Abstract
Background
Bronchiectasis is a chronic respiratory disease characterised by abnormal and irreversible dilatation of the smaller airways and associated with a mortality rate greater than twice that of the general population. Antibiotics serve as front‐line therapy for managing bacterial load, but their use is weighed against the development of antibiotic resistance. Dual antibiotic therapy has the potential to suppress infection from multiple strains of bacteria, leading to more successful treatment of exacerbations, reduced symptoms, and improved quality of life. Further evidence is required on the efficacy of dual antibiotics in terms of management of exacerbations and extent of antibiotic resistance.
Objectives
To evaluate the effects of dual antibiotics in the treatment of adults and children with bronchiectasis.
Search methods
We identified studies from the Cochrane Airways Group Specialised Register (CAGR), which includes the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Allied and Complementary Medicine (AMED), and PsycINFO, as well as studies obtained by handsearching of journals/abstracts. We also searched the following trial registries: US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov, and the World Health Organization (WHO) International Clinical Trials Registry Platform. We imposed no restriction on language of publication. We conducted our search in October 2017.
Selection criteria
We searched for randomised controlled trials comparing dual antibiotics versus a single antibiotic for short‐term (< 4 weeks) or long‐term management of bronchiectasis diagnosed in adults and/or children by bronchography, plain film chest radiography, or high‐resolution computed tomography. Primary outcomes included exacerbations, length of hospitalisation, and serious adverse events. Secondary outcomes were response rates, emergence of resistance to antibiotics, systemic markers of infection, sputum volume and purulence, measures of lung function, adverse events/effects, deaths, exercise capacity, and health‐related quality of life. We did not apply outcome measures as selection criteria.
Data collection and analysis
Two review authors independently screened the titles and abstracts of 287 records, along with the full text of seven reports. Two studies met review inclusion criteria. Two review authors independently extracted outcome data and assessed risk of bias. We extracted data from only one study and conducted GRADE assessments for the following outcomes: successful treatment of exacerbation; response rates; and serious adverse events.
Main results
Two randomised trials assessed the effectiveness of oral plus inhaled dual therapy versus oral monotherapy in a total of 118 adults with a mean age of 62.8 years. One multi‐centre trial compared inhaled tobramycin plus oral ciprofloxacin versus ciprofloxacin alone, and one single‐centre trial compared nebulised gentamicin plus systemic antibiotics versus a systemic antibiotic alone. Published papers did not report study funding sources.
Effect estimates from one small study with 53 adults showed no evidence of treatment benefit with oral plus inhaled dual therapy for the following primary outcomes at the end of the study: successful management of exacerbation ‐ cure at day 42 (odds ratio (OR) 0.66, 95% confidence interval (CI) 0.22 to 2.01; 53 participants; one study; very low‐quality evidence); number of participants with Pseudomonas aeruginosa eradication at day 21 (OR 2.33, 95% CI 0.66 to 8.24; 53 participants; one study; very low‐quality evidence); and serious adverse events (OR 0.48, 95% CI 0.08 to 2.87; 53 participants; one study; very low‐quality evidence). Similarly, researchers provided no evidence of treatment benefit for the following secondary outcomes: clinical response rates ‐ relapse at day 42 (OR 0.57, 95% CI 0.12 to 2.69; 53 participants; one study; very low‐quality evidence); microbiological response rate at day 21 ‐ eradicated (OR 2.40, 95% CI 0.67 to 8.65; 53 participants; one study; very low‐quality evidence); and adverse events ‐ incidence of wheeze (OR 5.75, 95% CI 1.55 to 21.33). Data show no evidence of benefit in terms of sputum volume, lung function, or antibiotic resistance. Outcomes from a second small study with 65 adults, available only as an abstract, were not included in the quantitative data synthesis. The included studies did not report our other primary outcomes: duration; frequency; and time to next exacerbation; nor our secondary outcomes: systemic markers of infection; exercise capacity; and quality of life. We did not identify any trials that included children.
Authors' conclusions
A small number of studies in adults have generated high‐quality evidence that is insufficient to inform robust conclusions, and studies in children have provided no evidence. We identified only one dual‐therapy combination of oral and inhaled antibiotics. Results from this single trial of 53 adults that we were able to include in the quantitative synthesis showed no evidence of treatment benefit with oral plus inhaled dual therapy in terms of successful treatment of exacerbations, serious adverse events, sputum volume, lung function, and antibiotic resistance. Further high‐quality research is required to determine the efficacy and safety of other combinations of dual antibiotics for both adults and children with bronchiectasis, particularly in terms of antibiotic resistance.
Plain language summary
Dual antibiotics for bronchiectasis
Background to the question
Bronchiectasis is a lung disease involving abnormal airways, leading to repeated chest infections, and associated with a mortality rate more than twice that of the general population. Although previously considered a relatively rare disease, numbers appear to be increasing, particularly for those over 75 years in low/middle‐income countries. Antibiotics are the main therapy for chest infection, but their use must be weighed against potential side effects and the risk of increasing resistance to antibiotic therapy. One strategy to improve response and/or reduce antibiotic resistance involves giving two antibiotic agents at the same time: dual antibiotic therapy. This review therefore aimed to evaluate the effects of dual antibiotics for treatment of adults and children with bronchiectasis.
Study characteristics
In October 2017, we identified two relevant studies comparing oral plus inhaled dual therapy versus oral therapy alone. They included a total of 118 adults with an average age of 62.8 years. One study compared inhaled tobramycin plus oral ciprofloxacin with oral ciprofloxacin, and the second study compared inhaled gentamicin plus a systemic (affecting the whole body, rather than just the lungs) antibiotic with a systemic antibiotic alone. Only a research summary was available for the latter. Published papers did not report study funding sources
Main results
Results from one small trial of 53 adults show no evidence of treatment benefit with oral plus inhaled dual therapy in terms of successful treatment of exacerbations, the occurrence of serious unwanted events, amount of phlegm, lung function, or resistance to antibiotic treatment. However, we found insufficient evidence to permit confident conclusions about their use.
Quality of the evidence
The overall quality of the evidence was very poor, largely because one of the studies was not well described and included few participants. Information on exacerbations, exercise ability, and quality of life was not reported. We did not identify any trials that compared other types of dual antibiotic therapy, and we found none that included children. Therefore uncertainty remains concerning the use of dual antibiotics, and further high‐quality studies are needed to examine the role of dual antibiotics in the treatment of adults and children with bronchiectasis.
Summary of findings
Summary of findings for the main comparison. Oral + inhaled dual therapy compared with oral monotherapy for bronchiectasis.
| Oral + inhaled dual therapy compared with oral monotherapy for bronchiectasis | ||||||
| Patient or population: bronchiectasis Setting: United Kingdom and United States Intervention: oral + inhaled dual therapy Comparison: oral monotherapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with oral monotherapy | Risk with oral + inhaled dual therapy | |||||
| Successful treatment of exacerbation: number of participants cured. Outcome assessed on day 42 | 444 per 1000 | 346 per 1000 (150 to 617) | OR 0.66 (0.22 to 2.01) | 53 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Successful treatment of exacerbation: number of participants with P aeruginosa eradication. Outcome assessed on day 21 | 185 per 1000 | 346 per 1000 (130 to 652) | OR 2.33 (0.66 to 8.24) | 53 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Serious adverse events. Outcome assessed on day 42 | 148 per 1000 | 77 per 1000 (14 to 333) | OR 0.48 (0.08 to 2.87) | 53 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Treatment response: relapse (day 42). Outcome assessed on day 42 | 185 per 1000 | 115 per 1000 (27 to 379) | OR 0.57 (0.12 to 2.69) | 53 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Microbiological response: eradicated. Outcome assessed on day 21 | 200 per 1000 | 375 per 1000 (143 to 684) | OR 2.40 (0.67 to 8.65) | 49 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Hospitalisations | Not estimable | (0 studies) | ‐ | |||
| Death | Not estimable | (0 studies) | ‐ | |||
| Quality of life | Not estimable | (0 studies) | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one point for high risk of bias from incomplete outcome data
bDowngraded 2 points owing to imprecision (wide confidence intervals crossing the line of no effect and few events)
Background
Description of the condition
Bronchiectasis not attributable to cystic fibrosis has been described as non‐CF bronchiectasis but, in accordance with current clinical guidelines, we will referred to it as "bronchiectasis" throughout this review (Polverino 2017). Bronchiectasis is a persistent respiratory condition associated with progressive destruction of the airways due to a 'vicious cycle' of recurrent bacterial infection, pulmonary inflammation, and consequent structural damage (Cole 1997; Pasteur 2010). The pathological process of bronchiectasis leads to disruption of the normal epithelial barrier, which consequently allows inhaled pathogens to both colonise the airways and cause clinical episodes of infection (Cole 1986). In severe cases, this cycle of infection may lead to repeated hospitalisation, chronic respiratory failure, and death. An understanding of the cycle is central to the management of bronchiectasis, as strategies to arrest both inflammatory and bacterial components are required to limit progression of lung injury. Approximately half of presenting cases are idiopathic, but the most common cause is a previous chest infection, such as bacterial pneumonia or tuberculosis (Pasteur 2010). Diagnosis is based on identification of one or more abnormally dilated bronchi on high‐resolution computerised tomography (HRCT) with characteristic symptoms including breathlessness, chronic productive cough, and recurrent lower respiratory tract infection (Chang 2010; Pasteur 2010; Polverino 2017). Patients colonised with Pseudomonas aeruginosa and those with a high annual exacerbation rate show accelerated decline in lung function, reduced health‐related quality of life (measured via St George's Respiratory Questionnaire (SGRQ)), increased risk of hospitalisation, and increased mortality risk (Evans 1996; Martinez Garcia 2007; Wilson 1997). Low forced expiratory volume in one second (FEV1) % predicted, a higher proportion of affected lobes, and increased breathlessness are associated with increased risks of hospitalisation and mortality (Chalmers 2014; Martinez Garcia 2014; Seitz 2010).
