Abstract
Background
Glucocorticoids are commonly used for croup in children. This is an update of a Cochrane Review published in 1999 and previously updated in 2004 and 2011.
Objectives
To examine the effects of glucocorticoids for the treatment of croup in children aged 0 to 18 years.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, Issue 2, 2018), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (1946 to 3 April 2018), and Embase (Ovid) (1996 to 3 April 2018, week 14), and the trials registers ClinicalTrials.gov (3 April 2018) and the World Health Organization International Clinical Trials Registry Platform (ICTRP, 3 April 2018). We scanned the reference lists of relevant systematic reviews and of the included studies.
Selection criteria
We included randomised controlled trials (RCTs) that investigated children aged 0 to 18 years with croup and measured the effects of glucocorticoids, alone or in combination, compared to placebo or another pharmacologic treatment. The studies needed to report at least one of our primary or secondary outcomes: change in croup score; return visits, (re)admissions or both; length of stay; patient improvement; use of additional treatments; and adverse events.
Data collection and analysis
One author extracted data from each study and another verified the extraction. We entered the data into Review Manager 5 for meta‐analysis. Two review authors independently assessed risk of bias for each study using the Cochrane 'Risk of bias' tool and the certainty of the body of evidence for the primary outcomes using the GRADE approach.
Main results
We added five new RCTs with 330 children. This review now includes 43 RCTs with a total of 4565 children. We assessed most (98%) studies as at high or unclear risk of bias. Compared to placebo, glucocorticoids improved symptoms of croup at two hours (standardised mean difference (SMD) ‐0.65, 95% confidence interval (CI) ‐1.13 to ‐0.18; 7 RCTs; 426 children; moderate‐certainty evidence), and the effect lasted for at least 24 hours (SMD ‐0.86, 95% CI ‐1.40 to ‐0.31; 8 RCTs; 351 children; low‐certainty evidence). Compared to placebo, glucocorticoids reduced the rate of return visits or (re)admissions or both (risk ratio 0.52, 95% CI 0.36 to 0.75; 10 RCTs; 1679 children; moderate‐certainty evidence). Glucocorticoid treatment reduced the length of stay in hospital by about 15 hours (mean difference ‐14.90, 95% CI ‐23.58 to ‐6.22; 8 RCTs; 476 children). Serious adverse events were infrequent. Publication bias was not evident. Uncertainty remains with regard to the optimal type, dose, and mode of administration of glucocorticoids for reducing croup symptoms in children.
Authors' conclusions
Glucocorticoids reduced symptoms of croup at two hours, shortened hospital stays, and reduced the rate of return visits to care. Our conclusions have changed, as the previous version of this review reported that glucocorticoids reduced symptoms of croup within six hours.
Plain language summary
Glucocorticoids for croup in children
Review question
We assessed the effectiveness of glucocorticoids for croup in children to determine if they reduced croup symptoms; minimised return visits to care; shortened length of stay; reduced the need for additional treatments; or had side effects.
Background
Croup causes the throat and windpipe to swell, resulting in hoarseness, a barking cough, and noisy breathing. Glucocorticoids can reduce swelling, making it easier to breathe.
This review was previously published in 1999 and updated in 2004 and 2011.
Search date
We searched for articles published up to 3 April 2018.
Study characteristics
We included 43 studies with 4565 children aged up to 18 years published from 1964 to 2013. The glucocorticoids investigated included beclomethasone, betamethasone, budesonide, dexamethasone, fluticasone, and prednisolone. Most studies (26, 60%) compared any glucocorticoid to placebo. Of these, 15 (58%) tested dexamethasone compared to placebo. Three studies compared 0.6 mg/kg to 0.15 mg/kg dosages of dexamethasone, a common clinical question. Half of the studies (22, 51%) described outpatients who presented to emergency departments or outpatient clinics, and 18 (42%) took place in North America, eight (19%) in Europe, seven (16%) in Asia, and 10 (23%) in Australia. Twenty‐six (60%) studies compared glucocorticoids to fake treatment (placebo); four (10%) compared glucocorticoids to epinephrine; 11 (26%) compared one glucocorticoid to another; three (7%) compared one glucocorticoid to a combination of glucocorticoids; five (12%) compared glucocorticoids given in different ways; and four (9%) compared glucocorticoids given in different amounts.
Study funding sources
Funding sources included government (12%), academic or research institute (7%), industry (19%), or foundations (7%). More than half of studies (56%) did not report funding sources.
Key results
Glucocorticoids improved croup symptoms at two hours (moderate‐certainty evidence), and the effect lasted at least 24 hours (low‐certainty evidence). Glucocorticoids reduced rates of return visits, admissions, and readmissions (moderate‐certainty evidence). When treated with placebo, 204 of every 1000 children will return for medical care. When treated with glucocorticoids, 74 to 153 of every 1000 children will return for medical care. Glucocorticoids reduced length of stay by 15 hours (range 6 to 24 hours), but made no difference in need for additional treatments. Of studies that compared glucocorticoids to placebo, 50% collected data on side effects. Four studies reported rare occurrences of secondary infections (e.g. pneumonia, ear infection). Most other side effects were not severe (e.g. emotional distress, hyperactivity, vomiting). We are not certain which type, amount, and administration mode (oral, inhaled, injected) of glucocorticoids is best for reducing symptoms of croup in children.
Quality of the evidence
Most studies (98%) had methods problems, reporting issues, or both.
Summary of findings
Background
Description of the condition
Croup (laryngotracheobronchitis) is a common cause of upper airway obstruction in children and is characterised by hoarseness, a barking cough, and inspiratory stridor. These symptoms are thought to occur as a result of oedema of the larynx and trachea, which has been triggered by a recent viral infection. Parainfluenza virus type 1 is the pathogen most commonly identified in cases of croup (Rihkanen 2008). Although croup is a self limiting illness, it is a large burden on healthcare systems because of frequent visits to doctors and emergency departments and, when necessary, hospitalisations. Each year, about 3% of children are diagnosed with croup, usually between the ages of six months and three years (Johnson 2009). During a six‐year period in Alberta, Canada, 20,019 infants aged under two years visited emergency departments for 27,355 episodes of croup, of whom 8.0% were admitted to hospital (Rosychuk 2010). Population‐based studies indicate that less than 5% of children with croup are typically admitted to the hospital for treatment (Bjornson 2008).
Description of the intervention
Since the late 1980s it has been recognised that glucocorticoids provide some clinical benefit for children with croup. In 1989, a meta‐analysis by Kairys 1989 showed that treatment with glucocorticoids resulted in significantly greater clinical improvement after 12 hours and a reduced incidence of intubations as compared to placebo. In 2000, a meta‐analysis by Griffin 2000 demonstrated that treatment with glucocorticoids resulted in greater improvements in croup score and fewer hospital admissions compared to placebo. Although racemic adrenaline (epinephrine), or L‐adrenaline, has been shown to provide temporary relief to children with croup, it is not thought to have long‐term benefits (Bjornson 2008; Johnson 2009; Waisman 1992). In the past, the standard management of croup included mist treatment (i.e. treatment with humidified air), although there is little evidence of its effectiveness (Bjornson 2008; Lavine 2001; Moore 2007; Neto 2002; Scolnik 2006).
How the intervention might work
Localised inflammation of the upper airway caused by a virus is believed to cause croup symptoms. The narrowest part of the airway is the subglottic region. Even small amounts of oedema in this region can significantly increase the work of breathing in young children because airway resistance increases dramatically. Poiseuille's law states that airway resistance is related to the radius of the airway to the power of four (Loftis 2006). Glucocorticoids are believed to reduce the degree of inflammation and swelling through their anti‐inflammatory properties, leading to decreased effort in breathing for the child.
Why it is important to do this review
This Cochrane Review was first published in the Cochrane Library in 1999 and included 24 randomised controlled trials (RCTs) that examined the effectiveness of glucocorticoids for children with croup (Ausejo 2000). Since then, there has been continued interest in the use of glucocorticoids to treat young people with croup, and new RCTs have been published. The review was updated in 2004 (Russell 2004) and 2011 (Russell 2011) to include RCTs published since the first version of this review was completed (Ausejo 2000). Areas of uncertainty remained regarding the use of glucocorticoids in children with croup, for example glucocorticoids provided via different modes of administration (oral, nebulised, intramuscular) and the role of glucocorticoids in mild cases of croup. The validity of the results in previous versions of the review was threatened by publication bias. The incorporation of new RCTs may influence the presence of publication bias.
It was important to update this review to incorporate newly published evidence. Furthermore, previous versions of this review did not extract and analyse data for all relevant primary and secondary outcomes, and did not incorporate complete assessments of study‐level risk of bias or outcome‐level certainty of evidence. These elements are now recognised as critical to interpreting the findings of a systematic review. Moreover, data on adverse events, a potentially important outcome, were not extracted. For the first time in this update, we have extracted and analysed data for all primary and secondary outcomes, judged risk of bias for all included studies, and assessed the certainty of the evidence for the primary outcomes using GRADE principles. We have also presented data on adverse events.
Objectives
To examine the effects of glucocorticoids for the treatment of croup in children aged 0 to 18 years.
Methods
Criteria for considering studies for this review
Types of studies
We only included RCTs. Studies that met our inclusion criteria were included regardless of language, publication status, trial conduct and reporting quality, or risk of bias. We excluded all other study designs.
Types of participants
We included studies of children aged 0 to 18 years diagnosed with croup, pseudo croup, or laryngotracheitis. We defined croup as a syndrome consisting of hoarseness, barking cough, and stridor, in which alternative diagnosis of acute stridor was excluded. We included both inpatients and outpatients; we considered children admitted to the emergency department to be outpatients.
Types of interventions
We included studies where the experimental intervention was one or more glucocorticoid, provided via any mode of administration. We placed no restrictions on the type or dose of glucocorticoid administered. We included any study where the comparator was placebo or any other active pharmacologic treatment. Comparisons included: any glucocorticoid compared to placebo; any glucocorticoid compared to epinephrine; one glucocorticoid compared to one or a combination of other glucocorticoids; glucocorticoids given by different modes of administration; glucocorticoids given in different doses. We excluded studies if none of the treatment groups received one or more glucocorticoid.
Types of outcome measures
We included RCTs that measured and reported on one or more of our primary or secondary outcomes, as follows.
Primary outcomes
Change in clinical croup score from baseline to 2, 6, 12, and/or 24 hours.
Return visits or (re)admissions to the hospital or both.
Secondary outcomes
Length of stay in the hospital or emergency department.
Patient improvement at 2, 6, 12, and/or 24 hours (yes or no, as reported in the individual studies).
The use of additional treatments, including: antibiotics, epinephrine, intubation/tracheostomy, mist tent, and/or supplemental glucocorticoids.
Any adverse events.
We excluded studies that did not report on any of the primary or secondary outcomes.
Search methods for identification of studies
Electronic searches
We searched electronic sources using a strategy developed by a research librarian on 3 April 2018 (Appendix 1). We included subject headings and key words for croup and glucocorticoids, and restricted the search to RCTs. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, Issue 2, 2018), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, Ovid MEDLINE Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE (1946 to 3 April 2018), and Embase (Ovid) (1996 to 3 April 2018, week 14).
An interim update search of the following databases was performed by previous review authors in 2014: CENTRAL (the Cochrane Library 2014, Issue 8), MEDLINE (1966 to September week 1, 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (12 September 2014), and Embase (1974 to September 2014) (Appendix 2). The results for the 2014 searches are included in this update of the review, and previous versions of the review have been used as sources of studies (Ausejo 1999; Ausejo 2000; Russell 2004; Russell 2011).
Searching other resources
We searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch) on 3 April 2018 (Appendix 1). We scanned the reference lists of relevant systematic reviews identified during screening and of the included studies to identify additional relevant primary studies. A separate author group searched the same trial registries as part of the interim update search on 16 September 2014.
Data collection and analysis
Selection of studies
The unique records identified via the search following the removal of duplicates were stored in EndNote reference management software (EndNote). These records were transferred to a Microsoft Excel workbook for screening (Microsoft Excel). Two review authors (AG, CJ) independently screened the records, first by title and abstract (when available), and then by full text. During title and abstract screening, the review authors marked each record as 'include', 'exclude', or 'unsure'. All records marked 'include' or 'unsure' by either review author were selected for full‐text screening. During full‐text screening, the review authors again marked each candidate record as 'include', 'exclude', or 'unsure'. The review authors then convened to reach consensus on which studies should be included. In the case of uncertainty, a third review author (MG) or an independent third party provided arbitration. Members of another author group screened the records identified in the 2014 interim update search following a similar process.
Data extraction and management
Two review authors (AG, MG) extracted data from the English and French language studies using a Microsoft Excel workbook designed by the review author team (Microsoft Excel). Data from the Spanish and Danish language studies were extracted by native or second‐language speakers external to the author team. From each included study, we extracted characteristics of the children (inpatient or outpatient; croup severity), croup score used (if relevant), and experimental and control interventions (type of drug, route of administration, dosage). We also collected data on the primary and secondary outcome measures when available. We extracted croup‐related return visits or (re)admissions or both if specified in the publication. If the reason for a return visit and/or (re)admission was not specified, we extracted all return visits, admissions, and readmissions. In some studies, admissions, return visits, and readmissions were reported. In this case, we extracted the data as follows: admissions when reported with or without return visits and/or readmissions; return visits if reported with or without readmissions, and admissions was not reported; and readmissions if neither admissions nor return visits was reported. One review author (AG, MG, or CJ) verified the data extracted from each individual study to identify and correct errors or omissions. No additional data were obtained from trial authors for this update.
We extracted data from graphs using Plot Digitizer open source software from 12 studies (Amir 2006; Cetinkaya 2004; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Husby 1993; Kuusela 1988; Roberts 1999). Plot Digitizer and similar tools enable data to be extracted from graphs with greater efficiency and interrater reliability compared to manual extraction (Burda 2017; Kadic 2016). Two review authors (AG, MG) independently extracted data from the available graphs and convened to reach consensus on the values obtained. When the extracted values were very similar, we took the mean of both authors' estimates. When the values did not coincide, we re‐extracted the data to resolve errors in estimation.
Assessment of risk of bias in included studies
We used the Cochrane 'Risk of bias' tool (2010 version) to assess risk of bias for all included studies (Higgins 2011b). Following Cochrane standards, we judged the risk of bias for each record as low, unclear, or high among seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other bias. We determined overall risk of bias as follows: low when all domains were judged as low risk; unclear when one or more domains were judged as unclear risk and no domains were judged as high risk; and high when one or more domains were judged as high risk. Two review authors (AG, MG) independently assessed risk of bias for each included study. For the Danish and Spanish language studies, native or second‐language speakers extracted relevant text snippets from the studies and translated these to English to facilitate the 'Risk of bias' assessments. In the case of disagreement, the review authors discussed the judgements for each domain and overall until consensus was reached. When the review authors could not reach consensus, a third party external to the review team was consulted to facilitate a decision.
When available, we used trial registers to complement the assessment of risk of bias. We searched for trials registered on ClinicalTrials.gov, the WHO ICTRP, the ISRCTN registry, and via Google. We identified trial register records for two (5%) of 43 included studies (Fifoot 2007; Garbutt 2013).
Measures of treatment effect
We entered all search results into Review Manager 5 for analysis (Review Manager 2014). Croup scores were reported as the Westley score (Westley 1978), the telephone outpatient (TOP) score (Bjornson 2016), the Downes and Raphaelly score (Downes 1975), or various author‐created scales. We therefore used standardised mean differences (SMDs) to combine the outcome for any croup score. A treatment effect (difference between treatment means) divided by its measurement variation (e.g. a pooled standard deviation) gives the SMD. We did not find effect estimates to be significantly different between Westley and other croup scores, so we included studies that reported any croup score in the subgroup analyses. Of note, a decrease in Westley score of one point from baseline is thought to be a clinically important change.
We expressed length of stay as mean differences (MDs) and calculated an overall MD. We calculated risk ratios (RRs) for binary data (i.e. return visits and/or (re)admissions, patient improvement, use of additional treatments). We calculated risk differences (RDs) where outcomes had zero events in both groups. For return visits and/or (re)admissions, we calculated the number needed to treat for an additional beneficial outcome (NNTB) for significant results. Because there was substantial variation in control group event rates between studies, we reported the NNTB for the mean control group rate, as well as for the smallest and largest control group rate observed.
We reported data on adverse events narratively.
Unit of analysis issues
We calculated the change from baseline croup score in 28 (65%) studies where the change from baseline measures was not reported directly (Alshehr 2005; Amir 2006; Cetinkaya 2004; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Johnson 1996; Klassen 1994; Klassen 1998; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Rittichier 2000; Roberts 1999; Roorda 1998; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982).
We pooled counts, means, and variances using standard formulae for six (14%) studies that contained more than one experimental treatment group (Cetinkaya 2004; Eboriadou 2010; Fifoot 2007; Geelhoed 1995c; Johnson 1998; Luria 2001). One study by Geelhoed (Geelhoed 1995a; Geelhoed 1995b), and another by Skowron (Skowron 1966a; Skowron 1966a&b; Skowron 1966b), presented the results of two individual trials in one publication. We treated these as separate comparisons in the analyses and used pooled counts only when they were reported as such in the publications.
Dealing with missing data
When they were not directly reported, we estimated the variances for continuous data in accordance with the work of Abrams 2005 and Follmann 1992. Using standard formulae, we imputed standard deviations from standard errors in three (7%) studies (Alshehr 2005; Johnson 1998; Von Mühlendahl 1982), ranges in three (7%) studies (Alshehr 2005; Roorda 1998; Super 1989), 95% confidence intervals (CIs) in two (5%) studies (Fitzgerald 1996; Klassen 1998), and interquartile ranges (IQRs) in three (7%) studies (Johnson 1996; Klassen 1994; Klassen 1998). For the change in croup score from baseline, when not directly reported (n = 14, 33%), we derived the variance of the change assuming a correlation of 0.5 between pre‐ and post‐treatment scores (Alshehr 2005; Amir 2006; Chub‐Uppakarn 2007; Fitzgerald 1996; Johnson 1996; Klassen 1994; Klassen 1998; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Roorda 1998; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982).
For 11 (26%) studies where there were inadequate data from which to impute variances for change in croup score or length of stay, we substituted average variances from other studies in the main analysis (Cetinkaya 2004; Dobrovoljac 2012; Eboriadou 2010; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Kuusela 1988; Massicotte 1973; Roberts 1999; Skowron 1966a; Skowron 1966b). When the number of studies with imputed data within a meta‐analysis is relatively small, Furukawa and colleagues assert that variance data can be safely borrowed from other studies and still provide accurate results (Furukawa 2006). For certain outcomes only one study was included in the comparison, and that study did not report a variance estimate. In this case, we did not calculate a point estimate of effect (Cetinkaya 2004; Duman 2005; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Rittichier 2000).
We substituted medians for means in eight (19%) studies (Alshehr 2005; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Johnson 1996; Klassen 1994; Klassen 1998; Super 1989; Von Mühlendahl 1982). When data for our prespecified time points (2, 6, 12, and 24 hours from baseline) were not reported, we used time points close to these if available. We substituted one hour for two hours in one study (Dobrovoljac 2012); four hours for six hours in 12 (28%) studies (Alshehr 2005; Amir 2006; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Massicotte 1973); five hours or discharge for six hours in one study (Johnson 1998); and 14 hours for 12 hours in one study (Massicotte 1973).
Assessment of heterogeneity
We assessed heterogeneity quantitatively with the Chi² test for heterogeneity and the I² statistic (Higgins 2002). The I² statistic indicates the per cent variability due to between‐study (or interstudy) variability as opposed to within‐study (or intrastudy) variability. We considered an I² of less than 40% to be low (potentially unimportant), 30% to 60% to be moderate, 50% to 90% to be substantial, and 75% to 100% to be considerable (see Higgins 2011a, Section 9.5.2).
Assessment of reporting biases
In addition to visually inspecting the funnel plots, we used the rank correlation test and weighted regression for the detection of publication bias (Begg 1994; Egger 1997; Light 1984). We used more than one method because the relative merits of the methods are not well established.
Data synthesis
We used random‐effects models to combine treatment effects regardless of quantified heterogeneity for the analyses of all outcomes.
GRADE and 'Summary of findings' tables
We created 'Summary of findings' tables for our two main comparisons (any glucocorticoid compared to placebo, and any glucocorticoid compared to epinephrine) for the primary outcomes: change in croup score at 2, 6, 12, and 24 hours from baseline, and return visits or (re)admissions or both. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence as it relates to the studies that contributed data to the meta‐analyses (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), employing GRADEpro GDT 2015 software. We justified all decisions to downgrade the certainty of the evidence using footnotes, and made comments to aid the readers' understanding where necessary. Of note, we downgraded single‐study analyses for inconsistency, because there was no evidence of consistency. We also created 'Summary of findings' tables for the remaining comparisons. To not detract from the two main comparisons, these are included in the Additional tables section.
Subgroup analysis and investigation of heterogeneity
We explored heterogeneity between studies using subgroup analyses for the primary outcomes of change in croup score from baseline to 2, 6, 12, and 24 hours, and return visits or (re)admissions or both, using the Chi² test for subgroup differences in meta‐analysis. We explored heterogeneity by croup score, by inpatient or outpatient status, and by glucocorticoid.
Sensitivity analysis
In some analyses, we imputed variance data for most of the included RCTs (e.g. any glucocorticoid compared to placebo, change in croup score after two hours). We undertook sensitivity analyses for these and all other analyses containing imputed variance data using the largest, smallest, and average variances from the other included RCTs. As per the protocol for the review, we did not undertake any additional sensitivity analyses.
Results
Description of studies
Results of the search
An interim search in 2014 identified 30 unique studies. The previous authors screened the studies, and added one new included study, Dobrovoljac 2012, and one new excluded study, Faghihinia 2007. The previous authors classified three trials as awaiting classification (Eboriadou 2010; Garbutt 2013; Mohammadzadeh 2014).
Our 2018 searches identified 79 records. This included 69 records retrieved via the database searches; five records identified via the trial register searches; three trials awaiting classification from the 2014 search (Eboriadou 2010; Garbutt 2013; Mohammadzadeh 2014); and two trials identified via other means (Chub‐Uppakarn 2007; Soleimani 2013). We identified Soleimani 2013 via a search of Google.ca. This study was not indexed in any of the databases that we otherwise searched. We identified Chub‐Uppakarn 2007 following a scan of the list of excluded studies from the previous version of this review, and it appeared to meet our inclusion criteria.
After removing duplicates, we screened 55 records by title and abstract and excluded 44 records. We screened the remaining 11 records by full text and included four studies (Chub‐Uppakarn 2007; Eboriadou 2010; Garbutt 2013; Soleimani 2013). We also included one new ongoing study (ACTRN12609000290291). We added five excluded studies: Gill 2017 and Roked 2015 were not randomised trials; the intervention was not a glucocorticoid in Eghbali 2016 and Mohammadzadeh 2014; and Faraji‐Goodarzi 2018 did not report any relevant outcomes. We excluded one new ongoing study as it reported no relevant outcomes and was terminated due to inadequate recruitment (NCT01748162).
A flow diagram illustrating the study selection process for the 2018 search is shown in Figure 1. We added five new RCTs with 330 children (Chub‐Uppakarn 2007; Dobrovoljac 2012; Eboriadou 2010; Garbutt 2013; Soleimani 2013). We included 43 studies (involving a total of 4565 children) in this 2018 updated review (see Characteristics of included studies). Two of the included studies reported on the findings of two individual RCTs each (Geelhoed 1995a; Geelhoed 1995b; Skowron 1966a; Skowron 1966a&b; Skowron 1966b). We presented these as separate comparisons in the analyses and only pooled the data when they were presented as such in the publication (Skowron 1966a&b). One of the included studies was published in both English and Danish (Husby 1993). We used the English report to complete the data extraction and 'Risk of bias' appraisal.
1.
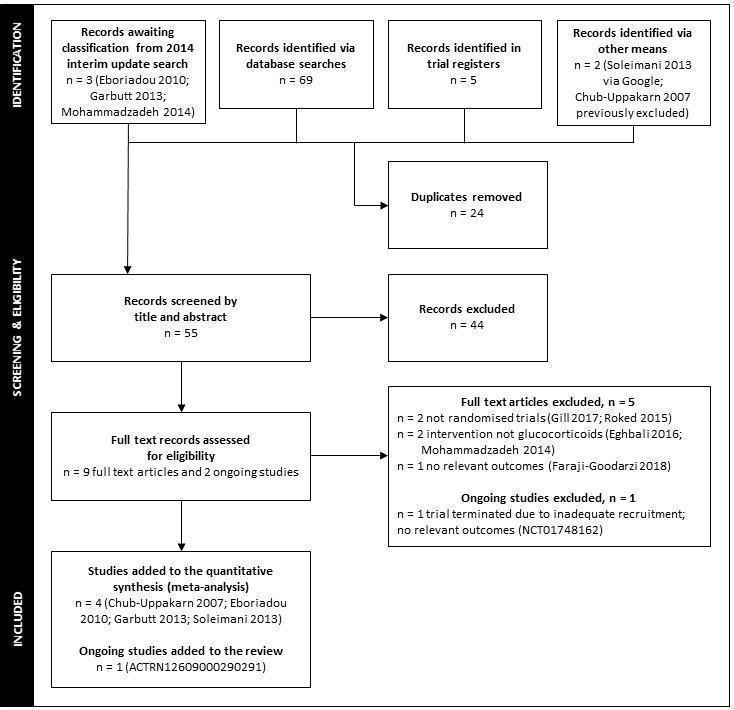
Flow diagram of study selection for the 2018 update searches. In 2014, the review authors identified 30 studies in an interim update search. The review authors included one new study, Dobrovoljac 2012, and excluded one new study, Faghihinia 2007.
Included studies
Participant and trial characteristics
Forty studies (93%) were published in English, and one each in French (Massicotte 1973), Spanish (Martinez Fernandez 1993), and Danish (Vad Pedersen 1998). Three studies (7%) included children with mild croup (Bjornson 2004; Geelhoed 1996; Luria 2001). Sample sizes tended to be small with a median of 72 (interquartile range (IQR) 54 to 99) children. Twenty‐two studies (51%) assessed outpatient children (n = 21 emergency department visits; n = 1 physician office visits) (Alshehr 2005; Amir 2006; Bjornson 2004; Cetinkaya 2004; Cruz 1995; Dobrovoljac 2012; Donaldson 2003; Duman 2005; Eboriadou 2010; Fifoot 2007; Garbutt 2013; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1996; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Luria 2001; Rittichier 2000; Soleimani 2013; Sparrow 2006). Twenty‐one studies (49%) assessed hospitalised children (Chub‐Uppakarn 2007; Eden 1964; Eden 1967; Fitzgerald 1996; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; James 1969; Koren 1983; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Roorda 1998; Skowron 1966a; Skowron 1966b; Super 1989; Tibballs 1992; Vad Pedersen 1998; Von Mühlendahl 1982).
Thirty‐one studies (72%) were two‐armed trials (Alshehr 2005; Amir 2006; Bjornson 2004; Chub‐Uppakarn 2007; Cruz 1995; Dobrovoljac 2012; Donaldson 2003; Eden 1964; Eden 1967; Fitzgerald 1996; Garbutt 2013; Geelhoed 1996; Geelhoed 2005; Godden 1997; Husby 1993; James 1969; Johnson 1996; Klassen 1994; Klassen 1996; Koren 1983; Leipzig 1979; Massicotte 1973; Rittichier 2000; Roberts 1999; Roorda 1998; Soleimani 2013; Sparrow 2006; Super 1989; Tibballs 1992; Vad Pedersen 1998; Von Mühlendahl 1982); seven studies (16%) were three‐armed trials (Duman 2005; Eboriadou 2010; Fifoot 2007; Geelhoed 1995c; Johnson 1998; Klassen 1998; Luria 2001); and three studies (7%) were four‐armed trials (Cetinkaya 2004; Kuusela 1988; Martinez Fernandez 1993). Two studies (5%) included two individual two‐armed trials each (Geelhoed 1995a; Geelhoed 1995b; Skowron 1966a; Skowron 1966b).
Characteristics of the comparisons
Twenty‐six studies (60%) investigated any glucocorticoid compared to placebo. Of these, 15 (58%) investigated dexamethasone (Bjornson 2004; Cruz 1995; Dobrovoljac 2012; Eden 1967; Geelhoed 1996; James 1969; Johnson 1996; Koren 1983; Kuusela 1988; Leipzig 1979; Luria 2001; Martinez Fernandez 1993; Skowron 1966a&b; Super 1989; Von Mühlendahl 1982); four (15%) investigated budesonide (Godden 1997; Husby 1993; Klassen 1994; Roberts 1999); three (12%) investigated prednisolone (Eden 1964; Massicotte 1973; Tibballs 1992); one (4%) investigated fluticasone (Roorda 1998); and three (12%) investigated both dexamethasone and budesonide (Cetinkaya 2004; Geelhoed 1995c; Johnson 1998). Four studies (10%) investigated any glucocorticoid compared to epinephrine. Of these, one investigated budesonide (Fitzgerald 1996); two investigated dexamethasone (Kuusela 1988; Martinez Fernandez 1993); and one investigated both dexamethasone and beclomethasone (Eboriadou 2010).
Eleven studies (26%) investigated one glucocorticoid compared to another glucocorticoid. Of these, six investigated dexamethasone compared to budesonide (Cetinkaya 2004; Duman 2005; Geelhoed 1995c; Johnson 1998; Klassen 1998; Vad Pedersen 1998); one investigated dexamethasone compared to betamethasone (Amir 2006); one investigated dexamethasone compared to beclomethasone (Eboriadou 2010); and three investigated dexamethasone compared to prednisolone (Fifoot 2007; Garbutt 2013; Sparrow 2006). Three studies investigated one glucocorticoid compared to a combination of glucocorticoids. Of these, one investigated dexamethasone and budesonide compared to a combination of dexamethasone and budesonide (Klassen 1998), and two investigated dexamethasone compared to a combination of dexamethasone and budesonide (Geelhoed 2005; Klassen 1996).
Five studies (12%) investigated dexamethasone using different modes of administration. Of these, four investigated oral compared to intramuscular dexamethasone (Cetinkaya 2004; Donaldson 2003; Rittichier 2000; Soleimani 2013), and one investigated oral compared to nebulised dexamethasone (Luria 2001). Four studies investigated dexamethasone given in different doses. Of these, three investigated 0.60 mg/kg compared to 0.15 mg/kg dexamethasone (Alshehr 2005; Chub‐Uppakarn 2007; Fifoot 2007), and one investigated both 0.60 mg/kg compared to 0.30 mg/kg and 0.30 mg/kg compared to 0.15 mg/kg dexamethasone (Geelhoed 1995a; Geelhoed 1995b).
Reported outcomes: primary outcomes
Fifteen studies (35%) reported a two‐hour change in croup score (Amir 2006; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Husby 1993; Johnson 1996; Roberts 1999; Roorda 1998); 20 (47%) reported a six‐hour change in croup score (Alshehr 2005; Amir 2006; Chub‐Uppakarn 2007; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Godden 1997; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Kuusela 1988; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Roorda 1998; Vad Pedersen 1998; Von Mühlendahl 1982); 12 (28%) reported a 12‐hour change in croup score (Alshehr 2005; Chub‐Uppakarn 2007; Fitzgerald 1996; Geelhoed 1995c; Godden 1997; Kuusela 1988; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Super 1989; Vad Pedersen 1998; Von Mühlendahl 1982); and 11 (26%) reported a 24‐hour change in croup score (Alshehr 2005; Cetinkaya 2004; Fitzgerald 1996; Godden 1997; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Rittichier 2000; Roberts 1999; Roorda 1998; Super 1989). Of the 29 studies (67%) that reported a change in croup score, 17 (59%) used a validated score (the Westley score or a modified Westley score) (Alshehr 2005; Amir 2006; Cetinkaya 2004; Chub‐Uppakarn 2007; Dobrovoljac 2012; Duman 2005; Fifoot 2007; Godden 1997; Husby 1993; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Rittichier 2000; Roorda 1998; Super 1989); 11 (38%) used author‐created scales (Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 2005; Kuusela 1988; Leipzig 1979; Martinez Fernandez 1993; Massicotte 1973; Roberts 1999; Vad Pedersen 1998; Von Mühlendahl 1982); and one used the score by Downes 1975 (Eboriadou 2010). The studies by Bjornson 2004 and Garbutt 2013 used another validated score, the telephone outpatient (TOP) score, to measure clinical improvement. The TOP score is a two‐item, three‐point score used to assess the presence of stridor and barky cough by asking parents about their child's symptoms in the previous 24 hours (Bjornson 2016). Twenty‐six studies (60%) reported return visits or (re)admissions to the hospital or both (Alshehr 2005; Amir 2006; Bjornson 2004; Cruz 1995; Donaldson 2003; Duman 2005; Eboriadou 2010; Fifoot 2007; Fitzgerald 1996; Garbutt 2013; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996; Geelhoed 2005; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Luria 2001; Rittichier 2000; Roberts 1999; Skowron 1966a; Skowron 1966a&b; Skowron 1966b; Soleimani 2013; Sparrow 2006; Vad Pedersen 1998).
Reported outcomes: secondary outcomes
A total of 12 studies (28%) reported length of stay in the hospital or emergency department (Alshehr 2005; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Klassen 1998; Kuusela 1988; Leipzig 1979; Roorda 1998; Skowron 1966a; Skowron 1966a&b; Skowron 1966b; Sparrow 2006; Super 1989). Twelve studies (28%) reported patient improvement. Of these, one reported improvement after two hours (Roberts 1999); eight reported improvement after six hours (Eden 1964; Eden 1967; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Massicotte 1973; Roberts 1999); six reported improvement after 12 hours (Eden 1964; Eden 1967; James 1969; Massicotte 1973; Roberts 1999; Super 1989); and seven reported improvement after 24 hours (Cruz 1995; Donaldson 2003; Eden 1964; Eden 1967; James 1969; Roberts 1999; Super 1989). About two‐thirds of the included studies (n = 29) reported the use of additional treatments. Of these, 11 reported intubation/tracheotomies (Chub‐Uppakarn 2007; Eden 1967; Fitzgerald 1996; Geelhoed 1995c; Godden 1997; James 1969; Johnson 1996; Johnson 1998; Leipzig 1979; Roorda 1998; Skowron 1966a; Skowron 1966a&b; Skowron 1966b); four reported the use of antibiotics (Husby 1993; James 1969; Koren 1983; Rittichier 2000); 13 reported the use of supplemental glucocorticoids (Dobrovoljac 2012; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Johnson 1996; Klassen 1994; Klassen 1996; Klassen 1998; Rittichier 2000; Roorda 1998; Super 1989; Vad Pedersen 1998); 20 reported the use of epinephrine (Amir 2006; Dobrovoljac 2012; Donaldson 2003; Duman 2005; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 2005; Godden 1997; Johnson 1996; Johnson 1998; Klassen 1994; Klassen 1996; Klassen 1998; Koren 1983; Rittichier 2000; Roberts 1999; Sparrow 2006; Super 1989; Tibballs 1992); and five reported the use of a mist tent (Alshehr 2005; Johnson 1996; Klassen 1996; Rittichier 2000; Super 1989). Twenty‐two studies reported collecting adverse events data. Of these, seven reported serious adverse events following the administration of glucocorticoids (namely secondary bacterial infections, e.g. pneumonia, otitis media) (Alshehr 2005; Bjornson 2004; Johnson 1996; Klassen 1998; Kuusela 1988; Roberts 1999; Super 1989), and 15 reported no serious adverse events (Chub‐Uppakarn 2007; Duman 2005; Eden 1967; Fifoot 2007; Fitzgerald 1996; Garbutt 2013; Husby 1993; James 1969; Johnson 1998; Klassen 1994; Leipzig 1979; Roorda 1998; Sparrow 2006; Tibballs 1992; Vad Pedersen 1998).
Excluded studies
One study was excluded following the 2014 interim update search. Faghihinia 2007 did not report any usable results. We excluded four studies following the searches in 2018 (Figure 1). Roked 2015 and Gill 2017 were not randomised trials; Eghbali 2016 and Mohammadzadeh 2014 were randomised trials that did not investigate glucocorticoids; and Faraji‐Goodarzi 2018 was a randomised trial that did not report any relevant outcomes. There were no relevant outcomes for an ongoing randomised trial (NCT01748162), and the trial was terminated due to inadequate recruitment (see Characteristics of excluded studies).
We edited the excluded studies list to remove legacy excluded studies that evidently did not meet the inclusion criteria (e.g. letters, commentaries, summaries, case studies). We made this change to comply with current Cochrane standards for methods and reporting. We excluded 33 studies in this 2018 updated review.
Ongoing studies
We identified one ongoing study (ACTRN12609000290291). We will assess this study for inclusion in a future update.
Risk of bias in included studies
We judged overall risk of bias to be low in Garbutt 2013, unclear in 30 studies (Alshehr 2005; Bjornson 2004; Chub‐Uppakarn 2007; Cruz 1995; Donaldson 2003; Eden 1964; Eden 1967; Fifoot 2007; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1996; Geelhoed 2005; Godden 1997; Husby 1993; James 1969; Johnson 1996; Johnson 1998; Klassen 1996; Klassen 1998; Koren 1983; Kuusela 1988; Leipzig 1979; Luria 2001; Martinez Fernandez 1993; Massicotte 1973; Roorda 1998; Skowron 1966a&b; Sparrow 2006; Super 1989; Tibballs 1992; Von Mühlendahl 1982), and high in 12 studies (Amir 2006; Cetinkaya 2004; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Fitzgerald 1996; Geelhoed 1995c; Klassen 1994; Rittichier 2000; Roberts 1999; Soleimani 2013; Vad Pedersen 1998). The 'Risk of bias' tables in Characteristics of included studies show the rationales for our 'Risk of bias' decisions. Figure 2 and Figure 3 show the 'Risk of bias' judgements for the included studies.
