front of the neck. Participants were instructed to put their chins down to touch their chests or necks. In some cases, they were assisted with gentle pressure on their head. Some of the participants with dementia needed repetitions of the instructions. People were excluded if they could not perform a chin down intervention Experimental: chin down posture with regular thin liquids (15 cPs) (3 swallows of 3 mL from spoon and 3 self‐regulated swallows from 8 oz cup filled with 6 oz thin liquid) Control: normal head posture. Regular thin liquids (15 cPs) (3 swallows of 3 mL from spoon and 3 self‐regulated swallows from 8 oz cup filled with 6 oz thin liquid) |
|
| Outcomes | Elimination of aspiration on videofluoroscopy | |
| Length of follow‐up | Immediate effects of three interventions for aspiration of thin liquids only | |
| Funding source | No funding source is provided. Robbins 2008 acknowledges National Institute on Deafness and Other Communication Disorders, National Institute of Health, Novartis, E‐Z‐EM, American Speech Hearing Association, and Communication Sciences Disorders Clinical Trials Research Group. The thin, nectar and honey thick liquids were produced by E‐Z‐EM Corporation (Lake Success, NY; Varibar) specifically for the study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | This is a cross‐over design. Patients meeting entry criteria for the study were referred for videofluoroscopy. Aspiration on one or more of the six trial swallows on videofluoroscopy qualified the patient for the study and randomisation. Patients were randomised in the radiography suite. Randomisation was stratified by age at enrolment and by diagnosis (dementia/Parkinson's disease). Participants were then randomised to a sequence of three interventions. The authors report that one additional patient was randomised two weeks short of the minimum eligibility age of 50 years, six patients were incorrectly stratified, and two patients were given all three interventions but not in the randomly assigned sequence. All were included in the analysis. It is unclear if these two patients had dementia. There is insufficient information on randomisation of participants to specific sequences of interventions. |
| Allocation concealment (selection bias) | Unclear risk | There is insufficient information on allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | There is insufficient information on blinding of participants, personnel delivering the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Videofluoroscopy exams and outcome data were scored by experienced raters, however blinding of the raters was also not possible due to the nature of the intervention of the chin down posture and modified fluids (Brandt 2006). There is no information on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From a total of 711 participants, 351 participants with dementia were randomised to order of presentation of interventions. All completed the study. There were no intervention withdrawals and no trial group changes as it was a cross‐over design with interventions delivered in one data collection session. There was no missing data. Authors supplied all data on request. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but the published report includes the prespecified outcomes. All analysed outcomes are included in the report. Aspiration frequency, and rates of aspiration on each intervention are also provided for study participants, subgrouped by diagnosis. |
| Other bias | High risk | There is a possibility of a carry‐over effect from the thickened liquid interventions. There is no reference to the washout period between interventions in this study. Thicker consistencies may result in residue remaining in the oropharynx after a swallow. This residue may increase the likelihood of aspiration in subsequent swallows. Fatigue of swallow may also have occurred in this cross‐over trial design. The authors noted that participants were significantly more likely to aspirate on honey thick liquids when this consistency was presented last in the sequence of interventions than when this consistency was presented first in the sequence. This may be due to a fatigue effect. |
Robbins 2008.
