Abstract
Background
Granulosa cell tumour is a rare gynaecological tumour of the ovary with recurrences many years after initial diagnosis and treatment. Evidence‐based management of granulosa cell tumour of the ovary is limited, and treatment has not been standardised. Surgery, including fertility‐sparing procedures for young women, has traditionally been the standard treatment. Adjuvant treatments following surgery have been based on non‐randomised trials. A combination of bleomycin, etoposide and cisplatin (BEP) has traditionally been used for treatment of advanced and/or recurrent disease that cannot be optimally managed surgically.
Objectives
To evaluate the effectiveness and safety of different treatment modalities offered in current practice for the management of primary, residual and recurrent adult‐onset granulosa cell tumours (GCTs) of the ovary.
Search methods
We searched the Cochrane Gynaecological Cancer Group Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE up to December 2013. We also searched registers of clinical trials, abstracts of scientific meetings and reference lists of included studies.
Selection criteria
We searched for randomised controlled trials (RCTs), quasi‐RCTs and observational studies that examined women with adult‐onset granulosa cell tumours of the ovary (primary and recurrent). For non‐randomised studies, we included studies that used multivariate analysis to adjust for baseline characteristics.
Data collection and analysis
Two review authors independently abstracted data and assessed risk of bias. Studies were heterogeneous with respect to treatment comparisons, so data were not synthesised in meta‐analyses, and methods for assessing heterogeneity were not needed. Risk of bias in included studies was assessed by using the six core items used to assess RCTs and by evaluating four additional criteria specifically addressing risk of bias in non‐randomised studies.
Main results
Five retrospective cohort studies (535 women with a diagnosis of GCT) that used appropriate statistical methods for adjustment were included in the review.
Two studies, which carried out multivariate analyses that attempted to identify factors associated with better outcomes (in terms of overall survival), reported no apparent evidence of a difference in overall survival between surgical approaches, whether a participant underwent lymphadenectomy or received adjuvant chemotherapy or radiotherapy. Only percentage of survival for all participants combined was reported in two trials and was not reported at all in one study.
One study showed that women who received postoperative radiotherapy had lower risk of disease recurrence compared with those who underwent surgery alone (adjusted hazard ratio (HR) 0.3, 95% confidence interval (CI) 0.1 to 0.6, P value 0.04). Three studies reportedthat there was no evidence of differences in disease recurrence based on execution and type of adjuvant chemotherapy or on type of surgery or surgical approach, other than that surgical staging may be important. One study described no apparent evidence of a difference in disease recurrence between fertility‐sparing surgery and conventional surgery. Recurrence‐free survival was not reported in one study.
Toxicity and adverse event data were incompletely reported in the five studies. None of the five studies reported on quality of life (QoL). All studies were at very high risk of bias.
Authors' conclusions
One study showed a lower recurrence rate with the use of adjuvant radiotherapy, although this study was at high risk of bias and the results should be interpreted with caution. After evaluating the five small retrospective studies, we are unable to reach any firm conclusions as to the effectiveness and safety of different types and approaches of surgery, including conservative surgery, as well as adjuvant chemotherapy or radiotherapy, for management of GCTs of the ovary. The available evidence is very limited, and the review provides only low‐quality evidence. Further research is very likely to have an important impact on our confidence in the estimate of effect and may alter our findings.
Ideally, multinational RCTs are needed to answer these questions. The disease is relatively rare and generally has a good prognosis. RCTs are challenging to conduct, but three ongoing trials have been identified, demonstrating that they are feasible, although two of these studies are single‐arm trials. The study that may be able to provide answers to the question of which chemotherapeutic regimen should be selected for management of sex cord stromal tumours is an ongoing, randomised, phase 2 study, led by the Gynaecological Oncology Group to compare the efficacy of carboplatin and paclitaxel versus standard BEP. These investigators are also looking into the value of inhibin A and inhibin B as predictive biomarkers. Additional trials are required to assess toxicity and QoL associated with different treatment regimens as well as the safety of conservative surgical options.
Plain language summary
Are any effective treatment options available for the management of granulosa cell tumour of the ovary?
Background
Granulosa cell tumours (GCTs) of the ovary are rare ovarian tumours (2% to 5% of all ovarian cancers). Most ovarian tumours arise from the outer surface layer of the ovary, but GCTs arise from granulosa cells (sex cord cells) within the ovaries that produce oestrogen (primary female sex hormones). These tumours grow relatively slowly and can recur 10 to 15 years after primary treatment. If women with these tumours want to have children, the surgeon usually removes only the diseased ovary. However, standard treatment has consisted of surgery to remove tubes, ovaries and uterus, as most women develop GCTs around the time of the menopause, when fertility is no longer a matter of concern.
Review question
Previous studies have assessed chemotherapy (different combination regimens) with or without radiotherapy following surgery. This review aimed to examine the effects of various treatment methods, including fertility‐sparing surgery, on the survival of women with GCT of the ovary.
Main findings
Five retrospective studies (including 535 women with a diagnosis of GCT) met our inclusion criteria.
Two studies, which attempted to identify factors associated with better outcomes (in terms of overall survival), suggested that no apparent evidence could be found of differences in overall survival between surgical approaches (including whether the surgery was keyhole or open) whether a patient underwent lymphadenectomy (removal of lymph nodes) or received adjuvant chemotherapy or radiotherapy. Only percentage of survival for all women combined was reported in two trials and was not reported at all in one study.
One study showed that women who received postoperative radiotherapy had lower risk of disease recurrence compared with those who underwent only surgery. In three studies, no apparent evidence to suggest that disease recurrence was associated with type of adjuvant chemotherapy or type of surgery, although surgical staging may be important. In one study, disease recurrence was not noted to be different between patients who underwent fertility‐sparing surgery, where only the affected fallopian tube and ovary were removed, and those treated with conventional surgery, in which both tubes and ovaries were removed. Given the high overall survival rate, fertility‐sparing surgery may be an important treatment option for young patients wishing to have children in the future. Recurrence‐free survival was not reported in one study.
Toxicity and adverse event data were incompletely reported in the five studies. None of the five studies reported on quality of life. All studies were at very high risk of bias (low quality).
Quality of the evidence
All five studies were retrospective (looked at past findings) and were at very high risk of bias (low quality); therefore future studies should look at current evidence in randomised studies on adult GCT of the ovary. Three randomised studies comparing chemotherapy are ongoing. The study that may be able to answer the question regarding best choice of chemotherapy in sex cord stromal tumours is an ongoing randomised study comparing the efficacy of two drugs (carboplatin and paclitaxel) versus standard chemotherapy (BEP ‐ bleomycin, etoposide, cisplatin).
Overall, the evidence in this review is of low quality, which may seriously weaken confidence in the results. Further research is very likely to have an important impact on evidence provided in the future. The effectiveness and safety of different ways of treating patients with adult‐onset granulosa cell tumour of the ovary have not yet been assessed by high‐quality studies. Such trials are required to assess toxicity and quality of life associated with different treatments and to assess the safety of the types of surgery used. Generally, current evidence is not robust enough to allow recommendations for changes in clinical practice.
Background
Description of the condition
Granulosa cell tumours (GCTs) of the ovary (unless otherwise stated, GCTs throughout this review refers to the adult‐onset type) are classified under the category of sex ‐cord stromal tumours, which have an age‐standardised rate ranging between countries from 0 to 0.9 cases per 100,000 women (aged 20 to 74 years) per year (Parkin 2003). GCTs account for 70% to 85% of malignant sex cord stromal tumours (Freeman 2006; Schumer 2003) but overall constitute only 2% to 5% of all ovarian tumours (Aboud 1997; Malmstrom 1994; Schumer 2003; Uygun 2003). Adult‐onset GCTs most frequently occur during the perimenopausal or early postmenopausal period (Aboud 1997; Abu‐Rustum 2006; Schumer 2003) and are more likely to be unilateral than bilateral.
The clinical presentation of GCTs may involve detection of a pelvic mass, but most women present with postmenopausal bleeding or irregular perimenopausal bleeding. GCTs secrete hormones, hence women present with symptoms caused by the production of oestrogen (primary female sex hormones), follicle‐regulating protein, folliculin and the inhibin hormone. These act primarily to inhibit the secretion of follicle‐stimulating hormone by the anterior pituitary gland, which releases many hormones that affect growth, sexual development, metabolism and the system of reproduction (Gershenson 2004). Thus, it is usually the general gynaecologist or endocrinologist who initiates investigations for GCTs.
Patients with GCTs of the ovary are often treated with surgery alone. Although patients often present at an early stage, adult‐onset GCTs are malignant and can metastasise, recur and cause death. Around a quarter of patients develop recurrent tumours and require further treatment (Uygun 2003). One‐third of recurrences present more than five years after initial treatment, and one‐fifth after 10 years (Aboud 1997). Reported five‐year overall survival for patients with stage I disease ranges from 75% to 95%, with many studies demonstrating survival rates in excess of 90% (Mangili 2013). However, the five‐year overall survival rate is reduced to between 55% and 75% for patients with stage II tumour and drops to 22% to 50% for those with stage III/IV tumour (Colombo 2007). The overall 10‐year survival rate has been reported as between 85% and 95% (Ayhan 2009; Freeman 2006; Kim 2006; Savage 1998), and reported disease mortality rates are approximately 20% (Evans 1980; Fox 1975; Homesley 1999). More than 70% of women with recurrent disease die from their disease (Colombo 2007; Malmstrom 1994).
Pathologically, the grade of malignancy of GCTs is difficult to determine (Aboud 1997). Unfortunately, no histological signs are accurate for predicting poor prognosis. The only absolute indicative factor for prognosis is the presence of extraovarian spread at the time of initial diagnosis (Fox 1992). Tumour size and mitotic rate are associated with poor prognosis (Fox 1975).
Description of the intervention
Current modalities for management
(A) Surgery
A vast majority of cases are International Federation of Gynecology and Obstetrics (FIGO) (Benedet 2000) stage I disease (Fox 1975; Pankratz 1978). However, as staging may be incomplete at the time of primary surgery, this has been disputed (Abu‐Rustum 2006) because nodal metastases are rarely reported at primary diagnosis (Abu‐Rustum 2006). As a result, many patients do not receive further treatment after initial surgery, and recurrence can occur decades after primary diagnosis (Schumer 2003; Stuart 2003), possibly because the natural history of the tumour is relatively indolent and residual disease may take many years to become clinically apparent. Even if a GCT is confined to the ovary, hyperoestrogenism over a prolonged time may lead to endometrial hyperplasia and cancer. The reported co‐incidence of endometrial hyperplasia in GCTs of the ovary is 24% to 80%, and that of adenocarcinoma is 10% (Hoskins 1992).
Surgery has been the mainstay of treatment for GCTs (Schumer 2003). Advanced tumour stage and the presence of residual disease are associated with poor prognosis (Abu‐Rustum 2006). Complete surgical resection with total abdominal hysterectomy and bilateral salpingo‐oophorectomy is often performed (Kim 2006), and some sources suggest that primary treatment has the biggest influence on relapse and survival rates (Evans 1980; Haba 1993). This reinforces the argument for initial comprehensive surgical exploration and staging (Abu‐Rustum 2006). Staging of sex cord stromal tumours is generally similar to that of epithelial ovarian tumours, as defined by FIGO. This involves a vertical midline laparotomy incision that allows adequate exploration and biopsy of the upper abdomen and its contents (omentum, underside of diaphragm, paracolic gutters and bowel serosa). It may include a partial omentectomy, evaluation of para‐aortic and pelvic lymph nodes, peritoneal biopsies and peritoneal washings for cytology (Schumer 2003). However, the prognostic significance of positive cytology or surface involvement in GCTs has not been well defined, unlike in epithelial tumours (Schumer 2003). Few recent studies have addressed the value of lymphadenectomy in GCTs, and the rate of lymph node metastasis in sex cord stromal tumours is extremely low so can be safely omitted (Park 2012; Thrall 2011). Controversy has arisen regarding the management of younger women who desire to retain fertility and have disease apparently confined to one ovary (stage Ia) (Schumer 2003)—this describes more than 90% of such cases (Colombo 2007). If fertility‐sparing surgery is performed, an endometrial biopsy is recommended to rule out concomitant endometrial cancer (Schumer 2003).
(B) Non‐surgical treatment
(i) A variety of opinions have been expressed on chemotherapy as adjuvant treatment for newly diagnosed disease and/or as primary treatment for recurrent disease (Brown 2005). Various chemotherapy regimens have been explored, with reported response rates varying widely from 20% to 100% (Al‐Badawi 2002; Brown 2004; Brown 2005; Gershenson 2004; Homesley 1999; Savage 1998; Schumer 2003). Regimens used for GCTs are mainly platinum‐based, with combinations of bleomycin, cisplatin, etoposide, vinblastine, actinomycin‐D, 5‐fluorouracil, doxorubicin and cyclophosphamide (Chiara 1993; Colombo 1986; Colombo 1999; Gershenson 1996; Homesley 1999; Pautier 2008; Pecorelli 1999; Savage 1998; Zambetti 1990). Others suggest the use of carboplatin and taxanes such as paclitaxel as a single agent or in combination with platinum (Brown 2004; Brown 2005; Jacobs 1982; Tresukosol 1995). Optimal surgical staging (with omission of lymphadenectomy) followed by six cycles of a BEP (bleomycin, etoposide and cisplatin) chemotherapeutic regimen was found to have a significant effect on disease‐free survival (DFS) in a large, retrospective study (Park 2012).
