Abstract
Background
In most Western countries, obstetricians and midwives induce labour in about 25% of pregnant women. Oxytocin is an effective drug for this purpose, but associated with serious adverse effects of which uterine tachysystole, fetal distress and the need for immediate delivery are the most common. Various administration regimens such as reduced or pulsatile dosing have been suggested to minimise these. Discontinuation in the active phase of labour, i.e. when contractions are well‐established and the cervix is dilated at least 5 cm is another method which may reduce adverse effects.
Objectives
To assess whether birth outcomes can be improved by discontinuation of intravenous (IV) oxytocin, initiated in the latent phase of induced labour, once active phase of labour is established.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (31 January 2018), Scopus, ClinicalTrials.gov, and the WHO International Clinical Trials Registry Platform (ICTRP) (23 January 2018) together with reference checking, citation searching, and contact with study authors to identify additional studies.
Selection criteria
Randomised controlled trials (RCTs) comparing discontinued IV with continuous IV oxytocin in the active phase of induced labour.
No exclusion criteria were applied in terms of parity, maternal age, ethnicity, co‐morbidity status, labour setting, gestational age, and prior caesarean delivery.
Studies comparing different dosage regimens are outside the scope of this review.
Data collection and analysis
We used standard Cochrane methods.
Main results
We found 10 completed RCTs involving 1888 women. One additional trial is ongoing. The included trials were conducted in hospital settings between February 1998 and January 2016, two in Europe (Denmark, and Greece), two in Turkey, and one each in Israel, Iran, USA, Bangladesh, India, and Thailand. Most trials included full‐term singleton pregnancies with a fetus in vertex presentation. Some excluded women with cervical priming prior to induction and some excluded women with a history of prior caesarean delivery. When reported, the average age of the women ranged from 22 to 31 years, nulliparity from 45% to 68%, and pre‐pregnancy body mass index from 22 to 32.
Many of the included trials had design limitations and were judged to be at either high or unclear risk of bias across a number of 'Risk of bias' domains.
Four trials included a Consort flow diagram. In three, this gave details of participants delivered before the active phase of labour, and treatment compliance for those who reached that stage. One Consort diagram only provided the latter information. The data in many of the trials without such a flow diagram were implausibly compliant with treatment allocation, suggesting that there had been silent post randomisation exclusions of women delivered before the active phase of labour. We therefore conducted a secondary analysis (not in our protocol) of caesarean section among women who reached the active phase of labour and were therefore eligible for the intervention.
Our analysis by 'intention‐to‐treat' found that, compared with continuation of IV oxytocin stimulation, discontinuation of IV oxytocin may reduce the caesarean delivery rate, risk ratio (RR) 0.69, 95% confidence interval (CI) 0.56 to 0.86, 9 trials, 1784 women, low‐level certainty. However, restricting our analysis to women who reached the active phase of labour (using 'reached active phase' as our denominator) suggests there is probably little or no difference between groups (RR 0.92, 95% CI 0.65 to 1.29, 4 trials, 787 women, moderate‐certainty evidence).
Discontinuation of IV oxytocin probably reduces the risk ofuterine tachysystole combined with abnormal fetal heart rate (FHR) compared with continued IV oxytocin (RR 0.15, 95% CI 0.05 to 0.46, 3 trials, 486 women, moderate‐level certainty). We are uncertain about whether or not discontinuation increases the risk of chorioamnionitis (average RR 2.32, 95% CI 0.99 to 5.45, 1 trial, 252 women, very low‐level certainty). Discontinuation of IV oxytocin may have little or no impact on the use of analgesia and epidural during labour compared to the use of continued IV oxytocin (RR 1.04 95% CI 0.95 to 1.14, 3 trials, 556 women, low‐level certainty). Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs) are probably reduced by discontinuing IV oxytocin (RR 0.65, 95% CI 0.51 to 0.83, 7 trials, 1390 women, moderate‐level certainty). Compared to continuing IV oxytocin, discontinuing IV oxytocin probably has little or no impact on the incidence of Apgar < 7 at five minutes (RR 0.78, 95% CI 0.27 to 2.21, 4 trials, 893 women, low‐level certainty), or and acidotic cord gasses at birth (arterial umbilical pH < 7.10), (RR 1.03, 95% CI 0.50 to 2.13, 4 trials, 873 women, low‐level certainty).
Many of this review's maternal and infant secondary outcomes (including maternal and neonatal mortality) were not reported in the included trials.
Authors' conclusions
Discontinuing IV oxytocin stimulation after the active phase of labour has been established may reduce caesarean delivery but the evidence for this was low certainty. When restricting our analysis to those trials that separately reported participants who reached the active phase of labour, our results showed there is probably little or no difference between groups. Discontinuing IV oxytocin may reduce uterine tachysystole combined with abnormal FHR.
Most of the trials had 'Risk of bias' concerns which means that these results should be interpreted with caution. Our GRADE assessments ranged from very low certainty to moderate certainty. Downgrading decisions were based on study limitations, imprecision and indirectness.
Future research could account for all women randomised and, in particular, note those who delivered before the point at which they would be eligible for the intervention (i.e. those who had caesareans in the latent phase), or because labour was so rapid that the infusion could not be stopped in time.
Future trials could adopt the outcomes listed in this review including maternal and neonatal mortality, maternal satisfaction, and breastfeeding.
Plain language summary
Discontinuation of intravenous oxytocin used to stimulate uterine contractions in the active phase of induced labour
What is the issue?
Oxytocin is a natural hormone, which causes the uterus (womb) to have regular, painful contractions and labour to start. It is available as an intravenous (into a vein (IV)) drug and infused slowly to artificially stimulate labour if doctors or midwives feel that it is necessary to accelerate the birth of the baby, or if the mother requests it. In Western countries, about one in four pregnant women have labour induced, usually with prostaglandin drugs either alone or in combination with oxytocin.
Risks associated with using IV oxytocin to stimulate uterine contractions include the woman having contractions that are too long or too frequent (uterine hyperstimulation), which can lead to changes in the baby’s heart rate and the need for emergency caesarean. This review examines whether stopping IV oxytocin once labour is well‐established (i.e. the cervix is dilated more than halfway) reduces the associated risks for mother and baby compared to continuing with IV oxytocin.
Why is this important?
Stopping oxytocin infusion once active labour has started could result in a more natural childbirth, particularly if the risk of uterine overstimulation and need for immediate caesarean section is reduced. Also, the overall total dose of oxytocin the mother received would be reduced, which could lead to fewer adverse effects (e.g. maternal nausea, vomiting and headache, or changes to the baby's heart rate).
What evidence did we find?
We searched for evidence (January 2018) and found 10 randomised controlled studies (1888 women and their babies) conducted between February 1998 and January 2016 at hospitals in Denmark, Greece, Turkey, Israel, Iran, USA, Bangladesh, India, and Thailand. We cannot be confident in the results because of study design limitations and how the findings were reported.
Stopping IV oxytocin during active labour may reduce the number of women who have a caesarean section (nine trials, 1784 women). However, when we performed another analysis including only those women who were actually in active labour, we found that there is probably little or no difference between the two groups (four trials, 787 women).
Discontinuing IV oxytocin probably reduces the risk of women having contractions that become too long or too strong resulting in changes to the baby’s heart rate (three trials, 486 women). We are uncertain about whether stopping IV oxytocin or not affects the risk of having a bacterial infection of the membranes or sac inside the womb) (one trial, 252 women). Stopping IV oxytocin during labour may have little or no impact on women’s use of analgesia and epidural compared to women who continued to receive IV oxytocin (three trials, 556 women).
There were probably fewer babies in the discontinued IV oxytocin group with abnormal cardiotocography results (an electronic method of measuring both the women’s contractions and the baby’s heartbeat) compared to women who continued to receive IV oxytocin (seven trials, 1390 women).
Compared to continued IV oxytocin, discontinuing IV oxytocin probably has little or no impact on the number of babies with a low score on a standard test of well‐being for newborn babies (Apgar), five minutes after being born (four trials, 893 women), or another other measure of infant well‐being involving analysing blood taken from the umbilical cord once (four trials, 873 women).
The included trials did not report on many of this review's outcomes, including death of the mother or her baby.
What does this mean?
Stopping oxytocin after the active phase of labour has started may reduce the number of women with contractions that become too long or too strong resulting in changes to the baby’s heart rate, and the risk of having a caesarean. However, the possible reduction in the risk of caesarean may be an artefact of poor study design.
Better quality trials are needed. These could include in the analysis those women who did not reach the active phase of labour because their babies were delivered earlier by caesarean, and those whose labour was so rapid that the oxytocin could not be stopped in time, i.e. analysis should be by 'intention‐to‐treat".
Future studies could include the outcomes listed in this review, including women's satisfaction.
Summary of findings
Summary of findings for the main comparison. Discontinued intravenous (IV) oxytocin stimulation compared with continued IV oxytocin stimulation in the active phase for induction of labour.
| Discontinued intravenous (IV) oxytocin stimulation compared with continued IV oxytocin during the active phase for induction of labour | ||||||
|
Patient or population: pregnant women in latent phase of labour stimulated with oxytocin for with induction of labour. No exclusion criteria were applied in terms of parity, maternal age, ethnicity, co‐morbidity status, labour setting, gestational age, or prior caesarean delivery. Setting: hospital settings Country of trials: two trials in Europe (Denmark and Greece), two in Turkey, one trial in Iran, Israel, USA, Bangladesh, India, and Thailand Intervention: discontinuation of IV oxytocin during active phase of labour Comparison: continuation of IV oxytocin during active phase of labour | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Corresponding risk | Assumed risk | |||||
| Disontinued oxytocin | Continued oxytocin | |||||
| Caesarean delivery | Low‐risk population | RR 0.69 (0.56 to 0.86) | 1784 (9) |
⊕⊕⊝⊝ low1 | ||
| 121 per 1000 (98 to 150) | 175 per 1000 | |||||
| Caesarean delivery after active phase has begun, using "reached active phase" as the denominator | Low‐risk population | RR 0.92 (0.65 to 1.29) | 787 (4) |
⊕⊕⊕⊝ moderate2 | This outcome was not prespecified in our protocol | |
|
136 per 1000 (96 to 191) |
148 per 1000 | |||||
| Uterine tachysystole combined with abnormal fetal heart rate | Low‐risk population |
RR 0.15 95% CI 0.05 to 0.46 |
486 (3) |
⊕⊕⊕⊝ moderate3 | ||
| 14 per 1000 (5 to 43) | 93 per 1000 | |||||
| Chorioamnionitis | Low‐risk population |
RR 2.32 95% CI 0.99 to 5.45 |
252 (1) |
⊕⊝⊝⊝ very low4,5,6 | ||
| 127 per 1000 (18 to 300) | 55 per 1000 | |||||
| Use of analgesia and epidural during labour | Low‐risk population |
Average RR 1.04 95% CI 0.95 to 1.14 |
556 (3) |
⊕⊕⊝⊝ low7,8 | ||
|
716 per 1000 (654 to 785) |
688 per 1000 | |||||
| Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs) | Low‐risk population |
RR 0.65 95% CI 0.51 to 0.83 |
1390 (7) | ⊕⊕⊕⊝ moderate10 | ||
| 125 per 1000 (98 to 160) | 193 per 1000 | |||||
| Apgar score at five minutes below seven | Low‐risk population | RR 0.78 95% CI 0.27 to 2.21 | 893 (4) |
⊕⊕⊝⊝ low11,12 | ||
|
11 per 1000 (4 to 24) |
15 per 1000 | |||||
| Acidotic cord gasses (arterial umbilical pH < 7.10) | Low‐risk population | RR 1.03 95% CI 0.50 to 2.13 | 873 (4) |
⊕⊕⊝⊝ low11,12 | ||
|
32 per 1000 (16 to 67) |
32 per 1000 | |||||
- Downgraded (‐2) for very serious study limitations ‐ the majority of trials were high risk of bias for blinding, and/or selection bias. The majority of trials were unclear risk of bias for methods of randomisation and/or allocation concealment (lack of details or poor methods used). Randomisation performed too early and early caesarean deliveries prior to intervention, favouring discontinuation.
- Downgraded (‐1) serious study limitations ‐ Two trials were high risk of bias for blinding (performance and detection bias) and one was at unclear risk. Some trial reports lacked details of methods of randomisation/allocation concealment.
- Downgraded (‐1) serious study limitations ‐ Two trials were high risk of bias for blinding (performance and detection bias) and the other two were at unclear risk. One trial was high risk for attrition bias and one trial was unclear risk. Two trials were high risk for selection bias and the other trial was at unclear risk. Some trial reports lacked details of methods of randomisation/allocation concealment.
- Downgraded (‐1) serious study limitations (risk of bias). The trial reported lack of details relating to randomisation or allocation concealment, was at a high risk of detection and performance bias, and high risk of other bias.
- Downgraded (‐1) for serious imprecision (wide confidence intervals, evidence based on a single study with small sample size)
- Downgraded (‐1) for indirectness of evidence (indirect outcome measure)
- Downgraded (‐1) for serious study limitations. All trials at high risk of bias for selection and reporting bias. Two trials unclear allocation concealment and one trial unclear for sequence generation. One trial had high risk for attrition bias and one trial unclear. One trial high risk for other bias and one trial unclear.
- Downgraded (‐1) for imprecision (substantial heterogeneity in the analysis).
- Downgraded (‐1) for heterogeneity (I2 = 90%) leading to exclusion of one trial from the meta‐analysis.
- Downgraded (‐1) for serious study limitations (risk of bias) ‐ The majority of trials reports lacked details about allocation concealment (unclear risk) and one trial was at a high risk of bias. One trial was also unclear about methods of sequence generation. Three trials were at a high risk of performance and detection bias and two trials were at an unclear risk. Two trials were at a high risk of attrition bias, three trials were at an unclear risk. Nearly all trials were at a high risk of selective reporting bias and most trials were at an unclear risk of other bias.
- Downgraded (‐1) for serious study limitations ‐ one trial was unclear for sequence generation and two were unclear for allocation concealment. One trial was high risk for allocation concealment. Two trials were unclear for performance and detection bias and one trial was unclear. One trial was high risk for attrition bias and one was unclear. Two trials were high risk for selective reporting bias and one was unclear. One trial was high risk for other bias and two were unclear.
- Downgraded (‐1) due to serious imprecision ‐ low events, wide confidence intervals.
Background
Description of the condition and the intervention
Oxytocin (Syntocinon® ‐ Orphan Biovitrum) was first synthesised in 1954 (Hertog 2001). Since then it has become one of the most widely used medications for induction of labour (Simpsons 2009), often in combination with prostaglandins, Foley catheter and/or artificial rupture of membranes. It is used in approximately one in four labouring women (Oscarsson 2006; Selin 2009). It is usually administered intravenously with the dose titrated against the contraction strength and frequency.
