Abstract
Background
Poorly controlled asthma and preventable exacerbations place a significant strain on healthcare, often requiring additional medications, hospital stays or treatment in the emergency department.
Long‐acting beta2‐agonists (LABA) are the preferred add‐on treatment for adults with asthma whose symptoms are not well controlled on inhaled corticosteroids (ICS), but have important safety concerns in asthma. Long‐acting muscarinic antagonists (LAMA) have confirmed efficacy in chronic obstructive pulmonary disease and are now being considered as an alternative add‐on therapy for people with uncontrolled asthma.
Objectives
To assess the efficacy and safety of adding a LAMA to ICS compared with adding a LABA for adults whose asthma is not well controlled on ICS alone.
Search methods
We searched the Cochrane Airways Group's Specialised Register (CAGR) from inception to April 2015, and imposed no restriction on language of publication. We searched additional resources to pick up unpublished studies, including ClinicalTrials.gov, World Health Organization trials portal, reference lists of primary studies and existing reviews, and manufacturers' trial registries. The most recent search was conducted in April 2015.
Selection criteria
We searched for parallel and cross‐over RCTs in which adults whose asthma was not well controlled with ICS alone were randomised to receive LAMA add‐on or LABA add‐on for at least 12 weeks.
Data collection and analysis
Two review authors independently screened the electronic and additional searches and extracted data from study reports. We used Covidence for duplicate screening, extraction of study characteristics and numerical data, and risk of bias ratings.
The pre‐specified primary outcomes were exacerbations requiring oral corticosteroids (OCS), quality of life and serious adverse events.
Main results
We included eight studies meeting the inclusion criteria, but four double‐blind, double‐dummy studies of around 2000 people dominated the analyses. These four trials were between 14 and 24 weeks long, all comparing tiotropium (usually Respimat) with salmeterol on top of medium doses of ICS.
Studies reporting exacerbations requiring OCS showed no difference between the two add‐ons, but our confidence in the effect was low due to inconsistency between studies and because the confidence intervals (CI) included significant benefit of either treatment (odds ratio (OR) 1.05, 95% CI 0.50 to 2.18; 1753 participants; 3 studies); three more people per 1000 might have an exacerbation on LAMA, but the CIs ranged from 29 fewer to 61 more. Imprecision was also an issue for serious adverse events and exacerbations requiring hospital admission, rated low (serious adverse events) and very low quality (exacerbations requiring hospital admission), because there were so few events in the analyses.
People taking LAMA scored slightly worse on two scales measuring quality of life (Asthma Quality of Life Questionnaire; AQLQ) and asthma control (Asthma Control Questionnaire; ACQ); the evidence was rated high quality but the effects were small and unlikely to be clinically significant (AQLQ: mean difference (MD) ‐0.12, 95% CI ‐0.18 to ‐0.05; 1745 participants; 1745; 4 studies; ACQ: MD 0.06, 95% CI 0.00 to 0.13; 1483 participants; 3 studies).
There was some evidence to support small benefits of LAMA over LABA on lung function, including on our pre‐specified preferred measure trough forced expiratory volume in one second (FEV1) (MD 0.05 L, 95% CI 0.01 to 0.09; 1745 participants, 4 studies). However, the effects on other measures varied, and it is not clear whether the magnitude of the differences were clinically significant.
More people had adverse events on LAMA but the difference with LABA was not statistically significant.
Authors' conclusions
Direct evidence of LAMA versus LABA as add‐on therapy is currently limited to studies of less than six months comparing tiotropium (Respimat) to salmeterol, and we do not know how they compare in terms of exacerbations and serious adverse events. There was moderate quality evidence that LAMAs show small benefits over LABA on some measures of lung function, and high quality evidence that LABAs are slightly better for quality of life, but the differences were all small. Given the much larger evidence base for LABA versus placebo for people whose asthma is not well controlled on ICS, the current evidence is not strong enough to say that LAMA can be substituted for LABA as add‐on therapy.
The results of this review, alongside pending results from related reviews assessing the use of LAMA in other clinical scenarios, will help to define the role of these drugs in asthma and it is important that they be updated as results from ongoing and planned trials emerge.
Keywords: Adult; Female; Humans; Male; Middle Aged; Administration, Inhalation; Adrenal Cortex Hormones; Adrenal Cortex Hormones/administration & dosage; Adrenergic beta‐2 Receptor Agonists; Adrenergic beta‐2 Receptor Agonists/administration & dosage; Asthma; Asthma/drug therapy; Double‐Blind Method; Drug Therapy, Combination; Drug Therapy, Combination/methods; Muscarinic Antagonists; Muscarinic Antagonists/administration & dosage; Quality of Life; Randomized Controlled Trials as Topic; Salmeterol Xinafoate; Salmeterol Xinafoate/administration & dosage; Tiotropium Bromide; Tiotropium Bromide/administration & dosage
Plain language summary
Is it better to add long‐acting muscarinic antagonists or long‐acting beta2‐agonists to inhaled corticosteroids for people with uncontrolled asthma?
Main point
Differences between long‐acting muscarinic antagonists (LAMA) and long‐acting beta2‐agonists (LABA) are mostly small or uncertain, based on studies less than six months in duration. The current evidence is not strong enough to support using LAMA instead of LABA for people whose asthma is not controlled on inhaled corticosteroids.
Why is the question important?
People who have asthma that is not well controlled often have attacks that require extra treatment and time in hospital.
LABA are inhaled drugs that can improve symptoms and reduce the likelihood of asthma attacks when inhaled corticosteroids are not helpful alone, but they can have serious side effects. LAMA, another type of inhaled drug that is already used for other lung diseases, are a possible new treatment option for this group of people with asthma.
How did we answer the question?
We looked for randomised controlled studies (clinical studies where people are randomly put into one of two or more treatment groups) that compared LAMA with LABA, both on top of inhaled corticosteroids, for at least 12 weeks. Two people looked through all of the possible published and unpublished studies that we found from several databases and websites, to find a list of studies that looked at the question we were interested in. The most recent searches were done in April 2015.
What did we find out?
We could not tell whether people taking LAMA were more or less likely to need oral corticosteroids for an asthma attack than people taking LABA because not many people needed them and the studies showed different results; overall three more people in 1000 might have an asthma attack on LAMA, but the real result could be anywhere between 29 fewer and 61 more than if you took a LABA. Similarly, too few people in the studies had serious side effects or asthma attacks that required urgent medical treatment to judge whether one treatment was better than the other.
The studies showed that LAMAs might be a bit better than LABA for lung function (how well your lungs work), and LABAs slightly better for quality of life, but the differences were small and we could not tell if one was better than the other for most outcomes.
The results were mostly based on four good studies of around 2000 people, which were between 14 and 24 weeks of duration. All of the studies looked at a LAMA drug called tiotropium.
Summary of findings
Summary of findings for the main comparison. Long‐acting muscarinic antagonists (LAMA) add‐on compared with long‐acting beta2‐agonists (LABA) add‐on for adults with asthma.
| Long‐acting muscarinic antagonists (LAMA) add‐on compared with long‐acting beta2‐agonists (LABA) add‐on for adults with asthma | ||||||
|
Patient or population: adults with asthma not well controlled on ICS
Settings: outpatient
Intervention: LAMA add‐on
Comparison: LABA add‐on Time point: calculated as the mean duration of the studies contributing to each analysis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk3 | Corresponding risk | |||||
| LABA add‐on | LAMA add‐on | |||||
|
Exacerbations (OCS) 23 weeks |
59 per 1000 | 62 per 1000 (30 to 120) | OR 1.05 (0.50 to 2.18) | 1755 (3 RCTs) | ⊕⊕⊝⊝ Low 1,2 | No clear benefit of 1 add‐on over the other |
|
AQLQ total
1 = severely impaired; 7 = not impaired at all 22 weeks |
The mean score in the LABA group was 5.60 | The mean score in the LAMA group was 0.12 worse (0.18 worse to 0.05 worse) | ‐ | 1745 (4 RCTs) | ⊕⊕⊕⊕ High | Small LABA benefit; MCID = 0.5 so difference was unlikely to be clinically significant |
|
Serious adverse events 22 weeks |
25 per 1000 | 21 per 1000 (10 to 42) | OR 0.84 (0.41 to 1.73) | 2012 (4 RCTs) | ⊕⊕⊝⊝ Low 5 | No clear benefit of 1 add‐on over the other |
|
Exacerbations (hospital) 22 weeks |
8 per 1000 | 6 per 1000 (1 to 23) | OR 0.72 (0.18 to 2.92) | 2022 (4 RCTs) | ⊕⊝⊝⊝ Very low 4,5 | No clear benefit of 1 add‐on over the other |
|
Trough FEV1 (L)6 (higher is better) 22 weeks |
The mean trough FEV1 in the LABA group was 0.07 L | The mean trough FEV1 in the LAMA group was 0.05 L better (0.01 better to 0.09 better) | ‐ | 1745 (4 RCTs) | ⊕⊕⊕⊝ Moderate 4 | Small LAMA benefit |
|
ACQ total 0 = no impairment; 6 = maximum impairment 23 weeks |
The mean score in the LABA group was 1.31 | The mean score in the LAMA group was 0.06 higher (0 higher to 0.12 higher) | ‐ | 1483 (3 RCTs) | ⊕⊕⊕⊕ High | Small LABA benefit; MCID = 0.5 so difference was unlikely to be clinically significant |
|
Adverse events (all) 23 weeks |
519 per 1000 | 544 per 1000 (498 to 592) | OR 1.11 (0.92 to 1.35) | 1839 (3 RCTs) | ⊕⊕⊕⊝ Moderate 1 | More people on LAMA had an adverse event but the difference with LABA was not statistically significant |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; CI: confidence interval; FEV1: forced expiratory volume in 1 second; ICS: inhaled corticosteroid; LABA: long‐acting beta2‐agonist; LAMA: long‐acting muscarinic antagonist; MCID: minimal clinically important difference; OCS: oral corticosteroids; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Confidence intervals include important benefit on either treatment (‐1 imprecision).
2 I2 = 50%, which was not statistically significant (P value = 0.16), but visual inspection of the forest plot showed opposite directions of effect of the pooled twin trials and the cross‐over study (‐1 inconsistency)
3 For continuous outcomes, the assumed risk was calculated as a weighted mean of the control group scores (NCT00565266 not included in the AQLQ or ACQ calculation because the study reported change from baseline and the remaining studies reported endpoint data). For dichotomous outcomes, it was the pooled control group event rate of all included studies.
4 There was some statistical heterogeneity in these outcomes (exacerbations requiring hospital admission: I2 = 20%, P value = 0.29; trough FEV1: I2 = 46%, P value = 0.14), which was not statistically significant, but visual inspection of the forest plots showed clear variation in study results (‐1 inconsistency).
5 Very wide confidence intervals; small number of events in the analysis (‐2 imprecision).
6 Other lung function outcomes showed mixed results: small benefit of LAMA on trough PEF (moderate quality), possible but non‐significant benefit of LAMA on peak FEV1 (very low quality) and trough FVC (moderate quality), no effect on peak FVC (moderate quality), LABA benefit on percentage predicted FEV1 (very low quality).
Background
Description of the condition
Asthma is a common and potentially serious chronic disease of the airways, which causes difficulty breathing due to narrowing of the airways, thickening of the airway walls and increased mucous production (GINA 2014). Asthma is recognised as a heterogeneous disease, but commonly causes symptoms including wheezing, shortness of breath, chest tightness and cough that vary over time in their occurrence, frequency and intensity (GINA 2014).
Around the world and particularly in low‐ and middle‐income countries, asthma is frequently undiagnosed and untreated (Global Asthma Report 2011), and remains a significant cause of avoidable morbidity and mortality in high‐income countries such as the UK (BTS/SIGN 2014; NRAD 2014), imposing "a substantial burden on patients, their family and the community" (GINA 2014). World Health Organization estimates suggest 300 million people are affected worldwide, with direct treatment costs and indirect costs of lost productivity among the highest for non‐communicable diseases (Global Asthma Report 2011). Prevalence estimates vary, and changes over time have been linked to various factors including air pollution, tobacco legislation, diet and prevalence of other atopic diseases (Anderson 2005).
The two broad aims of asthma treatment are to maintain daily symptom control and prevent acute worsening of symptoms known as asthma attacks or exacerbations. To achieve this, medication, usually given via an inhaler, is started at the most appropriate level based on the severity and frequency of symptoms according to treatment steps laid out in guidelines (e.g. GINA 2014). Depending on symptom control and frequency of exacerbations when treatment has been commenced, therapy can be stepped up by increasing dose or adding medications to recapture control, or stepped down to maintain people at the lowest effective therapy and minimise adverse effects.
Description of the intervention
The lowest treatment step in most guidelines is the sole use of a short‐acting bronchodilating inhaler on an as‐needed basis (e.g. salbutamol), which is often sufficient to treat mild or intermittent asthma symptoms. Regular use of low‐dose inhaled corticosteroids (ICS) is the primary recommended preventer therapy for people with persistent asthma who remain inadequately controlled on as‐needed medication alone (BTS/SIGN 2014; GINA 2014). Regular ICS improves lung function and reduces the need for reliever medications (Adams 2008a; Adams 2008b). However, some people with asthma will continue to have symptoms and asthma attacks on ICS alone and guidelines suggest a range of treatment options for this group of people (GINA 2014 step three and above). Long‐acting beta‐agonists (LABA), such as formoterol and salmeterol, are the current preferred add‐on therapy (BTS/SIGN 2014; Ducharme 2008; GINA 2014), as they have often small but statistically significant benefits on a range of outcomes over other treatment options such as increasing ICS dose (Ducharme 2010), adding theophylline (Tee 2009), or adding a leukotriene receptor antagonist (Chauhan 2014). Despite these confirmed benefits, LABA have been linked to increased morbidity and mortality in asthma (Cates 2014; Nelson 2006; Salpeter 2006), leading to warnings from the US Food and Drug Administration and the UK Medicines and Healthcare Products Regulatory Agency to highlight the increased risk of serious adverse events (FDA 2010; MHRA 2014). While the risks are reduced when LABA are used as an add‐on treatment to ICS (Cates 2014; Ernst 2006), it is still unclear whether the risk of adverse events remains higher than with ICS alone (Ducharme 2008).
ICS also carry risks and add‐on drugs that allow their dose to be kept low are often seen as preferable to high‐dose monotherapy. Prolonged use of higher doses of ICS carries the risk of serious unwanted effects including growth retardation in children, decreased bone density, eye disorders, sleep problems and anxiety (NICE 2013).
Long‐acting muscarinic antagonists (LAMA), a class of drugs with confirmed effectiveness in chronic obstructive pulmonary disease (COPD) (Karner 2014), are now being considered as an alternative to LABA add‐on therapy for adults with asthma requiring more than ICS alone. Tiotropium, the first LAMA to be licensed in COPD and the most widely used, has added benefits over LABA in terms of frequency of exacerbations and hospital admissions for COPD, but not in terms of mortality or overall hospital admissions (Chong 2012). Evidence for the safety and efficacy of aclidinium bromide and glycopyrronium bromide, two LAMA formulations that have been licensed for use in COPD, is emerging but less well established (Ni 2014).
How the intervention might work
LAMA block receptors of the neurotransmitter acetylcholine on airway smooth muscle, glands and nerves, preventing muscle contraction and mucous secretion (Moulton 2011). The action on these receptors helps to alleviate symptoms of breathlessness, coughing and wheezing that characterise asthma (Lipworth 2014). These characteristics of LAMA, and the overlap in pathophysiology and symptoms of asthma and COPD (Gosens 2006), have led to their testing in asthma as an add‐on therapy for people who do not achieve adequate control from standard‐dose ICS alone, thus avoiding prolonged exposure to higher doses of ICS.
