Abstract
Background
This review adds to a series of reviews looking at primary medical management options for patients with chronic rhinosinusitis.
Chronic rhinosinusitis is common and characterised by inflammation of the lining of the nose and paranasal sinuses leading to nasal blockage, nasal discharge, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. Antifungals have been suggested as a treatment for chronic rhinosinusitis.
Objectives
To assess the effects of systemic and topical antifungal agents in patients with chronic rhinosinusitis, including those with allergic fungal rhinosinusitis (AFRS) and, if possible, AFRS exclusively.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; Cochrane Central Register of Controlled Trials (CENTRAL); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 17 November 2017.
Selection criteria
Randomised controlled trials (RCTs) with at least a two‐week follow‐up period comparing topical or systemic antifungals with (a) placebo, (b) no treatment, (c) other pharmacological interventions or (d) a different antifungal agent. We did not include post‐surgical antifungal use.
Data collection and analysis
We used the standard Cochrane methodological procedures. Our primary outcomes were disease‐specific health‐related quality of life (HRQL), patient‐reported disease severity and the significant adverse effects of hepatic toxicity (systemic antifungals). Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse effects of gastrointestinal disturbance (systemic antifungals) and epistaxis, headache or local discomfort (topical antifungals). We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results
We included eight studies (490 adult participants). The presence of nasal polyps on examination was an inclusion criterion in three studies, an exclusion criterion in one study and the remaining studies included a mixed population. No studies specifically investigated the effect of antifungals in patients with AFRS.
Topical antifungal treatment versus placebo or no intervention
We included seven studies (437 participants) that used amphotericin B (six studies; 383 participants) and one that used fluconazole (54 participants). Different delivery methods, volumes and concentrations were used.
Four studies reported disease‐specific health‐related quality of life using a range of instruments. We did not meta‐analyse the results due to differences in the instruments used, and measurement and reporting methods. At the end of treatment (one to six months) none of the studies reported statistically significant differences between the groups (low‐quality evidence ‐ we are uncertain about the result).
Two studies reported disease severity using patient‐reported symptom scores. Meta‐analysis was not possible. At the end of treatment (8 to 13 weeks) one study showed no difference and the second found that patients in the placebo group had less severe symptoms (very low‐quality evidence ‐ we are very uncertain about the result).
In terms of adverse effects, topical antifungals may lead to more local irritation compared with placebo (risk ratio (RR) 2.29, 95% confidence interval (CI) 0.61 to 8.62; 312 participants; 5 studies; low‐quality evidence) but little or no difference in epistaxis (RR 0.97, 95% CI 0.14 to 6.63; 225 participants; 4 studies, low‐quality evidence) or headache (RR 1.26, 95% CI 0.60 to 2.63; 195 participants; 3 studies; very low‐quality evidence).
None of the studies found a difference in generic health‐related quality of life (one study) or endoscopic score (five studies) between the treatment groups. Three studies investigated CT scan; two found no difference between the groups and one found a significant decrease in the mean percentage of air space occluded, favouring the antifungal group.
Systemic antifungal treatment versus placebo or no treatment
One study (53 participants) comparing terbinafine tablets against placebo reported that there may be little or no difference between the groups in disease‐specific health‐related quality of life or disease severity score (both low‐quality evidence). Systemic antifungals may lead to more hepatic toxicity events (RR 3.35, 95% CI 0.14 to 78.60) but fewer gastrointestinal disturbances (RR 0.37, 95% CI 0.04 to 3.36), compared to placebo, although the evidence was of low quality.
This study did not find a difference in CT scan score between the groups. Generic health‐related quality of life and endoscopic score were not measured.
Other comparisons
We found no studies that compared antifungal agents against other treatments for chronic rhinosinusitis.
Authors' conclusions
Due to the very low quality of the evidence, it is uncertain whether or not the use of topical or systemic antifungals has an impact on patient outcomes in adults with chronic rhinosinusitis compared with placebo or no treatment. Studies including specific subgroups (i.e. AFRS) are lacking.
Plain language summary
Topical or systemic antifungal therapy for chronic rhinosinusitis
Review question
We reviewed the evidence for the benefits and harms of antifungal treatment in patients with chronic rhinosinusitis including those with allergic fungal rhinosinusitis (AFRS).
Background
Chronic rhinosinusitis is a common condition characterised by inflammation of the nose and paranasal sinuses (a group of air‐filled spaces behind the nose, eyes and cheeks). Patients with chronic rhinosinusitis have at least two of the following symptoms for at least 12 weeks: either a blocked nose and/or discharge from their nose (runny nose) and one of either pain/pressure in their face or a reduced sense of smell (hyposmia). Some people also have nasal polyps, which are grape‐like swellings of the normal nasal lining inside the nasal passage and sinuses. Some people with chronic rhinosinusitis with nasal polyps are allergic to airborne fungus and this can cause a specific type of condition called allergic fungal rhinosinusitis (AFRS).
Fungal spores are commonly found in the nose as they are in the air we breathe. It is not clear if fungus plays a role in all cases of chronic rhinosinusitis but there is evidence that it may have a role in a subset of patients. Antifungal treatments work to kill fungal spores or to stop them growing. Antifungal treatments for chronic rhinosinusitis are used either topically (put into the nose) or taken systemically (by mouth).
Study characteristics
We included eight studies (490 adult participants). Seven studies (437 participants) investigated topical antifungals (nasal sprays or irrigations) and one study (53 participants) investigated systemic antifungals (tablets). All studies compared antifungals to placebo or no treatment. Most studies were well conducted and there was a mix of patients with chronic rhinosinusitis both with, and without, nasal polyps.
Key results and quality of the evidence
At the end of at least four weeks treatment, none of the studies found that patients using antifungals (topical or systemic) had a better quality of life or less severe symptoms than patients who used placebo or had no treatment.
Not many participants in the studies reported having adverse effects. Topical antifungals may lead to more nasal irritation compared with placebo. It is uncertain if patients taking topical antifungals have more headaches or nosebleeds than with placebo.
For systemic antifungals, it is uncertain if patients using antifungals have more problems with their liver (hepatic toxicity) than with placebo. Systemic antifungals may lead to fewer patients with gastrointestinal disturbances compared to placebo.
We found no studies that compared antifungal treatment with other treatments for chronic rhinosinusitis.
We assessed the quality of the evidence as either low (further research is very likely to have an important impact on our confidence in the result) or very low (any estimate of the result is very uncertain), as some of the results are only from one or two studies, which do not have a lot of participants. Moreover, the different studies reported outcomes using different measurement scales making it difficult to draw conclusions.
Conclusions
Due to the very low quality of the evidence, it is uncertain whether or not the use of topical or systemic antifungals has an impact on patient outcomes in adults with chronic rhinosinusitis compared with placebo or no treatment. More trials are needed to assess well‐defined patient populations (such as the AFRS subgroup) and to evaluate other antifungals that have not been assessed in randomised controlled trials.
Summary of findings
Background
This review will update and replace a previously published review 'Topical and systemic antifungal therapy for the symptomatic treatment of chronic rhinosinusitis' (Sacks 2011).
Description of the condition
Chronic rhinosinusitis is characterised by inflammation of the nose and paranasal sinuses. It is defined by the presence of two or more symptoms, one of which must be nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip) and one of facial pain/pressure and/or reduction or loss of sense of smell. Symptoms must have continued for at least 12 weeks. In addition, people must have either mucosal changes within the ostiomeatal complex or sinuses (or both) as evidenced by a computerised tomography (CT) scan and/or endoscopic signs of at least one of the following: nasal polyps, mucopurulent discharge primarily from the middle meatus or oedema/mucosal obstruction primarily in the middle meatus (EPOS 2012).
Two major phenotypes of chronic rhinosinusitis have been identified based on the presence or absence of nasal polyps on examination. Nasal polyps are tumour‐like hyperplastic swellings of the nasal mucosa, most commonly originating from within the ostiomeatal complex (Larsen 2004). Chronic rhinosinusitis with nasal polyps (CRSwNP) is diagnosed when polyps are seen (on direct or endoscopic examination) bilaterally in the middle meatus. The acronym CRSsNP is used for the condition in which no polyps are present.
Although the aetiology of chronic rhinosinusitis is not fully understood, it may involve abnormalities in the host response to irritants, commensal and pathogenic organisms and allergens, obstruction of sinus drainage pathways, abnormalities of normal mucociliary function, loss of the normal mucosal barrier or infection. Two typical profiles may be observed with respect to inflammatory mediators; in eosinophilic chronic rhinosinusitis, which is typically associated with nasal polyps, high levels of eosinophils, immunoglobulin E (IgE) and interleukin (IL)‐5 may be found, while in neutrophilic chronic rhinosinusitis, more often associated with chronic rhinosinusitis without polyps, neutrophils predominate, with elevated interferon (IFN) gamma, IL‐8 and tumour necrosis factor (TNF) (EPOS 2012).
While treatment decisions should be made based on an understanding of the patient's chronic rhinosinusitis phenotype and likely aetiology, in practice treatment may be initiated without knowledge of the polyp status, particularly in primary care. This review (and most of its companion reviews) consider patients with and without polyps together in the initial evaluation of treatment effects. However, subgroup analyses explore potential differences between them.
There is much debate regarding the role of fungus in the aetiology of chronic rhinosinusitis. Intranasal fungus can be demonstrated in nearly all diseased and normal sinuses (Braun 2003; Lackner 2005; Ponikau 1999). The definition and categorisation of fungal rhinosinusitis is still controversial but the most commonly accepted system divides the condition into two: invasive and non‐invasive disease, based on histopathological evidence of tissue invasion by fungi (Chakrabarti 2009). Invasive fungal disease is a unique entity and represents angioinvasive fungal propagation in the immunocompromised host setting. This is not the common presentation of chronic rhinosinusitis experienced by the vast majority of chronic sinusitis patients. Treatments for invasive fungal sinusitis usually include surgery followed by medical treatment (EPOS 2012).
Non‐invasive fungal rhinosinusitis can be divided into two categories: a fungus ball (also known as mycetoma) and allergic fungal rhinosinusitis (AFRS). A fungus ball is a fungal collection in an abnormal sinus that usually produces only mild symptoms and can be surgically removed. Patients with fungus balls will not be included in this review.
AFRS is a well‐recognised subgroup of chronic rhinosinusitis, in which an IgE mediated hypersensitivity to fungal elements drives the inflammatory process. Allergic fungal rhinosinusitis is generally diagnosed using the Bent‐Kuhn criteria (type I hypersensitivity confirmed by history, skin tests or serology; nasal polyposis; characteristic CT scan (double density sign); eosinophilic mucus without fungal invasion into sinus tissue; positive fungal stain of sinus contents removed intraoperatively or during office endoscopy) (Bent 1994). A more recent derivation of this was proposed by Philpott et al whereby immunocompetence replaces type I hypersensitivity, reflecting the group of characteristic patients seen in rhinologic practice (Philpott 2011). Following on from this, there is some evidence that a much broader group of patients with chronic rhinosinusitis with an eosinophilic inflammation may be mediated by fungal elements and a subsequent cascade of immune effects through non‐classical pathways (Sok 2006). Furthermore, since Bent and Kuhn defined their subgroup of AFRS, further parallel groups have been defined including eosinophilic fungal rhinosinusitis (EFRS) and eosinophilic mucinous rhinosinusitis (EMRS). Patients with eosinophilic fungal rhinosinusitis have been defined as those who meet the Bent‐Kuhn criteria for AFRS except for the IgE mediated hypersensitivity to a fungal allergen. Patients with eosinophilic mucinous rhinosinusitis are defined as those who meet the Bent‐Kuhn criteria for AFRS except that they have no positive fungal culture or smear.
Chronic rhinosinusitis represents a common source of ill health; 11% of UK adults reported chronic rhinosinusitis symptoms in a worldwide population study (Hastan 2011). Symptoms have a major impact on quality of life, reportedly greater in several domains of the SF‐36 than angina or chronic respiratory disease (Erskine 2015; Gliklich 1995). Acute exacerbations, inadequate symptom control and respiratory disease exacerbation are common. Complications are rare, but may include visual impairment, bone erosion and expansion, and intracranial infection (EPOS 2012). Chronic rhinosinusitis affects an increasing proportion of the adult population until the sixth decade of life and then declines (Chen 2003).
The most commonly used interventions for chronic rhinosinusitis are used either topically (sprayed into the nose) or systemically (by mouth) and include steroids, antibiotics and saline. In the late 1990s some centres advocated the use of topical antifungals in chronic rhinosinusitis patients (Ponikau 1999). Since then there has been increasing controversy and contrasting papers have both advocated and refuted the use of both topical and systemic antifungal agents in the management of these patients (Ebbens 2007). A carefully defined population of patients with AFRS (and its derivatives) is likely to benefit most from the use of antifungals, however trials specifically in this group have been less prevalent.
Description of the intervention
Antifungal agents can be used as systemic medications (orally or intravenously) or as topical preparations delivered directly to the nose and sinuses. Topical treatments can be given using different delivery systems such as douching, nebulisation, atomisation, inhalation, irrigation, spray, drops or powder insufflations.
We will include all antifungals used in the management of inflammatory disease of the paranasal sinuses, both systemic and topical. Examples of antifungal agents include amphotericin B, gluconazole, itraconazole, voriconazole and ketoconazole. These agents may be fungistatic or fungicidal depending on the drug concentration and the susceptibility of the fungus.
How the intervention might work
Antifungal agents work in one of two ways, either as fungicides that kill the fungal spores, or as fungistatics that inhibit the growth and reproduction of the spores. Although good research demonstrates an interaction of the immune system with fungus in chronic rhinosinusitis (Ponikau 2007), this does not necessarily imply that fungus is the key aetiological factor and that antifungals will thus be effective in managing the disease. In chronic rhinosinusitis it may be that inappropriate immune activation may be the driving pathologic mechanism and fungal elements are only the innocent target of the process. Fungus is commonly found in our environment and thus freely available to inhale into the nose (Lackner 2005).
When taken orally (systemic) certain classes of antifungals, such as the azoles, have the potential for adverse effects such as gastrointestinal disturbances and they have also been associated with serious adverse effects, particularly with regard to hepatic and renal toxicity. Topical amphotericin is expensive and also associated with potential adverse effects such as headache and local irritations (Ebbens 2006).
Why it is important to do this review
The previous Cochrane Review and other more recent systematic reviews have concluded that there is no convincing evidence to support the use of antifungals in chronic rhinosinusitis (Mistry 2014; Sacks 2011). However, the authors of these reviews have commented on the clinical diversity of the included populations within the trials, particularly with regard to diagnosis. Often the population includes patients with both chronic rhinosinusitis and AFRS, as this distinction is ambiguous in some trials. It is important to understand whether there is a difference in treatment effect between these two populations. Similarly, the existing reviews include a heterogeneous population of people with respect to sinus surgery prior to the start of the trial.
We will not include studies designed to evaluate interventions in the immediate peri‐surgical period, which are focused on assessing the impact of the intervention on the surgical procedure or on modifying the post‐surgical results (preventing recurrence of chronic rhinosinusitis symptoms).
This review is one of a suite of Cochrane Reviews looking at common management options for patients with chronic rhinosinusitis (Chong 2016a; Chong 2016b; Chong 2016c; Head 2016a; Head 2016b; Head 2016c), and we have used the same methods and outcome measures as have been used across these reviews.
This systematic review will aim to look at the balance of benefits and harms for both systemic and topical antifungal agents in the treatment of patients with chronic rhinosinusitis.
Objectives
To assess the effects of systemic and topical antifungal agents in patients with chronic rhinosinusitis, including those with allergic fungal rhinosinusitis (AFRS) and, if possible, AFRS exclusively.
The review excludes patients in the immediate post‐surgical period (within six weeks of sinus surgery).
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
randomised controlled trials, including cluster‐randomised trials and quasi‐randomised trials (cross‐over trials were only included if the data from the first phase were available); and
patients were followed up for at least two weeks.
We excluded studies with the following design characteristics:
randomised patients by side of nose (within‐patient controlled) because it is difficult to ensure that the effects of any of the interventions considered can be localised; or
perioperative studies, where the sole purpose of the study was to investigate the effect of the intervention on surgical outcome.
Types of participants
Patients (adults and children) with chronic rhinosinusitis, whether with polyps or without polyps. This included the subgroups of people with a diagnosis of allergic fungal rhinosinusitis (AFRS), eosinophilic fungal rhinosinusitis (EFRS) or eosinophilic mucinous rhinosinusitis (EMRS).
We excluded studies that included a majority of patients with:
cystic fibrosis;
aspirin‐exacerbated respiratory disease (aka Samter's triad);
antrochoanal polyps (benign polyps originating from the mucosa of the maxillary sinus);
malignant polyps and inverted papilloma;
primary ciliary dyskinesia;
invasive fungal disease in the sinuses;
fungal balls (sinus mycelia);
a history of surgery for nasal polyps within six weeks of entry to the study.
Fungus can be demonstrated in almost all diseased and normal sinuses (Lackner 2005), thus we did not set associated fungus confirmed either histologically or on culture as an inclusion criterion. The immunological role of the fungus and the host is still an area of ongoing research.
Patients with chronic rhinosinusitis were included if they fulfilled the criteria defined by EPOS (EPOS 2012).
In order to identify patients with AFRS/EFRS for subgroup analysis, we used the modified Bent‐Kuhn criteria (Philpott 2011), where a patient must fulfil the following criteria:
type I hypersensitivity for fungal spore(s) confirmed by history, skin tests or serology OR immunocompetence;
nasal polyposis;
characteristic CT scan (double density sign);
eosinophilic mucus without fungal invasion into sinus tissue;
positive fungal stain of sinus contents removed intraoperatively or during office endoscopy.
We identified patients with EMRS for subgroup analysis if they met the criteria for AFRS (above) except that they did not have a positive fungal culture/smear.
Types of interventions
We included the following groups of topical or systemic antifungals:
polyene antifungals (e.g. amphotericin);
imidazole, triazole and thiazole antifungals (e.g. itraconazole);
allylamines;
echinocandins.
We included both topically applied and systemic antifungals in the review. We included any dose and delivery method. The minimum duration of treatment was 28 days.
Comparisons
The comparators were:
placebo or no intervention;
another class of antifungals;
-
the same type of antifungal, which is either:
given for a different duration;
given at a different dose;
-
other treatments for chronic rhinosinusitis, including:
intranasal corticosteroids;
oral/systemic steroids;
antibiotics;
nasal saline irrigation.
Concurrent treatments were allowed if they were used in both treatment arms; they included, for example:
nasal saline irrigation only;
intranasal corticosteroids only;
intranasal corticosteroids plus antibiotics;
intranasal corticosteroids plus nasal irrigation plus oral steroids;
other combinations.
Comparison pairs
There were multiple possible comparison pairs due to the large number of interventions allowed.
The main comparison pairs of interest were:
topical antifungalsversus no antifungal intervention or placebo;
systemic antifungals versus no antifungal intervention or placebo;
topical antifungals versus no intervention or placebo alongside intranasal steroids or other standard treatment in all arms of the trial.
Other possible comparison pairs were:
antifungals versus intranasal steroids;
antifungals versus oral/systemic steroids;
antifungals class A versus antifungals class B;
antifungal A with duration of treatment X versus antifungal A with duration of treatment Y;
antifungal A at dose X versus antifungal A at dose Y.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20.
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales). In the absence of validated symptom score data, patient‐reported individual symptom scores were reported for the following symptoms: nasal obstruction/blockage/congestion, nasal discharge (rhinorrhoea), facial pressure/pain, loss of sense of smell (adults) and cough (children).
Significant adverse effects: hepatic toxicity (systemic antifungals).
Secondary outcomes
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments.
Other adverse effects: gastrointestinal disturbances, allergic reactions (systemic antifungals).
Other adverse effects: epistaxis, headache, local discomfort (e.g. itching, mild burning) (topical antifungals).
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Mackay/Lund‐Kennedy).
Computerised tomography (CT) scan score (e.g. Lund‐Mackay).
Both short‐term (at the end of treatment) and long‐term effects are important therefore we evaluated outcomes at the end of treatment or within four weeks, at four weeks to six months, six to 12 months and more than 12 months. For adverse effects we analysed data from the longest time periods.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 17 November 2017.
Electronic searches
The Information Specialist searched for published, unpublished and ongoing studies by running searches in the following databases from their inception:
the Cochran ENT Trials Register (searched via the Cochrane Register of Studies 17 November 2017);
the Cochrane Register of Studies Online (searched 17 November 2017);
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 20 November 2017);
Ovid Embase (1974 to 20 November 2017);
Ovid CAB Abstracts (1910 to 20 November 2017);
EBSCO CINAHL (1982 to 20 November 2017);
LILACS, lilacs.bvsalud.org (searched 20 November 2017);
KoreaMed (searched via Google Scholar 20 November 2017);
IndMed, www.indmed.nic.in (searched 20 November 2017);
PakMediNet, www.pakmedinet.com (searched 20 November 2017);
Web of Knowledge, Web of Science (1945 to 20 November 2017);
ClinicalTrials.gov, (searched via the Cochrane Register of Studies and ClinicalTrials.gov 21 November 2017);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP), www.who.int/ictrp (searched 20 November 2017);
ISRCTN, www.isrctn.com (searched 20 November 2017).
The subject strategies for databases were modelled on the search strategy designed for CENTRAL (Appendix 1). Where appropriate, these were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011)).
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE, theCochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
Data collection and analysis
Selection of studies
At least two review authors (KH, LYC, SS) independently screened all titles and abstracts of the studies obtained from the database searches to identify potentially relevant studies. At least two review authors (KH, LYC, CP, CH) evaluated the full text of each potentially relevant study to determine whether it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and methodological input where necessary.
Data extraction and management
At least two review authors (KH, SS, LYC) independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data. If we had found differences between publications of a study, we would have contacted the original authors for clarification. We would have used data from the main paper(s) if no further information was found.
We included key characteristics of the studies, such as study design, setting, sample size, population and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers. For this review, this included:
presence or absence of allergic fungal rhinosinusitis (AFRS), eosinophilic fungal rhinosinusitis (EFRS) and eosinophilic mucinous rhinosinusitis (EMRS);
presence or absence of nasal polyps and baseline nasal polyp score where appropriate;
presence of eosinophilic chronic rhinosinusitis;
whether the patient has had previous sinus surgery.
We also noted down whether studies only selected patients with known AFRS and how this was identified.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis; i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from measurement scales such as SNOT‐22 and EQ‐5D as continuous data.
For binary data: the numbers of participants experiencing an event and the number of patients assessed at the time point.
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted into binary data.
We prespecified the time points of interest for the outcomes in this review. While studies may have reported data at multiple time points, we only extracted the longest available data within the time points of interest. For example, for 'short' follow‐up periods, our time point is defined as 'three to six months' post‐randomisation. If a study reported data at three, four and six months, we only extracted and analysed the data for the six‐month follow‐up.
