Abstract
Background
Cryptococcal disease remains one of the main causes of death in HIV‐positive people who have low cluster of differentiation 4 (CD4) cell counts. Currently, the World Health Organization (WHO) recommends screening HIV‐positive people with low CD4 counts for cryptococcal antigenaemia (CrAg), and treating those who are CrAg‐positive. This Cochrane Review examined the effects of an approach where those with low CD4 counts received regular prophylactic antifungals, such as fluconazole.
Objectives
To assess the efficacy and safety of antifungal drugs for the primary prevention of cryptococcal disease in adults and children who are HIV‐positive.
Search methods
We searched the CENTRAL, MEDLINE PubMed, Embase OVID, CINAHL EBSCOHost, WHO International Clinical Trials Registry Platform (WHO ICTRP), ClinicalTrials.gov, conference proceedings for the International AIDS Society (IAS) and Conference on Retroviruses and Opportunistic Infections (CROI), and reference lists of relevant articles up to 31 August 2017.
Selection criteria
Randomized controlled trials of adults and children, who are HIV‐positive with low CD4 counts, without a current or prior diagnosis of cryptococcal disease that compared any antifungal drug taken as primary prophylaxis to placebo or standard care.
Data collection and analysis
Two review authors independently assessed eligibility and risk of bias, and extracted and analysed data. The primary outcome was all‐cause mortality. We summarized all outcomes using risk ratios (RR) with 95% confidence intervals (CI). Where appropriate, we pooled data in meta‐analyses. We assessed the certainty of the evidence using the GRADE approach.
Main results
Nine trials, enrolling 5426 participants, met the inclusion criteria of this review. Six trials administered fluconazole, while three trials administered itraconazole.
Antifungal prophylaxis may make little or no difference to all‐cause mortality (RR 1.07, 95% CI 0.80 to 1.43; 6 trials, 3220 participants; low‐certainty evidence). For cryptococcal specific outcomes, prophylaxis probably reduces the risk of developing cryptococcal disease (RR 0.29, 95% CI 0.17 to 0.49; 7 trials, 5000 participants; moderate‐certainty evidence), and probably reduces deaths due to cryptococcal disease (RR 0.29, 95% CI 0.11 to 0.72; 5 trials, 3813 participants; moderate‐certainty evidence). Fluconazole prophylaxis may make no clear difference to the risk of developing clinically resistant Candida disease (RR 0.93, 95% CI 0.56 to 1.56; 3 trials, 1198 participants; low‐certainty evidence); however, there may be an increased detection of fluconazole‐resistant Candida isolates from surveillance cultures (RR 1.25, 95% CI 1.00 to 1.55; 3 trials, 539 participants; low‐certainty evidence). Antifungal prophylaxis was generally well‐tolerated with probably no clear difference in the risk of discontinuation of antifungal prophylaxis compared with placebo (RR 1.01, 95% CI 0.91 to 1.13; 4 trials, 2317 participants; moderate‐certainty evidence). Antifungal prophylaxis may also make no difference to the risk of having any adverse event (RR 1.07, 95% CI 0.88 to 1.30; 4 trials, 2317 participants; low‐certainty evidence), or a serious adverse event (RR 1.08, 95% CI 0.83 to 1.41; 4 trials, 888 participants; low‐certainty evidence) when compared to placebo or standard care.
Authors' conclusions
Antifungal prophylaxis reduced the risk of developing and dying from cryptococcal disease. Therefore, where CrAG screening is not available, antifungal prophylaxis may be used in patients with low CD4 counts at diagnosis and who are at risk of developing cryptococcal disease.
12 April 2019
Up to date
All studies incorporated from most recent search
All eligible published studies found in the last search (31 Aug, 2017) were included
Plain language summary
Preventing cryptococcal disease in HIV‐positive people
What is the aim of this review?
The aim of this Cochrane Review was to find out if taking an antifungal drug regularly, such as fluconazole, prevented HIV‐positive people who have low cluster of differentiation 4 (CD4) cell counts, from getting cryptococcal disease, and what the potential complications were. Cochrane researchers collected and analysed all relevant studies to answer this question, and found nine trials that looked at this question.
Key messages
We found that regularly taking antifungal medication prevented HIV‐positive people who had low CD4 counts from developing cryptococcal disease. We also found that primary prophylaxis probably reduced the number of people dying specifically from cryptococcal disease. However it probably did not reduce the number of people dying overall.
What was studied in the review?
Cryptococcal disease is one of the leading causes of death for HIV‐positive people who have low CD4 counts. The current recommended strategy in most countries to prevent people from developing cryptococcal disease, is to screen eligible patients with a blood test that picks up early signs of disease. We looked at trials that studied whether taking antifungal prophylaxis stopped people from dying or developing cryptococcal disease. We also looked at the side effects of the antifungal drug and whether it caused resistance to antifungal drugs in other fungal infections, such as thrush.
What are the main results of the review?
We found nine trials that included 5426 participants. These trials were conducted in Australia, Canada, South Africa, the UK, the USA,Thailand, and sub‐Saharan Africa. Seven trials were conducted before the availability of modern antiretroviral therapy. The participants in two large trials received modern HIV treatment regimens.
We found that antifungal prophylaxis may have no effect on death overall, although it reduced the risk of those with low CD4 counts developing cryptococcal disease by 71%. Prophylaxis with an antifungal probably also reduced deaths specifically from cryptococcal disease. There may be an increased risk of the vaginal tract becoming colonized with fluconazole‐resistant Candida organisms if someone takes prophylaxis, however, this may not necessarily result in an increased risk of clinical disease that doesn't respond to treatment. Generally, there were few side effects of taking antifungal prophylaxis, and it was well‐tolerated when compared to placebo.
How up to date is this review?
The review authors searched for studies that had been published up to 31 August 2017.
Summary of findings
Summary of findings for the main comparison. Antifungal prophylaxis versus no antifungal prophylaxis for preventing cryptococcal disease in HIV‐positive people.
| Antifungal prophylaxis versus no antifungal prophylaxis | |||||
| Patient or population: people who are HIV‐positive Setting: global Intervention: antifungal prophylaxis Comparison: no antifungal prophylaxis | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (trials) | Certainty of the evidence (GRADE) | |
| Risk with no antifungal prophylaxis | Risk with antifungal prophylaxis | ||||
| All‐cause mortality | 111 per 1000 | 119 per 1000 (89 to 159) | RR 1.07 (0.80 to 1.43) | 3220 (6 RCTs) | ⊕⊕⊝⊝ Lowa,b,c |
| Cryptococcal disease occurrence | 30 per 1000 | 9 per 1000 (5 to 15) | RR 0.29 (0.17 to 0.49) | 5000 (7 RCTs) | ⊕⊕⊕⊝ Moderated,e |
| Mortality due to cryptococcal disease | 11 per 1000 | 3 per 1000 (1 to 9) | RR 0.29 (0.11 to 0.72) | 3813 (5 RCTs) | ⊕⊕⊕⊝ Moderatee,f |
| Clinical resistance of Candida species to fluconazole | 49 per 1000 | 46 per 1000 (28 to 77) | RR 0.93 (0.56 to 1.56) | 1198 (3 RCTs) | ⊕⊕⊝⊝ Lowg,h |
| Microbiological resistance of Candida to fluconazole: surveillance sampling | 348 per 1000 | 435 per 1000 (348 to 539) | RR 1.25 (1.00 to 1.55) | 539 (3 RCTs) | ⊕⊕⊝⊝ Lowi,j |
| Treatment discontinuation | 259 per 1000 | 262 per 1000 (236 to 293) | RR 1.01 (0.91 to 1.13) | 2317 (4 RCTs) | ⊕⊕⊕⊝ Moderateb |
| Any serious adverse event | 153 per 1000 | 165 per 1000 (127 to 215) | RR 1.08 (0.83 to 1.41) | 888 (4 RCTs) | ⊕⊕⊝⊝ Low b,c,k |
| Any adverse events | 320 per 1000 | 342 per 1000 (281 to 415) | RR 1.07 (0.88 to 1.30) | 2317 (4 RCTs) | ⊕⊕⊝⊝ Lowb,l |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio; ART: antiretroviral therapy | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aNot downgraded for inconsistency. I² statistic = 39% bDowngraded two for indirectness. Participants in most of the included studies did not receive current standard ART regimens, nor did they receive them in a time period consistent with current practice. cNot downgraded for imprecision as narrow CIs around absolute risk dDowngraded by one for indirectness. In the largest study, which contributed 47.2% to the pooled estimate of effect, participants received current standard of care in type and time from diagnosis to ART (Hakim 2017). eNot downgraded for imprecision; although there were few events, CIs around absolute risk were narrow, containing only clinically appreciable benefit fDowngraded by one for indirectness. Most trials were unclear in how they attributed death to cryptococcal disease. In the largest study, which contributed 68.8% to the pooled estimate of effect, participants received current standard of care in type and time from diagnosis to ART (Hakim 2017). gDowngraded one for inconsistency. Clinical heterogeneity in how clinical resistance was defined hDowngrade one for imprecision. Few events in intervention and control groups. iDowngraded one for indirectness. Surveillance sampling did not directly relate to clinical disease. jDowngraded one for imprecision. Broad CIs around absolute risk contained clinically appreciable harm and no appreciable effect. kDowngraded one for indirectness. Studies did not clearly define grading of serious adverse events. lDowngraded one for inconsistency. Unexplained heterogeneity of I² statistic = 64%.
Background
Description of the condition
Cryptococcal disease is an opportunistic infection that is common among people who are HIV‐positive with low cluster of differentiation 4 (CD4) cell counts. In 2014, the global prevalence was 6% (Rajasingham 2017). It is a leading cause of morbidity and mortality, both before and after initiation of anti‐retroviral therapy (ART) in patients with low CD4 counts (Jarvis 2010). It is mostly caused by infection with Cryptococcus neoformans. Cryptococcus gattii is responsible in some cases. Patients may present with meningitis, pneumonia, or in some rare cases, cutaneous, ophthalmic, or prostatic lesions (Skolnik 2017). Cryptococcal meningitis is the commonest presentation of HIV‐related cryptococcal disease in adults. It is the leading cause of meningitis in adults in sub‐Saharan Africa, and accounts for 15% of HIV‐related deaths globally (Rajasingham 2017). The case fatality rate in sub‐Saharan Africa ranges from 35% to 65%, compared with 10% to 20% in most high‐income countries (Lessells 2011). While high‐income countries have seen considerable reduction in the incidence of cryptococcal meningitis following increased access to ART (Mirza 2003), low‐income countries have not experienced the same decline (Tenforde 2017; Wall 2014; Williamson 2017). This may be attributed to late diagnosis of HIV and delays in starting ART in these settings (Kambugu 2008). In some settings, over 50% of HIV‐positive people and presenting with cryptococcal meningitis are ART‐experienced (Rhein 2016).
There are various diagnostic tools available for the detection of cryptococcal disease. Cryptococcal meningitis can be diagnosed through cerebrospinal fluid (CSF) microscopy, culture, or cryptococcal antigen detection. A positive cryptococcal antigen (CrAg) test does not confer diagnosis, as HIV‐positive people with advanced disease can be CrAg‐positive weeks to months before the development of cryptococcal meningitis. India ink microscopy of CSF is the commonest technique, but has reduced sensitivity if the fungal burden is low. CSF culture, considered the gold standard, has a higher yield than India ink, but may also have poorer sensitivity with low fungal burdens. CSF cryptococcal antigen testing is highly sensitive and specific for cryptococcal meningitis, and is available as a point‐of‐care rapid test. Blood culture, or serum or plasma cryptococcal antigen testing, can be used to detect disseminated infection (CDC 2017). Pulmonary cryptococcal disease can be detected through cryptococcal antigen testing of bronchoalveolar fluid; however, the sensitivity of this test is low, and the definitive diagnosis is made through histopathology, cytopathology, or culture of respiratory specimens or biopsies.
Description of the intervention
Prophylaxis for the prevention of opportunistic infections, such as Pneumocytis (PJP) is an integral component of HIV care, and has been shown to reduce HIV‐associated mortality among people with low CD4 counts (WHO 2016). When primary prophylaxis for cryptococcal disease is administered, typically, antifungals are used. A previous version of this Cochrane Review showed that primary prophylaxis with fluconazole or itraconazole reduced the incidence of cryptococcal disease, but had no effect on mortality (Chang 2005).
Oral fluconazole is well‐absorbed and well‐tolerated, without significant adverse events (McLachlan 1996). It is commonly used for secondary prophylaxis of cryptococcal meningitis after successful treatment, to prevent relapse (WHO 2011). Long periods of monotherapy for primary or secondary prophylaxis may increase the risk of cryptococcal resistance to fluconazole (Apisarnthanarak 2008b; Cheong 2013), especially in patients whose CD4 cell counts are falling (Kontoyiannis 2002). A systematic review showed that primary fluconazole prophylaxis may result in increased risk of colonization with susceptible dose‐dependent or resistant yeasts; however, no effect was seen on the risk of resistant systemic fungal infection (Brion 2007). The concern remains that with widespread use of antifungal prophylaxis, resistant fungal strains will render antifungals ineffective, resulting in refractory or relapsed cases of cryptococcal meningitis in HIV‐positive people.
Oral itraconazole does not absorb as well as fluconazole, and its bioavailability is markedly influenced by gastric contents. Erratic absorption with the capsule formulation, and high rates of gastrointestinal intolerance with the oral solution, have led to decreased use of this antifungal agent in recent years (Pound 2011). In addition, drug interactions mediated through the cytochrome P450 enzyme system may further limit the use of itraconazole as part of a multi‐drug regimen (Pierard 2000).
How the intervention might work
There are two broad approaches to preventing cryptococcal disease. The first method (primary prophylaxis) consists of treating all those with a low CD4 count with prophylactic antifungals, while simultaneously initiating ART. This prevents cryptococcal disease during the period of immune recovery. The second method of controlling cryptococcal disease involves screening and pre‐emptive treatment. This method has been recommended by the World Health Organization (WHO), and relies on the ability to detect cryptococcal antigen in the blood. Patients who are HIV‐positive, and have severe disease with low CD4 counts, are tested for the presence of cryptococcal antigen in blood; if positive, they are investigated for cryptococcal disease, and treated with antifungals (WHO 2011).