2.
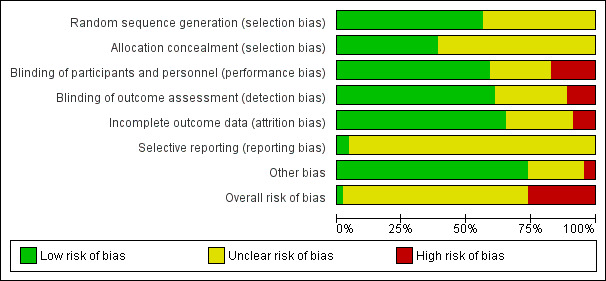
Risk of bias graph: Review authors' judgements of risk of bias for each domain and overall presented as percentages across all included studies.
3.
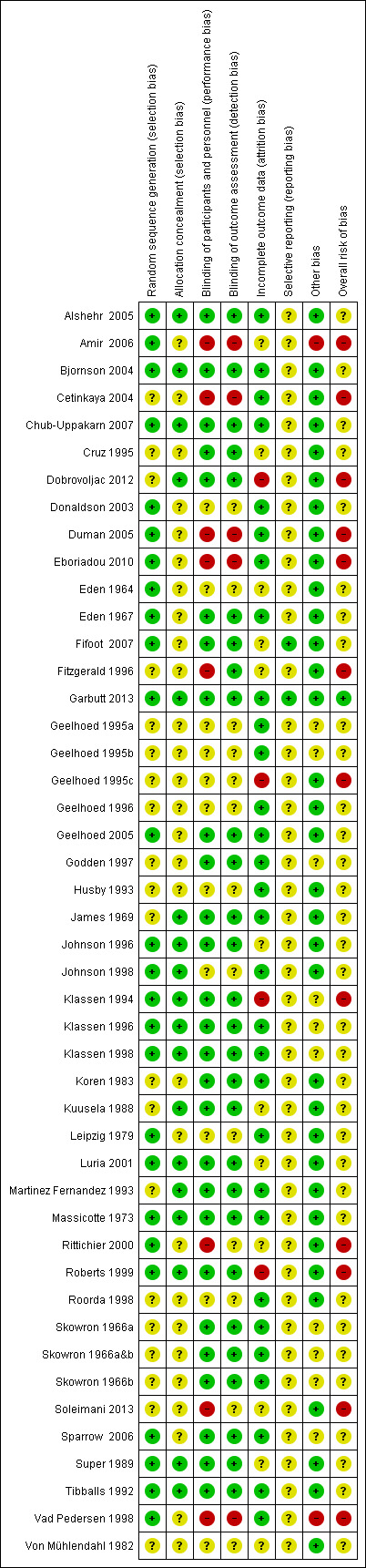
Risk of bias summary: Review authors' judgements of risk of bias among seven domains and overall for each included study.
Allocation
We judged risk of bias for random sequence generation to be low in 26 studies (60%) and unclear in 17 studies (40%). The 16 studies at unclear risk of bias were described as randomised, however the method for generating the randomisation sequence was not clear or not reported (Cetinkaya 2004; Cruz 1995; Dobrovoljac 2012; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996; Godden 1997; Husby 1993; James 1969; Koren 1983; Kuusela 1988; Martinez Fernandez 1993; Roorda 1998; Skowron 1966a&b; Soleimani 2013; Von Mühlendahl 1982). Randomisation was adequately described in the remaining 26 studies. We judged risk of bias for allocation concealment to be low in 18 studies (42%) and unclear in 25 studies (58%). For the 25 studies at unclear risk of bias, there was insufficient information reported in the publication to determine whether or not the groups to which the children were allocated could have been foreseen (Amir 2006; Cetinkaya 2004; Cruz 1995; Donaldson 2003; Duman 2005; Eboriadou 2010; Eden 1964; Eden 1967; Fifoot 2007; Fitzgerald 1996; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996; Geelhoed 2005; Godden 1997; Husby 1993; Koren 1983; Leipzig 1979; Rittichier 2000; Roorda 1998; Skowron 1966a&b; Soleimani 2013; Sparrow 2006; Vad Pedersen 1998; Von Mühlendahl 1982). Allocation concealment was adequate in the remaining 18 studies.
Blinding
We judged risk of bias for blinding of participants and personnel to be low in 25 (58%), unclear in 10 (23%), and high in eight (19%) studies. Of the eight studies at high risk of bias, four appeared to be open‐label (Amir 2006; Duman 2005; Rittichier 2000; Vad Pedersen 1998). Cetinkaya 2004 did not explicitly describe any measures taken to blind the participants and personnel from treatment assignment, and any blinding could have been broken. The personnel were not blinded in the study by Fitzgerald 1996. In Eboriadou 2010, the treatments were clearly distinguishable, and the method for blinding was not described even though the study was termed "double‐blind". In Soleimani 2013, only the outcome assessor was blinded. Of the 10 studies assessed as at unclear risk of bias, six were described as double‐blind without any further details about who was blinded and how (Eden 1964; Geelhoed 1996; Husby 1993; Leipzig 1979; Roorda 1998; Von Mühlendahl 1982). In Donaldson 2003, Geelhoed 1995a, Geelhoed 1995c, and Johnson 1998, blinding was attempted, but we judged that the blinding could have been broken; however, it was unclear how often this may have occurred. The remaining studies included satisfactory descriptions of how participants and personnel were blinded.
We judged risk of bias for blinding of outcome assessment to be low in 26 (60%), unclear in 12 (28%), and high in five (12%) studies. For 21 studies (49%), there was no mention of a third‐party outcome assessor, so the judgement for outcome assessment was carried over from blinding of participants and personnel (Cetinkaya 2004; Chub‐Uppakarn 2007; Cruz 1995; Dobrovoljac 2012; Duman 2005; Eboriadou 2010; Eden 1964; Eden 1967; Geelhoed 1995a; Geelhoed 1995b; Geelhoed 1995c; Geelhoed 1996; Geelhoed 2005; Godden 1997; Husby 1993; Koren 1983; Kuusela 1988; Luria 2001; Martinez Fernandez 1993; Massicotte 1973; Sparrow 2006; Tibballs 1992). Of the remaining studies, we judged two as at high risk of bias because the outcome assessors were not blinded (Amir 2006; Vad Pedersen 1998). We judged six studies as at unclear risk of bias. In Donaldson 2003, Johnson 1998, and Rittichier 2000, blinding of the outcome assessors was attempted, but we judged that the blinding could have been broken, although it was unclear how often this may have occurred. The studies by Leipzig 1979, Roorda 1998, and Von Mühlendahl 1982 were described as double‐blind, but it was unclear if the outcome assessors were blinded. In Soleimani 2013, the outcome assessor was described as blinded, but it was unclear how or if the blinding could have been broken. The remaining studies included satisfactory descriptions of how the outcome assessors were blinded.
Incomplete outcome data
We judged risk of bias for incomplete outcome data to be low in 27 (63%), unclear in 12 (28%), and high in four (9%) studies. The four studies at high risk of bias reported large losses to follow‐up that were imbalanced between groups (Dobrovoljac 2012; Geelhoed 1995c; Klassen 1994; Roberts 1999). Dobrovoljac 2012 and Roberts 1999 used the last observation carried forward method to estimate endpoint outcome values. For the studies at unclear risk of bias, in one study the number of children analysed was not reported (Amir 2006), and in six studies it was either unclear to which group the children who were lost to follow‐up had been allocated, or whether or not the losses to follow‐up were balanced between groups (Cruz 1995; Eden 1964; Johnson 1996; Kuusela 1988; Rittichier 2000; Soleimani 2013; Von Mühlendahl 1982). In four studies, losses to follow‐up ranged from 13% to 17% (Fifoot 2007; Luria 2001; Soleimani 2013; Super 1989). In Fitzgerald 1996, loss to follow‐up was 5%, and the last value observation forward method was used to estimate endpoint outcome values. We judged risk of bias due to incomplete outcome data not to be a concern for the remainder of the studies.
Selective reporting
We judged risk of bias for selective reporting to be low in two (5%) and unclear in 41 (95%) studies. For the two studies at low risk of bias (Fifoot 2007; Garbutt 2013), the outcomes in the trial registers matched those reported in the publications. For the remaining 41 studies, no protocol or trial registry was cited in the publication or located via online searches. In all cases, the outcomes reported in the methods matched those reported in the results section of the publications.
Other potential sources of bias
We judged risk of bias from other sources to be low in 34 (79%), unclear in seven (16%), and high in two (5%) studies. For the two studies at high risk of bias, there was a baseline imbalance in croup score (Amir 2006; Vad Pedersen 1998). For six of the studies at unclear risk of bias, there was potential for bias in participant selection because some children were not enrolled due to manpower constraints, failure of the emergency department to contact the research team, or because the emergency department was busy (Geelhoed 1995a; Geelhoed 1995b; Godden 1997; Klassen 1994; Klassen 1996; Klassen 1998; Sparrow 2006). For one study at unclear risk of bias, baseline data were not presented, therefore it was not possible to estimate whether or not baseline imbalances existed between the groups (Skowron 1966a&b).
Effects of interventions
Summary of findings for the main comparison. Any glucocorticoid compared to placebo for croup.
| Any glucocorticoid compared to placebo for croup | ||||||
| Patient or population: children with croup Intervention: any glucocorticoid Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Placebo | Any glucocorticoid | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 2 hours) | The mean change in croup score was ‐1.50 to ‐0.81. | The mean change in croup score was 0.65 standard deviations more (1.13 more to 0.18 more). | ‐ | 426 (7 RCTs) | ⊕⊕⊕⊝ MODERATEa | A standard deviation of 0.65 represents a moderate difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 6 hours) | The mean change in croup score was ‐3.23 to ‐0.65. | The mean change in croup score was 0.76 standard deviations more (1.12 more to 0.40 more). | ‐ | 959 (11 RCTs) | ⊕⊕⊕⊝ MODERATEb | A standard deviation of 0.76 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 12 hours) | The mean change in croup score was ‐7.62 to ‐1.00. | The mean change in croup score was 1.03 standard deviations more (1.53 more to 0.53 more). | ‐ | 571 (8 RCTs) | ⊕⊕⊕⊝ MODERATEc | A standard deviation of 1.03 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 24 hours) | The mean change in croup score was ‐2.56 to ‐1.05. | The mean change in croup score was 0.86 standard deviations more (1.40 more to 0.31 more). | ‐ | 351 (8 RCTs) | ⊕⊕⊝⊝ LOWd | A standard deviation of 0.86 represents a large difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.52 (0.36 to 0.75) | 1679 (10 RCTs) | ⊕⊕⊕⊝ MODERATEe | ||
| 204 per 1000 | 106 per 1000 (74 to 153) | |||||
| Adverse events | 13/26 (50%) studies reported collecting adverse events data, and 8/13 (62%) reported no serious adverse events. Bjornson 2004 reported 7 instances of pneumonia (3/359, 0.83% in the dexamethasone group and 4/361, 1.11% in the placebo group). Johnson 1996 reported 1 child with neutropenia consistent with bacterial tracheitis in the dexamethasone group (1/28, 3.57%). Kuusela 1988 reported 7 secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy: 5/35, 14% in the dexamethasone group and 2/16, 12.5% in the placebo group. Super 1989 reported 1 child with pneumonitis in the placebo group (1/13, 7.7%) and 2 children with pneumonia in the dexamethasone group (2/16, 12.5%). Roberts 1999 reported 1 instance of exacerbated symptoms, 5 children with emotional distress, 2 with vomiting, and 1 instance of eye irritation in the budesonide group (9/42, 21.4%) and 3 instances of exacerbated symptoms, 6 children with emotional distress, 3 with vomiting, 2 rashes, and 1 instance each of eye irritation and tongue irritation in the placebo group (16/40, 40%). | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 81%), and variation in point estimates. bWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 83%), and variation in point estimates and in direction of effects for one study. cWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 86%), and variation in point estimates. dWe downgraded by two levels for inconsistency. There was considerable heterogeneity (I² = 81%), and variation in point estimates. The confidence intervals did not overlap for some studies. There was variation in the direction of effects. eWe downgraded by one level for inconsistency. There was substantial heterogeneity (I² = 52%), and variation in point estimates.
Summary of findings 2. Any glucocorticoid compared to epinephrine for croup.
| Any glucocorticoid compared to epinephrine for croup | ||||||
| Patient or population: children with croup Intervention: any glucocorticoid Comparison: epinephrine | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Epinephrine | Any glucocorticoid | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 2 hours) | The mean change in croup score was ‐4.24 to ‐3.74. | The mean change in croup score was 0.77 standard deviations less (0.24 more to 1.77 less). | ‐ | 130 (2 RCTs) | ⊕⊕⊝⊝ LOWa, b | A standard deviation of 0.77 represents a large difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 6 hours) | The mean change in croup score was ‐1.25 to ‐1.10. | The mean change in croup score was 0.10 standard deviations more (1.18 more to 0.97 less). | ‐ | 63 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWc, d | A standard deviation of 0.10 represents a minimal difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 12 hours) | The mean change in croup score was ‐3.86 to ‐1.45. | The mean change in croup score was 0.07 standard deviations more (0.57 more to 0.43 less). | ‐ | 129 (3 RCTs) | ⊕⊕⊕⊝ MODERATEe | A standard deviation of 0.07 represents a minimal difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 24 hours) | The mean change in croup score was ‐4.40 to ‐2.01. | The mean change in croup score was 0.17 standard deviations less (0.18 more to 0.51 less). | ‐ | 129 (3 RCTs) | ⊕⊕⊕⊝ MODERATEf | A standard deviation of 0.17 represents a small difference between groups. |
| Return visits or (re)admissions or both | Study population | RD 0.00 (‐0.04 to 0.04) | 130 (2 RCTs) | ⊕⊕⊕⊝ MODERATEg | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse events | 3/4 (75%) studies reported collecting adverse events data. Fitzgerald 1996 reported no serious adverse events. Kuusela 1988 reported 5 cases of secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy in the dexamethasone group (5/16, 31.3%). Eboriadou 2010 reported 4 cases of tremor and tachycardia (4/25, 16%) in the epinephrine group. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). **We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for inconsistency. There was considerable heterogeneity (I² = 87%), and variation in point estimates. There was minimal overlap of the confidence intervals. bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and a clinically important benefit for epinephrine compared to glucocorticoids. cWe downgraded by two levels for inconsistency. There was considerable heterogeneity (I² = 78%), and variation in point estimates and in the direction of effects. dWe downgraded by one level for imprecision. The sample size was small (did not meet optimal information size). The effect estimate included both the null effect and a clinically important effect for glucocorticoids compared to epinephrine. eWe downgraded by one level for imprecision. The sample size was small (did not meet optimal information size). fWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). gWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
Any glucocorticoid compared to placebo
See Table 1
Primary outcomes
1. Change in clinical croup score
Compared to placebo, glucocorticoids resulted in significantly greater reductions in croup score after two (standardised mean difference (SMD) ‐0.65, 95% confidence interval (CI) ‐1.13 to ‐0.18; P = 0.007; I² = 81%; 7 RCTs; 426 children; moderate‐certainty evidence; Analysis 1.1), six (SMD ‐0.76, 95% CI ‐1.12 to ‐0.40; P < 0.001; I² = 83%; 11 RCTs; 959 children; moderate‐certainty evidence; Analysis 1.2), 12 (SMD ‐1.03, 95% CI ‐1.53 to ‐0.53; P < 0.001; I² = 86%; 8 RCTs; 571 children; moderate‐certainty evidence; Analysis 1.3), and 24 hours (SMD ‐0.86, 95% CI ‐1.4 to ‐0.31; P = 0.002; I² = 81%; 8 RCTs; 351 children; low‐certainty evidence; Analysis 1.4).
1.1. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 1 Croup score (change baseline ‐ 2 hours) by score.
1.2. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 2 Croup score (change baseline ‐ 6 hours) by score.
1.3. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 3 Croup score (change baseline ‐ 12 hours) by score.
1.4. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 4 Croup score (change baseline ‐ 24 hours) by score.
There were no significant subgroup differences in reductions in croup score by score (Westley 1978 or otherwise) (Analysis 1.1; Analysis 1.2; Analysis 1.3; Analysis 1.4) or by inpatient or outpatient status (Analysis 1.5; Analysis 1.6; Analysis 1.7) at any time point. At two hours, there was no subgroup difference in effect by glucocorticoid (Chi² = 5.65; P = 0.06; I² = 64.6%; Analysis 1.8). At six hours, there was a significant subgroup difference in effect by glucocorticoid (Chi² = 11.46; P = 0.009; I² = 73.8%; Analysis 1.9), accounted for by the larger reduction in croup score for prednisolone (SMD ‐1.87, 95% CI ‐2.62 to ‐1.13; P < 0.001; 1 RCT; 42 children) compared to budesonide (SMD ‐0.81, 95% CI ‐1.04 to ‐0.58; P < 0.001; I² = 0%; 5 RCTs; 333 children) and dexamethasone (SMD ‐0.62, 95% CI ‐1.17 to ‐0.08; P = 0.03; I² = 85%; 6 RCTs; 567 children). Fluticasone did not show an effect (SMD 0.06, 95% CI ‐0.89 to 1.02; P = 0.90; 1 RCT; 17 children). At 12 hours, there was a significant subgroup difference in effect by glucocorticoid (Chi² = 10.08; P = 0.006; I² = 80.2%; Analysis 1.10), accounted for by the larger reduction in croup score for prednisolone (SMD ‐2.40, 95% CI ‐3.26 to ‐1.55; P < 0.001; 1 RCT; 39 children) compared to budesonide (SMD ‐0.97, 95% CI ‐1.26 to ‐0.68; P < 0.001; I² = 0%; 3 RCTs; 209 children) and dexamethasone (SMD ‐0.85, 95% CI ‐1.55 to ‐0.15; P = 0.02; I² = 84%; 5 RCTs; 323 children). At 24 hours, there was a significant subgroup difference in effect by glucocorticoid (Chi² = 9.02; P = 0.01; I² = 77.8%; Analysis 1.11). Although larger reductions in croup score were observed with budesonide (SMD ‐1.40, 95% CI ‐1.88 to ‐0.93; P < 0.001; I² = 0%; 2 RCTs; 89 children) and dexamethasone (SMD ‐0.89, 95% CI ‐1.55 to ‐0.22; P = 0.009; I² = 81%; 6 RCTs; 245 children) compared to placebo, fluticasone did not show an effect (SMD 0.21, 95% CI ‐0.75 to 1.17; P = 0.67; 1 RCT; 17 children).
1.5. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 5 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient.
1.6. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 6 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient.
1.7. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 7 Croup score (change baseline ‐ 24 hours) by inpatient/outpatient.
1.8. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 8 Croup score (change baseline ‐ 2 hours) by glucocorticoid.
1.9. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 9 Croup score (change baseline ‐ 6 hours) by glucocorticoid.
1.10. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 10 Croup score (change baseline ‐ 12 hours) by glucocorticoid.
1.11. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 11 Croup score (change baseline ‐ 24 hours) by glucocorticoid.
2. Return visits or (re)admissions to the hospital or both
Compared to placebo, glucocorticoids reduced the rate of return visits or (re)admissions to the hospital or both (risk ratio (RR) 0.52, 95% CI 0.36 to 0.75; P < 0.001; I² = 52%; 10 RCTs; 1679 children; moderate‐certainty evidence; Analysis 1.12). There were no significant subgroup differences in effect by glucocorticoid (budesonide or dexamethasone, Analysis 1.13), by inpatient or outpatient status (Analysis 1.12), or by croup severity (mild or moderate croup, Analysis 1.14).
1.12. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 12 Return visits or (re)admissions or both by inpatient/outpatient.
1.13. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 13 Return visits or (re)admissions or both by glucocorticoid.
1.14. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 14 Return visits or (re)admissions or both by croup severity.
The number needed to treat for an additional beneficial outcome (NNTB) is shown in Table 3. For the mean placebo group rate (30.62%), the NNTB was 7 children (95% CI 5 to 12). For the smallest placebo group rate (2.06%), the NNTB was 102 children (95% CI 78 to 179). For the largest placebo group rate (72.00%), the NNTB was 3 children (95% CI 2 to 5).
1. Number needed to treat for an additional beneficial outcome for return visits or (re)admissions or both for any glucocorticoid compared to placebo.
| Baseline rate (%) | NNTB (95% CI) |
| Mean baseline rate | |
| 30.62 | 7 (5 to 12) |
| Smallest baseline rate | |
| 2.06 | 102 (78 to 179) |
| Largest baseline rate | |
| 72.00 | 3 (2 to 5) |
NNTB: number needed to treat for an additional beneficial outcome
Secondary outcomes
1. Length of stay in the hospital or emergency department
Compared to those given a placebo, children treated with glucocorticoids spent significantly fewer hours in the hospital (mean difference (MD) ‐14.90, 95% CI ‐23.58 to ‐6.22; P < 0.001; I² = 54%; 8 RCTs; 476 children; Analysis 1.15). All of the included studies investigated inpatients. There was no significant subgroup difference in effect by glucocorticoid (budesonide, dexamethasone, or fluticasone; Analysis 1.16).
1.15. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 15 Length of stay by inpatient/outpatient.
1.16. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 16 Length of stay by glucocorticoid.
2. Patient improvement
Only one study investigated patient improvement two hours after the administration of glucocorticoids compared to placebo. Roberts 1999 studied 82 hospitalised children aged six months to eight years with moderate to severe croup who were given budesonide or placebo, and observed no significant difference in improvement after two hours (RR 1.81, 95% CI 0.96 to 3.40; P = 0.07; 1 RCT; 82 children; Analysis 1.17). Compared to placebo, glucocorticoids were associated with improvement in a significantly greater proportion of children after six (RR 1.45, 95% CI 1.12 to 1.88; P = 0.005; I² = 34%; 6 RCTs; 332 children; Analysis 1.18); 12 (RR 1.33, 95% CI 1.09 to 1.62; P = 0.005; I² = 53%; 6 RCTs; 340 children; Analysis 1.19); and 24 hours (RR 1.28, 95% CI 1.01 to 1.61; P = 0.04; I² = 75%; 5 RCTs; 251 children; Analysis 1.20).
1.17. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 17 Improvement (at 2 hours) by inpatient/outpatient.
1.18. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 18 Improvement (at 6 hours) by inpatient/outpatient.
1.19. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 19 Improvement (at 12 hours) by inpatient/outpatient.
1.20. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 20 Improvement (at 24 hours) by inpatient/outpatient.
Only inpatients were included in the 12‐hour analysis (Analysis 1.19). There were no significant subgroup differences in estimates of effect by inpatient or outpatient status at six or 24 hours (Analysis 1.18; Analysis 1.20). There were no significant subgroup differences in effect by glucocorticoid at six (budesonide, dexamethasone, or prednisolone; Analysis 1.21), 12 (budesonide, dexamethasone, or prednisolone; Analysis 1.22), or 24 hours (dexamethasone or prednisolone; Analysis 1.23).
1.21. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 21 Improvement (at 6 hours) by glucocorticoid.
1.22. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 22 Improvement (at 12 hours) by glucocorticoid.
1.23. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 23 Improvement (at 24 hours) by glucocorticoid.
3. The use of additional treatments
There was no significant difference between children treated with glucocorticoids and those given placebo in the use of antibiotics (risk difference (RD) 0.00, 95% CI ‐0.04 to 0.04; P = 1.00; I² = 0%; 3 RCTs; 202 children; Analysis 1.24); the use of epinephrine (RD ‐0.03, 95% CI ‐0.08 to 0.01; P = 0.16; I² = 45%; 9 RCTs; 709 children; Analysis 1.25); the rate of intubation/tracheostomy (RD 0.00, 95% CI ‐0.01 to 0.01; P = 0.79; I² = 0%; 11 RCTs; 1090 children; Analysis 1.26); the use of a mist tent (RD ‐0.20, 95% CI ‐0.87 to 0.47; P = 0.55; I² = 95%; 2 RCTs; 84 children; Analysis 1.27); or the use of supplemental glucocorticoids (RR 0.61, 95% CI 0.36 to 1.03; P = 0.07; I² = 10%; 6 RCTs; 305 children; Analysis 1.28).
1.24. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 24 Additional treatments: antibiotics.
1.25. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 25 Additional treatments: epinephrine.
1.26. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 26 Additional treatments: intubation/tracheostomy.
1.27. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 27 Additional treatments: mist tent.
1.28. Analysis.
Comparison 1 Any glucocorticoid compared to placebo, Outcome 28 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
Of the 26 studies that investigated any glucocorticoid compared to placebo, 13 reported collecting adverse events data. Of these, eight reported no serious adverse events (Eden 1967; Husby 1993; James 1969; Johnson 1998; Klassen 1994; Leipzig 1979; Roorda 1998; Tibballs 1992). Bjornson 2004 reported seven instances of pneumonia (3/359, 0.83% in the dexamethasone group and 4/361, 1.11% in the placebo group). Johnson 1996 reported one child with neutropenia consistent with bacterial tracheitis in the dexamethasone group (1/28, 3.57%). Kuusela 1988 reported seven secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy: 5/35, 14% in the dexamethasone group and 2/16, 12.5% in the placebo group. Super 1989 reported one child with pneumonitis in the placebo group (1/13, 7.7%) and two children with pneumonia in the dexamethasone group (2/16, 12.5%). Roberts 1999 reported one instance of exacerbated symptoms, five children with emotional distress, two with vomiting, and one instance of eye irritation in the budesonide group (9/42, 21.4%) and three instances of exacerbated symptoms, six children with emotional distress, three with vomiting, two rashes, and one instance each of eye irritation and tongue irritation in the placebo group (16/40, 40%).
Any glucocorticoid compared to epinephrine
See Table 2
Primary outcomes
1. Change in clinical croup score
Compared to epinephrine, the change in croup score following treatment with glucocorticoids was not significantly different after two (SMD 0.77, 95% CI ‐0.24 to 1.77; P = 0.13; I² = 87%; 2 RCTs; 130 children; low‐certainty evidence; Analysis 2.1); six (SMD ‐0.10, 95% CI ‐1.18 to 0.97; P = 0.85; I² = 78%; 2 RCTs; 63 children; very low‐certainty evidence; Analysis 2.2); 12 (SMD ‐0.07, 95% CI ‐0.57 to 0.43; P = 0.78; I² = 47%; 3 RCTs; 129 children; moderate‐certainty evidence; Analysis 2.3); or 24 hours (SMD 0.17, 95% CI ‐0.18 to 0.51; P = 0.35; I² = 0%; 3 RCTs; 129 children; moderate‐certainty evidence; Analysis 2.4).
2.1. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 1 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient.
2.2. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 2 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient.
2.3. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 3 Croup score (change baseline ‐ 12 hours) by inpatient/outpatient.
2.4. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 4 Croup score (change baseline ‐ 24 hours) by inpatient/outpatient.
The analyses at six (Analysis 2.2), 12 (Analysis 2.3), and 24 hours (Analysis 2.4) included only inpatients. At two hours, there was a significant subgroup difference in effect by inpatient or outpatient status (Chi² = 7.44, P = 0.006; I² = 86.6%; Analysis 2.1). For outpatients, glucocorticoids were significantly less effective at reducing the croup score compared to epinephrine after two hours (SMD 1.29, 95% CI 0.73 to 1.84; P < 0.001; 1 RCT; 64 children). No significant difference was detected between the two treatments for inpatients (SMD 0.26, 95% CI ‐0.22 to 0.75; P = 0.29; 1 RCT; 66 children).
At two hours, there was a significant subgroup difference in effect by glucocorticoid (Chi² = 7.37, P = 0.03; I² = 72.9%; Analysis 2.5). Epinephrine was significantly more effective at reducing croup score compared to beclomethasone (SMD 1.41, 95% CI 0.62 to 2.19; P < 0.001; 1 RCT; 33 children) and dexamethasone (SMD 1.13, 95% CI 0.35 to 1.91; P = 0.005; 1 RCT; 31 children). At this time point, there was no difference in the reduction in croup score between budesonide and epinephrine (SMD 0.26, 95% CI ‐0.22 to 0.75; P = 0.29; 1 RCT; 66 children). The 12‐ and 24‐hour analyses investigated budesonide and dexamethasone, and there were no significant subgroup differences in effect (Analysis 2.6; Analysis 2.7).
2.5. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 5 Croup score (change baseline ‐ 2 hours) by glucocorticoid.
2.6. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 6 Croup score (change baseline ‐ 12 hours) by glucocorticoid.
2.7. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 7 Croup score (change baseline ‐ 24 hours) by glucocorticoid.
2. Return visits or (re)admissions to the hospital or both
Eboriadou 2010 and Fitzgerald 1996 investigated return visits and readmissions, respectively, following the administration of glucocorticoids (dexamethasone and beclomethasone, and budesonide, respectively) compared to epinephrine. Neither study reported any events (RD 0.00, 95% CI ‐0.04 to 0.04; P = 1.00; I² = 0%; 2 RCTs; 130 children; moderate‐certainty evidence; Analysis 2.8).
2.8. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 8 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
Kuusela 1988 investigated length of stay for 32 children hospitalised with croup who were treated with dexamethasone, epinephrine, a combination of dexamethasone and epinephrine, or placebo. There was no significant difference in hours spent in the hospital between children treated with dexamethasone and those treated with epinephrine (MD ‐10.00, 95% CI ‐33.89 to 13.89; P = 0.41; 1 RCT; 32 children; Analysis 2.9).
2.9. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 9 Length of stay by inpatient/outpatient.
2. Patient improvement
We identified no studies that reported on patient improvement for this comparison.
3. The use of additional treatments
Fitzgerald 1996 investigated the use of additional treatments for children aged six months to six years admitted to the hospital with croup who were treated with budesonide or epinephrine. There was no significant difference in the proportion of children who required additional epinephrine (RR 0.30, 95% CI 0.03 to 2.69; P = 0.28; 1 RCT; 66 children; Analysis 2.10) between groups. No child was intubated (RD 0.00, 95% CI ‐0.06 to 0.06; P = 1.00; 1 RCT; 66 children; Analysis 2.11). There was no significant difference in the proportion of children who required supplemental glucocorticoids (RR 0.83, 95% CI 0.48 to 1.43; P = 0.49; 1 RCT; 66 children; Analysis 2.12) between groups.
2.10. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 10 Additional treatments: use of epinephrine.
2.11. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 11 Additional treatments: intubation/tracheostomy.
2.12. Analysis.
Comparison 2 Any glucocorticoid compared to epinephrine, Outcome 12 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
Of the four studies that investigated glucocorticoids compared to epinephrine, three reported collecting adverse events data. Fitzgerald 1996 reported no serious adverse events. Kuusela 1988 reported five cases of secondary bacterial infections (pneumonia, sinusitis, otitis media) requiring antibiotic therapy in the dexamethasone group (5/16, 31.3%). Eboriadou 2010 reported four cases of tremor and tachycardia (4/25, 16%) in the epinephrine group.
Dexamethasone compared to budesonide
See: dexamethasone compared to budesonide for croup (Table 4).
2. Dexamethasone compared to budesonide for croup.
| Dexamethasone compared to budesonide for croup | ||||||
| Patient or population: children with croup Intervention: dexamethasone Comparison: budesonide | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Budesonide | Dexamethasone | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 6 hours) | The mean change in croup score was ‐2.93 to ‐2.00. | The mean change in croup score was 0.46 standard deviations more (0.79 more to 0.13 more). | ‐ | 326 (4 RCTs) | ⊕⊕⊝⊝ LOWa, b | A standard deviation of 0.46 represents a moderate difference between groups. |
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 12 hours) | The mean change in croup score was ‐3.07 to ‐2.33. | The mean change in croup score was 0.75 standard deviations more (1.19 more to 0.30 more). | ‐ | 84 (2 RCTs) | ⊕⊕⊝⊝ LOWc, d | A standard deviation of 0.75 represents a large difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.69 (0.40 to 1.22) | 374 (5 RCTs) | ⊕⊕⊕⊝ MODERATEe | ||
| 122 per 1000 | 84 per 1000 (49 to 149) | |||||
| Adverse events | 4/6 (67%) studies reported collecting adverse events data, and 3/4 (75%) studies reported no serious adverse events (Duman 2005; Johnson 1998; Vad Pedersen 1998). Klassen 1998 reported 1 case of oral thrush in the budesonide group (1/65, 1.5%), and 1 case of hives and 1 case of violent behaviour in the dexamethasone group (2/69, 2.9%). | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for risk of bias. The contributing studies were at high (n = 2) and unclear (n = 2) risk of bias. Allocation concealment was unclear in two studies; blinding was unclear in two studies; and one study was unblinded. There was a baseline imbalance in croup score in one study. bWe downgraded by one level for inconsistency. There was substantial heterogeneity (I² = 51%), and variation in point estimates. cWe downgraded by one level for risk of bias. The contributing studies were at high risk of bias. Allocation concealment was unclear in both studies; blinding was unclear in one study, and the other study was unblinded. There was a baseline imbalance in croup score in one study. dWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). eWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included a null effect as well as considerable benefit for dexamethasone compared to budesonide.
Primary outcomes
1. Change in clinical croup score
Compared to budesonide, dexamethasone resulted in a significantly greater reduction in croup score after six (SMD ‐0.46, 95% CI ‐0.79 to ‐0.13; P = 0.006; I² = 51%; 4 RCTs; 326 children; low‐certainty evidence; Analysis 3.1) and 12 hours (SMD ‐0.75, 95% CI ‐1.19 to ‐0.30; P = 0.001; I² = 0%; 2 RCTs; 84 children; low‐certainty evidence; Analysis 3.2). The analysis at 12 hours (Analysis 3.2) included only inpatients. At six hours, there was no significant subgroup difference in effect by inpatient or outpatient status (Analysis 3.1).
3.1. Analysis.
Comparison 3 Dexamethasone compared to budesonide, Outcome 1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient.
3.2. Analysis.
Comparison 3 Dexamethasone compared to budesonide, Outcome 2 Croup score (change baseline ‐ 12 hours) by inpatient/outpatient.
2. Return visits or (re)admissions to the hospital or both
There was no significant difference in the rate of return visits or (re)admissions to the hospital or both when children were treated with dexamethasone compared to budesonide (RR 0.69, 95% CI 0.40 to 1.22; P = 0.20; I² = 0%; 5 RCTs; 374 children; moderate‐certainty evidence; Analysis 3.3). There were no significant subgroup differences in effect by inpatient or outpatient status (Analysis 3.3).
3.3. Analysis.
Comparison 3 Dexamethasone compared to budesonide, Outcome 3 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was no significant difference in hours spent in the hospital or emergency department between children treated with dexamethasone and those treated with budesonide (SMD ‐0.29, 95% CI ‐0.72 to 0.14; P = 0.19; I² = 45%; 2 RCTs; 184 children; Analysis 3.4). There was no significant subgroup difference in effect by inpatient or outpatient status (Analysis 3.4).
3.4. Analysis.
Comparison 3 Dexamethasone compared to budesonide, Outcome 4 Length of stay by inpatient/outpatient.
2. Patient improvement
Klassen 1998 investigated response to treatment, defined as a two‐point improvement in croup score, among 198 children aged three months to five years who were treated with budesonide, dexamethasone, or a combination of budesonide and dexamethasone in the emergency department for croup. There was no difference in response to treatment between those treated with dexamethasone and those treated with budesonide (RR 1.12, 95% CI 0.93 to 1.34; P = 0.22; 1 RCT; 134 children; Analysis 3.5).
3.5. Analysis.
Comparison 3 Dexamethasone compared to budesonide, Outcome 5 Improvement (at 6 hours) by inpatient/outpatient.
3. The use of additional treatments
Compared to those treated with budesonide, children treated with dexamethasone were at a significantly reduced risk of needing treatment with epinephrine (RR 0.45, 95% CI 0.21 to 0.96; P = 0.04; I² = 0%; 4 RCTs; 321 children; Analysis 3.6). Geelhoed 1995c and Johnson 1998 investigated the need for intubation/tracheostomy among children treated with dexamethasone or budesonide for croup. There were no events in either study (RD 0.00, 95% CI ‐0.04 to 0.04; P = 1.00; I² = 0%; 2 RCTs; 145 children; Analysis 3.7). There was no significant difference in the need for additional glucocorticoids between children treated with dexamethasone and those treated with budesonide (RR 0.48, 95% CI 0.18 to 1.32; P = 0.15; I² = 0%; 3 RCTs; 240 children; Analysis 3.8).
3.6. Analysis.
Comparison 3 Dexamethasone compared to budesonide, Outcome 6 Additional treatments: epinephrine.
3.7. Analysis.
Comparison 3 Dexamethasone compared to budesonide, Outcome 7 Additional treatments: intubation/tracheostomy.
3.8. Analysis.
Comparison 3 Dexamethasone compared to budesonide, Outcome 8 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
Of the six studies investigating dexamethasone compared to budesonide, three (50%) reported no serious adverse events (Duman 2005; Johnson 1998; Vad Pedersen 1998). Klassen 1998 reported one case of oral thrush in the budesonide group (1/65, 1.5%), and one case of hives and one case of violent behaviour in the dexamethasone group (2/69, 2.9%).