Extracting data from figures
Where values for primary or secondary outcomes were shown as figures within the paper we contacted the study authors to try to obtain the raw values. When the raw values were not provided, we extracted information from the graphs using an online data extraction tool (http://arohatgi.info/WebPlotDigitizer/app/), using the best quality version of the relevant figures available.
Assessment of risk of bias in included studies
At least two review authors (KH, SS, LYC) independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), and we used the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
We summarised the effects for dichotomous outcomes (e.g. proportion of patients with symptom resolution) as risk ratios (RR) with confidence intervals (CIs). For the key outcomes that we presented in the 'Summary of findings' table, we also expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also planned to calculate the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk will typically be either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium risk population' or, alternatively, (b) the average risk of the control groups in the included studies is used as the 'study population' (Handbook 2011). If a large number of studies had been available, and where appropriate, we had also planned to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD). If different scales were used to measure the same outcome we used the standardised mean difference (SMD), and we provided a clinical interpretation of the SMD values.
Unit of analysis issues
This review did not use data from phase II of cross‐over studies or from studies where the patient was not the unit of randomisation, i.e. studies where the side (right versus left) was randomised.
If we had found cluster‐randomised trials, we planned to analyse these according to the methods in section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We contacted study authors via email whenever the outcome of interest was not reported, if the methods of the study suggested that the outcome had been measured. We did the same if not all data required for meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, the only other imputations that we had planned were calculations relating to disease severity (measured by patient‐reported symptom scores) as we thought that some studies may have measured individual symptoms rather than using validated instruments (see 'Imputing total symptom scores' below). We extracted and analysed data for all outcomes using the available case analysis method.
Imputing total symptom scores
Where a paper did not present information for the total disease severity in terms of patient‐reported symptom scores but presented data for the results of individual symptoms, we would have used the symptoms covering the important domains of the EPOS chronic rhinosinusitis diagnosis criteria (EPOS 2012), in order to calculate a total symptom score. The EPOS 2012 criteria for chronic rhinosinusitis require at least two symptoms. One of the symptoms must be either nasal blockage or nasal discharge; other symptoms can include facial pressure/pain, loss of sense of smell (for adults) or cough (for children). Where mean final values or changes from baseline were presented in the paper for the individual symptoms we would have sum these to calculate a 'total symptom score'. We would have calculated standard deviations for the total symptom score as if the symptoms were independent, random variables that were normally distributed. We acknowledge that there would have been likely to be a degree of correlation between the individual symptoms, however we would have used this process as the magnitude of correlation between the individual symptoms is not currently well understood (no evidence found). If the correlation is high, the summation of variables as discrete variables is likely to give a conservative estimate of the total variance of the summed final score. If the correlation is low, this method of calculation will underestimate the standard deviation of the total score. However, the average patient‐reported symptom scores have a correlation coefficient of about 0.5; if this is also applicable to chronic rhinosinusitis symptoms, the method used should have had minimal impact (Balk 2012). As this method of calculation does not take into account weighting of different symptoms (no evidence found), we would have downgraded all the disease severity outcomes in GRADE for lack of use of validated scales.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included trials for potential differences between studies in the types of participants recruited, interventions or controls used and the outcomes measured.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as between‐study publication bias and within‐study outcome reporting bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results were mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias in a meta‐analysis is likely to occur. We tried to find further information from the study authors. If no further information could be obtained, we noted this as being a high risk of bias. Where there was insufficient information to judge the risk of bias, we noted this as an unclear risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We planned to create a funnel plot if sufficient studies (more than 10) were available for an outcome. If we had observed asymmetry of the funnel plot, we would have conducted more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel method. If we had found time‐to‐event data we had planned to analyse it using the generic inverse variance method.
If we had found continuous data from different studies that were suitable for meta‐analysis, and if all the data were from the same scale, we would have pooled mean values obtained at follow‐up with change outcomes and reported this as a MD. However, if the data were from different scales, we would have used the SMD as an effect measure and we would not have pooled change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We planned to conduct some subgroup analyses regardless of whether statistical heterogeneity was observed, as these are widely suspected to be potential effect modifiers. For this review, this included:
Presence of allergic fungal rhinosinusitis (as defined by the modified Bent‐Kuhn criteria; see Types of participants), EFRS and EMRS. Patients with AFRS may respond differently to antifungal agents as in AFRS an IgE mediated hypersensitivity to fungal elements drives the inflammatory process.
Phenotype of patients: whether patients have chronic rhinosinusitis without nasal polyps, chronic rhinosinusitis with nasal polyps, they are a mixed group or the status of polyps is not known or not reported. We planned to undertake the subgroup analysis as although there appears to be a considerable overlap between the two forms of chronic rhinosinusitis with regards to inflammatory profile, clinical presentation and effect of treatment (Cho 2012; DeMarcantonio 2011; Ebbens 2010; Fokkens 2007; Ragab 2004; Ragab 2010; van Drunen 2009), there is some evidence pointing to differences in the respective inflammatory profiles (Kern 2008; Keswani 2012; Tan 2011; Tomassen 2011; Zhang 2008; Zhang 2009), and potentially even differences in treatment outcome (Ebbens 2011). The role of fungi in the pathology is also unclear and this makes it uncertain whether antifungals will have similar effects.
Eosinophilic versus non‐eosinophilic chronic rhinosinusitis. Some researchers hypothesise that patients with eosinophilic chronic rhinosinusitis will form an eosinophilic reaction towards the fungi present in their sinonasal mucin. It is proposed that this reaction will subsequently be involved in the inflammatory response (Ponikau 1999).
We planned to present the main analyses of this review according to the subgroup of presence of AFRS. We intended to present all other subgroup analysis results in tables.
When studies had a mixed group of patients, we planned to analyse the study as one of the subgroups (rather than as a mixed group) if more than 80% of patients belonged to one category. For example, if 81% of patients had AFRS, we would have analysed the study as that subgroup.
In addition to the subgroups above, we planned to conduct the following subgroup analyses in the presence of statistical heterogeneity:
patient age (children versus adults);
dose;
duration of treatment;
method of delivery;
class of antifungal agent.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
impact of model chosen: fixed‐effect versus random‐effects model;
risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that had a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed));
how outcomes were measured: we planned to investigate the impact of including data where the validity of the measurement was unclear.
If any of these investigations found a difference in the size of the effect or heterogeneity, we would have mentioned this in the Effects of interventions section.
GRADE and 'Summary of findings' table
Using the GRADE approach, at least two review authors (KH, SS, LYC) independently rated the overall quality of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we will apply this in the interpretation of results. There are four possible ratings: 'high', 'moderate', 'low' and 'very low'. A rating of 'high' quality evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision;
publication bias.
The 'Summary of findings' tables present only the top priority outcomes (disease‐specific health‐related quality of life, disease severity score, adverse effects and generic quality of life score). We did not include the outcomes endoscopic score or CT scan score in the 'Summary of findings' tables.
Results
Description of studies
Results of the search
The searches retrieved a total of 1496 references after removal of duplicates. We identified two additional references from other sources. We screened the titles and abstracts and subsequently removed 1413 references. We assessed 85 full texts for eligibility. We excluded 65 references, 38 without presenting reasons. Most of these studies were the wrong study design (literature review, systematic review, letter). We excluded 23 studies (27 records), with reasons (see Excluded studies).
We included eight studies (15 references) (see Included studies). We did not identify any ongoing studies.
There are four studies (five references) awaiting assessment (Deka 2007; Frigas 2007; Lopatin 2004; Stergiou 2007). These are presented only as abstracts and although we attempted to contact the authors to determine if the trial was published in full, no response was received.
We did not identify any ongoing studies.
A flow chart of study retrieval and selection is provided in Figure 1.
1.
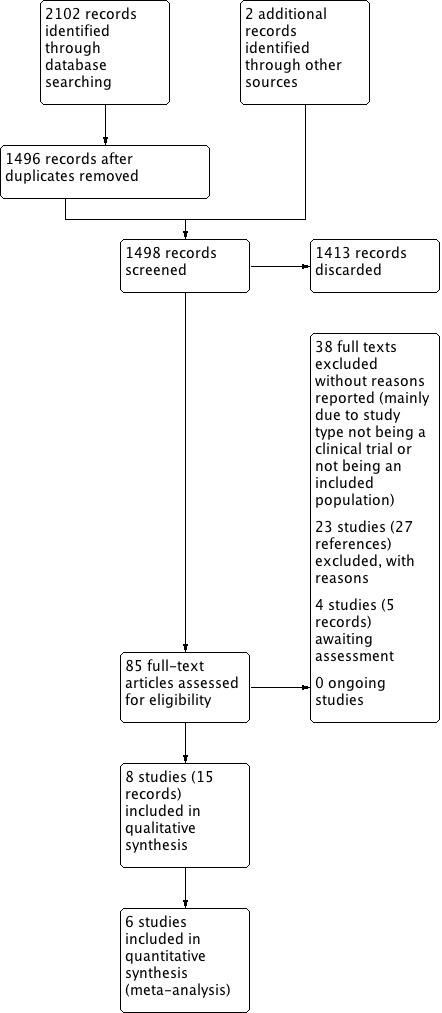
Study flow diagram.
Included studies
We included eight studies in the review. More details about the included studies can be found in Characteristics of included studies and a summary can be found Table 3.
1. Summary of study characteristics.
| Ref ID | Population | Intervention | Adjuvant treatment | ||||
| Inclusion (n) | Polyps | AFRS | Intervention | Method of delivery | Treatment duration | ||
|
Corradini 2006 (Italy) |
Nasal polyps + positive fungal infection (48) |
100% | 0% | Amphotericin B (3 mg/mL) | Inhalation: 0.24 mL/day 6 times per week for 1 month (daily total = 0.8 mg AMB) 0.16 mL/day 6 times per week for undefined time (total daily dose = 0.5 mg AMB) |
Undefined ‐ 19 months? | Medical polypectomy and lysine acetylsalicylate (NSAID) 4 mg/day |
|
Ebbens 2006 (Belgium, UK, Spain, Netherlands) |
Chronic rhinosinusitis ± nasal polyps (116) |
82%% | 0% | Amphotericin B (0.1 mg/mL) |
Irrigation: 25 mL solution applied to each nostril twice daily using an Emcur (Rhinicur) nasal douching device (total daily dose = 10 mg AMB) | 13 weeks | Antibiotics, INCS and systemic steroids were allowed, with restrictions. 68% of participants used INCS. |
|
Hashemian 2016 (Iran) |
Chronic rhinosinusitis ± nasal polyps unresponsive to treatment (54) |
44% | NR | Fluconazole (2 mg/mL (0.2%)) |
Nasal drops: 2 mg/mL (6 drops per day, 2 times a day) (total daily dose = 1.2 mg fluconazole) | 8 weeks | All patients used INCS (fluticasone) |
|
Liang 2008 (Taiwan) |
Chronic rhinosinusitis without nasal polyps (70) | 0% | NR | Amphotericin B (0.04 mg/mL) |
Irrigation: 250 mL (0.04 mg/mL solution) in each nostril once daily using a Sanvic SH903 pulsatile irrigator (total daily dose = 20 mg AMB) | 4 weeks | No adjunct treatment was allowed |
|
Ponikau 2005 (USA) |
Chronic rhinosinusitis unresponsive to treatment (30) 100% with positive fungal culture |
NR | NR | Amphotericin B (0.25 mg/mL) |
Irrigation: 20 mL (0.25 mg/mL solution) in each nostril twice daily using a bulb syringe (total daily dose = 20 mg AMB) | 6 months | Participants continued with current treatment regimen (50% used INCS) |
|
Shin 2004 (South Korea) |
Chronic rhinosinusitis patients with nasal polyps (41) |
100% | 0% | Amphotericin B (high: 0.1 mg/mL; low: 0.05 mg/mL) |
Irrigation: 10 mL of the solution into each nostril twice daily with a syringe High‐dose: 0.1 mg/mL (total daily dose = 4 mg AMB) Low‐dose 0.05 mg/mL (total daily total = 2 mg AMB) |
4 weeks | Not reported |
|
Weschta 2004 (Germany) |
Chronic rhinosinusitis with nasal polyps referred for surgery (78) |
100% | 0% | Amphotericin B (3 mg/mL) |
Nasal spray: 2 puffs per nostril (0.2 mL per nostril), 4 times daily (total daily dose = 4.8 mg) | 8 weeks | Participants continued with current treatment regimen (40% used INCS) |
| Systemic antifungals | |||||||
|
Kennedy 2005 (USA) |
Chronic rhinosinusitis unresponsive to treatment (53) 77% with positive fungal culture |
NR | NR | Terbinafine | Oral: 625 mg/day | 6 weeks | Participants continued with current treatment regimen ‐ regimen was kept consistent |
AFRS: allergic fungal rhinosinusitis; AMB: amphotericin B; INCS: intranasal corticosteroids; NR: not reported
None of the studies reported eosinophilic chronic rhinosinusitis status.
Design
All of the included studies were parallel‐group randomised controlled trials (RCTs). Six studies had two study arms, one study had three study arms (Shin 2004) and one study had four study arms (Corradini 2006), although in each case only two arms were relevant to this review. Six of the studies blinded participants and healthcare professionals to treatment group (Ebbens 2006; Hashemian 2016; Kennedy 2005; Liang 2008; Ponikau 2005; Weschta 2004).
Sample size
There were 490 participants relevant to this review in the included studies. The sample sizes in the studies ranged from 30 to 116 participants. Only one study included more than 80 participants.
Setting
Seven of the studies were single‐centre, conducted in six countries: two from the USA and one each from Germany, Iran, Italy, South Korea and Taiwan. One study was multi‐centre and conducted at six sites in four countries (Belgium, the Netherlands, Spain and the UK) (Ebbens 2006). The settings of all studies were secondary or tertiary ear, nose and throat (ENT) clinics.
Population
Age
Six studies only included adults (aged 18 years or older), one study included participants from the age of 12 years (Liang 2008), and one study did not provide any information on the age of participants (Corradini 2006). In the seven studies providing information, the mean ages of participants ranged from 39 to 53 years. No studies included children under 12 years.
Sex
Seven studies provided details of the sex of participants and all included males and females. The percentage of male participants in the studies ranged from 33.6% to 70.8%. Corradini 2006 did not provide any information on the sex of participants.
Diagnosis
One study included patients with nasal polyps and a positive fungal culture but did not mention a formal diagnosis of chronic rhinosinusitis (Corradini 2006). All remaining studies included patients with chronic rhinosinusitis diagnosed using appropriate methods. Three studies included participants who were unresponsive to previous medical therapy for chronic rhinosinusitis (Hashemian 2016; Kennedy 2005; Ponikau 2005). All participants in two studies (Corradini 2006; Ponikau 2005) and 77% of participants in Weschta 2004 had an initial fungal culture at the start of the trial. This was not measured in the other studies.
Nasal polyps
Two studies did not provide details about whether participants had polyps (Kennedy 2005; Ponikau 2005), three studies used nasal polyps as an inclusion criterion (Corradini 2006; Shin 2004; Weschta 2004), and one study excluded patients with nasal polyps (Liang 2008). The remaining two studies reported polyps in 43.8% (Hashemian 2016) and 81.9% (Ebbens 2006) of participants.
Allergic fungal rhinosinusitis (AFRS)
Four studies excluded patients with AFRS (Corradini 2006; Ebbens 2006; Shin 2004; Weschta 2004). The other studies did not report whether patients were diagnosed with AFRS.
Intervention
Topical antifungals
Seven studies investigated the use of topical antifungal agents: amphotericin B (six studies) and fluconazole (one study). A range of different delivery methods, concentrations, frequencies and durations were used in the studies and further details can be found in Table 3. It was noticeable that the daily doses of topical antifungal used in the studies were generally lower than would be expected. Whilst there is no formal guidance for topical use (such as in the British National Formulary; BNF 2018), rhinology clinical practice dose regimens for amphotericin B would be approximately 20 mg per day. Of the six studies using this agent, four used 10 mg/day or less, so half of the 'usual' daily dose or less.
Systemic antifungals
Kennedy 2005 (53 participants) used systemic terbinafine tablets (625 mg/day) for six weeks, which is considered to be a high daily dose. For reference, the British National Formulary recommends a dose of 250 mg/day for terbinafine (BNF 2018).
Use of adjuvant treatments
Intranasal corticosteroids were used routinely in one study (Hashemian 2016), and the current treatment regimen was continued in three studies (Ebbens 2006; Kennedy 2005; Ponikau 2005; Weschta 2004). Adjuvant treatments were not allowed in Liang 2008 and not reported in another study (Shin 2004). All participants in Corradini 2006 underwent a medical polypectomy with 40 mg triamcinolone retard intramuscularly three times every 10 days (total dose 120 mg) and continued with lysine acetylsalicylate (4 mg/day; six times/week). Further details are provided in Table 3.
Comparison
All included studies compared the effects of topical antifungals (seven studies; 437 participants) or systemic antifungals (one study, 53 participants) with placebo or no treatment.
Topical antifungals compared with placebo or no treatment
Six studies compared topical antifungals to placebo solution (Ebbens 2006; Hashemian 2016; Liang 2008; Ponikau 2005; Shin 2004; Weschta 2004). Corradini 2006 compared topical antifungal agents with no treatment.
Systemic antifungals compared with placebo or no treatment
One study (53 participants) compared terbinafine tablets with placebo tablets (Kennedy 2005).
Outcomes
Neither Corradini 2006 nor Shin 2004 presented any primary or secondary efficacy outcomes as defined for this review, with the former reporting polyps recurrence at 20 months and the latter investigating the cytokine protein content of nasal polyps. The adverse effects results from these studies are included in the review, however.
Primary outcomes
Disease‐specific health‐related quality of life
Five studies presented this information, using three different scales. Details of the range and direction of the instruments are provided in Table 4.
2. Summary of disease severity score results.
| Ref ID | Instrument details |
How reported (time point) |
Results |
Difference between groups Notes |
|
| Antifungal | Placebo | ||||
| Topical antifungals | |||||
| Ebbens 2006 | RSOM‐31 Range: 0 to 775a Lower score = better QOL |
Change from baseline (13 weeks) |
Baseline: 150 Mean change: 17.0 SD: 86.4 N: 59 |
Baseline: 176 Mean change: ‐3.6 SD: 100.4 N: 57 |
P = 0.35 Small relative changes (17 and 3.6 points on a scale of 0 to 775) |
| Hashemian 2016 | SNOT‐20 Range: 0 to 100 Lower score = better QOL |
Endpoint (8 weeks) |
Baseline: 36.29 After treatment: 27.25 SD: 15.88 N: 24 |
Baseline: 41.33 After treatment: 28.71 SD: 18.24 N: 24 |
P = 0.76 Large SD values compared to mean may be an indication that the data are skewed |
| Liang 2008 | Chinese RSOM‐31 Range: 0 to 775a Lower score = better QOL |
Endpoint (4 weeks) |
Median baseline: 201.5 Median after treatment: 65.5 Range: 10 to 466 N: 32 |
Median baseline: 227 Median after treatment: 121.5 Range: 8 to 405 N: 32 |
P = 0.091 Unequal distribution of median within the range values indicate the data are likely to be skewed |
| Ponikau 2005 | SNOT‐20 Range: 0 to 5 Lower score = better QOL |
Change from baseline (6 months) |
Median baseline: 2.3 Median change: ‐0.3 Range: ‐1.3 to 0.3 N: 10 |
Median baseline: 1.8 Median change: ‐0.3 Range: ‐1.8 to 0.8 N: 14 |
P = 0.72 Data reported as medians and ranges indicating possibility of skewed data, although the median appears to sit in middle of range values |
| Systemic antifungals | |||||
| Kennedy 2005 | Rhinosinusitis Disability Index (RSDI) Range: 0 to 120 Lower score = better QOL |
Unclear (9 weeks) |
Values for the results were not given | Authors state that "no differences were observed” at any time point measured | |
IQR: interquartile ranges; N: number of participants; QOL: quality of life; RSOM‐31: Rhinosinusitis Outcome Measure‐31; SD: standard deviation; SNOT‐20: Sino‐Nasal Outcome Test‐20
a) The range is not explicitly stated in the paper but is assumed to be from 0 to 775, which is the general range for the RSOM‐31 instrument including the importance scale. b) The range is not explicitly stated in the paper but is the standard range for the RSDI instrument.
Rhino‐sinusitis Disability Index (RSDI): Kennedy 2005 (nine weeks).
Sino‐Nasal Outcomes Test (SNOT‐20): Ponikau 2005 (three and six months); Hashemian 2016 (eight weeks; it is unclear whether a Persian/Iranian version was used or what the impact of this was on validation).
Rhinosinusitis Outcome Measure‐31 (RSOM‐31): Ebbens 2006 (13 weeks); Liang 2008 (two and four weeks; Chinese version).
Weschta 2004 used a "rhinosinusitis quality of life" score (RQL) but as we could find no details on whether this instrument had undergone any validation, we did not include the results.
Disease severity
Three studies presented information on disease severity:
Patient's overall evaluation of sinusitis measured on a four‐point scale (although the authors did not provide information on whether higher or lower scores indicated worse symptoms) (Kennedy 2005).
Sum of the following individual symptoms each measured on a visual analogue scale (VAS) of 0 to 10 cm (higher score = worse symptoms): nasal blockage, facial pain, smell disturbance, nasal discharge and sneezing. The sum of individual symptom values was calculated, with a final range of 0 to 50 (Weschta 2004).
Sum of the following individual symptoms each measured on a VAS of 0 to 10 cm (reported as a range of 0 to 100; higher score = worse symptoms): nasal blockage, rhinorrhoea, facial pain, postnasal drip and anosmia (loss of sense of smell). The sum of individual symptom values was calculated, with a final range of 0 to 500 (Ebbens 2006).
Significant adverse effects: hepatic toxicity (systemic antifungals)
Kennedy 2005, the only study that investigated systemic antifungal agents, measured the number of patients with increased aspartate aminotransferase (AST), alanine aminotransferase (ALT) or gamma‐glutamyl (GGT) levels although no definition of 'increased' was provided.
Secondary outcomes
Generic health‐related quality of life
Only Ebbens 2006 measured generic health‐related quality of life. They used the short form‐36 (SF‐36) questionnaire and separated the results into the physical and mental component scores (range = 0 to 100, lower score = worse quality of life).
Other adverse effects: gastrointestinal disturbances, allergic reactions (systemic antifungals)
This was reported in Kennedy 2005, the only study investigating systemic antifungals.
Other adverse effects: epistaxis, headache, local discomfort (e.g. itching, mild burning) (topical antifungals)
Five of the six studies investigating topical antifungals reported other adverse effects such as epistaxis, headache and local discomfort (Ebbens 2006; Hashemian 2016; Ponikau 2005; Shin 2004; Weschta 2004).
Endoscopic score (nasal polyps size score or endoscopy score, e.g. Lund‐Kennedy)
Five studies reported the results of nasal endoscopy. Three studies assessed the extent of nasal polyps:
Scored each nostril on a scale of 0 to 4 (0 = no polyps, 4 = polypoid changes below the lower edge of the inferior turbinate); total range = 0 to 8 (Hashemian 2016; Ponikau 2005).