The most commonly reported adverse effect of LAMA for airways disease is dry mouth, with others including constipation or diarrhoea, cough and headache (BNF). All LAMA for maintenance treatment of airways disease are delivered via inhalers, either by powder (HandiHaler, Genuair, Breezhaler) or soft mist delivery (Respimat), and are not suitable for use as rescue medication.
In COPD, there is conflicting evidence regarding the safety of tiotropium delivered via the Respimat device, with one observational study finding it increases the risk of death, particularly from cardiac events, compared with both placebo and tiotropium via the HandiHaler device (Verhamme 2013). Another large randomised trial including over 17,000 people with COPD found no significant differences in long‐term safety between the two devices (Wise 2013). As yet, it is unclear whether differential safety profiles will be seen in people with asthma.
Why it is important to do this review
Only one preparation of LAMA (Spiriva Respimat 2.5 mcg) has been granted a UK license for use in severe asthma alongside LABA and ICS (eMC 2014). Following its demonstrated efficacy in COPD (Karner 2014), clinical trials are emerging testing various LAMA regimens against the existing treatment options. One study found that nearly 30% of people who were uncontrolled on fluticasone remained so with the guideline recommended addition of LABA (Bateman 2004), suggesting there is a need for additional therapeutic options. Therefore, it is important to assess the efficacy and safety of LAMA add‐on against LABA add‐on, since LABA add‐on is the preferred step‐up treatment when ICS alone are ineffective (GINA 2014).
Three other reviews are currently being produced to assess 1. LAMA add‐on compared with increasing ICS dose (Kew 2014), 2. LAMA add‐on compared with no change to ICS dose (Allison 2014), and 3. LAMA add‐on as triple therapy with LABA plus ICS compared with LABA plus ICS alone (Kew 2015a).
Objectives
To assess the efficacy and safety of adding a LAMA to ICS compared with adding a LABA for adults whose asthma is not well controlled on ICS alone.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel or cross‐over randomised controlled trials (RCTs) of at least 12 weeks' duration. We included studies reported as full‐text, abstract only and unpublished data.
We did not exclude studies on the basis of blinding.
Types of participants
We included adults (aged 18 years or older) whose asthma is not well controlled with ICS alone. We excluded trials that included participants with other chronic respiratory co‐morbidities (e.g. COPD, bronchiectasis).
If studies included adults and adolescents or children under 12 and data were not reported separately, we included them if the mean age in both groups was over 18 years.
Types of interventions
We included trials comparing the addition of LAMA add‐on with LABA add‐on to any dose of ICS.
Studies involving the addition of the following LAMAs at any dose:
tiotropium (Spiriva HandiHaler or Respimat);
aclidinium bromide (Eklira Genuair);
glycopyrronium bromide (Seebri Breezhaler).
Eligible comparison groups were randomised to receive the same dose of ICS as the intervention group, with the addition of any of the following LABAs:
formoterol 12 or 24 mcg twice daily
salmeterol 50 mcg twice daily
vilanterol 22 mcg once daily
Since LABAs are available as single inhalers or in combination inhalers with ICS (e.g. Symbicort, Seretide, Dulera, Relvar), we included either formulation as long as the ICS was comparable to the dose given alongside the LAMA in the intervention group.
We included studies that allowed participants to continue using their usual short‐ or long‐acting medications (e.g. salbutamol, terbutaline and ipratropium, leukotriene receptor antagonists), provided any non‐randomised LAMA or LABA were stopped during the study run‐in.
Types of outcome measures
Primary outcomes
Exacerbations requiring oral corticosteroids.
Quality of life (measured on a validated asthma scale, e.g. Asthma Quality of Life Questionnaire; AQLQ).
Any serious adverse event.
Secondary outcomes
Exacerbations requiring hospitalisation.
Lung function (in particular, trough forced expiratory volume in one second (FEV1)).
Asthma control (measured on a validated scale, e.g. Asthma Control Questionnaire (ACQ) or Asthma Control Test).
Any adverse events.
Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion for the review.
If exacerbations were reported as a composite of more than one definition (e.g. people with one or more exacerbation requiring hospitalisation or emergency department visit), we analysed these separately.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group's Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (see Appendix 1 for further details). We searched all records in the CAGR using the search strategy in Appendix 2.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov), the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) and industry trial registries. We searched all databases from their inception to April 2015, and we imposed no restriction on language of publication. Searches were conducted in April 2015.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
We searched for errata or retractions from included studies published in full‐text on PubMed (www.ncbi.nlm.nih.gov/pubmed) on 18 February 2015.
Data collection and analysis
Selection of studies
Using Covidence, two review authors (KK and DE) independently screened titles and abstracts for inclusion of all the potential studies that we identified as a result of the search. We retrieved the full‐text study reports/publication and two review authors (KK and DE) independently screened the full text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third review author (DA or AB). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table.
Data extraction and management
We used a data collection form in Covidence for study characteristics and outcome data, which was piloted on at least one study in the review. Two review authors (KK and DE) extracted study characteristics from included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals and date of study.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (KK and DE) independently extracted outcome data from included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third review author (DA or AB). One review author (KK) transferred data into Review Manager 5 (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (DE) spot‐checked study characteristics for accuracy against the trial reports.
Assessment of risk of bias in included studies
Two review authors (KK and DE) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (DA or AB). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgment in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different from a participant‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contribute to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to this published protocol and reported any deviations in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratios (OR) and continuous data as mean difference (MD) or standardised mean difference (SMD) with their 95% confidence intervals (CI). We entered data presented as a scale with a consistent direction of effect. We narratively described skewed data reported as medians and interquartile ranges. We analysed data from cross‐over trials using generic inverse variance (GIV) and only if double‐counting of participants has been accounted for. If raw data and adjusted analyses (e.g. accounting for baseline differences) were both presented, we used the adjusted analyses. When data published in peer‐reviewed papers was different to that given on clinicaltrials.gov, we cross‐checked them and contacted the study sponsor or trial author for more information if there was a discrepancy in the effect.
We undertook meta‐analyses only where meaningful (i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense).
Where multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) were combined in the same meta‐analysis, we halved the control group to avoid double counting.
If change from baseline and endpoint scores were available for continuous data, we used change from baseline unless the majority of studies reported endpoint scores. If a study reported outcomes at multiple time points, we used the end‐of‐study measurement.
When an analysis using only participants who completed the trial and an analysis that imputed data for participants who were randomised but did not provide endpoint data (e.g. last observation carried forward) were both available, we used the analysis that imputed data.
For dichotomous outcomes, we assumed equivalence of treatments if the OR estimate and its 95% CI were between the pre‐defined arbitrary limits of 0.9 and 1.1.
Unit of analysis issues
For dichotomous outcomes, we used participants rather than events as the unit of analysis (i.e. number of adults admitted to hospital rather than number of admissions per adult). However, if exacerbations were reported as rate ratios we analysed them on this basis. For cross‐over trials, we requested data in the format shown in Appendix 3 for dichotomous outcomes in order to control for intercorrelation of matched pairs (Elbourne 2002). For continuous data in cross‐over trials, we entered data using GIV from suitable adjusted analyses to account for the trial's design.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results using a sensitivity analysis.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. If we identified substantial heterogeneity (e.g. I2 greater than 30%), we reported it and explored possible causes by pre‐specified subgroup analysis.
Assessment of reporting biases
We were not able to pool more than 10 trials, so were unable to examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We used a random‐effects model for all analyses as we expected variation in effects due to differences in study populations and methods. We performed sensitivity analyses using fixed‐effect.
'Summary of findings' table
We created a 'Summary of findings' table for all outcomes named in this protocol. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for the pre‐specified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) using GRADEpro software (Brozek 2008). We justified all decisions to downgrade or upgrade the quality of studies using footnotes and we made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned the following subgroup analyses for the primary outcomes, using the formal test for subgroup differences in Review Manager 5 (RevMan 2014):
Duration of therapy (six months or less, more than six months).
Corticosteroid dose (according to GINA 2014 ‐ defined low, medium and high cut‐offs).
Dose and type of LABA (e.g. formoterol 24 mcg, salmeterol 50 mcg).
Dose and type of LAMA (e.g. tiotropium HandiHaler 18 mcg, tiotropium Respimat 5 mcg).
Sensitivity analysis
We planned sensitivity analyses for the primary outcomes by excluding the following:
studies at high risk of bias for blinding of participants and personnel;
unpublished data (i.e. no peer‐reviewed full paper available);
cross‐over trials.
Results
Description of studies
Results of the search
We identified 76 records in the electronic database searches, and 121 additional records by searching clinicaltrials.gov, reference lists of other publications and drug company trial registries. We identified 54 of the total 197 as duplicates and screened titles and abstracts for the remaining 143. We excluded 104 at this stage. We retrieved full texts for the remaining 39, and we excluded 23 at this stage, which related to 19 excluded studies. The main reason for exclusion was the wrong comparator being used (11 publications), such as ICS alone (relevant to a separate review). Other reasons for exclusion were 'too short' (i.e. less than 12 weeks' duration) (six publications), wrong population (three) and wrong intervention (one). One study was not an RCT, and one was withdrawn prior to enrolment of participants. The remaining 16 citations related to eight studies, which were included in the qualitative synthesis. Figure 1 shows the trial flow.
1.

Study flow diagram.
Included studies
Eight studies met all the inclusion criteria and were included in the review, but only four could be included in the quantitative synthesis. The four studies appearing in at least one meta‐analysis randomised 2049 people with asthma to the treatment arms compared in this review. One of the remaining four studies was terminated (NCT00706446), one did not report any results on clinicaltrials.gov (NCT01290874), and the two others did not report data that could be pooled with the other studies (Rajanandh 2014; Rajanandh 2015). Data that could not be combined in meta‐analysis are described narratively in Effects of interventions. Summary study characteristics including study duration and location, treatments received and blinding, are presented in Table 2.
1. Summary characteristics of included studies.
| Study ID | Country | Total number participants | Duration (weeks) | Design | ICS | Add‐ons | Mean age (years) | % predicted FEV1 |
| NCT00350207 | International | 262 | 16 | P, DB/DD | Budesonide 400‐1000 mcg | 1) Tio Respimat 5 mcg once daily 2) Salmeterol 50 mcg twice daily |
43.5 42.3 |
74.1 75.6 |
| NCT00565266* | US | 210 | 14 | C, DB/DD | Beclomethasone dipropionate 80 mcg x2 | 1) Tio HandiHaler 18 mcg once daily 2) Salmeterol 50 mcg twice daily |
42.2 | 71.5 |
|
NCT00706446 No data |
US | 355** | 52** | P, OL | Variable | 1) Tiotropium (dose/ type NR) 2) Salmeterol or formoterol (dose NR) |
NR | NR |
| NCT01172808 | International | 801 | 24 | P, DB/ DD | Continued stable, medium dose | 1) Tio Respimat 2.5 mcg once daily 2) Tio Respimat 5 mcg once daily 3) Salmeterol 50 mcg twice daily |
43.7 44.4 42.6 |
NR |
| NCT01172821 | International | 776 | 24 | P, DB/DD | Continued stable, medium dose | 1) Tio Respimat 2.5 mcg once daily 2) Tio Respimat 5 mcg once daily 3) Salmeterol 50 mcg twice daily |
43.0 44.3 41.5 |
NR |
|
NCT01290874 No data |
US | Unclear | 52 | P, OL | NR | 1) Tio HandiHaler 18 mcg once daily 2) Salmeterol 50 mcg or formoterol 12 mcg twice daily |
NR | NR |
| Rajanandh 2014 | India | 84 | 13 | P, OL | Budesonide 400 mcg | 1) Tio HandiHaler 18 mcg once daily 2) Formoterol 12 mcg twice daily |
40.4 37.2 |
66.9 66.6 |
| Rajanandh 2015 | India | 172 | 26 | P, OL | Budesonide 400 mcg | 1) Tio HandiHaler 18 mcg once daily 2) Formoterol 12 mcg twice daily |
37.4 38.4 |
66.1 66.2 |
Total number participants is the number randomised to the groups of interest for this review. Age and % predicted FEV1 are presented as mean values.
C: cross‐over; DB/DD: double‐blind, double‐dummy; FEV1: forced expiratory volume in 1 second; ICS: inhaled corticosteroids; NR: not reported; OL: open label; P: parallel.
* Cross‐over study so characteristics are for the whole population; every participant received each treatment for 14 weeks with a 2‐week washout period.
** Planned enrolment and duration ‐ study was terminated.
Design and duration
All eight studies were RCTs of at least 12 weeks' duration. All but one of the studies had a parallel design, and the remaining was a three‐period cross‐over (NCT00565266). Three studies were international trials conducted at multiple sites across various countries (NCT00350207; NCT01172808; NCT01172821), three were conducted in the US (NCT00565266; NCT00706446; NCT01290874), and two were conducted in India (Rajanandh 2014; Rajanandh 2015). Overall, six of the eight studies lasted six months or less. Two were one‐year long studies, but neither reported any data (NCT00706446; NCT01290874).
Four of the studies were double‐blind, double‐dummy designs, and four were open‐label; none of the open‐label studies contributed data to the meta‐analyses.
Participant inclusion and exclusion criteria
All of the studies listed detailed inclusion and exclusion criteria within their published reports or on a trial registration website. All studies recruited men and women of at least 18 years of age, and some stipulated an upper age limit of 60 or 65 years. Other inclusion criteria that were common across studies included currently not smoking and a smoking history of less than 10 pack‐years, informed consent, contraception measures for women, and ability to use study devices and perform the necessary procedures. The diagnosis of asthma required across studies was defined differently, and sometimes only a 'clinical history' with no specific criteria stated. In general, though, reversibility to short‐acting beta2‐agonists (SABA), percentage predicted FEV1 above 40% and the need for daily controller medication was required. One study recruited only black people (NCT01290874), and another study recruited people who were homozygous for arginine at the 16th amino acid position of the beta2‐adrenergic receptor (NCT00350207).
Exclusion criteria that were common across studies included a range of other "significant" medical illnesses, often to be judged so by the study investigators (commonly including cancers, myocardial infarction, heart failure and arrhythmia). Also common were the exclusion of other lung diseases (commonly COPD, bronchiectasis, cystic fibrosis and tuberculosis), thoracotomy with pulmonary resection and current or recent participation in pulmonary rehabilitation. Studies also generally excluded participants with a recent respiratory tract infection or exacerbation of asthma (within four weeks), and people who had been classified as having life‐threatening asthma within five years of study entry. Pregnant or nursing women were generally excluded from participation, as were people with hypersensitivity or contraindications to any component of the study drugs, and people with current prior alcohol or drug misuse. Exclusion due to the use of other asthma medications were varied, but generally participants were not included if they were taking, or had recently taken, other long‐acting medications or drugs given to people with very severe asthma (e.g. OCS), and anti‐immunoglobulin (Ig)E medications such as omalizumab.
Participant baseline characteristics
Baseline characteristics, with the exception of percentage male and mean age, were generally poorly reported across studies. Mean percentage predicted FEV1 at baseline was between 66% and 76% in the three studies reporting it (NCT00350207; NCT00565266; Rajanandh 2014; Rajanandh 2015). Mean ages were all between 37 and 45 years. The proportion of men and women was fairly balanced within studies reporting this information, and across studies the percentage of men ranged between 33% (NCT00565266) and 65% (LAMA group of Rajanandh 2014).
Characteristics of the interventions
All of the studies compared the LAMA, tiotropium, to salmeterol or formoterol, both used as an add‐on drug to ICS. NCT01172808 and NCT01172821 were multi‐arm twin trials that included separate arms for two doses of tiotropium Respimat, 2.5 mcg daily and 5 mcg daily. NCT00350207 used tiotropium at 5 mcg daily; NCT00565266, NCT01290874, Rajanandh 2014, and Rajanandh 2015 used tiotropium HandiHaler 18 mcg daily, but only one of these contributed data to at least one meta‐analysis. The remaining study, which was terminated (NCT00706446), did not state the type and dose of tiotropium and did not contribute any data.