Dexamethasone compared to beclomethasone
See: dexamethasone compared to beclomethasone for croup (Table 5)
3. Dexamethasone compared to beclomethasone for croup.
| Dexamethasone compared to beclomethasone for croup | |||||
| Patient or population: children with croup Intervention: dexamethasone Comparison: beclomethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Beclomethasone | Dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RD 0.00 (‐0.09 to 0.09) | 39 (1 RCT) | ⊕⊕⊝⊝ LOWa, b | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Adverse events | Eboriadou 2010 reported no adverse events related to the glucocorticoids. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
Primary outcomes
1. Change in clinical croup score
We identified no studies that investigated the change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Eboriadou 2010 investigated return visits to the emergency department among 39 children aged six months to five years treated with dexamethasone or beclomethasone for croup. No children returned for additional care (RD 0.00, 95% CI ‐0.09 to 0.09; P = 1.00; 1 RCT; 39 children; low‐certainty evidence; Analysis 4.1).
4.1. Analysis.
Comparison 4 Dexamethasone compared to beclomethasone, Outcome 1 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
We identified no studies that investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement
We identified no studies that investigated clinical improvement for this comparison.
3. The use of additional treatments
We identified no studies that investigated the use of additional treatments for this comparison.
4. Any adverse events
Eboriadou 2010 investigated dexamethasone compared to beclomethasone and reported no adverse events related to the glucocorticoids.
Dexamethasone compared to betamethasone
See: dexamethasone compared to betamethasone for croup (Table 6)
4. Dexamethasone compared to betamethasone for croup.
| Dexamethasone compared to betamethasone for croup | |||||
| Patient or population: children with croup Intervention: dexamethasone Comparison: betamethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Betamethasone | Dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (follow‐up: 2 hours) | The mean change in croup score from 1 study was ‐1.68. | The mean change in croup score was 1.38 units more (2.58 more to 0.18 more). | ‐ | 52 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa, b, c |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (follow‐up: 6 hours) | The mean change in croup score from 1 study was ‐1.89. | The mean change in croup score was 1.53 units more (2.75 more to 0.31 more). | ‐ | 52 (1 RCT) | ⊕⊝⊝⊝ VERY LOWd, e, f |
| Return visits or (re)admissions or both | Study population | RR 0.95 (0.67 to 1.34) | 52 (1 RCT) | ⊕⊝⊝⊝ VERY LOWg, h | |
| 731 per 1000 | 694 per 1000 (490 to 979) | ||||
| Adverse events | Amir 2006 did not report collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias. Allocation concealment was unclear, and the study was not blinded. There was a baseline imbalance in croup score. bWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. cWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). dWe downgraded by one level for risk of bias. The one contributing study was at high risk of bias. Allocation concealment was unclear, and the study was not blinded. There was a baseline imbalance in croup score. eWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. fWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). gWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. hWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and appreciable benefit or harm for dexamethasone compared to betamethasone.
Primary outcomes
1. Change in clinical croup score
Amir 2006 investigated the reduction in croup score for 52 children aged six months to six years who visited the emergency department and were treated with dexamethasone or betamethasone for croup. Compared to betamethasone, dexamethasone resulted in a significantly greater reduction in croup score after two (MD ‐1.38, 95% CI ‐2.58 to ‐0.18; P = 0.02; 1 RCT; 52 children; very low‐certainty evidence; Analysis 5.1) and six hours (MD ‐1.53, 95% CI ‐2.75 to ‐0.31; P = 0.01; 1 RCT; 52 children; very low‐certainty evidence; Analysis 5.2).
5.1. Analysis.
Comparison 5 Dexamethasone compared to betamethasone, Outcome 1 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient.
5.2. Analysis.
Comparison 5 Dexamethasone compared to betamethasone, Outcome 2 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient.
2. Return visits or (re)admissions to the hospital or both
Amir 2006 investigated re‐examinations by a primary care physician among 52 children aged six months to six years treated with dexamethasone or betamethasone in the emergency department for croup. There was no significant difference in the rate of re‐examinations between the dexamethasone and betamethasone groups (RR 0.95, 95% CI 0.67 to 1.34; P = 0.76; 1 RCT; 52 children; very low‐certainty evidence; Analysis 5.3).
5.3. Analysis.
Comparison 5 Dexamethasone compared to betamethasone, Outcome 3 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
We identified no studies that investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement
We identified no studies that investigated clinical improvement for this comparison.
3. The use of additional treatments
Amir 2006 investigated the need for epinephrine among 52 children aged six months to six years treated with dexamethasone or betamethasone in the emergency department for croup. The risk for needing epinephrine was significantly higher for those treated with dexamethasone compared to those treated with betamethasone (RR 2.11, 95% CI 1.18 to 3.76; P = 0.01; 1 RCT; 52 children; Analysis 5.4).
5.4. Analysis.
Comparison 5 Dexamethasone compared to betamethasone, Outcome 4 Additional treatments: use of epinephrine.
4. Any adverse events
We identified no studies that investigated adverse events for this comparison.
Dexamethasone compared to prednisolone
See: dexamethasone compared to prednisolone for croup (Table 7)
5. Dexamethasone compared to prednisolone for croup.
| Dexamethasone compared to prednisolone for croup | |||||
| Patient or population: children with croup Intervention: dexamethasone Comparison: prednisolone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Prednisolone | Dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (follow‐up: 6 hours) | The mean change in croup score from 1 study was ‐2.35. | The mean change in croup score was 0.19 units less (0.17 more to 0.55 less). | ‐ | 99 (1 RCT) | ⊕⊕⊝⊝ LOWa, b |
| Return visits or (re)admissions or both | Study population | RR 0.39 (0.19 to 0.79) | 306 (3 RCTs) | ⊕⊕⊕⊝ MODERATEc | |
| 200 per 1000 | 78 per 1000 (38 to 158) | ||||
| Adverse events | Fifoot 2007, Garbutt 2013, and Sparrow 2006 reported no serious adverse events related to the glucocorticoids. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). cWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
Primary outcomes
1. Change in clinical croup score
Fifoot 2007 investigated the reduction in croup score for 99 children aged six months to six years who visited the emergency department and were treated with dexamethasone or prednisolone for croup. There was no significant difference in the reduction in croup score six hours following treatment with prednisolone or dexamethasone (MD 0.19, 95% CI ‐0.17 to 0.55; P = 0.30; 1 RCT; 99 children; low‐certainty evidence; Analysis 6.1).
6.1. Analysis.
Comparison 6 Dexamethasone compared to prednisolone, Outcome 1 Croup (change baseline ‐ 6 hours) by inpatient/outpatient.
2. Return visits or (re)admissions to the hospital or both
Compared to prednisolone, dexamethasone significantly reduced the rate of return visits to medical care (RR 0.39, 95% CI 0.19 to 0.79; P = 0.009; 3 RCTs; 306 children; moderate‐certainty evidence; Analysis 6.2). The analysis included only outpatients.
6.2. Analysis.
Comparison 6 Dexamethasone compared to prednisolone, Outcome 2 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
Sparrow 2006 investigated hours spent in the emergency department among 133 children aged three to 142 months who were treated with dexamethasone or prednisolone for croup. There was no significant difference in length of stay between the two treatment groups (MD 0.50, 95% CI ‐0.55 to 1.55; P = 0.35; 1 RCT; 133 children; Analysis 6.3).
6.3. Analysis.
Comparison 6 Dexamethasone compared to prednisolone, Outcome 3 Length of stay by inpatient/outpatient.
2. Patient improvement
We identified no studies that investigated clinical improvement for this comparison.
3. The use of additional treatments
There was no significant difference in the need for epinephrine between children treated with dexamethasone and those treated with prednisolone (RR 0.69, 95% CI 0.26 to 1.85; P = 0.46; I² = 0; 2 RCTs; 232 children; Analysis 6.4). Fifoot 2007 investigated the need for additional glucocorticoids among children aged six months to six years treated in the emergency department with dexamethasone or prednisolone for croup. The need for additional glucocorticoids was not significantly different between children treated with dexamethasone and those treated with prednisolone (RR 0.92, 95% CI 0.34 to 2.48; P = 0.86; 1 RCT; 86 children; Analysis 6.5).
6.4. Analysis.
Comparison 6 Dexamethasone compared to prednisolone, Outcome 4 Additional treatments: epinephrine.
6.5. Analysis.
Comparison 6 Dexamethasone compared to prednisolone, Outcome 5 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
Fifoot 2007, Garbutt 2013, and Sparrow 2006 investigated dexamethasone compared to prednisolone and reported no serious adverse events.
Budesonide and dexamethasone compared to dexamethasone
See: budesonide and dexamethasone compared to dexamethasone for croup (Table 8)
6. Budesonide and dexamethasone compared to dexamethasone for croup.
| Budesonide and dexamethasone compared to dexamethasone for croup | ||||||
| Patient or population: children with croup Intervention: budesonide and dexamethasone Comparison: dexamethasone | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments** | |
| Dexamethasone | Budesonide and dexamethasone | |||||
| Change in croup score. Assessed with different scores in different studies. Lower scores mean fewer symptoms. (follow‐up: 6 hours) | The mean change in croup score was ‐3.24 to ‐1.80. | The mean change in croup score was 0.05 standard deviations less (0.19 more to 0.30 less). | ‐ | 255 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | A standard deviation of 0.05 represents a minimal difference between groups. |
| Return visits or (re)admissions or both | Study population | RR 0.91 (0.45 to 1.83) | 254 (3 RCTs) | ⊕⊕⊝⊝ LOWb | ||
| 100 per 1000 | 91 per 1000 (45 to 183) | |||||
| Adverse events | 1/3 (33%) studies reported collecting adverse events data. Klassen 1998 reported no adverse events in the dexamethasone or dexamethasone and budesonide group. | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
**We used Cohen's interpretation of effect sizes to determine the magnitude of the difference between groups (0.2 represents a small effect, 0.5 represents a medium effect, 0.8 represents a large effect). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). bWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and a significant benefit or harm for dexamethasone and budesonide compared to dexamethasone alone.
Primary outcomes
1. Change in clinical croup score
There was no significant difference in the reduction in croup score after six hours for children treated with combined dexamethasone and budesonide compared to those treated with dexamethasone alone (SMD 0.05, 95% CI ‐0.19 to 0.30; P = 0.67; I² = 0%; 3 RCTs; 255 children; moderate‐certainty evidence; Analysis 7.1). There was no significant difference in effect by inpatient or outpatient status (Analysis 7.1).
7.1. Analysis.
Comparison 7 Budesonide and dexamethasone compared to dexamethasone, Outcome 1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient.
2. Return visits or (re)admissions to the hospital or both
There was no significant difference in the rate of admissions or return visits between children treated with combined dexamethasone and budesonide compared to those treated with dexamethasone alone (RR 0.91, 95% CI 0.45 to 1.83; P = 0.79; I² = 0%; 3 RCTs; 254 children; low‐certainty evidence; Analysis 7.2). There was no significant subgroup difference in effect by inpatient or outpatient status (Analysis 7.2).
7.2. Analysis.
Comparison 7 Budesonide and dexamethasone compared to dexamethasone, Outcome 2 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
There was no significant difference in hours spent in the hospital or emergency department among children treated with dexamethasone and budesonide compared to those treated with dexamethasone alone (MD 0.44, 95% CI ‐0.05 to 0.92; P = 0.08; I²= 0%; 2 RCTs; 204 children; Analysis 7.3). There were no significant subgroup differences in effect by inpatient or outpatient status (Analysis 7.3).
7.3. Analysis.
Comparison 7 Budesonide and dexamethasone compared to dexamethasone, Outcome 3 Length of stay by inpatient/outpatient.
2. Patient improvement
After six hours, there was no significant difference in the clinical improvement of children treated with dexamethasone and budesonide compared to those treated with dexamethasone alone (RR 1.11, 95% CI 0.65 to 1.90; P = 0.70; 2 RCTs; 183 children; Analysis 7.4). This analysis included only outpatients (Analysis 7.4).
7.4. Analysis.
Comparison 7 Budesonide and dexamethasone compared to dexamethasone, Outcome 4 Improvement (at 6 hours) by inpatient/outpatient.
3. The use of additional treatments
There was no significant difference in the need for epinephrine (RR 1.42, 95% CI 0.27 to 7.39; P = 0.67; I² = 0%; 2 RCTs; 183 children; Analysis 7.5); a mist tent (RR 1.07, 95% CI 0.69 to 1.65; P = 0.77; 1 RCT; 50 children; Analysis 7.6); supplemental glucocorticoids (RR 1.10, 95% CI 0.07 to 16.66; P = 0.95; I² = 66%; 2 RCTs; 182 children; Analysis 7.7) among children who were treated with dexamethasone and budesonide compared to those treated with dexamethasone alone.
7.5. Analysis.
Comparison 7 Budesonide and dexamethasone compared to dexamethasone, Outcome 5 Additional treatments: epinephrine.
7.6. Analysis.
Comparison 7 Budesonide and dexamethasone compared to dexamethasone, Outcome 6 Additional treatments: mist tent.
7.7. Analysis.
Comparison 7 Budesonide and dexamethasone compared to dexamethasone, Outcome 7 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
Klassen 1998 reported no adverse events in the dexamethasone group or dexamethasone and budesonide group.
Budesonide and dexamethasone compared to budesonide
See: budesonide and dexamethasone compared to budesonide for croup (Table 9).
7. Budesonide and dexamethasone compared to budesonide for croup.
| Budesonide and dexamethasone compared to budesonide for croup | |||||
| Patient or population: children with croup Intervention: budesonide and dexamethasone Comparison: budesonide | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Budesonide | Budesonide and dexamethasone | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (follow‐up: 6 hours) | The mean change in croup score from 1 study was ‐2.30. | The mean change in croup score was 0.20 units more (0.59 more to 0.19 less). | ‐ | 129 (1 RCT) | ⊕⊕⊝⊝ LOWa, b |
| Return visits or (re)admissions or both | Study population | RD 0.00 (‐0.03 to 0.03) | 129 (1 RCT) | ⊕⊕⊝⊝ LOWc, d | |
| 0 per 1000 | 0 per 1000 (0 to 0) | ||||
| Adverse events | Klassen 1998 reported 1 case of oral thrush in the budesonide group (1/65, 1.5%) and no adverse events in the dexamethasone and budesonide group. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RD: risk difference | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). cWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. dWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
Primary outcomes
1. Change in clinical croup score
Klassen 1998 investigated children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was no significant difference in the reduction in croup score after six hours for children treated with combined dexamethasone and budesonide compared to those treated with budesonide alone (MD ‐0.20, 95% CI ‐0.59 to 0.19; P = 0.32; 1 RCT; 129 children; low‐certainty evidence; Analysis 8.1).
8.1. Analysis.
Comparison 8 Budesonide and dexamethasone compared to budesonide, Outcome 1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient.
2. Return visits or (re)admissions to the hospital or both
Klassen 1998 investigated return visits to the emergency department among children aged three months to five years treated with dexamethasone, budesonide, or a combination of the two for croup. There were no events in the budesonide group or the dexamethasone and budesonide group (RD 0.00, 95% CI ‐0.03 to 0.03; P = 1.00; 1 RCT; 129 children; low‐certainty evidence; Analysis 8.2).
8.2. Analysis.
Comparison 8 Budesonide and dexamethasone compared to budesonide, Outcome 2 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
Klassen 1998 investigated hours spent in the emergency department among children aged three months to five years treated with dexamethasone, budesonide, or a combination of the two for croup. There was no significant difference in the length of stay for children treated with dexamethasone and budesonide compared to those treated with budesonide alone (MD 0.25, 95% CI ‐0.36 to 0.86; P = 0.42; 1 RCT; 129 children; Analysis 8.3).
8.3. Analysis.
Comparison 8 Budesonide and dexamethasone compared to budesonide, Outcome 3 Length of stay by inpatient/outpatient.
2. Patient improvement
Klassen 1998 investigated response to treatment, defined as a two‐point reduction in croup score, among children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was no significant difference in the response to treatment among children treated with dexamethasone and budesonide compared to those treated with budesonide alone (RR 0.97, 95% CI 0.79 to 1.20; P = 0.80; 1 RCT; 129 children; Analysis 8.4).
8.4. Analysis.
Comparison 8 Budesonide and dexamethasone compared to budesonide, Outcome 4 Improvement (at 6 hours) by inpatient/outpatient.
3. The use of additional treatments
Klassen 1998 investigated the need for additional treatments among children aged three months to five years treated in the emergency department with dexamethasone, budesonide, or a combination of the two for croup. There was no significant difference in the need for epinephrine (RR 1.02, 95% CI 0.15 to 6.99; P = 0.99; 1 RCT; 129 children; Analysis 8.5) or supplemental glucocorticoids (RR 1.31, 95% CI 0.52 to 3.29; P = 0.57; 1 RCT; 129 children; Analysis 8.6) among children treated with dexamethasone and budesonide compared to those treated with budesonide alone.
8.5. Analysis.
Comparison 8 Budesonide and dexamethasone compared to budesonide, Outcome 5 Additional treatments: epinephrine.
8.6. Analysis.
Comparison 8 Budesonide and dexamethasone compared to budesonide, Outcome 6 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
Klassen 1998 reported one case of oral thrush in the budesonide group (1/65, 1.5%) and no adverse events in the dexamethasone and budesonide group.
Oral compared to intramuscular dexamethasone
See: oral dexamethasone compared to intramuscular dexamethasone for croup (Table 10)
8. Oral dexamethasone compared to intramuscular dexamethasone for croup.
| Oral dexamethasone compared to intramuscular dexamethasone for croup | |||||
| Patient or population: children with croup Intervention: oral dexamethasone Comparison: intramuscular dexamethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Intramuscular dexamethasone | Oral dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RR 0.81 (0.58 to 1.12) | 440 (3 RCTs) | ⊕⊕⊕⊝ MODERATEa | |
| 259 per 1000 | 210 per 1000 (150 to 290) | ||||
| Adverse events | None of the studies reported collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for imprecision. The effect estimate included both a null effect and substantial benefit for oral compared to intramuscular dexamethasone.
Primary outcomes
1. Change in clinical croup score
We identified no studies that investigated the change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
There was no significant difference in the rate of return visits or admissions following treatment with oral dexamethasone compared to intramuscular dexamethasone (RR 0.81, 95% CI 0.58 to 1.12; P = 0.21; I² = 0%; 3 RCTs; 440 children; moderate‐certainty evidence; Analysis 9.1). The analysis included only outpatients (Analysis 9.1).
9.1. Analysis.
Comparison 9 Oral compared to intramuscular dexamethasone, Outcome 1 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
We identified no studies that investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement
Donaldson 2003 investigated clinical improvement, defined as parents' assessment that their child's condition had improved at least somewhat after 24 hours, among children aged three to 84 months treated in the emergency department with oral or intramuscular dexamethasone for croup. There was no significant difference in the rate of clinical improvement between the oral and intramuscular dexamethasone groups (RR 1.07, 95% CI 0.95 to 1.19; P = 0.27; 1 RCT; 95 children; Analysis 9.2).
9.2. Analysis.
Comparison 9 Oral compared to intramuscular dexamethasone, Outcome 2 Improvement (at 24 hours) by inpatient/outpatient.
3. The use of additional treatments
There was no significant difference in the need for antibiotics (RR 0.14, 95% CI 0.02 to 1.15; P = 0.07; 1 RCT; 277 children; Analysis 9.3); epinephrine (RR 0.94, 95% CI 0.71 to 1.24; P = 0.64; I² = 0%; 2 RCTs; 372 children; Analysis 9.4); a mist tent (RR 1.34, 95% CI 0.31 to 5.89; P = 0.70; 1 RCT; 277 children; Analysis 9.5); or supplemental glucocorticoids (RR 1.10, 95% CI 0.50 to 2.41; P = 0.81; 1 RCT; 277 children; Analysis 9.6) among children treated with oral dexamethasone compared to those treated with intramuscular dexamethasone.
9.3. Analysis.
Comparison 9 Oral compared to intramuscular dexamethasone, Outcome 3 Additional treatments: antibiotics.
9.4. Analysis.
Comparison 9 Oral compared to intramuscular dexamethasone, Outcome 4 Additional treatments: epinephrine.
9.5. Analysis.
Comparison 9 Oral compared to intramuscular dexamethasone, Outcome 5 Additional treatments: mist tent.
9.6. Analysis.
Comparison 9 Oral compared to intramuscular dexamethasone, Outcome 6 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
We identified no studies that investigated adverse events for this comparison.
Oral compared to nebulised dexamethasone
See: oral dexamethasone compared to nebulised dexamethasone for croup (Table 11).
9. Oral dexamethasone compared to nebulised dexamethasone for croup.
| Oral dexamethasone compared to nebulised dexamethasone for croup | |||||
| Patient or population: children with croup Intervention: oral dexamethasone Comparison: nebulised dexamethasone | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Nebulised dexamethasone | Oral dexamethasone | ||||
| Return visits or (re)admissions or both | Study population | RR 0.39 (0.17 to 0.89) | 176 (1 RCT) | ⊕⊕⊝⊝ LOWa, b | |
| 209 per 1000 | 81 per 1000 (35 to 186) | ||||
| Adverse events | None of the studies reported collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size).
Primary outcomes
1. Change in clinical croup score
We identified no studies that investigated the change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Luria 2001 investigated returns to medical care of children aged six months to six years following treatment with oral or nebulised dexamethasone in the emergency department for croup. There were significantly fewer return visits to medical care among those treated with oral dexamethasone compared to those treated with nebulised dexamethasone (RR 0.39, 95% CI 0.17 to 0.89; P = 0.03; 1 RCT; 176 children; low‐certainty evidence; Analysis 10.1).
10.1. Analysis.
Comparison 10 Oral compared to nebulised dexamethasone, Outcome 1 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
We identified no studies that investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement
We identified no studies that investigated clinical improvement for this comparison.
3. The use of additional treatments
We identified no studies that investigated the use of additional treatments for this comparison.
4. Any adverse events
We identified no studies that investigated adverse events for this comparison.
Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg
See: dexamethasone 0.30 mg/kg compared to dexamethasone 0.15 mg/kg for croup (Table 12)
10. Dexamethasone 0.30 mg per kg compared to dexamethasone 0.15 mg per kg for croup.
| Dexamethasone 0.30 mg per kg compared to dexamethasone 0.15 mg per kg for croup | |||||
| Patient or population: children with croup Intervention: dexamethasone 0.30 mg per kg Comparison: dexamethasone 0.15 mg per kg | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Dexamethasone 0.15 mg per kg | Dexamethasone 0.30 mg per kg | ||||
| Return visits or (re)admissions or both | Study population | RR 0.94 (0.06 to 14.27) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa, b | |
| 34 per 1000 | 32 per 1000 (2 to 492) | ||||
| Adverse events | Geelhoed 1995b did not report collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. bWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included significant benefit, the null effect, and potential harm for 0.30 mg/kg compared to 0.15 mg/kg dexamethasone.
Primary outcomes
1. Change in clinical croup score
We identified no studies that investigated the change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Geelhoed 1995b investigated re‐presentations to medical care for croup among children aged greater than three months treated in the emergency department with 0.30 mg/kg or 0.15 mg/kg dexamethasone. There was no significant difference in the rate of re‐presentations to medical care among children treated with 0.30 mg/kg compared to those treated with 0.15 mg/kg dexamethasone (RR 0.94, 95% CI 0.06 to 14.27; P = 0.96; 1 RCT; 60 children; very low‐certainty evidence; Analysis 11.1).
11.1. Analysis.
Comparison 11 Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 1 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
We identified no studies that investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement
We identified no studies that investigated clinical improvement for this comparison.
3. The use of additional treatments
Geelhoed 1995b investigated the need for additional treatments among children aged greater than three months treated in the emergency department with 0.30 mg/kg or 0.15 mg/kg dexamethasone for croup. There was no difference between the two treatments in the need for epinephrine (RR 0.43, 95% CI 0.19 to 0.98; P = 0.05; 1 RCT; 60 children; Analysis 11.2). No child required supplemental glucocorticoids (RD 0.00, 95% CI ‐0.06 to 0.06; P = 1.00; 1 RCT; 60 children; Analysis 11.3).
11.2. Analysis.
Comparison 11 Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 2 Additional treatments: epinephrine.
11.3. Analysis.
Comparison 11 Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg, Outcome 3 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
We identified no studies that investigated adverse events for this comparison.
Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg
See: dexamethasone 0.60 mg/kg compared to dexamethasone 0.30 mg/kg for croup (Table 13)
11. Dexamethasone 0.60 mg per kg compared to dexamethasone 0.30 mg per kg for croup.
| Dexamethasone 0.60 mg per kg compared to dexamethasone 0.30 mg per kg for croup | |||||
| Patient or population: children with croup Intervention: dexamethasone 0.60 mg per kg Comparison: dexamethasone 0.30 mg per kg | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Dexamethasone 0.30 mg per kg | Dexamethasone 0.60 mg per kg | ||||
| Return visits or (re)admissions or both | Study population | RR 1.40 (0.25 to 7.81) | 60 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa, b | |
| 69 per 1000 | 97 per 1000 (17 to 539) | ||||
| Adverse events | Geelhoed 1995a did not report collecting adverse events data. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. bWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included significant benefit, the null effect, and potential for harm for 0.60 mg/kg compared to 0.30 mg/kg dexamethasone.
Primary outcomes
1. Change in clinical croup score
We identified no studies that investigated the change in croup score for this comparison.
2. Return visits or (re)admissions to the hospital or both
Geelhoed 1995a investigated re‐presentations to medical care for croup among children aged greater than three months treated in the emergency department with 0.60 mg/kg or 0.30 mg/kg dexamethasone. There was no significant difference in the rate of re‐presentations to medical care among children treated with 0.60 mg/kg compared to 0.30 mg/kg dexamethasone (RR 1.40, 95% CI 0.25 to 7.81; P = 0.70; 1 RCT; 60 children; very low‐certainty evidence; Analysis 12.1).
12.1. Analysis.
Comparison 12 Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 1 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
We identified no studies that investigated length of stay in the hospital or emergency department for this comparison.
2. Patient improvement
We identified no studies that investigated clinical improvement for this comparison.
3. The use of additional treatments
Geelhoed 1995a investigated the need for additional treatments among children aged greater than three months treated in the emergency department with 0.60 mg/kg or 0.30 mg/kg dexamethasone for croup. There was no difference between the two treatments in the need for epinephrine (RR 0.78, 95% CI 0.27 to 2.28; P = 0.65; 1 RCT; 60 children; Analysis 12.2) or supplemental glucocorticoids (RR 2.81, 95% CI 0.12 to 66.40; P = 0.52; 1 RCT; 60 children; Analysis 12.3).
12.2. Analysis.
Comparison 12 Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 2 Additional treatments: epinephrine.
12.3. Analysis.
Comparison 12 Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg, Outcome 3 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
We identified no studies that investigated adverse events for this comparison.
Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg
See: dexamethasone 0.60 mg/kg compared to dexamethasone 0.15 mg/kg for croup (Table 14)
12. Dexamethasone 0.60 mg per kg compared to dexamethasone 0.15 mg per kg for croup.
| Dexamethasone 0.60 mg per kg compared to dexamethasone 0.15 mg per kg for croup | |||||
| Patient or population: children with croup Intervention: dexamethasone 0.60 mg per kg Comparison: dexamethasone 0.15 mg per kg | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | |
| Dexamethasone 0.15 mg per kg | Dexamethasone 0.60 mg per kg | ||||
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (follow‐up: 2 hours) | The mean change in croup score from 1 study was ‐1.05. | The mean change in croup score was 0.15 units more (0.29 more to 0.01 more). | ‐ | 41 (1 RCT) |
⊕⊕⊝⊝ LOWa, b |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (follow‐up: 6 hours) | The mean change in croup score was ‐3.10 to ‐2.09. | The mean change in croup score was 0.33 units more (0.50 more to 0.16 more). | ‐ | 178 (3 RCTs) | ⊕⊕⊕⊝ MODERATEc |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (follow‐up: 12 hours) | The mean change in croup score was ‐3.50 to ‐2.95. | The mean change in croup score was 0.17 units less (1.45 more to 1.78 less). | ‐ | 113 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWd, e |
| Change in croup score. Assessed with the Westley croup score. Lower scores mean fewer symptoms. (follow‐up: 24 hours) | The mean change in croup score from 1 study was ‐4.00. | The mean change in croup score was 0.50 units less (0.14 less to 0.86 less). | ‐ | 72 (1 RCT) | ⊕⊕⊝⊝ LOWf, g |
| Return visits or (re)admissions or both | Study population | RR 0.92 (0.54 to 1.55) | 129 (2 RCTs) | ⊕⊕⊝⊝ LOWh | |
| 288 per 1000 | 265 per 1000 (155 to 446) | ||||
| Adverse events | Alshehr 2005 reported 1 case of bacterial tracheitis and 2 cases of bronchopneumonia in the 0.60 mg/kg dexamethasone group (3/36, 8.3%) and no adverse events in the 0.15 mg/kg dexamethasone group. Chub‐Uppakarn 2007 and Fifoot 2007 reported no adverse events from 0.15 mg/kg or 0.60 mg/kg dexamethasone. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. bWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). cWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). dWe downgraded by two levels level for inconsistency. There was considerable heterogeneity (I² = 99%), and variation in point estimates. The 95% confidence intervals did not overlap. eWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included both the null effect and appreciable benefit and harm for 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. fWe downgraded by one level for inconsistency. As there was only one study in the analysis, there was no evidence of consistency. gWe downgraded by one level for imprecision. The sample size was small (did not meet the optimal information size). hWe downgraded by two levels for imprecision. The sample size was small (did not meet the optimal information size). The effect estimate included the null effect as well as appreciable benefit and harm for 0.60 mg/kg compared to 0.15 mg/kg dexamethasone.
Primary outcomes
1. Change in clinical croup score
Children treated with 0.60 mg/kg dexamethasone experienced significantly greater reductions in croup score after two hours (MD ‐0.15, 95% CI ‐0.29 to ‐0.01; P = 0.04; 1 RCT; 41 children; low‐certainty evidence; Analysis 13.1) and six hours (MD ‐0.33, 95% CI ‐0.50 to ‐0.16; P < 0.001; I² = 4%; 3 RCTs; 178 children; moderate certainty evidence; Analysis 13.2) compared to those treated with 0.15 mg/kg dexamethasone. Twelve hours after treatment, there was no significant difference in the change in croup score for children treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone (MD 0.17, 95% CI ‐1.45 to 1.78; P = 0.84; I² = 99%; 2 RCTs; 113 children; very low‐certainty evidence; Analysis 13.3). There was a significant subgroup difference in effect by inpatient or outpatient status after 12 hours (Chi² = 72.89, P < 0.001; I² = 98.6%; Analysis 13.3). Among inpatients, Chub‐Uppakarn 2007 found a significantly greater reduction in croup score in children treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone (MD ‐0.65, 95% CI ‐0.80 to ‐0.50; P < 0.001; 1 RCT; 41 children). Among outpatients, Alshehr 2005 found a significantly smaller reduction in croup score in children treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone (MD 1.00, 95% CI 0.65 to 1.35; P < 0.001; 1 RCT; 72 children). After 24 hours, Alshehr 2005 found that children treated with 0.60 mg/kg dexamethasone had a significantly smaller reduction in croup score compared to those treated with 0.15 mg/kg dexamethasone (MD 0.50, 95% CI 0.14 to 0.86; P = 0.007; 1 RCT; 72 children; low‐certainty evidence; Analysis 13.4).
13.1. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 1 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient.
13.2. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 2 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient.
13.3. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 3 Croup score (change baseline ‐ 12 hours) by inpatient/outpatient.
13.4. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 4 Croup score (change baseline ‐ 24 hours) by inpatient/outpatient.
2. Return visits or (re)admissions to the hospital or both
Data from two studies showed no significant difference in return visits and admissions when children were treated with 0.60 mg/kg dexamethasone compared to 0.15 mg/kg dexamethasone (RR 0.92, 95% CI 0.54 to 1.55; P = 0.75; I² = 0%; 2 RCTs; 129 children; low‐certainty evidence; Analysis 13.5) (Alshehr 2005; Fifoot 2007). All children in the analysis were outpatients.
13.5. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 5 Return visits or (re)admissions or both by inpatient/outpatient.
Secondary outcomes
1. Length of stay in the hospital or emergency department
Alshehr 2005 investigated length of stay in the emergency department or outpatient clinic for children aged three months to nine years treated with 0.60 mg/kg or 0.15 mg/kg dexamethasone for croup. There was no significant difference in hours spent in the emergency department or outpatient clinic between children treated with 0.60 mg/kg or 0.15 mg/kg dexamethasone (MD 2.00, 95% CI ‐2.16 to 6.16; P = 0.35; 1 RCT; 72 children; Analysis 13.6).
13.6. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 6 Length of stay by inpatient/outpatient.
2. Patient improvement
We identified no studies that investigated clinical improvement for this comparison.
3. The use of additional treatments
Alshehr 2005, Chub‐Uppakarn 2007, and Fifoot 2007 investigated the need for additional treatments among children treated with 0.60 mg/kg or 0.15 mg/kg dexamethasone as outpatients for croup. Fifoot 2007 found no difference between the two treatments in the need for epinephrine (RR 1.10, 95% CI 0.07 to 16.80; P = 0.95; 1 RCT; 65 children; Analysis 13.7). Chub‐Uppakarn 2007 reported no need for intubation among children in either treatment group (RD 0.00, 95% CI ‐0.09 to 0.09; P = 1.00; 1 RCT; 41 children; Analysis 13.8). Alshehr 2005 found no difference between the two treatments in the need for a mist tent (RR 1.13, 95% CI 0.69 to 1.84; P = 0.64; 1 RCT; 72 children; Analysis 13.9). Fifoot 2007 found no difference between the two treatments in the need for supplemental glucocorticoids (RR 0.89, 95% CI 0.27 to 2.97; P = 0.85; 1 RCT; 57 children; Analysis 13.10).
13.7. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 7 Additional treatments: epinephrine.
13.8. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 8 Additional treatments: intubation/tracheotomy.
13.9. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 9 Additional treatments: mist tent.
13.10. Analysis.
Comparison 13 Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg, Outcome 10 Additional treatments: supplemental glucocorticoids.
4. Any adverse events
Alshehr 2005 reported one case of bacterial tracheitis and two cases of bronchopneumonia in the 0.60 mg/kg dexamethasone group (3/36, 8.3%) and no adverse events in the 0.15 mg/kg dexamethasone group. Chub‐Uppakarn 2007 and Fifoot 2007 reported no adverse events from 0.60 mg/kg or 0.15 mg/kg dexamethasone.
Publication bias
Considering the analysis for change in croup score after six hours for any glucocorticoid compared to placebo, we found no indication of publication bias following visual inspection of the funnel plot. The Egger's test for small‐study effects (weighted regression) was not statistically significant (P = 0.08). The Begg's test for small‐study effects (rank correlation) was also not statistically significant (P = 0.48; continuity corrected P = 0.53).
Considering the analysis for return visits or (re)admissions or both for any glucocorticoid compared to placebo, the funnel plot appeared asymmetrical, indicating publication bias. The Egger's test for small‐study effects was not statistically significant (P = 0.23). The Begg's test for small‐study effects was also not statistically significant (P = 0.21; continuity corrected P = 0.25).
We did not test for publication bias for any of the other comparisons or outcomes, as there were insufficient numbers of included studies.
Sensitivity analysis
In cases where variance data for individual studies were unavailable (i.e., Analysis 1.1, Analysis 1.2, Analysis 1.3, Analysis 1.4, Analysis 1.15, Analysis 2.1, Analysis 2.4, Analysis 3.1, and Analysis 7.1), we imputed variance data based on other studies present in the same analysis. We conducted sensitivity analyses by imputing from either the largest, smallest, or average variances of the other included studies in each analysis. We found no difference in the magnitude of effect for any analyses based on the different possibilities for imputed variances (largest, smallest, or average). For this reason, we have presented only the analyses where we used the average of the variances of the other included studies to impute missing variances.
Discussion
Summary of main results
Any glucocorticoid compared to placebo
Twenty‐six studies investigated glucocorticoids compared to placebo. Glucocorticoids significantly reduced the symptoms of croup within two hours of treatment, and the effect lasted at least 24 hours. The effect was dependent on the glucocorticoid administered. Budesonide and dexamethasone reduced the symptoms of croup within two hours of treatment, and the effect lasted at least 24 hours. One trial showed that prednisolone reduced the symptoms of croup within six hours, and the effect lasted at least 12 hours. One trial showed that fluticasone did not significantly reduce the symptoms of croup after two, six, and 24 hours compared to placebo. We have moderate certainty about the effect of glucocorticoids compared to placebo for reducing the symptoms of croup from two to 12 hours, as there was considerable between‐study heterogeneity in effect estimates (Table 1). We have low certainty about the effect of glucocorticoids compared to placebo for reducing the symptoms of croup after 24 hours, as there was considerable between‐study heterogeneity in the magnitude and direction of the effect (Table 1).
Compared to placebo, both budesonide and dexamethasone significantly reduced the rate of return visits and/or (re)admissions to the hospital or emergency department. We have moderate certainty about the effect of glucocorticoids for reducing the rate of return visits or (re)admissions or both, as there was considerable between‐study heterogeneity in effect estimates (Table 1).
Compared to placebo, glucocorticoids reduced the length of stay in the hospital by approximately 15 hours and resulted in clinical improvement in a significantly greater proportion of children after six hours. The effect lasted at least 24 hours. There was no significant difference in the need for additional treatments between children treated with glucocorticoids and those treated with placebo. Treatment with glucocorticoids was infrequently associated with serious adverse events.