Both methods have advantages and disadvantages. Primary prophylaxis has been shown to be effective at reducing the incidence of cryptococcal meningitis at a population level, but is less cost effective (Micol 2010). Prior to this review, the use of prophylactic antifungals in cryptococcal antigen negative patients with low CD4 counts was only recommended by the WHO if a prolonged delay in ART initiation was likely. This recommendation was based on the lack of a consistent survival benefit associated with primary prophylaxis, costs associated with providing prophylaxis to a large number of people, and concerns over drug resistance and congenital anomalies (WHO 2011).
The focus of this review was solely on the effects of primary prophylaxis with an antifungal agent. However, these are not, and should not, be considered mutually exclusive interventions.
The optimal CD4 count level at which primary antifungal prophylaxis should be initiated is unclear. Different studies have reported initiating treatment at < 50 cells/µL (Micol 2010), < 100 cells/µL (Chetchotisakd 2004; Micol 2010), < 200 cells/µL (Parkes‐Ratanshi 2011), and < 300 cells/µL (Smith 2001), with varying cost‐effectiveness.
Why it is important to do this review
The previous published version of this review showed that primary antifungal prophylaxis with either itraconazole or fluconazole was effective in reducing the incidence of cryptococcal disease in adults with advanced HIV disease. However, the effect on overall mortality was unclear (Chang 2005). Since the review's publication, a number of new, relevant trials have been published. Another review, which included observational studies in addition to randomized controlled trials (RCT), similarly concluded that primary antifungal prophylaxis could prevent cryptococcal meningitis, but not reduce all‐cause mortality (Ssekitoleko 2013). However, the scope of the review was limited to the adult population, and publications in English, in peer‐reviewed journals, with an outdated literature search.
In order to provide updated high‐quality evidence, we restricted our studies to RCTs, included paediatric populations, and non‐English publications, and conducted searches of the grey literature. The outputs of this review can contribute to the formulation of future guideline recommendations for the prevention of cryptococcal disease in adults and children who are HIV‐positive.
Objectives
To assess the efficacy and safety of antifungals for the primary prevention of cryptococcal disease in adults and children who are HIV‐positive.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs.
Types of participants
Adults and children who are HIV‐positive, with low CD4 cell counts, without a current or prior diagnosis of cryptococcal disease.
Types of interventions
Interventions
Triazole antifungals, used as primary prophylaxis to prevent fungal infections. We considered drugs within this class approved for clinical use, such as itraconazole, fluconazole, voriconazole, posaconazole, and isavuconazole.
Control
Placebo or no antifungal intervention.
Types of outcome measures
Primary outcomes
All‐cause mortality: number of deaths from any cause/number randomized
Secondary outcomes
-
Cryptococcal disease:
number of HIV‐positive people diagnosed/number randomized
-
including episodes of: antigenaemia, meningitis, or pneumonia during the follow‐up period
diagnosis of antigenaemia: serum cryptococcal antigen test, blood culture
diagnosis of meningitis: CSF India ink staining, CSF culture, CSF cryptococcal antigen test
diagnosis of pneumonia: culture, histopathology, or cytopathology of respiratory specimens
Deaths due to cryptococcal disease: number of deaths attributed to a diagnosis of cryptococcal meningitis
Adherence: number categorized as adherent by authors/number randomized
Cryptococcal antifungal drug resistance: number categorized as resistant by authors/number randomized
Infections caused by Candida species resistant to the prophylactic antifungal drug: number with infections by resistant Candida/number randomized
Treatment discontinuation: number discontinuing regimen due to adverse events, patient choice, pregnancy, or for any other reason. This was only assessed in trials with placebo control arms.
-
Adverse events:
number with any reported adverse event/number randomized
in addition, severe (grades 3 to 5) hepatotoxicity (elevated ALT and AST), anaemia, rash, diarrhoea, nausea, and vomiting (categorized according to the Division of AIDS Table for Grading severity of Adult and Paediatric adverse events) will be evaluated as the number with severe adverse events/number randomized for each of these events (DAIDS 2014).
Search methods for identification of studies
We attempted to identify all relevant studies, regardless of language or publication status. We included all studies that addressed one or more of our outcomes.
Electronic searches
We searched the following databases on 31 August 2017: the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, issue 8), published in the Cochrane Library; MEDLINE PubMed; Embase OVID, and CINAHL EBSCOHost, using the search strategies in Appendix 1.
We also searched the WHO International Clinical Trials Registry Platform (WHO ICTRP; www.who.int/ictrp/en/) and ClinicalTrials.gov (https://clinicaltrials.gov/ct2/home) on 31 August 2017, to identify ongoing trials.
Searching other resources
Grey literature
We actively searched for grey literature, by contacting researchers in the field and searching for publications regardless of language.
We searched abstracts from the Conference on Retroviruses and Opportunistic Infections (CROI) and the International AIDS (IAS) conferences. We searched conference outputs from 2015, 2016, and 2017.
Reference lists
We checked the reference lists of all studies identified by the above methods for other potentially relevant studies. We also searched the reference lists and included studies of other systematic reviews.
Correspondence
We contacted researchers working in the field for unpublished and ongoing trials.
Data collection and analysis
Selection of studies
Two review authors (AA and SJ) independently screened the titles and abstracts of the search results to identify studies relevant to this review. We resolved disagreements through consultation with the third review author (IEW). We retrieved full‐text articles of potentially eligible trials. We included studies that met the predefined inclusion criteria. We resolved disagreements by discussion with the third review author.
Data extraction and management
Two review authors (AA and SJ) independently extracted data from the included trials, using a standardized data extraction form, which we created and piloted. For each trial, we extracted the study design, risk of bias, participant characteristics (age, gender, ethnicity, baseline CD4+ T cell count and viral load, use of ART, time to ART, cryptococcal antigen status, endemicity of cryptococcus), trial setting, interventions (antifungal type, dose, and duration), duration of follow‐up, treatment discontinuations, adverse events, and reported outcomes.
We resolved disagreements in data extraction through consultation with the third review author (IEW). One author entered all the extracted data into Review Manager 5 (RevMan 2014). Another review author independently checked the entered data for accuracy. We contacted authors of primary trials for missing data.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each included study, using the Cochrane ‘Risk of bias' assessment tool (Higgins 2011). We resolved disagreements through consultation with the third review author. We contacted trial authors for clarification when the risk of bias was unclear. We summarized the results of the risk of bias for each included trial in the ‘Risk of bias' tables.
Measures of treatment effect
We measured the treatment effect for dichotomous outcomes using risk ratios (RR). We calculated 95% confidence intervals (CI) for all outcomes. We performed meta‐analysis where there were sufficient combinable data.
Unit of analysis issues
We analysed the data at the level of the individual.
Dealing with missing data
We performed all analyses on an intention‐to‐treat basis, using the total number of participants randomized as the denominator.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plots for CIs overlap, and by using the Chi² test for heterogeneity. We quantified the heterogeneity using the I² statistic. We used the approach set out in the Cochrane Handbook for Systematic Reviews of Interventions for statistical tests of heterogeneity. We interpreted I² in the context of (i) magnitude and direction of effects and (ii) strength of evidence for heterogeneity (e.g. P value from the Chi² test, or a CI for I²). We classified heterogeneity as defined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
We interrogated possible sources of heterogeneity, using subgroup analysis. Where we were unable to explain significant heterogeneity through subgroup analysis, we considered this when we assessed certainty of evidence with the GRADE criteria.
Assessment of reporting biases
No analysis included more than 10 trials, so we were unable to assess for publication bias.
Data synthesis
We analysed the data using Review Manager 5 (RevMan 2014). We used the random‐effects model for all meta‐analyses, as we considered the different studies to be estimating different, yet related, intervention effects (Higgins 2011). Where considerable unexplained heterogeneity was detected, we did not pool the results.
Subgroup analysis and investigation of heterogeneity
We investigated potential sources of heterogeneity by performing subgroup analyses for all‐cause mortality and cryptococcal disease outcomes on the following.
CD4+ threshold for initiation of prophylaxis
CrAg status at baseline
Timing of ART initiation
Type of ART
Type of antifungal medication
Sensitivity analysis
We included all randomized trials in the meta‐analysis, regardless of their risk of bias.
We had intended to conduct sensitivity analyses for the primary outcome by excluding trials with a high or unclear risk of bias for the following.
Attrition (> 20%)
Sequence generation
Allocation concealment
Assessing the certainty of the evidence
We evaluated the certainty of the evidence using the GRADE approach. We generated ‘Summary of findings' tables using GRADEpro GDT (GRADEpro GDT).
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
See Figure 1: Study flow diagram
1.
Study flow diagram.
Results of the search
We retrieved 1069 records from our searches conducted between 1 January 1980 and 31 August 2017, using the terms in our search strategy in Appendix 1. We identified 3 additional records through other sources. After removing duplicates, we identified 1045 records, which we screened for relevance against our inclusion criteria. We identified 41 records for full‐text screening; of these, we included nine randomized controlled trials (RCT) in 17 reports. The selection process is depicted in Figure 1.
Included studies
We included nine RCTs (17 records). See the ‘Characteristics of included studies' tables.
We also summarized key characteristics of these studies in Table 2, to aid interpretation of the data.
1. Key characteristics of included studies.
| Study ID | Country | Number randomized | Age (years) | CD4 threshold(cells/µL) | Triple ART regimen | Intervention | Time to ART | Excluded CrAg +ve | CrAG status at baseline |
| Chariyalertsak 2002 | Thailand | 129 | Mean 33 (range 22 to 58) | < 200 | No | Itraconazole 200 mg daily + CTX | NR | No | NR |
| Chetchotisakd 2004 | Thailand | 90 | Range: 20 to 53 | < 100 | No | Fluconazole 400 mg weekly | NR | Yes | CrAG‐ve: 90/90 |
| Goldman 2005 | USA | 829 | Median 38 (range: 19 to 71) | < 150 | No | Fluconazole 200 mg three times per week | NR | No | NR |
| Hakim 2017 | Uganda, Zimbabwe, Malawi, Kenya | 1805 | Median 36 (IQR 29 to 42) | < 100 | Yes | Enhanced prophylaxis: fluconazole 100 mg daily + CTX + INH daily + immediate albendazole + 5 days of azithromycin |
5 days (2 to 8) | No | CrAG+ve: 133/1781 |
| McKinsey 1999 | USA | 295 | Median 36 to 37 | < 150 | No | Itraconazole 200 mg daily | NR | No | NR |
| Parkes‐Ratanshi 2011 | Uganda | 1519 | Mean 36 | < 200 | Yes | Fluconazole 200 mg 3 times per week | 11 weeks (median; IQR 7 to 17 weeks); fluconazole 82 days; placebo 87 days | Yes | CrAG‐ve: 1519/1519 |
| Revankar 1998 | USA | 62 | NR | < 350 | Unknown | Fluconazole 200 mg daily | NR | No | NR |
| Schuman 1997 | USA | 323 | Mean 37 | < 300 | No | Fluconazole 200 mg weekly | NR | No | NR |
| Smith 2001 | Australia, Canada, South Africa, UK | 374 | Mean 38 (SD 8) | < 300 | No | Itraconazole 200 mg daily | NR | No | NR |
Abbreviations: NR: not reported; ART: antiretroviral therapy; CTX: co‐trimoxazole; CD4: cluster of differentiation 4; IQR: interquartile range; +ve: positive; ‐ve: negative.
Design
We included nine RCTs, with a total of 5426 participants. Two trials were conducted in Thailand (Chariyalertsak 2002; Chetchotisakd 2004), four in the USA (Goldman 2005; McKinsey 1999; Revankar 1998; Schuman 1997), one in Uganda (Parkes‐Ratanshi 2011), and two were multi‐centre trials conducted in Uganda, Zimbabwe, Malawi, and Kenya (Hakim 2017), and Australia, Canada, South Africa, and the UK (Smith 2001).
Participants
Most trials included both adults and adolescents, older than 13 years. One trial included adolescents over 15 years (Parkes‐Ratanshi 2011). One trial also included children older than five years (Hakim 2017).
Six trials did not report on the cryptococcal antigen (CrAg) status of their participants at baseline (Chariyalertsak 2002; Goldman 2005; McKinsey 1999; Revankar 1998; Schuman 1997; Smith 2001). Chetchotisakd 2004 and Parkes‐Ratanshi 2011 reported on the CrAg status of their participants at baseline, but excluded the CrAg‐positive patients. Hakim 2017 reported on the CrAg status of participants at baseline, but did not exclude the CrAg‐positive patients.
Full inclusion and exclusion criteria are presented in the ‘Characteristics of included studies' table.
Interventions
Six trials randomly assigned HIV‐positive participants to the antifungal study drug or placebo (Chariyalertsak 2002; Chetchotisakd 2004; McKinsey 1999; Parkes‐Ratanshi 2011; Schuman 1997; Smith 2001). Two studies randomized participants to continuous administration of antifungal prophylaxis or antifungals, as needed for the treatment of candidiasis (Goldman 2005; Revankar 1998). Hakim 2017 assigned participants randomly to either standard prophylaxis for Pneumocystis jiroveci pneumonia (PJP) with trimethoprim‐sulfamethoxazole or an enhanced prophylaxis package consisting of 12 weeks of fluconazole (100 mg once a day), one dose of albendazole 400 mg, five days of azithromycin (500 mg once a day), 12 weeks of trimethoprim–sulfamethoxazole (trimethoprim 160 mg once a day and sulfamethoxazole 800 mg once a day), isoniazid 300 mg once a day, and pyridoxine 25 mg once a day for 12 weeks.
The choices and doses of antifungal used included itraconazole 200 mg daily (Chariyalertsak 2002; McKinsey 1999; Smith 2001), fluconazole 100 mg daily (Hakim 2017), fluconazole 200 mg daily (Revankar 1998), fluconazole 200 mg three times per week (Goldman 2005; Parkes‐Ratanshi 2011), fluconazole 200 mg weekly (Schuman 1997), and fluconazole 400 mg weekly (Chetchotisakd 2004).
Five included studies did not report if participants received co‐trimoxazole prophylaxis (Chetchotisakd 2004; Goldman 2005; McKinsey 1999; Revankar 1998; Schuman 1997). Seventy‐five percent of participants in the treatment arm and 65% of participants in the placebo arm received co‐trimoxazole prophylaxis in Smith 2001. One study reported that participants were offered co‐trimoxazole according to national guidelines (Parkes‐Ratanshi 2011). All participants in two trials received standard co‐trimoxazole prophylaxis (Chariyalertsak 2002; Hakim 2017).