The LABA used for comparison was salmeterol 50 mcg twice daily in NCT00350207NCT00565266, NCT01172808 and NCT01172821, the four studies comprising most of the analyses. NCT00706446 and NCT01290874 allowed salmeterol 50 mcg or formoterol 12 mcg, both twice daily, but neither contributed data to the analyses. Rajanandh 2014 and Rajanandh 2015 used formoterol 12 mcg twice daily.
The ICS used in the intervention and comparison groups varied. Some studies included the ICS as part of the randomised treatment issued by the investigators, and others stipulated a specific dose regimen as part of the inclusion criteria. NCT00350207 used budesonide at 400 to 1000 mcg (low to medium dose), NCT00565266 used beclomethasone dipropionate 80 mcg twice daily (low dose), and Rajanandh 2014 and Rajanandh 2015 used budesonide 400 mcg (low dose). The twin studies, NCT01172808 and NCT01172821, asked participants to continue their usual ICS at a stable, medium dose. NCT00706446 allowed ICS at variable dosing based on the person's prior dose and the physician's judgement, and NCT01290874 did not describe the ICS used.
Outcomes and analysis structure
We have presented pooled data without subgroups for all of the pre‐specified outcomes under the Comparison 1, LAMA add‐on versus LABA add‐on. Continuous data were available as means and standard error for most of the studies, so could be entered using the Review Manager 5 calculator (RevMan 2014). However, the continuous data for NCT00565266 had to be entered as adjusted between‐group differences to account for the trial's cross‐over design, so outcomes to which the study contribute data were analysed with GIV.
In general, four studies made up the majority of the analyses: three parallel (NCT00350207; NCT01172808; NCT01172821) and one cross‐over (NCT00565266). Two of these studies included two doses of tiotropium that were merged in the main analysis and dealt with separately for the primary outcomes in subgroups in Comparison 2 (adjusting for double counting of the control group). The two dose groups from these two studies were also compared head‐to‐head for the primary outcomes in Comparison 3 (LAMA dose head‐to‐head).
Exacerbations were generally poorly reported. Much of the data analysed for 'Exacerbations requiring hospital admission' were extracted from serious adverse events coded as 'asthma' using Medical Dictionary for Regulatory Activities (MedDRA) terminology. This was deemed appropriate since ClinicalTrials.gov defines serious adverse events as those that "result in death, require either inpatient hospitalisation or the prolongation of hospitalisation, are life‐threatening, result in a persistent or significant disability/incapacity or result in a congenital anomaly/birth defect". However, we did not include non‐serious adverse event data coded as 'asthma' in the primary outcome 'Exacerbations requiring OCS', as the definition was not sufficiently specific and may have included events that were either more or less severe. For this reason, we analysed the asthma adverse event data as a post‐hoc outcome separate from the pre‐specified 'Exacerbations requiring OCS'.
Four studies reported asthma‐related quality of life on the AQLQ, and Rajanandh 2015 used the St George's Respiratory Questionnaire (SGRQ). We reported the SGRQ data separately in the results rather than combining it using SMD with the AQLQ data so the results are easier to interpret on the relevant scales.
The studies used several measures of lung function that could not be compiled meaningfully in a single meta‐analysis (trough peak expiratory flow (PEF), trough and peak FEV1, percentage predicted FEV1, and trough and peak forced vital capacity (FVC)). Rajanandh 2014 and Rajanandh 2015 both measured percentage predicted FEV1, although only Rajanandh 2014 data in a format that could have been entered into a meta‐analysis, so these data have been summarised narratively. We chose to analyse different measures of lung function separately and have described the data for each in the results. Trough FEV1 was pre‐specified as our preferred measure.
Excluded studies
We examined the full‐text reports for 19 studies, which we ultimately excluded. We excluded seven studies, with 11 associated records, because they used the wrong comparator for this review (Kerstjens 2012; NCT00772538; NCT00776984; NCT01316380; NCT01340209; NCT02066298; NCT02127697). All of these compared a LAMA with placebo, either alone or on top of other treatments such as ICS or LABA plus ICS. One study considered the effects of a smoking cessation programme among people with asthma, and was not relevant to the research question (NCT01696214). We excluded six studies because they were shorter than the pre‐specified 12 weeks. Two were single‐dose studies (CTRI/2008/091/000306; JPRN‐UMIN000010352), and four administered treatments for two to three weeks (EUCTR2006‐003385‐34‐NL; NCT00557700; NCT01573624; NCT01641692). Some of these studies also used the wrong comparator for this review. Three studies recruited the wrong population for this review: two studied people with COPD (JPRN‐UMIN000003618; JPRN‐UMIN000005459), and one recruited adolescents rather than adults with asthma (Vogelberg 2014). We excluded one study because it used an observational rather than randomised controlled design (NCT00557180), and one because the protocol was withdrawn before any participants were enrolled (NCT00546234).
Risk of bias in included studies
Overall, several studies were given high risk of bias ratings, particularly in the blinding domains and selective reporting, and there was some uncertainty in others, mostly due to insufficient reporting. However, most of the high risk of bias judgements were associated with studies that did not contribute data to the meta‐analyses. Risk of bias judgements are explained for each study in the Characteristics of included studies table, and Figure 2 shows an overview.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Reporting within the clinicaltrials.gov records was not detailed enough in most cases to assess this domain fully, but prior contact with the study sponsors and additional contact for this review confirmed standard practices of the industry‐funded trials warranting low risk of bias judgements (using computerised codes and automated allocation systems). We were unsure of the allocation procedures in the two trials contributing no data to the analyses. Rajanandh 2014 and Rajanandh 2015 described allocation concealment with opaque envelopes.
Blinding
Three studies were open label and were rated high risk of bias for both performance and detection bias; placebos were not used to blind the participants and personnel from group allocation and there was no information in any of the reports to suggest that outcome assessment was blinded to control for detection bias. However, two of the open‐label studies did not feature in any of the meta‐analyses, and the other only contributed data to two secondary outcomes. As such, it is unlikely that the majority of results were affected by bias related to lack of blinding.
Incomplete outcome data
Two studies were considered high risk of bias for attrition bias. Three studies analysed participants who completed the trial, and did not attempt to impute values for participants who dropped out, which was over 25% of the population in Rajanandh 2014 and just under 20% in each of the relevant groups in Rajanandh 2015; one other stated that an intention‐to‐treat analysis was undertaken, but the numbers stated for each outcome suggested 20% to 21% were not accounted for. Two included studies did not report attrition (NCT00706446; NCT01290874). The three remaining studies, which dominated the analyses, had low and even rates of attrition and were rated low risk of bias.
Selective reporting
Two of the included studies were rated high risk of bias due to missing outcomes or insufficient reporting of data to allow meta‐analysis. In two others, it was unclear whether all outcomes had been reported due to lack of clarity in the listing of outcomes. One study reported data as stated in the protocol and were rated low risk of bias (NCT00350207), authors of another study provided additional data that changed our rating from unclear to low (NCT00565266), and missing data for two other studies were subsequently published in a pooled report, which changed the ratings from high to low (NCT01172808; NCT01172821).
Other potential sources of bias
No additional sources of bias were identified in four studies, which were all rated low risk of bias. The three studies contributing the majority of data to the analyses were given unclear ratings, mainly because they were all funded by Boehringer Ingelheim and because there was minimal baseline information about the participants to judge whether the groups were well balanced. The remaining study was rated high risk of bias because it was terminated without a description why, and without any interim results.
Effects of interventions
See: Table 1
Primary outcomes
Exacerbations requiring oral corticosteroids
Three studies reported exacerbations requiring OCS, two of which were twin trials reported as a pooled result (NCT01172808; NCT01172821). Three more people per 1000 would have an exacerbation on LAMA compared with LABA, but the CIs ranged from 29 fewer to 61 more. The effect was too imprecise to determine whether one treatment reduced these exacerbations more than the other (OR 1.05, 95% CI 0.50 to 2.18; 1753 participants; 3 studies; low quality; Analysis 1.1). The evidence was downgraded due to this uncertainty, and for inconsistency because the two results (pooled twin trials and the cross‐over study) gave different directions of effect.
1.1. Analysis.
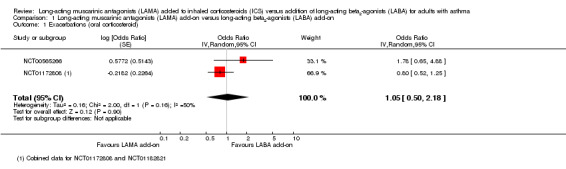
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 1 Exacerbations (oral corticosteroid).
We also analysed data extracted from the non‐serious adverse event tables that were recorded as 'asthma'. It is unclear what sort of event qualified under this outcome, but three studies reported data in this way. The pooled effect was more precise and did not show that LAMA or LABA reduced these events more than the other (OR 0.95, 95% CI 0.74 to 1.22; 1839 participants; 3 studies; I2 = 0%; moderate quality; Analysis 1.13). Evidence for this additional post‐hoc analysis was rated moderate quality after being downgraded once for indirectness, as it was a proxy outcome with uncertainties about the definitions used.
1.13. Analysis.
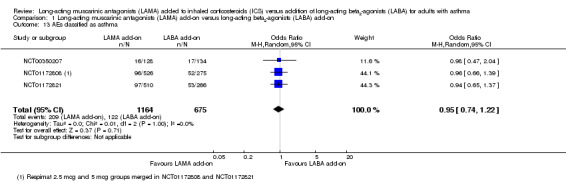
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 13 AEs classified as asthma.
Quality of life
People treated with LAMA add‐on scored slightly worse than LABA add‐on for quality of life measured on the AQLQ (MD ‐0.12, 95% CI ‐0.18 to ‐0.05; 1745 participants; 4 studies; I2 = 0%; Analysis 1.2). The difference was statistically significant but both CIs fell well below the established minimal clinically important difference (MCID) of 0.5 on the AQLQ, so it is unlikely to be a clinically meaningful difference. The evidence was rated high quality, as the effect was relatively precise and consistent, and the studies were of good methodological quality.
1.2. Analysis.
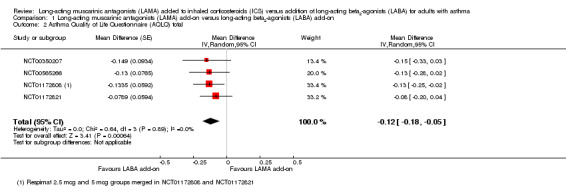
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 2 Asthma Quality of Life Questionnaire (AQLQ) total.
Rajanandh 2015 measured quality of life using the SGRQ but only presented data graphically. Total scores in both groups were significantly improved after six months, but the mean score in the LAMA group was worse than the LABA group. There was no information about variance and whether the difference was statistically significant.
Any serious adverse event
The CIs were too wide to determine whether serious adverse events were more likely with LAMA or LABA because so few events occurred in the studies (OR 0.84, 95% CI 0.41 to 1.73; 2012 participants; 4 studies; I2 = 23%; low quality; Analysis 1.3). The estimate suggested 4 fewer people per 1000 would have serious adverse events on LAMA, but the CIs ranged from 17 fewer to 15 more, and the evidence was downgraded twice for this reason.
1.3. Analysis.
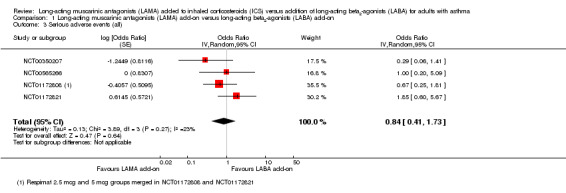
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 3 Serious adverse events (all).
Secondary outcomes
Exacerbations requiring hospitalisation
The evidence for this more serious form of exacerbation was very low quality, mostly because events were rare in the studies. There were slightly fewer exacerbations in the LAMA add‐on groups, but the CIs were too wide to judge whether LAMA or LABA were more effective, and visually there was inconsistency between study results (OR 0.72, 95% CI 0.18 to 2.92; 2022 participants; 4 studies; I2 = 20%; very low quality; Analysis 1.4).
1.4. Analysis.
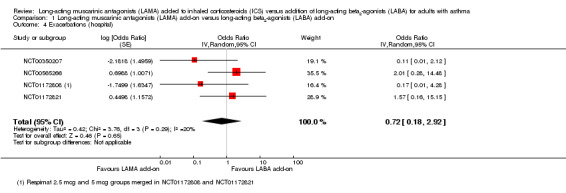
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 4 Exacerbations (hospital).
Lung function
We downgraded all of the lung function outcomes, with the exception of trough FEV1, which was our preferred measure, because they were reported by the same few studies and we were unsure of their relevance to the question on top of our preferred measure (i.e. indirectness).
Forced expiratory volume in one second
Trough FEV1 was higher in people given LAMA add‐on compared with people given LABA add‐on, but again this effect was relatively small and there was heterogeneity between study results (downgraded once) (MD 0.05 L, 95% CI 0.01 to 0.09; 1745 participants; 4 studies; I2 = 46%; moderate quality; Analysis 1.5). Three studies reported peak FEV1 but two were within three hours of bronchodilation and one immediately after four puffs of albuterol, so we did not pool the data. Rajanandh 2014 reported percentage predicted FEV1 and showed that LAMA add‐on was less effective than LABA add‐on, but they did not report when the measurement was taken (i.e. pre‐ or post‐bronchodilator), so it may not have been a fair comparison given the faster onset of formoterol. Rajanandh 2015 also measured percentage predicted FEV1 and suggested that tiotropium was less effective than formoterol, but did not report data sufficiently to combine it with Rajanandh 2014 in a meta‐analysis.
1.5. Analysis.
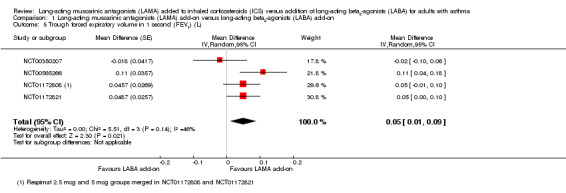
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 5 Trough forced expiratory volume in 1 second (FEV1) (L).
Peak expiratory flow
People treated with LAMA add‐on had slightly better trough PEF than those given LABA add‐on, but the difference was small (MD 5.78 L/minute, 95% CI 0.86 to 10.71; 1745 participants; 4 studies; I2 = 0%; moderate quality; Analysis 1.7).
1.7. Analysis.
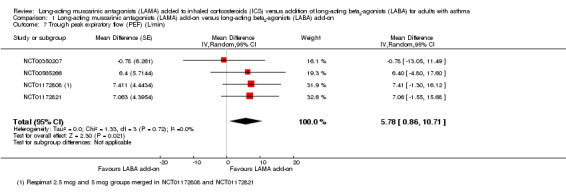
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 7 Trough peak expiratory flow (PEF) (L/min).
Forced vital capacity
Trough FVC was slightly higher in people taking LAMA add‐on compared with people taking LABA add‐on, but the CIs included the possibility that LABA were better (MD 0.03 L, 95% CI ‐0.02 to 0.07; 1745 participants; 3 studies; I2 = 0%; Analysis 1.8). The two studies reporting peak FVC did not detect a difference between the two add‐on therapies, with the CIs including benefit on either treatment (MD ‐0.00 L, 95% CI ‐0.04 to 0.03; 1483 participants; 2 studies; I2 = 4%; Analysis 1.9). The evidence for both FVC outcomes was only downgraded for indirectness (see above), and rated moderate quality.