Bacteria most commonly associated with infective exacerbations include non‐typeable Haemophilus influenzae, P aeruginosa, Streptococcus pneumoniae, Staphylococcus aureus, and Moraxella catarrhalis (Foweraker 2011). The microbiological profile differs between adults and children, and Pseudomonas is more common among adults. Pseudomonas is resistant to many oral antibiotics and is very difficult to eradicate, but it is prevalent in only 0 to 6% of children. Colonising pathogens such as Pseudomonas, H influenzae and M catarrhalis also commonly display antimicrobial resistance as the result of frequent exposure to antimicrobial agents. The main aims of therapeutic management include preservation of lung function; reduction in symptoms, such as cough, breathlessness, and expectoration; reduction in the number and duration of exacerbations; and improvement in quality of life (Lavery 2005; Pasteur 2010).
Global prevalence estimates are confounded by variable diagnostic strategies (Weycker 2005), as well as by higher prevalence rates in developing countries (Habesoglu 2011), but the global burden of bronchiectasis is increasing, with mortality rates rising by 3% per year in England and Wales between 2001 and 2007 (Roberts 2010), and hospitalisations increasing by 3% per year in the United States over a nine‐year period (Seitz 2010). Both Roberts 2010 and Seitz 2012 reported higher prevalence rates among women and in people over 60 years of age. More recent studies suggest that prevalence may be increasing more rapidly than was previously estimated. In Germany in 2013, prevalence was estimated at 67 cases per 100,000 general population (Ringshausen 2015). In the UK from 2004 to 2013, incidence rates rose by approximately 63%, with an increase from 21.2 to 35.2 in women, and from 18.2 to 26.9 in men, per 100,000 person‐years (Quint 2016). Similarly, point prevalence rose from 350.5 to 566.1 in women, and from 301.2 to 485.5 in men, per 100,000 head of population, with approximately 262,900 adults in the UK living with bronchiectasis in 2013. The disease has a significant impact on paediatric populations: Younger children and those with more frequent exacerbations experience worse quality of life (Kapur 2012a). Bronchiectasis is more common in some ethnic groups, for example, southwest Alaskan children (16:1000) and Australian indigenous children (15:1000) (Chang 2002). Furthermore, one study reported an estimated incidence of 3.7 per 100,000 per year among children younger than 15 years of age in New Zealand. This equates to a prevalence of 1:3000 children overall and 1:625 in Pacific children (Twiss 2005). It also demonstrates that the incidence rate among children in New Zealand is almost seven times higher than among those in Finland (Twiss 2005). Average mortality rates per 100,000 general population in Europe are estimated at 0.3 in 27 of the 28 EU countries (ranging from 0.01 in Germany to 1.18 in the UK) and at 0.2 in nine non‐EU countries (ranging from 0.01 in Azerbaijan to 0.67 in Kyrgyzstan), on the basis of 2005 to 2009 data (European Lung White Book 2013). More recent UK estimates suggest that age‐adjusted mortality rates are 2.26 times higher in women and 2.14 times higher in men compared with the general population (Quint 2016).
Description of the intervention
The lungs of patients with bronchiectasis are commonly colonised by bacteria, and treatment with antibiotics can help to decrease bacterial load while reducing systemic inflammation (Kapur 2012). Antibiotics are used to reduce bacterial burden and to tackle the cycle of infection and lung damage, consequently helping to reduce the impact and frequency of chest infection and the frequency and duration of hospital admissions, while also reducing mortality (Cole 1986; Pasteur 2010). Antibiotics can be administered on a short‐term (< 4 weeks) or longer‐term (≥ 4 weeks) basis via various modes, including oral, inhaled, and intravenous routes, with specific choice of antibiotic informed by analysis of sputum bacteriology. Antibiotics serve as front‐line therapy for management of bacterial load, but their use is weighed against potential adverse effects and increasing concerns about antibiotic resistance (Pasteur 2010).
'Combination' or 'dual' antibiotic therapy for bronchiectasis is defined as the combination of two or more antibiotics, rather than as use of a single antibiotic (monotherapy), irrespective of the route of administration or the duration of therapy. Dual antibiotic therapy is commonly administered therapeutically over a short duration (up to four weeks), rather than prophylactically for prevention, and is commonly used to treat patients with acute exacerbations whose lungs are colonised by multiple strains of bacteria with different patterns of antibiotic resistance, when monotherapy is unlikely to be effective. Dual therapy may also be used when the clinician is concerned about increasing the risk of antibiotic resistance, for example, when antibiotics have been prescribed frequently or for a prolonged duration. British Thoracic Society guidelines recommend the use of combination antibiotics when patients present with multiple pathogens (Pasteur 2010).
How the intervention might work
Chronic bacterial airway colonisation commonly occurs in patients with bronchiectasis; high bacterial load is associated with increased inflammation and symptoms and worse quality of life (McShane 2013). It has been hypothesised that inflammation contributes to progression of bronchiectasis, and evidence suggests that the presence of bacteria in the airways promotes inflammation (Haworth 2014). Bronchiectatic airways are commonly colonised by multiple bacteria or different strains of the same bacteria, some of which may not be positively cultured in the laboratory. Bacterial load can be reduced through treatment with systemic antibiotics (Rubin 2014), and various antibiotic strategies have been used to reduce bacterial load and reinfection, including short‐term (< 4 weeks) therapy for acute exacerbations and longer‐term (≥ 4 weeks) prophylactic therapy for frequent exacerbations characterised by chronic sputum purulence (Chalmers 2012; Evans 2003). Although longer‐term antibiotics are not recommended for routine treatment (Valery 2012; Wu 2014), they may be considered for treatment of patients with frequent exacerbations (three or more per year requiring antibiotic therapy) (Pasteur 2010). Dual antibiotic therapy for exacerbations could reduce bacterial load and levels of inflammation, consequently improving clinically meaningful outcomes, such as length of exacerbation, frequency of exacerbation, disease progression, and mortality.
Why it is important to do this review
The benefits and risks of dual antibiotics given for management of acute exacerbations and for prophylaxis are currently unclear. It is important to weigh the benefits of dual antibiotics in terms of bacterial eradication and suppression of bacterial load against the risks of enhanced antibiotic resistance and exposure to side effects associated with multiple antibiotic therapy.
This review summarises available evidence on the use of dual antibiotics for patients with bronchiectasis to inform clinical practice and future research needs. This review has been conducted alongside two other, closely related reviews: Macrolide antibiotics for bronchiectasis (Kelly 2018) andHead‐to‐head trials of antibiotics for bronchiectasis (Kaehne 2017).
Objectives
To evaluate the effects of dual antibiotics in the treatment of adults and children with bronchiectasis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) reported as full text, those published as abstract only, and unpublished data.
Types of participants
We included adults and children with a clinical diagnosis of bronchiectasis confirmed by plain film chest radiography or HRCT. We excluded studies in which participants had received continuous or high‐dose antibiotics immediately before the study began or a diagnosis of cystic fibrosis (CF), sarcoidosis, or active allergic bronchopulmonary aspergillosis.
Types of interventions
We included studies comparing dual antibiotics versus a single antibiotic, provided that both arms included a common route of administration. We planned to analyse short‐course (< 4 weeks) and long‐term (≥ 4 weeks) dual antibiotics separately. This review focused on comparisons of antimicrobial agents and therefore excluded comparisons of macrolides owing to their anti‐inflammatory properties. Potential comparison groups for dual therapy versus monotherapy included the following.
Oral dual therapy versus oral monotherapy.
Intravenous dual therapy versus intravenous monotherapy.
Oral + inhaled dual therapy versus oral monotherapy.
Oral + intravenous dual therapy versus oral monotherapy.
Inhaled + intravenous dual therapy versus inhaled monotherapy.
Inhaled + oral dual therapy versus inhaled monotherapy.
Intravenous + inhaled dual therapy versus intravenous monotherapy.
Intravenous + oral dual therapy versus inhaled monotherapy.
We included studies that compared one combination of antibiotics versus another if a comparison was made between different classes of antibiotics in combination (e.g. cephalosporin A + aminoglycoside A vs cephalosporin B + inhaled aminoglycoside B) or between different administration routes for antibiotics from the same class (e.g. IV cephalosporin + IV aminoglycoside vs IV cephalosporin + inhaled aminoglycoside).
Types of outcome measures
Primary outcomes
Successful treatment of exacerbation
Length of exacerbation
Length of hospitalisation
Time to next exacerbation
Frequency of exacerbations
Serious adverse event ‐ We used the definitions from Hansen 2015 to describe serious adverse events, which were those that resulted in death or life‐threatening events; requirement for hospitalisation or prolongation of existing hospitalisation; persistent or significant disability; or congenital anomalies, or events that were considered medically important.