Participants in the Hakim 2017 and Parkes‐Ratanshi 2011 trials were all anti‐retroviral therapy (ART)‐naïve at the start of follow‐up, and then received current standard ART triple therapy, initiated during the trial. Participant therapies in the Hakim 2017 trial initiated ART at a median of five days, as would be expected under the current standard of care. Participants in the Parkes‐Ratanshi 2011 study initiated ART at a median of 11 weeks. Five trials included participants that were on a mix of a non‐current standard ART regimen and no ART at baseline (Chariyalertsak 2002; Goldman 2005; McKinsey 1999; Schuman 1997; Smith 2001). HIV‐positive participants in Chetchotisakd 2004 were all ART‐naïve at baseline, but they did not report which ART regimen they initiated. One trial did not report the ART status of its participants (Revankar 1998).
Outcome measures
Seven studies reported death as an outcome (Chariyalertsak 2002; Chetchotisakd 2004; Goldman 2005; Hakim 2017; McKinsey 1999; Parkes‐Ratanshi 2011; Smith 2001); we included six of these studies in our analysis. Hakim 2017 reported all‐cause mortality; however, the co‐interventions used in this study, as described in Table 2, could possibly have confounded any effect measured. Therefore, we did not include this study in our meta‐analysis for this outcome. The CD4 cell count thresholds for initiation of antifungal prophylaxis varied from < 100 cells/µL to < 300 cells/µL. Duration of follow‐up varied from 22 weeks to 42 months.
Seven studies reported the incidence of cryptococcal disease (Chariyalertsak 2002; Chetchotisakd 2004; Goldman 2005; Hakim 2017; McKinsey 1999; Parkes‐Ratanshi 2011; Smith 2001). Six studies measured cryptococcal disease occurrence, and used standard prophylaxis, consisting solely of an antifungal or placebo as an adjunct to standard care.
Five studies reported mortality due to cryptococcal disease (Chariyalertsak 2002; Chetchotisakd 2004; Hakim 2017; McKinsey 1999; Parkes‐Ratanshi 2011). In these studies, there was variable reporting of the method of diagnosis of death due to cryptococcal disease. Hakim 2017 measured cryptococcal disease occurrence and used enhanced prophylaxis, which included co‐interventions, as described in Table 2. We did not deem these co‐interventions to be active on mortality due to cryptococcal disease, and so included this study in the pooled estimate.
Only Chariyalertsak 2002 reported adherence to antifungal prophylaxis.
Four studies reported clinically defined Candida resistance in patients enrolled in trials (Chariyalertsak 2002; Goldman 2005; Revankar 1998; Schuman 1997). Chariyalertsak 2002 compared Itraconazole to placebo, while Goldman 2005, Revankar 1998, and Schuman 1997 compared fluconazole to placebo. We identified four studies that reported microbiologically‐defined resistance in Candida species isolated from patients enrolled in trials (Goldman 2005; McKinsey 1999; Revankar 1998; Schuman 1997).
Four studies reported discontinuation of antifungal prophylaxis compared to placebo for any reason, and adverse events (Chariyalertsak 2002; McKinsey 1999; Parkes‐Ratanshi 2011; Smith 2001).
Excluded studies
We excluded 22 studies after assessing the full‐text articles (see ‘Characteristics of excluded studies' table).
Studies awaiting classification
We were unable to retrieve the full‐text reports of two studies to assess them for inclusion (Smith 1999, Anonymous 1998).
Risk of bias in included studies
We have presented the ‘Risk of bias' summary, which represents the review authors' judgements about each risk of bias item for each included study in Figure 2. We have summarized our findings for each domain below:
2.

‘Risk of bias' summary: review authors' judgements about each ‘Risk of bias' item for each included study
Allocation
Computer‐generated randomization lists were used by Chariyalertsak 2002; Hakim 2017; and Smith 2001. Random lists were generated using permuted blocks in Parkes‐Ratanshi 2011; Revankar 1998; and Schuman 1997. Methods for sequence generation were not explicitly stated in Goldman 2005 and McKinsey 1999. No methods for sequence generation were described for Chetchotisakd 2004.
There was adequate concealment of treatment allocation in three of the nine trials (Chariyalertsak 2002; Hakim 2017; Parkes‐Ratanshi 2011). The remaining six did not record any method of allocation concealment.
Blinding
We judged all nine trials to be free of the risk of performance bias, as all the participants received either the study medication or matching placebo. Hakim 2017 was an open label trial, however, we judged our main outcomes to be objective assessments, and therefore not prone to performance bias.
We judged two of the nine trials as having unclear risk of detection bias (Goldman 2005; Schuman 1997).
Incomplete outcome data
We judged Revankar 1998 as having high risk of attrition bias, because a disproportionate number of participants in the intervention and control groups were excluded from the trial, based on death within three months of enrolment.
McKinsey 1999 and Chetchotisakd 2004 were assessed as having unclear risk of attrition bias, because neither trial recorded any loss to follow‐up data.
The remaining six trials were judged as having low risk of attrition bias.
Selective reporting
We assessed the risk of bias from selective outcome reporting to be unclear in Chetchotisakd 2004, as the authors did not report loss to follow‐up, drop out rates, or adverse events in detail. The other eight trials were assessed at low risk.
Other potential sources of bias
We assessed the risk of bias as high in the Revankar 1998 study, because baseline characteristics and baseline ART status were not described. Four trials were judged as having unclear risk, because there was insufficient information available to make an assessment on whether the funding received from pharmaceutical companies impacted the study design or analyses (Chariyalertsak 2002; Chetchotisakd 2004; McKinsey 1999; Smith 2001). We judged four trials at low risk of other potential sources of bias (Goldman 2005; Hakim 2017; Parkes‐Ratanshi 2011; Schuman 1997).
Effects of interventions
See: Table 1
Primary outcomes
All‐cause mortality
Antifungal prophylaxis had no consistent effect on all‐cause mortality (risk ratio (RR) 1.07, 95% CI 0.80 to 1.43; six trials, 3220 participants; Analysis 1.1). We could not include data for this outcome from the most recent trial, which initiated ART a mean of five days after screening, as there were co‐interventions in the intervention arm that would have confounded the effect on mortality (Hakim 2017).
1.1. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 1 All‐cause mortality.
Subgroup analyses
There was little difference in pooled effect estimates when we subdivided all‐cause mortality by: CD4 threshold for prophylaxis (I² statistic = 0%; Analysis 1.2), baseline CrAG status (I² statistic = 0%; Analysis 1.3), time‐to‐initiation of ART (I² statistic = 0%; Analysis 1.4), ART regimens (I² statistic = 0%; Analysis 1.5), or type of antifungal drug (I² statistic = 0%; Analysis 1.6).
1.2. Analysis.
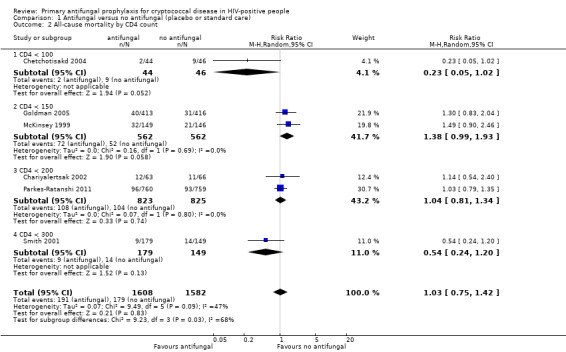
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 2 All‐cause mortality by CD4 count.
1.3. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 3 All‐cause mortality by baseline CrAG status.
1.4. Analysis.
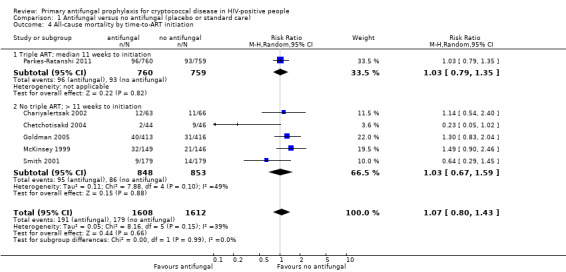
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 4 All‐cause mortality by time‐to‐ART initiation.
1.5. Analysis.
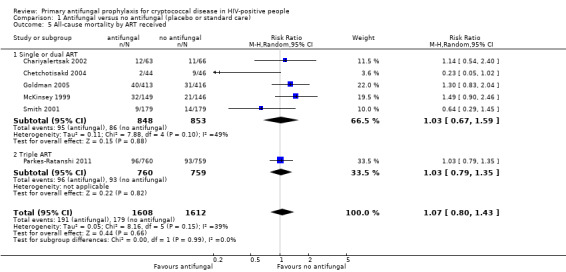
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 5 All‐cause mortality by ART received.
1.6. Analysis.
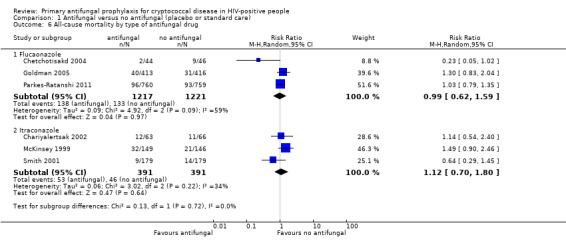
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 6 All‐cause mortality by type of antifungal drug.
Secondary outcomes
Cryptococcal disease occurrence
We excluded unconfirmed, suspected cases of cryptococcal disease from our analysis. Hakim 2017 measured cryptococcal disease occurrence, and used enhanced prophylaxis, which included co‐interventions described in Table 2. We did not deem these co‐interventions to be active on cryptococcal disease, so included this study in the pooled estimate.
The seven studies that measured cryptococcal disease identified 91 cases. Most of the studies did not report the source of the cryptococcal infection, simply referring to invasive cryptococcal disease. All 10 cases in Chetchotisakd 2004 were confirmed cases of cryptococcal meningitis; Smith 2001 reported one case of cryptococcal pneumonia and one case of cryptococcal meningitis. Parkes‐Ratanshi 2011 confirmed 11 cases of cryptococcal meningitis, five participants with invasive cryptococcal disease and positive blood cultures, and three participants who became CrAg‐positive after starting prophylaxis. Hakim 2017 reported 32 new cases of cryptococcal infection: 22 cases of cryptococcal meningitis, and one case of cryptococcal fungaemia in the standard prophylaxis arm, and nine cases of cryptococcal meningitis in the enhanced prophylaxis arm.
Meta‐analysis showed a large reduction in the risk of developing cryptococcal disease in those who received antifungal prophylaxis. Participants on antifungal prophylaxis were 71% less likely to develop cryptococcal disease than those receiving placebo or standard care (RR 0.29, 95% CI 0.17 to 0.49; seven trials, 5000 participants; Analysis 1.7). Benefit of antifungal prophylaxis was seen consistently across the included studies, although this was not statistically significant at a 95% level of confidence in four of the studies.
1.7. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 7 Cryptococcal disease occurrence.
Subgroup analyses
There was no clear difference in effect estimates when we subgrouped cryptococcal disease occurrence by: CD4 threshold for prophylaxis (I² 0%; Analysis 1.8), ART regimen (I² statistic = 0%; Analysis 1.9), or type of antifungal drug (I² 0%; Analysis 1.10 ). Subgrouping by time‐to‐initiation of ART showed a similar benefit of prophylaxis across all subgroups, with a small amount of heterogeneity (I² statistic = 36.9%; Analysis 1.11). There was no clear difference between subgroups by baseline CrAG status (I² statistic = 0%; Analysis 1.12). Proportionally fewer participants who were CrAg‐negative at baseline went on to develop cryptococcal disease (regardless of treatment arm) compared to CrAg‐positive cases. Few participants and one study contributed data to the baseline CrAg‐positive subgroup analysis (Hakim 2017).
1.8. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 8 Cryptococcal disease occurrence by CD4 count.
1.9. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 9 Cryptococcal disease occurrence by ART received.
1.10. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 10 Cryptococcal disease occurrence by type of antifungal drug.
1.11. Analysis.
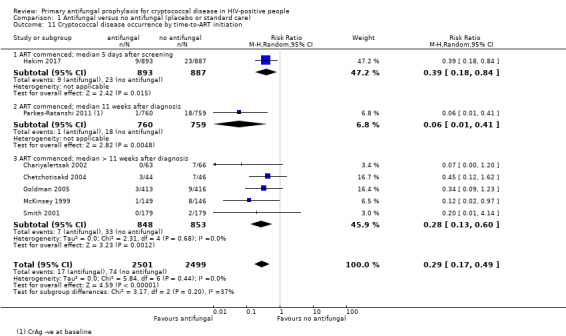
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 11 Cryptococcal disease occurrence by time‐to‐ART initiation.
1.12. Analysis.
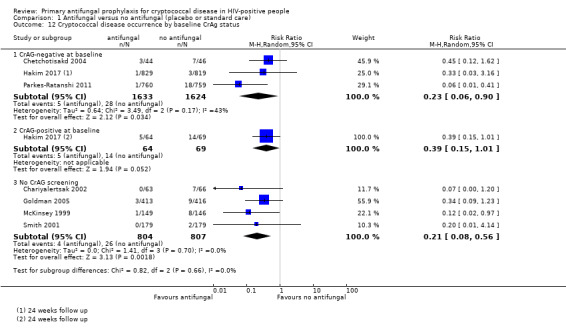
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 12 Cryptococcal disease occurrence by baseline CrAg status.
Cryptococcal‐specific mortality
People taking antifungal prophylaxis were less likely to die from cryptococcal disease (RR 0.29, 95% CI 0.11 to 0.72; five trials, 3813 participants; Analysis 1.13).
1.13. Analysis.
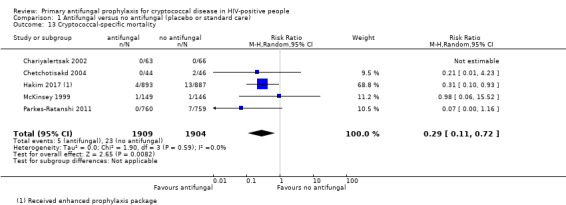
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 13 Cryptococcal‐specific mortality.
No clear difference was seen in studies that excluded participants who tested CrAG‐positive, and those on current standard ART regimens (one nucleoside reverse transcriptase inhibitor and two non‐nucleoside reverse transcriptase inhibitors).
Adherence
Chariyalertsak 2002 (129 participants) reported no significant difference in adherence between participants receiving antifungals and placebo. Ninety‐two per cent of those receiving antifungals adhered to the regimen, while 85% of those receiving placebo adhered.
Cryptococcal antifungal drug resistance
We did not identify any studies that reported cryptococcal antifungal resistance.