Scored each nostril on a scale of 0 to 3 (0 = no polyps; 3 = polyps fill whole nasal cavity); total range = 0 to 6 (Weschta 2004).
Two studies provided a more general endoscopic score:
Amount of mucosal disease measured by nasal secretions, nasal polyps and nasal crusting, each on a scale of 0 to 2 (0 = absent, 2 = severe) in predefined areas (e.g. middle meatus, ethmoid region). Sum scores were calculated by adding all independent values for both nostrils but the total possible range was not given (Ebbens 2006).
Measured oedema, discharge, polyps, crusting and scarring, graded from 0 (normal) to 2 (severely diseased); total range = 0 to 10 (Liang 2008).
Computerised tomography (CT) scan score (e.g. Lund‐Mackay)
Four studies measured CT score using five different measures; two investigated the percentage change in opacification and three used variations of the Lund‐Mackay score:
Change in opacification:
Percentage change from baseline in CT opacification score (Kennedy 2005).
Percentage change from baseline in inflammatory mucosal thickening, which occluded the nasal and paranasal cavities (Ponikau 2005).
Three studies used modified versions of the Lund‐Mackay scoring system:
Each of the five major left and right sinuses were scored on a six‐point opacification scale (0 = no opacification; 5 = total opacification; total range of 0 to 50) (Kennedy 2005).
Each sinus, nasal passage and both osteomeatal complexes were assessed for mucosal thickening on a four‐point scale (0 to 3; 0 = lower severity; total range of 0 to 30) (Hashemian 2016).
Each of the five major left and right sinuses were scored on a five‐point opacification scale (0 = no opacification, 4 = complete opacification; total range of 0 to 40) (Weschta 2004).
None of the studies using modified scores refer to validation papers.
Excluded studies
We excluded 23 studies (27 records), with reasons. See Characteristics of excluded studies for more details.
We excluded 13 studies (16 papers) because although they were randomised controlled trials (RCTs) all of the participants underwent surgery either before or during the trial (Gerlinger 2009; Gupta 2007; IRCT138706101138N1; Jiang 2015; Khalil 2011; Lopatin 2007; NCT02285283; Nikakhlagh 2015; Panda 2012; Ravikumar 2011; Rojita 2017; Somu 2015; Zhang 2012). One study gave antifungals pre‐operatively but the control group underwent surgery immediately and no pre‐operative results were available (Verma 2016).
We excluded eight studies (nine papers) due to the study design: six were case series where all participants received an antifungal agent (Chan 2008; Hashemi 2014; Helbling 2006; Hofman 2004; Joshi 2007; Ricchetti 2002b); one study (two papers) related to a non‐randomised trial comparing an antifungal agent with placebo (Ricchetti 2002); and one study randomised participants by side of nose (Thamboo 2011).
We excluded one study as the participants were randomised to antifungal agents or endoscopic surgery (Patro 2015).
Risk of bias in included studies
See Figure 2 for the 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for the 'Risk of bias' summary.
2.
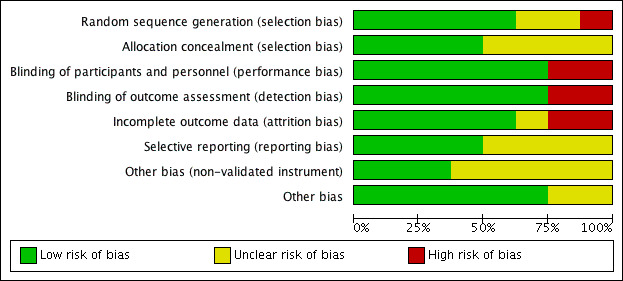
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
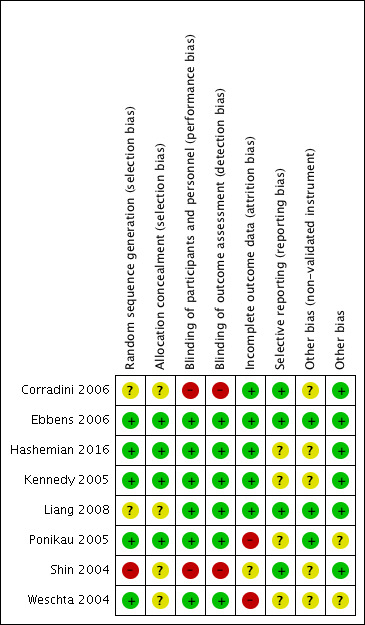
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We rated one study as having a high risk of selection bias as it was unclear from a statement in the paper whether the participants were randomly selected to the study or randomly allocated to treatment group (Shin 2004). Two studies stated that the patients were 'randomly' allocated to treatment group but provided no details on the methods used (Corradini 2006; Liang 2008). All other studies were at low risk of bias for sequence generation.
Allocation concealment
Four studies did not mention any methods used to ensure that the allocation of patients to treatment groups was not unduly influenced (Corradini 2006; Liang 2008; Shin 2004; Weschta 2004). All other studies reported methods for ensuring allocation concealment, which included automated randomisation, no knowledge of block size and allocation by someone independent to the study.
Blinding
Performance bias
Two studies did not mention blinding and so we judged them to be at high risk of bias for this domain (Corradini 2006; Shin 2004); however, one of these did have a control arm that used an 'inert' solution (Shin 2004). All of the remaining studies were blinded and we judged them to be at low risk of bias.
Detection bias
Similar to performance bias we assessed the same two studies to be at high risk of detection bias (Corradini 2006; Shin 2004). We judged the other studies to be at low risk of bias.
Incomplete outcome data
We assessed two studies to be at high risk of attrition bias. Ponikau 2005 reported that 20% of patients (6/30) did not complete the study; five of those who dropped out were from the intervention group compared to one in the placebo group. Weschta 2004 also reported a high and unbalanced dropout rate (38% from the antifungal arm compared with 18% from the control arm); five participants (13%) in the treatment arm dropped out due to "intolerance of the study medication". We felt Shin 2004 to be at unclear risk of attrition bias as the information regarding those who were eligible for the trial but did not participate, and whether there were any participants that did not finish the trial, was not clearly presented. We judged the remaining five studies to be at low risk of attrition bias.
Selective reporting
We assessed three studies as at unclear risk of bias due to selective reporting:
In Kennedy 2005, some of the outcomes mentioned in the methods section were described as "not statistically different" in the paper but results were not reported.
Some of the outcomes in the methods section in Weschta 2004 were only reported vaguely in the results. For example, for endoscopic score the paper states, "The median endoscopy scores were almost identical in the AMB and control groups (4 vs 4) and did not change remarkably after treatment." In addition, a difference in adverse effects between the groups was reported but details of the type of event and the number of patients was not provided.
A protocol was available for Hashemian 2016, where endoscopic score is listed as an outcome (IRCT138811063186N1). This outcome was not reported in the published paper. In addition, standard deviations were not given and results for adverse effects were not well reported although they were provided following personal communication.
We assessed the remaining five studies to be at a low risk of bias. We identified no protocols through any sources for these studies but all of the outcomes as presented in the methods sections were reported in the results sections.
Other potential sources of bias
Unvalidated instruments
We assessed five studies as having an 'unclear' risk of bias due to the use of potentially unvalidated measurement instruments.
Kennedy 2005 refers to a 'modified' version of the (validated) Lund‐Mackay scoring system but does not provide a reference to the modifications and the impact on the validation.
The Hashemian 2016 study, conducted in Iran, used the validated SNOT‐20 instrument but no details were presented for any validation with regards to language translation. Neither Corradini 2006 nor Shin 2004 reported any outcomes of interest and we classified them as having 'unclear' risk of bias.
Weschta 2004 used their own instrument called the "rhinosinusitis quality of life score (RQL)", which was modified from the mini Rhinoconjunctivitis Quality of Life Questionnaire (miniRQLQ) developed for people with rhinoconjunctivitis due to allergy (Juniper 1991). The modifications reduced the total number of questions by half without details or evidence of whether the modification validation affected the face validity or responsiveness of the instrument to detect changes. Due to the lack of information regarding the validity of the instrument for chronic rhinosinusitis patients, we did not include data for this outcome in the results.
The remaining studies used validated instruments and we assessed them to be at low risk of bias.
Other
Ponikau 2005 reported imbalances in age and duration of chronic rhinosinusitis between the groups with the people allocated to the antifungal treatment group being older and having had chronic rhinosinusitis for a longer time. The paper does not indicate whether there was a statistical difference between the groups and so we rated the study as having an unclear risk of bias. In Weschta 2004, the paper identifies that "...dropouts were accounted for by recruitment of additional patients." It was unclear how many patients this was relevant for and whether the process was randomised and allocation concealment protected.
We assessed the six remaining studies as at low risk of bias.
Funding and declarations of interest
Funding
Three studies reported funding sources. One study was funded by a pharmaceutical company (Kennedy 2005). Two studies reported funding from academic or governmental sources (Hashemian 2016; Ponikau 2005). The remaining five studies did not present information on funding sources (Corradini 2006; Ebbens 2006; Liang 2008; Shin 2004; Weschta 2004).
Declarations of interest
Ponikau 2005 declared that one of the funding organisations owned a patent for which the first author was listed as the inventor and that a license agreement had been signed with Accentia Pharmaceutical Inc. The patent states: "the invention involves administrating an antifungal agent such that it contact mucus [sic]in an amount, at a frequency, and for a duration effective to prevent, reduce, or eliminate non‐invasive fungus‐induced rhinosinusitis."
In two studies, although declarations were not explicitly stated, two had affiliations with pharmaceutical companies. Ebbens 2006 declared that three of the authors had consultancy arrangements with pharmaceutical companies, and three authors had Novartis as their affiliation in Kennedy 2005.
One study explicitly reported that the authors declared no conflicts of interest (Hashemian 2016), and no information was presented in four studies (Corradini 2006; Liang 2008; Shin 2004; Weschta 2004).
Effects of interventions
Summary of findings for the main comparison. Topical antifungal versus placebo/no treatment for chronic rhinosinusitis.
| Topical antifungal versus placebo/no treatment for chronic rhinosinusitis | ||||||
|
Patient or population: chronic rhinosinusitis Intervention: topical antifungal Comparison: placebo/no treatment | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without topical antifungal | With topical antifungal | Difference | ||||
| Heath‐related quality of life (HRQL) Assessed with: various instruments Follow‐up: range 4 weeks to 6 months № of participants: 312 (5 RCTs) | 4 studies (252 participants) using different disease‐specific quality of life instruments reported no statistically significant difference between the groups receiving topical antifungal and placebo in terms of change from baseline or endpoint values | ⊕⊕⊝⊝ LOW 1 | Topical antifungals may lead to little or no difference in disease‐specific health‐related quality of life, compared to placebo, for patients with chronic rhinosinusitis | |||
| Disease severity score Assessed with: various scales Follow‐up: range 8 weeks to 13 weeks № of participants: 176 (2 RCTs) | 2 studies (all patients with chronic rhinosinusitis with nasal polyps) reported a disease severity score using different symptoms. Ebbens 2006 (116 participants) reported mean change from baseline and found that both the placebo and antifungal group only had small mean changes from baseline, which were not statistically significant between the groups (P = 0.31). Weschta 2004 (60 participants) reported the median disease severity scores at the end of treatment. They found that the median symptom score in the placebo group was significantly lower (fewer symptoms) than the topical antifungal group (P < 0.05).3 | ⊕⊝⊝⊝ VERY LOW 2 | It is uncertain whether topical antifungals improve disease severity scores compared to placebo for people with chronic rhinosinusitis | |||
| Generic HRQL (change from baseline) Assessed with: SF‐36 physical component (higher = better) Scale from: 0 to 100 Follow‐up: mean 13 weeks № of participants: 116 (1 RCT) | — | The mean change from baseline in the SF‐36 physical component score without topical antifungals was 1.4 points | — | MD 0.8 points lower (3.66 lower to 2.06 higher) | ⊕⊕⊝⊝ LOW 7 | There may be little or no difference in generic quality of life (physical component) between topical antifungals and placebo for patients with chronic rhinosinusitis |
| Generic HRQL (change from baseline) Assessed with: SF‐36 mental component (higher = better) Scale from: 0 to 100 Follow‐up: mean 13 weeks № of participants: 116 (1 RCT) | — | The mean change from baseline in SF‐36 mental component score without topical antifungal was 1.9 points | — | MD 2.2 points lower (5.46 lower to 1.06 higher) | ⊕⊕⊝⊝ LOW 7 | There may be little or no difference in generic quality of life (physical component) between the use of topical antifungals and placebo for patients with chronic rhinosinusitis |
| Adverse effects ‐ epistaxis Follow‐up: range 4 weeks to 6 months № of participants: 225 (4 RCTs) | RR 0.97 (95% CI 0.14 to 6.63) | Study population | ⊕⊕⊝⊝ LOW 4 | It is uncertain whether topical antifungals increase the risk of epistaxis compared to placebo for patients with chronic rhinosinusitis | ||
| 1.9% | 1.8% (0.3 to 12.5) | 0.1% fewer (1.6 fewer to 10.6 more) | ||||
| Adverse effects ‐ headache Follow‐up: range 4 weeks to 6 months № of participants: 195 (3 RCTs) | RR 1.26 (95% CI 0.60 to 2.63) | Study population | ⊕⊝⊝⊝ VERY LOW 5 | It is uncertain whether topical antifungals increase the risk of headache compared to placebo for patients with chronic rhinosinusitis | ||
| 11.0% | 13.8% (6.6 to 28.9) | 2.9% more (4.4 fewer to 17.9 more) | ||||
| Adverse effects ‐ local irritation Follow‐up: range 4 weeks to 6 months № of participants: 312 (5 RCTs) | RR 2.29 (95% CI 0.61 to 8.62) | Study population | ⊕⊕⊝⊝ LOW 6 | Topical antifungals may lead to more local irritation events compared to placebo for patients with chronic rhinosinusitis | ||
| 0.7% | 1.5% (0.4 to 5.6) | 0.8% more (0.3 fewer to 5 more) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HRQL: health‐related quality of life; MD: mean difference; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by two levels due to imprecision: there was some evidence to suggest that the data were skewed in three of the five studies, reducing our confidence in the results. Furthermore, the validity of some instruments was unclear.
2Downgraded by one level due to inconsistency: the results of the two studies appeared to differ from each other. Downgraded by one level due to indirectness: all of the included population had nasal polyps, which may not be representative of all chronic rhinosinusitis patients. Downgraded by two levels due to imprecision: the data from one study had wide confidence intervals and the other study presented only median and interquartile range (IQR) values.
3Ebbens 2006 measured the symptoms of nasal blockage, rhinorrhoea, facial pain, postnasal drip and anosmia. Weschta 2004 measured the symptoms of nasal blockage, facial pain, smell disturbance, nasal discharge and sneezing.
4Downgraded by two levels due to imprecision: only one trial reported any events (two events in treatment group), resulting in very wide confidence intervals. Poor reporting of epistaxis results in the trials.
5Downgraded by one level due to inconsistency: adverse effects were generally poorly reported and definitions were likely to be different between studies as the event rates were very different between studies. Downgraded by two levels due to imprecision. Only one trial reported any events and the confidence intervals were very wide.
6Downgraded by two levels due to imprecision: small numbers of events lead to wide confidence intervals, which include a clinically important increase and a clinically important decrease in adverse effects.
7Downgraded by two levels due to imprecision: results come from one study. A minimally important difference has been identified as three points for the SF‐36 and so the confidence intervals include a potentially clinically important effect.
Summary of findings 2. Systemic antifungal versus placebo/no treatment for chronic rhinosinusitis.
| Systemic antifungal versus placebo/no treatment for chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis Intervention: systemic antifungal Comparison: placebo/no treatment | ||||||
| Outcomes | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Certainty of the evidence (GRADE) | What happens | ||
| Without systemic antifungal | With systemic antifungal | Difference | ||||
| Heath‐related quality of life (HRQL) Assessed with: Rhinosinusitis Disability Index (RSDI) Follow‐up: 6 weeks № of participants: 53 (1 RCT) | Values for the RSDI results were not provided in the paper but the authors state that "no differences were observed” at any time point measured | ⊕⊕⊝⊝ LOW 1 | Systemic antifungals may lead to little or no difference in disease‐specific health‐related quality of life, compared with placebo, for patients with chronic rhinosinusitis | |||
| Disease severity score Assessed with: overall evaluation of sinusitis measured on a 4‐point scale Follow‐up: 6 weeks № of participants: 53 (1 RCT) | Symptoms of facial pain/pressure, facial congestion and nasal discharge were measured. No values were reported but the authors state that "no differences were observed [between the treatment groups]". | ⊕⊕⊝⊝ LOW 1 | Systemic antifungals may lead to little or no difference in disease severity score, compared with placebo, for patients with chronic rhinosinusitis | |||
| Adverse effects ‐ hepatic toxicity Follow‐up: 6 weeks № of participants: 53 (1 RCT) |
RR 3.35 (95% CI 0.14 to 78.60) | Study population | ⊕⊕⊝⊝ LOW 2 | Systemic antifungal agents may lead to more hepatic toxicity events compared with placebo for patients with chronic rhinosinusitis | ||
| No events were reported | — | — | ||||
| Adverse effects ‐ gastrointestinal disturbances
Follow‐up: 6 weeks
№ of participants: 53 (1 RCT) |
RR 0.37 (95% CI 0.04 to 3.36) | Study population | ⊕⊕⊝⊝ LOW3 | Systemic antifungal agents may lead to more gastrointestinal disturbances compared with placebo for patients with chronic rhinosinusitis | ||
| 10.7% | 4.0% (0.4 to 36.0) | 6.7% fewer (10.3 fewer to 25.3 more) | ||||
| Adverse effects ‐ allergic reactions | No study reported this outcome | |||||
| Generic health‐related quality of life | No study reported this outcome | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio; RSDI: Rhinosinusitis Disability Index | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded by one level due to imprecision: results come from one small study (44 participants). Downgraded by one level due to risk of bias: the paper does not present quantitative results and so is at risk of selective outcome reporting.
2Downgraded by two levels due to imprecision: results come from one small study (44 participants) reporting one event in the systemic antifungal group, leading to very wide confidence intervals.
3Downgraded by two levels due to imprecision: results come from one small study (44 participants) reporting three events in the placebo group and one event in the systemic antifungal group, leading to wide confidence intervals.
Comparison 1: Topical antifungals versus placebo or no treatment
Seven studies (437 participants) were included in this comparison (Corradini 2006; Ebbens 2006; Hashemian 2016; Liang 2008; Ponikau 2005; Shin 2004; Weschta 2004).
Primary outcomes
Disease‐specific health‐related quality of life
Although four studies reported disease‐specific health‐related quality of life using validated instruments, the data were difficult to interpret for a number of reasons:
A variety of different instruments were used and the length of scales (e.g. RSOM‐31) was not reported in some studies.
Some studies suggested that the scoring system or scale had been modified from the validated version but did not provide full details. Weschta 2004 did not use a validated instrument, which biases the results towards not detecting a difference due to the loss of validity and ability to detect differences.
There were a variety of ways in which the data were reported in the studies, for example change from baseline versus endpoints, means and standard deviations versus medians with ranges.
The data were likely to be not normally distributed in at least three of the studies.
Considering of all of these factors we have summarised the results narratively and presented them in full in Table 4.
All four of the studies reported no statistically significant difference between groups.
Ebbens 2006 (116 participants) reported the change from baseline using the Rhinosinusitis Outcome Measure‐31 (RSOM‐31; range: 0 to 775, lower = better quality of life) at 13 weeks as means with standard deviations. Neither group had a large mean change from baseline values (3.6 points and 17 points change on a scale of 775 in the antifungal and placebo groups, respectively) but there was no significant difference between the groups (P = 0.35).
Hashemian 2016 (48 participants) reported the endpoint values using the SNOT‐20 quality of life instrument (range 0 to 100; lower = better quality of life) at eight weeks. The standard deviations (provided from personal correspondence with the authors) suggest that the data may be skewed based on their size compared to the mean. There was no difference between the groups at the end of treatment (P = 0.76).
Ponikau 2005 (24 participants) reported the change from baseline using the SNOT‐20 quality of life instrument at six months. The results were presented as medians with ranges, which may be because of the small sample size or because the authors felt the data to be skewed. There was no statistically significant difference in change from baseline values between the groups (P = 0.72, Wilcoxon rank sum test).
Liang 2008 (64 participants) reported the endpoint values using the Chinese RSOM‐31 values at four weeks. The results were presented as medians with ranges and the data appeared to be highly skewed. The median score was lower in the antifungal group but the result was not significant (P = 0.091).
Disease severity (combined or individual symptom scores)
Two studies (176 participants), recruiting only patients with chronic rhinosinusitis with nasal polyps, reported disease severity as the sum of five individual symptom scores.
Ebbens 2006 (116 participants) measured the symptoms of nasal blockage, rhinorrhoea, facial pain, postnasal drip and anosmia, each on a visual analogue scale (VAS) of 0 to 10 cm (converted to 0 to 100). The paper presents the mean and standard deviation for the mean change in total symptom score (range: 0 to 500) after 13 weeks of treatment. Both antifungal and placebo groups experienced small reductions in symptom score with the following mean change from baseline (standard deviation (SD)) values: placebo group: ‐21.1 (101.2); antifungal group: ‐3.1 (82.8). The difference between the groups is not significant (P = 0.31)
Weschta 2004 (60 participants) measured the symptoms nasal blockage, facial pain, smell disturbance, nasal discharge and sneezing, each on a scale of 0 to 10. The paper presents the median and interquartile ranges for the total symptom score (range: 0 to 50) after eight weeks of treatment. There is no indication that the results are significantly skewed. After treatment, the median symptom score was lower (less severe symptoms) in the control group (16.5; 12.0 to 24.0) compared to the group allocated to antifungal treatment (26.0; 21.3 to 29.8). This result was statistically significant (P < 0.005).
Significant adverse effects: hepatic toxicity (systemic antifungals)
This outcome was not relevant for the analysis of topical antifungals.
Secondary outcomes
Generic health‐related quality of life
Ebbens 2006 (116 participants) reported generic health‐related quality of life using the short form‐36 (SF‐36), although they reported the physical and mental component scores separately (0 to 100, lower scores = better quality of life) and did not report an overall score. The mean difference in mean change from baseline values between the antifungal and placebo groups after 13 weeks of treatment was ‐0.80 for the physical component (95% confidence interval (CI) ‐3.66 to 2.06) and ‐2.20 for the mental component score (95% CI ‐5.46 to 1.06). It is uncertain whether there is a difference between the groups (Analysis 1.1).
1.1. Analysis.
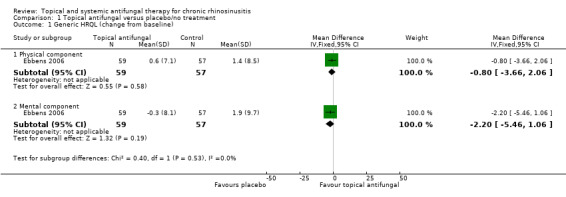
Comparison 1 Topical antifungal versus placebo/no treatment, Outcome 1 Generic HRQL (change from baseline).