Despite this titration, a common complication of its use is uterine tachysystole, more than five contractions in 10 minutes averaged over a period of 30 minutes (ACOG 2009). When the contractions are too frequent or too long, the relaxation period between them is too short, which may lead to lack of sufficient oxygen supply for the child causing abnormal fetal heart rate (FHR), which may require immediate delivery by caesarean or instrumental delivery (Oláh 2015).
The use of oxytocin almost doubles the probability of uterine tachysystole combined with FHR changes and thereby leads to an increased risk of immediate intervention (Bakker 2007). Oxytocin use also increases the risk of uterine rupture (Cahill 2008) and postpartum haemorrhage (Grotegut 2011). A pilot study suggests oxytocin increases the risk of unsuccessful breastfeeding (Fernandez 2012). One study found an inverse association between oxytocin use and urinary stress incontinence (Svare 2014). The use of oxytocin during labour may result in several maternal adverse effects including hypotension, tachycardia (heart rate > 100 beats per minute), arrhythmias (irregular heart rhythm), nausea, vomiting, headache, and flushing (Dansereau 1999). Furthermore, prolonged duration of oxytocin use decreases the efficacy of labour induction and increases the rate of maternal complication rates, due to down‐regulation or de‐sensitization of oxytocin receptors in the myometrium (Phaneuf 2000). Rarely, excessive dosage of oxytocin may cause water retention, hyponatraemia, myocardial ischaemia, seizures, and coma (Oscarsson 2006).
Dahlen 2013 has questioned whether oxytocin usage could have long‐term adverse effects on behavioural development of children, since oxytocin crosses the placenta barrier (Malek 1996).
There is a growing concern about the use of oxytocin infusion for labour induction and acceleration (Oláh 2015). According to a survey of liability cases, approximately 50% of these claims affecting maternity services involve alleged misuse of oxytocin (Clark 2008). For these reasons, oxytocin is considered one of the 12 most dangerous, medications used in hospital (ISMP List 2014).
One way of a safer oxytocin use could be to stop the infusion at the active phase of labour instead of continuing the infusion until delivery of the placenta. However, there is not a consensus on this (Vlachos 2015).
The comparison of interest is continued oxytocin (standard care) versus discontinued oxytocin or placebo once the active phase of labour is established. One definition of the active phase of labour is a combination of regular contractions with a cervical examination that confirms complete effacement and dilatation of at least 6 cm (ACOG 2014). Another definition of the active phase of labour is regular painful contractions combined with progressive cervical dilatation from 4 cm (NICE 2017).
We will use the definition of active phase of labour as described by the trial authors.
Resumption of oxytocin will be accepted in the case of slow progression of labour, as defined by the trial authors. The trial authors' definitions of oxytocin solutions and dosages will be used.
How the intervention might work
Trials have been published supporting experiences from clinical practice that labour progresses when the active phase of labour is established without further oxytocin stimulation, thereby reducing maternal and neonatal complications (Daniel‐Spiegel 2004). Discontinuation of oxytocin infusion once active labour is achieved enables the possibility for a more natural childbirth mechanism after an artificial induction of labour with synthetic oxytocin. Discontinuation may reduce the risk of uterine tachysystole and the concomitant risk of reduced fetal oxygen supply causing fetal distress and the need for immediate delivery (Saccone 2017; Vlachos 2015). Thus, in theory, discontinuation of oxytocin infusion during the active phase could be more effective and safer than conventional continuous administration/infusion.
Why it is important to do this review
The extent of oxytocin use and the potential risk of both maternal and fetal adverse effects of oxytocin emphasise the need for determining the optimal oxytocin regimen during induction of labour. The potential adverse effects of oxytocin are correlated with considerable socio‐economic and human costs (Clark 2008). Reducing the duration of oxytocin stimulation during labour will probably reduce the risk of acute caesarean delivery, the number of newborn with asphyxial sequelae and the number of maternal and neonatal adverse events during labour and delivery, and this in turn will reduce the risk of expensive litigation.
Trials comparing continuation or stopping oxytocin are difficult to run and analyse because it is often difficult to randomise women at the point in active labour at which stopping is an option. Recruitment and randomisation earlier in labour, either at the point of induction or when oxytocin is begun to accelerate labour, is more practicable. But some women allocated to "stopping in the active phase" will never reach that point because they will be delivered by caesarean earlier, and others will not have the oxytocin stopped because they proceed through the active phase too quickly. To ensure that these "unavoidable non‐compliance" cases are recorded, it is even more important than usual, that reports of trials of this intervention have a detailed CONSORT participant flow diagram.
Objectives
To assess whether birth outcomes can be improved by discontinuation of intravenous (IV) oxytocin, initiated in the latent phase of induced labour, once active phase of labour is established.
Methods
Criteria for considering studies for this review
Types of studies
Randomised control trials (RCTs) comparing continuous intravenous (IV) oxytocin infusion with discontinued administration of oxytocin in the active phase of induced labour. Cluster‐RCTs were eligible for inclusion but none were identified.
Quasi‐randomised RCTs and trials using a cross‐over design are not eligible for inclusion in this review. Abstracts without full‐text publication were only to be included if the corresponding author could provide us with the necessary data.
After a review of the identified studies, we discovered that many studies had no CONSORT participant flow diagram, and we could not confidently rule out the possibility of post‐randomisation exclusions.
Types of participants
Pregnant women receiving oxytocin stimulation for induction of labour during the latent phase of labour. No exclusion criteria were applied in terms of parity, maternal age, ethnicity, co‐morbidity status, labour setting, gestational age, or prior caesarean delivery.
Types of interventions
Intravenous oxytocin stimulation replaced by saline and/or discontinued when the active phase of labour was established (defined by the individual authors) versus continued IV oxytocin stimulation until delivery, regardless of the oxytocin dosage regimen used.
Studies comparing different dosage regimens or pulsatile oxytocin dosage regimens were not included in this review.
Types of outcome measures
Primary outcomes
Caesarean delivery
Secondary outcomes
Maternal
Pre‐specified in our protocol
Duration of the active phase of labour (as defined by the trial authors)
Postpartum haemorrhage (as defined by the trial authors)
Uterine tachysystole combined with abnormal fetal heart rate (FHR)
Uterine tachysystole
Chorioamnionitis
Maternal mortality
Maternal admission to intensive care unit
Use of analgesia and epidural during labour
Uterine rupture/scar dehiscence
Episiotomy
Third‐ or fourth‐degree perineal tear
Retained placenta/manual removal
Postnatal blood transfusion
Length of hospital stay
Breastfeeding (any, as defined by the trial authors)
Maternal satisfaction
Not pre‐specified in our protocol
Vaginal instrumental delivery
Caesarean delivery after the active phase of labour has begun
Fetal
Pre‐specified in our protocol
Intrapartum fetal death
Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs)
Apgar score at five minutes below seven
Acidotic cord gasses at birth (arterial umbilical pH < 7.10)
Need for intubation within the first 24 hours postpartum
Neonatal morbidity (e.g. seizures, birth asphyxia, neonatal encephalopathy, infection requiring antibiotics), excluding congenital malformations
Neonatal death within the first 24 hours postpartum
Childhood disability
Not pre‐specified in our protocol
Neonatal admission to the neonatal intensive care unit (NICU)
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (31 January 2018).
The Register is a database containing over 24,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched Scopus, ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (23 January 2018) (see Appendix 1 for search terms used).
Searching other resources
We searched the reference lists of retrieved studies and contacted study authors. We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
We identified and removed duplicate reports of individual trials by integrating the search results with a reference management package (www.covidence.org). Review author Sidsel Boie and Jannet Bakker independently assessed for inclusion all the potential studies identified; the titles and abstracts were assessed and exclusions made. We obtained the full text of potentially applicable studies, linked multiple communications relating to the same study, and assessed the full text against the eligibility criteria for inclusion in the review. We resolved any disagreement at each stage through discussion and, when required, we consulted the rest of the review team. The review authors were not blinded to the study details such as the trial authors' names, institution, and journal of publication or results during the study selection process. We contacted the investigators of potentially eligible studies to provide supplementary information to assist with the final decision regarding the studies inclusion in the review. In this way, we were able to obtain further information from three trials (Bor 2016; Chopra 2015; Diven 2012). We also asked the study authors to provide us with unpublished data, if necessary. We described the excluded study and the primary reason for exclusion in the review.
Data extraction and management
From the eligible studies, Sidsel Boie and Jannet Bakker extracted data using a pre‐designed data form. Bor 2016 was classified by Jannet Bakker, BY Van Der Goes and Jim Thornton. We resolved discrepancies through discussion and, when required, we consulted the review team. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above‐mentioned outcomes was unclear, we attempted to contact authors of the original reports to provide further details.
For each study, the following data were extracted: setting, dates, sample size, exclusion criteria, inclusion criteria, trial dates, cervical dilatation at the time of establishing the intervention, oxytocin dosage regimens used, recruited proportion, study completion rates, outcome measurements, a list of adjusted confounders sources of funding, and trialists' declarations of interest.
Assessment of risk of bias in included studies
Sidsel Boie, Jannet Bakker and Jim Thornton independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving the review team.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were performed blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for the different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or was supplied by the trial authors, we included the missing data in the analyses undertaken.
We assessed the methods as:
low risk of bias (no more than 10% of missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
high risk of bias (trials which were unregistered or registered after completion);
low risk of bias (trials registered during the recruitment phase, so long as the infusion was double‐blind, i.e. so long as we could be confident that interim results had not been inspected);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this review, we assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparison of discontinued IV oxytocin versus continued IV oxytocin.
Caesarean delivery
Maternal: uterine tachysystole combined with abnormal FHR
Maternal: chorioamnionitis
Maternal: use of analgesia and epidural during labour
Intrapartum CTG abnormalities (suspicious/pathological CTG)
Apgar score at five minutes below seven
Neonatal: acidotic cord gasses at birth (arterial umbilical pH < 7.10)
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create a ’Summary of findings’ table. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence were downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CIs).
Continuous data
For continuous data, we used the mean difference as the outcome (duration of the active phase) is measured in the same way between trials.
Unit of analysis issues
Cluster‐randomised trials
No cluster‐randomised trials were identified.
For future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their standard errors (SEs) using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) by using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials and other unit of analysis issues
None of the included studies were cross‐over or had more than two intervention groups.
Future updates: it is unlikely that cross‐over designs will be a valid study design for Pregnancy and Childbirth reviews, and so will be excluded.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we included all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number of women randomised minus any participants whose outcomes were known to be missing.
However, for the outcome 'caesarean delivery after the active phase of labour had begun', it is difficult to know how to deal with participants who delivered post randomisation but before reaching the active phase. Such participants not only failed to get their allocated intervention, but could not have done so because they never became eligible. We therefore present the results of this analysis in two ways:
using the denominator of the whole group as randomised (Analysis 1.10);
using women who reached the active phase as the denominator (Analysis 1.11).
1.10. Analysis.
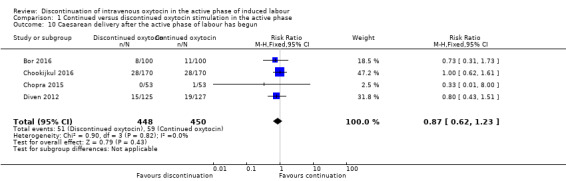
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 10 Caesarean delivery after the active phase of labour has begun.
1.11. Analysis.
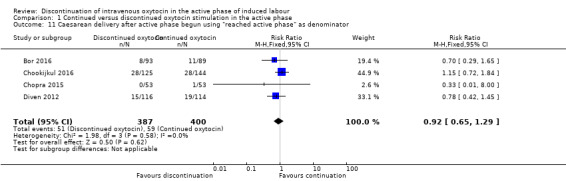
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 11 Caesarean delivery after active phase begun using "reached active phase" as denominator.
Assessment of heterogeneity
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a T² was greater than zero, or if there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Since nine trials contributed data for the meta‐analysis of the primary outcome, we were unable to investigate reporting biases (such as publication bias) using a funnel plot. However there is a risk of the small‐study effect and publication bias, many of the included trials are small and favours discontinuation.
In future updates, we will investigate reporting bias using a funnel plot and assess it visually. In case the visual assessment suggests asymmetry, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out the statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data unless there was significant heterogeneity. We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials examined the same intervention, and the trials’ populations and methods were judged to be sufficiently similar. Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or where substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. In future updates, if the average treatment effect was not clinically meaningful, we did not combine trials.
Subgroup analysis and investigation of heterogeneity
None of the predefined subgroups were reported in the trials, therefore none of the prespecified subgroup analysis were made.
In future updates, if we identify substantial heterogeneity, we will use subgroup and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, we will use random‐effects analysis to produce it.
We will carry out the following subgroup analyses.
Parity: nulliparous women versus multiparous women.
Gestational age: term (> 37 weeks) versus preterm (< 37 weeks).
Previous caesarean delivery: women with no prior caesarean section versus women with repeat caesarean section.
Subgroup analysis is planned to be restricted to the review's primary outcome.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned to conduct sensitivity analyses if there was substantive heterogeneity observed for the primary outcome. We did not observe any substantive heterogeneity for the primary outcome and therefore no sensitivity analyses were used. For future updates, we will undertake sensitivity analysis on any aspect of the included trials methodology that could have influenced the results of the meta‐analysis such as participant eligibility criteria for inclusion in each study, random sequence generation, and allocation concealment. Where full details of eligibility criteria are not available or where components are rated as “high risk of bias”, we will exclude the study from a repeat meta‐analysis to determine their impact on the overall intervention effect. We consider studies with a low risk of incomplete outcome data 'high quality' and will include them in the repeat analysis. We will exclude conference abstracts. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Results
Description of studies
Results of the search
See: Figure 1.
1.
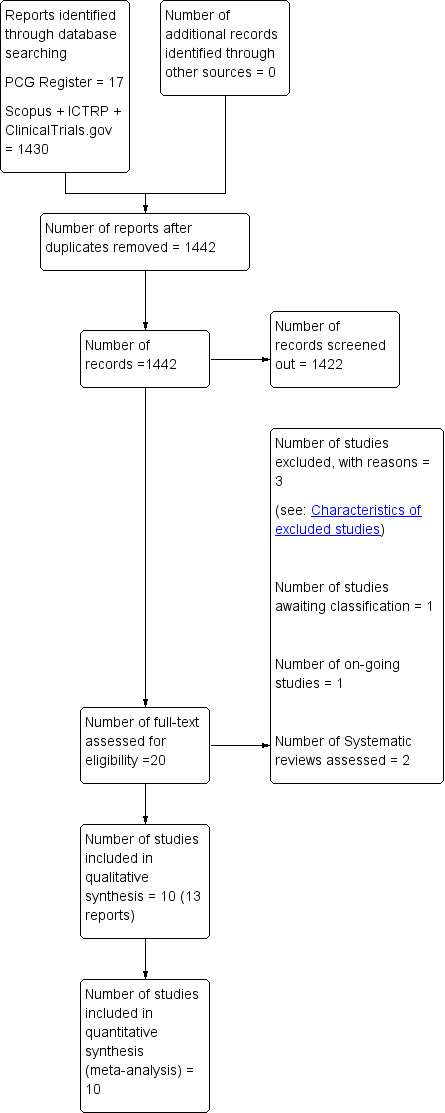
Study flow diagram.
