Abstract
Background
Misoprostol is an orally active prostaglandin. In most countries misoprostol is not licensed for labour induction, but its use is common because it is cheap and heat stable.
Objectives
To assess the use of oral misoprostol for labour induction in women with a viable fetus.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (17 January 2014).
Selection criteria
Randomised trials comparing oral misoprostol versus placebo or other methods, given to women with a viable fetus for labour induction.
Data collection and analysis
Two review authors independently assessed trial data, using centrally‐designed data sheets.
Main results
Overall, there were 75 trials (13,793 women); these were of mixed quality.
In nine trials comparing oral misoprostol with placebo (1109 women), women using oral misoprostol were more likely to give birth vaginally within 24 hours (risk ratio (RR) 0.16, 95% confidence interval (CI) 0.05 to 0.49; one trial; 96 women) and less likely to undergo caesarean birth (RR 0.72, 95% CI 0.54 to 0.95; 8 trials; 1029 women). Differences in ‘uterine hyperstimulation with fetal heart rate changes’ were compatible with no effect (RR 2.71, 95% CI 0.84 to 8.68; 7 trials; 669 women).
Ten trials compared oral misoprostol with vaginal prostaglandin (dinoprostone) (3,240 women). There was little difference in the frequency of: vaginal birth within 24 hours (RR 1.10, 95% CI 0.99 to 1.22; 5 trials; 2,128 women), uterine hyperstimulation with fetal heart rate changes (RR 0.95, 95% CI 0.59 to 1.53; 7 trials; 2,352 women), and caesarean birth (RR 0.92, 95% CI 0.81 to 1.04; 10 trials; 3240 women).
Five trials compared administration of oral misoprostol with intracervical prostaglandin E2 (681 women). Oral misoprostol was associated with fewer instances of failure to achieve vaginal birth within 24 hours (RR 0.78, 95% CI 0.63 to 0.97; 3 trials; 452 women) but more frequent uterine hyperstimulation with fetal heart rate changes (RR 3.57, 95% CI 1.11 to 11.54; 3 trials; 490 women). The available data for this comparison were however limited and the differences in caesarean birth were small (RR 0.85, 95% CI 0.63 to 1.16; 5 trials; 742 women).
Nine trials compared oral misoprostol with intravenous oxytocin (1282 women). There were no obvious differences in the frequency of: vaginal birth within 24 hours (RR 0.79, 95% CI 0.59 to 1.05; 6 trials; 789 women), or uterine hyperstimulation with fetal heart rate changes (RR 1.30, 95% CI 0.43 to 3.91; 7 trials; 947 women). There were, however, fewer caesarean births with oral misoprostol (RR 0.77, 95% CI 0.60 to 0.98; 9 trials; 1282 women).
Thirty‐seven trials compared oral misoprostol with vaginal misoprostol (6417 women). There was little difference in the frequency of: vaginal birth within 24 hours (RR 1.08, 95% CI 0.86 to 1.36; 14 trials; 2,448 women), uterine hyperstimulation with fetal heart rate changes (RR 0.71, 95% CI 0.47 to 1.08; 29 trials; 5,503 women), and caesarean birth (RR 0.93, 95% CI 0.81 to 1.07; 35 trials; 6,326 women).
The incidence of serious neonatal or maternal morbidity or death was rare and no meaningful results were available for any of the comparisons.
Authors' conclusions
Oral misoprostol as an induction agent is effective at achieving vaginal birth. It is more effective than placebo, as effective as vaginal misoprostol and vaginal dinoprostone, and results in fewer caesarean sections than oxytocin alone.
Where misoprostol remains unlicensed for the induction of labour, many practitioners will prefer to use a licensed product like dinoprostone. If using oral misoprostol, the evidence suggests that the dose should be 20 to 25 mcg in solution. Given that safety is the primary concern, the evidence supports the use of oral regimens over vaginal regimens. This is especially important in situations where the risk of ascending infection is high and the lack of staff means that women cannot be intensely monitored.
Plain language summary
Oral misoprostol for induction of labour
Oral misoprostol is effective at inducing (starting) labour. It is more effective than placebo, as effective as vaginal misoprostol and vaginal dinoprostone, and results in fewer caesarean sections than oxytocin. However, there are still not enough data from randomised controlled trials to determine the best dose to ensure safety.
Induction of labour in late pregnancy is used to prevent complications when the pregnant woman or her unborn child are at risk. Reasons for induction include being overdue, pre‐labour rupture of membranes and high blood pressure. Prostaglandins are hormones that are naturally present in the uterus (womb); they soften the cervix and stimulate contractions in labour. The artificial prostaglandin E2 dinoprostone can be administered vaginally to induce labour but it is unstable at room temperature and is expensive. Oral misoprostol is a cheap and heat stable prostaglandin E1 synthetic analogue originally developed for the treatment of stomach ulcers.
The search for trials took place in January 2014. This review of 75 randomised controlled trials (13,793 women) found that oral misoprostol appears to be at least as effective as current methods of induction.
Nine trials (1,282 women) showed that oral misoprostol was equivalent to intravenous infusion of oxytocin. There were no obvious differences in the number of women who had a vaginal birth within 24 hours, or the number of women who experienced uterine hyperstimulation with changes to the baby's heart rate, although there were fewer caesarean sections in the group of women who were given oral misoprostol.
For the 37 thirty seven trials (6,417 women) that compared oral and vaginal misoprostol, there was little difference in the number of women who had a vaginal birth within 24 hours, uterine hyperstimulation with changes to the baby's heart rate, or caesarean section.
In 10 trials (3,240 women) comparing oral misoprostol with a vaginal prostaglandin (dinoprost), there was little difference in the frequency of vaginal birth within 24 hours, uterine hyperstimulation with changes to the baby's heart rate, or caesarean section.
The nine trials that compared oral misoprostol with placebo (1,109 women) and found that oral misoprostol is more effective than placebo for inducing labour. Women in the oral misoprostol group were more likely to have vaginal birth within 24 hours, and less likely to have a caesarean section. There was little difference between groups in terms of the number of women who experienced uterine hyperstimulation with changes to the baby's heart rate.
Five trials compared oral misoprostol with intracervical (inserted into the entrance of the womb) prostaglandin E2 (681 women). Oral misoprostol was associated with fewer instances of failure to achieve vaginal birth within 24 hours but more frequent uterine hyperstimulation with changes to the baby's heart rate. The available data for this comparison was limited and the differences in caesarean birth were small.
Overall, the incidence of serious illness or death of the mother or her baby was rare and no meaningful results were available for any of the comparisons in this review.
Using oral misoprostol to induce labour is effective at achieving vaginal birth. It is more effective than placebo, as effective as vaginal misoprostol and vaginal dinoprostone, and results in fewer caesarean sections than using oxytocin alone.
In some countries where misoprostol is not licenced for the purpose of inducing labour, many clinicians may prefer to use some other licensed product such as dinoprostone. Where oral misoprostol is used, evidence suggests that an appropriate dose may be 20 to 25 mcg in solution. Given that safety is the primary concern, the evidence supports the use of oral regimens over vaginal regimens. This is particularly important in settings where the mother is at a higher risk of infection and where there may be insufficient staff to closely monitor the mother and her baby.
Background
This review is one of a series of reviews of methods of labour induction using a standardised protocol. For more detailed information on the rationale for this methodological approach, please refer to the currently published 'generic' protocol (Hofmeyr 2009). The generic protocol describes how a number of standardised reviews will be combined to compare various methods of preparing the cervix of the uterus and inducing labour.
Induction of labour is a common clinical situation. The reasons for an induction are either clinical (post‐term pregnancy, prelabour rupture of membranes, hypertensive disorders) or social (parents' and clinicians' convenience).
Prostaglandins have been widely used for labour induction, particularly if the cervix is not 'favourable' (Keirse 1993). Prostaglandin E2 (PGE2 or dinoprostone) appears to be the prostaglandin of choice when used vaginally in the form of gel, tablets or pessaries. Unfortunately, vaginal PGE2 preparations are expensive and unstable at room temperature.
Oral administration of prostaglandins is less effective (Keirse 1989) and has been virtually abandoned, mainly due to gastrointestinal side effects. However, interest in oral prostaglandins has increased with the introduction of a new synthetic prostaglandin E1 analogue ‐ misoprostol. This drug is used mainly for the prevention and treatment of nonsteroidal anti‐inflammatory drug‐induced ulcers of the digestive tract. It is relatively cheap and stable at room temperature. Used for this indication, oral misoprostol is usually given in two to four doses of 200 micrograms (mcg) per day and the time to maximum concentration is 10 to 20 minutes (versus 60 to 80 minutes in the case of vaginal administration, Abdel‐Aleem 2003). Side effects are dose dependent and are mainly confined to the digestive tract, such as diarrhoea and nausea (Garris 1989).
Uterine contractions induced by misoprostol are often strong enough to expel products of conception in early pregnancy. Widespread use of misoprostol in Brazil for termination of pregnancy (Costa 1993) resulted in the identification of teratogenic effects including abnormalities of extremities (clubfoot, agenesis of the feet and hands, syndactyly, constriction rings) and Mobius sequence (loss of function of the motor cranial nerves, especially VI, VII and XII) (Fonseca 1991; Gonzales 1999; Pastuszak 1998). These defects affect less than 1% of exposed fetuses (Philip 2002). Maternal death from uterine rupture at 16 weeks' gestation after self‐medication with misoprostol has been reported (Costa 1993).
Randomised trials, which have evaluated the effectiveness of a vaginally administered misoprostol for labour induction with a viable fetus, have been reviewed for The Cochrane Library (Hofmeyr 2010). Oral use is particularly attractive because of easy and non‐invasive administration. However, there are inherent risks of such an approach. An overdose, causing uterine hyperstimulation may precipitate labour, and may be life‐threatening for both mother and fetus. It is therefore, important that oral misoprostol is evaluated in a systematic fashion to assess if it can be recommended for routine clinical practice.
Despite having been widely studied for several reproductive health indications, misoprostol's licence has never been extended. This is thought to be due to the manufacturer's concerns about potential adverse publicity generated by the powerful US anti‐abortion lobby. Off‐label drug use is common in obstetrics, and includes many drugs, which would be considered mandatory in everyday practice (e.g. corticosteroids to prevent neonatal respiratory distress syndrome after premature labour and oxytocin 10 international units intramuscularly to prevent postpartum haemorrhage). Despite this, many practitioners are concerned about the potential legal consequences of using an off‐label drug should a serious adverse event occur, especially if an effective licensed alternative is available (Weeks 2005). Now that the patent for misoprostol has expired, generic misoprostol products licensed for reproductive health indications have become available in various parts of the world such as Brazil, France and Egypt. The arrival of licensed products will ease the medico‐legal concerns of those wishing to use misoprostol for induction of labour.
Description of the condition
Induction of labour is used to bring an end to pregnancy when the benefits of giving birth at that time outweigh the risks of the induction process.
Description of the intervention
Oral misoprostol is given as a regular medication to pregnant women either as a tablet or in solution. The dosage and form of the medication varies, but it is generally used only until the woman's cervix is fully ripened. If further uterine stimulation is required after this, an intravenous infusion of oxytocin is generally used until birth, although some studies have continued the use of low‐dose misoprostol.
How the intervention might work
Similar to other prostaglandins, oral misoprostol acts on the uterus to soften (or 'ripen') the cervix and to stimulate contractions.
Why it is important to do this review
Oral misoprostol is a low‐cost and effective method for induction which is in common use in many parts of the world. Until recently, the drug was not licensed for use in pregnancy and so the drug manufacturers had neither formally evaluated it nor packaged the drug for induction of labour. There has been confusion therefore over which misoprostol regimen to use, and the use of excessive doses in late pregnancy has been thought to have been responsible for serious complications including uterine rupture. A formal evaluation of the many published studies is therefore very important to guide national and international recommendations for its appropriate use.
Objectives
To determine, from randomised controlled trials, the effectiveness and safety of oral misoprostol for third trimester induction of labour.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing oral misoprostol for labour induction with placebo/no treatment or other methods for labour induction. Quasi‐randomised trials were not included. This review includes only induction methods listed above oral misoprostol on a predefined list of methods of labour induction (seeData collection and analysis). We have included only trials that have some form of random allocation to the study groups and report at least one of the prestated outcomes. We have also included studies that compare various oral misoprostol regimens.
In this review we make no distinction between cervical ripening and induction of labour if the aim was to achieve vaginal birth as safely as possible. However, we excluded the studies if the primary aim of intervention was to 'facilitate' spontaneous onset of labour over a long period of time (for example, oral misoprostol every other day between 40 and 42 weeks' gestation).
Types of participants
Pregnant women due for third trimester induction of labour who carry a viable fetus.
Types of interventions
Oral misoprostol compared with placebo/no treatment or six other methods for labour induction placed above oral misoprostol on a predefined list (seeData collection and analysis):
placebo/no treatment;
vaginal prostaglandin E2;
intracervical prostaglandin E2;
oxytocin alone;
amniotomy alone;
amniotomy + oxytocin;
vaginal misoprostol.
We will not discuss any of the comparisons listed above without relevant trials in this review. For the details of this selection strategy, please refer to Data collection and analysis.
In previously published versions of this review, we proposed to compare low‐, medium‐ and high‐dose regimens. We defined low‐dose regimens as less than 50 micrograms (mcg), medium‐dose as 50 to 100 mcg inclusive and high‐dose as more than 100 mcg. These arbitrary groups proved impractical because most trials used either 25 mcg, 50 mcg or 100 mcg doses. In order to study dose‐related effects, we decided to group regimens into: (i) 0 to 25 mcg, (ii) 26 to 50 mcg, (iii) 51 to 199 mcg and (iv) 200 mcg or more. We acknowledge that this change has been driven to some extent by the trials' data and is therefore a potential source of bias. Also, the same dose can be given at varying intervals (usually between two and six hours) and these differences could influence the primary outcomes. 'Low‐dose' regimens with two‐hourly administration may result in a higher cumulative dose over 24 hours than 'high‐dose' regimens. However, the plasma half‐life of oral misoprostol is short (20 to 40 minutes) and, therefore, it would appear that dose is more important than frequency. Consequently, at least at this point in time, we have not planned analyses based on frequency of administration.
Types of outcome measures
Two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic) prespecified the clinically relevant outcomes for trials of methods of cervical ripening/labour induction. After discussion a consensus has been reached by all registered Cochrane Pregnancy and Childbirth Group review authors with an interest in labour induction.
Primary outcomes
We chose five primary outcomes as being most representative of the clinically important measures of ineffectiveness and complications: (1) vaginal delivery not achieved within 24 hours (includes all caesarean sections); (2) uterine hyperstimulation with fetal heart rate (FHR) changes; (3) caesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but fewer babies with severe morbidity. However, in the context of labour induction at term this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. We will explore the incidence of individual components as secondary outcomes.
Secondary outcomes
Secondary outcomes relate to measures of clinical ineffectiveness, complications and satisfaction.
Measures of ineffectiveness: (6) cervix unfavourable/unchanged after 12 to 24 hours; (7) oxytocin augmentation.
Complications: (8) uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia; (11) instrumental vaginal delivery; (12) meconium‐stained liquor; (13) Apgar score less than seven at five minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side effects (all); (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side effects; (23) postpartum haemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction: (26) woman not satisfied; (27) caregiver not satisfied.
'Uterine rupture' includes all clinically significant ruptures of unscarred or scarred uteri. We have excluded asymptomatic scar dehiscence noted incidentally at the time of surgery.
While we have sought all of the above outcomes, only those with data appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the reviews we use the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting two minutes or more) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with fetal heart rate changes such as persistent decelerations, tachycardia or decreased short‐term variability).
Outcomes are included in the analysis if data were available according to original allocation and reasonable measures were taken to minimise observer bias.
Only outcomes with available data appear in the analysis tables. We have, nevertheless, extracted and reported data on outcomes that were not prestated above. However, we have clearly labelled them as such ('not prespecified'). In order to minimise the risk of bias, we have based the conclusions solely on the prestated outcomes.
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Pregnancy and Childbirth Group’s Trials Register (17 January 2014).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions, and included data from abstracts so long as there was sufficient information on the study method, population and outcomes.
Data collection and analysis
To avoid duplication of data in a series of reviews on interventions for labour induction as described in the generic protocol for methods for cervical ripening and labour induction in late pregnancy (Hofmeyr 2009), the labour induction methods were listed in a specific order, from one to 27. Each review included comparisons between one of the methods (from two to 27) with only those methods above it on the list. Thus, this review of oral misoprostol (8) could include comparisons with any of the following: (1) placebo/no treatment; (2) vaginal prostaglandins; (3) intracervical prostaglandins; (4) intravenous oxytocin; (5) amniotomy; (6) intravenous oxytocin with amniotomy; (7) vaginal misoprostol. The current list is as follows:
(1) placebo/no treatment; (2) vaginal prostaglandins (Kelly 2009); (3) intracervical prostaglandins (Boulvain 2008); (4) intravenous oxytocin (Alfirevic 2009); (5) amniotomy (Bricker 2000); (6) intravenous oxytocin with amniotomy (Bimbashi 2012; Howarth 2001); (7) vaginal misoprostol (Hofmeyr 2010); (8) oral misoprostol (this review); (9) mechanical methods including extra‐amniotic Foley catheter (Jozwiak 2012); (10) membrane sweeping (Boulvain 2005); (11) extra‐amniotic prostaglandins (Hutton 2001); (12) intravenous prostaglandins (Luckas 2000); (13) oral prostaglandins (French 2001); (14) mifepristone (Hapangama 2009); (15) oestrogens with or without amniotomy (Thomas 2001); (16) corticosteroids (Kavanagh 2006a); (17) relaxin (Kelly 2013); (18) hyaluronidase (Kavanagh 2006b); (19) castor oil, bath, and/or enema (Kelly 2013a); (20) acupuncture (Smith 2013); (21) breast stimulation (Kavanagh 2005); (22) sexual intercourse (Kavanagh 2001); (23) homoeopathic methods (Smith 2003); (24) nitric oxide donors (Kelly 2011); (25) buccal or sublingual misoprostol (Muzonzini 2004); (26) hypnosis (Nishi 2013); (27) other methods for induction of labour.
The reviews were analysed by the following clinical categories of participants:
previous caesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favourable, unfavourable or undefined.
For most reviews, the initial data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with the Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews. From 2001, the data extraction was no longer conducted centrally.
The trials were initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, a standardised data extraction form was developed and then piloted for consistency and completeness. This pilot process involved the researchers at the CESU and previous review authors in the area of induction of labour. For a description of the methods used to carry out the initial reviews, seeHofmeyr 2009.
For this update we used the following methods when assessing the reports identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy. We resolved any disagreement through discussion with the third author.
Data extraction and management
We designed a form to extract data. For eligible studies, at least two review authors extracted the data using the agreed form. We resolved discrepancies through discussion with the third author. We entered data into Review Manager software (RevMan 2012) and checked it for accuracy.
When information regarding any of the above is unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion or by involving the third author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We did not analyse any continuous data in this update. In future updates, if we include continuous data, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not include any cluster‐randomised trials in this update. If included in future updates, we will include cluster‐randomised trials in the analyses along with individually‐randomised trials. We will adjust their sample sizes using the methods described in the Handbook [Section 16.3.4 or 16.3.6] using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity or subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not eligible for inclusion in this review.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either the Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Funnel plots were not used to assess reporting bias.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful we did not provide a summary statistic.
If random‐effects analyses were used, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Where the data allowed, the results were analysed by the following clinical categories of participants:
previous caesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favourable, unfavourable or undefined.
Subgroup analysis and investigation of heterogeneity
If we identified substantial heterogeneity, we investigated it using subgroup and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it (see details in Data synthesis).
In the main analysis ("all women") for each comparison (i.e. not on the clinical categories) we carried out the following subgroup analyses:
dose‐related effects of oral misoprostol, regimens: 0 to 25 mcg versus 26 to 50 mcg versus 51 to 199 mcg versus 200 mcg or more.
The subgroup analysis was conducted for all outcomes within the main analysis.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2012). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In the event of significant heterogeneity, we carried out sensitivity analyses to explore the effect of trial quality (judged according to the method and reporting of allocation concealment), with poor‐quality studies (allocation concealment inadequate or not reported) being excluded from the analyses in order to assess whether this explained the heterogeneity. No other sensitivity analysis was conducted.
Results
Description of studies
Results of the search
We have included 76 studies in the current review, containing a total of 14,412 women. Only 160 women in three studies had undergone previous caesarean section (Carlan 2001 (V50); How 2001 (V25); Patil 2005).
Included studies
Placebo/no treatment as comparator
Nine studies were categorised under this comparison. Only two studies compared oral misoprostol with no treatment (expectant management) (Javaid 2008; Rath 2007). In both studies only women with ruptured membranes were included and those randomised to the expectant group had no treatment for 20 to 24 hours. Seven studies compared oral misoprostol with placebo. Two of these studies used initial 50 mcg doses (Cheung 2006; Levy 2007); three studies used initial 100 mcg doses (Hoffman 2001; Lo 2003; Lyons 2001); and two used 200 mcg doses (Beigi 2003; Ngai 1996). In Cheung 2006, women were randomised three ways: to placebo, 50 mcg or 100 mcg misoprostol. As 50 mcg is the most commonly recommended dose for oral misoprostol, we chose to include only the outcomes for the placebo versus 50 mcg dose. We have also included the outcomes for the 50 mcg versus 100 mcg doses in that section of the review. In Hoffman 2001 women in both arms received dinoprostone after 12 hours if not in active labour. The participants all had ruptured membranes at term except in Beigi 2003, where women with intact membranes were also included.
Dinoprostone as comparator
In 10 studies, the comparison was with the prostaglandin dinoprostone administered vaginally (Dallenbach 2003 (T); Dodd 2006; Gherman 2001a; Henrich 2008 (T); Hofmeyr 2001(T); le Roux 2002 (V50); Majoko 2002 (V50); Moodley 2003; Shetty 2004; Tessier 1997). The studies included only women with intact membranes, except for Hofmeyr 2001(T), which also included women with ruptured membranes and Henrich 2008 (T) and Tessier 1997 did not specify the membrane status for the included women. The initial dosage of misoprostol was 50 mcg or less in all studies except for Shetty 2004, where 100 mcg was used. In Majoko 2002 (V50), the initial dose was 10 mcg and each successive four‐hourly dose was doubled to a maximum of 400 mcg. In the studies by Dallenbach 2003 (T) and Hofmeyr 2001(T), women received 20 mcg oral misoprostol (dissolved in water) two‐hourly for two doses and then 40 mcg two‐hourly. Doses could also be given in labour if contractions slowed. In the studies by Moodley 2003 and Dodd 2006, women received 20 mcg of oral solution two‐hourly. A third arm of the Moodley 2003 study, where women received an initial 25 mcg of vaginal misoprostol followed by 20 mcg oral misoprostol, is not included in this review.
Oral misoprostol was compared with intracervical dinoprostone in the studies by Bartha 2000 and Patil 2005 (both 200 mcg dose), as well as Langenegger 2005, Sheela 2007 (V25) and Nagpal 2009 (50 mcg dose). All studies included only women with intact membranes except for Nagpal 2009 which included only women with ruptured membranes.
In the Tessier 1997 study, 23 of the 267 women had undergone a caesarean section in a previous pregnancy.
Oxytocin as comparator
Nine studies compared oral misoprostol with oxytocin. In six, the women had ruptured membranes at term (Al‐Hussaini 2003; Butt 1999; Crane 2003; Dodd 2006a (T); Mozurkewich 2003; Ngai 2000), whilst in two other trials women with intact membranes were also included (Nigam 2004; Wing 2004), and one study included a mix of women with ruptured and intact membranes (Aalami‐Harandi 2013 (T)). In most trials the dose was 100 mcg. However, in Dodd 2006a (T) (abstract form only), an hourly dose of titrated oral solution starting with 5 mcg was used; in Butt 1999 and Nigam 2004, 50 mcg was used; and in Crane 2003, 75 mcg was used. Aalami‐Harandi 2013 (T) used a 200 mcg tablet of misoprostol dissolved in 200 mL water, and administered 25 mL (25 mcg) every two hours.
Vaginal misoprostol as comparator
The most common comparison in this review is with vaginal misoprostol (37 studies). The figure in brackets after the study reference indicates the initial dosage of vaginal misoprostol used in the comparison. In 16 studies the participants had intact membranes (Adair 1998 (V50); Bennett 1998 (V50); Carlan 2001 (V50); Fisher 2001 (V50); Khazardoost 2011 (V25); Kwon 2001 (V50); le Roux 2002 (V50); Majoko 2002 (V50); Mehrotra 2010 (V50); Nopdonrattkaoon 2003 (V50); Paungmora 2004 (V50); Pongsatha 2005 (V50); Sheela 2007 (V25); Shetty 2001 (V50); Shetty 2003 (V25); Wing 1999 (V25)) and in three studies they had ruptured membranes (Jindal 2011 (V50); Puga 2001 (V50); Rizvi 2007 (V25)). Eleven studies included women with both intact and ruptured membranes (Cheng 2008 (T) (V25); Colon 2005 (V25); Deshmukh 2013 (V50); Dyar 2000 (V50); Hall 2002 (V25); Pais'wong 2008 (V25); Rahman 2013 (V25); Souza 2013(V25)(T); Toppozada 1997 (V100); Uludag 2005 (V50); Wing 2000 (V25)), whilst in six studies the membrane status was not clarified (Adam 2005 (V50); Elhassan 2007 (V50); Schneider 2004 (V25); Sheikher 2009 (V25); Sitthiwattanawong 1999; Sultana 2006 (V100)). Most trials used a 50 mcg dose of oral misoprostol (16 studies), but three used 20 to 25 mcg (Cheng 2008 (T) (V25); How 2001 (V25); Souza 2013(V25)(T)), 11 used 100 mcg (Hall 2002 (V25); Khazardoost 2011 (V25); Paungmora 2004 (V50); Pongsatha 2005 (V50); Puga 2001 (V50); Rizvi 2007 (V25); Shetty 2003 (V25); Sultana 2006 (V100); Toppozada 1997 (V100; Uludag 2005 (V50); Wing 2000 (V25)) and two used 200 mcg (Adair 1998 (V50); Carlan 2001 (V50)). In Majoko 2002 (V50), the initial dose was 10 mcg and each successive four‐hourly dose was doubled, to a maximum of 400 mcg. In Colon 2005 (V25), the initial dose was 50 mcg, but increased to 100 mcg for subsequent doses. The dosage of vaginal misoprostol varied from 25 mcg to 100 mcg. The initial dose of vaginal misoprostol is stated in mcg after the year of article publication.
Two studies (Carlan 2001 (V50); How 2001 (V25)) included women who had previous caesarean sections (131 and 27 respectively).
Sublingual misoprostol as comparator
There was just one study (Elhassan 2007 (V50)) which compared 50 mcg of oral, vaginal and sublingual misoprostol (50 women in each group).
Vaginal dinoprostone insert as comparator
One study (Rouzi 2014) compared titrated oral misoprostol solution at 20 mcg with a 10 mcg dinoprostone vaginal, inserted for a maximum of 24 hours.
Other comparisons
There were several other randomised trials comparing different oral misoprostol protocols. In one study Kipikasa 2005 compared 25 mcg and 50 mcg doses every three days for nine days. This study was conducted on an outpatient basis. Two studies compared 50 mcg and 100 mcg doses (Cheung 2006; Shetty 2002) and two studies by the same team examined frequency of dosaging. They initially compared 50 mcg given four‐hourly or six‐hourly (Pongsatha 2001) and then conducted a similar study comparing 100 mcg three‐hourly and six‐hourly (Pongsatha 2002). In this review, we have grouped together the dosage frequency studies with dosage subgroups.
One study compared a regimen of oral misoprostol 25 mcg given three‐hourly until in labour (with oxytocin only if contractions settled) with a regimen of two oral misoprostol tablets followed by routine oxytocin (De 2006). Another examined two management policies for women with ruptured membranes at term (Shetty 2002a). One group received oral misoprostol 50 mcg four‐hourly at recruitment and the other had conservative management for 24 hours followed by PGE2 1 to 2 mg six‐hourly. The study by Thaisomboon 2012 (T) included only women with intact membranes. In this study, one group received hourly misoprostol solution 20 mcg (200 mcg tablet dissolved in 200 mL of tap water). If no uterine contractions were achieved after the first four doses the dose was doubled to 40 mcg up to a maximum of eight doses. The other group received misoprostol solution orally 50 mcg every four hours (20 mL of 200 mcg misoprostol in 80 mL water). The study was double blinded by the use of 20 mL of placebo solution between doses.
Excluded studies
Four exclusions are potentially controversial. We have excluded the study by Windrim 1997 because 138 women in the comparator group were induced with four different methods (intracervical dinoprostone, vaginal dinoprostone, oxytocin, vaginal misoprostol). We felt that these methods are too different to be compared to oral misoprostol as one intervention. Ascher‐Walsh 2000 used oral misoprostol to achieve spontaneous onset of labour, i.e. oral misoprostol or placebo were given in three‐day intervals between 40 and 42 weeks of pregnancy. This review with its clinical outcomes concentrates on methods to achieve vaginal birth as quickly as possible, so we decided to exclude this trial.The study by Zvandasara 2008 was excluded as it included women with intrauterine fetal death (four women). Finally,the study by Rasheed 2007 (V50) included data on 25 women who were not randomised.These women were undergoing induction of labour with oral misoprostol (their normal unit induction method) just before the trial started. We therefore included their data with the oral misoprostol arm within the data set from the 285 women who were in the randomised group. The additional data has resulted in an uneven balance between the groups (165 versus 145), and the data derived from those randomised alone are not retrievable from the manuscript.
A number of studies were excluded as they used a combination of techniques. We excluded the studies by Kadanali 1996 and Bricker 2008 because they used vaginal misoprostol followed by oral administration. These studies are therefore included in the Cochrane review of vaginal misoprostol by Hofmeyr 2003. The study by Neto 1988 concentrated on the uterine effects of oral and vaginal misoprostol. Five women received oral misoprostol in the dose of 200 mcg four‐hourly, five women received 400 mcg four‐hourly and five women received a single 200 mcg tablet vaginally. The reported outcomes included initiation, dynamics and duration of uterine contractions. Clinical outcomes were not reported and we have therefore excluded the study. We excluded Bozhinova 2007 as the comparison of oral misoprostol was with combined vaginal and sublingual misoprostol. Delaney 2001 was excluded as women had an artificial rupture of membranes performed at study entry and the oxytocin or oral misoprostol was only used for those who were not in labour one hour later.
We excluded Ho 2010 as it is a study of labour augmentation in women with 'failure to progress in labour' rather than labour induction.
As reported above, the study by Moodley 2003 had three arms. We have included outcomes from the groups treated with oral misoprostol and vaginal dinoprostone in this review, but not from the third group, where women received a mixture of oral and vaginal misoprostol.
Several studies are still ongoing (DebBarma 2013; Gherman 2002; Pranuthi 2011), awaiting translation or have not been not been reported fully yet (Atkinson 2000; Bonebrake 2001; Butler 2004; Getgan 2003; Goedken 2000; Madhavi 2011; Niroomanesh 2011; Pearson 2002; Saldivar 2001; Tuipae 1999; Vijitrawiwat 2003; Yazdani 2012; Young 2001).
Risk of bias in included studies
See Figure 1; Figure 2 for a summary of 'Risk of bias' assessments.
1.
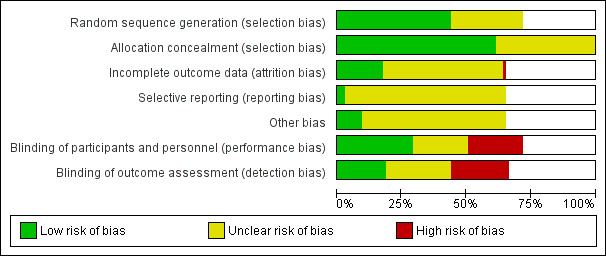
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
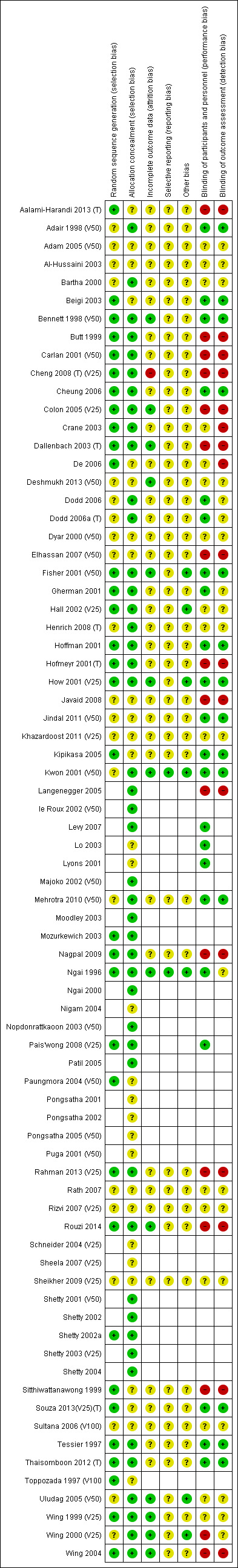
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Thirty‐three trials were assessed as being low risk of bias (Aalami‐Harandi 2013 (T); Beigi 2003; Bennett 1998 (V50); Butt 1999; Carlan 2001 (V50); Cheng 2008 (T) (V25); Cheung 2006; Colon 2005 (V25); Crane 2003; Dallenbach 2003 (T); De 2006; Fisher 2001 (V50); Gherman 2001; Hall 2002 (V25); Hoffman 2001; Hofmeyr 2001(T); How 2001 (V25); Kipikasa 2005; Mozurkewich 2003; Nagpal 2009; Ngai 1996; Pais'wong 2008 (V25); Paungmora 2004 (V50); Rahman 2013 (V25); Rouzi 2014; Shetty 2002; Sitthiwattanawong 1999; Souza 2013(V25)(T); Tessier 1997; Thaisomboon 2012 (T); Toppozada 1997 (V100; Wing 1999 (V25); Wing 2004) for sequence generation. The method of randomisation was unclear in 21 trials (Adair 1998 (V50); Adam 2005 (V50); Al‐Hussaini 2003; Bartha 2000; Deshmukh 2013 (V50); Dodd 2006; Dodd 2006a (T); Dyar 2000 (V50); Elhassan 2007 (V50); Henrich 2008 (T); Javaid 2008; Jindal 2011 (V50); Khazardoost 2011 (V25); Kwon 2001 (V50); Mehrotra 2010 (V50); Rath 2007; Rizvi 2007 (V25); Sheikher 2009 (V25); Sultana 2006 (V100); Uludag 2005 (V50); Wing 2000 (V25))
Allocation concealment
Forty‐six trials were low risk of bias for allocation concealment (Adair 1998 (V50); Bartha 2000; Bennett 1998 (V50); Butt 1999; Carlan 2001 (V50); Cheng 2008 (T) (V25); Cheung 2006; Colon 2005 (V25); Crane 2003; Dallenbach 2003 (T); Dodd 2006; Dodd 2006a (T); Fisher 2001 (V50); Gherman 2001a; Hall 2002 (V25); Henrich 2008 (T); Hoffman 2001; Hofmeyr 2001(T); How 2001 (V25); Kwon 2001 (V50); Langenegger 2005; le Roux 2002 (V50); Levy 2007; Majoko 2002 (V50); Mehrotra 2010 (V50); Moodley 2003; Mozurkewich 2003; Nagpal 2009; Ngai 1996; Ngai 2000; Nopdonrattkaoon 2003 (V50); Pais'wong 2008 (V25); Patil 2005; Rahman 2013 (V25); Rouzi 2014; Shetty 2001 (V50); Shetty 2002; Shetty 2002a; Shetty 2003 (V25); Shetty 2004; Thaisomboon 2012 (T); Tessier 1997; Uludag 2005 (V50); Wing 1999 (V25); Wing 2000 (V25); Wing 2004). The following trials were assessed as unclear risk of bias for this domain (Aalami‐Harandi 2013 (T); Adam 2005 (V50); Al‐Hussaini 2003; Beigi 2003; De 2006; Deshmukh 2013 (V50); Dyar 2000 (V50); Elhassan 2007 (V50); Javaid 2008; Jindal 2011 (V50); Khazardoost 2011 (V25); Kipikasa 2005; Lo 2003; Lyons 2001; Nigam 2004; Paungmora 2004 (V50); Pongsatha 2001; Pongsatha 2002; Puga 2001 (V50); Rath 2007; Rizvi 2007 (V25); Schneider 2004 (V25); Sheela 2007 (V25); Sheikher 2009 (V25); Sitthiwattanawong 1999; Souza 2013(V25)(T); Sultana 2006 (V100); Toppozada 1997 (V100).
Blinding
Of the studies we included, 14 were at low risk of bias for both performance and detection bias (Adair 1998 (V50); Beigi 2003; Bennett 1998 (V50); Cheung 2006; Fisher 2001 (V50); Hoffman 2001; How 2001 (V25); Jindal 2011 (V50);Kipikasa 2005; Kwon 2001 (V50);Mehrotra 2010 (V50); Souza 2013(V25)(T); Tessier 1997; Thaisomboon 2012 (T)). Seven trials were at low risk performance bias but unclear risk of detection bias (Dodd 2006; Dodd 2006a (T); Lo 2003; Levy 2007; Lyons 2001; Ngai 1996; Pais'wong 2008 (V25)).