Any glucocorticoid compared to epinephrine
Four studies investigated glucocorticoids compared to epinephrine. There was no significant difference in the reduction in symptoms of croup for children treated with epinephrine compared to those treated with glucocorticoids two, six, 12, or 24 hours following their administration. After two hours, the effect was dependent on the glucocorticoid administered. Epinephrine resulted in significantly greater reductions in symptoms of croup compared to beclomethasone and dexamethasone. There was no significant difference in the reduction in croup symptoms between epinephrine and budesonide two hours after treatment. We have very low to moderate certainty about the effect of glucocorticoids compared to epinephrine for reducing the symptoms of croup. The sample sizes were small for the six‐, 12‐, and 24‐hour analyses, and there was considerable between‐study heterogeneity in effect estimates for the six‐hour analysis. For the two‐hour analysis, there was considerable between‐study heterogeneity in the magnitude and direction of the effect estimates; the sample size for the comparison was small; and the pooled effect estimate was imprecise (Table 2).
There was no significant difference in the rate of return visits or (re)admissions or both following treatment with glucocorticoids compared with epinephrine. We have moderate certainty about the effect of glucocorticoids compared to epinephrine for reducing the rate of return visits or (re)admissions or both, as the sample size did not meet the optimal information size and the contributing studies reported no events (Table 2).
There was no significant difference in length of stay in the hospital for children treated with glucocorticoids compared to those treated with epinephrine, nor were there any significant differences in the need for additional treatments. One study reported a 31.3% rate of secondary bacterial infections among children treated with dexamethasone. Another study reported a 16% rate of tremor and tachycardia among children treated with epinephrine.
One glucocorticoid compared to another glucocorticoid
Eleven studies investigated one glucocorticoid compared to another glucocorticoid. Compared to budesonide, dexamethasone resulted in significantly greater reductions in symptoms of croup after six and 12 hours. We have low certainty about the effect of budesonide compared to dexamethasone for reducing the symptoms of croup, as the contributing studies were all at high or unclear risk of bias; for the six‐hour analysis there was substantial between‐study heterogeneity in effect estimates; and for the 12‐hour analysis the sample size did not meet the optimal information size (Table 4). Compared to betamethasone, dexamethasone resulted in significantly greater reductions in symptoms of croup after two and six hours. We have very low certainty about the effect of dexamethasone compared to betamethasone for reducing the symptoms of croup, as only one study contributed to the analysis, and it was at high risk of bias and had a small sample size (Table 6). There was no significant difference in the reduction in symptoms of croup six hours following treatment with dexamethasone compared to prednisolone. We have low certainty about the effect of dexamethasone compared to prednisolone for reducing the symptoms of croup, as only one study contributed to the analysis and it had a small sample size (Table 7).
There was no significant difference between dexamethasone and budesonide, dexamethasone and beclomethasone, and dexamethasone and betamethasone in the rate of return visits or (re)admissions or both. We have moderate certainty about the effect of dexamethasone compared to budesonide for reducing the rate of return visits or (re)admissions or both, as few events were reported, and the effect estimate included the null effect as well as considerable benefit for dexamethasone compared to budesonide (Table 4). We have low certainty about the effect of dexamethasone compared to beclomethasone for reducing the rate of return visits or (re)admissions or both, as only one study contributed to the analysis and it had a small sample size and reported no events (Table 5). We have very low certainty about the effect of dexamethasone compared to betamethasone for reducing the rate of return visits or (re)admissions or both, as only one small study contributed to the analysis, and the effect estimate included the null effect as well as appreciable benefit and harm (Table 6). Compared to prednisolone, dexamethasone significantly reduced the rate of return visits or (re)admissions or both. We have moderate certainty about the effect of dexamethasone compared to prednisolone for reducing the rate of return visits or (re)admissions or both, as the sample size did not reach the optimal information size (Table 7).
There was no significant difference in length of stay in the hospital or emergency department between children treated with dexamethasone compared to budesonide, or with dexamethasone compared to prednisolone. One study showed no significant difference in clinical improvement between children treated with dexamethasone and those treated with budesonide. Compared to those treated with budesonide, children treated with dexamethasone were at a significantly reduced risk for needing epinephrine. There was no difference between children treated with dexamethasone and budesonide in the need for intubation or supplemental glucocorticoids. Compared to those treated with betamethasone, children treated with dexamethasone were at a significantly increased risk for needing epinephrine. There was no significant difference between children treated with dexamethasone and those treated with prednisolone in the need for epinephrine or supplemental glucocorticoids. Adverse events were infrequently reported.
One glucocorticoid compared to a combination of glucocorticoids
Three studies investigated one glucocorticoid compared to a combination of glucocorticoids. There was no significant difference in the reduction in symptoms of croup for children treated with dexamethasone compared to combined dexamethasone and budesonide, nor for children treated with budesonide compared to combined budesonide and dexamethasone. We have moderate certainty about the effect of dexamethasone compared to dexamethasone and budesonide for reducing the symptoms of croup, as the sample size for the analysis did not meet the optimal information size (Table 8). We have low certainty about the effect of budesonide compared to budesonide and dexamethasone for reducing the symptoms of croup (Table 9), as only one small study contributed to the analysis.
There was no significant difference in the rate of return visits or (re)admissions to the hospital or both following treatment with dexamethasone compared to combined dexamethasone and budesonide, nor following treatment with budesonide compared to combined budesonide and dexamethasone. We have low certainty about the effect of dexamethasone compared to dexamethasone and budesonide for reducing the rate of return visits or (re)admissions or both (Table 8), as the sample size for the analysis did not meet the optimal information size; there were few events; and the estimate was imprecise. We have low certainty about the effect of budesonide compared to dexamethasone and budesonide for reducing the rate of return visits or (re)admissions or both (Table 9), as only one small study contributed to the analysis.
There was no significant difference in hours spent in the hospital or emergency department, in clinical improvement, or in the need for additional treatments for children treated with dexamethasone compared to those treated with combined dexamethasone and budesonide, nor for children treated with budesonide compared to combined budesonide and dexamethasone. Only one study collected adverse events data, which included one case (1.5%) of oral thrush in the budesonide group and no events in the budesonide and dexamethasone group (Klassen 1998).
Glucocorticoids given by different modes of administration
Five studies investigated dexamethasone given by different modes of administration. There was no significant difference in the rate of return visits or (re)admissions or both for children treated with oral dexamethasone compared to intramuscular dexamethasone. There was a significantly reduced rate of return visits or (re)admissions or both for children treated with oral dexamethasone compared to those treated with nebulised dexamethasone. We have moderate certainty about the effect of oral compared to intramuscular dexamethasone for reducing the rate of return visits or (re)admissions or both, as the contributing studies reported few events and the estimate was imprecise (Table 10). We have low certainty about the effect of oral compared to nebulised dexamethasone for reducing the rate of return visits or (re)admissions or both because only one study contributed to the analysis, and the sample size did not meet the optimal information size (Table 11).
There was no significant difference in clinical improvement or in the need for additional treatments among children treated with oral dexamethasone compared to those treated with intramuscular dexamethasone. None of the studies comparing dexamethasone given by different modes of administration reported collecting adverse events data.
Dexamethasone given in different doses
Four studies investigated dexamethasone given in different doses. One study reported a significantly greater reduction in croup score after two hours for inpatients treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone (Chub‐Uppakarn 2007). After six hours, there was a significantly greater reduction in croup score for children treated with 0.60 mg/kg dexamethasone compared to those treated with 0.15 mg/kg dexamethasone. After 12 hours, there was no significant difference in the change in croup score among children treated with 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. The effect differed significantly by inpatient and outpatient status. In inpatients, the 0.60 mg/kg dose resulted in a significantly greater reduction in croup score after 12 hours, whereas in outpatients, the 0.15 mg/kg dose was more effective. One study investigated change in croup score after 24 hours for inpatients treated with 0.60 mg/kg or 0.15 mg/kg dexamethasone (Alshehr 2005). Those treated with 0.15 mg/kg experienced significantly greater reductions in croup score after 24 hours compared to those treated with 0.60 mg/kg dexamethasone. We have very low to moderate certainty about the effect of 0.60 mg/kg dexamethasone compared to 0.15 mg/kg dexamethasone for reducing croup score (Table 14). The two‐hour analysis included only one study with a small sample size. The six‐hour analysis included three studies, but the sample size did not meet the optimal information size. In the 12‐hour analysis, there was considerable between‐study heterogeneity in effect estimates, and the sample sizes did not meet the optimal information size. In the 12‐hour analysis, the pooled effect estimate included the null effect as well as appreciable benefit and harm. The 24‐hour analysis included only one study with a small sample size.
There was no significant difference in the rate of return visits or (re)admissions or both for children treated with 0.30 mg/kg compared to 0.15 mg/kg dexamethasone; 0.60 mg/kg compared to 0.30 mg/kg dexamethasone; or 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. We have very low certainty about the effect of 0.30 mg/kg compared to 0.15 mg/kg dexamethasone (Table 12) and 0.60 mg/kg compared to 0.30 mg/kg dexamethasone (Table 13) for reducing the rate of return visits or (re)admissions or both for croup, as the analysis included only one small study that reported few events, and the effect estimate included significant benefit, the null effect, and potential for harm. We have low certainty about the effect of 0.60 mg/kg compared to 0.15 mg/kg dexamethasone for reducing the rate of return visits or (re)admissions or both because the sample size did not meet the optimal information size, and the effect estimate included significant benefit, the null effect, and potential for harm (Table 14).
One study reported no significant difference in the hours spent in the emergency department between children treated with 0.60 mg/kg compared to those treated with 0.15 mg/kg dexamethasone (Alshehr 2005). There was no significant difference in the need for additional treatments between children treated with 0.30 mg/kg compared to 0.15 mg/kg dexamethasone; 0.60 mg/kg compared to 0.30 mg/kg dexamethasone; or 0.60 mg/kg compared to 0.15 mg/kg dexamethasone. Adverse events were infrequently reported for the 0.15 mg/kg and 0.60 mg/kg doses of dexamethasone.
Overall completeness and applicability of evidence
We searched for RCTs that compared glucocorticoids to placebo or any other active pharmacologic treatment for croup. The number of included studies was large (n = 43), and 26 (60%) of these investigated glucocorticoids compared to placebo. Only four studies investigated glucocorticoids compared to epinephrine; 11 investigated one glucocorticoid compared to another glucocorticoid; three investigated one glucocorticoid compared to a combination of glucocorticoids; five investigated glucocorticoids given by different modes of administration; and four investigated glucocorticoids given in different doses. Most (67%) of the studies reported a change in croup score for at least one time point, and 59% used the Westley croup score (Westley 1978), which has been shown to be a valid and reliable measure of croup severity. Most (51%) of the studies investigated outpatients presenting to emergency departments or outpatient clinics, generally with mild to moderate croup. A review of utilisation data from Alberta, Canada indicated that at least two‐thirds of children who present to emergency care for croup have mild symptoms (Johnson 2003). Only 1% to 5% of children with croup are admitted to hospital (Johnson 2003), so studies of inpatients were over‐represented in this review. We have therefore presented subgroup analyses by inpatient or outpatient setting. We found no evidence of publication bias for our two primary outcomes: change in croup score (at six hours) and return visits or (re)admissions to the hospital or both for glucocorticoids compared to placebo.
Quality of the evidence
This systematic review included 43 RCTs of 4565 children. Most of the studies were at unclear or high overall risk of bias (98%). The method for generating the randomisation sequence was unclear in 40% of studies, which were often described as "randomised" without any further methodological details. Whether or not the allocation sequence was adequately concealed between randomisation and assignment to treatment groups was unclear in 58% of studies. We were unable to ascertain whether the conduct of these studies was methodologically flawed. However, based on the information provided in the publications, we cannot exclude the possibility of selection bias. Empirically, selection bias has been associated with exaggerated estimates of treatment effects (Jüni 2001; Wood 2008). Inadequate allocation concealment is more likely to result in biased estimates of treatment effects when the outcomes of a study are subjective (Wood 2008). Croup score, one of our primary outcomes, is typically assessed by the healthcare provider, and interobserver variability has been reported to be fair to moderate (Chan 2001). Hartling 2014 demonstrated that the association between selection bias and the estimate of treatment effects may not hold true for RCTs in child health. We are therefore uncertain as to how selection bias may have impacted our results.
Almost half (42%) of the included studies were at unclear or high risk of bias for blinding of participants and personnel, and 40% were at unclear or high risk of bias for blinding of outcome assessors. Many of the studies judged as at unclear risk of bias for the blinding domains were described as "blind" or "double‐blind"; however, details about who was blinded or how, or both, were omitted from the publications. Although it is possible that these studies were well conducted but inadequately reported, we cannot confidently exclude the potential for performance and detection bias. In eight (19%) studies, participants and personnel were not blinded. All but one of these studies investigated glucocorticoids given via different modes of administration (e.g. orally, intramuscularly, nebulised), therefore blinding participants and personnel to the treatment assignment would not have been feasible. Studies that are not blinded or that are inadequately blinded can result in exaggerated estimates of treatment effects (Wood 2008). This association may not be true for RCTs in child health (Hartling 2014), therefore we are uncertain as to how the inclusion of unblinded or inadequately blinded trials may have impacted our results.
Most studies (63%) were at low risk of bias for incomplete outcome data. Although most (95%) were at unclear risk of bias for selective reporting, only 10 (23%) of the included studies were published after the International Committee of Medical Journal Editors released a statement announcing mandatory registration for trials published in their member journals (De Angelis 2004). The outcomes reported in the results matched those reported in the methods sections of the publications for all studies. All included studies except Tibballs 1992 reported on important markers of treatment success: change in croup score, patient improvement, or return visits or (re)admissions or both. We therefore do not believe that selective reporting substantially impacted the results of this review. It is also unlikely that publication bias influenced our findings. Baseline imbalances in croup score could have biased the results of two studies, favouring the betamethasone group in the study by Amir 2006 (compared to dexamethasone) and the budesonide group in the study by Vad Pedersen 1998 (compared to dexamethasone).
For any glucocorticoid compared to placebo, we detected between‐study heterogeneity in point estimates of effect as well as heterogeneity in the pooled estimates of effect by glucocorticoid for change in croup score. For this reason, we downgraded the certainty of the evidence for the change in croup score after 2, 6, 12, and 24 hours. With respect to the estimates for individual glucocorticoids, after two hours the between‐study estimates for budesonide were heterogeneous. Two studies showed a clear benefit for dexamethasone, while Johnson 1996 showed the potential for no difference in effect between dexamethasone and placebo. Between‐study estimates for the effectiveness of budesonide compared to placebo after 6, 12, and 24 hours showed a consistent beneficial effect. For dexamethasone, between‐study estimates were highly heterogeneous at all time points and included the potential for benefit, no effect, or harm compared to placebo. In future updates of this review, we may use meta‐regression analyses to explore factors that may explain at least some of the heterogeneity that we have observed (e.g. the 'effective' dosage of the active comparator). If such an analysis is deemed important to clinicians and researchers, it should be planned and documented a priori before future updates of this review. Only one very small study (N = 17) investigated croup score for fluticasone compared to placebo 2, 6, and 24 hours after treatment (Roorda 1998). Another single study (N = 42) investigated croup score for prednisolone compared to placebo six and 12 hours after treatment (Massicotte 1973). We caution drawing conclusions based on the evidence from these small, single studies.
Accounting for the pooled estimates of effect by glucocorticoid, two hours following their administration the test for subgroup differences between the effects of budesonide, dexamethasone, and fluticasone approached statistical significance (P = 0.06). While the effect estimate for fluticasone (based on one study) compared to placebo was not statistically significant, the pooled effect estimate for budesonide was highly statistically significant (P = 0.005), and that for dexamethasone approached statistical significance (P = 0.06). Six hours following their administration, there was a significant subgroup difference in effect between budesonide, dexamethasone, fluticasone, and prednisolone (P = 0.009). This was accounted for by the fact that the effect estimate for prednisolone (based on one study) was substantially larger compared to the pooled estimates for budesonide and dexamethasone, and fluticasone (based on one study) had no statistically significant effect (P = 0.90). There was a significant subgroup difference in effect between budesonide, dexamethasone, and prednisolone (P = 0.006) 12 hours following administration. This was accounted for by the fact that the effect estimate for prednisolone (based on one study) was substantially larger compared to the pooled estimates for budesonide and dexamethasone. There was a significant subgroup difference in effect between budesonide, dexamethasone, and fluticasone (P = 0.01) 24 hours following administration. This was accounted for by the fact that the effect estimate for fluticasone (based on one study) indicated no statistically significant effect, while the pooled estimates for budesonide and dexamethasone both showed beneficial effects.
For any glucocorticoid compared to placebo, we also downgraded the certainty of the evidence for return visits or (re)admissions or both for inconsistency. There is little evidence that publication bias influenced our results for return visits or (re)admissions or both.
Similar threats to the certainty of the evidence were present in the other 12 comparisons in this review, including concerns regarding risk of bias, inconsistency, and imprecision. Aside from the comparison of glucocorticoids versus placebo, where seven to 11 RCTs made up the analyses for the primary outcomes, all other comparisons contained between one and five included studies. In combination with the fact that the studies mostly included small samples of children (median n = 72, IQR 54 to 99), many analyses had to be downgraded for inadequate precision as optimal information size criteria were not met. We downgraded a number of the analyses for inconsistency, as only one study was included in the analysis and we therefore could not ascertain any consistency in the findings. In fact, many of the analyses contained only one or two small RCTs. We caution drawing strong conclusions from the results of these few small studies. There exist very few within‐study comparisons of one glucocorticoid compared to another, of glucocorticoids given by different modes of administration, or of different doses of the same glucocorticoid.
Potential biases in the review process
In order to reduce the risk that we would miss relevant studies, we collaborated with a research librarian who conducted a thorough and extensive search of the online literature. We imposed no language restrictions on the search, and we included three non‐English language studies. In previous versions of this review, the review authors attempted to locate additional literature by contacting study authors. In this update, we scanned the references lists of the included studies and relevant systematic reviews identified during study selection to identify any additional studies that we may have missed. Two review authors (AG, CJ) independently screened the retrieved articles for eligibility, and reached consensus on the included studies or consulted a third‐party arbitrator when necessary. We did not re‐run historical searches or screen for articles published prior to 2014, assuming based on previous reports that these had been conducted following rigorous methods. With regard to the new outcomes, change in croup score after two hours, patient improvement after two hours, and adverse events, we extracted this information for all articles including those that had been previously included in the review.
One review author (AG or MG) extracted data from the included studies, and another review author verified the data for completeness and to identify errors (AG, CJ, or MG). Two review authors (AG, MG) independently assessed risk of bias in the included studies and certainty of evidence (GRADE) for the primary outcomes, and reached consensus on the assessments or consulted a third‐party arbitrator when necessary. As we relied heavily on the publications when carrying out our 'Risk of bias' assessment, it is very possible that our judgements are not reflective of trial conduct. In the context of this review, it was not feasible to contact the trial authors for more detailed information, because most included studies were published more than 10 years ago. Reporting standards have evolved since the early 2000s. The CONSORT statement, which guides the complete and transparent reporting of trials, was first published in 1996 and updated in 2001 and 2010 (Moher 2012). Journal endorsement of CONSORT is associated with improved reporting of clinical trials, however reporting remains sub‐optimal (Turner 2012). Although we assessed almost all of the trials as being at unclear or high risk of bias, we downgraded the certainty of the evidence only if most trials in the analysis were at high risk of bias, and it appeared that the potential bias would likely impact the effect estimate. Of note, we also downgraded single‐trial analyses for inconsistency, as we could not ascertain any consistency in the findings. A similar approach is recommended by the Agency for Healthcare Research and Quality (AHRQ) for systematic reviews of healthcare interventions produced by the Evidence‐based Practice Center (EPC) program, whereby single‐study analyses are judged as 'unknown' for consistency (Berkman 2015). This resulted in lower certainty of evidence appraisals for single‐trial analyses, which we believe to be more representative of the available data. In determining the certainty of the evidence, we could not assess publication bias for most analyses because they included fewer than eight studies.
We performed many calculations and imputations to create a usable data set. We chose change from baseline measures rather than final value measures to alleviate any possible concerns with imbalanced confounders. Consistent with previous versions of this review, we assumed a correlation coefficient of 0.5 when imputing change‐from‐baseline standard deviations.
Since standard deviations within groups were largely homogeneous, our substitution for missing standard deviations with the arithmetic average of the reported standard deviations was reasonable. In our sensitivity analyses, we found no indication that our results would have been substantially different if larger or smaller standard deviations had been imputed.
For the change in croup score, we substituted medians for means in eight studies. One study reported both medians and means (Donaldson 2003), and six studies reported ranges or IQRs. We chose to assume that the distribution of the data was approximately normal, because the means and the medians reported in the study by Donaldson 2003 were proximal; the ranges were relatively centred on the median or mean; and most studies reported statistics that required an assumption of normality. Hence, the medians should roughly approximate the means for change in croup score.
Agreements and disagreements with other studies or reviews
Griffin 2000 conducted a systematic review examining nebulised steroids compared to placebo for the treatment of croup. The authors found that nebulised steroids significantly improved croup score at five hours (RR 1.48, 95% CI 1.27 to 1.74) and significantly reduced the need for hospital admission (RR 0.56, 95% CI 0.42 to 0.75). The authors concluded that nebulised steroids are an effective treatment option for children presenting to the emergency department with croup. Our review, which includes glucocorticoids given via any mode of administration and trials published up to April 2018, resulted in similar findings.
Authors' conclusions
Implications for practice.
For children with croup, glucocorticoids (compared with placebo) reduce symptoms at two hours, and this benefit lasts at least 24 hours. Dexamethasone and budesonide were the most widely studied glucocorticoids. Even though the findings for dexamethasone compared to placebo were inconsistent, data from four head‐to‐head trials show that dexamethasone is more effective than budesonide at reducing the symptoms of croup six and 12 hours after treatment, as well as the need for epinephrine. For children with croup, treatment with glucocorticoids reduces hours spent in the hospital or emergency department, and the rate of return visits or (re)admissions or both. We did not find any differences between dexamethasone and budesonide in rates of return visits or (re)admissions or both. There appears to be no additional benefit from combined dexamethasone and budesonide in comparison to using either treatment alone. There were insufficient data to draw conclusions about the role of other glucocorticoids (e.g. fluticasone, prednisolone) for reducing the symptoms of croup. Few serious adverse events were reported related to short‐term treatment with glucocorticoids for croup.
Implications for research.
Previous versions of this review asserted that additional trials assessing the effectiveness of dexamethasone and budesonide compared to placebo are not warranted. The cumulative meta‐graph by year for change in croup score six hours after treatment (Figure 4) shows that the standardised mean difference for the effect of glucocorticoids compared to placebo has been stable. Accordingly, we located no new studies published since 1999 that reported on this outcome for this comparison. For return visits or (re)admissions or both, the cumulative meta‐graph by year (Figure 5) indicates that the pooled risk ratio has also been relatively constant. No new trials reporting on this outcome for this comparison have been published since 2004.
4.
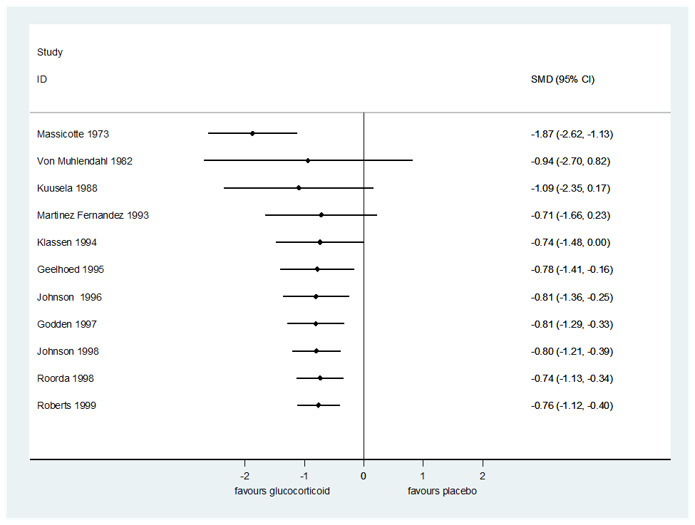
Cumulative meta‐graph by year for change in croup score six hours after treatment for any glucocorticoid compared to placebo.
5.
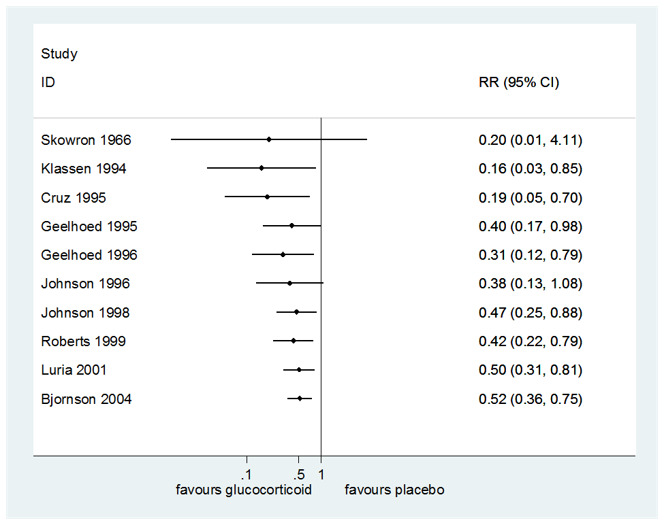
Cumulative meta‐graph by year for return visits or (re)admissions or both for any glucocorticoid compared to placebo.
The present update is the first time that study‐level risk of bias and outcome‐level certainty of the evidence have been assessed in the context of this review. These assessments revealed that for any glucocorticoid compared to placebo, the certainty of the evidence was moderate for change in croup score from baseline to 12 hours and return visits or (re)admissions or both, owing to considerable between‐study heterogeneity in effect estimates. The certainty of the evidence was low for change in croup score at 24 hours due to substantial between‐study heterogeneity in effect estimates and in the direction of effect estimates. We concur that the evidence appears stable, and it is unlikely that additional research will substantially impact the pooled estimates of effects. It is possible that a large, rigorously conducted trial that adheres to present‐day standards for reporting and measures change in croup score using a valid and reliable measure (e.g. Westley score) may help to further improve the credibility of the evidence base. This may be especially true for dexamethasone, for which the between‐study heterogeneity in effects was substantial at all time points investigated.
There were very few within‐study comparisons of dexamethasone provided via different modes of administration (oral, intramuscular, or nebulised), or of the effectiveness of different doses of dexamethasone. The studies that do exist did not report change in croup score or patient improvement. No within‐study comparisons by dosage have been published for budesonide. While dexamethasone is the mainstay of treatment for croup in children (Petrocheilou 2014), as we have shown, budesonide is an effective alternative. Studies of the effectiveness of dexamethasone given via different modes of administration, and of both dexamethasone and budesonide provided in different doses are thus warranted to inform clinical practice. These should preferably report on improvement in symptoms using a valid and reliable score (e.g. Westley score). Direct comparisons of the effectiveness of different glucocorticoids were few. In future updates of this review, additional analyses (e.g. indirect comparisons, network meta‐analysis) may be of interest to evaluate the comparative effectiveness of different glucocorticoids in light of the paucity of head‐to‐head trials. These additional analyses should be planned and documented a priori if they are deemed of interest to researchers and clinicians. Trials that compare one and multiple days of glucocorticoid treatment would also be of interest. While symptoms may resolve quickly for most children who have mild to even moderate croup, severely ill children who are hospitalised and suffer symptoms for a longer duration may experience different effects from glucocorticoid treatment.
There is a need for increased attention to the importance of trial outcomes to children and their families. Collecting data on patient‐important outcomes will ensure that trials reporting on glucocorticoids for croup are relevant to those who experience the condition. There currently exists little research investigating family‐ and patient‐important outcomes related to croup or other acute respiratory infections in children (Dyson 2017). We are therefore not certain that the outcomes presented herein are of interest to children and families. There is a need for research to identify appealing and effective methods of engaging children and their families in patient‐centred outcomes research (Dyson 2017). From there, the identification of patient‐important outcomes will help to inform future research priorities.
The results from this review demonstrate that glucocorticoids can substantially reduce symptoms of croup and the rate of return visits or (re)admissions to hospital or both. Nonetheless, there is evidence that significant proportions of children with croup do not receive glucocorticoids (Knapp 2008). Research is required to identify barriers to glucocorticoid treatment, and to establish effective knowledge translation strategies to narrow the evidence‐to‐practice gap.
Feedback
Taste of oral steroids may be a problem
Summary
A recent letter in the Lancet has questioned the results of a study on oral prednisolone for wheeze in young children on the basis that (amongst other things) oral prednisolone tastes very bitter and may not have been taken well by the children in the study.(1)
Whilst the authors have replied that they overcame the problem by asking parents to mix the powder with the child's favorite juice, I have had comments from parents in the past that their children did not like the taste of soluble prednisolone tablets, and I gather that dexamethasone solution is also very bitter.
For this reason I have abandoned the use of prednisolone and dexamethasone in children with croup or acute asthma, and use soluble betamethasone tablets instead. Betamethasone and dexamethasone are equal in potency and both are more potent than oral prednisolone; the British National Formulary states that the equivalent dose is that 5 mg of prednisolone is equivalent to 750 µg betamethasone (which equates to one and a half 500 µg tablets). It should also be noted that dexamethasone oral solution costs about 10 times as much as betamethasone tablets!
My extrapolation of the results of this review to the use of betamethasone in primary care is based on two assumptions. Firstly that betamethasone is equivalent to dexamethasone, and secondly that the outpatient trials in secondary care contain patients that are similar to those presenting in primary care. I wonder if the authors agree that this is reasonable?
Reference
1. Weinberger M, Ahrens R. Oral prednisolone for viral wheeze in young children. Lancet 2004;363(9405):330
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
In response to Dr. Cates' comment regarding the use of betamethasone for the treatment of croup, we are unable to conclude that betamethasone is efficacious for the treatment of croup.
Among the included studies, only Klassen et al (1998) reported the results of the blinding methodology. Children were randomized to identically tasting and appearing budesonide, dexamethasone or both treatments. Research assistants and parents were asked to identify which study medication the child received. The responses were similar and this indicates that blinding was successful. In addition, Klassen has conducted RCTs using intravenous dexamethasone with a 70% sucrose solution. This has been very well‐tolerated with a very low incidence of vomiting. Paediatric croup and asthma trials have shown that when compared to prednisone/prednisolone, oral dexamethasone combined with flavoured syrup is both well‐tolerated and an inexpensive treatment.
To date, we are not aware of any RCTs in children with croup that compared betamethasone to placebo or an active treatment, such as dexamethasone. Although betamethasone is theoretically as potent as dexamethasone, there is no actual empirical data to prove this. Therefore, we cannot judge the equivalency, or the tolerability, of betamethasone versus dexamethasone. Perhaps a randomized controlled trial should be conducted that directly compares betamethasone to dexamethasone so the palatability and equivalency can be assessed.
In response to the second stated assumption, there are guidelines for generalising results of trials to clinical practice and physicians need to carefully consider the comparability of participants in any one study to their own patients.1
1Guyatt G, Haynes B, Jaeschke R, Cook D, Greenhalgh T, Meade M, Green L, Naylor C, Wilson M, McAlister F, Richardson M. Introduction: the philosophy of evidence‐based medicine. In: Guyatt G, Rennie D, editors. Users' guides to the medical literature: a manual for evidence‐based clinical practice. Chicago: AMA Press; 2002. pp. 3‐12.
Kelly Russell Terry Klassen David Johnson
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Contributors
Chris Cates
What's new
| Date | Event | Description |
|---|---|---|
| 3 April 2018 | New search has been performed | New authors joined the team to update the review. We updated the searches and included five new trials. Three were newly identified trials (Dobrovoljac 2012; Garbutt 2013; Soleimani 2013); one was previously excluded (Chub‐Uppakarn 2007); and one was previously awaiting classification (Eboriadou 2010). We excluded six new trials, Eghbali 2016; Faghihinia 2007; Faraji‐Goodarzi 2018; Gill 2017; Mohammadzadeh 2014; Roked 2015, and one ongoing trial (NCT01748162). We included one new ongoing trial (ACTRN12609000290291). In this update we assessed the risk of bias and the certainty of the evidence. We added two new primary outcomes: change in croup score after two hours, and patient improvement after two hours. We added adverse events as a secondary outcome and 'Summary of findings' tables. We added two new comparisons: oral compared to nebulised dexamethasone, and dexamethasone compared to beclomethasone. |
| 3 April 2018 | New citation required and conclusions have changed | Our conclusions have changed. The previous version of this review concluded that glucocorticoids, as compared to placebo, reduce croup symptoms within six hours and that the effect lasts 12 hours. We conclude that glucocorticoids, as compared to placebo, reduce croup symptoms within two hours and that the effect lasts at least 24 hours. |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 16 September 2014 | New citation required but conclusions have not changed | Review was updated, and conclusions remain unchanged. |
| 16 September 2014 | New search has been performed | Searches updated. We included one new trial, Dobrovoljac 2012, and excluded one new trial (Faghihinia 2007). We added a two‐hour croup score and a two‐hour improvement outcome. |
| 1 December 2011 | Amended | Grammatical correction made to the Plain language summary. |
| 18 July 2011 | Amended | Analysis 5.2 contained an error, as the negative signs for the change in croup scores at six hours were not included. The mean difference remains non‐significant. |
| 23 July 2010 | New search has been performed | Searches conducted. We added seven new trials since the 2004 publication (Alshehr 2005; Amir 2006; Cetinkaya 2004; Duman 2005; Fifoot 2007; Geelhoed 2005; Sparrow 2006). We excluded three new trials (Chub‐Uppakarn 2007; Custer 2005; Schooff 2005). |
| 20 May 2010 | New citation required but conclusions have not changed | New authors joined the team to update the review. The conclusions remain unchanged. |
| 16 August 2008 | Amended | Converted to new review format |
| 3 February 2004 | Feedback has been incorporated | Feedback incorporated. |
| 7 April 2003 | New search has been performed | Searches conducted. |
| 17 August 1997 | New search has been performed | Searches conducted. |
Acknowledgements
We thank: Robin Featherstone (MLIS, Alberta Research Centre for Health Evidence, University of Alberta, Canada) for implementing the update searches; Gabrielle Zimmermann (PhD, Department of Pediatrics, University of Alberta, Canada) and Devonne Brandys (MPH, Department of Pediatrics, University of Alberta, Canada) for translating and extracting data from the Danish and Spanish articles, respectively; Bita Mesgarpour (PharmD, PhD, National Institute for Medical Research Development, Iran) for screening an excluded Persian article; and Jennifer Pillay (BSc, Alberta Research Centre for Health Evidence, University of Alberta, Canada) for assisting with the GRADE certainty of evidence appraisals. We also thank Monica Ausejo Segura, Stantel Gushue, James Kellner, David Moher, Yuanyuan Liang, Ba' Pham, Kelly Russell, Antonio Saenz, Lisa Tjosvold, Kathleen Wagontall, and Natasha Wiebe for their work on previous versions of this review. Finally, we thank the following people for commenting on this updated draft: Janet Yarrow, Auxiliadora Fraiz, Gina Neto, Mark Jones, and Lubna Al‐Ansary.