Secondary outcomes
Response rates as defined by study authors (e.g. diary cards of physician global assessment)
Sputum volume and purulence
Measures of lung function (e.g. forced expiratory volume in one second (FEV1))
Systemic markers of infection (e.g. leucocyte count, C‐reactive protein (CRP), erythrocyte sedimentation rate (ESR))
Adverse events (e.g. cardiac arrhythmias, GI symptoms, hearing impairment, nephrotoxicity)
Deaths
Emergence of resistance to antibiotics
Exercise capacity (e.g. Six‐Minute Walk Distance (6MWD))
Quality of life (e.g. St George's Respiratory Questionnaire (SGRQ))
Adverse/side effects
Reporting one or more of these outcomes was not a study inclusion criterion for this review.
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (crso.cochrane.org).
Weekly searches of MEDLINE Ovid SP 1946 to date.
Weekly searches of Embase Ovid SP 1974 to date.
Monthly searches of PsycINFO Ovid SP 1967 to date.
Monthly searches of Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO 1937 to date.
Monthly searches of Allied and Complementary Medicine (AMED) EBSCO.
Handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings, are provided in Appendix 1. See Appendix 2 for search terms used to identify studies for inclusion in this review.
We will search the following trials registries.
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov).
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
We searched the Cochrane Airways Trials Register and additional sources from inception to October 2017, with no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We also searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed) and reported the search date.
Data collection and analysis
Selection of studies
Two review authors (LF and SG) independently screened titles and abstracts of all studies identified for inclusion as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve.' The same two review authors independently screened retrieved full‐text study reports or publications for inclusion and recorded reasons for exclusion of ineligible studies. They reported no disagreements with regard to study selection. We recorded the study selection process using a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram and study details using Characteristics of excluded studies tables (Moher 2009).
Data extraction and management
We used a data collection form, piloted on at least one study in the review, to record study characteristics and outcome data. One review author (RA) extracted the following study characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study setting, withdrawals, dates of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant medications, excluded medications.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (DL and LF) independently extracted outcome data from included studies and noted in the Characteristics of included studies table when outcome data were not reported in a useable way. We resolved disagreements by consensus or by consultation with a third review author (SS or SJM). One review author (DL) transferred data into Review Manager (RevMan 2014). We double‐checked that data had been entered correctly by comparing data presented in the systematic review with those provided in the study reports. A second review author (RA) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (DL and LF) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved disagreements by discussion with another review author (SS). We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from risk of bias for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account risk of bias for studies that contributed to those outcomes.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Felix 2017).
Measures of treatment effect
We analysed dichotomous data as odds ratios, and continuous data as mean differences or standardised mean differences. We entered data presented as a scale with a consistent direction of effect.
We intended to undertake meta‐analyses only when this was meaningful (i.e. when treatments, participants, and the underlying clinical question were similar enough for pooling to make sense). However, data were available from only one included study, and meta‐analysis was not possible. Nevertheless, we included the data narratively in the review.
We planned to narratively describe skewed data reported as medians and interquartile ranges.
If multiple arms had been reported in a single trial, we planned to include only the relevant arms. Similarly, if two comparisons (e.g. drug A vs placebo and drug B vs placebo) had been combined in the same meta‐analysis, we would have halved the control group to avoid double‐counting. None of the included studies included more than two study arms.
Unit of analysis issues
In all included studies, the unit of analysis was the participant. In terms of exacerbation rates and hospitalisation rates, we focused on the number of events experienced by the participant during the trial and analysed the results using rate ratios when possible.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data (e.g. when a study was identified as abstract only). When this was not possible, and the missing data were considered a serious source of bias, we had planned to perform a sensitivity analysis to explore the impact of including such studies in the overall assessment of results.
One of the included studies was an abstract for which contact details for the principal investigator were not reported. We contacted the institution to which the authors were affiliated to obtain more information.
Assessment of heterogeneity
We planned to use the I² statistic to measure heterogeneity among studies in meta‐analyses; in the presence of substantial heterogeneity, we would have explored possible causes by performing prespecified subgroup analyses. As we conducted no meta‐analyses, we did not assess heterogeneity.
Assessment of reporting biases
Only two studies met the inclusion criteria; thus we identified fewer than the recommended minimum number of eight studies required to create a funnel plot to explore possible small‐study and publication biases.
We were not able to pool the included studies and therefore were unable to explore small‐study and publication biases.
Data synthesis
We planned to use a random‐effects model for meta‐analyses and to perform a sensitivity analysis using a fixed‐effect model, but this was not possible, as we were unable to pool data from the included studies.
'Summary of findings' table
We created a 'Summary of findings' table using the following primary and secondary outcomes: exacerbations, hospitalisations, serious adverse events, response rates, deaths, and quality of life. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of evidence related to included studies that contributed data on our prespecified outcomes. We followed methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and we used GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes and inserted comments to aid the reader's understanding of grades when necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Duration: short (< 4 weeks) or longer (≥ 4 weeks).
Type of antibiotic: aminoglycosides, beta‐lactams, chloramphenicol, fluoroquinolones, macrolides, tetracyclines.
Children versus adults.
Pseudomonas colonisation versus no Pseudomonas colonisation.
We planned to use the following outcomes.
Exacerbations.
Hospitalisations.
Serious adverse events.
We planned to use the formal test for subgroup interactions provided in Review Manager (RevMan 2014).
However data available from the two included studies were insufficient to permit subgroup analyses.
Sensitivity analysis
We planned to evaluate the impact of methodological quality of included studies using the following domains to remove studies at high or unclear risk of bias: random sequence generation and allocation concealment. Data were insufficient to undertake sensitivity analyses.
Results
Description of studies
Results of the search
A systematic search, conducted in October 2017, identified 287 unique records of potentially relevant trials. Following inspection of titles and abstracts, review authors considered 280 records as irrelevant. We obtained full texts for the remaining seven records and included two studies that met review eligibility criteria (Bilton 2006; Hossain 2010). See Characteristics of included studies. We excluded five records with reasons (documented in Excluded studies) and summarised the selection process in the study flow diagram (Figure 1).
1.
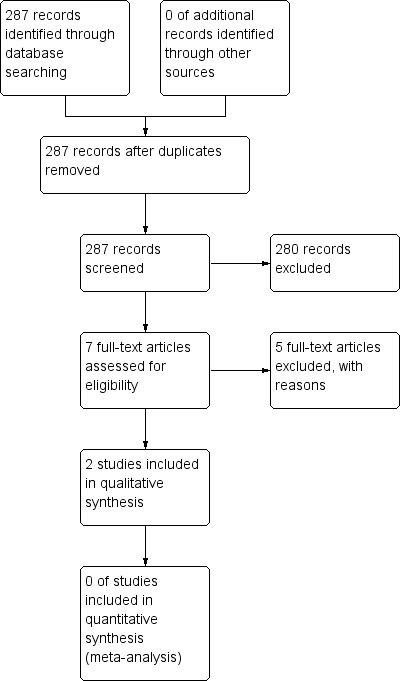
Study selection flow diagram.
One included study originally aimed to test the use of nebulised tobramycin to manage exacerbations of bronchiectasis (Bilton 2006). However, in keeping with recommendations of the research ethics committee (communication with authors), investigators redesigned the trial to test the effectiveness of tobramycin inhalation plus oral ciprofloxacin compared with placebo inhalation plus oral ciprofloxacin. We included the redesigned published study in this review.
Included studies
Methods
Both of the included studies were RCTs (Bilton 2006; Hossain 2010). Bilton 2006 was conducted in multiple centres across UK and USA, and Hossain 2010 was conducted at a single centre in Bangladesh.
Ten participants withdrew from Bilton 2006 (five in the oral + inhaled dual therapy (O + I) group, five in the oral monotherapy (O) group).
Participants
The two studies included a total of 118 participants (Bilton 2006 = 53; Hossain 2010 = 65). Adults (72% female) aged 18 to 80 years with a mean age of 62.8 years (± 11.5 years) participated in Bilton 2006. Bronchiectasis was confirmed by HRCT scan, and researchers excluded from the study those with CF, allergic bronchopulmonary aspergillosis, active tuberculosis, glucose‐6‐phosphate dehydrogenase deficiency, significant renal disease, or change in steroid therapy within 2 weeks of the acute exacerbation. In addition, eligibility criteria included a history of chronic P aeruginosa, confirmed by sputum culture, during the previous 12 months and at screening. Furthermore, the P aeruginosa isolate had to demonstrate sensitivity to ciprofloxacin (minimum inhibitory concentration ≤ 4 µg/mL) at study enrolment. The second study was reported as an abstract alone and did not provide detailed information on participant characteristics or study inclusion/exclusion criteria (Hossain 2010).
Smoking history
In Bilton 2006, one participant was a current smoker (placebo/oral ciprofloxacin group) and 20 participants (13 placebo/oral ciprofloxacin; 7 tobramycin/oral ciprofloxacin) were former smokers. Hossain 2010 did not report smoking history.
Interventions
Both studies assessed the effectiveness of oral plus inhaled dual therapy versus oral monotherapy.
Bilton 2006 randomised participants to receive either tobramycin inhalation solution (300 mg per 5 mL of inhalation solution) plus oral ciprofloxacin (750 mg), or placebo (1.25 mg of quinine sulphate per 5 mL of inhalation solution) plus oral ciprofloxacin (750 mg), twice daily for 14 days.
Hossain 2010 randomised participants to receive either nebulised gentamicin plus a systemic antibiotic or nebulised placebo plus a systemic antibiotic. Study authors did not report information on frequency and dose.