Fifteen trials were at a high risk of both performance and detection bias (Aalami‐Harandi 2013 (T); Butt 1999; Carlan 2001 (V50); Cheng 2008 (T) (V25); Colon 2005 (V25); Dallenbach 2003 (T); Elhassan 2007 (V50); Hofmeyr 2001(T); Javaid 2008; Langenegger 2005; Nagpal 2009; Rahman 2013 (V25); Rouzi 2014; Sitthiwattanawong 1999; Wing 2004). Two trials were at unclear risk of performance bias but high risk of detection bias (Crane 2003; De 2006) and one trial was at high risk of performance bias and unclear risk of detection bias (Wing 2004). Fifteen trials were at unclear risk of both performance and detection bias (Adam 2005 (V50); Al‐Hussaini 2003; Bartha 2000; Deshmukh 2013 (V50); Dyar 2000 (V50); Hall 2002 (V25); Henrich 2008 (T); Khazardoost 2011 (V25); Rath 2007; Rizvi 2007 (V25); Sheikher 2009 (V25); Sultana 2006 (V100); Uludag 2005 (V50); Wing 1999 (V25); Wing 2000 (V25)).
Where the treatment was not blinded there is a real possibility of bias, both in clinical decision making and assessment of outcomes. A clinician with a prior belief that oral misoprostol is effective and safe might be less likely to perform a caesarean section in case of fetal distress or slow labour. On the other hand, a clinician who is anxious about possible risks of the new treatment may be more likely to intervene.
Incomplete outcome data
There was wide variation in the outcomes reported in the studies, and some were not consistent with the outcomes in this review. So whilst 'vaginal birth within 24 hours' is an important summary primary outcome in this review, it is rarely used by trialists, many of whom prefer a continuous variable of time to birth (which is not one of the Cochrane outcomes). Similarly not all published papers use the strict Cochrane definition of hyperstimulation with or without fetal heart rate abnormalities despite these being important outcomes. In assessing these outcomes, the authors have had to use judgement in matching the reported outcomes (for example "hyperstimulation") with the predefined Cochrane outcomes. Increasingly, however, trialists are turning to the Cochrane reviews for their selection of outcomes, and so recent high‐quality studies are well matched to the Cochrane outcomes set.
Selective reporting
There is a wide variation in reported outcomes, but no evidence of selective reporting was found.
Other potential sources of bias
None.
Effects of interventions
(1) Comparison with placebo (analyses 1 to 4)
Nine trials with 1109 participating women in total compared oral misoprostol with placebo or no treatment.
Primary outcomes: women using oral misoprostol were more likely to give birth vaginally within 24 hours (although this was only assessed in one trial of 96 women (risk ratio (RR) 0.16, 95% confidence interval (CI) 0.05 to 0.49) in which women in both arms were given dinoprostone after 12 hours), Analysis 1.1. Oral misoprostol was associated with lower caesarean section (CS) rates (RR 0.72, 95% CI 0.54 to 0.95), Analysis 1.3.
1.1. Analysis.
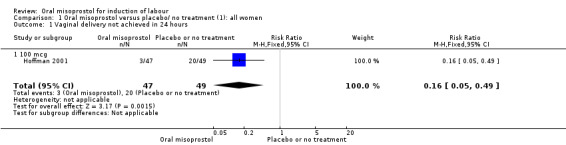
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
1.3. Analysis.
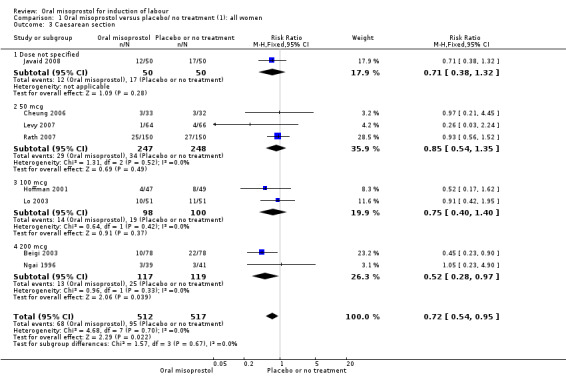
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 3 Caesarean section.
Secondary outcomes: oral misoprostol was also associated with less oxytocin augmentation (RR 0.42, 95% CI 0.37 to 0.49), Analysis 1.7, fewer admissions to the neonatal intensive care unit (RR 0.42, 95% CI 0.32 to 0.56), Analysis 1.14, and lower rates of epidural use (RR 0.81, 95% CI 0.69‐0.96) Analysis 1.6 There were no other clinically important differences in prespecified outcomes, including measures of uterine hyperstimulation. Most of the women studied had ruptured membranes, and analysis confined to this subgroup demonstrated similar findings to the overall analysis.
1.7. Analysis.
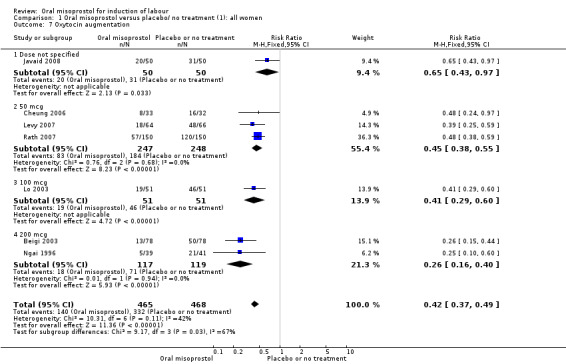
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 7 Oxytocin augmentation.
1.14. Analysis.
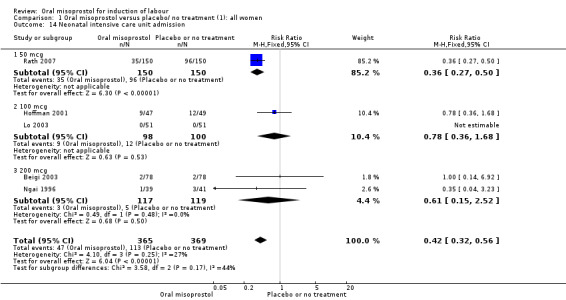
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 14 Neonatal intensive care unit admission.
Subgroups: in the subgroup of all women with ruptured membranes, there was a significant increase in the number of women with a vaginal birth within 24 hours (one study of 96 women, RR 0.16, 95% CI 0.05 to 0.49), Analysis 3.1. In the subgroups of all women with intact membranes and primigravid women with ruptured membranes, there were no significant changes in the primary outcomes.
3.1. Analysis.
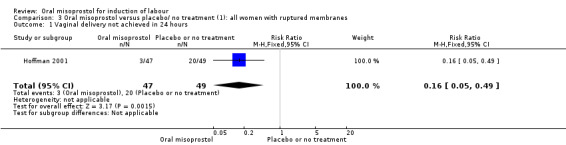
Comparison 3 Oral misoprostol versus placebo/ no treatment (1): all women with ruptured membranes, Outcome 1 Vaginal delivery not achieved in 24 hours.
(2) Comparison with vaginal dinoprostone (analyses 5 to 11)
A total of 3,240 women were randomised to oral misoprostol or vaginal dinoprostone in 10 trials. The most common dose of misoprostol used in these studies was 20 mcg oral solution given two‐hourly.
Primary outcomes: there was no effect on CS rate in those treated with oral misoprostol (RR 0.92, 95% CI 0.81 to 1.04), Analysis 5.3 or in the number of women not achieving vaginal birth within 24 hours (RR 1.09, 95% CI 0.99 to 1.20), Analysis 5.1,
Secondary outcomes: women treated with misoprostol more frequently had an unchanged cervix after 12 to 24 hours (RR 1.41, 95% CI 1.01 to 1.96), Analysis 5.6. There were no other statistically significant differences between the groups in any of the outcomes, including hyperstimulation rates and frequency of meconium‐stained liquor.
5.6. Analysis.
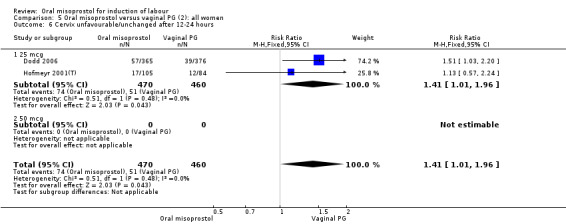
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
Subgroups: six trials (n = 2,129) provided the data for a subgroup of women with intact membranes. The data show that women induced with misoprostol have fewer CSs (RR 0.85, 95% CI 0.73 to 0.99), Analysis 6.3. However, they had slower labours with increased rates of unchanged cervix after 12 to 24 hours (RR 1.51, 95% CI 1.03 to 2.20), Analysis 6.6 and a lower rate of vaginal birth within 24 hours (RR 1.12, 95%CI 1.01‐1.25) Analysis 6.1. There were no other significant differences.
6.3. Analysis.
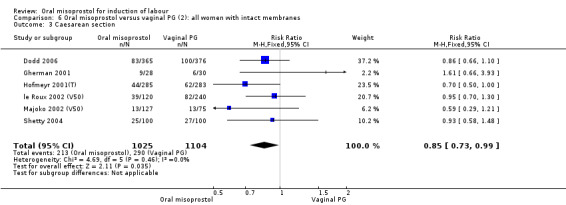
Comparison 6 Oral misoprostol versus vaginal PG (2): all women with intact membranes, Outcome 3 Caesarean section.
6.6. Analysis.
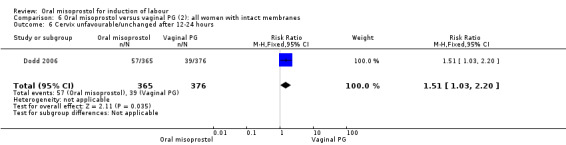
Comparison 6 Oral misoprostol versus vaginal PG (2): all women with intact membranes, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
Only two trials provided the data for women with ruptured membranes and there were only data for two outcomes. The number of women who did not achieve vaginal birth within 24 hours was reduced in the misoprostol group (RR 0.60, 95% CI 0.37 to 0.97), Analysis 7.1, i.e. induction with oral misoprostol achieved vaginal birth within the first 24 hours more frequently compared with vaginal dinoprostone. There were no other significant differences in primary outcomes between the subgroups.
7.1. Analysis.
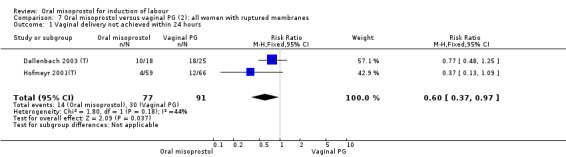
Comparison 7 Oral misoprostol versus vaginal PG (2): all women with ruptured membranes, Outcome 1 Vaginal delivery not achieved within 24 hours.
Only one study recruited women with previous CSs (n = 267) of which 23 had previous CS. No uterine ruptures were reported.
(3) Comparison with intracervical prostaglandin E2 (analyses 12, 13 and 14)
This comparison was found in five trials, involving 681 women randomised to oral misoprostol or intracervical dinoprostone. Four trials included only women with intact membranes and one included only women with ruptured membranes. Three used misoprostol doses of 50 mcg (352 women) and two used 200 mcg (two trials, 390 women).
Primary outcomes: the rate of uterine hyperstimulation with fetal heart rate (FHR) changes was significantly higher in those treated with misoprostol (RR 3.57, 95% CI 1.11 to 11.54), Analysis 12.2. However, significantly more women in the oral misoprostol group achieved vaginal birth within 24 hours (39% versus 49%, RR 0.78, 95% CI 0.63 to 0.97), Analysis 12.1.
12.2. Analysis.
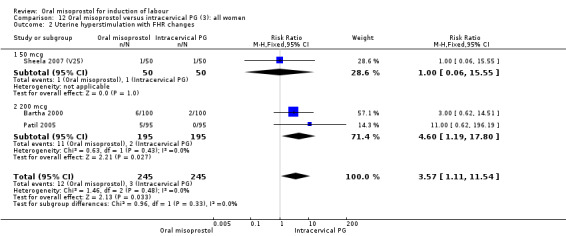
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
12.1. Analysis.
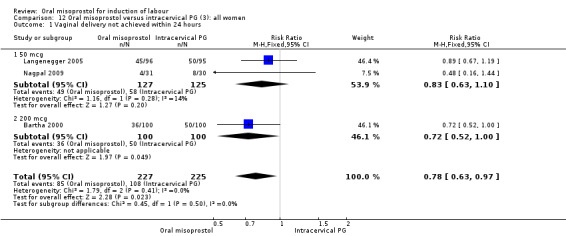
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
Secondary outcomes: the rate of uterine hyperstimulation without FHR changes was significantly higher in those treated with misoprostol (RR 6.25, 95% CI 1.14 to 34.31), Analysis 12.8. Only one study considered maternal dissatisfaction, and this was lower in those treated with oral misoprostol (RR 0.51, 95% CI 0.27 to 0.96), Analysis 12.19. There were no other differences between the groups.
12.8. Analysis.
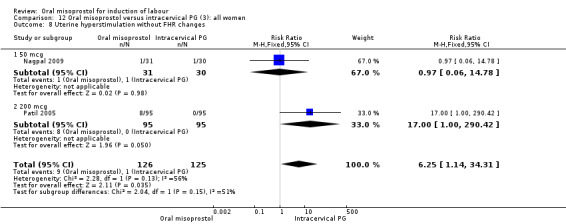
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 8 Uterine hyperstimulation without FHR changes.
12.19. Analysis.
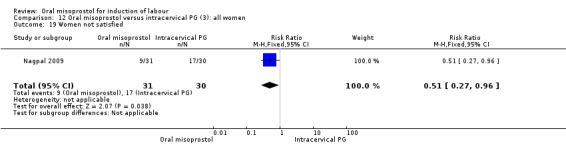
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 19 Women not satisfied.
Subgroups: the subgroups for those with intact membranes found the oral misoprostol group to have a significantly higher rate of uterine hyperstimulation with FHR changes (RR 4.60, 95% CI 1.19 to 17.80), Analysis 13.2. There was an increase in the number of women with a vaginal birth within 24 hours but this was not statistically significant (RR 0.81, 95% CI 0.65 to 1.00), Analysis 13.1. In the subgroup with ruptured membranes, there was only one study and this showed no significant difference in any of the primary outcomes.
13.2. Analysis.
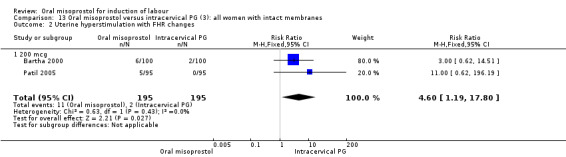
Comparison 13 Oral misoprostol versus intracervical PG (3): all women with intact membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
13.1. Analysis.
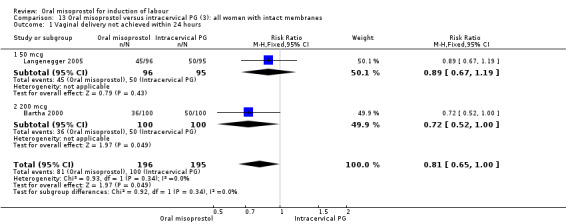
Comparison 13 Oral misoprostol versus intracervical PG (3): all women with intact membranes, Outcome 1 Vaginal delivery not achieved within 24 hours.
There was substantial heterogeneity for the 'need for oxytocin' outcome (I² = 80%), Analysis 12.7. Sensitivity and subgroup analysis indicated that this was not related to the poor quality study or dose.
12.7. Analysis.
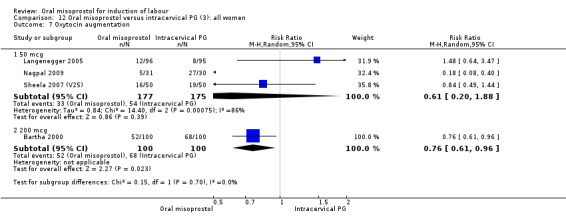
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 7 Oxytocin augmentation.
(4) Comparison with intravenous oxytocin (analyses 15, 16, 17, 18 and 19)
Nine trials including 1282 women have compared oral misoprostol with intravenous oxytocin. Five studies used 100 mcg (818 women), two studies used 50 mcg (178 women), one used 20 mcg solution (30 women) and one study used 25 mcg solution (256 women).
Primary outcomes: the CS rate was significantly lower in women who received oral misoprostol (RR 0.77, 95% CI 0.60 to 0.98), Analysis 15.3, but there were no significant differences seen in any of the other primary outcomes. However, the meta‐analysis of uterine hyperstimulation syndrome indicated substantial heterogeneity, Analysis 15.2. This may be explained by the trial by Crane 2003. which used a dose of 75 mcg four‐hourly. That study found significantly less hyperstimulation with misoprostol, whilst the remainder found no difference.
15.3. Analysis.
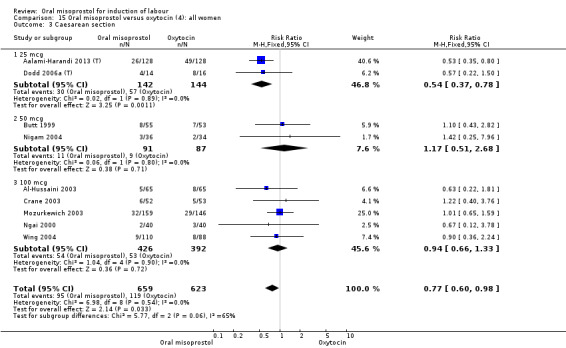
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 3 Caesarean section.
15.2. Analysis.
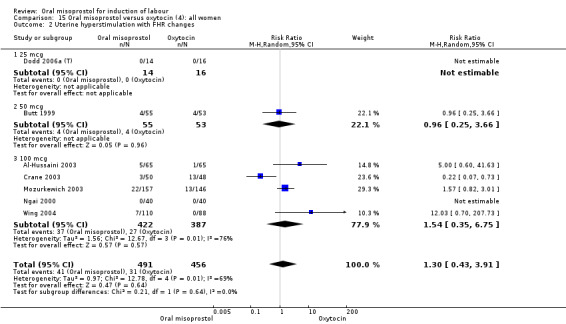
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
Secondary outcomes: meconium staining of the liquor was seen more frequently in the misoprostol group (RR 1.65, 95% CI 1.04 to 2.60), Analysis 15.11, but this was not reflected in significant differences in any adverse fetal or neonatal outcomes.There were no other statistically significant differences between the oral misoprostol and intravenous oxytocin groups (except for oxytocin use, which was naturally higher in the oxytocin group).
15.11. Analysis.
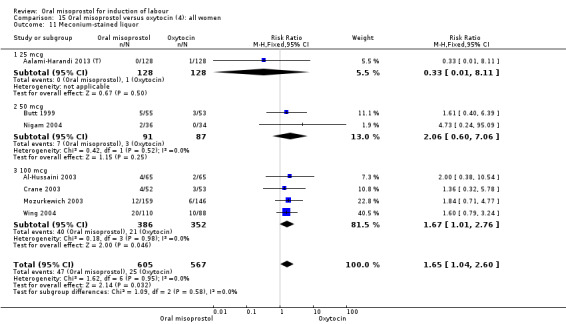
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 11 Meconium‐stained liquor.
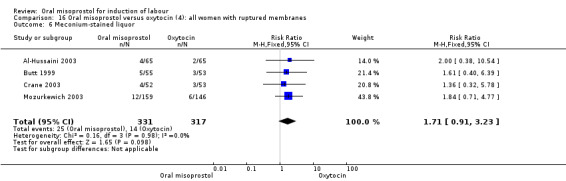
Subgroups: in six of the trials (758 women) there were outcome data for women with ruptured membranes. In this subgroup, the percentage of women with meconium‐stained liquor (and the RR) was similar to that in the overall results (Analysis 15.11), but the reduced numbers meant that the difference was not statistically significant (RR 1.71, 95% CI 0.91 to 3.23), Analysis 16.6.
16.6. Analysis.
Comparison 16 Oral misoprostol versus oxytocin (4): all women with ruptured membranes, Outcome 6 Meconium‐stained liquor.
There were no data reported for the subgroup of women with intact membranes.
(5) Comparison with sublingual misoprostol (analysis 20)
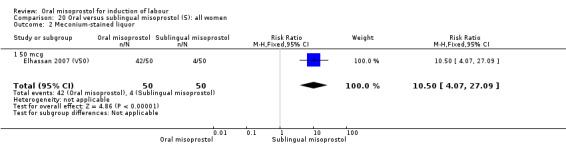
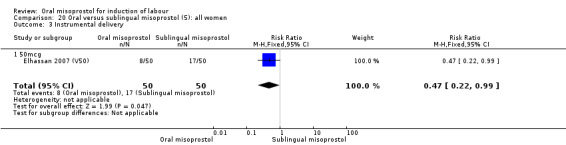
There was just one study of poor methodological quality in this comparison which compared 50 mcg of oral, vaginal and sublingual misoprostol (50 in each group, Elhassan 2007 (V50)). There was no significant difference in CS rate, but the oral misoprostol group had significantly higher rates of meconium‐stained liquor than the sublingual misoprostol group (RR 10.50, 95% CI 4.07 to 27.09), Analysis 20.2, and significantly lower rates of instrumental vaginal birth (RR 0.47, 95% CI 0.22 to 0.99), Analysis 20.3.
20.2. Analysis.
Comparison 20 Oral versus sublingual misoprostol (5): all women, Outcome 2 Meconium‐stained liquor.
20.3. Analysis.
Comparison 20 Oral versus sublingual misoprostol (5): all women, Outcome 3 Instrumental delivery.
(6) Comparison with vaginal misoprostol (analyses 21 to 29)
This was the most commonly studied comparison, with 37 trials and 6417 randomised women.
Primary outcomes: the overall outcome for the rate of uterine hyperstimulation with FHR changes was highly heterogenous. The interaction test indicated that there was a significant difference between dosage subgroups (Test for subgroup differences: Chi² = 18.50, df = 3 (P = 0.0003), I² = 83.8%) with less hyperstimulation in the 25 mcg oral misoprostol dose (RR 0.30, 95% CI 0.07 to 1.19), rising to an increase in hyperstimulation with the 200 mcg oral misoprostol dose (RR 1.61, 95% CI 1.07 to 2.43), Analysis 21.2. Sensitivity analysis revealed that the overall result was not affected by the removal of low‐quality studies.
21.2. Analysis.
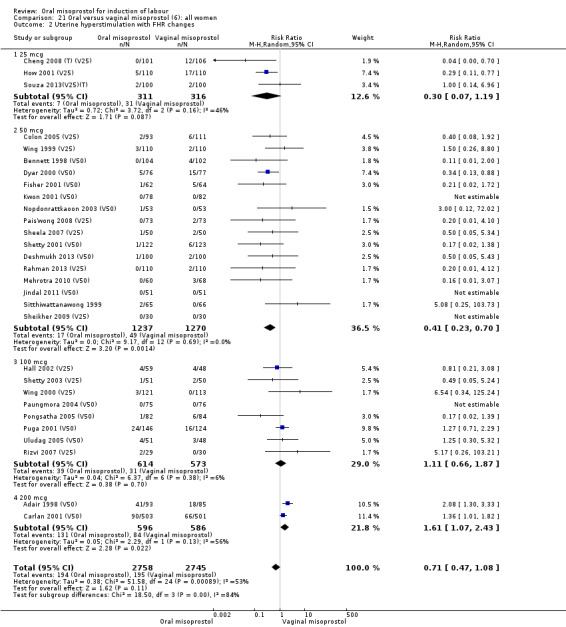
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
The overall outcome for vaginal birth within 24 hours was also highly heterogenous, Analysis 21.1, but the interaction test did not indicate a subgroup difference in relation to oral misoprostol dosage. Sensitivity analysis with removal of the low‐quality studies did not explain the heterogeneity.
21.1. Analysis.
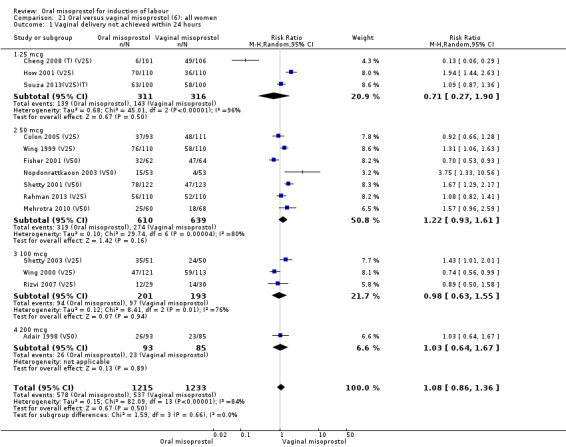
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
The overall outcomes for the CS rate was also heterogenous (Heterogeneity: Tau² = 0.06; Chi² = 60.78, df = 34 (P = 0.003); I² = 44%), Analysis 21.3. The heterogeneity was not affected by the removal of low‐quality studies and the interaction test showed that there was no significant interaction between dosage subgroups.
21.3. Analysis.
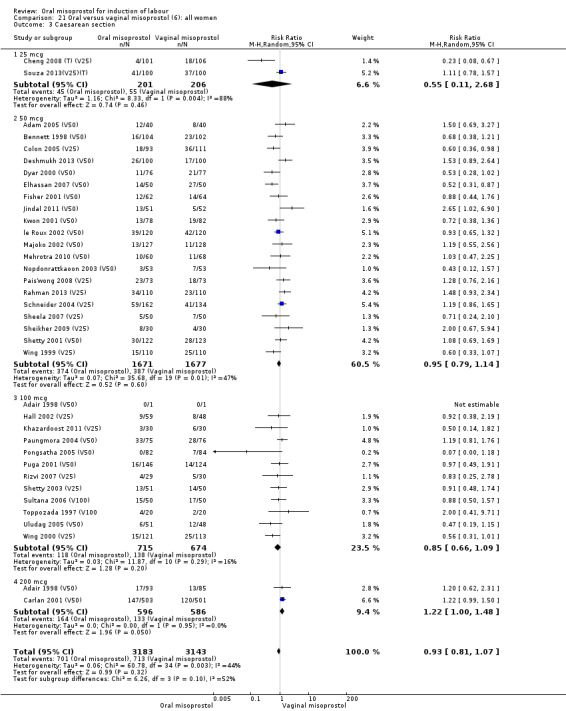
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 3 Caesarean section.
There were no other significant findings in the two remaining primary outcomes.
Secondary outcomes: when all the data are considered together, the only statistically significant differences without heterogeneity were a reduction in the Apgars of less than seven at five minutes in the oral misoprostol group (RR 0.60, 95% CI 0.44 to 0.82), Analysis 21.13, and a decrease in postpartum haemorrhage (RR 0.57, 95% CI 0.34 to 0.95), Analysis 21.21. However, the oral misoprostol group had an increase in meconium‐stained liquor (RR 1.22, 95% CI 1.03 to 1.44), Analysis 21.12.
21.13. Analysis.
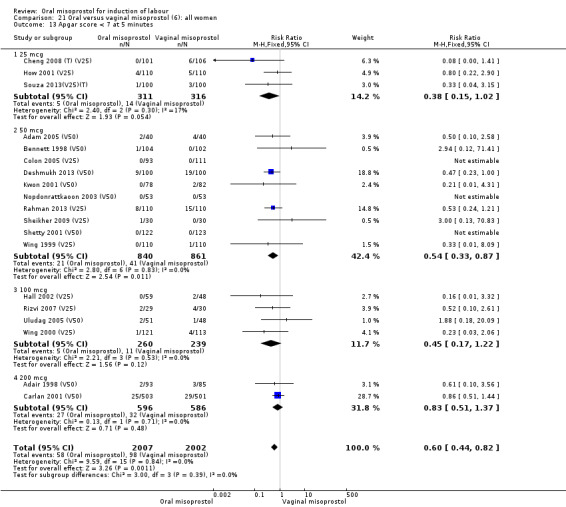
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 13 Apgar score < 7 at 5 minutes.
21.21. Analysis.
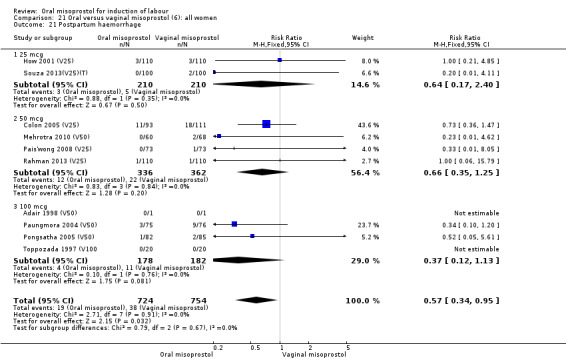
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 21 Postpartum haemorrhage.
21.12. Analysis.
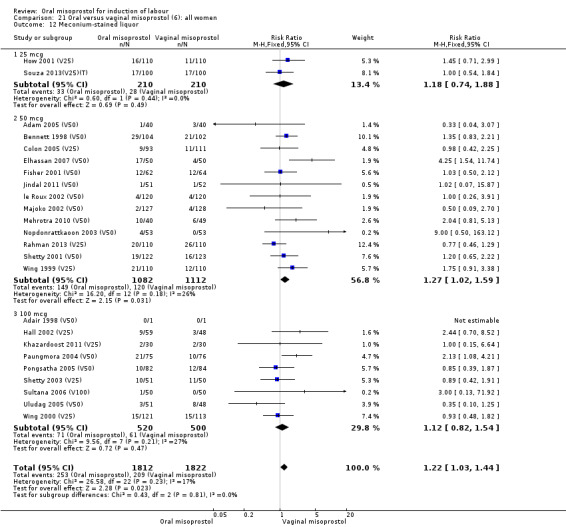
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 12 Meconium‐stained liquor.
The overall outcome for the rate of uterine hyperstimulation without FHR changes was also highly heterogenous, Analysis 21.8. The heterogeneity was not affected by the removal of low‐quality studies and the interaction test showed that there was no significant interaction between dosage subgroups.
21.8. Analysis.
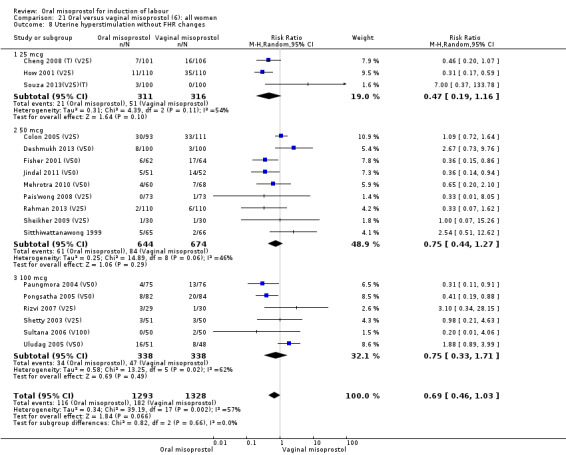
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 8 Uterine hyperstimulation without FHR changes.
The overall outcome for the use of oxytocin was also highly heterogenous, and the interaction test showed that there was a significant dosage subgroup interaction (Test for subgroup differences: Chi² = 9.10, df = 3 (P = 0.03), I² = 67.0%), Analysis 21.7. However, this was not in a dose‐dependent manner. The heterogeneity was not affected by the removal of low‐quality studies.
21.7. Analysis.
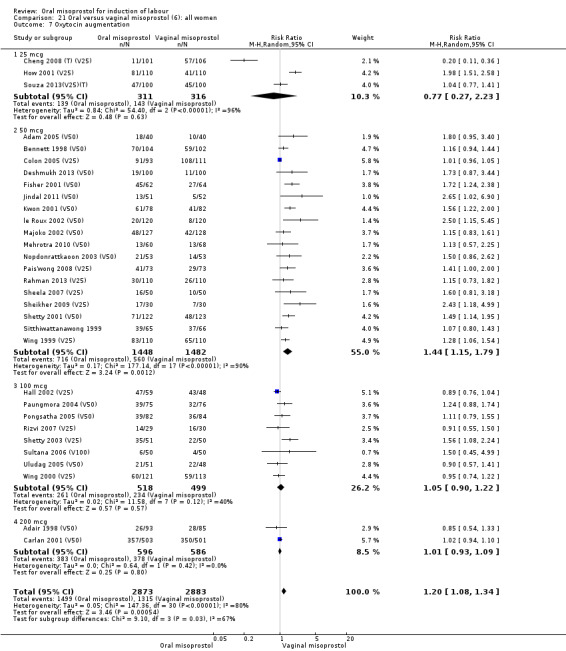
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 7 Oxytocin augmentation.
No uterine ruptures were reported in the women who had previous CSs.
Subgroups:
Intact membranes (analyses 22, 24, 27)
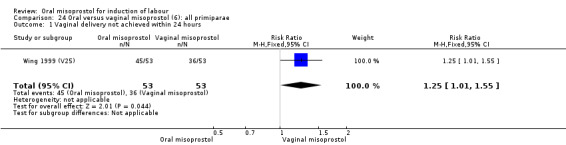
Seventeen of the 34 trials gave data for women with intact membranes. As with the overall data, there was no difference in any of the primary outcomes. The outcomes for vaginal birth not achieved in 24 hours and uterine hyperstimulation with FHR changes showed significant heterogeneity. Only one study examined the effect on vaginal birth within 24 hours in women with intact membranes (Wing 1999 (V25)) according to parity. That study showed a significant worsening in primigravid women given oral misoprostol (RR 1.25, 95% CI 1.01 to 1.55), Analysis 24.1, but not in multiparous women (RR 1.41, 95% CI 0.94 to 2.11), Analysis 27.1.
24.1. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 1 Vaginal delivery not achieved within 24 hours.
27.1. Analysis.
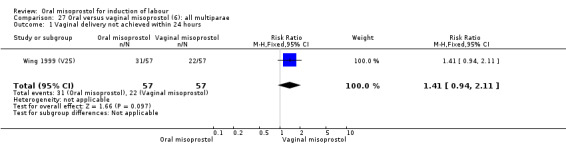
Comparison 27 Oral versus vaginal misoprostol (6): all multiparae, Outcome 1 Vaginal delivery not achieved within 24 hours.
Fifteen trials that reported uterine hyperstimulation with FHR changes showed no significant change in hyperstimulation in the oral misoprostol group (average RR 0.83, 95% CI 0.46 to 1.52; Heterogeneity: Tau² = 0.33; Chi² = 22.75, df = 11 (P = 0.02); I² = 52%), Analysis 22.2. However, there was significant heterogeneity in the results, with a significant association with dosage. The two 200 mcg studies had a significantly higher rate of uterine hyperstimulation with FHR changes and removing them from the analysis removed the overall heterogeneity and left the results as (RR 0.41, 95% CI 0.19 to 0.91).
22.2. Analysis.
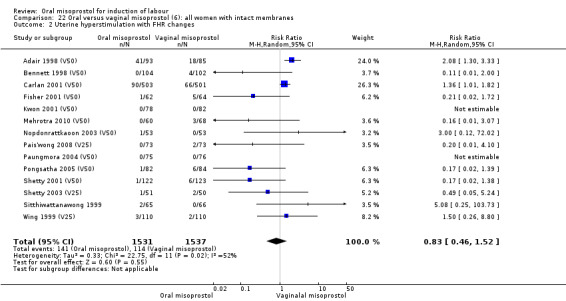
Comparison 22 Oral versus vaginal misoprostol (6): all women with intact membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
Ruptured membranes (analysis 25)
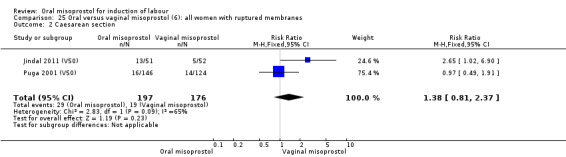
Only two trials reported women with ruptured membranes as a subgroup (Jindal 2011 (V50); Puga 2001 (V50)). The only two reported primary outcomes (CS and uterine hyperstimulation with FHR changes) showed no significant difference between oral and vaginal routes, Analysis 25.1; Analysis 25.2.
25.1. Analysis.
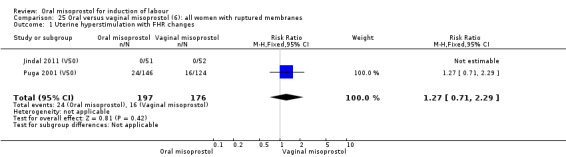
Comparison 25 Oral versus vaginal misoprostol (6): all women with ruptured membranes, Outcome 1 Uterine hyperstimulation with FHR changes.
25.2. Analysis.
Comparison 25 Oral versus vaginal misoprostol (6): all women with ruptured membranes, Outcome 2 Caesarean section.
Primiparae (analyses 24)
Only three trials reported outcomes for this subgroup. An increase in the number of women who did not birth vaginally within 24 hours was seen in the trial by Wing 1999 (V25), in which a 50 mcg dose was used in primips with intact membranes and an unfavourable cervix (RR 1.25, 95% CI 1.01 to 1.55), Analysis 24.1.
Multiparae (analyses 27)
Only two trials analysed this subgroup of women separately (Hall 2002 (V25); Toppozada 1997 (V100)) and they found no difference in the four reported outcomes, Analysis 27.1; Analysis 27.2; Analysis 27.3; Analysis 27.4.
27.2. Analysis.
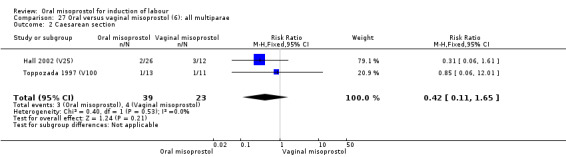
Comparison 27 Oral versus vaginal misoprostol (6): all multiparae, Outcome 2 Caesarean section.
27.3. Analysis.
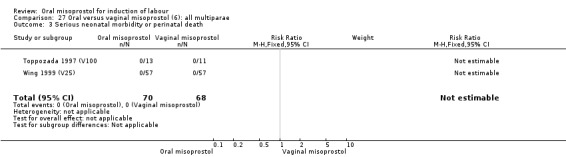
Comparison 27 Oral versus vaginal misoprostol (6): all multiparae, Outcome 3 Serious neonatal morbidity or perinatal death.
27.4. Analysis.
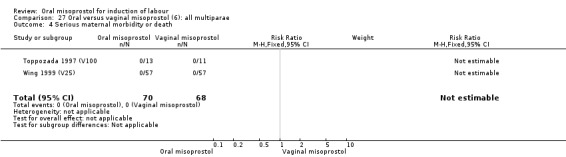
Comparison 27 Oral versus vaginal misoprostol (6): all multiparae, Outcome 4 Serious maternal morbidity or death.
