Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a condition associated with high morbidity, mortality and cost to the community. Patients often report symptomatic improvement with short‐acting beta‐2 agonists (SABA) and anticholinergic bronchodilator medications, and both are recommended in COPD guidelines. These medications have different mechanisms of action and therefore could have an additive effect when combined.
Objectives
To compare the relative efficacy and safety of regular long term use (at least four weeks) of ipratropium bromide and short‐ acting beta‐2 agonist therapy in patients with stable COPD.
Search methods
The Cochrane Airways Group Specialised Register of Trials was searched. Bibliographies were checked to identify relevant cross‐references. Drug companies were contacted for relevant trial data. The searches are current to July 2008.
Selection criteria
All randomised controlled trials comparing at least 4 weeks of treatment with an anticholinergic agent (ipratropium bromide) alone or in combination with a beta‐2 agonist (short acting) versus the beta‐2 agonist alone, delivered via metered dose inhaler or nebuliser, in non‐asthmatic adult subjects with stable COPD.
Data collection and analysis
Data extraction and study quality assessment was performed independently by three reviewers. Authors of studies and relevant manufacturers were contacted if data were missing.
Main results
Eleven studies (3912 participants) met the inclusion criteria of the review. Small benefits of ipratropium over a short‐acting beta‐2 agonist were demonstrated on lung function outcomes. There were small benefits in favour of ipratropium on quality of life (HRQL), as well as a reduction in the requirement for oral steroids. Combination therapy with ipratropium plus a short‐acting beta‐2 agonist conferred benefits over a short‐acting beta‐2 agonist alone in terms of post‐bronchodilator lung function. There was no significant benefit of combination therapy in subjective improvements in HRQL, but again there was a reduction in the requirement for oral steroids.
Authors' conclusions
The available data from the trials included in this review suggest that the advantage of regular long term use of ipratropium alone or in combination with a short‐acting beta‐2 agonist or over a beta‐2 agonist alone are small, if the aim is to improve lung function, symptoms and exercise tolerance. Until further data are available, the strategy of providing a short‐acting beta‐2 agonist on a PRN basis, and then either continuing with the short‐acting beta‐2 agonist regularly or conducting an "n of 1" trial of regular beta‐2 agonist or regular anticholinergic to determine the treatment that gives the best relief of symptoms (and continuing with it), would seem cost effective. This strategy does need formal evaluation. Patient preference is also important, as is the relative importance of avoiding the use of systemic corticosteroids.
Plain language summary
Ipratropium bromide versus short acting beta‐2 agonists for stable chronic obstructive pulmonary disease
This review looks at studies that compare the regular use for at least four weeks of different types of inhaled short‐acting bronchodilator medication in people with chronic obstructive pulmonary disease (COPD, or emphysema/chronic bronchitis). There were eleven trials included. There were no major differences seen between the responses to ipratropium and salbutamol, or the combination. Where there were benefits, they were small and would not support a general recommendation for the use of ipratropium bromide or a combination with beta‐2 agonist over a beta‐2 agonist alone in COPD. People with COPD could use the short‐acting bronchodilator that gives them the most improvement in their symptoms.
Background
Chronic Obstructive Pulmonary Disease (COPD) is a chronic, slowly progressive condition characterised by airflow limitation that is at best only partially reversible. It is a condition associated with high mortality, morbidity and cost to the community. COPD encompasses two distinct processes of chronic bronchitis and emphysema, however in both, airflow is limited by structural changes. Emphysema is a destructive process of the alveolar structures and chronic bronchitis affects both large and small airways and is characterised by chronic excess mucus secretion which is accompanied by a chronic cough. It is generally recognised that the single most important cause of COPD is cigarette smoking and that smoking cessation is the only intervention that reduces the accelerated rate of decline in lung function observed in COPD.
Despite the lack of major reversibility of airways obstruction, patients often report symptomatic improvement with short‐acting beta‐2 agonist and anticholinergic bronchodilator medications. These agents are widely used and are recommended in management guidelines for symptomatic COPD, even though they do not slow the decline in lung function (ATS/ERS 2004; NICE/BTS 2004).
Anticholinergic medicines (such as ipratropium bromide) act on muscarinic receptors, whereas beta‐2 agonists (such as salbutamol) act via the adrenergic system to cause bronchodilation. These bronchodilators can be delivered in several ways, e.g. by metered dose inhaler, dry powder device or by nebulisation. Some studies demonstrate that ipratropium bromide is at least as effective as short acting beta‐2 agonists (Chapman 1991; Matera 1995; Nisar 1992). Ipratropium bromide may be more efficacious than beta‐2 agonists in the predominantly elderly COPD patient population, as there is possibly a decline in response to beta‐2 agonists with increasing age (van Schayck 1991), possibly due to reduced receptor numbers (Ullah 1981). In addition, beta‐2 agonists potentially have more side‐effects such as tachycardia and tremor than anticholinergic medicines.
The aim of this review was to compare the relative efficacy and safety of regular long term use of shorter‐acting anti‐cholinergic medications alone or in combination with a short acting beta‐2 agonist (SABA) compared with SABA alone, for patients with stable COPD. The relative effects of ipratropium and long‐acting beta‐agonists are considered in a separate review (Appleton 2006).
Objectives
To compare the relative efficacy and safety of regular long term use (at least four weeks) of ipratropium bromide and short‐acting beta‐2 agonist therapy in patients with stable COPD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCT). Included studies had to have data on at least one of the outcome variables.
Types of participants
Non‐asthmatic adult subjects with stable COPD as defined by the British Thoracic Society (BTS 1997). These guidelines specify COPD as a tobacco smoking related, chronic, slowly progressive disorder characterised by airways obstruction ( FEV1 <80% predicted and FEV1/FVC ratio <70%) which does not change markedly over several months and where the impairment is largely fixed but is partially reversible by bronchodilator or other therapy.
'Stable' was defined as no recent infections, exacerbations, hospitalisation in the past month.
Studies which included subjects with severe, concurrent other diseases, including cardiac, liver and renal disease were excluded.
Types of interventions
This review, therefore, is limited to studies considering ipratropium bromide (IpB), used regularly for at least four weeks in a stated dose, delivered via metered dose inhaler (MDI) or nebuliser, in an outpatient setting, in a randomised comparison with a short‐acting beta‐2 agonist (SABA). Studies were included if they compared:
1) Ipratropium bromide versus SABA 2) Ipratropium bromide + SABA versus SABA alone
The review protocol specified anticholinergic bronchodilators, and the study duration criteria had originally stipulated eight weeks, but this was revised down to four weeks minimum duration as there were significant large multi‐centre RCTs of four and six weeks duration identified which the authors believed should not be omitted from this review. This review was intended to examine all anti‐cholinergic agents (including ipratropium bromide, oxitropium bromide, tiotropium bromide or atropine methonitrate) versus beta‐2 agonists. However, owing to the expanding literature concerning tiotropium it was felt that the scope of the review would become too extensive to be useful in helping to guide clinical practice. The efficacy of tiotropium bromide in comparison with long‐acting beta‐2 agonists to is examined in a separate Cochrane review (Barr 2005).
Types of outcome measures
1) Lung function ‐ including FEV1, FVC, PEF 2) Health status [health related quality of life scores (HRQL)] 3) Dyspnoea scores. These were measured directly, at rest or during exercise, or indirectly by self‐report in symptom diaries. 4) Exercise capacity ‐ six minute walk distance (6MWD), shuttle walk test 5) Adverse and haemodynamic effects ‐ blood pressure and pulse rate effects from the medication 6) Use of other medication such as rescue bronchodilators, corticosteroids or theophylline 7) Acute exacerbations
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and hand searching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'COPD' were searched using the following terms:
(ipratropium or oxitropium or atropine or atrovent or oxivent or respontin ) AND (((beta* AND agonist*) AND short*) OR ((beta* AND adrenergic*) AND short*) OR (bronchodilator* AND short*) or fenoterol or metaproterenol or salbutamol or albuterol)
Searches are current to July 2008.
Searching other resources
Handsearches of abstracts from meetings of the American and British Thoracic Societies, and the European Respiratory Society were conducted. Bibliographies were checked to identify relevant cross‐references. Authors and drug companies were contacted for relevant trial data.
Online databases of unpublished trial summaries were searched (http://ctr.gsk.co.uk; http://www.clinicalstudyresults.org).
The only anticholinergic agent studied in trials that met inclusion criteria was ipratropium bromide.
Data collection and analysis
Selection of studies
All identified citations were reviewed to identify potentially relevant studies. Full text versions of these potential studies were assessed by three reviewers to determine if they met the inclusion criteria. Differences were resolved by discussion. Those that met inclusion criteria were assessed for study quality.
Data extraction and management
Data for trials was extracted independently by three reviewers and entered into the Cochrane Collaboration software program (Review Manager). Standard errors (when available) were converted to standard deviations.
Assessment of risk of bias in included studies
We judged the risk of bias for each study (high, low or unclear) based on the process of allocation (generation of allocation schedule and its concealment to investigators/participants).
1) Trial quality was assessed using the following: (a) Cochrane approach to concealment of allocation. I. Grade A: adequate ii. Grade B: unclear iii. Grade C: clearly inadequate
(b) Additional assessment was performed using the Jadad five point scale (Jadad 1996) I. The study was described as randomised (yes:1, no;0) ii. Method of randomisation was described and was appropriate (yes:1, no:‐1) iii. The study was described as double blind (yes:1, no:0) iv. The method of blinding was described and was appropriate (yes:1, no:‐1) v. The was a description of withdrawals and drop outs (yes:1, no:0)
Dealing with missing data
Authors and drug companies were contacted in an attempt to obtain missing and raw data. The data able to be retrieved is entered in MetaView. In some cases there is no measure of spread of data, although means are known. These trials are listed in MetaView as having SD as zero, and in this case these studies are not included in the meta‐analysis.
Assessment of heterogeneity
We assessed the degree of statistical variation over what would be expected with the play of chance with the I square measurement. We undertook secondary analysis where this exceeded 20%.
Data synthesis
Results of the analyses for continuous outcomes are expressed as a mean difference (MD) together with 95% confidence interval (CI) or a standardised mean difference (SMD) SMDs for outcomes where there was variation in the method of reporting of those outcomes. For dichotomous outcomes, odds ratio (OR) are used.
Sensitivity analysis
Sensitivity analysis using risk of bias as a categorising variable was planned. If the heterogeneity was not explained in terms of study quality the following subgroup analyses were to be conducted:
I) Type of different short‐acting beta‐2 adrenoceptor agonists. ii) Delivery system (e.g. metered dose inhaler versus nebuliser)
Results
Description of studies
Results of the search
Thirty studies reported in 35 references were identified and selected for possible inclusion in the review.
Eleven studies recruiting 3912 participants were included in the review. For full details of the included studies, see Characteristics of included studies.
Included studies
Ipratropium bromide versus short acting beta‐2 agonist (SABA)
We identified eight studies published in 1986‐1998, which compared the effects of ipratropium bromide (IpB) versus SABA. These included data available from three combination studies in which the IpB/beta‐2 agonist combination was compared with the combination components (Combivent 1994; Combivent 1997; Gross 1998).
Type of SABA
The beta‐2 agonist in two studies was metaproterenol (Friedman 1996; Tashkin 1986), and in another, (Hodzhev 1999) the beta‐2 agonist was fenoterol. The remaining five studies (Combivent 1994; Combivent 1997; Colice 1996; Rennard 1996; Gross 1998) compared salbutamol (albuterol) with IpB.
Type of drug delivery
IpB and the SABA were delivered as solutions by a nebuliser in four studies (Combivent 1997; Colice 1996; Friedman 1996; Gross 1998), and by MDI in four studies (Tashkin 1986; Rennard 1996; Combivent 1994; Hodzhev 1999).
Duration of these studies
The duration of these studies was 30 days (Hodzhev 1999), 42 days (parallel phase, following crossover phase of study, Gross 1998), 85 days (Combivent 1994; Combivent 1997; Colice 1996; Friedman 1996) and 90 days (Tashkin 1986; Rennard 1996).
Drug dose
The dose of IpB administered was 0.5 mg in all nebulised solution studies, as listed above and 21 mcg and 42 mcg in the two MDI studies (Combivent 1994 and Rennard 1996 respectively).
The dose of beta‐2 agonist was as follows, 2.5 mg (Colice 1996; Gross 1998), 3 mg (Combivent 1997; Rennard 1996:649A), 15 mg salbutamol solutions (Rennard 1996:593A), and 15 mg metaproterenol solution (Friedman 1996). In the MDI studies the dose of salbutamol was 240 mcg (Rennard 1996) and metaproterenol‐ 1500 mcg (Tashkin 1986).
The sample sizes in the eight studies ranged from N of 44 (Hodzhev 1999) to 863 (Gross 1998). While 3146 people participated in these studies, data was contributed for meta‐analysis by a maximum of 1572 participants (IpB = 795, beta‐2 agonist = 777) owing to incomplete reporting of data (and 3 studies that included combination arms as mentioned above). The mean age of study participants was 64 years with a mean baseline FEV1 of 1.00 litres. The mean gender distribution across these studies was 65% male:35% female. The range of baseline FEV1 reported in four of eight studies was 0.18‐3.3 litres. No data was given on smoking status of subjects other than having a smoking history of at least 10 pack years, with the exception of Tashkin 1986 who reported that participants smoked between 1.5 and 2.5 packs daily
Ipratropium bromide plus short‐acting beta‐2 agonist versus short‐acting beta‐2 agonist
We identified seven studies published between 1994 and 1999 which compared the effects of a combination of IpB and beta‐2 agonist versus beta‐2 agonist alone.
Type of SABA
The beta‐2 agonist used in these studies was salbutamol (Combivent 1994; Combivent 1997; Campbell 1999; Gross 1998; Levin 1996; Rennard 1996) and metaproterenol (Tashkin 1996). Three of these were three arm studies (Combivent 1994; Combivent 1997; Gross 1998), where the combination was compared with each component.