The search of the Cochrane Pregnancy and Childbirth Group’s Trials Register retrieved 17 trial reports. The additional search of Scopus, ClinicalTrials.gov and ICTRP retrieved 1430 hits. The total number of hits (without duplicates) was 1442; 1422 were screened out and 20 were further examined for this review. We included 10 studies (13 reports) and excluded three studies. We found one ongoing trial (NCT02553226) and two published systematic reviews (Saccone 2017; Vlachos 2015). Figure 1 shows the assessment process for selection of the studies.
All first and last authors of the original reports were requested to provide additional details. We were able to obtain additional information from three trials (Bor 2016; Chopra 2015; Diven 2012).
One study is awaiting classification (Abdelhamid 2010). We are trying to obtain a full‐text copy of this trial.
Included studies
Design
The included 10 trials were all reported to be randomised controlled trials. Two were reported as double‐blinded (Chookijkul 2016; Ustunyurt 2007). One trial reported blinding of the participants (Chopra 2015) but not personnel.
Sample sizes
The trials had sample sizes of 100 to 342 women. Outcome data were reported for 1888 women giving birth to 1888 live‐born children.
Setting
Two trials were conducted in Europe; in Denmark (Bor 2016) and in Greece (Rashwan 2011). The other trials originated from the USA (Diven 2012), Bangladesh (Begum 2013), Iran (Bahadoran 2011), India (Chopra 2015), Thailand (Chookijkul 2016), Israel (Daniel‐Spiegel 2004), and Turkey (Ozturk 2015; Ustunyurt 2007).
The trials were conducted between February 1998 and January 2016.
Bahadoran 2011 recruited participants in the period April 2009 to September 2009. Begum 2013 conducted the trial from June 2004 to December 2004. Bor 2016 recruited participants between May 2009 and May 2012. Chookijkul 2016 enrolled the trial in the period February 2014 to January 2015. Chopra 2015 conducted the trial during 2009 and 2010. Daniel‐Spiegel 2004 recruited participants between February 1998 and February 2000. Diven 2012 conducted the trial between February 2009 and September 2011. Ozturk 2015 conducted the trial between April 2005 and September 2005. Rashwan 2011 recruited participants in the period September 2008 to June 2010. Ustunyurt 2007 conducted the trial between October 2004 and August 2005.
Participants
All trials enrolled women with a singleton pregnancy. One trial (Rashwan 2011) included pregnancies after 35 gestational weeks, whereas the all others included term pregnancies. Women with a history of prior caesarean delivery were excluded in five trials (Begum 2013; Chookijkul 2016; Chopra 2015; Diven 2012; Ustunyurt 2007), and women receiving cervical ripening with, e.g. prostaglandin or Foley catheter were excluded in one trial (Bahadoran 2011). Mean maternal age was 22 to 31 years (10 trials, 1888 women) (Table 2). The proportion of nulliparous women was 45% to 68% (five trials, 1258 women) (Table 3). Pre‐pregnancy body mass index (BMI) was 22 m2/kg to 32 m2/kg (seven trials, 1544 women) (Table 4). Mean birthweight was 2850 g to 3705 g, (eight trials, 1640 women) (Table 5).
1. Maternal age.
| Trial ID | ||
| Discontinuation mean ±SD | Continuation mean ±SD | |
| Bahadoran 2011 | 25.1 (±3.1) | 25.9 (±3.3) |
| Begum 2013 | Not reported | |
| Bor 2016 | 26 (23 to 30)* | 31.0 (27 to 35)* |
| Chookijkul 2016 | 24.9 (±6.6) | 25.5 (±6.2) |
| Chopra 2015 | 25.9 years (18 to 37)** | 26.5 (20 to 40)** |
| Daniel‐Spiegel 2004 | 30.1 (±6.1) | 29.5 (±6.1) |
| Diven 2012 | 27.7 (±5.7) | 27.1 (±5.6) |
| Ozturk 2015 | 23 (±3.0) | 22 (±3.0) |
| Rashwan 2011 | 24.1 (±3.9) | 23.2 (±3.2) |
| Ustunyurt 2007 | 24.6 (±4.9) | 24.8 (±5.2) |
* Median (IQR)
** Mean (min to max)
2. Parity.
| Trial ID | ||||
| Discontinuation | Continuation | |||
| Nulliparous % (n) | Parous % (n) | Nulliparous % (n) | Parous % (n) | |
| Bahadoran 2011 | Not reported | |||
| Begum 2013 | Not reported | |||
| Bor 2016 | 46% (n = 46) | 54% (n = 54) | 45% (45) | 55% (55) |
| Chookijkul 2016 | 59.4% (n = 101) | 40.6% (n = 69) | 58.8% (100) | 41.2% (70) |
| Chopra 2015 | 51% (n = 26) | 49% (n = 25) | 47% (25) | 53% (28) |
| Daniel‐Spiegel 2004 | Not sufficient reported | |||
| Diven 2012 | 51.2% (n = 64) | 48.8% (n = 61) | 49.6% (63) | 50.4% (64) |
| Ozturk 2015 | Not reported | |||
| Rashwan 2011 | Not sufficient reported | |||
| Ustunyurt 2007 | 67.9% (n = 114) | 32.1% (n = 54) | 63.2% (110) | 36.8% (64) |
3. Pre‐pregnancy BMI.
| Trial ID | ||
| Discontinuation mean ±SD | Continuation mean ±SD | |
| Bahadoran 2011 | 22 ±2.4 | 22 ±2.1 |
| Begum 2013 | Not reported | |
| Bor 2016 | 26 (23 to 30)* | 25 (22 to 32)* |
| Chookijkul 2016 | 26.8 ±4.1 | 27.3 ±4.8 |
| Chopra 2015 | 25.7 (20.5 to 31)** | 27.7 (23.8 to 30.6)** |
| Daniel‐Spiegel 2004 | Not reported | |
| Diven 2012 | 31.0 ±7.4 | 31.7 ±7.3 |
| Ozturk 2015 | Not reported | |
| Rashwan 2011 | 26.3 ±3.9 | 25.7 ±3.2 |
| Ustunyurt 2007 | 23.0 ±3.4 | 23.2 ±3.3 |
* Median (IQR)
** Mean (min to max)
4. Birthweight.
| Trial ID | ||
| Discontinuation mean ±SD | Continuation mean ±SD | |
| Bahadoran 2011 | 3198 ±288 | 3172 ±266 |
| Begum 2013 | 3340 ±40 | 3400 ±40 |
| Bor 2016 | 3705 (3347 to 4000)* | 3600 (3212 to 4055)* |
| Chookijkul 2016 | 3128.0 ±403.9 | 3159.4 ±399.7 |
| Chopra 2015 | 2850 (1800 to 4025)** | 2870 (1800 to 3800)** |
| Daniel‐Spiegel 2004 | 3391 ±513 | 3299 ±525 |
| Diven 2012 | 3475 (2715–4650) *** | 3475 (2345–4495)*** |
| Ozturk 2015 | Not reported | |
| Rashwan 2011 | Not reported | |
| Ustunyurt 2007 | 3289 ±388 | 3242 ±397 |
* Median (IQR)
** Mean (min to max)
*** Median (range)
Interventions and comparisons
The intervention was assigned at the active phase of labour in all trials. The women were randomised to one of two intervention arms: continued or discontinued oxytocin stimulation (no infusion or placebo‐isotonic saline). Women were randomised when admitted for labour induction (Diven 2012), when the oxytocin stimulation was initiated (Bahadoran 2011; Begum 2013; Bor 2016; Daniel‐Spiegel 2004), or at the active phase of labour (Chopra 2015). Four trials did not report when the women were randomised (Chookijkul 2016; Ozturk 2015; Rashwan 2011; Ustunyurt 2007).
The definition of the active phase was based on frequency of contractions (three to five per 10 minutes or regular) and cervical dilatation (4 cm to 6 cm) (10 trials). One trial did not define the active phase of labour (Rashwan 2011) (Table 6).
5. Definition of active phase of labour.
| Trial ID | ||
| Cervix dilatation | Uterine contractions | |
| Bahadoran 2011 | 4 cm and 80 % effacement or 5 cm without considering effacement |
poor defined: Effective contractions |
| Begum 2013 | ≥ 5 cm | 3‐4 per 10 minutes |
| Bor 2016 | ≥ 5 cm | 3‐5 per 10 minutes |
| Chookijkul 2016 | ≥ 4 cm | poor defined: Good contractions |
| Chopra 2015 | 4‐6 cm | 3‐5 per 10 minutes |
| Daniel‐Spiegel 2004 | ≥ 5 cm | 3‐5 per 10 minutes |
| Diven 2012 | ≥ 4 cm. | 3‐5 per 10 minutes |
| Ozturk 2015 | ≥ 5 cm | 3‐5 per 10 minutes |
| Rashwan 2011 | not defined | 3‐5 per 10 minutes |
| Ustunyurt 2007 | ≥ 5 cm | 3‐4 per 10 minutes |
The concentration of the oxytocin infusion was 5 IU (International Units) diluted in 500 mL isotonic saline in all trials. The initial dose, the incremental dose, and the time for adjusting the dose varied, and for all trials the procedure corresponded to a low‐dose regimen (Budden 2014).
Oxytocin infusion was discontinued prior to birth in 4.6% to 8.7% of the women with continuous oxytocin stimulation, due to non‐reassuring fetal heart rate (FHR). The drip was re‐started in 3.8% to 46.4% of the women with discontinued oxytocin stimulation, due to lack of progression (Table 7).
6. Non‐compliance as per protocol.
| Trial ID | ||
| Discontinued group (had oxytocin restarted due to lack of progression |
Continued group (had oxytocin discontinued due to non‐reassuring FHR) |
|
| Bahadoran 2011 | Not reported | Not reported |
| Begum 2013 | 4% (n = 2) | 6% (n = 3) |
| Bor 2016 | 36% (n = 36) | Not reported |
| Chookijkul 2016 | 9.4% (n = 16) | 8.8% (n = 15) |
| Chopra 2015 | 3.8% (n = 2) | Not reported |
| Daniel‐Spiegel 2004 | 7.6% (n = 4) | 7.6% (n = 4) |
| Diven 2012 | 46.4% (n = 58) | Not reported |
| Ozturk 2015 | Not reported | Not reported |
| Rashwan 2011 | Not reported | Not reported |
| Ustunyurt 2007 | 6.5% (n = 11) | 4.6% (n = 8) |
Outcomes
Primary outcome ‐ caesarean delivery
Nine of the included trials reported this review's primary outcome (Begum 2013; Bor 2016; Chookijkul 2016; Chopra 2015; Daniel‐Spiegel 2004; Diven 2012; Ozturk 2015; Rashwan 2011; Ustunyurt 2007).
Maternal secondary outcomes
Duration of active phase of labour
Eight trials contributed with data for the duration of the active phase of labour (Bahadoran 2011; Bor 2016; Chopra 2015; Daniel‐Spiegel 2004; Diven 2012; Ozturk 2015; Rashwan 2011; Ustunyurt 2007). Three trials reported the duration by median and interquartile range (IQR) (Bor 2016; Chopra 2015; Diven 2012). The other five trials reported the duration by mean and SD (Bahadoran 2011; Daniel‐Spiegel 2004; Ozturk 2015; Rashwan 2011; Ustunyurt 2007). Bor 2016, Chopra 2015 and Diven 2012 kindly provided unpublished data on mean duration of labour, and these data were included in the meta‐analysis.
The reported mean duration of the active phase of labour varies greatly between trials and some trials reported large standard deviations (SDs), suggesting that the numbers are not normally distributed.
Postpartum haemorrhage (as defined by author)
Postpartum blood loss of 500 mL or more was reported in five trials (Begum 2013; Bor 2016; Chookijkul 2016; Chopra 2015; Diven 2012). Chopra 2015 kindly provided additional unpublished data to this outcome measure.
Uterine tachysystole combined with abnormal FHR
The definition varied between the studies. Data from three trials using the definition tachysystole (> 5 contractions per 10 minutes) with abnormal FHR change, were used for the meta‐analysis of this outcome (Bor 2016; Chopra 2015; Rashwan 2011).
Uterine tachysystole
We defined tachysystole as > 5 contractions per 10 minutes.
Four trials reported data for this outcome (Begum 2013; Bor 2016; Daniel‐Spiegel 2004; Ustunyurt 2007).
Chorioamnionitis
One trial reported this outcome (Diven 2012). The definition of chorioamnionitis was maternal temperature ≥ 100.4o F and maternal tachycardia, fetal tachycardia, or both.
Use of analgesia and epidural during labour
Epidural use was reported in four trials (Bor 2016; Diven 2012; Daniel‐Spiegel 2004; Rashwan 2011). No other type of analgesia during labour was reported. Epidural use may differ substantially between countries and birth sites due to different national guidelines and local practices.
None of the trials described if the epidural was established prior to the intervention or not.
Howver, one might assume that it was standard procedure to have an epidural in Rashwan 2011, since all participants in the continuation arm received one. We assume that due to the open‐labelled design, the staff and women in the discontinuation arm agreed upon 'wait to see' if it is necessary.
Third‐ and fourth‐degree perineal tear
One trial (Bor 2016) reported data for this outcome.
Other maternal secondary outcomes that were not pre‐specified in our protocol
Vaginal instrumental delivery
Instrumental delivery included vaginal delivery by ventouse or forceps.
This outcome was reported in five trials (Begum 2013; Bor 2016; Chookijkul 2016; Chopra 2015; Daniel‐Spiegel 2004).
Caesarean delivery after the active phase of labour has begun
One trial (Chopra 2015), performed randomisation at the time of intervention, and therefore the reported caesarean deliveries were all performed in the active phase of labour. Three trials (Bor 2016; Chookijkul 2016; Diven 2012), provided detailed CONSORT flow diagrams illustrating the number of caesarean deliveries in the active phase.
Fetal secondary outcomes
Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs)
Seven trials (Begum 2013; Bor 2016; Chookijkul 2016; Chopra 2015; Daniel‐Spiegel 2004; Rashwan 2011; Ustunyurt 2007) reported on this outcome.