(7) Hourly 20 mcg titrated oral misoprostol versus four‐hourly oral misoprostol 50 mcg (analysis 30)
One study of 64 women compared the effect of using hourly 20 mcg solution and four‐hourly 50 mcg solution (Thaisomboon 2012 (T)). There were no differences in any of the 12 outcomes, Analysis 30.1; Analysis 30.2; Analysis 30.3; Analysis 30.4; Analysis 30.5; Analysis 30.6; Analysis 30.7; Analysis 30.8; Analysis 30.9; Analysis 30.10; Analysis 30.11; Analysis 30.12.
30.1. Analysis.
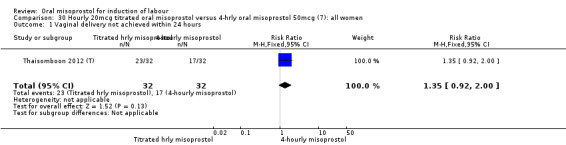
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
30.2. Analysis.
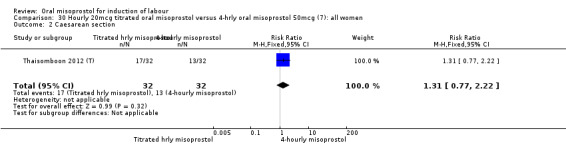
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 2 Caesarean section.
30.3. Analysis.
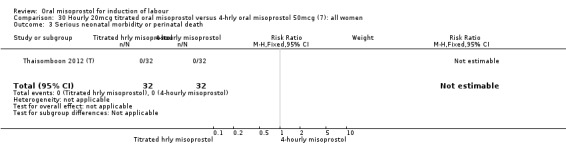
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 3 Serious neonatal morbidity or perinatal death.
30.4. Analysis.
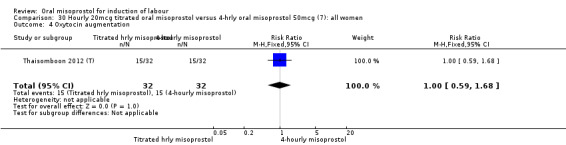
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 4 Oxytocin augmentation.
30.5. Analysis.
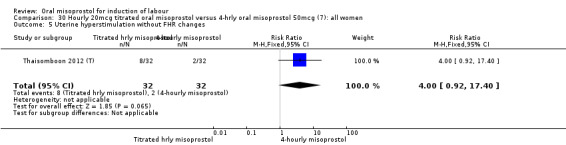
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 5 Uterine hyperstimulation without FHR changes.
30.6. Analysis.
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 6 Uterine rupture.
30.7. Analysis.
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 7 Apgar score < 7 at 5 minutes.
30.8. Analysis.
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 8 Neonatal intensive care unit admission.
30.9. Analysis.
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 9 Nausea.
30.10. Analysis.
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 10 Vomiting.
30.11. Analysis.
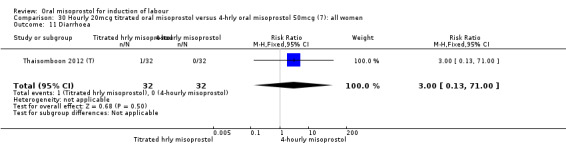
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 11 Diarrhoea.
30.12. Analysis.
Comparison 30 Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women, Outcome 12 Postpartum haemorrhage.
(8) Oral misoprostol 25 mcg three‐daily versus 50 mcg three‐daily (analysis 31)
In one study (Kipikasa 2005), single oral doses of misoprostol were given every three days to women as outpatients. In this study there was no significant difference in any of the reported outcomes, Analysis 31.1; Analysis 31.2; Analysis 31.3; Analysis 31.4; Analysis 31.5; Analysis 31.6; Analysis 31.7; Analysis 31.8; Analysis 31.9; Analysis 31.10; Analysis 31.11; Analysis 31.12; Analysis 31.13; Analysis 31.14; Analysis 31.15.
31.1. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 1 Uterine hyperstimulation with FHR changes.
31.2. Analysis.
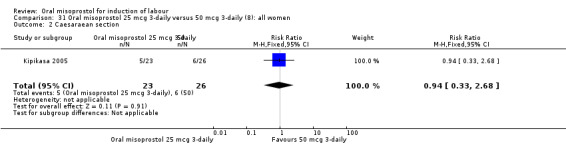
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 2 Caesaraean section.
31.3. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 3 Serious maternal morbidity or death.
31.4. Analysis.
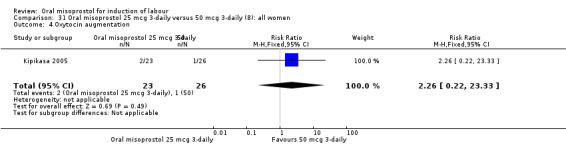
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 4 Oxytocin augmentation.
31.5. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 5 Uterine hyperstimulation without FHR changes.
31.6. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 6 Uterine rupture.
31.7. Analysis.
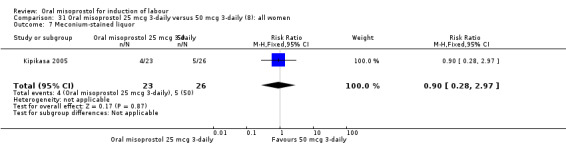
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 7 Meconium‐stained liquor.
31.8. Analysis.
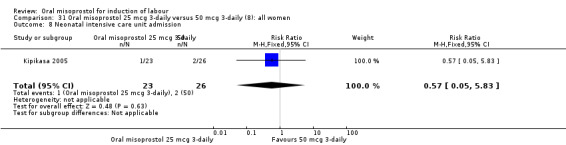
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 8 Neonatal intensive care unit admission.
31.9. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 9 Perinatal death.
31.10. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 10 Nausea.
31.11. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 11 Maternal side effects(all).
31.12. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 12 Nausea.
31.13. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 13 Vomiting.
31.14. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 14 Diarrhoea.
31.15. Analysis.
Comparison 31 Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women, Outcome 15 Shivering.
(9) Oral misoprostol 50 mcg versus 100 mcg (analyses 32, 33, 34)
Two studies containing 317 women addressed the relationship between the 50 and 100 mcg doses (Cheung 2006; Shetty 2002). There were no significant differences between the 50 mcg and 100 mcg oral doses in any of the 11 outcomes assessed.
(10) Oral misoprostol 50 mcg, three‐ to four‐hourly versus six‐hourly (analyses 35, 36)
Two trials compared the outcomes when women were given oral tablets either three‐ to four‐hourly or six‐hourly. One study with 89 women (Pongsatha 2001) compared 50 mcg oral regimen four‐ or six‐hourly whilst the other, with 133 women (Pongsatha 2002), compared 100 mcg given orally three‐ or six‐hourly. There were no differences in any of the outcomes studied when the data were combined, although there was significant heterogeneity in results for the outcomes of uterine hyperstimulation with FHR changes and oxytocin augmentation. In the study using 100 mcg, there was significantly less need for oxytocin augmentation when the misoprostol was given three‐ to four‐hourly compared to when it was used six hourly (RR 0.72, 95% CI 0.54 to 0.96), Analysis 35.3.2.
35.3. Analysis.
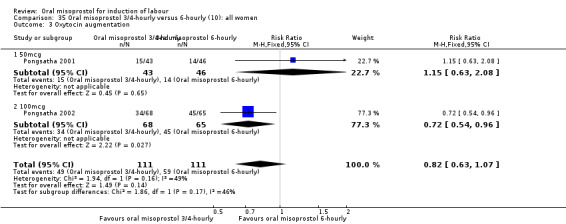
Comparison 35 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women, Outcome 3 Oxytocin augmentation.
(11) Oral misoprostol three‐hourly versus oral misoprostol x two then routine oxytocin (analyses 37 and 38)
One study compared the use of oral misoprostol 25 mcg used three‐hourly until in labour with a policy of two oral misoprostol doses (25 mcg) followed by routine oxytocin (De 2006). There were no statistically significant differences in any of the 10 outcomes.
(12) Oral misoprostol versus delayed vaginal prostaglandins (analysis 39)
Another small study of 61 women compared two policies for dealing with ruptured membranes at term (Shetty 2002a). One group had immediate oral misoprostol (50 mcg) whilst the other waited for 24 hours and then had vaginal prostaglandins (1 mg or 2 mg depending on Bishop Score). The only statistically significant outcome was in the 'vaginal birth not achieved in 24 hours' outcome, which was reduced in the immediate oral misoprostol group (RR 0.52, 95% CI 0.32 to 0.83), Analysis 39.1.
39.1. Analysis.
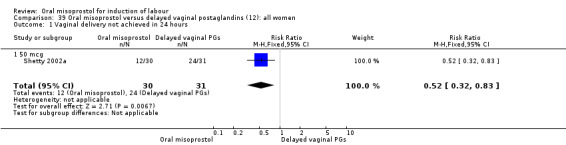
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
(13) Oral misoprostol versus dinoprostone vaginal insert (analysis 40)
One study compared the use of hourly titrated oral misoprostol with 10 mcg dinoprostone vaginal insert placed for a maximum of 24 hours (Rouzi 2014). There were no statistically significant differences in any of the 10 outcomes.
Discussion
Efficacy compared to other induction agents
Oral misoprostol is more effective than placebo and equivalent to intravenous oxytocin, vaginal misoprostol and vaginal dinoprostone for the induction of labour in women. Oral misoprostol results in fewer caesarean sections than oxytocin alone. In the comparisons with oxytocin infusions, there seems to be a higher rate of meconium staining at all dosages, but this was not associated with any adverse effect on the fetus. It is possible that the meconium staining could be a direct effect of the misoprostol on the fetal gut. Gastrointestinal stimulation leading to diarrhoea is a well‐described side effect of oral misoprostol in adults, and small amounts of misoprostol in the fetus could lead to the expulsion of meconium and hence meconium‐stained liquor. This effect is also seen with vaginal misoprostol, but it appears to be smaller. This may be due to the lower peak serum concentrations that occur following vaginal administration.
There have been concerns in the past about uterine hyperstimulation with oral misoprostol. In studies where most women were given low dose oral misoprostol (20‐25 mcg two‐hourly), the hyperstimulation rates were the same as for vaginal dinoprostone (i.e. around 5% overall and 2.9% with fetal heart rate (FHR) abnormalities).
There were fewer studies comparing oral misoprostol and intracervical dinoprostone, but these found that the oral misoprostol (at doses of 50 and 200 mcg) resulted in stronger contractions than the dinoprostone. This led to higher rates of hyperstimulation and a more rapid labour, but with no effect on fetal outcomes.
Whilst no increase in adverse fetal outcomes was seen in these studies, this meta‐analysis contains too few studies to exclude a difference in adverse outcomes which are rare (see additional Table 1 for numbers needed). However, the comparable safety data on vaginal dinoprostone are also limited. There are nearly 14,000 women in the trials in this review, which compares favourably with the Cochrane review of the current standard treatment vaginal dinoprostone (PGE2) with 10,000 women (Kelly 2009).
1. Sample size calculation.
| Outcomes | Baseline rate | ? Important change | Rel. Risk | Total sample size |
| Failure to achieve vaginal birth within 24 hours | 30% | 21% | 0.7 | 778 |
| Caesarean section (CS) | 10% | 7% | 0.7 | 1294 |
| Hyperstimulation | 1% | 0.7% | 0.7 | 30,716 |
| Perinatal mortality and morbidity | 0.5% | 0.35% | 0.7 | 61,686 |
| Uterine rupture in women with previous CS | 0.5% | 0.35% | 0.7 | 61,686 |
| Maternal death or serious morbidity | 0.2% | 0.14% | 0.7 | 154,598 |
Should misoprostol be used orally or vaginally?
The large variety of doses of both oral and vaginal misoprostol used in the direct comparisons make it very difficult to interpret these data. The only consistent findings were a reduction in low Apgar score at five minutes in those given oral misoprostol (but there is no corresponding reduction in special baby care unit admission), lower rates of postpartum haemorrhage, and an increase in meconium‐stained liquor. The cause of the improved Apgars in those given oral misoprostol is not clear, but it may relate to the hyperstimulation rates, which were generally lower in those given low‐dose oral misoprostol. The relative acceptability of the oral and vaginal routes also need to be considered alongside this clinical data. Satisfaction was only considered in one study of 200 women (Colon 2005 (V25)), and in this study only two women (one in each group) expressed dissatisfaction. However, other clinical studies comparing oral and vaginal misoprostol have found increased satisfaction with the oral route.
In summary, there is some evidence that the oral route may result in improved clinical outcomes over the vaginal route. Given the likely greater acceptability for women using an oral route, it is the authors' opinion that the oral route should be preferred over the vaginal route.
What is the optimal dose of oral misoprostol?
The comparison with vaginal misoprostol is complicated by the wide variation in dosages used for both vaginal and oral misoprostol. Hence a comparison between 50 mcg oral misoprostol and 100 mcg vaginal misoprostol may give very different outcomes to a comparison between 200 mcg oral misoprostol and 25 mcg vaginal misoprostol. This is especially important given that both efficacy and side effects are a function of the ability of misoprostol to produce effective uterine contractions. The optimal dose will have to balance the desire for short induction to birth interval with impact of strong, effective uterine contractions on the fetal wellbeing and uterine muscle integrity.
An attempt to assess the effect of dose on effectiveness and safety has been made with the subgroup analyses by various doses of oral misoprostol. This has resulted in multiple subgroup analyses with a high potential for spurious results (type I error). To protect against this, we have analysed further the subgroups with significant heterogeneity within the results. We have used the interaction test (a form of chi‐squared test) to assess the association with dosage. We chose uterine hyperstimulation without FHR changes as the outcome most closely representing a bioassay of misoprostol activity. The relative strength of various misoprostol dosages given vaginally and orally is best seen in the graph for outcome 21.8, where similar hyperstimulation rates are seen when the dose of oral misoprostol is about double that of the vaginal doses. Similarly, the use of the same doses orally and vaginally results in generally lower hyperstimulation rates in the oral group. This suggests that equivalent biological activity is seen when the dose of oral misoprostol is about double that of the vaginal dose. Given that the Cochrane review of vaginal misoprostol suggests a dosage of 25 mcg vaginal misoprostol, it might be expected that the optimal dose (i.e. that which is equivalent to vaginal dinoprostone) would be 50 mcg. In the comparison with vaginal dinoprostone, most trials used oral misoprostol dosages of 20 to 25 mcg and it is the trials using this dose in which the CS rate may be decreased (Analysis 5.3, Analysis 6.3). Overall, therefore, the results of this review suggest that the optimal dosage of oral misoprostol is 20 to 25 mcg given two‐hourly.
5.3. Analysis.
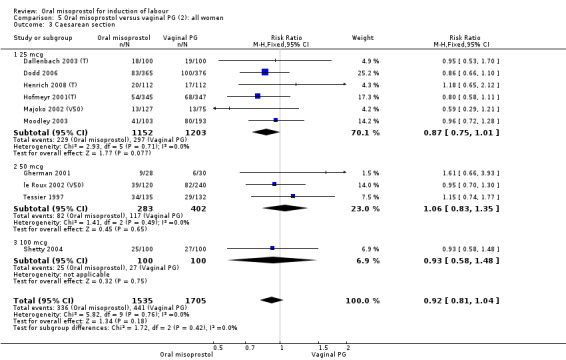
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 3 Caesarean section.
Obtaining a misoprostol dosage of 25 mcg tablets by cutting a 200 mcg tablet into eighths is difficult and imprecise. To get around this, the studies that used 20 to 25 mcg have generally dissolved the 200 mcg tablet in 200 mL water and administered 20 mL. Bioassays suggest that misoprostol remains active for at least 24 hours in this form (Matonhodze 2005). Whilst this will undoubtedly produce a more precise dose, there are ongoing issues around the storage of the unused solution. In view of the lack of good information about the stability of misoprostol in solution and the risks involved in having unused solution sitting around in containers on a labour ward, the best advice would be to discard any unused solution after administering each dose. The recent availability of misoprostol 25 mcg tablets is welcome and should overcome many of these problems.
Risks of induction
Some observational studies have reported a high uterine rupture rate when vaginal misoprostol was given to women who have had previous CSs. There is less evidence for oral misoprostol, but there were rupture rates of 10% (one of 10) and 9.7% (four of 41) in two studies that used oral misoprostol for induction (Aslan 2004; Gherman 2001a). Whilst the lack of ruptures in the 158 women in this review's randomised trials who underwent induction is encouraging (Carlan 2001 (V50); How 2001 (V25)), it would take a trial of over 60,000 women to evaluate whether there was an increase in the 0.5% background scar rupture rate in women undergoing a trial of vaginal birth (Table 1).
There is no consensus on what constitutes an acceptable risk of labour induction in the absence of life‐threatening conditions for mother and baby. It is likely that most parents and clinicians would not be prepared to accept a 0.5% to 1% increase in serious adverse outcomes. In fact, it is likely that women would be prepared to spend more time on delivery suite if this means a safer labour, but there is a noticeable lack of data on this. Indeed, most studies in this review did not assess women's views or satisfaction rates.
Currently available data are nowhere near large enough to address the issue of safety of either the induction process (Table 1) ar the long‐term follow‐up of babies exposed to misoprostol. It is important to reiterate here that 'no evidence of difference' in this Cochrane review does not mean 'evidence of no difference'. In other words, when even more randomised data become available, small but important clinical differences between various regimens may become apparent, i.e. statistically significant.
Trials or meta‐analyses that have adequate power to address the issue of rare adverse fetal outcomes will need to include an excess of 30,000 women. Until the logistics and financial resources are available to conduct such studies, alternative ways must be found of assessing the risk. Registries of women using oral misoprostol for induction may be a way of achieving this.
Summary of main results
In nine trials comparing oral misoprostol with placebo (1109 women, almost all with ruptured membranes), women using oral misoprostol were more likely to give birth vaginally within 24 hours (risk ratio (RR) 0.16, 95% confidence interval (CI) 0.05 to 0.49; one study in which all women received dinoprostone after 12 hours), needed less oxytocin (seven studies; 933 women) and had a lower CS rate (eight studies 1029 women).
Ten trials compared oral misoprostol with vaginal prostaglandin (dinoprostone) (3,240 women). There was little difference in the frequency of: vaginal birth within 24 hours (5 trials; 2,128 women), uterine hyperstimulation with fetal heart rate changes (7 trials; 2,352 women), and caesarean birth (10 trials; 3240 women), but evidence of slower inductions (cervix unfavourable/unchanged after 12‐24 hours).
Five trials compared administration of oral with intracervical misoprostol (681 women). Oral misoprostol was associated with fewer instances of failure to achieve vaginal birth within 24 hours (3 trials; 452 women) but more frequent uterine hyperstimulation with fetal heart rate changes (3 trials; 490 women). The available data for these comparisons was however limited and the differences in caesarean birth were small (5 trials; 742 women).
Nine trials compared oral misoprostol with intravenous oxytocin (1282 women). There were no obvious differences in the frequency of: vaginal birth within 24 hours (6 trials; 789 women), or uterine hyperstimulation with fetal heart rate changes (7 trials; 947 women). There were, however, fewer caesarean births with oral misoprostol (RR 0.77, 95% CI 0.60 to 0.98; 9 trials; 1282 women). Oral misoprostol was associated with increased rates of meconium‐stained liquor (seven trials; 1172 women).
Thirty‐seven trials compared oral misoprostol with vaginal misoprostol (6417 women). There was little difference in the frequency of: vaginal birth within 24 hours (14 trials; 2,448 women), uterine hyperstimulation with fetal heart rate changes (29 trials; 5,503 women), and caesarean birth (35 trials; 6,326 women). However, there were fewer babies born with a low Apgar score in the oral misoprostol group (19 studies, 4009 women) and fewer women with postpartum haemorrhage (10 trials, 1478 women), but increases in meconium‐stained liquor (24 studies, 3634 women).
Overall completeness and applicability of evidence
With 75 studies included in the review, there has been the opportunity to examine an extensive range of misoprostol regimens and comparisons with other induction methods. The studies have also been conducted in a wide range of settings, making the results applicable internationally. The studies have all however been conducted in settings where the participants can be closely monitored. This will reduce the risk to the baby of any uterine hyperstimulation, but means that the results may not be applicable to setting where fetal monitoring is not available. In those settings, priority should be given to induction methods with low rates of hyperstimulation and/or fetal risks.
Quality of the evidence
There is adequate high‐quality evidence within the 75 studies to make robust recommendations. There is however, a wide spread of oral misoprostol regimens examined in this review and a large variation in the quality of studies and reports. So whilst the quality of evidence for some comparisons is very robust (for example, oral misoprostol versus vaginal misoprostol), for other less common comparisons the strength of the recommendations must be lower (e.g. in those comparisons of different misoprostol regimens). With so many comparisons within the review, there is also a risk of statistical type 1 error, that is a false‐positive result. The results in which there are very few studies, or heterogeneity, or those in which the meta‐analysis result is of borderline statistical significance must therefore be treated with caution, and we have sought to do this.
Potential biases in the review process
Both review authors Z Alfirevic and AD Weeks have been involved in misoprostol research for many years, running some of the studies themselves and knowing many of the other people who have run other studies. There is therefore the potential to be biased in their assessment of the strength and importance of studies. The robustness of the methodology along with the peer review process will help to prevent any important effect on the results or recommendations of the review.
Agreements and disagreements with other studies or reviews
This review is one of several reviews within The Cochrane Library on induction techniques, but the only one that directly addresses the use of oral misoprostol. The World Health Organization, in preparation for their recommendations for the induction of labour, conducted an extensive review into all forms of induction of labour (WHO 2011). Their conclusion was that "oral misoprostol (25 mcg, two‐hourly) is recommended for induction of labour" along with other methods including dinoprostone, vaginal misoprostol (25 mcg) and cervical balloon catheter. A review focusing specifically on low‐dose oral misoprostol (20 to 25 mcg) was carried out using the Cochrane methodology by Kundodyiwa et al (Kundodyiwa 2009). The findings were similar to this review and the authors concluded that "low‐dose oral misoprostol solution (20 mcg) administered every two hours seems at least as effective as both vaginal dinoprostone and vaginal misoprostol, with lower rates of caesarean birth and uterine hyperstimulation, respectively".
Authors' conclusions
Implications for practice.
Oral misoprostol as an induction agent is effective at achieving vaginal birth. It is more effective than placebo, as effective as vaginal misoprostol and vaginal dinoprostone, and results in fewer caesarean sections than oxytocin alone. Oral misoprostol 20 to 50 mcg is as effective as vaginal misoprostol, and has lower rates of low Apgar scores and postpartum haemorrhage.
There had been concerns about high rates of hyperstimulation with oral misoprostol, despite the fact that this had never been shown to cause any increase in adverse fetal outcomes. With low doses of oral misoprostol, this does not appear to be a problem. Rates of hyperstimulation are equivalent to both placebo and the current gold standard, vaginal dinoprostone. Comparisons with vaginal misoprostol are made difficult by the wide variety of doses of both oral and vaginal misoprostol used, which results in significant heterogeneity.
Although there were no reported uterine ruptures in the 160 women in this review who were induced with misoprostol having had a previous caesarean sections, observational studies suggest that the uterine rupture rate is high with misoprostol, even when used in low doses. We therefore continue to recommend that it should not be used for women with previous caesarean section scars.
In deciding whether to change to oral misoprostol from dinoprostone, practitioners will need to balance the advantages of low‐dose oral misoprostol (reduced caesarean section rate, low cost, heat stability and oral administration) against the problems resulting from the lack of a 25 mcg oral formulation (inaccuracies in dosage and the risks of making up the dosage oneself). If using oral misoprostol, the evidence suggests that the dose 20 to 25 mcg in solution and report serious adverse outcomes. Given that the primary consideration should be safety of induction, the evidence supports the use of oral regimens (using a maximum of 50 mcg) over vaginal regimens. This is especially important in situations where the risk of ascending infection is high and staffing levels mean that women undergoing induction cannot be intensely monitored (i.e. having one‐to‐one care and electronic fetal monitoring).
Implications for research.
Methodologically sound clinical trials continue to be a priority, both in developing and industrialised countries. Ideally, these trials should be blinded and be limited to low‐dose oral misoprostol (20 to 50 mcg). A randomised examination comparing different regimens would be helpful to determine whether the labour intensive one‐ to two‐hourly administration is necessary, and the relative benefits of oral solution and the newly available 25 mcg tablets. Unblinded studies are prone to biased reporting of outcomes such as randomisation (induction) to birth interval, uterine hyperstimulation, fetal distress and maternal side effects. Also, in unblinded trials the threshold to perform caesarean section may vary according to the clinician's prior beliefs on the safety of misoprostol. On the other hand, administration of vaginal placebo may increase the number of vaginal examinations, influence clinical decision making and introduce bias in the assessment of women's and clinicians' views.
Any proposed dose regimen includes a trade‐off between rapid birth and uterine hyperstimulation. Qualitative studies are required to assess how much emphasis women place on a short labour compared with increased perinatal risks that may be associated with shorter labours. Given that this trade‐off may vary depending on personality and culture, a flexible approach to dosage may be appropriate.
Only large, pragmatic trials with adequate sample size or large registries will be able to address the risks of uterine hyperstimulation, uterine rupture, serious neonatal and maternal morbidity, and long‐term safety. The contact author would be happy to collect the information on serious adverse outcomes associated with the use of oral misoprostol (maternal and perinatal deaths, uterine rupture) and report them, with permission, in future updates of this review.
Feedback
Thornton, 1 October 2014
Summary
The authors are to be congratulated on their detailed presentation of the randomised trials of the various doses of oral misoprostol. This detail matters because, as they recognise, comparing drugs for induction of labour requires consideration of the doses used and their bio‐equivalence as well as routes of administration.
However, their two main conclusions are not justified by the data they so carefully present, namely:
That oral misoprostol is more effective than placebo, and results in fewer Caesareans than vaginal dinoprostone, and
That a low oral dose of 20‐25 micrograms should be used, being as effective as vaginal dinoprostone and having lower rates of low Apgar scores and postpartum haemorrhage.
Broadly the evidence for effectiveness/equivalence is based on studies using higher doses of oral misoprostol (>25 micrograms). But the evidence that the oral route causes fewer complications is almost entirely restricted to studies using low oral doses. To describe this in detail:
1. Evidence that oral misoprostol is more effective than placebo
Analysis 1.1: The reduction in “vaginal delivery not achieved within 24 hours” with oral misoprostol is based on one trial of 100 micrograms. There is no evidence in trials with lower doses.
Analysis 1.6: No evidence for reduction in “cervix unfavourable/unchanged” with any dose. Note this outcome is omitted in Comparison 1 probably in error. Outcome 6 should be cervix unchanged (see page 5 and other tables p 82 ff). Instead ‘epidural analgesia’ is the label for outcome 6 here (page 82). Epidural appears again where it should, as Outcome 10, but with another dose and other figures. I therefore assume that Outcome 6 should be cervix unchanged on page 82.
Analysis 1.7: The reduction in “oxytocin augmentation” with oral misoprostol is based on one trial with dose not specified, and six trials of ≥50 micrograms. There is no evidence for the 25 mcg dose.
2. Evidence that the oral route is associated with fewer Caesarean sections than vaginal dinoprostone
Analysis 5.3: The seven trials of the 25 microgram dose showed reduced Caesareans. The four trials of a higher dose show no significant difference.
Evidence that oral misoprostol 25 mcg is as effective as vaginal dinoprostone
Analysis 5.6: Oral misoprostol 25 mcg is inferior to vaginal dinoprostone with regard to cervix unfavourable/unchanged after 12‐24 hours and inconclusive with regard to the outcomes vaginal delivery not achieved within 24 hours (analysis 5.1) and oxytocin augmentation (analysis 5.7).
3. Evidence that oral misoprostol 25 mcg has lower rates of low Apgar score and postpartum haemorrhage
Analysis 5.13 and 22: There is no evidence that oral misoprostol 25 mcg or any other dose reduces these outcomes in comparison with vaginal dinoprostone.
4. Evidence that oral misoprostol causes less uterine hyper‐stimulation with fetal heart rate changes
Analysis 5.2: Five trials of 25 micrograms failed to show reduced hyper‐stimulation with fetal heart rate changes compared with vaginal dinoprostone. The two trials of 50 micrograms and the single trial of 100 micrograms show a large, albeit not statistically significant, increase in uterine hyper‐stimulation with fetal heart rate changes.
Analysis 21.2: Here, in comparison with vaginal misoprostol, the evidence is less clear cut and does not follow the 25 microgram cut‐off. Nevertheless, 16 trials with the 50 micrograms dose show reduced uterine hyper‐stimulation with fetal heart rate changes, but the two trials with the 200 micrograms show an increase in uterine hyper‐stimulation with fetal heart rate changes.
One interpretation of these data would be that low oral doses of misoprostol (20 to 25 mcg) are probably safe, but there is little or no evidence that they are effective. Higher oral doses are probably effective, but they appear to have broadly similar or increased rates of hyper‐stimulation and Caesarean section as the vaginal route comparators. The conclusion that the oral route is both effective AND results in less hyper‐stimulation only follows from a crude combination of all the different dosage regimes. An important problem with this approach is that conclusions on various outcomes are based on different sets of studies leading to different sets of dosage regimens. This is particularly problematic when a safety conclusion is based on a set of studies dominated by a low dose and effectiveness is based on studies dominated by higher doses. The specific claim that the low dose of 25 micrograms is effective is not supported by the data.
Of course all these conclusions need to be tempered by the fact that nearly all the trials were small, open label, investigator‐led studies, and used with different vaginal comparator doses.
A more conservative, but probably fairer interpretation, would be that despite 76 trials in 14,412 women, we still do not know the risk/benefit ratio of oral misoprostol in its various doses as compared to it or other induction agents administered by the vaginal route.
Comment submitted by Jim Thornton, October 2014
Reply
The feedback from Thornton (1st October 2014) is wide‐ranging and the answers are provided in detail below.
“The authors are to be congratulated on their detailed presentation of the randomised trials of the various doses of oral misoprostol. This detail matters because, as they recognise, comparing drugs for induction of labour requires consideration of the doses used and their bio‐equivalence as well as routes of administration.
However, their two main conclusions, namely (1) that the oral route is more effective than placebo, and results in fewer Caesareans than vaginal dinoprostone, and (2) that a low oral dose of 20‐25 micrograms should be used being as effective as vaginal misoprostol and having lower rates of low Apgar scores and postpartum haemorrhage, are not justified by the data they so carefully present.
Broadly the evidence for effectiveness/equivalence is based on studies using higher doses of oral misoprostol (>25 micrograms). But the evidence that the oral route causes fewer complications is almost entirely restricted to studies using low oral doses.
I describe it in detail below.
Evidence that oral misoprostol is more effective than placebo
Comparison 1 outcome 1. The reduction in “vaginal delivery not achieved within 24 hours” with oral misoprostol is based on one trial of 100 micrograms. No evidence in trials with lower doses.
Comparison 1 outcome 6. No evidence for reduction in “cervix unfavourable/unchanged” with any dose. Note this outcome is omitted in Comparison 1 probably in error. Outcome 6 should be cervix unchanged (see page 5 and other tables p 82 ff). Instead epidural analgesia is the label for outcome 6 here (page 82). Epidural appears again where it should, as Outcome 10, but with another dose and other figures. I therefore assume that Outcome 6 should be cervix unchanged on page 82
Thornton has correctly identified an error, but on review of the original data both 1.6 and 1.10 do both relate to epidural use. They have now been merged so as to combine the two studies in meta‐analysis.
Comparison 1 outcome 7. The reduction in “oxytocin augmentation” with oral misoprostol is based on one trial dose not specified, and six trials of >=50 micrograms. There is no evidence for the 25 mcg dose.
It is correct that there is little evidence of the efficacy of low dose misoprostol against placebo. However, very few studies are conducted against placebo due to the previously proven efficacy of induction of the technique. So the question pertinent to this review is ‘how should one induce labour?’ not ‘whether one should do an induction’ (which a placebo‐controlled study would provide an answer to). In the same way, a review of CS incision techniques could be placebo controlled, but it would not be helpful as there would be no incision to analyse. It is for this reason that there are very few placebo‐controlled studies of induction techniques and, once researchers have demonstrated the benefits of induction, the research moves on to compare the new technique with the current gold standard.
Evidence that the oral route is associated with fewer Caesarean sections than vaginal dinoprostone
Comparison 5, outcome 3. The 7 trials of the 25 microgram dose showed reduced Caesareans. The four trials of a higher dose show no significant difference.
This outcome has now changed as a duplicate publication has been excluded (see above). But throughout the review we argue that the optimal dosage of oral misoprostol is 20‐25mcg and this analysis supports that conclusion.
Evidence that oral misoprostol 25 mcg is as effective as vaginal dinoprostone.
Comparison 5 outcome 6. Oral misoprostol 25 mcg is inferior to vaginal dinoprostone with regard to cervix unfavourable/unchanged after 12‐24 hours and inconclusive with regard to outcome 1 ‐ vaginal delivery not achieved within 24 hours and outcome 7 – oxytocin augmentation.
We repeatedly acknowledge in the text that although the CS rates appears to be decreased in comparison to dinoprostone, the induction process appears to be slower. Given that the primary aim of induction is to achieve a safe vaginal birth we do not think that this is a major problem.
Evidence that oral misoprostol 25 mcg has lower rates of low Apgar score and postpartum haemorrhage
Comparison 5 outcomes 13 and 22. There is no evidence that oral misoprostol 25 mcg or any other dose reduces these outcomes in comparison with vaginal dinoprostone.
We agree. The reduction in low Apgar scores and postpartum haemorrhage are seen in the comparison with vaginal misoprostol, and this is already clear in the analyses and text.
Evidence that oral misoprostol causes less uterine hyper‐stimulation with fetal heart rate changes.
Comparison 5, outcome 2. Five trials of 25 micrograms failed to show reduced hyper‐stimulation with fetal heart rate changes compared with vaginal dinoprostone. The two trials of 50 micrograms and the single trial of 100 micrograms show a large, albeit not statistically significant, increase in uterine hyper‐stimulation with fetal heart rate changes.
Comparison 21, outcome 2. Here, in comparison with vaginal misoprostol, the evidence is less clear cut and does not follow the 25 microgram cut‐off. Nevertheless, 16 trials with the 50 micrograms dose show reduced uterine hyper‐stimulation with FHR changes, but the 2 trials with the 200 micrograms show an increase in uterine hyper‐stimulation with FHR changes.
This is correct. But we have not suggested that oral misoprostol has lower outcomes of this outcome. Indeed in the ‘Implications for Practice’ we state that low doses of oral misoprostol appear to have equivalent rates of hyperstimulation to dinoprostone, and that the data against vaginal misoprostol is ‘difficult’ due to significant heterogenicity. In the text 'effects of interventions comparison 6' we summarise the evidence against vaginal misoprostol by saying that there does appear to be related to dose with lower rates in lower doses of oral misoprostol.
One interpretation of these data would be that low oral doses (20 to 25 mcg) are probably safe, but there is little or no evidence that they are effective.
See comments above. This review did not set out to provide evidence for efficacy of induction per se, but to compare different methods.
Higher oral doses are probably effective, but they appear to have broadly similar or increased rates of hyper‐stimulation and Caesarean section as the vaginal route comparators.
We agree that the outcomes with high dose misoprostol are not as good, which is why we recommend low dose oral misoprostol.
The conclusion that the oral route is both effective AND results in less hyper‐stimulation only follows from a very crude combination of all the different dosage regimes. An important problem with this approach is that conclusions on various outcomes are based on different sets of studies leading to different sets of dosage regimens. This is particularly problematic when a safety conclusion is based on a set of studies dominated by a low dose and effectiveness is based on studies dominated by higher doses. The specific claim that the low dose of 25 micrograms is effective is not supported by the data.
With respect, we disagree for the reasons given above.
Of course all these conclusions need to be tempered by the fact that nearly all the trials were small, open label, investigator‐led studies, and used with different vaginal comparator doses.
A more conservative, but probably fairer interpretation, would be that despite 76 trials in 14,412 women, we still do not know the risk/benefit ratio of oral misoprostol in its various doses as compared to it or other induction agents administered by the vaginal route.”
Contributors
Andrew Weeks
Rydahl and Clausen, 11 March 2015
Summary
The Cochrane Handbook chapter 10 section 2.2.1 (1) cautions that duplicate data may overestimate the effect of an intervention. We have identified duplicate data within this Cochrane Review (2). In the analysis of oral misoprostol versus vaginal prostaglandin effect on Caesarean section (analysis 5.3)(2), data are reported from seven low dose studies (25 mcg misoprostol). We reread the reports of these seven studies, and in this process found duplicate data in two that calls into question the conclusions of this review. The two studies are Hofmeyer 2001 (3) and Matonhodze 2003 (4). Our view that there are duplicate data from these two trials presented in the review is based on several factors. While authorship between the two papers is not identical, the two studies do share the first authors Hofmeyer and Matonhodze. Also, Hofmeyer 2001 is a multicentre study from the UK and South Africa comparing titrated oral misoprostol with vaginal prostaglandin for women with intact or ruptured membranes (3), whilst Matonhodze 2003 was a three arm trial comparing titrated oral misoprostol with vaginal prostaglandin and Foley catheter for women with intact membranes (4). The methods section of both papers state that a sub‐set of the South African study population was used twice. Hofmeyer 2001 states: “At the South African sites women with intact membranes were randomly allocated to three groups, the third being for induction of labor with an extra‐amniotic Foley catheter bulb. The result of this comparison will be reported separately” (3). Matonhodze 2003 (4) says “A randomized study design was used, nested within the larger trial. Those women with intact membranes enrolled at the South African sites were randomly allocated to Foley catheter/oral misoprostol, oral misoprostol alone or vaginal dinoprostone. This report presents the results of that three‐way comparison”. This paper notes that the larger trial referred to is Hofmeyer2001 (4).