| Methods | Multicentre trial Randomised, controlled, parallel design trial. The study period was 9 June 1998 to 9 December 2005. |
|
| Participants | Country: USA 515 participants were eligible for this study and 260/515 had dementia only. These are the focus of the analysis. All had participated in the Logemann 2008 study. All people with dementia had a physician‐identified diagnosis of dementia. Stage and severity of dementia was measured using the Bedford Alzheimer Nursing Severity Scale (Volicer 1994). Inclusion criteria
Exclusion criteria
Gender: 155 male; 105 female Age: range: 51‐95 years; mean age: 81.18 years; SD: 8.18 years Type of dementia: 88/260 participants had Alzheimer's disease, 75/260 had single or multistroke dementia, and 97/260 were identified as "other" types of dementia Race: white not of hispanic origin = 204/260; black not of hispanic origin = 33/260; hispanic = 8/260; Asian or Pacific Islander = 14/260; American Indian/Alaskan Native = 0; Other = 1 Feeding method: 22/260 participants had a gastrostomy; 238/260 did not have a gastrostomy Educational level: 2/260 = no formal education; 57/260 = some grammar school (grades 1‐8); 58/260 = some high school (grades 9‐12); 68/260 = high school graduate or equivalent; 46/260 = one or more years of college; 18/260 = one or more years of graduate education; missing data = 11 131 participants were assigned to the regular thin liquids (15 cPs) using the chin down posture intervention 66 participants were assigned to the nectar thick liquids (300 cPs) in a neutral head position intervention 63 participants were assigned to the honey thick liquids (3000 cPs in a neutral head position intervention |
|
| Interventions | Participants were randomly assigned to one of three interventions Intervention 1 Nectar thick liquids (300 cPs) with no postural adjustment Intervention 2 Honey thick liquids (3000 cPs) with no postural adjustment Intervention 3 Chin down posture while consuming thin liquids (15 cPs) Chin down was defined as touching the chin to the front of the neck. Participants were instructed to put their chins down to touch their chests or necks. In some cases, they were assisted with gentle pressure on their head. Some of the participants with dementia needed repetitions of the instructions. People were excluded if they could not perform a chin down intervention. |
|
| Outcomes |
|
|
| Length of follow‐up | Follow‐up was limited to a three‐month period after intervention | |
| Funding source | Grant support was obtained from the National Institute of Deafness and Other Communication Disorders, National Institutes of Health (DC03206). Additional support was provided by Novartis and E‐Z‐EM to the American Speech‐Language‐Hearing Association, Communication Sciences and Disorders Clinical Trials Research Group. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequences for primary assignment (chin down posture versus thickened fluids) were developed by a statistician at the statistical and data centre. The sequences were stratified by participant age and diagnosis and included randomly assigned block sizes of 32, 40 and 48 within each of the four strata. If a participant was assigned to a thickened liquid, a second randomisation was done to assign the participant to the 'nectar thick' liquids group or 'honey thick' liquids group with equal probability. |
| Allocation concealment (selection bias) | Low risk | This is described in detail by Robbins 2008. Participants were randomly assigned centrally by a telephone system controlled by the statistical and data centre at the EMMES Corporation (Rockville, Maryland). A study speech language pathologist called a central telephone number and entered participant criteria when prompted to by using the telephone keys. If the participant was eligible, an intervention was assigned and a summary page that included intervention assignment and meal monitoring was faxed to the speech language pathologist. Randomisation sequences for primary assignment (chin down posture versus thickened liquids) were developed by a statistician at the statistical and data centre. The sequences were stratified by participant age (50 to 79 years or 80 to 95 years) and diagnosis and included randomly assigned block sizes of 32, 40, or 48 within each of the four strata. If a participant was assigned to thickened liquids, a second randomisation was done to assign the participant to the 'nectar thick' liquids group or 'honey thick' liquids group with equal probability. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neither the participants nor direct caregivers were blinded to intervention assignment. The outcome measurements are not likely to be influenced by the lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes in Robbins 2008 required objective documentation of a pneumonia event. Speech language pathologists, other healthcare professionals involved in the study, the study chair, the principal investigator of the CSDRG and staff at the co‐ordinating centre with day‐to‐day responsibility for working with the sites to resolve data queries and missing forms were blinded to any outcome reporting or analyses during the trial (Brandt 2006). The risk of bias for Robbins 2008 therefore is rated as low. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information regarding participant follow‐up and reasons for incomplete data were provided. Authors supplied all data on request. |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available but the published report includes the prespecified outcomes. All analysed outcomes are included in the report. |
| Other bias | High risk | There are two important risks for bias in the design and implementation of the study.