(ii) Radiotherapy has been proposed for recurrent disease, for residual disease or for palliative care (Choan 2006; Disaia 1978; Engle 1958; Gershenson 1987; Hauspy 2011; Lee 1999; Malmstrom 1994; Ohel 1983; Savage 1998; Wolf 1999). However, response rates have varied substantially, mainly because of the absence of sectional imaging, especially in older studies (Choan 2006).
(iii) Hormonal therapies such as gonadotrophin‐releasing hormone (GnRH) agonists (GnRHa) (Fishman 1996; Homesley 1999; Kauppila 1992; Liu 2001; Lovell 2002; Martikainen 1989; Maxwell 1994), GnRH antagonists (Ameryckx 2005), progestogens and anti‐oestrogens, such as megestrol (Briasoulis 1997; Gershenson 1987) and aromatase inhibitors, which inhibit the conversion of androstenedione to oestrone, have also been suggested as primary or adjuvant treatment (Freeman 2006; Hardy 2005; Korach 2009; Rao 2005; Schumer 2003).
Each of the above treatment modalities may have adverse effects and toxicities, which range from minor perioperative morbidity and mild haematological and neurological symptoms to major postoperative morbidity, severe post chemotherapy symptoms and radiotherapy reactions. In severe cases, adverse effects can be fatal.
Tumour markers
Inhibin and anti‐müllerian hormones are produced by granulosa cells and have been used as tumour markers for initial diagnosis, for assessment of response to treatment and for follow‐up. Their effectiveness as tumour markers for guidance on adjuvant therapy, follow‐up and recurrence is unclear, as evidence comes from studies at high risk of bias (Boggess 1997; Geerts I 2009; Mom 2007). Targeted therapies are an emerging treatment modality for ovarian cancer, and identification of the FOXL‐2 mutation (D'Angelo 2011; Jamieson 2010) in the pathogenesis of GCT of the ovary is now under evaluation. Consequently, recruitment of participants is now under way for a trial investigating the effects of ketoconazole on adult GCT of the ovary (NCT01584297).
Long‐term follow‐up has recently emerged as an independent predictor of relapse, prompting recommendations for lifelong follow‐up, even in early‐stage GCT of the ovary (Mangili 2013).
Why it is important to do this review
Optimum management for granulosa cell tumour (GCT) of the ovary varies, and relatively little evidence has been reported by small trials and cohort studies. To our knowledge, no systematic reviews have examined the management of adult GCT of the ovary. Treatment is usually individualised because of the rarity of the disease. Although staging of GCT of the ovary follows that of epithelial ovarian cancer, its pathology, recurrence rate and management differ and remain challenging for the oncologist.
Furthermore, no standard follow‐up has been determined for the management of adult GCTs of the ovary. This systematic review was conducted to establish and summarise existing evidence and to highlight gaps in the literature.
Objectives
To evaluate the effectiveness and safety of different treatment modalities offered in current practice for the management of primary, residual and recurrent adult‐onset granulosa cell tumours (GCTs) of the ovary.
Methods
Criteria for considering studies for this review
Types of studies
For completeness, we searched for relevant randomised controlled trials (RCTs) and quasi‐RCTs, but as we did not expect to find any, the following types of non‐randomised studies (NRSs) with concurrent comparison groups were also included.
Non‐randomised trials, prospective and retrospective cohort studies and case series of 10 or more patients.
Case‐control studies and case series of fewer than 10 patients were excluded.
To minimise the effects of selection bias in non‐randomised studies (systematic differences between baseline characteristics of the groups compared), we included only studies that applied statistical adjustments for baseline characteristics using multivariate analyses (e.g. adjusting for age, stage, performance status, grade).
Types of participants
Adult women (when authors of studies mentioned that participants were 'adults' or had 'adult‐onset GCTs' or mentioned age 16 years and over) diagnosed with GCT of any stage (as defined by the authors who designed the reporting trial).
Primary (pretreatment).
Residual (as reported by study authors, e.g. more than 2 cm residual disease following primary treatment).
Recurrent (as reported by study authors, e.g. at least six months after disease‐free interval).
Studies that included women with co‐existing tumours were excluded from the review, unless investigators reported separately the effects of treatment for women without co‐existing tumours. We understand that co‐existing tumours, such as endometrial and other ovarian pathology, may have been present that may be considered a result of the oestrogen‐producing GCT, but we believe that including such cases would introduce bias in interpretation of outcomes.
The three categories above were analysed separately. If tumour‐node‐metastasis (TNM) staging was used, this was converted to FIGO staging. The staging methods used, as well as reasons for incomplete staging, were specified, either from the study report or by contacting the study authors. However, in cases where this information was difficult or not possible to obtain, this fact was clearly stated and data analysed appropriately when more than one study was included in this comparison group. In such circumstances, the study was excluded if inclusion was deemed inappropriate by consensus of the review authors.
If the exact FIGO staging was difficult to identify but early, locally advanced and advanced outside the pelvis stages were provided, this was also considered.
Types of interventions
Any surgical intervention in the treatment of GCTs versus any other surgical intervention (e.g. total pelvic clearance versus fertility‐sparing surgery, laparoscopic surgery versus laparotomy).
Any surgical intervention in the treatment of GCTs versus any other non‐surgical intervention (e.g. placebo, no treatment, chemotherapy, radiotherapy, hormonal treatment, alternative therapy such as mistletoe or acupuncture) given singly or in combination.
Any non‐surgical intervention versus any other non‐surgical intervention, given singly or in combination.
Adjuvant intervention (chemotherapy/radiotherapy/hormonal treatment) given singly or in combination versus primary intervention alone, provided the study protocol specified that treatment and control groups received the same treatment, except for the adjuvant intervention being evaluated.
Any of the above comparisons were considered for each primary tumour, residual tumour and recurrent tumour, if data were available.
Types of outcome measures
Primary outcomes
Death from all causes.
Recurrence (tumour identification after presumed complete treatment of primary)—localised (pelvis) or distant (extrapelvic) recurrence. It was anticipated that these data would be reported in several ways, including recurrence, time to progression, progression‐free interval and disease‐free survival (DFS).
Secondary outcomes
Response to treatment (Miller 1981).
Quality of life (QoL), as measured by a validated scale.
-
Adverse events (classified according to CTCAE 2010 and Cornis 2007):
direct surgical morbidity (injury (bladder, ureter, vascular, small bowel and colon), presence and complications of adhesions, febrile morbidity, intestinal obstruction, haematoma, local infection, excessive operative time),
surgically related systemic morbidity (chest infection, thromboembolic events (deep vein thrombosis and pulmonary embolism), cardiac events (cardiac ischaemia and cardiac failure), cerebrovascular accident,
delayed recovery: prolonged hospital admission, unscheduled admission,
chemotherapy toxicity,
radiotherapy toxicity,
other adverse effects not categorised above (e.g. effects of hormonal treatment, psychosocial, sexual).
Grades of toxicity were extracted and grouped as follows.
Haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage).
Gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver disease, proctitis).
Genitourinary.
Skin (stomatitis, mucositis, alopecia, allergy).
Neurological (peripheral and central disorders).
Pulmonary
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
See Cochrane Gynaecological Cancer Group methods used in reviews.
The following electronic databases were searched:
Cochrane Gynaecological Cancer Collaborative Review Group Trials Register.
Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 11, part of The Cochrane Library.
MEDLINE: 1948 to 2013 November week 3.
EMBASE: 1980 to 2013 week 50.
CENTRAL, MEDLINE and EMBASE search strategies based on terms related to the review topic are presented in Appendix 1, Appendix 2 and Appendix 3, respectively.
All relevant articles found were identified on PubMed, and the 'related articles' feature was used to carry out a further search for newly published articles.
Searching other resources
Unpublished and grey literature
A Google search was conducted to look for Internet‐based resources and open‐access publications. Metaregister (http://www.controlled‐trials.com/rct), Physicians Data Query (http://www.nci.nih.gov), http://www.clinicaltrials.gov and http://www.cancer.gov/clinicaltrials were searched for ongoing trials.
Conference proceedings and abstracts were searched through ZETOC (http://zetoc.mimas.ac.uk) and WorldCat Dissertations.
Handsearching
The citation lists of included studies, key textbooks and previous systematic reviews were handsearched.
Reports of conferences were handsearched in the following sources:
International Gynecological Cancer Society (IGCS).
European Sociey of Gynaecological Oncology (ESGO).
Society of Gynecologic Oncologists (SGO).
British Gynaecological Cancer Society (BGCS).
Australian Society of Gynaecologic Oncologists (ASGO).
American Society of Clinical Oncology (ASCO).
European Society of Medical Oncology (ESMO).
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were removed and remaining references were examined by two review authors (MG, SS). Those studies that clearly did not meet the inclusion criteria were excluded, and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers was assessed independently by two review authors (MG, SS). Reasons for exclusion were documented. Disagreements were resolved by consensus or through arbitration (AB). Review authors were not blinded to authors of articles or to journals.
Data extraction and management
Two review authors (MG, SS) independently abstracted data from each included study using a specially designed data collection form, with differences resolved by discussion or by a third review author when necessary (AB). Authors of relevant articles were contacted to obtain missing data. This information is presented in the Characteristics of included studies and helped to provide a context for interpreting study results.
For included studies, data were abstracted as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data on the following were recorded.
Trial characteristics and setting
We recorded the type of study design and noted the country (or countries) in which the trial was performed and whether it was a single‐centre or multicentre trial. The median (or mean) duration of follow‐up was recorded. We also recorded author name, year of publication, source of study funding and journal citation (including language), as well as assessment of risk of bias in the study (see later).
Characteristics of study participants
Age.
Parity.
Tumour size.
Histological grade (mitotic index, lymph vascular space invasion).
FIGO stage (surgical or radiological).
Performance status.
Previous therapies.
Exclusion criteria.
Characteristics of interventions
Mode of intervention: surgery, chemotherapy, radiotherapy, hormonal treatment, no intervention.
Type: primary or adjuvant.
-
Co‐interventions.
Other than the intervention under study, protocols were equivalent in treatment and control groups.
Issue of co‐intervention was not considered.
Co‐intervention definitely exists.
Other characteristics: optimal debulking (defined as no residual nodule 2 cm or smaller) at surgery, drug used, dosage, administration route/frequency/planned number of cycles).
Outcomes
-
Details of outcomes are reported (see above), including method of assessment and time intervals (see below).
For each outcome: outcome definition (with diagnostic criteria, if relevant).
Unit of measurement (if relevant).
For scales: upper and lower limits, and whether high or low score is good.
Results: number of participants allocated to each intervention group.
For each outcome of interest: sample size and missing participants.
Time points at which outcomes were collected and reported.
Abstraction of outcome data from each study
For time‐to‐event data (overall survival (OS) and DFS), we abstracted the log of the hazard ratio (HR) and its variance from trial reports. If these were not presented, we attempted to abstract the data required to estimate them using Parmar's methods (Parmar 1998) (e.g. number of events in each arm and log‐rank P value comparing relevant outcomes in each arm, relevant data from Kaplan‐Meir survival curves). If it was not possible to estimate the log HR, we attempted to abstract the number of participants in each treatment arm who experienced the outcome of interest at 3, 5 and 10 years or the longest period stated in the trial and the number of participants assessed, in order to estimate a risk ratio (RR).
When possible, all data abstracted were those relevant to an intention‐to‐treat (ITT) analysis.
We contacted the authors of the primary research to verify the data and to obtain further data when the report was incomplete. When this was not possible, we stated so in the text of the review.
Assessment of risk of bias in included studies
The risk of bias in included RCTs was assessed in accordance with the guidelines provided in The Cochrane Collaboration tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included assessment of the following.
Sequence generation.
Allocation concealment.
Blinding (of participants, healthcare providers and outcome assessors).
-
Incomplete outcome data.
-
We recorded the proportions of participants whose outcomes were not reported at the end of the study. We coded the satisfactory level of loss to follow‐up for each outcome as:
low risk of bias, if fewer than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
high risk of bias, if more than 20% of participants were lost to follow‐up or reasons for loss to follow‐up were different between treatment arms; and
unclear risk of bias, if loss to follow‐up was not reported.
-
Selective reporting of outcomes.
Other possible sources of bias.
We applied (MG, AB) the risk of bias tool independently, and differences were resolved by discussion. Results were summarised in both a risk of bias graph and a risk of bias summary (Figure 1; Figure 2). Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias.
1.
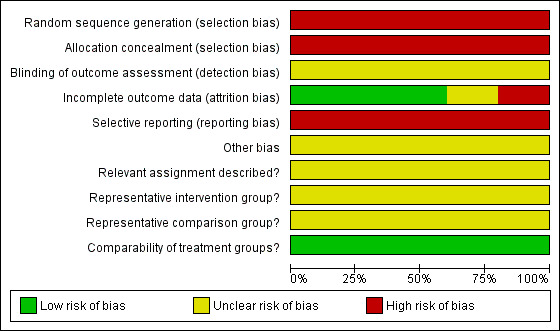
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
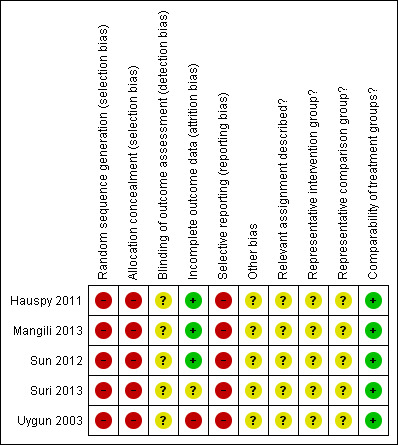
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Risk of bias in non‐randomised controlled trials was assessed in accordance with four additional criteria and largely followed the Newcastle‐Ottawa Scale and a subsequent publication, which was based in part on this scheme (Taggart 2001). We removed the use of stars, which were awarded for each criterion, as we did not want to assess risk of bias in included studies based on a scoring system.