Appendices
Appendix 1. Search strategies for the 2018 update
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present
Search strategy
Adrenal Cortex Hormones/ (60364)
2 Beclomethasone/ (2922)
3 exp Betamethasone/ (6916)
4 Budesonide/ (4068)
5 Cortisone/ (19496)
6 Corticosterone/ (24404)
7 Cortodoxone/ (839)
8 Dexamethasone/ (48406)
9 exp Glucocorticoids/ (180113)
10 Hydrocortisone/ (68362)
11 Hydroxycorticosteroids/ (373)
12 exp Methylprednisolone/ (18208)
13 Prednisolone/ (31247)
14 Prednisone/ (37573)
15 Pregnenolone/ (4281)
16 Pregnenediones/ (2129)
17 Tetrahydrocortisol/ (268)
18 Triamcinolone/ (3619)
19 adrenal cortex hormone*.tw,kf,nm. (60630)
20 becl?met*.tw,kf,nm. (3790)
21 betamet?asone*.tw,kf,nm. (7296)
22 budesonide*.tw,kf,nm. (5626)
23 clobetasol*.tw,kf,nm. (1588)
24 corticoid*.tw,kf,nm. (5892)
25 corticosteroid*.tw,kf,nm. (92787)
26 corticosterone*.tw,kf,nm. (32273)
27 cortisone*.tw,kf,nm. (23088)
28 cortodoxone*.tw,kf,nm. (839)
29 dexamet?asone*.tw,kf,nm. (66433)
30 glucocortico*.tw,kf,nm. (103275)
31 hydrocortisone*.tw,kf,nm. (73211)
32 hydroxycorticosteroid*.tw,kf,nm. (6722)
33 hydroxypregnenolone*.tw,kf,nm. (960)
34 methylprednisolone*.tw,kf,nm. (24326)
35 prednisolone*.tw,kf,nm. (42949)
36 prednisone*.tw,kf,nm. (50091)
37 pregnenedione*.tw,kf,nm. (2134)
38 pregnenolone*.tw,kf,nm. (6983)
39 tetrahydrocortisol*.tw,kf,nm. (483)
40 triamcinolone*.tw,kf,nm. (10882)
41 or/1‐40 [Combined MeSH & text words for glucocorticoids] (446199)
42 exp Laryngitis/ (3890)
43 (croup* or pseudocroup*).tw,kf. (1613)
44 (laryngo tracheo bronch* or laryngotracheobronch*).tw,kf. (520)
45 (laryngo tracheit* or laryngotracheit*).tw,kf. (807)
46 laryngit*.tw,kf. (1906)
47 or/42‐46 [Combined MeSH & text words for croup] (6129)
48 and/41,47 [Combined concepts for glucocorticoids & croup] (582)
49 randomized controlled trial.pt. (456662)
50 controlled clinical trial.pt. (92277)
51 randomized.ab. (406831)
52 placebo.ab. (187642)
53 drug therapy.fs. (2003729)
54 randomly.ab. (287587)
55 trial.ab. (422510)
56 groups.ab. (1778613)
57 or/49‐56 (4169633)
58 exp animals/ not humans.sh. (4438451)
59 57 not 58 [Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision) ; Lefebvre C, et al. Retrieved: http://handbook.cochrane.org/chapter_6/6_searching_for_studies.htm] (3603352)
60 and/48,59 [RCT filter applied to glucocorticoid & croup results] (370)
61 (2017* or 2018*).dt. (1532741)
62 and/60‐61 [Update date limit applied] (13)
63 remove duplicates from 62 (12)
Ovid Embase 1996 to 2018 Week 14
Search strategy
1 beclomethasone/ (4929)
2 betamethasone/ (10019)
3 budesonide/ (16983)
4 corticosteroid/ (165448)
5 corticosterone/ (18405)
6 cortisone/ (6825)
7 cortodoxone/ (926)
8 dexamethasone/ (99875)
9 exp glucocorticoid/ (471958)
10 hydrocortisone/ (75173)
11 hydroxycorticosteroid/ (78)
12 methylprednisolone/ (66440)
13 prednisolone/ (84613)
14 prednisone/ (111548)
15 pregnane derivative/ (1213)
16 pregnenolone/ (2440)
17 steroid hormone/ (6806)
18 tetrahydrocortisol/ (301)
19 adrenal cortex hormone*.tw,kw. (1003)
20 becl?met*.tw,kw. (2724)
21 betamet?asone*.tw,kw. (4062)
22 budesonide*.tw,kw. (6811)
23 clobetasol*.tw,kw. (1235)
24 corticoid*.tw,kw. (3831)
25 corticosteroid*.tw,kw. (106431)
26 corticosterone*.tw,kw. (18551)
27 cortisone*.tw,kw. (4870)
28 cortodoxone*.tw,kw. (4)
29 dexamet?asone*.tw,kw. (50038)
30 glucocortico*.tw,kw. (64349)
31 hydrocortisone*.tw,kw. (9577)
32 hydroxycorticosteroid*.tw,kw. (187)
33 hydroxypregnenolone*.tw,kw. (297)
34 methylprednisolone*.tw,kw. (16949)
35 prednisolone*.tw,kw. (25664)
36 prednisone*.tw,kw. (31429)
37 pregnenedione*.tw,kw. (4)
38 pregnenolone*.tw,kw. (2909)
39 tetrahydrocortisol*.tw,kw. (266)
40 triamcinolone*.tw,kw. (6622)
41 or/1‐40 [Combined Emtree & text words for glucocorticoids] (638411)
42 croup/ (1261)
43 laryngitis/ (2179)
44 laryngotracheobronchitis/ (367)
45 pseudocroup/ (69)
46 (croup* or pseudocroup*).tw,kw. (1216)
47 laryngit*.tw,kw. (1441)
48 (laryngo tracheit* or laryngotracheit*).tw,kw. (385)
49 (laryngo tracheo bronch* or laryngotracheobronch*).tw,kw. (245)
50 or/42‐49 [Combined Emtree & text words for croup] (4776)
51 and/41,50 [Combined concepts for glucocorticoids & croup] (977)
52 crossover procedure/ (50498)
53 double blind procedure/ (121483)
54 randomized controlled trial/ (447481)
55 single blind procedure/ (29482)
56 allocat*.tw,kw. (112943)
57 assign*.tw,kw. (289117)
58 (cross over* or crossover*).tw,kw. (72474)
59 (doubl* adj blind*).tw,kw. (140493)
60 factorial*.tw,kw. (28709)
61 placebo*.tw,kw. (221607)
62 random*.tw,kw. (1153942)
63 (singl* adj blind*).tw,kw. (17413)
64 volunteer*.tw,kw. (178200)
65 or/52‐64 [Recommended terms for finding trials in Embase from Cochrane Handbook Chapter 6.3.2.2; Retrieved: http://handbook.cochrane.org/chapter_6/6_3_2_2_what_is_in_the_cochrane_central_register_of_controlled.htm] (1706442)
66 exp animals/ not exp humans/ (2612490)
67 65 not 66 (1545695)
68 and/51,67 [RCT filter applied to glucocorticoid & croup results] (147)
69 (2017* or 2018*).dp,dd,dc. (2250336)
70 ("2017" or "2018").yr. (1571837)
71 68 and (69 or 70) (9)
72 remove duplicates from 71 (9)
CENTRAL via Wiley Cochrane Library
Search strategy
#1 [mh ^"Adrenal Cortex Hormones"] 2468
#2 [mh ^Beclomethasone] 1007
#3 [mh Betamethasone] 31
#4 [mh ^Budesonide] 1461
#5 [mh ^Cortisone] 89
#6 [mh ^Corticosterone] 37
#7 [mh ^Cortodoxone] 28
#8 [mh ^Dexamethasone] 2927
#9 [mh Glucocorticoids] 4253
#10 [mh ^Hydrocortisone] 5262
#11 [mh ^Hydroxycorticosteroids] 10
#12 [mh Methylprednisolone] 57
#13 [mh ^Prednisolone] 2122
#14 [mh ^Prednisone] 3149
#15 [mh ^Pregnenolone] 17
#16 [mh ^Pregnenediones] 568
#17 [mh ^Tetrahydrocortisol] 11
#18 [mh ^Triamcinolone] 273
#19 "adrenal cortex hormone*":ti,ab,kw 2633
#20 becl?met*:ti,ab,kw 2353
#21 (betametasone* or betamethasone*):ti,ab,kw 1929
#22 budesonide*:ti,ab,kw 3746
#23 clobetasol*:ti,ab,kw 506
#24 corticoid*:ti,ab,kw 456
#25 corticosteroid*:ti,ab,kw 15013
#26 corticosterone*:ti,ab,kw 101
#27 cortisone*:ti,ab,kw 405
#28 cortodoxone*:ti,ab,kw 46
#29 (dexametasone* or dexamethasone*):ti,ab,kw 7687
#30 glucocortico*:ti,ab,kw 7186
#31 hydrocortisone*:ti,ab,kw 8605
#32 hydroxycorticosteroid*:ti,ab,kw 114
#33 hydroxypregnenolone*:ti,ab,kw 8
#34 methylprednisolone*:ti,ab,kw 3945
#35 prednisolone*:ti,ab,kw 5229
#36 prednisone*:ti,ab,kw 7607
#37 pregnenedione*:ti,ab,kw 586
#38 pregnenolone*:ti,ab,kw 54
#39 tetrahydrocortisol*:ti,ab,kw 32
#40 triamcinolone*:ti,ab,kw 2072
#41 {or #1‐#40} 48795
#42 [mh Laryngitis] 119
#43 (croup* or pseudocroup*):ti,ab,kw 175
#44 ("laryngo tracheo bronch*" or laryngotracheobronch*):ti,ab,kw 17
#45 ("laryngo tracheit*" or laryngotracheit*):ti,ab,kw 12
#46 laryngit*:ti,ab,kw 190
#47 {or #42‐#46} 353
#48 #41 and #47 128
#49 #41 and #47 Publication Year from 2017 to 2018 4
Trial registry: ClinicalTrials.gov (https://clinicaltrials.gov/)
Search strategy:
Advanced search >
Search Terms: croup OR laryngitis OR laryngotracheobronchitis OR laryngotracheitis
Age: Child
Intervention: Anti‐Inflammatory Agents
World Health Organization International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/)
Search strategy
Advanced search >
Title: croup OR laryngitis OR laryngotracheobronchitis OR laryngotracheitis
Search for clinical trials in children
Recruitment Status is: ALL
Appendix 2. Search strategies for the 2014 interim update search
MEDLINE (Ovid)
Search strategy
1 exp Laryngitis/ 2 laryngit*.tw. 3 croup*.tw. 4 laryngotracheobronchit*.tw. 5 (pseudocroup* or pseudo‐croup*).tw. 6 laryngotracheit*.tw. 7 or/1‐6 8 exp Glucocorticoids/ 9 glucocorticoid*.tw,nm. 10 Adrenal Cortex Hormones/ 11 corticosteroid*.tw,nm. 12 corticoid*.tw,nm. 13 Hydrocortisone/ 14 hydrocortisone.tw,nm. 15 hydroxypregnenolone.tw,nm. 16 Pregnenolone/ 17 pregnenolone.tw,nm. 18 Tetrahydrocortisol/ 19 tetrahydrocortisol.tw,nm. 20 Hydrocortisone/ 21 hydrocortisone.tw,nm. 22 Cortodoxone/ 23 cortodoxone.tw,nm. 24 Cortisone/ 25 cortisone.tw,nm. 26 Corticosterone/ 27 corticosterone.tw,nm. 28 Hydroxycorticosteroids/ 29 hydroxycorticosteroid*.tw,nm. 30 triamcinolone.tw,nm. 31 prednisone.tw,nm. 32 prednisolone.tw,nm. 33 paramethasone.tw,nm. 34 methylprednisolone.tw,nm. 35 dexamethasone.tw,nm. 36 clobetasol.tw,nm. 37 beclomethasone.tw,nm. 38 betamethasone.tw,nm. 39 Pregnenediones/ 40 pregnenedione*.tw,nm. 41 budesonide*.tw,nm. 42 or/8‐41 43 7 and 42
Embase.com
Search strategy
#21 #12 AND #20 #20 #15 NOT #19 #19 #16 NOT #18 #18 #16 AND #17 #17 'human'/de #16 'animal'/de OR 'animal experiment'/de OR 'nonhuman'/de #15 #13 OR #14 #14 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti #13 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #12 #3 AND #11 #11 #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 #10 hydrocortisone:ab,ti OR hydroxypregnenolone:ab,ti OR pregnenolone:ab,ti OR tetrahydrocortisol:ab,ti OR cortodoxone:ab,ti OR cortisone:ab,ti OR corticosterone*:ab,ti OR hydroxycorticosteroid*:ab,ti OR triamcinolone:ab,ti OR prednisone:ab,ti OR prednisolone:ab,ti OR paramethasone:ab,ti OR methylprednisolone:ab,ti OR dexamethasone:ab,ti OR clobetasol:ab,ti OR beclomethasone:ab,ti OR betamethasone:ab,ti OR pregnenedione*:ab,ti OR budesonide*:ab,ti #9 'pregnenolone derivative'/de OR 'tetrahydrocortisol'/de OR 'hydrocortisone'/de OR 'cortodoxone'/de OR 'cortisone'/de OR 'corticosterone'/de OR 'hydroxycorticosteroid'/de OR 'triamcinolone'/de OR 'prednisone'/de OR 'prednisolone'/de OR 'paramethasone'/de OR 'methylprednisolone'/de OR 'dexamethasone'/de OR 'clobetasol'/de OR 'beclometasone'/de OR 'pregnane derivative'/de OR 'betamethasone'/de OR 'budesonide'/de #8 corticoid*:ab,ti 2 #7 corticosteroid*:ab,ti #6 'corticosteroid'/de #5 glucocorticoid*:ab,ti #4 'glucocorticoid'/exp #3 #1 OR #2 #2 laryngit*:ab,ti OR croup:ab,ti OR pseudocroup*:ab,ti OR 'pseudo‐croup':ab,ti OR laryngotracheit*:ab,ti #1 'laryngitis'/de OR 'croup'/de OR 'laryngotracheobronchitis'/de OR 'pseudocroup'/de
MEDLINE In‐Process & Other Non‐Indexed Citations
Search strategy
1 laryngit*.tw. 2 croup.tw. 3 laryngotracheobronchit*.tw. 4 (pseudocroup* or pseudo‐croup*).tw. 5 laryngotracheit*.tw. 6 or/1‐5 7 glucocorticoid*.tw. 8 corticosteroid*.tw. 9 corticoid*.tw. 10 hydrocortisone.tw. 11 hydroxypregnenolone.tw. 12 pregnenolone.tw. 13 tetrahydrocortisol.tw. 14 hydrocortisone.tw. 15 cortodoxone.tw. 16 cortisone.tw. 17 corticosterone.tw. 18 hydroxycorticosteroid*.tw. 19 triamcinolone.tw. 20 prednisone.tw. 21 prednisolone.tw. 22 paramethasone.tw. 23 methylprednisolone.tw. 24 dexamethasone.tw. 25 clobetasol.tw. 26 beclomethasone.tw. 27 betamethasone.tw. 28 pregnenedione*.tw. 29 budesonide*.tw. 30 or/7‐29 31 6 and 30
Data and analyses
Comparison 1. Any glucocorticoid compared to placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Croup score (change baseline ‐ 2 hours) by score | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.18] |
| 1.1 Westley score | 5 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.44, 0.01] |
| 1.2 Non‐Westley score | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.93, ‐0.10] |
| 2 Croup score (change baseline ‐ 6 hours) by score | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.12, ‐0.40] |
| 2.1 Westley score | 5 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.79 [‐1.02, ‐0.56] |
| 2.2 Non‐Westley score | 6 | 623 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.43, ‐0.18] |
| 3 Croup score (change baseline ‐ 12 hours) by score | 8 | 571 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.03 [‐1.53, ‐0.53] |
| 3.1 Westley score | 2 | 113 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.54 [‐2.56, ‐0.53] |
| 3.2 Non‐Westley score | 6 | 458 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐1.45, ‐0.30] |
| 4 Croup score (change baseline ‐ 24 hours) by score | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.40, ‐0.31] |
| 4.1 Westley score | 4 | 169 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.72, ‐0.37] |
| 4.2 Non‐Westley score | 4 | 182 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.56, 0.16] |
| 5 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.13, ‐0.18] |
| 5.1 Inpatient | 5 | 301 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.44, ‐0.16] |
| 5.2 Outpatient | 2 | 125 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.93, 0.29] |
| 6 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.76 [‐1.12, ‐0.40] |
| 6.1 Inpatient | 8 | 723 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.22, ‐0.23] |
| 6.2 Outpatient | 3 | 236 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.84 [‐1.11, ‐0.56] |
| 7 Croup score (change baseline ‐ 24 hours) by inpatient/outpatient | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.40, ‐0.31] |
| 7.1 Inpatient | 7 | 291 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.82 [‐1.46, ‐0.19] |
| 7.2 Outpatient | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.09 [‐1.71, ‐0.48] |
| 8 Croup score (change baseline ‐ 2 hours) by glucocorticoid | 7 | 426 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.10, ‐0.22] |
| 8.1 Budesonide | 4 | 246 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.01 [‐1.71, ‐0.30] |
| 8.2 Dexamethasone | 3 | 163 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.00, 0.03] |
| 8.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.52, 1.42] |
| 9 Croup score (change baseline ‐ 6 hours) by glucocorticoid | 11 | 959 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐1.07, ‐0.41] |
| 9.1 Budesonide | 5 | 333 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.04, ‐0.58] |
| 9.2 Dexamethasone | 6 | 567 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐1.17, ‐0.08] |
| 9.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.89, 1.02] |
| 9.4 Prednisolone | 1 | 42 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.87 [‐2.62, ‐1.13] |
| 10 Croup score (change baseline ‐ 12 hours) by glucocorticoid | 8 | 571 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.04 [‐1.51, ‐0.56] |
| 10.1 Budesonide | 3 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.26, ‐0.68] |
| 10.2 Dexamethasone | 5 | 323 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.55, ‐0.15] |
| 10.3 Prednisolone | 1 | 39 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐3.26, ‐1.55] |
| 11 Croup score (change baseline ‐ 24 hours) by glucocorticoid | 8 | 351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.41, ‐0.37] |
| 11.1 Budesonide | 2 | 89 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐1.88, ‐0.93] |
| 11.2 Dexamethasone | 6 | 245 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.55, ‐0.22] |
| 11.3 Fluticasone | 1 | 17 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.75, 1.17] |
| 12 Return visits or (re)admissions or both by inpatient/outpatient | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.75] |
| 12.1 Inpatient | 3 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.30] |
| 12.2 Outpatient | 7 | 1356 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.80] |
| 13 Return visits or (re)admissions or both by glucocorticoid | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.36, 0.72] |
| 13.1 Budesonide | 4 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.19, 0.90] |
| 13.2 Dexamethasone | 8 | 1454 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.81] |
| 14 Return visits or (re)admissions or both by croup severity | 10 | 1679 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.76] |
| 14.1 Mild croup | 3 | 1068 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.30, 0.95] |
| 14.2 Moderate croup | 7 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.26, 0.86] |
| 15 Length of stay by inpatient/outpatient | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.90 [‐23.58, ‐6.22] |
| 15.1 Inpatient | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.90 [‐23.58, ‐6.22] |
| 16 Length of stay by glucocorticoid | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐14.55 [‐22.70, ‐6.41] |
| 16.1 Budesonide | 2 | 131 | Mean Difference (IV, Random, 95% CI) | ‐15.29 [‐26.89, ‐3.69] |
| 16.2 Dexamethasone | 6 | 328 | Mean Difference (IV, Random, 95% CI) | ‐18.25 [‐27.87, ‐8.62] |
| 16.3 Fluticasone | 1 | 17 | Mean Difference (IV, Random, 95% CI) | 4.80 [‐12.34, 21.94] |
| 17 Improvement (at 2 hours) by inpatient/outpatient | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.96, 3.40] |
| 17.1 Inpatient | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.96, 3.40] |
| 18 Improvement (at 6 hours) by inpatient/outpatient | 6 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| 18.1 Inpatient | 4 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.96, 1.90] |
| 18.2 Outpatient | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [1.16, 2.74] |
| 19 Improvement (at 12 hours) by inpatient/outpatient | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 19.1 Inpatient | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 20 Improvement (at 24 hours) by inpatient/outpatient | 5 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.01, 1.61] |
| 20.1 Inpatient | 4 | 213 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.98, 1.43] |
| 20.2 Outpatient | 1 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [1.14, 3.51] |
| 21 Improvement (at 6 hours) by glucocorticoid | 6 | 332 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [1.12, 1.88] |
| 21.1 Budesonide | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [1.19, 2.32] |
| 21.2 Dexamethasone | 2 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.76, 2.72] |
| 21.3 Prednisolone | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.69, 2.62] |
| 22 Improvement (at 12 hours) by glucocorticoid | 6 | 340 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.09, 1.62] |
| 22.1 Budesonide | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.08, 1.84] |
| 22.2 Dexamethasone | 3 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.52 [1.06, 2.18] |
| 22.3 Prednisolone | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.85, 1.55] |
| 23 Improvement (at 24 hours) by glucocorticoid | 5 | 251 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [1.01, 1.61] |
| 23.1 Dexamethasone | 4 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.05, 1.84] |
| 23.2 Prednisolone | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.91, 1.20] |
| 24 Additional treatments: antibiotics | 3 | 202 | Risk Difference (M‐H, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 25 Additional treatments: epinephrine | 9 | 709 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 26 Additional treatments: intubation/tracheostomy | 11 | 1090 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.01] |
| 27 Additional treatments: mist tent | 2 | 84 | Risk Difference (M‐H, Random, 95% CI) | ‐0.20 [‐0.87, 0.47] |
| 28 Additional treatments: supplemental glucocorticoids | 6 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.36, 1.03] |
Comparison 2. Any glucocorticoid compared to epinephrine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient | 2 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.77 [‐0.24, 1.77] |
| 1.1 Inpatient | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.75] |
| 1.2 Outpatient | 1 | 64 | Std. Mean Difference (IV, Random, 95% CI) | 1.29 [0.73, 1.84] |
| 2 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.18, 0.97] |
| 2.1 Inpatient | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.18, 0.97] |
| 3 Croup score (change baseline ‐ 12 hours) by inpatient/outpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 3.1 Inpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 4 Croup score (change baseline ‐ 24 hours) by inpatient/outpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 4.1 Inpatient | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 5 Croup score (change baseline ‐ 2 hours) by glucocorticoid | 2 | 130 | Std. Mean Difference (IV, Random, 95% CI) | 0.88 [0.13, 1.63] |
| 5.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.22, 0.75] |
| 5.2 Dexamethasone | 1 | 31 | Std. Mean Difference (IV, Random, 95% CI) | 1.13 [0.35, 1.91] |
| 5.3 Beclomethasone | 1 | 33 | Std. Mean Difference (IV, Random, 95% CI) | 1.41 [0.62, 2.19] |
| 6 Croup score (change baseline ‐ 12 hours) by glucocorticoid | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.43] |
| 6.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.47, 0.50] |
| 6.2 Dexamethasone | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐1.09, 0.82] |
| 7 Croup score (change baseline ‐ 24 hours) by glucocorticoid | 3 | 129 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.18, 0.51] |
| 7.1 Budesonide | 1 | 66 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.27, 0.70] |
| 7.2 Dexamethasone | 2 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.38, 0.61] |
| 8 Return visits or (re)admissions or both by inpatient/outpatient | 2 | 130 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8.1 Inpatient | 1 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 8.2 Outpatient | 1 | 64 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 9 Length of stay by inpatient/outpatient | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐33.89, 13.89] |
| 9.1 Inpatient | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐33.89, 13.89] |
| 10 Additional treatments: use of epinephrine | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 2.69] |
| 11 Additional treatments: intubation/tracheostomy | 1 | 66 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 12 Additional treatments: supplemental glucocorticoids | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.48, 1.43] |
Comparison 3. Dexamethasone compared to budesonide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 4 | 326 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.79, ‐0.13] |
| 1.1 Inpatient | 2 | 97 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.04, ‐0.22] |
| 1.2 Outpatient | 2 | 229 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.90, 0.18] |
| 2 Croup score (change baseline ‐ 12 hours) by inpatient/outpatient | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.19, ‐0.30] |
| 2.1 Inpatient | 2 | 84 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.19, ‐0.30] |
| 3 Return visits or (re)admissions or both by inpatient/outpatient | 5 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.40, 1.22] |
| 3.1 Inpatient | 2 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.14, 2.79] |
| 3.2 Outpatient | 3 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.38, 1.30] |
| 4 Length of stay by inpatient/outpatient | 2 | 184 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.72, 0.14] |
| 4.1 Inpatient | 1 | 50 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.14, ‐0.00] |
| 4.2 Outpatient | 1 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.46, 0.22] |
| 5 Improvement (at 6 hours) by inpatient/outpatient | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.34] |
| 5.1 Outpatient | 1 | 134 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.93, 1.34] |
| 6 Additional treatments: epinephrine | 4 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.96] |
| 7 Additional treatments: intubation/tracheostomy | 2 | 145 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.04, 0.04] |
| 8 Additional treatments: supplemental glucocorticoids | 3 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.18, 1.32] |
Comparison 4. Dexamethasone compared to beclomethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return visits or (re)admissions or both by inpatient/outpatient | 1 | 39 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 1.1 Outpatient | 1 | 39 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
Comparison 5. Dexamethasone compared to betamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.58, ‐0.18] |
| 1.1 Outpatient | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.58, ‐0.18] |
| 2 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.75, ‐0.31] |
| 2.1 Outpatient | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.75, ‐0.31] |
| 3 Return visits or (re)admissions or both by inpatient/outpatient | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.34] |
| 3.1 Outpatient | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.67, 1.34] |
| 4 Additional treatments: use of epinephrine | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.18, 3.76] |
Comparison 6. Dexamethasone compared to prednisolone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Croup (change baseline ‐ 6 hours) by inpatient/outpatient | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.17, 0.55] |
| 1.1 Outpatient | 1 | 99 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.17, 0.55] |
| 2 Return visits or (re)admissions or both by inpatient/outpatient | 3 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.19, 0.79] |
| 2.1 Outpatient | 3 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.19, 0.79] |
| 3 Length of stay by inpatient/outpatient | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.55, 1.55] |
| 3.1 Outpatients | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 0.5 [‐0.55, 1.55] |
| 4 Additional treatments: epinephrine | 2 | 232 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.26, 1.85] |
| 5 Additional treatments: supplemental glucocorticoids | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.34, 2.48] |
Comparison 7. Budesonide and dexamethasone compared to dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 3 | 255 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.19, 0.30] |
| 1.1 Inpatient | 1 | 72 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.30, 0.63] |
| 1.2 Outpatient | 2 | 183 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.32, 0.39] |
| 2 Return visits or (re)admissions or both by inpatient/outpatient | 3 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.83] |
| 2.1 Inpatient | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.46, 2.29] |
| 2.2 Outpatient | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.13, 2.60] |
| 3 Length of stay by inpatient/outpatient | 2 | 204 | Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.05, 0.92] |
| 3.1 Inpatient | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐6.75, 4.15] |
| 3.2 Outpatient | 1 | 133 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.04, 0.94] |
| 4 Improvement (at 6 hours) by inpatient/outpatient | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.65, 1.90] |
| 4.1 Outpatient | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.65, 1.90] |
| 5 Additional treatments: epinephrine | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.27, 7.39] |
| 6 Additional treatments: mist tent | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.69, 1.65] |
| 7 Additional treatments: supplemental glucocorticoids | 2 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 16.66] |
Comparison 8. Budesonide and dexamethasone compared to budesonide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 1 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.59, 0.19] |
| 1.1 Outpatient | 1 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.59, 0.19] |
| 2 Return visits or (re)admissions or both by inpatient/outpatient | 1 | 129 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 2.1 Outpatient | 1 | 129 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.03, 0.03] |
| 3 Length of stay by inpatient/outpatient | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.36, 0.86] |
| 3.1 Outpatient | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.36, 0.86] |
| 4 Improvement (at 6 hours) by inpatient/outpatient | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.20] |
| 4.1 Outpatient | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.79, 1.20] |
| 5 Additional treatments: epinephrine | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.15, 6.99] |
| 6 Additional treatments: supplemental glucocorticoids | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.52, 3.29] |
Comparison 9. Oral compared to intramuscular dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return visits or (re)admissions or both by inpatient/outpatient | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Outpatient | 3 | 440 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.12] |
| 2 Improvement (at 24 hours) by inpatient/outpatient | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.95, 1.19] |
| 2.1 Outpatient | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.95, 1.19] |
| 3 Additional treatments: antibiotics | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.02, 1.15] |
| 4 Additional treatments: epinephrine | 2 | 372 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.24] |
| 5 Additional treatments: mist tent | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.31, 5.89] |
| 6 Additional treatments: supplemental glucocorticoids | 1 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.50, 2.41] |
Comparison 10. Oral compared to nebulised dexamethasone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return visits or (re)admissions or both by inpatient/outpatient | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.17, 0.89] |
| 1.1 Outpatient | 1 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.17, 0.89] |
Comparison 11. Dexamethasone 0.30 mg/kg compared to 0.15 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return visits or (re)admissions or both by inpatient/outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.27] |
| 1.1 Outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.06, 14.27] |
| 2 Additional treatments: epinephrine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.19, 0.98] |
| 3 Additional treatments: supplemental glucocorticoids | 1 | 60 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
Comparison 12. Dexamethasone 0.60 mg/kg compared to 0.30 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return visits or (re)admissions or both by inpatient/outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.25, 7.81] |
| 1.1 Outpatient | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.25, 7.81] |
| 2 Additional treatments: epinephrine | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.27, 2.28] |
| 3 Additional treatments: supplemental glucocorticoids | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.12, 66.40] |
Comparison 13. Dexamethasone 0.60 mg/kg compared to 0.15 mg/kg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Croup score (change baseline ‐ 2 hours) by inpatient/outpatient | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.29, ‐0.01] |
| 1.1 Inpatient | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.29, ‐0.01] |
| 2 Croup score (change baseline ‐ 6 hours) by inpatient/outpatient | 3 | 178 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.50, ‐0.16] |
| 2.1 Inpatient | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.54, ‐0.22] |
| 2.2 Outpatient | 2 | 137 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.50, 0.35] |
| 3 Croup score (change baseline ‐ 12 hours) by inpatient/outpatient | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐1.45, 1.78] |
| 3.1 Inpatient | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐0.80, ‐0.50] |
| 3.2 Outpatient | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 1.0 [0.65, 1.35] |
| 4 Croup score (change baseline ‐ 24 hours) by inpatient/outpatient | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.14, 0.86] |
| 4.1 Outpatient | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.14, 0.86] |
| 5 Return visits or (re)admissions or both by inpatient/outpatient | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.55] |
| 5.1 Outpatient | 2 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.54, 1.55] |
| 6 Length of stay by inpatient/outpatient | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐2.16, 6.16] |
| 6.1 Outpatient | 1 | 72 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐2.16, 6.16] |
| 7 Additional treatments: epinephrine | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.07, 16.80] |
| 8 Additional treatments: intubation/tracheotomy | 1 | 41 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.09, 0.09] |
| 9 Additional treatments: mist tent | 1 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.69, 1.84] |
| 10 Additional treatments: supplemental glucocorticoids | 1 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.27, 2.97] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alshehr 2005.
| Methods | Randomised double‐blind trial | |
| Participants |
Study period: September 1998 to December 2002 Setting: emergency rooms and outpatient clinics in 3 medical institutes, Abha City, Saudi Arabia Inclusion criteria: children aged 3 months to 9 years who had been given a diagnosis of croup and had persistent, moderately severe respiratory distress (Westley croup score > 3) Exclusion criteria: symptoms or signs suggesting another cause of stridor; history of chronic pulmonary disease; severe systemic disease; immune dysfunction; stridor or intubation for more than 1 month; glucocorticoid therapy in the last 4 weeks before study entry Baseline characteristics (N = 72): proportion male: treatment: 56%; comparator: 53% mean (SD) age, months: treatment: 16.8 (12); comparator: 17.6 (13) median (range) Westley croup score: treatment: 5.0 (3 to 6); comparator: 4.5 (3 to 6) |
|
| Interventions | Treatment (N = 36): single dose 0.15 mg/kg oral dexamethasone Comparator (N = 36): single dose 0.6 mg/kg oral dexamethasone |
|
| Outcomes | Change in Westley croup score from baseline to 4, 12, and 24 hours; hospitalisation; length of stay in hospital; use of mist tent | |
| Notes | All children received mist therapy throughout the observation period; funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A blocked randomization code was produced by random‐number generating software" |
| Allocation concealment (selection bias) | Low risk | Quote: "To make the study drugs indistinguishable from each other, they were packaged in opaque containers and diluted on the same amount of solution." "A blocked randomization code was produced ... and the code was not broken until after the study ended and all decisions regarding data analysis were finalized" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" "To make the study drugs indistinguishable from each other, they were packaged in opaque containers and diluted on the same amount of solution." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double‐blind" "to make the study drugs indistinguishable from each other, they were packaged in opaque containers and diluted on the same amount of solution" "the code was not broken until after the study ended and all the decisions regarding data analysis were finalized" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 14% (N = 12) of children recruited were excluded prior to randomisation. All randomised children were followed up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Amir 2006.
| Methods | Randomised controlled trial | |
| Participants |
Study period: November 2002 to March 2003 Setting: emergency department of Schneider Children’s Medical Center, Israel Inclusion criteria: children aged 6 months to 6 years with a clinical picture of mild to moderate acute laryngotracheitis presenting to the emergency department. Mild to moderate croup defined as a Westley croup score of 1 to 11. Exclusion criteria: spasmodic croup; acute epiglottitis; bacterial tracheitis; pneumonia; foreign body aspiration; chronic lung disease; congenital or acquired anatomical airway anomalies; immunosuppressed or immunocompromised; treated before arrival at the emergency department with inhaled bronchodilators or corticosteroids in any form; exposed to varicella in the previous 28 days; contradictions to corticosteroid treatment Baseline demographics (N = 52): proportion male: treatment: 73%; comparator: 27% mean (SD) age in months: treatment: 31.1 (20.4); comparator: 26.7 (16.8) mean (SD) Westley croup score: treatment: 3.6 (2.6); comparator: 2.0 (2.5) |
|
| Interventions | Treatment (N = 26): single 0.60 mg/kg (maximum 10 mg) dose of intramuscular dexamethasone Comparator (N = 26): single 0.40 mg/kg dose of oral betamethasone Treatments were of equivalent potency; supplemental oxygen was provided to children in whom air saturation was < 95%. |
|
| Outcomes | Change in Westley croup score from baseline to 2 and 4 hours; return visits to the emergency department; use of epinephrine | |
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study participants were assigned by a random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding described. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: used a third‐party outcome assessor. No blinding. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: the number of children analysed is not reported. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | High risk | Comment: baseline imbalance in croup score |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Bjornson 2004.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: September 2001 to April 2002 and September 2002 to February 2003 Setting: 4 paediatric emergency departments (Alberta Children’s Hospital in Calgary, Stollery Children’s Health Centre in Edmonton, Winnipeg Children’s Hospital in Winnipeg, or Children’s Hospital of Eastern Ontario in Ottawa) in Canada Inclusion criteria: children aged 3 months to 9 years with mild croup based on an initial medical evaluation. Mild croup was defined as onset within the past 72 hours of a seal‐like, barking cough and a Westley croup score of ≤ 2. Exclusion criteria: symptoms or signs of another cause of stridor; history of congenital or acquired stridor, asthma, exposure to varicella within the previous 21 days, chronic pulmonary disease, severe systemic disease, or known immune dysfunction; treatment with corticosteroids within the past 2 weeks; treatment of respiratory distress with epinephrine prior to enrolment; those enrolled in another clinical trial in the past 4 weeks; parents unable to speak either English or French; lack of a telephone at home; a prior visit to the emergency department because of croup during this episode of the disease Baseline demographics (N = 720): proportion male: treatment: 61%; control: 61% mean (SD) age in months: treatment: 35 (23); control: 35 (23) Westley croup score: treatment: 38% score of 0, 38% score of 1, 24% score of 2; control: 38% score of 0, 43% score of 1, 19% score of 2 |
|
| Interventions | Treatment (N = 359): single 0.60 mg/kg (maximum of 20 mg) dose of oral dexamethasone Control (N = 361): single dose of oral placebo (10 mL distilled water) Both treatment and placebo included 50 mL of wild cherry‐flavoured syrup. |
|
| Outcomes | Return visits to any healthcare provider within 7 days | |
| Notes | Funding source: Canadian Institutes of Health Research; Alberta Children's Hospital Foundation; Children's Hospital of Eastern Ontario Research Institute; Stollery Children's Hospital Foundation; Cumberland Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization scheme, stratified by centre, used random permuted blocks of 6‐20 children to ensure the comparable assignment of eligible patients." |
| Allocation concealment (selection bias) | Low risk | Quote: "Codes were secured at each center's pharmacy until enrolment and all decisions regarding data analysis had been finalized." The preparations were "not distinguishable" and "packaged in sequentially numbered, sealed bags." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind" "Parents were unable to determine which preparation their child had received" "Preparations were not distinguishable by appearance, volume, weight, taste, or smell." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "double blind" "Preparations were not distinguishable by appearance, volume, weight, taste, or smell and were packaged in identical syringes in sequentially numbered, sealed bags" "biostatistician who was not otherwise involved in the study performed the data analysis." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing data imputed using intention‐to‐treat analysis. 5% (N = 37) protocol deviations equally distributed between groups. 2% (N = 13) had incomplete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Cetinkaya 2004.