The definitions of CTG abnormalities varied between studies.
Abnormalities in FHR: Begum 2013, Bor 2016, and Chookijkul 2016 defined abnormal CTG as fetal tachycardia or bradycardia, and/or minimal to absent baseline variability, and/or recurrent variable or late decelerations.
Non‐reassuring FHR pattern: Daniel‐Spiegel 2004, Rashwan 2011, Ustunyurt 2007, and Rashwan 2011 used the definition according to the American guidelines (ACOG 2009). However, two studies (Begum 2013; Chopra 2015), did not offer a detailed definition of non‐reassuring FHR pattern.
Apgar score at five minutes below seven
Four trials (Bor 2016; Chopra 2015; Diven 2012; Ustunyurt 2007) reported this outcome. Chopra 2015 kindly provided additional unpublished data for this outcome.
Arterial acidotic cord gasses at birth pH < 7.10
Four trials (Bor 2016; Chopra 2015; Diven 2012; Ustunyurt 2007) reported this outcome.
Chopra 2015 and Diven 2012 kindly provided additional unpublished data for this outcome measure.
Other fetal secondary outcomes that were not pre‐specified in our protocol
Neonatal admission to the neonatal intensive care unit (NICU)
Six trials reported this outcome (Begum 2013; Bor 2016; Chookijkul 2016; Diven 2012; Rashwan 2011; Ustunyurt 2007).
Sources of trial funding
Sources of trial funding for Bor 2016: The Central Denmark Region Committees on Health Research Foundation.
Sources of trial funding for Chookijkul 2016: Bhumibol Adulyadej Hospital Research Fund.
One trial reported having no sources of trial funding: Chopra 2015.
Information on sources of trial funding was absent in seven of the trials (Bahadoran 2011; Begum 2013; Daniel‐Spiegel 2004; Diven 2012; Ozturk 2015; Rashwan 2011; Ustunyurt 2007).
Trialists' declarations of interest
Trial authors in six of the trials reported having no interest to declare (Bahadoran 2011; Bor 2016; Chookijkul 2016; Chopra 2015; Diven 2012;Ozturk 2015).
Trial authors' declaration of interest was absent in four of the included trials (Begum 2013; Daniel‐Spiegel 2004; Rashwan 2011; Ustunyurt 2007).
Trial registration
Two trials (Bor 2016; Diven 2012), were registered prior to recruitment of participants was initiated. One trial (Chookijkul 2016), was registered after completion of the recruitment. Seven trials (Bahadoran 2011; Begum 2013; Chopra 2015; Daniel‐Spiegel 2004; Ozturk 2015; Rashwan 2011; Ustunyurt 2007) were not registered.
Excluded studies
Three studies were excluded. One trial (Pacheco 2006) did not meet the inclusion criteria; women received oxytocin for augmentation in active phase of labour. One trial was excluded since the intervention was probably not initiated when the active phase of labour was established (D'Souza 1986). The third trial was excluded because of the use of alternate weeks for the random sequence generation (Girard 2009).
Risk of bias in included studies
Allocation
Random sequence generation
We found a low risk of selection bias with regard to the random sequence generation in seven trials (Bahadoran 2011; Begum 2013; Bor 2016; Chookijkul 2016; Chopra 2015; Daniel‐Spiegel 2004; Ustunyurt 2007). Three trials did not provide information on how the random sequences were generated and were classified as having an unclear risk of bias (Diven 2012; Ozturk 2015; Rashwan 2011).
Allocation concealment
One trial was at low risk of selection bias for allocation concealment since randomisation was centralised in real time (Bor 2016). Five trials (Bahadoran 2011; Begum 2013; Chookijkul 2016; Chopra 2015; Daniel‐Spiegel 2004) used sealed opaque envelopes, but none reported that the envelopes were numbered nor any envelope losses. We therefore judged these as at uncertain risk of selection bias for allocation concealment. Two trials did not adequately report the methods used to conceal allocation, thus we considered the risk to be unclear (Diven 2012; Rashwan 2011). Two trials had a high risk of bias because they used a closed box (Ozturk 2015) or open random allocation (Ustunyurt 2007) to allocate the women.
Blinding
Two trials (Chookijkul 2016; Ustunyurt 2007) were at low risk of performance and detection bias. The trials reported being double‐blinded with referral to staff, participants, and outcome assessor. The intervention in both arms included an intravenous (IV) infusion (oxytocin or placebo). The infusion was prepared by a third person (Ustunyurt 2007) or by a pharmacist (Chookijkul 2016).
Five trials (Begum 2013; Bor 2016; Daniel‐Spiegel 2004; Diven 2012; Ozturk 2015) were conducted as open‐label and were at high risk of performance and detection bias.
Bahadoran 2011 was at high risk of detection bias, duration of labour stages is subjective and time from randomisation to birth was not reported.
Three trials had an unclear risk of performance bias, as they reported only blinding of the participants (Chopra 2015), or did not provide sufficient information on their blinding procedure (Bahadoran 2011; Rashwan 2011).
Incomplete outcome data
Three trials (Bor 2016; Daniel‐Spiegel 2004; Diven 2012) were at low risk of attrition bias because of low (less than 10%) and balanced incomplete outcome data. Four trials were at high risk of attrition bias because of post‐randomisation exclusions (Bahadoran 2011; Ozturk 2015), or no reporting of missing values (Chookijkul 2016; Chopra 2015). Three trials were at unclear risk (Begum 2013; Rashwan 2011; Ustunyurt 2007).
Selective reporting
One trial (Diven 2012) had low risk of reporting bias based on the information provided in the registries and the publications. Eight trials (Bahadoran 2011; Begum 2013; Chookijkul 2016; Chopra 2015; Daniel‐Spiegel 2004; Ozturk 2015; Rashwan 2011; Ustunyurt 2007) were not registered in a trial registry, therefore the risk of reporting bias was high. One trial (Bor 2016) had unclear risk of reporting bias, since the secondary outcomes were not reported in the registered protocol.
Other potential sources of bias
One trial (Bor 2016), has low risk of other potential sources of bias although randomisation was performed when oxytocin infusion was initiated and not when continued or discontinued; the Consort flow diagram documents both caesarean births before the active phase and trial compliance with treatment allocation.
Two trials (Bahadoran 2011; Begum 2013) had high risk of other potential sources of bias due to randomisation being performed prior to intervention was due to be initiated; randomisation could be hours or even days prior to intervention.
One trial (Diven 2012) had a high risk of bias due to early termination of the study.
Six trials (Chookijkul 2016; Chopra 2015; Daniel‐Spiegel 2004; Ozturk 2015; Rashwan 2011; Ustunyurt 2007) were of unclear risk, due to insufficient detail to make judgement for other sources of bias.
Figure 2 summarises our ’Risk of bias’ assessments, which we used to assess study quality in the ’Summary of findings’ tables (see Table 1). We have also included full details of ’Risk of bias’ assessments in Figure 3.
2.
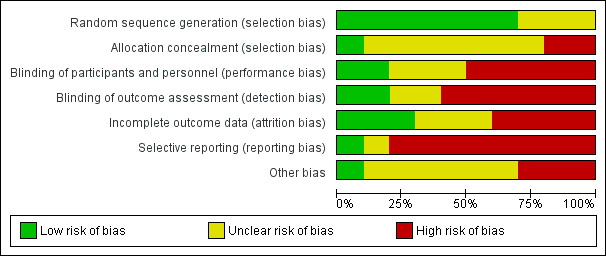
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
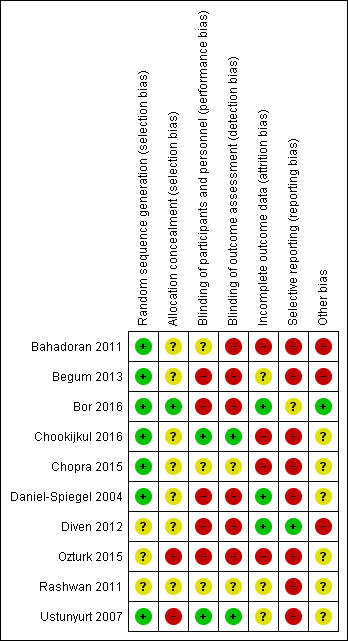
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
See Table 1.
In this review we included 10 trials involving 1888 women. We conducted one comparison and 13 meta‐analyses (see Data and analyses for further information)
Comparison 1: discontinued intravenous (IV) oxytocin versus continued IV oxytocin stimulation during the active phase of labour
Primary outcomes
Caesarean delivery
Discontinuation of oxytocin in the active phase of induced labour may reduce the caesarean delivery rate (risk ratio (RR) 0.69; 95% confidence interval (CI) 0.56 to 0.86; 9 trials, 1784 women; low level of certainty; Analysis 1.1). The quality of the evidence was downgraded from high to low due to the risk of bias (Table 1). We found no evidence of statistical heterogeneity.
1.1. Analysis.
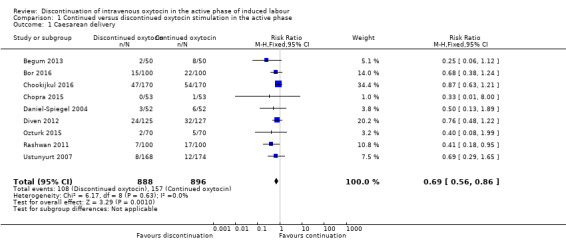
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 1 Caesarean delivery.
Secondary outcomes
Maternal
Duration of the active phase of labour (as defined by author)
We found the duration of the active phase of labour might be slightly prolonged when oxytocin was discontinued compared to continued (mean difference (MD) 26 minutes; 95% CI 5 to 46 minutes, 9 trials, 1336 women; Analysis 1.2).
1.2. Analysis.
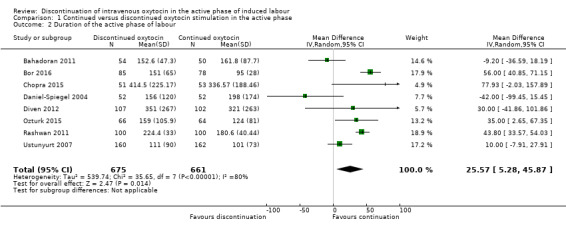
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 2 Duration of the active phase of labour.
Substantial heterogeneity was observed in this analysis and we used a random‐effects model (I² = 80%, Tau² = 539.74; Chi² test for heterogeneity P < 0.0001). This is plausible since it is rarely possible to be precise about the time of onset of the active phase of labour. It is likely that different trials used different estimates based on maternal or midwife recall of the time when contractions became regular and strong, on a particular cervical dilatation, or on what was recorded in the medical records. All these different estimates are plausibly influenced by knowledge of whether oxytocin was discontinued. The only unbiased estimation of labour duration would be the time from randomisation to delivery, but this was not reported in any trial. The apparent prolongation of labour after discontinuation should therefore be treated with caution.
Postpartum haemorrhage (as defined by the trial authors)
We found that discontinuation of oxytocin probably makes little or no difference to the risk of postpartum haemorrhage of 500 mL or more ‐ 26/496 in the discontinued group and 37/500 in the continued oxytocin group (RR 0.72, 95% CI 0.45 to 1.13, 5 trials, 996 women; Analysis 1.3). We found no evidence of substantial heterogeneity.
1.3. Analysis.
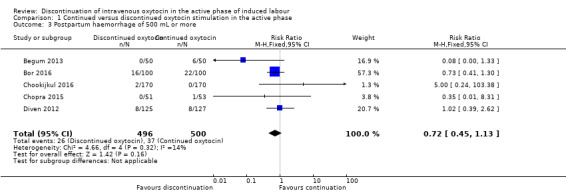
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 3 Postpartum haemorrhage of 500 mL or more.
Uterine tachysystole combined with abnormal fetal heart rate (FHR)
Tachysystole combined with abnormal FHR is probably reduced if oxytocin is discontinued (3/240 versus 23/246) (RR 0.15; 95% CI 0.05 to 0.46; 3 trials, 486 women; moderate level of certainty; Analysis 1.4). There was no substantial heterogeneity. The quality of the evidence was downgraded for risk of bias (Table 1).
1.4. Analysis.
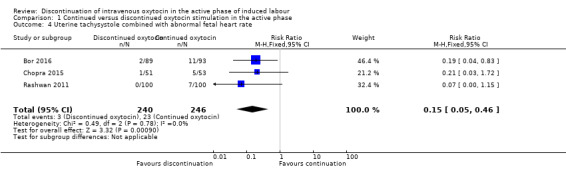
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 4 Uterine tachysystole combined with abnormal fetal heart rate.
Uterine tachysystole
The risk of uterine tachysystole is probably reduced if oxytocin is discontinued as compared to continued (RR 0.45; 95% CI 0.30 to 0.68; 4 trials, 728 women; Analysis 1.5). We found no evidence of substantial heterogeneity.
1.5. Analysis.
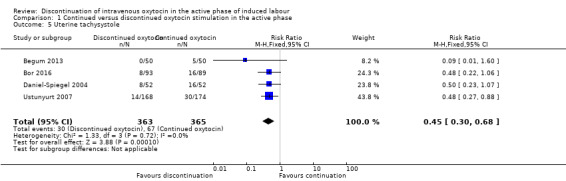
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 5 Uterine tachysystole.
Chorioamnionitis
We are uncertain whether discontinuation of oxytocin affects the risk of chorioamnionitis with data from just one small trial (252 women). There were 16/125 women with chorioamnionitis in the discontinued group and 7/127 women in the continued oxytocin group (RR 2.32, 95% CI 0.99 to 5.45, 1 trial, 252 women, very low level of certainty; Analysis 1.6). Downgrading decisions were based on risk of bias, serious imprecision and indirectness of evidence.
1.6. Analysis.
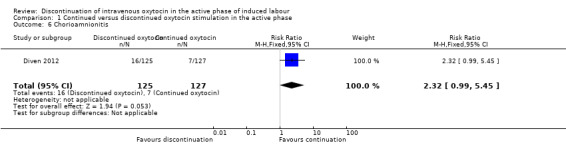
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 6 Chorioamnionitis.
Maternal mortality
No trials reported data for this outcome.
Maternal admission to intensive care unit
No trials reported data for this outcome.
Use of analgesia and epidural during labour
We decided to exclude an outliner (Rashwan 2011) from our meta‐analysis, due to extreme evidence of heterogeneity (I2 = 90%, Tau² = 2.20, Chi² test for heterogeneity P < 0.00001). The heterogeneity was only related to one trial, where every women in the continued group gets an epidural, which raises the suspicion that the two groups were not getting the same treatment as per protocol. When excluding the outliner (Rashwan 2011) from the analysis, we found no evidence of heterogeneity.