Cohort selection.
-
Were relevant details provided regarding criteria for assignment of participants to treatments?
Yes.
No.
Unclear.
-
Was the group of women who received the experimental intervention representative?
Yes, if representative of women with GCT of the ovary.
No, if the group of participants was selected.
Unclear, if selection of the group was not described.
-
Was the group of women who received the comparison intervention representative?
Yes, if drawn from the same population as the intervention group.
No, if drawn from a different source.
Unclear, if selection of the group was not described.
Comparability of treatment groups.
-
Were no differences noted between the two groups, or were differences controlled for, in particular with reference to age, stage, grade, primary or recurrent disease and performance status?
Yes, if at least three of these characteristics were reported and any reported differences were controlled for.
No, if the two groups differed and differences were not controlled for.
Unclear, if fewer than three of these characteristics were reported, even if no other differences were noted between groups and other characteristics had been controlled for.
Measures of treatment effect
We planned to use the following measures of the effects of treatment.
For time‐to‐event data, we used the HR, if possible.
Dealing with missing data
We did not impute missing outcome data for any of the outcomes.
Data synthesis
We identified five included studies, but it was not possible to perform meta‐analyses because although all were at very high risk of bias, heterogeneity was evident in terms of treatment comparisons, outcomes and consistency in the reporting of outcomes. Therefore it was not relevant to assess heterogeneity between results of studies, and we were unable to assess reporting biases using funnel plots or to conduct subgroup analyses or sensitivity analyses.
Results
Description of studies
Results of the search
The search strategy identified 1334 unique references. Two review authors independently read the abstracts and articles; those that obviously did not meet the inclusion criteria were excluded at this stage. Twenty‐four articles were retrieved in full and translated into English when appropriate; updated versions of relevant studies were identified. A further two references were available in abstract form only and reported on the same study at two different conferences. The full‐text screening of these 26 references resulted in exclusion of 20 of them for the reasons described in the table Characteristics of excluded studies. However, six references, which reported on five studies (Hauspy 2011; Mangili 2013; Sun 2012; Suri 2013; Uygun 2003), were identified as having met our inclusion criteria and are described in Characteristics of included studies (see PRISMA flow chart for further details of the study selection process; Figure 3).
3.
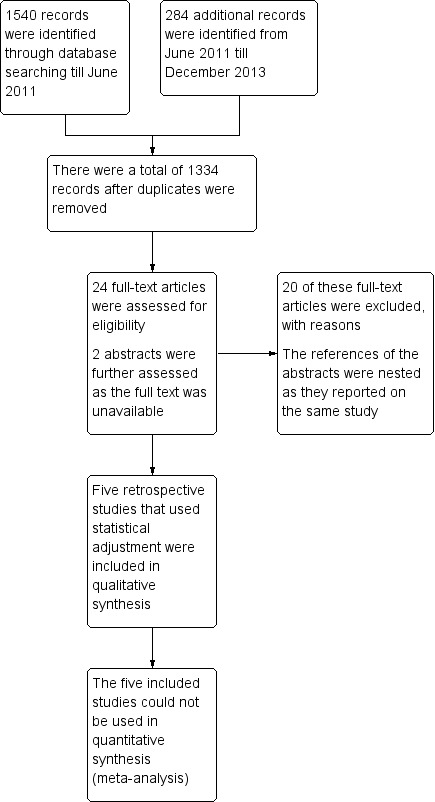
PRISMA flow diagram.
Searches of the grey literature did not identify any additional studies.
Included studies
Study design
The five included retrospective cohort studies (Hauspy 2011; Mangili 2013; Sun 2012; Suri 2013; Uygun 2003) provided data on 535 women with a diagnosis of GCT; diagnosis appeared to be pathologically confirmed in 522 of these women, and analyses focused on 362 women. It is unclear from the Suri 2013 abstract whether analyses included data on the 160 women who did not receive adjuvant chemotherapy, as the abstract reports only the results of the 41 women who received different forms of adjuvant chemotherapy.
Two studies were single‐centre studies (Hauspy 2011; Uygun 2003), and three were multicentre studies (Mangili 2013; Sun 2012; Suri 2013).
The single‐centre Hauspy 2011 study looked into the management of 103 women with GCT of the ovary from 1961 to 2006. All 103 women had been treated surgically. In this study women who received adjuvant radiotherapy (31/103) were compared with those who did not. The single‐centre Uygun 2003 study assessed 45 women between 1979 and 1998 who underwent unilateral salpingo‐oophorectomy (4/45) and compared them with those who underwent bilateral salpingo‐oophorectomy (41/45). This study also compared women who underwent chemotherapy (30/45) as adjuvant treatment and those who received no adjuvant chemotherapy because they had no macroscopic residual disease. In addition, women who received external beam radiotherapy (11/45) as adjuvant treatment were compared with those who did not.
The multicentre studies of Mangili 2013, Sun 2012 and Suri 2013 examined 97, 176 and 201 (41 of which received adjuvant chemotherapy) women with GCT of the ovary from 1965 to 2008, from 1984 to 2010 and from 1995 to 2010, respectively.
The Italian multicentre MITO‐9 retrospective study (Mangili 2013) included women treated at or referred after primary treatment to MITO centres; all 97 women had undergone some form of surgery, and effects of surgical approach, lymphadenectomy and adjuvant treatment were compared in the form of Cox models. Participants were excluded if they had a concomitant diagnosis of another malignancy that was not a GCT or an endometrial carcinoma.
Sun 2012 compared women who underwent fertility‐sparing surgery (98/176) versus conventional staging surgery (78/176). Women who received chemotherapy as an adjuvant treatment (28/176) were compared with those who did not.
Suri 2013 was available in abstract form only and set out to determine the effects of obesity and the efficacy of two adjuvant chemotherapy regimens in terms of progression‐free survival (PFS) with ovarianGCTs.
Participant characteristics
None of the five studies reported on parity, histological grade or performance status or provided details of whether women had received previous therapy. Median tumour size was not reported in three studies (Mangili 2013; Suri 2013; Uygun 2003).
Median age in the Hauspy 2011 study was 47 years, and median tumour size was 10 cm (range 1 to 28 cm). Around three‐quarters of the women had stage I disease. Median follow‐up was 73 months, and by the end of the study, 38% of participants had developed recurrent disease and 12 women had died (10 of these women died of GCT).
Mean and median age in the Mangili 2013 study was 52 and 51 years, respectively (range 27 to 82). Seventy (72%) women had stage I GCT of the ovary, and a further 11/97 (11%) had 'apparent' stage I (Ix) disease. Median follow‐up was 88 months (range six to 498). At the time of last follow‐up, five participants (4.5%) had died of disease, six (5.5%) were alive with evidence of disease, 80 (75%) were alive with no evidence of disease and 14 (13%) were lost to follow‐up; two deaths (1.9%) were considered related to other causes.
Women in the Sun 2012 study had a median age of 46 years and a mean tumour size of 10.4 cm (range 0.2 to 40). Most of the women in this study (77%) had stage I disease. Median follow‐up was 60.7 months, and a recurrence rate similar to that of Uygun 2003 was reported (21%). In the Sun 2012 study, 137/176 (78%) women had stage I disease; nine of 176 (5%) had stage II, 10/176 (6%) had stage III and one of 176 (0.5%) had stage IV disease. In 19/176 (11%) women, disease stage was not known.
Median age in Suri 2013 was 47 years (range 37 to 58). Most women had stage I disease (86%). No differences in body mass index (BMI), age or race were noted between groups. Median follow‐up time was 41 months (range 0.2 to 350). A total of 17 of 41 participants (41%) with disease recurrence had received a chemotherapy regimen.
Median age in Uygun 2003 was 46 years, and 52% of women had stage I to II disease. Median follow‐up was 84 months, and the recurrence rate was 21%. Mean survival with early and late‐stage disease was 122 and 34 months, respectively.
Outcomes
Review authors used multivariate analyses to attempt to determine which factors were associated with better outcomes in terms of overall and disease‐free survival or both.
The authors in the Hauspy 2011 study reported DFS in univariate and multivariate analyses but reported OS rates only at 5, 10 and 15 years of follow‐up. The HR in the multivariate model for disease recurrence was adjusted for postoperative radiotherapy, tumour size, stage, unilateral or bilateral salpingo‐oophorectomy, presence of endometrial cancer and rupture. Median DFS was also reported. Adverse events and other important secondary outcomes were not reported in any of the studies, other than toxicity, which was reported in Uygun 2003.
Univariate and multivariate Cox regression analyses were performed in the Mangili 2013 study to identify independent predictors of recurrence and survival. To define prognostic parameters for relapse and survival, variables regarding participant characteristics were dichotomised in the following manner: age younger than 50 or older than 50 years of age; juvenile GCT subtype versus adult GCT histology; tumour size smaller than 10 cm versus larger than 10 cm; primary surgery at MITO centres or elsewhere; conservative versus radical surgery; laparoscopic versus laparotomic approach; complete staging versus incomplete staging; residual disease at primary surgery versus no residual disease; execution of lymphadenectomy versus no lymph node dissection; stage I of disease versus advanced stage of disease and adjuvant treatment versus no postsurgical treatment. Variables with P value less than 0.05 on univariate analysis were selected for multivariate analysis.
The Suri 2013 study analysed PFS in women with GCTs who received different chemotherapy regimens. Hazard ratios for recurrence were estimated by univariate and multivariate Cox regression models. In univariate analysis, BMI of 30 or greater was associated with worse PFS (HR 1.74, 95% confidence interval (CI) 1.03 to 2.92). The exact variables used in the Cox models for PFS were not explicitly reported, but it is likely that variables on demographics including age, race and BMI and clinical data including stage and adjuvant treatment were assessed.
Uygun 2003 reported OS in both a univariate model and a multivariate model that was adjusted for age, menopausal status, parity, chemotherapy and radiotherapy. Overall response rate in the chemotherapy arm was reported, along with mild toxicity and treatment‐related deaths (none). The numbers of deaths have been documented by cause.
The Sun 2012 study reported overall 5‐ and 10‐year survival rates. Recurrence rates were also reported in univariate and multivariate analyses. The multivariate Cox model for disease recurrence was adjusted for age, initial stage, presence of residual tumour after initial surgery, need for adjuvant chemotherapy, tumour size and type of surgery.
Ongoing studies
Ketoconazole as Inhibitor of the Enzyme CYP17 in Locally Advanced or Disseminated Granulosa Cell Tumour of Ovary (not yet recruiting) (NCT01584297).
Phase II Study of Paclitaxel in Patients With Ovarian Stromal Cancer (still recruiting) (NCT00006227).
Phase II Randomized Study of Paclitaxel and Carboplatin Versus Bleomycin Sulfate, Etoposide Phosphate, and Cisplatin in Patients With Advanced or Recurrent Sex Cord‐Stromal Tumors of the Ovary (NCT01042522).
Excluded studies
Of the 26 references retrieved in full text, 20 were excluded for the following reasons.
Six studies included participants younger than 16 years of age with juvenile‐onset GCT of the ovary. In some cases, the quality of the studies was good and comparisons between treatments were made; these are discussed in Agreements and disagreements with other studies or reviews (Colombo 1986; Engle 1958; Evans 1980; Kietlinska 1993; Savage 1998; Sehouli 2004).
Seven studies did not apply statistical adjustments for baseline characteristics using multivariate analysis because the sample size was too small or for various other reasons not specified (Al‐Badawi 2002; Alberti 1984; Baumann 1992; Pankratz 1978; Pecorelli 1999; Pectasides 1992; Wolf 1999).
Three studies reported interventions alone with no comparison group (Fotopoulou 2010; Nosov 2009; Pautier 2008).
Three studies analysed fewer than 10 women (Chiara 1993; Tao 2009; Zambetti 1990).
One study examined the management of sex cord stromal tumours, and separate analysis for granulosa cell ovarian tumours was not carried out (Homesley 1999).
For further details of all excluded studies, see Characteristics of excluded studies.
Risk of bias in included studies
The five included studies (Hauspy 2011; Mangili 2013; Sun 2012; Suri 2013; Uygun 2003) were at very high risk of bias as they satisfied, at most, only 2 of the 10 criteria used to assess risk of bias (see Figure 1; Figure 2).
All were retrospective analyses, so the methods of sequence generation and concealment of allocation (relevant only to RCTs) were deemed to be unsatisfactory. None of the five studies reported details of assignment of participants to groups. Thus it was unclear whether the two intervention groups in each study were representative of women with GCT of the ovary. None of the studies reported whether outcome assessors were blinded. It was unclear whether additional bias may have been present in any of the studies, but it did seem that outcomes may have been selectively reported, as none of the studies had adequate and complete reporting of overall survival data using appropriate methods, or studies did not report the HR, although DFS was by and large well reported. A multivariate analysis was performed in all five studies, with adjustment for important prognostic factors, so the two groups in each study were deemed to be comparable. At least 90% of participants were assessed at the endpoint in three studies (Hauspy 2011; Mangili 2013; Sun 2012), but this item was scored as having high risk of bias in the Uygun 2003 study, as less than 80% of participants were assessed at endpoint, and as having unclear (so potentially high) risk of bias in Suri 2013, as information was insufficient to permit judgement based on the abstract.
Effects of interventions
Overall survival
Hauspy 2011 reported a median follow‐up of 73 months. Five‐, 10‐, and 15‐year OS rates were 93%, 91% and 87%, respectively.
