Abstract
Background
Acute rhinosinusitis is an acute infection of the nasal passages and paranasal sinuses that lasts less than four weeks. Diagnosis of acute rhinosinusitis is generally based on clinical signs and symptoms in ambulatory care settings. Technical investigations are not routinely performed, nor are they recommended in most countries. Some trials show a trend in favour of antibiotics, but the balance of benefit versus harm is unclear.
We merged two Cochrane Reviews for this update, which comprised different approaches with overlapping populations, resulting in different conclusions. For this review update, we maintained the distinction between populations diagnosed by clinical signs and symptoms, or imaging.
Objectives
To assess the effects of antibiotics versus placebo or no treatment in adults with acute rhinosinusitis in ambulatory care settings.
Search methods
We searched CENTRAL (2017, Issue 12), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1950 to January 2018), Embase (January 1974 to January 2018), and two trials registers (January 2018). We also checked references from identified trials, systematic reviews, and relevant guidelines.
Selection criteria
Randomised controlled trials of antibiotics versus placebo or no treatment in people with rhinosinusitis‐like signs or symptoms or sinusitis confirmed by imaging.
Data collection and analysis
Two review authors independently extracted data about cure and side effects and assessed the risk of bias. We contacted trial authors for additional information as required.
Main results
We included 15 trials involving 3057 participants. Of the 15 included trials, 10 appeared in our 2012 review, and five (631 participants) are legacy trials from merging two reviews. No new studies were included from searches for this update. Overall, risk of bias was low. Without antibiotics, 46% of participants with rhinosinusitis, whether or not confirmed by radiography, were cured after 1 week and 64% after 14 days. Antibiotics can shorten time to cure, but only 5 to 11 more people per 100 will be cured faster if they receive antibiotics instead of placebo or no treatment: clinical diagnosis (odds ratio (OR) 1.25, 95% confidence interval (CI) 1.02 to 1.54; number needed to treat for an additional beneficial outcome (NNTB) 19, 95% CI 10 to 205; I² = 0%; 8 trials; high‐quality evidence) and diagnosis confirmed by radiography (OR 1.57, 95% CI 1.03 to 2.39; NNTB 10, 95% CI 5 to 136; I² = 0%; 3 trials; moderate‐quality evidence). Cure rates with antibiotics were higher when a fluid level or total opacification in any sinus was found on computed tomography (OR 4.89, 95% CI 1.75 to 13.72; NNTB 4, 95% CI 2 to 15; 1 trial; moderate‐quality evidence). Purulent secretion resolved faster with antibiotics (OR 1.58, 95% CI 1.13 to 2.22; NNTB 10, 95% CI 6 to 35; I² = 0%; 3 trials; high‐quality evidence). However, 13 more people experienced side effects with antibiotics compared to placebo or no treatment (OR 2.21, 95% CI 1.74 to 2.82; number needed to treat for an additional harmful outcome (NNTH) 8, 95% CI 6 to 12; I² = 16%; 10 trials; high‐quality evidence). Five fewer people per 100 will experience clinical failure if they receive antibiotics instead of placebo or no treatment (Peto OR 0.48, 95% CI 0.36 to 0.63; NNTH 19, 95% CI 15 to 27; I² = 21%; 12 trials; high‐quality evidence). A disease‐related complication (brain abscess) occurred in one participant (of 3057) one week after receiving open antibiotic therapy (clinical failure, control group).
Authors' conclusions
The potential benefit of antibiotics to treat acute rhinosinusitis diagnosed either clinically (low risk of bias, high‐quality evidence) or confirmed by imaging (low to unclear risk of bias, moderate‐quality evidence) is marginal and needs to be seen in the context of the risk of adverse effects. Considering antibiotic resistance, and the very low incidence of serious complications, we conclude there is no place for antibiotics for people with uncomplicated acute rhinosinusitis. We could not draw conclusions about children, people with suppressed immune systems, and those with severe sinusitis, because these populations were not included in the available trials.
Plain language summary
Antibiotics for sinus infection of short duration in adults
Review question
Do antibiotics cure sinus infection faster than no antibiotics in adults?
Background
A sinus is a cavity situated in the head. Adults with short‐duration sinus infection experience stuffy nose and thick, yellow discharge from the nose. People with sinus infection can feel slime in the back of the throat, facial pain, pain when bending forward, and pain in the upper teeth or when chewing. A short‐duration sinus infection may be suspected following physical examination and questions about symptoms. Blood examination or images of the sinuses can support diagnosis, but are not routinely recommended in most countries. Short‐duration sinus infections are mostly caused by viruses. Nevertheless, physicians tend to prescribe antibiotics, which should only be used to treat bacterial infections. Taking antibiotics unnecessarily results in antibiotic resistance against bacterial infections. We investigated whether antibiotics cure adults with short‐duration sinus infection faster than a dummy drug (placebo) or no treatment.
Search date
18 January 2018.
Study characteristics
We included 15 studies in which adults with short‐duration sinus infection, whether or not confirmed by imaging, randomly received antibiotics, or a dummy drug or no treatment, in ambulatory care settings. The studies included a total of 3057 adults whose average age was 36 years; about 60% were female. Participants were followed until they were cured. Trial duration ranged from 8 to 28 days.
Study funding sources
Seven studies received financial support from government or academic institutions; six received grants from the pharmaceutical industry; and five did not state sources of support.
Key results
Without antibiotics, almost half of all participants were cured after one week, and two out of three were cured after 14 days. Five (diagnosis based on symptoms described to a doctor) to 11 (diagnosis confirmed by x‐ray) more people per 100 were cured faster with antibiotics. A computed tomography (CT) scan could better predict who would benefit from antibiotics, but routine use would cause health problems related to radiation exposure. Ten more people per 100 were relieved faster of thick, yellow discharge from the nose with antibiotics compared to a dummy drug or no treatment. Thirteen more people per 100 experienced side effects (mostly concerning stomach or intestines) with antibiotics compared to a dummy drug or no treatment. Compared with people who initially started antibiotics, five more people per 100 in the dummy drug or no treatment group had to start antibiotics because their condition worsened. Serious complications (e.g. brain abscess) were rare.
We found that antibiotics are not a first‐choice treatment for adults with short‐duration sinus infection. We found no evidence relating to adults with severe sinusitis or with reduced immunity, or to children.
Quality of evidence
We found high‐quality evidence when the diagnosis was based on symptoms described to a doctor. We downgraded evidence quality to moderate when diagnosis was confirmed by x‐ray or CT scan because the number of participants was small, which makes the estimates less reliable.
Summary of findings
Summary of findings for the main comparison. Antibiotics compared to placebo for acute rhinosinusitis in adults.
| Antbiotics compared to placebo for acute rhinosinusitis in adults | ||||||
| Patient or population: acute rhinosinusitis in adults, whether clinically diagnosed or confirmed by imaging Settings: general practice (11 studies), otolaryngology outpatient clinics of university hospitals (2 studies), medical centre (1 study), unknown (2 studies) Intervention: antibiotics Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Antibiotics | |||||
| Cure in adults with clinically diagnosed acute rhinosinusitis | 55 per 100 | 60 per 100 (56 to 65) | OR 1.25 (1.02 to 1.54) | 1687 (8 studies) | ⊕⊕⊕⊕ High | Combination of sinusitis‐like symptoms. Most frequently used clinical symptoms: nasal discharge, facial pain, and common cold or upper respiratory tract infection. NNTB 19 (95% CI 10 to 205) |
| Cure in adults with acute rhinosinusitis confirmed by radiography1 | 51 per 100 |
62 per 100 (52 to 72) |
OR 1.57 (1.03 to 2.39) |
394 (3 studies) |
⊕⊕⊕⊝ Moderate2, 3 |
Clinical suspicion + radiography, using various criteria:
NNTB 10 (95% CI 5 to 136) |
| Cure in adults with acute rhinosinusitis confirmed by computed tomography1,4 | 11 per 100 |
39 per 100 (18 to 64) |
OR 4.89 (1.75 to 13.72) |
127 (1 study) |
⊕⊕⊕⊝ Moderate5 | Clinical suspicion + computed tomography, using as a criterion presence of fluid level or total opacification in any sinus NNTB 4 (95% CI 2 to 15) |
| Severity or duration of different clinical symptoms: resolution of purulent secretion | 60 per 100 | 70 per 100 (63 to 77) | OR 1.58 (1.13 to 2.22) | 660 (3 studies) | ⊕⊕⊕⊕ High | NNTB 10 (95% CI 6 to 35) |
| Side effects: total | 15 per 100 | 28 per 100 (24 to 33) |
OR 2.21 (1.74 to 2.82) |
1816 (10 studies) | ⊕⊕⊕⊕ High | NNTH 8 (95% CI 6 to 12) |
| Side effects: diarrhoea | 10 per 100 | 18 per 100 (13 to 24) |
Peto OR 2.00 (1.41 to 2.85) |
1210 (7 studies) | ⊕⊕⊕⊕ High | NNTH 13 (95% CI 8 to 29) |
| Clinical failure | 11 per 100 | 6 per 100 (4 to 8) |
Peto OR 0.48 (0.36 to 0.63) |
2603 (12 studies) | ⊕⊕⊕⊕ High | NNTH 19 (95% CI 15 to 27) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Only outcomes that could be pooled were presented.
1High heterogeneity (I² = 41%) for the outcome of cure in adults with acute rhinosinusitis confirmed by imaging led us to split the outcome into cure in adults with acute rhinosinusitis confirmed by radiography and cure in adults with acute rhinosinusitis confirmed by computed tomography. 2There was a high risk of blinding bias in Axelsson 1970. Blinding was not reported and was probably not applied. Placebo group participants did not receive tablets, only nose drops. It was not possible to blind sinus irrigation as an intervention. Only group 2 participants underwent radiological evaluation every second day. Group 3 participants received a longer course of tablets than group 4. For this reason, we downgraded the quality of evidence from high to moderate. Omitting this trial from the meta‐analysis did not substantially change the overall result; therefore, we did not downgrade the quality of the evidence further. 3Three trials reported cure in adults with acute rhinosinusitis confirmed by radiography, and confidence intervals were wide. We downgraded the quality of the evidence to moderate. 4Lindbaek 1996 and Lindbaek 1998 presented study results from two distinct groups (those with fluid level or total opacification in any sinus on computed tomography and those with only mucosal thickening on computed tomography, respectively). Consequently, the results of the two trials were very different (I² = 84%). We opted to report only the results from Lindbaek 1996 because the beneficial effect of antibiotics was clearly present only in this subgroup. 5Only one trial (N = 127) reported on cure in adults with acute rhinosinusitis confirmed by computed tomography. We downgraded the quality of the evidence to moderate because of the low number of participants despite this being a well‐conducted trial.
Background
We merged two Cochrane Reviews for this update (De Sutter 2012; Ahovuo‐Saloranta 2014).
Description of the condition
Acute rhinosinusitis is defined as an acute infection of the nasal passages and the paranasal sinuses lasting fewer than four weeks (Ah‐See 2007; Lanza 1997). It is one of the most common diagnoses made in ambulatory care and continues to be a clinical challenge (Blackwell 2014; Lethbridge‐Cejku 2006; McCaig 1995; Okkes 2005; Schappert 1998; Willet 1994). Although guidelines have long recommended restricted use of antibiotics for rhinosinusitis, antibiotics continue to be prescribed for 67% to 100% of people with suspected acute rhinosinusitis (Gulliford 2014 (UK); Rún 2015 (Denmark, Iceland); Fleming‐Dutra 2016 (USA)).
Rhinosinusitis is a more exact term than sinusitis since it takes into account that inflammation of the sinuses is unlikely to occur without inflammation of the mucous membranes of the nose. In this review, the term 'sinusitis' was used when inflammation of a specific sinus (confirmed by radiology or ultrasound) was mentioned (e.g. maxillary sinusitis). Sinusitis was often used in older studies when referring to rhinosinusitis.
Typical signs and symptoms of acute rhinosinusitis include purulent nasal discharge, postnasal drip, sinus pain at palpation, unilateral facial pain, and maxillary toothache (Autio 2015; Axelsson 1972; Williams 1993). However, there is no convincing evidence that people with these clinical findings would benefit from antibiotic treatment (Young 2008). Bacterial infections can also be self limiting. Imaging investigations, such as x‐ray and computed tomography (CT), have been used to demonstrate fluid in the sinuses (air‐fluid level or total opacity). Sinus ultrasound has also been used for this purpose in Scandinavia (Varonen 2000). However, radiological methods cause radiation, are not readily available in ambulatory care settings, and cannot differentiate between viral and bacterial infections. Rhinosinusitis could be confirmed by sinus puncture (Lindbaek 2002), but this is not a feasible ambulatory care method. Acute rhinosinusitis remains a clinical diagnosis with a non‐specific clinical picture.
Description of the intervention
We investigated the effectiveness of antibiotics versus placebo or no treatment in adults with acute rhinosinusitis, whether diagnosed clinically or by imaging.
Two previous Cochrane Reviews ('Antibiotics for acute maxillary sinusitis in adults' and 'Antibiotics for clinically diagnosed acute rhinosinusitis in adults') described the effect of antibiotics for acute rhinosinusitis (Ahovuo‐Saloranta 2014; De Sutter 2012). The reviews studied the same condition but looked at different populations: people diagnosed by imaging versus people diagnosed clinically according to their signs and symptoms (Ahovuo‐Saloranta 2014; De Sutter 2012). As different approaches resulted in different conclusions, we therefore merged these reviews while maintaining the relevant distinction between the two populations. We omitted comparison between antibiotics, as assessed by Ahovuo‐Saloranta 2014. Rather than clinical trials, local up‐to‐date antibiotic resistance patterns should guide clinicians in making the best choice of a particular antibiotic and dose in the subgroup of people with suspected bacterial rhinosinusitis.
Two other Cochrane Reviews focused on antibiotic treatment for people with acute infections of the nose, sinuses, or both (Kenealy 2013; Morris 2002). Kenealy and colleagues looked at the effect of antibiotics in people with symptoms of acute upper respiratory tract infection lasting less than seven days, or acute purulent rhinitis of less than 10 days duration (Kenealy 2013). The authors concluded that there was insufficient evidence to warrant the use of antibiotics for common cold or for persisting acute purulent rhinitis in children or adults (Kenealy 2013). Morris and colleagues considered antibiotic treatment in children with persistent nasal discharge (Morris 2002). The authors concluded that antibiotics have some benefit in the short and medium term in children with purulent rhinorrhoea for more than 10 days, or in older children with radiologically confirmed rhinosinusitis (Morris 2002).
How the intervention might work
Acute rhinosinusitis can be caused by viral or bacterial infections. Acute viral rhinosinusitis is a viral upper respiratory tract infection (or common cold) which, in most cases, also involves the sinuses. Gwaltney 1994 showed that 87% of people with a common cold also have sinus abnormalities on CT scan. Antibiotics are unnecessary in viral rhinosinusitis (Hickner 2001), and people prescribed an antibiotic can develop bacterial resistance to that antibiotic (Costelloe 2010).
Few people (0.5% to 2%) develop bacterial rhinosinusitis (Berg 1986; Gwaltney 1996). Antibiotics may be indicated for bacterial rhinosinusitis to speed up recovery or to prevent suppurative complications. Identifying people with bacterial rhinosinusitis on a clinical basis is challenging (Ebell 2017; Lindbaek 2002). Bacterial origin may be more likely if symptoms last for more than a week (Gwaltney 2005). Consequently, the notions of 'viral' and 'bacterial' are not very workable in daily practice, and there is a pressing need to identify who would benefit from antibiotics (Lanza 1997).
Why it is important to do this review
Diagnosis of most people with acute rhinosinusitis who present in ambulatory care settings is based on clinical signs and symptoms. In most countries, technical investigations are not routinely performed, nor are they recommended (Brazzelli 2003; Hickner 2001; Low 1997). Except for the Cochrane Reviews that are part of this amalgamation (Ahovuo‐Saloranta 2014; De Sutter 2012), two other previously published Cochrane Reviews did not focus exclusively on adults or people with suspected rhinosinusitis (Kenealy 2013; Morris 2002). Results from those reviews could therefore not indicate if this population should be treated with antibiotics. Individual trials show a trend in favour of antibiotics for this population, but the balance of benefit versus harm is unclear.
Objectives
To assess the effects of antibiotics versus placebo in adults with acute rhinosinusitis in ambulatory care settings.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) comparing antibiotics with placebo or no treatment in participants with rhinosinusitis‐like signs or symptoms, whether confirmed by imaging or not. We considered trials including participants with an upper respiratory tract infection or common cold if most participants had rhinosinusitis‐like symptoms, or if participants with rhinosinusitis‐like symptoms could be analysed separately.
We excluded the following studies.
Trials in which participants were included on the basis of a laboratory investigations such as measurement of C‐reactive protein (CRP) or erythrocyte sedimentation rate (ESR), bacteriological or cytological investigations.
Studies comparing one antibiotic with another and trials comparing antibiotics versus other medications.
Trials in which more than 50% of participants were considered to have a common cold.
Trials in which participants had signs and symptoms for more than 30 days.
Trials in which participants were not randomised, or trials that did not include a placebo arm.
Types of participants
We considered all trials in which adults with acute rhinosinusitis, whether clinically diagnosed or confirmed by imaging, were randomly assigned to treatment with an antibiotic, placebo, or no treatment. The clinical diagnosis of acute rhinosinusitis was based on the presence of clinical signs or symptoms that are associated with the presence of fluid in the sinuses in diagnostic studies or that are mentioned in clinical practice guidelines as indicating rhinosinusitis. These included: started with a common cold or experienced both phases of the illness (i.e. catches a cold, feels better after a few days, then feels worse again), purulent nasal discharge, unilateral maxillary pain, pain in the upper teeth, pain when chewing, postnasal drip, pain on bending forward, and duration of symptoms for more than seven days.
We limited participants to adults (aged 18 years or over); the Cochrane Review by Morris 2002 reviewed studies on children. We limited the duration of symptoms to 30 days or less to exclude participants with subacute or chronic rhinosinusitis, where the infection was probably not the primary cause of inflammation (Bachert 2003).
Types of interventions
We included only RCTs that compared antibiotic therapy versus placebo or no treatment. We included trials that permitted concurrent use of other medications if participants were allowed equal access in both the antibiotic and placebo groups.
Types of outcome measures
Primary outcomes
-
Cure in people with:
clinically diagnosed rhinosinusitis;
rhinosinusitis confirmed by imaging.
Secondary outcomes
Ratings of measures of overall well‐being.
-
Severity or duration of different clinical symptoms:
resolution of purulent secretion;
resolution of pain;
illness duration;
restriction of daily activities.
-
Use of concomitant medications:
analgesics;
nasal decongestants.
Side effects.
Clinical failure.
Serious adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases up to 18 January 2018 for this update:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 12), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, in the Cochrane Library using the strategy in Appendix 1;
MEDLINE via Ovid (from January 1950 to January 2018) using the strategy in Appendix 1; and
Embase via Elsevier (from January 1974 to January 2018) using the strategy in Appendix 2.
We searched the following trials registries on 18 January 2018:
the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch) (Appendix 3); and
ClinicalTrials.gov (clinicaltrials.gov) (Appendix 4).
We did not restrict the results by language or publication status.
Searching other resources
We scrutinised the reference lists of identified trials, systematic reviews, and relevant guidelines for other eligible trials.
Data collection and analysis
Selection of studies
Two review authors (ML, ADS) independently screened titles and abstracts of all studies identified as a result of the search for studies that were potentially eligible for inclusion in the review. We retrieved the full‐text study reports, and two review authors (ML, ADS) independently screened the full texts to identify studies for inclusion, and identify and record reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion or by consulting a third review author (MVD) where necessary. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of excluded studies table (Moher 2009). We did not impose any language restrictions.
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. Two review authors (ML, ADS) extracted the following study characteristics from the included studies.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (ML, ADS) independently extracted outcome data from the included studies. We noted in the Characteristics of included studies table if outcome data were not reported in a usable way. There were no disagreements. One review author (ML) transferred data into the Review Manager 5 file (Review Manager 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (ADS) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (ML, ADS) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Any disagreements were resolved by discussion. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes, where necessary. Where information on risk of bias related to unpublished data or correspondence with a trialist, we planned to note this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol and reported any deviations from it in the Differences between protocol and review section. We ensured that current Cochrane methods were applied.