1.8. Analysis.
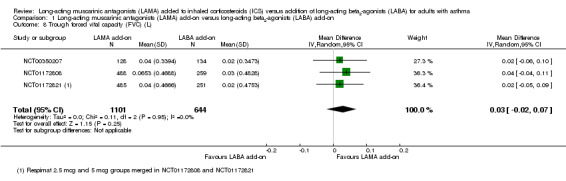
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 8 Trough forced vital capacity (FVC) (L).
1.9. Analysis.
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 9 Peak FVC (L).
Asthma control
Three studies reporting the ACQ showed that the asthma of people taking LAMA add‐on were slightly less controlled than people taking LABA add‐on (MD 0.06, 95% CI 0.00 to 0.13; 1483 participants; 3 studies; I2 = 0%; Analysis 1.10). As with the AQLQ, the evidence was rated high quality but the effect and its CIs were not in the range of the MCID (also 0.5), and touched the line of no effect, so the difference between the treatments was unlikely to be of clinical significance.
1.10. Analysis.
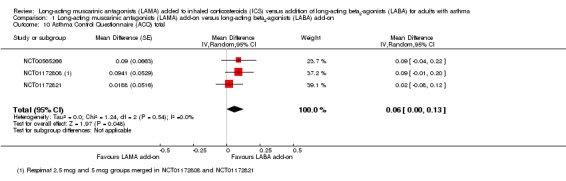
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 10 Asthma Control Questionnaire (ACQ) total.
The two twin studies reported the number of people meeting criteria for 'response' on the ACQ, defined as people whose score improved by at least the MCID. The studies detected no difference between the groups, and the CIs were too wide to infer equivalence of the two treatments (OR 0.91, 95% CI 0.73 to 1.13; 1563 participants; 2 studies; I2 = 0%; Analysis 1.11).
1.11. Analysis.
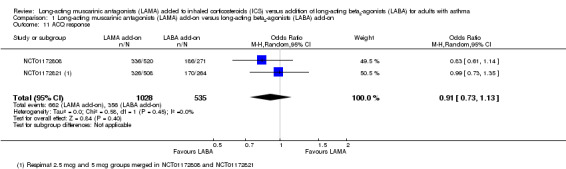
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 11 ACQ response.
Any adverse events
More people taking LAMA had an adverse event than people taking LABA, but the difference was not statistically significant (OR 1.11, 95% CI 0.92 to 1.35; 1839 participants; 3 studies; I2 = 0%; Analysis 1.12). The CIs were relatively tight but not so much that equivalence of the two treatments could be concluded.
1.12. Analysis.
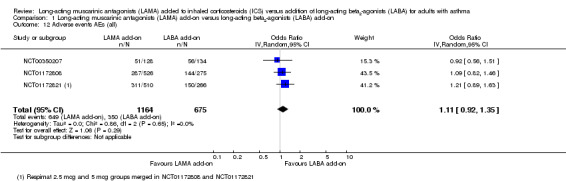
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 12 Adverse events AEs (all).
Subgroup analyses
Duration of therapy
The four studies reporting data for the primary outcomes were all less than six months' duration, so we could not perform a subgroup analysis as planned.
Corticosteroid dose
We did not conduct a subgroup analysis on the basis of corticosteroid dose because there was not a clear comparison to be made between the four studies contributing data to the primary outcomes. NCT01172808 and NCT01172821 allowed any stable medium dose of corticosteroid, NCT00565266 allowed low‐dose beclomethasone, and NCT00350207 allowed any dose between 400 and 1000 mcg budesonide but did not report the mean dose taken during the study.
Dose and type of long‐acting beta2‐agonists
All four studies reporting data for the primary outcomes used salmeterol 50 mcg twice daily in the comparison group, so there was no subgroup comparison to be made.
Dose and type of long‐acting muscarinic antagonists
A subgroup analysis of the exacerbations (OCS) data comparing dose and type of LAMA showed some differences in effect that were not statistically different from each other (test for subgroup differences: I2 = 25%, P value = 0.26; Analysis 2.1). Since so few studies reported the outcome, it was difficult to judge whether there was a true difference between the two Respimat doses and HandiHaler 18 mcg.
2.1. Analysis.
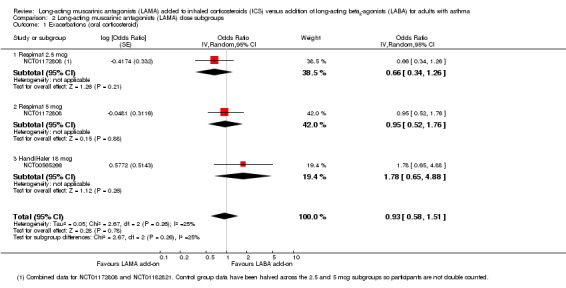
Comparison 2 Long‐acting muscarinic antagonists (LAMA) dose subgroups, Outcome 1 Exacerbations (oral corticosteroid).
The same four studies appeared in analyses for the AQLQ and serious adverse events outcomes. There was no evidence of significant subgroup differences between Respimat 2.5 mcg, Respimat 5 mcg, and HandiHaler 18 mcg in either analysis (Analysis 2.2 and Analysis 2.3; test for subgroup differences I2 = 0% in both cases).
2.2. Analysis.
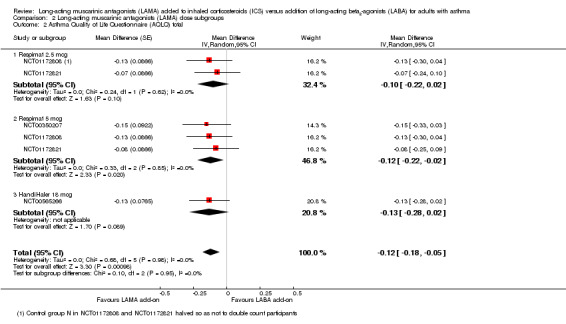
Comparison 2 Long‐acting muscarinic antagonists (LAMA) dose subgroups, Outcome 2 Asthma Quality of Life Questionnaire (AQLQ) total.
2.3. Analysis.
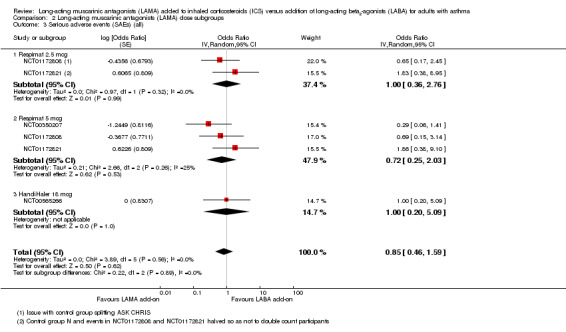
Comparison 2 Long‐acting muscarinic antagonists (LAMA) dose subgroups, Outcome 3 Serious adverse events (SAEs) (all).
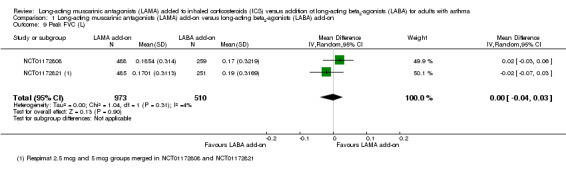
For all three primary outcomes, we also conducted a head‐to‐head comparison of Respimat 2.5 mcg versus Respimat 5 mcg using the two trials including both doses (NCT01172808; NCT01172821). The head‐to‐head comparisons showed no statistically significant difference with regards to exacerbations requiring OCS, although fewer occurred in the lower‐dose group OR 0.69, 95% CI 0.40 to 1.22; 1036 participants; 1 study; Analysis 3.1). The doses were similar with respect to AQLQ score (MD 0.01, 95% CI ‐0.09 to 0.10; 973 participants; 2 studies; I2 = 0%; Analysis 3.2) and rates of serious adverse events (OR 1.09, 95% CI 0.47 to 2.49; 1036 participants; 2 studies; I2 = 0%; Analysis 3.3).
3.1. Analysis.
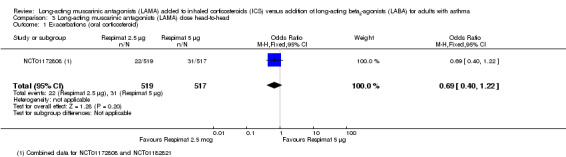
Comparison 3 Long‐acting muscarinic antagonists (LAMA) dose head‐to‐head, Outcome 1 Exacerbations (oral corticosteroid).
3.2. Analysis.
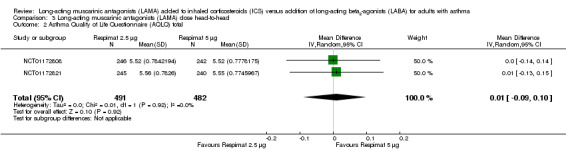
Comparison 3 Long‐acting muscarinic antagonists (LAMA) dose head‐to‐head, Outcome 2 Asthma Quality of Life Questionnaire (AQLQ) total.
3.3. Analysis.
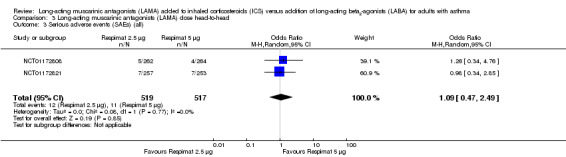
Comparison 3 Long‐acting muscarinic antagonists (LAMA) dose head‐to‐head, Outcome 3 Serious adverse events (SAEs) (all).
Sensitivity analyses
Studies at high risk of bias for blinding of participants and personnel
We rated the four open‐label studies included in the review at high risk of bias for blinding but they did not contribute data to the primary outcomes, so could not be excluded in a sensitivity analysis.
Unpublished data
We included no conference abstracts. With the exception of additional data provided by the authors of NCT00565266, which was removed in the cross‐over sensitivity analysis (below), all of the data included in the primary outcomes were available in peer‐reviewed reports or publicly available websites.
Cross‐over trials
We removed the cross‐over study, NCT00565266, from the three primary outcomes in a sensitivity analysis based on study design.
Removing NCT00565266 from the 'Exacerbations requiring OCS' analysis left only the pooled twin trials, which showed a more favourable effect for LAMA than the pooled result (Analysis 4.1), but the conclusions remained the same. It was not possible to determine whether one treatment was more effective than the other. The AQLQ result was very similar with the cross‐over trial removed (Analysis 4.2), and the serious adverse event effect was slightly larger in magnitude but even more imprecise without the cross‐over data (Analysis 4.3).
4.1. Analysis.
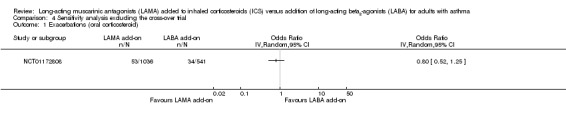
Comparison 4 Sensitivity analysis excluding the cross‐over trial, Outcome 1 Exacerbations (oral corticosteroid).
4.2. Analysis.
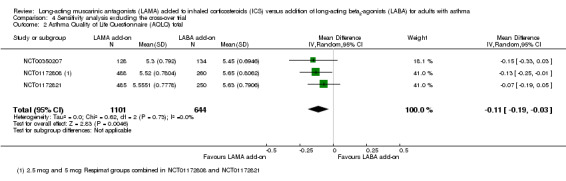
Comparison 4 Sensitivity analysis excluding the cross‐over trial, Outcome 2 Asthma Quality of Life Questionnaire (AQLQ) total.
4.3. Analysis.
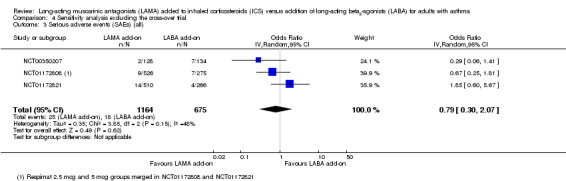
Comparison 4 Sensitivity analysis excluding the cross‐over trial, Outcome 3 Serious adverse events (SAEs) (all).
Discussion
Summary of main results
We included eight studies meeting the inclusion criteria, but four double‐blind, double‐dummy studies dominated the analyses, looking primarily at tiotropium (Respimat) versus salmeterol. Trials ranged between 12 and 52 weeks, but the main four trials were all under six months' duration and contributed data from about 2000 people to most of the analyses.
There was low quality evidence with regards to exacerbations requiring treatment with OCS with the direction of effect slightly in favour of LABA over LAMA but with very wide CIs. In absolute terms, 3 more people per 1000 had an exacerbation on LAMA compared with LABA, but the CIs ranged from 29 fewer to 61 more. Imprecision was also an issue for serious adverse events (rated low quality) and exacerbations requiring hospital admission (rated very low quality), because there were too few events in the analyses to determine whether one treatment was better than the other.
People treated with LAMA scored slightly worse on two scales measuring quality of life (AQLQ) and asthma control (ACQ); the evidence was rated high quality but the differences were below the MCID on both scales.
There was some evidence to support small benefits of LAMA on lung function, including on our pre‐specified preferred measure trough FEV1. However, this was not the case for all the measures that we considered; the effects were not always statistically significant or in favour of LAMA, and it was not clear whether the magnitude of the differences were clinically significant.
More people had adverse events with LAMA but the difference with LABA was not statistically significant.
Overall completeness and applicability of evidence
The current evidence base to address this question was incomplete in several respects. We were unable to assess the robustness of the data with most of the planned sensitivity and subgroup analyses because too few studies reported the primary outcomes. All of the studies used the LAMA, tiotropium, mostly delivered via the Respimat rather than HandiHaler, so we do not know whether our findings will apply to other LAMA drugs such as glycopyrronium and aclidinium (neither of which are licensed for use in asthma at present). Similarly, since all the studies in the analyses used salmeterol as the LABA comparator, there is a possibility that formoterol or one of the once‐daily preparations (e.g. indacaterol or vilanterol) would give different results. Furthermore, there is currently no comparison of LAMA add‐on with combination formoterol plus budesonide in the 'SMART' approach as maintenance and reliever therapy, for which there is evidence of added efficacy compared with current best practice or higher doses of corticosteroid in combination therapy (Cates 2013; Kew 2013). The SMART approach is unique to formoterol plus budesonide due to the fast‐onset properties of formoterol, which may represent a benefit over LAMA add‐on, and this is an area for potential comparison. Overall, the use of LAMA is relatively new for asthma, with only one license extension for Spiriva Respimat used in combination with LABA plus ICS currently granted in the UK. As such, the research base is likely to grow in coming years and future versions of this review may be more able to form meaningful conclusions that account for the possible effect moderators we outlined in our planned subgroup analyses.
As it stands, the evidence base directly comparing LAMA plus ICS with LABA plus ICS is small, and so clinicians considering the use of LAMA as an alternative to LABA will likely turn to the larger evidence bases for each drug against ICS alone. This evidence base is particularly strong for LABA, with the most recent review including 77 studies of over 20,000 people compared with four studies of about 2000 people for LAMA. This evidence bias towards the older and more commonly used LABA class of drugs has highlighted the reliable evidence for LABA add‐on both in terms of its efficacy (Ducharme 2008), and safety concerns (Cates 2014), but comparing LAMA and LABA add‐on in this indirect way is not as reliable as using randomised trials comparing them directly.
In terms of the conduct of the included studies in this review, the three open‐label studies reported very few data between them, which meant the four multi‐site double‐blind, double‐dummy studies dominated the findings. While this means the meta‐analyses are less prone to biases from within the studies, it may represent a reporting bias towards industry‐funded multicentre studies (three of the four were international trials funded by Boehringer‐Ingelheim). These studies are likely to be highly controlled and conducted to standards that may not be representative of normal medical care with respect to service provision and inhaler adherence. Another downside of these studies is their fairly strict inclusion and exclusion criteria, which may leave an uncertainty of the risk and benefits of LAMA and LABA used in certain patient subgroups not represented in the trials. For example, the cardiac adverse effects of tiotropium that have been documented in COPD trials may be dangerous for people with asthma with cardiac or renal co‐morbidity (MHRA 2010), particularly older people with significant smoking histories.
