Abstract
Background
Dietary antioxidants, such as vitamin C, in the epithelial lining and lining fluids of the lung may be beneficial in the reduction of oxidative damage (Arab 2002). They may therefore be of benefit in reducing symptoms of inflammatory airway conditions such as asthma, and may also be beneficial in reducing exercise‐induced bronchoconstriction, which is a well‐recognised feature of asthma and is considered a marker of airways inflammation. However, the association between dietary antioxidants and asthma severity or exercise‐induced bronchoconstriction is not fully understood.
Objectives
To examine the effects of vitamin C supplementation on exacerbations and health‐related quality of life (HRQL) in adults and children with asthma or exercise‐induced bronchoconstriction compared to placebo or no vitamin C.
Search methods
We identified trials from the Cochrane Airways Group's Specialised Register (CAGR). The Register contains trial reports identified through systematic searches of a number of bibliographic databases, and handsearching of journals and meeting abstracts. We also searched trial registry websites. The searches were conducted in December 2012.
Selection criteria
We included randomised controlled trials (RCTs). We included both adults and children with a diagnosis of asthma. In separate analyses we considered trials with a diagnosis of exercise‐induced bronchoconstriction (or exercise‐induced asthma). We included trials comparing vitamin C supplementation with placebo, or vitamin C supplementation with no supplementation. We included trials where the asthma management of both treatment and control groups provided similar background therapy. The primary focus of the review is on daily vitamin C supplementation to prevent exacerbations and improve HRQL. The short‐term use of vitamin C at the time of exacerbations or for cold symptoms in people with asthma are outside the scope of this review.
Data collection and analysis
Two review authors independently screened the titles and abstracts of potential studies, and subsequently screened full text study reports for inclusion. We used standard methods expected by The Cochrane Collaboration.
Main results
A total of 11 trials with 419 participants met our inclusion criteria. In 10 studies the participants were adults and only one was in children. Reporting of study design was inadequate to determine risk of bias for most of the studies and poor availability of data for our key outcomes may indicate some selective outcome reporting. Four studies were parallel‐group and the remainder were cross‐over studies. Eight studies included people with asthma and three studies included 40 participants with exercise‐induced asthma. Five studies reported results using single‐dose regimes prior to bronchial challenges or exercise tests. There was marked heterogeneity in vitamin C dosage regimes used in the selected studies, compounding the difficulties in carrying out meaningful analyses.
One study on 201 adults with asthma reported no significant difference in our primary outcome, health‐related quality of life (HRQL), and overall the quality of this evidence was low. There were no data available to evaluate the effects of vitamin C supplementation on our other primary outcome, exacerbations in adults. One small study reported data on asthma exacerbations in children and there were no exacerbations in either the vitamin C or placebo groups (very low quality evidence). In another study conducted in 41 adults, exacerbations were not defined according to our criteria and the data were not available in a format suitable for evaluation by our methods. Lung function and symptoms data were contributed by single studies. We rated the quality of this evidence as moderate, but further research is required to assess any clinical implications that may be related to the changes in these parameters. In each of these outcomes there was no significant difference between vitamin C and placebo. No adverse events at all were reported; again this is very low quality evidence.
Studies in exercise‐induced bronchoconstriction suggested some improvement in lung function measures with vitamin C supplementation, but theses studies were few and very small, with limited data and we judged the quality of the evidence to be low.
Authors' conclusions
Currently, evidence is not available to provide a robust assessment on the use of vitamin C in the management of asthma or exercise‐induced bronchoconstriction. Further research is very likely to have an important impact on our confidence in the estimates of effect and is likely to change the estimates. There is no indication currently that vitamin C can be recommended as a therapeutic agent in asthma. There was some indication that vitamin C was helpful in exercise‐induced breathlessness in terms of lung function and symptoms; however, as these findings were provided only by small studies they are inconclusive. Most published studies to date are too small and inconsistent to provide guidance. Well‐designed trials with good quality clinical endpoints, such as exacerbation rates and health‐related quality of life scores, are required.
Plain language summary
Vitamin C for asthma and exercise‐induced breathlessness
Review question
This review considered the question of whether vitamin C may be helpful for people with asthma or exercise‐induced breathlessness.
Background
Asthma is an inflammatory lung condition characterised by the narrowing of airways and is associated with wheezing, breathlessness, cough and chest tightness. Vitamin C has been suggested as a possible treatment for asthma.
Study characteristics
Eleven studies on 419 people with asthma or exercise‐induced breathlessness were included in this review comparing vitamin C compared to placebo (no vitamin C). Most studies were in adults and one small study was in children. The small number of studies available for review and their different designs meant that we were only able to describe individual studies, rather than pooling the results together to get an average from the trials. The study design was not well described in most study reports and therefore it was impossible to determine risk of bias for most of the studies. There was very little data available in the trials for our key outcomes and this may indicate some selective outcome reporting.
Key results
There was no indication of benefit from the studies that considered vitamin C in relation to asthma. However, it is not possible to form any clear conclusions on the basis of those studies at this stage. The review concludes that there is insufficient evidence currently available to evaluate the use of vitamin C as a treatment in asthma. Larger, well‐designed research is needed to provide clearer guidance. There was some indication that vitamin C was helpful in exercise‐induced breathlessness in terms of how easily people breathe and their symptoms; however, as these findings were provided by only very small studies they do not provide complete answers to guide treatment.
Quality of the evidence
Details of the way patients were allocated to receive vitamin C or not were not clearly described in 10 of the 11 studies and we considered this carefully in the review in relation to our level of uncertainty in interpreting the results. Taking this into account, together with the imprecision of the results, we judged the estimates of the usefulness of vitamin C as a treatment to be of either low or moderate quality in relation to asthma.
Additionally, for exercise‐induced breathlessness the three studies providing data to the review were small and we are mindful of the need to draw very cautious conclusions about the results.
This plain language summary is current as of December 2012.
Summary of findings
Summary of findings for the main comparison. Summary of findings: Vitamin C supplementation compared with placebo for asthma.
| Vitamin C supplementation compared with placebo for asthma | ||||
|
Patient or population: adults and children with asthma Settings: community Intervention: vitamin C supplementation Comparison: placebo | ||||
| Outcomes | Analyses in study report | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Health‐related quality of life (HRQL) | There was no evidence of a difference in HRQL between the groups after 16 weeks treatment as measured by the available SF‐36 data | 201 adults (1 study) | ⊕⊕⊝⊝ low 1, 2 | Authors' note: "SF‐36 data on physical functioning were incomplete and this section was excluded from analysis" |
| Asthma exacerbations | No exacerbations in either group | 16 children (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| Lung function: FEV1 (ml) (change from 0 to 16 weeks) | MD ‐11 (95% CI ‐92 to 70) | 201 adults (1 study) | ⊕⊕⊕⊝ moderate1 | The 95% CI excludes a clinically important mean difference in FEV1, so the outcome was not downgraded for imprecision |
| Asthma symptoms (change from 0 to 16 weeks) | MD 0 (95% CI ‐0.2 to 0.1) | 201 adults (1 study) | ⊕⊕⊕⊝ moderate1 | |
| Adverse events | No adverse events in either group | 41 adults (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. FEV1: forced expiratory volume in one second; MD: mean difference | ||||
1One point deducted to reflect selective reporting as data reported by only one trial. 2One point deducted because results were not available from all components of the quality of life instrument which reduces our confidence in this result. 3We deducted two points for imprecision and unclear randomisation.
Summary of findings 2. Summary of findings: Vitamin C supplementation compared with placebo for exercise ‐induced bronchoconstriction/asthma.
| Vitamin C supplementation compared with placebo for exercise‐induced bronchoconstriction/asthma | ||||
|
Patient or population: adults and children with exercise‐induced bronchoconstriction/asthma Settings: community Intervention: vitamin C supplementation Comparison: placebo | ||||
| Outcomes | Analyses in study report | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Health‐related quality of life (HRQL) | See comment | See comment | See comment | No between‐group data reported |
| Asthma exacerbations | See comment | See comment | See comment | Not reported |
|
Lung function: FEV1 % drop Postexercise |
Reported that the maximum percentage drop in FEV1 postexercise on the vitamin C diet was ‐6.4% (95% CI ‐12.0% to ‐0.8%; the effect size using omega‐squared (ES) was 0.40) which is indicative of an attenuated EIB response. This was significantly different (P < 0.05) from the maximum drop of ‐12.9% (95% CI ‐18.6% to ‐12.3%) on placebo | 8 adults (1 study) | ⊕⊕⊝⊝ low1,2 | Cross‐over study |
| Asthma symptoms | Reported that a significant improvement (P < 0.05) in mean asthma symptom scores was observed (6.3; 95% CI 5.8 to 6.8) on the vitamin C diet compared to the placebo diet (5.8; 95% CI 5.1 to 6.2) | 8 adults (1 study) | ⊕⊕⊝⊝ low1,2 | Cross‐over study |
| Adverse events | See comment | See comment | See comment | Not reported |
| GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate. CI: confidence interval; EIB: exercise‐induced bronchoconstriction; FEV1: forced expiratory volume in one second | ||||
1One point deducted for selective reporting as data provided by only one small trial. 2One point deducted to reflect the 'Risk of bias' assessment for this trial (unclear randomisation).
Background
Description of the condition
Asthma is a chronic inflammatory airways disease that is associated with episodic symptoms of breathlessness, wheeze and chest tightness. The spectrum of severity of this condition is wide, ranging from no or minimal symptoms to it being disabling or life‐threatening. It can affect all age groups although it often starts in children. It is estimated that 300 million people suffer from asthma worldwide and it is predicted that this number will increase to 400 million by 2025 (WHO 2007). Between 2001 and 2009 the number of people in the US with asthma increased by five million, from 20 to 25 million (CDC 2012; CDCP 2011); during 2008 to 2010 there were higher asthma prevalence rates among Alaska Native (9.4%), American Indian (also 9.4%), black (11.2%) and multiple‐race (14.1%) people than white people (7.7%), and for Asian people the rate was 5.2% (CDCP 2011). Prevalence rates are slightly higher among children (10%) than adults (8%) in the US (CDCP 2011).
There are quality of life (Clayton 2005) and financial (Wu 2007) implications for many people with asthma. Over 65,000 hospital admissions for asthma were recorded In the period between 2005 and 2006 in the UK (NHS 2011) and in the US approximately 10 million people experience asthma exacerbations each year (Krishnan 2006).
National (e.g. BTS/SIGN 2011; NIH 2007) and international (e.g. GINA 2011) guidelines have been produced for the management of asthma.
Exercise‐induced bronchoconstriction describes narrowing of airways during or following exercise and is associated with exercise‐induced symptoms of breathlessness, wheeze and cough. It is a common symptom in asthma and is a marker of the presence of airways inflammation. However, up to 20% of individuals with exercise‐induced bronchoconstriction do not have a diagnosis of asthma (Parsons 2013) and therefore may represent a discreet clinical entity.
Description of the intervention
There is some evidence that a lower level of fruit consumption is associated with paediatric wheezing (Chatzi 2007; Okoko 2007). Benefits of fruit intake to adults with asthma have also been reported, suggesting that this aspect of diet may be a modifiable risk factor for asthma symptoms (Patel 2006).
In the UK consumption of fresh fruit and vegetables declined between the 1950s and early 1990s (Seaton 1994). In the US fruit and vegetable consumption remained at the same level between 1994 and 2005 (Blanck 2008), and there are numerous recent examples of national initiatives to promote consumption of fresh fruit and vegetables both in Europe (EUFIC 2012) and worldwide (WHO 2012). It has been hypothesised that this reduction in fruit intake in a 'western diet' is associated with the increase in prevalence of asthma and its severity in the developed countries (Misso 2005; Patel 2006). This association may be related to a reduction in intake of the dietary antioxidants, including vitamin C, and hence vitamin C supplementation may ameliorate some of the symptoms of asthma.
How the intervention might work
Vitamin C is a recognised dietary antioxidant and it has been suggested that dietary antioxidants in the epithelial lining and lining fluids of the lung may be beneficial in the reduction of oxidative damage (Arab 2002). A reduction in the intake of naturally occurring antioxidants, such as vitamin C, may result in greater exposure to reactive oxygen species and hence result in inflammation. This may be implicated in the aetiology of asthma or in its severity. Misso 2005 reported particularly low vitamin C intake in males with severe asthma, and noted the need for more research to assess the benefits of vitamin C supplementation in patients with severe asthma. However, the association of dietary antioxidants with asthma severity is not fully understood. Misso 2005 noted, for example, that whilst there was some indication of an association between vitamin C intake and asthma severity in men, data from women, who had a significantly higher vitamin C intake than men in the sample, did not indicate a similar pattern. Alternative mechanisms for the potential action of vitamin C in asthma are its effects on the arachidonic acid pathway (Cohen 1997) or its antiviral properties (Anah 1980).
Why it is important to do this review
This review aims to establish whether vitamin C supplementation may have a positive role in the management of asthma in children and adults.
Objectives
To examine the effects of vitamin C supplementation on exacerbations and health‐related quality of life (HRQL) in adults and children with asthma or exercise‐induced bronchoconstriction compared to placebo or no vitamin C.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We planned to include studies reported in full text, those published as an abstract only and also unpublished data.
Types of participants
We included both adults and children with a diagnosis of asthma. In separate analyses we considered trials with a diagnosis of exercise‐induced bronchoconstriction (or exercise‐induced asthma).
Types of interventions
We included trials comparing vitamin C supplementation with placebo, or vitamin C supplementation with no supplementation. The primary focus of the review is on daily vitamin C supplementation to prevent exacerbations and improve HRQL. The short‐term use of vitamin C at the time of exacerbations or for cold symptoms in people with asthma are outside the scope of this review and we planned to exclude clinical trials addressing these issues if they were identified in our searches.
Types of outcome measures
Primary outcomes
Health‐related quality of life.
Asthma exacerbation as defined by hospital admissions or treatment with a course of oral corticosteroids.
Secondary outcomes
Measures of lung function: forced expiratory flow in one second (FEV1), peak expiratory flow rate (PEFR) including changes related to exercise.
Asthma symptoms.
Adverse events reported to be related to vitamin C supplementation.
Reporting one or more of the outcomes listed here in the trial was not an inclusion criterion for the review.
In the separate analysis of patients with a diagnosis of exercise‐induced bronchoconstriction (or exercise‐induced asthma) we focused on measures of lung function: FEV1, PEFR.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group's Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy in Appendix 2.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO trials portal (www.who.int/ictrp/en/) using search terms based on those in Appendix 2. We searched all databases from their inception up to December 2012, with no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references, and we searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed). We reported the date this was done within the review.
Data collection and analysis
Selection of studies
Two review authors (SJM and AH) independently screened the titles and abstracts of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We included trials where the asthma management of both treatment and control groups provided similar background therapy. We retrieved the full‐text study reports/publication and two review authors (SJM and AH) independently screened the full text, identified studies for inclusion and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion and consulted a third member of the author team (MW). We identified and excluded duplicates and collated multiple reports of the same study so that each included study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and 'Characteristics of excluded studies' table.
1.