Discontinuation of IV oxytocin may have little or no impact on the use of analgesia and epidural during labour compared to the use of continued IV oxytocin (RR 1.04 95% CI 0.95 to 1.14, 3 trials, 556 women, low level of certainty; Analysis 1.7). Downgrading decisions were based on risk of bias and imprecision.
1.7. Analysis.
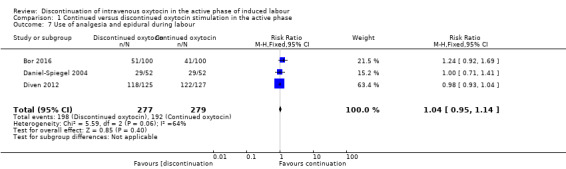
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 7 Use of analgesia and epidural during labour.
Uterine rupture/scar dehiscence
No trials reported data for this outcome.
Episiotomy
No trials reported data for this outcome.
Third‐ or fourth‐degree perineal tear
In terms of third‐ or fourth‐degree perineal tear, we only have data from one small trial (199 women) ‐ there was one women (out of 99) in the discontinued IV oxytocin group with this outcome and four women (out of 100) in the continued IV oxytocin group (RR 0.25, 95% CI 0.03 to 2.22; Analysis 1.8).
1.8. Analysis.
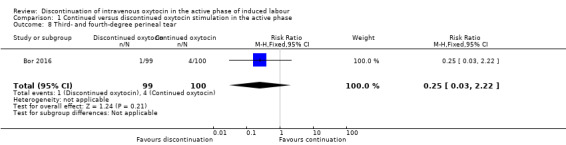
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 8 Third‐ and fourth‐degree perineal tear.
Retained placenta/manual removal
No trials reported data for this outcome.
Postnatal blood transfusion
No trials reported data for this outcome.
Length of hospital stay
No trials reported data for this outcome.
Breastfeeding (any, as defined by the trial authors)
No trials reported data for this outcome.
Maternal satisfaction
No trials reported data for this outcome.
Additional outcomes not pre‐specified in our protocol
Vaginal instrumental delivery
It is unclear whether discontinuation affects vaginal instrumental delivery ‐ there were 33 instrumental births out of the 423 women in the discontinued oxytocin group and 31 out of 425 women with instrumental vaginal birth in the continued oxytocin group (RR 1.07, 95% CI 0.67 to 1.72, 5 trials, 848 women; Analysis 1.9). We found no evidence of statistical heterogeneity.
1.9. Analysis.
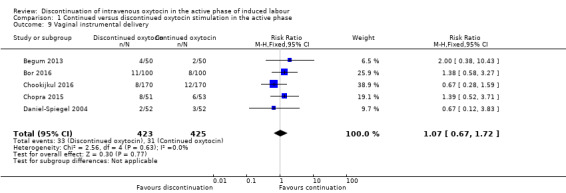
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 9 Vaginal instrumental delivery.
Caesarean delivery after the active phase of labour has begun
Discontinuation of oxytocin has an uncertain effect on the rate of caesarean delivery after the active phase has been reached (RR 0.87, 95% CI 0.62 to 1.23, 4 trials, 898 women; Analysis 1.10). Similarly, if the analysis is performed with only those women who reach the active phase included in the denominator, the results suggest there is probably little or no difference between groups (RR 0.92; 95% CI 0.65 to 1.29; 4 trials, 787 women; moderate‐certainty evidence; Analysis 1.11). Downgrading decisions were due to study limitations (risk of bias). We found no evidence of statistical heterogeneity in either of these analyses.
These results contrast with our main analysis for caesarean delivery (Analysis 1.1).
Fetal
Intrapartum fetal death
No trials reported data for this outcome.
Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs)
Discontinuation probably reduces the risk of abnormal FHR patterns (RR 0.65; 95% CI 0.51 to 0.83; 7 trials, 1390 participants, moderate level of certainty; Analysis 1.12). The quality of the evidence was downgraded for risk of bias (Table 1). We found evidence of heterogeneity (I2 = 26%, Tau² = 0.04, Chi² test for heterogeneity P < 0.23).
1.12. Analysis.
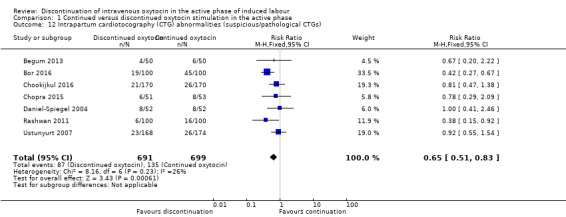
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 12 Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs).
Apgar score at five minutes below seven
Compared to continuing IV oxytocin, discontinuing IV oxytocin probably has little or no impact on the incidence of Apgar < 7 at five minutes (RR 0.78; 95% CI 0.27 to 2.21; 4 trials, 893 women, low level of certainty; Analysis 1.13). The quality of the evidence was downgraded for risk of bias and serious imprecision
1.13. Analysis.
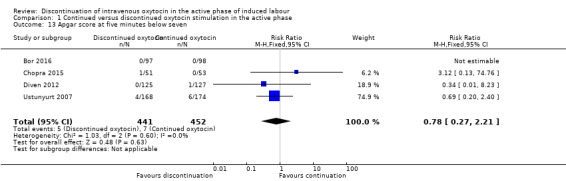
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 13 Apgar score at five minutes below seven.
Acidotic cord gasses at birth (arterial umbilical pH < 7.10)
Compared to continuing IV oxytocin, discontinuing IV oxytocin probably has little or no impact on the risk of acidotic cord gasses at birth (arterial umbilical pH < 7.10), (RR 1.03, 95% CI 0.50 to 2.13, 4 trials, 873 women, low level of certainty; Analysis 1.14). The quality of the evidence was downgraded for risk of bias and serious imprecision.
1.14. Analysis.
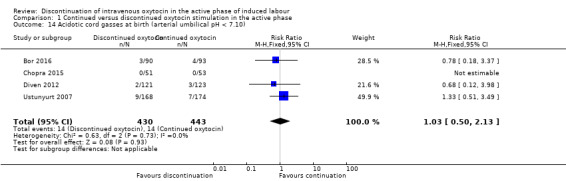
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 14 Acidotic cord gasses at birth (arterial umbilical pH < 7.10).
Need for intubation within the first 24 hours postpartum
No trials reported data for this outcome.
Neonatal morbidity (e.g. seizures, birth asphyxia, neonatal encephalopathy, infection requiring antibiotics), excluding malformations
No trials reported data for this outcome.
Neonatal death within the first 24 hours postpartum
No trials reported data for this outcome.
Childhood disability
No trials reported data for this outcome.
Additional outcomes not pre‐specified in our protocol
Neonatal admission to the neonatal intensive care unit (NICU)
It is unclear how discontinuation affects neonatal admissions to the NICU, however there were no signs that discontinuation may lead to a major increase in the risk (RR 0.75, 95% CI 0.49 to 1.13; 7 trials, 1434 newborns; Analysis 1.15). We found no evidence of heterogeneity.
1.15. Analysis.
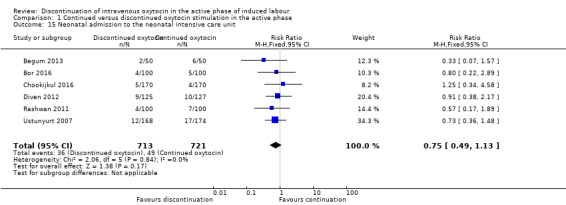
Comparison 1 Continued versus discontinued oxytocin stimulation in the active phase, Outcome 15 Neonatal admission to the neonatal intensive care unit.
Discussion
Summary of main results
This review compares the discontinuation of intravenous (IV) oxytocin versus the continuation of IV oxytocin during the active phase of induced labour. We included 10 randomised controlled trials (RCTs) involving 1888 women, with all trials to a varying degree contributing data to our meta‐analyses.
On analysis by 'intention‐to‐ treat' compared with continuation of oxytocin stimulation, discontinuation appears to reduce the caesarean delivery rate. However the, difference is largely due to reduced caesarean deliveries in the latent phase of labour, which could not plausibly be as a result of the intervention. When we restricted our analysis to women who had actually reached the active phase of labour, the effect was no longer evident.
The risk of uterine tachysystole combined with abnormal fetal heart rate (FHR) is probably reduced when oxytocin is discontinued. It is uncertain whether discontinuation increases the risk of chorioamnionitis because the certainty of this evidence is very low, and it is also uncertain how discontinuation affects the use of analgesia and epidural during labour.
There were no substantial differences between discontinued IV oxytocin and continued IV oxytocin in respect of the other secondary outcomes in this review for which we had data,including postpartum haemorrhage of 500 mL or more, third‐ or fourth‐degree perineal tear, vaginal instrumental delivery, and admission to neonatal intensive care (NICU).
Discontinuation of IV oxytocin may have little or no impact on the use of analgesia and epidural during labour compared to the use of continued IV oxytocin. Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs) are probably reduced by discontinuing IV oxytocin. Compared to continuing IV oxytocin, discontinuing IV oxytocin probably has little or no impact on the incidence of Apgar < 7 at five minutes or acidotic cord gasses at birth (arterial umbilical pH < 7.10).
Overall completeness and applicability of evidence
There is insufficient evidence to completely address the objectives of this review. The presented results relate to women with a singleton vertex pregnancy, scheduled for induction of labour, and stimulated with oxytocin corresponding to a low‐dose regimen.
Caesarean delivery (the primary outcome in this review) was reported in nine of the 10 included trials. However in terms of the secondary outcomes in this review, substantially fewer trials reported outcome data and there were no data for many of our secondary outcomes, in particular, maternal and neonatal mortality, maternal satisfaction, breastfeeding establishment and duration, and childhood disability. Some of the outcomes (i.e. chorioamnionitis and acidotic cord gasses) were only reported in one or two trials
The external validity can be questioned mainly due to two circumstances, reported caesarean delivery rate differs according to the setting from 1% (India, Chopra 2015) to 29.7% (Thailand, Chookijkul 2016), and the policy for induction of labour may also vary according to the setting (e.g. the use of medications or balloon catheter for cervical ripening, or rupture of membranes prior to oxytocin stimulation). Our review is not necessarily applicable to the discontinuation of oxytocin used for acceleration of spontaneous labour as opposed to induction.
Quality of the evidence
This review included data from 10 randomised controlled trials including 1888 women performed in nine countries from 1998 to 2016. To our knowledge, there is one ongoing trial eligible for inclusion in this review (NCT02553226). When this review is updated, we will incorporate the new findings when published.
For our primary outcome, caesarean delivery rate, we found high consistency. However, we consider the risk of selection bias (random sequences generation and allocation bias) and attrition bias likely to alter the results (Figure 2; Figure 3). Two trials were double‐blinded, and together they weighted approximately 42% of the risk estimate of the primary outcome. The other trials were not blinded, hence the proportion of information from studies at high risk of bias is sufficient to affect the interpretation of the result. Seven of the nine trials in Analysis 1.1 had a high risk of reporting bias, due to no protocol registered in a trial register prior to conducting the trial, which seriously weakens confidence in the result. Furthermore, it became clear after data collection that a number of trials had randomised participants either before, or early in labour, and that many caesareans had been performed before the active phase, i.e. the caesareans were due to reasons which could not conceivably have been related to the experimental intervention. In the two largest trials there was a large imbalance in these early caesareans favouring discontinuation.
Overall, many of the included trials had design limitations and were judged to be at either high or unclear risk of bias across a number of risk of bias domains.
We examined the quality of the overall body of the evidence for the main comparison of discontinued IV oxytocin stimulation versus continued IV oxytocin stimulation in the active phase of labour using GRADE. Our GRADE assessments ranged from very low certainty to moderate certainty. Downgrading decisions were based on study limitations, imprecision and indirectness ‐ see (Table 1).
Our findings of reduced uterine tachysystole with FHR changes with IV oxytocin discontinuation are highly plausible. However, the effects on other outcomes should be interpreted with caution.
Potential biases in the review process
We made every attempt to minimise bias. We searched multiple databases without language or date restrictions to limit bias by identifying all relevant studies. We based our review on published literature, but did not systematically search for grey literature. For all included trials, we made contact with authors to seek clarification or further information. Three of 11 authors responded adequately. Furthermore, we were unable to retrieve the full text of one identified abstract (Abdelhamid 2010).
Two review authors independently appraised studies for inclusion, and extracted data in order to minimise bias. We attempted to use a systematic and transparent process to assess the quality of the evidence relating to specific outcomes and to produce ’Table 1.
Agreements and disagreements with other studies or reviews
Our overall conclusion questions whether discontinuation of IV oxytocin is associated with a possible reduction in the risk of caesarean section among women who reach the active phase of labour and further analysis suggests this may be an artefact of poor study design. This conclusion is in contrast with other non‐Cochrane systematic reviews on this topic.
The first (Vlachos 2015), included seven trials also included in the present review (Bahadoran 2011; Begum 2013; Daniel‐Spiegel 2004; Diven 2012; Ozturk 2015; Rashwan 2011; Ustunyurt 2007). We included an additional three trials. However, the review by Vlachos 2015 did not analyse caesarean delivery among women who reached the active phase of labour.
The second review by Saccone 2017, included nine trials (Bahadoran 2011; Begum 2013; Bor 2016; Chopra 2015; Daniel‐Spiegel 2004; Diven 2012; Ozturk 2015; Rashwan 2011; Ustunyurt 2007 that are also included in our review, but also included the Chookijkul 2016 trial. Again, the Saccone 2017 review did not analyse caesarean delivery among women who reached the active phase of labour. We note that for abnormal FHR, Saccone 2017 appeared to have used different data in three trials (Bor 2016; Chopra 2015; Diven 2012). The data used from Bor 2016 appears to be data from a row above the correct data in table 2 of the trial report. The data from Chopra 2015 appears to be extracted from the text in the result section (second last paragraph in the section on page 4 of the trial report. Chopra 2015, kindly provided us with the data separately for this outcome. The data that Saccone 2017 used from Diven 2012 appears to be the data on the number of women who have been induced due to abnormal FHR. Diven 2012 was unable to provide the data on this outcome.
Authors' conclusions
Implications for practice.
Discontinuing oxytocin stimulation after the active phase of labour has been established may reduce uterine tachysystole with fetal heart rate (FHR) changes, and caesarean delivery, but the quality of evidence is low. When analysis was restricted to those trials that separately reported participants who reached the active phase of labour, discontinuation had little or no effect on caesarean delivery. Our findings do not necessarily apply to the discontinuation of oxytocin used for acceleration of spontaneous labour as opposed to induction.
Implications for research.
Future research studies need to account for all women randomised and in particular note those delivered before the point at which they could be eligible for the intervention (caesareans in the latent phase) or because labour was so rapid that the infusion could not be stopped in time.