We chose to look specifically at adults in this review and the studies recruited similar populations with respect to demographics, where this information was available. The inclusion criteria and baseline characteristics of the included populations would suggest the results are most applicable to non‐smoking adults with moderate asthma (percentage predicted FEV1 around 70%), who are experiencing symptoms on their ICS controller inhaler. Studies consistently excluded participants with histories of life‐threatening asthma and other medical illnesses, so it is unclear how this evidence may apply to these more complex populations.
Quality of the evidence
Of all the outcomes that we analysed, we rated only two high quality. The most common reason for downgrading evidence was imprecision, with several outcomes being downgraded once, and two were downgraded twice for this reason; this often precluded any meaningful conclusion on the relative benefits of the treatments, as the CIs included significant benefit of both, even if the direction of the estimate favoured one treatment. This imprecision was partly due to the relatively small number of trials that could be included in most of the meta‐analyses, and because some of the outcomes that we considered were rare (serious adverse events and exacerbations requiring hospital admission in particular).
Our primary exacerbation outcome, those requiring a course of OCS, was only reported by one study, which made the estimate imprecise. The outcome is also likely to be affected by publication bias, as two studies planned to report time to exacerbation data but omitted the analyses or any related information because "less than 50% of patients in each treatment group experienced an asthma exacerbation" (NCT01172808; NCT01172821). We learnt through correspondence with the study sponsor that additional data may be available once full manuscripts are published for these trials in 2015.
Two studies by the same author team had not been published in peer‐reviewed journals and no data were available on the registration website (NCT00706446; NCT01290874), which may represent a level of publication bias in all the analyses. It is unclear how these studies may have affected results if they had been completed or reported fully, as the number of randomised participants was not given. Rajanandh 2014 reported only one outcome that was pre‐specified in this review, percentage predicted FEV1, and Rajanandh 2015 reported only percentage predicted FEV1 and SGRQ, and these may have been affected by bias related to their open‐label design; they also could not be combined with the measures reported by other studies in the review, so the evidence was very low quality.
Heterogeneity was rarely an issue in the analyses, which may be due to the four studies contributing data to the analyses all being well‐controlled trials that were similar with respect to several of the expected effect moderators (e.g. type of LAMA and LABA compared, background ICS dose, age and study duration). It is for this reason that the applicability of the findings may be compromised.
Potential biases in the review process
We conducted the review to the standards set by MECIR 2013, and in accordance with the published protocol wherever possible (Kew 2015). Any deviations from the protocol have been logged in the Differences between protocol and review section, and were largely a result of insufficient data as described above.
It is unlikely that we missed any relevant studies, as a skilled information specialist conducted the main electronic searches, which were supplemented by extensive supplementary searches of several other resources (drug company trial registries and reference lists of associated studies and reviews), in addition to those required by MECIR 2013 (clinicaltrials.gov, WHO trials portal). By searching these additional resources, we identified one study that had been terminated and another that did not reported any data, which illustrates the possibility of publication bias.
We also attempted to contact all trial authors for additional or missing data and study information where this was not available in the published reports, and authors of the cross‐over trial provided us with re‐formatted data in a way that accounted for intercorrelation of matched pairs (Elbourne 2002). Entering data with these transformations, and entering continuous data using GIV is more accurate for this type of trial, and may explain subtle differences between the results of our review and others. Even so, we tested the robustness of the results by removing the cross‐over data from the primary outcomes in a sensitivity analysis, and conclusions were not affected.
Agreements and disagreements with other studies or reviews
Several systematic reviews have considered the use of tiotropium for asthma compared with a range of possible treatment options (Befekadu 2014; Rashid 2014; Rodrigo 2015; Tian 2014), some of which considered LABA add‐on as a comparator.
Rodrigo 2015 performed meta‐analyses of several treatment strategies including ICS plus LAMA versus ICS plus LABA and concluded, "the use of tiotropium in patients poorly controlled despite the use of medium to high doses of ICS was not inferior to salmeterol". There were some differences in the meta‐analytic methods used, but the effects were based on the same four studies and showed a broadly similar pattern to the results of this review, with clinically small benefits of tiotropium over LABA on some measures of lung function, small benefits of LABA over tiotropium on the AQLQ, and mostly non‐significant effects on other measures. The authors interpreted this as evidence of non‐inferiority, which may not be justified because there was no pre‐specified margin or necessary conditions to be met to reach this conclusion.
Befekadu 2014 provided a narrative synthesis of evidence for tiotropium in asthma, referring only to NCT00350207 and NCT00565266 because the twin trials had not yet been published. Their conclusions were more in line with our own interpretation, highlighting the possible benefits of tiotropium over salmeterol on lung function, which supports further investigation, and acknowledging the inconsistencies and imprecision in study findings overall.
Authors' conclusions
Implications for practice.
Direct evidence of long‐acting muscarinic antagonists (LAMA) versus long‐acting beta2‐agonists (LABA) as add‐on therapy is currently limited to studies of less than six months comparing tiotropium Respimat to salmeterol, and we do not know how they compare in terms of exacerbations and serious adverse events. There is moderate quality evidence that LAMAs show small benefits over LABA on some measures of lung function, and high quality evidence that LABAs are slightly better for quality of life, but the differences were all small. Given the much larger evidence base for LABA versus placebo for people whose asthma is not well controlled on ICS, the current evidence is not strong enough to say that LAMA can be substituted for LABA as add‐on therapy.
Implications for research.
The results of this review, alongside pending results from related reviews assessing the use of LAMA in other clinical scenarios, will help to define the role of these drugs in asthma and should be updated as results from known ongoing trials emerge.
What's new
| Date | Event | Description |
|---|---|---|
| 11 September 2018 | Amended | New literature search run and screened, but not fully incorporated. References added to Studies awaiting classification. One new trial with 80 participants eligible for inclusion (Zhang 2018), one full report of included study that will allow inclusion of approximately 1000 Black people with asthma ‐ an important subgroup (Wechsler 2015). Several new references to already included study which may contain new relevant data (MezzoTinA). |
Acknowledgements
We are grateful to Liz Stovold for designing and running the electronic searches.
Rebecca Normansell was the Editor for this review and commented critically on the review.
The Background and Methods section of this protocol/review is based on a standard template used by Cochrane Airways Group.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, the National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (T he Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 Muscarinic* NEXT Antagonist*
#6 LAMA:TI,AB
#7 Glycopyrronium*
#8 NVA237
#9 Seebri OR Breezhaler
#10 Aclidinium*
#11 LAS34273
#12 Turdorza or Pressair or Eklira or Genuair
#13 tiotropium*
#14 Spiriva
#15 umeclidinium*
#16 GSK573719
#17 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16
#18 MeSH DESCRIPTOR Adrenergic beta‐Agonists
#19 long* NEAR beta* NEAR agonist*
#20 LABA:TI,AB
#21 *formoterol
#22 salmeterol
#23 vilanterol
#24 #18 or #19 or #20 or #21 or #22 or #23
#25 #4 and #17 and #24
[Note: in search line #1, MISC2 denotes the field in which the reference has been coded for condition, in this case, asthma]
Appendix 3. Requested dichotomous data format for cross‐over trials
| Event on LABA | Event on LAMA | ||
| Frequency | No | Yes | Total |
| No | |||
| Yes | |||
| Total | |||
Data and analyses
Comparison 1. Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations (oral corticosteroid) | 2 | Odds Ratio (Random, 95% CI) | 1.05 [0.50, 2.18] | |
| 2 Asthma Quality of Life Questionnaire (AQLQ) total | 4 | Mean Difference (Random, 95% CI) | ‐0.12 [‐0.18, ‐0.05] | |
| 3 Serious adverse events (all) | 4 | Odds Ratio (Random, 95% CI) | 0.84 [0.41, 1.73] | |
| 4 Exacerbations (hospital) | 4 | Odds Ratio (Random, 95% CI) | 0.72 [0.18, 2.92] | |
| 5 Trough forced expiratory volume in 1 second (FEV1) (L) | 4 | Mean Difference (Random, 95% CI) | 0.05 [0.01, 0.09] | |
| 6 Peak FEV1 (L) | 3 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 7 Trough peak expiratory flow (PEF) (L/min) | 4 | Mean Difference (Random, 95% CI) | 5.78 [0.86, 10.71] | |
| 8 Trough forced vital capacity (FVC) (L) | 3 | 1745 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.02, 0.07] |
| 9 Peak FVC (L) | 2 | 1483 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.04, 0.03] |
| 10 Asthma Control Questionnaire (ACQ) total | 3 | Mean Difference (Random, 95% CI) | 0.06 [0.00, 0.13] | |
| 11 ACQ response | 2 | 1563 | Odds Ratio (M‐H, Random, 95% CI) | 0.91 [0.73, 1.13] |
| 12 Adverse events AEs (all) | 3 | 1839 | Odds Ratio (IV, Random, 95% CI) | 1.11 [0.92, 1.35] |
| 13 AEs classified as asthma | 3 | 1839 | Odds Ratio (M‐H, Random, 95% CI) | 0.95 [0.74, 1.22] |
1.6. Analysis.
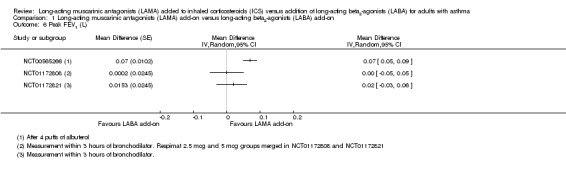
Comparison 1 Long‐acting muscarinic antagonists (LAMA) add‐on versus long‐acting beta2‐agonists (LABA) add‐on, Outcome 6 Peak FEV1 (L).
Comparison 2. Long‐acting muscarinic antagonists (LAMA) dose subgroups.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations (oral corticosteroid) | 2 | Odds Ratio (Random, 95% CI) | 0.93 [0.58, 1.51] | |
| 1.1 Respimat 2.5 mcg | 1 | Odds Ratio (Random, 95% CI) | 0.66 [0.34, 1.26] | |
| 1.2 Respimat 5 mcg | 1 | Odds Ratio (Random, 95% CI) | 0.95 [0.52, 1.76] | |
| 1.3 HandiHaler 18 mcg | 1 | Odds Ratio (Random, 95% CI) | 1.78 [0.65, 4.88] | |
| 2 Asthma Quality of Life Questionnaire (AQLQ) total | 4 | Mean Difference (Random, 95% CI) | ‐0.12 [‐0.18, ‐0.05] | |
| 2.1 Respimat 2.5 mcg | 2 | Mean Difference (Random, 95% CI) | ‐0.1 [‐0.22, 0.02] | |
| 2.2 Respimat 5 mcg | 3 | Mean Difference (Random, 95% CI) | ‐0.12 [‐0.22, ‐0.02] | |
| 2.3 HandiHaler 18 mcg | 1 | Mean Difference (Random, 95% CI) | ‐0.13 [‐0.28, 0.02] | |
| 3 Serious adverse events (SAEs) (all) | 4 | Odds Ratio (Random, 95% CI) | 0.85 [0.46, 1.59] | |
| 3.1 Respimat 2.5 mcg | 2 | Odds Ratio (Random, 95% CI) | 1.00 [0.36, 2.76] | |
| 3.2 Respimat 5 mcg | 3 | Odds Ratio (Random, 95% CI) | 0.72 [0.25, 2.03] | |
| 3.3 HandiHaler 18 mcg | 1 | Odds Ratio (Random, 95% CI) | 1.0 [0.20, 5.09] |
Comparison 3. Long‐acting muscarinic antagonists (LAMA) dose head‐to‐head.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations (oral corticosteroid) | 1 | 1036 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.40, 1.22] |
| 2 Asthma Quality of Life Questionnaire (AQLQ) total | 2 | 973 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.09, 0.10] |
| 3 Serious adverse events (SAEs) (all) | 2 | 1036 | Odds Ratio (M‐H, Random, 95% CI) | 1.09 [0.47, 2.49] |
Comparison 4. Sensitivity analysis excluding the cross‐over trial.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exacerbations (oral corticosteroid) | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2 Asthma Quality of Life Questionnaire (AQLQ) total | 3 | 1745 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.19, ‐0.03] |
| 3 Serious adverse events (SAEs) (all) | 3 | 1839 | Odds Ratio (IV, Random, 95% CI) | 0.79 [0.30, 2.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
NCT00350207.
| Methods |
Study design: RCT Study grouping: parallel group Open label: no Cluster RCT: no |
|
| Participants |
Baseline characteristics LAMA add‐on
LABA add‐on
Inclusion criteria: people homozygous for arginine at the 16th amino acid position of the beta2‐adrenergic receptor (B16 Arg/Arg); informed consent form; men or women outpatients aged 18‐65 years; documented history of asthma; current non‐smokers or ex‐smokers with a cigarette smoking history of < 10 pack‐years; maintenance treatment with ICS with a total daily dose of 400‐1000 mcg budesonide or equivalent Exclusion criteria: significant disease other than asthma; recent history (i.e., ≤ 6 months) of myocardial infarction; hospitalised for heart failure within 1 year; any unstable or life‐threatening cardiac arrhythmia or cardiac arrhythmia requiring intervention or a change in drug therapy within the past year; malignancy with resection, radiotherapy or chemotherapy within 5 years (treated basal cell carcinoma allowed); COPD, history of life‐threatening pulmonary obstruction, cystic fibrosis or bronchiectasis; known active TB; thoracotomy with pulmonary resection; current or recent (6 weeks) pulmonary rehabilitation |
|
| Interventions |
Intervention characteristics LAMA add‐on
LABA add‐on
|
|
| Outcomes |
Continuous
Dichotomous
|
|
| Identification |
Sponsorship source: Boehringer Ingelheim with collaboration from Pfizer Country: Austria, Belgium, Denmark, Finland, France, Germany, Greece, Italy, Russia, Slovakia, South Africa, Spain, Turkey, and the UK Setting: 109 investigational sites in 14 countries Comments: none Authors name: Leonardo Fabbri (corresponding), Eric D Bateman (first author) Institution: Cape Town, South Africa, Frankfurt and Biberach, Germany, and Modena, Italy Email: leonardo.fabbri@unimore.it Address: Bateman: Department of Medicine, University of Cape Town; Fabbri: Section of Respiratory Diseases, University of Modena and Reggio Emilia Modena |
|
| Notes | Pre‐treatment: "Demographic characteristics were well balanced across treatment groups, with slightly more female patients in the tiotropium group and slightly more patients who have never smoked in the salmeterol group" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was in blocks of 3 with no stratification. The randomisation schedule was generated with a validated system (PMX CTM Release 3.3.0 HP2; Propack Data GmbH, Karlsruhe, Germany)" |
| Allocation concealment (selection bias) | Unclear risk | Not sufficiently described in the available reports but previous contact with study sponsors confirmed that a concealed allocation system was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Blinding was achieved with a double‐blind, double‐dummy design with matching placebos" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blinding was achieved with a double‐blind, double‐dummy design with matching placebos" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out was between 4.5% and 6.2% across groups. All but 1 participant (placebo group) were included in the efficacy analyses through imputation Reasons for non‐completion of study were provided and were balanced between groups |
| Selective reporting (reporting bias) | Low risk | Outcomes were well reported in the published paper and fully reported on clinicaltrials.gov |
| Other bias | Unclear risk | "Demographic characteristics were well balanced across the treatment groups, with slightly more female patients in the tiotropium group and slightly more patients who had never smoked in the salmeterol group" Industry sponsored trial with data analyses performed by sponsor. Minimal demographic/baseline characteristics reported |
NCT00565266.