Other adverse effects: gastrointestinal disturbances, allergic reactions (systemic antifungals)
This outcome is not relevant for topical antifungals.
Other adverse effects: epistaxis, headache, local discomfort (e.g. itching, mild burning) (topical antifungals)
Epistaxis
Only Ebbens 2006 (116 participants) specifically reported epistaxis as an adverse effect, which was reported by two participants in each group. Three other studies stated that no participants had adverse effects (other than local discomfort) in either treatment group (Corradini 2006; Ponikau 2005; Shin 2004); it is therefore assumed that no participants had epistaxis (risk ratio (RR) 0.97, 95% CI 0.14 to 6.63; 4 studies; 225 participants) (Analysis 1.2).
1.2. Analysis.
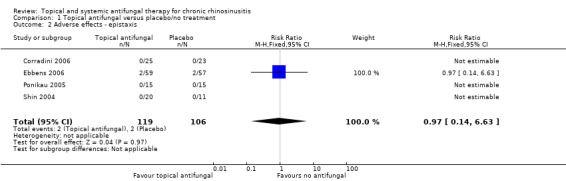
Comparison 1 Topical antifungal versus placebo/no treatment, Outcome 2 Adverse effects ‐ epistaxis.
Headache
Only Ebbens 2006 (116 participants) reported headache as an adverse effect, although Ponikau 2005 specifically stated that they would not be reporting headache as it was a symptom of chronic rhinosinusitis as well as a possible adverse effect. Two studies stated that no participants had adverse effects (other than local discomfort) in either treatment group (Corradini 2006; Shin 2004), so it is assumed that no participants had headache (RR 1.26, 95% CI 0.60 to 2.63; 3 studies; 195 participants) (Analysis 1.3).
1.3. Analysis.
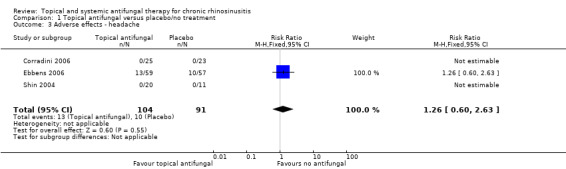
Comparison 1 Topical antifungal versus placebo/no treatment, Outcome 3 Adverse effects ‐ headache.
Local discomfort
Five studies reported data on local irritation that could be included in a meta‐analysis. Where irritation was observed in the antifungal treatment arm it was described as a 'slight burning sensation' (Hashemian 2016), 'nasal burning' (Ponikau 2005), or 'skin itching' (Liang 2008) (RR 2.29, 95% CI 0.61 to 8.62; 5 studies; 312 participants) (Analysis 1.4).
1.4. Analysis.
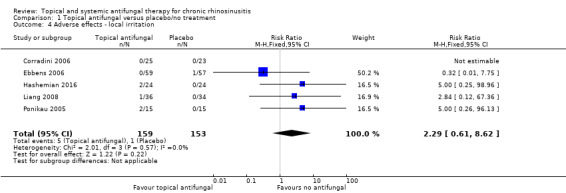
Comparison 1 Topical antifungal versus placebo/no treatment, Outcome 4 Adverse effects ‐ local irritation.
Furthermore, two studies made statements about local irritation but the numbers for each group were not available. Weschta 2004 identified significantly more participants in the amphotericin B group who reported "nasal burning" (P < 0.005) and Shin 2004 indicated that some participants reported "mild nasal discomfort due to a burning sensation" but did not report how many people this affected or to which group they were allocated.
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Kennedy)
Extent of polyps
Three studies presented data for the extent of nasal polyps after treatment.
Hashemian 2016 (54 participants) assessed polyp size in each nostril using a range of 0 to 4 (0 = no polyp, 4 = polypoid changes below the lower edge of the inferior turbinate; total range: 0 to 8). No significant difference in final polyp score between the groups was reported at eight weeks (P = 0.38).
Ponikau 2005 (30 participants), using the same scale as Hashemian 2016, reported the change in the extent of polyps from baseline in each treatment group as medians and ranges. They identified no significant difference between the treatment arms at three months (P = 0.47) but a significantly larger reduction in polyp size in the antifungals group compared to placebo (P = 0.038) at six months.
Weschta 2004 (78 participants) assessed polyp size in each nasal cavity using a range of 0 to 3 (0 = no polyps; 3 = polyps fill whole nasal cavity; total range: 0 to 6). The results are not well reported but the authors state: "The median endoscopy scores were almost identical in the AMB and control groups (4 vs 4) and did not change remarkably after treatment.”
Endoscopy score
Two studies used an endoscopy score to compare the groups after treatment.
Ebbens 2006 assessed the amount of mucosal disease by measuring nasal secretions, nasal polyps and nasal crusting each on a scale of 0 to 2 (0 = absent, 2 = severe) in predefined areas (e.g. middle meatus, ethmoid region). Sum scores were calculated by adding all independent values for both nostrils but the total possible range is not given in the paper. The data are presented as mean change in endoscopy scores from baseline values. The authors found no difference between the groups (P = 0.64) after 13 weeks treatment.
Liang 2008 measured oedema, discharge, polyps, crusting and scarring, all graded from 0 (normal) to 2 (severely diseased). The total range is not provided but is likely to be 0 to 10. The data are presented as the median endoscopy scores at the end of treatment. The authors found no difference between the groups (P = 0.944) after four weeks treatment.
Computerised tomography (CT) scan score (e.g. Lund‐Mackay)
Three studies reported CT scan scores, although different scales were used:
Hashemian 2016 (48 participants) measured mucosal thickening, scored on scale of 0 to 3 (0 = no thickening) for each of the frontal, maxillary, sphenoid and ethmoid sinuses, the nasal passages and ostiomeatal complexes. Each of the scores was summed to give a final range from 0 to 30 points (from personal communication with authors). The study showed that there was no difference in CT scores between the topical antifungal and placebo groups (standardised mean difference (SMD) ‐0.22, 95% CI ‐0.79 to 0.34) (Analysis 1.5).
Weschta 2004 (60 participants) used the Lund‐Kennedy score, which measures opacification on a scale of 0 to 4 (0 = not opacified) for each of the of the maxillary, anterior and posterior ethmoidal, sphenoidal and frontal sinuses. Each of the scores was summed to give a final range from 0 to 40 points. After treatment, the median CT scan scores in the antifungal treatment group were 26.5 (interquartile range (IQR) 19.5 to 35.8) and in the control group were 26.5 (IQR 23.0 to 32.0). This result was not statistically significant (P > 0.2).
Ponikau 2005 used digitised coronal CT scans to measure the percentage of airspace occluded by inflammatory mucosal thickening. There was a significant decrease in the mean percentage of air space occluded between the group receiving topical antifungals (‐8.8%, standard deviation (SD) 13.6) and the placebo group (2.5%, SD 10.3).
1.5. Analysis.
Comparison 1 Topical antifungal versus placebo/no treatment, Outcome 5 CT score.
Subgroup analyses
We had planned to present subgroup analyses by presence of allergic fungal rhinosinusitis and eosinophilic status. However, these factors were not well presented in the studies and so subgroup analysis was not possible. The presence of nasal polyps was reported but as only Liang 2008 exclusively included recruited patients without nasal polyps and meta‐analysis was not possible for the primary outcome, we did not complete subgroup analyses. We planned to investigate the other factors identified in the methods (patient age, dose, duration of treatment, method of delivery, class of antifungal agent) in the event of statistical heterogeneity, but this situation did not occur.
Comparison 2: Systemic antifungals versus placebo or no treatment
One study was included in this comparison (Kennedy 2005; 53 participants), which compared terbinafine tablets with placebo tablets in patients with chronic rhinosinusitis (unknown polyps status) for six weeks.
Primary outcomes
Disease‐specific health‐related quality of life
Kennedy 2005 (53 participants) measured disease‐specific health‐related quality of life using the Rhinosinusitis Disability Index (RSDI). Values for the RSDI results were not given but the authors state "no differences were observed" at any time point measured.
Disease severity (combined or individual symptom scores)
Kennedy 2005 (53 participants) measured the symptoms of facial pain/pressure, facial congestion and nasal discharge. No values were reported but the authors state that no differences between the groups were observed.
Significant adverse effects: hepatic toxicity (systemic antifungals)
Although one patient in the terbinafine group had increased aspartate aminotransferase (AST), alanine aminotransferase (ALT) or gamma‐glutamyl (GGT) levels, the paper goes on to state that "No clinically significant difference between treatment groups was observed in liver function tests (LFT) at week 3 or week 6" (RR 3.35, 95% CI 0.14 to 78.60; 53 participants) (Analysis 2.1).
2.1. Analysis.
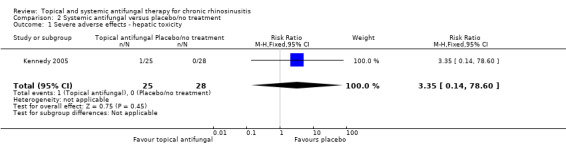
Comparison 2 Systemic antifungal versus placebo/no treatment, Outcome 1 Severe adverse effects ‐ hepatic toxicity.
Secondary outcomes
Generic health‐related quality of life
This outcome was not reported in the included study.
Other adverse effects: gastrointestinal disturbances, allergic reactions (systemic antifungals)
Kennedy 2005 (53 participants) reported that one person experienced gastrointestinal disorders in the terbinafine group compared with three people in the placebo group (RR 0.37, 95% CI 0.04 to 3.36) (Analysis 2.2).
2.2. Analysis.
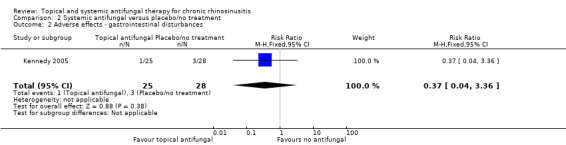
Comparison 2 Systemic antifungal versus placebo/no treatment, Outcome 2 Adverse effects ‐ gastrointestinal disturbances.
Other adverse effects: epistaxis, headache, local discomfort (e.g. itching, mild burning) (topical antifungals)
This outcome is not relevant for systemic antifungals.
Endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Kennedy)
This outcome was not reported in the included study.
Computerised tomography (CT) scan score (e.g. Lund‐Mackay)
Kennedy 2005 reported the CT scan score in two ways: the percentage change from baseline in the total opacification score (higher = worse) (mean difference (MD) ‐0.14, 95% CI ‐19.22 to 18.94; 49 participants) (Analysis 2.3) and the percentage change from baseline total in obstruction score of the frontal recess, middle meatus infundibulum and sphenoethmoid recess (higher = worse) (MD ‐4.40, 95% CI ‐40.12 to 31.32; 47 participants) (Analysis 2.4). No statistical difference was observed in either group and large standard deviations indicate very large variations in the results.
2.3. Analysis.
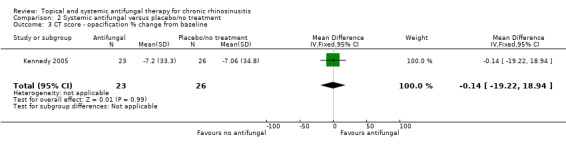
Comparison 2 Systemic antifungal versus placebo/no treatment, Outcome 3 CT score ‐ opacification % change from baseline.
2.4. Analysis.
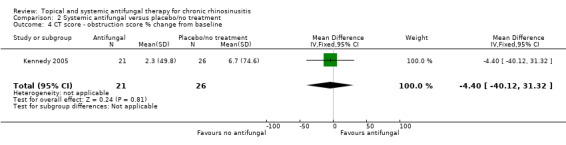
Comparison 2 Systemic antifungal versus placebo/no treatment, Outcome 4 CT score ‐ obstruction score % change from baseline.
Subgroup analyses
As only one study was included in this comparison, subgroup analyses were not possible.
Discussion
Summary of main results
Topical antifungals versus placebo
Seven studies (437 participants) comparing antifungals with placebo or no treatment were included. There were a variety of different administration methods used from low‐volume nasal sprays to high‐volume nasal irrigation. The inclusion criteria of the studies ranged from excluding patients with nasal polyps (one study) to including only patients with nasal polyps (three studies). It was difficult to analyse the data as the outcomes were measured using different instruments (some with potential validation issues) and the results were reported in different ways (means and medians).
The efficacy outcomes of both disease‐specific and generic health‐related quality of life and disease severity as measured by patient‐reported symptoms did not appear to differ between the topical antifungals and placebo/no treatment groups. With regards to adverse effects there may have been more local irritation events in the group receiving antifungal agents compared with placebo. It is uncertain if there was a difference between the groups with regards to developing headaches or epistaxis. No differences were found between the groups in CT scan scores or endoscopy scores.
There was considerable variation in the doses of antifungals used in the studies. The dose of amphotericin B used ranged from 0.8 mg/day to 20 mg/day with varying concentrations, dosing regimens and delivery methods. The dose of fluconazole used was 1.2 mg per day. In many cases the dose was considered to be low.
Systemic antifungals versus placebo
One study (53 participants) compared systemic antifungals (terbinafine tables) against placebo. No statistically significant difference between the groups was observed in disease‐specific health‐related quality of life, disease severity as measured by patient‐reported symptoms or CT scan scores. One patient in the systemic antifungals group had elevated liver function tests but fewer people reported gastrointestinal disturbances in the systemic antifungal group compared to the placebo group, although the results were not significantly different between the groups in either case. The dose of terbinafine used in the study was over twice the recommended daily dose in the British National Formulary (BNF 2018), with the rationale being that the dosing was as used for invasive fungal sinusitis. This study may have been limited by use of the CT scan scores as the primary outcome measure; radiological changes correlate poorly with symptom scores.
Overall completeness and applicability of evidence
The evidence included a wide range of participants with chronic rhinosinusitis including those with and without nasal polyps. The included populations were representative of the average chronic rhinosinusitis population. However, the presence of allergic fungal rhinosinusitis, eosinophilic fungal rhinosinusitis or eosinophilic mucinous rhinosinusitis was not well reported within the papers and in fact these patients were excluded in some of the studies.
Six of the seven studies that reported the age of the participants only included adults in their trial populations. The seventh study extended their inclusion criteria to include children from the age of 12 years. No evidence exists for children below 12 years, although chronic rhinosinusitis is predominantly a disease of adulthood.
Quality of the evidence
We assessed the evidence included within this review to be of low or very low quality. Although, for the most part, we did not consider the risk of bias in the studies to be very high and they were generally well conducted, the studies were typically very small (30 to 116 participants) with only one study having more than 80 participants. The results of the studies were often poorly reported using a range of different instruments and methods to measure the same outcome. In particular, the validity of the instruments used to measure quality of life and symptom scores was of concern. Some studies appeared to have modified validated instruments meant for other populations without referencing the further validation, with potential adverse consequences for the validity and reliability of the results. Even when a validated instrument was cited, it was unclear if a validated instrument for the particular language and setting had been applied (Wild 2005).
Potential biases in the review process
Due to the differences in the instruments used for measuring the primary efficacy outcome (disease‐specific health‐related quality of life) and the ways in which this outcome had been reported (means with standard deviations versus medians with ranges), we made the decision not to try to meta‐analyse the results. We had concerns that some of the data were from skewed distributions and felt that completing a meta‐analysis may lead to spurious conclusions. There was some thought that there may be 'sub' populations within the overall trial population who might respond differently to the antifungal treatment but not enough information was available to be able to investigate this.
The definition of the population inclusion criteria excluded patients who had recently undergone surgery. However, it is noted that allergic fungal rhinosinusitis is often identified during or even after surgery and so by excluding the post‐surgical population we may have missed some of these studies.
Agreements and disagreements with other studies or reviews
One recent paper, published after the final date of the literature search, compared amphotericin B with placebo in 80 patients with chronic rhinosinusitis (20% of whom had nasal polyps) (Yousefi 2017). Their results are consistent with the findings of this review in that their study found no statistically significant difference between the groups at three months for any of the outcomes: patient‐reported symptom severity (nasal obstruction, post‐nasal drip, sense of smell and facial pain), health‐related quality of life or CT scores. The lack of any difference most probably represents the fact that 80% of participants had chronic rhinosinusitis without nasal polyps, where fungal aetiology is unlikely to play a role.
The results of the previous Cochrane Review and another more recent systematic review reach the same conclusions as this review (Mistry 2014; Sacks 2011). Both agree that there is no convincing evidence to support the use of antifungals in chronic rhinosinusitis. The authors of previous reviews share our concern regarding the clinical diversity of the included populations within the trials, particularly with regard to diagnosis, with acknowledgement that the population often includes patients with both chronic rhinosinusitis and allergic fungal rhinosinusitis.
Authors' conclusions
Implications for practice.
Due to the very low quality of the evidence, it is uncertain whether or not the use of topical or systemic antifungals has an impact on patient outcomes in adults with chronic rhinosinusitis compared with placebo or no treatment. There is no evidence available to assess the efficacy of antifungal agents for specific subgroups of chronic rhinosinusitis, such as allergic fungal rhinosinusitis, eosinophilic fungal rhinosinusitis or eosinophilic mucinous rhinosinusitis, but this finding is very much limited by the study designs, which did not focus specifically on these specific fungal subgroups and also had marked variation in the treatment regimens.
The evidence in this review is for patients who did not undergo surgery.
Implications for research.
As of November 2017, we have found eight studies of topical or systemic antifungal agents for patients with chronic rhinosinusitis who did not have surgery. There is low‐quality evidence (i.e. we are uncertain about the estimates) that there is little or no difference between antifungals (topical or systemic) and placebo or no treatment, in terms of quality of life or patient‐reported symptom scores. The quality of the evidence for adverse effects is low or very low due to inadequate reporting methods and small study sizes.
We considered the potential for future research into the use of antifungal agents and feel that this area of research might not be prioritised above research for other standard interventions as identified by the other reviews in this suite (Chong 2016a; Chong 2016b; Chong 2016c; Head 2016a; Head 2016b; Head 2016c). If research is carried out, open questions remain about the use of topical and systemic antifungals in patients with specific subtypes of chronic rhinosinusitis: allergic fungal rhinosinusitis, eosinophilic fungal rhinosinusitis or eosinophilic mucinous rhinosinusitis.
This review is one of a suite of reviews on medical treatments for chronic rhinosinusitis, each of which features its own research recommendations. Across all reviews, key features of future research are as follows:
Trials should be adequately powered and imbalances in prognostic factors (for example, prior sinus surgery) must be accounted for in the statistical analysis.
Study participants should be diagnosed with chronic rhinosinusitis using the EPOS 2012 criteria and should primarily be recruited based on their symptoms. Different patient phenotypes (that is, those with and without nasal polyps) should be recognised and trials should use stratified randomisation within these subgroups or focus on one or other of the phenotypes. In addition, subcategories of chronic rhinosinusitis such as allergic fungal rhinosinusitis, eosinophilic fungal rhinosinusitis and eosinophilic mucinous rhinosinusitis should be well defined and diagnosed at the start of the trial with stratification at randomisation. Ideally multi‐centre studies focused on these fungal subgroups would be more useful in addressing the role of both topical and systemic antifungals; some of the excluded case series suggest that an effect may be present.
Studies should focus on outcomes that are important to patients and use validated instruments to measure these. Validated chronic rhinosinusitis‐specific health‐related quality of life questionnaires exist, for example the Sino‐Nasal Outcome Test‐22 (SNOT‐22). Patients may find dichotomised outcomes easiest to interpret; for example the percentage of patients achieving a minimal clinically important difference (MCID) or improvement for that outcome. Such MCIDs or cut‐off points should be included in the study protocol and clearly outlined in the methods section.
Trials and other high‐quality studies should use consistent outcomes and adhere to reporting guidelines, such as CONSORT, so that results can be compared across future trials. There is now a core outcome set for chronic rhinosinusitis trials that should guide research teams in setting these trials henceforth (CHROME 2017).
Notes
This review will update and replace the previously published review 'Topical and systemic antifungal therapy for the symptomatic treatment of chronic rhinosinusitis' (Sacks 2011).
Acknowledgements
We would like to acknowledge Samantha Cox for her input into the Search methods for identification of studies section and Jenny Bellorini for her help with copy editing the review.
We would like to acknowledge Tess Cooper who assisted with the initial screening of abstracts and Peta‐Lee Sacks for her help and assistance in reviewing and editing the protocol.