Future trials should adopt the outcomes as listed in this review including neonatal and maternal mortality, maternal satisfaction, breastfeeding and longer‐term child outcomes.
Acknowledgements
Three trial authors (Bor 2016; Chopra 2015; Diven 2012) kindly provided additional information and unpublished data for the review and thereby improved the quality of the review.
We acknowledge BY Van Der Goes for assessing the trial conducted and published by some of the review authors (Bor 2016).
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of Cochrane Pregnancy and Childbirth's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms
Search terms
SCOPUS:
Oxytocin AND Labour
ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP):
oxytocin AND labo(u)r
Sidsel Boie worked with a librarian experienced in performing systematic literature searches to perform this search and the detail of the full search is documented in the study flow diagram, Figure 1.
Data and analyses
Comparison 1. Continued versus discontinued oxytocin stimulation in the active phase.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean delivery | 9 | 1784 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.56, 0.86] |
| 2 Duration of the active phase of labour | 8 | 1336 | Mean Difference (IV, Random, 95% CI) | 25.57 [5.28, 45.87] |
| 3 Postpartum haemorrhage of 500 mL or more | 5 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.45, 1.13] |
| 4 Uterine tachysystole combined with abnormal fetal heart rate | 3 | 486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.05, 0.46] |
| 5 Uterine tachysystole | 4 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.30, 0.68] |
| 6 Chorioamnionitis | 1 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.99, 5.45] |
| 7 Use of analgesia and epidural during labour | 3 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.95, 1.14] |
| 8 Third‐ and fourth‐degree perineal tear | 1 | 199 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.22] |
| 9 Vaginal instrumental delivery | 5 | 848 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.67, 1.72] |
| 10 Caesarean delivery after the active phase of labour has begun | 4 | 898 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.62, 1.23] |
| 11 Caesarean delivery after active phase begun using "reached active phase" as denominator | 4 | 787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.29] |
| 12 Intrapartum cardiotocography (CTG) abnormalities (suspicious/pathological CTGs) | 7 | 1390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.51, 0.83] |
| 13 Apgar score at five minutes below seven | 4 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.27, 2.21] |
| 14 Acidotic cord gasses at birth (arterial umbilical pH < 7.10) | 4 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.50, 2.13] |
| 15 Neonatal admission to the neonatal intensive care unit | 6 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bahadoran 2011.
| Methods | Randomised controlled trial. | |
| Participants | Inclusion: gestational age > 36 weeks, physician‐prescribed induction, age of 18 to 35 years, pre‐pregnancy BMI < 26, singleton fetus with vertex presentation and anterior oxiput, a Bishop score > 5, no use of medicine other than painkillers and antibiotics, no use of prostaglandins or other induction methods, no placenta abruption, and correct heart rate pattern. Exclusion (after randomisation): hypertonic uterus, fetal distress prior to the intervention, allergy towards the study medication, insufficient progression (< 0.5 cm cervical dilatation/hour). Setting: Iranian University Hospitals, April 2009 to September 2009 (period of inclusion). Number of included participants: 60 continued and 60 discontinued oxytocin, 10 women were excluded after inclusion. The paper contains no CONSORT flow diagram. Participants were recruited at induction but the randomisation envelopes were not opened until the in active phase (4 cm or 5 cm). It is not noted if envelopes were returned unopened if labour proceeded to rapidly to allow opening or if caesarean was done before the active phase. According to the text, 5 individuals in each group Quote: "exited from the study due to lack of progression". However, both results tables 2 and 3 include only 50 individuals in the control group and 54 in the experimental one. This suggests the 10 and 6, respectively exited. |
|
| Interventions |
5 units of oxytocin in 500 cc ringer serum. Starting dose 6 mU/minute and then increased every 30 minute by 6 mU until effective labour contractions were achieved. No available information on maximal dosage. |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Trial funding: no information. The trial was not registered. Trial authors reported no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Closed envelopes but the lack of consort flow diagram makes judging participant flow difficult. Envelopes were not reported to be numbered and there was no statement about missing envelopes. Not clear if unopened envelopes were returned to the sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The shift supervisor opened the envelope, prepared the infusion and replaced the oxytocin drip with either placebo or a new oxytocin drip. Only the shift supervisor was aware of the assigned intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessments were performed by 3 persons using a validated tool for evaluating the stages. The duration of the various stages of labour is subjective. Randomisation to birth interval, which would be unbiased, was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No information of the number of eligible women. Outcome data were missing for 10/120 women (8.3%). No flow diagram was included. |
| Selective reporting (reporting bias) | High risk | No protocol was published prior to article. No clinical outcomes were reported. |
| Other bias | High risk | Randomisation was performed when oxytocin infusion was initiated and not when continued or discontinued, and there was no Consort flow diagram. |
Begum 2013.
| Methods | Randomised controlled trial. | |
| Participants | Inclusion: women with a singleton fetus in vertex presentation at ≥ 37 weeks to 40 weeks of gestation and induction due to prolonged pregnancy (40‐42 weeks), premature rupture of membranes, or mild pre‐eclampsia/hypertension ≥ 39 weeks. Exclusion: non‐vertex presentation, previous caesarean, multiple pregnancy/hydramnios, cervical dilatation of > 3 cm on admission, congenital fetal anomaly, fetal distress on admission, estimated fetal weight > 4 kg, severe maternal disease/complication (i.e. pre‐eclampsia/eclampsia, APH, diabetes, or heart disease). Setting: Chittagong Medical College & Hospital, Bangladesh, June to December 2004 (period of inclusion). Number of included participants: 50 with continued versus 50 with discontinued oxytocin. No Consort trial flow diagram was provided. Primigravid and multiparous participants were both included and induced with different dose oxytocin regimens. But the rate of each parity was not reported. Participants were recruited prior to induction of labour and quote: "when the cervix was not ripened per vaginal prostaglandin was given first". The rate of prostaglandin use by group was not reported. |
|
| Interventions |
5 IU and 2.5 IU in 500 mL ringer lactate solution or 5% dextrose in normal saline in primigravid and multigravid patients. Starting dose 10 drops/minute. Drip was increased every half hour until there was effective contractions and 5 cm cervical dilatation was reached. Maximum dose: 20 mu/minute. |
|
| Outcomes |
|
|
| Notes | Sources of trial funding: no information provided. The trial was not registered. Information on declaration of interest is absent from the trial report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were opened before dividing the patients in 2 groups. Envelopes were not reported to be numbered and there was no statement about missing envelopes Lack of CONSORT flow diagram. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and no placebo used. Prostaglandin was administered after envelope opening but the rate by group was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on the number of eligible women. No flow diagram presented. |
| Selective reporting (reporting bias) | High risk | No protocol was published prior to article. Unregistered trial. |
| Other bias | High risk | Randomisation was performed when oxytocin infusion was initiated and not when continued or discontinued. |
Bor 2016.
| Methods | Randomised controlled trial. | |
| Participants | Inclusion: women with induction or augmentation of labour with oxytocin. Exclusion: < 18 years of age, unable to give written informed consent, cervical dilation of more than 4 cm, multiple pregnancies, more than 1 prior caesarean section, non‐vertex presentation, persistent pathological cardiotocography before oxytocin infusion, an estimated fetal weight of more than 4250 g. Setting: Regional hospital of Randers, Denmark, May 2009 to May 2012 (period of inclusion). Number of included participants: 100 women in the continued group versus 100 women in the discontinued group. |
|
| Interventions |
The oxytocin infusion (5 IU oxytocin diluted in 500 mL of isotonic saline) was initiated at 3.3 mIU/minute and increased every 20 minutes by 3.3 mIU/minute until there was 3 to 5 contractions every 10 minutes) achieved. The maximal dose of oxytocin infusion was 30 mU/minute. |
|
| Outcomes | Primary
Secondary
|
|
| Notes | Sources of trial funding: the Central Denmark Region Committees on Health Research Foundation. Trial authors declare no conflict of interest. Trial registered 22 December 2006. Non‐compliance 36% in the discontinued group. GCP‐monitored trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a computer‐generated randomisation program. |
| Allocation concealment (selection bias) | Low risk | Centralised real time randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding and no placebo used. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although there was no blinding, outcomes such as mode of delivery are objective, other outcomes are subjective and at risk of bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat principle are followed. Missing values are described (up to 10% for some outcomes). Numbers and reasons provided for each group. |
| Selective reporting (reporting bias) | Unclear risk | Protocol published on clinicaltrialregister.eu (in Danish) (2006‐006956‐36). Primary outcome: duration of labour and duration of various phases of labour. Secondary outcomes not reported in the protocol, nor on EUdract trial register. |
| Other bias | Low risk | Although randomisation was performed when oxytocin infusion was initiated and not when continued or discontinued, the Consort flow diagram documents both caesarean births before the active phase and trial compliance with treatment allocation. |
Chookijkul 2016.
| Methods | Randomised trial. | |
| Participants | Inclusion: women in the latent phase of labour and with a need for labour induction or augmentation, gestational ages of 37 to 42 weeks, cephalic presentation, estimated fetal weight < 4000 g, Bishop score > 4. Exclusion: malpresentation, previous uterine surgery, placenta previa, placental abruption, persistent non‐reassuring fetal heart rate‐pattern, PROM, active phase of labour, fetal abnormalities, severe maternal diseases (i.e. pre‐eclampsia, diabetes, HIV, heart disease). Setting: Bhumibol Adulyadej Hospital, Thailand, February 2014 to January 2015 (period of inclusion). Number of included participants: 170 in the continued group and 170 in the discontinued group. |
|
| Interventions |
When there was a cervical dilation of 4 cm, the infusion was replaced with infusion containing either oxytocin or placebo. Amniotomy was not performed prior to stimulation. No available information on infusion rate/dosage. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | The difference in caesarean rate observed in this trial was entirely in caesareans performed in the latent phase (26 versus 19). i.e. on women who had not experienced the trial intervention because labour had not proceeded that far. Among women who reached the active phase, who presumably had the oxytocin infusion stopped or not, the caesareans did not differ (28 versus 28). Discontinued group 9.4% non‐compliance (had oxytocin restarted due to lack of progression). Sources of trial funding: Bhumibol Adulyadej Hospital Research Fund. Trial authors declare no conflict of interest. Trial was registered May 03, 2015, after the trial had completed in January 2015. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Sealed and opaque envelopes, not numbered. Perfect balance (170 per group) between groups suggests no post randomisation exclusions (due to lost envelopes) and no post randomisation compliance problems, i.e. no women delivered before the envelope could be opened and the investigational infusion connected and started. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Oxytocin and placebo was prepared by 1 pharmacist who did not attend or take responsibility for attending cases. It was labelled by running numbers. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Oxytocin and placebo was prepared by 1 pharmacist who did not attend or take responsibility for attending cases. It was labelled by running numbers. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat principles were followed. Missing values are not reported. Trial registration after completion. Secondary outcomes not fully specified on trial register. Operative vaginal delivery (forceps or ventouse), cord pH, perinatal and neonatal deaths, not reported. PPH reported but not defined. Apgar scores at 1 and 5 minutes reported only as quote: "equally excellent in both groups". |
| Selective reporting (reporting bias) | High risk | No protocol was published prior to article. Trial was registered May 03, 2015, after the trial had completed in January 2015. |
| Other bias | Unclear risk | No other source of bias noted. |
Chopra 2015.
| Methods | Randomised controlled trial. | |
| Participants | Inclusion: induction of labour, singleton fetus in cephalic presentation, 36–42 weeks of gestation. Exclusion: associated medical‐surgical disorders, previous uterine scar, parity > 3, fetal major congenital malformations or intrauterine demise, persistent non‐reassuring fetal heart rate pattern. Setting: Post Graduate Institute of Medical Education and Research, Chandigarh, India 2009‐2010 (period of inclusion). Number of included participants: 51 in discontinued group versus 53 in continued group. |
|
| Interventions |
Oxytocin infusion was initiated at a rate of 3 mlU/minute and was increased every 30 minutes by 3 mlU/minute until regular contractions at a rate of 3–5 contractions/10 minutes were achieved. The maximum dose of oxytocin was 42 mu/minute. Infusion of oxytocin was incremental until 4 cm to 6 cm cervical dilation, which, along with 3–5 contractions in 10 minutes, marked the active stage of labour. At cervical dilatation of 4 cm to 6 cm, amniotomy was performed in those with intact membranes and the patients were randomised. |
|
| Outcomes |
|
|
| Notes | No funding was provided for the trial. Trial authors declare no conflict of interest. The trial was not registered. Discontinued group 3.8% non‐compliance (had oxytocin restarted due to lack of progression). Additional data on outcomes not reported in the paper were provided by the author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation programme. |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes were used. Envelopes were not numbered. No flow diagram or record of missing envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Possible blinding of participants. Participants in discontinued group receive isotonic saline when active phase is established. No blinding of personnel is described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No blinding is described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No flow diagram to illustrate allocation. No information on the number of eligible women. 2% post‐randomisation exclusion. 106 included participants. Data are reported for 104 participants (51 in discontinued group versus 53 in continued group). Missing values are not reported. Additional data on postpartum haemorrhage, need for resuscitation, incidence of neonatal respiratory distress, neonatal hypoglycaemia, Apgar scores and neonatal hyperbilirubinaemia provided by author since only reported as quote: "not significantly different between the two groups" in the publication. |
| Selective reporting (reporting bias) | High risk | No published protocol prior to article. |
| Other bias | Unclear risk | No other source of bias noted. |
Daniel‐Spiegel 2004.