| Methods |
Study design: RCT Study grouping: cross‐over Open label: no Cluster RCT: no |
|
| Participants |
Baseline characteristics ICS + LAMA add‐on
ICS + LABA add‐on
Inclusion criteria: ≥ 18 years; clinical history consistent with asthma; FEV1 > 40% of predicted value; asthma confirmed by beta‐agonist reversibility to 4 puffs of albuterol 12% + or methacholine PC20 ≤ 8 mg/mL (not on ICS), or ≤ 16 mg/mL (on ICS); need for daily controller therapy (i.e. ICS, leukotriene modifiers with or without LABA) based on prescription in last year, symptoms for > twice a week; if on ICS up to fluticasone 100 mcg , stable dose for ≥ 2 weeks; non‐smoker for ≥ 1 year, and history < 10 pack‐years; willing to use an effective form of contraception throughout the study; ability to measure morning PEF on schedule and complete study diary correctly at least 75% of the time; ≥ 75% adherence with study medication during run‐in; no asthma exacerbation requiring OCS or additional asthma medications (including an increased dose of ICS) during run‐in Exclusion criteria: lung disease or significant medical illness other than asthma, including COPD and chronic bronchitis; established or suspected vocal cord dysfunction; history of respiratory tract infection within 4 weeks; history of a significant asthma exacerbation within 4 weeks; history of life‐threatening asthma requiring treatment with intubation and mechanical ventilation within 5 years; hyposensitisation therapy other than an established maintenance regimen; inability to use inhalers; pregnant |
|
| Interventions |
Intervention characteristics ICS + LAMA add‐on
ICS + LABA add‐on
|
|
| Outcomes |
Continuous
Dichotomous
|
|
| Identification |
Sponsorship source: National Heart, Lung, and Blood Institute Country: US Setting: 10 university medical centres in the US Authors name: Vernon M. Chinchilli, PhD Institution: Penn State Hershey College of Medicine Email: vchinchi@psu.edu Address: (+1) 717‐531‐4262 |
|
| Notes | Continuous outcomes: Continuous outcomes were extracted as contrasts to be entered in generic inverse variance as this is the most appropriate for cross‐over trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Communication with trial authors: "the Data Coordinating Center (DCC) generated the randomization scheme via the statistical software package SAS" |
| Allocation concealment (selection bias) | Low risk | Communication with trial authors: "the network pharmacist constructed the blinded drug packets according to the randomization scheme, and then the drug packets were shipped to the clinical centers. The DCC developed a web‐based system in which the study coordinator at a clinical center logged into the website whenever an eligible patient was ready for randomization, entered the appropriate information into the randomization module, and then was notified by the randomization module as to the appropriate drug packet for that eligible patient" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: participants and personnel were blinded to knowledge of which intervention participants received. The clinical trial register (clintrial.gov) stated, "Masking: Double Blind (Subject, Caregiver, Investigator, Outcomes Assessor)." The primary manuscript states "In a three‐way, double‐blind, triple‐dummy cross‐over trial......" and the methods specify that placebo inhalers were used [for blinding purposes] |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: blinding on clinicaltrials.gov described as subject, caregiver, investigator, outcomes assessor |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We were provided with the ITT dataset; however, comparison with the dataset based on people who had an event or completed follow‐up showed little or no difference on the overall results |
| Selective reporting (reporting bias) | Low risk | Comment: the primary outcome and the majority of secondary outcomes (i.e. as specified in the protocol\clintrials.gov record) were reported for the research hypothesis of interest. The secondary biomarker outcomes were not reported but did not influence assessment of safety or efficacy. Data were obtained for dichotomous outcomes in 2 x 2 tables in order to account for the trial's cross‐over design in the analysis |
| Other bias | Low risk | Quote: "the company had no role in the performance of the trial, the analysis or interpretation of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication" |
NCT00706446.
| Methods |
Study design: RCT Study grouping: parallel group Open label: yes Cluster RCT: no |
|
| Participants |
Baseline characteristics No full text available and no results posted on clinicaltrials.gov. The following baseline characteristics were not available for either group
Inclusion criteria: clinical history consistent with asthma; current prescription for a LABA, either alone or in combination with an ICS; informed consent; non‐smoker (total lifetime smoking history < 10 pack‐years); no known contraindication to inhaled tiotropium (e.g. narrow angle glaucoma, history of bladder neck obstruction or significant symptoms related to prostatic hypertrophy) Exclusion criteria: lung disease other than asthma; established or suspected diagnosis of vocal cord dysfunction; significant unstable medical illness (other than asthma); history of life‐threatening asthma within 5 years; history of respiratory tract infection within 4 weeks; hyposensitisation therapy other than an established maintenance regimen; current use of, or allergy to, tiotropium; pregnancy or lactation; if able to bear children, not using acceptable contraception; inability to use inhalers; inability to participate over the 1‐year period |
|
| Interventions |
Intervention characteristics LAMA add‐on
LABA add‐on
|
|
| Outcomes | No full text available and no results posted on clinicaltrials.gov | |
| Identification |
Sponsorship source: Brigham and Women's Hospital with collaboration from Harvard Clinical Research Institute and Massachusetts General Hospital Country: USA Setting: 2 centres Comments: study terminated Authors name: Elliot Israel, MD Institution: Brigham and Women's Hospital Email: eisrael@partners.org Address: Brigham and Women's Hospital Respiratory, 75 Francis St, Boston MA 02115 |
|
| Notes | Study terminated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Allocation was randomised ‐ no details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not applicable, study did not complete |
| Selective reporting (reporting bias) | Unclear risk | Study did not complete and hence no outcomes are reported. No interim results reported, or information regarding the decision to terminate |
| Other bias | High risk | Study terminated prior to completion |
NCT01172808.
| Methods |
Study design: RCT Study grouping: parallel group Open Label: no Cluster RCT: no |
|
| Participants |
Baseline characteristics LAMA add‐on (low)
LAMA add‐on (high)
LABA add‐on
Inclusion criteria: informed consent; men or women aged 18‐75 years; ≥ 3 months of asthma at enrolment; diagnosed before 40.5 years of age, confirmed with FEV1 increase of ≥ 12% and ≥ 200 mL after salbutamol; on maintenance treatment with a medium, stable dose of ICS for ≥ 4 weeks; ACQ (≥ 1.5) prior to randomisation; pre‐bronchodilator FEV1 60‐90% of predicted normal at screening; variation of absolute FEV1 of screening (pre‐bronchodilator) as compared with visit 2 (pre‐dose) must be within ± 30%; non‐smoker for at least 1 year, and history < 10 pack‐years; able to use inhalers and perform trial procedures correctly Exclusion criteria: lung disease or significant medical illness other than asthma; clinically relevant abnormal screening, haematology or blood chemistry; hospitalised for cardiac failure during the past year; any unstable or life‐threatening cardiac arrhythmia; known active TB; resection, radiotherapy or chemotherapy within 5 years for malignancy (treated basal cell carcinoma allowed); thoracotomy with pulmonary resection; significant alcohol or drug abuse within 2 years; current or recent (6 weeks) pulmonary rehabilitation; known hypersensitivity to the study drugs or any other components of the delivery systems; pregnant or nursing women; women of childbearing potential not using effective contraception; investigational drug, beta‐blockers, tiotropium, oral or patch beta‐adrenergic blockers, OCS or "experimental" drugs for asthma not recommended by international guidelines within 4 weeks; anti‐IgE antibodies (e.g. omalizumab) within 6 months; cromone, methylxanthines or phosphodiesterase‐4 inhibitors within 2 weeks; asthma exacerbation or respiratory tract infection within 4 weeks; previously randomised in this trial or in the respective twin trial (205.419) or currently participating in another trial |
|
| Interventions |
Intervention characteristics LAMA add‐on (low)
LAMA add‐on (high)
LABA add‐on
|
|
| Outcomes |
Continuous
Dichotomous
|
|
| Identification |
Sponsorship source: Boehringer Ingelheim Country: US, Brazil, China, Guatemala, India, Japan, Latvia, Mexico, Peru, Poland, Russian Federation Setting: 114 Boehringer Ingelheim investigational sites in 11 countries Comments: no publications listed, available only on manufacturer's website and clinicaltrials.gov IDs: 205.418, NCT01172808 Authors name: Boehringer Ingelheim Institution: N/A Email: clintriage.rdg@boehringer‐ingelheim.com Address: Boehringer Ingelheim Pharmaceuticals, tel: (+1) 800‐243‐0127 |
|
| Notes |
Pre‐treatment: minimal baseline characteristics reported, no differences noted TWIN TRIAL WITH NCT01172821 (205.419) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as 'randomised' on the clinicaltrials.gov record. Previous contact with study sponsors confirmed standard practice with computerised codes |
| Allocation concealment (selection bias) | Low risk | Described as 'randomised' on the clinicaltrials.gov record. Previous contact with study sponsors confirmed concealed automated allocation systems are used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Masking described as 'double‐blind' in the clinicaltrials.gov record. Details of inhalers used made it clear that inhalers were double dummy to maintain the blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind but no specific details about outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out was < 10% in all groups and the full analysis set was used for all safety and efficacy analyses. "There was 1 patient in the TIO R5 group randomised but not treated" |
| Selective reporting (reporting bias) | Low risk | Study results were reported on clinicaltrials.gov but did not give time to first exacerbation as "less than 50% of patients in each treatment group experienced an asthma exacerbation". Numbers in each group having exacerbations were not reported but were subsequently reported in a publication as a pooled result with NCT01172821 |
| Other bias | Unclear risk | Data were provided by Boehringer Ingelheim, who sponsored the study and manufacturer of tiotropium Respimat. Minimal demographic/baseline characteristics reported |
NCT01172821.
| Methods |
Study design: RCT Study grouping: parallel group Open label: no Cluster RCT: no |
|
| Participants |
Baseline characteristics LAMA add‐on (low)
LABA add‐on
LAMA add‐on (high)
Inclusion criteria: informed consent; men or women aged 18‐75 years; ≥ 3 months' asthma at enrolment; diagnosed before 40.5 years, confirmed with FEV1 increase of ≥ 12% and ≥ 200 mL after salbutamol; on maintenance treatment with a medium, stable dose of ICS for ≥ 4 weeks; ACQ (≥ 1.5) prior to randomisation; pre‐bronchodilator FEV1 60‐90% of predicted normal at screening; variation of absolute FEV1 of screening (pre‐bronchodilator) as compared with visit 2 (pre‐dose) must be within ± 30%; non‐smoker for ≥ 1 year, and history < 10 pack‐years; able to use inhalers and perform trial procedures correctly Exclusion criteria: lung disease or significant medical illness other than asthma; clinically relevant abnormal screening, haematology or blood chemistry; hospitalised for cardiac failure during the past year; any unstable or life‐threatening cardiac arrhythmia; known active TB; resection, radiotherapy or chemotherapy within 5 years for malignancy (treated basal cell carcinoma allowed); thoracotomy with pulmonary resection; significant alcohol or drug abuse within 2 years; current or recent (6 weeks) pulmonary rehabilitation; known hypersensitivity to the study drugs or any other components of the delivery systems; pregnant or nursing women; women of childbearing potential not using effective contraception; investigational drug, beta‐blockers, tiotropium, oral or patch beta‐adrenergics, OCS or "experimental" drugs for asthma not recommended by international guidelines within 4 weeks; anti‐IgE antibodies (e.g. omalizumab) within 6 months; cromone, methylxanthines or phosphodiesterase‐4 inhibitors within 2 weeks; asthma exacerbation or respiratory tract infection within 4 weeks; previously randomised in this trial or in the respective twin trial (205.419) or currently participating in another trial |
|
| Interventions |
Intervention characteristics LAMA add‐on (low)
LABA add‐on
LAMA add‐on (high)
|
|
| Outcomes |
Continuous
Dichotomous
|
|
| Identification |
Sponsorship source: Boehringer Ingelheim with collaboration from Pfizer Country: US, Brazil, China, Guatemala, India, Japan, Latvia, Mexico, Peru, Poland, Russian Federation Setting: 125 investigational sites in 11 countries IDs: 205.419, NCT01172821 Authors name: Thomas B Casale Institution: University of South Florida Email: casalej@ceighton.edu Address: Morsani College of Medicine, Tampa, FL |
|
| Notes |
Pretreatment: minimal baseline characteristics reported, no differences noted TWIN TRIAL WITH NCT01172808 (205.418) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Described as 'randomised' on the clinicaltrials.gov record. Previous contact with study sponsors confirmed standard practice with computerised codes |
| Allocation concealment (selection bias) | Low risk | Described as 'randomised' on the clinicaltrials.gov record. Previous contact with study sponsors confirmed concealed automated allocation systems are used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was double‐blind, double‐dummy design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study was double‐blind, double‐dummy design |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "There was 1 patient in the TIO R2.5 and 1 patient in the TIO R5 group randomised but not treated." Drop‐out ranged between 4.7 and 6.4 across groups and 99.8% were included using imputation for the full analysis set (FAS) |
| Selective reporting (reporting bias) | Low risk | Study results were reported on clinicaltrials.gov but did not give time to first exacerbation as "less than 50% of patients in each treatment group experienced an asthma exacerbation". Numbers in each group having exacerbations were not reported but were subsequently reported in a publication as a pooled result with NCT01172821 |
| Other bias | Unclear risk | Study was sponsored by Boehringer Ingelheim who manufacture 1 of the investigational drugs (tiotropium). Minimal demographic/baseline characteristics reported |
NCT01290874.
| Methods |
Study design: RCT Study grouping: parallel group Open label: yes Cluster RCT: no |
|
| Participants |
Baseline characteristics No full text available and no results posted on clinicaltrials.gov. The following baseline characteristics were not available for either group
Inclusion criteria: black people (self identified, with ≥ 1 biological parent identified as black; men or women aged 18‐75 years; ability to provide informed consent; clinical history consistent with asthma for > 1 year; ability to perform pulmonary function tests; FEV1 > 40% of predicted; receiving ICS/LABA combination therapy, or ICS moderate‐dose monotherapy; baseline ACQ > 1.25; non‐smoker for past year (total lifetime smoking history < 10 pack‐years) Exclusion criteria: use of equivalent of inhaled fluticasone > 1000 mcg daily; chronic use of OCS or Anti‐IgE for asthma; lung disease other than asthma or diagnosis of vocal cord dysfunction; significant unstable medical illness (other than asthma); pregnancy, lactation, or an unwillingness to maintain effective contraception; significant exacerbation of asthma or respiratory tract infection within 4 weeks; life‐threatening asthma within 5 years; hyposensitisation therapy other than an established maintenance regimen; use of inhaled anticholinergic therapy (ipratropium, tiotropium) within 1 month; known contraindication to inhaled tiotropium (e.g. narrow angle glaucoma, history of bladder neck obstruction or significant symptoms related to prostatic hypertrophy); inability to speak and read English |
|
| Interventions |
Intervention characteristics LAMA add‐on
LABA add‐on
|
|
| Outcomes | No full text available and no results posted on clinicaltrials.gov | |
| Identification |
Sponsorship source: Brigham and Women's Hospital with collaboration from Olmsted Medical Center, American Academy of Family Physicians National Research Network, and Harvard Clinical Research Institute Country: US Setting: 13 medical centres and university sites in the US Comments: no results posted and no publications identified Authors name: Elliot Israel, MD Institution: Brigham and Women's Hospital Email: eisrael@partners.org Address: 75 Francis St Boston, MA 02115, US |
|
| Notes | None | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, no other details |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was open label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of number enrolled, number of withdrawals or number included in the analyses |
| Selective reporting (reporting bias) | High risk | No data published. No publications provided or results posted on clinicaltrials.gov |
| Other bias | Low risk | None noted |
Rajanandh 2014.