We are indebted to Professor Motokazu Yanagi for translating and identifying a primary studies for exclusion from this review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
| CENTRAL (via CRS Web) | MEDLINE (Ovid) | Embase (Ovid) | Web of Science (Web of Knowledge) |
| #1 MESH DESCRIPTOR Rhinitis EXPLODE ALL TREES #2 MESH DESCRIPTOR Paranasal Sinus Diseases EXPLODE All TREES #3 MESH DESCRIPTOR Paranasal Sinuses EXPLODE All TREES #4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis):TI,AB,KY #5 (kartagener* near syndrome*):TI,AB,KY #6 (inflamm* near sinus*):TI,AB,KY #7 ((maxilla* or frontal*) near sinus*):TI,AB,KY #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #9 MESH DESCRIPTOR Chronic Disease EXPLODE All TREES #10 MESH DESCRIPTOR Recurrence EXPLODE All TREES #11 MESH DESCRIPTOR Fungi EXPLODE All TREES #12 MESH DESCRIPTOR Mycetoma EXPLODE All TREES #13 (chronic or persis* or recurrent* or fung* or eosinophil* or mycetoma* or Maduromycos* or Actinomycetoma* or Eumycetoma*):TI,AB,KY #14 #9 OR #10 OR #11 OR #12 OR #13 #15 #8 AND #14 #16 (CRSsNP or AFS or AFRS):TI,AB,KY #17 ((sinusitis or rhinitis) near (chronic or persis* or recurrent* or fung*)):TI,AB,KY #18 #15 OR #16 OR #17 #19 MESH DESCRIPTOR Nasal Polyps EXPLODE All TREES #20 MESH DESCRIPTOR Paranasal Sinus Diseases EXPLODE ALL TREES WITH QUALIFIERS MI #21 MESH DESCRIPTOR Rhinitis EXPLODE ALL TREES WITH QUALIFIERS MI #22 MESH DESCRIPTOR Paranasal Sinuses EXPLODE ALL TREES WITH QUALIFIERS MI #23 MESH DESCRIPTOR Nasal Mucosa EXPLODE ALL TREES WITH QUALIFIERS MI #24 MESH DESCRIPTOR Nose EXPLODE ALL TREES #25 MESH DESCRIPTOR Nose Diseases EXPLODE ALL TREES #26 #24 OR #25 #27 MESH DESCRIPTOR Polyps EXPLODE ALL TREES #28 #26 AND #27 #29 (rhinopolyp* or CRSwNP):TI,AB,KY #30 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) near (papilloma* or polyp* or fung*)):TI,AB,KY #31 #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #28 OR #29 OR #30 #32 MESH DESCRIPTOR Antifungal Agents EXPLODE All TREES #33 (antifung* or "anti fung*" or fungastic or fungicidal or Fungizone or Amphocil or Zonal or Diflucan or Triflucan or hexal or Fluco* or Flunazul or Fungata or Lavisa or Loitin or Neofomiral or oxifungol or Solacap or 49858 of BÈagyne or 51211 or Sporanox or Orungal):TI,AB,KY #34 MESH DESCRIPTOR Mycoses EXPLODE ALL TREES WITH QUALIFIERS DT,TH #35 MESH DESCRIPTOR Venturicidins EXPLODE All TREES #36 MESH DESCRIPTOR Trimetrexate EXPLODE All TREES #37 MESH DESCRIPTOR Triacetin EXPLODE All TREES #38 MESH DESCRIPTOR Tolnaftate EXPLODE All TREES #39 MESH DESCRIPTOR Tomatine EXPLODE All TREES #40 MESH DESCRIPTOR Thymol EXPLODE All TREES #41 MESH DESCRIPTOR Sodium Benzoate EXPLODE All TREES #42 MESH DESCRIPTOR Sirolimus EXPLODE All TREES #43 MESH DESCRIPTOR Salicylic Acid EXPLODE All TREES #44 MESH DESCRIPTOR Pentamidine EXPLODE All TREES #45 MESH DESCRIPTOR Nystatin EXPLODE All TREES #46 MESH DESCRIPTOR Nifuratel EXPLODE All TREES #47 MESH DESCRIPTOR Natamycin EXPLODE All TREES #48 MESH DESCRIPTOR Mycobacillin EXPLODE All TREES #49 MESH DESCRIPTOR Monensin EXPLODE All TREES #50 MESH DESCRIPTOR Miconazole EXPLODE All TREES #51 MESH DESCRIPTOR Mepartricin EXPLODE All TREES #52 MESH DESCRIPTOR Lucensomycin EXPLODE All TREES #53 MESH DESCRIPTOR Ketoconazole EXPLODE All TREES #54 MESH DESCRIPTOR Itraconazole EXPLODE All TREES #55 MESH DESCRIPTOR Hexetidine EXPLODE All TREES #56 MESH DESCRIPTOR Griseofulvin EXPLODE All TREES #57 MESH DESCRIPTOR Flucytosine EXPLODE All TREES #58 MESH DESCRIPTOR Fluconazole EXPLODE All TREES #59 MESH DESCRIPTOR Econazole EXPLODE All TREES #60 MESH DESCRIPTOR Echinocandins EXPLODE All TREES #61 MESH DESCRIPTOR Dichlorophen EXPLODE All TREES #62 MESH DESCRIPTOR Cyclosporine EXPLODE All TREES #63 MESH DESCRIPTOR Cycloheximide EXPLODE All TREES #64 MESH DESCRIPTOR Clotrimazole EXPLODE All TREES #65 MESH DESCRIPTOR Filipin EXPLODE All TREES #66 MESH DESCRIPTOR Cerulenin EXPLODE All TREES #67 MESH DESCRIPTOR Candicidin EXPLODE All TREES #68 MESH DESCRIPTOR Brefeldin A EXPLODE All TREES #69 MESH DESCRIPTOR Benzoates EXPLODE All TREES #70 MESH DESCRIPTOR Azaserine EXPLODE All TREES #71 MESH DESCRIPTOR Antimycin A EXPLODE All TREES #72 MESH DESCRIPTOR Amphotericin B EXPLODE All TREES #73 (acivicin or ajoene or amorolfin or Amphotericin or anidulafungin or Antimycin or artemether or aureobasidin or Azaserine or bafilomycin or Benzoates or bifonazole or blasticidin or Brefeldin or butenafine or butoconazole):TI,AB,KY #74 (Candicidin or candidin or captax or caspofungin or Cerulenin or ciclopirox or cilofungin or Clotrimazole or compactin or cordycepin or cryptophycin or Cycloheximide or Cyclosporine or (decanoic near acid) or (diallyl near trisulfide) or Dichlorophen or diucifon or echinocandin or Echinocandins or Econazole or Ethonium):TI,AB,KY #75 (fenticonazole or ferroin or Filipin or Flucytosine or glyphosate or Griseofulvin or hamycin or Hexetidine or hydroxyitraconazole or (ICI near "195739") or isoconazole or Itraconazole or iturin or jasplakinolide or Ketoconazole or lactoferricin or lapachol or lawsone or leptomycin or Lucensomycin):TI,AB,KY #76 (Mepartricin or methylamphotericin or micafungin or Miconazole or miltefosine or Monensin or monorden or mucidin or muconaldehyde or Mycobacillin or myxothiazol or n‐hexanal or naftifine or Natamycin or Nifuratel or nikkomycin or nitroxoline or Nystatin or oxiconazole or papulacandin or (pelargonic near acid) or Pentamidine or polygodial or (polyoxin near D) or posaconazole or (potassium near iodate) or pradimicin or protegrin‐1 or purothionin or pyochelin or pyrithione or Pyrrolnitrin):TI,AB,KY #77 (rhizoxin or Rutamycin or (salicylhydroxamic near acid) or (Salicylic near Acid) or saperconazole or (Sch near "39304") or sertaconazole or sinefungin or Sirolimus or (Sodium near Benzoate) or squalestatin or sulconazole or terbinafine or terconazole or thermozymocidin or Thymol or tioconazole or Tolnaftate or Tomatine or Triacetin or trichostatin or Trimetrexate or troclosene or (usnic near acid) or Venturicidins or vibunazole or voriconazole or wortmannin):TI,AB,KY #78 #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 #79 #31 AND #78 |
1 exp Rhinitis/ 2 exp Paranasal Sinuses/ 3 exp Paranasal Sinus Diseases/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).ab,ti. 5 (kartagener* adj3 syndrome*).ab,ti. 6 (inflamm* adj3 sinus*).ab,ti. 7 ((maxilla* or frontal*) adj3 sinus*).ab,ti. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp Chronic Disease/ 10 exp Recurrence/ 11 exp Fungi/ 12 exp Mycetoma/ 13 (chronic or persis* or recurrent* or fung* or eosinophil* or mycetoma* or Maduromycos* or Actinomycetoma* or Eumycetoma*).ab,ti. 14 9 or 10 or 11 or 12 or 13 15 8 and 14 16 (CRSsNP or AFS or AFRS).ab,ti. 17 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent* or fung*)).ab,ti. 18 15 or 16 or 17 19 exp Nasal Polyps/ 20 exp Paranasal Sinus Diseases/mi [Microbiology] 21 exp rhinitis/mi [Microbiology] 22 exp Nasal Mucosa/mi [Microbiology] 23 exp Paranasal Sinuses/mi [Microbiology] 24 exp Nose/ 25 exp Nose Diseases/ 26 24 or 25 27 exp Polyps/ 28 26 and 27 29 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp* or fung*)).ab,ti. 30 (rhinopolyp* or CRSwNP).ab,ti. 31 18 or 19 or 20 or 21 or 22 or 23 or 28 or 29 or 30 32 exp Antifungal Agents/ or exp Amphotericin B/ or exp Antimycin A/ or exp Azaserine/ or exp Benzoates/ or exp Brefeldin A/ or exp Candicidin/ or exp Cerulenin/ or exp Clotrimazole/ or exp Cycloheximide/ or exp Cyclosporine/ or exp Dichlorophen/ or exp Echinocandins/ or exp Econazole/ or exp Filipin/ or exp Fluconazole/ or exp Flucytosine/ or exp Griseofulvin/ or exp Hexetidine/ or exp Itraconazole/ or exp Ketoconazole/ or exp Lucensomycin/ or exp Mepartricin/ or exp Miconazole/ or exp Monensin/ or exp Mycobacillin/ or exp Natamycin/ or exp Nifuratel/ or exp Nystatin/ or exp Pentamidine/ or exp Rutamycin/ or exp Salicylic Acid/ or exp Sirolimus/ or exp Sodium Benzoate/ or exp Thymol/ or exp Tomatine/ or exp Tolnaftate/ or exp Triacetin/ or exp Trimetrexate/ or exp Venturicidins/ 33 exp Mycoses/dt, th [Drug Therapy, Therapy] 34 (acivicin or ajoene or amorolfin or Amphotericin or anidulafungin or Antimycin or artemether or aureobasidin or Azaserine or bafilomycin or Benzoates or bifonazole or blasticidin or Brefeldin or butenafine or butoconazole).ab,ti,nm. 35 (Candicidin or candidin or captax or caspofungin or Cerulenin or ciclopirox or cilofungin or Clotrimazole or compactin or cordycepin or cryptophycin or Cycloheximide or Cyclosporine or (decanoic adj3 acid) or (diallyl adj3 trisulfide) or Dichlorophen or diucifon or echinocandin or Echinocandins or Econazole or Ethonium or fenticonazole or ferroin or Filipin or Fluconazole or Flucytosine or glyphosate or Griseofulvin or hamycin or Hexetidine or hydroxyitraconazole or (ICI adj3 "195739") or isoconazole or Itraconazole or iturin or jasplakinolide or Ketoconazole or lactoferricin or lapachol or lawsone or leptomycin or Lucensomycin or Mepartricin or methylamphotericin or micafungin or Miconazole or miltefosine or Monensin or monorden or mucidin or muconaldehyde or Mycobacillin or myxothiazol or n‐hexanal or naftifine or Natamycin or Nifuratel or nikkomycin or nitroxoline or Nystatin or oxiconazole or papulacandin or (pelargonic adj3 acid) or Pentamidine or polygodial or (polyoxin adj3 D) or posaconazole or (potassium adj3 iodate) or pradimicin or protegrin‐1 or purothionin or pyochelin or pyrithione or Pyrrolnitrin or rhizoxin or Rutamycin or (salicylhydroxamic adj3 acid) or (Salicylic adj3 Acid) or saperconazole or (Sch adj3 "39304") or sertaconazole or sinefungin or Sirolimus or (Sodium adj3 Benzoate) or squalestatin or sulconazole or terbinafine or terconazole or thermozymocidin or Thymol or tioconazole or Tolnaftate or Tomatine or Triacetin or trichostatin or Trimetrexate or troclosene or (usnic adj3 acid) or Venturicidins or vibunazole or voriconazole or wortmannin).ab,ti,nm. 36 (antifung* or "anti fung*" or fungastic or fungicidal or Fungizone or Amphocil or Zonal or Diflucan or Triflucan or hexal or Fluco* or Flunazul or Fungata or Lavisa or Loitin or Neofomiral or oxifungol or Solacap or 49858 of Beagyne or "51211" or Sporanox or Orungal).ab,ti,nm. 37 32 or 33 or 34 or 35 or 36 38 31 and 37 |
1 exp rhinitis/ 2 exp Paranasal Sinuses/ 3 exp Paranasal Sinus Diseases/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).ab,ti. 5 (kartagener* adj3 syndrome*).ab,ti. 6 (inflamm* adj3 sinus*).ab,ti. 7 ((maxilla* or frontal*) adj3 sinus*).ab,ti. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp Chronic Disease/ 10 exp Recurrence/ 11 exp Fungi/ 12 exp Mycetoma/ 13 (chronic or persis* or recurrent* or fung* or eosinophil* or mycetoma* or Maduromycos* or Actinomycetoma* or Eumycetoma*).ab,ti. 14 9 or 10 or 11 or 12 or 13 15 8 and 14 16 (CRSsNP or AFS or AFRS).ab,ti. 17 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent* or fung*)).ab,ti. 18 15 or 16 or 17 19 exp Nasal Polyps/ 20 exp Nose/ 21 exp Nose Diseases/ 22 20 or 21 23 exp Polyps/ 24 22 and 23 25 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp* or fung*)).ab,ti. 26 (rhinopolyp* or CRSwNP).ab,ti. 27 18 or 19 or 24 or 25 or 26 28 exp Antifungal Agents/ 29 exp Amphotericin B/ 30 exp Antimycin A/ 31 exp Azaserine/ 32 exp Benzoates/ 33 exp Brefeldin A/ 34 exp Candicidin/ 35 exp Cerulenin/ 36 exp Clotrimazole/ 37 exp Cycloheximide/ 38 exp Cyclosporine/ 39 exp Dichlorophen/ 40 exp Echinocandins/ 41 exp Econazole/ 42 exp Filipin/ 43 exp Fluconazole/ 44 exp Flucytosine/ 45 exp Griseofulvin/ 46 exp Hexetidine/ 47 exp Itraconazole/ 48 exp Ketoconazole/ 49 exp Lucensomycin/ 50 exp Mepartricin/ 51 exp Miconazole/ 52 exp Monensin/ 53 exp Mycobacillin/ 54 exp Natamycin/ 55 exp Nifuratel/ 56 exp Nystatin/ 57 exp Pentamidine/ 58 exp Rutamycin/ 59 exp Salicylic Acid/ 60 exp Sirolimus/ 61 exp Sodium Benzoate/ 62 exp Thymol/ 63 exp Tomatine/ 64 exp Tolnaftate/ 65 exp Triacetin/ 66 exp Trimetrexate/ 67 exp Venturicidins/ 68 exp mycosis/dt, th [Drug Therapy, Therapy] 69 (acivicin or ajoene or amorolfin or Amphotericin or anidulafungin or Antimycin or artemether or aureobasidin or Azaserine or bafilomycin or Benzoates or bifonazole or blasticidin or Brefeldin or butenafine or butoconazole).tw. 70 (Candicidin or candidin or captax or caspofungin or Cerulenin or ciclopirox or cilofungin or Clotrimazole or compactin or cordycepin or cryptophycin or Cycloheximide or Cyclosporine or (decanoic adj3 acid) or (diallyl adj3 trisulfide) or Dichlorophen or diucifon or echinocandin or Echinocandins or Econazole or Ethonium or fenticonazole or ferroin or Filipin or Fluconazole or Flucytosine or glyphosate or Griseofulvin or hamycin or Hexetidine or hydroxyitraconazole or (ICI adj3 "195739") or isoconazole or Itraconazole or iturin or jasplakinolide or Ketoconazole or lactoferricin or lapachol or lawsone or leptomycin or Lucensomycin or Mepartricin or methylamphotericin or micafungin or Miconazole or miltefosine or Monensin or monorden or mucidin or muconaldehyde or Mycobacillin or myxothiazol or n‐hexanal or naftifine or Natamycin or Nifuratel or nikkomycin or nitroxoline or Nystatin or oxiconazole or papulacandin or (pelargonic adj3 acid) or Pentamidine or polygodial or (polyoxin adj3 D) or posaconazole or (potassium adj3 iodate) or pradimicin or protegrin‐1 or purothionin or pyochelin or pyrithione or Pyrrolnitrin or rhizoxin or Rutamycin or (salicylhydroxamic adj3 acid) or (Salicylic adj3 Acid) or saperconazole or (Sch adj3 "39304") or sertaconazole or sinefungin or Sirolimus or (Sodium adj3 Benzoate) or squalestatin or sulconazole or terbinafine or terconazole or thermozymocidin or Thymol or tioconazole or Tolnaftate or Tomatine or Triacetin or trichostatin or Trimetrexate or troclosene or (usnic adj3 acid) or Venturicidins or vibunazole or voriconazole or wortmannin).tw. 71 (antifung* or "anti fung*" or fungastic or fungicidal or Fungizone or Amphocil or Zonal or Diflucan or Triflucan or hexal or Fluco* or Flunazul or Fungata or Lavisa or Loitin or Neofomiral or oxifungol or Solacap or 49858 of Beagyne or "51211" or Sporanox or Orungal).tw. 72 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 73 27 and 72 |
S1 TOPIC: ((rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis)) S2 TOPIC: ((kartagener* near/3 syndrome*)) S3 TOPIC: ((inflamm* near/3 sinus*)) S4 TOPIC: ((maxilla* near/3 sinus*)) S5 TOPIC: ((frontal* near/3 sinus*)) S6 #5 OR #4 OR #3 OR #2 OR #1 S7 TOPIC: ((chronic or persis* or recurrent* or fung* or eosinophil* or mycetoma* or Maduromycos* or Actinomycetoma* or Eumycetoma*)) S8 #7 AND #6 S9 TOPIC: (CRSsNP or AFS or AFRS) S10 TOPIC: (sinusitis near/3 chronic) S11 TOPIC: (sinusitis near/3 persis*) S12 TOPIC: (sinusitis near/3 recurrent*) S13 TOPIC: (sinusitis near/3 fung*) S14 TOPIC: (rhinitis near/3 fung*) S15 TOPIC: (rhinitis near/3 recurrent*) S16 TOPIC: (rhinitis near/3 persis*) S17 TOPIC: (rhinitis near/3 chronic) S18 #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 S19 TOPIC: (nose near/3 papilloma*) S20 TOPIC: (nose near/3 polyp*) S21 TOPIC: (nose near/3 fung*) S22 TOPIC: (nasal near/3 fung*) S23 TOPIC: (nasal near/3 polyp*) S24 TOPIC: (nasal near/3 papilloma*) S25 TOPIC: (rhino* near/3 papilloma*) S26 TOPIC: (rhino* near/3 polyp*) S27 TOPIC: (rhino* near/3 fung*) S28 TOPIC: (rhinitis near/3 fung*) S29 TOPIC: (rhinitis near/3 polyp*) S30 TOPIC: (rhinitis near/3 papilloma*) S31 TOPIC: (sinus* near/3 papilloma*) S32 TOPIC: (sinus* near/3 polyp*) S33 TOPIC: (sinus* near/3 fung*) S34 TOPIC: (sinonasal near/3 fung*) S35 TOPIC: (sinonasal near/3 polyp*) S36 TOPIC: (sinonasal near/3 papilloma*) S37 TOPIC: (rhinopolyp* or CRSwNP) S38 #37 OR #36 OR #35 OR #34 OR #33 OR #32 OR #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 S39 TOPIC: (acivicin or ajoene or amorolfin or Amphotericin or anidulafungin or Antimycin or artemether or aureobasidin or Azaserine or bafilomycin or Benzoates or bifonazole or blasticidin or Brefeldin or butenafine or butoconazole) S40 TOPIC: (Candicidin or candidin or captax or caspofungin or Cerulenin or ciclopirox or cilofungin or Clotrimazole or compactin or cordycepin or cryptophycin or Cycloheximide or Cyclosporine) S41 TOPIC: (decanoic near/3 acid) S42 TOPIC: (diallyl near/3 trisulfide) S43 TOPIC: (Dichlorophen or diucifon or echinocandin or Echinocandins or Econazole or Ethonium or fenticonazole or ferroin or Filipin or Fluconazole or Flucytosine or glyphosate or Griseofulvin or hamycin or Hexetidine or hydroxyitraconazole) S44 TOPIC: (ICI near/3 "195739") S45 TOPIC: (isoconazole or Itraconazole or iturin or jasplakinolide or Ketoconazole or lactoferricin or lapachol or lawsone or leptomycin or Lucensomycin or Mepartricin or methylamphotericin or micafungin or Miconazole or miltefosine or Monensin or monorden or mucidin or muconaldehyde or Mycobacillin or myxothiazol or n‐hexanal or naftifine or Natamycin or Nifuratel or nikkomycin or nitroxoline or Nystatin or oxiconazole or papulacandin) S46 TOPIC: (pelargonic near/3 acid) S47 TOPIC: (Pentamidine or polygodia) S48 TOPIC: (polyoxin near/3 "D") S49 TOPIC: (potassium near/3 iodate) S50 TOPIC: (posaconazole or pradimicin or protegrin‐1 or purothionin or pyochelin or pyrithione or Pyrrolnitrin or rhizoxin or Rutamycin) S51 TOPIC: (salicylhydroxamic near/3 acid) S52 TOPIC: (Salicylic near/3 Acid) S53 TOPIC: (Sch near/3 "39304") S54 TOPIC: (saperconazole or sertaconazole or sinefungin or Sirolimus) S55 TOPIC: (Sodium near/3 Benzoate) S56 TOPIC: (squalestatin or sulconazole or terbinafine or terconazole or thermozymocidin or Thymol or tioconazole or Tolnaftate or Tomatine or Triacetin or trichostatin or Trimetrexate or troclosene) S57 TOPIC: (usnic near/3 acid) S58 TOPIC: (Venturicidins or vibunazole or voriconazole or wortmannin) S59 TOPIC: (antifung* or "anti fung*" or fungastic or fungicidal or Fungizone or Amphocil or Zonal or Diflucan or Triflucan or hexal or Fluco* or Flunazul or Fungata or Lavisa or Loitin or Neofomiral or oxifungol or Solacap or 49858 of Beagyne or "51211" or Sporanox or Orungal) S60 #59 OR #58 OR #57 OR #56 OR #55 OR #54 OR #53 OR #52 OR #51 OR #50 OR #49 OR #48 OR #47 OR #46 OR #45 OR #44 OR #43 OR #42 OR #41 OR #40 OR #39 S61 #60 AND #38 |
| CINAHL (EBSCO) | ICTRP | ClinicalTrials.gov | LILACS |
| S36 S29 AND S35 S35 S30 OR S31 OR S32 OR S33 OR S34 S34 TX (antifung* or "anti fung*" or fungastic or fungicidal or Fungizone or Amphocil or Zonal or Diflucan or Triflucan or hexal or Fluco* or Flunazul or Fungata or Lavisa or Loitin or Neofomiral or oxifungol or Solacap or 49858 of Beagyne or "51211" or Sporanox or Orungal) S33 TX (Candicidin or candidin or captax or caspofungin or Cerulenin or ciclopirox or cilofungin or Clotrimazole or compactin or cordycepin or cryptophycin or Cycloheximide or Cyclosporine or (decanoic N3 acid) or (diallyl N3 trisulfide) or Dichlorophen or diucifon or echinocandin or Echinocandins or Econazole or Ethonium or fenticonazole or ferroin or Filipin or Fluconazole or Flucytosine or glyphosate or Griseofulvin or hamycin or Hexetidine or hydroxyitraconazole or (ICI N3 "195739") or isoconazole or Itraconazole or iturin or jasplakinolide or Ketoconazole or lactoferricin or lapachol or lawsone or leptomycin or Lucensomycin or Mepartricin or methylamphotericin or micafungin or Miconazole or miltefosine or Monensin or monorden or mucidin or muconaldehyde or Mycobacillin or myxothiazol or n‐hexanal or naftifine or Natamycin or Nifuratel or nikkomycin or nitroxoline or Nystatin or oxiconazole or papulacandin or (pelargonic N3 acid) or Pentamidine or polygodial or (polyoxin N3 D) or posaconazole or (potassium N3 iodate) or pradimicin or protegrin‐1 or purothionin or pyochelin or pyrithione or Pyrrolnitrin or rhizoxin or Rutamycin or (salicylhydroxamic N3 acid) or (Salicylic N3 Acid) or saperconazole or (Sch N3 "39304") or sertaconazole or sinefungin or Sirolimus or (Sodium N3 Benzoate) or squalestatin or sulconazole or terbinafine or terconazole or thermozymocidin or Thymol or tioconazole or Tolnaftate or Tomatine or Triacetin or trichostatin or Trimetrexate or troclosene or (usnic N3 acid) or Venturicidins or vibunazole or voriconazole or wortmannin) S32 TX (acivicin or ajoene or amorolfin or Amphotericin or anidulafungin or Antimycin or artemether or aureobasidin or Azaserine or bafilomycin or Benzoates or bifonazole or blasticidin or Brefeldin or butenafine or butoconazole) S31 (MH "Mycoses/DT/TH") S30 (MH “Antifungal Agents+”) or (MH “Amphotericin B+”) or (MH “Antimycin A+”) or (MH “Azaserine+”) or (MH “Benzoates+”) or (MH “Brefeldin A+”) or (MH “Candicidin+”) or (MH “Cerulenin+”) or (MH “Clotrimazole+”) or (MH “Cycloheximide+”) or (MH “Cyclosporine+”) or (MH “Dichlorophen+”) or (MH “Echinocandins+”) or (MH “Econazole+”) or (MH “Filipin+”) or (MH “Fluconazole+”) or (MH “Flucytosine+”) or (MH “Griseofulvin+”) or (MH “Hexetidine+”) or (MH “Itraconazole+”) or (MH “Ketoconazole+”) or (MH “Lucensomycin+”) or (MH “Mepartricin+”) or (MH “Miconazole+”) or (MH “Monensin+”) or (MH “Mycobacillin+”) or (MH “Natamycin+”) or (MH “Nifuratel+”) or (MH “Nystatin+”) or (MH “Pentamidine+”) or (MH “Rutamycin+”) or (MH “Salicylic Acid+”) or (MH “Sirolimus+”) or (MH “Sodium Benzoate+”) or (MH “Thymol+”) or (MH “Tomatine+”) or (MH “Tolnaftate+”) or (MH “Triacetin+”) or (MH “Trimetrexate+”) or (MH “Venturicidins+”) S29 S18 OR S19 OR S20 OR S21 OR S26 OR S27 OR S28 S28 TX (rhinopolyp* or CRSwNP) S27 TX ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) N3 (papilloma* or polyp* or fung*)) S26 S24 AND S25 S25 (MH "Polyps+") S24 S22 OR S23 S23 (MH "Nose Diseases+") S22 (MH "Nose+") S21 (MH "Rhinitis+/MI") OR (MH "Nasal Mucosa+/MI") S20 (MH "Paranasal Sinus Diseases+/MI") OR (MH "Paranasal Sinuses+/MI") S19 (MH "Nasal Polyps+") S18 S15 OR S16 OR S17 S17 TX ((sinusitis or rhinitis) n3 (chronic or persis* or recurrent* or fung*)) S16 TX (CRSsNP or AFS or AFRS) S15 S8 AND S14 S14 S9 OR S10 OR S11 OR S12 OR S13 S13 TX (chronic or persis* or recurrent* or fung* or eosinophil* or mycetoma* or Maduromycos* or Actinomycetoma* or Eumycetoma*) S12 (MH "Mycetoma+") S11 (MH "Fungi+") S10 (MH "Chronic Disease+") S9 (MH "Recurrence+") S8 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 S7 TX ((maxilla* or frontal*) n3 sinus*) S6 TX (inflamm* n3 sinus*) S5 TX kartagener* n3 syndrome* S4 TX rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis S3 (MH "Paranasal Sinus Diseases+") S2 (MH "Paranasal Sinuses+") S1 (MH "Rhinitis+") |