Measures of treatment effect
We entered outcome data for each study into the data tables in Review Manager 5 to calculate the treatment effects (Review Manager 2014). We used odds ratio for dichotomous outcomes, and mean differences or standardised mean differences for continuous outcomes.
We conducted meta‐analyses only where this was meaningful, that is the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
Unit of analysis issues
In trials with multiple treatment groups, we compared event rates in the antibiotic treatment arms (intervention) with placebo event rates (control). We did not include cluster‐RCTs.
Dealing with missing data
Where numerical outcome data were missing and could not be obtained from the authors, these were calculated from other available statistics, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Assessment of heterogeneity
We assessed variability among studies for statistical heterogeneity using Cochran's test for heterogeneity and the I² statistic. The I² statistic describes the percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error. We considered a value greater than 50% to represent substantial heterogeneity, in which case we used a random‐effects model.
Assessment of reporting biases
We planned to construct funnel plots to assess the likelihood of publication bias if 10 studies or more were available for analysis.
Data synthesis
We pooled data from studies judged to be clinically homogeneous using Review Manager 5 software (Review Manager 2014). If more than one study provided usable data in any single comparison, we performed a meta‐analysis.
GRADE and 'Summary of findings' table
We created Table 1 using the following outcomes.
Cure in adults with clinically diagnosed rhinosinusitis.
Cure in adults with rhinosinusitis confirmed by radiography.
Cure in adults with rhinosinusitis confirmed by CT scan.
Resolution of purulent secretion.
Side effects: general.
Side effects: diarrhoea.
Clinical failure.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b),using GRADEpro GDT software (GRADEpro GDT 2015). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Clinically diagnosed rhinosinusitis.
Rhinosinusitis confirmed by imaging.
We used the Chi² test to test for subgroup interactions using Review Manager 5 software (Review Manager 2014).
Sensitivity analysis
We carried out the following sensitivity analyses.
Excluding studies at higher risk of bias.
Assessing the influence of missing data: adding dropouts as failures, successes or as having the same cure rate as control group.
Adding participants who were 'improved' to those who were cured.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
For the 2018 update, after deleting duplicates, we identified 524 new records from electronic searches. We rejected 354 records on the basis of title or keyword assessment, 52 records after assessing abstracts, and three records following full‐text record assessment. We rejected 115 trials based on information from trials registers (WHO ICTRP or ClinicalTrials.gov). No new studies were added for this update as a result of 2018 searches (Figure 1).
1.
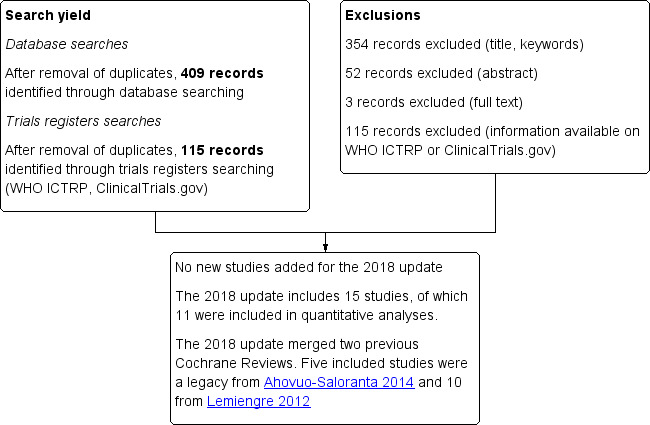
Study flow diagram.
Included studies
Because we merged two Cochrane Reviews (Ahovuo‐Saloranta 2014; De Sutter 2012), we revised search results from De Sutter 2012, and included five studies (631 participants) from Ahovuo‐Saloranta 2014 (Axelsson 1970; Lindbaek 1996; Lindbaek 1998; Rantanen 1973; Van Buchem 1997a; Van Buchem 1997b). (Van Buchem 1997b was a Dutch translation of Van Buchem 1997a; we used data from Van Buchem 1997a). We retained 10 trials (2450 participants) that were included in De Sutter 2012 (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Meltzer 2005; Merenstein 2005; Norrelund 1978; Stalman 1997; Varonen 2003; Williamson 2007).
We included 15 trials involving a total of 3057 participants for this update.
Design
With one exception, all included trials were randomised controlled trials (RCTs) that compared an antibiotic with a placebo. Axelsson 1970 (most probably) compared antibiotic treatment to no treatment.
Sample sizes
Ten trials involving 2450 participants concerned clinically diagnosed rhinosinusitis (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Meltzer 2005; Merenstein 2005; Norrelund 1978; Stalman 1997; Varonen 2003; Williamson 2007).
Five trials involving 631 participants concerned rhinosinusitis confirmed by imaging (radiology investigation: 431 participants (Axelsson 1970; Rantanen 1973; Van Buchem 1997a); CT scan: 200 participants (Lindbaek 1996; Lindbaek 1998)). In addition, Kaiser 2001 identified a subgroup of 82 participants in which rhinosinusitis was confirmed by radiography.
Setting
Eleven trials recruited participants from ambulatory care settings (Bucher 2003; De Sutter 2002; Garbutt 2012; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Norrelund 1978; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007). One trial also enrolled walk‐in and non‐referred participants from otolaryngology outpatient clinics of the university hospital (Bucher 2003). Kaiser 2001 recruited participants from an outpatient clinic of a university hospital. Meltzer 2005 enrolled participants from 14 medical centres worldwide, but settings were not described. Axelsson 1970 and Rantanen 1973 did not describe study settings.
Participants
Inclusion criteria
All included studies used clinical signs and symptoms to enrol participants. The three most common inclusion criteria were nasal discharge (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Meltzer 2005; Merenstein 2005; Norrelund 1978; Stalman 1997; Varonen 2003; Williamson 2007), facial pain (Bucher 2003; Garbutt 2012; Meltzer 2005; Merenstein 2005; Norrelund 1978; Stalman 1997; Varonen 2003; Williamson 2007), and common cold or upper respiratory tract infection (De Sutter 2002; Kaiser 2001; Stalman 1997; Varonen 2003). Two studies included participants with pus in the nasal cavity on rhinoscopy (Bucher 2003; Kaiser 2001), but this symptom was a clinical criterion for inclusion in three trials (Merenstein 2005; Varonen 2003; Williamson 2007).
Five studies used imaging criteria to include participants: confirmed secretion on radiography (Axelsson 1970), homogenous shadows in the sinuses or a fluid level on radiography (Rantanen 1973), more than 5 mm mucosal thickening, opacity or fluid level on radiography (Van Buchem 1997a), presence of fluid level or total opacification in any sinus on CT (Lindbaek 1996), and presence of mucosal thickening without fluid levels or total opacification on CT (Lindbaek 1998). In these trials, participants were preselected on clinical suspicion of having sinusitis. No further details about the clinical criteria used to select participants were provided. Kaiser 2001 used the presence, in at least one sinus, of an air‐fluid level, a complete opacity, or a mucosal thickening of 10 mm as a criterion to identify participants for their subgroup of participants with radiologically confirmed maxillary sinusitis.
Exclusion criteria
Common exclusion criteria were recent antibiotic use (Axelsson 1970; Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Rantanen 1973; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007), severe illness (Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Stalman 1997), symptoms of complicated rhinosinusitis (De Sutter 2002; Garbutt 2012; Varonen 2003), long‐lasting symptoms before inclusion (Bucher 2003; De Sutter 2002; Lindbaek 1996; Lindbaek 1998; Stalman 1997; Van Buchem 1997a; Varonen 2003), chronic ear, nose, and throat disease (Bucher 2003; Kaiser 2001; Meltzer 2005; Van Buchem 1997a; Varonen 2003; Williamson 2007), comorbidity (De Sutter 2002; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Stalman 1997; Van Buchem 1997a; Williamson 2007), previous sinus surgery (Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Varonen 2003), immune deficiency (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Merenstein 2005), allergy for study medication (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Meltzer 2005; Merenstein 2005; Norrelund 1978; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007), pregnancy or lactation (Bucher 2003; De Sutter 2002; Norrelund 1978; Stalman 1997; Varonen 2003; Williamson 2007), and inability to follow the protocol (language or mental problems) (Bucher 2003; De Sutter 2002; Garbutt 2012; Stalman 1997; Van Buchem 1997a). Due to the occurrence of a brain abscess in a placebo group participant, after 2000, Bucher 2003 excluded people with CRP levels greater than 100 mg/L or between 50 and 99 mg/L as a safety measure is there was clinical deterioration or CRP increase greater than 100 mg/L within three days of inclusion. No participants had to be excluded due to this new exclusion criterion. Axelsson 1970 excluded participants who were recently treated with nasal decongestants. Kaiser 2001 excluded participants with a positive pharyngeal culture for Streptococcus pyogenes. Lindbaek and colleagues excluded participants who misused alcohol or narcotics and those who had rheumatic disease (Lindbaek 1996; Lindbaek 1998). Garbutt 2012 excluded participants who rated their symptoms as very mild or mild. Stalman 1997 excluded participants who used xylometazoline nose drops for more than seven days, received antacid or iron treatment, or were referred to an ear, nose, and throat specialist.
Characteristics of the participants
The average age of participants was approximately 36 years. Norrelund 1978 did not report participants' mean age, but we calculated a median age of between 30 and 39 years. Axelsson 1970, Lindbaek 1996, and Lindbaek 1998 permitted younger participants, but the mean age of the study population was comparable to the average (33 years in Axelsson 1970, 38.6 years in Lindbaek 1996, and 39.7 years in Lindbaek 1998).
The male‐to‐female ratio was about 5:8.
The mean duration of symptoms before inclusion was around or at least seven days in seven trials (De Sutter 2002; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Stalman 1997; Williamson 2007). Participants had symptoms for about four to five days before inclusion in two trials (Bucher 2003; Kaiser 2001). The mean duration of symptoms at baseline was longer in two studies (11 days in Garbutt 2012 and 15.4 days in Van Buchem 1997a). Axelsson 1970, Norrelund 1978, Rantanen 1973, and Varonen 2003 did not report the mean duration of symptoms before inclusion.
Interventions
Treatment group
Nine studies compared amoxicillin to placebo (De Sutter 2002; Garbutt 2012; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Van Buchem 1997a; Varonen 2003; Williamson 2007). Of these nine studies, five had more than one treatment arm, and three compared several antibiotic courses to placebo (penicillin V and amoxicillin (Lindbaek 1996; Lindbaek 1998); amoxicillin, penicillin V, and doxycycline (Varonen 2003)).
Two studies compared an antibiotic course and/or corticosteroid spray to placebo (Meltzer 2005; Williamson 2007) Meltzer 2005 compared mometasone furoate nasal spray once daily only, mometasone furoate nasal spray twice daily only, and amoxicillin only. Williamson 2007 compared budesonide nasal spray only, amoxicillin only, budesonide nasal spray and amoxicillin.
Norrelund 1978 compared pivampicillin to placebo; Kaiser 2001 compared azithromycin to placebo; Rantanen 1973 and Stalman 1997 compared doxycycline to placebo; and Bucher 2003 compared amoxicillin/clavulanic acid to placebo. Axelsson 1970 compared irrigation, phenoxymethylpenicillin and lincomycin to no treatment.
Treatment arms without antibiotic treatment were excluded from analyses (irrigation arm (44 participants) in Axelsson 1970; mometasone furoate nasal spray once daily only (243 participants), mometasone furoate nasal spray twice daily (235 participants) in Meltzer 2005).
All antibiotics were administered orally.
Co‐interventions
Ten studies permitted nasal decongestants and analgesics (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Norrelund 1978; Stalman 1997; Van Buchem 1997a; Varonen 2003). Meltzer 2005 did not permit use of nasal decongestants. Six studies prescribed nasal decongestants for all participants (Axelsson 1970; Bucher 2003; Garbutt 2012; Norrelund 1978; Rantanen 1973; Van Buchem 1997a). Two studies did not describe use of nasal decongestants (Merenstein 2005; Williamson 2007). Four studies did not describe use of analgesics (Axelsson 1970; Merenstein 2005; Rantanen 1973; Williamson 2007). One study prescribed cough syrup (dextromethorphan hydrobromide or guaifenesin) for all participants (Garbutt 2012).
Outcomes
Primary outcome: cure
Definitions of cure and time of evaluation varied among trials that used cure as primary outcome (Axelsson 1970; Bucher 2003; De Sutter 2002; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Norrelund 1978; Rantanen 1973; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007). This was reflected in variations in cure rates in placebo groups (clinical diagnosis: 30% to 74%; diagnosis confirmed by imaging: 11% to 59%).
Clinically diagnosed rhinosinusitis
Eight trials defined cure or improvement as primary outcome (Bucher 2003; De Sutter 2002; Kaiser 2001; Merenstein 2005; Norrelund 1978; Stalman 1997; Varonen 2003; Williamson 2007). The common denominator of all definitions was the resolution or improvement of major symptoms, evaluated only by the participant (Bucher 2003; De Sutter 2002; Merenstein 2005; Varonen 2003; Williamson 2007), or by the participant and the investigator (Kaiser 2001; Norrelund 1978; Stalman 1997).
The two remaining trials used change on a symptom score as the main outcome measure: Garbutt 2012 used the mean change in Sino‐Nasal Outcome Test‐16 score, a validated and responsive measure, to assess the effect of treatment on disease‐specific quality of life at day 3, and Meltzer 2005 used the mean AM/PM major symptom score (sum of scores for rhinorrhoea, postnasal drip, nasal decongestion/stuffiness, sinus headache, and facial pain/pressure/tenderness on palpation over the paranasal sinuses) over days 2 to 15 of the treatment phase as a primary outcome measure.
Rhinosinusitis confirmed by imaging
Six studies defined cure as primary outcome (Axelsson 1970; Kaiser 2001 (subgroup of participants with radiologically confirmed maxillary sinusitis); Lindbaek 1996; Lindbaek 1998; Rantanen 1973; Van Buchem 1997a). The common denominator in all definitions was resolution or improvement of major symptoms, evaluated by the participant alone (Axelsson 1970; Lindbaek 1996; Lindbaek 1998; Van Buchem 1997a), or by the participant and the investigator (Kaiser 2001; Rantanen 1973). However, Rantanen 1973 evaluated sinus recovery rather than participants.
Secondary outcomes
Some trials provided information on effects on purulent secretion (Bucher 2003; De Sutter 2002; Meltzer 2005; Norrelund 1978; Stalman 1997), pain (De Sutter 2002; Meltzer 2005; Stalman 1997; Van Buchem 1997a; Williamson 2007), malaise (De Sutter 2002; Merenstein 2005; Van Buchem 1997a; Williamson 2007), illness duration (Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Norrelund 1978; Varonen 2003; Williamson 2007), restriction of daily activities (Bucher 2003; De Sutter 2002; Garbutt 2012; Stalman 1997;Williamson 2007), intake of analgesics (De Sutter 2002; Norrelund 1978; Stalman 1997; Varonen 2003), intake of nasal decongestants (Stalman 1997; Varonen 2003), side effects (Axelsson 1970; Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Meltzer 2005; Merenstein 2005; Norrelund 1978; Stalman 1997; Van Buchem 1997a; Varonen 2003), clinical failure (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007), and serious adverse events (Bucher 2003; Garbutt 2012; Williamson 2007).
Seven studies collected laboratory samples (Bucher 2003; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Rantanen 1973; Van Buchem 1997a; Varonen 2003). Four studies obtained nasopharyngeal secretions for culture (Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Varonen 2003). Rantanen 1973 performed a sinus puncture. Two studies measured CRP, leukocytes and neutrophils (Bucher 2003; Van Buchem 1997a). Only Kaiser 2001 reported interaction between culture result, cure and treatment group. Kaiser 2001 found that participants in the antibiotic group with positive culture had lower symptom scores (P = 0.002) and a higher rate of symptom resolution on day 7 (73% versus 47%; P = 0.007) and a higher cure rate on day 8 (65% versus 41%; P = 0.032) compared to placebo group participants. There was no significant difference in symptom resolution on day 7 in the culture‐negative group between antibiotic and placebo group participants (63% versus 69%; P = 0.75).
Excluded studies
We had previously excluded five studies. Three RCTs included participants with clinical symptoms of acute rhinosinusitis and specific bacteriological criteria (Gananca 1973; Gananca 1977; Hadley 2010). One excluded study had included participants with clinical symptoms (maxillary pain) and raised values of either C‐reactive protein (CRP) or erythrocyte sedimentation rate (ESR) (Hansen 2000a; Hanssen 2000b was a Danish translation of Hansen 2000a). Haye 1998 was excluded because participants with empyema (defined as complete opacity or an air‐fluid level, or a mucosal thickness of 6 mm or more measured at the upper lateral border of the maxillary sinus) were withheld. See Characteristics of excluded studies.
Risk of bias in included studies
'Risk of bias' assessments are reported in Characteristics of included studies and graphically presented in Figure 2 and Figure 3.
2.
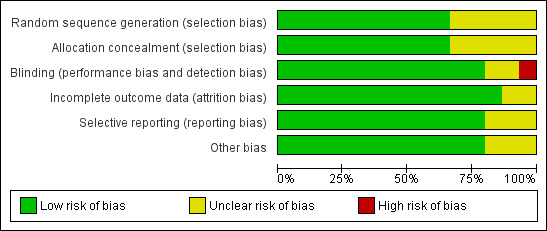
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The risk of selection bias was low in nine studies (Bucher 2003; De Sutter 2002; Garbutt 2012; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Van Buchem 1997a; Williamson 2007), and unclear in four studies (Axelsson 1970; Kaiser 2001; Norrelund 1978; Rantanen 1973; Stalman 1997; Varonen 2003).
Ten studies reported adequate allocation sequencing (Bucher 2003; De Sutter 2002; Garbutt 2012; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Stalman 1997; Van Buchem 1997a; Williamson 2007). Four studies used block randomisation (Garbutt 2012; Merenstein 2005; Stalman 1997; Williamson 2007); two used unrestricted randomisation (De Sutter 2002; Van Buchem 1997a); and four studies combined blocked and stratified randomisation (Bucher 2003; Lindbaek 1996; Lindbaek 1998; Meltzer 2005). Six studies used a computerised random number generator (Bucher 2003; De Sutter 2002; Garbutt 2012; Meltzer 2005; Merenstein 2005; Van Buchem 1997a; Stalman 1997). Lindbaek 1996 and Lindbaek 1998 used dice. Williamson 2007 used random number tables to select the blocks. Four studies presented insufficient information about the sequence generation process to inform assessment (Axelsson 1970; Kaiser 2001; Norrelund 1978; Varonen 2003). Axelsson 1970 and Kaiser 2001 reported only random assignment. Two studies reported using a block randomisation procedure but did not specify the process to select blocks (Norrelund 1978; Varonen 2003).
Ten trials concealed allocation adequately (Bucher 2003; De Sutter 2002; Garbutt 2012; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Van Buchem 1997a; Varonen 2003; Williamson 2007). Four trials did not provide information on methods used to blind participants and investigators enrolling participants (Axelsson 1970; Norrelund 1978; Rantanen 1973; Stalman 1997). One study reported only that the medication boxes or envelopes were identical for drugs and placebo, but did not state use of sequential numbering (Kaiser 2001).
Blinding
The risk of performance and detection bias was low in 12 studies (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007), unclear in 2 studies (Norrelund 1978; Rantanen 1973), and high in 1 study (Axelsson 1970).
Eleven trials blinded allocated intervention adequately (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007). The intervention and placebo tablets were identical in colour, shape, and taste, and blinding of participants and investigators was assured in these studies. Two studies indicated double‐blinding, but did not provide information about the blinding procedure (Norrelund 1978; Rantanen 1973). Meltzer 2005 did not provide precise information on how the randomisation result was concealed, but the method of random sequence generation (computer‐randomised code) and information on double‐dummy design gave the impression that concealment had been fulfilled (additional information requested but not received from the trial authors). Axelsson 1970 did not mention blinding in the methods section, and close examination of the study design led us to strongly believe that there was no blinding: placebo group participants did not take tablets, only nose drops; it is not possible to blind sinus irrigation as an intervention; only group 1 participants received radiological evaluation every second day; and group 2 participants took a longer course of tablets than group 3. We therefore graded the risk of bias for this domain as high for Axelsson 1970.