Mangili 2013 reported a median follow‐up of 88 months. The authors reported disease‐specific OS at 5 and 10 years of 97% and 95%, respectively. Only older age at diagnosis (over 50 years), advanced stage of disease and residual tumour at completion of surgery were associated with a poor prognosis in univariate analyses, and multivariate analysis showed age and stage to be independent indicators of poor prognosis for survival. Surgical approach (laparoscopy vs laparotomy) and whether a participant underwent lymphadenectomy or received adjuvant treatment (mostly chemotherapy) did not appear to impact survival according to univariate analyses and were not considered in the multivariate model.
Uygun 2003 reported a median follow‐up of 84 (six to 141) months. According to univariate analysis, only two factors were significant for OS: stage at initial diagnosis and presence of residual disease following surgery. Age, menopausal status, parity, chemotherapy and radiotherapy did not influence OS. In a multivariate analysis, only stage at diagnosis remained significant (P value < 0.01). The overall 5‐year survival rate was 55%, and median survival after recurrence was 21 months. No treatment‐related deaths were reported.
Sun 2012 reported a median follow‐up period of 61 months. Overall 5‐ and 10‐year disease‐specific survival rates were 96.5% and 94%, respectively.
Overall survival was not reported in the Suri 2013 study.
Recurrence and disease‐free survival
Unilateral versus bilateral salpingo‐oophorectomy
In the Hauspy 2011 study, the authors reported no apparent evidence of a difference in the risk of recurrence or death between women who underwent unilateral salpingo‐oophorectomy and those who had bilateral salpingo‐oophorectomy (adjusted HR 1.7, 95% CI 0.8 to 3.6, P value 0.14).
Conservative surgery or staging surgery (and type of adjuvant chemotherapy)
Sun 2012 reported a median follow‐up period of 61 months. The recurrence rate was 21%. Following univariate Cox regression modelling, recurrence was associated with advanced‐stage (P value 0.02) residual tumour following surgery (P value < 0.01) and adjuvant chemotherapy (P value 0.02). After multivariate analysis, only residual tumour following surgery and tumour size (13.5 cm) were associated with recurrence. Age at diagnosis, menopausal status, BMI, gravidity and parity, surgical extent (fertility‐sparing or non‐conservative staging surgery) and type of adjuvant chemotherapy were not associated with disease recurrence (P value > 0.05).
Mangili 2013 found that surgical treatment outside the MITO centre and incomplete surgical staging (HR 1.23, 95% CI 1.02 to 2.28) were markers for recurrence in both univariate and multivariate analyses. However, surgical approach (laparoscopy versus laparotomy), type of surgery (conservative versus radical), execution of adjuvant chemotherapy and performance of lymphadenectomy were not associated with recurrence.
Suri 2013 reported a median follow‐up period of 41 months. Forty‐one (20%) participants received adjuvant chemotherapy; of these, only one of 14 (7%) participants who received paclitaxel/carboplatin (PC) had recurrent disease compared with 10 of 16 (63%) participants who received BEP and 6 of 11 (55%) who received other chemotherapy regimens (HR 4.5, 95% CI 0.55 to 36.99, P value 0.16), but no apparent evidence of a difference was reported.
Adjuvant radiotherapy versus salpingo‐oophorectomy alone
In the Hauspy 2011 study, women who received radiotherapy after salpingo‐oophorectomy (31/103) were compared with women who underwent salpingo‐oophorectomy alone. Women who received postoperative radiotherapy had a lower risk of recurrence or death compared with women who underwent salpingo‐oophorectomy alone (HR 0.3, 95% CI 0.1 to 0.6, P value 0.04). Median DFS was 125 months overall and 251 months in women who received radiotherapy.
Toxicity
No cases of moderate or severe toxicity were reported in the Uygun 2003 study, which included women who received chemotherapy and radiotherapy.
In the Suri 2013 study, women treated with PC had fewer recurrences than those treated with BEP, and the study authors claim that PC has a more favourable therapeutic index (measure of relative safety of the drug) than BEP.
Discussion
Summary of main results
Five retrospective cohort studies (including 535 women with a diagnosis of GCT) met our inclusion criteria, but all studies were at very high risk of bias.
In two studies (Mangili 2013; Uygun 2003), which carried out multivariate analyses that were undertaken to identify the factors associated with better outcomes (in terms of overall survival), no evidence suggested a difference in overall survival associated with surgical approaches and whether a participant underwent lymphadenectomy or received adjuvant chemotherapy or radiotherapy. Only percentage survival for all participants combined was reported in two trials (Hauspy 2011; Sun 2012) and was not reported at all in one study (Suri 2013).
One study (Hauspy 2011) showed that women who received postoperative radiotherapy had lower risk of disease recurrence compared with those who were treated with surgery alone (adjusted HR 0.3, 95% CI 0.1 to 0.6, P value 0.04). In three studies (Mangili 2013; Sun 2012; Suri 2013), no apparent evidence showed differences in disease recurrence associated with execution and type of adjuvant chemotherapy or with type of surgery or surgical approach, other than that surgical staging may be important. In one study (Hauspy 2011), no apparent evidence revealed a difference in disease recurrence between fertility‐sparing surgery and surgery performed to remove both tubes and ovaries. Recurrence‐free survival was not reported in the Uygun 2003 study.
Specific adverse effects of surgery and toxicity due to chemotherapy or radiotherapy were not adequately documented in any of the five studies, but mild toxicity due to chemotherapy was reported in Uygun 2003. In the Suri 2013 study, PC was reported to have a more favourable therapeutic index (measure of relative safety of the drug) compared with BEP. Safety and toxicity data of chemotherapeutic regimes are vital in allowing a full assessment of interventions, especially in the Uygun 2003 and Suri 2013 studies, as all of the women who received chemotherapy were given a combination of cisplatin, cyclophosphamide and doxorubicin—PC or BEP.
Quality of life (QoL) data were not documented in any of the five studies. Data on QoL and on complications following surgery would have provided valuable information.
Overall completeness and applicability of evidence
Although the five included studies met our inclusion criteria, the effectiveness and safety of different types and approaches of surgery (including fertility‐sparing surgery; conservative, radical and staging surgery and laparoscopic or laparotomic approach), as well as of chemotherapy and radiotherapy, for the management of adult‐onset GCT of the ovary need further evaluation, and no real inferences can be made at this time. Clinical practice is unlikely to change on the basis of these five studies. Although one study assessed the effects of postoperative radiotherapy and showed lower risk of recurrence in women who received radiotherapy, this study was at very high risk of bias.
We were unable to fully address our objectives in this review; types of interventions and outcomes (primary and secondary) specified in the review were incompletely reported. No studies primarily compared fertility‐sparing surgery versus non‐conservative staging surgery or toxicity data versus different chemotherapeutic regimens. None of the studies provided any documentation with regard to the nature of follow‐up or any guidance on tumour markers.
Overall, the evidence is of low quality (GRADE Working Group 2004), as we are very uncertain about each of the survival estimates for all of the comparisons examined, and these biases and uncertainties in the review may seriously weaken confidence in the results. Further research is very likely to have an important impact on our confidence in the estimate of effect and may further inform our findings. The effectiveness and safety of different treatment modalities for the management of adult‐onset granulosa cell tumours of the ovary have not been assessed in RCTs or even in good quality non‐randomised studies. Although all five included studies used statistical adjustment, which may minimise the effects of selection bias, the fact that all studies were at very high risk of bias based on a thorough assessment of quality items makes conclusions based on this potentially unreliable evidence uncertain. If the GRADE approach is used (GRADE Working Group 2004), any sort of recommendations for the use of any treatment modality in this setting on the basis of available observational studies would be very unlikely because the benefits and potential harms of these treatments are unclear and point estimates were not reported in many cases, so consistency of point estimates and accuracy of these estimates (examination of 95% CIs) could not be assessed (only P values of statistically significant results were reported from Cox models for survival in Mangili 2013, Uygun 2003 and Sun 2012 studies in most cases). It is unclear what effect the design of these studies and the selection of co‐variates used in statistical adjustment had on the validity of the findings. However, these adjustments did appear to be sensible, so overall we deemed the evidence to be of low quality rather than of very low quality, despite the other limitations outlined above. In some patients, radiotherapy could be considered as a possible treatment option independent of the quality assessment; it has been suggested that for some women, radiotherapy following salpingo‐oophorectomy may delay time to disease recurrence, but the associated toxicity has not been reported.
Quality of the evidence
All five studies were retrospective in nature, at very high risk of bias and likely to be underpowered, given the modest numbers in each study and the multiple adjustments used. HRs should be used for survival outcomes, and an HR was reported explicitly only for DFS in the Hauspy 2011 study and for the comparison of PC and BEP in Suri 2013. Currently, the quality of the evidence is low; therefore further evidence from good quality prospective studies is needed.
The objective of this review was to evaluate the effectiveness and safety of different treatment modalities for the management of primary and recurrent granulosa cell tumours of the ovary. We were unable to address these specific objectives adequately, as the quality of the evidence was low (GRADE Working Group 2004), all five included studies were at very high risk of bias and survival outcomes were poorly and incompletely reported (see Overall completeness and applicability of evidence). Other important outcomes such as QoL, adverse events, toxicity and details of tumour markers were not reported at all or were reported to an inadequate level. We were unable to deduce valid conclusions on types of surgery and on adjuvant treatment options.
The five studies met all of our inclusion criteria, but the results should be interpreted with caution; the findings of this review are unlikely to change clinical practice, although further research is very likely to have an important impact, so this outcome could change if future trials are included in a subsequent update. Each of the included studies has multiple limitations, with poor and incomplete reporting of outcomes a matter of particular concern. RCTs are challenging in this area, but we did identify one relevant comparative ongoing trial (NCT01042522) that compared the efficacy of carboplatin and paclitaxel with that of BEP, so they are feasible (see Agreements and disagreements with other studies or reviews). Ideally, multicentre RCTs with long‐term follow‐up comparing other treatment modalities are needed to define the role of treatment for ovarian GCTs.
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature, and all studies were sifted and data extracted independently by at least two review authors. We suspected that we would find no relevant RCTs. Therefore, we attempted to ensure that we did not overlook any relevant evidence by searching a wide range of non‐randomised studies of reasonable quality design.
The greatest threat to the validity of the review is likely to be publication bias: Studies that did not find benefits of different treatment modalities may not have been published. We were unable to assess this possibility, as we did not find an adequate number of studies that met the inclusion criteria.
Agreements and disagreements with other studies or reviews
Several relevant prospective phase 2 trials had good methodological quality, but unfortunately, these were one‐arm trials and did not include a comparison group. A prospective clinical trial (Pecorelli 1999) led by the European Organization of Research and Cancer Gynecological Cooperative Group/Gynecological Cancer Cooperative Group (EORTC/GCCG) group assessed cisplatin (P), vinblastine (V) and bleomycin (B) as a combination regimen in recurrent or advanced GCT of the ovary and confirmed therapeutic activity of the PVB regimen. This was a good quality trial, which focused on chemotherapeutic toxicity and response rate outcomes. A phase 3 trial to confirm these potentially promising findings would be welcomed. The phase 2 trial led by the Gynaecological Oncology Group (GOG) (Homesley 1999) examined the efficacy and toxicity of bleomycin (B), etoposide (E) and cisplatin (P) for ovarian stromal malignancies. Most of the women had GCT of the ovary, and this trial showed BEP to be an active regimen as a first‐line chemotherapeutic combination for ovarian sex cord stromal tumours. The results of this trial are still applied in clinical practice, with the BEP regimen commonly used as the primary adjuvant treatment for management of GCT of the ovary. The previously used vinblastine was replaced by etoposide because of toxicity, but a phase 3 RCT is sought to confirm these findings.
One ongoing study following the emerging theory of FOXL‐2 mutations in GCT of the ovary is examining the effect of ketoconazole, which acts as an inhibitor of the enzyme CYP17. However, this is a single‐arm phase 2 trial that will not be able to offer comparable data (NCT01584297).
Another phase 2 study led by the Gynaecological Oncology Group is a single‐arm study assessing the effect of paclitaxel in women with ovarian stromal cancers. This study includes not only women with GCTs but also women with all ovarian stromal cancers that have not been amenable to surgery or women who have had recurrent disease after a single chemotherapeutic treatment. This study may help in guiding clinicians regarding treatment for recurrent cases (NCT00006227), but again, unless a phase 3 trial takes precedence, a gap in the evidence will remain.
The study that may provide answers to the question of the best choice of chemotherapeutic regimen in sex cord stromal tumours is an ongoing randomised phase 2 study (NCT01042522) that is comparing the efficacy of carboplatin and paclitaxel with that of the standard BEP; it is being led by the GOG. Women who have undergone surgery with stages II to IVA recurrent disease and women with residual disease measuring greater than 1 cm following primary surgery are eligible. Moreover, this study is also looking into the value of inhibin A and inhibin B as predictive biomarkers.
Given the paucity of assessable data and the lack of RCTs, we recognise that available data are of limited value, and it is unlikely that clinical practice will change. However, we have highlighted gaps in the literature and have summarised existing evidence for the treatment and management of GCTs. We believe that in the absence of robust evidence, this review is important for facilitating decision making and outlining treatment options open to women. We recognise that GCTs are rare tumours; hence we have highlighted the need to conduct good quality studies and/or trials and have acknowledged the difficulties involved in doing so. These are rare cancers, and the published data reflect decades of “research,” mainly with small, phase 2, non‐randomised studies or case series from centres worldwide.
Authors' conclusions
Implications for practice.