Type of drug delivery
Drugs were delivered by MDI's in three studies (Combivent 1994; Campbell 1999; Rennard 1996) and in the remaining four studies, the IpB and beta‐2 agonists were delivered as solutions by a nebuliser (Combivent 1997; Gross 1998; Levin 1996; Tashkin 1996).
Drug dose
The dose of IpB administered was 0.5 mg in all nebulised solution studies (Levin 1996; Combivent 1997; Gross 1998; Tashkin 1996) and 42 mcg, 21 mcg and 18 mcg in the two MDI studies (Rennard 1996; Combivent 1994; Campbell 1999 respectively).
The dose of beta‐2 agonist was as follows, 2.5 mg (Gross 1998; Levin 1996), 3 mg salbutamol solution (Combivent 1997), and 15 mg metaproterenol solution (Tashkin 1996). The doses of salbutamol aerosol delivered by MDI were 240 mcg, 100 and 90 mcg (Rennard 1996; Combivent 1994; Campbell 1999 respectively).
Duration of studies
The duration of these studies was 29 days (Campbell 1999) 42 days (parallel phase, following crossover phase of study, Gross 1998), and 85 days (4 studies) and 90 days (Rennard 1996).
The sample sizes in the seven studies ranged from an N of 195 (Levin 1996) to 863 (Gross 1998). A total of 3189 people participated in these studies, however, data was contributed for analysis by a maximum of 1858 participants (combination N = 942, beta‐2 agonist N = 916). Participants had a mean age of 65 years and mean baseline FEV1 of 1.00 litres (6 studies), and percent predicted FEV1=37%, (5 studies). The mean proportions of males and females were 65% and 35% respectively. The range of baseline FEV1 reported in four of six studies was 0.18‐3.3 litres. No data was given on current smoking status of subjects other than having a smoking history of at least 10 pack years.
The degree of FEV1 reversibility (after inhalation of a short acting beta‐2 agonist) was not measured in any study, with the exception of Tashkin 1986 where the demonstration of a significant bronchospastic component (increase of at least 15% of baseline FEV1) in the absence of a history of asthma or current allergic disease (as determined by IgE and levels of blood eosinophils) was an inclusion criteria. Three studies (Combivent 1994; Combivent 1997; Friedman 1996) included subjects whose baseline FEV1 exceeded inclusion criteria of FEV1 <65% of predicted, by up to 11.5 %.
Excluded studies
Nineteen studies failed to meet the inclusion criteria for the following reasons: comparison with placebo and not beta‐2 agonist (2), review articles (2), studies were of too short a duration (10), not RCTs (4), study participants had asthma only (1).
Risk of bias in included studies
We present an assessment of the risk of bias for included studies in Figure 1. This gives an overview of our judgements of the degree to which the studies are at risk of bias.
1.
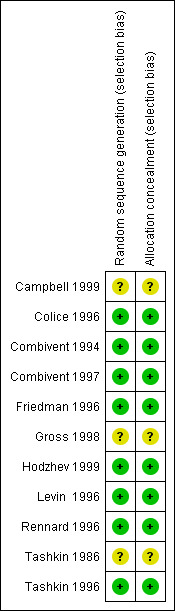
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Eight of the eleven studies were rated A according to the Cochrane approach to concealment of allocation after correspondence with Boehringer Ingelheim and one author of a study (Hodzhev 1999). This study, although small and unblinded designed primarily to determine benefits in individual patients, the investigators were initially blinded to the randomisation/ allocation of participants. Two studies were rated B (Gross 1998; Campbell 1999; Tashkin 1986) as none of these reported randomisation procedures in sufficient detail to determine whether a satisfactory attempt to control selection bias had been made, and this information was not able to be obtained from the authors.
After correspondence with the authors or Boehringer Ingelheim, in terms of Jadad quality assessment scores, four studies scored 5 (Combivent 1994; Combivent 1997; Rennard 1996; Tashkin 1996); three studies scored 4 because these did not describe withdrawals and dropouts adequately (Colice 1996; Friedman 1996; Levin 1996) and Hodzhev 1999 scored 3 because this study was unblinded. Because information was unable to be obtained from the authors, two studies scored 4 as the randomisation method was unclear (Gross 1998; Tashkin 1986). Campbell 1999 scored 2 (randomisation and blinding method unclear, withdrawals and drop outs not adequately described).
Effects of interventions
Boehringer Ingelheim provided missing data for all ipratropium (alone or combination) versus short acting beta‐2 agonist studies. We could not obtain data for the studies by Gross 1998 and Campbell 1999.
In most studies, lung function was the major outcome and was measured in terms of FEV1 and FVC. For each of these lung function parameters, there was a measure of mean pre‐dose FEV1 and FVC, mean peak change in FEV1, FVC from the test day baseline and mean area under the FEV1 and FVC curves (AUC) above test day baseline FEV1 and FVC respectively.
Generally, statistical heterogeneity was not an issue in this review (I2 value was negligible or low) with the exception of outcome 8 (Number of subjects experiencing medication‐related adverse events) in the comparison of Ipratropium bromide versus short‐acting beta‐2 agonist.
Ipratropium bromide versus short‐acting beta‐2 agonist [or SABA, including salbutamol (albuterol), metaproterenol, fenoterol, delivered via MDI or nebuliser]
I) Lung Function
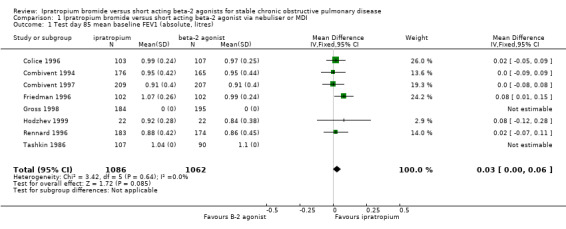
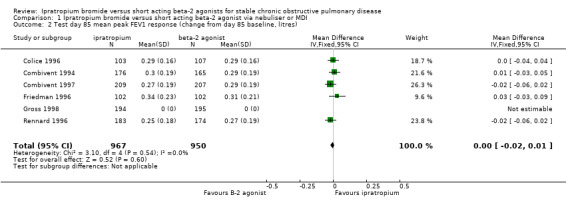
Mean test day baseline FEV1 Six studies reported the effects of treatment with IpB or SABA (5 studies of 3 months duration, 1 study of one month duration). There was a small difference (of borderline statistical significance) between treatments in the baseline/pre‐bronchodilator FEV1 measured at the end of the study (MD: 0.03 litres, 95% CI: 0.00, 0.06). After 90 days of therapy Tashkin 1986 reported no significant difference between treatments. Mean peak change from test day baseline FEV1 Five studies reported the effects of 3 months treatment with IpB or SABA. There was no significant difference between treatments in the mean peak change in FEV1 (MD: 0 litres, 95% CI: ‐0.02, 0.01).
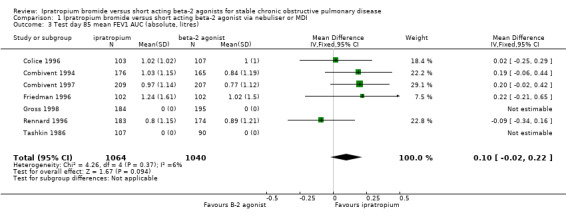
Mean Area under the FEV1 curve (FEV1 AUC) above test day baseline FEV1 Five studies reported the effects of 3 months treatment with IpB or SABA. There was no significant difference between treatments in the mean area under the FEV1 curve above test day baseline (MD: 0.10 litres, 95% CI: ‐0.02, 0.22). Tashkin 1986 reported a significant difference favouring ipratropium.
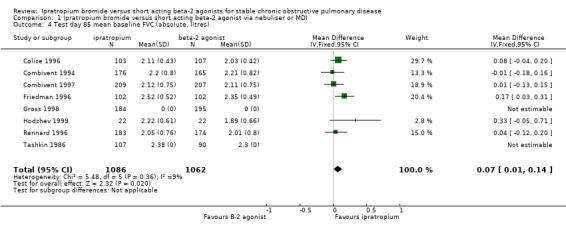
Mean test day baseline FVC Six studies reported the effects of treatment with IpB or SABA (5 studies of 3 months duration, 1 study of one month duration). IpB treatment was associated with a small but significant improvement in the baseline FVC measured at the end of the study compared with SABA. (MD: 0.07 litres, 95% CI: 0.01, 0.14). After 90 days of therapy Tashkin 1986 reported no significant difference between treatments.
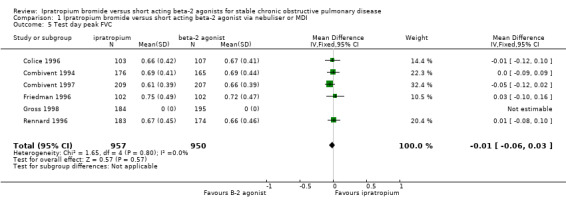
Mean test day peak change in FVC Five studies reported the effects of 3 months treatment with IpB or SABA. There was no significant difference between treatments in the mean peak change in FVC (MD: ‐0.01 litres, 95% CI: ‐0.06, 0.03).
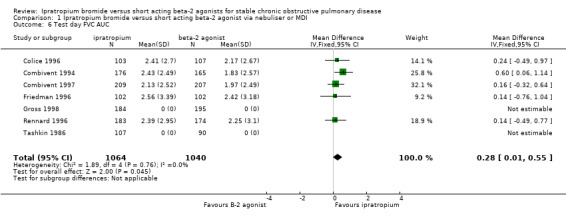
Mean area under the FVC curve (FVC AUC) above test day baseline FVC Five studies reported the effects of 3 months treatment with IpB or SABA. The difference between IpB and SABA significantly favoured ipratropium (MD: 0.28 litres, 95% CI: 0.01, 0.55). Tashkin 1986 reported a significant difference favouring ipratropium. Peak Expiratory Flow (PEF) Unpublished PEF data has been sought from Boehringer Ingelheim. However, no statistically or clinically significant changes were demonstrated in morning peak flows (four studies) and evening peak flows (two studies) between treatment groups after 85 days of treatment. Evening PEF readings were reported (Friedman 1996) to be significantly higher in the IpB group (286 ml), than in the metaproterenol group (270 ml) on day 85 (data extracted from graph).
ii) HRQL measured using the Chronic Respiratory Disease Questionnaire (CRQ)
Clinically meaningful improvements (0.5 unit change or greater) after 85 days of treatment compared with day 1 were reported in the mean CRQ Dyspnoea and Fatigue domains within the IpB group in two studies (Colice 1996; Friedman 1996) and in the Emotion domain in one study (Colice 1996). However, Colice 1996 also demonstrated a clinically meaningful improvement in the mean CRQ Dyspnoea domain in the salbutamol group after 85 days of treatment compared with day 1.
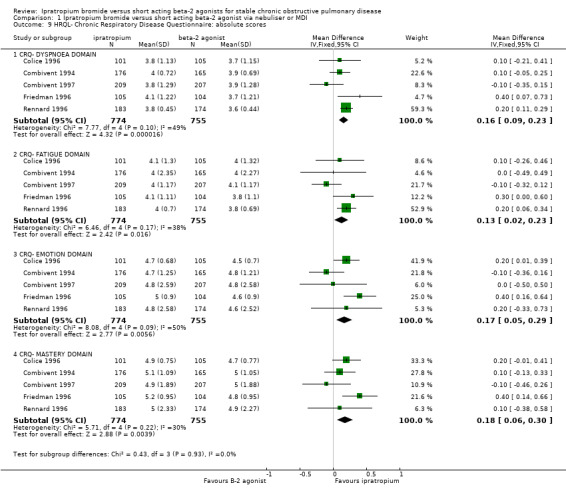
Meta‐analysis of "between treatment group" differences showed small, statistically significant differences in CRQ domain scores between treatments. These favoured IpB treatment and were found in all four domains of the CRQ: Dyspnoea: MD: 0.16 units (95% CI: 0.09, 0.23); Fatigue: MD: 0.13 units (95% CI: 0.02, 0.23); Emotion: MD: 0.17 units (95% CI: 0.04, 0.29); Mastery: MD: 0.18 units (95% CI: 0.06, 0.30).
iii) Dyspnoea scores
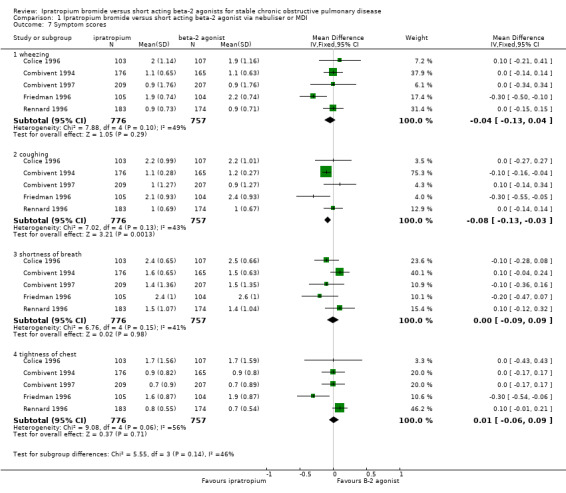
Five studies reported the effects of 3 months treatment with IpB or SABA on self reported symptoms scores. Symptoms scores did not change significantly over time within any treatment group (Friedman 1996; Combivent 1994; Combivent 1997) and meta‐analysis showed no statistically significant differences between treatments for: Wheezing: MD: ‐0.04 (95% CI: ‐0.13, 0.04); Shortness of Breath: MD: 0.00 (95% CI: ‐0.09, 0.09); Tightness of Chest: MD: 0.01 (95% CI: ‐0.06, 0.09). SABA treatment however, was associated with a small reduction in the scores for Coughing: MD: ‐0.08 (95% CI: ‐0.13, ‐0.03).