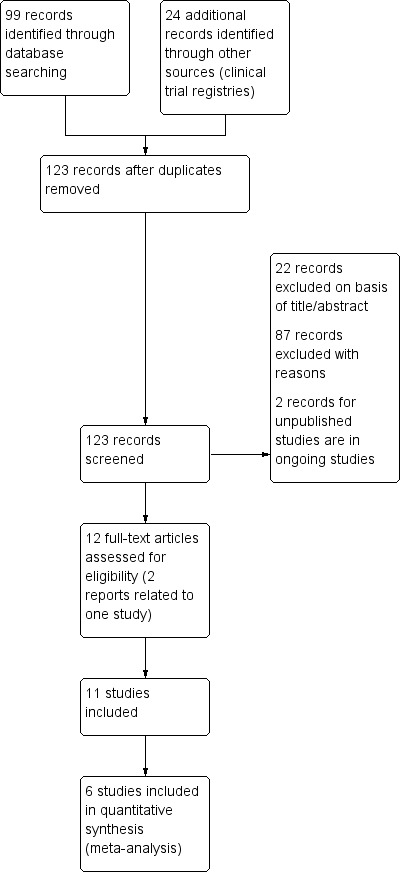
Study flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data which we piloted on one study in the review. Two review authors (SJM and AH) extracted study characteristics from the included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (SJM and AH) independently extracted outcome data from the included studies. We have noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus or by involving a third author (MW). One review author (SJM) transferred data into the Review Manager (RevMan) file. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (AH) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (SJM and AH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another author (MW). We assessed the risk of bias according to the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. We considered blinding separately for different key outcomes where necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to a published protocol and would have reported any deviations from it in the 'Differences between protocol and review' section of the systematic review.
Measures of treatment effect
We analysed dichotomous data as odds ratio and continuous data as mean difference or standardised mean difference. We entered data presented as a scale with a consistent direction of effect.
We undertook meta‐analyses only where this was meaningful, i.e. if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We planned to describe skewed data reported as medians and interquartile ranges narratively. The issue did not arise in the included trials.
Where multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (e.g. drug A versus placebo and drug B versus placebo) had been combined in the same meta‐analysis, we planned to halve the control group to avoid double‐counting. The issue did not arise in our evaluation of the included trials.
Unit of analysis issues
In all studies the unit of analysis was the patient. In repeated measures studies (cross‐over trials) we have reported within‐patient differences. As no statistical aggregation of studies was possible in this review we have reported results as presented in the trial reports.
Dealing with missing data
We planned to contact investigators or study sponsors in order to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as abstract only). Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis; however this issue did not arise.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. If we had identified substantial heterogeneity we planned to report it and explore possible causes by prespecified subgroup analysis. This was, however, not an issue as there were no opportunities to aggregate studies statistically.
Assessment of reporting biases
If we had been able to pool more than 10 trials, we would have created and examined a funnel plot to explore possible small study biases. However, this was not an issue in this review.
Data synthesis
We planned to use a fixed‐effect model and perform a sensitivity analysis with a random‐effects model. However, these concerns did not arise with the included studies. We planned to analyse dichotomous outcomes using odds ratio and continuous data as mean difference; however, there was no opportunity for statistical aggregation of studies and the results reported in the review were obtained directly from the trial reports.
'Summary of findings' table
We created two 'Summary of findings' tables using the following outcomes:
health‐related quality of life;
asthma exacerbation as defined by hospital admissions or treatment with a course of oral corticosteroids;
measures of lung function: FEV1, PEFR;
asthma symptoms;
adverse events.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it relates to the studies which contributed data to the review for the prespecified outcomes. We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the GRADEpro software. We have justified all decisions to downgrade or upgrade the quality of studies using footnotes and we have included comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses:
age (children under 12 years of age versus adults and adolescents);
severity (requirement for regular preventative treatment or not).
We planned to use the following outcomes in subgroup analyses:
health‐related quality of life;
asthma exacerbation as defined by hospital admissions or treatment with a course of oral corticosteroids;
measures of lung function: FEV1, PEFR;
asthma symptoms;
adverse events.
There was, however, no opportunity to pursue these planned analyses as studies could not be aggregated.
We planned to use the formal test for subgroup interactions in Review Manager (Review Manager (RevMan)) if the requirement to do so had arisen.
Sensitivity analysis
We planned to carry out the following sensitivity analyses:
study quality as defined by standard Cochrane 'Risk of bias' criteria;
use of random‐effects meta‐analysis instead of fixed‐effect.
However, the need to do this did not arise as it was not possible to combine data from different trials in any single analysis.
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of the included studies in this review. We avoided making recommendations for practice and our implications for research suggests priorities for future research and outlines uncertainties in the area.
Results
Description of studies
Results of the search
We identified 99 records through Cochrane Airways Group database searches and a further 24 from other sources. Further details are provided in Figure 1.
Included studies
Eleven studies met our inclusion criteria (Anah 1980; Anderson 1983; Cohen 1997; Fogarty 2003; Kordansky 1979; Malo 1986; O'Sullivan 2000; Nadi 2012; Schachter 1982; Schertling 1990; Tecklenburg 2007). Eight of these (Anah 1980; Anderson 1983; Fogarty 2003; Kordansky 1979; Malo 1986; O'Sullivan 2000; Nadi 2012; Schertling 1990) focused on asthma and three (Cohen 1997; Schachter 1982; Tecklenburg 2007) on exercise‐induced bronchoconstriction. In total there were 419 participants (379 for asthma and 40 for exercise‐induced bronchoconstriction).
In 10 studies the participants were adults and only one (Anderson 1983) focused on a paediatric group (16 children). Four of the 11 studies were parallel‐group in design (Anah 1980; Anderson 1983; Fogarty 2003; Nadi 2012) (318 participants in total), and the remainder (Kordansky 1979; Malo 1986; O'Sullivan 2000; Schachter 1982; Schertling 1990; Tecklenburg 2007) were cross‐over design studies (101 participants in total).
However, only six of the 11 studies on 338 participants provided data that could be included in our analyses (Anah 1980; Anderson 1983; Fogarty 2003; Nadi 2012; Schachter 1982; Tecklenburg 2007). The majority were parallel‐group in design (Anah 1980; Anderson 1983; Fogarty 2003; Nadi 2012 (318 participants in total)) and the remaining two cross‐over studies (Schachter 1982; Tecklenburg 2007) contributed eight and 12 participants respectively. These two cross‐over trials (Schachter 1982; Tecklenburg 2007) contributed to the exercise‐induced asthma/bronchoconstriction analyses reported in Table 3. The four parallel‐group studies contributed to the analysis of asthma outcomes reported in Table 4.
1. Outcomes: chronic asthma.
| Vitamin C supplementation versus control (asthma) | |||||
| Outcome | Studies | N | Age | Study ID | Study reported result |
| Health‐related quality of life | 1 | 201 participants randomised Vitamin C: 95 (72 completed (76%)) Placebo: 106 (82 completed (77%)) | Vitamin C mean: 42 years Placebo mean: 40 years | Fogarty 2003 | No significant difference in HRQL between the groups after 16 weeks treatment as measured by the available SF‐36 data* |
| Asthma exacerbations | 1 | 16 children Vitamin C: 7 No treatment: 9 | Vitamin C mean: 9.3 years (± 2.4) Control mean: 9.2 years (± 1.9) | Anderson 1983 | No exacerbations in either group |
| FEV1 (L) (at one month) | 1 | 60 participants Vitamin C: 30 Placebo: 30 | Vitamin C mean: 48.38 years (± 9.03) Placebo mean: 40.53 years (± 10.48) | Nadi 2012 | No direct comparison between vitamin C and placebo reported. Before versus after treatment comparison in vitamin C (P = 0.65) and in placebo (P = 0.044) |
| FEV1 (ml) (change from 0 to 16 weeks) | 1 | 201 participants randomised Vitamin C: 95 (72 completed (76%)) Placebo: 106 (82 completed (77%)) | Vitamin C mean: 42 years Placebo mean: 40 years | Fogarty 2003 | No significant difference between vitamin C and placebo: MD ‐11 (95% CI ‐92 to 70), P = 0.78 |
| FEV1 (% predicted) | 1 | 10 participants (cross‐over design) | Very limited details in trial report (conference abstract) | O'Sullivan 2000 | No significant change in FEV1 after vitamin C administration (95 ± 2.7% versus 94.2 ± 3.2%) |
| PEFR (L/min) (change from 0 to 16 weeks) AM | 1 | 201 participants randomised Vitamin C: 95 (72 completed (76%)) Placebo: 106 (82 completed (77%)) | Vitamin C mean: 42 years Placebo mean: 40 years | Fogarty 2003 | No significant difference between vitamin C and placebo: MD 0.9 (95% CI ‐11.8 to 13.5), P = 0.89 |
| PEFR (L/min) (change from 0 to 16 weeks) PM | 1 | 201 participants randomised Vitamin C: 95 (72 completed (76%)) Placebo: 106 (82 completed (77%)) | Vitamin C mean: 42 years Placebo mean: 40 years | Fogarty 2003 | No significant difference between vitamin C and placebo: MD 2.2 (95% CI ‐10.0 to 14.3), P = 0.73 |
| Asthma symptoms (change from 0 to 16 weeks) | 1 | 201 participants randomised Vitamin C: 95 (72 completed (76%)) Placebo: 106 (82 completed (77%)) | Vitamin C mean: 42 years Placebo mean: 40 years | Fogarty 2003 | No significant difference between vitamin C and placebo: MD 0 (95% CI ‐0.2 to 0.1), P = 0.33 |
| Adverse events | 1 | 41 participants Vitamin C: 22 Placebo: 19 | Vitamin C mean: 26.5 years (range 15 to 42) Placebo mean: 27.8 years (range 15 to 46) | Anah 1980 | No adverse events in either group |
*Authors note: "SF‐36 data on physical functioning were incomplete and this section was excluded from analysis".
FEV1: forced expiratory volume in one second HRQL: health‐related quality of life PEFR: peak expiratory flow rate
2. Outcomes: exercise‐induced bronchoconstriction/asthma.
| Vitamin C supplementation versus placebo (exercise‐induced bronchoconstriction/asthma) | |||||
| Outcome | Studies | N | Age | Study ID | Study reported result |
|
Health‐related quality of life |
0 | 0 | — | — | — |
|
Asthma exacerbations |
0 | 0 | — | — | — |
|
FEV1 (L) (change scores) | |||||
| Immediately after exercise | 1 | 12 participants (cross‐over design) | Mean age 26 years (± 5) |
Schachter 1982 | No significant difference between vitamin C and placebo Vitamin C: mean +0.21 (standard error (SE) ± 0.06) Placebo mean +0.08 (SE ± 0.08) t = 1.46 (P = 0.18) |
| 5 minutes after exercise | 1 | 12 participants (cross‐over design) | Mean age 26 years (± 5) | Schachter 1982 | No significant difference between vitamin C and placebo Vitamin C: mean ‐ 0.24 (SE ± 0.06) Placebo mean: ‐0.44 (SE ± 0.14) t = 2.13 (P = 0.057) |
| Post‐bronchodilator | 1 | 12 participants (cross‐over design) | Mean age 26 years (± 5) | Schachter 1982 | Post‐bronchodilator scores significantly better on vitamin C Vitamin C: mean + 0.43 (SE ± 0.12) Placebo mean + 0.22 (SE ± 0.10) t = 3.42 (P < 0.01) |
| FEV1 % drop postexercise |
1 | 8 participants (cross‐over design) | Mean age: 24.5 years (4.8) | Tecklenburg 2007 | A significant advantage in favour of vitamin C Reported maximum % drop in FEV1 postexercise on vitamin C diet was ‐6.4% (95% CI ‐12.0 to ‐0.8%; effect size using omega‐squared (ES) 0.40); indicative of an attenuated EIB response. This was significantly different (P < 0.05) from the maximum drop of ‐12.9% (95% CI ‐18.6 to ‐12.3%) on placebo |
|
PEF (change scores) | |||||
| Immediately after exercise | 1 | 12 participants (cross‐over design) | Mean age 26 years (± 5) | Schachter 1982 | A significant advantage in favour of vitamin C Vitamin C: mean +0.59 (SE ± 0.16) Placebo mean +0.10 (SE ± 0.25) t = 2.3 (P < 0.05) |
| 5 minutes after exercise | 1 | 12 participants (cross‐over design) | Mean age 26 years (± 5) | Schachter 1982 | No significant difference between vitamin C and placebo Vitamin C: mean ‐0.73 (SE ± 0.28) Placebo mean ‐0.95 (SE ± 0.40) t = 0.90 (NS) |
| Post‐bronchodilator | 1 | 12 participants (cross‐over design) | Mean age 26 years (± 5) | Schachter 1982 | A significant advantage in favour of vitamin C Vitamin C: mean +0.83 (SE ± 0.26) Placebo mean +0.39 (SE ± 0.29) t = 2.69 (P < 0.05) |
|
Asthma symptoms |
1 | 8 participants (cross‐over design) |
Mean age: 24.5 years (4.8) |
Tecklenburg 2007 | A significant advantage in favour of vitamin C
Reported asa significant improvement (P < 0.05) in mean asthma symptom scores (mean score 6.3; 95% CI 5.8 to 6.8) on the vitamin C diet compared to the placebo diet (mean score 5.8; 95% CI 5.1 to 6.2) |
| Adverse events | 0 | 0 | — | — | — |
*Tecklenburg 2007 used Asthma Quality of Life Questionnaire symptom score component. These data are reported in the table under symptoms.
CI: confidence interval FEV1: forced expiratory volume in one second NS: non‐significant SE: standard error
There was marked heterogeneity in vitamin C dosage regimes used in the selected studies.
Six studies used regular regimes with differing dosages varying from 1 g (Anah 1980; Anderson 1983; Fogarty 2003; Nadi 2012) and 1.5 g (Tecklenburg 2007) to 5 g once daily (Schertling 1990). Duration of treatment was also very variable, ranging from two weeks to six months.
Five studies (Cohen 1997; Kordansky 1979; Malo 1986; O'Sullivan 2000; Schachter 1982) reported results using single‐dose regimes prior to bronchial challenges or exercise tests. The dosages varied from 500 mg (Kordansky 1979; Schachter 1982) to 2 g (Cohen 1997; Malo 1986; O'Sullivan 2000).
Anah 1980 was funded by the Research Grant Committee of the College of Medical Sciences, University of Benin. Fogarty 2003 was funded by the NHS National Research and Development Programme on Asthma Management administered by the National Asthma Campaign. Kordansky 1979 was funded by the National Institute of Allergy and Infectious Diseases and Tecklenburg 2007 was funded, in part, by the Gatorade Sports Science Institute (Gatorade is a sports drinks). The source of funding was unspecified in the trial reports for Anderson 1983; Cohen 1997; Malo 1986; Nadi 2012; O'Sullivan 2000; Schachter 1982 and Schertling 1990
Excluded studies
We excluded 83 references for the following reasons: 77 (93%) did not compare vitamin C with placebo or no treatment; three (4%) were non‐randomised, one (1%) was not focused on participants with asthma, one (1%) was a summary of an excluded study and the remaining study (1%) was retracted by the authors following publication. Full details can be found in Characteristics of excluded studies.
Risk of bias in included studies
Full details of our 'Risk of bias' judgements can be found in Characteristics of included studies and an overview of our judgements can be seen in Figure 2.
2.