After evaluating only five small retrospective studies, we are unable to reach valid conclusions as to the effectiveness and safety of different types and approaches of surgery such as fertility‐sparing surgery, as well as adjuvant chemotherapy or radiotherapy, for the management of GCTs of the ovary in clinical practice. One study showed improved disease‐free survival with the use of adjuvant radiotherapy, but because of high risk of bias, it is difficult to make meaningful clinical decisions based on these data. In the other studies that reported both overall and disease‐free survival, no apparent survival advantage was seen with adjuvant chemotherapy, radiotherapy or no further treatment after surgery. No clear recommendations about the type of surgery that should be offered can be made on the basis of the available data.
Implications for research.
The available evidence is very limited, and the quality of this evidence in this review is low. Further research is very likely to have an important impact on our confidence in the estimate of effect and may alter our findings. Ideally, multinational RCTs are needed to answer these questions. Although the disease is relatively rare and generally has a good prognosis, RCTs are challenging, but the fact that we identified three ongoing trials shows that they are feasible, albeit two of the trials are single‐arm trials. The study that may provide answers to the question of the best choice of chemotherapeutic regimen in sex cord stromal tumours is an ongoing randomised phase 2 study that is comparing the efficacy of carboplatin and paclitaxel with that of the standard BEP; it is being led by the GOG. Moreover, this study is also looking into the value of inhibin A and inhibin B as predictive biomarkers. Additional trials are required to assess toxicity and QoL with the use of different treatment regimens and to assess the safety of conservative surgical options.
What's new
| Date | Event | Description |
|---|---|---|
| 17 July 2018 | Amended | Next stage expected date amended. |
| 28 June 2018 | Review declared as stable | Low usage of review and currently not a priority to update. |
Acknowledgements
We thank Jo Morrison for clinical and editorial advice, Jane Hayes for designing the search strategy and Gail Quinn and Clare Jess for their contributions to the editorial process.
We thank Margaret Cruickshank and David Parkin for providing expert advice.
Additionally, we would like to thank Mary Cairns for adding input to the earlier published protocol.
We would also like to thank Heather Dickinson who provided expert methodological and statistical input during the protocol development and detailed peer review comments at the full review stage.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, the NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL: 2013, Issue 11
MeSH descriptor Granulosa Cell Tumor explode all trees
granulosa cell* and (tumor* or tumour* or malignan* or cancer* or carcinom* or neoplasm*)
(#1 OR #2)
MeSH descriptor Ovary explode all trees
ovar*
(#4 OR #5)
(#3 AND #6)
Appendix 2. MEDLINE search strategy
MEDLINE Ovid: 1948 to 2013 November week 3
exp Granulosa Cell Tumor/
(granulosa cell* and (tumor* or tumour* or malignan* or cancer* or carcinoma* or neoplas*)).mp.
1 or 2
exp Ovary/
ovar*.mp.
4 or 5
6 and 3
randomised controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
surgery.fs.
radiotherapy.fs.
therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
exp Cohort Studies/
cohort*.mp.
exp case‐control studies/
(case and control).mp.
or/8‐22
7 and 23
(animals not (humans and animals)).sh.
24 not 25
key: mp=title, original title, abstract, name of substance word, subject heading word pt=publication type ab=abstract fs=floating subheading sh=subject heading
Appendix 3. EMBASE search strategy
EMBASE Ovid: 1980 to 2013 week 50
exp granulosa cell tumor/
(granulosa cell* and (tumor* or tumour* or malignan* or cancer* or carcinoma* or neoplas*)).mp.
1 or 2
exp ovary/
ovar*.mp.
4 or 5
3 and 6
exp controlled clinical trial/
randomized.ab.
placebo.ab.
dt.fs.
su.fs.
rt.fs.
th.fs.
randomly.ab.
trial.ab.
groups.ab.
exp cohort analysis/
cohort*.mp.
exp case control study/
(case and control).mp.
or/8‐21
7 and 22
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name ab=abstract fs=floating subheading
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hauspy 2011.
| Methods | A retrospective cohort study from a single institution in Canada | |
| Participants | Women with GCT of ovary between 1961 and 2006 at Princess Margaret Hospital University Health Network, Toronto, were identified from the institutional database. 103 participants were evaluated. Women who had postoperative radiotherapy both after surgery and after recurrence were compared with those who did not receive radiotherapy All 103 women had primary surgery Median age was 47 years (range 22 to 77) Information on parity was not reported Median tumour size was 10 cm (range 1 to 28) Histological grade (e.g. mitotic index, lymph vascular space invasion) was not reported 78/103 (77%) women had stage I disease; 11/103 (11%) had stage II; 10/103 (10%) had stage III and in four of 103 (4%), stage was not available Details on whether women had received previous therapy were not provided |
|
| Interventions | Women who had undergone unilateral salpingo‐oophorectomy were compared with women who were treated with bilateral salpingo‐oophorectomy Adjuvant radiotherapy (n = 31) was compared with no radiotherapy Of those who received adjuvant radiotherapy, 23 were given whole abdominal radiotherapy (the upper abdomen received a 23‐Gy mid‐plane dose in 22 fractions, and the pelvis was given a 45‐Gy mid‐plane dose in 29 fractions) and eight received pelvic radiotherapy (median dose to the central axis, 41 Gy, given in 21 daily fractions) |
|
| Outcomes | OS at five, 10 and 15 years; DFS and DFS rate at five and 10 years HR was reported from a Cox model for DFS and was adjusted for adjuvant radiotherapy, tumour size, stage, salpingo‐oophorectomy, endometrial cancer and rupture |
|
| Notes | After a median follow‐up of 73 months (range, 1 to 399), 39 (38%) had recurrent disease, and 12 women had died. Two women died of second primary cancers: one of bladder cancer and one of breast cancer. The remaining 10 women died of GCT. Median DFS was 125 months (95% CI 102 to 165). The DFS rate was 81% (95% CI 73% to 90%) and 52% (95% CI 40% to 67%) at five and 10 years, respectively. The five‐, 10‐, and 15‐year overall survival rate was 93%, 91% and 87%, respectively Women with lower‐stage disease had better disease outcomes than women with higher‐stage disease. Estimated HR was 0.4 for stage I versus stage III (95% CI 0.1 to 0.9, P value 0.02). Neither the largest tumour diameter nor the presence of residual disease after surgery was a significant factor (P value 0.78 and P value 0.23, respectively) for predicting DFS Median DFS was 251 months (95% CI 142 to 293) for women treated with adjuvant RT compared with 112 months (95% CI 94 to 139 months) for women who did not receive radiotherapy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly assigned |
| Allocation concealment (selection bias) | High risk | Not randomly assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For survival outcomes: % analysed: 103/114 (90%) "Information on the presenting symptoms was available for 78 patients" (78/114 (68%) or 78/103 (76%) for those actually included in the study). As this does not represent the management aspect, despite being less than 80%, it still classes as low risk |
| Selective reporting (reporting bias) | High risk | DFS was reported using appropriate statistical technique (HR from Cox model), but OS was reported at five‐, 10‐ and 15‐year time points |
| Other bias | Unclear risk | Information was insufficient to assess whether any additional form of bias may have been present |
| Relevant assignment described? | Unclear risk | "Between 1961 and 2006, 114 patients were registered with a diagnosis of GCT. The patients included in the present study were the 103 patients for whom histologic slides were available for review and the diagnosis of GCT was confirmed" |
| Representative intervention group? | Unclear risk | It appeared that women may have been more likely to have received radiotherapy if they had more advanced disease, but this was difficult to confirm from Table 5 in the paper |
| Representative comparison group? | Unclear risk | This was difficult to deduce from Table 5, as 79% of those at early stage still received radiotherapy compared with 90% who did not |
| Comparability of treatment groups? | Low risk | Multivariate Cox model was used for survival outcomes and was adjusted for important baseline factors |
Mangili 2013.
| Methods | An Italian multicentre retrospective study aimed at describing clinical characteristics and treatment strategies for GCTs of the ovary (MITO‐9 study) | |
| Participants | Women with GCT of the ovary between 1965 and 2008 treated or referred to MITO centres after primary treatment were identified retrospectively. 97 women were evaluated. Women were excluded if they had a concomitant diagnosis of another malignancy that was not a GCT or an endometrial carcinoma. To be included in the analysis, participants needed to have at least one clinical visit at either Institution with a review of their pathology at the corresponding institution All 97 women underwent initial primary surgery Mean and median age in the study was 52 and 51 years, respectively (range 27 to 82) Information on parity was not reported Median tumour size was not reported Histological grade (e.g. mitotic index, lymph vascular space invasion) was not reported 70/97 (72%) women had stage I disease; 11/97 (11%) had 'apparent' stage I (Ix) disease; six of 97 (6%) had stage II; eight of 97 had stage III and two of 97 (2%) had stage IV disease Details on whether women had received previous therapy were not provided |
|
| Interventions | The study authors reported a Cox model for overall and recurrence‐free survival, and the following comparisons of relevance were included.
Surgery was the first treatment for all women. Fertility‐sparing surgery, defined as preservation of the uterus and one ovary, was performed in young women desiring to preserve fertility, only in cases of disease confined to one ovary. Radical surgery, including total abdominal hysterectomy, bilateral salpingo‐oophorectomy and complete tumour debulking, was the standard procedure if fertility was not an issue Women with advanced‐stage GCT (stages II, III and IV) received postoperative treatment All participants were incorporated in a prolonged surveillance programme with periodic clinical, serologic and radiologic follow‐up at a MITO centre, given the tendency of these tumours to recur several years after initial diagnosis |
|
| Outcomes | Univariate and multivariate Cox regression analyses were performed to identify independent predictors of recurrence and survival. To define prognostic parameters for relapse and survival, variables regarding women's characteristics were dichotomised in the following manner: age < 50 or > 50 years; juvenile GCT subtype versus adult GCT histology; tumour size measuring < 10 or > 10 cm; primary surgery at MITO centres or elsewhere; conservative versus radical surgery; laparoscopic versus laparotomic approach; complete staging versus incomplete staging; residual disease at primary surgery versus no residual disease; execution of lymphadenectomy versus no lymph node dissection; stage I of disease versus advanced stage of disease and adjuvant treatment versus no postsurgical treatment. Variables with P value < 0.05 on univariate analysis were selected for multivariate analysis Older age at diagnosis (> 50 years), advanced stage of disease and residual tumour at the end of surgery were associated with a poor prognosis. At multivariate analysis, age (HR 5.52, 95% CI 2.42 to 6.86) and stage (HR 10.25, 95% CI 8.21 to 16.64) were independent poor prognostic indicators for survival. Histology, number of mitoses, mass diameter, surgical approach (laparoscopy vs laparotomy), surgical staging, lymphadenectomy, treatment at a referral tertiary centre and postoperative treatment were not statistically significantly associated with death Surgical treatment outside the MITO centre (HR 3.32, 95% CI 1.35 to 8.15) and incomplete surgical staging (HR 1.23, 95% CI 1.02 to 2.28) retained significant predictive value for recurrence in both univariate and multivariate analyses. Surgical approach (laparoscopy vs laparotomy), type of surgery (conservative vs radical), execution of adjuvant chemotherapy and performance of lymphadenectomy were not associated with recurrence |
|
| Notes | In the study population, > 95% of women received continued follow‐up at MITO centres, and > 50% of them had more than 10 years of follow‐up Five‐year OS rates were 99% and 95% for women < 50 and > 50 years old (log‐rank test, P value 0.036). Five‐year OS rates were 98.7% and 75% for participants with stage I to II and III to IV, respectively (log‐rank test, P value < 0.001) Median follow‐up was 88 months (range six to 498). A total of 33 women had at least one episode of disease recurrence, with median time to recurrence of 53 months (range nine to 332) At the time of last follow‐up, five participants (4.5%) had died of disease, six (5.5%) were alive with evidence of disease, 80 (75%) were alive with no evidence of disease and 14 (13%) were lost to follow‐up; two deaths (1.9%) were considered related to other causes. After non–GCT‐related death was excluded, estimated OS after five and 10 years was 97% and 95%, respectively; the five‐year and 10‐year DFS rate was 91.8% and 71.6%, respectively |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly assigned |
| Allocation concealment (selection bias) | High risk | Not randomly assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For survival outcomes: % analysed: 97/97 (100%) |
| Selective reporting (reporting bias) | High risk | Magnitude of estimates that were not significant was not reported |
| Other bias | Unclear risk | Information was insufficient to assess whether any additional form of bias may have been present |
| Relevant assignment described? | Unclear risk | "A series of 97 patients diagnosed with primary GCTs of the ovary treated or referred after primary treatment to MITO centres from 1965 to 2008 were retrospectively analysed" |
| Representative intervention group? | Unclear risk | Information was insufficient to assess this item, as no breakdown was performed by treatment arm |
| Representative comparison group? | Unclear risk | Information was insufficient to assess this item, as no breakdown was performed by treatment arm |
| Comparability of treatment groups? | Low risk | Multivariate Cox model was used for survival outcomes and was adjusted for important baseline factors, although the magnitude of effect size was not reported for variables that were not significant |
Sun 2012.