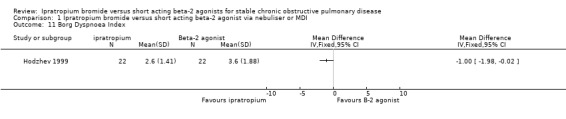
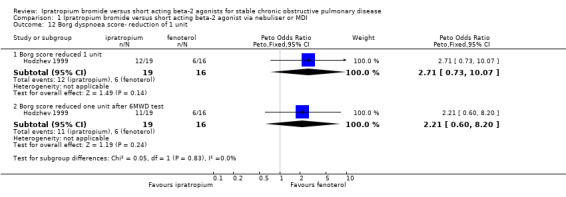
Borg Dyspnoea Scores were reported by one small unblinded study (Hodzhev 1999). For the IpB treated group, at the end of 30 days treatment, there were significant changes from baseline in Borg scores before and after the six minute walk test. This was not observed for the fenoterol group in the proportions of people reporting less dyspnoea (reduction in score of 1 unit) between treatments, before or after the six minute walk test.
iv) Exercise Capacity
One study (Hodzhev 1999) reported data on the effect of IpB versus fenoterol after 30 days of treatment in terms of the six minute walk test distance. This small unblinded study found that ipratropium treatment was associated with a significant increase from baseline in the distance walked in the six minute walk test. Another study (Gross 1998) from which data was not obtainable, reported no significant difference in the distance walked in a six minute walk test between the IpB and salbutamol treated groups during the parallel phase (day 43‐84) of the trial.
v) Number of subjects experiencing medication‐related adverse events
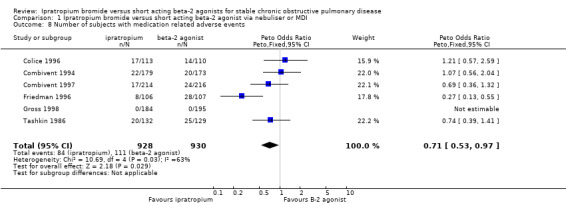
Pooled analysis of five studies indicated that regardless of delivery method (MDI, nebulised), fewer subjects receiving IpB experienced medication‐related adverse events compared with subjects receiving SABA, either salbutamol or metaproterenol (Peto OR = 0.71, 95% CI:0.53, 0.97). Significant statistical heterogeneity was demonstrated in this analysis (I2 =63% with either fixed or random effects models) which appeared to be driven by the study comparing nebulised IpB with metaproterenol (Friedman 1996). Removal of Friedman 1996 resolved the heterogeneity but there was no longer a significant difference between treatments for this outcome. Subgroup analysis based on type of beta‐2 agonist showed that there was no benefit of IpB treatment, when compared with salbutamol, delivered via MDI or nebuliser (Peto OR: 0.94, 95% CI:0.64, 1.39). However, IpB treatment was associated with significantly less adverse events compared to metaproterenol (Peto OR: 0.47, 95% CI:0.29, 0.76; I2 =72%), however the I2 was high.
vi) Haemodynamic effects
No clinically or statistically significant different changes in mean blood pressure and pulse rate recorded during the first three hours after treatment were reported (8 studies). No clinically significant acute or long‐term ECG changes were noted.
vii) Number of subjects increasing/adding systemic (oral) steroids
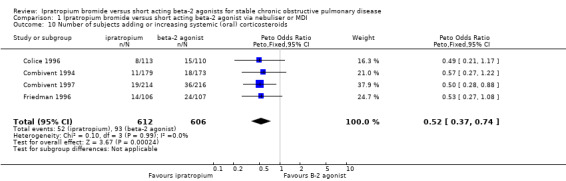
Meta‐analysis of four studies indicated that regardless of delivery method (MDI, nebulised), significantly fewer subjects receiving IpB added or increased oral steroid use compared with subjects receiving SABA, either salbutamol or metaproterenol (Peto OR = 0.52, 95% CI:0.37, 0.74). This gave a NNT of 15 (95% CI 12 to 28, Figure 2).
2.
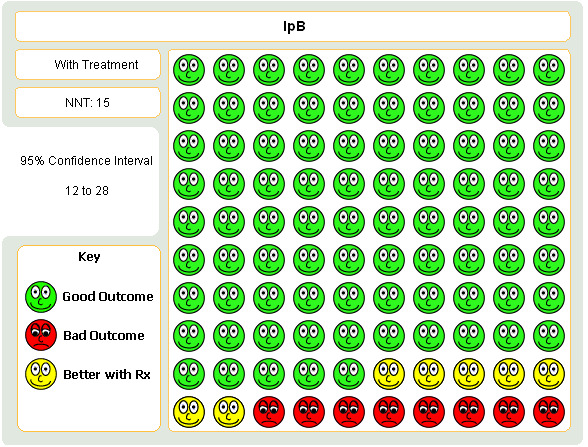
Graphic to demonstrate that in the treatment of COPD with either ipratropium or short‐acting beta‐agonist, 15 people would need to be treated ipratropium in order to prevent one person requiring a course oral steroids.
Ipratropium plus short‐acting beta‐2 agonist versus short‐acting beta‐2 agonist
I)Lung Function Mean test day baseline FEV1 Five studies of 3 months duration reported the effects of treatment with IpB combination therapy versus SABA alone. There was no significant difference between treatments in the baseline FEV1 measured at the end of the study (MD= 0.00 litres, 95% CI: ‐0.03, 0.03). One study (Campbell 1999) for which data was not able to be obtained, reported no significant differences between treatments after 29 days of treatment.
Peak change from test baseline FEV1 Five studies reported the mean peak FEV1 response to the IpB combination or SABA. There was a small significant increase in the mean peak response in FEV1 in the combination group (MD= 0.07 litres, 95% CI: 0.05, 0.08). Data was not able to be obtained for two studies. One study (Gross 1998) reported the mean peak FEV1 response after 42 days of approximately 0.3 litres for combination and 0.23 litres for salbutamol alone, but the statistical significance is unclear. This data was extracted from a graph. Campbell 1999 also reported the mean peak FEV1 response after 29 days of treatment was significantly greater for the combination therapy (0.34 litres) compared to the SABA (0.27 litres) No P value was given however.
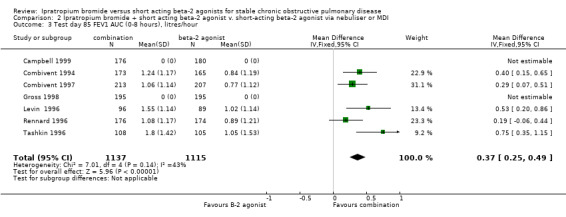
Area under the FEV1 curve above test day baseline (FEV1 AUC) Data available from five studies showed that at the end of the treatment period, the IpB combination treated groups demonstrated a significantly greater FEV1‐AUC than the SABA groups (MD: 0.37 litres/hr, 95% CI:0.24, 0.49).
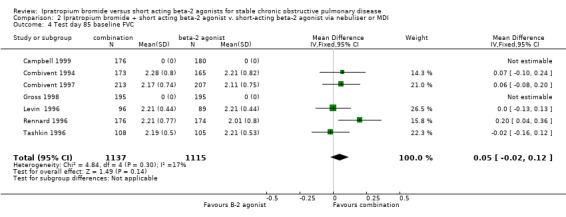
Mean test day baseline FVC Five studies showed that after 85 days of treatment, there were no significant differences between IpB combination and SABA therapy in mean baseline FVC (MD: 0.05 litres, 95% CI: ‐0.21, 0.12).
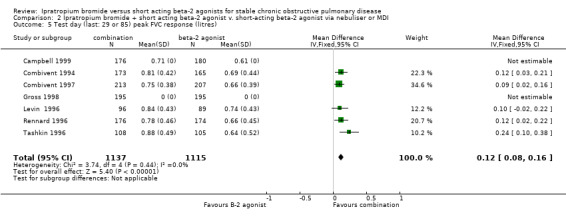
Peak change from test day baseline FVC Five studies reported increases in mean peak FVC response after three months treatment with IpB combination therapy or SABA. Combination treatment was associated with significant increases in peak FVC response compared to SABA treatment (MD: 0.12 litres, 95% CI: 0.08, 0.17). Campbell 1999 also reported the mean peak FVC response after 29 days of treatment was significantly greater for the IpB combination therapy (0.71 litres) compared to the SABA group (0.61 litres). No P value was given however.
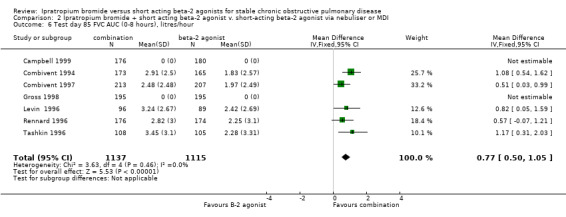
Area under the FVC curve above test day baseline (FVC‐AUC) Data from five studies showed that the FVC‐AUC was significantly greater on IpB combination therapy compared to SABA treatment (MD: 0.77 litres/hr, 95% CI: 0.50, 1.05)
Peak Expiratory Flow (PEF) Four studies reported no significant differences in mean daily morning and evening PEF recordings between treatment groups over 12 weeks study duration, except for one study (Combivent 1997) which reported that evening measurements were significantly higher in the combination group than in the salbutamol group over the 12 week study.
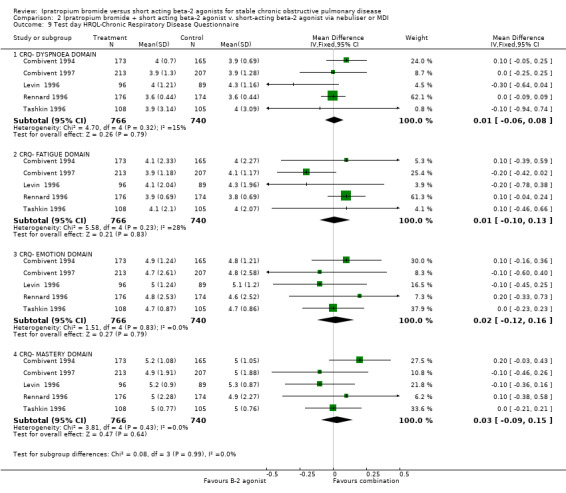
ii) HRQL measured using the Chronic Respiratory Disease Questionnaire (CRQ)
Two studies (Combivent 1997; Rennard 1996) reported that CRQ scores improved slightly/modestly for the entire study group and one study (Tashkin 1996) reported that neither treatments had a demonstrable effect on HRQL scores. It can be assumed that clinically meaningful improvements (0.5 unit change or greater) were not obtained as a result of either treatment.
Data subsequently obtained from five studies showed no significant differences in CRQ scores between treatment groups for the CRQ domains of Dyspnoea (MD= 0.01 units, 95% CI: ‐0.06, 0.08) Fatigue (MD= 0.02 units, 95% CI: ‐0.09, 0.13) Emotion (MD= 0.02 units, 95% CI: ‐0.12, 0.16) or Mastery (MD= 0.03 units, 95% CI: ‐0.09, 0.14).
iii) Dyspnoea Scores
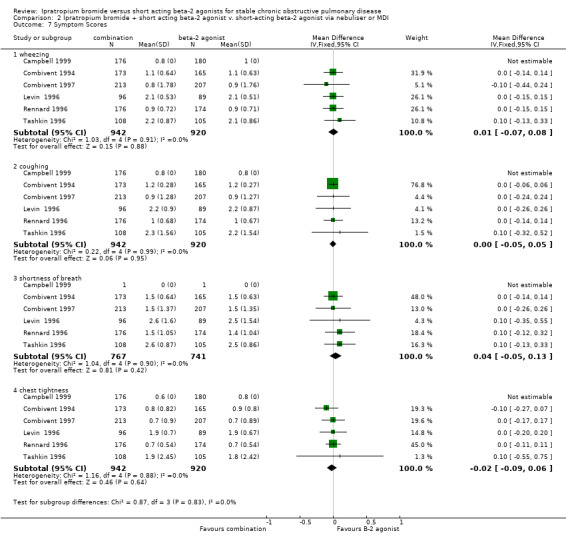
Self reported symptoms scores did not change over the three month duration of five studies and there were no significant differences between treatment groups for :
Wheezing (MD= 0.01, 95% CI: ‐0.07, 0.08),
Coughing (MD= 0.00, 95% CI: ‐0.05, 0.05),
Shortness of Breath (MD= 0.04, 95% CI: ‐0.05, 0.13), or
Chest Tightness (MD= ‐0.02, 95% CI: ‐0.09, 0.6)
One study (Campbell 1999) from which data was unobtainable, reported a significant improvement in symptoms (wheezing and shortness of breath) in the IpB combination group, compared with the salbutamol group.
iv) Exercise Capacity
The only study to measure this outcome reported no significant difference in 6 MWD between treatments during the parallel phase of the study (Gross 1998).
v) Number of subjects experiencing medication related adverse events
Pooled analysis indicated no significant difference in the number of subjects experiencing medication related adverse events when IpB plus beta‐2 agonist was compared with beta‐2 agonist treatment only (Peto OR: 1.16, 95% CI: 0.86, 1.57).
vi) Haemodynamic effects
Four studies reported no statistically or clinically significant changes in blood pressure or pulse rate over 85 days. No clinically significant acute or long‐term ECG changes were noted in three studies.
vii) Number of subjects increasing/adding systemic (oral) steroids
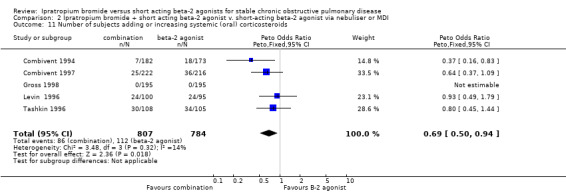
Pooled analysis of four studies indicated that regardless of delivery method (MDI, nebulised), fewer subjects receiving IpB/SABA combination added or increased oral steroid use compared with subjects receiving SABA ‐salbutamol or metaproterenol, (Peto OR = 0.69, 95% CI:0.5, 0.94). This gave a NNT of 20 (95% CI 12 to 108; see Figure 3)
3.