| Methods | Randomised controlled trial | |
| Participants | Inclusion: induction of labour for post date pregnancy (> 42 weeks), ruptured membranes for more then 24 hours, oligohydramnios (amniotic fluid index < 5 cm), intrauterine growth restriction, diabetes, or a sporadic non‐reassuring fetal heart rate pattern. Exclusion: non‐vertex presentation, past history of more than 1 caesarean delivery, multiple pregnancies, non‐reassuring fetal heart rate before induction of labour and estimated fetal weight of more than 4250 g. Setting: Ha`Emek Medical Center, Afula, Israel from 1st February 1998 to 29th February 2000 (period of inclusion). Number of included participants: 104 women (52 continued and 52 discontinued group). The CONSORT flow diagram (fig 1) suggests that all 52 women allocated to the quote: "discontinue oxytocin in the active phase" group had oxytocin discontinued, despite all of them being recruited and randomised before 3 cm dilatation. There is no mention of any caesarean births before the active phase, nor of any rapid labour such that there was insufficient time to discontinue the oxytocin. |
|
| Interventions |
Induction of labour was started by oxytocin infusion of 1 mIU/minute (5 IU of oxytocin was diluted in 500 mL of 0.9% NaCl). The dose was increased every 20 minutes by 1 mIU/minute until regular contractions at a rate of 3–5 per 10 minutes were reached. The maximal allowed dose of oxytocin was 20 mIU/minute. In group I, infusion of oxytocin was incremental until 5 cm dilatation, and was maintained at the same level until delivery. In group II, infusion of oxytocin was discontinued when cervical dilatation reached 5 cm. |
|
| Outcomes |
|
|
| Notes | Discontinued group: 7.6% had oxytocin restarted due to lack of progression. Sources of trial funding: no information provided. Information on declaration of interest is absent from the trial report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence. |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes were used. Not numbered. No record of missing envelopes. Quote: "The envelopes were opened before dividing the patients in two groups." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No post‐randomisation exclusions. 200 assessed for eligibility. 96 did not meet inclusion criteria or refused participation. 104 were randomised. |
| Selective reporting (reporting bias) | High risk | No published protocol prior to article. The trial was unregistered. Apart from mode of delivery and birthweight, and a statement that quote: "no maternal or fetal complications were recorded", no maternal or fetal complications were reported. |
| Other bias | Unclear risk | Randomisation was performed when oxytocin was initiated at less than 3 cm. The Consort flow diagram implies that no caesarean births occurred before the active phase and that every woman who reached the active phase in the intervention group had time for the active phase to be diagnosed, for the oxytocin to be stopped and actually had the oxytocin stopped. |
Diven 2012.
| Methods | Randomised controlled trial. | |
| Participants | Inclusion: induction of labour (regardless of indication for induction, Bishop score, parity, or cervical ripening prior to stimulation), singleton gestation > 37 weeks. Exclusion: multiple gestations, previous caesarean delivery, active labour, documented fetal anomalies. Setting: Lehigh Valley Health Network, Allentown, PA, USA, February 2009 to August 2011 (trial inclusion period). Number of included participants: 252 women (125 discontinued group and 127 continued group). |
|
| Interventions |
Intravenous infusion of oxytocin, 30 IU in 500 mL of 0.9% NaCl, titrated to target 3‐5 contractions in a 10‐minute period. The continued group followed a standard institutional oxytocin protocol in which usual practice is to continue oxytocin until delivery, unless there was an indication to stop the infusion. The discontinued group had oxytocin discontinued once the patient was deemed to be in active labour by the obstetrician. Active labour was defined by the clinician’s assessment of regular uterine contractions with a cervical examination that confirmed dilatation of 4 cm. |
|
| Outcomes | Primary outcome
Secondary outcomes
Maternal
Neonatal
|
|
| Notes | Discontinued group: 24.8% never had oxytocin discontinued when they reached active phase of labour and 46.4% had oxytocin restarted due to lack of progression. Sources of trial funding: no information provided. Trial authors declare no conflict of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation was random, but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram illustrates enrolment. No post‐randomisation exclusions. Intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Protocol published on clinicaltrials.gov (NCT00957593). Primary outcome registered as mode of delivery and secondary outcome: perinatal outcome (not further defined). All pre‐specified outcomes in the article were reported. |
| Other bias | High risk | According to the power calculation, 304 participants were to be included, but recruitment was terminated at 252 participants due to low inclusion rate and high number of protocol violations (> 70% in the intervention group). |
Ozturk 2015.
| Methods | Randomised controlled trial. | |
| Participants | Inclusion: nulliparity, gestational age between 36 and 42 weeks, singleton fetus, vertex presentation, expected fetal weight < 4000 g, no contraindication for labour induction or augmentation. Exclusion: Setting: Ministry of Health Ankara Etlik Zu¨beyde Hanım Maternity and Women’s Health Training and Research Hospital, Ankara, Turkey, April 2005 to September 2005 (period of inclusion). Number of included participants: 130 (64 continued group versus 66 in discontinued group). |
|
| Interventions |
In the continued group, incremental oxytocin infusion was administered until 5 cm cervical dilatation and then was maintained at the same level until delivery. In the discontinued group, the infusion was discontinued at the onset of the active phase of labour (defined as cervical dilatation of 5 cm). The starting dose of oxytocin was 1 to 2 mIU/minute and the dose was increased by 2 mIU/minute every 15 minutes. The maximum dose for oxytocin was 40 mIU/minute until regular contractions at a rate of 3–5 every 10 minutes were achieved. The oxytocin solution was prepared with 0.9% NaCl at a 1% concentration. |
|
| Outcomes | Duration of active phase
|
|
| Notes | The trial was unregistered. Sources of trial funding: no information provided. Trial authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information provided: participants were randomly chosen. |
| Allocation concealment (selection bias) | High risk | A number was attributed to each patient and the numbers were randomly selected from a closed box to constitute the study and control groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7% post‐randomisation exclusions (70 were assigned to each group, analyses were conducted on 66 and 64 in each group). |
| Selective reporting (reporting bias) | High risk | No protocol submitted prior to trial initiation and the trial was unregistered. |
| Other bias | Unclear risk | No other source of bias noted. The timing of randomisation was not described. No Consort flow diagram was reported. |
Rashwan 2011.
| Methods | Randomised controlled trial. | |
| Participants | Inclusion: induction of labour, maternal age 20‐30 years, gestational age 36‐42 weeks, vertex presentation, Bishop score > 4, no indication for caesarean section (inadequate pelvis, cephalopelvic disproportion, persistent non‐reassuring fetal heart rate, scarred uterus), no chronic or pregnancy‐induced illness. Setting: Kasr Al‐Aini Maternity Hospital, Cairo, Egypt, 1 st of September 2008 to the 15th of June 2010 (period of inclusion). Number of included participants: 200 women (continued group 100 women (60 nulliparous and 40 multiparous) versus discontinued group 100 women (60 nulliparous and 40 multiparous)). |
|
| Interventions |
In group I, oxytocin was continued throughout labour. In group II, oxytocin was discontinued when the active phase of labour was established (not defined by the authors). Induction of labour was started in all patients using the low‐dose protocol suggested by ACOG by oxytocin intravenous drip infusion at a rate of 1 mIU/minute (5 IU of oxytocin was diluted in 500 mL of 0.9% NaCl). The dose was increased every 20 minutes by 1 mIU/minute until regular contractions at a rate of 3 to 5 per 10 minutes were reached. The maximal allowed dose of oxytocin was 20 mIU/minute. |
|
| Outcomes | Primary outcome
Secondary outcome
|
|
| Notes | Trial was unregistered. Sources of trial funding: no information provided. Information on declaration of interest is absent from the trial report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of outcome assessor is not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data on number of eligible women. No Consort flow diagram. Timing of randomisation in relation to labour not stated. No statement about post‐randomisation exclusions. |
| Selective reporting (reporting bias) | High risk | No protocol published prior to trial initiation. Trial was unregistered. |
| Other bias | Unclear risk | No other sources of bias noted |
Ustunyurt 2007.
| Methods | Randomised controlled trial. | |
| Participants | Inclusion: live fetus, cephalic presentation, sonographically estimated fetal weight < 4000 g, 37 to 42 weeks of gestation. Exclusion: malpresentation, placenta previa, previous caesarean section, multiple gestation, persistent non‐reassuring fetal heart rate. Setting: Zekai Tahir Burak Women’s Health Education and Research Hospital.October, Ankara, Turkey, 2004 to August 2005 (period of inclusion). Number of included participants: 342 women (174 women in continued group versus 168 in discontinued group). |
|
| Interventions |
When in the active phase (5 cm cervical dilation and regular contractions at 3‐minute intervals), the infusion was stopped and the patients were randomised. Labour induction was started in all patients using an oxytocin infusion of 2 mIU/minute with a prepared solution of 5 IU of oxytocin diluted in 500 mL of 0.9% NaCl. The dose was increased every 15 minutes by 2 mIU/minute until regular contractions at a rate of 3 to 5 per 10 minutes were achieved. Amniotomy was performed in women with intact membranes at the beginning of the active phase. |
|
| Outcomes | Primary outcome
Secondary outcomes Maternal
Neonatal
|
|
| Notes | Discontinued group: 6.5% had oxytocin restarted due to lack of progression. Trial was unregistered. Sources of trial funding: no information provided. Information on declaration of interest is absent from the trial report. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table. |
| Allocation concealment (selection bias) | High risk | The patients were randomised by residents who did not participate in monitoring of patients. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The patients were randomised and solutions were prepared by residents who did not participate in monitoring of patients. Other residents that monitored the patients did not know anything about the protocol that was used for induction. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Other residents that monitored the patients did not know anything about the protocol that was used for induction. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data on number of eligible women. No Consort flow diagram. No statement about post‐randomisation exclusions. |
| Selective reporting (reporting bias) | High risk | No protocol published prior to trial. Trial unregistered. |
| Other bias | Unclear risk | No other sources of bias noted. |
APH: BMI: body mass index; IU: international units; NaCl: sodium chloride, NICU: neonatal intensive care unit; PPH: postpartum haemorrhage; PROM: premature rupture of membranes
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| D'Souza 1986 | Uncertain time of the intervention: the definition Intervention when uterine activity was satisfactory, defined as (1500‐2000 kPa/15 minutes). No remarks on cervical dilatation nor using the term 'active phase of labour' in relation to intervention start. There is uncertainty about whether the intervention correlates to the active phase of labour. |
| Girard 2009 | Quasi‐RCT: randomisation is based on an alternate‐week basis. |
| Pacheco 2006 | Wrong intervention: women receiving oxytocin stimulation for augmentation in the active phase of labour. Oxytocin is not used for induction of labour and not started in the latent phase. |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Abdelhamid 2010.
| Methods | Randomised controlled trial. |
| Participants | Women admitted for induction or augmentation and treated with oxytocin. Inclusion criteria Singleton pregnancy GA 37‐43 weeks Parity: 2‐8 Uneventful course of pregnancy, without medical complication |
| Interventions | Continuous stimulation until delivery versus discontinuation of stimulation when active phase is reached, defined as 5 cm of dilatation. |
| Outcomes | No information provided in abstract. |
| Notes | Only abstract available. Unable to locate original work. |
GA: gestational age
Characteristics of ongoing studies [ordered by study ID]
NCT02553226.
| Trial name or title | CONDISOX‐ Continued versus discontinued oxytocin stimulation of labour in a double‐blind randomised controlled trial. |
| Methods | Double‐blind multicentre randomised controlled trial. |
| Participants | 1200 women stimulated with Syntocinon® infusion for induction of labour (with or without cervical priming by prostaglandin). |
| Interventions | Latent phase: stimulation will be given according to national guidelines: initially 20 mL/hour of 10 IE Syntocinon® diluted in 1000 mL 0,9% NaCl. The dose rate will be increased every 20 minutes by 20 mL/hour until appropriate uterine activity of 3 to 5 contractions per 10 minutes is achieved. The maximum allowed dose rate 180 mL/hour for induction of labour. Active phase: the woman will be included in the study and randomised, when the active phase of labour is established (cervical dilatation ≥ 6 cm, ≥ 3 contractions per 10 minutes, and rupture of membranes). When the active phase of labour is established is oxytocin infusion replaced with project medicine.
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | April 2016 |
| Contact information | Sidsel Boie, Department of Obstetrics and Gynecology, Randers Regional Hospital, Skovlyvej 1, 8930 Randers NØ, Denmark. e‐mail: sidselboie@clin.au.dk |
| Notes | www.condisox.dk (available in Danish and English) clinicaltrials.gov Identifier: NCT02553226 |
NaCl: sodium chloride, NICU: neonatal intensive care unit
Differences between protocol and review
There are some differences between our published protocol (Boie 2016) and the full review. These are outlined below.
Outcomes
'Uterine hyperstimulation' has been rephrased to 'Uterine tachysystole combined with abnormal fetal heart rate'.
The reported postpartum haemorrhage volume in the trials was of 500 mL and not 1000 mL as per protocol pre‐specified, therefore we changed the outcome measure for postpartum haemorrhage (defined by trial authors).
'Vaginal instrumental delivery by ventouse or forceps' was included in the review and the meta‐analysis.
We added the outcome 'caesarean delivery after the active phase of labour had begun' and included the trials that reported the timing of caesarean deliveries. This outcome is reported in two analyses, one using the whole group of randomised women as denominator and one using women who reached the active phase as denominator.
‘Apgar score less than seven at five minutes’ has been rephrased to ‘Apgar score at five minutes below seven’.
We changed the outcome 'breastfeeding' to 'breastfeeding (any, as defined by the trial authors)'.
The reported pH limit in the trials was < 7.10, not as pre‐specified < 7.00 and therefore we changed the outcome for 'Acidotic cord gasses (arterial umbilical pH < 7.10)'. We acknowledge the fact that a clinically relevant pH often is below 7, however we feel obligated to report the findings on pH < 7.10.
We added the outcome neonatal admission to the NICU since this outcome was reported in more than half of the included trials.
Sensitivity analysis
Our original plan was not to perform a sensitivity analysis by high or low risk of bias. However, it became clear during data abstraction that many trials had either no CONSORT flow diagram (Bahadoran 2011; Begum 2013; Chopra 2015; Ozturk 2015; Rashwan 2011; Ustunyurt 2007), or a flow diagram that implied an implausible 100% compliance with active phase discontinuation in the experimental group, despite recruiting and randomising at less than 3 cm dilatation (Daniel‐Spiegel 2004). One trial Chookijkul 2016), had a flow diagram which indicated those caesarean deliveries before the active phase. Two trials (Bor 2016; Diven 2012), had a flow diagram that included both caesarean deliveries before the active phase and the numbers of failure to discontinue oxytocin because of too rapid a labour.
Our plan had been to conduct all analysis by 'intention‐to‐treat'. However, it became clear after data collection that a number of trials had randomised participants either before, or early in labour, and that many caesareans had been performed before the active phase, i.e. the caesareans were due to reasons which could not conceivably have been related to the experimental intervention. In the two largest trials there was a large imbalance in these early caesareans favouring discontinuation. We therefore added a secondary outcome "caesarean delivery after the active phase of labour had begun". This was only possible in trials that reported the timing of caesareans (Bor 2016; Chookijkul 2016; Chopra 2015; Diven 2012).
Contributions of authors
Designing of the protocol: Sidsel Boie drafted the original protocol, which was finalised after feedback from Julie Glavind, Pinar Bor, Niels Uldbjerg, Ben Mol, Irene De Graaf, Adeline Velu and Jannet Bakker.
Jannet Bakker and Sidsel Boie performed selection of the included trials, supported by the rest of the review team. Sidsel Boie contacted the authors of the included trials for additional information. Data extractions forms were filled in by Sidsel Boie, Jannet Bakker and Jim Thornton. Sidsel Boie and Pinar Bor did not participate in the data extraction nor the 'Risk of bias' assessment of Bor 2016, due to their authorship. Niels Uldbjerg and Sidsel Boie performed the data analyses and the first interpretation of the data supported by the rest of the review team.