| Methods | Retrospective multicentre cohort study in Taiwan | |
| Participants | Women with GCT of the ovary between 1984 and 2010 from multiple medical centres in Taiwan were identified from the medical records. 176 women with GCT of the ovary were evaluated, all of whom had undergone surgery. Women who had received complete surgery with no gross residual were compared with those treated with incomplete surgery. Comparisons were made between women who had received chemotherapy and those who had not Median age was 46 years Information on parity was not reported Mean tumour size was 10.4 cm (range 0.2 to 40) Histological grade (e.g. mitotic index, lymph vascular space invasion) was not reported 137/176 (78%) women had stage I disease; nine of 176(5%) had stage II; 10/176 (6%) had stage III and one of 176 (0.5%) had stage IV disease. In 19/176 (11%) women, stage was not known Details on whether women had received previous therapy were not provided |
|
| Interventions | Women who underwent complete surgery without gross tumour were compared with women who had undergone incomplete surgery. Among these women, those who had received chemotherapy were compared with those who had not. Chemotherapeutic regimens were documented only for recurrent cases and consisted mainly of a BEP regimen | |
| Outcomes | Overall five‐ and 10‐year survival rates were 96.5% and 94%, respectively. The recurrence rate was 21%. Median time to relapse was 57.6 months (range two to 166 months). In the univariate analysis, initial stage, presence of residual tumour after initial surgery, need for adjuvant chemotherapy and tumour size were associated with disease recurrence. In the multivariate analysis, only the presence of residual tumour after initial surgery and tumour size (> 13.5 cm) were significantly associated with recurrence | |
| Notes | Greater recurrence was seen in the adjuvant chemotherapy group than in the group without adjuvant chemotherapy. No significant difference in recurrence was seen between women who underwent conservative surgery and those who underwent staging surgery Tumour size and cutoff point were evaluated using the receiver operator characteristic curve |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly assigned |
| Allocation concealment (selection bias) | High risk | Not randomly assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For survival outcomes: % analysed: 176/176 (100%) |
| Selective reporting (reporting bias) | High risk | Recurrence was reported using appropriate statistical technique (HR from Cox model), but OS was reported at five‐ and 10‐year time points |
| Other bias | Unclear risk | Information was insufficient to assess whether any additional form of bias may have been present |
| Relevant assignment described? | Unclear risk | "176 Taiwanese women with pathologically confirmed GCTs diagnosed between 1984 and 2010 were analysed. A retrospective review of patient medical records was conducted" |
| Representative intervention group? | Unclear risk | Information was insufficient to assess this item, as no breakdown was performed by treatment arm |
| Representative comparison group? | Unclear risk | Information was insufficient to assess this item, as no breakdown was performed by treatment arm |
| Comparability of treatment groups? | Low risk | Multivariate Cox model was used for recurrence‐free survival and was adjusted for important baseline factors |
Suri 2013.
| Methods | A retrospective analysis of women diagnosed with GCTs set out to determine the effects of obesity and the efficacy of two adjuvant chemotherapy regimens on progression=free survival (PFS) in ovarian GCTs. The publication was available only in abstract form | |
| Participants | Multi‐institution retrospective analysis of 201 women (of whom 41 received different types of adjuvant chemotherapy) diagnosed with GCTs between 1995 and 2010 was conducted Median age was 47 years (range 37 to 58) Information on parity was not reported Mean tumour size was not reported Histological grade (e.g. mitotic index, lymph vascular space invasion) was not reported Most women had stage I disease (86%), 20 (10%) had stage II/III disease and eight (4%) were unstaged Details on whether women had received previous therapy were not provided Median BMI was 29 (range 24 to 35) 109 (57%) women were Caucasian, 68 (36%) were African American and 13 (7%) were of other ethnicity No differences in BMI, age and race were noted between groups |
|
| Interventions | Forty‐one (20%) women received adjuvant chemotherapy. The median number of cycles of chemotherapy was six (range three to six) for paclitaxel/carboplatin (PC) and for bleomycin/etoposide/cisplatin (BEP) (range three to six); other chemotherapy regimens had a median of three cycles (range one to 12) | |
| Outcomes | This large study analysed PFS in women with GCTs who received different chemotherapy regimens. Hazard ratios for recurrence were estimated by univariate and multivariate Cox regression models. The exact variables used in the Cox models for PFS were not explicitly reported, but it is likely that variables on demographics including age, race and body mass index (BMI) and clinical data including stage and adjuvant treatment were assessed In univariate analysis, BMI >= 30 was associated with worse PFS (HR 1.74, 95% CI 1.03 to 2.92) Only one of 14 (7%) participants who received PC had recurrent disease, 10 of 16 (63%) participants who received BEP had recurrence and six of 11 (55%) participants who received other chemotherapy regimens had recurrence (HR 4.5, 95% CI 0.55 to 36.99, P value 0.16) Women treated with PC had fewer recurrences than those treated with BEP. PC was described as having a more favourable therapeutic index (measure of relative safety of the drug) as compared with BEP. The study authors stated that these 'encouraging' results for PC await confirmation from the ongoing prospective non‐inferiority trial comparing PC versus BEP in women with ovarian sex cord stromal tumours |
|
| Notes | Median follow‐up time was 41 months (range 0.2 to 350) The study authors concluded that obesity is a modifiable risk factor that was associated with worse PFS. Therefore, they stated that studies evaluating weight reduction programmes in participants with GCT should be conducted to determine whether this intervention will improve survival outcomes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly assigned |
| Allocation concealment (selection bias) | High risk | Not randomly assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | High risk | Only published abstract is available, but it appears that overall survival was not reported |
| Other bias | Unclear risk | Information was insufficient to assess whether any additional form of bias may have been present |
| Relevant assignment described? | Unclear risk | Multi‐institution retrospective analysis of participants diagnosed with GCTs between 1995 and 2010 was conducted |
| Representative intervention group? | Unclear risk | Information was insufficient to permit judgement, as only published abstract is available |
| Representative comparison group? | Unclear risk | Information was insufficient to permit judgement, as only published abstract is available |
| Comparability of treatment groups? | Low risk | Hazard ratios for recurrence were estimated by univariate and multivariate Cox regression models |
Uygun 2003.
| Methods | Retrospective single‐centre cohort study in Turkey | |
| Participants | 47 women with adult GCT of the ovary were evaluated from the tumour registry at Istanbul between the period of January 1979 to December 1998. Only 45 were included because data from the remaining two were lacking. TAH/BSO/debulking surgery were compared with unilateral salpingo‐oophorectomy. Effects of chemotherapy and radiotherapy versus no adjuvant therapy were compared Median age was 46 years (range 16 to 71) Information on parity was not reported Details of tumour size were not reported Histological grade (e.g. mitotic index, lymph vascular space invasion) was not reported 23/45 (52%) were in clinical stages I to II and 22/45 (48%) were in stages III to IV. After surgery, 20/45 (44%) with stage IIC and above disease had residual disease. Nine of 45 (20%) had recurrence and most had stage III disease Details on whether participants had received previous therapy were not provided |
|
| Interventions | OS was compared in women with residual disease and with no residual disease. OS was also compared between early and advanced stages; in women who had or had not received chemotherapy and in women who had or had not received radiotherapy. Seven of nine participants who had recurrence were treated with chemotherapy and/or radiotherapy. Four of 45 had USO, and the rest had complete surgery | |
| Outcomes | OS at five years was 55%. 20/45 deaths were reported, of which 18 were due to GCT progression. Only 14 women were available for evaluation for therapeutic response to chemotherapy. The regimen used was cisplatin(IV dose of 60 to 75 mg/m2), doxorubicin (50 to 60 mg/m2) and cyclophosphamide (750 mg/m2) every three weeks. Median number of chemotherapy cycles was six (range two to nine). Five of 14 had complete response, and five of 11 had partial response. Overall response rate was 71%. Mild toxicity and no treatment‐related deaths were reported. 11/45 received radiotherapy. Partial response was seen in only six of 11 women | |
| Notes | No significant difference in OS was seen in women who received chemotherapy (P value 0.37) or radiotherapy (P value 0.29). The only significant factors were stage (P value < 0.001) and residual disease (P value < 0.001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomly assigned |
| Allocation concealment (selection bias) | High risk | Not randomly assigned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30/45 women had received chemotherapy, but response rate was documented for only 14 women. It has been documented that 15/30 had received chemotherapy as an adjuvant treatment and 15 for macroscopic residual disease. Response rate for the remaining 15 women if available could have had an effect on the outcome |
| Selective reporting (reporting bias) | High risk | OS was reported only at five years. HR and DFS were not mentioned |
| Other bias | Unclear risk | Information was insufficient to assess whether any additional form of bias may have been present |
| Relevant assignment described? | Unclear risk | Between 1979 and 1998, 47 women with GCT from the Institute of Oncology at the University of Istanbul were obtained from the surgical epicrisis, pathology reports, radiotherapy cards and follow‐up reports. 45/47 were available for evaluation for the study, as data were not available for the other two |
| Representative intervention group? | Unclear risk | Information was insufficient to assess this item, as no breakdown was performed by treatment arm |
| Representative comparison group? | Unclear risk | Information was insufficient to assess this item, as no breakdown was performed by treatment arm |
| Comparability of treatment groups? | Low risk | A multivariate model was reported; we assume that this was a Cox proportional hazards model, although this was not explicitly stated |
BEP: bleomycin, etoposide and cisplatin. BMI: body mass index. BSO: bilateral Salpingo Oophorectomy. CI: confidence interval. DFS: disease‐free survival. GCT: granulosa cell tumour. HR: hazard ratio. OS: overall survival. PC: paclitaxel and carboplatin. PFS: progression‐free survival. TAH: total Abdominal Hysterectomy USO: unilateral salpingo‐oophorectomy
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Badawi 2002 | Multivariate analysis not done |
| Alberti 1984 | Full paper not available and abstract could not be extracted |
| Baumann 1992 | Statistical adjustment not used |
| Chiara 1993 | Intervention used with no comparison group and to only nine participants |
| Colombo 1986 | One of 11 participants had juvenile‐onset GCT and statistical adjustment not used |
| Engle 1958 | Included women < 16 years old and multivariate analysis not used |
| Evans 1980 | Included women < 16 years old. Statistical adjustment not used |
| Fotopoulou 2010 | No comparison groups in the study |
| Homesley 1999 | GOG study looking at the management of all sex cord stromal tumours rather than GCT alone; looked into the bleomycin, etoposide and cisplatin regimen (BEP) and did not use comparison groups |
| Kietlinska 1993 | Age range 15 to 84 and no statistical adjustment |
| Nosov 2009 | Few juvenile GCTs included. No comparison groups included |
| Pankratz 1978 | Full paper not retrieved but abstract suggests that statistical adjustment was not carried out |
| Pautier 2008 | No comparison groups in the study |
| Pecorelli 1999 | Multivariate analysis not used |
| Pectasides 1992 | Multivariate analysis not used |
| Savage 1998 | Age range 13 to 77 years and statistical adjustment not done |
| Sehouli 2004 | Age range three to 83 years and no comparison groups found |
| Tao 2009 | Effects of bevacizumab looked at in only seven women |
| Wolf 1999 | Multivariate analysis not used |
| Zambetti 1990 | Only seven women included in the study |
BEP: bleomycin, etoposide and cisplatin. GCT: granulosa cell tumour.
Characteristics of ongoing studies [ordered by study ID]
NCT00006227.
| Trial name or title | Paclitaxel in Treating Patients With Ovarian Stromal Cancer |
| Methods | Open‐label phase 2 trial |
| Participants | Women with biopsy‐proven ovarian stromal tumours not amenable to surgery, or with residual disease > 1 cm or with recurrent disease after one chemotherapeutic treatment |
| Interventions | Women receive paclitaxel IV over three hours on day one. Treatment continues every 21 days in the absence of disease progression or unacceptable toxicity |
| Outcomes | Frequency of complete clinical response, PFS and OS |
| Starting date | November 2000 |
| Contact information | ClinicalTrials.gov identifier: NCT00006227 |
| Notes |
NCT01042522.
| Trial name or title | Paclitaxel and Carboplatin or Bleomycin Sulfate, Etoposide Phosphate, and Cisplatin in Treating Patients With Advanced or Recurrent Sex Cord Ovarian Stromal Tumors |
| Methods | Randomised phase 2 trial comparing two treatment regimens |
| Participants | Women over 18 years of age with biopsy‐proven sex cord ovarian stromal tumours; newly diagnosed, stage IIA to IVB disease with surgery; recurrent disease in chemotherapy‐naive women |
| Interventions | Experimental: Arm I women receive paclitaxel IV over three hours and carboplatin IV over one hour on day one. Treatment is repeatedevery 21 days for six courses in the absence of disease progression or unacceptable toxicity Experimental: Arm II women receive bleomycin sulphate IV on day one and etoposide IV over one hour and cisplatin IV over 30 minutes on days one through five. Treatment is repeated every 21 days for four courses in the absence of disease progression or unacceptable toxicity |
| Outcomes | PFS as primary outcome; secondary outcomes include tumour response rate, OS, toxicity, use of inhibinA/B as a predictive biomarker, changes in biomarkers in response to treatment |
| Starting date | February 2010 |
| Contact information | ClinicalTrials.gov identifier: NCT01042522 |
| Notes |
NCT01584297.
| Trial name or title | Ketoconazole as Inhibitor of the Enzyme CYP17 in Locally Advanced or Disseminated Granulosa Cell Tumour of Ovary |
| Methods | Open phase 2 clinical trial |
| Participants | Women > 18 years of age with performance status of zero or one, metastatic or unresectable disease |
| Interventions | Women will receive ketoconazole 400 mg three times a day. Study treatment period six months or up to progression of disease, unacceptable toxicity, death or withdrawal from the study for any reason |
| Outcomes | Overall response rate, clinical benefit, PFS, OS, quality of life and safety profile |
| Starting date | October 2012 |
| Contact information | ClinicalTrials.gov identifier: NCT01584297 |
| Notes |
Differences between protocol and review
We added the following study constraint in the types of studies section, as it was apparent that selection bias would lead to considerably distorted results.
To minimise the effects of selection bias (systematic differences between baseline characteristics of the groups compared), we included only studies that applied statistical adjustments for baseline case mix using multivariate analyses (e.g. adjusting for age, stage, performance status, grade).