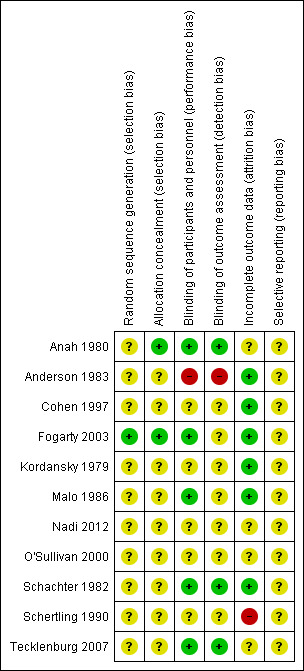
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged only one trial as low risk with regard to random sequence generation (Fogarty 2003) and assessed the remainder as unclear in this respect. We evaluated two trials as low risk in terms of allocation concealment (Anah 1980; Fogarty 2003) with the remaining nine in the unclear category.
Blinding
We assessed five studies as low risk with regard to performance bias (Anah 1980; Fogarty 2003; Malo 1986; Schachter 1982; Tecklenburg 2007). We judged five to be in the unclear category (Cohen 1997; Kordansky 1979; Nadi 2012; O'Sullivan 2000; Schertling 1990) and one (the only paediatric study in the review) was in the high risk of bias category (Anderson 1983). Our assessment of detection bias led to similar judgements as in the performance bias although two studies (Fogarty 2003; Malo 1986) were placed in the unclear rather than the low category and there were, therefore, only three studies in the low risk of bias category, seven in unclear and one which we assessed as high risk of bias.
Incomplete outcome data
We judged six studies to be at low risk of bias (Anderson 1983; Cohen 1997; Fogarty 2003; Kordansky 1979; Malo 1986; Schachter 1982). One study (Schertling 1990) was in the high risk of bias category, and we rated the remaining four as unclear in terms of attrition bias.
Selective reporting
We rated all studies as unclear in terms of reporting bias, but we had concerns about the SF‐36 data from Fogarty 2003, as SF‐36 data on physical functioning were noted by the authors to be incomplete and this section was excluded from the analysis.
Effects of interventions
Asthma
Health‐related quality of life
We found no health‐related quality of life (HRQL) data in the included studies that could be included in our analyses. However, Fogarty 2003 narratively reported no evidence of a difference in HRQL between the vitamin C and placebo groups after 16 weeks of treatment in terms of the available SF‐36 data; we rated the quality of this evidence as low (Table 1). Details of data reported in the included trials relevant to our prespecified outcomes are included in Table 3. No mean difference or confidence interval is reported in the paper.
Asthma exacerbation as defined by hospital admissions or treatment with a course of oral corticosteroids
Only one small study, involving 16 children, reported data for asthma exacerbations (as defined by hospital admissions or treatment with a course of oral corticosteroids) and there were no events in either the vitamin C or control group (Anderson 1983); we rated the quality of this evidence as very low (Table 1). Although data on exacerbations were provided for 22 adults on vitamin C and 19 adults on placebo in Anah 1980, the data were not reported in a format consistent with our prespecified criteria (defined by hospital admissions or treatment with a course of oral corticosteroids). Anah 1980 reported nine asthma attacks in the vitamin C group and 35 asthma attacks in the placebo group; these data include mild, moderate and severe asthma attacks as defined by the authors. There were two severe asthma attacks in the vitamin C group and eight in the placebo group, one moderate asthma attack in the vitamin C group and 16 in the placebo group, and six mild attacks in the vitamin C group and 11 in the placebo group. It is unclear how many asthma attacks were experienced by each participant.
Measures of lung function: FEV1, PEFR
In five studies there was no significant difference between vitamin C and placebo in FEV1 outcomes (Fogarty 2003; Kordansky 1979; Malo 1986; Nadi 2012; O'Sullivan 2000).
FEV1 (L) at one month was reported in one study with 60 participants (Nadi 2012) and based on within‐group analyses (paired t‐tests). A slight but significant advantage was reported for change in FEV1 in the placebo group (before 1.63 ± 0.68, after 1.82 ± 0.78, P = 0.044) but not in the vitamin C group (before 1.40 ± 0.56, after 1.44 ± 0.59, P = 0.65). Data were not reported in a form that enabled us to do an appropriate between‐group analysis, given pretreatment imbalances for this outcome.
Change in FEV1 (ml) from zero to 16 weeks was reported in one study with 201 participants(Fogarty 2003). There was no significant difference between placebo and vitamin C (MD 11 ml; 95% CI ‐70 to 92); we rated the quality of this evidence as moderate, as the confidence interval excluded a clinically important change in FEV1 on vitamin C (Santanello 1999; Table 1).
FEV1 outcomes were recorded in Kordansky 1979. However, they could not be included in our analysis as the data were not reported; there is an indication in the trial report that no significant difference was detected for this outcome between vitamin C and placebo.
FEV1 data were also collected in Malo 1986. The data were not reported in a format that we could transfer to a meta‐analysis, however it is indicated in the trial report that there was no significant difference between vitamin C and placebo for this outcome.
FEV1 % predicted was reported in O'Sullivan 2000 (a conference abstract) but insufficient details were reported to transfer to a meta‐analysis. The authors reported that "There was no significant change in FEV1 after vitamin C administration (95 ± 2.7% versus 94.2 ± 3.2%)".
In Fogarty 2003 and Schertling 1990, the only two studies reporting PEFR outcomes, there was no significant difference between vitamin C and placebo.
PEFR data collected at both AM and PM were reported by one study with 201 participants (Fogarty 2003). There was no significant difference between vitamin C and placebo with respect to AM (MD 0.90 L/min; 95% CI ‐11.8 to 13.5) or PM scores (MD 2.20 L/min; 95% CI ‐10.0 to 14.3). This study has a narrow confidence interval and therefore implies no clinically important change in PEFR with vitamin C.
In relation to PEFR, Schertling 1990 reported that there were no statistically significant differences, or clinically relevant differences, between vitamin C and placebo. Insufficient details were reported to transfer to a meta‐analysis.
Asthma symptoms
Data were provided by one study with 201 participants (Fogarty 2003) regarding the change in symptom scores between zero and 16 weeks. There was no significant difference between vitamin C and placebo (MD 0.0; 95% CI ‐0.2 to 0.1); we rated the quality of this evidence as moderate (Table 1).
With regard to asthma symptoms, Schertling 1990 reported that there were no statistically significant differences, or clinically relevant differences, between vitamin C and placebo
Adverse events
Only one parallel‐group study (Anah 1980) with 41 participants explicitly reported adverse event data. There were no adverse events in either in the vitamin C or the placebo groups; we rated the quality of this evidence as very low (Table 1).
In Schertling 1990, a cross‐over trial with 29 participants, one patient reported nausea during the ascorbic acid period, and a few patients noted other mild symptoms during the study, but the authors report "no relevant differences for occurrences between two groups / testing periods".
Withdrawals
Only one study specifically reported withdrawals. Fogarty 2003 documented a withdrawal rate of 24%, mostly for non‐medical reasons with a small number withdrawing due to asthma deterioration or "other medical reasons" which were not specified.
Exercise‐induced asthma/bronchoconstriction
Health‐related quality of life
None of the three included studies reported between‐group differences in HRQL in relation to exercise‐induced asthma/bronchoconstriction. Tecklenburg 2007 provided data from the symptoms component of the Asthma Quality of Life Questionnaire (AQLQ), reporting a significant improvement on the vitamin C diet (mean score 6.3; 95% CI 5.8 to 6.8) versus placebo (mean score 5.8; 95% CI 5.1 to 6.2) (P < 0.05). However, we are mindful that this measure may be more relevant to asthma studies considering the effects of vitamin C supplementation over longer periods and arguably of less relevance in short‐term studies investigating more immediate outcomes. Data reported in the included trials relevant to our prespecified outcomes are included in Table 4.
Asthma exacerbation as defined by hospital admissions or treatment with a course of oral corticosteroids
This outcome, in relation to exercise‐induced asthma/bronchoconstriction, was not reported in the included studies. Again this outcome is of particular importance in asthma trials measuring the effects of vitamin C supplementation over longer periods and less relevant in contexts where more immediate outcomes are informative.
Measures of lung function: FEV1, PEFR
The change in FEV1 (L) following exercise, compared with FEV1 (L) before exercise, was reported in a small cross‐over study with 12 participants (Schachter 1982). The comparison of vitamin C versus placebo change scores was recorded immediately following exercise (MD 0.13; vitamin C 0.21 (standard error (SE) 0.06) versus placebo 0.08 (SE 0.08), P = 0.18), at five minutes after exercise (MD 0.20; vitamin C ‐0.24 (SE 0.06) versus placebo ‐0.44 (SE 0.14), P = 0.057) and following postexercise bronchodilator (MD 0.21; vitamin C 0.43 (SE 0.12) versus placebo 0.22 (SE 0.10), P < 0.01). The authors report significantly greater increases in post‐bronchodilator pulmonary function in patients on vitamin C compared with placebo).
The maximum % drop in FEV1 scores following exercise was reported in a very small cross‐over study with eight participants (Tecklenburg 2007). The authors reported that the maximum percentage drop in FEV1 postexercise on the vitamin C diet was 6.4% (95% CI 12.0% to 0.8%; effect size using omega‐squared (ES) 0.40), which is indicative of an attenuated exercise‐induced bronchoconstriction response. We rated the quality of this evidence as low (Table 1). This was significantly different (P < 0.05) from the maximum drop of 12.9% (95% CI 18.6% to 12.3%) on the placebo diet. There are uncertainties regarding the reported CI for the placebo diet and the data were not reported in a form that enabled us to do a between‐group analysis
Categorical outcomes relating to FEV1 were reported in Cohen 1997, a cross‐over trial. In the vitamin C group 11 of the 20 participants demonstrated less deterioration in their postexercise change scores (with a % fall in FEV1 no greater than 15%), however in the placebo group all participants had a % fall greater than 15%. These data suggest a protective effect of vitamin C in exercise‐induced asthma.
Postexercise change in PEFR was also reported by Schachter 1982. In a comparison of vitamin C versus placebo change scores recorded immediately following exercise there was a significant difference favouring vitamin C (MD 0.49 L/second; vitamin C 0.59 (SE0.16) versus placebo 0.10 (SE 0.25), P < 0.05). However, at five minutes after exercise this difference was not significant (MD 0.22; vitamin C ‐0.73 (SE 0.28) versus placebo ‐0.95 (SE 0.40), non‐significant). Following postexercise bronchodilator there was a significant difference favouring vitamin C (MD 0.44 L/second; vitamin C 0.83 (SE 0.26) versus placebo 0.39 (SE 0.29), P < 0.05).
Asthma symptoms
One very small cross‐over study with eight participants (Tecklenburg 2007) reported asthma symptom data using the AQLQ and showed a significant improvement in patients on the vitamin C diet (MD .6.3; 95% CI 5.8 to 6.80) versus the placebo diet (MD 5.8; 95% CI 5.1 to 6.2, P < 0.05); we rated the quality of this evidence as low (Table 1). The minimally important difference on this scale is regarded as 0.5 units.
Adverse events
None of the included studies explicitly reported adverse event data.
Withdrawals
No study specifically reported withdrawals.
Discussion
Summary of main results
Eleven studies were identified as meeting the inclusion criteria for this review: vitamin C versus no vitamin C supplementation. All but one of the studies were in adults, and the studies varied considerably with respect to severity (Characteristics of included studies). The small number of studies available for review and their disparate designs meant that only descriptions of the individual studies were possible with no prospect of aggregating the current studies available. We are also mindful that only three of the 11 included studies were published in the last 10 years (Fogarty 2003; Nadi 2012; Tecklenburg 2007) and the management context for the most of the studies may not be comparable to the treatment patients receive today
Given the significant impact exacerbations of asthma may have on lung function and quality of life, this is an important endpoint for studies in this condition. None of the studies provided robust evidence of vitamin C supplementation influencing asthma exacerbations or health‐related quality of life. Only one study (Anah 1980) used exacerbation rate as a primary outcome, but the data could not be included in our review as the definition of exacerbation did not meet our inclusion criteria and there was a lack of statistical analysis. This study reported a reduction in the numbers of severe exacerbations, defined as asthma attacks requiring hospital admission. However, no statistical analysis was presented to allow comparison of the number of participants with one or more exacerbations in each group, or the exacerbation rates between groups.
Fogarty 2003 was the largest included trial with 201 participants and looked at changes in symptom scores in patients with a clinical diagnosis of asthma taking supplements of vitamin C, magnesium or placebo. No significant difference in symptom scores was demonstrated during the period of this study, either as an individual outcome or in combination with others.
The fundamental importance of considering the impact of exacerbations (Fitzgerald 2006) and health‐related quality of life (Wilson 2012) in relation to asthma has been well appreciated for several years, and these outcomes are commonly assessed in clinical trials of asthma. It is therefore particularly disappointing that there is very little information in the trials relating to these outcomes.
Nine studies used lung function (including FEV1, PEFR or measures of bronchial hyperreactivity) as their primary outcome measures. There was some evidence from very small studies of improved lung function but it would be necessary to replicate these findings in larger trials for any robust conclusions to be drawn. The largest study (Fogarty 2003), which was at lowest risk of bias, reported narrow confidence intervals for FEV1 and PEFR; this implies no clinically important effect of vitamin C on these measures of lung function.
Table 3 clarifies the data available from the trials including our prespecified primary and secondary outcomes for asthma. There was a paucity of data for our primary outcomes (health‐related quality of life and asthma exacerbations) and the available data for measures of lung function and symptoms scores reported in the table do not indicate a significant benefit of vitamin C supplementation. The strength of the evidence is generally low (Table 1) and most of the studies contributing data are very small, with disparate approaches. It is therefore not feasible to draw firm conclusions either for or against the use of supplementary vitamin C in the management of asthma. In summary, we would suggest that currently there is insufficient robust evidence from the available trials to evaluate the benefits of vitamin C supplementation and that such judgements should be reserved until data are available from further adequately powered studies.
Our concerns about the available data for the impact of vitamin C supplementation on exercise‐induced bronchoconstriction/asthma are similar. The data are tabulated in Table 4. There is some evidence of a significant benefit in post‐bronchodilator FEV1 and PEFR; however, in each case these data are contributed only by a single small study. There is also suggestive evidence of a benefit in symptoms scores, but these data are drawn from only one small study. As with the primary and secondary outcomes for asthma, the strength of evidence is of a low order (Table 2). In summary, there is a need for additional adequately powered studies, with appropriate outcome measures, to bring more clarity to the assessment of the role of vitamin C supplementation in exercise‐induced bronchoconstriction/asthma.
Overall completeness and applicability of evidence
The primary outcomes chosen, exacerbation rates and health‐related quality of life, are important in assessing asthma control and the impact of treatment. Data for these clinically significant endpoints were lacking, with no studies reporting complete health‐related quality of life data and only one using exacerbations as a primary endpoint.
Measures of lung function were used more frequently, including exercise‐induced changes in lung function or bronchial challenge tests. However, the studies were small and disparate in design and it is not possible to draw conclusions about overall clinical effectiveness from the results.