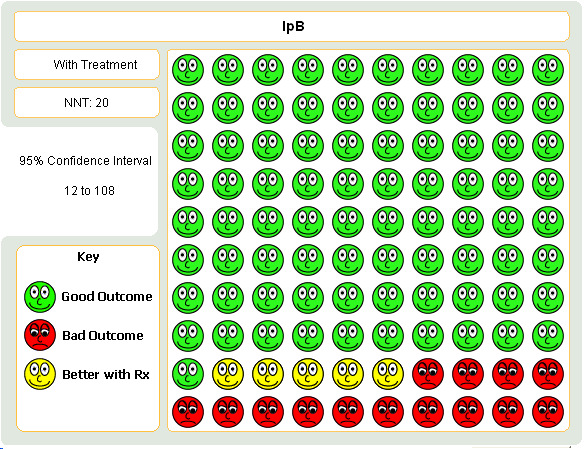
Graphic to demonstrate that in the treatment of COPD with either combination ipratropium and short‐acting beta‐agonist or short‐acting beta‐agonist alone, 20 people would need to be treated ipratropium in order to prevent one person requiring a course oral steroids.
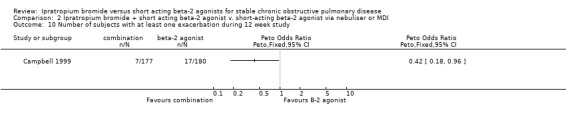
viii) Number of subjects experiencing exacerbations
One study (Campbell 1999) indicated that the number of subjects experiencing at least one exacerbation of COPD was reduced in subjects treated with combination therapy (nebulised) compared with those receiving salbutamol alone (Peto OR: 0.42, 95% CI:0.18, 0.96).
Discussion
Based on the available data, compared with treatment with SABA, long term treatment with IpB alone, (of one to three months duration) resulted in no significant improvements post‐bronchodilator FEV1 measurements, but there was a small benefit in pre‐bronchodilator FEV1 of borderline statistical significance. There was a small increase in pre‐bronchodilator FVC and the post‐ bronchodilator increase in FVC area under the curve over 8 hours approached statistical significance. These data suggest that the benefits of ipratropium over SABA are small, in terms of lung function.
Ipratropium combination therapy offered statistically and clinically significant benefits over SABA in post‐bronchodilator lung function outcomes. Funnel plot asymmetry was suggestive of publication bias, and further studies would help to establish the extent to which the pooled effect estimate may be affected by under‐reporting of non‐significant data (Figure 4). The largest benefits were seen in terms of the FEV1 and FVC area under the curve, over the test day baseline measured over 8 hours. These benefits were far above the cut off of 0.12 litres for clinical significance (Rossi 2002) and demonstrate a role for maintenance therapy with the combination. The observed benefits are consistent with at least an additive effect given that the drugs have different pharmacological actions. It is perhaps important to note that in the ipratropium versus beta‐2 agonist comparison, mean post‐bronchodilator FEV1 responses ranging from 0.2‐0.3 L (24‐29%) were observed in both groups. Combination studies demonstrated a significant difference between groups in terms of peak change in FEV1 (in the order of 28‐40% and 24‐31% over baseline for the combination and beta‐2 agonist groups respectively), and this degree of reversibility demonstrated in both treatment groups is higher than expected for subjects with COPD according to older definitions i.e.‐reversibility of airways obstruction. The findings are probably generalisable to COPD patients defined by more recent criteria i.e. partially reversible, given that asthmatics were excluded based upon having a history of asthma, atopy and eosinophilia (ATS/ERS 2004; NICE/BTS 2004).
4.
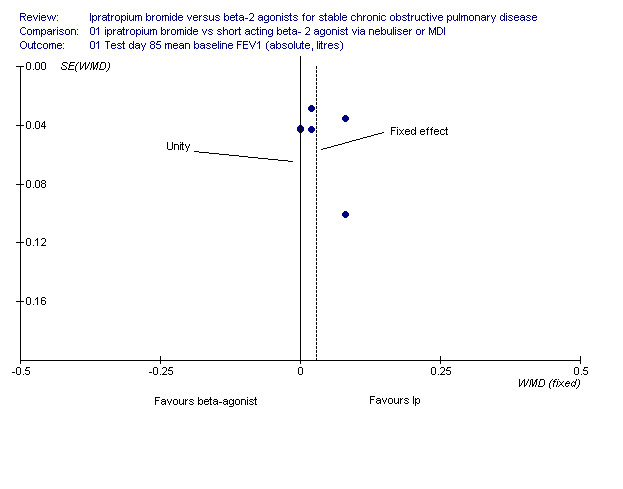
Funnel plot demonstrating possible publication bias
It is also important to note that reductions in the FEV1 AUC and FVC AUC response were observed for both treatments at the end of the study compared with test day one responses. Tachyphylaxis to the FEV1 response in IpB and combination treated groups ranged from 5‐26% and 22‐28% respectively of the day one response. Tachyphylaxis to the beta‐2 agonist across studies ranged from 16‐36%. It is uncertain whether these reductions compared with baseline are significant for any treatment group. The response to IpB (22‐26%) or the combination (38‐52%) remained consistently higher than the response to beta‐2 agonist across all studies.
Generally, neither ipratropium alone or combination therapy resulted in significant reductions in symptom scores with the exception of two studies which also reported significant improvements in CRQ scores. These two studies also reported significantly higher physicians global evaluation scores in the IpB groups compared with the beta‐2 agonist groups. However, the level of symptoms reported during the studies were "mild" and physicians global evaluations indicated that subjects were fair to good despite poor spirometric values. The significant improvement in symptoms (wheezing and shortness of breath) in the combination group in one study (Campbell 1999) is difficult to explain given the similarity of participants' baseline FEV1 as a % of predicted across studies. In spite of the apparent lack of impact on symptoms, there was an impact on the requirement of oral steroid therapy which favoured IpB when compared with SABA alone (NNT of 15), and also in combination with SABA (NNT of 20). The HRQL outcomes also seem to be incongruent with the bronchodilator responses seen in the participants in these studies. The subjects on combination therapy who experienced significant lung function benefits, experienced no significant HRQL improvements (measured using the CRQ), suggesting no subjective benefit of adding IpB. Paradoxically, inconsistent "within group" and "between group" improvements in CRQ domain scores at the threshold of clinical relevancy (Redelmeier 1996) were demonstrated in two of six studies in subjects receiving IpB (which conferred no lung function benefits over a SABA), in Dyspnoea, Fatigue and Emotion domains. However, subjects in the SABA arm of one study also demonstrated improvements.
There are several considerations that should be borne in mind when assessing the effects of IpB on the subjective outcomes in this review. The first consideration is the relationship between quality of life and requirement of oral steroids as a proxy for exacerbations, and the second is the relationship between quality of life and the frequency of adverse events. There were significant differences in favour of IpB as a monotherapy in the requirement of oral steroids and the number of adverse events, and suggests that it is relative effects of therapy in these two variables that account for the otherwise apparent discordance between lung function outcome and HRQL. If the frequency of exacerbations influences health status measurements (Spencer 2004), then it is possible that the reduced need for oral steroids in the IpB groups reflects a significant reduction in severe exacerbations requiring steroids, with an associated difference in the capacity for daily activity between IpB and beta‐agonist. There was strong agreement between the studies in the size and direction of this effect in both sets of comparisons. Interestingly however, the significant reduction in oral steroids favouring combination therapy was not accompanied by an improvement in quality of life, and the impact on adverse events was more equivocal. This suggests that the frequency of adverse events between these therapies may also have a significant impact on quality of life, particularly where the effect sizes are small. Furthermore, the mild symptoms at baseline seem inconsistent with the impaired lung function in people recruited to these studies but may be a consequence of people adapting their lifestyles to limit their activities and therefore symptoms. If this is the case then HRQL improvements may be difficult to detect in subjects who are "mildly" symptomatic at the outset of therapy. The effect sizes reported in Friedman 1996 are larger than those reported in other studies for FEV1, symptom scores, and quality of life despite the similar disposition of the participants at baseline. We have performed a series of post‐hoc sensitivity analyses on these outcomes (see Table 1). This study randomised participants to IpB or metaproterenol and consideration of adverse events leaves the validity of this finding in question. The adverse events for the beta‐agonist group in Friedman 1996 are higher than those reported for the beta‐agonist groups in the other trials and the more impressive effect of IpB could be explained by poorer control in participants who are intolerant of the metaproterenol, leading to a larger mean difference on symptoms and CRQ domains when compared with estimates from the other studies.
1. Sensitivty analyses.
| Outcome | Effect size & I2 | Study removed | Effect size & I2 |
| Comparison 01:01; Test day 85 mean baseline FEV1 | 0.03 [0, 0.06]; I2: 0% | Friedman 1996 | 0.01 [‐0.02, 0.05]; I2: 0% |
| Comparison 01; 07 Wheezing subgroup | ‐0.05 [‐0.13, 0.03]; I2: 52.9% | Friedman 1996 | 0.01 [‐0.08, 0.1]; I2: 0% |
| Comparison 01; 07 Coughing subgroup | ‐0.08 [‐0.13, 0.04]; I2: 43.8% | Friedman 1996 | ‐0.08 [‐0.13, ‐0.03]; I2: 26.1% |
| Comparison 01; 07 Shortness of breath subgroup | 0 [‐0.09, 0.08]; I2: 41.5% | Friedman 1996 | 0.02 [‐0.07, 0.11]; I2: 35.8% |
| Comparison 01; 07 Tightness of chest subgroup | 0.02 [‐0.06, 0.09]; I2: 53.3% | Friedman 1996 | 0.05 [‐0.03, 0.13]; I2: 0% |
| Comparison 01:09; CRQ dyspnoea domain | 0.16 [0.09, 0.23]; I2: 49.9% | Friedman 1996 | 0.15 [0.08, 0.22]; I2: 48.6% |
| Comparison 01:09; CRQ fatigue domain | 0.16 [0.07, 0.25]; I2: 52.2% | Friedman 1996 | 0.11 [0, 0.22]; I2 42.7% |
| Comparison 01:09; CRQ emotion domain | 0.16 [0.04, 0.29]; I2: 52.3% | Friedman 1996 | 0.09 [‐0.05, 0.23]; I2: 19.7% |
| Comparison 01:09; CRQ mastery domain | 0.18 [0.06, 0.3]; I2: 33.5% | Friedman 1996 | 0.11 [‐0.03, 0.25]; I2: 0% |
The lung function benefits seen with combination therapy might be expected to confer some exercise capacity gain (i.e. increase in six minute walk distance). However, none were seen and this is an outcome which should be measured in future studies.
Any marginal benefits of IpB or combination therapy over SABAs need to be weighed against the drug costs as IpB and the combination are more expensive than SABA therapy alone. The cost issue is particularly relevant for nebulised therapy. In this regard, it is important that IpB treatment has been shown to be at least as safe as SABAs in terms of adverse events and haemodynamic effects, which is supported by this review. A pharmacoeconomic evaluation of combination inhalation aerosol therapy compared with components was conducted in 1999 to determine the costs of health care resource utilisation associated with COPD exacerbations (Friedman 1999). This used data from 1067 patients from two RCTs (Combivent 1994; Rennard 1996). Ipratropium and combination therapy was associated with significantly less exacerbations of COPD than beta‐2 agonist (salbutamol) therapy. The length of hospital stay (and costs per patient) were 103 days ($US 269) for salbutamol, 20 days for ipratropium ($156) and 46 days ($197) for combination therapy. Total costs were significantly higher for the salbutamol group. However there was no significant difference in the costs between the ipratropium and combination therapy groups. It is not known whether such cost savings associated with exacerbations of COPD are generalisable to the clinical setting of stable COPD, and a study that provided a detailed, prospective, cost effectiveness analysis would be welcomed.
A role for "N=1 randomised trials" to identify patients who actually benefit from anticholinergic therapy has been suggested (van Weel 1998). Patient preference, acquired with methodological validity, may be valuable to determine which drug or drug combination is appropriate for which patient. The relevance of patient preference has been demonstrated in a randomised double‐blind crossover study (Blosser 1995) comparing the effects of ipratropium 36 mcg q.i.d. and salbutamol 180 mcg q.i.d. for seven days in 15 patients with COPD, which reported that the mean FEV1 after seven days therapy was not significantly different between the treatments. No difference in exercise tolerance or dyspnoea scores were reported at the end of the treatment interval. However, in a subjective evaluation of the treatments, 7 subjects favoured ipratropium, 7 favoured albuterol and one had no preference. Importantly, only 5 of the 15 subjects preferred the drug to which they showed greatest reversibility in FEV1. As this review has shown that there is little objective difference between any of the bronchodilator strategies, therapy should be targeted to those patients shown to benefit from it.
Given the general acceptance and recommendation of IpB/beta‐2 agonist combination therapy for the treatment of patients with COPD, the superiority of the type of beta‐2 agonist, whether short ‐ or long‐acting remains to be determined. To date only one study (D'Urzo 2001), a randomised, double blind, cross‐over trial, has been reported, comparing IpB (40 mcg q.i.d.) plus formoterol (12 mcg b.d.) and IpB plus salbutamol (200 mcg q.i.d.). Evaluation after 3 weeks of treatment demonstrated statistically significant increases in mean pre‐bronchodilator PEF (12 litres/min) and FEV1 (0.120 litres) values with the formoterol/ipratropium combination compared with the salbutamol/ipratropium combination. Post‐bronchodilator FEV1 values were also shown to be significantly increased with the formoterol combination (in the order of 0.150 litres). These marginal clinical benefits will need to be weighed against the additional costs of adding a long‐acting beta‐2 agonist to ipratropium.