Infections caused by Candida species resistant to the prophylactic antifungal drug triazole
(a) Clinical resistance
Schuman 1997 compared fluconazole to placebo for the prevention of candidiasis. Two open label trials compared the continuous use of fluconazole prophylaxis for symptomatic treatment of clinical Candida disease (Goldman 2005; Revankar 1998). Clinical resistance was largely defined as participants who developed Candida disease that did not respond to treatment with fluconazole; the exact definition varied between studies, as described in Table 3. We subgrouped the results of this analysis by antifungal therapy.
2. Clinically defined resistance to fluconazole and itraconazole.
| Description of studies | 2 X 2 table | |||||
| Study ID | Aims of study | Definition of clinical resistance | Prophylaxis given | Intervention received | Number of participants with clinical disease resistant to fluconazole | Number of participants randomized |
| Clinically defined resistance (episodes of clinical resistance per number of patients randomised): fluconazole | ||||||
| Goldman 2005 | To compare fluconazole to standard care for the prevention of Candida infections. | Clinical endpoint defined as persistent or refractory candidiasis* | Fluconazole 200 mg three times weekly | Continuous fluconazole | 18 | 413 |
| Standard care | 18 | 416 | ||||
| Revankar 1998 | To compare fluconazole to standard care for the prevention of Candida infections. | Clinical resistance was defined as the presence of resistant isolates (MIC > 16 µg/mL) that affected response to therapy | Fluconazole 200 mg daily | Continuous fluconazole | 2 | 16 |
| Standard care | 5 | 28 | ||||
| Schuman 1997 | To compare fluconazole to placebo for prevention of mucosal candidiasis in HIV‐positive women. | Clinical resistance not defined | Fluconazole 200 mg once weekly | Fluconazole | 6 | 162 |
| Placebo + Standard care | 7 | 161 | ||||
| Clinically defined resistance (episodes of clinical resistance per number of patients randomised): itraconazole | ||||||
| Chariyalertsak 2002 | To compare Itraconazole prophylaxis to placebo for the prevention of deep fungal infections | Clinical resistance defined as candidiasis that did not respond to treatment* | Itraconazole 200 mg daily | Itraconazole | 1 | 63 |
| Placebo + Standard care | 0 | 66 | ||||
*Full details of definition of clinical disease available in Characteristics of included studies
Subgroup analyses
Neither fluconazole prophylaxis (RR 0.93, 95% CI 0.56 to 1.56; three trials, 1198 participants; Analysis 1.14) nor itraconazole prophylaxis (RR 3.14, 95% CI 0.13 to 75.69; one trial, 129 participants; Analysis 1.14) showed a clear effect on the risk of developing Candida disease clinically resistant to the antifungal agent.
1.14. Analysis.
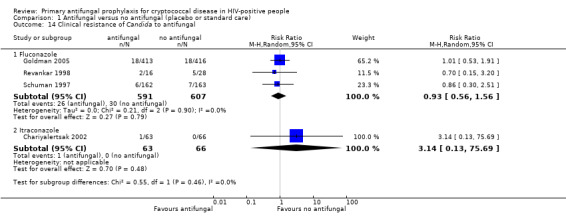
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 14 Clinical resistance of Candida to antifungal.
(b) Microbiological resistance
Three studies monitored resistance by taking surveillance cultures obtained from mucosal swabs, and reporting all strains of Candida resistant to fluconazole (Goldman 2005; Revankar 1998; Schuman 1997). Goldman 2005 and Revankar 1998 reported resistance in oropharyngeal swabs, and Schuman 1997 reported results from vaginal swabs. One study only reported Candida albicans isolates (McKinsey 1999). McKinsey 1999 used itraconazole, and reported both resistance to itraconazole and cross‐resistance to fluconazole, from swabs of any mucosa, from participants with clinical disease. We defined resistance to fluconazole as a minimum inhibitory concentration (MIC) > 16 µg/mL. All studies reported this. Schuman 1997 reported participants with a MIC > 16 µg/mL as ‘dose‐dependent susceptible'. They reported absolute resistance as MIC > 64 µg/mL. For this analysis, we combined participants with these results to form an aggregate number of events with MIC > 16 µg/mL (Table 4). There was marked qualitative heterogeneity between studies that reported on this outcome, as sampling methods, antifungal drug, and Candida species detected differed markedly between McKinsey 1999 and the remaining studies. As a result, we chose not to pool estimates across all three studies.
3. Microbiologically defined resistance of Candida to fluconazole.
| Description of studies | 2 X 2 table | |||||
| Study ID | Study aims | Type of isolate | Organism reported | Intervention received | Number of participants with at least 1 isolate resistant to fluconazole (MIC, > 16 µg/mL) | Number of participants with at least one sample where Candida was isolated |
| Microbiologically defined resistance of Candida to fluconazole (number of patients with at least one resistant isolate): fluconazole received | ||||||
| Schuman 1997 | To compare fluconazole to placebo for prevention of mucosal candidiasis in HIV‐positive women | Vaginal mucosal surveillance cultures taken 3 monthly | All Candida species combined | Fluconazole | 29 | 88 |
| Placebo + Standard care | 21 | 79 | ||||
| Goldman 2005 | To compare fluconazole to standard care for the prevention of Candida infections | Surveillance swab obtained at end of the study | All Candida species combined | Continuous fluconazole | 50 | 110 |
| Standard care | 79 | 218 | ||||
| Revankar 1998 | To compare fluconazole to standard care for the prevention of Candida infections | Isolates obtained from clinical disease and 3 monthly surveillance swabs | All Candida species combined | Continuous fluconazole | 9 | 16 |
| Standard care | 13 | 28 | ||||
| Microbiologically‐defined resistance of Candida to fluconazole (number of patients with at least one resistant isolate): itraconazole received, cross‐resistance to fluconazole reported | ||||||
| McKinsey 1999 | To compare Itraconazole to placebo for the prevention of deep fungal infections (including cryptococcal disease) | Vaginal and oesophageal mucosal isolates from clinical disease occurrences | C. albicans only (Other species not reported) | Itraconazole | 9/40* patients had isolates reported as ‘not susceptible' | 40 |
| Placebo + Standard care | 2/55* patients had isolates reported as ‘not susceptible' | 55 | ||||
*Itraconazole received, cross resistance to fluconazole reported.
Subgroup analyses
Among the three studies using fluconazole prophylaxis and surveillance sampling, antifungal prophylaxis was found to increase the risk of developing microbiological resistance to fluconazole in all Candida species (RR 1.25, 95% CI 1.00 to 1.55; three trials, 539 participants; Analysis 1.15). In the subgroup, which included one study in which itraconazole prophylaxis was used and samples were obtained from clinical disease, we found that antifungal prophylaxis increased the risk of developing microbiological cross‐resistance to fluconazole among C. albicans species (RR 6.19, 95% CI 1.41 to 27.10; one trial, 95 participants; Analysis 1.15; McKinsey 1999).
1.15. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 15 Microbiological resistance of Candida to fluconazole.
Treatment discontinuation
Four studies reported the discontinuation of antifungal prophylaxis compared to placebo for any reason (Chariyalertsak 2002; McKinsey 1999; Parkes‐Ratanshi 2011; Smith 2001). The reasons included serious adverse events, hepatotoxicity, pregnancy, use of contraindicated medications (such as rifampicin), and patient decision (Table 5). We found no clear difference between those who discontinued antifungal prophylaxis compared to placebo (RR 1.01, 95% CI 0.91 to 1.13; four trials, 2317 participants; Analysis 1.16).
4. Reasons for discontinuation of antifungal prophylaxis.
| Treatment discontinuation (cause) | Antifungal group | Placebo group |
| Chariyalertsak 2002 (N = 129) | ||
| Access disallowed medicationsa | 3 (2.3%) | 3 (2.3%) |
| Adverse events | 2 (1.6%) | 1 (0.7%) |
| Hepatotoxicity | 1 (0.7%) | 1 (0.7%) |
| Patient choice | 14 (11%) | 9 (6.9%) |
| McKinsey 1999 (N = 295) | ||
| Adverse events | 13 (4.4%) | 5 (1.7%) |
| Patient choice | 27 (9.1%) | 36 (12%) |
| Parkes‐Ratanshi 2011 (N = 1519) | ||
| Loss to follow‐up | 31 (2%) | 19 (1.3%) |
| Patient choice | 11 (0.7%) | 4 (0.3%) |
| Safety concerns | 59 (3.8%) | 59 (3.8%) |
| Smith 2001 (N = 374) | ||
| Access disallowed medicationsa | 15 (4%) | 3 (0.8%) |
| Adverse event | 31 (8.3%) | 29 (7.8%) |
| Hepatotoxicity | 2 (0.5%) | 3 (0.8%) |
| Patient choice | 33 (8.8%) | 46 (12%) |
| Pregnancy | 0 (0%) | 1 (0.3%) |
| Other | 37(9.9%) | 42 (11%) |
aWe defined this as the number of participants who had to discontinue the study medication because of the need to take other medication that interfered with itraconazole serum levels.
1.16. Analysis.
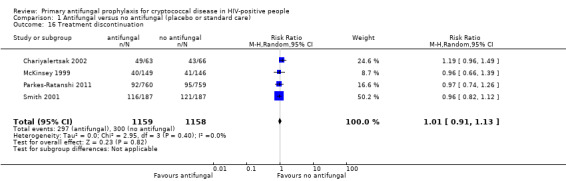
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 16 Treatment discontinuation.
Adverse events
We excluded Hakim 2017 from the analysis of adverse events, as unpicking the effects of the co‐interventions delivered in this trial was not possible.
(a) Serious adverse events
Four studies reported serious adverse events (Chariyalertsak 2002; Chetchotisakd 2004; McKinsey 1999; Smith 2001). These were measured as the number of patients experiencing at least one serious adverse event. One study reported no adverse events in either group (Chetchotisakd 2004). All studies were conducted before 2004, and as such, the participants were on a mix of older anti‐retroviral drugs, described in Table 2. There was no clear difference in the occurrence of serious adverse events between participants receiving antifungal prophylaxis and those receiving placebo. (RR 1.08, 95% CI 0.83 to 1.41; four trials, 888 participants; Analysis 1.17)
1.17. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 17 Any serious adverse event.
(b) Any adverse event
Four studies reported any adverse events (Chariyalertsak 2002; McKinsey 1999; Parkes‐Ratanshi 2011; Smith 2001).Three out of the four studies were conducted before 2004, and as such, the participants were on a mix of older anti‐retroviral drugs, described in Table 2. Adverse events were measured as the number of patients experiencing at least one adverse event. There was no clear difference in the occurrence of adverse events between participants receiving antifungal prophylaxis and those receiving placebo (RR 1.07, 95% CI 0.88 to 1.30; 4 trials; 2317 participants; Analysis 1.18).
1.18. Analysis.
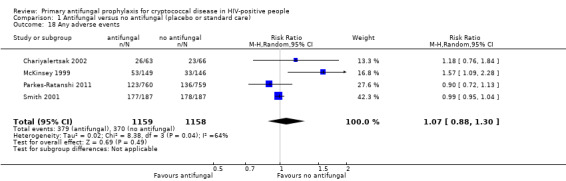
Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 18 Any adverse events.
No clear difference was found between groups for any of the most commonly reported adverse events (Analysis 1.19).
1.19. Analysis.

Comparison 1 Antifungal versus no antifungal (placebo or standard care), Outcome 19 Common adverse events.
Diarrhoea (RR 1.31, 95% CI 0.32 to 5.29; 2 trials, 424 participants)
Abdominal pain (RR 0.91, 95% CI 0.56 to 1.46; 2 trials, 1814 participants)
Nausea (RR 0.97, 95% CI 0.64 to 1.47; 2 trials, 1814 participants)
Rash (RR 1.03, 95% CI 0.56 to 1.9; 4 trials, 2317 participants)
Discussion
Summary of main results
See Table 1.
Nine trials, enrolling 5426 participants, met the inclusion criteria of this Cochrane Review.
Antifungal primary prophylaxis alone may make little or no difference to all‐cause mortality (low‐certainty evidence). For cryptococcal‐specific outcomes, prophylaxis probably reduces the risk of developing cryptococcal disease (moderate‐certainty evidence), and probably reduces deaths due to cryptococcal disease (moderate‐certainty evidence). It may make no clear difference to the risk of developing clinically‐resistant Candida disease (low‐certainty evidence); however, there may be an increased risk of having Candida resistant to fluconazole isolated by surveillance cultures (low‐certainty evidence). Antifungal prophylaxis was generally well‐tolerated, with no clear difference in the risk of needing to discontinue antifungal prophylaxis compared with placebo (moderate‐certainty evidence), and no clear difference in the risk of having any adverse event (low‐certainty evidence) or a serious adverse event (low‐certainty evidence).
Potential benefits of antifungal prophylaxis
Antifungal prophylaxis probably reduces the risk of developing cryptococcal disease. It also probably reduces the risk of dying from cryptococcal disease.
Potential harms of antifungal prophylaxis
Antifungal prophylaxis is well tolerated, with no clear difference in the occurrence of adverse events, and probably no clear difference in treatment discontinuations. There may be an increased risk of developing fluconazole resistant Candida species, although this may not translate to disease resistant to treatment. In the absence of cryptococcal antigen (CrAg) screening programmes and high CrAg prevalence, primary prophylaxis could under‐treat CrAg‐positive people who are HIV‐positive with high titres and subclinical meningitis. Itraconazole potentially interacts with common first‐line antiretrovirals (tenofovir, efavirenz) rendering it less suitable for widespread use compared to fluconazole, where there are no interactions with current first line antiretrovirals (HIV drug interactions 2018).
Overall completeness and applicability of evidence
We included nine trials that evaluated the efficacy and safety of interventions for preventing cryptococcal infection in HIV‐positive people. Four of these trials were conducted in low‐ and middle‐income countries, while the remaining five were conducted in high‐income countries. All participants were adults, even though several studies included children and adolescents in eligibility criteria.
Several studies included in this review were older and less relevant to the contemporary HIV experience, due to changes in antiretroviral therapy (ART) treatment regimens and timing of ART initiation in recent years. Only two trials included participants who received currently recommended triple ART (Hakim 2017; Parkes‐Ratanshi 2011), and in only one of these was ART initiated within one to two weeks of HIV diagnosis, as would be the current practice, particularly in patients with low CD4 cell counts (Hakim 2017). In addition, three studies used itraconazole prophylaxis, which is less commonly used, due to substantial drug interactions (Chariyalertsak 2002; McKinsey 1999; Smith 2001). Hakim 2017 evaluated a combination of interventions that included antifungals, antibiotics, and anthelmintics, compared with standard prophylaxis for pneumocystis using only co‐trimoxazole. Despite the finding that several studies did not represent the current HIV care experience, the protective effect of prophylaxis was consistent across all study populations, including those receiving the current standard of HIV care.