|
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Keller 2003 | Quasi‐experimental study investigating whether body weight can be maintained or improved in dementia residents of special care units using a comprehensive intervention strategy. Study excluded as food/fluid consistency was not modified |
| Weijenberg 2013 | Research protocol investigating increased masticatory activity and quality of life in elderly persons with dementia |
Differences between protocol and review
We planned to include cross‐over trials in the review only if the data from the first intervention period were reported, and we planned to use this trial data. The rationale for this decision was that dementia and its associated dysphagia are both progressive, and therefore any trials covering a longer period of time within the cross‐over trial may be attributed to some progression of the condition. However, although Logemann 2008 used a cross‐over design, we decided to include this study as there was no time delay between interventions and the intervention presentation was randomised. This trial also mimics clinical practice and to exclude it would not highlight the immediate and longer‐term differences associated with the intervention on patient outcomes.
We aimed to minimise reporting biases through a comprehensive search for studies, inclusion of unpublished studies and use of trial registries. We aimed to reduce or eliminate reporting biases by considering the following: publication bias, time lag bias, duplicate publication bias, location bias, citation bias, language bias or outcome reporting bias. This was not possible given that only two individual trials were eligible for inclusion.
We did not include subgroup analysis of types of dementia in the review.
Contributions of authors
E Flynn drafted the review with support from M Walshe, and C Smith. E Flynn developed the Background section. M Walshe provided a methodological perspective and advice on writing the review. E Flynn, M Walshe and C Smith provided advice on content. E Flynn secured funding for the review. E Flynn, M Walshe and C Smith commented on all sections of the review. E Flynn, M Walshe, and C Smith reviewed the final version prior to submission. C Walsh provided statistical advice.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Health Research Board, Ireland, Ireland.
Eadaoin Flynn is funded by the Health Research Board: Cochrane Fellowship CTF.2012.6 (H01474)
-
NIHR, UK.
This review was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health
Declarations of interest
Eadaoin Flynn ‐ none known Christina H Smith ‐ none known Cathal D Walsh ‐ none known Margaret Walshe ‐ none known
New
References
References to studies included in this review
Logemann 2008 {published and unpublished data}
- Logemann JA, Gensler G, Robbins JA, Lindblad AS, Brandt D, Hind JA, et al. A randomized study of three interventions for aspiration of thin liquids in patients with Dementia or Parkinson's Disease. Journal of Speech, Language, and Hearing Research 2008;51(1):173‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robbins 2008 {published and unpublished data}
- Robbins JA, Gensler G, Hind J, Logemann JA, Lindblad AS, Brand D, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: A randomized trial. Annals of Internal Medicine 2008;7:509‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Keller 2003 {published data only}
- Keller HH, Gibbs AJ, Boudreau LD, Goy RE, Pattillo MS, Brown HM. Prevention of weight loss in dementia with comprehensive nutritional treatment. Journal of the American Geriatrics Society 2003;51(7):945‐52. [DOI] [PubMed] [Google Scholar]
Weijenberg 2013 {published data only}
- Weijenberg RA, Lobbezoo F, Knol DL, Tomassen J, Scherder EJA. Increased masticatory activity and quality of life in elderly persons with dementia‐a longitudinal matched cluster randomized single‐blind multicenter intervention study. BioMed Central Neurology 2013;13(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Alagiakrishnan 2013