The conclusions in this review of the comparative effects of bronchodilators on simple spirometric outcomes are perhaps limited by the accumulating evidence which suggests that spirometric measurements of FEV1 and FVC may not be the best measures of bronchodilator response in COPD. The role of spirometry in evaluating therapeutic responses has been reviewed (O'Donnell 2000). In advanced COPD, exertional dyspnoea has been correlated with the level of dynamic lung hyperinflation (DH) (O'Donnell 1997; Belman 1996; O'Donnell 1998) as measured by inspiratory capacity (IC). Furthermore, a RCT using cross‐over study design with three week treatment arms of ipratropium and placebo (O'Donnell 1999) showed that of the available spirometric parameters which indirectly measure reduced lung hyperinflation, IC correlated better than expiratory flow measurements, with reduced dyspnoea and improvements in exercise tolerance. An increases in IC of 10% predicted (0.3 L) was associated with a significant increase (>25%) in exercise endurance time. Perhaps, most significantly, the improvements in IC and exercise tolerance after ipratropium treatment occurred in a proportion of subjects (31%) who showed little or no improvement in FEV1 (<10% predicted). Future critical evaluation of the benefits of bronchodilator therapy will require the incorporation of measurements of lung hyperinflation in spirometric assessments, in addition to measures of symptoms, exercise tolerance and HRQL and longer trial duration to detect critical events such as hospitalisations due to exacerbations.
Authors' conclusions
Implications for practice.
Long term combination therapy over 12 weeks with ipratropium plus a short‐acting beta‐2 agonist was associated with some clinically meaningful post‐bronchodilator outcomes compared to beta‐2 agonist treatment but these were not reflected in subjective improvements in HRQL or symptom scores. Ipratropium or beta‐2 agonist therapy, appeared to offer similar, small, but clinically relevant HRQL benefits (using the CRQ) in the absence of lung function benefits.
As none of the treatments was associated with lowering of baseline FEV1 or FVC responses, the routine first line use of ipratropium alone or the combination, in preference to short‐acting beta‐2 agonist therapy, is difficult to rationalise on the basis of sustaining lung function in patients with COPD. The observed significant post‐bronchodilator increases in FEV1 and FVC with ipratropium, combination, and short acting beta‐2 agonist treated groups, suggest that at least some of the study populations may have had a significant reversible component to their airways disease (present in around half of COPD patients). The generalisability of the observed "mean" benefits to the poorly reversible population is unclear. The observed reduction in the need for oral corticosteroids in people treated with ipratropium alone or in combination with a SABA is an important finding and suggests a benefit in terms of reducing exacerbations in COPD. It may also be a consideration if patients have relative contraindications to steroid use. However, this needs formal assessment in long‐term studies.
Until further data is available, the strategy of providing a beta‐2 agonist on a PRN basis, and then, when the patient requires regular treatment, either continuing with the beta agonist regularly or conducting an "n of 1" trial of regular beta‐2 agonist or regular anticholinergic to determine the treatment that gives the best relief of symptoms (and continuing with it), would seem cost effective, although this needs formal evaluation. Patient preference is an important factor which requires consideration when agents perform similarly. Given the available evidence, this review does not support the routine first line use of ipratropium alone or the combination, over initial short acting beta‐2 agonist therapy alone, for patients with symptomatic COPD over a range of severities.
Implications for research.
The relative value of ipratropium therapy alone or in combination with short acting beta‐2 agonists needs still to be determined in studies which measure outcomes such as measures of inspiratory capacity or dynamic hyperinflation in combination with other measures such as exercise tolerance, dyspnoea scores, HRQL and effects on exacerbation rates. Studies should incorporate measures of health utilisation measures and need to be of longer duration to capture effects on exacerbation rates. The important issue of patient preferences need to be considered in future studies, as does the question of how therapy is escalated in COPD. It is arguable however that given the emergence of the long‐acting anti‐cholinergic tiotropium on to the market, that future studies should focus on the evaluation of this therapy using relevant outcomes, for long term use in COPD, including comparisons with existing effective therapies. Its relative cost effectiveness also needs to be considered.
Feedback
Concerns over use of medication‐related AEs rather than all cause AEs, 6 August 2018
Summary
We have thoroughly reviewed the meta‐analysis and one of the outcomes analyzed has caught our attention. We are concerned with analysis 1.8 which analyzed “medication” related adverse events because this requires a subjective assessment by the assessor and one would never know for sure whether there is a cause‐effect relationship between the intervention and adverse event. For reasons such as loss of blinding and other possible biases, this could lead to underestimation of adverse events. In the context of an RCT it would be reasonable to include “any” adverse events because any adverse events unrelated to the interventions would be balanced between the two arms. If you were to perform an analysis on adverse events, it would be ideal to include both an analysis on “medication” related adverse events and “any” adverse events, however, if only one was to be chosen then “any” adverse events would be preferred. If the analysis had assessed “any” adverse events, which were reported by the majority of the studies included, it would have allowed inclusion of Gross 1998 and would have provided a more accurate estimate. We feel this is an important concern, as the Cochrane handbook also supports being cautious in the terminology used when certain data are extracted from studies. The data from Tashkin 1986 used for the analysis were “number with more than 1 adverse experience” which is not consistent with the other four studies analyzed. Additionally, the heterogeneity of the studies was 63% based on I2, therefore, the variance between study results is more likely to be due to actual differences rather than chance alone. It was clearly mentioned that heterogeneity was driven by Friedman 1996 with nebulized ipratropium, and when removed, there was no statistically significant difference between treatments in this outcome. However, your conclusion did not discuss what the implications of this would be in clinical practice. Would it be safe to say that ipratropium is associated with a reduced risk of adverse events compared to SABA or is there caution in stating nebulized ipratropium is better. Furthermore, the initial objective of the review was to assess both efficacy and safety, however your conclusion only addressed efficacy and not safety. Thanks for your time and consideration, Do you have any affiliation with or involvement in any organisation with a financial interest in the subject matter of your comment?I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment
Reply
Triggered by this feedback, the editorial team are assessing the need for this review to be updated. We will screen an updated literature search, and will add our views to this review in due course. Emma Dennett, Managing Editor, Cochrane Airways.
Contributors
Su Qiong R. Liang, Karyn Bagri and Aaron M. Tejani
Fraser Health Authority of British Columbia
What's new
| Date | Event | Description |
|---|---|---|
| 20 August 2018 | Amended | Feedback comment added to the review. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 27 April 2009 | Amended | Technical problem identified; resolved by programmer |
| 4 July 2008 | New search has been performed | Search re‐run; no new studies found |
| 3 July 2008 | Amended | Converted to new review format. |
| 15 February 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to express our thanks to Nicole Pushlar from Boerhinger Ingelheim US for providing us with extensive unpublished data. Thanks also to Elisabeth Strom for supplying unpublished data, and Dr Hodzhev for responding to our requests for clarification. We are grateful to Professor Vasily Vlassov for translating the Russian articles excluded from the review, and to Drs Phillippa Poole and Chris Cates for their excellent editorial assistance.
Data and analyses
Comparison 1. Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test day 85 mean baseline FEV1 (absolute, litres) | 8 | 2148 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.00, 0.06] |
| 2 Test day 85 mean peak FEV1 response (change from day 85 baseline, litres) | 6 | 1917 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 3 Test day 85 mean FEV1 AUC (absolute, litres) | 7 | 2104 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.02, 0.22] |
| 4 Test day 85 mean baseline FVC (absolute, litres) | 8 | 2148 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.01, 0.14] |
| 5 Test day peak FVC | 6 | 1907 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.06, 0.03] |
| 6 Test day FVC AUC | 7 | 2104 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.01, 0.55] |
| 7 Symptom scores | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 wheezing | 5 | 1533 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.13, 0.04] |
| 7.2 coughing | 5 | 1533 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.13, ‐0.03] |
| 7.3 shortness of breath | 5 | 1533 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.09, 0.09] |
| 7.4 tightness of chest | 5 | 1533 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.09] |
| 8 Number of subjects with medication related adverse events | 6 | 1858 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.53, 0.97] |
| 9 HRQL‐ Chronic Respiratory Disease Questionnaire: absolute scores | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 CRQ‐ DYSPNOEA DOMAIN | 5 | 1529 | Mean Difference (IV, Fixed, 95% CI) | 0.16 [0.09, 0.23] |
| 9.2 CRQ‐ FATIGUE DOMAIN | 5 | 1529 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.02, 0.23] |
| 9.3 CRQ‐ EMOTION DOMAIN | 5 | 1529 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.05, 0.29] |
| 9.4 CRQ‐ MASTERY DOMAIN | 5 | 1529 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [0.06, 0.30] |
| 10 Number of subjects adding or increasing systemic (oral) corticosteroids | 4 | 1218 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.37, 0.74] |
| 11 Borg Dyspnoea Index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12 Borg dyspnoea score‐ reduction of 1 unit | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 12.1 Borg score reduced 1 unit | 1 | 35 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.71 [0.73, 10.07] |
| 12.2 Borg score reduced one unit after 6MWD test | 1 | 35 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.21 [0.60, 8.20] |
| 13 Six minute walk distance | 2 | 423 | Mean Difference (IV, Fixed, 95% CI) | 62.60 [‐15.65, 140.85] |
| 14 Six minute walk distance test‐improved at least 30 metres | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 1 Test day 85 mean baseline FEV1 (absolute, litres).
1.2. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 2 Test day 85 mean peak FEV1 response (change from day 85 baseline, litres).
1.3. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 3 Test day 85 mean FEV1 AUC (absolute, litres).
1.4. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 4 Test day 85 mean baseline FVC (absolute, litres).
1.5. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 5 Test day peak FVC.
1.6. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 6 Test day FVC AUC.
1.7. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 7 Symptom scores.
1.8. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 8 Number of subjects with medication related adverse events.
1.9. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 9 HRQL‐ Chronic Respiratory Disease Questionnaire: absolute scores.
1.10. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 10 Number of subjects adding or increasing systemic (oral) corticosteroids.
1.11. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 11 Borg Dyspnoea Index.
1.12. Analysis.
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 12 Borg dyspnoea score‐ reduction of 1 unit.
1.13. Analysis.
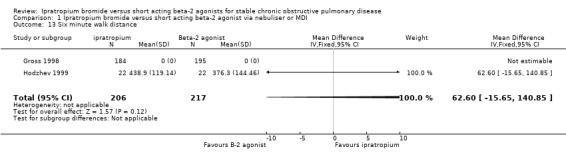
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 13 Six minute walk distance.
1.14. Analysis.
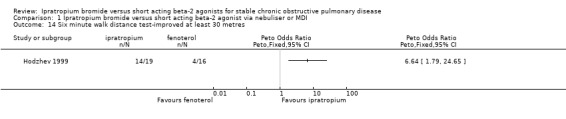
Comparison 1 Ipratropium bromide versus short acting beta‐2 agonist via nebuliser or MDI, Outcome 14 Six minute walk distance test‐improved at least 30 metres.
Comparison 2. Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Test day 85 baseline FEV1(absolute, litres) | 7 | 2252 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.03, 0.04] |
| 2 Test day (last:29 or 85) peak FEV1 response (litres) | 7 | 2248 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [0.05, 0.09] |
| 3 Test day 85 FEV1 AUC (0‐8 hours), litres/hour | 7 | 2252 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [0.25, 0.49] |
| 4 Test day 85 baseline FVC | 7 | 2252 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐0.02, 0.12] |
| 5 Test day (last: 29 or 85) peak FVC response (litres) | 7 | 2252 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [0.08, 0.16] |
| 6 Test day 85 FVC AUC (0‐8 hours), litres/hour | 7 | 2252 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [0.50, 1.05] |
| 7 Symptom Scores | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 wheezing | 6 | 1862 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.07, 0.08] |
| 7.2 coughing | 6 | 1862 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.05, 0.05] |
| 7.3 shortness of breath | 6 | 1508 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.05, 0.13] |
| 7.4 chest tightness | 6 | 1862 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.09, 0.06] |
| 8 Six minute walk distance (m) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Test day HRQL‐Chronic Respiratory Disease Questionnaire | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 CRQ‐ DYSPNOEA DOMAIN | 5 | 1506 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.06, 0.08] |
| 9.2 CRQ‐ FATIGUE DOMAIN | 5 | 1506 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.10, 0.13] |
| 9.3 CRQ‐ EMOTION DOMAIN | 5 | 1506 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.12, 0.16] |
| 9.4 CRQ‐ MASTERY DOMAIN | 5 | 1506 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.09, 0.15] |
| 10 Number of subjects with at least one exacerbation during 12 week study | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 11 Number of subjects adding or increasing systemic (oral) corticosteroids | 5 | 1591 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.50, 0.94] |
| 12 Number of subjects experiencing adverse event related to medication | 5 | 1558 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.86, 1.57] |
2.1. Analysis.
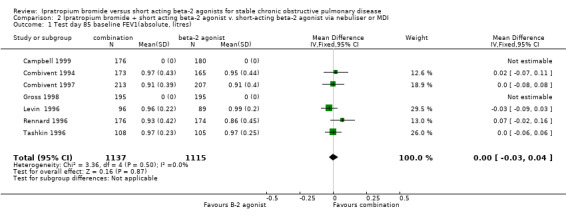
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 1 Test day 85 baseline FEV1(absolute, litres).
2.2. Analysis.
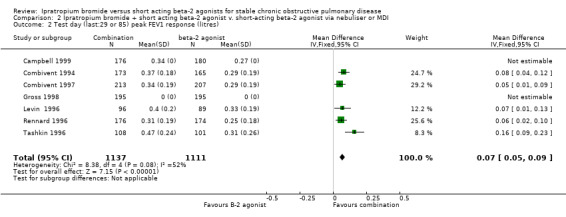
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 2 Test day (last:29 or 85) peak FEV1 response (litres).
2.3. Analysis.
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 3 Test day 85 FEV1 AUC (0‐8 hours), litres/hour.
2.4. Analysis.
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 4 Test day 85 baseline FVC.
2.5. Analysis.
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 5 Test day (last: 29 or 85) peak FVC response (litres).
2.6. Analysis.
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 6 Test day 85 FVC AUC (0‐8 hours), litres/hour.
2.7. Analysis.
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 7 Symptom Scores.
2.8. Analysis.

Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 8 Six minute walk distance (m).
2.9. Analysis.
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 9 Test day HRQL‐Chronic Respiratory Disease Questionnaire.
2.10. Analysis.
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 10 Number of subjects with at least one exacerbation during 12 week study.
2.11. Analysis.
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 11 Number of subjects adding or increasing systemic (oral) corticosteroids.