Matonhodze 2003 therefore reused data from the Hofmeyer 2001 study for women from South Africa with intact membranes (4). The number of participants in the two studies differed slightly: Hofmeyer 2001 reports missing data for 4 participants from South Africa, Matonhodze 2003 does not report on missing data, which may explain the small difference between the two study populations. We re‐calculated analysis 5.3 excluding the possible duplicate data from Matonhodze 2003 (4). Returning to the issue of duplicate data resulting in an overestimation of effects (1), we believe this to be the case here. The Cochrane review found oral misoprostol to be a superior method for induction of labour with a risk ratio for the effect on Caesarean section of 0.83 with 95% CI 0.72 to 0.96 (2). In our analysis the risk ratio is 0.87 with 95% CI 0.75 to 1.01; a result that no longer reaches statistical significance. The review concludes “oral misoprostol as an induction agent is effective at achieving vaginal birth. It is more effective than placebo, as effective as vaginal misoprostol and results in fewer caesarean sections than vaginal dinoprostone or oxytocin” (2). However, if we are correct about the duplicate data this would change the conclusion about Caesarean section, and would have further potential consequences as Matonhodze 2003 (4) is used in several other analyses (5.1, 5.2, 5.9, 5.11, 5.13, 5.14, 5.16, 6.1, 6.2 and 6.3). This Cochrane Review has informed policy and practice around the world. Recently, the Council for the Use of Expensive Hospital Medicines in Denmark (RADS) used this review to recommend low dose oral misoprostol for the induction of labour (5). We reviewed this recommendation and submitted our analysis to the Danish Minister of Health (6), our comments are included in the official report (6). We urge the authors of this review to consider our comments without delay.
Comment submitted by Eva Rydahl and Jette Aaroe Clausen, March 2015 References (1) Higgins J, Green S. (2011). Cochrane handbook for systematic review of interventions (5.1.0 ed.) The Cochrane Collaboration. (2) Alfirevic Z, Aflaifel N, and Weeks A. (2014). Oral misoprostol for induction of labour. The Cochrane Database of Systematic Reviews, 6, CD001338. doi:10.1002/14651858.CD001338.pub3. (3) Hofmeyr GJ, Alfirevic Z, Matonhodze B, Brocklehurst P, Campbell E, and Nikodem VC. (2001). Titrated oral misoprostol solution for induction of labour: A multi‐centre, randomised trial. BJOG : An International Journal of Obstetrics and Gynaecology, 108:952‐959. (4) Matonhodze BB, Hofmeyr GJ, and Levin J. (2003). Labour induction at term‐‐a randomised trial comparing foley catheter plus titrated oral misoprostol solution, titrated oral misoprostol solution alone, and dinoprostone. South African Medical Journal, 93:375‐379. (5) Rådet for Anvendelse af Dyr Sygehusmedicin (RADS) (2014) Behandlingsvejledning for prostaglandiner til igangsættelse af fødsler. [Guideline for the use of prostaglandins for induction of labor] Accessed 03/11/15 on http://www.regioner.dk/sundhed/medicin/r%C3%A5det+for+anvendelse+af+dyr+sygehusmedicin+rads/behandlingsvejledninger (6) Clausen JA, Rydahl E. (2015) Analyse af RADS´vejledning vedrørende protaglandiner til igangsættelse af fødsel [Analysis of RADS recommendations on prostaglandines for induction of labor]; Department of Midwifery, Metropolitan University College, Copenhagen, Denmark. Accessed 03/11/15 on http://www.ft.dk/samling/20141/almdel/suu/bilag/278/1503680/index.htm;
Reply
This is correct and we have now excluded the offending study (Matonhodze 2003 is now listed as an additional report of Hofmeyr 2001(T)). The analyses have therefore changed and we have amended the text accordingly.
Contributors
Andrew Weeks
What's new
| Date | Event | Description |
|---|---|---|
| 26 April 2018 | Feedback has been incorporated | The authors have responded to Feedback 1 and Feedback 2. |
| 21 March 2018 | Amended | The review has been amended following Feedback 1 and Feedback 2. In the previous version of this review, we erroneously included a study (Matonhodze 2003) that was actually a subset of another multicentre included study (Hofmeyr 2001(T)). This has now been rectified and we have adjusted the results of the comparison with vaginal dinoprostone. Consequently, the results and discussion have been amended accordingly. This review will no longer be updated in its current form and Published notes has been updated with more information about this. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 13 April 2015 | Feedback has been incorporated | Feedback 2 submitted by Eva Rydahl and Jette Aaroe Clausen. |
| 9 April 2015 | Feedback has been incorporated | Feedback 1 submitted by Jim Thornton. |
| 17 January 2014 | New citation required but conclusions have not changed | Nineteen new studies included: (Aalami‐Harandi 2013 (T); Deshmukh 2013 (V50); Elhassan 2007 (V50); Henrich 2008 (T); Javaid 2008; Jindal 2011 (V50); Khazardoost 2011 (V25); Kipikasa 2005; Mehrotra 2010 (V50); Nagpal 2009; Rahman 2013 (V25); Rath 2007; Rizvi 2007 (V25); Rouzi 2014; Sheikher 2009 (V25); Sitthiwattanawong 1999; Souza 2013(V25)(T); Sultana 2006 (V100); Thaisomboon 2012 (T)). |
| 17 January 2014 | New search has been performed | Search updated and updated meta‐analysis and text. |
| 21 September 2009 | Amended | Search updated. Twenty‐two reports added to Studies awaiting classification. |
| 5 June 2008 | New search has been performed | Search updated. Fifteen new studies have been added. |
| 5 June 2008 | Amended | Converted to new review format. |
| 31 October 2005 | New search has been performed | Search updated; 28 new trials have been added to the review and the text has been updated. |
| 31 October 2005 | New citation required and conclusions have changed | Previously, with only 13 trials in total, most of the comparisons showed no major differences. Now, with over 8600 women randomised in 41 trials, differences between the induction methods are emerging. Oral misoprostol is now found to be more effective than placebo, as effective as oxytocin (in women with ruptured membranes) and possibly more effective than vaginal dinoprostone. With higher doses of misoprostol, uterine hyperstimulation is a problem. A 50 mcg oral dose is as effective as vaginal misoprostol and there is a lower rate of side effects. It may therefore be preferable. As with all methods of induction there remain unanswered questions about safety. For rarer outcomes such as uterine rupture or stillbirth the meta‐analysis is still too small to demonstrate significant differences. Whilst these doubts remain, the use of products licensed for induction (e.g. dinoprostone and oxytocin) is recommended. |
Notes
This review will no longer be updated in its current form. This review will be superseded by a new review on low‐dose oral misoprostol for induction of labour ‐ the protocol for that review is now published in PROSPERO.
Acknowledgements
We acknowledge the helpful suggestions made by Graham Howarth, Anke Gaussmann, Justus Hofmeyr and Caroline Crowther on the earlier versions of this review.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Data and analyses
Comparison 1. Oral misoprostol versus placebo/ no treatment (1): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.49] |
| 1.1 100 mcg | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.49] |
| 2 Uterine hyperstimulation with FHR changes | 7 | 669 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.84, 8.68] |
| 2.1 50 mcg | 2 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.15 [0.25, 105.31] |
| 2.2 100 mcg | 3 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.54, 8.87] |
| 2.3 200 mcg | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.15 [0.13, 75.08] |
| 3 Caesarean section | 8 | 1029 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.54, 0.95] |
| 3.1 Dose not specified | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.38, 1.32] |
| 3.2 50 mcg | 3 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.54, 1.35] |
| 3.3 100 mcg | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.40, 1.40] |
| 3.4 200 mcg | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.28, 0.97] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 200 mcg | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 200 mcg | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Epidural analgesia | 2 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.69, 0.96] |
| 6.1 50 mcg | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.03] |
| 6.2 100 mcg | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.44, 1.01] |
| 7 Oxytocin augmentation | 7 | 933 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.37, 0.49] |
| 7.1 Dose not specified | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.43, 0.97] |
| 7.2 50 mcg | 3 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.38, 0.55] |
| 7.3 100 mcg | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.29, 0.60] |
| 7.4 200 mcg | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.16, 0.40] |
| 8 Uterine hyperstimulation without FHR changes | 5 | 784 | Risk Ratio (M‐H, Random, 95% CI) | 4.78 [0.73, 31.23] |
| 8.1 50 mcg | 2 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.39, 10.87] |
| 8.2 100 mcg | 2 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 13.0 [1.77, 95.73] |
| 8.3 200 mcg | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Postpartum haemorrhage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 5 | 379 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.37, 1.17] |
| 11.1 50 mcg | 3 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.30, 3.32] |
| 11.2 100 mcg | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.13, 1.19] |
| 11.3 200 mcg | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.31, 1.74] |
| 12 Meconium‐stained liquor | 4 | 561 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.66, 1.89] |
| 12.1 50 mcg | 2 | 365 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.65, 2.60] |
| 12.2 100 mcg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.31, 3.11] |
| 12.3 200 mcg | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.27, 2.62] |
| 13 Apgar score < 7 at 5 minutes | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.24, 2.26] |
| 13.1 100 mcg | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.22] |
| 13.2 200 mcg | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.26, 3.95] |
| 14 Neonatal intensive care unit admission | 5 | 734 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.32, 0.56] |
| 14.1 50 mcg | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.27, 0.50] |
| 14.2 100 mcg | 2 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.36, 1.68] |
| 14.3 200 mcg | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.15, 2.52] |
| 15 Perinatal death | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 15.1 Dose not specified | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.99] |
| 15.2 200 mcg | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Nausea | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.49] |
| 16.1 200 mcg | 1 | 156 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.49] |
| 17 Vomiting | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.42, 6.93] |
| 17.1 200 mcg | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.70 [0.42, 6.93] |
1.2. Analysis.
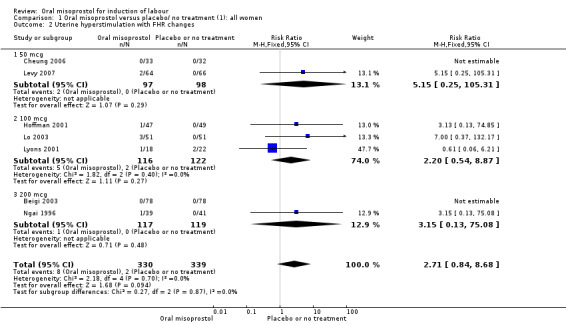
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
1.4. Analysis.
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 4 Serious neonatal morbidity or perinatal death.
1.5. Analysis.
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 5 Serious maternal morbidity or death.
1.6. Analysis.
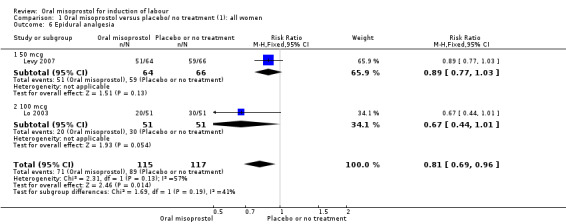
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 6 Epidural analgesia.
1.8. Analysis.
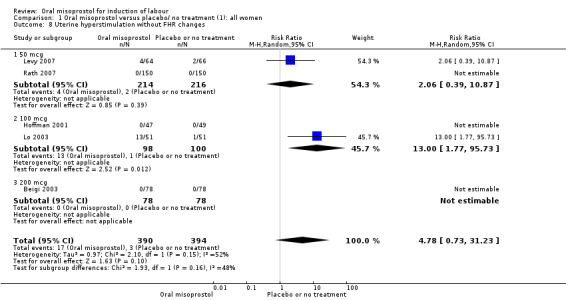
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 8 Uterine hyperstimulation without FHR changes.
1.11. Analysis.
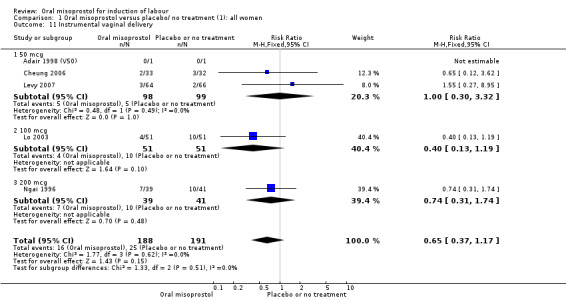
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 11 Instrumental vaginal delivery.
1.12. Analysis.
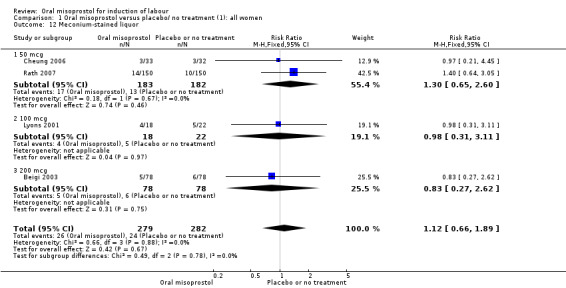
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 12 Meconium‐stained liquor.
1.13. Analysis.
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 13 Apgar score < 7 at 5 minutes.
1.15. Analysis.
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 15 Perinatal death.
1.16. Analysis.
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 16 Nausea.
1.17. Analysis.
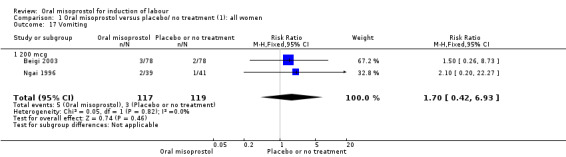
Comparison 1 Oral misoprostol versus placebo/ no treatment (1): all women, Outcome 17 Vomiting.
Comparison 2. Oral misoprostol versus placebo/ no treatment (1): all women with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.06, 6.21] |
2.1. Analysis.
Comparison 2 Oral misoprostol versus placebo/ no treatment (1): all women with intact membranes, Outcome 1 Uterine hyperstimulation with FHR changes.
Comparison 3. Oral misoprostol versus placebo/ no treatment (1): all women with ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.05, 0.49] |
| 2 Uterine hyperstimulation with FHR changes | 5 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.62 [1.01, 21.19] |
| 3 Caesarean section | 8 | 875 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.09] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.2. Analysis.
Comparison 3 Oral misoprostol versus placebo/ no treatment (1): all women with ruptured membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
3.3. Analysis.
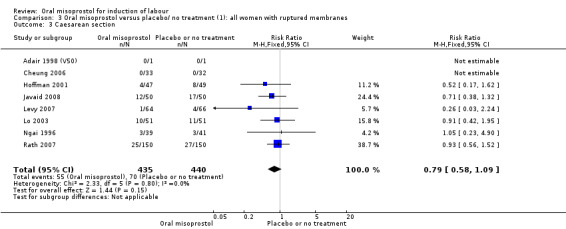
Comparison 3 Oral misoprostol versus placebo/ no treatment (1): all women with ruptured membranes, Outcome 3 Caesarean section.
3.4. Analysis.
Comparison 3 Oral misoprostol versus placebo/ no treatment (1): all women with ruptured membranes, Outcome 4 Serious neonatal morbidity or perinatal death.
3.5. Analysis.
Comparison 3 Oral misoprostol versus placebo/ no treatment (1): all women with ruptured membranes, Outcome 5 Serious maternal morbidity or death.
Comparison 4. Oral misoprostol versus placebo/ no treatment (1): all primiparae with ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 132.17] |
| 2 Caesarean section | 2 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.39, 1.64] |
| 3 Serious maternal morbidity or death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
Comparison 4 Oral misoprostol versus placebo/ no treatment (1): all primiparae with ruptured membranes, Outcome 1 Uterine hyperstimulation with FHR changes.
4.2. Analysis.
Comparison 4 Oral misoprostol versus placebo/ no treatment (1): all primiparae with ruptured membranes, Outcome 2 Caesarean section.
4.3. Analysis.
Comparison 4 Oral misoprostol versus placebo/ no treatment (1): all primiparae with ruptured membranes, Outcome 3 Serious maternal morbidity or death.
Comparison 5. Oral misoprostol versus vaginal PG (2): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 5 | 2128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.99, 1.22] |
| 1.1 25 mcg | 4 | 1928 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.99, 1.25] |
| 1.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 100 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.83, 1.29] |
| 2 Uterine hyperstimulation with FHR changes | 7 | 2352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.59, 1.53] |
| 2.1 25 mcg | 4 | 1827 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.51, 1.41] |
| 2.2 50 mcg | 2 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.26, 9.01] |
| 2.3 100 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 102.85] |
| 3 Caesarean section | 10 | 3240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.04] |
| 3.1 25 mcg | 6 | 2355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.75, 1.01] |
| 3.2 50 mcg | 3 | 685 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.83, 1.35] |
| 3.3 100 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.58, 1.48] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 25 mcg | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 2 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 25 mcg | 2 | 1436 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 2 | 930 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.01, 1.96] |
| 6.1 25 mcg | 2 | 930 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [1.01, 1.96] |
| 6.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Oxytocin augmentation | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 25 mcg | 3 | 1239 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.73, 1.30] |
| 7.2 50 mcg | 3 | 685 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.97, 1.69] |
| 7.3 100 mcg | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.98, 1.66] |
| 8 Uterine hyperstimulation without FHR changes | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 25 mcg | 5 | 2123 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.32, 2.03] |
| 8.2 50 mcg | 2 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.29, 1.76] |
| 8.3 100 mcg | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 7.0 [0.37, 133.78] |
| 9 Ruptured uterus | 7 | 2757 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 25 mcg | 5 | 2130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 50 mcg | 2 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 2 | 799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.98, 1.19] |
| 10.1 25 mcg | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.98, 1.22] |
| 10.2 50 mcg | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.18] |
| 11 Instrumental vaginal delivery | 6 | 2524 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.88, 1.24] |
| 11.1 25 mcg | 4 | 1857 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.83, 1.30] |
| 11.2 50 mcg | 2 | 467 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.73, 1.45] |
| 11.3 100 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.71, 1.75] |
| 12 Meconium‐stained liquor | 8 | 2548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.86, 1.32] |
| 12.1 25 mcg | 4 | 1463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.89, 1.54] |
| 12.2 50 mcg | 4 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.67, 1.44] |
| 12.3 100 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.41, 1.60] |
| 13 Apgar score < 7 at 5 minutes | 6 | 2181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.32, 1.10] |
| 13.1 25 mcg | 4 | 1856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.35, 1.25] |
| 13.2 50 mcg | 2 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.16] |
| 14 Neonatal intensive care unit admission | 9 | 3181 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 14.1 25 mcg | 6 | 2354 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.10] |
| 14.2 50 mcg | 2 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.19, 1.53] |
| 14.3 100 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.12] |
| 15 Neonatal encephalopathy | 2 | 1101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 25 mcg | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 50 mcg | 1 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Perinatal death | 4 | 1899 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.97] |
| 16.1 25 mcg | 3 | 1632 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.06, 15.97] |
| 16.2 50 mcg | 1 | 267 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal side effects (all) | 3 | 1632 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.36] |
| 17.1 25 mcg | 3 | 1632 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.77, 1.36] |
| 17.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Nausea | 3 | 1023 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.44, 1.11] |
| 18.1 25 mcg | 2 | 965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.41, 1.07] |
| 18.2 50 mcg | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [0.21, 22.35] |
| 19 Vomiting | 3 | 1632 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.79, 1.54] |
| 19.1 25 mcg | 2 | 1432 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.71, 1.56] |
| 19.2 50 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.68, 2.35] |
| 20 Diarrhoea | 2 | 965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.27, 1.87] |
| 20.1 25 mcg | 2 | 965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.27, 1.87] |
| 20.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Shivering | 2 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.37] |
| 21.1 25 mcg | 2 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.81, 1.37] |
| 21.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Postpartum haemorrhage | 3 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 22.1 25 mcg | 3 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.11] |
| 22.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Serious maternal complications | 1 | 692 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.1 25 mcg | 1 | 692 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23.2 50 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24 Hyperpyrexia | 2 | 965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 25 mcg | 2 | 965 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
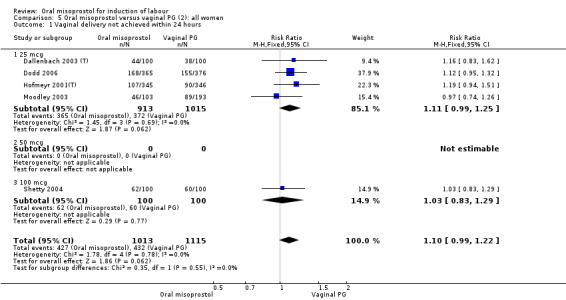
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
5.2. Analysis.
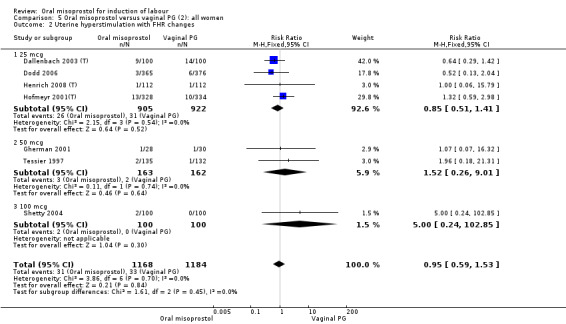
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
5.4. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 4 Serious neonatal morbidity or perinatal death.
5.5. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 5 Serious maternal morbidity or death.
5.7. Analysis.
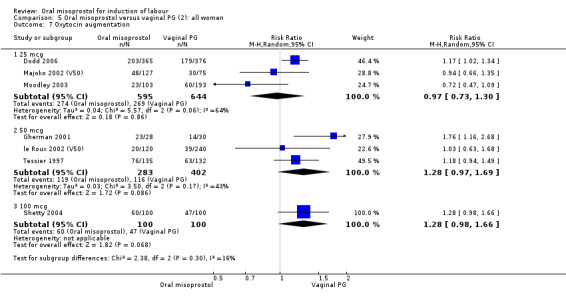
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 7 Oxytocin augmentation.
5.8. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 8 Uterine hyperstimulation without FHR changes.
5.9. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 9 Ruptured uterus.
5.10. Analysis.
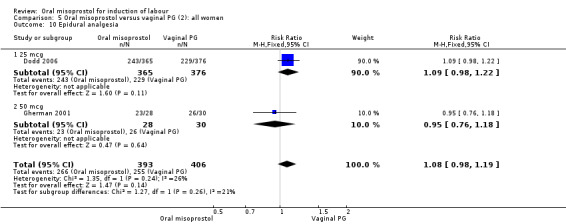
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 10 Epidural analgesia.
5.11. Analysis.
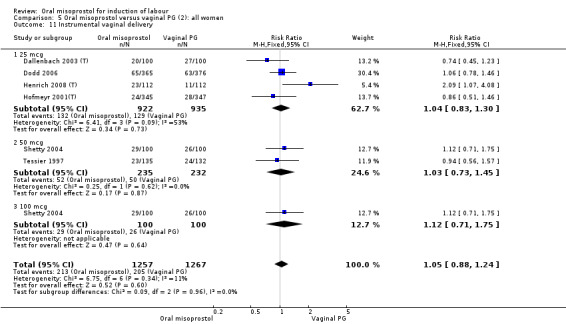
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 11 Instrumental vaginal delivery.
5.12. Analysis.
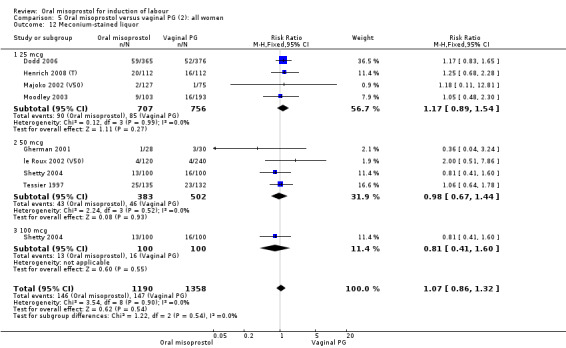
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 12 Meconium‐stained liquor.
5.13. Analysis.
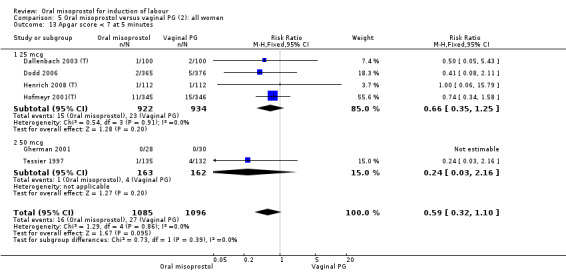
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 13 Apgar score < 7 at 5 minutes.
5.14. Analysis.
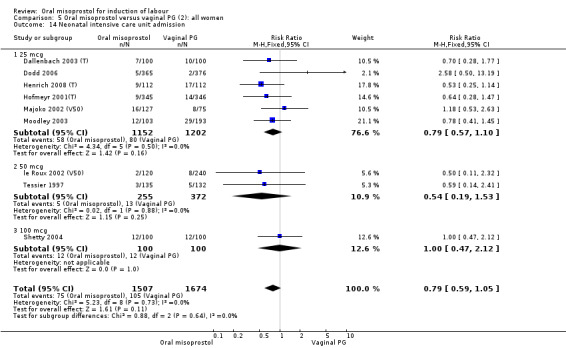
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 14 Neonatal intensive care unit admission.
5.15. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 15 Neonatal encephalopathy.
5.16. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 16 Perinatal death.
5.17. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 17 Maternal side effects (all).
5.18. Analysis.
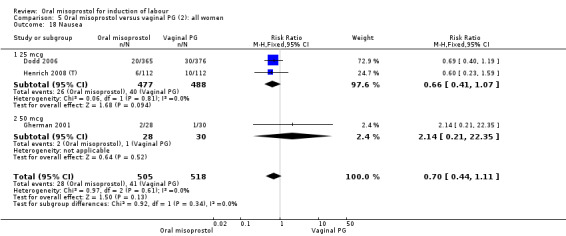
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 18 Nausea.
5.19. Analysis.
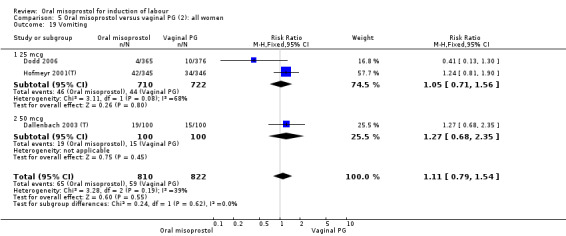
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 19 Vomiting.
5.20. Analysis.
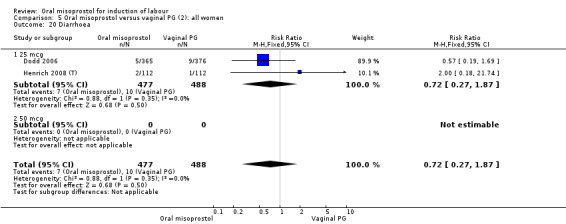
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 20 Diarrhoea.
5.21. Analysis.
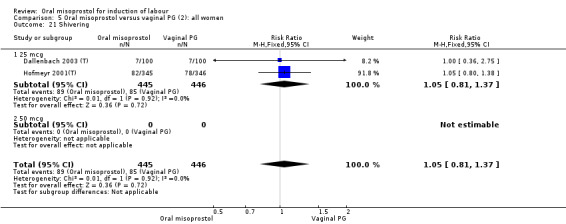
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 21 Shivering.
5.22. Analysis.
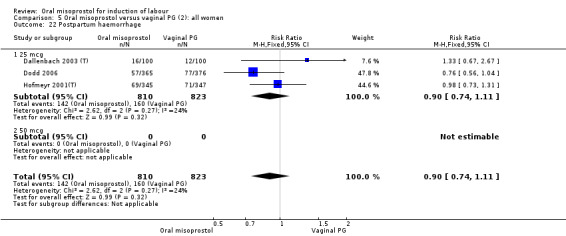
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 22 Postpartum haemorrhage.
5.23. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 23 Serious maternal complications.
5.24. Analysis.
Comparison 5 Oral misoprostol versus vaginal PG (2): all women, Outcome 24 Hyperpyrexia.
Comparison 6. Oral misoprostol versus vaginal PG (2): all women with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 4 | 1666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.01, 1.25] |
| 2 Uterine hyperstimulation with FHR changes | 4 | 1218 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.51, 3.09] |
| 3 Caesarean section | 6 | 2129 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.73, 0.99] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 25 mcg | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 25 mcg | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.03, 2.20] |
6.1. Analysis.
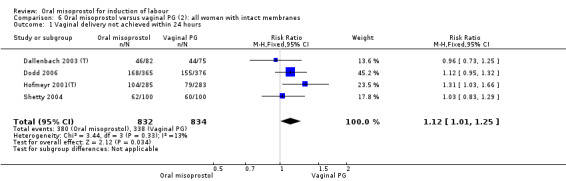
Comparison 6 Oral misoprostol versus vaginal PG (2): all women with intact membranes, Outcome 1 Vaginal delivery not achieved within 24 hours.
6.2. Analysis.
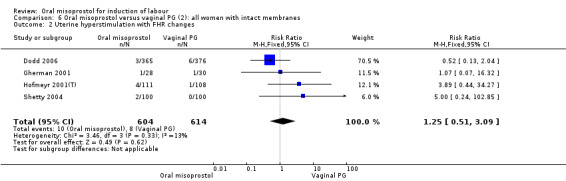
Comparison 6 Oral misoprostol versus vaginal PG (2): all women with intact membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
6.4. Analysis.
Comparison 6 Oral misoprostol versus vaginal PG (2): all women with intact membranes, Outcome 4 Serious neonatal morbidity or perinatal death.
6.5. Analysis.
Comparison 6 Oral misoprostol versus vaginal PG (2): all women with intact membranes, Outcome 5 Serious maternal morbidity or death.
Comparison 7. Oral misoprostol versus vaginal PG (2): all women with ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.37, 0.97] |
| 2 Caesarean section | 1 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.81, 5.20] |
7.2. Analysis.
Comparison 7 Oral misoprostol versus vaginal PG (2): all women with ruptured membranes, Outcome 2 Caesarean section.
Comparison 8. Oral misoprostol versus vaginal PG (2): all women with unfavourable cervices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.37] |
8.1. Analysis.
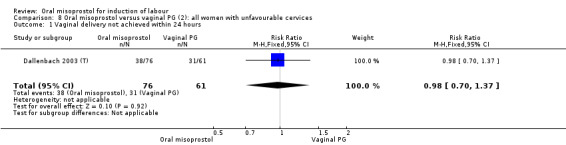
Comparison 8 Oral misoprostol versus vaginal PG (2): all women with unfavourable cervices, Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 9. Oral misoprostol versus vaginal PG (2): all women with favourable cervices.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.25] |
9.1. Analysis.
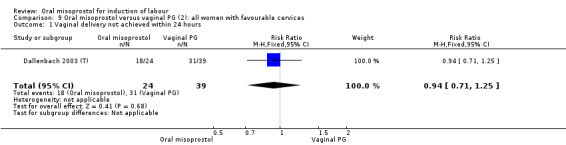
Comparison 9 Oral misoprostol versus vaginal PG (2): all women with favourable cervices, Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 10. Oral misoprostol versus vaginal PG (2): all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 437 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.86, 1.79] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.22, 12.46] |
| 3 Caesarean section | 2 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.56, 1.37] |
10.1. Analysis.
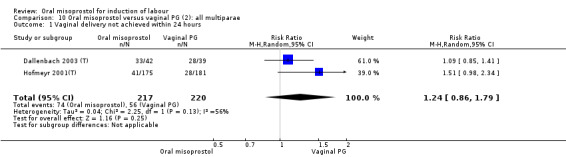
Comparison 10 Oral misoprostol versus vaginal PG (2): all multiparae, Outcome 1 Vaginal delivery not achieved within 24 hours.
10.2. Analysis.
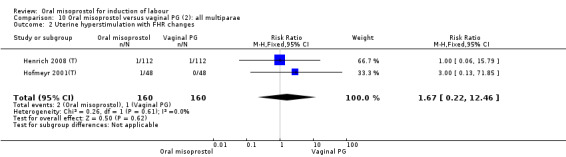
Comparison 10 Oral misoprostol versus vaginal PG (2): all multiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
10.3. Analysis.
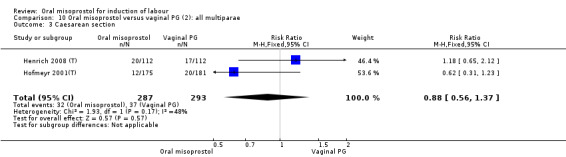
Comparison 10 Oral misoprostol versus vaginal PG (2): all multiparae, Outcome 3 Caesarean section.
Comparison 11. Oral misoprostol versus vaginal PG (2): all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 453 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.27] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.29, 25.44] |
| 3 Caesarean section | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.62, 1.24] |
11.1. Analysis.
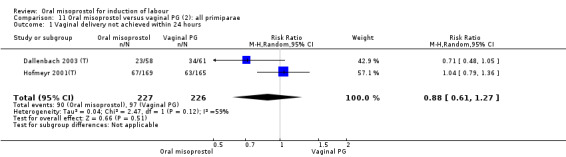
Comparison 11 Oral misoprostol versus vaginal PG (2): all primiparae, Outcome 1 Vaginal delivery not achieved within 24 hours.
11.2. Analysis.
Comparison 11 Oral misoprostol versus vaginal PG (2): all primiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
11.3. Analysis.
Comparison 11 Oral misoprostol versus vaginal PG (2): all primiparae, Outcome 3 Caesarean section.
Comparison 12. Oral misoprostol versus intracervical PG (3): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 3 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.63, 0.97] |
| 1.1 50 mcg | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.10] |
| 1.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.52, 1.00] |
| 2 Uterine hyperstimulation with FHR changes | 3 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.11, 11.54] |
| 2.1 50 mcg | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.55] |
| 2.2 200 mcg | 2 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.6 [1.19, 17.80] |
| 3 Caesarean section | 5 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.16] |
| 3.1 50 mcg | 3 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.59, 1.45] |
| 3.2 200 mcg | 2 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.22] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12‐24 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.03] |
| 6.1 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.43, 1.03] |
| 7 Oxytocin augmentation | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 50 mcg | 3 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.20, 1.88] |
| 7.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.61, 0.96] |
| 8 Uterine hyperstimulation without FHR changes | 2 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.25 [1.14, 34.31] |
| 8.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.06, 14.78] |
| 8.2 200 mcg | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.0 [1.00, 290.42] |
| 9 Uterine rupture | 4 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 50 mcg | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 200 mcg | 2 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Perinatal death | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.1 50 mcg | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 3 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.62, 1.44] |
| 11.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.06] |
| 11.2 200 mcg | 2 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.63, 1.49] |
| 12 Meconium‐stained liquor | 3 | 452 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.96, 2.36] |
| 12.1 50 mcg | 2 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.84, 3.03] |
| 12.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.77, 2.67] |
| 13 Apgar score < 7 at 5 minutes | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.07] |
| 13.1 50 mcg | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.07] |
| 13.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care unit admission | 4 | 552 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.48, 2.06] |
| 14.1 50 mcg | 3 | 352 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.06] |
| 14.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.51, 2.36] |
| 15 Neonatal encephalopathy | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 50 mcg | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal death | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 50 mcg | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal side effects (all) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.43] |
| 17.1 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.43] |
| 18 Postpartum haemorrhage | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.05, 5.06] |
| 19 Women not satisfied | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.27, 0.96] |
12.3. Analysis.
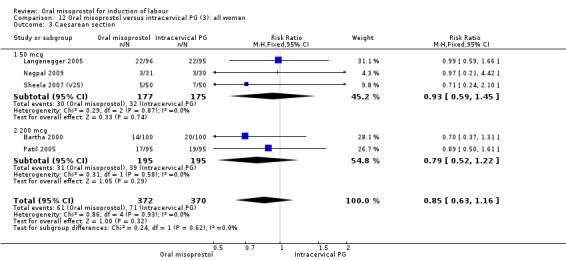
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 3 Caesarean section.
12.4. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 4 Serious neonatal morbidity or perinatal death.
12.5. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 5 Serious maternal morbidity or death.
12.6. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 6 Cervix unfavourable/unchanged after 12‐24 hours.
12.9. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 9 Uterine rupture.
12.10. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 10 Perinatal death.
12.11. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 11 Instrumental vaginal delivery.
12.12. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 12 Meconium‐stained liquor.
12.13. Analysis.
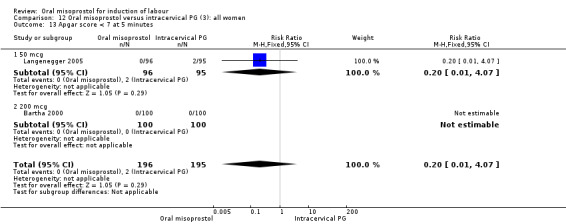
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 13 Apgar score < 7 at 5 minutes.
12.14. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 14 Neonatal intensive care unit admission.
12.15. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 15 Neonatal encephalopathy.
12.16. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 16 Maternal death.
12.17. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 17 Maternal side effects (all).
12.18. Analysis.
Comparison 12 Oral misoprostol versus intracervical PG (3): all women, Outcome 18 Postpartum haemorrhage.
Comparison 13. Oral misoprostol versus intracervical PG (3): all women with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.65, 1.00] |
| 1.1 50 mcg | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.67, 1.19] |
| 1.2 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.52, 1.00] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.6 [1.19, 17.80] |
| 2.1 200 mcg | 2 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.6 [1.19, 17.80] |
| 3 Caesarean section | 3 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.62, 1.20] |
| 3.1 50 mcg | 1 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.59, 1.66] |
| 3.2 200 mcg | 2 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.22] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.1 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 200 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.3. Analysis.
Comparison 13 Oral misoprostol versus intracervical PG (3): all women with intact membranes, Outcome 3 Caesarean section.