Incomplete outcome data
The risk of attrition bias was low in 13 studies (Axelsson 1970; Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Norrelund 1978; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007), and unclear in 2 studies (Meltzer 2005; Rantanen 1973).
The overall post‐randomisation dropout rate was 5.1%. Rantanen 1973 did not report post‐randomisation dropout rates.
The ratio of participants with missing data to participants with events is a good marker of bias due to incomplete data (Higgins 2011a). In the 13 included studies with cure as the primary outcome, the ratio ranged from 0.01 to 0.33 (Axelsson 1970; Bucher 2003; De Sutter 2002; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Norrelund 1978; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007). However, the ratio was low (0.09) for Garbutt 2012, who reported "significant improvement at day 10." The risk of bias due to dropout was low in these 13 studies. We could not calculate the ratio of participants with missing data to participants with events for Meltzer 2005, because the primary outcome was not cure, but a difference in symptom scores. However, the post‐randomisation dropout rate was low (2.6% at day 15) in Meltzer 2005 and probably did not cause bias.
Two studies performed sensitivity analyses. In De Sutter 2002 and Williamson 2007, different scenarios did not reveal a significant difference in cure rate between the intervention and control group. Garbutt 2012 conducted a sensitivity analysis for participants who completed the study drug and those with symptoms for seven days or more and 28 days or less. Varonen 2003 imputed dropouts as treatment failures.
Ten studies followed the intention‐to‐treat (ITT) principle for analysis of the main outcome (Bucher 2003; De Sutter 2002; Garbutt 2012; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Stalman 1997; Van Buchem 1997a; Varonen 2003). Four trials included only participants with complete outcome data (Axelsson 1970; Kaiser 2001; Norrelund 1978; Williamson 2007).
Selective reporting
The risk of reporting bias was low in 12 studies (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Rantanen 1973; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007), and unclear in 3 studies (Axelsson 1970; Meltzer 2005; Norrelund 1978).
Twelve studies predefined primary and secondary endpoints (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Rantanen 1973; Stalman 1997; Varonen 2003; Williamson 2007). Definitions of primary outcomes were unclear in two studies (Axelsson 1970; Norrelund 1978). Norrelund 1978 predefined symptoms, side effects, and medication intake that were to be recorded, but provided the definition of cure for the first time in the results section of the report. Meltzer 2005 reported most outcomes of interest incompletely; data could not be pooled with other trials. Young 2008 performed an individual participant data meta‐analysis and had the results of an unpublished Schering‐Plough trial. This trial had the same design as Meltzer 2005, but had a lower odds ratio. This could suggest selective reporting in Meltzer 2005.
Other potential sources of bias
The risk of other potential sources of bias was low in 12 studies (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Merenstein 2005; Norrelund 1978; Stalman 1997; Varonen 2003; Williamson 2007), and unclear in 3 studies (Axelsson 1970; Rantanen 1973; Van Buchem 1997a).
No included studies contained design‐specific risks of bias or were stopped early. Two studies had small, unimportant imbalances of baseline participant characteristics (Stalman 1997; Williamson 2007). Rantanen 1973 did not describe participants' characteristics at baseline, and Axelsson 1970 provided only limited information about these characteristics. One blinded trial broke blinding 12 times due to side effects (3 participants) or clinical failure (9 participants) (Lindbaek 1996). Following the ITT principle, these participants were included in the analyses in the groups to which they were originally randomised (Lindbaek 1996). Van Buchem 1997a possibly selected participants with worse symptoms, since only 20% of participants with possible maxillary sinusitis entered the trial.
Study protocols for participants in intervention and placebo groups were similar. There was a low risk of bias due to increased or different diagnostic activity.
Seven studies were financially supported by government or academic institutions (Garbutt 2012; Lindbaek 1996; Lindbaek 1998; Merenstein 2005; Stalman 1997; Varonen 2003; Williamson 2007). Researchers in six studies received grants from pharmaceutical industry sources (Bucher 2003; De Sutter 2002; Meltzer 2005; Stalman 1997;Varonen 2003; Williamson 2007). Five studies did not state sources of support (Axelsson 1970; Kaiser 2001; Norrelund 1978; Rantanen 1973; Van Buchem 1997a).
More than 276 practices recruited participants. Two trials recruited participants from one site (Kaiser 2001; Merenstein 2005). Eight trials recruited participants from multiple sites, with an average of 9.9 participants per practice (range 3.6 to 15.8, 25th percentile = 6.5, 75th percentile = 15.5) (Bucher 2003; De Sutter 2002; Garbutt 2012; Meltzer 2005; Norrelund 1978; Stalman 1997; Varonen 2003; Williamson 2007). The number of participating practices was not reported in five trials (Axelsson 1970; Lindbaek 1996; Lindbaek 1998; Rantanen 1973; Van Buchem 1997a).
Effects of interventions
See: Table 1
Primary outcome
1. Cure
Without antibiotics, 46% of participants with rhinosinusitis, whether or not confirmed by radiography, were cured after one week (Bucher 2003; Kaiser 2001; Norrelund 1978; Williamson 2007), and 64% after 14 days (Bucher 2003; Merenstein 2005; Van Buchem 1997a; Varonen 2003; Williamson 2007).
1.1 Clinically diagnosed rhinosinusitis
The ITT population included 2450 participants (10 trials). We analysed data from 1687 participants (69%). We excluded Meltzer 2005 (499 participants) and Garbutt 2012 (166 participants) because the proportion of participants cured at a specific time point was not reported as cure was not their main outcome measure. Stalman 1997 reported only the total cure rate for both groups and stated there was no difference between groups. We used the same percentages in both groups for pooling.
Despite choices made by some trial authors, we considered participants who started other antibiotics as treatment failures, not dropouts. The total dropout rate was 5.3%.
Almost half (47%) of participants were cured after one week (Bucher 2003; Kaiser 2001; Norrelund 1978; Williamson 2007), 51% after 10 days (De Sutter 2002; Stalman 1997; Williamson 2007), and 71% after 14 days (Bucher 2003; Merenstein 2005; Varonen 2003; Williamson 2007), irrespective of treatment group.
The estimated odds ratio (OR) for the overall treatment effect of antibiotics relative to placebo was 1.25 (95% confidence interval (CI) 1.02 to 1.54; number needed to treat for an additional beneficial outcome (NNTB) 19, 95% CI 10 to 205; I² = 0%; high‐quality evidence; Analysis 1.1.1; Figure 4).
1.1. Analysis.
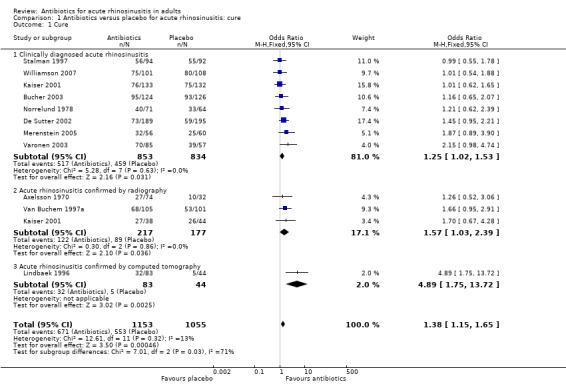
Comparison 1 Antibiotics versus placebo for acute rhinosinusitis: cure, Outcome 1 Cure.
4.
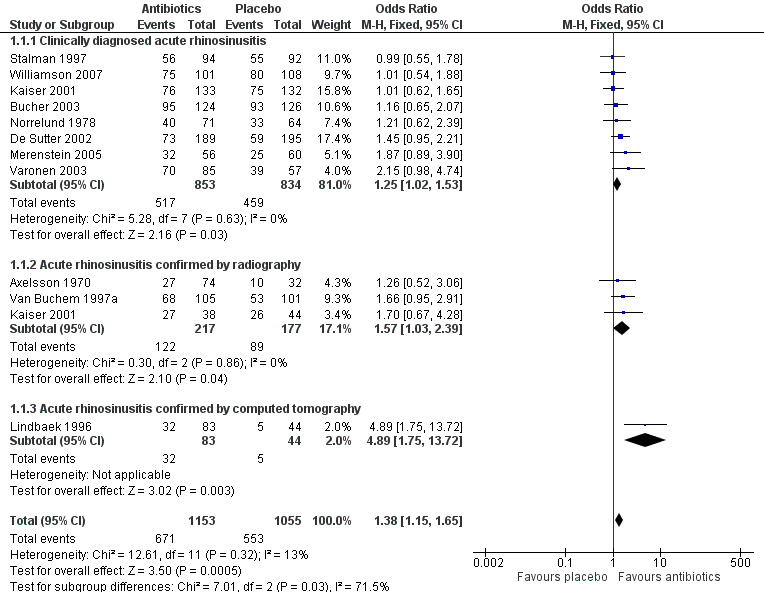
Forest plot of comparison: 1 Antibiotics versus placebo in acute rhinosinusitis, outcome: 1.1 Cure.
We categorised studies into three groups: cure assessed at one week (Bucher 2003; Kaiser 2001; Norrelund 1978; Williamson 2007); cure assessed at around day 10 (De Sutter 2002; Stalman 1997; Williamson 2007); and cure assessed at day 14 (Bucher 2003; Merenstein 2005; Varonen 2003; Williamson 2007). Heterogeneity among studies was very low (0% at 1 week and 10 days; 6% at 14 days).
There were no significant differences between treatment groups: after one week, the OR for cure was 1.07 (95% CI 0.81 to 1.41; Analysis 3.1.1); after 10 days OR for cure was 1.19 (95% CI 0.92 to 1.53; Analysis 3.1.2); and after 14 days OR for cure was 1.37 (95% CI 0.98 to 1.91; Analysis 3.1.3). Meltzer 2005 did not find any difference in symptom score between the antibiotic and placebo groups at day 15, so we assumed that adding data from this study would not change our overall result. Garbutt 2012 found a significant difference in symptom score at day 7, favouring amoxicillin (mean difference (MD) 0.19, 95% CI 0.02 to 0.35). This study also provided data about "significantly improved" participants. Including these data (Analysis 3.1.4 and Analysis 3.1.5) did not substantially change the overall result.
3.1. Analysis.
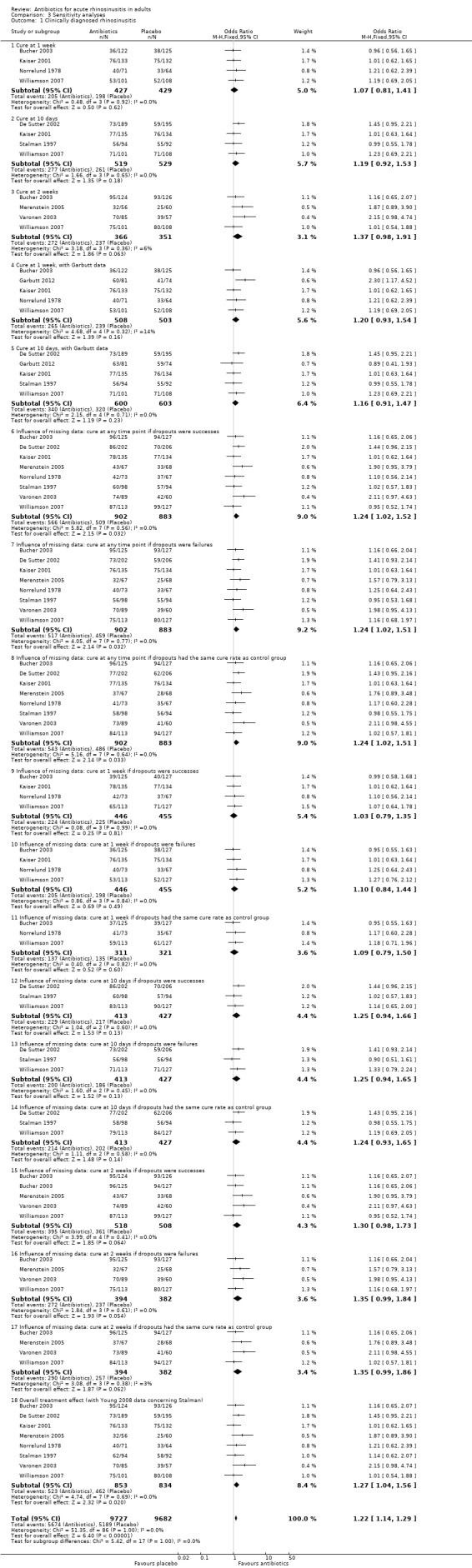
Comparison 3 Sensitivity analyses, Outcome 1 Clinically diagnosed rhinosinusitis.
We used three methods to impute data to assess the influence of missing data on the overall results: assuming the outcomes of participants for whom no outcome was recorded as cured; not cured; or according to the cure rate observed in the control group. Twelve analyses revealed no clear differences for the baseline analyses (Analysis 3.1.6 to Analysis 3.1.17).
Excluding studies that included ITT analyses removed antibiotic benefit (OR 1.06, 95% CI 0.76 to 1.47) (Bucher 2003; De Sutter 2002; Merenstein 2005; Stalman 1997; Varonen 2003). Pooling studies with ITT analyses confirmed benefit of antibiotics (OR 1.39, 95% CI 1.07 to 1.79).
Pooling studies in which participants declared themselves as cured endorsed the benefit of antibiotics (OR 1.40, 95% CI 1.08 to 1.82) (Bucher 2003; De Sutter 2002; Merenstein 2005; Varonen 2003; Williamson 2007). Pooling studies in which the investigator decided if the participant was cured showed no benefit of antibiotics (OR 1.05, 95% CI 0.76 to 1.46) (Kaiser 2001; Norrelund 1978; Stalman 1997).
Studies that included only participants with pus on rhinoscopy revealed no benefits with antibiotics (OR 1.07, 95% CI 0.74 to 1.56) (Bucher 2003; Kaiser 2001).
1.2 Rhinosinusitis confirmed by imaging
The ITT population included 713 participants, and we analysed data from 652 participants (91.4%). We excluded 61 participants from the Rantanen 1973 study because the primary outcome was sinus recovery progress instead of cure.
Four trials included participants on the basis of clinical signs and symptoms (Bucher 2003; De Sutter 2002; Kaiser 2001; Varonen 2003); radiographs were taken, but only one study used images to assess cure rates (Kaiser 2001). All participants underwent sinus ultrasound in Varonen 2003. The impact of ultrasound result cure rates was not reported.
We considered participants who started other antibiotics as treatment failures, not dropouts. The total dropout rate was 4.3%. Rantanen 1973 did not report post‐randomisation dropout rates.
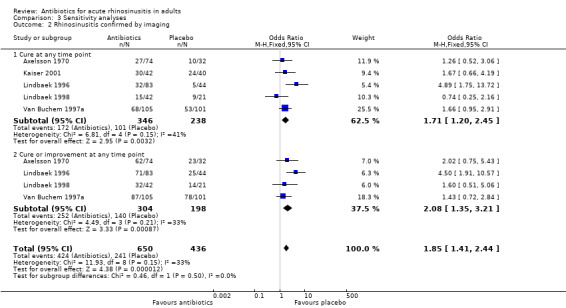
Cure was evaluated at day 8 (Kaiser 2001), day 10 (Axelsson 1970; Lindbaek 1996; Lindbaek 1998), or day 14 (Van Buchem 1997a). The estimated OR for the overall treatment effect of antibiotics relative to placebo was 1.71 (95% CI 1.20 to 2.45; NNTB 8, 95% CI 5 to 23; I² = 41%; Analysis 3.2.1). Heterogeneity was high, so we looked for outliers and split analyses for participants with rhinosinusitis confirmed by radiography or CT.
3.2. Analysis.
Comparison 3 Sensitivity analyses, Outcome 2 Rhinosinusitis confirmed by imaging.
However, treatment effects among those who underwent CT differed significantly from effects in participants selected by radiography. Pooling these data was therefore not possible, and analyses were performed separately.
Three studies used radiography to confirm maxillary sinusitis (Axelsson 1970; Kaiser 2001; Van Buchem 1997a). The estimated OR was 1.57 (95% CI 1.03 to 2.39; NNTB 10, 95% CI 5 to 136; I² = 0%; moderate‐quality evidence; Analysis 1.1.2; Figure 4). Omitting Axelsson 1970, which was assessed as at high risk of bias due to lack of blinding, did not have a significant impact on this result (OR 1.67, 95% CI 1.04 to 2.70, NNBT 9, 95%CI 5 to 104).
Lindbaek 1996 and Lindbaek 1998 reported on two distinct participant groups who underwent CT examination: those with fluid level or total opacification in any sinus on CT, and those with mucosal thickening on CT. The effect of antibiotics relative to placebo was only significant in the group with fluid level or total opacification in any sinus on CT (Lindbaek 1996: estimated OR 4.89, 95% CI 1.75 to 13.72; NNTB 4, 95% CI 2 to 15; moderate‐quality evidence; Analysis 1.1.3; Figure 4; ) (Lindbaek 1998: estimated OR 0.74, 95% CI 0.25 to 2.16).
Four studies reported on cure or improvement (Axelsson 1970; Lindbaek 1996; Lindbaek 1998; Van Buchem 1997a). The estimated OR for overall treatment effect of antibiotics relative to placebo was 2.08 (95% CI 1.35 to 3.21; NNTB 8, 95% CI 6 to 18; I² = 33%; Analysis 3.2.2). Heterogeneity was high. Studies that used radiography to confirm maxillary sinusitis indicated no difference in improvement rates between participants who received antibiotics versus those who received placebo (estimated OR 1.59, 95% CI 0.90 to 2.80; I² = 0%, NNTB 9, 95% CI 5 to 40) (Axelsson 1970; Van Buchem 1997a). The effect of antibiotics relative to placebo on 'improvement' was significant only in participants with fluid level or total opacification in any sinus on CT (Lindbaek 1996: estimated OR 4.5, 95% CI 1.91 to 10.57; NNTB 4, 95% CI 3 to 7) (Lindbaek 1998: estimated OR 1.60, 95% CI 0.51 to 5.06).
Secondary outcomes
1. Ratings of measures of overall well‐being
Three studies investigated whether participants' general feeling of illness improved faster with antibiotics (De Sutter 2002; Merenstein 2005; Van Buchem 1997a). It was not possible to pool data for meta‐analysis because De Sutter 2002 used data from a diary, Merenstein 2005 compared Likert scores at different time points, and Van Buchem 1997a looked at differences in symptom scores for "sickness" after one and two weeks. Only Van Buchem 1997a found a marginal but significant difference in symptom score for sickness after two weeks evaluated by the investigator (mean change 1.2 for placebo versus 0.8 for antibiotics, "P < 0.05" reported by Van Buchem 1997a). This finding did not persist when the degree of sickness was evaluated by the participant.
Williamson 2007 found no significant interaction between baseline severity (feeling unwell and level of daily activity restriction) and treatment group (antibiotic versus placebo).
2. Severity or duration of different clinical symptoms
2.1. Resolution of purulent secretion
Five studies reported outcome data for purulent secretion (De Sutter 2002; Meltzer 2005; Norrelund 1978; Stalman 1997; Van Buchem 1997a). We extracted data from one study on day 8 (Norrelund 1978), and two studies on day 10 (De Sutter 2002; Stalman 1997). De Sutter 2002 provided data upon request. Meltzer 2005 published only least‐square means data. Outcomes were reported by participants in two studies (De Sutter 2002; Meltzer 2005), the investigator in two studies (Norrelund 1978; Stalman 1997), and by both participants and investigators in one study (Van Buchem 1997a).
The estimated OR for resolution of purulent secretion was 1.58, irrespective of endpoint timing (95% CI 1.13 to 2.22; NNTB 10, 95% CI 6 to 35; I² = 0%; high‐quality evidence; Analysis 2.1).