| Methods |
Study design: RCT Study grouping: parallel group Open label: yes Cluster RCT: no |
|
| Participants |
Baseline characteristics LAMA add‐on
LABA add‐on
Inclusion criteria: aged 18‐60 years; clinically diagnosed as having mild‐to‐moderate persistent asthma Improvement in FEV1 > 12% after bronchodilator inhalation; written informed consent Exclusion criteria: clinically significant renal, respiratory (other than asthma), cardiac, gastrointestinal, hepatic, endocrine or haematological disorders; cancer; unresolved upper respiratory tract infection within the past 3 weeks; suspected hypersensitivity to study therapy or excipients; pregnancy or lactation; any other concurrent illness; any major surgery; and receipt of any oral, inhaled or parenteral forms of corticosteroid during the month before the study |
|
| Interventions |
Intervention characteristics LAMA add‐on
LABA add‐on
|
|
| Outcomes |
Continuous
No dichotomous outcomes reported |
|
| Identification |
Sponsorship source: SRM University Country: India Setting: Department of Pulmonary Medicine, SRM Medical College Hospital and Research Centre Registration ID: CTRI/2012/08/002915. This is a pilot study for a subsequent paper that is as yet not fully published. Authors name: Muhasaparur G. Rajanandh Institution: SRM College of Pharmacy, Tamil Nadu, India Email: mgrpharm@gmail.com Address: SRM College of Pharmacy, SRM University, Kattankulathur, Chennai, Kancheepuram, TAMIL NADU603 203, India |
|
| Notes |
Pre‐treatment: "No significant differences in baseline characteristics were found between the groups (P>0.05)" This is a PILOT STUDY for a subsequent paper which is as yet not fully published |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was generated using Random allocation software, version 1.0 |
| Allocation concealment (selection bias) | Low risk | "Concealment of optimization codes was done by serially numbered, opaque envelope model" Envelopes were sealed (CTRI website) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. No description of measures taken to blind outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Per protocol analysis was performed." Those that did not complete the trial were not included in the analyses (over 25% of the total population) |
| Selective reporting (reporting bias) | Unclear risk | The main trial was retrospectively registered (CTRI/2012/08/002915) but the planned outcomes of the pilot study are not detailed. Lung function and rescue medication were the main focus of the paper and were well reported |
| Other bias | Low risk | None noted |
Rajanandh 2015.
| Methods |
Study design: RCT Study grouping: parallel group Open label: yes Cluster RCT: no |
|
| Participants |
Baseline characteristics LAMA add‐on
LABA add‐on
Inclusion criteria: aged 18‐60 years, both men and women diagnosed clinically with mild‐to‐moderate persistent asthma, with an improvement in FEV1 > 12% after bronchodilator inhalation. Written informed consent was obtained from all participants prior to the study Exclusion criteria: participants with clinically significant renal, respiratory (other than asthma) cardiac, gastrointestinal, hepatic, endocrine disorders, haematological disorders, cancer or any other concurrent illness; participants who had undergone major surgery; unresolved upper respiratory tract infection within past 3 weeks of the pre‐study visit; corticosteroids during the month prior to the study; known or suspected hypersensitivity to study therapy or excipients; unwilling to give informed consent; pregnant and lactating women |
|
| Interventions |
Intervention characteristics LAMA add‐on
LABA add‐on
|
|
| Outcomes |
Continuous
No dichotomous outcomes were listed |
|
| Identification |
Sponsorship source: SRM University Country: India Setting: Department of Pulmonary Medicine, SRM Medical College Hospital and Research Centre Registration ID: CTRI/2012/08/002915 Authors name: Muhasaparur G. Rajanandh Institution: SRM College of Pharmacy, Tamil Nadu, India Email: mgrpharm@gmail.com Address: SRM College of Pharmacy, SRM University, Kattankulathur, Chennai, Kancheepuram, TAMIL NADU603 203, India |
|
| Notes | Contacted author November 2014 ‐ awaiting full publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation list was generated using Random allocation software, version 1.0 |
| Allocation concealment (selection bias) | Low risk | "Concealment of optimization codes was done by serially numbered, opaque envelope model" Envelopes were sealed (CTRI website) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label. No description of measures taken to blind outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 18% dropped out of tiotropium group and 19% dropped out of formoterol groups. "Per protocol analysis was performed" |
| Selective reporting (reporting bias) | High risk | The main trial was retrospectively registered (CTRI/2012/08/002915). All 4 outcomes were reported in the paper, although could not in sufficient detail to allow meta‐analysis (i.e. without group means and variance, or with details of a group comparison with level of statistical significance) |
| Other bias | Low risk | None noted |
ACQ: Asthma Control Questionnaire; AE: adverse event; AQLQ: Asthma Quality of Life Questionnaire; COPD: chronic obstructive pulmonary disease; ED: emergency department; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; HFA: hydrofluoroalkane; ICS: inhaled corticosteroids; ITT: intention to treat; L: litres; LABA: long‐acting beta2‐agonists; LAMA: long‐acting muscarinic antagonists; mcg: micrograms; MDI: metered dose inhaler; min: minute; N/A: not available; NR: not reported; OCS: oral corticosteroids; PC20: histamine provocative concentration causing a 20% drop in FEV1; PEF: peak expiratory flow; RCT: randomised controlled trial; SAE: serious adverse event; SD: standard deviation; SE: standard error; TB: tuberculosis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| CTRI/2008/091/000306 | Too short ‐ single dose of tiotropium Status: not recruiting |
| EUCTR2006‐003385‐34‐NL | Too short Status: authorised |
| JPRN‐UMIN000003618 | Wrong participant population (COPD not asthma) Status: not recruiting |
| JPRN‐UMIN000005459 | Wrong participant population (COPD not asthma) Status: not recruiting |
| JPRN‐UMIN000010352 | Too short ‐ single dose of tiotropium Status: not recruiting |
| Kerstjens 2012 | Wrong comparator |
| NCT00546234 | Study withdrawn prior to enrolment |
| NCT00557180 | Wrong study design ‐ observational Status: not recruiting |
| NCT00557700 | Too short (20 days) |
| NCT00772538 | Wrong comparator (vs. placebo ‐ i.e. ICS alone) |
| NCT00776984 | Wrong comparator (vs. ICS alone, and all participants required to be taking a LABA) |
| NCT01316380 | Wrong comparator (vs. ICS alone, and all participants required to be taking a LABA) |
| NCT01340209 | Wrong comparator (vs. ICS alone) |
| NCT01573624 | Too short (14 days) |
| NCT01641692 | Too short (14 days per cross‐over period) |
| NCT01696214 | Wrong intervention (smoking cessation) |
| NCT02066298 | Wrong comparator (tiotropium vs. ICS and placebo, not LAMA + ICS or LABA) |
| NCT02127697 | Wrong comparator (glycopyrronium bromide vs. placebo, not with ICS or against a LABA) |
| Vogelberg 2014 | Wrong participant population ‐ adolescents |
COPD: chronic obstructive pulmonary disease; ICS: inhaled corticosteroid; LABA: long‐acting beta2‐agonists; LAMA: long‐acting muscarinic antagonist.
Differences between protocol and review
We conducted additional searches of manufacturers' trial registry websites. We used Covidence for sifting, and extraction of study characteristics and outcome data. We were not able to pool more than 10 trials, so were unable to examine a funnel plot to explore possible small‐study and publication biases.
The four studies reporting data for the primary outcomes were all less than six months' duration, so we could not perform a duration subgroup analysis as planned.
We analysed data for an additional outcome, 'Adverse events classified as asthma', because the preferred data for 'Exacerbations requiring OCS' were not available in most trials.
Contributions of authors
Kayleigh Kew wrote the background and methods.
Kayleigh Kew, Debbie Allison and David Evans screened the searches,
Kayleigh Kew and David Evans made final decisions for study inclusion or exclusion and extracted data.
Kayleigh Kew constructed the analyses and interpreted the results, with consultation from David Evans, Debbie Allison and Anne Boyter.
All review authors edited and approved the final version of the document.
Sources of support
Internal sources
St George's, University of London (Kayleigh Kew), UK.
External sources
-
National Institute for Healthcare Research, UK.
Evidence to guide care in adults and children with asthma, 13/89/14
Declarations of interest
Kayleigh Kew: none known.
David Evans: none known.
Debbie Allison: none known.
Anne Boyter: none known.
Edited (no change to conclusions)
References
References to studies included in this review
NCT00350207 {published data only}
- Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16‐Arg/Arg patients with asthma. Journal of Allergy and Clinical Immunology 2011;128(2):315‐22. [DOI] [PubMed] [Google Scholar]
- NCT00350207. A 16‐week randomised, placebo‐controlled, double‐blind, double‐dummy, parallel‐group study comparing the efficacy and safety of tiotropium inhalation solution delivered by the Respimat Inhaler (2 actuations of 2.5 mcg once daily) with that of salmeterol from the hydrofluoroalkane metered dose inhaler (2 actuations of 25 mcg twice daily) in moderate persistent asthma patients with the B16‐Arg/Arg genotype. clinicaltrials.gov/show/NCT00350207 (accessed 18 December 2014).
NCT00565266 {published and unpublished data}
- Lane M. Tiotropium bromide in asthma patients: an alternative to inhaled long‐acting beta‐agonists?. Journal of the Royal College of Physicians of Edinburgh 2010;40(4):321‐2. [DOI] [PubMed] [Google Scholar]
- NCT00565266. Asthma Clinical Research Network (ACRN) Trial ‐ Tiotropium bromide as an alternative to increased inhaled corticosteroid in patients inadequately controlled on a lower dose of inhaled corticosteroid (TALC). clinicaltrials.gov/show/NCT00565266 (accessed 14 January 2015).
- Peters SP, Bleecker ER, Kunselman SJ, Icitovic N, Moore WC, Pascual R, et al. Predictors of response to tiotropium versus salmeterol in asthmatic adults. Journal of Allergy and Clinical Immunology 2013;132(5):1068‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SP, Kunselman SJ, Icitovic N, Moore WC, Pascual R, Ameredes BT, et al. Tiotropium bromide step‐up therapy for adults with uncontrolled asthma. New England Journal of Medicine 2010;363(18):1715‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00706446 {published data only}
- NCT00706446. Genotype stratified treatment with anticholinergic vs. beta‐agonist (long acting) and exacerbations (GABLE). clinicaltrials.gov/show/NCT00706446 (accessed 18 December 2014).
NCT01172808 {published data only}
- NCT01172808. A phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat® Inhaler (2.5 and 5 µg once daily) compared with placebo and salmeterol HFA MDI (50 µg twice daily) over 24 Weeks in moderate persistent asthma. clinicaltrials.gov/show/NCT01172808 (accessed 18 December 2014).
NCT01172821 {published data only}
- Casale T, Bleecker E, Meltzer E, Pizzichini E, Schmidt O, Bateman E, et al. Phase III trials to investigate tiotropium as add‐on therapy to inhaled corticosteroids for patients with symptomatic asthma: trial design and planned statistical analyses. Allergy 2013;68:377. [Google Scholar]
- Casale TB, Bateman ED, Dahl R, Pizzichini E, Vandewalker ML, Virchow JC, et al. Tiotropium Respimat (R) add‐on therapy reduces airflow obstruction in patients with symptomatic moderate asthma, independent of T(H)2 inflammatory status. Journal of Allergy and Clinical Immunology 2014;133(2):Ab5. [Google Scholar]
- NCT01172821. A phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat® Inhaler (2.5 and 5 µg once daily) compared with placebo and salmeterol HFA MDI (50 µg twice daily) over 24 Weeks in moderate persistent asthma. clinicaltrials.gov/show/NCT01172821 (accessed 18 December 2014).
NCT01290874 {published data only}
- NCT01290874. Blacks and exacerbations on LABA vs. tiotropium (BELT). clinicaltrials.gov/show/NCT01290874 (accessed 18 December 2014).
Rajanandh 2014 {published data only}
- Rajanandh MG, Nageswari AD, Ilango K. Pulmonary function assessment in mild to moderate persistent asthma patients receiving montelukast, doxofylline, and tiotropium with budesonide: a randomized controlled study. Clinical Therapeutics 2014;36(4):526‐33. [DOI] [PubMed] [Google Scholar]
Rajanandh 2015 {published data only}
- Ilango K. A clinical trial to study the effects of few anti asthmatic drug combinations on asthma patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2012/08/002915 (accessed 8 January 2015).
- Rajanandh MG, Nageswari AD, Ilango K. Assessment of montelukast, doxofylline, and tiotropium with budesonide for the treatment of asthma: which is the best among the second‐line treatment? A randomized trial. Clinical Therapeutics 2015;37(2):41826. [DOI] [PubMed] [Google Scholar]
- Rajanandh MG, Nageswari AD, Ilango K. Assessment of various second‐line medications in addition to inhaled corticosteroid in asthma patients: a randomized trial. Clinical and Experimental Pharmacology and Physiology 2014;41(7):509‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
CTRI/2008/091/000306 {published data only}
- Chest Research Foundation. A clinical trial comparing bronchodilator effects of two drugs, tiotropium bromide and formoterol fumarate each over 24 hours in subjects with asthma. apps.who.int/trialsearch/Trial2.aspx?TrialID=CTRI/2008/091/000306 (accessed 8 January 2015).
EUCTR2006‐003385‐34‐NL {published data only}
- Boehringer Ingelheim Pharma GmbH, Co. KG. The effect of tiotropium‐bromide on deep inspiration‐induced bronchodilation and airway responsiveness in asthma ‐ tiotropium‐bromide and lung mechanics. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006‐003385‐34‐NL (accessed 8 January 2015).
JPRN‐UMIN000003618 {published data only}
- Saitama Medical University. Randomized clinical study with tiotropium and formoterol/budesonide in COPD with asthma. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000003618 (accessed 8 January 2015).
JPRN‐UMIN000005459 {published data only}
- EACC (Ehime Asthma COPD Conference). Add‐on therapy for COPD receiving inhaled bronchodilators. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000005459 (accessed 8 January 2015).
JPRN‐UMIN000010352 {published data only}
- Fukuoka National Hospital. Study for effects of tiotropium on smokers and non‐smokers with bronchial asthma. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000010352 (accessed 8 January 2015).
Kerstjens 2012 {published data only}
- Corren J, Frew A, Engel M, Schmidt H, Moroni‐Zentgraf P, Kerstjens HAM. Tiotropium as add‐on therapy to ICS+LABA in patients with symptomatic severe asthma: spirometric assessment over 24 hours [Abstract]. American College of Chest Physicians 2013;144(4):91A. [Google Scholar]
- Kerstjens HAM, Engel M, Dahl R, Paggiaro P, Beck E, Vandewalker M, et al. Tiotropium in asthma poorly controlled with standard combination therapy. New England Journal of Medicine 2012;367(13):1198‐207. [DOI] [PubMed] [Google Scholar]
- Vandewalker ML, Engel M, Schmidt H, Siebold W, Moroni‐Zentgraf P, Kerstjens H, et al. Efficacy of tiotropium in patients with asthma in relation to allergic status [Abstract]. Journal of Allergy and Clinical Immunology 2013;131:AB1. [Google Scholar]
NCT00546234 {published data only}
- NCT00546234. Assessing treatment options for smokers with asthma. www.clinicaltrials.gov/ct2/show/NCT00546234 (accessed 8 January 2015).
NCT00557180 {published data only}
- National Jewish Health. Examining the link between obesity, inflammation, and response to asthma medications. clinicaltrials.gov/show/NCT00557180 (accessed 8 January 2015).
NCT00557700 {published data only}
- NCT00557700. Study to investigate the effect of inhaled tiotropium bromide on neurokinin‐A induced bronchoconstriction in patients with mild‐to‐moderate asthma. www.clinicaltrials.gov/ct2/show/NCT00557700 (accessed 8 January 2015).