13.4. Analysis.
Comparison 13 Oral misoprostol versus intracervical PG (3): all women with intact membranes, Outcome 4 Serious neonatal morbidity or perinatal death.
13.5. Analysis.
Comparison 13 Oral misoprostol versus intracervical PG (3): all women with intact membranes, Outcome 5 Serious maternal morbidity or death.
Comparison 14. Oral misoprostol versus intracervical PG (3): all women with ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.16, 1.44] |
| 1.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.16, 1.44] |
| 2 Caesarean section | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.21, 4.42] |
| 2.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.21, 4.42] |
14.1. Analysis.
Comparison 14 Oral misoprostol versus intracervical PG (3): all women with ruptured membranes, Outcome 1 Vaginal delivery not achieved in 24 hours.
14.2. Analysis.
Comparison 14 Oral misoprostol versus intracervical PG (3): all women with ruptured membranes, Outcome 2 Caesarean section.
Comparison 15. Oral misoprostol versus oxytocin (4): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 6 | 789 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.59, 1.05] |
| 1.1 25 mcg | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.38, 0.80] |
| 1.2 50 mcg | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 100 mcg | 3 | 433 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.81, 2.14] |
| 2 Uterine hyperstimulation with FHR changes | 7 | 947 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.43, 3.91] |
| 2.1 25 mcg | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 50 mcg | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.25, 3.66] |
| 2.3 100 mcg | 5 | 809 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.35, 6.75] |
| 3 Caesarean section | 9 | 1282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.98] |
| 3.1 25 mcg | 2 | 286 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.37, 0.78] |
| 3.2 50 mcg | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.51, 2.68] |
| 3.3 100 mcg | 5 | 818 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.33] |
| 4 Serious neonatal morbidity or perinatal death | 2 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.69] |
| 4.1 25 mcg | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.02, 1.69] |
| 4.2 100 mcg | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 100 mcg | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Oxytocin augmentation | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.39, 0.69] |
| 6.1 100 mcg | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.39, 0.69] |
| 7 Uterine hyperstimulation without FHR changes | 4 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.42] |
| 7.1 100 mcg | 4 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.59, 1.42] |
| 8 Uterine rupture | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.10, 58.33] |
| 8.1 100 mcg | 2 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [0.10, 58.33] |
| 9 Epidural analgesia | 3 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.08] |
| 9.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 50 mcg | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.25] |
| 9.3 100 mcg | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.11] |
| 10 Instrumental vaginal delivery | 5 | 854 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.66, 1.35] |
| 10.1 25 mcg | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.2 50 mcg | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.48, 1.95] |
| 10.3 100 mcg | 3 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.62, 1.42] |
| 11 Meconium‐stained liquor | 7 | 1172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.04, 2.60] |
| 11.1 25 mcg | 1 | 256 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.11] |
| 11.2 50 mcg | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.60, 7.06] |
| 11.3 100 mcg | 4 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.01, 2.76] |
| 12 Apgar score < 7 at 5 minutes | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.42, 4.58] |
| 12.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 50 mcg | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.82 [0.24, 98.13] |
| 12.3 100 mcg | 3 | 608 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.24, 3.80] |
| 13 Neonatal intensive care unit admission | 6 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.85, 1.77] |
| 13.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 50 mcg | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.51, 2.82] |
| 13.3 100 mcg | 4 | 688 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.82, 1.85] |
| 14 Neonatal encephalopathy | 3 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 50 mcg | 2 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 100 mcg | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Perinatal death | 3 | 493 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.11, 67.13] |
| 15.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 50 mcg | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 100 mcg | 2 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.76 [0.11, 67.13] |
| 16 Nausea | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.37, 2.22] |
| 16.1 100 mcg | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.37, 2.22] |
| 17 Vomiting | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.43, 3.18] |
| 17.1 100 mcg | 2 | 235 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.43, 3.18] |
| 18 Diarrhoea | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 100 mcg | 1 | 105 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Postpartum haemorrhage | 3 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.25, 1.73] |
| 19.1 25 mcg | 1 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.53] |
| 19.2 100 mcg | 2 | 410 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.06, 13.31] |
15.1. Analysis.
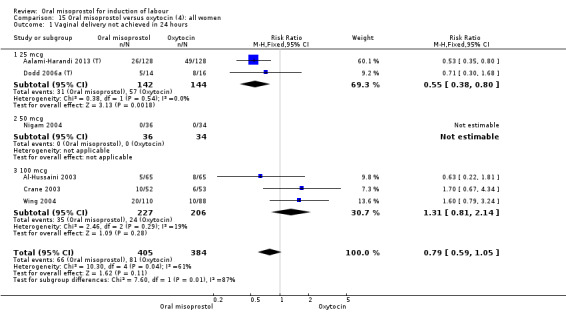
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
15.4. Analysis.
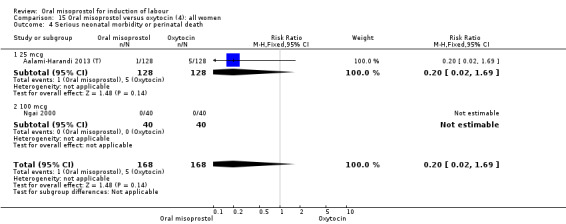
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 4 Serious neonatal morbidity or perinatal death.
15.5. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 5 Serious maternal morbidity or death.
15.6. Analysis.
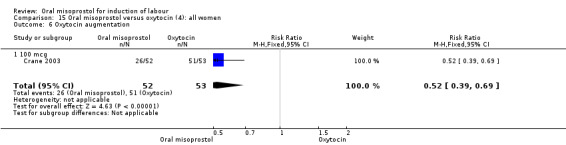
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 6 Oxytocin augmentation.
15.7. Analysis.
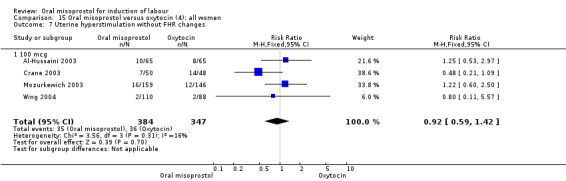
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 7 Uterine hyperstimulation without FHR changes.
15.8. Analysis.
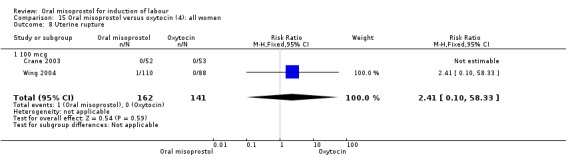
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 8 Uterine rupture.
15.9. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 9 Epidural analgesia.
15.10. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 10 Instrumental vaginal delivery.
15.12. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 12 Apgar score < 7 at 5 minutes.
15.13. Analysis.
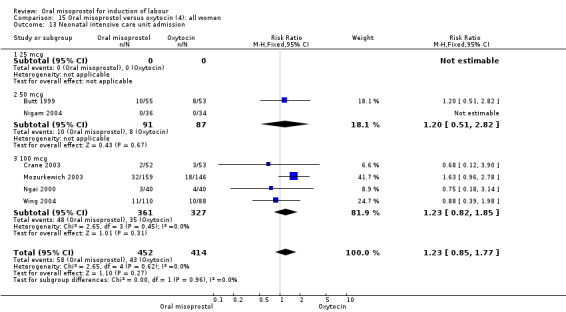
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 13 Neonatal intensive care unit admission.
15.14. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 14 Neonatal encephalopathy.
15.15. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 15 Perinatal death.
15.16. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 16 Nausea.
15.17. Analysis.
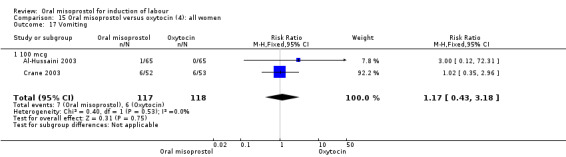
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 17 Vomiting.
15.18. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 18 Diarrhoea.
15.19. Analysis.
Comparison 15 Oral misoprostol versus oxytocin (4): all women, Outcome 19 Postpartum haemorrhage.
Comparison 16. Oral misoprostol versus oxytocin (4): all women with ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 3 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.56, 1.64] |
| 2 Uterine hyperstimulation with FHR changes | 6 | 749 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.33, 3.05] |
| 3 Caesarean section | 6 | 758 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.66, 1.28] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Meconium‐stained liquor | 4 | 648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.91, 3.23] |
16.1. Analysis.
Comparison 16 Oral misoprostol versus oxytocin (4): all women with ruptured membranes, Outcome 1 Vaginal delivery not achieved in 24 hours.
16.2. Analysis.
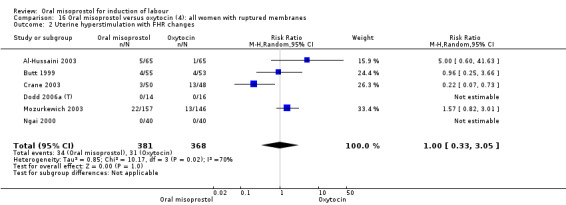
Comparison 16 Oral misoprostol versus oxytocin (4): all women with ruptured membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
16.3. Analysis.
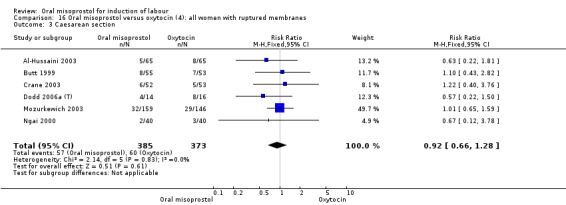
Comparison 16 Oral misoprostol versus oxytocin (4): all women with ruptured membranes, Outcome 3 Caesarean section.
16.4. Analysis.
Comparison 16 Oral misoprostol versus oxytocin (4): all women with ruptured membranes, Outcome 4 Serious neonatal morbidity or perinatal death.
16.5. Analysis.
Comparison 16 Oral misoprostol versus oxytocin (4): all women with ruptured membranes, Outcome 5 Serious maternal morbidity or death.
Comparison 17. Oral misoprostol versus oxytocin (4): all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.82, 3.01] |
| 2 Caesarean section | 2 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.67, 1.52] |
17.1. Analysis.
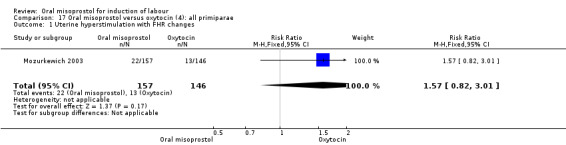
Comparison 17 Oral misoprostol versus oxytocin (4): all primiparae, Outcome 1 Uterine hyperstimulation with FHR changes.
17.2. Analysis.
Comparison 17 Oral misoprostol versus oxytocin (4): all primiparae, Outcome 2 Caesarean section.
Comparison 18. Oral misoprostol versus oxytocin (4): all primiparae with ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.82, 3.01] |
| 2 Caesarean section | 1 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.65, 1.59] |
18.1. Analysis.
Comparison 18 Oral misoprostol versus oxytocin (4): all primiparae with ruptured membranes, Outcome 1 Uterine hyperstimulation with FHR changes.
18.2. Analysis.
Comparison 18 Oral misoprostol versus oxytocin (4): all primiparae with ruptured membranes, Outcome 2 Caesarean section.
Comparison 19. Oral misoprostol versus oxytocin (4): all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 141 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.15, 3.54] |
19.1. Analysis.
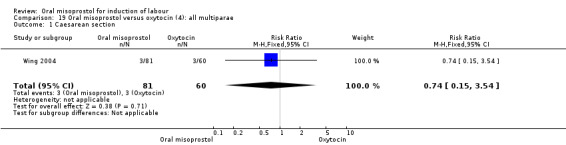
Comparison 19 Oral misoprostol versus oxytocin (4): all multiparae, Outcome 1 Caesarean section.
Comparison 20. Oral versus sublingual misoprostol (5): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.74, 3.26] |
| 1.1 50 mcg | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.56 [0.74, 3.26] |
| 2 Meconium‐stained liquor | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.5 [4.07, 27.09] |
| 2.1 50 mcg | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.5 [4.07, 27.09] |
| 3 Instrumental delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.22, 0.99] |
| 3.1 50mcg | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.22, 0.99] |
20.1. Analysis.
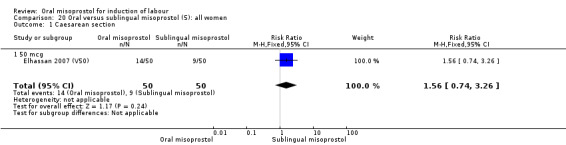
Comparison 20 Oral versus sublingual misoprostol (5): all women, Outcome 1 Caesarean section.
Comparison 21. Oral versus vaginal misoprostol (6): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 14 | 2448 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.86, 1.36] |
| 1.1 25 mcg | 3 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.27, 1.90] |
| 1.2 50 mcg | 7 | 1249 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.93, 1.61] |
| 1.3 100 mcg | 3 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.63, 1.55] |
| 1.4 200 mcg | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.64, 1.67] |
| 2 Uterine hyperstimulation with FHR changes | 29 | 5503 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.47, 1.08] |
| 2.1 25 mcg | 3 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.07, 1.19] |
| 2.2 50 mcg | 16 | 2507 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.23, 0.70] |
| 2.3 100 mcg | 8 | 1187 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.66, 1.87] |
| 2.4 200 mcg | 2 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.07, 2.43] |
| 3 Caesarean section | 35 | 6326 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 3.1 25 mcg | 2 | 407 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.11, 2.68] |
| 3.2 50 mcg | 20 | 3348 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.79, 1.14] |
| 3.3 100 mcg | 12 | 1389 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.66, 1.09] |
| 3.4 200 mcg | 2 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.00, 1.48] |
| 4 Serious neonatal morbidity or perinatal death | 10 | 1772 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.60, 1.33] |
| 4.1 25 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 50 mcg | 6 | 1203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.60, 1.33] |
| 4.3 100 mcg | 2 | 191 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 200 mcg | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 9 | 1389 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 5.1 25 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 50 mcg | 6 | 971 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 20.90] |
| 5.3 100 mcg | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.4 200 mcg | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Serious maternal complications | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 25mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Oxytocin augmentation | 31 | 5756 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [1.08, 1.34] |
| 7.1 25 mcg | 3 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.27, 2.23] |
| 7.2 50 mcg | 18 | 2930 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.15, 1.79] |
| 7.3 100 mcg | 8 | 1017 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.90, 1.22] |
| 7.4 200 mcg | 2 | 1182 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.93, 1.09] |
| 8 Uterine hyperstimulation without FHR changes | 18 | 2621 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.46, 1.03] |
| 8.1 25 mcg | 3 | 627 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.19, 1.16] |
| 8.2 50 mcg | 9 | 1318 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.44, 1.27] |
| 8.3 100 mcg | 6 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.33, 1.71] |
| 9 Uterine rupture | 8 | 2276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 25 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 50 mcg | 6 | 1072 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 200 mcg | 1 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 5 | 1859 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.96, 1.11] |
| 10.1 25 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.77] |
| 10.2 50 mcg | 3 | 655 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.19] |
| 10.3 200 mcg | 1 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.11] |
| 11 Instrumental vaginal delivery | 18 | 3485 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.86, 1.22] |
| 11.1 25 mcg | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.96] |
| 11.2 50 mcg | 11 | 1767 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.30] |
| 11.3 100 mcg | 5 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.92, 2.27] |
| 11.4 200 mcg | 1 | 1004 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 12 Meconium‐stained liquor | 24 | 3634 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.03, 1.44] |
| 12.1 25 mcg | 2 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.74, 1.88] |
| 12.2 50 mcg | 13 | 2194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.02, 1.59] |
| 12.3 100 mcg | 9 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.82, 1.54] |
| 13 Apgar score < 7 at 5 minutes | 19 | 4009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.44, 0.82] |
| 13.1 25 mcg | 3 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.15, 1.02] |
| 13.2 50 mcg | 10 | 1701 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.33, 0.87] |
| 13.3 100 mcg | 4 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.22] |
| 13.4 200 mcg | 2 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.51, 1.37] |
| 14 Neonatal intensive care unit admission | 22 | 4170 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.86, 1.20] |
| 14.1 25 mcg | 2 | 427 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.27, 1.64] |
| 14.2 50 mcg | 10 | 1648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.80, 1.32] |
| 14.3 100 mcg | 9 | 913 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.25] |
| 14.4 200 mcg | 2 | 1182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.85, 1.75] |
| 15 Neonatal encephalopathy | 5 | 1014 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 15.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 50 mcg | 4 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.10] |
| 15.3 100 mcg | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Perinatal death | 9 | 1434 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.2 50 mcg | 4 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 100 mcg | 5 | 459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 200 mcg | 1 | 178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal side effects (all) | 3 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.53, 1.50] |
| 17.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 50 mcg | 1 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.49, 1.55] |
| 17.3 100 mcg | 2 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.29, 3.21] |
| 18 Nausea | 9 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.94, 1.60] |
| 18.1 25 mcg | 3 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.03, 2.47] |
| 18.2 50 mcg | 4 | 777 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 18.3 100 mcg | 2 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.49, 18.78] |
| 19 Vomiting | 10 | 1635 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.90, 1.79] |
| 19.1 25 mcg | 3 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [0.90, 2.93] |
| 19.2 50 mcg | 5 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.62, 1.53] |
| 19.3 100 mcg | 2 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [0.63, 14.59] |
| 20 Diarrhoea | 14 | 2300 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.78, 1.95] |
| 20.1 25 mcg | 3 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.63, 3.63] |
| 20.2 50 mcg | 7 | 1226 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.58, 1.92] |
| 20.3 100 mcg | 4 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.42, 5.85] |
| 21 Postpartum haemorrhage | 10 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.34, 0.95] |
| 21.1 25 mcg | 2 | 420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.17, 2.40] |
| 21.2 50 mcg | 4 | 698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.35, 1.25] |
| 21.3 100 mcg | 4 | 360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.12, 1.13] |
| 22 Woman not satisfied | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.08, 18.82] |
| 22.1 25 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.2 50 mcg | 1 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.08, 18.82] |
| 23 Vaginal delivery not achieved within 24 hours (subgroup by quality) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 23.1 High quality | 13 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.81, 1.37] |
| 23.2 Unknown/poor quality | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.50, 1.58] |
| 24 Shivering | 3 | 539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.43, 1.80] |
| 24.1 25 mcg | 1 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.07, 16.56] |
| 24.2 50 mcg | 2 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.41, 1.82] |
| 25 Uterine hyperstimulation with FHR changes (subgroup by quality) | 28 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 25.1 High quality | 18 | 3911 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.13] |
| 25.2 Unknown/poor quality | 10 | 1392 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.28, 1.49] |
| 26 Caesarean section (subgroup by quality) | 34 | 5948 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.79, 1.06] |
| 26.1 High quality | 19 | 4010 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.71, 1.03] |
| 26.2 Unknown/poor quality | 15 | 1938 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.31] |
21.4. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 4 Serious neonatal morbidity or perinatal death.
21.5. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 5 Serious maternal morbidity or death.
21.6. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 6 Serious maternal complications.
21.9. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 9 Uterine rupture.
21.10. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 10 Epidural analgesia.
21.11. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 11 Instrumental vaginal delivery.
21.14. Analysis.
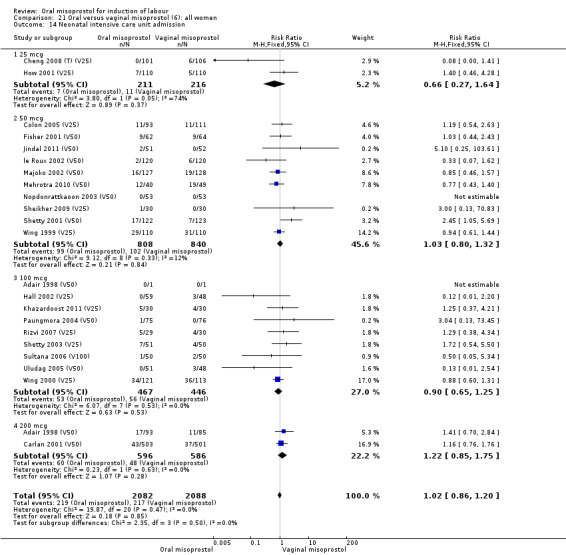
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 14 Neonatal intensive care unit admission.
21.15. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 15 Neonatal encephalopathy.
21.16. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 16 Perinatal death.
21.17. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 17 Maternal side effects (all).
21.18. Analysis.
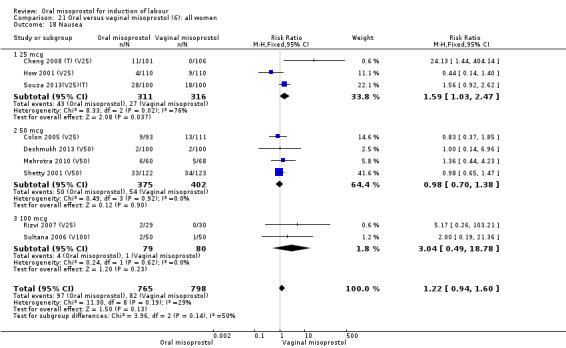
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 18 Nausea.
21.19. Analysis.
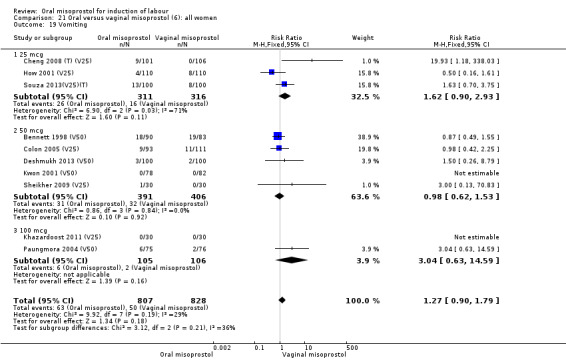
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 19 Vomiting.
21.20. Analysis.
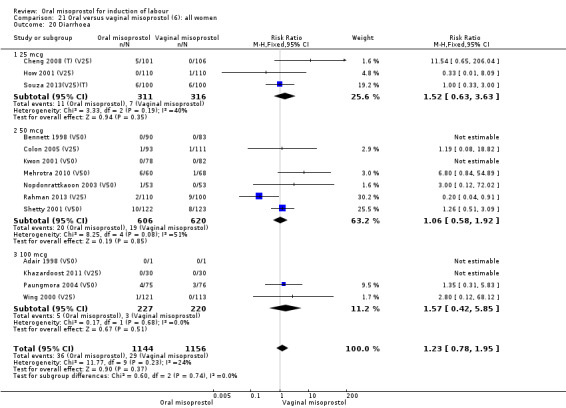
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 20 Diarrhoea.
21.22. Analysis.
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 22 Woman not satisfied.
21.23. Analysis.
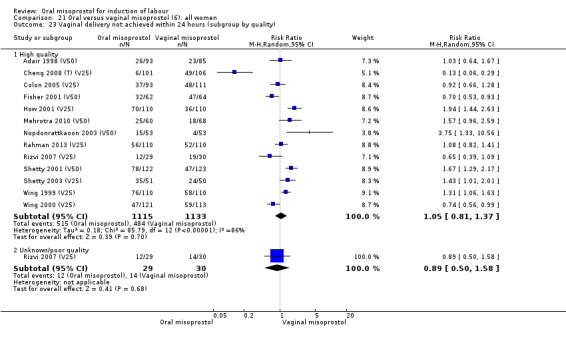
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 23 Vaginal delivery not achieved within 24 hours (subgroup by quality).
21.24. Analysis.
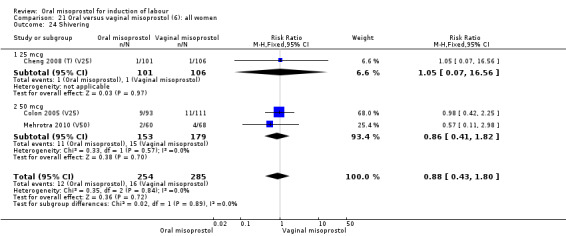
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 24 Shivering.
21.25. Analysis.
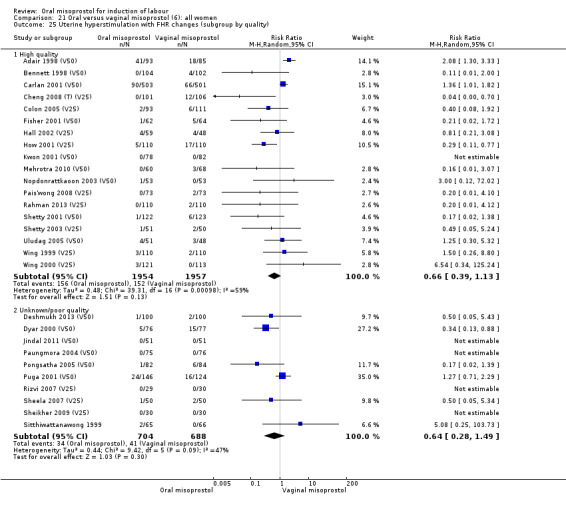
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 25 Uterine hyperstimulation with FHR changes (subgroup by quality).
21.26. Analysis.
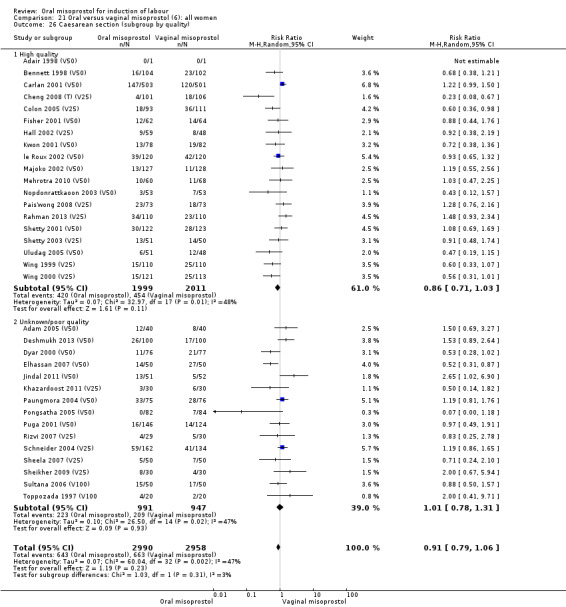
Comparison 21 Oral versus vaginal misoprostol (6): all women, Outcome 26 Caesarean section (subgroup by quality).
Comparison 22. Oral versus vaginal misoprostol (6): all women with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 7 | 1104 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.98, 1.76] |
| 2 Uterine hyperstimulation with FHR changes | 14 | 3068 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.52] |
| 3 Caesarean section | 17 | 3494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.89, 1.14] |
| 4 Serious neonatal morbidity or perinatal death | 4 | 755 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 4 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
22.1. Analysis.
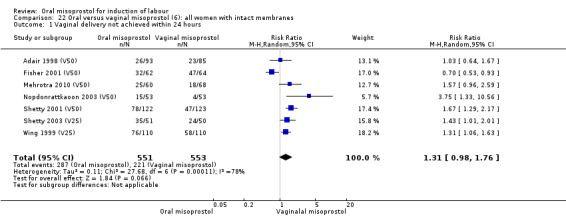
Comparison 22 Oral versus vaginal misoprostol (6): all women with intact membranes, Outcome 1 Vaginal delivery not achieved within 24 hours.
22.3. Analysis.
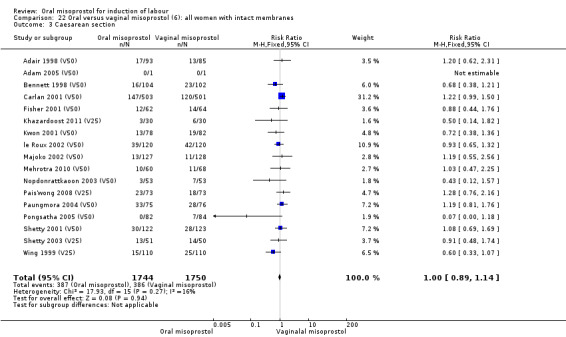
Comparison 22 Oral versus vaginal misoprostol (6): all women with intact membranes, Outcome 3 Caesarean section.
22.4. Analysis.
Comparison 22 Oral versus vaginal misoprostol (6): all women with intact membranes, Outcome 4 Serious neonatal morbidity or perinatal death.
22.5. Analysis.
Comparison 22 Oral versus vaginal misoprostol (6): all women with intact membranes, Outcome 5 Serious maternal morbidity or death.
Comparison 23. Oral versus vaginal misoprostol (6): all primiparae with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.01, 1.55] |
| 2 Serious neonatal morbidity or perinatal death | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Serious maternal morbidity or death | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
23.1. Analysis.
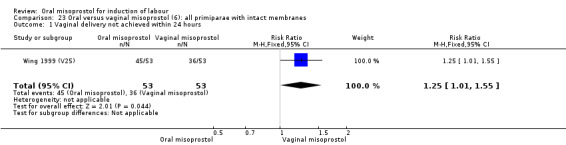
Comparison 23 Oral versus vaginal misoprostol (6): all primiparae with intact membranes, Outcome 1 Vaginal delivery not achieved within 24 hours.
23.2. Analysis.
Comparison 23 Oral versus vaginal misoprostol (6): all primiparae with intact membranes, Outcome 2 Serious neonatal morbidity or perinatal death.
23.3. Analysis.
Comparison 23 Oral versus vaginal misoprostol (6): all primiparae with intact membranes, Outcome 3 Serious maternal morbidity or death.
Comparison 24. Oral versus vaginal misoprostol (6): all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.01, 1.55] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.43] |
| 3 Caesarean section | 3 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.01, 2.58] |
| 4 Serious neonatal morbidity or perinatal death | 3 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.67, 1.59] |
| 5 Serious maternal morbidity or death | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Oxytocin augmentation | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.87, 3.44] |
| 7 Uterine hyperstimulation without FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [0.73, 9.76] |
| 8 Instrumental vaginal delivery | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.23, 1.59] |
| 9 Apgar score < 7 at 5 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.23, 1.00] |
| 10 Nausea | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.96] |
| 11 Vomiting | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.79] |
24.2. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 2 Uterine hyperstimulation with FHR changes.
24.3. Analysis.
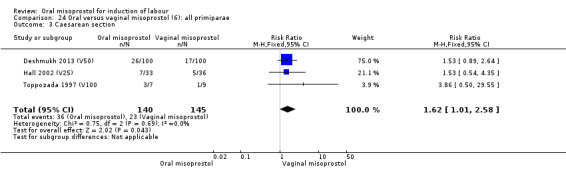
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 3 Caesarean section.
24.4. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 4 Serious neonatal morbidity or perinatal death.
24.5. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 5 Serious maternal morbidity or death.
24.6. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 6 Oxytocin augmentation.
24.7. Analysis.
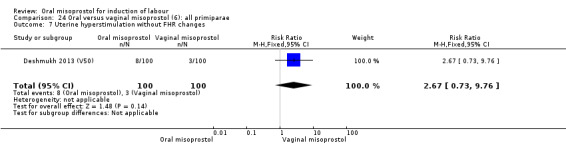
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 7 Uterine hyperstimulation without FHR changes.
24.8. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 8 Instrumental vaginal delivery.
24.9. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 9 Apgar score < 7 at 5 minutes.
24.10. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 10 Nausea.
24.11. Analysis.
Comparison 24 Oral versus vaginal misoprostol (6): all primiparae, Outcome 11 Vomiting.
Comparison 25. Oral versus vaginal misoprostol (6): all women with ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.71, 2.29] |
| 2 Caesarean section | 2 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.81, 2.37] |
Comparison 26. Oral versus vaginal misoprostol (6): all primiparae with unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 106 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.01, 1.55] |
| 2 Caesarean section | 2 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.76, 4.71] |
| 3 Serious neonatal morbidity or perinatal death | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Serious maternal morbidity or death | 2 | 122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
26.1. Analysis.
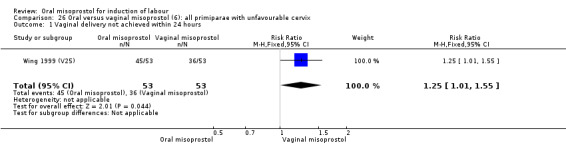
Comparison 26 Oral versus vaginal misoprostol (6): all primiparae with unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
26.2. Analysis.
Comparison 26 Oral versus vaginal misoprostol (6): all primiparae with unfavourable cervix, Outcome 2 Caesarean section.
26.3. Analysis.
Comparison 26 Oral versus vaginal misoprostol (6): all primiparae with unfavourable cervix, Outcome 3 Serious neonatal morbidity or perinatal death.
26.4. Analysis.
Comparison 26 Oral versus vaginal misoprostol (6): all primiparae with unfavourable cervix, Outcome 4 Serious maternal morbidity or death.
Comparison 27. Oral versus vaginal misoprostol (6): all multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.94, 2.11] |
| 2 Caesarean section | 2 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.65] |
| 3 Serious neonatal morbidity or perinatal death | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Serious maternal morbidity or death | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 28. Oral versus vaginal misoprostol (6): all multiparae with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.94, 2.11] |
28.1. Analysis.
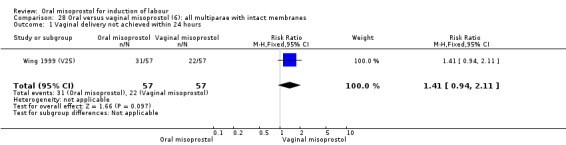
Comparison 28 Oral versus vaginal misoprostol (6): all multiparae with intact membranes, Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 29. Oral versus vaginal misoprostol (6): all multiparae with unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.94, 2.11] |
| 2 Caesarean section | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.06, 12.01] |
| 3 Serious neonatal morbidity or perinatal death | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Serious maternal morbidity or death | 2 | 138 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
29.1. Analysis.
Comparison 29 Oral versus vaginal misoprostol (6): all multiparae with unfavourable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
29.2. Analysis.
Comparison 29 Oral versus vaginal misoprostol (6): all multiparae with unfavourable cervix, Outcome 2 Caesarean section.
29.3. Analysis.
Comparison 29 Oral versus vaginal misoprostol (6): all multiparae with unfavourable cervix, Outcome 3 Serious neonatal morbidity or perinatal death.
29.4. Analysis.
Comparison 29 Oral versus vaginal misoprostol (6): all multiparae with unfavourable cervix, Outcome 4 Serious maternal morbidity or death.
Comparison 30. Hourly 20mcg titrated oral misoprostol versus 4‐hrly oral misoprostol 50mcg (7): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.92, 2.00] |
| 2 Caesarean section | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.77, 2.22] |
| 3 Serious neonatal morbidity or perinatal death | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Oxytocin augmentation | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.59, 1.68] |
| 5 Uterine hyperstimulation without FHR changes | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.92, 17.40] |
| 6 Uterine rupture | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Apgar score < 7 at 5 minutes | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Neonatal intensive care unit admission | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Nausea | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Vomiting | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Diarrhoea | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.00] |
| 12 Postpartum haemorrhage | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 31. Oral misoprostol 25 mcg 3‐daily versus 50 mcg 3‐daily (8): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesaraean section | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.33, 2.68] |
| 3 Serious maternal morbidity or death | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Oxytocin augmentation | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.22, 23.33] |
| 5 Uterine hyperstimulation without FHR changes | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Uterine rupture | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Meconium‐stained liquor | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.28, 2.97] |
| 8 Neonatal intensive care unit admission | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.05, 5.83] |
| 9 Perinatal death | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Nausea | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Maternal side effects(all) | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Nausea | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Vomiting | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Diarrhoea | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Shivering | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 32. Oral misoprostol 50 mcg versus 100 mcg (9): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.95, 1.40] |
| 2 Uterine hyperstimulation with FHR changes | 2 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.09] |
| 3 Caesarean section | 2 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.73, 1.70] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.01] |
| 5 Oxytocin augmentation | 2 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.46] |
| 6 Epidural analgesia | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.66, 1.30] |
| 7 Instrumental vaginal delivery | 2 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.59, 1.38] |
| 8 Meconium‐stained liquor | 2 | 317 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.71, 1.90] |
| 9 Apgar score < 7 at 5 minutes | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.69] |
| 10 Neonatal intensive care unit admission | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.39, 1.63] |
| 11 Nausea | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.71, 1.55] |
| 12 Diarrhoea | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
32.1. Analysis.
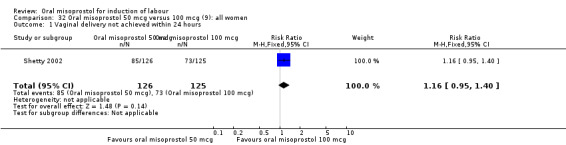
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
32.2. Analysis.
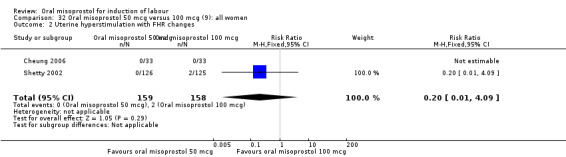
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
32.3. Analysis.
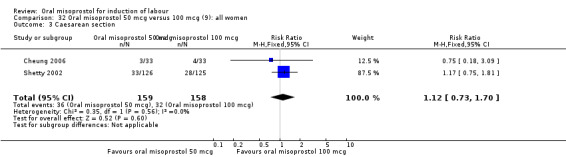
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 3 Caesarean section.
32.4. Analysis.
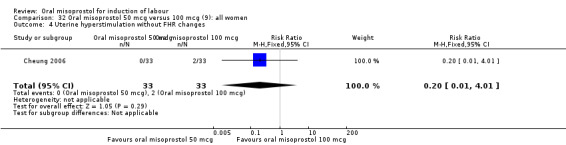
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 4 Uterine hyperstimulation without FHR changes.
32.5. Analysis.
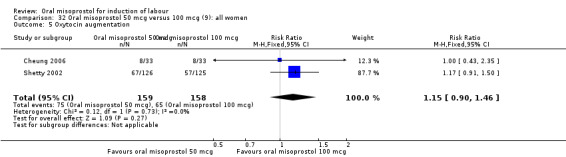
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 5 Oxytocin augmentation.