We had initially specified in the protocol that we would assess the risk of bias in non‐randomised controlled trials in accordance with the Newcastle‐Ottawa Scale. However, after publication of the protocol, we identified a subsequent publication that was based in part on this scheme (Taggart 2001), and we created a modified version that we thought more adequately addressed risk of bias in the five included studies. A degree of repetition was noted in the Newcastle‐Ottawa Scale, with core risk of bias items including assessment of attrition bias and items included that we thought were of lesser importance.
Only five studies met the inclusion criteria for the review, and none of these findings could be pooled, so we were unable to perform any quantitative synthesis. Should more studies be identified for updates of the review, the following methods will be employed.
Data on outcomes will be extracted in this way.
For time‐to‐event (overall and recurrence‐free survival) data, we will extract the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these are not reported, we will attempt to estimate them from other reported statistics using the methods of Parmar 1998.
For dichotomous outcomes (e.g. adverse events, deaths), we will extract the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint to estimate a risk ratio (RR).
For continuous outcomes (e.g. quality of life measures), we will extract the final value and standard deviation of the outcome of interest and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up to estimate the mean difference between treatment arms and its standard error.
When possible, all data extracted will be those relevant to an intention‐to‐treat analysis, in which participants are analysed in the groups to which they were assigned.
The time points at which outcomes were collected and reported will be noted.
Data will be abstracted independently by two review authors (MG, AB) onto a data abstraction form specially designed for the review. Differences between review authors will be resolved by discussion or by appeal to a third review author if necessary.
Measures of treatment effect
We will use the following measures of the effects of treatment.
For time‐to‐event data, we will use the HR, if possible. The HR summarises the chances of survival in women who received one type of treatment compared with the chances of survival in women who received another type of treatment. However, the logarithm of the HR, rather than the HR itself, is generally used in meta‐analyses.
For dichotomous outcomes, we will use the RR.
For continuous outcomes, we will use mean differences between treatment arms if all trials measured the outcome on the same scale; otherwise standardised mean differences will be used.
Dealing with missing data
If data are missing or only imputed data are reported, we will contact study authors to request data on outcomes only among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between studies will be assessed by visual inspection of forest plots, by estimation of the percentage of heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, if possible, by subgroup analyses (see below). If evidence of substantial heterogeneity is found, possible reasons for this will be investigated and reported.
Assessment of reporting biases
Funnel plots corresponding to meta‐analysis of the primary outcome will be examined to assess the potential for small‐study effects. When evidence of small‐study effects is found, publication bias will be considered as only one of a number of possible explanations. If these plots suggest that treatment effects may not be sampled from a symmetrical distribution, as assumed by the random‐effects model, sensitivity analyses will be performed using fixed‐effect models.
Data synthesis
When sufficient clinically similar studies are available, their adjusted results will be pooled in meta‐analyses.
For time‐to‐event data, HRs will be pooled using the generic inverse variance facility of RevMan 5.
For dichotomous outcomes, the RR was calculated for each study, and all RRs were then pooled.
For continuous outcomes, mean differences (or standardised mean differences) between treatment arms at the end of follow‐up will be pooled.
If any studies have multiple treatment groups, the ‘shared’ comparison group will be divided into the number of treatment groups and comparisons between each treatment group, and the split comparison group will be treated as independent comparisons.
Random‐effects models with inverse variance weighting will be used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
Subgroup analyses will be performed with trials grouped by:
stage of disease; and
primary or recurrent disease.
Factors such as age, stage, type of intervention and length of follow‐up will be considered in interpretation of any heterogeneity.
Sensitivity analysis
Sensitivity analyses will be performed while excluding studies at high risk of bias.
Contributions of authors
MG and SS searched for relevant studies and individually examined each potentially relevant full‐text reference. MG and AB extracted data on risk of bias items. MG, after collation of all references, worked on development of the full review alongside AB and SS. AB drafted methodological and statistical sections of the review, as well as various sections of the discussion. MG and SS drafted clinical sections of the review, added expertise and drafted the discussion. SS, second review author for the review, provided methodological, clinical, policy and consumer perspectives on writing the review, contributed to the discussion from a gynaecological oncology perspective, provided general advice and helped to draft the review after peer review comments were received.
Sources of support
Internal sources
Department of Obstetrics & Gynaecology, University of Aberdeen, UK.
Department of Obstetrics and Gynaecology, James Cook University Hospital, Middlesbrough, UK.
Department of Obstetrics and Gynaecology, NHS Greater Glasgow and Clyde, UK.
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration Programme Grant Scheme CPG‐10/4001/12
Declarations of interest
None.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Hauspy 2011 {published data only}
- Hauspy J, Beiner ME, Harley I, Rosen B, Murphy J, Chapman W, et al. Role of adjuvant radiotherapy in granulosa cell tumors of the ovary. International Journal of Radiation Oncology, Biology, Physics 2011;79(3):770–4. [DOI] [PubMed] [Google Scholar]
Mangili 2013 {published data only}
- Mangili G, Ottolina J, Gadducci A, Giorda G, Breda E, Savarese A, et al. Long‐term follow‐up is crucial after treatment for granulosa cell tumours of the ovary. British Journal of Cancer 2013;109:29–34. [DOI: 10.1038/bjc.2013.241] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2012 {published data only}
- Sun H‐D, Lin H, Jao MS, Wang KL, Liou W‐S, Hung Y‐C, et al. A long‐term follow‐up study of 176 cases with adult‐type ovarian granulosa cell tumors. Gynecologic Oncology 2012;124:244–9. [DOI] [PubMed] [Google Scholar]
Suri 2013 {published data only (unpublished sought but not used)}
- Suri A. Effects of obesity and adjuvant chemotherapy regimens on progression free survival in patients with ovarian granulosa cell tumors. Gynecologic Oncology Conference: 2013 Annual Meeting of the Western Association of Gynecologic Oncologists Seattle, WA United States. June 2013.
- Suri A. The impact of obesity and adjuvant chemotherapy regimens on women with ovarian granulosa cell tumors. Gynecologic Oncology Conference: 44th Annual Meeting of the Society of Gynecologic Oncology Los Angeles, CA United States. March 2013.
Uygun 2003 {published data only}
References to studies excluded from this review
Al‐Badawi 2002 {published data only}
- Al‐Badawi IA, Brasher PMA, Ghatage P, Nation JG, Schepansky A, Stuart GCE. Postoperative chemotherapy in advanced ovarian granulosa cell tumors. International Journal of Gynecological Cancer. United States: Department of Gynecology, Tom Baker Cancer Center, 1331‐29th Street NW, Calgary, Alberta T2N 4N2, Canada., 2002; Vol. 12, issue 1:119‐23. [DOI] [PubMed]
Alberti 1984 {published data only}
Baumann 1992 {published data only}
- Baumann D, Donat H, Bohme M, Lenz E. Clinical experiences with treatment of granulosa cell tumors. Zentralblatt fur Gynakologie. GERMANY: Abteilung fur Gynakologische Onkologie der Klinik fur Gynakologie und Geburtshilfe, Medizinischen Akademie Magdeburg., 1992; Vol. 114, issue 7:361‐4. [PubMed]
Chiara 1993 {published data only}
- Chiara S, Merlini L, Campora E, Bruzzone M, Giudici S, Rosso R, et al. Cisplatin‐based chemotherapy in recurrent or high risk ovarian granulosa‐cell tumor patients. European Journal of Gynaecological Oncology 1993;14(4):314‐7. [PubMed] [Google Scholar]
Colombo 1986 {published data only}
- Colombo N, Sessa C, Landoni F, Sartori E, Pecorelli S, Mangioni C. Cisplatin, vinblastine, and bleomycin combination chemotherapy in metastatic granulosa cell tumor of the ovary. Obstetrics & Gynecology 1986;67(2):265‐8. [DOI] [PubMed] [Google Scholar]
Engle 1958 {published data only}
- Engle RB. Roentgen treatment of granulosa cell carcinoma of the ovary. American Journal of Roentgenology, Radium Therapy & Nuclear Medicine 1958;80(5):793‐8. [PubMed] [Google Scholar]
Evans 1980 {published data only}
- Evans AT 3rd, Gaffey TA, Malkasian GD Jr, Annegers JF. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstetrics & Gynecology 1980;55(2):231‐8. [PubMed] [Google Scholar]
Fotopoulou 2010 {published data only}
- Fotopoulou C, Savvatis K, Braicu EI, Brink‐Spalink V, Darb‐Esfahani S, Lichtenegger W, et al. Adult granulosa cell tumors of the ovary: tumor dissemination pattern at primary and recurrent situation, surgical outcome. Gynecologic Oncology November 2010;119(2):285‐90. [DOI] [PubMed] [Google Scholar]
Homesley 1999 {published data only}
- Homesley HD, Bundy BN, Hurteau JA, Roth LM. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies. Gynecologic Oncology 1999;72(2):131‐7. [DOI] [PubMed] [Google Scholar]
Kietlinska 1993 {published data only}
- Kietlińska Z, Pietrzak K, Drabik M. The management of granulosa‐cell tumors of the ovary based on long‐term follow up. European Journal of Gynaecologic Oncology 1993;14:118‐27. [PubMed] [Google Scholar]
Nosov 2009 {published data only}
- Nosov V, Silva I, Tavassoli F, Adamyan L, Farias‐Eisner R, Schwartz PE. Predictors of recurrence of ovarian granulosa cell tumors. International Journal of Gynecologic Cancer 2009;19(4):628‐33. [DOI] [PubMed] [Google Scholar]
Pankratz 1978 {published data only}
- Pankratz E, Boyes DA, White GW, Galliford BW, Fairey RN, Benedet JL. Granulosa cell tumors. A clinical review of 61 cases. Obstetrics and Gynecology. UNITED STATES, 1978; Vol. 52, issue 6:718‐23. [PubMed]
Pautier 2008 {published data only}
- Pautier P, Gutierrez‐Bonnaire M, Rey A, Sillet‐Bach I, Chevreau C, Kerbrat P, et al. Combination of bleomycin, etoposide, and cisplatin for the treatment of advanced ovarian granulosa cell tumors. International Journal of Gynecologic Cancer 2008;18(3):446‐52. [DOI] [PubMed] [Google Scholar]
Pecorelli 1999 {published data only}
- Pecorelli S, Wagenaar HC, Vergote IB, Curran D, Beex LV, Wiltshaw E, et al. Cisplatin (P), vinblastine (V) and bleomycin (B) combination chemotherapy in recurrent or advanced granulosa(‐theca) cell tumours of the ovary. European Journal of Cancer 1999;35(9):1331‐7. [DOI] [PubMed] [Google Scholar]
Pectasides 1992 {published data only}
- Pectasides D, Alevizakos N, Athanassiou AE. Cisplatin‐containing regimen in advanced or recurrent granulosa cell tumours of the ovary. Annals of Oncology 1992;3(4):316‐8. [DOI] [PubMed] [Google Scholar]
Savage 1998 {published data only}
- Savage P, Constenla D, Fisher C, Shepherd JH, Barton DP, Blake P, et al. Granulosa cell tumours of the ovary: demographics, survival and the management of advanced disease. Clinical Oncology (Royal College of Radiology) 1998;10(4):242‐5. [DOI] [PubMed] [Google Scholar]
Sehouli 2004 {published data only}
- Sehouli J, Drescher FS, Mustea A, Elling D, Friedmann W, Kühn W, et al. Granulosa cell tumor of the ovary: 10 years follow‐up data of 65 patients. Anticancer Research 2004;24(2C):1223‐9. [PubMed] [Google Scholar]
Tao 2009 {published data only}
- Tao X, Sood AK, Deavers MT, Schmeler KM, Nick AM, Coleman RL, et al. Anti‐angiogenesis therapy with bevacizumab for patients with ovarian granulosa cell tumors. Gynecologic Oncology. United States: Department of Gynecologic Oncology, The University of Texas M. D. Anderson Cancer Center, Houston, TX 77030‐1439, USA., 2009; Vol. 114, issue 3:431‐6. [DOI] [PMC free article] [PubMed]
Wolf 1999 {published data only}
- Wolf JK, Mullen J, Eifel PJ, Burke TW, Levenback C, Gershenson DM. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecologic Oncology 1999;73(1):35‐41. [DOI] [PubMed] [Google Scholar]
Zambetti 1990 {published data only}
- Zambetti M, Escobedo A, Pilotti S, Palo G. Cis‐platinum/vinblastine/bleomycin combination chemotherapy in advanced or recurrent granulosa cell tumors of the ovary. Gynecologic Oncology. UNITED STATES: Division of Medical Oncology, Institute Nazionale Tumori, Milan, Italy., 1990; Vol. 36, issue 3:317‐20. [DOI] [PubMed]
References to ongoing studies
NCT00006227 {published data only}
- LL (Principal investigator). Paclitaxel in treating patients with ovarian stromal cancer. http://clinicaltrials.gov/show/NCT00006227.
NCT01042522 {published data only}
- Brown J. Paclitaxel and carboplatin or bleomycin sulfate, etoposide phosphate, and cisplatin in treating patients with advanced or recurrent sex cord‐ovarian stromal tumors. http://clinicaltrials.gov/show/NCT01042522.
NCT01584297 {published data only}
- Garcia‐Donas J (Study Director). Ketoconazole as inhibitor of the enzyme CYP17 in locally advanced or disseminated granulosa cell tumour of ovary. http://clinicaltrials.gov/show/NCT01584297.