2.1. Analysis.
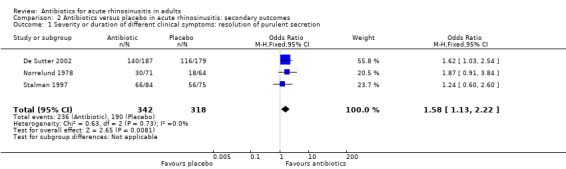
Comparison 2 Antibiotics versus placebo in acute rhinosinusitis: secondary outcomes, Outcome 1 Severity or duration of different clinical symptoms: resolution of purulent secretion.
We could not pool some data on purulent secretion. Norrelund 1978 found that 75% of participants in the antibiotic group and 56% in the placebo group had at least 50% reduction in secretion on day 8 (OR 2.29, 95% CI 1.11 to 4.74; NNTB 6, 95% CI 4 to 40; P = 0.002). Furthermore, De Sutter 2002 found a significant mean change in mean score on the symptom "thick nasal discharge" between baseline and 10‐day follow‐up (P ≤ 0.001). These results were confirmed by Meltzer 2005, who found a significant difference in least‐square means for rhinorrhoea between days 2 and 15 (P ≤ 0.01). Van Buchem 1997a found a significant difference in symptom score for secretion at the right side after one week (reported by the participant) (mean change 1.0 for placebo versus 1.2 for antibiotics, "P < 0.05" reported by Van Buchem 1997a), but this difference disappeared after two weeks. Evaluation of secretion at clinical examination did not confirm this finding.
2.2. Resolution of pain
Five studies provided outcome data for pain (De Sutter 2002; Meltzer 2005; Stalman 1997; Van Buchem 1997a; Williamson 2007). Unfortunately, as the outcome measures were too different and raw data were not available, pooling of data was not possible.
Considering pain in general, no study found a difference in pain duration between the antibiotic and placebo groups (De Sutter 2002; Stalman 1997; Williamson 2007). Full resolution of pain occurred between day 4 and day 7 in most participants. Also, when considering specific types of pain such as unilateral facial pain (De Sutter 2002), pain on bending forward (De Sutter 2002; Stalman 1997), pain in upper teeth or when chewing (De Sutter 2002; Stalman 1997), facial pain, pressure, or tenderness (De Sutter 2002; Meltzer 2005), and sinus headache (De Sutter 2002; Meltzer 2005), none of the trials detected a significant difference in pain duration when comparing antibiotic and placebo groups. Evaluating differences in symptom scores, Van Buchem 1997a found no differences after one and two weeks for frontal pain, maxillary pain, headache on bending, or tapping pain.
2.3. Illness duration
Five studies calculated the mean illness duration (Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Norrelund 1978; Varonen 2003). All studies compared illness duration between antibiotic and placebo groups. We could not pool the data because the standard deviations were not available. Kaiser 2001 reported the mean illness duration for participants with or without Streptococcus pneumoniae,Haemophilus influenzae, orMoraxella catarrhalis in their nasopharyngeal secretions (with bacteria: five days in the azithromycin group versus seven days in the placebo group; without bacteria: six days in the azithromycin group versus six days in the placebo group), but not for the total group. Norrelund 1978 found a subjective improvement after an average of 3.5 days in the antibiotic group compared with 3.7 days in the placebo group. They did not mention if this was a significant difference, but we can assume that it was not. Varonen 2003 also did not find a significant difference: the mean illness duration in participants taking antibiotics was 6.0 days, compared with 6.4 days in the placebo group (P = 0.66). Lindbaek 1996 found that participants with a fluid level or total opacification in any sinus on CT were cured seven days faster with antibiotic treatment than without (median time of the sinusitis episode: nine days in the amoxicillin group, 11 days in the penicillin group, and 17 days in the placebo group). Participants with only mucosal thickening on CT showed no significant difference in illness duration across the intervention groups (median time of the sinusitis episode: 10 days in the placebo and amoxicillin groups and 13.5 days in the placebo group) (Lindbaek 1998).
2.4. Restriction of daily activities
Four studies collected data on the restriction of daily activities due to rhinosinusitis (Bucher 2003; De Sutter 2002; Garbutt 2012; Stalman 1997). Pooling of data was not possible because the outcome measures were too different. None of the studies found a significant difference in activity restriction between the antibiotic and placebo groups.
Williamson 2007 found no significant interaction between baseline severity (feeling unwell and level of restriction on daily activity) and treatment group (antibiotic versus placebo).
3. Use of concomitant medications
3.1. Analgesics
Ten studies allowed the use of analgesics, that is paracetamol, in Bucher 2003, De Sutter 2002, Garbutt 2012, Lindbaek 1996, Lindbaek 1998, Norrelund 1978, Stalman 1997, Van Buchem 1997a, and Varonen 2003, and/or ibuprofen, in De Sutter 2002, Kaiser 2001, Norrelund 1978, and Varonen 2003. Five of these studies also recorded the use of analgesics (De Sutter 2002; Garbutt 2012; Norrelund 1978; Stalman 1997; Varonen 2003). It was not possible to pool the data because the raw data were not available or the outcome measures were too different. There was no effect of antibiotics on the use of analgesics in four studies (De Sutter 2002; Garbutt 2012; Norrelund 1978; Stalman 1997). Varonen 2003 revealed that participants receiving placebo used analgesics more often than those receiving antibiotics (43% in the placebo group and 26% in the antibiotic group, P = 0.03).
3.2. Nasal decongestants
Eleven studies allowed the use of xylometazoline nose drops (Axelsson 1970; Bucher 2003; De Sutter 2002; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Norrelund 1978; Rantanen 1973; Stalman 1997; Van Buchem 1997a; Varonen 2003). Six studies prescribed nasal decongestants for every participant (Axelsson 1970; Bucher 2003; Garbutt 2012; Norrelund 1978; Rantanen 1973; Van Buchem 1997a). Garbutt 2012 permitted the use of pseudoephedrine‐sustained action. Merenstein 2005 did not mention if nose drops were permitted. Corticosteroid nose drops were part of the intervention in two studies (Meltzer 2005; Williamson 2007). Meltzer 2005 explicitly stated that use of concomitant medication that could interfere with the study medication was not permitted.
Only two studies registered intake of nose drops (vasoconstrictors), Stalman 1997 and Varonen 2003, and antihistamines, Varonen 2003. Pooling of data was not possible because the outcome measures were too dissimilar. Neither study found a significant difference between groups in use of these medications. Garbutt 2012 found no difference between groups in use of pseudoephedrine‐sustained action.
4. Side effects
The side effects described in the trials were nausea, vomiting, abdominal pain, stomach pain, diarrhoea, skin rash, dizziness, fatigue, hot flushes, jittery feeling, dry mouth, headache, epistaxis, and vaginal discharge or pruritus. The most common side effects were gastrointestinal.
We pooled data from 10 trials on side effects in general (Axelsson 1970; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Merenstein 2005; Norrelund 1978; Stalman 1997; Van Buchem 1997a; Varonen 2003). De Sutter 2002 reported only data about diarrhoea. We did not add data from Lindbaek 1998 because the only side effects reported in this study were those that caused participants to stop their study medication. Of the participants who experienced side effects, 68.3% received antibiotics. This difference was statistically significant (OR 2.21, 95% CI 1.74 to 2.82; number needed to treat for an additional harmful outcome (NNTH) 8, 95% CI 6 to 12; I² = 16%; high‐quality evidence; Analysis 2.2; Figure 5).
2.2. Analysis.

Comparison 2 Antibiotics versus placebo in acute rhinosinusitis: secondary outcomes, Outcome 2 Side effects: total.
5.
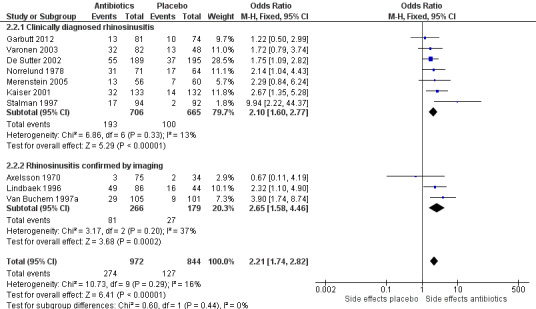
Forest plot of comparison: 2 Antibiotics versus placebo in acute rhinosinusitis whether or not confirmed by imaging, outcome: 2.2 Side effects: general.
More specifically, we could pool data on diarrhoea from seven trials (Axelsson 1970; De Sutter 2002; Garbutt 2012; Lindbaek 1996; Merenstein 2005; Stalman 1997; Varonen 2003). Garbutt 2012 reported only the percentage of diarrhoea for both groups and stated there was no difference between groups. We used the same percentages in both groups for pooling.
Of participants who received antibiotics, 16.7% reported diarrhoea, versus 9.6% of participants who received placebo. This result was statistically significant (Peto OR 2.00, 95% CI 1.41 to 2.85); NNTH 13, 95% CI 8 to 29; I² = 20%; high‐quality evidence; Analysis 2.3). We could not pool the results of Bucher 2003 because the raw data were not available, but their results were consistent with ours (OR 3.89, 95% CI 2.09 to 7.25 at day 7; OR 1.71, 95% CI 0.91 to 3.23 at 14 days).
2.3. Analysis.
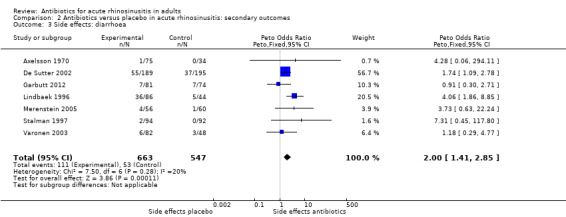
Comparison 2 Antibiotics versus placebo in acute rhinosinusitis: secondary outcomes, Outcome 3 Side effects: diarrhoea.
Meltzer 2005 only mentioned that there were no differences in treatment‐emergent side effects among the treatment groups. In that trial, five participants in the amoxicillin group and six in the placebo group discontinued treatment because of side effects. Williamson 2007 and Rantanen 1973 did not provide any information for this outcome.
5. Clinical failure
We pooled data on clinical failure from 12 trials (Axelsson 1970; Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Meltzer 2005; Stalman 1997; Van Buchem 1997a; Varonen 2003; Williamson 2007). In eight trials, clinical failure was assessed as an abnormal course of rhinosinusitis (exacerbation, ongoing symptoms, respiratory complications, treatment failure) leading to commence or extend antibiotic therapy. The number of treatment failures in the control and active treatment groups were compared (Bucher 2003; De Sutter 2002; Garbutt 2012; Kaiser 2001; Lindbaek 1996; Lindbaek 1998; Van Buchem 1997a; Varonen 2003). Axelsson 1970 reported numbers of participants who deteriorated at day 5 and 10. Meltzer 2005 and Stalman 1997 reported numbers of participants who met the criteria for treatment failure, but did not report whether these were prescribed open antibiotic therapy. Williamson 2007 reported the number of participants that withdrew because of treatment failure.
The pooled result showed that clinical failure occurred less frequently in participants receiving antibiotics compared to placebo (6.1% versus 11.2%, Peto OR 0.48, 95% CI 0.36 to 0.63; NNTH 19, 95% CI 15 to 27; I² = 21%; high‐quality evidence; Analysis 2.4; Figure 6).
2.4. Analysis.
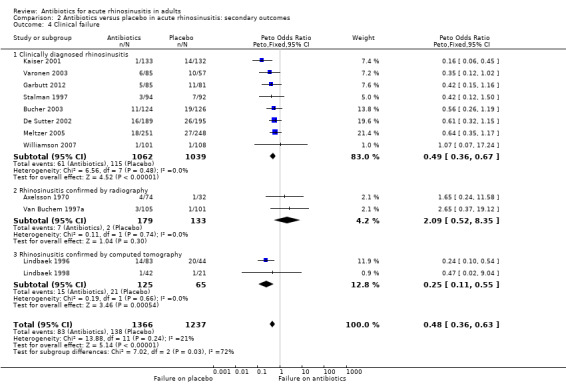
Comparison 2 Antibiotics versus placebo in acute rhinosinusitis: secondary outcomes, Outcome 4 Clinical failure.
6.
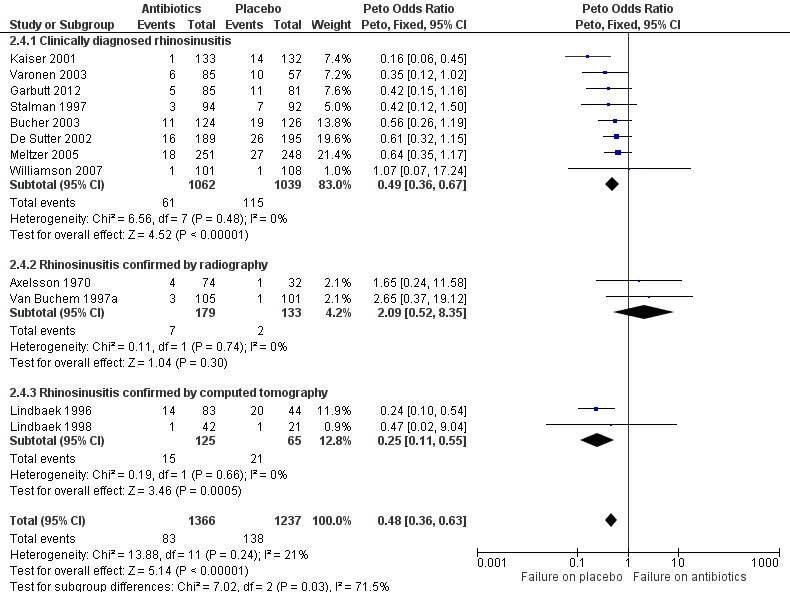
Forest plot of comparison: 2 Antibiotics versus placebo in acute rhinosinusitis: secondary outcomes, outcome: 2.4 Clinical failure.
6. Serious adverse events
Only one serious disease‐related adverse event occurred in the placebo group (Bucher 2003): after two weeks of symptomatic treatment, a participant who was treated for one week with amoxicillin‐clavulanate (1 g twice daily, open antibiotic therapy) experienced a brain abscess caused by an amoxicillin‐clavulanate‐sensitive strain of Streptococcus milleri. The participant was operated on and recovered but was reported to have a residual frontal syndrome.
There were two additional serious adverse events in the placebo group: one myocardial infarction and one severe depressive episode (Bucher 2003). Both were thought to be neither disease nor drug related. Other trials did not report any serious adverse events, which means that serious complications in participants with clinically diagnosed acute rhinosinusitis are rare.
Discussion
Summary of main results
The intention‐to‐treat (ITT) population included 3057 participants (15 trials). Without treatment, almost half of participants with acute rhinosinusitis, whether or not confirmed by radiography, recovered within one week, and two of three participants within 14 days. Antibiotics may slightly shorten the time to cure, but only 5 (diagnosis based on symptoms, range 1 to 10) to 11 (diagnosis confirmed by x‐ray, range 1 to 21) more participants per 100 would achieve cure faster by taking antibiotics instead of placebo. When a fluid level or total opacification was present on computed tomography (CT), 28 more participants per 100 (range 7 to 53) achieved cure faster with antibiotics. Antibiotics do not reduce the time to pain relief or the general feeling of illness. People who take antibiotics do not resume daily activities earlier and do not take less analgesics or nasal decongestants than people treated with placebo. In people with purulent rhinorrhoea, 10 more people per 100 (range 3 to 17) would experience a faster resolution of nasal discharge by taking antibiotics. However, we found that 13 more people per 100 (range 9 to 18) would experience side effects of the treatment. This potential harm needs to be compared to the possible benefit of people with purulent rhinorrhoea taking antibiotics. Five fewer people per 100 (range 3 to 7) would experience clinical failure if they took antibiotics instead of placebo (Table 1). This review did not investigate the effect of antibiotics in people with proven positive bacterial sinus cultures.
Overall completeness and applicability of evidence
We investigated if antibiotic therapy could speed up the recovery process in people with acute rhinosinusitis, whether clinically diagnosed or confirmed by imaging. The main symptoms used for participant inclusion among the included studies were the presence of nasal discharge, facial pain, and a common cold or upper respiratory tract infection. This is in line with the clinical presentation of rhinosinusitis in patients in ambulatory care settings. We included studies where participant inclusion depended on clinical symptoms and abnormalities on radiography or CT. The reason for this was that in some countries, imaging is used to confirm diagnosis of rhinosinusitis. Based on included study populations, we are reasonably confident that this review covers the general population of people with rhinosinusitis‐like symptoms. We could draw no conclusions about the efficacy of antibiotics in children, people with suppressed immune systems, or those with serious diseases (e.g. very high fever, prolonged symptoms, septic symptoms such as tachycardia, sweating, and low blood pressure) and people referred to an ear, nose, and throat specialist because of the serious course of the disease or fear of complications, since trials did not include these groups of patients, and it is unlikely that they will be included in future placebo‐controlled trials.
Quality of the evidence
We used GRADEpro GDT 2015 to assess evidence quality for each outcome (Table 1).
We assessed high‐quality evidence for the following outcomes: cure in adults with clinically diagnosed acute rhinosinusitis; severity or duration of different clinical symptoms ‐ resolution of purulent secretion; general side effects; side effects relating to diarrhoea; and clinical failure. We assessed moderate‐quality evidence for cure in adults with acute rhinosinusitis confirmed by radiography, downgrading the quality of the evidence due to few trials reporting this outcome (n = 3) and wide confidence intervals. It is unlikely that participants in Axelsson 1970 were blinded: the study report not provide any information about blinding and give the study design, it is highly likely that "treatment" was compared to "no treatment". Therefore we assessed this study as at high risk of bias for this domain. Nevertheless, omitting the results of Axelsson 1970 did not substantially changed the odds ratios (OR 1.67, 95% CI 1.04 to 2.70, NNTB 9, 95%CI 5 to 104). For this reason, we did not further downgrade evidence quality for this outcome.
Only one study with few participants (N = 127) reported cure in adults with acute rhinosinusitis confirmed by CT (Lindbaek 1996). Although this was a robust trial, we downgraded the quality of the evidence to moderate because of the limited number of participants.
All but three included studies reported on the main review outcome of rhinosinusitis cure (Garbutt 2012; Meltzer 2005; Rantanen 1973). The analyses show a consistent result.
Potential biases in the review process
We carried out thorough searches in several different databases on 18 January 2018. We used a predefined selection procedure for including and excluding studies and followed this strategy consistently. We documented reasons for exclusion. We predefined the research questions and answered them in the same sequence.
We assessed the included studies and summarised information in the Characteristics of included studies table. We assessed risk of bias rigorously. It is possible we evaluated three studies as poor undeservedly, but this does not mean that the studies were performed incorrectly (Rantanen 1973; Meltzer 2005; Norrelund 1978); rather, we evaluated them as unclear risk of bias due to insufficient information reported in the articles.
Some studies did not report raw data. In such cases, we estimated numbers of events by multiplying the percentage with the total number of participants in the group to make pooling of results possible. Stalman 1997 reported only the total cure rate for both groups and stated there was no difference between groups. We used the same percentages in both groups for pooling. This assumption could be imprecise; however, omitting these data did not substantially change odds ratios. Young 2008 performed a meta‐analysis on clinically diagnosed acute rhinosinusitis using individual patient data meta‐analysis (IPDMA). Young 2008 had raw data from Stalman 1997 at his disposal, and reported that the exact cure rate was 63% in the placebo group and 66% in the antibiotic group when the primary outcome was assessed. Using these cure rates in our analysis did not substantially change odds ratios (OR 1.27, 95% CI 1.04 to 1.56; number needed to treat for an additional beneficial outcome (NNTB) 18, 95% CI 10 to 104; Analysis 3.1.18). Garbutt 2012 reported only the percentage of diarrhoea for both groups and stated there was no difference between groups. We used the same percentages in both groups for pooling. Omitting these data from the analyses did not substantially change the odds ratios (OR 2.19, 95% CI 1.51 to 3.18; NNTB 11, 95% CI 7 to 24).
Definitions of cure varied among trials, and this raised questions about the comparability of studies; however, the underlying interpretation of cure was similar. As well as calculating the overall treatment effect, we divided the studies into three groups (cure at one week, 10 days, and 14 days) to assess the effect at various time points.