Outcomes
Primary outcomes
Successful treatment of exacerbation
Bilton 2006 reported this outcome as a cure rate using the following definition:resolution or improvement of symptoms of acute exacerbation (day 21 ‐ primary outcome).
Hossain 2010 reported this outcome using the following categorisation: resolved ‐ resolution of symptoms and signs (S/S) of acute exacerbation; improved ‐ not fully resolved; not improved ‐ no change or deterioration of S/S.
Length of hospitalisation
Only Hossain 2010 reported this outcome.
Serious adverse events
Only Bilton 2006 reported this outcome. This study reported the proportions of participants who required hospitalisation and treatment for worsening symptoms such as dyspnoea, cough, chest pain, or tightness that was associated with bronchiectasis and chronic infection.
Length of exacerbation, time to next exacerbation, frequency of exacerbations
The included studies did not report any of the above remaining primary outcomes.
Secondary outcomes
Response rates as defined by study authors (e.g. diary cards of physician global assessment)
Bilton 2006 classified response as follows at day 21: failed ‐ participants with persistent or worsening symptoms of exacerbations, hospitalisation, or administration of additional antibiotic; or indeterminate ‐ participants with missing data and those without a definitive cure or failure rate; or relapse (classified on day 42) ‐ including those who were cured on day 21 but required further treatment with additional antibiotics.
Bilton 2006 also classified response rates according to microbiological results at day 21. Researchers classified sputum culture as "eradicated" (no P aeruginosa infection and/or inability to produce sputum), "persistent" (with P aeruginosa infection and/or treatment with additional antibiotics for continued infection), "superinfected" (new pathogen and new or worsening symptoms of infection), or "indeterminate" (unable to classify).
Sputum volume and purulence
Only Bilton 2006 reported sputum volume. Neither of the included studies reported sputum purulence.
Measures of lung function (e.g. forced expiratory volume in one second (FEV1))
Bilton 2006 measured FEV1 (L) at baseline and at 7 and 14 days. Hossain 2010 also measured FEV1 (values not given) but did not report the details.
Serious adverse events (e.g. cardiac arrhythmias, GI symptoms, hearing impairment, nephrotoxicity)
Both of the included studies reported serious adverse events (Bilton 2006; Hossain 2010).
Deaths
Bilton 2006 and Hossain 2010 did not report this outcome measure, and it remains unclear whether any deaths occurred during the study period. In Bilton 2006, 7 of 10 withdrawals were due to adverse events, but study authors did not provide follow‐up data, so we do not know whether any deaths occurred in this group.
Emergence of resistance to antibiotics
Bilton 2006 reported emergence of P aeruginosa resistance to ciprofloxacin and tobramycin.
Adverse/side effects
Bilton 2006 reported adverse effects of study medications.
Systemic markers of infection, exercise capacity, quality of life
Included studies did not report any of the above outcomes.
Notes
Neither of the included studies reported information on power calculation to inform sample size, trial registration, funders, or the role of sponsors. Trial authors did not provide conflict of interest statements. Bilton 2006 reported that researchers obtained ethical approval for their trial.
Excluded studies
We recorded reasons for exclusion of five studies following examination of full text reports (see Characteristics of excluded studies). Two studies did not meet study inclusion criteria for the intervention, as the comparison arm was not given monotherapy (Orriols 1999; Orriols 2015). We excluded the remaining three studies because participants were not exclusively patients with bronchiectasis and we were unable to contact trial authors to obtain information on these participants alone (Takamoto 1994; Vergnon 1985; Watanabe 1990).
Risk of bias in included studies
Two independent review authors (DL and LF) agreed on judgements reported under the 'Risk of bias' section at the end of each Characteristics of included studies table. Figure 2 and Figure 3 also provide a summary of the risk of bias in both included studies.
2.
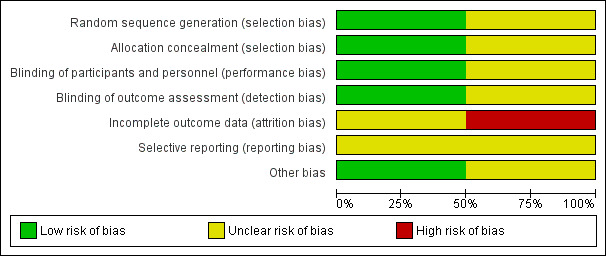
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
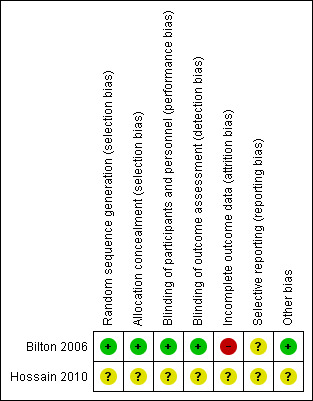
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged Bilton 2006 as having low risk of bias, as the randomisation sequence was computer generated. However, we judged Hossain 2010 as having unclear risk because insufficient information was provided in the abstract.
We judged Bilton 2006 as having low risk of bias for allocation concealment following confirmation from trial authors that assignment was concealed via an independent central allocation process. We judged Hossain 2010 as having unclear risk owing to insufficient information provided in the abstract.
Blinding
We judged Bilton 2006 as having low risk of bias for this domain. The principal investigator confirmed that drugs were supplied by a pharmaceutical company in identical opaque vials, and that both drugs had a similar taste. We judged Hossain 2010 as having unclear risk owing to insufficient information provided in the abstract.
We judged Bilton 2006 as having low risk of bias following confirmation from the principal investigator that outcome assessors were blinded to group allocation. We judged Hossain 2010 as having unclear risk owing to insufficient information provided in the abstract.
Incomplete outcome data
We judged Bilton 2006 as having high risk of attrition bias because reasons for missing outcome data were not balanced between intervention groups. We judged Hossain 2010 as having unclear risk owing to insufficient information provided in the abstract.
Selective reporting
We classified risk of selective reporting bias as unclear for both of the included studies because information on which to base a clear judgement was insufficient (Bilton 2006; Hossain 2010).
Other potential sources of bias
We judged Bilton 2006 as having low risk of bias for this domain, as no other sources of bias were identified. We judged Hossain 2010 as having unclear risk because information provided in the abstract was insufficient.
Effects of interventions
See: Table 1
Primary outcomes
Successful treatment of exacerbation
Bilton 2006 reported no differences between groups in the number of participants cured at day 21 (OR 0.42, 95% CI 0.14 to 1.30; one study; 53 adults; Analysis 1.1) or at day 42 (OR 0.66, 95% CI 0.22 to 2.01; one study; 53 adults; Analysis 1.2). Researchers noted no differences between groups in terms of number of participants with P aeruginosa eradication at day 21 (OR 2.33, 95% CI 0.66 to 8.24; Analysis 1.3). In Hossain 2010, participants receiving dual therapy had an enhanced recovery rate compared with those receiving systemic antibiotics alone at three time points (P = 0.05; P = 0.02; P = 0.02), but which of the four follow‐up time points (day 3, 7, 14, or 21) is referred to remains unclear. The study report was available only as an abstract and did not provide any quantitative data other than the P value.
1.1. Analysis.
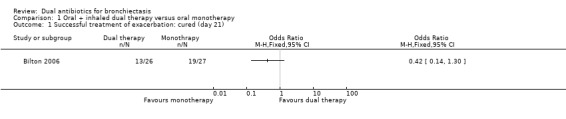
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 1 Successful treatment of exacerbation: cured (day 21).
1.2. Analysis.
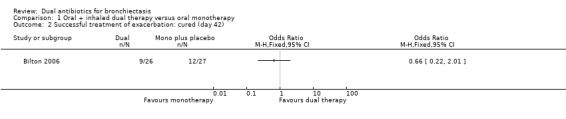
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 2 Successful treatment of exacerbation: cured (day 42).
1.3. Analysis.
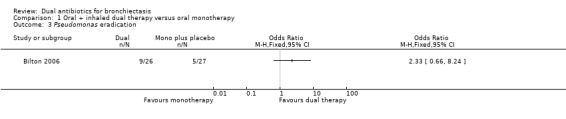
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 3 Pseudomonas eradication.
According to our GRADE assessment, we judged this outcome as very low quality (Table 1).
Length of hospitalisation
Hossain 2010 reported that dual therapy reduced hospital stay among participants in the intervention group; however, the abstract did not include any quantitative data.
Serious adverse events
Bilton 2006 reported no differences between groups in frequency of serious adverse events (OR 0.48, 95% CI 0.08 to 2.87; one study; 53 adults; Analysis 1.4). Four participants receiving monotherapy and two receiving dual therapy required hospitalisation for worsening symptoms such as dyspnoea, cough, and chest pain or tightness.
1.4. Analysis.
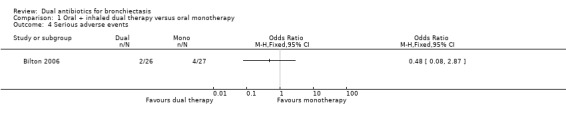
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 4 Serious adverse events.
According to our GRADE assessment, we judged this outcome as very low quality (Table 1).
Length of exacerbation, time to next exacerbation, frequency of exacerbations
None of the included studies reported any of the above outcomes.
Secondary outcomes
Response rates
Response to treatment ‐ failure or relapse
Treatment responses were not different between groups at day 21 for the classification of treatment failure (OR 2.75, 95% CI 0.79 to 9.62; one study; 53 adults; Analysis 1.5); nor at day 42 for the classifications of treatment relapse (OR 0.57, 95% CI 0.12 to 2.69; one study; 53 adults; Analysis 1.6) and treatment failure (OR 2.75, 95% CI 0.79 to 9.62; one study; 53 adults; Analysis 1.7). Relapse rates were not measured at day 21.