2.12. Analysis.
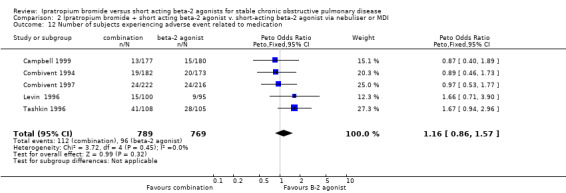
Comparison 2 Ipratropium bromide + short acting beta‐2 agonist v. short‐acting beta‐2 agonist via nebuliser or MDI, Outcome 12 Number of subjects experiencing adverse event related to medication.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Campbell 1999.
| Methods | RCT: 29 day (two arm) parallel group MDI study. Randomisation: unclear Blinding: double blind Excluded: not described. Withdrawals: not adequately described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis: not stated Jadad Score:2 | |
| Participants | Setting: USA, multi‐centre (17) study. 357 subjects with stable COPD. mean age: 65.6 years male/female: n= 251/103 (not equal to 357) mean baseline FEV1: 36.2% predicted. No range given. Inclusion criteria:>or =40 years of age, > 10 year pack history of smoking, stable disease, FEV1 < or = 65% predicted, FEV1/FVC < or =70 %, and using 2 prescribed therapeutic medication for COPD control in 3 months prior to entry into trial. Exclusion criteria: history of asthma, allergic rhinitis or atopy, total blood eosinophil count > 300,000/ml. | |
| Interventions | 1) a combination of albuterol (90 mcg) and ipratropium (18 mcg), (2 puffs), four times daily. 2 ) 90 mcg albuterol (2 puffs), four times daily. All medication administered via MDI for 29 days. | |
| Outcomes | Primary outcomes measured on Test Day 1 and 29 : pulmonary function tests i.e. 1)Test Day baseline FEV1. 2) Test Day peak change in FEV1. 3) Test Day AUC (0‐6 hr) FEV1. Secondary Outcomes included: I) the above responses for FVC measured day 1 and 29. Measured on fortnightly visits to the clinic to monitor: 1) COPD symptoms (wheeze, dyspnoea, cough, chest tightness) recorded daily in symptom diary on a scale of 0‐3. 2) adverse events. | |
| Notes | No SDs published, but sought from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Colice 1996.
| Methods | RCT: 85 day (two arm) parallel group inhalation solution study. Randomisation: computer generated Blinding: double blind Excluded: not described. Withdrawals: described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis: not stated Jadad Score:4 | |
| Participants | Setting: USA, multi‐centre (11) study. 223 subjects with stable COPD. mean age: 64 years male/female: 60% / 40% mean baseline FEV1: 1.00 Litres or 39% predicted. No range given. Inclusion criteria: > or = 40 years of age, >10 year pack history of smoking, stable disease, FEV1 < or = 65% predicted, FEV1/FVC < or =70 % Exclusion criteria: history of asthma, allergic rhinitis or atopy, total blood eosinophil count >500/mm3, recent use of large doses of corticosteroids, recent MI (3 years), use of beta blockers, chronic home oxygen, other significant disease. | |
| Interventions | 1) 0.5 mg ipratropium 0.1% inhalation solution, three times daily. 2) 2.5 mg albuterol 0.5% inhalation solution, three times daily. Medication was provided in 2.5ml vials and delivered by nebuliser, for 85 days. | |
| Outcomes | Primary outcomes measured on Test Day 1, 43, 85 : pulmonary function tests i.e. 1)Test Day baseline FEV1, FVC 2) Test Day peak change in FEV1, FVC 3) Test Day AUC above test day baseline (0‐8 hr) FEV1, FVC. Secondary Outcomes included: I) HRQL using the Chronic Respiratory Disease Questionnaire (CRQ) measured day 1, 43 and 85. Secondary Outcomes measured on fortnightly clinic visits to monitor: 2) Daily PEFR‐ measured morning and evening and recorded in diary. 3) adverse events. 4) concomitant medication. | |
| Notes | Unpublished standard errors obtained from Boehringer Ingelheim and converted to standard deviations. Study quality details provided by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Third party randomisation |
Combivent 1994.
| Methods | RCT: 12 week (three arm) parallel group MDI study. Randomisation: computer generated Blinding: double blind Excluded: not described. Withdrawals: described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis. Jadad Score:5 | |
| Participants | Setting: USA, multi‐centre (24) study.
534 subjects with stable COPD.
mean age: 63.4 years
male/female: 65% / 35%
mean baseline FEV1: 0.99 Litres or 37% predicted.
range: 0.29‐2.78 litres or 11.5‐76.2% predicted. Inclusion criteria:> or= 40 years of age, stable, FEV1 < or =65% predicted, FEV1/FVC < or =70 %, >10 year pack history of smoking, and using 2 prescribed therapeutic medication for COPD control in 3 months prior to entry into trial. Exclusion criteria: history of asthma, allergic rhinitis or atopy, total blood eosinophil count >500/mm3, daily use of >10mg oral prednisone within 1 month before entry. |
|
| Interventions | 1) a combination of albuterol (100 mcg) and ipratropium (21 mcg), (2 puffs), four times daily. 2 ) 21 mcg ipratropium (2 puffs), four times daily. 3) 100 mcg albuterol (2 puffs), four times daily. All medication administered via MDI for 85 days. | |
| Outcomes | Primary outcomes measured on Test Day 1, 29, 57, 85 : pulmonary function tests i.e. 1)Test Day baseline FEV1, FVC 2) Test Day peak change in FEV1, FVC 3) Test Day AUC (0‐8 hr) FEV1, FVC Secondary Outcomes measured on fortnightly visits: 1) Daily PEFR‐ measured morning and evening and recorded in diary. 2) COPD symptoms (wheeze, dyspnoea, cough, chest tightness) recorded daily in symptom diary. | |
| Notes | Inclusion criteria state FEV < 65%. FEV1 range at baseline= 11.5% ‐ 76.2% predicted, suggesting some subjects with only mild disease. Unpublished standard errors obtained from Boehringer Ingelheim and converted to standard deviations. Study quality details provided by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Third party randomisation |
Combivent 1997.
| Methods | RCT: 12 week (three arm) parallel group inhalation solution study. Randomisation: computer generated Blinding: double blind Excluded: not described. Withdrawals: described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis. Jadad Score:5 | |
| Participants | Setting: USA, multi‐centre (25) study.
652 subjects with stable COPD.
mean age: 64.9 years
male/female: 65% / 35%
mean baseline FEV1: 0.91 Litres or 34% predicted.
range: 0.18‐2.49 litres or 9.2‐64.4% predicted. Inclusion criteria:> or =40 years of age, > or =10 year pack history of smoking, stable disease, FEV1 < or =65% predicted, FEV1/FVC < or =70 %, > or =10 year pack history of smoking, and using 2 prescribed therapeutic medication for COPD control in 3 months prior to entry into trial. Exclusion criteria:history of asthma, allergic rhinitis or atopy, total blood eosinophil count >500/mm3. |
|
| Interventions | 1) a combination of albuterol (3.0 mg) and ipratropium (0.5 mg) inhalation solution , three times daily. 2 ) ipratropium inhalation solution (0.5 mg) , three times daily. 3) albuterol inhalation solution (3mg), three times daily. Medication was provided in identical 2.5ml vials and delivered by nebuliser, for 85 days. | |
| Outcomes | Primary outcomes measured on Test Day 1, 29, 57, 85 : pulmonary function tests i.e. 1)Test Day baseline FEV1, FVC 2) Test Day peak change in FEV1, FVC 3) Test Day AUC (0‐8 hr) FEV1, FVC Secondary Outcomes measured on fortnightly visits to monitor: 1) Daily PEFR‐ measured morning and evening and recorded in diary. 2) HRQL using the Chronic Respiratory Disease Questionnaire (CRQ). 3) COPD symptoms (wheeze, dyspnoea, cough, chest tightness) recorded daily in symptom diary. 4) adverse events 5)concomitant medication | |
| Notes | Unpublished standard errors obtained from Boehringer Ingelheim and converted to standard deviations. Study quality details provided by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Third party randomisation |
Friedman 1996.
| Methods | RCT: 85 day (two arm) parallel group inhalation solution study. Randomisation: computer generated Blinding: double blind Excluded: not described. Withdrawals: not adequately described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis not stated. Jadad Score:4 | |
| Participants | Setting: USA, multi‐centre (9) study.
213 subjects with stable COPD.
mean age: 61.5 years
male/female: 76% / 24%
Mean baseline FEV1: 1.07 litres or 37% predicted.
range: 0.3‐2.3 litres or 12‐72% predicted. Inclusion criteria: > or = 40 years of age, stable disease, FEV1 < or = 65% predicted, FEV1/FVC < or =70 %, > 10 year pack history of smoking. Exclusion criteria: history of asthma, allergic rhinitis or atopy, total blood eosinophil count >500/mm3, other significant disease, recent MI (< 1 year). |
|
| Interventions | 1) 0.5 mg ipratropium 0.167% inhalation solution, three times daily. 2) 15 mg metaproterenol 5% inhalation solution, three times daily. Medication was provided in 2.0 ml vials and delivered by nebuliser, for 85 days. | |
| Outcomes | Primary outcomes measured on Test Day 1, 43, 85 : pulmonary function tests i.e. 1) Test Day baseline FEV1, FVC 2) Test Day peak change in FEV1, FVC 3) Test Day AUC above test day baseline (0‐8 hr) FEV1, FVC. Secondary Outcomes included: I) HRQL using the Chronic Respiratory Disease Questionnaire (CRQ) measured day 1, 43 and 85. Secondary Outcomes measured on fortnightly clinic visits to monitor: 2) Daily PEFR‐ measured morning and evening and recorded in diary. 3) COPD symptoms (wheeze, dyspnoea, cough, chest tightness) 4) adverse events. 5) concomitant medication. | |
| Notes | Unpublished standard errors obtained from Boehringer Ingelheim and converted to standard deviations. Study quality details provided by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Third party randomisation |
Gross 1998.
| Methods | Six week 3 arm cross over, followed by 6 week parallel phase. Drug administered during parallel phase was the drug administered in the last arm of the crossover. Randomisation: unclear Blinding: double blind Excluded: not described. Withdrawals: described. Power calculation: not given. Intention to treat analysis not stated. Jadad Score:4 | |
| Participants | Setting: USA, multi‐centre (60) study. 863 subjects with stable chronic bronchitis. mean age: 66.3 years male/female: 62% / 38% mean baseline FEV1: 1.15 Litres. range: 0.4‐3.30 litres Inclusion criteria: FEV1 >25% and < or = 65% predicted, at least 40 years of age, using at least one bronchodilator for COPD control in 3 months prior to entry into trial, >10 year pack history of smoking, able to complete 6 minute walk and abstain from use of salmeterol, theophylline and beta‐2 agonists. Exclusion criteria: history of allergic rhinitis, atopy, asthma, polycythaemia, cor pulmonale, hypoxia, any parenchymal disease not attributable to COPD, recent hospitalisation (2 months) for pulmonary exacerbation, unstable angina, recent MI (6 months), hypersensitivity to components of study drugs, narrow angle glaucoma. | |
| Interventions | 1) 2.5 mg albuterol, four times daily, 2) 0.5 mg ipratropium, four times daily 3) a combination of albuterol (2.5 mg) and ipratropium (0.5 mg). Medication was provided in 3.0 ml vials and delivered by nebuliser, for 42 days during the parallel phase of the study. | |
| Outcomes | Outcomes measured on Test Day 1, 14, 28, 42, 56, 70, 84: Primary outcome: Pulmonary function testing i.e. 1) Change from pre‐dose FEV1 to peak FEV1, measured within 8 hours of dosing on test days 14, 28 and 42 during the cross‐over phase. Secondary outcomes: 1)Change from pre‐dose FEV1 and FVC to peak FEV1 and FVC measured within 8 hours of dosing 2) FEV1 and FVC AUC (0‐8 hr) 3) six minute walk measured after 2 weeks of treatment on each of the study medications on days 14, 28 and 42 of the crossover phase, and day 84, after 6 weeks of parallel phase. 4) adverse events. | |
| Notes | No SDs published, but sought from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Third party randomisation |
Hodzhev 1999.
| Methods | RCT: 30 day parallel group study. Randomisation: block randomisation using a random number table. Blinding: unblinded Excluded: not described Withdrawals: described Baseline characteristics: comparable Power calculation: not given. Jadad Score:3 | |
| Participants | Setting: Medical Institute, Bulgaria. 44 subjects with stable, severe COPD (FEV1 < 35% pred). Only subjects with non‐reversible obstruction included in the study. fenoterol n=22 ipratropium n=22 mean age: 63 years mean FEV1% predicted (SD): fenoterol‐ 24.6 (1.5); ipratropium‐22.6 (1.3). Exclusion criteria: History of asthma, allergic rhinitis or atopy; Eosinophilia; Active lung tuberculosis or lung carcinoma; cardiovascular disorders; disorders of locomotor apparatus; Anemias; renal, liver or metabolic disorders; Hypertrophy of the prostate and glaucoma; patients with supporting inhalator and peroral corticosteroid therapy. | |
| Interventions | 1) 40 mcg ipratropium, four times daily. 3) 100 mcg fenoterol, four times daily. All medication administered via MDI for 30 days. | |
| Outcomes | Outcomes: 1) Forced expiratory parameters (FEV1, FVC) 2) Dyspnoea according to American Thoracic Society Dyspnoea Scale and Borg Scale. 3) Exercise tolerance using the six minute walk distance test. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Third party randomisation |
Levin 1996.
| Methods | RCT: 85 day (two arm) parallel group inhalation solution study. Randomisation: computer generated Blinding: double blind Excluded: not described. Withdrawals: not adequately described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis not stated. Jadad Score:4 | |
| Participants | Setting: USA, multi‐centre (8) study.