Two studies excluded CrAg‐positive patients prior to randomization (Chetchotisakd 2004; Parkes‐Ratanshi 2011). One study reported baseline CrAg status after trial completion (Hakim 2017). Among CrAg‐negative participants, antifungal prophylaxis continued to show a protective effect. However, there were far fewer occurrences of cryptococcal disease overall among those who were CrAg‐negative at baseline, compared to those who were CrAg‐positive.
We found no trials that reported on resistance of Cryptococcus isolates, and this is an important gap in our understanding of the adverse effects of antifungal prophylaxis.
There was some evidence that antifungal prophylaxis may increase the number of resistant Candida species in surveillance samples; however, it is unclear if this translates to clinically meaningful Candida resistance, as no clear effect was demonstrated on the risk of developing clinically resistant Candida disease. However, the certainty of the evidence contributing to these analyses was low, making it difficult to draw firm conclusions on the impact of antifungal prophylaxis on Candida resistance.
The data on adverse events from these trials were graded as low quality, and as a result, we should also interpret the finding of no clear difference between treatment arms with caution. However, moderate‐quality evidence suggested that treatment discontinuation did not clearly differ between study arms, suggesting that adverse events may in fact not differ between the groups.
Certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach and presented our findings in the Table 1. Three of the included studies were designed as open label studies. We did not consider this biased the outcomes measured, as our primary outcome, and most of the secondary outcomes, were objectively measured. Certainty ranged from moderate to low across all the reported outcomes. Reasons for downgrading included: the majority of participants not receiving the current standard of care relating to type of ART, and time from diagnosis to initiation, indirectness related to the subjective assessment of mortality due to cryptococcal disease, few events, unclear grading of serious adverse events, and unexplained substantial heterogeneity related to the assessment of adverse events. Many of the trials we found were older and less relevant to current HIV care; we considered this in our approach to GRADEing indirectness.
Potential biases in the review process
We minimized biases in the conduct of this review by adhering to the standard methodology described in Cochrane Handbook for Systematic reviews of Interventions. We conducted a comprehensive literature search with no language restrictions. Two authors independently scanned the search results for potentially eligible studies. Two review authors independently assessed full‐text articles of potentially eligible trials, and two review authors independently extracted data from the nine included trials.
We recognized that there were limitations and potential biases in measuring mortality due to cryptococcal disease, due to the risk of misdiagnosis. However, we chose to include this outcome to give a better reflection of the effect of the intervention on cryptococcal disease. We took this into account in our assessment of the certainty of the evidence.
Resistance to fluconazole is one of the main concerns and criticisms of antifungal prophylaxis, but microbiological resistance detected in surveillance cultures did not necessarily translate to clinical disease; however, the review would have been somewhat incomplete if we did not present all the evidence that was available on this issue. Again, this was taken into account in our assessment of the certainty of the evidence.
We further amended our inclusion criteria to include studies with co‐interventions. We minimized the confounding effect of these co‐interventions by only including trials with outcomes where the co‐interventions were considered to have minimal or no impact on the outcome being measured. For example, Hakim 2017 reported a reduction in all‐cause mortality; however, there were important co‐interventions that would have had an effect on mortality, so these data were not included in the analysis for this outcome.
These differences are detailed in the Differences between protocol and review section.
Agreements and disagreements with other studies or reviews
The findings from this review were consistent with those of previous published reviews, which both showed that antifungal prophylaxis may have made little or no difference to all‐cause mortality, but reduced the occurrence of cryptococcal disease (Chang 2005; Ssekitoleko 2013). However, the findings from this review are more relevant to current HIV populations.
One study included in the Chang 2005 review did not meet our inclusion criteria. We also included two studies published after the Chang 2005 review (Hakim 2017; Parkes‐Ratanshi 2011). Furthermore, we considered outcomes related to resistance in trials looking at prevention of Candida infection, which were not included in the Chang 2005 review (Goldman 2005; Revankar 1998; Schuman 1997) .
Authors' conclusions
Implications for practice.
Primary prophylaxis with either fluconazole or itraconazole probably reduces the risk of developing cryptococcal disease. Prophylaxis also probably reduces the risk of death due to cryptococcal disease, however, this may not have translated to a reduction in all‐cause mortality in the trials identified. Clinicians and policy makers will have to consider the benefit of providing antifungal prophylaxis in the context of cryptococcal disease prevalence, cost, consistent drug supply, and the availability of cryptococcal antigen (CrAg) screening in their setting. Antifungal primary prophylaxis could be considered a part of differentiated packages of care for those who are diagnosed late with low cluster of differentiation 4 (CD4) cell counts, and those at risk of cryptococcal disease, particularly where CrAg screening is unavailable.
Implications for research.
The authors do not believe that further research is required to show the efficacy of primary antifungal prophylaxis in reducing the occurrence of cryptococcal disease, particularly among patients where CrAg status is unknown. The cost‐benefit of providing antifungal prophylaxis to CrAg‐negative patients remains an area of debate, due to the low occurrence of cryptococcal disease in this group. Further analyses of the cost effectiveness and feasibility of implementing this intervention in different settings are needed, as well as comparisons between the primary prophylaxis strategy and the strategy of CrAg screening plus pre‐emptive antifungal therapy for those who screened positive.
What's new
| Date | Event | Description |
|---|---|---|
| 28 August 2018 | New search has been performed | This is an update of a review last published in 2005 (Chang 2005). The review author team updated the protocol extensively, and differences are highlighted in the ‘Differences between protocol and review' section. |
| 28 August 2018 | New citation required and conclusions have changed | Nine trials (5426 participants) met the inclusion criteria of this review update.One study included in the Chang 2005 review did not meet our inclusion criteria. We also included two studies published after the Chang 2005 review (Hakim 2017; Parkes‐Ratanshi 2011). We considered outcomes related to resistance in trials looking at prevention of Candida infection, which were not included in the Chang 2005 review. The findings of this review update are consistent with those of previous published reviews, which both showed that antifungal prophylaxis may have made little or no difference to all‐cause mortality, but reduced the occurrence of cryptococcal disease (Chang 2005; Ssekitoleko 2013). However, the findings from this review are more relevant to current HIV populations. |
Acknowledgements
The Academic Editor of this review was Professor Mical Paul.
We thank the Cochrane Infectious Disease Group for their support and help in streamlining the review process and Paul Garner for his help as Co‐ordinating Editor.
We thank Vittoria Lutje and Joy Oliver for helping with the search strategy. We want to thank Marcel Kitenge for his contribution and assistance with conference and additional searches.
Ajibola Awotiwon, Ingrid Eshun‐Wilson, and Samuel Johnson were supported by the Effective Health Care Research Consortium. This Consortium and the CIDG editorial base is funded by UK aid from the UK Government for the benefit of low‐ and middle‐income countries (Grant: 5242). The views expressed in this publication do not necessarily reflect UK government policy.
SJ was supported by a funding agreement from Cochrane CRG support programme (Cochrane UK).
Graeme Meintjes was supported by the Wellcome Trust (098316), and the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (Grant No 64787).
Appendices
Appendix 1. Search strategies
Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor: [HIV Infections] explode all trees
#2 MeSH descriptor: [HIV] explode all trees
#3 hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome):ti,ab,kw (Word variations have been searched)
#4 MeSH descriptor: [Lymphoma, AIDS‐Related] this term only
#5 MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only
#6 #1 or #2 or #3 or #4 or #5 Publication Year from 1980 to 2017
#7 prevent* or prophyl* or chemoprevent* or chemoprophyla*:ti,ab,kw (Word variations have been searched)
#8 MeSH descriptor: [Antifungal Agents] explode all trees
#9 azole* or fluconazole or amphotericin or flucytosine or voriconazole or diflucan or itraconazole:ti,ab,kw (Word variations have been searched)
#10 #8 or #9#6 and 7 and #10
MEDLINE PubMed
| Search | Query |
| #17 | Search (((((((("Cryptococcosis"[Mesh] OR "Meningitis, Cryptococcal"[Mesh]) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) OR ((cryptococcosis OR cryptococcoses OR torulosis OR toruloses OR cryptococcal OR cryptococal OR cryptococcus OR toruloma OR torulomas) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) AND ((prevent* [Title/Abstract] OR prophyl* [Title/Abstract] OR chemoprevent* [Title/Abstract] OR chemoprophyla* [Title/Abstract] or primary [Title/Abstract]) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) AND (((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) AND ((((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab]))))) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Publication date from 1980/01/01 |
| #16 | Search ((("Cryptococcosis"[Mesh] OR "Meningitis, Cryptococcal"[Mesh]) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) OR ((cryptococcosis OR cryptococcoses OR torulosis OR toruloses OR cryptococcal OR cryptococal OR cryptococcus OR toruloma OR torulomas) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Publication date from 1980/01/01 |
| #6 | Search ((((((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) AND ((((Antifungal agents[mh] OR azole*[tiab] OR fluconazole[tiab] OR amphotericin[tiab] OR flucytosine[tiab] OR voriconazole[tiab] OR diflucan[tiab] OR itraconazole[tiab] OR rifampin[tiab] OR 5‐FC[tiab]))) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) AND ((prevent* [Title/Abstract] OR prophyl* [Title/Abstract] OR chemoprevent* [Title/Abstract] OR chemoprophyla* [Title/Abstract]) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] ))) AND ((((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab]))))) AND ( "1980/01/01"[PDat] : "3000/12/31"[PDat] )) Sort by: PublicationDate Filters: Publication date from 1980/01/01 |
| #5 | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) Sort by: PublicationDate Filters: Publication date from 1980/01/01 |
| #4 | Search ((Antifungal agents[mh] OR azole*[tiab] OR fluconazole[tiab] OR amphotericin[tiab] OR flucytosine[tiab] OR voriconazole[tiab] OR diflucan[tiab] OR itraconazole[tiab] OR rifampin[tiab] OR 5‐FC[tiab])) Sort by: PublicationDate Filters: Publication date from 1980/01/01 |
| #3 | Search prevent* [Title/Abstract] OR prophyl* [Title/Abstract] OR chemoprevent* [Title/Abstract] OR chemoprophyla* [Title/Abstract] Sort by: PublicationDate Filters: Publication date from 1980/01/01 |
| #2 | Search ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])))) Sort by: PublicationDate Filters: Publication date from 1980/01/01 |
| #1 | Search ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])))) Sort by: PublicationDate |
Embase
1 ‘human immunodeficiency virus infection'/exp or ‘human immunodeficiency virus infection'.mp. or ‘human immunodeficiency virus'/exp or ‘human immunodeficiency virus'.mp. or ‘human immunodeficiency virus':ab,ti.mp. or ‘human immuno+deficiency virus':ab,ti.mp. or ‘human immunedeficiency virus':ab,ti.mp. or ‘human immune+deficiency virus':ab,ti.mp. or hiv:ab,ti.mp. or ‘hiv‐1':ab,ti.mp. or ‘hiv‐2':ab,ti.mp. or ‘acquired immunodeficiency syndrome':ab,ti.mp. or ‘acquired immuno+deficiency syndrome':ab,ti.mp. or ‘acquired immunedeficiency syndrome':ab,ti.mp. or ‘acquired immune+deficiency syndrome':ab,ti.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] (391379)
2 ‘randomized controlled trial'/de or ‘randomized controlled trial'.mp. or random*:ab,ti.mp. or trial:ti.mp. or allocat*:ab,ti.mp. or factorial*:ab,ti.mp. or placebo*:ab,ti.mp. or assign*:ab,ti.mp. or volunteer*:ab,ti.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] (614214)
3 ‘crossover procedure'/de or ‘crossover procedure'.mp. or ‘double‐blind procedure'/de or ‘double‐blind procedure'.mp. or ‘single‐blind procedure'/de or ‘single‐blind procedure'.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] (206231)
4 (crossover or cross‐over).ab. or (crossover or cross‐over).ti. (89952)
5 2 or 3 or 4 (713029)
6 antifungal agent.mp. or exp antifungal agent/ (336774)
7 (fluconazole or amphotericin or flucytosine or voriconazole or diflucan or itraconazole or rifampin or 5‐FC).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword, floating subheading word] (106224)
8 (prevent* or prophyl* or chemoprevent* or chemoprophyla*:).ab. or (prevent* or prophyl* or chemoprevent* or chemoprophyla*:).ti. (1772805)
9 prophylaxis/ (100999)
10 6 or 7 (348179)
11 8 or 9 (1793846)
12 1 and 5 and 10 and 11 (152)
CINAHL EBSCOHost
| Search ID# | Search Terms |
| S4 | S1 AND S2 AND S3 |
| S3 | TI ( prevent* or prophyl* or chemoprevent* or chemoprophyla* ) OR AB ( prevent* or prophyl* or chemoprevent* or chemoprophyla* ) |
| S2 | MH antifungal agents OR ( fluconazole or amphotericin or flucytosine or voriconazole or diflucan or itraconazole or rifampin or 5‐FC ) |
| S1 | MH hiv infection OR MH hiv OR TX ( hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency sy ... |
Data and analyses
Comparison 1. Antifungal versus no antifungal (placebo or standard care).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 6 | 3220 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.80, 1.43] |
| 2 All‐cause mortality by CD4 count | 6 | 3190 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.75, 1.42] |
| 2.1 CD4 < 100 | 1 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.05, 1.02] |
| 2.2 CD4 < 150 | 2 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.99, 1.93] |
| 2.3 CD4 < 200 | 2 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.81, 1.34] |
| 2.4 CD4 < 300 | 1 | 328 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.24, 1.20] |
| 3 All‐cause mortality by baseline CrAG status | 6 | 3220 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.80, 1.43] |
| 3.1 CrAG‐negative at baseline | 2 | 1609 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.14, 2.43] |
| 3.2 No CrAG screening | 4 | 1611 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.91, 1.63] |
| 4 All‐cause mortality by time‐to‐ART initiation | 6 | 3220 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.80, 1.43] |
| 4.1 Triple ART; median 11 weeks to initiation | 1 | 1519 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.35] |
| 4.2 No triple ART; > 11 weeks to initiation | 5 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.59] |
| 5 All‐cause mortality by ART received | 6 | 3220 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.80, 1.43] |
| 5.1 Single or dual ART | 5 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.67, 1.59] |
| 5.2 Triple ART | 1 | 1519 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.35] |
| 6 All‐cause mortality by type of antifungal drug | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Flucaonazole | 3 | 2438 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.62, 1.59] |
| 6.2 Itraconazole | 3 | 782 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.70, 1.80] |
| 7 Cryptococcal disease occurrence | 7 | 5000 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.49] |
| 8 Cryptococcal disease occurrence by CD4 count | 7 | 5000 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.49] |
| 8.1 CD4 < 100 | 2 | 1870 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.78] |
| 8.2 CD4 < 150 | 2 | 1124 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.08, 0.76] |
| 8.3 CD4 < 200 | 2 | 1648 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.31] |
| 8.4 CD4 < 300 | 1 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.14] |
| 9 Cryptococcal disease occurrence by ART received | 7 | 5000 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.49] |
| 9.1 No triple ART | 5 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.60] |
| 9.2 Triple ART | 2 | 3299 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.03, 1.30] |
| 10 Cryptococcal disease occurrence by type of antifungal drug | 7 | 5000 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.49] |
| 10.1 Fluconazole | 4 | 4218 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.16, 0.62] |
| 10.2 Itraconazole | 3 | 782 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.03, 0.51] |
| 11 Cryptococcal disease occurrence by time‐to‐ART initiation | 7 | 5000 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.17, 0.49] |
| 11.1 ART commenced; median 5 days after screening | 1 | 1780 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.18, 0.84] |
| 11.2 ART commenced; median 11 weeks after diagnosis | 1 | 1519 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.41] |
| 11.3 ART commenced; median > 11 weeks after diagnosis | 5 | 1701 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.60] |
| 12 Cryptococcal disease occurrence by baseline CrAg status | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 CrAG‐negative at baseline | 3 | 3257 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.06, 0.90] |
| 12.2 CrAG‐positive at baseline | 1 | 133 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.15, 1.01] |
| 12.3 No CrAG screening | 4 | 1611 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.08, 0.56] |
| 13 Cryptococcal‐specific mortality | 5 | 3813 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.11, 0.72] |
| 14 Clinical resistance of Candida to antifungal | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 Fluconazole | 3 | 1198 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.56, 1.56] |
| 14.2 Itraconazole | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 3.14 [0.13, 75.69] |
| 15 Microbiological resistance of Candida to fluconazole | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 Surveillance sampling, fluconazole used, all Candida species | 3 | 539 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.00, 1.55] |
| 15.2 Sampling from clinical disease, itraconazole used, C. albicans only | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 6.19 [1.41, 27.10] |
| 16 Treatment discontinuation | 4 | 2317 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.91, 1.13] |
| 17 Any serious adverse event | 4 | 888 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.83, 1.41] |
| 18 Any adverse events | 4 | 2317 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.88, 1.30] |
| 19 Common adverse events | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Diarrhoea | 2 | 424 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.32, 5.29] |
| 19.2 Abdominal pain | 2 | 1814 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.56, 1.46] |
| 19.3 Nausea | 2 | 1814 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.64, 1.47] |
| 19.4 Rash | 4 | 2317 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.56, 1.91] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chariyalertsak 2002.