1.5. Analysis.
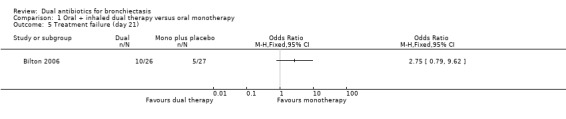
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 5 Treatment failure (day 21).
1.6. Analysis.
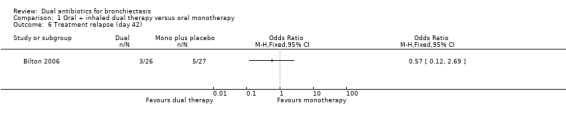
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 6 Treatment relapse (day 42).
1.7. Analysis.
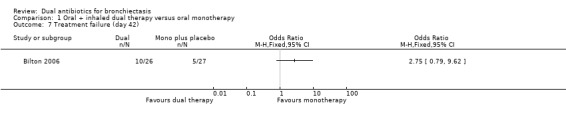
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 7 Treatment failure (day 42).
Microbiological response ‐ eradicated, persistent, superinfected
Microbiological response was not different between groups at day 21 for the following classifications: eradicated (OR 2.40, 95% CI 0.67 to 8.65; Analysis 1.8); persistent (OR 0.37, 95% CI 0.11 to 1.26; Analysis 1.9); and superinfected (OR 3.26, 95% CI 0.13 to 83.90; Analysis 1.10).
1.8. Analysis.
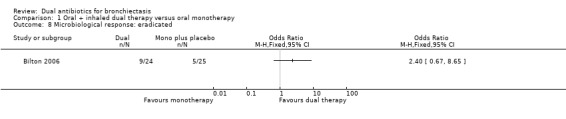
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 8 Microbiological response: eradicated.
1.9. Analysis.
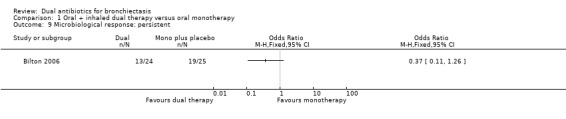
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 9 Microbiological response: persistent.
1.10. Analysis.
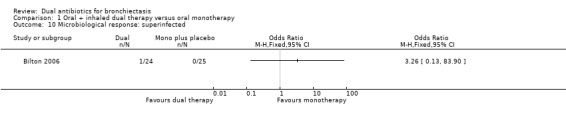
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 10 Microbiological response: superinfected.
According to our GRADE assessment, we judged this outcome as very low quality (Table 1).
Sputum volume and purulence
Bilton 2006 reported no statistically significant differences in mean sputum volume at days 7 and 14 with dual therapy compared with monotherapy.
Measures of lung function
Researchers noted no statistically significant differences in FEV1 between groups in the included studies. Bilton 2006 reported mean FEV1 (L) graphically for all data collection points (days 7 and 14), but it was not possible for review authors to accurately retrieve the raw data. Hossain 2010 did not report further details.
Systemic markers of infection
None of the included studies reported this outcome.
Adverse events
Researchers noted no differences between groups in the number of people who experienced an adverse event in Bilton 2006 (OR 0.21, 95% CI 0.02 to 2.03; Analysis 1.11). However, the incidence of wheeze was significantly higher in the dual therapy group compared with the monotherapy group (OR 5.75, 95% CI 1.55 to 21.33; Analysis 1.12). Data show no differences between groups in terms of adverse events arising from the use of study medications, although it is unclear from the paper whether this relates specifically to the antibiotic interventions (OR 1.45, 95% CI 0.49 to 4.31; Analysis 1.13). Hossain 2010 reported that five participants in the dual therapy group developed wheeze and chest tightness following administration of nebulised gentamicin. It remains unclear whether any of the participants in the monotherapy group experienced an adverse event.
1.11. Analysis.
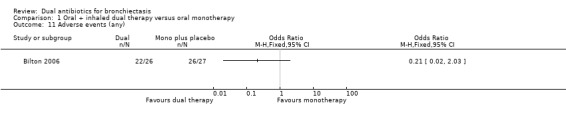
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 11 Adverse events (any).
1.12. Analysis.
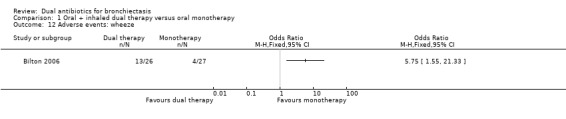
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 12 Adverse events: wheeze.
1.13. Analysis.
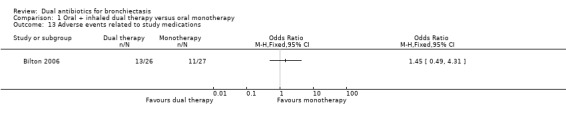
Comparison 1 Oral + inhaled dual therapy versus oral monotherapy, Outcome 13 Adverse events related to study medications.
Deaths
Neither of the included studies explicitly reported any deaths,
Emergence of resistance to antibiotics
Data show no differences between groups in the development of antibiotic resistance in Bilton 2006. One patient receiving dual tobramycin+ciprofloxacin therapy entered the study with tobramycin‐susceptible P aeruginosa strains that became resistant by the end of the study. No participants receiving ciprofloxacin monotherapy developed tobramycin‐resistantP aeruginosa strains during the study.
Exercise capacity
Neither Bilton 2006 nor Hossain 2010 reported this outcome.
Quality of life
Neither of the two included studies reported this outcome.
Discussion
Summary of main results
Two randomised trials met the inclusion criteria for this systematic review (Bilton 2006; Hossain 2010); both assessed the effectiveness of oral plus inhaled dual therapy versus oral monotherapy, and both were conducted in adults. Bilton 2006 was a multi‐centre trial conducted in UK and USA, and Hossain 2010 was a single‐centre study conducted in Bangladesh. Only an abstract was available for Hossain 2010, and outcomes were described narratively or results were reported as the P value alone; therefore we were unable to include these data in the quantitative data synthesis.
We found no evidence of treatment effect with oral plus inhaled dual therapy for all outcomes reported in the summary of findings, including successful treatment of exacerbations, serious adverse events, and response rates, although the effect estimate was based on one small study of 53 adults (Bilton 2006). Similarly, we found no evidence of effect on sputum volume, lung function, adverse events, or antibiotic resistance.
Overall completeness and applicability of evidence
Of the pre‐defined potential comparison groups, we identified only one group (oral plus inhaled dual therapy vs oral monotherapy) for inclusion in the review. The comparison included only two small studies with a total of 118 participants. Our search did not identify any other comparisons that met our study selection criteria. The two included studies did not report some of our primary outcomes (length, frequency, and time to next exacerbation) and did not report some of our secondary outcomes (systemic markers of infection, exercise capacity, and quality of life). It is particularly important to measure the impact of this chronic condition on health‐related quality of life from the patient's perspective. Furthermore, we were unable to obtain quantitative data for pooled analyses or to adequately assess risk of bias for one study, as findings were available only in a conference abstract. We did not identify any studies that evaluated the use of dual antibiotics in children, or that assessed long‐term (more than four weeks) use of dual antibiotic therapy; we identified insufficient studies to permit assessment of effects by class of antibiotic. Similarly, we found insufficient studies to conduct sensitivity analyses. Our findings therefore are limited by scant available data, and we were unable to evaluate all outcomes planned in the protocol.
Quality of the evidence
We judged overall quality of the evidence as very low for outcomes included in the GRADE assessment. Only one comparison ‐ oral plus inhaled dual antibiotic versus oral antibiotic alone ‐ was assessed, and included studies did not report several of the outcomes that we planned to include in our GRADE assessment. We judged the quality of evidence as very low for the three outcomes included in the GRADE assessment (treatment of exacerbations, response rate, and serious adverse events). All outcomes were limited by incomplete outcome data. We considered effects as imprecise owing to wide confidence intervals that crossed the line of no effect and inclusion of few events and small sample sizes.
Potential biases in the review process
We used a comprehensive systematic search, conducted by a highly experienced information specialist, to identify potentially eligible studies. We also searched multiple resources including electronic databases, journals, conference proceedings, reference lists of included studies, citations of included studies, and trial registries. Nevertheless, we recognise the possibility of publication bias in this review, which could lead to overestimation or underestimation of effects of the intervention in terms of the different outcomes included in this review. Trials showing no, or negative, effects are less likely to be offered for publication, and, if offered, they are less likely to be accepted, resulting in a biased set of data available for review. We were able to extract quantitative data from only one study and were unable to assess the presence of publication bias through formal testing.
Furthermore, some papers may have been misclassified as not eligible for inclusion in the review. However, two review authors independently assessed all studies, and a third review author verified selection, so we are confident that we assessed studies excluded from the analyses on the basis of consistent and appropriate criteria. We double‐checked all data to avoid extraction and transcription errors.
We contacted the investigators of both included studies to request further information on trial methods and outcome data. The author of one trial, published as a full‐text paper, provided clarification on randomisation procedures. We did not receive a response from the authors of the study that was published only as an abstract. We also contacted the author of one excluded study published in a non‐English language but did not receive a response. We obtained translations of two non‐English language studies that we excluded following inspection of the translated text. We were unable to conduct planned subgroup or sensitivity analyses owing to the small number of included studies.