Sidsel Boie drafted the initial version of the review, which was finalised after feedback from Julie Glavind, Adeline Velu, Pinar Bor, Niels Uldbjerg, Ben Mol, Irene De Graaf, Jannet Bakker and Jim Thornton. Sidsel Boie is the guarantor for this review.
Sources of support
Internal sources
Randers Regional Hospital, Department of Obstetrics and Gynaecology, Denmark.
Aarhus University, Clinical Medicine, Denmark.
Aarhus University Hospital, Department of Obstetrics and Gynaecology, Denmark.
The University of Adelaide, Department of Obstetrics and Gynaecology, Australia.
Academic Medical Center, Department of Obstetrics and Gynaecology, Amsterdam, Netherlands.
External sources
No sources of support supplied
Declarations of interest
Sidsel Boie is co‐author on one of the included trials (Bor 2016) and primary investigator of an ongoing double‐blind multicentre randomised controlled trial (RCT) on the topic (NCT02553226). She has/will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments has been/will be carried out by other members of the team who are not directly involved in the trial.
Adeline V Velu: none known.
Julie Glavind: Trygfonden Denmark provided postdoctoral salary support at Aarhus University Hospital from April‐September 2014. She currently receives salary support (as a registrar) from Central Denmark Region. She is an investigator of an ongoing double‐blind multicentre RCT on the topic (NCT02553226). She will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments will be carried out by other members of the team who are not directly involved in the trial.
Ben Willem J Mol and his institution have received payment for consultancy from ObsEva Geneva. Ben has also received payment for review preparation from European Journal of Obstetrics & Gynecology from ESHRE Munich and Prebic Geneva in respect of travel/accommodation/meeting expenses for various non‐commercial scientific meetings
Niels Uldbjerg is an investigator on the ongoing randomised trial, the CONDISOX trial (NCT02553226) that may be considered for inclusion in a future update of this review. He will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments will be carried out by other members of the team who are not directly involved in the trial.
Irene de Graaf: none known.
Pinar Bor is principle investigator on one of the included trials (Bor 2016), and an investigator on a ongoing double‐blind multicentre RCT on the topic (NCT02553226). She has/will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments has been/will be carried out by other members of the team who are not directly involved in the trial.
Jannet JH Bakker is one of the authors of the ongoing randomised trial, the CONDISOX trial (NCT02553226) that may be considered for inclusion in a future update of this review. She will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments will be carried out by other members of the team who are not directly involved in the trial.
JIm Thornton is the chair of the trial steering committee for the ongoing randomised trial, the CONDISOX trial (NCT02553226) that may be considered for inclusion in a future update of this review. He will not be involved in any decisions relating to this review ‐ assessment for inclusion, risk of bias, data extraction and GRADE quality assessments will be carried out by other members of the team who are not directly involved in the trial.
New
References
References to studies included in this review
Bahadoran 2011 {published data only}
- Bahadoran P, Falahati J, Shahshahan Z, Kianpour M. The comparative examination of the effect of two oxytocin administration methods of labor induction on labor duration stages. Iranian Journal of Nursing and Midwifery Research 2011;16(1):100‐5. [PMC free article] [PubMed] [Google Scholar]
Begum 2013 {published data only}
- Begum LN, Sultana M, Nahar S, Begum R, Barua S. A randomized clinical trial on the need of continuing oxytocin infusion in active phase of induced labour. Chattagram Maa‐O‐Shishu Hospital Medical College Journal 2013;12(2):23‐30. [Google Scholar]
Bor 2016 {published data only}
- Bor P, Ledertoug S, Boie S, Knoblauch NO, Stornes I. Continuation versus discontinuation of oxytocin infusion during the active phase of labour: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2016;123(1):129‐35. [DOI] [PubMed] [Google Scholar]
Chookijkul 2016 {published data only}
- Chookijkul L, Prommas S, Pariyawateekul P, Orungrote N, Smanchat B, Suwannarurk K. Cesarean section rate in oxytocin infusion between continuous until delivery and discontinuation at active phase of labor: A randomized controlled study. Thai Journal of Obstetrics and Gynaecology 2016;24:73‐80. [Google Scholar]
- Chookijkul L, TCTR20150503001. Cesarean section rate in oxytocin infusion between continuous until delivery and discontinuation at active phase of labor: a randomized controlled study. http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=1381 (first received 3 May 2015).
Chopra 2015 {published data only}
- Chopra S, SenGupta SK, Jain V, Kumar P. Stopping oxytocin in active labor rather than continuing it until delivery: a viable option for the induction of labor. Oman Medical Journal 2015;30(5):320‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta SK, Jain V, Chopra S, Kumar P. Oxytocin discontinuation in active phase: the effects. BJOG: an international journal of obstetrics and gynaecology 2014;121(Suppl 2):88. [Google Scholar]
Daniel‐Spiegel 2004 {published data only}
- Daniel‐Spiegel E, Weiner Z, Ben‐Shlomo I, Shalev E. For how long should oxytocin be continued during induction of labour?. BJOG: an International Journal of Obstetrics and Gynaecology 2004;111(4):331‐4. [DOI] [PubMed] [Google Scholar]
Diven 2012 {published data only}
- Diven L, Gogle J, Eid S, Smulian J, Quinones J. Induction of labor with oxytocin: should oxytocin be held?. American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S144. [DOI] [PubMed] [Google Scholar]
- Diven LC, Rochon ML, Gogle J, Eid S, Smulian JC, Quinones JN. Oxytocin discontinuation during active labor in women who undergo labor induction. American Journal of Obstetrics and Gynecology 2012;207(6):471.e1‐471e8. [DOI] [PubMed] [Google Scholar]
Ozturk 2015 {published data only}
- Ozturk FH, Yilmaz SS, Yalvac S, Kandemir O. Effect of oxytocin discontinuation during the active phase of labor. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(2):196‐8. [DOI] [PubMed] [Google Scholar]
Rashwan 2011 {published data only}
- Rashwan ASSA, Gaafar HM, Mohamed AMM. Comparative study between continuous use of oxytocin infusion throughout the active phase of labor versus its discontinuation and its effect on the course of labor. Medical Journal of Cairo University 2011;79(2):121‐5. [Google Scholar]
Ustunyurt 2007 {published data only}
- Ustunyurt E, Ugur M, Ustunyurt BO, Iskender TC, Ozkan O, Mollamahmutoglu L. Prospective randomized study of oxytocin discontinuation after the active stage of labor is established. Journal of Obstetrics and Gynaecology Research 2007;33(6):799‐803. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
D'Souza 1986 {published data only}
- D'Souza SW, Lieberman B, Cadman J, Richards B. Oxytocin induction of labour: hyponatraemia and neonatal jaundice. European Journal Obstetrics & Gynecology and Reproductive Biology 1986;22:309‐17. [DOI] [PubMed] [Google Scholar]
Girard 2009 {published data only}
- Girard B, Vardon D, Creveuil C, Herlicoviez M, Dreyfus M. Discontinuation of oxytocin in the active phase of labor. Acta Obstetricia et Gynecologica Scandinavica 2009;88(2):172‐7. [DOI] [PubMed] [Google Scholar]
Pacheco 2006 {published data only}
- Pacheco LD, Rosen MP, Gei AF, Saade GR, Hankins GD. Management of uterine hyperstimulation with concomitant use of oxytocin and terbutaline. American Journal of Perinatology 2006;23(6):377‐80. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Abdelhamid 2010 {published data only}
- Abdelhamid EE. For how long should oxytocin be continued during induction of labour?. Jamahiriya Medical Journal 2010;10(4):251‐3. [CENTRAL: CN‐01016774] [Google Scholar]
References to ongoing studies
NCT02553226 {published data only}
- NCT02553226. Continued versus discontinued oxytocin stimulation of labour in a double‐blind randomised controlled trial. clinicaltrials.gov/ct2/show/NCT02553226 (first received 17 September 2015).
Additional references
ACOG 2009
- Anon. ACOG Practice Bulletin No. 107: Induction of labor. Obstetrics and Gynecology 2009;114(2 Pt 1):386‐97. [PUBMED: 19623003] [DOI] [PubMed] [Google Scholar]
ACOG 2014
Bakker 2007
- Bakker PC, Kurver PH, Kuik DJ, Geijn HP. Elevated uterine activity increases the risk of fetal acidosis at birth. American Journal of Obstetrics and Gynecology 2007;196(4):313‐6. [DOI] [PubMed] [Google Scholar]
Budden 2014
- Budden A, Chen L, Henry A. High‐dose versus low‐dose oxytocin infusion regimens for induction of labour at term. Cochrane Database of Systematic Reviews 2014, Issue 10. [DOI: 10.1002/14651858.CD009701.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2008
- Cahill AG, Waterman BM, Stamilio DM, Odibo AO, Allsworth JE, Evanoff B, et al. Higher maximum doses of oxytocin are associated with an unacceptably high risk for uterine rupture in patients attempting vaginal birth after cesarean delivery. American Journal of Obstetrics and Gynecology 2008;199(1):32e1‐5. [DOI: 10.1016/j.ajog.2008.03.001] [DOI] [PubMed] [Google Scholar]
Clark 2008
- Clark SL, Belfort MA, Dildy GA, Meyers JA. Reducing obstetric litigation through alterations in practice patterns. Obstetrics and Gynecology 2008;112(6):1279‐83. [PUBMED: 19037036] [DOI] [PubMed] [Google Scholar]
Dahlen 2013
- Dahlen HG, Kennedy HP, Anderson CM, Bell AF, Clark A, Foureur M, et al. The EPIIC hypothesis: intrapartum effects on the neonatal epigenome and consequent health outcomes. Medical Hypotheses 2013; Vol. 80, issue 5:656‐62. [PUBMED: 23414680] [DOI] [PMC free article] [PubMed]
Dansereau 1999
- Dansereau J, Joshi AK, Helewa ME, Doran AT, Lange ER, Luther IR. Double‐blind comparison of carbetocin versus oxytocin in prevention of uterine atony after caesarean section. American Journal of Obstetrics and Gynecology 1999;180(3 Pt 1):670‐6. [DOI] [PubMed] [Google Scholar]
Dencker 2010
- Dencker A, Taft C, Bergvist L, Lifa H, Berg M. Childbirth experience questionnaire (CEQ): development and evaluation of a multidimensional instrument. BMC pregnancy and Childbirth 2010; Vol. 10, issue 81. [DOI: 10.1186/1471-2393-10-81] [DOI] [PMC free article] [PubMed]
Fernandez 2012
- Fernandez O, Marin GM, Malalana MA, Fernandez‐Cañadas MA, Lopez SF, Costareli V. Newborn feeding behaviour depressed by intrapartum oxytocin: a pilot study. Acta Paediatrica 2012;101(7):749‐54. [DOI] [PubMed] [Google Scholar]
Grotegut 2011
- Grotegut CA, Paglia MJ, Johnson LN, Thames B, James AH. Oxytocin exposure in women with postpartum hemorrhage secondary to uterine atony. American Journal of Obstetrics and Gynecology 2011;204(1):56.e1‐56.e6. [DOI: 10.1016/j.ajog.2010.08.023] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hertog 2001
- Hertog CE, Groot AN, Dongen PW. History and use of oxytocics. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2001;94(1):8‐12. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ISMP List 2014
- List of High‐Alert Medications in Acute Care Settings. Institute for Safe Medication Practices (ISMP) (http://www.ismp.org/tools/highalertmedications.pdf) 2014.
Malek 1996
- Malek A, Blann E, Mattison DR. Human placental transport of oxytocin. Journal of Maternal and Fetal Medicine 1996;5(5):245‐55. [PUBMED: 8930795] [DOI] [PubMed] [Google Scholar]
NICE 2017
- National Collaborating Centre for Women's and Children's Health (UK) et al. Intrapartum care: care of healthy women and their babies during childbirth. National Institute for Health and Care Excellence December 2014 (updated February 2017). [PUBMED: 25950072] [PubMed]
Oláh 2015
- Oláh KSJ, Steer PJ. The use and abuse of oxytocin. Obstetrician & Gynaecologist 2015;17(4):265‐71. [DOI: 10.1111/tog.12222] [DOI] [Google Scholar]
Oscarsson 2006
- Oscarsson ME, Amer‐Wahlin I, Rydhstroem H, Källén K. Outcome in obstetric care related to oxytocin use. A population‐based study. Acta Obstetricia et Gynecologica Scandinavica 2006;85(9):1094‐8. [DOI] [PubMed] [Google Scholar]
Phaneuf 2000
- Phaneuf S, Rodriguez Liñares B, TambyRaja RL, MacKenzie IZ, López Bernal A. Loss of myometrial oxytocin receptors during oxytocin‐induced and oxytocin‐augmented labour. Journal of Reproduction and Fertile 2000;120(1):91‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saccone 2017
- Saccone G, Ciardulli A, Baxter J, Quiñones, J, Diven L, Bor P, et al. Discontinuing oxytocin infusion in the active phase of labor. Obstetrics and Gynecology 2017;30(5):1090‐6. [DOI: 10.1097/AOG.0000000000002325S] [DOI] [PubMed] [Google Scholar]
Selin 2009
- Selin L, Almstrom E, Wallin G, Berg M. Use and abuse of oxytocin for augmentation of labor. Acta Obstetricia et Gynecologica Scandinavica 2009;88(12):1352‐7. [DOI] [PubMed] [Google Scholar]
Simpsons 2009
- Simpson KR, Knox GE. Oxytocin as a high‐alert medication: implications for perinatal patient safety. MCN. American Journal of Maternal Child Nursing 2009;34(1):8‐15. [DOI] [PubMed] [Google Scholar]
Svare 2014
- Svare JA, Hansen BB, Lose G. Risk factors for urinary incontinence 1 year after the first vaginal delivery in a cohort of primiparous Danish women. International Urogynecology Journal 2014;25(1):47‐51. [DOI] [PubMed] [Google Scholar]
Vlachos 2015
- Vlachos DE, Pergialiotis V, Papantoniou N, Trompoukis S, Vlachos GD. Oxytocin discontinuation after the active phase of labor is established. Journal of Maternal‐Fetal and Neonatal Medicine 2015;28(12):1421‐7. [DOI: 10.3109/14767058.2014.955000] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Boie 2016
- Boie S, Velu AV, Glavind J, Mol BWJ, Uldbjerg N, Graaf I, Bor P, Bakker JJH. Discontinuation of intravenous oxytocin in the active phase of induced labour. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD012274] [DOI] [PMC free article] [PubMed] [Google Scholar]