32.6. Analysis.
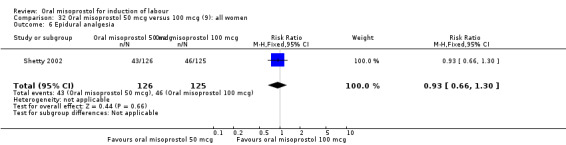
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 6 Epidural analgesia.
32.7. Analysis.
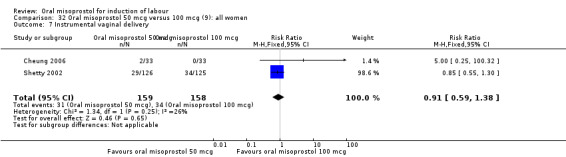
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 7 Instrumental vaginal delivery.
32.8. Analysis.
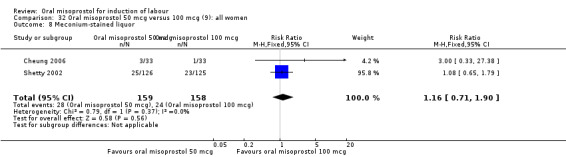
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 8 Meconium‐stained liquor.
32.9. Analysis.
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 9 Apgar score < 7 at 5 minutes.
32.10. Analysis.
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 10 Neonatal intensive care unit admission.
32.11. Analysis.
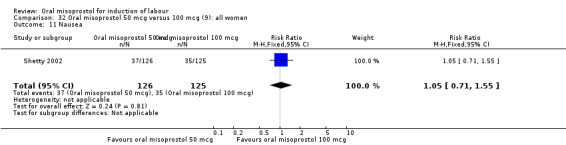
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 11 Nausea.
32.12. Analysis.
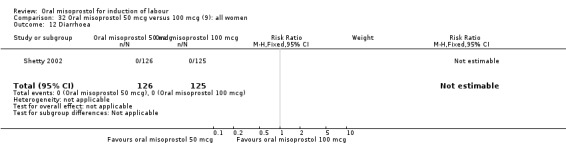
Comparison 32 Oral misoprostol 50 mcg versus 100 mcg (9): all women, Outcome 12 Diarrhoea.
Comparison 33. Oral misoprostol 50 mcg versus 100 mcg (9): all women with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.95, 1.40] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.09] |
| 3 Caesarean section | 1 | 251 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.75, 1.81] |
33.1. Analysis.
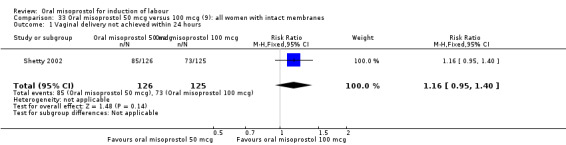
Comparison 33 Oral misoprostol 50 mcg versus 100 mcg (9): all women with intact membranes, Outcome 1 Vaginal delivery not achieved within 24 hours.
33.2. Analysis.
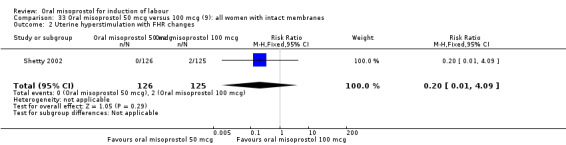
Comparison 33 Oral misoprostol 50 mcg versus 100 mcg (9): all women with intact membranes, Outcome 2 Uterine hyperstimulation with FHR changes.
33.3. Analysis.
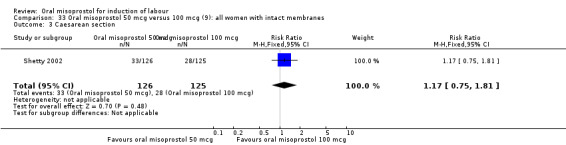
Comparison 33 Oral misoprostol 50 mcg versus 100 mcg (9): all women with intact membranes, Outcome 3 Caesarean section.
Comparison 34. Oral misoprostol 50 mcg versus 100 mcg (9): all women with ruptured membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Caesarean section | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.09] |
34.1. Analysis.
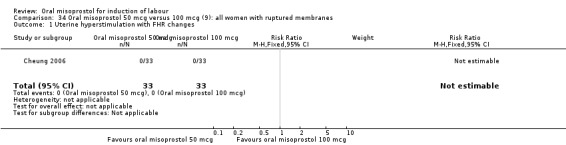
Comparison 34 Oral misoprostol 50 mcg versus 100 mcg (9): all women with ruptured membranes, Outcome 1 Uterine hyperstimulation with FHR changes.
34.2. Analysis.
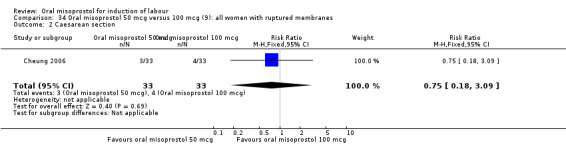
Comparison 34 Oral misoprostol 50 mcg versus 100 mcg (9): all women with ruptured membranes, Outcome 2 Caesarean section.
Comparison 35. Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 2 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.07, 4.30] |
| 1.1 50mcg | 1 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.42] |
| 1.2 100mcg | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.25, 8.31] |
| 2 Caesarean section | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.71, 2.80] |
| 2.1 50 mcg | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.49, 5.30] |
| 2.2 100mcg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.56, 3.06] |
| 3 Oxytocin augmentation | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.63, 1.07] |
| 3.1 50mcg | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.63, 2.08] |
| 3.2 100mcg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.54, 0.96] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.25, 8.31] |
| 4.1 100mcg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.25, 8.31] |
| 5 Instrumental vaginal delivery | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.61] |
| 5.1 50mcg | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.16, 2.53] |
| 5.2 100mcg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.14, 2.30] |
| 6 Meconium‐stained liquor | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [0.13, 76.60] |
| 6.1 50mcg | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [0.13, 76.60] |
| 7 Nausea | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.04, 1.31] |
| 7.1 50mcg | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.51] |
| 7.2 100mcg | 1 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.59] |
| 8 Diarrhoea | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.51] |
| 8.1 50mcg | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.01, 8.51] |
35.1. Analysis.
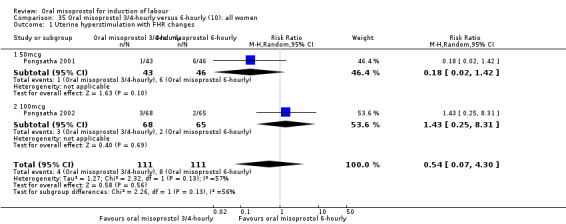
Comparison 35 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women, Outcome 1 Uterine hyperstimulation with FHR changes.
35.2. Analysis.
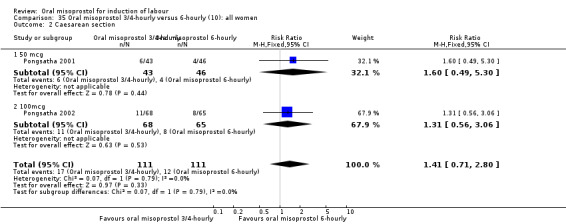
Comparison 35 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women, Outcome 2 Caesarean section.
35.4. Analysis.
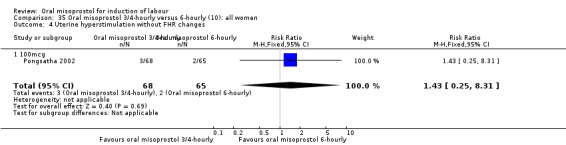
Comparison 35 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women, Outcome 4 Uterine hyperstimulation without FHR changes.
35.5. Analysis.
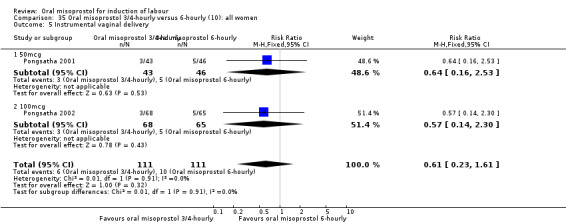
Comparison 35 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women, Outcome 5 Instrumental vaginal delivery.
35.6. Analysis.
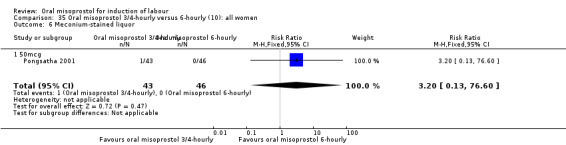
Comparison 35 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women, Outcome 6 Meconium‐stained liquor.
35.7. Analysis.
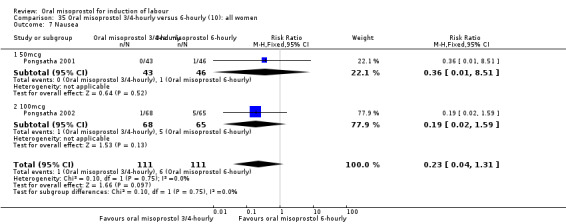
Comparison 35 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women, Outcome 7 Nausea.
35.8. Analysis.
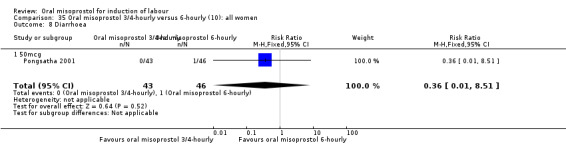
Comparison 35 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women, Outcome 8 Diarrhoea.
Comparison 36. Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women with intact membranes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 2 | 222 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.07, 4.30] |
| 2 Caesarean section | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.71, 2.80] |
36.1. Analysis.
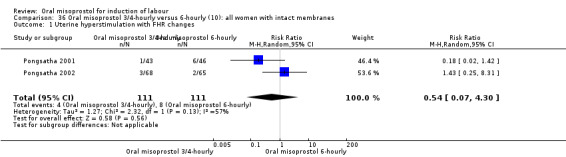
Comparison 36 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women with intact membranes, Outcome 1 Uterine hyperstimulation with FHR changes.
36.2. Analysis.
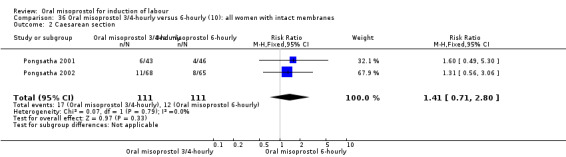
Comparison 36 Oral misoprostol 3/4‐hourly versus 6‐hourly (10): all women with intact membranes, Outcome 2 Caesarean section.
Comparison 37. Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.56, 1.42] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.44, 1.73] |
| 4 Uterine hyperstimulation without FHR changes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.77] |
| 5 Instrumental vaginal delivery | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.81] |
| 6 Apgar score < 7 at 5 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Neonatal intensive care unit admission | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.77] |
| 8 Postpartum haemorrhage | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Diarrhoea | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Vomiting | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.81] |
37.1. Analysis.
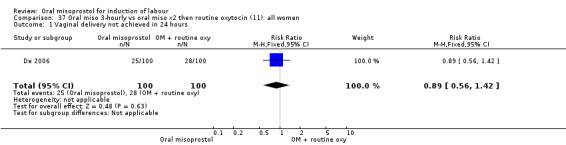
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 1 Vaginal delivery not achieved in 24 hours.
37.2. Analysis.
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
37.3. Analysis.
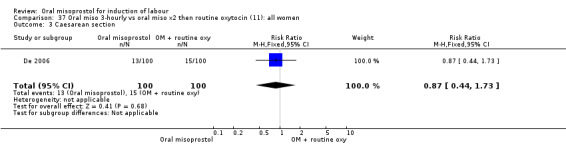
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 3 Caesarean section.
37.4. Analysis.
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 4 Uterine hyperstimulation without FHR changes.
37.5. Analysis.
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 5 Instrumental vaginal delivery.
37.6. Analysis.
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 6 Apgar score < 7 at 5 minutes.
37.7. Analysis.
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 7 Neonatal intensive care unit admission.
37.8. Analysis.
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 8 Postpartum haemorrhage.
37.9. Analysis.
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 9 Diarrhoea.
37.10. Analysis.
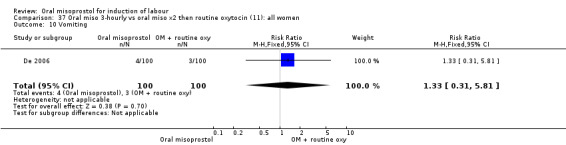
Comparison 37 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all women, Outcome 10 Vomiting.
Comparison 38. Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.59, 1.47] |
| 2 Caesarean section | 1 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.89] |
38.1. Analysis.
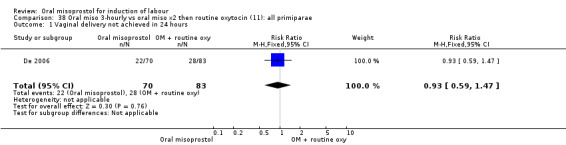
Comparison 38 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all primiparae, Outcome 1 Vaginal delivery not achieved in 24 hours.
38.2. Analysis.
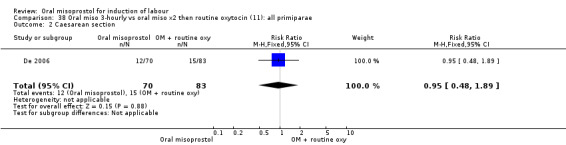
Comparison 38 Oral miso 3‐hourly vs oral miso x2 then routine oxytocin (11): all primiparae, Outcome 2 Caesarean section.
Comparison 39. Oral misoprostol versus delayed vaginal postaglandins (12): all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved in 24 hours | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.32, 0.83] |
| 1.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.32, 0.83] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.33, 3.21] |
| 3.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.33, 3.21] |
| 4 Oxytocin augmentation | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.44, 1.49] |
| 4.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.44, 1.49] |
| 5 Uterine hyperstimulation without FHR changes | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.16, 6.87] |
| 5.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.16, 6.87] |
| 6 Instrumental vaginal delivery | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.61, 2.60] |
| 6.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.61, 2.60] |
| 7 Apgar score < 7 at 5 minutes | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Neonatal intensive care unit admission | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.13, 73.16] |
| 8.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.10 [0.13, 73.16] |
| 9 Nausea | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.20] |
| 9.1 50 mcg | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.22, 2.20] |
| 10 Oxytocin augmentation | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
39.2. Analysis.
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 2 Uterine hyperstimulation with FHR changes.
39.3. Analysis.
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 3 Caesarean section.
39.4. Analysis.
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 4 Oxytocin augmentation.
39.5. Analysis.
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 5 Uterine hyperstimulation without FHR changes.
39.6. Analysis.
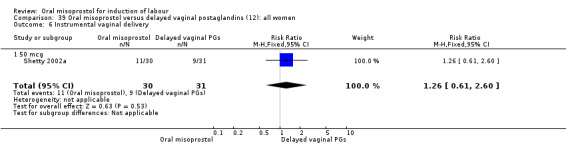
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 6 Instrumental vaginal delivery.
39.7. Analysis.
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 7 Apgar score < 7 at 5 minutes.
39.8. Analysis.
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 8 Neonatal intensive care unit admission.
39.9. Analysis.
Comparison 39 Oral misoprostol versus delayed vaginal postaglandins (12): all women, Outcome 9 Nausea.
Comparison 40. Oral misoprostol versus dinoprostone vaginal insert.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine hyperstimulation with FHR changes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.46, 4.23] |
| 2 Caesarean section | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.24, 1.05] |
| 3 Serious neonatal morbidity/perinatal death | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.88, 55.60] |
| 4 Oxytocin augmentation | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.58, 1.06] |
| 5 Uterine hyperstimulation without FHR changes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.50, 3.28] |
| 6 Meconium‐stained liquor | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.53] |
| 7 Apgar score < 7 at 5 minutes | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Perinatal death | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.71] |
| 9 Nausea | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 28.23] |
| 10 Vomiting | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.62] |
40.1. Analysis.
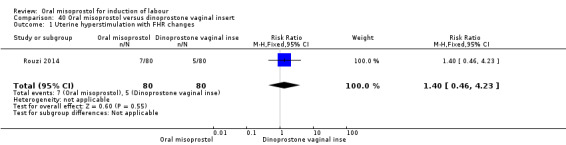
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 1 Uterine hyperstimulation with FHR changes.
40.2. Analysis.
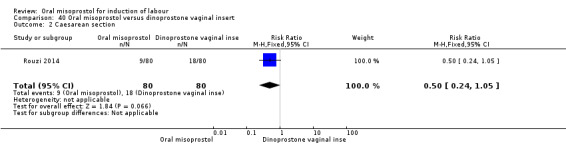
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 2 Caesarean section.
40.3. Analysis.
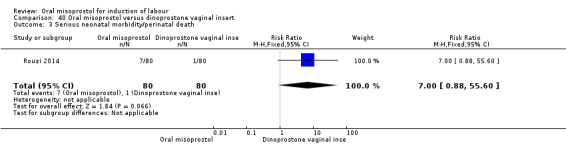
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 3 Serious neonatal morbidity/perinatal death.
40.4. Analysis.
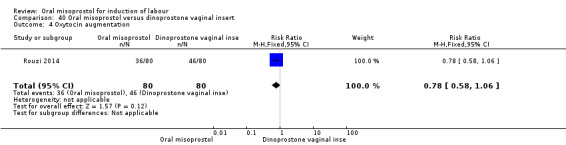
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 4 Oxytocin augmentation.
40.5. Analysis.
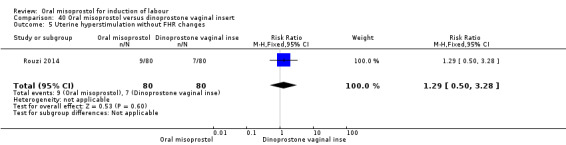
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 5 Uterine hyperstimulation without FHR changes.
40.6. Analysis.
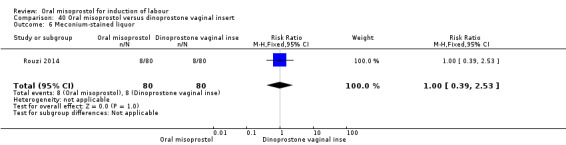
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 6 Meconium‐stained liquor.
40.7. Analysis.
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 7 Apgar score < 7 at 5 minutes.
40.8. Analysis.
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 8 Perinatal death.
40.9. Analysis.
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 9 Nausea.
40.10. Analysis.
Comparison 40 Oral misoprostol versus dinoprostone vaginal insert, Outcome 10 Vomiting.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aalami‐Harandi 2013 (T).
| Methods | Computer‐generated randomisation. | |
| Participants | 260 with singleton cephalic pregnancy and a live fetus at term with BS < 6. | |
| Interventions | Group 1: oral misoprostol 200 mcg dissolved in 200 mL of water. 25 mL was administered every 2 hrs until contractions started for a maximum 12 doses. Group 2: oxytocin at infusion rate of 2 mU/min increased by 2 mU/min every 15 minutes to a maximum dose 38 mU/min. |
|
| Outcomes | Labour and delivery outcomes.
Limited neonatal outcomes. Limited side effect outcomes. |
|
| Notes | 4 postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded trial. |
Adair 1998 (V50).
| Methods | Sequentially numbered opaque envelopes. | |
| Participants | 178 women with intact membranes and unfavourable cervix (BS < 7). Each woman had either medical or obstetric complication requiring delivery. | |
| Interventions | Oral misoprostol 200 mcg and 1/2 tablet placebo vaginally or oral placebo tablet and a 1/2 tablet of 100 mcg misoprostol (50 mcg) vaginally. Doses were repeated every 6 hours (maximum 3) or until labour was established. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisation‐no more details. |
| Allocation concealment (selection bias) | Low risk | Placebo controlled trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised double blinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Randomised double blinded study. |
Adam 2005 (V50).
| Methods | "Randomized", but method not specified. | |
| Participants | 80 women with singleton pregnancies and BS < 5. | |
| Interventions | Oral or vaginal misoprostol 50 mcg 6‐hourly (max 4 doses). | |
| Outcomes | Brief maternal and fetal outcomes. | |
| Notes | Short report only ‐ few details. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized", but method not specified. |
| Allocation concealment (selection bias) | Unclear risk | Method was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Al‐Hussaini 2003.
| Methods | Methods state that "women were randomised" ‐ no further details. | |
| Participants | 130 women with ruptured membranes for over 24 hours at greater than 37 weeks. | |
| Interventions | Oral misoprostol 100 mcg 6‐hourly x 2 or iv oxytocin. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Bartha 2000.
| Methods | Sequentially‐numbered, opaque, sealed envelopes. | |
| Participants | 200 women with intact membranes and unfavourable cervix (BS < 6). | |
| Interventions | 200 mcg of oral misoprostol as a single dose or 0.5 mg dinoprostone intracervically every 6 hours (maximum 4 doses). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. Uterine activity. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque, sealed envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Beigi 2003.
| Methods | "Pharmacy prepared and distributed the medication according to the schedule"; study described as double‐blind. | |
| Participants | 160 women requiring induction at term; 50 had ruptured membranes and 79 were nulliparous. | |
| Interventions | Oral misoprostol 200 mcg or placebo as a single dose followed 12 hours later by iv oxytocin if not in labour. | |
| Outcomes | Labour and birth outcomes. Neonatal outcomes. | |
| Notes | 4 women were excluded after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was performed using a computer‐generated random number table. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study described as double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study described as double‐blinded. |
Bennett 1998 (V50).
| Methods | Sequentially‐numbered, sealed envelopes stratified according to BS to the low or high group. | |
| Participants | 206 women with intact membranes. | |
| Interventions | Either 50 mcg oral tablet with vaginal placebo or oral placebo with 50 mcg vaginal tablet. Medication was given every 4 hours. | |
| Outcomes | Labour and delivery outcomes. Uterine activity. Neonatal outcomes. Maternal side effects. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables with randomization in blocks of four. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed envelopes stratified according to BS to the low or high group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women presented. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The induction method was concealed from all caregivers, participants, and investigators until data analysis was completed. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The induction method was concealed from all caregivers, participants, and investigators until data analysis was completed. |
Butt 1999.
| Methods | Sequentially‐numbered, opaque, sealed envelopes. Stratification by parity (nulliparous, multiparous). | |
| Participants | 108 women with PROM at term. 72 women were nulliparous and 57 had BS < 7. | |
| Interventions | Oral misoprostol 50 mcg every 4 hours or iv oxytocin. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. Uterine activity. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers with randomisation in blocks of four and six. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque, sealed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blind. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blind. |
Carlan 2001 (V50).
| Methods | Sequentially‐numbered, opaque envelopes in blocks of 50. | |
| Participants | 1004 women with indications for induction and intact membranes and BS < 7 at over 24 weeks' gestation. 598 women were nulliparous. 131 had previous CS. | |
| Interventions | Oral misoprostol 200 mcg every 6 hours for 2 doses, then 300 mcg every 6 hours for 4 doses or vaginal misoprostol 50 mcg every 6 hours for 2 doses then 100 mcg every 6 hours for 4 doses. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation was performed. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes in blocks of 50 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded |
Cheng 2008 (T) (V25).
| Methods | Sequentially‐numbered, opaque envelopes containing allocation. Unblinded. | |
| Participants | 220 women of 34‐42 weeks with BS < 7. Indication was post‐term pregnancy in 54%, 10% had ruptured membranes. | |
| Interventions | Vaginal misoprostol 25 mcg 4‐hourly versus titrated oral misoprostol solution (200 mcg in 200 mL). The solution was given hourly with 20 mcg for 4 doses, then 40 mcg and 60 mcg until adequate contractions occurred. If the contractions became inadequate, the misoprostol solution could be restarted at 10 mcg increasing to 20 and 40 mcg. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | 13 women who underwent caesarean section without medical indication or who had epidural analgesia were excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The treatment arm allocation was determined using a computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes containing allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 women who underwent caesarean section without medical indication or who had epidural analgesia were excluded. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was unblinded. |
Cheung 2006.
| Methods | Sequentially‐numbered, opaque envelopes containing powder of misoprostol or placebo. | |
| Participants | 98 women with ruptured membrane for under 6 hours at term with no labour pain. | |
| Interventions | 3 groups ‐ oral placebo vs oral misoprostol 50 mcg vs oral misoprostol 100 mcg, all given 4‐hourly for up to 6 doses. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | Double‐blind study. 2 case report forms lost so excluded from the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computerized random number generator was used. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes containing powder of misoprostol or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Everyone was blinded to which treatment each subject received, until the end of the study, when the envelope number code was deciphered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Everyone was blinded to which treatment each subject received, until the end of the study, when the envelope number code was deciphered. |
Colon 2005 (V25).
| Methods | Sequentially numbered, opaque envelopes. | |
| Participants | 204 women at 32‐42 weeks with BS < 6 and ruptured or intact membranes. | |
| Interventions | Oral misoprostol 50 mcg (x 1) then 100 mcg (x 3) all given 4‐hourly or vaginal misoprostol 25 mcg 4‐hourly x 4 max. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | 8 postrandomisation exclusions who "did not meet entry criteria". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment arm allocation was determined by the use of a computer‐generated table of random numbers |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded |
Crane 2003.
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 105 women at term with ruptured membranes and uncomplicated pregnancies. | |
| Interventions | Oral misoprostol 75 mcg 4‐hourly or iv oxytocin infusion. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation in blocks of 4 and 6. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Physicians managing labor were not blinded to study group allocation. |
Dallenbach 2003 (T).
| Methods | Sequentially‐numbered, opaque envelopes in randomly‐sized blocks. | |
| Participants | 202 women with healthy fetuses at term and with unfavourable cervices (BS </= 6). | |
| Interventions | Dinoprostone gel 2 mg 6 hours apart or titrated oral misoprostol (20 mcg every 2 hrs x 2 then 40 mcg every 2 hrs x 10 until 3 contractions every 10 minutes, max dose 475 mcg). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. Maternal side effects. | |
| Notes | 2 exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random allocation scheme was derived from a computer‐generated list of numbers, with randomly permuted blocks between 2 and 6. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes in randomly‐sized blocks. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all included women reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and clinicians were not blinded to the allocated treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Women and clinicians were not blinded to the allocated treatment |
De 2006.
| Methods | Unclear. | |
| Participants | 200 women over 34 weeks with a BS > 5. Previous CS or parity > 2 excluded. | |
| Interventions | Oral misoprostol 25 mcg 3‐hourly until contracting 3 in 10 (median 3 doses). Oxytocin after only if contractions settled or oral misoprostol 25 mcg 3‐hourly x 2 followed by routine oxytocin. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. Maternal side effects. | |
| Notes | No exclusions. No CTGs used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Women were randomised by a computer‐generated table. |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Obstetric registrars and residents monitored uterine contractions and fetal heart rate by intermittent auscultation every 30 min. These staff were not blinded to treatment allocation |
Deshmukh 2013 (V50).
| Methods | Participants were "randomly assigned". No other details available. | |
| Participants | 200 primigravida with singleton pregnancies between 34‐42 weeks and with BS of < 5 or less. | |
| Interventions | Oral or vaginal misoprostol, 50 mcg every 6 hours for a maximum of 4 doses. | |
| Outcomes | Labour and delivery outcomes.
Limited neonatal outcomes. Limited side effect outcomes. |
|
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were "randomly assigned". No other details available. |
| Allocation concealment (selection bias) | Unclear risk | B‐unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Dodd 2006.
| Methods | Double blind with identical treatment packs sequentially numbered in variable blocks, stratified for parity and centre. | |
| Participants | 741 women at term with intact membranes and BS < 7. | |
| Interventions | Oral misoprostol 20 mcg solution 2‐hourly (max x 6) or vaginal dinoprostone 1 mg (primips) or 2 mg (parous) 6‐hourly (max x 2), each with placebo. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. Maternal side effects. | |
| Notes | Analysed by intention‐to‐treat. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial ‐ no further details. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Dodd 2006a (T).
| Methods | Double‐blind placebo‐controlled ‐ but no further details. | |
| Participants | 30 women at term having had artificial or spontaneous rupture of membranes. | |
| Interventions | Oral misoprostol solution given hourly (initially 5 mcg then 10 mcg and 20 mcg ‐ maximum not specified), or iv oxytocin infusion. Both groups also got placebo. | |
| Outcomes | Only primary outcome data given. | |
| Notes | Abstract. The numbers given only as percentages. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised trial ‐ no further details |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Dyar 2000 (V50).
| Methods | Unclear. | |
| Participants | 153 women with unfavourable cervix (BS < 7). | |
| Interventions | Vaginal or oral misoprostol, 50 mcg every 4 hours for a maximum of 6 doses. Oral dose was increased to 100 mcg after 2 doses if there was no significant response. | |
| Outcomes | Time‐to‐delivery interval. Tachysystole, hyperstimulation, CS. | |
| Notes | Abstract. The numbers given only as percentages. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Elhassan 2007 (V50).
| Methods | Open randomisation. No other details available. | |
| Participants | 150 women with singleton live pregnancies and BS < 5. | |
| Interventions | 3 groups, open to operator and patient. 50 mcg of misoprostol either oral (group 1), vaginal (group 2) or sublingual (group 3). | |
| Outcomes | Limited labour and delivery outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. . |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. "An open, randomised, controlled clinical trial was conducted." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
Fisher 2001 (V50).
| Methods | Sealed‐opaque envelopes. | |
| Participants | 124 women (76 primips) with intact membranes at any gestation (mean 41 weeks, range 33‐36, IUGR and vaginal bleeding excluded), all with BS < 9. | |
| Interventions | Oral misoprostol 50 mcg every 3 hours for 48 hours or vaginal misoprostol 50 mcg every 6 hours for 48 hours. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | 2 exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study pharmacist prepared the envelopes by using random number tables with randomisation in blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelope. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all enrolled women reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | None noted. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study participants, nurses, residents and attending staff were unaware of the block randomisation and group assignment until completion of the trial. |
Gherman 2001.
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 60 women over 24 weeks' gestation (mean 39 weeks) with BS </= 6 and intact membranes. 82% and 73% were nulliparous in the 2 groups. | |
| Interventions | Oral misoprostol 50 mcg 4‐hourly x 6 or PGE2 tablets (melted and mixed in surgical lubricant) 2 mg 4‐hourly x 6. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | 2 exclusions. Abstract states PGE2 dose of 4 mg, method section has 2 mg. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number table, in permuted block design. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Sequentially‐numbered, opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patient and providers were blinded to group allocation sequence and group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Primary care givers were blinded to the group allocation until the time of induction. |
Hall 2002 (V25).
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 107 women at term with BS < 5. 28 had ruptured membranes and 69 were nulliparous. | |
| Interventions | Oral misoprostol 100 mcg followed after 3‐4 hours by 200 mcg repeated every 3‐4 hours until in labour; or vaginal misoprostol 25 mcg followed after 3‐4 hours by 50 mcg repeated every 3‐4 hours until in labour. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation was performed. A series of consecutively numbered opaque envelopes with each envelope containing an even or odd number was generated |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Sequentially‐numbered, opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
Henrich 2008 (T).
| Methods | Participants were "randomised" and a "closed envelope extracted from a box after gaining consent". No other details available. | |
| Participants | 224 women with singleton pregnancies at over 37 weeks. Women with BS of 7 or more were excluded. | |
| Interventions | Misoprostol‐group took at first 25μg orally, then 50μg, and on a third administration, 100 μg, each at a time, in a time span of four hours, up to a maximal dose of 175 μg the first day, and one of 300 μg in the second and third days. Minprostin‐group took each 6th hour 3 mg of Dinoprostone as vaginal tablets | |
| Outcomes | Labour and delivery outcomes.
Neonatal outcomes. Maternal side‐effect outcomes. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Low risk | A ‐ closed envelope. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Hoffman 2001.
| Methods | Sequentially‐numbered, opaque envelopes containing drug or placebo. | |
| Participants | 103 women without complications at term with ruptured membranes. 43 were nulliparous. | |
| Interventions | Oral misoprostol 100 mcg or placebo 6‐hourly for 2 doses followed by dinoprostone gel 1 mg 6‐hourly until in labour. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | 7 postrandomisation exclusions. Placebo looked and tasted different to misoprostol. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred by using computer generated numbers. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate: Sequentially‐numbered, opaque envelopes containing drug or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study was double blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study was double blinded. |
Hofmeyr 2001(T).
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 695 women in whom the decision has been made to induce labour with dinoprostone regardless of membrane and cervical status. Women with previous CS, twins and breech presentation were excluded. | |
| Interventions | Titrated oral misoprostol versus vaginal dinoprostone 2 mg. Oral misoprostol was administered as solution (200 mcg tablet dissolved in 200 mL of water). Initial 2‐3 doses were 20 mcg increased to 40 mcg every 2 hours. Further doses were not given if contractions were judged to be clinically adequate. Vaginal dinoprostone was given as a 2 mg gel followed by another dose 6 hours later. In both groups oxytocin was started if there was no response after 24 hours. | |
| Outcomes | Labour and birth outcomes. Neonatal outcomes. Maternal side effects. | |
| Notes | 5 women lost to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes were prepared using a computer‐ generated list of allocation. Allocations were balanced between groups using random block size (Betwwen two and six). |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded. |
How 2001 (V25).
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 330 women with singleton pregnancies at over 32 weeks and with BS of < 6 (intact and ruptured membranes included). Women with more than 1 previous CS excluded ‐ 27 women had 1 previous CS. | |
| Interventions | 3 groups, blinded to operator and patient. Misoprostol 25 mcg pv and 25 mcg po OR misoprostol 25 mcg pv and placebo po or misoprostol 25 mcg po with placebo pv. All doses given 4‐hourly up to 12 doses. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. Maternal side effects. | |
| Notes | The combined oral and vaginal group was not considered in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group allocation was predetermined in the pharmacy by means of a computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women reported. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blinded study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded study. |
Javaid 2008.
| Methods | Participants were randomised " open randomisation". No other details available. | |
| Participants | 100 women at term with singleton pregnancies and ruptured membranes. | |
| Interventions | Oral misoprostol versus expectant management for 24 hours. | |
| Outcomes | Limited labour and delivery outcomes. | |
| Notes | Dose of oral misoprostol not specified. The numbers given only as percentages. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. "It was an open randomized comparative study." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
Jindal 2011 (V50).
| Methods | "Identical placebos". Randomised and double‐blind; a coded list indicted which allocation the woman was to receive ‐ the tablets (active and placebo) we then taken from the appropriate 'vial'. | |
| Participants | 103 women at term with singleton pregnancies and BS equal or less than 4. All women with ruptured membranes < 4 hours duration. | |
| Interventions | Oral or vaginal misoprostol, both 50 mcg 4‐hourly, max 6 doses | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. |
|
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear whether the allocation was concealed or whether could be seen from the list. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. "At the end of the study decoding was done according to the decoding list which determined finally which women received active drug misoprostol vaginally and which received orally, accordingly grouped as vaginal or oral for analysis." |
Khazardoost 2011 (V25).
| Methods | Participants were "randomised". No other details available. | |
| Participants | 60 women at term with intact membranes and BS < 6. | |
| Interventions | A single dose of oral misoprostol 100 mcg or vaginal misoprostol 25 mcg | |
| Outcomes | Delivery outcomes. Neonatal outcomes. Maternal side effect outcomes. |
|
| Notes | Data on oxytocin augmentation were excluded due to translation error. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Kipikasa 2005.
| Methods | Participants were "assigned randomly" "by means of a computer programme". A research nurse made the group allocation. | |
| Participants | 49 women with singleton pregnancies at least 40 weeks' gestation. All women with BS of 5 or less. | |
| Interventions | Oral misoprostol either 25 or 50 mcg every 3 days for a maximum 3 course over 9 days. | |
| Outcomes | Labour and delivery outcomes. limited neonatal outcomes. |
|
| Notes | Misoprostol given as outpatient basis. " the study was conducted as a pilot". The tables state n = 29 in the 50 mcg group, but the text and the percentages within the tables all use n = 26. For the review we assumed n = 26. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Using computer number generator. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants and key study personnel ensured. "Physicians managing labor were blinded concerning study group assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
Kwon 2001 (V50).
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 167 women at term with intact membranes who were unsuitable for amniotomy. | |
| Interventions | Oral or vaginal misoprostol, both 50 mcg 6‐hourly, max 8 doses. | |
| Outcomes | Labour and delivery outcomes. Minimal neonatal outcomes. Maternal side effects. | |
| Notes | 7 women excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Envelopes were prepared by the study pharmacist using random number tables with randomisation in blocks of four. No more details described. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelope. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women presented. |
| Selective reporting (reporting bias) | Low risk | Selective reporting not apparent. |
| Other bias | Low risk | None noted. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The study participants, attending staff and residents were unaware of the blocked randomisation and group assignment was concealed until the envelope was opened |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blinded randomised controlled trial. |
Langenegger 2005.