Agreements and disagreements with other studies or reviews
There are no previous versions of this review. We included in this review two small trials that assessed the effectiveness of oral plus inhaled dual therapy versus oral monotherapy alone, with a total of 118 adult participants and no children. We highlighted the paucity of evidence in this area in relation to all important outcomes and, in accordance with this lack of evidence, identified no published reviews of the relevant benefits and risks of combined antibiotics compared with monotherapy in bronchiectasis. A review of single versus combination intravenous antibiotics for eradicating Pseudomonas aeruginosa in people with cystic fibrosis found no significant benefit associated with a beta‐lactam or aminoglycoside monotherapy compared with a beta‐lactam‐aminoglycoside combination upon examination of poor quality evidence (Elphick 2005). However, recent bronchiectasis guidelines emphasise differences in treatment responses between bronchiectasis and cystic fibrosis, and although the guidelines provide no specific recommendations for dual therapy, they do offer recommendations for use of antibiotics in people with this condition (Polverino 2017). This review cannot inform robust recommendations for practice owing to insufficient high‐quality evidence, and review authors found no evidence related to the role of dual antibiotics in the treatment of children.
Authors' conclusions
Implications for practice.
This systematic review identified minimal published evidence to guide clinical practice on the routine use of dual antibiotics for treatment of patients with bronchiectasis.
Only two published trials with 118 adult participants met our inclusion criteria; these studies evaluated the addition of nebulised aminoglycosides to systemic antibiotics. Bilton and colleagues investigated the addition of nebulised tobramycin to oral ciprofloxacin for treatment of 53 adult patients with acute Pseudomonas aeruginosa infection in bronchiectasis. Researchers found no evidence of treatment benefit with oral plus inhaled dual therapy in terms of successful treatment of exacerbations, serious adverse events, sputum volume, lung function, and antibiotic resistance. Hossain reported the results of a single‐centre placebo‐controlled comparison of systemic antimicrobials versus the addition of nebulised gentamicin, but we were unable to include this study in the quantitative synthesis. Both studies reported a higher incidence of wheeze with dual therapy. Overall, we have very low confidence in the outcomes presented.
Review authors have identified the need for better quality evidence on the benefits and risks of dual antibiotics to guide clinical practice in the treatment of patients with bronchiectasis.
Implications for research.
Our review highlights the need for additional long‐term randomised placebo‐controlled trials to determine the effectiveness of dual antibiotics versus single antibiotics for treatment of adults and children with bronchiectasis. The two included studies compared oral plus inhaled dual therapy versus oral therapy alone, but no trials have compared other combinations of modes of administration (e.g. intravenous and inhaled; different antibiotics delivered via a common mode of administration, such as two inhaled antibiotics). Some evidence from observational studies of dual antibiotic regimens suggests that including nebulised antibiotics is more effective than providing intravenous regimens alone for eradicating Pseudomonas aeruginosa. (Orriols 1999), This and other comparisons require formal testing in randomised controlled trials to establish the relative benefits of different types of dual therapy. The overall quality of evidence derived from the two included studies is very poor, and we found no data on our primary outcomes of duration, frequency, or time to next exacerbation, nor on our secondary outcomes of microbiological infection measures, exercise capacity, and quality of life. Furthermore neither of the included trials enrolled children or investigated long‐term (more than four weeks) use of dual antibiotic therapy. Future high‐quality studies should consider both short‐ and long‐term antibiotic management for adults and children including our prespecified review outcomes, especially health‐related quality of life, and should report data on adults and children separately. We also consider it important to assess the relative risks and benefits of continuous versus cyclical antibiotic administration, but this question will be addressed in a separate review.
Acknowledgements
We would like to thank Edge Hill University for support provided for this review. We would also like to thank Cochrane Airways for its support, and Nobuyuki Horita and Hiraku Tsujimoto for their translations of Watanabe 1990 and Takamoto 1994.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
Rebecca Normansell was the Editor and commented critically on the review.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| CENTRAL (the Cochrane Library) | Monthly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Bronchiectasis search
1. exp Bronchiectasis/
2. bronchiect$.mp.
3. bronchoect$.mp.
4. kartagener$.mp.
5. (ciliary adj3 dyskinesia).mp.
6. (bronchial$ adj3 dilat$).mp.
7. or/1‐6
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter (Lefebvre 2011) were adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 BRONCH:MISC1
#2 MeSH DESCRIPTOR Bronchiectasis Explode All
#3 bronchiect*
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Anti‐Bacterial Agents Explode 1
#6 antibiotic* or anti‐biotic*
#7 anti‐bacteri* or antibacteri*
#8 *cillin
#9 *mycin or micin*
#10 *oxacin
#11 *tetracycline
#12 macrolide*
#13 quinolone*
#14 trimethoprim
#15 ceph*
#16 sulpha*
#17 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 #4 and #17
[In search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, bronchiectasis]
Data and analyses
Comparison 1. Oral + inhaled dual therapy versus oral monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Successful treatment of exacerbation: cured (day 21) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Successful treatment of exacerbation: cured (day 42) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Pseudomonas eradication | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Serious adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Treatment failure (day 21) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Treatment relapse (day 42) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Treatment failure (day 42) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Microbiological response: eradicated | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Microbiological response: persistent | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Microbiological response: superinfected | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Adverse events (any) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Adverse events: wheeze | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Adverse events related to study medications | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bilton 2006.
| Methods |
Aims: to test the effect of adding tobramycin inhaled solution to oral ciprofloxacin for the treatment of acute exacerbations of non‐cystic fibrosis bronchiectasis in patients with P aeruginosa infection Design: a double‐blind, randomised, active comparator, parallel‐design study Total study duration: 42 days from randomisation, not including the 28‐day pre‐screening run‐in period. Elapsed time between pre‐screening and randomisation differed for each participant as participants were randomised during an exacerbation Number of study centres and locations: multiple (17 centres): 5 in the United Kingdom, 12 in the United States Study setting: home (participants received the first dose of study drug on day 1 in the presence of study personnel but took subsequent doses at home) Methods of recruitment: unclear Study start and end dates: not reported Withdrawals: 10 participants withdrew from the study (5 from inhaled tobramycin + oral ciprofloxacin group, 5 from oral ciprofloxacin group). Seven withdrawals (2 from inhaled tobramycin + oral ciprofloxacin group and 5 from oral ciprofloxacin group) were due to adverse events. Among these, 3 participants (1 from inhaled tobramycin + oral ciprofloxacin group, and 2 from oral ciprofloxacin group) withdrew owing to possibly drug‐related adverse events, and 4 in the oral ciprofloxacin group withdrew on day 21 or later owing to non‐drug‐related adverse events. Three participants in the inhaled tobramycin + oral ciprofloxacin group withdrew from the study owing to "unsatisfactory therapy responses" after receiving 8, 8, and 15 days of dosing, respectively. One participant from the inhaled tobramycin + oral ciprofloxacin group who withdrew at day 8 owing to "unsatisfactory therapeutic responses" also had an adverse event. Analysis by intent to treat: yes. It was reported that "safety and efficacy analysis was performed on the study population, which included all randomised participants who had received at least one dose of study medication." |
|
| Participants |
53 adults were randomised Inclusion criteria: history of chronic P aeruginosa lung infection, confirmed by a sputum culture that was positive for P aeruginosa both within 12 months before screening and at the time of screening. In addition, P aeruginosa had to show ciprofloxacin sensitivity (mic < 4) at the time of study enrolment. Participants who did not experience an acute exacerbation within 2 months of the screening visit were rescreened for study eligibility Exclusion criteria: cystic fibrosis, allergic bronchopulmonary aspergillosis, active tuberculosis, glucose‐6‐phosphate dehydrogenase deficiency, significant renal disease, change in steroid therapy within 2 weeks of exacerbation Mean age: inhaled tobramycin + oral ciprofloxacin: 61.4 years; placebo/ciprofloxacin: 63.7 years Age range: 18 to 80 years Gender: inhaled tobramycin + oral ciprofloxacin group: 20 women, 6 men; placebo/ciprofloxacin group: 18 women, 9 men Diagnostic criteria: men and women between 18 and 80 years of age with bronchiectasis confirmed by central reading of an HRCT scan Severity of condition: not stated Baseline lung function: placebo/ciprofloxacin group: FEV1 51.4% predicted, FVC 70.4% predicted. Inhaled tobramycin + oral ciprofloxacin group: FEV1 53.2% predicted, FVC 70% predicted Smoking history: placebo/ciprofloxacin group: current smokers 0, former smokers 10. Inhaled tobramycin + oral ciprofloxacin group: current smokers 1, former smokers 7 Baseline imbalances: No significant imbalances were identified. 13% of ciprofloxacin‐resistant participants were excluded from the study |
|
| Interventions |
Inhaled tobramycin + oral ciprofloxacin group (n = 26) Ciprofloxacin Dose: 750 mg Delivery mode: oral Frequency: twice daily Duration: 14 days Tobramycin inhaled solution Dose: 300 mg per 5 mL Delivery mode: aerosolised with the use of a jet nebuliser Frequency: twice daily Duration: 14 days Ciprofloxacin + placebo group (n = 27) Ciprofloxacin Dose: 750 mg Delivery mode: oral Frequency: twice daily Duration: 14 days Placebo Dose: 1.25 mg quinine sulphate per 5 mL Delivery mode: aerosolised with the use of a jet nebuliser Frequency: twice daily Duration: 14 days Adherence: Study personnel were present during administration of the first dose on day 1 Run‐in phase: 28 days Run‐out phase: none Participants were not allowed to use inhaled tobramycin within 28 days before screening or between screening and exacerbation event. Maintenance therapy with antibiotics, including aerosolised antibiotics other than inhaled tobramycin, was allowed up until the time of exacerbation; no changes were permitted within 14 days before the exacerbation and during the study period |
|
| Outcomes | Participants kept a structured respiratory symptoms diary Primary Clinical efficacy: assessed on days 14, 21, and 42, and classified as follows. Day 14
Day 21
Day 42
Sputum microbiology was classified on day 21 as follows, based on sputum culture findings.