One of the two studies identified in our searches, but for which data are currently not posted (IRCT138904224359N1a), may meet our inclusion criteria in the update of this review. However, we note that it will not contribute to our primary outcomes as it does not focus on health‐related quality of life or asthma exacerbations. We also note that the other ongoing trial (NCT01057615a) may well not meet our inclusion criteria as the comparison appears to be essentially between vitamin C supplementation and fish oil, rather than vitamin C supplementation versus placebo, and the trial specifically focuses on exercise‐induced bronchoconstriction. However, a more adequate assessment will be possible when the full details of the trial are reported
There is currently only a single small study in children (Anderson 1983 including 16 children), so we currently have very limited evidence to assess the impact of vitamin C on asthma in children.
Quality of the evidence
Only one of the 11 trials meeting our inclusion criteria included over 100 participants (Fogarty 2003) and on average the sample size per trial was just 38. In the remaining 10 included studies the sample sizes ranged from six to 60 and the average per trial was just 22. Only six of the 11 studies provided data that could be included in our tables (Table 3; Table 4) and for each outcome we could include data from only one study in each case. There is, therefore, a worrying paucity of data available for this review and there is a substantial risk that there may have been reporting bias. With respect to the quality of the included research, in terms of our 'Risk of bias' assessment using Cochrane criteria, we categorised only one trial as low risk with regard to random sequence generation (Fogarty 2003) and we evaluated the remainder as unclear. In terms of allocation concealment we evaluated three trials as low risk of bias (Anah 1980; Fogarty 2003; Schertling 1990) with the remaining eight assessed as unclear. In terms of both the quantity and quality of available data, with respect to our 'Risk of bias' assessment relating to randomisation procedures, it is clear that any robust interpretation of these data would be inappropriate and this conclusion is supported by our assessment of performance bias where we judged only five studies (Anah 1980; Fogarty 2003; Schachter 1982; Schertling 1990; Tecklenburg 2007) to be low risk of bias. We judged five to be in the unclear category (Cohen 1997; Kordansky 1979; Malo 1986; O'Sullivan 2000; Nadi 2012) with the remaining trial in the high risk of bias category (Anderson 1983). The evaluation of detection bias was consistent with our assessment of performance bias, apart from Schertling 1990 which we judged in this context as unclear rather than low risk of bias.
We primarily downgraded for risk of bias due to the inadequate description of study design across the included studies and the risk of selective reporting as noted above. For a number of outcomes we also downgraded for imprecision as the results came from small trials or were otherwise indeterminate. The notable exception to this was FEV1, where the estimated effect excludes a clinically meaningful difference of 120mL (Table 1).
Potential biases in the review process
A possibility of publication bias in this review is acknowledged, whereby a potential failure to identify unpublished negative trials could conceivably lead to the positive effects of vitamin C supplementation being overestimated and, similarly, any failure to identify unpublished positive trials may have led to a conservative assessment of the treatment benefits. These issues are present in most systematic reviews and we believe that a very significant proportion of the research addressing this clinical question has been identified through the Cochrane Airways Group's comprehensive systematic database searches, in the identification of both published and unpublished trials meeting our inclusion criteria.
We also acknowledge the possibility of study selection bias. We endeavoured to minimise this risk by two review authors independently evaluating all identified studies, and we are confident that trials judged as failing to meet our inclusion criteria were assessed on a consistent basis.
Agreements and disagreements with other studies or reviews
Our conclusions are very similar to a previous Cochrane review on vitamin C for asthma (Kaur 2009). However, in Kaur 2009 the primary outcomes were lung function and symptom scores as opposed to exacerbations and health‐related quality of life in this review. Two additional studies meeting the inclusion criteria were identified in this review (Nadi 2012; Schertling 1990). However, our conclusions are consistent with those drawn in the 2009 review where the authors argue that "further methodologically strong and large‐scale randomised controlled trials are needed in order to address the question of the effectiveness of vitamin C in asthma". We hope that such research will be conducted and that particular emphasis will be given to the assessment of efficacy with respect to any impact on asthma exacerbations and health‐related quality of life.
Authors' conclusions
Implications for practice.
The studies of vitamin C supplementation in asthma included in this review provide only very limited evidence with which to evaluate the use of vitamin C in stable asthma or exercise‐induced bronchoconstriction/asthma. There is currently no indication that vitamin C can be recommended as a therapeutic agent in asthma or in the prevention of exercise‐induced bronchoconstriction. The studies are generally too small to provide clinical recommendations. In addition, clinically important endpoints such as exacerbation rates and health‐related quality of life scores are lacking.
Implications for research.
Our conclusions are in line with a previous Cochrane review considering the use of vitamin C in stable asthma (Kaur 2009). We agree that larger, more robust research is required to clarify the use of this supplement.
Well‐designed and adequately powered double‐blind, placebo‐controlled trials are required in both adults and children. These trials should include clinically meaningful endpoints including exacerbation rates, health‐related quality of life measures, robust symptom scores and the use of rescue medications. In addition changes to lung function parameters, including markers of bronchial hyperreactivity and spirometric changes, should be examined and their clinical implications reported.
We concur with Hemila 2013 that more research would be helpful with respect to exercise‐induced asthma. More specific trials are required to examine the inconclusive evidence reported for the use of vitamin C in exercise‐induced asthma and to clarify its influence on bronchial hyperreactivity.
Future studies should report adverse effects of vitamin C, which to date have not been adequately reported.
What's new
| Date | Event | Description |
|---|---|---|
| 12 June 2018 | Amended | A search update was run on 9 May 2018 in the Cochrane Airways Trial Register by Elizabeth Stovold (Information specialist, Cochrane Airways). Elizabeth Stovold and Rebecca Normansell (CoEd Cochrane Airways) screened the update search. The search returned 28 records. Twenty six records were excluded on the basis of title and abstract. Two were retrieved in full text. We found two small studies (Akhtar 2016; Hemila 2011 (subsequently retracted)) added to Characteristics of studies awaiting classification. We have decided not to update the review at this point in time. |
Acknowledgements
We would particularly like to acknowledge the excellent support and assistance from Emma Welsh, Liz Stovold and Emma Jackson of the Cochrane Airways Review Group, together with the greatly appreciated guidance from Chris Cates (Cochrane Airways Review Group Co‐ordinating Editor). We are also most grateful to Uwe Wollina, Katja Boehm, Dariusz Wozniak, Alieksei Seniukovich and Zhirajr Mokini Poturljan for the translation of non‐English studies; and to Professor Peter Barnes and Dr Al Biltagi who provided helpful feedback on two trials. The support provided by librarians Judith Scammel, Jane Appleton and Hilary Garrett at St George's University, London, is also greatly appreciated.
Milo Puhan was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy for Cochrane Airways Group Register
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Ascorbic Acid
#6 ascorbic* near acid*
#7 vitamin* NEAR C
#8 antioxidant*
#9 #5 or #6 or #7 or #8
#10 #4 and #9
[In search line #1, MISC1 denotes the field in the reference record where the record has been coded for condition, in this case, asthma.]
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anah 1980.
| Methods | Randomised, double‐blind controlled trial. Parallel‐group design | |
| Participants | 41 participants: vitamin C: 22, placebo: 19
Age: vitamin C: mean 26.5 (range 15 to 42), placebo mean 27.8 (range 15 to 46)
Sex: vitamin C: males 12 (55%), placebo 10 (53%)
PEFR: vitamin C: mean 274.1 (range 200 to 340), placebo mean 279.2 (range 200 to 325) Stated as recruited from Asthma Clinic. Each patient had the following investigations done on admission to the trial: haemoglobin or packed cell volume (PCV), white blood cell count (WBC) with eosinophil count (%), stool microscopy, peak expiratory flow rate (PEFR), plasma ascorbic acid. All had asthma for at least 4 years, but in remission. Most on maintenance therapy with bronchodilators. One on low‐dose oral steroids. Maintenance therapy continued in trial. All had history of attacks in rainy season and trial ran in rainy season Inclusion criteria: one criterion for selection was the increase in exacerbation during the rainy season. These exacerbations were precipitated by respiratory infection. Selection of participants for the study was simple: any person who agreed to participate after a full explanation of the procedure was accepted, unless he/she lived outside the city. If he/she could attend at regular intervals, including reporting to the hospital emergency service in the event of a severe attack at any time of the day or night, he/she was accepted Exclusion criteria: those with complicating bronchitis and/or emphysema were not admitted into the study group The trial was conducted in Benin City, Nigeria |
|
| Interventions | 1 g of ascorbic acid as 1 effervescent tablet once daily versus matching placebo. The tablets were dissolved in a small quantity of water. 2 identical effervescent tablets were provided for the trial by Roche (Nig.) Limited Most were on maintenance therapy with bronchodilators. One patient was on a small dose of oral steroids (5 mg of prednisolone daily) on entry into the study. All remained on whatever drugs sustained their remission |
|
| Outcomes | Asthma attacks (reporting precludes us from including these data in our definition of exacerbations as hospital admission or course of oral steroids). Stool microscopy. Eosinophil count. Data collected at 4, 8, 12, 14 weeks | |
| Notes | 14‐week trial Funded by the Research Grant Committee of the College of Medical Sciences, University of Benin Effervescent tablets were provided by Roche (Nig.) Limited |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Low risk | Coded allocation to A (vitamin C group) or B (placebo group) which was decoded on completion of the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. 2 identical effervescent tablets were provided for the trial by Roche (Nig.) Limited |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. 2 identical effervescent tablets were provided for the trial by Roche (Nig.) Limited |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No indication of withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes not reported (genotype, stool microscopy and eosinophil count data not reported as the authors judged the data to be unreliable) |
Anderson 1983.
| Methods | Randomised controlled trial, parallel‐group design | |
| Participants | 16 children: vitamin C: 7, control: 9
Age: vitamin C: 9.3 (2.4), control: 9.2 (1.9)
Severity: vitamin C: 4 moderate and 3 severe, control: 6 moderate and 3 severe
Sex: vitamin C: 7 males (100%), control: 5 males (56%) Children with difficulty in asthma management who were randomly selected. Diagnostic criteria: MMEFR (resting maximal mid‐expiratory flow rate): average 66% for moderate and 36% for severe Inclusion criteria: history of recurrent respiratory infections and asthma confirmed by lung function studies Exclusion criteria: none specified in trial report Paediatric Respiratory Clinic H F Verwoerd Hospital, Pretoria |
|
| Interventions | 1 g ascorbic acid (Redoxon) as a single dose in the morning (as adjunct to standard therapy) versus nothing (as an adjunct to standard therapy) for 6 months Co‐medication: each child received 3 daily doses of sodium cromoglycate (Ludamol) and either fenoterol (Berotec) or salbutamol (Ventolin). None received glucocorticoids during the study and none was hospitalised during the trial |
|
| Outcomes | Exacerbations (requiring glucocorticoids or hospitalisation), IgE (serum), antibodies to streptolysin 0 (ASO), polymorphonuclear leucocyte (PMNL) migration, secretory immunoglobulin A (IgA), serum immunoglobulin and total haemolytic complement levels | |
| Notes | Funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study compared ascorbic acid to no intervention and therefore the trial was unblinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No statement that assessors were unblinded or any attempt so to do, but measurements objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data |
| Selective reporting (reporting bias) | Unclear risk | No stated primary objective; some outcomes not reported |
Cohen 1997.
| Methods | Randomised, double‐blind trial, cross‐over design | |
| Participants | 20 patients with asthma received both vitamin C and placebo in a randomised, cross‐over design
Mean age 13.8 (7 to 28)
Sex: 13 males (65%) Reported as: all participants demonstrated exercise‐induced asthma (EIA) by having a decline of at least 15% in their forced expiratory volume in 1 second after a standard exercise test on a motorised treadmill (model Q50, Quinton Instrument Co, Seattle, Wash). The patients were advised to refrain from taking their regular medication, which consisted of inhaled steroids, antihistamines and bronchodilators, 12 hours before the test Baseline FEV1 (L) (before exercise): 2.36 (0.85); baseline FEV1 (L) (after exercise): 1.74 (0.72) Baseline FEV1 (% predicted) (before exercise): 86 (12); baseline FEV1 (% predicted) (after exercise): 63 (13) The setting was a university hospital in Israel |
|
| Interventions | Reported as: 2 g of oral ascorbic acid versus placebo 1 hour before a 7‐minute treadmill exercise session. 8 minutes after exercise pulmonary function tests were performed. 1 week later participants received the alternative intervention | |
| Outcomes | Development of exercise‐induced asthma (EIA), FEV1 (L) and FEV1 (% predicted) | |
| Notes | Summary of FEV1; a single large dose of ascorbic acid before exercise prevented the development of EIA in 9 of 20 patients and reduced the airways’ responsiveness to exercise in 2 other patients Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blind but method of blinding (whether it was a matched placebo) is unclear in trial report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind but method of blinding (whether it was a matched placebo) is unclear in trial report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Information included in trial report from all 20 participants |
| Selective reporting (reporting bias) | Unclear risk | No indication of reporting bias |
Fogarty 2003.
| Methods | Randomised, placebo‐controlled, double‐blind, parallel‐group | |
| Participants | 201 adults randomised: vitamin C: 95 (72 completed (76%)), placebo: 106 (82 completed (77%))
Age: vitamin C: 42 years, placebo: 40 years
Sex: vitamin C: 37 males (39%), placebo: 42 (40%) Baseline lung function: mean FEV1 (L) vitamin C: 2.8 (SD not reported), placebo: 2.8 (SD not reported) Baseline lung function: mean % predicted FEV1 (SD): vitamin C: 85.3 (15.7), placebo: 83.3 (17.5) Inclusion criteria: stated as: patients aged 18 to 60 years with a physician diagnosis of asthma and using at least one dose of an inhaled corticosteroid daily for the last 6 months were identified from computerised records Exclusion criteria: stated as: participants currently taking oral corticosteroids or diuretics, had used vitamin C, magnesium or calcium supplements within 3 months, had experienced exacerbation of asthma within 4 weeks, had a cumulative smoking history of 10 pack‐years or more, or were pregnant or planning a pregnancy Patients recruited from 24 GP practices, Nottingham, UK |
|
| Interventions | Run‐in period: consenting individuals entered a 3‐week run‐in period. Participants who complied with the measurement protocol and experienced no exacerbation of asthma during the run‐in period then proceeded into the supplementation study Interventions: vitamin C 1 g/day (5.6 mmol) plus magnesium placebo versus vitamin C placebo and magnesium placebo. A third group (not included in this review) received 450 mg/day magnesium chelate. No patient included in the vitamin C versus placebo comparison was receiving magnesium |
|
| Outcomes | Stated as: FEV1, forced vital capacity (FVC), inhaled dose of methacholine causing a 20% fall in FEV1 (PD20) to a maximum dose of 12.25 mmol methacholine (and values above this were censored), and average morning and evening peak flow, average daily bronchodilator use and daily symptom score recorded in a diary for the preceding 2 weeks. Study included a composite measure as their primary outcome. Data collected at the beginning and after 4, 8, 12 and 16 weeks of supplementation (but only reported at 16 weeks in trial report) We did not include data in our analyses from Fogarty 2006 (an additional study report related to this trial) due to the loss of randomisation at that stage |
|
| Notes | 16‐week trial Funded by: NHS National Research and Development Programme on Asthma Management administered by the National Asthma Campaign |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned using a random number generator, in blocks also stratified by a regular corticosteroid dose, defined as low or high |
| Allocation concealment (selection bias) | Low risk | Allocation by an individual code number, decoded after the last participant completed the trial. Randomisation and dispensing performed independently from the recruitment of trial participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Randomised to vitamin C 1 g/day (5.6 mmol) plus magnesium placebo, magnesium amino chelate (Lamberts Health Care, Tunbridge Wells, UK) 450 mg/day (27.6 mmol) plus vitamin C placebo, or double‐matched placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear in trial report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal listed in study report. Loss to follow‐up, but taken into account. ITT analysis and as treated analysis reported: precise details of ITT imputation not given: "assuming deterioration in all outcomes in participants who withdrew". 66/300 withdrew (some from magnesium group not included in this review) |
| Selective reporting (reporting bias) | Unclear risk | Primary outcome measure reported, but secondary outcomes not all reported and incomplete reporting of SF‐36 data |
Kordansky 1979.