rhinitis AND fungal OR rhinitis AND antifungal OR sinusitis AND fungal OR sinusitis AND antifungal or CRS AND fungal OR CRS AND antifungal OR AFRS AND antifungal OR AFRS AND fungal OR rhinosinusitis AND fungal OR rhinosinusitis AND antifungal |
via Cochrane Register of Studies 1 rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis AND INSEGMENT 2 kartagener* near syndrome* AND INSEGMENT 3 sinus* or rhinitis* or sinonasal AND INSEGMENT 4 (nose or nasal or rhino*) AND (papilloma* or polyp* or fung*) AND INSEGMENT 5 rhinopolyp* or CRSwNP AND INSEGMENT 6 CRSsNP or AFS or AFRS AND INSEGMENT 7 #1 OR #2 OR #3 OR #4 OR #5 AND INSEGMENT 8 antifung* or "anti fung*" or fungastic or fungicidal or Fungizone or Amphocil or Zonal or Diflucan or Triflucan or hexal or Fluco* or Flunazul or Fungata or Lavisa or Loitin or Neofomiral or oxifungol or Solacap or 49858 of Béagyne or 51211 or Sporanox or Orungal AND INSEGMENT 9 acivicin or ajoene or amorolfin or Amphotericin or anidulafungin or Antimycin or artemether or aureobasidin or Azaserine or bafilomycin or Benzoates or bifonazole or blasticidin or Brefeldin or butenafine or butoconazole AND INSEGMENT 10 Candicidin or candidin or captax or caspofungin or Cerulenin or ciclopirox or cilofungin or Clotrimazole or compactin or cordycepin or cryptophycin or Cycloheximide or Cyclosporine or (decanoic near acid) or (diallyl near trisulfide) or Dichlorophen or diucifon or echinocandin or Echinocandins or Econazole or Ethonium AND INSEGMENT 11 fenticonazole or ferroin or Filipin or Flucytosine or glyphosate or Griseofulvin or hamycin or Hexetidine or hydroxyitraconazole or (ICI near "195739") or isoconazole or Itraconazole or iturin or jasplakinolide or Ketoconazole or lactoferricin or lapachol or lawsone or leptomycin or Lucensomycin AND INSEGMENT 12 Mepartricin or methylamphotericin or micafungin or Miconazole or miltefosine or Monensin or monorden or mucidin or muconaldehyde or Mycobacillin or myxothiazol or n‐hexanal or naftifine or Natamycin or Nifuratel or nikkomycin or nitroxoline or Nystatin or oxiconazole or papulacandin or (pelargonic near acid) or Pentamidine or polygodial or (polyoxin near D) or posaconazole or (potassium near iodate) or pradimicin or protegrin‐1 or purothionin or pyochelin or pyrithione or Pyrrolnitrin AND INSEGMENT 13 rhizoxin or Rutamycin or (salicylhydroxamic near acid) or (Salicylic near Acid) or saperconazole or (Sch near "39304") or sertaconazole or sinefungin or Sirolimus or (Sodium near Benzoate) or squalestatin or sulconazole or terbinafine or terconazole or thermozymocidin or Thymol or tioconazole or Tolnaftate or Tomatine or Triacetin or trichostatin or Trimetrexate or troclosene or (usnic near acid) or Venturicidins or vibunazole or voriconazole or wortmannin AND INSEGMENT 14 #8 OR #9 OR #11 OR #10 OR #12 OR #13 AND INSEGMENT 15 #7 AND #14 AND INSEGMENT 16 (NCT*):AU AND INSEGMENT 17 #15 AND #16 AND INSEGMENT via ClinicalTrials.gov ( rhinitis OR sinusitis OR rhinosinusitis OR nasosinusitis OR pansinusitis OR ethmoiditis OR sphenoiditis OR CRSsNP OR AFS OR AFRS OR rhinopolyps OR CRSwNP OR nasal AND polyp OR nose AND polyp OR fungal AND sinus OR fungus AND sinus OR rhino AND polyp ) AND ( Antifungal OR antifungus OR “anti fungal” OR “anti fungus” OR fungastic OR fungicidal OR Fungizone OR Amphocil OR Zonal OR Diflucan OR Triflucan OR hexal OR Fluco OR Flunazul OR Fungata OR Lavisa OR Loitin OR Neofomiral OR oxifungol OR Solacap OR 49858 of Béagyne OR 51211 OR Sporanox OR Orungal OR acivicin OR ajoene OR amorolfin OR Amphotericin OR anidulafungin OR Antimycin OR artemether OR aureobasidin OR Azaserine OR bafilomycin OR Benzoates OR bifonazole OR blasticidin OR Brefeldin OR butenafine OR butoconazole OR Candicidin OR candidin OR captax OR caspofungin OR Cerulenin OR ciclopirox OR cilofungin OR Clotrimazole OR compactin OR cORdycepin OR cryptophycin OR Cycloheximide OR CyclospORine OR decanoic AND acid OR diallyl AND trisulfide OR DichlORophen OR diucifon OR echinocandin OR Echinocandins OR Econazole OR Ethonium OR fenticonazole OR ferroin OR Filipin OR Flucytosine OR glyphosate OR Griseofulvin OR hamycin OR Hexetidine OR hydroxyitraconazole OR ICI AND "195739" OR isoconazole OR Itraconazole OR iturin OR jasplakinolide OR Ketoconazole OR lactoferricin OR lapachol OR lawsone OR leptomycin OR Lucensomycin OR Mepartricin OR methylamphotericin OR micafungin OR Miconazole OR miltefosine OR Monensin OR monORden OR mucidin OR muconaldehyde OR Mycobacillin OR myxothiazol OR n‐hexanal OR naftifine OR Natamycin OR Nifuratel OR nikkomycin OR nitroxoline OR Nystatin OR oxiconazole OR papulacandin OR pelargonic AND acid OR Pentamidine OR polygodial OR polyoxin AND D OR posaconazole OR potassium AND iodate OR pradimicin OR protegrin‐1 OR purothionin OR pyochelin OR pyrithione OR Pyrrolnitri OR rhizoxin OR Rutamycin OR salicylhydroxamic AND acid OR Salicylic AND Acid OR saperconazole OR Sch AND "39304" OR sertaconazole OR sinefungin OR Sirolimus OR Sodium AND Benzoate OR squalestatin OR sulconazole OR terbinafine OR terconazole OR thermozymocidin OR Thymol OR tioconazole OR Tolnaftate OR Tomatine OR Triacetin OR trichostatin OR Trimetrexate OR troclosene OR usnic AND acid OR Venturicidins OR vibunazole OR voriconazole OR wortmannin ) AND EXACT "Interventional" [STUDY‐TYPES] |
TW:rhinit* OR TW:sinusit* OR TW:rhinosinusitis OR TW:rinit* OR (TW:nose AND TW:polyp*) OR (TW:nasal AND TW:polyp*) OR (TW: polipos AND TW:nasa*) OR TW:CRSsNP OR TW:CRSwNP OR TW:CRS OR TW:AFRS |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| General comments/notes (internal for discussion): |
| Flow chart of trial | ||
| Group A (Intervention) | Group B (Comparison) | |
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 |
||
| No. dropped out (no follow‐up data for any outcome available) |
||
| No. excluded from analysis1 (for all outcomes) ‐ Reason 1 ‐ Reason 2 |
||
| 1This should be the people who received the treatment and were therefore not considered 'dropouts' but were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). | ||
| Information to go into 'Characteristics of included studies' table | |
| Methods | X arm, double/single/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster‐RCT, with x duration of treatment and x duration of follow‐up |
| Participants |
Location: country, no of sites etc. Setting of recruitment and treatment: Sample size:
Participant (baseline) characteristics:
Inclusion criteria:[state diagnostic criteria used for CRS, polyps score if available] Exclusion criteria: |
| Interventions |
Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment Comparator group (n = y): Use of additional interventions (common to both treatment arms): |
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
| Funding sources | 'No information provided'/'None declared'/State source of funding |
| Declarations of interest | 'No information provided'/'None declared'/State conflict |
| Notes | |
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Quote: "…" Comment: |
|
| Allocation concealment (selection bias) | Quote: "…" Comment: |
|
| Blinding of participants and personnel (performance bias) | Quote: "…" Comment: |
|
| Blinding of outcome assessment (detection bias) | Quote: "…" Comment: |
|
| Incomplete outcome data (attrition bias) | Quote: "…" Comment: |
|
| Selective reporting (reporting bias) | Quote: "…" Comment: |
|
| Other bias (see section 8.15) Insensitive/non‐validated instrument? |
Quote: "…" Comment: |
|
| Other bias (see section 8.15) | Quote: "…" Comment: |
| Findings of study: continuous outcomes | |||||||
| Results (continuous data table) | |||||||
| Outcome | Group A | Group B | Other summary stats/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
| Disease‐specific HRQL (instrument name/range) Time point: |
|||||||
| Generic HRQL (instrument name/range) Time point: |
|||||||
| Symptom score (overall) (instrument name/range) Time point: |
|||||||
|
Added total ‐ if scores reported separately for each symptom (range) Time point: |
|||||||
| Nasal blockage/obstruction/congestion (instrument name/range) |
|||||||
| Nasal discharge (instrument name/range) |
|||||||
| Facial pain/pressure (instrument name/range) |
|||||||
| Smell (reduction) (instrument name/range) |
|||||||
| Headache (instrument name/range) |
|||||||
| Cough (in children) (instrument name/range) |
|||||||
| Polyp size (instrument name/range) |
|||||||
| CT score (instrument name/range) |
|||||||
| Comments: | |||||||
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/intervention | Group A | Group B | Other summary stats/notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
| Renal/hepatic toxicity | Systemic antifungals | |||||
| Headache | Topical antifungals | |||||
| Gastrointestinal disturbances (diarrhoea, nausea, vomiting, stomach irritation) |
Topical antifungals Systemic antifungals |
|||||
| Epistaxis | Topical antifungals | |||||
| Local discomfort | Topical antifungals | |||||
| Anaphylaxis or other serious allergic reactions | Systemic antifungals | |||||
| Comments: | ||||||
Data and analyses
Comparison 1. Topical antifungal versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Generic HRQL (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Physical component | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐0.80 [‐3.66, 2.06] |
| 1.2 Mental component | 1 | 116 | Mean Difference (IV, Fixed, 95% CI) | ‐2.20 [‐5.46, 1.06] |
| 2 Adverse effects ‐ epistaxis | 4 | 225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.63] |
| 3 Adverse effects ‐ headache | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.60, 2.63] |
| 4 Adverse effects ‐ local irritation | 5 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [0.61, 8.62] |
| 5 CT score | 1 | 48 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.22 [‐0.79, 0.34] |
Comparison 2. Systemic antifungal versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Severe adverse effects ‐ hepatic toxicity | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.35 [0.14, 78.60] |
| 2 Adverse effects ‐ gastrointestinal disturbances | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.04, 3.36] |
| 3 CT score ‐ opacification % change from baseline | 1 | 49 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐19.22, 18.94] |
| 4 CT score ‐ obstruction score % change from baseline | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐4.4 [‐40.12, 31.32] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Corradini 2006.
| Methods | 4‐arm, non‐blinded, parallel‐group RCT, with unclear duration of treatment and 20 months duration of follow‐up | |
| Participants |
Location: Italy, 1 site Setting of recruitment and treatment: university hospital Sample size: 48
Participant (baseline) characteristics:
Inclusion criteria: nasal polyposis with fungal infection. Confirmed via medical history and physical examination, skin prick tests, measurement of specific IgE and nasal lavage. Exclusion criteria: patients with nasal polyps but without evidence of fungal infection |
|
| Interventions |
Intervention (n = 23): amphotericin B (50 mg × 15 mL of 5% glucose solution), inhalation
Comparator group (n = 25): no antifungal treatment Use of additional interventions (common to both treatment arms): Medical polypectomy: 40 mg of triamcinolone retard intramuscularly 3 times every 10 days (total dose 120 mg) Lysine acetylsalicylate (LAS): after a nasal provocation test with LAS patients were treated with LAS inhalation (4 mg/day; 6 times/week) (treatment duration at this dose is assumed to be 19 months) |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: none reported Secondary outcomes: none reported Other outcomes reported by the study: Polyp recurrence at 20 months, sensitisation to allergens |
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Adverse effects were not reported as an outcome but there is one statement reading "LAS and amphotericin B treatment was well tolerated by all patients and no adverse reactions were observed.” This paper presents a 4‐arm study Group A: surgical endoscopic transnasal ethmoidectomy then topical endonasal treatment with LAS – this group is not included as all participants underwent surgery Group B: medical polypectomy with triamcinolone retard IM, then topical endonasal treatment with LAS (included in this review) Group C: surgical endoscopic transnasal ethmoidectomy then topical endonasal treatment with LAS and amphotericin B – this group is not included as all patients underwent surgery Group D: medical polypectomy with triamcinolone retard IM, then topical endonasal treatment with LAS and amphotericin B (included in this review) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "were randomly assigned" Comment: no information about methods used |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: 4 different treatment arms with different treatment regimens. Blinding is not likely to have been completed. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: blinding of outcome assessment was reported but it was assumed that it was not completed as there is no mention of blinding nor placebo control in the paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: it appears that all of the people who were randomised were included in the results. No discussion of withdrawals, which is surprising in a 20‐month study. |
| Selective reporting (reporting bias) | Low risk | Comment: no published protocol on ClinicalTrials.gov or European Trials Register. It appears that all of the outcomes presented in the methods are reported in the results section. |
| Other bias (non‐validated instrument) | Unclear risk | Comment: no outcomes of interest for this review. Standard endoscopy and imaging instruments presumed to have been used, but no further information. |
| Other bias | Low risk | Comment: no other bias identified |
Ebbens 2006.
| Methods | 2‐arm, double‐blind, multi‐centre, parallel‐group RCT, with 13‐week duration of treatment and follow‐up | |
| Participants |
Location: 4 countries (Belgium, the Netherlands, Spain, UK); 6 sites Setting of recruitment and treatment: 6 tertiary care otorhinolaryngology clinics Sample size: 116
Participant (baseline) characteristics:
Inclusion criteria: patients older than 18 years and 1)clinical signs and symptoms related to CRS and/or NP (nasal congestion, nasal discharge, headache and/or facial pain) that are present persistently or recurrently (i.e. intermittent or present > 6 weeks after the last surgical procedure) for a total period of at least 6 months; 2) endoscopic signs of CRS and/or NP; 3) previous history of ESS sinus CT scan score of 5 according to the Lund‐Mackay scoring system performed within a period of 2 months before randomisation Exclusion criteria: patients with allergic fungal sinusitis were not eligible to enrol Other reasons for exclusion were: 1) nasal infections that can be explained by anatomical defects, immunoglobulin deficiency, complement deficiency, cystic fibrosis, Wegener, sarcoidosis, vasculitis or chronic granulomatous disease; 2) AIDS or known to be HIV‐positive; 3) positive culture for Mycobacterium spp; 4) osteoporosis; 5) chronic renal and/or hepatic failure; 6) female patients who are pregnant or lactating; 7) inadequate use of contraceptive precautions; 8) administration of homeopathic preparations to the nose or paranasal sinuses; 9) chronic use of systemic steroids; 10) use of nasal decongestants or local antibiotics; 11) oral antifungal therapy; 12) immunosuppressive therapy; 13) previous randomisation into the study; 14) enrollment in other investigational drug trials; 15) psychiatric, addictive or any other disorder compromising the ability truly to give informed consent; 16) concerns for compliance with the protocol procedures |
|
| Interventions |
Intervention (n = 59): amphotericin B; in sterile water containing 2.5% glucose, resulting in a clear yellow solution. 25 mL solution (100 µg/mL) applied to each nostril twice daily using an Emcur (Rhinicur) nasal douching device. Total daily dose = 10 mg amphotericin. Treatment duration = 13 weeks. Comparator group (n = 57): placebo nasal lavage (dissolving 3.4 mL/L Cernevit in sterile water containing 2.5% glucose), resulting in a clear yellow solution. Cernevit, a multivitamin preparation for use intravenously, was chosen as placebo for its colour and absence of toxic effects on nasal mucosa. Treatment duration = 13 weeks. Use of additional interventions (common to both treatment arms): Intranasal corticosteroids: allowed when used consistently during the whole trial period (group 1: 41 (70%); group 2: 38 (67%)) Antibiotics: were allowed at clinical exacerbation (either amoxicillin/clavulanic acid 500/125 mg 3 times daily or ciprofloxacin 750 mg twice daily combined with clindamycin 600 mg 3 times daily), but only after aerobic and anaerobic cultures were performed by suction and injection in a port‐a‐cul (group 1: 12 (20%); group 2: 10 (18%)) Systemic steroids: were allowed for a maximum period of 14 days when prescribed for a disease other than upper airway pathology (group 1: 1 (2%); group 2: 0 (0%)) (Combined antibiotic and systemic treatment required in group 1: 3 (5%); group 2: 2 (4%)) |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | No information provided | |
| Declarations of interest |
The rest of the authors declared that they have no conflict of interest |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated…using a computer‐generated randomization schedule (block length of 4) provided by the Department of Biostatistics, ... Separate randomization lists were generated for each participating center and given to each pharmacy department. Patient numbers were sequentially assigned in time for each participating center." Comment: well‐described randomisation process |
| Allocation concealment (selection bias) | Low risk | Quote: "Separate randomization lists were generated for each participating center and given to each pharmacy department." "Numbered light‐rejecting bottles containing either amphotericin B or placebo were prepared and dispensed by an independent pharmacist in each participating center to each patient on randomization." Comment: well‐described process for concealing allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "No difference in appearance, taste, or smell between placebo and amphotericin B solutions could be detected." Comment: independent randomisation and allocation. Efforts made to make treatments as similar as possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Randomization codes were revealed to the researchers only when recruitment and data collection were complete." Comment: all outcome assessment was completed blind to the allocation of treatment group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8/59 (13.6%) and 9/57 (15.8%) of participants dropped out in the amphotericin B and placebo groups, respectively. Reasons for dropout were similar between the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol was identified on the US or European Clinical Trials Registry. All outcomes as reported in the methods section are reported (as baseline values and change from baseline) in the results section. |
| Other bias (non‐validated instrument) | Low risk | Comment: authors used RSOM‐31, SF‐36 and visual analogue scales, which are validated instruments |
| Other bias | Low risk | Comment: no additional sources of bias were identified |
Hashemian 2016.