| Methods | Randomised controlled trial | |
| Participants |
Study period: not reported Setting: paediatric emergency department at the Şişlu Etfal Education and Research Hospital in Istanbul, Turkey Inclusion criteria: children aged 6 to 36 months who were admitted to the paediatric emergency clinic with a diagnosis of croup, defined as the acute (< 48 h) onset of stridor, chest wall retraction, barking cough, and hoarse voice Exclusion criteria: epiglottitis; reactive airway exacerbation; foreign body aspiration; acute bacterial pneumonia, acquired or congenital upper airway anomalies; intubated in the previous month; received steroids within the preceding 2 weeks Baseline characteristics (N = 60): proportion males: 67% age: not reported Westley croup score: not reported |
|
| Interventions | All children received 5 to 6 L/min of moisturised oxygen for 20 minutes upon arriving at the hospital, along with a single dose of 0.16 mg/kg of salbutamol. Treatment 1 (N = 15): 500 µg nebulised budesonide, a single dose of oral placebo (multivitamin syrup), and 2 mL intramuscular placebo (saline) Treatment 2 (N = 15): 2 mL nebulised placebo (saline), a single dose oral placebo (multivitamin syrup), and 0.60 mg/kg (maximum 8 mg) intramuscular dexamethasone Treatment 3 (N = 15): 2 mL nebulised placebo (saline), a single dose of 0.60 mg/kg (maximum 8 mg) oral dexamethasone, 2 mL intramuscular placebo (saline) Control (N = 15): 2 mL intramuscular placebo (saline), 2 mL nebulised salbutamol solution with saline, and oral placebo (multivitamin syrup) |
|
| Outcomes | Change in Westley croup score from baseline to 24 hours | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: blinding not explicitly described; it appears there was an attempt to blind parents and children by using similar‐appearing oral, nebulised, and intramuscular placebos. Unclear if personnel were blinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of blinding or third‐party outcome assessors. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Chub‐Uppakarn 2007.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: March 2001 to October 2003 Setting: paediatric ward of Hatyai Hospital in the southern part of Thailand Inclusion criteria: children aged 6 months to 5 years who were admitted to the paediatric ward with moderate to severe croup. Westley croup score 4 to 7 Exclusion criteria: history of contact with chicken pox within the preceding 3 weeks; history of congenital or acquired stridor; chronic pulmonary disease; asthma; severe systemic disease or known immune dysfunction; treatment with corticosteroids within the preceding 2 weeks; treatment with epinephrine for respiratory distress before enrolment Baseline demographics (N = 41): proportion male: treatment 1: 55%; treatment 2: 86% mean (SE) age in months: treatment 1: 16.9 (2.0); treatment 2: 18.8 (2.6) mean (SD) Westley croup score: treatment 1: 4.26 (0.22); treatment 2: 4.60 (0.25) |
|
| Interventions | Treatment 1 (N = 21): single 0.15 mg/kg dose (maximum 3 mg) of intramuscular dexamethasone Treatment 2 (N = 20): single 0.60 mg/kg dose (maximum 12 mg) of intramuscular dexamethasone |
|
| Outcomes | Change in Westley croup score at 2, 6, and 12 hours; intubations; adverse events | |
| Notes | All children were treated with a single nebulisation of epinephrine (1:1000) 1 mL in 0.9% saline 3 mL at baseline. Funding source: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer‐generated randomization scheme used random permuted blocks of four children" |
| Allocation concealment (selection bias) | Low risk | Quote: "codes were secured at the hospital pharmacy until enrolment and all decisions regarding data analysis had been finalized" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the preparations of dexamethasone suspension consisted of 10 mL of dexamethasone phosphate injection in concentrations of 1.2 and 0.3 mg/mL The preparations were packaged in identical containers by a hospital pharmacist and were not distinguishable in appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Cruz 1995.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: November 1992 to December 1993 Setting: emergency department at the Children's Hospital of Michigan, USA Inclusion criteria: children aged 6 months to 5 years reporting to the emergency department with a clinical diagnosis of acute laryngotracheitis or viral croup. Westley croup score of at least 2 and able to be managed as outpatients Exclusion criteria: history of prior intubation; structural airway anomalies; those requiring more than 1 racemic epinephrine treatment; hospitalisation; β‐agonist therapy; received steroids in the past 24 hours Baseline demographics (N = 45): proportion male: treatment: 74%; control: 63% median (SD) age in months: treatment: 18.0 (19.0); control: 21.0 (8.0) median (range) Westley croup score: treatment: 3 (2 to 5); control: 3 (2 to 5) |
|
| Interventions | Treatment (N = 19): single 0.60 mg/kg (maximum 10 mg) dose of intramuscular dexamethasone Control (N = 19): equal volume of placebo (normal saline) |
|
| Outcomes | Return visits to the emergency department; patient improvement 24 hours after discharge | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" "Both the patients and the investigators were blinded to the content of the syringe" "the drug code was broken only after the last patient had completed the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 16% (N = 7) were excluded or lost to follow‐up, unclear if losses were balanced between groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Dobrovoljac 2012.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency department of the Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children over 6 months of age presenting to the emergency department with mild to moderate croup (harsh cough with or without stridor, Westley croup score 1 to 6) Exclusion criteria: Westley croup score < 1; severe croup (Westley croup score > 6) requiring epinephrine upon arrival; received steroids within the last week; other significant coexisting illnesses Baseline demographics (N = 70): proportion male: treatment: 69%; control: 66% mean (SD) age in months: treatment: 37.1 (22.6); control: 27.4 (25.7) mean (SD) Westley croup score: treatment: 2.7 (0.8); control: 2.8 (1.0) |
|
| Interventions | Treatment (N = 35): single 0.15 mg/kg (1 mg/mL solution) dose of oral dexamethasone Control (N = 35): same volume of placebo solution All children also received 0.15 mg/kg oral dexamethasone at 60 minutes (after study completion). |
|
| Outcomes | Change in Westley croup score from baseline to 2 hours; use of epinephrine and supplemental glucocorticoids | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by the pharmacy department and the randomization list was kept concealed from emergency physicians, nurses and parents until the end of the trial." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the PMH pharmacy ensured that the two preparations could not be differentiated" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 6% loss to follow‐up due to worsening condition, all in the placebo group (N = 4, 11%). Used the last observation carried forward method, which could have biased results in favour of the treatment group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Donaldson 2003.
| Methods | Randomised double‐blind trial | |
| Participants |
Study period: January 1999 to December 1999 Setting: emergency department of William Beaumont Hospital, USA Inclusion criteria: children aged 3 to 84 months with history of inspiratory stridor or a barky cough and a Westley croup score of ≥ 2 after 10 to 15 minutes of cool mist therapy in the emergency department Exclusion criteria: Westley croup score < 2; signs suggesting another cause for stridor such as epiglottitis, bacterial tracheitis, foreign body, chronic lung disease; severe comorbidities; inability of parents to give informed consent; glucocorticoid therapy within 4 weeks of presenting Baseline demographics (N = 96): proportion male: treatment 1: 73%; treatment 2: 57% mean (SD) age in months: treatment 1: 23.2 (17.9); treatment 2: 28.9 (17.7) mean (SD) Westley croup score: treatment 1: 3.5 (1.8); treatment 2: 3.5 (1.7) |
|
| Interventions | Treatment 1 (N = 49): 0.60 mg/kg intramuscular dexamethasone and oral placebo (syrup) Treatment 2 (N = 46): 0.60 mg/kg oral dexamethasone and intramuscular placebo (direct pressure with hub of syringe on thigh) |
|
| Outcomes | Unscheduled revisits; parent‐reported symptom relief after 24 hours; use of epinephrine | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "block randomization method from a random number generator performed by the department of Pharmacy" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "In both groups, neither the parents nor the treating physicians were present in the treatment room during the administration of medications" "The emergency medicine faculty... were blinded to the route of administration of the drug" "If the child vomited while in the ED, the treatment given was unblinded" Comment: blinding was attempted but could be broken if the child vomited while in the emergency department. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: third‐party outcome assessor described as blinded. Because blinding of children and parents could have been broken, the assessors could have become unblinded during conversation with parents. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: used intention‐to‐treat analysis. 1% (N = 1) loss to follow‐up, unclear from which group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Duman 2005.
| Methods | Randomised controlled trial | |
| Participants |
Study period: September 2002 to September 2003 Setting: paediatric emergency department of Dokuz Eylül University Faculty of Medicine, Izmir, Turkey Inclusion criteria: children aged more than 6 months presenting to the emergency department with a history of inspiratory stridor, barking cough, hoarseness and signs of inspiratory distress, and a Westley croup score of ≥ 2 Exclusion criteria: Westley croup score < 2, and with suggested other causes for stridor (epiglottitis, bacterial tracheitis, foreign body aspiration); past history of laryngoscopy, tracheal intubation, chronic lung disease, or severe comorbidities; immediate intubation or transfer to intensive care; corticosteroid therapy within 4 weeks of presentation; history of tuberculosis personally or in the family; chickenpox within the preceding 21 days; known immunodeficiency Baseline demographics (N = 76): proportion male: treatment 1: 77%; treatment 2: 90%; comparator: 77% mean (SD) age in months: treatment 1: 41.5 (25.5); treatment 2: 43.3 (24.7); comparator: 34.8 (22.4) mean (SD) Westley croup score: treatment 1: 5.3 (1.2); treatment 2: 5.5 (1.8); comparator: 5.0 (1.3) |
|
| Interventions | Treatment 1 (N = 31): 2.5 mL (0 to 20 kg) or 5.0 mL (20 to 40 kg) nebulised epinephrine with same volume normal saline and 0.60 mg/kg intramuscular dexamethasone Treatment 2 (N = 19): 2.5 mL (0 to 20 kg) or 5.0 mL (20 to 40 kg) nebulised epinephrine with same volume normal saline and 2 mg nebulised budesonide Comparator (N = 26): cool mist therapy and 0.60 mg/kg intramuscular dexamethasone In all groups, the drug was administered for a period of 20 minutes using a nebuliser with oxygen at a rate of 6 to 7 L/min through a face mask. |
|
| Outcomes | Change in Westley croup score from baseline to 2 hours; admissions from the emergency department; use of epinephrine | |
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to treatment according to a randomisation list produced at the beginning of the study. Patients were randomised in blocks of three." Comment: assumed that the blocked randomisation was computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no blinding. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no blinding. No description of a third‐party outcome assessor. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Eboriadou 2010.
| Methods | Randomised double‐blinded controlled trial | |
| Participants |
Study period: January 2000 to December 2001 Setting: emergency department at a hospital in Greece Inclusion criteria: children aged 6 months to 5 years presenting to the emergency department with a clinical diagnosis of viral croup by a history of a short period of viral upper respiratory symptoms followed by hoarseness or barking cough and clinical evidence of hoarseness, barking cough, or stridor in the emergency department; modified Downes and Raphaelly croup score ≥ 2; could be managed as outpatients Exclusion criteria: known structural airways anomalies; acute epiglottitis; bacterial tracheitis; pneumonia; foreign body aspiration or past history of laryngoscopy; history of prior intubation; chronic airway obstruction; received steroids in the past 24 hours; required more than 1 treatment with nebulised L‐epinephrine or hospitalisation Baseline characteristics (N = 64): proportion males: treatment 1: 80%; treatment 2: 58%; treatment 3: 65% mean age in years: treatment 1: 2.6; treatment 2: 3.2; treatment 3: 3.4 mean modified Downes and Raphaelly croup score: treatment 1: 5.13; treatment 2: 5.89; treatment 3: 3.95 |
|
| Interventions | Treatment 1 (N = 25): single 5 mL (1:1000 mg/mL) dose of nebulised L‐epinephrine via nebuliser with oxygen at a rate of 5 L/minute Treatment 2 (N = 19): single 0.60 mg/kg (maximum 8 mg) dose of intramuscular dexamethasone Treatment 3 (N = 20): single 200 µg dose of inhaled beclomethasone dipropionate delivered via AeroChamber device Supplemental oxygen was used for children with oxygen saturation values < 95%. |
|
| Outcomes | Change in modified Downes and Raphaelly croup score from baseline to 2 hours; return visits to the emergency department | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were assigned by a random numbers table to receive..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: described as "double‐blind", though the interventions were clearly distinguishable, and the mechanism of blinding was not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Eden 1964.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: hospital in USA Inclusion criteria: children hospitalised for treatment of acute croup, including all children with acute respiratory infections characterised by hoarseness, inspiratory stridor, and a barking cough Exclusion criteria: not reported Baseline characteristics (N = 50): proportion male: not reported age: not reported croup score: not reported |
|
| Interventions | All children received as routine therapy oxygen, increased humidity, and tetracycline. Treatment (N = 25): 1 mg/kg intramuscular methyl prednisolone every 6 hours for 24 hours Control (N = 25): 1 mg/kg placebo (control) preparation every 6 hours for 24 hours |
|
| Outcomes | Patient improvement at 6, 12, 24 hours | |
| Notes | Funding source: Upjohn Company (supplied drugs for the trial) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were divided into two groups according to a table of random sampling" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "The composition of each preparation was unknown to the investigators until the end of the study" Comment: described as double‐blind. Investigators blinded, but unclear if participants or personnel (or both) were blinded because who administered the treatments is not stated. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor, unclear who performed the measurements. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 6% (N = 3) lost to follow‐up due to inadequate evaluation. All losses were in 1 group, but unclear which group. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Eden 1967.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: hospital in USA Inclusion criteria: children hospitalised with acute croup, including those presenting with acute respiratory infections characterised by barking cough, hoarseness, sternal retractions, and respiratory stridor Exclusion criteria: not reported Baseline demographics (N = 50): proportion male: treatment: 60%; control: 80% age in years (%): treatment: 68% 0 to 2 years, 28% 2 to 4 years, 4% > 4 years; control: 56% 0 to 2 years, 28% 2 to 4 years, 16% > 4 years croup score: not reported |
|
| Interventions | Treatment (N = 25): 0.1 mL/kg per dose schedule (0.1 mg/kg) intramuscular dexamethasone every 6 hours for 48 hours (total daily dose of 0.40 mg/kg) Control (N = 25): volume of 0.1 mL/kg per dose schedule of placebo (control) preparation intramuscularly every 6 hours for 48 hours |
|
| Outcomes | Patient improvement at 6, 12, 24 hours; tracheostomy | |
| Notes | Funding source: dexamethasone (Decadron) by Merck Sharp & Dohme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were divided into two groups according to a table of random sampling" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" "The composition of each preparation was unknown to the investigators until the end of the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Fifoot 2007.
| Methods | Randomised double‐blind trial | |
| Participants |
Study period: July 2004 to October 2005 Setting: emergency department of Mater Childrens' Hospital, Brisbane, Australia Inclusion criteria: children aged 6 months to 6 years presenting to the emergency department with croup (Westley croup score ≥ 2) with parents available for telephone follow‐up 1 week following enrolment Exclusion criteria: chronic respiratory disease (excluding asthma); severe croup (Westley croup score > 7); known allergy or relative contraindication to steroids (varicella or exposure to varicella within the past 3 weeks, history of tuberculosis, diabetes, or hypertension, known immunodeficiency); treatment with steroids in the preceding week or with nebulised adrenaline en route or immediately on arrival in the emergency department Baseline demographics (N = 99): proportion male: treatment 1: 79%; treatment 2: 65%; treatment 3: 80% mean (SD) age in years: treatment 1: 1.76 (1.52); treatment 2: 1.53 (1.31); treatment 3: 1.74 (1.61) mean (SD) Westley croup score: treatment 1: 3.15 (0.89); treatment 2: 2.71 (0.84); treatment 3: 2.81 (0.87) |
|
| Interventions | Treatment 1 (N = 34): single 1 mg/kg dose of oral prednisolone Treatment 2 (N = 34): single 0.15 mg/kg dose of oral dexamethasone Treatment 3 (N = 31): single 0.60 mg/kg dose of oral dexamethasone Children who did not tolerate oral therapy after 2 attempts received nebulised budesonide. |
|
| Outcomes | Change in Westley croup score from baseline to 2 and 4 hours; re‐presentations with croup; use of epinephrine and use of supplemental glucocorticoids | |
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized (using a computer‐generated sequence)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Recruiting staff and study investigators were blinded to treatment assignments. MCH pharmacists prepared each steroid agent as a solution, such that each child would receive an identical volume of preparation... flavoured to standardize taste and palatability" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Recruiting staff and study investigators were blinded to treatment assignments. MCH pharmacists prepared each steroid agent as a solution, such that each child would receive an identical volume of preparation... flavoured to standardize taste and palatability" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 13% (N = 13) loss to follow‐up. Losses balanced between groups. Did not use intention‐to‐treat analysis for the telephone follow‐up outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: no deviations from protocol detected. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Fitzgerald 1996.
| Methods | Randomised double‐blind trial | |
| Participants |
Study period: not reported Setting: emergency departments of 3 paediatric tertiary referral hospitals, Sydney, Australia Inclusion criteria: children aged 6 months to 6 years admitted to the emergency department based on a decision by the medical staff regarding croup severity. To be included in study children were required to have acute (viral) or spasmodic croup and a minimum Westley croup score of 6. Exclusion criteria: significant past or present systemic disease; pre‐existing known airway abnormalities; confirmed hypersensitivity to budesonide or L‐adrenaline; suspected epiglottitis; foreign body aspiration; bronchiolitis or asthma; need for immediate intubation or transfer to intensive care; treated with glucocorticoids in the 4 weeks prior to the study Baseline demographics (N = 67): proportion males: not reported mean (SD) age in months: treatment 1: 20.9 (12.7); treatment 2: 24.9 (12.5) mean (SD) modified Westley croup score: treatment 1: 7.1 (1.2); treatment 2: 7.7 (1.1) |
|
| Interventions | Treatment 1 (N = 35): single 2 mg/4 mL dose of nebulised budesonide Treatment 2 (N = 31): single 4 mg/4 mL dose of nebulised L‐epinephrine |
|
| Outcomes | Change in modified Westley croup score from baseline to 2, 12, and 24 hours; readmission to the hospital; intubation, use of epinephrine, and use of additional steroids | |
| Notes | Funding source: Astra Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: described as double‐blind, but nursing staff (personnel) were unblinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The study medication was administered by nursing staff and the investigator was not present when the medication was placed in the opaque nebulizer bowl" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 5% (N = 3) loss to follow‐up. All‐patients‐treated analysis excluded N = 1 child (1.5%). 13 children who received medications appropriately did not remain for the entire 24 hours. Last value extended for those who recovered before the 24‐hour period, however no children returned or were readmitted; unclear how this may have affected the findings. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Garbutt 2013.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: 26 October 2009 to 16 April 2010 and 6 September 2010 to 29 April 2011 Setting: 10 offices of primary care practitioners in St Louis, MO, USA Inclusion criteria: children aged 1 to 8 years with croup symptoms for ≤ 48 hours and a clinical diagnosis of mild or moderate croup at an office visit, based on symptoms in the past 24 to 36 hours Exclusion criteria: diagnosis of severe croup or impending respiratory failure; prior treatment with epinephrine or oral corticosteroids for this croup episode; symptoms or signs suggesting other cause of stridor; chronic respiratory disease including asthma; known contraindication to steroid use; parent not in the same household as the child in the subsequent 4 days, could not participate in telephone interviews, or was not English speaking Baseline demographics (N = 87): proportion male: treatment: 61%; comparator: 68% mean (SD) age in years: treatment: 2.67 (1.43); comparator: 3.11 (1.58) mean (SD) Westley croup score: treatment : 0.4 (0.7); comparator: 0.6 (0.8) mean (SD) Telephone Outpatient Score: treatment: 2.2 (0.9); comparator: 2.0 (0.9) |
|
| Interventions | Treatment (N = 41): single 2 mg/kg (maximum 60 mg/day) dose of oral prednisolone once per day for 3 days Comparator (N = 46): single 0.60 mg/kg (maximum 18 mg) dose of oral dexamethasone, followed by 2 days of placebo comparable in appearance, smell, and taste |
|
| Outcomes | Additional health care for croup within 11 days of randomisation | |
| Notes | Funding source: National Center for Research Resources (National Institutes of Health); National Institutes of Health Roadmap for Medical Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomized blocks were used to assign subjects to treatment groups, with randomization stratified by site. Computer generated random numbers determined how the two treatments were allocated" |
| Allocation concealment (selection bias) | Low risk | Quote: "Study drug packages were prepared offsite by the pharmacist" "The pharmacist... packaged the bottles in a sealed opaque envelope" "For allocation concealment, the drug formulation ensured the volume of the weight‐based dose was equivalent for each medication." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The pharmacist... packaged the bottles in a sealed opaque envelope" "comparable in appearance, smell and taste" "patients, parents, PCPs, and study team members were blinded to treatment assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The pharmacist... packaged the bottles in a sealed opaque envelope" "comparable in appearance, smell and taste" "patients, parents, PCPs, and study team members were blinded to treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. Losses to follow‐up on days 1, 2, 3, 4, and 11 were 7%, 9%, 9%, 3%, and 2% (non‐cumulative). Participation in follow‐up interviews was balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Comment: no deviations from protocol detected. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Low risk | Comment: all domains judged as low risk. |
Geelhoed 1995a.
| Methods | Randomised double‐blind controlled trial (trial A; see Geelhoed 1995b for trial B) | |
| Participants |
Study period: July 1994 to August 1994 Setting: Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months admitted to the hospital with a diagnosis of croup (acute onset of inspiratory stridor, chest wall retractions, barking cough, and hoarse voice) and a minimum modified Westley croup score of 3 Exclusion criteria: other acute or chronic medical problems; modified Westley croup score < 3 (mild croup); families without a telephone or with limited English language abilities; any kind of steroid therapy in the past week; pre‐existing upper airway condition; history of prolonged stridor; those presenting with a clinical picture suggesting a diagnosis other than croup; admitted directly to the intensive care unit with severe croup Baseline demographics (N = 60): proportion male: treatment: 62%; comparator: 81% mean (SD) age in months: treatment: 35 (19); comparator: 42 (27) mean modified Westley croup score: treatment: 3.8; comparator: 3.7 |
|
| Interventions | Treatment (N = 29): single 0.30 mg/kg (maximum 6 mg) dose of oral dexamethasone Comparator (N = 31): single 0.60 mg/kg (maximum 12 mg) of oral dexamethasone |
|
| Outcomes | Change in modified Westley croup score from baseline to 2 and 4 hours; re‐presentations with croup; length of hospital stay; use of epinephrine and use of additional glucocorticoids | |
| Notes | Study reports on 2 comparisons. Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "treatments were administered in a double blind fashion" "If the child was withdrawn from the study, their study code was broken." Comment: described as double‐blind. Unclear who was blinded. Code could be broken, but unclear how frequently this occurred. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. 2% (N = 1) in trial A withdrew, 5% (N = 3) in trial A lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: some children were not enrolled when the emergency department was busy, potential to bias participant selection. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Geelhoed 1995b.
| Methods | Randomised double‐blind controlled trial (trial B; see Geelhoed 1995a for trial A) | |
| Participants |
Study period: August 1994 to December 1994 Setting: Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months admitted to the hospital with a diagnosis of croup (acute onset of inspiratory stridor, chest wall retractions, barking cough, and hoarse voice) and a minimum modified Westley croup score of 3 Exclusion criteria: other acute or chronic medical problems; modified Westley croup score < 3 (mild croup); families without a telephone or with limited English language abilities; any kind of steroid therapy in the past week; pre‐existing upper airway condition; history of prolonged stridor; those presenting with a clinical picture suggesting a diagnosis other than croup; admitted directly to the intensive care unit with severe croup Baseline demographics (N = 60): proportion male: treatment: 90%; control: 74% mean (SD) age in months: treatment: 38 (34); comparator: 32 (23) mean modified Westley croup score: treatment: 4.0; comparator: 3.7 |
|
| Interventions | Treatment (N = 29): single 0.15 mg/kg (maximum 3 mg) dose of oral dexamethasone Comparator (N = 31): single 0.30 mg/kg (maximum 6 mg) dose of oral dexamethasone |
|
| Outcomes | Change in modified Westley croup score from baseline to 2 and 4 hours; re‐presentations with croup; length of hospital stay; use of epinephrine and use of additional glucocorticoids | |
| Notes | Study reports on 2 comparisons. Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "treatments were administered in a double blind fashion" "If the child was withdrawn from the study, their study code was broken." Comment: described as double‐blind. Unclear who was blinded. Code could be broken, but unclear how frequently this occurred. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. 8% (N = 5) in trial B lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: some children were not enrolled when the emergency department was busy, potential to bias participant selection. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Geelhoed 1995c.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children aged 3 months and older admitted to hospital with a diagnosis of croup (acute onset of inspiratory stridor, chest wall retractions, barking cough, hoarse voice) and a minimum croup score of 3 Exclusion criteria: croup score < 3 (mild croup); caregivers did not consent; family did not have a telephone; caregivers had limited English language abilities; received steroid therapy in the past week; pre‐existing conditions of the upper airway or prolonged stridor; clinical examination suggested a diagnosis other than croup; admitted directly to the intensive care unit with severe croup Baseline demographics (N = 80): proportion male: treatment 1: 52%; treatment 2: 85%; control: 80% mean (SD) age in months: treatment 1: 35 (35); treatment 2: 33 (30); control: 30 (23) mean croup score: treatment 1: 3.8; treatment 2: 3.7; control: 3.8 |
|
| Interventions | Treatment 1 (N = 23): single dose (0.60 mg/kg) oral dexamethasone and 4 mL nebulised saline Treatment 2 (N = 27): single 2 mg (4 mL) dose nebulised budesonide and placebo Control (N = 30): single dose oral placebo and 4 mL of nebulised saline |
|
| Outcomes | Change in croup score from baseline to 2, 4, and 12 hours; re‐presentations with croup; length of hospital stay; use of epinephrine, use of supplemental glucocorticoids, and intubations | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "treatments were administered in a double blind fashion" "If the attending doctors considered patients to be severely ill or failing to improve, they could be withdrawn at any time to receive steroids, at which time their study code was broken." Comment: described as double‐blind. Unclear who was blinded. Code could be broken. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: did not use intention‐to‐treat analysis for the telephone follow‐up. 17% (N = 9) withdrew, more in placebo than in treatment group (23% compared to 9%). Additional 10% (N = 8) lost to follow‐up, unclear from which group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Geelhoed 1996.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency department of Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months presenting to the emergency department with a diagnosis of croup (acute onset of inspiratory stridor, barking cough, hoarseness, and chest wall retractions) not severe enough to warrant admission Exclusion criteria: other acute or chronic medical problems; families that did not have a telephone or had limited English language abilities; received any type of steroids in the preceding week; pre‐existing upper airway condition; history of prolonged stridor; clinical picture that suggested a diagnosis other than croup Baseline demographics (N = 100): proportion male: treatment: 68%; control: 72% mean (SD) age in months: treatment: 37 (23); control: 45 (26) mean croup score: treatment: 0.9; control: 0.9 |
|
| Interventions | Treatment (N = 50): single 0.15 mg/kg dose of oral dexamethasone Control (N = 50): single dose of oral placebo |
|
| Outcomes | Reattendance at the emergency department with croup | |
| Notes | Funding source: no external funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Treatments were given double blind" Comment: no further explanation given. Unclear who was blinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 4% (N = 4) lost to follow‐up. Equal between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Geelhoed 2005.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency department of Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months who presented to the emergency department with a diagnosis of croup (acute onset of inspiratory stridor, chest wall retractions, barking cough, hoarse voice) and no other acute or chronic medical problems requiring admission. Croup was defined as the acute onset of inspiratory stridor, barking cough, hoarse voice, and chest wall retractions Exclusion criteria: other acute or chronic medical problems; families that did not have a telephone or had limited English language abilities; received any type of steroids in the preceding week; pre‐existing upper airway condition; history of prolonged stridor; clinical picture that suggested a diagnosis other than croup Baseline demographics (N = 72): proportion male: treatment: 72%; control: 67% mean (SD) age in months: treatment: 35 (22); control: 36 (30) mean (SD) croup score: treatment: 4.1 (0.8); control: 4.1 (0.8) |
|
| Interventions | Treatment (N = 36): single 0.15 mg/kg dose of oral dexamethasone and 2 mg of nebulised budesonide Control (N = 36): single 0.15 mg/kg dose of oral dexamethasone and a dose of equivalent volume of nebulised placebo (saline) |
|
| Outcomes | Change in croup score from baseline to 2 and 4 hours; readmissions for croup; length of stay in hospital | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomized to 1 of 2 groups based on hospital pharmacy computer‐generated numbers." |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "a nurse who had no further part in the management of the child administered treatment. Other staff and subjects were blinded to the nebulized treatments given." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1% (N = 1) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Godden 1997.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: November 1993 to April 1995 Setting: paediatric wards of Poole NHS Trust Hospital, Dorset, England Inclusion criteria: children admitted to hospital with a clinical diagnosis of croup based on the modified Westley croup score Exclusion criteria: receiving bronchodilators or received systemic steroids within the previous month Baseline demographics (N = 89): proportion male: treatment: 72%; control: 64% mean (range) age in months: treatment: 35.7 (7 to 116); control: 37.4 (7 to 93) mean (SD) modified Westley croup score (N = 87): treatment: 5.30 (3.44); control: 5.15 (3.70) |
|
| Interventions | Treatment (N = 47): initial 2 mg (4 mL) dose of nebulised budesonide, followed by a repeating dose of 1 mg every 12 hours Control (N = 42): initial 4 mL dose of nebulised placebo (normal saline), followed by a repeating dose of 2 mL placebo (normal saline) every 12 hours Both treatment and placebo delivered via an opaque nebuliser chamber, driven by wall oxygen at a rate of 8 L/min. |
|
| Outcomes | Change in modified Westley croup score from baseline to 2, 4, 12, and 24 hours; length of stay in hospital; use of epinephrine and intubation | |
| Notes | Baseline croup score not presented for 2 children due to prior treatment with nebulised L‐epinephrine; funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "trial solution was supplied in an opaque respule within a sealed silver foil packet." "The patient initially received 4 mL of a solution containing either normal saline vehicle or 4mg (4mL) of budesonide, via an opaque nebuliser chamber." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 8% (N = 7) withdrew, equal between groups (9% in study group, 7% in control group). |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: some possible participants were not enrolled due to manpower constraints, which could potentially have biased participant selection. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Husby 1993.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1990 to December 1991 Setting: Department of Paediatrics, Kolding Hospital, Denmark Inclusion criteria: children admitted to hospital with croup (inspiratory stridor, cough, and respiratory distress), with a modified Westley croup score > 5 and informed parental consent Exclusion criteria: clinical condition consistent with epiglottitis, foreign body aspiration, bronchiolitis, or asthma; received local or systemic steroid treatment or epinephrine Baseline demographics (N = 36) (1 child excluded before placebo was administered): proportion male: treatment: 80%; control: 75% median (range) age in years: treatment: 1.6 (0.6 to 4.9); control: 1.1 (0.4 to 4.2) median (range) modified Westley croup score: treatment: 8 (6 to 10); control: 8 (6 to 12) |
|
| Interventions | Treatment (N = 20): 2, 1000 µg (2 mL 500 µg/mL) doses of nebulised budesonide, 30 minutes apart Control (N = 16): 2, 2 mL doses of placebo (0.9% saline), 30 minutes apart Both treatment and placebo were given with a dynamic flow rate of 8 L/min. |
|
| Outcomes | Change in modified Westley croup score from baseline to 2 hours; use of antibiotics | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as double‐blind, no further explanation. Insufficient information provided to judge. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 child omitted as did not receive treatment due to technical problems. No other missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
James 1969.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: September 1965 to November 1966 (excluding 2 periods during May and August 1966, total of 6 weeks) Setting: children's division of the Los Angeles County‐University of Southern California Medical Center, USA Inclusion criteria: children admitted to hospital with a diagnosis of croup or laryngotracheobronchitis (dyspnoea with inspiratory stridor, subcostal, suprasternal, or sternal retractions, and a barking, seal‐like cough) Exclusion criteria: those with very mild stridor at admission; history of persistent or congenital stridor; suspected diagnosis of acute epiglottitis; clinical or roentgenographic evidence of an associated pneumonitis Baseline demographics (N = 88): proportion male: treatment: 76%; control: 66% median age in months: treatment: 17; control: 12 modified Westley croup score: not reported |
|
| Interventions | Treatment (N = 45): single 4 mg/mL dose of intramuscular dexamethasone sodium phosphate Control (N = 43): single 4 mg/mL dose of placebo solution identical in appearance Both the treatment and placebo administered 0 to 3 hours after admission. |
|
| Outcomes | Patient improvement at 12 and 24 hours; use of antibiotics and tracheostomy | |
| Notes | Funding source: Merck Sharp & Dohme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Low risk | Quote: "dexamethasone... and a placebo solution of identical appearance were provided in randomly numbered vials. As each patient was admitted to the study, he received the predetermined dose of medication from the next bottle in the series, after which the bottle was discarded." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "...solution of identical appearance were provided in randomly numbered unlabeled vials" "All of the evaluations were completed before the drug code was broken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "...solution of identical appearance were provided in randomly numbered unlabeled vials" "All of the evaluations were completed before the drug code was broken" Comment: outcome measures assessed by staff and in some cases also the investigator. Both staff and investigator appear to have been blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Johnson 1996.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1989 to November 1991 Setting: emergency department of The Hospital for Sick Children, Toronto, Canada Inclusion criteria: children presenting to the emergency department between 8:00 a.m. and midnight with signs and symptoms consistent with acute laryngotracheitis, including seal‐like barking cough and inspiratory stridor that had developed 2 to 72 hours before they were seen; persistent moderate respiratory distress (modified Westley croup score 2.5 to 5) after having been treated for at least 30 minutes with humidified oxygen provided by plastic tubing aimed toward the nose and mouth; written parental informed consent Exclusion criteria: history of congenital stridor or endotracheal intubation longer than 1 month; signs or symptoms suggesting another cause of stridor (e.g. bacterial tracheitis, epiglottitis, supraglottic foreign body, spasmodic croup); presence of marked expiratory wheeze that responded to treatment with bronchodilators; history of chronic respiratory problems such as asthma requiring routine daily treatment with bronchodilators; presence of a severe systemic disease that would affect the decision to admit or discharge; history of receiving corticosteroids in the last 2 weeks or racemic epinephrine hydrochloride in the last 4 hours Baseline demographics (N = 55): proportion male: treatment: 71%; control: 56% median (25th, 75th percentile) age in months: treatment: 15 (11, 29); control: 17 (9, 22) median (25th, 75th percentile) modified Westley croup score: treatment: 4 (3, 4); control: 4 (3, 4) |
|
| Interventions | Treatment (N = 28): single 10 mg (< 8 kg body weight), 15 mg (8 to 12 kg), or 20 mg (> 12 kg) dose of nebulised parenteral dexamethasone sodium phosphate solution (10 mg/mL) mixed with normal saline to make 4 mL provided with 100% oxygen at a flow rate of 6 to 7 L/min Control (N = 27): placebo (normal saline) provided in the same fashion All children were also treated with humidified oxygen supplied by plastic tubing aimed toward the child's nose and mouth throughout the study period. |
|
| Outcomes | Change in modified Westley croup score from baseline to 2 and 4 hours; hospitalisation rate; improvement at 4 hours; use of epinephrine, use of mist tent, intubation, and use of additional glucocorticoids | |
| Notes | Funding source: Pediatric Consultants, The Hospital For Sick Children, Toronto, Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized in blocks of 10" Comment: block randomisation assumed to be computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Quote: "the randomized code was generated and held by the hospital pharmacy until after enrolment of all patients." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The pharmacy dispensed the appropriate medication in a vial specially prepared for this study, based on a randomization schedule" "To ensure blinding of investigators, staff, and parents, nebulizer containers were covered during and after nebulization" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The pharmacy dispensed the appropriate medication in a vial specially prepared for this study, based on a randomization schedule" "To ensure blinding of investigators, staff, and parents, nebulizer containers were covered during and after nebulization" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 16% (N = 9) protocol deviations not included in the analysis. More losses in the intervention group, but unclear if this was significant |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Johnson 1998.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: September 1993 to May 1996 Setting: emergency departments of The Hospital for Sick Children in Toronto and Alberta Children's Hospital in Calgary, Canada Inclusion criteria: children aged from 3 months to 9 years presenting to the emergency department between 3:00 p.m. and 6:00 a.m. who were given a diagnosis of croup (acute onset of inspiratory stridor associated with a seal‐like barking cough) with persistent moderately severe respiratory distress (modified Westley croup score of 3 to 6) after being treated with humidified oxygen for 30 minutes Exclusion criteria: signs and symptoms suggesting another cause of stridor (e.g. epiglottitis, bacterial tracheitis, supraglottic foreign body); parents who were unable to speak English well enough to give informed consent; history of chronic pulmonary disease, severe systemic disease, immune dysfunction, stridor, intubation for more than 1 month; glucocorticoid therapy in the 4 weeks prior to entering the study Baseline demographics (N = 144): proportion males: 69% mean (SD) age in months: 24 (18) mean (SD) modified Westley croup score: treatment 1: 3.8 (0.9); treatment 2: 4.0 (0.9); control: 3.8 (0.8) |
|
| Interventions | Treatment 1 (N = 48): single 4 mg dose of nebulised budesonide Treatment 2 (N = 47): single 0.6 mg/kg dose of intramuscular dexamethasone Control (N = 49): single dose of nebulised placebo suspension All children received 0.5 mL of 2.25% racepinephrine and normal saline combined with either treatment or placebo (total volume 8 mL) via nebuliser with oxygen from a wall outlet at a rate of 6 to 7 L/min through a face mask held tightly to the child's face over a period of 20 minutes. |
|
| Outcomes | Change in modified Westley croup score from baseline to 5 hours or discharge; rate of hospitalisation; use of epinephrine, use of supplemental glucocorticoids, intubations | |
| Notes | Funding source: Astra Pharma | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A blocked randomization code was produced by random‐number generating software." |
| Allocation concealment (selection bias) | Low risk | Quote: "A blocked randomization code... provided only to the pharmacy at the hospital" "The pharmacies prepared sequential patient packets containing study drugs that were sealed and were identical in appearance and weight." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "To make the nebulized study drugs indistinguishable from each other, they were packaged in opaque containers and discharged directly into a colored nebulizer" "to maintain masking, the study nurse temporarily took the parents away from their child while an emergency staff nurse not otherwise involved in the care of the child injected the dexamethasone into the child's thigh, placed a bandage over the injection site (all children received a bandage whether or not they received dexamethasone), and initiated nebulization." Comment: blinding was attempted, but it could have been broken. Unmasking occurred in 3 cases. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: outcome assessor described as blinded, but blinding could have been broken. Unmasking occurred in 3 cases. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: used intention‐to‐treat analysis, including 17% (N = 25) with protocol deviations. 2% (N = 1) from the treatment group were lost to follow‐up because parents could not be reached. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Klassen 1994.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1992 to October 1993 Setting: emergency department at the Children's Hospital of Eastern Ontario, Canada Inclusion criteria: children aged from 3 months to 5 years presenting to the emergency department between 9:00 a.m. and midnight (except holidays) with mild to moderate croup consisting of hoarseness, inspiratory stridor, and barking cough, and a Westley croup score ≥ 2 after breathing humidified oxygen for at least 15 minutes Exclusion criteria: diagnosis of epiglottitis or chronic upper or lower airway disease (not including asthma); corticosteroids administered within the past 2 weeks; severe croup (defined as a Westley croup score of 8 or higher or requiring treatment with racemic epinephrine immediately on arrival) Baseline demographics (N = 54): proportion males: treatment: 63%; control: 74% mean (SD) age in years: treatment: 1.8 (1.2); control: 2.2 (1.4) median (25th, 75th percentile) Westley croup score: treatment: 4 (3, 5); control: 4 (3, 5) |
|
| Interventions | Treatment (N = 27): single 2 mg (4 mL) dose of nebulised budesonide Control (N = 27): single 4 mL dose of nebulised placebo (0.9% saline solution) Both treatment and placebo administered by an updraft nebuliser with a continuous flow of oxygen at 5 to 6 L/min. |
|
| Outcomes | Change in Westley croup score from baseline to 4 hours; admissions to the hospital; 2‐point improvement in croup score at 4 hours; use of epinephrine, use of supplemental glucocorticoids | |
| Notes | Funding source: Ontario Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 10 by the pharmacy department, with a random number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization list was kept concealed from the research assistants, parents and emergency physicians and from the child's regular physician until the end of the trial." "the pharmacy provided both budesonide and normal saline in opaque brown syringes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "the pharmacy provided both budesonide and normal saline in opaque brown syringes to ensure blinding. The research assistants then placed the study drug directly into an opaque nebulizer reservoir." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the pharmacy provided both budesonide and normal saline in opaque brown syringes to ensure blinding. The research assistants then placed the study drug directly into an opaque nebulizer reservoir." Comment: research assistants blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Did not use intention‐to‐treat analysis. 7% (N = 4) lost, all from the placebo group (15%) due to worsening condition or lack of satisfaction with treatment. Unbalanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: 24 children were not enrolled because the emergency department failed to contact the study team; this could potentially have biased participant selection. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Klassen 1996.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1993 to April 1994 Setting: emergency department of the Children's Hospital of Eastern Ontario, Canada Inclusion criteria: children aged 3 months to 5 years presenting to the emergency department with mild to moderate croup (hoarseness, inspiratory stridor, barking cough) and a modified Westley croup score ≥ 3 after at least 15 minutes of mist therapy Exclusion criteria: diagnosis of epiglottitis, chronic upper or lower airway disease (excluding asthma), severe croup (modified Westley croup score ≥ 8); received glucocorticoids within the previous 2 weeks; needed immediate racemic epinephrine on arrival Baseline demographics (N = 50): proportion males: treatment: 68%; control: 76% mean (SD) age in years: treatment: 1.2 (0.7); control: 1.8 (1.3) mean (SD) modified Westley croup score: treatment: 4.4 (1.1); control: 4.1 (0.9) |