| Methods | Randomised controlled trial, cross‐over design | |
| Participants | 6 adults (2 women and 4 men) ranging in age from 20 to 34 selected at random
Participants with ragweed‐sensitive asthma, defined by skin prick positivity. Tested out of the ragweed season. Asymptomatic and not on treatment Inclusion criteria: ragweed‐sensitive asthmatics Trial conducted in Baltimore, USA |
|
| Interventions | 500 mg once daily (2 capsules) vitamin C for 7 days versus lactose placebo (2 capsules) for 7 days Co‐medication: during the study period, these patients were not receiving bronchodilators nor were they on corticosteroid therapy |
|
| Outcomes | PD20 to ragweed extract challenge FEV1, PD35 SGaw, tested on day 7, 3 hours after dose of placebo/vitamin C The FEV1 data were not reported in a format that we could use in our analyses |
|
| Notes | 7‐day cross‐over trial Funded by the National Institute of Allergy and Infectious Diseases |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as double‐blind but details of blinding (whether an identical placebo pill was used) were not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study described as double‐blind but details of blinding (whether an identical placebo pill was used) were not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 participants completed both periods of the cross‐over. No information on withdrawals included in trial report |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes summarised. Sample dose‐response curves given. No tables of summary statistics |
Malo 1986.
| Methods | Randomised controlled trial, cross‐over design | |
| Participants | 16 adults with asthma meeting the American Thoracic Society criteria
Age range 19 to 59, mean 43.1 (11.9) years
Sex: 3 men (19%)
Mean duration of asthma: 10.5 (14.6) years
Baseline lung function: mean % predicted FEV1 (SD): 86.6 (19.6) Department of Chest Medicine, Hopital du Sacre‐Coeur, Montreal, Canada |
|
| Interventions | Run‐in: medication was withheld as follows: inhaled beta2‐adrenergic agents for 8 hours and sustained release theophylline derivatives for 12 hours Intervention: 250 ml of a transparent and odourless sweet liquid in which was dissolved either 2 g ascorbic acid or placebo. 1 hour later spirometry was measured and histamine challenge done until PC20 reached Co‐medication: all participants received beta2‐adrenergic bronchodilators and/or sustained‐release theophylline derivatives and 6 were also receiving beclomethasone |
|
| Outcomes | FEV1, FVC, PC20 during histamine challenge The FEV1 data were not reported in a format that we could use in our analyses |
|
| Notes | Funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised according to a 4.3.1 two‐treatment cross‐over design, but details of random sequence generation were not included in trial report |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study described as double‐blind and details of blinding are provided as "The active and placebo medications consisted of 250 mL of a transparent and odourless sweet liquid in which was dissolved or not, respectively, 2 gm of ascorbic acid. These solutions of similar taste were prepared and coded by the hospital pharmacy" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 participants completed both periods of the cross‐over. No information on withdrawals included in trial report |
| Selective reporting (reporting bias) | Unclear risk | Trial report does not include complete details of outcomes |
Nadi 2012.
| Methods | Randomised, double‐blind trial, parallel‐group design | |
| Participants | 60 participants with severe asthma: vitamin C: 30, placebo: 30 described as "matched"
Age: vitamin C: 48.38 (± 9.03), placebo: 40.53 (± 10.48) Baseline lung function: mean FEV1 L (SD) vitamin C: 1.40 (0.56), placebo: 1.63 (0.68) Inclusion criteria: bronchial asthma, where the diagnosis was established through demonstrating reversible airway obstruction. Severe asthma (stable asthma step 4, according to 2004 Global strategy for asthma management and prevention guideline) Exclusion criteria: smokers and patients with other chronic diseases were excluded from the study Participants recruited from Ekbatan hospital in Hamadan (Iran) |
|
| Interventions | 1000 mg vitamin C daily versus placebo Co‐interventions: standard asthma treatment (according to stepwise therapy in 4th step of bronchial asthma) in both groups, in which patients were controlled appropriately |
|
| Outcomes | FEV1, FVC and FEV1/FVC leukocyte vitamin C level. Data recorded at baseline and 1 month | |
| Notes | 1‐month trial Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not described. Participants were randomly divided into 2 groups, A and B (including 30 patients in each group), and were matched according to their age, weight, height, gender, BMI and drug consumption |
| Allocation concealment (selection bias) | Unclear risk | Described as "Both patients and physician were unaware of details of patient’s distributions", however no further details available in trial report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind – however details of the placebo (whether it was matched to the intervention) are not included in trial report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind – however details of the placebo (whether it was matched to the intervention) are not included in trial report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details of withdrawals in trial report |
| Selective reporting (reporting bias) | Unclear risk | No indication of reporting bias |
O'Sullivan 2000.
| Methods | Randomised, double‐blind, controlled trial, cross‐over design | |
| Participants | 10 participants diagnosed with mild asthma according to America Thoracic Society criteria. Very limited details in trial report (conference abstract) Location of trial not included in abstract |
|
| Interventions | Each participant completed 2 treatment periods with ingestion of either 2 g of ascorbic acid or placebo 45 minutes prior histamine broncho‐provocation | |
| Outcomes | FEV1 and bronchial hyper‐responsiveness to inhaled histamine | |
| Notes | Funding source not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Study described as double‐blind but unclear whether an identical placebo was used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No indication of withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No indication of reporting bias |
Schachter 1982.
| Methods | Randomised, double‐blind, controlled trial, cross‐over design | |
| Participants | 12 adults with exercise‐induced asthma, never on corticosteroids or admitted to hospital
Mean age 26 (5) years
Sex: 5 males (41.7%) Baseline lung function: mean % predicted FEV1 (SD): 75 (18.1) Participants were diagnosed with asthma as defined by the American Thoracic Society’s criteria Inclusion criteria: all 12 participants gave a characteristic description of exercise‐induced bronchoconstriction (EIB). Those participants who demonstrated sufficient EIB (20% reduction in maximal expiratory flow at 40% of the vital capacity below total lung capacity on the partial expiratory flow‐volume curve (MEF40%(P)) after exercise were invited to proceed to the remaining challenges Trial conducted in New Haven, Connecticut, USA |
|
| Interventions | All 12 participants were instructed to take no medications for at least 8 hours prior to each challenge experiment. They were similarly instructed to refrain from foods or beverages containing large amounts of methylxanthines or ascorbic acid (coffee, fruits, juices, etc.) Intervention: single‐dose vitamin C (500 mg) orally 1 hour before exercise challenge versus placebo (sucrose). The vitamin C and placebo were given in identical capsules in a double‐blind random order |
|
| Outcomes | FEV1, FVC, maximal expiratory flow (MEF) and PEFR. Measured at before exercise, immediately after exercise, 5 minutes after exercise and post‐bronchodilator following exercise | |
| Notes | Trial conducted on 2 subsequent days. Participants underwent exercise challenge 90 minutes post‐dose. No details of washout period provided in report Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Vitamin C and placebo in identical capsules |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind. Vitamin C and placebo in identical capsules |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data provided for 12 participants |
| Selective reporting (reporting bias) | Unclear risk | No indication of reporting bias |
Schertling 1990.
| Methods | Randomised, double‐blind, controlled trial, cross‐over design | |
| Participants | 29 participants with infection‐related bronchial asthma
Age: aged 18 to 60 years
Sex: 18 men (62%) and 11 women Inclusion criteria: 18 years of age or older with infection‐associated asthma Exclusion criteria: inhaled and systemic corticosteroids, kidney diseases, acute and severe purulent infections Participants recruited from an ambulatory healthcare centre in Berlin, East Germany |
|
| Interventions | 2‐week run‐in period Intervention: 5 g/day oral ascorbic acid in 3 sugar‐coated pills versus oral placebo (identical capsules) 2 weeks on first intervention, 1 week washout period and 2 weeks on second intervention The order of the test periods was chosen randomly |
|
| Outcomes | Daily asthma symptom score; 4 measurements per day of expiratory peak flow throughout the entire study; 3 checks throughout study of bronchial hyperreactivity, using histamine provocation; broncho‐alveolar lavage at the end of testing periods, with determination of alveolar differential cell count and measurement of metabolic activity of broncho‐alveolar cells, using chemiluminescence; global assessment of effectiveness and tolerance by doctor and patient | |
| Notes | 2 weeks on daily oral ascorbic acid or placebo Funding source not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The sequence of testing periods was chosen randomly. Details of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details in trial report |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient details in trial report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information included in trial report about masking for data collectors/analysers |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data provided on 23 or 24 participants |
| Selective reporting (reporting bias) | Unclear risk | None apparent |
Tecklenburg 2007.
| Methods | Randomised, double‐blind, trial, cross‐over design | |
| Participants | 8 participants
Mean age: 24.5 (4.8)
Sex: 2 males (25%) All participants were diagnosed with clinically treated mild‐to‐moderate persistent asthma with an FEV1 greater than 70% of predicted and documented EIB (usual diet) as indicated by a drop of greater than 10% in postexercise FEV1 compared with pre‐exercise values. Physician‐diagnosed asthma. All participants had a history of chest tightness, shortness of breath and intermittent wheezing following exercise Baseline lung function: mean FEV1 L (SD) 3.82 (0.37) Baseline lung function: mean % predicted FEV1 (SD): 97.0 (6.1) Inclusion criteria: exercise‐induced bronchoconstriction Participants: university students and the local community Indiana, USA |
|
| Interventions | Run‐in: participants were asked to discontinue taking leukotriene receptor antagonists (LTRAs; N = 3 montelukast (Singulairs)) 12 h prior to testing, 10, 13, 14 and 4 days prior to testing to abstain from taking combined inhaled corticosteroids (ICS) and long‐acting b2‐agonists (N = 2, fluticasone propionate (Flovents) and salmeterol (Advairs)) or combined long‐acting b2‐agonists and LTRAs (N = 3 salmeterol (Advairs) and montelukast (Singulairs)). In addition, all participants were asked to refrain from taking caffeine, and to avoid physical activity for 12 h and 24 h, respectively, before exercise testing. Participants were asked to abstain from taking antioxidant supplements other than those given during the course of the study. Participants were advised to avoid foods that were high in vitamin C during the study Intervention: pharmaceutical grade ascorbic acid supplement (1500 mg/day: 3 500 mg capsules)(NOW foods, Bloomingdale, IL) versus a matched (colour/size) placebo (3 capsules/day of sucrose)(NOW foods, Bloomingdale, Illinois) |
|
| Outcomes | Pulmonary function (FEV1) pre‐ and postexercise, symptoms, exhaled nitric oxide and urine analysis | |
| Notes | Participants were randomised to active treatment or placebo for 2 weeks. 1‐week wash‐out period. Participants crossed over to the alternative condition for the following 2 weeks Study was funded, in part, by the Gatorade Sports Science Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo manufactured by the same company as the active treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Matching placebo manufactured by the same company as the active treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No indication of withdrawals |
| Selective reporting (reporting bias) | Unclear risk | No indication of reporting bias |
Abbreviations
ASO: antibodies to streptolysin O BMI: body mass index EIA: exercise‐induced asthma EIB: exercise‐induced bronchoconstriction FEV1: forced expiratory volume in one second FVC: forced vital capacity IgA: immunoglobulin A ITT: intention‐to‐treat MEF: maximal expiratory flow MEF40%(P): maximal expiratory flow at 40% of the vital capacity below total lung capacity on the partial expiratory flow‐volume curve MEFR: mid‐expiratory flow rate mmol: millimoles MMEFR: resting maximal mid‐expiratory flow rate PC20: histamine provocative concentration causing a 20% drop in FEV1 PCV: packed cell volume PEFR: peak expiratory flow rate PD35 sGaw: a 35% reduction in specific airway conductance PMNL: polymorphonuclear leucocyte WBC: white blood cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Biltagi | This study was retracted by the authors in 2012 due to data analysis issues. Dr Al‐Biltagi has confirmed that there are no plans to re‐analyse the data and that the research is due to be repeated Retraction. Omega‐3 fatty acids, vitamin C and Zn supplementation in asthmatic children: a randomised self‐controlled study. Acta Paediatrica 2012;101(8):891 |
| Aliyali 2010 | Study did not compare vitamin C to placebo/no treatment |
| Bagnato 1999 | Study did not compare vitamin C to placebo/no treatment |
| Baumann 2005 | Study did not compare vitamin C to placebo/no treatment |
| Bede 2008 | Study did not compare vitamin C to placebo/no treatment |
| Belousova 2006 | Study did not compare vitamin C to placebo/no treatment |
| Bernorio 1996 | Study did not compare vitamin C to placebo/no treatment |
| Bime 2012 | Not a trial of vitamin C |
| Cakmak 2004A | Study did not compare vitamin C to placebo/no treatment |
| Carlsten 2011 | Study did not compare vitamin C to placebo/no treatment |
| Carlsten 2011a | Study did not compare vitamin C to placebo/no treatment |
| Chazan 1981 | Study not randomised |
| Covar 2010 | Study did not compare vitamin C to placebo/no treatment |
| Cristofalo 1999 | Study did not compare vitamin C to placebo/no treatment: combination intervention as vitamin C given with N‐acetylcysteine |
| Cuomo 2004 | Study did not compare vitamin C to placebo/no treatment: intervention was a mixture of antioxidants |
| Daniliak 1995 | Study did not compare vitamin C to placebo/no treatment |
| Dauletbaev 2001 | Study did not compare vitamin C to placebo/no treatment; not randomised |
| De Lucia 1991 | Study did not compare vitamin C to placebo/no treatment |
| Dunstan 2007 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Echazarreta 2000 | Study did not compare vitamin C to placebo/no treatment |
| Echazarreta 2005 | Study did not compare vitamin C to placebo/no treatment |
| Ensom 2003 | Study did not compare vitamin C to placebo/no treatment |
| Falk 2005 | Study did not compare vitamin C to placebo/no treatment |
| Greenough 2010 | Participants not diagnosed with asthma; a prophylactic study |
| Gvozdjakova 2005 | Study did not compare vitamin C to placebo/no treatment |
| Gvozdjakova 2005b | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Gvozdjakova 2006 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Hernandez 2009 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Hosseini 2001 | Study did not compare vitamin C to placebo/no treatment |
| Houdard 1969 | Study did not compare vitamin C to placebo/no treatment |
| Jabbari 2005 | Study did not compare vitamin C to placebo/no treatment |
| Jahnova 2002 | Study did not compare vitamin C to placebo/no treatment |
| Kiss 2000 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Kligler 2011 | Study did not compare vitamin C to placebo/no treatment |
| Kolpakova 2007 | Study did not compare vitamin C to placebo/no treatment |
| Kongerud 2003 | Study did not compare vitamin C to placebo/no treatment |
| Kriukov 2003 | Study not focused on participants with asthma: evaluation of antioxidants in the prophylaxis of respiratory diseases in young servicemen |
| Kurth 2008 | Study did not compare vitamin C to placebo/no treatment |
| Labhe 2001 | Study did not compare vitamin C to placebo/no treatment |
| Lau 2004 | Study did not compare vitamin C to placebo/no treatment |
| Lipman, 1964 | Study did not compare vitamin C to placebo/no treatment |
| Lisitsa 2007 | Study did not compare vitamin C to placebo/no treatment |
| Liu 2003 | Study did not compare vitamin C to placebo/no treatment: intervention was a combination of vitamins C and E |
| Ma 2013 | Study did not compare vitamin C to placebo/no treatment: intervention was enhanced diet and antioxidants |
| Medvedeva 2002 | Study did not compare vitamin C to placebo/no treatment |
| Mohsenin 1983 | Not a randomised controlled trial |
| Murphy 2002 | Study did not compare vitamin C to placebo/no treatment |
| Neuman 1999 | Study did not compare vitamin C to placebo/no treatment |
| Neuman 2000 | Study did not compare vitamin C to placebo/no treatment |
| Nikitin 1993 | Study did not compare vitamin C to placebo/no treatment. |
| Olekhnovich 1982 | Study did not compare vitamin C to placebo/no treatment: the comparison was between vitamin E (intramuscular or orally) and lecithin versus lecithin, and there is no indication of randomisation |
| Onur 2011 | Study did not compare vitamin C to placebo/no treatment |
| Panahi 2012 | Study did not compare vitamin C to placebo/no treatment |
| Panina 2002 | Study did not compare vitamin C to placebo/no treatment |
| Pearson 2004 | Study did not compare vitamin C to placebo/no treatment |
| Peden 2005 | Study did not compare vitamin C to placebo/no treatment |
| Pennings 1999 | Study did not compare vitamin C to placebo/no treatment |
| Peters 2001 | Study did not compare vitamin C to placebo/no treatment |
| Prieto 1988 | Study did not compare vitamin C to placebo/no treatment |
| Ratanamaneechat 2010 | Study did not compare vitamin C to placebo/no treatment |
| Romieu 1998 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2001 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2002 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2002a | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2003 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2003a | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2004 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2004a | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2004b | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Romieu 2004c | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Shaheen 2007 | Study did not compare vitamin C to placebo/no treatment |
| Shimizu 1996 | Study did not compare vitamin C to placebo/no treatment |
| Smith 2004 | Study did not compare vitamin C to placebo/no treatment |
| Szlagatys 2005 | Study did not compare vitamin C to placebo/no treatment |
| Ting 1983 | Not a randomised controlled trial |
| Trenca 2001 | Study did not compare vitamin C to placebo/no treatment: combination intervention |
| Tug 2007 | Study did not compare vitamin C to placebo/no treatment |
| Tunnicliffe 2003 | Study did not compare vitamin C to placebo/no treatment |
| Varshavskii 2003 | Study did not compare vitamin C to placebo/no treatment |
| Vazquez‐Armenta 2012 | Not a trial of vitamin C, but of alpha‐lipoic acid |
| Voelker 1997 | A summary report of a trial (almost certainly Trenca 2001) using a combination intervention |
| Voitsekhovskaia 2007 | Study did not compare vitamin C to placebo/no treatment |
| Wood 2007 | Study did not compare vitamin C to placebo/no treatment |
| Wood 2008 | Study did not compare vitamin C to placebo/no treatment |
| Wood 2012 | Study did not compare vitamin C to placebo/no treatment |
| Wood 2012a | Study did not compare vitamin C to placebo/no treatment |
| Yamamoto 2013 | Not a trial of vitamin C, but of N‐acetylcysteine supplementation to people exposed to diesel exhaust |
Characteristics of ongoing studies [ordered by study ID]
IRCT138904224359N1.