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 200 women with "indications for induction" at over 34 weeks with intact membranes. Previous CS excluded. | |
| Interventions | Oral misoprostol 50 mcg 4‐hourly (max x 6) or intracervical dinoprostone 0.5 mg 6‐hourly (max x 4). Dosages could be repeated after a 24‐hour rest period. | |
| Outcomes | Labour and delivery outcomes. Data on hyperstimulation given at numerous points, no totals available. | |
| Notes | 9 women excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
le Roux 2002 (V50).
| Methods | Sequentially‐numbered, sealed opaque envelopes. | |
| Participants | 573 women with singleton pregnancies at over 34 weeks and intact membranes and BS < 7. | |
| Interventions | 3 groups. Oral misoprostol 50 mcg 6‐hourly (max x 4) or vaginal misoprostol 50 mcg 6‐hourly (max x 4) or dinoprostone gel 1 mg 6‐hourly (max x 2). | |
| Outcomes | Labour and delivery outcomes. Minimal neonatal outcomes. | |
| Notes | 93 women (16%) had protocol violations and were excluded from the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Levy 2007.
| Methods | Double‐blind study: "coded drug boxes, prepared by pharmacy". | |
| Participants | 130 women at term with ruptured membranes for under 4 hours, no contractions and BS < 6. | |
| Interventions | Oral misoprostol 50 mcg or placebo, each 4‐hourly (max x 3). All given oxytocin at 12 hours if not in active labour. | |
| Outcomes | Labour and delivery outcomes. No neonatal outcomes. | |
| Notes | No exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
Lo 2003.
| Methods | "Identical placebos". | |
| Participants | 102 nulliparous women at term with ruptured membranes and cervices less than 2 cm dilated. | |
| Interventions | Oral misoprostol 100 mcg or placebo given 4‐hourly for 2 doses only or placebo. | |
| Outcomes | Labour and delivery outcomes. Minimal neonatal outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
Lyons 2001.
| Methods | Unclear. | |
| Participants | 40 women at 40‐42 weeks with intact membranes and BS < 6 (parity unclear). | |
| Interventions | Oral misoprostol 100 mcg or placebo every 24 hours for 3 days. | |
| Outcomes | Minimal outcomes reported. | |
| Notes | Abstract form only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo used. |
Majoko 2002 (V50).
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 406 women with singleton pregnancies at over 36 weeks with intact membranes. | |
| Interventions | 4 groups. Miso 50 mcg pv 8‐hourly x 2 or PGE2 gel 3 mg pv 8‐hourly x 2 or graduated oral miso (10, 20, 40, 80, 160, 90) increased every 4 hours or extra‐amniotic PGF2a with Foley balloon catheter. | |
| Outcomes | CS, oxytocin augmentation, uterine rupture, mec. liquor and NICU admission only. | |
| Notes | Membrane status not specified in paper, but clarified with author. Error with randomisation codes meant that intention to randomise 2:1 in favour of misoprostol was not achieved. Data on catheter with extra‐amniotic PGF2a in mechanical methods review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Mehrotra 2010 (V50).
| Methods | Sequentially‐numbered folded forms. | |
| Participants | 128 women with singleton pregnancy at term with BS equal to or less than 6. All women with intact membranes. | |
| Interventions | Oral misoprostol 50 mcg or 50 mcg vaginal misoprostol, every 4 hours until regular contractions or for a maximum of 200 mcg. | |
| Outcomes | Labour and delivery outcomes.
Neonatal outcomes. Maternal side effect outcomes. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the study subject and the clinicians taking care of their labour and deliveries were blinded to the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. "The cervical assessment and misoprostol administration on recruitment of cases and during the process of labour and delivery was carried out by a different physician." |
Moodley 2003.
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 400 women with indication for induction at any gestation, alive or dead and with any membrane status. | |
| Interventions | 3 groups. Oral miso 20 mcg 2‐hourly (max x 4) or dinoprostone 1 mg 6‐hourly (max x 3), or vaginal miso 25 mcg x 1 followed by oral misoprostol 20 mcg 2‐hourly (max x 3). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | Combined oral and vaginal misoprostol group not included in analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Mozurkewich 2003.
| Methods | Multicentre study with central randomisation via internet site. | |
| Participants | 306 nulliparous women at term with ruptured membranes. | |
| Interventions | Oral misoprostol 100 mcg 6‐hourly x 2 followed by iv oxytocin or immediate iv oxytocin. | |
| Outcomes | Labour and delivery outcomes including randomisation to delivery interval. Neonatal outcomes. | |
| Notes | 1 postrandomisation exclusion. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Nagpal 2009.
| Methods | Randomisation was by computer‐generated numbers, opaque envelopes. After randomisation patients and staff were aware of the group allocation. | |
| Participants | 61 women with a singleton pregnancy at term and prelabour spontaneous rupture of membranes confirmed by speculum examination and BS of 5 or less. All women had a reactive non‐stress test on admission. | |
| Interventions | Oral misoprostol 50 mcg 4‐hourly (max x 3) or intracervical dinoprostone gel 0.5 mg 2 doses 6 hours apart. | |
| Outcomes | Labour and delivery outcomes.
Limited neonatal outcomes. Limited side effect outcomes. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number. |
| Allocation concealment (selection bias) | Low risk | A ‐ Adeqate. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | After randomisation, patients and staff were aware of the group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. After randomisation, patients and staff were aware of the group allocation. |
Ngai 1996.
| Methods | Randomisation was by the sealed envelope method. The stratification was by parity (nulliparas, multiparas). | |
| Participants | 82 women with a singleton pregnancy at term and prelabour spontaneous rupture of membranes confirmed by speculum examination. All women had a reactive non‐stress test on admission. | |
| Interventions | 200 mcg oral misoprostol powder or placebo (vitamin B6). If no response after 12 hours labour was induced with oxytocin. |
|
| Outcomes | Changes in the BS, need for oxytocin for induction, interval from recruitment to onset of uterine activity and delivery, mode of delivery, neonatal outcome. | |
| Notes | 2 women excluded from the primary analysis (one breech and one without baseline cervical assessment). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Stratified randomisation between multipara and nullipara |
| Allocation concealment (selection bias) | Low risk | Each patient was given an envelope, chosen according to the stratified randomisation between multiparas and nulliparas |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women reported |
| Selective reporting (reporting bias) | Low risk | Selective reporting not apparent |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The envelopes were coded by the hospital pharmacy and the codes were broken only at the end of the study, so that both the medical staff and the patient were blinded to assignment |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessor not described |
Ngai 2000.
| Methods | Randomisation was by sealed envelopes. | |
| Participants | 86 women with term PROM not in labour after 12 hours. | |
| Interventions | Oral misoprostol 100 mcg every 4 hours (max 3 doses) or iv oxytocin. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. Uterine activity. | |
| Notes | 6 women excluded from the primary analysis (one breech and 5 without baseline cervical assessment). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Nigam 2004.
| Methods | Randomisation method not stated. | |
| Participants | 70 women at term with need for induction, membrane status not defined. | |
| Interventions | Oral misoprostol 50 mcg 4‐hourly or iv oxytocin 2 mU/mL in increasing doses. | |
| Outcomes | CS and vaginal delivery not achieved in 24 hours, meconium‐stained liquor, NICU admission and neonatal encephalopathy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nopdonrattkaoon 2003 (V50).
| Methods | Randomisation in sequentially‐numbered envelopes. | |
| Participants | 106 women at term with intact membranes and BS </= 4. 46 were nulliparous. | |
| Interventions | Oral misoprostol 50 mcg 4‐hourly x 6, or vaginal misoprostol 50 mcg (in 2 mL of 1% carboxy methyl cellulose) repeated 4‐hourly x 6. (If remained unfavourable for ARM then dinoprostone allowed after a rest period). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Pais'wong 2008 (V25).
| Methods | Double‐blind randomised study. Medication kept in opaque packets. Unclear whether the randomisation envelopes were sequentially numbered or whether a envelope was randomly picked from a selection of 4 ("randomised in blocks of 4"). | |
| Participants | 146 women at term with singleton pregnancies, intact membranes and a BS < 7. Women with an estimated fetal weight of > 4 kg, previous CS or parity > 5 excluded. | |
| Interventions | A single dose of 50 mcg oral misoprostol and vitamin B6 placebo vaginally or 25 mcg vaginal misoprostol with vitamin B6 oral placebo. The single dose of either was followed by iv oxytocin 6 hours later. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
Patil 2005.
| Methods | Randomisation in sequentially‐numbered envelopes ‐ number generation not specified. | |
| Participants | 190 women (all parities) with intact membranes and BS < 7. Included 2 women with a single CS each. | |
| Interventions | Oral misoprostol 200 mcg 8‐hourly (max x 3) or intracervical dinoprostone 0.5 mg 8‐hourly (max x 3). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Paungmora 2004 (V50).
| Methods | Computer‐generated numbers, but allocation unclear. | |
| Participants | 153 women of all parities at term with intact membranes and BS < 7. | |
| Interventions | Oral misoprostol 100 mcg 6‐hourly (max x 8) or vaginal misoprostol 50 mcg 6‐hourly (max x 8). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | 2 excluded when found to be breech after randomisation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers, but allocation unclear. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pongsatha 2001.
| Methods | Unclear. | |
| Participants | 89 women at over 34 weeks with intact membranes and unfavourable cervices (BS < 4). 58 were primiparous. | |
| Interventions | Oral misoprostol 50 mcg every 4 or 6 hours until labour/SROM/ARM possible. Both max 48 hours. | |
| Outcomes | Labour and delivery outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pongsatha 2002.
| Methods | Unclear. | |
| Participants | 133 women at over 34 weeks with intact membranes and unfavourable cervices (BS < 4). 33 were primiparous. | |
| Interventions | Oral misoprostol 100 mcg every 3 hours for max 8 doses or every 6 hours for max 4 doses. | |
| Outcomes | Labour and delivery outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pongsatha 2005 (V50).
| Methods | Participants were "randomised". No other details available. | |
| Participants | 166 women of all parities of 34‐42 weeks with intact membranes and BS < 5. | |
| Interventions | Oral misoprostol 100 mcg 3‐hourly (max x 8) or vaginal misoprostol 50 mcg 4‐hourly (max x 6). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Puga 2001 (V50).
| Methods | Unclear. | |
| Participants | 270 women over 35 weeks with ruptured membranes (parity unclear). | |
| Interventions | Oral misoprostol 100 mcg (3 doses, interval not stated) or vaginal misoprostol 50 mcg (3 doses ‐ interval not stated). | |
| Outcomes | CS and uterine hyperstimulation rates only. | |
| Notes | Abstract form only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rahman 2013 (V25).
| Methods | Random number table. | |
| Participants | 228 women at term with a singleton live fetus and a BS equal to or less than 6 were included. | |
| Interventions | 2 groups: oral misoprostol 50 mcg 4‐hourly (max x 5), and intra‐vaginal misoprostol 25 mcg 4‐hourly (max x 5). | |
| Outcomes | Labour and delivery outcomes.
Neonatal outcomes. Side effect outcomes. |
|
| Notes | 8 postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐blinded trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Non‐blinded trial. |
Rath 2007.
| Methods | Participants were "randomised". No other details available. | |
| Participants | 300 women at term with a singleton cephalic fetus. All women with ruptured membranes. | |
| Interventions | Oral misoprostol 50 mcg 4‐hourly (max x 6), the control group received expectant management for 20‐24 hrs, then oxytocin. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Rizvi 2007 (V25).
| Methods | Participants were "randomised". No other details available. | |
| Participants | 59 women primi and second gravida with BS < 4. "women with ruptured membrane were also participated" | |
| Interventions | Oral misoprostol 100 mcg or vaginal misoprostol 25 mcg 4‐hourly for 24 hours. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Rouzi 2014.
| Methods | Computerised randomisation in sealed, opaque envelopes. | |
| Participants | 160 women </= 34 weeks with BS < 6. | |
| Interventions | Group 1 received titrated oral misoprostol at 1 mcg/1 mL (prepared from 200 mcg misoprostol tablet dissolved in 200 mL of water). The starting dose was 20 mcg hourly for 2 doses. in the absence of contractions the dose was increased to 30 mcg hourly for 3 doses then 40 mcg for one dose then 50 mcg for 1 dose and 60 mcg hourly for 4 doses. The misoprostol was stopped once there were 2‐3 contractions every 10 minutes. If the frequency fell below this then an oxytocin infusion was used. Group 2 received 10 mg dinoprostone vaginal insert, which was inserted manually for a maximum of 24 hours or until the establishment of regular uterine contractions and was then removed. |
|
| Outcomes | Labour and delivery outcomes.
Neonatal outcomes. Limited side effect outcomes. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation in sealed, opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no women lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. Original protocol not seen. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial. |
Schneider 2004 (V25).
| Methods | Unclear. | |
| Participants | 311 women with "medical and obstetrical complications". No other details. | |
| Interventions | Oral misoprostol 50 mcg 4‐hourly or vaginal misoprostol 25 mcg 4‐hourly. | |
| Outcomes | CS rate and neonatal encephalopathy only. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sheela 2007 (V25).
| Methods | Participants were "randomised". No other details available. | |
| Participants | 150 women at term with a singleton fetus and a BS < 5 were included. All women had intact membranes. | |
| Interventions | 3 groups: Oral misoprostol 50 mcg 6‐hourly (max x 5), vaginal misoprostol 25 mcg 6‐hourly (max x 5) or intracervical dinoprostone gel 0.5 mcg 12‐hourly (max x 3). Once in active labour, the prostaglandins were stopped and replaced with amniotomy and iv oxytocin. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sheikher 2009 (V25).
| Methods | Participants were "randomly assigned". No other details available. | |
| Participants | 90 women at term with a singleton live fetus and a BS equal to or less than 5 were included. | |
| Interventions | 3 groups: Oral misoprostol 50 mcg 4‐hourly (max x 5), vaginal misoprostol 25 mcg 4‐hourly (max x 5) or intracervical Foley's catheter. | |
| Outcomes | Labour and delivery outcomes.
Neonatal outcomes. limited side effect outcomes. |
|
| Notes | Intracervical Foley's catheter group data not included in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | B‐unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Shetty 2001 (V50).
| Methods | Randomisation in sequentially‐numbered envelopes. | |
| Participants | 245 women at term with BS < 8 (149 were nulliparous, 116 had BS < 4). | |
| Interventions | Oral or vaginal misoprostol 50 mcg 4‐hourly (max 5 doses). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | Status of membranes at recruitment never mentioned, but intact membranes implied. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Shetty 2002.
| Methods | Randomisation in sealed, opaque envelopes. | |
| Participants | 251 women at term with intact membranes and BS < 7 (168 were nulliparous). | |
| Interventions | Oral misoprostol 50 or 100 mcg 4‐hourly (max 5 doses). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Shetty 2002a.
| Methods | Computerised randomisation in sealed, opaque envelopes. | |
| Participants | 61 women of all parities at term with ruptured membranes and no sign of labour. | |
| Interventions | Oral misoprostol 50 mcg 4‐hourly (max x 5) given at recruitment, or conservative management for 24 hours followed by PGE2 1‐2 mg 6‐hourly (max x 3) until BS > 7 when given oxytocin. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No exclusions. Note PGE2 use delayed for 24 hours. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Shetty 2003 (V25).
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 101 women at term with intact membranes and BS < 8. 57 were nulliparous. | |
| Interventions | Oral misoprostol 100 mcg every 4 hours (max 5 doses) or vaginal misoprostol 25 mcg every 4 hours (max 5 doses). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Shetty 2004.
| Methods | Sequentially‐numbered, opaque envelopes. | |
| Participants | 200 women at > 36 weeks with intact membranes and BS < 8. | |
| Interventions | Oral misoprostol 100 mcg 4‐hourly (max x 5) or vaginal PGE2 gel 3 mg (max 5 doses). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Sitthiwattanawong 1999.
| Methods | Open‐label randomised trial. Block of four randomisation method. No other details. | |
| Participants | 131 women with singleton pregnancy and a live fetus and > 34 weeks of gestation. All patient with intact membranes and BS equal to or less than 4. | |
| Interventions | Same dosing regimen of misoprostol was used for both oral and vaginal groups. 50 mcg every 4‐hours until 3 in 10 contractions or ruptured membranes. | |
| Outcomes | Limited labour and delivery outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block of 4 randomisation. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding. |
Souza 2013(V25)(T).
| Methods | Computerised randomisation. | |
| Participants | 200 women with singleton pregnancy and a live fetus and > 37 weeks of gestation. All patient with BS equal to or less than 6. | |
| Interventions | Group 1 received titrated oral misoprostol solution starting with 20 mcg/hour; the dose increased by 20 mcg/hour every 6 hours up to 80 mcg/hour for a maximum 48 doses. Group 2 received vaginal misoprostol 25 mcg every 6 hours for a maximum 8 doses. |
|
| Outcomes | Labour and birth outcomes.
Neonatal outcomes. Side effect outcomes. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation. |
| Allocation concealment (selection bias) | Unclear risk | B‐unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple‐blinded trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triple‐blinded trial. |
Sultana 2006 (V100).
| Methods | Unclear‐the exact method of randomisation is not given ‐ women were "randomly selected and assigned to one of two equal groups". Elsewhere in the text the study is described as a "randomised trial". | |
| Participants | 100 women with singleton pregnancy and a live fetus at 37‐42 weeks of gestation with BS of 4 or less. | |
| Interventions | Same dosing regimen of misoprostol was used for both oral and vaginal groups. 100 mcg every 4 hours until progressive labour or for a max. 600 mcg. | |
| Outcomes | Labour and delivery outcomes.
Neonatal outcomes. Maternal side effect outcomes. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk’. |
Tessier 1997.
| Methods | A computer‐generated random number list with allocation to labelled envelopes containing the study mediation/placebo. A double‐blinded study. | |
| Participants | 267 women with an indication for induction of labour. 11% of the participants had a previous CS. | |
| Interventions | Vaginal PGE2 gel (2 mg) or 50 mcg of oral misoprostol every 6 hours for maximum of 4 doses. Each woman received also a placebo gel or tablet. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | Unpublished study. The study was conducted as an outpatient basis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number. |
| Allocation concealment (selection bias) | Low risk | A computer‐generated random number list with allocation to labelled envelopes containing the study mediation/placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk'. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All members of the healthcare team and the patients were blinded to the treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
Thaisomboon 2012 (T).
| Methods | Sequentially‐numbered opaque envelops using random number tables with computer‐generated randomisation in block of 4. | |
| Participants | 64 women with term singleton fetus and obstetric or medical indication for induction of labour. All women with BS < 6 and intact membranes. 70 women were initially recruited but 6 excluded when they requested a CS. | |
| Interventions | 20 mL of misoprostol solution (200 mcg tablet dissolved in 200 mL of tap water) every hour. If uterine contractions were not achieved after the first 4 doses the dose was doubled to 40 mcg, up to a maximum of 8 doses. The other group received misoprostol solution orally 50 mcg every 4 hours Placebo was used on the other hours so as to double‐blind the study. | |
| Outcomes | Labour and delivery outcomes.
Neonatal outcomes. Maternal side effect outcomes |
|
| Notes | 6 patients were excluded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number tables. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered opaque envelops using random number tables with computer‐generated randomisation in block of 4. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of ‘Low risk’ or ‘High risk'. |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A double‐blind clinical trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
Toppozada 1997 (V100.
| Methods | The exact method of randomisation is not given ‐ women were randomly selected and assigned to one of two equal groups according to a computer‐generated table. | |
| Participants | 40 women with singleton pregnancy and a live fetus at 37‐42 weeks of gestation scheduled for induction of labour because of diabetes, hypertension or post‐term pregnancy. Cervix was unfavourable in all women (BS </= 4). Status of membranes (ruptured or intact) is not given. | |
| Interventions | One group (n = 20) received oral misoprostol (100 mcg). If there was no response within 3 hours, the majority of women were given 200 mcg of oral misoprostol. The total permitted dose was 1000 mcg. The mean total dose was 510 mcg (SD = 137.27 mcg). In the vaginal misoprostol group (n = 20) initial dose was 100 mcg, followed by an assessment 3 hours later. If the response was judged to be adequate, additional 100 mcg were given every 3 hours until cervix was more than 5 cm dilated. If there was no response to the first vaginal tablet, another 100 mcg were given vaginally 3 hours later. If there was no response after the second dose, the third dose was doubled (200 mcg). Maximum permitted dose was 1000 mcg. The mean total dose in this group was 385 mcg (SD = 142.44 mcg). | |
| Outcomes | Interval from onset of induction to onset of contractions and to delivery, changes in the BS, CTG abnormalities including uterine tachysystole and hypertonus, mode of delivery, duration of the third stage, maternal side effects. | |
| Notes | A positive response was defined as three uterine contractions per 10 minutes each lasting 45 seconds and inducing changes in the BS. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Uludag 2005 (V50).
| Methods | Sequentially‐numbered cards in sealed envelopes drawn from box ‐ even numbers allocated vaginal, odd numbers allocated oral misoprostol. | |
| Participants | 99 women at 32‐42 weeks with BS < 6. Both intact and ruptured membranes included. | |
| Interventions | Oral misoprostol 100 mcg or vaginal misoprostol 50 mcg ‐ both given 4‐hourly to a maximum of 6 doses. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered cards in sealed envelopes drawn from box ‐ even numbers allocated vaginal, odd numbers allocated oral misoprostol. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women reported |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Low risk | None reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
Wing 1999 (V25).
| Methods | Sequentially‐numbered, sealed envelopes. | |
| Participants | 220 women with intact membranes and unfavourable cervix. | |
| Interventions | Oral misoprostol was given 50 mcg every 4 hours to a maximum dose of 300 mcg. Vaginal misoprostol was given 25 mcg every four hours to a maximum dose of 150 mcg. | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | No postrandomisation exclusions. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The subjects were assigned by means of a computerized random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Group allocation was predetermined and placed in consequentially‐numbered, sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for all women reported. No postrandomisation exclusions. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
Wing 2000 (V25).
| Methods | Randomised trial | |
| Participants | 236 women with indication for labour induction and cervix with BS < 8. | |
| Interventions | Misoprostol was given either orally (100 mcg 4‐hourly, max 6 doses) or vaginally (25 mcg every 4 hours, max 6 doses). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | Two women excluded from primary analysis (1 woman had "serial" induction and 1 requested removal from the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two women were excluded from data analysis because of deviation from study protocol. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’. |
| Other bias | Low risk | None noted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | The study was not blinded. |
Wing 2004.
| Methods | Sequentially‐numbered, sealed envelopes. | |
| Participants | 200 women at any gestation with favourable cervices (BS > 6) and intact or recently ruptured membranes (less than 24 hours). | |
| Interventions | Oral misoprostol 100 mcg 4‐hourly (max x 6) or iv oxytocin infusion ("standard regimen"). | |
| Outcomes | Labour and delivery outcomes. Neonatal outcomes. | |
| Notes | 2 women in misoprostol group accidentally received extra‐amniotic saline. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Treatment allocation was determined with the use of a computer generated random number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially‐numbered, sealed envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only two women were excluded from analysis because of deviation from study protocol. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
| Other bias | Unclear risk | Insufficient information about the sequence generation process to permit judgement of ‘Low risk’ or ‘High risk’ |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The study was not blinded. |
ARM: artificial rupture of membranes BS: Bishop score CS: caesarean section CTG: cardiotocography FHR: fetal heart rate hr(s): hour(s) IUGR: intrauterine growth restriction iv: intravenous max: maximum mcg: micrograms mU/min: milliunits per minute mU/mL: milliunits per millilitre mec: meconium mg: milligram NICU: Neonatal intensive care unit PGE2: prostaglandin cream po: oral administration ('per oram') PROM: premature rupture of membranes pv: vaginal administration ('per vaginum') SD: standard deviation SROM: spontaneous rupture of membranes vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbassi 2008 | Not a randomised trial ‐ quasi‐experimental study. |
| Ascher‐Walsh 2000 | This study compared outpatient cervical ripening regimens at 40‐41 weeks' gestation. 100 mcg oral misoprostol, 200 mcg oral misoprostol or placebo were given every 3 days until 42 weeks. At 42 weeks labour was induced either with oxytocin or vaginal dinoprostone. This protocol differs substantially from the standard protocols, i.e. its primary aim is to achieve spontaneous onset of labour. The aim of the protocols included in this review is to achieve vaginal birth quickly and safely. |
| Ayaz 2008 | Not a randomised trial, quasi‐experimental study. |
| Bozhinova 2007 | Women who received vaginal misoprostol were also given sub‐lingual misoprostol at a dose of 50 mg every 4 hours "til the regular birth activity was reached". |
| Bricker 2008 | In the intervention group oral misoprostol was preceded by a dose of vaginal misoprostol. |
| Delaney 2001 | Abstract only. Women in both groups were subjected to amniotomy and the intervention was only introduced for those not in labour 1 hour later. |
| Hassan 2005 | Not a randomised trial ‐ alternate women allocated to each group. |
| Ho 2010 | The objective of the study was to compare titrated oral misoprostol to intravenous oxytocin for labour augmentation among women at 36 to 42 weeks of gestation with spontaneous onset of active labour. |
| Kadanali 1996 | In this study, the initial dose of misoprostol (100 mcg) was administered vaginally followed by oral administration (100 mcg every 2 hours). This study is included in the Cochrane review on vaginal misoprostol. |
| Neto 1988 | In this study, 15 women were divided in three groups: (i) oral misoprostol (400 mcg every 4 hours), (ii) oral misoprostol (200 mcg every 4 hours) and (iii) vaginal misoprostol (200 mcg once). The authors reported only outcomes related to the uterine activity, i.e. administration to contractions interval and strength and duration of uterine contractions. |
| Rasheed 2007 (V50) | This study of 310 women included 25 non‐randomised women in one arm. These women were using the unit's standard protocol (which was the same as the oral misoprostol arm study protocol) at the start of the study and so their data were included in the results. There is no analysis available without the inclusion of these non‐randomised participants. |
| Robinson 2011 | Ongoing study designed as non‐randomised study. |
| Thigpen 2004 | Abstract only. Vaginal misoprostol compared with oral misoprostol combined with transcervical Foley catheter. Study to be included in mechanical methods review. |
| Windrim 1997 | The comparator group in this study was managed according to the hospital's established induction protocol. This meant that women in the comparator group were induced either with intracervical dinoprostone (0.5 mg) or intravaginal dinoprostone 1 mg every 6 hours, or intravaginal dinoprostone 2 mg every 6 hours, or dilute oxytocin infusion. The exact numbers of women per method were not reported. In addition, 11 women in the comparator group were induced with vaginal misoprostol (50 mcg every 5 hours). We felt that the interventions in the comparator group were sufficiently different to be 'lumped' together. |
| Zvandasara 2008 | In addition to live fetuses the study also included 4 cases of IUFD (1 in the oral misoprostol group and 3 in the vaginal misoprostol group). |
IUFD: intrauterine fetal death mcg: micrograms mg: milligram PROM: prelabour rupture of membranes
Characteristics of studies awaiting assessment [ordered by study ID]
Atkinson 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Bonebrake 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Butler 2004.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Getgan 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Goedken 2000.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Madhavi 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Niroomanesh 2011.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Not in English ‐ awaiting translation. |
Pearson 2002.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Saldivar 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Tuipae 1999.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Vijitrawiwat 2003.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Yazdani 2012.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Article not in English ‐ awaiting translation. |
Young 2001.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting more data as available as abstract only at present. |
Characteristics of ongoing studies [ordered by study ID]
DebBarma 2013.
| Trial name or title | |
| Methods | Random table or by lottery. |
| Participants | Women with full term pregnancy having alive fetus with vertex presentation and Bishops less than 5. |
| Interventions | Oral misoprostol 25 mcg 4‐hourly max 5 doses. |
| Outcomes | The time interval from induction to vaginal birth (primary outcome), rate of vaginal birth, caesarean section rate, uterine tachysystole and neonatal outcome (secondary outcomes). |
| Starting date | No date specified. |
| Contact information | |
| Notes |
Gherman 2002.
| Trial name or title | Trial name not known. |
| Methods | |
| Participants | Interim report on first 75 women published in abstract form. |
| Interventions | Oral misoprostol 50 mcg or 100 mcg given 4‐hourly for a maximum of 6 doses. |
| Outcomes | Unclear. |
| Starting date | |
| Contact information | |
| Notes |
Pranuthi 2011.
| Trial name or title | |
| Methods | |
| Participants | Interim report on 64 women published in abstract form. |
| Interventions | Oral misoprostol 25 mcg 4‐hourly Max 6 doses. |
| Outcomes | The number of vaginal births, induction to birth interval, oxytocin requirement, indication of caesarean section, side effect and perinatal outcome. |
| Starting date | |
| Contact information | |
| Notes |
mcg: micrograms
Differences between protocol and review
In previously published versions of this review, we proposed to compare low‐, medium‐ and high‐dose regimens. We defined low‐dose regimens as less than 50 micrograms (mcg), medium‐dose as 50 to 100 mcg inclusive and high‐dose as more than 100 mcg. These arbitrary groups proved impractical because most trials used either 25 mcg, 50 mcg or 100 mcg doses. In order to study dose‐related effects, we decided to group regimens into: (i) 0 to 25 mcg, (ii) 26 to 50 mcg, (iii) 51 to 199 mcg and (iv) 200 mcg or more. We acknowledge that this change has been driven to some extent by the trials' data and is therefore a potential source of bias. Also, the same dose can be given at varying intervals (usually between two and six hours) and these differences could influence the primary outcomes. 'Low‐dose' regimens with two‐hourly administration may result in a higher cumulative dose over 24 hours than 'high‐dose' regimens. However, the plasma half‐life of oral misoprostol is short (20 to 40 minutes) and, therefore, it would appear that dose is more important than frequency. Consequently, at least at this point in time, we have not planned analyses based on frequency of administration.
The methods have been updated to current Cochrane Pregnancy and Childbirth Group standards.
Contributions of authors
Zarko Alfirevic (ZA) produced the protocol and the original review in 2001. He also supervised the subsequent updates. Andrew Weeks joined ZA in 2004; he collated the new data and amended the results, discussions and conclusions in 2005 and 2007. Nasreen Aflaifel joined the team for the update in 2013, adding the new data and updating the results. All authors reviewed and accepted the final paper.
Sources of support
Internal sources
The University of Liverpool, UK.
External sources
No sources of support supplied
Declarations of interest
Zarko Alfirevic is one of the principal investigators of a trial included in this review. He acted as an adviser and co‐investigator on the Phase III trials to companies involved in the development of misoprostol products for labour induction, but payments were on a one‐off basis with no regular or long‐lasting personal relationships with any organisation. Neither he, nor his immediate family, hold any shares or stocks in any company. Andrew Weeks runs the www.misoprostol.org website as a service to provide accurate information to women about misoprostol use. He is principal investigator for an MRC funded phase 3 study of oral misoprostol for labour induction. Neither he nor his family have financial interests that would gain from an increased use of misoprostol.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Aalami‐Harandi 2013 (T) {published data only}
- Aalami‐Harandi R, Karamali M, Moeini A. Induction of labor with titrated oral misoprostol solution versus oxytocin in term pregnancy: randomized controlled trial. Revista Brasileira de Ginecologia e Obstetricia 2013;35(2):60‐5. [DOI] [PubMed] [Google Scholar]
Adair 1998 (V50) {published data only}
- Adair CD, Weeks JW, Barrilleaux PS, Philibert L, Edwards MS, Lewis DF. Labor induction with oral versus vaginal misoprostol: a randomized, double‐blind trial. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S93. [DOI] [PubMed] [Google Scholar]
- Adair CD, Weeks JW, Barrilleaux S, Edwards M, Burlison K, Lewis DF. Oral or vaginal misoprostol administration for induction of labor: a randomised, double‐blind trial. Obstetrics & Gynecology 1998;92(5):810‐3. [DOI] [PubMed] [Google Scholar]
Adam 2005 (V50) {published data only}
- Adam I, Hassan OA, Elhassan EM. Oral misoprostol vs. vaginal misoprostol for cervical ripening and labour induction. International Journal of Gynecology & Obstetrics 2005;89:142‐3. [DOI] [PubMed] [Google Scholar]
Al‐Hussaini 2003 {published data only}
- Al‐Hussaini TK, Abdel‐Aal SA, Youssef MA. Oral misoprostol vs. intravenous oxytocin for labor induction in women with prelabor rupture of membranes at term. International Journal of Gynecology & Obstetrics 2003;82(1):73‐5. [DOI] [PubMed] [Google Scholar]
Bartha 2000 {published data only}
- Bartha JL, Comino‐Delgado R, Garcia‐Benasach F, Martinez‐Del‐Fresno P, Moreno‐Corral LJ. Oral misoprostol and intracervical dinoprostone for cervical ripening and labor induction: a randomized comparison. Obstetrics & Gynecology 2000;96:465‐9. [DOI] [PubMed] [Google Scholar]
Beigi 2003 {published data only}
- Beigi A, Kabiri M, Zarrinkoub F. Cervical ripening with oral misoprostol at term. International Journal of Gynecology & Obstetrics 2003;83:251‐5. [DOI] [PubMed] [Google Scholar]
Bennett 1998 (V50) {published data only}
- Bennett K, Butt K, Crane J, Hutchens D, Young D. Misoprostol for labour induction at term. Society of Obstetricians and Gynaecologists of Canada 54th Annual Meeting; 1998 June; Victoria, Canada. 1998:11.
- Bennett KA, Butt K, Crane JMG, Hutchens D, Young DC. A masked randomized comparison of oral and vaginal administration of misoprostol for labor induction. Obstetrics & Gynecology 1998;92(4 Suppl 1):481‐6. [DOI] [PubMed] [Google Scholar]
Butt 1999 {published data only}
- Butt KD, Bennett KA, Crane JM, Hutchens D, Young DC. Randomized comparison of oral misoprostol and oxytocin for labor induction in term prelabor membrane rupture. Obstetrics & Gynecology 1999;94(6):994‐9. [DOI] [PubMed] [Google Scholar]
Carlan 2001 (V50) {published data only}
- Carlan SJ, Bouldin S, Blust D, O'Brien WF. Safety and efficacy of misoprostol orally and vaginally: a randomized trial. Obstetrics & Gynecology 2001;98:107‐12. [DOI] [PubMed] [Google Scholar]
Cheng 2008 (T) (V25) {published data only}
- Cheng SY, Ming H, Lee JC. Titrated oral compared with vaginal misoprostol for labor induction: a randomized controlled trial. Obstetrics & Gynecology 2008;111(1):119‐25. [DOI] [PubMed] [Google Scholar]
Cheung 2006 {published data only}
- Cheung PC, Yeo EL, Wong KS, Tang LC. Oral misoprostol for induction of labor in prelabor rupture of membranes (PROM) at term: a randomized control trial. Acta Obstetricia et Gynecologica Scandinavica 2006;85(9):1128‐33. [DOI] [PubMed] [Google Scholar]
Colon 2005 (V25) {published data only}
- Colon I, Clawson K, Hunter K, Druzin ML, Taslimi MM. Prospective randomized clinical trial of inpatient cervical ripening with stepwise oral misoprostol vs vaginal misoprostol. American Journal of Obstetrics and Gynecology 2005;192:747‐52. [DOI] [PubMed] [Google Scholar]
- Colon I, Clawson K, Taslimi M, Druzin M. Prospective randomized clinical trial of inpatient cervical ripening with stepwise oral misoprostol. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S15. [DOI] [PubMed] [Google Scholar]
Crane 2003 {published data only}
- Crane J, Delaney T, Hutchens D. Oral misoprostol labor induction in term prelabor membrane rupture. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S168. [DOI] [PubMed] [Google Scholar]
- Crane JMG, Delaney T, Hutchens D. Oral misoprostol for premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 2003;189:720‐4. [DOI] [PubMed] [Google Scholar]
Dallenbach 2003 (T) {published data only}
- Dallenbach P, Boulvain M, Viardot C, Irion O. Oral misoprostol or vaginal dinoprostone for labor induction: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2003;188(1):162‐7. [DOI] [PubMed] [Google Scholar]
- Dallenbach P, Boulvain M, Viardot C, Irion O. Oral misoprostol or vaginal dinoprostone for labor induction? A randomized controlled trial [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S108. [DOI] [PubMed] [Google Scholar]
De 2006 {published data only}
- De A, Bagga R, Gopalan S. The routine use of oxytocin after oral misoprostol for labour induction in women with an unfavourable cervix is not of benefit. Australian and New Zealand Journal of Obstetrics and Gynaecology 2006;46(4):323‐9. [DOI] [PubMed] [Google Scholar]
Deshmukh 2013 (V50) {published data only}
- Deshmukh VL, Yelikar KA, Waso V. Comparative study of efficacy and safety of oral versus vaginal misoprostol for induction or labour. Journal of Obstetrics and Gynaecology of India 2013;63(5):321‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodd 2006 {published data only}
- Dodd J, Crowther C, Ronbinson J. Misoprostol for the induction of labour at term: a randomised controlled trial. Australian and New Zealand Journal of Obstetrics and Gynaecology 2005;45:347‐8. [Google Scholar]
- Dodd JM, Crowther CA, Robinson JS. Factors associated with adverse maternal health outcomes following induction of labour at term: analyses from a randomised trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:86.
- Dodd JM, Crowther CA, Robinson JS. Oral misoprostol for induction of labour at term: randomised controlled trial. BMJ 2006;332(7540):509‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd JM, Crowther CA, Robinson JS. Time of commencing induction of labour: a nested randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:85.
Dodd 2006a (T) {published data only}
- Dodd JM, Crowther CA, Robinson JS. Oral misoprostol versus intravenous oxytocin for induction of labour following artificial or spontaneous rupture of membranes: a randomised controlled trial. Perinatal Society of Australia and New Zealand 10th Annual Congress; 2006 April 3‐6; Perth, Australia. 2006:258.