195 subjects with stable COPD.
mean age: 64.0 years
male/female: 63% / 37%
mean baseline FEV1: 1.02 Litres or 39% predicted. No range given. Inclusion criteria: > or = 40 years of age, stable disease, FEV1 < or = 65% predicted, FEV1/FVC < or =70 %, > or =10 year pack history of smoking. Exclusion criteria: history of asthma, allergic rhinitis or atopy, total blood eosinophil count >500/mm3, recent MI (< 1 year), other significant disease, viral infection or febrile illness in past month. |
|
| Interventions | 1) a combination of albuterol (2.5 mg, 3.0 ml) solution plus ipratropium (0.5 mg) solution. 2) albuterol (2.5 mg, 3.0 ml) solution, plus placebo, three times daily. Medication delivered via nebuliser. | |
| Outcomes | Primary outcomes measured on Test Days 1, 43 and 85 1) Peak change in FEV1 2) FEV1 AUC above test day baseline 3) baseline FEV1 Secondary Outcomes included: 1)Peak change in FVC 2) FVC AUC (0‐8 hr) above test day baseline 3) baseline FVC 4) HRQL using the Chronic Respiratory Disease Questionnaire (CRQ) measured day 1, 43 and 85. Secondary Outcomes measured on fortnightly clinic visits to monitor: 1) Daily PEFR‐ measured morning and evening and recorded in diary. 2) COPD symptoms. 3) adverse events. 4) concomitant medication. | |
| Notes | Unpublished standard errors obtained from Boehringer Ingelheim and converted to standard deviations. Study quality details provided by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Third party randomisation |
Rennard 1996.
| Methods | RCT: 90 day MDI study Randomisation: computer generated Blinding: double blind Excluded: not described. Withdrawals: described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis not stated. Jadad Score:5 | |
| Participants | Setting: USA.
357 subjects with COPD.
mean age: 65.1 years
male : female= 70% : 30%
mean baseline FEV1: 0.90 Litres
range not given. Inclusion criteria: > or = 40 years of age, >10 year pack history of smoking, stable disease, FEV1 < or = 65% predicted, FEV1/FVC < or =70 % predicted. Exclusion criteria: history of asthma, allergic rhinitis or atopy, elevated blood eosinophil count, daily use of >10mg oral prednisone and use of cromolyn sodium. |
|
| Interventions | 1) ipratropium 42 mcg, 4 x times daily 2) albuterol 240 mcg, x times daily Medication delivered via MDI for 90 days | |
| Outcomes | Primary outcomes measured beginning and end of trial : pulmonary function tests i.e. 1) Change in Test Day baseline FEV1, FVC. 2) Change in Test Day AUC (0‐4 hr) FEV1, FVC. 3) HRQL: CRQ. 4) COPD symptoms. | |
| Notes | Combination study but only subjects treated with beta‐2 agonist alone or ipratropium alone were included. Unpublished data for all 3 arms, including standard errors obtained from Boehringer Ingelheim. SEs were converted to SDs. Study not described as double‐blind but study quality details obtained from author. Allocation concealment details provided by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Third party randomisation |
Tashkin 1986.
| Methods | RCT: 90 day (two arm) parallel group MDI study. Randomisation: unclear Blinding: double blind Excluded: not described. Withdrawals: described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis not stated. Jadad Score:4 | |
| Participants | Setting: USA.
261 stable subjects with COPD.
mean age: 61.5 years
male : female=84% : 16%
mean baseline FEV1: 1.09 Litres or 36% predicted. Inclusion criteria: > or = 37 years of age, > or =10 year pack history of smoking, stable disease, FEV1 < or = 75% predicted, FEV1/FVC < or =70 %. Subjects had to demonstrate significant reversible airflow obstruction with a bronchodilator (at least 15%). Exclusion criteria: history of asthma, allergic rhinitis or atopy, total blood eosinophil count >500/microlitre, recent respiratory tract infection, febrile disease, serious other significant disease (cardiac, renal, hepatic, endocrine, metabolic disease, TB, Prostatic hypertrophy, narrow angle glaucoma, hypersensitivity to test drugs. |
|
| Interventions | 1) 40 mcg ipratropium, 4 times daily. 2) 1500 mcg metaproterenol 4 times daily. Medication was delivered by MDI, for 85 days. | |
| Outcomes | Outcomes measured on Days 1, 45 and 90: 1) pulmonary function i.e. Test Day baseline FEV1, FVC. Peak change in FEV1, FVC from test day baseline. % change in FEV1, FVC from test day baseline. FEV1, FVC AUC (0‐6 hr). 2) Symptoms (wheeze, dyspnoea, cough, chest tightness), medications were recorded in a daily diary card during run‐in period and study. 3) Global evaluations. 5) 12 lead ECG, BP. | |
| Notes | Subjects had to demonstrate significant reversible airflow obstruction with a bronchodilator (at least 15%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised; other information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Tashkin 1996.
| Methods | RCT: 85 day (two arm) parallel group inhalation solution study. Randomisation: computer generated Blinding: double blind Excluded: not described. Withdrawals: described. Baseline characteristics: comparable Power calculation: not given. Intention to treat analysis not stated. Jadad Score:5 | |
| Participants | Setting: USA, multi‐centre (8) study.
213 stable subjects with COPD.
mean age: 60.6 years
male : female= 67% : 33%
mean baseline FEV1: 1.07 Litres or 37.4% predicted.
range:0.36‐2.37 litres or 13‐64% predicted. Inclusion criteria: > or = 40 years of age, > or =10 year pack history of smoking, stable disease, FEV1 < or = 65% predicted, FEV1/FVC < or =70 %. Exclusion criteria: history of asthma, allergic rhinitis or atopy, total blood eosinophil count >500/mm3, recent MI (< 1 year), other significant disease, recent respiratory tract infection, narrow angle. |
|
| Interventions | 1) 0.5 mg ipratropium 0.167% inhalation solution (0.3 ml), plus 15 mg metaproterenol 0.6% inhalation solution (2.5 ml), three times daily. 2) placebo (0.3 ml diluent) plus 15 mg metaproterenol 0.6% inhalation solution (2.5 ml), three times daily. Medication was delivered by nebuliser, for 85 days. | |
| Outcomes | Outcomes measured on Days 1, 43 and 85: 1) pulmonary function i.e. Test Day baseline FEV1, FVC. 2) peak change in FEV1, FVC from test day baseline. 3) % change in FEV1, FVC from test day baseline. 4) FEV1, FVC AUC (0‐8 hr). 5) HRQL: Chronic Respiratory Disease Questionnaire (CRQ). Recorded in a daily diary card during run‐in period and study: 6) daily morning and evening PEFR. 7) COPD symptoms (wheeze, dyspnoea, cough, chest tightness). 8) additional medication use. | |
| Notes | Testing for reversible airflow obstruction with a bronchodilator was not required. Unpublished standard errors obtained from Boehringer Ingelheim and converted to standard deviations. Study quality details provided by Boehringer Ingelheim. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Third party, off site. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bauer 1975 | Comparison of ipratropium bromide and placebo. |
| Brown 1984 | Time course study over 5 hours on three separate days. |
| Dejaegher 1984 | Time course study over seven hours, conducted on two days, in patients with reversible airways obstruction. |
| Disse 1999 | Review article. |
| Heimer 1991 | Time course study over one hour. |
| Hidalgo 1983 | Time course study on one day. |
| Kheir 1993 | Time course study over six hours, on three days. |
| Khristoliubova 1999 | Not a randomised controlled trial |
| Lees 1980 | Time course study on three separate days. |
| Leitch 1978 | Short duration study. |
| Lien 1980 | Comparison of ipratropium bromide and placebo. |
| Matera 1996 | Time course study over 12 hours on four separate days. |
| Nardini 1996 | Review |
| Nishimura 1992 | Treatment given for two weeks only. |
| Petro 1981 | Short study over 4 weeks not randomised or blinded and measured airways resistance only. Long term study of fenoterol/ipratropium combination over twelve months was uncontrolled, i.e. pre‐post study design. |
| Pierce 1982 | Study conducted in subjects with reversible airways disease due to asthma. |
| Shmelev 1999 | Not a randomised controlled trial |
| Simanenkov 1998 | Not a randomised controlled trial |
| Tang 1984 | Study conducted in subjects with reversible airways obstruction on three consecutive days. |
Contributions of authors
SA: Second draft of protocol, study assessment, data extraction, first draft of review, manuscript revisions LP: Protocol draft review, study assessment, data extraction, manuscript review TJ: Protocol draft review, study assessment, data extraction, manuscript review BA: Manuscript review BS: Protocol draft review, study assessment PP: Review editing JM: First draft of protocol TL: Write‐up and manuscript revisions
Sources of support
Internal sources
No sources of support supplied
External sources
Nederlands Astma Fonds, Netherlands.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Campbell 1999 {published data only}
- Campbell S. For COPD a combination of ipratropium bromide and albuterol sulfate is more effective than albuterol base. Archives Internal Medicine 1999;159(Jan 25):156‐60. [DOI] [PubMed] [Google Scholar]
Colice 1996 {published and unpublished data}
- Brown D, et al. A randomized, double blind, parallel, multi‐centre comparison of inhalation solution with albuterol inhalation solution following single‐dose and chronic administration (85 days) in patients with chronic obstructive pulmonary disease. Boehringer Ingelheim unpublished report USA U91‐0865 1991.
- Colice GL. Nebulized bronchodilators for outpatient management of stable chronic obstructive pulmonary disease. The American Journal of Medicine 1996;100(1A):11S‐18S. [DOI] [PubMed] [Google Scholar]
- Rennard SI, Serby CW, Ghafouri M, Johnson PA, Friedman M. Extended therapy with ipratropium is associated with improved lung function in patients with COPD. A retrospective analysis of data from seven clinical trials. Chest 1996;110:62‐70. [DOI] [PubMed] [Google Scholar]
Combivent 1994 {published and unpublished data}
- "COMBIVENT" Inhalation Aerosol Study Group. In chronic obstructive pulmonary disease, a combination of ipratropium and albuterol is more effective than either agent alone. Chest 1994;105(5):1411‐9. [DOI] [PubMed] [Google Scholar]
- Alexander KM, et al. A randomized, double blind, parallel, multi‐centre comparison of Combivent (ipratropium bromide and albuterol sulfate) inhalation aerosol with its components following single‐dose and chronic administration (85 days) in patients with chronic obstructive pulmonary disease. Boehringer Ingelheim unpublished report: USA U92‐0462. 1992.
Combivent 1997 {published data only}
- Alexander KM, et al. A randomized, double blind, parallel, multi‐centre comparison of Combivent (ipratropium bromide and albuterol sulfate) inhalation solution with its components following single‐dose and chronic administration (85 days) in patients with chronic obstructive pulmonary disease. Boehringer Ingelheim unpublished report: USA U92‐0801 1992.
- Rennard SI, Serby CW, Ghafouri M, Johnson PA, Friedman M. Extended therapy with ipratropium is associated with improved lung function in patients with COPD. A retrospective analysis of data from seven clinical trials. Chest 1996;110:62‐70. [DOI] [PubMed] [Google Scholar]
- The COMBIVENT Inhalation Study Group. Routine nebulized ipratropium and albuterol together are better than either alone in COPD. Chest 1997;112(6):1514‐21. [DOI] [PubMed] [Google Scholar]
Friedman 1996 {published and unpublished data}
- Brown D, et al. A randomized, double blind, parallel, multi‐centre comparison of Atrovent (ipratropium bromide) inhalation solution with metaproterenol inhalation solution following single‐dose and chronic administration (85 days) in patients with chronic obstructive pulmonary disease. Boehringer Ingelheim unpublished report USA U91‐0866. 1991.