| Trial name or title | Effect of Vitamin E and C supplements on improving asthma |
| Methods | Randomised controlled trial |
| Participants | History of at least 1 year asthma, age 18 to 50 years Exclusion criteria: suffering from diseases other than asthma, smoking, consumption of contraceptive pills |
| Interventions | 800 mg of vitamin C daily for 12 weeks versus placebo (study also includes a vitamin E condition and a vitamin E + vitamin C condition) |
| Outcomes | Asthma severity and serum levels |
| Starting date | November 2010 |
| Contact information | Dr Hesamodin Nabavi Zadeh Yasuj University of Medical Sciences |
| Notes | Study description stated as: "The purpose of this double blind clinical trial is to determine the effect of vitamin E & C, alone and in combination, on the improvement of asthma. 80 asthmatic patients, referred to Mofateh Special Clinics and Yasuj Imam Sajjad Hospital, will be randomly assigned into one of following groups to receive 400 mg Vitamin E per day, 800 mg vitamin C, 400 mg Vitamin E as well as 800 mg vitamin C, or placebo for 12 weeks. Holms‐Rahe questionnaire was used to investigate daily stress. Dietary intake is measured using 24‐hour dietary recalls questionnaire and respiratory factors are measured by peak flow meter and Spiro meter. Severity of asthma and the serum levels of vitamin C and E are measured before and after the intervention." Study results not posted at time of completion of this review |
NCT01057615.
| Trial name or title | Effect of Fish Oil and Vitamin C on Exercise‐Induced Bronchoconstriction and Airway Inflammation in Asthma |
| Methods | Randomised controlled trial |
| Participants | Diagnosis of asthma, based on medication use as well as history and symptoms as outlined in the NHLBI Guidelines for the Diagnosis and Management of Asthma |
| Interventions | Fish oil versus vitamin C versus fish oil + vitamin C |
| Outcomes | Pulmonary function and exhaled nitric oxide to measure airway inflammation |
| Starting date | January 2010 |
| Contact information | Timothy D Mickleborough, PhD Indiana University |
| Notes | Study description stated as: "The aim of this study is to extend previous findings that nutritional supplementation or dietary modification can ameliorate exercise‐induced bronchoconstriction. It has been shown in separate studies that fish oil and ascorbic acid (vitamin C) individually protect against EIB by improving pulmonary function and reducing airway inflammation. The main aim of this study is to determine the comparative and additive effects of fish oil and ascorbic acid supplementation on EIB and airway inflammation in asthmatic individuals" Study results not posted at time of completion of this review |
Contributions of authors
SJM drafted the protocol, with input from AH and MW. SJM and AH independently selected studies for inclusion, with support from MW, and independently extracted the data for inclusion in the meta‐analyses. SJM and AH independently evaluated each included study for risk of bias. SJM and AH completed the analyses and results sections. MW completed the discussion and conclusions with input from SJM and AH. SJM and AH assessed the quality of evidence for the 'Summary of findings' tables with input from Milo Puhan and Chris Cates.
Sources of support
Internal sources
-
SJM, UK.
NIHR and St George's University of London
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Anah 1980 {published data only}
- Anah CO, Jarike LN, Baig HA. High dose ascorbic acid in Nigerian asthmatics. Tropical and Geographical Medicine 1980;32(2):132‐7. [] [PubMed] [Google Scholar]
Anderson 1983 {published data only}
- Anderson R, Hay I, Wyk HA, Theron A. Ascorbic acid in bronchial asthma. South African Medical Journal 1983;63(17):649‐52. [] [PubMed] [Google Scholar]
Cohen 1997 {published data only}
- Cohen HA, Neuman I, Nahum H. Blocking effect of vitamin c in exercise‐induced asthma. Archives of Pediatrics & Adolescent Medicine 1997;151(4):367‐70. [] [DOI] [PubMed] [Google Scholar]
Fogarty 2003 {published data only}
- Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, et al. Corticosteroid sparing effects of vitamin C and magnesium in asthma: a randomised trial. Respiratory Medicine 2006;100(1):174‐9. [] [DOI] [PubMed] [Google Scholar]
- Fogarty A, Lewis SA, Scrivener SL, Antoniak M, Pacey S, Pringle M, et al. Oral magnesium and vitamin C supplements in asthma: A parallel group randomized placebo‐controlled trial. Clinical and Experimental Allergy 2003;33(10):1355‐9. [] [DOI] [PubMed] [Google Scholar]
Kordansky 1979 {published data only}
- Kordansky DW, Rosenthal RR, Norman PS. The effect of vitamin C on antigen‐induced bronchospasm. Journal of Allergy and Clinical Immunology 1979;63(1):61‐4. [] [DOI] [PubMed] [Google Scholar]
Malo 1986 {published data only}
- Malo JL, Cartier A, Pineau L, L'Archevêque J, Ghezzo H, Martin RR. Lack of acute effects of ascorbic acid on spirometry and airway responsiveness to histamine in subjects with asthma. Journal of Allergy and Clinical Immunology 1986;78(6):1153‐8. [] [DOI] [PubMed] [Google Scholar]
Nadi 2012 {published data only}
- Nadi E, Tavakoli F, Zeraati F, Goodarzi MT, Hashemi SH. Effect of vitamin C administration on leukocyte vitamin C. Acta Medica Iranica 2012;50(4):233‐8. [] [PubMed] [Google Scholar]
O'Sullivan 2000 {published data only}
- O'Sullivan S, Doyle S, Cormican L, Gunaratnam C, Poulter LW, Burke CM. Attenuation of bronchial hyperresponsiveness to histamine by vitamin C in asthmatic subjects [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A106. [] [Google Scholar]
Schachter 1982 {published data only}
- Schachter EN, Schlesinger A. The attenuation of exercise‐induced bronchospasm by ascorbic acid. Annals of Allergy 1982;49(3):146‐51. [] [PubMed] [Google Scholar]
Schertling 1990 {published data only}
- Schertling M, Winsel K, Muller S, Henning RD, Meiske W, Slapke J. Action of ascorbic acid on clinical course of infection related bronchial asthma and on reactive oxygen metabolites by BAL cells [Einfluß von Ascorbinsäure auf den klinischen Verlaufdes infektbedingten Asthma bronchiale und die Bildung von reaktiven Sauerstoffmetabolitendurch BAL‐Zellen]. Zeitschrift für Klinische Medizin 1990;45(20):1770‐4. [] [Google Scholar]
Tecklenburg 2007 {published data only}
- Tecklenburg SL, Mickleborough TD, Fly AD, Bai Y, Stager JM. Ascorbic acid supplementation attenuates exercise‐induced bronchoconstriction in patients with asthma. Respiratory Medicine 2007;101(8):1770‐8. [] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Al‐Biltagi {published data only}
- Retraction. Omega‐3 fatty acids, vitamin C and Zn supplementation in asthmatic children: a randomized self‐controlled study. Acta Paediatrica 2012; Vol. 101, issue 8:891. [] [PubMed]
- Al‐Biltagi MA, Baset AA, Bassiouny M, Kasrawi MA, Attia M. Omega‐3 fatty acids, vitamin C and Zn supplementation in asthmatic children: a randomized self‐controlled study. Acta Paediatrica 2009;98(4):737‐42. [] [DOI] [PubMed] [Google Scholar]
Aliyali 2010 {published data only}
- Aliyali M, Poorhasan Amiri A, Sharifpoor A, Zalli F, Amiri AP. Effects of N‐acetylcysteine on asthma exacerbation. Iranian Journal of Allergy, Asthma, and Immunology 2010;9(2):103‐9. [] [PubMed] [Google Scholar]
Bagnato 1999 {published data only}
- Bagnato GF, Gulli S, Pasquale R, Giacobbe O, Spatari G, Purello d'Ambrosio F. Effect of inhaled glutathione on airway response to 'fog' challenge in asthmatic patients. Respiration 1999;66(6):518‐21. [] [DOI] [PubMed] [Google Scholar]
Baumann 2005 {published data only}
- Baumann JM, Rundell KW, Evans TM, Levine AM. Effects of cysteine donor supplementation on exercise‐induced bronchoconstriction. Medicine and Science in Sports and Exercise 2005;37(9):1468‐73. [] [DOI] [PubMed] [Google Scholar]
Bede 2008 {published data only}
- Bede O, Nagy D, Surányi A, Horváth I, Szlávik M, Gyurkovits K. Effects of magnesium supplementation on the glutathione redox system in atopic asthmatic children. Inflammation Research 2008;57(6):279‐86. [] [DOI] [PubMed] [Google Scholar]
Belousova 2006 {published data only}
- Belousova CG, Geppe NA, Bolevich SB, Soodaeva SK, Climanov IA, Kolosova NG. Free radical status and antioxidant defense in children with asthma treated by inhaled corticosteroids combined with prolonged b2‐agonist [Abstract]. European Respiratory Journal 2006;28(Suppl 50):483s [P2790]. [] [Google Scholar]
Bernorio 1996 {published data only}
- Bernorio S, Pecis M, Zucchi A, Guerra G, Migliorini V, Negri L, et al. Glutathione in bronchial hyperresponsiveness. Journal of Aerosol Medicine 1996;9(2):207‐13. [] [Google Scholar]
Bime 2012 {published data only}
- Bime C, Wei CY, Holbrook J, Smith LJ, Wise RA. Association of dietary soy genistein intake with lung function and asthma control: a post‐hoc analysis of patients enrolled in a prospective multicentre clinical trial. Primary Care Respiratory Journal 2012;21(4):398‐404. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cakmak 2004A {published data only}
- Cakmak G, Demir T, Gemicioglu B, Aydemir A, Serdaroglu E, Donma O. The effects of add‐on zafirlukast treatment to budesonide on bronchial hyperresponsiveness and serum levels of eosinophilic cationic protein and total antioxidant capacity in asthmatic patients. Tohoku Journal of Experimental Medicine 2004;204(4):249‐56. [] [DOI] [PubMed] [Google Scholar]
Carlsten 2011 {published data only}
- Carlsten C, Pui M, MacNutt M, DyBuncio A, Lay J, Alexis N. Effect of antioxidant supplementation on diesel exhaust (de)‐associated changes in airway reactivity and innate immunity: a controlled crossover exposure study in humans [Abstract]. American Journal of Respiratory and Critical Care Medicine 2011;183(Meeting Abstracts):A3279. [] [Google Scholar]
Carlsten 2011a {published data only}
- Carlsten C, Pui M, MacNutt M, Lay J, Alexis N. Antioxidant supplementation attenuates changes in innate immunity associated with diesel exhaust (DE) in the lung: A controlled crossover exposure study [Abstract]. European Respiratory Society Annual Congress; 2011 Sep 24‐28; Amsterdam. Amsterdam, 2011; Vol. 38, issue 55:611s [3443]. []
Chazan 1981 {published data only}
- Chazan R, Droszcz W. Phagocytic activity of the peripheral blood granulocytes in patients treated with triamcinolone acetonide and vitamin C [Aktywnosc fagocytarna granulocytow krwi obwodowej osob leczonych acetonidem triamcinolonu, w trakcie stosowania witaminy C]. Pneumonologia Polska 1981;49(8‐9):621‐6. [] [PubMed] [Google Scholar]
Covar 2010 {published data only}
- Covar R, Gleason M, MacOmber B, Stewart L, Szefler P, Engelhardt K, et al. Impact of a novel nutritional formula on asthma control and biomarkers of allergic airway inflammation in children. Clinical and Experimental Allergy 2010;40(8):1163‐74. [] [DOI] [PubMed] [Google Scholar]
Cristofalo 1999 {published data only}
- Cristofalo MG, Savojardo M, Pecorella G, Galiano S, Lo Coco L, Parisi A, et al. The end of antioxiding treatment for pulmonary illness. Our experience [Ruolo degli antiossidanti nel danno polmonare. Nostre esperienze]. Medicina Dello Sport 1999;52(3):165‐75. [] [Google Scholar]
Cuomo 2004 {published data only}
- Cuomo B, Fasoli L, Guerrea T, Cosettini M, Don M, Saretta F, et al. Efficacy of antioxidant supplementation diet in asthmatic children [abstract]. European Respiratory Journal 2004;24(Suppl 48):167s. [] [Google Scholar]
Daniliak 1995 {published data only}
- Daniliak IG, Kogan AK, Bolevich S. Aevit and glutamic acid in the treatment of patients with bronchial asthma [Aevit i glutaminovaia kislota v lechenii bol'nykh bronkhial'noÄ astmoÄ]. Klinicheskaia Meditsina 1995;73(5):50‐3. [] [PubMed] [Google Scholar]
Dauletbaev 2001 {published data only}
- Dauletbaev N, Rickmann J, Viel K, Buhl R, Wagner TO, Bargon J. Glutathione in induced sputum of healthy individuals and patients with asthma. Thorax 2001;56(1):13‐8. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Lucia 1991 {published data only}
- Lucia F, Bonavia M, Crimi E, Scaricabarozzi I, Brusasco V. Antiinflammatory‐antioxidant treatment with a methane sulfonanilide in allergen‐induced asthma. Annals of Allergy 1991;66(5):424‐9. [] [PubMed] [Google Scholar]
Dunstan 2007 {published data only}
- Dunstan JA, Breckler L, Hale J, Lehmann H, Franklin P, Lyons G, et al. Supplementation with vitamins C, E, beta‐carotene and selenium has no effect on anti‐oxidant status and immune responses in allergic adults: A randomized controlled trial. Clinical and Experimental Allergy 2007;37(2):180‐7. [] [DOI] [PubMed] [Google Scholar]
Echazarreta 2000 {published data only}