| Methods | Study design: randomized controlled trial (RCT) | |
| Participants |
Inclusion criteria: 18 to 60 years, documented HIV infection, Karnofsky score of > 70 (normal activity possible with effort), absolute CD4 lymphocyte count of < 200 cells/µL, and residence in the Chiang Mai area. Exclusion criteria: history of systemic fungal infections, use of systemic antifungal therapy within 2 weeks before study entry, history of active tuberculosis, pregnancy or breastfeeding, a history of intolerance to triazole compounds, failure to use a medically approved and effective method of birth control, inability to take oral medications, use of a medication with a known interaction with itraconazole, and serum aminotransferase levels at > 5 times the upper limit of normal. Number randomized: 129 Descriptive baseline data:
Dropouts during study period: 0 |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Country: Thailand Setting: hospital Dates: March 1998 to February 2000 (recruitment) Funding: Funded by Janssen Pharmaceuticals Others: Study was stopped in March 2000 after the first patient completed 104 weeks of follow‐up, when an interim analysis showed significant difference in the occurrence of systemic fungal infections between the two groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The patients were randomly assigned to receive itraconazole or placebo in a 1:1 ratio. Randomization was performed by the drug manufacturer (Janssen Pharmaceutical) with a computerized randomization list based on a block size of 6. |
| Allocation concealment (selection bias) | Low risk | The medication was packaged in sequentially numbered boxes that were dispensed to successive patients. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A prospective, randomized, placebo‐controlled, double‐blind study was conducted to compare the safety and efficacy of itraconazole (200 mg per day) with that of placebo." Placebo was identical in appearance to the study drug |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study was described as double‐blind. The authors did not explicitly state that the outcome assessors were blinded. However, the outcomes we assessed in this review were mostly objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available, however, no suggestion of selective reporting seen. |
| Other bias | Unclear risk | Grant received from Janssen Pharmaceuticals; Janssen also randomized participants. No information on specific conflicts of interests provided. |
Chetchotisakd 2004.
| Methods | Study design: RCT | |
| Participants |
Inclusion criteria: adult patients (> 14 years old) with documented HIV infection and CD4 counts < 100 cells/µL Exclusion criteria: systemic fungal infection, allergy or intolerance to fluconazole, liver enzymes > 5 times the normal limit, positive serum cryptococcal antigen, and pregnancy and lactation in women Number randomized: 90 Descriptive baseline data:
Dropouts during study period: not reported |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: Thailand Setting: hospital Dates: February 2000 to August 2001 (recruitment) Funding: not reported Others: study was terminated because of the national policy that fluconazole should be used in practice. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No method recorded |
| Allocation concealment (selection bias) | Unclear risk | No method recorded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" patients received placebo or study medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study was described as double‐blind. The authors did not explicitly state that the outcome assessors were blinded. However, the outcomes we assessed in this review were mostly objective. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up and dropout rates not recorded |
| Selective reporting (reporting bias) | Unclear risk | Loss to follow‐up, dropout rates, and adverse events not reported in detail ("No serious adverse reaction related to medication was seen during the study") |
| Other bias | Unclear risk | No information provided on conflicts of interest |
Goldman 2005.
| Methods | Study design: open label RCT | |
| Participants |
Inclusion criteria: documentation of HIV infection, a CD4+ T cell count of less than or equal to 150 cells/mm³ within 30 days before study entry, age over 13, weight > 40 kg, experienced one episode of oesophageal candidiasis in 6 months before randomization. Exclusion criteria: Pregnant, prior resistant Candida infection, azole allergy or intolerance, development of 3 episodes of OPC within 12 weeks before study entry, history of EC, need for systemic antifungal therapy, receipt of 11 months of continuous systemic or oral topical antifungal therapy within the past 3 months, severe liver disease, treated for oppurtunistic infection 14 days prior to randomization, subjects receiving medications contraindicated with fluconazole. Number randomized: 829 Descriptive baseline data:
Dropouts during study period: fluconazole‐continuous therapy 13%; fluconazole‐episodic 8.9%; combined 11% |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: multi‐centre ‐ USA Setting: hospitals Dates: May 1997 to April 2000 (recruitment) Funding: Trial was funded by the National Institute of Allergy and Infectious Diseases, National Institutes of Health and Pfizer. Others: Defnition of clinically resistant Candida infection: "A subject was considered to have an fluconazole resistant infection if (1) signs or symptoms of oesophageal candidiasis (EC) worsened after 7 days of therapy and either endoscopically confirmed EC or worsening oropharyngeal candidiasis (OPC) occurred, accompanied by oesophageal symptoms; (2) OPC remained after 14 days of therapy for EC; or (3) OPC or confirmed EC was present after 21 days of therapy" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described. “Eligible subjects were randomised at a ratio of 1:1 to undergo 1 of 2 different management strategies” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open label, outcomes measured not prone to performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open label trial so blinding of clinical assessors not possible. No blinding of laboratory staff assessed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for treatment discontinuation or attrition addressed in comprehensive flow diagram 184/416 in episodic arm prematurely discontinued randomized strategy 205/413 in continuous arm prematurely discontinued randomized strategy Attrition balanced between arms – majority exited due to non‐compliance (balanced between arms) |
| Selective reporting (reporting bias) | Low risk | Protocol available. All expected outcomes reported. |
| Other bias | Low risk | Funding information reported and conflicts of interests addressed |
Hakim 2017.
| Methods | Study design: open label RCT | |
| Participants |
Inclusion criteria: HIV‐positive adults and children who were 5 years of age or older, who had not received previous ART, and who had a CD4+ count of fewer than 100 cells per cubic millimetre. Exclusion criteria: pregnancy or breast‐feeding, had received single‐dose nevirapine to prevent mother to‐child transmission of HIV, or had any contraindications to the trial drugs. Number randomized: 1805 Descriptive baseline data:
Dropouts during study period: 3.1%: Standard prophylaxis (24); Enhanced prophylaxis (18) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: multicentre; Uganda, Zimbabwe, Malawi, and Kenya Setting: Urban and peri‐urban centres Dates: June 2013 to April 2015 (recruitment) Funding: supported by the Joint Global Health Trials Scheme of the Medical Research Council (MRC), the U.K. Department for International Development, the Wellcome Trust, and the PENTA Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “computer generated sequential randomisation list with variably sized permuted blocks was prepared by the trial statistician and incorporated securely into the online trial database.” |
| Allocation concealment (selection bias) | Low risk | “The list was concealed until eligibility was confirmed by staff members at the local centre, who then performed the randomisation” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “open label”; “ all nurses and physicians were aware of the trial‐group assignments” Although study was unblinded, this was unlikely to have an impact on the outcome we extracted from this study – cryptococcal disease |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was not blinded – however, diagnosis of cryptococcal meningitis is not very subjective and we did not think this would have introduced bias, in addition, secondary outcomes were evaluated by a review board. “An end‐point review committee whose members were unaware of trial‐group assignment and trial drugs received used protocol defined criteria and grading tables to adjudicate all the secondary clinical outcomes that were reported by the trial physicians” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% were lost to follow up or withdrew consent after randomization |
| Selective reporting (reporting bias) | Low risk | These were not the results of the full study – patients were also randomized to receive raltegravir and additional nutrition. However all results relevant to the antifungal prophylaxis portion of the study were reported. The protocol was available for review. |
| Other bias | Low risk | Of note, patients also were randomized to receive raltegravir or nutritional supplements, which may have impacted some of the outcomes, but unlikely to impact diagnosis of cryptococcal meningitis. |
McKinsey 1999.
| Methods | Study design: RCT | |
| Participants |
Inclusion criteria: age > 13 years, HIV (western blot or enzyme immunoassay), life expectancy > 1 year, no life‐threatening infection or malignancy other than Kaposi sarcoma, CD4 < 150, and residence in a city with high prevalence of histoplasmosis. Exclusion criteria: Use of investigational drug in last 1 month, pregnancy or lactation, failure to use contraception, history of intolerance, unable to take medications orally, active fungal infection, and use of medication with interaction Number randomized: 295 Descriptive baseline data:
Dropouts during study period: not reported |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: USA Setting: multi‐centre: urban (Kansas, Indianapolis, Nashville, Memphis) Dates: June 1993 to April 1995 (recruitment) Funding: The study was supported by the National Institute of Allergy and Infectious Diseases and the Janssen Research Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization not explicitly stated, although it is stated that each site had an independent randomization code. "Randomisation was stratified by site, and each site in the study had an independent randomisation code." |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was described as double‐blind and they received a placebo capsule, which was identical in appearance to itraconazole |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study was described as double‐blind. The authors did not explicitly state that the outcome assessors were blinded. However, the outcomes we assessed in this review were mostly objective. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up was not reported. |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported. No protocol available. |
| Other bias | Unclear risk | Funded by the National Institute of Allergy and Infectious Diseases and by Janssen Research Foundation. No information provided on role of funding on study design or outcomes assessed. |
Parkes‐Ratanshi 2011.
| Methods | Study design: RCT | |
| Participants |
Inclusion criteria: ART‐naïve adults (> 15 years) with laboratory confirmation of HIV infection (Murex HIV‐1.2.0, Murex Biotech; HIV Uni‐form II plus O, Biomerieux; Cambridge Biotech HIV‐1 Western blot) and a CD4 count less than 200 cells/µL (FACSCount Becton Dickinson, USA) Exclusion criteria: serum cryptococcal antigen (CrAg; Remel, Lexana, USA) titre > 1:8 on 2 occasions, pregnancy or lactation, liver transaminases (LFT) > 3 x upper limit of normal (ULN), and moribund patients. Number randomized: 1519 Descriptive baseline data:
Dropouts during study period: fluconazole (4%); placebo (2.5%) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: Uganda Setting: multi‐centre ‐ hospitals and clinics Dates: Sept 2004 to Feb 2008 (recruitment) Funding: The trial was funded by the Medical Research Council, UK, and the Rockefeller Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “An independent statistician prepared a list for 1:1 randomisation to fluconazole or matching placebo in random permuted blocks of size 40.” |
| Allocation concealment (selection bias) | Low risk | “Trial drug was packaged and labelled by an independent clinician and pharmacist. Participants were allocated to sequential trial numbers on enrolment and received the corresponding sealed trial drug pack.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients received matching placebo or study medication |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The (EPRC) had access to participants’ files, hospital notes, verbal autopsy data, and retrospective CrAg results, but were blind to treatment group.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3.3% of participants were lost to follow‐up and 1% withdrew consent. |
| Selective reporting (reporting bias) | Low risk | Trial was registered on controlled‐trials.com |
| Other bias | Low risk | “This research was supported by the Medical Research Council, UK, and the Rockefeller Foundation. Neither had a role in design, analysis, or writing of this paper.” |
Revankar 1998.
| Methods | Study design: open label RCT | |
| Participants |
Inclusion criteria: HIV‐positive patients, CD4 < 350, evidence of active oropharyngeal candidiasis by potassium hydroxide (KOH) preparation and culture and currently not taking any azole compound. Exclusion criteria: known hypersensitivity to azole compounds, were unable to take oral medications, pregnancy, serum alanine aminotransferase/aspartate aminotransferase ratio more than 10 times normal, serum alkaline phosphatase level more than 5 times normal, bilirubin level was more than 3 times normal. Number randomized: 62 Descriptive baseline data:
Dropouts during study period: fluconazole‐continuous (5%); fluconazole‐intermittent (9.5%) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: USA Setting: tertiary health centre Dates: not reported Funding: the trial was funded by the National Institute of Dental Research, the National Institute of Health for the Frederic C. Bartter General Clinical Research Center and Pfizer Inc. Others: resistance was defined as a rise in MIC > 16 µg/mL from initial culture, the emergence of new, resistant (MIC > 16 µg/mL) species any time after the initial culture, or an increase in the proportion of resistant isolates from 10% to at least 50% in a species. Patients who had resistant isolates at the initial culture could be considered to have developed resistance if either of the latter two criteria were present. Microbiological resistance was defined as simply the presence of resistant isolates (MIC > 16 µg/mL). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was by permuted blocks with a block size of six |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Open label trial – assessment of Candida resistance may be prone to performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of lab staff not discussed, assessment of Candida resistance may be subjective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss to follow up < 20% (8%) Those who died at < 3 months were excluded from analysis. 4 in intervention group and 16 in control group were excluded based on death < 3 months. |
| Selective reporting (reporting bias) | Low risk | No protocol available; all expected outcomes reported. |
| Other bias | High risk | Baseline characteristics not described. No description of baseline ART status. |
Schuman 1997.