NCT00772538 {published data only}
- NCT00772538. A phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat® inhaler (5 mcg/day) over 48 weeks as add‐on controller therapy on top of usual care in patients with severe persistent asthma. www.clinicaltrials.gov/ct2/show/NCT00772538 (accessed 8 January 2015).
NCT00776984 {published data only}
- NCT00776984. A phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat® inhaler (5 mcg/day) over 48 weeks as add‐on controller therapy on top of usual care in patients with severe persistent asthma. www.clinicaltrials.gov/ct2/show/NCT00776984 (accessed 8 January 2015).
NCT01316380 {published data only}
- NCT01316380. A phase III, randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate efficacy and safety of tiotropium inhalation solution delivered via Respimat® inhaler (2.5 ug and 5 ug once daily) compared to placebo over 12 weeks in mild persistent asthma. www.clinicaltrials.gov/show/NCT01316380 (accessed 15 January 2015).
- Paggiaro P, Engel M, Tudoric N, Forstner B, Radeczky E, Zubek V, et al. Phase III trial of tiotropium as add‐on therapy to low‐dose inhaled corticosteroids for patients with symptomatic mild persistent asthma: Design and planned analyses [Abstract]. European Respiratory Journal 2013;42 (Suppl 57):877s. [Google Scholar]
- Paggiaro P, Halpin DMG, Buhl R, Engel M, Zubek V, Blahova Z, et al. Tiotropium Respimat add‐on to inhaled corticosteroids improves lung function in patients with symptomatic mild asthma: results from a phase III trial. Journal of Allergy and Clinical Immunology 2014;133(2):AB4. [Google Scholar]
NCT01340209 {published data only}
- NCT01340209. A phase III randomised, double‐blind, placebo‐controlled, parallel‐group trial to evaluate safety and efficacy of tiotropium inhalation solution delivered via Respimat inhaler (2.5 and 5 µg once daily) compared with placebo over 52 weeks in patients with moderate to severe persistent asthma. www.clinicaltrials.gov/show/NCT01340209 (accessed 15 January 2015).
NCT01573624 {published data only}
- NCT01573624. Evaluate the safety, efficacy and dose response of GSK573719 in combination with fluticasone furoate in subjects with asthma (ILA115938). clinicaltrials.gov/show/NCT01573624 (accessed 18 December 2014).
NCT01641692 {published data only}
- NCT01641692. A multi‐national, randomized, double‐blind, placebo‐controlled, 3‐period crossover study with GSK 573719 as monotherapy in adult subjects with asthma. www.clinicaltrials.gov/ct2/show/NCT01641692 (accessed 8 January 2015).
NCT01696214 {published data only}
- NCT01696214. SAPS: Smoking Asthmatics Pilot Study. clinicaltrials.gov/show/NCT01696214 (accessed 18 December 2014).
NCT02066298 {published data only}
- NCT02066298. Steroids in eosinophil negative asthma. www.clinicaltrials.gov/ct2/show/NCT02066298 (accessed 8 January 2015).
NCT02127697 {published data only}
- NCT02127697. A randomized, double‐blind, parallel group, 52‐week study evaluating the efficacy, safety and tolerability of NVA237 in patients with poorly controlled asthma. www.clinicaltrials.gov/ct2/show/NCT02127697 (accessed 8 January 2015).
Vogelberg 2014 {published data only}
- Vogelberg C, Engel M, Moroni‐Zentgraf P, Leonaviciute‐Klimantaviciene M, Sigmund R, Downie J, et al. Once‐daily tiotropium in adolescents with symptomatic asthma despite inhaled corticosteroid treatment: a dose‐ranging study [Abstract]. Clinical and Translational Allergy 2014;4((Suppl 1)):2. [Google Scholar]
References to studies awaiting assessment
MezzoTinA {published data only}
- Casale TB, Dahl R, Virchow JC, Engel M, Moroni‐Zentgraf P, Luehmann R, et al. Tiotropium respimat add‐on therapy reduces exacerbation risk in patients with symptomatic moderate to severe asthma, independent of t helper 2 status. A. Morikawa, Gunma University, Gunma, Japan: American Thoracic Society, 2015; Vol. 191:A4193 MISC1 ‐ AST.
- DE Doherty, M Castro, P Moroni‐Zentgraf, M Engel, R Luehmann, R Buhl. Tiotropium respimat add‐on therapy improves lung function, as measured by peak expiratory flow, in adult patients across severities of symptomatic asthma. A. Morikawa, Gunma University, Gunma, Japan: American Thoracic Society, 2015; Vol. 191:A4258.
- Dahl R, Bateman ED, Casale T, Pizzichini E, Vandewalker M, Virchow JC, et al. Once‐daily tiotropium Respimat as add‐on to at least medium‐ to high‐dose ICS, with or without LABA, improves lung function in patients with symptomatic asthma, independent of allergic status. 33rd congress of the european academy of allergy and clinical immunology. 2014:515.
- Dahl R, Casale T, Bleecker E, Pizzichini E, Engel M, Moroni‐Zentgraf P, et al. Efficacy of tiotropium Respimat in adults with moderate asthma, by baseline LTRA use. European Respiratory Journal 2015;46. [Google Scholar]
- Dahl R, Casale T, Pizzichini E, Vandewalker M, Virchow JC, Engel M, et al. Once‐daily tiotropium Respimat as add‐on to at least medium‐to high‐dose ICS, with or without LABA, improves lung function in patients with symptomatic asthma, independent of allergic status. Thorax 2014;69:A177‐A178. [Google Scholar]
- FitzGerald JM, Engel M, Moroni‐Zentgraf P, Luehmann R, Bleecker ER. Once‐daily tiotropium respimat add‐on therapy improves symptom control across severities of symptomatic asthma, independent of allergic status. A. Morikawa, Gunma University, Gunma, Japan: American Thoracic Society, 2015; Vol. 191:A4263.
- FitzGerald JM, Kerstjens H, Paggiaro P, Ohta K, Ichinose M, Moroni‐Zentgraf P, et al. Once‐daily tiotropium respimat add‐on to ICS+/‐LABA improves control across asthma severities. European Respiratory Journal 2014;44. [Google Scholar]
- Frith P, Price D, Bateman E, Paggiaro P, Kaplan A, Engel M, et al. Efficacy of once‐daily tiotropium respimat 5UG from five phase III trials in adults with symptomatic asthma. Respirology 2015;20:137. [Google Scholar]
- Greos L, Berger W, Moroni‐Zentgraf P, Engel M, Luehmann R, Kerstjens H. Once‐daily tiotropium respimat add‐on to medium‐dose inhaled corticosteroids improves lung function and asthma control in adult patients with moderate symptomatic asthma, independent of prior long‐acting‐2‐agonist use. Chest 2015;148(4). [Google Scholar]
- Vandewalker ML, Karpel J, Bensch G, Ford L, Engel M, Sigmund R, et al. Once‐daily tiotropium Respimat add‐on to maintenance therapy improves lung function in patients with moderate or severe symptomatic asthma, independent of T helper 2 inflammatory status. Annals of Allergy, Asthma & Immunology 2014;113(5):A105‐A106. [Google Scholar]
- Yang WH, Casale T, Bateman ED, Dahl R, Pizzichini E, Vandewalker M, et al. Tiotropium respimat add‐on therapy reduces airflow obstruction in patients with symptomatic moderate asthma, independent of TH2 inflammatory status. Allergy, Asthma, and Clinical Immunology Vol. 10, issue 2:A52.
Wechsler 2015 {published data only}
- NCT01290874. Blacks and exacerbations on LABA vs. tiotropium (BELT). Http://clinicaltrials.gov/show/nct01290874 2014.
- Wechsler ME, Yawn BP, Fuhlbrigge AL, Pace WD, Pencina MJ, Doros G, et al. Anticholinergic vs long‐acting β2‐agonist in combination with inhaled corticosteroids in Black adults with asthma: The BELT randomized clinical trial. JAMA 2015;314(16):1720‐1730. [DOI] [PubMed] [Google Scholar]
Zhang 2018 {published data only}
- Zhang L, Huang G, Jin L. Therapeutic effects of a long‐acting cholinergic receptor blocker, tiotropium bromide, on asthma. Medical Science Monitor 2018;24:944‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 2008a
- Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW. Beclomethasone versus placebo for chronic asthma. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD002738.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2008b
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] [DOI] [Google Scholar]
Allison 2014
- Allison DE, Kew KM, Boyter AC. Long‐acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS for adults with asthma. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD011397] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 2005
- Anderson HR. Prevalence of asthma. BMJ 2005;330:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 2004
- Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJ, Pauwels RA, et al. Can guideline‐defined asthma control be achieved? The gaining optimal asthma control study. American Journal of Respiratory and Critical Care Medicine 2004;170(8):836‐44. [DOI] [PubMed] [Google Scholar]
Befekadu 2014
- Befekadu E, Onofrei C, Colice GL. Tiotropium in asthma: a systematic review. Journal of Asthma and Allergy 2014;7:11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
BNF
- Joint Formulary Committee. British National Formulary. www.medicinescomplete.com (accessed 29 August 2014).
Brozek 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEPro Version 3.2 for Windows. McMasters, Canada, 2008.
BTS/SIGN 2014
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. BTS/SIGN British guideline on the management of asthma, October 2014. www.brit‐thoracic.org.uk/guidelines‐and‐quality‐standards/asthma‐guideline/ (accessed 16 February 2015).
Cates 2013
- Cates CJ, Karner C. Combination formoterol and budesonide as maintenance and reliever therapy versus current best practice (including inhaled steroid maintenance), for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD007313.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2014
- Cates CJ, Wieland LS, Oleszczuk M, Kew KM. Safety of regular formoterol or salmeterol in adults with asthma: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD010314.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chauhan 2014
- Chauhan BF, Ducharme FM. Addition to inhaled corticosteroids of long‐acting beta2‐agonists versus anti‐leukotrienes for chronic asthma. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD003137.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2012
- Chong J, Karner C, Poole P. Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD009157.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- The Alfred Hospital, Monash University, National ICT Australia and the University of London. Covidence. Version 1. Melbourne: Alfred Health, 2013.
Ducharme 2008
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 5. [DOI: 10.1002/14651858.CD005535.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ducharme 2010
- Ducharme FM, Ni Choinin M, Greenstone I, Lasserson TJ. Addition of long‐acting beta2‐agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD005533.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington H, Vale A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31:140‐9. [DOI] [PubMed] [Google Scholar]
eMC 2014
- Electronic Medicines Compendium. License extension 19th September 2014: Spiriva Respimat 2.5 micrograms solution for inhalation. www.medicines.org.uk/emc/history/20134 (accessed 24 October 2014).
Ernst 2006
- Ernst P, McIvor A, Ducharme FM, Boulet LP, Fitzgerald M, Chapman KR, et al. Safety and effectiveness of long‐acting inhaled beta‐agonist bronchodilators when taken with inhaled corticosteroids. Annals of Internal Medicine 2006;146(9):692‐4. [DOI] [PubMed] [Google Scholar]
FDA 2010
- United States Food, Drug Administration. FDA Drug Safety Communication: New safety requirements for long‐acting inhaled asthma medications called long‐acting beta‐agonists (LABAs). www.fda.gov/drugs/drugsafety/ (accessed 29 August 2014).
GINA 2014
- Global Initiative for Asthma. From the Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2014. www.ginasthma.org/ (accessed 29 August 2014).
Global Asthma Report 2011
- International Union Against Tuberculosis and Lung Disease. The Global Asthma Report 2011. theunion.org/what‐we‐do/publications/technical/global‐asthma‐report (accessed 1 September 2014).
Gosens 2006
- Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respiratory Research 2006;7(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Karner 2014
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009285.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kew 2013
- Kew KM, Karner C, Mindus SM, Ferrara G. Combination formoterol and budesonide as maintenance and reliever therapy versus combination inhaler maintenance for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD009019.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kew 2014
- Kew KM, Allison DE, Evans DJW, Boyter AC. Long‐acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus higher dose ICS for adults with asthma. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD011437] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kew 2015a
- Kew KM, Dahri K. Long‐acting muscarinic antagonists (LAMA) added to combination long‐acting beta2‐agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 5. [DOI: 10.1002/14651858.CD011721] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipworth 2014
- Lipworth BJ. Emerging role of long acting muscarinic antagonists for asthma. British Journal of Clinical Pharmacology 2014;77(1):55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
MECIR 2013
- MECIR. Methodological Expectations for the conduct of Cochrane Intervention Reviews. editorial‐unit.cochrane.org/mecir (accessed 3 February 2015).
MHRA 2010
- Medicines and Healthcare Products Regulatory Agency (MHRA). Drug safety update: tiotropium. www.gov.uk/drug‐safety‐update/tiotropium (accessed 21 April 2015).
MHRA 2014
- Medicines and Healthcare Products Regulatory Agency. Asthma long‐acting ß2 agonists: use and safety. www.gov.uk/government/publications/asthma‐long‐acting‐ss2‐agonists‐use‐and‐safety (accessed 16 February 2015).
Moulton 2011
- Moulton BC, Fryer AD. Muscarinic receptor antagonists, from folklore to pharmacology; finding drugs that actually work in asthma and COPD. British Journal of Clinical Pharmacology 2011;163(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nelson 2006
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM, SMART Study Group. The salmeterol multicenter asthma research trial. Chest 2006;129:15‐26. [DOI] [PubMed] [Google Scholar]
Ni 2014
- Ni H, Soe Z, Moe S. Aclidinium bromide for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD010509.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. NICE advice [KTT5] high‐dose inhaled corticosteroids in asthma. www.nice.org.uk/Advice/ktt5 (accessed 1 September 2014).
NRAD 2014
- Royal College of Physicians. Why asthma still kills: the national review of asthma deaths. www.rcplondon.ac.uk/sites/default/files/why‐asthma‐still‐kills‐full‐report.pdf (accessed 1 September 2014).
Rashid 2014
- Rashid Q, Klein R. Tiotropium in the treatment of patients with asthma. Southern Medical Journal 2014;107(5):330‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodrigo 2015
- Rodrigo GJ, Castro‐Rodríguez JA. What is the role of tiotropium in asthma? A systematic review with meta‐analysis. Chest 2015;147(2):388‐96. [DOI] [PubMed] [Google Scholar]
Salpeter 2006
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta‐analysis: effect of long‐acting beta‐agonists on severe asthma exacerbations and asthma related deaths. Annals of Internal Medicine 2006;144:904‐12. [DOI] [PubMed] [Google Scholar]
Tee 2009
- Tee A, Koh MS, Gibson PG, Lasserson TJ, Wilson A, Irving LB. Long‐acting beta2‐agonists versus theophylline for maintenance treatment of asthma. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001281.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tian 2014
- Tian JW, Chen JW, Chen R, Chen X. Tiotropium versus placebo for inadequately controlled asthma: a meta‐analysis. Respiratory Care 2014;59(5):654‐66. [DOI] [PubMed] [Google Scholar]
Verhamme 2013
- Verhamme KM, Afonso A, Romio S, Stricker BC, Brusselle GG, Sturkenboom MC. Use of tiotropium Respimat Soft Mist Inhaler versus HandiHaler and mortality in patients with COPD. European Respiratory Journal 2013;42(3):606‐15. [DOI] [PubMed] [Google Scholar]
Wise 2013
- Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, et al. Tiotropium Respimat Inhaler and the risk of death in COPD. New England Journal of Medicine 2013;369:1491‐501. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Kew 2015
- Kew KM, Allison DE, Evans DJW, Boyter AC. Long‐acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long‐acting beta2‐agonists (LABA) for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011438] [DOI] [PMC free article] [PubMed] [Google Scholar]