- Alagiakrishnan K, Bhanji R, Kurian M. Evaluation and management of oropharyngeal dysphagia in different types of dementia: A systematic review. Archives of Gerontology and Geriatrics 2013;56:1‐9. [DOI] [PubMed] [Google Scholar]
Atherton 2007
- Atherton M, Bellis‐Smith N, Cichero JA, Suter M. Texture‐modified foods and thickened fluids as used for individuals with dysphagia: Australian standardised labels and definitions. Nutrition & Dietetics 2007;64:53‐76. [Google Scholar]
Bonilha 2013
- Bonilha HS, Blair J, Carnes B, Huda W, Humphries K, McGrattan K, et al. Preliminary investigation of the effect of pulse rate on judgements of swallowing impairment and treatment recommendations. Dysphagia 2013;28(4):928‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brandt 2006
- Brandt DK, Hind JA, Robbins JA, Lindblad AS, Gensler G, Gill G, et al. Communication Sciences and Disorders Clinical Trials Research Group. Challenges in the design and conduct of a randomized study of two interventions for liquid aspiration. Clinical Trials 2006;3:457–68. [DOI] [PubMed] [Google Scholar]
Brush 1998
- Brush JA, Camp CJ. Spaced retrieval during dysphagia therapy: A case study. Clinical Gerontologist 1998;19:96‐9. [Google Scholar]
Bulow 2003
- Bülow M, Olsson R, Ekberg O. Videoradiographic analysis of how carbonated thin liquids and thickened liquids affect the physiology of swallowing in subjects with aspiration on thin liquids. Acta Radiologica 2003;44(4):366‐72. [DOI] [PubMed] [Google Scholar]
Cahill 2012
- Cahill S, O'Shea E, Pierce M. Creating excellence in dementia care: A research review for Ireland's national dementia strategy [2012]. Dementia Services Information and Development Centre. Available from: https://health.gov.ie/blog/publications/creating‐excellence‐in‐dementia‐care‐a‐research‐review‐for‐irelands‐national‐dementia‐strategy/ (accessed 20 September 2018).
Chang 2011
- Chang C, Roberts BL. Strategies for feeding patients with dementia: How to individualize assessment and intervention based on observed behavior. American Journal of Nursing 2011;111(4):36‐44. [DOI] [PubMed] [Google Scholar]
Cichero 2013
- Cichero JAY, Steele C, Duivestein J, Clave P, Chen J, Kayashita J, et al. The need for international terminology and definitions for texture‐modified foods and thickened liquids used in dysphagia management: Foundations of a Global Initiative. Current Physical Medicine and Rehabilitation Reports 2013;1:280‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dantas 1990
- Dantas RO, Kern MK, Massey BT, Dodds WJ, Kahrilas PJ, Brasseur JG, et al. Effect of swallowed bolus variables on oral and pharyngeal phases of swallowing. American Journal of Physiology 1990;258:675‐81. [DOI] [PubMed] [Google Scholar]
Daviglus 2010
- Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Cox NJ, et al. National Institutes of Health State‐of‐the‐Science Conference Statement: Preventing Alzheimer's disease and cognitive decline. Annals of Internal Medicine 2010;153:176‐81. [DOI] [PubMed] [Google Scholar]
Easterling 2008
- Easterling CS, Robbins E. Dementia and dysphagia. Geriatric Nursing 2008;29(4):275‐85. [DOI] [PubMed] [Google Scholar]
Ekberg 2002
- Ekberg O, Handy S, Woisard V, Wuttge‐Hannig A, Ortega P. Social and psychological burden of dysphagia: Its impact on diagnosis and treatment. Dysphagia 2002;17:139‐46. [DOI] [PubMed] [Google Scholar]
Ferri 2005
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005;366:2112‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2005
- Garcia JA, Chambers E, Matta Z, Clark M. Viscosity Measurements of Nectar and Honey‐thick Liquids: Product, Liquid, and Time Comparisons. Dysphagia 2005;20:325‐35. [DOI] [PubMed] [Google Scholar]
Garcia 2010
- Garcia JM, Chambers E. Managing dysphagia through diet modifications. American Journal of Nursing 2010;110(11):27‐33. [DOI] [PubMed] [Google Scholar]
Geeganage 2012