| Methods | Study design: RCT | |
| Participants |
Inclusion criteria: age >13 years, HIV (western blot or enzyme immunoassay), CD4 < 300 Exclusion criteria: history of Candida oesophagitis, receiving systemic antifungals, known intolerance of azoles, current pregnancy or lactating Number randomized: 323 Descriptive baseline data:
Dropouts during study period: fluconazole (5%); placebo (10%) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: USA Setting: multicentre: urban, 14 sites participating in the community programmes for clinical research Dates: May 1992 to January 1994 Funding: The trial was supported by the National Institute of Allergies and Infectious Diseases (NIAD) Others: Open label fluconazole was permitted for candidiasis prophylaxis was permitted after two oropharyngeal episodes or one episode of vaginal or oesophageal |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomly assigned to received weekly fluconazole or placebo using a permuted block scheme with randomly mixed block sizes of two and four" |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as "double blind", and no subjective outcomes assessed |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of laboratory assessors analysing Candida isolates not described, assessment of Candida resistance may be subjective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "95% of surviving patients receiving fluconazole and 90% of patients receiving placebo attended follow‐up 6 months after finishing the trial |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in the methods section were reported. No protocol available. |
| Other bias | Low risk | "Staff members from NIAID (funding body) were part of the protocol team but had no role in decision to publish the study |
Smith 2001.
| Methods | Study design: RCT | |
| Participants |
Inclusion criteria: documented HIV‐1 infection and average of two CD4 counts of < 300 cells/mL within the past 4 months Exclusion criteria: women who were pregnant or not using reliable contraception, severe hepatic impairment, known hypersensitivity to azole compounds, a history of previous systemic fungal infection (including oesophageal candidosis) or any fungal infection unresponsive to azole therapy and use of systemic antifungal agents, rifabutin, rifampicin, phenytoin, terfenadine, astemizole, anticholinergic agents, or H2 antagonists. Number randomized: 374 participants Descriptive baseline data:
Dropouts during study period: itraconazole (9%); placebo (6%) |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
Location: multicentre; Australia, Canada, South Africa, UK Setting: clinic Dates: January 1994 to October 1997 Funding: The trial was funded by the Janssen Research Foundation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by a computer generated code |
| Allocation concealment (selection bias) | Unclear risk | No description of methods of allocation concealment documented |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blind. Patients received matching placebo or study medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study was described as double‐blind. The authors did not explicitly state that the outcome assessors were blinded. However, the outcomes we assessed in this review were mostly objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 10% loss to follow‐up over 2 years. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported, no protocol available |
| Other bias | Unclear risk | Role of Janssen research foundation in design of study and any analysis unclear |
Abbreviations: CD4: cluster of differentiation 4; OPC: oropharyngeal candidiasis; EC: oesophageal candidiasis.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 1995 | This was an editorial report of another study. |
| Anonymous 2001 | This was a systematic review. |
| Apisarnthanarak 2008a | This was a retrospective study. |
| Chaiwarith 2011 | This was a retrospective cohort study. |
| Chaiwarith 2013 | The patients included in this study were on secondary prophylaxis for cryptococcal infection. |
| Geletko 1996 | This was a cross‐over study. |
| Havlir 1998 | The comparator in this study was not placebo or no intervention. |
| Jüst‐Nubling 1991 | This study did not report on any of the outcomes we were interested in for this review. |
| Manfredi 1997 | This was a retrospective study. |
| Manosuthi 2005 | This was a retrospective cohort study. |
| Manosuthi 2006 | This was a retrospective cohort study. |
| Mfinanga 2015 | The intervention evaluated in this study was community support combined with serum cryptococcal antigen screening. |
| Micol 2010 | This was a cost‐effectiveness study. |
| Mylonakis 1998 | This was an editorial report of another study. |
| Penzak 1998 | This was an editorial report. |
| Powderly 1995 | The comparator was not placebo or no intervention. |
| Singh 1996 | The participants in this study were not randomized. |
| Stevens 1991 | This study did not report on any of the outcomes we were interested in. |
| Svoboda 1995 | This was a narrative review. |
| Thurey 2008 | This was a systematic review. |
| Wakeham 2010 | This study did not report on any of the outcomes we were interested in for this review. |
| White 1993 | This was a narrative review. |
Characteristics of studies awaiting assessment [ordered by study ID]
Anonymous 1998.
| Methods | Not known |
| Participants | HIV‐positive women |
| Interventions | Not known |
| Outcomes | Not known |
| Notes | Abstract and full‐text unavailable for screening |
Smith 1999.
| Methods | RCT |
| Participants | Number of participants (N): 70 participants |
| Interventions | 1. Itraconazole 200 mg daily 2. Placebo |
| Outcomes | 1. Treatment discontinuation 2. Adverse events |
| Notes | Full text unavailable for screening |
Differences between protocol and review
This is an update of a previous Cochrane review (Chang 2005). The new review author team extensively revised the protocol, which is available on the CIDG website at cidg.cochrane.org/our‐reviews under the subheading ‘Related content'.
Several outcomes that were not originally included in the protocol were added during the review process. This included mortality due to cryptococcal disease and microbiological resistance in Candida species. We included these outcomes to clarify the benefits and harms of the intervention.
Adherence was reported as described in the trial.
Several outcomes measures changed from rate to proportion. There was no intention of analysing these outcomes as rates and the teams intention was always to look at proportions, however incorrect wording was used in the published protocol and this was corrected in the final review.
We counted cases of cryptococcal disease in the studies if the investigators referred to them as confirmed cases. We did not count cases that the authors referred to as suspected. We also didn't rely on the study authors specifically describing the method of diagnosis.
We included studies that didn't specify the method of cryptococcal diagnosis.
We included studies that gave co‐trimoxazole prophylaxis in both groups, as we decided that in order for the review to be relevant in today's setting, we would need to include studies where standard HIV co‐interventions, such as co‐trimoxazole and isoniazid prophylaxis were provided.
We included studies that provided other co‐interventions with antifungal prophylaxis. We felt this was necessary in order to include the most recent and applicable evidence. We minimized the confounding effect of the co‐interventions as described previously.
Candida resistance to fluconazole was assessed by microbiological assessment and not restricted to clinical diseases. We used an MIC > 16 µg/mL to define resistance to fluconazole, according to the majority of the study definitions.
We amended the comparator to placebo or no antifungal intervention in response to peer review comments.
We amended our subgroup analyses in response to peer review comments to include the following subgroups for all‐cause mortality and cryptococcal disease occurrence.
CD4+ threshold for initiation of prophylaxis
CrAg status at baseline
Timing of ART initiation
Type of ART
Type of antifungal drug
Contributions of authors
AA, SJ, and IEW planned and drafted the protocol, SJ and AA screened and extracted all data, SJ AA and IEW performed all analyses and GRADEd the outcomes.
AA and SJ drafted the final review. IEW commented on the final review.
GM and GR reviewed and edited the protocol, assisted with methodological and clinical queries, and commented on the final review.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
-
Department for International Development, UK.
Grant: 5242
Declarations of interest
AA has no known conflicts of interest. SJ has no known conflicts of interest. GR has no known conflicts of interest. GM has no known conflicts of interest. IEW has no known conflicts of interest.
These authors contributed equally to this work
These authors contributed equally to this work
Unchanged
References
References to studies included in this review
Chariyalertsak 2002 {published data only}
- Chariyalertsak S, Supparatpinyo K, Sirisanthana T, Nelson KE. A controlled trial of itraconazole as primary prophylaxis for systemic fungal infections in patients with advanced human immunodeficiency virus infection in Thailand. Clinical Infectious Diseases 2002;34(2):277‐84. [PUBMED: 11740718] [DOI] [PubMed] [Google Scholar]
Chetchotisakd 2004 {published data only}
- Chetchotisakd P, Sungkanuparph S, Thinkhamrop B, Mootsikapun P, Boonyaprawit P. A multicentre, randomized, double‐blind, placebo‐controlled trial of primary cryptococcal meningitis prophylaxis in HIV‐infected patients with severe immune deficiency. HIV Medicine 2004;5(3):140‐3. [PUBMED: 15139978] [DOI] [PubMed] [Google Scholar]
Goldman 2005 {published data only}
- Goldman M, Cloud GA, Wade KD, Reboli AC, Fichtenbaum CJ, Hafner R, et al. A randomized study of the use of fluconazole in continuous versus episodic therapy in patients with advanced HIV infection and a history of oropharyngeal candidiasis: AIDS Clinical Trials Group Study 323/Mycoses Study Group Study 40. Clinical Infectious Diseases 2005;41(10):1473‐80. [DOI] [PubMed] [Google Scholar]
- NCT00000951. A study to compare the use of fluconazole as continuous therapy versus periodic therapy in HIV‐positive patients with recurrent thrush [A Phase IV randomized study of the use of fluconazole as chronic suppressive therapy versus episodic therapy in HIV positive subjects with recurrent oropharyngeal candidiasis]. clinicaltrials.gov/ct2/show/NCT00000951 (first posted 31 August 2001).
Hakim 2017 {published data only}
- Hakim J, Musiime V, Szubert AJ, Mallewa J, Siika A, Agutu C, et al. Enhanced prophylaxis plus antiretroviral therapy for advanced HIV infection in Africa. New England Journal of Medicine 2017;377(3):233‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN43622374. Reduction of early mortality in HIV‐infected African adults and children starting antiretroviral therapy [Reduction of Early mortALITY in HIV‐infected African adults and children starting antiretroviral therapy: a randomised controlled trial]. isrctn.com/ISRCTN43622374 (first received 28 September 2011).
McKinsey 1999 {published data only}
- Goldman M, Cloud GA, Smedema M, LeMonte A, Connolly P, McKinsey DS, et al. Does long‐term itraconazole prophylaxis result in in vitro azole resistance in mucosal Candida albicans isolates from persons with advanced human immunodeficiency virus infection? The National Institute of Allergy and Infectious Diseases Mycoses study group. Antimicrobial Agents and Chemotherapy 2000;44(6):1585‐7. [PUBMED: 10817713] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monte AM, Goldman M, Smedema ML, Connolly PA, McKinsey DS, Cloud GA, et al. DNA fingerprinting of serial Candida albicans isolates obtained during itraconazole prophylaxis in patients with AIDS. Medical Mycology 2001;39(2):207‐13. [PUBMED: 11346270] [DOI] [PubMed] [Google Scholar]
- McKinsey DS, Wheat LJ, Cloud GA, Pierce M, Black JR, Bamberger DM, et al. Itraconazole prophylaxis for fungal infections in patients with advanced human immunodeficiency virus infection: randomized, placebo‐controlled, double‐blind study. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Clinical Infectious Diseases 1999;28(5):1049‐56. [PUBMED: 10452633] [DOI] [PubMed] [Google Scholar]
Parkes‐Ratanshi 2011 {published data only}
- ISRCTN76481529. Primary prevention of invasive cryptococcal disease using fluconazole prophylaxis in Human Immunodeficiency Virus (HIV) infected Ugandans. isrctn.com/ISRCTN76481529 (first received 18 May 2001).