Secondary Pulmonary function tests: FEV1 (L) assessed on days 0, 7, and 14 Adverse event rate Post hoc analysis: concordance of clinical and microbiological outcomes at day 21, which included clinical efficacy (cured) and P aeruginosa eradication |
|
| Notes |
Power calculation: not reported Trial registration: not reported Conflicts of interest: not reported Funders: not reported Role of the sponsors: not reported Ethical approval: achieved at each participating centre Conclusions: "The addition of an inhaled tobramycin solution to therapy with oral ciprofloxacin for the treatment of acute exacerbations of bronchiectasis due to P aeruginosa improved microbiological outcome and was concordant with clinical outcome; the inability to demonstrate an additional clinical benefit may have been due to emergent wheeze resulting from treatment" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "randomisation was computer generated with sites telephoning the company's central number in order to allocate the specific numbered vials" |
| Allocation concealment (selection bias) | Low risk | Correspondence with the principal investigator of the study confirmed that random sequence was allocated centrally |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from correspondence): "patients and the research team were blinded as the placebo was supplied by the company who made TOBI (by then I think it was Novartis) and the placebo contained something to make it taste the same as TOBI" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "assessment of endpoints was also blind as none of the patients filling in symptom questionnaires and none of the lab staff working on the microbiology knew which treatment a patient was on" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 5 participants in the inhaled tobramycin + oral ciprofloxacin group who withdrew from the study, 2 withdrew owing to drug‐related adverse events and 3 because of an "unsatisfactory therapeutic response" after receiving at least 8 days of study drug. In the oral ciprofloxacin group, only 1 participant withdrew owing to drug‐related adverse events and the remaining 4 withdrawals were attributed to non‐drug‐related adverse events Comment: Reasons for missing outcome data were imbalanced between the 2 groups and may have been related to the intervention |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available to permit a judgement of 'low risk' or 'high risk.' Trial authors acknowledge that important outcomes such as time‐to‐next‐exacerbation and time‐to‐next‐ exacerbation‐requiring‐hospitalisation were not assessed in this study |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hossain 2010.
| Methods |
Aims: to investigate the efficacy of adding nebulised gentamicin to systemic antibiotics to improve clinical outcomes during an exacerbation of bronchiectasis. The clinical outcome was categorised as follows: resolved, improved but not fully resolved, or not improved Design: randomised controlled trial Total study duration: 21 days Number of study centres and locations: single; Dhaka, Bangladesh Study setting: National Institute of Diseases of the Chest Methods of recruitment: unclear Study start and end dates: not reported Analysis by intent to treat: not stated Withdrawals: 11 in total, 3 from the nebulised gentamicin + systemic antibiotic group and 8 from the placebo + systemic antibiotic group. Withdrawals were due to failure to attend follow‐up clinic Exact withdrawals are unclear owing to inconsistent reporting in the abstract: nebulised gentamicin + systemic antibiotic group started with 35 participants, with 3 withdrawals leaving 32, but the abstract states that 30 participants completed the study in the nebulised gentamicin + systemic antibiotic group |
|
| Participants | 65 adults were randomised Settings: National Institute of Diseases of the Chest Country: Bangladesh Inclusion criteria: diagnosis of bronchiectasis deemed to be exacerbating and treated with systemic antibiotics Exclusion criteria: not reported Methods of recruitment: not reported Mean age: not reported Age range: not reported Gender: not reported Diagnostic criteria: not reported Severity of condition: not reported Baseline lung function : not reported Smoking history : not reported Baseline imbalances: not reported |
|
| Interventions |
Nebulised gentamicin + systemic antibiotic group (n = 35) Dose: not stated Placebo + systemic antibiotic group (n = 30) Dose: not stated Adherence: not reported Run‐in phase: not reported Run‐out phase: not reported |
|
| Outcomes |
Primary: exacerbation status classified at days 3, 7, 14, and 21 as follows.
Secondary: not reported Post hoc analysis: not reported |
|
| Notes |
Power calculation: not reported Trial registration: unclear Conflicts of interest: not reported Funders: not reported Role of the sponsors: not reported Ethical approval: not reported Conclusions: "(1) Addition of nebulized gentamicin to systemic antibiotic improves clinical efficacy compared to only systemic antibiotic. (2) It can reduce hospital stay when used as adjuvant with systemic antibiotic for the treatment of exacerbation of bronchiectasis" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of the randomisation process were not reported |
| Allocation concealment (selection bias) | Unclear risk | Details of the allocation process were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was not described as blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The assessment was not described as blinded. The outcome assessment included severity of exacerbation, but it is not clear how this was assessed or by whom |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 from intervention group and 8 from placebo group did not attend follow‐up visits, but no further details are reported |
| Selective reporting (reporting bias) | Unclear risk | Only data reported in an abstract were available |
| Other bias | Unclear risk | The abstract did not report baseline values, so the potential for baseline imbalances was unclear. Similarly, no data were provided on route of administration of systemic antibiotics nor on adherence |
FEV1: forced expiratory volume in one second; FVC: forced vital capacity; HRCT: high‐resolution computed tomography; MIC: minimum inhibitory concentration; TOBI:Tobramycin
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Orriols 1999 | Did not meet inclusion criteria for the intervention. Nebulised dual antibiotic vs symptomatic treatment (i.e. comparison arm was not given a monotherapy antibiotic) |
| Orriols 2015 | Did not meet inclusion criteria for the intervention. The study compared nebulised dual antibiotics with symptomatic treatment (i.e. comparison arm was not given a monotherapy antibiotic) |
| Takamoto 1994 | The intervention was not exclusively restricted to participants with bronchiectasis, and it is unclear whether the study was randomised. We were unable to contact trial authors for clarification on study design or data on participants with bronchiectasis alone |
| Vergnon 1985 | The intervention was not exclusively restricted to participants with bronchiectasis, and we were unable to contact trial authors for data on these participants alone |
| Watanabe 1990 | The intervention was not exclusively restricted to participants with bronchiectasis, and it is unclear whether the study was randomised. We were unable to contact trial authors for clarification on study design or data on participants with bronchiectasis alone |
Differences between protocol and review
We have noted no differences between the review and the planned protocol, although we found insufficient evidence to conduct all pre‐planned analyses.
Contributions of authors
All review authors contributed to the background, discussion, and conclusions. Lambert Felix, Stephen Milan, and Sally Spencer contributed to the methods and results sections.
Haley Harrison searched trial registries. Seamus Grundy and Lambert Felix contributed to screening of searches and identifying the included studies. Ross Armstrong, Dave Lynes, and Lambert Felix extracted data and completed the risk of bias assessment. Dave Lynes transferred data into Review Manager. Lambert Felix and Sally Spencer undertook data analyses.
Sources of support
Internal sources
-
Edge Hill University, UK.
Funded a part‐time review author (LF) to support a series of reviews on bronchiectasis
External sources
The review authors declare that no such funding was received for this systematic review, UK.
Declarations of interest
Sally Spencer, Dave Lynes, and Ross Armstrong are named co‐investigators on a study funded by Edge Hill University to develop a series of reviews on bronchiectasis; Lambert Felix is a part‐time review author supported by this funding; Haley Harrison received funding support through an NIHR internship. The remaining review authors did not receive funding for this systematic review.