- Friedman M. A multicenter study of nebulized bronchodilator solutions in chronic obstructive pulmonary disease. The American Journal of Medicine 1996;100(1A):30s‐39s. [DOI] [PubMed] [Google Scholar]
Gross 1998 {published data only}
- Gross N, Tashkin D, Miller R, Oren J, Coleman W, Linberg S, et al. Inhalation by nebulization of albuterol‐ipratropium combination (Dey Combination) is superior to either agent alone in the treatment of chronic obstructive pulmonary disease. Respiration 1998;65:354‐62. [DOI] [PubMed] [Google Scholar]
Hodzhev 1999 {published data only}
- Hodzhev B, Kostianev S, Todorov I, Belev G, Kartev S. Individual results of treatment of COPD with low doses of fenoterol compared with treatment with ipratropium bromide. Folia Med (Plovdiv) 1999;41:46‐52. [PubMed] [Google Scholar]
Levin 1996 {published and unpublished data}
- Levin DC, Little KS, Laughlin KR, Galbraith JM, Gustman PM, et al. Addition of anticholinergic solution prolongs bronchodilator effect of Beta2 agonists in patients with chronic obstructive pulmonary disease. The American Journal of Medicine 1996;100(1A):40s‐48s. [DOI] [PubMed] [Google Scholar]
Rennard 1996 {published and unpublished data}
- Rennard SI, Serby CW, Ghafouri M, Johnson PA, Friedman M. Extended therapy with ipratropium is associated with improved lung function in patients with COPD. A retrospective analysis of data from seven clinical trials. Chest 1996;110:62‐70. [DOI] [PubMed] [Google Scholar]
Tashkin 1986 {published data only}
- Tashkin DP, Ashutosh K, Bleeker ER, Britt EJ, Cugell DW, Cumminsky JM, et al. Comparison of the anticholinergic bronchodilator ipratropium bromide with metaproterenol in chronic obstructive pulmonary disease. A 90‐Day Multi‐Centre Study. The American Journal of Medicine 1986;81(Suppl 5A):81‐90. [DOI] [PubMed] [Google Scholar]
Tashkin 1996 {published and unpublished data}
- Tashkin DP, Bleeker E, Braun S, Campbell S, DeGraff AC, Hudgell DW, et al. Results of a multicentre study of nebulised inhalant bronchodilator solutions. The American Journal of Medicine 1996;100(1A):62s‐69s. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bauer 1975 {published data only}
- Bauer VP, Kummer F. The action of a tropic acid ester on bronchospasm:A double‐blind study [Zur wirkung eines tropasaure‐esters auf den bronchospasmus]. Wiener Klinische Wochenschrift 1975;87(4):132‐6. [PubMed] [Google Scholar]
Brown 1984 {published data only}
- Brown IG, Chan CS, Kelly CA, Dent AG, Zimmerman PV. Assessment of the clinical usefulness of nebulised ipratropium bromide in patients with chronic airflow limitation. Thorax 1984;39:272‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dejaegher 1984 {published data only}
- Dejaegher Ph, Demedts M, Rochette F, Veken J. Effects of 40ug and 400ug of ipratropium bromide in obstructive lung disease. Acta Clinica Belgica 1984;39(3):136‐40. [DOI] [PubMed] [Google Scholar]
Disse 1999 {published data only}
- Disse B, Speck GA, Rominger KL, Witek TJ, Hammer R. Tiotropium (SPIRIVA): Mechanistical considerations and clinical profile in obstructive lung disease. Life Sciences 1999;64(6/7):457‐64. [DOI] [PubMed] [Google Scholar]
Heimer 1991 {published data only}
- Heimer D, Brami JL, Liberman D, Bark H. Comparison of a B2 adrenergic agonist and an anticholinergic agent given by sequential inhalation in patients with severe chronic obstructive pulmonary disease. Israel Journal of Medical Science 1991;27:307‐10. [PubMed] [Google Scholar]
Hidalgo 1983 {published data only}
- Banos Hidalgo P, Ramos Martos A, Cabrera Moreno R, Luque Leal J, Palcios Giner YA. Comparacion en estudio doble ciego de la asociacion de un aerosol de salbutamol y bromuro de ipratropio y de su efecto individual en bronquitis cronica y asma bronquial. Revista Clinica Espanola 1983;171(4):265‐8. [PubMed] [Google Scholar]
Kheir 1993 {published data only}
- Kheir A, Ying Y, Hannhart B, Duvivier C, Peslin R, Polu JM. Comparison de l'effet bronchodilatateur at du site d'action du fenoterol et de l'ipratropium chez bronchiteux chroniques obstructifs [A comparison of the bronchodilator effect and the site of action of fenoterol and of ipratropium in bronchitis with airflow obstruction]. Revue des Maladies Respiratoires 1993;10:9‐15. [PubMed] [Google Scholar]
Khristoliubova 1999 {published data only}
- Khristoliubova EI, Volkova LI, Shcherbakova IV. Long‐term atrovent treatment of chronic obstructive bronchitis. Klinicheskaia Meditsina (Mosk) 1999;77(12):51‐2. [PubMed] [Google Scholar]
Lees 1980 {published data only}
- Lees AW, Allan GW, Smith J. Nebulised ipratropium bromide and salbutamol in chronic bronchitis. British Journal of Clinical Practice 1980;34(11‐12):340‐2. [PubMed] [Google Scholar]
Leitch 1978 {published data only}
- Leitch AG, Hopkins JM, Ellis DA, Merchant S, McHardy GJR. The effect of aerosol ipratropium bromide and salbutamol on exercise tolerance in chronic bronchitis. Thorax 1978;33:711‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lien 1980 {published data only}
- Lien J Th. Klinisk sammenligning av Atrovent og placebo hos pasienter med kronisk ostruktiv lungesykdom. Idsskr Nor Loegeforen 1980;100:1193‐5. [PubMed] [Google Scholar]
Matera 1996 {published data only}
- Matera MG, Caputi M, Cazzola M. A combination with clinical recommended dosages of salmeterol and ipratropium is not more effective than salmeterol alone in patients with chronic obstructive pulmonary disease. Respiratory Medicine 1996;90:497‐9. [DOI] [PubMed] [Google Scholar]
Nardini 1996 {published data only}
- Nardini S. Inhaled antimuscarinic agents and COPD. Monaldi Archives for Chest Disease 1996;51(1):52‐3. [PubMed] [Google Scholar]
Nishimura 1992 {published data only}
- Nishimura K, Koyama H, Izumi T. A comparison of bronchodilating drugs for the treatment of stable COPD. Nihon Hyobu Shikkan Gakkai Zasshi 1992;30(5):835‐43. [PubMed] [Google Scholar]
Petro 1981 {published data only}
- Petro W, Nakhosteen JA, Konietzko N. Bronchodilator effect of a combination of a beta‐adrenergic agonist and a cholinergic antagonist [Broncholyse unter kombinierter anwendung von Beta‐adrenergen agonisten und cholinergen antagonisten]. Prax Pneumol 1981;35:779‐82. [Google Scholar]
Pierce 1982 {published data only}
- Pierce RJ, Holmes PW, Campbell AH. Use of ipratropium bromide in pateints with severe airways obstruction. Australian and New Zealand Journal of Medicine 1982;12:38‐43. [DOI] [PubMed] [Google Scholar]
Shmelev 1999 {published data only}
- Shmelev EI, Khmel'kova NG, Nonikov DV, Melent'eva EM, Abubikirov AF, Makarova VL, et al. Experience in long‐term use of berodual in the treatment of patients with chronic obstructive bronchitis. Terapevticheskii Arkhiv 1999;71(3):22‐4. [PubMed] [Google Scholar]
Simanenkov 1998 {published data only}
- Simanenkov VI, Il'iashevich IG, Liparteliani BG, Ledovaia AV. Long‐term atrovent treatment of chronic obstructive bronchitis [Dlitel'noe lechenie atroventom pri khronicheskom obstruktivnom bronkhite]. Terapevticheskii Arkhiv 1998;70(9):77‐9. [PubMed] [Google Scholar]
Tang 1984 {published data only}
- Tang OT, Flatley M. A comparison of effects of inhaling a combined preparation of fenoterol with ipratropium bromide (Duovent) with those of fenoterol and salbutamol. Postgraduate Medical Journal 1984;60:24‐7. [PubMed] [Google Scholar]
Additional references
Anthonisen 1986
- Anthonisen NR, Wright EC. Bronchodilator response in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1986;133:814‐9. [PubMed] [Google Scholar]
Anthonisen 1994
- Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: The Lung Health Study. JAMA 1994;272:1497‐505. [PubMed] [Google Scholar]
Appleton 1999
- Appleton S, Smith B, Veale A, Bara A. Regular long‐acting beta‐2 adrenoceptor agonists for chronic obstructive pulmonary disease (Cochrane review). The Cochrane Database of Systematic Reviews 1999, Issue 4. [Google Scholar]
Appleton 2006
- Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, et al. Ipratropium bromide versus long‐acting beta‐2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 3. [Art. No.: CD006101. DOI: 10.1002/14651858.CD006101] [DOI] [PMC free article] [PubMed] [Google Scholar]
ATS 1995
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1995;152:s78‐s121. [PubMed] [Google Scholar]
ATS/ERS 2004
- Celli BR, MacNee W, ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. European Respiratory Journal 2004;23:932‐46. [DOI] [PubMed] [Google Scholar]
Barnes 1993
- Barnes PJ. Theoretical aspects of anticholinergic treatment. In: Gross NJ editor(s). Anticholinergic therapy in obstructive airways disease. London: Franlkin Scientific Publications, 1993. [Google Scholar]
Barr 2005
- Barr RG, Bourbeau J, Camargo CA, Ram FS. Tiotropium for stable chronic obstructive pulmonary disease (Cochrane review). Cochrane Database of Systematic Reviews 2005, Issue 2. [Art. No.: CD002876. DOI: 10.1002/14651858.CD002876.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Belman 1996
- Belman M, Botnick W, Shin J. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1996;153:967‐75. [DOI] [PubMed] [Google Scholar]
Blosser 1995
- Blosser SA, Maxwell SL, Reeves‐Hoche MK, Localio AR, Zwillich CW. Is an anti‐cholinergic agent superior to a beta‐2 agonist in improving dyspnea and exercise limitation in COPD?. Chest 1995;108:730‐5. [DOI] [PubMed] [Google Scholar]
Boyd 1997
- Boyd G, Morice AH, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1997;10:815‐21. [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society. Chronic Obstructive Pulmonary Disease Guidelines. Thorax 1997;52(Suppl 5):S2‐S28. [Google Scholar]
Calverly 2003
- Calverly PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003;58:659‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chapman 1991
- Chapman KR. Clinical implications of anticholinergic bronchodilator therapy in COPD. Res Clin Forums 1991;13(2):43‐50. [Google Scholar]
Cwealth of Aust 2000
- Commonwealth of Australia. Schedule of Pharmaceutical Benefits Effective from May 2001; GENERAL Respiratory system. http://www.health.gov.au/pbs/scripts/dispgp.cfm 2000.
D'Urzo 2001
- D'Urzo A, Salvo M, Ramirez‐Rivera A, Almeida J, Sichletidis L, Rapatz G, Kottakis J. In patients with COPD, treatment with a combination of formoterol and ipratropium is more effective than a combination of salbutamol and ipratropium: A 3 week, randomized, double‐blind, with‐in patient, multicentre study. Chest 2001;119:1347‐56. [DOI] [PubMed] [Google Scholar]
Ferguson 2000
- Ferguson G. Recommendations for the management of COPD. Chest 2000;117(2 Suppl):23s‐28s. [DOI] [PubMed] [Google Scholar]
Friedman 1999
- Friedman M, Serby C, Menjoge S, Wilson D, Hilleman D, Witek T. Pharmacoeconomic evaluation of a combination of ipratropium plus albuterol compared with ipratropium alone and albuterol alone. Chest 1999;115:635‐41. [DOI] [PubMed] [Google Scholar]
GOLD 2003
- National Heart, Lung, and Blood Institute. [Global Initiative on Obstructive Lung Disease (GOLD) Pocket Guide (updated 2003)]. www.goldcopd.com [accessed 16/02/04].
GOLD 2004
- National Heart, Lung, and Blood Institute. Global Initiative for Chronic Obstructive Pulmonary Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2004). www.goldcopd.com [accessed 21/03/05].
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomised controlled trials: Is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Lipworth 1989
- Lipworth B, Tregaskis B, McDevitt D. Beta‐adrenoceptor responses to inhaled salbutamol in the elderly. British Journal of Pharmacology 1989;28:725‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahler 2002
- Mahler DA, Wire P, Horstman D, Chang CN, Yates J, Fischer T, Shah T. Effectiveness of fluticasone propionate and salmeterol combination delivered via the Diskus device in the treatment of chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2002;166:1084‐91. [DOI] [PubMed] [Google Scholar]
Matera 1995
- Matera MG, Cazzola M, Vinciguerraaa A, et al. Comparison of the bronchodilating effects of salmeterol, salbutamol and ipratropium bromide in patients with chronic obstructive pulmonary disease. Pulmonary Pharmacology 1995;8:267‐71. [DOI] [PubMed] [Google Scholar]
NICE/BTS 2004
- National Institute for Clinical Excellence (NICE). Chronic obstructive pulmonary disease: national clinical guideline for management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004; Vol. 59, issue Suppl I. [PMC free article] [PubMed]
Nisar 1992
- Nisar M, Earis JE, Pearson MG, Calverly PMA. Acute bronchodilator trials in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1992;146:555‐9. [DOI] [PubMed] [Google Scholar]
O'Donnell 1997
- O'Donnell D, Bertley J, Chau L, Webb K. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiological mechanisms. American Journal of Respiratory & Critical Care Medicine 1997;155:109‐15. [DOI] [PubMed] [Google Scholar]
O'Donnell 1998
- O'Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation and endurance during exercise in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1998;158:1557‐65. [DOI] [PubMed] [Google Scholar]
O'Donnell 1999
- O' Donnell D, Lam M, Webb K. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1999;160:542‐9. [DOI] [PubMed] [Google Scholar]
O'Donnell 2000
- O' Donnell D. Assessment of bronchodilator efficacy in symptomatic COPD. Is spirometry useful?. Chest 2000;117(2 Suppl):42s‐47s. [DOI] [PubMed] [Google Scholar]
Pauwels 2000
- Pauwels R. National and international guidelines for COPD: The need for evidence. Chest 2000;117(2 Suppl):20s‐22s. [DOI] [PubMed] [Google Scholar]
Redelmeier 1996
- Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. Journal of Clinical Epidemiology 1996;49(11):1215‐19. [DOI] [PubMed] [Google Scholar]
Rossi 2002
- Rossi A, Kristufek P, Levine BE, Thomson MH, Till D, Kottakis J, et al. Comparison of the efficacy, tolerability, and safety of formoterol dry powder and oral, slow‐release theophylline in the treatment of COPD. Chest 2002;121:1058‐69. [DOI] [PubMed] [Google Scholar]
Snider 1985
- Snider GL, Klenerman J, Thurlbeck WM, Bengali ZH. The definition of emphysema: Report of a National Heart and Blood Institute, Division of Lung Disease, Workshop. American Review of Respiratory Disease 1985;132:182‐5. [DOI] [PubMed] [Google Scholar]
Spencer 2004
- Spencer S, Calverley PM, Burge PS, Jones PW. Impact of preventing exacerbations on deterioration of health status in COPD. European Respiratory Journal 2004;23(5):698‐702. [DOI] [PubMed] [Google Scholar]
Tashkin 2003
- Tashkin D, Kesten S. Long‐term treatment benefits with tiotropium in COPD patients with and without short‐term bronchodilator responses. Chest 2003;123:1441‐49. [DOI] [PubMed] [Google Scholar]
Ullah 1981
- Ullah MI, Newman GB, Saunders KB. Influence of age in response to ipratropium and salbutamol in asthma. Thorax 1981;36:523‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ulrik 1995
- Ulrik C. Efficacy of inhaled salmeterol in the management of smokers with chronic obstructive pulmonary disease: a single centre, randomised, double blind, placebo controlled, cross over study. Thorax 1995;50:750‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Schayck 1991
- Schayck CP, Folgering H, Harbers H, et al. Effects of allergy and age on responses to salbutamol and ipratropium bromide in moderate asthma and chronic bronchitis. Thorax 1991;46:355‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Weel 1998
- Weel C. Combining nebulised ipratropium and albuterol improved FEV1 but did not improve symptoms or quality of life in COPD. Evidence‐Based Medicine 1998:120. [Google Scholar]