- Echazarreta AL, Sala E, Rahman I, Peinado V, Barbera JA, Roca J, et al. Effects of platelet‐activating factor challenge on oxidant/antioxidant balance and exhaled nitric oxide in asthma. European Respiratory Journal 2000;16(Suppl 31):266s. [] [Google Scholar]
Echazarreta 2005 {published data only}
- Echazarreta AL, Rahman I, Peinado V, Barberà JA, Roca J, MacNee W, et al. Lack of systemic oxidative stress during PAF challenge in mild asthma. Respiratory Medicine 2005;99(5):519‐23. [] [DOI] [PubMed] [Google Scholar]
Ensom 2003 {published data only}
- Ensom MH, Chong G, Zhou D, Beaudin B, Shalansky S, Bai TR. Estradiol in premenstrual asthma: a double‐blind, randomized, placebo‐controlled, crossover study. Pharmacotherapy 2003;23(5):561‐71. [] [DOI] [PubMed] [Google Scholar]
Falk 2005 {published data only}
- Falk B, Gorev R, Zigel L, Ben‐Amotz A, Neuman I. Effect of lycopene supplementation on lung function after exercise in young athletes who complain of exercise‐induced bronchoconstriction symptoms. Annals of Allergy, Asthma & Immunology 2005;94(4):480‐5. [] [DOI] [PubMed] [Google Scholar]
Greenough 2010 {published data only}
- Greenough A, Shaheen SO, Shennan A, Seed PT, Poston L. Respiratory outcomes in early childhood following antenatal vitamin C and E supplementation. Thorax 2010;65(11):998‐1003. [] [DOI] [PubMed] [Google Scholar]
Gvozdjakova 2005 {published data only}
- Gazdik F, Gazdikova K, Jahnova E, Pijak MR. Sparing effect of coenzyme Q10, a‐tocopherol and ascorbic acid on the consumption of corticosteroids in allergic asthmatics [Abstract]. Allergy & Clinical Immunology International 2003;1 Suppl:Abstract No: P‐2‐38. [] [Google Scholar]
Gvozdjakova 2005b {published data only}
- Gvozdjáková A, Kucharská J, Bartkovjaková M, Gazdíková K, Gazdík FE. Coenzyme Q10 supplementation reduces corticosteroids dosage in patients with bronchial asthma. BioFactors (Oxford, England) 2005;25(1‐4):235‐40. [] [DOI] [PubMed] [Google Scholar]
Gvozdjakova 2006 {published data only}
- Gazdik F, Gvozdjakova A, Kucharska J, Kucharikova Z, Jahnova E, Gazdikova K. Coenzyme Q(10) supplementary therapy decreased corticoid consumption in patients with bronchial asthma. Advances in Immunopathology & Respiratory Allergy 2006;13(1):29‐33. [] [Google Scholar]
Hernandez 2009 {published data only}
- Hernandez M, Zhou H, Zhou B, Robinette C, Crissman K, Hatch G, et al. Combination treatment with high‐dose vitamin C and alpha‐tocopherol does not enhance respiratory‐tract lining fluid vitamin C levels in asthmatics. Inhalation Toxicology 2009;21(3):173‐81. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hosseini 2001 {published data only}
- Hosseini S, Pishnamazi S, Sadrzadeh SMH, Farid F, Farid R, Watson RR. Pycnogenol in the management of asthma. Journal of Medicinal Food 2001;4(4):201‐9. [] [DOI] [PubMed] [Google Scholar]
Houdard 1969 {published data only}
- Houdard YP. Trial of cortibucline. Marseille Medical 1969;106(11):993‐7. [] [PubMed] [Google Scholar]
Jabbari 2005 {published data only}
- Jabbari Azad F, Rafatpanah H, Farid R, Watson R, Hosseini S, Tavallei A, et al. Phycnogenol in the management of asthma [Abstract]. XIX World Allergy Organization Congress, June 26‐July 1, Munich, Germany 2005:Abstract 268. []
Jahnova 2002 {published data only}
- Jahnova E, Horvathova M, Gazdik F, Weissova S. Effects of selenium supplementation on expression of adhesion molecules in corticoid‐dependent asthmatics. Bratislavske Lekarske Listy 2002;103(1):12‐6. [] [PubMed] [Google Scholar]
Kiss 2000 {published data only}
- Kiss A, Horvath IM, Chung KF, Barnes PJ, Horvath IM, Chung KF. Vitamin C and E supplementation in mild asthma ‐ assessment by non‐invasive methods. European Respiratory Journal 2000;16(Suppl 31):40s. [] [Google Scholar]
Kligler 2011 {published data only}
- Kligler B, Homel P, Blank AE, Kenney J, Levenson H, Merrell W. Randomized trial of the effect of an integrative medicine approach to the management of asthma in adults on disease‐related quality of life and pulmonary function. Alternative Therapies in Health and Medicine 2011;17(1):10‐5. [] [PubMed] [Google Scholar]
Kolpakova 2007 {published data only}
- Kolpakova AF. [Effects of combined therapy with domestic inhalational anti‐asthmatic drugs on oxidant status of patients with bronchial asthma]. [Russian]. Terapevticheskii Arkhiv 2007;79(3):41‐4. [] [PubMed] [Google Scholar]
Kongerud 2003 {published data only}
- Kongerud J, Crissman K, Hatch G, Alexis N. Ascorbic acid is decreased in induced sputum of mild asthmatics. Inhalation Toxicology 2003;15:101‐9. [] [DOI] [PubMed] [Google Scholar]
Kriukov 2003 {published data only}
- Kriukov EV, Novozhenov VG. Efficacy of antioxidants in the prophylaxis of respiratory diseases in young servicemen. Voenno‐meditsinskii Zhurnal 2003;324(8):28‐31. [] [PubMed] [Google Scholar]
Kurth 2008 {published data only}
- Kurth T, Barr RG, Gaziano JM, Buring JE. Randomised aspirin assignment and risk of adult‐onset asthma in the Women's Health Study. Thorax 2008;63(6):514‐8. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Labhe 2001 {published data only}
- Labhe RU, Mani UV, Iyer UM, Mishra M, Jani K, Bhattacharya A. The effect of spirulina in the treatment of bronchial asthma. Journal of Nutraceuticals, Functional & Medical Foods 2001;3(4):53‐61. [] [Google Scholar]
Lau 2004 {published data only}
- Lau BHS, Riesen SK, Truong KP, Lau EW, Rohdewald P, Barreta RA. Pycnogenol as an adjunct in the management of childhood asthma. Journal of Asthma 2004;41(8):825‐32. [] [DOI] [PubMed] [Google Scholar]
Lipman, 1964 {published data only}
- Lipman WH, Simon SW. A clinical evaluation of a new aerosol for use in the treatment of bronchial asthma. Annals of Allergy 1964;22:460‐7. [] [PubMed] [Google Scholar]
Lisitsa 2007 {published data only}
- Lisitsa AV, Soodaeva SK, Klimanov IA, Chuchalin AG. The dynamics of blood plasma antioxidant activity in patients with asthma exacerbation during the course of phosphogliv admission [Abstract]. European Respiratory Journal 2007;30(Suppl 51):616s [P3618]. [] [Google Scholar]
Liu 2003 {published data only}
- Liu YL, Zhao X, Gong JQ, Hao XH. Efficacy of antioxidants in treatment of asthma. Acta Acadumiae Medicinae CPAPF 2003;14(6):342‐3. [] [Google Scholar]
Ma 2013 {published data only}
- Ma J, Strub P, Lavori PW, Buist AS, Camargo CA, Nadeau KC, et al. DASH for asthma: A pilot study of the DASH diet in not‐well‐controlled adult asthma. Contemporary Clinical Trials 2013;35(2):55‐67. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Medvedeva 2002 {published data only}
- Medvedeva IV, Lapik SV, Gur'eva SA, Savina IA. Clinical and biochemical aspects of flixotide administration in patients with moderate bronchial asthma. Terapevticheskii Arkhiv 2002;74(3):21‐5. [] [PubMed] [Google Scholar]
Mohsenin 1983 {published data only}
- Mohsenin V, Dubois AB, Douglas JS. Effect of ascorbic acid on response to methacholine challenge in asthmatic subjects. American Review of Respiratory Disease 1983;127(3):143‐7. [] [DOI] [PubMed] [Google Scholar]
Murphy 2002 {published data only}
- Murphy JD, Ferguson CS, Brown KR, Harms CA. The effect of dietary antioxidants on lung function in exercise induced asthmatics [Abstract]. Medicine and Science in Sports and Exercise 2002;34(5 Suppl 1):S155. [] [Google Scholar]
Neuman 1999 {published data only}
- Neuman I, Nahum H, Ben‐Amotz A. Prevention of exercise‐induced asthma by a natural isomer mixture of beta‐carotene. Annals of Allergy, Asthma & Immunology 1999;82(6):549‐53. [] [DOI] [PubMed] [Google Scholar]
Neuman 2000 {published data only}
- Neuman I, Nahum H, Ben‐Amotz A. Reduction of exercise‐induced asthma oxidative stress by lycopene, a natural antioxidant. Allergy 2000;55(12):1184‐9. [] [DOI] [PubMed] [Google Scholar]
Nikitin 1993 {published data only}
- Nikitin AV, Kashin AV, Karpukhina EP. Correction of antioxidant defense in patients with bronchial asthma by the method of intravascular laser irradiation [Korrektsiia antioksidantnoi zashchity u bol'nykh bronkhial'noi astmoi metodom endovaskuliarnogo lazernogo oblucheniia]. Problemy Tuberkuleza 1993;1993(3):46‐7. [] [PubMed] [Google Scholar]
Olekhnovich 1982 {published data only}
- Olekhnovich VM, Krylov VI, Sorogin VP, Zhogin SV. Therapeutic effectiveness of antioxidants in complex treatment of bronchial asthma in children [Terapevticheskaia effektivnost' antioksidantov v kompleksnom lechenii bronkhial'noi astmy u detei]. Pediatriia 1982;2:34‐5. [] [PubMed] [Google Scholar]
Onur 2011 {published data only}
- Onur E, Kabaroğlu C, Gunay T, Var A, Yilmaz T, Dündar P, et al. The beneficial effects of physical exercise on antioxidant status in asthmatic children. Allergologia et Immunopathologia 2011;39(2):90‐5. [] [DOI] [PubMed] [Google Scholar]
Panahi 2012 {published data only}
- Panahi Y, Tavana S, Sahebkar A, Masoudi H, Madanchi N. Impact of adjunctive therapy with Chlorella vulgaris extract. Scientia Pharmaceutica 2012;80(3):719‐30. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Panina 2002 {published data only}
- Panina NT, Yakovleva NG, Kotenko TV, Danilov LN, Panina NT, Yakovleva NG, et al. Role of antioxidant and trace element complex in outpatient care of bronchial asthma [Abstract]. European Respiratory Journal 2002;20(Suppl 38):52s. [] [Google Scholar]
Pearson 2004 {published data only}
- Pearson PJ, Lewis SA, Britton J, Fogarty A. Vitamin E supplements in asthma: a parallel group randomised placebo controlled trial. Thorax 2004;59(8):652‐6. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peden 2005 {published data only}
- Peden DB. The role of vitamins E and C in maintaining lung health in people with asthma. ClinicalTrials.gov 2005. []
Pennings 1999 {published data only}
- Pennings HJ, Borm PJ, Evelo CT, Wouters EF. Changes in levels of catalase and glutathione in erythrocytes of patients with stable asthma, treated with beclomethasone dipropionate. European Respiratory Journal 1999;13(6):1260‐6. [] [DOI] [PubMed] [Google Scholar]
Peters 2001 {published data only}
- Peters EA, Hiltermann JTN, Stolk J. Effect of apocynin on ozone‐induced airway hyperresponsiveness to methacholine in asthmatics. Free Radical Biology & Medicine 2001;31(11):1442‐7. [] [DOI] [PubMed] [Google Scholar]
Prieto 1988 {published data only}
- Prieto L, Juyol M, Paricio A, Martínez MA, Palop J, Castro J. Oral challenge test with sodium metabisulfite in steroid‐dependent asthmatic patients. Allergologia et Immunopathologia 1988;16(6):393‐6. [] [PubMed] [Google Scholar]
Ratanamaneechat 2010 {published data only}
- Ratanamaneechat S, George D, Kham A, Baran J, Nagle K, Naples R, et al. Serum superoxide dismutase activity as a predictor of adverse events after H1N1 vaccination [Abstract]. American Journal of Respiratory and Critical Care Medicine 2010;181(Meeting Abstracts):A6791. [] [Google Scholar]
Romieu 1998 {published data only}
- Romieu I, Meneses F, Ramirez M, Gerber M, Dekker D, Walda I, et al. Acute effect of ozone on respiratory function among participant in a randomized trial of antioxidant supplementation. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A48. [] [DOI] [PubMed] [Google Scholar]
Romieu 2001 {published data only}
- Romieu I, Sienra Monge JJ, Ramirez M, Reyes Ruiz NI, Rio Navarro BE, Ruiz Navarro MX, et al. Impact of antioxidant supplementation on the respiratory health of asthmatic children exposed to high levels of particulate and ozone in Mexico City. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A40. [] [Google Scholar]
Romieu 2002 {published data only}
- Romieu I, Sienra‐Monge JJ, Ramírez‐Aguilar M, Téllez‐Rojo MM, Moreno‐Macías H, Reyes‐Ruiz NI, et al. Antioxidant supplementation and lung functions among children with asthma exposed to high levels of air pollutants. American Journal of Respiratory and Critical Care Medicine 2002;166(5):703‐9. [] [DOI] [PubMed] [Google Scholar]
Romieu 2002a {published data only}
- Romieu I, Sienra‐Monge J, Ramirez M, Moreno‐Macias H, Rio‐Navarro B, Ruiz‐Navarro M, et al. Impact of antioxidant supplementation in the inflammatory response in asthmatic children exposed to high levels of ozone in Mexico City [abstract]. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A437. [] [Google Scholar]
Romieu 2003 {published data only}
- Ramirez M, Morena H, Sienra JJ, Reyes N, Rio BE, Hatch G, et al. Modulation of air pollution impact on peak expiratory flow by serum levels of a‐tocopherol and ascorbic acid among asthmatic children in Mexico City [abstract]. American Thoracic Society 99th International Conference 2003:B091 Poster 917. []
Romieu 2003a {published data only}
- Romieu I, Sienra JJ, Ramirez M, Moreno H, Reyes NI, Rio BE, et al. Antioxidant supplementation and generic susceptibility to ozone: a randomized controlled trial of children with asthma [abstract]. American Thoracic Society 99th International Conference 2003:C014. []