| Methods | 2‐arm, double‐blind, single‐centre, parallel‐group RCT, with 8 weeks duration of treatment and follow‐up | |
| Participants |
Location: Iran, 1 site Setting of recruitment and treatment: secondary care, hospital ENT clinic Sample size: 54
Participant (baseline) characteristics:
Inclusion criteria: adults (age > 18 years) with CRS diagnosed according to the American Academy of Otolaryngology ‐ Head and Neck Surgery (AAO‐HNS) criteria, which had not been responsive to routine medical treatments Exclusion criteria: patients who were pregnant, lactating or suffered from a major illness (such as cardiovascular disease, acute renal or liver disease, cancer or active malignancy). Known sensitivity to fluconazole; immune compromised patients; patients with acute complication of CRS; superimposition of ARS (fever, acute pain, pressure on face); antibiotic use in recent 7 days; systemic antifungal use in recent 7 days and systemic steroid use in recent 30 days |
|
| Interventions |
Intervention (n = 27): fluconazole nasal drops 0.2% (12 drops per day, 2 times a day). Total daily dose = 1.2 mg fluconazole. Treatment duration = 8 weeks. Comparator group (n = 27): placebo nasal drops (12 drops per day, 2 times a day). Treatment duration = 8 weeks. Use of additional interventions (common to both treatment arms): Fluticasone nasal spray 50 µg (2 puffs per day, 2 times a day) |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | "Academic research fund was provided by Hamadan University of Medical Sciences" | |
| Declarations of interest | "The authors declare no conflicts of interest at all." | |
| Notes | Registered in Iranian Registry of Clinical Trials: IRCT138811063186N1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by tossing a coin by an independent third party (ward secretary)." Comment: adequate randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "… the bottles were coded by a third party who wrote down the codes in a table and the third party himself decoded the bottles at the end of the study." Comment: randomisation completed by a 3rd party and clinicians were handed coded bottles |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…drug and placebo were exactly identical in terms of their appearance and could not be identified neither by the clinician nor the patient." Comment: adequate details in paper to demonstrate that sufficient efforts were made to prevent the participants knowing their allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "…drug and placebo were exactly identical in terms of their appearance and could not be identified neither by the clinician nor the patient." Comment: adequate details in paper to demonstrate that sufficient efforts were made to prevent the participants knowing their allocation for the outcome of SNOT‐20. For CT scan and endoscopic score it is assumed that these were completed by blinded clinician. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 6/54 (11%) of randomised participants did not complete the study. There was no difference in the number of people dropping out between the groups. The reasons for dropping out were "exacerbation of disease" (1 person) and voluntary refusal to continue study (5 people) |
| Selective reporting (reporting bias) | Unclear risk | Comment: although the protocol is available (IRCT138811063186N1), endoscopic score is not listed as an outcome. Furthermore, the method for reporting endoscopic score and CT scan score are not reported in the published paper. Standard deviations for the data are not given in the paper. The results for adverse effects are not well described. |
| Other bias (non‐validated instrument) | Unclear risk | Comment: although SNOT‐20 is a validated tool in CRS, it is unclear whether an Iranian version was used. No information on validity of the version was used with regards to translation and cultural adaptation. No details were given regarding the criteria used for endoscopic score and CT scan score and so it is not possible to say whether these were validated instruments. |
| Other bias | Low risk | Comment: no other bias identified |
Kennedy 2005.
| Methods | 2‐arm, double‐blinded, multicentre, parallel‐group RCT, with 6‐week duration of treatment and 9‐week duration of follow‐up | |
| Participants |
Location: United States; unclear number of sites Setting of recruitment and treatment: not reported Sample size: 53
Participant (baseline) characteristics:
Inclusion criteria: all patients were required to have signs and symptoms of CRS for a period of greater than 3 months before screening and to have failed previous medical therapy. Diagnosis of CRS was based on AAO‐HNS definitions. Patients were required to have CT scan evidence of sinusitis (more than 25% opacification/mucoperiosteal thickening in at least 2 of the major paranasal sinuses). Exclusion criteria: sinus surgery within the 3 months before screening |
|
| Interventions |
Intervention (n = 25): terbinafine, tablets, 625 mg/day, 6 weeks Comparator group (n = 28): identical looking placebo tablets, 6 weeks Use of additional interventions (common to both treatment arms): Use of systemic antibiotics, oral and nasal steroids, anti‐leukotriene inhibitors or antihistamines was allowed during the trial, but the regimen was kept consistent from 6 weeks before randomisation through to the end of the study |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | Novartis pharmaceutical corporation | |
| Declarations of interest | No information provided. Authors acknowledge Novartis employee for preparation of the manuscript. Three authors have Novartis as their affiliation. | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using a validated system that automated the random assignment of treatment codes." Comment: automatic randomisation |
| Allocation concealment (selection bias) | Low risk | Comment: as randomisation was automated it is assumed that the allocation to treatment group was adequately concealed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the patient and investigator were blinded to the treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both the patient and investigator were blinded to the treatment assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All randomized patients who took at least one dose of study medication and had at least one post baseline assessment were used in the efficacy analysis (intention to treat [ITT] population)." Comment: although withdrawals from the trial overall were 9/53 (17.0%), of which 4/25 (16%) were from the terbinafine and 5/28 (18%) were from the placebo group, the reasons are provided and are equal between the groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol mentioned within the paper and no protocol found on clinicaltrials.gov Some outcomes mentioned in methods section are just reported as "not statistically different" in the paper but results are not reported |
| Other bias (non‐validated instrument) | Unclear risk | Quote:"CT scans were graded for extent of opacification at baseline and end of week 6 using a modification (total opacification=50) of the Lund‐Mackay scoring system." Comment: unclear whether the modified version of the Lund‐Mackay scoring system had been validated |
| Other bias | Low risk | Comment: no other sources of bias were identified |
Liang 2008.
| Methods | 2‐arm, double‐blinded, single‐centre, parallel‐group RCT, with 4 weeks duration of treatment and follow‐up | |
| Participants |
Location: Taiwan, 1 site Setting of recruitment and treatment: outpatient ENT clinic Sample size: 70
Participant (baseline) characteristics:
Inclusion criteria: people over 12 years old with a diagnosis of CRS based on the definition included in a report published by the Chronic Rhinosinusitis Task Force in 2003. The inclusion criteria were typical nasal symptoms for > 12 weeks, nasal endoscopy that showed mucosal swelling or purulent discharge and positive findings on sinus x‐ray films. Exclusion criteria: nasal polyps, pregnant or immunocompromised, history of sinus surgery, or had taken antibiotics or antifungal agents within 1 week before enrolling in the study |
|
| Interventions |
Intervention (n = 36): amphotericin B, 20 mg of amphotericin B in 500 mL of normal saline, used as a nasal irrigation using a Sanvic SH903 pulsatile irrigator, 250 mL for each nostril, once daily. Total daily dose = 20 mg amphotericin B. Treatment duration = 4 weeks. Comparator group (n = 34): placebo (with a yellowish dye), 4 mL of placebo solution in 500 mL of normal saline, used as a nasal irrigation using a Sanvic SH903 pulsatile irrigator, 250 mL for each nostril, once daily. Treatment duration = 4 weeks. Use of additional interventions (common to both treatment arms): Patients were NOT allowed to use oral antibiotics, oral antifungals, oral steroids or oral antihistamines. Participants were also told not to use nasal sprays. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | Non‐parametric tests were used for quality of life score and endoscopic scores | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly allocated" Comment: not enough information to determine whether this was a low risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Comment: not enough information to determine |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" Comment: although there is a lack of information the paper does explain how the placebo solution was made to look like the amphotericin solution (addition of dye) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" Comment: not enough information to determine whether the outcome measure of nasal endoscopy was completed by someone who had knowledge of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the dropout rate was low at 6/70 (8.6%). There was no difference in the dropout rate or reasons for dropout between the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol could be found on clinicaltrials.gov or the Chinese clinical trial registry. Results for all outcomes as presented in the methods sections are presented in the results as median values with ranges. |
| Other bias (non‐validated instrument) | Low risk | Comment: the study used the RSOM‐31 instrument for health‐related quality of life and the paper did provide the reference to the validation paper relating to the validation of the Chinese version. It is not clear what the scoring system used was. The Lund‐Mackay endonasal scoring system is a validated, widely used scale. References are given for the validation papers. |
| Other bias | Low risk | Comment: no additional sources of bias were identified |
Ponikau 2005.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with 6 months duration of treatment and follow‐up | |
| Participants |
Location: USA, 1 site Setting of recruitment and treatment: Otorhinolaryngology Department, Mayo Sample size: 30
Participant (baseline) characteristics:
Inclusion criteria: adults > 18 years meeting the American Academy of Otorhinolaryngology diagnosis of CRS. CRS symptoms for > 3 months. Demonstrated mucosal thickening on coronal CT scans > 5 mm in 2 or more sinuses and on nasal endoscopy (DAS). Exclusion criteria: acute bacterial exacerbation of CRS, acute complication of CRS, antibiotic therapy or systemic antifungal use in last 7 days, systemic steroid use in the last 3 months Known hypersensitivity to amphotericin B, female patients who are pregnant or lactating, immunocompromised patients (HIV, post transplant, diabetes), acute respiratory illnesses (within the last 7 days), acute complication of CRS (i.e. abscess), acute bacterial exacerbation of CRS (acute pain, acute pressure, fever, pus on discharge), orbital or central nervous system complications of CRS |
|
| Interventions |
Intervention (n = 15): 20 mL amphotericin B solution (250 µg/mL) to each nostril twice a day by using a bulb syringe, for 6 months. Total daily dose = 20 mg amphotericin B. Comparator group (n = 15): 20 mL sterile water placebo solution (identical in appearance to the intervention arm) to each nostril twice a day using a bulb syringe, for 6 months Use of additional interventions (common to both treatment arms): Both groups continued with their current treatment regimen but were instructed to record any change. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | "Supported by grants from the National Institutes of Health, R01 AI49235, and by the Mayo Foundation for Education and Research.” | |
| Declarations of interest | "The Mayo Foundation for Education and Research owns US Patent 6,555,566 (Methods and materials for treating and preventing inflammation of mucosal tissue). Dr Ponikau is listed as an inventor. A license agreement has been signed with Accentia Pharmaceutical, Inc. No other relevant conflicts exist.” | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"The Division of Biostatistics, Mayo Clinic Rochester (Minn), generated the randomization schedule by using a block randomization scheme (block size of 4). Investigators were unaware of the block size." Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: "Investigators were unaware of the block size. The pharmacist produced numbered bottles with each patient’s study number, containing either amphotericin B or placebo, according to the randomization schedule." Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote:"No difference in the appearance, taste, or smell could be detected [between the intervention and placebo solutions]." Comment: adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: [For primary outcome]"The reproducibility of this method was independently confirmed by 3 blinded investigators" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 6/30 (20%) patients did not complete the study. The reasons are provided but 5 were from the intervention group and 1 from the placebo group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was identified on clinicaltrials.gov. As well as presenting the raw results the paper presents "percentage improved", which was not stated in the methods section. No mention of how adverse effects were measured in the methods section. |
| Other bias (non‐validated instrument) | Low risk | Comment: study used a validated tool (SNOT‐20) for the primary outcome |
| Other bias | Unclear risk | Comment: as a single‐centre trial, there is a possibility of selection bias and a lack of generalisability. There were also imbalances in age and duration of CRS between the 2 groups, but the statistical significance of these was not reported. |
Shin 2004.
| Methods | 3‐arm, non‐blinded, parallel‐group trial (unclear randomisation), with 4 weeks duration of treatment and follow‐up | |
| Participants |
Location: South Korea, single site Setting of recruitment and treatment: Department of Otolaryngology Sample size: 41
Participant (baseline) characteristics:
Inclusion criteria: diagnosis of CRS was based on the 1996 Task Force on Rhinosinusitis criteria. CT scan of the paranasal sinuses and endoscopy was used to confirm the presence of nasal polyps. All of the participants had a negative skin prick test and a negative multiple allergosorbent test chemiluminescent assay. Exclusion criteria: patients who had received systemic or topical steroids or antibiotics or who had a history of allergy, asthma or other systemic diseases |
|
| Interventions |
High‐dose antifungal group 1 (n = 16): amphotericin B dissolved in sterile water at a concentration of 100 mg/L. Intranasal administration of 10 mL of the solution into each nostril twice daily with a syringe. Total daily dose = 4 mg amphotericin B. Treatment duration = 4 weeks. Low‐dose antifungal group 2 (n = 14): amphotericin B dissolved in sterile water at a concentration of 50 mg/L. Intranasal administration of 10 mL of the solution into each nostril twice daily with a syringe. Total daily dose = 2 mg amphotericin B. Treatment duration = 4 weeks. Comparator group (n = 11): normal saline, 10 mL of the solution was administered into each nostril twice daily. Treatment duration = 4 weeks. Use of additional interventions (common to both treatment arms): none listed |
|
| Outcomes |
Outcomes of interest in the review: No primary outcomes reported No secondary outcomes reported Other outcomes reported by the study: Cytokine protein contents of nasal polyps (IL‐5, IL‐8, INF‐y, RANTES) |
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomly selected based on their willingness to participate” Comment: it is unclear if this 'randomisation' was to the study (i.e. not an RCT) or to the treatment group. No randomisation methods are given. Due to a lack of information about baseline characteristics, selection bias is possible |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment. Lack of information about baseline characteristics. Participant selection is possible. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: the study does not mention that it was blinded. There was a control group but the control treatment (intranasal saline) is likely to look different to the intervention groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: the study does not mention if the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: outcome data were available for all participants who completed. However, the paper does not provide information about the number of people who were potentially eligible for the trial, or who started and did not finish. |
| Selective reporting (reporting bias) | Low risk | Comment: no protocol for the trial was available on clinicaltrials.gov or the WHO clinical trials registry All of the outcomes that were reported in the methods are presented in the results section |
| Other bias (non‐validated instrument) | Unclear risk | Comment: no outcomes of interest were reported |
| Other bias | Low risk | Comment: no other sources of bias were identified |
Weschta 2004.
| Methods | 2‐arm, double‐blind, single‐centre, parallel‐group RCT, with 8 weeks duration of treatment and follow‐up | |
| Participants |
Location: Germany, 1 site Setting of recruitment and treatment: Department of Otorhinolaryngology and Head and Neck Surgery Sample size: 78
Participant (baseline) characteristics:
Inclusion criteria: 1)age > 18 years, 2) recent CT scan of paranasal sinuses, 3) symptom score > 14 (max 30), 4) endoscopy score > 2 (max 6), 5) CT score > 19 (max 40) Exclusion criteria: 1) current participation in other clinical study, 2) pregnancy or breast‐feeding, 3) mental impairment or severe illnesses, 4) hypersensitivity to study medication, 5) history of immotile cilia syndrome or cystic fibrosis, 6) urgent need for or recent paranasal surgery, 7) recent start on specific antiallergic immunotherapy, corticosteroid therapy, antihistamines, acetylsalicylic acid desensitisation, 8) discontinuous study medication intake, 9) antimycotic or immunosuppressive therapy, 9) clinical suspicion of AFRS |
|
| Interventions |
Intervention (n = 39): amphotericin B (3 mg/mL), nasal spray, 2 puffs per nostril (200 µL per nostril), 4 times daily. Total daily dose = 4.8 mg amphotericin. Treatment duration = 8 weeks. Comparator group (n = 39): control nasal spray: saline solution containing tartrazine, chinin sulfate, 1‐(4‐sulfo‐1‐phenylazo)‐2‐naphthol‐6‐sulfo acid, choline in 5% glucose solution, 2 puffs per nostril, 4 times daily. Treatment duration = 8 weeks. Use of additional interventions (common to both treatment arms): Patients were allowed to continue with medication as before providing the dose was stable. Topical or systemic corticosteroids were used by 61% in the intervention and 50% in the control group. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Funding sources | No information provided | |
| Declarations of interest | No information provided | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated to the 2 treatment arms by the Department of Biometry and Medical Documentation, University of Ulm." Comment: no further information provided about method of randomisation |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of methods used to conceal allocation of patients. It does mention that healthcare professionals were kept blind to the treatment allocation until the end of the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Active drug and control sprays were manufactured by the pharmacy of the University Hospital of Ulm. They were indistinguishable in color, taste, smell, and nasal sensations during application." "To assure blinding of investigators, the mild irritant chinin sulfate was added to the control spray. Neither patients nor investigators were aware of the kind of treatment during the entire study period." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: although this is not discussed in detail, the flow chart on page 1124 clearly shows that "unblinding" occurred after the data analysis was completed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 15/39 (38%) participants dropped out from the intervention arm; 7/39 (18%) dropped out of the control arm. Reasons for the dropouts were provided; most in the intervention group were due to intolerance of the study medication |
| Selective reporting (reporting bias) | Unclear risk | Comment: the protocol for the study could not be identified through clinicaltrials.gov or the European trials registry. All of the outcomes as reported in the methods section were reported in the results section although for some only vague figures are given. For example, for endoscopic score the paper states "The median endoscopy scores were almost identical in the AMB and control groups (4 vs 4) and did not change remarkably after treatment." A big difference in adverse effects between the groups is reported but details of the events and the number of patients is not provided. |
| Other bias (non‐validated instrument) | Unclear risk | Comment: for disease‐specific quality of life the study modified an existing questionnaire developed for patients with allergy ‐ the mini Rhinoconjunctivitis Quality of Life Questionnaire (mRQLQ) "rhinosinusitis quality of life score (RQL)". However, the paper does not provide any link to any validation of the modified instrument, and no publications on the validation of the RQL were found by the review authors. The remaining instruments used were well‐accepted, validated instruments (Lund Mackay, VAS used for symptoms). |
| Other bias | Unclear risk | Comment: baseline characteristics were balanced with the exception of gender. The procedure for additional recruitment of patients to compensate for dropouts was not reported. |
AFRS: allergic fungal rhinosinusitis ALT: alanine aminotransferase AMB: amphotericin B ARS: acute rhinosinusitis AST: aspartate aminotransferase CT: computerised tomography CRS: chronic rhinosinusitis ENT: ear, nose and throat ESS: endoscopic sinus surgery F: female GGT: gamma‐glutamyl transpeptidase IM: intramuscular LAS: lysine acetylsalicylate M: male NP: nasal polyps RANTES: regulated on activation, normal T cell expressed and secreted RCT: randomised controlled trial RSOM‐31: Rhinosinusitis Outcome Measure‐31 SD: standard deviation SNOT‐20: Sino‐Nasal Outcome Test‐20 VAS: visual analogue scale WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chan 2008 | STUDY DESIGN: Case series |
| Gerlinger 2009 | POPULATION: Post‐surgical population ‐ all participants underwent surgery at the start of the trial |
| Gupta 2007 | POPULATION: Post‐surgical population ‐ all participants had surgery at the start of the trial prior to randomisation |
| Hashemi 2014 | STUDY DESIGN: Case series |
| Helbling 2006 | STUDY DESIGN: Case series |
| Hofman 2004 | STUDY DESIGN: Case series |
| IRCT138706101138N1 | POPULATION: Post‐surgical population ‐ all patients underwent surgery at the start of the trial |
| Jiang 2015 | POPULATION: Post‐surgical population ‐ all patients underwent surgery 1 month prior to randomisation (6‐week limit) |
| Joshi 2007 | STUDY DESIGN: Case series |
| Khalil 2011 | POPULATION: Post‐surgical population ‐ all patients underwent surgery at the start of the trial |
| Lopatin 2007 | POPULATION: Post‐surgical population ‐ all patients underwent surgery at the start of the trial |
| NCT02285283 | POPULATION: Post‐surgical population ‐ all patients will undergo surgery. Clinical trial protocol ‐ no information regarding whether this trial has completed. |
| Nikakhlagh 2015 | POPULATION: Post‐surgical population ‐ all participants underwent surgery before the start of the trial (within 6 weeks) |
| Panda 2012 | POPULATION: Post‐surgical population ‐ all patients underwent surgery at the start of the trial |
| Patro 2015 | COMPARISON: All participants in the control group underwent surgery immediately |
| Ravikumar 2011 | POPULATION: Post‐surgical population ‐ all participants underwent surgery as part of the trial. |
| Ricchetti 2002 | STUDY DESIGN: Non‐randomised trial |
| Ricchetti 2002b | STUDY DESIGN: Case series |
| Rojita 2017 | POPULATION: Post‐surgical population ‐ all patients underwent surgery at the start of the trial |
| Somu 2015 | POPULATION: Post‐surgical population ‐ all patients underwent surgery during the trial |
| Thamboo 2011 | STUDY DESIGN: Randomised by side of nose INTERVENTION: Honey (with antimicrobial and antifungal properties) |
| Verma 2016 | POPULATION: Control group underwent immediate surgery. No pre‐operative comparisons were made. |
| Zhang 2012 | POPULATION: Post‐surgical population ‐ all patients underwent surgery during the trial |
Characteristics of studies awaiting assessment [ordered by study ID]
Deka 2007.
| Methods | Prospective randomised controlled trial |
| Participants | 88 patients with allergic fungal sinusitis |
| Interventions | Group 1: amphotericin B nasal lavage and corticosteroid nasal spray; Group 2: corticosteroid nasal spray alone |
| Outcomes | Improvement of nasal symptoms, nasal endoscopy score |
| Notes | Abstract only. Contacted authors for more information but no response was received. Abstract published in 2007; it is unlikely that this study will be published in full. |
Frigas 2007.
| Methods | Prospective, double‐blind, placebo‐controlled trial |
| Participants | 8 patients with chronic rhinosinusitis and mild asthma |
| Interventions | Group 1: 200 mg of itraconazole, twice daily for 4 weeks; Group 2: placebo tablets, twice daily for 4 weeks |
| Outcomes | Chronic rhinosinusitis symptoms, sinus CT scan |
| Notes | Abstract only. Contacted authors for more information but no response was received. Abstract published in 2007; it is unlikely that this study will be published in full. |
Lopatin 2004.
| Methods | Unclear |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Abstract only; it is unlikely that this study will be published in full. Unable to obtain the full abstract ‐ title of paper only. |
Stergiou 2007.
| Methods | Prospective randomised controlled trial |
| Participants | Chronic sinusitis |
| Interventions | Group 1: amphotericin B suspension; Group 2: placebo solution Treatment duration = 4 months |
| Outcomes | Chronic rhinosinusitis symptoms |
| Notes | Registered protocol and abstract of trial protocol only. Trial protocol was last updated in 2007 and no results are provided. Unclear if patients all underwent surgery at the start of the trial. |
CT: computerised tomography
Contributions of authors
Karen Head wrote the review text with the help of the other authors.
Lee Yee Chong, Claire Hopkins and Carl Philpott reviewed and edited the review text.
Lee Yee Chong and Karen Head completed initial screening of abstracts, Lee Yee Chong, Karen Head and Steve Sharp completed screening of the updated search.
Karen Head, Lee Yee Chong, Claire Hopkins and Carl Philpott reviewed the full‐text papers for inclusion.
Karen Head and Steve Sharp completed the data extraction.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Infrastructure funding for Cochrane ENT
Declarations of interest
Karen Head: none known.
Steve Sharp: none known.
Lee Yee Chong: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus, and am a trustee of the patient charity Fifth Sense.