- Geeganage C, Beevan J, Ellender S, Bath PM. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD000323.pub2] [DOI] [PubMed] [Google Scholar]
Groher 2010
- Groher ME, Crary MA. Dysphagia: Clinical Management for Adults and Children. Missouri: Mosby: Elsevier, 2010. [Google Scholar]
Gräsbeck 2003
- Gräsbeck A, Horstmann V, Englund E, Passant U, Gustafsun L. Evaluation of predictors of mortality in frontotemporal dementia—methodological aspects. International Journal of Geriatric Psychiatry 2003;18(7):586‐93. [DOI] [PubMed] [Google Scholar]
Guigoz 1996
- Guigoz Y, Vellas B, Garry PJ. Assessing the nutritional status of the elderly: the Mini Nutritional Assessment as part of the geriatric evaluation. Nutrition Review 1996;54:59‐65. [DOI] [PubMed] [Google Scholar]
Hanson 2012
- Hanson B, O’Leary MT, Smith CH. The effect of saliva on the viscosity of thickened drinks. Dysphagia 2012;27(1):10‐9. [DOI] [PubMed] [Google Scholar]
Hao Wang 2016
- Wang CH, Charlton B, Kohlwes J. The horrible taste of nectar and honey ‐ inappropriate use of thickened liquids in dementia. JAMA Internal Medicine 2016;176(6):735‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org Cochrane Handbook for Systematic Reviews of Interventions.
Hind 2012
- Hind J, Divyak E, Zielinski J, Taylor A, Hartman M, Gangnon R, et al. Comparison of standardized bariums with varying rheological parameters on swallowing kinematics in males. Journal of Rehabilitation Research & Development 2012;49:1399–404. [DOI] [PubMed] [Google Scholar]
Hudson 2000
- Hudson HM, Daubert CR, Mills RH. The interdependency of protein‐energy malnutrition, aging, and dysphagia. Dysphagia 2000;15(1):31‐8. [DOI] [PubMed] [Google Scholar]
IASLT/INDI 2009
- IASLT and Irish Nutrition and Dietetic Institute. Irish consistency descriptors for modified fluids and food. www.iaslt.ie/info/policy.php 2009.
IDDSI 2016
- The International Dysphagia Diet Standardisation Initiative. The International Dysphagia Diet Standardisation Initiative. iddsi.org/resources/framework/ 2016. [DOI] [PMC free article] [PubMed]
Koss 1998
- Koss E, Gilmore C. Environmental interventions and functional abilities of AD patients. In: Vellas B, Filten J, Frisoni G editor(s). Research and Practice in Alzheimer's disease. New York: Springer, 1998:185‐91. [Google Scholar]
Kuo 2009
- Kuo S, Rhodes R, Mitchell S, Mor V, Teno J. Natural history of feeding tube use in nursing home residents with advanced dementia. Journal of the American Medical Directors Association 2009;10(4):264‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Langmore 2002
- Langmore SE, Skarupski KA, Park PS, Fries BE. Predictors of aspiration pneumonia in nursing home residents. Dysphagia 2002;17(4):298‐307. [DOI] [PubMed] [Google Scholar]
Langmore 2007
- Langmore SE, Olney RK, Lomen‐Hoerth C, Miller BL. Dysphagia in patients with frontotemporal lobar dementia. Archives of Neurology 2007;64:58‐62. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanvill J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Available from www.cochrane‐handbook.org: The Cochrane Collaboration.
Matta 2006
- Matta Z, Chambers E, Garcia JM, Helverson JM. Sensory characteristics of beverages prepared with commercial thickeners used for dysphagia diets. Journal of American Dietetics Association 2006;106(7):1049‐54. [DOI] [PubMed] [Google Scholar]
Matthews 2013
- Matthews FA, Arthur A, Barnes LE, Bond J, Jagger C. A two‐decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013;26:1405‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michou 2012
- Michou E, Mastan A, Ahmed S, Mistry S, Hamdy S. Examining the role of carbonation and temperature on water swallowing performance: A swallowing reaction‐time study. Chemical Senses 2012;37(9):799‐807. [DOI] [PubMed] [Google Scholar]
Ministry of Health Labour and Welfare 2009
- Ministry of Health Labour and Welfare. Food for special dietary uses. Japanese Government [2009] Available from https://www.mhlw.go.jp/english/topics/foodsafety/fhc/03.html (accessed 20 September 2018).