New
References
References to studies included in this review
Bilton 2006 {published data only}
- Bilton D, Henig N, Morrissey B, Gotfried M. Addition of inhaled tobramycin to ciprofloxacin for acute exacerbations of Pseudomonas aeruginosa infection in adult bronchiectasis. Chest 2006;130(5):1503‐10. [PUBMED: 17099030] [DOI] [PubMed] [Google Scholar]
Hossain 2010 {published data only}
- Hossain A, Ahamed M, Sharkar ZH. Change in clinical outcome of exacerbation of bronchiectasis on addition of nebulized gentamicin to systemic antibiotic. Respirology 2010;15(Suppl 2):38 (OS 08‐01). [Google Scholar]
References to studies excluded from this review
Orriols 1999 {published data only}
- Orriols R, Roig J, Ferrer J, Sampol G, Rosell A, Ferrer A, et al. Inhaled antibiotic therapy in non‐cystic fibrosis patients with bronchiectasis and chronic bronchial infection by Pseudomonas aeruginosa. Respiratory Medicine 1999;93(7):476‐80. [PUBMED: 10464834] [DOI] [PubMed] [Google Scholar]
Orriols 2015 {published data only}
- Orriols R, Hernando R, Ferrer A, Terradas S, Montoro B. Eradication therapy against Pseudomonas aeruginosa in non‐cystic fibrosis bronchiectasis. Respiration 2015;90(4):299‐305. [PUBMED: 26340658] [DOI] [PubMed] [Google Scholar]
Takamoto 1994 {published data only}
- Takamoto M, Ishibashi T, Toyoshima H, Tanaka H, Tamaru N, Watanabe K, et al. Imipenem/cilastatin sodium alone or combined with amikacin sulfate in respiratory infections. Japanese Journal of Antibiotics 1994;47(9):1131‐44. [PubMed] [Google Scholar]
Vergnon 1985 {published data only}
- Vergnon JM, Vincent M, Ros A, Brun Y, Brune J. [Comparative clinical trial of cefoperazone versus ampicillin + tobramycin in severe bronchopulmonary and pleural infectious pathology] [Essai clinique comparatif cefoperazone contre ampicilline + tobramycine en pathologie infectieuse broncho‐pulmonaire et pleurale grave]. Revue de Pneumologie Clinique 1985;41(3):205‐11. [PUBMED: 4048752] [PubMed] [Google Scholar]
Watanabe 1990 {published data only}
- Watanabe A, Motomiya M, Nukiwa T, Nakai Y, Honda Y, Konno K, et al. A well‐controlled comparative clinical study of the combination regimen of ciprofloxacin plus erythromycin for the treatment of repeated acute exacerbations of chronic respiratory tract infections. Chemotherapy 1994;42(10):1194‐201. [Google Scholar]
Additional references
Chalmers 2012
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short‐ and long‐term antibiotic treatment reduces airway and systemic inflammation in non–cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2012;186(7):657‐65. [DOI] [PubMed] [Google Scholar]
Chalmers 2014
- Chalmers JD, Goeminne P, Aliberti S, Melissa J. McDonnell MJ, Lonni S, et al. The bronchiectasis severity index: an international derivation and validation study. American Journal of Respiratory and Critical Care Medicine 2014;189(5):576‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2002
- Chang AB, Grimwood K, Mulholland EK, Torzillo PJ. Bronchiectasis in indigenous children in remote Australian communities. Medical Journal of Australia 2002;177(4):200‐4. [PUBMED: 12175325] [DOI] [PubMed] [Google Scholar]
Chang 2010
- Chang AB, Bell SC, Byrnes CA, Grimwood K, Holmes P, King PT, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand. Medical Journal of Australia 2010;193(6):356‐65. [DOI] [PubMed] [Google Scholar]
Cole 1986
- Cole PJ. Inflammation: a two‐edged sword ‐ the model of bronchiectasis. European Journal of Respiratory Diseases Supplement 1986;147:6‐15. [PubMed] [Google Scholar]
Cole 1997
- Cole P. The damaging role of bacteria in chronic lung infection. Journal of Antimicrobial Chemotherapy 1997;40(Suppl A):5‐10. [DOI] [PubMed] [Google Scholar]
Elphick 2005
- Elphick HE, Tan AA. Single versus combination intravenous antibiotic therapy for people with cystic fibrosis. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002007.pub2] [DOI] [PubMed] [Google Scholar]
European Lung White Book 2013
- Gibson GJ, Loddenkemper R, Sibille Y, Lundbäck B, editor(s). European Lung White Book: Respiratory Health and Disease in Europe. www.erswhitebook.org/ (accessed before 11 October 2017).
Evans 1996
- Evans SA, Turner SM, Bosch BJ, Hardy CC, Woodhead MA. Lung function in bronchiectasis: the influence of P seudomonas aeruginosa. European Respiratory Journal 1996;9(8):1601‐4. [DOI] [PubMed] [Google Scholar]
Evans 2003
- Evans DJ, Greenstone M. Long‐term antibiotics in the management of non‐CF bronchiectasis ‐ do they improve outcome?. Respiratory Medicine 2003;97(7):851‐8. [DOI] [PubMed] [Google Scholar]
Foweraker 2011
- Foweraker JWD. Microbiology of non‐CF bronchiectasis. European Respiratory Society Monograph 2011;52:68‐96. [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 15 July 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Habesoglu 2011
- Habesoglu MA, Ugurlu AO, Eyuboglu FO. Clinical, radiologic, and functional evaluation of 304 patients with bronchiectasis. Annals of Thoracic Medicine 2011;6(3):131‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2015
- Hansen MP, Thorning S, Aronson JK, Beller EM, Glasziou PP, Hoffmann TC, et al. Adverse events in patients taking macrolide antibiotics versus placebo for any indication. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haworth 2014
- Haworth CS, Foweraker JE, Wilkinson P, Kenyon RF, Bilton D. Inhaled colistin in patients with bronchiectasis and chronic Pseudomonas aeruginosa infection. American Journal of Respiratory and Critical Care Medicine 2014;189:975–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Kaehne 2017
- Kaehne A, Milan SJ, Felix LM, Spencer S, Sheridan E, Marsden PA. Head‐to‐head trials of antibiotics for non‐cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD012590] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kapur 2012
- Kapur N, Grimwood K, Masters IB, Morris PS, Chang AB. Lower airway microbiology and cellularity in children with newly diagnosed non‐CF bronchiectasis. Pediatric Pulmonology 2012;47:300–7. [DOI] [PubMed] [Google Scholar]
Kapur 2012a
- Kapur N, Masters IB, Newcombe P, Chang AB. The burden of disease in pediatric non‐cystic fibrosis bronchiectasis. Chest 2012;141(4):1018‐24. [PUBMED: 21885727] [DOI] [PubMed] [Google Scholar]
Kelly 2018
- Kelly C, Chalmers JD, Crossingham I, Relph N, Felix LM, Evans DJ, et al. Macrolide antibiotics for bronchiectasis. Cochrane Database of Systematic Reviews 2018, Issue 3. [DOI: 10.1002/14651858.CD012406.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavery 2005
- Lavery K, Bradley JM, Elborn JS. Bronchiectasis: challenges in diagnosis and management. International Journal of Respiratory Care 2005;1:92‐8. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Martinez Garcia 2007
- Martinez‐Garcia MA, Soler‐Cataluna JJ, Perpina‐Tordera M, Roman‐Sanchez P, Soriano J. Factors associated with lung function decline in adult patients with stable non‐cystic fibrosis bronchiectasis. Chest 2007;132(5):1565‐72. [DOI] [PubMed] [Google Scholar]
Martinez Garcia 2014
- Martinez‐Garcia MA, Gracia J, Relat MV, Giron RM, Carro LM, Rosa Carrillo D, et al. Multidimensional approach to non‐cystic fibrosis bronchiectasis: the FACED score. European Respiratory Journal 2014;43:1357‐67. [DOI] [PubMed] [Google Scholar]
McShane 2013
- McShane PJ, Naureckas ET, Tino G, Strek ME. Non‐cystic fibrosis bronchiectasis. American Journal of Respiratory and Critical Care Medicine 2013;188:647–56. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasteur 2010
- Pasteur MC, Bilton D, Hill AT. British Thoracic Society Bronchiectasis Non‐CF Guideline Group. British Thoracic Society guideline for non CF bronchiectasis. Thorax 2010;65:i1–i58. [DOI] [PubMed] [Google Scholar]
Polverino 2017
- Polverino E, Goeminne PC, McDonnell MJ, Aliberti S, Marshall SE, Loebinger MR, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. European Respiratory Journal 2017;50(3):1700629. [DOI: 10.1183/13993003.00629-2017] [DOI] [PubMed] [Google Scholar]
Quint 2016
- Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population‐based cohort study. European Respiratory Journal 2016;47(1):186‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ringshausen 2015
Roberts 2010
- Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respiratory Medicine 2010;104:981‐5. [DOI] [PubMed] [Google Scholar]
Rubin 2014
- Rubin BK, Williams RW. Aerosolized antibiotics for non‐cystic fibrosis bronchiectasis. Respiration 2014;88:177–84. [DOI] [PubMed] [Google Scholar]
Seitz 2010
- Seitz AE, Olivier KN, Steiner CA, Montes de Oca R, Holland SM, Prevots DR. Trends and burden of bronchiectasis‐associated hospitalizations in the United States, 1993‐2006. Chest 2010;138(4):944‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seitz 2012
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000‐2007. Chest 2012;142(2):432‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Twiss 2005
- Twiss J, Metcalfe R, Edwards E, Byrnes C. New Zealand national incidence of bronchiectasis "too high" for a developed country. Archives of Disease in Childhood 2005;90(7):737‐40. [PUBMED: 15871981] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valery 2012
- Valery P, Morris P, Grimwood K, Torzillo P, Byrnes C, Masters IB, et al. Azithromycin for Indigenous children with bronchiectasis: study protocol for a multi‐centre randomized controlled trial. BMC Paediatrics 2012;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weycker 2005
- Weycker D, Edelsberg J, Oster G, Tino G. Prevalence and economic burden of bronchiectasis. Clinical Pulmonary Medicine 2005;12:205‐9. [Google Scholar]
Wilson 1997
- Wilson CB, Jones PW, O'Leary CJ, Hansell DM, Cole PJ, Wilson R. Effect of sputum bacteriology on the quality of life of patients with bronchiectasis. European Respiratory Journal 1997;10:1754‐60. [DOI] [PubMed] [Google Scholar]
Wu 2014
- Wu Q, Shen W, Cheng H, Zhou X. Long‐term macrolides for non‐cystic fibrosis bronchiectasis: a systematic review and meta‐analysis. Respirology 2014;19(3):321–9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Felix 2017
- Felix LM, Grundy S, Milan SJ, Armstrong R, Harrison H, Lynes D, et al. Dual antibiotics for non‐cystic fibrosis bronchiectasis. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD012514] [DOI] [PMC free article] [PubMed] [Google Scholar]