Additional references
Aboud 1997
- Aboud E. Adult granulosa cell tumours of the ovary. European Journal of Gynaecological Oncology 1997;XVIII(6):520‐2. [PubMed] [Google Scholar]
Abu‐Rustum 2006
- Abu‐Rustum N, Restivo A, Ivy J, Soslow R, Sabbatini P, Sonoda Y, et al. Retroperitoneal nodal metastasis in primary and recurrent granulosa cell tumours of the ovary. Gynecologic Oncology 2006;103:31‐4. [DOI] [PubMed] [Google Scholar]
Ameryckx 2005
- Ameryckx L, Fatemi H, Sutter P, Amy J. GnRH antagonist in the adjuvant treatment of a recurrent ovarian granulosa cell tumor: a case report. Gynecologic Oncology 2005;99(3):764‐6. [DOI] [PubMed] [Google Scholar]
Ayhan 2009
- Ayhan A, Salman M, Velipasaoglu M, Sakinci M, Yuce K. Prognostic factors in adult granulosa cell tumors of the ovary: a retrospective analysis of 80 cases. Journal of Gynecologic Oncology 2009;20(3):158‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Benedet 2000
- Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. International Journal of Gynaecology and Obstetrics 2000;70(2):209‐62. [PubMed] [Google Scholar]
Boggess 1997
- Boggess JF, Soules MR, Goff BA, Greer BE, Cain JM, Tamimi HK. Serum inhibin and disease status in women with ovarian granulosa cell tumors. Gynecologic Oncology 1997;64:64‐9. [DOI] [PubMed] [Google Scholar]
Briasoulis 1997
- Briasoulis E, Karavasilis V, Pavlidids N. Megestrol activity in recurrent adult type granulosa cell tumour of the ovary. Annals of Oncology 1997;8:811‐2. [DOI] [PubMed] [Google Scholar]
Brown 2004
- Brown J, Shvartsman H, Deavers M, Burke T, Munsell M, Gershenson D. The activity of taxanes in the treatment of sex cord‐stromal tumors. Journal of Clinical Oncology 2004;22(17):3517‐23. [DOI] [PubMed] [Google Scholar]
Brown 2005
- Brown J, Shvartsman H, Deavers M, Ramondetta L, Burke T, Munsell M, et al. The activity of taxanes compared with bleomycin, etoposide, and cisplatin in the treatment of sex cord‐stromal ovarian tumours. Gynecologic Oncology 2005;97(2):489‐96. [DOI] [PubMed] [Google Scholar]
Choan 2006
- Choan E, Samant R, Kee Fung M, Le T, Hopkins L, Senterman M. Palliative radiotherapy for recurrent granulosa cell tumour of the ovary: a report of 3 cases with radiological evidence of response. Gynecologic Oncology 2006;102:406‐10. [DOI] [PubMed] [Google Scholar]
Colombo 1986
- Colombo N, Sessa C, Landoni F, Sartori E, Pecorelli S, Mangioni C. Cisplatin, vinblastine, and bleomycin combination chemotherapy in metastatic granulosa cell tumor of the ovary. Obstetrics and Gynecology 1986;67:265‐8. [DOI] [PubMed] [Google Scholar]
Colombo 1999
Colombo 2007
- Colombo N, Parma G, Zanagnolo V, Insinga A. Management of ovarian stromal cell tumors. Journal of Clinical Oncology 2007;25(20):2944‐51. [DOI] [PubMed] [Google Scholar]
Cornis 2007
- Cornis R, Group Chair Eastern Cooperative Oncology Group (ECOG). ECOG common toxicity criteria. http://www.ecog.org/general/common_tox.html (accessed March 2014).
CTCAE 2010
- National Institutes of Health: National Cancer Institute. Common terminology criteria for adverse events v4.03: June 14, 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010‐06‐14_QuickReference_5x7.pdf.
D'Angelo 2011
- D'Angelo E, Mozos A, Nakayama D, Espinosa I, Catasus L, Prat J, et al. Prognostic significance of FOXL2 mutation and mRNA expression in adult and juvenile granulosa cell tumors of the ovary. Modern Pathology 2011;24(10):1360‐7. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks J, Altman D, Bradburn M. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman D editor(s). Systematic Reviews in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publishing Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Disaia 1978
- Disaia P, Saltz A, Kagan A, Rich W. A temporary response of recurrent granulosa cell tumor to adriamycin. Obstetrics and Gynecology 1978;52:355‐8. [PubMed] [Google Scholar]
Fishman 1996
- Fishman A, Kudelka A, Tresukosol D, Edwards C, Freedman R, Kaplan A, et al. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. Journal of Reproductive Medicine 1996;41(6):393‐6. [PubMed] [Google Scholar]
Fox 1975
- Fox H, Agrawal K, Langley F. A clinicopathologic study of 92 cases of granulosa cell tumours of the ovary with special reference to the factors affecting prognosis. Cancer 1975;35:231. [DOI] [PubMed] [Google Scholar]
Fox 1992
- Fox H, Buckley C. Pathology of malignant gonadal stromal tumours of ovary. In: Coppleson M editor(s). Gynaecologic Oncology. Vol. 59, Edinburgh: Churchill Livingstone, 1992:947. [Google Scholar]
Freeman 2006
- Freeman S, Modesitt S. Anastrozole therapy in recurrent adult granulosa cell tumors: a report of 2 cases. Gynecologic Oncology 2006;103(2):755‐8. [DOI] [PubMed] [Google Scholar]
Geerts I 2009
- Geerts I, Vergote I, Neven P, Billen J. The role of Inhibins B and antimullerian hormone for diagnosis and follow‐up of granulosa cell tumors. International Journal of Gynecological Cancer 2009;19(5):847‐55. [DOI] [PubMed] [Google Scholar]
Gershenson 1987
- Gershenson D, Copeland L, Kavanagh J, Stringer C, Saul P, Wharton J. Treatment of metastatic stromal tumors of the ovary with cisplatin, doxorubicin and cyclophosphamide. Obstetrics and Gynecology 1987;70:765‐9. [PubMed] [Google Scholar]
Gershenson 1996
- Gershenson D, Morris M, Burke T, Levenback C, Matthews C, Wharton J. Treatment of poor prognosis sex‐cord stromal tumors of the ovary with the combination of bleomycin, etoposide and cisplatin. Obstetrics and Gynecology 1996;87:527‐31. [DOI] [PubMed] [Google Scholar]
Gershenson 2004
- Gershenson D, McGuire W, Gore M, Quinn M, Thomas G. Gynaecologic Cancers: Controversies in Management. Philadelphia, PA: Elsevier, 2004. [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haba 1993
- Haba R, Miki H, Kobayashi S, Ohmori M. Combined analysis of flow cytometry and morphometry of ovarian granulosa cell tumor. Cancer 1993;72:3258‐62. [DOI] [PubMed] [Google Scholar]
Hardy 2005
- Hardy R, Bell J, Nicely C, Reid G. Hormonal treatment of a recurrent granulosa cell tumor of the ovary: case report and review of literature. Gynecologic Oncology 2005;96:865‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Homesley 1999
- Homesley H, Bundy B, Hurteau J, Roth L. Bleomycin, etoposide, and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: a Gynecologic Oncology Group study. Gynecologic Oncology 1999;2(72):131‐7. [DOI] [PubMed] [Google Scholar]
Hoskins 1992
- Hoskins W, Rubin S. Malignant gonadal stromal tumours of ovary: clinical features and management. In: Coppleson M editor(s). Gynaecologic Oncology. Vol. 60, Edinburgh: Churchill Livingstone, 1992:961. [Google Scholar]
Jacobs 1982
- Jacobs A, Deppe G, Cohen C. Combination chemotherapy of ovarian granulosa cell tumor with cis‐platinum and doxorubicin. Gynecologic Oncology 1982;14:294‐7. [DOI] [PubMed] [Google Scholar]
Jamieson 2010
- Jamieson S, Butzow R, Andersson N, Alexiadis M, Unkila‐Kallio L, Anttonen M, et al. The FOXL2 C134W mutation is characteristic of adult granulosa cell tumors of the ovary. Modern Pathology 2010;23(11):1477‐85. [DOI] [PubMed] [Google Scholar]
Kauppila 1992
- Kauppila A, Bangah M, Burger H, Martikainen H. GnRH agonist analog therapy in advanced/ recurrent granulosa cell tumors: further evidence of a role of inhibin in monitoring response to treatment. Gynecologic Endocrinology 1992;6(4):271‐4. [DOI] [PubMed] [Google Scholar]
Kim 2006
- Kim YM, Jung M, Kim K, Kim YT, Nam J, Mok J. Adult granulosa cell tumor of the ovary: 35 cases in a single Korean institute. Acta Obstetrica et Gynecologica 2006;85:112‐5. [DOI] [PubMed] [Google Scholar]
Korach 2009
- Korach J, Tamar P, Mario B, Tima D, Eddie F, Gilad B. Promising effect of aromatase inhibitors on recurrent granulosa cell tumors. International Journal of Gynecological Cancer 2009;19(5):830‐3. [DOI] [PubMed] [Google Scholar]
Lee 1999
- Lee I, Levin W, Chapman W, Goldberg R, Murphy K, Milosevic M. Radiotherapy for the treatment of metastatic granulosa cell tumor in the mediastinum: a case report. Gynecologic Oncology 1999;73:455‐60. [DOI] [PubMed] [Google Scholar]
Liu 2001
- Liu J, Hyden‐Granskog C, Voutilainen R. Gonadotrophins inhibit and activin induces expression of inhibin/ activin beta subunit mRNA in cultured human granulosa‐luteal cells. Molecular Human Reproduction 2001;7(4):319‐23. [DOI] [PubMed] [Google Scholar]
Lovell 2002
- Lovell T, Gladwell R, Groome N, Knight P. Modulatory effects of gonadotrophins and insulin‐like growth factor on the secretion of inhibin A and progesterone by granulosa cells from chicken preovulatory (F1‐F3) follicles. Reproduction 2002;123(2):291‐300. [PubMed] [Google Scholar]
Malmstrom 1994
- Malmstrom H, Hogberg T, Risberg B, Simonsen E. Granulosa cell tumors of the ovary: prognostic factors and outcome. Gynecologic Oncology 1994;52:50‐5. [DOI] [PubMed] [Google Scholar]
Martikainen 1989
- Martikainen H, Penttinen J, Huhtaniemi I, Kauppila A. Gonadotropin‐releasing hormone analog therapy effective in ovarian granulosa cell malignancy. Gynecologic Oncology 1989;35(3):406‐8. [DOI] [PubMed] [Google Scholar]
Maxwell 1994
- Maxwell G, Soisson A, Miles P. Failure of gonadotropin releasing hormone therapy in patients with metastatic ovarian sex cord stromal tumors. Oncology 1994;51(4):356‐9. [DOI] [PubMed] [Google Scholar]
Miller 1981
- Miller A, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207‐14. [DOI] [PubMed] [Google Scholar]
Mom 2007
- Mom CH, Engelen MJA, Willemse PHB, Gietema GA, Hoor KA, Vries EG, et al. Granulosa cell tumors of the ovary: the clinical value of serum inhibin A and B levels in a large single center cohort. Gynecologic Oncology 2007;2:365‐72. [DOI] [PubMed] [Google Scholar]
Newcastle‐Ottawa Scale
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomized studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed March 2014).
Ohel 1983
- Ohel G, Kaneti H, Schenker J. Granulosa cell tumors in Israel: a study of 172 cases. Gynecologic Oncology 1983;15:278‐86. [DOI] [PubMed] [Google Scholar]
Park 2012
- Park JY, Jin KL, Kim DY, Kim JH, Kim YM, et al. Surgical staging and adjuvant chemotherapy in the management of patients with adult granulosa cell tumors of the ovary. Gynecologic Oncology 2012;125(1):80‐6. [DOI] [PubMed] [Google Scholar]
Parkin 2003
- Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents, vol VIII. IARC Scientific Publication No. 155. Vol. CD‐ROM C15 I‐VIII: updated database, Lyon, France: International Agency for Research on Cancer, 2003. [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Rao 2005
- Rao G, Miller D. Clinical applications of hormonal therapy in ovarian cancer. Current Treatment Options in Oncology 2005;6(2):97‐102. [DOI] [PubMed] [Google Scholar]
Savage 1998
- Savage P, Constenla D, Fisher C, Shepherd J, Barton D, Blake P, et al. Granulosa cell tumors of the ovary: demographics, survival and the management of advanced disease. Clinical Oncology 1998;10:242‐5. [DOI] [PubMed] [Google Scholar]
Schumer 2003
- Schumer S, Cannistra S. Granulosa cell tumour of the ovary. Journal of Clinical Oncology 2003;21(6):1180‐9. [DOI] [PubMed] [Google Scholar]
Stuart 2003
- Stuart G, Dawson L. Update on granulosa cell tumours of the ovary. Current Opinion in Obstetrics and Gynecology 2003;15(1):33‐7. [DOI] [PubMed] [Google Scholar]
Taggart 2001
- Taggart DP, D'Amico R, Altman DG. Effect of arterial revascularisation on survival: a systematic review of studies comparing bilateral and single internal mammary arteries. The Lancet 2001;358:870‐5. [DOI] [PubMed] [Google Scholar]
Thrall 2011
- Thrall MM, Paley P, Pizer E, Garcia R, Goff BA. Patterns of spread and recurrence of sex cord‐stromal tumors of the ovary. Gynecologic Oncology 2011;122(2):242‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tresukosol 1995
- Tresukosol D, Kudelka A, Edwards C, Charnsangavej C, Narboni N, Kavanagh J. Recurrent ovarian granulosa cell tumor: a case report of a dramatic response to Taxol. International Journal of Gynecological Cancer 1995;5:156‐9. [DOI] [PubMed] [Google Scholar]
Wolf 1999
- Wolf J, Mullen J, Eifel P, Burke T, Levenback C, Gerhenson D. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecologic Oncology 1999;73:35‐41. [DOI] [PubMed] [Google Scholar]