- Parkes‐Ratanshi R, Wakeham K, Kamali A, Levin J, Coutinho A, Whitworth J, et al. Successful primary prevention of cryptococcal disease using fluconazole prophylaxis in HIV‐infected Ugandan adults (cryptopro): o26. HIV Medicine 2009;10:8‐9. [Google Scholar]
- Parkes‐Ratanshi R, Wakeham K, Levin J, Namusoke D, Whitworth J, Coutinho A, et al. Primary prophylaxis of cryptococcal disease with fluconazole in HIV‐positive Ugandan adults: a double‐blind, randomised, placebo‐controlled trial. Lancet Infectious Diseases 2011;11(12):933‐41. [PUBMED: 21982529] [DOI] [PMC free article] [PubMed] [Google Scholar]
Revankar 1998 {published data only}
- Revankar SG, Kirkpatrick WR, McAtee RK, Dib OP, Fothergill AW, Redding SW, et al. A randomized trial of continuous or intermittent therapy with fluconazole for oropharyngeal candidiasis in HIV‐infected patients: clinical outcomes and development of fluconazole resistance. American Journal of Medicine 1998;1:7‐11. [DOI] [PubMed] [Google Scholar]
Schuman 1997 {published data only}
- Schuman P, Capps L, Peng G, Vazquez J, el‐Sadr W, Goldman AI, et al. Weekly fluconazole for the prevention of mucosal candidiasis in women with HIV infection. A randomized, double‐blind, placebo‐controlled trial. Terry Beirn Community Programs for Clinical Research on AIDS. Annals of Internal Medicine 1997;126(9):689‐96. [DOI] [PubMed] [Google Scholar]
- Vazquez JA, Peng G, Sobel JD, Steele‐Moore L, Schuman P, Holloway W, et al. Evolution of antifungal susceptibility among Candida species isolates recovered from human immunodeficiency virus‐infected women receiving fluconazole prophylaxis. Clinical Infectious Diseases 2001;33(7):1069‐75. [DOI] [PubMed] [Google Scholar]
- Vazquez JA, Sobel JD, Peng G, Steele‐Moore L, Schuman P, Holloway W, et al. Evolution of vaginal Candida species recovered from human immunodeficiency virus‐infected women receiving fluconazole prophylaxis: the emergence of Candida glabrata? Terry Beirn Community Programs for Clinical Research in AIDS (CPCRA). Clinical Infectious Diseases 1999;28(5):1025‐31. [DOI] [PubMed] [Google Scholar]
Smith 2001 {published data only}
- Smith DE, Bell J, Johnson M, Youle M, Gazzard B, Tchamouroff S, et al. A randomized, double‐blind, placebo‐controlled study of itraconazole capsules for the prevention of deep fungal infections in immunodeficient patients with HIV infection. HIV Medicine 2001;2(2):78‐83. [PUBMED: 11737382] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 1995 {published data only}
- Anonymous. Fluconazole may have edge in preventing infections. AIDS Alert 1995;10(5):67‐8. [Google Scholar]
Anonymous 2001 {published data only}
- Anonymous. Antifungal drug fluconazole found to be effective in preventing thrush in people who are HIV positive. AHRQ Research Activities 2001;250:15. [Google Scholar]
Apisarnthanarak 2008a {published data only}
- Apisarnthanarak A, Mundy LM. The impact of primary prophylaxis for cryptococcosis on fluconazole resistance in Candida species. Journal of Acquired Immune Deficiency Syndromes 2008;47(5):644‐5. [DOI] [PubMed] [Google Scholar]
Chaiwarith 2011 {published data only}
- Chaiwarith R, Fakthongyoo A, Praparattanapan J, Boonmee D, Sirisanthana T, Supparatpinyo K. Itraconazole vs fluconazole as a primary prophylaxis for fungal infections in HIV‐infected patients in Thailand. Curr HIV Research 2011;9(5):334‐8. [DOI] [PubMed] [Google Scholar]
Chaiwarith 2013 {published data only}
- Chaiwarith R, Praparattanapan J, Nuntachit N, Kotarathitithum W, Supparatpinyo K. Discontinuation of primary and secondary prophylaxis for opportunistic infections in HIV‐infected patients who had CD4+ cell count 3 but undetectable plasma HIV‐1 RNA: an open‐label randomized controlled trial. AIDS Patient Care and STDs 2013;27(2):71‐6. [DOI] [PubMed] [Google Scholar]
Geletko 1996 {published data only}
- Geletko SM, Segarra M, Mayer KH, Fiore TC, Bettencourt FA, Flanigan TP, et al. Electronic compliance assessment of antifungal prophylaxis for human immunodeficiency virus‐infected women. Antimicrobial Agents and Chemotherapy 1996;40(6):1338‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Havlir 1998 {published data only}
- Havlir DV, Dube MP, McCutchan JA, Forthal DN, Kemper CA, Dunne MW, et al. Prophylaxis with weekly versus daily fluconazole for fungal infections in patients with AIDS. Clinical Infectious Diseases 1998;27(6):1369‐75. [DOI] [PubMed] [Google Scholar]
Jüst‐Nubling 1991 {published data only}
- Jüst‐Nubling G, Gentschew G, Meissner K, Odewald J, Staszewski S, Helm EB, et al. Fluconazole prophylaxis of recurrent oral candidiasis in HIV‐positive patients. European Journal of Clinical Microbiology & Infectious Diseases 1991;10(11):917‐21. [DOI] [PubMed] [Google Scholar]
Manfredi 1997 {published data only}
- Manfredi R, Mastroianni A, Coronado OV, Chiodo F. Fluconazole as prophylaxis against fungal infection in patients with advanced HIV infection. Archives of Internal Medicine 1997;157(1):64‐9. [PubMed] [Google Scholar]
Manosuthi 2005 {published data only}
- Manosuthi W, Chumpathat N, Chaovavanich A, Sungkanuparph S. Safety and tolerability of nevirapine‐based antiretroviral therapy in HIV‐infected patients receiving fluconazole for cryptococcal prophylaxis: a retrospective cohort study. BMC Infect Diseases 2005;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manosuthi 2006 {published data only}
- Manosuthi W, Sungkanuparph S, Thongyen S, Chumpathat N, Eampokalap B, Thawornwan U, et al. Antifungal susceptibilities of Cryptococcus neoformans cerebrospinal fluid isolates and clinical outcomes of cryptococcal meningitis in HIV‐infected patients with/without fluconazole prophylaxis. Journal of the Medical Association of Thailand 2006;89(6):795‐802. [PubMed] [Google Scholar]
Mfinanga 2015 {published data only}
- Mfinanga S, Chanda D, Kivuyo SL, Guinness L, Bottomley C, Simms V, et al. Cryptococcal meningitis screening and community‐based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open‐label, randomised controlled trial. Lancet 2015;385(9983):2173‐82. [DOI] [PubMed] [Google Scholar]
Micol 2010 {published data only}
- Micol R, Tajahmady A, Lortholary O, Balkan S, Quillet C, Dousset JP, et al. Cost‐effectiveness of primary prophylaxis of AIDS associated cryptococcosis in Cambodia. PLoS One 2010;5(11):e13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mylonakis 1998 {published data only}
- Mylonakis E, Flanigan TP. Editorial response: Antifungal prophylaxis with weekly fluconazole for patients with AIDS. Clinical Infectious Diseases 1998;27(6):1376‐8. [DOI] [PubMed] [Google Scholar]
Penzak 1998 {published data only}
- Penzak SR, Gubbins PO. Preventing and treating azole‐resistant oropharyngeal candidiasis in HIV‐infected patients. American Journal of Health‐System Pharmacy 1998;55(3):279‐83. [DOI] [PubMed] [Google Scholar]
Powderly 1995 {published data only}
- Powderly WG, Finkelstein D, Feinberg J, Frame P, He W, Horst C, et al. A randomised trial comparing fluconazole with clotrimazole troches for the prevention of fungal infections in patients with advanced human immunodeficiency virus infection. NIAID AIDS Clinical Trials Group. New England Journal of Medicine 1995;332(11):700‐5. [DOI] [PubMed] [Google Scholar]
Singh 1996 {published data only}
- Singh N, Barnish MJ, Berman S, Bender B, Wagener MM, Rinaldi MG, et al. Low‐dose fluconazole as primary prophylaxis for cryptococcal infection in AIDS patients with CD4 cell counts of ≤ 100/mm³: demonstration of efficacy in a positive, multicenter trial. Clinical Infectious Diseases 1996;23(6):1282‐6. [DOI] [PubMed] [Google Scholar]
Stevens 1991 {published data only}
- Stevens DA, Greene SI, Lang OS. Thrush can be prevented in patients with acquired immunodeficiency syndrome and the acquired immunodeficiency syndrome‐related complex. Randomized, double‐blind, placebo‐controlled study of 100‐mg oral fluconazole daily. Archives of Internal Medicine 1991;151(12):2458‐64. [PUBMED: 1747004] [PubMed] [Google Scholar]
Svoboda 1995 {published data only}
- Svoboda J. Prophylaxis of opportunistic infections in HIV infection. Journal of Community Health 1995;20(2):203‐7. [DOI] [PubMed] [Google Scholar]
Thurey 2008 {published data only}
- Thurey J, Molyneux E. Evidence behind the WHO guidelines: Hospital Care for Children: the usefulness of azole prophylaxis against cryptococcal meningitis in HIV‐positive children. Journal of Tropical Pediatrics 2008;54(6):361‐3. [DOI] [PubMed] [Google Scholar]
Wakeham 2010 {published data only}
- Wakeham K, Parkes‐Ratanshi R, Watson V, Ggayi AB, Khoo S, Lalloo DG. Co‐administration of fluconazole increases nevirapine concentrations in HIV‐infected Ugandans. Journal of Antimicrobial Chemotherapy 2010;65(2):316‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 1993 {published data only}
- White MH. Antifungal prophylaxis. Current Opinion in Infectious Diseases 1993;6(6):737‐839. [Google Scholar]
References to studies awaiting assessment
Anonymous 1998 {published data only}
- Anonymous. Preventing mucosal candidiasis in HIV‐infected women. Emergency Medicine 1998;30(2):112‐5. [Google Scholar]
Smith 1999 {published data only}
- Smith D, Midgley J, Gazzard B. A randomised, double‐blind study of itraconazole versus placebo in the treatment and prevention of oral or oesophageal candidosis in patients with HIV infection. International Journal of Clinical Practice 1999;53(5):349‐52. [PUBMED: 10695098] [PubMed] [Google Scholar]
Additional references
Apisarnthanarak 2008b
- Apisarnthanarak A, Jirayasethpong T, Sa‐nguansilp C, Thongprapai H, Kittihanukul C, Kamudamas A, et al. Antiretroviral drug resistance among antiretroviral‐naive persons with recent HIV infection in Thailand. HIV Medicine 2008;9(5):322‐5. [PUBMED: 18400079] [DOI] [PubMed] [Google Scholar]
Brion 2007
- Brion LP, Uko SE, Goldman DL. Risk of resistance associated with fluconazole prophylaxis: systematic review. Journal of Infection 2007;54(6):521‐9. [PUBMED: 17239952] [DOI] [PubMed] [Google Scholar]
CDC 2017
- Panel on Opportunistic Infections in HIV‐Infected Adults, Adolescents. Guidelines for the prevention and treatment of opportunistic infections in HIV‐infected adults and adolescents: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at aidsinfo.nih.gov/contentfiles/lvguidelines/adult_oi.pdf (accessed 3 Sept 2017).
Cheong 2013
- Cheong JW, McCormack J. Fluconazole resistance in cryptococcal disease: emerging or intrinsic?. Medical Mycology 2013;51(3):261‐9. [PUBMED: 22989195] [DOI] [PubMed] [Google Scholar]
DAIDS 2014
- DAIDS Grading Table Working Group. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events. Version 2.0. Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services. November 2014. Available from rsc.tech‐res.com/docs/default‐source/safety/daids_ae_grading_table_v2_nov2014.pdf.
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 6 August 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
HIV drug interactions 2018
- Liverpool HIV drug interactions. HIV drug interaction checker. www.hiv‐druginteractions.org/checker (accessed 22 May 2018).
Jarvis 2010
- Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: findings from 4961 suspected cases. BMC Infectious Diseases 2010;10:67. [PUBMED: 20230635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kambugu 2008
- Kambugu A, Meya DB, Rhein J, O'Brien M, Janoff EN, Ronald AR, et al. Outcomes of cryptococcal meningitis in Uganda before and after the availability of highly active antiretroviral therapy. Clinical Infectious Diseases 2008;46(11):1694‐701. [PUBMED: 18433339] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kontoyiannis 2002
- Kontoyiannis DP, Lewis RE. Antifungal drug resistance of pathogenic fungi. Lancet 2002;359(9312):1135‐44. [PUBMED: 11943280] [DOI] [PubMed] [Google Scholar]
Lessells 2011
- Lessells RJ, Mutevedzi PC, Heller T, Newell ML. Poor long‐term outcomes for cryptococcal meningitis in rural South Africa. South African Medical Journal 2011;101(4):251‐2. [PUBMED: 21786729] [DOI] [PubMed] [Google Scholar]
McLachlan 1996
- McLachlan AJ, Tett SE. Pharmacokinetics of fluconazole in people with HIV infection: a population analysis. British Journal of Clinical Pharmacology 1996;41(4):291‐8. [PUBMED: 8730974] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirza 2003
- Mirza SA, Phelan M, Rimland D, Graviss E, Hamill R, Brandt ME, et al. The changing epidemiology of cryptococcosis: an update from population‐based active surveillance in 2 large metropolitan areas, 1992‐2000. Clinical Infectious Diseases 2003;36(6):789‐94. [PUBMED: 12627365] [DOI] [PubMed] [Google Scholar]
Pierard 2000
- Pierard GE, Arrese JE, Pierard‐Franchimont C. Itraconazole. Expert Opinion on Pharmacotherapy 2000;1(2):287‐304. [DOI] [PubMed] [Google Scholar]
Pound 2011
- Pound MW, Townsend ML, Dimondi V, Wilson D, Drew RH. Overview of treatment options for invasive fungal infections. Medical Mycology 2011;49(6):561‐80. [PUBMED: 21366509] [DOI] [PubMed] [Google Scholar]
Rajasingham 2017
- Rajasingham R, Smith RM, Park BJ, Jarvis JN, Govender NP, Chiller TM, et al. Global burden of disease of HIV‐associated cryptococcal meningitis: an updated analysis. Lancet Infectious Diseases 2017;17(8):873‐81. [PUBMED: 28483415] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rhein 2016
- Rhein J, Morawski BM, Hullsiek K, Nabeta H, Kiggundu R, Tugume L, et al. Efficacy of adjunctive sertraline for the treatment of HIV‐associated cryptococcal meningitis: an open‐label dose‐ranging study. Lancet Infectious Diseases 2016;16(7):809‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skolnik 2017
- Skolnik K, Huston S, Mody CH. Cryptococcal lung infections. Clinical Chest Medicine 2017;38:451‐64. [DOI] [PubMed] [Google Scholar]
Ssekitoleko 2013
- Ssekitoleko R, Kamya MR, Reingold AL. Primary prophylaxis for cryptococcal meningitis and impact on mortality in HIV: a systematic review and meta‐analysis. Future Virology 2013;8(9):917‐30. [PUBMED: 24368930] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tenforde 2017
- Tenforde MW, Mokomane M, Leeme T, Patel RKK, Lekwape N, Ramodimoosi C, et al. Advanced HIV disease in Botswana following successful antiretroviral therapy rollout: incidence of and temporal trends in cryptococcal meningitis. Clinical Infectious Diseases 2017;65(5):779‐86. [PUBMED: 28505328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wall 2014
- Wall EC, Everett DB, Mukaka M, Bar‐Zeev N, Feasey N, Jahn A, et al. Bacterial meningitis in Malawian adults, adolescents, and children during the era of antiretroviral scale‐up and Haemophilus influenzae type B vaccination, 2000‐2012. Clinical Infectious Diseases 2014;58(10):e137‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV‐infected adults, adolescents and children: World Health Organization. December 2011. Available from apps.who.int/iris/bitstream/handle/10665/44786/9789241502979_eng.pdf?sequence=1. [PubMed]
WHO 2016
- World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd edition. World Health Organization. 2016. Available from apps.who.int/iris/bitstream/handle/10665/208825/9789241549684_eng.pdf?sequence=1.
Williamson 2017
- Williamson PR, Jarvis JN, Panackal AA, Fisher MC, Molloy SF, Loyse A, et al. Cryptococcal meningitis: epidemiology, immunology, diagnosis and therapy. Nature Reviews. Neurology 2017;13(1):13‐24. [PUBMED: 27886201] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chang 2004
- Chang LW, Phipps WT, Rutherford GW. Antifungal interventions for the primary prevention of cryptococcal disease in adults with HIV. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004773] [DOI] [PubMed] [Google Scholar]
Chang 2005
- Chang LW, Phipps WT, Kennedy GE, Rutherford GW. Antifungal interventions for the primary prevention of cryptococcal disease in adults with HIV. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD004773.pub2] [DOI] [PubMed] [Google Scholar]