National Dysphagia Diet Task Force 2002
- National Dysphagia Diet Task Force, American Dietetic Association. National Dysphagia Diet: Standardization for Optimal Care. In: Judith Clayton editor(s). National Dysphagia Diet: Standardization for Optimal Care. United States of America: Diana Faulhaber, 2002. [Google Scholar]
National Patent Safety Agency 2011
- National Patient Safety Agency, Royal College Speech and Language Therapists, British Dietetic Association, National Nurses Nutrition Group & Hospital Caterers Association. Dysphagia diet food texture descriptions. www.ndr‐ik.org/Generalnews/dysphagia‐diet‐food‐texture‐descriptors.html 2011.
Newman 2016
- Newman R, Vilardell N, Clave P, Speyer R. Effect of bolus viscosity on the safety and efficacy of swallowing and kinematics of the swallow response in patients with oropharyngeal dysphagia. Dysphagia 2016;31(2):232‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2006
- National Institute for Health and Care Excellence. Dementia: Supporting people with dementia and their carers in health and social care. London: NICE, 2006. [Google Scholar]
Nunes 2016
- Nunes G, Santos C, Santos C, Fonseca J. Percutaneous endoscopic gastrostomy for nutritional support in dementia patients. Aging Clinical Experimental Research 2016;28:983‐9. [DOI] [PubMed] [Google Scholar]
Payne 2011
- Payne C, Methven L, Fairfield C, Bell A. Consistently inconsistent: Commercially available starch‐based dysphagia products. Dysphagia 2011;26(1):27‐33. [DOI] [PubMed] [Google Scholar]
Prince 2013
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferro C. The global prevalence of dementia: A systematic review and meta analysis. Alzheimer's & Dementia 2013;9:63‐75. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenbek 1996
- Rosenbek JC, Robbins J, Roecker EV, Coyle JL, Woods JL. A Penetration‐Aspiration Scale. Dysphagia 1996;11:93‐8. [DOI] [PubMed] [Google Scholar]
Royal College of Physicians 2010
- Royal College of Physicians, British Society of Gastroenterology. Oral feeding difficulties and dilemmas: A guide to practical care, particularly towards the end of life. London: Royal College of Physicians, 2010. [Google Scholar]
Ryan 2016
- Ryan R, Hill S. How to GRADE the quality of the evidence. Cochrane Consumers and Communication Group available at cccrg.cochrane.org/author‐resources Version 1.0 2016.