|
| Interventions | All children received a single 0.60 mg/kg dose of oral dexamethasone at entry to the study. Treatment (N = 25): single 2 mg (4 mL) dose of nebulised budesonide Control (N = 25): single 4 mL dose of placebo (0.9% saline solution) Both treatment and control delivered by an updraft nebuliser with a continuous flow of oxygen at a rate of 5 to 6 L/min. |
|
| Outcomes | Change in modified Westley croup score from baseline to 4 hours; admissions to the hospital; 2‐point improvement in croup score at 4 hours; use of epinephrine, use of supplemental glucocorticoids, and use of mist tent | |
| Notes | Funding source: Ontario Ministry of Health | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 10 by the pharmacy department, using a random numbers table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed... by the pharmacy department" "the pharmacy provided both budesonide and normal saline in opaque, brown syringes." "the randomization code was revealed only after all patients had completed the trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Budesonide is slightly opaque; therefore, to conceal its identity, the pharmacy provided both budesonide and normal saline in opaque, brown syringes. The research assistants placed the drugs directly into an opaque nebulizer reservoir. Once nebulized, the drugs were indistinguishable by sight and smell." "Both the research assistants and the physicians caring for the patients in the emergency department were blinded to treatment assignment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Budesonide is slightly opaque; therefore, to conceal its identity, the pharmacy provided both budesonide and normal saline in opaque, brown syringes. The research assistants placed the drugs directly into an opaque nebulizer reservoir. Once nebulized, the drugs were indistinguishable by sight and smell." "Both the research assistants and the physicians caring for the patients in the emergency department were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2% (N = 1) in the placebo group required racemic epinephrine and was excluded; 2% (N = 1) lost to follow‐up in the treatment group because parent could not be contacted for follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: 33 children were not enrolled because the study team was not contacted; this could potentially have biased participant selection. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Klassen 1998.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1995 to April 1996 and October 1996 to January 1997 Setting: emergency departments of the Children's Hospital of Eastern Ontario, Ottawa, or the Winnipeg Children's Hospital, Winnipeg, Canada Inclusion criteria: children aged 3 months to 5 years who presented to the emergency department with croup (hoarseness, inspiratory stridor, and barking cough) and Westley croup score of ≥ 2 following at least 15 minutes of mist therapy; parents available for telephone follow‐up a week after enrolling in the study Exclusion criteria: epiglottitis; chronic respiratory disease (except asthma); severe croup (Westley croup score ≥ 8); racemic epinephrine treatment upon arriving at the emergency department; glucocorticoids in the last 2 weeks; history of tuberculosis in child or household; chickenpox or exposure to it within the past 21 days; known immunodeficiency Baseline demographics (N = 198): proportion males: treatment 1: 77%; treatment 2: 62%; treatment 3: 64% median (25th, 75th percentile) age in years: treatment 1: 1.5 (1.0, 2.2); treatment 2: 1.3 (0.8, 2.1); treatment 3: 1.6 (1.0, 2.5) mean (95% CI) Westley croup score: treatment 1: 3.5 (3.2 to 3.7); treatment 2: 3.6 (3.3 to 3.8); treatment 3: 3.8 (3.5 to 4.0) |
|
| Interventions | Treatment 1 (N = 65): single 2 mg (4 mL) dose of nebulised budesonide plus the appropriate volume of oral placebo (clear syrup solution) Treatment 2 (N = 69): single 4 mL dose of nebulised placebo (saline solution) plus 0.6 mg/kg oral dexamethasone Treatment 3 (N = 64): single 4 mL dose of nebulised budesonide plus 0.6 mg/kg of oral dexamethasone |
|
| Outcomes | Change in Westley croup score from baseline to 4 hours (or until discharge); admissions to hospital; length of stay in the emergency department; 2‐point improvement in croup score at 4 hours; use of epinephrine and use of additional glucocorticoids | |
| Notes | Funding source: Ontario Ministry of Health; Emergency Health Services, Toronto, Ontario; Manitoba Medical Services Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A central pharmacy... randomized individuals to the 3 groups, using computer‐generated random numbers in random blocks of 6 or 9" |
| Allocation concealment (selection bias) | Low risk | Quote: "the list was kept at a central pharmacy until the end of the study to ensure allocation concealment. Because the drugs were packaged identically and identified only by a sequential study number, the research assistant who administered the intervention remained unaware of the next group assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Dexamethasone syrup and placebo dexamethasone syrup were identical in taste and appearance... All solutions were packaged in brown syringes and the research assistant instilled either solution directly into an opaque nebulizer reservoir." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Dexamethasone syrup and placebo dexamethasone syrup were identical in taste and appearance... All solutions were packaged in brown syringes and the research assistant instilled either solution directly into an opaque nebulizer reservoir." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: intention‐to‐treat analysis used. 1% (N = 1) loss to follow‐up in the treatment group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: 10 children were not enrolled because the study team was not contacted; this could potentially have biased participant selection. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Koren 1983.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: January 1979 to January 1980 Setting: paediatric division of the Chaim Sheba Medical Center, Tel Hashomer, Israel Inclusion criteria: children aged from 8 months to 8 years hospitalised with croup (all with inspiratory stridor, dyspnoea, subcostal and/or suprasternal retraction, and a barking cough); informed consent given by the parents Exlcusion criteria: evidence of associated bronchitis, bronchopneumonia, and acute epiglottitis Baseline demographics (N = 78): proportion males:laryngotracheitis (LT) group: treatment: 59%; control: 56%; spasmodic croup (SC) group: treatment: 57%; control: 64% mean age in years, months:LT group: treatment: 2 years, 5 months; control: 2 years, 7 months; SC group: treatment: 2 years, 6 months; control: 2 years, 8 months croup score: not measured |
|
| Interventions | Children in all groups were sedated via rectal administration of 75 mg/kg of body weight of chloral hydrate. Treatment (N = 40): single 0.60 mg/kg dose of intramuscular dexamethasone sodium phosphate (4 mg/mL) Control (N = 38): single dose of intramuscular placebo solution of identical appearance |
|
| Outcomes | Use of epinephrine and use of antibiotics | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Dexamethasone sodium phosphate and a placebo solution of identical appearance were administered" "All evaluations were completed before the study code was opened" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Kuusela 1988.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1984 to March 1985 and January 1986 to April 1986 Setting: Department of Paediatrics, Tampere University Central Hospital, Finland Inclusion criteria: children diagnosed and admitted for acute laryngitis (croup) Exclusion criteria: not reported Baseline demographics (N = 72): proportion males: treatment 1: 74%; treatment 2: 88%; treatment 3: 88%; control: 67% mean (SD) age in years: treatment 1: 2.9 (1.5); treatment 2: 2.3 (1.7); treatment 3: 2.8 (1.8); control: 3.2 (2.7) croup score: not measured |
|
| Interventions | All children received initial treatment in the emergency department, then were transferred to a ward where they were placed in a humid room and given oral fluids at a minimum of 20 mL/kg body weight over the next 4 hours. Treatment 1 (N = 19): single 0.6 mg/kg (0.12 mL/kg, maximum 2 mL) dose of intramuscular dexamethasone plus at least 1 0.25 mL/5 kg (maximum 1.5 mL) dose of nebulised L‐epinephrine (2.25% epinephrine base with 0.5% chlorobutanol as preservative). Additional doses of nebulised L‐epinephrine every 2 hours as needed Treatment 2 (N = 16): single 0.6 mg/kg (0.12 mL/kg, maximum 2 mL) dose of intramuscular dexamethasone plus at least 1 0.25 mL/5 kg (maximum 1.5 mL) dose of nebulised placebo solution. Additional doses of nebulised placebo every 2 hours as needed Treatment 3 (N = 16): single 0.6 mg/kg (0.12 mL/kg, maximum 2 mL) dose of intramuscular placebo plus at least 1 0.25 mL/5 kg (maximum 1.5 mL) dose of nebulised L‐epinephrine (2.25% epinephrine base with 0.5% chlorobutanol as preservative). Additional doses of nebulised L‐epinephrine every 2 hours as needed Control (N = 21): single 0.6 mg/kg (0.12 mL/kg, maximum 2 mL) dose of intramuscular placebo plus at least 1 0.25 mL/5 kg (maximum 1.5 mL) dose of nebulised placebo solution. Additional doses of nebulised placebo every 2 hours as needed |
|
| Outcomes | Change in clinical score based on dyspnoea and cough scale from baseline to 6, 12, and 24 hours; length of stay in hospital | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Low risk | Quote: "the ampules were unlabelled, numbered, and randomized; the code was not available for investigators until the end of the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo preparation consisted of the corresponding diluent supplied in similar ampules." "the code was not available for the investigators until the end of the study" "The active solution and the placebo preparation were identically packed in individual randomized vials containing 10 ml" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: did not use intention‐to‐treat analysis. 8% (N = 6) excluded due to protocol violations, unclear what group they were in. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Leipzig 1979.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: November 1976 to March 1978 Setting: Pediatric Service of the State University Hospital or the Crouse‐Irving Memorial Hospital, Syracuse, NY, USA Inclusion criteria: all children admitted to hospital with a diagnosis of croup with disease of sufficient severity on a predetermined scoring system; consent of the child's physician and parents Exclusion criteria: not reported Baseline demographics (N = 30): proportion males: not reported mean age in months: treatment: 21.3; control: 21.0 mean (SD) croup score: treatment: 8.46 (1.45); control: 8.14 (1.46) |
|
| Interventions | Treatment (N = 16): 2 0.30 mg/kg doses of intramuscular dexamethasone (4 mg/mL) Control (N = 14): 2 doses of intramuscular placebo (sterile saline) (1 dose initially and another 2 hours later) |
|
| Outcomes | Change in croup score from baseline to 24 hours; length of stay at hospital; intubation | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "assigned from a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Vials had been previously prepared containing either dexamethasone (4 mg/L) or sterile saline. They were marked only with a number, assigned from a table of random numbers" Comment: described as double‐blind. Unclear who was blinded and who prepared the vials. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Vials had been previously prepared containing either dexamethasone (4 mg/L) or sterile saline. They were marked only with a number, assigned from a table of random numbers" Comment: described as double‐blind. Unclear who was blinded and who prepared the vials. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Luria 2001.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: September 1995 to December 1997 Setting: emergency departments at either Children's Hospital Medical Center in Cincinnati or Children's Hospital in Columbus, OH, USA Inclusion criteria: children aged 6 months to 6 years presenting to the emergency department with mild croup (barky cough, stridor and/or hoarseness for < 48 hours) and having a viral prodrome consisting of fever, cough, or rhinorrhoea (in an attempt to exclude children with spasmodic croup) Exclusion criteria: treated with corticosteroids 14 days prior to enrolling in the study; a clinical picture consistent with spasmodic croup; history of prolonged endotracheal intubation; history of chronic respiratory illness (i.e. asthma or cystic fibrosis); a condition associated with airway abnormalities; those without a working telephone or those with a severe disease (i.e. received nebulised racemic epinephrine or corticosteroids at the order of the emergency department physician or had < 94% oxygen saturation) Baseline demographics (N = 264): proportion males: treatment 1: 72%; treatment 2: 64%; control: 65% mean (range) age in months: treatment 1: 28 (6 to 70); treatment 2: 31 (6 to 71); control: 26 (6 to 71) mean (range) modified Westley croup score: treatment 1: 1.6 (0 to 6); treatment 2: 1.6 (0 to 5); control: 1.7 (0 to 5) |
|
| Interventions | Treatment group 1 (N = 85): single 0.60 mg/kg (maximum 10 mg) dose of oral dexamethasone (1 mg/mL) plus nebulised placebo solution Treatment group 2 (N = 91): single dose of oral placebo plus a 160 μg dose of nebulised dexamethasone sodium phosphate Control (N = 88): single dose of oral placebo plus nebulised placebo solution Nebulised study preparations were delivered with a nebuliser that had a fill volume of 3 mL and the oxygen flow set at 5 to 6 L/min. |
|
| Outcomes | Return visits to the emergency department | |
| Notes | Funding source: the Bremer Foundation at the Ohio State University, Columbus, OH, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization was performed in blocks of 15 by the study pharmacist at each enrolling site with the use of a random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "The study pharmacist then assembled numbered "croup kits" containing study preparations that reflected the results of the randomization." "The study physician retrieved the lowest numbered kit when enrolling a new subject to maintain the randomization order. Only pharmacists knew the results of the randomization." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The kits were sealed to prevent any tampering and were kept in the EDs... Only the study pharmacists knew the results of the randomization... All oral study preparations were mixed 1:1 with a commercially available grape flavouring to minimize taste bias." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: used intention‐to‐treat analysis, including N = 9 protocol deviations. 16% (N = 43) loss on day 7 for the telephone follow‐up (14% in the oral treatment group, 15% in the nebulised treatment group, 19% in the placebo group) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Martinez Fernandez 1993.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1989 to September 1990 Setting: Children's Hospital in Spain Inclusion criteria: children hospitalised with symptoms suggestive of croup (acute laryngitis, laryngotracheobronchitis, spasmodic croup) Exclusion criteria: child's croup judged by the physician to be too severe Baseline characteristics (N = 66): proportion males: not reported age: not reported mean (SD) croup score: treatment 1: 3.5 (1.7); treatment 2: 2.9 (1.4); treatment 3: 3.3 (1.1); control: 3.2 (1.5) |
|
| Interventions | Treatment 1 (N = 15): single dose of intramuscular placebo, plus 0.14% nebulised L‐epinephrine initially and every 4 hours as needed Treament 2 (N = 16): single 0.5 mg/kg dose of intramuscular dexamethasone, plus nebulised placebo (saline) initially and every 4 hours as needed Treatment 3 (N = 18): single 0.5 mg/kg dose of intramuscular dexamethasone, plus 0.14% nebulised L‐epinephrine initially and every 4 hours as needed Control (N = 17): single dose of intramuscular placebo, plus nebulised placebo (saline) initially and every 4 hours as needed All children received humidified oxygen and fluid therapy. |
|
| Outcomes | Change in croup score from baseline to 6, 12, and 24 hours | |
| Notes | Written in Spanish; funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Low risk | Comment: treatments shipped in pre‐numbered ampoules, unlabeled and randomly ordered by the pharmacy. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: described as double‐blind. Treatments shipped in pre‐numbered ampoules, unlabeled and randomly ordered by the pharmacy. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Massicotte 1973.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: December 1971 to March 1972 Setting: L'Hôpital Sainte‐Justine, Montreal, Quebec, Canada Inclusion criteria: children admitted to hospital with severe croup (croup score ≥ 9) Exclusion criteria: mild or moderate croup (croup score < 9); require tracheotomy on admission (altered consciousness, peribuccal cyanosis); those with epiglottitis, foreign body aspiration, diphtheria, pharyngeal abscess, acute or chronic medical conditions; corticosteroids in the past 48 hours, allergy to penicillin or ampicillin Baseline characteristics (N = 42): proportion males: treatment: 80%; control: 80% age: not reported, most < 4 years mean croup score:acute onset: treatment: 12.55; control: 12.33; progressive onset: treatment: 12.38; control: 12.27 |
|
| Interventions | All children were placed in a humidified room and received intravenous saline; all children additionally received 100 mg ampicillin/24 hours in 4 doses (1 every 6 hours) over the course of 10 days. Treatment (N = 25): single 4 mg/kg dose of intravenous methyl‐prednisolone initially (40 mg for 6 to 8 kg; 60 mg for 9 to 12 kg; 80 mg for 13 to 16 kg; 120 mg for 17 to 20 kg), followed by repeated doses at 4 and 8 hours, if needed Control (N = 17): single 4 mg/kg dose of intravenous placebo (lactose) initially, followed by repeated doses at 4 and 8 hours, if needed |
|
| Outcomes | Change in croup score from baseline to 4 and 14 hours; patient improvement at 4 and 14 hours | |
| Notes | Written in French; funding source: Upjohn Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: used a random numbers table |
| Allocation concealment (selection bias) | Low risk | Comment: the treatments were identical in appearance, containing either methyl‐prednisolone or placebo. Administration was double‐blind, and the code was not broken until the last child had completed the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: the treatments were identical in appearance, containing either methyl‐prednisolone or placebo. Administration was double‐blind, and the code was not broken until the last child had completed the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Rittichier 2000.
| Methods | Randomised controlled trial | |
| Participants |
Study period: October 1996 to June 1999 Setting: emergency department at the Children's Hospital in Denver, CO, USA Inclusion criteria: children aged 3 months to 12 years presenting to the emergency department with moderate croup (hoarseness and barky cough associated with either a history/presence of stridor at rest, and/or retractions) of < 48 hours onset of illness (defined as onset of barky cough) Exclusion criteria: children with epiglottitis; foreign body aspiration; reactive airway exacerbation; acute bacterial pneumonia; acquired or congenital upper airway anomalies such a tracheomalacia; immunocompromised; history of steroid exposure in the previous 2 weeks; children with mild croup (history or presence of a barky cough without the presence/history of the associated stridor or retractions); children with severe croup who had altered mental status, severe retractions, cyanosis associated with their croup; admitted to the hospital during the initial emergency department visit, either before consideration or after being enrolled in the study Baseline demographics (N = 277): proportion male: treatment 1: 70%; treatment 2: 69% median (SD) age in years: treatment 1: 2.03 (1.81); treatment 2: 2.01 (1.84) mean croup score: treatment 1: 2.09; treatment 2: 1.95 |
|
| Interventions | All children were given a cool mist therapy per emergency department protocol. Treatment 1 (N = 139): single 0.60 mg/kg (maximum 8 mg) dose of intramuscular dexamethasone Treatment 2 (N = 138): single 0.60 mg/kg (maximum 8 mg) dose of oral dexamethasone |
|
| Outcomes | Change in Westley croup score from baseline to 24 hours; unscheduled return visits to the emergency department; use of epinephrine, use of additional glucocorticoids, use of mist tent, and use of antibiotics | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a random allocation chart based on a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomization code was held by the nursing staff in the ED" Comment: randomisation code was held by nurses in the ED, and enrolment was performed by physicians, fellows, and residents. Unclear whether they could have determined the allocation sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Nurses administered the dexamethasone either orally or intramuscularly per hospital protocol." Comment: no blinding of participants and personnel. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "To help with blinding of the physician... Band‐Aids were placed on all patients whether they received PO or IM medicine." "Caretakers were contacted... by a caller who was blinded to the route of administration... Caretakers were instructed to not disclose the route of administration of the medicine to the caller." Comment: blinding was attempted but could have been broken. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 16% lost to follow‐up (N = 13 protocol deviations; N = 27 could not be reached, of which data for N = 10 were lost in a hospital move). Unclear if losses were balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Roberts 1999.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: April 1994 to April 1996 Setting: infectious diseases ward of the Women's and Children's Hospital in North Adelaide, Australia Inclusion criteria: children aged 6 months to 8 years admitted with croup (inspiratory stridor, barking cough, hoarse voice, and respiratory distress) and a croup score of ≥ 4; stable croup (2 scores taken 15 minutes apart within 1 point of each other); written informed consent from the parent Exclusion criteria: suspected epiglottitis, foreign body aspiration, bronchiolitis, pneumonia or active asthma; intubation due to airways disease in the previous 12 months; acute wheezing; treatment with corticosteroids in the previous 4 weeks; treatment with adrenaline in the previous week; significant past or present pulmonary, cardiovascular, renal, hepatic, gastrointestinal, neurological, or endocrine disease that could interfere with the study; children unable to inhale the nebuliser mist for at least 1.5 minutes Baseline demographics (N = 82): proportion males: treatment: 76%; control: 78% mean (SD) age in years: treatment: 2.3 (1.4); control: 2.2 (1.0) mean (SD) croup score: treatment: 6.4 (1.5); control: 6.3 (1.4) |
|
| Interventions | Treatment (N = 42): 2 mg/4 mL dose of nebulised budesonide every 12 hours for a maximum of 4 doses Control (N = 40): dose of nebulised placebo (same formulation but without budesonide) every 12 hours for a maximum of 4 doses Both treatment and placebo driven by an air or oxygen flow of 6 L/min. |
|
| Outcomes | Change in croup score from baseline to 2, 6, 12, 24 hours; revisits to the hospital for follow‐up; 2‐point improvement in croup score at 2, 6, and 12 hours; use of epinephrine | |
| Notes | Used a croup score similar to Leipzig 1979 with alterations to stridor assessment, oxygen saturation, and temperature; funding source: Astra Draco | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer‐generated randomization in blocks of six were performed by Astra Draco" |
| Allocation concealment (selection bias) | Low risk | Quote: "All study medication was packaged identically, identified only by a study number... the randomisation code was kept at Astra Craco and broken only after study completion, hence all treatment decisions were made without awareness of study allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All respules and all ventstreams used in the study were made of an opaque plastic to conceal any differences between the active and placebo doses" "The randomization code was kept at Astra Draco and only broken after study completion" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All respules and all ventstreams used in the study were made of an opaque plastic to conceal any differences between the active and placebo doses" "The randomization code was kept at Astra Draco and only broken after study completion" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 20% (N = 10) lost due to withdrawals, protocol deviations, inability to contact parents. Used the last value extended principle for analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Roorda 1998.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1995 to March 1997 Setting: hospital in the Netherlands Inclusion criteria: children aged 4 to 52 months hospitalised with moderate croup Exlusion criteria: received systemic steroids, any bronchodilators, or antibiotics in the previous 48 hours Baseline demographics (N = 17): proportion male: treatment: 89%; control: 63% mean (range) age in months: treatment: 29 (6 to 44); control: 38 (4 to 52) mean (range) modified Westley croup score: treatment: 3.1 (1 to 5); control: 2.9 (1 to 8) |
|
| Interventions | Treatment (N = 9): 2 doses of 1000 µg of fluticasone propionate administered by metred dose inhaler (4 puffs of 250 µg), 30 minutes apart Control (N = 8): placebo administered in a similar fashion |
|
| Outcomes | Change in modified Westley croup score from baseline to 2, 6, and 24 hours; length of stay in hospital; use of additional glucocorticoids and intubation | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as double‐blind. Unclear who was blinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: described as double‐blind. Unclear who was blinded. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Skowron 1966a.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: December 1964 to March 1965 Setting: tracheitis ward of The Hospital for Sick Children, Toronto, Canada Inclusion criteria: children hospitalised with croup Exclusion criteria: not reported Baseline demographics (N = 200 in total, N = 94 for 1.0 mL dexamethasone compared to placebo, 6 excluded): proportion males: 77% mean age in years: 2.3 croup score: not measured |
|
| Interventions | 1.0 mL (4 mg) dexamethasone compared to placebo (see Skowron 1966b for 1.5 mL (6 mg) dexamethasone compared to placebo) All children were placed in a croupette with moist air, given twice daily intramuscular crystalline sodium penicillin (825,000 IU/day) and streptomycin sulphate (0.5 g), as well as secobarbital, 3/4 grain per rectum on admission for children over 6 months. Treatment (N = 41): 1.0 mL (4 mg, based on approximately 0.4 mg/kg) subcutaneous dexamethasone every 6 hours for a total of 4 doses Control (N = 53): 1.0 mL placebo every 6 hours for a total of 4 doses |
|
| Outcomes | Readmissions to the hospital; length of stay in the hospital; tracheotomy | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators. As each child was admitted to the series, he received subcutaneously either material A or material B, according to a random selection code" Comment: bottles were not sequentially numbered, but instead labelled A or B. Unclear where the random selection code was held |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators." "After the results were documented, the code was broken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators." "After the results were documented, the code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3% (N = 6) lost due to protocol deviations |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: no baseline data presented, impossible to judge if baseline imbalances existed. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Skowron 1966a&b.
| Methods | See Skowron 1966a and Skowron 1966b | |
| Participants | See Skowron 1966a and Skowron 1966b | |
| Interventions | See Skowron 1966a and Skowron 1966b | |
| Outcomes | See Skowron 1966a and Skowron 1966b | |
| Notes | See Skowron 1966a and Skowron 1966b | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Skowron 1966a and Skowron 1966b |
| Allocation concealment (selection bias) | Unclear risk | See Skowron 1966a and Skowron 1966b |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See Skowron 1966a and Skowron 1966b |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See Skowron 1966a and Skowron 1966b |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Skowron 1966a and Skowron 1966b |
| Selective reporting (reporting bias) | Unclear risk | See Skowron 1966a and Skowron 1966b |
| Other bias | Unclear risk | See Skowron 1966a and Skowron 1966b |
| Overall risk of bias All outcomes | Unclear risk | See Skowron 1966a and Skowron 1966b |
Skowron 1966b.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: December 1964 to March 1965 Setting: tracheitis ward of The Hospital for Sick Children, Toronto, Canada Inclusion criteria: children hospitalised with croup Exclusion criteria: not reported Baseline demographics (N = 200 in total, N = 100 for 1.5 mL dexamethasone compared to placebo): proportion males: 77% mean age in years: 2.3 croup score: not measured |
|
| Interventions | 1.5 mL (6 mg) dexamethasone compared to placebo (see Skowron 1966a for 1.0 mL dexamethasone compared to placebo) All children were placed in a croupette with moist air, given twice daily intramuscular crystalline sodium penicillin (825,000 IU/day) and streptomycin sulphate (0.5 g), as well as secobarbital, 3/4 grain per rectum on admission for children over 6 months. Treatment (N = 56): 1.5 mL (6 mg, based on approximately 0.5 mg/kg) subcutaneous dexamethasone every 6 hours for a total of 4 doses Control (N = 44): 1.5 mL placebo every 6 hours for a total of 4 doses |
|
| Outcomes | Readmissions to the hospital; length of stay in the hospital; tracheotomy | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators. As each child was admitted to the series, he received subcutaneously either material A or material B, according to a random selection code" Comment: bottles were not sequentially numbered, but instead labelled A or B. Unclear where the random selection code was held |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators." "After the results were documented, the code was broken" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The test material was provided in labelled vials, the content of which was unknown to the investigators." "After the results were documented, the code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3% (N = 6) lost due to protocol deviations |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: no baseline data presented, impossible to judge if baseline imbalances existed. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Soleimani 2013.
| Methods | Randomised single‐blind controlled trial | |
| Participants |
Study period: January 2009 to March 2010 Setting: emergency department at Ali‐Ebne Abitaleb Hospital, Iran Inclusion criteria: children aged 6 months to 6 years admitted to the emergency department with barking cough, stridor, hoarseness, and respiratory distress Exclusion criteria: chronic pulmonary disease; severe croup (croup score > 7); recurrent croup; allergy to corticosteroids; contraindication of corticosteroid (history of tuberous sclerosis, history of varicella infection during the past 3 weeks); history of corticosteroid administration during the last 4 weeks; foreign body; epiglottitis; bacterial tracheitis; immune deficiency Baseline demographics (N = 68): proportion males: 53% mean (SD) age in months: 26.3 (1.5) mean (SD) croup score: treatment 1: 1.81 (0.59); treatment 2: 2.03 (0.47) |
|
| Interventions | Treatment 1 (N = 36): single dose 0.60 mg/kg intramuscular dexamethasone Treatment 2 (N = 32): single dose 0.60 mg/kg oral dexamethasone |
|
| Outcomes | Return visits or (re)admissions or both | |
| Notes | Not indexed in the databases searched (located via a search of Google.ca); funding source: Zahedan University of Medical Sciences, Zahedan, Iran | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as "randomly divided"; unclear how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: participants and personnel were not blinded. Treatments were clearly distinguishable. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: outcome assessor described as blinded. Unclear how they were blinded and if the blinding could have been broken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 15% loss to follow‐up; unclear from which group children were lost. No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Sparrow 2006.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: emergency department of Princess Margaret Hospital for Children, Perth, Australia Inclusion criteria: children older than 3 months who presented to the emergency department with mild to moderate croup defined by clinical symptoms (acute onset of inspiratory stridor and a hoarse voice accompanied by a barking cough) and a modified Taussig croup score of < 5 Exlcusion criteria: children whose families did not have a telephone; limited English language proficiency; received steroids Baseline demographics (N = 133): proportion males: treatment: 74%; comparator: 63% mean (SD) age in months: treatment: 45 (31.6); comparator: 37 (28.8) mean (SD) modified Taussig croup score: treatment: 2.0 (1.2); comparator: 2.0 (1.3) |
|
| Interventions | Treatment (N = 65): single 1 mg/kg dose of oral prednisolone Comparator (N = 68): single 0.15 mg/kg dose of oral dexamethasone |
|
| Outcomes | Unscheduled re‐presentations to medical care; time spent in the emergency department; use of epinephrine | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computer generated orders randomised into blocks of 10" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The PMH pharmacy ensured that the two steroid preparations could not be differentiated, the code being held by the pharmacy. Bottles were simply labelled solution A or solution B" Comment: bottles were not sequentially numbered, but instead labelled A or B. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Bottles were simply labelled solution A or solution B, and following randomization... given by a nurse who took no further part in the child's care." "the code was not broken until all data were collected and all follow up completed" "similar taste and appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Unclear risk | Comment: many children not approached because the emergency department was busy in the winter; this could potentially have biased participant selection. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Super 1989.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: October 1983 to April 1985 Setting: Cleveland Metropolitan General Hospital or Rainbow Babies and Childrens Hospital, OH, USA Inclusion criteria: children admitted to hospital with a diagnosis of croup (barking cough, inspiratory stridor, hoarseness) with a viral prodrome consisting of rhinorrhoea, cough, or fever and a modified Westley croup score ≥ 3 after 30 minutes of mist therapy Exclusion criteria: clinical picture consistent with acute epiglottitis, spasmodic croup, or pneumonia; history of a chronic illness except asthma; history of tracheal intubation or laryngoscopy Baseline demographics (N = 29): proportion male: treatment: 68%; control: 62% mean (SD) age in months: treatment: 15.5 (5); control: 15.8 (12) median (range) modified Westley croup score: treatment: 4.5 (3 to 7); control: 5.0 (3 to 6) |
|
| Interventions | All children received mist therapy for at least 30 minutes. Treatment (N = 16): single 0.6 mg/kg dose of parenteral dexamethasone Control (N = 13): single dose of parenteral placebo (saline) |
|
| Outcomes | Change in modified Westley croup score from baseline to 12 and 24 hours; length of stay in hospital; 2‐unit improvement in croup score at 12 and 24 hours; use of supplemental glucocorticoids and use of mist tent | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned, by a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "either parenterally administered dexamethasone or saline solution of the same color, volume and consistency as the dexamethasone. Randomization and drug preparation were done in the pharmacy" "The drug code was broken only after the last patient completed the study" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Neither the patient nor the investigators knew whether the patient received dexamethasone or placebo. The drug code was broken only after the last patient completed the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Neither the patient nor the investigators knew whether the patient received dexamethasone or placebo. The drug code was broken only after the last patient completed the study." "All decisions regarding the data analysis were made before the drug code was broken. Whenever possible these decisions were made in favour of the null hypothesis" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 17% (N = 5) lost to follow‐up (19% in treatment group due to early discharge and protocol deviation, 15% in placebo group due to early discharge or missed observations) |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Tibballs 1992.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: not reported Setting: Royal Children's Hospital, Melbourne, Australia Inclusion criteria: hospitalised children aged 6 months or older who required endotracheal intubation for upper airway obstruction caused by croup (defined as coryzal symptoms, fever, barking cough, hoarse voice, retraction, inspiratory stridor, or cyanosis developing over several days) Exclusion criteria: children younger than 6 months old; congenital airway anomalies; previous intubations; spasmodic croup (sudden onset without preceding fever or symptoms of upper respiratory tract infection) Baseline demographics (N = 70, 3 excluded): proportion males: treatment: 63%; control: 66% mean (range) age in months: treatment: 19 (6 to 99); control: 19 (6 to 83) croup score: not measured |
|
| Interventions | All children received endotracheal intubation under inhalational anaesthesia with halothane, first with an oral endotracheal tube, in order to secure the airway rapidly and assess the diameter, and second substituted with a nasal tube. Humidification was provided with heat and moisture exchangers, with oxygen added as required. The tube was aspirated routinely every 1 to 2 hours to remove secretions. Treatment (N = 38): 1 mg/kg nasogastric prednisolone within 24 hours of intubation and then every 12 hours until 24 hours after extubation Control (N = 32, 3 excluded): 1 mg/kg of placebo within 24 hours of intubation and then every 12 hours until 24 hours after extubation |
|
| Outcomes | Use of epinephrine | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "order determined by a table of random numbers" |
| Allocation concealment (selection bias) | Low risk | Quote: "Unidentified placebo and prednisolone were supplied by the pharmacy in an order determined by a table of random numbers" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind" "Unidentified placebo and prednisolone were supplied by the pharmacy" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no description of a third‐party outcome assessor. Carried over judgement from blinding of participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 4% (N = 3) lost because of exclusion due to bacterial infection or protocol deviations, all in the placebo group |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
Vad Pedersen 1998.
| Methods | Randomised controlled trial | |
| Participants |
Study period: October 1989 to September 1990 Setting: paediatric department of Esbjerg Centralsygehus (Central Hospital), Denmark Inclusion criteria: children hospitalised with croup based on a modified Westley croup score ≥ 3 (including chest wall retractions, barking cough, respiratory frequency, and stridor) Exclusion criteria: required immediate intensive care; clinical suspicion of epiglottitis; cyanosis; croup recurrence; had received local or systemic steroid treatment; being treated with carbamazepine, phenobarbital, or rifampicin Baseline demographics (N = 59, 2 excluded): proportion males: 63% mean age in months: treatment 1: 23.8; treatment 2: 24.1 mean (SD) croup score: treatment 1: 3.67 (1.02); treatment 2: 4.17 (0.99) |
|
| Interventions | Treatment 1 (N = 27): 2, 1000 μg doses of inhaled budesonide at 30‐minute intervals Treatment 2 (N = 29): single 0.6 mg/kg (0.15 mL/kg) dose of intramuscular dexamethasone |
|
| Outcomes | Change in croup score from baseline to 6 and 12 hours; return visits to the hospital; use of supplemental glucocorticoids | |
| Notes | Written in Danish; funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: block randomisation with varying block size |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: unblinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: unblinded. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3% (N = 2) who were randomised were excluded due to protocol deviations. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | High risk | Comment: baseline imbalance in croup score |
| Overall risk of bias All outcomes | High risk | Comment: at least 1 domain judged as high risk. |
Von Mühlendahl 1982.
| Methods | Randomised double‐blind controlled trial | |
| Participants |
Study period: January 1979 to April 1980 Setting: 3 paediatric clinics in West Berlin, Germany Inclusion criteria: children admitted to hospital with a diagnosis of pseudo‐croup to 1 of 3 paediatric clinics in West Berlin Exclusion criteria: children who were already somnolent or cyanotic at admission (stage III or IV or pseudo‐croup) Baseline demographics (N = 406; 349 included in the evaluation): proportion males: not reported age distribution: treatment: 15 were < 1 year; 50 were 1 to 1 11/12 years; 96 were 2 to 5 11/12 years; 15 were 6 to 10 11/12 years control: 11 were < 1 year; 44 were 1 to 1 11/12 years; 107 were 2 to 5 11/12 years; 11 were 6 to 10 11/12 years croup score: treatment: 77 had a score of 1 to 3; 99 had a score ≥ 4 control: 67 had a score of 1 to 3; 106 had a score ≥ 4 |
|
| Interventions | Treatment (N = 176): single dose 6 mg dose oral dexamethasone Control (N = 173): single dose 6 mg oral placebo |
|
| Outcomes | Change in croup score from baseline to 6 and 12 hours | |
| Notes | Funding source: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided to judge. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: described as double‐blind. Unclear who was blinded. Subjective outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: described as double‐blind. Unclear who was blinded. Subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: of 406 children, 57 (14%) failed to complete the study. 24 (7%) were eliminated due to protocol violation; 19 (5%) received further doses of steroids; and 4 (1%) developed measles. Unclear what group the lost children were in. Did not use intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol identified. All prespecified outcomes from methods appear in results. |
| Other bias | Low risk | Comment: no other sources of bias identified. |
| Overall risk of bias All outcomes | Unclear risk | Comment: at least 1 domain judged as unclear risk, and no domains judged as high risk. |
CI: confidence interval ED: emergency department SD: standard deviation SE: standard error
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anene 1996 | Randomised controlled trial; children were not diagnosed with croup |
| Bollobas 1965 | Not a randomised controlled trial; children were not diagnosed with croup |
| Cichy 1983 | Not a randomised controlled trial |
| Connolly 1969 | Randomised controlled trial; children were not diagnosed with croup |
| Couser 1992 | Randomised controlled trial; children were not diagnosed with croup |
| Eghbali 2016 | Randomised controlled trial; the intervention was L‐epinephrine |
| Faghihinia 2007 | Randomised controlled trial; no usable results were presented (not clear how many children were in each group) |
| Faraji‐Goodarzi 2018 | Randomised controlled trial; no relevant outcomes |
| Flisberg 1973 | Not a randomised controlled trial |
| Freezer 1990 | Not a randomised controlled trial; study was a retrospective chart review |
| Gill 2017 | Not a randomised controlled trial |
| Goddard 1967 | Randomised controlled trial; children were not diagnosed with croup |
| Haque 1981 | Not a randomised controlled trial; children in the control group received no treatment |
| Havaldar 1997 | Not a randomised controlled trial; children were not diagnosed with croup |
| Kelley 1992 | Not a randomised controlled trial; study was a retrospective chart review |
| Kunkel 1996 | Not a randomised controlled trial; intervention was epinephrine |
| Ledwith 1995 | Not a randomised controlled trial; intervention was epinephrine |
| Martensson 1960 | Not a randomised controlled trial |
| McDonogh 1994 | Not a randomised controlled trial; study was a retrospective chart review |
| Mohammadzadeh 2014 | Randomised controlled trial; intervention was epinephrine |
| NCT01748162 | Randomised controlled trial; did not report any relevant outcomes |
| Novik 1960 | Not a randomised controlled trial |
| Osváth 1994 | Not a randomised controlled trial |
| Prendergast 1994 | Not a randomised controlled trial; intervention was epinephrine |
| Rizos 1998 | Not a randomised controlled trial |
| Roked 2015 | Not a randomised controlled trial; study was a retrospective chart review |
| Ross 1969 | Not a randomised controlled trial; study was a retrospective chart review |
| Serra 1997 | Not a randomised controlled trial |
| Sumboonnanonda 1997 | Randomised controlled trial; children in the control group received no treatment |
| Sussman 1964 | Randomised controlled trial; children were not diagnosed with croup |
| Tal 1983 | Randomised controlled trial; children were not diagnosed with croup |
| Tellez 1991 | Randomised controlled trial; children were not diagnosed with croup |
| Wilhelmi 1976 | Not a randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12609000290291.
| Trial name or title | Oral prednisolone compared to oral dexamethasone (in 2 different doses) in children with croup: randomised clinical trial comparing the improvement in Westley croup score |
| Methods | Randomised controlled trial (parallel) |
| Participants |
Inclusion criteria: children aged 6 months to 10 years with a clinical diagnosis of croup (laryngotracheitis, laryngotracheobronchitis); weight no more than 20 kg; parents could be contacted by telephone; parents speak English Exclusion criteria: high clinical suspicion of an alternative diagnosis; known allergy to prednisolone or dexamethasone; immunosuppressive disease or treatment; steroid therapy in the past 14 days; enrolment in the present study in the past 14 days (i.e. repeat enrolment) |
| Interventions | Treatment 1: single 1 mg/kg dose of oral prednisolone Treatment 2: single 0.15 mg/kg dose of oral dexamethasone |
| Outcomes | Westley croup score; unscheduled medical re‐attendance/readmission; length of stay in hospital; use of additional treatments |
| Starting date | 18 March 2009 |
| Contact information | Dr Colin Parker. Princess Margaret Hospital for Children, Roberts Road Subiaco 6008 Western Australia, Australia. Colin.Parker@health.wa.gov.au |
| Notes | Trial registration at the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (ACTRN12609000290291); funding source: Princess Margaret Hospital Foundation |
Differences between protocol and review
New authors joined the review group for the 2018 update: Allison Gates, Michelle Gates, Ben Vandermeer, Cydney Johnson, and Lisa Hartling. We added an age range for children in our inclusion criteria (0 to 18 years). We extracted data for all outcomes from all included studies. We also extracted data for three new outcomes: change in croup score after two hours, patient improvement after two hours, and any adverse events. We used the 2010 Cochrane 'Risk of bias' tool to assess risk of bias for all included studies. We used GRADE to assess the certainty of the body of evidence. We added 'Summary of findings' tables for 13 comparisons. We excluded legacy excluded studies that evidently did not meet the inclusion criteria. We undertook the aforementioned changes to ensure that the updated review complies with current Cochrane standards for methods and reporting. We added "in children" to the review title following feedback from a peer reviewer.