Dyar 2000 (V50) {published data only}
- Dyar TR, Greig P, Cummings R, Nichols K. The efficacy and safety of oral versus vaginal misoprostol for the induction of term labor. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S135. [Google Scholar]
Elhassan 2007 (V50) {published data only}
- Elhassan EM, Nasr AM, Adam I. Sublingual compared with oral and vaginal misoprostol for labor induction. International Journal of Gynecology & Obstetrics 2007;97(2):153‐4. [DOI] [PubMed] [Google Scholar]
Fisher 2001 (V50) {published data only}
- Fisher S, Davies G, Mackenzie P. Oral versus vaginal misoprostol for induction of labour: a double‐blind, placebo‐controlled randomised trial. American Journal of Obstetrics and Gynecology 2001;184(1):S117. [DOI] [PubMed] [Google Scholar]
- Fisher S, Mackenzie V, Davies G. Oral versus vaginal misoprostol for induction of labor: a double‐blind randomized controlled trial. American Journal of Obstetrics and Gynecology 2001;185:906‐10. [DOI] [PubMed] [Google Scholar]
Gherman 2001 {published data only}
- Browning J, Gherman RB. Oral misoprostol versus intravaginal prostaglandin E2 for preinduction cervical ripening: a randomized trial. Obstetrics & Gynecology 2000;95(4 Suppl):76S. [PubMed] [Google Scholar]
- Gherman R, Browning J, O'Boyle A, Murphy Goodwin T. Oral misoprostol vs. intravaginal prostaglandin e2 for preinduction cervical ripening. Journal of Reproductive Medicine 2001;46(7):641‐6. [PubMed] [Google Scholar]
Hall 2002 (V25) {published data only}
- Hall R, Duarte‐Gardea M, Harlass F. Oral versus vaginal misoprostol for labor induction. Obstetrics & Gynecology 2002;99(6):1044‐8. [DOI] [PubMed] [Google Scholar]
Henrich 2008 (T) {published data only}
- Henrich W, Dudenhausen JW, Hanel C, Chen FC. Oral misoprostol against vaginal dinoprostone for labor induction at term: a randomized comparison [Prospektiv randomisierte Studie zum Vergleich von Misoprostol oral mit Dinoproston vaginal bei der Geburtseinleitung am Termin]. Zeitschrift fur Geburtshilfe und Neonatologie 2008;212(5):183‐8. [DOI] [PubMed] [Google Scholar]
Hoffman 2001 {published data only}
- Hoffman R, Anthony J, Fawcus J. Oral misoprostol vs. placebo in the management of prelabor rupture of membranes at term. International Journal of Gynecology & Obstetrics 2001;72:215‐21. [DOI] [PubMed] [Google Scholar]
- Hoffman RAM, Fawcus S, Anthony J. Oral misoprostol versus placebo in the management of prelabour rupture of membranes at term. Women's Health ‐ into the new millenium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. 1999:65.
Hofmeyr 2001(T) {published and unpublished data}
- Hofmyer GJ, Alfirevic Z, Matonhodze B, Brocklehurst P, Campbell E, Nikodem C. Titrated oral misoprostol solution for labour induction: a multi‐centre, randomised trial. BJOG: an international journal of obstetrics and gynaecology 2001;108:952‐9. [DOI] [PubMed] [Google Scholar]
- Matonhodze B, Alfirevic Z, Hofmeyr J, Brocklehurst P. Titrated oral misoprostol for labour induction: a randomised trial [abstract]. Prenatal and Neonatal Medicine 2000;5(Suppl 2):148. [Google Scholar]
- Matonhodze B, Alfirevic Z, Hofmeyr J, Campbell L, Brocklehurst P. Titrated oral misoprostol for labour induction: a random allocation trial. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S19. [DOI] [PubMed] [Google Scholar]
- Matonhodze BB, Hofmeyr GJ, Levin J. Labour induction at term‐‐a randomised trial comparing Foley catheter plus titrated oral misoprostol solution, titrated oral misoprostol solution alone, and dinoprostone. South African Medical Journal 2003;93(5):375‐9. [PubMed] [Google Scholar]
How 2001 (V25) {published data only}
- How H, Leaseburge L, Khoury J, Siddiqi T, Sibai B. Is there an ideal route of misoprostol administration for cervical ripening and labor induction [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S118. [DOI] [PubMed] [Google Scholar]
- How H, Leaseburge L, Khoury J, Siddiqi T, Spinnato J, Sibai B. A comparison of various routes and dosages of misoprostol for cervical ripening and the induction of labor. American Journal of Obstetrics and Gynecology 2001;185:911‐5. [DOI] [PubMed] [Google Scholar]
Javaid 2008 {published data only}
- Javaid MK, Hassan S, Tahira T. Management pre labour rupture of the membranes at term; induction of labor compared with expectant. Professional Medical Journal 2008;15(2):216‐9. [Google Scholar]
Jindal 2011 (V50) {published data only}
- Jindal P, Avasthi K, Kaur M. A comparison of vaginal vs. oral misoprostol for induction of labor‐double blind randomized trial. Journal of Obstetrics and Gynecology of India 2011;61(5):538‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khazardoost 2011 (V25) {published data only}
- Khazardoost S, Hakimi P, Noorzadeh M, Shafaat M. Misoprostol for cervical ripening: a clinical trial in 60 pregnant women. Tehran University Medical Journal 2011;68(10):595‐9. [Google Scholar]
Kipikasa 2005 {published data only}
- Kipikasa JH, Adair CD, Williamson J, Breen JM, Medford LK, Sanchez‐Ramos L. Use of misoprostol on an outpatient basis for postdate pregnancy. International Journal of Gynecology & Obstetrics 2005;88:108‐11. [DOI] [PubMed] [Google Scholar]
Kwon 2001 (V50) {published data only}
- Kwon J, Davies G, MacKenzie V. A comparison of oral and vaginal misoprostol for induction of labour at term: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2001;108:23‐6. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Mackenzie VP, Davies GAL. A comparison of oral and vaginal misoprostol for induction of labour. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S128. [DOI] [PubMed] [Google Scholar]
Langenegger 2005 {published data only}
- Langenegger EJ, Odendaal HJ, Grove D. Oral misoprostol versus intracervical dinoprostone for induction of labour. International Journal of Gynecology & Obstetrics 2005;88:242‐8. [DOI] [PubMed] [Google Scholar]
le Roux 2002 (V50) {published data only}
- Roux P, Olarogun J, Penny J, Anthony J. Oral and vaginal misoprostol compared with dinoprostone for induction of labor: a randomized controlled trial. Obstetrics & Gynecology 2002;99(2):201‐5. [DOI] [PubMed] [Google Scholar]
Levy 2007 {published data only}
- Levy R, Vaisbuch E, Furman B, Brown D, Volach V, Hagay ZJ. Induction of labor with oral misoprostol for premature rupture of membranes at term in women with unfavorable cervix: a randomized, double‐blind, placebo‐controlled trial. Journal of Perinatal Medicine 2007;35:126‐9. [DOI] [PubMed] [Google Scholar]
- Levy R, Vaisbuch E, Furman B, Doitch H, Oron S, Hagay Z. Prospective randomized clinical trial of immediate induction of labor with oral misoprostol for prelabor rupture of the membranes in women with unfavorable cervix at term. American Journal of Obstetrics and Gynecology 2005;193(6 Suppl):S44. [Google Scholar]
Lo 2003 {published data only}
- Lo J, Alexander J, McIntire D, Leveno K. Efficacy of oral misoprostol in nulliparous women with premature rupture of membranes. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S204. [Google Scholar]
- Lo J, Alexander J, McIntire D, Leveno K. Randomized trial of oral misoprostol in nulliparous women with premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S204. [Google Scholar]
- Lo JY, Alexander JM, McIntire DD, Leveno KJ. Ruptured membranes at term: randomized, double‐blind trial of oral misoprostol for labor induction. Obstetrics & Gynecology 2003;101(4):685‐9. [DOI] [PubMed] [Google Scholar]
Lyons 2001 {published data only}
- Lyons C, Rumney P, Huang W, Morrison E, Thomas S, Nageotte M, et al. Outpatient cervical ripening with oral misoprostol post‐term: induction rates decreased. American Journal of Obstetrics and Gynecology 2001;184(1):S116. [Google Scholar]
Majoko 2002 (V50) {published data only}
- Majoko F, Zwizwai M, Nystrom L, Lindmark G. Vaginal misoprostol for induction of labour: a more effective agent than prostaglandin f2 alpha gel and prostaglandin e2 pessary. Central African Journal of Medicine 2002;48(11‐12):123‐8. [PubMed] [Google Scholar]
Mehrotra 2010 (V50) {published data only}
- Mehrotra S, Singh U, Gupta HP. A prospective double blind study using oral versus vaginal misoprostol for labour induction. Journal of Obstetrics & Gynaecology 2010;30(5):461‐4. [DOI] [PubMed] [Google Scholar]
Moodley 2003 {published data only}
- Moodley J, Venkatachalam S, Songca P. Misoprostol for cervical ripening at and near term ‐ a comparative study. South African Journal of Obstetrics and Gynaecology 2003;9(2):34‐7. [PubMed] [Google Scholar]
- Moodley J, Venkatachalam S, Songca P. Misoprostol for cervical ripening at and near term‐‐a comparative study. South African Medical Journal 2003;93:371‐4. [PubMed] [Google Scholar]
Mozurkewich 2003 {published data only}
- Mozurkewich E, Horrocks J, Daley S, Oeyen P, Sarvis A, Halvorson M, et al. The misoprostol study: a randomized controlled trial of misoprostol for premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S168. [Google Scholar]
- Mozurkewich E, Horrocks J, Daley S, Oeyen P, Halvorson M, Johnson M, et al. The MisoPROM study: a multicenter randomized comparison of oral misoprostol and oxytocin for premature rupture of membranes at term. American Journal of Obstetrics and Gynecology 2003;189:1026‐30. [DOI] [PubMed] [Google Scholar]
Nagpal 2009 {published data only}
- Nagpal MB, Raghunandan C, Saili A. Oral misoprostol versus intracervical prostaglandin E2 gel for active management of premature rupture of membranes at term. International Journal of Gynecology & Obstetrics 2009;106(1):23‐6. [DOI] [PubMed] [Google Scholar]
Ngai 1996 {published data only}
- Ngai CSW, To WWK, Lao T, Ho PC. Cervical priming with oral misoprostol in prelabour rupture of membranes at term. 27th British Congress of Obstetrics and Gynaecology;1995 July 4‐7, Dublin, Ireland. 1995:A479.
- Ngai SW, To WK, Lao T, Ho PC. Cervical priming with oral misoprostol in pre‐labor rupture of membranes at term. Obstetrics & Gynecology 1996;87:923‐6. [DOI] [PubMed] [Google Scholar]
Ngai 2000 {published data only}
- Jackson N, Paterson‐Brown S. Labour characteristics and uterine activity: misoprostol compared with oxytocin in women at term with prelabour rupture of the membranes [letter]. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1181‐2. [DOI] [PubMed] [Google Scholar]
- Ngai SW, Chan YM, Lam SW, Lao T. Prospective randomised study to compare misoprostol and oxytocin for labour induction in prelabour rupture of membranes in term pregnancy. British Journal of Obstetrics and Gynaecology 1998;105 Suppl 17:82. [Google Scholar]
- Ngai SW, Chan YM, Lam SW, Lao TT. Labour characteristics and uterine activity: misoprostol compared with oxytocin in women at term with prelabour rupture of membranes. British Journal of Obstetrics and Gynecology 2000;107(2):222‐7. [DOI] [PubMed] [Google Scholar]
Nigam 2004 {published data only}
- Nigam A, Singh VK, Dubay P, Pandey K, Bhagioliwal A, Prakash A. Misoprostol vs. oxytocin for induction of labor at term. International Journal of Gynecology & Obstetrics 2004;86:398‐400. [DOI] [PubMed] [Google Scholar]
Nopdonrattkaoon 2003 (V50) {published data only}
- Nopdonrattakoon L. A comparison between intravaginal and oral misoprostol for labor induction: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2003;29(2):87‐91. [DOI] [PubMed] [Google Scholar]
Pais'wong 2008 (V25) {published data only}
- Paisarntantiwong R, Getgan M. A comparison between single dose of 50 microg oral misoprostol and 25 microg vaginal misoprostol for labor induction. Journal of the Medical Association of Thailand 2005;88(Suppl 2):S56‐S62. [PubMed] [Google Scholar]
Patil 2005 {published data only}
- Patil PK, Swamy MK, Rao Radhika K. Oral misoprostol vs intra‐cervical dinoprostone for cervical ripening and labour induction. Journal of Obstetrics and Gynaecology of India 2005;55(2):128‐31. [Google Scholar]
Paungmora 2004 (V50) {published data only}
- Paungmora N, Herabutaya Y, O‐Prasertsawat P. A comparison of oral and vaginal misoprostol for induction of labour at term: a randomised controlled trial. Thai Journal of Obstetrics and Gynaecology 2003;15(4):272. [DOI] [PubMed] [Google Scholar]
- Paungmora N, Herabutya Y, O‐Prasertsawat P, Punyavachira P. Comparison of oral and vaginal misoprostol for induction of labor at term: a randomized controlled trial. Journal of Obstetrics & Gynaecology Research 2004;30(5):358‐62. [DOI] [PubMed] [Google Scholar]
Pongsatha 2001 {published data only}
- Pongsatha S, Tongsong T, Somsak T. A comparison between 50 mcg oral misoprostol every 4 hours and 6 hours for labor induction: a prospective randomized controlled trial. Journal of the Medical Association of Thailand 2001;84(7):989‐94. [PubMed] [Google Scholar]
- Somsak T, Pongsatha S. A comparison between oral misoprostol 50 micrograms every 4 hours and every 6 hours for labor induction. Thai Journal of Obstetrics and Gynaecology 2000;12(4):334. [Google Scholar]
Pongsatha 2002 {published data only}
- Pongsatha S, Sirisukkasem S, Tongsong T. A comparison of 100ug oral misoprostol every 3 hours and 6 hours for labor induction: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2002;28(6):308‐12. [DOI] [PubMed] [Google Scholar]
Pongsatha 2005 (V50) {published data only}
- Pongsatha S, Vijittrawiwat A, Tongsong T. A comparison of labor induction by oral and vaginal misoprostol. International Journal of Gynecology & Obstetrics 2005;88:140‐1. [DOI] [PubMed] [Google Scholar]
Puga 2001 (V50) {published data only}
- Puga O, Nien J, Gomez R, Medina L, Carstens M, Gonzalez R, et al. Premature rupture of membranes after 35 weeks: a randomized clinical trial of induction of labor with oral versus vaginal administration of misoprostol. American Journal of Obstetrics and Gynecology 2001;184(1):S85. [Google Scholar]
Rahman 2013 (V25) {published data only}
- Rahman H. Comparative evaluation of 50ug oral misoprostol and 25ug intra‐vaginal misoprostol for induction of labour at term. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:316.
- Rahman H, Pradhan A, Kharka L, Renjhen P, Kar S, Dutta S. Comparative evaluation of 50 microgram oral misoprostol and 25 microgram intravaginal misoprostol for induction of labour at term: a randomized trial. Journal of Obstetrics and Gynaecology Canada : JOGC 2013;35(5):408‐16. [DOI] [PubMed] [Google Scholar]
Rath 2007 {published data only}
- Rath DM, Manas K. Induction of labor with oral misoprostol in women with prelabor rupture of membranes at term. Journal of Obstetrics and Gynecology of India 2007;57(6):505‐8. [Google Scholar]
Rizvi 2007 (V25) {published data only}
- Rizvi S, Umber F, Yusuf AW. Labour induction at term; oral versus intravaginal misoprostol. Annals of King Edward Medical College 2007;13(1):119‐21. [Google Scholar]
Rouzi 2014 {published data only}
- Rouzi AA. Randomized clinical trial between titrated oral dose of misoprostol and Propess for induction of labor. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12611000420943 (accessed 22 January 2013) 2011.
- Rouzi AA, Alsibiani S, Mansouri N, Alsinani N, Darhouse K. Randomized clinical trial between hourly titrated oral misoprostol and vaginal dinoprostone for induction of labor. American Journal of Obstetrics and Gynecology 2014;210(1):56.e1‐6. [DOI] [PubMed] [Google Scholar]
Schneider 2004 (V25) {published data only}
- Schneider M, Ramsey R, Kao L, Bennett KA. Misoprostol is effective for induction of labor in high risk pregnant women: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S73. [Google Scholar]
Sheela 2007 (V25) {published data only}
- Sheela CN, Mhaskar A, George S. Comparison of vaginal misoprostol and oral misoprostol with intracervical dinoprostone gel for labor induction at term. Journal of Obstetrics and Gynaecology of India 2007;57(4):327‐30. [Google Scholar]
Sheikher 2009 (V25) {published data only}
- Sheikher C, Suri N, Kholi U. Comparative evaluation of oral misoprostol, vaginal misoprostol and intracervical Foley's catheter for induction of labour at term. JK Science 2009;11(2):75‐7. [Google Scholar]
Shetty 2001 (V50) {published data only}
- Shetty A, Danielian P, Templeton A. A comparison of oral and vaginal misoprostol in the induction of labour at term: a random allocation trial. Journal of Obstetrics and Gynaecology 2000;20(Suppl 1):S19. [DOI] [PubMed] [Google Scholar]
- Shetty A, Danielian P, Templeton A. A comparison of oral and vaginal misoprostol tablets in induction of labour at term. BJOG: an international journal of obstetrics and gynaecology 2001;108:238‐43. [DOI] [PubMed] [Google Scholar]
- Shetty A, Danielian P, Templeton A. A comparison of oral and vaginal tablets in the induction of labor at term. XVI FIGO World Congress of Obstetrics & Gynecology. Book 4; 2000 Sept 3‐8; Washington DC, USA. 2000:28‐9.
- Shetty A, Danielian P, Templeton A. Oral versus vaginal misoprostol in the induction of labour at term: a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2000;107:813. [Google Scholar]
Shetty 2002 {published data only}
- Shetty A, Martin R, Danielian P, Templeton A. A comparison of two dosage regimens of oral misoprostol for labor induction at term. Acta Obstetricia et Gynecologica Scandinavica 2002;81:337‐42. [DOI] [PubMed] [Google Scholar]
- Shetty A, Martin R, Danielian P, Templeton A. A comparison of two dose regimens of oral misoprostol in the induction of labour at term: a random allocation controlled trial [abstract]. Journal of Obstetrics & Gynaecology 2001;21(1):91. [Google Scholar]
Shetty 2002a {published data only}
- Shetty A, Stewart K, Stewart G, Rice P, Danielian P, Templeton A. Active management of term prelabour rupture of membranes with oral misoprostol. BJOG: an international journal of obstetrics and gynaecology 2002;109:1354‐8. [DOI] [PubMed] [Google Scholar]
Shetty 2003 (V25) {published data only}
- Livingstone I, Acharya S, Shetty A, Rice P, Danielian P, Templeton A. 100ug of oral misoprostol versus 25ug of vaginal misoprostol in term labour induction ‐ a randomised comparison. Journal of Obstetrics and Gynaecology 2004;24(1):106. [DOI] [PubMed] [Google Scholar]
- Shetty A, Livingstone I, Acharya S, Rice P, Danielian P, Templeton A. Oral misoprostol (100ug) versus vaginal misoprostol (25ug) in term labor induction: a randomized comparison. Acta Obstetricia et Gynecologica Scandinavica 2003;82(12):1103‐6. [DOI] [PubMed] [Google Scholar]
Shetty 2004 {published data only}
- Shetty A, Livingstone I, Acharya S, Danielian P, Rice P, Templeton A. A randomised comparison of oral misoprostol and vaginal prostaglandin E2 tablets in labour induction at term. BJOG: an international journal of obstetrics and gynaecology 2003;110:963. [DOI] [PubMed] [Google Scholar]
- Shetty A, Livingstone I, Acharya S, Danielian P, Templeton A, Rice P. A randomised comparison of oral misoprostol and vaginal prostaglandin E2 tablets in labour induction at term. BJOG: an international journal of obstetrics and gynaecology 2004;111:436‐40. [DOI] [PubMed] [Google Scholar]
Sitthiwattanawong 1999 {published data only}
- Sitthiwattanawong W. A comparison between oral and intravaginal administration of 50 microgram misoprostol for cervical ripening and induction of labor. Thai Journal of Obstetrics and Gynaecology 2000;12(4):352. [Google Scholar]
- Sitthiwattanawong W, Pongsatha S. Oral misoprostol for cervical ripening and labour induction: a randomized controlled trial. Thai Journal of Obstetrics and Gynaecology 1999;11(2):87‐92. [Google Scholar]
Souza 2013(V25)(T) {published data only}
- Rolland Souza A. [Titrated oral suspension compared with vaginal misoprostol for labor induction: a randomized controlled trial] [Misoprostol em solução oral titulada escalonada versus via vaginal para indução do trabalho de parto: ensaio clínico randomizado]. Revista Brazileira de Ginecologia e Obstetricia 2011;33(9):270. [Google Scholar]
- Rolland de Souza A. Oral misoprostol titrated solution versus vaginal misoprostol for induction of labour: randomized controlled trial. http://clinicaltrials.gov/ct2/show/record/NCT00992524 (accessed 22 January 2013) 2011.
- Souza AS, Feitosa FE, Costa AA, Pereira AP, Carvalho AS, Paixao RM, et al. Titrated oral misoprostol solution versus vaginal misoprostol for labor induction. International Journal of Gynecology and Obstetrics 2013;123(3):207‐12. [DOI] [PubMed] [Google Scholar]
Sultana 2006 (V100) {published data only}
- Sultana N, Rouf S, Rashid M. Oral versus vaginal misoprostol for induction of labour. Journal of Bangladesh College of Physicians and Surgeons 2006;24(2):44‐9. [Google Scholar]
Tessier 1997 {published data only}
- * Tessier F, Danserau J. Oral misoprostol versus vaginal dinoprostone for labor induction: a double‐blind randomized controlled trial. Personal communication 1997.
- Tessier F, Dansereau J. A double‐blind randomized controlled trial comparing oral misoprostol to vaginal prostaglandin E2 gel for the induction of labour at or near term. American Journal of Obstetrics and Gynecology 1997;176(1):S111. [Google Scholar]
Thaisomboon 2012 (T) {published data only}
- Thaisomboon A, Russameecharoen K, Wanitpongpan P, Phattanachindakun B, Changnoi A. Comparison of the efficacy and safety of titrated oral misoprostol and a conventional oral regimen for cervical ripening and labor induction. International Journal of Gynecology and Obstetrics 2012;116(1):13‐6. [DOI] [PubMed] [Google Scholar]
Toppozada 1997 (V100 {published data only}
- Toppozada MK, Anwar MYM, Hassan HA, El‐Gazaerly WS. Oral or vaginal misoprostol for induction of labour. International Journal of Gynecology & Obstetrics 1997;56:135‐9. [DOI] [PubMed] [Google Scholar]
Uludag 2005 (V50) {published data only}
- Uludag S, Saricali FS, Madazli R, Cepni I. A comparison of oral and vaginal misoprostol for induction of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 2005;122:57‐60. [DOI] [PubMed] [Google Scholar]
Wing 1999 (V25) {published data only}
- Wing D, Ham D, Paul RH. A comparison of orally administered misoprostol with vaginally administered misoprostol for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1999;180(5):1155‐60. [DOI] [PubMed] [Google Scholar]
- Wing DA, Ham D, Paul RH. A comparison of orally administered misoprostol to vaginally administered misoprostol for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S127. [DOI] [PubMed] [Google Scholar]
Wing 2000 (V25) {published data only}
- Wing DA, Park MR, Paul RH. A randomised comparison of oral and intravaginal misoprostol for labor induction. Obstetrics & Gynecology 2000;95(6 Pt 1):905‐8. [DOI] [PubMed] [Google Scholar]
Wing 2004 {published data only}
- Wing DA, Fassett MJ, Guberman C, Tran S, Parrish A, Guinn D. A comparison of orally administered misoprostol to intravenous oxytocin for labor induction in women with favorable cervix examinations. American Journal of Obstetrics and Gynecology 2004;190:1689‐96. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abbassi 2008 {published data only}
- Abbassi RM, Sirichand P, Rizvi S. Safety and efficacy of oral versus vaginal misoprostol use for induction of labour at term. Journal of the College of Physicians and Surgeons Pakistan 2008;18(10):625‐9. [DOI] [PubMed] [Google Scholar]
Ascher‐Walsh 2000 {published data only}
- Ascher‐Walsh C, Burke B, Baxi L. Outpatient management of prolonged pregnancy with misoprostol: a randomised, double‐blind, placebo‐controlled study, prelim.data. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S520. [Google Scholar]
Ayaz 2008 {published data only}
- Ayaz A, Saeed S, Farooq MU, Ahmad F, Bahoo LA, Ahmad I. Pre‐labor rupture of membranes at term in patients with an unfavorable cervix: active versus conservative management. Taiwanese Journal of Obstetrics & Gynecology 2008;47(2):192‐6. [DOI] [PubMed] [Google Scholar]
Bozhinova 2007 {published data only}
- Bozhinova S. Is it already time to legalize the usage of cytotec (misoprostol) in the obstetrics' practice?. Akusherstvo i Ginekologiia 2007;46(9):56‐61. [PubMed] [Google Scholar]
Bricker 2008 {published data only}
- Bricker L, Peden H, Alfirevic Z. The PROMMIS trial: a multicentre randomised trial to evaluate a low dose misoprostol regimen for induction of labour in the presence of prelabour rupture of the amniotic membranes [abstract]. Journal of Obstetrics and Gynaecology 2007;27(Suppl 1):S22‐3. [Google Scholar]
- Bricker L, Peden H, Tomlinson AJ, Al‐Hussaini TK, Idama T, Candelier C, et al. Titrated low‐dose vaginal and/or oral misoprostol to induce labour for prelabour membrane rupture: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2008;115(12):1503‐11. [DOI] [PubMed] [Google Scholar]
Delaney 2001 {published data only}
- Delaney T, Crane J, Hutchens D, Fanning C, Young D. Oral misoprostol labor induction in patients with a favorable cervix. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S202. [Google Scholar]
Hassan 2005 {published data only}
- Hassan AA. A comparison of oral misoprostol tablets and vaginal prostaglandin E2 pessary in induction of labour at term. Journal of the College of Physicians & Surgeons Pakistan (JCPSP) 2005;15(5):284‐7. [PubMed] [Google Scholar]
Ho 2010 {published data only}
- Ho M, Cheng SY, Li TC. Titrated oral misoprostol solution compared with intravenous oxytocin for labor augmentation: a randomized controlled trial. Obstetrics & Gynecology 2010;116(3):612‐8. [DOI] [PubMed] [Google Scholar]
Kadanali 1996 {published data only}
- Kadanali S, Kucukozkan T, Zor N, Kumptepe Y. Comparison of labor induction with misoprostol versus oxytocin/prostaglandin E2 in term pregnancy. International Journal of Gynecology & Obstetrics 1996;55:99‐104. [DOI] [PubMed] [Google Scholar]
Neto 1988 {published data only}
- Neto CM, Delbin AL, Do Val Junior R. Tocographic pattern caused by misoprostol [Padrao tocografico desencadeado pelo misoprostol]. Revista Paulista de Medicina 1988;106(4):205‐8. [PubMed] [Google Scholar]
Rasheed 2007 (V50) {published data only}
- Rasheed R, Alam AA, Younus S, Raza F. Oral versus vaginal misoprostol for labour induction. JPMA ‐ Journal of the Pakistan Medical Association 2007;57(8):404‐7. [PubMed] [Google Scholar]
Robinson 2011 {published data only}
- Robinson D. Efficacy and safety of titrated oral misoprostol solution for labor induction at term. http://clinicaltrials.gov/ct2/show/record/NCT01070472 (accessed 22 January 2013) 2011.
Thigpen 2004 {published data only}
- Thigpen B, Bofill J, Bufkin L, Woodring T, Moore L, Morrison J. A randomized controlled trial comparing vaginal misoprostol to cervical foley plus oral misoprostol for cervical ripening and labor induction [abstract]. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S18. [Google Scholar]
Windrim 1997 {published data only}
- Windrim R, Bennett K, Mundle W, Young DC. Oral administration of misoprostol for labor induction: a randomized controlled trial. Obstetrics & Gynecology 1997;89:392‐7. [DOI] [PubMed] [Google Scholar]
Zvandasara 2008 {published data only}
- Zvandasara P, Saungweme G, Mlambo J, Chidembo W, Madzivanzira N, Mwanjira C. Induction of labour with titrated oral misoprostol suspension. A comparative study with vaginal misoprostol. Central African Journal of Medicine 2008;54(9‐12):43‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Atkinson 2000 {published data only}
- Atkinson MW, Van Kessel, Benedetti T. The use of low dose oral misoprostol to induce labour in the third trimester. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S129. [Google Scholar]
Bonebrake 2001 {published data only}
- Bonebrake R, Haag T, Fleming A, Temp M, Haynatzki G. Vaginal misoprostol is more effective with fewer side effects than oral misoprostol for cervical ripening and induction of labor [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S204. [Google Scholar]
Butler 2004 {published data only}
- Butler B, Crane J, Delaney T. Induction of labour with misoprostol in women at term with an unfavorable cervix: a randomized comparison of oral and vaginal administration. American Journal of Obstetrics and Gynecology 2004;191(6 Suppl 1):S190. [Google Scholar]
Getgan 2003 {published data only}
- Getgan M, Paisarntantiwong R, Sripramote M. A randomized comparison between 50 micrograms orally and misoprostol 25 micrograms vaginally for cervical ripening and induction of labor. Thai Journal of Obstetrics and Gynaecology 2003;15(4):276. [Google Scholar]
Goedken 2000 {published data only}
- Goedken J, Poehlmann S, Paul M. A blinded randomized controlled trial of misoprostol, dinoprostone, and oxytocin for labor induction. Obstetrics & Gynecology 2000;95(4 Suppl):73S. [Google Scholar]
Madhavi 2011 {published data only}
- Madhavi N, Jahan A. Prospective randomised comparative study of labour with misoprostol vs oxytocin in pre labour rupture of membranes. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:106.
Niroomanesh 2011 {published data only}
- Niroomanesh S, Dadashaliha M, Akrami M. Titrated oral misoprostol solution compared with oxytocin for induction of labor in women with unfavorable cervix. Tehran University Medical Journal 2011;69(7):413‐9. [Google Scholar]
Pearson 2002 {published data only}
- Pearson M, Hollier L, Shah A, Yeomans E. A randomized comparison of oral misoprostol versus intravenous oxytocin for induction of labor with term premature rupture of membranes. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S174. [Google Scholar]
Saldivar 2001 {published data only}
- Saldivar D, Triana H, Soria A, Guzman A, Cabero L, Farran I, et al. Oral misoprostol versus intracervical dinoprostone for induction of labour in women with an unfavourable cervix [Misoprostol oral vs dinoprostona intracervical en la induccion del trabajo en pacientes con cervix desfavorable]. Journal of Perinatal Medicine 2001;29 Suppl 1(Pt 1):293. [Google Scholar]
Tuipae 1999 {published data only}
- Tuipae S, Khooarmornpattana S. Effectiveness of oral misoprostol for cervical priming in term pre‐labor rupture of membranes (PROM). Thai Journal of Obstetrics and Gynaecology 1999;11(4):276. [Google Scholar]
Vijitrawiwat 2003 {published data only}
- Vijitrawiwat A, Pongsatha S. A comparison between oral misoprostol 100 micrograms every 3 hours and vaginal misoprostol 50 micrograms every 4 hours for labor induction. Thai Journal of Obstetrics and Gynaecology 2003;15(4):285. [Google Scholar]
Yazdani 2012 {published data only}
- Yazdani SH, Bouzari Z, Farahi S, Tabary AM. Oral misoprostol with oxytocin versus oxytocin alone for labor induction in pre‐labor rupture of membranes (PROM) at term pregnancy. Journal of Babol University of Medical Sciences 2012;14(3):7‐12. [Google Scholar]
Young 2001 {published data only}
- Young D, Delaney T, Armson T, Fanning C. Lower dose vaginal and oral misoprostol in labor induction ‐ rct. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S203. [Google Scholar]
References to ongoing studies
DebBarma 2013 {published data only}
- DebBarma AM. A comparative study of misoprostol oral versus vaginal route for induction of labour. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4251 (accessed 22 January 2013).
Gherman 2002 {published data only}
- Gherman R. A randomized double‐blind comparison of oral misoprostol dosing regimens for cervical ripening. Obstetrics & Gynecology 2002;99(4 Suppl):47S. [Google Scholar]
Pranuthi 2011 {published data only}
- Pranuthi R, Padmaja A, Padmaja P. Comparison of oral misoprostol with vaginal misoprostol for induction of labour. 54th All India Congress of Obstetrics and Gynaecology; 2011 January 5‐9; Hyderabad, Andhra Pradesh, India. 2011:118.
Additional references
Abdel‐Aleem 2003
- Abdel‐Aleem H, Villar J, Gulmezoglu AM, Mostafa SA, Youssef AA, Shokry M, et al. The pharmacokinetics of the prostaglandin E1 analogue misoprostol in plasma and colostrum after postpartum oral administration. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;108(1):25‐8. [DOI] [PubMed] [Google Scholar]
Alfirevic 2009
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003246.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aslan 2004
- Aslan H, Unlu E, Agar M, Ceylan Y. Uterine rupture associated with misoprostol labor induction in women with previous cesarean delivery. European Journal of Obstetrics & Gynecology and Reproductive Biology 2004;113:45‐8. [DOI] [PubMed] [Google Scholar]
Bimbashi 2012
- Bimbashi A, Duley L, Ndoni E, Dokle A. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2012, issue 4. [DOI: 10.1002/14651858.CD009821; CD009821] [DOI]
Boulvain 2005
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Costa 1993
- Costa SH, Vessey MP. Misoprostol and illegal abortion in Rio de Janeiro, Brazil. Lancet 1993;341:1258‐61. [DOI] [PubMed] [Google Scholar]
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
Fonseca 1991
- Fonseca W, Alencar AJC, Mota FSB, Coelho HLL. Misoprostol and congential malformation. Lancet 1991;338:56. [DOI] [PubMed] [Google Scholar]
French 2001
- French L. Oral prostaglandin E2 for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garris 1989
- Garris RE, Kirkwood CF. Misoprostol: a prostaglandin E1 analogue. Clinical Pharmacology 1989;8:627‐44. [PubMed] [Google Scholar]
Gherman 2001a
- Gherman RB, Heath T. Trial of labor after cesarean delivery: a pilot study of oral misoprostol for preinduction cervical ripening. Obstetrics & Gynecology 2001;97(Suppl 1):S68. [Google Scholar]
Gonzales 1999
- Gonzales CH, Marques Dias MJ. Congenital malformation in children exposed to misoprostol in utero. Frontiers in Fetal Health 1999;1(1):15. [Google Scholar]
Hapangama 2009
- Hapangama D, Neilson JP. Mifepristone for induction of labour. Cochrane Database of Systematic Reviews 2009, issue 3. [DOI: 10.1002/14651858.CD002865.pub2] [DOI] [PMC free article] [PubMed]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2003
- Hofmeyr GJ, Gülmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD000941] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD000941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howarth 2001
- Howarth GR, Botha DJ. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2001
- Hutton E, Mozurkewich E. Extra‐amniotic prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jozwiak 2012
- Jozwiak M, Bloemenkamp KWM, Kelly AJ, Mol BWJ, Irion O, Boulvain M. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD001233.pub2] [DOI] [PubMed] [Google Scholar]
Kavanagh 2001
- Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2005
- Kavanagh J, Kelly AJ, Thomas J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003392.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006a
- Kavanagh J, Kelly AJ, Thomas J. Corticosteroids for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003100.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006b
- Kavanagh J, Kelly AJ, Thomas J. Hyaluronidase for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003097.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Keirse 1989
- Keirse MJNC, Chalmers I. Methods for inducing labour. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:1057‐79. [Google Scholar]
Keirse 1993
- Keirse MJNC. Prostaglandins in preinduction cervical ripening: meta‐analysis of worldwide clinical experience. Journal of Reproductive Medicine 1993;38:89‐98. [PubMed] [Google Scholar]
Kelly 2009
- Kelly AJ, Malik S, Smith L, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003101.pub2] [DOI] [PubMed] [Google Scholar]
Kelly 2011
- Kelly AJ, Munson C, Minden L. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD006901.pub2] [DOI] [PubMed] [Google Scholar]
Kelly 2013
- Kelly AJ, Kavanagh J, Thomas J. Relaxin for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2013a
- Kelly AJ, Kavanagh J, Thomas J. Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003099.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kundodyiwa 2009
- Kundodyiwa TW, Alfirevic Z, Weeks AD. Low‐dose oral misoprostol for induction of labor: a systematic review.. Obstet Gynecol 2009;113 (2, Pt 1):374‐383. [DOI] [PubMed] [Google Scholar]
Luckas 2000
- Luckas M, Bricker L. Intravenous prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matonhodze 2005
- Matonhodze BB. Induction of labour in an under‐resourced environment. Johannesburg, South Africa: University of Witwatersrand, 2005. [Google Scholar]
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishi 2013
- Nishi D, Shirakawa MN, Ota E, Hanada N, Mori R. Hypnosis for induction of labour. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010852] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pastuszak 1998
- Pastuszak AL, Schuler L, Speck‐Martins CE, Coelho KFA, Cordello S, Vargas F, et al. Use of misoprostol during pregnancy and Mobious' syndrome in infants. New England Journal of Medicine 1998;338:1881‐5. [DOI] [PubMed] [Google Scholar]
Philip 2002
- Philip NM, Shannon C, Winikoff B. Misoprostol and teratogenicity: reviewing the evidence. Critical Issues in Reproductive Health. New York: Population Council, 2002. [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Smith 2003
- Smith CA. Homoeopathy for induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003399] [DOI] [PubMed] [Google Scholar]
Smith 2013
- Smith CA, Crowther CA, Grant SJ. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD002962.pub3] [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas J, Kelly AJ, Kavanagh J. Oestrogens alone or with amniotomy for cervical ripening or induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003393] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weeks 2005
- Weeks AD, Fiala C, Safar P. Misoprostol and the debate over off‐label drug use. BJOG: an international journal of obstetrics and gynaecology 2005;112:269‐72. [DOI] [PubMed] [Google Scholar]
WHO 2011
- WHO. WHO Recommendations for Induction of Labour. Geneva: World Health Organization, 2011. [PubMed] [Google Scholar]
References to other published versions of this review
Alfirevic 2001
- Alfirevic Z. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001338] [DOI] [PubMed] [Google Scholar]
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]