Romieu 2004 {published data only}
- Romieu I, Sienra JJ, Ramirez M, Moreno H, Rio BE, Hatch G, et al. Antioxidant supplementation and inflammatory responses among young asthmatics exposed to high levels of air pollutants [Abstract]. European Respiratory Journal 2004; Vol. 24, issue Suppl 48:623s. []
Romieu 2004a {published data only}
- Romieu I, Sienra‐Monge JJ, Ramirez‐Aguilar M, Moreno‐Macias H, Reyes‐Ruiz NI, Rio‐Navarro BE. Incidence of wheezing and genetic polymorphism of GSTM1 among asthmatic children supplemented with antioxidants in Mexico City [Abstract]. American Thoracic Society 100th International Conference, May 21‐26, 2004, Orlando 2004:C24 Poster 701. []
Romieu 2004b {published data only}
- Romieu I, Sienra‐Monge JJ, Ramírez‐Aguilar M, Moreno‐Macías H, Reyes‐Ruiz NI, Estela del Río‐Navarro B, et al. Genetic polymorphism of GSTM1 and antioxidant supplementation influence lung function in relation to ozone exposure in asthmatic children in Mexico City. [see comment]. Thorax 2004; Vol. 59, issue 1:8‐10. [] [PMC free article] [PubMed]
Romieu 2004c {published data only}
- Sienra‐Monge JJ, Ramirez‐Aguilar M, Moreno‐Macias H, Reyes‐Ruiz NI, Río‐Navarro BE, Ruiz‐Navarro MX, et al. Antioxidant supplementation and nasal inflammatory responses among young asthmatics exposed to high levels of ozone. Clinical and Experimental Immunology 2004;138(2):317‐22. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaheen 2007 {published data only}
- Shaheen SO, Newson RB, Rayman MP, Wong AP, Tumilty MK, Phillips JM, et al. Randomised, double blind, placebo‐controlled trial of selenium supplementation in adult asthma. Thorax 2007;62(6):483‐90. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shimizu 1996 {published data only}
- Shimizu T, Maeda S, Arakawa H, Mochizuki H, Tokuyama K, Morikawa A. Relation between theophylline and circulating vitamin levels in children with asthma. Pharmacology 1996;53(6):384‐9. [] [DOI] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Smith LJ, Holbrook JT, Wise R, Blumenthal M, Dozor AJ, Mastronarde J, et al. Dietary intake of soy genistein is associated with lung function in patients with asthma. Journal of Asthma 2004;41(8):833‐43. [] [DOI] [PubMed] [Google Scholar]
Szlagatys 2005 {published data only}
- Szlagatys‐Sidorkiewicz A, Korzon M, Malaczynska T, Renkel J, Popadiuk S, Woźniak M. The antioxidative‐prooxidative balance in children with asthma treated with inhaled corticosteroids and long acting beta2‐agonists [Rownowaga antyoksydacyjno‐prooksydacyjna u dzieci z astma leczonych kortykosteroidami wziewnymi i dlugo dzialajacymi beta2‐mimetykami]. Pneumonologia I Alergologia Polska 2005;73(2):178‐81. [] [PubMed] [Google Scholar]
Ting 1983 {published data only}
- Ting S, Mansfield LE, Yarbrough J. Effects of ascorbic acid on pulmonary functions in mild asthma. Journal of Asthma 1983;20(1):39‐42. [] [DOI] [PubMed] [Google Scholar]
Trenca 2001 {published data only}
- Trenca CA, Koenig JQ, Williams PV. Dietary antioxidants and ozone‐induced bronchial hyperresponsiveness in adults with asthma. Archives of Environmental Health 2001;56(3):242‐9. [] [DOI] [PubMed] [Google Scholar]
Tug 2007 {published data only}
- Tug T, Godekmerdan A, Sari N, Karatas F, Erdem ES. Effects of supportive treatment such as antioxidant or leukotriene receptor antagonist drugs on inflammatory and respiratory parameters in asthma patients. Clinical Pharmacology and Therapeutics 2007;81(3):371‐6. [] [DOI] [PubMed] [Google Scholar]
Tunnicliffe 2003 {published data only}
- Tunnicliffe WS, Harrison RM, Kelly FJ, Dunster C, Ayres JG. The effect of sulphurous air pollutant exposures on symptoms, lung function, exhaled nitric oxide, and nasal epithelial lining fluid antioxidant concentrations in normal and asthmatic adults. Occupational and Environmental Medicine 2003;60(11):e15. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
Varshavskii 2003 {published data only}
- Varshavskii BIa, Trubnikov GV, Galaktipmpva LP, Koreniak NA, Koledeznaia IL, Oberemok AN. Oxidant‐antioxidant status of patients with bronchial asthma during inhalation and systemic glucocorticoid therapy [Russian] [Oksidantno‐antioksidantnyi status bol'nykh bronkhial'noi astmoi pri ingaliatsionnoi u sustenbii gkuyjijirtujiudbii terapii]. Terapevticheskii Arkhiv 2003;75(3):21‐4. [] [PubMed] [Google Scholar]
Vazquez‐Armenta 2012 {published data only}
- Vazquez‐Armenta G, Barragan‐Alvarez C, Loza‐Valdes A, Ramos‐Marquez ME, Siller‐Lopez F. Protein carbonyl and sputum eosinophil number decrease with alpha‐lipoic acid adjuvant therapy in asthma patients [Abstract]. Free Radical Biology & Medicine 2012;53(Suppl 2):S117. [] [Google Scholar]
Voelker 1997 {published data only}
- Voelker R. Antioxidants and asthma. JAMA 1997;277(24):1926. [] [Google Scholar]
Voitsekhovskaia 2007 {published data only}
- Voitsekhovskaia IuG, Skesters A, Orlikov GA, Silova AA, Rusakova NE, Larmane LT, et al. Assessment of some oxidative stress parameters in bronchial asthma patients beyond add‐on selenium supplementation. [Russian]. Biomeditsinskaia Khimiia 2007;53(5):577‐84. [] [PubMed] [Google Scholar]
Wood 2007 {published data only}
- Wood LG, Garg ML, Powell H, Gibson PG. Dietary lycopene reduces neutrophilic airway inflammation in asthma [Abstract]. Thoracic Society of Australia and New Zealand Annual Scientific Meeting, 25‐28 March 2007, Auckland. 2007:Abstract TP002. []
Wood 2008 {published data only}
- Wood LG, Garg ML, Powell H, Gibson PG. Lycopene‐rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radical Research 2008;42(1):94‐102. [] [DOI] [PubMed] [Google Scholar]
Wood 2012 {published data only}
- Wood LG, Garg ML, Smart J, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: A randomized clinical trial [Abstract]. Respirology 2012;17(Suppl 1):45. [] [DOI] [PubMed] [Google Scholar]
Wood 2012a {published data only}
- Wood LG, Garg ML, Smart JM, Scott HA, Barker D, Gibson PG. Manipulating antioxidant intake in asthma: A randomized controlled trial. American Journal of Clinical Nutrition 2012;96(3):534‐43. [] [DOI] [PubMed] [Google Scholar]
Yamamoto 2013 {published data only}
- Yamamoto M, Singh A, Sava F, Pui M, Tebbutt SJ, Carlsten C. MicroRNA expression in response to controlled exposure to diesel exhaust: Attenuation by the antioxidant N‐acetylcysteine in a randomized crossover study. Environmental Health Perspectives 2013;121(6):670‐5. [] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Akhtar 2016 {published data only}
- Akhtar R, Jain S, Kumar A, Bhatia S, Joshi JC, Singh D, et al. Evaluation of therapeutic efficacy of ascorbic acid in patients with bronchial asthma. Journal of young pharmacists : JYP 2016;8(3):214‐9. [Google Scholar]
Hemila 2011 {published data only}
- Hemila H, Al‐Biltagi M, Baset AA. Vitamin C and asthma in children: modification of the effect by age, exposure to dampness and the severity of asthma. Clinical and translational allergy 2011;1(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hemilä H, Al‐Biltagi M, Baset AA. Retraction: Vitamin C and asthma in children: modification of the effect by age, exposure to dampness and the severity of asthma. Clin Transl Allergy 2012;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
IRCT138904224359N1 {published data only}
- Effect of vitamin E and C supplements on improving asthma. Iranian Registry of Clinical Trials 2010. [URL: http://www.irct.ir/searchresult.php?id=4359&number=1]
NCT01057615 {published data only}
- Effect of fish oil and vitamin C on exercise‐induced bronchoconstriction and airway inflammation in asthma. ClinicalTrials.gov 2011. [URL: http://clinicaltrials.gov/show/NCT01057615]
Additional references
Arab 2002
- Arab L, Steck‐Scott S, Fleishauer AT. Lycopene and the lung. Experimental Biology and Medicine 2002;227(10):894‐9. [DOI] [PubMed] [Google Scholar]
Blanck 2008
- Blanck HM, Gillespie C, Kimmons JE, Seymour JD, Serdula MK. Trends in fruit and vegetable consumption among U.S. men and women, 1994–2005. Preventing Chronic Disease 2008;5(2):1‐10. [PMC free article] [PubMed] [Google Scholar]
BTS/SIGN 2011
- British Guideline on the Management of Asthma. A national clinical guideline. British Thoracic Society (www.brit‐thoracic.org.uk) and Scottish Intercollegiate Guidelines Network (www.sign.ac.uk) Revised May 2011 (accessed 12 October 2013).
CDC 2012
- Centers for Disease Control and Prevention. Trends in Asthma Prevalence, Health Care, and Mortality in the United States, 2001‐2010. CDC (http://www.cdc.gov/nchs/data/databriefs/db94.htm) (accessed 12 October 2013).
CDCP 2011
- Centers for Disease Control and Prevention. Vital signs. www.cdc.gov/vitalsigns/asthma/ (accessed 12 October 2013).
Chatzi 2007
- Chatzi L, Apostolaki G, Bibakis I, Skypala I, Bibaki‐Liakou V, Tzanakis N, et al. Protective effect of fruits, vegetables and the Mediterranean diet on asthma and allergies among children in Crete. Thorax 2007;62:677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clayton 2005
- Clayton S. Paediatric asthma: overcoming barriers to an improved quality of life. British Journal of Nursing 2005;14(2):80–5. [DOI] [PubMed] [Google Scholar]
EUFIC 2012
- European Food Information Council. Fruit and vegetable consumption in Europe – do Europeans get enough?. www.eufic.org/article/en/expid/Fruit‐vegetable‐consumption‐Europe/ (accessed 12 October 2013).
Fitzgerald 2006
- Fitzgerald JM, Gibson PG. Asthma exacerbations. 4: Prevention. Thorax 2006;61:992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2011
- Global Initiative for Asthma (GINA). GINA Report, Global Strategy for Asthma Management and Prevention. Updated December 2011. www.ginasthma.org (accessed 12 October 2013).
Hemila 2013
- Hemila H. Vitamin C may alleviate exercise‐induced bronchoconstriction: a meta‐analysis. BMJ Open 2013;3:e002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org (accessed 12 October 2013).
Kaur 2009
- Kaur B, Rowe BH, Stovold E. Vitamin C supplementation for asthma. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000993.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krishnan 2006
- Krishnan V, Diette GB, Rand CS, Bilderback AL, Merriman B, Hansel NN, et al. Mortality in patients hospitalized for asthma exacerbations in the United States. American Journal of Respiratory and Critical Care Medicine 2006;174(6):633‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Misso 2005
- Misso NLA, Brooks‐Wildhaber J, Ray S, Vally H, Thompson PJ. Plasma concentrations of dietary and non dietary antioxidants are low in severe asthma. European Respiratory Journal 2005;26:257–64. [DOI] [PubMed] [Google Scholar]
NHS 2011
- Hospital episode statistics. www.hesonline.nhs.uk (accessed 12 October 2013).
NIH 2007
- Guidelines for the Diagnosis and Management of Asthma (EPR 3). www.nhlbi.nih.gov/guidelines/asthma/ 2007 (accessed 12 October 2013).
Okoko 2007
- Okoko BJ, Burney PB, Newson RB, Potts JF, Shaheen SO. Childhood asthma and fruit consumption. European Respiratory Journal 2007;29:1161–8. [DOI] [PubMed] [Google Scholar]
Parsons 2013
- Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, et al. An official American Thoracic Society clinical practice guideline: exercise‐induced bronchoconstriction. American Journal of Respiratory and Critical Care Medicine 2013;187(9):1016‐27. [DOI] [PubMed] [Google Scholar]
Patel 2006
- Patel BD, Welch AA, Bingham SA, Luben RN, Day NE, Khaw K‐T, et al. Dietary antioxidants and asthma in adults. Thorax 2006;61:388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Santanello 1999
- Santanello N C, Zhang J, Seidenberg B, Reiss T F, Barber B L. What are minimal important changes for asthma measures in a clinical trial?. Eur Respir J 1999;14:23‐7. [DOI] [PubMed] [Google Scholar]
Seaton 1994
- Seaton A, Godden DJ, Brown K. Increase in asthma: a more toxic environment or a more susceptible population?. Thorax 1994;49(2):171‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2007
- Bousquet J, Khaltaev N (editors). World Health Organization. Global Surveillance, Prevention and Control of Chronic Respiratory Diseases: A Comprehensive Approach. Geneva: WHO Press, 2007. [ISBN 978 92 4 156346 8] [Google Scholar]
WHO 2012
- World Health Organization. Promoting fruit and vegetable consumption around the world. www.who.int/dietphysicalactivity/fruit/en/index2.html (accessed 12 October 2013).
Wilson 2012
- Wilson SR, Rand CS, Cabana MD, Foggs MB, Halterman JS, Olson L, et al. Asthma outcomes: Quality of life. Journal of Allergy and Clinical Immunology 2012;129(3):S88–S123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2007
- Wu F, Takaro TK. Childhood asthma and environmental interventions. Environmental Health Perspectives 2007;115(6):971–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