Contributions of authors
Allison Gates (AG): study selection, data extraction and verification, 'Risk of bias' assessment, GRADE, manuscript preparation. Michelle Gates (MG): data extraction and verification, 'Risk of bias' assessment, GRADE, manuscript preparation. Ben Vandermeer (BV): statistical adviser, statistical analyses, contribution to the manuscript. Cydney Johnson (CJ): study selection, data extraction and verification, contribution to the manuscript. Lisa Hartling (LH): methodological adviser, contribution to the manuscript. David W Johnson (DJ): clinical adviser, contribution to the manuscript. Terry P Klassen (TPK): clinical adviser, contribution to the manuscript.
Sources of support
Internal sources
-
Alberta Research Centre for Health Evidence (ARCHE), University of Alberta, Canada.
Funding for the completion of this update, and for previous versions of this review
-
TRanslating Emergency Knowledge for Kids (TREKK), Manitoba, Canada.
Funding for the completion of this update
-
Canadian Coordinating Office for Health Technology Assessment (CCOHTA), Ottawa, Canada.
Funding for previous versions of this review
-
Children's Hospital of Eastern Ontario Research Institute (CHEO RI), Ottawa, Canada.
Funding for previous versions of this review
-
Thomas C. Chalmers Centre for Systematic Reviews, Ottawa, Canada.
Funding for previous versions of this review
-
Fondo de Investigaciones Sanitarias, Instituto de Salud Carlos III, Madrid, Spain.
Funding for previous versions of this review
-
Instituto Nacional de la Salud (INSALUD), Madrid, Spain.
Funding for previous versions of this review
External sources
-
Alberta Heritage Foundation for Medical Research, Canada.
Funding for previous versions of this review
Declarations of interest
Allison Gates: None known. Michelle Gates: None known. Ben Vandermeer: None known. Cydney Johnson: None known. Lisa Hartling: None known. David W Johnson: is an author of three of the included studies (Bjornson 2004; Johnson 1996; Johnson 1998). Terry P Klassen: is an author of four of the included studies (Bjornson 2004; Klassen 1994; Klassen 1996; Klassen 1998).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Alshehr 2005 {published data only}
- Alshehr M, Almegamsi T, Hammdi A. Efficacy of a small dose of oral dexamethasone in croup. Biomedical Research 2005;65(1):65‐72. [Google Scholar]
Amir 2006 {published data only}
- Amir L, Hubermann H, Halevi A, Mor M, Mimouni M, Waisman Y. Oral betamethasone versus intramuscular dexamethasone for the treatment of mild to moderate viral croup. Pediatric Emergency Care 2006;22(8):541‐4. [DOI] [PubMed] [Google Scholar]
Bjornson 2004 {published and unpublished data}
- Bjornson C, Klassen TP, Williamson J, Brant R, Plint A, Bulloch B, et al. A randomized trial of single dose of oral dexamethasone for mild croup. New England Journal of Medicine 2004;351(13):1306‐13. [DOI] [PubMed] [Google Scholar]
Cetinkaya 2004 {published data only}
- Cetinkaya F, Tufekci BS, Kutluk G. A comparison of nebulized budesonide, and intramuscular, and oral dexamethasone for treatment of croup. International Journal of Pediatric Otorhinolaryngology 2004;68(4):453‐6. [DOI] [PubMed] [Google Scholar]
Chub‐Uppakarn 2007 {published data only}
- Chub‐Uppakarn S, Sangsupawanich P. A randomized comparison of dexamethasone 0.15 mg/kg versus 0.6 mg/kg for the treatment of moderate to severe croup. International Journal of Pediatric Otorhinolaryngology 2007;71(3):473‐7. [DOI] [PubMed] [Google Scholar]
Cruz 1995 {published data only}
- Cruz MN, Stewart G, Rosenberg N. Use of dexamethasone in the outpatient management of acute laryngotracheitis. Pediatrics 1995;96(2 Pt 1):220‐3. [PubMed] [Google Scholar]
Dobrovoljac 2012 {published data only}
- Dobrovoljac M, Geelhoed G. How fast does oral dexamethasone work in mild to moderately severe croup? A randomized double‐blinded clinical trial. Emergency Medicine Australasia 2012;24(1):79‐85. [DOI] [PubMed] [Google Scholar]
Donaldson 2003 {published data only}
- Donaldson D, Poleski D, Knipple E, Filips K, Reetz L, Pascula RG, et al. Intramuscular versus oral dexamethasone for the treatment of moderate‐to‐severe croup: a randomized double‐blind trial. Academy of Emergency Medicine 2003;10(1):16‐21. [DOI] [PubMed] [Google Scholar]
Duman 2005 {published data only}
- Duman M, Ozdemir D, Atasever S. Nebulized L‐epinephrine and steroid combination in the treatment of moderate to severe croup. Clinical Drug Investigation 2005;25(3):183‐9. [DOI] [PubMed] [Google Scholar]
Eboriadou 2010 {published data only}
- Eboriadou M, Chryssanthopoulou D, Stamoulis P, Damianidou L, Haidopoulou K. The effectiveness of local corticosteroids therapy in the management of mild to moderate viral croup. Minerva Pediatrica 2010;62(1):23‐8. [PubMed] [Google Scholar]
Eden 1964 {published data only}
- Eden A, Larkin VP. Corticosteroid treatment of croup. Pediatrics 1964;33:768‐9. [PubMed] [Google Scholar]
Eden 1967 {published data only}
- Eden AN, Kaufman A, Yu R. Corticosteroids and croup. Controlled double‐blind study. JAMA 1967;200(5):403‐4. [PubMed] [Google Scholar]
Fifoot 2007 {published data only}
- Fifoot AA, Ting JY. Comparison between single‐dose oral prednisolone and oral dexamethasone in the treatment of croup: a randomized, double‐blinded clinical trial. Emergency Medicine Australasia 2007;19(1):51‐8. [DOI] [PubMed] [Google Scholar]
Fitzgerald 1996 {published data only}
- Fitzgerald D, Mellis C, Johnson M, Allen H, Cooper P, Asperen P. Nebulized budesonide is as effective as nebulized adrenaline in moderately severe croup. Pediatrics 1996;97(5):722‐5. [PubMed] [Google Scholar]
Garbutt 2013 {published data only}
- Garbutt JM, Conion B, Sterkel R, Baty J, Schechtman KB, Mandrell K, et al. The comparative effectiveness of prednisolone and dexamethasone for children with croup: a community‐based randomized trial. Clinical Pediatrics 2013;52(11):1014‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geelhoed 1995a {published data only}
- Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatric Pulmonology 1995;20(6):362‐8. [DOI] [PubMed] [Google Scholar]
Geelhoed 1995b {published data only}
- Geelhoed GC, Macdonald WB. Oral dexamethasone in the treatment of croup: 0.15 mg/kg versus 0.3 mg/kg versus 0.6 mg/kg. Pediatric Pulmonology 1995;20(6):362‐8. [DOI] [PubMed] [Google Scholar]
Geelhoed 1995c {published data only}
- Geelhoed GC, Macdonald WB. Oral and inhaled steroids in croup: a randomized, placebo‐controlled trial. Pediatric Pulmonology 1995;20(6):355‐61. [DOI] [PubMed] [Google Scholar]
Geelhoed 1996 {published data only}
- Geelhoed GC, Turner J, Macdonald WB. Efficacy of a small single dose of oral dexamethasone for outpatient croup: a double blind placebo controlled clinical trial. BMJ 1996;313(7050):140‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Geelhoed 2005 {published data only}
- Geelhoed GC. Budesonide offers no advantage when added to oral dexamethasone in the treatment of croup. Pediatric Emergency Care 2005;21(6):359‐62. [DOI] [PubMed] [Google Scholar]
Godden 1997 {published data only}
- Godden CW, Campbell MJ, Hussey M, Cogswell JJ. Double blind placebo controlled trial of nebulised budesonide for croup. Archives of Disease in Childhood 1997;76(2):155‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Husby 1993 {published data only}
- Husby S, Agertoft L, Mortensen S, Pedersen S. Treatment of croup with nebulised steroid (budesonide): a double blind, placebo controlled study. Archives of Disease in Childhood 1993;68(3):352‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen S, Agertoft L, Husby S, Pedersen S. Pseudocroup treated with inhaled steroid (budesonide). A double‐blind placebo‐controlled trial. Ugeskr Laeger 1994;156(45):6661‐3. [PubMed] [Google Scholar]
James 1969 {published data only}
- James JA. Dexamethasone in croup. A controlled study. American Journal of Diseases of Children 1969;117(5):511‐6. [DOI] [PubMed] [Google Scholar]
Johnson 1996 {published data only}
- Johnson DW, Schuh S, Koren G, Jaffee DM. Outpatient treatment of croup with nebulized dexamethasone. Archives of Pediatrics & Adolescent Medicine 1996;150(4):349‐55. [DOI] [PubMed] [Google Scholar]
Johnson 1998 {published data only}
- Johnson DW, Jacobson S, Edney PC, Hadfield P, Mundy ME, Schuh S. A comparison of nebulized budesonide, intramuscular dexamethasone, and placebo for moderately severe croup. New England Journal of Medicine 1998;339(8):498‐503. [DOI] [PubMed] [Google Scholar]
Klassen 1994 {published data only}
- Klassen TP, Feldman ME, Watters LK, Sutcliffe T, Rowe PC. Nebulized budesonide for children with mild‐to‐moderate croup. New England Journal of Medicine 1994;331(5):285‐9. [DOI] [PubMed] [Google Scholar]
Klassen 1996 {published data only}
- Klassen TP, Watters LK, Feldman ME, Sutcliffe T, Rowe PC. The efficacy of nebulized budesonide in dexamethasone‐treated outpatients with croup. Pediatrics 1996;97(4):463‐6. [PubMed] [Google Scholar]
Klassen 1998 {published data only}
- Klassen TP, Craig WR, Moher D, Osmond MH, Pasterkamp H, Sutcliffe T, et al. Nebulized budesonide and oral dexamethasone for treatment of croup: a randomized controlled trial. JAMA 1998;279(20):1629‐32. [DOI] [PubMed] [Google Scholar]
Koren 1983 {published data only}
- Koren GF. Corticosteroid treatment of laryngotracheitis v spasmodic croup in children. American Journal of Diseases of Children 1983;137(10):941‐4. [DOI] [PubMed] [Google Scholar]
Kuusela 1988 {published data only}
- Kuusela AL, Vesikari T. A randomized double‐blind, placebo‐controlled trial of dexamethasone and racemic epinephrine in the treatment of croup. Acta Paediatrica Scandinavica 1988;77(1):99‐104. [DOI] [PubMed] [Google Scholar]
Leipzig 1979 {published data only}
- Leipzig B, Oski FA, Cummings CW, Stockman JA, Swender P. A prospective randomized study to determine the efficacy of steroids in treatment of croup. Journal of Pediatrics 1979;94(2):194‐6. [DOI] [PubMed] [Google Scholar]
Luria 2001 {published data only}
- Luria JW, Gonzalez‐del‐Rey JA, DiGuilio GA, McAneney CM, Olsen JJ, Ruddy RM. Effectiveness of oral or nebulized dexamethasone for children with mild croup. Archives of Pediatrics & Adolescent Medicine 2001;155(12):1340‐5. [DOI] [PubMed] [Google Scholar]
Martinez Fernandez 1993 {published data only}
- Martinez Fernandez A, Sanchez GE, Rica EI, Echaniz UI, Alonso DM, Vilella CM, et al. Randomized double‐blind study of treatment of croup with adrenaline and/or dexamethasone in children. Anales Españoles de Pediatria 1993;38:29‐32. [PubMed] [Google Scholar]
Massicotte 1973 {published data only}
- Massicotte P, Tetreault L. Evaluation of methyl‐prednisolone in the treatment of acute laryngitis in children. Unión Médicale du Canada 1973;102(10):2064‐72. [PubMed] [Google Scholar]
Rittichier 2000 {published data only}
- Rittichier KK, Ledwith CA. Outpatient treatment of moderate croup with dexamethasone: intramuscular versus oral dosing. Pediatrics 2000;106(6):1344‐8. [DOI] [PubMed] [Google Scholar]
Roberts 1999 {published data only}
- Roberts GW, Master VV, Staugas RE, Raftos JV, Parsons DW, Coulthard KP, et al. Repeated dose inhaled budesonide versus placebo in the treatment of croup. Journal of Paediatrics and Child Health 1999;35(2):170‐4. [DOI] [PubMed] [Google Scholar]
Roorda 1998 {published data only}
- Roorda RJ, Walhof CM. Effects of inhaled fluticasone propionate administered with metered dose inhaler and spacer in mild to moderate croup: a negative preliminary report. Pediatric Pulmonology 1998;25(2):114‐7. [DOI] [PubMed] [Google Scholar]
Skowron 1966a {published data only}
- Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Canadian Medical Association Journal 1966;94(11):528‐31. [PMC free article] [PubMed] [Google Scholar]
Skowron 1966a&b {published data only}
- Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Canadian Medical Association Journal 1966;94(11):528‐31. [PMC free article] [PubMed] [Google Scholar]
Skowron 1966b {published data only}
- Skowron PN, Turner JA, McNaughton GA. The use of corticosteroid (dexamethasone) in the treatment of acute laryngotracheitis. Canadian Medical Association Journal 1966;94(11):528‐31. [PMC free article] [PubMed] [Google Scholar]
Soleimani 2013 {published data only}
- Soleimani G, Daryadel A, Moghadam AA, Sharif MR. The comparison of oral and IM dexamethasone efficacy in croup treatment. Journal of Comprehensive Pediatrics 2013;4(4):175‐8. [Google Scholar]
Sparrow 2006 {published data only}
- Sparrow A, Geelhoed G. Prednisolone versus dexamethasone in croup: a randomised equivalence trial. Archives of Disease in Childhood 2006;91(7):580‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Super 1989 {published data only}
- Super DM, Cartelli NA, Brooks LJ, Lembo RM, Kumar ML. A prospective randomized double‐blind study to evaluate the effect of dexamethasone in acute laryngotracheitis. Journal of Pediatrics 1989;115(2):323‐9. [DOI] [PubMed] [Google Scholar]
Tibballs 1992 {published data only}
- Tibballs J, Shann FA, Landau LI. Placebo‐controlled trial of prednisolone in children intubated for croup. Lancet 1992;340(8822):745‐8. [DOI] [PubMed] [Google Scholar]
Vad Pedersen 1998 {published data only}
- Vad Pedersen L, Dahl M, Falk‐Petersen HE, Larsen SE. Inhaled budesonide versus intramuscular dexamethasone in the treatment of pseudocroup [Inhaleret budesonid versus dexamethasone i.m. til behandling at pseudocroup]. Ugeskrift for Laeger 1998;160(15):2253‐6. [PubMed] [Google Scholar]
Von Mühlendahl 1982 {published data only}
- Mühlendahl KE, Kahn D, Spohr HL, Dressler F. Steroid treatment of pseudo‐croup. Helvetica Paediatrica Acta 1982;37(5):431‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Anene 1996 {published data only}
- Anene O, Meert KL, Uy H, Simpson P, Sarnaik AP. Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double‐blind, placebo‐controlled trial. Critical Care Medicine 1996;24(10):1666‐9. [DOI] [PubMed] [Google Scholar]
Bollobas 1965 {published data only}
- Bollobas B. On local adrenocortical hormone treatment of rhinolaryngologic diseases. Zeitschrift für Laryngologie, Rhinologie, Otologie und Ihre Grenzgebiete 1965;44(7):476‐81. [PubMed] [Google Scholar]
Cichy 1983 {published data only}
- Cichy M, Pawlik J. Treatment of subglottic laryngitis in children. Otolaryngologia Polska 1983;37(1):11‐3. [PubMed] [Google Scholar]
Connolly 1969 {published data only}
- Connolly JH, Field C, Glasgow J. A double blind trial of prednisolone in epidemic bronchiolitis due to respiratory syncytial virus. Acta Pediatrica Scandinavica 1969;58(2):116‐20. [DOI] [PubMed] [Google Scholar]
Couser 1992 {published data only}
- Couser RJ, Ferrara TB, Falde B, Johnson K, Schilling CG, Hoekstra RE. Effectiveness of dexamethasone in preventing extubation failure in preterm infants at increased risk for airway edema. Journal of Pediatrics 1992;121(4):591‐6. [DOI] [PubMed] [Google Scholar]
Eghbali 2016 {published data only}
- Eghbali A, Sabbagh A, Bagheri B, Taherahmade H, Kahbazi M. Efficacy of nebulized L‐epinephrine for treatment of croup: a randomized, double‐blind study. Fundamental & Clinical Pharmacology 2016;30(2016):70‐5. [DOI: 10.1111/fcp.12158] [DOI] [PubMed] [Google Scholar]
Faghihinia 2007 {published data only}
- Faghihinia J. A comparison between intramuscular dexamethasone and fluticasone propionate inhaler in treatment of croup. World Allergy Organization Journal 2007;S104:327. [Google Scholar]
Faraji‐Goodarzi 2018 {published data only}
- Faraji‐Goodarzi M, Taee N, Mohammadi‐Kamalvand M. Comparison of the effect of cold drink and dexamethasone, and their combined effect on children with croup. Drug Research 2018;68(4):185‐8. [DOI] [PubMed] [Google Scholar]
Flisberg 1973 {published data only}
- Flisberg K, Olsholt R. Pseudocroup with stridor. Acta Oto‐Laryngologica 1973;76(4):295‐9. [DOI] [PubMed] [Google Scholar]
Freezer 1990 {published data only}
- Freezer N, Butt W, Phelan P. Steroids in croup: do they increase the incidence of successful extubation?. Anaesthesia and Intensive Care 1990;18(2):224‐8. [DOI] [PubMed] [Google Scholar]
Gill 2017 {published data only}
Goddard 1967 {published data only}
- Goddard JE Jr, Phillips OC, Marcy JH. Betamethasone for prophylaxis of postintubation inflammation: a double‐blind study. Anesthesia and Analgesia 1967;46(3):348‐53. [PubMed] [Google Scholar]
Haque 1981 {published data only}
- Haque KN. Efficacy of dexamethasone in acute laryngotracheobronchitis (croup). Saudi Medical Journal 1981;2(3):143‐5. [Google Scholar]
Havaldar 1997 {published data only}
- Havaldar PV. Dexamethasone in laryngeal diphtheritic croup. Annals of Tropical Paediatrics 1997;17(1):21‐3. [DOI] [PubMed] [Google Scholar]
Kelley 1992 {published data only}
- Kelley PB, Simon JE. Racemic epinephrine use in croup and disposition. American Journal of Emergency Medicine 1992;10(3):181‐3. [DOI] [PubMed] [Google Scholar]
Kunkel 1996 {published data only}
- Kunkel NC, Baker MD. Use of racemic epinephrine, dexamethasone, and mist in the outpatient management of croup. Pediatric Emergency Care 1996;12(3):156‐9. [DOI] [PubMed] [Google Scholar]
Ledwith 1995 {published data only}
- Ledwith CA, Shea LM, Mauro RD. Safety and efficacy of nebulized racemic epinephrine in conjunction with oral dexamethasone and mist in the outpatient treatment of croup. Annals of Emergency Medicine 1995;25(3):331‐7. [DOI] [PubMed] [Google Scholar]
Martensson 1960 {published data only}
- Martensson B, Nilsson G, Torbjar J. The effect of corticosteroids in the treatment of pseudo‐croup. Acta Oto‐Laryngologica 1960;158(Suppl):62‐71. [DOI] [PubMed] [Google Scholar]
McDonogh 1994 {published data only}
- McDonogh AJ. The use of steroids and nebulised adrenaline in the treatment of viral croup over a seven year period at a district hospital. Anaesthesia and Intensive Care 1994;22(2):175‐8. [DOI] [PubMed] [Google Scholar]
Mohammadzadeh 2014 {published data only}
- Mohammadzadeh I, Noorouzi AR, Nakhjavani N, Barari‐Savadkoohi R, Mohammadpor‐Mir A, Alizadeh‐Navaei R. The effect of dexamethasone and nebulised L‐epinephrine in treatment of croup. Journal of Babol University of Medical Sciences 2014;16(2):12‐6. [Google Scholar]
NCT01748162 {published data only}
- NCT01748162. Management of recurrent croup. clinicaltrials.gov/ct2/show/NCT01748162 (first received 12 December 2012).
Novik 1960 {published data only}
- Novik A. Corticosteroid treatment of non‐diphtheric croup. Acta Oto‐Laryngologica 1960;158(Suppl):20‐2. [PubMed] [Google Scholar]
Osváth 1994 {published data only}
- Osváth P, Kelenhegyi K, Szánthó A. Management of childhood pseudocroup with budesonide inhalation. Orvosi Hetilap 1994;135(46):2535‐7. [PubMed] [Google Scholar]
Prendergast 1994 {published data only}
- Prendergast M, Jones JS, Hartman D. Racemic epinephrine in the treatment of laryngotracheitis: can we identify children for outpatient therapy?. American Journal of Emergency Medicine 1994;12(6):613‐6. [DOI] [PubMed] [Google Scholar]
Rizos 1998 {published data only}
- Rizos J, DiGravio B, Sehl M, Tallon J. The disposition of children with croup treated with racemic epinephrine and dexamethasone in the emergency department. Journal of Emergency Medicine 1998;16(4):535‐9. [DOI] [PubMed] [Google Scholar]
Roked 2015 {published data only}
- Roked F, Atkinson M, Hartshorn S. Best practice: one or two doses of dexamethasone for the treatment of croup?. Archives of Disease in Childhood 2015;100(Suppl 3):A40‐1. [Google Scholar]
Ross 1969 {published data only}
- Ross JA. Special problems in acute laryngotracheobronchitis. Laryngoscope 1969;79(7):1218‐26. [DOI] [PubMed] [Google Scholar]
Serra 1997 {published data only}
- Serra A, Bonarrigo A, Cupido GF, Manciagli M, Pantalena V, Raso D, et al. Experience with flunisolide in inflammatory diseases in otorhinolaryngology. Multicentric trial [Impiego di flunisolide nel trattamento di laringiti e sinusiti]. Otorinolaringologia 1997;47(3):137‐44. [Google Scholar]
Sumboonnanonda 1997 {published data only}
- Sumboonnanonda A, Suwanjutha S, Sirinavin S. Randomized controlled trial of dexamethasone in infectious croup. Journal of the Medical Association of Thailand 1997;80(4):262‐5. [PubMed] [Google Scholar]
Sussman 1964 {published data only}
- Sussman S, Grossman M, Magoffin R, Schieble J. Dexamethasone (16 alpha‐methyl, 9 alpha fluoroprednisolone) in obstructive respiratory tract infections in children. Pediatrics 1964;34:851‐5. [PubMed] [Google Scholar]
Tal 1983 {published data only}
- Tal A, Bavilski C, Yohai D. Dexamethasone and salbutamol in the treatment of acute wheezing in infants. Pediatrics 1983;71(1):13‐8. [PubMed] [Google Scholar]
Tellez 1991 {published data only}
- Tellez DW, Galvis AG, Storgion SA, Amer HN, Hoseyni M, Deakers TW. Dexamethasone in the prevention of postextubation stridor in children. Journal of Pediatrics 1991;118(2):289‐94. [DOI] [PubMed] [Google Scholar]
Wilhelmi 1976 {published data only}
- Wilhelmi J. High dosage rectal prednisone therapy (Rectodelt 100) in viral croup of the small child. Medizinische Monatsschrift 1976;30(10):467‐9. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12609000290291 {published data only}
- ACTRN12609000290291. A comparison of oral prednisolone and oral dexamethasone in children with croup: a prospective, randomised, double blinded multicentre trial [Oral prednisolone vs oral dexamethasone (in two different doses) in children with croup: randomised clinical trial comparing the improvement in Westley Croup Score]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=83722 (first received 18 May 2009).
Additional references
Abrams 2005
- Abrams KR, Gillies CL, Lambert PC. Meta‐analysis of heterogeneously reported trials assessing change from baseline. Statistics in Medicine 2005;24:3823‐44. [DOI: 10.1002/sim.2423] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PubMed] [Google Scholar]
Berkman 2015
- Berkman ND, Lohr KN, Ansari MT, Balk EM, Kane R, McDonagh M, et al. Grading the strength of a body of evidence when assessing health care interventions: an EPC update. Journal of Clinical Epidemiology 2015;68(11):1312‐24. [DOI] [PubMed] [Google Scholar]
Bjornson 2008
- Bjornson C, Johnson DW. Croup. Lancet 2008;371(9609):329‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjornson 2016
- Bjornson C, Williamson J, Johnson D. Telephone Out Patient Score: the derivation and validation of a telephone follow‐up assessment tool for use in clinical research in children with croup. Pediatric Emergency Care 2016;32(5):290‐7. [DOI] [PubMed] [Google Scholar]
Burda 2017
- Burda BU, O'Connor EA, Webber EM, Redmond N, Perdue LA. Estimating data from figures with a Web‐based program: considerations for a systematic review. Research Synthesis Methods 2017;8(3):258‐62. [DOI: 10.1002/jrsm.1232] [DOI] [PubMed] [Google Scholar]
Chan 2001
- Chan AKJ, Langley JM, LeBlanc JC. Interobserver variability of croup scoring in clinical practice. Paediatrics & Child Health 2001;6(6):347‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Angelis 2004
- Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. New England Journal of Medicine 2004;351(12):1250‐1. [DOI] [PubMed] [Google Scholar]
Downes 1975
- Downes JJ, Raphaelly RC. Pediatric intensive care. Anesthesiology 1975;43:238‐50. [DOI] [PubMed] [Google Scholar]
Dyson 2017
- Dyson MP, Shave K, Fernandes RM, Scott SD, Hartling L. Outcomes in child health: exploring the use of social media to engage parents in patient‐centered outcomes research. Journal of Medical Internet Research 2017;19(3):e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EndNote [Computer program]
- Clarivate Analytics. EndNote. Version X7. Philadelphia: Clarivate Analytics, 2013.
Follmann 1992
- Follmann D, Elliott P, Suh l, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [DOI: 10.1016/j.jclinepi.2005.06.006] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT. Version (accessed prior to 23 November 2017). Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Griffin 2000
- Griffin S, Ellis S, Fitzgerald‐Barron A, Rose J, Egger M. Nebulized steroid in the treatment of croup: a systematic review of randomised controlled trials. British Journal of General Practice 2000;50(451):135‐41. [PMC free article] [PubMed] [Google Scholar]
Hartling 2014
- Hartling L, Hamm MP, Fernandes RM, Dryden D, Vandermeer B. Quantifying bias in randomized controlled trials in child health: a meta‐epidemiological study. PLoS ONE 2014;9(2):e88008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI: 10.1136/bmj.d5928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2003
- Johnson D, Williamson J. Health care utilization by children with croup in Alberta. Pediatric Research 2003;53:83A. [Google Scholar]
Johnson 2009
- Johnson DW. Croup. BMJ Clinical Evidence 2009;2009:pii: 0321. [Google Scholar]
Jüni 2001
- Jüni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kadic 2016
- Kadic AJ, Vucic K, Dosenovic S, Sapunar D, Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. Journal of Clinical Epidemiology 2016;74(2016):119‐23. [DOI: 10.1016/j.clinepi.2016.01.002] [DOI] [PubMed] [Google Scholar]
Kairys 1989
- Kairys SW, Olmstead EM, O'Connor GT. Steroid treatment of laryngotracheitis: a meta‐analysis of the evidence from randomized trials. Pediatrics 1989;83(5):683‐93. [PubMed] [Google Scholar]
Knapp 2008
- Knapp JF, Simon SD, Sharma V. Quality of care for common pediatric respiratory illnesses in United States emergency departments: analysis of 2005 National Hospital Ambulatory Medical Care Survey Data. Pediatrics 2008;122(6):1165‐70. [DOI] [PubMed] [Google Scholar]
Lavine 2001
- Lavine E, Scolnik D. Lack of efficacy of humidification in the treatment of croup. Why do physicians persist in using an unproven modality?. Canadian Journal of Emergency Medicine 2001;3(3):209‐12. [DOI] [PubMed] [Google Scholar]
Light 1984
- Light RS, Pillemar DB. Summing Up: The Science of Reviewing Research. Cambridge: Harvard University Press, 1984. [Google Scholar]
Loftis 2006
- Loftis L. Acute infectious upper airway obstructions in children. Seminars in Pediatric Infectious Diseases 2006;17(1):5‐10. [DOI] [PubMed] [Google Scholar]
Microsoft Excel [Computer program]
- Microsoft Corporation. Microsoft Excel. Version 2016. Redmond: Microsoft Corporation, 2016.
Moher 2012
- Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. International Journal of Surgery 2012;10(1):28‐55. [DOI] [PubMed] [Google Scholar]
Moore 2007
- Moore M, Little P. Humidified air inhalation for treating croup: a systematic review and meta‐analysis. Family Practice 2007;24(4):295‐301. [DOI] [PubMed] [Google Scholar]
Neto 2002
- Neto GM, Kentab O, Klassen TP, Osmond MH. A randomized controlled trial of mist in the acute treatment of moderate croup. Academic Emergency Medicine 2002;9(9):873‐9. [DOI] [PubMed] [Google Scholar]
Petrocheilou 2014
- Petrocheilou A, Tanou K, Kalampouka E, Malakasioti G, Giannios C, Kaditis AG. Viral croup: diagnosis and a treatment algorithm. Pediatric Pulmonology 2014;49(5):421‐9. [DOI] [PubMed] [Google Scholar]
Plot Digitizer [Computer program]
- SourceForge. Plot Digitizer. SourceForge, accessed 4 December 2017.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rihkanen 2008
- Rihkanen H, Beng ER, Nieminen T, Komsi K‐L, Raty R, Saxen H, et al. Respiratory viruses in laryngeal croup of young children. Journal of Pediatrics 2008;152(5):661‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosychuk 2010
- Rosychuk RJ, Klassen TP, Metes D, Voaklander DC, Senthilselvan A, Rowe BH. Croup presentations to emergency departments in Alberta, Canada: a large population‐based study. Pediatric Pulmonology 2010;45(1):83‐91. [DOI] [PubMed] [Google Scholar]
Scolnik 2006
- Scolnik D, Coates AL, Stephens D, Silva Z, Lavine E, Schuh S. Controlled delivery of high vs low humidity vs mist therapy for croup in emergency departments: a randomized controlled trial. JAMA 2006;295(11):1274‐80. [DOI] [PubMed] [Google Scholar]
Turner 2012
- Turner L, Shamseer L, Altman DG, Schulz KF, Moher D. Does use of the CONSORT Statement impact the completeness of reporting of randomised controlled trials published in medical journals? A Cochrane review. Systematic Reviews 2012;1:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waisman 1992
- Waisman Y, Klein BL, Boenning DA, Young GM, Chamberlain JM, O'Donnell R, et al. Prospective randomized double‐blind study comparing L‐epinephrine and racemic epinephrine aerosols in the treatment of laryngotracheitis (croup). Pediatrics 1992;89(2):302‐6. [PubMed] [Google Scholar]
Westley 1978
- Westley CR, Cotton EK, Brooks JG. Nebulized racemic epinephrine by IPPB for the treatment of croup. American Journal of Diseases in Children 1978;132(5):484‐7. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Lotte Gluud L, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: a meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ausejo 1999
- Ausejo M, Saenz A, Pham B, Kellner JD, Johnson DW, Moher D, et al. The effectiveness of glucocorticoids in treating croup: meta‐analysis. BMJ 1999;319(7210):595‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ausejo 2000
- Ausejo M, Saenz A, Pham B, Kellner JD, Johnson DW, Moher D, et al. Glucocorticoids for croup. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001955.pub2] [DOI] [PubMed] [Google Scholar]
Russell 2004
- Russell K, Wiebe N, Saenz A, Ausejo Segura M, Johnson D, Hartling L, et al. Glucocorticoids for croup. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001955.pub2] [DOI] [PubMed] [Google Scholar]
Russell 2011
- Russell KF, Liang Y, O'Gorman K, Johnson DW, Klassen TP. Glucocorticoids for croup. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD001955.pub3] [DOI] [PubMed] [Google Scholar]