For the primary outcome of cure, we checked if inputting missing data in three different ways changed the overall result (Analysis 3.1.6 to Analysis 3.1.17).
For the secondary outcome, resolution of purulent secretion, data were collected once by the participant, and once by the clinician (inspection). Due to the low number of studies, we did not take this into account.
There was important variation in choices of antibiotics and dosage schedules. This may be due to differences in antibiotic resistance at different time points and in different countries. We assumed that the trial authors' choice of antibiotics was suitable for their countries and local resistance patterns at that point in time, and that this did not influence the effect of the antibiotic treatment on cure rates. However, since the trials did not perform bacterial cultures, we cannot prove this.
As we considered only studies that included adults without bacteriological cultures, the proportion of adults with bacterial rhinosinusitis is unknown. However, as described, we wanted to focus on adults who visited general practitioners with acute rhinosinusitis symptoms and who are treated empirically, with or without imaging. This review answers the important clinical question of whether these people should be treated with antibiotics or not.
Agreements and disagreements with other studies or reviews
We compared our meta‐analysis results with those from Rosenfeld 2007, Young 2008, Falagas 2008, Fokkens 2012, and Burgstaller 2016.
Rosenfeld 2007 included trials based on a clinical diagnosis, as well as trials that used technical investigations to establish the diagnosis (Gananca 1973 (bacteriology); Hansen 2000a (elevated CRP or ESR); Haye 1998 (radiography); Lindbaek 1996 (CT); Lindbaek 1998 (CT); Van Buchem 1997a (radiography). Rosenfeld 2007 excluded Norrelund 1978 because of a language barrier. Williamson 2007 and Garbutt 2012 were not yet published. This study group found a modest antibiotic benefit for people with uncomplicated acute rhinosinusitis 7 to 12 days after entering a clinical trial (absolute risk difference 0.15, 95% CI 0.04 to 0.25; risk ratio 1.28, P = 0.007; NNTB 7), based on data from Bucher 2003, De Sutter 2002, Gananca 1973, Hansen 2000a, Haye 1998, Kaiser 2001, Lindbaek 1996, Lindbaek 1998, and Stalman 1997. However, there was a high level of heterogeneity (I² = 80%). The forest plot shows that the benefit of antibiotics is higher in studies with an inclusion based on specific technical investigations (bacteriology, diagnostic algorithm, including CRP, CT; (NNTB 3)). The risk difference of studies that enrolled participants with a negative imaging or based strictly on clinical criteria was 0.03 (95% CI ‐0.02 to 0.08; NNTB 12.5 to 50). This is in accordance with our results. At 14 to 15 days, there was no longer any statistical benefit (results based on data from Bucher 2003; Meltzer 2005; Merenstein 2005; Van Buchem 1997a). Benefits were offset by a relative increase of 83% in adverse events (number needed to treat for an additional harmful outcome (NNTH) 9), which is similar to our calculations.
Young 2008 performed a IPDMA on clinically diagnosed acute rhinosinusitis. An IPDMA is the best way of performing subgroup analyses given that individual patient data from a number of RCTs can be obtained. As well as investigating the effect of antibiotics in the total group, the authors were able to investigate the effect of antibiotics in subgroups, such as people with at least seven days of rhinosinusitis‐like symptoms, as guidelines advocate prescribing antibiotics for this patient group (Hickner 2001). Young 2008 completed this IPDMA by further analysis of the effect of antibiotics in people with specific signs and symptoms, with the aim of identifying people who benefit most from antibiotic therapy. Young 2008 included the same trials, except for Norrelund 1978, because they could not get the raw data for the IPDMA, and Garbutt 2012 because this trial was not yet published.Young 2008 included the raw data of Meltzer 2005 and of an identical unpublished trial run by Schering‐Plough. The estimated OR for the overall treatment effect of antibiotics relative to placebo was 1.37 (95% CI 1.13 to 1.66; NNTB 15, 95% CI 7 to 190, data IPDMA). The ORs of analyses of aggregated data of Young 2008 were similar (OR 1.35, 95% CI 1.15 to 1.59). This OR for overall treatment effect in clinically diagnosed rhinosinusitis is slightly higher than in this review, probably due to the favourable results of Meltzer 2005. Young 2008 found that older people and people reporting severe symptoms or longer duration of symptoms (six days or more) took longer to cure but were no more likely to benefit from treatment than other people. For other patient‐reported symptoms (previous common cold or two stages of illness, pain on bending, unilateral facial pain, pain in the teeth, and purulent nasal discharge), estimates were not sufficiently precise to draw any conclusion about their prognostic value. Participants with purulent discharge in the pharynx, ascertained by a physician, seemed to cure more slowly and to have some non‐significant benefits from antibiotics (OR 1.60, 95% CI 0.95 to 2.76; NNTB 8, 95% CI 4 to 47). The same was found for people with a higher temperature (OR 1.28, 95% CI 0.87 to 1.88). As we did not have the raw data, we could not perform this subgroup analyses. Young 2008 did not investigate adverse events.
Falagas 2008 included trials in adults or children based on clinical diagnosis and/or specific technical investigations (extra trials: Gananca 1973 (bacteriology); Garbutt 2001 (children); Hansen 2000a (elevated CRP or ESR); Haye 1998 (radiography); Kristo 2005 (children); Lindbaek 1996 (CT); Lindbaek 1998 (CT); Wald 1986 (children)).Falagas 2008 excluded Norrelund 1978 due to a language barrier. For Kaiser 2001, Falagas 2008 only took into account the subgroup of people with rhinosinusitis confirmed by radiography. Falagas 2008 did not include Garbutt 2012 because this study was not yet published. Falagas 2008 made a distinction between "cured or improved" and "cured". Considering the different definitions, "cure" or "improvement" seem to be a subjective interpretation of how a patient feels at one time point, expressed in resolution of symptoms, restriction in daily activities, or feeling cured; therefore, we did not choose to make this distinction in this review. Taking cure as an outcome measure, Falagas 2008 found a higher cure rate in people taking antibiotics compared to placebo (OR 1.82, 95% CI 1.34 to 2.46; NNTB 7, 95% CI 5 to 14). In this analysis, the diagnosis was made on clinical criteria only in only 4 of the 12 trials. Falagas 2008 omitted the trials by Kaiser 2001, Meltzer 2005, Stalman 1997, and Varonen 2003 because these trials did not report rates for cure (as defined by Falagas 2008) separately. As in Rosenfeld 2007, the forest plot revealed heterogeneity (I² = 50%): the trials with inclusion based on specific technical investigations (CT or laboratory tests) showed more benefit for antibiotics than the trials with inclusion on a clinical basis. Furthermore, taking cure or improvement as an outcome measure, Falagas 2008 found that people with rhinosinusitis benefit from antibiotics (OR 1.64, 95% CI 1.35 to 2.00; NNTB 11, 95% CI 8 to 17). However, for the Meltzer 2005 study, Falagas 2008 used the number of treatment failures as the number of people who were not cured, which makes the cure rate artificially high. Heterogeneity was low, but the same trend (more technical investigations, more benefit of antibiotics) can be seen. Falagas 2008 put their positive result into perspective by comparing the NNTB of 7 (95% CI 5 to 14) to a NNTH (adverse events) of 9 (95% CI 5 to 30), a number that is, irrespective of the different kinds of studies included in the analysis, comparable to our results. Falagas 2008 found no significant difference in cure or improvement for adults versus children, imaging versus clinical criteria for inclusion, assessment at 7 to 11 versus 14 to 15 days, or year of publication.
Burgstaller 2016 included original studies that compared treatment of any antibiotic with placebo in people with symptoms and signs of acute rhinosinusitis lasting for seven or more days. The reason given by Burgstaller 2016 for this inclusion criterion was the recommendation of the 'European position paper on rhinosinusitis and nasal polyps' (Fokkens 2012). Fokkens 2012 recommend antibiotic treatment only in people with duration of symptoms of more than 10 days; however, as no original study included this kind of participant, Burgstaller 2016 used seven days or more as their criterion. Only Garbutt 2012 (clinical criteria), Merenstein 2005 (clinical criteria), Hadley 2010 (radiography and bacteriology), Lindbaek 1996 (CT), and Lindbaek 1998 (CT) were selected. Burgstaller 2016 looked separately at improvement and cure, finding a significantly higher proportion of people with improvement of symptoms after 3 and 7 days in people treated with antibiotics compared to placebo (OR 2.78, 95% CI 1.39 to 5.58 at day 3, based on data from Garbutt 2012, Hadley 2010, Lindbaek 1996, and Lindbaek 1998; and OR 2.29, 95% CI 1.19 to 4.41 at day 7, based on data from Garbutt 2012). There was no relevant difference in the rate of cure at day 10 (OR 1.92, 95% CI 0.63 to 5.8, based on data from Lindbaek 1996 and Lindbaek 1998) or at day 14 (OR 1.57, 95% CI 0.79 to 3.13, based on data from Merenstein 2005). It was difficult to compare results because there was minimal overlap in pooled studies. Furtermore, we questioned if symptoms lasting for seven days or more is a good criterion for selecting participants who could benefit from antibiotics. Lindbaek 2002 reviewed the clinical diagnosis of acute purulent sinusitis in general practice. The strongest predictor for the diagnosis of purulent sinusitis is the presence of purulent secretion in the nasal cavity. Pain in the teeth and an elevated ESR were associated in two of the four studies. Two phases in the illness history, ineffectiveness of decongestants and transillumination of the sinuses, might be of some value. We found no evidence that people with symptoms lasting seven days or more before consulting their physician are more likely to have bacterial sinusitis. Of the 440 people in Lindbaek 1996 and Lindbaek 1998 combined, 202/254 (80%) of people with CT‐confirmed sinusitis were symptomatic for more than seven days, while 131/186 (70%) of people without CT‐confirmed sinusitis were symptomatic for more than seven days. The difference is statistically significant (P = 0.03), but has not been analysed in a multivariate logistic regression. This means that about 60% of people presenting to general practices with sinusitis symptoms for more than seven days would have CT‐confirmed sinusitis, and 40% would have a prolonged viral infection. But of people presenting with symptoms of seven days or less duration, about 50% will have a CT‐confirmed sinusitis, and about 50% viral upper respiratory tract infections (Lindbaek 2015 [pers comm]). Consequently, despite only 20% of people with CT‐confirmed sinusitis having symptoms for seven or fewer days (Hickner 2001), duration of symptoms is a poor predictor of acute bacterial sinusitis.
In conclusion, people with clinically diagnosed rhinosinusitis and rhinosinusitis confirmed by imaging (radiography, CT) or laboratory findings (CRP, bacteriology) constitute different groups, and we are convinced that they cannot be pooled in one analysis, as performed by Burgstaller 2016, Falagas 2008, and Rosenfeld 2007. The separate results are very relevant: when a clinician makes a clinical diagnosis, the one analysis is applicable; when a decision is made to confirm the diagnosis by imaging, the other analysis is applicable. This would be clear for any clinician and avoids confusion.
Authors' conclusions
Implications for practice.
Our review showed that there could be a beneficial therapeutic effect of antibiotics in adults with clinically diagnosed acute rhinosinusitis. But this effect is small, and only around 5 more people per 100 will be cured faster if they receive antibiotics instead of placebo. The lack of effect of antibiotics could be related to the non‐specificity of the clinical presentation of acute bacterial sinusitis. Performing radiography increases this number from 5 to 11 per 100. In adults with a fluid level or total opacification present on computed tomography, 28 more people per 100 cured faster with antibiotics. In clinically diagnosed rhinosinusitis, the subgroup of people with purulent discharge could benefit slightly more than those without. However, the benefits need to be seen in the context of the risk of experiencing adverse effects, especially of a gastrointestinal nature, and health risks of radiation exposure. Five fewer people per 100 will experience clinical failure if they receive antibiotics instead of placebo. This review addresses mainly people assessed in a ambulatory care setting and excluded people who were investigated further with laboratory tests. Considering the worldwide high antibiotic prescription rate for rhinosinusitis, growing antibiotic resistance, and the very low incidence of complications, we can conclude that there is no place for the use of antibiotics in adults with uncomplicated acute rhinosinusitis. For subgroups that are potentially more vulnerable, such as children, adults with a suppressed immune system, and adults with severe sinusitis (e.g. very high fever, prolonged symptoms, findings suggestive of severe sepsis such as hypotension) and people referred to an ear, nose, and throat specialist because of confirmed or perceived complications, no evidence is available from randomised, placebo‐controlled trials. Complications are so rare (1/3057 participants in our review) that only case reports can give information about their course, hence evidence that serious complications can be prevented by giving antibiotics early is lacking.
Implications for research.
Despite the availability of several studies and meta‐analyses, there is still insufficient clinical data to enable subgroup analysis of people who probably could benefit more from antibiotics, for example people with high fever, severe facial pain, or rhinorrhoea. It may be unlikely that such data will become available, as they pose ethical (exposing vulnerable people to placebo treatment) and feasibility (a relatively small group that would take a long time to collect data) issues. Better recording of routinely collected data and morbidity, as well as adverse drug reaction registers, might be helpful in answering this question. The possibility of C‐reactive protein (CRP) measurement or bacteriological investigations to select people that benefit from antibiotics should be further explored.
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2018 | New citation required but conclusions have not changed | Two Cochrane Reviews, 'Antibiotics for acute maxillary sinusitis in adults' (Ahovuo‐Saloranta 2014) and 'Antibiotics for clinically diagnosed acute rhinosinusitis in adults' (De Sutter 2012), described the effect of antibiotics for acute rhinosinusitis. Although both reviews studied the same condition, they studied different populations: people diagnosed according to clinical signs, confirmed or not by imaging or bacterial culture (Ahovuo‐Saloranta 2014), and people diagnosed according to clinical signs and symptoms (De Sutter 2012). Different approaches resulted in different conclusions, which was confusing for clinicians. We therefore merged these Cochrane Reviews whilst maintaining the relevant distinction between populations diagnosed by clinical signs and symptoms, or imaging. |
| 18 January 2018 | New search has been performed | Searches updated. As a consequence of merging two Cochrane Reviews, we incorporated five trials from Ahovuo‐Saloranta 2014 in the analyses (Axelsson 1970; Lindbaek 1996; Lindbaek 1998; Rantanen 1973; Van Buchem 1997a). The comparison between antibiotics, as published by Ahovuo‐Saloranta 2014, was omitted. Instead of clinical trials, local up‐to‐date antibiotic resistance patterns should guide clinicians in making the best choice for the appropriate antibiotic and dose in the subgroup of people with suspected bacterial rhinosinusitis. No new studies were added as a result of the update. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 2 April 2014 | New search has been performed | Searches updated. |
| 7 April 2008 | Amended | Converted to new review format |
Acknowledgements
We would like to thank Sarah Thorning, David Honeyman, and Justin Clark for help in searching the literature, and Managing Editors Elizabeth Dooley and Ann Jones for all their patience and help.
We wish to acknowledge the following people for commenting on draft versions of this review: Roy Buffery, Mireya Aznar, Nelcy Rodriguez, Raghda Rashad, Noorin Bhimani, Jack Anon, Helena Liira, Pakpoom Supiyaphun, Conor Teljeur, Bruce Arroll, Sree Nair, Dilip Raghavan, Meenu Sing, Abimbola Farinde, José Luis Ferrero Albert, Terry Neeman, Devada Singh‐Franco, Shunjie Chua, Cheow Peng Ooi, Theo Verheij, and Allen Cheng.
We would like to thank Dr Morten Lindbaek for providing additional information about his trial.
We would also like to thank everyone who contributed to De Sutter 2012 and Ahovuo‐Saloranta 2014.
And last but not least, we wish to thank Anneli Ahovuo‐Saloranta and James Young for their great contributions to previous published versions of this review.