Satizabal 2016
- Satizabal CL, Beiser AL, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. New England Journal of Medicine 2016;374:523‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:332. [DOI] [PubMed] [Google Scholar]
Sdravou 2012
- Sdravou K, Walshe M, Dagdilelis L. Effects of carbonated liquids on oropharyngeal swallowing measures in people with neurogenic dysphagia. Dysphagia 2012;27(2):240‐50. [DOI] [PubMed] [Google Scholar]
Shanahan 1993
- Shanahan TK, Logemann JA, Rademaker AW, Pauloski BR, Kahrilas PJ. Chin down posture effect on aspiration in dysphagic patients. Archives of Physical Medicine and Rehabilitation 1993;74:736‐9. [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith HA, Kindell J, Baldwin RC, Waterman D, Makin AJ. Swallowing problems and dementia in acute hospital settings: practical guidance for the management of dysphagia. Clinical Medicine 2009;9(6):544‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steele 2015
- Steele CM, Alsanei WA, Ayanikalath S, Barbon CE, Chen J, Cichero JA, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: A systematic review. Dysphagia 2015;30(1):2‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stratton 2004
- Stratton RJ, Hackston A, Longmore D, Dixon R, Price S, Stroud M, et al. Malnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘Malnutrition Universal Screening Tool (‘MUST’) for adults. British Journal of Nutrition 2004;92:799‐808. [DOI] [PubMed] [Google Scholar]
Suh 2009
- Suh MK, Kim H, Na DL. Dysphagia in patients with dementia: Alzheimer versus vascular. Alzheimer Disease and Associated Disorders 2009;23(2):178‐84. [DOI] [PubMed] [Google Scholar]
Swan 2015
- Swan K, Speyer R, Heijnen BJ, Wagg B, Cordier R. Living with oropharyngeal dysphagia: effects of bolus modification on health‐related quality of life—a systematic review. Quality of Life Research 2015;24:2447. [DOI] [PubMed] [Google Scholar]
Takizawa 2016
- Takizawa C, Gemmell E, Kenworthy J, Speyer R. A systematic review of the prevalence of oropharyngeal dysphagia in stroke, Parkinson's disease, Alzheimer's disease, head injury and pneumonia. Dysphagia 2016;31:434‐41. [DOI] [PubMed] [Google Scholar]
Tolstrup Anderson 2013
- Tolstrup Anderson U, Beck AM, Kjaersgaard A, Hansen T, Poulsen I. Systematic review and evidence based recommendations on texture modified foods and thickened fluids for adults (> 18 years) with oropharyngeal dysphagia. e‐SPEN Journal 2013;8(4):127‐34. [Google Scholar]
Troche 2008
- Troche MS, Sapienza CM, Rosenbek JC. Effects of bolus consistency on timing and safety of swallow in patients with Parkinson's disease. Dysphagia 2008;23:26‐32. [DOI] [PubMed] [Google Scholar]
Vilardell 2016
- Vilardell N, Rofes L, Arreola V, Speyer R, Clave P. A comparative study between modified starch and xanthan gum thickeners in post stroke oropharyngeal dysphagia. Dysphagia 2016;31:169‐79. [DOI] [PubMed] [Google Scholar]
Volicer 1994
- Volicer L, Hurley AC, Lathi DC, Kowall NW. Measurement of severity in advanced Alzheimer's disease. Journal of Gerontology 1994;49:223‐26. [DOI] [PubMed] [Google Scholar]
Wada 2001
- Wada H, Nakajoh K, Satoh‐Nakagawa T, Suzuki T, Ohrui T, Arai H, et al. Risk factors of aspiration pneumonia in Alzheimer's disease patients. Gerontology 2001;47(5):271‐6. [DOI] [PubMed] [Google Scholar]
Walshe 2014
- Walshe M. Oropharyngeal dysphagia in neurodegenerative disease. Journal of Gastroenterology and Hepatology Research 2014;3(10):1265‐71. [Google Scholar]
Weiner 2009
- Weiner M, Lipton AF. Textbook of Alzheimer's Disease and Other Dementias. 1st Edition. Arlington VA: American Psychiatric Publishing, 2009. [Google Scholar]
Welch 1993
- Welch MV, Logemann JA, Rademaker AW, Kahrilas PJ. Changes in pharyngeal dimensions effected by chin tuck. Archives of Physical Medicine and Rehabilitation 1993;74:178‐81. [PubMed] [Google Scholar]
Wendin 2010
- Wendin K, Ekman S, Bulow M, Ekberg O, Johansson D, Rothenberg E, et al. Objective and quantitative definition of modified food textures based on sensory and rheological methodology. Food & Nutrition Research 2010;54:5134‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Flynn 2014
- Flynn EP, Smith CH, Walsh CD, Walshe M. Modifying the consistency of food and fluids for swallowing difficulties in dementia. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD011077] [DOI] [PMC free article] [PubMed] [Google Scholar]