New
References
References to studies included in this review
Corradini 2006 {published data only}
- Corradini C, Ninno M, Buonomo A, Nucera E, Paludetti G, Alonzi C, et al. Amphotericin B and lysine acetylsalicylate in the combined treatment of nasal polyposis associated with mycotic infection. Journal of Investigational Allergology & Clinical Immunology 2006;16(3):188‐93. [CENTRAL: CN‐00570817; CRS: 1400041; EMBASE: 2006292109; PUBMED: 16784013] [PubMed] [Google Scholar]
Ebbens 2006 {published data only}
- Ebbens FA, Bachert C, Mullol J, Hellings P, Lund V, Fokkens W. Amphotericin B nasal lavages equally as effective as placebo. Clinical Otolaryngology and Allied Sciences 2006;31(2):169. [CENTRAL: CN‐00596982; CRS: 1421655] [Google Scholar]
- Ebbens FA, Georgalas C, Luiten S, Drunen CM, Badia L, Scadding GK, et al. The effect of topical amphotericin B on inflammatory markers in patients with chronic rhinosinusitis: a multicenter randomized controlled study. Laryngoscope 2009;119(2):401‐8. [CENTRAL: CN‐00681619; CRS: 1486764; PUBMED: 19160404] [DOI] [PubMed] [Google Scholar]
- Ebbens FA, Scadding GK, Badia L, Hellings PW, Jorissen M, Mullol J, et al. Amphotericin B nasal lavages: not a solution for patients with chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2006;118(5):1149‐56. [CENTRAL: CN‐00573476; CRS: 1402692; EMBASE: 2006535603; PUBMED: 17088142] [DOI] [PubMed] [Google Scholar]
Hashemian 2016 {published and unpublished data}
- Hashemian F, Hashemian F, Molaali N, Rouini M, Roohi E, Torabian S. Clinical effects of topical antifungal therapy in chronic rhinosinusitis: a randomized, double‐blind, placebo‐controlled trial of intranasal fluconazole. EXCLI Journal. Germany: Leibniz Research Centre for Working Environment and Human Factors (Ardeystr.67, Dortmund D‐44139, Germany. E‐mail: excli@ifado.de), 2016; Vol. 15:95‐102. [CENTRAL: CN‐01138520; CRS: 1869028; EMBASE: 20160151286; PUBMED: 27065776] [DOI] [PMC free article] [PubMed]
- IRCT138811063186N1. Evaluation of the therapeutic effect of fluconazol drop on chronic rhinosinusitis [Evaluation of the therapeutic effect of fluconazol drop on chronic rhinosinusitis in patients referred to otorhinolarnyngology clinic in Hamadan]. http://www.irct.ir/searchresult.php?id=3186&number=1 (first received 2 August 2011). [CENTRAL: CN‐01039355; CRS: 1769954]
Kennedy 2005 {published data only}
- Kennedy DW, Kuhn FA, Hamilos DL, Zinreich SJ, Butler D, Warsi G, et al. Treatment of chronic rhinosinusitis with high‐dose oral terbinafine: a double blind, placebo‐controlled study. Laryngoscope 2005;115(10):1793‐9. [CENTRAL: CN‐00531103; CRS: 1372731; EMBASE: 2005469506; PUBMED: 16222197] [DOI] [PubMed] [Google Scholar]
Liang 2008 {published data only}
- Liang KL, Su MC, Shiao JY, Tseng HC, Hsin CH, Lin JF, et al. Amphotericin B irrigation for the treatment of chronic rhinosinusitis without nasal polyps: a randomized, placebo‐controlled, double‐blind study. American Journal of Rhinology 2008;22(1):52‐8. [CENTRAL: CN‐00630195; CRS: 1447377; EMBASE: 20083148186; PUBMED: 18284860] [DOI] [PubMed] [Google Scholar]
Ponikau 2005 {published data only}
- Ponikau JU, Sherris DA, Weaver A, Kita H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: a randomized, placebo‐controlled, double‐blind pilot trial. Journal of Allergy and Clinical Immunology 2005; Vol. 115, issue 1:125‐31. [CENTRAL: CN‐00502168; CRS: 1346380; EMBASE: 2005024192; PUBMED: 15637558] [DOI] [PubMed]
- Sherris DA, Ponikau JU, Weaver A, Frigas E, Kita H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: a prospective, randomized, placebo‐controlled trial. Journal of Allergy and Clinical Immunology 2004;113(2 Suppl):2629. [CENTRAL: CN‐00597119; CRS: 1421766] [DOI] [PubMed] [Google Scholar]
Shin 2004 {published data only}
- Shin SH, Ye MK. Effects of topical amphotericin B on expression of cytokines in nasal polyps. Acta Oto‐Laryngologica 2004;124(10):1174‐7. [CENTRAL: CN‐00505471; CRS: 1349677; PUBMED: 15768813] [DOI] [PubMed] [Google Scholar]
Weschta 2004 {published data only}
- Riechelmann H. Fungus and nasal polyps. 3rd International Consensus Conference on Nasal Polyposis; 2004 Apr 23‐25; Brussels, Belgium. 2004. [CENTRAL: CN‐00519649; CRS: 1362346]
- Weschta M, Formanek M, Herbert R. Topical antimycotic treatment after nasal polyps. 106th Annual Meeting of the American Academy of Otolaryngology ‐ Head and Neck Surgery Foundation (AAO‐HNS). 2002; Vol. 127:P85.
- Weschta M, Rimek D, Formanek M, Podbielski A, Riechelmann H. Effect of nasal antifungal therapy on nasal cell activation markers in chronic rhinosinusitis. Archives of Otolaryngology‐‐Head & Neck Surgery 2006;132(7):743‐7. [CENTRAL: CN‐00566587; CRS: 1395911; PUBMED: 16847182] [DOI] [PubMed] [Google Scholar]
- Weschta M, Rimek D, Formanek M, Polzehl D, Podbielski A, Riechelmann H. Topical antifungal treatment of chronic rhinosinusitis with nasal polyps: a randomized, double‐blind clinical trial. Journal of Allergy and Clinical Immunology 2004;113(6):1122‐8. [CENTRAL: CN‐00467988; CRS: 1317522; PUBMED: 15208594] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chan 2008 {published data only}
- Chan KO, Genoway KA, Javer AR. Effectiveness of itraconazole in the management of refractory allergic fungal rhinosinusitis. Journal of Otolaryngology ‐ Head & Neck Surgery 2008;37(6):870‐4. [CRS: 3474502] [PubMed] [Google Scholar]
Gerlinger 2009 {published data only}
- Gerlinger I, Fittler A, Fonai F, Patzko A, Mayer A, Botz L. Postoperative application of amphotericin B nasal spray in chronic rhinosinusitis with nasal polyposis, with a review of the antifungal therapy. European Archives of Oto‐rhino‐laryngology 2009;266(6):847‐55. [CENTRAL: CN‐00671532; CRS: 1478049; EMBASE: 20093152029; PUBMED: 18953552] [DOI] [PubMed] [Google Scholar]
- Gerlinger I, Fittler A, Mayer A, Patzko A, Fonay F, Pytel J, et al. Postoperative application of amphotericin B nasal spray in chronic rhinosinusitis with nasal polyposis. Can recidive polyposis be prevented? [Amphotericin b‐tartalmu orrspray posztoperativ alkalmazasa orrpolyposissal jaro kronikus rhinosinusitis eseteiben. Megelozheto‐e a recidiva?]. Orvosi Hetilap 2008;149(37):1737‐46. [CENTRAL: CN‐00913521; CRS: 1682078; EMBASE: 2008472251; PUBMED: 18805757] [DOI] [PubMed] [Google Scholar]
Gupta 2007 {published data only}
- Gupta RP, Bahadur S, Thakar A, Handa K, Sarkaar C. Management protocols of allergic fungal sinusitis. Indian Journal of Otolaryngology and Head and Neck Surgery 2007;59(1):35‐40. [CENTRAL: CN‐00690768; CRS: 1495357; EMBASE: 20083312958] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashemi 2014 {published data only}
- Hashemi M, Fereidani A, Berjis N, Okhovat SA, Eshaghain A. Effectiveness of itraconazole on clinical symptoms and radiologic findings in patients with recurrent chronic rhinosinusitis and nasal polyposis. Advanced Biomedical Research 2014;3:162. [CRS: 7062504; PUBMED: 25221765] [DOI] [PMC free article] [PubMed] [Google Scholar]
Helbling 2006 {published data only}
- Helbling A, Baumann A, Hanni C, Caversaccio M. Amphotericin B nasal spray has no effect on nasal polyps. Journal of Laryngology and Otology 2006;120(12):1023‐5. [CENTRAL: CN‐00597184; CRS: 1421814; PUBMED: 16965642] [DOI] [PubMed] [Google Scholar]
Hofman 2004 {published data only}
- Hofman T, Skrobisz W, Hofman A, Hofman M. The estimation of efficacy of fluconazole treatment in patients with chronic sinusitis. Mikologia Lekarska 2004;11(4):297‐301. [CRS: 3474505] [Google Scholar]
IRCT138706101138N1 {published data only}
- IRCT138706101138N1. Effects of intranasal amphotericin‐B irrigation on recurrence of massive nasal polyposis [Effects of intranasal amphotericin‐B irrigation on recurrence of massive nasal polyposis after endoscopic sinus surgery and comparison with nasal corticosteroid sprays]. http://www.irct.ir/searchresult.php?id=1138&number=1 (first received 30 September 2010). [CENTRAL: CN‐01039346; CRS: 1769945]
Jiang 2015 {published data only}
- Jiang R‐S, Twu C‐W, Liang K‐L. Effect of 200 mug/ml amphotericin b nasal irrigation on postfunctional endoscopic sinus surgery care. Otolaryngology ‐ Head and Neck Surgery 2017;157(1 Suppl 1):P159. [Google Scholar]
- Jiang RS, Hsu SH, Liang KL. Amphotericin B nasal irrigation as an adjuvant therapy after functional endoscopic sinus surgery. American Journal of Rhinology & Allergy 2015;29(6):435‐40. [CENTRAL: CN‐01134399; CRS: 1864908; EMBASE: 20160037074; PUBMED: 26637583] [DOI] [PubMed] [Google Scholar]
Joshi 2007 {published data only}
- Joshi RR, Bhandary S, Khanal B, Singh RK. Fungal maxillary sinusitis: a prospective study in a tertiary care hospital of eastern Nepal. Kathmandu University Medical Journal (KUMJ) 2007;5(2):195‐8. [PubMed] [Google Scholar]
Khalil 2011 {published data only}
- Khalil Y, Tharwat A, Abdou AG, Essa E, Elsawy AH, Elnakib O, et al. The role of antifungal therapy in the prevention of recurrent allergic fungal rhinosinusitis after functional endoscopic sinus surgery: a randomized, controlled study. Ear, Nose, & Throat Journal 2011;90(8):E1‐7. [CENTRAL: CN‐00804489; CRS: 1600866; EMBASE: 2011499609; PUBMED: 21853425] [DOI] [PubMed] [Google Scholar]
Lopatin 2007 {published data only}
- Lopatin AS, Akulich II, Kochetkov PA. Long‐term systemic antifungal therapy for nasal polyposis. European Archives of Oto‐rhino‐laryngology 2007;264(Suppl 1):S282. [CENTRAL: CN‐00596972; CRS: 1421646] [Google Scholar]
NCT02285283 {published data only}
- NCT02285283. RCT of itraconazole for fungal sensitive chronic rhinosinusitis with nasal polyps [Randomized double‐blinded controlled trial of oral antifungal for the treatment of fungal sensitive chronic rhinosinusitis with nasal polyps]. clinicaltrials.gov/show/NCT02285283 (first received 6 November 2014). [CENTRAL: CN‐01039767; CRS: 1770363]
Nikakhlagh 2015 {published data only (unpublished sought but not used)}
- IRCT201204049375N1. The effect of itraconazole in the treatment of allergic fungal sinusitis [A placebo‐controlled double‐blind randomized trial of itraconazole for allergic fungal sinusitis ‐ allergic fungal sinusitis (AFS)]. http://www.irct.ir/searchresult.php?id=9375&number=1 (first received 9 April 2013). [CENTRAL: CN‐01012798; CRS: 1743415]
- Nikakhlagh S, Khodadadi A, Kanani M, Karampour LS, Saki N. The effect of the oral itraconazole on the management of allergic fungal sinusitis. Biomedical and Pharmacology Journal 2015;8:85‐9. [CENTRAL: CN‐01138882; CRS: 1869390; EMBASE: 20160128607] [Google Scholar]
Panda 2012 {published data only}
- Panda NK, Francis AA, Chakrabarti A, Virk RS. Oral itraconazole as an adjunct to steroids in AFRS. Otolaryngology ‐ Head and Neck Surgery 2012;147(2 Suppl):P114. [CENTRAL: CN‐01415741; CRS: 7062506] [Google Scholar]
Patro 2015 {published data only}
- Patro SK, Verma RK, Panda NK, Chakrabarti A, Singh P. Efficacy of preoperative itraconazole in allergic fungal rhinosinusitis. American Journal of Rhinology & Allergy 2015;29(4):299‐304. [CENTRAL: CN‐01259222; CRS: 5167418; PUBMED: 26163250] [DOI] [PubMed] [Google Scholar]
Ravikumar 2011 {published data only}
- Ravikumar A, Kumar P. Role of itraconazole in sinonasal polyposis. Otolaryngology ‐ Head and Neck Surgery 2011;145:127‐8. [Google Scholar]
Ricchetti 2002 {published data only}
- Ricchetti A, Landis BN, Giger R, Aparicio H, Lacroix J‐S. Comparison of the effect of intra‐nasal lavages versus spray with amphotericin B on nasal polyposis. 19th Congress of the European Rhinologic Society; 2002 Jun 16‐19; Ulm, Germany. 2002:152. [CENTRAL: CN‐00404508; CRS: 1265415]
- Ricchetti A, Landis BN, Giger R, Zheng C, Lacroix JS. Effect of local antifungal treatment on nasal polyposis. Otorhinolaryngologia Nova 2002;12:48‐51. [CENTRAL: CN‐00452864; CRS: 1305264] [Google Scholar]
Ricchetti 2002b {published data only}
- Ricchetti A, Landis BN, Maffioli A, Giger R, Zeng C, Lacroix JS. Effect of anti‐fungal nasal lavage with amphotericin B on nasal polyposis. Journal of Laryngology and Otology 2002;116(4):261‐3. [CRS: 7062668; PUBMED: 11945184] [DOI] [PubMed] [Google Scholar]
Rojita 2017 {published data only}
- Rojita M, Samal S, Pradhan P, Venkatachalam VP. Comparison of steroid and itraconazole for prevention of recurrence in allergic fungal rhinosinusitis: a randomized controlled trial. Journal of Clinical and Diagnostic Research 2017;11(4):MC01‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Somu 2015 {published data only}
- Somu L, Saravanam PK, Ravikumar. Role of itraconazole in sino nasal polyposis. Journal of Evolution of Medical and Dental Sciences 2015;4(70):12112‐9. [CRS: 7062671] [Google Scholar]
Thamboo 2011 {published data only}
- Thamboo A, Thamboo A, Philpott C, Javer A, Clark A. Single‐blind study of manuka honey in allergic fungal rhinosinusitis. Journal d'Oto‐rhino‐laryngologie et de Chirurgie Cervico‐faciale 2011;40(3):238‐43. [CENTRAL: CN‐00795839; CRS: 1592410; PUBMED: 21518647] [PubMed] [Google Scholar]
Verma 2016 {published data only}
- Verma RK, Patro SK, Francis AA, Panda NK, Chakrabarti A, Singh P. Role of preoperative versus postoperative itraconazole in allergic fungal rhinosinusitis. Medical Mycology 2016;6:614‐23. [DOI] [PubMed] [Google Scholar]
Zhang 2012 {published data only}
- Zhang Z, Zhang J, Luo W, Jiang H. Surgery added with fluconazole in treatment of fungal rhinosinusitis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke za Zhi [Journal of Clinical Otorhinolaryngology, Head, and Neck Surgery] 2012;26(15):692‐3, 696. [CENTRAL: CN‐00850362; CRS: 1626206; PUBMED: 23167181] [PubMed] [Google Scholar]
References to studies awaiting assessment
Deka 2007 {published data only (unpublished sought but not used)}
- Deka RC, Chokkalingam V, Vishnoi RK, Kumar R. Topical amphotericin B and steroid in AFS. Otolaryngology ‐ Head and Neck Surgery 2007;137(2 Suppl 1):40. [CENTRAL: CN‐00614784; CRS: 1435672] [Google Scholar]
Frigas 2007 {published data only (unpublished sought but not used)}
- Frigas E. A pilot, prospective, double blind, placebo‐controlled treatment trial with itraconazole orally in patients with chronic rhinosinusitis and asthma. Journal of Allergy and Clinical Immunology 2007;119(1):560. [CENTRAL: CN‐00725113; CRS: 1526958] [Google Scholar]
Lopatin 2004 {published data only}
- Lopatin AS, Akulich II, Kochetkov PA. Systemic antifungals in the treatment of nasal polyposis: do they work?. 3rd International Consensus Conference on Nasal Polyposis; 2004 Apr 23‐25; Brussels, Belgium. 2004. [CENTRAL: CN‐00519616; CRS: 1362322]
Stergiou 2007 {published data only}
- NCT00425620. Amphotericin B suspension in refractory chronic sinusitis [A prospective, randomized, double‐blind, placebo‐controlled, multicenter, parallel‐group study of intranasal amphotericin B suspension in patients with refractory, postsurgical chronic sinusitis (CS)]. http://clinicaltrials.gov/ct2/show/nct00425620 (first received 23 January 2007). [CENTRAL: CN‐00861830; CRS: 1636508]
- Stergiou A, Casciano R, Katz L, Etschmaier M, Sherris D, Tichenor W, et al. Intranasal amphotericin B in patients with refractory, postsurgical chronic sinusitis: a phase III pivotal clinical trial design. XXVI Congress of the European Academy of Allergology and Clinical Immunology (EAACI); 2007 Jun 9‐13; Goteburg, Sweden. 2007:Abstract No. 45. [CENTRAL: CN‐00597001; CRS: 1421672]
Additional references
Balk 2012
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical Assessment of Within‐arm Correlation Imputation in Trials of Continuous Outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
Bent 1994
- Bent JP 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngology ‐ Head and Neck Surgery 1994;111(5):580‐5. [DOI] [PubMed] [Google Scholar]
BNF 2018
- Joint Formulary Committee. British National Formulary (online). London: BMJ Group and Pharmaceutical Press (http://www.medicinescomplete.com) (accessed 23 May 2018).
Braun 2003
- Braun H, Buzina W, Freudenschuss K, Beham A, Stammberger H. Eosinophilic fungal rhinosinusitis: a common disorder in Europe?. Laryngoscope 2003;113(2):264‐9. [DOI] [PubMed] [Google Scholar]
Chakrabarti 2009
- Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Buzina W, Kita H, et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope 2009;119(9):1809‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2003
- Chen Y, Dales R, Lin M. The epidemiology of chronic rhinosinusitis in Canadians. Laryngoscope 2003;113(7):1199‐205. [DOI] [PubMed] [Google Scholar]
Cho 2012
- Cho SH, Hong SJ, Han B, Lee SH, Suh L, Norton J, et al. Age‐related differences in the pathogenesis of chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2012;129(3):858‐60.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016a
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011993.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016b
- Chong LY, Head K, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011996.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016c
- Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011995.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
CHROME 2017
- Hopkins C, Hettige R, Soni‐Jaiswal A, Lakhani R, Carrie S, Cervin A, et al. CHronic Rhinosinusitis Outcome MEasures (CHROME) – developing a core outcome set for trials of interventions in chronic rhinosinusitis. Rhinology 2018;56(1):22‐32. [DOI] [PubMed] [Google Scholar]
DeMarcantonio 2011
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. [DOI] [PubMed] [Google Scholar]
Ebbens 2007
- Ebbens FA, Georgalas C, Rinia AB, Drunen CM, Lund VJ, Fokkens WJ. The fungal debate: where do we stand today?. Rhinology 2007;45(3):178‐89. [PubMed] [Google Scholar]
Ebbens 2010
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. [DOI] [PubMed] [Google Scholar]
Ebbens 2011
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOS 2012
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. [PubMed] [Google Scholar]
Erskine 2015
- Erskine SE, Verkerk MM, Notley C, Williamson IG, Philpott, CM. Chronic rhinosinusitis: patient experiences of primary and secondary care – a qualitative study. Clinical Otolaryngology 2015;41:8‐14. [DOI] [PubMed] [Google Scholar]
Fokkens 2007
- Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. [PubMed] [Google Scholar]
Gliklich 1995
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hastan 2011
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. [DOI] [PubMed] [Google Scholar]
Head 2016a
- Head K, Chong LY, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011992.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2016b
- Head K, Chong LY, Piromchai P, Hopkins C, Philpott C, Schilder AGM, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011994.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2016c
- Head K, Chong LY, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Short‐course oral steroids alone for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011991.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Juniper 1991
- Juniper EF, Guyatt GH. Development and testing of a new measure of health status for clinical trials in rhinoconjunctivitis. Clinical and Experimental Allergy 1991;21:77‐83. [DOI] [PubMed] [Google Scholar]
Kern 2008
- Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi‐Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. American Journal of Rhinology 2008;22(6):549‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keswani 2012
- Keswani A, Chustz RT, Suh L, Carter R, Peters AT, Tan BK, et al. Differential expression of interleukin‐32 in chronic rhinosinusitis with and without nasal polyps. Allergy 2012;67(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lackner 2005
- Lackner A, Stammberger H, Buzina W, Freudenschuss K, Panzitt T, Schosteritsch S, et al. Fungi: a normal content of human nasal mucus. American Journal of Rhinology 2005;19(2):125‐9. [PubMed] [Google Scholar]
Larsen 2004
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. [DOI] [PubMed] [Google Scholar]
Mistry 2014
- Mistry SG, Kumar BN. The value of antifungal therapy in allergic fungal rhinosinusitis. Rhinology 2014;52(1):9‐18. [DOI] [PubMed] [Google Scholar]
Philpott 2011
- Philpott CM, Clark A, Javer AR. Allergic fungal rhinosinusitis ‐ a new staging system. Rhinology 2011;49:318‐23. [DOI] [PubMed] [Google Scholar]
Ponikau 1999
- Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, Gaffey TA, et al. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clinic Proceedings 1999;74(9):877‐84. [DOI] [PubMed] [Google Scholar]
Ponikau 2007
- Ponikau JU, Sherris DA, Kita H. The role of ubiquitous airborne fungi in chronic rhinosinusitis. Clinical Allergy and Immunology 2007;20:177‐84. [PubMed] [Google Scholar]
Ragab 2004
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. [DOI] [PubMed] [Google Scholar]
Ragab 2010
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sok 2006
- Sok JC, Ferguson BJ. Differential diagnosis of eosinophilic chronic rhinosinusitis. Current Allergy and Asthma Reports 2006;6:203‐14. [DOI] [PubMed] [Google Scholar]
Tan 2011
- Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 2011;128(6):1198‐206.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tomassen 2011
- Tomassen P, Zele T, Zhang N, Perez‐ Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. [DOI] [PubMed] [Google Scholar]
van Drunen 2009
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. [DOI] [PubMed] [Google Scholar]
Wild 2005
- Wild D, Grove A, Martin M, Eremenco S, McElroy S, Verjee‐Lorenz A, et al. Principles of good practice for the translation and cultural adaptation process for patient‐reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value in Health 2005;8(2):94‐104. [DOI] [PubMed] [Google Scholar]
Yousefi 2017
- Yousefi J, Akhavan A, Hoseini‐Motlagh R, Banaei‐Boroujeni S, Panahi Y, Hossein Khosravi M. Effect of amphotericin B on treatment of chronic rhinosinusitis: a double‐blind randomized clinical trial. Razavi International Journal of Medicine 2017;5(4):e64550. [Google Scholar]
Zhang 2008
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Sacks 2011
- Sacks PL, Harvey RJ, Rimmer J, Gallagher RM, Sacks R. Topical and systemic antifungal therapy for the symptomatic treatment of chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD008263.pub2] [DOI] [PubMed] [Google Scholar]