Appendices
Appendix 1. MEDLINE and CENTRAL search strategy
MEDLINE (Ovid)
1 exp Sinusitis/ 2 sinusit*.tw. 3 Rhinitis/ 4 rhinit*.tw. 5 rhinosinusit*.tw. 6 nasosinusit*.tw. 7 ((suppurative or purulent) adj2 (nasal discharge or rhinitis or rhinorrhoea or rhinorrhoea)).tw. 8 or/1‐7 9 exp Anti‐Bacterial Agents/ 10 antibacterial*.tw. 11 antibiotic*.tw. 12 exp Amoxicillin/ 13 amoxicillin*.tw,nm. 14 Ampicillin/ 15 ampicillin*.tw,nm. 16 Azithromycin/ 17 azithromycin.tw,nm. 18 Cefaclor/ 19 cefaclor.tw,nm. 20 exp Cefadroxil/ 21 cefadroxil.tw,nm. 22 cefatrizine.tw,nm. 23 Cefuroxime/ 24 cefuroxim*.tw,nm. 25 Cephalexin/ 26 cephalexin*.tw,nm. 27 Cephalosporins/ 28 cephalosporin*.tw,nm. 29 Ciprofloxacin/ 30 ciprofloxacin*.tw,nm. 31 Clarithromycin/ 32 clarithromycin*.tw,nm. 33 Clindamycin/ 34 clindamycin*.tw,nm. 35 Doxycycline/ 36 doxycyclin*.tw,nm. 37 Erythromycin/ 38 erythromycin*.tw,nm. 39 Fluoroquinolones/ 40 fluoroquinolone*.tw,nm. 41 levofloxacin.tw,nm. 42 Lincomycin/ 43 lincomycin*.tw,nm. 44 Macrolides/ 45 macrolide*.tw,nm. 46 Minocycline/ 47 minocyclin*.tw,nm. 48 Miocamycin/ 49 (miocamycin* or miokamycin*).tw,nm. 50 moxifloxacin*.tw,nm. 51 norfloxacin.tw,nm. 52 Norfloxacin/ 53 Ofloxacin/ 54 ofloxacin.tw,nm. 55 Penicillins/ 56 penicillin*.tw,nm. 57 Quinolones/ 58 quinolone*.tw,nm. 59 Spiramycin/ 60 spiramycin.tw,nm. 61 telithromycin.tw,nm. 62 tetracyclines/ or tetracycline/ 63 tetracycline*.tw,nm. 64 Trimethoprim‐Sulfamethoxazole Combination/ 65 trimethoprim‐sulfamethoxazole combination.tw,nm. 66 cotrimoxazole*.tw,nm. 67 or/9‐66 68 8 and 67
Appendix 2. Embase (Elsevier) search strategy
#21 #12 AND #20 #20 #15 NOT #19 #19 #16 NOT #18 #18 #16 AND #17 #17 'human'/de #16 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #15 #13 OR #14 #14 crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR placebo*:ab,ti OR (doubl* NEXT/1 blind*):ab,ti OR allocat*:ab,ti OR random*:ab,ti OR trial:ti #13 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #12 #6 AND #11 #11 #7 OR #8 OR #9 OR #10 #10 amoxicillin:ab,ti OR ampicillin*:ab,ti OR azithromycin:ab,ti OR cefaclor:ab,ti OR cefadroxil:ab,ti OR cefatrizine:ab,ti OR cefuroxim*:ab,ti OR cephalexin*:ab,ti OR cephalosporin*:ab,ti OR ciprofloxacin*:ab,ti OR clarithromycin*:ab,ti OR clindamycin:ab,ti OR doxycyclin*:ab,ti OR erythromycin*:ab,ti OR fluoroquinolone*:ab,ti OR levofloxacin*:ab,ti OR lincomycin*:ab,ti OR macrolide*:ab,ti OR minocyclin*:ab,ti OR miocamycin*:ab,ti OR miokamycin*:ab,ti OR moxifloxacin*:ab,ti OR norfloxacin*:ab,ti OR ofloxacin*:ab,ti OR penicillin*:ab,ti OR quinolone*:ab,ti OR spiramycin*:ab,ti OR telithromycin*:ab,ti OR tetracyclin*:ab,ti OR trimethoprim*:ab,ti OR cotrimoxazol*:ab,ti #9 'amoxicillin'/de OR 'ampicillin'/de OR 'azithromycin'/de OR 'cefaclor'/de OR 'cefadroxil'/de OR 'cefuroxime'/de OR 'cefalexin'/de OR 'cephalosporin'/de OR 'ciprofloxacin'/de OR 'clarithromycin'/de OR 'clindamycin'/de OR 'doxycycline'/de OR 'erythromycin'/de OR 'lincomycin'/de OR 'macrolide'/de OR 'quinolone derivative'/de OR 'minocycline'/de OR 'miokamycin'/exp OR 'norfloxacin'/de OR 'ofloxacin'/de OR 'penicillin derivative'/de OR 'spiramycin'/de OR 'tetracycline derivative'/de OR 'cotrimoxazole'/de #8 antibiotic*:ab,ti #7 'antibiotic agent'/exp #6 #1 OR #2 OR #3 OR #4 OR #5 #5 ((suppurative OR purulent) NEAR/2 ('nasal discharge' OR rhinitis OR rhinorrhea OR rhinorrhoea)):ab,ti #4 rhinit*:ab,ti OR rhinosinusit*:ab,ti OR nasosinusit*:ab,ti #3 'rhinitis'/de OR 'rhinosinusitis'/de #2 sinusit*:ab,ti #1 'sinusitis'/exp
Appendix 3. WHO International Clinical Trials Registry Platform (ICTRP) search strategy
sinusit* AND antibacterial* OR rhinit* AND antibacterial* OR rhinosinusit* AND antibacterial* OR nasosinusit* AND antibacterial* OR suppurative nasal discharge AND antibacterial* OR purulent nasal discharge AND antibacterial* OR suppurative rhinorrhoea AND antibacterial* OR purulent rhinorrhoea AND antibacterial* OR suppurative rhinorrhea AND antibacterial* OR purulent rhinorrhea AND antibacterial* OR sinusit* AND antibiotic* OR rhinit* AND antibiotic* OR rhinosinusit* AND antibiotic* OR nasosinusit* AND antibiotic* OR suppurative nasal discharge AND antibiotic* OR purulent nasal discharge AND antibiotic* OR suppurative rhinorrhoea AND antibiotic* OR purulent rhinorrhoea AND antibiotic* OR suppurative rhinorrhea AND antibiotic* OR purulent rhinorrhea AND antibiotic*
Appendix 4. ClinicalTrials.gov search strategy
(sinusitis OR rhinitis OR rhinosinusitis OR nasosinusitis OR ((suppurative OR purulent) AND nasal discharge OR rhinorrhoea OR rhinorrhea)) AND (antibacterial OR anti‐bacterial OR antibiotic OR antibiotics)
Data and analyses
Comparison 1. Antibiotics versus placebo for acute rhinosinusitis: cure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cure | 11 | 2208 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.15, 1.65] |
| 1.1 Clinically diagnosed acute rhinosinusitis | 8 | 1687 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.02, 1.53] |
| 1.2 Acute rhinosinusitis confirmed by radiography | 3 | 394 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.03, 2.39] |
| 1.3 Acute rhinosinusitis confirmed by computed tomography | 1 | 127 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.89 [1.75, 13.72] |
Comparison 2. Antibiotics versus placebo in acute rhinosinusitis: secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severity or duration of different clinical symptoms: resolution of purulent secretion | 3 | 660 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.13, 2.22] |
| 2 Side effects: total | 10 | 1816 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.74, 2.82] |
| 2.1 Clinically diagnosed rhinosinusitis | 7 | 1371 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.10 [1.60, 2.77] |
| 2.2 Rhinosinusitis confirmed by imaging | 3 | 445 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.58, 4.46] |
| 3 Side effects: diarrhoea | 7 | 1210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.00 [1.41, 2.85] |
| 4 Clinical failure | 12 | 2603 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.36, 0.63] |
| 4.1 Clinically diagnosed rhinosinusitis | 8 | 2101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.36, 0.67] |
| 4.2 Rhinosinusitis confirmed by radiography | 2 | 312 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [0.52, 8.35] |
| 4.3 Rhinosinusitis confirmed by computed tomography | 2 | 190 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.11, 0.55] |
Comparison 3. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinically diagnosed rhinosinusitis | 9 | 19409 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.14, 1.29] |
| 1.1 Cure at 1 week | 4 | 856 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.81, 1.41] |
| 1.2 Cure at 10 days | 4 | 1048 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.92, 1.53] |
| 1.3 Cure at 2 weeks | 4 | 717 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.98, 1.91] |
| 1.4 Cure at 1 week, with Garbutt data | 5 | 1011 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.93, 1.54] |
| 1.5 Cure at 10 days, with Garbutt data | 5 | 1203 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.91, 1.47] |
| 1.6 Influence of missing data: cure at any time point if dropouts were successes | 8 | 1785 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.02, 1.52] |
| 1.7 Influence of missing data: cure at any time point if dropouts were failures | 8 | 1785 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.02, 1.51] |
| 1.8 Influence of missing data: cure at any time point if dropouts had the same cure rate as control group | 8 | 1785 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.02, 1.51] |
| 1.9 Influence of missing data: cure at 1 week if dropouts were successes | 4 | 901 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.79, 1.35] |
| 1.10 Influence of missing data: cure at 1 week if dropouts were failures | 4 | 901 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.84, 1.44] |
| 1.11 Influence of missing data: cure at 1 week if dropouts had the same cure rate as control group | 3 | 632 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.79, 1.50] |
| 1.12 Influence of missing data: cure at 10 days if dropouts were successes | 3 | 840 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.66] |
| 1.13 Influence of missing data: cure at 10 days if dropouts were failures | 3 | 840 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.65] |
| 1.14 Influence of missing data: cure at 10 days if dropouts had the same cure rate as control group | 3 | 840 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.93, 1.65] |
| 1.15 Influence of missing data: cure at 2 weeks if dropouts were successes | 4 | 1026 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.98, 1.73] |
| 1.16 Influence of missing data: cure at 2 weeks if dropouts were failures | 4 | 776 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.84] |
| 1.17 Influence of missing data: cure at 2 weeks if dropouts had the same cure rate as control group | 4 | 776 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.99, 1.86] |
| 1.18 Overall treatment effect (with Young 2008 data concerning Stalman) | 8 | 1687 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.04, 1.56] |
| 2 Rhinosinusitis confirmed by imaging | 5 | 1086 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.41, 2.44] |
| 2.1 Cure at any time point | 5 | 584 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.20, 2.45] |
| 2.2 Cure or improvement at any time point | 4 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.08 [1.35, 3.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Axelsson 1970.
| Methods | Study design: RCT Study duration: not reported |
|
| Participants | Setting: not reported Country: Sweden Health status: clinically suspected sinusitis with secretion confirmed on radiography (different projections were used to prove fluid in the sinus, and thereby called an "affected sinus"). Completely opaque maxillary sinuses were only accepted if the secretion was confirmed by a single diagnostic irrigation. Number: treatment (78 (38 penicillin V and 40 lincomycin)); control (34) Age: mean 33 (range 13 to 80 years) Sex: 60% women Exclusion criteria: history of nasal allergy and people recently treated with nasal decongestants or antibiotics |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: concomitant use of oxymetazoline (three drops in each nostril three times daily) was prescribed for all treatment arms. |
|
| Outcomes | Primary outcomes:
Secondary outcome:
Correlation between subjective and radiological improvement |
|
| Notes | Funding source: not stated Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: it is unclear if participants were a convenience sample or a consecutive series of eligible patients. Participants were randomly divided into 4 treatment groups. No details about randomisation (simple, unrestricted, restricted) were provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided about randomisation list or numbering or appearance of drug containers |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: blinding not reported, but probably not done since 1. The placebo group did not take tablets, only nose drops. 2. It was not possible to blind "sinus irrigation" as an intervention (group 1) 3. Only group 1 underwent radiological evaluation every second day 4. Group 2 received a longer course of tablets than group 3 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate: 6/98 (6.1%)
The reasons for missing data: not reported. The ratio of participants with missing data to participants with events: 0.16 Compliance with treatment: not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol was described in the methods. The primary endpoints (cure, improvement) were not adequately defined. Nevertheless, study authors reported which outcomes were recorded. |
| Other bias | Unclear risk | Comment: it appears that groups were balanced, although information on demographic characteristics was limited (similar according to mean age, radiological state). |
Bucher 2003.
| Methods | Study design: RCT Study duration: from November 1 to April 30 of 1997 to 2001 (4 winter seasons) |
|
| Participants | Setting: general practice and the internal medicine and otolaryngology outpatient clinics of the University Hospital Basel (only walk‐in patients and not referred patients) (24 general practices and 2 outpatient clinics) Country: Switzerland Health status: people presenting with a history of repeated purulent nasal discharge and maxillary or frontal unilateral or bilateral pain for at least 48 hours, but less than 1 month, and presence of pus under rhinoscopy (this last criterion was withdrawn after the first winter season). Number:
Age: mean 37 Sex: 54% women Exclusion criteria: age younger than 18, an upper respiratory tract infection or use of antibiotics for any reason within the previous 4 weeks, an upper respiratory tract infection or intermittent fever that persisted for more than 4 weeks, pathologic features or malformation of nasal cavities or the pharynx, immunosuppressive treatment, HIV infection, allergy to amoxicillin‐clavulanate, pregnancy or breastfeeding, and no fluency in one of the national languages. After 2000, an extra exclusion criterion was introduced because of a brain abscess in the placebo group: (1) people with a CRP level greater than 100 mg/L (2) people with a CRP level between 50 and 99 mg/L if 3 days after inclusion clinical worsening or an increase in CRP level higher than 100 mg/L occurred. |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: decongestant therapy (xylometazoline hydrochloride spray) and paracetamol tablets (500 mg with a maximum dose of 6 tablets a day) were provided. Steam inhalation was allowed. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding source: not stated Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: a computer random number generator was used. Stratified randomisation: general practice or outpatient clinic as stratification unit, participants randomised in blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Comment: tablets were provided in identical, numbered containers. The allocation sequence was performed by a statistician who was not involved in the final analysis. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: tablets of equal size, colour, and taste. All study physicians and the study nurse were blinded to the treatment given to each participant. Data were entered by the study nurse. Randomisation code was kept at the 24‐hour emergency call centre in Basel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Postrandomisation dropout rate: 2/252 (0.8%)
Reasons for missing data: loss of follow‐up (1 participant) or adherence problems (1 participant). 1 participant with a serious adverse event was considered as a dropout by the authors but included in this review as a failure. Ratio of participants with missing data to participants with events: 0.01 Comment:
|
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. |
| Other bias | Low risk | Comment:
|
De Sutter 2002.
| Methods | Study design: RCT Study duration: from October 1998 to December 1999 |
|
| Participants | Setting: general practice (69 practices) Country: Belgium Health status: adults presenting with a respiratory tract infection and purulent rhinorrhoea Number:
Age: mean 37 in amoxicillin group and 39 in placebo group Sex: 54% women Exclusion criteria: allergy to penicillin or ampicillin, having received antibiotic therapy within the previous week, symptoms lasting more than 30 days, abnormality on clinical chest examination, complications of sinusitis (facial oedema or cellulitis; orbital, visual, meningeal, or cerebral signs), pregnancy or lactation, comorbidity that might impair immune competence, and inability to follow the protocol because of language or mental health problems |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: decongestant therapy (xylometazoline 1% nose drops) and paracetamol or ibuprofen |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding source: a grant from Eurogenerics NV, Brussels Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: assignment via a computer‐generated random number list to receive antibiotics or placebo |
| Allocation concealment (selection bias) | Low risk | Comment: the randomisation list was kept at the pharmacy of the University Hospital. The randomisation list was accessible to the participating family physician only in case of a serious adverse event. The trial medication was supplied in numbered, uniform cardboard boxes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: capsules had the same size, colour, and shape for active and placebo treatment. To assess effectiveness of masking, participants and family physician guessed their treatment group at day 10. Data were encoded and entered without knowledge of allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Postrandomisation dropout rate: 32/416 (7,6%)
Reasons for missing data: loss to follow‐up or withdrawal without knowing if they were cured or not. Ratio of participants with missing data to participants with events: 0.18 Withdrawal with "known" illness course:
Open antibiotic therapy (after 10 days follow‐up)
Sensitivity analysis performed. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. |
| Other bias | Low risk | Comment:
|
Garbutt 2012.
| Methods | Study design: RCT Study duration: between November 1, 2006 and May 1, 2009 |
|
| Participants | Setting: ambulatory care (10 offices) Country: USA Health status: people presenting with a history of maxillary pain or tenderness in the face or teeth, purulent nasal secretions, and rhinosinusitis symptoms for 7 days or more and 28 days or less that were not improving or worsening, or rhinosinusitis symptoms lasting for less than 7 days that had significantly worsened after initial improvement. Symptoms had to be moderate, severe, or very severe. Numbers:
Age: median 32 in the amoxicillin group, 31 in the placebo group Sex: 64% women Exclusion criteria: allergy to penicillin or amoxicillin, prior antibiotic treatment within 4 weeks, complications of sinusitis, a comorbidity that could impair their immune response, cystic fibrosis, requiring an antibiotic for a concurrent condition, pregnancy, and people who rated their symptoms as very mild or mild |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: to be used as needed during 5 to 7 days (except if contra‐indications)
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Funding source: grant U01‐AI064655‐01A1 from the National Institute of Allergy and Infectious Diseases. This institute did not have a role in the design and conduct of the study; in the collection, management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript. Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: blocked randomisation scheme. Computer‐generated random numbers were used to determine how the 2 study drugs were allocated to the consecutively numbered study treatment packages. Randomisation occurred when the research assistant assigned the treatment package. |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation was performed in advance by the investigational pharmacist who did not participate in participants' enrolment or outcome assessment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: the tablets were similar in appearance and taste and dispensed in the same fashion. Research assistants were blinded to group assignment. The percentage of participants who guessed their treatment assignment correctly did not differ by study group (36% in amoxicillin group and 37% in placebo group, P = 0.2). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate: 11/166 (6.6%) due to missing data
Discontinuation of treatment rate: 23/166 (13.9%)
Treatment with other antibiotics: 16/166 (9.6%)
Ratio of participants with missing data to participants with events (outcome: significant improvement after 10 days): 0.09. Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. Sensitivity analysis for participants who completed 10 days of treatment with the study drug and those with symptoms for 7 days or more and 28 days or less. Findings were consistent with the primary analysis. |
| Other bias | Low risk | Comments:
|
Kaiser 2001.
| Methods | Study design: RCT Study duration: unknown |
|
| Participants | Setting: outpatient clinic of the University of Geneva Hospital Country: Switzerland Health status: people presenting with common cold or acute sinusitis and had a history of rhinorrhoea of less than 4 weeks and a confirmed upper respiratory tract infection at physical examination, including rhinoscopy. Number:
Age: median 35 Sex: 52% women (gender of 4 dropouts not reported) Exclusion criteria: high fever (> 38.5 °C) and an overall clinical impression that antibiotic treatment was absolutely required (˜ 4% of the screened population), chronic ear, nose, and throat disease, a positive pharyngeal culture for Streptococcus pyogenes, known allergy to macrolides, antibiotic treatment in the previous 10 days, immunosuppression, and underlying pulmonary disease |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: ibuprofen and nasal drops containing oxymetazoline was offered to all participants. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Definitions of cure:
Subgroup analysis: predefined subset of participants with and without Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis |
|
| Notes | Funding source: not stated Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: random assignment. No further information available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: drugs and placebo were in identical containers. No further information available. No information about the centralisation of randomisation or the numbering of the containers |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: drugs and placebo had the same shape and taste. Participants and investigators were blinded to the treatment administered. This investigator remained blinded to bacteriological and radiological results, even if an open antibacterial treatment was deemed necessary. The sinus radiograph (occipitomental view) was interpreted independently by 2 radiologists blinded to the clinical results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate: 4/269 (1.5%)
Ratio of participants with missing data to participants with events: 0.03 Open antibiotic treatment (treatment failure): 15/265 (5.7%).
Comment: it appeared that all these participants receiving open antibiotic therapy were included in the analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. |
| Other bias | Low risk | Comment:
|
Lindbaek 1996.
| Methods | Study design: randomised, double‐blind trial Study duration: January to May 1994 and November 1994 to May 1995 |
|
| Participants | Setting: general practice Country: Norway Health status: clinical diagnosis of acute sinusitis, confirmed by computed tomography (presence of fluid level or total opacification in any sinus, independently scored by 2 experienced radiologists). Ear, nose, and throat comorbidity was not assessed. Number:
Age: mean 38.6 (range 16 to 74) Sex: 65% women Exclusion criteria: age 15 or under, pregnancy, ongoing antibiotic treatment, immunosuppressive treatment, previous operations in the nose or sinus region, misuse of alcohol or narcotics, rheumatic disease, and allergy to penicillin. Participants with symptoms for more than 30 days were excluded because of possible chronic sinusitis. Participants with high fever and strong pain were not included because of ethical considerations. |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: concomitant use of nasal decongestants and paracetamol was allowed. Comment: For this review, group 1 and 2 were combined in the analyses. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding source: government Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: restricted randomisation (blocks of 3 within each of 6 subgroups). A dice was used to generate the random allocation. Stratified randomisation: according to clinical severity score (breakpoint 9.0) and localisation of the sinusitis (unilateral maxillary, bilateral maxillary or in at least 1 of the remaining sinus regions). If maxillary sinusitis in combination with sinusitis in 1 of the other sinus regions: stratification to 1 of the maxillary sinusitis groups |
| Allocation concealment (selection bias) | Low risk | Comment: the statistician sent the randomisation list to the company that produced the medication boxes with numbers according to the list. The author received the numbered boxes for each of the subgroups from the company (information from the main investigator). Tablets appeared similar. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: the trial was double‐blind (at participant, general practitioner, and radiologist level). The randomisation codes were broken after the whole study was finished. If another antibiotic was prescribed because of clinical failure (evaluation day 10), the randomisation code was not broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate at day 10: 3/130 (2.3%)
The ratio of participants with missing data to participants with events: 0.07. Discontinuation of trial medication rate: 12/130 (9.2%).
Treatment compliance was not reported. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. |
| Other bias | Low risk | Comment:
|
Lindbaek 1998.
| Methods | Study design: RCT Study duration: January to May 1994 and November 1994 to May 1995 |
|
| Participants | Setting: general practice Country: Norway Health status: clinical diagnosis of acute sinusitis (> 7 and < 30 days), confirmed by computed tomography (presence of mucosal thickening of 5 mm without fluid levels or total opacification, independently scored by 2 experienced radiologists). Ear, nose, and throat comorbidity was not assessed. Number:
Age: mean 39.7 (range 16 to 83) Sex: 61% women Exclusion criteria: age 15 or under, pregnancy, ongoing antibiotic treatment, immunosuppressive treatment, previous operations in the nose or sinus region, misuse of alcohol or narcotics, rheumatic disease, and allergy to penicillin. Participants with symptoms for more than 30 days were excluded because of possible chronic sinusitis. Participants with high fever and strong pain were not included because of ethical considerations. |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: concomitant use of nasal decongestants and paracetamol was allowed. Comment: For this review, group 1 and 2 were combined in the analyses. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding source: government Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: restricted randomisation (blocks of 3 within each of 6 subgroups). A dice was used to generate the random allocation. Stratified randomisation: according to clinical severity score (breakpoint 9.0) and localisation of the sinusitis (unilateral maxillary, bilateral maxillary or in at least 1 of the remaining sinus regions). If maxillary sinusitis in combination with sinusitis in 1 of the other sinus regions: stratification to 1 of the maxillary sinusitis groups. |
| Allocation concealment (selection bias) | Low risk | Comment: the statistician sent the randomisation list to the company that produced the medication boxes with numbers according to the list. The author received the numbered boxes for each of the subgroups from the company (information from the main investigator). Tablets appeared similar. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: the trial was double‐blind (at participant, general practitioner, and radiologist level). The randomisation codes were broken after the whole study was finished. If another antibiotic was prescribed because of clinical failure (evaluation day 10), the randomisation code was not broken. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/70 participants were taken out of the study because of bad‐quality CT scans. Post‐randomisation dropout rate at day 10: 5/68 (7.4%)
Ratio of participants with missing data to participants with events: 0.11 Discontinuation of trial medication rate: 5/63
Treatment compliance: not reported |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. |
| Other bias | Low risk | Comment: detailed description of demographic characteristics and sinusitis severity rating with which to assess the comparability of the groups at baseline |
Meltzer 2005.
| Methods | Study design: RCT Study duration: January to September 2003 |
|
| Participants | Setting: not specified (71 medical centres) Country: 14 countries Health status: people presenting with signs and symptoms of acute rhinosinusitis for ≥ 7 days but ≤ 28 days and major symptom score ≥ 5 but ≤ 12 at screening and baseline visits with no more than 3 of the 5 following symptoms rated severe at the baseline visit: rhinorrhoea, postnasal drip, nasal congestion/stuffiness, sinus headache and facial pain/pressure/tenderness on palpation over the paranasal sinuses Numbers:
Age: age 35.9 in treatment group 3, 34.4 in control group Sex: 66% women Exclusion criteria: signs or symptoms suggestive for fulminant bacterial sinusitis (fever ≥ 101 °F/38.3 °C, persistent severe unilateral facial or tooth pain, facial swelling, dental involvement, or a worsening of symptoms after initial improvement), chronic rhinosinusitis (or sinus or nasal surgery for this condition within 6 months before screening), otitis or atrophic rhinitis, nasal polyps noted on anterior rhinoscopic examination, symptomatic seasonal allergic rhinitis (after pollen exposure during the study), an allergy to corticosteroids, any other condition that would interfere with study evaluations, unstable asthma or with a history of exacerbations within 30 days before screening or forced expiratory volume in 1 second (FEV1) < 65% of predicted within 3 months before screening |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: forbidden (nasal saline, nasal cromolyn sodium ipratropium bromide, corticosteroids (excluding oral inhaled corticosteroids for mild to moderate persistent asthma), antihistamines, decongestants, leukotriene pathway modifiers, analgesics, non‐steroidal anti‐inflammatory drugs). |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Funding source: Schering‐Plough Research Institute Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects ... were randomized in a 1:1:1:1 ratio to 4 treatment arms" Quote: "Randomisation was performed according to a computer‐generated code, stratified on the basis of duration of rhinosinusitis symptoms before baseline (7 to 14 days and 15 to 28 days)" |
| Allocation concealment (selection bias) | Low risk | Quote: "The study was conducted in accordance with the Declaration of Helsinki and guidelines on Good Clinical Practice." Comment: the randomisation schedule for blinding of treatments was maintained by the sponsor and was disclosed only after the study completion and database closure. A set of sealed envelopes corresponding to the individual participant supplies, which contained the identification of the test drug, was provided to each site to enable the investigator to identify the treatment assignment of an individual participant, in the event of an emergency that requires this knowledge, without compromising the blinding of other study participants. These envelopes were returned to the sponsor, and open envelopes were accompanied by a written explanation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: a double‐dummy blinding technique was used during the treatment phase. Participants units were numbered from 0001 to 3000. All study drugs dispensed were labelled with the study number, packaging requisition number, treatment unit number, and the investigational use statement with the instructions for proper storage conditions. Placebo or amoxicillin were identical in appearance. Mometasone furoate nasal spray and placebo spray were identical in appearance. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation dropout rate: 13/499 (2.6%) at day 15, 26/499 (5.2%) at day 29 (4/503 participants were excluded after randomisation since they did not meet the protocol criteria for entry. These were not considered as drop outs)
Reasons for missing data: loss to follow‐up (13 lost to follow‐up after treatment phase, 13 during follow‐up phase) Ratio of participants with missing data to participants with events: not calculable, because the primary outcome was not 'cure' but a difference in symptom scores Discontinuation of treatment: 49/499 (9,8%)
Reasons for discontinuation of treatment: adverse events, treatment failure, lost to follow‐up, wish to discontinue, non‐compliance with the protocol The authors stated that the analyses were based on an ITT population. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. The outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. |
| Other bias | Low risk | Comment:
|
Merenstein 2005.
| Methods | Study design: RCT Study duration: 1 October 2001 to 31 March 2003 |
|
| Participants | Setting: ambulatory care (1 suburban ambulatory care office) Country: USA Health status: people presenting with at least 1 cardinal feature described by the clinical prediction rule and having symptoms for at least 7 days
Numbers:
Age: mean 35.1 in the amoxicillin group and 32.6 in the placebo group Sex: 69% women Exclusion criteria: antibiotic treatment within the past month in the history, allergy to penicillin, sinus surgery in history, compromised immunity, pneumonia in history, and streptococcal pharyngitis in history |
|
| Interventions | Treatment group:
Control group:
|
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Funding source: supported by a grant from the American Academy of Family Physicians and the American Academy of Family Physicians Foundation Joint AAFP/F‐AAFP Grant Awards Program. Support was also provided by the Capitol Area Primary Care Research Network. Contact with study authors for additional information: about random sequence generation, allocation concealment, blinding Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: they used stratified randomisation with each physician representing the strata, and participants were randomised in block sizes of 6. A computer random number generator was used to create the permuted blocks. A biostatistician who was not employed by Georgetown University performed the allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Comment: prior to the start of the trial, envelopes containing amoxicillin or placebo were prepared by the pharmacy, and each envelope was labelled with a study ID. The envelopes given to each participant contained 40 capsules, either placebo or amoxicillin, with instructions to take twice daily for 10 days. The randomisation codes were sent to the Pharmacy Department at Georgetown and were kept in a locked cabinet. Participants were consecutively enrolled over the 18‐month enrolment period. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: the envelopes were opaque, and the pills within were identical in appearance, size, shape, colour, and taste. All study physicians, participants, and research co‐ordinators were blinded to the treatment given to each participant. Through this process allocation concealment was achieved over the entire course of the enrolment period; neither physician nor participant could determine which treatment the next participant would receive. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate: 19/135 (14%)
Reasons for missing data: loss to follow‐up (only baseline data collected) Ratio of participants with missing data to participants with events: 0.33 The authors state that the primary analyses were performed using the ITT principle. The dropouts were counted as "not improved" in the ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. The analysis of the subgroups was not specified in the methods section. |
| Other bias | Low risk |
|
Norrelund 1978.
| Methods | Study design: RCT Study duration: between January 10, 1977 and June, 30, 1977 |
|
| Participants | Setting: general practice (19 general practitioners) Country: Denmark Health status: participants showing at least 3 symptoms, including at least 1 of the main symptoms
Number:
Age: older than 14 years, mean unknown Sex: 61% women Exclusion criteria: penicillin allergy, pregnancy |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: concomitant use of nasal decongestants allowed (xylometazoline 0.1% nasal spray, 4 times daily). |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Funding source: not stated Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: blocked randomisation (each doctor had been sent a box containing 10 glasses, of which half in random sequence contained an active ingredient). No information about the process of selecting the blocks |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about the centralisation of randomisation or the numbering of the glasses |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Comment: the glasses contained pivampicillin or identical‐looking placebo tablets. No information about the blinding of healthcare providers and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate: 5/140 (3.6%)
The ratio of participants with missing data to participants with events: 0.07. No ITT analysis: participants who needed referral to an ENT specialist or discontinued medication because of side effects were allowed to be removed from the trial. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were not predefined. Nevertheless, they predefined which symptoms, side effects, and medication intakes they would register. The definition of 'cure' is described for the first time in the results section. |
| Other bias | Low risk | Comment:
|
Rantanen 1973.
| Methods | Study design: RCT Study duration: not reported |
|
| Participants | Setting: "outpatients" Country: not specified Health status: participants were diagnosed with acute maxillary sinusitis. The diagnosis was based on anamnestic data, clinical examination, and irrigation findings (rated macroscopically with respect to the amount and quality of the secretion: purulent secretion, mucous secretion, no secretion). X‐ray examination (4 projections) revealed homogenous shadows in the sinuses or fluid level. Secretion for bacteriological examination was withdrawn through a puncture needle under sterile conditions. Number: treatment (27); control (34) Age: mean 34 years Sex: 64% women Exclusion criteria: cases of sinusitis who were treated previously |
|
| Interventions | Treatment group:
Control group:
Co‐interventions (for all participants):
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
Separate analyses were performed for sinuses with bacterial inflammation and sterile inflammation. |
|
| Notes | Funding source: not stated Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided about assignment. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided about randomisation list. Information about numbering or appearance of drug containers is lacking. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The examiners did not know to which of the therapeutic schemes each participant belonged." Comment: double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "All participants recovered completely within 4 weeks." Comment:
|
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. "Cure" was measured for each "sinus" instead of each participant, which was confusing and made pooling with other studies impossible. |
| Other bias | Unclear risk | Comment: insufficient power (number of participants far too low) |
Stalman 1997.
| Methods | Study design: RCT Study duration: between September 1, 1993 and August 31, 1995 |
|
| Participants | Setting: general practice (12 family practices) Country: the Netherlands Health status: people with symptoms of an upper respiratory tract infection for at least 5 days, and 3 main symptoms or 2 main symptoms and 1 other symptom
Number:
Age: mean 37 Sex: 65% women Exclusion criteria: people with xylometazoline nose drop treatment lasting more than 7 days, comorbidity (diabetes mellitus, heart failure, immune deficiency), pregnancy or breastfeeding, symptoms lasting longer than 3 months, antibiotic treatment in the previous 4 weeks, allergy to doxycycline, severe illness resulting from a sinusitis in 1 of the other sinuses, antiacid or iron treatment, referral to an ENT specialist, inability to speak Dutch |
|
| Interventions | Treatment group:
Control group:
Co‐interventions (for all participants):
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funding source: supported by grants from the Nederlandse organisatie voor Wetenschappelijk Onderzoek and Pharbita Ltd Contact with study authors for additional information: none. Young 2008 had contact with the study authors and obtained the exact cure rates at day 10. Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: participants were assigned to doxycyline or placebo treatment in blocks of 4 according to a computer‐generated randomisation schedule. |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about centralisation of randomisation, numbering of drug containers, or opaque, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: doxycycline and placebo appeared and tasted the same. Blinding of participants and treatment team was maintained throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate at day 10: 6/192 (3.1%)
Ratio of participants with missing data to participants with events: 0.05 Discontinuation of trial medication rate: 20/186 (10.7%)
All these participants were included in the analysis following the ITT principle. |
| Selective reporting (reporting bias) | Low risk | The study protocol is described in the methods section. The primary and secondary endpoints were predefined. Only the definition of "improvement" was not stated clearly. |
| Other bias | Low risk | Comment:
|
Van Buchem 1997a.
| Methods | Study design: RCT Study duration: between 1 March 1993 and 1 March 1994 |
|
| Participants | Setting: general practice Country: the Netherlands Health status: adults with a clinical diagnosis of maxillary sinusitis (history, physical examination), for whom antibiotic therapy was considered, confirmed by radiograph (> 5 mm mucosal thickening, opacity or air‐fluid level). Ear, nose, and throat comorbidity was assessed; approximately 12% had allergic disease. Number:
Age: mean 34 Sex: 63% women Exclusion criteria: other nasal disorders (e.g. nasal polyps), concurrent bronchitis, current episodes of longer than 3 months, antibiotic treatment during the previous month, known hypersensitivity to amoxicillin, hepatic, renal, or immunological disorder, and coagulation abnormalities |
|
| Interventions | Treatment group:
Control group:
Co‐interventions (for all participants):
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Bacteriological outcomes were not assessed. |
|
| Notes | Funding source: not stated Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: unrestricted randomisation (computer‐generated list used for allocation) Quote: "The randomisation of allocation of the amoxicillin or placebo (distributed in identical bottles) was computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation of participants was carried out and capsules were provided by the hospital pharmacy in the hospital to which participants were referred to the ENT specialist." Quote: "During the trial, the code of allocation schedule was kept in the office of the head of the hospital pharmacy, and was broken prematurely only if severe clinical development or severe adverse effects occurred" Comment: the capsules with amoxicillin or placebo looked and tasted identical and were prescribed in the same frequency and for the same duration. The capsules were distributed in identical bottles. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: nature of the medication blinded for the pharmacist's assistant, participant, and ENT specialist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate: 8/214 (3.7%)
Reason for missing data: not attending follow‐up visit Ratio of participants with missing data to participants with events: 0.07 Discontinuation of trial medication rate: 1/210 (0.5%)
Treatment compliance reported as 98% assessed by pills taking by participants. Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. |
| Other bias | Unclear risk | Comment:
|
Varonen 2003.
| Methods | Study design: RCT Study duration: from November 1998 to October 1999 |
|
| Participants | Setting: ambulatory care (9 practices) Country: Finland Health status: people with an upper respiratory tract infection and having at least 3 main symptoms and 1 clinical sign
Numbers:
Age: mean 40.6 in treatment group and 38.1 in control group Sex: 70% women (2 unknown sex) Exclusion criteria were acute maxillary sinusitis symptoms lasting over 30 days, antibiotics during the previous month, allergy to study medications, pregnancy, breastfeeding, exacerbation of a diagnosed chronic maxillary sinusitis, previous paranasal sinus surgery, clinical suspicion of dental or frontal sinusitis or pansinusitis, suspicion of a severe complication, and previous sinus surgery. |
|
| Interventions | Treatment group:
Control group:
Co‐interventions: allowed if the physician considered them necessary.
For this review, group 1, 2, and 3 were combined in the analyses. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Funding source: Stakes, the National Research and Development Centre for Welfare and Health covered the administrative and travel costs of this study. Leiras‐Schering and SmithKline Beecham provided the study medication. Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The treatments were previously randomised in blocks of 20 consecutive participants at the Military Pharmacy in Helsinki." |
| Allocation concealment (selection bias) | Low risk | Quote: "The study medications were coded with 6‐number individual codes." Quote: "During the trial, the senior researcher kept the code and was the primary contact in the case of adverse effects or severe complications. All study centres also had the code in a closed envelope to be opened only if the senior researcher could not be reached." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: the medication bottles were identically sealed. Quote: "Physicians, participants, and the main researcher remained blinded to the treatments until the recruitment was ended." Comment: the result of the ultrasound was not disclosed to the participant. Comment: the main researcher did not know the participant's history, treatment, or the result of the ultrasound examination while interviewing the participant 14 to 16 days after inclusion. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate: 8/150 (5,3%)
Ratio of participants with missing data to participants with events: 0.07 Comment:
|
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. |
| Other bias | Low risk | Number of participants per health centre: 15.8 |
Williamson 2007.
| Methods | Study design: RCT (factorial design) Study duration: from November 2001 to November 2005 |
|
| Participants | Setting: ambulatory care (58 family practices, 74 family physicians) Country: UK Health status: people presenting with uncomplicated acute illness (< 28 days) and at least 2 symptoms and 1 clinical sign of sinusitis (according to the Berg and Carenfelt criteria: purulent nasal discharge predominating on 1 side, local facial pain predominating on 1 side, purulent nasal discharge on both sides, pus in the nasal cavity) Numbers:
Age: mean 43 in amoxicillin group and 42 in placebo group Sex: 72.5% women Exclusion criteria: < 2 of the Berg and Carenfelt criteria (low probability of sinusitis), history of recurrent sinusitis (≥ 2 attacks of acute sinusitis in the previous 12 months), significant morbidities (poorly controlled diabetes or heart failure), pregnant or breastfeeding, allergies, a history of adverse reactions to either medications, and receiving antibiotics or steroids in the previous month |
|
| Interventions | Treatment group:
Control group:
For this review, we reduced the 4 treatment arms to 2 (factorial design): treatment group 1 and 2 (treatment group) versus treatment group 3 and control group (control group) |
|
| Outcomes | Primary outcome:
Secondary outcome:
|
|
| Notes | Funding source: supported by the UK Department of Health. The UK Department of Health did not participate in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. Contact with study authors for additional information: none Other notes:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Recruitment plan was for 4 recruited cases per family physician (1 block randomised pack of 4 per physician and 2 physicians per practice)." Quote: "The packs were made up using random number tables." Quote: "Randomisation was performed at the level of the patient." Quote: "Each randomized pack therefore consisted of an auditable sequence of the 4 possible combinations of the 2 interventions and physicians were instructed to use the packs in sequence." |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent person to the trial team was employed for distribution using the random sequence and trial code." Quote: "The code break was kept in a sealed envelope in a locked filing cabinet at the university throughout the study period." Quote: "All drug containers and all trial materials were identifiable only by the randomisation code number." Comment: blind‐sequenced trial packs. Quote: "The sealed, opaque, numbered packages contained physician instructions and either active or placebo drugs that were distributed in batches in randomised blocks of 4." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Neither the antibiotic nor the nasal steroid spray was recognisable as active or placebo medication, identical in taste and appearance." Comment: no significant difference in participant's belief in the effectiveness of the treatment allocated (0‐to‐5 scales) for the antibiotic tablet versus placebo tablet (P = 0.07), or for steroid spray versus placebo spray (P = 0.25). Comment: the single code break envelope was not opened until all data collection had been completed and all variables had been entered into the database. All outcome assessments were recorded on a central database and checked and verified when necessary by a research fellow blinded to treatment grouping. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post‐randomisation dropout rate: 31/240 (12.9%) due to loss to follow‐up
Reasons for missing data: loss to follow‐up 2 additional participants withdrew (1 in the amoxicillin group and 1 in the placebo group) because of ongoing symptoms; we considered these as failures in our review. Ratio of participants with missing data to participants with events: 0.23 Comment:
|
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is described in the methods section. The primary and secondary endpoints were predefined. |
| Other bias | Low risk | Comment:
|
CRP: C‐reactive protein CT: computed tomography ENT: ear, nose, and throat ITT: intention‐to‐treat RCT: randomised controlled trial SNOT: Sino‐Nasal Outcome Test VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gananca 1973 | Randomised, double‐blind, placebo‐controlled trial. Acute sinusitis diagnostic criteria did not fulfil inclusion criteria for this review (clinical symptoms and signs and bacteriologic criteria). |
| Gananca 1977 | Randomised, double‐blind, placebo‐controlled trial. Acute sinusitis diagnostic criteria did not fulfil inclusion criteria for this review (clinical symptoms and signs and bacteriologic criteria). |
| Hadley 2010 | Prospective, multicentre, placebo‐controlled, randomised, double‐blind phase IIIb clinical trial. Acute sinusitis diagnostic criteria did not fulfil inclusion criteria for this review (clinical symptoms and signs, confirmed by radiography; analysis only performed for participants with positive sinus culture). |
| Hansen 2000a | Randomised, double‐blind, placebo‐controlled trial. Acute sinusitis diagnostic criteria did not fulfil inclusion criteria for this review (clinical symptoms (maxillary pain) and raised values of either C‐reactive protein or erythrocyte sedimentation rate. |
| Haye 1998 | Randomised, double‐blind, placebo‐controlled trial. Acute sinusitis diagnostic criteria did not fulfil inclusion criteria for this review (exclusion of participants with empyema (defined as complete opacity or an air‐fluid level, or a mucosal thickness of 6 mm or more as measured at the upper lateral border of the maxillary sinus)). |
Differences between protocol and review
Two Cochrane Reviews, 'Antibiotics for acute maxillary sinusitis in adults' and 'Antibiotics for clinically diagnosed acute rhinosinusitis in adults' (Ahovuo‐Saloranta 2014; De Sutter 2012), described the effects of antibiotics for acute rhinosinusitis. Although both reviews studied the same condition, they evaluated different populations, namely participants who were diagnosed by imaging (Ahovuo‐Saloranta 2014), versus participants who were diagnosed by clinical signs and symptoms (De Sutter 2012). Different approaches resulted in different conclusions, which was confusing for clinicians. We therefore merged these Cochrane Reviews while maintaining the relevant distinction between both populations. The comparison between antibiotics, as published by Ahovuo‐Saloranta 2014, was omitted. Instead of clinical trials, local up‐to‐date antibiotic resistance patterns should guide clinicians in making the best choice regarding which antibiotic and dose in the subgroup of people with suspected bacterial rhinosinusitis should be prescribed.
Contributions of authors
An De Sutter (ADS) wrote the first draft of the protocol. Marieke Lemiengre (ML) wrote the first draft of the amalgamation of the separate reviews Ahovuo‐Saloranta 2014 and De Sutter 2012. ADS, Mieke van Driel (MVD), Dan Merenstein (DM), Helena Liira (HL), and Marjukka Mäkelä (MM) commented on the draft and suggested changes that consequently led to a new version.
Sources of support
Internal sources
-
Ghent University, Belgium.
Salary
External sources
No sources of support supplied
Declarations of interest
Marieke B Lemiengre: none known. Mieke L van Driel: Co‐author of De Sutter 2002. No other conflicts of interest known. Dan Merenstein: Main investigator of Merenstein 2005. No other conflicts of interest known. Helena Liira: Main investigator of Varonen 2003. No other conflicts of interest known. Marjukka Mäkelä: Co‐author of Varonen 2003. No other conflicts of interest known. An IM De Sutter: Main investigator of De Sutter 2002. No other conflicts of interest known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Axelsson 1970 {published data only}
- Axelsson A, Chidekel N, Grebelius N, Jensen C. Treatment of acute maxillary sinusitis. A comparison of four different methods. Acta Otolaryngologica 1970;70(1):71‐6. [DOI] [PubMed] [Google Scholar]
Bucher 2003 {published data only}
- Bucher HC, Tschudi P, Young J, Periat P, Welge‐Luussen A, Zust H, et al. Effect of amoxicillin‐clavulanate in clinically diagnosed acute rhinosinusitis: a placebo‐controlled, double‐blind, randomized trial in general practice. Archives of Internal Medicine 2003;163(15):1793‐8. [PUBMED: 12912714] [DOI] [PubMed] [Google Scholar]
De Sutter 2002 {published data only}
- Sutter AI, Meyere MJ, Christiaens TC, Driel ML, Peersman W, Maeseneer JM. Does amoxicillin improve outcomes in patients with purulent rhinorrhea? A pragmatic randomized double‐blind controlled trial in family practice. Journal of Family Practice 2002;51(4):317‐23. [PUBMED: 11978253] [PubMed] [Google Scholar]
Garbutt 2012 {published data only}
- Garbutt JM, Banister C, Spitznagel E, Piccirillo JF. Amoxicillin for acute rhinosinusitis: a randomized controlled trial. JAMA 2012;307(7):685‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaiser 2001 {published data only}
- Kaiser L, Morabia A, Stalder H, Ricchetti A, Auckenthaler R, Terrier F, et al. Role of nasopharyngeal culture in antibiotic prescription for patients with common cold or acute sinusitis. European Journal of Clinical Microbiology and Infectious Diseases 2001;20(7):445‐51. [DOI] [PubMed] [Google Scholar]
Lindbaek 1996 {published data only}
- Lindbaek M, Hjortdahl P, Johnsen ULC. Randomised, double blind, placebo controlled trial of penicillin V and amoxycillin in treatment of acute sinus infections in adults. BMJ 1996;313(7053):325‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindbaek 1998 {published data only}
- Lindbaek M, Kaastad E, Dolvik S, Johnsen U, Laerum E, Hjortdahl P. Antibiotic treatment of patients with mucosal thickening in the paranasal sinuses, and validation of cut‐off points in sinus CT. Rhinology 1998;36(1):7‐11. [PubMed] [Google Scholar]
Meltzer 2005 {published data only}
- Meltzer EO, Bachert C, Staudinger H. Treating acute rhinosinusitis: comparing efficacy and safety of mometasone furoate nasal spray, amoxicillin, and placebo. Journal of Allergy and Clinical Immunology 2005;116(6):1289‐95. [PUBMED: 16337461] [DOI] [PubMed] [Google Scholar]
- Svensson J, Lundberg J, Olsson P, Stjärne P, Tennvall GR. Cost‐effectiveness of mometasone furoate nasal spray in the treatment of acute rhinosinusitis. Primary Care Respiratory Journal 2012;21(4):412‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merenstein 2005 {published data only}
- Merenstein D, Whittaker C, Chadwell T, Wegner B, D'Amico F. Are antibiotics beneficial for patients with sinusitis complaints? A randomized double‐blind clinical trial. Journal of Family Practice 2005;54(2):144‐51. [PUBMED: 15689289] [PubMed] [Google Scholar]
Norrelund 1978 {published data only}
- Norrelund N. Treatment of sinusitis in general practice. A controlled investigation of pivampicillin (Pondocillin) [Behandling af sinuitis i almenpraksis. En kontrolleret undersogelse over pivampicillin (Pondocillin)]. Ugeskrift for Laeger 1978;140(45):2792‐5. [PUBMED: 362655] [PubMed] [Google Scholar]
Rantanen 1973 {published data only}
- Rantanen T, Arvilommi H. Double‐blind trial of doxicycline in acute maxillary sinusitis. A clinical and bacteriological study. Acta Otolaryngologica 1973;76(1):58‐62. [DOI] [PubMed] [Google Scholar]
Stalman 1997 {published data only}
- Stalman W, Essen GA, Graaf Y, Melker RAC. The end of antibiotic treatment in adults with acute sinusitis‐like complaints in general practice? A placebo‐controlled double‐blind randomized doxycycline trial. British Journal of General Practice 1997;47(425):794‐9. [PUBMED: 9463979] [PMC free article] [PubMed] [Google Scholar]
Van Buchem 1997a {published data only}
- Buchem FL, Knottnerus JA, Schrijnemaekers VJ, Peeters MF. Primary‐care‐based randomised placebo‐controlled trial of antibiotic treatment in acute maxillary sinusitis. Lancet 1997;349(9053):683‐7. [DOI] [PubMed] [Google Scholar]
Varonen 2003 {published data only}
- Varonen H, Kunnamo I, Savolainen S, Makela M, Revonta M, Ruotsalainen J, et al. Treatment of acute rhinosinusitis diagnosed by clinical criteria or ultrasound in primary care. A placebo‐controlled randomised trial. Scandinavian Journal of Primary Health Care 2003;21(2):121‐6. [PUBMED: 12877377] [DOI] [PubMed] [Google Scholar]
Williamson 2007 {published data only}
- Williamson IG, Rumsby K, Benge S, Moore M, Smith PW, Cross M, et al. Antibiotics and topical nasal steroid for treatment of acute maxillary sinusitis: a randomized controlled trial. JAMA 2007;298(21):2487‐96. [PUBMED: 18056902] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gananca 1973 {published data only}
- Gananca M, Trabulsi LR. The therapeutic effects of cyclacillin in acute sinusitis: in vitro and in vivo correlations in a placebo‐controlled study. Current Medical Research and Opinion 1973;1(6):362‐8. [DOI] [PubMed] [Google Scholar]
Gananca 1977 {published data only}
- Gananca MM, Gasel JJ. A double‐blind study with the association tetracycline, acetylsalicylic acid, phenacetin, caffeine and phenyltoloxamine in the treatment of acute sinusitis. Folha Medica 1977;75(5):583‐5. [Google Scholar]
Hadley 2010 {published data only}
- Hadley JA, Mosges R, Desrosiers M, Haverstock D, Veenhuyzen D, Herman‐Gnjidic Z. Moxifloxacin five‐day therapy versus placebo in acute bacterial rhinosinusitis. Laryngoscope 2010;120(5):1057‐62. [DOI] [PubMed] [Google Scholar]
Hansen 2000a {published data only}
- Hansen JG, Schmidt H, Grinsted P. Randomised, double blind, placebo controlled trial of penicillin V in the treatment of acute maxillary sinusitis in adults in general practice. Scandinavian Journal of Primary Health Care 2000;18(1):44‐7. [DOI] [PubMed] [Google Scholar]
Haye 1998 {published data only}
- Haye R, Lingaas E, Hoivik HO, Odegard T. Azithromycin versus placebo in acute infectious rhinitis with clinical symptoms but without radiological signs of maxillary sinusitis. European Journal of Clinical Microbiology and Infectious Diseases 1998;17(5):309‐12. [DOI] [PubMed] [Google Scholar]
Additional references
Ah‐See 2007
- Ah‐See KW, Evans AS. Sinusitis and its management. BMJ 2007;334:358‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Autio 2015
- Autio TJ, Koskenkorva T, Närkiö M, Leino TK, Koivunen P, Alho OP. Diagnostic accuracy of history and physical examination in bacterial acute rhinosinusitis. Laryngoscope 2015;125(7):1541‐6. [DOI: 10.1002/lary.25247] [DOI] [PubMed] [Google Scholar]
Axelsson 1972
- Axelsson A, Chidekel N. Symptomatology and bacteriology correlated to radiological findings in acute maxillary sinusitis. Acta Oto‐laryngologica 1972;74(1):118‐22. [DOI] [PubMed] [Google Scholar]
Bachert 2003
- Bachert C, Hörmann K, Mösges R, Rasp G, Riechelmann H, Müller R, et al. An update on the diagnosis and treatment of sinusitis and nasal polyposis. Allergy 2003;58(3):176‐91. [DOI] [PubMed] [Google Scholar]
Berg 1986
- Berg O, Carenfelt C, Rystedt G, Anggärd A. Occurence of asymptomatic sinusitis in common cold and other acute ENT‐infections. Rhinology 1986;24(3):223‐5. [PubMed] [Google Scholar]
Blackwell 2014
- Blackwell DL, Lucas JW, Clarke TC. Summary health statistics for U.S. adults: national health interview survey, 2012. Vital and Health Statistics. Series 10, Data from the National Health Survey 2014;10(260):1‐161. [PUBMED: 24819891] [PubMed] [Google Scholar]
Brazzelli 2003
- Brazzelli MG, Astin MP. Making the Best Use of Department of Clinical Radiology. Guidelines for Doctors. 5th Edition. London: Royal College of Radiologists, 2003. [Google Scholar]
Burgstaller 2016
- Burgstaller JM, Steurer J, Holzmann D, Geiges G, Soyka MB. Antibiotic efficacy in patients with a moderate probability of acute rhinosinusitis: a systematic review. European Archives of Oto‐rhino‐laryngology 2016;273(5):1067‐77. [DOI: 10.1007/s00405-015-3506-z; PUBMED: 25597034] [DOI] [PubMed] [Google Scholar]
Costelloe 2010
- Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta‐analysis. BMJ 2010;340:c2096. [DOI: 10.1136/bmj.c2096] [DOI] [PubMed] [Google Scholar]
Ebell 2017
- Ebell MH, Hansen JG. Proposed clinical decision rules to diagnose acute rhinosinusitis among adults in primary care. Annals of Family Medicine 2017;15(4):347‐54. [DOI: 10.1370/afm.2060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falagas 2008
- Falagas ME, Giannopoulou KP, Vardakas KZ, Dimopoulos G, Karageorgopoulos DE. Comparison of antibiotics with placebo for treatment of acute sinusitis: a meta‐analysis of randomised controlled trials. Lancet Infectious Diseases 2008;8(9):543‐52. [DOI] [PubMed] [Google Scholar]
Fleming‐Dutra 2016
- Fleming‐Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010‐2011. JAMA 2016;315(17):1864‐73. [DOI: 10.1001/jama.2016.4151] [DOI] [PubMed] [Google Scholar]
Fokkens 2012
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50(1):1‐298. [DOI] [PubMed] [Google Scholar]
Garbutt 2001
- Garbutt JM, Goldstein M, Gellman E, Shannon W, Littenberg B. A randomized, placebo‐controlled trial of antimicrobial treatment for children with clinically diagnosed acute sinusitis. Pediatrics 2001;107(4):619‐25. [PUBMED: 11335733] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT. Version (accessed prior to 10 May 2018). Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Gulliford 2014
- Gulliford MC, Dregan A, Moore MV, Ashworth M, Staa Tv, McCann G, et al. Continued high rates of antibiotic prescribing to adults with respiratory tract infection: survey of 568 UK general practices. BMJ Open 2014;4(10):e006245. [DOI: 10.1136/bmjopen-2014-006245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gwaltney 1994
- Gwaltney JM Jr, Phillips CD, Miller RD, Riker D. Computed tomographic study of the common cold. New England Journal of Medicine 1994;330(1):25‐30. [DOI] [PubMed] [Google Scholar]
Gwaltney 1996
- Gwaltney JM Jr. Acute community‐acquired sinusitis. Clinical Infectious Diseases 1996;23(6):1209‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gwaltney 2005
- Gwaltney JM Jr. Sinusitis. In: Mandell GL, Bennett JE, Dolin R editor(s). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. Philadelphia: Elsevier, 2005:772‐83. [Google Scholar]
Hanssen 2000b
- Hansen JG, Schmidt H, Grinsted P. [Penicillin treatment of acute maxillary sinusitis in adults. A randomized, double‐blind, placebo‐controlled trial from general practice].. Ugeskr Laeger 2000;162(40):5351‐3. [PubMed] [Google Scholar]
Hickner 2001
- Hickner JM, Bartlett JG, Besser RE, Gonzales R, Hoffman JR, Sande MA. Principles of appropriate antibiotic use for acute rhinosinusitis in adults: background. Annals of Internal Medicine 2001;134(6):498‐505. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kenealy 2013
- Kenealy T, Arroll B. Antibiotics for the common cold and acute purulent rhinitis. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD000247.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kristo 2005
- Kristo A, Uhari M, Luotonen J, Ilkko E, Koivunen P, Alho OP. Cefuroxime axetil versus placebo for children with acute respiratory infection and imaging evidence of sinusitis: a randomized, controlled trial. Acta Paediatrica 2005;94(9):1208‐13. [PUBMED: 16278986] [DOI] [PubMed] [Google Scholar]
Lanza 1997
- Lanza DC, Kennedy DW. Adult rhinosinusitis defined. Otolaryngology Head and Neck Surgery 1997;117(3 Suppl 2):1‐7. [DOI] [PubMed] [Google Scholar]
Lethbridge‐Cejku 2006
- Lethbridge‐Cejku M, Rose D, Vickerie J. Summary health statistics for US adults: national health interview survey, 2004. Vital and Health Statistics. Series 10, Data from the National Health Survey 2006;10(228):19‐22. [PubMed] [Google Scholar]
Lindbaek 2002
- Lindbaek M, Hjortdahl P. The clinical diagnosis of acute purulent sinusitis in general practice ‐ a review. British Journal of General Practice 2002;52(479):491‐5. [PMC free article] [PubMed] [Google Scholar]
Lindbaek 2015 [pers comm]
- Lindbaek M. Illness duration of 7 days and purulent sinusitis: a question [personal communication]. Lemiengre MB, De Sutter AI 12 February 2015.
Low 1997
- Low DE, Desroires M, McSherry J, Garber G, Williams JW, Remy H, et al. A practical guide for the diagnosis and treatment of acute sinusitis. Canadian Medical Association Journal 1997;156(Suppl 6):1‐14. [PubMed] [Google Scholar]
McCaig 1995
- McCaig LF, Hughes JM. Trends in antimicrobial drug prescribing among office‐based physicians in the United States. JAMA 1995;273(3):214‐9. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Morris 2002
- Morris P, Leach A. Antibiotics for persistent nasal discharge (rhinosinusitis) in children. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD001094.pub2] [DOI] [PubMed] [Google Scholar]
Okkes 2005
- Okkes I, Oskam S, Boven K. EFP. Episodes of care in Dutch family practice. Epidemiological data based on the routine use of the International Classification of Primary Care (ICPC) in the Transition Project of the Academic Medical Center, University of Amsterdam (1985 to 2003). www.transhis.nl. Login required. Amsterdam: Academic Medical Center/University of Amsterdam, Department of Family Medicine, (accessed prior to 10 August 2018).
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenfeld 2007
- Rosenfeld RM, Singer M, Jones S. Systematic review of antimicrobial therapy in patients with acute rhinosinusitis. Otolaryngology‐Head and Neck Surgery 2007;137(3 Suppl):S32‐45. [DOI] [PubMed] [Google Scholar]
Rún 2015
- Rún Sigurðardóttir N, Nielsen AB, Munck A, Bjerrum L. Appropriateness of antibiotic prescribing for upper respiratory tract infections in general practice: comparison between Denmark and Iceland. Scandinavian Journal of Primary Health Care 2015;33(4):269‐74. [DOI: 10.3109/02813432.2015.1114349] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schappert 1998
- Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1996. Vital and Health Statistics. Series 13, Data from the National Health Survey 1998;13(134):1‐37. [PubMed] [Google Scholar]
Van Buchem 1997b
- Buchem FL, Knottnerus JA, Schrijnemaekers VJJ, Peeters MF. No benefit of antibiotic treatment in acute maxillary sinusitis: a randomized double‐blind placebo‐controlled study. Nederlands Tijdschrift voor Geneeskunde 1997;141(49):2405‐11. [Google Scholar]
Varonen 2000
- Varonen H, Mäkelä M, Savolainen S, Läärä E, Hilden J. Comparison of ultrasound, radiography, and clinical examination in the diagnosis of acute maxillary sinusitis: a systematic review. Journal of Clinical Epidemiology 2000;53(9):940‐8. [PUBMED: 11004420 ] [DOI] [PubMed] [Google Scholar]
Wald 1986
- Wald ER, Chiponis D, Ledesma‐Medina J. Comparative effectiveness of amoxicillin and amoxicillin‐clavulanate potassium in acute paranasal sinus infections in children: a double‐blind, placebo‐controlled trial. Pediatrics 1986;77(6):795‐800. [PUBMED: 3520469] [PubMed] [Google Scholar]
Willet 1994
- Willet LR, Carson JL, Williams JW Jr. Current diagnosis and management of sinusitis. Journal of General Internal Medicine 1994;9(1):38‐45. [DOI] [PubMed] [Google Scholar]
Williams 1993
- Williams JWJ, Simel DL. Does this patient have sinusitis? Diagnosing acute sinusitis by history and physical examination. JAMA 1993;270(10):1242‐6. [PubMed] [Google Scholar]
Young 2008
- Young J, Sutter A, Merenstein D, Essen GA, Kaiser L, Varonen H, et al. Antibiotics for adults with clinically diagnosed acute rhinosinusitis: a meta‐analysis of individual patient data. Lancet 2008;371(9616):908‐14. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ahovuo‐Saloranta 2011
- Ahovuo‐Saloranta A, Rautakorpi U‐M, Borisenko OV, Liira H, Williams JW Jr, Mäkelä M. Antibiotics for acute maxillary sinusitis. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD000243.pub2] [DOI] [PubMed] [Google Scholar]
Ahovuo‐Saloranta 2014
- Ahovuo‐Saloranta A, Rautakorpi UM, Borisenko OV, Liira H, Williams JW Jr, Mäkelä M. Antibiotics for acute maxillary sinusitis in adults. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD000243.pub3] [DOI] [PubMed] [Google Scholar]
De Sutter 2006
- Sutter AIM, Lemiengre M, Merenstein D, Young J, Driel ML. Antibiotics for clinically diagnosed acute rhinosinusitis in adults. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD006089] [DOI] [PubMed] [Google Scholar]
De Sutter 2012
- Sutter AIM, Lemiengre MB, Merenstein D, Young J, Driel ML. Antibiotics for clinically diagnosed acute rhinosinusitis in adults. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD006089.pub3] [DOI] [PubMed] [Google Scholar]
Lemiengre 2012
- Lemiengre MB, Driel ML, Merenstein D, Young J, Sutter AIM. Antibiotics for clinically diagnosed acute rhinosinusitis in adults. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD006089.pub4] [DOI] [PubMed] [Google Scholar]
Williams 1997
- Williams J Jr, Aguilar C, Makela M, Cornell J, Hollman D, Chiquette E, et al. Antibiotic therapy for acute sinusitis: a systematic literature review. Cochrane Database of Systematic Reviews 1997, Issue 1. [DOI: 10.1002/14651858.CD000243] [DOI] [Google Scholar]
Williams 1999
- Williams JW Jr, Aguilar C, Makela M, Cornell J, Hollman DR, Chiquette E, et al. Antimicrobial therapy for acute maxillary sinusitis. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD000243.pub2] [DOI] [Google Scholar]
Williams 2003
- Williams JW Jr, Aguilar C, Cornell J, Chiquette E, Dolor RJ, Makela M, et al. Antibiotics for acute maxillary sinusitis. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD000243] [DOI] [PubMed] [Google Scholar]


